UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
(Mark One)
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES ACT OF 1934 |
OR
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report: ______________________________
For the transition period from___________________to___________________
Commission file number:
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant’s name into English)
(Jurisdiction of incorporation)
(Address of principal executive offices)
E-mail:
Telephone: +
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered, pursuant to Section 12(b) of the Act
Title of each class |
| Trading Symbol(s) |
| Name of each exchange on which registered |
representing three ordinary shares | The | |||
The |
* | Not for trading, but only in connection with the listing of the American depositary shares on The NASDAQ Stock Market LLC. |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report:
As of December 31, 2022, there were
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
☐ Yes ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
☐ Yes ☒
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ | Accelerated filer ☐ | Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act.
† | The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012. |
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ | Other ☐ | |||
by the International Accounting Standards Board ☒ |
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
☐ Yes ☐ No
TABLE OF CONTENTS
| Page | ||
ii | |||
iv | |||
1 | |||
1 | |||
1 | |||
1 | |||
47 | |||
47 | |||
95 | |||
110 | |||
121 | |||
124 | |||
125 | |||
125 | |||
137 | |||
138 | |||
140 | |||
140 | |||
Material Modifications to the Rights of Security Holders and Use of Proceeds | 140 | ||
141 | |||
142 | |||
142 | |||
143 | |||
143 | |||
Purchases of Equity Securities by the Issuer and Affiliated Purchasers | 143 | ||
143 | |||
143 | |||
143 | |||
144 | |||
144 | |||
144 | |||
145 | |||
i
INTRODUCTION
Unless otherwise indicated or the context otherwise requires, references in this annual report to:
| ● | “ADSs” are to our American depositary shares, each of which represents three of our ordinary shares; |
| ● | “ADV” are to Peak Asia Investment Holdings V Limited and/or its affiliates as the case may be; |
| ● | “AIH” are to Aesthetic Medical International Holdings Group Limited, a Cayman Islands exempted company; and we” “us” “our company” “the Company” and “our” are to Aesthetic Medical International Holdings Group Limited and its subsidiaries, and in the context of describing our consolidated financial information; |
| ● | “China” or the “PRC” are to the People’s Republic of China, including Hong Kong, Macau and Taiwan, unless when refer to specific laws and regulations adopted by Mainland China; and “Mainland China” are to the People’s Republic of China excluding Hong Kong, Macau and Taiwan; |
| ● | “Contractual Arrangements” are to a series of contractual arrangements entered into by us with Dr. Zhou Pengwu, Shenzhen Pengai Hospital Investment Management Co., Ltd., the Relevant Subsidiaries and Ms. Ding Wenting in 2018, 2019, 2020, 2021, 2022 with respect to the Target Equity Interests, as detailed in “Item 4. Information on the Company—4.A. History and Development of the Company—Contractual Arrangements with respect to Target Equity Interests”; |
| ● | “Hawyu” are to Hawyu (HK) Limited and/or its affiliates as the case may be; |
| ● | “Jiechuang” are to Hainan Oriental Jiechuang Investment Partnership (Limited Partnership) and/or its affiliates as the case may be; |
| ● | “Lafang” are to Lafang China Co., Ltd. and/or its affiliates as the case may be; |
| ● | “MY Universe” are to MY Universe (HK) Limited and/or its affiliates as the case may be; |
| ● | “Wanda” are to Australia Wanda International Company Limited and/or its affiliates as the case may be; |
| ● | “Relevant Subsidiaries” are to, collectively, Shenzhen Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd., Guangzhou Pengai Aesthetic Medical Hospital Co., Ltd., Guangzhou Pengai Xiuqi Aesthetic Medical Clinic Co., Ltd., Shanghai Pengai Aesthetic Medical Outpatient Department Co., Ltd., Shanghai Jiahong Aesthetic Medical Outpatient Department Co., Ltd., Yantai Pengai Cosmetic Surgery Hospital Co., Ltd., Beijing Aomei Yixin Investment Consultant Co., Ltd., and Beijing Aomei Yixin Investment Consultant Co., Ltd. Pengai Aesthetic Medical Clinic (collectively “Relevant Subsidiaries”), which were held by Dr. Zhou Pengwu directly or indirectly as to 27%, 26%, 19%, 12%, 24%, 25% and 25% respectively, as of December 31, 2022. Changsha Pengai Aesthetic Medical Hospital Co., Ltd. and Nanchang Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd. were divested in October 2022 and Hangzhou Pengai Aesthetic Medical Outpatient Department was ceased operation in August 2022 due to underperformance. Shenzhen Miaoyan Aesthetic Medical Clinic was divested due to impact of COVID-19 pandemic in Shenzhen in December 2022. |
| ● | “RMB” and “renminbi” are to the legal currency of Mainland China; |
| ● | “shares” or “ordinary shares” are to our ordinary shares, par value US$0.001 per share; |
| ● | “Target Equity Interests” are to these equity interests held by Dr. Zhou Pengwu in the Relevant Subsidiaries, which constitute all of Dr. Zhou Pengwu’s shareholdings in the Relevant Subsidiaries as of December 31, 2022. |
| ● | “US$,” “U.S. dollars,” “$,” and “dollars” are to the legal currency of the United States; and |
ii
We present our financial results in RMB. We make no representation that any RMB or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or RMB, as the case may be, at any particular rate, or at all. The PRC government imposes control over its foreign currency reserves in part through direct regulation of the conversion of RMB into foreign exchange and through restrictions on foreign trade. This annual report contains translations of certain foreign currency amounts into U.S. dollars for the convenience of the reader. Unless otherwise specified, all translations of renminbi amounts into U.S. dollar amounts in this press release are made at RMB6.8972 to US$1.00, which was the exchange rate on December 30, 2022 as set forth in the H.10 statistical release of the Board of Governors of the Federal Reserve System.
We completed an initial public offering of our ADSs at an initial offering price of US$12.00 per ADS on October 29, 2019. On January 19, 2023, we transferred the listing of our ADSs from the Nasdaq Global Market to the Nasdaq Capital Market. The ADSs, each representing three of our ordinary shares, par value US$0.001 per share, are traded on the Nasdaq Capital Market under the symbol “AIH.”
iii
FORWARD-LOOKING INFORMATION
This annual report contains forward-looking statements that reflect our current expectations and views of future events. All statements other than statements of current or historical facts are forward-looking statements. These forward-looking statements are made under the “safe harbor” provisions of the U.S. Private Securities Litigations Reform Act of 1995.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,” “potential,” “continue” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include statements relating to:
● | our business prospects, trends and conditions in the industry and markets in which we operate; |
● | the potential impact of an infectious disease caused by a novel coronavirus (“COVID-19”) to our business operations and the economy in China and elsewhere generally; |
● | our goals, business strategies and plans to achieve these strategies; |
● | our expected growth in, and market size of, our services in the markets we operate; |
● | expected changes in our revenue, costs and expenditures; |
● | our ability to offer new services in the markets and the industry we operate; |
● | our ability to continue to develop new technologies and/or update our existing technologies; |
● | growth of and trends of competition in our industry; |
● | general economic, political and business conditions in the markets in which we operate; |
● | changes to the regulatory environment and general outlook in the industry and markets in which we operate; |
● | the performance of the global financial markets, including changes in our ability to access the capital markets and changes in the levels of interest rates; |
● | our liquidity and financial condition; |
● | our relationship with, and other conditions affecting, our customers; |
● | our expectation regarding the use of the remainder of proceeds from our initial public offering; |
● | our dividend policy; and |
● | other factors beyond our control. |
These factors should not be construed as exhaustive and should be read with the other cautionary statements in this annual report, including but not limited to those listed under “Item 3. Key Information—3.D. Risk Factors.”
iv
These forward-looking statements involve various risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. We operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for our management to predict all risk factors and uncertainties, nor can we assess the impact of all factors on our business. Our actual results could be materially different from our expectations. Important risks and factors that could cause our actual results to be materially different from our expectations are generally set forth in this annual report. You should read thoroughly this annual report and the documents that we refer to with the understanding that our actual future results may be materially different from and worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements.
This annual report contains certain data and information that we obtained from various government and private publications. Statistical data in these publications also include projections based on a number of assumptions. Our industry may not grow at the rate projected by market data, or at all. Failure of this market to grow at the projected rate may have a material and adverse effect on our business and the market price of our ADSs. In addition, the rapidly evolving nature of our industry results in significant uncertainties for any projections or estimates relating to the growth prospects or future condition of our market. Furthermore, if any one or more of the assumptions underlying the market data are later found to be incorrect, actual results may differ from the projections based on these assumptions. You should not place undue reliance on these forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. The forward- looking statements made in this annual report relate only to events or information as of the date on which the statements are made in this annual report. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this annual report and the documents that we refer to in this annual report and have filed as exhibits to the registration statement, of which this annual report is a part, completely and with the understanding that our actual future results may be materially different from what we expect.
v
PART I.
Item 1.Identity of Directors, Senior Management and Advisers
Not applicable.
Item 2.Offer Statistics and Expected Timetable
Not applicable.
Item 3.Key Information
Our Holding Company Structure and Their Respective Individual Shareholders
AIH is not a Chinese operating company but a Cayman Islands holding company with operations conducted by its subsidiary, Peng Yida Business Consulting (Shenzhen) Co., Ltd. and through contractual arrangements with individuals and entities based in China and this structure involves unique risks to investors. Investors are purchasing securities of a Cayman Islands holding company rather than securities of our subsidiaries that have substantive business operations in China and the Cayman Island holding company controls our subsidiaries in China primarily by direct equity ownership of up to, but not including, 70.0% of the share interests of the subsidiaries.
Chinese law imposes various restrictions on the direct foreign investment in PRC companies, including a limitation of 70% or less of equity interests or the rights held by foreign entities in PRC medical institutions. Consequently, contractual arrangements are entered into to provide investors with more exposure to foreign investment in seven Relevant Subsidiaries. We own 65% or more direct equity interest in each of the Relevant Subsidiaries and as a result we are able to control and consolidate such subsidiaries as a result of our direct majority equity ownership, among other things, and do not depend on the contractual arrangements in relation to the minority equity interest to control and consolidate such subsidiaries. See “Item 4. Information on the Company—4.A. History and Development of the Company—Contractual Arrangements with respect to Target Equity Interests” in this annual report. Nevertheless, the contractual arrangements related to Target Equity Interest, involves unique risks to investors in the ADSs and ordinary shares of our Company. Such contractual arrangements have not been tested in court. The regulatory authorities in China could disallow such arrangements, which may have negative impact on our business and reduce the value of our securities. Contractual arrangements may not be as effective as direct ownership. See “Item 3. Key Information—3.D. Risk Factors - Risks relating to our corporate structure – If the PRC government deems that the Contractual Arrangements do not comply with PRC laws and regulations, or if these laws and regulations or their interpretations change in the future, we could be subject to severe penalties or be forced to relinquish our interests received through the Contractual Arrangements” “ – The Contractual Arrangements may not be as effective in providing control as direct ownership and Relevant Subsidiaries or Dr. Zhou Pengwu may fail to perform their respective obligations under the Contractual Arrangements” and “ – Any failure by our Relevant Subsidiaries and their other respective shareholders, including Dr. Zhou Pengwu, to perform their obligations under our contractual arrangements with them would have a material adverse effect on our business” in this annual report.
As we are a holding company with substantive business operations in China, you should pay special attention to other disclosures included in this annual report and risk factors included herein. In particular, any failure of us to fully comply with new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer the ADSs, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause the ADSs to significantly decline in value or become worthless. See “Item 3. Key Information – 3.D. Risk Factors – Uncertainties regarding the enforcement of laws and that rules and regulations in China can change quickly with little advance notice, which could materially and adversely affect us.” In addition, the PRC government has significant authority to exert influence on the ability of a company with substantive operations in China, such as us, to conduct its business, accept foreign investments or list on a U.S. or other foreign exchanges. For example, we face risks associated with regulatory approvals of offshore offerings, anti-monopoly regulatory actions, oversight on cybersecurity and data privacy. Due to our business operations in Hong Kong, the regulatory actions related to data security or anti-monopoly concerns in Hong Kong may also impact our ability to conduct our business, accept foreign investments or list on a U.S. or foreign exchange. See “Item 3. Key Information – 3.D. Risk Factors – Risks Related to Doing Business in China.
1
In this annual report, “AIH” refer to Aesthetic Medical International Holdings Group Limited, and “we” “us” “our company” “the Company” and “our” refer to Aesthetic Medical International Holdings Group Limited and its subsidiaries, and in the context of describing our consolidated financial information.
The Holding Foreign Companies Accountable Act
In particular, the Public Company Accounting Oversight Board (“PCAOB”) is currently unable to inspect our auditor in relation to their audit work performed for our financial statements and the inability of the PCAOB to conduct inspections over our auditor deprives our investors with the benefits of such inspections. Our ADSs will be prohibited from trading in the United States under the Holding Foreign Companies Accountable Act, or the HFCAA, if the PCAOB is unable to inspect or fully investigate auditors located in China. On December 2, 2021, the U.S. Securities and Exchange Commission, or the SEC, adopted final amendments implementing the disclosure and submission requirements of the HFCAA, pursuant to which the SEC will identify an issuer as a “Commission Identified Issuer” if the issuer has filed an annual report containing an audit report issued by a registered public accounting firm that the PCAOB has determined it is unable to inspect or investigate completely, and will then impose a trading prohibition on an issuer after it is identified as a Commission-Identified Issuer for three consecutive years. On December 16, 2021, PCAOB issued the HFCAA Determination Report, according to which our auditor is subject to the determinations that the PCAOB is unable to inspect or investigate completely. In May 2022, in connection with its implementation of the HFCAA, the SEC conclusively named our Company as a “Commission-Identified Issuer” following the filing of our annual report on Form 20-F for fiscal year 2021 with the SEC on May 16, 2022. In June 2021, the Senate passed the Accelerating Holding Foreign Companies Accountable Act (the “AHFCAA”), which was signed into law on December 29, 2022, amending the HFCAA and requiring the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchange if its auditor is not subject to PCAOB inspections for two consecutive years instead of three consecutive years. On August 26, 2022, the PCAOB announced that it had signed a Statement of Protocol (the “SOP”) with the China Securities Regulatory Commission and the Ministry of Finance of China. The SOP, together with two protocol agreements governing inspections and investigations, establishes a specific, accountable framework to make possible complete inspections and investigations by the PCAOB of audit firms based in mainland China and Hong Kong, as required under U.S. law. On December 15, 2022, the PCAOB announced that it was able to secure complete access to inspect and investigate PCAOB-registered public accounting firms headquartered in mainland China and Hong Kong completely in 2022. The PCAOB Board vacated its previous 2021 determinations that the PCAOB was unable to inspect or investigate completely registered public accounting firms headquartered in mainland China and Hong Kong. However, whether the PCAOB will continue to conduct inspections and investigations completely to its satisfaction of PCAOB-registered public accounting firms headquartered in mainland China and Hong Kong is subject to uncertainty and depends on a number of factors out of our, and our auditor’s, control, including positions taken by authorities of the PRC. The PCAOB is expected to continue to demand complete access to inspections and investigations against accounting firms headquartered in mainland China and Hong Kong in the future and states that it has already made plans to resume regular inspections in early 2023 and beyond. The PCAOB is required under the HFCAA to make its determination on an annual basis with regards to its ability to inspect and investigate completely accounting firms based in the mainland China and Hong Kong. The possibility of being a “Commission-Identified Issuer” and risk of delisting could continue to adversely affect the trading price of our securities. Should the PCAOB again encounter impediments to inspections and investigations in mainland China or Hong Kong as a result of positions taken by any authority in either jurisdiction, the PCAOB will make determinations under the HFCAA as and when appropriate. See “Risk Factors – Risks Related to Doing Business in China – Our ADSs will be prohibited from trading in the United States under the Holding Foreign Companies Accountable Act, or the HFCAA, in 2024 if the PCAOB is unable to inspect or fully investigate auditors located in China, or in 2023 if the proposed changes to the law are enacted AHFCAA is enacted. The delisting of our ADSs, or the threat of their being delisted, may materially and adversely affect the value of your investment. In May 2022, in connection with its implementation of the HFCAA, the SEC conclusively named our Company as a ‘Commission-Identified Issuer’ following the filing of our annual report for fiscal year 2021 on Form 20-F with the SEC on May 16, 2022.”
The PRC government has recently published new policies that significantly affected certain industries, and we cannot rule out the possibility that it will in the future release regulations or policies regarding the industry where we operate, which could adversely affect our business, financial condition and results of operations. Furthermore, the PRC government has recently indicated an intent to exert more oversight and control over overseas securities offerings and other capital markets activities and foreign investment in China-based companies like us. These risks could result in a material change in our operations and the value of our ordinary shares or the ADSs, or could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or become worthless. For more information on various risks related to doing business in China, see “Item 3. Key Information—3.D. Risk Factors” section.
2
As of the date of this prospectus, AIH, our Cayman Islands holding company and other subsidiaries of our Company have not received any inquiry, notice, warning or sanctions regarding the offering from the CSRC or any other PRC governmental authorities. However, since these statements and regulatory actions by the PRC government are newly published and detailed official guidance and related implementation rules have not been issued or taken effect, it is uncertain how soon the regulatory bodies in Mainland China will finalize implementation measures, and the impact the modified or new laws and regulations will have on the daily business operations of our PRC subsidiaries, our ability to accept foreign investments and list on an U.S. or other foreign exchange. For more information on various risks related to doing business in China, see “Item 3. Key Information—3.D. Risk Factors” section. As of the date of this annual report, AIH, our Cayman Islands holding company and other subsidiaries of our Company have not received any inquiry, notice, warning or sanctions regarding the offering from the CSRC or any other PRC governmental authorities. However, since these statements and regulatory actions by the PRC government are newly published and detailed official guidance and related implementation rules have not been issued or taken effect, it is uncertain how soon the regulatory bodies in China will finalize implementation measures, and the impact the modified or new laws and regulations will have on the daily business operations of our PRC subsidiaries, our ability to accept foreign investments and list on an U.S. or other foreign exchange. For more information on various risks related to doing business in China, see the “Risk Factors” sections of this annual report.
Permissions Required from the PRC Authorities for Our Operations
We conduct our business primarily through our subsidiaries and consolidated affiliated entities in Mainland China. Our operations in China are governed by PRC laws and regulations. As of the date of this annual report, none of our PRC subsidiaries is required to obtain additional licenses or permits beyond a regular business license for their operations. Each of our PRC subsidiaries is required to obtain a regular business license from the local branch of the State Administration for Market Regulation. Each of our PRC subsidiaries has obtained a valid business license for its respective business scope, and no application for any such license has been denied.
3.A.Selected Financial Data
[Reserved]
3.B.Capitalization and Indebtedness
Not applicable.
3.C.Reasons for the Offer and Use of Proceeds
Not applicable.
3.D.Risk Factors
Summary Risk Factors
Our business is subject to a number of risks, including risks that may prevent us from achieving our business objectives or may adversely affect our business, financial condition, results of operations, cash flows, and prospects. These risks are discussed more fully below and include, but are not limited to:
Risks relating to our business and our industry
● | any future occurrence of force majeure events, natural disasters or outbreaks of contagious diseases in the PRC; |
● | our ability to obtain sufficient funding for our expansion plans; |
● | any decrease in supply or fluctuation in the cost of supplies; |
● | our ability to maintain proper inventory levels for our operations; |
3
● | the strength of our brand and reputation; |
● | increasing ongoing compliance costs in a heavily regulated industry; |
● | our compliance with PRC laws and regulations on medical advertisement; |
● | possible non-compliance incidents; |
● | customer complaints, claims and legal proceedings in the regular course of our operations from time to time; |
● | our ability to recruit and retain an adequate number of managers, doctors, nurses, image consultants and other support staff in our treatment centers; |
● | our ability to maintain the quality of the pharmaceuticals, medical equipment, medical supplies, injection materials, skincare products, implants and consumables we use; and |
● | unfavorable market perception of the overall aesthetic medical industry. |
Risks relating to our corporate structure
● | the Contractual Arrangements being compliant with PRC laws and regulations; |
● | the Contractual Arrangements being ineffective in providing control; |
● | potential conflicts of interest with Dr. Zhou Pengwu; |
● | the Contractual Arrangements being subject to scrutiny by the PRC tax authorities; and |
● | our PRC medical centers being deemed as “Sino-Foreign Equity Medical Institutions.” |
Risks relating to doing business in the PRC
● | increased labor costs and potential non-compliance caused by the enforcement of the PRC Labor Contract Law; |
● | changes in the PRC’s economic, political and social conditions, as well as governmental policies; |
● | uncertainties with respect to the PRC legal system and changes in laws, regulations and policies in China; |
● | changes in international trade policies and barriers to trade or the emergence of a trade war; |
● | potential liabilities under the FCPA, Chinese anti-unfair competition laws and relevant tax laws; |
● | restrictions on currency exchange; |
● | potential liability or penalties and limitation on our ability to inject capital into these subsidiaries due to PRC regulations relating to investments in offshore companies by PRC residents; and |
● | the risks related to the enactment of the Holding Foreign Companies Accountable Act. |
Risks relating to the ADSs
● | our broad discretion to determine how to use the remaining net proceeds from our initial public offering; |
4
● | the ability by our chief executive officer and his spouse to control and exert significance influence over our company; |
● | provisions in our fourth amended and restated articles of association (as revised) limiting your ability to influence corporate matters; |
● | our reliance on exemptions from certain corporate governance requirements due to our “controlled company” status; and |
● | an increased risk of securities class action litigation. |
In addition, our corporate structure and being based in and having the majority of our operations in China pose following significant regulatory, liquidity and enforcement risks to investors, which are described in further details in the “Risk Factors” section of this annual report, including risks that any actions by the Chinese government to exert more oversight and control over securities that are listed overseas or foreign investment in China-based issuers could significantly limit or completely hinder our ability to continue to offer securities to investors and cause the value of our securities to significantly decline or be worthless. See “Risk Factors”.
Risks relating to our business and our industry
Any future occurrence of force majeure events, natural disasters or outbreaks of contagious diseases in the PRC could prevent us from effectively serving our customers and thus adversely affect our results of operations.
Any occurrence of force majeure events, natural disasters or outbreaks of epidemics, including those caused by avian influenza or swine influenza, may restrict business activities in the areas affected and materially and adversely affect our business and results of operations. Since early 2013, there have been outbreaks of highly pathogenic avian flu, caused by the H7N9 virus, in certain parts of China, and in early 2009, there were reports of outbreaks of a highly pathogenic swine flu, caused by the H1N1 virus, in certain regions of Asia and Europe.
An outbreak of COVID-19 continues to spread within the PRC and globally. The new strain of coronavirus is considered highly contagious and may pose a serious public health threat. On January 30, 2020, the World Health Organization reportedly declared this COVID-19 outbreak a health emergency of international concern. In March 2020, the World Health Organization declared the COVID-19 a pandemic. Since the COVID-19 outbreak, the PRC government imposed various strict measures with the aim to contain the virus including, but not limited to, travel restrictions, mandatory quarantine requirements, and postponed resumption of business operations. In response to the outbreak of COVID-19, we postponed the resumption of operations of aesthetic treatment centers in China after the Chinese New Year holiday in 2020. During the temporary shutdown of our treatment centers, we could not carry out any business procedures. Since late February 2020, we have actively taken proper precautionary measures to contain the spread of the pandemic, and gradually re-opened our aesthetic medical treatment centers. By the end of March 2022, all of our aesthetic treatment centers had resumed their operations. We also adopted a thorough disease prevention scheme and implemented measures including, but not limited to, regularly sterilizing and ventilating our facilities, staggering employee lunch time, monitoring the body temperature of employees, and keeping track of the travel history and health condition of employees and their immediate family members. We also provided COVID-19 prevention training sessions to our employees and procured essential disease prevention supplies to ensure the safety of all employees, maintain the normal operation of our treatment centers, and protect the well being of our customers. We have applied these COVID-19 prevention measures consistently throughout 2021 and up till the measures of COVID-19 were adjusted from Class A infectious diseases to Class B infectious diseases in December 2022.
In October 2022, we divested two underperforming treatment centers, Changsha Pengai Aesthetic Medical Hospital Co., Ltd. and Nanchang Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd., and received cash inflows of RMB4.6 million and RMB3.0 million, respectively. In December 2022, we also divested Shenzhen Miaoyan Aesthetic Medical Clinic, another underperforming treatment center without cash inflows. In August 2022, we ceased the operations of Hangzhou Pengai Aesthetic Medical Outpatient Department due to underperformance.
5
The COVID-19 pandemic has had a material adverse impact on our business, financial condition and results of operations. Our results of operations and overall operating conditions improved as the COVID-19 pandemic was largely contained in China. Nonetheless, we expect the COVID-19 pandemic to continue to have an impact on our business, financial condition and results of operations given the uncertainties surrounding the COVID-19 pandemic. We cannot assure you that the COVID-19 pandemic could be eliminated or fully contained in the near future, or that similar outbreaks would not occur at all. If COVID-19 and its resulting disruption to our business continue over an extended period of time, it could have a material adverse impact on our business, financial condition and results of operations in the long run. For discussion on liquidity and capital resources, see “Item 5. Operating and Financial Review and Prospects—5.B. Liquidity and Capital Resources.”
As of the date of this annual report, the COVID-19 pandemic has been largely under control in China, although cases of infection still come up from time to time. However, the global spread of the COVID-19 pandemic still persists in many countries. We are closely monitoring the development of the COVID-19 pandemic and continuously evaluating any further potential impact on our business, results of operations and financial condition, which we believe will depend on the duration and degree of the pandemic. If the outbreak persists or escalates, we may be subject to further negative impact on our business operations and financial condition.
Moreover, the PRC has experienced natural disasters like earthquakes, floods and droughts in the past few years. Any future occurrence of several natural disasters in the PRC may materially and adversely affect its economy and therefore our business. An outbreak of contagious diseases, and other adverse public health developments in China, would have a material adverse effect on our business operations. These could include restrictions on our ability to provide services to our customers, as well as cause temporary closure of our treatment centers. Such closures or service restrictions would severely disrupt our operations and adversely affect our financial condition and results of operations. We have not adopted any written preventive measures or contingency plans to combat any future occurrence of force majeure events, natural disasters or outbreaks of contagious diseases.
If we fail to obtain sufficient funding for our expansion plans, our business and growth prospects may be adversely affected.
Since the COVID-19 virus could be spread to the countryside and cause a second wave of infections, we expect the COVID-19 pandemic to continue to subject us to uncertainties, which may negatively affect our future cash flow in to an extent that we cannot predict at this time, and we can only try our best to continue to maintain and improve our cash flow. For discussion of liquidity and capital resources, see “Item 5. Operating and Financial Review and Prospects—5.B. Liquidity and Capital Resources.” However, we have considered our cash flow from future operations and available borrowing facilities to conclude that we have sufficient financial resources to meet its financial obligations as and when they fall due in the coming 12 months. Given our current credit status and the current availability of capital, we believe that we will not encounter any major difficulties in obtaining additional borrowings. We may, however, require additional cash resources to finance our continued growth or other future developments, including any marketing initiatives or investments we may decide to pursue. The amount and timing of such additional financing needs will vary depending on the timing of our new treatment centers openings, investments in acquired treatment centers and the amount of cash flow from our operations. If our resources are insufficient to satisfy our cash requirements, we may seek additional financing. To the extent that we raise additional financing by selling additional equity, our shareholders may experience dilution. To the extent we engage in debt financing, the incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that may, among other things, restrict our operations or our ability to pay dividends. Servicing such debt obligations could also be burdensome to our operations. If we fail to service the debt obligations or are unable to comply with such debt covenants, we could be in default under the relevant debt obligations and our liquidity and financial conditions may be materially and adversely affected.
Our ability to obtain additional capital on acceptable terms is subject to a variety of uncertainties, some of which are beyond our control, including general economic and capital market conditions, credit availability from banks or other lenders, the consents of our prior creditors, receipt of necessary PRC government approvals, investors’ confidence in us, the performance of the aesthetic medical treatment industry in general, and our operating and financial performance in particular. We cannot assure you that future financing will be available in amounts or on terms acceptable to us, if at all. In the event that financing is not available or is not available on terms acceptable to us, our business, results of operations and growth prospects may be adversely affected.
6
We have not entered into any long-term supply agreements with our suppliers. A decrease in supply, or an increase in the cost, or quality supplies may adversely affect our business, financial condition and results of operations.
For the years ended December 31, 2020, 2021 and 2022, our cost of inventories and consumables amounted to RMB163.9 million, RMB175.0 million, and RMB160.2 million (US23.2 million), respectively, representing 45.8%, 46.5% and 50.9% of our total cost of sales and services rendered for the same periods, respectively. Consistent with industry practice, we have not entered into any long-term supply agreements with our suppliers and we cannot assure you that our suppliers will continue to supply to us on commercially reasonable terms, or at all. If any of our suppliers fail to supply sufficient quantities or experience fluctuations in market prices of the same, for example, due to temporary operation suspension caused by the outbreak of COVID-19, we may have to obtain replacements for such supplies from alternate suppliers. We cannot assure you that we will be able to do so in a timely manner at commercially reasonable terms. Any such disruption in supply may adversely affect the operations of our treatment centers, which may in turn adversely affect our business, results of operations, financial condition and prospects. In addition, should the prices of supplies increase significantly, we cannot assure you that we would be able to pass on any increase in purchase costs to our customers. Any substantial fluctuation in market prices of the supplies required in our operations may significantly increase our costs, resulting in us reducing, suspending or ceasing provision of certain types of services, thereby reducing our sales and profit.
Although the price of most comsumables historically remained flat, we cannot guarantee that a shortage of supply will not arise in the future, or that our suppliers will not increase their prices. In the event that we are not able to source from any supplier on commercially reasonable terms, or at all, our business, financial condition and results of operation will be adversely affected.
We may not be able to maintain proper inventory levels for our operations.
To ensure adequate inventory supply, we must forecast inventory needs and place orders with our suppliers based on our estimates of future demand for particular products. We may not be able to accurately forecast demand for supplies because of the difficulties of estimating the demand for our services. In 2020, 2021 and 2022, our average inventory turnover days were 31.3 days, 30.6 days and 33.4 days, respectively.The volatile economic environment and fast-evolving demands and preferences of our customers have made accurate projection of inventory levels increasingly challenging.
Inventory levels in excess of customer demand may result in inventory obsolescence, a decline in inventory values, inventory write-downs or write-offs, or expiration of products, which would cause our gross margin to suffer and could impair the strength of our brand. High inventory levels may also require us to commit substantial capital resources, preventing us from using them for other important business purposes. Conversely, if we underestimate customer demand or if our suppliers fail to provide supplies to us in a timely manner, we may experience inventory shortages. Such inventory shortages might result in unfilled customer needs, damage to our reputation, and have a negative impact on customer relationships and reduce our sales. We cannot assure you that we will be able to maintain proper inventory levels for our operations and such failure may have an adverse effect on our business, financial condition, results of operations and prospects.
We depend significantly on the strength of our brand and reputation, and any damage to our brand or reputation could materially and adversely impact our business and results of operations.
Our brand and reputation are critical to our success in China’s rapidly growing aesthetic medical industry. We believe that our success and continued growth depends on the public perception of our brand name and our ability to protect and promote our brand name. Many factors which are important to help maintain and enhance our brand, are beyond our control and may negatively impact our brand and reputation. Such factors include:
● | our ability to effectively control the quality of the services performed by our doctors and other medical staff, and to monitor the performance of such personnel as we continue to expand; |
● | our ability to maintain a convenient, standardized and reliable customer experience as customer preferences evolve and as we expand our service offerings; and |
● | our ability to increase brand awareness among existing and potential customers through various means of marketing and promotional activities. |
7
Our failure to develop, maintain and enhance our brand and reputation may materially and adversely affect the level of market recognition of, and trust in, our services, which could result in decreased sales and loss of customers leading to a material adverse effect on our results of operations and cash flows.
In addition, allegations against us have appeared in online forums and news articles. These allegations have included claims of medical malpractice, dissatisfaction with treatment results, inappropriate sales tactics, arbitrary treatment service prices and the use of illegal pharmaceuticals. Any negative review, comment or allegation regarding our company, treatment centers or services by the media, our customers, our former employees or the public in the media or on online social networks may harm our brand, public image and reputation, which in turn may result in a loss of customers and business partners and have a material adverse effect on our business, financial condition, results of operations and prospects.
Furthermore, our customers may have expectations regarding the degree of improvement of their physical appearance resulting from our services. However, we cannot guarantee the results of our services since results vary depending on factors such as the medical history of our customers, their adherence to our pre-procedure and post-procedure instructions, their respective responses to procedures, unknown allergies and other factors beyond our control. It is also an inherent risk that the results of our services may lead to undesirable or unexpected outcomes, such as complications and injuries, or otherwise fail to meet our customers’ expectations. Such undesirable or unexpected outcomes may result in customer dissatisfaction, requests for refunds, or complaints, claims or legal actions against us, which may lead to negative publicity. Any negative publicity may adversely harm our brand image and reputation and cause a deterioration in the level of market recognition of and trust in our services, thereby resulting in decreased sales and potential loss of customers and business partners as well as physicians and staff, and therefore have a material adverse effect on our business, results of operations, financial condition and prospects.
8
We conduct our business in a heavily regulated industry and incur ongoing compliance costs as well as face penalties for non-compliance.
We conduct our business in a heavily regulated industry. The rules and regulations relate mainly to the licensing of treatment centers, the quality and the licensing of medical facilities, equipment and services, the pricing, procurement and usage of pharmaceuticals, and the licensing, practice and number of medical professionals. For more details, please see the section headed “Item 4. Information on the Company—4.B. Business Overview” in this annual report. Accordingly, our treatment centers are subject to periodic licensing renewal requirements and inspections by various government agencies and departments at the provincial and municipal level. In addition, any changes in laws and regulations could require us to obtain additional licenses, permits, approvals or certificates, impose additional conditions or requirements for the renewal of the licenses of the treatment centers, or result in the invalidation of our currently owned licenses.To comply with national and regional policies and regulations of the aesthetic medical industry implemented by the PRC government in 2021, we upgraded our medical risk control system, which included 1) established a supervisory team to update the Board the progress of risk control progress of the treatment centers; 2) strengthened the allocation of medical staffs, medical workplaces and facilities for various treatment operations, as well as the supervision and management of the operation procedures; 3) improved the emergency response procedures of each treatment center, and would regularly carry out drills; and 4) set up self-examination team and reward and penalty mechanism at each treatment center to review the surgical and customer records, ensuring operational compliance.
In the past, some of our treatment centers were subject to administrative warnings and penalties due to certain non-compliance incidents. Although we took measures to improve the internal control on the procurement of medical facilities, as a result of the growth in our operations and the increasing number of treatment centers we manage, as well as the tightened PRC laws for consumer protection, we have experienced, and will continue to experience, non-compliance incidents. For instance, several of our treatment centers were subject to administrative penalties due to their failure to comply with certain regulations on medical advertisements, and several of our treatment centers were subject to administrative penalties due to the medical professionals’ malpractice, operation beyond the permitted scope of licenses or without licenses, use of unqualified medical facilities or pharmaceuticals, use of expired disinfectant, non-compliance with regulations on disposal of medical waste, failure to evaluate occupational hazards, non-compliance with statistic regulations, non-compliance with fire protection regulations, failure to timely file the required annual report of certain treatment centers to the relevant local branch of the State Administration of Market Regulation, failure to meet standards of hygiene, failure to fulfill inspection requirements of the licensing and medical facilities, and non-compliance with tax regulations, failure to meet the standard of drafting and preservation of medical case record, non-compliance with regulations on prevention and handling of medical disputes and non-compliance with regulations on publicity of service items and fees. In addition, certain of our treatment centers may be subject to administrative penalties due to failure to comply with fire code and environmental regulations. Failure to comply with fire protection design review and inspection requirements could lead to the temporary closure of certain treatment centers. Also, certain information concerning some foreign doctors (if any) might be published on the website of one of our treatment centers, which could have inadvertently created the impression that those foreign doctors were employed by us.
In addition, we have experienced incidents of non-compliance which did not result in administrative warnings or penalties but could adversely impact our reputation, create additional compliance costs for us or otherwise impair our business and operations. For example, while we require that all human placenta extract products procured and used in our treatment centers are domestically produced, registered by the National Medical Products Administration, or the NMPA, which is formerly know as China Food and Drug Administration, and legally compliant, we have had instances in the past where certain of our treatment centers used imported human placenta extract products that were unregistered with the NMPA.
In June 2018, the business license of our treatment center in Shanghai was temporarily suspended for performing dental implants without the appropriate license, which we promptly rectified.
We cannot guarantee that any future incidents of non-compliance would not result in temporary or permanent suspension of any of the licenses, permits, approvals and certificates necessary for our business. If we fail to obtain or renew any necessary licenses, permits, approvals and certificates, or are found to be non-compliant with any of these laws, regulations or rules, we may be unable to provide relevant medical services, suffer reputational harm, face penalties, suspension of operations or even revocation of operating licenses or criminal liability, depending on the nature of the findings, any of which could materially and adversely affect our business, financial condition and results of operations.
9
If we are unable to fully comply with PRC laws and regulations on medical advertisement, our brand image, results of operations and financial conditions could suffer significantly.
As a medical aesthetic service provider, we must comply with the PRC Advertisement Law, the PRC Administrative Measures on Medical Advertisement and other relevant advertising laws and regulations and constantly monitor our advertising content. According to the Administrative Measures on Medical Advertisement and the Notice on Further Strengthening the Management of Medical Advertisements, or the Administrative Measures on Medical Advertisement, we must obtain a medical advertisement approval certificate before publishing any medical advertisement. The content in the published advertisement shall be consistent with what has been approved and recorded in the medical advertisement approval certificate. In addition, the Administrative Measures on Medical Advertisement explicitly stipulate that such medical advertisements shall not include any specific treatment method, any guarantees of the treatment, any name or image of any patient, any particular medical professional, nor use any medical research institution or its personnel or any public association or organization to suggest any treatment is valid. For violations of these laws and regulations, the PRC competent health administrative authority and Chinese medicine administrative authority may issue warnings and require remediation. If the violations are more severe, they may impose measures such as suspension of business until the violations have been remedied, revocation of the license for operating a particular medical department, or even revocation of the Medical Institution Practicing License. In addition, the competent administration for industry and commerce (now known as the administration for market regulation) may also suspend the business and the business licenses of institutions that are repetitive and serious offenders in accordance with the PRC Advertisement Law. In 2021, the regulators has strengthened the supervision of the aesthetic medical industry and proposed various guidelines to ensure the healthy development of the aesthetic medical industry, including regulating advertisements as well as cracking down illegal manufacturing and sales of the aesthetic medical products. See “Item 4. Information on the Company—4.B. Business Overview—Regulation—Regulations on medical advertising in the PRC.”
In the past, some of our treatment centers have received administrative penalties for non-compliance with these laws and regulations. For instance, several of our treatment centers have been penalized for publishing medical advertisements without having obtained relevant medical advertisement approval certificates, not strictly following the scope and manners as approved and recorded in the relevant medical advertisement approval certificates, and publishing medical advertisements with expressions explicitly prohibited by these laws and regulations, publishing medical advertisements without showing the relevant licensing number on the relevant medical advertisement approval certificate, or publishing medical advertisements with untrue or misleading expressions. The results of such non-compliance actions ranged from warnings, fines, deduction of medical institution practice points, suspension of publication of advertisements, remediation, reduction of adverse impact and withdrawal of advertisement, among other things. Any violation of these laws and regulations on medical advertisements, if material or not rectified, may subject us to administrative penalties, impair our brand image, and materially and adversely impact our business, financial condition, results of operations and prospects.
Our internal control system and compliance team may not be able to prevent all possible non-compliance incidents.
We are subject to a number of regulations pertaining to the licensing of our treatment centers, the quality and the licensing of medical facilities, equipment and services, the pricing and procurement and usage of pharmaceuticals, and the licensing, conduct and number of medical professionals. We have established internal control process intended to ensure all of our employees and contractors comply with the relevant laws and regulations applicable to us. However, we cannot assure you that such controls will be effective to prevent all instances of non-compliance. Any failure of our internal controls could have a material adverse effect on our business, financial condition and results of operations.
10
We are subject to customer complaints, claims and legal proceedings in the regular course of our operations from time to time, which could result in significant costs and materially and adversely affect our brand image, reputation and results of operations.
We rely on our doctors and other medical staff in our treatment centers to make appropriate decisions regarding the treatment of our customers. However, we cannot assure you that every employee at our treatment centers will always act in accordance with the appropriate professional standard of care. Any deviation from the appropriate standard of care by our doctors and other medical staff, or any failure to properly manage our treatment centers’ activities, may result in unsatisfactory treatment outcomes, patient injuries or, in extreme cases, deaths. Given the nature of the aesthetic medical industry and subjectiveness of the level of satisfaction with services provided, we are also susceptible to other types of complaints associated with our services from time to time. These include claims relating to (i) dissatisfaction with our customer service, (ii) disputes over charges, (iii) over-promising of treatment outcome, (iv) dissatisfaction with post-treatment recovery periods, and (v) general dissatisfaction with treatment results. In addition, due to the fact that the number of procedures we performed has increased over the years as part of our growth, the absolute number of such complaints, allegations and other claims, regardless of merits, has increased and may continue to increase. Such complains, allegations and claims, if not managed properly, could have a material adverse effect on our reputation, business, results of operations, financial condition and prospects.
With respect to settlement of client complaints, customers generally accept complimentary gifts, refunds, services or supplemental operations at no additional cost to settle their complaints. However, we may also be required to pay monetary compensation to settle customer complaints and medical disputes. In addition, we may be subject to third-party liability claims and may be required to pay compensation to patients who suffer from unexpected adverse reactions to treatment received in our treatment centers, even if we were not at fault. As of the date of this annual report, we were named as the defendant in 11 ongoing litigations for our consolidated businesses in the PRC. While we believe many of the claims against us do not have merits, and plan to firmly defend our rights, we cannot assure that we will be able to achieve satisfactory outcome in those proceedings. The maximum amount of the damages claimed by the plaintiffs in those 11 proceedings in aggregate amounted to RMB18.2 million. Furthermore, some dissatisfied customers may even resort to extreme actions.
We may be subject to similar customer complaints, serious incidents or lawsuits in the future and may not successfully prevent or address all customer complaints in the future. Any complaint, claim or legal proceeding, regardless of merit, if widely disseminated, could affect our corporate image and reputation in the industry, divert management resources and cause us to incur extra costs to handle these complaints and litigation matters. A settlement or successful claim against us can also result in significant costs, damages, compensation and reputational damage and adversely affect our business, results of operations and financial condition.
If we are unable to recruit and retain an adequate number of managers, doctors, nurses, image consultants and other support staff in our treatment centers, our service quality and business strategy may suffer.
Our performance is largely dependent on the talent and efforts of highly skilled medical professionals. Our future success will in part depend on our ability to identify, hire and retain highly qualified medical professionals of all areas of our treatment centers. The recruitment of qualified physicians is competitive in the PRC due to their shortage. The near-term supply of specialist physicians is limited due to the length of training required, including academic study and clinical training, which can take more than eight years for certain medical specialties, as well as additional certification and licensing requirements for certain specialties. Competition for such qualified professionals is intense. We believe that physicians generally consider the following key factors when selecting medical institutions to join: reputation and culture, the efficiency of hospital management, the quality of facilities and support staff, the number of patient visits, compensation, training programs and location. Our treatment centers may not effectively compete with other aesthetic medical treatment centers or clinics in hiring qualified professionals. We use physicians who also practice at other hospitals or treatment centers, as PRC regulations allow licensed physicians to register and practice at multiple medical institutions in the same provincial administrative area. If the PRC government imposes restrictions on such practice in the future, we may not be able to retain our current base of multi-site practice physicians. If we are unable to successfully recruit or retain seasoned and qualified physicians, our business, financial condition and results of operations may be adversely affected.
Our success is also dependent on our ability to recruit and retain qualified medical institution administrators and medical professionals. It has become increasingly costly to recruit and retain medical professionals in recent years and there is no guarantee that we will be able to recruit and retain sufficient medical professionals in the future. If we do not succeed in attracting an appropriate number of qualified treatment center managers, nurses, image consultants or other support staff, our service quality and our ability to execute our business strategy may suffer. A shortage of skilled professionals could also require us to pay higher wages, which would reduce our profits and have a material and adverse effect on our operating results and financial performance.
11
If our physicians and other medical professionals do not obtain and maintain appropriate licenses, we may be subject to penalties against our treatment center, which could adversely affect our business.
Medical practice in China is strictly regulated. Physicians, nurses and medical technicians who practice at medical institutions must hold practicing licenses and may only practice within the scope of their licenses and at the specific medical institutions at which their licenses are registered. Please see “Item 4. Information on the Company—4.B. Business Overview—Regulation” for more details. In practice, it takes some time for physicians, nurses and medical technicians to transfer their licenses from one medical institution to another or add any further service scope or another medical institution to their permitted practicing institutions. From time to time, some of our physicians, nurses and other medical professionals could be required to make such amendments to their licenses due to changes to the location or nature of their work. We cannot assure you that all of our medical professionals have completed the transfer of their licenses and related government procedures in a timely manner or at all. In addition, we cannot assure you that our physicians, nurses and other medical professionals will always strictly follow the requirements and will not practice outside the permitted scope of their respective licenses. Our failure to properly manage the employment of our physicians, nurses and other medical professionals may subject us to administrative penalties against our treatment centers, which could adversely affect our business.
We may fail to maintain the quality of the pharmaceuticals, medical equipment, medical supplies, injection materials, skincare products, implants and consumables we use. If these products do not meet the required standards, we could be exposed to liabilities and our business operations and reputation could suffer.
Although we have adopted a series of measures for selecting suppliers, such as maintaining and updating a list of qualified suppliers, we cannot guarantee that all of the pharmaceuticals, medical equipment, medical supplies, injection materials, skincare products, implants and consumables we use are free of defects or substantially meet the relevant quality standards. We were involved in a lawsuit related to one of our patients who suffered severe complications after receiving a Botox injection at one of our treatment centers. Although the court found that all liability rested with the manufacturer, we cannot assure you that similar incidents will not occur in the future, or that such incidents will not materially and adversely affect us. Moreover, we launched new skincare products called The Four Beauty in January 2020, which are manufactured by a third party and sold in our treatment centers. We also launched 6 new non-surgical aesthetic medical treatment solutions with the support of new products and equipments in 2022. If the products provided by our suppliers are defective, of poor quality, or cause any adverse drug reaction, we could be subject to liability claims, complaints or related adverse publicity that could result in the imposition of penalties or even suspension of licenses by relevant authorities or compensation awarded by courts against us. We may also need to find suitable replacement products, which may lower our profit margins and result in delays in services to our customers.
Our suppliers are also subject to extensive laws and regulations. If our suppliers violate applicable laws and regulations, our reputation or procurement may be materially and adversely affected. PRC laws and regulations require us to procure materials from qualified suppliers, with requisite licenses, permits or filings to operate their business. We cannot ensure that all of our suppliers maintain all the licenses required or the validity of their licenses at all times. We have been fined with insignificant amounts by regulators in the past for failure to maintain proper records of our suppliers’ qualification expiration dates. It is possible we may in the future fail to comply with this requirement if, for example, our suppliers lose appropriate qualifications without our knowledge, which could result in penalties and fines. See “Item 4. Information on the Company—4.B. Business Overview—Regulation.” In addition, we may be exposed to reputational damage or even liabilities for defective goods provided by our suppliers or negative publicity associated with our suppliers, and our results of operations could suffer as a result.
Our business, financial condition, results of operations and prospects may be adversely affected by an unfavorable market perception of the overall aesthetic medical industry.
Aesthetic medical services have been gaining popularity in recent years. However, many consumers remain cautious about the risks inherent in aesthetic medical procedures. Media influences, peer perceptions, research indicating adverse health effects of aesthetic medical procedures or otherwise could lead to deterioration in the market perception of aesthetic medical treatments and to less demand for aesthetic medical services. In addition, if any allegation surfaces in the media or in social media forums of any accident, ineffectiveness of treatment, poor service standards or mishandling of sensitive personal information by any operator of aesthetic medical services, regardless of merit, the entire aesthetic medical industry and any industry participant including us could experience reputational harm. Our business, financial condition, results of operations and prospects may be materially and adversely affected as a result.
12
We face intense competition, and if we do not compete successfully against new or existing competitors, we may lose our market share and our profitability may be adversely affected.
We compete with private aesthetic hospitals and clinics and aesthetic medical departments in public general hospitals located in the same geographic areas as our treatment centers. We will also compete with future market entrants as the rapid growth of the aesthetic medical industry in the PRC may attract more domestic or international players to enter. Some of our existing and potential competitors may have competitive advantages, such as significantly greater financial, marketing or other resources and may be able to mimic and adopt our business model. We compete for customers primarily on the basis of location, price, the range and the quality of services that we offer and our brand name. We cannot assure you that we will be able to successfully compete against new or existing competitors. Any inability to successfully compete with new or existing competitors may prevent us from increasing or sustaining our revenue and profitability level and result in a loss of market share.
If we are unable to adapt to changing aesthetic medical trends and our customers’ changing needs, we will not be able to compete effectively, which may materially and adversely affect our business, financial condition and results of operations.
The aesthetic medical market requires us to closely monitor the trends in the market and the needs of our customers, which may require us to introduce new products, technologies, devices, solutions, service categories and treatment procedures and enhance our existing services and procedures. We have active dialogue and exchange of information with experts from well-respected aesthetic medical institutions overseas such as the United States, Europe, Singapore, Japan and South Korea to learn and adopt aesthetic medical solutions, standards and technologies. We must maintain strong relationships with leading overseas aesthetic medical institutions to ensure that we are accessing the latest technology and quickly and cost-effectively responding to our customers’ changing needs. We may be required to incur development and acquisition costs to keep pace with new technologies, implement technological innovations or to replace obsolete technologies. If we fail to identify, develop and introduce new products, solutions, service categories, features, enhancements and technologies on a timely and cost-effective basis, demand for our services may decrease and we may not be able to compete effectively or attract customers, which may materially and adversely affect our business and results of operations.
Our revenue is particularly sensitive to changes in economic conditions.
Demand for our aesthetic medical services and the resulting spending by our customers are particularly sensitive to changes in general economic conditions and our customers’ disposable incomes. We cannot assure you that the local economy in the places where we operate can sustain stable growth in consumer spending. In response to an actual or perceived economic downturn, people may reduce their spending on aesthetic medical services, which may materially and adversely affect our ability to generate revenue from these services, and our financial condition and results of operations.
If we are unable to manage our growth effectively, we may not be able to capitalize on new business opportunities and our business and financial results may be materially and adversely affected.
We have significantly expanded our business before 2021, but in 2021 the Company decided to strategically shift its development strategy from rapid national expansion to a more focused, stable and sustainable growth. As of December 31, 2022, out of our existing network of eleven treatment centers, we have established six treatment centers, acquired six treatment centers, and obtained minority stakes in one treatment center. Our organization may become larger and more complex with the addition of treatment centers in the future. Our expansion may require a significant amount of time from our management and substantial operational, financial and other resources.
To manage our growth and expansion, and to attain and maintain profitability, we will continue to place significant demands on our management and on our administrative, operational and financial personnel and infrastructure. Our success also depends on our ability to recruit, train and retain additional qualified management personnel and professionals as well as other administrative, sales and marketing personnel. To accommodate our growth, we need to continue managing our relationships with our suppliers and customers. All of these endeavors will require substantial management attention and effort and significant additional expenditure. We cannot assure you that we will be able to manage any future growth effectively and efficiently, and any failure to do so may materially and adversely affect our ability to capitalize on new business opportunities, which in turn may have a material adverse effect on our business and financial results.
13
On January 10, 2023, we entered into a share purchase agreement with a non-affiliated third party, under which we transferred 100% of the equity of our wholly-owned subsidiary, Shenzhen Miaoyan Medical Technology Investment Co., Ltd. to this third party for a consideration of RMB1.9 million due to our strategy to further focus on mature and profitable institutions. As of the date of this annual report, the transaction has not been closed.
We have operations outside of China and intend to expand our international operations, which exposes us to significant risks.
We currently have operations throughout China and our network also covers Singapore. Our business strategy includes further expanding our operations into new markets outside of China. Our company may become larger and more complex with our intended plans to expand into new geographic areas, through a combination of acquisitions and organic growth. The success of our business therefore depends, in large part, on our ability to operate successfully from geographically disparate locations and to further expand our international operations and sales. Operating in international markets requires significant resources and management attention and subjects us to regulatory, economic and political risks that are different from those that we face in China. We cannot assure you that further international expansion will be successful.
Among the risks we believe are most likely to affect us include:
● | lack of control, difficulties, inefficiencies and costs associated with staffing and managing foreign operations; |
● | potential distraction of management from our China operations; |
● | greater difficulty collecting accounts receivable and longer payment cycles; |
● | compliance with local laws and regulations and unexpected changes in regulatory requirements; |
● | reduced protection for intellectual property rights in some countries; |
● | the effectiveness of our policies and procedures designed to ensure compliance with similar anti-corruption, anti-money laundering, and sanction laws; |
● | possible tariffs and trade restrictions; |
● | foreign taxes; |
● | fluctuations in currency exchange rates; |
● | natural disasters, contagious disease outbreaks, travel restrictions, and war or terrorist activities; and |
● | political and economic instability. |
Our failure to manage any of these risks successfully could harm our operations, reduce our revenue, and have other adverse effects on our operating results.
Execution of our strategies to grow our business depends on our ability to successfully expand into new geographic areas in a timely, cost-effective and non-disruptive manner.
Execution of our strategies to grow our business depends partly on our ability to expand into new geographic areas in a timely, cost-effective and non-disruptive manner, through a combination of acquisitions and organic growth. Our ability to successfully expand into new markets depends on many factors including, among others, our ability to:
● | identify suitable geographic markets for the type of services we offer; |
● | identify local consumer preferences; |
14
● | address local market competition; |
● | negotiate acceptable lease terms, including desirable tenant allowances; |
● | hire, train and retain a growing workforce of doctors and other personnel; |
● | successfully integrate new treatment centers into our existing control structure and operations, including our information technology systems; and |
● | secure financing or maintain sufficient capital to invest in new treatment centers or making acquisitions. |
If we fail to expand into new markets in a timely and cost-effective manner, whether through organic growth or through acquisitions, our overall business growth strategy and prospects could be materially and adversely affected.
We may be unable to identify or execute acquisition opportunities, businesses we acquire may have unknown or contingent liabilities, and we may not realize the benefits we anticipate from such acquisitions, which may materially and adversely affect our business, financial condition, results of operations and prospects.
Our success depends on our ability to continually enhance and broaden our service offerings in response to changing customer demands, competitive pressures and innovation. We may consider opportunities to partner with or acquire other businesses, products or technologies that may enhance our services platform or technology, expand the breadth of our operations or customer base or advance our business strategies. We may not be able to identify suitable targets for acquisition, or negotiate commercially acceptable terms for acquisitions. Even if we are able to identify suitable targets, such acquisitions can be difficult, time consuming and costly and we may not be able to secure necessary financing for the acquisitions. Businesses that we acquire may have unknown or contingent liabilities, including liabilities for failure to comply with relevant laws, rules and regulations. In acquiring the treatment centers, we typically require sellers to indemnify us for any claims and liabilities that are in connection with or arise from a time prior to our acquisitions.
However, we could suffer reputational harm for actual or alleged inferior service or harm that occurred at treatment centers prior to our acquisition and need to respond to claims initially as unsatisfied customers will likely pursue their claims against the treatment centers and us. Depending on the circumstances of the sellers, the indemnities we receive from them may not be sufficient or forthcoming, due to bankruptcy or disappearance of sellers or otherwise. Historically, certain entities we acquired failed to comply with certain regulations and the sellers did not provide sufficient indemnities. As a result, we may be liable for historical defects. Even if we have received indemnities, our reputation may be harmed because of the sellers’ historical defects. We maintain contact with the sellers of the treatment centers we acquired post acquisitions. Some of the sellers of our acquired treatment centers remain as minority shareholders of these subsidiaries. Moreover, any financial mitigation we receive from sellers of these treatment centers may not mitigate against any reputational harm we may suffer as a result of these claims. In addition, future acquisitions and subsequent integration of newly acquired assets and businesses into our own could be expensive and time-consuming, require significant attention from our management and could result in a diversion of resources from our existing business, which in turn could have an adverse effect on our business operations. Acquired assets or businesses may not generate the financial results we expect. If we are not able to identify, capture or execute opportunities to expand our operations successfully through acquisitions, or suffer reputational or financial harm caused by unknown or contingent liabilities of the treatment centers we acquire, our business, financial condition, results of operations and prospects could be materially and adversely affected.
15
Newly opened and acquired treatment centers may not achieve operating results as anticipated, which could materially and adversely affect our results of operations.
It typically takes newly opened and acquired treatment centers a period of time to achieve a utilization rate comparable to our existing treatment centers, due to factors such as time needed to build customer awareness in the local community and to integrate new treatment centers’ operations into our existing infrastructure. The treatment centers we acquired did not or may not achieve the business or financial performance as we expect. The opening and acquisition of new treatment centers involve regulatory approvals and reviews by various authorities in China, including health authorities. We may not be able to obtain all the required approvals, permits or licenses for opening and acquisitions of treatment centers in a timely manner or at all. We may not be able to fully utilize the newly opened and acquired treatment centers as anticipated due to our inability or material delay in obtaining the required approvals, permits or licenses and any substantial increase in costs to ramp up operations and utilization. In addition, the operating results generated at the newly opened and acquired treatment centers may not be comparable to the operating results generated at any of our existing treatment centers. The treatment centers may even operate at a loss, which could materially and adversely affect our results of operations. We may elect to dispose of the acquired treatment centers subsequently and recognize losses. In 2021, we transferred our interest in or closed the operation of seven treatment centers. In 2022, we divested three underperforming treatment centers and closed the operation of one treatment center. As a result, our results of operations may fluctuate from year to year. Therefore, period-to-period comparisons of our operating results may not be meaningful and you should not rely on them to predict our future performance or the price of our ADSs.
We are dependent on certain key members of our senior management and other key employees, and our business, financial condition, results of operations and prospects will suffer greatly if we lose their services.
We are dependent on certain key members of our senior management team, some of whom have been with us since our inception, to manage our current operations and meet future business challenges. In particular, we rely on the expertise, experience and leadership of Dr. Zhou Pengwu, our chairman and chief executive officer and Ms. Ding Wenting, our vice-chairwoman and spokesperson.
We do not maintain key personnel insurance. Competition for competent candidates in the industry is intense and the pool of competent candidates is limited. The loss of any key members of our senior management team could materially disrupt our operations and delay the implementation of our business strategies. In addition, we may not be able to locate suitable or qualified replacements, and may incur additional expenses to recruit and train new personnel, which could severely limit our business and growth. In addition, if any member of our senior management team or key employees joins a competitor or forms a competing business, we may lose know-how, trade secrets, customers and key professionals and staff. We have entered into employment agreements, confidentiality and non-compete agreements with all of the key members of our management team. We cannot assure you, however, the extent to which any of these agreements will be enforceable under the applicable laws. For more details, see “—Risks relating to doing business in the PRC—Uncertainties with respect to the PRC legal system and changes in laws, regulations and policies in China could materially and adversely affect us.” If, as a result of similar incidents, or events, we may lose the services of key members of our senior management, which could have a material adverse effect on our business, financial condition and results of operations.
We may not be able to adequately protect our intellectual property rights, which could harm our brand and our business.
We believe our trademarks and other intellectual property rights are crucial to our success. Our principal intellectual property rights include our trademarks for the “Pengai” brand. Although we rely on the registration of trademarks and applicable laws to protect our intellectual property rights, these measures may not be sufficient to prevent misappropriation of our intellectual property rights. There is no assurance that third parties will not infringe on our intellectual property rights. Our efforts to enforce or defend our intellectual property rights may not be adequate, may require significant attention from our management and may be costly. We may have to initiate legal proceedings to defend the ownership of our trademarks or brand against any infringement by third parties, which may be costly and time-consuming, and we might be required to devote substantial management time and resources in an attempt to achieve a favorable outcome. Furthermore, the outcome of any legal actions to protect our intellectual property rights may be uncertain. If we are unable to adequately protect or safeguard our intellectual property rights, our business, financial condition, results of operations and prospects may be adversely affected.
16
In addition, as permitted by the PRC laws, other parties may register trademarks which may look similar to our registered trademarks under certain circumstances, which may cause confusion among consumers. We may not be able to prevent other parties from using trademarks that are similar to ours and our consumers may confuse our treatment centers with others using similar trademarks. In such case, the goodwill and value of our trademarks and the public perception of our brand and our image may be adversely affected. A negative perception of our brand and image could have a material and adverse effect on our sales, and therefore on our business, financial condition, results of operations and prospects.
Our business is subject to seasonality.
Our operating results are exposed to seasonal fluctuations in demand for our services. We usually experience relatively higher customer visits in the second half of each financial year, which is mainly because we experience a higher number of customer visits for the period from October to December, primarily due to the Chinese National Day holiday, Christmas, and in anticipation of the subsequent New Year and Chinese New Year holidays. As such, our revenue is usually slightly higher in the second half of the year.
Our historical financial and operating results may not be indicative of future performance, and we may not be able to achieve and sustain the historical level of revenue and profitability.
Our historical results may not be indicative of future performance. Our financial and operating results may not meet the expectations of public market analysts or investors, which could cause the price of the ADSs to decline. Our revenue, cost, expenses and operating results may vary from period to period in response to a variety of factors beyond our control, including general economic conditions, new trends in the aesthetic medical market and our ability to control costs and operating expenses. Our operations largely depend on our ability to retain current customers and attract new ones, encourage more spending by our customers, continue adopting innovative technologies and introducing new services in response to customer demand, increase brand awareness through marketing and promotional activities and take advantage of any growth in the relevant markets. We cannot assure you that we will achieve any of them in the future. We believe that period-to-period comparisons of our operating results in the past may not be indicative of our future performance and you should not rely on them to predict the future performance of our operating results or ADSs.
Landlords’ lack of required permits, failure to renew leases, failure to fulfill the procedure of leasing or substantial increases in rents may materially and adversely affect our business and financial performance.
Most real estate for our treatment centers is leased. The availability of commercially attractive locations for our treatment centers is important to our business. Upon the expiry of each of the lease agreements, we have to negotiate terms of renewal with the relevant landlord. As there has been a general increase in rent for commercial properties in the PRC in recent years, and as a majority of our treatment centers are located on premises leased from independent third parties in central urban locations, there is no guarantee that we can renew the leases or negotiate new leases on similar or favorable terms (including, without limitation, on similar tenure and with similar rent) in the future, if at all, or that the leases will not be terminated early by the landlords. In the event that we are required to seek alternative locations for our treatment centers, there is no guarantee that we can secure comparable locations or negotiate leases on comparable terms. In addition, the use of certain of our leased buildings is inconsistent with the permitted use of such buildings or such land and some landlords or lessors did not obtain sufficient approvals, permits and registrations from the governmental authorities and the other third parties. If any challenge from any government authority or a third party arises, our lease agreement may be invalidated and we may also be required to relocate our operations. Furthermore, certain of our lease agreements have not been filed with the PRC authorities, which may subject us to fines if the relevant PRC authority requires us to rectify and we fail to do so within a specified period of time. In addition, certain of our leased properties are subject to mortgage. We may be forced to relocate in the event that the mortgagees are entitled to exercise their rights under the relevant mortgages. If we were forced to relocate, we might not be able to relocate to desirable locations in a timely and cost-effective manner. All these factors may have an adverse impact upon our business operation, financial position and our future potential growth.
17
We are not in full compliance with social insurance, housing provident funds and income tax contributions.
We are required to make social insurance, housing provident funds and income tax contributions for the benefit of employees of our PRC subsidiaries under PRC laws and regulations. Our PRC subsidiaries have not made social insurance, housing provident funds and income tax contributions in full for all of their employees. Companies operating in the PRC are also required to withhold individual income tax on employees’ salaries based on the actual salary of each employee upon payment. Our PRC subsidiaries have not fully withheld the individual income tax in accordance with the relevant PRC laws and regulations.
Under the relevant PRC laws and regulations, the relevant PRC authorities may determine that we need to make supplemental social insurance and housing fund contributions and that we are subject to fines, supplementary payment and legal sanctions in relation to our failure to make social insurance and housing fund contributions in full for our employees, in which case our business, financial position or operation may be adversely affected. With respect to the underwithheld individual income tax, we may be required by relevant PRC authorities to make up sufficient withholding and pay late fees and fines. In addition, some of our PRC subsidiaries make social insurance for employees of other PRC subsidiaries, in which case we are subject to fines and adverse record on our credit files. If so, our business, financial position or operation may be adversely affected.
Our business is subject to professional and other liabilities for which we may not be insured.
We are exposed to potential liabilities that are inherent to the aesthetic medical and healthcare industries. Our treatment centers have been the subject of such claims in the past. To protect our business from the cost of any such claims, we maintain product liability insurance that we believe to be appropriate for our operations as of the date of this annual report. However, we may face liabilities that exceed our available insurance coverage or arise from claims outside the scope of our insurance coverage. Our insurance policies are subject to annual renewal, and we may not be able to obtain product liability insurance in the future on acceptable terms or at all. In addition, our insurance premiums could be subject to increases in the future, which may be material. If the coverage limits are inadequate to cover our liabilities or our insurance costs continue to increase as a result of liability claims or other litigation, then our business, financial condition and results of operations may be adversely affected.
We could be exposed to risks related to our management of customers’ medical data.
Our treatment centers collect and maintain medical data and treatment records of our customers. PRC laws and regulations generally require medical institutions and their medical personnel to protect the privacy of their customers and prohibit unauthorized disclosure of personal information. Such medical institutions and their medical personnel will be liable for damage caused by divulging the customers’ private or medical records without consent. We have taken measures to maintain the confidentiality of our customers’ medical records, including encrypting such information in our information technology system so that it cannot be viewed without proper authorization and setting internal rules requiring our employees to maintain the confidentiality of our customers’ medical records. However, these measures may not always be effective in protecting our customers’ medical records. Our information technology systems could be breached through hacking. Personal information could be leaked due to any theft or misuse of personal information due to misconduct or negligence. In addition, although we do not make the customers’ medical records available to the public, we use such data on an aggregated basis after redacting personally identifiable information for marketing purposes.
We are subject to governmental regulation and other legal obligations related to the protection of personal data, privacy and information security in the regions where we do business, and there has been and may continue to be a significant increase in such laws that restrict or control the use of personal data. See “Item 4. Information on the Company—B. Business Overview—Regulations—Regulations related to data security and privacy protection.” In mainland China, the Personal Information Security Specification (2020 Version) came into force on October 1, 2020.
18
On June 10, 2021, the Standing Committee of the National People’s Congress of China, or the SCNPC, promulgated the PRC Data Security Law, which took effect in September 2021. The PRC Data Security Law imposes data security and privacy obligations on entities and individuals carrying out data activities, and introduces a data classification and hierarchical protection system based on the importance of data in economic and social development, and the degree of harm it will cause to national security, public interests, or legitimate rights and interests of individuals or organizations when such data is tampered with, destroyed, leaked, illegally acquired or used. The PRC Data Security Law also provides for a national security review procedure for data activities that may affect national security and imposes export restrictions on certain data an information.
In early July 2021, regulatory authorities in Mainland China launched cybersecurity investigations with regard to several China-based companies that are listed in the United States. The Chinese cybersecurity regulator announced on July 2 that it had begun an investigation of a major China-based transportation platform and two days later ordered that the company’s app be removed from smartphone app stores. On July 5, 2021, the Chinese cybersecurity regulator launched the same investigation on two other Internet platforms.
On July 6, 2021, the General Office of the Communist Party of China Central Committee and the General Office of the State Council jointly issued the Opinions on Severely Cracking Down on Illegal Securities Activities According to Law, which stressed on, among other things, require (i) speeding up the revision of the provisions on strengthening the confidentiality and management relating to overseas issuance and listing of securities and (ii) improving the laws and regulations relating to data security, cross-border data flow, and management of confidential information.
On August 17, 2021, the State Council promulgated the Regulations on the Protection of the Security of Critical Information Infrastructure, or the Regulations, which took effect on September 1, 2021. Pursuant to the Regulations, critical information infrastructure refers to any important network facilities or information systems of an important industry or field such as public communication and information service, energy, transport, water conservation, finance, public services, e-government affairs and national defense science, and other industries and sectors that may endanger national security, people’s livelihood and public interest in case of damage, function loss or data leakage. In addition, relevant administration departments of each critical industry and sector are responsible for formulating eligibility criteria and determining the critical information infrastructure in the respective industry or sector. The operators will be informed about the final determination as to whether they are categorized as “critical information infrastructure operators”.
On August 20, 2021, the SCNPC promulgated the Personal Information Protection Law of the PRC, or the Personal Information Protection Law, which took effect on November 1, 2021. As the first systematic and comprehensive law specifically for the protection of personal information in the PRC, the Personal Information Protection Law provides, among others, that (i) an individual’s consent shall be obtained to use sensitive personal information, such as biometric characteristics and individual location tracking, (ii) personal information operators using sensitive personal information shall notify individuals of the necessity of such use and impact on the individual’s rights, and (iii) where personal information operators reject an individual’s request to exercise his or her rights, the individual may file a lawsuit with a People’s Court.
In addition, on December 28, 2021, the Cyberspace Administration of China (“CAC”), the National Development and Reform Commission (“NDRC”), and several other administrations jointly issued the revised Measures for Cybersecurity Review, which became effective and replaced the existing Measures for Cybersecurity Review on February 15, 2022. Furthermore, the CAC released the draft of the Regulations on Network Data Security Management on November 14, 2021 for public consultation, or the Draft Regulations, which among other things, stipulates that a data processor listed overseas must conduct an annual data security review by itself or by engaging a data security service provider and submit the annual data security review report for a given year to the municipal cybersecurity department before January 31 of the following year. If the draft Regulations on Network Data Security Management are enacted in the current form, we, as an overseas listed company, will be required to carry out an annual data security review and comply with the relevant reporting obligations. The Draft Regulations is released for public comment only, and its provisions and anticipated adoption or effective date may be subject to change and thus its interpretation and implementation remain substantially uncertain.
19
On February 24, 2023, the China Securities Regulatory Commission, or the CSRC, jointly with other relevant governmental authorities, promulgated the Provisions on Strengthening Confidentiality and Archives Management of Overseas Securities Issuance and Listing by Domestic Enterprises, or the Confidentiality and Archives Management Provisions, which takes effect on March 31, 2023. According to the Confidentiality and Archives Management Provisions, domestic companies, whether offering and listing securities overseas directly or indirectly, must strictly abide the applicable laws and regulations when providing or publicly disclosing, either directly or through their overseas listed entities, documents and materials to securities services providers such as securities companies and accounting firms or overseas regulators in the process of their overseas offering and listing. If such documents or materials contain any state secrets or government authorities work secrets, domestic companies must obtain the approval from competent governmental authorities according to the applicable laws, and file with the secrecy administrative department at the same level with the approving governmental authority. Furthermore, the Confidentiality and Archives Management Provisions also provides that securities companies and securities service providers shall also fulfill the applicable legal procedures when providing overseas regulatory institutions and other relevant institutions and individuals with documents or materials containing any state secrets or government authorities work secrets or other documents or materials that, if divulged, will jeopardize national security or public interest. Since the Confidentiality and Archives Management Provisions was promulgated recently, substantial uncertainties still exist with respect to the interpretation and implementation of such provisions and how they will affect us.
On July 7,2022, the CAC promulgated Measures for the Security Assessment of Outbound Data Transfer, which became effective on September 1, 2022 and provides that a data processor is required to apply for security assessment for cross-border data transfer in any of the following circumstances:(i)where a data processor provides critical data to offshore entities and individuals; (ii)where a critical information infrastructure operator or a data processor which processes personal information of more than one million individuals provides personal information to offshore entities and individuals; (iii)where a data processor has provided personal information in the aggregate of more than 100,000 individuals or sensitive personal information of more than 10,000 individuals in total to offshore entities and individuals since January 1 of the previous year; or (iv)other circumstances prescribed by the CAC for which application for security assessment for cross-boarder transfer of data is required.
The relevant regulatory authorities in Mainland China continue to regulate and strengthen the protection of personal data, privacy and information security, and may impose additional requirements from time to time. The relevant regulatory authorities also release, from time to time, their monitoring results and requires relevant enterprises listed in such notices to rectify their non-compliance. There are uncertainties as to the interpretation and application of laws in one jurisdiction which may be interpreted and applied in a manner inconsistent to another jurisdiction and may conflict with our current policies and practices or require changes to the features of our business and operation. If we are unable to address any information protection concerns, any compromise of security that results unauthorized disclosure or transfer of personal data, or to comply with the then applicable laws and regulations, we may incur additional costs and liability and result in governmental enforcement actions, litigation, fines and penalties or adverse publicity and could cause our users and clients to lose trust in us, which could have a material adverse effect on our business, results of operations, financial condition and prospects. We may also be subject to new laws, regulations or standards or new interpretations of existing laws, regulations or standards, including those in the areas of data security and data privacy, which could require us to incur additional costs and restrict our business operations.
Although we believe our current usage of customers’ medical records is in compliance with applicable laws and regulations governing the use of such information, any change in such laws and regulations could affect our ability to use medical data and subject us to liability for the use of such data. Failure to protect customers’ medical records, or any restriction on or liability as a result of, our use of medical data, could have a material adverse effect on our business.
A technological failure, security breach or other disruptions of our computer network infrastructure and centralized information technology systems may interrupt our business.
Our computer network infrastructure and information technology systems are critical for our operation, such as billing, financial and budgeting data, customer records and inventory. We regularly maintain, upgrade and enhance the capabilities of our information technology systems to meet operational needs. Any failure associated with our information technology systems, including those caused by power disruption or loss, natural disasters, computer viruses or hackers, network failures or other unauthorized tampering, may cause interruptions in our ability to provide services to our clients, keep accurate records, and maintain proper business operations, which may adversely affect our business, financial condition and results of operations.
20
In addition, a variety of our software systems are hosted by third-party service providers whose security and information technology systems are subject to similar risks. The failure of our or our service providers’ information technology could disrupt our entire operation or result in decreased sales, increased overhead costs and product shortages, all of which could have a material adverse effect on our reputation, business, financial condition, results of operations and prospects.
The significant amount of advertising and marketing expenses spent on enhancing our brand awareness may not be effective and may adversely affect our results of operations.
We plan to enhance our brand recognition among our existing and target customers along with our expansion into new geographic areas. Our sales and marketing strategies are key to enhancing our brand awareness. In connection with our strategic expansion into new markets, we normally increase advertising of our brand through various advertising media platforms, which requires significant expenses before these newly opened treatment centers reach expected profit levels, thereby affecting our overall profitability. In 2020, 2021 and 2022, our advertising and marketing expenses were RMB303.9 million, RMB255.5 million and RMB132.2 million (US$19.2 million), respectively. We expect the trend of increased advertising and marketing spending to continue as we spend on an increasingly diverse array of online and mobile search engines, marketing and social media platforms. If we do not adequately spend on such platforms, or effectively select or utilize the right platforms, we may not generate the desired result from our advertising spending. Moreover, it may take months or several years to implement our sales and marketing strategies and such time is hard to predict. As a result, we cannot guarantee that our marketing spending or the marketing strategies we have adopted or plan to adopt will have their anticipated effect or generate sustainable revenue and profit.
If we fail to maintain and further develop our direct sales force and partner networks, our business could suffer. Additionally, our third-party partners may not effectively promote our services or may engage in activities that could harm our reputation and sales of our products and services.
We rely on our direct sales force and partner networks to generate sales, including referral arrangements with businesses in the beauty industry such as hairdressers. However, if we fail to maintain or develop our sales force and partner networks, or if any of our partners are ineffective in promoting our services or engage in activities that harm our reputation, it could have a material adverse effect on our ability to generate sales, which could have a material adverse effect on our business, financial condition and results of operations.
We may grant employee share options and other share-based compensation awards in the future. Any additional grant of employee share options and other share-based compensation awards in the future may have a material adverse effect on our results of operations.
We have adopted and may in the future adopt employee share option plans for the purpose of granting share-based compensation awards to our employees, officers, directors and other eligible persons to incentivize their performance and align their interests with ours. For more information on these share incentive plans, see “Item 6. Directors, Senior Management and Employees—6.B. Compensation—Share Incentive Plan” and “Item 6. Directors, Senior Management and Employees—6.B. Compensation— Performance Incentive Plan.” As a result of these grants and potential future grants, we expect to continue to incur significant share-based compensation expenses in the future. We are required to account for share-based compensation in accordance with IFRS 2—Share-based Payment, which generally requires a company to recognize, as an expense, the fair value of share options and other equity incentives to employees based on the fair value of equity awards on the date of the grant, with the compensation expense recognized over the period in which the recipient is required to provide service in exchange for the equity award. If we grant options or other equity incentives in the future, we could incur significant compensation charges and our results of operations could be adversely affected. The expenses associated with share-based compensation will decrease our profitability, and the additional awards issued under share-based compensation plans will dilute the ownership interests of our shareholders, including holders of our ADSs. However, if we limit the scope of our share-based compensation plan, we may not be able to attract or retain key personnel who expect to be compensated by such share-based awards.
