UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 20-F
| REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) or 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||||||
| OR | ||||||||
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended | ||||||||
| OR | ||||||||
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from __________ to __________ | ||||||||
| OR | ||||||||
| SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Date of event requiring the shell company report __________ | ||||||||
Commission file number: 001-31269
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant's name into English)
(Jurisdiction of incorporation or organization)
(Address of principal executive office)
(Name, Telephone, Email and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||||||||
| SIX Swiss Exchange | ||||||||||||||||||||
Securities registered or to be registered pursuant to Section 12(g) of the Act. None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act. None
Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of the period covered by the annual report. 493,244,479
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definitions of "large accelerated filer," "accelerated filer," and "emerging growth company" and in Rule 12b-2 of the Exchange Act.
| ☒ | Accelerated Filer | ☐ | Non-accelerated Filer | ☐ | Emerging Growth Company | ||||||||||||||||||
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term "new or revised financial accounting standard" refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b) ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP | ☐ | ☒ | Other | ☐ | |||||||||||||
| as issued by the International Accounting Standards Board | |||||||||||||||||
If "Other" has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
| INDEX | Page | |||||||
| Introduction and Use of Certain Terms | ||||||||
| Market Information | ||||||||
| Special Note About Forward-Looking Statements | ||||||||
| PART I | ||||||||
| Item 1. | Identity of Directors, Senior Management and Advisers | |||||||
| Item 2. | Offer Statistics and Expected Timetable | |||||||
| Item 3. | Key Information | |||||||
| Item 4. | Information on the Company | |||||||
| Item 4A. | Unresolved Staff Comments | |||||||
| Item 5. | Operating and Financial Review and Prospects | |||||||
| Item 6. | Directors, Senior Management and Employees | |||||||
| Item 7. | Major Shareholders and Related Party Transactions | |||||||
| Item 8. | Financial Information | |||||||
| Item 9. | The Offer and Listing | |||||||
| Item 10. | Additional Information | |||||||
| Item 11. | Quantitative and Qualitative Disclosures About Market Risk | |||||||
| Item 12. | Description of Securities Other than Equity Securities | |||||||
| PART II | ||||||||
| Item 13. | Defaults, Dividend Arrearages and Delinquencies | |||||||
| Item 14. | Material Modifications to the Rights of Security Holders and Use of Proceeds | |||||||
| Item 15. | Controls and Procedures | |||||||
| Item 16A. | Audit Committee Financial Expert | |||||||
| Item 16B. | Code of Ethics | |||||||
| Item 16C. | Principal Accountant Fees and Services | |||||||
| Item 16D. | Exemptions from the Listing Standards for Audit Committees | |||||||
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers | |||||||
| Item 16F. | Change in Registrant's Certifying Accountant | |||||||
| Item 16G. | Corporate Governance | |||||||
| Item 16H. | Mine Safety Disclosure | |||||||
| Item 16I. | Disclosure regarding Foreign Jurisdictions that Prevent Inspections | |||||||
| Item 16J. | Insider Trading Policies | |||||||
| Item 16K. | Cybersecurity | |||||||
| PART III | ||||||||
| Item 17. | Financial Statements | |||||||
| Item 18. | Financial Statements | |||||||
| Item 19. | Exhibits | |||||||
| Consolidated Financial Statements of Alcon Inc. | ||||||||
INTRODUCTION AND USE OF CERTAIN TERMS
Alcon Inc. publishes Consolidated Financial Statements expressed in US dollars. Our Consolidated Financial Statements responsive to Item 18 of this Annual Report filed on Form 20-F with the US Securities and Exchange Commission (the "Annual Report") are prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"). "Item 5. Operating and Financial Review and Prospects", together with “Item 4.B. Business Overview” and “Item 6.D. Employees”, constitute the Operating and Financial Review ("Rapport annuel"), as defined by the Swiss Code of Obligations.
Unless the context requires otherwise, the words "we", "our", "us", "Alcon", "Company" and similar words or phrases in this Annual Report refer to Alcon Inc. and its consolidated subsidiaries. The words "Novartis" and "Novartis Group" refer to Novartis AG and its consolidated affiliates. In this Annual Report, references to the "eye care market" are to the Surgical and Vision Care markets in which we participate, including the sale of ophthalmic surgical devices, contact lenses and ocular health products, but not including the sale of spectacles and prescription ophthalmic pharmaceutical products other than glaucoma pharmaceutical products; references to "United States dollars", "US dollars", "USD" or "$" are to the lawful currency of the United States of America, and references to "CHF" are to Swiss francs, the lawful currency of Switzerland; references to "International" are to the entire world except the United States of America, unless the context otherwise requires; references to "associates" are to our employees; references to the "SEC" are to the US Securities and Exchange Commission; references to the "FDA" are to the US Food and Drug Administration; references to "EMA" are to the European Medicines Agency, an agency of the EU; references to the "NYSE" are to the New York Stock Exchange; references to the "SIX" are to the SIX Swiss Exchange; references to "ATIOL" mean advanced technology intraocular lenses; and references to "Alcon shares" or "our shares" are to Alcon ordinary shares, nominal value CHF 0.04 per share, with ticker symbol "ALC."
All product names appearing in italics are trademarks owned by or licensed to Alcon or its subsidiaries. Product names identified by a "®" or a "™" are trademarks that are not owned by or licensed to Alcon or its subsidiaries and are the property of their respective owners.
MARKET INFORMATION
This Annual Report contains certain industry and market data that were obtained from third-party sources, such as industry surveys and industry publications, including, but not limited to, publications by Market Scope, GfK and Nielsen. This Annual Report also contains other industry and market data, including market sizing estimates, growth and other projections and information regarding our competitive position, prepared by our management on the basis of such industry sources and our management's knowledge of and experience in the industry and markets in which we operate (including management's estimates and assumptions relating to such industry and markets based on that knowledge). Our management has developed its knowledge of such industry and markets through its experience and participation in these markets.
In addition, industry surveys and industry publications generally state that the information they contain has been obtained from sources believed to be reliable but that the accuracy and completeness of such information is not guaranteed and that any projections they contain are based on a number of significant assumptions. Forecasts, projections and other forward-looking information obtained from these sources involve risks and uncertainties and are subject to change based on various factors, including those discussed in the section "Special Note About Forward-Looking Statements" below. You should not place undue reliance on these statements.
1
SPECIAL NOTE ABOUT FORWARD-LOOKING STATEMENTS
This Annual Report contains, and our officers and representatives may from time to time make, certain “forward-looking statements” within the meaning of the safe harbor provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as “anticipate,” “intend,” “commitment,” “look forward,” “maintain,” “plan,” “goal,” “seek,” “target,” “assume,” “believe,” “project,” “estimate,” “expect,” “strategy,” “future,” “likely,” “may,” “should,” “will” and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding our liquidity, revenue, gross margin, operating margin, effective tax rate, foreign currency exchange movements, earnings per share, our plans and decisions relating to various capital expenditures, capital allocation priorities and other discretionary items such as our market growth assumptions, our social impact and sustainability plans, targets, goals and expectations, and generally, our expectations concerning our future performance. You should not place undue reliance on these statements.
Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties and risks that are difficult to predict such as:
•cybersecurity breaches or other disruptions of our information technology systems;
•compliance with data privacy, identity protection and information security laws, particularly with the increased use of artificial intelligence;
•the impact of a disruption in our global supply chain or important facilities, particularly when we single-source or rely on limited sources of supply;
•our ability to forecast sales demand and manage our inventory levels and the changing buying patterns of our customers;
•our ability to manage social impact and sustainability matters;
•our reliance on outsourcing key business functions;
•global and regional economic, financial, monetary, legal, tax, political and social change;
•our success in completing and integrating strategic acquisitions;
•the success of our research and development efforts, including our ability to innovate to compete effectively;
•our ability to comply with the US Foreign Corrupt Practices Act of 1977 and other applicable anti-corruption laws;
•pricing pressure from changes in third party payor coverage and reimbursement methodologies;
•our ability to properly educate and train healthcare providers on our products;
•our ability to protect our intellectual property;
•our ability to comply with all laws to which we may be subject;
•the ability to obtain regulatory clearance and approval of our products as well as compliance with any post-approval obligations, including quality control of our manufacturing;
•the effect of product recalls or voluntary market withdrawals;
•the accuracy of our accounting estimates and assumptions, including pension and other post-employment benefit plan obligations and the carrying value of intangible assets;
•the impact of unauthorized importation of our products from countries with lower prices to countries with higher prices;
•our ability to service our debt obligations;
•the need for additional financing through the issuance of debt or equity;
•the effects of litigation, including product liability lawsuits and governmental investigations;
•supply constraints and increases in the cost of energy;
2
•our ability to attract and retain qualified personnel;
•legislative, tax and regulatory reform;
•the impact of being listed on two stock exchanges;
•the ability to declare and pay dividends;
•the different rights afforded to our shareholders as a Swiss corporation compared to a US corporation;
•the effect of maintaining or losing our foreign private issuer status under US securities laws; and
•the ability to enforce US judgments against Swiss corporations.
Some of these factors are discussed in more detail in this Annual Report, including under “Item 3. Key Information—3.D. Risk Factors”, “Item 4. Information on the Company” and “Item 5. Operating and Financial Review and Prospects”. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this Annual Report as anticipated, believed, estimated or expected. We provide the information in this Annual Report as of the date of its filing. We do not intend, and do not assume any obligation, to update any information or forward-looking statements set out in this Annual Report as a result of new information, future events or otherwise.
3
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
1.A. DIRECTORS AND SENIOR MANAGEMENT
Not Applicable.
1.B. ADVISERS
Not Applicable.
1.C. AUDITORS
Not Applicable.
4
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not Applicable.
5
ITEM 3. KEY INFORMATION
3.A. [RESERVED]
3.B. CAPITALIZATION AND INDEBTEDNESS
Not Applicable.
3.C. REASONS FOR THE OFFER AND USE OF PROCEEDS
Not Applicable.
6
3.D. RISK FACTORS
You should carefully consider the risks described below, together with all of the other information included in this Annual Report, in evaluating Alcon and our securities. The following risk factors could adversely affect our business, financial condition and results of operations and the price of our securities.
Risks Related to Our Business Generally
Significant cybersecurity breaches could disrupt business operations, result in the loss of critical and confidential information and adversely impact our reputation and results of operations.
We are heavily dependent on critical, complex and interdependent information technology systems, including internet-based systems, to support our business processes. We are also increasingly seeking to develop or acquire technology-based products to improve patient welfare in a variety of ways, which could also result in us gathering personal information about patients and others electronically.
The size and complexity of our information technology systems, and, in some instances, their age, make them potentially vulnerable to external or internal security incidents, breakdowns, malicious intrusions, cybercrimes, including state-sponsored cybercrimes, malware, misplaced or lost data, programming or human errors or other similar events. For our technology-based products that are increasingly connected to the internet, failure to update software that runs on our medical devices (whether the failure is caused by us or our customers) could increase the vulnerability of those devices to attacks by criminals, which could adversely impact a healthcare facility’s operations, patient safety, data confidentiality and data integrity. Like many companies, our technology landscape has become more complex as we also rely on our third party partners to be cyber resilient. Furthermore, because cyberthreats continue to evolve and become more sophisticated, it is becoming increasingly difficult to detect and successfully defend against them, particularly because there is strong competition to hire a limited pool of individuals with a cybersecurity skill set. Consequently, there is a risk that a cybersecurity breach remains undetected for a period of time.
We have experienced certain adverse incidents and expect to continue to experience them in the future and, as the external cyberattack threat only keeps growing, we may not be able to prevent future breakdowns or breaches in our systems (or those of our third party partners) and we may not be able to prevent such events from having a material adverse effect on our business, financial condition, results of operations or reputation.
A cybersecurity breach could negatively impact important business processes, such as the conduct of scientific research and clinical trials, the submission of the results of such efforts to health authorities in support of requests for product approvals, the functioning of our manufacturing and supply chain processes, our compliance with legal obligations and our other key business activities, including our associates' ability to communicate with one another and with third parties. These risks are heightened when our office-based associates work from home. Such potential information technology issues could also lead to the loss of important information such as trade secrets or other intellectual property and could accelerate the development or manufacturing of competing products by third parties. Furthermore, malfunctions in software or in devices that make significant use of information technology, including our surgical equipment, could lead to a risk of harm to patients.
Cybersecurity breaches, technology disruptions, privacy violations, or similar issues could expose personal information and interrupt our operations or the operations of our customers, which could result in enforcement actions or liability, including potential government fines, claims for damages, remediation costs and shareholders' litigation. Any such events could require us to expend significant resources beyond those we already invest to further modify or enhance our protective measures, to remediate any damage and to enable the continuity of our business.
Data privacy, identity protection and information security compliance may require significant resources, and our failure to comply with applicable law could lead to significant liability.
Our routine business operations, including through the use of information technologies such as the internet, social media, artificial intelligence, mobile technologies and technology-based medical devices like our surgical equipment, increasingly involve our collecting, storing, accessing, and processing personal data and other information about patients, vendors, customers, associates, collaborators and others that are subject to privacy and security laws, regulations and customer-imposed controls. Failure to protect that information could expose such people's personal information to unauthorized persons. As we transform into a more digital organization through the launch of products and services such as MARLO, SmartCataract and other new technology and data driven projects, our risk increases. Any such event could give rise to significant potential liability and reputational harm, including potentially substantial monetary penalties.
Artificial intelligence-based software is increasingly being used in the healthcare industry. As with many developing and potentially disruptive technologies, artificial intelligence-based software presents risks and challenges that could affect its
7
further development, adoption, and use, and therefore our business. For example, algorithms may be flawed; data sets may be insufficient, of poor quality, or contain biased information; and inappropriate or controversial data practices by data scientists, engineers, and end-users could impair results. If the analyses that artificial intelligence applications assist in producing are deficient or inaccurate, we could be subjected to competitive harm, potential legal liability, and brand or reputational harm. Also, as artificial intelligence continues to become more advanced, cyberattackers could use artificial intelligence to develop malicious code, sophisticated phishing attempts and convincing deep fakes. A deep fake is a manipulation of content or the voices or images of actual people, including our senior management, to maliciously publish false messages that appear to be authentic. Such messages may harm our reputation or be used to commit fraud against us, which may in turn have an adverse impact on our revenue and profits. Furthermore, use of artificial intelligence-based software may lead to the release of confidential information which may impact our ability to realize the benefit of our intellectual property.
We are subject to certain privacy laws and regulations that continue to evolve, including Swiss privacy laws, the EU's General Data Protection Regulation ("GDPR"), the California Consumer Privacy Act ("CCPA") and the US Health Insurance Portability and Accountability Act ("HIPAA") with respect to some of our products and services. In addition, there are different and potentially conflicting data privacy laws in effect in the various jurisdictions in which we operate and we must understand and comply with each law and standard in each of these jurisdictions while ensuring the data is secure. In addition, we must make significant efforts to ensure that any international transfers of personal data are done in compliance with applicable law. Failure to comply with these laws could lead to significant monetary liability and reputational damage.
Disruptions in our global supply chain or important facilities could cause production interruptions, delays and inefficiencies.
We are engaged in manufacturing and sourcing of products and materials on a global scale. Our operations and those of our suppliers could be disrupted by a number of factors, including: disruptions in logistics; strikes and other labor disputes; loss or impairment of key manufacturing sites; loss of key suppliers; supplier capacity constraints; raw material and product quality or safety issues; inflation; industrial accidents or other occupational health and safety issues; the impact on our suppliers of tighter credit or capital markets; epidemics and pandemics; natural and man-made disasters, including climatic events (including any potential effect of climate change), power grid failures, acts of war or terrorism, workplace violence, political unrest, fires or explosions; changes in public policy, law or public opinion affecting the availability of key components in our manufacturing process; and other external factors over which we have no control.
In addition, we single-source or rely on limited sources of supply for some components, raw materials and production services, such as sterilization, viscoelastics and active pharmaceutical ingredients (“API”), used in the production of our products. The loss of one of these suppliers or the inability of any such supplier to meet performance and quality specifications, requested quantities or delivery schedules could cause our sales and profitability to decline and have a negative impact on our customer relations. Moreover, a price increase from a supplier where we do not have a supply alternative could cause our profitability to decline if we cannot increase our prices to our customers. To ensure sufficient supply, we may determine that we need to provide financing to some subset of our supplier base, such as the financing arrangement with Lifecore Biomedical, Inc. we entered into in May 2023, which could increase our financial exposure to such suppliers.
In past years, we have incurred shortages of critical components. For example, beginning in 2022 and continuing through mid-2023, our contact lens care business was impacted by a shortage of components used to manufacture the bottles. In 2021, there was a global shortage of semiconductor chips, which are an essential component to the manufacture of our equipment. These types of shortages have resulted, and may continue to result, in delays in the manufacture of our products, increased costs to source alternative supplies, harm to our reputation, loss of business to competitors, and otherwise materially and adversely affect our business and operations.
Finally, in some cases, we manufacture our products at a single manufacturing facility. In many cases, regulatory approvals of our products are limited to a specifically approved manufacturing facility. If we fail to produce enough of a product at a facility, or if our manufacturing process at that facility is disrupted, we may be unable to deliver that product to our customers on a timely basis. Problems may arise during the manufacturing process for a variety of reasons, including technical, labor or other difficulties, equipment malfunction, contamination, failure to follow specific protocols and procedures, destruction of or damage to any facility (as a result of a natural or man-made disaster, use and storage of hazardous materials or other events), power grid failures or other reasons. In the event of a quality control issue, we may voluntarily, or our regulators may require us to, close a facility indefinitely. If any such problems arise, we may be unable to purchase substitute products from third-party manufacturers to make up any resulting shortfall in the production of a product, as such third-party manufacturers may only exist in limited numbers or appropriate substitutes may not be available. This risk is particularly relevant with respect to products for which we represent a substantial portion of the market, such as vitreoretinal equipment and other vitreoretinal-related products including viscoelastic. A failure to deliver
8
products on a timely basis could lead to customer dissatisfaction and damage to our reputation. Significant delays in the delivery of our products or a delay in the delivery of a key product could also negatively impact our sales and profitability.
Our inability to forecast demand accurately may adversely affect our sales and earnings and add to sales variability from quarter to quarter.
We balance the need to maintain inventory levels that are sufficient to ensure competitive lead times against the risk of inventory obsolescence because of changing customer requirements, fluctuating commodity prices, changes to our products, product transfers or the life cycle of our products. To successfully manage our inventories, we must estimate demand from our customers and produce products in sufficient quantity that substantially corresponds to that demand. If we fail to adequately forecast demand for any product, or fail to determine the optimal product mix for production purposes, we may face production capacity issues in manufacturing sufficient quantities of a given product. In addition, failures in our information technology systems or human error could also lead to inadequate forecasting of our overall demand or product mix.
As the number of unique products (SKUs) we offer grows, particularly an increasing number of IOL and contact lens styles with varying diopters, the demand forecasting precision required for us to avoid production capacity issues will also increase. Accordingly, the continued proliferation of unique SKUs in our surgical and vision care portfolios could increase the risk of product unavailability and lost sales. Moreover, an increasing number of SKUs could increase global inventory requirements, especially for consigned products such as IOLs, negatively impacting our working capital performance and leading to write-offs due to obsolescence and expired products.
Compounding the risk of inaccurate forecasts, the manufacturing process for our products has lengthy lead times to acquire and install new equipment and product lines to ramp up production. Thus, if we fail to adequately forecast demand, then we may be unable to scale production in a timely manner to meet unexpected higher demand.
Finally, a significant portion of our vision care products are sold to major healthcare distributors and major retail chains in certain markets. Consequently, our sales and quarterly growth comparisons, as well as our estimates for required inventory levels, may be affected by fluctuations in the buying patterns of such buyers. These fluctuations may result from seasonality, pricing, a recall of a competitor’s product, large retailers' and distributors' buying decisions or other factors. If we overestimate demand and produce too much of a particular product, we face a risk of inventory obsolescence, leaving us with inventory that we cannot sell profitably or at all. By contrast, if we underestimate demand and produce insufficient quantities of a product, we could be forced to choose between producing additional unexpected quantities of that product at a higher price or foregoing sales.
Social impact and sustainability matters may impact our business and reputation.
In addition to the importance of our financial performance, investors, investor advocacy groups, lenders, and other market participants are increasingly judging companies by their performance on a variety of social impact and sustainability ("SIS") matters, which are considered to contribute to the long-term sustainability of companies’ performance. To help judge a company’s SIS performance, a variety of organizations rate a company’s SIS performance based on a variety of SIS topics, and the results of these assessments are widely publicized. In addition, some investors now use SIS criteria to determine whether Alcon qualifies for inclusion in their investment portfolio while investment in funds that specialize in companies that perform well in SIS assessments are increasingly popular. Topics taken into account in such assessments include, among others, our efforts and impacts on climate change and human rights, diversity and inclusion, ethics and compliance with law, the role of our board of directors in supervising various sustainability issues and the public’s ability to access our products and solutions.
We are frequently asked by investors to set ambitious SIS goals and provide new and more robust disclosure on goals, progress toward goals and other matters of interest. In addition, a number of our customers, particularly EU and UK governments, have adopted, or may adopt, procurement policies that impose sustainability standards. Our ability to sell to these customers, including the ability to win public tenders, may depend, in part, on whether we can meet, and provide evidence of meeting, those sustainability standards. In response, we have adapted the tracking and reporting of our corporate responsibility program to various evolving SIS frameworks, and we have established and announced goals and other objectives related to SIS matters. These goal statements reflect our current plans and aspirations and, due to various factors many of which are beyond our control, we may be unable to achieve them. Our efforts to accomplish and accurately report on these goals and objectives present numerous operational, reputational, financial, legal and other risks, any of which could have a material negative impact, including on our reputation and stock price.
The standards for tracking and reporting on SIS matters are relatively new, have not been harmonized and continue to evolve. Our selection of disclosure frameworks that seek to align with various reporting standards may change from time to time and may result in a lack of consistent or meaningful comparative data from period to period. In addition, the US, Swiss, European, and other regulatory authorities have imposed, and may continue to impose, mandatory disclosure requirements with respect to SIS matters, and such standards may change over time, which could result in significant
9
revisions to our current goals, reported progress in achieving such goals, or ability to achieve such goals in the future. In addition, enhancements to our processes and controls to reflect evolving reporting standards and third party assurance of our data, processes and controls may be costly and require additional resources.
While there has been a strong interest from investors, investor advocacy groups, lenders, and other market participants for us to advance our performance in SIS matters, there has also been a strong backlash against certain corporate SIS initatives with opposition coming from government officials, consumers and others. Reconciling the two movements to satisfy everyone may not be possible. If our SIS practices do not meet evolving expectations and standards, then our reputation, our ability to attract or retain associates, our ability to compete, and our attractiveness as an investment could be negatively impacted. Similarly, our failure or perceived failure to pursue or fulfill our goals, targets and objectives or to satisfy various reporting standards within the timelines we announce, or at all, could also have similar negative impacts and expose us to government enforcement actions and private litigation.
Our reliance on outsourcing key business functions to third parties heightens the risks faced by our businesses.
We outsource the performance of certain key business functions to third parties and invest a significant amount of effort and resources into doing so. Such outsourced functions can include research and development collaborations, clinical trial activities, manufacturing operations, human resources, warehousing and distribution activities, certain finance functions, submission of regulatory applications, marketing activities, data management and others. Outsourcing of services to third parties could expose us to suboptimal quality of service delivery or deliverables and potentially result in repercussions such as missed deadlines or other timeliness issues, erroneous data, supply disruptions, non-compliance (including with applicable legal or regulatory requirements and industry standards) and/or reputational harm, with potential negative effects on our results.
Ultimately, if the third parties, fail to meet their obligations to us, we may lose our investment in the collaborations and fail to receive the expected benefits of these arrangements. Contractual remedies may be inadequate to compensate us for the damage to our business or lost profits. In addition, many of the companies to which we outsource key business functions may have more limited resources compared to us, and, in particular, may not have internal compliance resources comparable to those within our organization. Should any of these third parties fail to carry out their contractual duties or regulatory obligations or fail to comply with the law, including laws relating to anti-bribery laws and export and trade controls, or should they act inappropriately in the course of their performance of services for us, there is a risk that we could be held responsible for their acts, that our reputation may suffer and that penalties may be imposed upon us. Any such failures by third parties could have a material adverse effect on our business, financial condition, results of operations or reputation.
Changing economic and financial environments in many countries and increasing global political and social instability may adversely impact our business.
We sell our products in more than 140 countries. As a result, local and regional economic and financial environments and political and social conditions throughout the world influence and affect our results of operations and business.
Unpredictable political and social conditions currently exist in various parts of the world, particularly in emerging markets, including a backlash against free trade, anti-immigrant sentiment, social unrest, a refugee crisis, food and water shortages, terrorism and the risk of direct conflicts between nations. In addition, the current trade environment is extremely volatile, including the imposition of trade tariffs, trade or economic sanctions, or other restrictions. Changes in trade policy vis-à-vis countries that we operate in could affect our ability to sell products and/or increase the cost of doing business in such countries. For example, we expect that the ongoing trade dispute between the US and China, which has been exacerbated over tensions involving Taiwan, could potentially have an adverse effect on the export of our surgical equipment to China. In other cases, economic nationalism programs that require governments to purchase products made in their own country, such as the policies China has recently enacted, can make it difficult for us to compete. Further, significant conflicts continue in parts of the Middle East, including conflicts involving Israel, Saudi Arabia and Iran, and with respect to places such as North Korea, Ukraine, and Taiwan. Collectively, such difficult conditions could, among other things, disturb the international flow of goods and increase the costs and difficulties of international transactions.
In addition, local economic conditions may adversely affect the ability of payors, as well as our distributors, customers, suppliers and service providers, to pay for our products, or otherwise to buy necessary inventory or raw materials, and to perform their obligations under agreements with us. Although we make efforts to monitor these third parties' financial condition and their liquidity, our ability to do so is limited, and some of them may become unable to pay their bills in a timely manner, or may even become insolvent, which could negatively impact our business and results of operations. These risks may be elevated with respect to our interactions with fiscally-challenged government payors, or with third parties with substantial exposure to such payors. For example, we have significant outstanding receivable balances that are dependent upon either direct or indirect payment by various governmental and non-governmental entities across the world. The ultimate payment of these receivables is dependent on the ability of these governments to maintain liquidity primarily through borrowing capacity, particularly in the EU. If certain governments are not able to maintain access to
10
liquidity through borrowing capacity, the ultimate payment of their respective portion of outstanding receivables could be at risk and impact profits and cash flow.
Further, in many emerging markets, average income levels are relatively low, government reimbursement for the cost of healthcare products and services is limited and prices and demand are sensitive to general economic conditions. These challenges may prevent us from realizing the expected benefits of our investments in such emerging markets, which could have an adverse impact on our business, financial condition and results of operations.
Economic conditions in our markets may also deteriorate due to epidemics or pandemics; natural and man-made disasters, including climatic events (including any potential effect of climate change), power grid failures, acts of war or terrorism, inflation, political unrest, fires or explosions; and other external factors over which we have no control.
To the extent that economic and financial conditions directly affect consumers, some of our businesses, including the elective surgical and contact lens businesses, may be particularly sensitive to declines in consumer spending, as the costs of elective surgical procedures and discretionary purchases of contact lenses are typically borne by individuals with limited reimbursement from their medical insurance providers or government programs. For example, while cataract surgery involving our monofocal IOLs is generally fully covered by medical insurance providers or government reimbursement programs, implantation of certain of our ATIOL products may only be partially covered, with the individual paying out-of-pocket for the non-covered component. Accordingly, individuals may be less willing to incur the costs of these private pay or discretionary procedures or purchases in weak or uncertain economic conditions and may elect to forgo such procedures or products or to trade down to more affordable options.
We may not successfully complete and integrate strategic acquisitions to expand or complement our business.
As part of our growth strategy, we regularly evaluate and pursue external investments, alliances, license arrangements, acquisitions and other transactions, which we collectively refer to as "BD&L" transactions, to expand or complement our business. For example, in 2022, we closed the acquisitions of Ivantis, Inc. and Aerie Pharmaceuticals, Inc., as well as the product acquisitions from Kala Pharmaceuticals, Inc. These and other ventures may bring new technologies, products or customers to enhance our position in the ophthalmic industry. We may be unable to identify suitable acquisition candidates at attractive prices or at all. Acquisition activities can be thwarted by overtures from competitors for the targeted candidates and governmental regulation (including market concentration limitations and other competition laws).
Further, even if we are successful in completing an acquisition, we could face risks relating to our ability to:
•successfully integrate the venture due to corporate cultural differences, difficulties in retaining key personnel, customers and suppliers, coordination with other products and changing market preferences;
•maintain uniform standards, controls, procedures and policies throughout acquired companies, including effective integration of acquired companies into our internal control over financial reporting;
•achieve expected synergies and obtain the desired financial or strategic benefits from acquisitions within the anticipated time periods, if at all; and
•successfully enter categories and markets in which we may have limited or no prior experience.
Moreover, acquisitions demand significant company resources and could divert management's attention from our existing business, result in liabilities being incurred that were not known at the time of acquisition or create tax or accounting issues. Furthermore, acquisitions or ventures could also result in potentially dilutive issuances of equity securities, the incurrence of debt, the assumption of contingent liabilities, an increase in expenses related to certain assets and increased operating expenses, all of which could adversely affect our financial condition and results of operations. Significant judgment is required to determine which transactions will result in optimal returns, and to the extent that the economic benefits associated with any of our acquisitions or investments do not meet our expectations, we may be required to record impairment charges related to goodwill, intangible assets or other assets associated with such transactions.
We often enter into option agreements to acquire early-stage technologies, which may fail in the development process or proof-of-concept stage. Even if such a failure occurs before we exercise our option to acquire the technology, we may have already made a significant investment in the failed technology. Further, if we complete the acquisition, we may not be able to successfully integrate the acquired technology into our business or otherwise use it to develop commercialized products. If we fail to timely recognize or address these matters or to devote adequate resources to them, we may fail to achieve our growth strategy or otherwise not realize the intended benefits of an acquisition.
We operate in a highly competitive industry and if we fail to innovate, we may be unable to maintain our position in the markets in which we compete and unable to build and expand our markets.
Our industry is highly competitive and, in both our surgical and vision care businesses, we face intense competition. For example, in our surgical business, we face a mixture of competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of specialized products. Development by other
11
companies of new or improved products, processes or technologies, including digital solutions, may make our products or proposed products less competitive or obsolete. In contact lenses, we face intense competition from existing competitors' products and expect increased competition from contact lens manufacturers in Asia. New market entrants and existing competitors are also challenging distribution models with innovation in non-traditional, disruptive models such as direct-to-consumer, internet and other e-commerce sales opportunities, which could adversely impact the importance of the traditional eye care professional ("ECP") channel in which we have a significant presence and may lead to greater pricing pressure. In ocular health, particularly in pharmaceuticals where we have just recently re-entered, we face competitors with more established R&D capabilities, greater experience in preclinical testing and human clinical trials, and a wider product portfolio. Our vision care business also competes with manufacturers of eyeglasses and providers of other forms of vision correction including ophthalmic surgery. New drug discoveries have the potential to disrupt core elements of our surgical and vision care businesses.
While we currently enjoy leading positions within our industry, our success highly depends on our ability to maintain or build on those leading positions. We cannot predict the timing or impact of the introduction of competitive products, including new market entries, “generic” versions of our approved products, or private label products that treat the same conditions as those of our products. To compete effectively, we must continue to create, invest in or acquire advanced technology, incorporate this technology into our proprietary products, obtain regulatory approvals in a timely manner where required and manufacture and successfully market our products. See “–We may not successfully complete and integrate strategic acquisitions to expand or complement our business” and “–Our research and development efforts may not succeed in bringing new products to market or may fail to do so in a cost-efficient manner or in a manner sufficient to grow our business, replace lost sales or take advantage of new technologies.”
Shifts in industry market share can occur in connection with product issues, physician advisories, safety alerts and publications about our products. New products from our competitors may be safer or more effective, more convenient to use, have better insurance coverage or reimbursement levels or be more effectively marketed than our own products. Specifically in the case of pharmaceuticals, the generic versions of our competitors’ branded products or our own branded products may be sold at a substantially lower price than our own products. Further, in the current environment of managed care, consolidation among healthcare providers, increased competition and declining reimbursement rates, unless we innovate, we must increasingly compete on the basis of price.
Finally, our financial performance also depends on our ability to successfully build and expand our markets. For example, while we currently expect our key markets to grow, particularly in multifocal contact lenses and ATIOLs, the size of the markets in which we compete may not increase above existing levels, we may not be able to regain or gain market share, expand our market penetration or the size of the market for our products or compete effectively, and the number of procedures in which our products are used may not increase above existing levels. Decreases in market sizes or our market share and declines in average selling prices or procedural volumes could materially adversely affect our results of operations or financial condition. Furthermore, our failure to expand our markets beyond existing levels could impact our ability to grow in line with or above current industry standards. Moreover, our ability to respond to competitive pressures will depend on our ability to decrease our costs, maintain gross margins and operating results, achieve manufacturing efficiencies and maintain manufacturing capacity.
Our research and development efforts may not succeed in bringing new products to market or may fail to do so in a cost-efficient manner or in a manner sufficient to grow our business, replace lost sales or take advantage of new technologies.
Our ability to continue to maintain and grow our business, to replace sales lost due to competition and to bring to market products that take advantage of new and potentially disruptive technologies depends heavily on the success of our research and development activities. Our success relies on our ability to identify and successfully develop cost-effective new products that address unmet medical and consumer needs. To accomplish this, we commit substantial financial, human and capital resources to product research and development, both through our internal dedicated resources and through BD&L transactions. Developing and marketing new products involves a costly, lengthy and uncertain process. Even when our new product development projects make it to market, there have been, and in future may be, instances where projects are subsequently discontinued for technical, clinical, regulatory or commercial reasons. In spite of our investments, our research and development activities and external investments may not produce commercially successful new products that will enable us to replace sales lost to our competitors or increase revenue to grow our business. We may not be able to successfully identify and obtain value from our external business development and strategic collaborative efforts. In addition, our new products may cannibalize a portion of the revenues we derive from existing products, therefore driving replacement revenue instead of incremental revenue.
Further, even if we are able to secure regulatory approval and achieve initial commercial success of our products, our products may abruptly cease to be commercially viable due to the discovery of adverse health effects. See "-We may implement product recalls or voluntary market withdrawals of our products."
12
If we are unable to maintain a cost-effective flow of successful new products sufficient to maintain and grow our business, cover any sales erosion due to competition and take advantage of market opportunities, this lack of innovation could have a material adverse effect on our business, financial condition or results of operations. For a description of the government approval processes which must be followed to market our products, see "-Regulatory clearance and approval processes for our products are expensive, time-consuming and uncertain, and the failure to obtain and maintain required regulatory clearances and approvals could prevent us from commercializing our products" and "Item 4. Information on the Company-4.B. Business Overview-Government Regulation".
If we fail to comply with applicable anti-corruption and anti-bribery laws, export control laws, trade sanction laws, or other global trade laws, we could be subject to penalties and civil and/or criminal sanctions and our business could be materially adversely affected.
We have extensive international operations and sell our product in more than 140 countries, including in countries that are perceived to have heightened levels of public sector corruption. Operating in such jurisdictions subjects us to increased scrutiny and heightens the risk of violating worldwide anti-bribery laws, including those that prohibit companies and their intermediaries from making improper payments to government officials or other third parties for the purpose of obtaining or retaining business, such as the Foreign Corrupt Practices Act of 1977, as amended ("FCPA"), and laws that prohibit commercial bribery. Scrutiny over such improper payments can suddenly increase. For example, in 2023, China launched a comprehensive anti-corruption campaign focused on the healthcare industry, including hospitals and pharmaceutical companies. Our internal control policies and procedures may not always protect us from reckless or criminal acts committed by our associates or agents.
In addition, we are required to comply with various global trade laws that apply to our worldwide operations, including import laws and export control and economic sanctions laws, which may affect our transactions with certain customers. In certain circumstances, export control and economic sanctions regulations may prohibit the export of certain products or services. In other circumstances, we may be required to obtain an export license before exporting the item.
Compliance with the various import laws that apply to our businesses can restrict our access to, and increase the cost of obtaining, certain products and at times can interrupt our supply of imported inventory. Any noncompliance by us with applicable laws and regulations or the failure to maintain, renew or obtain necessary permits and licenses could result in criminal, civil and administrative penalties and could have an adverse effect on our results of operations. For example, as a result of Russia's invasion of Ukraine, the US, Swiss, EU and UK governments, among others, have developed coordinated sanctions and export control measure packages including: comprehensive financial sanctions against major Russian banks (including SWIFT cut off); additional designations of Russian individuals with significant business interests, involvement in Russian military activities, or government connections; and enhanced export controls and trade sanctions targeting Russia's imports of a wide range of goods as a whole. While not material to our overall sales, we have continued to ensure that patients and eye care professionals in Russia and Belarus have sustained, equal access to our eye care products and services. Our business must be conducted in compliance with applicable economic and trade sanction laws and regulations, many of which are changed or strengthened frequently often without much notice. Any violation of the applicable global trade laws could result in government investigations, adverse media coverage and criminal or civil sanctions, which could disrupt our business and adversely affect our reputation and business, results of operations, cash flows and financial condition.
Changes in third-party payor coverage and reimbursement methodologies and potential regulatory price controls may adversely impact our ability to sell our products at prices necessary to support our current business strategy.
The prices, sales and demand for some of our products, in particular our surgical and pharmaceutical products, could be adversely affected by the increased emphasis managed care organizations and governments continue to place on reducing health care costs. In addition, some third-party payors will not provide reimbursement for a new product until we demonstrate the innovative value or improved patient outcomes of the new product, which could impact our ability to grow the market for sales of the product. For our pharmaceutical products, we must compete to be placed on formularies of managed care organizations. Exclusion of a product from a formulary can lead to reduced usage in the managed care organization. There have also been recent initiatives by third-party payors to challenge the prices charged for medical products. Physicians, eye care professionals and other healthcare providers may be reluctant to purchase our products if they do not receive adequate reimbursement from third-party payors to cover the cost of those products and for procedures performed using those products. This risk can be heightened in times of higher inflation if reimbursement rates do not keep pace with increasing costs. Reductions in the prices for our products in response to these trends could reduce our profit margins, which would adversely affect our ability to invest and grow our business.
Governmental programs that typically reimburse at predetermined fixed rates may also decrease or otherwise limit amounts available through reimbursement. For example, in the EU, member states impose controls on whether products are reimbursable by national or regional health service providers and on the prices at which products are reimbursed under state-run healthcare schemes. Some member states operate reference pricing systems in which they set national reimbursement prices by reference to those in other member states. Countries implementing a volume-based
13
procurement process, such as the one initiated in China in 2018, can lead to decreased prices. The US recently passed the Inflation Reduction Act, which makes significant changes to how drugs are covered and paid for under the Medicare program, including the creation of financial penalties for drugs whose prices rise faster than the rate of inflation, redesign of the Medicare Part D program to require manufacturers to bear more of the liability for certain drug benefits and the introduction of government price-setting for certain Medicare Part D drugs starting in 2026. Other governmental funding restrictions, legislative proposals and interpretations of policy may negatively impact amounts available through reimbursement, including by restricting payment increases to hospitals and other providers through reimbursement systems, or by restricting whether reimbursement is available for our products at all.
We expect that additional health care reform measures will be adopted in the future in the countries in which we operate, including those initiatives affecting coverage and reimbursement for our products, any of which could limit the amounts that governments will pay for health care products and services, which could adversely affect the growth of the market for our products or the demand for our products, or result in additional pricing pressures. We cannot predict the effect such reforms or the prospect of their enactment may have on our business.
If we fail to properly educate and train healthcare providers on our products, then customers may not buy our products.
We market our surgical and certain of our vision care products including pharmaceutical products to healthcare providers, including ECPs, public and private hospitals, ambulatory surgical centers, eye clinics and ophthalmic surgeons' offices and group purchasing organizations and our other vision care products to retailers and distributors. We have developed, and strive to maintain, strong relationships with members of each of these groups who assist in product research and development and advise us on how to satisfy the full range of consumer and surgeon needs. We rely on these groups to recommend our products to their patients and to other members of their organizations.
Contact lens and lens care consumers have a tendency not to switch products regularly and are repeat consumers. As a result, the success of these products relies on an ECP’s initial recommendation of our products, which may be based on our ability to educate the ECP on our products. Even if we are successful at educating ECPs on our products, ECPs may continue to lose influence in the consumer's selection of contact lenses, which would cause our business to become more dependent upon the success of educating consumers directly. If we had to increase our direct-to-consumer marketing, we could potentially face challenges in maintaining our good relationships with ECPs, who may view our direct-to-consumer marketing as a threat to their business.
In our surgical business and with our pharmaceutical products, ECPs, including ophthalmic surgeons, play a significant role in determining the course of treatment and, ultimately, the type of products that will be used to treat a patient for cataracts, vitreoretinal conditions, refractive errors, glaucoma and dry eye, among other things. As a result, it is important for us to properly and effectively market our products to ECPs. Acceptance of our products also depends on our ability to train ECPs and their clinical staff on the safe and appropriate use of our products, which takes time. This training process may take longer than expected and may therefore affect our ability to increase sales. Following completion of training, we rely on the trained ECPs to advocate the benefits of our products in the broader marketplace. Convincing ECPs to dedicate the time and energy necessary for adequate training is challenging, and we may not be successful in these efforts. If we are not successful in convincing ECPs of the merits of our products or educating them on the use of our products, they may not use our products and we will be unable to fully commercialize or profit from such products.
Even if we protect our intellectual property to the fullest extent permitted by applicable law, our competitors and other third parties could develop and commercialize products similar or identical to ours, which could impair our ability to compete.
We rely on a combination of patents, trademarks, designs and copyrights to protect our intellectual property. The scope, strength and duration of those intellectual property rights can vary significantly from product to product and country to country. We also rely on a variety of trade secrets, know-how and other confidential information to supplement these protections. In the aggregate, these intellectual property rights are of material importance to our business.
The protections afforded by these intellectual property rights may limit the ability of competitors to commercialize products covered by the applicable intellectual property rights, but they do not prevent competitors from marketing alternative products that compete with our products. In addition, these intellectual property rights may be challenged by third parties and regulatory agencies, and intellectual property treated as trade secrets and protected through confidentiality agreements may be independently developed by third parties and/or subject to misappropriation by others. Furthermore, in certain countries, particularly in emerging markets, due to ambiguities in the law and enforcement difficulties, intellectual property rights may not be as effective as in Western Europe or the US.
For our pharmaceutical products, we face challenges from third parties seeking to manufacture and market generic versions of our pharmaceutical products prior to the expiration of the applicable patents covering those products. In the US, manufacturers of generic versions of pharmaceutical products may challenge the validity, or claim non-infringement, of our pharmaceutical products through the Abbreviated New Drug Application, or ANDA, process with the FDA and
14
related ANDA litigation. Loss of patent protection for one of our pharmaceutical products would generally lead to a significant and rapid loss of sales for that product as lower priced generic versions of that drug become available.
Therefore, even if we protect our intellectual property to the fullest extent permitted by applicable law, competitors and other third parties may nonetheless develop and commercialize products similar or identical to ours, which could impair our ability to compete and have an adverse effect on our business, financial condition and results of operations.
We are a multinational business that operates in numerous tax jurisdictions.
We conduct operations in multiple tax jurisdictions, and the tax laws of those jurisdictions generally require that the transfer prices between affiliated companies in different jurisdictions be the same as those between unrelated companies dealing at arm's length and that such prices are supported by contemporaneous documentation. While we believe that we operate in compliance with applicable transfer pricing laws and intend to continue to do so, our transfer pricing procedures are not binding on applicable tax authorities. If tax authorities in any of these jurisdictions were to successfully challenge our transfer prices as not reflecting arm's length transactions, they could require us to adjust our transfer prices and thereby reallocate our income to reflect these revised transfer prices, which could result in a higher overall tax liability to us and possibly interest and penalties.
Additionally, the integrated nature of our worldwide operations can produce conflicting claims from tax authorities in different countries as to the profits to be taxed in the individual countries. The majority of the jurisdictions in which we operate have double tax treaties with other foreign jurisdictions, which provide a framework for mitigating the impact of double taxation on our revenues and capital gains. However, mechanisms developed to resolve such conflicting claims are largely untested, can be expected to be very lengthy and do not always contain a mandatory dispute resolution clause.
In recent years, tax authorities around the world have increased their scrutiny of company tax filings and have become more rigid in exercising any discretion they may have. As part of this, the Organization for Economic Co-operation and Development ("OECD") has proposed certain changes to the International tax standards that have resulted and will continue to result in local tax law changes under its Base Erosion and Profit Shifting ("BEPS") Action Plans to address issues of transparency, coherence and substance. Most recently, the OECD released its plans, including model rules and guidelines, for proposing further amendments to the international tax standards, including the standard referred to as Pillar One, which contains a new attribution of the right to tax corporate profits where customers are located and the standard referred to as Pillar Two ("Pillar Two"), which consists of a mechanism ensuring that all corporate profits would be subject to a 15% minimum taxation level in each country in which they operate. Currently, countries are drafting or have enacted legislation to implement Pillar Two rules with some effective dates as early as January 1, 2024. We are continuing to follow Pillar Two legislative developments to evaluate the full future impact it could have on our consolidated results of operations, financial position and cash flows; however, we do expect Pillar Two to lead to an increase of our tax expense and effective tax rate. Moreover, recommendations by the OECD could require companies to disclose more information to tax authorities on operations around the world, which could lead to greater audit scrutiny. On August 16, 2022, the Inflation Reduction Act was enacted in the US, which introduced, among other items, a new minimum corporate income tax on certain large corporations and increased funding for the Internal Revenue Service.
In general, tax reform efforts, including with respect to tax base or rate, transfer pricing, intercompany dividends, cross border transactions, controlled corporations and limitations on tax relief allowed on the interest on intercompany debt, will require us to continually assess our organizational structure and could lead to an increased risk of international tax disputes, an increase in our effective tax rate and an adverse effect on our financial condition.
Financial markets, including inflation and volatile exchange rates, are unpredictable, which could lead to unexpected impacts to our earnings, the return on our financial investments and the value of some of our assets.
Financial markets may adversely affect our earnings, the return on our financial investments and the value of some of our assets. For example, inflation rates in the US and EU were at multi-decade highs in 2022 and remained elevated in 2023, which have caused the cost to manufacture our products to increase. Specifically, since 2022, we have experienced inflationary pressure on the costs of labor, electronic components, resins and freight. Our business results depend, in part, on our continued ability to manage these inflationary pressures through pricing actions and productivity initiatives, while maintaining and improving margins and market share. Increasing prices to match the levels of inflation we are currently experiencing may cause some of our customers, particularly in the elective surgical and contact lens businesses where patients typically do not receive reimbursement from their medical insurance providers or government programs, to decrease their purchases or opt for a lower cost alternative. Failure to manage these inflationary pressures could adversely impact our results of operations or cash flows. In addition, higher inflation has resulted in, and may continue to result in, rising interest rates. Our business could be adversely impacted by increases in the cost of borrowing from rising interest rates. Rising interest rates increase the borrowing costs on new debt, including debt we may refinance.
Changes in exchange rates between the US dollar, our reporting currency, and other currencies can also result in significant increases or decreases in our reported sales, costs and earnings as expressed in US dollars, and in the reported
15
value of our assets, liabilities and cash flows. As we have experienced since 2022, if the US dollar strengthens relative to the currencies of the foreign countries in which we operate, our consolidated financial position and results of operations may be negatively impacted as amounts in foreign currencies will generally translate into fewer US dollars. Despite any measures we may undertake in the future to reduce, or hedge against, foreign currency exchange risks, because a significant portion of our earnings and expenditures are in currencies other than the US dollar, and the fact that our expenditures in Swiss francs and US dollars are significantly higher than our revenue in Swiss francs and US dollars, respectively, any such exchange rate volatility may negatively and materially impact our business, results of operations and financial condition, and may impact the reported value of our net sales, earnings, assets and liabilities. Additionally, some of our customers are required to pay us in US dollars. When the US dollar is particularly strong, our customer's debts to us are more difficult to repay, particularly if the customer is unable to obtain US dollars. For more information on the effects of currency fluctuations on our Consolidated Financial Statements and on how we manage currency risk, see "Item 5. Operating and Financial Review and Prospects-5.A. Operating Results-Effects of Currency Fluctuations" and "Item 11. Quantitative and Qualitative Disclosures About Market Risk".
Countries facing financial difficulties, including countries experiencing high inflation rates and highly indebted countries facing large capital outflows, may impose controls on the exchange of foreign currency. Such exchange controls could limit our ability to distribute retained earnings from our local affiliates or to pay intercompany payables due from those countries.
We are subject to laws targeting fraud and abuse in the healthcare industry.
We are subject to various global laws pertaining to healthcare fraud and abuse, including state and federal anti-kickback laws and physician self-referral laws. For example, the US federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering, arranging for or recommending the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs, and in some cases, private insurance. These US laws have been interpreted to apply to arrangements between manufacturers, on the one hand, and prescribers, purchasers, formulary managers and other healthcare-related professionals, on the other hand. US law provides that a claim for federal healthcare program reimbursement for items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Pricing and rebate programs for covered outpatient drugs reimbursed under federal healthcare programs must comply with the Medicaid drug rebate requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, the Veterans Health Care Act of 1992, as amended, and the Deficit Reduction Act of 2005, as amended. The statutes and regulations governing the various price reporting requirements are complex and have changed over time, and the US government has not given clear guidance on many issues. In addition, recent statutory and regulatory developments have not yet been applied by the government or courts to specific factual situations. We believe that we are in compliance with all applicable government price reporting requirements, but there is the potential that the Centers for Medicare & Medicaid Services ("CMS"), other regulatory and law enforcement agencies or a court could arrive at different interpretations, with adverse financial or other consequences for us. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws. Some European Union bodies and most European Union member states and Japan impose controls and restrictions that are similar in nature or effect to those described above.
In recent years, the US government and several US states have enacted legislation requiring medical device companies to establish marketing compliance programs and file other periodic reports. Similar legislation is being considered in other US states. Many of these requirements are new and uncertain, and available guidance is limited. We could face enforcement action, fines and other penalties and could receive adverse publicity, all of which could harm our business, if it is alleged that we have failed to fully comply with such laws and regulations. Similarly, if the physicians or other providers or entities that we do business with are found to have not complied with applicable laws, they may be subject to sanctions, which could also have a negative impact on our business.
Depending on the circumstances, failure to meet these applicable legal and regulatory requirements can result in civil litigation, criminal prosecution, fines or other penalties, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, private "qui tam" actions brought by individual whistleblowers in the name of the government, or refusal to allow us to enter into supply contracts, including government contracts, any of which could have a material adverse effect on our business, financial condition or results of operations.
16
Regulatory clearance and approval processes for our products are expensive, time-consuming and uncertain, and the failure to obtain and maintain required regulatory clearances and approvals could prevent us from commercializing our products.
Our businesses are subject to varying degrees of governmental regulation in the countries in which we operate, and the general trend is toward increasingly stringent regulation. The exercise of broad regulatory powers by the FDA continues to result in increases in the amounts of testing and documentation required for the commercialization of regulated products and a corresponding increase in the expense of product introduction. Similar trends are also evident in the EU and in other markets throughout the world. Compliance with these laws and regulations is costly and materially affects our business. Among other effects, health care regulations substantially increase the time, difficulty and costs incurred in obtaining and maintaining approval to market newly developed and existing products.
Most of our products are regulated as medical devices or pharmaceuticals and face difficult development and approval processes in most jurisdictions we operate in, particularly in the US and EU; however other products may be regulated as other categories such as lasers, dietary supplements and medical foods. We discuss these regulations more thoroughly "Item 4. Information on the Company-4.B. Business Overview-Government Regulation-Product Approval and Monitoring".
The process of developing new products and obtaining necessary FDA clearance or approval, CE marking, or other regulatory marketing authorization is lengthy, expensive and uncertain. Our potential products could take a significantly longer time than we expect to gain marketing authorization or may never gain such marketing authorization. Regulatory authorities may require additional testing or clinical data to support marketing authorization, delaying authorization and market entry of our products. Even if the FDA or another regulatory agency or notified body approves a product, the approval may limit the indicated uses for a product, may otherwise limit our ability to promote, sell and distribute a product or may require post-marketing studies or impose other post-marketing obligations.
We may be unable to successfully maintain the registrations, licenses, clearances or other authorizations we have received or may receive in the future. We also routinely make minor modifications to our products, labeling, instructions for use, manufacturing process and packaging that may trigger a requirement to notify regulatory authorities or to update such registrations or authorizations. This may subsequently require us to manage multiple versions of individual products around the world, depending on the status of any re-registration approvals. Managing such multiple versions may require additional inventory in the form of "bridging stock", extensive redress operations and inventory increases that could exceed our manufacturing capacity or supply chain ability at the time. This could result in prolonged product shortages that could negatively impact our sales, both in terms of any unavailable products and the potential loss of customers that opt for another supplier.
Legislative and regulatory reforms may impact our ability to develop and commercialize our products.
The global regulatory environment is increasingly stringent and unpredictable. Unexpected changes can have an adverse impact on our business, financial condition and results of operations.
First, it has been, and will continue to be, costly and onerous to comply with changes and new requirements relating to the regulatory approval process or postmarket requirements applicable to our products in various jurisdictions. As discussed in "Item 4. Information on the Company-4.B. Business Overview-Government Regulation-Product Approval and Monitoring" the EU has made recent changes to its regulatory regime (the "EU MDR"), which imposes stricter requirements for the marketing and sale of medical devices. As of May 2021, all new medical devices marketed in the EU require certification according to these new requirements. Devices certified pursuant to the Medical Device Directives before May 2020 with valid CE certificates have been given a timeline to meet the new requirements and can be placed on the market until May 2027 or 2028 (depending on the product classification), provided that the manufacturer of the legacy product has submitted an application for a conformity assessment by May 2024. In addition, several countries that did not have regulatory requirements for medical devices have established such requirements in recent years, and other countries have expanded, or plan to expand, their existing regulations. While certain countries may harmonize their regulations in the future, requirements continue to differ significantly among countries. Further, the FDA is also pursuing various efforts to modernize its regulation of devices, including potential changes to the 510(k) pathway and establishing an alternative pathway that permits reliance on objective performance criteria. We expect this global regulatory environment to continue to evolve, which could impact the cost of, the time needed to approve and, ultimately, our ability to maintain existing approvals or obtain future approvals for, our products. Due to the number of medical devices we market, it is possible not all products will be certified by the current EU MDR deadline, and some products may be rationalized if considered too costly to certify.
Second, new legislation and new regulations and interpretations of existing health care statutes and regulations are frequently adopted, any of which could affect our future business and results of operations. For example, in the US, there have been a number of health care reform legislative and regulatory measures proposed and adopted at the federal and state government levels that affect the health care system generally and that have had significant impact on our business.
17
Third, if certain countries, including the US, change their regulations to no longer require a prescription for the purchase of contact lenses then there would be a significant impact on the way we market and distribute contact lenses because it would limit the role of the ECP as an intermediary. Such changes could require us to incur significant costs to update our marketing and distribution methodologies and could adversely affect the sales of our vision care products.
Finally, within our surgical business, a considerable portion of our sales and sales growth rely on patient-pay premium technologies, in markets where access to these technologies has been established. For example, in the US, two landmark rulings issued by the CMS established a bifurcated payment system for certain of our ATIOLs pursuant to which part of the cost of the cataract surgery with such ATIOLs would be reimbursed under Medicare, with the remaining cost paid out-of-pocket. For more details, see "Item 4. Information on the Company-4.B. Business Overview-Our Products-Surgical". To the extent regulatory bodies in the US, such as CMS, or other health authorities outside the US, decide to amend the regulations governing patient-pay reimbursement for advanced technologies, our sales and sales growth could be negatively impacted.
We may implement product recalls or voluntary market withdrawals of our products.
The manufacturing and marketing of our products, including surgical equipment and instruments and pharmaceuticals, involve an inherent risk that our products may prove to be defective and cause a health risk. We are also subject to laws and regulations requiring us to report adverse events associated with our products. Such adverse events and potential health risks identified in our monitoring efforts or from ongoing clinical studies may lead to voluntary or mandatory market actions, including recalls, product withdrawals or changes to the instructions for using our products.
Governmental authorities throughout the world, including the FDA, have the authority to require the recall of certain of our commercialized products in the event of material deficiencies or defects in, for example, design, labeling or manufacture.
We may also voluntarily initiate certain field actions, such as a correction or removal of our products in the future as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. If a correction or removal of one of our products is initiated to reduce a health risk posed by the product, or to remedy a violation of the Federal Food, Drug, and Cosmetic Act ("FDCA") caused by the product that may present a risk to health, the correction or removal must be reported to the FDA. Similarly, field actions conducted for safety reasons in the European Economic Area must be reported to the regulatory authority in each country where the field action occurs.
A recall of one of our products or a similar competing product manufactured by another manufacturer could impair sales and subsequent regulatory approvals of other similar products we market and lead to a general loss of customer confidence in our products. A product recall could also lead to a health authority inspection or other regulatory action or to us being named as a defendant in lawsuits.
The manufacture of our products is highly regulated and complex.
The manufacture of our product portfolio is complex and heavily regulated by governmental health authorities around the world, including the FDA. Whether our products are manufactured at our own dedicated manufacturing facilities or by third parties, we must ensure that all manufacturing processes comply with current Good Manufacturing Practices, quality system requirements and other applicable regulations, such as national and local environmental, health, and safety laws, as well as with our own high quality standards. In recent years, health authorities have substantially intensified their scrutiny of manufacturers' compliance with such requirements.
Any significant failure by us or our third-party suppliers to comply with these requirements or the health authorities' expectations may cause us to shut down our production facilities or production lines or we could be prevented from importing our products from one country to another. Moreover, if we fail to properly plan for manufacturing capacity, the complexity of our manufacturing process could lead to a long lead time to increase capacity. Any of these events could lead to product shortages, or to our being entirely unable to supply products to customers and consumers for an extended period of time. Such shortages or shutdowns have led to, and could continue to lead to, significant losses of sales revenue and to potential third-party litigation. In addition, health authorities have in some cases imposed significant penalties for such failures to comply with regulatory requirements. A failure to comply fully with regulatory requirements could also lead to a delay in the approval of new products to be manufactured at the impacted site.
If we fail to comply with applicable environmental, health and safety laws and regulations, we may face significant administrative, civil or criminal fines, penalties or other sanctions. In addition, we may incur substantial costs to comply with current or future environmental, health and safety laws and regulations, which have tended to become more stringent over time, including any potential laws and regulations that may be implemented in the future to address global climate change concerns. Compliance with current or future environmental, health and safety laws and regulations may increase our costs or impair our research, development or production efforts.
18
We may be subject to penalties if we fail to comply with post-approval legal and regulatory requirements and our products could be subject to restrictions or withdrawal from the market.
The research, development, testing, manufacturing, sale and marketing of our products are subject to extensive governmental regulation. Government regulation includes inspection of and controls over testing, manufacturing, safety and environmental controls, efficacy, labeling, advertising, marketing, promotion, record keeping, tracking, reporting, distributing, import, export, samples, electronic records and electronic signatures.
Among other requirements, we are required to comply with applicable adverse event and malfunction reporting requirements for our products. For example, for our medical device products, in the US, we are required to report to the FDA any incident in which one of our marketed devices may have caused or contributed to a death or serious injury or has malfunctioned and the malfunction of the device or a similar device that we market would be likely to cause or contribute to death or serious injury if the malfunction were to recur. In addition, all manufacturers placing medical devices on the market in the European Economic Area are legally required to report any serious or potentially serious incidents involving devices produced or sold by the manufacturer to the relevant authority in those jurisdictions where any such incident occurred.
Our advertising and promotional activities are also subject to stringent regulatory rules and oversight. The marketing approvals from the FDA and other regulators of certain of our products are, or are expected to be, limited to specific uses. We are prohibited from marketing or promoting any uncleared or unapproved use of our product, referred to as "off-label" use. In addition to promoting our products in a manner consistent with our clearances and approvals, we must have adequate substantiation for the claims we make for our products. If any of our claims are determined to be false, misleading or deceptive, we could be subject to enforcement action. As Alcon and our associates increasingly use social media to communicate, and given the speed of dissemination of information online, there is a heightened risk that Alcon or one of our associates sends a message that may be deemed inappropriate or prohibited by a regulatory authority. In addition, unsubstantiated claims also present a risk of consumer class action or consumer protection litigation and competitor challenges. In the past, we have had to change or discontinue promotional materials because of regulatory agency requests, and we are exposed to that possibility in the future.
Failure to comply with statutes and regulations administered by the FDA and other regulatory bodies or failure to adequately respond to any notices of violation or any similar reports could result in, among other things, any of the following enforcement actions:
•warning letters or untitled letters issued by the FDA;
•fines, civil penalties, in rem forfeiture proceedings, injunctions, consent decrees and criminal prosecution;
•detention of imported products;
•delays in approving, or refusal to approve, our products;
•withdrawal or suspension of approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies;
•product recall or seizure;
•operating restrictions or interruption of production; and
•inability to export to certain countries.
If any of these items were to occur, it could result in unanticipated expenditures to address or defend such actions, could harm our reputation and could adversely affect our business, financial condition and results of operations.
Goodwill and other intangible assets on our books may lead to significant noncash impairment charges.
We carry a significant amount of goodwill and other intangible assets on our Consolidated Balance Sheet, primarily due to the value of the Alcon brand name, but also intangible assets associated with our technologies, acquired research and development, currently marketed products and marketing know-how. As a result, we may incur significant noncash impairment charges if the fair value of the intangible assets and the groupings of cash generating units containing goodwill would be less than their carrying value on our Consolidated Balance Sheet at any point in time. There were no impairment charges in 2023. Impairment charges in 2022 and 2021 amounted $62 million and $225 million, respectively.
For a detailed discussion of how we determine whether an impairment has occurred, what factors could result in an impairment and the impact of impairment charges on our results of operations, see "Note 2. Selected Accounting Policies-Goodwill and intangible assets" to our Consolidated Financial Statements.
19
We may be underestimating our future pension and other post-employment benefit plan obligations.
We sponsor pension and other post-employment benefit plans in various forms. While most of our plans are now defined contribution plans, certain of our associates remain under defined benefit plans. For these defined benefit plans, we are required to make significant assumptions and estimates about future events in calculating the present value of expected future plan expenses and liabilities. These include assumptions used to determine the discount rates we apply to estimated future liabilities and rates of future compensation increases. Assumptions and estimates we use may differ materially from the actual results we experience in the future due to changing market and economic conditions, higher or lower withdrawal rates or longer or shorter life spans of participants, among other variables. For example, at December 31, 2023, a decrease in the interest rate we apply in determining the present value of expected future total defined benefit plan obligations (consisting of pension and other post-employment benefit obligations) of one-quarter of one percent would have increased our year-end defined benefit obligation by $27 million. Any differences between our assumptions and estimates and our actual experience could require us to make additional contributions to our pension funds. Further, additional employer contributions might be required if plan funding falls below the levels required by local rules.
Unauthorized or illegal distribution may harm our business and reputation.
Our products may be subject to competition from lower priced versions of our products intended to be sold in countries where there are government imposed price controls or other market dynamics that make the products lower priced. Despite government regulations aimed at limiting such imports, the volume of imports may continue to rise in certain countries. This importation may adversely affect our profitability in the US and elsewhere and could become more significant in the future. Foreign currency exchange rate fluctuations can exacerbate this risk by causing price differentials and arbitrage opportunities. For example, when the US dollar is particularly strong against most other world currencies, our products sold outside the US may be brought into the US by a reseller and sold to our customers at a lower price than we would have charged, yet the reseller may still make a profit.
In addition, our industry continues to be challenged by the vulnerability of distribution channels to counterfeiting. Reports of increased levels of counterfeiting could materially affect consumer confidence in the authentic product and harm our business or lead to litigation.
Our existing debt may limit our flexibility to operate our business or adversely affect our business and our liquidity position.
We have outstanding debt of $4.7 billion as of December 31, 2023, and we may incur additional indebtedness in the future for various reasons, including fluctuations in operating results, capital expenditures and potential acquisitions.
Our indebtedness may:
•make it difficult for us to satisfy our obligations, including making interest payments on our debt obligations;
•require us to dedicate a portion of our cash flows to payments on our debt, reducing our ability to use our cash flows to fund capital expenditures, BD&L or other strategic transactions, working capital and other general operational requirements, or to pay dividends to our shareholders;
•limit our flexibility to plan for and react to changes in our business;
•negatively impact our credit rating and increase the cost of servicing our debt;
•place us at a competitive disadvantage relative to some of our competitors that have less debt than us;
•increase our vulnerability to general adverse economic and industry conditions, including changes in interest rates or a downturn in our business or the economy; and
•make it difficult to refinance our existing debt or incur new debt on terms that we would consider to be commercially reasonable, if at all.
The occurrence of any one of these events could have a material adverse effect on our business, financial condition or result of operations or cause a significant decrease in our liquidity and impair our ability to pay amounts due on our indebtedness. Further, to lower inflation, governmental and regulatory agencies have been enacting changes to monetary policy and interest rates, which have led to, and can lead to further, increases to borrowing costs.
We may need to obtain additional financing, which may not be available or, if it is available, may not be on favorable terms and may result in dilution of our then-existing shareholders.
We may need to raise additional funds to:
•finance unanticipated working capital requirements or refinance our existing indebtedness;
•develop or enhance our infrastructure and our existing products and services;
20
•engage in mergers and acquisitions or other strategic BD&L transactions;
•fund strategic relationships; and
•respond to competitive pressures.
If we raise additional funds by issuing equity or convertible debt securities, the percentage ownership of our then-existing shareholders may be diluted, and holders of these securities may have rights, preferences or privileges senior to those of our then-existing shareholders. Further, the use of financing to invest in research and development, business acquisitions, and capital expenditures may not generate the expected returns or cash flows. Significant judgment is required to determine which investments will result in optimal returns, and we could make investment that are ultimately less profitable than those investments we do not select.
Litigation and governmental investigations may harm our business or otherwise distract our management.
We, from time to time, are, and may in the future be, subject to various investigations and legal proceedings that arise or may arise involving product liability, sales and marketing practices, commercial disputes, employment, wrongful discharge, antitrust or competition, securities, health and safety, environmental, tax, international trade, privacy, intellectual property, including Hatch-Waxman litigation, and anti-bribery regulations, such as the FCPA, including compliance with ongoing reporting obligations to the government resulting from any settlements. See “Item 8. Financial Information-8.A. Consolidated Statements and Other Financial Information-Legal Proceedings”.
Substantial, complex or extended litigation could cause us to incur large expenditures, affect our ability to market and distribute our products and distract our management. For example, intellectual property litigation in which we are named as a defendant from time to time could result in significant damage awards and injunctions that could prevent the manufacture and sale of the affected products or require us to make significant royalty payments to continue to sell the affected products. In 2022, we entered into a confidential settlement agreement with a competitor where we each exchanged cross-licenses of certain intellectual property and we made a one-time payment of $199 million. In 2020, Hoya Corporation filed suit against us alleging that our UltraSert Pre-Loaded Delivery System infringes their US patents. On January 11, 2024, the court granted our motion for summary judgment of non-infringement with respect to three of the six asserted patents and certain claims of the other three asserted patents, and also granted our motion for summary judgment with respect to Hoya’s claim that Alcon’s alleged infringement was willful. This matter was fully and finally resolved prior to the trial scheduled to begin on February 20, 2024.
Lawsuits by associates, shareholders, customers or competitors, or potential indemnification obligations and limitations of our director and officer liability insurance, could be very costly and substantially disrupt our business. Disputes with such companies or individuals from time to time are not uncommon, and we may be unable to resolve such disputes on terms favorable to us.
Even meritless claims could subject us to adverse publicity, hinder us from securing insurance coverage in the future or require us to incur significant legal costs. As a result, significant claims or legal proceedings to which we are a party could have a material adverse effect on our business, prospects, financial condition and results of operations.
Failure to comply with law, legal proceedings and government investigations may have a significant negative effect on our results of operations.
We are obligated to comply with the laws of all of the countries around the world in which we operate and sell products. These laws cover an extremely wide and growing range of activities. Such legal requirements can vary from country to country and new requirements may be imposed on us from time to time as government and public expectations regarding acceptable corporate behavior change, and enforcement authorities modify interpretations of legal and regulatory provisions and change enforcement priorities. In addition, our associates, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, in violation of such laws and public expectations.
For example, we are faced with increasing pressures, including new laws and regulations from around the world, to be more transparent with respect to how we do business, including with respect to our interactions with healthcare professionals and organizations. These laws and regulations include requirements that we disclose payments or other transfers of value made to healthcare professionals and organizations, including by our associates or third parties acting on our behalf, as well as with regard to the prices for our products. We are also subject to certain privacy laws, including Swiss privacy laws, the EU's General Data Protection Regulation and the California Consumer Privacy Act, which include significant penalties for non-compliance.
In addition, we have significant activities in a number of developing countries around the world, both through our own associates and through third parties retained to assist us. In some of these countries, a culture of compliance with law may not be as fully developed as in other countries.
21
To help us in our efforts to comply with the many requirements that impact us, we have a significant global ethics and compliance program in place, and we devote substantial time and resources to efforts to conduct our business in a lawful and publicly acceptable manner. Nonetheless, our ethics and compliance program may be insufficient or associates may fail to comply with the training they received, and any actual or alleged failure to comply with law or with heightened public expectations could lead to substantial liabilities that may not be covered by insurance, or to other significant losses, and could affect our business, financial position and reputation.
In particular, in recent years, there has been a trend of increasing government investigations, legal proceedings and law enforcement activities against companies and executives operating in our industry. Increasingly, such activities can involve criminal proceedings and can retroactively challenge practices previously considered to be acceptable.
For additional information, see “Item 8. Financial Information-8.A. Consolidated Statements and Other Financial Information-Legal Proceedings."
Such proceedings are inherently unpredictable, and large judgments or penalties sometimes occur. As a consequence, we may in the future incur judgments or penalties that could involve large cash payments, including the potential repayment of amounts allegedly obtained improperly and other penalties, including enhanced damages. In addition, such proceedings may affect our reputation, create a risk of potential exclusion from government reimbursement programs and may lead to civil litigation. As a result, having taken into account all relevant factors, we may in the future enter into major settlements of such claims without bringing them to final legal adjudication by courts or other such bodies, despite having potentially significant defenses against them, in order to limit the risks they pose to our business and reputation. Such settlements may require us to pay significant sums of money and to enter into corporate integrity or similar agreements intended to regulate company behavior for a period of years, which can be costly to operate under.