21
A material weakness was identified in our internal control over financial reporting. If we fail to develop and maintain an effective system of internal control over financial reporting, we may be unable to accurately report our financial results or prevent fraud and our ability to comply with applicable regulations could be impaired.
Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2020. In making this assessment, it used the criteria established within the Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) (2013 framework). Based on this assessment, our management has concluded that, as of December 31, 2020, our internal control over financial reporting was not effective. We and our independent registered public accounting firm identified one material weakness in our internal control over financial reporting. The material weakness identified was related to a lack of an effective control procedure to evaluate accounting of complex non-routine transactions. In the fiscal year 2021, we have not identified any material weakness in our internal control over financial reporting.
As defined in the standards established by the U.S. Public Company Accounting Oversight Board, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
We are a public company in the United States and are subject to the Sarbanes-Oxley Act of 2002. Section 404 of the Sarbanes-Oxley Act, or Section 404, requires that we include a report from management on the effectiveness of our internal control over financial reporting in our annual report on Form 20-F beginning with our annual report for the fiscal year ended December 31, 2020. In addition, once we cease to be an “emerging growth company” as such term is defined in the JOBS Act, our independent registered public accounting firm must attest to and report on the effectiveness of our internal control over financial reporting. Our management may conclude that our internal control over financial reporting is not effective. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us. In addition, a public company’s reporting obligations may place a significant strain on our management, operational and financial resources and systems for the foreseeable future. We may be unable to timely complete our evaluation testing and any required remediation.
During the course of documenting and testing our internal control procedures, in order to satisfy the requirements of Section 404, we may identify other weaknesses and deficiencies in our internal control over financial reporting. In addition, if we fail to maintain the adequacy of our internal control over financial reporting, as these standards are modified, supplemented or amended from time to time, we may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404. If we fail to achieve and maintain an effective internal control environment, we could suffer material misstatements in our financial statements and fail to meet our reporting obligations, which would likely cause investors to lose confidence in our reported financial information. This could in turn limit our access to capital markets, harm our results of operations, and lead to a decline in the trading price of our ADSs. Additionally, ineffective internal control over financial reporting could expose us to increased risk of fraud or misuse of corporate assets and subject us to potential delisting from the stock exchange on which we list, regulatory investigations and civil or criminal sanctions. We may also be required to restate our financial statements for prior periods.
In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal controls over financial reporting, we will need to expend significant resources and provide significant management oversight. Implementing any appropriate changes to our internal controls may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective in maintaining the adequacy of our internal control.
If we are unable to conclude that we have effective internal controls over financial reporting, investors may lose confidence in our operating results, the price of the ADSs could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, the ADSs may not be able to remain listed on the Nasdaq Capital Market.
22
Risks relating to our corporate structure
If the PRC government deems that the Contractual Arrangements do not comply with PRC laws and regulations, or if these laws and regulations or their interpretations change in the future, we could be subject to severe penalties or be forced to relinquish our interests received through the Contractual Arrangements.
According to the Industry Catalog for Guiding Foreign Investment (Revision 2015), or the Foreign Investment Catalog 2015, which became effective in April 2015, amended in June 2017, and replaced by the Special Administrative Measures (Negative List) for Access of Foreign Investment (2018 Edition) in June 2018, and the Special Administrative Measures (Negative List) for Access of Foreign Investment (2021 version), or the Negative List, promulgated by the NDRC and the Ministry of Commerce of the PRC, or the MOFCOM jointly on December 27, 2021 and came into effect on January 1, 2022, PRC law only allows foreign investment in PRC medical institutions through joint venture entities, and the foreign shareholding in these entities is limited to 70.0%, which percentage was stipulated in the Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions, or the JV Interim Measures. We historically held more than 70.0% in nine of our PRC subsidiaries after the effective date of the Foreign Investment Catalog 2015, namely Yantai Pengai Jiayan Cosmetic Surgery Hospital Co., Ltd., Hangzhou Pengai Aesthetic Medical Clinic Co., Ltd., Chongqing Pengai Aesthetic Medical Hospital Co., Ltd., Changsha Pengai Aesthetic Medical Hospital Co., Ltd., Shanghai Pengai Aesthetic Medical Clinic Co., Ltd, Jiangsu Liangyan Hospital Management Co., Ltd., and Beijing Aomei Yixin Investment Consultant Co., Ltd., Guangzhou Pengai Xiuqi Aesthetic Medical Clinic Co., Ltd., and Shanghai Jiahong Aesthetic Medical Clinic Co., Ltd. We decreased our shareholdings in these PRC subsidiaries to 70.0% by transferring excess equity interests to Dr. Zhou Pengwu and certain of our employees in 2018, 2019, 2020 and 2021, subject to the completion of registration of share transfer to Dr. Zhou and the equity pledge. In connection with such equity transfer to Dr. Zhou and Dr. Zhou Pengwu’s shareholding, in Shenzhen Pengai Xiuqi Aesthetic Medical Hospital, Guangzhou Pengai Aesthetic Medical Hospital Co., Ltd., Hangzhou Pengai Aesthetic Medical Clinic Co., Ltd., Jinan Pengai Cosmetic Surgery Hospital Co., Ltd., and Jiangsu Liangyan Hospital Management Co., Ltd., Beijing AomeiYixin Investment Consultant Co., Ltd. which he acquired from other shareholders in 2018, 2019, 2020 and 2021, we entered into a series of Contractual Arrangements with Dr. Zhou Pengwu and the Relevant Subsidiaries, among other parties, with respect to the Target Equity Interests. For details of the Contractual Arrangements, see “Item 4. Information on the Company—4.A. History and Development of the Company—Contractual Arrangements with respect to Target Equity Interests.”
In the opinion of Han Kun Law Offices, our PRC legal counsel, the execution, delivery and performance of each of the Contractual Arrangements by the parties thereto, and the consummation of the transactions contemplated thereunder do not and will not result in any violation of any explicit requirement under applicable PRC Laws currently in effect. However, we have been advised by our PRC legal counsel that there are substantial uncertainties regarding the interpretation and application of current and future PRC laws, regulations and rules, including those relating to foreign investment restrictions and the Foreign Investment Law (as defined below). There can be no assurance that the PRC regulatory authorities will take a view that is consistent with the opinion of our PRC legal counsel.
The Chinese regulatory authorities could disallow our structure, which could result in a material change in our operations and the value of our securities could decline or become worthless. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China” for recent regulatory initiatives implemented by the relevant PRC government entities. In addition, any actions by the Chinese government to exert more oversight and control over securities that are listed overseas or foreign investment in China-based issuers could significantly limit or completely hinder our ability to continue to offer securities to investors and cause the value of our securities to significantly decline or be worthless. In addition, it is uncertain whether any new PRC laws or regulations relating to Contractual Arrangements will be adopted, or if adopted, what they would provide.
If our ownership structure, Contractual Arrangements or businesses of the Relevant Subsidiaries are found to be in violation of any existing or future PRC laws or regulations, or if the Relevant Subsidiaries fail to obtain or maintain any of the required permits or approvals, the relevant PRC regulatory authorities would have broad discretion to take action in dealing with such violations or failures, including:
● | revoking the Contractual Arrangements; |
● | imposing fines, confiscating the income from the Relevant Subsidiaries, or imposing other requirements with which we may not be able to comply; |
● | discontinuing or restricting our operations in the PRC; |
23
● | imposing conditions or requirements with which we may not be able to comply; or |
● | taking other regulatory or enforcement actions that could be harmful to our business. |
Any of these actions could materially and adversely affect our business, financial condition and results of operations. If any of these occurrences results in our failure to receive the economic benefits of the Target Equity Interests, we may not be able to consolidate the above in our consolidated financial statements in accordance with IFRS as issued by IASB.
The Contractual Arrangements may not be as effective in providing control as direct ownership and Relevant Subsidiaries or Dr. Zhou Pengwu may fail to perform their respective obligations under the Contractual Arrangements.
We own and hold 70.0% of equity interests in the Relevant Subsidiaries and will rely on the Contractual Arrangements to control the Target Equity Interests in the Relevant Subsidiaries. The Contractual Arrangements may not be as effective as direct ownership in controlling over the Target Equity Interests. If the Relevant Subsidiaries and/or Dr. Zhou Pengwu fail to perform their respective obligations under the Contractual Arrangements, we may have to incur substantial costs and expend additional resources to enforce such Contractual Arrangements, and rely on legal remedies under PRC laws, including contractual remedies, which may not be sufficient or effective. These legal remedies may not be as effective as direct ownership in controlling the Target Equity Interests. All the agreements under the Contractual Arrangements are governed by and interpreted in accordance with PRC laws, and disputes arising from these Contractual Arrangements will be resolved through arbitration in Mainland China. However, the legal framework and system in Mainland China are not as developed as in some other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could limit our ability to enforce Contractual Arrangements. Meanwhile, there are very few precedents and little formal guidance as to how Contractual Arrangements should be interpreted or enforced under PRC law. Significant uncertainties remain regarding the ultimate outcome of such arbitration. In addition, under PRC law, rulings by arbitrators are final, parties cannot appeal the arbitration results in courts, and if the losing parties fail to carry out the arbitration awards within a prescribed time limit, the prevailing parties may only enforce the arbitration awards in the PRC courts through arbitration award recognition proceedings, which would require additional expenses and cause a delay. If we fail to enforce Contractual Arrangements, or if we suffer significant delay or face other obstacles in the process of enforcing Contractual Arrangements, our control over the Target Equity Interests may be negatively affected.
Any failure by our Relevant Subsidiaries and their other respective shareholders, including Dr. Zhou Pengwu, to perform their obligations under our contractual arrangements with them would have a material adverse effect on our business.
If our Relevant Subsidiaries and their other respective shareholders, including Dr. Zhou Pengwu, fail to perform their respective obligations under the Contractual Arrangements, we may have to incur substantial costs and expend additional resources to enforce such arrangements. We may also have to rely on legal remedies under PRC law, including seeking specific performance or injunctive relief, and claiming damages, which we cannot assure will be effective under PRC law. For example, if other shareholders of our Relevant Subsidiaries refuse to transfer their equity interest in our Relevant Subsidiaries to us or our designee if we exercise the purchase option pursuant to the contractual arrangements, or if they otherwise act in bad faith toward us, then we may have to take legal actions to compel them to perform their contractual obligations. In addition, if any third parties claim any interest in such shareholders’ equity interests in our Relevant Subsidiaries, our ability to exercise shareholders’ rights or foreclose the share pledge according to the Contractual Arrangements may be impaired.
All of the Contractual Arrangements are governed by and interpreted in accordance with PRC law, and disputes arising from the contractual arrangements will be resolved through arbitration in Mainland China. The legal system in the PRC is not as developed as in some other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could limit our ability to enforce these contractual arrangements. See “—Risks Related to Doing Business in China— Uncertainties with respect to the PRC legal system and changes in laws, regulations and policies in China could materially and adversely affect us.” Meanwhile, there are very few precedents and little formal guidance as to how contractual arrangements in the context of a variable interest entity should be interpreted or enforced under PRC law. There remain significant uncertainties regarding the ultimate outcome of such arbitration should legal action become necessary. In addition, under PRC law, rulings by arbitrators are final, parties cannot appeal the arbitration results in courts, and if the losing parties fail to carry out the arbitration awards within a prescribed time limit, the prevailing parties may only enforce the arbitration awards in PRC courts through arbitration award recognition proceedings, which would require additional expenses and delay. In the event we are unable to enforce the Contractual Arrangements, or if we suffer significant delays or other obstacles in the process of enforcing these Contractual Arrangements, we may not be able to exert effective or complete control over our Relevant Subsidiaries or enjoy the economic benefit from such Relevant Subsidiaries, and our ability to conduct our business may be negatively affected.
24
If the custodians or authorized users of our controlling non-tangible assets, including chops and seals, fail to fulfill their responsibilities, or misappropriate or misuse these assets, our business and operations may be materially and adversely affected.
Under PRC law, legal documents for corporate transactions, including agreements and contracts that our business relies on, are executed using the chop or seal of the signing entity or with the signature of a legal representative whose designation is registered and filed with the relevant local branch of the SAMR. We generally execute legal documents by affixing chops or seals, rather than having the designated legal representatives sign the documents.
We have three major types of chops, corporate chops, contract chops and finance chops. We use corporate chops generally for documents to be submitted to government agencies, such as applications for changing business scope, directors or company name, and for legal letters. We use contract chops for executing leases and commercial contracts. We use finance chops generally for making and collecting payments, including issuing invoices. Use of corporate chops and contract chops must be approved by our legal department and administrative department, and use of finance chops must be approved by our finance department. The chops of our subsidiary (including our Relevant Subsidiaries) are generally held by the relevant entities so that documents can be executed locally. Although we usually utilize chops to execute contracts, the registered legal representatives of our subsidiaries or affiliated entities and their subsidiaries have the apparent authority to enter into contracts on behalf of such entities without chops, unless such contracts set forth otherwise.
In order to maintain the physical security of our chops, we generally have them stored in secured locations accessible only to the designated key employees of our legal, administrative or finance departments. Our designated legal representatives generally do not have access to the chops. Although we have approval procedures in place and monitor our key employees, including the designated legal representatives of our subsidiaries and affiliated entities and their subsidiaries, the procedures may not be sufficient to prevent all instances of abuse or negligence. There is a risk that our key employees or designated legal representatives could abuse their authority, for example, by binding our subsidiaries and affiliated entities and their subsidiaries with contracts against our interests, as we would be obligated to honor these contracts if the other contracting party acts in good faith in reliance on the apparent authority of our chops or signatures of our legal representatives. If any designated legal representative obtains control of the chop in an effort to obtain control over the relevant entity, we would need to have a shareholder or board resolution to designate a new legal representative and to take legal action to seek the return of the chop, apply for a new chop with the relevant authorities, or otherwise seek legal remedies for the legal representative’s misconduct. If any of the designated legal representatives obtains and misuses or misappropriates our chops and seals or other controlling intangible assets for whatever reason, we could experience disruption to our normal business operations. We may have to take corporate or legal action, which could involve significant time and resources to resolve while distracting management from our operations, and our business and operations may be materially and adversely affected.
Dr. Zhou Pengwu may have potential conflicts of interest with us, which may materially and adversely affect our control over the Target Equity Interests.
We have control over the Relevant Subsidiaries by holding 70.0% of equity interest in each of the Relevant Subsidiaries, and we control the Target Equity Interests directly held by Dr. Zhou Pengwu through the Contractual Arrangements. Dr. Zhou Pengwu may have potential conflicts of interest with us due to his role as director of our company and director of certain of the Relevant Subsidiaries, as what is in the best interests of the Relevant Subsidiaries may not be in the best interests of our company. Dr. Zhou Pengwu may breach or refuse to renew the existing Contractual Arrangements we have with him and the Relevant Subsidiaries, which would materially and adversely affect our control over the Target Equity Interests and our ability to receive economic benefits in connection with the Target Equity Interests. If Dr. Zhou Pengwu breaches Contractual Agreements or otherwise has disputes with us, we may have to initiate arbitration or other legal proceedings, which involve significant uncertainty. Such disputes and proceedings may significantly distract our management’s attention, adversely affect our ability to control the Target Equity Interests and otherwise result in negative publicity and adversely affect the reputation of our treatment centers. There is no assurance that the outcome of any such dispute or proceeding will be in our favor.
25
Our Contractual Arrangements with the Relevant Subsidiaries may be subject to scrutiny by the PRC tax authorities and they may determine that we owe additional taxes, which could negatively affect our financial condition and the value of your investment.
Under applicable PRC laws and regulations, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities, and additional taxes and interest may be imposed. The PRC enterprise income tax law requires every enterprise in Mainland China to submit its annual enterprise income tax return together with a report on transactions with its related parties to the relevant tax authorities. The tax authorities may impose reasonable adjustments on taxation if they have identified any related party transactions that are inconsistent with arm’s length principles. We may be subject to material and adverse tax consequences if the PRC tax authorities determine that the Contractual Arrangements between Shenzhen Pengai Investment, Dr. Zhou Pengwu and the Relevant Subsidiaries were not entered into on an arm’s length basis and make special tax adjustments to the tax positions of Shenzhen Pengai Investment and Dr. Zhou Pengwu.
In addition, if Shenzhen Pengai Investment exercises the option to purchase all or any part of the Target Equity Interests, the equity interest transfer price may be subject to review and tax adjustment by the relevant tax authority. Dr. Zhou Pengwu will be subject to personal income tax on the difference between the equity interest transfer price and the amount he has paid to obtain the Target Equity Interests. According to the exclusive option agreement under the Contractual Arrangements, Dr. Zhou Pengwu may pay the remaining amount to Shenzhen Pengai Investment, and the amount to be received by Shenzhen Pengai Investment may also be subject to enterprise income tax. Such tax amounts could be substantial, and our financial condition may be adversely affected as a result.
Furthermore, the PRC tax authorities may impose any late payment fees and other penalties on the Relevant Subsidiaries for the adjusted but unpaid taxes according to the applicable regulations. Our financial position could be materially and adversely affected if any of the Relevant Subsidiaries’ tax liabilities increase or if late payment fees and other penalties are imposed.
If the PRC government deems our PRC medical centers as “Sino-Foreign Equity Medical Institutions,” we could be subject to certain liabilities for failing to comply with the JV Interim Measures.
Since the Foreign Investment Catalog 2015 became effective in April 2015, PRC law only allows foreign investment in PRC medical institutions through joint venture entities, and the foreign shareholding in these entities is limited to 70.0%, the percentage stipulated in the JV Interim Measures. We own and hold our equity interests in the PRC treatment centers indirectly through Pengai Investment. Since the JV Interim Measures do not address the equity percentage of a medical institution held indirectly by a non-PRC entity through its subsidiary in the PRC, we do not believe that we, both prior to and after we decreased our indirect shareholding in the Relevant Subsidiaries to 70.0%, are in violation of any explicit provision of the JV Interim Measures. However, we cannot assure you that the PRC regulatory authorities will not take a different view in the future. In the case that the PRC government authorities deem our PRC medical centers as a Sino-Foreign Equity Medical Institution, we may face liabilities for not meeting the requirements or administrative procedures for Sino-Foreign Equity Medical Institutions historically.
26
Risks relating to doing business in the PRC
The enforcement of the PRC Labor Contract Law, and other labor-related regulations in the PRC may increase our labor costs and limit our flexibility to use labor. Our failure to comply with PRC labor-related laws may expose us to penalties.
On June 29, 2007, the Standing Committee of the National People’s Congress of China enacted the PRC Labor Contract Law, which became effective on January 1, 2008 and was amended on December 28, 2012. The PRC Labor Contract Law introduces specific provisions related to fixed-term employment contracts, part-time employment, probation, consultation with labor unions and employee assemblies, employment without a written contract, dismissal of employees, severance, and collective bargaining, which together represent enhanced enforcement of labor laws and regulations. According to the PRC Labor Contract Law, an employer is obliged to sign an unfixed-term labor contract with any employee who has worked for the employer for 10 consecutive years and will reach the statutory retirement age within ten years. Further, if an employee requests or agrees to renew a fixed-term labor contract that has already been entered into twice consecutively, the resulting contract must have an unfixed term, with certain exceptions. The employer must pay economic compensation to an employee where a labor contract is terminated or expires in accordance with the PRC Labor Contract Law, except for certain situations which are specifically regulated. As a result, our ability to terminate employees is significantly restricted. In addition, the government has issued various labor-related regulations to further protect the rights of employees. According to such laws and regulations, employees are entitled to annual leave ranging from 5 to 15 days and are able to be compensated for any untaken annual leave days in the amount of three times their daily salary, subject to certain exceptions. In the event that we decide to change our employment or labor practices, the PRC Labor Contract Law and its implementation rules may also limit our ability to effect those changes in a manner that we believe to be cost-effective. In addition, as the interpretation and implementation of these new regulations are still evolving, our employment practices may not be at all times deemed in compliance with the new regulations. If we are subject to severe penalties or incur significant liabilities in connection with labor disputes or investigations, our business and financial conditions may be adversely affected.
Companies operating in Mainland China are required to participate in various government sponsored employee benefit plans, including certain social insurance, housing funds and other welfare-oriented payment obligations, and contribute to the plans in amounts equal to certain percentages of salaries, including bonuses and allowances, of their employees up to a maximum amount specified by the local government from time to time. The requirement to maintain employee benefit plans has not been implemented consistently by local governments in Mainland China given the different levels of economic development in different locations. We did not pay, or were not able to pay, certain past social security and housing fund contributions in strict compliance with the relevant PRC regulations for and on behalf of our employees due to differences in local regulations and inconsistent implementation or interpretation by local authorities in the PRC and varying levels of acceptance of the housing fund system by our employees. We may be subject to fines and penalties for our failure to make payments in accordance with the applicable PRC laws and regulations. We may be required to make up the contributions for these plans as well as to pay late fees and fines. We have not made any accruals for the interest on underpayments and penalties that may be imposed by the relevant PRC government authorities in the financial statements. If we are subject to penalties, late fees or fines in relation to the underpaid employee benefits, our financial condition and results of operations may be adversely affected.
The PRC’s economic, political and social conditions, as well as governmental policies, could affect the business environment and financial markets in China, our ability to operate our business, our liquidity and our access to capital.
Most of our operations are conducted in China. Accordingly, our business, results of operations, financial condition and prospects may be influenced to a significant degree by economic, political, legal and social conditions in China. China’s economy differs from the economies of developed countries in many respects, including with respect to the amount of government involvement, level of development, growth rate, control of foreign exchange allocation of resources, an evolving regulatory resources, and a lack of sufficient transparency in the regulatory process. While the PRC economy has experienced significant growth over the past 40 years, growth has been uneven across different regions and among various economic sectors of China. The PRC government has implemented various measures to encourage economic development and guide the allocation of resources. Some of these measures may benefit the overall PRC economy, but may have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are currently applicable to us. In addition, in the past the PRC government implemented certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity in China, which may adversely affect our business and results of operation. More generally, if the business environment in China deteriorates from the perspective of domestic or international investment, our business in China may also be adversely affected.
27
Uncertainties with respect to the PRC legal system and changes in laws, regulations and policies in China could materially and adversely affect us.
We conduct our business primarily through our subsidiaries in China. PRC laws and regulations govern our operations in China. Our subsidiaries are generally subject to laws and regulations applicable to foreign investments in China, which may not sufficiently cover all of the aspects of our economic activities in China. In addition, the implementation of laws and regulations may be in part based on government policies and internal rules that are subject to the interpretation and discretion of different government agencies (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not always be aware of any potential violation of these policies and rules. Such unpredictability regarding our contractual, property and procedural rights could adversely affect our business and impede our ability to continue our operations. Furthermore, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. These uncertainties could materially and adversely affect our business and results of operations.
In January 2015, the MOFCOM, published a discussion draft of the proposed Foreign Investment Law, or the 2015 Draft Foreign Investment Law. The National People’s Congress published another discussion draft of the Foreign Investment Law and its amendment, or the 2018 Draft Foreign Investment Law, on December 2018 and January 2019 respectively. On March 15, 2019, the National People’s Congress approved the Foreign Investment Law, which has come into effect since January 1, 2020, or the 2019 Foreign Investment Law. Among other things, the 2015 Draft Foreign Investment Law expands the definition of foreign investment and introduces the principle of “actual control” in determining whether a company should be treated as a foreign invested enterprise, or FIE. Once an entity falls within the definition of FIE, it may be subject to foreign investment “restrictions” or “prohibitions” set forth in a “negative list” to be separately issued by the State Council. If an FIE proposes to conduct business in an industry subject to foreign investment “restrictions” in the “negative list,” the FIE must go through a pre-approval process. The 2019 Foreign Investment Law have revised the definition of “foreign investment” and removed all references to the definitions of “actual control” or “variable interest entity structure” under the 2015 Draft Foreign Investment Law, and have further specified that all “foreign investments” shall be conducted pursuant to the negative list issued or approved to be issued by the State Council.
Notwithstanding the above, the 2019 Foreign Investment Law stipulates that foreign investment includes foreign investors investing in China through any other methods under laws, administrative regulations or provisions prescribed by the State Council. There are possibilities that the Contractual Arrangements adopted by us will be deemed as a form of foreign investment, at which time it will be uncertain whether our investment in treatment centers in China and the Contractual Arrangements will be deemed to be in violation of the foreign investment regulations and how they will be handled. Therefore, there is no guarantee that the Contractual Arrangements, our business and operation will not be materially and adversely affected in the future due to the changes in PRC laws and regulations. In addition, any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention.
Due to our status as a Cayman Islands holding company, in compliance with PRC law, we own no more than 70% equity interest in our PRC operating subsidiaries and Contractual Arrangements are entered into in relation to the minority equity interest. We are able to control and consolidate our PRC operating subsidiaries as a result of our direct majority equity ownership, among other things, and do not depend on the contractual arrangements in relation to the minority equity interest to control and consolidate our PRC operating subsidiaries. However, in the event that the Chinese regulatory authorities disallowed the contractual arrangements, it may negatively impact our operations or the value of our securities, although the negative impact may not be material, given that the affected equity interest would be no more than 30% of the total equity interest.
Given recent statements by the Chinese government indicating an intent to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers, any such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or become worthless.
The General Office of the Central Committee of the Communist Party of China and the General Office of the State Council jointly issued the Opinions on Severely Cracking Down on Illegal Securities Activities According to Law, or the Opinions, which was made available to the public on July 6, 2021. The Opinions emphasized the need to strengthen the administration over illegal securities activities, and the need to strengthen the supervision over overseas listings by Chinese companies. Effective measures, such as promoting the construction of relevant regulatory systems, will be taken to deal with the risks and incidents of China-concept overseas listed companies. As of the date of this annual report, we have not received any inquiry, notice, warning, or sanctions from PRC government authorities in connection with the Opinions.
28
As such, the Company’s business segments may be subject to various government and regulatory interference in the provinces in which they operate. We could be subject to regulation by various political and regulatory entities, including various local and municipal agencies and government sub-divisions. We incur increased costs necessary to comply with existing and newly adopted laws and regulations or penalties for any failure to comply. Additionally, the governmental and regulatory interference could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless.
On December 24, 2021, the China Securities Regulatory Commission, or the CSRC, together with other relevant government authorities in Mainland China issued the Provisions of the State Council on the Administration of Overseas Securities Offering and Listing by Domestic Companies (Draft for Comments) and the Measures for the Filing of Overseas Securities Offering and Listing by Domestic Companies (Draft for Comments), both of which were open for public comments until January 23, 2022. On February 17, 2023, the CSRC issued the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Enterprises, or the Trial Measures, which take effect on March 31, 2023. On the same date, the CSRC circulated Supporting Guidance Rules No. 1 through No. 5, Notes on the Trial Measures, Notice on Administration Arrangements for the Filing of Overseas Listings by Domestic Enterprises and relevant CSRC Answers to Reporter Questions, or collectively, the Guidance Rules and Notice, on CSRC’s official website. The Trial Measures, together with the Guidance Rules and Notice, reiterate the basic principles of the Draft Administrative Provisions and Draft Filing Measures and impose substantially the same requirements for the overseas securities offering and listing by domestic enterprises. Under the Trial Measures and the Guidance Rules and Notice, domestic enterprises conducting overseas securities offering and listing, either directly or indirectly, shall complete filings with the CSRC pursuant to the Trial Measures’ requirements within three working days following the submission of an application for initial public offering or listing. Starting from March 31, 2023, enterprises that have been listed overseas or satisfy all of the following conditions shall be deemed as “Grandfathered Issuers” and are not required to complete the overseas listing filing immediately, but shall complete filings as required if they conduct refinancing or are involved in other circumstances that require filing with the CSRC: (i) the application for indirect overseas offering or listing shall have been approved by the relevant overseas regulatory authority or stock exchange prior to March 31, 2023 (as the SEC does not approve or disapprove of an offering, this requirement is interpreted to be the SEC’s declaration of the registration statement to be effective with respect to this offering), (ii) the enterprise is not required to reapply for the approval of the relevant overseas regulatory authority or stock exchange, and (iii) such overseas securities offering or listing shall be completed before September 30, 2023. Any future securities offerings and listings outside of mainland China by our company, including but not limited to follow-on offerings, refinancing, secondary listings, and going private transactions, will be subject to the filing requirements with the CSRC under the Trial Measures, and we cannot assure you that we will be able to comply with such filing requirements in a timely manner, or at all.
If it is determined that any approval, filing or other administrative procedure from the CSRC or other PRC governmental authorities is required for any future offering or listing, we cannot assure that we can obtain the required approval or accomplish the required filings or other regulatory procedures in a timely manner, or at all. If we fail to obtain the relevant approval or complete the filings and other relevant regulatory procedures, we may face sanctions by the CSRC or other PRC regulatory agencies, which may include fines and penalties on our operations in China, limitations on our operating privileges in China, restrictions on or prohibition of the payments or remittance of dividends by our PRC subsidiaries in China, or other actions that could have a material and adverse effect on our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our ADSs. As of the date of this annual report, we have not received any inquiry or notice or any objection to this offering from the CSRC, the CAC or any other PRC governmental authorities that have jurisdiction over our operations. However, given the current regulatory environment in the PRC, there remains uncertainty regarding the interpretation and enforcement of PRC laws, which can change quickly with little advance notice subject to any future actions of the PRC authorities. If the CSRC or other regulatory authorities later promulgate new rules or explanations requiring that we obtain their approvals or accomplish the required filing or other regulatory procedures for this offering, we may be unable to obtain a waiver of such approval requirements, if and when procedures are established to obtain such a waiver. Any failure of us to fully comply with new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer the ADSs, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause the ADSs to significantly decline in value or become worthless.
We have been closely monitoring the development in the regulatory landscape in Mainland China, particularly regarding the requirement of approvals, including on a retrospective basis, from the CSRC, the CAC or other PRC authorities, as well as regarding any annual data security review or other procedures that may be imposed on us. If any approval, review or other procedure is in fact required, we are not able to guarantee that we will obtain such approval or complete such review or other procedure timely or at all. For any approval that we may be able to obtain, it could nevertheless be revoked and the terms of its issuance may impose restrictions on our operations and offerings relating to our securities.
29
It may be difficult for overseas regulators to conduct investigations or collect evidence within China.
Shareholder claims or regulatory investigation that are common in the United States generally are difficult to pursue as a matter of law or practicality in China. For example, in China, there are significant legal and other obstacles to providing information needed for regulatory investigations or litigation initiated outside China. Although the authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such cooperation with the securities regulatory authorities in the Unities States may not be efficient in the absence of mutual and practical cooperation mechanism. Furthermore, according to Article 177 of the PRC Securities Law, which became effective in March 2020, no foreign securities regulator is allowed to directly conduct investigations or evidence collection activities within the PRC territory. The Trial Measures and the Draft Provisions on Confidentiality and Archives Administration also repeat the above principle and rule. While detailed interpretation of or implementation rules under Article 177 have yet to be promulgated, the inability for a foreign securities regulator to directly conduct investigations or evidence collection activities within China may further increase the difficulties you face in protecting your interests.
Changes in international trade policies and barriers to trade or the emergence of a trade war may dampen growth in China and other markets where we operate, and our business operations and results may be negatively impacted.
International trade disputes could result in tariffs and other protectionist measures that could adversely affect our business. Tariffs could increase our operating costs as well as the cost of the goods and products which could affect our customer’s discretionary spending level. In addition, any escalation in existing trade tensions or the advent of a trade war, or news and rumors of the escalation of a potential trade war, could affect consumer confidence and have a material adverse effect on our business, results of operations and, ultimately, the trading price of our ADSs.
Political tensions between the United States and China have escalated due to, among other things, the COVID-19 outbreak, the PRC National People’s Congress’ passage of Hong Kong national security legislation, sanctions imposed by the U.S. Department of Treasury on certain officials of the Hong Kong Special Administrative Region and the central government of the PRC, and the executive orders issued by U.S. President in August 2020 that prohibit certain transactions with ByteDance Ltd., Tencent Holdings Ltd. and the respective subsidiaries of such companies. In addition, on December 31, 2020, the New York Stock Exchange commenced proceedings to delist securities of three major telecommunications service providers in China in light of an executive order prohibiting any transaction in publicly traded securities of certain China-based companies by any U.S. person. There remains uncertainty as to whether the New York Stock Exchange or relevant regulatory authorities will finally determine the executive order or additional regulatory rules or orders to be applicable and proceed with the delisting proceedings against these companies or any other China-based issuers listed in the United States. Rising political tensions could reduce levels of trades, investments, technological exchanges and other economic activities between the two major economies, which would have a material adverse effect on global economic conditions and the stability of global financial markets. Any of these factors could have a material adverse effect on our business, prospects, financial condition and results of operations. Furthermore, there have been media reports on deliberations within the U.S. government regarding potentially limiting or restricting China-based companies from accessing U.S. capital markets. If any such deliberations were to materialize, the resulting legislation may have a material and adverse impact on the stock performance of China-based issuers listed in the United States. It is currently unclear whether the proposed or additional legislations would be enacted that would have the effect of potentially limiting or restricting China-based companies from accessing U.S. capital markets.
We may be exposed to liabilities under the U.S. Foreign Corrupt Practices Act, or the FCPA, Chinese anti-unfair competition laws and relevant tax laws, and any determination that we have violated these laws could have a material adverse effect on our business or our reputation.
We are subject to the FCPA. The FCPA generally prohibits us from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. We are also subject to the anti-bribery laws of other jurisdictions, particularly China. As our business expands, the applicability of the FCPA and other anti-bribery laws to our operations will increase. Our procedures and controls to monitor anti-bribery compliance may fail to protect us from reckless or criminal acts committed by our employees or agents. If we, due to either our own deliberate or inadvertent acts or those of others, fail to comply with applicable anti-bribery laws, our reputation could be harmed and we could incur criminal or civil penalties, other sanctions and/or significant expenses, which could have a material adverse effect on our business, including our financial condition, results of operations, cash flows and prospects.
30
Some of our PRC subsidiaries may pay commissions to or reimburse the expenses and costs incurred by individuals or entities if they recommend customers. Pursuant to the Anti-Unfair Competition Law of the PRC, amended and effective on April 23, 2019, commissions to suppliers are permitted if the parties properly reflect such commissions in their financial records. We are also required to withhold individual income tax in the case the referee is an individual. We have reflected such commissions on our financial records and withheld individual income tax based on our understanding. However, as we do not regard the reimbursement of expenses and costs as part of the commissions, reimbursement paid to the referee may not be reflected on our financial records or have individual income tax withheld. There is no assurance that the relevant PRC authorities will take a view consistent with our understanding. As of the date of this annual report, we have not received any penalty decisions from any relevant PRC authorities. However, we cannot assure you that we will not be subject to penalty due to allegations of violating relevant commercial bribery or anti-unfair competition laws or tax laws in the future by the local authorities in the regions where we have operations or that we will be able to defend ourselves against such allegations. If we become subject to penalties or fines for violating relevant commercial bribery or anti-unfair competition laws and/or tax laws, our business and reputation may be adversely affected.
Restrictions on currency exchange may limit our ability to receive and use financing in foreign currencies, including the remaining proceeds from our initial public offering, effectively.
Our PRC subsidiaries’ ability to obtain foreign exchange is subject to significant foreign exchange controls and, in the case of transactions under the capital account, requires the approval of and/or registration with PRC government authorities, including the Chinese State Administration of Foreign Exchange, or SAFE. In particular, if we finance our PRC subsidiaries by means of foreign debt from us or other foreign lenders, the amount is not allowed to, among other things, exceed the statutory limits and such loans must be registered with the local counterpart of the SAFE. If we finance our PRC subsidiaries by means of additional capital contributions, the amount of these capital contributions must first be registered or filed by the relevant government authority.
In the light of the various requirements imposed by PRC regulations on loans to, and direct investment in, PRC entities by offshore holding companies, we cannot assure you that we will be able to complete the necessary government registrations, filings or obtain the necessary government approvals on timely basis, if at all, with respect to future loans or capital contributions by us to our PRC subsidiaries. If we fail to complete such registrations, filings, or obtain such approval, our ability to use the proceeds we receive from our initial public offering and to capitalize or otherwise fund our PRC operations may be negatively affected, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC resident beneficial owners or our wholly foreign-owned subsidiaries in China to liability or penalties, limit our ability to inject capital into these subsidiaries, limit these subsidiaries’ ability to increase their registered capital or distribute profits to us, or may otherwise adversely affect us.
In October 2005, SAFE promulgated the Circular of the State Administration of Foreign Exchange on Issues Concerning the Regulation of Foreign Exchange in Equity Finance and Return Investments by Domestic Residents through Offshore Special Purpose Vehicles, or SAFE Circular 75, that requires PRC citizens or residents to register with SAFE or its local branch in connection with their establishment or control of an offshore entity established for the purpose of overseas equity financing involving a roundtrip investment whereby the offshore entity acquires or controls onshore assets or equity interests held by the PRC citizens or residents.
31
In July 2014, SAFE promulgated the Circular on Issues Concerning the Foreign Exchange Administration over the Overseas Investment and Financing and Round-trip Investment by Domestic Residents via Special Purpose Vehicles, or SAFE Circular 37 and overruled SAFE Circular 75 on the same date. SAFE Circular 37 requires PRC residents or entities to register with of the SAFE or its local branches in connection with their direct establishment or indirect control of an offshore entity, for the purpose of overseas investment and financing, with such PRC residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or interests, referred to in SAFE Circular 37 as a “special purpose vehicle.” The term “control” under SAFE Circular 37 is broadly defined as the operation rights, beneficiary rights or decision-making rights acquired by the PRC residents in the offshore special purpose vehicles by such means as acquisition, trust, proxy, voting rights, repurchase, convertible bonds or other arrangements. SAFE Circular 37 further requires amendment to the registration in the event of any changes with respect to the basic information of or any significant changes with respect to the special purpose vehicle. According to the Circular of Further Simplifying and Improving the Policies of Foreign Exchange Administration Applicable to Direct Investment released in February 2015 by SAFE, local banks will examine and handle foreign exchange registration for overseas direct investment, including the initial foreign exchange registration and amendment registration under SAFE Circular 37 from June 2015. If the shareholders of the offshore holding company who are PRC residents do not complete their registration with the local SAFE branches, our wholly foreign-owned subsidiaries may be prohibited from distributing their profits and proceeds from any reduction in capital, share transfer or liquidation to the offshore company, and the offshore company may be restricted in its ability to contribute additional capital to our wholly foreign-owned subsidiaries. Moreover, failure to comply with SAFE registration and amendment requirements described above could result in liability under PRC law for evasion of applicable foreign exchange restrictions.
To our knowledge, besides Dr. Zhou and Ms. Ding, who have fulfilled the registration under SAFE Circular 75, and besides Shanghai Qiangshi Business Information Consultant Co., Ltd., who has fulfilled the procedure of overseas investment for the purpose of purchasing and holding our company’s ordinary shares through Wise Sunny Limited, we are not aware of any PRC residents or entities who hold direct or indirect interests in our company and are subject to SAFE filing or registration requirements, and we will request PRC residents who we know hold direct or indirect interests in our company, if any, to make the necessary applications, filings and amendments as required under SAFE Circular 37 and other related rules. However, we may not be informed of the identities of all the PRC residents or entities holding direct or indirect interest in our company, and we cannot provide any assurance that these PRC residents or entities will comply with our request to make or obtain any applicable registrations or comply with other requirements under SAFE Circular 37 or other related rules. The failure or inability of our PRC resident or entities shareholders to comply with the registration procedures set forth in these regulations may subject us to fines and legal sanctions, restrict our cross-border investment activities, limit the ability of our wholly foreign-owned subsidiaries in China to distribute dividends and the proceeds from any reduction in capital, share transfer or liquidation to us, and we may also be prohibited from injecting additional capital into our wholly foreign-owned subsidiaries. Moreover, failure to comply with the various foreign exchange registration requirements described above could result in liability under PRC law for circumventing applicable foreign exchange restrictions. As a result, our business operations and our ability to distribute profits to you could be materially and adversely affected.
32
PRC regulations establish complex procedures for some acquisitions of Chinese companies by foreign investors, which could make it more difficult for us to pursue growth through acquisitions in Mainland China.
PRC regulations and rules concerning mergers and acquisitions including the Rules on Mergers and Acquisitions of Domestic Companies by Foreign Investors, or the M&A Rules, and other recently adopted regulations and rules with respect to mergers and acquisitions established additional procedures and requirements that could make merger and acquisition activities by foreign investors more time consuming and complex. For example, the M&A Rules require that the MOFCOM be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise, if (i) any important industry is concerned, (ii) such transaction involves factors that have or may have impact on the national economic security, or (iii) such transaction will lead to a change in control of a domestic enterprise which holds a famous trademark or PRC time-honored brand. Moreover, according to the Anti-Monopoly Law of PRC, which was last amended on June 24, 2022 and became effective on August 1, 2022, and the Provisions of the State Council on the Threshold of Filings for Undertaking Concentrations, or the Prior Notification Rules issued by the State Council in August 2008 and amended on September 2018, the concentration of business undertakings by way of mergers, acquisitions or contractual arrangements that allow one market player to take control of or to exert decisive impact on another market player must also be notified in advance to the MOFCOM when the threshold is crossed and such concentration shall not be implemented without the clearance of prior notification. In addition, the Circular of the General Office of the State Council on the Establishment of Security Review System for the Merger and Acquisition of Domestic Enterprises by Foreign Investors that became effective in March 2011, and the Rules on Implementation of Security Review System for the Merger and Acquisition of Domestic Enterprises by Foreign Investors, or the Security Review Rules issued by the MOFCOM that became effective in September 2011 specify that mergers and acquisitions by foreign investors that raise “national defense and security” concerns and mergers and acquisitions through which foreign investors may acquire the de facto control over domestic enterprises that raise “national security” concerns are subject to strict review by the MOFCOM, and the rules prohibit any activities attempting to bypass a security review by structuring the transaction through, among other things, trusts, entrustment or contractual control arrangements. In the future, we may grow our business by acquiring complementary businesses. Complying with the requirements of the above-mentioned regulations and other relevant rules to complete such transactions could be time consuming, and any required approval processes, including obtaining approval from the MOFCOM or its local counterparts may delay or inhibit our ability to complete such transactions. We believe that our business is not in an industry that raises “national defense and security” or “national security” concerns. However, the MOFCOM or other government agencies may publish explanations in the future determining that our business is in an industry subject to the security review, in which case our future acquisitions in the PRC, including those by way of entering into contractual control arrangements with target entities, may be closely scrutinized or prohibited. Our ability to expand our business or maintain or expand our market share through future acquisitions would as such be materially and adversely affected.
Our business benefits from certain financial incentives and discretionary policies granted by local governments. Expiration of, or changes to, these incentives or policies would have an adverse effect on our results of operations.
In the past, local governments in Mainland China granted certain financial incentives from time to time to our PRC subsidiaries as part of their efforts to encourage the development of local businesses. The Circular on the Relevant Tax Policies in Respect of Medical and Hygiene Institutions issued by the State Administration of Taxation, or the SAT and Ministry of Finance that became effective in July 2000 and amended in 2009, specifies that to support the development of profitable medical institution, the following preferential policy shall be applied to the income derived by profitable medical institution as is directly used to improve the medical and hygiene service conditions within three years after the date of obtaining practice registration: (1) the self-produced preparation for its own use shall be exempted from any value-added tax; and (2) the property, land, vehicles and vessels for the profitable medical institution’s own use shall be exempted from real estate tax, land-use tax of cities and towns and operation tax of vehicle and ship. Upon the expiration of the term of three years for exempting from tax, the tax collection shall be restored. The Circular on Comprehensively Promoting the Pilot Program of the Collection of Value-added Tax in Lieu of Business Tax issued by the State Administration of Taxation, or the SAT, and Ministry of Finance that became effective in May 2016, specifies that medical institutions which provide the medical services are exempted from value-added tax during the pilot scheme period for levying VAT in place of business tax. The timing, amount and criteria of government financial incentives are determined within the sole discretion of the local government authorities and cannot be predicted with certainty before we actually receive any financial incentive. We generally do not have the ability to influence local governments in making these decisions. Local governments may decide to reduce or eliminate incentives at any time. In addition, some of the government financial incentives are granted on a project basis and subject to the satisfaction of certain conditions, including compliance with the applicable financial incentive agreements and completion of the specific project therein. We cannot guarantee that we will satisfy all relevant conditions, and if we do so we may be deprived of the relevant incentives. We cannot assure you of the continued availability of the government incentives currently enjoyed by us. Any reduction or elimination of incentives would have an adverse effect on our results of operations.
33
If we are classified as a PRC resident enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders.
The PRC Enterprise Income Tax Law, or the EIT Law effective as of January 1, 2008 and amended on December 2018 and the Regulation on the Implementation of the EIT Law, effective as of January 1, 2008 and amended on April 2019, define the term “de facto management bodies” as “bodies that substantially carry out comprehensive management and control on the business operation, employees, accounts and assets of enterprises.” Under the EIT Law, an enterprise incorporated outside of PRC whose “de facto management bodies” are located in PRC is considered a “resident enterprise” and will be subject to a uniform 25% enterprise income tax (EIT) rate on its global income. On April 22, 2009, PRC’s State Administration of Taxation, or the SAT, in the Notice Regarding the Determination of Chinese-Controlled Offshore-Incorporated Enterprises as PRC Tax Resident Enterprises on the Basis of De Facto Management Bodies, or SAT Circular 82, further specified certain criteria for the determination of what constitutes “de facto management bodies.” If all of these criteria are met, the relevant foreign enterprise may be regarded to have its “de facto management bodies” located in China and therefore be considered a PRC resident enterprise. These criteria include: (i) the enterprise’s day-to-day operational management is primarily exercised in Mainland China; (ii) decisions relating to the enterprise’s financial and human resource matters are made or subject to approval by organizations or personnel in China; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholders’ meeting minutes are located or maintained in China; and (iv) 50% or more of voting board members or senior executives of the enterprise habitually reside in Mainland China. Although SAT Circular 82 only applies to foreign enterprises that are majority-owned and controlled by PRC enterprises, not those owned and controlled by foreign enterprises or individuals, the determining criteria set forth in SAT Circular 82 may be adopted by the PRC tax authorities as the test for determining whether the enterprises are PRC tax residents, regardless of whether they are majority-owned and controlled by PRC enterprises.
We believe that neither our company nor any of our subsidiaries outside of Mainland China is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities, and uncertainties remain with respect to the interpretation of the term “de facto management body.” If the PRC tax authorities determine that our company or any of its subsidiaries outside of Mainland China is a PRC resident enterprise for enterprise income tax purposes, that entity would be subject to a 25% enterprise income tax on its global income. In addition, we will also be subject to PRC enterprise income tax reporting obligations. If such entity derives income other than dividends from its wholly-owned subsidiaries in Mainland China, a 25% EIT on its global income may increase our tax burden. Dividends paid to a PRC resident enterprise from its wholly-owned subsidiaries in Mainland China may be regarded as tax-exempt income if such dividends are deemed to be “dividends between qualified PRC resident enterprises” under the EIT Law and its implementation rules. However, we cannot assure you that such dividends will not be subject to PRC withholding tax, as the PRC tax authorities, which enforce the withholding tax, have not yet issued relevant guidance.
In addition, if we are classified as a PRC resident enterprise for PRC tax purposes, we may be required to withhold tax at a rate of 10% from dividends we pay to our shareholders, including the holders of the ADSs, that are non-resident enterprises. In addition, non-resident enterprise shareholders (including the ADS holders) may be subject to a 10% PRC withholding tax on gains realized on the sale or other disposition of ADSs or ordinary shares, if such income is treated as sourced from within Mainland China. Furthermore, gains derived by our non-PRC individual shareholders from the sale of our shares and ADSs may be subject to a 20% PRC withholding tax. Furthermore, if we are deemed a PRC resident enterprise, dividends paid to our non-PRC individual shareholders (including the ADS holders) and any gain realized on the transfer of ADSs or ordinary shares by such shareholders may be subject to PRC tax at a rate of 20% (which, in the case of dividends, may be withheld at source by us), if such gains are deemed to be from PRC sources. These rates may be reduced by an applicable tax treaty, but it is unclear whether non-PRC shareholders of our company would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as a PRC resident enterprise. Any such tax may reduce the returns on your investment in the ADSs or ordinary shares.
34
We may rely on dividends and other distributions on equity paid by our PRC subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.
We are a holding company, and we may rely on dividends and other distributions on equity paid by our PRC subsidiaries for our cash and financing requirements, including the funds necessary to pay dividends and other cash distributions to our shareholders or to service any debt we may incur. If any of our PRC subsidiaries incur debt on its own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us. Under PRC laws and regulations, Peng Yi Da, a wholly foreign-owned enterprise, may pay dividends only out of its respective accumulated profits as determined in accordance with PRC accounting standards and regulations. In addition, a wholly foreign-owned enterprise is required to set aside at least 10% of its accumulated after-tax profits each year, if any, to fund a certain statutory reserve fund, until the aggregate amount of such fund reaches 50% of its registered capital. Such reserve funds cannot be distributed to us as dividends. At its discretion, a wholly foreign-owned enterprise may allocate a portion of its after-tax profits based on PRC accounting standards to an enterprise expansion fund, or a staff welfare and bonus fund.
Our PRC subsidiaries generate primarily all of their revenue in renminbi, which is not freely convertible into other currencies. As result, any restriction on currency exchange may limit the ability of our PRC subsidiaries to use their renminbi revenues to pay dividends to us.
In response to the persistent capital outflow in Mainland China and renminbi’s depreciation against U.S. dollar in the fourth quarter of 2016, China’s People’s Bank of China, or the PBOC, and the SAFE have promulgated a series of capital control measure over recent months, including stricter vetting procedures for China-based companies to remit foreign currency for overseas investments, dividends payments and shareholder loan repayments.
The PRC government may continue to strengthen its capital controls, and more restrictions and substantial vetting process may be put forward by SAFE for cross-border transactions falling under both the current account and the capital account. Any limitation on the ability of our PRC subsidiaries to pay dividends or make other kinds of payments to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends, or otherwise fund and conduct our business.
We and our shareholders face uncertainties in the PRC with respect to indirect transfers of equity interests in PRC resident enterprises.
The indirect transfer of equity interest in PRC resident enterprises by a non-PRC resident enterprise, or Indirect Transfer, is potentially subject to income tax in Mainland China at a rate of 10% on the gain if such transfer is considered as not having a commercial purpose and is carried out for tax avoidance. The SAT has issued several rules and notices to tighten the scrutiny over acquisition transactions in recent years. SAT Circular 7 sets out the scope of Indirect Transfers, which includes any changes in the shareholder’s ownership of a foreign enterprise holding PRC assets directly or indirectly in the course of a group’s overseas restructuring, and the factors to consider in determining whether an Indirect Transfer has a commercial purpose. An Indirect Transfer satisfying all the following criteria will be deemed to lack a bona fide commercial purpose and be taxable under PRC laws: (i) 75% or more of the equity value of the intermediary enterprise being transferred is derived directly or indirectly from the PRC taxable assets; (ii) at any time during the one-year period before the indirect transfer, 90% or more of the asset value of the intermediary enterprise (excluding cash) is comprised directly or indirectly of investments in mainland China, or 90% or more of its income is derived directly or indirectly from China; (iii) the functions performed and risks assumed by the intermediary enterprise and any of its subsidiaries that directly or indirectly hold the PRC taxable assets are limited and are insufficient to prove their economic substance; and (iv) the non-PRC tax payable on the gain derived from the indirect transfer of the PRC taxable assets is lower than the potential PRC income tax on the direct transfer of such assets. Nevertheless, a non-resident enterprise’s buying and selling shares or ADSs of the same listed foreign enterprise on the public market will fall under the safe harbor available under SAT Circular 7 and will not be subject to PRC tax pursuant to SAT Circular 7.
On October 17, 2017, the State Administration of Taxation issued the Circular on Issues of Tax Withholding regarding Non-PRC Resident Enterprise Income Tax at Source, or SAT Circular 37, which came into effect on December 1, 2017 and amended on June 15, 2018. SAT Circular 37 further clarifies the practice and procedure of the withholding of nonresident enterprise income tax.
35
However, as these rules and notices are relatively new and there is a lack of clear statutory interpretation, we face uncertainties regarding the reporting required for and impact on future private equity financing transactions, share exchange or other transactions involving the transfer of shares in our company by investors that are non-PRC resident enterprises, or the sale or purchase of shares in other non-PRC resident companies or other taxable assets by us. For example, the PRC tax authorities may consider that our current offering involves an indirect change of shareholding in our PRC subsidiaries and therefore it may be regarded as an Indirect Transfer under SAT Circular 7 or SAT Circular 37. Although we believe no SAT Circular 7 or SAT Circular 37 reporting is required on the basis that the current offering has commercial purposes and is not conducted for tax avoidance, the PRC tax authorities may pursue us to report under SAT Circular 7 or SAT Circular 37 and request that we and our PRC subsidiaries assist in the filing. As a result, we and our subsidiaries may be required to expend significant resources to provide assistance and comply with SAT Circular 7 or SAT Circular 37, or establish that we or our non-resident enterprises should not be subject to tax under SAT Circular 7 or SAT Circular 37, for the current offering or other transactions, which may have an adverse effect on our and their financial condition and day-to-day operations.
Any failure to comply with PRC regulations regarding the registration requirements for our employee equity incentive plans may subject us to fines and other legal or administrative sanctions, which could adversely affect our business, financial condition and results of operations.
We have adopted a share incentive plan and a performance incentive plan pursuant to which we may grant share options or other equity incentives to our directors, employees or consultants in the future. In February 2012, the SAFE promulgated the Notices on Issues Concerning the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plans of Overseas Publicly Listed Companies, or the Stock Option Rules. In accordance with the Stock Option Rules and relevant rules and regulations, PRC citizens or non-PRC citizens residing in China for a continuous period of not less than one year, who participate in any stock incentive plan of an overseas publicly listed company, subject to a few exceptions, are required to register with SAFE through a domestic qualified agent, which could be a PRC subsidiary of such overseas publicly listed company, and complete certain procedures. We and our employees who are PRC citizens or who reside in China for a continuous period of not less than one year and who participate in our stock incentive plan will be subject to such regulation. However, any failure to comply with the SAFE registration requirements may subject them to fines and legal sanctions and may limit the ability of our PRC subsidiaries to distribute dividends to us. We also face regulatory uncertainties that could restrict our ability to adopt additional incentive plans for our directors and employees under PRC law.
Our ADSs may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect auditors who are located in China. The delisting of our ADSs, or the threat of their being delisted, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections deprives our investors with the benefits of such inspections.
The Holding Foreign Companies Accountable Act, or the HFCAA, was enacted on December 18, 2020. The HFCAA states if the SEC determines that we have filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years beginning in 2021, the SEC shall prohibit our shares or ADSs from being traded on a national securities exchange or in the over the counter trading market in the U.S.
On March 24, 2021, the SEC adopted interim final rules to implement the disclosure and submission requirements of the HFCAA. An identified issuer shall be required to comply with these rules if the SEC identifies it as having a “non-inspection” year under a process to be subsequently established by the SEC. On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, which was signed into law on December 29, 2022, amending the HFCAA and requiring the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchange if its auditor is not subject to PCAOB inspections for two consecutive years instead of three consecutive years. On September 22, 2021, the PCAOB adopted a final rule implementing the HFCAA, which provides a framework for the PCAOB to use when determining, as contemplated under the HFCAA, whether the PCAOB is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and disclosure requirements in the HFCAA. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions. On December 16, 2021, the PCAOB issued determinations that it is unable to inspect or investigate completely registered public accounting firms headquartered in mainland China and in Hong Kong, because of positions taken by PRC authorities in those jurisdictions.
36
On August 26, 2022, the PCAOB announced that it had signed a Statement of Protocol (the “SOP”) with the China Securities Regulatory Commission and the Ministry of Finance of China. The SOP, together with two protocol agreements governing inspections and investigations (together, the “SOP Agreement”), establishes a specific, accountable framework to enable complete inspections and investigations by the PCAOB of audit firms based in mainland China and Hong Kong, as required under U.S. law. The SOP Agreement remains unpublished and is subject to further explanation and implementation. Pursuant to the fact sheet with respect to the SOP Agreement disclosed by the SEC, the PCAOB shall have sole discretion to make its selection of audit firms for inspection or investigation and the PCAOB inspectors and investigators shall have a right to see all audit documentation without redaction. Under the PCAOB’s rules, a reassessment of a determination under the HFCAA may result in the PCAOB reaffirming, modifying or vacating the determination.
On December 15, 2022, the PCAOB announced that it had secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. The PCAOB Board vacated its December 2021 determinations that the PCAOB was unable to inspect or investigate completely registered public accounting firms headquartered in mainland China and Hong Kong. The PCAOB will continue to demand to inspect and investigate completely in mainland China and Hong Kong in the future and is planning to resume regular inspections in early 2023 and beyond, as well as to continue pursuing ongoing investigations and initiate new investigations as needed. However, the PCAOB’s ability to continue to satisfactorily inspect or investigate registered public accounting firms headquartered in mainland China and Hong Kong is subject to uncertainties and depends on a number of factors beyond our and our auditor’s control.
We appointed Union Power HK, a public accounting firm registered with PCAOB as our independent registered public accounting firm, effective January 28, 2022. Union Power HK, the independent registered public accounting firm that issues the audit report included elsewhere in this annual report, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards.
The SEC is assessing how to implement other requirements of the HFCAA, including the listing and trading prohibition requirements described above.
Such uncertainty in the implementation of HFCAA and the regular inspections and assessments carried out by PCAOB could cause the market price of our ADSs to be materially and adversely affected, and our securities could be delisted or prohibited from being traded “over-the-counter” earlier than would be required by the HFCAA. If our securities are unable to be listed on another securities exchange by then, such a delisting would substantially impair your ability to sell or purchase our ADSs when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of our ADSs. Trading in our securities may also be prohibited under the HFCAA if the PCAOB determines that it cannot inspect or investigate completely our auditor, and as a result an exchange may delist our securities, causing the value of our ADSs to decline significantly.
Risks relating to the ADSs
We have broad discretion to determine how to use the remaining net proceeds from our initial public offering and may use them in ways that may not enhance our results of operations or the price of the ADSs.
Although we currently intend to use the remaining net proceeds from our initial public offering in the manner described in the section titled “Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds—14.E. Use of Proceeds” in this annual report, our management will have broad discretion over the use of net proceeds from our initial public offering, and we could spend the net proceeds from our initial public offering in ways the holders of the ADSs may not agree with, or that do not yield a favorable return. Because of the number and variability of factors that will determine our use of the net proceeds from our initial public offering, our use of these proceeds may differ substantially from our current plans. The failure by our management to apply these funds effectively could have a material adverse effect on our business, financial condition and results of operation. You will not have the opportunity, as part of your investment decision, to assess whether proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the net proceeds of our initial public offering.
37
Our ADSs may be delisted from the Nasdaq Capital Market as a result of our failure of meeting the Nasdaq Capital Market continued listing requirements.
Our ADSs are currently listed on the Nasdaq Capital Market under the symbol “AIH”. In order to maintain the listing of our ADSs on the Nasdaq Capital Market, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, minimum share price, and certain corporate governance requirements. There can be no assurances that we will be able to comply with such applicable listing standards.
On June 2, 2022, we received a staff determination letter from the Nasdaq notifying us that, based on our stockholders’ equity as reported in our annual report for the year ended December 31, 2021, we did not meet the minimum stockholders’ equity requirement for continued listing on the Nasdaq Global Market under Nasdaq Listing Rule 5450(b)(1)(A) (the “Rule”). As provided in the Rule, we have 45 calendar days to submit a play to regain compliance with the minimum stockholders’ equity requirement.
On December 1, 2022, we received a staff determination letter from the Nasdaq notifying us that because we have not regained compliance with the Rule, unless we request an appeal of this determination, our ADSs would be scheduled for delisting from the Nasdaq Global Market. We requested a hearing before the Nasdaq Hearings Panel and subsequently submitted an application to transfer the listing of our ADSs from the Nasdaq Global Market to the Nasdaq Capital Market. The Listing Qualifications of Nasdaq approved our request to the transfer listing on January 17, 2023. Subsequently, our ADSs have been listed on the Nasdaq Capital Market uninterruptedly under the symbol “AIH” since January 19, 2023. However, there can be no assurance that we will be able to successfully maintain compliance with the several Nasdaq continued listing requirements in the future.
Our chief executive officer, Dr. Zhou Pengwu, and his spouse, Ms. Ding Wenting, are able to control and exert significance influence over our company, and their interest may be different from or conflict with that of the holders of our ADSs.
Our chief executive officer, Dr. Zhou Pengwu, and his spouse, Ms. Ding Wenting, jointly control more than 50% of the voting power of our company. Although currently each of Dr. Zhou Pengwu and Ms. Ding Wenting disclaims beneficial ownership of any of our ordinary shares beneficially owned by the other, they might act as a group in the future. In addition, one of our directors, Dr. Zhou Yitao, is the son of Dr. Zhou Pengwu. In addition to the elections of our directors, Dr. Zhou Pengwu and Ms. Ding Wenting are and will continue to be able to exert a significant degree of influence or actual control over other management and affairs and control matters requiring an approval from a majority of shareholders, including the merger, consolidation or sale of all or substantially all of our assets, and any other significant transaction. Dr. Zhou Pengwu’s and Ms. Ding Wenting’s interest might not always be aligned with the interests of our other shareholders.
Certain provisions in our fourth amended and restated articles of association (as revised) may limit your ability to influence corporate matters and could discourage others from pursuing any change of control transactions.
Our fourth amended and restated articles of association (as revised), currently in effect, provides, among other things, that certain actions including the voluntary delisting of the our securities, a Change of Control Event (as defined in our fourth amended and restated articles of association (as revised)) and certain transfers or other disposals of any assets, businesses or securities of us or any of our subsidiaries, can only be approved by shareholders’ resolutions. For more details, please see “Item 6. Directors, Senior Management and Employees—6.C. Board Practices—Appointment of directors by our principal shareholders” and “Item 10. Additional Information—10.B. Memorandum and Articles of Association—Ordinary Shares—Reserved Matters.”
As a result of these provisions in our fourth amended and restated articles of association (as revised), although holders of our ordinary shares have the same voting rights, certain shareholders such as Dr. Zhou Pengwu, ADV, MY Universe, and Hawyu will have considerable influence over certain corporate governance matters such as decisions regarding mergers, consolidations and the sale of all or substantially all of our assets and appointment of chief officers. Such principal shareholders may take actions that are not in the best interest of us or our other shareholders.
These provisions may also discourage, delay or prevent a change in control of our company, which could have the effect of depriving our other shareholders of the opportunity to receive a premium for their shares as part of a sale of our company and may reduce the price of our ADSs. These provisions may limit your ability to influence corporate matters and could discourage others from pursuing any potential merger, takeover or other change of control transactions that holders of our ordinary shares and ADSs may view as beneficial.
38
We might be a “controlled company” as defined under the Nasdaq Stock Market Rules in the future. As a result, we would be entitled to rely on exemptions from certain corporate governance requirements and holders of our ordinary shares or ADSs may not have the same protections generally available to stockholders of other companies listed on stock exchanges in the United States.
Our chief executive officer, Dr. Zhou Pengwu, and his spouse, Ms. Ding Wenting cumulatively hold more than 50% of the voting power for the election of our directors. Although currently each of Dr. Zhou Pengwu and Ms. Ding Wenting disclaims beneficial ownership of any of our ordinary shares beneficially owned by the other, they might file a formal notice to act as a group in the future, under which situation we would be a “controlled company” as defined under Rule 5615(c)(1) of the Nasdaq Stock Market Rules. If we are deemed as a “controlled company,” we would qualify for exemptions from several of corporate governance requirements under the Nasdaq listing standards, including requirements that:
● | a majority of the board of directors consist of independent directors; |
● | compensation of officers be determined or recommended to the board of directors by a majority of its independent directors or by a compensation committee comprised solely of independent directors; and |
● | director nominees be selected or recommended to the board of directors by a majority of its independent directors or by a nominating committee that is composed entirely of independent directors. |
Accordingly, to the extent that we choose to rely on one or more of these exemptions, our shareholders would not be afforded the same protections generally as shareholders of other Nasdaq- listed companies as long as Dr. Zhou Pengwu and Ms. Ding Wenting file a formal notice to act as a group, controlling more than 50% of the voting power of our company, and our board determines to rely upon one or more of such exemptions.
We may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
If securities analysts do not publish favorable research about our business or if they publish negative evaluations of our business, the price of the ADSs could decline.
The trading market for the ADSs will rely in part on the research and reports that industry or financial analysts publish about us or our business. We may never obtain research coverage by industry or financial analysts. If no or few analysts commence coverage of us, the trading price of our stock would likely decrease. Even if we do obtain analyst coverage, if one or more of the analysts covering our business downgrade their evaluations of our stock, the price of our stock could decline. If one or more of these analysts cease to cover our stock, we could lose visibility in the market for our stock, which in turn could cause our stock price to decline.
We are an “emerging growth company” as defined in the Securities Act, and we cannot be certain if the reduced disclosure requirements applicable to us as an “emerging growth company” will make the ADSs less attractive to investors.
We are eligible to be treated as an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. As a result, our shareholders may not have access to certain information that they may deem important. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if our total annual gross revenue exceeds US$1.07 billion, if we issue more than US$1.0 billion in non-convertible debt securities during any three-year period, or if the market value of our ordinary shares held by non-affiliates exceeds US$700.0 million. We cannot predict if investors will find the ADSs less attractive because we may rely on these exemptions. If some investors find the ADSs less attractive as a result, there may be a less active trading market for the ADSs and our stock price may be more volatile.
39
As a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to a domestic U.S. issuer, which may limit the information publicly available to our shareholders.
As a foreign private issuer, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act and therefore there may be less publicly available information about us than if we were a U.S. domestic issuer. For example, we are not subject to the proxy rules in the United States and disclosure with respect to our annual general meetings will be governed by the Cayman Islands requirements. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules thereunder. Therefore, our shareholders may not know on a timely basis when our officers, directors and principal shareholders purchase, or sell, our ordinary shares or ADSs.
As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq corporate governance standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.
As a foreign private issuer, we are permitted to take advantage of certain corporate governance requirements under the Nasdaq listing standards that allow us to follow Cayman Islands law for certain governance matters. Certain corporate governance practices in the Cayman Islands may differ significantly from corporate governance listing standards as, except for general fiduciary duties and duties of care, Cayman Islands law had no corporate governance regime which prescribes specific corporate governance standards as of the date of this annual report. For example, we have followed and intend to continue to follow Cayman Islands corporate governance practices in lieu of the corporate governance requirements of the Nasdaq Capital Market that listed companies must have a majority of independent directors. Cayman Islands law does not impose a requirement that our board of directors consist of a majority of independent directors. Nor does Cayman Islands law impose specific requirements on the establishment of a compensation committee or nominating committee or nominating process. To the extent we choose to follow home country practice in the future, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers.
Because we are incorporated under Cayman Islands law and conduct our operations primarily in emerging markets, you may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts may be limited.
We are an exempted company incorporated under the laws of the Cayman Islands. Our corporate affairs are governed by our memorandum and articles of association, the Companies Act (As Revised) of the Cayman Islands, or the Companies Act, and the common law of the Cayman Islands. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from the common law of England, the decisions of whose courts are of persuasive authority, but are not binding, on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedent in some jurisdictions in the United States. In particular, the Cayman Islands has a less developed body of securities laws than the United States. Some U.S. states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law than the Cayman Islands. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the United States.
There is uncertainty as to whether the courts of the Cayman Islands would (1) recognize or enforce against us judgments of courts of the United States based on certain civil liability provisions of U.S. securities laws, or (2) entertain original actions brought in the Cayman Islands against us or our directors or officers that are predicated upon the federal securities laws of the United States or the securities laws of any state in the United States.
The uncertainty with regard to Cayman Islands law relates to whether a judgment obtained from the United States courts under the civil liability provisions of the securities laws will be determined by the courts of the Cayman Islands as penal or punitive in nature. If such a determination is made, the courts of the Cayman Islands will not recognize or enforce the judgment against a Cayman Islands company. Because the courts of the Cayman Islands have yet to rule on whether such judgments are penal or punitive in nature, it is uncertain whether they would be enforceable in the Cayman Islands.
40
There is no statutory recognition in the Cayman Islands of judgments obtained in the United States, although the Cayman Islands will generally recognize as a valid judgment, a final and conclusive judgment in personam obtained in the federal or state courts in the United States under which a sum of money is payable (other than a sum of money payable in respect of multiple damages, taxes or other charges of a like nature or in respect of a fine or other penalty) or, in certain circumstances, an in personam judgment for non-monetary relief, and would give a judgment based thereon provided that (i) such courts had proper jurisdiction over the parties subject to such judgment; (ii) such courts did not contravene the rules of natural justice of the Cayman Islands; (iii) such judgment was not obtained by fraud; (iv) the enforcement of the judgment would not be contrary to the public policy of the Cayman Islands; (v) no new admissible evidence relevant to the action is submitted prior to the rendering of the judgment by the courts of the Cayman Islands; and (vi) there is due compliance with the correct procedures under the laws of the Cayman Islands.
In addition, we conduct substantially all of our business operations in emerging markets, including China, and substantially all of our directors and senior management are based in China. The SEC, U.S. Department of Justice and other authorities often have substantial difficulties in bringing and enforcing actions against non-U.S. companies and non-U.S. persons, including company directors and officers, in certain emerging markets, including China. Additionally, our public shareholders may have limited rights and few practical remedies in emerging markets where we operate, as shareholder claims that are common in the United States, including class action securities law and fraud claims, generally are difficult or impossible to pursue as a matter of law or practicality in many emerging markets, including China. For example, in China, there are significant legal and other obstacles for the SEC, the DOJ and other U.S. authorities to obtaining information needed for shareholder investigations or litigation. Although the competent authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, the regulatory cooperation with the securities regulatory authorities in the United States has not been efficient in the absence of a mutual and practical cooperation mechanism. In China, without the consent of the competent PRC securities regulators and relevant authorities, no organization or individual may provide the documents and materials relating to securities business activities to foreign securities regulators.
As a result of all of the above, our public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors or large shareholders than they would as public shareholders of a company incorporated in the United States.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our ordinary shares are directly or indirectly held by residents of the U.S. and we fail to meet additional requirements necessary to maintain our foreign private issuer status. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq listing standards. If we lost our foreign private issuer status, we would incur significant additional legal, accounting and other expenses that we do not incur as a foreign private issuer, in order to maintain a listing on a U.S. securities exchange.
We do not currently intend to pay dividends on our securities, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of the ADSs.
We have never declared or paid any dividends on our ordinary shares. We currently intend to invest our future earnings, if any, to fund our growth. Therefore, you are not likely to receive any dividends on your ADSs at least in the near term, and the success of an investment in ADSs will depend upon any future appreciation in its value. Consequently, investors may need to sell all or part of their holdings of ADSs after price appreciation, which may never occur, to realize any future gains on their investment. There is no guarantee that the ADSs will appreciate in value or even maintain the price at which our investors purchased their ADSs.
41
There is no public market for our ordinary shares, and you may not be able to resell the ADSs at or above the price you paid, or at all.
Our ADSs are listed on the Nasdaq Capital Market, while there is no public market for our ordinary shares. Our ordinary shares will not be listed on any other exchange, or quoted for trading on any over-the-counter trading system, in the United States. The initial public offering price for the ADSs were determined by negotiations between us and the underwriters and may bear no relationship to the market price for the ADSs after our initial public offering. We cannot assure you that an active trading market for the ADSs will develop or maintain, or that the market price of the ADSs will increase above the initial public offering price. If an active trading market for the ADSs does not develop or maintain, the market price and liquidity of the ADSs will be materially and adversely affected.
The market price for the ADSs may be volatile which could result in substantial loss to you.
The market price for the ADSs is likely to be highly volatile and subject to wide fluctuations in response to factors, including the following:
● | announcements of competitive developments; |
● | regulatory developments affecting us, our customers or our competitors; |
● | reputation of players in our industry or companies headquartered in China; |
● | announcements regarding litigation or administrative proceedings involving us; |
● | actual or anticipated fluctuations in our period-to-period operating results; |
● | changes in financial estimates by securities research analysts; |
● | additions or departures of our executive officers; |
● | fluctuations of exchange rates between the RMB and the U.S. dollar; |
● | release or expiry of lock-up or other transfer restrictions on our outstanding ordinary shares or ADSs; and |
● | sales or perceived sales of additional ordinary shares or ADSs. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. For example, since August 2008, multiple exchanges in the United States and other countries and regions, including China, experienced sharp declines in response to the growing credit market crisis and the recession in the United States. As recently as August 2015, the exchanges in China experienced a sharp decline. Prolonged global capital markets volatility may affect overall investor sentiment towards the ADSs, which would also negatively affect the trading prices for the ADSs.
42
Fluctuations in currency exchange rates may have a material adverse effect on our results of operations and the value of your investment.
The value of the renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions. On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the renminbi to the U.S. dollar, and the renminbi appreciated more than 20% against the U.S. dollar over the following three years. Between July 2008 and June 2010, this appreciation halted, and the exchange rate between the renminbi and U.S. dollar remained within a narrow band. In June 2010, the PBOC, announced that the PRC government would increase the flexibility of the exchange rate, and thereafter allowed the renminbi to appreciate slowly against the U.S. dollar within the narrow band fixed by the PBOC. However, more recently, on August 11, 12 and 13, 2015, the PBOC significantly devalued the renminbi by fixing its price against the U.S. dollar 1.9%, 1.6%, and 1.1% lower than the previous day’s value, respectively. On October 1, 2016, the renminbi joined the International Monetary Fund’s basket of currencies that make up the Special Drawing Right, along with the U.S. dollar, the Euro, the Japanese yen and the British pound. In the fourth quarter of 2016, the renminbi depreciated significantly while the U.S. dollar surged and China experienced persistent capital outflows. With the development of the foreign exchange market and progress towards interest rate liberalization and renminbi internationalization, the PRC government may in the future announce further changes to the exchange rate system. There is no guarantee that the renminbi will not appreciate or depreciate significantly in value against the U.S. dollar in the future. It is difficult to predict how market forces, PRC and U.S. government’s policies and regulations may impact the exchange rate between the renminbi and the U.S. dollar in the future.
Significant revaluation of the renminbi may have a material adverse effect on your investment. For example, to the extent that we need to convert U.S. dollars into renminbi for our operations, appreciation of the renminbi against the U.S. dollar would have an adverse effect on the renminbi amount we would receive from the conversion. Conversely, if we decide to convert our renminbi into U.S. dollars for the purpose of making payments for dividends on our ordinary shares or ADSs or for other business purposes, appreciation of the U.S. dollar against the renminbi would have a negative effect on the U.S. dollar amount available to us. In addition, appreciation or depreciation in the value of the renminbi relative to U.S. dollars would affect our financial results reported in U.S. dollar terms regardless of any underlying change in our business or results of operations.
Very limited hedging options are available in Mainland China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may decide to enter into hedging transactions in the future, the availability and effectiveness of these hedges may be limited and we may not be able to adequately hedge our exposure or at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert renminbi into foreign currency.
Substantial future sales or perceived sales of the ADSs in the public market could cause the price of the ADSs to decline.
Sales of the ADSs in the public market after our initial public offering, or the perception that these sales could occur, could cause the market price of the ADSs to decline. As of December 31, 2022, we had 94,044,740 ordinary shares outstanding, including 29,852,856 ordinary shares represented by ADSs. All ADSs sold in our initial public offering are freely transferable without restriction or additional registration under the Securities Act. The remaining ordinary shares outstanding after our initial public offering became available for sale, subject to restrictions as applicable under Rule 144 under the Securities Act, upon the expiration of the 180-day lock-up arrangements entered into among us, the underwriters and other relevant parties on April 30, 2020. We cannot predict what effect, if any, market sales of securities held by our significant shareholders or any other shareholder, or the availability of these securities for future sale, will have on the market price of the ADSs.
43
Holders of ADSs have fewer rights than shareholders and must act through the depositary to exercise their rights.
Holders of ADSs do not have the same rights as our registered shareholders. As a holder of our ADSs, you will not have any direct right to attend general meetings of our shareholders or to cast any votes at such meetings. As an ADS holder, you will only be able to exercise the voting rights carried by the underlying ordinary shares indirectly by giving voting instructions to the depositary in accordance with the provisions of the deposit agreement. Under the deposit agreement, you may vote only by giving voting instructions to the depositary. Upon receipt of your voting instructions, the depositary will try, as far as is practicable, to vote the ordinary shares underlying your ADSs in accordance with your instructions. If we ask for your instructions, then upon receipt of your voting instructions, the depositary will try to vote the underlying ordinary shares in accordance with these instructions. If we do not instruct the depositary to ask for your instructions, the depositary may still vote in accordance with instructions you give, but it is not required to do so. You will not be able to directly exercise your right to vote with respect to the underlying ordinary shares unless you withdraw the shares, and become the registered holder of such shares prior to the record date for the general meeting. When a general meeting is convened, you may not receive sufficient advance notice of the meeting to withdraw the shares underlying your ADSs and become the registered holder of such shares to allow you to attend the general meeting and to vote directly with respect to any specific matter or resolution to be considered and voted upon at the general meeting. In addition, under our memorandum and articles of association currently in effect, for the purposes of determining those shareholders who are entitled to attend and vote at any general meeting, our directors may close our register of members and/or fix in advance a record date for such meeting, and such closure of our register of members or the setting of such a record date may prevent you from withdrawing the ordinary shares underlying your ADSs and becoming the registered holder of such shares prior to the record date, so that you would not be able to attend the general meeting or to vote directly. If we ask for your instructions, the depositary will notify you of the upcoming vote and will arrange to deliver our voting materials to you. We have agreed to give the depositary notice of shareholder meetings sufficiently in advance of such meetings. Nevertheless, we cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote the underlying ordinary shares represented by your ADSs. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for their manner of carrying out your voting instructions. This means that you may not be able to exercise your right to direct how the shares underlying your ADSs are voted and you may have no legal remedy if the shares underlying your ADSs are not voted as you requested. In addition, in your capacity as an ADS holder, you will not be able to call a shareholders’ meeting.
Except in limited circumstances, the depositary for our ADSs gives us a discretionary proxy to vote the ordinary shares underlying your ADSs if you do not vote at shareholders’ meetings, which could adversely affect your interests.
Under the deposit agreement for the ADSs, if you do not vote, the depositary gives us a discretionary proxy to vote the ordinary shares underlying your ADSs at shareholders’ meetings unless:
● | we have instructed the depositary that we do not wish a discretionary proxy to be given; |
● | we have informed the depositary that there is substantial opposition as to a matter to be voted on at the meeting; |
● | a matter to be voted on at the meeting would have a material adverse impact on shareholders; or |
● | the voting at the meeting is to be conducted via a show of hands unless voting by poll is required by the applicable listing rules or our articles of association. |
The effect of this discretionary proxy is that you cannot prevent our ordinary shares underlying your ADSs from being voted, except under the circumstances described above. This may make it more difficult for shareholders to influence the management of our company. In contrast, holders of our ordinary shares will not be subject to this discretionary proxy.
44
You may not receive distributions on the ADSs or any value for them if such distribution is illegal or impractical or if any required government approval cannot be obtained in order to make such distribution available to you.
Although we do not have any present plan to pay any dividends, the depositary of the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities underlying the ADSs, after deducting its fees and expenses and any applicable taxes and governmental charges. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities whose offering would require registration under the Securities Act but are not so properly registered or distributed under an applicable exemption from registration. The depositary may also determine that it is not reasonably practicable to distribute certain property. In these cases, the depositary may determine not to distribute such property. We have no obligation to register under the U.S. securities laws any offering of ADSs, ordinary shares, rights or other securities received through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, ordinary shares, rights or anything else to holders of ADSs. This means that you may not receive distributions we make on our ordinary shares or any value for them if it is illegal or impractical for us to make them available to you. These restrictions may cause a material decline in the value of the ADSs.
Your right to participate in any future rights offerings may be limited, which may cause dilution to your holdings.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make rights available to you in the United States unless we register the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Also, under the deposit agreement, the depositary bank will not make rights available to you unless either both the rights and any related securities are registered under the Securities Act, or the distribution of them to ADS holders is exempted from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. If the depositary does not distribute the rights, it may, under the deposit agreement, either sell them, if possible, or allow them to lapse. Accordingly, you may be unable to participate in our rights offerings and may experience dilution in your holdings.
We may be classified as a passive foreign investment company, which could result in adverse U.S. federal income tax consequences to U.S. holders of the ADSs or ordinary shares.
Depending upon the value of the ADSs and ordinary shares and the nature and composition of our assets and income over time, we could be classified as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Based on the value of our outstanding ADSs and ordinary shares and the composition of our assets and income, we do not believe we were a PFIC for the taxable year ending December 31, 2022. However, there can be no assurance that we will not be a PFIC for the current taxable year. In addition, there can be no assurance that we will not be a PFIC for any future taxable year. PFIC status is a factual determination that must be tested each taxable year and will depend on the composition of our assets and income in each such taxable year.
We will be classified as a PFIC for any taxable year if either (i) at least 75% of our gross income for the taxable year is passive income or (ii) at least 50% of the value of our assets (based on a quarterly value of the assets during the taxable year) is attributable to assets that produce or are held for the production of passive income. In determining the average percentage value of our gross assets, the aggregate value of our assets will generally be deemed to be equal to our market capitalization (determined by the sum of the aggregate values of our outstanding equity) plus our liabilities. Accordingly, we could become a PFIC if our market capitalization were to decrease significantly while we hold substantial cash, cash equivalents or other assets that produce or are held for the production of passive income. In addition, because there are uncertainties in the application of the relevant PFIC rules, it is possible that the Internal Revenue Service, or IRS, may challenge our classification of certain income and assets as non-passive or our valuation of our tangible and intangible assets, which could result in a determination that we were a PFIC for the current or subsequent taxable years.
If we were classified as a PFIC in any taxable year in which a U.S. Holder (as defined in “Item 10. Additional Information—10.E. Taxation—United States Federal Income Taxation”) holds the ADSs or ordinary shares, the U.S. Holder would generally be subject to additional taxes and interest charges on certain “excess distributions” we make and on the gain, if any, recognized on the disposition or deemed disposition of such U.S. Holder’s ADS or ordinary shares, even if we are no longer a PFIC in the year of distribution or disposition. Moreover, such U.S. Holder would also be subject to special U.S. tax reporting requirements. For more information on the U.S. tax consequences to U.S. Holders that would result from our classification as a PFIC, see “Item 10. Additional Information—10.E. Taxation—United States federal income taxation—Passive foreign investment company.”
45
You may have difficulty enforcing judgments obtained against us.
We are a company incorporated under the laws of the Cayman Islands, and substantially all of our assets are located outside the United States. Substantially all of our current operations are conducted in the PRC. In addition, some of our directors and officers are nationals and residents of countries other than the United States. A substantial portion of the assets of these persons are located outside the United States. As a result, it may be difficult for you to effect service of process within the United States upon these persons. It may also be difficult for you to enforce in U.S. courts judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors, some of whom currently reside in the United States and whose assets are located outside the United States. In addition, there is uncertainty as to whether the courts of the Cayman Islands or the PRC would recognize or enforce judgments of U.S. courts against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state.
The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between Mainland China and the country where the judgment is made or on principles of reciprocity between jurisdictions. Mainland China does not have any treaties or other forms of reciprocity with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States.
You may be subject to limitations on transfers of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
Your rights to pursue claims against the depositary as a holder of ADSs are limited by the terms of the deposit agreement.
Under the deposit agreement, any action or proceeding against or involving the depositary, arising out of or based upon the deposit agreement or the transactions contemplated thereby or by virtue of owning the ADSs may only be instituted in a state or federal court in New York, New York, and you, as a holder of our ADSs, will have irrevocably waived any objection which you may have to the laying of venue of any such proceeding, and irrevocably submitted to the exclusive jurisdiction of such courts in any such action or proceeding.
ADS holders may not be entitled to a jury trial with respect to claims arising under the deposit agreement, which could result in less favorable outcomes to the plaintiff(s) in any such action.
The deposit agreement governing the ADSs representing our ordinary shares provides that the federal or state courts in the City of New York have exclusive jurisdiction to hear and determine claims arising under the deposit agreement and in that regard, to the fullest extent permitted by law, ADS holders waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our ordinary shares, the ADSs or the deposit agreement, including any claim under the U.S. federal securities laws.
If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable state and federal law. To our knowledge, as of the date of this annual report, the enforceability of a contractual pre-dispute jury trial waiver in connection with claims arising under the federal securities laws had not been finally adjudicated by the United States Supreme Court. In determining whether to enforce a contractual pre-dispute jury trial waiver provision, courts will generally consider whether a party knowingly, intelligently and voluntarily waived the right to a jury trial. We believe that a contractual pre-dispute jury trial waiver provision is generally enforceable, including under the laws of the State of New York, which govern the deposit agreement. It is advisable that you consult legal counsel regarding the jury waiver provision before investing in the ADSs.
46
If you or any other holders or beneficial owners of ADSs bring a claim against us or the depositary in connection with matters arising under the deposit agreement or the ADSs, including claims under federal securities laws, you or such other holder or beneficial owner may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us and / or the depositary. If a lawsuit is brought against us and/or the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have had, including results that could be less favorable to the plaintiff(s) in any such action.
Nevertheless, if this jury trial waiver provision is not enforced, to the extent a court action proceeds, it would proceed under the terms of the deposit agreement with a jury trial. No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any holder or beneficial owner of ADSs or by us or the depositary of compliance with any substantive provision of the U.S. federal securities laws and the rules and regulations promulgated thereunder.
Techniques employed by short sellers may drive down the market price of the ADSs.
Short selling is the practice of selling securities that the seller does not own but rather has borrowed from a third party with the intention of buying identical securities back at a later date to return to the lender. The short seller hopes to profit from a decline in the value of the securities between the sale of the borrowed securities and the purchase of the replacement shares, as the short seller expects to pay less in that purchase than it received in the sale. As it is in the short seller’s interest for the price of the security to decline, many short sellers publish, or arrange for the publication of, negative opinions regarding the relevant issuer and its business prospects in order to create negative market momentum and generate profits for themselves after selling a security short. These short attacks have, in the past, led to selling of shares in the market.
Public companies that have substantially all of their operations in China have been the subject of short selling. Notably, short sellers issued negative research reports about certain public companies based in Mainland China in early 2020, which attracted much attention of global investors. Much of the scrutiny and negative publicity has centered on allegations of a lack of effective internal control over financial reporting resulting in financial and accounting irregularities and mistakes, inadequate corporate governance policies or a lack of adherence thereto and, in many cases, allegations of fraud. As a result, many of these companies are now conducting internal and external investigations into the allegations and, in the interim, are subject to shareholder lawsuits and/or SEC enforcement actions.
It is not clear what effect such negative publicity could have on us. The negative publicity against other public companies based in Mainland China could also harm investors’ confidence in Chinese companies in general, and thus affect our reputation and the market price of our ADSs. If we were to become the subject of any unfavorable allegations, whether such allegations are proven to be true or untrue, we could have to expend a significant amount of resources to investigate such allegations and/or defend ourselves. While we would strongly defend against any such short seller attacks, we may be constrained in the manner in which we can proceed against the relevant short seller by principles of freedom of speech, applicable state law or issues of commercial confidentiality. Such a situation could be costly and time-consuming, and could distract our management from growing our business. Even if such allegations are ultimately proven to be groundless, allegations against us could severely impact our business operations, and any investment in the ADSs could be greatly reduced or even rendered worthless.
Item 4.Information on the Company
4.A.History and Development of the Company
We commenced operations in 1997 through Shenzhen Pengcheng Clinic, which became Pengcheng Hospital in 2003. Since then, we have expanded our business operations through acquisitions and organic growth. In particular, we established our second flagship hospital, Shenzhen Pengai Hospital, in 2005.
In May 2011, our company was incorporated in the Cayman Islands under the name of Pengai Hospital Management Corporation as our offshore holding company. Following certain name changes, our company changed its name to Aesthetic Medical International Holdings Group Limited in July 2018.
Peng Oi Investment (Hong Kong) Holdings Limited (formerly known as Peng Cheng Investment (Hong Kong) Holdings Limited) was incorporated in Hong Kong in July 2004. In July 2011, we acquired the entire equity interest in Peng Oi Investment (Hong Kong) Holdings Limited from Dr. Zhou Pengwu and Ms. Ding Wenting.
47
Dragon Jade Holdings Limited was incorporated in the British Virgin Islands, or the BVI in January 2014. Later in 2014, we transferred to Dragon Jade Holdings Limited our entire equity interest in Peng Oi Investment (Hong Kong) Holdings Limited in exchange for Dragon Jade Holdings Limited to issue and allot one fully paid share of US$1.00 to our company. Upon completion of the share swap, Dragon Jade Holdings Limited became a direct wholly-owned subsidiary of our company.
Peng Yida Business Consulting (Shenzhen) Co., Ltd. Is a direct wholly-owned subsidiary of Peng Oi Investment (Hong Kong) Holdings Limited, which was established in the PRC in December 2010.
Shenzhen Pengai Hospital Investment Management Co., Ltd. (formerly known as Shenzhen Pengcheng Hospital Investment Co., Ltd.), or Shenzhen Pengai Investment, is a direct wholly-owned subsidiary of Peng Yida Business Consulting (Shenzhen) Co., Ltd., which was established in the PRC in December 2004. Shenzhen Pengai Investment is the holding company of our operating subsidiaries in the PRC.
We acquired the entire equity interest in Newa Medical Aesthetics Limited in October 2015 to further extend our footprint to Hong Kong.
We incorporated Stargaze Wealth Limited in the BVI in April 2017 and Aesthetic Medical International Holdings (Singapore) Pte. Ltd., formerly known as China Aesthetic Healthcare Holding (Singapore) Pte. Ltd., in Singapore in May 2017 to invest in our Singapore medical center.
We have continuously expanded our footprint by investment in new treatment centers or acquisition of existing ones. In 2019, we established five and acquired three new treatment centers (in two of which we held minority equity interest before 2019) in cooperation with business partners. In 2020, we established one and acquired four new treatment centers, excluding treatment centers from Hanfei. For example, in November 2019, Nanchang Pengai Xiuqi was established with a registered capital of RMB15.0 million, which will replace Nanchang Pengai Aesthetic Medical Clinic Co., Ltd., or Nanchang Pengai, as we aim to enhance our business footprint in Jiangxi Province. In 2021, to further leverage our extensive operational experiences and resources in the Hong Kong and Mainland China and the Yangtz River Delta area in Mainland China, our wholly-owned subsidiary Guangzhou Pengai Aesthetic Medical Hospital Co., Ltd. Entered into a definitive equity transfer agreement (the “Agreement”) with a shareholder of Guangzhou Meixi Aesthetic Medical Clinic Company Limited (“Meixi”) on October 17, 2021. Pursuant to the Agreement, we purchased 73% of equity interests in Meixi from its existing shareholder. Meixi’s name was changed to Guangzhou Pengai Xiuqi Aesthetic Medical Clinic Co., Ltd. (“Guangzhou Xiuqi”), and its financial statement was consolidated by us in November 2021. Currently, Guangzhou Xiuqi operates a non-surgical satellite clinic with a gross floor area of approximately 900 square meters and nine consulting rooms. As of the date of this annual report, we have a strategically established network, comprising 11 treatment centers including 10 wholly- or majority-owned centers. Our treatment centers spread across major cities in mainland China, with major focus in the Guangdong-Hong Kong-Macau Greater Bay area and and the Yangtz River Delta area in Mainland China. We have completed the transfer of our equity interest in each of Changsha Pengai, Nanchang Pengai to business partners and the shutdown of Shenzhen Miaoyan as part of our region development plan, which are not counted in the number of our treatment centers in this annual report.
In May 2021, we entered into definite share subscription agreement for the issuance of 5,329,410 ordinary shares to Lafang China Co., Ltd. (“Lafang”), a daily consumer products producer in China, at a sale price of US$8.50 every three ordinary shares (equivalent to US$8.50 per American Depositary Share of the Company), which amounted to US$15.1 million in proceeds. In May 2022, we entered into an amendment to the above share subscription agreement with Lafang. Pursuant to this amendment, we issued 21,413,276 ordinary shares to Hawyu (HK) Limited, a wholly-owned subsidiary of Lafang, at a sale price of RMB4.67 per ordinary share (equivalent to approximately US$2.1 per American Depositary Share based on the exchange rate as of May 31, 2022), which amounted to RMB100 million (US$15 million) in proceeds. The private placement was closed in July 2022. We utilized the money from this private placement to fund our business development and working capital.
In July 2022, we entered into a Subscription Agreement for the issuance of 36,402,570 ordinary shares to Jiechuang or its designated affiliates at a sale price of RMB4.67 per ordinary share (equivalent to approximately US$2.1 per American Depositary Share based on the exchange rate as of July 20, 2022), which amounted to RMB170 million (US$25 million) in proceeds. The private placement was closed in February 2023 through the issuance and allotment of 36,402,570 ordinary shares to MY Universe (HK) Limited as designated by Jiechuang. We utilized the money from this private placement to fund our business development and working capital.
48
Concurrently with the Subscription Agreement with Jiechuang, we entered into a Share Purchase Agreement with Dr. Pengwu Zhou and Ms. Wenting Ding (the “Founders”), certain of our existing shareholders controlled by the Founders, and Wanda. Pursuant to the Share Purchase Agreement, Seefar Global Holdings Limited, Jubilee Set Investments Limited, and Pengai Hospital Management Corporation (collectively referred as to the “Sellers”) agreed to sell and Wanda as the buyer agreed to purchase, an aggregate of 21,321,962 of our ordinary shares (the “Sale Share”) for the total consideration in USD that is equivalent of RMB100 million, representing a price of RMB4.67 per Sale Share. The Founders entered into the Share Purchase Agreement as parties to the Share Purchase Agreement and guarantors for the Sellers.
Concurrently with the Subscription Agreement with Jiechuang, we entered into a Shareholders’ Agreement with the Founders, Seefar, Jubilee and certain other parties. The Shareholders’ Agreement governs, among other things, the appointment of our board of directors (the “Directors”) and senior management, the notice, quorum and Directors’ voting arrangement of board meetings, certain lock-up commitments of the Founders and their affiliates and pre-emptive rights mechanisms for our ordinary shares. Pursuant to the Shareholders’ Agreement, we shall deliver two separate warrants to purchase our ordinary shares to Seefar and Wanda, respectively, on the date of completion of closings of both the Share Transfer and subscription of ordinary shares by Jiechuang. Upon the exercise and fulfillment of the terms of the Seefar and Wanda Warrants, we shall issue and allot 4,655,386 ordinary shares to Seefar and 6,423,983 ordinary shares to Wanda.
Concurrently with the Subscription Agreement with Jiechuang, we entered into a Cooperation Agreement with Peak Asia Investment Holdings V Limited (“ADV”) and its affiliate, the Founders, Wanda and Jiechuang. The Cooperation Agreement provides, among other things, that: (i) upon the Closing, ADV shall, subject to the requisite approvals being obtained and continuing in force, convert the outstanding Principal Amount (as defined in the Convertible Note issued to ADV on September 17, 2020 (the “Note”)) and the Conversion Catch-up Amount (as defined in the Note), at a conversion price that is equal to the USD equivalent of RMB4.203 per ordinary share; and (ii) we shall execute and deliver to ADV the warrant for the purchase of our shares to ADV on the date of the Cooperation Agreement (such warrant, the “Warrant”). The Warrant shall be effective on and from the Closing and shall be exercisable into ordinary shares of the Company in accordance with the terms thereof. The warrant exercise price shall be equal to the USD equivalent of RMB4.67 per ordinary share and may be settled, subject to the terms and conditions of Warrant, by way of cashless settlement and/or set-off against the Exit Payment (as defined in the Exit Payments Agreement entered into by ADV, the Company, the Founders on September 15, 2020). The Warrant shall contain customary registration rights and the Warrant Shares shall be freely transferable on the exercise of the Warrant.
4.B.Business Overview
Mission
Our mission is to bring beauty, health and confidence to everyone by delivering safe, high quality aesthetic medical services. We plan to achieve the mission by maintaining and strengthening our leading market position and brand in the aesthetic medical treatment market in China and by growing our presence globally.
Overview
We are a leading provider of aesthetic medical services in China. Leveraging over 20 years of clinical experience, we provide one-stop aesthetic service offerings that include (1) surgical aesthetic treatments, such as eye surgery, rhinoplasty, breast augmentation and liposuction, (2) non-surgical aesthetic treatments which comprise minimally invasive treatments and energy-based treatments such as laser, ultrasound and ultraviolet light treatments, and (3) other aesthetic services such as cosmetic dentistry, as well as general medical services. As one of the market leaders in China, we believe we are well-positioned to benefit from the favorable tailwinds, including growing social acceptance of aesthetic medical services.
49
We generate our revenue primarily from providing aesthetic treatments. We have grown our network significantly since our operation commenced in 1997. As of the date of this annual report, we have a strategically established network comprising 11 treatment centers (including 10 wholly- or majority-owned centers). Our treatment centers spread across major cities in China, with major focus in the Guangdong-Hong Kong-Macau Greater Bay area and the Yangtz River Delta area in Mainland China. As we continue to expand our network, one of our core business strategies is to develop our “flagship” medical institutions, which are typically large-scale full-service treatment centers that contribute a significant proportion of our revenue and are staffed with our most experienced doctors. We believe that developing flagship medical institutions will not only improve our brand awareness in the surrounding area, but also enable us to provide high-end, specialized, and complex medical services to customers referred from smaller-scale treatment centers within our network. We currently have two flagship medical institutions, i.e., Pengcheng Hospital and Shenzhen Pengai. Our scalable business model is built on our highly standardized operating procedures across a centralized network, which we believe has allowed us to quickly and successfully expand. We intend to continue to expand our network into new cities throughout China and selected global markets through organic growth as well as strategic acquisitions, and strategically restructure and focus more on our core markets in the form of Guangdong-Hong Kong-Macao Greater Bay Area and the Yangtze Delta Area, where we see larger growth potential.
Since our inception, we have been committed to delivering high quality services to our customers. Our doctors have rich experience in providing both surgical and non-surgical aesthetic medical services. We believe our team of highly qualified and experienced medical professionals, as well as our stringent safety controls, have underpinned our strong reputation as we continue to attract and retain customers and receive industry recognition for our high quality services. We have received a number of high-profile awards. In 2020, we co-published the 2020 White Paper for the Aesthetic Medical Service Industry in China, along with Forbes China, which was a recognition of our leading position in the industry. In 2021, we have received a number of high-profile awards, including “Agency of the Year Award for Outstanding Performance” by Aesthetic Medical Industry Notes, “Annual Quality, Safe and Secure Aesthetic Medical Agency in 2021” by Shenzhen Association of Plastics and Aesthetics, and “the Best New Digital Media Operation Innovation Award in 2021” by Allergan. In addition, in September 2021, we launched a preliminary plan for business strategies and restructing to optimize our business operations, aiming to achieve a more focused market delineation, steadier development and healthier portfolio. In 2022, the famous high-end hyaluronic acid supplier Allergan gave us the award of CoolSculpting Strategic Partner of the Year 2022 in recognition of our industry-leading role in high-quality compliance operations.
Business model
We generate revenue primarily from our core business providing surgical aesthetic treatment services and non-surgical aesthetic treatment services, comprising minimally invasive treatments and energy-based treatments (surgical and non-surgical treatments together contributed to 94.7%, 89.4% and 91.8% of our revenue in 2020, 2021 and 2022, respectively), as well as non-core business providing general healthcare and other aesthetic services (contributed to 5.3%, 10.6% and 8.2% of our revenue in 2020, 2021 and 2022, respectively). We offer a wide range of surgical and non-surgical aesthetic treatments to cater to the various needs of our customers. The following table sets forth our revenue by service offering for the years indicated:
For the year ended December 31, |
| ||||||||||||||||
| 2020 |
| 2021 |
| 2022 |
| |||||||||||
| RMB |
|
| RMB |
| US$ |
|
| RMB |
| US$ |
|
| ||||
(in thousands, except percentages) |
| ||||||||||||||||
Non-surgical aesthetic medical services |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
Minimally invasive aesthetic treatments | 234,562 |
| 26.0 | % | 177,187 |
| 27,805 |
| 27.4 | % | 223,891 | 32,461 |
| 33.4 | % | ||
Energy-based treatments | 218,108 |
| 24.2 | % | 157,061 |
| 24,646 |
| 24.3 | % | 258,394 | 37,464 |
| 38.6 | % | ||
Sub-total | 452,670 |
| 50.2 | % | 334,248 |
| 52,451 |
| 51.7 | % | 482,285 | 69,925 |
| 72.0 | % | ||
Surgical aesthetic medical services | 401,645 |
| 44.5 | % | 243,070 |
| 38,143 |
| 37.7 | % | 132,628 | 19,229 |
| 19.8 | % | ||
General healthcare services and other aesthetic medical services | 47,258 |
| 5.3 | % | 68,275 |
| 10,714 |
| 10.6 | % | 55,178 | 8,000 |
| 8.2 | % | ||
Total | 901,573 |
| 100.0 | % | 645,593 |
| 101,308 |
| 100 | % | 670,091 | 97,154 |
| 100 | % | ||
50
In addition, the following table sets forth our other key operating metrics, such as the number of procedures performed and the average spending per aesthetic procedure, for the years indicated:
For the year ended December 31, |
| ||||||
Key operating metrics |
| 2020 |
| 2021 |
| 2022 |
|
Number of doctors (full-time and part-time) |
| 230 |
| 169 | 126 | ||
Number of surgical treatments |
| 92,922 |
| 46,568 | 30,124 | ||
Average spending per surgical procedure (in RMB) |
| 3,140 |
| 5,220 | 4,403 | ||
Number of non-surgical treatments |
| 413,235 |
| 424,239 | 514,409 | ||
Average spending per non-surgical procedure (in RMB) |
| 928 |
| 788 | 938 | ||
Total number of aesthetic treatments |
| 506,157 |
| 470,807 | 544,533 | ||
% surgical treatments |
| 18.4 | % | 9.9 | % | 5.5 | % |
% non-surgical treatments |
| 81.6 | % | 90.1 | % | 94.5 | % |
Overview of aesthetic services
Non-surgical aesthetic medical services
Our non-surgical aesthetic medical services primarily comprise (1) energy-based treatments and (2) minimally invasive aesthetic treatments. Depending on the complexity, materials used and scope of the procedure, our treatments vary significantly in price. The following table provides a summary of the key services we provide and information in 2022.
|
| Price range per | ||
standard | ||||
procedure | ||||
Level 1 classification |
| Level 2 classification |
| (RMB) |
|
|
| ||
Energy-based treatment |
| Epidermal energy-based treatments |
| 200-150,340 |
| Mesotherapy |
| 100 -262,295 | |
Minimally invasive treatment |
| Botox injection |
| 500-30,400 |
| Hyaluronic acid injection |
| 500-277,000 | |
| Thread lift |
| 2,000-60,400 |
To ensure the consistency quality and safety in our procedures, we utilize a standardized process for the treatments. These aesthetic procedures are typically completed within one hour, with recovery time of up to one week, depending on the type of procedure and the customer’s physical conditions.
In 2022, we launched 6 new non-surgical aesthetic medical treatment solutions with the support of new products and equipments, as to Multi-dimensional Pigmentation Clearing, Non-invasive Body Shaping, Collagen Ion Cannon, 6G laying Thin, Acne bacteria Balance, and White Moonlight. We introduced various well-known aesthetic medical products, such as Rejuran produced by Pharma Research Products Co., Ltd., CureWhite produced by IMEIK Technology Development Co., Ltd., Ellanse produced by Huadong Medicine Co., Ltd., Loviselle produced by Changchun SinoBiomaterials Co., Ltd., and purchased advanced energy-based treatment equipment to further expand our service offerings, including the Alma Opus Plasma, and Solta Medical Thermage FLX. We are committed to continuously developing constructive treatment solutions for our customers in the future.
Minimally invasive aesthetic treatments
Minimally invasive aesthetic treatments are non-surgical procedures that can help individuals improve their appearance with minimal or no incisions. These treatments are typically performed in fifteen minutes to two hours with minimal pain and mild bruising or swelling, whilst achieving immediate effects. Recovery time varies depending upon the service type, but generally the healing period is between one to two weeks.
To ensure consistency of quality and safety, all our injection treatments follow a standardized procedure that involves the application of numbing cream, disinfection of the treatment area which has been identified and marked by our doctors and injection at the treatment area with a sterilized syringe. After the injection, the treated area is cleaned, shaped and iced.
51
Energy-based treatments
We offer a range of epidermal energy-based treatments using high-grade equipment from China, Germany, Israel and the United States. Our standardized procedures for energy-based treatments, which typically take less than one hour, involve the following steps: facial cleansing, application of numbing cream or anesthetic to the treatment area, disinfection, methodical application of the energy-based treatment, including testing on a small area before adjusting the level of energy-based intensity accordingly. To achieve and maintain optimal results, customers may choose to repeat the procedure, depending on the type of procedure and customer reaction to the treatment.
Mesotherapy is catagorized within energy-based treatment due to naming conventions. We offer a wide range of mesotherapy treatments using injection materials from low end to high end according to customer spending power and skin needs.
Surgical aesthetic medical services
We provide customary surgical aesthetic medical services, including double eye lid surgery, rhinoplasty, breast augmentation, liposuction and facelifts, among others. Surgical aesthetic treatments typically involve local or full anesthesia, and an extended recovery time. All of our surgical aesthetic treatments are performed in fully equipped operating rooms in our treatment centers. Depending on the complexity of the procedure and the physicians involved, our treatments vary significantly in price. The following table sets forth our main surgical aesthetic treatments and information in 2022.
Price range per | ||||
standard | ||||
procedure | ||||
Treatment |
| Description |
| (RMB) |
Eye surgery |
| Change the shape or appearance of the eyes, eyelids |
| 1,500-39,600 |
Nose surgery / rhinoplasty |
| Change the shape or appearance of the nose by adding fillers or cartilage |
| 2,000-81,700 |
Breast augmentation |
| Procedures designed to enlarge/reduce or change the shape of the breasts |
| 3,980-236,698 |
Liposuction |
| Procedure in which excess fatty tissue is removed from a specific part of the body through suction |
| 2,000-317,879 |
Due to their complexity, we require all our surgical aesthetic treatments to be attended (or performed) by doctors with a high level of experience. To ensure the consistency of quality and safety in our procedures we utilize a standardized process for the treatment. These procedures are typically completed within one to three hours, with recovery time ranging from one to twelve months, depending on the type of procedure and the customer’s physical conditions.
General healthcare services and other aesthetic medical services
We provide a range of medical services through our Pengcheng Hospital, primarily in internal medicine and traditional Chinese medicine. We also provide other aesthetic medical services, primarily in aesthetic dentistry, aesthetic traditional Chinese medicine and hair loss treatments. Our aesthetic traditional Chinese medical treatments, unlike western medical treatments, which are clinically proven, include herbal oral medications, dietary advice, herbal poultices and foot baths, ultraviolet negative ion beauty treatments, ultrasounds, iontophoresis (use of local electric current) and acupuncture, cupping, massage and skin scraping (known as “gua sha” in China). Historically we have provided additional general healthcare services, including otolaryngology, bromhidrosis treatment and abortion, which we have discontinued.
52
Aesthetic services process
We have developed, improved and implemented highly standardized operational procedures across our treatment centers through years of industry experience and accumulated know-how. Our process focuses on providing customized services and high quality customer experiences. The following diagram illustrates a typical aesthetic service process, which involves image consultants, doctors, nurses and customer service personnel.
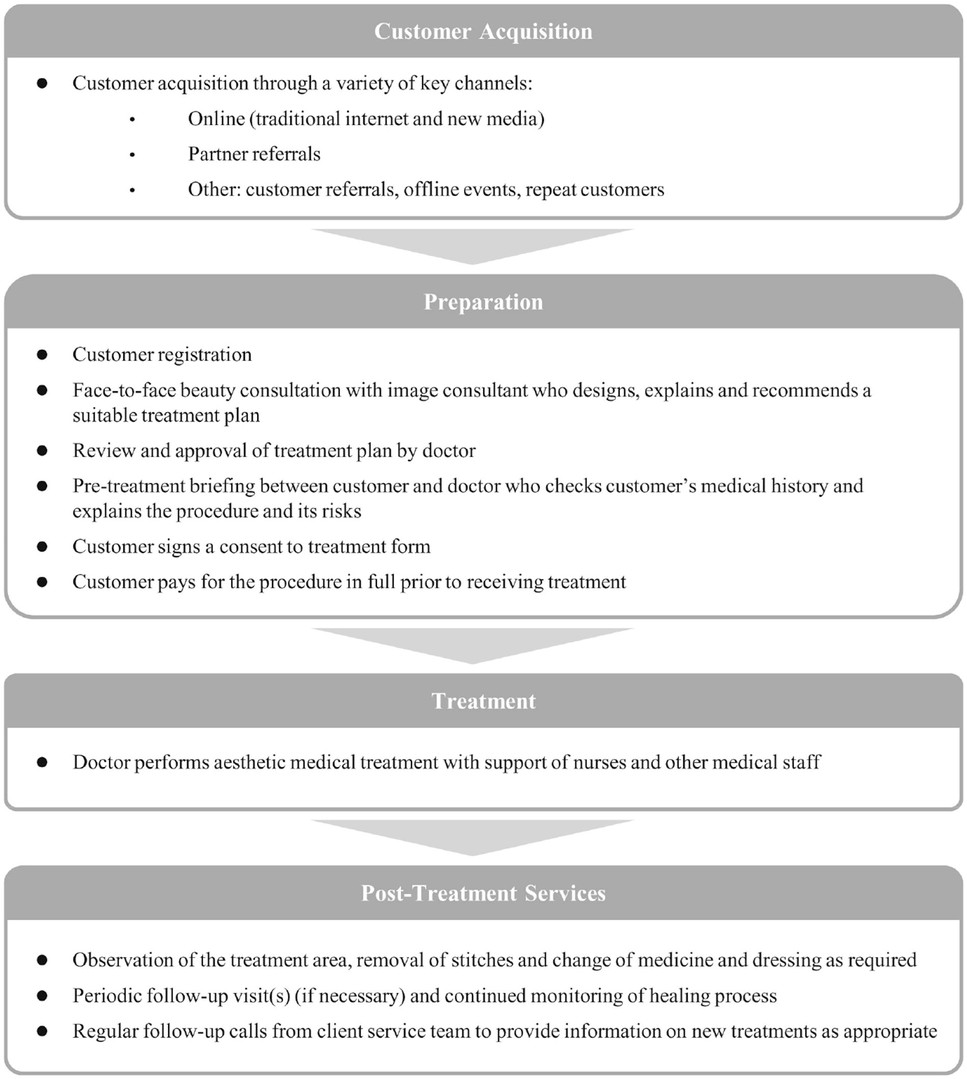
53
In 2022, we implemented new internal operational protocols by establishing new standard operating procedure (SOP) to ensure customers receive better and consistent services across all its treatment centers. Employees were also required to record all the medical treatments handled, with detailed operating procedures as well as the marketing strategies used for internal reference. Through case studies of these records, we were able to improve our service quality, and provide the most standardized and suitable solutions to customers. In addition, we have been working with an experienced consulting team on a training program for our front-line employees, including reception, customer services, operating managers, e-commerce staff and assistant medical practitioners. We believe that these measures would lay a solid foundation for our future business growth and expansion, in a way that the infrastructure would allow easy business replication. To better evaluate the service quality of all our centers, we engaged mystery shoppers, who discretely visit each treatment center with a budget of RMB1,000 and provide assessments on user experience for management’s review. As at the date of this annual report, 29 mystery shoppers’ assessments and feedback have been received. We believe these measures can improve service quality of all centers, which support business scalability and sustainable growth in the long run.
Our treatment centers
As of the date of this annual report, we have an extensive presence in mainland China and Singapore, with locations in 8 cities. All of our treatment centers are wholly- or majority-owned with the exception of Singapore Mendis. The following map illustrates our network in mainland China.
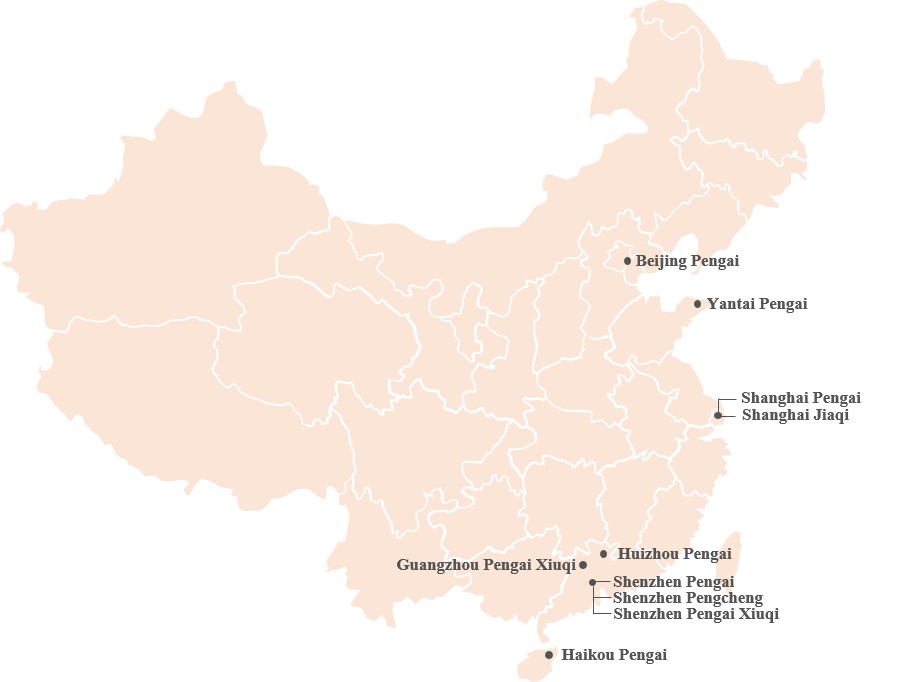
In addition to the network in mainland China, we have entered the Singapore markets, with the investment in Singapore Mendis in 2017.
54
Our current network comprises 11 treatment centers across mainland China and Singapore, including two flagship hospitals. Based on relevant Chinese regulations, the treatment centers in our network can be classified as the following:
● | Hospital, which meets the minimum standard for Grade I general hospital, including certain prescribed clinical and medical departments, and medical personnel requirements; or |
● | Outpatient department, which meets certain prescribed clinical department and medical personnel requirements and typically comprises aesthetic treatment consultation rooms, aesthetic surgery, aesthetic dermatology and dentistry, amongst other departments. |
● | Clinic, which meets certain prescribed clinical department and medical personnel requirements and typically comprises one or two of the departments of aesthetic surgery, aesthetic dermatology, aesthetic dentistry, aesthetic traditional Chinese medicine. |
For more information on the definitions of each type of medical institution, please refer to “—Regulation—Regulations on the administration and classification of medical institutions.”
One of our core strategies is to develop our flagship hospitals, which are large-scale, full-service hospitals located at the epicenter of our regional network hubs and accompanied by our smaller-scale treatment centers in the surrounding vicinity. Our flagship hospitals contribute a meaningful proportion of our revenue and are staffed with our most experienced doctors.
The premises of the majority of our treatment centers are leased, while Pengai Huizhou and part of Pengcheng Hospital are our owned properties. The following table provides a summary of our treatment centers, including key operational data, as of December 31, 2022.
| Date of | Classification |
|
| ||||||||
establishment / | in accordance |
| ||||||||||
Acquired / | acquisition / | with Chinese | GFA | Beneficial |
| |||||||
Treatment center(10) |
| invested |
| investment |
| regulation |
| (sq.m.) |
| Interest |
| |
Shenzhen Pengcheng (L) |
| Established |
| December 2003 |
| Hospital |
| 8,391 | 100.0 | % | ||
Shenzhen Pengai(L) |
| Established |
| November 2005 |
| Hospital |
| 6,182 | 100.0 | % | ||
Haikou Pengai(M) |
| Established |
| March 2011 |
| Hospital |
| 2,169 | 87.0 | % | ||
Huizhou Pengai(M) |
| Established |
| June 2011 |
| Hospital |
| 1,670 | 67.5 | % | ||
Shanghai Pengai(L) |
| Acquired |
| January 2014 |
| Outpatient Department |
| 1,048 | 80.0 | %(3) | ||
Shenzhen Pengai Xiuqi(L) |
| Established |
| May 2017 |
| Hospital |
| 1,902 | 94.0 | %(7)(9) | ||
Singapore Mendis(S) |
| Invested |
| November 2017 |
| N/A |
| 125 | 22.2 | % | ||
Yantai Pengai(M) |
| Established |
| June 2018 |
| Hospital |
| 1,983 | 95.0 | %(5) | ||
Shanghai Jiahong(M) |
| Acquired |
| March 2020 |
| Outpatient Department |
| 1,097 | 64.0 | %(2) | ||
Beijing Pengai(S) |
| Acquired |
| January 2021 |
| Clinic |
| 392 | 95.0 | %(6)(8) | ||
Guangzhou Pengai Xiuqi(M) |
| Acquired |
| November 2021 |
| Outpatient Department |
| 900 | 70.1 | %(4) | ||
Notes:
* | To streamline the management of our network of treatment centers, we strategically categoriz our treatment centers based on a number of factors, including, among other things, the cities they are located in and the size of the treatment centers. In the table above, large, medium and small treatment centers are denoted with (L), (M) and (S), respectively. |
(1) | Indicates flagship hospitals. |
(2) | Includes 12.0% equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
(3) | Includes 15.0% equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
55
(4) | Includes 19% equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
(5) | Includes 24% equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
(6) | Includes 25% equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
(7) | Includes 27% equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
(8) | Includes 25% of its parent company’s equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
(9) | Includes 26% of its parent company’s equity interest held by Dr. Zhou Pengwu, for which we are entitled to relevant economic benefits pursuant to the Contractual Arrangements. |
(10) | English names of treatment centers for the indicative purpose only. |
In 2022, we generated 76.5% of our revenue from our top five treatment centers. The following table sets forth the revenue from these five treatment centers in 2020, 2021 and 2022.
For the year ended December 31, |
| ||||||||||||||
| 2020** |
| 2021 |
| 2022 |
| |||||||||
RMB |
| RMB |
| RMB |
| USD |
|
| |||||||
(in thousands) |
| % |
| (in thousands) |
| % |
| (in thousands) |
| (in thousands) |
|
| |||
Shenzhen Pengai* | 190,166 | 26.4 | % | 143,005 | 22.2 | % | 187,757 | 27,222 | 28.0 | % | |||||
Shenzhen Pengcheng* | 171,840 |
| 23.9 | % | 208,955 |
| 32.4 | % | 210,497 |
| 30,519 |
| 31.4 | % | |
Changsha Pengai | 59,407 |
| 8.2 | % | 50,567 |
| 7.8 | % |
|
| |||||
Chongqing Pengai* |
| 25,026 |
| 3.9 | % | ||||||||||
Shenzhen Yuexin | 33,157 |
| 4.6 | % | 32,430 |
| 4.9 | % | |||||||
Shanghai Pengai | 29,839 |
| 4.1 | % | 40,655 | 5,894 | 6.1 | % | |||||||
Shenzhen Pengai Xiuqi | 37,106 | 5,380 | 5.5 | % | |||||||||||
Yantai Pengai |
|
| 36,763 | 5,330 | 5.5 | % | |||||||||
Subtotal top five treatment centers | 484,409 |
| 67.3 | % | 459,983 |
| 71.2 | % | 512,778 |
| 74,346 |
| 76.5 | % | |
Total | 720,202 |
| 100 | % | 645,593 |
| 100 | % | 670,091 |
| 97,154 |
| 100 | % | |
* | Indicates flagship hospitals |
** | Without considering the one-off impact from Hanfei |
Summary information on our flagship hospitals
Shenzhen Pengai
Founded in November 2005, Shenzhen Pengai Hospital was established as our second flagship hospital and was our second largest revenue contributor in 2022. Shenzhen Pengai Hospital has a gross floor area of approximately 6,182 sq.m. with 7 surgical rooms and 38 treatment rooms. In the third quarter of 2022, we began renovating the first floor of Shenzhen Pengai to provide customers with a better service experience. The renovation was completed by the end of this year. As of December 31, 2022, Shenzhen Pengai Hospital had 7 major departments including plastic surgery, aesthetic dermatology, aesthetic traditional Chinese medicine, aesthetic dentistry, anesthesiology, medical laboratory, and medical imaging.
Shenzhen Pengai Hospital serves as the training base for the Dalian Medical University College of Aesthetic and Plastic Surgery. As of December 31, 2022, Shenzhen Pengai Hospital had 301 employees, including 39 doctors, 49 nurses, 19 other medical staff and 194 non-medical staff.
56
In February 2022, Shenzhen Pengai announced the successful completion of its recent renovation project, which has transformed its first floor into a state-of-the-art reception center to offer customers a comfortable and innovative environment for their aesthetics needs. On March 8, a Brand Upgrade & 2023 Aesthetic Medical Industry Standardization Initiative Ceremony was successfully held in Shenzhen Pengai. The senior officers of our group, leaders of the China Association of Plastic Surgery, national strategic partners, industry experts and media partners gathered in Shenzhen Pengai to witness the strategic upgrade of the Pengai Aesthetic medical brand and explore the new concept of healthy development of the industry.
|
|
Pengcheng Hospital
Founded in 1997, Pengcheng Hospital became our first flagship hospital in 2003 to provide both aesthetic medical services and general healthcare and other aesthetic services. Pengcheng Hospital was also our largest revenue contributor in 2021. As of the date of this annual report, this treatment center has a gross floor area of approximately 8,391 sq.m. with 7 surgical rooms and 59 treatment rooms. It is equipped with advanced medical devices, such as Thermage radiofrequency systems, Body-Jet liposuction systems and Aesculap flexible neuroendoscopy systems. As of December 31, 2022, Pengcheng Hospital had 19 major clinical departments, including plastic surgery, aesthetic dermatology, aesthetic traditional Chinese medicine, aesthetic dentistry, surgical, gynecology, internal medicine, otorhinolaryngology, laboratory testing, radiology, anesthesiology etc. As of December 31, 2022, Pengcheng Hospital had 408 employees, including 39 doctors, 62 nurses, 35 other medical staff and 272 non-medical staff.
|
|
Integration
Efficient and effective integration of new treatment centers into our network is an important component of our business strategies and a driver of our success. Crucial to successful integration of acquired treatment centers is our post-acquisition integration plan, which involves a dedicated post-acquisition taskforce that conducts a detailed review of the acquired treatment center’s equipment, systems and personnel. In particular, we focus on ensuring that the medical professionals at the acquired treatment centers comply with our group-wide protocols and meet our quality standards. In order to retain these personnel, we offer a compelling employment package that includes ongoing training and a performance-based compensation scheme that aligns their interest with those of the treatment centers.
57
Aside from service protocols and quality of staff, we also maintain standardized indoor decor to lend consistency to the look and feel of our treatment centers. We regularly review and adjust the mix and focus of clinical departments to leverage each treatment center’s unique strengths to enhance its reputation and profile.
Our customers
Our customers are primarily female individuals residing in China. The majority of our customers are aged between 18 and 30, and this age cohort represented 46.0% of our customer base in 2022, while customers in the 31 to 40 and above 40 age cohorts represented 35.2% and 18.0% of our customer base in 2022, respectively.
The following table provides a snapshot of our active customer base.
For the year ended December 31, |
| ||||||||||||
| 2020* |
| 2021 |
| 2022 |
| |||||||
| Number of |
|
| Number of |
|
| Number of |
|
| ||||
| customers |
| % |
| customers | % |
| customers |
| % |
| ||
New customers | 95,724 | 39.5 | % | 56,455 | 23.4 | % | 47,907 | 22.2 | % | ||||
Repeat customers | 146,834 |
| 60.5 | % | 185,213 |
| 76.6 | % | 168,138 | 77.8 | % | ||
Total active customers | 242,558 | 100 | % | 241,668 |
| 100 | % | 216,045 | 100 | % | |||
* | Without considering the one-off impact from Hanfei |
Our active customer base, defined as customers who have received at least one procedure in the relevant year, was 242,558, 241,668, and 216,045 respectively, in 2020, 2021 and 2022, representing a CAGR of -10.6%. Our treatments are transactional in nature and we have not entered into any long-term agreements with our customers.
Customer service
Our customer service team plays a critical role in the service process and is responsible for following up with our customers (including courtesy check-in calls), collecting customer feedback and handling general customer complaints.
A key component of the services we provide to our customers is the consultation with our image consultants, who provide personalized treatment plan to each customer prior to him/her undertaking the aesthetic treatment. Our image consultants provide advice on the type of treatment, how it should be executed and other factors relevant to achieving an optimal aesthetic outcome that addresses the customer’s needs. These consultations help to enhance the aesthetic outcome of treatments and are highly valued by our customers.
Customer feedback system
In order to enhance customer loyalty and establish long-term customer relationships, we have implemented a customer feedback system which enables us to improve our treatments and services to meet customers’ needs. We collect feedback through various channels, including our customer service hotline, the comment collection box at each treatment center and face-to-face communication with the staff at our treatment centers. Our staff is required to keep records of all feedback, whether through centralized handling of feedback made through our service hotline at our company headquarters in Shenzhen, daily inspection of the comment collection box at each treatment center or records prepared after customer interviews. These records are reviewed by the customer service managers at each treatment center before thorough analysis and appropriate follow-up actions are undertaken.
Due to the nature of our business and the subjective nature of the level of satisfaction with aesthetic medical services, we receive complaints from time to time. Customer complaints are usually in relation to staff attitude, dispute over pricing (competitor offering an equivalent service for less), over-promise, longer than expected recovery time or general dissatisfaction with treatment results, which includes potential side effects. The majority of customer complaints relate to general dissatisfaction with treatment results—this type of complaint represented 99.2%, 98.6% and 100.0% of the total number of complaints for the years ended December 31, 2020, 2021 and 2022, respectively.
58
The overall number of complaints we typically receive is low—we received 256, 231 and 151 complaints for the years ended December 31, 2020, 2021 and 2022, respectively. The total number of complaints received represented 0.05%, 0.05% and 0.03% of the total number of aesthetic treatments provided for the years ended December 31, 2020, 2021 and 2022, respectively. In particular, the percentage of the number of customer complaints attributable to general dissatisfaction with treatment results out of the total number of aesthetic treatment procedures we performed in 2020, 2021 and 2022, respectively, was level at approximately 0.05%, 0.05% and 0.03%.
Management of complaints
Our customer service department is responsible for handling customer complaints. In order to ensure prompt and proper handling of customer complaints, we have implemented strict internal guidelines which are administered at the treatment center level. Where (i) a customer demands compensation for an amount of more than RMB10,000 or a refund for an amount of more than RMB50,000, (ii) a customer has raised the same issue more than three times, (iii) we may be exposed to reputational damage or operational disruption (in particular if media may become involved) or (iv) they relate to potential wrongdoing of a treatment center or its personnel which may constitute medical malpractice according to the Medical Malpractice Regulation, the complaint would be escalated to the designated customers’ complaint settlement operations team under our medical safety control department for specific follow up.
We generally offer free treatments and/or partial or full refunds to settle complaints. Occasionally, we may provide additional compensation to settle customer complaints on a case-by-case basis.
In order to ensure the highest level of customer satisfaction and to maintain our strong brand name, we have taken a relatively generous approach in offering such refunds and compensation historically. In 2020, 2021 and 2022, the amount of refunds and compensation incurred was RMB3.5 million, RMB3.5 million and RMB2.8 million (US$0.4 million), respectively, representing 0.5%, 0.5% and 0.4% of our total revenue for the corresponding period. We believe that the number of complaints we received and the amount of refunds and compensation incurred were insignificant, considering the size of our operation, the nature of our industry, and the relatively generous approach we took in offering such refunds and compensation. We seek to further enhance the quality of our service offerings and upkeep our rigorous medical standards, to continue to maintain the customer complaints we received at a low level.
Our suppliers
As of December 31, 2022, we had approximately 684 suppliers, providing us with a diverse selection of medical equipment, supplies and medical consumables. Our suppliers fall into five key categories: medical devices, energy-based equipment, implants, injection materials and other medical consumables. In general, we have worked with our major suppliers for more than ten years and believe we maintain strong relationships with them. In 2020, 2021 and 2022, our five largest suppliers in each respective year in aggregate accounted for 31.2%, 26.4% and 24.3%, respectively, of our total amount of purchases, and our single largest supplier accounted for 9.7%, 6.9% and 6.0% of our total amount of purchases, during the same periods, respectively.
We have a system for selecting reliable and quality suppliers, with a selection and review process based on qualification of the business and/or products, pricing, reputation, service quality, delivery schedule and product offering. We maintain multiple suppliers for key categories of purchases to ensure continuity and quality of supply. Payment terms with the majority of our suppliers are on open account. Certain suppliers grant us credit periods ranging from 20 to 60 days, although we generally pay on delivery.
During the three years ended December 31, 2022, we did not encounter any major problems in sourcing despite not having long-term contracts with our suppliers, nor did we encounter any business disruption due to supply shortages or delays. In any event, we believe any shortage or delay in the supply of implants, injection materials and medical consumables will not have any material impact on us as we are able to switch to other suppliers with comparable quality and prices.
To the knowledge of our directors, none of our directors or any shareholder who owns more than 5% of our issued share capital, nor any of their respective associates, has any ownership interest in any of our five largest suppliers.
59
Our equipment
We are committed to utilizing high quality equipment for our services. We owned a wide variety of energy-based treatment and non-surgical aesthetic equipment. Our key equipment includes the Lumenis M22, Alma Harmony XL pro, Candela Picoway, Fotona4D Pro, Peninsula MicroUltra, Peninsula Gold Microneedle, Alma Accent XL, Alma BeautiFill, and play an important role in achieving high quality aesthetic outcomes for our customers. We source our energy-based equipment and related components from our suppliers in the United States, Europe, and China. In 2021 and 2022, we entered into sale-and-lease-back arrangements with a third party financial lease service provider for a term of two years with an aggregate principal amount of RMB127.7 million and RMB90.1 million, respectively. The net book value of equipment under such arrangements accounts for approximately 16% and 37% of the total value of our equipment in 2021 and 2022, respectively. We believe finance lease is a common approach for financing activities.
Sales and marketing
As of December 31, 2022, we had an effective, disciplined sales and marketing team of 118 who, in addition to a base salary, generate commissions based on the number and types of procedures sold. We have strict policies governing our sales and marketing activities to ensure the consistency of our offering and our customers’ experience. For example, we have standardized service catalogs, sales scripts and price lists throughout the network, with management and/or supervisor approval required for deviations such as discounts. In addition, prior to effecting each sale transaction, the treatments proposed by our sales staff are reviewed and approved by our doctors to ensure feasibility and compliance with internal policies. Customers then pay in full prior to receiving the treatments, using a range of payment methods.
We also have a dedicated marketing team responsible for analyzing market intelligence, organizing advertising activities, evaluating marketing campaigns and assisting in the preparation of promotional materials. Given the size of Chinese market, marketing efforts for each of our treatment centers are primarily initiated and implemented by the marketing personnel situated at each treatment center with the support of our headquarters. Each treatment center’s marketing efforts are tailored to customers in the region where the treatment center is based. These marketing efforts are focused on (1) informing customers of the comprehensive aesthetic medical services that we offer; and (2) emphasizing the high level of customer satisfaction. For example, we hold themed marketing events when opening new treatment centers and introducing new treatments. In addition, we also host conferences and invite our potential clients and promote our services.
In the second quarter of 2022, in response to irregular epidemic prevention and control measures during the outbreak of COVID-19, we have developed new customer acquisition strategies through the establishment of online and offline collaboration with renowned enterprises to target potential customers with high level of consumption. These new B2B methods have complemented our existing B2C marketing initiatives and helped optimize our overall marketing efficiency. While two treatment centers in Shanghai were temporarily closed while the city was under lockdown from mid-March to early June, 2022, we leveraged our online marketing channels and private domain reach to promote our brands and treatment packages. The campaign proved to be successful, as the Group recorded a rapid rebound in business volume once the lockdown was eased. Taking Shanghai Peng’ai as an example (an aesthetic outpatient department), since the resumption of operation, the number of customers has rebounded to 107% of June 2021.
In addition to the private domain channel introduced in the second quarter, we started collaboration with key opinions leaders (KOLs) and tapped into livestreaming e-commerce. The campaign was successful and effective in expanding target audience to younger generation who has strong demand in self-care and aesthetic related consumption.
Based on our extensive experience in China, we have obtained an in-depth understanding of the needs and preferences of our customers. Our extensive local knowledge allows us to carry out a wide range of creative marketing events and/or initiatives which cater to local preferences.
Competition
The aesthetic medical services market in China is highly competitive and fragmented with numerous market participants. Our competitors include major privately-owned multi-site operators in China such as Mylike, Yestar, Aist and Evercare. We believe the principal competitive factors in this market are price and quality of service, variety of services rendered, convenience and proximity of treatment center location to place of business or residence, brand recognition and reputation, targeted marketing and customized services.
60
We also face intense competition in our general healthcare service business. We compete primarily with other treatment centers in our areas of operation. Key competitive factors include healthcare service quality, reputation, convenience and price. We expect new competitors in the general healthcare service industry will continue to emerge given the state of China healthcare reform and the central and local governments’ supportive policies towards public healthcare reform and private capital investment in the healthcare services industry. For a discussion of risks relating to competition, see “Item 3. Key Information—3.D. Risk Factors—Risk relating to our business and our industry—We face intense competition, and if we do not compete successfully against new or existing competitors, we may lose our market share and our profitability may be adversely affected.”
Intellectual property
As of December 31, 2022, we had (i) 37 registered trademarks, 32 of which are in mainland China and 5 in Hong Kong, (ii) 40 registered domain names, of which all are registered in mainland China, and (iii) 2 registered patents, of which all are registered in mainland China. Our principal intellectual property rights are our registered trademarks such as “鹏爱” (Pengai), as well as our patented technologies, which include a device and method for measuring facial symmetry and a device and method for mandibular shaping.
We recognize the importance of protecting and enforcing our intellectual property rights. We have registered all the principal trademarks, patents and internet domain names in China that are necessary for us to carry out our business operations. We will take the necessary legal action to protect our intellectual property rights if we discover any infringement of those rights.
As of the date of this annual report, we were not aware of any material infringement of our intellectual property rights and we believe that we have taken reasonable measures to prevent infringement of our own intellectual property rights. As of the date of this annual report, we did not have any pending or, to our knowledge, threatened claims against us or any of our subsidiaries relating to the infringement of any intellectual property rights owned by third parties. For risks related to any failure to protect our intellectual property rights, see “Item 3. Key Information—3.D. Risk Factors—Risk relating to our business and our industry—We may not be able to adequately protect our intellectual property rights, which could harm our brand and our business.”
In order to maintain our substantial market position in the aesthetic medical services market in China, we have active dialogue and exchange of information and experts with well-respected aesthetic medical institutions in the United States, Europe, South Korea and Japan to learn about and adopt new aesthetic medical treatments and advanced technologies. Any knowledge gleaned from these exchanges is shared with our medical staff via our training programs. We believe this allows us to strengthen our technological advantage in this market and anticipate and respond quickly to our customers’ needs and improve the quality, safety and efficiency of our services.
We maintain product liability insurance for the medical devices and energy-based equipment. We do not maintain business interruption insurance or key employee insurance for our executive officers or professional malpractice liability insurance for key doctors and medical staff in most of our aesthetic medical treatment centers because the non-surgical aesthetic treatments, which contribute a major portion of our revenue, are less invasive, quicker to recover and therefore less risky.
The premiums that we paid for our insurance were RMB 573,426, RMB541,358 and RMB149,034 (US$21,608) in 2020, 2021 and 2022, respectively, representing 0.08%, 0.08% and 0.02% of our revenue for those periods, respectively. During the three years ended December 31, 2022, we did not submit any material professional malpractice liability insurance claims. However, we might be subject to liabilities that exceed our insurance coverage. See “Item 3. Key Information—3.D. Risk Factors—Risk relating to our business and our industry— Our business is subject to professional and other liabilities for which we may not be insured.”
Regulation
Substantially all of our operations are in PRC and our business is subject to supervision and regulation by the PRC government. Below sets forth a summary of the most significant laws, rules, regulations and policies related to the aesthetic medical industry and our business in the PRC.
61
Regulations on the institutional reforms of medical institutions
Promotion Law on Basic Medical and Healthcare
The Promotion Law on Basic Medical and Healthcare, which were promulgated on December 28, 2019 by the Standing Committee of the National People’s Congress and became effective on June 1, 2020, stipulates specific support for socially-run medical care institutions, granting socially-run medical care institutions similar rights enjoyed by public hospitals. Prior to the its promulgation, the State also supported the subdivision of disciplines among socially-run medical care institutions (including ophthalmology, orthopedics, stomatology, gynecology, pediatrics, oncology, psychiatry and medical cosmetology, etc.), gradually cancelled staffing among public hospitals, lessened entry barriers for socially-run medical care institutions and enhanced supervision, and introduced a series of policies and regulations encouraging social capital invested or funded elderly healthcare institutions or rehabilitation institutions, thereby demonstrating the support of the State for socially-run medical care institutions.
Opinions on Further Promoting the Reform of Medicine and Health System
The Opinions on Further Promoting the Reform of Medicine and Health System, or the Opinions, which were promulgated by the Central Committee of the Communist Party of the PRC and the State Council and became effective on March 17, 2009, advocate a range of measures to reform medical institutions in China and to establish a basic medicine and health system covering urban and rural residents. Measures aimed at reforming medical institutions include: (i) separating governmental agencies from public medical institutions; (ii) separating for-profit medical institutions from not-for-profit medical institutions; (iii) separating sponsorship from operations of public hospitals; and (iv) separating pharmaceutical dispensing from pharmaceutical prescription. The Opinions include proposals for the establishment and improvement of corporate governance systems of public medical institutions, and checks and balances in decision-making, execution and supervision processes between organizers and operators of public medical institutions. The Opinions also promote private capital investment in medical institutions (including investments by foreign investors), the development of private medical institutions and the reform of public medical institutions (including those established by state-owned enterprises) through private capital investment.
Notice on Further Encouraging and Guiding Private Capital to Invest in Medical Institutions
The Notice of the State Council on Forwarding the Opinions of the National Development and Reform Commission, or the NDRC, the Ministry of Health and the other three departments, on Further Encouraging and Guiding Private Capital to Invest in Medical Institutions, which was promulgated by the General Office of the State Council and became effective on November 26, 2010, states that the PRC government encourages and supports investments by private investors in medical institutions of various types. For example, private investors are permitted to apply for approval to establish for-profit or not-for-profit medical institutions. They are also encouraged to participate in the reform of existing public hospitals, including those established by state-owned enterprises, by converting them into private not-for-profit medical institutions in order to systematically reduce the proportion of public hospitals in the system, and to set up hospital management companies to provide specialized services.
In addition, private medical institutions with experience in the provision of healthcare services and a good reputation may be selected as participants in the restructuring of hospitals established by state- owned enterprises through pilot reform programs. They are also encouraged to modernize their hospital management, establish standardized corporate governance structures, improve their cost control and quality management systems, and employ professional managers to manage their hospital, such as by engaging or authorizing domestic or overseas medical institutions with professional experience to participate in the management of their hospitals to improve efficiencies, as well as improve their clinical research and build up their research and development teams. Medical institutions are also encouraged to develop into large, sophisticated, technology-intensive medical groups and adopt brand-focused development strategies to build good reputations.
Several Opinions on Promoting the Development of Healthcare Service Industry
Several Opinions on Promoting the Development of Healthcare Service Industry, or the 2013 Opinions, was promulgated by the State Council and became effective on September 28, 2013. The 2013 Opinions encourage the private sector to invest in the healthcare service industry by various means including new establishments and participation in restructuring, and also encourage private capital to invest in not-for-profit medical institutions for providing basic healthcare services. The 2013 Opinions also propose the idea of relieving the requirements for Sino-foreign equity/cooperative joint venture medical institutions and expanding the eligibility for wholly foreign-invested medical institutions in the pilot program.
62
Several Opinions on Accelerating the Development of Medical Institutions with Social Capital
Several Opinions on Accelerating the Development of Medical Institutions with Social Capital, which was promulgated by the National Health and Family Planning Commission and the State Administration of Traditional Chinese Medicine and became effective on December 30, 2013, specifies the policies that support the development of privately-funded medical institutions, which include (i) gradual relief of foreign capital investment in medical institutions, (ii) reduction of the requirements in the service sector, allowing social capital investment in the areas which are not explicitly prohibited, (iii) reduction of the requirements for the deployment and use of large medical equipment in private hospitals, (iv) improvement of supporting policies for the development of private hospitals in areas such as medical insurance and price control, and (v) acceleration of the approval process regarding the establishment and operation of private hospitals.
Several Policies and Measures Regarding Promoting Private Investment in Medical Institutions and Accelerating Development
Several Policies and Measures Regarding Promoting Private Investment in Medical Institutions and Accelerating Development, which was promulgated by the General Office of the State Council and effective on June 11, 2015, provides for (i) elimination and cancellation of administrative authorities’ unreasonable requirements for pre-examination and pre-approval regarding the establishment of medical institutions, and the reduction in the time required for making such examination and approval, (ii) reasonable control of the number and scale of public medical institutions and the exploration of the space for development of the medical institutions through private capital investments, (iii) support for the listing and financing of eligible and qualified for-profit medical institutions funded by private capital investments, and (iv) encouragement for private investors with managerial experience in medical institutions to participate in the management of public medical institutions in various forms such as through hospital management groups and subject to a clear distribution of power and responsibilities.
Opinions on Encouraging Social Forces to Provide Multi-layered and Diverse Healthcare Services
Opinions on Encouraging Social Forces to Provide Multi-layered and Diverse Healthcare Services, which were promulgated by the General Office of the State Council on May 16, 2017, stipulate the policies to actively support social forces to go deep into the niche service market, such as specialized medical services, expand the effective supply of services, and foster professionalized advantages. A number of competitive branded service agencies will be formed at a rapid pace for such specialties including but not limited to aesthetic medical services.
Decisions of the Central Committee of the Communist Party of the PRC on Several Major Issues Concerning Comprehensively Deepening Reforms
The Decisions of the Central Committee of the Communist Party of the PRC on Several Major Issues Concerning Comprehensively Deepening Reforms, which was promulgated by the Central Committee of the Communist Party of the PRC and became effective on November 12, 2013, promotes private sector investment in the healthcare service industry and in other services industries that are poorly funded or in need of diversification. It also permits doctors to have a multi-site practice and allows privately funded medical institutions to benefit from the medical insurance system.
Regulations on the administration and classification of medical institutions
Administrative Measures on Medical Institutions and its Implementing Measures
The Administrative Measures on Medical Institutions, which was promulgated on February 26, 1994 by the State Council, came into effect on September 1, 1994 and was amended on February 6, 2016 and March 29, 2022, effective on May 1, 2022, and the Implementation Measures of the Administrative Measures on Medical Institutions, which was promulgated by the Ministry of Health on August 29, 1994, came into effect on September 1, 1994, and was amended in 2006, 2008, and 2017, require that the establishment of medical institutions to be reviewed and approved by healthcare administrative departments at or above the county level and an Approval Letter of Establishment of Medical Institution to be obtained from competent healthcare administrative departments. In addition, any entity or individual that intends to establish a medical institution must follow the application approval procedures and register with the relevant healthcare administrative authorities to obtain a Medical Institution Practicing License.
63
Law on Maternal and Infant Healthcare and Its Implementation Measures
The Law on Maternal and Infant Healthcare, which was promulgated by the Standing Committee of the National People’s Congress on October 27, 1994, came into effect on June 1, 1995, and was amended on August 27, 2009 and November 4, 2017, and the Implementation Measures of the Law of the People’s Republic of China on Maternal and Infant Healthcare, which was promulgated by the State Council and came into effect on June 20, 2001 and was amended on November 17, 2017, require medical institutions engaged in (i) genetic disease diagnosis and prenatal diagnosis, (ii) pre-marital medical examinations, or (iii) midwifery services, ligature operations or operations for termination of gestation, to be licensed by the public health administrative authority of different levels as required to obtain the corresponding qualification certificates.
Administrative Measures for the Examination of Medical Institutions (For Trial Implementation)
The Administrative Measures for the Examination of Medical Institutions (For Trial Implementation), which was promulgated by the Ministry of Health and came into effect on June 15, 2009, stipulates that a medical institution’s Medical Institution Practicing License is subject to periodic examinations and verifications by the registration authorities. Such examinations and verifications are based on records and points generated by point management system for malpractice of medical institutions, established by local health administrative departments. The verification period is three years for general hospitals, traditional Chinese medicine hospitals, western medicine and traditional Chinese medicine hospitals, ethnic minority medicine and specialized hospitals, as well as sanitariums, rehabilitation hospitals, maternity and children’s healthcare centers, emergency centers, clinical laboratories and specialized disease prevention institutions equipped with more than 100 beds, and one year for other medical institutions. In the event that a medical institution fails to apply for verification as required and post re-verification procedures are unsuccessful, the registration authorities may cancel such medical institution’s Medical Institution Practicing License.
Administrative Measures for Aesthetic Medical Services
The Administrative Measures for Aesthetic Medical Services, which was promulgated by the Ministry of Health on January 22, 2002, came into effect on May 1, 2002, and was amended in 2009 and 2016, requires applicants for establishing aesthetic medical institutions or aesthetic medical departments within medical institutions to meet certain requirements. Aesthetic medical institutions may not commence operation before obtaining a Medical Institution Practicing License. Service items provided by aesthetic medical institutions or aesthetic medical departments within medical institutions must be approved by designated specialized academic associations and filed with relevant authorities.
Classification Catalog of Aesthetic Medical Items
The Classification Catalog of Aesthetic Medical Items, which was promulgated by the Ministry of Health and came into effect on December 11, 2009, classifies aesthetic medical services into four categories: (i) aesthetic surgical items (further divided into four grades) (ii) aesthetic dentistry items (iii) aesthetic dermatological items, and (iv) aesthetic Chinese medicine items. Provincial-level counterparts of the Ministry of Health may adjust the catalog based on local circumstances. Every medical institution will be registered with one or more categories from the day of establishment and is only allowed to conduct medical activities specifically provided under such categories.
Opinions on Implementing Classification Administration of Urban Medical Institutions
The Opinions on Implementing Classification Administration of Urban Medical Institutions, which was promulgated jointly by the Ministry of Health, State Administration of Chinese Traditional Medicine, the Ministry of Finance and National Development and Reform Commission on July 18, 2000 and came into effect on September 1, 2000, states that whether a medical institution is classified as not-for-profit or for-profit will be based on such medical institution’s business objectives, service purposes and implementation of various financial, taxation, pricing and accounting policies, provided that governments may not operate for-profit medical institutions. Medical institutions have to file written statements regarding their not-for-profit or for-profit status with relevant healthcare authorities when completing the application, registration and re-examination procedures in accordance with relevant laws, and the handling authority of health will, jointly with other relevant authorities, decide the not-for-profit/for-profit status for such medical institution based on the source of its investment and the nature of its business.
64
Categories of Medical Institutions in the PRC
According to the Basic Standards for Medical Institutions (For Trial Implementation), which was promulgated on September 2, 1994 and partly abrogated on August 2, 2010, March 15, 2011 and December 5, 2011, and the Interim Measures for the Assessment of Medical Institutions promulgated by the Ministry of Health on September 21, 2011, medical institutions in the PRC are divided into three classes (Class I, II and III) with regard to their medical practice conditions, including but not limited to, the amount of registered beds, treatment departments, personnel, properties, equipment, completeness of their internal rules and regulations as well as payment of registered capital.
(i)Grade I general hospitals must be at a minimum equipped with, among others, (a) 20 to 99 hospital beds, (b) clinical departments including emergency rooms, internal medicine, surgery, obstetrics and gynecology, preventive healthcare section; (c) medical departments including pharmacy, laboratory, x-ray room, and a disinfection supply room, and (d) 0.7 relevant health technicians per hospital bed, three doctors, five nurses, health technicians of medicament, inspection and radiation and one doctor of chief level or above.
(ii)Outpatient departments must be at a minimum be equipped with, among others, (a) no less than five clinical departments including three clinical departments that are either emergency rooms, internal medicine or surgery and two clinical departments that are either obstetrics and gynecology, pediatrics, traditional Chinese medicine, ophthalmology, otolaryngology, stomatology and prevention and health; (b) medical departments including pharmacy, laboratory, X-ray room, treatment room, disposal room and disinfection supply room at least and (c) no less than five doctors including one doctor at the associate-chief level and above, one doctor for each clinical department, five nurses including one nurse with nurse-in-charge title and above and one health technician with relevant specialty for each medical laboratory.
Basic Standard for Aesthetic Medical Institution and Aesthetic Medical Department (For Trial Implementation)
The Basic Standard for Aesthetic Medical Institution and Aesthetic Medical Department (For Trial Implementation), which was promulgated by the Ministry of Health and came into effect on September 2, 1994, and was revised on August 2, 2010, March 15, 2011 and December 5, 2011, specifies basic standards that aesthetic medical hospitals, aesthetic medical outpatient departments, aesthetic medical clinics and aesthetic medical specialty departments should meet, such as the required number of beds, clinical departments and medical personnel. Certain basic standards for each type of aesthetic medical institutions are set forth below.
(i)Aesthetic medical hospitals must be equipped with, among others, (a) more than 20 hospital beds, 12 aesthetic treatment beds and four dentistry complex therapy chairs, (b) clinical departments including an aesthetic treatment consultation room, aesthetic surgery, aesthetic dentistry, aesthetic dermatology, aesthetic traditional Chinese medicine, aesthetic treatment room and anesthesiology, and (c) at least 1.03 relevant healthcare technicians per bed (chair), 0.4 nurse per bed (chair), six doctors at the associate-chief level and above with relevant specialties, two nurses with nurse-in-charge title and above and one attending specialist doctor for each department.
(ii)Aesthetic medical outpatient departments must be at a minimum equipped with, among others, (a) four aesthetic treatment beds, two operation tables, two dentistry complex therapy chairs and two observation beds, (b) clinical departments including an aesthetic treatment consultation room, aesthetic surgery, aesthetic dentistry and aesthetic dermatology (may also have an aesthetic traditional Chinese medicine department and aesthetic treatment rooms), and (c) at least 2.4 relevant healthcare technicians per operation table, 1.03 relevant healthcare technicians and 0.4 nurse per observation bed and dentistry complex therapy chair, five registered doctors including at least one doctor at the associate-chief level and above with relevant specialties and one registered nurse, and one attending specialist doctor for each department.
(iii)Aesthetic medical clinics must be at a minimum equipped with, among others, (a) two aesthetic treatment beds, or one operation table and one observation bed or one dentistry complex therapy chair, (b) no more than two clinical departments that are aesthetic surgery, aesthetic dermatology, aesthetic dentistry and aesthetic traditional Chinese medicine; (c) at least one attending specialist doctor and one nurse for each department.
65
(iv)Aesthetic medical specialty departments must be at a minimum equipped with, among others, (a) four aesthetic treatment beds, one operation table, one dentistry complex therapy chair and one observation bed, (b) clinical departments including aesthetic treatment consultation room and aesthetic treatment room, and at least two clinical departments that are either aesthetic surgery, aesthetic dermatology, aesthetic dentistry and aesthetic traditional Chinese medicine, and (c) at least 2.4 relevant healthcare technicians per operation table, 1.03 relevant healthcare technicians and 0.4 nurse per observation bed and dentistry complex therapy chair, one attending specialist doctor and one registered nurse for each department.
Circular on Further Strengthening Comprehensive Regulatory Enforcement in the Medical Beauty Industry
On April 3, 2020, the State Administration for Market Regulation, National Health Commission of the PRC, National Medical Products Administration of the PRC, Office of the Central Cyberspace Affairs Commission, among others, jointly promulgated the Circular on Further Strengthening Comprehensive Regulatory Enforcement in the Medical Beauty Industry, which stipulate that medical beauty services shall be implemented by a surgeon or physician in charge or the practicing physician under the guidance of the surgeon or physician in charge in accordance with the registered medical beauty service items in the medical institutions that set up medical beauty related subjects. No organization or individual shall carry out medical beauty services without meeting the legal conditions. Medical beauty institutions shall purchase drugs and medical devices in enterprises with production and operation qualifications. Medical beauty advertisements belong to medical advertisements, and non-medical institutions shall not publish medical advertisements.
Special Rectification Work Plan for Cracking Down on Illegal Medical Beauty Services
On May 28, 2021, the State Administration for Market Regulation, State Administration of Traditional Chinese Medicine, National Health Commission of the PRC, National Medical Products Administration of the PRC, Office of the Central Cyberspace Affairs Commission, among others, jointly promulgated the Special Rectification Work Plan for Cracking Down on Illegal Medical Beauty Services, which stipulate that in order to further safeguard the legitimate rights and interests of consumers and protect people’s health and life safety, the foresaid departments are scheduled to carry out special rectification work against illegal medical and beauty services nationwide from June to December 2021. The work tasks mainly include: (i) severely crack down on illegal activities related to medical beauty, (ii) strictly standardize the behavior of medical beauty service, (iii) severely crack down on the illegal manufacture, sale of drugs and medical devices, and (iv) seriously investigate and prosecute illegal advertising and internet information.
Regulations on the supervision over pharmaceuticals and medical services in medical institutions
Pharmaceutical Administration Law of the People’s Republic of China and Its Implementation Regulations
The Pharmaceutical Administration Law of the People’s Republic of China, which was promulgated by the Standing Committee of the National People’s Congress on September 20, 1984, came into effect on July 1, 1985, and was amended in 2001, 2013, 2015 and 2019, and the Regulations for the Implementation of the Pharmaceutical Administration Law of the People’s Republic of China, which was promulgated by the State Council on August 4, 2002, came into effect on September 15, 2002, and was amended on February 6, 2016 and March 2, 2019, provide that medical institutions may only purchase pharmaceuticals from licensed enterprises engaged in pharmaceutical manufacturing and distribution (except for the purchase of those Chinese herbal medicines that are not administrated by the approval number system). Also, medical institutions must establish an examination and inspection system to verify the qualification certificates and other specifications of the pharmaceuticals they purchase, and may not purchase or use pharmaceuticals that do not satisfy the relevant requirements. In addition, medical institutions must adopt and implement a pharmaceutical storage system and take necessary measures to ensure the quality of the pharmaceuticals such as providing refrigeration or be freeze-proof, damp-proof, pest-proof, and mouse-proof. Medical institutions are also required to retain certain qualified pharmaceutical technician personnel. Non-pharmaceutical technician personnel are prohibited from directly engaging in drug-related technical work. Obtaining an Import Permit Certificate or an Export Permit Certificate issued by the drug administration department of the State Council is also required for the import and export of narcotics and permitted psychotropic substances.