Any such judgments or settlements, and any accruals that we may take with respect to potential judgments or settlements, could have a material adverse impact on our business, financial condition or results of operations, as well as on our reputation.
Continued energy supply constraints and increases in the cost of energy, including as a result of the ongoing conflict in Ukraine, could adversely impact our results of operations.
We use natural gas and electricity to operate our manufacturing plants, and these operations can be directly affected by volatility in the cost and availability of energy, which is often subject to factors outside of our control. Global conflicts such as the ongoing conflicts between Russia and Ukraine and conflicts in the Middle East can impact global energy markets leading to high volatility and increased prices for natural gas and electricity. Energy shortages could lead to additional price increases, energy supply rationing, or temporary reduction in operations or closure of our manufacturing plants leading to an inability to meet demand and harm to our reputation with healthcare providers and patients, all of which could have a material adverse impact on our business or results of operations.
We may be unable to attract and retain qualified personnel.
We are highly dependent upon skilled personnel in key parts of our organization, and we invest heavily in recruiting, training and retaining qualified individuals. The loss of the service of key members of our organization—including senior members of our scientific and management teams, high-quality researchers and development specialists and skilled personnel in developing countries—could delay or prevent the achievement of major business objectives.
In addition, our ability to hire qualified personnel also depends on the flexibility to reward superior performance and to pay competitive compensation. Laws, regulations and customary practice on executive compensation, including legislation and customary practice in our home country, Switzerland, may restrict our ability to attract, motivate and retain the required level of qualified personnel. For example, pay benchmarks for Swiss and other European companies may be inconsistent with the current market in the US, making it more difficult to recruit talent in the US, which has a large concentration of medical device talent. Further, certain associates are required to travel frequently between Switzerland and the US. These associates may be unwilling or unable to make such a commitment. Finally, changes to immigration policies in the numerous countries in which we operate, including the US, as well as restrictions on global travel as a result of local or global public health crises requiring quarantines or other precautions to limit exposure to infectious diseases, may limit our ability to hire or retain talent in, or transfer talent to, specific locations.
Finally, our business, particularly the manufacturing of our products, requires a substantial number of personnel. Any failure to retain stable and dedicated labor by us may lead to disruption to our business operations, including the manufacturing of our products. Due to the tight labor market, we have experienced, and expect to continue to experience, increases in labor costs to remain competitive in retaining talent. If we are unable to manage and control our labor costs, our business, financial condition and results of operations may be materially and adversely affected.
22
Risks related to the Ownership of our Shares
Your percentage ownership in Alcon may be diluted in the future.
In the future, your percentage ownership in Alcon may be diluted because of equity issuances from acquisitions, capital markets transactions or otherwise, including equity awards that we may grant to our directors, officers and associates under our associate participation plans. These additional issuances will have a dilutive effect on our earnings per share, which could adversely affect the market price of our shares.
Our maintenance of two exchange listings could result in pricing differentials of our ordinary shares between the two exchanges.
Our shares trade on the NYSE in US dollars and on the SIX in Swiss francs, which may result in price differentials between the two exchanges for a variety of factors, including fluctuations in the US dollar/Swiss franc exchange rate and differences in trading schedules.
We may not pay or declare dividends.
Although Alcon expects that it will continue to recommend the payment of a regular cash dividend based upon the prior year’s core net income, we may not pay or declare dividends in the future. The declaration, timing and amount of any dividends to be paid by Alcon will be subject to the approval of shareholders at the relevant General Meeting of shareholders. The determination by the Board as to whether to recommend a dividend and the approval of any such proposed dividend by the shareholders will depend upon many factors, including our financial condition, earnings, corporate strategy, capital requirements of our operating subsidiaries, covenants, legal requirements and other factors deemed relevant by the Board and shareholders.
In addition, any dividends that we may declare will be denominated in Swiss francs. Consequently, exchange rate fluctuations will affect the US dollar equivalent of dividends received by holders of shares held via Depository Trust Company ("DTC") or shares directly registered with Computershare Trust Company, N.A. in the US. If the value of the Swiss franc decreases against the US dollar, the value of the US dollar equivalent of any dividend will decrease accordingly.
See "Item 8. Financial Information-8.A. Consolidated Statements and Other Financial Information-Dividend Policy" for more information.
As a foreign private issuer, we are subject to different US securities laws and rules than a domestic issuer, which may limit the information publicly available to US shareholders.
We report under the Securities Exchange Act of 1934, as amended ("Exchange Act"), as a non-US company with foreign private issuer status. Because we qualify as a foreign private issuer under the Exchange Act and although we are subject to Swiss laws and regulations with regard to such matters and intend to continue to furnish quarterly financial information to the SEC, we are exempt from certain provisions of the Exchange Act that are applicable to US domestic public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act, (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. Foreign private issuers are also exempt from Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. In addition, as a foreign private issuer, we are entitled to rely on exceptions from certain corporate governance requirements of the NYSE. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
Furthermore, we prepare our financial statements under IFRS. There are, and may continue to be, certain significant differences between IFRS and US Generally Accepted Accounting Principles, or US GAAP, including but not limited to potentially significant differences related to the accounting and disclosure requirements relating to defined benefit pension plans and other post-employment benefits, nonfinancial assets, taxation, and recognition and impairment of long-lived assets. As a result, our financial information and reported earnings for historical or future periods could be significantly different if they were prepared in accordance with US GAAP, and you may not be able to meaningfully compare our financial statements under IFRS with those companies that prepare financial statements under US GAAP.
We may lose our foreign private issuer status.
We are a foreign private issuer and therefore we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to US domestic issuers. To maintain our status as a foreign private issuer, either (a) a majority of our shares must be directly or indirectly owned of record by non-residents of the US or (b)(i) a majority of our executive officers or directors may not be United States citizens or residents, (ii) more than 50 percent of our assets cannot be located in the US and (iii) our business must be administered principally outside the US.
23
If we were to lose our foreign private issuer status, we would be required to comply with the Exchange Act reporting and other requirements applicable to US domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. For instance, we would be required to change our basis of accounting from IFRS to US GAAP, which we expect would be difficult and costly and could also result in potentially material changes to historical financial statements previously prepared on the basis of IFRS. We may also be required to make changes in our corporate governance practices in accordance with various SEC and NYSE rules. The regulatory and compliance costs to us under US securities laws could be significantly higher than the costs we will incur as a foreign private issuer. As a result, a loss of foreign private issuer status would increase our legal and financial compliance costs and would make some activities highly time-consuming and costly. If we were required to comply with the rules and regulations applicable to US domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance, and we could be required to accept reduced coverage or incur substantially higher costs to obtain coverage.
Our status as a Swiss corporation means that our shareholders enjoy certain rights that may limit our flexibility to raise capital, issue dividends and otherwise manage ongoing capital needs.
Swiss law reserves for approval by shareholders certain corporate actions over which a board of directors would have authority in some other jurisdictions. For example, shareholders must approve the payment of dividends. Swiss law also requires that our shareholders themselves resolve to, or authorize our Board to, increase, or decrease, our share capital. As permitted under Swiss law, our shareholders authorized our Board in 2023 (i) to increase or decrease our issued share capital from time to time without additional shareholder approval by 10% (capital increase) or by 5% (capital decrease) of the issued share capital at the time of the authorization in May 2023 (capital range) and (ii) to increase our issued share capital from time to time without additional shareholder approval by 10% of the issued share capital at the time of the authorization in May 2023 in connection with financial instruments (conditional share capital). The authorization under the capital range expires in May 2028 and must be renewed by the shareholders from time to time thereafter in order to be available for raising capital or capital reduction. Subject to specific exceptions, including exceptions explicitly described in Alcon’s articles of incorporation, Swiss law grants to existing shareholders (i) subscription rights to subscribe for new issuances of shares and (ii) advance subscription rights to subscribe for new shares to be issued in connection with convertible bonds or similar instruments with conversion or option rights. A resolution adopted at a shareholders' meeting by a qualified majority of two-thirds of the votes represented, and the absolute majority of the nominal value of the shares represented, may restrict or exclude, or allow the Board to restrict or exclude, such subscription or advance subscription rights in certain limited circumstances. In addition to provide more flexibility in the structuring of the share capital, the recent Swiss corporate law reform also permits notably the payment of interim dividends and the denomination of the share capital in foreign currency, both subject to shareholders' approval; however, any change regarding the currency of our share capital would require an amendment to Alcon’s articles of incorporation. Despite these changes, Swiss law still does not provide as much flexibility in the various rights and regulations that can attach to different categories of shares as do the laws of some other jurisdictions. These Swiss law requirements relating to our capital management may limit our flexibility, and situations may arise where greater flexibility would have provided benefits to our shareholders.
It may be difficult to enforce US judgments against us.
We are organized under the laws of Switzerland. As a result, it may not be possible for investors to effect service of process within the US upon us or to enforce judgments against us obtained in US courts, including judgments in actions predicated upon the civil liability provisions of the federal securities laws of the US. We have been advised by our Swiss counsel that there is doubt as to the enforceability in Switzerland of original actions, or in actions for enforcement of judgments of US courts, of civil liabilities to the extent predicated upon the federal and state securities laws of the US. Original actions against persons in Switzerland based solely upon the US federal or state securities laws are governed, among other things, by the principles set forth in the Swiss Federal Act on Private International Law. This statute provides that the application of provisions of non-Swiss law by the courts in Switzerland shall be precluded if the result is incompatible with Swiss public policy. Also, mandatory provisions of Swiss law may be applicable regardless of any other law that would otherwise apply.
Switzerland and the US do not have a treaty providing for reciprocal recognition and enforcement of judgments in civil and commercial matters. The recognition and enforcement of a judgment of the courts of the US in Switzerland are governed by the principles set forth in the Swiss Federal Act on Private International Law. This statute provides in principle that a judgment rendered by a non-Swiss court may be enforced in Switzerland only if:
•the non-Swiss court had jurisdiction pursuant to the Swiss Federal Act on Private International Law;
•the judgment of such non-Swiss court has become final and non-appealable;
•the judgment does not contravene Swiss public policy;
24
•the court procedures and the service of documents leading to the judgment were in accordance with the due process of law; and
•no proceeding involving the same position and the same subject matter was first brought in Switzerland, or adjudicated in Switzerland, or was earlier adjudicated in a third state and this decision is recognizable in Switzerland.
25
ITEM 4. INFORMATION ON THE COMPANY
4.A. HISTORY AND DEVELOPMENT OF THE COMPANY
General Corporate Information
Alcon is a stock corporation (Aktiengesellschaft) organized under the laws of Switzerland in accordance with article 620 et seq. of the Swiss Code of Obligations and registered with the register of commerce of the Canton of Fribourg, Switzerland (“Commercial Register”) under registration number CHE‑234.781.164. Alcon is registered in the Commercial Register under each of Alcon AG, Alcon SA and Alcon Inc., all of which are stated in Alcon's Articles of Incorporation (our "Articles of Incorporation") as our corporate name. Alcon was formed for an unlimited duration, effective as of September 21, 2018, the date of the registration of Alcon in the Commercial Register. On April 9, 2019, Alcon’s shares were listed on the SIX and the NYSE under the ticker symbol “ALC.”
Alcon is domiciled in Fribourg, Switzerland and our registered office is located at Rue Louis‑d’Affry 6, 1701 Fribourg, Switzerland. Our headquarters is located in Geneva, Switzerland at the following address: Chemin de Blandonnet 8, 1214 Vernier, Geneva, Switzerland. Our telephone number is +41 58 911 2110. Our principal website is www.alcon.com. The information contained on our website is not a part of this Form 20‑F.
General Development of Business
Alcon was originally founded in 1945 by pharmacists Robert Alexander and William Conner, who opened a small pharmacy under the “Alcon” name in Fort Worth, Texas. In 1947, Alcon was first incorporated and began manufacturing specialty pharmaceutical products to address ocular health needs. In the succeeding years, Alcon began operating internationally with the opening of an office in Canada and first formed its surgical division.
In 1977, Alcon was acquired by a Swiss subsidiary of Nestlé S.A. and, consequently, Alcon began operating as a wholly owned subsidiary of Nestlé until 2002. On March 20, 2002, Nestlé completed an initial public offering of approximately 25% of Alcon's outstanding common shares upon which Alcon was publicly listed and traded on the NYSE under the symbol “ACL”. In a series of transactions, Nestlé then sold all of its remaining interest in Alcon to Novartis from 2008 to 2010, and Novartis then acquired the remaining publicly held shares of Alcon through a merger of Alcon into Novartis on April 8, 2011, creating the Alcon division within Novartis.
In connection with the Novartis acquisition of Alcon, Novartis merged its then‑existing contact lens and contact lens care unit, CIBA Vision, and certain of its ophthalmic pharmaceutical products into Alcon and moved Alcon's generic ophthalmic pharmaceutical business into the Sandoz division within Novartis. Prior to Alcon's separation from Novartis, Novartis moved certain pharmaceutical products owned by Alcon into Novartis.
On April 9, 2019, Novartis completed the legal and structural separation of Alcon into a stand-alone company through a spin-off transaction, upon which Alcon became a stand-alone, independent company.
Since the spin-off, Alcon has focused on launching innovative new products, investing in manufacturing line expansion and pursuing adjacencies such as pharmaceuticals.
Significant Acquisitions, Dispositions and other Events
Significant Investments
In 2012, we began a multi‑year software implementation project to standardize our processes, enhance data transparency and globally integrate our fragmented and aging information technology systems across our commercial, supply and manufacturing operations worldwide, through a new foundation of Systems, Applications and Products in Data Processing ("SAP"), which is an ERP software platform. We paid a total of approximately $806 million relating to the implementation of the new ERP system, the payment of which was substantially complete by December 31, 2022.
In addition, we have made significant investments in certain of our manufacturing facilities to enhance our production capabilities. For more information, see “Item 4.D. Property, Plants and Equipment—Major Facilities”.
26
Acquisitions
In the past three years, we have entered into certain acquisition transactions, including (i) the acquisition of 100% of the outstanding shares and equity of Aerie Pharmaceuticals, Inc. (“Aerie”) on November 21, 2022, (ii) the acquisition of 100% of the outstanding shares and equity of Ivantis, Inc. ("Ivantis") on January 7, 2022, and (iii) the acquisition of exclusive US commercialization rights to Simbrinza (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% from Novartis on June 8, 2021. For further details on certain of our significant transactions in 2023, 2022 and 2021, see Notes 3 and 21 to the Consolidated Financial Statements.
Debt Issuances
In connection with the spin-off, we borrowed an aggregate of approximately $3.2 billion under various unsecured loan facilities. We then paid to Novartis approximately $3.1 billion to satisfy certain intercompany indebtedness owed by Alcon and its subsidiaries to Novartis and its affiliates.
In September 2019, Alcon Finance Company, an indirect wholly owned subsidiary of the Company (“AFC”), issued unsecured senior notes in the aggregate principal sum of $2.0 billion, all of which were guaranteed by the Company and the proceeds were used to repay part of the unsecured loan facilities.
In May 2020, AFC issued $750 million in unsecured senior notes, which were guaranteed by the Company. See Note 16 to our Consolidated Financial Statements for additional information.
2022 Euro Bond Issuance
On May 31, 2022, Alcon Finance B.V., an indirect, wholly owned subsidiary of the Company ("AFBV"), issued Euro denominated senior notes due in 2028 (the "Series 2028 Notes"), which are guaranteed by the Company. The Series 2028 Notes are unsecured senior obligations of AFBV issued and closed in a public offering. The total principal amount of the Series 2028 Notes is 500 million euros, and the proceeds were used to repay part of the unsecured loan facilities funded in 2019. The Series 2028 Notes were issued at 99.476% with 2.375% interest payable annually in May, beginning in May 2023. For more information on the Series 2028 Notes, see Note 16 to our Consolidated Financial Statements.
2022 Bridge Loan Facility
On September 14, 2022, the Company and AFC entered into a facility agreement with J.P. Morgan Securities PLC as arranger, J.P. Morgan Chase Bank, N.A., London Branch as original lender, bookrunner and underwriter, and J.P. Morgan SE as agent (the "2022 Bridge Loan Facility Agreement"). The 2022 Bridge Loan Facility Agreement provided for a $900 million unsecured term loan facility (the "2022 Bridge Loan Facility") for the purposes of financing or refinancing (i) the consideration payable for the Aerie acquisition, (ii) any existing indebtedness of Aerie and its subsidiaries and (iii) related fees and expenses in connection with the foregoing. On November 21, 2022, in connection with the consummation of the Aerie acquisition, $775 million of the financing commitments of the lenders under the 2022 Bridge Loan Facility were drawn, the proceeds of which were used for the Aerie acquisition. The 2022 Bridge Loan Facility was repaid in full with the proceeds of the 2022 Notes described below and is no longer available to us for borrowings. For more information on the 2022 Bridge Loan Facility, see Note 16 to our Consolidated Financial Statements.
2022 US Bond Issuance
On December 6, 2022, AFC issued senior notes (“2022 Notes”) in the principal amounts of $700 million and $600 million with maturity dates in 2032 and 2052, respectively, which are guaranteed by the Company. The 2022 Notes are unsecured senior obligations of AFC issued in a private placement. The total principal amount of the 2022 Notes is $1.3 billion, and the proceeds were used to repay the 2022 Bridge Loan Facility and the remaining principal of the unsecured loan facilities funded in 2019. The 2022 Notes consist of the following:
•Series 2032 Notes - $0.7 billion due in 2032 issued at 99.458%, 5.375% interest is payable twice per year in June and December, beginning in June 2023.
•Series 2052 Notes - $0.6 billion due in 2052 issued at 99.674%, 5.750% interest is payable twice per year in June and December, beginning June 2023.
For more information on the 2022 Notes, see Note 16 to our Consolidated Financial Statements.
27
Transformation Program
On November 19, 2019, we announced a multi-year transformation program to better align our organizational structure with the scope of Alcon's business operations globally. We created four shared business centers designed to create efficiencies for reinvestment into key growth drivers. The transformation program was originally projected to deliver annual run-rate savings of approximately $200 to $225 million, to be reinvested into key growth drivers, with an original projected cost of the program of $300 million by 2023. On November 15, 2022, we announced additional transformation initiatives to deliver incremental efficiencies. As a result, we projected incremental run-rate savings of approximately $100 million, with incremental program costs of approximately $125 million. As expected, this program was completed by year-end 2023. Through December 31, 2023, the total expense recognized with respect to the transformation program was in line with estimates.
War on Ukraine
Beginning in February 2022, as a result of the war on Ukraine by Russia, economic sanctions and export controls were imposed by much of the world on Russian financial institutions and businesses. These sanctions could adversely impact net sales, create disruptions in the global supply chain, increase the risk of cyberattacks, and potentially have an adverse impact on the global economy, financial markets, energy markets, currency rates and otherwise. As a result of the global impacts, we have experienced volatility in currency translation effects. Our manufacturing and procurement exposure in Russia and Ukraine is limited as our operations consist mainly of associates in local functions, including sales and customer support. Refer to "Item 3. Key Information-3.D. Risk Factors-Changing economic and financial environments in many countries and increasing global political and social instability may adversely impact our business."
For the years ended December 31, 2023 and 2022, net sales in Russia and Ukraine amounted to approximately 2% of consolidated net sales. Total assets in Russia and Ukraine amounted to $82 million as of December 31, 2023. As of December 31, 2023, operations previously impacted by the war on Ukraine continued operating to the extent practicable and permitted by law.
Additional Information
The SEC maintains an internet website at www.sec.gov that contains reports, proxy and information statements and other information regarding companies that file documents electronically with the SEC. Our internet website is www.alcon.com. The information included on our internet website or the information that might be accessed through such website is not included in this Annual Report and is not incorporated into this Annual Report by reference.
28
4.B. BUSINESS OVERVIEW
Overview
Alcon is the global leader in eye care with $9.4 billion in net sales during the year ended December 31, 2023. We research, develop, manufacture, distribute and sell a full suite of eye care products within two key businesses: Surgical and Vision Care. Based on sales for the year ended December 31, 2023, we are the number one company by global market share in the ophthalmic surgical market and in the vision care market. We employ over 25,000 associates operating in 56 countries and serving consumers and patients in over 140 countries. We believe our market leading position and global footprint allow us to benefit from economies of scale, maximize the potential of our commercialized products and pipeline and will permit us to effectively grow the market and expand into new product categories.
Our Surgical business is focused on ophthalmic products for cataract surgery, vitreoretinal surgery, refractive laser surgery and glaucoma surgery. Our broad surgical portfolio includes implantables, consumables and surgical equipment required for these procedures and supports the end-to-end needs of the ophthalmic surgeon. Our Vision Care business comprises daily disposable, reusable and color-enhancing contact lenses and a comprehensive portfolio of ocular health products, including products for dry eye, ocular allergies, glaucoma, and contact lens care, as well as ocular vitamins and redness relievers. Alongside our world-class products, Alcon provides best-in-class service, training, education and technical support for our customers.
Our Surgical and Vision Care businesses are complementary and benefit from synergies in research and development, manufacturing, distribution and consumer awareness and education. This allows us to position ourselves as a trusted partner for eye care products across the continuum of care from retail consumers, to optometry, to surgical ophthalmology. For example, in research and development, we can apply our expertise in material and surface chemistry to develop innovative next-generation products for both our IOL and contact lens product lines. Similarly, our global commercial footprint and expertise as a global organization provide us with product development, manufacturing, distribution and commercial promotion and marketing knowledge that can be applied to both of our businesses.
We are dedicated to providing innovative products that enhance quality of life by helping people See Brilliantly. Our strong foundation is based on our longstanding success as a trusted brand, our legacy of industry firsts and advancements, our leading positions in the markets in which we compete and our continued commitment to substantial investment in innovation. With more than 75 years of history in the ophthalmic industry, we believe the Alcon brand name is synonymous with innovation, quality, service and leadership among eye care professionals worldwide.
Our Markets
Overview
We currently operate in the global ophthalmic surgical and vision care markets, which are large, dynamic and growing. As the world population grows and ages, the need for quality eye care is expanding and evolving, and we estimate that the size of the eye care market in which we operate is approximately $34 billion and is projected to grow mid-single digits per year from 2023 to 2028.
Although it is estimated that 90% of all visual impairments are currently preventable, treatable or curable, we operate in markets that have substantial unmet medical and consumer needs. For example, based on market research, it is estimated that there are currently 65 million people with moderate to severe vision impairment due to cataracts, 1.8 billion who suffer from presbyopia, 153 million with uncorrected refractive errors, 146 million with diabetic retinopathy, 103 million living with glaucoma and approximately 1.4 billion who suffer from symptoms of dry eye, among other unaddressed ocular health conditions. In addition, there are 1 billion people living with some form of unaddressed visual impairment. Below is a brief description of these ocular disorders.
29

Our Surgical and Vision Care products are targeted at addressing many of these unmet medical and consumer needs. We expect the surgical and vision care markets to continue to grow, driven by multiple factors and trends, including:
•Aging population with growing eye care needs: A growing aging population continues to drive the increased prevalence of eye care conditions worldwide, as the number of persons aged 60 years or over is expected to more than double by 2050, rising from 962 million globally in 2017 to 2.1 billion in 2050.
•Innovation improving the quality of eye care: Technology innovation in eye care is driving an increased variety of products that more effectively treat eye conditions. The importance of vision correction and preservation, the high return on healthcare spend and the improved patient outcomes are leading to increased coverage and reimbursement opportunities from governmental and private third-party payors, expanding patient access to such eye care products.
•Increasing wealth and growth from emerging economies: It is estimated that by 2030 the global middle class population could exceed 5 billion people with the majority of growth coming in emerging markets. This major demographic shift is generating a large, new customer base with increased access to eye care products and services along with the resources to pay for them. The expansion of training opportunities for eye care professionals in emerging markets is also leading to increased patient awareness and access to premium eye care products and surgical procedures, facilitating their growth.
•Increasing prevalence of myopia, progressive myopia and digital eye strain: It is estimated that by 2050, half of the world's population (nearly five billion people) will be myopic. Further, the modern work environment, along with leisure preferences, have increased the number of hours people spend in front of a screen, adversely impacting vision and increasing the risk of progressive myopia and digital eye strain.
The Surgical Market
The surgical market in which we operate is estimated to be $13 billion and is projected to grow mid-single digits per year from 2023 to 2028. The surgical market includes sales of implantables, consumables and surgical equipment, including associated technical, clinical and service support and training. Surgical implantables are medical devices designed to remain in the eye, such as monofocal, ATIOLs and stents placed in the eye during cataract surgery. Consumables include hand-held instruments, surgical solutions, equipment cassettes, patient interfaces and other disposable items typically used during a single ophthalmic surgical procedure. Finally, surgical equipment includes multi-use surgical consoles, lasers and diagnostic instruments used across procedures to enable surgeons to visualize and conduct ophthalmic surgeries. Market growth is expected to be driven mainly by:
▪An aging population causing increased global demand in cataract and vitreoretinal procedures;
▪Higher uptake of premium patient-pay technologies, such as where ATIOL penetration is only approximately 13% outside the US versus approximately 19% in the US;
30
▪Increased adoption of advanced technologies, such as improved diagnostic instruments, surgical options for glaucoma management and the growing use of phacoemulsification during cataract removal, which is utilized in over 65% of cases in emerging markets versus over 95% in the US; and
▪Additionally, diabetes is increasing in prevalence, with an estimated global prevalence of 10.5% of adults, growing to more than 12% by 2045.
The Vision Care Market
The vision care market in which we operate is estimated to be approximately $21 billion and is projected to grow mid-single digits per year from 2023 to 2028. The vision care market is comprised of products designed for use by eye care professionals and consumers. Products are largely categorized across two product lines: contact lenses and ocular health. Market growth is expected to be driven mainly by:
•Fast growing daily disposable SiHy contact lens and premium reusable lens segment fueled by better material, improved health and comfort and enhanced vision acuity;
•Advancements in specialty lenses combined with increasing demand for toric, multifocal, multifocal toric and cosmetic lenses, which command an approximately 15-30% pricing premium over spherical lenses, allowing patients to continue wearing contact lenses as they become older and helping to expand the market;
•A significant population of approximately 1.4 billion people worldwide who suffer from symptoms of dry eye, but do not have clinical signs of dry eye, over 750 million people who have both symptoms and clinical signs of dry eye, and over 600 million people who are at risk of developing dry eye in that they have clinical signs, but are not yet suffering from dry eye symptoms;
•A rising number of elderly people worldwide such that primary open-angle glaucoma (POAG) now affects an estimated 68 million people and ocular hypertension, often a predecessor to POAG, is estimated to affect another 43 million people;
•Growing access and consumption of vision care products in emerging markets such as Asia, which had an estimated single-digit contact lens penetration as compared to double digits in the developed world; and
•Increasing consumer access through the expansion of distribution models, including internet sales and other direct-to-consumer channels.
Our Business
Overview
With $9.4 billion in net sales during the year ended December 31, 2023, we are the global leader in eye care. Our broad range of products represents one of the most complete portfolios in the ophthalmic industry and comprises high-quality and technologically advanced products across all major product categories in the surgical and vision care markets. Our Surgical and Vision Care products are used in treating multiple ocular health conditions and offer leading eye care solutions for patients throughout their lives.
31
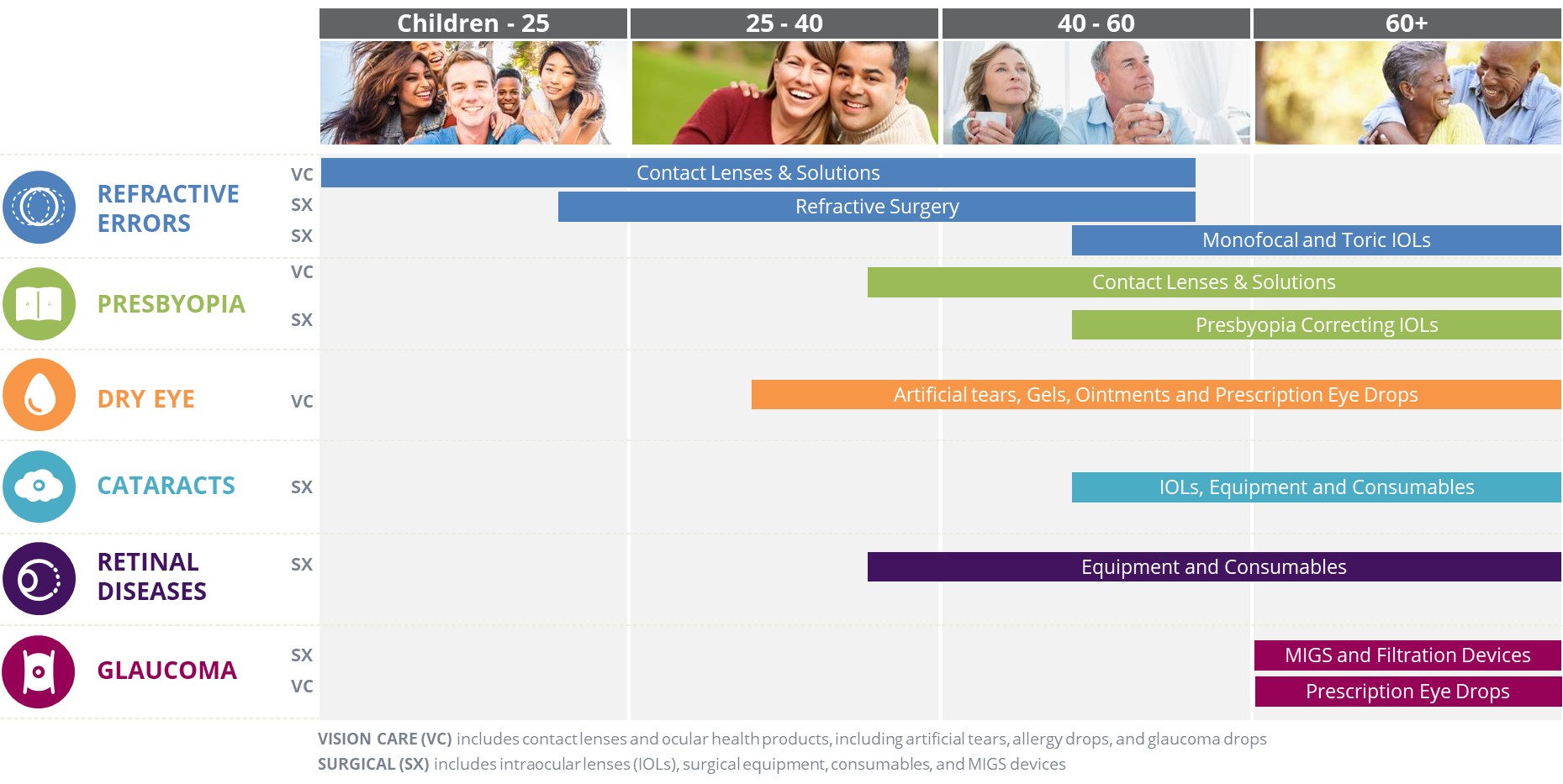
Our leadership position across most of our product categories enhances our ability to extend our product offering through the launch of new and innovative products and to expand our geographic reach into ophthalmic markets worldwide. Our Surgical business had approximately $5.3 billion in net sales of implantables, consumables and equipment, as well as services and other surgical products, and our Vision Care business had approximately $4.1 billion in net sales of our contact lens and ocular health products, during the year ended December 31, 2023.

We believe the Alcon brand name is synonymous with innovation, quality, service and leadership among eye care professionals worldwide. In each of our markets, we rely on our strong relationships with eye care professionals and consumers to attract and retain customers and expand the market. We customize our selling efforts with the goal of surrounding eye care professionals with Alcon representatives who can help address each aspect of a customer's needs.
32
Our field force supplements the direct promotion of our products by providing customers with access to clinical education programs, hands on training, data from clinical studies and technical service assistance.
We have 17 state-of-the-art manufacturing facilities that employ our proprietary technologies and know-how. We believe our global footprint, knowledge base in manufacturing, state-of-the-art facilities and capacity planning enable us to handle increased levels of product demand and product complexity. Furthermore, our global manufacturing and supply chain allows us to leverage economies of scale and reduce cost per unit as we ramp up production.
We believe we have made one of the largest commitments to research and development of any surgical and vision care company, with over 1,800 associates worldwide researching and developing treatments for vision conditions and eye diseases, and have sought innovation from both internal and external sources. In 2023, we invested $828 million in research and development. In addition to our in-house research and development capabilities, we also consider external innovation opportunities and routinely screen for companies developing emerging technologies that we believe could enhance our existing product offerings or develop into innovative new products. We intend to continue to pursue acquisition, licensing and collaboration opportunities as part of our goal of remaining a market leader in innovation.
Our Surgical Business
We hold the number one position in the global surgical market, offering implantable products, consumables and equipment for use in surgical procedures to address cataracts, vitreoretinal conditions, refractive errors and glaucoma. Our Surgical business has the most complete line of ophthalmic surgical devices in the industry, creating a "one-stop shop" for our customers that we consider to be a key differentiator for our business. For the year ended December 31, 2023, our Surgical business had $5.3 billion in net sales.
Our Vision Care Business
Our Vision Care business consists of an extensive portfolio of contact lens and ocular health products, aimed at helping consumers see better. Our product lines include daily disposable, reusable and color-enhancing contact lenses. We also offer a comprehensive portfolio of ocular health products, including products for dry eye, glaucoma, contact lens care and ocular allergies, as well as ocular vitamins and redness relievers. With $4.1 billion in vision care net sales for the year ended December 31, 2023, we aim to continue to innovate across our vision care portfolio to improve the lives of consumers and eye care professionals around the world.
Our Strengths
We have a strong foundation based on robust industry expertise, leading brands and excellence in customer service, backed by more than 75 years of history as a trusted brand. Our strengths include:
•Global leader in highly attractive markets with the most complete brand portfolio. With $9.4 billion in net sales in the year ended December 31, 2023, we are the leader in an attractive eye care market, which is supported by favorable population megatrends and is expected to grow mid-single digits per year from 2023 to 2028. Our Surgical business is the market leader in sales of ophthalmic equipment used in the operating room and is supported by the largest installed base of equipment worldwide, which we use to cross-promote our surgical consumables and IOLs. In our Vision Care business, our extensive portfolio of contact lens and ocular health products includes well-recognized brands such as TOTAL, PRECISION, Systane, Pataday and Opti-Free. We believe our global leadership position and extensive brand portfolio allow us to benefit and build on the robust fundamentals driving growth in our markets.
•Innovation-focused with market leading development capabilities and investment. We believe we have made one of the largest commitments to research and development in the eye care market, with proven research and development capabilities in the areas of optical design, material and surface chemistry, automation and equipment platforms. Currently, we employ over 1,800 individuals dedicated to our research and development efforts, including physicians, doctors of optometry and PhDs. In addition, we actively seek opportunities to collaborate with third parties on advanced technologies to support our eye care business.
•Global scale and reach supported by high-quality manufacturing network. We have an extensive global commercial footprint that provides us with the scale and reach to support future growth, maximize the potential of new launches, enter new geographies efficiently and to take advantage of the large, dynamic and growing surgical and vision care markets. Our commercial footprint, which includes operations in 56 countries, reaches consumers and patients in over 140 countries and is supported by over 3,900 sales force associates, 17 state-of-the-art manufacturing facilities employing our proprietary technologies and know-how and our extensive global regulatory capability. Our extensive sales and distribution network, supported by our market leadership position
33
and focus on innovation and customer experience, enhances our ability to expand our geographic reach and extend our product offerings through the launch of new and innovative products worldwide.
•Outstanding customer relationships and a trusted reputation for customer service, training and education. We believe that maintaining the highest levels of service excellence in our customer experience is a critical success factor in our industry. In our Vision Care business, we regularly meet with eye care practitioners to gain feedback and insights on our products and consumers' needs. We also provide training support at over 70 state-of-the-art interactive training centers around the world, as well as through numerous digital and event-based training programs that we provide for practitioners, clinical support staff, students, residents, patients and consumers. In each of our businesses, we have built and maintained our relationships with key participants to establish our trusted reputation in the industry.
•World leading expertise in eye care led by a first-class management team. Our expertise in eye care is driven by our more than 75-year history in the industry and is supported by a high-quality workforce of more than 25,000 associates. We believe our institutional knowledge provides a competitive advantage because our associates' industry expertise, relationships with our customers and understanding of the development, manufacture and sale of our products helps us to better identify new customer needs, assess markets for entry and identify promising technologies. In addition, we believe the diverse experience of our management team in running complex businesses allows them to add significant value to our company. In particular, we benefit from having a management team with an extensive background in the eye care industry. Led by David J. Endicott, our Chief Executive Officer, our management team's deep knowledge of eye care has created excitement among our workforce for our mission.
Our Strategy
Our going-forward strategy builds on five key pillars in order to generate sustainable and profitable growth:
•Maximize the potential of our near-term portfolio by growing key products. In Surgical, we plan to maintain our leading position in the IOL market as we continue to launch our ATIOLs on our new Clareon platform. In addition, we expect improved diagnostics and new optical designs will address historical barriers to ATIOL adoption to further grow this patient-pay market. We will also continue to invest behind our presbyopia-correcting products (e.g., PanOptix, Vivity), execute on the development of our next generation equipment ecosystem for the operating room (e.g., UNITY VCS) and office (e.g., UNITY Dx), leading to integration and intra-operability, and expand our reach in surgical glaucoma with the Hydrus microstent. In Vision Care, we intend to maintain and grow our leading position in most of our product categories through increased eye care professional and consumer education, supported by continuous production innovation. We intend to expand our position in the daily disposable category behind our DAILIES TOTAL1 and PRECISION1 family of products and trade patients up to a premium offering in the reusable segment with the TOTAL30 family of products, and our planned PRECISION7, 1-week replacement lens with cutting edge technology on comfort and visual performance. We also continue to pursue cutting edge presbyopia solutions through new design lenses to existing multi-focal lenses to significantly improve visual performance and comfort for presbyopic patients and improve fitting and reduce chair time for the optometrist. The presbyopia segment could become an estimated $5 billion market in the future if we are able to reduce dropout rate of presbyopic patients and increase penetration among non-contact lens users. We also aim to expand the dry eye product market by leveraging our well-recognized Systane family of eye drops and increasing investment in dry eye education and awareness, as well as address the allergy relief market with the Pataday family of products, where we see a significant unmet need and an opportunity for robust market growth.
•Accelerate innovation and deliver the next wave of technologies. We are committed to accelerating innovation by continuing to be one of the market leaders in investment in ophthalmic research and development. The research and development activities of our Surgical business are focused on expanding our ATIOL portfolio to optimize the function of the IOL in restoring vision and reducing outcome variability, including through the use of advanced optics, adjustable materials and accommodating lenses. We are also developing next-generation lasers, robotics and other equipment for cataract, vitreoretinal and laser-refractive surgery, as well as improved visualization equipment. In our Vision Care business, our focus is on developing and launching new contact lens materials, coatings and designs to improve visual performance and to extend our product lines and improve patient comfort, as well as on new products to expand our portfolio of dry eye diagnostic and treatment, presbyopia and ocular health products. Finally, we expect to continue to supplement our internal innovation investments by identifying and executing on attractive BD&L opportunities with leading academic institutions and early-stage companies.
34
•Capture opportunities to expand markets and pursue adjacencies. We believe there is a significant opportunity for growth in markets around the world due to under-penetration of both premium surgical devices, such as ATIOLs, and of our Vision Care portfolio. We intend to facilitate this growth by continued investment in promotion and customer education across all of our markets. In emerging markets in particular, we believe that the growing number of eye care professionals and dedicated eye hospitals, increased levels of affluence, improving technology access and better patient awareness will increase the adoption of our products. By acquiring Ivantis in early 2022, we expanded our Surgical portfolio to include the Hydrus microstent, a minimally invasive glaucoma surgery (or MIGS) device for the treatment of mild-to-moderate glaucoma. We are also expanding into the ophthalmic pharmaceutical space through the acquisitions of the exclusive US commercialization rights for Simbrinza from Novartis in June 2021 and Eysuvis and Inveltys from Kala Pharmaceuticals, Inc. in July 2022, and most recently the acquisition of Aerie Pharmaceuticals, Inc. in November 2022. The Aerie transaction adds on-market products Rocklatan and Rhopressa as well as a pipeline of products, including AR-15512 (a Phase 3 product candidate for dry eye disease), and R&D capabilities to expand our ophthalmic pharmaceutical presence. In addition, we believe we have significant opportunities to expand into adjacent product categories in which Alcon has not significantly participated in the past, through a combination of internal development efforts and potential mergers and acquisitions activity. These opportunities include pharmaceuticals, office-based diagnostics, surgical visualization and consumer driven ocular health products, where we expect our eye care expertise and global commercial footprint will allow us to attract and retain new customers.
•Support new business models to expand customer experience. In Surgical, we intend to continue to identify new business models that benefit healthcare providers and improve access to leading Alcon products and technologies. For example, in the future, we may pursue value-based business models that reward improved patient outcomes, as well as models that contract the entire procedure versus individual products. In Vision Care, where e-commerce entries have created some disruption of traditional sales channels, we believe that digital technology can address pain points experienced in existing paths to purchase. We intend to continue investing and innovating in digital capabilities to develop new business models in response to channel shifts and the increase in direct-to-consumer influence.
•Leverage infrastructure to improve operating efficiencies and margin profile over time. With the significant organizational and infrastructure investments we have made over the last several years, we believe we have established a stable foundation that will allow us to continue to enhance the productivity of our commercial resources. We expect to drive significant top line growth and increase operating leverage through improved sales mix, further supply chain efficiency initiatives and support new lower-cost manufacturing platforms to meaningfully improve our core operating income margins over time. We are also optimizing our end to end processes and systems to ensure streamlined and efficient operations and improved customer experience.
Our Industry
Selected Conditions that are Treated by Eye Surgery and Surgical Products
Cataracts
A cataract is the progressive clouding of the normally transparent natural lens in the eye. This clouding is usually caused by the aging process, although it can also be caused by heredity, diabetes, environmental factors and, in some cases, medications. As cataracts grow, they typically result in blurred vision and increased sensitivity to light. Cataract formations occur at different rates and may affect one or both eyes. Cataract surgery is one of the most frequently performed surgical procedures. According to the National Eye Institute, cataracts are the leading cause of blindness worldwide even though effective surgical treatment exists. Currently, surgical removal of the clouded lens followed by insertion of a transparent artificial replacement lens, called an IOL, is the preferred treatment for cataracts. The clouded lens is usually removed through a process known as phacoemulsification. During phacoemulsification, an ophthalmic surgeon makes a small surgical incision in the cornea (approximately 2-3 millimeters wide) and inserts an ultrasonic probe that breaks up, or emulsifies, the clouded lens while a hollow needle removes the pieces of the lens. Once the clouded lens is removed, the surgeon inserts an intraocular lens through the same surgical incision. An ATIOL is a type of IOL that also corrects for refractive errors, like presbyopia and astigmatism.
35
Retinal Disorders
Vitreoretinal procedures involve surgery on the back portion of the eye, namely the retina and surrounding structures. Vitrectomy is the removal of the gel-like substance, known as vitreous, that fills the back portion of the eye. Removal of the vitreous allows a vitreoretinal surgeon to operate directly on the retina or on membranes or tissues that have covered the retina. These procedures typically treat conditions such as diabetic retinopathy, retinal detachment or tears, macular holes, complications of surgery on the front of the eye, diabetic macular edema, trauma, tumors and pediatric disorders. Vitreoretinal surgery can also involve electronic surgical equipment, lasers and hand-held microsurgical instruments as well as gases and liquids that are injected into the eye.
Refractive Errors
Refractive errors, such as myopia, commonly known as near-sightedness, hyperopia, commonly known as far-sightedness, and astigmatism, a condition in which images are not focused at any one point, result from an inability of the cornea and the lens to focus images on the retina properly. If the curvature of the cornea is incorrect, light passing through it onto the retina is not properly focused and a blurred image results. For many years, eyeglasses and contact lenses were the only solutions for individuals afflicted with common visual impairments; however, they are not always convenient or attractive solutions. Laser refractive surgery offers an alternative to eyeglasses and contact lenses. Excimer lasers, which are low-temperature lasers that remove tissue without burning, are currently used to correct refractive errors by removing small amounts of tissue to reshape the cornea. These lasers remove tissue precisely without the use of heat and without affecting the surrounding tissue. In the LASIK procedure, the surgeon uses either a femtosecond laser or an automated microsurgical instrument, called a microkeratome, to create a thin corneal flap that remains hinged to the eye. The corneal flap is then folded back and excimer laser pulses are applied to the exposed layer of the cornea to change the shape of the cornea. The corneal flap is then returned to its normal position. LASIK has become the most commonly practiced form of laser refractive surgery globally.
Presbyopia
Presbyopia occurs when the natural crystalline lens inside the eye becomes less flexible and loses the ability to focus on close objects. Presbyopia is a vision condition that accompanies the natural aging process of the eye. It cannot be prevented and affects nearly two billion people worldwide. Although the onset of presbyopia among patients may seem to occur suddenly, generally becoming noticeable when patients reach their mid- to late 30s or early to mid-40s, sight reduction typically occurs gradually over time and continues for the rest of the patient's life. Some signs of presbyopia include difficulty reading materials held close to the reader, blurred vision while viewing a computer screen and eye fatigue along with headaches when reading. Presbyopia can be accompanied by other common vision conditions, such as myopia, hyperopia and astigmatism. Presbyopia, while most commonly managed with reading glasses, can be addressed surgically by the implantation of an ATIOL that allows for the correction of presbyopia at the time of cataract surgery.
Glaucoma
Glaucoma, a group of eye conditions that damage the optic nerve, is the second leading cause of blindness worldwide. While elevated intraocular pressure was historically considered to be synonymous with glaucoma, it is now known that many patients with glaucoma have normal intraocular pressure. Treating glaucoma is typically aimed at lowering intraocular pressure for patients with normal or elevated pressure.
Most commonly, glaucoma is managed using medication (e.g., drops). For cases requiring additional intervention, laser-based procedures and conventional surgical techniques, such as filtration surgery and tube shunts, have typically been used to lower IOP. Filtration surgeries, such as trabeculectomy, involve the creation of a new channel to drain aqueous humor from inside the eye. Similarly, tube shunts establish a route for fluid to exit through an implanted device. Device and procedure-based surgical intervention, known as MIGS which generally work to increase aqueous outflow, has been experiencing rapid adoption among both glaucoma and cataract specialists.
Selected Conditions and Eye Care Considerations that are Addressed by Vision Care Products
Refractive Errors
Refractive errors such as myopia, hyperopia, astigmatism and presbyopia are commonly addressed by the use of contact lenses. Presbyopia, for example, can be addressed by the use of multifocal and multifocal toric contact lenses.
36
Dry Eye Disease
Dry eye disease is a ubiquitous, complex and multifactorial condition, and its effect on patients ranges from intermittent and irritating discomfort to a serious, chronic, progressive and irreversible vision-threatening disorder. The incidence of dry eyes rises with age, and longer life spans and aging populations throughout the world are key contributors to increased demand for treatment. Evolving patterns of work and play also contribute to increased demand for treatment, as more people spend significant amounts of time working on computers and other digital devices. Wealthier, professional and urban population segments are expanding in rapidly emerging economies and other developing nations, and these populations have greater access to health care and more resources with which to acquire treatment. In addition, more sophisticated diagnostic tools and a greater variety of dry eye products and treatments, such as artificial tear products, are offering improved effectiveness and greater relief as they simultaneously stimulate demand.
Infections and Contamination due to Inadequate Contact Lens Care
Proper care of contact lenses through compliance with disinfection regimens is important in reducing the risk of infection and irritation associated with the use of reusable contact lenses, as contact lenses are subject to contamination from cosmetics, grease, bacteria, soaps, hand lotions and atmospheric pollutants, and from proteins contained in natural tears. When used properly, contact lens care products remove such contaminants from the surface of the contact lens. In addition, lens rewetting drops may be used to rehydrate the lens during wear and to clear away surface material.
Ocular Allergies
Allergic conjunctivitis occurs when the conjunctiva of the eye becomes swollen from inflammation due to a reaction to pollen, dander, mold or other allergy-causing substances. When the eyes are exposed to allergy-causing substances, which can vary from person-to-person and are often dependent on geography, a substance called histamine is released by the body and causes blood vessels in the conjunctiva to swell. "Allergy eyes" can become red and itchy very quickly. Seasonal Allergic Conjunctivitis ("SAC") is the most common type of eye allergy. People affected by SAC experience symptoms during certain seasons of the year. Allergy eye can be treated with various ocular health products including medications, such as antihistamines, and combinations of antihistamines and redness relievers.
Glaucoma
Glaucoma is commonly managed using prescription eye drops to reduce intraocular pressure for patients with normal or elevated pressure.
Our Products
We research, develop, manufacture, distribute and sell eye care products. Our broad range of products represents one of the strongest portfolios in the eye care industry, with high-quality and technologically advanced products across all major product categories in ophthalmic surgical devices and vision care. We are organized into two global business segments: Surgical and Vision Care.
Surgical
We hold the number one position in the global ophthalmic surgical market, offering implantable products, consumables and equipment for use in surgical procedures to address cataracts, vitreoretinal conditions, refractive errors and glaucoma. Our Surgical portfolio includes equipment, instrumentation and diagnostics, IOLs and other implantables and a broad line of consumables, including viscoelastics, surgical solutions, incisional instruments, surgical custom packs and other products. For the year ended December 31, 2023, net sales for our implantables, consumables and equipment and other surgical products were $1.7 billion, $2.7 billion and $0.9 billion, respectively.
Cataract, vitreoretinal, refractive and glaucoma surgeries are generally performed in hospitals or ambulatory surgery centers and are supported through a network of eye clinics, ophthalmic surgery offices and group purchasing organizations. The primary ophthalmic surgical procedures for cataract, vitreoretinal and glaucoma surgery are broadly reimbursed in most mature markets. Third-party coverage or patient co-pay options are also available for refractive laser correction and ATIOLs. Finally, a growing private pay market for premium surgical devices provides a mutually beneficial environment for patients, providers and medical device companies by allowing patients to pay the non-reimbursable cost of a procedure associated with selecting premium devices, such as ATIOLs.
Our installed base of equipment is core to our market leading position in our Surgical business, with best-in-class platforms in cataract and vitreoretinal equipment and the largest installed base of cataract phacoemulsification consoles, vitrectomy consoles and refractive lasers in the industry. These platforms each have long buying cycles that last
37
approximately seven to ten years and act as anchoring technologies that drive recurring sales of our consumables and help cross-promote sales of our implantable devices.
Sustainable patient access to quality eye care is core to our business. Alcon has invested significant resources to innovate new technologies, expand reimbursement pathways (public and/or private insurance) and teach new skills to clinicians around the world to improve patient outcomes and eye care access. Across our Surgical portfolio, we sell a tiered offering of products intended to meet the specific needs of customers in markets around the world at different price points. Newly launched offerings that bring considerable technology innovation to the market are typically introduced at a price premium to offset the cost of research and development. As these products age and/or competitive products advance, prices typically trend downward, requiring continuous innovation cycles to maintain and/or grow our margins. We also develop specific products to match customer needs in different customer segments, for example, premium-tier and mid-tier surgical consoles that can be manufactured and sold at different price points in different markets. Likewise, we have introduced the Legion system to help fill the gap in access to phacoemulsification surgery. This affordable system brings some of the advanced features of the Centurion system, combined with the greater serviceability, durability and portability to developing markets.

Our strong installed base of equipment and extensive clinician relationships drive sales of our IOLs and consumables. We consider the quality and breadth of our portfolio to be a key differentiator as a "one-stop-shop" offering for our customers, synonymous with quality, reliability and accessibility. Our Cataract Refractive Suite covers every stage of the surgical workflow from clinical planning to cataract removal and post-operative optimization.
We sell Centurion, our vision system for cataract surgery in most major markets. This system includes Active Fluidics technology, an automated system that optimizes anterior chamber stability by allowing surgeons to proactively set and maintain target IOP within the eye during the cataract removal procedure, thereby delivering an unprecedented level of intraoperative control.
We also sell the LenSx Laser System in select major markets. The first femtosecond laser to receive FDA clearance for use in cataract surgery, LenSx is used to create incisions in the cornea, create a capsulorhexis and complete lens fragmentation as part of the cataract procedure. This enables surgeons to perform some of the most delicate manual steps of cataract surgery with image-guided visualization and micron precision.
Our Verion reference unit and Verion digital marker together form an advanced surgical planning, imaging and guidance technology designed to provide greater accuracy and efficiency during cataract surgery. Our ORA System provides key intra-operative measurements to improve the placement precision of an implanted IOL during cataract surgery, for example, by aligning the rotation of a toric IOL to the axis of astigmatism. Post-operatively, our ORA System aids with outcomes analysis and ongoing optimization for improved outcomes.
Our ARGOS biometer offers an integrated image-guided solution for every step of the surgical process from post-operative measurement to surgical planning and intra-operative guidance for optimal IOL positioning. ARGOS biometer provides efficient measurement, simplified workflow, precise measurement via swept-source OCT (SS-OCT), and integration with the rest of our cataract refractive suite.
In 2021, we launched our first application, SMARTCataract, to our digital health platform, SMART Solutions, enabling remote cataract surgical planning and automated transfer of data from diagnostic devices to OR equipment, reducing time spent manually entering patient data into individual devices.
Finally, the NGENUITY 3D visualization system provides surgeons improved visualization by combining a high-dynamic 3D camera, advanced high-speed image optimization, polarizing surgeon glasses and an ultra-high definition 4K OLED 3D display that offers improved depth perception. Within visualization, we also sell the LuxOR surgical ophthalmic microscope with its proprietary ILLUMIN-i technology, which provides an expanded illumination field with a 6x-larger, highly stable red reflex zone.
38

An IOL is a tiny, artificial lens for the eye, which replaces the eye's natural lens that is removed during cataract surgery. We have a longstanding record of innovation within the IOL market. Our AcrySof IOL is the most implanted IOL in the world. AcrySof IOLs are made of the first material specifically engineered for use in intraocular lens.
In 2005, we introduced a new class of IOLs to correct presbyopia with our multifocal AcrySof ReSTOR offering. In 2006, we launched the AcrySof Toric IOL, designed to correct various levels of preexisting astigmatism in cataract patients. In 2009, we launched the AcrySof IQ Toric lens globally, incorporating the aspheric technology into a toric design.
In recent years, presbyopia correction lenses have evolved to include trifocal designs. Beginning in 2015, we launched the AcrySof IQ PanOptix trifocal IOL in select markets. This novel diffractive optic sends light to three foci to support near, intermediate and distance vision. Beginning in 2019, we also launched the AcrySof IQ Vivity non-diffractive extended depth of focus ("EDoF") IOL in select markets. This optic design allows for extended range of vision and presbyopia correction with the visual disturbance profile of a monofocal IOL. In 2022, we launched Clareon PanOptix and Clareon Vivity in North America, Asia, and the EU, combining our leading trifocal and EDoF optic designs with a new material with an advanced design that enables sharp, crisp vision, low edge glare and unsurpassed optic clarity.
We have also introduced several innovations to the delivery device used for introducing an IOL into the capsular bag during cataract surgery. Our UltraSert pre-loaded IOL delivery system combines the control of a manually loaded device with the safety and convenience of a disposable, pre-loaded injector to optimize the implantation of the AcrySof IQ Aspheric IOL into the cataract patient's eye.
In 2017, we received a European CE Mark for the Clareon IOL with the AutonoMe delivery system followed by FDA approval in 2020. AutonoMe is the first automated, disposable, pre-loaded IOL delivery system that enables precise delivery of the IOL into the capsular bag in patients undergoing cataract surgery. The new device is being introduced with the Clareon IOL.
Our ATIOLs (those that correct for refractive errors such as presbyopia and astigmatism) provide significant visual benefits to patients above standard monofocal IOLs. Accordingly, the price for these ATIOLs is higher than the price for monofocal styles. This impacts the market penetration of ATIOLs in the majority of countries, as patients must pay incremental charges above the cost of traditional cataract surgery to obtain an ATIOL and, in some markets, must pay out-of-pocket for the entire surgical procedure and the ATIOL.
In the US, our monofocal IOLs are generally fully covered by medical insurance providers or government reimbursement programs, whereas certain of our ATIOLs may only be partially covered. This payment model was established by two landmark rulings issued by CMS in May 2005 and January 2007. The CMS rulings provide Medicare beneficiaries a choice between cataract surgery with a monofocal IOL, which would be reimbursed as a covered benefit under Medicare, or cataract surgery with an ATIOL, which would be partially reimbursed under Medicare and partially paid out-of-pocket. Many commercial insurance plans mirror the CMS rulings, although commercial plans may vary based on third-party payor. The bifurcated payment for the implantation of ATIOLs has increased the market acceptance of our ATIOLs in the US. Outside the US, payment and reimbursement models vary widely from country to country, generally depending on the policy adopted by the relevant local healthcare authority on coverage and payment.
In addition to IOLs, we offer devices to treat glaucoma. In 2018, the Hydrus microstent device was launched in the US by Ivantis, which we acquired in 2022. Hydrus is approved and marketed in a number of major markets, including the US. The microstent is implanted into the Schlemm's canal to enhance outflow, reducing eye pressure for the treatment of primary open angle glaucoma (POAG).
Our EX-PRESS glaucoma filtration device is approved and marketed for refractory glaucoma in the US, Europe, Canada, Australia, China and many other markets. The device shunts aqueous from the anterior chamber to a subconjuntival reservoir in a similar fashion as a trabeculectomy without removal of any sclera or iris tissue.
39

To provide convenience, efficiency and value for ophthalmic surgeons, Alcon offers Custom Pak surgical procedure packs for use in ophthalmic surgery. Unlike conventional surgical procedure packs, our Custom Pak surgical procedure packs allow individual surgeons to customize the products included in their pack, which results in less waste in the environment. Our Custom Pak surgical procedure packs include both our single-use products as well as third-party items not manufactured by Alcon. We believe that our Custom Pak offering allows ophthalmic surgeons to improve their efficiency in the operating room, while avoiding the complexity and cost of having to kit surgical items for each respective procedure. We offer more than 10,000 configurations of our Custom Pak surgical procedure packs globally.
 Our vitreoretinal surgical product offering is one of the most comprehensive in the industry for surgical procedures for the back of the eye. We currently market our vitreoretinal surgical products in substantially all of the countries in which we sell products.
Our vitreoretinal surgical product offering is one of the most comprehensive in the industry for surgical procedures for the back of the eye. We currently market our vitreoretinal surgical products in substantially all of the countries in which we sell products.For vitrectomy procedures, we sell our Constellation vision system globally. We believe this system delivers a higher level of control to the physician through higher vitreous cutting rates and embedded laser technology. The Constellation vision system platform continues to drive our market share in the global premium segment of vitrectomy packs.
In addition to our Constellation vision system, we also sell a full line of vitreoretinal products, including procedure packs, lasers and hand-held microsurgical instruments, as well as our Grieshaber and MIVS lines of disposable retinal surgery instruments. We also sell a full line of scissors, forceps and micro-instruments in varying gauge sizes, as well as a range of medical grade vitreous tamponades, which replace vitreous humor during many retinal procedures.
We continue to advance our portfolio with smaller gauge (27+) instruments and higher cut speed vitrectomy probes. We also sell Hypervit high speed vitrectomy probes, which operate at a speed of 20,000 cuts per minute ("cpm"). This increased speed helps reduce traction that can cause iatrogenic tears and post-operative complications.

Our refractive products include lasers, disposable patient interfaces used during laser vision correction procedures, technology fees and diagnostic devices necessary to plan the refractive procedures. Our WaveLight refractive suite includes the EX500 excimer laser, designed to reshape the cornea, and the FS200 femtosecond laser, designed to create a corneal flap and to deliver laser refractive therapy as part of the LASIK refractive procedure.
Our refractive portfolio also includes Contoura Vision, powered by the WaveLight Toplyzer VARIO diagnostic device, and the recently launched WaveLight Plus, our most advanced LASIK treatment designed to provide surgeons with the ability to perform more personalized laser procedures for patients with near-sightedness or near-sightedness with astigmatism.
Vision Care
Our Vision Care portfolio comprises daily disposable, reusable and color-enhancing contact lenses, as well as a comprehensive portfolio of ocular health products, including products for dry eye, glaucoma, contact lens care and ocular
40
allergies, as well as ocular vitamins and redness relievers. For the year ended December 31, 2023, net sales of our contact lens and ocular health products were $2.4 billion and $1.7 billion, respectively.
We serve our customers and patients through optometrists, ophthalmologists and other eye care professionals, retailers, optical chains and pharmacies, as well as distributors that resell directly to smaller retailers and eye care professionals, who sell the products to end-users. The vision care market is primarily private pay, with patients substantially paying for contact lenses and ocular health products out-of-pocket. Partial reimbursement is available in some countries for visits to eye care professionals and a portion of either spectacle or contact lens costs.
Sales of our contact lens and ocular health products are influenced by optometrist, ophthalmologist and other eye care professional recommendations, our marketing and consumer education efforts and consumer preferences. In addition to price, contact lenses compete on functionality, design and comfort, while ocular health products compete largely on product attributes, brand familiarity and professional recommendations. For our contact lens and ocular health products, we typically compete in the premium price segments of the market and we use improvements in functionality, design and consumer convenience to maintain our pricing position over time.

Alcon is the number two company in the branded contact lens market based on market share in 2023. We are the number one manufacturer of daily disposable SiHy lenses in the US in 2023. This position is driven largely by our core brands TOTAL, PRECISION, DAILIES AquaComfort PLUS and Air Optix.
Our TOTAL product line with its water gradient technology reduces end-of-day dryness, as the water content approaches nearly 100% at the outermost surface of the lens, and is designed to be a super-premium lens positioned to compete at the highest levels across the contact lens market. Our TOTAL product line includes DAILIES TOTAL1, the first and only water gradient disposable contact lens in the market. We launched TOTAL30, our premium offering in the reusable segment, in 2021 to encourage patients to trade up to a next generation, water gradient technology. We also launched TOTAL30 toric and multifocal in 2023 to complete the TOTAL30 product family. DAILIES TOTAL1 in the multifocal offering provides a platform for expanding the presbyopia market, which we believe is a potential multibillion dollar opportunity for market participants.
PRECISION1, our new mainstream daily disposable silicone-hydrogel ("SiHy") lens with aqueous extraction and surface treatment, is priced in between the super-premium DAILIES TOTAL1 and the more value-conscious DAILIES AquaComfort PLUS. PRECISION1 is designed for long lasting performance and delivers precise vision, dependable comfort and ease of handling. Following a successful introduction of PRECISION1 spherical lenses, we introduced PRECISION1 for Astigmatism, a toric lens designed for astigmatic patients. In the US and EU, PRECISION1 for Astigmatism features the PRECISION BALANCE 8|4 lens design for a stable lens-wearing experience. Studies show that 47% of patients have astigmatism that needs correction, but less than 15% wear toric contact lenses. As a result, we believe the launch of PRECISION1 for Astigmatism provides a significant opportunity to attract new contact lens wearers and maximize retention. We are working to launch our first 1-week replacement lens, PRECISION7, with cutting edge ActivFlo re-wetting system for clear vision and outstanding comfort. Wearers will benefit from an easier replacement schedule and having a fresh lens more often.
DAILIES AquaComfort PLUS, our most affordable daily disposable contact lens in monofocal, astigmatism-correcting and multifocal options, delivers dependable performance by working with tears to release moisture with every blink. This lens is designed for value-conscious wearers who want the flexibility and simplicity of a daily disposable lens.
Air Optix, our more affordable monthly replacement product line, features SiHy contact lenses in monofocal, astigmatism-correcting and multifocal options, as well as Air Optix Colors and Air Optix plus HydraGlyde contact lenses. Air Optix plus HydraGlyde brings together two innovative technologies—SmartShield technology and HydraGlyde moisture matrix—for a unique combination of deposit protection and longer-lasting lens surface moisture.
We continue to experience market growth driven by trade-up to premium lenses, expansion of toric and multifocal specialty lenses, as well as increasing penetration in emerging markets.
41

Our key brands in our ocular health portfolio include the Systane family of artificial tear and related dry eye products, Pataday family of eye allergy products, as well as the Opti-Free and Clear Care lines of multi-purpose and hydrogen peroxide disinfecting solutions, respectively.
Alcon currently holds a market leading position in artificial tears. We continue to focus on core product performance while increasing promotion behind a best-in-class innovation portfolio under the brand leadership of Systane artificial tears. The Systane portfolio is a comprehensive offering of ocular health solutions, most of which are indicated for the temporary relief of burning and irritation due to dryness of the eye. The Systane portfolio includes products for daily and nighttime relief, as well as products for discomfort associated with contact lens wear. In 2021, we continued significant international expansion for Systane Ultra multi-dose preservative-free ("MDPF") and Systane Hydration MDPF. In 2022, we launched a preservative-free formulation of Systane Complete. By adding the option of MDPF presentations to our portfolio, we address a key need by many eye care practitioners for effective dry eye relief without preservatives. We plan to continue to extend our Systane line with a new formulation that combines the strength of Systane Complete nano-lipids with hyaluronic acid to provide relief from more severe dry eye symptoms.
Previously available only by prescription, in 2020, we began to offer the Pataday family of allergy relief eye drops over-the-counter in the US. Pataday Twice Daily Relief, Pataday Once Daily Relief and Pataday Once Daily Extra Strength eye drops each contains olopatadine, the number one doctor-prescribed active ingredient for eye allergy relief.
In 2021, we began our expansion into the ophthalmic pharmaceutical space by acquiring the exclusive US commercialization rights to Simbrinza, a fixed combination of a carbonic anhydrase inhibitor and an alpha-2 adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure ("IOP") in patients with open-angle glaucoma or ocular hypertension. We then acquired Eysuvis, a corticosteroid indicated for the short‑term (up to two weeks) treatment of the signs and symptoms of dry eye disease, and Inveltys, a corticosteroid indicated for the treatment of post-operative inflammation and pain following ocular surgery, from Kala Pharmaceuticals, Inc. in July 2022. In November 2022, to complement our previous acquisitions, we acquired Aerie Pharmaceuticals, Inc. The Aerie transaction adds on-market products Rhopressa, a once-daily eye drop that contains netarsudil, a Rho kinase (ROCK) inhibitor specifically designed to target a diseased trabecular meshwork believed to be the main cause of elevated IOP in open-angle glaucoma and ocular hypertension, and Rocklatan, a once-daily eye drop that is a fixed-dose combination of latanoprost, a prostaglandin analog (PGA), and netarsudil, as well as a pipeline of products, including AR-15512, a Phase 3 product candidate for dry eye disease, and R&D capabilities to expand our ophthalmic pharmaceutical presence.
Alcon is also a market leader in contact lens care in both multi-purpose (Opti-Free PureMoist) and hydrogen peroxide solutions (Clear Care and AOSEPT PLUS). The vast majority of our contact lens care products are comprised of disinfecting solutions to remove harmful micro-organisms on contact lenses, with a smaller amount of sales coming from contact lens rewetting drops to improve wearing comfort for contact lenses. We benefit from strong synergies between our contact lens business and our contact lens care products.
Finally, our ocular health portfolio also includes artificial tear and related dry eye products marketed under the Tears Naturale and Genteal brands, products for the temporary relief of ocular itching due to ocular allergies marketed under the Naphcon-A and Zaditor brands and vitamins for the maintenance of general ocular health marketed under the ICAPS and Vitalux brands.
Our ocular health portfolio is typically over the counter, but certain of our ocular health products are regulated as pharmaceuticals and require a prescription.
Principal Markets
Alcon serves consumers and patients in over 140 countries worldwide. The US is our largest market with 46% of our net sales in 2023. See Note 4 to the Consolidated Financial Statements for net sales by segment and geography for each of the last three fiscal years. US sales of the vast majority of our products are not subject to material changes in seasonal demand; however, sales of certain of our vision care products, including those for allergies and dry eye, are subject to seasonal variation. In addition, sales of our surgical equipment are also subject to variation based on hospital or clinic purchasing cycles.
42
Research and Development
We believe we have made one of the largest commitments to research and development in the eye care market, with proven research and development capabilities in the areas of clinical research and development, optical design, material and surface chemistry, software development, automation and equipment platforms. We invested approximately $828 million, $702 million and $842 million in research and development in 2023, 2022 and 2021, respectively. Currently, our research and development organization employs over 1,800 individuals dedicated to our research and development efforts, including physicians, doctors of optometry and PhDs. Our researchers have extensive experience in the field of ophthalmology and frequently have academic or practitioner backgrounds to complement their product development expertise.
We organize cross-functional development teams to drive new innovations to our customers and our patients around the world. New projects for our Surgical and Vision Care pipelines originate either from concepts developed internally by staff scientists and engineers, ideas from eye care professionals or through strategic partnerships with academic institutions or other companies. As the global leader in eye care, Alcon recognizes the need to support a cohesive ecosystem comprised of both disruptive and incremental innovation from internal and external sources. During 2023, we have successfully completed the Alcon Seed Fund’s ("ASF") pilot phase and finalized its new operating model. The ASF was introduced in 2022 and designed to complement and support Alcon’s existing mechanisms for external innovation, which include the Alcon Research Institute and BD&L. ASF considered over 400 companies in 2023 alone and has executed 14 deals from inception through December 31, 2023. ASF aims to power the innovation ecosystem to find innovative ways to address the most relevant unmet needs.
In 2023, we launched innovations to address patient and customer needs, including TOTAL30 for Astigmatism, TOTAL30 Multifocal for patients with presbyopia and WaveLight Plus for personalized vision correction. We also expanded AlconScience.com internationally to connect healthcare professionals to regionally relevant scientific information and data.
We regularly review and refine our operating model to optimize for efficiency and productivity. Across our Surgical and Vision Care pipelines, we have more than 90 pipeline projects in process as of December 31, 2023, including 59 that have achieved positive proof of concept or are undergoing regulatory review. In addition to our in-house research and development capabilities, as part of our efforts to pursue strategic research and development partnerships with third parties, our dedicated business development team has completed over 75 BD&L transactions since 2018.