66
Measures for the Supervision and Administration of Pharmaceuticals in Medical Institutions (for Trial Implementation)
The Measures for the Supervision and Administration of Pharmaceuticals in Medical Institutions (for Trial Implementation), which was promulgated by the China Food and Drug Administration, or the CFDA, on October 11, 2011 and came into effect the same date, requires medical institutions to purchase pharmaceuticals from enterprises qualified for the production or distribution of pharmaceuticals and comply with certain standards regarding the storage, safekeeping, preparation and use of such pharmaceuticals. Any pharmaceutical prepared by a medical institution must be used by and for that same medical institution only. Medical institutions are prohibited from selling prescription pharmaceuticals directly to the public via mail, online or over the counter.
Measures for the Classification and Administration of Prescription Pharmaceuticals and Non-prescription Pharmaceuticals (For Trial Implementation)
The Measures for the Classification and Administration of Prescription Pharmaceuticals and Non-prescription Pharmaceuticals (For Trial Implementation), which was promulgated by State Drug Administration on June 18, 1999 and came into effect on January 1, 2000, sets forth the principals for the classification and administration of prescription pharmaceuticals and non-prescription pharmaceuticals based on their types, specifications, indications, dosage and supply channels. According to this measure, prescription pharmaceuticals may only be produced, purchased and used by licensed doctors’ prescription and licensed associate doctors, while non-prescription pharmaceuticals may be produced, purchased and used by anyone at their discretion without licensed doctors or licensed associate doctors’ prescription.
Regulations on Centralized Pharmaceutical Procurement by Medical Institutions
The Opinions on Further Regulating Centralized Pharmaceutical Procurement by Medical Institutions, which was jointly promulgated by the Ministry of Health and five other departments on January 17, 2009, and the Interpretations of Issues Related to the Opinions on Further Regulating Centralized Pharmaceutical Procurement by Medical Institutions, which was jointly promulgated by the Ministry of Health and five other departments on June 19, 2009, as well as the Administrative Measures for Supervision on Centralized Pharmaceutical Purchase and the Standards of Centralized Pharmaceutical Procurement Work for Medical Institutions jointly promulgated by the Ministry of Health and six other departments on June 2, 2010 and July 7, 2010, respectively, provide the general framework and detailed operational procedures regarding the centralized pharmaceutical procurement mechanism that requires non-profit medical institutions established by governments or state-owned enterprises to procure pharmaceuticals through the non-profit centralized pharmaceutical procurement platform organized by the competent governmental authorities. Medical institutions of other forms, are also encouraged to participate in the centralized pharmaceutical procurement system.
Regulations on Prices of Pharmaceuticals and Medical Services
The Notice on Issuing Opinions on Reforming the Price Formation Mechanism of Drugs and Medical Services, which was jointly promulgated by the NDRC, the Department of Health, and the Ministry of Human Resources and Social Security, came into effect on November 9, 2009. It provides that the price for basic healthcare services provided by non-profit medical institutions have to be set by government-directed pricing guidelines, while the price for healthcare services provided by for-profit medical institutions and certain special categories of healthcare services provided by non-profit medical institutions may be determined by the market.
According to the Notice of Issues Related to the Implementation of Market Price Adjustment by Non-public Medical Institutions, which was promulgated and implemented on March 25, 2014 by the National Development and Reform Commission, National Health and Family Planning Commission and the Ministry of Human Resources and Social Security, private for-profit medical institutions may set their own pricing for medical services, but such pricing must be determined according to principles of fairness, legitimacy and good faith and maintained at a relatively stable price level within a specific period. In addition, prices of medical services and pharmaceuticals must be displayed publicly to patients through various means, and medical institutions must take the initiative to receive social supervision.
67
The Opinions on Promoting Pharmaceutical Pricing Reform, which was promulgated by the National Development and Reform Commission, National Health and Family Planning Commission, Ministry of Human Resources and Social Security and four other departments on May 4, 2015 and came into effect on the same date, sets forth that from June 1, 2015, except for narcotic drugs and Class I psychotropic drugs, the pricing of drugs formulated by the government will be abolished. The pricing of narcotic drugs and Class I psychotropic drugs is still temporarily regulated by the NDRC which sets the maximum ex-factory and maximum retail prices until new regulation on pricing is implemented. The pricing for other drugs may be determined by manufacturers and sellers on their own based on the cost of production and operation as well as market supply and demand.
According to the Pilot Scheme of Deepening the Reform of Medical Services Price, which was promulgated and implemented on August 25, 2021 by the National Health Commission of the PRC and seven other departments, private for-profit medical institutions may set their own pricing for medical services, but such pricing must be reasonable pricing and determined in accordance with principles of fairness, legitimacy and good faith, and consistency between quality and price, and those included in the medical insurance fund to be paid shall be managed in accordance with the medical insurance agreements.
Administrative Measures for Pharmaceutical Importation
The Administrative Measures for Pharmaceutical Importation, which was jointly promulgated by the Ministry of Health and the General Administration of Customs on August 18, 2003 and amended on August 24, 2012, provides that imported pharmaceuticals may be registered and cleared for customs only after obtaining an Imported Pharmaceutical Registration Certificate, a Pharmaceutical Product Registration Certificate or an Imported Pharmaceutical Permit, as applicable.
Administrative Measures on Drug Recalls
The Administrative Measures on Drug Recalls, which was promulgated by National Medical Products Administration on October 24, 2022 and came into effect on November 1, 2022, requires that drug manufacturers, drug distributors and drug users shall actively assist the drug marketing authorization holder, or the holder, in investigating and evaluating the drugs with possible quality problems or other potential safety hazards, take the initiative to cooperate with the holder in fulfilling the obligation of recalls, timely transmit and give feedback of the information on drug recalls according to the recall plan, control and recall the drugs with quality problems or other potential safety hazards. Where a drug manufacturer, drug distributor or drug user finds that the drugs manufactured, sold or used by it may have quality problems or other potential safety hazards, it shall notify the holder in a timely manner, suspend the production, release, sale and use of the drugs when necessary, and report to the drug regulatory department of the government of the province, autonomous region or centrally-administered municipality where it is located. The information in the notice and report shall be authentic. The holder, drug manufacturer, drug distributor and drug user shall establish and implement a drug traceability system as required, keep complete purchase and sale records, and ensure the traceability of the drugs on the market. Drug manufacturers, drug distributors and drug users shall cooperate with the holder in the investigation of the quality problems or other potential safety hazards of the drugs concerned, and provide relevant materials.
Administrative Measures for the Control of Radioactive Pharmaceuticals
The Administrative Measures for the Control of Radioactive Pharmaceuticals, which was promulgated by the State Council, came into effect on January 13, 1989, and was amended on January 8, 2011 and March 1, 2017, requires medical institutions to comply with relevant national regulations and rules concerning radioisotope health protection when using radioactive pharmaceuticals. Any medical institution wishing to use radioactive pharmaceuticals must obtain a License for the Use of Radioactive Pharmaceuticals of the corresponding grade from the supervision and administration department of pharmaceuticals at the provincial, regional or municipal levels, as applicable. The License for the Use of Radioactive Pharmaceuticals is valid for five years and is of varying grades based on the technical skill and professional level of the radiological personnel and the equipment conditions of such medical institution. In addition, the preparation or use of radioactive preparations must meet the relevant provisions of the Pharmaceutical Administration Law of the People’s Republic of China and the implementing rules thereof.
68
Regulations on the Administration of Narcotic Pharmaceuticals and Psychotropic Substances
The Regulations on the Administration of Narcotic Pharmaceuticals and Psychotropic Substances, which was promulgated by the State Council on August 3, 2005 and was amended on December 7, 2013 and February 6, 2016, provides that if a medical institution needs to use any narcotic pharmaceutical or Class I psychotropic substance, such medical institution must, upon approval by the competent public health department, obtain the Seal Card for the Purchase and Use of Narcotic Pharmaceuticals and Class I Psychotropic Substances, or the Seal Card. If a medical institution with a Pharmaceutical Preparation Certificate for Medical Institutions and a Seal Card needs to dispense for clinical use any narcotic pharmaceutical or psychotropic substance that is not available on the market, the preparation of such pharmaceutical or psychotropic substance will be subject to approval by the competent provincial, regional or municipal pharmaceutical regulatory department where the medical institution is located. Such prepared narcotic pharmaceutical or psychotropic substance may only be dispensed by the medical institution that prepared it and may only be used in the institution itself and may not be marketed.
Measures for the Administration of Prescriptions
The Measures for the Administration of Prescriptions, which was promulgated on February 14, 2007 and came into effect on May 1, 2007 by the Ministry of Health, provides the standards and format of prescriptions, the principles of prescription writing that doctors must abide by, and other regulations related to the provision and preparation of prescriptions. In accordance with the Measures for the Administration of Prescriptions, a registered doctor must acquire the prescription rights in the medical institution where he or she is registered. The prescription prescribed by the assistant doctor is then valid upon the signature or personal seal of the doctor in the same medical institution.
Regulations on the supervision over medical equipment in medical institutions
Regulations on the Supervision and Administration of Medical Devices
The Regulations on the Supervision and Administration of Medical Devices, or the Regulations on Medical Devices, which was promulgated by the State Council on January 4, 2000, amended on March 7, 2014, May 4, 2017 and February 9, 2021 and took effect on June 1, 2021, regulates the management of medical device manufacturing and the supervision, distribution and use of medical devices as well as relevant legal obligations. Pursuant to the Regulations on Medical Devices, medical device enterprises and medical institutions may only purchase medical devices from properly licensed enterprises. Medical institutions may not use unregistered, uncertified, expired, ineffective or outdated medical devices. Disposable medical devices may not be used repeatedly and medical institutions must destroy used disposable medical devices in accordance with relevant rules and keep records such disposal.
Administrative Measures on Radiotherapy
According to the Administrative Measures on the Radiotherapy, which was promulgated by the Ministry of Health on January 24, 2006, came into effect on March 1, 2006 and was amended on January 19, 2016, medical institutions engaged in radio diagnosis and radiotherapy must be equipped with the conditions required for conducting radio diagnosis and radiotherapy, and apply for a License for Radiotherapy issued by the competent public health administrative authorities. Medical institutions may not conduct radio diagnosis and radiotherapy if they fail to obtain the License for Radio-diagnosis and Radiotherapy or to register with radio diagnosis and radiotherapy on the Medical Institution Practicing License. During the course of radiotherapy, medical institutions must take protective measures in accordance with the relevant laws and regulations.
Regulations on the Safety and Protection of Radioisotopes and Radiation-emitting Devices and Measures for Administration of the Safety Licensing of Radioactive Isotopes and Radioactive Equipment
The Regulations on the Safety and Protection of Radioisotopes and Radiation-emitting Devices, which was promulgated by the State Council on September 14, 2005, came into effect on December 1, 2005 and was revised on July 29, 2014 and March 2, 2019, and the Measures for Administration of the Safety Licensing of Radioactive Isotopes and Radioactive Equipment, which was promulgated by the Ministry of Environmental Protection (currently known as the Ministry of Ecology and Environment) on January 18, 2006 and amended on December 6, 2008, December 12, 2017, August 22, 2019 and January 4, 2021, respectively, require any entity engaging in the production, sale or use of radioisotopes or radiation-emitting devices of different categories to obtain a Safety License for Radiation. In addition, medical institutions using radioisotopes or radiation-emitting devices for diagnosis and treatment are required to obtain a permit for radiological diagnostic and therapeutic techniques and medical radiation.
69
Administrative Measures on the Deployment and Use of Large Medical Equipment (for Trial Implementation)
The Administrative Measures on the Deployment and Use of Large Medical Equipment (for Trial Implementation), which was jointly promulgated by the National Health Commission and the State Drug Administration and became effective on May 22, 2018, provides that the State implements classified and categorized allocation plans and license management to large medical equipment according to the Catalog of Large Medical Equipment. Large medical equipment refers to medical equipment with complicated technology, large capital input, high operating cost, great impact on medical expenses and included in the catalog management. Medical institutions that intend to purchase large medical equipment must apply to the competent health administrative authorities and purchase the approved large medical equipment upon the receipt of a License for the Deployment of Large Medical Equipment.
Administrative Measures for Medical Consumables for Medical Institutions (for Trial Implementation)
Administrative Measures for Medical Consumables for Medical Institutions (for Trial Implementation) or the Measures for Medical Consumables for Medical Institutions was jointly promulgated by the National Health Commission and the National Administration of Traditional Chinese Medicine on June 6, 2019 and came into effect on September 1, 2019. Measures for Medical Consumables for Medical Institutions provide for the definition and classification of medical consumables, and state that whole-process administration will be imposed on work related to medical consumables, including selection, procurement, receiving inspections, storage, distribution, clinical use, monitoring, and evaluation. Measures for Medical Consumables for Medical Institutions state that medical consumables will be subject to unified procurement, adding that clinical use of medical consumables is administered at three different levels, and for this purpose, medical consumables are divided into three classes, i.e. Class I, Class II and Class III. Class I medical consumables are utilized by medical technicians, while Class II medical consumables are used by qualified medical technicians after they have received the necessary training, and Class III medical consumables, as stipulated by relevant administrative provisions on medical technologies, are used by those medical technicians who obtain the qualification to make relevant technical operations. Furthermore, Measures for Medical Consumables for Medical Institutions require that medical consumables usable by medical institutions shall fall in a limited range of varieties, to limited specifications and in a limited quantity, and the number of enterprises supplying the medical consumables with the same or similar functions should be controlled as well.
Regulations on medical personnel of medical institutions
Physicians Law of the People’s Republic of China
The Physicians Law of of the People’s Republic of China, promulgated by the Standing Committee of the National People’s Congress on August 20, 2021 and came in effect on March 1, 2022, which replaced the Law on Medical Practitioners of the People’s Republic of China, promulgated by the Standing Committee of the National People’s Congress on June 20, 1998. The Physicians Law of of the People’s Republic of China provides that physicians in the PRC must obtain qualification licenses for their medical profession.Qualified physicians and qualified assistant physicians must register with the relevant health administrative authorities at or above the county level. After registration, physicians may practice in their registered institution within the registered practicing categories and practicing scope.
Administrative Measures for the Registration of Medical Practitioners
Administrative Measures for the Registration of Medical Practitioners, which was promulgated by the National Health and Family Planning Commission on February 28, 2017 and came into effect on April 1, 2017, provides that the State has established an information system for medical practitioner administration to carry out e-registration and online administration. Upon gaining a qualification license to practice as a doctor, medical practitioners may conduct activities related to medical care, disease prevention or healthcare according to their registered area, category and scope of practice. Medical practitioners serving in several institutions within the same place of practice shall determine a specific institution as the main practicing institution, and apply for registration with the competent health and family planning administrative authority that approves the practice of the institution. For other institutions where the physician intends to practice, the physicians must apply with the administrative departments of health and family planning that approves the practice of such institutions, indicating the locations and names of such institutions.
70
Trial Regulations on the Position of Health Technical Personnel
The Trial Regulations on the Position of Health Technical Personnel, which was promulgated by the Ministry of Health on February 22, 1981 and came into effect on the same date, classifies health technical positions into four types: medical, medicine, nursing and technician. The chief medical/ medicine/nursing/technical physicians and associate-chief medical/medicine/nursing/technical physicians are classified as senior technical positions. The attending medical/medicine/nursing/technical physicians are classified as intermediate technical positions. The medical/medicine/nursing/technical physicians and the medical/medicine/nursing/technical staff are classified as primary technical positions. Health technical position qualifications at various levels are reviewed respectively, by the corresponding senior/ intermediate/primary position review committee. Senior position review committee is generally created by departments in the State Council, provinces, autonomous regions or municipalities directly under the central government. The aforementioned authorities may authorize competent subordinate entities to constitute senior position review committees, and such subordinate entities should apply for approval regarding the establishment of senior position review committees from the nominating department. The competence to constitute intermediate/primary position review committees is determined by departments in the State Council, provinces, autonomous regions or municipalities directly under the central government.
Regulations on Nurses
The Regulations on Nurses, which was promulgated by the State Council on January 31, 2008, came into effect on May 12, 2008 and was amended on March 27, 2020, provides that a nurse shall apply to the competent health administrative department that approves the establishment of the medical institution in which he or she intends to practice or that processes the record-filing of the said medical institution and obtain a Nurse’s Practicing Certificate. The practicing nurse registration is valid for five years. The number of nurses at a medical institution may not be less than the standard number set by the public health administrative authority of the State Council.
Administrative Measures for Aesthetic Medical Services
The Administrative Measures for Aesthetic Medical Services, which was promulgated by the Ministry of Health on January 22, 2002, came into effect on May 1, 2002, and was amended on February 13, 2009 and January 19, 2016, requires medical practitioners of aesthetic medical services to obtain the qualification license of aesthetic medical chief doctor or provide aesthetic medical clinical services under supervision of licensed chief doctors. An aesthetic medical chief doctor is required to have, among others, (a) a doctor’s qualification and registration with relevant authority, (b) working experience with relevant clinical specialties, and (c) aesthetic medical training or an advanced education certificate or no less than one year of clinical working experience in the aesthetic medical services.
Personnel providing aesthetic medical nursing services must also meet relevant requirements including but not limited to (a) nurse qualifications and registrations with relevant authorities, (b) no less than two years of nursing working experience, and (c) aesthetic medical nursing training or advanced education certificates or not less than six months of clinical nursing working experience in the aesthetic medical services. The chief doctor responsible for aesthetic surgeries must also have at least six years of clinical working experience in the aesthetic surgery departments or plastic surgery departments. The chief doctor responsible for aesthetic dentistry treatment must also have at least five years of clinical working experience in aesthetic dentistry departments or stomatology departments. The chief doctor responsible for aesthetic traditional Chinese medicine treatment or aesthetic dermatological treatment must also have at least three years of clinical working experience in traditional Chinese medicine or dermatology.
Regulations on Physicians’ Multi-sited Practice
Several Opinions on Accelerating the Development of Medical Institutions with Social Capital, which was promulgated on December 30, 2013 by the National Health and Family Planning Commission and the State Administration of Traditional Chinese Medicine, specifically states that multi-sited practice of physicians is permitted. Medical personnel can orderly move among medical institutions owned by different entities.
The Notice on Issuing Opinions on Promoting and Regulating Physicians’ Multi-sited Practice, which was jointly promulgated by the National Health and Family Planning Commission, National Development and Reform Commission, and the Ministry of Human Resources and Social Security on November 5, 2014 and came into effect on the same date, requires that physicians with multi-sited practice to meet the following criteria: (1) be qualified for intermediate or higher professional and technical positions, and have been engaged in the same profession for no less than five years, (2) be healthy and be competent for multi-sited practice, and (3) have no unqualified record in the periodic assessments of physicians in the last two consecutive cycles. It also states that registration management applies to multi-sited practices.
71
Interim Administrative Measures for Temporary Medical Practicing in Mainland China by Foreign Doctors
The Interim Administrative Measures for Temporary Medical Practicing in Mainland China by Foreign Doctors, which was promulgated by the Department of Health on October 7, 1992, came into effect on January 1, 1993, and was amended on November 28, 2003 and January 19, 2016, provides that temporary medical practice in China by foreign doctors refers to clinical diagnosis and treatment business activities that are carried out for a period of less than one year by foreign doctors licensed in other countries who are invited to come to or employed in Mainland China. Foreign doctors wishing to engage in temporary medical practice in Mainland China must be registered and issued a License of Short-term Medical Practicing by Foreign Doctors. The effective period for a short-term practicing foreign doctor permit in China does may exceed one year, but foreign doctors may re-apply for such registration upon the expiration of their previous registration.
Notice on Issues Related to Strengthening the Management of Medical Aesthetic In-charge Physicians
According to the Notice on Issues Related To Strengthening the Management of Medical Aesthetic In-charge Physicians, which were promulgated by the National Health and Family Planning Commission on March 17, 2017, medical institutions to carry out aesthetic medical service, should comply with the Administrative Measures for Aesthetic Medical Services, to check and ratify the specialty of the medical aesthetic in-charge physicians of the medical institution.
Management Measures of Qualification and Practice of Aesthetic Medical Attending Doctors (for Trial Implementation)
The Management Measures of Qualification and Practice of Aesthetic Medical Attending Doctors (for Trial Implementation), which was promulgated by the Health and Family Planning Commission of Guangdong Province on March 17, 2014 and became effective on May 1, 2014, provides that practicing doctors conducting aesthetic medical activities in Guangdong Province should obtain the Qualification Certificate of Aesthetic Medical Attending Physicians in Guangdong Province before conducting aesthetic medical diagnosis and treatment activities. After obtaining the Qualification Certificate of Aesthetic Medical Attending Physicians in Guangdong Province, they may only conduct aesthetic medical diagnosis and treatment activities in accordance with the approved category of aesthetic medical doctors, the registered practice place and the practice scope. Doctors without the Qualification Certificate of Aesthetic Medical Attending Physicians in Guangdong Province shall engage in aesthetic medical activities under the guidance of attending doctors holding such qualification certificate, and shall not independently conduct aesthetic medical activities. Attending doctors conducting aesthetic medical activities that are beyond the registered doctor’s category or are out of the registered practice place will be prohibited from conducting aesthetic medical activities for 6 months and will be required to attend trainings on laws and regulations.
Regulations on anti-corruption and anti-commercial bribery
The PRC governmental departments have formulated relevant laws and regulations for standardizing the anti-corruption and anti-commercial bribery in the medical treatment and healthcare industry.
Code of Conduct for the Practitioners of Medical Institutions
The Code of Conduct for the Practitioners of Medical Institutions, or the Code, was jointly promulgated by the Ministry of Health, the State Food and Drug Administration, the State Administration of Traditional Chinese Medicine and came into effect on June 26, 2012, providing the basic rules of conduct for practitioners at medical institutions, managerial persons, doctors, nurses, pharmaceutical technicians, medical technicians and other relevant persons. The Code applies to practitioners of all levels and classes at medical institutions. Pursuant to the Code, practitioners in medical institutions must perform their duties honestly, be self-disciplined, and abide by medical ethics. Accordingly, such practitioners may not ask for or illegally receive any property from patients, receive improper benefits by leveraging their positions, receive such rebates or commissions, in various form or titles, offered by personnel in creating or operating an enterprise related to medical equipment and machinery, pharmaceuticals, and chemical agents, participate in operational entertainment arranged, organized or paid by such personnel, gain access to or acquire the basic medical protection fund by cheating or facilitate such improper acquisition by others, violate the laws by participating in advertisement for medical treatments and marketing and promotion of pharmaceuticals or machinery for medical treatment, or re-sell a registration number for treatment at a profit.
72
Nine Criteria for Incorrupt Practices of Staff of Medical Institutions
In accordance with the Notice on Printing and Distributing of the “Nine Criteria” for Incorrupt Practices of Staff of Medical Institutions, which was promulgated and implemented by the National Health Commission, the National Healthcare Security Administration, and the State Administration of Traditional Chinese Medicine and effective on November 12, 2021, medical institutions are required to implement the policy of the “Nine Criteria”, including (1) staff of medical institutions shall receive remuneration according to law and based on the services they provided and shall not accept commercial commissions, (2) staff of medical institutions shall strictly follow the principle of good faith and shall not participate in insurance fraud, (3) staff of medical institutions shall practice medicine in accordance with norms and shall not conduct excessive diagnosis and treatment, (4) staff of medical institutions shall abide by working procedures and shall not accept donations in violation of regulations, (5) staff of medical institutions shall abide by the requirement of confidentiality and shall not disclose patients’ privacy, (6) staff of medical institutions shall follow the needs of diagnosis and treatment, and shall not seeking profits in referral of patients, (7) staff of medical institutions shall maintain the order of diagnosis and treatment and shall not undermine the fairness in medical treatment, (8) staff of medical institutions shall build harmonious relations and shall not accept “red packets” from patients, and (9) staff of medical institutions shall keep the bottom line of contacts and shall not accept kickbacks from enterprises. Staff of medical institutions, including but not limited to health professionals, management personnel, logistics personnel and other social practitioners who provide services within the medical institutions and are managed by the medical institutions, shall abide by the relevant requirements of the Nine Criteria, subject themselves to management and strictly implement relevant requirements. Any such stuff who violates the above requirements shall be punished in accordance with laws and regulations within administrative authority.
Regulations on Governance of Commercial Bribery in the Purchase and Sales of Medicine
In accordance with the Implementation Opinions on Launching Special Governance on Commercial Bribery in Medicine Purchase and Sale, promulgated and implemented by the Ministry of Health and the State Administration of Traditional Chinese Medicine and effective April 21, 2006 and the Provisions on the Establishment of Adverse Records of Commercial Briberies in the Purchase and Sale of Medicine, which was promulgated by the National Health and Family Planning Commission on December 25, 2013 and implemented on March 1, 2014, the focus of the specialized governance framework regarding commercial bribery to procure medicine are on: (i) the act of the leaders and the relevant personnel of medical institutions of receiving property or rebate granted by the manufacturing and business enterprises and their marketing and sales personnel during the procurement of medicines, medical equipment and medical consumables, (ii) the act of the medical professionals of medical institutions of receiving property or commission granted by the manufacturing and business enterprises and their marketing and sales personnel during the clinical, diagnosis and treatment, and (iii) the act of medical institutions of receiving property granted by the manufacturing and business enterprises and their marketing and sales personnel. Personnel in violation of the relevant provisions will be punished by confiscation of the illegal goods and revocation of their business licenses. If the act constitutes a crime, criminal liability on the relevant parties will also be pursued according to laws.
Regulations on medical malpractice
The PRC Civil Code
The PRC Civil Code, which was promulgated by the National People’s Congress on May 28, 2020, came into effect on January 1, 2021 provides that contracting parties must perform their obligations in full as agreed. The parties shall, under the principle of good faith, perform such obligations according to the nature and purpose of the contract as well as trading practices.
A lawfully established contract is legally binding on the contracting parties, each of whom must perform its own obligations in accordance with terms of the contract, and no party may unilaterally modify or terminate the contract.
The PRC Civil Code provides that, if a medical institution or the medical personnel of a medical institution are at fault for damage inflicted on a patient during the course of diagnosis and treatment, the medical institution will be liable for compensation. The medical institution is responsible for the damage caused to the patient due to the failure of the medical personnel to fulfill their statutory obligations in the course of diagnosis and treatment. Medical institutions and their medical personnel must protect the privacy of their patients and will be liable for damage caused by divulging the patients’ private or medical records without consent.
73
Interpretations of the Supreme People’s Court on Several Issues concerning the Application of Law in Hearing Cases Involving Disputes over the Liability for Medical Damage
According to the Interpretations of the Supreme People’s Court on Several Issues concerning the Application of Law in Hearing Cases Involving Disputes over the Liability for Medical Damage, which was promulgated by the Supreme People’s Court on December 13, 2017, became effective on December 14, 2017 and was amended on December 29, 2020, after a patient has filed a lawsuit against some of the medical institutions where he or she was treated, such medical institutions are allowed to file an application to bring in other medical institutions where the patient has received treatment as joint defendants or third parties. The people’s court may also bring related parties into the lawsuit according to the law when the court deems it necessary.
Regulations on Handling Medical Malpractice
The Regulations on Handling Medical Malpractice, which was promulgated by the State Council on April 4, 2002 and came into effect on September 1, 2002, provides a legal framework and detailed provisions regarding the prevention, identification, disposition, compensation and penalties of or relating to cases involving personal injury to patients caused by medical institutions or medical personnel due to malpractice. Medical accidents shall, according to the seriousness of personal injuries to patients, be classified into four grades. The criteria for specific grades shall be formulated by the health administration department of the State Council.
Trial Administrative Measures of the Health Department of Guangdong Province on Point Deductions for Medical Institution Malpractice
The Trial Administrative Measures of the Health Department of Guangdong Province on Point Deductions for Medical Institution Malpractice, or the Guangdong Measures, which was promulgated and came into effect on January 4, 2011 by the Health Department of Guangdong Province, specifies certain circumstances under which a medical institution’s malpractice points will be deducted and the number of malpractice points to be deducted under each circumstance. A medical institution’s malpractice points may be subject to deductions each year, and when the annual renewal of the Medical Institution Practicing License is completed, the deducted malpractice points are reset to zero. From the date of renewal, if there are more than 24 points deducted within a year, the health administrative department will report the point deductions to its supervising department. Medical institutions at all levels must comply with the Guangdong Measures, provided that such medical institutions have legitimately obtained a Medical Institutions Practicing License within the administrative area of Guangdong Province.
Regulations on Point Accumulation and Deduction for Medical Institution Malpractice in Shanghai
On January 30, 2007, the Health Department of Shanghai promulgated the Notice on Improving the Management of Point Accumulation for Medical Institution Malpractice in Shanghai, which came into effect on the same date, establishing the archive management system and laying out detailed procedures for archive management. The Administrative Measures of Shanghai on Point Deduction for Medical Institutions Malpractice, or the Shanghai Measures which was promulgated on October 15, 2018 and came into effect on January 1, 2019 by the Health Department of Shanghai, specifies certain circumstances under which a medical institution’s malpractice points will be deducted and the number of points to be deducted under each circumstance. A medical institution’s malpractice points may be subject to deduction each examination and verification period following the issuance date of the Medical Institution Practicing License or the qualification date of the previous examination and verification period. Upon expiration of each deduction period, the deducted malpractice points are reset to zero. Where a medical institution has a one-year examination and verification period and its malpractice points are deducted by more than 12 during such period, or where a medical institution has a three-year examination and verification period and its malpractice points are deducted by more than 36 during such period (1) if it is within 3 months before the expiration of the current examination and verification period, the registration authority should order such medical institution to apply for examination and verification immediately and should extend the examination and verification period of such medical institution by one to six months. The medical institution must then apply for re-examination and verification upon the expiration of the extended examination and verification period, whereas, in the event that the malpractice points are deducted by more than 6 during the extended examination and verification period, the medical institution will fail the re-examination and verification and its Medical Institution Practicing License will be revoked by the registration authority; (2) the legal representative and principal responsible person of such medical institution shall take the examination regarding relevant laws and regulations of health care organized by the health administrative department. If such persons do not take the examination or fail the examination, such medical institution will be deemed unqualified for re-examination and re-verification, and its Medical Institution Practicing License will be revoked by the registration authority. Medical institutions at all levels in Shanghai must comply with the Shanghai Measures, provided that such medical institutions have legitimately obtained a Medical Institutions Practicing License within the administrative area of Shanghai.
74
Regulations on Point Accumulation for Medical Institution Malpractice in Beijing
The Health Department of Beijing promulgated the Trial Administrative Measures of Beijing on Point Accumulation for Medical Institution Malpractice on February 22, 2008, or the Beijing Measures, which came into effect on March 20, 2008, specifying certain circumstances under which a medical institution’s malpractice points will be deducted and the number of points to be deducted under each circumstance. Medical institutions at all levels in Beijing must comply with the Beijing Measures, provided that such medical institutions have filed for practice registration with the health administrative department and legitimately obtained a Medical Institutions Practicing License within the administrative area of Beijing. On January 16, 2017, the Beijing Health and Family Planning Commission and Beijing Administration of Traditional Chinese Medicine jointly promulgated the Notice on Further Improving the Management of Point Accumulation for Medical Institution Malpractice, which came into effect on the same date, stating that the verification and examination of medical institutions may be postponed if a certain number of malpractice points of such medical institutions are deducted.
Administrative Measures of Hainan Province on Point Deduction for Medical Institution Malpractice
The Administrative Measures of Hainan Province on Point Deduction for Medical Institution Malpractice, which was promulgated on March 14, 2019 and came into effect on April 1, 2019 by the Health Commission of Hainan Province, specifies certain circumstances under which a medical institution’s malpractice points will be deducted and the number of points to be deducted under each circumstance. The malpractice points of a medical institution may be deducted in each year following the issuance date of such medical institution’s Medical Institution Practicing License. The registration authority may extend the examination and verification period of medical institutions by six months under the following circumstances: (1) for a medical institution that has a one-year examination and verification period, its malpractice points are deducted by more than 12 points during such period, or (2) for a medical institution that has a three-year examination and verification period, its malpractice points are deducted by more than 36 points during such period. If the malpractice points of the aforementioned medical institutions are deducted by more than 6 points during the extended examination and verification period, the registration authority may deem it as a failed examination and verification situation and revoke the Medical Institution Practicing License. All medical institutions holding a Medical Institution Practicing License issued in Hainan Province must comply with these measures.
Administrative Measures of Shandong Province on Point Deduction for Medical Institution Malpractice
Administrative Measures of Shandong Province on Point Deduction for Medical Institution Malpractice, which was promulgated on March 14, 2018 and came into effect on May 1, 2018 by the Health and Family Planning Commission of Shandong Province, specifies certain circumstances under which a medical institution’s malpractice points will be deducted and the number of points that will be deducted under each circumstance. The malpractice points of a medical institution may be deducted in each year following the issuance date of its Medical Institution Practicing License. Upon expiration of the one-year deduction period, the malpractice points are reset to zero. For a medical institution with a one-year examination and verification period, if its malpractice points are deducted by more than 18 points during such period, the registration authority may extend the examination and verification period of such medical institution by six months. The extended six-month examination and verification period will also be made to a medical institution with a three-year examination and verification period if its malpractice points are deducted by more than 80 during the three-year period. The aforesaid measures are applicable to all kinds of public and private medical institutions at all levels that have obtained the Medical Institution Practicing License according to laws within the administrative areas of Shandong Province.
75
Administrative Measures of Jiangxi Province on Point Deduction for Medical Institution Malpractice (2022 version)
According to Administrative Measures of Jiangxi Province on Point Deduction for Medical Institution Malpractice (2022 version), which was promulgated by the Department of Health of Jiangxi Province on March 2, 2022, the malpractice points of a medical institution may be deducted on a calendar year basis. Upon the end of each calendar year, a new deduction period shall start. Where the malpractice points deducted in a calendar year are less than 6, the competent health department shall conduct a warning interview with the person in charge of the medical institution as the case may be. Where the malpractice points deducted in a calendar year are greater than or equal to 6 but less than 12, the competent health department will include the medical institution as a key review and key supervision target when it implements administrative licensing and daily supervision and inspection. Where the malpractice points deducted in a calendar year are greater than or equal to 12 but less than 24, the competent health department shall postpone the acceptance of the relevant medical institution’s hospital-review related applications in the calendar year, and cancel the medical institution’s qualification for excellence appraisal. Where the malpractice points deducted in a calendar year are greater than or equal to 24, or the medical institution has more than three departments (or second- and third-level diagnosis and treatment subjects) been deducted more than 8 malpractice points, (1) the competent health department shall cancel the original evaluation level of the medical institution and withdraw the certificate and relevant logo, (2) if the medical institution is within 3 months from the expiration of the examination and verification period, the registration authority shall order the medical institution to apply for examination and verification immediately, and give the medical institution 1 to 6 montn’s deferred examination and verification period. The medical institution shall practice in accordance with the relevant regulations during the deferred examination and verification period. If the medical institution has accumulated 10 or more malpractice points during the deferred examination and verification period, it shall be deemed unqualified when re-verifying, and the registration authority shall cancel its Medical Institution Practicing License or record-filing certificate.
Interim Administrative Measures of Hunan Province on Point Deduction for Medical Institution Malpractice
According to the Interim Administrative Measures of Hunan Province on Point Deduction for Medical Institution Malpractice, which was promulgated by the Department of Health of Hunan Province on November 11, 2011, the malpractice points of a medical institution may be deducted in each year following the issuance date of the Medical Institution Practicing License. Upon expiration of the 12-month deduction period, the malpractice points are reset to zero. If the malpractice points are deducted by more than 12 within one year, the registration authority will extend its examination and verification period by one to six months. If the malpractice points are deducted by six or more during the extended examination and verification period, the medical institution will fail the examination.
Regulations on Preventing and Handling Medical Disputes
The Regulations on Preventing and Handling Medical Disputes, which was promulgated by the State Council on July 31, 2018 and come into effect on October 1, 2018, and the Measures for Complaint Management of Medical Institutions, which was promulgated by the National Health Committee on March 6, 2019 and came into effect on April 10, 2019, require medical institutions to establish a sound system for receipt of complaints, set up a unified complaint management department or be equipped with full-time (or part-time) staff, and post medical dispute resolution measures, procedures and contacts in a conspicuous place in the medical institution to make it convenient for patients to submit complaints or feedback. For medical institutions that have not established a system to receive complaints, or such system fails to comply with the relevant requirements, it may receive rectification order, warning from and be imposed fine by the competent health administrative department, while related staff and medical personnel directly responsible may be punished under serious circumstances. If any crime is constituted, the violator shall be subject to criminal liability in accordance with the law.
76
Regulations on medical advertising in the PRC
Advertisement Law of the People’s Republic of China
The Advertisement Law of the People’s Republic of China, or the Advertisement Law, which was promulgated by the Standing Committee of National People’s Congress on October 27, 1994, came into effect on February 1, 1995, and was amended on April 24, 2015, October 26, 2018 and April 29, 2021, provides that advertisements may not contain false or misleading content or deceive or mislead consumers. Advertisements related to pharmaceuticals and medical devices must be reviewed by relevant authorities in accordance with relevant rules before being distributed or broadcasted through movies, television, newspapers, journals or other means. The Advertisement Law further requires that any advertisement for medical treatment, pharmaceuticals or medical devices to not contain: (i) any assertion or guarantee regarding efficacy or safety, (ii) any statement on cure rate or effective rate, (iii) any comparison with the efficacy and safety of other pharmaceuticals or medical devices or with other medical institutions, (iv) any use of endorsements or testimonials, or (v) other items as prohibited by laws and administrative regulations.
Administrative Measures for Internet Advertisement
The Administrative Measures for Internet Advertisement, which was promulgated by the State Administration of Market Regulation on March 24, 2023 and will come into effect on May 1, 2023, provides that internet advertisement must be identifiable and can make consumers recognize it as an advertisement. For goods or services ranked according to bidding, advertisement publishers shall indicate the word “advertisement” prominently in order to make a clear distinction with the natural search results. No entity and individual may publish any advertisement of prescription pharmaceuticals by means of the internet unless otherwise provided by laws and regulations. No advertisement of any medical treatment, pharmaceuticals, medical devices, pesticides, veterinary medicines, dietary supplements, foods for special medical purpose or other advertisements which are subject to review by advertisement review authorities as required by laws and regulations may be released unless it has passed such review. Internet advertisements which are subject to review shall be released strictly in accordance with the contents which have passed such review, and shall not be edited, spliced or modified. Where the contents of advertisements which have passed review need to be modified, a new application for advertisement review shall be submitted.
Administrative Measures on Medical Advertisement
The Administrative Measures on Medical Advertisement, which was jointly promulgated by the State Administration of Industry and Commerce and the Ministry of Health on September 27, 1993, and was amended on November 10, 2006 and came into effect on January 1, 2007, requires medical advertisements to be reviewed by relevant health authorities and a Medical Advertisement Approval Certificate to be obtained before such advertisement may be released by a medical institution. Medical Advertisement Review Certificate has an effective term of one year and may be renewed upon application.
Notice on Further Strengthening the Management of Medical Advertisements
The Notice on Further Strengthening the Management of Medical Advertisements, which was promulgated by the Ministry of Health and came into effect on July 17, 2008, emphasizes severe censorship, regulation and punishment on medical institutions’ non-compliance of medical advertisement. In case that a medical institution fails to correct its non-compliance of medical advertisement after two or more warnings, or that such non-compliance results in any patient suffering personal injuries or property losses, the medical institution shall be ordered to suspend its business for rectification, or the license for operating particular medical department or even the Medical Institution Practice License of such institution will be revoked in accordance with the provisions of the Administrative Measures on Medical Advertisement.
Enforcement Guidance for the Medical Cosmetology Advertising
The Enforcement Guidance for the Medical Cosmetology Advertising, which was promulgated by the State Administration for Market Regulation on November 1, 2021 and came into effect on the same day, stipulates that the market regulatory departments shall rectify the chaos of all kinds of medical beauty advertisements in accordance with the laws and regulations, strive to solve the problems of great harmfulness and concentrated public response, and crack down a number of illegal advertising activities.
77
Regulations on environmental protection related to medical institutions
Regulations on the Management of Medical Waste and the Management Measures for Medical Waste of Medical Institutions
The Regulations on the Management of Medical Waste, which was promulgated by the State Council on June 16, 2003, came into effect on the same date and was revised on January 8, 2011, and the Management Measures for Medical Waste of Medical Institutions, which was promulgated by the Ministry of Health on October 15, 2003 and came into effect on the same date, require medical institutions to timely deliver medical waste to a specially designated location for centralized disposal of medical waste and categorize the medical waste in accordance with the Classified Catalog of Medical Waste. High-risk waste such as the culture medium or specimens of pathogens and the preserving liquid of bacteria strains or virus strains must be sterilized on the spot before disposal. Sewage generated by any medical institution and excretion of its patients or patients suspected of infectious diseases must be sterilized in accordance with the relevant laws, rules and regulations, and must not be discharged into sewage treatment system until the relevant standards are met.
Regulations on Urban Drainage and Sewage Treatment
The Regulations on Urban Drainage and Sewage Treatment, which was promulgated by the State Council on October 2, 2013 and came into effect on January 1, 2014, requires urban entities and individuals to dispose sewage through urban drainage facilities covering their geographical area in accordance with relevant rules. Companies or other entities engaging in medical activities must apply for a Sewage Disposal Drainage License before disposing sewage into urban drainage facilities. Sewage- disposing entities and individuals are required to pay a sewage treatment fee in accordance with relevant rules.
Measures for the Administration of Permits for the Discharge of Urban Sewage into Drainage Network
The Measures for the Administration of Permits for the Discharge of Urban Sewage into Drainage Network, which was promulgated by the Ministry of Housing and Urban-Rural Development on January 22, 2015 and came into effect on March 1, 2015, and was revised on February 1, 2023, provides that enterprises engaging in industry, construction, catering and discharging sewage into the urban drainage network must apply for and obtain a License for Drainage.
Catalog of Classified Management of Pollutant Discharge Permits for Stationary Pollution Sources (2019 Edition)
The Catalog of Classified Management of Pollutant Discharge Permits for Stationary Pollution Sources (2019 Edition), which was promulgated by the Ministry of Ecology and Environment on December 20, 2019 and came into effect on the same date, provides that medical institutions shall either apply for a pollutant discharge permit or submit a pollutant discharge registration form through the online platform based on the standards set therein.
Regulations on consumer protection and right of personality
The principal legal provisions for the protection of consumer interests are set out in the Consumer Protection Law, which was promulgated by the Standing Committee of the National People’s Congress on October 31, 1993, became effective as of January 1, 1994 and was amended on October 25, 2013. According to the Consumer Protection Law, the rights and interests of the consumers who buy or use commodities or receive services for the purposes of daily consumption are protected and all manufacturers and sellers involved must ensure that the products and services will not cause damage to the consumers’ personal safety and properties. Violations of the Consumer Protection Law may result in the imposition of fines. In addition, the violating business operator may be ordered to suspend operations and its business license may be revoked. Criminal liability may be imposed in serious cases.
In accordance with the PRC Civil Code, the legitimate rights and interests of civil parties include reputational rights and the right to one’s image, and parties that infringe on the civil rights and interests of others will be held liable for tortious acts.
The Measures for Penalties against Infringements on Consumers’ Rights (2020 Revision) promulgated by State Administration of Industry and Commerce on January 5, 2015 and amended on October 23, 2020, provides that, business operator shall covenant with customer regarding the quantity, quality, price, term, after-sale service, liability, etc. under prepaid arrangement. In the event of failure to provide goods or services as agreed, business operator shall perform as requested by the customer or refund the prepaid payment and bear the interest of prepaid payment and other necessary and reasonable costs paid by the customer.
78
Regulations on fire prevention
Fire Prevention Law
According to The Fire Prevention Law of the PRC, or the Fire Prevention Law, which was promulgated by the Standing Committee of the National People’s Congress on April 29, 1998, came into effect on May 1, 2009 and was amended on October 28, 2008, April 23, 2019 and April 29, 2021 the Ministry of Public Security and its local counterparts at or above county level must monitor and administer fire prevention affairs. The Fire Prevention Law also provides that the fire prevention design or construction of a construction project must conform to the national fire prevention technical standards, and the construction entity must submit its fire prevention design documents to the fire prevention department of the public security authority for approval or filing purposes.
Upon the completion of a construction project to which a fire prevention design has been applied, according to the requirements of the Fire Prevention Law, such project must go through an acceptance check on fire prevention by, or filed with, the relevant fire prevention departments of public security authorities. For each public assembly venue, the construction entity or entity using such venue must, prior to use and operation of any business thereof, apply for a safety inspection on fire prevention with the relevant fire prevention department under the public security authority at or above the county level where the venue is located, and such place cannot be put into use and operation if it fails to pass the safety inspection on fire prevention or fails to conform to the safety requirement for fire prevention after such inspection.
Regulations related to foreign investment in the PRC
Company Law of the People’s Republic of China
The Company Law of the People’s Republic of China, which was promulgated by the Standing Committee of National People’s Congress on December 29, 1993, came into effect on July 1, 1994, and was amended in 1999, 2004, 2005, 2013 and 2018, provides that companies established in the PRC may either be limited liability companies or companies limited by shares. Each company has the status of a legal person and owns its own assets. Assets of a company may be used in full for the company’s liability. The Company Law applies to foreign-invested companies unless relevant laws provide otherwise.
Foreign Investment Law of the People’s Republic of China
On March 15, 2019, the National People’s Congress approved the Foreign Investment Law, which became effective on January 1, 2020, and replaced the Sino-foreign Equity Joint Venture Enterprise Law, the Sino-foreign Cooperative Joint Venture Enterprise Law and the Wholly Foreign-invested Enterprises Law. On December 26, 2019, the State Council issued the Regulations on Implementing the Foreign Investment Law of the PRC, which came into effect on January 1, 2020, and replaced the Regulations on Implementing the Sino-Foreign Equity Joint Venture Enterprise Law, Provisional Regulations on the Duration of Sino-Foreign Equity Joint Venture Enterprise Law, the Regulations on Implementing the Wholly Foreign-Invested Enterprise Law, and the Regulations on Implementing the Sino-foreign Cooperative Joint Venture Enterprise Law.
Under the Foreign Investment Law, the State shall implement the management systems of pre-establishment national treatment and negative list for foreign investment, according to which the treatment given to foreign investors and their investments during the investment access stage shall be not lower than that given to their domestic counterparts, and the State shall give national treatment to foreign investment beyond the negative list where special administrative measures for the access of foreign investment in specific fields is specified. Besides, the State shall protect foreign investors’ investment, earnings and other legitimate rights and interests within the territory of Mainland China in accordance with the law. The State will take measures to prompt foreign investment such as ensuring fair competition for foreign-invested enterprises to participate in government procurement activities, and protection of intellectual property rights of foreign investors and foreign-invested enterprises.
On December 30, 2019, the Ministry of the Commerce and the State Administration of Market Regulation issued the Measures for the Reporting of Foreign Investment Information, which came into effect on January 1, 2020 and replaced the Provisional Administration Measures for the Registration of the Formation and Changes of Foreign Invested Enterprises. Since January 1, 2020, for foreign investors carrying out investment activities directly or indirectly in Mainland China, the foreign investors or foreign-invested enterprises shall submit investment information to the commerce authorities pursuant to these measures.
79
Interim Provisions on Investment Made by Foreign-Invested Enterprises in the PRC
The Interim Provisions on Investment Made by Foreign-Invested Enterprises in the PRC, which was jointly promulgated by the Ministry of Commerce and the State Administration of Industry and Commerce on July 25, 2000, and was amended and came into effect on October 28, 2015, provides that the provisions of the Interim Provisions Guiding Foreign Investment Direction and the Industry Catalog for Guiding Foreign Investment will govern foreign-invested enterprises’ investment in the PRC. Foreign-invested enterprises are not permitted to invest in any sector prohibited to receive foreign investment. Where a foreign-invested enterprise makes an investment in a restricted sector, the foreign- invested enterprise must file an application with the provincial commercial department of the place where the investee company is located. The relevant company registration authority will decide, in accordance with the relevant provisions of the Company Law and the Regulations on the Administration of Company Registration of the PRC, whether to approve the registration or not. If the registration is approved, a Business License of an Enterprise Legal Person will be issued with the designation “Invested by a Foreign-Invested Enterprise” added. The foreign-invested enterprise is required to report the establishment of the invested company within 30 days of the date of its establishment to the original examination and approval authority for record-filing.
Regulations on Mergers and Acquisitions of Domestic Companies by Foreign Investors
The Regulations on Mergers and Acquisitions of Domestic Companies by Foreign Investors, which was jointly promulgated by the Ministry of Finance, the State Assets Supervision and Administration Commission, the State Administration for Taxation, the State Administration of Industry and Commerce, the China Securities Regulatory Commission, and the State Administration of Foreign Exchange on August 8, 2006, came into effect on September 8, 2006 and was amended on June 22, 2009, requires foreign investors acquiring domestic companies by means of asset acquisition or equity acquisition to comply with relevant foreign investment industry policies and be subject to approval by relevant commerce authorities.
Catalog of Industries for Encouraged Foreign Investment and 2021 Negative List
The current regulation regime of foreign investment in the PRC, setting aside special arrangements adopted in pilot free trade zones, preliminarily consists of three principal legal documents, i.e. the Catalog of Industries for Encouraged Foreign Investment (2022 Edition), or the 2022 Encouraged Catalog, which was promulgated jointly by the Ministry of Commerce and the National Development and Reform Commission, on October 26, 2022 and became effective on January 1, 2023, the Special Administrative Measures for Access of Foreign Investment (Negative List) (2021 Edition), which was promulgated jointly by the Ministry of Commerce and the National Development and Reform Commission, on Decemember 27, 2021 and became effective on January 1, 2022, or the 2021 Negative List, and the Provisions Guiding Foreign Investment Direction, which was promulgated by the State Council on February 11, 2002 and came into effect on April 1, 2002. These three legal documents collectively classify all foreign investment projects into four categories: (1) encouraged projects, (2) permitted projects, (3) restricted projects, and (4) prohibited projects. If the industry in which the investment is to occur falls into the encouraged category, foreign investment, in certain cases, may receive preferential policies or benefits. If it falls into the restricted category, foreign investment may be conducted in accordance with applicable legal and regulatory restrictions. If falls into the prohibited category, foreign investment of any kind will not be allowed.
The 2022 Encouraged Catalog and 2021 Negative List govern investment activities in the PRC by foreign investors and classify industries into three categories with regard to foreign investment: “encouraged”, “restricted” and “prohibited”. Industries not listed in the Catalog are generally deemed as falling into a fourth category, “permitted”, unless specifically restricted by other PRC laws. For some restricted industries, foreign investors can only conduct investment activities through equity or contractual joint ventures, while in other cases PRC partners are required to hold the majority interests in such joint ventures. In addition, some projects in the restricted category are subject to higher-level governmental approvals. Foreign investors are not allowed to invest in industries in the prohibited category. According to the current 2022 Encouraged Catalog and 2021 Negative List, investment into medical institutions falls within the restricted category. Foreign investment in healthcare institutions is restricted to the form of Sino-foreign cooperation or joint venture.
80
Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions
The Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions, which was promulgated by the Ministry of Health and the Ministry of Foreign Trade and Economic Cooperation on May 15, 2000 and came into effect on July 1, 2000, allows foreign investors to partner with Chinese medical entities to establish a medical institution in the PRC by means of an equity joint venture or a cooperative joint venture. Establishment of equity joint venture or cooperative joint venture must meet certain requirements, including that total investment may not be less than RMB20 million, and that the equity percentage of the Chinese partner in the joint venture may not be less than 30%. Establishment of an equity joint venture or cooperative joint venture will be subject to approval by relevant authorities.
Notice on Further Encouraging and Guiding Private Capital to Invest in Medical Institutions
Pursuant to the Notice on Further Encouraging and Guiding Private Capital to Invest in Medical Institutions, which was promulgated on November 26, 2010, foreign investors are permitted to establish for-profit or not-for-profit medical institutions in the PRC as foreign-invested projects. Overseas medical institutions, enterprises and other economic organizations are permitted to establish medical facilities together with domestic medical institutions, enterprises or other economic organizations in the form of equity or cooperative joint ventures, and the restrictions on equity proportion for foreign capital will be gradually removed. A pilot program will be introduced and gradually expanded to permit eligible foreign investors to establish wholly foreign- owned medical institutions.
Additional Provisions to the Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions
The Additional Provisions to the Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions, which was jointly promulgated by the Ministry of Commerce and the Ministry of Health on December 30, 2007 and came into effect on January, 2008, provides that the total investment by Hong Kong or Macau service providers in establishing equity or cooperative medical institutions in the PRC may not be less than RMB10 million. Hong Kong and Macau service providers must also comply with the Closer Economic Partnership Arrangement between Mainland China and Hong Kong and Arrangement regarding Establishing Closer Economic Partnership between Mainland China and Macau, respectively. The Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions may be applied to equity or cooperative medical institutions in which by Hong Kong or Macau service providers have invested to the extent not provided under the Additional Provisions to the Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions.
Additional Provisions (Second) to the Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions
The Additional Provisions (Second) to the Interim Administrative Measures on Sino-Foreign Equity Medical Institutions and Sino-Foreign Cooperative Medical Institutions, which was jointly promulgated by the Ministry of Commerce and the Ministry of Health on December 7, 2008 and came into effect on January 1, 2009, allows Hong Kong and Macau service providers to establish wholly- owned clinics within the Guangdong Province without any limitation on total investment. Hong Kong and Macau service providers may also partner with Chinese medical entities to establish a clinic in the Guangdong Province by means of equity joint ventures or cooperative joint ventures without any limitation on the total investment or equity percentage.
81
Regulations related to labor protection in the PRC
Labor Law of the PRC
The Labor Law of the PRC, which was promulgated by the Standing Committee of the National People’s Congress on July 5, 1994, which came into effect on January 1, 1995 and was amended on August 27, 2009 and December 29, 2018, provides that an employer must develop and improve its rules and regulations to protect the rights of its workers, such as by developing and improving its labor safety and health system, stringently implementing national protocols and standards on labor safety and health, conducting labor safety and health education for workers, guarding against labor accidents and reducing occupational hazards. Labor safety and health facilities must comply with relevant national standards. An employer must provide workers with the necessary labor protection equipment that comply with labor safety and health conditions required under national regulations, as well as provide regular health checks for workers that are engaged in operations with occupational hazards. Workers engaged in special operations must first receive specialized training and obtain the pertinent qualifications. Employers must also develop a vocational training system. Vocational training funds must be set aside and used in accordance with national regulations, and vocational training for workers must be carried out systematically based on the actual conditions of the company.
Labor Contract Law of the PRC and its implementation regulations
The Labor Contract Law, which was promulgated by the Standing Committee of the National People’s Congress on June 29, 2007, came into effect on January 1, 2008, and was amended on December 28, 2012, and the Implementation Regulations on Labor Contract Law, which was promulgated by the State Council and effective on September 18, 2008, regulate the relation between employers and employees and contain specific provisions involving the terms of the labor contract. Labor contracts must be made in writing and may, after reaching an agreement upon due negotiations, be for a fixed-term, an un-fixed term, or conclude upon the completion of certain work assignments. An employer may legally terminate a labor contract and dismiss its employees after reaching an agreement upon due negotiations with the employee or by fulfilling the statutory conditions.
Laws and Regulations on the Supervision over the Social Security and Housing Funds
According to the Temporary Regulations on the Collection and Payment of Social Insurance Premium, the Regulations on Work Injury Insurance, the Regulations on Unemployment Insurance and the Trial Measures on Employee Maternity Insurance of Enterprises, enterprises in the PRC must provide beneficial plans for their employees, that include basic pension insurance, unemployment insurance, maternity insurance, work injury insurance and medical insurance. An enterprise must also provide social insurance by processing social insurance registration with local social insurance agencies, and must pay or withhold relevant social insurance premiums for or on behalf of the employees. The Law on Social Insurance, which was promulgated on October 28, 2010 and came into effect on July 1, 2011 and was amended on December 29, 2018, regulates basic pension insurance, unemployment insurance, maternity insurance, work injury insurance and medical insurance, and has elaborated in detail the legal obligations and liabilities of employers who do not comply with relevant laws and regulations on social insurance. The Regulations on the Administration of Housing Provident Fund, which was promulgated and came into effect on April 3, 1999, and was amended on March 24, 2002 and March 24, 2019, provides that housing provident fund contributions paid by an individual employee and housing provident fund contributions paid by his or her employer all belong to the individual employee.
Program of Deepening the Reform of the Communist Party and Organs of State
The Program of Deepening the Reform of the Communist Party and Organs of State, which was promulgated by the Central Committee of the Communist Party of the PRC on March 21, 2018, provides that in order to improve the efficiency of collection and management of social insurance funds, the basic pension insurance premium, the basic medical insurance premium, the unemployment insurance premium and other social insurance premiums should be levied by tax authorities.
Reform Program of the Collection and Management of State Taxes and Local Taxes
The Reform Program of the Collection and Management of State Taxes and Local Taxes, which was promulgated by the General Office of the Central Committee of the Communist Party of the PRC and the General Office of the State Council on July 20, 2018, explicitly provides that from January 1, 2019, the basic pension insurance premium, the basic medical insurance premium, the unemployment insurance premium, the employment injury insurance premium, the maternity insurance premium and other social insurance premiums should be levied by tax authorities.
82
Notice on Opinions of Imposing Overdue Fine of Social Insurance Premiums for Enterprise Employees in Guangdong Province
The Notice on Opinions of Imposing Overdue Fine of Social Insurance Premiums for Enterprise Employees in Guangdong Province, which was promulgated by the Local Taxation Bureau and the Department of Human Resources and Social Security of Guangdong Province on April 16, 2018 and amended on June 15, 2018, provides that if employers declare and pay the overdue social insurance premium that were due before July 1, 2011, the taxation bureau may collect the overdue amount first, and the collection of overdue fine generated after July 1, 2011 will be postponed. In addition, if employers declare and pay the overdue social insurance premium that were due after July 1, 2011, without postponing the collection of the overdue fine from the taxation bureau at or above the county level, the overdue fine will be collected along with the outstanding amount that is in arrears.
Interim Measures for the Administration of Arrear Collection of Social Insurance Premiums
The Interim Measures for the Administration of Arrear Collection of Social Insurance Premiums, which was promulgated by the Local Taxation Bureau of Guangdong Province on March 5, 2014 and became effective on May 1, 2014 and amended on June 15, 2018, provides that for overdue amounts due before July 1, 2011, if employers take the initiative to, or comply with the taxation bureau’s orders to pay such overdue amounts, the overdue fine should not be collected. For overdue amounts that were due after July 1, 2011, employers should pay such overdue amounts along with the overdue fine with the rate of five over ten thousand (5/10000) per day.
Notice on Proper Handling the Payment of Basic Pension Premiums for Employees of Enterprises
The Notice on Proper Handling of the Payment of Basic Pension Premiums for Employees of Enterprises, which was promulgated by the Department of Human Resources and Social Security and the Finance Department of Hunan Province and became effective December 26, 2017, provides that for arrears due before December 31, 2016, employers and employees should pay the overdue amount in accordance with the original payment threshold and payment proportion, along with the overdue fine. For overdue amounts that were due after January 1, 2017, employers and employees should pay the overdue amount in accordance with the payment threshold calculated according to the employees’ historical salaries and the payment proportion of the time, along with the overdue fine.
Regulations related to taxation in the PRC
Enterprise Income Tax
According to the Enterprise Income Tax Law, or the EIT Law, which was promulgated by the National People’s Congress on March 16, 2007, came into effect on January 1, 2008, and was amended on February 24, 2017 and December 29, 2018 and the Implementation Regulations on the EIT Law, which was promulgated by the State Council on December 6, 2007 and came into effect on January 1, 2008 and amended on April 23, 2019, a uniform income tax rate of 25% will be applied to domestic enterprises, foreign-invested enterprises and foreign enterprises that have established production and operation facilities in the PRC. These enterprises are classified as either resident enterprises or non-resident enterprises. Resident enterprises refer to enterprises that are established in accordance with PRC laws, or that are established in accordance with the laws of foreign countries but whose actual or de facto control is administered from within the PRC. Non-resident enterprises refer to enterprises that are set up in accordance with the laws of foreign countries and whose actual administration is conducted outside the PRC, but who (whether or not through the establishment of institutions in the PRC) derive income from the PRC. Under the EIT Law and relevant implementing regulations, a uniform corporate income tax rate of 25% is imposed on these institutions. However, if non-resident enterprises have not established institutions in the PRC, or if they have established institutions in the PRC but there is no actual relationship between the relevant income derived in the PRC and the institutions set up by them, the enterprise income tax is set at the rate of 10%.
83
International Tax Treaties and Withholding Tax
According to the Treaty on the Avoidance of Double Taxation and Tax Evasion between Mainland and Hong Kong, or the Tax Treaty, if the non-PRC parent company of a PRC enterprise is a Hong Kong resident which beneficially owns a 25% or more interest in the PRC enterprise, the 10% withholding tax rate applicable under the EIT Law may be lowered to 5% for dividends and 7% for interest payments once approvals have been obtained from the relevant tax authorities. Factors impacting the determination of beneficial ownership is indicated in the Announcement of the State Administration of Taxation on Issues concerning “Beneficial Owners” in Tax Treaties, which was issued by the State Administration of Taxation on February 3, 2018 and came into effect on April 1, 2018, according to which negative impacts will be generally imposed to the recognition of a beneficial owner if the applicant is obliged to pay more than 50% of its income to third country (region) residents within 12 months of receiving the income, or the business activities undertaken by the applicant do not constitute substantive business activities. Pursuant to the Notice on the Issues concerning the Application of the Dividend Clauses of Tax Agreements, which was promulgated by the State Administration of Taxation and came into effect on February 20, 2009, the non-resident taxpayer or the withholding agent is required to obtain and keep sufficient documentary evidence proving that the recipient of the dividends meets the relevant requirements for enjoying a lower withholding tax rate under a tax treaty.
Administrative Measures for Convention Treatment for Non-resident Taxpayers
Pursuant to the Administrative Measures for Convention Treatment for Non-resident Taxpayers, which was promulgated by the State Taxation Administration on October 14, 2019 and came into effect on January 1, 2020, any non-resident taxpayer fulfilling conditions for enjoying the convention treatment may be entitled to the convention treatment on its own when filing a tax return or making a withholding declaration through a withholding agent, subject to collection and preservation of relevant materials for future reference in accordance with the Measures and the subsequent administration by the tax authorities.
Value-Added Tax
The Temporary Regulations on Value-added Tax, which was promulgated by the State Council on December 13, 1993, came into effect on January 1, 1994, and was amended in 2008, 2016, and 2017, and the Detailed Implementing Rules of the Temporary Regulations on Value-added Tax, which was promulgated by the Ministry of Finance and became effective on December 25, 1993, and was amended on December 15, 2008 and October 28, 2011, require all taxpayers selling goods, providing processing, repairing or replacement services or importing goods in the PRC to pay value-added tax. Unless otherwise specified, the value-added tax, or VAT, rate is 17% for taxpayers selling or importing goods, selling labor service, or providing tangible movable property leasing service. The applicable rate for the export of goods by taxpayers is zero unless otherwise provided by the State Council. According to the Notice on Adjusting value-added tax Rates promulgated by the Ministry of Finance and the State Administration of Tax on 4 April 2018, the tax rate of 17% applicable to any taxpayer’s VAT taxable sale activities shall be adjusted to 16%. According to the Announcement of the Ministry of Finance, the State Taxation Administration and the General Administration of Customs on Relevant Policies for Deepening the Value-Added Tax Reform jointly promulgated by the MOF, the SAT and the General Administration of Customs of the PRC on March 20, 2019 and became effective on April 1, 2019, the VAT tax rates of 16% applicable to the VAT taxable sale or import of goods by taxpayer shall be adjusted to 13%.
According to the Trial Scheme for the Conversion of Business Tax to Value-added Tax, which was promulgated by the Ministry of Finance and the State Administration of Taxation on November 16, 2011, the government launched gradual taxation reforms starting from January 1, 2012, whereby it collected value-added tax in lieu of business tax on a trial basis in regions and industries showing strong economic performance, such as transportation and certain modern service industries. Furthermore, according to the Notice of the Ministry of Finance and the State Administration of Taxation on Overall Implementation of the Pilot Program of Replacing Business Tax with Value-added Tax promulgated on March 23, 2016, last revised on March 20, 2019 and became effective on April 1, 2019, all business tax payers in the living service industry must pay VAT in lieu of business tax beginning on May 1, 2016. A Pilot taxpayer may enjoy VAT tax preference in accordance with the relevant provisions in the remaining period of preferential tax policies, if a taxpayer has enjoyed business tax preferences according to the relevant policies before the implementation date of pilot collection of VAT in lieu of business tax.
84
Notice on Relevant Tax Policies on Medical and Health Institutions
The Notice on Relevant Tax Policies on Medical and Health Institutions, which was promulgated by the Ministry of Finance and the State Administration of Tax on July 10, 2000, came into effect on the same date and amended on May 18, 2009, provides that incomes of for-profit medical institutions are taxable in accordance with relevant rules. Nevertheless, for-profit medical institution is granted a three-year tax exemption period commencing on the date of the issuance of the practice license if its revenue is directly used to improve medical and health conditions. During the no-tax period: (1)self-produced preparations for self-use by for-profit medical institutions are exempted from value- added tax, and (2) properties, land and vehicles for self-use by for-profit medical institutions are exempted from property tax, urban land use tax and vehicle and vessel usage tax. Pharmaceutical retail enterprise spun-off from pharmacy of for-profit medical institutions is subject to applicable taxations.
Regulations related to foreign exchange in the PRC
Administrative Regulations of the PRC on Foreign Exchange
Administrative Regulations of the PRC on Foreign Exchange, which was promulgated by the State Council on January 29, 1996, came into effect on April 1, 1996, and was amended on January 14, 1997 and August 5, 2008, provides that foreign exchange receipts of domestic institutions or individuals may be transferred back into the PRC or deposited abroad. The State Administration of Foreign Exchange, or the SAFE, may specify relative conditions and other requirements in accordance with the international receipts, payments status and requirements of foreign exchange control. Foreign exchange receipts under the current items may be retained or sold to financial institutions engaged in the settlement or sale of foreign exchange. Domestic institutions or individuals that make direct investments abroad or are engaged in the distribution or sale of valuable securities or derivative products overseas should register according to SAFE regulations. Such institutions or individuals subject to prior approval or record-filing with other competent authorities must obtain the required approval or finish record-filing prior to foreign exchange registration. The exchange rate for RMB follows a managed floating exchange rate system based on market demand and supply.
Foreign Exchange Settlement, Sales and Payment
The Administrative Regulation of the People’s Bank of China Regarding Foreign Exchange Settlement, Sales and Payment, which was promulgated by the People’s Bank of China on June 20, 1996 and came into effect on July 1, 1996, provides that foreign exchange receipts under the current account of foreign-invested enterprises may be retained to the fullest extent specified by the foreign exchange bureau. Any portion in excess of such amount must be sold to a designated foreign exchange bank or sold in a foreign exchange swap center.
Notice of Issues concerning Foreign Exchange Administration over the Overseas Investment and Financing and Round-trip Investment by Domestic Residents via Special Purpose Vehicles
On July 4, 2014 the SAFE issued the Notice of Issues concerning Foreign Exchange Administration over the Overseas Investment and Financing and Round-trip Investment by Domestic Residents via Special Purpose Vehicles, or Circular 37, which provides that (i) a domestic resident (domestic institution or domestic resident individual), must register with the local branch of the SAFE before it contributes the assets of or its equity interest into a special purpose vehicle for the purpose of investment and financing as defined in the implementing guidelines of Circular 37 (the definition of domestic resident also includes certain foreigner without legal identity certificate of the PRC while habitually resides in Mainland China for economic interests), and (ii) when the special purpose vehicle undergoes a change of basic information, such as change of a PRC resident natural person shareholder, name or operating period, or a material event, such as change in share capital of a PRC resident natural person, merger or split, the domestic resident must promptly register such change with the local branch of the SAFE.
Notice on Further Improving and Adjusting the Policies Related to Foreign Exchange Administration in Direct Investment
The Notice on Further Improving and Adjusting the Policies Related to Foreign Exchange Administration in Direct Investment, which was promulgated by the SAFE on November 19, 2012, came into effect on December 17, 2012 and was amended on May 4, 2015, October 10, 2018 and December 30, 2019, respectively, improves foreign exchange administration in direct investment by repealing or adjusting certain approval items for foreign exchange administration in direct investment.
85
Notice on Reforming the Management Approach regarding the Settlement of Foreign Exchange Capital of Foreign-invested Enterprises
The Notice on Reforming the Management Approach regarding the Settlement of Foreign Exchange Capital of Foreign-invested Enterprises, or Circular 19, was promulgated by the SAFE on March 30, 2015, came into effect on June 1, 2015, was amended on December 30, 2019 and was partly replaced by the Notice on Reforming and Regulating Policies on the Control over Foreign Exchange Settlement of Capital Accounts, or Circular 16, on June 9, 2016. According to Circular 19, the foreign exchange capital of foreign invested enterprises will be subject to the willingness settlement of foreign exchange capital, or the Willingness Foreign Exchange Settlement. The Willingness Foreign Exchange Settlement means that foreign exchange capital in the capital account of a foreign invested enterprise of which the rights and interests of monetary contribution has been confirmed by the local foreign exchange bureau (or the entry registration of monetary contribution by the banks) can be settled at the banks based on the actual operational needs of the foreign invested enterprise. The proportion of the Willingness Foreign Exchange Settlement of the foreign exchange capital of a foreign invested enterprise is temporarily determined as 100%. The RMB fund converted from the foreign exchange capital shall be kept in a designated account. If a foreign invested enterprise needs to make further payments from such account, the enterprise needs to provide supporting documents and go through the review process with the banks. Furthermore, Circular 19 and Circular 16 provides that the use of capital by foreign-invested enterprises must comply with the principles of authenticity and self-use within the business scope of enterprises. The capital of a foreign invested enterprise and capital in RMB obtained by the foreign invested enterprise from foreign exchange settlement may not be used for the following purposes: (1) direct or indirect payment beyond the business scope of the enterprises or a payment prohibited by relevant laws and regulations, (2) direct or indirect investment in securities or investments other than banks’ principal-secured products unless otherwise provided by relevant laws and regulations, (3) direct or indirect grant of loans to non-affiliated enterprises (unless permitted by the scope of business), and (4) paying the expenses related to the purchase of real estate that is not for self-use (except for real estate enterprises).
Circular on Further Promoting Cross-border Trade and Investment Facilitation
On October 23, 2019, the SAFE issued the Circular on Further Promoting Cross-border Trade and Investment Facilitation, or SAFE Circular 28. Among others, SAFE Circular 28 relaxes the prior restrictions and allows the foreign-invested enterprises without equity investment as in their approved business scope to use their capital obtained from foreign exchange settlement to make domestic equity investment as long as the investments are real and in compliance with the foreign investment-related laws and regulations. In addition, SAFE Circular 28 stipulates that qualified enterprises in certain pilot areas may use their capital income from registered capital, foreign debt and overseas listing, for the purpose of domestic payments without providing authenticity certifications to the relevant banks in advance for those domestic payments.
Regulations related to data security and privacy protection
The PRC Constitution states that PRC law protects the freedom and privacy of communications of citizens and prohibits infringement of these rights. The PRC Civil Code provides in a stand-alone chapter of right of personality and reiterate that the personal information of a natural person shall be protected by the law. Any organization or individual shall legitimately obtain such person information of others in due course on a need-to-know basis and ensure the safety and privacy of such information, and refrain from excessively handling or using such information.
86
Data Security Law of the PRC
The SCNPC promulgated the Data Security Law of the PRC, or the Data Security Law on June 10, 2021, which took effect on September 1, 2021. The Data Security Law sets forth data security and privacy related compliance obligations on entities and individuals carrying out data related activities and clarifies the scope of data to cover a wide range of information records generated from all aspects of production, operation and management of government affairs and enterprises in the process of the gradual transformation of digitalization, and requires that data collection shall be conducted in a legitimate and proper manner, and theft or illegal collection of data is not permitted. Data processors shall establish and improve the whole-process data security management rules, organize, and implement data security trainings as well as take appropriate technical measures and other necessary measures to protect data security. In addition, data processing activities shall be conducted based on the graded protection system for cybersecurity. For example, a processor of important data shall designate the personnel and the management body responsible for data security, carry out risk assessments for its data processing activities and file the risk assessment reports with the competent authorities. Monitoring of the data processing activities shall be strengthened, and remedial measures shall be taken immediately in case of discovery of risks regarding data security related defects or bugs. In case of data security incidents, responding measures shall be taken immediately, and disclosure to users and report to the competent authorities shall be made in a timely manner.
Personal Information Protection Law of the PRC
The Personal Information Protection Law of the PRC, or the PIPL, which was promulgated by the National People’s Congress of the PRC on August 20, 2021 and took effect on November 1, 2021, provides detailed rules for processing personal information and further improves the personal information protection system, it aims at protecting the personal information rights and interests, regulating the processing of personal information, ensuring the orderly and free flow of personal information in accordance with the law and promoting the reasonable use of personal information.
Cybersecurity Law of the PRC
On November 7, 2016, the National People’s Congress of the PRC promulgated the Cybersecurity Law of the PRC, or the Cybersecurity Law, which became effective on June 1, 2017. Pursuant to Cybersecurity Law, the “personal information” refers to all kinds of information recorded by electronic or otherwise that can be used to independently identify or be combined with other information to identify individuals’ personal information including but not limited to: individuals’ names, dates of birth, ID numbers, biologically identified personal information, addresses and telephone numbers, etc. The Cybersecurity Law also provides that: (i) to collect and use personal information, network operators shall follow the principles of legitimacy, rightfulness, and necessity, disclose rules of data collection and use, clearly express the purposes, means and scope of collecting and using the information, and obtain the consent of the persons whose data is gathered; (ii) network operators shall neither gather personal information unrelated to the services they provide, nor gather or use personal information in violation of the provisions of laws and administrative regulations or the scopes of consent given by the persons whose data is gathered; and shall dispose of personal information they have saved in accordance with the provisions of laws and administrative regulations and agreements reached with users; (iii) network operators shall not divulge, tamper with or damage the personal information they have collected, and shall not provide the personal information to others without the consent of the persons whose data is collected. However, if the information has been processed and cannot be recovered and thus it is impossible to match such information with specific persons, such circumstance is an exception.
Measures for the Security Assessment of Outbound Data Transfer
On July 7,2022, the CAC promulgated Measures for the Security Assessment of Outbound Data Transfers, which became effective on September 1,2022 and provide that a data processor is required to apply for security assessment for cross-border data transfer in any of the following circumstances:(i)where a data processor provides critical data to offshore entities and individuals; (ii)where a critical information infrastructure operator or a data processor which processes personal information of more than one million individuals provides personal information to offshore entities and individuals; (iii)where a data processor has provided personal information in the aggregate of more than 100,000 individuals or sensitive personal information of more than 10,000 individuals in total to offshore entities and individuals since January 1 of the previous year; or (iv)other circumstances prescribed by the CAC for which application for security assessment for cross-boarder transfer of data is required.
87
Measures for the Standard Contract for Outbound Transfer of Personal Information
The Measures for the Standard Contract for Outbound Transfer of Personal Information, which was promulgated by the CAC on February 22, 2023 and will come into effect on June 1, 2023, provides the basic principles and requirements on the outbound transfer of personal information outside the PRC under the standard contract provided by the CAC. Pursuant to the Measures for the Standard Contract for Outbound Transfer of Personal Information, any personal information handler transferring personal information abroad by entering into the standard contract shall meet all of the following conditions and conduct a personal information protection impact assessment: (i) it is not a critical information infrastructure operator; (ii) it processes the personal information of less than 1 million individuals; (iii) it has cumulatively transferred abroad the personal information of less than 100,000 individuals since January 1 of the previous year; and (iv) it has cumulatively transferred abroad the sensitive personal information of less than 10,000 individuals since January 1 of the previous year. The Measures also provides that the personal information handler shall not carry out the outbound transfer until the standard contract enters into force, and shall file a reocrd with the cyberspace authority at the provincial level within 10 working days from the effective date of the standard contract executed.
Administrative Measures for the Cybersecurity of Medical and Healthcare Institution
The Administrative Measures for the Cybersecurity of Medical and Healthcare Institution, or the measures, which were promulgated by the National Health Commission, the National Administration of Traditional Chinese Medicine, and the National Bureau of Disease Control and Prevention on August 8,2022 and became effective on the same day. The measures require all the medical and health institutions to set up data life-cycle management systems and user participation-based cybersecurity management systems, including but not limited to strengthening system construction, implementing daily network maintenance and monitoring, conducting annual self-inspection and rectification, and classifying and grading data assets. Where personal information and data are leaked or major cybersecurity incidents occur in violation of the measures, the case shall be handled in accordance with the Cybersecurity Law, the Cryptography Law, Promotion Law on Basic Medical and Healthcare, the Personal Information Protection Law, the Regulations for the Security Protection of Critical Information Infrastructure, cybersecurity classified protection system and other laws and regulations.
Regulations on personal information protection related to medical institutions
According to the Regulations for Medical Institutions on Medical Records Management released on November 20, 2013, and effective from January 1, 2014, the medical institutions and medical practitioners shall strictly protect the privacy information of patients. Any leakage of patients’ medical records for non-medical, non-teaching or non-research purposes is prohibited. The National Health and Family Planning Commission released the Measures for Administration of Population Health Information (Trial) on May 5, 2014, referring the medical health service information as the population healthcare information, which is not allowed to be stored in a server located outside the territory of Mainland China. Pursuant to the Management Measures of Standards, Safety and Service of National Health and Medical Big Data (Trial), promulgated by the National Health Commission on July 12, 2018, the medical institutions should establish relevant safety management systems, operation instructions and technical specifications to safeguard the safety of healthcare big data generated in the process of health management service or prevention and cure service of diseases. It also stipulates that such healthcare big data should be stored in onshore servers and shall not be provided overseas without safety assessment.
Opinions on Severely Cracking Down on Illegal Securities Activities According to Law
On July 6, 2021, the General Office of the Communist Party of China Central Committee and the General Office of the State Council jointly issued the Opinions on Severely Cracking Down on Illegal Securities Activities According to Law, which stressed on, among other things, improving laws and regulations on data security, cross-border data flow and management of confidential information, speeding up the revisions to regulations on strengthening the confidentiality and document management of securities issuance and listing outside the mainland of the PRC to increase the accountability of entities listed outside the mainland of the PRC to information security, and enhancing standardized management of mechanism and procedure for cross-border data transfer, enhancing the cooperation of cross-border audit supervision.
88
Cybersecurity Review Measures
On December 28, 2021, the CAC issued the Cybersecurity Review Measures 2021, which became effective on February 15, 2022 and replaced the Cybersecurity Review Measures 2020. The scope of review under the Cybersecurity Review Measures 2021 extends to critical information infrastructure operators that intend to purchase internet products and services and network platform operators engaging in data processing activities, which affect or may affect national security. According to Article 7 of the Measures, network platform operators who possess personal information of over a million users shall apply to the Cybersecurity Review Office for cybersecurity reviews before listing in a foreign country. Besides, the Cybersecurity Review Measures 2021 also provide that if the relevant authorities consider that certain network products and services and data processing activities affect or may affect national security, the authorities may initiate a cybersecurity review even if the operators do not have an obligation to report for a cybersecurity review under such circumstances. The Cybersecurity Review Measures 2021 also elaborate the factors to be considered when assessing the national security risks of the relevant activities, including among others, risks of core data, important data or a large amount of personal information being stolen, leaked, destroyed, and illegally used or illegally exited the country, risks of critical information infrastructure, core data, important data or a large amount of personal information data being affected, controlled and maliciously used by foreign governments after a listing, and risks associated with Internet information security.
4.C.Organizational Structure
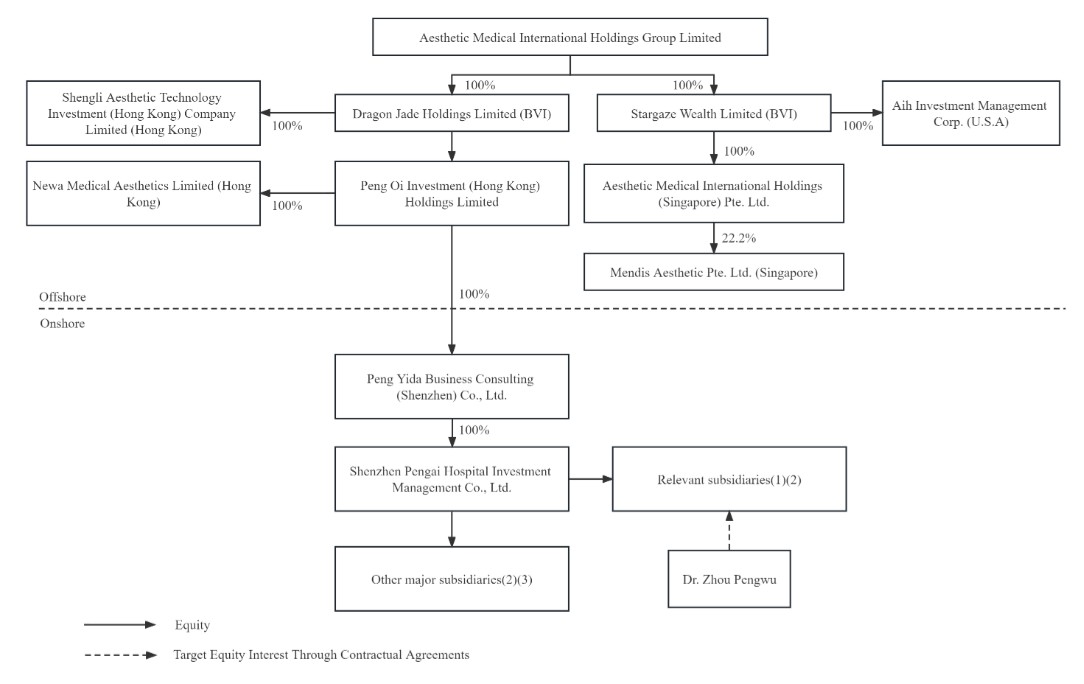
89
(1) | As of the date of this annual report, Shenzhen Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd., Guangzhou Pengai Aesthetic Medical Hospital Co., Ltd., Guangzhou Pengai Xiuqi Aesthetic Medical Clinic Co., Ltd., Shanghai Pengai Aesthetic Medical Outpatient Department Co., Ltd., Shanghai Jiahong Aesthetic Medical Outpatient Department Co., Ltd., Yantai Pengai Cosmetic Surgery Hospital Co., Ltd., Beijing Aomei Yixin Investment Consultant Co., Ltd., and Beijing Aomei Yixin Investment Consultant Co., Ltd. Pengai Aesthetic Medical Clinic, collectively “Relevant Subsidiaries”, or each a “Relevant Subsidiary,” are held by Dr. Zhou Pengwu, our chairman and chief executive officer, as to 27%, 26%, 19%, 12%, 24%, 25% and 25% respectively. Changsha Pengai Aesthetic Medical Hospital Co., Ltd. and Nanchang Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd. were divested and Hangzhou Pengai Aesthetic Medical Outpatient Department was ceased operation in August 2022 due to underperformance. Shenzhen Miaoyan Aesthetic Medical Clinic was divested due to impact of COVID-19 pandemic in Shenzhen in December 2022. due to underperformance in October 2022. Shenzhen Miaoyan Aesthetic Medical Clinic was divested due to impact of COVID-19 pandemic in December 2022. We refer to these equity interests held by Dr. Zhou Pengwu as the “Target Equity Interests,” which constitute all of Dr. Zhou Pengwu’s shareholdings in the Relevant Subsidiaries. Shenzhen Pengcheng Hospital, Shenzhen Pengai Aesthetic Medical Hospital, Haikou Pengai Aesthetic Medical Hospital Co., Ltd. were established before the effective date of the Foreign Investment Catalogue 2015 and thus, it is not subject to the 70% foreign shareholding restrictions. |
(2) | As of the date of this annual report, except for the Target Equity Interests representing all of Dr. Zhou Pengwu’s equity interests in the Relevant Subsidiaries which we consolidate through Contractual Arrangements as further described below, the remaining equity interest in our PRC operating subsidiaries is held by unaffiliated third parties and certain of our employees. |
(3) | Other major subsidiaries are those subsidiaries other than Relevant Subsidiaries under Shenzhen Pengai Hospital Investment Management Co., Ltd. that hold our treatment centers. The table below sets forth other major subsidiaries as of the date of this annual report and our beneficial interest in such subsidiaries. |
Subsidiaries |
| Beneficial Interest | |
Haikou Pengai Aesthetic Medical Hospital Co., Ltd. |
| 87.0 | % |
Huizhou Pengai Aesthetic Medical Hospital Co., Ltd. |
| 67.5 | % |
Shenzhen Pengai Aesthetic Medical Hospital |
| 100.0 | % |
Shenzhen Pengcheng Hospital |
| 100.0 | % |
Contractual Arrangements with respect to Target Equity Interests
Pursuant to the Foreign Investment Catalog 2015, which became effective in April 2015, foreign investors in PRC medical institutions can only conduct investment activities through joint ventures. The foreign shareholding in these entities is limited to 70.0% as stipulated in the JV Interim Measures since the effective date of the Foreign Investment Catalog 2015. We historically held more than 70.0% equity interest in certain of our PRC subsidiaries that are medical institutions which we acquired or established after the effective date of the Foreign Investment Catalog 2015. Due to such restriction, we had decreased our shareholding to no more than 70.0% in such PRC subsidiaries by transferring excessive equity interests to Dr. Zhou Pengwu and certain of our employees in 2018. In connection with such equity transfer and Dr. Zhou’s shareholding in Hangzhou Pengai Aesthetic Medical Clinic Co., Ltd., Jinan Pengai Cosmetic Surgery Hospital Co., Ltd., Shenzhen Pengai Xiuqi Aesthetic Medical Hospital, Guangzhou Pengai Aesthetic Medical Hospital Co., Ltd. and Chongqing Pengai Aesthetic Medical Hospital Co., Ltd. which he subsequently acquired from other shareholders in 2018 and 2019, and Shanghai Pengai Aesthetic Medical Clinic Co., Ltd., Jiangsu Liangyan Hospital Management Co., Ltd. and Beijing AomeiYixin Investment Consultant Co., Ltd. in 2020, we entered into a series of Contractual Arrangements with Dr. Zhou Pengwu, Shenzhen Pengai Investment, the Relevant Subsidiaries and Ms. Ding Wenting in 2018, 2019, 2020, 2021, and 2022 with respect to the Target Equity Interests except for Changsha Pengai Aesthetic Medical Hospital Co., Ltd. and Nanchang Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd., which were divested due to underperformance in Octorber 2022. Hangzhou Pengai Aesthetic Medical Outpatient Department was ceased operation in August 2022 due to underperformance. While we have disposed Chongqing Pengai Aesthetic Medical Hospital Co., Ltd and Jiangsu Liangyan Hospital Management Co., Ltd. in December 2021 and Changsha Pengai Aesthetic Medical Hospital Co., Ltd. and Nanchang Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd. in the third quarter of 2022 and Shenzhen Miaoyan Aesthetic Medical Clinic in the fourth quarter of 2022, these Contractual Arrangements enable us to (i) exercise control over the Target Equity Interests in the Relevant Subsidiaries; (ii) receive economic benefits from the Target Equity Interests in the Relevant Subsidiaries; and (iii) have an exclusive option to purchase all or part of the Target Equity Interests when and to the extent permitted by PRC laws.
90
Our revenue generated from the Relevant Subsidiaries aggregately accounted for approximately 11.6%, 32.3% and 31.1% of our total revenue in 2020, 2021 and 2022, respectively. Our revenue is not affected by the Contractual Arrangement as we own 65% or more direct equity interest in each of the Relevant Subsidiary and do not rely on the Contractual Arrangements to exercise control over the Relevant Subsidiaries. We incurred net loss of RMB76.1 million in the year ended December 31, 2022. Excluding the Contractual Arrangement in the Relevant Susbsidies, a net loss of RMB77.5 million would be attributable to equity holders of our Company.
Treatment centers | Share percentage registered under the China State Administration for Market Regulation | Treatment centers' revenue and net profit with Contractual Arrangement in 2022 (RMB) | Treatment centers' net profit without Contractual Arrangement in 2022 (RMB) | |
Revenue | Net profit/(loss) | Net profit/(loss) | ||
Shenzhen Pengai Xiuqi | 67% | 37,106,276 | 2,903,217 | 2,119,348 |
Guangzhou Pengai Xiuqi | 70% | 6,648,596 | 1,154,771 | 854,530 |
Shanghai Pengai | 65% | 41,584,743 | (2,410,425) | (2,048,861) |
Hangzhou Pengai | 70% | 10,623,716 | 2,435,491 | 1,704,844 |
Beijing Pengai | 70% | 13,229,867 | (2,062,021) | (1,546,516) |
Yantai Pengai | 71% | 36,762,712 | 1,513,187 | 1,150,022 |
Jinan Pengai | 70% | 0 | 0 | 0 |
Group consolidated statements without Contractual Arrangement in 2022 (RMB) | ||
Revenue | Net profit/(loss) | Profit/(loss) attributable to owners of the Company |
670,091,179 | (77,404,383) | (77,545,068) |
With respect to our control over the Target Equity Interests, the Contractual Arrangements may not be as effective as direct ownership. If the Relevant Subsidiaries and/or Dr. Zhou Pengwu fail to perform their respective obligations under the Contractual Arrangements, we may have to incur substantial costs and expend additional resources to enforce such Contractual Arrangements, and rely on legal remedies under PRC laws, including contractual remedies, which may not be sufficient or effective. See “Item 3. Key Information—3.D. Risk Factors—Risks relating to our corporate structure—The Contractual Arrangements may not be as effective in providing control as direct ownership and the Relevant Subsidiaries or Dr. Zhou Pengwu may fail to perform their respective obligations under the Contractual Arrangements.”
There are also substantial uncertainties regarding the interpretation and application of current and future PRC laws, regulations and rules regarding the status of the rights of our Cayman Islands holding company with respect to the Contractual Arrangements. It is uncertain whether any new PRC laws or regulations relating to Contractual Arrangements will be adopted or if adopted, what they would provide. If we or any of our Relevant Subsidiaries is found to be in violation of any existing or future PRC laws or regulations, or fail to obtain or maintain any of the required permits or approvals, the relevant PRC regulatory authorities would have broad discretion to take action in dealing with such violations or failures. See “Item 3. Key Information—D. Risk Factors—Risks Relating to Our Corporate Structure—If the PRC government deems that the Contractual Arrangements do not comply with PRC laws and regulations, or if these laws and regulations or their interpretations change in the future, we could be subject to severe penalties or be forced to relinquish our interests received through the Contractual Arrangements.”
The following is a summary of the currently effective Contractual Arrangements with respect to the Target Equity Interests.
Loan agreement
Shenzhen Pengai Investment, as the lender, entered into certain loan agreements with Dr. Zhou Pengwu, as the borrower. Pursuant to each of these loan agreements, Shenzhen Pengai Investment agrees to extend a loan to Dr. Zhou Pengwu in an equivalent amount to the purchase price to be paid by Dr. Zhou Pengwu for acquiring the Target Equity Interests. Pursuant to each of these loan agreements, Dr. Zhou Pengwu shall repay the loan by transferring the current and future economic interest of the Target Equity Interests to Shenzhen Pengai Investment.
91
Economic interest transfer agreement
Dr. Zhou Pengwu, Shenzhen Pengai Investment and each of the Relevant Subsidiaries entered into certain economic interest transfer agreements. Pursuant to each of these economic interest transfer agreements, the economic interest in relation to the Target Equity Interests currently held and subsequently acquired by Dr. Zhou Pengwu, including but not limited to (i) incomes arising from the disposal of the Target Equity Interests (including derivative equity interest of the Target Equity Interests) under any circumstance; (ii) dividends and bonus obtained on the basis of the Target Equity Interests (including derivative equity interest of the Target Equity Interests) under any circumstance; (iii) residual assets and other economic profits allocated after the liquidation of the Relevant Subsidiary, and (iv) any other cash income, property and economic benefit arising from the Target Equity Interests (including derivative equity interest of the Target Equity Interests), shall be transferred to Shenzhen Pengai Investment. Upon the execution of each economic interest transfer agreement, the repayment obligation of Dr. Zhou Pengwu under each loan agreement is deemed fully discharged.
Exclusive option agreement
Dr. Zhou Pengwu, Shenzhen Pengai Investment and each of the Relevant Subsidiaries entered into certain exclusive option agreements. Pursuant to these exclusive option agreements, Dr. Zhou Pengwu irrevocably granted Shenzhen Pengai Investment an exclusive right to purchase, or have its designated person(s) to purchase, at its discretion, all or part of his equity interest in the Relevant Subsidiary, and the purchase price shall be the lowest price permitted by applicable PRC law. Each of Dr. Zhou Pengwu and the Relevant Subsidiary undertakes that, among others, without the prior written consent of Shenzhen Pengai Investment, he or it shall or shall cause the Relevant Subsidiary not to declare any dividends or distribute any residual profits, change or amend its articles of association, increase or decrease its registered capital, or change its structure of registered capital in other manners. In the event that Dr. Zhou Pengwu increases its capital injection into the Relevant Subsidiary, Dr. Zhou Pengwu undertakes and confirms that any additional equity so acquired shall be subject to the purchase option. Unless terminated by Shenzhen Pengai Investment at its sole discretion, the exclusive option agreement will remain effective until all equity interest in the Relevant Subsidiary held by Dr. Zhou Pengwu are transferred or assigned to Shenzhen Pengai Investment or its designated person(s).
Equity interest pledge agreement
Dr. Zhou Pengwu as pledgor, Shenzhen Pengai Investment as pledgee, and each of the Relevant Subsidiaries entered into certain equity interest pledge agreements. Pursuant to these equity interest pledge agreements, Dr. Zhou Pengwu has pledged all of the Target Equity Interests in Relevant Subsidiaries and agreed to pledge all future equity interest in the Relevant Subsidiary acquired by him to Shenzhen Pengai Investment to guarantee the performance by Dr. Zhou Pengwu and the Relevant Subsidiary of their respective obligations under the loan agreement, the economic interest transfer agreement, the exclusive option agreement and the power of attorney. If the Relevant Subsidiary or Dr. Zhou Pengwu breaches any obligations under these agreements, Shenzhen Pengai Investment, as pledgee, will be entitled to dispose of the pledged equity and have priority to be compensated by the proceeds from the disposal of the pledged equity. Dr. Zhou Pengwu shall not permit the existence of any security interest or other encumbrance on the pledged equity interest or any portion thereof, without the prior written consent of Shenzhen Pengai Investment and the Relevant Subsidiary shall not assent to or assist in such actions. These equity interest pledge agreements will remain effective until Dr. Zhou Pengwu discharge all the obligations under the loan agreement, the economic interest transfer agreement, the exclusive option agreement and the power of attorney and the full payment of all direct, indirect and derivative losses and losses of anticipated profits, suffered by the pledgee, incurred as a result of any breach by Dr. Zhou Pengwu or the Relevant Subsidiary under these agreements or invalidity, revocation and termination of any of these agreements.
Power of attorney
Pursuant to relevant power of attorney executed by Dr. Zhou Pengwu, he has irrevocably authorized Shenzhen Pengai Investment or its designated person(s) to exercise all of such shareholder’s voting and other rights associated with the Target Equity Interests in each of the Relevant Subsidiaries, including but not limited to, the right to attend shareholder meetings, the right to vote, the right to sell, transfer, pledge or depose of the Target Equity Interests and the right to appoint legal representatives, directors and other management. The proxy agreement remains effective as long as Dr. Zhou Pengwu remains a shareholder of the Relevant Subsidiary, unless Pengai Investment has given contrary written instructions.
92
Spousal consent letter
Pursuant to relevant spousal consent letters executed by Ms. Ding Wenting, she unconditionally and irrevocably agreed that the equity interest in each of the Relevant Subsidiaries held or to be held by Dr. Zhou Pengwu and registered or to be registered in his name will be disposed of pursuant to the loan agreement, the economic interest transfer agreement, the exclusive option agreement and the power of attorney. Ms. Ding Wenting agreed not to assert any rights over the equity interest in the Relevant Subsidiaries held or to be held by Dr. Zhou Pengwu. In addition, in the event that Ms. Ding Wenting obtains any equity interest in each of the Relevant Subsidiaries for any reason, she agreed to be bound by the Contractual Arrangements.
In the opinion of Han Kun Law Offices, our PRC counsel, (i) the above Contractual Arrangements currently do not result in any violation of the applicable PRC laws or regulations currently in effect; and (ii) the agreements under the Contractual Arrangements among Dr. Zhou Pengwu, Shenzhen Pengai Investment and Relevant Subsidiaries are governed by PRC laws or regulations, are valid and binding upon each party to such arrangements and enforceable against each party thereto in accordance with their terms and applicable PRC laws and regulations currently in effect, and do not contravene applicable PRC laws or regulations currently in effect.
There are substantial uncertainties regarding the interpretation and application of current or future PRC laws and regulations. We have been further advised by our PRC legal counsel that if the PRC government finds that the above Contractual Arrangements or the agreements under the Contractual Arrangements that establish the structure for operating aesthetic medical services, do not comply with PRC government restrictions on foreign investment in such businesses, we could be subject to severe penalties including being prohibited from continuing operations. For a description of the risks related to these Contractual Arrangements and our corporate structure, please see “Item 3. Key Information—3.D. Risk Factors—Risks relating to our corporate structure.”
On July 20, 2022, the Company, Dr. Pengwu Zhou and Ms. Wenting Ding (the “Founders”), certain existing shareholders of the Company controlled by the Founders and Australia Wanda International Company Limited (“Wanda”) entered into a Share Purchase Agreement. Pursuant to the Share Purchase Agreement, Seefar Global Holdings Limited (“Seefar”), Jubilee Set Investments Limited (“Jubilee”), and Pengai Hospital Management Corporation (collectively referred as to the “Sellers”) agreed to sell and Wanda as the buyer agreed to purchase, an aggregate of 21,321,962 ordinary shares of the Company (the “Sale Share”) for the total consideration in USD that is equivalent of RMB100 million, representing a price of RMB4.67 per Sale Share (the “Share Transfer”). The Founders entered into the Share Purchase Agreement as parties to the Share Purchase Agreement and guarantors for the Sellers. The Share Transfer is expected to be closed in May 2023.
On July 20, 2022, the Company, the Founders and Hainan Oriental Jiechuang Investment Partnership (Limited Partnership) (“Jiechuang”) as the investor entered into a Subscription Agreement. Pursuant to the Subscription Agreement, Jiechuang agrees to subscribe an aggregate of 36,402,570 newly issued ordinary shares of the Company (the “Subscription Shares”) for the total consideration in USD that is equivalent of RMB170 million, representing a subscription price of RMB4.67 per ordinary share (equivalent to approximately US$2.08 per American Depository Shares (“ADS”) of the Company based on the exchange rate as of the date of this press release). The actual subscription price in terms of the U.S. dollar per ADS is subject to change based on the exchange rate one day prior to the closing date. The Founders entered into the Subscription Agreement as parties to the Agreement and guarantors for the Company. Shenzhen Lafang Investment Management Co., Ltd. (“Lafang Investment”) and Shenzhen Venture Capital M&A Fund Management (Shenzhen) Co., Ltd. are the general partners of Jiechuang, where Lafang Investment serves as the executive partner. Shenzhen Capital Group Co., Ltd. is a limited partner of Jiechuang. The transaction was closed in February 2023.
On July 20, 2022, the Company, the Founders, Seefar, Jubilee and certain other parties thereto entered into a Shareholders’ Agreement. The Shareholders’ Agreement governs, among other things, the appointment of the Company’s board of directors (the “Directors”) and senior management, the notice, quorum and Directors’ voting arrangement of board meetings, certain lock-up commitments of the Founders and their affiliates and pre-emptive rights mechanisms for the Company’s ordinary shares. Pursuant to the Shareholders’ Agreement, the Company shall deliver two separate warrants to purchase ordinary shares of the Company to Seefar and Wanda, respectively, on the date of completion of closings of both the Share Transfer and subscription of ordinary shares by Jiechuang.
93
On July 20, 2022, Peak Asia Investment Holdings V Limited (“ADV”) and its affiliate, the Company, the Founders, Wanda and Jiechuang entered into a Cooperation Agreement. The Cooperation Agreement provides, among other things, that:
| ● | ADV shall, with respect to each annual and extraordinary meeting of the Company, (a) be present at such meeting or otherwise cause all ordinary shares and American Depositary Shares beneficially owned by ADV (the “Covered Shares”) to be counted as present for the purpose of establishing a quorum, and respond to each request by the Company for written consent; (b) vote (or consent), or cause to be voted at such meeting (or validly execute and return and cause such consent to be granted with respect to), all Covered Shares (i) in favor of the proposed transactions contemplated in the Cooperation Agreement, the adoption of the transaction agreements pertaining to the proposed transactions contemplated in the Cooperation Agreements and any other matters necessary for consummation of the proposed transactions, and (ii) against (a) any proposal or transaction that competes with proposed transactions, and (b) any other action that would impede, interfere with, delay, postpone or adversely affect the proposed transactions. |
| ● | ADV has not granted, and shall not grant at any time prior to the Closing, a proxy or power of attorney with respect to any Covered Shares which is inconsistent with ADV’s obligations pursuant to the Cooperation Agreement. |
| ● | ADV has not granted, and shall not grant at any time prior to the Closing, a proxy or power of attorney with respect to any Covered Shares which is inconsistent with ADV’s obligations pursuant to the Cooperation Agreement. |
| ● | Upon the Closing, ADV shall, subject to the requisite approvals being obtained and continuing in force, convert the outstanding Principal Amount (as defined in the Convertible Note issued to ADV on September 17, 2020 (the “Note”)) and the Conversion Catch-up Amount (as defined in the Note), at a conversion price that is equal to the USD equivalent of RMB4.203 per ordinary share. |
| ● | The Company shall execute and deliver to ADV the warrant for the purchase of shares of the Company to ADV on the date of the Cooperation Agreement (such warrant, the “Warrant”). The Warrant shall be effective on and from the Closing and shall be exercisable into ordinary shares of the Company in accordance with the terms thereof (such ordinary shares, the “Warrant Shares”). The warrant exercise price shall be equal to the USD equivalent of RMB4.67 per ordinary share and may be settled, subject to the terms and conditions of Warrant, by way of cashless settlement and/or set-off against the Exit Payment (as defined in the Exit Payments Agreement entered into by ADV, the Company, the Founders on September 15, 2020). The Warrant shall contain customary registration rights and the Warrant Shares shall be freely transferable on the exercise of the Warrant. |
| ● | The Cooperation Agreement shall come into effect from July 20, 2022, and each party may terminate the Cooperation Agreement upon the earlier of the following: (a) if the Closing does not occur by the Outside Date; and (b) any of the transaction agreements is terminated. “Outside Date” means (i) December 31, 2022; (ii) if all the conditions, other than the satisfaction of the PRC regulatory condition in respect of the proposed transaction (including approvals/registrations/filings required for outward foreign direct investment and antitrust approvals/filings), are satisfied or waived by December 31, 2022, March 31, 2023; or (iii) such other date as agreed between the parties to the Cooperation Agreement. |
4.D.Property, Plant and Equipment
As of the date of this annual report, we own building ownership rights to certain buildings on four different parcels of land in Shenzhen, Huizhou and New York with a total gross floor area of 2,697 sq.m. Shenzhen Pengai Investment, one of our subsidiaries, is the owner of these buildings. Shenzhen Pengai Investment has obtained the relevant property rights certificates.
Two of our owned properties, which are located in Luohu District, Shenzhen, and Huicheng District, Huizhou, Guangdong, are leased to Pengcheng Hospital and Huizhou Pengai for the operation of their businesses, respectively.
The majority of the properties on which we operate in mainland China and Singapore are leased. As of the date of this annual report, we own four different properties with a total gross floor area of 3,905 sq.m and we leased properties in 11 different locations with an aggregate gross floor area of 22,962 sq.m.
94
The following table sets forth the number of lease agreements by expiry year.
Number of | ||
Expiry year |
| leases |
2023 |
| 1 |
2024 |
| — |
2025+ |
| 17 |
Item 4.A. Unresolved Staff Comments
None.
Item 5.Operating and Financial Review and Prospects
You should read the following discussion together with our financial statements and the related notes appearing elsewhere in this annual report. Some of the information contained in this discussion or set forth elsewhere in this annual report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should read “Item 3. Key Information—3.D. Risk Factors” and “Forward-Looking Information” for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
5.A.Operating Results
Overview
We generate revenue primarily from the provision of the following services, which represent our core business:
● | Surgical aesthetic treatments, commonly referred to as plastic surgery in the United States, such as eye surgery, rhinoplasty, breast augmentation and liposuction; and |
● | Non-surgical aesthetic treatments, which comprise minimally invasive aesthetic treatments and energy-based treatments. |
We also generate revenue from our non-core business of providing general healthcare and other aesthetic services. General healthcare comprises a range of medical services, such as internal medicine and traditional Chinese medicine, and other aesthetic medical treatments comprise aesthetic dentistry, aesthetic traditional Chinese medicine and hair loss treatments.
Factors affecting our results of operations
We believe our financial condition and operations are affected by the following factors:
Our expansion through organic growth and acquisitions
From January 1, 2019 to December 31, 2021, organic growth and the acquisition of new treatment centers were important contributors to our revenue growth. In 2020, we opened one and acquired four new treatment centers, excluding treatment centers from Hanfei as the Hanfei acquisition was terminated in 2021. These treatment centers, to the extent that they started operations, contributed RMB24.2 million additional revenue in 2020. In October 2020, the Group entered into an agreement to acquire 95% of equity interest of Beijing Aomei Yixin Investment Consultant Co., Ltd. (“Beijing Aomei Yixin”) with consideration of RMB11.5 million. The transaction has been completed in January 2021 and Beijing Aomei Yixin becomes a subsidiary of the Company since then. In 2021, we acquired one treatment center, the “Guangzhou Meixi Aesthetic Medical Clinic Company Limited” whose name was then changed into “Guangzhou Pengai Xiuqi Aesthetic Medical Clinic Co., Ltd”. In August 2022, Beijing Haiyue Xingguang Aesthetic Medical Clinic’s name was changed into “Beijing Aomei Yixin Investment Consultant Co., Ltd. Pengai Aesthetic Medical Clinic”.
95
Futher Divestment of Treatment Centers to Enhance Operating Efficiency
In 2022, we strived to enhance operating efficiency through various measures, including spinning off our underperforming treatment centers to focus on our development in the Hong Kong and Mainland China. In October 2022, we divested two underperforming treatment centers, Changsha Pengai Aesthetic Medical Hospital Co., Ltd. and Nanchang Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd., and received cash inflows of RMB4.6 million and RMB3.0 million, respectively. In December 2022, we also divested Shenzhen Miaoyan Aesthetic Medical Clinic, another underperforming treatment center, without cash inflows. In August 2022, Hangzhou Pengai Aesthetic Medical Outpatient Department was ceased operation due to underperformance.
On January 10, 2023, we entered into a share purchase agreement with a non-affiliated third party, under which we transferred 100% of the equity of our wholly-owned subsidiary, Shenzhen Miaoyan Medical Technology Investment Co., Ltd. to this third party for a consideration of RMB1.9 million due to our strategy to further focus on mature and profitable institutions. As of the date of this annual report, the transaction has not been closed.
Number of procedures performed and average spending per procedure
Revenue from surgical and non-surgical aesthetic medical services is affected by the number of such procedures performed and the average spending per procedure. The number of procedures is generally affected by market demand, the number of our treatment centers and our marketing efforts. Average spending per procedure is generally affected by our product and service mix, customers’ consumption habits, our marketing strategies and our pricing policy. The provision of general healthcare services has recently been affected by the current medical regulatory environment in China, which is not favorable to privately owned general hospitals. As a result, we have decided to decrease the general healthcare services we provide. However, we will continue to focus on other aesthetic medical services, and expect to continue generating revenue from these services.
Due to the outbreak of COVID-19 and the relevant quarantine requirements, we postponed the resumption of operations of aesthetic treatment centers in China after the Chinese New Year holiday in 2020. During the temporary shutdown of our treatment centers, we could not perform procedures. By the end of March 2020, all of our aesthetic treatment centers had resumed their operations. However, we experienced a significantly decrease in market demand for aesthetic medical services in the first half of 2020. While the COVID-19 pandemic has been largely contained in China, and the number of procedures we performed has picked up since the second half of 2020, the negative impact of the COVID-19 pandemic on the market demand and our business still existed in 2021 and 2022. In December 2022, China relaxed many restrictions including scrapping the use of Covid tracking app and lifting domestic travel restrcitions, which may case a second wave of infections. We will continue to monitor and evaluate the financial impacts to us. For more details, see “Item 3. Key Information—3.D. Risk Factors—Risks Relating to Our Business and Industry—Any future occurrence of force majeure events, natural disasters or outbreaks of contagious diseases in the PRC could prevent us from effectively serving our customers and thus adversely affect our results of operations.”
Cost of sales and services rendered
Our ability to control costs and expenses affects our profitability. Salaries in the medical treatment industry in China have generally been increasing in recent years, and we seek to offer competitive wages and other benefits to recruit and retain quality medical professionals. In addition, our cost of inventories and consumables has been relatively significant during the past few years, comprising 45.8%, 46.5%, and 50.9% of our cost of sales and services rendered in 2020, 2021 and 2022, respectively. Cost of inventories and consumables is affected primarily by the number of procedures we perform, but the costs of materials we use in our services have been declining over time during the past few years.
Successful marketing of our services
In order to enhance public recognition of our brand and new treatment procedures or services in both existing and new markets, we advertise through various media platforms. We utilize various marketing tools, including advertising on traditional media and online channels, hosting promotional events and circulating promotional materials, to attract new customers, retain our existing customers and increase customers’ spending. We have been seeking to optimize the marketing channels we use, and to deepen our cooperation with new media marketing channels such as So-Young, in order to improve the efficiency of our sales and marketing efforts. Our advertising and marketing expenses were RMB381.2 million, RMB 255.5million and RMB132.2 million (US$19.2 million) in 2020, 2021 and 2022, respectively, accounting for 42.3%, 39.6% and 19.7% respectively, of our revenue for the same periods. The effectiveness of our marketing and promotional efforts will directly impact our revenue and profitability.
96
Ability to introduce and develop innovative aesthetic medical service techniques
Success in the aesthetic medical market requires us to monitor closely market trends and customer preferences, introduce new treatment procedures and services, and enhance our existing services and procedures. We regularly consult with experts at well-respected foreign aesthetic medical institutions in the United States, Europe, South Korea and Japan to learn and adopt new aesthetic medical solutions, standards and technologies. Our ability to adopt the latest technologies and quickly and cost-effectively respond to our customers’ ever changing preferences are key to our success.
Seasonality
Our operating results are exposed to seasonal fluctuations of demand for our services. During2020 and 2021, we have experienced relatively higher customer visits in the second half of each financial year, which was mainly because we experienced a higher number of customer visits for the period from October to December, primarily due to the Chinese National Day holiday, Christmas, and the subsequent New Year and Chinese New Year holidays. As such, our revenue was slightly higher in the second half of each financial year during 2020 and 2021. Our revenue was slightly lower in the second half of 2022 caused by a rise in the number of people infected with COVID-19 in December.
Favorable Policies
On July 26, 2022, the Development and Reform Commission of Shenzhen Municipality issued an action plan for promoting the healthcare and aesthetic medical industry in the city, with an aim to strengthen the operating standards and enhance service quality of the aesthetic medical enterprises. Pursuant to the action plan, aesthetic medical enterprises which are newly accredited by the Joint Commission on Accreditation of Healthcare Organizations shall receive a project subsidy of 20% of the total investment, up to RMB 10 million, on merit basis. Enterprises which help standardize the aesthetic medical services in Shenzhen shall also be giving a maximum subsidy of RMB0.5 million on merit basis. It is expected that these will accelerate the market consolidation as non-complying practitioners will be eliminated. The policies are expected to benefit leading aesthetic medical companies of high operational quality and compliance status such as AIH.
Certain statements of comprehensive income line items
Revenue
We have one reporting segment and generate revenue from three service offerings: (i) non- surgical aesthetic medical services, comprising minimally invasive aesthetic treatments and energy-based treatments, (ii) surgical aesthetic medical services, and (iii) general healthcare services and other aesthetic medical services. We regard our non-surgical and surgical aesthetic medical services as our core business that we believe will have the greatest impact on our future results of operations. The following table sets forth a breakdown of our revenue, as an amount and as a percentage of total revenue, by service offering for the periods indicated.
For the year ended December 31, |
| ||||||||||||||
| 2020 |
| 2021 |
| 2022 |
| |||||||||
| RMB |
|
| RMB |
|
| RMB |
| US$ |
|
| ||||
Non-surgical aesthetic medical services | |||||||||||||||
Minimally invasive aesthetic treatments | 234,562 | 26.0 | % | 177,187 | 27.4 | % | 223,891 | 32,461 | 33.4 | % | |||||
Energy-based treatments | 218,108 | 24.2 | % | 157,061 | 24.3 | % | 258,394 | 37,464 | 38.6 | % | |||||
Sub-total | 452,670 | 50.2 | % | 334,248 | 51.7 | % | 482,285 | 69,925 | 72.0 | % | |||||
Surgical aesthetic medical services | 401,645 | 44.5 | % | 243,070 | 37.7 | % | 132,628 | 19,229 | 19.8 | % | |||||
General healthcare services and other aesthetic medical services | 47,258 | 5.3 | % | 68,275 | 10.6 | % | 55,178 | 8,000 | 8.2 | % | |||||
Total | 901,573 | 100.0 | % | 645,593 | 100.0 | % | 670,091 | 97,154 | 100.0 | % | |||||
We had a total revenue of RMB670.1 million (US$97.1 million) in 2022, representing an increase of 3.8% from 2021, primarily due to (i) the increasing customer demand for non-surgical aesthetic medical treatments, (ii) better quality and well-organized services, (iii) more optimal operation strategies as well as staffing arrangement.
97
Our revenue primarily depends on the number of aesthetic procedures performed and average spending by our customers per aesthetic procedure. The following table sets forth certain of our operating data for the periods indicated.
| For the year ended December 31, | |||||
| 2020* |
| 2021 |
| 2022 | |
Number of aesthetic procedures performed | ||||||
Non-surgical aesthetic medical services | ||||||
Minimally invasive aesthetic treatments | 117,181 | 97,156 | 102,293 | |||
Energy-based treatments | 296,054 | 327,083 | 412,116 | |||
Sub-total | 413,235 | 424,239 | 514,409 | |||
Surgical aesthetic medical services | 92,922 | 46,568 | 30,124 | |||
* | Without considering the one-off impact from Hanfei. |
| For the year ended December 31, | |||||||
| 2020* | 2021 |
| 2022 | ||||
| RMB |
| RMB |
| RMB |
| US$ | |
Average spending by our customers per aesthetic procedure |
|
|
|
|
|
|
|
|
Non-surgical aesthetic medical services |
| 928 |
| 788 | 938 |
| 136 | |
Minimally invasive aesthetic treatments |
| 1,594 |
| 1,824 | 2,189 |
| 317 | |
Energy-based treatments |
| 665 |
| 480 | 627 |
| 91 | |
Surgical aesthetic medical services |
| 3,140 |
| 5,220 | 4,403 |
| 638 | |
* | Without considering the one-off impact from Hanfei. |
From 2020 to 2022, average spending by our customers per aesthetic procedure had generally declined due to a number of factors. For example, as part of our marketing strategy, we encourage potential customers to try out our services by offering a small number of low-ASP treatments; in addition, in order to increase utilization of our equipment, we from time to time offer promotional events to attract new customers; and as such we may adjust our pricing policy in response to increased competition and the decrease in cost of materials, such as hyaluronic acid. These measures have negatively contributed to our overall average spending by customers per procedure, but we believe these measures are beneficial to our overall profitability and long-term growth.
Aesthetic medical services (surgical and non-surgical)
Our aesthetic medical services consist of surgical and non-surgical aesthetic medical services. Our revenue from aesthetic medical services was RMB854.3 million, RMB577.3 million and RMB614.9 million (US$89.2 million) in 2020, 2021 and 2022, respectively. Revenue from aesthetic medical services as a percentage of our total revenue was 94.7%, 89.4% and 91.8% in 2020, 2021 and 2022, respectively. The total revenue from aesthetic medical services for 2022 increased by 6.5% primarily due to the increasing customer demand for non-surgical aesthetic medical treatments.
● | Non-surgical aesthetic medical services. Our non-surgical aesthetic medical services primarily consist of services such as injection treatments, energy-based treatments and thread lifts. Our revenue from non-surgical aesthetic medical services was RMB452.7 million, RMB334.2 million and RMB482.3 million (US$69.9 million) in 2020, 2021 and 2022, respectively. Revenue from non-surgical aesthetic medical services as a percentage of our total revenue was 50.2%, 51.7% and 72.0% in 2020, 2021 and 2022, respectively. The total revenue from non-surgical aesthetic medical services for 2022 increased by 44.3%. |
● | Surgical aesthetic medical services. Our surgical aesthetic medical services primarily consist of services such as eye surgery (for example, double eyelid surgery), nose surgery (rhinoplasty), breast augmentation and liposuction. Our revenue from surgical aesthetic medical services was RMB401.6 million, RMB243.1 million and RMB132.6 million (US$19.2 million) in 2020, 2021 and 2022, respectively. Revenue from aesthetic medical services as a percentage of our total revenue was 44.5%, 37.7% and 19.8% in 2020, 2021 and 2022, respectively. The total revenue from surgical aesthetic medical services for 2022 decreased by 45.5%. |
98
General healthcare services and other aesthetic medical services
General healthcare services and other aesthetic medical services primarily consist of a range of medical services, such as internal medicine, urology, gynecology and obstetrics treatments, as well as various other aesthetic medical treatments, such as dentistry, dermatology and hair loss treatments. Our revenue from general healthcare services and other aesthetic medical services was RMB47.3 million, RMB68.3 million and RMB55.2 million (US$8.0 million), respectively, representing 5.3%, 10.6% and 8.2% of our total revenue, respectively, in 2020, 2021 and 2022. The total revenue from general healthcare services and other aesthetic medical services for 2022 decreased by 19.2% primarily due to the divestment of treatment centers during the fiscal year 2021 and 2022.
Cost of sales and services rendered
Our cost of sales and services rendered primarily consists of employee benefit expenses for our medical staff, cost of inventories and consumables, inspection fee paid to third party inspection service providers, as well as operating lease and rental expenses, depreciation and amortization, and utilities and office expenses incurred in relation to our medical staff.
In 2020, 2021 and 2022, our cost of sales and services rendered amounted to RMB356.8 million, RMB376.1 million and RMB314.5 million (US$45.6 million), respectively, representing 39.6%, 58.3% and 46.9% of our total revenue, respectively.
Gross margin
The table below sets forth a breakdown of our gross margin by service offering for the periods indicated.
| Gross margin |
| |||||
for the year ended December 31, |
| ||||||
| 2020 |
| 2021 |
| 2022 |
| |
Non-surgical aesthetic medical services |
| 59.4 | % | 39.4 | % | 55.3 | % |
Minimally invasive aesthetic treatments |
| 62.1 | % | 43.1 | % | 54.5 | % |
Energy-based treatments |
| 56.5 | % | 35.4 | % | 56.0 | % |
Surgical aesthetic medical services |
| 63.0 | % | 50.9 | % | 48.6 | % |
General healthcare services and other aesthetic medical services |
| 48.0 | % | 20.6 | % | 44.0 | % |
Overall |
| 60.4 | % | 41.7 | % | 53.1 | % |
The overall increase in gross margin from 2021 to 2022 is mainly due to the divestment of treatment centers that failed to make profit or did not meet our requirements during the fiscal year 2021 and 2022, which optimized our cost structure.
Selling expenses
Selling expenses primarily consist of advertising and marketing expenses, and the employee benefit expenses for our sales team. In 2020, 2021 and 2022, our selling expenses amounted to RMB510.6 million, RMB404.7 million and RMB226.5 million (US$32.8 million), respectively, representing 56.6%, 62.7% and 33.8% of our revenue in the corresponding period. The selling expenses would have decreased by 44.0% to RMB226.5 million (US$63.5 million) from RMB404.7 million in 2021, primarily due to (i) the divestment of treatment centers during the fiscal year 2021 and 2022, (ii) our strategic shift of marketing focus to a lower-cost online marketing channels, such as private domain customer reach and social media livestreaming.
General and administrative expenses
General and administrative expenses primarily consist of employee benefit expenses for our management team, as well as the utility and office expense and rental expense incurred in relation to our administrative staff. In 2020, 2021 and 2022, our general and administrative expenses amounted to RMB230.6 million, RMB207.0 million and RMB150.4 million (US$21.8 million), respectively, representing 25.6%, 32.1% and 22.4% of our revenue in the corresponding period. The general and administrative expenses in 2022 decreased by 27.3%, primarily due to (i) the divestment of treatment centers during the fiscal year 2021 and 2022, (ii) the optimization of administrative expenses and management labor costs.
99
Fair value loss of convertible note
We designated our convertible note as a financial liability at fair value through profit or loss. It was initially recognized at fair value. It is carried at fair value with changes in fair value recognized in profit or loss. We recorded a fair value loss of the convertible note of RMB1.6 million, RMB4.2 million and RMB26.5 million (US$3.8 million) in 2020, 2021 and 2022, respectively. The convertible note was redeemed in November 2019. In September 2020, we entered into a two-and-a-half year Convertible Note Purchase Agreement in a principal amount of USD10 million with ADV, of which USD5 million was actually drew down. In July 2022, we entered into a Share Purchase Agreement, a Subscription Agreement, a Shareholders’ Agreement and a Cooperation Agreement (collectively “Financing Agreements”) with Shenzhen Lafang Investment Management Co., Ltd., ADV and other parties, under which we settled with ADV on the above Convertible Note Purchase Agreement. According to the Financing Agreements, the above Convertible Note held by ADV will no longer accrue interest after July 2022 and ADV undertakes to covert the outstanding principal amount as well as interest into our ordinary shares at the price of RMB4.203 per ordinary share without cash payment upon the final settlement of the Financing Agreements. We also entered into a Warrant Agreement with ADV, under which we were required to pay USD2.7 million to ADV to settle the financial liability under the Exit Payment term in the Convertible Note Purchase Agreement. ADV agreed to postpone our cash payment date of USD2.7 million to December 2024 or, at its discretion, convert such amount at the price of RMB4.67 per ordinary share at any time during this period.
Impairment of non-current assets
We designated our goodwill, tradenames and property, plant and equipment as a non-financial asset. They were tested annually for impairment. We recorded an impairment of non-current assets of RMB33.0 million and RMB314.0 million in 2020 and 2021 respectively. We recognized no impairment loss in 2022, representing an decrease from RMB314.0 million in 2021. The impairment was identified in 2021 primarily due to our strategic restructuring and impairment assessments at cash-generating unit level and noted several recoverable amounts, which were lower than their carrying amounts for certain cash-generating units. Relevant impairments are non-operating and non-cash items.
Income tax expense
Our income tax expense consists of current income taxation and deferred income tax credit or charge. Our subsidiaries established and operated in the PRC are subject to PRC enterprise income tax at the rate of 25%. The following table shows a breakdown of our income tax expense for the periods indicated.
For the year ended on | ||||||||
December 31, | ||||||||
| 2020 |
| 2021 |
| 2022 | |||
| RMB |
| RMB |
| RMB |
| US$ | |
(in thousands) | ||||||||
Current income taxation: |
|
|
|
|
| |||
—PRC enterprise income tax | 486 | 8,208 | 3,308 |
| 480 | |||
Deferred income tax (credit)/charge | (13,073) | (20,006) | (21,375) |
| (3,099) | |||
Total | (12,587) | (11,798) | (18,067) |
| (2,619) | |||
Our effective tax rates were (4.9)%, (1.7)% and (56.0)% for the years ended December 31, 2020, 2021 and 2022, respectively, which fluctuation was mainly due to the tax loss not recognised and non-taxable fair value changes in relation to our convertible redeemable preferred shares, convertible note, exchangeable note liabilities, and derivative financial instruments, as well as certain other non-tax deductible expenses such as the interest expense on convertible note, share-based compensation expenses, and professional fees in relation to our financing activities but are not capitalized.
100
The income tax expense as a percentage of adjusted profit before income tax is presented as a supplemental disclosure because it is used by us to analyze the fluctuation of income tax expenses. However, adjusted profit before income tax is a non-IFRS financial measure and should not be considered in isolation or construed as an alternative to profit/(loss) before income tax as an indicator normally included in IFRS-based financial results. Adjusted profit before income tax presented in this annual report may not be comparable to other similarly titled measurements of other companies. The following table provides a quantitative reconciliation of adjusted profit before income tax to its most directly comparable IFRS measurement, profit/(loss) before income tax:
For the year ended December 31, | |||||||||
| 2020 |
| 2021 |
| 2022 | ||||
| RMB |
| RMB |
| RMB |
| US$ | ||
(in thousands) | |||||||||
(Loss)/Profit before income tax | (259,492) |
| (681,314) |
| (94,173) | (13,654) | |||
Adjusted for: | |||||||||
Fair value loss of convertible note | 1,599 |
| 4,240 |
| 26,506 | 3,843 | |||
Interest expense on convertible note | — |
| — |
| — | — | |||
Share-based compensation expenses | 78,967 |
| 35,463 |
| 27,528 | 3,991 | |||
Professional fees | 18,581 |
| 30,570 |
| 8,888 | 1,289 | |||
IT - related expenses paid to a related party | — |
| — |
| — | 1,420 | |||
Compensation to suppliers | — |
| — |
| 9,794 | ||||
Impairment of non-current assets | 32,969 |
| 313,959 |
| — | — | |||
Loss on disposal of subsidiaries | 1,531 |
| 21,558 |
| 11,330 | 1,643 | |||
Loss on disposal of an associate | 927 |
| — |
| — | — | |||
Donation | 1,330 |
| — |
| — | — | |||
Adjusted profit/(loss) before income tax for the year | (123,588) |
| (275,524) |
| (10,127) | (1,468) | |||
Effective tax rate based on adjusted profit before income tax | 10.2 | % | 4.3 | % | (56.0) | % | |||
Our income tax expense as a percentage of the adjusted profit before income tax for the years ended December 31, 2020, 2021 and 2022 were 10.2%, 4.3% and -56.0%, respectively.
Taxation
Cayman Islands
We are an exempted company with limited liability incorporated in the Cayman Islands. Under Cayman Islands law, we are not subject to income or capital gains tax in the Cayman Islands.
Hong Kong
Hong Kong profits tax rate is 16.5%. During the periods under review, we did not incur any profit tax as there was no estimated assessable profit that was subject to Hong Kong profit tax.
PRC
Pursuant to the EIT Law, a uniform 25% EIT rate is generally applied to both foreign-invested except where a special preferential rate applies. Our PRC subsidiaries were subject to this uniform 25% EIT rate during the periods under review.
Under the EIT Law, the profits of a foreign-invested enterprise that are distributed to its immediate holding company outside the PRC will be subject to a withholding tax rate of 10%. Pursuant to a special arrangement between Hong Kong and the PRC, such rate is reduced to 5% if a Hong Kong resident enterprise owns over 25% of a PRC company during the 12 consecutive months preceding the receipt of the dividends and is determined by the competent PRC tax authority to have satisfied the relevant conditions and requirements.
101
Results of operations
The following table sets forth our results of operations for the periods indicated in absolute amounts and as a percentage of revenue.
For the year ended December 31, | ||||||||
| 2020 |
| 2021 |
| 2022 | |||
| RMB |
| RMB |
| RMB |
| US$ | |
(in thousands) | ||||||||
Revenue | 901,573 | 645,593 | 670,091 |
| 97,154 | |||
Cost of sales and services rendered | (356,796) | (376,092) | (314,505) |
| (45,599) | |||
Gross profit | 544,777 | 269,501 | 355,586 |
| 51,555 | |||
Selling expenses | (510,608) | (404,683) | (226,474) |
| (32,836) | |||
General and administrative expenses | (230,646) | (206,971) | (150,379) |
| (21,803) | |||
Finance income | 1,185 | 113 | 155 |
| 22 | |||
Finance costs | (29,189) | (27,230) | (21,635) |
| (3,136) | |||
Other gains, net | 600 | 6,074 | (24,920) |
| (3,613) | |||
Fair value loss of convertible note | (1,599) | (4,240) | (26,506) |
| (3,843) | |||
Impairment of non-current assets | (32,969) | (313,959) | — |
| — | |||
Share of profits/(losses) of investments accounted for using the equity method | (1,043) | 81 | — |
| — | |||
(Loss)/profit before income tax | (259,492) | (681,314) | (94,173) |
| (13,654) | |||
Income tax expense | 12,587 | 11,798 | 18,067 |
| 2,619 | |||
(Loss)/profit for the year | (246,905) | (669,516) | (76,106) |
| (11,034) | |||
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
Revenue
Our revenue increased by 3.8% to RMB670,091 million (US$97,154 million) in 2022 from RMB645.6 million in 2021.
Non-surgical aesthetic medical services
Our revenue from non-surgical aesthetic medical services increased by 44.3% to RMB482.3 million (US$69.9 million) in 2022 from RMB334.2 million in 2021, among which revenue from energy-based treatments increased by 64.5% to RMB258.4 million (US$37.5 million) from RMB157.1 million in 2021, and revenue from minimally invasive aesthetic medical services increased by 26.4% to RMB223.9 million (US$32.5 million) in 2022 from RMB177.2 million in 2021.
Surgical aesthetic medical services
Our revenue from surgical aesthetic medical services decreased by 45.4% to RMB132,628 million (US$19,229 million) from RMB243.1 million in 2021.
General healthcare services and other aesthetic medical services
Our revenue from general healthcare services and other aesthetic medical services decreased by 19.2% to RMB55.2 million (US$8.0 million) from RMB68.3 million in 2021. We will continue to offer general healthcare services and other aesthetic medical services, and expect to continue earning revenue from these services.
Cost of sales and services rendered
Our cost of sales and services rendered decreased by 16.4% to RMB314.5 million (US$45.6 million) from RMB376.1 million in 2021.
102
Gross profit
As a result of the foregoing, our gross profit increased by 31.9% to RMB355.6 million (US$51.6 million) from RMB269.5 million in 2021. Our gross margin increased to 53.1% from 41.7% in 2021 primarily due to the divestment of treatment centers that failed to reach our expected profitability levels during the fiscal year 2021 and 2022.
Non-surgical aesthetic medical services
Our gross margin for non-surgical aesthetic medical services increased to 55.3% from 39.4% in 2021. Our gross margin for minimally invasive aesthetic treatments increased to 54.5% from 43.1% in 2021 and our gross margin for energy-based treatments increased to 56.0% from 35.4% in 2021.
In 2022, non-surgical aesthetic medical services took account for 90.0% of our total treatments in terms of the number of treatments, which increased from 84.1% in 2021, and the gross margin increased to 55.3% from 39.4% in 2021.
Surgical aesthetic medical services
Our gross margin for surgical aesthetic medical services decreased to 48.6% from 50.9% in 2021.
General healthcare services and other aesthetic medical services
Our gross margin for general healthcare services and other aesthetic medical services increased to 44.0% from 20.6% in 2021.
Selling expenses
Our selling expenses decreased by 44.0% to RMB226.5 million (US$32.8 million) in 2022 from RMB404.7 million in 2021. Selling expenses as a percentage of our total revenue decreased to 33.8% from 62.7% in 2021.
General and administrative expenses
Our general and administrative expenses decreased by 27.3% to RMB150.4 million (US$21.8 million) from RMB207.0 million in 2021.
Finance costs
Our finance costs decreased by 20.8% to RMB21.6 million (US$3.1 million) from RMB27.2 million in 2021, primarily due to decreased borrowings and decreased interest expenses for borrowings from the banks and other financial institutions.
Other gains, net
Our other gains, net decreased to RMB(24.9) million (US$(3.6) million) in 2022 from RMB6.1 million in 2021, mainly as a result of disposal and deregistration of subsidiaries, and the reclassification of investments in associate to asset held-for-sale.
Fair value loss of convertible note
We recorded a fair value loss of convertible note of RMB26.5 million (US$3.8 million) in 2022 compared to RMB4.2 million in 2021. On Septemer 17, 2020, we entered into a convertible note purchase agreement. As of December 31, 2022, the outstanding convertible note was RMB64.6 million.
Impairment of non-current assets
We recognized no impairment loss in respect of non-current assets in 2022, representing a significant decrease from RMB314.0 million in 2021. The change of non-current assets in 2022 was primarily because we performed impairment assessments at cash-generating unit level and found no recoverable amounts which were lower that their carrying amounts for certain cash-generating units. Relevant impairments are one-off and non-cash items.
103
Income tax expense/(credit)
We recorded an income tax credit of RMB18.1 million (US$2.6 million) as compared to an income tax credit of RMB11.8 million in 2021.
Loss/profit for the year
As a result of the foregoing, our net loss was RMB76.1 million (US$11.0 million) in 2022, compared with a net loss of RMB669.5 million in 2021.
Year Ended December 31, 2021 Compared to Year Ended December 31, 2020
For a detailed description of the comparison of our operating results for the year ended December 31, 2021 with those for the year ended December 31, 2020, see “Item 5.A. Operating and Financial Review and Prospects—Operating Results—Results of Operation— Year year ended December 31, 2021 compared to year ended December 31, 2020” of our Annual Report on Form 20-F filed with the Securities and Exchange Commission on May 16, 2022.
Critical accounting policies and significant judgments and estimates
We prepare our financial statements in conformity with IFRS, which requires us to make judgments, estimates and assumptions. We continually evaluate these estimates and assumptions based on the most recently available information, our own historical experience and various other assumptions that we believe to be reasonable under the circumstances. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from our expectations as a result of changes in our estimates.
Some of our accounting policies require a higher degree of judgment than others in their application and require us to make significant accounting estimates.
The selection of critical accounting policies, the judgments and other uncertainties affecting application of those policies and the sensitivity of reported results to changes in conditions and assumptions are factors that should be considered when reviewing our financial statements. We believe the following accounting policies involve the most significant judgments and estimates in the preparation of our financial statements.
Revenue recognition
Revenue is recognized when or as the control of the goods or services is transferred to the customer at an amount that reflects the consideration to which we expect to be entitled in exchange for those goods or services.
Revenue from provision of services, which includes non-surgical aesthetic medical services, comprising minimally invasive aesthetic treatments and energy-based treatments, surgical aesthetic medical services, and general healthcare services and other aesthetic medical services, is recognized at a point in time when the services have been rendered to customers. Minimally invasive aesthetic medical services, surgical aesthetic medical services and other aesthetic medical services are usually provided within a day, except for certain large-scale surgical aesthetic medical services in which customers are required to go through medical tests and screening for suitability of the surgery. The period of inpatient stays usually does not exceed more than a week. Most of our services do not require inpatient stays, though we do have inpatient facilities available upon patient request. With respect to energy- based treatments, we have certain packages that customers pay in advance. Payments for such energy-based treatments not yet rendered are recorded as contract liabilities in our consolidated balance sheets and are fully refunded to our customers upon their request. Revenue from the provision of general healthcare services and other aesthetic medical services is recognized at a point in time upon provision of such services for which the service period is usually within a day. We review the utilization pattern of contract liabilities and the treatment progress of individual customers on a regular basis to consider full recognition of the corresponding contract liabilities in profit or loss. Interest income is recognized using the effective interest method.
104
Purchase price allocation
We apply the acquisition method to account for business combinations. The application of business combination accounting requires the use of significant estimates and assumptions. The purchase method of accounting for business combinations requires us to estimate the fair value of assets acquired and liabilities assumed to properly allocate purchase price consideration between assets that are depreciated and amortized from goodwill. This exercise requires the use of management’s assumptions, which would not reflect unanticipated events and circumstances that may occur.
Goodwill impairment assessment
Goodwill arises on the acquisition of subsidiaries and represents the excess of the consideration transferred over our interest in fair value of the net identifiable assets, liabilities and contingent liabilities of the acquiree and the amount of the non-controlling interests in the acquiree.
Our management tests annually whether goodwill has suffered any impairment. For the purpose of impairment testing, goodwill has been allocated to individual cash-generating units, or CGUs, and is reviewed for impairment based on forecast operating performance and cash flows. The recoverable amounts of CGUs have been determined based on value-in-use calculations. These calculations require the use of estimates. The value-in-use calculations primarily use cash flow projections based on financial budgets approved by management. There are a number of assumptions and estimates involved in the preparation of cash flow projections for the period covered by the approved budgets. Key assumptions include the expected growth rates, timing of future capital expenditures and selection of discount rates to reflect the risks involved. Our management prepares the financial budgets reflecting actual and prior year performance and market development expectations. Our assessment indicated that reasonably possible changes in key assumptions on which value-in-use calculations are based would not cause the carrying amounts to exceed recoverable amounts of individual CGUs.
Fair value of series A preferred shares, convertible note and exchangeable note liabilities
We designated our series A preferred shares, convertible note and exchangeable note liabilities as financial liabilities at fair value through profit or loss. They were initially recognized at fair value.
Subsequent to initial recognition, our series A preferred shares, convertible note and exchangeable note liabilities are carried at fair value with changes in fair value recognized in profit or loss.
Our series A preferred shares, convertible note and exchangeable note liabilities are not traded in an active market and the fair value is determined by using valuation techniques. We use the market comparable approach or discounted cash flow method to determine the underlying equity value of our company and adopt the equity allocation method to determine the fair value of the series A preferred shares, convertible note and exchangeable note liabilities. The discounted cash flow method may include the use of cash flow projections based on financial forecasts prepared by management. The expected growth in revenue and gross margin, timing of future capital expenditures, weighted average cost of capital and terminal growth rate are based on actual and prior year performance as well as market expectations, which are key drivers of the underlying equity value of our company.
Estimated useful lives of property, plant and equipment
We determine the estimated useful lives and related depreciation charges for our property, plant and equipment with reference to the estimated periods that we intend to derive future economic benefits from the use of these assets. We perform periodic reviews of the estimated useful lives of property, plant and equipment, and revise the depreciation charges where estimated useful lives are different than those previously estimated.
Recently issued accounting standards
See note 2.2.2 to our audited consolidated financial statements appearing elsewhere in this annual report.
105
5.B.Liquidity and Capital Resources
Our principal sources of liquidity and capital resources have been, and are expected to continue to be, cash flow from operations, issuances of securities and bank borrowings. Our principal uses of cash have been, and we expect will continue to be, for working capital to support an increase in our scale of operations as well as investments for business expansion.
We had net current liabilities of RMB466.2 million as at December 31, 2022, which included current borrowings of RMB172.8 million. We had operating loss of RMB34.9 million and net operating cash outflows of RMB75.1 million for the year ended December 31, 2022, primarily due to the continued impact of COVID-19 outbreak and the divestment of loss-making treatment centers that negatively affected our overall profitability. After considering the gradual recovery of business post the COVID-19 outbreak, our expected cash flow from future operations taking into consideration of business growth, funds from bank borrowings and other sources of financing, we concluded that we have sufficient financial resources to meet our financial obligations as and when they fall due and continue our operation in the next 12 months, subject to any uncertainty of the development of COVID-19.
Given our current credit status and the current availability of capital, we believe that we will not encounter any major difficulties in obtaining additional bank borrowings. We plan to fund our future business plans, capital expenditures and related expenses as described in this annual report with cash from operations, the remaining net proceeds from our initial public offering and short-term and long-term indebtedness. We may, however, require additional cash resources due to changing business conditions or other future developments, including any investments or acquisitions we may decide to pursue. Also see “Item 3. Key Information—3.D. Risk Factors—Risks Relating to Our Business and Industry—Any future occurrence of force majeure events, natural disasters or outbreaks of contagious diseases in the PRC could prevent us from effectively serving our customers and thus adversely affect our results of operations.”
Cash flows
The following table presents selected cash flow data from our consolidated statements of cash flows for the periods indicated.
For the year ended December 31, | ||||||||
| 2020 |
| 2021 |
| 2022 | |||
| RMB |
| RMB |
| RMB |
| US$ | |
(in thousands) | ||||||||
Net cash generated from/(used in) operating activities | (843) | 51,091 | (75,126) |
| (10,892) | |||
Net cash (used in)/generated from investing activities | (127,368) | (11,525) | 3,111 |
| 451 | |||
Net cash generated from financing activities | 17,697 | (45,235) | 45,552 |
| 6,604 | |||
Net (decrease)/increase in cash and cash equivalents | (110,514) | (5,669) | (26,463) |
| (3,837) | |||
Cash and cash equivalents at beginning of the year | 154,490 | 44,384 | 39,289 |
| 5,696 | |||
Cash and cash equivalents at the end of the year | 44,384 | 39,289 | 12,161 |
| 1,763 | |||
Net cash generated from/(used in) operating activities
Our net cash used in operating activities in 2022 was RMB75.1 million (US$10.9 million) , primarily reflecting our loss before income tax as adjusted for non-cash items, including finance costs of RMB21.6 million (US$3.1 million), depreciation of property, plant and equipment of RMB61.1 million (US$8.9 million), share-based compensation of RMB27.5 million (US$4.0 million), fair value loss of convertible note of RMB26.5 million (US$3.8 million).
Our net cash generated from operating activities in 2021 was RMB51.1 million (US$8.0 million), primarily reflecting our loss before income tax as adjusted for non-cash items, including finance costs of RMB27.2 million (US$4.3 million), depreciation of property, plant and equipment of RMB93.2 million (US$14.6 million), share-based compensation of RMB35.5 million (US$5.6 million), impairment of non-current assets of RMB314.0 million (US$49.3 million), which was primarily goodwill, tradename and property, plant and equipment.
106
Our net cash used in operating activities in 2020 was RMB0.8 million (US$0.1 million), primarily reflecting our loss before income tax as adjusted for non-cash items, including finance costs of RMB29.2 million (US$4.5 million), depreciation of property, plant and equipment of RMB97.2 million (US$14.9 million), share-based compensation of RMB79.0 million (US$12.1 million), impairment of non-current assets of RMB33.0 million (US$5.1 million), which was primarily goodwill, tradename and property, plant and equipment.
Net cash generated from/(used in) investing activities
Our net cash generated from investing activities in 2022 was RMB3.1 million (US$0.4million). primarily reflecting net cash proceed from disposal of subsidiaries of RMB9.5 million (US$1.4 million) primarily due to the two business disposals in 2022, net cash used in transaction with related parties of RMB2.6 million (US$0.4 million).
Our net cash used in investing activities in 2021 was RMB31.5 million (US$4.9 million).
Our net cash used in investing activities in 2020 was RMB127.4 million (US$19.5 million), primarily reflecting purchase of and deposit paid for property, plant and equipment of RMB38.4 million (US$5.9 million), the deposit of long term investment of RMB21.3 million (US$3.3 million), and net cash used in business combinations of RMB70.0 million (US$10.7 million) primarily due to the four business acquisitions in 2020. If excluding the impact from Hanfei, our net cash used in investing activities in 2020 was RMB122.7 million (US$18.8 million).
Net cash generated/(used in) from financing activities
Our net cash generated from financing activities in 2022 was RMB45.6 million (US$6.6 million), primarily reflecting proceeds from borrowing of RMB276.3 million (US$40.1 million) and proceeds from share issue of RMB94.2(US$13.7 million), partially offset by repayment of borrowings of RMB 299.0 million (US$43.4 million)
Our net cash used in financing activities in 2021 was RMB45.2 million (US$7.1 million), primarily reflecting proceeds from borrowing of RMB240.9 million (US$37.8 million), partially offset by repayment of borrowings of RMB 221.6 million (US$34.8 million), and repayment of lease liabilities of RMB50.6 million (US$7.9 million) and interest paid of RMB12.8 million (US$2.0 million).
Our net cash generated from financing activities in 2020 was RMB17.7 million (US$2.7 million), primarily reflecting proceeds from borrowing of RMB276.6 million (US$42.4 million) and net proceeds from issuance of convertible note of RMB32.8 million (US$5.0 million), partially offset by repayment of borrowings of RMB217.7 million (US$33.4 million), and repayment of lease liabilities of RMB59.6 million (US$9.1 million) and interest paid of RMB10.5 million (US$1.6 million).
Indebtedness
Bank borrowings
The principal banks on our borrowings are Shanghai Pudong Development Bank, China Everbright Bank and Beijing of Bank. As of December 31, 2022, the outstanding principal balance of these loan was RMB36.2 million, RMB24.6 million and RMB21.3 million respectively.
Borrowings from other financial institution
In 2022, we entered into borrowings arrangements with third-party financial institution. As of December 31, 2022, the total outstanding principal balance of these borrowings was RMB90.1 million (US$13.1 million).
The loan and credit facility are guaranteed by our subsidiary and our related parties, and secured by some of their properties and/or our properties. The effective interest rate on these bank and other borrowings was 7.8% in 2022. Neither of these borrowings have any financial covenant requirements.
107
Convertible note
On September 17, 2020, we entered into a convertible note purchase agreement, and pursuant to which, we issued a convertible note at principal amount of US$5,000,000 (equivalent to approximately RMB33.4 million). The maturity date of such convertible note is March 17,2023. The convertible note is convertible into our ADSs at the option of the holder after six months from the issue date to the maturity date, unless we have elected to redeem the convertible note prior to conversion. We have the right to redeem all the outstanding convertible note after one year from the issue date. The holder has a right to redeem all outstanding convertible note at maturity date at a price equivalent to 12.5% internal rate of return compounded annually from the issue date to maturity date. We can elect to redeem all outstanding convertible note after one year from the issue date at a price equivalent to 15% internal rate of return compounded annually from the issue date to the redemption date.
Before the first anniversary of the issuance of the convertible note, the convertible note is redeemable at the option of us and by giving notice in writing to the convertible note holder. We monitor the convertible note on a fair value basis, which is in accordance with our risk management strategy and do not bifurcate any feature from its debt host instrument and designate the entire hybrid contract as a financial liability at fair value through profit or loss with the changes in the fair value recorded in consolidated statement of comprehensive income.
In September 2020, we entered into a two-and-a-half year Convertible Note Purchase Agreement in a principal amount of USD10 million with ADV, of which USD5 million was actually drew down. In July 2022, we entered into a Share Purchase Agreement, a Subscription Agreement, a Shareholders’ Agreement and a Cooperation Agreement (collectively “Financing Agreements”) with Shenzhen Lafang Investment Management Co., Ltd., ADV and other parties, under which we settled with ADV on the above Convertible Note Purchase Agreement. According to the Financing Agreements, the above Convertible Note held by ADV will no longer accrue interest after July 2022 and ADV undertakes to covert the outstanding principal amount as well as interest into our ordinary shares at the price of RMB4.203 per ordinary share without cash payment upon the final settlement of the Financing Agreements. We also entered into a Warrant Agreement with ADV, under which we were required to pay USD2.7 million to ADV to settle the financial liability under the Exit Payment term in the Convertible Note Purchase Agreement. ADV agreed to postpone our cash payment date of USD2.7 million to December 2024 or, at its discretion, convert such amount at the price of RMB4.67 per ordinary share at any time during this period.
Capital expenditures
Our capital expenditure in 2022 was RMB42.5 million (US$6.2 million), attributable to (i) purchase of property, plant and equipment and intangible assets, which mainly consists of machinery and equipment, including RMB1.8 million (US$0.3 million) for Pengcheng Hospital, RMB2.6 million (US$0.4 million) for Shenzhen Pengai, RMB5.3 million (US$0.8 million) for Shenzhen Xiuqi, RMB0.1 million (US$0 million) for Shanghai Jiahong, RMB3.7 million (US$0.5 million) for Nanchang Pengai Xiuqi, RMB9.2 million (US$1.3 million) for Pengyida, RMB4.8 million (US$0.7 million) for Guangzhou Pengai.
Our capital expenditure in 2021 was RMB33.4 million (US$5.2 million), attributable to (i) purchase of property, plant and equipment and intangible assets, which mainly consists of machinery and equipment, including RMB13.1 million (US$2.1 million) for Pengcheng Hospital, RMB13.3 million (US$2.1 million) for Shenzhen Pengai Hospital Investment Management Co., Ltd., RMB2.4 million (US$0.4 million) for Huizhou Pengai, RMB1.2 million (US$0.2 million) for Haikou Pengai RMB0.9 million (US$0.1 million) for Shenzhen Xiuqi.
Our capital expenditure in 2020 was RMB45.2 million (US$6.9 million), attributable to (i) purchase of property, plant and equipment and intangible assets, which mainly consists of leasehold improvements for our treatment centers, including RMB1.2 million (US$0.2 million) for Pengcheng Hospital, RMB8.3 million (US$1.3 million) for Shenzhen Pengai Hospital Investment Management Co., Ltd., RMB14.5 million (US$2.2 million) for Nanchang Pengai Xiuqi, RMB1.8 million (US$0.3 million) for Shanghai Pengai Jiayan, RMB1.8 million (US$0.3 million) for Peng Yida Business Consulting (Shenzhen) Co., Ltd. and RMB1.6 million (US$0.3 million) for Shenzhen Pengai, (ii) addition of machinery and equipment including RMB2.2 million (US$0.4 million) for Pengcheng Hospital, RMB1.7 million (US$0.3 million) for Shenzhen Pengai, RMB1.6 million (US$0.3 million) for Shenzhen Xiuqi.
We intend to fund our future capital expenditures with our existing cash balances and the remaining proceeds from our RMB170 million (US$25 million) financing. We will continue to make capital expenditures to meet the expected growth of our business.
108
Material Cash Requirements
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet financial guarantees or other off-balance sheet commitments to guarantee the payment obligations of any unconsolidated third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or product development services with us.
We did not have any off-balance sheet arrangements as of December 31, 2022.
Contractual Obligations
The following table sets forth our contractual obligations by specified categories as of December 31, 2022. Amounts we pay in future periods may vary from those reflected in the table.
Less than | More than | |||||||||
Total | 1 year | 1 to 2 years | 2 to 5 years | 5 years | ||||||
| RMB in thousands | |||||||||
Operating lease obligations(1) |
| 151,043 |
| 34,444 |
| 33,832 |
| 51,865 |
| 30,902 |
Borrowings |
| 201,876 |
| 172,766 |
| 24,533 |
| 4,577 |
| — |
Total |
| 352,919 |
| 207,210 |
| 58,365 |
| 56,442 |
| 30,902 |
Note:
(1) | We lease premises for aesthetic healthcare services and offices under non-cancellable operating agreements. The lease terms are between 3 and 9 years, and majority of lease agreements are renewable at the end of the lease period at market rate. |
Other than the contractual obligations set forth above, we do not have any contractual obligations that are long-term debt obligations, capital (finance) lease obligations, purchase obligations or other long-term liabilities reflected on our balance sheet as of December 31, 2022.
5.C.Research and Development, Patents and Licenses, etc.
We did not have in place research and development plans, and did not incur research and development expenses from January 1, 2019 to December 31, 2022.
5.D.Trend Information
The non-surgical aesthetic medical market in China recorded a compound annual growth rate of 29.2% from 2018 to 2022, outperforming than that of the entire industry, according to the research conducted by Ries Consulting, a positioning strategy consulting firm. The rapidly growing market is attributable to the steady growth of consumer disposable income and people’s growing acceptance of aesthetic medicine consumption, which was supported by the increased awareness of digital marketing advertisements. To ride on the market trend and further increase our market shares, we will continue to identify new treatment trends and introduce the latest services to the market while at the same time optimizing our sales and marketing strategies to increase customer outreach and conversion rate.
Other than the above, we are not aware of any trends, uncertainties, demands, commitments or events for the period from January 1, 2019 to December 31, 2022 that are reasonably likely to have a material effect on our net revenues, income, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
109
Item 6.Directors, Senior Management and Employees
6.A.Directors and Executive Officers
The following table sets forth information regarding our directors and executive officers as of the date of this annual report.
Name |
| Age |
| Positions(s) |
Zhou Pengwu* |
| 68 |
| Chairman of the Board of Directors and Chief Executive Officer |
Ding Wenting* |
| 58 |
| Vice-chairwoman of the Board of Directors and Chief Marketing Officer |
Hu Qing |
| 59 |
| Director and Chief Operating Officer |
Zhou Yitao* |
| 44 |
| Director and Chief Medical Technique Officer |
Wei Zhinan Nelson |
| 43 |
| Director |
Yan Hongfei |
| 38 |
| Director |
Cathy Peng |
| 58 |
| Independent Director |
Xue Hongwei |
| 44 |
| Independent Director |
Tsang Eric Chi Wai |
| 70 |
| Independent Director |
Wu Guanhua |
| 41 |
| Chief Financial Officer |
* | Ms. Ding Wenting is the wife of Dr. Zhou Pengwu and Dr. Zhou Yitao is the son of Dr. Zhou Pengwu. |
The following is a biographical summary of the experience of our directors and executive officers.
Dr. Zhou Pengwu is one of our founders and is our chairman of the board of directors and chief executive officer. Dr. Zhou has been a registered plastic surgeon since 1991. He has over 30 years of experience in aesthetic medicine and over 20 years of experience in managing aesthetic medical hospitals, beginning with the establishment of the Shenzhen Pengcheng Clinic in Shenzhen in 1997, which later became the Pengcheng Outpatient Department, then the Shenzhen Pengcheng General Hospital, and finally the Pengcheng Hospital. He acted in a variety of leadership positions in our company, including as the general manager and hospital administrator of the Pengcheng Hospital and Shenzhen Pengai Hospital, as well as chairman and general manager of Shenzhen Pengai Hospital Investment Management Co., Ltd., or Shenzhen Pengai Investment. Dr. Zhou also currently serves as an adjunct professor at the Aesthetic Medical School at Yichun University. In 2012, he was elected as vice-president of the Shenzhen Entrepreneur Association. In 2015, Dr. Zhou was elected as vice-chairman of the First Council of the Internet Aesthetic Medical Subcommittee of Chinese Cosmetic Surgery Association. He is also currently a vice-chairman member of the Fifth Council of the Aesthetic Medical Treatment Subcommittee of the Guangdong Medical Association and vice chairman of the Second Council of the Private Aesthetic Medical Treatment Institution of Chinese Cosmetic Surgery Association. In 2011, Dr. Zhou also participated in the drafting seminar of National Health and Family Planning Commission of the PRC for setting up standards for aesthetic medical treatment institutions. Dr. Zhou obtained an EMBA for hospital administrators from Peking University in October 2005. Dr. Zhou was awarded the “2017 Industry Leader Award” by China Council for the Promotion of International Trade Academy, China Economic Herald and China Business Association (Beijing) Entrepreneur Business Club. Dr. Zhou is the current spouse of Ms. Ding, our vice-chairwoman and the father of Dr. Zhou Yitao, a director of our company.