In 2023, we focused on integrating the Aerie R&D projects into our portfolio, defining the new organizational structure for pharmaceutical development and aligning processes and systems. The legacy Aerie R&D associates added significant technical expertise to our capabilities, enhancing our drug development expertise and our drug delivery capabilities. We continue to pursue AR-15512, a Phase 3 product candidate for dry eye disease, which is a topical transient receptor potential melastatin (TRPM8) agonist. TRPM8 agonism leads to the stimulation of tear production. Beyond AR-15512, we also acquired the worldwide ophthalmic rights to a bio-erodible, sustained-release, drug delivery technology called PRINT, which utilizes proprietary polymers capable of creating precisely-engineered, tunable ophthalmic implants through fully-scalable manufacturing processes. We are currently focused on incorporating axitinib, a pan-VEGF inhibitor, for neovascular age-related macular degeneration (wet AMD), a leading cause of vision loss and blindness for Americans aged 65 years and older. We believe the PRINT technology platform may also be useful In the future as we explore additional sustained-release applications using existing molecules. In connection with the product acquisition from Kala Pharmaceuticals, we also acquired AMPPLIFY, a topical drug delivery technology that helps improve delivery of certain molecules, such as loteprednol etabonate, over traditional suspension eyedrops by improving penetration of these drugs into target ocular surface tissues. Finally, we are conducting some limited earlier stage research related to diabetic macular edema (DME) and reformulations in glaucoma.
In the spirit of continuous improvement, Alcon has implemented several system transformations designed to make R&D projects faster and more efficient while making the company more agile in responding to changing regulations, audits and customer concerns. For example, we shifted our R&D team’s organizational structure, combining expertise into centralized functions to better support different product types. This centralization enables more standardized ways of working, consistency in data generation and agility in resource deployment. In addition, in early 2023, we celebrated the opening of a software development lab in our Alcon Global Services Center located in Bengaluru, India that allows us to enhance our capabilities with the "follow the sun" model. We also launched an enterprise digital platform that streamlines the path to regulatory submissions and simplifies product support activities.
Our research and development organization maintains an extensive network of relationships with top-tier scientists in academia and with leading healthcare professionals, surgeons, inventors and clinician-scientists working in ophthalmology. The principal purpose of these collaborative scientific interactions is to supplement our internal pipeline and leverage technological advancements in academia and the clinical setting.
43
While our primary focus is on delivering new products to our patients and customers, we also support the advancement of basic science through the Alcon Research Institute, which seeks to encourage, advance and support vision research. The Institute is one of the largest corporately funded research organizations devoted to vision research in the world. The Institute's activities are planned and directed by an autonomous Executive Steering Committee that is comprised of distinguished ophthalmologists and vision researchers. The Institute has worldwide representation and operates under the premise that improvements in the diagnosis and treatment of ocular diseases are dependent upon advances in basic science and clinical research carried out by independent investigators in institutions throughout the world.
Research and development activities within our Surgical business continue to focus on expanding intraocular lens capabilities to further improve surgical and refractive outcomes and on developing equipment and instrumentation for cataract, vitreoretinal, refractive and glaucoma surgeries, as well as new platforms for diagnostics and visualization. Our focus within the Vision Care business is on the research and development of new manufacturing platforms and novel contact lens materials, coatings and optical designs for various lens replacement schedules, with the ultimate goal of improving patient outcomes. In addition to our efforts to develop next-generation contact lens technologies, we are strengthening our ocular health portfolio with new products and novel technologies.
Marketing and Sales
Alcon conducts sales and marketing activities throughout the world. During the year ended December 31, 2023, 46% of our sales were in the US. We are present in every significant market in the world where ophthalmology and optometry are practiced, with operations in 56 countries supported by over 3,900 associates dedicated to direct sales and with products sold in over 140 countries.
Our global commercial capability is organized around sales and marketing organizations dedicated to our Surgical and Vision Care businesses and we customize these efforts to the medical practice needs of each customer. In addition to direct promotion of our products, our sales representatives provide customers with access to clinical education programs, data from clinical studies and technical service assistance. Our selling models also include focused efforts in key channels, including strategic accounts, key accounts and pharmacies.
In each of our markets, we rely on our strong relationships with ECPs to attract and retain customers. We engage healthcare professionals to serve as clinical consultants, to participate on advisory boards and to conduct presentations regarding our products. In addition, we have established or sponsor several long-standing programs that provide training and education to eye care professionals, including providing training support at over 70 state-of-the-art interactive training centers around the world. These facilities introduce ophthalmologists to our surgical equipment and cataract products through hands-on training in surgical techniques while exposing them to leading ophthalmologists.
In our Surgical business, our marketing efforts are supported by global advertising campaigns, claims from clinical registration and post-approval studies and by the participation of marketing and sales representatives in regional and global medical conferences. Technical service after the sale is provided using an integrated customer relationship management system in place in many markets. All of our technical service in the US, and a high percentage of that service outside the US, is provided by service technicians employed directly by Alcon. In countries where we do not have local operations or a scientific office, we use distributors to sell and handle the physical distribution of our products. Within our Surgical business, the practices of our marketing and sales representatives continue to change to meet emerging market trends, namely consolidation of providers, increasing pricing pressures, proliferation of smaller competitors, increasing demands for outcome evidence and a shift from relationship-based selling orientated toward physicians versus professional economic buyers focused on cost.
In our Vision Care business, we support our products with direct-to-consumer and ECP-oriented marketing campaigns, including advertising, promotions and other marketing materials, and with retailer-focused marketing and promotional materials. The fast-evolving landscape for our Vision Care business varies significantly by country. Three key trends in marketing and sales help drive the continuing evolution of our Vision Care business:
•Internet-based purchasing is increasing, as online players grow and the internet plays a bigger role as a source of consumer information and a platform for price referencing;
•Channel consolidation is accelerating, as chains grow in size and vertically integrate; and
•Independent eye care professionals vary in influence, as many align more closely with retailers.
We see an opportunity to leverage digital technology to address pain points experienced by consumers and patients in existing paths to purchase. We also intend to continue investing and innovating in digital capabilities to develop new business models and practice implementation support in response to channel shifts and increases in direct-to-consumer influence.
44
While we market all of our products by calling on medical professionals, direct customers and distribution methods differ across our business lines. Surgical products are sold directly to hospitals and ambulatory surgical centers, although we sell through distributors in certain markets outside the US where we do not have local operations or a scientific office. In many countries, contact lenses are available only by prescription. Our contact lenses can be purchased from eye care professionals, optical chains and large retailers, subject to country regulation. Our ocular health products can be found in major drugstores, pharmacies, food stores and mass merchandising and optical retail chains globally, with access subject to country regulations, including free-sale, pharmacy-only and prescription regulations. No single customer accounted for more than 10% of our global sales in 2023.
Manufacturing, Quality and Supplies
Manufacturing
We generally organize our manufacturing facilities along product categories, with most plants being primarily dedicated to the manufacture of either our Surgical or Vision Care product offerings. As of December 2023, we employed approximately 4,300 people to manufacture surgical products at nine facilities in the US, Belgium, Switzerland, Indonesia, Ireland and Germany and approximately 5,600 people to manufacture Vision Care products at eight facilities in the US, Germany, Singapore, Malaysia, and Ireland. Our functional division of plants reflects the unique differences in regulatory requirements governing the production of surgical medical devices as well as the different technical skills required of associates in these manufacturing environments. Except for our manufacturing site in Athlone, Ireland, which was acquired in late 2022, all of our manufacturing plants are ISO 13485:2016 and ISO 14001:2015 certified. Currently, we manufacture approximately 90% of our products internally and rely on third-party manufacturers for a limited number of products.
The goal of our supply chain strategy is to efficiently produce and distribute high quality products. To that end, we employ cost-reduction programs, known as continuous improvement programs, involving activities such as cycle-time reductions, efficiency improvements, automation, plant consolidations and procurement savings programs as a means to reduce manufacturing and component costs. For example, in Vision Care, in an effort to reduce the cost per contact lens, we have implemented programs designed to reduce the time it takes to ramp to peak production levels for the newly installed manufacturing lines. To comply with good manufacturing practices and to improve the skills of our associates, we train our direct labor manufacturing staff throughout the year. Our professional associates are trained in various aspects of management, regulatory and technical issues through a combination of in-house seminars, local university classes and trade meetings.
The manufacture of our products is complex, involves advanced technology and is heavily regulated by governmental health authorities around the world. Risks inherent to the medical device and pharmaceutical industries are part of our operations. If we or our third-party manufacturers fail to comply fully with regulations, there could be a product recall or other shutdown or disruption of our production activities. We have implemented a global manufacturing strategy to maximize business continuity in case of such events or other unforeseen catastrophic events.
Quality
Product quality and patient safety are vitally important for Alcon and our industry. Our customers and patients must always feel safe when using our products. Our Quality Management Systems group ("QMS") is responsible for establishing and maintaining a robust and compliant quality control system across Alcon. QMS regularly monitors industry trends, as well as global and regulatory changes, and adjusts our processes and procedures to adhere to current standards and best practices. In addition, our Quality Compliance group audits our internal processes and suppliers for compliance with approved processes and procedures.
Supplies
The components used in certain of our Surgical products, such as viscoelastics, and our ocular health products, such as our products for dry eye and pharmaceuticals, are sourced from facilities that meet the regulatory requirements of applicable health regulatory authorities. Because of the proprietary nature and complexity of the production of these components, a number of them are only available from a single or limited number of health regulatory authority-approved sources. The majority of active chemicals, biological raw materials and selected inactive chemicals used in our products are acquired pursuant to long term supply contracts. When we rely upon a sole source or limited sources of supply for certain components, we try to maintain a sufficient inventory consistent with prudent practice and production lead-times and to take other steps necessary to ensure our continued supply. The prices of our raw materials are generally stable; however, we continue to monitor established indices for key raw materials and negotiate any price impact with the supplier.
45
Human Capital Management
Alcon's culture is summarized in the Alcon Blueprint. The Alcon Blueprint includes Alcon's foundational principles and values and behaviors and serves as the bedrock for how we attract, develop and retain top talent. We seek diverse talent and perspectives that embody our values and contribute to our mission to help people to See Brilliantly. Our talent acquisition process encompasses all facets of sourcing, attracting, assessing, selecting and onboarding of new associates. Alcon focuses on the care and growth of associates through learning and development, performance feedback, career progression and a focus on associate engagement – all while ensuring competitive compensation and benefits. Our Chief Human Resources Officer, working with the Global Heads of Talent Acquisition, Talent Management, Total Rewards and Diversity and Inclusion, develops systems and processes to support Alcon's ability to attract and retain the best talent while fostering a culture of inclusion.
Intellectual Property
We strive to protect our investment in the research, development, manufacturing and marketing of our products through the use of patents, trademarks, copyrights, designs, trade secrets and other intellectual property. We own or have rights to a number of patents, trademarks, copyrights, designs, trade secrets and other intellectual property directly related and important to our businesses. As of December 31, 2023, we owned or had rights to approximately 2,300 patent families.
We believe that our patents are important to our business but that no single patent, or group of related patents, currently is of material importance in relation to our business as a whole. Our strategy is to develop patent portfolios for our research and development projects in order to obtain market exclusivity for the innovative features of our products in our major markets. The scope and duration of protection provided by a patent can vary significantly from country to country. However, even after the expiration of all patents covering a product, we may continue to derive commercial benefits from such product.
We routinely monitor the activities of our competitors and other third parties with respect to their use of our intellectual property. When appropriate, we will enforce our intellectual property rights to ensure that we are receiving the protections they afford us. Similarly, we will staunchly defend our right to develop and market products against unfounded claims of infringement by others. We will aggressively pursue or defend our position in the appropriate courts if the dispute cannot otherwise be promptly resolved.
In addition to our patents and pending patent applications in the US and selected non-US markets, we rely on proprietary know-how and trade secrets in our businesses and work to ensure the confidentiality of this information, including through the use of confidentiality agreements with associates and third parties. In some instances, we also acquire, or obtain licenses to, intellectual property rights that are important to our businesses from third parties.
All of our major products are sold under trademarks that we consider in the aggregate to be important to our businesses as a whole. We consider trademark protection to be particularly important in the protection of our investment in the sales and marketing of our vision care and contact lens and ocular health products. The scope and duration of trademark protection varies widely throughout the world.
We also rely on design and copyright protection in various jurisdictions to protect the physical appearance of our surgical and diagnostic equipment and the software and printed materials our business relies upon, including software used in our surgical and diagnostic equipment. The scope and duration of design and copyright protection for these materials also varies widely throughout the world.
Competition
The eye care industry is highly competitive and subject to rapid technological change and evolving industry requirements and standards. We compete with a number of different companies across our two business segments—Surgical and Vision Care. Companies within our industry compete on technological leadership and innovation, quality and efficacy of their products, relationships with eye care professionals and healthcare providers, breadth and depth of product offerings and pricing. The presence of these factors varies across our Surgical and Vision Care product offerings. Our principal competitors also sometimes form strategic alliances and enter into co-marketing agreements in an effort to better compete. We face strong local competitors in some markets, especially in developed markets, such as the US, Western Europe and Japan.
Surgical
The surgical market is highly competitive. Superior technology and product performance give rise to category leadership in the surgical market. Service and long term relationships are also key factors in this competitive environment. Surgeons
46
rely on the quality, convenience, value and efficiency of a product and the availability and quality of technical service. We primarily compete with Carl Zeiss Meditec AG, Bausch & Lomb Incorporated, Hoya Corporation, Glaukos Corporation and Johnson & Johnson in the surgical market.
We expect to compete against companies that offer alternative surgical treatment methodologies, including multifocal, tunable and accommodating ATIOL approaches, and companies that promote alternative approaches for responding to the conditions our products address. At any time, our known competitors and other potential market entrants may develop new devices or treatment alternatives that may compete directly with our products. In addition, they may gain a market advantage by developing and patenting competitive products or processes earlier than we can or by obtaining regulatory approvals / clearances or market registrations more rapidly than we can.
We believe that the principal competitive factors in our surgical market include:
•disruptive product technology;
•alternative treatment modalities;
•breadth of product lines and product services;
•ability to identify new market trends;
•acceptance by ophthalmic surgeons;
•customer service and clinical support;
•regulatory status and speed to market;
•price;
•product quality, reliability and performance;
•capacity to recruit engineers, scientists and other qualified associates;
•digital initiatives that change business models;
•reimbursement approval from governmental payors and private healthcare insurance providers; and
•reputation for technical leadership.
Shifts in industry market share can occur in connection with product issues, physician advisories, safety alerts and publications about our products. In the current environment of managed care, with consolidation among healthcare providers, increased competition and declining reimbursement rates, there is also increasing pressure on price.
Vision Care
The vision care market is also highly competitive, and our primary competitors are Johnson & Johnson, Bausch & Lomb Incorporated and The Cooper Companies, Inc. In addition, AbbVie, Inc. (Allergan) is a competitor in ocular health.
We believe our DAILIES TOTAL1 provides the most advanced daily disposable SiHy contact lens with its advanced "water gradient" technology and PRECISION1 provides a mainstream daily disposable SiHy lens with aqueous extraction and surface treatment. While daily disposable contact lenses remain appealing to many lens wearers, approximately two-thirds of contact lens wearers globally choose reusable lenses. Despite this preference, innovation within the reusable lens segment has lagged behind daily disposable lenses over the past 10 years. TOTAL30 and PRECSION7 product families are designed to change that by delivering a premium offering within the monthly and 1-week reusable space, respectively. We also compete with manufacturers of eyeglasses and with surgical procedures that correct visual defects. We believe that there are opportunities for contact lenses to attract new customers in the markets in which we operate, particularly in markets where the penetration of contact lenses in the vision correction market is low. Additionally, we compete with new market entrants with disruptive distribution models that could potentially innovate to challenge traditional models, including the eye care professional channel in which we have a significant presence. We also believe that laser vision correction is not a significant threat to our sales of contact lenses based on the growth of the contact lens market over the past decade and our involvement in the laser vision correction market through our Surgical business.
In ocular health, the market is characterized by competition for market share through the introduction of products that provide superior effectiveness and reduced burden for treating eye conditions. Recommendations from eye care professionals and customer brand loyalty, as well as our product quality and price, are key factors in maintaining market share in these products.
47
Government Regulation
Overview
Our businesses are subject to varying degrees of governmental regulation in the countries in which we operate, and the general trend is toward increasingly stringent regulation. In the US, the drug, device and dietary supplement industries have long been subject to regulation by various federal and state agencies, primarily as to product safety, efficacy, manufacturing, advertising, labeling and safety reporting. The exercise of broad regulatory powers by the FDA continues to result in increases in the amounts of testing and documentation required for the commercialization of regulated products and a corresponding increase in the expense of product introduction. Similar trends are also evident in the EU and in other markets throughout the world. In addition to market access regulation, our businesses are also subject to other forms of regulation, such as those relating to anti-bribery, data privacy and cybersecurity, social impact and sustainability and trade regulation matters. We are also subject to regulations related to environmental and safety matters, which are discussed in greater detail in "Item 4.D. Property, Plants and Equipment—Environmental Matters".
Product Approval and Monitoring
Most of our products are regulated as medical devices in the US and the EU. These jurisdictions each use a risk-based classification system to determine the type of information that must be provided to the local regulatory bodies in order to obtain the right to market a product. In the US, the FDA classifies devices into three classes: Class I (low risk), Class II (moderate risk) and Class III (high risk). Many of our devices are Class II or III devices that require premarket review by the FDA. The primary pathway for our Class II devices is FDA clearance of a premarket notification under section 510(k) of the FDCA. With a 510(k) submission, the manufacturer must submit a notification to the FDA that includes performance data that establish that the product is substantially equivalent to a "predicate device", which is typically another Class II previously-cleared device. Our Class III devices require FDA approval of a Premarket Approval application. With a Premarket Approval application, the manufacturer must submit extensive supporting evidence, including clinical data, sufficient to demonstrate a reasonable assurance that the device is safe and effective for its intended use.
In the EU, CE marking is required for all medical devices sold. Prior to affixing the CE Mark, the manufacturer must demonstrate that their device conforms to the relevant essential requirements of the EU's Medical Device Directive through a conformity assessment procedure. The nature of the assessment depends upon the classification of the device. The method of assessing conformity varies depending on the type and classification of the product. For most Class I devices, the assessment is a self-certification process by the manufacturer. For all other devices, the conformity assessment procedure requires review by a "notified body", which is authorized or licensed to perform conformity assessments by national device regulatory authorities. The conformity assessment procedures require a technical review of the manufacturer's product and an assessment of relevant clinical data. Notified bodies may also perform audits of the manufacturer's quality system. If satisfied that the product conforms to the relevant essential requirements, the notified body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity and application of the CE mark.
The EU published a new Medical Device Regulation, or EU MDR, in 2017, which imposes significant additional requirements on medical device manufacturers, including with respect to clinical evaluation, labeling, technical documentation and quality management systems. Medical devices placed on the market in the EU after May 2021 require certification according to these new requirements, except those legacy devices with valid CE certificates, issued pursuant to the Medical Device Directives before May 2020, which can be placed on the market until those certificates expire, at the latest in May 2024, provided there are no significant changes in the design or intended purpose of the device. In March 2023, the European Parliament officially approved and published the European Commission's proposal to extend the date of compliance out by three to four years depending on the class of medical device, which now extends the date of compliance from 2024 until 2027 or 2028, provided that the manufacturer of the legacy product has submitted a formal application for a conformity assessment by May 2024. This extension is intended to ensure that the various notified bodies have enough time to review legacy products for compliance with the new regulations. The additional requirements of the EU MDR legislation did not change; however the “sell off” date has been removed.
We also market products that are regulated in other product categories, including lasers, prescription and over-the-counter drug products, dietary supplements and medical foods. These products are also subject to extensive government regulation, which vary by jurisdiction. For example, in the US, our drug products must either be marketed in compliance with an applicable over-the-counter drug monograph or receive FDA approval of a New Drug Application. In the European Economic Area, our drug products must receive a marketing authorization from the competent regulatory authorities before they may be placed on the market. There are various application procedures available, depending on the type of product involved.
48
Clinical trials may be required to support the marketing of our drug or device products. In the US, clinical trials must be conducted in accordance with FDA requirements, including informed consent from study participants, and review and approval by an institutional review board ("IRB"), among other requirements. Additionally, FDA authorization of an Investigational Device Exemption ("IDE") application must be obtained for studies involving significant risk devices prior to commencing the studies. In the EU, clinical trials usually require the approval of an ethics review board and the prior notification to, or authorization of the study from, the regulatory authority in each country in which the trial will be conducted.
Regulations of the FDA and other regulatory agencies in and outside the US impose extensive manufacturing requirements as well as postmarket compliance and monitoring obligations on our business. The manufacture of our device, drug and dietary supplement products is subject to extensive and complex good manufacturing practice and quality system requirements, which govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, storage, handling and servicing of our products. We are also subject to requirements for product labeling and advertising, sampling, recordkeeping, reporting of adverse experiences and other information to identify potential problems with our marketed products, as well as recalls and field actions. We are also subject to periodic inspections for compliance with these requirements. We expect this regulatory environment will continue to require significant technical expertise and capital investment to ensure compliance.
Medical device, drug and dietary supplement manufacturers are also subject to taxes, as well as application, product, user, establishment and other fees.
Price Controls
The prices of our medical devices and drugs that require prescriptions or are reimbursed through payments to providers for services using our devices or drugs are subject to reimbursement programs and price control mechanisms that vary from country to country. Due to increasing political pressure and governmental budget constraints, we expect these programs and mechanisms to remain robust and to potentially even be strengthened or expanded. As a result, such programs and mechanisms could have a negative influence on the prices we are able to charge for our products, particularly those used in cataract, vitreoretinal and glaucoma surgeries.
Regulations Governing Reimbursement
In the US, patient access to our drug and device products that require a prescription or are included in provider service payments is determined in large part by the coverage and reimbursement policies of third-party health payors, including health insurers and government programs such as Medicare and Medicaid. Both government and commercial health insurers are increasingly focused on containing health care costs and have imposed, and are continuing to consider, additional measures that exert downward pressure on device and drug prices. For example, the US recently passed the Inflation Reduction Act, which makes significant changes to how drugs are covered and paid for under the Medicare program, including the creation of financial penalties for drugs whose prices rise faster than the rate of inflation, redesign of the Medicare Part D program to require manufacturers to bear more of the liability for certain drug benefits and the introduction of government price-setting for certain Medicare Part D drugs starting in 2026.
Outside the US, global trends toward cost-containment measures likewise may influence prices for our healthcare products in those countries. Adverse decisions relating to either coverage for our products or the amount of reimbursement for our products, could significantly reduce the demand for our products and the prices that our customers are willing to pay for them.
Health Care Fraud and Abuse; Anti-Bribery
We are subject to health care fraud and abuse and anti-bribery laws and regulations in the US and around the world, including state and federal anti-kickback, anti-self-referral and false claims laws in the US. In addition, the FCPA is increasingly used to prosecute relationships between US companies and healthcare providers outside of the US. These laws are complex and subject to evolving interpretation by government agencies and courts. For example, in the US, relationships between manufacturers of products paid for by federal and state healthcare programs and healthcare professionals are regulated by a series of federal and state laws and regulations, such as the Federal Anti-Kickback Statute (and similar US state laws), that restrict the types of permissible financial relationships with referral sources. In the US, the False Claims Act permits private litigants to pursue lawsuits that can trigger government investigations and result in substantial financial fines and penalties to the defendant, as well as payment of significant financial rewards to the successful private litigants. As discussed in "Item 4.B. Business Overview—Marketing and Sales", we engage in marketing activities targeted at healthcare professionals, which include among others the provision of training programs. If one or more of these activities were found to be in violation of fraud and abuse laws, anti-bribery laws and regulations or any
49
other law or governmental regulation, or there are changes to the interpretation of these laws, we could be subject to, among other things, civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations.
Data Privacy and Cybersecurity
We are subject to certain privacy laws and regulations that continue to evolve, including Swiss privacy laws, the EU's GDPR, the CCPA and the US's HIPAA with respect to some of our products, services an business operations. For example, the GDPR and HIPAA contain enhanced financial and other penalties for noncompliance. The US Federal Trade Commission and state Attorneys General are actively enforcing in the data privacy and cybersecurity space under existing consumer protection laws and new privacy laws such as the CCPA and FTC Breach Notification Rule. The FDA has also issued further guidance concerning cybersecurity for medical devices.
In addition, certain countries have issued or are considering data localization laws, which limit companies' ability to transfer protected data across country borders. Failure to comply with data privacy and cybersecurity laws and regulations can result in enforcement actions, including civil or criminal penalties.
Trade Regulation
The movement of products, services and investment across borders subject us to extensive trade regulations. A variety of laws and regulations in the countries in which we transact business apply to the sale, shipment and provision of goods, services and technology across borders. These laws and regulations govern, among other things, our import, export and other business activities. We are also subject to the risk that these laws and regulations could change in a way that would expose us to additional costs, penalties or liabilities. Some governments also impose economic sanctions against certain countries, persons or entities.
In addition to our need to comply with such regulations in connection with our direct activities, we also sell and provide goods, technology and services to agents, representatives and distributors who may export such items to customers and end-users. Failure by us or the third parties through which we do business to comply with applicable import, export control or economic sanctions laws and regulations may subject us to civil or criminal enforcement action and varying degrees of liability.
4.C. ORGANIZATIONAL STRUCTURE
Organizational Structure
See "Item 4.B. Business Overview” for additional information.
Significant Subsidiaries
See "Item 6.C. Board Practice” for additional information.
4.D. PROPERTY, PLANTS AND EQUIPMENT
Our corporate headquarters is located in Geneva, Switzerland. The principal office for our Swiss and international operations, which is also our registered office, is located in Fribourg, Switzerland, and the principal office for our US operations is located in Fort Worth, Texas.
We believe that our current manufacturing and production facilities have adequate capacity for our medium‑term needs. To ensure that we have sufficient manufacturing capacity to meet future production needs, we regularly review the capacity and utilization of our manufacturing facilities. The FDA and other regulatory agencies regulate the approval for use of manufacturing facilities for medical devices and pharmaceuticals, and compliance with these regulations requires a substantial amount of validation time prior to start‑up and approval. Accordingly, it is important to our business that we ensure we have sufficient manufacturing capacity to meet our future production needs.
50
Major Facilities
The following table sets forth our most significant production and research and development facilities:
| Location | Size of Site (in m2) | Major Activity | ||||||||||||
| Fort Worth, Texas | 315,200 | Production, research and development for Surgical and Vision Care businesses | ||||||||||||
| Grosswallstadt, Germany | 97,720 | Production, research and development for Vision Care business | ||||||||||||
| Johns Creek, Georgia | 84,825 | Production, research and development for Vision Care business | ||||||||||||
| Singapore | 69,000 | Production for Vision Care business | ||||||||||||
| Johor, Malaysia | 43,900 | Production for Vision Care business | ||||||||||||
| Irvine, California | 40,800 | Production, research and development for Surgical business | ||||||||||||
| Houston, Texas | 37,400 | Production for Surgical business | ||||||||||||
| Batam, Indonesia | 35,000 | Production for Surgical business | ||||||||||||
| Huntington, West Virginia | 32,980 | Production for Surgical business | ||||||||||||
| Sinking Spring, Pennsylvania | 21,800 | Production for Surgical business | ||||||||||||
| Cork, Ireland | 13,600 | Production for Surgical business | ||||||||||||
Erlangen/Pressath/Teltow, Germany | 10,700 | Production, research and development for Vision Care business | ||||||||||||
| Puurs, Belgium | 8,000 | Production for Surgical business | ||||||||||||
| Durham, North Carolina | 4,200 | Research and development for Vision Care business | ||||||||||||
| Schaffhausen, Switzerland | 4,100 | Production for Surgical business | ||||||||||||
| Athlone, Ireland | 3,410 | Production for Vision Care business | ||||||||||||
In August 2021, we launched an expansion project of our Grosswallstadt, Germany facility to add three additional contact lens production lines for an anticipated cost of $162 million. Through December 31, 2023, the total amount paid and committed was approximately $149 million. We expect to complete the project by early 2025.
In April 2021, we launched a further expansion of our Singapore facility to add four additional production lines for contact lenses. We expect to incur costs of $197 million. Through December 31, 2023, the total amount paid and committed was approximately $193 million. We expect to complete this project in 2024. We approved a further expansion in late 2021 to add three additional production lines and a new building for an expected cost of $280 million. Through December 31, 2023, the total amount paid and committed for this additional expansion was approximately $252 million. We expect to complete this expansion in 2026. In late 2023, we commenced an additional expansion to the Singapore facility to add three additional production lines for an expected cost of $156 million. Through December 31, 2023, the total amount paid and committed for this additional expansion was approximately $28 million. We expect to complete this project in 2028.
In September 2019, we launched an expansion of our Johns Creek, Georgia facility to add four production lines for contact lenses. This project was expanded in 2020 and was completed in 2023. Through December 31, 2023, the total amount paid and committed was approximately $240 million. In 2021, we launched an additional expansion to add two more production lines for contact lenses for $148 million. Through December 31, 2023, the total amount paid and committed was approximately $133 million. We expect to complete the project by early 2025. Also, in late 2021, we approved an additional expansion to add one more production line for contact lenses. This additional expansion is expected to cost approximately $73 million and be completed by 2026. Through December 31, 2023, the total amount paid and committed was approximately $54 million.
We funded each of the projects discussed above from working capital.
Environmental Matters
At Alcon, we believe that excellent environmental performance enables us to achieve our purpose of helping people See Brilliantly. We integrate core values of environmental protection into our business strategy to protect the environment, to add value to the business, manage risk and enhance our reputation.
We are committed to reducing the environmental impact of our operations, products and services. We strive to minimize waste and emissions, reuse and recycle materials and conserve natural resources, such as energy and water, across our value chain.
51
We are subject to laws and regulations concerning the environment, safety matters and regulation of chemicals in the countries where we manufacture and sell our products or otherwise operate our business. As a result, we have established internal policies and standards that aid our operations in systematically identifying relevant hazards, assessing and mitigating risks and communicating risk information. These internal policies and standards are in place to ensure our operations comply with relevant environmental, health and safety laws and regulations and that periodic audits of our operations are conducted. The potential risks we identify are integrated into our business planning, including investments in reducing safety and health risks to our associates and reducing our impact on the environment. We have also dedicated resources to monitor legislative and regulatory developments and emerging issues to anticipate future requirements and undertake policy advocacy when strategically relevant.
Each year, we publish on our website a Social Impact and Sustainability Report that provides additional details regarding our environmental sustainability strategy and highlights the steps we plan to undertake.
ITEM 4A. UNRESOLVED STAFF COMMENTS
None.
52
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
5.A. OPERATING RESULTS
This operating and financial review and prospects should be read together with the section captioned "Item 4. Information on the Company—4.B. Business Overview" and our Consolidated Financial Statements and the related notes to those financial statements. Among other things, those financial statements include more detailed information regarding the basis of preparation for the following information. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under "Item 3. Key Information —3.D. Risk Factors" and elsewhere in this Annual Report, Alcon's actual results may differ materially from those anticipated in these forward-looking statements. Please see "Special Note About Forward-Looking Statements."
“Item 5. Operating and Financial Review and Prospects”, together with “Item 4.B. Business Overview” and “Item 6.D. Employees”, constitute the Operating and Financial Review (“Rapport annuel”), as defined by the Swiss Code of Obligations.
Overview
Alcon researches, develops, manufactures, distributes and sells a full suite of eye care products within two segments: Surgical and Vision Care. The Surgical segment is focused on ophthalmic products for cataract surgery, vitreoretinal surgery, refractive laser surgery and glaucoma surgery, and includes implantables, consumables and surgical equipment required for these procedures. The Vision Care segment comprises daily disposable, reusable and color-enhancing contact lenses and a comprehensive portfolio of ocular health products, including products for dry eye, glaucoma, contact lens care and ocular allergies, as well as ocular vitamins and redness relievers.
We are dedicated to providing innovative products that enhance quality of life by helping people see better. Our strong foundation is based on our longstanding success as a trusted brand, our legacy of industry firsts and advancements, our leading positions in the markets in which we compete and our continued commitment to substantial investment in innovation. With more than 75 years of history in the ophthalmic industry, we believe the Alcon brand name is synonymous with innovation, quality, service and leadership among eye care professionals worldwide. We employ over 25,000 associates operating in 56 countries and serving consumers and patients in over 140 countries.
In 2023, Alcon's net sales to third parties amounted to $9.4 billion. The United States accounted for $4.3 billion, or 46%, of total net sales, Japan accounted for $0.6 billion, or 6%, of total net sales, China accounted for $0.5 billion or 6%, of total net sales, Switzerland accounted for $64 million, or 1%, of total net sales, and the rest of the world accounted for the remaining $3.9 billion of total net sales.
Basis of preparation
The Consolidated Financial Statements, which present our financial position, results of operations, comprehensive income and cash flows have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB") (“IFRS Accounting Standards”).
The preparation of the Consolidated Financial Statements requires management to make certain estimates and assumptions, either at the balance sheet date or during the year, that affect the reported amounts of assets and liabilities as well as revenues and expenses. Actual outcomes and results could differ from those estimates and assumptions.
For further information on the basis of preparation of the Consolidated Financial Statements, see Note 2 to the Consolidated Financial Statements.
53
Items you should consider when evaluating our Consolidated Financial Statements
Israel-Hamas war
In October 2023, Hamas launched an attack on Israel which led to an immediate military response. A state of war has officially been declared by the government of Israel. Net sales and total assets in Israel are not a material portion of our business. It is possible the impact of the war could expand beyond Israel to other parts of the Middle East, or have further global impacts. Refer to "Item 3. Key Information—3.D. Risk Factors—Changing economic and financial environments in many countries and increasing global political and social instability may adversely impact our business."
War on Ukraine
Beginning in February 2022, as a result of the war on Ukraine by Russia, economic sanctions and export controls were imposed by much of the world on Russian financial institutions and businesses. These sanctions could adversely impact net sales, create disruptions in the global supply chain, increase the risk of cyberattacks, and potentially have an adverse impact on the global economy, financial markets, energy markets, currency rates and otherwise. As a result of the global impacts, we have experienced volatility in currency translation effects. Our manufacturing and procurement exposure in Russia and Ukraine is limited as our operations consist mainly of associates in local functions, including sales and customer support. Refer to "Item 3. Key Information—3.D. Risk Factors—Changing economic and financial environments in many countries and increasing global political and social instability may adversely impact our business."
For the years ended December 31, 2023 and 2022, net sales in Russia and Ukraine amounted to approximately 2% of consolidated net sales. Total assets in Russia and Ukraine amounted to $82 million as of December 31, 2023. As of December 31, 2023, operations previously impacted by the war on Ukraine continued operating to the extent practicable and permitted by law.
Supply chain inflationary pressures
We have experienced inflationary pressures primarily in labor, utilities and services, electronic components, freight, resins, plastics and other raw materials, which we have managed with price increases and productivity initiatives. However, we expect gross margin to be impacted in the coming quarters as we sell inventory which was manufactured at higher costs.
Pillar Two income taxes
The Organization for Economic Cooperation and Development (“OECD") has published Global Anti-Base Erosion (“GloBE”) Model Rules, which include a minimum 15% tax rate by jurisdiction ("Pillar Two"). Various countries have enacted or intend to enact tax legislation to comply with Pillar Two rules. Alcon is within the scope of the OECD’s Pillar Two, which did not impact 2023 results but will impact Alcon’s financial results from January 1, 2024 onward.
Of the countries enacting Pillar Two legislation, we expect Switzerland to be the most impactful to Alcon. In December 2023, the Swiss government decided to partially implement Pillar Two by introducing a Qualified Domestic Minimum Top-up Tax (“QDMTT”) to reach the required taxation level of 15% on Pillar Two qualifying profits earned by companies domiciled in Switzerland effective from January 1, 2024. This QDMTT will not be applied to the Pillar Two qualifying profits earned by subsidiaries domiciled in tax jurisdictions outside of Switzerland. The implementation timing and specific provisions of any further Pillar Two tax regulations in Switzerland remain subject to further assessments at both the Federal and Cantonal levels. For the period ended December 31, 2023, we have applied the IASB amendment to IAS 12, Income Taxes, which provides a mandatory temporary exception from recognizing or disclosing deferred taxes related to Pillar Two such that there is no impact to the 2023 Consolidated Financial Statements. The future impact of Pillar Two for Alcon is estimated to increase Alcon's effective tax rate by approximately 1% to 2%. We are continuing to follow Pillar Two legislative developments to evaluate the potential future impact on our consolidated results of operations, financial position and cash flows beginning in 2024.
Estimation uncertainty
The preparation of Consolidated Financial Statements requires management to make certain estimates and assumptions, either at the balance sheet date or during the year that affect the reported amounts of assets and liabilities as well as revenues and expenses. Because of the inherent uncertainties, actual outcomes and results may differ from management's assumptions and estimates. See Note 2 to the Consolidated Financial Statements and the "Critical accounting policies and estimates" section within this Item 5.A.
54
Segment description
Alcon has two identified reportable segments: Surgical and Vision Care. Both segments are supported by Research and Development and Manufacturing and Technical Operations, whose results are incorporated into the respective segment contribution. Segment contribution excludes amortization and impairment charges for acquired product rights or other intangibles, general and administrative expenses for corporate activities, separation costs, transformation costs, fair value adjustments to contingent consideration liabilities, past service costs primarily for post-employment benefit plan amendments, acquisition and integration related costs, certain acquisition related items and certain other income and expense items. See Note 4 to the Consolidated Financial Statements.
In Surgical, Alcon researches, develops, manufactures, distributes and sells ophthalmic products for cataract surgery, vitreoretinal surgery, refractive laser surgery and glaucoma surgery. The surgical portfolio also includes implantables, consumables and surgical equipment required for these procedures and supports the end-to-end procedure needs of the ophthalmic surgeon. Alcon also provides services, training, education and technical support for the Surgical business. In 2023, the Surgical segment accounted for $5.3 billion, or 57%, of Alcon net sales to third parties, and contributed $1.5 billion, or 65%, of Alcon operating income (excluding unallocated income and expenses).
In Vision Care, Alcon researches, develops, manufactures, distributes and sells daily disposable, reusable, and color-enhancing contact lenses and a comprehensive portfolio of ocular health products, including products for dry eye, glaucoma, contact lens care and ocular allergies, as well as ocular vitamins and redness relievers. Alcon also provides services, training, education and technical support for the Vision Care business. In 2023, the Vision Care segment accounted for $4.1 billion, or 43%, of Alcon net sales to third parties, and contributed $777 million, or 35%, of Alcon operating income (excluding unallocated income and expenses).
Opportunity and risk summary
The surgical and vision care markets in which Alcon operates are large, dynamic and growing. As the world population grows and ages, the need for quality eye care is expanding and evolving. In addition, although it is estimated that 90% of all visual impairments are currently preventable, treatable or curable, we operate in markets that have substantial unmet medical and consumer needs. Our surgical and vision care products are targeted at addressing many of these unmet medical and consumer needs through products that are used in treating multiple ocular health conditions and offer leading eye care solutions for patients throughout their lives.
The surgical market in which we operate includes sales of implantables, consumables and surgical equipment, including associated technical, clinical and service support and training, and is projected to grow mid-single digits per year from 2023 to 2028. Growth drivers in the surgical market include: global growth of cataract and vitreoretinal procedures, driven by an aging population; higher uptake of premium patient-pay technologies; increased adoption of advanced technologies; and eye disease as a comorbidity linked to the global prevalence of diabetes.
The vision care market in which we operate is comprised of products designed for ocular care and consumer use, and is projected to grow mid-single digits per year from 2023 to 2028. Growth drivers in the vision care market include: better contact lens material, improved health and comfort and enhanced visual acuity; an expansion in the contact lens market driven by advancements in specialty lenses; a significant worldwide population who suffer from a combination of dry eye symptoms and signs as well as those who are at risk; global growth in primary open-angle glaucoma, driven by an aging population; growing access and consumption of vision care products in emerging markets; and increasing consumer access through the expansion of distribution models.
In each of our markets, we rely on our strong relationships with eye care professionals and consumers to attract and retain customers and expand the market. We believe we have made one of the largest commitments to research and development in the eye care market, which we expect to continue through internal innovation investments and identifying and executing on attractive acquisition, licensing and collaboration opportunities.
Alcon's future expectations are subject to various risks and uncertainties, including market dynamics in the surgical and vision care markets, general economic conditions, the pace of innovation in our industry, as well as successfully achieving our growth strategies and efficiency initiatives. These expectations were, in the view of management, prepared on a reasonable basis, reflect the best currently available estimates and judgments and present, to the best of management's knowledge and belief, the expected future financial performance of Alcon. However, this information is not fact and should not be relied upon as necessarily indicative of future results, and you are cautioned not to place undue reliance on the prospective financial information. There will likely be differences between Alcon expectations and the actual results and those differences could be material. Alcon's expectations may not be achieved and we do not undertake any obligation to release publicly the results of any future revisions we may make to our expectations. When considering Alcon's expectations, you should keep in mind the risk factors and other cautionary statements in "Item 3. Key Information—3.D Risk Factors" and "Special Note About Forward-Looking Statements."
55
Our financial results are affected to varying degrees by internal and external factors. For example, because of our heavy dependence on information technology systems, cybersecurity breaches or other disruptions of our information technology systems or our inability to comply with data privacy, identity protection or information security laws, particularly with the increased use of artificial intelligence, would significantly impact our business. Given our global operations, our compliance with anti-corruption laws is of heightened significance to our business. Litigation risk, including intellectual property and product liability lawsuits, and government investigations are additional risks our business faces.
The effect of a disruption in our global supply chain or important facilities, supply constraints, or an increase in the cost of energy would further impact our business. We also may be adversely affected by our inability to accurately forecast demand and manage our inventory levels and the changing buying patterns by our large distributor and retail customers. If we overestimate demand and produce too much of a particular product, we face a risk of inventory obsolescence. In addition, for certain materials, components and services, we rely on sole or limited sources of supply. Our customer relations could be negatively impacted by the loss of our significant suppliers or the inability of any such supplier to meet certain specifications or delivery schedules.
Further, our ability to manage social impact and sustainability matters to the satisfaction of everyone, some of which may have competing interests, may impact our results of operations. While we make significant efforts to attract and retain a high quality workforce, we may be unable to attract and retain qualified personnel. Our reliance on outsourcing key business functions adds additional risk.
Moreover, our ability to grow depends on the commercial success of our products and our ability to maintain our position in the highly competitive markets in which we operate. Our ability to grow also depends on the success of our research and development efforts and BD&L activities in bringing new products to market, as well as the commercial acceptance of our products. We have incurred debt that we must continue to service, and we may need additional financing in debt or equity.
Even if we protect our intellectual property to the fullest extent permitted by applicable law, competitors may market products that compete with our products. Increased pricing pressure in the healthcare industry in general as well as industry consolidation could also impact our ability to generate returns and invest for the future. Additionally, our products are subject to competition from lower priced versions of our products, and our industry continues to be challenged by the vulnerability of distribution channels to counterfeiting. Product recalls or voluntary market withdrawals in connection with defects or unanticipated use of our products could also have a material adverse effect upon our business.
Further, we strive to properly educate and train healthcare providers and rely on them to recommend our products to their patients and to other members of their organizations. Consumers in the eye health industry have a tendency not to switch products regularly and are repeat consumers, meaning that a physician's initial recommendation of our products, and a consumer's initial choice to use our products, have an impact on the success of our products.
Given our global presence, our operations and business results are also influenced and affected by the global economic and financial environment, including unpredictable political conditions and tax laws in various parts of the world. Additionally, a portion of our operations are conducted in emerging markets and are subject to risks and potential costs such as economic, political and social uncertainty, as well as relatively low average income levels and limited government reimbursement for the cost of healthcare products and services. Our operations and business results are also affected by the varying degrees of governmental regulation in the countries in which we operate, making the process of developing new products and obtaining necessary regulatory marketing authorization lengthy, expensive and uncertain. The manufacture of our products is also highly regulated. Any changes or new requirements related to the regulatory approval process or postmarket requirements applicable to our products in any jurisdiction could be costly and onerous. Finally, if any of our accounting estimates are inaccurate then our financial results would be adversely impacted.
For more details on these trends and how they could impact our results, see "Item 3. Key Information—3.D. Risk Factors".
56
Components of results of operations
Net sales to third parties
Revenue on the sale of Alcon products and services, which is recorded as "Net sales to third parties" in the Consolidated Income Statement, is recognized when a contractual promise to a customer (i.e., a performance obligation) has been fulfilled by transferring control over the promised goods and services to the customer, substantially all of which is at the point in time of shipment to or receipt of the products by the customer or when the services are performed. If contracts contain customer acceptance provisions, revenue would be recognized upon the satisfaction of acceptance criteria. The amount of revenue to be recognized is based on the consideration Alcon expects to receive in exchange for its goods and services, which may be fixed or variable. Variable consideration may include rebates, discounts including cash discounts, chargebacks, estimated payments for Medicare Part D prescription drug program coverage gap, patient co-pay program coupon utilization and sales returns. Variable consideration is only recognized when it is highly probable that a significant reversal of cumulative sales will not occur.
Surgical equipment may be sold together with other products and services under a single contract. The total consideration is allocated to the separate performance obligations based on the relative stand-alone selling price for each performance obligation. Revenue is recognized upon satisfaction of each performance obligation under the contract.
Other revenues
"Other revenues" mainly include revenue from contract manufacturing services which are recognized over time as the service obligations are completed. Associated costs incurred are recognized in "Cost of other revenues".
Inventories
Inventory is valued at the lower of acquisition or production cost determined on a first-in, first-out basis and net realizable value. This value is used for the "Cost of net sales" and "Cost of other revenues" in the Consolidated Income Statement. Unsalable inventory is fully written off in the Consolidated Income Statement under "Cost of net sales" and "Cost of other revenues".
Research & development
Internal research and development costs are fully charged to "Research & development" in the Consolidated Income Statement in the period in which they are incurred. Alcon considers that regulatory and other uncertainties inherent in the development of new products preclude the capitalization of internal development expenses as an intangible asset until marketing approval from a regulatory authority is obtained in relevant major markets, such as the United States, the European Union, Switzerland, China or Japan.
Critical accounting policies and estimates
Selected accounting policies are set out in Note 2 to the Consolidated Financial Statements, which are prepared in accordance with IFRS as issued by the IASB.
Given the uncertainties inherent in our business activities, we must make certain estimates and assumptions that require difficult, subjective and complex judgments. Because of uncertainties inherent in such judgments, actual outcomes and results may differ from our assumptions and estimates, which could materially affect our Consolidated Financial Statements.
Application of the following accounting policies requires certain assumptions and estimates that have the potential for the most significant impact on the Consolidated Financial Statements.
Impairment of goodwill and intangible assets
We review long-lived intangible assets for impairment whenever events or changes in circumstance indicate that the carrying amount may not be recoverable. Goodwill, the Alcon brand name and intangible assets not yet ready for use are not amortized but are reviewed for impairment at least annually. Our annual impairment testing date is Alcon's year-end, December 31.
A cash generating unit to which goodwill has been allocated (reportable segments) is considered impaired when its carrying amount, including the goodwill, exceeds its recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. If the recoverable amount of the reportable segment is less than its carrying amount, an impairment loss shall be recognized.
57
An intangible asset other than goodwill is considered impaired when its balance sheet carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. Usually, Alcon applies the fair value less costs of disposal method for its impairment assessment. In most cases, no direct or indirect observable market prices for identical or similar assets are available to measure the fair value less costs of disposal. Therefore, an estimate of fair value less costs of disposal is based on net present value techniques utilizing post-tax cash flows and discount rates. In the limited cases where the value in use method would be applied, net present value techniques would be applied using pre-tax cash flows and discount rates.
Fair value less costs of disposal reflects estimates of assumptions that market participants would be expected to use when pricing the asset or cash generating units, and for this purpose management considers the range of economic conditions that are expected to exist over the remaining useful life of the asset. The estimates used in calculating net present values involve significant judgment by management and include assumptions with measurement uncertainty, such as the following:
•Amount and timing of projected cash flows;
•Long-term sales forecasts, including sales growth rates;
•Timing and probability of regulatory and commercial success;
•Royalty rate for the Alcon brand name;
•Terminal growth rate; and
•Discount rate.
Generally, for intangible assets with a definite useful life Alcon uses cash flow projections for the whole useful life of these assets. For goodwill and the Alcon brand name, Alcon generally utilizes cash flow projections for a five-year period based on management forecasts, with a terminal value based on cash flow projections considering the long-term expected growth rates and impact of demographic trends of the population to which Alcon products are prescribed, for later periods. Probability-weighted scenarios are typically used.
Discount rates used consider Alcon estimated weighted average cost of capital adjusted for specific country and currency risks associated with cash flow projections to approximate the weighted average cost of capital of a comparable market participant.
Due to the above factors and those further described in the "Opportunity and risk summary" section above, actual cash flows and values could vary significantly from forecasted future cash flows and related values derived using net present value techniques.
For additional information on intangible assets and impairment charges recognized, see Note 9 to the Consolidated Financial Statements.
Goodwill and other intangible assets represent a significant part of our Consolidated Balance Sheet, primarily due to acquisitions. Although no significant additional impairments are currently anticipated, impairment evaluation could lead to material impairment charges in the future.
Business combinations
The acquisition method of accounting is used to account for all business combinations, regardless of whether equity instruments or other assets are acquired. Identifiable assets acquired and liabilities assumed in a business combination are measured initially at their fair values at the acquisition date. The excess of the consideration transferred over the fair value of the net identifiable assets acquired is recorded as goodwill, or directly in the income statement if it is a bargain purchase. Alcon primarily uses net present value techniques, utilizing post-tax cash flows and discount rates in estimating the fair value of identifiable assets acquired when allocating the purchase consideration paid for the acquisition. The estimates of the fair values involve significant judgment by management and include assumptions with measurement uncertainty, such as the following:
•Amount and timing of projected cash flows;
•Long-term sales forecasts;
•Timing and probability of regulatory and commercial success; and
•Discount rate.
Alcon may elect on a transaction-by-transaction basis to apply the optional concentration test to assess whether a transaction qualifies as a business. Under the test, when substantially all of the fair value of the gross assets acquired is
58
concentrated in a single identifiable asset or a group of similar identifiable assets, Alcon will account for the transaction as an asset purchase and not a business combination.
If the concentration test is not met, or Alcon elects not to apply this optional test, Alcon will perform an assessment focusing on the existence of inputs and processes that have the ability to create outputs to determine whether the transaction is an asset purchase or a business combination.
Contingent consideration
In a business combination, it is often necessary to recognize contingent future payments to previous owners, representing contractually defined potential amounts as a liability. Usually for Alcon these are linked to development or commercial milestones related to certain assets and are recognized as a financial liability at their fair value, which is then re-measured at each subsequent reporting date.
For the determination of the fair value of contingent consideration, various unobservable inputs are used. A change in these inputs might result in a significantly higher or lower fair value measurement. The inputs used are, among others, the timing and probability of regulatory and commercial success, sales forecast and assumptions regarding the discount rate, timing and different scenarios of triggering events. The significance and usage of these inputs to each contingent consideration may vary due to differences in the timing and triggering events for payments or in the nature of the asset related to the contingent consideration. These estimations typically depend on factors such as technical milestones or market performance and are adjusted for the probability of their likelihood of payment, and if material, are appropriately discounted to reflect the impact of time.
Changes in the fair value of contingent consideration liabilities in subsequent periods are recognized in the Consolidated Income Statement in "Cost of net sales" for currently marketed products and in "Research & development" for in-process research & development.
The effect of unwinding the discount over time is recognized in "Interest expense" in the Consolidated Income Statement.
Alcon accounts for variable or contingent consideration associated with asset acquisitions using the cost accumulation model. At the date of the asset acquisition, the intangible asset is initially recognized at the amount paid. Variable payments are subsequently capitalized as part of the cost of the asset when paid, on the basis that such payments represent the direct cost of acquisition.
Taxes
The estimated amounts for current and deferred tax assets or liabilities, including any amounts related to any uncertain tax positions, are based on currently known facts and circumstances. Tax returns are based on an interpretation of tax laws and regulations and reflect estimates based on these judgments and interpretations. The tax returns are subject to examination by the competent taxing authorities which may result in an assessment being made requiring payments of additional tax, interest or penalties. Inherent uncertainties exist in the estimates of the tax positions.
Research & development
Internal research & development costs are fully charged to "Research & development" in the Consolidated Income Statement in the period in which they are incurred. Alcon considers that regulatory and other uncertainties inherent in the development of new products preclude the capitalization of internal development expenses as an intangible asset until marketing approval from the regulatory authority is obtained in a relevant major market, such as the United States, the European Union, Switzerland, China or Japan.
Factors affecting comparability of period to period results of operations
The comparability of the period to period results of our operations can be impacted by significant transactions. Refer to Note 3 to the Consolidated Financial Statements for details related to significant transactions for the periods presented in the Consolidated Financial Statements.
59
Results of operations
In evaluating our performance, we consider not only the IFRS results, but also certain non-IFRS measures, including various "core" results and constant currency ("cc") results. These measures assist us in evaluating our ongoing performance from period to period and we believe this additional information is useful to investors in understanding the performance of our business. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information and reconciliation tables. These measures are not intended to be substitutes for the equivalent measures of financial performance prepared in accordance with IFRS and may differ from similarly titled non-IFRS measures of other companies.
Key figures
2023 compared to 2022 | 2022 compared to 2021 | ||||||||||||||||||||||||||||||||||||||||
| Change % | Change % | ||||||||||||||||||||||||||||||||||||||||
| ($ millions unless indicated otherwise) | 2023 | 2022 | $ | cc(1) (non-IFRS measure) | 2021 | $ | cc(1) (non-IFRS measure) | ||||||||||||||||||||||||||||||||||
| Net sales to third parties | 9,370 | 8,654 | 8 | 10 | 8,222 | 5 | 11 | ||||||||||||||||||||||||||||||||||
| Gross profit | 5,247 | 4,748 | 11 | 14 | 4,652 | 2 | 10 | ||||||||||||||||||||||||||||||||||
| Operating income | 1,039 | 672 | 55 | 77 | 580 | 16 | 59 | ||||||||||||||||||||||||||||||||||
| Operating margin (%) | 11.1 | 7.8 | 7.1 | ||||||||||||||||||||||||||||||||||||||
| Net income | 974 | 335 | 191 | 243 | 376 | (11) | 37 | ||||||||||||||||||||||||||||||||||
| Basic earnings per share ($) | 1.98 | 0.68 | 191 | 242 | 0.77 | (12) | 36 | ||||||||||||||||||||||||||||||||||
| Diluted earnings per share ($) | 1.96 | 0.68 | 188 | 241 | 0.76 | (11) | 37 | ||||||||||||||||||||||||||||||||||
Core results (non-IFRS measure)(1) | |||||||||||||||||||||||||||||||||||||||||
| Core operating income | 1,849 | 1,571 | 18 | 27 | 1,443 | 9 | 26 | ||||||||||||||||||||||||||||||||||
| Core operating margin (%) | 19.7 | 18.2 | 17.6 | ||||||||||||||||||||||||||||||||||||||
| Core net income | 1,360 | 1,108 | 23 | 34 | 1,063 | 4 | 23 | ||||||||||||||||||||||||||||||||||
| Core basic earnings per share ($) | 2.76 | 2.25 | 23 | 34 | 2.17 | 4 | 23 | ||||||||||||||||||||||||||||||||||
| Core diluted earnings per share ($) | 2.74 | 2.24 | 22 | 33 | 2.15 | 4 | 23 | ||||||||||||||||||||||||||||||||||
(1)Core results and constant currencies (cc) as presented in this table are non-IFRS measures. Alcon uses certain non-IFRS metrics when measuring performance, including when measuring current period results against prior periods. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information and reconciliation tables.
60
Commentary for the year ended December 31, 2022 compared to 2021 may be found in Item 5 of the Company's Annual Report on Form 20-F filed with the Securities and Exchange Commission ("SEC") on February 27, 2023 ("2022 Form 20-F").
Net sales by segment
| 2023 compared to 2022 | 2022 compared to 2021 | ||||||||||||||||||||||||||||||||||||||||
| Change % | Change % | ||||||||||||||||||||||||||||||||||||||||
| ($ millions unless indicated otherwise) | 2023 | 2022 | $ | cc(1) (non-IFRS measure) | 2021 | $ | cc(1) (non-IFRS measure) | ||||||||||||||||||||||||||||||||||
| Surgical | |||||||||||||||||||||||||||||||||||||||||
| Implantables | 1,703 | 1,725 | (1) | 2 | 1,522 | 13 | 20 | ||||||||||||||||||||||||||||||||||
| Consumables | 2,719 | 2,499 | 9 | 11 | 2,388 | 5 | 10 | ||||||||||||||||||||||||||||||||||
| Equipment/other | 892 | 821 | 9 | 12 | 793 | 4 | 10 | ||||||||||||||||||||||||||||||||||
| Total Surgical | 5,314 | 5,045 | 5 | 8 | 4,703 | 7 | 13 | ||||||||||||||||||||||||||||||||||
| Vision Care | |||||||||||||||||||||||||||||||||||||||||
| Contact lenses | 2,400 | 2,192 | 9 | 11 | 2,139 | 2 | 9 | ||||||||||||||||||||||||||||||||||
| Ocular health | 1,656 | 1,417 | 17 | 19 | 1,380 | 3 | 7 | ||||||||||||||||||||||||||||||||||
| Total Vision Care | 4,056 | 3,609 | 12 | 14 | 3,519 | 3 | 8 | ||||||||||||||||||||||||||||||||||
| Net sales to third parties | 9,370 | 8,654 | 8 | 10 | 8,222 | 5 | 11 | ||||||||||||||||||||||||||||||||||
(1)Constant currencies is a non-IFRS measure. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information.
Surgical
Surgical net sales were $5.3 billion, an increase of 5%. Excluding unfavorable currency impacts of 3%, Surgical net sales increased 8% in constant currencies.
•Implantables net sales were $1.7 billion, a decrease of 1%. Implantables net sales increased 5% excluding negative impacts of 3% from currency and 3% from the impact of an insurance reimbursement change in South Korea that took effect April 1, 2022. Growth in constant currencies was driven by intraocular lenses in other international markets. Implantables net sales increased 2% in constant currencies.
•Consumables net sales were $2.7 billion, an increase of 9%, reflecting favorable market conditions across geographies and price increases. Growth was partially offset by unfavorable currency impacts of 2%. Consumables net sales increased 11% in constant currencies.
•Equipment/other net sales were $892 million, an increase of 9%, driven by strong demand for cataract and vitreoretinal equipment in international markets and higher service revenues. Growth was partially offset by unfavorable currency impacts of 3%. Equipment/other net sales increased 12% in constant currencies.
Vision Care
Vision Care net sales were $4.1 billion, an increase of 12%, including 4% from products acquired in 2022. Excluding unfavorable currency impacts of 2%, Vision Care net sales increased 14% in constant currencies.
•Contact lenses net sales were $2.4 billion, an increase of 9%, driven by product innovation, including sphere and toric product launches, and price increases. Growth was partially offset by declines in legacy lenses and unfavorable currency impacts of 2%. Contact lenses net sales increased 11% in constant currencies.
•Ocular health net sales were $1.7 billion, an increase of 17%, primarily driven by the portfolio of eye drops, including acquired ophthalmic pharmaceutical products, price increases and recovery from supply chain challenges in contact lens care. Growth was partially offset by unfavorable currency impacts of 2%. Ocular health net sales increased 19% in constant currencies, including 10% from products acquired in 2022.
61
Operating income
2023 compared to 2022 | 2022 compared to 2021 | ||||||||||||||||||||||||||||
| Change % | Change % | ||||||||||||||||||||||||||||
| ($ millions unless indicated otherwise) | 2023 | 2022 | $ | cc(1) (non-IFRS measure) | 2021 | $ | cc(1) (non-IFRS measure) | ||||||||||||||||||||||
| Gross profit | 5,247 | 4,748 | 11 | 14 | 4,652 | 2 | 10 | ||||||||||||||||||||||
| Selling, general & administration | (3,209) | (3,068) | (5) | (5) | (3,076) | — | (4) | ||||||||||||||||||||||
| Research & development | (828) | (702) | (18) | (18) | (842) | 17 | 15 | ||||||||||||||||||||||
| Other income | 80 | 36 | 122 | 122 | 43 | (16) | (15) | ||||||||||||||||||||||
| Other expense | (251) | (342) | 27 | 27 | (197) | (74) | (75) | ||||||||||||||||||||||
| Operating income | 1,039 | 672 | 55 | 77 | 580 | 16 | 59 | ||||||||||||||||||||||
| Operating margin (%) | 11.1 | 7.8 | 7.1 | ||||||||||||||||||||||||||
Core results (non-IFRS measure)(1) | |||||||||||||||||||||||||||||
| Core gross profit | 5,917 | 5,381 | 10 | 13 | 5,216 | 3 | 11 | ||||||||||||||||||||||
| Core operating income | 1,849 | 1,571 | 18 | 27 | 1,443 | 9 | 26 | ||||||||||||||||||||||
| Core operating margin (%) | 19.7 | 18.2 | 17.6 | ||||||||||||||||||||||||||
(1) Core results and constant currencies are non-IFRS measures. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information and reconciliation tables.
Operating income was $1.0 billion (+55%, +77% cc), compared to $672 million in the prior year period. Operating margin increased 3.3 percentage points, reflecting improved underlying operating leverage from higher sales and manufacturing efficiencies. The current year period also included a $58 million benefit from the release of a contingent liability related to a recent acquisition. The prior year period was impacted by $90 million of legal settlement costs and intangible asset impairments of $62 million. Operating margin benefits were partially offset by increased inflationary impacts, increased investment in research and development, including spend following the acquisition of Aerie, higher amortization for intangible assets due to recent acquisitions, a shift in product mix in Surgical, including the impact from South Korea, and a negative 1.4 percentage point impact from currency. Operating margin increased 4.7 percentage points on a constant currencies basis.
Adjustments to arrive at core operating income in the current year period were $810 million, mainly due to $675 million of amortization, $139 million of transformation costs and $48 million of integration related expenses, partially offset by a $58 million benefit from the release of a contingent liability related to a recent acquisition. Adjustments to arrive at core operating income in the prior year period were $899 million, mainly due to $588 million of amortization, $119 million of transformation costs, $90 million of legal settlement costs, $64 million of acquisition and integration related expenses and $62 million in impairments of intangible assets.
Core operating income was $1.8 billion (+18%, +27% cc), compared to $1.6 billion in the prior year period. Core operating margin increased 1.5 percentage points, reflecting improved underlying operating leverage from higher sales and manufacturing efficiencies. Core operating margin benefits were partially offset by increased inflationary impacts, increased investment in research and development, including spend following the acquisition of Aerie, a shift in product mix in Surgical, including the impact from South Korea, and a negative 1.3 percentage point impact from currency. Core operating margin increased 2.8 percentage points on a constant currencies basis.
62
Segment contribution
For additional information regarding segment contribution, please refer to Note 4 to the Consolidated Financial Statements.
| 2023 compared to 2022 | 2022 compared to 2021 | ||||||||||||||||||||||||||||
| ($ millions unless indicated otherwise) | Change % | Change % | |||||||||||||||||||||||||||
| 2023 | 2022 | $ | cc(1) (non-IFRS measure) | 2021 | $ | cc(1) (non-IFRS measure) | |||||||||||||||||||||||
| Surgical segment contribution | 1,454 | 1,336 | 9 | 17 | 1,184 | 13 | 26 | ||||||||||||||||||||||
| As % of net sales | 27.4 | 26.5 | 25.2 | ||||||||||||||||||||||||||
| Vision Care segment contribution | 777 | 600 | 30 | 37 | 604 | (1) | 15 | ||||||||||||||||||||||
| As % of net sales | 19.2 | 16.6 | 17.2 | ||||||||||||||||||||||||||
| Not allocated to segments | (1,192) | (1,264) | 6 | 6 | (1,208) | (5) | (5) | ||||||||||||||||||||||
| Operating income | 1,039 | 672 | 55 | 77 | 580 | 16 | 59 | ||||||||||||||||||||||
Core adjustments (non-IFRS measure)(1) | 810 | 899 | 863 | ||||||||||||||||||||||||||
Core operating income (non-IFRS measure)(1) | 1,849 | 1,571 | 18 | 27 | 1,443 | 9 | 26 | ||||||||||||||||||||||
(1)Core results and constant currencies are non-IFRS measures. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information and reconciliation tables.
Surgical
Surgical segment contribution was $1.5 billion (+9%, +17% cc), compared to $1.3 billion in the prior year period. Segment contribution margin increased 0.9 percentage points, as improved underlying operating leverage from higher sales and manufacturing efficiencies was partially offset by a shift in product mix, including the impact from South Korea, and a negative 1.3 percentage point impact from currency. Segment contribution margin increased 2.2 percentage points on a constant currencies basis.
Vision Care
Vision Care segment contribution was $777 million (+30%, +37% cc), compared to $600 million in the prior year period. Segment contribution margin increased 2.6 percentage points, with improved underlying operating leverage from higher sales and manufacturing efficiencies, partially offset by increased investment in research and development following the acquisition of Aerie and increased inflationary impacts. Segment contribution margin benefits from higher margin ophthalmic pharmaceutical products following the acquisition of Aerie were offset by unfavorable product mix from launches of new silicone hydrogel daily contact lenses. There was also a negative 0.7 percentage point impact from currency. Segment contribution margin increased 3.3 percentage points on a constant currencies basis.
Not allocated to segments
Operating loss not allocated to segments totaled $1.2 billion (+6%, +6% cc), compared to $1.3 billion in the prior year period. The decrease in amounts not allocated was primarily driven by impairments of intangible assets and legal settlement costs in the prior year period. Additionally, the current year period included a $58 million benefit from the release of a contingent liability related to a recent acquisition, partially offset by higher amortization for intangible assets due to acquisitions and higher transformation costs.
63
Non-operating income & expense
| 2023 compared to 2022 | 2022 compared to 2021 | ||||||||||||||||||||||||||||
| Change % | Change % | ||||||||||||||||||||||||||||
| ($ millions unless indicated otherwise) | 2023 | 2022 | $ | cc(1) (non-IFRS measure) | 2021 | $ | cc(1) (non-IFRS measure) | ||||||||||||||||||||||
| Operating income | 1,039 | 672 | 55 | 77 | 580 | 16 | 59 | ||||||||||||||||||||||
| Interest expense | (189) | (134) | (41) | (41) | (120) | (12) | (13) | ||||||||||||||||||||||
| Other financial income & expense | (18) | (75) | 76 | 74 | (42) | (79) | (80) | ||||||||||||||||||||||
| Income before taxes | 832 | 463 | 80 | 112 | 418 | 11 | 70 | ||||||||||||||||||||||
| Taxes | 142 | (128) | nm | nm | (42) | nm | nm | ||||||||||||||||||||||
| Net income | 974 | 335 | 191 | 243 | 376 | (11) | 37 | ||||||||||||||||||||||
| Basic earnings per share ($) | 1.98 | 0.68 | 191 | 242 | 0.77 | (12) | 36 | ||||||||||||||||||||||
| Diluted earnings per share ($) | 1.96 | 0.68 | 188 | 241 | 0.76 | (11) | 37 | ||||||||||||||||||||||
Core results (non-IFRS measure)(1) | |||||||||||||||||||||||||||||
| Core taxes | (282) | (254) | (11) | (21) | (218) | (17) | (38) | ||||||||||||||||||||||
| Core net income | 1,360 | 1,108 | 23 | 34 | 1,063 | 4 | 23 | ||||||||||||||||||||||
| Core basic earnings per share ($) | 2.76 | 2.25 | 23 | 34 | 2.17 | 4 | 23 | ||||||||||||||||||||||
| Core diluted earnings per share ($) | 2.74 | 2.24 | 22 | 33 | 2.15 | 4 | 23 | ||||||||||||||||||||||
nm = not meaningful
(1)Core results and constant currencies are non-IFRS measures. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information and reconciliation tables.
Interest expense
Interest expense was $189 million, compared to $134 million in the prior year period. The current year period had increased financial debts following funding of the Aerie acquisition in November 2022 and less favorable interest rates.
Other financial income & expense
Other financial income & expense was a net expense of $18 million, compared to $75 million in the prior year period, primarily due to an increase in interest income and a reduction in foreign currency exchange losses.
Taxes
Tax benefit was $142 million, compared to a tax expense of $128 million in the prior year period. The current year tax was primarily driven by a $263 million discrete tax benefit associated with a long-term agreement with Swiss tax authorities in the fourth quarter of 2023 for the deductibility of a statutory expense in Switzerland (the "2023 Swiss Tax Agreement") and a net benefit of $36 million from other discrete tax items. The prior year expense was primarily driven by the recognition of tax expense for an Advanced Pricing Agreement between Swiss and US tax authorities related to fiscal years 2019 through 2022 (the "2022 APA"), partially offset by favorable discrete tax items.
Adjustments to arrive at core tax expense in the current year period were $424 million, primarily due to $263 million for the 2023 Swiss Tax Agreement and $155 million for the tax effect associated with operating income core adjustments. Adjustments to arrive at core tax expense in the prior year period were $126 million for the tax effect associated with operating income core adjustments, partially offset by core tax adjustments for discrete tax impacts for the 2022 APA for the fiscal years 2019 through 2021.
Core tax expense was $282 million, compared to $254 million in the prior year period. The average core tax rate was 17.2% compared to 18.6% in the prior year period. The current year average core tax rate benefited from the mix of pre-tax income/(loss) across geographical jurisdictions and discrete tax benefits. The prior year average core tax rate was impacted by the recognition of tax expense for the 2022 APA related to fiscal year 2022.
64
Net income and earnings per share
Net income was $974 million, compared to $335 million in the prior year period, primarily due to higher operating income, the tax benefit in the current year period compared to tax expense in the prior year period and lower other financial income & expense, partially offset by higher interest expense. The associated basic and diluted earnings per share were $1.98 and $1.96, respectively, compared to basic and diluted earnings per share of $0.68 in the prior year period.
Core net income was $1.4 billion, compared to $1.1 billion in the prior year period, primarily due to higher core operating income and lower other financial income & expense, partially offset by increases in interest expense and core tax expense. In addition, the current year reflects unfavorable currency impacts. The associated core basic and diluted earnings per share were $2.76 and $2.74, respectively, compared to $2.25 and $2.24, respectively, in the prior year period.