Ms. Ding Wenting is one of our founders and is currently our vice-chairwoman of the board of directors and chief marketing officer. Ms. Ding is primarily responsible for overall group sales, marketing and procurement. Ms. Ding has been acting in a variety of leadership positions in our company for over 20 years. From January 2002 to December 2005, Ms. Ding served as deputy administrator of Pengcheng Hospital. From March 2006 to December 2010, Ms. Ding served as deputy administrator of Shenzhen Pengai Hospital. Since December 2005, Ms. Ding has consecutively served as deputy general manager, vice-chairman and vice-president of Shenzhen Pengai Investment. Ms. Ding obtained an EMBA for hospital administrators from Peking University in August 2006. In February 2007, Ms. Ding received one-to-one training by Miki Takasaka, a master in the use of colors in Japan. In February 2007, Ms. Ding completed the foundation course in image consultancy offered by First Impressions. In April 2007, Ms. Ding completed the trainings and passed the examinations of the personal total image consulting course, personal color analysis course, personal style analysis course, and the fundamental personal image consultant course at Ximan Color Fashion Training Institute of Beijing Ximan Color Co., Ltd. In June 2007, Ms. Ding completed the course for color matching designers offered by the China Fashion & Color Association. In June 2008, Ms. Ding obtained a certificate in make-up artistry from Christian Chauveau’s Technical School of Artistic Make-up in Paris. In October 2012, Ms. Ding was appointed as the vice-president of the Shenzhen General Chamber of Commerce. Ms. Ding is the current spouse of Dr. Zhou Pengwu, our chairman and chief executive officer.
110
Ms. Hu Qing has served as a director of our company since March 2012. She is our chief operating officer, primarily responsible for the overall operations of our business. Ms. Hu has over 25 years of experience in the medical industry. From January 2004 to December 2005, Ms. Hu served as the deputy president of Shenzhen Pengcheng General Hospital. From December 2005 to December 2013, Ms. Hu consecutively served as the deputy hospital administrator, and general manager of Shenzhen Pengai Hospital. Since 2002, Ms. Hu has served as the vice-president, deputy general manager and director of Shenzhen Pengai Investment. Prior to joining us, Ms. Hu spent more than 13 years working in Japan and served as the director and the Secretary for the Beijing University of Traditional Chinese Medicine, Japanese Branch where she was in charge of daily operations, from April 1991 to March 2004. Ms. Hu received her bachelor’s degree in radio engineering from the South China Engineering School (currently known as the South China University of Technology) in July 1983 and obtained an EMBA for hospital administrators from Peking University in August 2006.
Dr. Zhou Yitao has served as a director of our company since March 2012. Dr. Zhou Yitao currently serves as our chief medical technique officer, primarily responsible for medical quality and safety. He has been the general manager of East China region of Shenzhen Pengai Investment since 2013, primarily responsible for our expansion in the East China region. From July 2012 to December 2013, Dr. Zhou Yitao served as the hospital administrator of Shanghai Nishizhen Aesthetic Medical Clinic Co., Ltd. From June 2010 to June 2012, he served as the hospital administrator of Nanchang Pengai. Since October 2012, he has been serving as the director of Shenzhen Pengai Investment. Dr. Zhou Yitao was designated as our executive director in July 2014. Dr. Zhou Yitao has over 15 years of experience in the medical industry. Prior to joining us, Dr. Zhou Yitao spent two years practicing medicine at the general surgery and plastic surgery departments of Pingxiang People’s Hospital and spent one year attending plastic surgery courses at each of the Orthopedic Hospital of Peking Union Medical College of Chinese Medical Science Institution from June 2004 to June 2005, the plastic surgery department of Shanghai No. 9 Hospital from October 2006 to November 2007, and the Navi Plastic Surgery Clinic in South Korea from March 2010 to September 2010. Dr. Zhou Yitao received his bachelor’s degree in clinical medicine from Hubei Medical University in July 2000 and a master’s degree in health administration from La Trobe University, Melbourne, Australia in October 2009. Dr. Zhou Yitao is the son of Dr. Zhou Pengwu, our chairman and chief executive officer, and Dr. Zhou Pengwu’s former spouse.
Mr. Wei Zhinan Nelson has served as a director of our company since June 2019. He has approximately 14 years of experience in the financial services industry across investment banking and principal investment, and currently covers the Greater China Region for ADV Partners Limited, or ADV. Prior to ADV, Mr. Wei was an executive director and the head of Greater China Region for 3M New Ventures, a separate investment entity of 3M Company (NYSE: MMM), where he led investments and mergers and acquisitions in technology, consumer, industrial, and healthcare sectors from January 2014 to June 2016. Prior to 3M New Ventures, Mr. Wei served as the vice president at Clarity China Partners, a private equity firm, from February 2011 to January 2014. Mr. Wei received his bachelor’s degree in economics from the University of California, Berkeley in May 2004.
Mr. Yan Hongfei has served as a director of our company since December 2016. He has approximately 8 years of experience in the financial services industry across principal investments and portfolio management, and covers the China market for ADV. Prior to ADV, Hongfei was vice president at Deutsche Bank Strategic Investment Group, the principal investment team of Deutsche Bank in China from September 2012 to May 2015. During his tenure with Deutsche Bank, Mr. Yan was responsible for deal sourcing, structuring, execution and post-investment management of both private equity and financing transactions. From October 2010 to July 2012, Mr. Yan was an analyst at KKR Capstone where he conducted post investment management for portfolio companies in consumer, retail, and environmental industries across Asia. Mr. Yan began his career with McKinsey & Company and served as an analyst focusing on providing strategic consulting in China from September 2008 to October 2010. Mr. Yan Hongfei received his master’s degree in biochemical engineering from Tsinghua University and received a double master’s degree in engineering from Ecole Centrale de Lille in June 2008.
Ms. Cathy Peng has served as our independent director since March 2020. Ms. Peng currently is the chief executive officer of ROCS Global Inc., which is an executive consulting firm headquartered in Silicon Valley and specialized in talent recruiting and management, innovation, ecosystem and globalization. Prior to that, she served as a managing partner at IntelliProGroup from September 2015 to September 2017. From January 2007 to March 2017, Ms. Peng served as the chief business development officer at Ethertronics Inc. From October 2003 to January 2007, Ms. Peng served as the general manager at Synocus International, a European management consulting firm. From May 1993 to August 2003, she served in positions such as the director of marketing & channel strategies at Motorola, Inc. As an entrepreneur, Ms. Peng was awarded as one of the Women of M2M at the Connected World Conference in 2014, Business Leader of Color by Chicago United Organization in 2013, and Emerging Executive Leader (Top 100 Under 50) by the Diversity MBA Magazine in 2011. As an educator, Ms. Peng is a guest lecturer and career advisor at Tsinghua University, and she has also delivered training and seminars at leading institutions such as University of Chicago and Stanford University. Ms. Peng obtained her EMBA degree from University of Chicago in the United States in March 2001.
111
Mr. Xue Hongwei has served as our independent director since October 2019. Mr. Xue founded Bybridge Consultancy Pte. Ltd. in 2015 and currently serves as its director. Mr. Xue previously served as a director of Mendis Aesthetics Pte. Ltd. From August 2014 to September 2015, Mr. Xue served as an accountant for Asia-Europe Foundation, a non-profit organization. From July 2013 to December 2013, Mr. Xue worked as a financial analyst at Schneider Electric (EPA:SU). From October 2012 to March 2013, Mr. Xue worked as a financial investment manager at China Sunergy Co., Ltd. (OTCMKTS: CSUNY). From October 2011 to September 2012, Mr. Xue Hongwei worked as an investment manager at China Electric Equipment Group. Mr. Xue is a member of the Association of Chartered Certified Accountants and a Chartered Accountant of Singapore. Mr. Xue received his combined bachelor’s and master’s degree in economics and business administration from Georg-August University of Goettingen, Germany, in August 2005.
Mr. Tsang Eric Chi Wai has served as independent director since October 2019. He also currently serves as the vice-chairman of Hong Kong All Stars Sports Association, a member of the Hong Kong Film Directors’ Guild-Committee; the chairman of Hong Kong Movie Stars Charities Limited, a non-profit organization in Hong Kong that provides assistance to the elderly, and the chairman of Caring For Children Foundation. Mr. Tsang is a Hong Kong actor, film director, producer and television host and has over 40 years of experience in the television and entertainment industry. He starred in Hong Kong films, gaining various acting awards and nominations. Mr. Tsang was awarded the Medal of Honor by the Hong Kong SAR Government in July 2008 and became a member of the 12th Chinese People’s Political Consultative Conference Standing Committee of Jiangmen, Guangdong in December 2011.
Mr. Wu Guanhua joined our company in 2012 and has served as our chief financial officer since August 2018. Mr. Wu Guanhua has over 10 years of experience in international corporate accounting, operations, financial and strategic management. From 2006 to 2008, Mr. Wu Guanhua served as project manager of MAZARS, a global audit, accounting and consulting group. Since March 2008, Mr. Wu Guanhua has served as a partner of Beijing Junyue Investment Consultancy Co., Ltd., a company specializing in consulting services for financing, listing procedures and business development strategies to small and medium enterprise in China. Mr. Wu obtained his bachelor’s degree in science from the University of Oxford Brooks in 2007. Mr. Wu is a certified accountant of The Association of Chartered Certified Accountants since 2007 and became a fellow member in 2012.
6.B.Compensation
Compensation of directors and executive officers
For the year ended December 31, 2022, we paid an aggregate of RMB6.4 million (US$0.9 million) in cash and other benefits to our directors and executive officers. We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our executive officers and directors. Our PRC subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, unemployment insurance and other statutory benefits and a housing provident fund.
For share incentive grants to our officers and directors, see “—6.B. Compensation—Share Incentive Plan.”
Employment agreements and indemnification agreements
We have entered into employment agreements with each of our senior executive officers. Under these agreements, each of our executive officers will be employed for a specified time period, which will be automatically extended unless either we or the executive officer gives prior notice to terminate such employment. We may terminate a senior executive officer’s employment for cause, at any time, without notice or remuneration, for certain acts of the officer, including, but not limited to, a conviction or plea of guilty to a felony, willful misconduct or a failure to perform agreed duties. We may also terminate an executive officer’s employment without cause by a 60-day prior written notice.
Each executive officer has agreed to hold, both during and after the termination or expiry of his or her employment agreement, in strict confidence and not to use, except as required in the performance of his or her duties in connection with the employment or pursuant to applicable law, any of our confidential information or trade secrets or the confidential or proprietary information of any third party received by us and for which we have confidential obligations. The senior executive officers have also agreed to disclose in confidence to us all inventions, designs and trade secrets which they conceive, develop or reduce to practice during the senior executive officer’s employment with us and to assign all right, title and interest in them to us, and assist us in obtaining and enforcing patents, copyrights and other legal rights for these inventions, designs and trade secrets.
112
In addition, each executive officer has agreed to be bound by non-competition and non-solicitation restrictions during the term of his or her employment and for one year following the termination of the employment. Specifically, each senior executive officer has agreed not to (i) approach our suppliers, customers, direct or end customers or contacts or other persons or entities introduced to the senior executive officer in his or her capacity as a representative of us for the purpose of doing business with such persons or entities that will harm our business relationships with these persons or entities; (ii) assume employment with or provide services to any of our competitors, or engage, whether as principal, partner, licensor or otherwise, any of our competitors, without our express consent; or (iii) seek directly or indirectly, to solicit the services of, or hire or engage, any person who is known to be employed or engaged by us; or (iv) otherwise interfere with our business or accounts.
We have entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we agree to indemnify them against certain liabilities and to reimburse them for expenses in connection with claims made by reason of their being a director or officer of our company.
Share Incentive Plan
In June 2019, our shareholders and board of directors approved the Aesthetic Medical International Holdings Group Limited Share Incentive Plan, or the Share Incentive Plan, to attract and retain the best available personnel, provide additional incentives to employees, directors and consultants, and promote the success of our business. The maximum aggregate number of ordinary shares that could be issued pursuant to all awards under the Share Incentive Plan was 5,940,452 shares. As of the date of this annual report, all options under the Share Incentive Plan to purchase ordinary shares have been granted and option to purchase 997,715 ordinary shares are outstanding.
The following paragraphs describe the principal terms of the Share Incentive Plan.
Types of Awards. The Share Incentive Plan permits the awards of options, share appreciation rights, restricted shares or any other type of awards approved by the plan administrator.
Plan Administration. The Share Incentive Plan will be administered by our board of directors, or one or more committees appointed by the board of directors, as the case may be, which will act as the plan administrator. The committee(s) or the full board of directors will determine the matters related to the Share Incentive Plan, including but not limited to: the participants to receive awards, the form, type and number of awards to be granted to each participant, and the terms and conditions of each award grant.
Award Agreement. Awards granted under the Share Incentive Plan are evidenced by an award agreement in writing, approved by the plan administrator, setting forth the terms of an award that has been duly authorized and approved.
Eligibility. We may grant awards to our board members, directors of one of our affiliates, officers, employees, consultants and other eligible persons.
Vesting Schedule. In general, the plan administrator in its sole discretion determines the vesting schedule, which is specified in the relevant award agreement.
Exercise of Options. The plan administrator in its sole discretion determines the exercise price for each award, which is stated in the relevant award agreement.
Transfer Restrictions. Awards may not be transferred in any manner by the participant other than in accordance with the exceptions provided in the Share Incentive Plan or the relevant award agreement or as otherwise determined by the plan administrator, such as transfers back to us, transfers by gift to one or more ‘family members’, transfers by will or the laws of descent and distribution.
Termination and Amendment of the Share Incentive Plan. Unless terminated earlier, the Share Incentive Plan has a term of ten years. Our board of directors or relevant committee appointed by the board of directors has the authority to amend or terminate the plan. However, no such action may adversely affect in any material way any awards previously granted unless agreed by the recipient.
As of the date of this annual report, our directors and executive officers as a group and our other employees as a group held outstanding options to purchase 997,715 ordinary shares of our company, at an exercise price of US$0.001 per share.
113
On June 1, 2019, we issued 5,940,452 ordinary shares to Shengli Family Limited at par value. We established Pengai Employees Trust pursuant to a declaration of trust dated June 17, 2019 among our company, as the settlor, Zedra Trust Company (Cayman) Limited, as the trustee, and Dr. Zhou Pengwu, as the enforcer, for the benefit of the grantees under our Share Incentive Plan. All of the issued shares of Shengli Family Limited have been transferred to Zedra Trust Company (Cayman) Limited as trust fund. Upon satisfaction of vesting conditions and request by relevant grantees, Shengli Family Limited will transfer the ordinary shares underlying the exercised share options to relevant grantees with the consent of the enforcer. Pursuant to the terms of the declaration of trust, the trustee shall not exercise the voting rights attached to those ordinary shares of our Company under its control without first obtaining written consent of the enforcer. Such shares are treated as treasury shares for accounting purposes only.
Performance Incentive Plan
In September 2019, our shareholders and board of directors approved the Aesthetic Medical International Holdings Group Limited 2019 Performance Incentive Plan, or the Performance Incentive Plan, to attract, motivate, retain and reward selected employees and other eligible persons, and to promote the success of our company. The maximum aggregate number of ordinary shares that could be issued pursuant to all awards under the Performance Incentive Plan is a number up to 10% of total issued and outstanding ordinary shares. On July 29, 2022, the Company issued 1,927,793 new ordinary shares to certain employees, representing an aggregate 2.0% of the total number of outstanding ordinary shares, at nil consideration in accordance with the 2019 Performance Incentive Plan proposed in September 2019. The purpose of this share option plan is to recognize contributions made by key employees on the negotiation and execution of the financing with Lafang China Co., Ltd. and Jiechuang, as well as to provide an incentive to retain a long-term relationship with the Company under the alignment of interests.
The following paragraphs describe the principal terms of the Performance Incentive Plan.
Types of Awards. The Performance Incentive Plan permits the awards of options, share appreciation rights, restricted shares, performance shares, share units, restricted share units, similar rights to purchase or acquire shares, cash awards or dividend equivalent rights.
Plan Administration. The Performance Incentive Plan will be administered by our board of directors, or one or more committees or subcommittees appointed by the board of directors, as the case may be, which will act as the plan administrator. The committee(s) or the full board of directors will determine the matters related to the Performance Incentive Plan, including but not limited to: the participants to receive awards, the form, type and number of awards to be granted to each participant, and the terms and conditions of each award grant.
Award Agreement. Awards granted under the Performance Incentive Plan are evidenced by an award agreement in writing, approved by the plan administrator, setting forth the terms of an award that has been duly authorized and approved.
Eligibility. We may grant awards to our board members, directors of one of our affiliates, officers, employees, consultants and other eligible persons.
Vesting Schedule. In general, the plan administrator in its sole discretion determines the vesting schedule, which is specified in the relevant award agreement.
Exercise of Options. The plan administrator in its sole discretion determines the exercise price for each award, which is stated in the relevant award agreement.
Transfer Restrictions. Awards may not be transferred in any manner by the participant other than in accordance with the exceptions provided in the Performance Incentive Plan or the relevant award agreement or as otherwise determined by the plan administrator, such as transfers back to us, transfers by gift to a ‘family member’ or former ‘family member’, transfers by will or the laws of descent and distribution, and transfers to a third party who provide financing under the plan administrator’s authorization for ‘cashless exercise’ procedures.
Termination and Amendment. Unless terminated earlier, the Performance Incentive Plan has a term of ten years. Our board of directors has the authority to amend or terminate the plan. However, no such action may adversely affect in any material way any awards previously granted unless agreed by the recipient.
114
6.C.Board Practices
Board of directors
Our board of directors consists of 9 directors. A director is not required to hold any shares in our company to qualify to serve as a director. Except as required by our corporate governance policies, a director who is in any way, whether directly or indirectly, interested in a contract or proposed contract with the Company shall declare the nature of his interest at a meeting of the directors. A director may vote with respect to any contract or any proposed contract or arrangement notwithstanding that he may be interested therein, and if he does so his vote shall be counted and he may be counted in the quorum at any meeting of our directors at which any such contract or proposed contract or arrangement is considered. Pursuant to our related party transaction policy currently effective, our audit committee, or in the event that any member of the audit committee is a related party in the transaction to be reviewed, any non-conflicted member(s) of our audit committee, shall have the authority to approve proposed related party transactions as defined thereunder, subject to exceptions under certain standing pre-approval. Our directors may exercise all the powers of our company to borrow money, mortgage or charge its undertaking, property and uncalled capital and to issue debentures or other securities whenever money is borrowed or as security for any debt, liability or obligation of our company or of any third party.
None of our directors has a service contract with us that provides for benefits upon termination of service.
Committees of the board of directors
We have three committees under the board of directors: an audit committee, a compensation committee, and a nominating and corporate governance committee. We have adopted a charter for each of the three committees. Each committee’s members and functions are described below.
Audit committee. Our audit committee currently consists of Mr. Xue Hongwei, Mr. Eric Chi Wai Tsang and Ms. Cathy Peng, and is chaired by Ms. Xue Hongwei. Each of them satisfies the “independence” requirements of Rule 5605(a)(2) of the Nasdaq Stock Market Rules and meets the independence standards under Rule 10A-3 under the Exchange Act. Our audit committee consists solely of independent directors that satisfy the Nasdaq and SEC requirements. We have determined that Mr. Xue Hongwei qualifies as an “audit committee financial expert” within the meaning of the SEC rules and possesses financial sophistication within the meaning of the Nasdaq Rules. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
● | selecting the independent registered public accounting firm and pre-approving all auditing and non-auditing services permitted to be performed by the independent registered public accounting firm; |
● | reviewing with the independent registered public accounting firm any audit problems or difficulties and management’s response; |
● | reviewing and approving all proposed related party transactions, as defined in Item 404 of Regulation S-K under the Securities Act; |
● | discussing the annual audited financial statements with management and the independent registered public accounting firm; |
● | reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies; |
● | annually reviewing and reassessing the adequacy of our audit committee charter; |
● | meeting separately and periodically with management and the independent registered public accounting firm; and |
● | reporting regularly to the board. |
115
Compensation committee. Our compensation committee currently consists of Ms. Cathy Peng, Mr. Xue Hongwei and Mr. Eric Chi Wai Tsang, and is chaired by Ms. Cathy Peng. Each of them satisfies the “independence” requirements of Rule 5605(a)(2) of the Nasdaq Stock Market Rules. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated upon. The compensation committee is responsible for, among other things:
● | reviewing the total compensation package for our executive officers and making recommendations to the board with respect to it; |
● | reviewing the compensation of our non-employee directors and making recommendations to the board with respect to it; and |
● | periodically reviewing and approving any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, and employee pension and welfare benefit plans. |
Nominating and corporate governance committee. Our nominating and corporate governance committee concurrently consists of Mr. Zhou Pengwu, Mr. Xue Hongwei and Ms. Cathy Peng, and is chaired by Zhou Pengwu. Mr. Xue and Ms. Peng satisfty the “independence” requirements of Rule 5605(a)(2) of the Nasdaq Stock Market Rules. The nominating and corporate governance committee assists the board in selecting individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things:
● | recommending nominees to the board for election or re-election to the board, or for appointment to fill any vacancy on the board; |
● | reviewing annually with the board the current composition of the board with regards to characteristics such as independence, age, skills, experience and availability of service to us; |
● | selecting and recommending to the board the names of directors to serve as members of the audit committee and the compensation committee, as well as of the nominating and corporate governance committee itself; and |
● | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
116
Appointment of directors by our principal shareholders
According to the Shareholders’ Agreement signed by and among Seefar Global Holdings Limited, Jubilee Set Investments Limited, Zhou Pengwu, Ding Wenting, ADV, Hawyu, Wanda, Jiechuang, the Board of Directors shall be subject to the following arrangements upon Closing (as defined in the Share Purchase Agreement among our company, Wanda, Seefar Global Holdings Limited and Jubilee Set Investments Limited dated 20 July 2022) (the “SPA Closing”).
| (d) | Chairman of the Board: The Board shall have two (2) co-chairmen, one of whom shall be the Executive Chairman who shall be the Director appointed by Wanda and Jiechuang, and the other of whom shall be the Non-executive Chairman and shall be Dr. Zhou Pengwu. (for the avoidance of doubt, Zhou Pengwu (current executive director of the Company) will be designated as a non-executive director of the Company on or about the date of the SPA Closing). The Executive Chairman shall take full charge of the Company’s daily management and business operation, while the Non-executive Chairman shall take charge of cultural affairs such as the dissemination of core values and shall not participate in any actual daily management and business operation of the Company. |
Duties of directors
Under Cayman Islands law, our directors have a fiduciary duty to act honestly in good faith with a view to our best interests. Our directors also have a duty to exercise the care, diligence and skill that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association, as amended and restated from time to time. Our company has the right to seek damages if a duty owed by our directors is breached. In limited exceptional circumstances, a shareholder has the right to seek damages if a duty owed by our directors is breached.
117
Our board of directors has all the powers necessary for managing, and for directing and supervising, our business affairs. The functions and powers of our board of directors include, among others:
● | convening shareholders’ annual and extraordinary general meetings and reporting its work to shareholders at such meetings; |
● | declaring dividends and distributions; |
● | appointing officers and determining the term of office of the officers; |
● | exercising the borrowing powers of our company and mortgaging the property of our company; and |
● | approving the transfer of shares in our company, including the registration of such shares in our share register. |
Terms of directors and executive officers
Pursuant to our fourth amended and restated articles of association (as revised),our directors may be elected by a resolution of our board of directors, or by an ordinary resolution of our shareholders. Our directors are not subject to a term of office and hold office until such time as they are removed from office by ordinary resolution of the shareholders. A director will cease to be a director if, among other things, the director (i) becomes bankrupt or has a receiving order made against him or suspends payment or compounds with his creditors; or (ii) dies or becomes of unsound mind; or (iii) resigns his office by notice in writing to us. Although there is no term limit, pursuant to our corporate governance guidelines, unless our board of directors determines in a particular instance that longer tenure is in the best interests of our company and its shareholders: (i) no director shall be nominated by our board of directors for re-election at an annual shareholders meeting coinciding with or next following his or her 72nd birthday, and (ii) inside directors shall retire on the date of their retirement or termination of employment with our company or its affiliates.
All of our executive officers are appointed by and serve at the discretion of our board of directors.
6.D.Employees
Overview
We had 1,550, 1,451 and 1,167 employees (full-time) in our wholly- or majority-owned medical institutions as of December 31, 2020, 2021 and 2022, respectively. The following table sets forth certain information about our employees by function as of December 31, 2022.
| Number of |
| Percent of total |
| |
Function(1) | Employees* | employees |
| ||
Management(2) |
| 6 |
| 0.3 | % |
Doctors(2) |
| 102 |
| 7.8 | % |
Other Medical Staff(3) |
| 226 |
| 21.1 | % |
Sales and Marketing |
| 118 |
| 10.8 | % |
Image Consultants |
| 193 |
| 17.2 | % |
Customer Service |
| 275 |
| 22.4 | % |
Finance and Accounting |
| 51 |
| 3.6 | % |
Human Resource and Administration |
| 136 |
| 11.9 | % |
Auxiliary Medical Staff(4) |
| 62 |
| 4.9 | % |
Total |
| 1,167 |
| 100 | % |
Notes:
118
Since our inception, we have not experienced any significant turnover of staff or any disruption to our business operations due to labor disputes as our employees are not represented by a labor union. There had been no complaints or claims from employees that materially and adversely affected our business operations during the historical period.
Our employees typically enter into standard employment contracts with us. Recruitment is centralized across our treatment centers based on identified employee needs. Each treatment center then enters into standard employment contracts with its own employees, while the decisions to employ the general manager and finance and accounting directors at each treatment center are made at the headquarters level.
We provide both in-house and external training for our employees to improve their skills and knowledge. Remuneration packages for our employees mainly comprise base salary, overtime allowance (if applicable) and a performance-related bonus. We set performance targets for our employees primarily based on their position and department and annually review their performance. The results of such reviews are used in their salary determinations, bonus awards, and promotion appraisals.
Qualified medical professionals
The qualification and expertise of doctors are vital to the quality of our healthcare services and our competitiveness. As of December 31, 2022, all of our 126 doctors (full-time and part-time) had obtained Doctor Qualification Certificates, and all our nursing staff have obtained Nurse Practice Certificates.
We place great emphasis on recruiting, training and retaining our employees. We maintain a high standard in selecting quality medical professionals and provide competitive compensation packages to them. Peer referral represents an important recruitment channel for us as a significant proportion of our doctors are sourced through this channel, indicative of our strong branding and reputation in the medical community. Our full-time doctors, who work for us exclusively during the term of their employment, are typically employed under three to five year employment contracts. We also have doctors who are contractors and not our employees. As part of ongoing development, we provide periodic training to our employees to enhance their skills and knowledge. Our administrative department regularly reviews the profiles of our doctors and prompts them to apply for their next professional rank when they become eligible. Our medical professionals are assigned to various departments in our treatment centers based on the scope of their licenses. Medical professionals are not allowed to practice in departments other than their designated department. The medical service department of each treatment center reviews the prescriptions issued by doctors and inspects each department to ensure no medical professionals practice beyond the scope of their licenses. As of the date of this annual report, there have been no material complaints or penalties in relation to our medical professionals practicing beyond the scope of their respective licenses.
As of December 31, 2022, our team of 126 doctors (including full-time and part-time) comprised 2 chief physicians, 28 associate-chief physicians, 49 attending physicians, 47 resident physicians in China. The majority of our doctors worked exclusively at our treatment centers while 24 of our doctors split their practice with other hospitals as of December 31, 2022. Our doctors typically receive compensation in the form of a salary plus bonus based on the number of procedures performed. Our doctors were supported by 224 other medical staff, including nurses and pharmacists, as of December 31, 2022.
6.E.Share Ownership
The following table sets forth information concerning the beneficial ownership of our ordinary shares as of December 31, 2022 by:
● | each of our directors and executive officers; and |
● | each person known to us to beneficially own 5% or more of our ordinary shares. |
119
The calculations in the table below are based on 130,582,310 ordinary shares outstanding as of the date of this annual report. Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
Ordinary Shares |
| ||||
Beneficially Owned |
| ||||
| Number |
| Percent |
| |
Executive officers and directors*: | |||||
Zhou Pengwu(1) |
| 33,549,180 |
| 25.7 | % |
Ding Wenting(2) |
| 36,119,572 |
| 25.7 | % |
Hu Qing(3) |
| 1,142,984 |
| 0.9 | % |
Zhou Yitao(3) |
| 849,000 |
| 0.7 | % |
Wei Zhinan Nelson(4) |
| — |
| — | |
Yan Hongfei(4) |
| — |
| — | |
Peng Cathy |
| — |
| — | |
Xue Hongwei |
| — |
| — | |
Ordinary Shares |
| ||||
Beneficially Owned |
| ||||
| Number |
| Percent |
| |
Tsang Eric Chi Wai |
| - |
| — | |
Wu Guanhua(3) |
| 1,491,597 |
| 1.1 | % |
All executive officers and directors as a group |
| 39,603,153 |
| 28.4 | % |
Principal shareholders: |
|
|
|
| |
MY Universe (HK) Limited(5) | 36,402,570 | 27.9 | % | ||
Hawyu (HK) Limited(6) | 21,413,276 | 16.4 | % | ||
Seefar Global Holdings Limited(7) |
| 14,837,711 |
| 11.4 | % |
Peak Asia Investment Holdings V Limited(4) |
| 15,576,960 |
| 11.9 | % |
Jubilee Set Investments Limited(2) |
| 15,490,692 |
| 11.9 | % |
Shengli Family Limited(8) |
| 1,285,198 |
| 1.0 | % |
Pengai Hospital Management Corporation(9) |
| 3,220,717 |
| 2.5 | % |
* | The business address of all directors and officers is 1122 Nanshan Boulevard, Nanshan District, Shenzhen, Guangdong Province, China. |
| (1) | Represents (i) 3,220,717 ordinary shares held by Pengai Hospital Management Corporation, a company incorporated in the British Virgin Islands, or BVI, which is owned by Dr. Zhou Pengwu and Ms. Ding Wenting as to 51.0% and 49.0%, respectively, as further disclosed in note (9) below; (ii) 14,837,771 ordinary shares held by Seefar Global Holdings Limited as further disclosed in note (7) below; (iii) 15,490,692 ordinary shares held by Jubilee Set Investments Limited as further disclosed in note (2) below, and (iv) 1,285,198 ordinary shares held by Shengli Family Limited as further disclosed in note (6) below. Dr. Zhou Pengwu and Ms. Ding Wenting are spouse and each of Dr. Zhou Pengwu and Ms. Ding Wenting may be deemed to have the beneficial ownership of the shares held by each other. |
| (2) | Represents 15,490,692 ordinary shares held by Jubilee Set Investments Limited. Jubilee Set Investments Limited is a company incorporated in the BVI, which is indirectly wholly-owned by TMF (Cayman) Ltd. acting as the trustee of the Wendy Trust. The Wendy Trust is a discretionary trust established by Ms. Ding Wenting as a settlor and its beneficiaries are Ms. Ding Wenting and her family members. Ms. Ding Wenting, as well as Dr. Zhou Pengwu, spouse of Ms. Ding and our chairman and chief executive officer, are deemed to have the beneficial ownership of the shares held by Jubilee Set Investments Limited. The registered address of Jubilee Set Investments Limited is Vistra Corporate Services Centre, Wickham’s Cay II, Road Town, Tortola, VG1110, BVI. |
| (3) | Represents shares that the person has the right to acquire within 60 days through exercise of share options. |
See “-6.B. Compensation-Share Incentive Plan” and “-Performance Incentive Plan” for a description of equity-based awards we have granted and may grant to our directors, officers and other individuals as a group.
120
| (4) | Represents 15,576,960 ordinary shares held by Peak Asia Investment Holdings V Limited. Peak Asia Investment Holdings V Limited is a company limited by shares and registered in the BVI. Peak Asia Investment Holdings V Limited is wholly owned by Beacon Technology Investment Holdings Limited, a company registered in Hong Kong, which in turn is wholly owned by ADV Opportunities Fund I, L.P., a Cayman exempted limited partnership. The general partner of ADV Opportunities Fund I, L.P. is ADV Opportunities Fund I, GP, L.P., a Cayman exempted limited partnership whose general partner is ADV Opportunities Fund I GP Ltd, a Cayman exempted company, which is wholly owned by ADV Partners Holdings Ltd, a Cayman exempted company. ADV Partners Holdings Ltd. is in turn wholly owned by Mr. Bradley Dean Landes, Mr. Suresh Eshwara Prabhala and Mr. Zhu Jianyi (Kenichi Shu). The registered address of Peak Asia Investment Holdings V Limited is Flemming House, Wickhams Cay, P.O. Box 662, Road Town, Tortola, BVI. Each of Mr. Wei Zhinan Nelson and Mr. Yan Hongfei was appointed as our director by ADV pursuant to our memorandum and articles of association then in effect before the completion of our initial public offering. For the purpose of this section, none of Mr. Wei Zhinan Nelson and Mr. Yan Hongfei is regarded as a beneficial owner of the shares held by ADV. |
| (5) | Represents 36,402,570 ordinary shares held by MY Universe. MY Universe is a company incorporated in Hong Kong, which is wholly owned by Jiechuang. Jiechuang is a company incorporated in Hainan Province, China, which is invested and managed by two general partners as to Shenzhen Lafang Investment Management Co., Ltd. and Shenzhen Venture Capital M&A Fund Management (Shenzhen) Co., Ltd. |
| (6) | Represents 21,413,276 ordinary shares held by Hawyu (HK) Limited. Hawyu (HK) Limited is a company incorporated in Hong Kong, which is wholly owned by Hainan Runming Biotechnology Co., Ltd. Hainan Runming Biotechnology Co., Ltd. is a company incorporated in Hainan Province, China, which is wholly owned by Lafang China Co., Ltd, a Chinese listed company incorporated in Guangdong Province,China. |
| (7) | Represents 14,837,771 ordinary shares held by Seefar Global Holdings Limited. Seefar Global Holdings Limited is a company incorporated in the BVI, which is indirectly wholly-owned by TMF (Cayman) Ltd. acting as the trustee of the Wan Shengda Trust. The Wan Shengda Trust is a discretionary trust established by Dr. Zhou Pengwu as a settlor and its beneficiaries are Dr. Zhou and his family members. Dr. Zhou Pengwu, as well as Ms. Ding Wenting, spouse of Dr. Zhou and our vice-chairwoman and chief marketing officer, are deemed to have the beneficial ownership of the shares held by Seefar Global Holdings Limited. Dr. Zhou Pengwu is entitled to exercise the voting power attached to our ordinary shares held by Seefar Global Holdings Limited. The registered address of Seefar Global Holdings Limited is Vistra Corporate Services Centre, Wickham’s Cay II, Road Town, Tortola, VG1110, BVI. |
| (8) | Represents 1,285,198 ordinary shares held by Shengli Family Limited, a company incorporated in the BVI, which is wholly owned by Zedra Trust Company (Cayman) Limited, the trustee of Pengai Employees Trust. We established Pengai Employees Trust pursuant to a declaration of trust dated June 17, 2019 among our company, as the settlor, Zedra Trust Company (Cayman) Limited, as the trustee, and Dr. Zhou Pengwu, as the enforcer, for the benefit of the grantees under our Share Incentive Plan. Upon satisfaction of vesting conditions and request by relevant grantees, Shengli Family Limited will transfer the ordinary shares underlying the exercised share options to relevant grantees with the consent of the enforcer. In addition, the voting rights attached to those ordinary shares held by Shengli Family Limited cannot be exercised without written consent of Dr. Zhou Pengwu. For details, see “—6.B. Compensation of Directors and Executive Officers—Share Incentive Plan.” The registered address of Shengli Family Limited is Sertus Incorporations (BVI) Limited, Sertus Chambers, P.O. Box 905, Quastisky Building, Road Town, Tortola, BVI. |
| (9) | Represents 3,220,717 ordinary shares held by Pengai Hospital Management Corporation, a company incorporated in the BVI. Pengai Hospital Management Corporation is owned by Dr. Zhou Pengwu and Ms. Ding Wenting, spouse of Dr. Zhou Pengwu, as to 51.0% and 49.0%, respectively. Dr. Zhou Pengwu is the sole director of Pengai Hospital Management Corporation, and has the sole power to exercise the voting and dispositive power over all of the Ordinary Shares held by Pengai Hospital Management Corporation. The registered address of Pengai Hospital Management Corporation is Intershore Chambers, P.O. Box 4342, Road Town, Tortola, BVI. |
Item 7.Major Shareholders and Related Party Transactions
7.A.Major Shareholders
Please refer to “Item 6. Directors, Senior Management and Employees—6.E. Share Ownership.”
121
7.B.Related Party Transactions
Agreements and transactions with investors
Convertible note
As at December 31, 2022 and the date of this annual report, the convertible note outstanding was RMB64.6 million. For details, see “Item 5. Operating and Financial Review and Prospects—5.A. Operating Results—Indebtedness—Convertible note.”
Exchangeable notes
As at December 31, 2022 and the date of this annual report, there was no exchangeable note outstanding. For details, see “Item 5. Operating and Financial Review and Prospects—5.A. Operating Results—Indebtedness—Exchangeable notes.”
Shareholder and note holder agreement
On November 15, 2018, we entered into an amended and restated shareholder and note holder agreement, with certain of our investors. Except for the registration rights, all the rights under the shareholder and note holder agreement terminated upon the completion of our initial public offering.
Pursuant to the shareholder and note holder agreement, we have granted certain registration rights to our investors. Such registration rights would terminate upon the earlier of (i) five years following the consummation of our initial public offering or (ii) such time at which all registrable securities held by a shareholder proposed to be sold may be sold under Rule 144 of the Securities Act in any 90-day period without registration in compliance with Rule 144 of the Securities Act. Set forth below is a description of the registration rights granted under the agreement. Set forth below is a description of the registration rights granted under the agreement.
Demand registration rights
At any time after the earlier of (i) December 8, 2020 or (ii) the date 12 months following the an initial public offering, upon a written request from the holders of at least 50% of the voting power of the registrable securities, we must file a registration statement covering the offer and sale of the registrable securities held by the requesting holders (a) representing at least twenty percent (20%) of the then outstanding registrable securities held by such holders, or (b) having an anticipated aggregate offering price, net of underwriting discounts and commissions, in excess of US$5,000,000, on any internationally recognized exchange that is reasonably acceptable to such requesting holders.
Registrable securities include, among others, our ordinary shares from the automatic conversion and re-designation of the preferred shares.
We are not obligated to effect a registration, among other things, if we have, within the 12-month period preceding the date of the request, already effected two registrations under the Securities. We have the right to defer filing of a registration statement for up to 90 days if our board of directors determines in good faith that the filing of a registration statement would be materially detrimental to us and our shareholders, but we cannot exercise the deferral right for more than 90 days on any one occasion or more than once in any 12-month period.
F-3 or S-3 registration rights
When we are eligible for registration on Form F-3 or S-3, upon a written request from any holder all registrable securities, we must effect a registration on Form F-3 or S-3 and any related qualification or compliance covering the offer and sale of the registrable securities.
122
We are not obligated to effect a Form F-3 or S-3 registration, among other things, if we have, within the 12-month period preceding the date of the request, already effected two registrations under the Securities Act or if the holders of registrable securities proposed to sell at an aggregate price to the public less than US$500,000. We have the right to defer filing of a registration statement for up to 90 days if our board of directors determines in good faith that the filing of a registration statement would be materially detrimental to us and our shareholders, but we cannot exercise the deferral right for more than 90 days on any one occasion or more than once in any 12-month period.
Piggyback registration rights
If we propose to file a registration statement under the Securities Act for purposes of effecting a public offering of our securities (including, but not limited to, registration statements relating to secondary offerings of our securities, but excluding registration statements relating to any employee benefit plan or a corporate reorganization), we must afford holders of registrable securities an opportunity to include in that registration all or any part of their registrable securities then held. We have the right to terminate or withdraw any registration initiated by us under the piggyback registration rights prior to the effectiveness of such registration whether or not any holder has elected to include securities in such registration. The underwriters of any underwritten offering have the right to limit the number of shares with registration rights to be included in the registration statement, subject to certain limitations.
Expenses of registration
We will pay all expenses relating to any demand, Form F-3 or S-3, or piggyback registration except for the underwriting discounts and selling commissions applicable to the sale of registrable securities and certain other limited exceptions.
Contractual Arrangements with respect to Target Equity Interests
See “Item 4. Information on the Company—4.C. Organizational Structure—Contractual Arrangements with respect to Target Equity Interests.”
Corporate guarantee
In 2018, our bank borrowings in total amount of RMB24.0 million (US$3.5 million) were guaranteed by our subsidiary and our related parties and secured by some of their properties and/or our properties.
As at December 31, 2022, the outstanding bank borrowing and other borrowings guaranteed by our related parties and secured by some of their properties and/or our properties was RMB201.9 million (US$29.3 million).
Lease agreement
In November 2017, Pengcheng Hospital entered into a lease agreement with Shenzhen Jiayan Investment Industrial Development Co., Ltd. or Shenzhen Jiayan, which is wholly owned by our directors, Dr. Zhou Pengwu and Ms. Ding Wenting. Pursuant to the lease agreement, Pengcheng Hospital will pay Shenzhen Jiayan a monthly rent of RMB188,521. The lease agreement expires in October 2022.
Loans to directors and executive officers
In 2018, we provided loans to certain of our directors and executive officers. The loans are interest-free and payable on demand. As of December 31, 2022 and the date of this annual report, there was no outstanding loan provided by us to our directors and executive officers and the balance due from our directors and executive officers were nil and nil, respectively.
Employment agreements and indemnification agreements
See “Item 6. Directors, Senior Management and Employees—6.B. Compensation—Employment agreements and indemnification agreements.”
123
Equity incentive plans
See “Item 6. Directors, Senior Management and Employees—6.B. Compensation—Share Incentive Plan” and “—Performance Incentive Plan” for a description of share options and restricted share units we have granted and may grant to our directors, officers and other individuals as a group.
7.C. Interests of Experts and Counsel
Not applicable.
Item 8.Financial Information
8.A.Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
Legal proceedings
As an aesthetic medical service and general healthcare service provider in China, we are subject to legal or arbitration proceedings, disputes or claims in the ordinary course of business. Except for those disclosed in “Item 3. Key Information—3.D. Risk Factors—Risks relating to our business and our industry—We are subject to customer complaints, claims and legal proceedings in the regular course of our operations from time to time, which could result in significant costs and materially and adversely affect our brand image, reputation and results of operations,” each of these proceedings had been settled as of the date of this annual report or is, in our view, immaterial in terms of their impact on our financial and operational conditions.
Save as disclosed above and based on the information available to us, we are not, as of the date of this annual report, engaged in any litigation, arbitration or claim of material importance, and no litigation, arbitration of claim of material importance is known to us to be pending or threatened by or against us that may have a material adverse effect on our business, financial condition and operating results.
Dividend Policy
We have not previously declared or paid cash dividends and we have no plan to declare or pay any dividends in the near future on our shares or the ADSs representing our ordinary shares. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
We are a holding company incorporated in the Cayman Islands. We rely principally on dividends from our PRC subsidiaries for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us. Dividend distributions from our PRC subsidiary to us are subject to PRC taxes, including withholding tax. In addition, regulations in the PRC currently permit payment of dividends of a PRC company only out of accumulated distributable after-tax profits as determined in accordance with its articles of association and the accounting standards and regulations in China. For more details, see “Item 4. Information of the Company—4.B. Business Overview—Regulation” and “Item 3. Key Information—3.D. Risk Factors—Risks relating to doing business in the PRC.”
Our board of directors has discretion as to whether to distribute dividends, subject to certain requirements of Cayman Islands law. In addition, subject to the provisions in our articles of association, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our board of directors. Under Cayman Islands law, a Cayman Islands company may pay a dividend out of either profit or share premium account, provided that in no circumstances may a dividend be paid if this would result in the company being unable to pay its debts as they fall due in the ordinary course of business. Even if our Board of Directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. If we pay any dividends on our ordinary shares, we will pay those dividends which are payable in respect of the ordinary shares represented by the ADSs to the depositary, as the registered holder of such ordinary shares, and the depositary then will pay such amounts to the ADS holders in proportion to the ordinary shares represented by the ADSs held by such ADS holders, subject to the terms of the deposit agreement, including the fees and expenses payable thereunder.
124
8.B. Significant Changes
Except as otherwise disclosed in this report, we have not experienced any significant changes since the date of the annual financial statements included herein.
Item 9. The Offer and Listing
9.A. Offering and Listing Details
Our ADSs, each representing three of our ordinary shares, par value US$0.001 per share, were listed on the Nasdaq Global Market under the symbol “AIH” from October 25, 2019 to January 19, 2023.
On June 2, 2022, we received a staff determination letter from the Nasdaq notifying us that, based on our stockholders’ equity as reported in our annual report for the year ended December 31, 2021, we did not meet the minimum stockholders’ equity requirement for continued listing on the Nasdaq Global Market under Nasdaq Listing Rule 5450(b)(1)(A) (the “Rule”). As provided in the Rule, we have 45 calendar days to submit a play to regain compliance with the minimum stockholders’ equity requirement. On December 1, 2022, we received a staff determination letter from the Nasdaq notifying us that because we have not regained compliance with the Rule, unless we request an appeal of this determination, our ADSs would be scheduled for delisting from the Nasdaq Global Market. We requested a hearing before the Nasdaq Hearings Panel and subsequently submitted an application to transfer the listing of our ADSs from the Nasdaq Global Market to the Nasdaq Capital Market. The Listing Qualifications of Nasdaq approved our request to the Transfer Listing on January 17, 2023. Subsequently, our ADSs have been listed on the Nasdaq Capital Market uninterruptedly under the symbol “AIH” since January 19, 2023.
9.B. Plan of Distribution
Not applicable.
9.C. Markets
See “—9.A. Offering and Listing Details.”
9.D. Selling Shareholders
Not applicable.
9.E. Dilution
Not applicable.
9.F. Expenses of the Issue
Not applicable.
Item 10.Additional Information
10.A.Share Capital
Not applicable.
10.B.Memorandum and Articles of Association
We are a Cayman Islands company and our affairs are governed by our fourth amended and restated memorandum and articles of association, as amended from time to time and the Companies Act (As Revised) of the Cayman Islands, which we refer to as the “Companies Act” below, and the common law of the Cayman Islands.
125
We incorporate by reference into this annual report our fourth amended and restated memorandum and articles of association, which was filed as Exhibit 3.2 to our registration statement on Form F-1 (File Number 333-234022) initially filed with the Securities and Exchange Commission on September 30, 2019 and declared effective on October 24, 2019. Our shareholders adopted our fourth amended and restated memorandum and articles of association by way of a special resolution passed on October 15, 2019, which became effective immediately prior to completion of our initial public offering of ADSs representing our ordinary shares, i.e., on October 29, 2019. The fourth amended and restated memorandum and articles of association was further amended by way of a special resolution passed at the annual general meeting of the Company held on 22 September 2022.
The following are summaries of material provisions of our fourth amended and restated memorandum and articles of association (as revised) and the Companies Act as they relate to the material terms of our ordinary shares.
Registered Office and Objects
Our registered office in the Cayman Islands is at the offices of Vistra (Cayman) Limited, P. O. Box 31119 Grand Pavilion, Hibiscus Way, 802 West Bay Road, Grand Cayman, KY1 - 1205 Cayman Islands.
According to clause 3 of our fourth amended and restated memorandum of association, the objects for which we are established are unrestricted and we shall have full power and authority to carry out any object not prohibited by the Companies Act or as the same may be revised from time to time, or any other law of the Cayman Islands.
Board of Directors
See “Item 6. Directors, Senior Management and Employees.”
Ordinary Shares
General
All of our outstanding ordinary shares are fully paid and non-assessable. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their ordinary shares. Under the Companies Act, we are not permitted to issue bearer shares. Our fourth amended and restated memorandum and articles prohibit us from issuing negotiable shares. Our company will issue only non-negotiable shares in registered form, which will be issued when registered in our register of members.
Dividends
Our board of directors may, from time to time, declare dividends on the shares subject to our fourth amended and restated memorandum and articles of association (as revised) and the Companies Act. The holders of our ordinary shares are entitled to receive such dividends. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our directors. No dividend may be declared and paid unless our directors determine that, immediately after the payment, we will be able to pay our debts as they fall due in the ordinary course of business and we have funds lawfully available for such purpose. Under Cayman Islands law, payment of the dividends may be made out of the following:
● | profits, realized or unrealized, or any reserve set aside from profits; |
● | “share premium account,” which represents the excess of the price paid to our company on issue of its shares over the par or “nominal” value of those shares, which is similar to the U.S. concept of additional paid in capital; or |
● | any other fund or account which can be authorized for this purposes in accordance with the Companies Act. |
However, no dividend shall bear interest against our company.
126
Voting rights
Holders of our ordinary shares may vote on all matters submitted to a vote of our shareholders, except as may otherwise be required by law. At any general meeting a resolution put to the vote of the meeting shall be decided on a show of hands unless voting by poll is required by Nasdaq rules or demanded by the chairman of the meeting or by one or more shareholder(s) at such general meeting.
Any ordinary resolution to be made by the shareholders requires the affirmative vote of a simple majority of the votes of the ordinary shares cast in a general meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes of the ordinary shares cast.
Under Cayman Islands law, some matters, such as amending the memorandum and articles of association, changing the name or resolving to be registered by way of continuation in a jurisdiction outside the Cayman Islands, require approval of shareholders by a special resolution.
No person will be entitled to vote at any general meeting or at any separate meeting of the holders of the ordinary shares unless the person is registered as of the record date for such meeting and unless all calls or other sums presently payable by the person in respect of ordinary shares in our company have been paid.
Transfer of ordinary shares
Subject to the restrictions contained in our fourth amended and restated articles of association (as revised), any of our shareholders may transfer all or any of his or her ordinary shares by an instrument of transfer in the usual or common form or any other form approved by our board of directors.
Our board of directors may, in its absolute discretion, decline to register any transfer of any ordinary share which is not fully paid up, or which is issued under any share incentive scheme for employees upon which a restriction on transfer imposed thereby still subsists, or on which we have a lien. Our board of directors may also decline to register any transfer of any ordinary share unless:
● | the instrument of transfer is lodged with us, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; |
● | the instrument of transfer is in respect of only one class of ordinary shares; |
● | the instrument of transfer is properly stamped, if required; |
● | in the case of a transfer to joint holders, the number of joint holders to whom the ordinary share is to be transferred does not exceed four; and |
● | a fee of such maximum sum as Nasdaq may determine to be payable or such lesser sum as our directors may from time to time require is paid to us in respect thereof. |
If our directors refuse to register a transfer, they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal.
The registration of transfers may, after compliance with any notice required of the Nasdaq, be suspended and the register of members closed at such times and for such periods as our board of directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register of members closed for more than 30 days in any year as our board may determine.
127
Winding up; liquidation
Pursuant to our fourth amended and restated articles of association (as revised), if our company shall be wound up the liquidator may, with the sanction of a special resolution of our company, divide amongst the shareholders in specie or kind the whole or any part of the assets of our company (whether they shall consist of property of the same kind or not) and may for such purpose set such value as he deems fair upon any property to be divided as aforesaid and may determine how such division shall be carried out as between the shareholders or different classes of shareholders. The liquidator may, with the like sanction, vest the whole or any part of such assets in trustees upon such trusts for the benefit of the contributories as the liquidator, with the like sanction shall think fit, but so that no shareholder shall be compelled to accept any shares or other securities whereon there is any liability.
Calls on ordinary shares and forfeiture of ordinary shares
Our board of directors may from time to time make calls upon shareholders for any amounts unpaid on their ordinary shares in a notice served to such shareholders at least 14 days prior to the specified time and place of payment. Any ordinary shares that have been called upon and remain unpaid are subject to forfeiture.
Repurchase, redemption and surrender of ordinary shares
The Companies Act and our fourth amended and restated articles of association (as revised) permit us to purchase our own shares. In accordance with our fourth amended and restated articles of association (as revised) and provided the necessary shareholders or board approval have been obtained, we may issue shares on terms that are subject to redemption, at our option or at the option of the holders of these shares, on such terms and in such manner, including out of capital, as may be determined by our board of directors. Under the Companies Act, the redemption or repurchase of any share may be paid out of our company’s profits or out of the proceeds of a fresh issue of shares made for the purpose of such redemption or repurchase, or out of capital (including share premium account and capital redemption reserve) if the company can, immediately following such payment, pay its debts as they fall due in the ordinary course of business. In addition, under the Companies Act no such share may be redeemed or repurchased (a) unless it is fully paid up, (b) if such redemption or repurchase would result in there being no shares outstanding, or (c) if the company has commenced liquidation. In addition, our company may accept the surrender of any fully paid share for no consideration.
Variations of rights of shares
All or any of the special rights attached to any class of shares may, subject to the provisions of the Companies Act, be varied or abrogated with the consent in writing of at least two thirds of the shares of that class or with the sanction of a special resolution passed at a general meeting of the holders of the shares of that class. The rights conferred upon the holders of the shares of any class issued shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such existing class of shares.
General meetings of shareholders
Shareholders’ meetings may be convened by a majority of our board of directors or our chairman or any director where required to do so pursuant to a requisition by one or more shareholders holding at the date of deposit of the requisition shares which carry in aggregate not less than 40% of all votes attaching to the issued and outstanding shares that carry the right to vote at general meetings. Advance notice of not less than seven calender days is required for the convening of our annual general shareholders’ meeting and any other general meeting of our shareholders. A quorum required for and throughout a meeting of shareholders consists of at least one shareholder entitled to vote and present in person or by proxy or (in the case of a shareholder being a corporation) by its duly authorized representative representing not less than one-third of our total number of shares in issue.
Inspection of books and records
The notice of registered office is a matter of public record. A list of the names of the current directors and alternate directors (if applicable) are made available by the Registrar of Companies in the Cayman Islands for inspection by any person on payment of a fee. The register of mortgages is open to inspection by creditors and members.
128
Holders of our ordinary shares have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we will provide our shareholders with annual audited financial statements. See “Where You Can Find More Information.”
No preemptive rights
Holders of ordinary shares will have no preemptive or preferential right to purchase any securities of our company under our fourth amended and restated articles of association (as revised) and the Companies Act.
Changes in capital
We may from time to time by ordinary resolution:
● | increase the share capital by such sum, to be divided into shares of such classes and amount, as the resolution shall prescribe; |
● | consolidate and divide all or any of our share capital into shares of a larger amount than our existing shares; |
● | sub-divide our existing shares, or any of them into shares of a smaller amount; or |
● | cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person and diminish the amount of our share capital by the amount of the shares so canceled. |
We may by special resolution, subject to any confirmation or consent required by the Companies Act, reduce our share capital or any capital redemption reserve in any manner permitted by law.
Exempted company
We are an exempted company with limited liability under the Companies Act. The Companies Act distinguishes between ordinary resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary company except that an exempted company:
● | does not have to file an annual return of its shareholders with the Registrar of Companies; |
● | is not required to open its register of members for inspection; |
● | does not have to hold an annual general meeting; |
● | may issue shares with no par value; |
● | may obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the first instance); |
● | may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands; |
● | may register as a limited duration company; and |
● | may register as a segregated portfolio company. |
“Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on the shares of the company.
129
Reserved matters
To the fullest extent permitted by law but in addition to such other requirements as may be provided in our fourth amended and restated articles of association (as revised) and the applicable laws, notwithstanding anything therein to the contrary, in no event shall the following actions be taken, or otherwise permitted, by or on behalf of us or any of our subsidiaries, unless and until approved by an ordinary resolution, or, if required by the Companies Act, a special resolution:
| ● | any voluntary delisting of our securities; |
| ● | a Change of Control Event (as defined in our fourth amended and restated articles of association ( as revised ) ); and |
| ● | any transfer or other disposal of any assets, businesses or securities of any of us and our subsidiaries, which are (i) outside the ordinary course of business of our relevant subsidiary; or (ii) which involve the transfer or disposal of 50% or greater of our and our subsidiaries’ consolidated assets or accounted for 50% or greater of our and our subsidiaries’ consolidated revenue, each as presented in our latest audited consolidated financial statements. |
10.C.Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report during the two years immediately preceding the date of this annual report.
10.D.Exchange Controls
Substantially all of our operations are in PRC and our business is subject to supervision and regulation by the PRC government. For PRC regulations that may affect the import or export of capital and the remittance of dividends, interest or other payments to non-PRC resident holders of our ordinary shares and ADSs, see “Item 4. Information on the Company—4.B. Business Overview—Regulation— Regulations related to foreign exchange in the PRC” and “—Regulations related to taxation in the PRC.”
10.E.Taxation
The following summary of Cayman Islands, PRC and U.S. federal income tax consequences of an investment in the ADSs or ordinary shares is based upon laws and relevant interpretations thereof in effect as of the date of this annual report, all of which are subject to change. This summary does not deal with all possible tax consequences relating to an investment in the ADSs or ordinary shares, such as the tax consequences under state, local and other tax laws, or tax laws of jurisdictions other than the Cayman Islands, the PRC and the United States. To the extent that the discussion relates to matters of PRC tax law, it represents the opinion of Han Kun Law Offices, our counsel as to PRC law.
Cayman Islands taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes levied by the government of the Cayman Islands that are likely to be material to holders of ordinary shares except for stamp duties which may be applicable on instruments executed in, or after execution brought within the jurisdiction of the Cayman Islands. No stamp duty is payable in the Cayman Islands on transfers of shares of Cayman Islands companies except those which hold interests in land in the Cayman Islands. The Cayman Islands is party to a double tax treaty with the United Kingdom in 2010 but is otherwise is not party to any double tax treaties which are applicable to any payments made by or to our company. There are no exchange control regulations or currency restrictions in the Cayman Islands.
Payments of dividends and capital in respect of our ordinary shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of our ordinary shares, nor will gains derived from the disposal of our ordinary shares be subject to Cayman Islands income or corporation tax.
130
Pursuant to Section 6 of the Tax Concessions Act (2011 Revision) of the Cayman Islands, we have obtained an undertaking from the Governor in Cabinet:
(1) | that no law which is enacted in the Cayman Islands imposing any tax to be levied on profits, income, gains or appreciations shall apply to us or our operations; and |
(2) | that the aforesaid tax or any tax in the nature of estate duty or inheritance tax shall not be payable on our shares debenture or other obligations. |
The undertaking for us is for a period of twenty years from July 29, 2014.
People’s Republic of China taxation
We are a holding company incorporated in the Cayman Islands. Under the EIT Law and its implementation rules, an enterprise established outside of Mainland China with a “de facto management body” within Mainland China is considered a “resident enterprise,” and will be subject to the enterprise income tax on its global income at the rate of 25%. The implementation rules define the term “de facto management body” as the body that exercises full and substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise. In 2009, the State Administration of Taxation issued SAT Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore is located in Mainland China. Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect the State Administration of Taxation’s general position on how the “de facto management body” text should be applied in determining the tax resident status of all offshore enterprises. According to SAT Circular 82, all offshore enterprises controlled by a PRC enterprise or a PRC enterprise will be regarded as a PRC tax resident by virtue of having its “de facto management body” in Mainland China only if all of the following conditions are met:
(i) | the primary location of the day-to-day operational management is in the PRC; |
(ii) | decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in the PRC; |
(iii) | the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder resolutions, are located or maintained in Mainland China; and |
(iv) | at least 50% of voting board members or senior executives habitually reside in Mainland China. |
We believe that none of Aesthetic Medical International Holdings Group Limited and its subsidiaries outside of Mainland China is a PRC resident enterprise for PRC tax purposes. Aesthetic Medical International Holdings Group Limited is not controlled by a PRC enterprise or PRC enterprise group, and we do not believe that Aesthetic Medical International Holdings Group Limited meets all of the conditions above. Aesthetic Medical International Holdings Group Limited is a company incorporated outside Mainland China. As a holding company, some of its key assets are located, and its records (including the resolutions of its board of directors and the resolutions of its shareholders) are maintained, outside Mainland China. For the same reasons, we believe our other subsidiaries outside of Mainland China are also not PRC resident enterprises. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.”
131
If the PRC tax authorities determine that Aesthetic Medical International Holdings Group Limited is a PRC resident enterprise for EIT purposes, we may be required to withhold tax at a rate of 10% on dividends we pay to our shareholders, including holders of the ADSs, that are non-resident enterprises. In addition, non-resident enterprise shareholders (including the ADS holders) may be subject to a 10% PRC withholding tax on gains realized on the sale or other disposition of ADS or ordinary shares, if such income is treated as sourced from within Mainland China. Furthermore, gains derived by our non-PRC individual shareholders from the sale of our shares and ADSs may be subject to a 20% PRC withholding tax. It is unclear whether our non-PRC individual shareholders (including the ADS holders) would be subject to any PRC tax (including withholding tax) on dividends received by such non-PRC individual shareholders in the event we are determined to be a PRC resident enterprise. If any PRC tax were to apply to dividends realized by non-PRC individuals, it will generally apply at a rate of 20%. The PRC tax liability may be reduced under applicable tax treaties. However, it is unclear whether non-PRC shareholders of Aesthetic Medical International Holdings Group Limited would be able to claim the benefits of any tax treaty between their country of tax residence and China in the event that Aesthetic Medical International Holdings Group Limited is treated as a PRC resident enterprise. See “Item 3. Key Information—3.D. Risk Factors—Risks related to doing business in China—If we are classified as a PRC resident enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders.”
Pursuant to the EIT Law and its implementation rules, if a non-resident enterprise has not set up an organization or establishment in Mainland China, or has set up an organization or establishment but the income derived has no actual connection with such organization or establishment, it will be subject to a withholding tax on its PRC-sourced income at a rate of 10%. Pursuant to the Arrangement between Mainland China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Tax Evasion on Income, the tax rate in respect to dividends paid by a PRC enterprise to a Hong Kong enterprise is reduced to 5% from a standard rate of 10% if the Hong Kong enterprise directly holds at least 25% of the PRC enterprise. Pursuant to the Notice of the State Administration of Taxation on the Issues concerning the Application of the Dividend Clauses of Tax Agreements, or SAT Circular 81, a Hong Kong resident enterprise must meet the following conditions, among others, in order to enjoy the reduced tax rate: (i) it must directly own the required percentage of equity interests and voting rights in the PRC resident enterprise; and (ii) it must have directly owned such percentage in the PRC resident enterprise throughout the 12 months prior to receiving the dividends. There are also other conditions for enjoying the reduced tax rate according to other relevant tax rules and regulations. Accordingly, our subsidiary Peng Oi Investment (Hong Kong) Holdings Limited may be able to enjoy the 5% tax rate for the dividends it receives from its PRC incorporated subsidiaries if they satisfy the conditions prescribed under SAT Circular 81 and other relevant tax rules and regulations and obtain the approvals as required. However, according to SAT Circular 81, if the relevant tax authorities determine our transactions or arrangements are for the primary purpose of enjoying a favorable tax treatment, the relevant tax authorities may adjust the favorable tax rate on dividends in the future.
If our Cayman Islands holding company, Aesthetic Medical International Holdings Group Limited, is not deemed to be a PRC resident enterprise, holders of the ADSs and ordinary shares who are not PRC residents will not be subject to PRC income tax on dividends distributed by us or gains realized from the sale or other disposition of our shares or ADSs.
United States federal income taxation
The following is a general discussion of certain U.S. federal income tax considerations relating to the ownership and disposition of the ADSs or ordinary shares by U.S. Holders (as defined below) that acquire the ADSs or ordinary shares and hold the ADSs or ordinary shares as “capital assets” (generally, property held for investment) under the U.S. Internal Revenue Code of 1986, as amended or the “Code”. This discussion does not address any aspect of U.S. federal gift or estate tax, alternative minimum tax, the Medicare tax on net investment income, or the state, local or non-U.S. tax consequences of an investment in the ADSs and ordinary shares. This discussion is based on the Code, existing, temporary and proposed regulations promulgated thereunder, published rulings, court decisions and, if applicable in the event that we are deemed to be a PRC resident enterprise under the PRC tax law, the income tax treaty between the United States and PRC (the “Treaty”), all as of the date hereof. These laws are subject to change, possibly on a retroactive basis. No ruling has been obtained and no ruling will be requested from the U.S. Internal Revenue Service (the “IRS”) with respect to any of the U.S. federal income tax consequences described below, and as a result, there can be no assurance that the IRS will not disagree with or challenge any of statements provided below.
This discussion is not a complete description of all tax considerations that may be relevant to particular investors in light of their individual circumstances or investors subject to special tax rules, such as:
● | broker or dealers in securities or currencies; |
132
● | dealers or traders in securities that elect to use a mark-to-market method of accounting for securities holdings; |
● | banks or certain financial institutions; |
● | insurance companies; |
● | tax-exempt organizations; |
● | individual retirement accounts and other tax deferred accounts; |
● | partnerships or other entities treated as partnerships or other pass-through entities for U.S. federal income tax purposes or persons holding ADSs or ordinary shares through any such entities; |
● | regulated investments companies or real estate investment trusts; |
● | persons that hold ADSs or ordinary shares as part of a hedge, straddle, constructive sale, conversion transaction or other integrated investment; |
● | persons whose functional currency for tax purposes is not the U.S. dollar; |
● | U.S. expatriates; |
● | persons that are resident or ordinarily resident in or have a permanent establishment in a jurisdiction outside the United States; |
● | persons who acquired the ADSs or ordinary shares pursuant to the exercise of any employee share option or otherwise as compensation; |
● | persons liable for alternative minimum tax; |
● | persons holding ADSs or ordinary shares in connection with a trade or business outside the United States; or |
● | persons that own or are deemed to own 10% or more of (i) the total combined voting power of all classes of our voting stock or (ii) the total value of all classes of our stock (including ADSs and ordinary shares). |
Prospective investors are urged to consult their own tax advisor concerning the particular U.S. federal income tax consequences to them of the ownership and disposition of the ADSs and ordinary shares, as well as the consequences to them arising under the laws of any other taxing jurisdictions.
For purposes of the U.S. federal income tax discussion below, a “U.S. Holder” is a beneficial owner of ADSs or ordinary shares who or that is:
● | An individual citizen or resident of the United States for U.S. federal income tax purposes; |
● | a corporation, or other entity classified as a corporation, that was created or organized in or under the laws of the United States or any state thereof or the District of Columbia; |
● | an estate the income of which is subject to U.S. federal income tax regardless of its source; or |
● | a trust if (i) a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (ii) the trust has a valid election in effect to be treated as a U.S. person. |
133
For U.S. federal income tax purposes, income earned through an entity or arrangement classified as a partnership for U.S. federal income tax purposes is attributed to its owners. Accordingly, if an entity or arrangement classified as a partnership for U.S. federal income tax purposes holds the ADSs or ordinary shares, the tax treatment of a partner in such a partnership will generally depend on the status of the partner and the activities of the partnership. Partnerships holding our ADSs or ordinary shares and their partners are urged to consult their tax advisors regarding the U.S. federal income tax consequences to them of an investment in ADSs or ordinary shares.
If a U.S. Holder holds ADSs, for U.S. federal income tax purposes, the U.S. Holder generally will be treated as the owner of the underlying ordinary shares that are represented by such ADSs. Accordingly, exchanges of ADSs for underlying ordinary shares represented by such ADSs will not be subject to U.S. federal income tax.
Taxation of distributions on ADSs and ordinary shares
The following discussion is subject to the discussion under “Passive Foreign Investment Company” below. If we make cash distributions and you are a U.S. Holder, the gross amount of any distributions with respect to your ADSs and ordinary shares (including the amount of any taxes withheld therefrom) will be includible in your gross income on the day you actually or constructively receive such income as dividend income if the distributions are made from our current or accumulated earnings and profits, determined under U.S. federal income tax principles. We do not intend to calculate our earnings and profits according to U.S. federal income tax principles. Thus, you should expect that distributions on the ADSs and ordinary shares, if any, will generally be treated as dividend income for U.S. federal income tax purposes. Dividends received on the ADSs or ordinary shares will not be eligible for the dividends received deduction allowed to corporations.
With respect to non-corporate U.S. Holders, certain dividends received from a qualified foreign corporation may be subject to reduced tax rates. A non-U.S. corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or in the preceding taxable year) generally will be treated as a qualified foreign corporation (i) if it is eligible for the benefits of a comprehensive tax treaty with the United States that includes an exchange of information program or (ii) with respect to any dividend it pays on stock which is readily tradable on an established securities market in the United States. We expect that the ADSs, which are listed on the Nasdaq Capital Market, will generally be considered to be readily tradable on an established securities market in the United States. Since we do not expect our ordinary shares to be listed on an established securities market, we do not believe that dividends we pay on our ordinary shares that are not represented by ADSs will meet the conditions required for the reduced tax rate. There can be no assurance that the ADSs will be considered readily tradable on an established securities market in later years. Non-corporate U.S. Holders of the ADSs that do not meet a minimum holding period requirement and certain other requirements will not be eligible for the reduced tax rate with respect to our dividends regardless of our status as a qualified foreign corporation. In the event that we are deemed to be a PRC resident enterprise under PRC tax law (see “Taxation—People’s Republic of China taxation), we may be eligible for the benefits of the Treaty. Dividends we pay on the ADSs or ordinary shares to non-corporate U.S. Holders during the course of a taxable year during which we are eligible for such benefits would be eligible for the reduced tax rate, in the case of ordinary shares regardless of whether they are represented by the ADSs. You should consult your own tax advisor regarding the availability of the reduced tax rate for dividends paid with respect to the ADSs and ordinary shares.
For U.S. foreign tax credit purposes, dividends we pay on the ADSs or ordinary shares generally will be treated as income from foreign sources and generally will constitute passive category income. In the event that we are deemed to be a PRC resident enterprise under the PRC Enterprise Income Tax Law, a U.S. Holder may be subject to PRC withholding taxes on dividends paid on our ADSs or ordinary shares (see “—Taxation—People’s Republic of China taxation”). For U.S. federal income tax purposes, the amount of the dividend income will include any amounts withheld in respect of PRC withholding tax. Depending on your individual facts and circumstances, you may be eligible, subject to a number of complex limitations, to claim a foreign tax credit in respect of foreign withholding taxes (to the extent that such withholding taxes are not refundable under the Treaty) that may be imposed on dividends received on the ADSs or ordinary shares. In lieu of claiming a credit, you may elect to deduct such PRC taxes in computing taxable income, subject to applicable limitations. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year. You should consult your own tax advisors as to your ability, and the various limitations on your ability, to claim foreign tax credits in connection with the receipt of dividends.
134
Sales and other dispositions of ADSs or ordinary shares
The following discussion is subject to the discussion under “Passive Foreign Investment Company” below. If you are a U.S. Holder, when you sell or otherwise dispose of ADSs or ordinary shares, you will recognize capital gain or loss in an amount equal to the difference between the amount realized on the sale or other disposition and your adjusted tax basis in the ADSs or ordinary shares. Your adjusted tax basis will generally be equal the amount you paid for the ADSs or ordinary shares. Any gain or loss you recognize will generally be long-term capital gain or loss if your holding period in the ADSs or ordinary shares is more than one year at the time of disposition. If you are a non-corporate U.S. Holder, including an individual, any such long-term capital gain will generally be eligible for a reduced rate of taxation. The deductibility of a capital loss is subject to limitations.
Gains from dispositions of the ADSs or ordinary shares may be subject to PRC tax if such gains are deemed as income derived from sources within Mainland China for PRC tax purposes (see “Taxation— People’s Republic of China taxation). In that case, a U.S. Holder’s amount realized would include the gross amount of the proceeds of the sale or disposition before deduction of the PRC tax. Any gain generally would constitute U.S. source income for foreign tax credit limitation purposes, which would generally limit the availability of foreign tax credits. However, in the event we are deemed to be a PRC resident enterprise, a U.S. Holder that is eligible for the benefits of the Treaty may be able to elect to treat its gain as foreign source gain for foreign tax credit purposes. If a U.S. Holder is not eligible for the benefits of the Treaty or fails to make the election to treat any gain as foreign source, then such U.S. Holder may not be able to use the foreign tax credit arising from any PRC tax imposed on the disposition of the ADSs or ordinary shares unless such credit can be applied (subject to applicable limitations) against United States federal income tax due on other income derived from foreign sources in the same income category (generally, the passive category). You should consult your own tax advisors regarding your eligibility for benefits under the Treaty and the creditability of any PRC tax on disposition gains in your particular circumstances.
Passive foreign investment company
If we were classified as a passive foreign investment company or “PFIC” in any taxable year in which you hold the ADSs or ordinary shares, as a U.S. Holder, you would generally be subject to adverse U.S. federal income tax consequences, in the form of increased tax liabilities (unless certain elections described below are timely made) and special U.S. tax reporting requirements.
In general, we will be classified as a PFIC for any taxable year if either (i) at least 75% of our gross income for the taxable year is passive income or (ii) at least 50% of the value of our assets (based on a quarterly value of the assets during the taxable year) is attributable to assets that produce or are held for the production of passive income (the “asset test”). For purposes of making the PFIC determination, we will be treated as owning our proportionate share of the assets and earning our proportionate share of the gross income of any other corporation of which we are, directly or indirectly, a 25% or greater shareholder (by value). For purposes of the asset test, any cash and cash invested in short-term, interest bearing, debt instruments, or bank deposits that are readily convertible into cash will generally count as producing passive income or held for the production of passive income.
Based the composition of our assets and income, including goodwill, which is based on the price of ADSs, we do not believe we were a PFIC for the taxable year ending December 31, 2022. However, there can be no assurance that we will not be a PFIC for the current taxable year. In addition, there can be no assurance that we will not be a PFIC for any future taxable year. PFIC status is a factual determination that tested each taxable year and will depend on the composition of our assets and income in each such taxable year. In particular, in determining the average percentage value of our gross assets, the aggregate value of our assets will generally be deemed to be equal to our market capitalization (the sum of the aggregate value of our outstanding equity) plus our liabilities. Accordingly, we could become a PFIC if our market capitalization were to decrease significantly while we hold substantial cash, cash equivalents or other assets that produce or are held for the production of passive income. In addition, because there are uncertainties in the application of the relevant rules, it is possible that the IRS may challenge our classification of certain income and assets as non-passive or our valuation of our tangible and intangible assets, which could result in a determination that we were a PFIC for the current or subsequent taxable years.
If we were a PFIC for any taxable year during which you held ADSs or ordinary shares, certain adverse U.S. federal income tax rules would apply. You would generally be subject to additional taxes and interest charges on certain “excess distributions” we make and on any gain realized on the disposition or deemed disposition of your ADSs or ordinary shares, regardless of whether we continue to be a PFIC in the year in which you receive an “excess distribution” or dispose of or are deemed to have disposed of, your ADSs or ordinary shares. Distributions in respect of your ADSs or ordinary shares during a taxable year would generally constitute “excess distributions” if, in the aggregate, they exceed 125% of the average amount of distributions with respect to your ADSs or ordinary shares over the three preceding taxable years or, if shorter, the portion of your holding period before such taxable year.
135
To compute the tax on “excess distributions” or any gain, (i) the “excess distribution” or the gain would be allocated ratably to each day in your holding period, (ii) the amount allocated to the current year and any tax year prior to the first taxable year in which we were a PFIC would be taxed as ordinary income in the current year, (iii) the amount allocated to other taxable years would be taxable at the highest applicable marginal rate in effect for that year, and (iv) an interest charge at the rate for underpayment of taxes for any period described under (iii) above would be imposed on the resulting tax liability on the portion of the “excess distribution” or gain that is allocated to such period. In addition, if we were a PFIC, no distribution that you receive from us would qualify for taxation at the reduced tax rate discussed in the “—Dividends on ADSs and Ordinary Shares” section above in the taxable year in which such distribution is made or in the preceding taxable year.
Under certain attribution rules, if we were a PFIC, you would be deemed to own your proportionate share of lower-tier PFICs, and would be subject to U.S. federal income tax on (i) a distribution on the shares of a lower-tier PFIC and (ii) a disposition of shares of a lower-tier PFIC, both as if you directly held the shares of such lower-tier PFIC. Under the PFIC rules, if we were considered a PFIC at any time that you hold ADSs or ordinary shares, we would continue to be treated as a PFIC with respect to your ADSs or ordinary shares unless we have ceased to be a PFIC and you have made a “deemed sale” election under the PFIC rules.