Effects of currency fluctuations
We prepare our Consolidated Financial Statements in US dollars. As a result, fluctuations in the exchange rates between the US dollar and other currencies can have a significant effect on our results of operations as well as on the reported value of our assets, liabilities and cash flows. This in turn may significantly affect reported earnings (both positively and negatively) and the comparability of period-to-period results of operations.
For purposes of our Consolidated Balance Sheet, we translate assets and liabilities denominated in other currencies into US dollars at the prevailing market exchange rates as of the relevant balance sheet date. For purposes of our Consolidated Income Statement and Consolidated Statement of Cash Flows, revenue, expense and cash flow items in local currencies are translated into US dollars at average exchange rates prevailing during the relevant period. As a result, even if the amounts or values of these items remain unchanged in the respective local currency, changes in exchange rates have an impact on the amounts or values of these items in our Consolidated Financial Statements.
Alcon manages its global currency exposure by engaging in hedging transactions where management deems appropriate as described in "Item 5.B. Liquidity and Capital Resources". The impact of ongoing macroeconomic conditions is currently unknown and could have a material adverse effect on our results of operations, cash flows or financial condition.
There is also a risk that certain countries could devalue their currency. If this occurs, it could impact the effective prices we are able to charge for our products and it could adversely impact both our Consolidated Income Statement and Consolidated Balance Sheet. Alcon is exposed to a potential adverse devaluation risk on its intercompany funding and total investment in certain subsidiaries operating in countries with exchange controls.
The hyperinflationary economies in which we operate are Argentina, Turkey and Venezuela. Argentina and Venezuela were hyperinflationary for all years presented. Turkey became hyperinflationary effective April 1, 2022, requiring retroactive implementation from January 1, 2022 of hyperinflationary accounting. Refer to Note 2 to the Consolidated Financial Statements for additional information.
65
Foreign exchange rates for foreign currency translation
The following tables set forth the foreign exchange rates of the US dollar against key currencies used for foreign currency translation when preparing the Consolidated Financial Statements:
Average for year | As of December 31 | ||||||||||||||||||||||||||||||||||
| ($ per unit unless indicated otherwise) | 2023 | 2022 | Change % | 2023 | 2022 | Change % | |||||||||||||||||||||||||||||
| AUD | 0.664 | 0.693 | (4) | 0.683 | 0.678 | 1 | |||||||||||||||||||||||||||||
| BRL | 0.200 | 0.194 | 3 | 0.206 | 0.189 | 9 | |||||||||||||||||||||||||||||
| CAD | 0.741 | 0.768 | (4) | 0.755 | 0.738 | 2 | |||||||||||||||||||||||||||||
| CHF | 1.113 | 1.047 | 6 | 1.189 | 1.081 | 10 | |||||||||||||||||||||||||||||
| CNY | 0.141 | 0.149 | (5) | 0.141 | 0.144 | (2) | |||||||||||||||||||||||||||||
| EUR | 1.081 | 1.051 | 3 | 1.107 | 1.065 | 4 | |||||||||||||||||||||||||||||
| GBP | 1.243 | 1.232 | 1 | 1.275 | 1.207 | 6 | |||||||||||||||||||||||||||||
| INR (100) | 1.211 | 1.272 | (5) | 1.203 | 1.208 | — | |||||||||||||||||||||||||||||
| JPY (100) | 0.712 | 0.760 | (6) | 0.707 | 0.757 | (7) | |||||||||||||||||||||||||||||
| RUB (100) | 1.171 | 1.432 | (18) | 1.111 | 1.380 | (19) | |||||||||||||||||||||||||||||
| KRW (1,000) | 0.765 | 0.774 | (1) | 0.775 | 0.793 | (2) | |||||||||||||||||||||||||||||
Average for year | As of December 31 | ||||||||||||||||||||||||||||||||||
| ($ per unit unless indicated otherwise) | 2022 | 2021 | Change % | 2022 | 2021 | Change % | |||||||||||||||||||||||||||||
| AUD | 0.693 | 0.752 | (8) | 0.678 | 0.726 | (7) | |||||||||||||||||||||||||||||
| BRL | 0.194 | 0.186 | 4 | 0.189 | 0.180 | 5 | |||||||||||||||||||||||||||||
| CAD | 0.768 | 0.798 | (4) | 0.738 | 0.785 | (6) | |||||||||||||||||||||||||||||
| CHF | 1.047 | 1.094 | (4) | 1.081 | 1.093 | (1) | |||||||||||||||||||||||||||||
| CNY | 0.149 | 0.155 | (4) | 0.144 | 0.157 | (8) | |||||||||||||||||||||||||||||
| EUR | 1.051 | 1.183 | (11) | 1.065 | 1.130 | (6) | |||||||||||||||||||||||||||||
| GBP | 1.232 | 1.376 | (10) | 1.207 | 1.351 | (11) | |||||||||||||||||||||||||||||
| INR (100) | 1.272 | 1.353 | (6) | 1.208 | 1.347 | (10) | |||||||||||||||||||||||||||||
| JPY (100) | 0.760 | 0.912 | (17) | 0.757 | 0.868 | (13) | |||||||||||||||||||||||||||||
| RUB (100) | 1.432 | 1.358 | 5 | 1.380 | 1.336 | 3 | |||||||||||||||||||||||||||||
| KRW (1,000) | 0.774 | 0.874 | (11) | 0.793 | 0.840 | (6) | |||||||||||||||||||||||||||||
66
The following table shows information concerning the rate of exchange of US dollar per Swiss franc based on exchange rate information found on Bloomberg Market System. The exchange rate in effect on February 21, 2024 as found on Bloomberg Market System was CHF 1.00 = USD 1.14.
| ($ per CHF) | Low(1) | High(1) | |||||||||
| January 2023 | 1.08 | 1.09 | |||||||||
| February 2023 | 1.06 | 1.07 | |||||||||
| March 2023 | 1.09 | 1.10 | |||||||||
| April 2023 | 1.11 | 1.12 | |||||||||
| May 2023 | 1.09 | 1.11 | |||||||||
| June 2023 | 1.11 | 1.12 | |||||||||
| July 2023 | 1.15 | 1.15 | |||||||||
| August 2023 | 1.13 | 1.14 | |||||||||
| September 2023 | 1.09 | 1.10 | |||||||||
| October 2023 | 1.10 | 1.11 | |||||||||
| November 2023 | 1.14 | 1.15 | |||||||||
| December 2023 | 1.18 | 1.20 | |||||||||
| January 2024 | 1.16 | 1.17 | |||||||||
| February 2024 (through February 21, 2024) | 1.13 | 1.14 | |||||||||
(1) Represents the lowest and highest, respectively, of the exchange rates on the last day of each month during the year.
Currency impact on key figures
The following table provides a summary of the currency impact on key company figures due to their conversion into US dollars, Alcon's reporting currency, of the financial data from entities reporting in non-US dollars.
2023 compared to 2022 | 2022 compared to 2021 | ||||||||||||||||||||||||||||||||||
| Change % | Percentage point currency impact | Change % | Percentage point currency impact | ||||||||||||||||||||||||||||||||
| $ | cc(1) (non-IFRS measure) | $ | cc(1) (non-IFRS measure) | ||||||||||||||||||||||||||||||||
| Net sales to third parties | 8 | 10 | (2) | 5 | 11 | (6) | |||||||||||||||||||||||||||||
| Gross profit | 11 | 14 | (3) | 2 | 10 | (8) | |||||||||||||||||||||||||||||
| Operating income | 55 | 77 | (22) | 16 | 59 | (43) | |||||||||||||||||||||||||||||
| Net income | 191 | 243 | (52) | (11) | 37 | (48) | |||||||||||||||||||||||||||||
| Basic earnings per share ($) | 191 | 242 | (51) | (12) | 36 | (48) | |||||||||||||||||||||||||||||
| Diluted earnings per share ($) | 188 | 241 | (53) | (11) | 37 | (48) | |||||||||||||||||||||||||||||
Core results (non-IFRS measure)(1) | |||||||||||||||||||||||||||||||||||
| Core operating income | 18 | 27 | (9) | 9 | 26 | (17) | |||||||||||||||||||||||||||||
| Core net income | 23 | 34 | (11) | 4 | 23 | (19) | |||||||||||||||||||||||||||||
| Core basic earnings per share ($) | 23 | 34 | (11) | 4 | 23 | (19) | |||||||||||||||||||||||||||||
| Core diluted earnings per share ($) | 22 | 33 | (11) | 4 | 23 | (19) | |||||||||||||||||||||||||||||
(1) Core results and constant currencies (cc) as presented in this table are non-IFRS measures. Alcon uses certain non-IFRS metrics when measuring performance, including when measuring current period results against prior periods. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information.
A 1% movement in the USD versus our basket of currencies would have resulted in a $46 million change in annual net sales and a $21 million change in both annual operating income and core operating income.
67
SUPPLEMENTARY INFORMATION - DEFINITIONS AND RECONCILIATIONS OF NON-IFRS MEASURES
Non-IFRS measures as defined by the Company
Alcon uses certain non-IFRS metrics when measuring performance, including when measuring current period results against prior periods, including core results, percentage changes measured in constant currencies, EBITDA, free cash flow, and net (debt)/liquidity.
Because of their non-standardized definitions, the non-IFRS measures (unlike IFRS measures) may not be comparable to the calculation of similar measures of other companies. These supplemental non-IFRS measures are presented solely to permit investors to more fully understand how Alcon management assesses underlying performance. These supplemental non-IFRS measures are not, and should not be viewed as, a substitute for IFRS measures.
Core results
Alcon core results, including core operating income and core net income, exclude all amortization and impairment charges of intangible assets, excluding software, net gains and losses on fund investments and equity securities valued at fair value through profit and loss ("FVPL"), fair value adjustments of financial assets in the form of options to acquire a company carried at FVPL, obligations related to product recalls, and certain acquisition related items. The following items that exceed a threshold of $10 million and are deemed exceptional are also excluded from core results: integration and divestment related income and expenses, divestment gains and losses, restructuring charges/releases and related items, legal related items, gains/losses on early extinguishment of debt or debt modifications, past service costs for post-employment benefit plans, impairments of property, plant and equipment and software, as well as income and expense items that management deems exceptional and that are or are expected to accumulate within the year to be over a $10 million threshold.
Taxes on the adjustments between IFRS and core results take into account, for each individual item included in the adjustment, the tax rate that will finally be applicable to the item based on the jurisdiction where the adjustment will finally have a tax impact. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions.
Alcon believes that investor understanding of its performance is enhanced by disclosing core measures of performance because, since they exclude items that can vary significantly from period to period, the core measures enable a helpful comparison of business performance across periods. For this same reason, Alcon uses these core measures in addition to IFRS and other measures as important factors in assessing its performance.
A limitation of the core measures is that they provide a view of Alcon operations without including all events during a period, such as the effects of an acquisition, divestment, or amortization/impairments of purchased intangible assets and restructurings.
Constant currencies
Changes in the relative values of non-US currencies to the US dollar can affect Alcon's financial results and financial position. To provide additional information that may be useful to investors, including changes in sales volume, we present information about changes in our net sales and various values relating to operating and net income that are adjusted for such foreign currency effects.
Constant currency calculations have the goal of eliminating two exchange rate effects so that an estimate can be made of underlying changes in the Consolidated Income Statement excluding:
•the impact of translating the income statements of consolidated entities from their non-US dollar functional currencies to the US dollar; and
•the impact of exchange rate movements on the major transactions of consolidated entities performed in currencies other than their functional currency.
Alcon calculates constant currency measures by translating the current year's foreign currency values for sales and other income statement items into US dollars, using the average exchange rates from the historical comparative period and comparing them to the values from the historical comparative period in US dollars.
68
For additional information on the effects of foreign currencies, refer to "Item 5.A. Operating Results—Effects of currency fluctuations" section.
EBITDA
Alcon defines earnings before interest, tax, depreciation and amortization ("EBITDA") as net income excluding income taxes, depreciation of property, plant and equipment (including any related impairment charges), depreciation of right-of-use assets, amortization of intangible assets (including any related impairment charges), interest expense and other financial income and expense. Alcon management primarily uses EBITDA together with net (debt)/liquidity to monitor leverage associated with financial debts. For a reconciliation of EBITDA to the most directly comparable measure presented in accordance with IFRS, see "Item 5.B. Liquidity and Capital Resources—EBITDA (non-IFRS measure)" section.
Free cash flow
Alcon defines free cash flow as net cash flows from operating activities less cash flow associated with the purchase or sale of property, plant and equipment. Free cash flow is presented as additional information because Alcon management believes it is a useful supplemental indicator of Alcon's ability to operate without reliance on additional borrowing or use of existing cash. Free cash flow is not intended to be a substitute measure for net cash flows from operating activities as determined under IFRS. For a reconciliation of free cash flow to the most directly comparable measure presented in accordance with IFRS, see "Item 5.B. Liquidity and Capital Resources—Free cash flow (non-IFRS measure)" section.
Net (debt)/liquidity
Alcon defines net (debt)/liquidity as current and non-current financial debt less cash and cash equivalents, current investments and derivative financial instruments. Net (debt)/liquidity is presented as additional information because management believes it is a useful supplemental indicator of Alcon's ability to pay dividends, to meet financial commitments and to invest in new strategic opportunities, including strengthening its balance sheet. For a reconciliation of net (debt)/liquidity to the most directly comparable measure presented in accordance with IFRS, see "Item 5.B. Liquidity and Capital Resources—Net (debt)/liquidity (non-IFRS measure)" section.
Growth rate and margin calculations
For ease of understanding, Alcon uses a sign convention for its growth rates such that a reduction in operating expenses or losses compared to the prior year is shown as a positive growth.
Gross margins, operating income margins and core operating income margins are calculated based upon net sales to third parties unless otherwise noted.
69
Reconciliation of IFRS results to core results (non-IFRS measure)
2023
| ($ millions except earnings per share) | IFRS results | Amortization of certain intangible assets(1) | Transformation costs(4) | Other items(7) | Core results (non-IFRS measure) | |||||||||||||||
| Gross profit | 5,247 | 663 | — | 7 | 5,917 | |||||||||||||||
| Operating income | 1,039 | 675 | 139 | (4) | 1,849 | |||||||||||||||
| Income before taxes | 832 | 675 | 139 | (4) | 1,642 | |||||||||||||||
Taxes(8) | 142 | (121) | (26) | (277) | (282) | |||||||||||||||
| Net income | 974 | 554 | 113 | (281) | 1,360 | |||||||||||||||
| Basic earnings per share ($) | 1.98 | 2.76 | ||||||||||||||||||
| Diluted earnings per share ($) | 1.96 | 2.74 | ||||||||||||||||||
Basic - weighted average shares outstanding (millions)(9) | 493.0 | 493.0 | ||||||||||||||||||
Diluted - weighted average shares outstanding (millions)(9) | 496.5 | 496.5 | ||||||||||||||||||
Refer to the associated explanatory footnotes at the end of the 'Reconciliation of IFRS results to core results (non-IFRS measure)' tables.
2022
| ($ millions except earnings per share) | IFRS results | Amortization of certain intangible assets(1) | Impairments(2) | Transformation costs(4) | Legal items(6) | Other items(7) | Core results (non-IFRS measure) | |||||||||||||||||||
| Gross profit | 4,748 | 572 | 59 | — | — | 2 | 5,381 | |||||||||||||||||||
| Operating income | 672 | 588 | 62 | 119 | 90 | 40 | 1,571 | |||||||||||||||||||
| Income before taxes | 463 | 588 | 62 | 119 | 90 | 40 | 1,362 | |||||||||||||||||||
Taxes(8) | (128) | (99) | (14) | (20) | (22) | 29 | (254) | |||||||||||||||||||
| Net income | 335 | 489 | 48 | 99 | 68 | 69 | 1,108 | |||||||||||||||||||
| Basic earnings per share ($) | 0.68 | 2.25 | ||||||||||||||||||||||||
| Diluted earnings per share ($) | 0.68 | 2.24 | ||||||||||||||||||||||||
Basic - weighted average shares outstanding (millions)(9) | 491.4 | 491.4 | ||||||||||||||||||||||||
Diluted - weighted average shares outstanding (millions)(9) | 494.4 | 494.4 | ||||||||||||||||||||||||
Refer to the associated explanatory footnotes at the end of the 'Reconciliation of IFRS results to core results (non-IFRS measure)' tables.
70
Reconciliation of IFRS results to core results (non-IFRS measure) - continued
2021
| ($ millions except earnings per share) | IFRS results | Amortization of certain intangible assets(1) | Impairments(2) | Separation costs(3) | Transformation costs(4) | Post-employ-ment benefits(5) | Legal items(6) | Other items(7) | Core results (non-IFRS measure) | |||||||||||||||||||||||
| Gross profit | 4,652 | 520 | 45 | — | — | — | — | (1) | 5,216 | |||||||||||||||||||||||
| Operating income | 580 | 529 | 225 | 36 | 68 | (16) | 50 | (29) | 1,443 | |||||||||||||||||||||||
| Income before taxes | 418 | 529 | 225 | 36 | 68 | (16) | 50 | (29) | 1,281 | |||||||||||||||||||||||
Taxes(8) | (42) | (95) | (51) | (6) | (13) | 2 | (12) | (1) | (218) | |||||||||||||||||||||||
| Net income | 376 | 434 | 174 | 30 | 55 | (14) | 38 | (30) | 1,063 | |||||||||||||||||||||||
| Basic earnings per share ($) | 0.77 | 2.17 | ||||||||||||||||||||||||||||||
| Diluted earnings per share ($) | 0.76 | 2.15 | ||||||||||||||||||||||||||||||
Basic - weighted average shares outstanding (millions)(9) | 490.0 | 490.0 | ||||||||||||||||||||||||||||||
Diluted - weighted average shares outstanding (millions)(9) | 493.4 | 493.4 | ||||||||||||||||||||||||||||||
Refer to the associated explanatory footnotes at the end of the 'Reconciliation of IFRS results to core results (non-IFRS measure)' tables.
71
Explanatory footnotes to IFRS to Core reconciliation tables
(1)Includes recurring amortization for all intangible assets other than software.
(2)Includes impairment charges related to intangible assets.
(3)Separation costs, primarily related to IT and third party consulting fees, following completion of the spin-off from Novartis.
(4)Transformation costs, primarily related to restructuring and third party consulting fees, for the multi-year transformation program. The transformation program was completed in the fourth quarter of 2023.
(5)Includes impacts from pension and other post-employment benefit plan amendments.
(6)For 2022, includes legal settlement costs.
For 2021, includes an increase in provisions for legal matters.
(7)For 2023, Gross profit includes the amortization of inventory fair value adjustments related to a recent acquisition, partially offset by fair value adjustments to contingent consideration liabilities. Operating income also includes the release of a contingent liability related to a recent acquisition and fair value adjustments to contingent consideration liabilities, partially offset by integration related expenses for a recent acquisition, the amortization of option rights and fair value adjustments of financial assets.
For 2022, Gross profit includes the amortization of inventory fair value adjustments related to recent acquisitions, partially offset by fair value adjustments to contingent consideration liabilities. Operating income also includes acquisition and integration related expenses, partially offset by fair value adjustments to contingent consideration liabilities and fair value adjustments of financial assets.
For 2021, Gross profit includes fair value adjustments to contingent consideration liabilities. Operating income also includes fair value adjustments to contingent consideration liabilities, partially offset by the amortization of option rights and fair value adjustments of financial assets.
(8)For 2023, total tax adjustments of $424 million include tax associated with operating income core adjustments and discrete tax items. Tax associated with operating income core adjustments of $810 million totaled $155 million with an average tax rate of 19.1%. Core tax adjustments for discrete tax items totaled $269 million, primarily due to a $263 million tax benefit associated with a long-term agreement related to deductibility of a statutory expense in Switzerland.
For 2022, total tax adjustments of $126 million include tax associated with operating income core adjustments, partially offset by discrete tax items. Tax associated with operating income core adjustments of $899 million totaled $166 million with an average tax rate of 18.5%. Core tax adjustments for discrete tax items totaled $40 million, primarily related to the recognition of an Advanced Pricing Agreement between US and Switzerland tax authorities for fiscal years 2019 through 2021.
For 2021, total tax adjustments of $176 million include tax associated with operating income core adjustments of $863 million with an average tax rate of 20.4%.
(9)Core basic earnings per share is calculated using the weighted-average shares of common stock outstanding during the period. Core diluted earnings per share also contemplate dilutive shares associated with unvested equity-based awards as described in Note 7 to the Consolidated Financial Statements.
72
5.B. LIQUIDITY AND CAPITAL RESOURCES
Our sources of funds have consisted principally of cash flows from operations, bank debt, credit facilities with lenders and issuance of senior notes. Our uses of those funds, other than for operations, have consisted principally of dividend payments, investments in capital expenditures, payments for long-term financial investments, purchases of intangible assets, acquisitions and associated expenses, repayment of financial debts and other obligations.
Potential future uses of our liquidity include capital expenditures, acquisitions, repayment of financial debts, dividend payments and other general corporate purposes. As of December 31, 2023, we had commitments for purchases of property, plant & equipment of $283 million.
We use the US Dollar as our reporting currency and are therefore exposed to foreign currency exchange movements, primarily in Euros, Japanese Yen, Chinese Renminbi, Canadian Dollars, Korean Won, Swiss Francs, Russian Rubles and emerging market currencies. The foreign currency exposure on the balance sheet is hedged with limited exception, but the impact of ongoing macroeconomic conditions is currently unknown and could have a material adverse effect on our results of operations, cash flows or financial condition. As of December 31, 2023 unsettled derivative positions included $2 million in unrealized gains and $10 million in unrealized losses.
All comments in this section relate to the year ended December 31, 2023 compared to 2022. Commentary for the year ended December 31, 2022 compared to 2021 may be found in Item 5 of the 2022 Form 20-F.
Cash flow and net (debt)/liquidity (non-IFRS measure)
| ($ millions) | 2023 | 2022 | |||||||||
| Net cash flows from operating activities | 1,388 | 1,217 | |||||||||
| Net cash flows used in investing activities | (1,094) | (1,865) | |||||||||
| Net cash flows used in financing activities | (211) | (8) | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | 31 | 61 | |||||||||
| Net change in cash and cash equivalents | 114 | (595) | |||||||||
| Change in derivative financial instrument assets | (6) | 5 | |||||||||
| Change in equity securities of public companies | — | (3) | |||||||||
| Change in current and non-current financial debts | (91) | (568) | |||||||||
| Change in net (debt) | 17 | (1,161) | |||||||||
| Net (debt) at January 1 | (3,660) | (2,499) | |||||||||
| Net (debt) at December 31 | (3,643) | (3,660) | |||||||||
Net cash flows from operating activities
Net cash flows from operating activities amounted to $1.4 billion in 2023, compared to $1.2 billion in the prior year period. The current year includes increased collections associated with higher sales and lower associate short-term incentive payments, which generally occur in the first quarter. Cash outflows in the current year include higher payments for revenue deductions, transformation and other operating expenditures, including increased investment in research and development. The current year cash outflows also include the settlement of legal proceedings with JJSVI, increased taxes paid due to the timing of payments, higher interest payments associated with increased financial debt outstanding and a negative impact of foreign currency rates on operating results. Both periods were impacted by changes in net working capital.
Changes in net working capital in the current year were mainly driven by increases in inventories and trade receivables and a decrease in trade payables, partially offset by the net change in other operating liabilities. The increase in inventories was primarily to meet expected upcoming demand. The increase in trade receivables was primarily driven by new receivables from higher sales outpacing collections. The decrease in trade payables was primarily driven by the timing of payments. The net change in other operating liabilities was primarily driven by higher accruals for short-term incentive benefits.
Changes in net working capital in the prior year period included increases in inventories and trade receivables, the net change in other operating assets and other operating liabilities and decreases in trade payables. The increase in
73
inventories was primarily driven by new product launches and higher raw materials and work in process at manufacturing sites to mitigate uncertainty caused by longer supply lead times. The increase in trade receivables was primarily driven by new receivables from higher sales outpacing collections. The net change in other operating assets was primarily driven by increases in long-term receivables and prepaid expenses. The net change in other operating liabilities was primarily driven by annual associate short-term incentive payments, payment of acquisition and integration costs related to Aerie and lower wage accruals due to the timing of payroll, partially offset by accruals for deductions from revenue. Trade payables decreased as the 2021 payables reflected increased discretionary spending in line with market recovery. Refer to Note 20 of the Consolidated Financial Statements for additional details regarding changes within net working capital in the current and prior year periods.
Net cash flows used in investing activities
Net cash flows used in investing activities amounted to $1.1 billion in 2023, compared to $1.9 billion in the prior year period. Cash outflows in the current year period include capital expenditures, payments for financial assets and purchases of intangible assets. Payments for financial assets primarily include a long-term note receivable related to new financing arrangements with Lifecore Biomedical, Inc. and certain of its affiliates (collectively, "Lifecore") in the second quarter of 2023 and long-term financial investments measured at fair value through other comprehensive income ("FVOCI"). Purchases of intangible assets primarily include intellectual property licenses. Refer to Note 17 of the Consolidated Financial Statements for additional information.
Cash outflows in the prior year period primarily included the acquisitions of Aerie, Ivantis and Eysuvis and Inveltys products, capital expenditures and purchases of long-term financial investments measured at FVOCI, partially offset by the sale of short-term investments obtained in the Aerie acquisition. Refer to Note 3 of the Consolidated Financial Statements for additional information on the acquisitions of Aerie, Ivantis and Eysuvis and Inveltys products.
Net cash flows used in financing activities
Net cash flows used in financing activities amounted to $211 million in 2023, compared to $8 million in the prior year period. Cash outflows in the current year period primarily include dividends paid to shareholders of Alcon Inc., lease payments and withholding taxes paid upon net settlements of equity-based compensation, partially offset by net proceeds from local debt facilities.
Cash outflows in the prior year period primarily included dividends paid to shareholders of Alcon Inc., lease payments and withholding taxes paid upon net settlements of equity-based compensation, partially offset by net cash inflows associated with financial debts. Net cash inflows associated with financial debts primarily included the issuance of Series 2028, Series 2032 and Series 2052 senior notes, issuance and repayment of the 2022 Bridge Loan Facility associated with the Aerie acquisition, repayment of the Facility B and Facility C term loans, payment of financial debts assumed in the Aerie acquisition and payments of certain local debt facilities. Refer to Notes 3, 16 and 20 of the Consolidated Financial Statements for additional information.
Free cash flow (non-IFRS measure)
The following is a summary of free cash flow for 2023, 2022 and 2021, together with a reconciliation to net cash flows from operating activities, the most directly comparable IFRS measure.
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Net cash flows from operating activities | 1,388 | 1,217 | 1,345 | ||||||||||||||
| Purchase of property, plant & equipment | (658) | (636) | (700) | ||||||||||||||
| Free cash flow | 730 | 581 | 645 | ||||||||||||||
Free cash flow amounted to an inflow of $730 million in 2023, compared to $581 million in the prior year period, due to increased cash flows from operating activities, partially offset by increased purchases of property, plant and equipment.
For additional information refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures".
74
Balance sheet
Assets
Total non-current assets were $24.0 billion as of December 31, 2023, in line with December 31, 2022. Intangible assets other than goodwill decreased $629 million primarily due to recurring amortization, partially offset by additions. Property, plant & equipment increased $344 million primarily due to capital expenditures and foreign currency translation effects, partially offset by depreciation. Financial assets increased $230 million primarily due to a long-term note receivable resulting from financing arrangements with Lifecore and purchases of long-term financial investments measured at FVOCI. Other non-current assets increased $55 million primarily due to deferred compensation plans assets and an investment in an associated company.
Total current assets were $5.6 billion as of December 31, 2023, an increase of $454 million when compared to $5.2 billion as of December 31, 2022. Inventories increased $213 million primarily to meet expected upcoming demand, partially offset by foreign currency translation effects. Cash and cash equivalents increased $114 million due to the net impact of operating, investing and financing activities as described in the preceding section. Our cash and cash equivalents are maintained at a number of financial institutions. To mitigate the risk of uninsured balances, we select financial institutions based on their credit ratings and financial strength, and we perform ongoing evaluations of these institutions to limit our concentration risk exposure. Trade receivables increased $97 million primarily driven by higher sales outpacing collections, partially offset by foreign currency translation effects.
Trade receivable balances include sales to wholesalers, retailers, doctor groups, private health systems, government agencies, pharmacy benefit managers, managed health-care organizations and government-supported healthcare systems. We closely monitor the level of trade receivables in the countries deemed to have an elevated credit risk. We consider macroeconomic and geopolitical environment, country profile and historical experience in addition to other relevant information when assessing the credit risk. Deteriorating credit risk factors may result in an increase in the average length of time that it takes to collect these trade receivables and may require Alcon to reevaluate the expected credit loss amount of these trade receivables in future periods or change the terms on which we operate. As of December 31, 2023, the amounts past due for more than one year in elevated credit risk countries are not significant.
The following table summarizes the aging of trade receivables as of December 31, 2023 and 2022:
| ($ millions) | 2023 | 2022 | ||||||
| Not overdue | 1,452 | 1,390 | ||||||
| Past due for not more than one month | 143 | 125 | ||||||
| Past due for more than one month but less than three months | 94 | 93 | ||||||
| Past due for more than three months but less than six months | 54 | 56 | ||||||
| Past due for more than six months but less than one year | 35 | 28 | ||||||
| Past due for more than one year | 36 | 38 | ||||||
| Provisions for doubtful trade receivables | (44) | (57) | ||||||
| Total trade receivables, net | 1,770 | 1,673 | ||||||
There is also a risk that certain countries could devalue their currency. Currency exposures are described in more detail in the "Item 5.A. Operating Results — Effects of currency fluctuations" section.
Liabilities
Total non-current liabilities were $6.5 billion as of December 31, 2023, a decrease of $240 million when compared to $6.8 billion as of December 31, 2022. Deferred tax liabilities decreased $267 million primarily due to a deferred tax asset recognized in the fourth quarter of 2023 related to a long-term agreement with Swiss tax authorities for the deductibility of a statutory expense in Switzerland. The deferred tax asset was recognized as a reduction to Alcon's net deferred tax liability in the Swiss tax jurisdiction. Financial debts increased $53 million primarily due to the refinancing of local debt facilities in Japan and foreign currency translation effects.
Total current liabilities were $2.5 billion as of December 31, 2023, a decrease of $258 million when compared to $2.7 billion as of December 31, 2022. Provisions and other current liabilities decreased $185 million primarily due to the JJSVI legal settlement payment, transformation payments and property tax payments, partially offset by higher accruals for incentive compensation. Current income tax liabilities decreased $61 million primarily due to the timing of payments. Trade payables decreased $50 million primarily due to the timing of payments.
75
Equity
Equity was $20.6 billion as of December 31, 2023, an increase of $947 million when compared to $19.7 billion as of December 31, 2022.
Net (debt)/liquidity (non-IFRS measure)
The following is a summary of net (debt) as of December 31, 2023 and December 31, 2022, together with a reconciliation to total financial debt, the most directly comparable IFRS measure.
| ($ millions) | 2023 | 2022 | |||||||||
| Current financial debt | (145) | (107) | |||||||||
| Non-current financial debt | (4,594) | (4,541) | |||||||||
| Total financial debt | (4,739) | (4,648) | |||||||||
| Less liquidity: | |||||||||||
| Cash and cash equivalents | 1,094 | 980 | |||||||||
| Derivative financial instruments | 2 | 8 | |||||||||
| Total liquidity | 1,096 | 988 | |||||||||
| Net (debt) | (3,643) | (3,660) | |||||||||
Net debt of $3.6 billion as of December 31, 2023 decreased $17 million compared to $3.7 billion as of December 31, 2022. Alcon's liquidity amounted to $1.1 billion as of December 31, 2023, compared to $988 million as of December 31, 2022. Total financial debt amounted to $4.7 billion as of December 31, 2023, compared to $4.6 billion as of December 31, 2022.
The average maturity of financial debts outstanding as of December 31, 2023 is 10.7 years, and 97% of Alcon's financial debt is at fixed interest rates. We believe that we have adequate liquidity to meet our needs.
In October 2023, the revolving credit facility was refinanced with a new revolving credit facility of $1.32 billion maturing five years after the date of the agreement. The revolving credit facility remained undrawn as of December 31, 2023 and February 27, 2024. Refer to Note 16 of the Consolidated Financial Statements for additional information.
For additional information regarding net (debt)/liquidity, which is a non-IFRS measure, see the explanation of non-IFRS measures in "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures".
EBITDA (non-IFRS measure)
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Net income | 974 | 335 | 376 | ||||||||||||||
| Taxes | (142) | 128 | 42 | ||||||||||||||
| Depreciation of property, plant & equipment | 385 | 330 | 323 | ||||||||||||||
| Depreciation of right-of-use assets | 91 | 76 | 81 | ||||||||||||||
| Amortization of intangible assets | 745 | 653 | 590 | ||||||||||||||
| Impairments of property, plant & equipment and intangible assets | — | 64 | 225 | ||||||||||||||
| Interest expense | 189 | 134 | 120 | ||||||||||||||
| Other financial income & expense | 18 | 75 | 42 | ||||||||||||||
| EBITDA | 2,260 | 1,795 | 1,799 | ||||||||||||||
76
Liquidity and financial debt by currency
The following table summarizes liquidity and financial debts by currency as of December 31, 2023 and 2022.
Liquidity (%)(1) | Financial debts (%)(2) | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| USD | 66 | 68 | 85 | 87 | |||||||||||||||||||
| EUR | 23 | 22 | 12 | 11 | |||||||||||||||||||
| CHF | — | 1 | — | — | |||||||||||||||||||
| JPY | — | — | 2 | 2 | |||||||||||||||||||
| Other | 11 | 9 | 1 | — | |||||||||||||||||||
| Total | 100 | 100 | 100 | 100 | |||||||||||||||||||
(1)Liquidity includes cash and cash equivalents and time deposits.
(2)Financial debts includes non-current and current financial debts.
5.C. RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES, ETC.
Alcon research & development expense totaled $828 million, $702 million and $842 million for the years 2023, 2022 and 2021, respectively. As described in the "Risk Factors" section and elsewhere in this Annual Report, we are subject to varying degrees of governmental regulation in the countries in which we operate, which makes the process of developing new products and obtaining necessary regulatory marketing authorization lengthy, expensive and uncertain. See "Item 3. Key Information—3.D. Risk Factors". For further information on Alcon research and development policies and additional product information, as well as a description of the regulatory approval process, see "Item 4. Information on the Company—4.B. Business Overview".
5.D. TREND INFORMATION
Please see "Item 5.A. Operating Results—Opportunity and risk summary" and "Item 4. Information on the Company—4.B. Business Overview" for trend information.
5.E. CRITICAL ACCOUNTING ESTIMATES
Please see “Item 5.A. Operating results—Critical accounting policies and estimates”.
77
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
6.A. DIRECTORS AND SENIOR MANAGEMENT
The information set forth under “Item 6.C. Board Practice—Corporate Governance—Board of Directors—Composition” and “Item 6.C. Board Practice—Corporate Governance—Executive Committee—Composition of the Executive Committee” is incorporated by reference.
78
6.B. COMPENSATION
Introduction
Dear Shareholder
On behalf of the Alcon Board of Directors ("Board") and Compensation Committee ("CC"), I am pleased to present the 2023 Compensation Report. This report outlines Alcon’s overall 2023 compensation framework and philosophy for the members of the Board as well as for the members of the Executive Committee of Alcon ("ECA") and provides a general outlook for our 2024 compensation structure.
This Compensation Report covers the financial year from January 2023 to December 2023.
2023 in Review
Once again, we are proud to have delivered on our goals to both our shareholders and our customers. Our global team of associates continue to work hard to achieve operational excellence and above all else, to help people See Brilliantly.
Business Overview
Alcon had another successful year in 2023. Our innovative product portfolio helped drive top line growth, and our associates were able to deliver above target financial metrics. In the face of continued macroeconomic pressures such as foreign currency, geopolitical risks and inflation, our team has proven once again that they are able to manage such challenges while delivering quality products to our customers. Highlights from the year are outlined below:
•Achieved exceptional Vision Care sales growth of +12%, or +14% on a constant currency1 basis versus 2022; we gained market share with our recent contact lens launches, benefited from the successful integration of newly acquired products and saw the continued success of Systane and Pataday;
•Increased equipment footprint in international markets, continued capture of consumables revenue, and maintained our global market-leading position in ATIOLs driving strong Surgical sales growth of +5%, or +8% on a constant currency basis versus 2022;
•Expanded operating margin by 330 basis points, or 470 basis points on a constant currency basis, and core operating margin1 by 150 basis points, or 280 basis points on a constant currency basis; with higher sales and operational efficiency delivering leverage and pricing actions and manufacturing efficiencies offsetting inflation, all while increasing investment in research and development;
•Drove shareholder value with diluted EPS of $1.96, representing growth of +188%, or +241% on a constant currency basis, versus 2022; core diluted EPS1 was $2.74, representing growth of +22%, or +33% on a constant currency basis, versus 2022; and
•Made progress on all four publicly stated long-term social and sustainability goals, including two social goals focused on access to vision improvement and two climate focused environmental goals and continue to be on track to achieve the commitments made in Alcon's 2022 Social Impact and Sustainability Report.
2023 ECA Compensation
The Board annually reviews the CEO and ECA's target compensation against our peer group. In 2023, the Board adjusted Mr. Endicott's long-term incentive ("LTI") target opportunity which is delivered entirely through performance share units with three-year cliff vesting. All other ECA members received increases aligned with the global budget for our broad-based associate population.
1 Constant currency growth, core operating margin and core diluted EPS are non-IFRS measures. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information.
79
2023 Incentive Payouts
Alcon's strong performance in 2023 had an impact on its incentive payouts. Robust operational and financial performance exceeded the target level for all short-term incentive ("STI") metrics (Sales, Core Operating Income and Free Cash Flow) yielding a business performance factor ("BPF") of 140% for all eligible Alcon associates. For the 2021-2023 long-term incentive ("LTI") performance stock unit ("PSU") award, Alcon exceeded target levels for all four metrics (Sales CAGR, Core EPS CAGR, Share of Peers and Innovation) resulting in a performance factor of 181%. Over the three year performance period, Alcon delivered shareholder value by growing market capitalization by 17% (USD 6 billion value).
2023 Annual General Meeting Vote and Engagement with Shareholders
At our 2023 Annual General Meeting (“AGM”), our 2022 Compensation Report received approval with support from 84% of the votes cast. While we are encouraged to receive robust shareholder support, we continued our efforts to engage with and gather feedback from our shareholders during 2023 regarding our executive compensation program.
Our engagement team included our Board Chair, Chief Human Resources Officer, General Counsel, Head of Investor Relations and Head of Social Impact and Sustainability. We heard directly from investors on a range of important topics including CEO compensation, executive compensation programs and broader Social Impact and Sustainability matters, which are summarized in the section "Shareholder Outreach".
2024 Outlook
As we head into 2024, the CC intends to maintain the same overall structure of ECA compensation as compared to 2023 including base salary, STI, LTI, benefits provision and continuation of robust share ownership requirements. The Board does not intend to make any changes to the CEO’s compensation.
2024 Annual General Meeting
In line with the Articles of Incorporation, we will ask our shareholders to cast a binding vote on the maximum aggregate amount of compensation for members of the Board for their term of office from the 2024 AGM to the 2025 AGM. We will also ask our shareholders to cast a binding vote on the maximum aggregate amount of compensation for members of the ECA for the 2025 financial year. In addition, we will ask our shareholders to endorse this 2023 Compensation Report in an advisory vote.
On behalf of the Board and the members of the CC, we thank you for your trust and investment in Alcon as well as your feedback and support.
Sincerely,
Karen May
Chair of the Compensation Committee
80
Compensation at a Glance
Shareholder Outreach
Our engagement team conducted a broad shareholder outreach during the second half of 2023. We continue to receive shareholder support for our management team and overall executive compensation program. We have summarized key highlights from our 2023 shareholder engagement and enhancements made in response to the shareholder feedback.
CEO target compensation adjustment – As previewed in the Outlook section of our 2022 Compensation Report, a Board commissioned peer group benchmarking study indicated Mr. Endicott’s target compensation opportunity to be below the range that the Board considers globally competitive. The Board has utilized a consistent blended peer group as a reference point for consideration of market competitive compensation since Alcon’s spin off. In addition to this, recent changes in the landscape for ophthalmic talent, including a recent competitor spin off, led the Board to assess potential vulnerabilities to competitive threats.
Prior to 2023, the last meaningful increase to our CEO’s target compensation was made in February 2020 in the form of an increase to the LTI target award. In 2021, he received a base salary increase aligned with the global budget for our broad-based associate population. No changes were made to his compensation in 2022.
The Board decided to bring the CEO compensation target opportunity closer to the peer group median and therefore, in 2023, the Board adjusted Mr. Endicott’s target compensation by 22% via an increase in his long-term incentive target award. The CEO's LTI consists entirely of performance share units with three-year cliff vesting which is aligned with shareholder expectations and Alcon's pay-for-performance philosophy. After the adjustment, Mr. Endicott’s target compensation opportunity is slightly below the peer group median, positioned at the bottom end of our US peers and at the top end of our international peers, maintaining the balance between Swiss domiciled companies and the medical device and ophthalmic talent base, which is primarily found in the US.
In conjunction with the CEO LTI target increase, the Board increased the CEO’s share ownership requirement from 5X to 6X of base salary.
During our outreach, our contacted shareholders were supportive of the target opportunity increase as a result of Alcon's performance under Mr. Endicott's leadership during his tenure, the challenging talent landscape and our Board's continued commitment to aligning shareholder interest with the CEO's target compensation. The Board does not intend to make any changes to the CEO’s target compensation in 2024 including his base salary and incentive targets.
Disclosure enhancement for 2021-2023 LTI payout table – We also heard feedback from our shareholders requesting additional transparency into our long-term incentives. Based on this feedback, we have included retrospective LTI targets for our financial metrics in the 2023 Compensation Report. In addition, we have included findings from a third-party assessment indicating the robustness of our financial target setting approach in the "2021-2023 Long-Term Incentive" section of the Compensation Report.
Progress against sustainability goals – As we progress our sustainability journey, we have made significant strides and are pleased to report progress toward meeting the two social goals focused on vision improvement and two climate focused environmental goals. We heard feedback to disclose the annual progress on our long term social impact and sustainability goals. The annual progress on the sustainability goals is disclosed as part of the CEO's individual performance goals and achievement of these goals is linked to CEO pay through the STI plan.
Benefits disclosure enhancement – We have provided additional details regarding the benefits we provide to our ECA members. We note that a large portion of the benefits (disclosed in “Other benefits”) provided are in the form of tax neutralization, in accordance with our global mobility policy. Generally, for associates on international assignments, over 70% of the benefit costs are related to maintaining the cost of living position neutral to their home base and of the 70%, over 75% of these costs are attributable to tax neutralization.
81
2023 ECA Compensation-Summary
We employ a strong pay-for-performance compensation program that motivates our senior executives to create long-term value for the Company and its shareholders.
The executive compensation program includes a balance of fixed and variable elements rewarding short-term and long-term performance through the delivery of cash payments and equity awards. Performance goals were aligned to the strategic plan in a mix of absolute and relative measures including financial and non-financial metrics. The current compensation program remains well suited to effectively align pay and performance.
The CC exercised no discretion with regard to our STI and LTI plans during 2023.
Exhibit 1
Annual Base Salary | Short-Term Incentive | Long-Term Incentive | Benefits | |||||||||||
Purpose | In line with global pay practices, reflects responsibilities, experience and skills | Rewards annual performance against key objectives | Rewards long-term value creation in line with Alcon’s strategy and business priorities | Retirement savings and insurance in line with local market practices and benefits associated with global mobility and international relocation | ||||||||||
Payment | Cash | Cash | Equity (Performance Stock Units) | Cash or in-kind contributions to retirement savings and insurance policies | ||||||||||
Performance period | — | One year | Three-year cliff vesting | — | ||||||||||
Performance measures | — | Three financial performance measures and an individual performance factor | Four equally weighted performance measures including financial, relative and innovation metrics | — | ||||||||||
Payout range | — | 0%-200% of the individual target award | 0%-200% of the number of Performance Stock Units granted | — | ||||||||||
Basis | Fixed | Variable | Variable | Fixed in proportion of pay | ||||||||||
82
Total Compensation for 2023
From January 1, 2023 to December 31, 2023, we awarded the ECA members the amounts set out below. The overall structure of ECA compensation remained the same as 2022 (base salary, STI, LTI and benefits).
For more detailed information, see section "ECA Compensation 2023" in this 2023 Compensation Report.
Exhibit 2
Compensation Gross Amounts | Fixed compensation | Variable compensation | Additional comp. | Totals in USD | Totals in CHF3 | |||||||||||||||||||||||||||||||||
From January 1, 2023 to December 31, 2023 | Annual base salary | Pension and insurance benefits | 2023 STI award | 2023-2025 LTI awards 1 | Other benefits | Total compen-sation | Total compen-sation | |||||||||||||||||||||||||||||||
David J. Endicott, CEO | 1,318,235 | 179,784 | 2,657,562 | 7,365,435 | 804,375 | 12,325,391 | 11,074,980 | |||||||||||||||||||||||||||||||
Other ECA members | 4,575,645 | 907,127 | 6,375,556 | 7,975,173 | 4,324,534 | 24,158,035 | 21,707,202 | |||||||||||||||||||||||||||||||
Totals in USD2 | 5,893,880 | 1,086,911 | 9,033,118 | 15,340,608 | 5,128,909 | 36,483,426 | ||||||||||||||||||||||||||||||||
Totals in CHF3 | 5,295,946 | 976,644 | 8,116,708 | 13,784,303 | 4,608,581 | 32,782,182 | ||||||||||||||||||||||||||||||||
1Performance Stock Units.
2Includes the CEO and six other ECA members.
3The amounts were converted at the rate of 1.0 CHF : 1.112904 USD.
83
2023 Board of Directors Compensation-Summary
We paid our Directors a fixed fee for services covering the term of their office from the 2023 Annual General Meeting ("2023 AGM") to the 2024 Annual General Meeting ("2024 AGM"). At the 2023 AGM, shareholders approved an increase to the Board fees, Board Chair fee and Committee membership fees as part of the Say-On-Pay ("SOP") budget increase. It was the first ever increase for the Board member fees.
The fixed compensation consists of a base fee for Board membership and additional fees for service on Board committees. Board members and the Board Chair receive fifty percent of their compensation in cash and fifty percent in unrestricted Alcon shares. On a voluntary basis, a Board member may opt to receive all or part of the cash portion in additional shares. Alcon does not provide any performance-based components of pay to the members of the Board.
Exhibit 3
Board function | CHF | USD1 | ||||||
Annual base fee: | ||||||||
Board Chair | 1,150,000 | 1,279,840 | ||||||
Board member base fee (Board retainer fee) | 205,000 | 228,145 | ||||||
Additional fees: | ||||||||
Vice Chair | 40,000 | 44,516 | ||||||
Chair of the Audit and Risk Committee | 70,000 | 77,903 | ||||||
| Chair of the Compensation Committee | 60,000 | 66,774 | ||||||
Chair of the Governance and Nomination Committee | 60,000 | 66,774 | ||||||
Chair of the Innovation Committee | 60,000 | 66,774 | ||||||
Member of the Audit and Risk Committee | 35,000 | 38,952 | ||||||
| Member of the Compensation Committee | 30,000 | 33,387 | ||||||
Member of the Governance and Nomination Committee | 30,000 | 33,387 | ||||||
Member of the Innovation Committee | 30,000 | 33,387 | ||||||
1The Board fees are converted at the rate of 1.0 CHF : 1.112904 USD.
Alcon Board Fee Payments in 2023
In 2023, Alcon paid the members of the Board the amounts described below.
For more details regarding the compensation paid to the individual members of the Board, see section "Board of Directors Compensation 2023" in this Compensation Report.
Exhibit 4
Payment in cash | Tax and other cash | Payment in shares | Number of shares | Other payments | Total fees | |||||||||||||||
Total fees paid in 20231 in USD | 465,483 | 781,363 | 2,621,189 | 34,445 | 42,926 | 3,910,961 | ||||||||||||||
Total fees paid in 2023 in CHF2 | 418,260 | 702,094 | 2,355,269 | 34,445 | 38,571 | 3,514,194 | ||||||||||||||
1Represents compensation for ten out of eleven members of the Board as David J. Endicott does not receive additional compensation for his service as a member of the Board.
2The payments in cash were made in Swiss Francs (CHF). For consistency all compensation payments are reported in USD in this report. The amounts were converted at the rate of 1.0 CHF : 1.112904 USD. All amounts are before the social security contributions and income tax deductions due by the Board member.
84
Corporate Governance
The Board makes decisions regarding Board compensation upon proposals from the CC. These proposals are based on analysis and review of board compensation practices, policies and benchmarking information. Similarly, the Board approves CEO compensation based on proposals from the CC. The CC decides compensation of the other ECA members based upon an analysis of relevant executive compensation practices, policies and benchmarking information.
The Board is responsible for approving the Compensation Report and for the proposal of the aggregate budget of Board compensation and ECA compensation to the shareholders at the AGM. The Corporate Governance Report contained in our 2023 Annual Report in "Item 6.C. Board Practice" provides further details regarding the responsibilities of the CC.
Adherence to Strong Governance Practices
The CC evaluates many governance factors when designing and establishing compensation for members of the ECA. It uses these mechanisms to help guide its decisions to ensure that the Company is rewarding long-term success, discouraging excessive risk-taking and aligning executive and shareholder interests.
Exhibit 5
| What we do | What we don’t do | ||||
•Provide a majority of executive pay in variable, rather than fixed compensation in order to ensure pay-for- performance | •No severance agreements | ||||
•Tie 100% of STI and LTI to appropriately ambitious performance metrics | •No single-trigger change in control payments | ||||
•Follow best practices in executive compensation design | •No change in control related excise tax gross ups | ||||
•Prohibit hedging, pledging and short sales of Company stock by executive officers and Directors | •No termination notice period in excess of twelve months | ||||
•Have robust share ownership requirements to reinforce alignment between executives and shareholders | •No stock option awards | ||||
•Include forfeiture and claw-back provisions for all variable compensation payments | •No active defined benefit pension plans | ||||
•Ensure that STI and LTI plans have target and maximum payout limits | •No guaranteed compensation | ||||
•Award all equity grants at market value | |||||
•Conduct ongoing investor outreach | |||||
85
ECA Compensation 2023
Compensation Governance
Authority for ECA Compensation Decisions
All decisions regarding CEO compensation and performance are made by the Board as a whole, excluding the CEO who is recused from such matters. The Board has delegated the authority to make compensation decisions for ECA members, excluding the CEO, to the CC.
The CEO makes recommendations to the CC on executive compensation policy and incentive plan design as well as proposals regarding the compensation and performance targets for ECA members. The CEO also proposes the assessment of performance achievements for ECA members. The CEO does not make proposals regarding his own compensation or performance.
Exhibit 6
Authority levels in ECA compensation | CEO | CC | Board | AGM | ||||||||||
ECA compensation policy and principles | R | A | ||||||||||||
CEO compensation and benefits | R | A | ||||||||||||
Other ECA member compensation and benefits | R | A | ||||||||||||
CEO performance targets and assessment of achievements | R | A | ||||||||||||
Other ECA members' performance targets and assessment of achievements | R | A | ||||||||||||
Share ownership requirements for the CEO and other members of the ECA | A | |||||||||||||
Maximum aggregate ECA compensation | R | P | A1 | |||||||||||
Incentive plan design and rules | R | P | A | |||||||||||
Compensation Report of the Company | R | P | A2 | |||||||||||
R Recommend P Propose A Approve
1binding vote
2advisory vote
Compensation Program
In the financial year 2023, Alcon’s ECA compensation framework included the strategic objectives of:
•Paying for performance and the execution of the Alcon strategy;
•Pursuing value for shareholders over the long-term;
•Creating alignment in the interests of executives and shareholders; and
•Motivating and retaining executives for the long-term.
The general principles for ECA compensation are defined in Articles 31 and 32 of our Articles of Incorporation (http://investor.alcon.com/governance//default.aspx). ECA compensation comprises fixed and variable elements. Fixed elements include an annual base salary and benefits. Variable compensation consists of STI and LTI plans, which are subject to performance measures and caps.
Pay-for-Performance
Variable compensation represents a majority of total compensation and affirms our pay-for-performance philosophy (see more information in Exhibits 16 and 21). Actual payout is contingent on the achievement of Company and individual performance goals. Performance metrics and goals are aligned with the Company’s business strategy and compensation philosophy, as well as long-term value creation for shareholders and are approved annually by the CC and the Board.
86
Peer Group
External peer compensation is an important market reference point for evaluating the competitive positioning of the members of the ECA, including our CEO.
The CC believes that a relevant set of peer companies that are similar to Alcon in size, industry, business mix and global footprint, enables shareholders to assess the appropriate levels and practices of compensation and allows for pay-for-performance comparisons. The CC approved the peer group in 2019 and no changes have been made to the peer group since its inception with the exception of Allergan, which was dropped from the peer group due to it's acquisition by AbbVie. Alcon’s revenue and market capitalization are above the median of the peer group companies.
Although Alcon is headquartered in Switzerland, a significant portion of our sales, management team and associate population are in the US. The US is the largest pool for both medical device and ophthalmology talent, and it is therefore critical that Alcon is able to attract and retain key talent from the US. As a result, our CC has selected a blended peer group of European and North American companies (42% European and 58% North American) to balance the European compensation structure with a need to attract and retain US talent. Based on our compensation philosophy, our desired competitive position is to stay near the median of the peer group. The 2023 peer group is outlined in Exhibit 7.
Exhibit 7
Global Peer Group | |||||
Agilent Technologies Inc. | Fresenius Medical Care | ||||
Align Technology Inc. | Givaudan | ||||
| BauschHealthCompanies Inc. | Lonza Group | ||||
| Baxter International Inc. | Merck KGaA | ||||
| Becton Dickinson & Company | Smith & Nephew | ||||
| Biogen Inc. | Stryker Corporation | ||||
| Boston Scientific | The Cooper Companies Inc. | ||||
Dentsply Sirona Inc. | UCB | ||||
Edwards Lifesciences Corporation | Zimmer Biomet Holding Inc. | ||||
| EssilorLuxottica | |||||
| Revenue* | Market Capitalization* | ||||
 |  | ||||
* Revenue and Market Capitalization data available as of December 31, 2023.
The annual total compensation of ECA members is targeted to the market median of benchmarks for comparable roles within this peer group. The CC considers compensation practices, structures and levels based on benchmarking information and advice provided by the CC’s independent external advisors (see more information under the section "Compensation Governance").
The CC and the Board review the compensation of the CEO and the other ECA members periodically and consider relevant benchmark information. The CC periodically reviews the peer group and may make adjustments to its composition as appropriate.
Forfeiture and Claw-back Rules
Any variable compensation paid or payable to ECA members is subject to forfeiture and claw-back rules under our STI and LTI plans, which allow the Company to retain unpaid or unvested compensation (forfeiture) or recover compensation already paid in cash or shares (claw-back). Such rules apply in cases where the action or behavior of an executive violates internal codes, guidelines or policies or conflicts with management standards, including Company and accounting rules and regulations or violates laws. The action to retain or recover variable compensation is subject to applicable laws of the jurisdiction involved. Alcon also adopted additional recoupment provisions in 2023 in accordance with the SEC rules and NYSE Listing Standards effective on October 2, 2023, which mandate the recovery of certain erroneously paid performance-based incentive compensation that may be received by our ECA members on or after October 2, 2023, if
87
Alcon has a qualifying financial restatement during the three completed fiscal years immediately prior to the fiscal year in which a financial restatement determination is made, subject to limited exceptions.
Share Ownership Requirements for ECA Members
The Board has established share ownership requirements for members of the ECA in order to align executives’ interests with those of shareholders. The ownership requirement is expressed as a multiple of the executive’s annual base salary and is in line with the practices of our peer group. Mr. Endicott's share ownership requirement was increased from 5X to 6X in conjunction with the LTI increase. The following Exhibit illustrates those requirements:
Exhibit 8
Leadership level | Share ownership requirement | ||||
David J. Endicott, CEO | 6 times annual base salary | ||||
Other members of the ECA | 3 times annual base salary | ||||
All members of the ECA must meet these requirements within five years of service from the commencement of ECA level role. If any of the ECA members fail to meet the requirement, or if they are not on track with the requirements, the CC may take several actions such as prohibiting the sale of Alcon shares until such time the requirement is met. At the end of 2023, each member of the ECA has met or is on track to meet the applicable ownership requirement.
Compensation Elements
Alcon’s compensation program has three broad components: annual base salary, variable compensation elements and employment benefits. Variable compensation elements are geared towards encouraging executives to deliver outstanding results and create sustainable shareholder value. They are also designed to prevent executives from taking excessive risks. The compensation program balances:
•fixed and variable compensation elements;
•short-term and long-term incentive compensation; and
•Company and individual performance.
Exhibit 9
Annual Base Salary
Annual Base Salary | Annual base salary (ABS) is set and reviewed considering: •Market value of the role •Benchmark information of peer companies •Market median within the peer companies •Executive’s role, performance, experience and potential •Business performance and the external environment | ||||
88
Exhibit 10
Variable Compensation
Short-Term Incentive | The STI is designed and delivers awards based on: Target value •Annual base salary (ABS)1 x STI target (% of ABS) = STI target value in USD/CHF Performance measurement •Measurement of financial performance (Business Performance Factor “BPF”) and individual performance (Individual Performance Factor “IPF”, see the description of the STI in the section "Short-Term Incentive" for more information) Payout •Performance period: 1 year •Range 0%-200% of the STI target value •Payout formula: STI target value x IPF x BPF = STI payout •Paid in the first quarter of the following year •Delivered in cash | ||||
Long-Term Incentive | The LTI is designed and delivers awards based on: Target value •Annual Base Salary (ABS) x LTI target (% of ABS) = LTI Target value in USD/CHF Target award •Target value divided by the Alcon share price at grant date = number of Performance Stock Units (PSUs) at target •Granted at the onset of the performance period Performance measurement •Measurement of metrics (see the description of the LTI in the section "Long-Term Incentive" for more information) Payout •Performance period: 3 years •Range 0%-200% of the target number of PSUs •Payout formula: Number of PSUs at target x PSU performance factor = number of PSUs vested •Cliff vesting of earned PSUs (i.e., all PSUs vest at the end of the performance period, subject to performance conditions) •Conversion of vested PSUs to Alcon shares •Payout delivered in unrestricted Alcon shares •Paid in the first quarter of the year following the performance period •PSUs carry dividend equivalents payable in cash at the end of the performance period based on the number of PSUs vested | ||||
1ABS earned during the financial year.
89
Short-Term Incentive
The short-term incentive compensation element is designed to reward the ECA members for their contribution towards achieving annual Company results and for their individual annual performance. The metrics used for the BPF are the same for all ECA members. The Individual Performance Factor "IPF" varies by individual. Based on this design, each member of the ECA participates in the overall Company’s success while also being rewarded for their individual contributions. The annual STI award value at target is based on a percentage of the ECA member’s annual base salary.
Exhibit 11
STI payout opportunity as a % of annual base salary | at target | at maximum | ||||||
David J. Endicott, CEO | 120 | % | 240 | % | ||||
Other members of the ECA (average) | 82 | % | 164 | % | ||||
The financial metrics for the short-term performance in 2023 are set out in the Exhibit below. The payout of STI is calculated by multiplying the target award by the BPF and IPF.
Exhibit 12
Metric | Financial Metrics 1 | Non-Financial Metric | ||||||||||||||||||||||||||||||||||||||||||
Third Party Net Sales | Core Operating Income | Free Cash Flow | Individual Performance | |||||||||||||||||||||||||||||||||||||||||
Definition | Measures the Company's Third Party Net Sales performance | Measures the Company’s profitability | Measures the Company’s capacity to realize cash | Measures the achievement of individual objectives (including Social Impact and Sustainability objectives) and individual values and behaviors | ||||||||||||||||||||||||||||||||||||||||
Rationale | Fosters the Company’s top line performance | Recognizes the primary indicator of profitability | Indicates the cash realized from operating activities | Considers individual contribution to the Company’s results | ||||||||||||||||||||||||||||||||||||||||
Weighting | 40% | 40% | 20% | 100% | ||||||||||||||||||||||||||||||||||||||||
Performance factors | BPF (total weightings of financial metrics 100%) | IPF | ||||||||||||||||||||||||||||||||||||||||||
Payout formula | ||||||||||||||||||||||||||||||||||||||||||||
ABS2 | X | STI Target | X | BPF | X | IPF | = | STI Payout | ||||||||||||||||||||||||||||||||||||
BPF maximum 150% x IPF maximum 150% = maximum 225% (capped at 200% of STI target) | ||||||||||||||||||||||||||||||||||||||||||||
Payout range | 0 - 200% | |||||||||||||||||||||||||||||||||||||||||||
1Financial achievements are measured in constant exchange rates to reflect operational performance and exclude the impact of acquisitions, divestitures and certain non-recurring items in accordance with the short-term incentive plan.
2ABS earned during the financial year.
Each ECA member has five individual performance goals with each having specific measurable objectives and initiatives. In 2023, the Board and CC continued to incorporate the achievement of social impact and sustainability objectives in determining the IPF for ECA members and overall STI payout. The five focus areas are as outlined below:
•Key strategic business and customer objectives;
•Advancing product innovation and delivery;
•Alcon's transformation program;
90
•Social impact and sustainability objectives, including environmental sustainability, diversity and inclusion, and company culture and talent programs; and
•Achieving a range of key financial and operational performance measures.
At the end of the year, the Board and CC assess each ECA member's achievement of performance objectives to determine their individual performance and IPF which directly impacts the final STI payout amount.
Performance levels, thresholds, targets and maximum values for the financial performance metrics are determined at the beginning of each one-year performance period. In line with good governance practice, the Board and the CC set targets that are appropriately ambitious and aligned with the Company’s business strategy and the Board’s strategic plan without encouraging the ECA member to take undue risks.
At the end of the performance period, the Board and the CC determine the financial performance achievement against the targets originally set and determine the BPF. In addition, they consider the IPF of each ECA member. The IPF is determined by the achievement of individual objectives and the demonstration of values and behaviors. The individual performance rating is the basis for determining the IPF (between 0% and 150%). The CEO and other ECA members are not present when their IPF is discussed and determined.
Long-Term Incentive
The long-term incentive program is designed to make a significant portion of ECA members' compensation contingent on long-term Company performance and to ensure alignment with shareholders’ interests. LTI awards consist of PSUs, which convert to shares at vesting, contingent on the achievement of the performance measures. The annual LTI grant value at target is based on a percentage of each ECA member's annual base salary.
Exhibit 13
LTI payout opportunity as a % of annual base salary | Below threshold | at target | at maximum1 | ||||||||
David J. Endicott, CEO | 0% | 575% | 1150% | ||||||||
Other members of the ECA (average) | 0% | 180% | 360% | ||||||||
1The maximum number of units that may be awarded is limited to 200% of the target number of units granted.
The metrics for the measurement of long-term performance are set out in the Exhibit below. The payout is calculated by adding the weighted achievements of the individual targets in a range from 0-200% and multiplying the number of PSUs granted by the resulting performance factor.
91
Exhibit 14
Metric | Third Party Net Sales CAGR1,2 | Core EPS CAGR2 | Share of Peers3 | Innovation4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Definition | Measures the Company's Third Party Net Sales performance | Measures the profitability by the earnings per share | Measures the Company’s market performance relative to competitors | Measures the key product pipeline and achievement of milestones | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Rationale | Fosters the Company’s Sales performance | Aligns ECA with shareholders by measuring earnings per share | Indicates relative competitive position against peers in terms of market share | Delivery of future products and key future growth drivers | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Weighting | 25% | 25% | 25% | 25% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Payout formula | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Metric 1 25% | + | Metric 2 25% | + | Metric 3 25% | + | Metric 4 25% | ||||||||||||||||||||||||||||||||||||||||||||||||||
ABS | X | LTI Target | X | Addition of weighted metrics = Performance Factor | = | Payout/Number of PSUs | ||||||||||||||||||||||||||||||||||||||||||||||||||
Weighted achievements of metrics = additive payout factor maximum 200% (cap) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Payout range | 0-200% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
1CAGR means Compound Annual Growth Rate.
2Financial achievements are measured in constant exchange rates to reflect operational performance. Impact of acquisitions, divestitures and certain non-recurring items are excluded from financial achievement in accordance with the long-term incentive plan.
3Metric “Share of peers” measures Alcon’s market share of key products in the Surgical and Vision Care segments relative to a peer group of competitors using third party syndicated data.
4The innovation metric includes 10 milestones which are generally related to timeline, cost of program development, total product cost and target product profile of achievements.
Net Sales CAGR - Measures Alcon’s Third Party Net Sales growth over a 3-year period. The goal setting process for the metric is defined by triangulating between Alcon’s internal strategic plan, expected market growth and investors' expectations.
Core EPS CAGR – Measures Alcon’s profitability growth over a 3-year period. Similar to Net Sales CAGR, goal setting process for this metric is also based on a triangulated approach of assessing Alcon’s internal strategic plan, expected market growth and investors' expectations.
Share of Peers - Measures Alcon’s market share of key product categories in the Surgical and Vision Care segments relative to a peer group of competitors. Market share performance is a relative performance measure based on independent, third-party market data, and is calculated as the change in share across the three-year period. Market share changes are weighted across multiple key product categories to develop a blended Alcon products share change for the three-year cycle.
Innovation – This metric is comprised of 10 milestones per cycle, typically five in both the Surgical and Vision Care segments. These milestones are approved by the Board’s Innovation Committee with each innovation cycle spanning a rolling three-year period with performance milestones in each year. At the completion of each cycle, the Board's Innovation Committee evaluates milestone achievement against performance tiers set at the beginning of the cycle. Milestones are linked to internal development programs, measuring against one of four performance areas:
•Timeline - measure the on-time completion of key product development activities;
•Program cost - measure budget adherence;
•Total product cost - measure the ability to meet unit cost targets; and
•Target product profile - measure the extent to which the product design achieved the intended benefits as measured by it first full year revenue
Similar to the performance target-setting and measurement of the STI award, the thresholds, targets and maximum values for the LTI performance metrics are determined at the onset of the three-year performance period. In line with good
92
governance practice, the Board and the CC set targets ensuring that they are appropriately ambitious and in support of the strategic plan but do not encourage undue risk taking.
At the end of the three-year performance period of each LTI award, the Board and the CC determine the performance achievements of each metric against the targets originally set.
At the end of the performance period of each LTI award, the Company intends to disclose in the applicable compensation report details of the final LTI payout.
Benefits
Alcon is a global company headquartered in Switzerland with multinational operations; the US serves as the largest pool for both medical device and ophthalmology talent. Of the seven ECA members, six are on Swiss employment contracts and one has an employment contract governed by US law. Six of our ECA members have been relocated to Switzerland from their home base and are supported with relocation benefits in line with our global mobility policy as highlighted below. Generally, for associates on international assignments, over 70% of the benefit costs are related to maintaining the cost of living position neutral to their home base and of the 70%, over 75% of these costs are attributable to tax neutralization.
All ECA members are enrolled in benefit plans providing for retirement income savings and insurance for disability and loss of life. These plans are in line with local market practices and legislation and are subject to the Company's plan rules and policies. The ECA members and the Company make statutory contributions.
Exhibit 15
Retirement savings and insurance contributions | Retirement and insurance benefits plan contributions provided in line with local market practice (most governed by legal provisions) - Company-paid: •Contributions to retirement savings •Insurance premiums for disability and survivor benefits •Health insurance (only in the US) •Contributions to mandatory social security systems | ||||
Other benefits | • Tax Equalization •Housing, schooling/education fees •Cost of living adjustments •International health insurance •Moving and Relocation allowance •Car allowance and other transportation expenses •Expense and representation allowance in line with Swiss market practice (covering small expenses) | ||||
93
CEO Compensation
It is the Board’s responsibility to attract and retain strong leadership talent and it considers management motivation and retention as a critical driver for Alcon’s long-term success. Alcon’s CEO, Mr. Endicott, has met or exceeded all the priorities that the Board set for him during his tenure. Below are a few key indicators of his performance:
•Delivered upon financial commitments to the investors since the spin off in 2019;
•Created value for shareholders through share price performance outpacing direct competitors since 2021;
•Delivered strong product pipeline with close to 60 product launches driving robust market share growth; over 70 products in the pipeline; and
•Achieved the two annual social goals focused on vision improvement and two climate focused environmental goals (details regarding the progress on the goals are included in the “2023 Short-Term Incentive” section).
As previewed in the Outlook section of our 2022 Compensation Report, a Board commissioned peer group benchmarking study indicated Mr. Endicott’s target compensation opportunity to be below the range that the Board considers globally competitive. In addition to this, recent changes in the landscape for ophthalmic talent, including a recent competitor spin off, led the Board to assess potential vulnerabilities to competitive threats.
The Board reviews the CEO’s compensation target opportunity against the blended peer group annually to ensure competitive target compensation levels are maintained. The Board has utilized a consistent blended peer group as a reference point for consideration of market competitive compensation since Alcon’s spin off. The Board strongly believes a set of peer companies that are similar to Alcon in size, complexity and representative of both US (availability of medical device and ophthalmic talent) and international companies, allows for relevant compensation comparisons.
Prior to 2023, the last meaningful increase to our CEO’s target compensation was made in February 2020 in the form of an increase to the LTI target award. In 2021, he received a base salary increase aligned with the global budget for our broad-based associate population. No changes were made to his compensation in 2022.
In 2023, the Board adjusted Mr. Endicott’s target compensation via an adjustment to his long-term incentive target opportunity from 430% to 575% representing a 22% increase, which consists entirely of performance share units with three-year cliff vesting. After this adjustment, Mr. Endicott’s target compensation remains slightly below the peer group median, positioned at the bottom end of our US peers and at the top end of our international peers, maintaining the balance between Swiss domiciled companies and the medical device and ophthalmic talent base, which is primarily found in the US. Additionally, Mr. Endicott’s target compensation opportunity continues to be aligned with Alcon’s pay-for-performance philosophy and is reflected in the pay mix as 87% of his pay is at risk with 72% of his at risk pay tied to the achievement of long-term strategic goals.
In conjunction with the LTI target increase, the Board increased the share ownership guidelines for the CEO from 5X to 6X of his base salary.
At target opportunity, the variable compensation represents 87% of the CEO’s total direct compensation. The average variable compensation of the other ECA members represents 72% of total direct compensation at target.
Exhibit 16 - Mix of Fixed and Variable Compensation at Target
| CEO | Other ECA members (excl. CEO) | |||||||
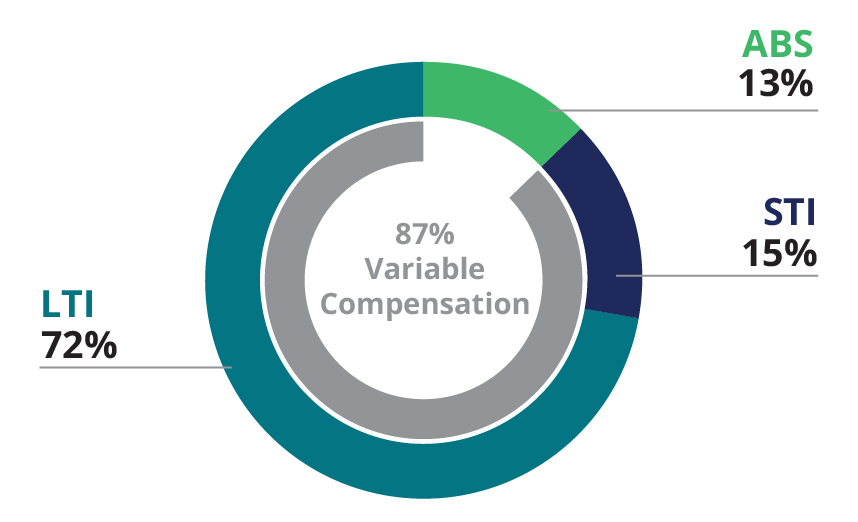 | 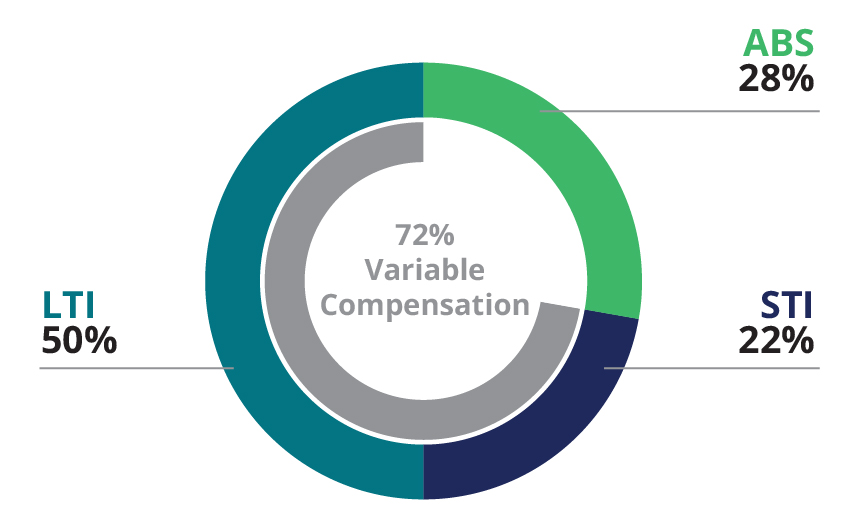 | |||||||
CEO ratios and average ECA members ratios are based on 2023 values of ABS, target STI and target 2023-2025 LTI. Mix excludes retirement, pension and insurance benefits as well as any other benefits.
94
Compensation Payments to the ECA Members
ECA Compensation Payments FY 2023
The following Exhibit 17 sets forth the total compensation received by the CEO (highest paid member of the ECA) and the aggregate total compensation received by all of the other ECA members for the period from January 1, 2023 to December 31, 2023. 2023 ECA payments are higher due to exceptional performance resulting in above target STI payout payout and the impact of year over year exchange rate.
The compensation Alcon paid to the ECA members in 2023 remained within the approved Say-On-Pay budget.
Exhibit 17
Compensation Gross Amounts | Fixed compensation | Variable compensation | Additional compen-sation | Totals in USD | Totals in CHF | ||||||||||||||||||||||||||||||||||||
From January 1, 2023 to December 31, 2023 | Annual base salary1 | Pension and insurance2 | 2023 short-term incentive3 | 2023-2025 long-term incentive4 | Other benefits5 | Total compen-sation6 | Total compen-sation6 | ||||||||||||||||||||||||||||||||||
David J. Endicott, CEO | 1,318,235 | 179,784 | 2,657,562 | 7,365,435 | 804,375 | 12,325,391 | 11,074,980 | ||||||||||||||||||||||||||||||||||
Aggregate amount of 6 other ECA members | 4,575,645 | 907,127 | 6,375,556 | 7,975,173 | 4,324,534 | 24,158,035 | 21,707,202 | ||||||||||||||||||||||||||||||||||
Totals in USD6 | 5,893,880 | 1,086,911 | 9,033,118 | 15,340,608 | 5,128,909 | 36,483,426 | |||||||||||||||||||||||||||||||||||
Totals in CHF | 5,295,946 | 976,644 | 8,116,708 | 13,784,303 | 4,608,581 | 32,782,182 | |||||||||||||||||||||||||||||||||||
1The total of Annual Base Salaries paid for the period from January 1, 2023 to December 31, 2023, including increases effective throughout the year, if applicable.
2The pension and insurance benefits are the actual contributions paid to benefit plans for the period from January 1 to December 31, 2023. It also includes the amount of USD 41,147 for mandatory contributions paid by Alcon to governmental social security systems for all ECA members, which provide the ECA members with the right to the maximum future insured government pension benefit. The aforementioned amount is a portion of a total amount of contributions of USD 1,469,798 paid by Alcon to the social security systems.
3The STI award disclosed is the amount earned for the performance year 2023. It will be paid in March 2024 in cash.
4The amounts of the 2023-2025 LTI awards represent the total value of the target number of PSUs granted to the CEO and six active ECA members on February 22, 2023. The value of the PSUs is based on the closing price of the underlying Alcon share on the date of grant of USD 72.09.
5The amounts of other benefits include the contractual Company-paid benefits, values of benefits in kind, payments made and payments or values to ECA members for the relevant period in 2023, including car allowance and other transportation expenses and benefits for international assignment (e.g, housing, schooling, tax equalization, cost of living adjustment and other international relocation benefits).
6Payments to ECA members were made in CHF and/or USD. The amounts were converted at the rate of 1.0 CHF : 1.112904 USD.
Alcon reports the 2023-2025 Long-Term Incentive Awards at the value at the time of grant in accordance with Swiss market practice. The basis for disclosure is the target value of the PSU at grant, reflecting the assumption that the awards will vest at 100% achievement, excluding any share price movement that may occur over the performance period. The future payout will be determined only after the conclusion of the performance period in three years (i.e. at the end of 2025) and the awards will vest in February 2026. The payout range is between 0% and 200% of the target number of PSUs.
95
ECA Compensation Payments FY 2022
The following Exhibit 18 sets forth the total compensation received by the CEO (highest paid member of the ECA) and the aggregate total compensation received by all of the other ECA members for the period from January 1, 2022 to December 31, 2022.
The compensation Alcon paid to the ECA members in 2022 remained within the approved Say-On-Pay budget.
Exhibit 18
Compensation Gross Amounts | Fixed compensation | Variable compensation | Additional compen-sation | Totals in USD | Totals in CHF | ||||||||||||||||||||||||||||||||||||
From January 1, 2022 to December 31, 2022 | Annual base salary1 | Pension and insurance2 | 2022 short-term incentive3 | 2022-2024 long-term incentive4 | Other benefits5 | Total compen-sation6 | Total compen-sation6 | ||||||||||||||||||||||||||||||||||
David J. Endicott, CEO | 1,240,379 | 168,035 | 2,143,375 | 5,568,577 | 1,272,305 | 10,392,671 | 9,924,481 | ||||||||||||||||||||||||||||||||||
Aggregate amount of 6 other ECA members | 4,218,777 | 775,456 | 5,213,820 | 7,814,585 | 4,619,890 | 22,642,528 | 21,622,482 | ||||||||||||||||||||||||||||||||||
Totals in USD6 | 5,459,156 | 943,491 | 7,357,195 | 13,383,162 | 5,892,195 | 33,035,199 | |||||||||||||||||||||||||||||||||||
Totals in CHF | 5,213,221 | 900,987 | 7,025,753 | 12,780,251 | 5,626,752 | 31,546,963 | |||||||||||||||||||||||||||||||||||
1The total of Annual Base Salaries paid for the period from January 1, 2022 to December 31, 2022, including increases effective throughout the year, if applicable.
2The pension and insurance benefits are the actual contributions paid to benefit plans for the period from January 1 to December 31, 2022. It also includes the amount of USD 37,765 for mandatory contributions paid by Alcon to governmental social security systems for all ECA members, which provide the ECA members with the right to the maximum future insured government pension benefit. The aforementioned amount is a portion of a total amount of contributions of USD 1,200,981 paid by Alcon to the social security systems.
3The STI award disclosed is the amount earned for the performance year 2022. It was paid in March 2023 in cash.
4The amounts of the 2022-2024 LTI awards represent the total value of the target number of PSUs granted to the CEO and six active ECA members on February 10, 2022. The value of the PSUs is based on the closing price of the underlying Alcon share on the date of grant of USD 77.73.
5The amounts of other benefits include the contractual Company-paid benefits, values of benefits in kind, payments made and payments or values to ECA members for the relevant period in 2022, including car allowance and other transportation expenses and benefits for international assignment (e.g. housing, schooling, tax equalization, cost of living adjustment and other international relocation benefits).
6Payments to ECA members were made in CHF and/or USD. The amounts were converted at the rate of 1.0 CHF : 1.047175 USD.
Outcome of Performance Awards 2023
2023 Short-Term Incentive
Alcon’s operational and financial performance exceeded the target level for all metrics. Sales out performance was driven by product innovation in both segments, strong commercial execution, better-than-expected Surgical demand in international market and better-than-expected contact lens share gains in the United States.
In the Surgical segment, Alcon's performance was driven by strong global demand for our consumables, including a faster market recovery in China than expected and an expanding equipment footprint with higher demand driven by international markets. As we continue to innovate and enhance our equipment offerings, we are confident that our devices will deliver the best and most streamlined experience for both patients and surgeons.
In the Vision Care segment, our innovative suite of lenses, including PRECISION1 and DAILIES TOTAL1, continued to outpace expectations, gain market share and drive performance. In addition, Systane and Pataday remain staples of our Ocular Health offering and once again saw higher demand than anticipated. Finally, thanks to the dedication and hard work from our supply chain and manufacturing teams, we were able to over-deliver contact lens care sales due to better product availability than initially planned.
Core operating income benefited from higher sales, manufacturing efficiencies and effective cost management resulting in improved operating leverage.
Free Cash Flow performance was above target primarily driven by our strong operating performance, partially offset by increased surgical inventory to ensure product availability.
96
In 2023, the CC commissioned a study with a third party advisor to assess Alcon's STI targets and annual STI target setting process against the peer group. The study highlighted Alcon’s overall relative pay and performance alignment and reaffirmed Alcon's robust goal setting process.
Exhibit 19 shows the weighting, target and payout level for the 2023 STI.
Exhibit 19
| Performance metric | Weighting | 2023 Target(1) ($ millions) | Payout Level(2) | Weighted Payout(3) | ||||||||||
| Third Party Net Sales | 40% | 9,363 | 123 | % | 49 | % | ||||||||
Core Operating Income(4) | 40% | 1,869 | 127 | % | 51 | % | ||||||||
Free Cash Flow(4) | 20% | 736 | 200 | % | 40 | % | ||||||||
| STI payout | 100% | 140 | % | |||||||||||
(1)Target is expressed at the exchange rates prevalent at the time of Board approval.
(2)Financial achievement is measured in constant exchange rates to reflect operational performance and excludes the impact of acquisitions, divestitures and certain non-recurring items in accordance with the short-term incentive plan.
(3)Rounded to the nearest whole %.
(4)Core Operating Income and Free Cash Flow are non-IFRS measures.
Each ECA member has five individual performance goals with each having specific measurable objectives and initiatives. In 2023, the Board and CC continued to incorporate the achievement of Social impact and sustainability goals in determining the IPF for ECA members and their overall STI payout. The five focus areas are as outlined below:
•Key strategic business and customer objectives;
•Advancing product innovation and delivery;
•Alcon's transformation program;
•Social impact and sustainability objectives, including environmental sustainability, diversity and inclusion and company culture and talent programs; and
•Achieving a range of key financial and operational performance measures.
At the end of the year, the Board and CC assess each ECA member's achievement of performance objectives to determine their individual performance and IPF which directly impacts the final STI payout amount.
For 2023, the CEO’s individual performance goals assessment is outlined below:
•Delivered strong financial results across the business; the Surgical segment grew +5% or +8% on a constant currency basis2 and the Vision Care segment grew +12% or +14% on a constant currency basis, both above reported market growth;
•Generated significant operating leverage through strong execution of operating plan; Core Operating Income2 grew +18% or +27% on a constant currency basis and Core Diluted Earnings per Share2 grew +22% to $2.74;
•Continued significant research and development investment into innovative eye care products with advancements in next-generation surgical equipment (UNITY), next-generation contact lenses (PRECISION7), and pharmaceuticals (AR-15512);
•Executed key business development and licensing deals to enhance our product flow, including improving reliability of a critical supplier and accelerating development of a surgical pipeline product;
•Completed our transformation program on-budget and on-time, re-deploying investment to R&D while creating improved operating leverage from the general and administrative functions;
•Accomplished all our diversity and inclusion goals, including diverse representation in management, while maintaining employee engagement and voluntary turnover scores above external industry benchmark;
•Achieved our annual social impact and sustainability targets: helped improve vision for over one million patients, screened 30,200 children and provided spectacles where required, exceeded our emission intensity goal by reducing utilization to 127 GHG/terajoules and achieved an estimated landfill diversion rate greater than 95.5% for non-hazardous waste from our manufacturing sites and distribution centers; and
2 Constant currency growth, core operating margin and core diluted EPS are non-IFRS measures. Refer to "Item 5.A. Operating Results—Supplementary Information—Definitions and Reconciliations of Non-IFRS Measures" section for additional information.
97
•Achieved a +14% fiscal year share price increase, exceeding our direct peer average, global industry peer average, and relevant healthcare and medical device market indices.
Based on the Board and CC’s assessment of the CEO’s performance against his individual goals in 2023, Mr. Endicott's IPF was assessed at 120% resulting in an overall STI payout of 168% (140% BPF X 120% IPF) of target. An average IPF of 118% was determined for the other ECA members resulting in an average STI payout of 166% of target.
2021-2023 Long-Term Incentive
The 2021-2023 LTI awards for the CEO and other ECA members vest in 2024. The Alcon LTI program for the ECA consists of 100% PSUs. Performance for the PSU consists of the following four metrics: Sales CAGR, Core EPS CAGR, Share of Peers and Innovation weighted equally. Alcon underwent a rigorous goal setting process to establish the construction of ambitious goals while balancing against incentivizing excessive risk taking. The CC considers a number of factors, both external and internal, including Alcon's forward-looking strategic plan, shareholders’ and analysts’ expectations regarding our future performance, general market outlook and the performance of our direct competitors to set targets that are appropriately challenging and aligned with shareholder expectations.
In 2023, the CC commissioned a study with a third party advisor to assess Alcon's targets for the in-flight PSU cycles and target setting process against the peer group. The findings of the study supported the robustness of Alcon's financial target setting process and reaffirms that Alcon's goals are challenging relative to shareholder expectations as well as to historical and peer performance.
Sales CAGR Metric
Over the three-year performance period, compounded average sales growth rate exceeded target on both a reported basis and a constant exchange rates basis. This strong performance is driven in part by the recovery of markets from the impact of COVID-19, but primarily by the strength of our innovative product offering. The Vision Care segment benefited from the success of its newest contact lenses, TOTAL30 and PRECISION1, along with the continued strength of the DAILIES TOTAL1 lens. In addition, the recent launches of toric and multifocal modalities in these brand families have further driven market share gains. Finally, the expansion of the Systane portfolio with Systane MDPF continues to drive strength in the artificial tears market. The Surgical segment has also seen exceptional equipment growth over the past three years, with our Phaco machines driving demand for replacements, upgrades and new placements. Our complete consumable portfolio continues to benefit from a healthy and growing eye care market and expanding equipment footprint. In the implantables market, PanOptix and Vivity have set the standard for premium ATIOLs and continue to lead in global market share.
Core EPS CAGR Metric
Over the three-year performance period, core EPS grew at a compounded average rate which exceeded the target on both a reported basis and a constant exchange rates basis. These strong results were driven by incremental sales and operating leverage, primarily in selling, general & administration. The careful execution of our transformation program has enabled the optimization of our cost structure, delivering leverage while we continued to invest in research & development, acquisitions and revenue generating activities. This performance was achieved despite macroeconomic challenges such as lingering impacts from the COVID-19 pandemic, higher costs from inflation and supply chain challenges.
Share-of-Peer Metric
In the Vision Care segment, our newest contact lenses, including PRECISION1, PRECISION1 for Astigmatism and TOTAL30 have driven market share gains across the 2021-2023 performance period. Alcon contact lenses have continued to grow ahead of the market since the beginning of the performance period. Systane has also outpaced the competition and gained market share over the performance period.
In the Surgical segment, Alcon grew ahead of the market, and ahead of planned targets, specifically in cataract consumables (phaco cassette paks and vit cassette paks), which reflect the share of procedures using Alcon equipment. Alcon also gained share in the monofocal IOL market, driven by the continued performance of the Clareon family of IOLs. Alcon maintained its global leadership in the advanced technology IOL category with its flagship products, PanOptix and Vivity.
Innovation Metric
We have continued to execute on our research and development strategy to meet innovation milestones for target product profile, timelines, total product cost and program costs set out for the 2021-2023 cycle. Innovation program achievements during this period included advancing key surgical equipment pipeline projects, progressing key Vision Care contact lens programs, as well as the commercial success of PRECISION1, TOTAL30 and Systane. Key milestones in the Surgical and Vision Care segments are outlined below:
98
•Achieved first year target product profile targets for new product launched for PRECISION1 for Astigmatism and SYSTANE COMPLETE, SYSTANE HYDRATION, and SYSTANE ULTRA MDPF in the Vision Care segment;
•Met timeline adherence for a next generation IOL delivery device in the Surgical segment and for multiple contact lens development programs in the Vision Care segment; and
•Delivered development cost targets for UNITY VCS/CS (next-generation cataract equipment platform) in the Surgical segment.
Exhibit 20
Performance metric | Weighting | Target | Payout Level | Weighted Payout % (0-200%)(1) | ||||||||||
Third Party Net Sales CAGR(2) | 25 | % | 7.2 | % | 200 | % | 50 | % | ||||||
Core EPS CAGR(2), (3) | 25 | % | 29.6 | % | 200 | % | 50 | % | ||||||
Share of Peers | 25 | % | Restricted Data(4) | 200 | % | 50 | % | |||||||
Innovation | 25 | % | Commercially Sensitive(5) | 125 | % | 31 | % | |||||||
PSU payout | 181 | % | ||||||||||||
(1)Rounded to the nearest whole %.
(2)Financial achievement is measured in constant exchange rates to reflect operational performance. Impact of acquisitions, divestitures and certain non-recurring items are excluded from financial achievement in accordance with the long-term incentive plan.
(3)Core EPS is a non-IFRS measure.
(4)Data is provided by third-party which restricts disclosure.
(5)Target not disclosed due to competitive nature of the metric.
Based on our results, the performance factor for the 2021-2023 PSU award was 181%. Over the three year performance period, Alcon delivered shareholder value by growing market capitalization by 17% (USD 6 billion value).
Fixed and Variable Compensation
The mix of fixed and variable compensation over the period from January 1, 2023 to December 31, 2023 is as follows:
Exhibit 21
Mix of Fixed and Variable Compensation at Actual 2023 STI Payout and 2023-2025 LTI at Grant
| CEO | Other ECA members (excl. CEO) | |||||||
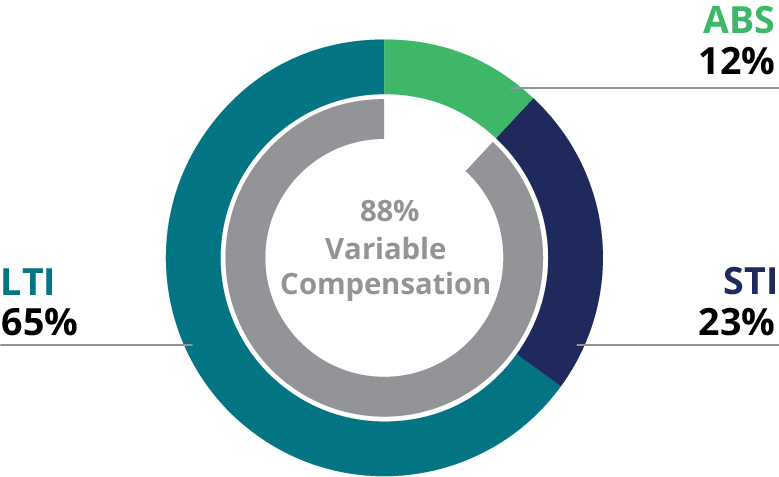 | 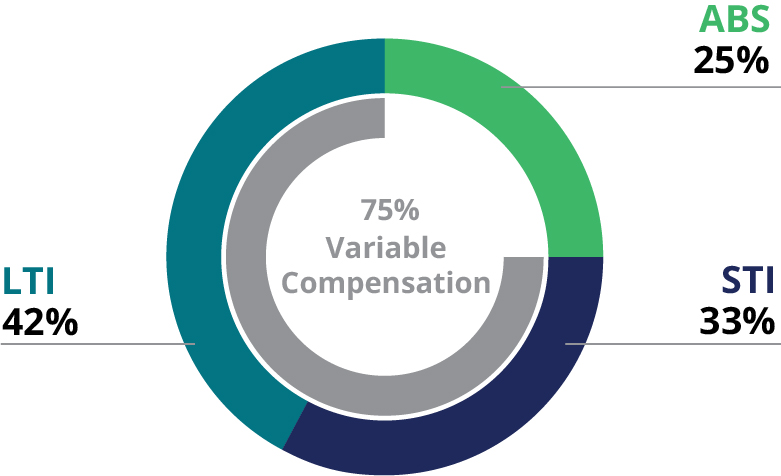 | |||||||
Average ratios are based on ABS, payout of 2023 STI (in March 2024) and grants of 2023-2025 LTI awards at grant value. Mix excludes retirement, pension and insurance benefits as well as any other benefits.
99
Equity Instruments Granted to the ECA Members
Equity Instruments Granted in FY 2023
The LTI awards (in PSUs) for the performance period 2023-2025 were granted on February 22, 2023 to the CEO and the six other members of the ECA. The number of PSUs are set out in Exhibit 22 below. The values of the awards are based on the closing price of the underlying Alcon share on the date of grant and disclosed in section "ECA Compensation Payments FY 2023", Exhibit 17.
Exhibit 22
Number of units granted to | 2023 PSUs based on the 2023-2025 LTI target Award1 | ||||
David J. Endicott, CEO | 102,170 | ||||
Other ECA members | 110,628 | ||||
Total | 212,798 | ||||
1The values of the awards in PSUs are disclosed under "ECA compensation payments FY 2023" (Exhibit 17).
Equity Instruments Granted in FY 2022
The LTI awards (in PSUs) for the performance period 2022-2024 were granted on February 10, 2022 to the CEO and the six other members of the ECA. The number of PSUs are set out in Exhibit 23 below. The values of the awards are based on the closing price of the underlying Alcon share on the date of grant and disclosed in section "ECA Compensation Payments FY 2022", Exhibit 18.
Exhibit 23
Number of units granted to | 2022 PSUs based on the 2022-2024 LTI target Award1 | ||||
David J. Endicott, CEO | 71,640 | ||||
Other ECA members | 100,535 | ||||
Total | 172,175 | ||||
1The values of the awards in PSUs are disclosed under "ECA compensation payments FY 2022" (Exhibit 18).
100
Share Ownership of the ECA Members
The number of Alcon shares or share-based units held by ECA members and “persons closely linked” (as defined below) to them as of each of December 31, 2023 and December 31, 2022 is set out in the Exhibit below. As of each of these dates, no ECA members, either individually or together with “persons closely linked”, owned 1% or more of the outstanding shares of Alcon.
Exhibit 24
| Number of units | December 31 | Vested shares | Unvested RSUs | Unvested target PSUs | Total | ||||||||||||
David J. Endicott | 2023 | 232,914 | - | 251,726 | 484,640 | ||||||||||||
| 2022 | 143,412 | 14,032 | 228,526 | 385,970 | |||||||||||||
Laurent Attias | 2023 | 19,637 | - | 33,886 | 53,523 | ||||||||||||
| 2022 | 16,959 | 2,033 | 33,314 | 52,306 | |||||||||||||
Ian Bell | 2023 | 40,243 | - | 54,562 | 94,805 | ||||||||||||
| 2022 | 22,701 | 2,853 | 49,810 | 75,364 | |||||||||||||
Leon Sergio Duplan Fraustro | 2023 | 21,166 | - | 61,072 | 82,238 | ||||||||||||
| 2022 | 13,119 | 2,833 | 59,156 | 75,108 | |||||||||||||
| Sue-Jean Lin | 2023 | 48,161 | 1,971 | 37,315 | 87,447 | ||||||||||||
| 2022 | 32,565 | 7,659 | 35,298 | 75,522 | |||||||||||||
Rajkumar Narayanan | 2023 | 34,609 | - | 41,634 | 76,243 | ||||||||||||
| 2022 | 24,283 | 2,321 | 37,803 | 64,407 | |||||||||||||
Tim C. Stonesifer | 2023 | 97,029 | - | 92,316 | 189,345 | ||||||||||||
| 2022 | 60,754 | 4,989 | 92,426 | 158,169 | |||||||||||||
Total | 2023 | 493,759 | 1,971 | 572,511 | 1,068,241 | ||||||||||||
| 2022 | 313,793 | 36,720 | 536,333 | 886,846 | |||||||||||||
101
Additional Disclosures
Employment Agreements
The Company and the members of the ECA entered into employment agreements for an indefinite period of time. Six of seven ECA members’ employment agreements are governed by Swiss law. The seventh ECA member’s employment agreement is governed by US law.
All employment contracts with ECA members provide for advanced notice of termination of employment, none of which exceed a 12-month period in accordance with our Articles of Incorporation. None of the employment agreements with the ECA members provide for any severance payment. For a description of Alcon’s change of control mechanisms, please see “Item 6. Directors Senior Management and Employees–6.C. Board Practice–Corporate Governance–“Executive Committee.”
Such employment agreements also prohibit the ECA member from competing against Alcon for a period up to 12 months after termination in accordance with our Articles of Incorporation.
Payments to Current or Former Members of the ECA
During 2023, no payments (or waivers of claims) other than those set out in Exhibit 17 (including the related notes) under section "ECA Compensation Payments FY 2023" were made to current or former members of the ECA or to “persons closely linked” to them.
Loans to Members of the ECA
Alcon’s Articles of Incorporation and corporate policies do not permit loans to current or former members of the ECA or to “persons closely linked” to them. As a result, no loans were granted in 2023, and none were outstanding as of December 31, 2023.
Persons Closely Linked
Persons closely linked to members of the ECA are (i) their spouse, (ii) their children below age 18, (iii) any legal entities that they own or otherwise control, (iv) any legal or natural person who is acting as their fiduciary or agent and (v) family trusts.
Compensation Expense 2023
The total expense for the year 2023 for compensation awarded to ECA members, using International Financial Reporting Standards (IFRS) measurement rules, is presented in Note 24 to the Company’s audited Consolidated Financial Statements. The numbers for compensation expense in Note 24 may differ from the numbers reported in this 2023 Compensation Report due to the accounting and disclosure standards applied.
Alcon Share-Based Units Awarded to Alcon Associates in 2023
In the financial year 2023, the total of approximately 2.3 million restricted shares, RSUs and target PSUs (all unvested) were granted, and approximately 2.0 million Alcon shares vested and were delivered to Alcon associates under the various equity-based incentive or participation plans. Current unvested equity instruments (restricted shares, RSUs and target PSUs) represent approximately 1% of issued shares. Alcon delivers treasury shares to associates to fulfill these obligations.
ECA Mandates Outside of Alcon
Pursuant to Swiss law, the Compensation Report must specify the functions of the ECA members in other for-profit undertakings. According to article 34 of Alcon's Articles of Incorporation, there are limitations as to the number of mandates outside of Alcon that each of our ECA members may have. As of December 31, 2023, the following external mandates in for-profit undertakings are subject to these limitations. Listed companies are denoted with an asterisk (*).
Exhibit 25
| Sue-Jean Lin | Arcutis Biotherapeutics, Inc.* | Board member | ||||||
| Other Members | None | |||||||
102
Board of Directors Compensation 2023
Compensation Framework
The Board compensation was set at a level that allowed for the attraction and appointment of high-caliber talent for Board roles with the relevant background and skills, including global experience in the medical devices and ophthalmology industries. The Board is comprised of both Swiss and international members.
Non-executive Board members are awarded a base fee. Further, they are entitled to additional fees for their roles of Chair and/or member on the Board committees. The Vice Chair also receives an additional fee. The Board Chair does not receive additional fees for work in committees. David J. Endicott, the CEO of Alcon, does not receive any fees for his Board membership. Mr. Endicott is compensated as a member of the ECA and his compensation is disclosed in section "ECA Compensation 2023." At the 2023 AGM, shareholders approved an increase to the Board fees, Board Chair fee and committee membership fees as part of the SOP budget increase. It was the first ever increase in Board member fees.
The following table sets out the compensation for the non-executive members of the Board from the 2023 AGM to the 2024 AGM:
Exhibit 26
Board function | CHF | USD1 | ||||||
Annual base fee: | ||||||||
Board Chair | 1,150,000 | 1,279,840 | ||||||
Board member base fee (Board retainer fee) | 205,000 | 228,145 | ||||||
Additional fees: | ||||||||
Vice Chair | 40,000 | 44,516 | ||||||
Chair of the Audit and Risk Committee | 70,000 | 77,903 | ||||||
| Chair of the Compensation Committee | 60,000 | 66,774 | ||||||
Chair of the Governance and Nomination Committee | 60,000 | 66,774 | ||||||
Chair of the Innovation Committee | 60,000 | 66,774 | ||||||
Member of the Audit and Risk Committee | 35,000 | 38,952 | ||||||
| Member of the Compensation Committee | 30,000 | 33,387 | ||||||
Member of the Governance and Nomination Committee | 30,000 | 33,387 | ||||||
Member of the Innovation Committee | 30,000 | 33,387 | ||||||
1The Board fees are converted at the rate of 1.0 CHF : 1.112904 USD.
In 2023, the following framework applied to the compensation of non-executive Board members:
•Fifty percent of the total fees is paid in shares on a mandatory basis in two installments: September 2023 and March 2024;
•Fifty percent of the total fees is paid in cash in four installments: June, September and December 2023 and March 2024;
•Each Board member may elect to receive up to one hundred percent of their fees in shares;
•The fees are paid in Swiss Francs;
•The shares delivered are unrestricted (free shares) listed on the SIX Swiss Exchange;
•The members of the Board are subject to share ownership requirements (as noted in Exhibit 27);
•Board members bear the full cost of their own social security contributions; and
•Board members do not receive variable compensation, in line with their focus on corporate strategy, supervision and governance. Their payment in shares is in unrestricted shares. They do not receive share options or other share-based instruments.
The general principles of compensation of the members of the Board are defined in our Articles of Incorporation. According to our Articles of Incorporation, Alcon may enter into agreements with members of the Board relating to their compensation for a fixed term of up to one year.
103
Share Ownership Requirements for Members of the Board
Board members are committed to align their interests with those of shareholders. The Board has set forth share ownership requirements which apply to the non-executive members of the Board.
Each member of the Board, including the Board Chair, is required to own Alcon shares that represent the value of his or her annual base fee. This requirement must be met within four years in office.
Exhibit 27
Board level | Share ownership requirement | ||||
Board Chair | 1 times annual base fee, within 4 years | ||||
Other Board members | 1 times annual base fee, within 4 years | ||||
Each member of the Board has met or is on track to meet the ownership requirement. Board members are prohibited from hedging or pledging their ownership positions in Alcon shares that are part of the share ownership requirement.
Compensation Governance
Authority for Board Compensation Decisions
Decisions regarding Board compensation are taken by the Board upon proposals from the CC. The CC's proposals are based on analysis and review of compensation practices, policies and benchmarking information provided by external compensation advisors.
The Board is responsible for approving the Compensation Report and for proposing the aggregate budget of Board compensation subject to a shareholders’ vote at the applicable AGM.
Exhibit 28
Authority levels in Board compensation | CC | Board | AGM | ||||||||
Board compensation policy and principles | P | A | |||||||||
Board Chair compensation | P | A | |||||||||
Other Board member compensation | P | A | |||||||||
Share ownership requirements for Board members | P | A | |||||||||
Maximum aggregate compensation of the Board members | R | P | A1 | ||||||||
Compensation Report of the company | R | P | A2 | ||||||||
R Recommend P Propose A Approve
1binding vote
2advisory vote
The Corporate Governance Report in "Item 6.C. Board Practice" of this Annual Report provides further details to the authorities of the CC.
104
Independence of Members of the Compensation Committee
Each of the members of the CC meets the independence criteria set forth in our Board Regulations. Effective from the 2023 AGM, the CC has been comprised of the following four members: Karen J. May (Chair), Thomas H. Glanzmann, Scott Maw and Ines Pöschel. At each AGM, the shareholders elect the members of the CC individually for a term of office of one year. The Board then nominates the CC Chair. Our Articles of Incorporation permit re-election to the CC. Alcon's 2023 Corporate Governance Report contained in Item 6.C. of the Alcon 2023 Annual Report, provides details regarding the members of the Board and the independence criteria for Board members. The Board Chair, the CEO and the Secretary of the Board attend the CC meetings by invitation. None is present when decisions relating to their respective interests are taken.
The Compensation Committee’s External Advisors
During 2023, the CC retained Willis Towers Watson ("WTW") as its external compensation advisor. For the same period, the CC also retained HCM International (Switzerland) ("HCM") for advice with regard to Swiss compensation matters. The CC appointed each of them in 2019 following a thorough process of evaluating proposals from various consulting firms. During 2023, WTW provided additional services to Alcon related to, among other things, consulting services related to compensation, pension and benefit programs. During the same period, HCM did not provide additional services to Alcon.
The CC conducted a review of the support received from the selected external advisors and is satisfied with the result of the work completed in 2023. At least annually, the CC will evaluate the quality of the consulting services received and the need to use specific advisors.
Compensation of the Members of the Board of Directors
Board Compensation FY 2023
The following Exhibit 29 sets out the total compensation received by non-executive members of the Board during 2023.
The disclosed compensation represents (i) the fees paid to the members of the Board in March 2023, which was the last installment of the fees for their term of office up to the 2023 AGM, and (ii) the fees paid up to December 31, 2023 for their term of office from the 2023 AGM to the 2024 AGM.
The installment of the fees paid in March 2023 completed the delivery of all fees due for the term of office from 2022 AGM to the 2023 AGM. The total of fees paid for that term remained within the approved budget.
The fees paid between the 2023 AGM and December 31, 2023 to the members of the Board of Directors are only a part of the total fees they will receive for the service on the Board during the term of office from the 2023 AGM to the 2024 AGM (three of four installments). In accordance with our normal payout schedule, a further payment of fees in cash and shares will be made in March 2024. Total 2023 Board fees are higher than in 2022 due to the increase to the Board fees, Board Chair fee and committee membership fees as part of the SOP budget increase approved by the shareholders at the 2023 AGM. The board fee structure did not change from 2022 to 2023.
The CEO of Alcon, David J. Endicott, is not included in this Exhibit 29 as he is not compensated for his Board membership. Mr. Endicott is compensated as a member of the ECA and his compensation is disclosed in section "ECA Compensation 2023."
105
Exhibit 29
Board members, functions1 | Payment in cash | Tax and other cash2 | Payment in shares3 | Number of shares4 | Other payments5 | Total fees 2023 | ||||||||||||||
F. Michael Ball Board Chair, member GNC | — | 292,219 | 876,330 | 11,497 | — | 1,168,549 | ||||||||||||||
Lynn D. Bleil Member ARC and IC | 130,484 | 55,297 | 110,529 | 1,458 | — | 296,310 | ||||||||||||||
Raquel C. Bono Member IC | 54,180 | 73,828 | 128,656 | 1,697 | — | 256,664 | ||||||||||||||
Arthur B. Cummings Member IC | 109,337 | 59,076 | 88,947 | 1,173 | 27,320 | 284,680 | ||||||||||||||
Thomas H. Glanzmann Chair IC, member GNC, CC | — | 19,574 | 328,208 | 4,323 | 5,202 | 352,984 | ||||||||||||||
D. Keith Grossman Vice Chair, Chair GNC, member IC | — | 90,443 | 271,250 | 3,575 | — | 361,693 | ||||||||||||||
Scott H. Maw Chair ARC, member CC | — | 83,511 | 250,361 | 3,303 | — | 333,872 | ||||||||||||||
Karen J. May Chair CC, member ARC | — | 81,454 | 244,070 | 3,218 | — | 325,524 | ||||||||||||||
Ines Pöschel Member GNC, CC | 140,863 | 12,298 | 135,497 | 1,786 | 5,202 | 293,860 | ||||||||||||||
Dieter P. Spälti Member ARC | 30,619 | 13,663 | 187,341 | 2,415 | 5,202 | 236,825 | ||||||||||||||
Total fees paid in 2023 in USD 6 | 465,483 | 781,363 | 2,621,189 | 34,445 | 42,926 | 3,910,961 | ||||||||||||||
Total fees paid in 2023 in CHF 7 | 418,260 | 702,094 | 2,355,269 | 34,445 | 38,571 | 3,514,194 | ||||||||||||||
1Board Committees: “ARC” Audit and Risk Committee; "CC" Compensation Committee; “GNC” Governance and Nomination Committee; “IC” Innovation Committee. F. Michael Ball does not receive an additional fee as a member of the GNC.
2These amounts represent the values of tax and, if applicable, social security due upon the allocation of shares, which were delivered in cash to the accounts. They were then deducted and paid to the applicable authorities. Further, the amounts include the residual amount of cash resulting from rounding down the number of shares to the next whole share.
3The amounts in USD represent the converted value in CHF based on the Alcon shares granted on March 1, 2023 at the closing price of CHF 63.54 per share on the date of grant and on September 1, 2023, at the closing price of CHF 73.20. The shares granted are listed on the SIX Swiss Exchange.
4The total number of shares reported were delivered to each Board member in (i) the second installment of the fee in shares in March 2023 (2022 AGM - 2023 AGM), and (ii) the first installment of the fee in shares (term 2023 AGM - 2024 AGM). The second and final installment in shares for the services from the 2023 AGM to the 2024 AGM will be delivered in March 2024.
5Includes (i) an amount of USD 20,810 for mandatory employer contributions paid by Alcon to governmental social security systems, which provides the relevant members of the Board with a right to the maximum future insured government pension benefit (this amount is a part of total mandatory employer contributions of USD 85,640 to the governmental social security systems) and (ii) USD 22,118 paid to Dr. Cummings (or his related entities) for consulting services, including assistance with clinical trials that Dr. Cummings, as an ophthalmologist, provided to Alcon (these services were unrelated to Dr. Cummings' board service).
6All amounts include the payments made and the shares delivered in March 2023 as installment of the fee for the term of office 2022 AGM - 2023 AGM.
7The payments in cash were made in Swiss Francs (CHF). For consistency they are reported in USD as all compensation in this 2023 Compensation Report. The amounts in CHF were converted to USD at the exchange rate of 1.0 CHF : 1.112904 USD. All amounts are before deductions of social security contributions and income tax paid by the Board members.
106
Board Compensation FY 2022
The following Exhibit 30 sets out the total compensation received by non-executive members of the Board during 2022.
The disclosed compensation represents (i) the fees paid to the members of the Board in March 2022, which was the last installment of the fees for their term of office up to the 2022 AGM, and (ii) the fees paid up to December 31, 2022 for their term of office from the 2022 AGM to the 2023 AGM.
The installment of the fees paid in March 2022 completed the delivery of all fees due for the term of office from 2021 AGM to the 2022 AGM. The total of fees paid for that term remained within the approved budget.
The fees paid between the 2022 AGM and December 31, 2022 to the members of the Board of Directors are only a part of the total fees they received for the service on the Board during the term of office from the 2022 AGM to the 2023 AGM (three of four installments). In accordance with our normal payout schedule, a further payment of fees in cash and shares was made in March 2023.
The CEO of Alcon, David J. Endicott, is not included in this Exhibit as he is not compensated for his Board membership.
Exhibit 30
Board members, functions1 | Payment in cash | Tax and other cash2 | Payment in shares3 | Number of shares4 | Other payments5 | Total fees 2022 | ||||||||||||||
F. Michael Ball Board Chair, member GNC | — | 248,801 | 746,015 | 10,738 | — | 994,816 | ||||||||||||||
Lynn D. Bleil Member ARC and IC | 136,133 | 34,074 | 102,059 | 1,469 | — | 272,266 | ||||||||||||||
Raquel C. Bono Member IC | 44,178 | 22,156 | 66,199 | 985 | — | 132,533 | ||||||||||||||
Arthur B. Cummings Member IC | 117,807 | 34,648 | 83,159 | 1,197 | 21,925 | 257,539 | ||||||||||||||
Thomas H. Glanzmann Chair IC, member GNC, CC | — | 19,018 | 295,135 | 4,248 | 4,775 | 318,928 | ||||||||||||||
D. Keith Grossman Vice Chair, Chair GNC, member IC | — | 82,532 | 247,328 | 3,560 | — | 329,860 | ||||||||||||||
Scott H. Maw Chair ARC, member CC | — | 74,049 | 221,778 | 3,197 | — | 295,827 | ||||||||||||||
Karen J. May Chair CC, member ARC | — | 74,669 | 223,776 | 3,221 | — | 298,445 | ||||||||||||||
Ines Pöschel Member GNC, CC | 124,352 | 6,949 | 137,038 | 1,966 | 4,775 | 273,114 | ||||||||||||||
Dieter P. Spälti Member ARC | 92,282 | 10,884 | 173,681 | 2,472 | 4,775 | 281,622 | ||||||||||||||
Total fees paid in 2022 in USD 6 | 514,752 | 607,780 | 2,296,168 | 33,053 | 36,250 | 3,454,950 | ||||||||||||||
Total fees paid in 2022 in CHF 7 | 491,562 | 580,400 | 2,192,726 | 33,053 | 34,617 | 3,299,305 | ||||||||||||||
1.Board Committees: “ARC” Audit and Risk Committee; "CC" Compensation Committee; “GNC” Governance and Nomination Committee; “IC” Innovation Committee. F. Michael Ball does not receive an additional fee as a member of the GNC.
2.These amounts represent the values of tax and, if applicable, social security due upon the allocation of shares, which were delivered in cash to the accounts. They were then deducted and paid to the applicable authorities. Further, the amounts include the residual amount of cash resulting from rounding down the number of shares to the next whole share.
3.The amounts in USD represent the converted value in CHF based on the Alcon shares granted on March 4, 2022 at the closing price of CHF 68.66 per share on the date of grant and on September 1, 2022, at the closing price of CHF 64.18. The shares granted are listed on the SIX Swiss Exchange.
4.The total number of shares reported were delivered to each Board member in (i) the second installment of the fee in shares in March 2022 (2021 AGM - 2022 AGM), and (ii) the first installment of the fee in shares (term 2022 AGM - 2023 AGM). The second and final installment in shares for the services from the 2022 AGM to the 2023 AGM was delivered in March 2023.
5.Includes (i) an amount of USD 19,101 for mandatory employer contributions paid by Alcon to governmental social security systems, which provides the relevant members of the Board with a right to the maximum future insured government pension benefit (this amount is a part of total mandatory employer contributions of USD 94,947 to the governmental social security systems) and (ii) USD 17,150 paid to Dr. Cummings (or his related entities) for consulting services, including assistance with clinical trials that Dr. Cummings, as an ophthalmologist, provided to Alcon (these services were unrelated to Dr. Cummings' board service).
6.All amounts include the payments made and the shares delivered in March 2022 as installment of the fee for the term of office 2021 AGM - 2022 AGM.
107
7.The payments in cash were made in Swiss Francs (CHF), for consistency they are reported in USD. The amounts in CHF were converted to USD at the exchange rate of 1.0 CHF : 1.047175 USD. All amounts are before deductions of social security contributions and income tax paid by the Board members.
Share Ownership of the Members of the Board of Directors
The number of Alcon shares held by members of the Board and “persons closely linked” to them as of December 31, 2023 are set out in the Exhibit below. As of this same date, no Board member, either individually or together with “persons closely linked”, owned 1% or more of the outstanding shares of Alcon. The CEO of Alcon and Board member, David J. Endicott, is not included in this Exhibit as his share ownership is disclosed in Exhibit 24.
The number of shares held as of December 31, 2022 is shown for comparison.
Exhibit 31
Board member | 2023 Total shares | 2022 Total shares | ||||||
| F. Michael Ball | 56,082 | 44,585 | ||||||
| D. Keith Grossman | 15,133 | 11,558 | ||||||
| Lynn D. Bleil | 9,656 | 8,198 | ||||||
| Raquel C. Bono | 2,794 | 1,097 | ||||||
| Arthur B. Cummings | 5,799 | 4,626 | ||||||
| Thomas H. Glanzmann | 20,406 | 16,083 | ||||||
| Scott H. Maw | 15,067 | 11,764 | ||||||
| Karen J. May | 25,483 | 22,265 | ||||||
| Ines Pöschel | 10,498 | 8,712 | ||||||
| Dieter P. Spälti | 24,228 | 18,813 | ||||||
| Total | 185,146 | 147,701 | ||||||
Additional Disclosures
Loans to Board Members
Alcon’s Articles of Incorporation and corporate policies do not permit loans to current or former members of the Board or to persons closely linked to them. No loans were granted in 2023, and none were outstanding as of December 31, 2023.
Other Payments to Current and Former Board Members
No payments (or waivers of claims) other than those set out in Exhibit 29 (including the related notes) under section "Board Compensation FY 2023" were made to current or former Board members or to persons closely linked to them.
Persons Closely Linked
Persons closely linked to members of the Board are (i) their spouse, (ii) their children below age 18, (iii) any legal entities that they own or otherwise control, (iv) any legal or natural person who is acting as their fiduciary or agent and (v) family trusts.
108
Mandates Outside of Alcon
Pursuant to Swiss law, the Compensation Report must specify the functions of the Board members in other for-profit undertakings. According to article 34 of Alcon's Articles of Incorporation, there are limitations as to the number of mandates outside of Alcon that each of our directors may have. As of December 31, 2023, the following external mandates in for-profit undertakings are subject to these limitations. Listed companies are denoted with an asterisk (*).
Exhibit 32
| F. Michael Ball | None | |||||||
| Lynn D. Bleil | Amicus Therapeutics* | Board member | ||||||
| Sonova Holding AG* | Board member | |||||||
| Stericycle, Inc.* | Board member | |||||||
| Raquel C. Bono | HealthVerity, Inc. | Board member | ||||||
| Humana, Inc.* | Board member | |||||||
| RCB Consulting | Principal | |||||||
| Steampunk, Inc. | Board member | |||||||
| TARA Mind, Inc. | Board member | |||||||
| Arthur B. Cummings | Arthur Cummings Eye Clinic Ltd. | Board member | ||||||
| Beacon Audiology Ltd. | Board member | |||||||
| Wellington Eye Clinic | Board member | |||||||
| David J. Endicott | None | |||||||
| Thomas H. Glanzmann | Glanzmann Enterprises AG | Board member | ||||||
| Grifols, S.A.* | Chairman and CEO | |||||||
| Medtech Ventures Partners | Partner | |||||||
| D. Keith Grossman | Nevro, Inc.* | Non-Executive Chairman | ||||||
| Outset Medical, Inc.* | Board member | |||||||
| Scott H. Maw | Avista Corporation* | Board member | ||||||
| Chipotle Mexican Grill, Inc. * | Board member | |||||||
| Karen J. May | Ace Hardware Corporation | Board member | ||||||
| Ines Pöschel | Belimo Holding AG* | Board member | ||||||
| dormakaba Holding AG* | Board member | |||||||
| Graubündner Kantonalbank* | Board member | |||||||
| Reichle Holding AG | Board member | |||||||
| Dieter P. Spälti | LBK Capital Group | CEO | ||||||
| Spectrum Value Management Ltd.1 | Vice Chairman | |||||||
Landmark Capital Ltd.1 | Chairman | |||||||
SCI-Schweiz Cement Industrie AG1 | Board member | |||||||
SEO Management AG1 | Board member | |||||||
| IHAG Holding AG | Vice Chairman | |||||||
1 Under common ownership
109
Outlook for 2024
Compensation Philosophy and Principles
The Company will continue to adopt a compensation philosophy which:
•Ensures a broadly competitive level of remuneration appropriate to each executive's scale of responsibility and individual performance;
•Considers the geographic and industry-specific nature of our talent pool and the medical device industry to attract, retain and motivate a world-class executive team to drive performance;
•Supports long-term value creation for shareholders;
•Aligns the compensation program for the senior executives with the broader management and employee population;
•Fully embraces Swiss governance expectations and follows principles of simplicity and transparency; and
•Links pay to achievement of social impact and sustainability goals through the STI plan.
Exhibit 33
Pay-for-performance | •Programs are designed to compensate short-term performance and long-term success •Rewards are achieved if financial and non-financial performance metrics are met | ||||
Alignment with shareholders | •A significant part of compensation is delivered in Alcon equity •Executives are expected to hold a significant level of Alcon shares | ||||
Market competitiveness | •Overall compensation is competitive with other companies in the medical device and other industries in which Alcon competes for talent •Total opportunity is targeted at market median | ||||
Motivation and retention | •Compensation is designed to attract, retain and motivate executives to achieve Company objectives •Compensation is reviewed periodically to ensure competitiveness and alignment to key strategic objectives | ||||
ECA Compensation
The CC is committed to a strong pay-for-performance framework to align executive compensation with shareholder interests. An anchor point of our philosophy is to offer market competitive compensation closer to the range of the median of our peer group. To achieve this goal, the CC continuously reviews and benchmarks Alcon's compensation against a global peer group (42% European, 58% North American, see "Peer Group" section for details). Based on the Company’s business strategy, compensation philosophy and the analysis of peer group compensation practices, below are the key features of ECA compensation for 2024:
•Same overall structure of ECA compensation as compared to 2023 (base salary, STI, LTI and benefits);
•No compensation increase for the CEO including base salary and short and long term incentive targets;
•Continuation of robust share ownership requirements; and
•No material changes to benefits provisions.
110
Board Compensation
At the 2023 AGM, shareholders approved an increase to the Board fees, Board Chair fee and committee membership fees as part of the SOP budget increase. It was the first ever increase in Board member fees.
The Board compensation framework will remain unchanged for the upcoming term of office 2024 AGM to the 2025 AGM, including:
•The overall framework of Board compensation from the 2023 AGM to the 2024 AGM will be carried forward to the term from the 2024 AGM to 2025 AGM;
•The Board Chair fee and Board member fees will remain unchanged; and
•The payment of fifty percent in shares (mandatory) and a voluntary election of a higher percentage in shares will continue.
Shareholder Vote at the 2024 AGM
In accordance with Article 29 of the Articles of Incorporation (http://investor.alcon.com/governance//default.aspx), the Board will ask shareholders at the 2024 AGM meeting to cast a binding vote on:
•The aggregate amount of compensation payable to non-executive members of the Board for their term of office from the 2024 AGM to the 2025 AGM; and
•The aggregate amount of compensation payable to ECA members in the financial year 2025.
In addition, the Board will ask shareholders to cast an advisory vote on the 2023 Compensation Report.
The procedures of voting on the compensation of ECA members and the Board are defined in our Articles of Incorporation which allow for an additional amount of compensation to be used when adding new members to the ECA.
The Exhibit below depicts the proposal for the 2024 AGM and the respective period of the compensation affected by the vote.
Exhibit 34
Compensation Proposals for Shareholder Approval at 2024 AGM
111
(This page has been left blank intentionally.)
112
6.C. BOARD PRACTICE
Corporate Governance
Group Structure and Shareholders
Operational Group Structure
The Company, with its registered office at Rue Louis-d’Affry 6, 1701 Fribourg, Switzerland, is a corporation organized under Swiss law and is the ultimate parent company of Alcon. As of December 31, 2023, the market capitalization of the Company was $38.533 billion (CHF 32.377 billion).
Alcon is the global leader in eye care with $9.4 billion in net sales during the year ended December 31, 2023. We research, develop, manufacture, distribute and sell a full suite of eye care products within two key businesses: Surgical and Vision Care. Our Surgical business is focused on ophthalmic products for cataract surgery, vitreoretinal surgery, refractive laser surgery and glaucoma surgery. Our Vision Care business is comprised of various contact lenses and a comprehensive portfolio of ocular health products, including products for dry eye, glaucoma, contact lens care and ocular allergies, as well as ocular vitamins and redness relievers. Further information is available under "Item 4. Information on the Company" and Note 4 to the Consolidated Financial Statements.
Listed and Non-listed Companies Belonging to the Alcon Group
The registered shares of the Company are listed on the SIX Swiss Exchange (Valor 43249246 / ISIN code CH0432492467) and the New York Stock Exchange (CUSIP code H01301128). The Company owns directly or indirectly all consolidated entities of Alcon, none of which has its shares otherwise listed.
The following table lists the most significant subsidiaries of the Company, including those entities with total assets or net sales to third parties in excess of 5% of the Company’s consolidated total assets or net sales to third parties, as applicable, at December 31, 2023. The referenced share capital may not reflect the taxable share capital and does not include any paid in surplus. Further information regarding the Company’s subsidiaries is disclosed in Note 27 of the Consolidated Financial Statements. The combination of the Company’s subsidiaries disclosed in the table below and in Note 27 of the Consolidated Financial Statements does not cover all subsidiaries of the Company.
| Country of Organization/ Entity Name | Equity Interest | Principal Place of Business | Share Capital | ||||||||
| China | |||||||||||
| Alcon (China) Ophthalmic Product Co., Ltd. | 100% | Beijing | USD 60,000,000 | ||||||||
| Japan | |||||||||||
| Alcon Japan Ltd. | 100% | Tokyo | JPY 500,000,000 | ||||||||
| Switzerland | |||||||||||
| Alcon Pharmaceuticals Ltd. | 100% | Fribourg | CHF 200,000 | ||||||||
| United States | |||||||||||
| Alcon Finance Corporation | 100% | Fort Worth, TX | USD 1 | ||||||||
| Alcon Laboratories, Inc. | 100% | Fort Worth, TX | USD 1 | ||||||||
| Alcon Research, LLC | 100% | Fort Worth, TX | — | ||||||||
| Alcon Vision, LLC | 100% | Fort Worth, TX | — | ||||||||
113
Significant Shareholders
According to the Alcon share register, the following nominee shareholders held more than 3% of the share capital of Alcon Inc. as of December 31, 2023:
| Holder | Number of Shares | Percentage | ||||||
| Cede & Co (DTC nominee), New York, NY (USA) | 104,529,837 | 20.92% | ||||||
| Nortrust Nominees Limited, London (UK) | 19,151,092 | 3.83% | ||||||
In addition, according solely to disclosure of shareholdings notifications filed with (i) Alcon and the SIX Swiss Exchange ("SIX Threshold Notifications") pursuant to the obligations set forth in the Swiss Federal Act on Financial Market Infrastructure and Market Conduct in Securities and Derivatives Trading ("FMIA") and the rules and regulations promulgated thereunder or (ii) the SEC, there are two shareholders that held shares representing at least 3% of the Company’s total share capital as of December 31, 2023, but were not registered with the Alcon share register. These shareholders are identified in the table below.
The information required to be included in the SIX Threshold Notifications regarding these shareholders varies from the information required to be included in beneficial ownership statements filed with the SEC (“SEC Notification”).
Interested persons can access the relevant SIX Threshold Notifications online at the SIX Swiss Exchange: https://www.ser-ag.com/en/resources/notifications-market-participants/significant-shareholders.html#/
The below table shows the information available to the Company, based on both notification regimes, with respect to shareholders reported to have significant positions in Alcon’s share capital as of December 31, 2023:
Holder | Number of shares and voting rights as per SIX Threshold Notification | Percentage as per SIX Threshold Notification1 | Number of shares beneficially owned as per SEC Notification2 | Percentage as per SEC Notification2 | ||||||||||
BlackRock, Inc. 50 Hudson Yards New York, NY 10001 | 24,679,2313 | 5.06 % | 36,716,1554 | 7.3%4 | ||||||||||
| UBS Group AG Bahnhofstrasse 45 PO Box CH-8021 Zurich, Switzerland | N/A | N/A | 25,782,426 | 5.16%5 | ||||||||||
1 Percentages indicated in this column have been established based on the share capital of the Company registered with the commercial register of the Canton of Fribourg on the date on which the respective disclosure obligation pursuant to the FMIA was triggered. Furthermore, according to the FMIA, this shareholder is required to notify Alcon and the SIX Swiss Exchange only at the time it reaches, exceeds or falls below any of the thresholds set forth in the FMIA; therefore, its shareholding as of December 31, 2023 may differ from the figures indicated as per the contents of the relevant SIX Threshold Notification.
2 In general, under SEC rules, "beneficial ownership", for the purposes of this column, refers to shares that an entity had the power to vote or the power to dispose of and shares that such entity or individual had the right to acquire within 60 days after December 31, 2023. Information in this column is current as of February 14, 2024.
3 Based solely on a SIX Threshold Notification dated November 9, 2019. This figure does not include its derivative position.
4 Based solely on a Schedule 13G filed with the SEC on February 1, 2024.
5 Based solely on a Schedule 13G filed with the SEC on February 13, 2024.
Cross-Shareholdings
Neither the Company nor any of its consolidated entities has any shareholdings exceeding 5% of the holdings of capital or voting rights in any entity that also has shareholdings exceeding 5% of the holdings of the capital or voting rights in the Company or any of its consolidated entities.
114
Capital Structure
Share Capital
As of December 31, 2023, the share capital of Alcon Inc. was CHF 19,988,000, fully paid-in and divided into 499,700,000 registered shares, each with a nominal value of CHF 0.04.
On May 5, 2023, Alcon's shareholders approved the introduction of a capital range and a conditional share capital in Alcon's Articles of Incorporation. Further information is available under the section "Capital Range and Conditional Share Capital" as stated below.
An authority to issue shares under an authorized share capital was granted by Alcon's shareholders on January 29, 2019 and expired on January 29, 2021. Within this timeframe, the Board resolved to increase the share capital in two successive transactions: (i) on November 19, 2019 by CHF 120,000 through the issuance of 3,000,000 new registered shares and (ii) on November 10, 2020 by CHF 320,000 through the issuance of 8,000,000 new shares.
Capital Range and Conditional Share Capital
Under the capital range, and until May 5, 2028 or an earlier expiry, the Board has the authority to increase or decrease the share capital ranging from CHF 18,988,600 (lower limit) to CHF 21,986,800 (upper limit). The capital increase or decrease may be effected by (A) issuing up to the lower of (i) 49,970,000 fully paid-in registered shares and (ii) 10% of the share capital at the time of increase or (B) cancelling up to 24,985,000 registered shares, as applicable. The Board is further authorized to withdraw or restrict subscription rights of existing shareholders and allocate such rights to third parties, the Company or any of its group companies, for the purposes of (a) raising equity capital, (b) acquisition transactions, (c) broadening the shareholders constituency in certain financial or investor market or (d) Board, executive management, employees, advisors or other participation programs. Further details, including the terms and conditions of a capital increase, or decrease, respectively, can be found in Articles 4a and 4c of the Articles of Incorporation, available under https://investor.alcon.com/governance/governance/default.aspx.
The Board can also rely on a conditional share capital instrument in its Articles of Incorporation through which the share capital may be increased in an amount not to exceed CHF 1,998,800, by the issuance of up to 49,970,000 fully paid-in registered shares through the voluntary or mandatory exercise of conversion, exchange, option, warrant, subscription or other rights granted to or imposed on shareholders or third parties alone or in connection with the issuance of bonds, notes, options, warrants or other similar securities or contractual obligations of the Company or its affiliates. The conditional share capital may be used for the same purposes as stated in the preceding paragraph in connection with the capital range. Further details, including the terms and conditions of a capital increase, can be found in Articles 4b and 4c of the Articles of Incorporation, available under https://investor.alcon.com/governance/governance/default.aspx.
The introduction of the capital range and the conditional share capital was approved by Alcon's shareholders on May 5, 2023. At year-end 2023, the Board had not made use of the authority under any of the capital range or conditional share capital provisions.
Changes in Capital
| 2023 | 2022 | 2021 | ||||||||||||
| Share capital in CHF | 19,988,000.00 | 19,988,000.00 | 19,988,000.00 | |||||||||||
| Registered shares | 499,700,000.00 | 499,700,000.00 | 499,700,000.00 | |||||||||||
| Nominal value per share in CHF | 0.04 | 0.04 | 0.04 | |||||||||||
Shares, Participation Certificates and Profit-sharing Certificates
The Company has a single class of shares, being registered shares in the form of uncertificated securities (in the sense of the Swiss Code of Obligations). A portion of these uncertificated shares is issued as intermediated securities (titres intermédiés) within the meaning of the Swiss Federal Intermediated Securities Act via the settlement system operated by SIX SIS, with the remaining shares directly held through Computershare Trust Company, N.A. in the US (including shares held through Computershare Trust Company, N.A. via DTC). All Alcon shares have equal voting rights and carry equal entitlements to dividends. No participation certificates (bons de participations) or profit-sharing certificates (bons de jouissance) have been issued.
115
Based solely upon shares registered in the Alcon share registry, as of December 31, 2023, approximately 19.12% of the Company's total share capital was held in Switzerland by 75,033 registered shareholders.
Limitations on Transferability and Nominees Registrations
The Articles of Incorporation of the Company do not provide for any limitation on transferability of shares or nominees registration.
Convertible Bonds and Options
As of December 31, 2023, Alcon did not have any convertible bonds, warrants, options or other securities granting rights to Alcon shares.
Board of Directors
Composition
The Board consists of eight to 13 members according to the Articles of Incorporation. As of December 31, 2023, the size of the Board was 11 members and the Board was comprised of the following members (ages listed are as of December 31, 2023):
 | F. Michael Ball, Chairman A seasoned healthcare executive with nearly four decades of experience with global healthcare companies, including nearly a decade as the chief executive officer of medical device and pharmaceutical companies, F. Michael Ball brings extensive executive leadership experience as well as in-depth industry and Alcon-specific knowledge to the Board. He previously held the position of Chief Executive Officer of Alcon, while it was a division of Novartis, and served as a member of the Novartis Executive Committee from February 1, 2016 until June 30, 2018. He previously served as Chief Executive Officer of Hospira, Inc. from 2011 to 2015. Prior to that, Mr. Ball held a number of senior leadership positions at Allergan, Inc., including President from 2006 to 2011. Before joining Allergan, Inc. in 1995, he held roles of increasing responsibility in marketing and sales at Syntex Corporation and Eli Lilly & Co. Mr. Ball served on the board of directors of several organizations, including Kythera Biopharmaceuticals Inc., Hospira, Inc., IntraLase Corp., AdvaMed and sTec, Inc. Mr. Ball has been a member of the board of directors of the Ophthalmology Foundation since 2021. He holds a Bachelor of Science and a Master of Business Administration from Queen’s University in Canada. Key Competencies: Global Business Operations, Healthcare Industry and Marketing | ||||
Age: 68 Citizenship: Canada and United States Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
116
 | Lynn D. Bleil An experienced healthcare industry consultant with nearly three decades of experience as a Senior Partner at McKinsey & Company combined with her valuable experience as a director of publicly-held healthcare and life sciences companies, Lynn D. Bleil brings to the Board extensive US and Swiss experience, strategy and leadership. Ms. Bleil has been a member of the boards of directors of Stericycle, Inc. since 2015 (where she chairs the Nominating & Governance Committee), Sonova Holding AG since 2016 and Amicus Therapeutics, Inc. since 2018. She is a former member of the board of directors of DST Systems Inc. and Auspex Pharmaceuticals, Inc. From 1985 through 2013, Ms. Bleil was a Senior Partner at McKinsey & Company. Ms. Bleil holds a Bachelor of Science in Chemical Engineering from Princeton University and a Master of Business Administration from the Stanford Graduate School of Business, both in the United States. Key Competencies: Financial, Healthcare Industry and Regulatory/Public Policy | ||||
Age: 60 Citizenship: United States Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
 | Raquel C. Bono, M.D. A board-certified trauma surgeon and retired Vice Admiral, US Navy Medical Corps, Raquel C. Bono, M.D. was the first female three-star admiral in the medical field in the history of the US Navy, as well as the first Asian-American woman promoted to Vice Admiral. Dr. Bono previously served as Chief Health Officer at Viking, Inc. from November 2020 until December 2023. From 2015 until October 2019, Dr. Bono served as the Chief Executive Officer and Director for the Defense Health Agency (DHA) where she led a joint, integrated combat support agency that enables all branches of the US military medical services to provide healthcare services to combatant commands. Before joining the DHA, Dr. Bono spent 25 years in healthcare leadership roles, including a distinguished career in the US Navy where she was honored with the Defense Distinguished Service Medal, three Defense Superior Service Medals, four Legion of Merit Medals, two Meritorious Service Medals and two Navy and Marine Corps Commendation medals. She has served on the board of directors of Humana, Inc. since September 2020 and has been a Principal at RCB Consulting since October 2019. Dr. Bono holds a Bachelor of Arts in Psychology from the University of Texas at Austin, a Master of Business Administration from Washington State University and a Doctor of Medicine from Texas Tech University Health Sciences Center. Key Competencies: Healthcare Industry, Government Relations and Regulatory/Public Policy | ||||
Age: 66 Citizenship: United States Year of initial appointment: 2022 Expiration of current term of office: 2024 | |||||
117
 | Arthur Cummings, M.D. As a native of South Africa with a large ophthalmology practice in Ireland whose opinion is frequently sought by innovators in ophthalmology, Arthur Cummings, M.D. brings to the Board an international perspective of a physician entrepreneur and practical first-hand knowledge of the innovation that ophthalmologists seek. Dr. Cummings has been a Consultant Ophthalmologist and member of the board of directors at Beacon Hospital, since 2007, and member of the board of directors, Owner and Medical Director at Wellington Eye Clinic, since 1998, both in Dublin, Ireland. Also, he has been a member of the board of directors and the Owner of Arthur Cummings Eye Clinic Ltd. since 2014. Dr. Cummings holds a Bachelor of Science in Medicine and Surgery (MB. ChB.) and a Master of Medicine in Ophthalmology (M. Med) from the University of Pretoria, South Africa. Dr. Cummings is a Fellow of the College of Surgeons in South Africa (FCS SA) in Ophthalmology and a Fellow of the Royal College of Surgeons of Edinburgh (FRCSEd) in Ophthalmology. Key Competencies: Healthcare Industry, Marketing and Technology | ||||
Age: 61 Citizenship: Ireland and South Africa Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
 | David J. Endicott A lifelong healthcare executive with leadership experience at global pharmaceutical and medical device companies, David J. Endicott is the Chief Executive Officer of Alcon and brings to the Board an in-depth knowledge of Alcon as well as the healthcare industry. He joined Alcon, when still operating as a division of Novartis, in July 2016 as President, Commercial and Innovation, and Chief Operating Officer. Prior to joining Alcon in 2016, Mr. Endicott was President of Hospira Infusion Systems, a Pfizer company. Before joining Hospira, Mr. Endicott served as an officer and executive committee member of Allergan, Inc. where he spent more than 25 years of his career in leadership roles across Europe, Asia, Latin America and the U.S. Mr. Endicott served on the board of directors of Zeltiq, Inc. and Orexigen Therapeutics, Inc. He currently serves on the board of AdvaMed. He holds an undergraduate degree in Chemistry from Whitman College and a Master’s degree in Business Administration from the University of Southern California, both in the United States. Key Competencies: Global Business Operations, Healthcare Industry and Marketing | ||||
Age: 58 Citizenship: United States Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
118
 | Thomas Glanzmann Thomas Glanzmann, a venture capital investor with Medtech Ventures Partners where he evaluates and invests in medical device companies, brings strategic insights and financial and risk management experience to the Board, as well as his decades long experience in the healthcare industry. Thomas Glanzmann is the Founder and has been a Partner at Medtech Ventures Partners since 2017. He was appointed Executive Chairman of Grifols S.A. in February 2023 and Chief Executive Officer in May 2023. Before those appointments, Mr. Glanzmann served as Vice Chairman from 2017 until 2023, as the Chairman of its Sustainability Committee from 2020 until 2023 and as a director since 2006. He was President and Chief Executive Officer of Gambro AB from 2006 to 2011 and Chief Executive Officer and Managing Director of HemoCue AB from 2005 to 2006. Mr. Glanzmann was Senior Advisor to the Executive Chairman and Acting Managing Director of the World Economic Forum from 2004 to 2005. From 1988 to 2004, Mr. Glanzmann worked in various positions at Baxter International Inc., including President of Baxter Bioscience, Chief Executive Officer of Immuno International Co., Ltd. and President of Europe Biotech Group. In 2004, he was a Senior Vice President and Corporate Officer of Baxter Healthcare Corporation and Baxter World Trade Corporation. He holds a Bachelor of Science in Political Science from Dartmouth College in the United States, a Master of Business Administration from the IMD Business School in Switzerland and a Board of Directors Certification from the UCLA Anderson School of Management in the United States. Key Competencies: Global Business Management, Healthcare Industry and Technology | ||||
Age: 65 Citizenship: Switzerland Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
 | D. Keith Grossman D. Keith Grossman, with nearly 40 years of experience with medical devices and supplies, including as Chief Executive Officer of publicly held medical device and technology companies, brings to the Board his executive and board leadership experience as well as operational and strategic planning expertise in the healthcare industry. He has been the Chair at Nevro, Inc. since March 2019, serving as in a non-executive capacity since October 2023. Mr. Grossman also served as Nevro's Chief Executive Officer and President from March 2019 until March 2023 and as Executive Chair of Nevro until October 2023. He has also been a member of the board of directors of Outset Medical, Inc. since 2014. He was President and Chief Executive Officer of Thoratec Corporation from 1996 to 2006 and from 2014 to 2015 and was a member of the board of directors from 1996 to 2015. Mr. Grossman was Chief Executive Officer and a member of the board of directors at Conceptus, Inc. from 2011 to 2013. He was Managing Director and Senior Advisor at TPG Capital, L.P. from 2007 to 2011. Mr. Grossman also served as a member of the board of directors of ViewRay, Inc. from 2018 to 2021, Zeltiq, Inc., as Lead Director, from 2013 to 2017, Intuitive Surgical, Inc. from 2004 to 2010 and Kyphon Inc. in 2007 and served on a number of private boards of directors. Mr. Grossman holds a Bachelor of Science in Animal Sciences from The Ohio State University and Master of Business Administration in Finance from Pepperdine Graziadio Business School at Pepperdine University, both in the United States. Key Competencies: Healthcare Industry, International Supply Chain and Technology | ||||
Age: 63 Citizenship: United States Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
119
 | Scott Maw An experienced financial executive with over three decades of experience at global companies, including the Chief Financial Officer of Starbucks Corporation, Scott Maw contributes to the Board his extensive understanding of complex financial analysis and reporting and internal controls over financial reporting of a global company. He has been a member of the board of directors of Avista Corporation since 2016, where he is the Chair of the Compensation Committee, and Chipotle Mexican Grill Inc. since 2019, where he is the Lead Independent Director and Chair of the Audit Committee. Mr. Maw is also member of the board of trustees of Gonzaga University. He was a member of the board of directors of Root, Inc. from 2020 until February 2023. Previously, he was Executive Vice President and Chief Financial Officer at Starbucks Corporation from 2014 until the end of 2018, Senior Vice President in Corporate Finance from 2012 to 2013 and Senior Vice President and Global Controller from 2011 to 2012. From 2010 to 2011, he was Senior Vice President and Chief Financial Officer of SeaBright Holdings, Inc. From 2008 to 2010, he was Senior Vice President and Chief Financial Officer of the Consumer Bank at JP Morgan Chase and Company. Prior to this, Mr. Maw held leadership positions in finance at Washington Mutual, Inc. from 2003 to 2008 and at GE Capital from 1994 to 2003. Mr. Maw holds a Bachelor of Business Administration in Accounting from Gonzaga University in the United States. Key Competencies: Financial, Global Business Operations and Consumer Industry | ||||
Age: 56 Citizenship: United States Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
 | Karen May Karen May, who possesses a unique combination of having been both a financial executive and a human resource executive of global companies, brings to the Board extensive operational, financial and human capital strategy experience. Ms. May has been a member of the board of directors of Ace Hardware Corporation, where she is Chair of the Audit and Finance Committee, since 2017. Previously, Ms. May was on the board of directors of MB Financial, Inc., where she served as Chair of the Compensation Committee until 2019. From 2012 to 2018, she was the Executive Vice President and Chief Human Resources Officer at Mondelez International, Inc. (previously known as Kraft Foods, Inc.). From 2005 to 2012, Ms. May was the Executive Vice President and Chief Human Resources Officer of Kraft Foods, Inc. Between 1990 and 2005, she held various positions in Human Resources and Finance at Baxter International Inc., including Corporate Vice President and Chief Human Resources Officer, Vice President, International Finance, and Vice President, Division Controller. Prior to Baxter International Inc., Ms. May was a Certified Public Accountant in the audit practice of Price Waterhouse. Ms. May holds a Bachelor of Science in Accounting from the University of Illinois in the United States. Key Competencies: Human Capital Management, Financial and Consumer Industry | ||||
Age: 65 Citizenship: United States Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
120
 | Ines Pöschel Ines Pöschel brings to the Board not only her experience as a Swiss lawyer, particularly in corporate governance, capital markets and mergers and acquisitions, but her extensive leadership roles in public policy with her appointments on government and public commissions. Ms. Pöschel has been a Partner at Kellerhals Carrard Zurich KlG since 2007. She has been a member of the board of directors of Graubündner Kantonalbank since 2018 and Belimo Holding AG and dormakaba Holding AG since 2023. She was a director of Implenia AG from 2016 until 2022. She earned an ESG Global Designation and Certificate from Competent Boards in 2023. From 2002 to 2007, Ms. Pöschel was a Senior Associate at Bär & Karrer AG. She was a Senior Manager at Andersen Legal LLC from 1999 to 2002. Since 2016, Ms. Pöschel has been a member of the Swiss Federal expert commission for commercial register. Ms. Pöschel has a Master's in Law from the University of Zurich in Switzerland, and passed the Swiss Bar Exam in 1996. Key Competencies: ESG, Legal/Governance and Regulatory/Public Policy | ||||
Age: 55 Citizenship: Switzerland Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
 | Dieter Spälti, Ph.D. As an executive of Spectrum Value Management Ltd., the family office of an iconic industrial Swiss family, Dr. Spälti has overseen all of its investments for two decades, which allows Dr. Spälti to bring to the Board significant financial and operational experience in addition to his previous consulting experience with numerous industrial, financial and technology firms in Europe, the US and Southeast Asia. Dr. Spälti served as Managing Partner at Spectrum Value Management Ltd., Switzerland from 2002 to 2006, he was then the Chief Executive Officer from 2006 to 2021 and he continues to serve as a member of their board of directors. Dr. Spälti is also member of the board of directors of IHAG Holding AG. He was a Vice Chairman and member of the board of directors at Holcim Ltd. from 2003 to 2022 and served, or continues to serve, on the board of directors of various non-listed Swiss and international companies that are controlled by the same beneficial owner. Dr. Spälti was a Partner at McKinsey & Company from 1993 to 2001. He holds a Ph.D. in Law from the University of Zurich, Switzerland. Key Competencies: Financial, Legal/Governance and Technology | ||||
Age: 62 Citizenship: Switzerland Year of initial appointment: 2019 Expiration of current term of office: 2024 | |||||
121
Independence and Executive Function
The independence of Board members is a key element of Alcon’s corporate governance framework. Therefore, Alcon has developed a strong set of independence criteria for its Board members based on international best practice standards, including the Swiss Code of Best Practices for Corporate Governance and the NYSE standards, which can be found in the Alcon Board Regulations, available under the investor relations portion of the Alcon website (https://investor.alcon.com/governance/governance/default.aspx).