If we were a PFIC in any year, you would generally be able to avoid the “excess distribution” rules described above by making a timely so-called “mark-to-market” election with respect to your ADSs provided they are “marketable.” The ADSs will be “marketable” as long as they remain regularly traded on a national securities exchange, such as the Nasdaq Capital Market. If you made this election in a timely fashion, you would generally recognize as ordinary income or ordinary loss the difference between the fair market value of your ADSs as of the close of any taxable year and your adjusted tax basis in such ADSs. Any income resulting from this election would generally be taxed at ordinary income rates. Any ordinary losses would be deductible, but only to the extent of the net amount of previously included income as a result of the mark-to-market election, if any. Your basis in the ADSs or ordinary shares would be adjusted to reflect any such income or loss. If you make a mark-to-market election with respect to the ADSs, but for a later taxable year either the ADSs no longer constitute “marketable stock” or we cease being a PFIC, you will not be subject to the mark-to market rules described above for such taxable year. The mark-to-market election will not be available for any lower tier PFIC that you may be deemed to own pursuant to the attribution rules discussed above. You should consult your own tax advisor regarding potential advantages and disadvantages to you of making a “mark-to-market” election with respect to your ADSs.
The PFIC rules provide for a separate election, referred to as a qualified electing fund election, which, if available, results in a tax treatment different (and generally less adverse than) the general PFIC tax treatment described above. That election, however, will not be available to you as we do not intend to provide the information you would need to make or maintain that election.
If you own the ADSs or ordinary shares during any taxable year that we are a PFIC, you will generally be required to file an annual report containing such information as the United States Treasury Department may require. You should consult with your own tax advisor concerning the U.S. federal income tax consequences of holding and disposing of the ADSs or ordinary shares if we are or become classified as a PFIC.
U.S. information reporting and backup withholding rules
Dividend payments with respect to the ADSs and ordinary shares and the proceeds received on the sale or other disposition of ADSs and ordinary shares may be subject to information reporting to the IRS and to backup withholding. Backup withholding will not apply, however, if (i) a U.S. Holder is an exempt recipient, or if (ii) the U.S. Holder provides a taxpayer identification number, certifying that the U.S. Holder is not subject to backup withholding. U.S. Holders who are required to establish their exempt status may be required to provide such certification on IRS Form W-9. Backup withholding is not an additional tax. Rather, any amounts withheld under the backup withholding rules from a payment to a U.S. Holder will be refunded or credited against such U.S. Holder’s U.S. federal income tax liability, provided that the required information is timely provided to the IRS.
Certain U.S. Holders who hold “specific foreign financial assets”, including stock of a non-U.S. corporation that is not held in an account maintained by a U.S. “financial institution” may be required to attach to their tax returns for the year certain specified information. A U.S. Holder who fails to timely furnish the required information may be subject to a penalty. You are advised to consult with its own tax advisor regarding the application of the U.S. information reporting and backup withholding rules to your particular circumstances.
136
PROSPECTIVE INVESTORS OF OUR ADSS AND ORDINARY SHARES SHOULD CONSULT WITH THEIR OWN TAX ADVISOR REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES RESULTING FROM OWNING OR DISPOSING OUR ADSS AND ORDINARY SHARES, INCLUDING THE APPLICABILITY AND EFFECT OF THE TAX LAWS OF ANY STATE, LOCAL OR NON-US JURISDICTION AND INCLUDING ESTATE, GIFT AND INHERITANCE LAWS.
10.F.Dividends and Paying Agents
Not Applicable.
10.G.Statement by Experts
Not Applicable.
10.H.Documents on Display
We previously filed with the SEC our registration statement on Form F-1 (Registration No. 333-234022), as amended, including the prospectus contained therein, to register our ordinary shares in relation to our initial public offering. We have also filed with the SEC a related registration statement on F-6 (Registration No. 333-234191) to register the ADSs.
We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Specifically, we are required to file annually a Form 20-F within four months after the end of each fiscal year, which is December 31. Copies of reports and other information, when so filed, may be inspected without charge and may be obtained at prescribed rates at the public reference facilities maintained by the Securities and Exchange Commission at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. The public may obtain information regarding the Washington, D.C. Public Reference Room by calling the Commission at 1-800-SEC-0330. The SEC also maintains a website at www.sec.gov that contains reports, proxy and information statements, and other information regarding registrants that make electronic filings with the SEC using its EDGAR system. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
We will furnish Deutsche Bank Trust Company Americas, the depositary of our ADSs, with our annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity with IFRS as issued by the IASB, and all notices of shareholders’ meetings and other reports and communications that are made generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and, upon our request, will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
In accordance with NASDAQ Stock Market Rule 5250(d), we will post this annual report on our website https://ir.aihgroup.net. In addition, we will provide hardcopies of our annual report to shareholders, including ADS holders, free of charge upon request.
10.I.Subsidiary Information
Not applicable.
Item 11.Quantitative and Qualitative Disclosures About Market Risk
In the normal course of business, we are exposed to various types of financial risks as described below.
Foreign exchange risk
Substantially all of our transactions are denominated in renminbi. As a result, we do not believe we are exposed to significant foreign exchange rate risks. However, as the proceeds of our initial public offering are denominated in U.S. dollars, any appreciation of the renminbi against the U.S. dollar will reduce the amount of proceeds we receive in terms of renminbi. On the other hand, a depreciation of the renminbi would adversely affect the value, translated or converted into U.S. dollars, of our net assets, earnings and any dividends we declare. We do not engage in hedging activities to manage such exchange rate risk.
137
With all other variables held constant, if the average exchange rate of RMB against US$ had strengthened or weakened by 5%, our post-tax results would increase or decrease by RMB682,000, RMB267,000 and RMB4,000 (US$580) in 2020, 2021 and 2022, respectively.
Credit risk
We have no significant concentrations of credit risk. The carrying amounts of pledged bank deposits, cash at banks, trade receivables, deposits and other receivables and amounts due from related companies included in the consolidated statements of financial position represent our maximum exposure to credit risk in relation to our financial assets. Our objective is to seek continual revenue growth while minimizing losses incurred due to increased credit risk exposure. The majority of our pledged deposits and cash at banks are deposited in major reputable financial institutions located in the PRC. Most of our revenue is settled by cash or credit cards. Our trade receivables are mainly due from financial institutions with sound financial standing. There has been no history of default in relation to these external parties. We do not expect any losses arising from non-performance by these counterparties.
Interest rate risk
Our interest rate risk relates primarily to our bank deposits and bank borrowings. Bank borrowings issued at variable rates expose us to cash flow interest rate risk. Bank borrowings issued at fixed rates expose us to fair value interest rate risk. We have not entered into interest rate swaps to hedge against our exposure to changes in fair values of our borrowings. We will, however, continue to monitor interest rate risk exposure and will consider hedging significant interest rate risk exposure should the need arise.
In 2020, 2021, interest rates on borrowings had been 10 basis points higher/ lower with all other variables held constant, our post-tax results for the year would have been RMB47,000, RMB48,070 lower/higher, respectively, mainly as a result of higher/lower interest expenses on floating rate borrowings. In 2022, the interest rate risk was effectively mitigated through a contractual agreement with banks that secured a fixed interest rate.
Liquidity risk
Our policy is to regularly monitor current and expected liquidity requirements to ensure that we maintain sufficient cash and cash equivalents and adequate amounts of committed credit facilities to meet our liquidity requirements in the short and long term. Our primary cash requirements have been the payment for operating expenses and we have historically met our working capital requirements primarily from cash generated from operations.
Price risk
We are exposed to price risk in respect of our convertible redeemable preferred shares, exchangeable note and convertible note carried at fair value with changes in fair value recognized in profit or loss. The fair value of the convertible redeemable preferred shares and exchangeable note is affected by changes in our market value. As the convertible redeemable preferred shares and exchangeable note were converted into ordinary shares immediately prior to the completion of our initial public offering, we currently are not exposed to this risk.
Item 12.Description of Securities Other Than Equity Securities
12.A.Debt Securities
Not applicable.
12.B.Warrants and Rights
Not applicable.
12.C.Other Securities
Not applicable.
138
12.D.American Depositary Shares
Deutsche Bank Trust Company Americas, an indirect wholly – owned subsidiary of Deutsche Bank A.G., with its principal office at 60 Wall Street, New York, NY 10005, United States of America, acts in its capacity as the depositary bank to our ADR program since October 2019.
As an ADS holder, you will be required to pay the following service fees to the depositary bank and certain taxes and governmental charges (in addition to any applicable fees, expenses, taxes and other governmental charges payable on the deposited securities represented by any of your ADSs):
Service |
| Fees |
● To any person to which ADSs are issued or to any person to which a distribution is made in respect of ADS distributions pursuant to stock dividends or other free distributions of stock, bonus distributions, stock splits or other distributions (except where converted to cash) | Up to US$0.05 per ADS issued | |
● Cancellation of ADSs, including the case of termination of the deposit agreement | Up to US$0.05 per ADS cancelled | |
● Distribution of cash dividends | Up to US$0.05 per ADS held | |
● Distribution of cash entitlements (other than cash dividends) and/or cash proceeds from the sale of rights, securities and other entitlements | Up to US$0.05 per ADS held | |
● Distribution of ADSs pursuant to exercise of rights | Up to US$0.05 per ADS held | |
● Distribution of securities other than ADSs or rights to purchase additional ADSs | Up to US$0.05 per ADS held | |
● Depositary services | Up to US$0.05 per ADS held on the applicable record date(s) established by the depositary bank |
As an ADS holder, you will also be responsible to pay certain fees and expenses incurred by the depositary bank and certain taxes and governmental charges (in addition to any applicable fees, expenses, taxes and other governmental charges payable on the deposited securities represented by any of your ADSs) such as:
● | Fees for the transfer and registration of ordinary shares charged by the registrar and transfer agent for the ordinary shares in the Cayman Islands (i.e., upon deposit and withdrawal of ordinary shares). |
● | Expenses incurred for converting foreign currency into U.S. dollars. |
● | Expenses for cable, telex and fax transmissions and for delivery of securities. |
● | Taxes and duties upon the transfer of securities, including any applicable stamp duties, any stock transfer charges or withholding taxes (i.e., when ordinary shares are deposited or withdrawn from deposit). |
● | Fees and expenses incurred in connection with the delivery or servicing of ordinary shares on deposit. |
● | Fees and expenses incurred in connection with complying with exchange control regulations and other regulatory requirements applicable to ordinary shares, deposited securities, ADSs and ADRs. |
● | Any applicable fees and penalties thereon. |
139
The depositary fees payable upon the issuance and cancellation of ADSs are typically paid to the depositary bank by the brokers (on behalf of their clients) receiving the newly issued ADSs from the depositary bank and by the brokers (on behalf of their clients) delivering the ADSs to the depositary bank for cancellation. The brokers in turn charge these fees to their clients. Depositary fees payable in connection with distributions of cash or securities to ADS holders and the depositary services fee are charged by the depositary bank to the holders of record of ADSs as of the applicable ADS record date.
The depositary fees payable for cash distributions are generally deducted from the cash being distributed or by selling a portion of distributable property to pay the fees. In the case of distributions other than cash (i.e., share dividends, rights), the depositary bank charges the applicable fee to the ADS record date holders concurrent with the distribution. In the case of ADSs registered in the name of the investor (whether certificated or uncertificated in direct registration), the depositary bank sends invoices to the applicable record date ADS holders. In the case of ADSs held in brokerage and custodian accounts (via DTC), the depositary bank generally collects its fees through the systems provided by DTC (whose nominee is the registered holder of the ADSs held in DTC) from the brokers and custodians holding ADSs in their DTC accounts. The brokers and custodians who hold their clients’ ADSs in DTC accounts in turn charge their clients’ accounts the amount of the fees paid to the depositary banks.
In the event of refusal to pay the depositary fees, the depositary bank may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder.
The depositary may make payments to us or reimburse us for certain costs and expenses, by making available a portion of the ADS fees collected in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary bank agree from time to time.
PART II.
Item 13.Defaults, Dividend Arrearages and Delinquencies
None.
Item 14.Material Modifications to the Rights of Security Holders and Use of Proceeds
14.A. — 14.D.Material Modifications to the Rights of Security Holders
See “Item 10. Additional Information” for a description of the rights of shareholders, which remain unchanged.
14.E.Use of Proceeds
The following “Use of Proceeds” information relates to the registration statement on Form F-1 (File No. 333-234022), as amended, including the annual report contained therein, which registered 7,500,000 ordinary shares represented by 2,500,000 ADSs and was declared effective by the SEC on October 24, 2019, for our initial public offering, which closed on October 29, 2019. Cantor Fitzgerald & Co., Haitong International Securities Company Limited and Prime Number Capital, LLC were the representatives of the underwriters.
For the period from October 24, 2019, the date that the registration statement on Form F-1 was declared effective by the SEC, to December 31, 2019, the total expenses incurred for our company’s account in connection with our initial public offering was approximately US$6.5 million, which included US$2.1 million in underwriting discounts and commissions for the initial public offering and approximately US$4.4 million in other costs and expenses for our initial public offering. None of the transaction expenses included payments to directors or officers of our company or their associates, persons owning more than 10% or more of our equity securities or our affiliates. None of the net proceeds from the initial public offering were paid, directly or indirectly, to any of our directors or officers or their associates, persons owning 10% or more of our equity securities or our affiliates.
For the period from October 24, 2019, the date that the registration statement on Form F-1 was declared effective by the SEC, to December 31, 2020, we used approximately US$28.2 million of the proceeds received from the initial public offering for purposes including redemption of certain convertible note, working capital, strategic acquisitions, establishment of new treatment centers and facility upgrades.
140
As a result, as of December 31, 2021, we used approximately all of the proceeds received from the initial public offering for the purposes mentioned.
Item 15.Controls and Procedures
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon that evaluation, our management, with the participation of our chief executive officer and chief financial officer, has concluded that, due to the outstanding material weakness described below, as of December 31, 2021, our disclosure controls and procedures were not effective in ensuring that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act was recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act of 1934. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements in accordance with International Financial Reporting Standards. Because of its inherent limitations, a system of internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate.
Internal Control over Financial Reporting
Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2020. In making this assessment, it used the criteria established within the Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) (2013 framework). Based on this assessment, our management has concluded that, as of December 31, 2020, our internal control over financial reporting was not effective. We and our independent registered public accounting firm identified one material weakness in our internal control over financial reporting. The material weakness identified related to a lack of an effective control procedure to evaluate accounting of complex non-routine transactions. As defined in the standards established by the U.S. Public Company Accounting Oversight Board, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness identified related to a lack of an effective control procedure to evaluate accounting of complex non-routine transactions. To remedy the identified material weakness, we have implemented, and plan to continue to implement the following measures:
● | formulating internal accounting and internal control guidance on evaluation of complex non-routine transactions; and |
● | establishing ongoing program to provide sufficient and appropriate IFRS trainings to our accounting staff. |
141
To remediate our identified material weakness, we have adopted a number of measures to improve our internal control over financial reporting. For example, we hired senior financial officers, each of whom has a solid understanding of and extensive work experience involving complex non-routine transactions. In 2020, we provided our accounting and finance staff with financial reporting and IFRS trainings. We will continue to provide our accounting and finance staff with trainings regularly, and we will continue to review and improve key controls over financial reporting. Our management concluded that the material weakness had not been remediated as of December 31, 2020. We are fully committed to continue to implement measures to remediate our material weakness in our internal control over financial reporting. However, the implementation of these measures may not fully address the deficiencies in our internal control over financial reporting. We are not able to estimate with reasonable certainty the costs that we will need to incur to implement these and other measures designed to improve our internal control over financial reporting. See “Item 3. Key Information—3.D. Risk factors—Risks relating to our business and our industry A material weakness was identified in our internal control over financial reporting. If we fail to develop and maintain an effective system of internal control over financial reporting, we may be unable to accurately report our financial results or prevent fraud.” In the fiscal year 2021 and 2022, we have not identified any material weakness in our internal control over financial reporting.
As a company with less than US$1.07 billion in revenues for our last fiscal year, we qualify as an “emerging growth company” pursuant to the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002 in the assessment of the emerging growth company’s internal control over financial reporting.
Attestation Report of the Registered Public Accounting Firm
Since we are an “emerging growth company” as defined under the JOBS Act, our independent registered public accounting firm has not conducted an audit of our internal control over financial reporting as we are exempt from the requirement to comply with the auditor attestation requirements that our independent registered public accounting firm attest to and report on the effectiveness of our internal control structure and procedures for financial reporting.
Changes in Internal Control over Financial Reporting
Other than as described above, no changes in our internal controls over financial reporting occurred during the period covered by this annual report that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
Item 16.A.Audit Committee Financial Expert
Our board of directors has determined that Xue Hongwei, an independent director and chair of our audit committee, qualifies as an “audit committee financial expert” within the meaning of the SEC rules and possesses financial sophistication within the meaning of the Nasdaq Rules.
Item 16.B.Code of Ethics
Our board of directors has adopted a code of ethics that applies to all directors, officers and employees of the Company, whether they work for the Company on a full-time, part-time, consultative or temporary basis. Certain provisions thereof apply specifically to our chief executive officer, chief financial officer, other chief senior officers, senior finance officer, controller, vice presidents and any other persons who perform similar functions for us. We have posted a copy of our code of business conduct and ethics on our website at https://ir.aihgroup.net.
142
Item 16.C.Principal Accountant Fees and Services
The following table sets forth the aggregate fees by the categories specified below in connection with certain professional services rendered by Union Power HK CPA Limited, our independent registered public accounting firm, for the periods indicated. We did not pay any other fees to our auditors during the periods indicated below.
Year Ended December 31, | ||||||
| 2020 |
| 2021 |
| 2022 | |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Audit fees(1) | 5,500 | 8,254 | 3,799 | |||
5,500 | 8,254 | 3,799 | ||||
(1) | “Audit fees” means the aggregate fees billed for professional services rendered by our principal auditors for the audit of our annual financial statements, including audit fees relating to our initial public offering in 2019. |
The policy of our audit committee is to pre-approve all auditing services and all non-audit services permitted to be performed by Union Power HK CPA Limited, our independent registered public accounting firm, including audit services and tax services as described above, other than those for de minimus services which are approved by our audit committee prior to the completion of the audit.
Item 16.D.Exemptions from the Listing Standards for Audit Committees
Not applicable.
Item 16.E.Purchases of Equity Securities by the Issuer and Affiliated Purchasers
In October 2020, our board of directors announced a share repurchase program pursuant to which we would repurchase up to US$6.0 million worth of our ADSs. 45,000 ADSs were repurchased in 2020, and 0 ADS was repurchased from January 1, 2021 up to December 31, 2022 in the open market pursuant to the applicable share repurchase programs. As of December 31, 2022, we have not retired the treasure shares of 45,000 ADSs.
Item 16.F | Change in Registrant’s Certifying Accountant |
Not applicable.
Item 16.G.Corporate Governance
As a foreign private issuer, we are permitted to take advantage of certain corporate governance requirements under the Nasdaq listing standards that allow us to follow Cayman Islands law for certain governance matters. Certain corporate governance practices in the Cayman Islands may differ significantly from corporate governance listing standards as, except for general fiduciary duties and duties of care, Cayman Islands law had no corporate governance regime which prescribes specific corporate governance standards as of the date of this annual report. For example, we have followed and intend to continue to follow Cayman Islands corporate governance practices in lieu of the corporate governance requirements of the Nasdaq Global Market that listed companies must have a majority of independent directors. Cayman Islands law does not impose a requirement that our board of directors consist of a majority of independent directors. Nor does Cayman Islands law impose specific requirements on the establishment of a compensation committee or nominating committee or nominating process. To the extent we choose to follow home country practice in the future, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers.
Item 16.H.Mine Safety Disclosure
Not applicable.
Item 16.I.Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
143
PART III.
Item 17.Financial Statements
We have elected to provide financial statements pursuant to Item 18.
Item 18.Financial Statements
The consolidated financial statements of Aesthetic Medical International Holdings Group Limited and its subsidiaries are included at the end of this annual report.
144
Item 19.Exhibits
Exhibit Number |
|
| |
|---|---|---|---|
1.1* | |||
2.1 | |||
2.2 | Form of registrant’s specimen American depositary receipt (included in Exhibit 2.1) | ||
2.3 | |||
2.4 | |||
2.5 | |||
2.6 | |||
2.7 | |||
2.8 | |||
4.1 | |||
4.2 | |||
4.3 | |||
4.4 | |||
4.5 | |||
4.6 | |||
4.7 |
145
Exhibit Number |
|
| |
|---|---|---|---|
4.8 | |||
4.9 | |||
4.10 | |||
4.11 | |||
4.12 | |||
4.13 | |||
4.14 | |||
4.15 | |||
4.16 | |||
4.17 | |||
4.18 | |||
4.19 | |||
4.20 |
146
Exhibit Number |
|
| |
|---|---|---|---|
4.21 | |||
4.22 | |||
4.23 | |||
4.24 | |||
4.25 | |||
4.26 | |||
4.27 | |||
4.28 | |||
4.29 | |||
4.30 | |||
4.31 | |||
4.32 | |||
4.33 |
147
Exhibit Number |
|
| |
|---|---|---|---|
4.34 | |||
4.35 | |||
4.36 | |||
4.37 | |||
4.38 | |||
4.39 | |||
4.40 | |||
4.41 | |||
4.42 | |||
4.43 | |||
4.44 | |||
4.45 | |||
4.46 | |||
4.47 |
148
Exhibit Number |
|
| |
|---|---|---|---|
4.48 | |||
4.49 | |||
4.50 | |||
4.51 | |||
4.52 | |||
4.53 | |||
4.54 | |||
4.55 | |||
4.56 | |||
4.57 | |||
4.58 | |||
4.59 | |||
4.60 | |||
4.61 |
149
Exhibit Number |
|
| |
|---|---|---|---|
4.62 | |||
4.63 | |||
4.64 | |||
4.65 | |||
4.66 | English translation of spousal consent letter dated April 23, 2021 by Ding Wenting | ||
4.67 | |||
4.68 | |||
4.69 | |||
4.70 | |||
8.1* | |||
11.1 | |||
12.1* | |||
12.2* | |||
13.1** | |||
13.2** | |||
15.1* | |||
15.2* | |||
101.INS* | XBRL Instance Document | ||
101.SCH* | XBRL Taxonomy Extension Scheme Document | ||
101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document | ||
101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document | ||
101.LAB* | XBRL Taxonomy Extension Label Linkbase Document | ||
101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document | ||
104* | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* | Filed herewith. |
** | Furnished herewith. |
150
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing its annual report on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Aesthetic Medical International Holdings Group Limited | |
|
| |
| By: | /s/ Zhou Pengwu |
| Name: | Zhou Pengwu |
Date: April 21, 2023 | Title: | Chairman and Chief Executive Officer |
151
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS FOR THE YEAR ENDED 31 DECEMBER 2021 and 2022
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (PCAOB ID: | F-2 |
F-4 | |
F-6 | |
F-8 | |
F-10 | |
F-11 |
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Aesthetic Medical International Holdings Group Limited.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Aesthetic Medical International Holdings Group Limited. and subsidiaries (the “Company”) as of December 31, 2022, the related consolidated statements of comprehensive income, changes in equity and cash flows for each of the three years in the period ended December 31, 2022, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2022, in conformity with International Financial Reporting Standards.
Material Uncertainty relating to Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2.1 to the consolidated financial statements, the Company had net current liabilities of RMB466.2 million and net liabilities of RMB125.9 million as at December 31, 2022, and together with other conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2.1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Group’s management. Our responsibility is to express an opinion on the Group’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Group in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Group is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
F-2
Impairment of goodwill in cash generating unit
Critical Audit Matter Description
As discussed in Note 2.9 (a) to the consolidated financial statements, goodwill is evaluated for impairment annually, or more frequently if events or circumstances indicate that the carrying value may not be recoverable. As discussed in Note 14, the management reviews the business performance of each cash generating unit (“CGU”) including the related goodwill and the recoverable amount of a CGU is determined based on a value-in-use calculation. As a result, impairment tests were performed over the CGU and the fair value of each CGU estimated using a discounted cash flow model covering a five-year period. The Company’s impairment test indicated that the goodwill had a carrying value that was not recoverable so there was no impairment for goodwill for the year ended December 31, 2022. The goodwill balance was RMB33.0 million of December 31, 2022.
We identified the valuation of goodwill as a critical audit matter. A high degree of subjective auditor judgment and specialized skills and knowledge was required to evaluate the projected revenue growth rates, projected earnings before interest, taxes, depreciation, and amortization (“EBITDA”) margins, and the discount rate used in the Company’s impairment test.
How We Addressed the Matter in Our Audit
Our audit procedures related to goodwill impairment to address this critical audit matter included the following, among others: We evaluated management’s determination of the projected revenue growth rates, projected EBITDA margins, and the discount rate. We assessed the Company’s ability to accurately prepare projections for the CGU by comparing the projected revenues from past periods to actual results for the same period. Additionally, we evaluated the reasonableness of the reporting unit projected revenue growth rates and projected EBITDA margins used in management’s discounted cash flow analysis by comparing projected amounts to past performance of the reporting unit. We assessed: (1) the reasonableness of the projected revenue growth rates and projected EBITDA margins by comparing the assumptions to relevant industry trends and current market indices of comparable entities (2) the reasonableness of the discount rate through testing the source information underlying the determination of the discount rate, and developing a range of independent estimates and comparing those to the discount rate applied by management.
Going concern
Critical Audit Matter Description
As discussed in Note 2.1 to the consolidated financial statements, the Company had net current liabilities of RMB466.2 million and net liabilities of RMB125.9 million as at December 31, 2022, and together with other conditions raise substantial doubt about its ability to continue as a going concern. Management seeks to the support from Binhua Wu who represents the major shareholder to satisfy the Company’s obligations as and when they become due for at least one year from the financial statements issuance date. However, the Company has not concluded that support from the major shareholder alleviate the substantial doubt related to its ability to continue as a going concern.
We determined that Company’s ability to continue as a going concern is a critical matter due to the estimation and uncertainty regarding the Company’s available funding and the risk of bias in management’s judgement and assumptions in their determination.
How We Addressed the Matter in Our Audit
Our audit procedures relating to the Company’s assertion on its ability to continue as a going concern included the following, among others: We inquired of Company management and reviewed Company records and documents to assess whether there are additional factors that contribute to the uncertainties disclosed. We assessed whether the Company’s identification of conditions and events that indicate there could be substantial doubt about its ability to continue as a going concern for a reasonable period of time was appropriate and adequately disclosed. We reviewed a cash flow projection prepared by management incorporating management’s plan and performed sensitivity analysis of significant assumptions to evaluate the changes in the cash flow projection that would result from changes in the assumptions.
/s/
We have served as the Company’s auditor since 2022.
April 21, 2023
F-3
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| Note |
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||||
Revenue |
| 5 |
| | |
| | |
Cost of sales and services rendered |
| 6 |
| ( | ( |
| ( | |
Gross profit |
|
| | |
| | ||
Selling expenses |
| 6 |
| ( | ( |
| ( | |
General and administrative expenses |
| 6 |
| ( | ( |
| ( | |
Operating result | ( | ( | ( | |||||
Finance income |
| 8 |
| | |
| | |
Finance costs |
| 8 |
| ( | ( |
| ( | |
Other gains/(losses), net |
| 9 |
| | |
| ( | |
Fair value loss of convertible note |
| 27 |
| ( | ( |
| ( | |
Impairment of non-current assets |
| 15 |
| ( | ( |
| — | |
Share of (losses)/profits of investments accounted for using the equity method |
| 16 |
| ( | |
| — | |
Loss before income tax |
|
| ( | ( |
| ( | ||
Income tax credit |
| 10 |
| | |
| | |
Loss for the year |
|
| ( | ( |
| ( | ||
Other comprehensive income/(loss): |
|
|
| |||||
Items that may be subsequently reclassified to profit or loss |
|
|
| |||||
Currency translation differences |
|
| ( | |
| ( | ||
Revaluation of investment properties upon transfer from property, plant and equipment | — | — | | |||||
Total other comprehensive income/(loss) for the year, net of tax |
|
| ( | |
| | ||
Total comprehensive income/(loss) for the year |
|
| ( | ( |
| ( | ||
Loss attributable to: |
|
|
| |||||
Owners of the Company |
|
| ( | ( |
| ( | ||
Non-controlling interests | ( | ( | | |||||
Loss for the year |
|
| ( | ( |
| ( |
The above consolidated statements of comprehensive income should be read in conjunction with the accompanying notes.
F-4
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| Note |
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||||
Loss per share for loss attributable to owners of the Company (in RMB per share) |
|
|
|
|
|
|
|
|
—Basic |
| 11 |
| ( |
| ( |
| ( |
—Diluted |
| 11 |
| ( |
| ( |
| ( |
Total comprehensive loss attributable to: |
|
|
|
|
| |||
Owners of the Company |
|
|
| ( |
| ( |
| ( |
Non-controlling interests |
|
|
| ( |
| ( |
| |
Total comprehensive loss for the year |
|
|
| ( |
| ( |
| ( |
The above consolidated statement of comprehensive income should be read in conjunction with the accompanying notes.
F-5
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
CONSOLIDATED BALANCE SHEETS
| Note |
| 2021 |
| 2022 | |
| RMB’000 | RMB’000 | ||||
ASSETS |
|
|
| |||
Non‑current assets |
|
|
| |||
Property, plant and equipment |
| 12 |
| |
| |
Investment properties |
| 14 |
| — |
| |
Intangible assets |
| 15 |
| |
| |
Investments accounted for using the equity method |
| 16 |
| |
| |
Prepayments and deposits |
| 17 |
| |
| |
Deferred income tax assets |
| 23 |
| |
| |
| |
| | |||
Current assets |
|
|
| |||
Inventories |
| 18 |
| |
| |
Trade receivables |
| 17 |
| |
| |
Other receivables, deposits and prepayments |
| 17 |
| |
| |
Amounts due from related parties |
| 35 |
| |
| |
Restricted cash | 19 | — | | |||
Asset held-for-sale | 20 | — | | |||
Cash and cash equivalents |
| 19 |
| |
| |
| |
| | |||
Total assets |
|
| |
| | |
EQUITY AND LIABILITIES |
|
|
| |||
Equity attributable to owners of the Company |
|
|
| |||
Share capital |
| 21a |
| |
| |
Treasury shares | 21b | ( | ( | |||
Accumulated losses |
|
| ( |
| ( | |
Other reserves |
| 22 |
| |
| |
| ( |
| ( | |||
Non‑controlling interests |
|
| ( |
| ( | |
Total equity |
|
| ( |
| ( |
The above consolidated balance sheets should be read in conjunction with the accompanying notes.
F-6
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
CONSOLIDATED BALANCE SHEETS (CONTINUED)
| Note |
| 2021 |
| 2022 | |
| RMB’000 | RMB’000 | ||||
LIABILITIES |
|
|
| |||
Non-current liabilities |
|
|
| |||
Borrowings |
| 24 |
| |
| |
Lease liabilities |
| 13 |
| |
| |
Convertible note |
| 27 |
| |
| — |
Deferred income tax liabilities | 23 |
| |
| — | |
| | |||||
Current liabilities |
|
|
| |||
Trade payables |
| 25 |
| |
| |
Accruals, other payables and provisions |
| 25 |
| |
| |
Contingent consideration and consideration payable | | | ||||
Amounts due to related parties |
| 35 |
| |
| — |
Contract liabilities |
| 26 |
| |
| |
Borrowings |
| 24 |
| |
| |
Convertible note | 27 | — | | |||
Lease liabilities |
| 13 |
| |
| |
Current income tax liabilities |
| |
| | ||
| | |||||
Total liabilities |
|
| |
| | |
Total equity and liabilities |
|
| |
| |
The above consolidated balance sheets should be read in conjunction with the accompanying notes.
F-7
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
Attributable to owners of the Company | ||||||||||||||
Non‑ | Total | |||||||||||||
Share | Treasury | Other reserves | Accumulated | controlling | equity/ | |||||||||
capital | shares | (Note 22) | losses | Sub‑total | interests | (deficit) | ||||||||
RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | ||||||||
Balance at 1 January 2020 |
| | ( | | ( | | | | ||||||
Comprehensive loss | ||||||||||||||
Loss for the year | — | — | — | ( | ( | ( | ( | |||||||
Currency translation differences | — | — | ( | — | ( | — | ( | |||||||
Total comprehensive income for the year | — | — | ( | ( | ( | ( | ( | |||||||
Transactions with owners | ||||||||||||||
Transfer to statutory reserve | — | — | | ( | — | — | — | |||||||
Transactions with non-controlling shareholders (Note 31) | — | — | | — | | ( | | |||||||
Share-based payment | — | — | | — | | — | | |||||||
Business combination (Note 29) | — | — | — | — | — | | | |||||||
Disposal of subsidiaries (Note 30) | — | — | — | — | — | ( | ( | |||||||
Dividend to non-controlling shareholders | — | — | — | — | — | ( | ( | |||||||
Share repurchase (Note 19) | — | ( | — | — | ( | — | ( | |||||||
Total transactions with owners | — | ( | | ( | | | | |||||||
F-8
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY (Continued)
Attributable to owners of the Company | Non- | |||||||||||||
Share | Treasury | Other reserves | Accumulated | controlling | Total | |||||||||
| capital |
| shares |
| (Note 22) |
| losses |
| Sub-total |
| interests |
| equity/(deficit) | |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Balance at 31 December 2020 | | ( | | ( | | | | |||||||
Balance at 31 December 2020 and 1 January 2021 | | ( | | ( | | | | |||||||
Comprehensive loss |
| |||||||||||||
Loss for the year |
| | | | ( | ( | ( | ( | ||||||
Currency translation differences |
| — | — | | — | | — | | ||||||
Total comprehensive loss for the year |
| — | — | | ( | ( | ( | ( | ||||||
Transactions with owners |
| |||||||||||||
Transactions with non-controlling shareholders (Note 31) |
| — | — | | — | | ( | | ||||||
Share-based payment |
| — | — | | — | | — | | ||||||
Business combination (Note 29) | — | — | — | — | — | | | |||||||
Disposal of subsidiaries (Note 30) | — | — | — | — | — | | | |||||||
Dividend to non-controlling shareholders | — | — | — | — | — | ( | ( | |||||||
Total transactions with owners |
| — | — | | — | | | | ||||||
Balance at 31 December 2021 | ( | | ( | ( | ( | ( | ||||||||
Balance at 31 December 2021 and 1 January 2022 | ( | | ( | ( | ( | ( | ||||||||
Comprehensive loss | ||||||||||||||
Loss for the year | — | — | — | ( | ( | | ( | |||||||
Currency translation differences | — | — | ( | — | ( | — | ( | |||||||
Revaluation of investment properties upon transfer from property, plant and equipment | — | — | | — | | — | | |||||||
Total comprehensive loss for the year | — | — | | ( | ( | | ( | |||||||
Transactions with owners | ||||||||||||||
Transactions with non-controlling shareholders (Note 31) | — | — | ( | — | ( | ( | ( | |||||||
Share-based payment | — | — | | — | | — | | |||||||
Disposal of subsidiaries (Note 30) | — | — | — | — | — | | | |||||||
Deregister of subsidiary | — | — | | — | | — | | |||||||
Issuance of shares(Note 20) | — | | — | | — | | ||||||||
Transaction costs related to issuance of shares | — | — | ( | — | ( | — | ( | |||||||
Total transactions with owners | — | | — | | | | ||||||||
Balance at 31 December 2022 | ( | | ( | ( | ( | ( | ||||||||
The above consolidated statements of changes in equity should be read in conjunction with the accompanying notes.
F-9
AESTHETIC MEDICAL INTERNATIONAL HOLDINGS GROUP LIMITED
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Note |
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||||
Cash flows from operating activities |
|
|
|
|
|
| ||
Cash generated from operations |
| 28 |
| | |
| ( | |
Income tax paid |
|
| ( | ( |
| ( | ||
Net cash (used in)/generated from operating activities |
|
| ( | |
| ( | ||
Cash flows from investing activities |
|
|
| |||||
Net cash used in business combinations |
| 29 |
| ( | ( |
| ( | |
Proceeds from disposal of property, plant and equipment |
|
| | |
| — | ||
Purchase of and deposit paid for property, plant and equipment |
|
| ( | ( |
| ( | ||
Purchase of and deposit paid for intangible assets |
|
| ( | ( |
| ( | ||
Deposit of long term investment |
|
| ( | — |
| — | ||
Deposit from investors | — | | — | |||||
Restricted cash |
|
| ( | |
| ( | ||
Balances with related parties |
|
| ( | |
| ( | ||
Interest income received |
|
| | |
| | ||
Proceeds from disposal of subsidiaries |
| 30 |
| | ( |
| | |
Proceeds from ceased operation of subsidiaries | — | — | ( | |||||
Net cash (used in)/(generated from) investing activities |
|
| ( | ( |
| | ||
Cash flows from financing activities |
|
|
| |||||
Proceeds from borrowings |
|
| | |
| | ||
Repayment of borrowings |
|
| ( | ( |
| ( | ||
Repayment of lease liabilities |
|
| ( | ( |
| ( | ||
Proceeds from other borrowings |
|
| | |
| | ||
Repayment of other borrowings |
|
| ( | ( |
| ( | ||
Proceeds from convertible note, net | | — | — | |||||
Interest paid |
|
| ( | ( |
| ( | ||
Issuance of shares |
|
| — | — |
| | ||
Dividends paid to non-controlling interests |
|
| ( | ( |
| ( | ||
Share repurchase | ( | — | — | |||||
Net cash generated from/(used in) financing activities | | ( | | |||||
Net decrease in cash and cash equivalents | ( | ( | ( | |||||
Cash and cash equivalents at beginning of the year |
|
| | |
| | ||
Effect of changes in foreign exchange rates |
|
| | |
| ( | ||
Cash and cash equivalents at end of the year |
| 19 |
| | |
| | |
Analysis of the balances of cash and cash equivalents at 31 December |
|
|
| |||||
Bank and cash balances |
|
| | |
| | ||
Less: Assets held-for-sale |
|
| — | — |
| — | ||
| 19 |
| | |
| |
The above consolidated statements of cash flow should be read in conjunction with the accompanying notes.
F-10
1 General information
Aesthetic Medical International Holdings Group Limited (the “Company”) was incorporated in the Cayman Islands on 27 May 2011 as an exempted company with limited liability under the Companies Law, Cap. 22 (Law 3 of 1961, as consolidated and revised) of the Cayman Islands. The address of its registered office is Offshore Incorporations (Cayman) Limited, Scotia Centre, 4th Floor, P.O. Box 2804, George Town, Grand Cayman KY1-1112, Cayman Islands.
The Company and its subsidiaries (together, the “Group”) are engaged in the provision of non-surgical aesthetic medical services, surgical aesthetic medical services, other aesthetic medical services and general healthcare services in the People’s Republic of China (the “PRC”). The principal activities of the subsidiaries are set out in Note 36.
The Company completed its initial public offering and listing of American Depositary Shares (“ADSs”) on the NASDAQ Global Market in October, 2019, and raised net proceeds of US$
These consolidated financial statement are presented in Renminbi (“RMB”) and rounded to the nearest thousand yuan, unless otherwise stated.
2 Summary of significant accounting policies
The principal accounting policies applied in the preparation of these consolidated financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated.
2.1 Basis of preparation
The consolidated financial statements of the Company have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board. These consolidated financial statements have been prepared under the historical cost convention, as modified by the revaluation of convertible note, investment properties and assets held for sale, which were carried at fair value.
Going Concern
The consolidated financial statements of the Company have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As a result, the Group had a loss of RMB
These factors raise substantial doubt about the Company’s ability to continue as a going concern. Besides continuing to negotiate with the banks, the Company is evaluating several measures of financing, such as external financings,which will also be considered. Currently, the Company intends to raise loans from financial institutions and potential funders to maintain its normal operations. The Company’s ability to continue as a going concern is primarily dependent on the Company’s ability to arrange adequate financing arrangements and generate cash flows from its operations.
The Company closed its private placement with Hainan Oriental Jiechuang Investment Partnership (“Jiechuang”) for aggregate gross proceeds of RMB
F-11
2 Summary of significant accounting policies (Continued)
2.2.1 New standards and amendments to standards adopted by the Group
The following new standards and amendments to standards are mandatory for accounting periods beginning on or after 1 January 2022. The adoption of these new standards and amendments to standards does not have a significant impact to the results and financial position of the Group:
Amendments to IFRS 3: Reference to the Conceptual Framework |
Amendments to IAS 16: Proceeds Before Intended Use |
Amendments to IFRS 1, IFRS 9, IFRS 16 and IAS 41: Annual Improvements to 2018-2021 cycle |
Amendments to IAS 37: Onerous Contracts - Costs of Fufilling a Contract |
Amendments to IFRS 9: Financial Instruments – Fees in the ’10 per cent’ test for derecognition of financial liabilities |
2.2.2 New standards, amendments to standards and interpretations not yet adopted
The following are new standards, amendments to standards and interpretations which have been issued but are not effective and have not been early adopted. The Group plans to adopt these new standards, amendments to standards and interpretations when they become effective:
Amendments to IFRS 10 and IAS 28 (2011): Sale or Contribution of Assets Between an Investor and Its Associate or Joint Venture |
| To be determined |
IFRS 17: Insurance Contracts and related amendments |
| 1 January 2023 |
Amendments to IAS 1: Classification of Liabilities as Current and Non-current |
| 1 January 2023 |
Amendments to IAS 1: Disclosure of accounting policies | 1 January 2023 | |
Amendments to IAS 8: Definition of accounting estimates | 1 January 2023 | |
Amendments to IAS 12: Deferred Tax Related to Assets and Liabilities Arising from a Single Transaction | 1 January 2023 |
F-12
2 Summary of significant accounting policies (Continued)
2.2 Changes in accounting policy and disclosures (Continued)
2.2.2 New standards, amendments to standards and interpretations not yet adopted (Continued)
Management is in the process of assessing the impact of these standards, amendments and interpretations to existing IFRS. None of these is expected to have a significant effect on the consolidated financial statements of the Group.
2.3 Subsidiaries
Consolidation
A subsidiary is an entity (including a structured entity) over which the Group has control. The Group controls an entity when the Group is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are consolidated from the date on which control is transferred to the Group. They are deconsolidated from the date that control ceases.
(a) | Business combinations |
The Group applies the acquisition method to account for business combinations. The consideration transferred for the acquisition of a subsidiary is the fair values of the assets transferred, the liabilities incurred to a assumed from the former owners of the acquiree and the equity interests issued by the Group. The consideration transferred includes the fair value of any asset or liability resulting from a contingent consideration arrangement. Identifiable assets acquired and liabilities and contingent liabilities assumed in a business combination are measured initially at their fair values at the acquisition date.
The Group recognises any non-controlling interest in the acquiree on an acquisition by acquisition basis. Non-controlling interests in the acquiree that are present ownership interests and entitle their holders to a proportionate share of the entity’s net assets in the event of liquidation are measured at either fair value or the present ownership interests’ proportionate share in the recognised amounts of the acquiree’s identifiable net assets. All other components of non-controlling interests are measured at their acquisition date fair value, unless another measurement basis is required by IFRS.
Acquisition-related costs are expensed as incurred.
If the business combination is achieved in stages, the acquisition date carrying value of the acquirer’s previously held equity interest in the acquiree is re-measured to fair value at the acquisition date; any gains or losses arising from such re-measurement are recognised in consolidated statement of comprehensive income.
Any contingent consideration to be transferred by the Group is recognised at fair value at the acquisition date. Subsequent changes to the fair value of the contingent consideration that is deemed to be an asset or liability is recognised in accordance with IFRS 9 in consolidated statement of comprehensive income. Contingent consideration that is classified as equity is not remeasured, and its subsequent settlement is accounted for within equity.
The excess of the consideration transferred, the amount of any non-controlling interest in the acquiree and the acquisition-date fair value of any previous equity interest in the acquiree over the fair value of the identifiable net assets acquired is recorded as goodwill. If the total of consideration transferred, non-controlling interest recognised and previously held interest measured is less than the fair value of the net assets of the subsidiary acquired in the case of a bargain purchase, the difference is recognised directly in the statement of comprehensive income.
F-13
2 Summary of significant accounting policies (Continued)
2.3 Subsidiaries (Continued)
Consolidation (Continued)
(a) | Business combinations (Continued) |
Intra-group transactions, balances and unrealised gains on transactions between group companies are eliminated. Unrealised losses are also eliminated unless the transaction provides evidence of an impairment of the transferred asset. When necessary, amounts reported by subsidiaries have been adjusted to conform with the Group’s accounting policies.
(b) | Changes in ownership interests in subsidiaries without change of control |
Transactions with non-controlling interests that do not result in a loss of control are accounted for as equity transactions - that is, as transactions with the owners of the subsidiary in their capacity as owners. The difference between fair value of any consideration paid and the relevant share acquired of the carrying amount of net assets of the subsidiary is recorded in equity. Gains or losses on disposals to non-controlling interests are also recorded in equity.
(c) | Disposal of subsidiaries |
When the Group ceases to have control, any retained interest in the entity is re-measured to its fair value at the date when control is lost, with the change in carrying amount recognised in consolidated statement of comprehensive income. The fair value is the initial carrying amount for the purposes of subsequently accounting for the retained interest as an associate, joint venture or financial asset. In addition, any amounts previously recognised in other comprehensive income in respect of that entity are accounted for as if the Group had directly disposed of the related assets or liabilities. It means the amounts previously recognised in other comprehensive income are reclassified to the consolidated statement of comprehensive income or transferred to another category of equity as specified/permitted by applicable IFRSs.
(d) | Contractual arrangements with respect to equity interests in certain PRC subsidiaries |
Since the Industry Catalog for Guiding Foreign Investment (Revision 2015) became effective in April 2015, PRC law only allows foreign investment in PRC medical institutions through joint venture entities, and the foreign shareholding in these entities is limited to
F-14
2 Summary of significant accounting policies (Continued)
2.3 Subsidiaries (Continued)
Consolidation (Continued)
(d) | Contractual arrangements with respect to equity interests in certain PRC subsidiaries (Continued) |
As of December 31, 2022, several of the above-mentioned subsidiaries have been divested or ceased operation of, including Changsha Pengai Aesthetic Medical Hospital Co., Ltd., Nanchang Pengai Xiuqi Aesthetic Medical Hospital Co., Ltd., Hangzhou Pengai Aesthetic Medical Outpatient Department and Shenzhen Miaoyan Aesthetic Medical Clinic.
The principal terms of the Contractual Arrangements are described below:
(i). Loan agreement
Shenzhen Pengai Investment, as the lender, entered into certain loan agreements with Dr. Zhou Pengwu, as the borrower. Pursuant to each of these loan agreements, Shenzhen Pengai Investment agrees to extend a loan to Dr. Zhou Pengwu in an equivalent amount to the purchase price to be paid by Dr. Zhou Pengwu for acquiring the Target Equity Interests. Pursuant to each of these loan agreements, Dr. Zhou Pengwu shall repay the loan by transferring the current and future economic interest of the Target Equity Interests to Shenzhen Pengai Investment.
(ii). Economic interest transfer agreement
Dr. Zhou Pengwu, Shenzhen Pengai Investment and each of the Relevant Subsidiaries entered into certain economic interest transfer agreements. Pursuant to each of these economic interest transfer agreements, the economic interest in relation to the Target Equity Interests currently held and subsequently acquired by Dr. Zhou Pengwu, including but not limited to (i) incomes arising from the disposal of the Target Equity Interests (including derivative equity interest of the Target Equity Interests) under any circumstance; (ii) dividends and bonus obtained on the basis of the Target Equity Interests (including derivative equity interest of the Target Equity Interests) under any circumstance; (iii) residual assets and other economic profits allocated after the liquidation of the Relevant Subsidiaries, and (iv) any other cash income, property and economic benefit arising from the Target Equity Interests (including derivative equity interest of the Target Equity Interests), shall be transferred to Shenzhen Pengai Investment. Upon the execution of each economic interest transfer agreement, the repayment obligation of Dr. Zhou Pengwu under each loan agreement is deemed fully discharged.
(iii). Exclusive option agreement
Dr. Zhou Pengwu, Shenzhen Pengai Investment and each of the Relevant Subsidiaries entered into certain exclusive option agreements. Pursuant to these exclusive option agreements, Dr. Zhou Pengwu irrevocably granted Shenzhen Pengai Investment an exclusive right to purchase, or have its designated person(s) to purchase, at its discretion, all or part of his equity interest in the Relevant Subsidiaries, and the purchase price shall be the lowest price permitted by applicable PRC law. Each of Dr. Zhou Pengwu and the Relevant Subsidiaries undertakes that, among others, without the prior written consent of Shenzhen Pengai Investment, he or it shall or shall cause the Relevant Subsidiaries not to declare any dividends or distribute any residual profits, change or amend its articles of association, increase or decrease its registered capital, or change its structure of registered capital in other manners. In the event that Dr. Zhou Pengwu increases his capital injection into the Relevant Subsidiaries, Dr. Zhou Pengwu undertakes and confirms that any additional equity so acquired shall be subject to the purchase option. Unless terminated by Shenzhen Pengai Investment at its sole discretion, the exclusive option agreement will remain effective until all equity interest in the Relevant Subsidiaries held by Dr. Zhou Pengwu is transferred or assigned to Shenzhen Pengai Investment or its designated person(s).
F-15
2 Summary of significant accounting policies (Continued)
2.3 Subsidiaries (Continued)
Consolidation (Continued)
(d) | Contractual arrangements with respect to equity interests in certain PRC subsidiaries (Continued) |
Pursuant to a relevant power of attorney executed by Dr. Zhou Pengwu, he has irrevocably authorized Shenzhen Pengai Investment or its designated person(s) to exercise all of such shareholder’s voting and other rights associated with the Target Equity Interests in each of the Relevant Subsidiaries, including but not limited to, the right to attend shareholder meetings, the right to vote, the right to sell, transfer, pledge or dispose of the Target Equity Interests and the right to appoint legal representatives, directors and other management. The proxy agreement remains effective as long as Dr. Zhou Pengwu remains a shareholder of the Relevant Subsidiaries, unless Shenzhen Pengai Investment has given contrary written instructions.
(v). Equity interest pledge agreement
Dr. Zhou Pengwu as pledgor, Shenzhen Pengai Investment as pledgee, and each of the Relevant Subsidiaries entered into certain equity interest pledge agreements. Pursuant to these equity interest pledge agreements, Dr. Zhou Pengwu has pledged all of the Target Equity Interests in Relevant Subsidiaries and agreed to pledge all future equity interest in the Relevant Subsidiaries acquired by him to Shenzhen Pengai Investment to guarantee the performance by Dr. Zhou Pengwu and the Relevant Subsidiaries of their respective obligations under the loan agreement, the economic interest transfer agreement, the exclusive option agreement and the power of attorney. If the Relevant Subsidiaries or Dr. Zhou Pengwu breach any obligations under these agreements, Shenzhen Pengai Investment, as pledgee, will be entitled to dispose of the pledged equity and have priority to be compensated by the proceeds from the disposal of the pledged equity. Dr. Zhou Pengwu shall not permit the existence of any security interest or other encumbrance on the pledged equity interest or any portion thereof, without the prior written consent of Shenzhen Pengai Investment and the Relevant Subsidiaries shall not assent to or assist in such actions. These equity interest pledge agreements will remain effective until Dr. Zhou Pengwu discharges all the obligations under the loan agreement, the economic interest transfer agreement, the exclusive option agreement and the power of attorney and the full payment of all direct, indirect and derivative losses and losses of anticipated profits, suffered by the pledgee, incurred as a result of any breach by Dr. Zhou Pengwu or the Relevant Subsidiaries under these agreements or invalidity, revocation and termination of any of these agreements.
(vi). Spousal consent letter
Pursuant to relevant spousal consent letters executed by Ms. Ding Wenting, she unconditionally and irrevocably agreed that the equity interest in each of the Relevant Subsidiaries held or to be held by Dr. Zhou Pengwu and registered or to be registered in his name will be disposed of pursuant to the loan agreement, the economic interest transfer agreement, the exclusive option agreement and the power of attorney. Ms. Ding Wenting agreed not to assert any rights over the equity interest in the Relevant Subsidiaries held or to be held by Dr. Zhou Pengwu. In addition, in the event that Ms. Ding Wenting obtains any equity interest in each of the Relevant Subsidiaries for any reason, she agreed to be bound by the Contractual Arrangements.
(e) | Risks in relation to the Contractual Arrangements |
In the opinion of the Company’s management, the Contractual Arrangements are in compliance with the current PRC laws and are legally binding and enforceable. However, uncertainties in the interpretation and enforcement of the PRC laws, regulations and policies could limit the Company’s ability to enforce these contractual arrangements.
F-16
2 Summary of significant accounting policies (Continued)
2.3 Subsidiaries (Continued)
Consolidation (Continued)
(e) | Risks in relation to the Contractual Arrangements (Continued) |
In January 2015, the Ministry of Commerce (“MOFCOM”), released for public comment a proposed PRC law, the Draft Foreign Investment Enterprises (“FIE”) Law, that appears to include contractual arrangements within the scope of entities that could be considered to be FIEs, that would be subject to restrictions under existing PRC law on foreign investment in certain categories of industry. Specifically, the Draft FIE Law introduces the concept of “actual control” for determining whether an entity is considered to be an FIE. In addition to control through direct or indirect ownership or equity, the Draft FIE Law includes control through contractual arrangements within the definition of “actual control”. If the Draft FIE Law is passed by the People’s Congress of the PRC and goes into effect in its current form, these provisions regarding control through contractual arrangements could be construed to include the Company’s contractual arrangements, and as a result, the Relevant Subsidiaries could become explicitly subject to the current restrictions on foreign investment in certain categories of industry. The Draft FIE Law includes provisions that would exempt from the definition of FIEs where the ultimate controlling shareholders are either entities organized under PRC law or individuals who are PRC citizens. The Draft FIE Law is silent as to what type of enforcement action might be taken. If the restrictions and prohibitions on FIEs included in the Draft FIE Law are enacted and enforced in their current form, the Company’s ability to use the contractual arrangements and the Company’s ability to conduct business through the contractual arrangements could be severely limited.
The Company’s ability to control the Target Equity Interests in the Relevant Subsidiaries also depends on the power of attorney exercised by Shenzhen Pengai Investment to vote on all matters requiring shareholders’ approvals in the Relevant Subsidiaries. As noted above, the Company believes these powers of attorney are legally binding and enforceable but may not be as effective as direct equity ownership. In addition, if the Company’s corporate structure or the contractual arrangements were found to be in violation of any existing PRC laws and regulations, the PRC regulatory authorities could, within their respective jurisdictions:
● | revoke the Contractual Arrangements; |
● | impose fines, confiscating the income from the Relevant Subsidiaries, or imposing other requirements with which the Company may not be able to comply; |
● | discontinue or restrict the Company’s operations in the PRC; |
● | impose conditions or requirements with which the Company may not be able to comply; or |
● | take other regulatory or enforcement actions that could be harmful to the Company’s business. |
The imposition of any of these restrictions or actions may result in a material adverse effect on the Company’s ability to conduct its business. In addition, if the imposition of any of these restrictions causes the Company to lose the right to direct the activities of the Relevant Subsidiaries or the right to receive their economic benefits, the Company may no longer be able to consolidate the financial statements of the Relevant Subsidiaries. In the opinion of management, the likelihood of losing the benefits in respect of the Company’s current ownership structure or the contractual arrangements is remote.
F-17
2 Summary of significant accounting policies (Continued)
2.4 Associates
An associate is an entity over which the Group has significant influence but not control, generally accompanying a shareholding of between
The Group’s share of post-acquisition profit or loss is recognised in the statement of comprehensive income, and its share of post-acquisition movements in other comprehensive income is recognised in other comprehensive income with a corresponding adjustment to the carrying amount of the investment. If the ownership interest in an associate is reduced but significant influence is retained, only a proportionate share of the amounts previously recognised in other comprehensive income is reclassified to profit or loss where appropriate. When the Group’s share of losses in an associate equals or exceeds its interest in the associate, including any other unsecured receivables, the Group does not recognise further losses, unless it has incurred legal or constructive obligations or made payments on behalf of the associate.
The Group determines at each reporting date whether there is any objective evidence that the investment in the associate is impaired. If this is the case, the Group calculates the amount of impairment as the difference between the recoverable amount of the associate and its carrying value and recognises the amount adjacent to ‘share of profit of investments accounted for using equity method’ in the consolidated statement of comprehensive income.
Profits and losses resulting from upstream and downstream transactions between the Group and its associate are recognised in the Group’s financial statements only to the extent of unrelated investor’s interests in the associates. Unrealised losses are eliminated unless the transaction provides evidence of an impairment of the asset transferred. Accounting policies of associates have been changed where necessary to ensure consistency with the policies adopted by the Group.
Gain or losses on dilution of equity interest in associates are recognised in the consolidated statement of comprehensive income.
2.5 Segment reporting
Operating segments are reported in a manner consistent with the internal reporting provided to the chief operating decision-maker, which is the Board of Directors. In the respective periods presented, the Company had
2.6 Foreign currency translation
(a) | Functional and presentation currency |
Items included in the consolidated financial statements of each of the Group’s entities are measured using the currency of the primary economic environment in which the entity operates (the “functional currency”). The consolidated financial statements are presented in RMB, which is the Company’s functional and the Group’s presentation currency.
F-18
2 Summary of significant accounting policies (Continued)
2.6 Foreign currency translation (Continued)
(b) | Transactions and balances |
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of the transactions or valuation where items are re- measured. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognised in consolidated statement of comprehensive income.
Exchange differences on Series A Preferred Shares, convertible note and exchangeable note liabilities were recorded in “fair value (loss)/gain of convertible redeemable preferred shares”, “fair value loss of convertible note” and “fair value (loss)/gain of exchangeable note liabilities”, respectively.
(c) | Group companies |
The results and financial position of all the Group entities (none of which has the currency of a hyper-inflationary economy) that have a functional currency different from the presentation currency are translated into the presentation currency as follows:
(i) | assets and liabilities for each balance sheet presented are translated at the closing rate at the reporting date; |
(ii) | income and expenses for each statement of comprehensive income are translated at average exchange rates (unless this average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case income and expenses are translated at the rate on the dates of the transactions); and |
(iii) | all resulting exchange differences are recognised in other comprehensive income. |
Goodwill and fair value adjustments arising on the acquisition of a foreign entity are treated as assets and liabilities of the foreign entity and translated at the closing rate. Currency translation differences arising are recognised in other comprehensive income.
2.7 Property, plant and equipment
Property, plant and equipment are stated at historical cost less depreciation. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
Subsequent costs are included in the asset’s carrying amount or recognised as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Group and the cost of the item can be measured reliably. The carrying amount of the replaced part is derecognised. All other repairs and maintenance are charged to the consolidated statement of comprehensive income during the financial period in which they are incurred.
F-19
2 Summary of significant accounting policies (Continued)
2.7 Property, plant and equipment (Continued)
Depreciation of property, plant and equipment is calculated using the straight-line method to allocate cost of each asset to their residual values over their estimated useful lives, as follows:
- Leasehold improvements |
| Shorter of remaining lease term and the estimated useful lives of the assets |
- Right of use assets | Shorter of the asset’s useful life and the lease term on a straight-line basis | |
- Machinery and equipment | ||
- Office equipment, furniture, fixture and motor vehicles | - | |
- Buildings | - |
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at the end of each reporting period.
An asset’s carrying amount is written down immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount.
Gains and losses on disposals are determined by comparing the proceeds with the carrying amount and are recognised within “general and administrative expenses” in the consolidated statement of comprehensive income.
Gains and losses on lease modification relating to the full termination of the leases are recognised within “general and administrative expenses” in the consolidated statement of comprehensive income.
2.8 Investment properties
Properties that are held for long – term rental yields or for capital appreciation or both, and that are not occupied by the companies in the Group, are classified as investment properties in the consolidated financial statements. Investment properties are carried at fair value basis and its movement will be reviewed annually.
If an owner-occupied property becomes an investment property because its use has changed, any difference resulting between the carrying amount and the fair value of this property at the date of transfer is recognised in equity as a revaluation of property, plant and equipment. However, if the fair value of the property at the date of transfer which results in a reversal of the previous impairment loss, the write-back is recognised in the consolidated income statement.
Subsequent expenditure is included to the asset’s carrying amount only when it is probable that future economic benefits associated with the item will flow to the Group and the cost of the item can be measured reliably. All other repairs and maintenance costs are expensed in consolidated statement of comprehensive income during the financial periods in which they are incurred.
(a) | Goodwill |
Goodwill arising on the acquisition of subsidiaries represents the excess of the consideration transferred, the amount of any non-controlling interest in the acquire and the acquisition- date fair value of any previous equity interest in the acquiree over the fair value of the identified net assets acquired.
For the purpose of impairment testing, goodwill acquired in a business combination is allocated to each of the cash-generating units (“CGUs”), or groups of CGUs, that is expected to benefit from the synergies of the combination. Each unit or group of units to which the goodwill is allocated represents the lowest level within the entity at which the goodwill is monitored for internal management purposes.
F-20
2 Summary of significant accounting policies (Continued)
2.9 Intangible assets (Continued)
(a) | Goodwill (Continued) |
Goodwill impairment reviews are undertaken annually or more frequently if events or changes in circumstances indicate a potential impairment. The carrying value of the CGU containing the goodwill is compared to the recoverable amount, which is the higher of value in use and the fair value less costs of disposal. Any impairment is recognised immediately as an expense and is not subsequently reversed. See note 15 for the goodwill impairment charges recognised in the respective years presented.
(b) | Computer software |
Acquired computer software licenses are capitalised on the basis of the costs incurred to acquire and bring the specific software into usage. These costs are amortised using the straight-line method over their estimated useful lives of
(c) | Medical licenses and tradenames |
Medical licenses and tradenames acquired through business combinations are initially recognised at fair value. Medical licences are amortised on a straight-line basis over respective license periods (ranged from to
2.10 Impairment of non-financial asset
Intangible assets that have an indefinite useful life or intangible assets not ready to use are not subject to amortisation and are tested annually for impairment. Assets that are subject to amortisation are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognised for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs of disposal and value in use. For purposes of periodic impairment assessment performed at each reporting date, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash-generating units). Non-financial assets other than goodwill that suffered an impairment are reviewed for possible reversal of the impairment at each reporting date.
2.11 Financial assets
2.11.1 Classification
The Group classifies its financial assets in the following measurement categories:
(i) | those to be measured subsequently at fair value (either through other comprehensive income, or through profit or loss), and |
(ii) | those to be measured at amortised cost. |
The classification depends on the Group’s business model for managing the financial assets and the contractual terms of the cash flows.
At initial recognition, the Group measures a financial asset at its fair value plus, in the case of a financial asset not at fair value through profit or loss, transaction costs that are directly attributable to the acquisition of the financial asset. Transaction costs of financial assets carried at fair value through profit or loss are expensed in profit or loss.
F-21
2 Summary of significant accounting policies (Continued)
2.11 Financial assets (Continued)
2.11.1 Classification (Continued)
For assets measured at fair value, gain and loss will either be recorded in profit or loss or other comprehensive income. For investments in debt instruments, this will depend on the business model in which the investment is held. For investments in equity instruments, this will depend on whether the Group has made an irrevocable election at the time of initial recognition to account for the equity investment at fair value through other comprehensive income.
The Group reclassifies debt investments when and only when its business model for managing those assets changes.
2.11.2 Recognition and measurement
Debt instruments
Subsequent measurement of debt instruments depends on the Group’s business model for managing the asset and the cash flow characteristics of the asset. There are two measurement categories into which the Group classifies its debt instruments:
| ● | Amortised cost: Assets that are held for collection of contractual cash flows where those cash flows represent solely payments of principal and interest are measured at amortised cost. A gain or loss on a debt investment that is subsequently measured at amortised cost and is not part of a hedging relationship is recognised in the consolidated statement of profit or loss when the asset is derecognised or impaired. Interest income from these financial assets is included in finance income using the effective interest rate method. |
| ● | Fair value through profit or loss: Assets that do not meet the criteria for amortised cost or financial assets at fair value through other comprehensive income are measured at fair value through profit or loss. A gain or loss on a debt investment that is subsequently measured at fair value through profit or loss and is not part of a hedging relationship is recognised in profit or loss and presented net in ‘other gains, net’ in the period in which it arises. |
Equity instruments
The Group subsequently measures all equity investments at fair value. Where the Group’s management has elected to present fair value gains and losses on equity investments in other comprehensive income, there is no subsequent reclassification of fair value gains and losses to profit or loss following the derecognition of the investment. Dividends from such investments continue to be recognised in profit or loss as other income when the Group’s right to receive payments is established.
Changes in the fair value of financial assets at fair value through profit or loss are recognised in other gains, net in the consolidated statement of comprehensive income as applicable. Impairment losses (and reversal of impairment losses) on equity investments measured at fair value through other comprehensive income are not reported separately from other changes in fair value.
2.11.3 Derecognition
Financial assets are derecognised when the rights to receive cash flows from the financial assets have expired or have been transferred and the Group has transferred substantially all the risks and rewards of ownership.
F-22
2 Summary of significant accounting policies (Continued)
2.12 Offsetting financial instruments
Financial assets and liabilities are offset and the net amount reported in the consolidated statements of financial position when there is a legally enforceable right to offset the recognised amounts and there is an intention to settle on a net basis or realise the asset and settle the liability simultaneously. The legally enforceable right must not be contingent on future events and must be enforceable in the normal course of business and in the event of default, insolvency or bankruptcy of the Company or the counterparty.
2.13 Impairment of financial assets
The Group has the following types of financial assets subject to IFRS 9’s expected credit loss model:
| ● | Trade receivables |
| ● | Other receivables and deposits |
| ● | Cash and cash equivalents |
| ● | Amounts due from related parties |
The Group assesses on a forward looking basis the expected credit losses associated with its assets carried at amortised cost.
For trade receivables, the Group applies the simplified approach permitted by IFRS 9, which requires expected lifetime losses to be recognised from initial recognition of the receivables.
Impairment on other receivables, deposits, amounts due from related parties are measured as either 12-month expected credit losses or lifetime expected credit loss, depending on whether there has been a significant increase in credit risk since initial recognition, then impairment is measured as lifetime expected credit losses.
To manage risk arising from pledged deposits and cash and cash equivalents, the Group only transacts with state-owned or reputable financial institutions. There has been no recent history of default in relation to these financial institutions.
2.14 Inventories
Inventories are stated at the lower of cost and net realisable value. Cost is determined using the weighted average method. Net realisable value is the estimated selling price in the ordinary course of business, less applicable variable selling expenses.
2.15 Cash and cash equivalents
In the consolidated statement of cash flows, cash and cash equivalents includes cash in hand and deposits held at call with banks.
2.16 Assets (or disposal groups) held-for-sale
Assets (or disposal groups) are classified as held for sale when their carrying amount is to be recovered principally through a sale transaction and a sale is considered highly probable. The assets (except for certain assets as explained below), (or disposal groups), are stated at the lower of carrying amount and fair value less costs to sell. Deferred tax assets, assets arising from employee benefits, financial assets (other than investments in subsidiaries and associates) and investment properties, which are classified as held for sale, would continue to be measured in accordance with the policies set out elsewhere in Note 2.
F-23
2 Summary of significant accounting policies (Continued)
2.17 Share capital
Ordinary shares are classified as equity. Mandatorily redeemable preference shares are classified as liabilities.
Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds.
2.18 Trade and other payables
Trade payables are obligations to pay for goods or services that have been acquired in the ordinary course of business from suppliers. Trade payables are classified as current liabilities if payment is due within one year or less (or in the normal operating cycle of the business if longer). If not, they are presented as non-current liabilities.
Trade and other payables are recognised initially at fair value and subsequently measured at amortised cost using the effective interest method.
2.19 Borrowings
Borrowings are recognised initially at fair value, net of transaction costs incurred. Borrowings are subsequently carried at amortised cost; any difference between the proceeds (net of transaction costs) and the redemption value is recognised in consolidated statement of comprehensive income over the periods of the borrowings using the effective interest method.
Fees paid on the establishment of loan facilities are recognised as transaction costs of the loan to the extent that it is probable that some or all of the facility will be drawn down. In this case, the fee is deferred until the draw-down occurs. To the extent there is no evidence that it is probable that some or all of the facility will be drawn down, the fee is capitalised as a pre-payment for liquidity services and amortised over the periods of the facility to which it relates.
Preference shares, if mandatorily redeemable at a specific date or redeemable at the option of the holder, are classified as liabilities. The dividends on these preference shares are recognised in the consolidated statement of comprehensive income as interest expense.
Borrowings are removed from the consolidated balance sheets when the obligation specified in the contract is discharged, cancelled or expired. The difference between the carrying amount of a financial liability that has been extinguished or transferred to another party and the consideration paid, including any non-cash assets transferred or liabilities assumed, is recognised in consolidated statement of comprehensive income as other income or finance costs.
Where the terms of a financial liability are renegotiated and the entity issues equity instruments to a creditor to extinguish all or part of the liability (debt for equity swap), a gain or loss is recognised in consolidated statement of comprehensive income, which is measured as the difference between the carrying amount of the financial liability and the fair value of the equity instruments issued.
Borrowings are classified as current liabilities unless the Group has an unconditional right to defer settlement of the liability for at least 12 months after the end of the reporting period.
The income tax expense for the year comprises current and deferred tax. Tax is recognised in the consolidated statement of comprehensive income, except to the extent that it relates to items recognised in other comprehensive income or directly in equity. In this case, the tax is also recognised in other comprehensive income or directly in equity.
F-24
2 Summary of significant accounting policies (Continued)
2.20 Current and deferred income tax (Continued)
(a) | Current income tax |
The current income tax charge is calculated on the basis of the tax laws enacted or substantively enacted at the reporting date in the countries where the Company’s subsidiaries and associates operate and generate taxable income. Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is subject to interpretation. It establishes provisions where appropriate on the basis of amounts expected to be paid to the tax authorities.
(b) | Deferred income tax |
Inside basis differences
Deferred income tax is recognised, using the liability method, on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the consolidated financial statements. However, deferred tax liabilities are not recognised if they arise from the initial recognition of goodwill, the deferred income tax is not accounted for if it arises from initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects neither accounting nor taxable profit or loss. Deferred income tax is determined using tax rates (and laws) that have been enacted or substantively enacted by the balance sheet date and are expected to apply when the related deferred income tax asset is realised or the deferred income tax liability is settled.
Deferred income tax assets are recognised only to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilised.
Outside basis differences
Deferred income tax liabilities are provided on taxable temporary differences arising from investments in subsidiaries and associates, except for deferred income tax liability where the timing of the reversal of the temporary difference is controlled by the Group and it is probable that the temporary difference will not reverse in the foreseeable future. Generally the Group is unable to control the reversal of the temporary difference of associates. Only when there is an agreement in place that gives the Group the ability to control the reversal of the temporary difference in the foreseeable futures, deferred tax liability in relation to taxable temporary difference arising from the associate’s undistributed profits is not recognised.
Deferred income tax assets are recognised on deductible temporary differences arising from investments in subsidiaries and associates only to the extent that it is probable the temporary difference will reverse in the future and there is sufficient taxable profit available against which the temporary difference can be utilised.
(c) Offsetting
Deferred income tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when the deferred income taxes assets and liabilities relate to income taxes levied by the same taxation authority on either the taxable entity or different taxable entities where there is an intention to settle the balances on a net basis.
F-25
2 Summary of significant accounting policies (Continued)
2.21 Employee benefits
(a) | Pension obligations |
The group companies incorporated in the PRC contribute based on certain percentage of the salaries of the employees to a defined contribution retirement benefit plan organised by relevant government authorities in the PRC on a monthly basis. The government authorities undertake to assume the retirement benefit obligations payable to all existing and future retired employees under these plans and the Group has no further obligation for post-retirement benefits beyond the contributions made. Contributions to these plans are expensed as incurred. Assets of the plans are held and managed by government authorities and are separate from those of the Group.
(b) | Profit-sharing and bonus plans |
The Group recognises a liability and an expense for bonuses, based on performance and takes into consideration the profit attributable to the Group’s shareholders after certain adjustments. The Group recognises a provision where contractually obliged or where there is a past practice that has created a constructive obligation.
(c) | Share based compensation |
The Group measures share-based compensation at the grant date based on the fair value of the equity awards determined using the Binomial option-pricing model. As the Group has granted share options with a service-only condition, the Group elected to recognize compensation expense over the period in which the recipient is required to provide service in exchange for the equity award.
2.22 Provisions
Provisions are recognised when the Group has a present legal or constructive obligation as a result of past events; it is probable that an outflow of resources will be required to settle the obligation; and the amount has been reliably estimated.
Where there are a number of similar obligations, the likelihood that an outflow will be required in settlement is determined by considering the class of obligations as a whole. A provision is recognised even if the likelihood of an outflow with respect to any one item included in the same class of obligations may be small.
Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the obligation. The increase in the provision due to passage of time is recognised as interest expense.
2.23 Revenue recognition
Revenue is recognised when or as the control of the goods or services is transferred to the customer at an amount that reflects the consideration to which the Group expects to be entitled in exchange for those goods or services. Depending on the terms of the contract and the applicable laws, services may be provided over time or at a point in time.
When control of goods or services is transferred over time, revenue is recognised over the period of the contract by reference to the progress towards complete satisfaction of that performance obligation. Otherwise, revenue is recognised at a point in time when the customer obtains control of the goods or services.
F-26
2 Summary of significant accounting policies (Continued)
2.23 Revenue recognition (Continued)
The progress towards complete satisfaction of the performance obligation is measured based on one of the following methods that best depict the Group’s performance in satisfying the performance obligation:
| ● | direct measurements of the value transferred by the Group to the customer; or |
| ● | the Group’s efforts or inputs to the satisfaction of the performance obligation. |
A contract asset is the Group’s right to consideration in exchange for goods and services that the Group has transferred to a customer. A receivable is recorded when the Group has an unconditional right to consideration. A right to consideration is unconditional if only the passage of time is required before payment of that consideration is due.
A contract liability is recognised when the Group has the obligation to transfer services to the customers for which the consideration received (or an amount of considerations is due) exceeds the measure of the remaining unsatisfied performance obligations.
The Group recognises the costs of obtaining and fulfilling a contract with a customers within the contract assets if the Group expects to recover those costs.
The following is a description of the accounting policy for the principal revenue streams of the Group:
(i) | Non-surgical aesthetic medical services, surgical aesthetic medical services and other aesthetic medical services |
The period of these services rendered is usually within a day, except for certain large-scale surgical aesthetic medical services in which customers receive inpatient treatment. The period of inpatient stays usually does not exceed more than a week.
The Group has certain aesthetic medical services package in 2022 for which customers pay in advance. The packages can be utilized for one year from the effective date. If the package has not been used within one year time period, any payment received is recorded as a contract liability in the consolidated balance sheet.
Revenue is recognised at a point in time when the respective services are rendered or a contract lability has been fulfilled. For certain aesthetic medical services package issued in 2022, revenue is recognised over time according to the proportion of the services performed as compared to the total services offered.
(ii) | General healthcare services |
Revenues are recognised upon the provision of the relevant services for which the service period is usually within a day.
Revenue is recognised at a point in time when the respective services are rendered.
Management reviews the utilisation pattern of contract liabilities and treatment progress of individual customers on a regular basis to determine full recognition of the corresponding contract liabilities in the consolidated statement of comprehensive income.
2.24 Interest income
Interest income is recognised using the effective interest method.
F-27
2 Summary of significant accounting policies (Continued)
2.25 Leases
The Group leases certain properties. Lease terms are negotiated on an individual basis and contain a wide range of different terms and conditions. The lease agreements do not impose any covenants.
Leases are recognised as a right-of-use asset and a corresponding liability at the date on which the leased asset is available for use by the Group. Each lease payment is allocated between the liability and finance cost. The finance cost is charged to profit or loss over the lease period so as to produce a constant periodic rate of interest on the remaining balance of the liability for each period.
Assets and liabilities arising from a lease are initially measured on a present value basis. Lease liabilities include the net present value of the following lease payments:
| ● | fixed payments (including in-substance fixed payments), less any lease incentives receivable; and |
| ● | payments of penalties for terminating the lease, if the lease reflects the lessee exercising that option |
The lease payments are discounted using the interest rate implicit in the lease, if that rate can be determined, or the Group’s incremental borrowing rate.
Right-of-use assets are measured at cost comprising the following:
| ● | the amount of the initial measurement of lease liability; |
| ● | any lease payments made at or before the commencement date less any lease incentives received; and |
| ● | any initial direct costs |
The right-of-use asset is depreciated over the shorter of the asset’s useful life and the lease term on a straight-line basis.
Payments associated with short-term leases and leases of low-value assets are recognised on a straight-line basis as an expense in profit or loss. Short-term leases are leases with a lease term of less than 12 months.
2.26 Compound financial instruments
Compound financial instruments of the Group is convertible note.
F-28
2 Summary of significant accounting policies (Continued)
2.26 Compound financial instruments (Continued)
(a) | Convertible notes in 2020 |
The Group designated the convertible note as a financial liability at fair value through profit or loss. Convertible note is initially recognised at fair value. Any directly attributable transaction costs are recognised as finance costs in the consolidated statement of comprehensive income.
Subsequent to initial recognition, the convertible note is carried at fair value with changes in fair value recognised in the consolidated statement of comprehensive income.
The convertible note is classified as a non-current liability unless the Group has an obligation to settle the liability within 12 months after the end of the reporting period.