The Board assesses the independence of its Board members on a regular basis, at least annually. As of December 31, 2023, all Board members, including the Chair, qualified as independent according to Alcon independence criteria, except for David J. Endicott.
Other than Mr. Endicott, who currently serves as Alcon’s Chief Executive Officer, no Board member was a member of the management of the Company or any other Alcon consolidated subsidiary in the last three financial years up to December 31, 2023.
No Board member has a significant business relationship with the Company or with any other Alcon consolidated subsidiary.
Mr. Endicott is an executive member of the Board by reason of his function as Chief Executive Officer of Alcon. All other members of the Board are non-executive directors since none of them carries out operational management tasks within Alcon.
As of December 31, 2023, none of the Board members held any official government functions or political posts.
Limitations of Number of Mandates
No member of the Board may hold more than ten additional mandates in other companies, of which no more than four shall be in other listed companies. Chairs of the board of directors of other listed companies count as two mandates. Mandates shall mean mandates in comparable functions at other organizations with an economic purpose. Mandates in different legal entities which are under joint control or same beneficial ownership are deemed one mandate. Further details can be found in Article 34 of the Articles of Incorporation, available under https://investor.alcon.com/governance/governance/default.aspx.
Elections and Terms of Office
The Board members, the Chair of the Board and the members of the Compensation Committee shall be elected individually by the General Meeting of Shareholders for a term of office lasting until completion of the next Annual General Meeting of Shareholders. The members of the Audit and Risk Committee, Governance and Nomination Committee and Innovation Committee are appointed by the Board. The chairperson of each of the Board Committees, including the Compensation Committee, is appointed by the Board.
There is no mandatory term limit for Board members.
The rules in the Articles of Incorporation reflect the statutory legal provisions regarding the appointment of the Chair, the members of the Board, the members of the Compensation Committee and the independent proxy.
Internal Organizational Structure
General Principles and Areas of Responsibilities
The Board constitutes itself in compliance with legal requirements and taking into consideration the resolutions of the General Meeting of Shareholders. It shall elect one or two Vice-Chairs. It shall appoint a secretary, who need not be a member of the Board.
The Board is the ultimate governance body of the Company. The Board is led by its independent Chair, F. Michael Ball. Mr. Ball leads the Board in representing the interest of the Company shareholders. Notably, he (i) provides leadership to the Board, (ii) supports the CEO, (iii) ensures an efficient way of working with the Board's Committees, the CEO and the Executive Committee, (iv) leads the annual performance assessment and (v) ensures an effective communication with the shareholders and the public.
The Vice Chair is D. Keith Grossman. In this role, Mr. Grossman leads the Board as long as the Chair is incapacitated.
122
The duties of Mr. Ball and Mr. Grossman in their respective functions are described in more detail in Articles 20 and 21, respectively, of the Alcon Board Regulations (https://investor.alcon.com/governance/governance/default.aspx).
The Board is responsible for the duties assigned to it by the Articles of Incorporation and the Alcon Board Regulations, which include the overall direction and supervision of management. It holds the ultimate decision-making authority for Alcon, with the exception of any decisions reserved to the shareholders. In performing its tasks, the Board follows the highest standards of ethics, integrity and governance. It undertakes annually a self-assessment process to evaluate its performance, the performance of its committees and the individual performance of its members.
Within the limits of the law and the Articles of Incorporation, the Alcon Board has delegated certain of its duties to the Executive Committee and the Board’s Committees.
Delegation to the Executive Committee
The Board has delegated to the Executive Committee the management of the business in accordance with the terms set forth in the Alcon Board Regulations. Such delegation has been formalized in Article 12 of the Alcon Board Regulations and further regulated in a set of internal regulations. Under the lead of the Chief Executive Officer, the Executive Committee is responsible for the management of the business and functions as a coordination committee, independent of any legal entity of the Alcon Group. A non-exhaustive list of the duties assigned to the Executive Committee can be found in Article 23 of the Alcon Board Regulations (https://investor.alcon.com/governance/governance/default.aspx).
Delegation to the Board’s Committees
The Board’s Committees enable the Board to work in an efficient and effective manner, ensuring a thorough review and discussion of matters, while giving the Board more time for deliberation and decision-making. For this purpose, the Board has delegated certain of its duties to each of its four permanent committees: the Audit and Risk Committee, the Compensation Committee, the Governance and Nomination Committee and the Innovation Committee. Details of the duties, responsibilities and decision-making powers of each committee can be found in the respective committee’s charter, contained in the Alcon Board Regulations, available under https://investor.alcon.com/governance/governance/default.aspx.
123
In 2023, the composition of the respective Board’s Committees was as follows:
Name | Audit and Risk Committee | Compensation Committee | Governance and Nomination Committee | Innovation Committee | ||||||||||
F. Michael Ball | Member | |||||||||||||
Lynn D. Bleil | Member | Member | ||||||||||||
Raquel Bono | Member | |||||||||||||
Arthur Cummings | Member | |||||||||||||
| David J. Endicott | ||||||||||||||
Thomas Glanzmann | Member | Member | Chair | |||||||||||
D. Keith Grossman | Chair | Member | ||||||||||||
Scott Maw | Chair | Member | ||||||||||||
Karen May | Member | Chair | ||||||||||||
Ines Pöschel | Member | Member | ||||||||||||
| Dieter Spälti | Member | |||||||||||||
Audit and Risk Committee
The Audit and Risk Committee consisted of four members in 2023, all of whom were determined by the Board to be independent and in possession of the financial literacy and accounting or related financial management expertise, as defined in the NYSE standards. The Audit and Risk Committee meets and consults regularly with the management, the Alcon Internal Audit function, the independent external auditors and external consultants. The Audit and Risk Committee regularly reports to the full Board on its decisions and deliberations.
The primary responsibilities of this committee include:
•supervising external auditors and selecting and nominating external auditors for election at the Annual General Meeting of shareholders;
•overseeing internal auditors;
•overseeing accounting policies, financial controls and compliance with accounting and internal control standards;
•approving quarterly financial statements and financial results releases;
•overseeing internal control and compliance processes and procedures;
•overseeing compliance with laws and external and internal regulations;
•ensuring that Alcon has implemented and maintained an appropriate and effective risk management system and process;
•ensuring that all necessary steps are taken to foster a culture of risk-adjusted decision-making without constraining reasonable risk-taking and innovation;
•approving guidelines and reviewing policies and processes; and
•reviewing with management, internal auditors and external auditors the identification, prioritization and management of risks; the accountabilities and roles of the functions involved in risk management; the risk portfolio; and the related actions implemented by management.
Compensation Committee
The Compensation Committee consisted of four members in 2023, all of whom were determined by the Board to be independent. The Compensation Committee meets and consults regularly with management and external consultants. The Compensation Committee regularly reports to the full Board on its decisions and deliberations.
The primary responsibilities of this committee include:
•developing a compensation philosophy in line with the principles set forth in the Articles of Incorporation and submit to the Board;
124
•providing oversight for Alcon's human capital strategy, including talent management, ECA members succession planning, diversity and inclusion initiatives and pay equity measures;
•designing, reviewing and recommending to the Board compensation policies and programs;
•reviewing and approving a peer group of companies for executive compensation comparisons;
•advising the Board on the compensation of Directors and the Chief Executive Officer of Alcon;
•determining the compensation of ECA members;
•supporting the Board in preparing the proposals to the General Meeting of Shareholders regarding the compensation of the members of the Board and ECA;
•preparing the annual compensation report and submitting it to the Board for approval;
•establishing executive and director stock ownership guidelines and stock trading policies and monitoring compliance with such policies; and
•overseeing communication and engagement on executive compensation matters with shareholders and their advisors.
Governance and Nomination Committee
The Governance and Nomination Committee consisted of four members in 2023. The Governance and Nomination Committee meets and consults regularly with management and external consultants. The Governance and Nomination Committee regularly reports to the full Board on its decisions and deliberations.
The primary responsibilities of this committee include:
•designing, reviewing and recommending corporate governance principles to the Board;
•overseeing Alcon's strategy and reputation regarding SIS matters (including climate change) and annually approving Alcon's Social Impact and Sustainability Report;
•establishing criteria and identifying candidates for election as Directors;
•assessing existing Directors and recommending to the Board whether they should stand for re-election;
•developing and reviewing an onboarding program for new Directors and an ongoing education plan for existing Directors;
•reviewing periodically the Articles of Incorporation with a view to reinforcing shareholder rights;
•reviewing periodically the composition and size of the Board and its committees;
•directing periodic assessments of the Board, directors and committees;
•reviewing annually the independence status of each Director; and
•reviewing directorships and agreements of Directors for conflicts of interest and dealing with conflicts of interest.
Alcon is committed to fostering a sustainable business that supports the well-being of our associates, customers, communities, and planet. The Social Impact and Sustainability (SIS) objectives of Alcon are integrated into its decision-making to deliver long-term value for its shareholders. Social Impact and Sustainability is of key importance within the Alcon governance framework and is subject to the oversight of the Board, acting principally through its Governance and Nomination Committee. Under the supervision of the Governance and Nomination Committee, the Alcon SIS Executive Steering Committee, supported by dedicated working groups, is tasked with the identification and management of environmental and social initiatives. The implementation of the related strategy and day-to-day activities are conducted by subject matter experts across the enterprise, under the leadership of the Global Head of SIS.
125

Innovation Committee
The Innovation Committee consisted of five members in 2023. The Innovation Committee meets and consults regularly with management. The Innovation Committee regularly reports to the full Board on its decisions and deliberations.
The primary responsibilities of this committee include:
•providing counsel to the Board and management in the area of technology, application of technology and new business models;
•reviewing and making recommendations to the Board on internal pipeline and external investments (e.g. potential acquisitions, equity investments, alliances and collaborations) relative to Alcon’s business portfolio, forecasted capital and operating capacity during the strategic and operating reviews;
126
•reviewing, evaluating and advising the Board on the strategic direction and competitiveness of the innovation pipeline through the evaluation of key innovation metrics;
•setting, reviewing, scoring and recommending for approval any innovation metrics/targets that may be incorporated into Alcon’s incentive compensation plans applicable to the ECA members;
•assisting the Board with oversight, risk management and evaluation of management’s criteria for selecting major new R&D and BD&L projects, assessing progress against major milestones, budget execution and post-launch revenue impact;
•reviewing, discussing and informing the Board of significant emerging science, technology, programs, issues or trends relevant to Alcon; and
•reviewing such other matters in relation to Alcon research and development, technology and innovation programs as the committee may, in its own discretion, deem desirable in connection with its responsibilities.
Frequency, Duration and Attendance of the Meetings of the Board of Directors and its Committees
The Board and its Committees are convened as often as the conduct of the business may require.
In 2023, the Board and its Committees met as follows:
Board of Directors | Audit and Risk Committee | Compensation Committee | Governance and Nomination Committee | Innovation Committee | |||||||||||||
Number of meetings | 6 | 9 | 6 | 5 | 5 | ||||||||||||
Approximate average duration1 | 7 hrs | 1 hr 40 min | 2 hrs 10 min | 1 hr 50 min | 2 hrs | ||||||||||||
Overall attendance | 100% | 100% | 100 % | 100 % | 100 % | ||||||||||||
1The approximate average duration does not includes dinners, lunches or breaks.
During 2023, each Board member attended the meetings of the Board and each Committee on which he or she serves, as represented below:
Meeting attendance | Board of Directors | Audit and Risk Committee | Compensation Committee | Governance and Nomination Committee | Innovation Committee | ||||||||||||
Number of Meetings 6 | Number of Meetings 9 | Number of Meetings 6 | Number of Meetings 5 | Number of Meetings 5 | |||||||||||||
F. Michael Ball | 6 | 5 | |||||||||||||||
Lynn D. Bleil | 6 | 9 | 5 | ||||||||||||||
Raquel Bono | 6 | 5 | |||||||||||||||
Arthur Cummings | 6 | 5 | |||||||||||||||
David J. Endicott | 6 | ||||||||||||||||
Thomas Glanzmann | 6 | 6 | 5 | 5 | |||||||||||||
D. Keith Grossman | 6 | 5 | 5 | ||||||||||||||
Scott Maw | 6 | 9 | 6 | ||||||||||||||
Karen May | 6 | 9 | 6 | ||||||||||||||
Ines Pöschel | 6 | 6 | 5 | ||||||||||||||
Dieter Spälti | 6 | 9 | |||||||||||||||
127
Board Evaluation and Education
The Governance and Nomination Committee and the Chair of the Board coordinate an annual self-evaluation of the Board and its Committees, which includes individual interviews with the Board Chair and the completion of a confidential survey by Board members. The Chair summarizes for the Board the results of the evaluation, and any findings are appropriately addressed. In addition, each Committee conducts its own self-evaluation annually. Periodically, the Governance and Nomination Committee will engage a third party to conduct the Board and Committee evaluation process. In 2024, a third party conducted the interviews with Board members and provided an assessment to the Chair and the full Board.
The Board recognizes the value of independent development and learning by its members. Therefore, it established a Director Education Program for its members, the purpose of which is to provide for internal and external speakers on trending topics, experiential learning of Alcon and its industry through site tours and product demonstrations and, at each Board member’s option, externally provided coursework. The intent of the Director Education Program is to ensure Board members are well-versed in matters related to Alcon, its business and the rapidly changing corporate governance environment.
Information and Control System of the Board vis-à-vis the Management
The Board ensures that it receives through several channels sufficient information from the Executive Committee to perform its supervisory duties and to make the decisions that are reserved to it by law, i.e. its non-delegable decisions.
Information to the Board of Directors
The Alcon Board Regulations confer to the members of the Board the right to have full and unrestricted access to management and employees of the Company and its subsidiaries in the execution of their duties. Also, the Chief Executive Officer regularly informs the Board regarding the performance of the business including risks and potential upsides to the operating plan. The Board and its Committees meet as often as required with the Chief Executive Officer and members of the Executive Committee or other members of the senior management. Further, the Board may invite, in accordance with the Alcon Board Regulations, external advisors to attend board or committee meetings in order to obtain a third party independent perspective on certain topics. Information is further communicated to the Board through regular reports (please refer to the section below “Alcon Management Information System”).
Alcon Management Information System
The Board receives monthly reports on the financial performance of the Company, including the performance of the Surgical and Vision Care segments. On a quarterly basis, prior to the release of each quarter’s results, the Board receives the Consolidated Financial Statement information and an outlook of the full-year results together with related commentary.
On an annual basis, the Board receives and approves the financial targets for the fiscal year. Mid-year, the Board meets for a strategic review of the business and approves the strategic plan for the next five years. Additionally, throughout the year, the Board directly or through its Committees also receives reports on, among other things:
•the enterprise risk management program and risk assessment reports;
•the compliance program;
•the internal audit function;
•manufacturing and technical operations;
•research and development and product pipeline;
•SIS matters;
•organic and inorganic innovation;
•commercial strategies and product launches;
•digital commerce opportunities;
•legal matters;
•competitive developments; and
•industry trends.
In matters of significance, the Board receives direct, immediate information.
128
Internal Audit
The purpose of the internal audit function is to review Alcon’s financial, operational, information technology and compliance activities to review compliance with laws, regulations and internal policies. It also supports Alcon’s efforts to maintain accurate and timely financial reporting while seeking to add value by suggesting improvements to Alcon’s operations and to assist Alcon in achieving its strategic and financial objectives. Internal audit is led by the Chief Audit Executive (“CAE”) who functionally reports to the Audit and Risk Committee. The CAE is responsible for the development, review and modification of Alcon’s internal audit policies and procedures. The CAE reviews effectiveness and efficiency of the internal control framework with existing policies and regulations and proposes remediation actions where deficiencies were identified. The CAE periodically submits to the Audit and Risk Committee reports on the activities of the internal audit function. In 2023, internal audit was involved in a total of 77 audit engagements. The results and remediation status of these audit engagements are reported to the Audit and Risk Committee on a periodic basis. At the final meeting for the year 2023, the Audit and Risk Committee reviewed and approved the Internal Audit plan for 2024.
Internal Control System
Alcon's internal control system is designed to provide reasonable assurance to the Board and management regarding the reliability of financial reporting and accounting policies and the preparation and the presentation of the Company’s financial statements. In 2023, Alcon's internal controls framework has been fully tested for effectiveness. The Audit and Risk Committee has ultimate responsibility to oversee the adequacy and effectiveness of internal control over financial reporting.
Risk Management
The Audit and Risk Committee has the responsibility to ensure the implementation of an appropriate and effective risk management system and process and to foster a culture of risk-adjusted decision-making without constraining reasonable risk taking and innovation. It approves guidelines and reviews policies and processes. In addition, the Audit and Risk Committee reviews with management, internal auditors and external auditors, the identification, prioritization and management of the risks, the accountabilities and roles of the functions involved with risk management, the risk portfolio and the related actions implemented by management. The Audit and Risk Committee informs the Executive Committee and the Board on a periodic basis on the risk management system and on the most significant risks and how these are managed. The CAE supports the Audit and Risk Committee and performs appropriate reviews of Alcon's risk management strategy.
Alcon’s key risk management tool is the Enterprise Risk Management ("ERM") program, the purpose of which is to help execute on Alcon’s strategy within the boundaries of regulations and improve the probability of achieving Alcon’s strategic and financial objectives. Alcon’s vision is to design a sustainable and appropriately scaled ERM program to proactively manage existing and emerging threats and opportunities to the business. The ERM program aims in particular to provide the business with the following: (i) operation discipline and rigor to enable business continuity, creation and preservation of value, (ii) forums for frequent risk discussions and escalation of relevant items with leadership and (iii) guidance, techniques and support to identify, assess (e.g. likelihood and impact), manage, monitor and report on major risks, including proper mitigation if necessary. The ERM program is under the supervision of a dedicated committee that is comprised of senior members of management and the members of the Audit and Risk Committee.
Compliance Function
As part of its global control system, Alcon has established a comprehensive global integrity and compliance program, under the supervision of the Audit and Risk Committee. The program is led by the Global Head, Integrity and Compliance under the functional leadership of Alcon’s General Counsel and is intended to help prevent, detect and mitigate compliance risk across the organization. The program is built on a culture and expectation of compliance at all levels. The fundamental elements of the program include dedicated resources to address compliance globally, formal compliance governance, a global intake process to receive questions and concerns (including through Alcon's Ethics Helpline), written standards, communications, training, multiple levels of risk-based auditing and monitoring, review of alleged misconduct and corrective/disciplinary actions for violations. The Audit and Risk Committee receives periodic updates on the performance of the Integrity and Compliance program and compliance related matters. The program also includes compliance committees, which have been established at the corporate, regional and country-levels and include participation by the Executive Committee and other senior leadership to provide strategic direction and oversight relating to the management of compliance risks for Alcon. Policies are reviewed and updated on a regular basis to address changes in laws and regulations and to strengthen compliance.
129
Executive Committee
Composition of the Executive Committee
As of December 31, 2023, the Executive Committee of Alcon was composed of the following members (ages listed are as of December 31, 2023):
 | David J. Endicott, Chief Executive Officer Please refer to the biography set forth under "Board of Directors." | ||||
Age: 58 Citizenship: United States | |||||
 | Laurent Attias, Head Corporate Development, Strategy, Business Development and Licensing (BD&L) and Mergers and Acquisitions (M&A) Laurent Attias is Head of Corporate Development, Strategy, BD&L and M&A where he leads the development of long-term strategic plans for the Surgical and Vision Care segments of Alcon and is responsible for Alcon’s BD&L, M&A, partnerships and alliance activities, a role he has held since 2015. Since 1994 when Mr. Attias joined Alcon, he has had various roles with increasing responsibility beginning with positions in Alcon’s Sales and Marketing functions and then holding the positions of Vice President, Refractive Sales and Marketing from 2002 to 2007; Vice President/General Manager of Alcon Canada from 2007 to 2009; Vice President, Central & Eastern Europe, Italy and Greece from 2009 to 2010; and President, Europe, Middle East and Africa (“EMEA”) from 2010 to 2012. From 2012 to 2015, as Senior Vice President of Global Commercial Franchises, Mr. Attias led all commercial execution and product pipeline activities of Alcon’s Surgical, Pharmaceutical and Vision Care franchises. Mr. Attias holds both a Bachelor of Business Administration in Marketing and a Master of Business Administration from Texas Christian University in the United States. | ||||
Age: 56 Citizenship: France and United States | |||||
130
 | Ian Bell, President, Global Business & Innovation Ian Bell has been the President, Global Business & Innovation since September 2021 where he oversees the development of new products and digital health solutions as well as the Alcon Surgical and Vision Care businesses. From January 2019 until he was appointed to his current role, he was President-International, overseeing the Europe, Russia, Middle East and Africa, Asia Pacific, Japan and Latin America and Caribbean markets. He joined Alcon in March 2016 as President of EMEA. From 2014 until joining Alcon, Mr. Bell served as Corporate Vice President and President of the EMEA region for Hospira, Inc. Mr. Bell was based in Singapore as the Corporate Vice President and President of Allergan, Inc.’s Asia Pacific region from 2008 to 2014. Mr. Bell joined Allergan in 2005 as Vice President and Managing Director of its neurosciences division for the EMEA region. He began his career at GlaxoSmithKline plc, where he held roles of increasing responsibility and scope in sales, marketing and strategy for more than 10 years. Mr. Bell holds the degree of Bachelor of Arts with honors in Economics from the University of York in the United Kingdom. | ||||
Age: 53 Citizenship: United Kingdom | |||||
 | Leon Sergio Duplan Fraustro, President North America Sergio Duplan has served as President-North America, overseeing the US and Canadian markets since 2015. Mr. Duplan joined Alcon in 2012 and served as Alcon’s President of Latin America and Canada for three years. Mr. Duplan began his career with Novartis in 2004, as Vice President of Sales in General Medicines, in Mexico then served as Head of Marketing and Sales for Latin America, General Medicines, Pharma from 2006 to 2008 and then Country Pharma Organization Head and Country President of Novartis Mexico from 2008 to 2012. Prior to joining Novartis, Mr. Duplan held several positions of increasing responsibility in sales, finance and country management at Procter & Gamble Company and Eli Lilly & Co. He is also a board member of The Alcon Foundation and Helen Keller International. Mr. Duplan holds a Bachelor's degree in Industrial Engineering from Universidad Iberoamericana in Mexico and a Master's of Business Administration from The Wharton School at the University of Pennsylvania in the United States. | ||||
Age: 56 Citizenship: Mexico and United States | |||||
131
 | Sue-Jean Lin, SVP, Chief Information & Transformation Officer Sue-Jean Lin is Senior Vice President, Chief Information and Transformation Officer where she leads the technology initiatives within Alcon and is responsible for leading the development and implementation of Alcon’s transformation program. Ms. Lin joined Alcon in August 2018 as Senior Vice President, Chief Information Officer. Prior to joining Alcon, Ms. Lin was Senior Vice President and Chief Information Officer for Hill-Rom Holdings, Inc., a global medical technology company. Prior to joining Hill-Rom in 2016, she spent more than two decades with Allergan, Inc., including as its Senior Vice President and Chief Information Officer, and before that, its Vice President of Finance & Regional Controller for the Europe, Middle East, Africa and Asia Pacific regions. In 2015, she also served as Interim Executive for Presbyterian Healthcare Services in the capacity of Senior Vice President and Chief Information Officer. She has served on the board of directors of Arcutis Biotherapeutics, Inc. since June 2021. Ms. Lin holds both a Bachelor’s degree in Accounting and a Master’s degree in Business Administration from the University of Nevada, Reno. She completed the Executive Leadership Program from the University of Southern California, Marshall School of Business, and holds a Cybersecurity Oversight certificate from the Software Engineering Institute of Carnegie Mellon University. | ||||
Age: 65 Citizenship: United Kingdom and United States | |||||
 | Rajkumar Narayanan, President, International Mr. Narayanan has been the President, International since September 2021 where he oversees the Europe, Russia, Middle East and Africa, Asia Pacific, China, Japan, Latin America and Caribbean markets. From April 2019 until he was appointed to his current role, he was Senior Vice President, Operational Strategy and Chief Transformation Officer and was responsible for leading the development and implementation of Alcon’s transformation program. He joined Alcon in June 2017 as President of the Asia Pacific region from Allergan, Inc., where he worked for more than 20 years in roles of increasing responsibility, including Senior Vice President Asia Pacific Region from 2014 to 2017; Vice President and Managing Director of the Medical Aesthetic Franchise for Europe Africa and Middle East from 2011 to 2014; and Vice-President, Greater China & Japan from 2008 to 2011. Prior to those roles, Mr. Narayanan was a part of Allergan’s Finance function in a number of country, region and corporate finance roles. Mr. Narayanan started his career in finance with Hindustan Unilever India in 1987. Mr. Narayanan holds a Bachelor of Science degree in Accounting and Finance from Mumbai University. He is also a Chartered Accountant and a Cost and Works Accountant in India. | ||||
Age: 59 Citizenship: United States | |||||
132
 | Tim C. Stonesifer, Chief Financial Officer Tim Stonesifer has been the Chief Financial Officer since April 2019. Prior to joining Alcon, he had served as Executive Vice President and Chief Financial Officer at Hewlett Packard Enterprise from November 2015 through September 2018. Prior to that role, Mr. Stonesifer acted as Senior Vice President and Chief Financial Officer, Enterprise Group at HP Co. since 2014. Before joining HP Co., he served as Chief Financial Officer of General Motors’ International Operations from 2011 to 2014. Previously, he served as Chief Financial Officer of Alegco Scotsman, a storage company, from 2010 to May 2011; Chief Financial Officer of Sabic Innovative Plastics (formerly GE Plastics) from 2007 to 2010; and various other positions at General Electric since joining the company in 1989. He has served on the board of directors of Insulet Corporation since January 2024. Mr. Stonesifer holds a Bachelor of Arts in Economics from the University of Michigan in the United States. | ||||
Age: 56 Citizenship: United States | |||||
Role of the Executive Committee
The members of the Executive Committee are appointed by the Board. In accordance with the Articles of Incorporation and the Alcon Board Regulations, the Board delegated the responsibility for the management of the business to the Executive Committee, under the lead of the Chief Executive Officer.
The Executive Committee shall in particular (i) develop strategies and policies and implement those upon approval by the Board, (ii) coordinate and monitor the group’s functions to achieve the business targets, (iii) ensure the efficient operation of the group, (iv) manage the proper provision and use of capacity and financial and other resources within the group and (v) ensure the development and succession of the senior management.
Alcon has not entered into any management agreements with any third parties pursuant to which Alcon would delegate any business management responsibilities to any such third parties.
As of December 31, 2023, none of the members of the Executive Committee held any official functions or political posts.
Limitations of Number of Mandates
No member of the Executive Committee may hold more than six additional mandates in other companies, of which no more than two additional mandates shall be in other listed companies. Each of these mandates shall be subject to approval by the Board. Members of the Executive Committee are not allowed to hold chairs of the board of directors of other listed companies. Mandates shall mean mandates in comparable functions at other organizations with an economic purpose. Mandates in different legal entities which are under joint control are deemed one mandate. Further details can be found in Article 34 of the Articles of Incorporation, available under https://investor.alcon.com/governance/governance/default.aspx.
Compensation, Shareholdings and Loans
Please refer to "Item 6.B - Compensation".
133
Shareholders’ Participations Rights
Voting-right Restrictions and Representation
Alcon has not imposed any restriction regarding share ownership or voting rights. Nominees shareholdings are not subject to any limitations. The right to vote at Alcon general meetings may only be exercised by a shareholder, usufructuary or nominee who is duly registered in Alcon share register on the record date for the applicable general meeting. Shareholders can be represented at general meetings by the independent proxy or by a third person authorized by written proxy who does not need to be a shareholder. As required by law, shareholders will also be given the opportunity to issue their voting instructions to the independent proxy electronically through an online voting platform.
Each Alcon share has the right to one vote. Shares held by the Company or any of its consolidated subsidiaries are not entitled to vote. Votes are taken either by a show of hands or by electronic voting, unless the General Meeting of Shareholders resolves to have a ballot or where a ballot is ordered by the chairman of the meeting. Further information can be found in Article 16 of the Articles of Incorporation, available under https://investor.alcon.com/governance/governance/default.aspx.
Statutory Quorums
Unless otherwise required by law, the General Meeting passes resolutions and elections with the absolute majority of the votes duly represented.
According to Article 704 of the Swiss Code of Obligation and as reflected in Article 18 of Alcon's Articles of Incorporation, the following shareholders’ resolutions require the approval of at least two thirds of the votes represented at a General Meeting of Shareholders: (1) an alteration of Alcon’s corporate purpose; (2) the combination of shares, (3) the creation of shares with increased voting powers; (4) the change of currency of the share capital; (5) an implementation of restrictions on the transfer of registered shares and the removal of such restrictions; (6) the introduction of shares with privileged voting rights; (7) the introduction of a capital range or the introduction of a conditional share capital; (8) an increase of the share capital through the conversion of equity surplus, against contributions in kind or by set-off against a claim and the grant of special rights; (9) a restriction or suspension of rights of option to subscribe to new shares; (10) the delisting of Alcon equity securities; (11) a provision of the Articles of Incorporation on holding the General Meeting abroad; (12) the introduction of an arbitration clause in the Articles of Incorporation; (13) a change of Alcon’s registered office; (14) Alcon’s dissolution; (15) any other matter that are reserved by law or the Articles of Incorporation; or (16) any amendment to the Articles of Incorporation which would create or eliminate a qualified majority requirement.
Article 704 of the Swiss Code of Obligations also provides that the introduction by the General Meeting of a casting vote for the person chairing the General Meeting requires a qualified majority.
Swiss law further provides for a qualified majority for certain special resolutions, such as in case of merger or demerger.
Convocation of General Meetings
The Annual General Meeting shall be held within six months after the close of the financial year of the Company. Extraordinary General Meetings may be convened upon request of the Alcon Board, the auditors or one or more shareholders representing in aggregate not less than 5% of the Company’s share capital. At least 20 days before the General Meeting, the invitation including the agenda is published in the Swiss Gazette of Commerce and can be mailed to the registered shareholders.
Agenda
One or more Alcon shareholders whose combined shareholdings represent an aggregate nominal value of at least 0.5% may demand that an item be included in the agenda of a General Meeting of Shareholders, or that a proposal relating to an agenda item be included in the notice convening the General Meeting of Shareholders. Such a demand must be made in writing at the latest 45 days before the meeting and shall specify the items and the proposals of such shareholder.
Registration in the Share Register
The share register of the Company is a non-public register, subject to confidentiality and privacy and data protections imposed on Alcon to protect registered shareholders. Alcon shares can be voted only if their relevant holder is registered
134
in the Alcon share register by the record date determined by the Board. The Articles of Incorporation do not provide for any specific rule regarding the closure of the share register.
Quiet Periods
The Company has strict internal policies regarding insider trading, in line with applicable regulations and international best practice standards.
Quiet Periods start fourteen days prior to the beginning of the last trading day of each calendar quarter and end following the first full trading day after the date of the release of the quarterly and/or annual results, unless otherwise designated by the Alcon Disclosure Committee. The Company has identified a certain number of Continuing Insiders, i.e. key individuals who may continuously be in possession of material non-public information, that are prohibited from trading in any Alcon securities during Quiet Periods and may trade in any such securities outside of Quiet Periods only with the prior written approval of the Company's corporate legal department.
In addition, Alcon associates may be designated Temporary Insiders in connection with confidential projects. In this capacity, they are prohibited to trade, during a certain period of time defined as a No Trading Period, in any securities of either Alcon or another company in which any such Alcon associate may have acquired material non-public information.
Changes of Control and Defense Measures
Duty to Make an Offer
Under the Swiss Financial Market Infrastructure Act, shareholders and groups of shareholders acting in concert who acquire more than 33.3% of Alcon shares would be under an obligation to make an offer to acquire all remaining Alcon shares. Alcon has neither opted out from the mandatory takeover offer obligation nor opted to increase the threshold for mandatory takeover offers in the Articles of Incorporation.
Clauses on Change of Control
In accordance with the Swiss rules against excessive compensation in listed companies, Alcon does not provide severance payments upon a change of control or “golden parachute” provisions in its agreements with its Directors, Executive Committee members or other members of senior management. Alcon’s Long Term Incentive Plan, which is applicable to all employee participants including Executive Committee members, provides for double trigger accelerated vesting of outstanding stock awards in the event a participant leaves the company for “good reason” or Alcon terminates the employee without “cause,” as such terms are defined in the plan, within two years following a change of control. If such a double trigger event occurs, the participant’s outstanding unvested awards would vest in full. In the case of Performance Share Units, awards less than 50% vested would vest at target and awards more than 50% vested would vest in accordance with Alcon’s actual performance, as determined by the Compensation Committee.
Auditors
Duration of the Mandate and Terms of Office of the Auditors
PricewaterhouseCoopers SA, Switzerland (“PwC Switzerland”), has been the statutory auditor of the Company since 2019 and conducts the audit activities required by Swiss law and the related SIX regulations. It was re-elected on May 5, 2023 for a term of one year for the 2023 financial year. Mike Foley has been the auditor in charge of the statutory audit since 2019. Alcon has a policy to rotate the lead audit partner of the statutory auditor at least once every five years.
Separately, on February 22, 2023, the Company appointed PricewaterhouseCoopers LLP , United States (“PwC US”) (PCAOB ID No. 238 ), for a term of one year, as its independent registered accounting firm to conduct the audit activities required by US law and the related NYSE regulations. PwC US performs the audit from offices located in Fort Worth, Texas . The appointment of PwC US does not require approval of the Company’s shareholders.
135
Auditing Fees and Additional Fees
The following table sets forth the amount of audit fees, audit-related fees, tax fees and all other fees billed or expected to be billed in aggregate by PwC Switzerland, PwC US and any other member firm of PricewaterhouseCoopers International Limited that rendered audit and related services to any member of Alcon, for the fiscal years ended December 31, 2023 and December 31, 2022:
| ($ millions) | 2023 | 2022 | ||||||
| Audit fees | 9.6 | 11.0 | ||||||
| Audit related fees | 0.6 | 0.4 | ||||||
| Tax fees | 0.1 | 0.1 | ||||||
| All other fees | 0.2 | 0.2 | ||||||
| Total | 10.5 | 11.7 | ||||||
Audit fees include fees billed for professional services rendered for audits of our annual consolidated and stand-alone financial statements, reviews of consolidated quarterly financial information and statutory audits of the Company (including in particular the Compensation Report) and our subsidiaries.
Audit-related fees include fees billed, as applicable, for assurance and related services such as due diligence, accounting consultations and audits in connection with mergers and acquisitions, employee benefit plan audits, internal control reviews and consultations concerning financial accounting and reporting standards, and other limited assurance services including in connection with sustainability reporting.
Tax fees include fees billed for professional services for tax compliance, tax advice and tax planning.
All other fees include non-audit and accounting research services and readiness assessments.
Control Measures over the Activities of the Auditors
The Board has delegated to the Audit and Risk Committee the oversight of the activities of the external auditors. The Audit and Risk Committee evaluates on an annual basis the qualifications and performance of our auditors and will determine whether PwC Switzerland should be proposed to the general meeting to stand for re-election. The criteria applicable to the performance assessment of our auditors include professional competence, sufficiency of resources to complete the audit mandate, independence and objectivity, capability to provide effective and pragmatic recommendations and coordination with the Audit and Risk Committee and other functions of the Alcon group, including internal audit.
Upon recommendation of the Audit and Risk Committee, the Board proposed that the shareholders accept the audited Consolidated Financial Statements of the Alcon group and the financial statements of the Company.
The Audit and Risk Committee is further responsible for the compensation of our auditors and pre-approve all auditing services, internal control-related services and non-audit services permitted under applicable statutory law, regulations and listing requirements.
In 2023, our auditors participated in nine meetings of the Audit and Risk Committee in order to discuss auditing matters and present the 2023 audit strategy and audit results. In addition, our auditors regularly meet in private session with the Audit and Risk Committee and individually with the Chair of the Audit and Risk Committee. Our auditors provide at least once a year to the Audit and Risk Committee a report regarding (i) the external auditor’s internal quality-control procedures, (ii) any material issues raised by quality-control reviews or any inquiry or investigation by governmental or professional authorities, (iii) any step taken to deal with such issues and (iv) all relationships between the external auditor and the Alcon group.
Information Policy
Alcon is committed to pursuing an open and transparent communication with shareholders, suppliers, customers, patients and associates. It publishes information in a professional manner in accordance with best practices and legal requirements.
136
Investor Relations
Effective communication with shareholders is an important part of Alcon's governance framework. Therefore, the Company is committed to actively engaging with shareholders and keeping them informed about Alcon's business, governance, strategy and performance, in accordance with applicable laws and regulations. Supported by the Investor Relations team, the Board Chair leads and supervises the annual shareholder outreach initiative, while the CEO and the CFO are responsible for the management of the activities necessary to maintain transparent and open shareholder relationships. The Company believes engagement and dialogue with the capital markets is crucial in securing support and confidence in management's leadership and Board's governance of Alcon. The Investor Relations team regularly organizes opportunities to learn about the Company through in-person and virtual meetings throughout the year, subject to its quiet period policy.
Communications
Financial information is published in the form of annual and quarterly financial results, in accordance with internationally recognized accounting standards. Related material, including annual reports, forms 20-F, quarterly results releases, investors presentations and conference call webcasts are available on the Alcon investor relations website. From time to time, Alcon issues press releases regarding business developments. Investors may subscribe to receive email distributions providing news and notifications about Alcon. The dissemination of material information about business developments is made in accordance with the rules of the SIX and the NYSE.
Information contained in reports and releases may only be deemed accurate in any material respect at the time of the publication. Past releases are not updated to reflect subsequent events.
Alcon's website provides regular information and updates about the Company at www.alcon.com. Detailed information regarding certain topics may be found as follows:
Topic | Website | ||||
Investor relations | https://investor.alcon.com | ||||
| Calendar | https://investor.alcon.com/news-and-events/events-and-presentations/default.aspx | ||||
Media releases | https://investor.alcon.com/news-and-events/press-releases/default.aspx | ||||
Leadership | https://investor.alcon.com/governance/leadership-team/default.aspx | ||||
Governance | https://investor.alcon.com/governance/governance/default.aspx | ||||
Financials | https://investor.alcon.com/financials/quarterly-results/default.aspx | ||||
Any information included on our internet websites or the information that might be accessed through such websites is not included in this Annual Report and is not incorporated into this Annual Report by reference.
Social Impact and Sustainability Report
Alcon publishes an annual Social Impact and Sustainability Report, which describes Alcon’s corporate responsibility strategy and highlights Alcon's approach to SIS matters. This report is available at https://www.alcon.com/about-us/social-impact-and-sustainability.
Differences in Corporate Governance Standards
According to the NYSE listing standards on corporate governance, listed foreign private issuers are required to disclose any significant ways in which their corporate governance practices differ from those governance practices that must be followed by NYSE-listed US domestic companies. We briefly summarize those differences in the following paragraphs.
Responsibility of the Audit Committee with regard to Independent Auditors
Our Audit and Risk Committee is responsible for the compensation, retention and oversight of our independent statutory auditors. It assesses the performance and qualification of our statutory auditors and submits its proposal for appointment, reappointment or removal of our statutory auditors to the full Board. As required by the Swiss Code of Obligations, our Board then submits its proposal to the shareholders for their vote at the Annual General Meeting. In contrast, under NYSE listing standards, the audit committee for US domestic companies is responsible for the appointment of the independent auditors.
137
Supervision of the Internal Audit Function
The CFO and the Audit and Risk Committee share the supervisory responsibility with respect to the internal audit function. In contrast, under NYSE standards, only the audit committee supervises the internal audit function.
Responsibility of the Compensation Committee for Performance Evaluations of Senior Management
In line with Swiss law, our Compensation Committee, together with the Board, proposes for shareholder approval at the Annual General Meeting the maximum aggregate amount of compensation for the Board and the maximum aggregate amount of fixed and variable compensation for the Executive Committee. Our shareholders elect each of the members of the Compensation Committee at the Annual General Meeting. In contrast, under NYSE standards, it is the responsibility of the compensation committee to evaluate senior management performance and to determine and approve, as a committee or together with the other independent directors, the compensation for senior officers and the board. US domestic companies listed on NYSE are only required to provide shareholders a periodic advisory non-binding vote on a company’s executive compensation practices.
Shareholders’ Votes on Equity Compensation Plans
Swiss law authorizes the Board to approve equity-based compensation plans. Shareholder approval is only mandatory if equity-based compensation plans require an increase in capital. No shareholder approval is required if shares for issuance under such plans are purchased by the issuer in the open market. In contrast, the NYSE standards require shareholder approval for the establishment of and material revisions to all equity compensation plans.
138
6.D. EMPLOYEES
The table below sets forth the breakdown of the total year-end number of our full-time equivalent employees by main category of activity for the past three years.
| For the year ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Production & Supply | 12,639 | 12,815 | 12,362 | ||||||||||||||
| Marketing & Sales | 8,124 | 8,124 | 7,893 | ||||||||||||||
| General & Administration | 2,166 | 2,133 | 2,180 | ||||||||||||||
| Research & Development (including support) | 2,386 | 2,106 | 1,954 | ||||||||||||||
| Total full-time equivalent employees | 25,315 | 25,178 | 24,389 | ||||||||||||||
Unions or works councils represent a significant number of our associates. We have not experienced any material work stoppages in recent years, and we consider our employee relations to be good.
6.E. SHARE OWNERSHIP
The information set forth under “Item 6.B. Compensation” is incorporated by reference. Also, refer to Note 23 to the Consolidated Financial Statements for a discussion of our equity-based compensation programs.
139
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
7.A. MAJOR SHAREHOLDERS
The information set forth under “Item 6. Directors, Senior Management and Employees—6.C. Board Practice—Corporate Governance” is incorporated by reference.
7.B. RELATED PARTY TRANSACTIONS
In December 2023, we acquired approximately 8.5% voting interest of an associated company for $10 million which was accounted for using the equity method based on Alcon's ability to exercise significant influence. Subsequent to the acquisition of the voting interest, Alcon paid $3 million to extend the duration of its option to acquire certain exclusive commercialization rights from the associated company and recognized an intangible asset in the Consolidated Balance Sheet. Long-term convertible notes due from the associated company included in Financial assets on the Consolidated Balance Sheet amounted to $11 million as of December 31, 2023.
7.C. INTERESTS OF EXPERTS AND COUNSEL
Not Applicable.
140
ITEM 8. FINANCIAL INFORMATION
8.A. CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION
Please refer to the financial statements beginning on page F-1 of this Annual Report.
Legal Proceedings
Please refer to Note 18 Provisions and other non-current liabilities of the financial statements beginning on page F-1 of this Annual Report.
Dividend Policy
Alcon expects that it will continue to recommend to shareholders the payment of a regular annual cash dividend based on the prior year’s core net income; however, the declaration, timing and amount, including potential increases, of any dividends will be subject to the approval of our shareholders at a General Meeting. The determination of the Board as to whether to recommend a dividend and the approval of any such proposed dividend by our shareholders will depend upon many factors, including our financial condition, earnings, corporate strategy, capital requirements of our operating subsidiaries, covenants, legal requirements and other factors deemed relevant by the Board and shareholders. For additional information, see "Item 3. Key Information—3.D. Risk Factors—Risks related to the Ownership of our Shares—We may not pay or declare dividends".
For information about deduction of the withholding tax or other duties from dividend payments, see "Item 10. Additional Information—10.E. Taxation—Swiss Taxation—Swiss Residents—Withholding Tax on Dividends" and "Item 10. Additional Information—10.E. Taxation—US Federal Income Taxation—Distributions on the Shares".
8.B. SIGNIFICANT CHANGES
A discussion of significant changes in our business can be found under "Item 4. Information on the Company —4.A. History and Development of the Company", "Item 4. Information on the Company — 4.B. Business Overview" and "Item 5. Operating and Financial Review and Prospects — 5.A. Operating Results".
141
ITEM 9. THE OFFER AND LISTING
9.A. OFFER AND LISTING DETAILS
Alcon Inc. shares are listed on the SIX and the NYSE as global registered shares under the trading ticker “ALC”. As such, they can be traded and transferred across applicable borders, without the need for conversion, with identical shares traded on different stock exchanges in different currencies. During 2023, the average daily trading volume of Alcon Inc. shares was approximately 0.9 million shares on both the SIX and the NYSE.
As of the date of this Annual Report, our shares are included in a number of indices, including the “Swiss Market Index”, or SMI, the principal Swiss index published by the SIX. This index contains 20 of the largest and most liquid stocks based on market capitalization and the most active stocks listed on the SIX. The SMI indicates trends in the Swiss stock market as a whole and is one of the most widely followed stock price indices in Switzerland.
9.B. PLAN OF DISTRIBUTION
Not Applicable.
9.C. MARKETS
See “Item 9.A. Offer and listing Details.”
9.D. SELLING SHAREHOLDERS
Not Applicable.
9.E. DILUTION
Not Applicable.
9.F. EXPENSES OF THE ISSUE
Not Applicable.
142
ITEM 10. ADDITIONAL INFORMATION
10.A. SHARE CAPITAL
Not Applicable.
10.B. MEMORANDUM AND ARTICLES OF ASSOCIATION
The following is a summary of certain provisions of our Articles of Incorporation ("Articles"), our Regulations of the Board of Directors ("Board Regulations") and of Swiss law, particularly, the Swiss CO, in each case as in force on December 31, 2023. This is not a summary of all the significant provisions of the Articles, the Board Regulations or of Swiss law and does not purport to be complete. This description is qualified in its entirety by reference to the Articles and the Board Regulations, which are an exhibit to this Form 20-F, and to Swiss law.
Company Purpose
Alcon, with its registered office at Rue Louis-d’Affry 6, 1701 Fribourg, Switzerland, is a corporation organized under Swiss law and registered under number CHE-234.781.164 and is the ultimate parent company of Alcon group. Our business purpose, as stated in article 2 of the Articles, is to acquire, hold, manage, sell direct and indirect participations in enterprises of any kind, in particular in the area of health care, medical devices, biology, chemistry, physics, information technology and related areas in Switzerland and abroad. Alcon may establish enterprises of any kind in Switzerland and abroad, hold equity interest in these enterprises, and conduct their management. Furthermore, Alcon may acquire, mortgage, operate or sell real estate and intellectual property rights in Switzerland or abroad. Alcon may provide loans, guarantees and other kinds of financing and security for Alcon group companies as well as borrow and invest money on the money and capital markets. Alcon may engage in all other types of activities or transactions and may take all measures that appear appropriate to promote the purpose of Alcon or that are related to the same. In pursuing its purpose, Alcon strives to create sustainable value.
Directors
(a) According to our Board Regulations, Directors may not participate in deliberations or resolutions on matters which affect, or reasonably might affect, the Director's interests, or the interests of a person close to the Director. Furthermore, the Swiss CO requires directors and members of senior management to safeguard the interests of the corporation and, in this connection, imposes a duty of care and a duty of loyalty on such individuals. This rule is generally interpreted to mean that directors and members of senior management are disqualified from participating in decisions which affect them personally. In addition, the Swiss CO sets forth that if, in connection with the conclusion of a contract, Alcon is represented by the person with whom it is concluding the contract, such contract shall be in writing.
(b) A Board of Directors ("Board" or "Board of Directors") resolution requires the affirmative majority of the votes cast. As with any Board resolution, Directors may not vote on their own compensation. The compensation of the Directors is subject to the approval of the aggregate amounts of such compensation by a shareholders' resolution under the Swiss CO.
(c) The Articles prohibit the granting of loans or credits to Directors.
(d) The Articles does not state retirement requirements for the Directors.
(e) Our Directors are not required to be shareholders under our Articles.
Shareholder Rights
Because Alcon has only one class of registered shares, the following information applies to all shareholders.
(a) The Swiss CO requires that, among other things, at least 5% of our annual profit be retained as general reserves, so long as these reserves amount to less than 20% of our registered share capital. Swiss law and the Articles permit us to accrue additional reserves.
Under the Swiss CO, we may only pay dividends out of balance sheet profits, out of reserves created for this purpose or out of free reserves. In any event, under the Swiss CO, while the Board may propose that a dividend be paid, we may only pay dividends upon shareholders' approval at a General Meeting of Shareholders. Our auditors must confirm that the
143
dividend proposal of our Board conforms with the Swiss CO and the Articles. Our Board intends to propose a dividend once each year. See "Item 8. Financial Information—Item 8.A. Consolidated Statements and Other Financial Information—Dividend Policy".
To the extent approved, dividends are usually due and payable shortly after the shareholders have passed a resolution approving the payment. Dividends which have not been claimed within five years after the due date revert to us, and are allocated to our general reserves. For information about deduction of the withholding tax or other duties from dividend payments, see "—Item 10.E. Taxation".
(b) Each share is entitled to one vote at a General Meeting of Shareholders. Voting rights may only be exercised for shares registered on the Alcon share register on the record date for the applicable General Meeting of Shareholders. In order to do so, the shareholder must file a share registration form with us, setting forth the shareholder's name, address and domicile (or, in the case of a legal entity, its registered office). If the shareholder has not timely filed the form, then the shareholder may not vote at, or participate in, General Meetings of Shareholders. Shareholders should contact their bank or broker if they wish to register their Alcon shares. Acquirers of Alcon shares that are registered on the Alcon U.S. share register maintained by Alcon's U.S. share registrar, Computershare Trust Company, N.A., should file a registration form with Computershare Trust Company, N.A.
Except as noted in the paragraph immediately below, shareholders' resolutions require the approval of a majority of the votes present or represented at a General Meeting of Shareholders. As a result, abstentions have the effect of votes against such resolutions. Some examples of shareholders' resolutions requiring a vote by such "absolute majority of the votes" are (1) amendments to the Articles; (2) elections of Directors, the Chair of the Board, the compensation committee members, the independent proxy and the statutory auditors; (3) approval of the management report, the consolidated financial statements and any other reports in accordance with the law or the Articles; (4) approval of the financial statements and appropriation of available earnings, such as the dividend, if any; (5) approval of the aggregate amounts of compensation of the Directors and the members of the ECA; (6) decisions to discharge Directors and the members of the ECA from liability for matters disclosed to the General Meeting of Shareholders; and (7) the repayment of the statutory capital reserve.
According to Article 704 of the Swiss CO and as reflected in Article 18 of Alcon's Articles of Incorporation, the following shareholders’ resolutions require the approval of at least two thirds of the votes represented at a General Meeting of Shareholders: (1) an alteration of Alcon’s corporate purpose; (2) the combination of shares, (3) the creation of shares with increased voting powers; (4) the change of currency of the share capital; (5) an implementation of restrictions on the transfer of registered shares and the removal of such restrictions; (6) the introduction of shares with privileged voting rights; (7) the introduction of a capital range or the introduction of a conditional share capital; (8) an increase of the share capital through the conversion of equity surplus, against contributions in kind or by set-off against a claim and the grant of special rights; (9) a restriction or suspension of rights of option to subscribe to new shares; (10) the delisting of Alcon equity securities; (11) a provision of the Articles of Incorporation on holding the General Meeting abroad; (12) the introduction of an arbitration clause in the Articles of Incorporation; (13) a change of Alcon’s registered office; (14) Alcon’s dissolution; (15) any other matter that are reserved by law or the Articles of Incorporation; or (16) any amendment to the Articles of Incorporation which would create or eliminate a qualified majority requirement. Article 704 of the Swiss CO further provides that the introduction by the General Meeting of a casting vote for the person chairing the General Meeting requires a qualified majority. Swiss law further provides for a qualified majority for certain special resolutions, such as in case of merger or demerger.
Our shareholders are required to annually elect all of the members of the Board, as well as the Chair of the Board, the members of the compensation committee and the independent proxy. The Articles do not provide for cumulative voting of shares.
At General Meetings of Shareholders, shareholders can be represented by the independent proxy or by a third person authorized by written proxy who does not need to be a shareholder. Each Alcon share has the right to one vote. Shares held by Alcon or any of its consolidated subsidiaries are not entitled to vote. Votes are taken either by a show of hands or by electronic voting, unless the General Meeting of Shareholders resolves to have a ballot or where a ballot is ordered by the chair of the meeting.
(c) Shareholders have the right to allocate the profit shown on our balance sheet and to distribute dividends by vote taken at the General Meeting of Shareholders, subject to the legal requirements described in "—Shareholder Rights".
(d) Under the Swiss CO, any surplus arising out of a liquidation of Alcon (i.e., after the settlement of all claims of all creditors) would be distributed to the shareholders in proportion to the paid in nominal value of their shares.
(e) The Swiss CO limits a corporation's ability to hold or repurchase its own shares. We and our subsidiaries may only repurchase shares if we have freely disposable equity available in the amount necessary for this purpose. The aggregate nominal value of all Alcon shares held by us and our subsidiaries may not exceed 10% of our registered share capital.
144
However, it is accepted that a corporation may repurchase its own shares beyond the statutory limit of 10%, if the repurchased shares are clearly earmarked for cancellation and such repurchase has been approved by our shareholders. In addition, we are required to recognize a minus position for own shares acquired by Alcon or, if our subsidiaries acquire our shares, create a special reserve on our balance sheet in each case in the amount of the purchase price of the acquired shares. Repurchased shares held by us or our subsidiaries do not carry any rights to vote at a General Meeting of Shareholders, but are entitled to the economic benefits generally connected with the shares.
Under the Swiss CO, we may not cancel treasury shares without either (i) the approval of a capital reduction by our shareholders or (ii) a capital reduction within the capital range.
(f) Not applicable.
(g) Since all of our issued and outstanding shares have been fully paid in, we can make no further capital calls on our shareholders. See "—Shareholder Rights" and "—Change in Control".
(h) See "—Change in Control".
Changes to Shareholder Rights
Under the Swiss CO, we may not issue new shares without the prior approval of a capital increase by our shareholders, subject to the existing capital range and conditional share capital of Alcon pursuant to the Articles, as approved by the shareholders. See "Item 10.A.—Share Capital" for additional information. If a capital increase is approved, then our shareholders would generally have certain pre-emptive rights to obtain newly issued shares in an amount proportional to the nominal value of the shares they already hold. These pre-emptive rights could be excluded in certain limited circumstances with the approval of a resolution adopted at a General Meeting of Shareholders by a supermajority of two thirds of the votes. In addition, we may not create shares with increased voting powers or place restrictions on the transfer of registered shares without the approval of a resolution adopted at a General Meeting of Shareholders by a supermajority of two thirds of the votes.
Shareholder Meetings
Under the Swiss CO and the Articles, we must hold an annual ordinary General Meeting of Shareholders within six months after the end of our financial year. General Meetings of Shareholders may be convened by the Board or, if necessary, by the statutory auditors. The Board is further required to convene an extraordinary General Meeting of Shareholders if so resolved by a General Meeting of Shareholders, or if so requested by shareholders holding an aggregate of at least 5% of the share capital, specifying the items for the agenda and their proposals. Shareholders holding shares with an aggregate nominal value of at least 0.5 percent of the share capital have the right to request that a specific proposal be put on the agenda, or that a proposal relating to an agenda item be included in the notice convening the General Meeting of Shareholders, and voted upon at the next General Meeting of Shareholders. Such request must be made in writing at the latest 45 days before the General Meeting of Shareholders. A General Meeting of Shareholders is convened by publishing a notice in the Swiss Official Gazette of Commerce (feuille officielle du commerce) at least 20 days prior to such meeting. Shareholders may also be informed by mail, e-mail or any other form that allows proof by text. There is no provision in the Swiss CO or the Articles requiring a quorum for the holding of a General Meeting of Shareholders. In addition, see "—Shareholder Rights" regarding conditions for exercising a shareholder's right to vote at a General Meeting of Shareholders.
Limitations
There are no limitations under the Swiss CO or our Articles on the right of non-Swiss residents or nationals to own or vote shares.
Change in Control
The Articles and the Board Regulations contain no provision that would have an effect of delaying, deferring or preventing a change in control of Alcon and that would operate only with respect to a merger, acquisition or corporate restructuring involving us or any of our subsidiaries.
According to the Swiss Merger Act, shareholders may pass a resolution to merge with another corporation at any time. Such a resolution would require the consent of at least two thirds of all votes present or represented at the necessary General Meeting of Shareholders.
Under the Swiss Financial Market Infrastructure Act, shareholders and groups of shareholders acting in concert who acquire more than 33.3% of our shares would be under an obligation to make an offer to acquire all remaining Alcon
145
shares. Alcon has neither opted out from the mandatory takeover offer obligation nor opted to increase the threshold for mandatory takeover offers in the Articles.
Disclosure of Shareholdings
Under the Swiss Financial Market Infrastructure Act, persons who directly, indirectly or in concert with other parties acquire or dispose of our shares or purchase or sale rights relating to our shares are required to notify us and the SIX of the level of their holdings whenever such holdings reach, exceed, or fall below certain thresholds—3%, 5%, 10%, 15%, 20%, 25%, 33.3%, 50% and 66.67%—of the voting rights represented by our share capital (whether exercisable or not). This also applies to anyone who has discretionary power to exercise voting rights associated with our shares. Following receipt of such notification we are required to inform the public by publishing the information via the electronic publication platform operated by the SIX.
An additional disclosure obligation exists under the Swiss CO which requires us to disclose, once a year in the notes to the financial statements published in our annual report, the identity of all of our shareholders (or related groups of shareholders) that hold a participation exceeding 5% of all voting rights.
Differences in the Law
See the references to Swiss law throughout this "—Item 10.B. Memorandum and Articles of Association."
Changes in Capital
The requirements of the Articles regarding changes in capital are not more stringent than the requirements of Swiss law.
Notices
According to the Articles, shareholder communications of Alcon shall be made in the Swiss Official Gazette of Commerce (feuille officielle du commerce). The Board of Directors may designate additional publication organs. Notices of Alcon to the shareholders may instead or in addition, at the election of the Board, be validly given by (i) mail, (ii) e-mail, or (iii) any other form that allows proof by text, to the most recent contact information of the shareholder or authorized recipient recorded.
Notices required under the Listing Rules will be published in electronic form on the website of SIX (currently https://www.ser-ag.com/en/resources/notifications-market-participants/official-notices.html#/).
10.C. MATERIAL CONTRACTS
2022 European Bond Offering
On May 31, 2022, AFBV completed an offering of the Series 2028 Notes. The Series 2028 Notes are senior unsecured obligations of AFBV and are fully and unconditionally guaranteed on a senior basis by the Company.
Interest is payable on the Series 2028 Notes on May 31 of each year, beginning on May 31, 2023. The Series 2028 Notes will mature on May 31, 2028.
AFBV may redeem the Series 2028 Notes, prior to March 31, 2028 (the date that is two months prior to their maturity date) at a redemption price equal to 100% of the principal amount of the Series 2028 Notes plus a “make-whole premium” and accrued and unpaid interest, if any, up to but excluding the redemption date. AFBV may also redeem the Series 2028 Notes, in whole, but not in part, on or after the date that is two months prior to their maturity date at a redemption price equal to 100% of their principal amount plus accrued and unpaid interest, if any, up to, but excluding the redemption date. If at any time, eighty percent (80%) or more of the aggregate principal amount of the Series 2028 Notes originally issued has been redeemed, purchased, or cancelled, AFBV may redeem or purchase, in whole, but not in part, the remaining outstanding Series 2028 Notes at the applicable “clean up price” as described in the terms and conditions of the Series 2028 Notes.
In addition, AFBV may redeem the Series 2028 Notes at any time prior to their respective maturities at a price equal to 100% of the outstanding principal amount of such Series 2028 Notes, plus accrued and unpaid interest, up to, but excluding the redemption date, if certain tax events occur that would notably obligate the Issuer to pay additional amounts as described in the terms and conditions of the Series 2028 Notes.
146
Subject to certain limitations, in the event of a change of control triggering event, AFBV will be required to make an offer to purchase the Series 2028 Notes at a price equal to 100% of the principal amount of the Series 2028 Notes, plus accrued and unpaid interest, if any, up to, but excluding the date of repurchase.
The terms and conditions of the Series 2028 Notes also contain certain limitations on AFBV’s ability to incur liens, as well as customary events of default.
2022 Bridge Loan Facility
On September 14, 2022, Alcon and Alcon Finance Corporation (the "Issuer"), an indirect, wholly owned subsidiary of Alcon, entered into a facility agreement with J.P. Morgan Securities PLC as arranger, J.P. Morgan Chase Bank, N.A., London Branch as original lender, bookrunner and underwriter, and J.P. Morgan SE as agent (the "2022 Bridge Loan Facility Agreement"). The 2022 Bridge Loan Facility Agreement provided for a $900 million unsecured term loan facility (the "2022 Bridge Loan Facility") for the purposes of financing or refinancing (i) the consideration payable for the Aerie acquisition, (ii) any existing indebtedness of Aerie and its subsidiaries and (iii) related fees and expenses in connection with the foregoing. The 2022 Bridge Loan Facility Agreement was an unsecured obligation of the Issuer and was fully guaranteed by the Company.
Borrowings under the 2022 Bridge Loan Facility bore interest at a rate equal to the aggregate of (i) the secured overnight financing rate as administered by the Federal Reserve Bank of New York, compounded daily in arrears, plus a credit adjustment spread (subject to a zero floor on such aggregate daily rate) and (ii) a margin that steps up from 0.30% to 1.40% based on the length of time elapsed since the completion of the Aerie acquisition.
On November 21, 2022, in connection with the consummation of the Aerie acquisition, $775 million of the financing commitments of the lenders under the 2022 Bridge Loan Facility were drawn, the proceeds of which were used to finance the equity portion of the consideration payable for the Aerie acquisition. On December 6, 2022, the 2022 Bridge Loan Facility was repaid in full with the proceeds of the 2022 Notes described below. None of the 2022 Bridge Loan Facility remains available to us for borrowings.
2022 US Bond Offering
On December 6, 2022, the Issuer completed an offering of the 2022 Notes. The 2022 Notes were issued under an Indenture, dated September 23, 2019 (the “Indenture”), by and among the Issuer, Alcon Inc. and Citibank, N.A., as trustee (the “Trustee”). The 2022 Notes are senior unsecured obligations of the Issuer and are fully and unconditionally guaranteed on a senior basis by the Company.
Interest is payable on the 2022 Notes on June 6 and December 6 of each year, beginning on June 6, 2023. The Series 2032 Notes will mature on December 6, 2032 and the Series 2052 Notes will mature on December 6, 2052.
The Issuer may redeem the Series 2032 Notes prior to September 6, 2032 (the date that is three months prior to their maturity date) and the Series 2052 Notes prior to June 6, 2052 (the date that is six months prior to their maturity date) at a redemption price equal to 100% of the principal amount of the applicable series of the 2022 Notes plus a “make-whole premium” and accrued and unpaid interest, if any, up to, but excluding the redemption date. The Issuer may also redeem the Series 2032 Notes on or after the date that is three months prior to their maturity date and the Series 2052 Notes on or after the date that is six months prior to their maturity date at a redemption price equal to 100% of their principal amount plus accrued and unpaid interest, if any, up to, but excluding the redemption date.
In addition, under certain circumstances, the Issuer may redeem any series of the 2022 Notes at its option, in whole, but not in part, for cash, at any time prior to their respective maturities at a price equal to 100% of the outstanding principal amount of such 2022 Notes, plus accrued and unpaid interest, up to, but excluding the redemption date.
Subject to certain limitations, in the event of a change of control triggering event, the Issuer will be required to make an offer to purchase each series of the 2022 Notes at a price equal to 101% of the principal amount of the 2022 Notes, plus accrued and unpaid interest, if any, up to, but excluding the date of repurchase.
The Indenture also contains certain limitations on the Issuer’s ability to incur liens, as well as customary events of default.
Acquisition Agreements
On November 5, 2021, Alcon exercised its option to purchase Ivantis, Inc. pursuant to an Option Agreement and Plan of Merger by and among Alcon Research, LLC, Ithaca Merger Sub, Inc., and Ivantis, Inc., dated as of November 9, 2018 (as subsequently amended, the "Ivantis Merger Agreement"). Pursuant to the Ivantis Merger Agreement, Alcon agreed to pay total upfront consideration of $479 million and potential contingent payments upon the achievement of certain regulatory and commercial milestones. As a result of the merger, which closed on January 7, 2022, Ivantis, Inc. became a wholly-
147
owned subsidiary of Alcon. The transaction expanded Alcon's Surgical portfolio to include the Hydrus microstent, a minimally-invasive glaucoma surgery (MIGS) device for the treatment of mild-to-moderate glaucoma.
On August 22, 2022, Alcon executed an Agreement and Plan of Merger (the "Aerie Merger Agreement") with Aerie Pharmaceuticals, Inc. ("Aerie"). Pursuant to the terms of the Aerie Merger Agreement, Alcon paid $15.25 per share to acquire all outstanding shares of Aerie's common stock. The total purchase consideration amounted to $744 million and total cash paid for the net identifiable assets recognized, net of cash acquired, was $666 million. Alcon also assumed debt of $316 million. This transaction was accounted for as a business combination that resulted in goodwill of $65 million under the preliminary purchase price allocation ("PPA") of the fair values of the acquired assets and assumed liabilities. As a result of the merger, which closed on November 21, 2022, Aerie became a wholly-owned subsidiary of Alcon. This transaction helps bolster Alcon’s presence in the ocular health space with its portfolio of commercial products and development pipeline within the Vision Care reportable segment. The PPA was subsequently finalized during the third quarter of 2023, resulting in adjusted goodwill of $21 million. Refer to Note 21.1 for additional information regarding the final PPA.
10.D. EXCHANGE CONTROLS
There are no Swiss governmental laws, decrees or regulations that restrict, in a manner material to Alcon, the export or import of capital, including any foreign exchange controls, or that generally affect the remittance of dividends or other payments to non-residents or non-citizens of Switzerland who hold Alcon shares.
10.E. TAXATION
The taxation discussion set forth below is intended only as a general summary and does not purport to be a complete analysis or listing of all potential tax considerations relevant to the ownership or disposition of our shares. The statements of US and Swiss tax laws set forth below are based on the laws and regulations in force as of the date of this Annual Report, including the current Convention Between the United States and the Swiss Confederation for the Avoidance of Double Taxation with Respect to Taxes on Income, entered into force on December 19, 1997 (the "Treaty"), and the US Internal Revenue Code of 1986, as amended (the "Code"), Treasury Regulations, rulings, judicial decisions and administrative pronouncements, all as in effect on the date hereof, and all of which are subject to change (possibly with retroactive effect) and to differing interpretations.
Swiss Taxation
The following is a general summary of certain tax consequences relating to owning and disposing of Alcon shares based on the Swiss tax laws and regulations and regulatory practices in force on the date of this Annual Report. Tax consequences are subject to changes in applicable law (or subject to changes in interpretation), including changes that could have a retroactive effect.
This is not a complete summary of the potential Swiss tax effects relevant to the Alcon shares nor does the summary take into account or discuss the tax laws of any jurisdiction other than Switzerland. For example, this summary does not address estate, gift, inheritance, capital or wealth taxes. It also does not take into account investors' individual circumstances. This summary does not purport to be a legal opinion or to address all tax aspects that may be relevant to any particular investor.
YOU ARE URGED TO CONSULT YOUR OWN TAX ADVISOR WITH RESPECT TO ACQUIRING, OWNING AND DISPOSING OF ALCON SHARES.
Swiss Residents
Withholding Tax on Dividends
Dividends that we pay and any similar cash or in-kind distributions we may make to a holder of our shares (including distributions of liquidation proceeds in excess of the nominal value, stock dividends and, under certain circumstances, proceeds from repurchases of shares by us in excess of the nominal value) are generally subject to a Swiss federal withholding tax (the "Withholding Tax") at a current rate of 35%. Under certain circumstances distributions out of capital contribution reserves made by shareholders after December 31, 1996 are exempt from the Withholding Tax. We are required to withhold this Withholding Tax from the gross distribution and to pay the Withholding Tax to the Swiss Federal Tax Administration. The Withholding Tax is refundable in full to Swiss residents who are the beneficial owners of the taxable distribution at the time it is resolved and duly report the gross distribution received on their personal tax return or in their financial statements for tax purposes, as the case may be.
148
Swiss Transfer Stamp Duty upon Transfer of Securities
The sale of our shares, whether by Swiss residents or Non-resident Holders, may be subject to federal securities Transfer Stamp Duty (Umsatzabgabe) of 0.15%, calculated on the gross sale proceeds, if the sale occurs through or with a Swiss bank or other Swiss securities dealer (Effektenhändler), as defined in the Swiss Federal Stamp Duty Act. The Transfer Stamp Duty has to be paid by the securities dealer and may be charged to the parties in a taxable transaction who are not securities dealers. In addition to this Transfer Stamp Duty, the sale of shares by or through a member of the SIX may be subject to a minor stock exchange levy.
Income Tax on Dividends
A Swiss Holder who holds Alcon shares as private assets ("Swiss Resident Private Shareholder") is required to report the receipt of dividends and similar distributions (including stock dividends and liquidation surplus) in its individual income tax returns and is subject to Swiss federal, cantonal and communal income tax on any net taxable income for the relevant tax period.
A Swiss Holder who is Swiss resident for tax purposes, a non-Swiss individual who is subject to Swiss income tax for reasons other than residency and a legal entity tax resident in Switzerland, in each case that holds Alcon shares as business assets, and a non-Swiss tax resident legal entity that holds Alcon shares as part of a Swiss permanent establishment or fixed place of business (each, a "Swiss Resident Commercial Shareholder") is required to recognize dividends and similar distributions (including stock dividends and liquidation surplus) on Alcon shares in its income statement for the relevant taxation period and is subject to Swiss federal, cantonal and communal individual or corporate income tax, as the case may be, on any net taxable earnings for such taxation period. The same tax treatment also applies to a Swiss Holder who, for income tax purposes, is classified as a "professional securities dealer" for reasons of, inter alia, frequent dealing, or leveraged investments, in shares and other securities. Swiss Resident Commercial Shareholders who are corporate taxpayers may be eligible for a participation deduction (Beteiligungsabzug) in respect of dividends if the Alcon shares held by them as part of a Swiss business have an aggregate market value of at least CHF 1 million.
Taxes upon Disposition of Alcon Shares
Capital gains realized on the sale or other disposal of Alcon shares held by a Swiss Resident Private Shareholder are generally not subject to any federal, cantonal or communal income taxation. However, gain realized upon a repurchase of shares by us may be characterized as taxable dividend income if certain conditions are met. Capital gains realized on shares held by a Swiss Resident Commercial Shareholder are, in general, included in the taxable income of such person.
Residents of Other Countries
Recipients of dividends and similar distributions on our shares who are neither residents of Switzerland for tax purposes nor holding shares as part of a business conducted through a permanent establishment situated in Switzerland ("Non-resident Holders") are not subject to Swiss income taxes in respect of such distributions. Moreover, gain realized by such recipients upon the disposal of our shares is not subject to Swiss income tax.
Non-resident Holders of our shares are, however, subject to the Withholding Tax on dividends and similar distributions mentioned above and under certain circumstances to the Transfer Stamp Duty described above. Such Non-resident Holders may be entitled to a partial refund of the Withholding Tax if the country in which they reside has entered into a bilateral treaty for the avoidance of double taxation with Switzerland. Non-resident Holders should be aware that the procedures for claiming treaty refunds (and the time frame required for obtaining a refund) may differ from country to country. Non-resident Holders should consult their own tax advisors regarding the receipt, ownership, purchase, sale or other dispositions of our shares and the procedures for claiming a refund of the Withholding Tax.
A Non-resident Holder of our shares will not be liable for any Swiss taxes other than the Withholding Tax described above and, if the transfer occurs through or with a Swiss bank or other Swiss securities dealer, the Transfer Stamp Duty described above. If, however, the shares of Non-resident Holders can be attributed to a permanent establishment or a fixed place of business maintained by such person within Switzerland during the relevant tax year, the shares may be subject to Swiss income taxes in respect of income and gains realized on the shares and such person may qualify for a full refund of the Withholding Tax based on Swiss tax law.
Residents of the United States
Non-resident Holders who are residents of the United States for purposes of the Treaty are eligible for a reduced rate of tax on dividends equal to 15% of the dividend, provided that such holders qualify for benefits under the Treaty and do not conduct business through a permanent establishment or fixed base in Switzerland to which our shares are attributable.
149
Such holders should consult their own tax advisors regarding their eligibility to claim the reduced rate and the procedures for claiming a refund of the amount of the Withholding Tax in excess of the 15% Treaty rate.
International Automatic Exchange of Information in Tax Matters
On November 19, 2014, Switzerland signed the Multilateral Competent Authority Agreement, which is based on article 6 of the OECD/Council of Europe administrative assistance convention and is intended to ensure the uniform implementation of automatic exchange of information (the "AEOI"). The Federal Act on the International Automatic Exchange of Information in Tax Matters (the "AEOI Act") entered into force on January 1, 2017. The AEOI Act is the legal basis for the implementation of the AEOI standard in Switzerland.
The AEOI is being introduced in Switzerland through bilateral agreements or multilateral agreements. The agreements have been, and will be, concluded on the basis of guaranteed reciprocity, compliance with the principle of specialty (i.e. the information exchanged may only be used to assess and levy taxes (and for criminal tax proceedings)) and adequate data protection. The United States is not a treaty state.
Based on such multilateral agreements and bilateral agreements and the implementing laws of Switzerland, Switzerland has begun to collect data in respect of financial assets (including shares) held in, and income derived thereon and credited to, accounts or deposits with a paying agent in Switzerland for the benefit of individuals resident in a EU member state or in a treaty state.
US Federal Income Taxation
The following discussion is a summary of the US federal income tax considerations generally applicable to the ownership and disposition of our shares. This summary is based on the Code, its legislative history, US Treasury Regulations, administrative guidance, published court decisions and the Treaty, all in effect as of the date hereof, and any of which may be repealed, revoked, or modified (possibly with retroactive effect) so as to result in US federal income tax consequences different from those discussed below. This summary is applicable to US Holders (as defined below) who are residents of the United States for purposes of the Treaty and who qualify for the full benefits of the Treaty. It applies only to US Holders that hold our shares as capital assets (generally, property held for investment purposes). This summary should not be construed to constitute legal or tax advice to any particular US Holder.
This summary does not apply to or address US Holders subject to special rules, including, without limitation, brokers, dealers in securities or currencies, traders in securities that elect to use a mark-to-market method of accounting for securities holdings, tax-exempt entities (including private foundations), insurance companies, banks, thrifts and other financial institutions, persons liable for alternative minimum tax, persons that hold an interest in an entity that holds our shares, persons that will own, or will have owned, directly, indirectly or constructively 10% or more (by vote or value) of our stock, persons that hold our shares as part of a straddle, hedge, conversion, constructive sale or other integrated transaction for US federal income tax purposes or persons whose functional currency is not the US dollar.
This summary does not purport to be a complete analysis of all of the potential US federal income tax considerations that may be relevant to US Holders in light of their particular circumstances. Further, it does not address any aspect of foreign, state, local or estate or gift taxation or the 3.8% Medicare tax imposed on certain net investment income. Each US Holder is urged to consult its tax advisor regarding the application of US federal taxation to its particular circumstances and the, state, local, non-US and other tax considerations of the ownership and disposition of our shares.
General
For purposes of this discussion, a “US Holder” is a beneficial owner of our shares that is, for US federal income tax purposes:
•an individual who is a citizen or resident of the United States;
•a corporation (or other entity or arrangement treated as a corporation for US federal income tax purposes) created in or organized under the laws of the United States, any state thereof or the District of Columbia;
•an estate the income of which is includable in gross income for US federal income tax purposes regardless of its source; or
•a trust (A) the administration of which is subject to the primary supervision of a US court and which has one or more US persons who have the authority to control all substantial decisions of the trust or (B) that has otherwise validly elected to be treated as a US person under the Code.
150
If a partnership (or other entity or arrangement treated as a partnership for US federal income tax purposes) is a beneficial owner of our shares, the tax treatment of a partner in the partnership that will generally depend upon the status of the partner and the activities of the partnership. Partnerships holding our shares and partners in such partnerships are urged to consult their tax advisors as to the particular US federal income tax consequences of an investment in our shares.
Distributions on the Shares
Subject to the passive foreign investment company (“PFIC”) rules discussed below, the gross amount of any distribution received by a US Holder with respect to our shares (including any amounts withheld to pay Swiss withholding taxes) generally will be included in the gross income of the US Holder as a dividend to the extent attributable to the Company’s current or accumulated earnings and profits, as determined under US federal income tax principles. The Company may not calculate its earnings and profits under US federal income tax rules. Accordingly, US Holders should expect that a distribution generally will be treated as a dividend for US federal income tax purposes. Unless the Company is treated as a PFIC for the taxable year in which it pays a distribution or in the preceding taxable year (see “Passive Foreign Investment Company Rules” below), the Company believes that it may qualify as a “qualified foreign corporation,” in which case distributions treated as dividends and received by non-corporate US Holders may be eligible for a preferential tax rate. Distributions on our shares generally will not be eligible for the dividends received deduction available to US Holders that are corporations.
The amount of any dividend paid in Swiss francs (including any amounts withheld to pay Swiss withholding taxes) will be included in the gross income of a US Holder in an amount equal to the US dollar value of the Swiss francs calculated by reference to the exchange rate in effect on the date the dividend is actually or constructively received by the US Holder, regardless of whether the Swiss francs are converted into US dollars on such date. A US Holder will have a tax basis in the Swiss francs equal to their US dollar value on the date of receipt. If the Swiss francs received are converted into US dollars on the date of receipt, the US Holder generally should not be required to recognize foreign currency gain or loss in respect of the distribution. If the Swiss francs received are not converted into US dollars on the date of receipt, a US Holder may recognize foreign currency gain or loss on a subsequent conversion or other disposition of the Swiss francs. Such gain or loss generally will be treated as US source ordinary income or loss.
A US Holder may be entitled to deduct or credit Swiss withholding tax imposed on dividends paid to a US Holder, subject to applicable limitations in the Code. The rules governing the foreign tax credit are complex. US Holders are urged to consult their own tax advisors regarding the availability of the foreign tax credit under their particular circumstances.
Sale, Exchange or Other Taxable Disposition of Our Shares
Subject to the PFIC rules discussed below, a US Holder generally will recognize a capital gain or loss on the sale, exchange or other taxable disposition of our shares in an amount equal to the difference between the amount realized for the shares and the US Holder’s adjusted tax basis in the shares. Any capital gain or loss will be long-term capital gain or loss if the ordinary shares have been held for more than one year. Individuals and other non-corporate US Holders who have long-term capital gains will generally be eligible for reduced tax rates. The deductibility of capital losses is subject to limitations. Any capital gain or loss recognized by a US Holder generally will be treated as US source gain or loss for US foreign tax credit purposes.
Passive Foreign Investment Company Rules
A foreign corporation will be considered a PFIC for any taxable year in which (i) 75% or more of its gross income is “passive income” or (ii) 50% or more of the average quarterly value of its assets produce (or are held for the production of) “passive income.” For this purpose, “passive income” generally includes interest, dividends, rents, royalties and certain gains. We currently do not believe that we were a PFIC in the taxable year ending December 31, 2023, nor do we anticipate that we will be a PFIC in subsequent taxable years. However, the determination of PFIC status is based on an annual determination that cannot be made until the close of the taxable year, involves extensive factual investigation, including ascertaining the fair market value of all of our assets on a quarterly basis and the character of each item of income that we earn, and is subject to uncertainty in several respects. Accordingly, we cannot assure you that we will not be treated as a PFIC for the taxable year ending December 31, 2023, or any subsequent taxable year, or that the IRS will not take a contrary position.
Required Disclosure with Respect to Foreign Financial Assets
Certain US Holders are required to report information relating to their holding an interest in our shares, subject to certain exceptions (including an exception for shares held in accounts maintained by certain financial institutions), by attaching a completed IRS Form 8938, Statement of Specified Foreign Financial Assets, with their tax return for each year in which they hold an interest in the shares. US Holders are urged to consult their tax advisors regarding information reporting requirements relating to their ownership of our shares.
151
10.F. DIVIDENDS AND PAYING AGENTS
Not Applicable.
10.G. STATEMENTS BY EXPERTS
Not Applicable.
10.H. DOCUMENTS ON DISPLAY
We maintain a website at the following address: www.alcon.com. The information on our website is not incorporated by reference in this Annual Report. We make available on or through our website certain reports and amendments to those reports that we file with or furnish to the SEC in accordance with the Exchange Act. We make this information available on our website free of charge as soon as reasonably practicable after we electronically file the information with, or furnish it to, the SEC.
You may read and copy any reports or other information that we file through the Electronic Data Gathering, Analysis and Retrieval (EDGAR) system through the SEC’s website on the internet at www.sec.gov.
We also make certain other documents available to the public (such as our Board committee charters, press releases and investor presentations) on our website (www.alcon.com).
Any statement in this Annual Report about any of our contracts or other documents is not necessarily complete. If the contract or document is filed as an exhibit to this Annual Report, the contract or document is deemed to modify the description contained in this Annual Report. You must review the exhibits themselves for a complete description of the contract or document.
Unless stated otherwise in this Annual Report, none of these documents form part of this Annual Report.
10.I. SUBSIDIARY INFORMATION
Not Applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The major financing risks faced by Alcon are managed by the Alcon treasury function. For information about the effects of currency and interest rate fluctuations and how we manage currency and interest risk, see "Item 5. Operating and Financial Review and Prospects—5.A. Operating Results" and "—5.B. Liquidity and Capital Resources". Please also see the information set forth under Note 17 to the Consolidated Financial Statements.
152
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
12.A. DEBT SECURITIES
Not Applicable.
12.B. WARRANTS AND RIGHTS
Not Applicable.
12.C. OTHER SECURITIES
Not Applicable.
12.D. AMERICAN DEPOSITARY SHARES
Not Applicable.
153
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
154
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None.
155
ITEM 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
As of December 31, 2023, the end of the period covered by this Annual Report, our management, including our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that as of December 31, 2023, the end of the period covered by this Annual Report, we maintained effective disclosure controls and procedures.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Our management, including our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of internal control over financial reporting using the criteria set forth in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the results of this evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2023.
Our independent registered public accounting firm, PricewaterhouseCoopers LLP, audited the effectiveness of our internal control over financial reporting. PricewaterhouseCoopers LLP's report as of December 31, 2023 is included in Item 18 of this Annual Report.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that occurred during the fiscal year ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
156
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our Board has determined that Lynn D. Bleil, Scott Maw, Karen May and Dieter Spälti, each of whom serves on our Audit and Risk Committee ("ARC"), are independent for purposes of serving on the audit committee under Rule 10A-3 and the listing standards promulgated by the New York Stock Exchange and are audit committee financial experts.
ITEM 16B. CODE OF ETHICS
Our Chief Executive Officer, Chief Financial Officer and Chief Accounting Officer are bound to adhere to our Code of Business Conduct, which applies to all of our associates and members of our Board. Our Code of Business Conduct is available on our website at www.alcon.com/about-us/responsible-business-practice.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information set forth under “Item 6. Directors, Senior Management and Employees—6.C. Board Practice—Corporate Governance—Auditors—Auditing Fees and Additional Fees" is incorporated by reference.
Policy on Audit and Risk Committee Pre-Approval of Services of Principal Accountant
The Audit and Risk Committee has established a written policy to pre-approve, on an annual basis, all anticipated audit and non-audit services provided by our independent auditors (“Pre-Approval Policy”). These services may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to 12 months from the date of pre-approval, and any pre-approval is detailed as to the particular service or category of services and is generally subject to a specific budget.
The Pre-Approval Policy provides that the independent auditors may not perform any services for Alcon unless the independent auditors are engaged pursuant to the Pre-Approval Policy. In addition, the Pre-Approval Policy prohibits the Audit and Risk Committee from pre-approving certain non-audit services that are prohibited from being performed by the independent auditors by applicable securities laws. Management is required to periodically report to the Audit and Risk Committee regarding the extent of services provided by the independent auditors. In 2023, all audit-related, tax and other services provided by PwC Switzerland, PwC US and any other firm of PricewaterhouseCoopers International Limited were pre-approved.
In connection with its review and evaluation of non-audit services, the Audit and Risk Committee is required to and does consider and conclude that the provision of the non-audit services is compatible with maintaining the independence of the independent auditor.
157
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not Applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
The following table sets forth purchases of our Ordinary Shares by us and our affiliated purchasers during the fiscal year ended December 31, 2023:
| Period | Total Number of Shares Purchased | Average Price Paid Per Share (USD) | Total Number of Shares Purchased as part of Publicly Announced Plans or Programs | Maximum Number (Or Approximate Dollar Value) of Shares that may yet be Purchased Under the Plans or Programs | ||||||||||
| January 1-31 | - | - | - | - | ||||||||||
| February 1-28 | - | - | - | - | ||||||||||
| March 1-31 | 3,000 | 64.77 | - | - | ||||||||||
| April 1-30 | - | - | - | - | ||||||||||
| May 1-31 | - | - | - | - | ||||||||||
| June 1-30 | - | - | - | - | ||||||||||
| July 1-31 | - | - | - | - | ||||||||||
| August 1-31 | - | - | - | - | ||||||||||
| September 1-30 | - | - | - | - | ||||||||||
| October 1-31 | - | - | - | - | ||||||||||
| November 1-30 | - | - | - | - | ||||||||||
| December 1-31 | - | - | - | - | ||||||||||
| Total | 3,000 | 64.77 | - | - | ||||||||||
158
ITEM 16F. CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT
Not Applicable.
ITEM 16G. CORPORATE GOVERNANCE
The information set forth under “Item 6. Directors, Senior Management and Employees—6.C. Board Practice—Corporate Governance—Differences in Corporate Governance Standards" is incorporated by reference.
ITEM 16H. MINE SAFETY DISCLOSURE
Not Applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not Applicable.
ITEM 16J. INSIDER TRADING POLICIES
Not Applicable.
ITEM 16K. CYBERSECURITY
Cybersecurity Risk Management and Strategy
We recognize the importance of timely and appropriately assessing, preventing, identifying and managing risks associated with Cybersecurity Threats, as such term is defined in Form 20-F, Part II, Item 16K(a). These risks include, among other things, potential operational risks; intellectual property theft; fraud; extortion; harm to associates, customers or patients; violation of privacy and other litigation and legal risk; and reputational risks. We have implemented cybersecurity processes, technologies and controls to aid in our efforts to assess, prevent, identify and manage such risks.
To identify and assess risks from Cybersecurity Threats, our enterprise risk management program considers Cybersecurity Threat risks alongside other company risks as part of our overall risk assessment process. Our internal audit team collaborates with subject matter specialists, as necessary, to gather insights for identifying and assessing Cybersecurity Threat risks, their likelihood and severity, and potential preventative measures and mitigations. We employ a range of
159
tools and services, including regular network and endpoint monitoring, vulnerability assessments, penetration testing and tabletop exercises to inform our risk identification, assessment and management.
We also have a cybersecurity-specific risk assessment process, which helps identify our Cybersecurity Threat risks by aligning our processes with industry cybersecurity frameworks, including the National Institute of Standards and Technology (“NIST”) and International Organization for Standardization (“ISO”) 27001 standards, as well as by engaging experts to attempt to infiltrate our Information Systems, as such term is defined in Form 20-F, Part II, Item 16K(a).
To provide for the availability of critical data and systems, maintain regulatory compliance, manage our risks from Cybersecurity Threats and to protect against, detect and respond to Cybersecurity Incidents, as such term is defined in Form 20-F, Part II, Item 16K(a), we undertake activities including:
▪The Alcon IT Security Incident Response procedure generally follows the NIST incident handling framework to help us identify, protect, detect, respond and recover when there is an actual or potential cybersecurity incident. Alcon’s incident response procedure also includes timely collaboration with appropriate Alcon associates from information security, data privacy and legal functions to appropriately identify and respond to any notification or other legal obligations related to such incidents, as applicable;
▪We monitor applicable data protection laws and best practices, and seek to implement, maintain and enhance our security safeguards and processes accordingly;
▪We regularly review our consumer facing policies and statements related to cybersecurity;
▪Where applicable, we seek to proactively inform our customers of substantive changes related to customer data handling;
▪We conduct annual data privacy, security and compliance training for all our associates, which includes data incident reporting;
▪We conduct annual cybersecurity management and incident training for associates involved in our systems and processes that handle sensitive data;
▪We perform multiple phishing email simulations for all associates and all contractors with access to corporate email systems to enhance awareness and responsiveness to such possible threats;
▪We perform regular tabletop exercises with our incident response provider to simulate a response to a cybersecurity incident and use the findings to improve our processes and technologies;
▪We have a group insurance plan to provide protection against the potential losses arising from a cybersecurity incident; and
▪We have an incident response engagement with a third-party to assist in an actual or potential cybersecurity incident.
Through policy, practice and contract, as applicable, we require associates, as well as third parties who provide services on our behalf, to treat Alcon data, including customer, patient, employee and other confidential and sensitive information, with care.
Our incident response plan coordinates the activities we take to prepare for, detect, respond to and recover from cybersecurity incidents, which include processes to triage, assess severity for, escalate, contain, investigate and remediate the incident, as well as to comply with potentially applicable legal obligations and mitigate potential brand, reputational or other damage.
As part of the above processes, our information security team regularly engages with Alcon's data privacy and legal teams, assessors, consultants, auditors and other third parties, including a regular maturity assessment by an independent Qualified Security Assessor to review our cybersecurity program to identify areas for continued focus, improvement and/or compliance.
Our processes also address Cybersecurity Threat risks associated with our use of third-party service providers, including those in our supply chain or who have access to Alcon data, including customer, patient, associate or other confidential or proprietary information, or Alcon systems or facilities. Third-party risks are included within our risk management assessment program, as well as our cybersecurity-specific risk identification program, both of which are discussed above. In addition, cybersecurity considerations affect the selection and oversight of our third-party service providers. We perform diligence on third parties that have access to our systems, data or facilities that house such systems or data, and continually monitor Cybersecurity Threat risks identified through such diligence. Additionally, we generally require those third parties that could introduce potentially heightened cybersecurity risk to us to agree by contract to manage their cybersecurity risks in specified ways, and to agree to be subject to cybersecurity audits, which we conduct as appropriate.
160
Our information security team partners with Alcon's data privacy and legal teams and other groups to timely determine whether and how risks from identified Cybersecurity Threats, including results from any previous Cybersecurity Incidents, have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations or financial conditions.
In the last three fiscal years, we have not experienced any material Cybersecurity Incidents and the expenses we have incurred from Cybersecurity Incidents were immaterial. We have not paid any penalties or settlements in the past three years.
For further discussion of risks from Cybersecurity Threats to us, see "Item 3. Key Information-3.D. Risk Factors-Significant cybersecurity breaches could disrupt business operations, result in the loss of critical and confidential information and adversely affect our reputation and results of operations."
Cybersecurity Governance
Cybersecurity is an important part of our risk management processes and an area of increasing focus for our Board and management.
Our Audit and Risk Committee of our Board is responsible for the oversight of risks from Cybersecurity Threats. At least annually, the Audit and Risk Committee receives an overview from management of our Cybersecurity Threat risk management and strategy processes covering topics such as data security posture, results from third-party assessments, progress towards predetermined risk-mitigation-related goals, our incident response plan and material Cybersecurity Threat risks or incidents and developments, as well as the steps management has taken to respond to such risks. In such sessions, the Audit and Risk Committee generally receives materials including a cybersecurity scorecard and other materials indicating current and emerging Cybersecurity Threat risks, and describing our ability to mitigate those risks, and discusses such matters with our Chief Information Security Officer ("CISO"). Members of the Audit and Risk Committee are also encouraged to regularly engage in ad hoc conversations with management on cybersecurity-related news events and discuss any updates to our cybersecurity risk management and strategy programs. Cybersecurity Threat risks are also considered during separate Board meeting discussions of important matters such as enterprise risk management, operational budgeting and strategic planning, business continuity planning, mergers and acquisitions, brand management and other relevant matters.
Our cybersecurity risk management and strategy processes, which are discussed in greater detail above, are led by our CISO who has over 30 years of prior relevant experience in various information technology roles involving managing global information security, application development and IT infrastructure organizations, developing cybersecurity strategy and implementing effective information and cybersecurity programs. Our CISO manages a team of associates who provide information assurance governance and consultation across all regions of our business. This team includes approximately 60 individuals holding various cybersecurity certifications. Our CISO and our information assurance team partner closely with our regional privacy officers, led by our Global Data Privacy Officer.
These members of management are informed about and monitor the prevention, mitigation, detection, classification and remediation of cybersecurity incidents through their management of, and participation in, the cybersecurity risk management and strategy processes described above, including the operation of our incident response plan.
As discussed above, these members of management report to Audit and Risk Committee about Cybersecurity Threat risks, among other cybersecurity related matters, at least annually.
161
Part III
ITEM 17. FINANCIAL STATEMENTS
See response to "Item 18. Financial Statements."
ITEM 18. FINANCIAL STATEMENTS
Please refer to the financial statements beginning on page F-1 of this Annual Report.
162
ITEM 19. EXHIBITS
Exhibit Number | Description | |||||||
| 1.1 | ||||||||
| 1.2 | ||||||||
| 2.1 | ||||||||
| 2.2 | Indenture by and among Alcon Finance Corporation, as Company, Alcon Inc,, as Guarantor, and Citibank, N.A., as Trustee, Paying Agent, Authenticating Agent and Registrar, dated September 23, 2019 - incorporated by reference to Exhibit 2.2 to the Annual Report on Form 20-F (File No. 001-31269) filed with the Securities and Exchange Commission on February 27, 2023 | |||||||
| 2.3 | Other than the indenture described in Exhibit 2.2, the total amount of long-term debt securities authorized under any instrument does not exceed 10% of the total assets of Alcon and its subsidiaries on a consolidated basis. We hereby agree to furnish to the SEC, upon its request, a copy of any instrument defining the rights of holders of long-term debt of Alcon or of its subsidiaries for which consolidated or unconsolidated financial statements are required to be filed. | |||||||
| 4.12 | ||||||||
| 4.13 | ||||||||
| 4.14 | ||||||||
| 4.15 | ||||||||
| 4.16 | ||||||||
| 8.1 | For a list of all principal subsidiaries of Alcon Inc., see "Item 18. Financial Statements-Note 27. Alcon subsidiaries and associated companies". | |||||||
| 12.1 | ||||||||
| 12.2 | ||||||||
| 13.1 | ||||||||
| 13.2 | ||||||||
| 15.1 | ||||||||
| 97.1 | ||||||||
| 101.INS | Inline XBRL Instance Document (embedded within Inline XBRL document) | |||||||
101.SCH | Inline XBRL Taxonomy Extension Schema | |||||||
101.CAL | Inline XBRL Taxonomy Extension Calculation | |||||||
101.DEF | Inline XBRL Taxonomy Extension Definition | |||||||
101.LAB | Inline XBRL Taxonomy Extension Label | |||||||
101.PRE | Inline XBRL Taxonomy Extension Presentation | |||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |||||||
163
Signatures
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this registration statement on its behalf.
| Alcon Inc. | |||||||||||
| By: | /s/ David J. Endicott | ||||||||||
| Name: | David J. Endicott | ||||||||||
| Title: | Authorized Representative | ||||||||||
| By: | /s/ Timothy C. Stonesifer | ||||||||||
| Name: | Timothy C. Stonesifer | ||||||||||
| Title: | Authorized Representative | ||||||||||
Date: February 27, 2024
164
CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC.
| Audited Consolidated Financial Statements | |||||
| Consolidated Income Statement | F-2 | ||||
| Consolidated Statement of Comprehensive Income | F-3 | ||||
| Consolidated Balance Sheet | F-4 | ||||
| Consolidated Statement of Changes in Equity | F-5 | ||||
| Consolidated Statement of Cash Flows | F-6 | ||||
| Notes to Consolidated Financial Statements of Alcon Inc. | F-7 | ||||
| Report of Independent Registered Public Accounting Firm | F-72 | ||||
F-1
CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC.
Consolidated Income Statement
(For the years ended December 31, 2023, 2022 and 2021)
| ($ millions except earnings per share) | Note | 2023 | 2022 | 2021 | |||||||||||||||||||
| Net sales to third parties | 4 | ||||||||||||||||||||||
| Other revenues | 4 | ||||||||||||||||||||||
| Net sales and other revenues | |||||||||||||||||||||||
| Cost of net sales | ( | ( | ( | ||||||||||||||||||||
| Cost of other revenues | ( | ( | ( | ||||||||||||||||||||
| Gross profit | |||||||||||||||||||||||
| Selling, general & administration | ( | ( | ( | ||||||||||||||||||||
| Research & development | ( | ( | ( | ||||||||||||||||||||
| Other income | |||||||||||||||||||||||
| Other expense | ( | ( | ( | ||||||||||||||||||||
| Operating income | |||||||||||||||||||||||
| Interest expense | 5 | ( | ( | ( | |||||||||||||||||||
| Other financial income & expense | 5 | ( | ( | ( | |||||||||||||||||||
| Income before taxes | |||||||||||||||||||||||
| Taxes | 6 | ( | ( | ||||||||||||||||||||
| Net income | |||||||||||||||||||||||
| Earnings per share ($) | |||||||||||||||||||||||
| Basic | 7 | ||||||||||||||||||||||
| Diluted | 7 | ||||||||||||||||||||||
| Weighted average number of shares outstanding (millions) | |||||||||||||||||||||||
| Basic | 7 | ||||||||||||||||||||||
| Diluted | 7 | ||||||||||||||||||||||
The accompanying Notes form an integral part of the Consolidated Financial Statements.
F-2
CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Consolidated Statement of Comprehensive Income
(For the years ended December 31, 2023, 2022 and 2021)
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Net income | |||||||||||||||||
| Other comprehensive income to be eventually recycled into the Consolidated Income Statement: | |||||||||||||||||
Currency translation effects, net of taxes(1) | ( | ( | |||||||||||||||
| Total of items to eventually recycle | ( | ( | |||||||||||||||
| Other comprehensive income never to be recycled into the Consolidated Income Statement: | |||||||||||||||||
Actuarial (losses)/gains from defined benefit plans, net of taxes(2) | ( | ||||||||||||||||
Fair value adjustments on equity investments, net of taxes(3) | ( | ( | |||||||||||||||
| Total of items never to be recycled | ( | ||||||||||||||||
| Total comprehensive income | |||||||||||||||||
(1)Amount is net of tax benefit of $1 million in 2023. Amount is net of tax expense of $0.4 million in 2022. Amount is net of tax benefit of $6 million in 2021.
(2)Amount is net of tax benefit of $8 million in 2023. Amounts are net of tax expense of $40 million and $11 million in 2022 and 2021, respectively.
(3)Amount is net of tax expense of $3 million in 2023. Amount is net of tax benefit of $1 million in 2022.
The accompanying Notes form an integral part of the Consolidated Financial Statements.
F-3
CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Consolidated Balance Sheet
(At December 31, 2023 and 2022)
| ($ millions) | Note | 2023 | 2022 | ||||||||||||||
| Assets | |||||||||||||||||
| Non-current assets | |||||||||||||||||
| Property, plant & equipment | 8 | ||||||||||||||||
| Right-of-use assets | 15 | ||||||||||||||||
| Goodwill | 9, 21.1 | ||||||||||||||||
| Intangible assets other than goodwill | 9 | ||||||||||||||||
| Deferred tax assets | 10 | ||||||||||||||||
| Financial assets | 11 | ||||||||||||||||
| Other non-current assets | 11 | ||||||||||||||||
| Total non-current assets | |||||||||||||||||
| Current assets | |||||||||||||||||
| Inventories | 12 | ||||||||||||||||
| Trade receivables | 13 | ||||||||||||||||
| Income tax receivables | |||||||||||||||||
| Cash and cash equivalents | 17 | ||||||||||||||||
| Other current assets | 14 | ||||||||||||||||
| Total current assets | |||||||||||||||||
| Total assets | |||||||||||||||||
| Equity and liabilities | |||||||||||||||||
| Equity | |||||||||||||||||
| Share capital | 7.1 | ||||||||||||||||
| Reserves | |||||||||||||||||
| Total equity | |||||||||||||||||
| Liabilities | |||||||||||||||||
| Non-current liabilities | |||||||||||||||||
| Financial debts | 16 | ||||||||||||||||
| Lease liabilities | 15 | ||||||||||||||||
| Deferred tax liabilities | 10 | ||||||||||||||||
| Provisions & other non-current liabilities | 18 | ||||||||||||||||
| Total non-current liabilities | |||||||||||||||||
| Current liabilities | |||||||||||||||||
| Trade payables | |||||||||||||||||
| Financial debts | 16 | ||||||||||||||||
| Lease liabilities | 15 | ||||||||||||||||
| Current income tax liabilities | |||||||||||||||||
| Provisions & other current liabilities | 19 | ||||||||||||||||
| Total current liabilities | |||||||||||||||||
| Total liabilities | |||||||||||||||||
| Total equity and liabilities | |||||||||||||||||
The accompanying Notes form an integral part of the Consolidated Financial Statements.
F-4
CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Consolidated Statement of Changes in Equity
(For the years ended December 31, 2023, 2022 and 2021)
| ($ millions) | Share capital | Other reserves | Fair value adjustments on equity investments | Actuarial (losses)/gains from defined benefit plans | Cumulative currency translation effects | Total value adjustments(1) | Equity | ||||||||||||||||
| Balance as of December 31, 2020 | ( | ( | ( | ||||||||||||||||||||
| Net income | — | ||||||||||||||||||||||
Other comprehensive income/(loss) | — | ( | ( | ( | |||||||||||||||||||
| Total comprehensive income | — | ( | ( | ||||||||||||||||||||
| Dividends | ( | — | ( | ||||||||||||||||||||
| Equity-based compensation | — | ||||||||||||||||||||||
Other movements(2) | |||||||||||||||||||||||
| Total other movements | — | — | — | ||||||||||||||||||||
| Balance as of December 31, 2021 | ( | ( | ( | ( | |||||||||||||||||||
| Net income | — | ||||||||||||||||||||||
| Other comprehensive income/(loss) | ( | ( | |||||||||||||||||||||
| Total comprehensive income | — | ( | ( | ||||||||||||||||||||
| Dividends | ( | — | ( | ||||||||||||||||||||
| Equity-based compensation | — | ||||||||||||||||||||||
Other movements(2) | — | ||||||||||||||||||||||
| Total other movements | — | ( | — | — | — | — | ( | ||||||||||||||||
| Balance as of December 31, 2022 | ( | ( | ( | ||||||||||||||||||||
| Net income | — | ||||||||||||||||||||||
Other comprehensive income/(loss) | ( | ( | ( | ( | |||||||||||||||||||
| Total comprehensive income | — | ( | ( | ( | |||||||||||||||||||
| Dividends | ( | — | ( | ||||||||||||||||||||
| Equity-based compensation | — | ||||||||||||||||||||||
Other movements(2) | |||||||||||||||||||||||
| Total other movements | — | ( | — | — | ( | ||||||||||||||||||
| Balance as of December 31, 2023 | ( | ( | ( | ||||||||||||||||||||
(1) "Total value adjustments" are presented net of the corresponding tax effects.
(2) Activity includes hyperinflationary accounting (see Note 2). The year ended December 31, 2023 also includes a reclassification to Other reserves related to the sale of an equity investment. The year ended December 31, 2021 primarily includes an adjustment to actuarial gains to recognize plan assets related to the separation of a pension plan in the spin-off from Novartis but which were not previously recorded.
The accompanying Notes form an integral part of the Consolidated Financial Statements.
F-5
CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Consolidated Statement of Cash Flows
(For the years ended December 31, 2023, 2022 and 2021)
| ($ millions) | Note | 2023 | 2022 | 2021 | |||||||||||||||||||
| Net income | |||||||||||||||||||||||
| Adjustments to reconcile net income to net cash flows from operating activities | |||||||||||||||||||||||
| Depreciation, amortization, impairments and fair value adjustments | 20.1 | ||||||||||||||||||||||
| Equity-based compensation expense | |||||||||||||||||||||||
| Non-cash change in current and non-current provisions and other non-current liabilities | |||||||||||||||||||||||
| Losses on disposal and other adjustments on property, plant & equipment and other non-current assets, net | |||||||||||||||||||||||
| Interest expense | |||||||||||||||||||||||
| Other financial income & expense | |||||||||||||||||||||||
| Taxes | ( | ||||||||||||||||||||||
| Interest received | |||||||||||||||||||||||
| Interest paid | ( | ( | ( | ||||||||||||||||||||
| Other financial payments | ( | ( | ( | ||||||||||||||||||||
| Taxes paid | ( | ( | ( | ||||||||||||||||||||
| Net cash flows before working capital changes and net payments out of provisions and other non-current liabilities | |||||||||||||||||||||||
| Net payments out of provisions and other cash movements in non-current liabilities | ( | ( | ( | ||||||||||||||||||||
| Change in net current assets and other operating cash flow items | 20.2 | ( | ( | ( | |||||||||||||||||||
| Net cash flows from operating activities | |||||||||||||||||||||||
| Purchase of property, plant & equipment | ( | ( | ( | ||||||||||||||||||||
| Purchase of intangible assets | ( | ( | ( | ||||||||||||||||||||
| Purchase of investment in associated company | 24 | ( | |||||||||||||||||||||
| Payments for financial assets | ( | ( | ( | ||||||||||||||||||||
| Proceeds from financial assets | |||||||||||||||||||||||
| Proceeds from sale of short-term investments | 21.1 | ||||||||||||||||||||||
| Acquisitions of assets, net of cash acquired | 21.2 | ( | ( | ||||||||||||||||||||
| Acquisition of business, net of cash acquired | 21.1 | ( | |||||||||||||||||||||
| Net cash flows used in investing activities | ( | ( | ( | ||||||||||||||||||||
| Dividends paid to shareholders of Alcon Inc. | 7.2 | ( | ( | ( | |||||||||||||||||||
| Repayment of current portion of non-current financial debts | 20.3 | ( | |||||||||||||||||||||
| Proceeds from current financial debts | 20.3 | ||||||||||||||||||||||
| Proceeds from non-current financial debts, net of issuance costs | 20.3 | ||||||||||||||||||||||
| Proceeds from 2022 Bridge Loan Facility, net of issuance costs | 20.3 | ||||||||||||||||||||||
| Repayment of non-current financial debts | 20.3 | ( | |||||||||||||||||||||
| Repayment of 2022 Bridge Loan Facility | 20.3 | ( | |||||||||||||||||||||
| Repayment of financial debts assumed in acquisition of business | 20.3 | ( | |||||||||||||||||||||
| Other changes in current financial debts | 20.3 | ( | ( | ||||||||||||||||||||
| Lease payments | 20.3 | ( | ( | ( | |||||||||||||||||||
| Payment of withholding taxes related to equity-based compensation | ( | ( | ( | ||||||||||||||||||||
| Other financing cash flows | ( | ( | |||||||||||||||||||||
| Net cash flows used in financing activities | ( | ( | ( | ||||||||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | ( | ||||||||||||||||||||||
| Net change in cash and cash equivalents | ( | ||||||||||||||||||||||
| Cash and cash equivalents at January 1 | |||||||||||||||||||||||
| Cash and cash equivalents at December 31 | |||||||||||||||||||||||
The accompanying Notes form an integral part of the Consolidated Financial Statements.
F-6
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC.
1. Description of business
Alcon Inc. (the "Company") and the subsidiaries it controls (collectively "Alcon") is a leading eye care company. Alcon is a global company specializing in the research, development, manufacturing and marketing of a broad range of eye care products within two businesses: Surgical and Vision Care. Alcon is a stock corporation organized under the laws of Switzerland, domiciled in Fribourg, Switzerland, with global headquarters located in Geneva, Switzerland. The shares of the Company are listed on the SIX Swiss Stock Exchange ("SIX") and on the New York Stock Exchange ("NYSE") under the symbol “ALC”.
The Consolidated Financial Statements of Alcon are comprised of the Consolidated Balance Sheet as of December 31, 2023 and 2022 and the Consolidated Income Statement, Consolidated Statement of Comprehensive Income, Consolidated Statement of Changes in Equity and Consolidated Statement of Cash Flows for each of the years ended December 31, 2023, 2022 and 2021.
2. Selected accounting policies
Basis of preparation
The accompanying Consolidated Financial Statements present our historical financial position, results of operations, comprehensive income and cash flows in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”) (“IFRS Accounting Standards”). Alcon's principal accounting policies are described in this Note.
Principles of consolidation and equity accounting
The Consolidated Financial Statements include the accounts of the Company and its wholly owned subsidiaries. In the event that the Company has an interest in another entity that is not wholly owned, the assets, liabilities, results of operations and cash flows of such entity are included in the Company's Consolidated Financial Statements, if the Company directly or indirectly has control over such entity. The Consolidated Financial Statements of the Company are prepared in accordance with IFRS as issued by the IASB. They are prepared in accordance with the historical cost convention except for items that are required to be accounted for at fair value. All intercompany transactions and accounts within Alcon were eliminated.
The Company's financial year-end is December 31, which is also the annual closing date of the individual entities' financial statements incorporated into the Consolidated Financial Statements.
Associated companies are all entities over which Alcon has a significant influence but not control or joint control. This is generally the case where Alcon holds between 20% and 50% of an entity's voting rights. Alcon can also have significant influence over an investee where it holds less than 20% of the voting rights if Alcon has significant transactions with the investee, Alcon has influence over the investee’s policy making decisions through Board representation, Alcon shares significant technical information with the investee or Alcon exchanges personnel with the investee. Investments in associated companies are accounted for using the equity method from the date when the investee is determined to be an associated company until the date when Alcon loses significant influence over the investee. Under the equity method, the investment is initially recognized at cost, and the carrying amount is subsequently increased or decreased, to recognize Alcon's share of profit or loss and other comprehensive income of the associated company after the date of initial recognition. Alcon eliminates its share of profit/(loss) from unrealized gains/(losses) from its transactions with associated companies against the carrying amount of the investment. Dividends received or receivable from associated companies are recognized as a reduction in the carrying amount of the investment.
The use of the equity method is discontinued from the date when the investee is determined to no longer be an associated company. The investment retained after cessation of the equity method is remeasured at fair value, and any gain or loss on such remeasurement and disposal of the investment is recognized in the Consolidated Income Statement.
F-7
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The carrying amounts of investments in associated companies are tested for impairment when triggering events are identified.
Use of estimates and assumptions
The preparation of Consolidated Financial Statements requires management to make certain estimates and assumptions, either at the balance sheet date or during the period that affect the reported amounts of assets and liabilities as well as revenues and expenses. Because of the inherent uncertainties, actual outcomes and results may differ from management's assumptions and estimates.
Foreign currencies
The Consolidated Financial Statements are presented in US dollars ("USD"). The functional currency of individual entities incorporated into the Consolidated Financial Statements is generally the local currency of the respective entity. The functional currency used for the reporting of certain Swiss entities is USD instead of their respective local currencies. This reflects the fact that the cash flows and transactions of these entities are primarily denominated in this currency.
For entities not operating in hyperinflationary economies, the entities results, financial position and cash flows that do not have USD as their functional currency are translated into USD using the following exchange rates:
•Income, expense and cash flows using for each month the average exchange rate, with the USD values for each month being aggregated during the year;
•Balance sheet using period-end exchange rates; and
•Resulting exchange rate differences are recognized in other comprehensive income.
The hyperinflationary economies in which Alcon operates are Argentina, Turkey and Venezuela. Argentina and Venezuela were hyperinflationary for all years presented. Turkey became hyperinflationary effective April 1, 2022, requiring retroactive implementation from January 1, 2022 of hyperinflationary accounting.
Acquisition of assets
Assets separately acquired are initially recognized on the balance sheet at cost if they meet the criteria for capitalization. The capitalized cost of the asset includes the purchase price and any directly attributable costs for bringing the asset into the condition to operate as intended. Expected costs for obligations to dismantle and remove property, plant and equipment when it is no longer used are included in their cost.
Property, plant and equipment
Property, plant and equipment are depreciated on a straight-line basis over their estimated useful lives. Freehold land is not depreciated. The related depreciation expense is included in the costs of the functions using the asset or "Cost of net sales" in the Consolidated Income Statement.
Property, plant and equipment are assessed for impairment at the cash generating unit ("CGU") level whenever there is an indication that the balance sheet carrying amount may not be recoverable using cash flow projections for the useful life.
The following table shows the respective useful lives for property, plant and equipment:
| Useful life | |||||
| Buildings and improvements | |||||
| Machinery and other equipment | |||||
| Machinery and equipment | |||||
| Furniture and vehicles | |||||
| Computer hardware | |||||
F-8
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Business combinations
The acquisition method of accounting is used to account for all business combinations, regardless of whether equity instruments or other assets are acquired. The consideration transferred for the acquisition of a subsidiary may include:
•Fair values of the assets transferred;
•Liabilities incurred to the former owners of the acquired business;
•Equity interests issued by the Company;
•Fair value of an asset or liability resulting from a contingent consideration arrangement; and
•Fair value of any pre-existing equity interest in the subsidiary.
Identifiable assets acquired and liabilities assumed in a business combination are measured initially at their fair values at the acquisition date. The excess of the consideration transferred over the fair value of the net identifiable assets acquired is recorded as goodwill, or directly in the income statement if it is a bargain purchase. Alcon primarily uses net present value techniques, utilizing post-tax cash flows and discount rates in estimating the fair value of identifiable assets acquired when allocating the purchase consideration paid for the acquisition. The estimates of the fair values involve significant judgment by management and include assumptions with measurement uncertainty such as the amount and timing of projected cash flows, long-term sales forecasts, the timing and probability of regulatory and commercial success and the discount rate.
Acquisition related costs are expensed as incurred.
Alcon may elect on a transaction-by-transaction basis to apply the optional concentration test to assess whether a transaction qualifies as a business. Under the test, when substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets, Alcon will account for the transaction as an asset purchase and not a business combination.
If the concentration test is not met, or Alcon elects not to apply this optional test, Alcon will perform an assessment focusing on the existence of inputs and processes that have the ability to create outputs to determine whether the transaction is an asset purchase or a business combination.
Goodwill and intangible assets
The annual impairment testing date is Alcon's financial year-end, December 31.
Goodwill
Goodwill arises in a business combination and is the excess of the consideration transferred to acquire a business over the underlying fair value of the net identified assets acquired. It is allocated to groups of CGUs which are usually represented by the reportable segments, which are the same as Alcon's operating segments. Goodwill is tested for impairment annually at the level of these groups of CGUs, and any impairment charges are recorded under "Other expense" in the Consolidated Income Statement.
Intangible assets available-for-use
Alcon has the following classes of available-for-use intangible assets: Currently marketed products, Marketing know-how, Technologies, Other intangible assets (including software) and the Alcon brand name.
Currently marketed products represent the composite value of acquired intellectual property, patents, and distribution rights and product trade names.
Marketing know-how represents the value attributable to the expertise acquired for marketing and distributing Alcon surgical products.
Technologies represent identified and separable acquired know-how used in the research, development and production processes.
Significant investments in internally developed and acquired software are capitalized and included in the "Other" category and amortized once available for use.
The Alcon brand name is shown separately as it is the only Alcon intangible asset that is available for use with an indefinite useful life. Alcon considers it appropriate that the brand name has an indefinite life since the branded products have a history of strong revenue and cash flow performance, and Alcon has the intent and ability to support the brand with spending to maintain its value for the foreseeable future.
F-9
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Except for the Alcon brand name, intangible assets available for use are amortized over their estimated useful lives on a straight-line basis and evaluated for potential impairment whenever facts and circumstances indicate that their carrying value may not be recoverable. The Alcon brand name is not amortized, but evaluated for potential impairment annually.
The following table shows the respective useful lives for available-for-use intangible assets and the location in the Consolidated Income Statement in which the respective amortization and any potential impairment charge is recognized:
| Useful life | Income statement location for amortization and impairment charges | |||||||
| Currently marketed products | "Cost of net sales" | |||||||
| Marketing know-how | "Cost of net sales" | |||||||
| Technologies | "Cost of net sales" or "Research and Development" | |||||||
| Other (including software) | In the respective functional expense | |||||||
| Alcon brand name | Not amortized, indefinite useful life | "Other expense" | ||||||
Acquired In-Process Research & Development ("IPR&D")
Acquired research and development intangible assets, which are still under development and have accordingly not yet obtained marketing approval, are recognized as IPR&D.
IPR&D is not amortized, but evaluated for potential impairment on an annual basis or when facts and circumstances warrant. IPR&D is considered impaired when its balance sheet carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its fair value less costs of disposal ("FVLCOD") and its value in use ("VIU"). Usually, Alcon applies the FVLCOD method for its impairment assessments. Under this approach when evaluating IPR&D for potential impairment, FVLCOD is estimated using net present value techniques utilizing post-tax cash flows and discount rates as there are no direct or indirect observable prices in active markets for identical or similar assets. The estimates used in calculating the net present values involve significant judgment by management and include assumptions with measurement uncertainty such as, the amount and timing of projected cash flows, long-term sales forecasts, discount rate, and the timing and probability of regulatory and commercial success. In the limited cases where the VIU method would be applied, net present value techniques would be applied using pre-tax cash flows and discount rates.
Any impairment charge is recorded in the Consolidated Income Statement under "Research & development".
Once a project included in IPR&D has been successfully developed it is transferred to the "Currently marketed products" category.
Impairment of goodwill, Alcon brand name and definite lived intangible assets
A CGU to which goodwill has been allocated (reportable segments) is considered impaired when its carrying amount, including the goodwill, exceeds its recoverable amount, which is defined as the higher of its FVLCOD and its VIU. If the recoverable amount of the reportable segment is less than its carrying amount, an impairment loss shall be recognized. The impairment loss shall be allocated to reduce the carrying amount of any goodwill allocated to the reportable segment first, with any remaining impairment loss allocated to other assets of the reportable segment on a pro-rata basis of their carrying amount.
An intangible asset other than goodwill is considered impaired when its balance sheet carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its FVLCOD and its VIU. If the recoverable amount of an asset is less than its carrying amount, the carrying amount of the asset shall be reduced to its recoverable amount. That reduction is an impairment loss. Usually, Alcon applies the FVLCOD method for its impairment assessment. In most cases, no direct or indirect observable market prices for identical or similar assets are available to measure the FVLCOD. Therefore, an estimate of FVLCOD is based on net present value techniques utilizing post-tax cash flows and discount rates. In the limited cases where the VIU method would be applied, net present value techniques would be applied using pre-tax cash flows and discount rates.
FVLCOD reflects estimates of assumptions that market participants would be expected to use when pricing the asset or CGUs, and for this purpose management considers the range of economic conditions that are expected to exist over the remaining useful life of the asset.
F-10
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The estimates used in calculating the net present values involve significant judgment by management and include assumptions with measurement uncertainty, such as the following:
•Amount and timing of projected cash flows;
•Long-term sales forecasts, including sales growth rates;
•Royalty rate for the Alcon brand name;
•Terminal growth rate; and
•Discount rate.
Other assumptions used in the net present values calculation include:
•Future tax rate;
•Actions of competitors (launch of competing products, marketing initiatives, etc.); and
•Outcome of R&D activities and forecast of related costs (future product developments).
Generally, for intangible assets with a definite useful life Alcon uses cash flow projections for the whole useful life of these assets. For goodwill and the Alcon brand name, Alcon generally utilizes cash flow projections for a five-year period based on management forecasts, with a terminal value based on cash flow projections considering the long-term expected growth rates and impact of demographic trends of the population to which Alcon products are prescribed, for later periods. Probability-weighted scenarios are typically used.
Discount rates used consider Alcon estimated weighted average cost of capital adjusted for specific country and currency risks associated with cash flow projections to approximate the weighted average cost of capital of a comparable market participant. Actual cash flows and values could vary significantly from forecasted future cash flows and related values derived using net present value techniques.
Cash and cash equivalents
Cash and cash equivalents includes cash on hand, deposits held at call with financial institutions, and other short-term and highly liquid investments. Other short-term and highly liquid investments are classified as cash and cash equivalents when original or weighted-average maturities are three months or less and amounts are readily convertible to known amounts of cash which are subject to an insignificant risk of changes in value. Bank overdrafts are usually presented within current financial debts on the Consolidated Balance Sheet except in cases where a right of offset has been agreed with a bank which then allows for presentation on a net basis.
Financial assets
Non-current financial assets such as the long-term note receivable, certain other financial assets, loans and long-term receivables from customers, primarily related to surgical equipment sales arrangements, VAT receivables, advances and other deposits, are carried at amortized cost, which reflects the time value of money, less any allowances for uncollectable amounts.
Alcon assesses on a forward-looking basis the expected credit losses associated with its non-current financial assets valued at amortized cost.
Purchased or originated credit-impaired financial assets are financial assets that are credit-impaired on initial recognition with one or more events that have a detrimental impact on the estimated future cash flows of those financial assets. The interest income of the financial assets is calculated by applying the credit-adjusted effective interest rate to the amortized cost of the financial asset. The calculation does not revert to the gross basis even if the credit risk of the financial asset subsequently improves so that the financial asset is no longer credit-impaired. Interest income is recognized in "Other financial income and expense" in the Consolidated Income Statement.
The lifetime expected credit loss ("ECL") of the purchased or originated credit-impaired financial assets is analyzed at inception and utilized in calculating the credit-adjusted effective interest rate, with no Day 1 impact on the carrying value of the financial assets. The value of any collateral related to the financial assets is considered in estimating the lifetime ECL at inception. For purchased or originated credit-impaired financial assets, a credit-adjusted effective interest rate is calculated by discounting the estimated future cash flows, including ECLs, to the amortized cost of the debt instrument on initial recognition. Any change in the lifetime ECL from inception would be reflected as a credit loss in the Consolidated Income statement.
For loans, VAT receivables, advances and other deposits valued at amortized cost, impairments, which are based on their expected credit losses, and exchange rate losses are included in "Other expense" in the Consolidated Income Statement
F-11
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
and exchange rate gains and interest income, using the effective interest rate method, are included in "Other income" in the Consolidated Income Statement.
For long-term receivables from customers, provisions for uncollectable amounts, which are based on their expected credit losses, are recorded as marketing and selling costs recognized in the Consolidated Income Statement within "Selling, general & administration" expenses.
Fund investments are valued at fair value through profit and loss ("FVPL"). Unrealized or realized gains and losses, including exchange gains and losses, are recognized in the Consolidated Income Statement in "Other income" for gains and "Other expense" for losses.
Equity investments, including equity securities and convertible notes receivable held as strategic investments are generally designated at the date of acquisition as financial assets valued at fair value through other comprehensive income ("FVOCI") with no subsequent recycling through profit and loss. Unrealized gains and losses, including exchange gains and losses, are recorded as a fair value adjustment in the Consolidated Statement of Comprehensive Income. They are reclassified to "Other reserves" when the equity investment is sold. If these equity securities and convertible notes receivable are not designated at the date of acquisition as financial assets valued at FVOCI, they are valued at FVPL, as described above for fund investments. Changes in fair value of options to acquire development stage companies are charged to Research and development expense.
Derivative financial instruments are initially recognized in the Consolidated Balance Sheet at fair value and are remeasured to their current fair value at the end of each subsequent reporting period. The valuation of forward exchange rate contracts and foreign exchange swaps are based on the discounted cash flow model, using interest curves and spot rates at the reporting date as observable inputs. Unsettled forward contracts and swaps are measured at fair value at quarter-end with changes in fair value recorded to the Consolidated Income Statement as unrealized gains or losses in "Other financial income & expense". Settled forward contracts and swaps are measured at maturity date at fair value with corresponding realized gains or losses recognized in the Consolidated Income Statement in "Other financial income & expense". No hedge accounting is applied for these arrangements.
Inventories
Inventory is valued at the lower of acquisition or production cost determined on a first-in, first-out basis and net realizable value. This value is used for the "Cost of net sales" and "Cost of other revenues" in the Consolidated Income Statement. Unsalable inventory is fully written off in the Consolidated Income Statement under "Cost of net sales" and "Cost of other revenues".
Trade receivables
Trade receivables are initially recognized at their invoiced amounts, including any related sales taxes less adjustments for estimated revenue deductions such as chargebacks and cash discounts.
Provisions for expected credit losses are established using an expected credit loss model ("ECL"). The provisions are based on a forward-looking ECL, which includes possible default events on the trade receivables over the entire holding period of the trade receivable. These provisions represent the difference between the trade receivable's carrying amount and the estimated net collectible amount. Charges for doubtful trade receivables are recorded as marketing and selling costs recognized in the Consolidated Income Statement within "Selling, general & administration" expenses.
Leases
As lessee, Alcon assesses whether a contract contains a lease at inception or modification of a contract based on whether the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. Alcon allocates contractual payments between lease and non-lease components based on their relative stand-alone price. Alcon recognizes a right-of-use asset and a corresponding lease liability for all arrangements in which it is a lessee, except for leases with a term of twelve months or less (short-term leases) and low value leases for which Alcon has elected the recognition exemptions allowed under IFRS 16.
Right-of-use assets
Right-of-use assets are initially recognized at cost, which is comprised of the amount of the initial measurement of the corresponding lease liabilities, adjusted for any lease payments made at or prior to the commencement date of the lease, lease incentives received and initial direct costs incurred, as well as any expected costs for obligations to dismantle and remove right-of-use assets when they are no longer used.
Right-of-use assets are depreciated on a straight-line basis over the shorter of the useful life of the right-of-use asset or the end of the lease term.
F-12
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Right-of-use assets are assessed for impairment whenever there is an indication that the balance sheet carrying amount may not be recoverable using cash flow projections for the useful life.
Lease liabilities
Lease liabilities are accounted for at amortized cost and are initially measured at the present value of future lease payments and are classified as current or non-current based on the due dates of the underlying principal payments. In determining the lease term, Alcon evaluates the renewal options and termination options reasonably certain to be exercised. Lease payments are discounted using the interest rate implicit in the lease or, if not readily determinable, the incremental borrowing rate Alcon would be expected to pay within the respective markets, on a borrowing with a similar term and security. Interest in the period is recorded within "Interest expense" in the Consolidated Income Statement.
Lease liabilities are remeasured for changes in estimated lease term, future lease payments arising from a change in an index or rate, amounts expected to be payable under a residual value guarantee, or in assessment of whether Alcon will exercise a purchase, extension or termination option. Changes to initial lease contract terms are assessed to determine their impact on the scope of lease, and any modifications increasing the scope of the lease are treated as new contracts under the initial measurement principles, while modifications that do not increase or that decrease the scope of the lease result in an adjustment to the right-of-use asset which is remeasured as of the date of the modification.
Principal payments made on lease liabilities and any initial direct costs paid are classified as financing cash outflows, while interest payments are classified as operating cash outflows.
Payments associated with short-term leases and leases of low-value assets are recognized on a straight-line basis as an expense in the Consolidated Income Statement and are classified as cash flows from operating activities.
Legal liabilities
Alcon is subject to contingencies arising in the ordinary course of business such as patent litigation and other product-related litigation, commercial litigation, and governmental investigations and proceedings. Provisions are recorded where a reliable estimate can be made of the probable outcome of legal or other disputes.
Contingent consideration
In a business combination, it is necessary to recognize contingent future payments to previous owners representing contractually defined potential amounts as a liability. Usually for Alcon, these are linked to development or commercial milestones related to certain assets and are recognized as a financial liability at their fair value, which is then re-measured at each subsequent reporting date.
For the determination of the fair value of a contingent consideration, various unobservable inputs are used. A change in these inputs might result in a significantly higher or lower fair value measurement. The inputs used are, among others, the timing and probability of regulatory and commercial success, sales forecast and assumptions regarding the discount rate, timing and different scenarios of triggering events. The significance and usage of these inputs to each contingent consideration may vary due to differences in the timing and triggering events for payments or in the nature of the asset related to the contingent consideration. These estimations typically depend on factors such as technical milestones or market performance and are adjusted for the probability of their likelihood of payment, and if material, appropriately discounted to reflect the impact of time.
Changes in the fair value of contingent consideration liabilities in subsequent periods are recognized in the Consolidated Income Statement in "Cost of net sales" for currently marketed products and in "Research & development" for IPR&D.
The effect of unwinding the discount over time is recognized in "Interest expense" in the Consolidated Income Statement.
Alcon accounts for variable or contingent consideration associated with asset acquisitions using the cost accumulation model. At the date of the asset acquisition, the intangible asset is initially recognized at the amount paid. Variable payments are subsequently capitalized as part of the cost of the asset when paid, on the basis that such payments represent the direct cost of acquisition.
Defined benefit pension plans and other post-employment benefits
The liability or asset recognized in the balance sheet in respect of defined benefit pension plans and other post-employment benefits is the present value of the defined benefit obligation at the end of the reporting period less the fair value of plan assets. The defined benefit obligation is calculated annually by independent actuaries using the projected unit credit method.
The present value of the defined benefit obligation is determined by discounting the estimated future cash outflows using interest rates of high-quality corporate bonds that are denominated in the currency in which the benefits will be paid, and
F-13
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
that have terms approximating the terms of the related obligation. In countries where there is no sufficient market for such bonds, the market rates on government bonds are used.
The current service cost for such post-employment benefit plans is included in the personnel expenses of the various functions where the associates are employed. The net interest on the net defined benefit liability is recognized as "Other expense" or "Other income". The net interest cost is calculated by applying the discount rate to the net balance of the defined benefit obligation and the fair value of plan assets. Past service cost is recognized as "Other expense" or "Other income" in the Consolidated Income Statement for the change in the present value of a defined benefit obligation for employee service in prior periods resulting from a plan amendment or a curtailment.
Remeasurement gains and losses arising from experience adjustments and changes in actuarial assumptions are recognized in the period in which they occur, directly in other comprehensive income.
Defined contribution plans
Financial debts
Financial debts are initially recognized at fair value, net of transaction costs incurred. Financial debts are subsequently measured at amortized cost. Any difference between the proceeds (net of transaction costs and discounts) and the redemption amount is recognized in the Consolidated Income Statement over the period of the financial debts using the effective interest method. Fees paid on the establishment of credit facilities are recognized as transaction costs of the financial debt to the extent that it is probable that some or all of the facility will be drawn down. In this case, the fee is deferred until the draw down occurs. To the extent that there is no evidence that it is probable that some or all of the facility will be drawn down, the fee is capitalized as a prepayment for liquidity services and amortized over the period of the facility to which it relates, and is recognized in "Other financial income & expense" in the Consolidated Income Statement.
Financial debts are derecognized from the balance sheet when the obligation specified in the contract is discharged, cancelled or expired. The difference between the carrying amount of a financial debt that has been extinguished and the consideration paid, including any non-cash assets transferred or liabilities assumed, is recognized in "Other financial income & expense" in the Consolidated Income Statement.
Interest paid on financial debts is classified as operating activities in the Consolidated Statement of Cash Flows. Proceeds and repayments of borrowings with due dates of three months or less are presented net as financing activities in the Consolidated Statement of Cash Flows. Financial debts are classified as current liabilities unless Alcon has an unconditional right and intent to defer the settlement of the liability for at least twelve months after the reporting period.
Revenue
Net sales to third parties
Revenue on the sale of Alcon products and services, which is recorded as "Net sales to third parties" in the Consolidated Income Statement, is recognized when a contractual promise to a customer (i.e., a performance obligation) has been fulfilled by transferring control over the promised goods and services to the customer, substantially all of which is at the point in time of shipment to or receipt of the products by the customer or when the services are performed. If contracts contain customer acceptance provisions, revenue would be recognized upon the satisfaction of acceptance criteria. The amount of revenue to be recognized is based on the consideration Alcon expects to receive in exchange for its goods and services. If a contract contains more than one performance obligation, the consideration is allocated based on the relative stand-alone selling price of each performance obligation.
Surgical equipment may be sold together with other products and services under a single contract and may be structured as an outright cash sale, an installment sale, or a lease. Surgical equipment installment sales and leases have a fixed payment amount which the customer may pay either in fixed intervals or as the customer purchases consumables and/or implantables. Revenues are recognized upon satisfaction of each of the performance obligations in the contract and the consideration is allocated based on the relative stand-alone selling price of each performance obligation.
•Surgical equipment revenue from outright cash sales and installment sales arrangements is recognized at the point in time when control is transferred to the customer. The current portion of long-term receivables from customers and long-term receivables from customers for installment sales arrangements are recorded in "Other current assets" (see "Current portion of long-term receivables from customers" in Note 14) and "Financial
F-14
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
assets" (see "Long-term receivables from customers" in Note 11), respectively. Financing income for installment sales arrangements longer than twelve months is recognized over the term of the arrangement in "Other income". Alcon applies the practical expedient under IFRS 15 to installment sales arrangements that are twelve months or less in duration.
•In addition to cash and installment sales, revenue is recognized under finance and operating lease arrangements. Leases in which Alcon transfers substantially all the risks and rewards incidental to ownership to the customer are treated as finance lease arrangements. Revenue from finance lease arrangements is recognized at amounts equal to the fair value of the equipment, which approximates the present value of the minimum lease payments under the arrangements. As interest rates embedded in lease arrangements are approximately market rates, revenue under finance lease arrangements is comparable to revenue for outright sales. Finance income for arrangements longer than twelve months is deferred and subsequently recognized based on a pattern that approximates the use of the effective interest method and recorded in "Other income". Operating lease revenue for equipment rentals is recognized on a straight-line basis over the lease term in "Net sales to third parties".
The consideration Alcon receives in exchange for its goods or services may be fixed or variable. Variable consideration is only recognized when it is highly probable that a significant reversal of cumulative sales will not occur. The most common elements of variable consideration are listed below:
•Rebates and discounts granted to government agencies, wholesalers, retail pharmacies, managed health-care organizations and other customers, estimated payments for Medicare Part D prescription drug program coverage gap (commonly called the "donut hole"), patient co-pay program coupon utilization, as well as chargebacks are provisioned and recorded as a deduction from revenue at the time the related revenues are recorded or when the incentives are offered. They are calculated based on historical experience, regulations, the specific terms in the individual agreements, product pricing, channels and payors.
•Cash discounts are offered to customers to encourage prompt payment and are provisioned and recorded as revenue deductions at the time the related sales are recorded.
•Sales returns provisions are recognized and recorded as revenue deductions when there is historical experience of Alcon agreeing to customer returns and Alcon can reasonably estimate expected future returns. In doing so, the estimated rate of return is applied, determined based on historical experience of customer returns and considering any other relevant factors. This is applied to the amounts invoiced, also considering the amount of returned products to be destroyed versus products that can be placed back in inventory for resale. Where shipments are made on a re-sale or return basis, without sufficient historical experience for estimating sales returns, revenue is only recorded when there is evidence of consumption or when the right of return has expired.