For the convertible note issued in 2020, it is redeemable at the option of the Company and the holder. This instrument can be converted into American Depository Receipts of the Company as set out in Note 27.
2.27 Dividend distribution
Dividend distribution to the Company’s shareholders is recognised as a liability in the Group’s consolidated financial statements in the period in which the dividends are approved by the Company’s Board of Directors.
3 Financial risk management
3.1 Financial risk factors
The Group’s activities expose it to a variety of financial risks: market risk (including currency risk, fair value interest rate risk and cash flow interest rate risk), credit risk and liquidity risk. The Group’s overall risk management programme focuses on the unpredictability of financial markets and seeks to minimise potential adverse effects on the Group’s financial performance.
(a) | Market risk |
(i) | Foreign exchange risk |
The majority of Group companies operate in the PRC and the majority of the transactions are denominated in RMB which is the Company’s and other Group Company’s functional currency, except for the US$ denominated convertible note.
With all other variables held constant, if the average exchange rate of RMB against US$ had strengthened or weakened by
F-29
3 | Financial risk management (Continued) |
3.1 Financial risk factors (Continued)
(a) | Market risk (Continued) |
(ii) | Credit risk |
The Group has no significant concentrations of credit risk. The carrying amounts of cash at banks, trade receivables, deposits and other receivables and amounts due from related parties included in the consolidated balance sheets represent the Group’s maximum exposure to credit risk in relation to its financial assets.
The majority of the Group’s cash at banks are deposited in major reputable financial institutions located in the PRC. Most of the Group’s revenue are settled by cash or credit cards. Trade receivables of the Group are mainly due from financial institutions with sound financial standing. There has been no history of default in relation to these external parties. Management does not expect any losses arising from non-performance by these counterparties.
Based on the Group’s historical experiences in collection of trade receivables, other receivables and amounts due from related parties, the directors consider the Group’s credit risk of these receivables to be low.
The Group considers that adequate provision for unrecoverable trade receivables, other receivables and amounts due from related parties has been made in the relevant accounting period after considering the Group’s experience in collection of trade receivables, other receivables and amounts due from related parties. Management does not expect any losses from non-performance by these counterparties.
(iii) | Cash flow and fair value interest-rate risk |
The Group’s income and operating cash flows are substantially independent of changes in market interest rates as the Group has no significant interest-bearing assets except for cash at banks. The Group’s exposures to changes in interest rates are mainly attributable to its borrowings and loans.
Borrowings issued at variable rates expose the Group to cash flow interest rate risk which is partially offset by cash held at variable rates.
At the reporting date, if interest rates on borrowings had been
(b) | Liquidity risk |
The Group is exposed to liquidity risk due to the impact of COVID–19 and it had net current liabilities of RMB
Prudent liquidity management implies maintaining sufficient cash and cash equivalents and the availability of funding through an adequate amount of committed credit facilities.
The Group’s primary cash requirements have been the payments for operating expenses and purchases of fixed assets. The Group mainly finances its working capital requirements through internal resources and proceeds from bank borrowings and issuance of ordinary shares.
F-30
3 Financial risk management (Continued)
3.1 Financial risk factors (Continued)
(b) | Liquidity risk (Continued) |
The Group’s policy is to regularly monitor current and expected liquidity requirements to ensure it maintains sufficient cash and cash equivalents and adequate amount of committed credit facilities to meet its liquidity requirements in the short and long term.
At the reporting date, the contractual undiscounted cash flows of the Group’s current financial liabilities approximate their respective carrying amounts due to their short maturities.
The table below analyses the Group’s non-derivative financial liabilities into relevant maturity groupings based on the remaining period at the reporting date to the contractual maturity date. The amounts disclosed in the table are the contractual undiscounted cash flows, including interest if applicable.
a. | Convertible note in 2020: The maximum exposure is |
| Less than |
| Between 1 and |
| Between 2 and |
| After |
| ||
1 year | 2 years | 5 years | 5 years | Total | ||||||
RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | ||||||
At 31 December 2021 |
|
|
|
|
|
|
|
|
|
|
Borrowings |
| |
| |
| — |
| — |
| |
Convertible note | — | | — | — | | |||||
Lease liabilities | | | | | | |||||
Trade payables |
| |
| — |
| — |
| — |
| |
Accruals and other payables (excluding accrued employee benefits, other taxes, provision and deposits received) |
| |
| — |
| — |
| — |
| |
Amounts due to related parties |
| |
| — |
| — |
| — |
| |
| |
| |
| |
| |
| | |
At 31 December 2022 |
|
|
|
|
|
|
|
|
|
|
Borrowings |
| | | | — | | ||||
Convertible note | | — | — | — | | |||||
Lease liabilities | | | | | | |||||
Trade payables |
| | — | — | — | | ||||
Accruals and other payables (excluding accrued employee benefits, other taxes, provision and deposits received) |
| | — | — | — | | ||||
| | | | | |
F-31
3 Financial risk management (Continued)
3.2 Capital risk management
The Group’s objectives when managing capital are to safeguard the Group’s ability to continue as a going concern in order to provide returns for shareholders and benefits for other stakeholders and to maintain an optimal capital structure to reduce the cost of capital.
In order to maintain or adjust the capital structure, the Group may adjust the dividend payments to shareholders, return capital to shareholders, issue new shares or obtain bank borrowings.
Consistent with others in the industry, the Group monitors capital on the basis of the gearing ratio. This ratio is calculated as net debt divided by total capital. Net debt is calculated as total borrowings (include current and non-current bank borrowings, convertible note as shown in the consolidated balance sheets) less cash and cash equivalents and restricted bank deposit. Total capital is calculated as “equity”, as shown in the consolidated balance sheets, plus net debt.
The gearing ratios at 31 December 2021 and 2022 were as follows:
| 2021 |
| 2022 | ||
RMB’000 | RMB’000 | ||||
Total borrowings |
| | | ||
Add: Convertible note (Note 27) |
| | | ||
Less: Cash and cash equivalents (Note 19) |
| ( | ( | ||
Restricted cash (Note 19) | — | ( | |||
Net debt |
| | | ||
Total equity |
| ( | |||
Total capital |
| | | ||
Gearing ratio |
| | % | | % |
3.3 Fair value estimation
The table below analyses financial instruments carried at fair value as at 31 December 2021 and 2022 by level of the inputs to valuation techniques used to measure fair value. Such inputs are categorised into three levels with a fair value hierarchy as follows:
| ● | Quoted prices (unadjusted) in active markets for identical assets or liabilities (level 1). |
| ● | Inputs other than quoted prices included within level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices) (level 2). |
| ● | Inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs) (level 3). |
F-32
3 Financial risk management (Continued)
3.3 Fair value estimation (Continued)
The following table presents the Group’s financial assets and liabilities that are measured at fair value as at 31 December 2022.
| Level 1 |
| Level 2 |
| Level 3 |
| Total | |
RMB’000 | RMB’000 | RMB’000 | RMB’000 | |||||
As at 31 December 2022 |
|
|
|
|
|
|
|
|
Assets | ||||||||
Investment properties (Note 14) | — | — | | | ||||
— | — | | | |||||
Liabilities |
|
|
|
| ||||
Financial liabilities at fair value through profit or loss |
|
|
|
| ||||
- Convertible note (Note 27) |
| — | — | | | |||
| — | — | | |
The fair value of financial instruments that are not traded in an active market (for example, over- the-counter derivatives) is determined by using valuation techniques. These valuation techniques maximise the use of observable market data where it is available and rely as little as possible on entity specific estimates. If all significant inputs required to fair value an instrument are observable, the instrument is included in level 2.
If one or more of the significant inputs is not based on observable market data, the instrument is included in level 3.
Specific valuation techniques used to value financial instruments include:
| ● | Quoted market prices or dealer quotes for similar instruments. |
| ● | Other techniques, such as discounted cash flow analysis including dividend growth model, are used to determine fair value for the remaining financial instruments. |
There were no significant transfers of financial assets between level 1, level 2 and level 3 fair value hierarchy classifications.
The following table presents the changes in level 3 liability instrument for the year ended 31 December 2021:
Convertible note | ||||
(Note 27) | Total | |||
RMB’000 | RMB’000 | |||
Opening balance |
| |
| |
Unrealised exchange difference |
| ( |
| ( |
Change in fair value |
| |
| |
Closing balance |
| |
| |
F-33
3 Financial risk management (Continued)
3.3 Fair value estimation (Continued)
The following table presents the changes in level 3 liability instrument for the year ended 31 December 2022:
Convertible note | ||||
(Note 27) | Total | |||
RMB’000 | RMB’000 | |||
Opening balance |
| |
| |
Change in fair value | | | ||
Closing balance |
| |
| |
4 Critical accounting estimates and judgments
Estimates and judgments are continually evaluated and are based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
The Group makes estimates and assumptions concerning the future. The resulting accounting estimates will, by definition, seldom equal the related actual results. The estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are addressed below.
(a) | Goodwill and propety, plant and equipment impairment assessment |
The Group tests annually whether goodwill and propery, plant and equipment have suffered any impairment, in accordance with the accounting policy stated in Note 2.9(a). The recoverable amounts of cash-generating units have been determined based on value-in-use calculations. These calculations require the use of estimates. The value-in-use calculations primarily use cash flow projections based on financial budgets approved by the Board of Directors. There is a number of assumptions and estimates involved in the preparation of cash flow projections for the periods covered by the approved budgets. Key assumptions include the expected growth rates, timing of future capital expenditures and selection of discount rates to reflect the risks involved.
Management prepares the financial budgets reflecting actual and prior year performance and market development expectations.
(b) | Purchase price allocation |
The application of business combination accounting requires the use of significant estimates and assumptions. The purchase method of accounting for business combinations requires the Group to estimate the fair value of assets acquired and liabilities assumed to properly allocate purchase price consideration between assets that are depreciated and amortised from goodwill. This exercise requires the use of management’s assumptions, which would not reflect unanticipated events and circumstances that may occur.
(c) | Fair values of convertible notes |
The convertible notes is not traded in an active market and the respective fair values are determined by using valuation techniques. The directors have used the market comparable approach to determine the underlying equity value of the Company and adopted equity allocation model to determine the fair values of the convertible notes.
F-34
4 Critical accounting estimates and judgments (Continued)
(d) | Estimated useful lives of property, plant and equipment and intangible assets |
The Group’s management determines the estimated useful lives and related depreciation and the amortisation charges for the Group’s property, plant and equipment and intangible assets with reference to the estimated periods that the Group intends to derive future economic benefits from the use of these assets. Management performs periodic review of the estimated useful lives of property, plant and equipment and intangible assets, and will revise the depreciation and amortisation charges where estimated useful lives are different than those previously estimated.
| (e) | Deferred tax assets |
Deferred tax assets are recognised for unused tax losses to the extent that it is probable that taxable profit will be available against which the losses can be utilized. Significant management judgement is required to determine the amount of deferred tax assets that can be recognised, based upon the likely timing and the level of future taxable profits, together with future tax planning strategies.
The Group has RMB
5 Revenue
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Non-surgical aesthetic medical services |
| |
| |
| |
Surgical aesthetic medical services |
| |
| |
| |
General healthcare services and other aesthetic medical services |
| |
| |
| |
| |
| |
| |
6 Expenses by nature
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Employee benefit expenses (Note 7) |
| |
| |
| |
Advertising and marketing expenses |
| |
| |
| |
Cost of inventories and consumables |
| |
| |
| |
Operating lease rental expenses |
| |
| |
| |
Amortisation and depreciation |
| |
| |
| |
Utilities and office expenses |
| |
| |
| |
Travelling and entertainment expenses |
| |
| |
| |
Bank charges |
| |
| |
| |
Loss on disposal of property, plant and equipment |
| |
| — |
| |
Legal and professional fees |
| |
| |
| |
Other expenses |
| |
| |
| |
| |
| |
| |
F-35
7 Employee benefit expenses
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Wages and salaries |
| |
| |
| |
Share-based compensation expenses | | | | |||
Pension costs - defined contribution plans |
| |
| |
| |
Other staff welfare expenses |
| |
| |
| |
| |
| |
| |
8 Finance income and costs
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Finance costs |
|
|
|
|
|
|
Interest expense on bank borrowings | ( | ( | ( | |||
Interest expense on other borrowings | ( | ( | ( | |||
Interest expense on lease liabilities |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( | |
Finance income |
|
|
|
| ||
Interest income on short-term bank deposits |
| |
| |
| |
Finance costs – net |
| ( |
| ( |
| ( |
9 Other gains (losses) net
The Company recognised other losses, net of RMB
F-36
10 Income tax expense
PRC corporate income tax have been provided for subsidiaries established and operating in Mainland China at the rate of
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Current tax |
|
|
|
|
|
|
PRC enterprise income tax |
| |
| |
| |
Deferred tax |
|
|
|
| ||
Origination and reversal of temporary differences (Note 23) |
| ( |
| ( |
| ( |
Income tax credit |
| ( |
| ( |
| ( |
The taxation on the Group’s loss before income tax differs from the theoretical amount that would arise using the taxation rate of PRC, the principal place of the Group’s operations, as follows:
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Loss before income tax |
| ( |
| ( |
| ( |
Calculated at a taxation rate of | ( |
| ( |
| ( | |
Expenses not tax deductible |
| |
| |
| |
Income not subject to tax |
| ( |
| — |
| — |
Utilisation of previously unrecognised tax losses |
| ( |
| — |
| — |
Recognition of previously unrecognised temporary difference |
| ( |
| — |
| — |
Tax losses not recognized |
| |
| |
| |
Difference in overseas tax rates |
| |
| ( |
| ( |
Over‑provision in respect of prior years |
| ( |
| ( |
| ( |
Income tax expense/(credit) |
| ( |
| ( |
| ( |
F-37
10 Income tax expense (Continued)
(a) | Cayman Islands Income Tax |
The Company is incorporated in the Cayman Islands as an exempted company with limited liability under the Companies Law of Cayman Islands and accordingly, is exempted from Cayman Islands income tax.
(b) | Hong Kong Profits Tax |
Hong Kong profits tax rate has been provided at the rate of
(c) | PRC Enterprise Income Tax (“EIT”) |
The income tax provision of the Group in respect of operations in the PRC has been calculated at the tax rate of
(d) | Singapore Corporation income tax |
The income tax provision of the Group in respect of operations in the Singapore has been calculated at the tax rate of
11 Loss per share
(a) | Basic loss per share |
Basic earnings per share (“EPS”) is calculated by dividing the loss attributable to owners of the Company by the weighted average number of ordinary shares in issue during the year.
(b) | Diluted loss per share |
Diluted loss per share is calculated by adjusting the weighted average number of ordinary shares outstanding to assume conversion of all dilutive potential ordinary shares.
For the year ended 31 December 2020, 2021 and 2022, the Company has
F-38
11 Loss per share (Continued)
(b) | Diluted Loss per share (Continued) |
The following table sets forth the computation of basic and diluted Loss per share:
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
(Note ii) | (Note ii) | (Note ii) | ||||
Numerator: |
|
|
|
|
| |
Loss attributable to owners of the Company – basic |
| ( |
| ( |
| ( |
Loss attributable to owners of the Company – diluted |
| ( |
| ( |
| ( |
Shares (denominator): |
|
|
| |||
Weighted average number of shares – basic |
| |
| |
| |
Weighted average number of shares – diluted |
| |
| |
| |
Loss per share – basic (RMB) |
| ( |
| ( |
| ( |
Loss per share – diluted (RMB) |
| ( |
| ( |
| ( |
Note i As the Group incurred losses for the years ended 31 December 2021 and 2022, share option and the conversion of convertible note would be anti-dilutive and therefore, related changes in fair value is not included in the computation of diluted loss per share. Diluted loss per share and basic loss per share are the same for the year ended 31 December 2021 and 2022.
F-39
12 Property, plant and equipment
Office equipment, | ||||||||||||
Machinery | furniture fixtures | |||||||||||
Leasehold | and | and motor | Right of use | |||||||||
| Buildings |
| improvements |
| equipment |
| vehicles |
| assets |
| Total | |
| RMB’000 | RMB’000 | RMB’000 | RMB’000 |
| RMB’000 |
| RMB’000 | ||||
At 31 December 2021 | ||||||||||||
Opening net book amount |
| |
| |
| |
| |
| |
| |
Additions | — | | | | | | ||||||
Impairment loss (Note 15) |
| — |
| ( |
| ( |
| ( |
| ( |
| ( |
Disposal |
| — |
| ( |
| ( |
| ( |
| ( |
| ( |
Business combination (Note 29) |
| — |
| |
| |
| — |
| |
| |
Disposal of subsidiaries (Note 30) |
| — |
| ( |
| ( |
| ( |
| ( |
| ( |
Depreciation charges |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Translation adjustments | — | ( | ( | ( | — | ( | ||||||
Closing net book amount |
| |
| |
| |
| |
| |
| |
Year ended 31 December 2021 |
|
|
|
|
|
| ||||||
At 31 December 2021 |
|
|
|
|
|
| ||||||
Cost |
| |
| |
| |
| |
| |
| |
Accumulated depreciation and impairment |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Net book amount |
| |
| |
| |
| |
| |
| |
At 31 December 2022 | ||||||||||||
Opening net book amount |
| | | | | | | |||||
Additions | | | | | | | ||||||
Disposal |
| — | — | ( | ( | ( | ( | |||||
Disposal of subsidiaries (Note 30) |
| — | ( | ( | ( | ( | ( | |||||
Depreciation charges |
| ( | ( | ( | ( | ( | ( | |||||
Transfer to Investment Properties | ( | — | — | — | — | ( | ||||||
Closing net book amount |
| | | | | | | |||||
Year ended 31 December 2022 |
| |||||||||||
At 31 December 2022 |
| |||||||||||
Cost |
| | | | | | | |||||
Accumulated depreciation and impairment |
| ( | ( | ( | ( | ( | ( | |||||
Net book amount |
| | | | | | |
As at 31 December 2021 and 2022, property, plant and equipment with net book value amounting to approximately RMB
F-40
13 Lease
(a) | Amounts recognised in the consolidated balance sheets: |
Right-of-use assets are classified as property, plant and equipment (Note 12).
| As at |
| As at |
| As at | |
31 December | 31 December | 31 December | ||||
2020 | 2021 | 2022 | ||||
RMB | RMB | RMB | ||||
Lease liabilities |
|
|
|
| ||
Current |
| | |
| | |
Non-current |
| | |
| | |
| | |
| |
(b) | Amounts recognised in the consolidated statements of comprehensive income: |
| 2020 |
| 2021 |
| 2022 | |
RMB | RMB | RMB | ||||
Interest expense (included in finance costs) (Note 8) |
| |
| |
| |
Expense relating to short-term leases (included in cost of goods sold and administrative expenses) |
| |
| |
| |
The total cash outflow for leases in 2022 was approximately RMB
14 Investment properties
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Fair value |
|
|
|
|
At 1 January |
| — |
| — |
Transfer from property, plant and equipment |
| — |
| |
At 31 December |
| — |
| |
Investment properties represents buildings held in the PRC.
Investment properties with fair value amounting to RMB
Amounts recognised in the consolidated income statements for investment properties:
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 |
| RMB’000 | |||
Rental income |
|
| — | — |
As at 31 December 2022, the fair values of the investment property are based on valuation performed by Dacheng Assessment, an accredited independent valuer. Dacheng Assessment is a specialist in valuing these types of investment properties in China. A valuation model in accordance with that recommended by the International Valuation Standards Committee has been applied.
F-41
15 Intangible assets
Computer | Medical | |||||||||
| Goodwill |
| software |
| licenses |
| Tradenames |
| Total | |
RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | ||||||
Year ended 31 December 2021 |
|
|
| |||||||
Opening net book amount |
| | | — |
| |
| | ||
Additions |
| — | | — |
| — |
| | ||
Disposal | — | ( | — | — | ( | |||||
Business combination (Note 29) |
| | | — |
| |
| | ||
Disposal of subsidiaries (Note 30) |
| ( | ( | — |
| ( |
| ( | ||
Amortisation |
| — | ( | — |
| ( |
| ( | ||
Impairment loss (Note) |
| ( | ( | — |
| ( |
| ( | ||
Closing net book amount | | | — | | | |||||
At 31 December 2021 |
|
|
| |||||||
Cost |
| | | |
| |
| | ||
Impairment | ( | ( | — | ( | ( | |||||
Accumulated amortisation |
| — | ( | ( |
| ( |
| ( | ||
Net book amount |
| | | — |
| |
| | ||
Year ended 31 December 2022 |
|
|
| |||||||
Opening net book amount |
| | | — | | | ||||
Additions |
| — | | — | — | | ||||
Disposal | — | ( | — | — | ( | |||||
Business combination (Note 29) |
| | — | — | — | | ||||
Disposal of subsidiaries (Note 30) |
| ( | — | — | — | ( | ||||
Amortisation |
| — | ( | — | — | ( | ||||
Closing net book amount | | | — | | | |||||
At 31 December 2022 |
| |||||||||
Cost |
| | | | | | ||||
Impairment | ( | ( | — | ( | ( | |||||
Accumulated amortisation |
| — | ( | ( | ( | ( | ||||
Net book amount |
| | | — | | |
Note: Goodwill of RMB
F-42
15 Intangible assets (Continued)
During the year ended 31 December 2021, the Company decided to strategically focus on treatment centres in East China, South China and Southwest China and devote much less resources to treatment centres in other regions of China. As a result, the Company recognized impairment of goodwill of RMB
During the year ended 31 December 2022, the Company has not recognized any impairment to property, plant and equipment and intangible assets.
Management reviews the business performance of each operating entity (also regarded as a CGU). Goodwill is allocated to relevant operating entities.
The recoverable amount of a CGU is determined based on a value-in-use calculation. It is calculated using pre-tax discounted cash flow projections based on financial budgets approved by management covering a
| 2021 |
| 2022 | |
Annual compound revenue growth rate |
|
| ||
Annual gross profit ratio |
|
| ||
Discount rate |
|
|
Management considers that because of their experience and expertise knowledge in the aesthetic medical treatment business in the PRC, a cash flow period of is reasonable.
Management determined budgeted gross margin based on past performance and its expectations of the market development. The average annual revenue growth rate used is consistent with the forecasts of the market from a industry study. The discount rate used is pre-tax and reflects specific risks relating to the entity. The cash flows beyond the periods are extrapolated using a
16 Investments accounted for using the equity method
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
At 1 January |
| |
| |
Acquisition |
| |
| |
Disposal | — | ( | ||
Impairment | ( | — | ||
Share of loss |
| |
| — |
Transfer to assets held-for-sale | — | ( | ||
At 31 December |
| |
| |
Set out below are the associates of the Group as at 31 December 2021 and 2022 which, in the opinion of the directors, are material to the Group. The associates as listed below have share capital consisting solely of ordinary shares, which are held directly by the Group. The places of establishment are also their principal places of business.
F-43
16 Investments accounted for using the equity method (Continued)
Nature of investments in associates as at 31 December 2021 and 2022:
Place of | % of ownership | Nature of the | Measurement | |||||||
Name of entity |
| establishment |
| 2021 |
| 2022 |
| relationship |
| method |
Moyan (Shenzhen) Network Technology Co., Ltd. |
| The PRC |
| | % | | % | Note 1 |
| Equity |
(美約(深圳)網路技術有限公司) |
|
|
|
|
|
|
|
|
|
|
Mendis Aesthetic PTE. Ltd. |
| Singapore |
| | % | — | % | Note 2 |
| Equity |
Jinan Pengai Meikang Aesthetic Medical Clinic Co., Ltd. |
|
|
|
|
|
|
|
| ||
(濟南鵬愛美康醫療美容診所有限公司) | The PRC | | % | — | % | Note 3 | Equity | |||
Shenzhen Huayanyuese Health Management Consulting Co., Ltd. | ||||||||||
(深圳市花顔悅色健康管理咨詢有限公司) |
| The PRC |
| | % | % | Note 4 |
| Equity | |
Shenzhen Pengai Lizhi Aesthetic Medical Clinic | ||||||||||
(深圳鹏爱荔枝医疗美容诊所) | The PRC | — | % | % | Note 5 | Equity | ||||
Note 1: Moyan (Shenzhen) Network Technology Co., Ltd. (“Moyan”) is engaged in internet marketing services.
Note 2: Mendis Aesthetic PTE. Ltd. (“Mendis”) is engaged in the provision of aesthetic medical services. The Company transformed the investment to assets held-for-sale during the year (Note 20).
Note 3: Jinan Pengai Meikang Aesthetic Medical Clinic Co., Ltd. (“Jinan Meikang”) is engaged in the provision of aesthetic medical services. The Company’s subsidiary Shenzhen Pengai Hospital Investment Management Co. Ltd. signed a share purchase agreement with Shandong Aiyue Yimei Medical Technology Co., Ltd. (“Aiyue”), where Aiyue purchased
Note 4: Shenzhen Huayanyuese Health Management Consulting Co., Ltd. is engaged in investment holding and provision of management services.
Note 5: Shenzhen Pengai Lizhi Aesthetic Medical Clinic (“Lizhi”) is engaged in the provision of aesthetic medical services. The Company’s subsidiary Shenzhen Miaoyan Medical Technology Investment Co., Ltd. ("Miaoyan Technology”) signed an investment cooperation agreement with Shenzhen Zimu Culture Investment Development Co., Ltd., where Miaoyan Technology subscribed
Moyan, Mendis, Jinan Pengai Meikang Aesthetic Medical Clinic Co., Ltd., Shenzhen Huayanyuese Health Management Consulting Co., Ltd. and Shenzhen Pengai Lizhi Aesthetic Medical Clinic are private companies and there are no quoted market prices available for their shares.
Summarised financial information for associates
Set out below are the summarised financial information for investments accounted for using the equity method:
F-44
16 Investments accounted for using the equity method (Continued)
Summarised balance sheet
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Current |
|
|
|
|
Cash and cash equivalents |
| |
| |
Other current assets (excluding cash and cash equivalents) |
| |
| |
Total current assets |
| |
| |
Financial liabilities (excluding trade payables) |
| ( |
| — |
Other current liabilities (including trade payables) |
| ( |
| ( |
Total current liabilities |
| ( |
| ( |
Total non-current assets |
| |
| |
Net assets |
| |
| |
Summarised statement of comprehensive income
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Revenue |
| |
| — |
Depreciation and amortisation |
| ( |
| — |
Interest expense |
| ( |
| — |
Loss before income tax |
| |
| — |
Income tax expense |
| — |
| — |
Loss for the year |
| |
| — |
The information above reflects the amounts presented in the financial statements of the associates (and not the Group’s share of those amounts) adjusted for differences in accounting policies between the Group and the associates.
17 Trade and other receivables, deposits and prepayments
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Trade receivables |
| |
| |
Other receivables |
| |
| |
Deposits |
| |
| |
Prepayments |
| |
| |
Advances to employees |
| |
| |
| |
| | |
Less: Non-current portion |
|
| ||
Prepayments and deposits |
| ( |
| ( |
Current portion |
| |
| |
The carrying amounts of trade and other receivables, deposits and prepayments are denominated in RMB and approximate their fair values.
Trade receivables are all aged within
F-45
18 Inventories
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Pharmaceuticals |
| |
| |
Medical consumables |
| |
| |
| |
| |
The cost of inventories recognised as expense and included in cost of inventories and consumables amounted to RMB
19 Restricted cash and cash and bank balances
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
Restricted cash |
| — |
| |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Cash and cash equivalents |
|
|
|
|
Cash at banks |
| |
| |
Time deposit | | — | ||
Cash on hand |
| |
| |
Total |
| |
| |
The analysis of bank and cash balances for the purpose of the consolidated statement of cash flows is as follows:
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Cash and cash equivalents |
| |
| |
The carrying amounts of the Group’s restricted bank deposit and cash and cash equivalents are denominated in the following currencies:
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
RMB |
| |
| |
US dollars |
| |
| |
Hong Kong dollars |
| |
| |
Singapore dollars |
| |
| |
| |
| |
Cash at banks earns interest at floating rates based on daily bank deposit rates. The Group’s balances of cash at bank which are denominated in RMB are deposited with banks in the PRC. The conversion of these RMB-denominated balances into foreign currencies and the remittance of funds out of the Mainland China are subject to the rules and regulations of foreign exchange control promulgated by the Government of the PRC.
F-46
20 Assets held-for-sale
The discontinued operation includes an investment in an associate of Mendis Aesthetic PTE. Ltd. (“Mendis”) with a carrying amount of RMB
In March 2022, the Group entered into a sales and purchase agreement to sell
The investment was reclassified as assets held-for-sale in March 2022. As at the date of reclassification, the carrying amount equals the fair value of the instrument, which is the consideration of the sales of shares. The Company recorded a loss on fair value of RMB
21a Share capital
Ordinary shares of USD | ||||||
| Number of |
| Nominal |
| Nominal | |
shares | value | value | ||||
USD’000 | RMB’000 | |||||
Authorised: |
| |||||
As at 1 January 2020, 31 December 2020, 1 January 2021, 31 December 2021, 1 January 2022 and 31 December 2022 |
| | | | ||
Issued and paid: |
| |||||
As at 1 January 2020, 31 December 2020, 1 January 2021, 31 December 2021, 1 January 2022 and 31 December 2022 |
| | | | ||
Issuance of shares to Hawyu (HK) Limited (Note i) | ||||||
Issuance of shares under Performance Incentive Plan (Note ii) | ||||||
As at 31 December 2022 | ||||||
Note i:
In May 2021, we entered into definite share subscription agreement for the issuance of
Note ii:
On 29 July 2022, the Company issued
21b Treasury Shares
| Number of |
| ||
shares | RMB’000 | |||
As at 1 January 2020, 31 December 2020, 1 January 2021, 31 December 2021, 1 January 2022 and 31 December 2022 |
F-47
21 Share capital (Continued)
Note i:
On 13 October 2020, the Company announced that its board of directors had approved a share repurchase program, under which the Company was authorized to repurchase in the open market up to US$
22 Other reserves
|
|
|
| Share‑based |
|
| ||||||
Capital | Merger | Statutory | compensation | Other | ||||||||
reserve | reserve | reserve | reserve | reserve | Total | |||||||
RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | |||||||
| Note (a) |
| Note (b) |
| Note (c) |
| Note (d) |
| Note (e) | |||
At 1 January 2020 | | ( | | | ( | | ||||||
Translation of foreign operations |
| — | — | — | — | ( | ( | |||||
Transfer to statutory reserve |
| — | — | | — | — | | |||||
Further acquisition of interests in a subsidiary (Note 31) |
| — | — | — | — | | | |||||
Partial disposal of interests in a subsidiary without loss of control (Note 31) |
| — | — | — | — | | | |||||
Share-based payment reserve |
| — | — | — | | — | | |||||
Share re-purchase | — | — | — | — | — | — | ||||||
At 31 December 2020 |
| | ( | | | ( | | |||||
At 31 December 2020 and 1 January 2021 | | ( | | | ( | | ||||||
Translation of foreign operations | — | — | — | — | | | ||||||
Further acquisition of interests in a subsidiary (Note 31) | | — | — | — | ( | ( | ||||||
Partial disposal of interests in a subsidiary without loss of control (Note 31) | — | — | — | — | | | ||||||
Share-based payment reserve | — | — | — | | — | | ||||||
At 31 December 2021 | | ( | | | ( | | ||||||
At 31 December 2021 and 1 January 2022 | | ( | | | ( | | ||||||
Translation of foreign operations | — | — | — | — | ( | ( | ||||||
Revaluation of investment properties upon transfer from property, plant and equipment | — | — | — | — | | | ||||||
Deregister of subsidiary | | — | — | — | — | | ||||||
Partial disposal of interests in a subsidiary without loss of control (Note 31) | — | — | — | — | ( | ( | ||||||
Issuance of shares | | — | — | — | ( | | ||||||
Transaction costs related to issuance of shares | ( | — | — | — | — | ( | ||||||
Share-based payment reserve | — | — | — | | — | | ||||||
At 31 December 2022 | | ( | | | ( | |
Notes
| (a) | Capital reserve |
The capital reserve mainly represents capital contribution made by an owner and shareholders of the Company.
F-48
22 Other reserves (Continued)
(b) Merger reserve
Merger reserve is mainly attributable to business combinations under common control.
(c) Statutory reserve
In accordance with the PRC regulations and the articles of association of the companies now comprising the Group, before distributing the net profit of each year, companies registered in the PRC are required to set aside
(d) Share-based compensation reserve
As at 1 January 2018, share-based compensation reserve amounting to RMB
On 1 June 2019, the Group adopted a share-based compensation plan. For the year ended 31 December 2022, share-based compensation expenses amounting to RMB
(e) Other reserve
Other reserve mainly represents the differences of carrying amount of non-controlling interests acquired, consideration paid to non-controlling interests and reduction of reserve as a result of the issuance of exchangeable note liabilities.
F-49
23 Deferred income tax
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
The deferred tax assets comprise attributable to: |
|
| ||
- Tax losses |
| |
| |
- Other temporary differences | | | ||
| | |||
The deferred tax liabilities comprise temporary differences attributable to: |
|
| ||
- Medical licenses and tradenames |
| ( |
| — |
- Others | — | — | ||
( | — |
Deferred income tax assets and liabilities are offset when there is a legally enforceable right to offset current income tax assets against current income tax liabilities and when the deferred income taxes relate to the same fiscal authority. The balances shown in the consolidated balance sheets are, after appropriate offsetting, as follows:
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Deferred income tax assets: |
|
|
| |
- Deferred income tax assets to be recovered after more than 12 months |
| |
| |
| |
| | |
Deferred income tax liabilities: |
|
| ||
- Deferred income tax liabilities to be settled after more than 12 months |
| ( |
| — |
| ( |
| — | |
Deferred income tax assets – net |
| |
| |
The movement in deferred income tax assets and liabilities during the year, without taking into consideration the offsetting of balances within the same taxation jurisdiction is as follows:
The gross movement on deferred income tax accounts is as follows:
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
At 1 January |
| |
| |
Disposal of subsidiaries (Note 30) |
| |
| ( |
Credited to consolidated statement of comprehensive income |
| |
| |
Translation adjustment | — | ( | ||
At 31 December |
| |
| |
F-50
23 Deferred income tax (Continued)
Deferred income tax assets
| Tax losses |
| Others |
| Total | |
RMB’000 | RMB’000 | RMB’000 | ||||
At 1 January 2021 |
| | | | ||
Disposal of a subsidiary (Note 30) |
| ( | ( | ( | ||
Credited to consolidated statement of comprehensive income |
| | ( | | ||
At 31 December 2021 and 1 January 2022 |
| | | | ||
Disposal of subsidiaries (Note 30) |
| ( | — | ( | ||
Credited to consolidated statement of comprehensive income | | ( | | |||
Translation adjustment | ( | — | ( | |||
At 31 December 2022 |
| | | |
Deferred income tax liabilities
| Medical licenses |
|
| |||
and tradenames | Others | Total | ||||
RMB’000 | RMB’000 | RMB’000 | ||||
At 1 January 2021 |
| | | | ||
Disposal of subsidiaries (Note 30) | ( | ( | ( | |||
At 31 December 2021 and 1 January 2022 |
| | — | | ||
Credited to consolidated statement of comprehensive income |
| ( | — | ( | ||
At 31 December 2022 |
| — | — | — |
Deferred income tax assets are recognised for tax loss carry-forwards to the extent that the realisation of the related tax benefit through future taxable profits is probable. The Group has unrecognised tax losses as at 31 December 2022 of RMB
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Tax loss expiring within 5 years |
| |
| |
F-51
24 Borrowings
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Non‑current |
|
|
|
|
Bank borrowings |
|
|
|
|
- secured |
| |
| |
Other borrowings | ||||
- secured | | | ||
| | |||
Current |
|
| ||
Bank borrowings |
|
| ||
- secured |
| |
| |
Other borrowings | ||||
- secured | | | ||
| | |||
| |
The carrying amounts of borrowings approximate their fair values and are denominated in RMB. The effective interest rates on the borrowings from banks was as
Bank borrowings are secured by the following:
(i) | property, plant and equipment and investment properties of the Group (Note 12 and Note 14); |
(ii) | personal properties provided by Dr. Zhou Pengwu and Ms. Ding Wenting (Note 35(d)); |
(iii) | personal guarantee provided by Dr. Zhou Pengwu, Dr. Zhou Yitao and Ms. Ding Wenting, Mr Zhou Xichun (Note 35(d)); and |
(iv) | corporate guarantee provided by a related company controlled by Dr. Zhou Pengwu (Note 35(d)). |
Other borrowings are secured by the following:
(i) | property, plant and equipment of the Group (Note 12); and |
(ii) | corporate guarantee provided by a related company controlled by Dr. Zhou Pengwu (Note 35(d)). |
25 Trade and accruals, other payables and provisions
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Trade payables |
| |
| |
Accrued employee benefits |
| |
| |
Accrued operating expenses |
| |
| |
Accrued professional service fees |
| |
| |
Deposits received |
| |
| |
Duty and tax payable other than corporate income tax |
| |
| |
Other payables to suppliers of plant and equipment |
| |
| |
Others |
| |
| |
| |
| |
F-52
The carrying amounts of trade and other payables are denominated in RMB. The carrying amounts approximate their fair values due to their short-term maturities.
26 Contract liabilities
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | |||
Advance receipt for treatment packages |
| |
| |
As at end of 31 December 2022, the contract liabilities are aged within one year from the date when the sales contracts in respect of treatment packages were entered into.
F-53
27 Convertible note
Convertible note in 2020
On 17 September 2020, the Company entered into a convertible note purchase agreement, pursuant to which, the Company issued a convertible note to ADV at a principal amount of US$
(a) | Conversion feature |
Convertible note is convertible into American Depository Receipts (“ADRs”) of the Company, at the option of the holder after 6 months from the issue date to the maturity date, unless the Company has previously elected to redeem the convertible note.
(b) | Redemption feature |
The Company has a right to redeem all of the outstanding convertible note after one year from the issue date. The holder has a right to redeem all outstanding convertible note at maturity date at a price equivalent to a
Before the first anniversary of the issuance of the convertible note, the convertible note is redeemable at the option of the Company and by giving notice in writing to the convertible note holder.
As a part of the financing plan of convertible note, on 17 September 2020, the Company also entered into an exit payment agreement with ADV to compensate ADV for a maximum of US$
F-54
27 Convertible note (Continued)
Convertible note in 2020 (Continued)
(c) | Fair value measurement |
The Group monitors the convertible note on a fair value basis which is in accordance with its risk management strategy and does not bifurcate any feature from its debt host instrument and designates the entire hybrid contract as a financial liability at fair value through profit or loss with the changes in the fair value recorded in consolidated statement of comprehensive income.
As at 31 December 2022, the directors used the binominal method.
The movement of the convertible note is set out as below:
| RMB’000 | |
For the year ended 31 December 2021 |
|
|
At 1 January 2021 |
| |
Unrealised exchange difference |
| ( |
Change in fair value |
| |
| ||
At 31 December 2021 |
| |
| ||
Fair value changes for the year included in consolidated statement of comprehensive income for liabilities held at the year end |
| |
| ||
For the year ended 31 December 2022 | ||
At 1 January 2022 | | |
Unrealised exchange difference | — | |
Change in fair value | | |
At 31 December 2022 | | |
Fair value changes for the year included in consolidated statement of comprehensive income for liabilities held at the year end | |
F-55
28 Cash generated from operations
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Loss before income tax |
| ( |
| ( |
| ( |
Adjustments for: |
|
|
| |||
Finance income |
| ( |
| ( |
| ( |
Finance costs |
| |
| |
| |
Amortisation of intangible assets (Note 15) |
| |
| |
| |
Depreciation of property, plant and equipment (Note 12) |
| |
| |
| |
Depreciation of investment properties (Note 14) |
| |
| — |
| — |
Loss on disposal of property, plant and equipment |
| |
| |
| |
Loss on disposal of intangible assets | — | | | |||
Share of losses/(profits) investments accounted for using the equity method | | ( | — | |||
Fair value loss of convertible note | | | | |||
Transaction cost related to issuance of convertible note | | — | — | |||
Reversal of contingent consideration | — | ( | — | |||
Share-based payment |
| |
| |
| |
Impairment of non-current assets | | | — | |||
Unrealised exchange difference | | ( | — | |||
Loss on disposal of associates |
| |
| — |
| |
Loss on disposal of subsidiaries (Note 30) |
| |
| |
| |
Loss on ceased operation of subsidiaries | — | — | — | |||
| ( |
| ( |
| | |
Changes in working capital: |
|
|
| |||
- Inventories |
| ( |
| ( |
| ( |
- Trade receivables |
| ( |
| |
| |
- Other receivables, deposits and prepayments |
| |
| |
| |
- Balances with related parties |
| — |
| — |
| — |
- Trade payables |
| |
| |
| |
- Accruals, other payables and provisions |
| |
| ( |
| ( |
- Contract liabilities |
| ( |
| |
| ( |
Cash flow from operating activities |
| |
| |
| ( |
This section sets out an analysis of net debt and the movements in net debt for the years:
Liabilities from financing activities | ||||||||||
Lease | Lease | |||||||||
Borrowing | Borrowing | liabilities | liabilities | |||||||
| due within |
| due after |
| due within |
| due after |
| ||
1 year | 1 year | 1 year | 1 year | Total | ||||||
Net debt | RMB’000 | RMB’000 | RMB’000 | RMB’000 | RMB’000 | |||||
As at 1 January 2021 | ( |
| ( | ( | ( |
| ( | |||
Cash flows |
| ( |
| ( | | |
| | ||
As at 31 December 2021 |
| ( |
| ( | ( | ( |
| ( | ||
As at 1 January 2022 |
| ( | ( | ( | ( | ( | ||||
Cash flows |
| ( | | | | | ||||
As at 31 December 2022 |
| ( | ( | ( | ( | ( | ||||
F-56
29 Business combination
| (a) | Guangzhou Pengai Xiuqi Aesthetic Medical Clinic Co., Ltd. |
On 14 October 2021, the Group acquired
As a result of the acquisition, the Group is expected to increase its presence in Guangzhou. The goodwill of RMB
The assets and liabilities recognised as a result of the acquisition are as follows:
| RMB’000 | |
Other receivables |
| |
Inventories |
| |
Property, plant and equipment |
| |
Net identifiable assets acquired |
| |
Add: goodwill (Note 15) |
| |
Net assets acquired |
| |
An analysis of net outflow of cash and cash equivalents in respect of the acquisition is as follows:
| RMB’000 | |
Cash consideration |
| ( |
Cash and cash equivalents |
| — |
Net outflow of cash and cash equivalents included in cash flows from investing activities |
| ( |
Acquisition-related costs have been charged to general and administrative expenses in consolidated statement of
comprehensive income for the year ended 31 December 2021.
For the year ended 31 December 2021, the revenue included in the consolidated statement of comprehensive income since 14 October 2021 contributed by Guang zhou Pengai Xiuqi was approximately RMB
The following table sets out the pro-forma revenue and profit after tax of the Group had the acquisition taken place
from 1 January 2021:
| 2021 | |
| RMB’000 | |
Pro forma consolidated statement of comprehensive income: |
|
|
Revenue |
| |
Loss after tax |
| ( |
F-57
29 Business combination (Continued)
| (b) | Beijing AomeiYixin Investment Consultant Co., Ltd. (“Beijing Aomei”) |
On 1 January 2021, the Group acquired
As a result of the acquisition, the Group is expected to increase its presence in Beijing. The goodwill of RMB
The assets and liabilities recognised as a result of the acquisition are as follows:
| RMB’000 | |
Cash and cash equivalents |
| |
Intangible assets |
| |
Trade receivables and other receivables |
| |
Inventories |
| |
Property, plant and equipment |
| |
Trade payables and other payables |
| ( |
Lease liabilities |
| ( |
Deferred income tax liabilities |
| ( |
Current income tax liabilities |
| ( |
Non-controlling interest |
| ( |
Net identifiable assets acquired |
| |
Add: goodwill (Note 15) |
| |
Net assets acquired |
| |
An analysis of net outflow of cash and cash equivalents in respect of the acquisition is as follows:
| RMB’000 | |
Cash consideration |
| — |
Cash and cash equivalents |
| |
Net outflow of cash and cash equivalents included in cash flows from investing activities |
| |
Acquisition-related costs have been charged to general and administrative expenses in the consolidated statement of comprehensive income for the year ended 31 December 2021.
For the year ended 31 December 2021, the revenue included in the consolidated statement of comprehensive income since 1 January 2021 contributed by Beijing Aomei was approximately RMB
| (c) | Shenzhen Miaoyan Aesthetic Medical Clinic |
On 30 May 2022, the Group acquired
As a result of the acquisition, the Group is expected to increase its presence in Shenzhen. The goodwill of RMB
F-58
29 Business combination (Continued)
| (c) | Shenzhen Miaoyan Medical Beauty Clinic (Continued) |
The assets and liabilities recognised as a result of the acquisition are as follows:
| RMB’000 | |
Property, plant and equipment |
| |
Prepayments |
| |
Inventories | | |
Trade and other receivables |
| |
Cash and cash equivalents |
| |
Trade payables and other payables |
| ( |
Net identifiable assets acquired |
| |
Add: goodwill (Note 15) |
| |
Net assets acquired |
| |
An analysis of net outflow of cash and cash equivalents in respect of the acquisition is as follows:
| RMB’000 | |
Cash consideration |
| ( |
Other payables | | |
Cash and cash equivalents |
| |
Net outflow of cash and cash equivalents included in cash flows from investing activities |
| ( |
Acquisition-related costs have been charged to general and administrative expenses in the consolidated statement of comprehensive income for the year ended 31 December 2022.
For the year ended 31 December 2022, the revenue included in consolidated statement of the comprehensive income since 30 May 2022 contributed by Shenzhen Miaoyan Aesthetic Medical Clinic was approximately RMB
The following table sets out the pro-forma revenue and profit after tax of the Group had the acquisition taken place from 1 January 2022:
| 2022 | |
| RMB’000 | |
Pro forma consolidated statement of comprehensive income: |
|
|
Revenue |
| |
Loss after tax |
| ( |
F-59
30 Disposals of subsidiaries
(a) | The Company entered into an Equity Transfer Agreement with an independent third-party regarding the sales of certain subsidiaries including Chongqing Pengai Aesthetic Medical Hospital Co., Ltd., Chengdu Pengai Yueji Aesthetic Medical Clinic Co., Ltd., Shenzhen Pengai Yuexin Aesthetic Medical Hospital, Shenzhen Pengai Yueji Aesthetic Medical Hospital, Xi’an New Pengai Yueji Aesthetic Medical Clinic Co., Ltd., Jiangsu Liangyan Hospital Management Co., Ltd., Yunnan Liangyan Aesthetic Medical Clinic Co., Ltd., Kunming Liangyan Aesthetic Medical Clinic Co., Ltd. and Kunming Liangyan Hospital Management Co., Ltd. Details of the net assets of these subsidiaries disposed of and their financial impacts are summarised as follows: |
| RMB’000 | |
Net assets disposed of: | ||
Property, plant and equipment |
| |
Intangible assets |
| |
Goodwill |
| — |
Deferred tax assets |
| — |
Inventories |
| |
Trade and other receivables |
| |
Cash and cash equivalents |
| |
Income tax payables |
| ( |
Trade payables and other payables |
| ( |
Borrowings |
| — |
Contract liabilities |
| ( |
Lease liabilities |
| — |
Non-controlling interests |
| ( |
Net assets of subsidiary |
| ( |
Loss on disposal of subsidiary |
| |
Consideration |
| ( |
Satisfied by: |
|
|
Cash consideration |
| — |
Settlement of pre-existing balances |
| ( |
| ( |
An analysis of the net outflow of cash and cash equivalents in respect of the disposal of these subsidiaries is as follows:
| 2021 | |
| RMB’000 | |
Cash received |
| — |
Cash and cash equivalents disposed of |
| ( |
Net cash inflow in respect of disposal |
| ( |
F-60
30 Disposals of subsidiaries (Continued)
(b) | Nanchang Peng’ai Xiuqi Aesthetic Medical Hospital Co., Ltd. |
In October 2022, the Group disposed of its entire equity interest in Nanchang Peng’ai Xiuqi Aesthetic Medical Hospital Co., Ltd. to a third party. Details of the net assets of Nanchang Peng’ai Xiuqi Aesthetic Medical Hospital Co., Ltd. disposed of and their financial impacts are summarised as follows:
| RMB’000 | |
Net assets disposed of: |
|
|
Property, plant and equipment |
| |
Deferred tax assets |
| |
Prepayments |
| |
Inventories |
| |
Trade and other receivables |
| |
Cash and cash equivalents |
| — |
Trade payables and other payables | ( | |
Lease liabilities |
| ( |
Non-controlling interests |
| |
Net assets of subsidiary | | |
Gain on disposal of subsidiary | | |
Cash consideration | |
An analysis of the net outflow of cash and cash equivalents in respect of the disposal of the Nanchang Peng’ai Xiuqi Aesthetic Medical Hospital Co., Ltd. is as follows:
| 2022 | |
| RMB’000 | |
Cash received |
| |
Net cash inflow in respect of disposal of Nanchang Peng'ai Xiuqi Aesthetic Medical Hospital Co., Ltd. |
| |
F-61
30 Disposals of subsidiaries (Continued)
(c) | Changsha Peng’ai Aesthetic Medical Hospital Co., Ltd. |
In October 2022, the Group disposed of its entire equity interest in Changsha Peng’ai Aesthetic Medical Hospital Co., Ltd. to a third party. Details of the net assets of Changsha Peng’ai Aesthetic Medical Hospital Co., Ltd. disposed of and their financial impacts are summarised as follows:
| RMB’000 | |
Net assets disposed of: |
|
|
Property, plant and equipment |
| |
Goodwill |
| |
Deferred tax assets |
| |
Prepayments |
| |
Inventories |
| |
Trade and other receivables |
| |
Cash and cash equivalents |
| |
Trade payables and other payables |
| ( |
Contract liabilities |
| ( |
Income tax liabilities |
| ( |
Lease liabilities |
| ( |
Non-controlling interest | | |
Net assets of subsidiary |
| |
Loss on disposal of subsidiary | ( | |
Cash consideration |
| |
An analysis of the net outflow of cash and cash equivalents in respect of the disposal of the Changsha Peng’ai Xiuqi Aesthetic Medical Hospital Co., Ltd. is as follows:
| 2022 | |
| RMB’000 | |
Cash received |
| |
Cash and cash equivalents disposed of |
| ( |
Net cash inflow in respect of disposal of Changsha Peng'ai Xiuqi Aesthetic Medical Hospital Co., Ltd. |
| |
31 Transactions with non-controlling interests
During the year ended 31 December 2020, 2021 and 2022, the Group completed the following transactions with non-controlling interests and the impact is as below:
|
| Debit to |
| Total net | ||
(Debit)/credit | non-controlling | (debit)/credit to | ||||
to other reserve | interests | Equity | ||||
| RMB’000 |
| RMB’000 |
| RMB’000 | |
For the year ended 31 December 2020 | ||||||
Acquisition of additional interests in a subsidiary: |
|
|
|
|
|
|
- Shanghai Pengai (Note a) |
| |
| ( |
| ( |
Disposal of interests in a subsidiary without loss of control: |
|
|
|
|
|
|
- Shanghai Jiahong (Note b) |
| ( |
| |
| — |
- Guangzhou Pengai (Note c) |
| |
| |
| |
| |
| ( |
| |
F-62
31 Transactions with non-controlling interests (Continued)
|
| Debit to |
| Total net | ||
(Debit)/credit | non-controlling | (debit)/credit to | ||||
to other reserve | interests | Equity | ||||
| RMB’000 |
| RMB’000 |
| RMB’000 | |
For the year ended 31 December 2021 | ||||||
Acquisition of additional interests in a subsidiary: |
|
|
|
|
|
|
- Huizhou Pengai (Note d) | ( | ( | ( | |||
- Changsha Pengai (Note e) | ( | | ( | |||
Disposal of interests in a subsidiary without loss of control: | ||||||
- Changsha Pengai (Note f) | | ( | | |||
|
|
|
|
| Debit to |
| Total net | ||
(Debit)/credit | non-controlling | (debit)/credit to | ||||
to other reserve | interests | Equity | ||||
| RMB’000 |
| RMB’000 |
| RMB’000 | |
For the year ended 31 December 2022 | ||||||
Acquisition of additional interests in a subsidiary: |
|
|
|
|
|
|
- Shenzhen Pengai Xiuqi(Note g) | ( | ( | ( | |||
- Guangzhou Pengai (Note h) | | ( | ( | |||
- Guangzhou Pengai Xiuqi (Note i) | | ( | — | |||
- Yantai Pengai (Note j) |
| |
| ( |
| ( |
|
|
|
(a) | Acquisition of interest in Shanghai Pengai |
| 2020 | |
| RMB’000 | |
Carrying amount of non-controlling interests acquired |
| ( |
Consideration paid to non-controlling interests |
| ( |
Excess of consideration paid to non-controlling interest recognised within equity |
| ( |
On 25 March 2020, the Group purchased an additional
(b) | Disposal of interest in Shanghai Jiahong without loss of control |
| 2020 | |
| RMB’000 | |
Carrying amount of non-controlling interests disposed of |
| ( |
Less: consideration received from non-controlling interest |
| — |
Gain on disposal within equity |
| ( |
On 9 December 2020, the Group disposed a
F-63
31 Transactions with non-controlling interests (Continued)
(c) | Disposal of interest in Guangzhou Pengai without loss of control |
| 2020 | |
| RMB’000 | |
Carrying amount of non-controlling interests disposed of |
| ( |
Less: consideration received from non-controlling interest |
| |
Gain on disposal within equity |
| |
On 7 December 2020, the Group disposed a
(d) | Acquisition of interest in Huizhou Pengai |
| 2021 | |
| RMB’000 | |
Carrying amount of non-controlling interests acquired |
| |
Consideration paid to non-controlling interests |
| ( |
Excess of consideration paid to non-controlling interest recognised within equity |
| ( |
On 13 September 2021, the Group purchased an additional
(e) | Acquisition of interest in Changsha Pengai |
| 2021 | |
| RMB’000 | |
Carrying amount of non-controlling interests acquired |
| ( |
Consideration paid to non-controlling interests |
| ( |
Excess of consideration paid to non-controlling interest recognised within equity |
| ( |
On 24 September 2021, the Group purchased an additional
(f) | Disposal of interest in Changsha Pengai without loss of control |
| 2021 | |
| RMB’000 | |
Carrying amount of non-controlling interests disposed of |
| |
Less: consideration received from non-controlling interest |
| |
Gain on disposal within equity |
| |
On 24 September 2021, the Group disposed a
F-64
31 Transactions with non-controlling interests (Continued)
(g) | Acquisition of interest in Shenzhen Pengai Xiuqi |
| 2022 | |
| RMB’000 | |
Carrying amount of non-controlling interests acquired |
| |
Consideration paid to non-controlling interests |
| ( |
Excess of consideration paid to non-controlling interest recognised within equity |
| ( |
On 30 May 2022, the Group purchased an additional
(h) | Acquisition of interest in Guangzhou Pengai |
| 2022 | |
| RMB’000 | |
Carrying amount of non-controlling interests acquired |
| ( |
Consideration paid to non-controlling interests |
| ( |
Excess of consideration paid to non-controlling interest recognised within equity |
| ( |
On 15 June 2022, the Group purchased an additional
(i) | Acquisition of interest in Guangzhou Pengai Xiuqi |
| 2022 | |
| RMB’000 | |
Carrying amount of non-controlling interests acquired |
| ( |
Consideration paid to non-controlling interests |
| — |
Excess of consideration paid to non-controlling interest recognised within equity |
| ( |
On 20 June 2022, the Group’s a equity interest in Guangzhou Peng Ai Xiuqi changed from
(j) |
| 2022 | |
RMB’000 | ||
Carrying amount of non-controlling interests acquired |
| ( |
Consideration paid to non-controlling interests |
| ( |
Excess of consideration paid to non-controlling interest recognised within equity |
| ( |
|
On 12 July 2022, the Group purchased an additional
F-65
32 Share based compensation
The Group has adopted a share-based compensation plan, namely the Aesthetic Medical International Holdings Group Limited Share Incentive Plan, (the “Share Incentive Plan”) and Performance Incentive Plan (the “Performance Incentive Plan”). The Share Incentive Plan was approved by the board of directors of the Company on 1 June 2019. According to the Share Incentive Plan,
One-fourth of granted options shall vest and become exercisable upon the first anniversary of the effective date of a registration statement covering any public offering of the Company’s securities (“First Vesting Date”). The remaining
The option holder may elect at any time to exercise any part or all of the vested options before the expiry date.
Movements in the number of options for the year ended 31 December 2021 and 2022 are as follows:
|
| Weighted- |
| Weighted- | ||
Number of | average | average grant | ||||
options | exercise price | date fair value | ||||
RMB | RMB | |||||
Outstanding as of 1 January 2021 | | | | |||
Vested during the year (note) | ( | |||||
Outstanding as of 31 December 2021 |
| | | | ||
Vested during the year (note) | ( | |||||
Outstanding as of 31 December 2022 | — | | |
Note: The Group vested the options to certain option holders in advance.
The fair value of share options were valued at grant date using Binomial option-pricing model. Assumptions used in the Binomial option-pricing model are presented below:
Risk free interest rate |
| | % |
Expected dividend yield |
| | |
Expected volatility |
| | % |
Exercise multiples |
| | |
Contractual life |
| |
Share options outstanding as of 31 December 2022 have the following expiry date and exercise prices:
Share options as of | ||||||
Grant date | Expiry date | Exercise price | 31 December 2022 | |||
1 June 2019 | 31 May 2029 | US$ | — | |||
Weighted average remaining contractual life of options outstanding at end of period: | Not applicable |
Performance Incentive Plan was approved by the board of directors in September 2019. On July 29, 2022, the Company issued
The share-based compensation expenses of approximately RMB
F-66
33 Summarised financial information of subsidiaries with material non-controlling interests
Set out below is the summarised financial information for each subsidiary that has non-controlling interests that are material to the Group.
As at 31 December 2020, 2021 and 2022, the non-controlling interests attributable to these entities accounted for
Summarised balance sheets
Huizhou Pengai | Shanghai Pengai | |||||||||||
2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |||||||
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Current |
|
|
|
|
|
|
|
|
|
|
|
|
- Assets |
| |
| |
| |
| |
| |
| |
- Liabilities |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Total current net assets/(liabilities) |
| |
| |
| |
| ( |
| ( |
| ( |
Non‑current |
|
|
|
|
|
|
| |||||
- Assets |
| |
| |
| |
| |
| |
| |
- Liabilities |
| — |
| ( |
| ( |
| ( |
| ( |
| ( |
Total non‑current net assets |
| |
| |
| |
| |
| |
| |
Net assets/(liabilities) |
| |
| |
| |
| |
| ( |
| ( |
Xiuqi Pengai | Haikou Pengai | |||||||||||
2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |||||||
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Current |
|
|
|
|
|
|
|
|
|
| ||
- Assets |
| | | |
| |
| |
| | ||
- Liabilities |
| ( | ( | ( |
| ( |
| ( |
| ( | ||
Total current net liabilities |
| ( | ( | ( |
| ( |
| ( |
| ( | ||
Non‑current |
|
|
|
| ||||||||
- Assets |
| | | |
| |
| |
| | ||
- Liabilities |
| — | — | — |
| ( |
| ( |
| ( | ||
Total non‑current net assets |
| | | |
| |
| |
| | ||
Net assets/(liabilities) |
| | | |
| |
| ( |
| ( | ||
F-67
33 Summarised financial information of subsidiaries with material non-controlling interests (Continued)
Summarised balance sheets (Continued)
Yantai Pengai | ||||||
2020 | 2021 | 2022 | ||||
RMB’000 | RMB’000 | RMB’000 | ||||
Current |
|
|
|
|
|
|
- Assets |
| |
| |
| |
- Liabilities |
| ( |
| ( |
| ( |
Total current net liabilities |
| ( |
| ( |
| ( |
Non-current |
|
|
|
|
|
|
- Assets |
| |
| |
| |
- Liabilities |
| ( |
| ( |
| ( |
Total non-current net assets |
| |
| |
| |
Net assets/(liabilities) |
| |
| ( |
| ( |
Summarised statements of comprehensive income
Huizhou Pengai | Shanghai Pengai | |||||||||||
2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |||||||
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Revenue |
| |
| |
| | |
| |
| | |
Profit/(loss) before income tax |
| |
| ( |
| | ( |
| ( |
| ( | |
Income tax (expense)/credit |
| ( |
| |
| | |
| |
| ( | |
Profit/(loss) and total comprehensive income/(loss) for the year |
| |
| ( |
| | ( |
| ( |
| ( | |
Total comprehensive income/(loss) allocated to non‑controlling interests |
| |
| ( |
| | ( |
| ( |
| ( | |
Dividend paid to non‑controlling interests |
| |
| |
| — | — |
| — |
| — | |
Xiuqi Pengai | Haikou Pengai | |||||||||||
2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |||||||
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Revenue |
| | | |
| |
| |
| | ||
(Loss)/Profit before income tax |
| ( | ( | |
| ( |
| ( |
| ( | ||
Income tax (expense)/credit |
| ( | | |
| |
| ( |
| ( | ||
(Loss)/Profit and total comprehensive (loss)/income for the year |
| ( | ( | |
| ( |
| ( |
| ( | ||
Total comprehensive (loss)/income allocated to non‑controlling interests |
| ( | ( | |
| ( |
| ( |
| ( | ||
Dividend paid to non‑controlling interests |
| — | — | — |
| |
| — |
| — | ||
F-68
33 Summarised financial information of subsidiaries with material non-controlling interests (Continued)
Summarised statements of comprehensive income (Continued)
Yantai Pengai | ||||||
| 2020 |
| 2021 |
| 2022 | |
RMB’000 | RMB’000 | RMB’000 | ||||
Revenue |
| |
| |
| |
Profit/(loss) before income tax |
| ( |
| ( |
| |
Income tax (expense)/credit |
| |
| |
| |
Profit/(loss) and total comprehensive income/(loss) for the year |
| ( |
| ( |
| |
Total comprehensive income/(loss) allocated to non-controlling interests |
| ( |
| ( |
| |
Dividend paid to non-controlling interests |
| — |
| — |
| — |
Summarised statements of cash flows
Huizhou Pengai | Shanghai Pengai | |||||||||||
2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |||||||
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
Cash generated from operations |
| |
| |
| ( |
| ( |
| |
| ( |
Income tax paid |
| ( |
| ( |
| ( |
| ( |
| ( |
| — |
Net cash generated from/(used in) operating activities |
| |
| |
| ( |
| ( |
| |
| ( |
Net cash used in investing activities |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Net cash used in financing activities |
| ( |
| ( |
| |
| ( |
| — |
| |
Net (decrease)/increase in cash and cash equivalents |
| ( |
| |
| |
| ( |
| |
| ( |
Cash and cash equivalents at beginning of the year |
| |
| |
| |
| |
| |
| |
Cash and cash equivalents at end of the year |
| |
| |
| |
| |
| |
| |
Xiuqi Pengai | Haikou Pengai | |||||||||||
2020 | 2021 | 2022 | 2020 | 2021 | 2022 | |||||||
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
|
| |
Cash generated from/(used in) operations |
| | ( | ( |
| |
| |
| | ||
Income tax paid |
| — | — | — |
| ( |
| ( |
| ( | ||
Net cash generated from/(used in) operating activities |
| | ( | ( |
| |
| |
| | ||
Net cash used in investing activities |
| ( | ( | ( |
| ( |
| ( |
| ( | ||
Net cash (used in)/generated from financing activities |
| ( | | ( |
| ( |
| ( |
| ( | ||
Net (decrease)/increase in cash and cash equivalents |
| ( | | ( |
| |
| |
| ( | ||
Cash and cash equivalents at beginning of the year |
| | | |
| |
| |
| | ||
Cash and cash equivalents at end of the year |
| | | |
| |
| |
| | ||
F-69
33 Summarised financial information of subsidiaries with material non-controlling interests (Continued)
Summarised statements of cash flows (Continued)
Yantai Pengai Jiayan | ||||||
|
| 2020 |
| 2021 |
| 2022 |
RMB’000 | RMB’000 | RMB’000 | ||||
Cash flows from operating activities | ||||||
Cash generated from operations |
| |
| |
| |
Income tax paid |
| — |
| — |
| — |
Net cash generated from operating activities |
| |
| |
| |
Net cash used in investing activities |
| ( |
| — |
| ( |
Net cash used in financing activities |
| ( |
| — |
| ( |
Net increase/ (decrease) in cash and cash equivalents |
| ( |
| |
| ( |
Cash and cash equivalents at beginning of the year |
| |
| |
| |
Cash and cash equivalents at end of the year |
| |
| |
| |
34 Commitments
(a) | Capital commitments |
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
Contracted but not provided for |
|
|
|
|
- Property, plant and equipment |
| — |
| — |
- Investments | — | — | ||
— | — |
(b) | Operating lease commitments |
The Group leases premises for aesthetic healthcare services and offices under non-cancellable operating agreements. The lease terms are between
From 1 January 2021, the Group has recognised right-of-use assets for these leases, except for short-term as stated in note 2.25 and note 13.
The future aggregate minimum lease payments under non-cancellable operating leases as follows:
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
Not later than 1 year |
| |
| |
(c) | Contingencies |
Certain subsidiaries of the Company have been named as defendants in two litigations in the PRC. The maximum amount of the damages claimed by the plaintiffs amounted to an aggregate of approximately RMB
F-70
35 Related party transactions
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Parties are also considered to be related if they are subject to common control, common significant influence or joint control. Members of key management and their close family member of the Group are also considered as related parties.
The following significant transactions were carried out between the Group and its related parties in addition to the related party information shown elsewhere in these consolidated financial statements. In the opinion of the directors of the Company, the related party transactions were carried out in the normal course of business and at terms negotiated between the Group and the respective related parties.
(a) | Transactions with related parties |
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
Depreciation, interest expense and building rental |
|
|
|
|
- A related company |
| |
| |
Medical Equipment rental and transactions |
|
|
| |
-A related company |
| |
| |
(b) | Balances with related parties |
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
Current (Note i) |
|
|
|
|
Amounts due from related parties |
|
|
|
|
Amounts due from related companies |
| — |
| |
Amounts due from non‑controlling interests |
| |
| |
| |
| | |
Amounts due to related parties |
|
|
| |
Amounts due to related companies |
| |
| — |
Amounts due to non‑controlling interests |
| — |
| — |
| |
| — |
Note i: Balances due from/to related parties are unsecured, interest free, repayable on demand and are denominated in RMB. Their carrying values approximate to their fair values.
(c) | Key management compensation |
Key management includes directors and senior managements. The compensation paid or payable to key management for employee services is shown below:
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
Basic salaries and bonus |
| |
| |
Share based compensation | | | ||
Pension costs - defined contribution plans |
| |
| |
| |
| |
F-71
35 Related party transactions (Continued)
(d) | Personal guarantees from Dr. Zhou Pengwu, Dr. Zhou Yitao, Zhou Xichun and Ms. Ding Wenting and corporate guarantee from a related company controlled by Dr. Zhou Pengwu |
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
Bank borrowings of the Group secured by personal guarantees from Dr. Zhou Pengwu, Dr. Zhou Yitao and Ms. Ding Wenting and corporate guarantee from a related company |
| |
| |
Bank borrowings of the Group secured by personal properties from Dr. Zhou Pengwu and Ms. Ding Wenting | | |
36 Particulars of the subsidiaries
The following is a list of the principal subsidiaries at 31 December 2021 and 2022:
Place of |
| ||||||||||
incorporation/ | Registered capital/ | Interest held |
| ||||||||
Name | establishment | issued share capital | Principal activities | 2021 | 2022 |
| |||||
Directly hold: |
|
|
|
|
|
|
|
|
|
| |
Dragon Jade Holdings Limited |
| British Virgin Islands (The “BVI”) |
| Registered capital of |
| Investment holding and provision of management services |
| | % | | % |
(龍翠控股有限公司) | |||||||||||
Stargaze Wealth Limited |
| BVI |
| Registered capital of |
| Investment holding and provision of management services |
| | % | | % |
(遙望星空有限公司) | |||||||||||
Indirectly hold: |
|
|
|
|
|
|
|
|
|
| |
Peng Oi Investment (Hong Kong) Holdings Limited |
| Hong Kong |
|
| Investment holding and provision of management services |
| | % | | % | |
(鵬愛投資(香港)集團有限公司) | |||||||||||
Peng Yida Business Consulting (Shenzhen) Co., Ltd. |
| The PRC |
| Registered capital of Hong Kong dollar |
| Investment holding and provision of management services |
| | % | | % |
(鵬意達商務諮詢(深圳)有限公司) | |||||||||||
Shenzhen Pengai Hospital Investment Management Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Investment holding and provision of management services |
| | % | | % |
(深圳鵬愛醫院投資管理有限公司) | |||||||||||
Aesthetic Medical International Holdings (Singapore) Pte. Ltd. |
| Singapore |
| Singapore dollars |
| Investment holding and provision of management services |
| | % | | % |
(中國醫美控股(新加坡)有限公司) | |||||||||||
Shenzhen Pengcheng General Hospital Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services and general healthcare services |
| | % | | % |
(深圳鵬程醫院有限公司) | |||||||||||
Shenzhen Pengai Aesthetic Medical Hospital Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
(深圳鵬愛醫療美容醫院有限公司) | |||||||||||
Shenzhen Pengai Beauty Promise Cosmetic Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Sales of cosmetic products |
| | % | | % |
(深圳市鵬愛美麗約定美容有限公司) | |||||||||||
Haikou Pengai Aesthetic Medical Hospital Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
(海口鵬愛醫療美容醫院有限公司) | |||||||||||
Huizhou Pengai Aesthetic Medical Hospital Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
(惠州鵬愛醫療美容醫院有限公司) | |||||||||||
Jinan Pengai Cosmetic Surgery Hospital Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
(濟南鵬愛美容整形醫院有限公司) | |||||||||||
Shanghai Pengai Medical Technology Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of medical aesthetic technical consulting and management services |
| | % | | % |
(上海鵬愛醫療科技有限公司) | |||||||||||
Shanghai Qiyue Medical Techonology Co., Ltd. | The PRC | Registered capital of RMB | Provision of medical aesthetic technical consulting and management services | | % | | % | ||||
(上海祺嶽醫療科技有限公司) | |||||||||||
Shengli Aesthetic Technology Investment,Hong Kong Company Limited | Hong Kong | Investment holding and provision of management services | % | % | |||||||
Aih Investment Management Corp. | U.S.A | Registered capital of | Investment holding and provision of management services | % | % | ||||||
F-72
36 Particulars of the subsidiaries (Continued)
Place of |
| ||||||||||
incorporation/ | Registered capital/ | Interest held |
| ||||||||
Name | establishment | issued share capital | Principal activities | 2021 | 2022 |
| |||||
Indirectly hold (Continued): | |||||||||||
Shenzhen Pengai Culture Broadcast Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of cultural, artistic activities, corporate image, exhibition planning and advertising service |
| | % | | % |
(深圳鵬愛文化傳播有限公司) | |||||||||||
Changsha Pengai Aesthetic Medical Hospital Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | — | |
(長沙鵬愛醫療美容醫院有限公司) | |||||||||||
Shanghai Pengai Aesthetic Medical Clinic Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
(上海鵬愛醫療美容門診部有限公司) | |||||||||||
Newa Medical Aesthetics Limited |
| Hong Kong |
|
| Provision of aesthetic medical services |
| | % | | % | |
Guangzhou Pengai Aesthetic Medical Hospital Co., Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
(廣州鵬愛醫療美容門診部有限公司) | |||||||||||
Shenzhen Pengai Xiuqi Aesthetic Medical Hospital Co. Ltd. |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
(廣州鵬愛醫療美容醫院有限公司) | |||||||||||
Hangzhou Pengai Aesthetic Medical Clinic Co., Ltd. (杭州鹏爱医疗美容门诊部有限公司) |
| The PRC |
| Registered capital of RMB |
| Provision of aesthetic medical services |
| | % | | % |
Shanghai Jiahong (formerly known as Shanghai Mingyue Yueji) Aesthetic Medical Clinic Co., Ltd. | The PRC | Registered capital of RMB | Provision of aesthetic medical services | | % | | % | ||||
Guangzhou Pengai Xiuqi Aesthetic Medical Clinic Co., Ltd. (廣州鵬愛秀琪醫療美容門診部有限公司) | The PRC | Registered capital of RMB | Provision of aesthetic medical services | | % | | % | ||||
Beijing AomeiYixin Investment ConsultantCo., Ltd. (北京澳美醫信投資顧問有限公司) | The PRC | Registered capital of RMB | Investment holding and provision of management services | | % | | % | ||||
Beijing Haiyue Xingguang Aesthetic Medical Clinic (北京海悅星光醫療美容診所) | The PRC | — | Provision of aesthetic medical services | | % | | % | ||||
Yantai Pengai Cosmetic Surgery Hospital Co., Ltd. (煙台鵬愛美容整形醫院有限公司) | The PRC | Registered capital of RMB | Provision of aesthetic medical services | | % | | % | ||||
37 Additional information: condensed financial statements of the Company
The condensed financial statements of Aesthetic Medical International Holdings Group Limited have been prepared in accordance with Securities and Exchange Commission Regulation S-X Rule 5-04 and Rule 12-04.
The Company records its investments in subsidiaries under the equity method of accounting. Such investments and loans to subsidiaries are presented on the condensed financial statements as “Investment in subsidiaries and due from subsidiaries” and the profit of the subsidiaries is presented as “Profit from subsidiaries” in the condensed statements of comprehensive income.
The footnote disclosures contain supplemental information relating to the operations of the Company and, as such, these financial statements should be read in conjunction with the notes to the consolidated financial statements. Certain information and footnote disclosures normally included in financial statements prepared in accordance with IFRS have been condensed or omitted.
As of December 31, 2021 and 2022, there were no material contingencies, significant provisions for long-term obligations, or guarantees of the Company, except for those, if any, which have been separately disclosed in the consolidated financial statements.
F-73
37 Additional information: condensed financial statements of the Company (Continued)
Condensed balance sheets of the parent company
2021 | 2022 | |||
| RMB’000 |
| RMB’000 | |
ASSETS |
|
|
|
|
Non‑current assets |
|
|
|
|
Investments in subsidiaries and due from subsidiaries |
| — |
| — |
Current assets |
|
| ||
Other receivables, deposits and prepayments |
| — |
| — |
Cash and cash equivalents |
| |
| |
| |
| | |
Total assets |
| |
| |
EQUITY AND LIABILITIES |
|
| ||
Equity attributable to owners of the Company |
|
| ||
Share capital and treasury shares |
| ( |
| ( |
Other reserves |
| ( |
| ( |
Total equity |
| ( |
| ( |
LIABILITIES |
|
| ||
Non‑current liabilities |
|
| ||
Convertible note |
| |
| — |
| |
| | |
Current liabilities |
|
| ||
Accruals, other payables and provisions |
| |
| |
Convertible note | — | | ||
| | |||
Total liabilities |
| |
| |
Total equity and liabilities |
| |
| |
F-74
37 Additional information: condensed financial statements of the Company (Continued)
Condensed statements of comprehensive income of the parent company
2020 | 2021 | 2022 | ||||
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Loss from subsidiaries |
| ( |
| ( |
| ( |
Selling expenses |
| ( |
| ( |
| — |
General and administrative expenses |
| ( |
| ( |
| ( |
Finance income |
| — |
| — |
| |
Finance costs |
| — |
| ( |
| — |
Other loss, net | — | ( | ( | |||
Fair value loss of convertible note |
| ( |
| ( |
| ( |
Loss before income tax |
| ( |
| ( |
| ( |
Income tax expense |
| — |
| — |
| — |
Loss for the year |
| ( |
| ( |
| ( |
Other comprehensive income/(loss): |
|
|
|
|
|
|
Items that may be subsequently reclassified to profit or loss |
|
|
|
|
|
|
Currency translation differences |
| ( |
| |
| ( |
Total other comprehensive income/(loss) for the year, net of tax |
| ( |
| |
| ( |
Total comprehensive loss for the year |
| ( |
| ( |
| ( |
Condensed statements of cash flows of the parent company
2020 | 2021 | 2022 | ||||
| RMB’000 |
| RMB’000 |
| RMB’000 | |
Net cash used in operating activities |
| ( |
| ( |
| ( |
Net cash used in investing activities |
| ( |
| — |
| — |
Net cash generated from financing activities |
| |
| — |
| |
Net increase/(decrease) in cash and cash equivalents |
| ( |
| ( |
| ( |
Cash and cash equivalents at beginning of the year |
| |
| |
| |
Cash and cash equivalents at end of the year |
| |
| |
| |
38 Subsequent events
On January 10, 2023, the Company entered into a share purchase agreement with a non-affiliated third party, under which it transferred
In February 2023, the Subscription Agreement between the Company and Jiechuang was closed through the issuance and allotment of
Concurrently with the Subscription Agreement with Jiechuang, the Company entered into a Share Purchase Agreement with Dr. Pengwu Zhou and Ms. Wenting Ding (the “Founders”), certain of our existing shareholders controlled by the Founders, and Wanda. Pursuant to the Share Purchase Agreement, Seefar Global Holdings Limited, Jubilee Set Investments Limited, and Pengai Hospital Management Corporation (collectively referred as to the “Sellers”) agreed to sell and Wanda as the buyer agreed to purchase, an aggregate of
F-75