Provisions for revenue deductions are adjusted to actual amounts as rebates, discounts, chargebacks, payment for Medicare Part D prescription drug program, patient co-pay program coupon utilization and returns are processed. The provision represents estimates of the related obligations, requiring the use of judgment when estimating the effect of these sales deductions.
Other revenues
"Other revenues" include revenue from contract manufacturing services which are recognized over time as the service obligations are completed and third party royalty income. Associated costs for contract manufacturing services are recognized in "Cost of other revenues".
Research & development
Internal research & development ("R&D") costs are fully charged to "Research & development" in the Consolidated Income Statement in the period in which they are incurred. Alcon considers that regulatory and other uncertainties inherent in the development of new products preclude the capitalization of internal development expenses as an intangible asset until marketing approval from a regulatory authority is obtained in a major market such as the United States, the European Union, Switzerland, China or Japan.
Payments made to third parties to in-license or acquire intellectual property rights and products, including initial upfront and subsequent milestone payments, are capitalized as intangible assets. If additional payments are made to the originator company to continue to perform R&D activities, an evaluation is made as to the nature of the payments. Such additional payments will be expensed if they are deemed to be compensation for subcontracted R&D services not resulting in an additional transfer of intellectual property rights to Alcon. Such additional payments will be capitalized if they are deemed to be compensation for the transfer to Alcon of additional intellectual property developed at the risk of the originator company. Subsequent internal R&D costs in relation to IPR&D and other assets are expensed until such time
F-15
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
that technical feasibility can be proven, as demonstrated by the receipt of marketing approval for the related product from a regulatory authority in a major market.
Equity-based compensation
Each of the periods presented include expense related to incentive compensation provided to eligible Alcon associates in the form of equity-settled or equity-based awards including restricted stock units ("RSUs") and performance stock units ("PSUs").
Alcon expenses the fair values of RSUs and PSUs granted to associates as compensation over the related vesting periods within the various functions where the associates are employed. The fair values of the awards are determined on their grant dates and are adjusted to account for the specific provisions of each of the corresponding grant agreements.
Alcon RSUs do not entitle the recipients to dividends. As such, the fair value upon grant is based on the Alcon share price at the grant date adjusted for potential future dividends to be paid within the holding period. The fair value of these grants, after making adjustments for assumptions related to their forfeiture during the vesting period, is expensed on a straight-line basis over the respective vesting period.
PSUs are subject to certain performance criteria being achieved during the vesting period and require plan participants to provide services during the vesting period. PSUs granted under Alcon's plans are subject to performance criteria based on internal performance metrics. The expense is determined taking into account assumptions concerning performance during the period relative to targets and expected forfeitures due to plan participants not meeting their service conditions. These assumptions are periodically adjusted. Any change in estimates for past services is recorded immediately as an expense or income in the Consolidated Income Statement and amounts for future periods are expensed over the remaining vesting period. As a result, at the end of the vesting period, the total charge during the whole vesting period represents the amount that will finally vest. The number of equity instruments that finally vest is determined at the vesting date.
If a plan participant leaves Alcon for reasons other than retirement, disability or death, then unvested restricted shares, RSUs and PSUs are forfeited, unless determined otherwise by the provision of the plan rules or by the Compensation Committee of the Company's Board of Directors, for example, in connection with a reorganization.
Restructuring charges
Restructuring provisions are recognized for the direct expenditures arising from the restructuring, where the plans are sufficiently detailed and where appropriate communication to those affected has been made.
Charges to increase restructuring provisions are included in "Other expense" in the Consolidated Income Statement. Corresponding releases are recorded in "Other income" in the Consolidated Income Statement.
Taxes
Taxes on income are expensed in the same periods as the revenues and expenses to which they relate and include any interest and penalties incurred during the period. Deferred taxes are determined using the comprehensive liability method and are calculated on the temporary differences that arise between the tax basis of an asset or liability and its carrying value in the balance sheet prepared for purposes of these Consolidated Financial Statements, except for those temporary differences related to investments in subsidiaries where the timing of their reversal can be controlled and it is probable that the difference will not reverse in the foreseeable future. Alcon recognizes deferred taxes for a new temporary difference where there are previously unrecognized temporary differences, instead of adjusting the amount of those unrecognized differences, for changes in the underlying economics where the initial recognition exemption applies. Since the retained earnings are reinvested, withholding or other taxes on eventual distribution of a subsidiary's retained earnings are only taken into account when a dividend has been planned.
The estimated amounts for current and deferred tax assets or liabilities, including any amounts related to any uncertain tax positions, are based on currently known facts and circumstances. Tax returns are based on an interpretation of tax laws and regulations and reflect estimates based on these judgments and interpretations. The tax returns are subject to examination by the competent taxing authorities which may result in an assessment being made requiring payments of additional tax, interest or penalties. Inherent uncertainties exist in the estimates of the tax positions.
The Organization for Economic Cooperation and Development (“OECD") has published Global Anti-Base Erosion (“GloBE”) Model Rules, which include a minimum 15% tax rate by jurisdiction ("Pillar Two"). For the period ended December 31, 2023, we have applied the IASB amendment to IAS 12, Income Taxes, which provides a mandatory temporary exception from recognizing or disclosing deferred taxes related to Pillar Two.
F-16
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Earnings per share
Basic earnings per share is based on the weighted average number of common shares outstanding. Diluted earnings per share is based on the weighted average number of common shares outstanding and all dilutive potential common shares outstanding.
New standards and interpretations not yet adopted
Effective January 1, 2024, Alcon will adopt Amendments to International Accounting Standards 1 ("IAS 1"), Presentation of Financial Statements, to clarify the criteria used in determining the classification on the balance sheet of a liability as non-current where an entity has the right to postpone settlement of the liability for at least twelve months after the reporting date. Adoption of the amendment will have an impact on the classification of certain of Alcon's financial debts currently classified as current due to Alcon's expectation to settle the liabilities within twelve months of the balance sheet date, even though Alcon has the right to roll over the financial debts for at least twelve months after the reporting date.
As of December 31, 2023, the current financial debts balance includes $82 million of financial debts that Alcon has a right to roll over for at least twelve months after balance sheet date that will be reclassified to non-current financial debts upon adoption of the Amendments to IAS 1.
3. Significant transactions
Significant transactions in 2023
There were no significant transactions during the twelve months ended December 31, 2023.
Significant transactions in 2022
Vision Care - Acquisition of Aerie Pharmaceuticals, Inc.
On November 21, 2022, Alcon acquired 100 % of the outstanding shares and equity of Aerie Pharmaceuticals, Inc. ("Aerie"), a pharmaceutical company focused on the discovery, development, manufacturing and commercialization of first-in-class ophthalmic therapies. Pursuant to the terms of the Agreement and Plan of Merger, Alcon paid $15.25 per share to acquire all outstanding shares of Aerie's common stock. The total purchase consideration amounted to $744 million and total cash paid for the net identifiable assets recognized, net of cash acquired, was $666 million. Alcon also assumed debt of $316 million. This transaction was accounted for as a business combination that resulted in goodwill of $65 million under the preliminary purchase price allocation ("PPA") of the fair values of the acquired assets and assumed liabilities. The total purchase consideration was funded with proceeds from a bridge loan facility agreement (the "2022 Bridge Loan Facility") on November 21, 2022. Refer to Note 16 for additional information regarding the 2022 Bridge Loan Facility. The PPA was subsequently finalized during the third quarter of 2023, resulting in adjusted goodwill of $21 million. Refer to Note 21.1 for additional information regarding the final PPA.
Series 2032 Notes and Series 2052 Notes issuance
On December 6, 2022, Alcon, through its wholly owned subsidiary Alcon Finance Corporation (“AFC”), completed a private offering of non-current financial debt consisting of $700 million of 5.375 % senior notes due 2032 and $600 million of 5.750 % senior notes due 2052. The funds borrowed through the issuance, together with cash, were used to repay the remaining $640 million Facility B term loan and the $775 million 2022 Bridge Loan Facility. Refer to Note 16 for additional information.
F-17
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Vision Care - Acquisition of Eysuvis and Inveltys products
On July 8, 2022, Alcon acquired two pharmaceutical ophthalmic eye drops, Eysuvis and Inveltys, from Kala Pharmaceuticals, Inc. The acquisition complements Alcon’s existing portfolio in the large and fast-growing dry eye category. Pursuant to the terms of the Asset Purchase Agreement, Alcon paid total upfront consideration of $60 million for Eysuvis and Inveltys, paid an additional amount to purchase certain related inventory and assumed certain liabilities of approximately $14 million for a purchase consideration of $79 million. In addition, Alcon agreed to potentially pay additional amounts upon achievement of certain commercial milestones if annual sales exceed defined targets that expire after 2029. The purchase consideration was allocated using the relative fair value approach primarily to currently marketed product intangible assets within the Vision Care reportable segment of $71 million and assumed liabilities of $14 million.
Series 2028 Notes issuance
On May 31, 2022, Alcon, through its wholly owned subsidiary Alcon Finance B.V. (“AFBV”), completed a public offering of $537 million (EUR500 million) of non-current EUR denominated financial debt consisting of 2.375 % senior notes due 2028. The funds borrowed through the issuance were used to repay the $376 million (EUR350 million) Facility C term loan in full and partially repay $160 million of the Facility B term loan. Refer to Note 16 for additional information.
Surgical - Acquisition of Ivantis, Inc.
On January 7, 2022, Alcon acquired 100 % of the outstanding shares and equity of Ivantis, Inc., a privately-held, US-based company and manufacturer of the Hydrus Microstent, a minimally-invasive glaucoma surgery (“MIGS”) device designed to lower intraocular pressure for open-angle glaucoma patients, for total upfront consideration of $479 million and additional amounts to be potentially paid upon achievement of development and commercial milestones. The acquisition expands Alcon’s surgical portfolio and is expected to help provide a platform for more growth in the glaucoma space. Refer to Note 21.2 for additional information regarding this transaction which was accounted for as an asset acquisition.
Significant transactions in 2021
Vision Care - Acquisition of Simbrinza US commercialization rights
On April 28, 2021, Alcon executed an Asset Purchase Agreement (“Agreement”) to acquire exclusive US commercialization rights to a pharmaceutical ophthalmic eye drop, Simbrinza (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2%, from Novartis. Under the terms of the Agreement, Alcon paid $355 million at closing on June 8, 2021 and recognized the intangible asset acquisition as currently marketed products within the Vision Care reportable segment. After closing, Alcon and Novartis immediately began a transition period during which Novartis sold Simbrinza on Alcon's behalf. The transition period concluded during the third quarter of 2021 and Alcon began to fully commercialize Simbrinza for the US market. Novartis retains all rights to Simbrinza® outside of the US.
F-18
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
4. Segment information
The segment information disclosed in these Consolidated Financial Statements reflects historical results consistent with the identifiable reportable segments of Alcon and financial information that the Chief Operating Decision Maker ("CODM") reviews to evaluate segmental performance and allocate resources among the segments. The CODM is the Executive Committee of Alcon.
The businesses of Alcon are divided operationally on a worldwide basis into two identified reportable segments, Surgical and Vision Care. Alcon's reportable segments are the same as its operating segments as Alcon does not aggregate any operating segments in arriving at its reportable segments. As indicated below, certain income and expenses are not allocated to segments.
Reportable segments are presented in a manner consistent with the internal reporting to the CODM. The reportable segments are managed separately due to their distinct needs and activities for research, development, manufacturing, distribution and commercial execution.
The Executive Committee of Alcon is responsible for allocating resources and assessing the performance of the reportable segments.
In Surgical, Alcon researches, develops, manufactures, distributes and sells ophthalmic products for cataract surgery, vitreoretinal surgery, refractive laser surgery and glaucoma surgery. The surgical portfolio also includes implantables, consumables and surgical equipment required for these procedures and supports the end-to-end procedure needs of the ophthalmic surgeon.
In Vision Care, Alcon researches, develops, manufactures, distributes and sells daily disposable, reusable, and color-enhancing contact lenses and a comprehensive portfolio of ocular health products, including products for dry eye, glaucoma, contact lens care and ocular allergies, as well as ocular vitamins and redness relievers.
Alcon also provides services, training, education and technical support for both the Surgical and Vision Care businesses.
The basis of preparation and the selected accounting policies mentioned in Note 2 are used in the reporting of segment results.
The Executive Committee of Alcon evaluates segmental performance and allocates resources among the segments primarily based on net sales and segment contribution.
Net identifiable assets are not assigned to the segments in the internal reporting to the CODM, and are not considered in evaluating the performance of the business segments by the Executive Committee of Alcon.
Segment contribution excludes amortization and impairment charges for acquired product rights or other intangibles, general and administrative expenses for corporate activities, separation costs, transformation costs, fair value adjustments to contingent consideration liabilities, past service costs primarily for post-employment benefit plan amendments, acquisition and integration related costs, certain acquisition related items and certain other income and expense items.
General & administration (corporate) includes the costs of the Alcon corporate headquarters, including all related corporate function costs.
Other income and expense items excluded from segment contribution include fair value adjustments of financial assets in the form of options to acquire a company carried at FVPL, net gains and losses on fund investments and equity securities valued at FVPL, restructuring costs, legal provisions and settlements and other income and expense items not attributed to a specific segment.
F-19
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Net sales and other revenues by segment
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Surgical | |||||||||||||||||
| Implantables | |||||||||||||||||
| Consumables | |||||||||||||||||
| Equipment/other | |||||||||||||||||
| Total Surgical net sales to third parties | |||||||||||||||||
| Vision Care | |||||||||||||||||
| Contact lenses | |||||||||||||||||
| Ocular health | |||||||||||||||||
| Total Vision Care net sales to third parties | |||||||||||||||||
| Total net sales to third parties | |||||||||||||||||
| Vision Care other revenues | |||||||||||||||||
| Total net sales and other revenues | |||||||||||||||||
Segment contribution and reconciliation to income before taxes
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Segment contribution | |||||||||||||||||
Surgical | |||||||||||||||||
Vision Care | |||||||||||||||||
| Total segment contribution | |||||||||||||||||
| Not allocated to segments: | |||||||||||||||||
| Amortization of intangible assets | ( | ( | ( | ||||||||||||||
| Impairment charges on intangible assets | ( | ( | |||||||||||||||
| General & administration (corporate) | ( | ( | ( | ||||||||||||||
| Separation costs | ( | ||||||||||||||||
| Transformation costs | ( | ( | ( | ||||||||||||||
| Fair value adjustments to contingent consideration liabilities | |||||||||||||||||
| Past service costs for post-employment benefit plan amendments | |||||||||||||||||
| Acquisition and integration related costs | ( | ( | |||||||||||||||
| Release of contingent liability related to a recent acquisition | |||||||||||||||||
| Other | ( | ( | ( | ||||||||||||||
| Operating income | |||||||||||||||||
| Interest expense | ( | ( | ( | ||||||||||||||
| Other financial income & expense | ( | ( | ( | ||||||||||||||
| Income before taxes | |||||||||||||||||
F-20
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Included in segment contribution are:
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Depreciation of property, plant & equipment: | |||||||||||||||||
Surgical | ( | ( | ( | ||||||||||||||
Vision Care | ( | ( | ( | ||||||||||||||
| Not allocated to segments | ( | ( | |||||||||||||||
| Total depreciation of property, plant & equipment | ( | ( | ( | ||||||||||||||
| Depreciation of right-of-use assets: | |||||||||||||||||
Surgical | ( | ( | ( | ||||||||||||||
Vision Care | ( | ( | ( | ||||||||||||||
| Total depreciation of right-of-use assets | ( | ( | ( | ||||||||||||||
| Impairment charges on property, plant & equipment, net: | |||||||||||||||||
Surgical | ( | ||||||||||||||||
| Total impairment charges on property, plant & equipment, net | ( | ||||||||||||||||
| Equity-based compensation: | |||||||||||||||||
Surgical | ( | ( | ( | ||||||||||||||
Vision Care | ( | ( | ( | ||||||||||||||
| Not allocated to segments | ( | ( | ( | ||||||||||||||
| Total equity-based compensation | ( | ( | ( | ||||||||||||||
Geographical information
The following table shows the United States, International and countries that accounted for more than 5% of at least one of the respective Alcon totals, for net sales for the years ended December 31, 2023, 2022 and 2021, and for selected non-current assets at December 31, 2023 and 2022:
Net sales(2) | Total of selected non-current assets(3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
($ millions unless indicated otherwise)(1) | 2023 | 2022 | 2021 | 2023 | 2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Country | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| United States | % | % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| International | % | % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| thereof: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Switzerland (country of domicile) | % | % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Japan | % | % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| China | % | % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | % | % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Company total | % | % | % | % | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1)International percentages may not sum due to rounding.
(2)Net sales to third parties by location of third-party customer.
(3)Includes property, plant & equipment, right-of-use assets, goodwill and other intangible assets. During 2023, Alcon retrospectively adjusted the provisional amounts that were recognized for the preliminary PPA at the Aerie acquisition date, resulting in Goodwill of $8,926 million as of December 31, 2022. The resulting total of selected non-current assets was $23,031 million as of December 31, 2022. Refer to Note 21.1 for more information regarding the PPA which was finalized in the third quarter of 2023.
No customer accounted for 10% or more of Alcon's net sales.
F-21
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
5. Interest expense and other financial income & expense
Interest expense
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Interest expense on financial debts | ( | ( | ( | ||||||||||||||
| Interest expense from discounting long-term liabilities | ( | ( | ( | ||||||||||||||
| Interest expense on lease liabilities | ( | ( | ( | ||||||||||||||
| Total interest expense | ( | ( | ( | ||||||||||||||
Other financial income & expense
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Interest income | |||||||||||||||||
| Loss on extinguishment of financial debt | ( | ||||||||||||||||
| Other financial expense | ( | ( | ( | ||||||||||||||
| Monetary loss from hyperinflation accounting | ( | ( | ( | ||||||||||||||
| Currency result, net | ( | ( | ( | ||||||||||||||
| Total other financial income & expense | ( | ( | ( | ||||||||||||||
6. Taxes
Income before taxes
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Switzerland | |||||||||||||||||
| Foreign | ( | ||||||||||||||||
| Total income before taxes | |||||||||||||||||
Current and deferred income tax income/(expense)
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Switzerland | ( | ( | ( | ||||||||||||||
| Foreign | ( | ( | ( | ||||||||||||||
| Current income tax (expense) | ( | ( | ( | ||||||||||||||
| Switzerland | |||||||||||||||||
| Foreign | ( | ( | |||||||||||||||
| Deferred tax income | |||||||||||||||||
| Total income tax income/(expense) | ( | ( | |||||||||||||||
F-22
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Analysis of tax rate
Alcon's overall applicable tax rate can change each year since it is calculated as the weighted average tax rate based on pre-tax income/(loss) of each subsidiary. The main elements contributing to the difference between Alcon's overall applicable tax rate and the effective tax rate are summarized in the below table.
| 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||
($ millions unless indicated otherwise)(1) | % | % | % | ||||||||||||||||||||||||||||||||
| Applicable tax rate | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
| Effect of disallowed expenditures | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
| Effect of equity-based compensation | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
| Effect of tax credits and allowances | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
Effect of deductibility of a statutory expense in Switzerland(2) | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
| Effect of adjustments to contingent consideration and other liabilities | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
Effect of changes in uncertain tax positions(3) | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
| Effect of previously unrecognized tax loss carryforward | ( | % | % | % | |||||||||||||||||||||||||||||||
| Effect of 2022 APA on prior years | ( | % | ( | % | % | ||||||||||||||||||||||||||||||
| Effect of non-deductible amortization | ( | % | ( | % | % | ||||||||||||||||||||||||||||||
| Effect of other items | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
| Effect of prior year items | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
| Effective tax rate | ( | % | ( | % | ( | % | |||||||||||||||||||||||||||||
(1)Percentages may not sum due to rounding.
(2)Includes agreements for fiscal years 2023, 2022 and 2021. 2023 also includes a long-term Swiss Tax Agreement; however, it is uncertain whether Alcon will obtain a similar treatment for future years for a portion of the benefit.
(3)Primarily includes the net effect of partial reserves and benefits recognized for the deductibility of statutory expenses in Switzerland. 2023 also includes the release of reserves in US and Germany following the conclusion of tax audits and other items. 2021 also relates to international transfer pricing.
Alcon has a substantial business presence in many countries and is therefore subject to different income and expense items that are non-taxable (permanent differences) or are taxed at different rates in those tax jurisdictions. This results in a difference between Alcon's applicable tax rate and effective tax rate as shown in the table above.
Fluctuations in taxes and effective tax rates are primarily due to the geographical pre-tax income and loss mix across certain tax jurisdictions relative to Alcon's consolidated income before taxes, changes in uncertain tax positions and certain non-recurring items.
The applicable tax rate was 20.2 % in 2023, compared to 22.5 % in 2022, primarily due to the mix of pre-tax income/(loss) across geographical jurisdictions. The effective tax rate was a 17.1 % benefit in 2023, compared to 27.6 % expense in 2022. The current year was primarily driven by a $263 million net benefit associated with the 2023 Swiss Tax Agreement (as defined below) and a net benefit of $36 million from other discrete tax items. The prior year expense was primarily driven by the 2022 APA (as defined below), partially offset by favorable discrete tax items.
The increase in the applicable tax rate for 2022 compared to 9.3 % in 2021 was primarily driven by more profit being taxable at the rate applicable in the US compared to Alcon's historical filing position as a result of the 2022 APA. The applicable tax rate in 2021 was impacted by pre-tax losses in certain tax jurisdictions.
Tax returns are subject to examination by competent taxing authorities, which may result in assessments being made requiring payments of additional tax, interest or penalties. Inherent uncertainties exist in the estimates of the tax positions.
F-23
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
2023 Swiss Tax Agreement
During the fourth quarter of 2023, Alcon entered into a long-term agreement with Switzerland tax authorities related to the deductibility of a statutory expense in Switzerland through March 31, 2039 (the "2023 Swiss Tax Agreement"). Alcon recorded a discrete tax benefit of $263 million in "Taxes" in the Consolidated Income Statement and corresponding deferred tax asset, net of reserves.
2022 Advanced Pricing Agreement
During the fourth quarter of 2022, Alcon recognized the impact of an Advanced Pricing Agreement between US and Switzerland tax authorities (the "2022 APA") related to the allocation and taxation of relevant Alcon profits between the US and Switzerland retroactive to 2019. The 2022 APA results in more profit being taxable at the rate applicable in the US compared to Alcon’s historical filing position. As a result, in the fourth quarter of 2022 Alcon recorded a discrete item of $37 million of income tax expense related to the 2019 through 2021 tax years and an increase of $64 million of income tax expense for the year ended December 31, 2022. The 2022 APA was agreed upon in the first quarter of 2023 and is valid through 2027.
Pillar Two income taxes
The OECD has published GloBE Model Rules, which include a minimum 15% tax rate by jurisdiction ("Pillar Two"). Various countries have enacted or intend to enact tax legislation to comply with Pillar Two rules. Alcon is within the scope of the OECD’s Pillar Two, which did not impact 2023 results but will impact Alcon’s financial results from January 1, 2024 onward.
Of the countries enacting Pillar Two legislation, we expect Switzerland to be the most impactful to Alcon. In December 2023, the Swiss government decided to partially implement Pillar Two by introducing a Qualified Domestic Minimum Top-up Tax (“QDMTT”) to reach the required taxation level of 15% on Pillar Two qualifying profits earned by companies domiciled in Switzerland effective from January 1, 2024. This QDMTT will not be applied to the Pillar Two qualifying profits earned by subsidiaries domiciled in tax jurisdictions outside of Switzerland. The implementation timing and specific provisions of any further Pillar Two tax regulations in Switzerland remain subject to further assessments at both the Federal and Cantonal levels. For the period ended December 31, 2023, we have applied the IASB amendment to IAS 12, Income Taxes, which provides a mandatory temporary exception from recognizing or disclosing deferred taxes related to Pillar Two such that there is no impact to the 2023 Consolidated Financial Statements. The future impact of Pillar Two for Alcon is estimated to increase Alcon's effective tax rate by approximately 1 % to 2 %. We are continuing to follow Pillar Two legislative developments to evaluate the potential future impact on our consolidated results of operations, financial position and cash flows beginning in 2024.
F-24
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
7. Share capital, dividends and earnings per share
7.1 Share capital
The share capital of the Company as of December 31, 2023 is CHF 20 million, which is comprised of 499.7 million registered shares, nominal value of CHF 0.04 per share.
The following table shows the movement in the shares:
(shares in millions)(1) | Common stock shares outstanding | Treasury stock shares | Total shares | ||||||||||||||
| January 1, 2021 | |||||||||||||||||
| Settlement of equity-based awards | ( | — | |||||||||||||||
| December 31, 2021 | |||||||||||||||||
| Settlement of equity-based awards | ( | — | |||||||||||||||
| December 31, 2022 | |||||||||||||||||
| Settlement of equity-based awards | ( | — | |||||||||||||||
| December 31, 2023 | |||||||||||||||||
(1)Totals may not sum due to rounding.
All of the Company's 6.4 million shares held in treasury as of December 31, 2023 may only be used to fulfill the future vesting of existing and future equity-based awards.
Capital range and conditional share capital
On May 5, 2023, Alcon's shareholders approved the introduction of a capital range and a conditional share capital in Alcon's Articles of Incorporation. Under the capital range, and until May 5, 2028 or an earlier expiry, the Company's Board of Directors (the "Board") has the authority to increase or decrease the share capital ranging from CHF 19 million (lower limit) to CHF 22 million (upper limit). The capital increase or decrease may be effected by (A) issuing up to the lower of (i) 50 million fully paid-in registered shares and (ii) 10 % of the share capital at the time of increase or (B) cancelling up to 25 million registered shares, as applicable. The Board is further authorized to withdraw or restrict subscription rights of existing shareholders and allocate such rights to third parties, the Company or any of its group companies, for the purposes of (a) raising equity capital, (b) acquisition transactions, (c) broadening the shareholders constituency in certain financial or investor market or (d) Board, executive management, employees, advisors or other participation programs.
The Board can also rely on a conditional share capital instrument in its Articles of Incorporation through which the share capital may be increased in an amount not to exceed CHF 2.0 million, by the issuance of up to 50 million fully paid-in registered shares through the voluntary or mandatory exercise of conversion, exchange, option, warrant, subscription or other rights granted to or imposed on shareholders or third parties alone or in connection with the issuance of bonds, notes, options, warrants or other similar securities or contractual obligations of the Company or its affiliates. The conditional share capital may be used for the same purposes as stated in the preceding paragraph in connection with the capital range. As of December 31, 2023, the Board had not made use of the authority under any of the capital range or conditional share capital provisions.
F-25
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
7.2 Dividends
On February 27, 2023, the Board proposed a dividend of CHF 0.21 per share, which was subsequently approved by the shareholders at the Annual General Meeting on May 5, 2023 and paid in May 2023 for an amount of $116 million.
On February 15, 2022, the Board proposed a dividend of CHF 0.20 per share, which was subsequently approved by the shareholders at the Annual General Meeting on April 27, 2022 and paid in May 2022 for an amount of $100 million.
On February 23, 2021, the Board proposed a dividend of CHF 0.10 per share, which was subsequently approved by the shareholders at the Annual General Meeting on April 28, 2021 and paid in May 2021 for an amount of $54 million.
7.3 Earnings per share
Basic earnings per share is computed by dividing net income for the period by the weighted average number of common shares outstanding during the period. For the years ended December 31, 2023, 2022 and 2021, the weighted average number of shares outstanding was 493.0 million, 491.4 million and 490.0 million shares, respectively.
The only potentially dilutive securities are the outstanding unvested equity-based awards under the Company's equity-based incentive plans, as described in Note 23. Except when the effect would be anti-dilutive, the calculation of diluted earnings per common share includes the weighted average net impact of unvested equity-based awards. For the years ended December 31, 2023, 2022 and 2021, the weighted average diluted number of shares outstanding was 496.5 million, 494.4 million and 493.4 million, respectively, which includes the potential conversion of 3.5 million, 3.0 million and 3.4 million unvested equity-based awards, respectively.
8. Property, plant & equipment
The following table summarizes the movements of property, plant & equipment in 2023:
| ($ millions) | Land | Buildings & improvements | Construction in progress | Machinery & other equipment | Total | ||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||
| January 1, 2023 | |||||||||||||||||||||||||||||
Additions | |||||||||||||||||||||||||||||
Disposals and derecognitions(1) | ( | ( | ( | ( | |||||||||||||||||||||||||
| Reclassifications for assets placed in service | ( | ||||||||||||||||||||||||||||
| Currency translation effects | |||||||||||||||||||||||||||||
| December 31, 2023 | |||||||||||||||||||||||||||||
| Accumulated depreciation | |||||||||||||||||||||||||||||
| January 1, 2023 | ( | ( | ( | ( | |||||||||||||||||||||||||
| Depreciation charge | ( | ( | ( | ||||||||||||||||||||||||||
Disposals and derecognitions(1) | |||||||||||||||||||||||||||||
| Currency translation effects | ( | ( | ( | ||||||||||||||||||||||||||
| December 31, 2023 | ( | ( | ( | ( | |||||||||||||||||||||||||
| Net book value at December 31, 2023 | |||||||||||||||||||||||||||||
(1)Derecognition of assets that are no longer used and are not considered to have a significant disposal value or other alternative use.
As of December 31, 2023, commitments for purchases of property, plant & equipment were $283 million.
F-26
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The following table summarizes the movements of property, plant & equipment in 2022:
| ($ millions) | Land | Buildings & improvements | Construction in progress | Machinery & other equipment | Total | ||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||
| January 1, 2022 | |||||||||||||||||||||||||||||
Additions | |||||||||||||||||||||||||||||
| Impact of business combination | |||||||||||||||||||||||||||||
Disposals and derecognitions(1) | ( | ( | ( | ( | |||||||||||||||||||||||||
| Reclassifications for assets placed in service | ( | ||||||||||||||||||||||||||||
| Currency translation effects | ( | ( | ( | ( | |||||||||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||||||||
| Accumulated depreciation | |||||||||||||||||||||||||||||
| January 1, 2022 | ( | ( | ( | ( | |||||||||||||||||||||||||
| Depreciation charge | ( | ( | ( | ||||||||||||||||||||||||||
| Impairment charge | ( | ( | |||||||||||||||||||||||||||
Disposals and derecognitions(1) | |||||||||||||||||||||||||||||
| Currency translation effects | |||||||||||||||||||||||||||||
| December 31, 2022 | ( | ( | ( | ( | |||||||||||||||||||||||||
| Net book value at December 31, 2022 | |||||||||||||||||||||||||||||
(1)Derecognition of assets that are no longer used and are not considered to have a significant disposal value or other alternative use.
F-27
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
9. Goodwill and other intangible assets
The following table summarizes the movements of goodwill and other intangible assets in 2023:
| Intangible assets other than goodwill | |||||||||||||||||||||||||||||||||||||||||||||||
| ($ millions) | Goodwill | Alcon brand name | Acquired in-process research & development | Technologies | Currently marketed products | Marketing know-how | Other intangible assets (including software) | Total | |||||||||||||||||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||||||||||||||||||||
| January 1, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||
| Impact of asset acquisitions | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| Additions | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
Disposals and derecognitions(1) | — | — | ( | — | ( | — | ( | ( | |||||||||||||||||||||||||||||||||||||||
| December 31, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||
| Accumulated amortization | |||||||||||||||||||||||||||||||||||||||||||||||
| January 1, 2023 | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||
| Amortization charge | — | — | — | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||
Disposals and derecognitions(1) | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| December 31, 2023 | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||
| Net book value at December 31, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||
(1) Derecognitions of assets that are no longer used or being developed and are not considered to have a significant disposal value or other alternative use.
The following table summarizes the allocation of the net book values of goodwill and other intangible assets by reportable segment at December 31, 2023:
| Intangible assets other than goodwill | |||||||||||||||||||||||||||||||||||||||||||||||
| ($ millions) | Goodwill | Alcon brand name | Acquired in-process research & development | Technologies | Currently marketed products | Marketing know-how | Other intangible assets (including software) | Total | |||||||||||||||||||||||||||||||||||||||
| Surgical | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Vision Care | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||
| Not allocated to segments | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| Net book value at December 31, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||
The Surgical and Vision Care reportable segments' CGUs, to which goodwill is allocated, are comprised of a group of smaller CGUs. The valuation method of the recoverable amount of the CGUs, to which goodwill is allocated, is based on the FVLCOD.
The Alcon brand name is an intangible asset with an indefinite life. The intangible asset is not allocated to the reportable segments as it is used to market the Alcon-branded products of both the Surgical and Vision Care businesses. Net sales of these products together are the grouping of CGUs, which is used to determine the recoverable amount. The valuation method is based on the FVLCOD.
F-28
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The following assumptions were used in the calculations for the recoverable amounts of goodwill and the Alcon brand name at December 31, 2023 and 2022:
| (As a percentage) | Surgical | Vision Care | |||||||||
| Terminal growth rate | |||||||||||
| Discount rate (post-tax) | |||||||||||
The Surgical and Vision Care reportable segments' terminal growth rate assumption of 3.0 % takes into consideration how the industry is expected to grow, analysis of industry expert reports, and expected relevant changes in demographics for various markets. The discount rates for both Surgical and Vision Care reportable segments consider Alcon's weighted average cost of capital, adjusted to approximate the weighted average cost of capital of comparable market participants. Both the terminal growth rates and the discount rates are consistent with external sources of information.
The FVLCOD, for all groupings of CGUs containing goodwill or indefinite life intangible assets, is reviewed for the impact of reasonably possible changes in key assumptions. In particular Alcon considered an increase in the discount rate, a decrease in the terminal growth rate and certain negative impacts on the forecasted cash flows. These reasonably possible changes in key assumptions did not indicate an impairment.
Refer to "Impairment of goodwill, Alcon brand name and definite lived intangible assets" and "Acquired In-Process Research & Development ("IPR&D")" in Note 2 for additional disclosures on how Alcon performs goodwill and intangible asset impairment testing.
The following table summarizes the movements of goodwill and other intangible assets in 2022:
| Intangible assets other than goodwill | |||||||||||||||||||||||||||||||||||||||||||||||
| ($ millions) | Goodwill | Alcon brand name | Acquired in-process research & development | Technologies | Currently marketed products | Marketing know-how | Other intangible assets (including software) | Total | |||||||||||||||||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||||||||||||||||||||
| January 1, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||
Impact of business combination(1) | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| Impact of asset acquisitions | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| Additions | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
Disposals and derecognitions(2) | — | — | ( | — | — | — | ( | ( | |||||||||||||||||||||||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||
| Accumulated amortization | |||||||||||||||||||||||||||||||||||||||||||||||
| January 1, 2022 | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||
| Amortization charge | — | — | — | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||
Disposals and derecognitions(2) | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Impairment charges | — | — | ( | — | ( | — | — | ( | |||||||||||||||||||||||||||||||||||||||
| December 31, 2022 | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||
| Net book value at December 31, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||
(1) During 2023, Alcon retrospectively adjusted the provisional amounts that were recognized for the preliminary PPA at the Aerie acquisition date, resulting in Goodwill of $8,926 million as of December 31, 2022. Refer to Note 21.1 for more information regarding the PPA which was finalized in the third quarter of 2023.
(2) Derecognitions of assets that are no longer used or being developed and are not considered to have a significant disposal value or other alternative use.
F-29
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The following table summarizes the allocation of the net book values of goodwill and other intangible assets by reportable segment at December 31, 2022:
| Intangible assets other than goodwill | |||||||||||||||||||||||||||||||||||||||||||||||
| ($ millions) | Goodwill | Alcon brand name | Acquired in-process research & development | Technologies | Currently marketed products | Marketing know-how | Other intangible assets (including software) | Total | |||||||||||||||||||||||||||||||||||||||
| Surgical | — | ||||||||||||||||||||||||||||||||||||||||||||||
Vision Care(1) | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||
| Not allocated to segments | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| Net book value at December 31, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||
(1) During 2023, Alcon retrospectively adjusted the provisional amounts that were recognized for the preliminary PPA at the Aerie acquisition date, resulting in Vision Care Goodwill of $4,382 million and total Goodwill of $8,926 million as of December 31, 2022. Refer to Note 21.1 for more information regarding the PPA which was finalized in the third quarter of 2023.
Intangible asset impairment charges
The following table shows the intangible asset impairment charges in 2023, 2022 and 2021:
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Surgical | ( | ( | |||||||||||||||
| Vision Care | ( | ( | |||||||||||||||
| Total | ( | ( | |||||||||||||||
For the year ended December 31, 2022, impairment charges recognized in the Consolidated Income Statement amounted to $62 million, primarily due to impairments of $61 million recognized in the second quarter. An impairment charge of $59 million was recognized in Cost of net sales for a currently marketed product CGU in the Surgical reportable segment due to higher forecasted research and development costs associated with product redesign and delayed launch date of the next generation product. The CGU was reduced to its recoverable amount of $15 million determined based on the VIU method at the time of impairment. VIU was estimated using net present value techniques utilizing pre-tax cash flows and a discount rate of 7.8 %. The remaining impairment charge of $2 million in the second quarter was recognized in Research & development to fully impair an acquired research & development intangible asset in the Vision Care reportable segment which will no longer be used.
For the year ended December 31, 2021, impairment charges recognized in the Consolidated Income Statement amounted to $225 million. Impairments of $180 million were recognized in Research & development in 2021. Of that amount, an impairment charge of $178 million was recognized in the third quarter of 2021 in Research & development to fully impair a CGU in the Surgical reportable segment upon a decision to suspend research and development efforts and commercialization of the product as Alcon prioritizes other products in the portfolio. An additional impairment charge of $2 million was recognized in the fourth quarter of 2021 in Research & development to fully impair a licensed technology in the Vision Care reportable segment, which will no longer be used in any future research and development activities. The remaining amount of $45 million relates to an impairment charge recognized in the first quarter of 2021 in Cost of net sales for a currently marketed product CGU in the Vision Care reportable segment due to lower expected sales. The CGU was reduced to its recoverable amount of $48 million determined based on the FVLCOD method at the time of impairment. FVLCOD was estimated using net present value techniques utilizing post-tax cash flows and a discount rate as there are no direct or indirect observable prices in active markets for identical or similar assets. The discount rate was consistent with the rate used in the annual goodwill impairment assessment.
F-30
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The estimates used in calculating net present values involve significant judgement by management and include assumptions with measurement uncertainty. The estimates include cash flow projections for a five-year period based on management forecasts, sales forecasts beyond the five-year period extrapolated using long-term expected growth rates, discount rates and future tax rates. Actual cash flows and values could vary significantly from forecasted future cash flows and related values derived using net present value techniques.
For FVLCOD, the estimates used are considered to be consistent with market participant assumptions. Since the cash flow projections are a significant unobservable input, the fair value of the CGUs were classified as Level 3 in the fair value hierarchy.
F-31
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
10. Deferred tax assets and liabilities
| ($ millions) | Property, plant & equipment | Intangible assets and deductible goodwill | Pensions and other benefit obligations of associates | Inventories | Tax loss carry-forwards | Other assets, provisions and accruals | Total | ||||||||||||||||||||||||||||||||||
| Gross deferred tax assets at December 31, 2022 | |||||||||||||||||||||||||||||||||||||||||
| Gross deferred tax liabilities at December 31, 2022 | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||||||||
| Net deferred tax balance at December 31, 2022 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| At December 31, 2022 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| (Charged)/credited to income | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Credited/(charged) to equity | ( | ( | |||||||||||||||||||||||||||||||||||||||
| Credited/(charged) to other comprehensive income | ( | ||||||||||||||||||||||||||||||||||||||||
| Net deferred tax balance at December 31, 2023 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Gross deferred tax assets at December 31, 2023 | |||||||||||||||||||||||||||||||||||||||||
| Gross deferred tax liabilities at December 31, 2023 | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||||||||
| Net deferred tax balance at December 31, 2023 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
The below table presents the Net deferred tax balance as of December 31, 2023 after offsetting $1.1 billion of deferred tax assets and liabilities within the same tax jurisdiction.
| ($ millions) | At December 31, 2023 | ||||
| Deferred tax assets | |||||
| Deferred tax liabilities | ( | ||||
| Net deferred tax liabilities | ( | ||||
F-32
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
| ($ millions) | Property, plant & equipment | Intangible assets | Pensions and other benefit obligations of associates | Inventories | Tax loss carry-forwards | Other assets, provisions and accruals | Total | ||||||||||||||||||||||||||||||||||
| Gross deferred tax assets at December 31, 2021 | |||||||||||||||||||||||||||||||||||||||||
| Gross deferred tax liabilities at December 31, 2021 | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||||||||
| Net deferred tax balance at December 31, 2021 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| At December 31, 2021 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| (Charged)/credited to income | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Credited/(charged) to equity | ( | ( | |||||||||||||||||||||||||||||||||||||||
| (Charged) to other comprehensive income | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Impact of business combination | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Impact of asset acquisitions | |||||||||||||||||||||||||||||||||||||||||
| Net deferred tax balance at December 31, 2022 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
| Gross deferred tax assets at December 31, 2022 | |||||||||||||||||||||||||||||||||||||||||
| Gross deferred tax liabilities at December 31, 2022 | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||||||||
| Net deferred tax balance at December 31, 2022 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||
The below table presents the Net deferred tax balance as of December 31, 2022 after offsetting $928 million of deferred tax assets and liabilities within the same tax jurisdiction.
| ($ millions) | At December 31, 2022 | ||||
| Deferred tax assets | |||||
| Deferred tax liabilities | ( | ||||
| Net deferred tax liabilities | ( | ||||
The below table presents deferred tax assets and deferred tax liabilities expected to have an impact on current taxes payable after more than twelve months.
| ($ billions) | At December 31, 2023 | At December 31, 2022 | |||||||||
| Deferred tax assets | |||||||||||
| Deferred tax liabilities | |||||||||||
For foreign unremitted earnings retained by consolidated entities for reinvestment, which amounted to $9
IFRS exceptions to recognizing taxable temporary differences include an exception to recognizing a deferred tax liability arising on the initial recognition of goodwill from acquisitions. As such, we have not provided a deferred tax for goodwill from acquisitions which amounted to $8.9
The gross value of capital loss carryforwards for which no deferred tax assets were recognized amounted to $131 million at December 31, 2023 ($120 million at December 31, 2022), most of which will expire in three years.
F-33
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Tax loss carryforwards are capitalized as deferred tax assets to the extent it is probable that sufficient taxable income will be available for the foreseeable future. The below tables presents the gross value of tax loss carryforwards that have or have not been recognized as deferred tax assets, with their expiry dates, as of December 31, 2023 and 2022.
| ($ millions) | Unrecognized | Recognized | Total at December 31, 2023 | ||||||||
Within five years | |||||||||||
More than five years | |||||||||||
Not subject to expiry | |||||||||||
Gross value of tax loss carryforwards | |||||||||||
| ($ millions) | Unrecognized | Recognized | Total at December 31, 2022 | ||||||||
Within five years | |||||||||||
More than five years | |||||||||||
Not subject to expiry | |||||||||||
Gross value of tax loss carryforwards | |||||||||||
11. Financial and other non-current assets
The below tables provide details related to Financial assets and Other non-current assets as of December 31, 2023 and 2022.
Financial assets
| ($ millions) | 2023 | 2022 | |||||||||
| Long-term note receivable and other financial assets measured at amortized cost | |||||||||||
Long-term financial investments measured at FVOCI(1) | |||||||||||
| Long-term financial investments measured at FVPL | |||||||||||
| Long-term receivables from customers | |||||||||||
| Non-current minimum lease payments from finance lease agreements | |||||||||||
| Long-term loans, VAT receivables, advances and security deposits | |||||||||||
| Total financial assets | |||||||||||
(1) Includes $11 million of Long-term convertible notes due from an associated company as of December 31, 2023. Refer to Note 24 for additional information.
F-34
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Minimum lease payments from finance lease agreements
The following table shows the receivables of the gross investments in finance leases and the net present value of the minimum lease payments, as well as unearned finance income, related to surgical equipment lease arrangements. The finance income is recorded in "Other income".
| 2023 | 2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ($ millions) | Total future payments | Unearned interest income | Present value | Provision | Net book value | Total future payments | Unearned interest income | Present value | Provision | Net book value | |||||||||||||||||||||||||||||||||||||||||||||||||
Not later than one year(1) | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Between one and five years | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Later than five years | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1) The current portion of the minimum lease payments is recorded in Trade receivables or Other current assets (to the extent not yet invoiced).
Other non-current assets
| ($ millions) | Note | 2023 | 2022 | ||||||||
| Deferred compensation plans | |||||||||||
| Prepaid post-employment benefit plans | |||||||||||
| Investment in associated company | 24 | ||||||||||
| Other non-current assets | |||||||||||
| Total other non-current assets | |||||||||||
12. Inventories
The amount of inventory recognized as an expense in "Cost of net sales" in the Consolidated Income Statement during 2023 amounted to $2.9 billion (2022: $2.7 billion, 2021: $2.5 billion). The amount of inventory recognized as an expense in "Cost of other revenues" in the Consolidated Income Statement during 2023 amounted to $67 million (2022: $59 million, 2021: $62 million).
| ($ millions) | 2023 | 2022 | ||||||
| Raw material, consumables | ||||||||
| Work in progress | ||||||||
| Finished products | ||||||||
| Total inventories | ||||||||
Alcon recognized inventory provisions and write-downs amounting to $206 million in 2023 (2022: $200 million, 2021: $220 million) and reversed inventory provisions amounting to $88 million in 2023 (2022: $72 million, 2021: $83 million). Inventory provisions mainly relate to the adjustment of inventory balances to their net realizable value based on the forecasted sales. Reversals are made when the products become salable.
F-35
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
13. Trade receivables
Trade receivable balances include sales to wholesalers, retailers, doctor groups, private health systems, government agencies, pharmacy benefit managers, managed health-care organizations and government-supported healthcare systems. We closely monitor the level of trade receivables in the countries deemed to have an elevated credit risk. We consider macroeconomic and geopolitical environment, country profile and historical experience in addition to other relevant information when assessing the credit risk. Deteriorating credit risk factors may result in an increase in the average length of time that it takes to collect these trade receivables and may require Alcon to reevaluate the expected credit loss amount of these trade receivables in future periods or change the terms on which we operate. As of December 31, 2023, the amounts past due for more than one year in elevated credit risk countries are not significant.
The following tables provide details related to Trade receivables as of December 31, 2023 and 2022, including trade receivables that are not overdue as specified in the payment terms and conditions established with Alcon's customers, as well as an analysis of overdue amounts, expected credit loss rates and related provisions for doubtful trade receivables:
| 2023 | ||||||||||||||
| ($ millions) | Gross trade receivables | Provision | Trade receivables, net | Expected credit loss rates | ||||||||||
| Not overdue | ( | % | ||||||||||||
| Past due for not more than one month | ( | % | ||||||||||||
| Past due for more than one month but less than three months | ( | % | ||||||||||||
| Past due for more than three months but less than six months | ( | % | ||||||||||||
| Past due for more than six months but less than one year | ( | % | ||||||||||||
| Past due for more than one year | ( | % | ||||||||||||
| Total | ( | |||||||||||||
| 2022 | ||||||||||||||
| ($ millions) | Gross trade receivables | Provision | Trade receivables, net | Expected credit loss rates | ||||||||||
| Not overdue | ( | % | ||||||||||||
| Past due for not more than one month | ( | % | ||||||||||||
| Past due for more than one month but less than three months | ( | % | ||||||||||||
| Past due for more than three months but less than six months | ( | % | ||||||||||||
| Past due for more than six months but less than one year | ( | % | ||||||||||||
| Past due for more than one year | ( | % | ||||||||||||
| Total | ( | |||||||||||||
The following table summarizes the movement in the provision for doubtful trade receivables:
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| January 1 | ( | ( | ( | ||||||||||||||
| Provisions for doubtful trade receivables charged to the Consolidated Income Statement | ( | ( | ( | ||||||||||||||
| Utilization of provisions for doubtful trade receivables | |||||||||||||||||
| Reversal of provisions for doubtful trade receivables | |||||||||||||||||
| Currency translation effects | ( | ||||||||||||||||
| December 31 | ( | ( | ( | ||||||||||||||
F-36
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Trade receivables include amounts denominated in the following major currencies:
| ($ millions) | 2023 | 2022 | |||||||||
| US dollar (USD) | |||||||||||
| Euro (EUR) | |||||||||||
| Japanese yen (JPY) | |||||||||||
| Chinese yuan (CNY) | |||||||||||
| Brazilian real (BRL) | |||||||||||
| Canadian dollar (CAD) | |||||||||||
| South Korean won (KRW) | |||||||||||
| Mexican peso (MXN) | |||||||||||
| Indian rupee (INR) | |||||||||||
| Australian dollar (AUD) | |||||||||||
| British pound (GBP) | |||||||||||
| Russian ruble (RUB) | |||||||||||
| Taiwan dollar (TWD) | |||||||||||
| Other currencies | |||||||||||
| Total trade receivables, net | |||||||||||
14. Other current assets
The following table provides details related to Other current assets as of December 31, 2023 and 2022:
| ($ millions) | 2023 | 2022 | |||||||||
| Current portion of long-term receivables from customers | |||||||||||
| Current portion of minimum lease payments from finance lease agreements | |||||||||||
| Current portion of long-term financial investments measured at FVPL | |||||||||||
| Prepaid expenses | |||||||||||
| VAT receivables | |||||||||||
| Other receivables, security deposits and current assets | |||||||||||
| Derivative financial instruments | |||||||||||
| Total other current assets | |||||||||||
F-37
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
15. Right-of-use assets and Lease liabilities
Right-of-use assets
Right-of-use assets as of December 31, 2023 and 2022 were comprised of the following:
| ($ millions) | 2023 | 2022 | ||||||
| Land | ||||||||
| Buildings | ||||||||
| Machinery & equipment and other assets | ||||||||
| Total right-of-use assets | ||||||||
Depreciation charges of $91 million, $76 million and $81 million for the years ended December 31, 2023, 2022 and 2021, respectively, are shown in the table below by underlying class of asset:
| ($ millions) | 2023 | 2022 | 2021 | ||||||||
| Land | |||||||||||
| Buildings | |||||||||||
| Machinery & equipment and other assets | |||||||||||
| Total depreciation of right-of-use assets | |||||||||||
Lease liabilities
Lease liabilities totaled $406 million as of December 31, 2023, including $71 million in current Lease liabilities and $335 million in non-current Lease liabilities. The contractual maturities of the undiscounted lease liabilities as of December 31, 2023 and 2022 are as follows:
| Lease liabilities undiscounted | ||||||||
| ($ millions) | 2023 | 2022 | ||||||
| Not later than one year | ||||||||
| Between one and five years | ||||||||
| Later than five years | ||||||||
| Total lease liabilities undiscounted | ||||||||
| Lease liabilities | ||||||||
| ($ millions) | 2023 | 2022 | ||||||
| Not later than one year | ||||||||
| Between one and five years | ||||||||
| Later than five years | ||||||||
| Total lease liabilities | ||||||||
F-38
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Additional disclosures
The following table provides additional disclosures related to Right-of-use assets and Lease liabilities:
| ($ millions) | 2023 | 2022 | 2021 | ||||||||
| Interest expense on lease liabilities | |||||||||||
| Expense on short-term, low value and variable leases | |||||||||||
| Total cash outflows for leases | |||||||||||
| Thereof: | |||||||||||
Lease liability payments(1) | |||||||||||
Interest payments(2) | |||||||||||
Short-term, low value and variable lease payments(2) | |||||||||||
(1) Reported as cash outflows from financing activities net of lease incentives received.
(2) Included within total net cash flows from operating activities.
16. Non-current and current financial debts
The below table summarizes non-current and current Financial debts outstanding as of December 31, 2023 and 2022.
| ($ millions) | 2023 | 2022 | ||||||
| Non-current financial debts | ||||||||
| Local facilities (Japan), floating rate debt due 2025 | ||||||||
| Revolving facility, floating rate due 2028 | ||||||||
| Total non-current financial debts | ||||||||
| Current financial debts | ||||||||
| Local facilities, floating rate: | ||||||||
| Japan | ||||||||
| All others | ||||||||
| Other short-term financial debts, floating rate | ||||||||
| Derivatives | ||||||||
| Total current financial debts | ||||||||
| Total financial debts | ||||||||
Interest expense recognized for Financial debts, excluding lease liabilities, was $162 million, $110 million and $95 million for the years ended December 31, 2023, 2022 and 2021, respectively. The weighted average interest rate on Financial debts was 3.5 % and 2.7 % in 2023 and 2022, respectively.
Series 2028 Notes issuance
On May 31, 2022, AFBV issued EUR denominated senior notes due in 2028 ("Series 2028 Notes"). The Series 2028 Notes are unsecured senior obligations of AFBV issued and closed in a public offering and rank equally in right of payment with the Series 2026, Series 2029, Series 2030 and Series 2049 notes. The total principal of the Series 2028 Notes is $553 million
F-39
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
(EUR500 million) as of December 31, 2023. The Series 2028 Notes were issued at 99.476 % with 2.375 % interest payable annually in May, beginning in May 2023. The Series 2028 Notes were issued at a discount totaling $3 million, which was recorded as a reduction to the carrying value of the Series 2028 Notes and will be amortized to Interest expense over the term of the Series 2028 Notes. AFBV incurred $3 million of debt issuance costs, which were recorded as a reduction to the carrying value of the Series 2028 Notes and will be amortized to Other financial income & expense over the term of the Series 2028 Notes.
On May 31, 2022, the funds borrowed through the issuance of the Series 2028 Notes were used to fully repay the $376 million (EUR350 million) Facility C term loan maturing in 2024 and repay $160 million of the $800 million Facility B term loan maturing in 2024. The transactions were accounted for as an extinguishment and partial extinguishment of a liability, respectively. Alcon recognized losses on extinguishment of $1 million associated with the write-off of unamortized deferred financing costs in Other financial income & expense during the second quarter of 2022.
2022 Bridge Loan Facility
On September 14, 2022, AFC executed a $900 million 2022 Bridge Loan Facility with J.P. Morgan Chase Bank, N.A. London Branch. The 2022 Bridge Loan Facility was fully guaranteed by the Company and was restricted for use in funding the acquisition of Aerie. On September 27, 2022, a Syndication Agreement was executed to add more financial institutions as new lenders, effective from September 28, 2022.
On November 21, 2022, in connection with the consummation of the Aerie acquisition, $775 million of the financing commitments were drawn with net proceeds of $771 million used for the acquisition of Aerie. AFC incurred $4 million of debt issuance costs, which were recorded as a reduction to the carrying value of the 2022 Bridge Loan Facility.
Series 2032 Notes and Series 2052 Notes issuance
On December 6, 2022, AFC issued senior notes due in 2032 ("Series 2032 Notes") and 2052 ("Series 2052 Notes"). The Series 2032 Notes and Series 2052 Notes are unsecured senior obligations of AFC issued and closed in a private offering and rank equally in right of payment with the Series 2026, Series 2028, Series 2029, Series 2030 and Series 2049 notes. The principal amounts of the Series 2032 Notes and Series 2052 Notes are $700 million and $600 million, respectively. The Series 2032 Notes and Series 2052 Notes were issued at a discount of $4 million and $2 million, respectively, which were recorded as a reduction to the carrying values of the Series 2032 Notes and Series 2052 Notes and will be amortized to Interest expense over the term of the notes. AFC incurred debt issuance costs of $4 million and $7 million for the Series 2032 Notes and Series 2052 Notes, respectively, which were recorded as a reduction to the carrying values of the Series 2032 Notes and Series 2052 Notes and will be amortized to Other financial income & expense over the term of the notes.
The Notes consist of the following:
• Series 2032 Notes - $700 million due in 2032 issued at 99.458 %, 5.375 % interest is payable twice per year in December and June, beginning in June 2023.
• Series 2052 Notes - $600 million due in 2052 issued at 99.674 %, 5.750 % interest is payable twice per year in December and June, beginning in June 2023.
Using the funds borrowed through the issuance of the Series 2032 Notes and Series 2052 Notes together with cash, the Company exercised its early redemption rights to fully repay the remaining $640 million Facility B term loan and to fully repay the drawn amount of $775 million under the 2022 Bridge Loan Facility, as required by the mandatory prepayment clause. Consequently, the undrawn commitment of the 2022 Bridge Loan Facility was cancelled. The transactions were accounted for as extinguishment of liabilities. Alcon recognized losses on extinguishment of $4 million associated with the write-off of unamortized deferred financing costs in Other financial income & expense during the fourth quarter of 2022.
Senior notes assumed in Aerie acquisition
As part of the Aerie acquisition, Alcon assumed Aerie's $316.2 million convertible senior notes due on October 1, 2024. The convertible notes were issued at 1.500 % interest payable semi-annually on April 1 and October 1 of each year. Following the delisting of Aerie on November 21, 2022, the senior notes were no longer convertible to equity. On December 20, 2022, Alcon made payments of $316.0 million to note holders. As of December 31, 2023, $0.2 million remained outstanding.
Series 2030 Notes issuance
On May 27, 2020, AFC issued senior notes due in 2030 (“Series 2030 Notes”). The Series 2030 Notes are unsecured senior obligations of AFC issued in a private placement and rank equally in right of payment with the Series 2026, Series 2029,
F-40
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
and Series 2049 notes. The total principal amount of the Senior 2030 Notes is $750 million. The Senior 2030 Notes were issued at 99.843 % with 2.600 % interest payable twice per year in May and November, beginning in November 2020. The Series 2030 Notes were issued at a discount totaling $1 million, which was recorded as a reduction to the carrying value of the Series 2030 Notes and will be amortized to Interest expense over the term of the Series 2030 Notes. AFC incurred $5 million of debt issuance costs, which were recorded as a reduction to the carrying value of the Series 2030 Notes and will be amortized to Other financial income & expense over the term of the Series 2030 Notes.
Revolving Credit Facility
On October 27, 2023, the Company and certain of its subsidiaries and a group of commercial banks entered into a refinancing agreement to replace the $1.0 billion unsecured committed multicurrency revolving credit facility maturing in March 2026. The new agreement consists of a $1.32 billion unsecured committed multicurrency revolving credit facility now maturing five years after the date of the agreement (the “Refinanced Revolving Facility Agreement”). The Refinanced Revolving Facility Agreement primarily bears interest rates equal to a term reference rate or a compounded reference rate, depending on currency, plus an applicable margin and a term reference rate credit adjustment spread, if applicable. It also includes relevant fallback mechanisms in case of rate unavailability. The Revolving Credit Facility remained undrawn as of December 31, 2023.
Local bilateral facilities
Alcon holds a number of local bilateral facilities in different countries with the largest share of borrowings in Japan. During the year ended December 31, 2022, changes in financial debts for local bilateral facilities primarily included the movement of balances from non-current to current and payment of certain local bilateral facilities in Japan. In addition, one local bilateral facility in Japan matured in February 2022 and was renewed for another one year term.
On February 14, 2023, three local bilateral facilities in Japan which matured in February 2023 were refinanced by three facilities with two year maturities. As of December 31, 2023, $82 million of the drawn local facilities balance was classified as current due to Alcon's expectation to settle the liabilities within twelve months of the balance sheet date. There was $49 million undrawn on the facilities in Japan as of December 31, 2023.
Guarantees
The Series 2026, 2028, 2029, 2030, 2032, 2049 and 2052 Notes, the three local bilateral facilities in Japan and the undrawn Revolving Credit Facility are guaranteed by the Company.
F-41
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Maturity of contractual undiscounted cash flows and interest payment commitments
The following table provides details on the maturity of the contractual undiscounted cash flows for Alcon's borrowings as of December 31, 2023 and 2022:
| 2023 | 2022 | ||||||||||||||||||||||
| ($ millions) | Nominal amount - Current and non-current financial debt | Derivatives | Total | Nominal amount - Current and non-current financial debt | Derivatives | Total | |||||||||||||||||
| Not later than one year | |||||||||||||||||||||||
| Between one and five years | |||||||||||||||||||||||
| Later than five years | |||||||||||||||||||||||
| Total contractual undiscounted cash flows | |||||||||||||||||||||||
| Unamortized debt discount and issuance costs | ( | ( | ( | ( | |||||||||||||||||||
| Total carrying value | |||||||||||||||||||||||
The following table provides details on the maturity of the future contractual interest payments commitments as of December 31, 2023 and 2022:
| ($ millions) | 2023 | 2022 | ||||||
| Not later than one year | ||||||||
| Between one and five years | ||||||||
| Later than five years | ||||||||
| Total cash flows | ||||||||
F-42
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
17. Financial instruments - additional disclosures
The below table provides detail related to financial instruments as of December 31, 2023 and December 31, 2022.
| ($ millions) | Note | 2023 | 2022 | ||||||||||||||
| Cash and cash equivalents | |||||||||||||||||
| Cash in current accounts | |||||||||||||||||
| Cash held in time deposits and money market funds | |||||||||||||||||
| Total cash and cash equivalents | |||||||||||||||||
| Financial assets - measured at fair value through other comprehensive income ("FVOCI") | |||||||||||||||||
| Long-term financial investments | 11 | ||||||||||||||||
| Total financial assets - measured at FVOCI | |||||||||||||||||
Financial assets - measured at amortized cost(1) | |||||||||||||||||
| Trade receivables | 13 | ||||||||||||||||
| Income tax receivables | |||||||||||||||||
| Other current assets (excluding prepaid expenses and other current assets measured at FVPL) | 14 | ||||||||||||||||
| Long-term note receivable and other financial assets | 11 | ||||||||||||||||
| Long-term receivables from customers | 11 | ||||||||||||||||
| Non-current minimum lease payments from finance lease agreements | 11 | ||||||||||||||||
| Long-term loans, VAT receivables, advances and security deposits | 11 | ||||||||||||||||
| Total financial assets - measured at amortized cost | |||||||||||||||||
| Financial assets - measured at fair value through profit and loss ("FVPL") | |||||||||||||||||
| Deferred compensation assets | 11 | ||||||||||||||||
| Current portion of long-term financial investments | 14 | ||||||||||||||||
| Derivative financial instruments | 14 | ||||||||||||||||
| Long-term financial investments | 11 | ||||||||||||||||
| Total financial assets - measured at FVPL | |||||||||||||||||
| Total financial assets | |||||||||||||||||
Financial liabilities - measured at amortized cost or cost(1) | |||||||||||||||||
| Current financial liabilities | |||||||||||||||||
| Financial debts | 16 | ||||||||||||||||
| Lease liabilities | 15 | ||||||||||||||||
| Trade payables | |||||||||||||||||
| Total current financial liabilities - measured at amortized cost or cost | |||||||||||||||||
| Non-current financial liabilities | |||||||||||||||||
| Financial debts | 16 | ||||||||||||||||
| Lease liabilities | 15 | ||||||||||||||||
| Total non-current financial liabilities - measured at amortized cost or cost | |||||||||||||||||
| Total financial liabilities - measured at amortized cost or cost | |||||||||||||||||
| Financial liabilities - measured at FVPL | |||||||||||||||||
| Contingent consideration liabilities | 18 | ||||||||||||||||
| Derivative financial instruments | 16 | ||||||||||||||||
| Total financial liabilities - measured at FVPL | |||||||||||||||||
| Total financial liabilities | |||||||||||||||||
| Net financial assets and financial liabilities | ( | ( | |||||||||||||||
(1) The carrying amount is a reasonable approximation of fair value, with the exception of the Series 2026, 2028, 2029, 2030, 2032, 2049 and 2052 Notes recorded in Non-current financial debts with a fair value of $4,347 million and carrying value of $4,566 million as of December 31, 2023 and a fair value of $4,145 million and carrying value of $4,541 million as of December 31, 2022. The fair value of notes was determined using Level 2 inputs. The notes were valued using a quoted market price for such notes, which have low trading volumes.
F-43
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Fair value by hierarchy
As required by IFRS, financial assets and liabilities recorded at fair value in the Consolidated Financial Statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. There are three hierarchical levels, based on an increasing amount of judgment associated with the inputs to derive fair value for these financial assets and liabilities, which are as follows:
Financial assets and liabilities carried at Level 1 fair value hierarchy are listed in active markets.
Financial assets and liabilities carried at Level 2 fair value hierarchy are valued using corroborated market data.
Level 1 financial assets include money market funds and deferred compensation assets. There were no financial liabilities carried at Level 1 fair value, and Level 2 financial assets and liabilities include derivative financial instruments.
Investments in money market funds are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices. The investments are classified as Cash & cash equivalents within the Consolidated Balance Sheet.
Deferred compensation investments for certain employee benefit plans are held in a rabbi trust and dedicated to pay the benefits under the associated plans but are not considered plan assets as the assets remain available to creditors of Alcon in certain events, including bankruptcy. Rabbi trust assets primarily consist of investments in mutual funds. These assets are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices.
Level 3 inputs are unobservable for the financial asset or liability. The financial assets and liabilities generally included in the Level 3 fair value hierarchy are equity securities and convertible notes receivable of private companies measured at FVOCI, fund investments, options to acquire private companies, and contingent consideration liabilities measured at FVPL.
F-44
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The following tables summarize financial assets and liabilities measured at fair value on a recurring basis or at amortized cost or cost as of December 31, 2023 and December 31, 2022.
December 31, 2023 | |||||||||||||||||||||||||||||
| ($ millions) | Level 1 | Level 2 | Level 3 | Valued at amortized cost or cost | Total | ||||||||||||||||||||||||
| Non-current financial assets | |||||||||||||||||||||||||||||
Long-term financial investments measured at FVOCI | — | ||||||||||||||||||||||||||||
| Long-term financial investments measured at FVPL | — | ||||||||||||||||||||||||||||
| Long-term note receivable and other financial assets measured at amortized cost | |||||||||||||||||||||||||||||
| Long-term receivables from customers | |||||||||||||||||||||||||||||
Deferred compensation assets(1) | — | ||||||||||||||||||||||||||||
| Non-current minimum lease payments from finance lease agreements | |||||||||||||||||||||||||||||
| Long-term loans, VAT receivables, advances and security deposits | |||||||||||||||||||||||||||||
| Non-current financial assets | |||||||||||||||||||||||||||||
| Current financial assets | |||||||||||||||||||||||||||||
| Money market funds | — | ||||||||||||||||||||||||||||
Current portion of long-term financial investments measured at FVPL(2) | — | ||||||||||||||||||||||||||||
Current portion of long-term receivables from customers(2) | |||||||||||||||||||||||||||||
Current portion of minimum lease payments from finance lease agreements(2) | |||||||||||||||||||||||||||||
VAT receivables(2) | |||||||||||||||||||||||||||||
Other receivables, security deposits and current assets(2) | |||||||||||||||||||||||||||||
Derivative financial instruments(2) | — | ||||||||||||||||||||||||||||
| Current financial assets | |||||||||||||||||||||||||||||
| Financial assets at fair value and amortized cost or cost | |||||||||||||||||||||||||||||
| Financial liabilities | |||||||||||||||||||||||||||||
| Contingent consideration liabilities | ( | — | ( | ||||||||||||||||||||||||||
| Non-current financial debt | ( | ( | |||||||||||||||||||||||||||
| Current financial debt | ( | ( | |||||||||||||||||||||||||||
| Derivative financial instruments | ( | — | ( | ||||||||||||||||||||||||||
| Financial liabilities at fair value and amortized cost | ( | ( | ( | ( | |||||||||||||||||||||||||
(1)Recorded in Other non-current assets.
(2)Recorded in Other current assets.
F-45
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
| December 31, 2022 | |||||||||||||||||||||||||||||
| ($ millions) | Level 1 | Level 2 | Level 3 | Valued at amortized cost or cost | Total | ||||||||||||||||||||||||
| Non-current financial assets | |||||||||||||||||||||||||||||
| Long-term financial investments measured at FVOCI | — | ||||||||||||||||||||||||||||
| Long-term financial investments measured at FVPL | — | ||||||||||||||||||||||||||||
| Long-term receivables from customers | |||||||||||||||||||||||||||||
Deferred compensation assets(1) | — | ||||||||||||||||||||||||||||
| Non-current minimum lease payments from finance lease agreements | |||||||||||||||||||||||||||||
| Long-term loans, advances and security deposits | |||||||||||||||||||||||||||||
| Non-current financial assets | |||||||||||||||||||||||||||||
| Current financial assets | |||||||||||||||||||||||||||||
| Money market funds | — | ||||||||||||||||||||||||||||
Current portion of long-term receivables from customers(2) | |||||||||||||||||||||||||||||
Current portion of minimum lease payments from finance lease agreements(2) | |||||||||||||||||||||||||||||
VAT receivables(2) | |||||||||||||||||||||||||||||
Other receivables, security deposits and current assets(2) | |||||||||||||||||||||||||||||
Derivative financial instruments(2) | — | ||||||||||||||||||||||||||||
| Current financial assets | |||||||||||||||||||||||||||||
| Financial assets at fair value and amortized cost or cost | |||||||||||||||||||||||||||||
| Financial liabilities | |||||||||||||||||||||||||||||
| Contingent consideration liabilities | ( | — | ( | ||||||||||||||||||||||||||
| Non-current financial debt | ( | ( | |||||||||||||||||||||||||||
| Current financial debt | ( | ( | |||||||||||||||||||||||||||
Derivative financial instruments | ( | — | ( | ||||||||||||||||||||||||||
| Financial liabilities at fair value and amortized cost | ( | ( | ( | ( | |||||||||||||||||||||||||
(1)Recorded in Other non-current assets.
(2)Recorded in Other current assets.
There were no transfers of financial instruments between levels in the fair value hierarchy during the years ended December 31, 2023 and December 31, 2022.
F-46
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Long-term note receivable and other financial assets measured at amortized cost
On May 22, 2023, Alcon entered into financing arrangements with a long-term supplier, Lifecore Biomedical, Inc. and certain of its affiliates (collectively, “Lifecore”). Alcon provided Lifecore total commitments of $150 million, primarily related to a $142 million senior term loan facility ("Long-term note receivable") maturing on May 22, 2029. The arrangements also include a sale and leaseback agreement for certain machinery and equipment. Transaction costs directly attributable to the acquisition of the financial assets amounting to $4 million were capitalized to financial assets at amortized cost.
The Long-term note receivable bears an annual fixed interest rate of 10 %, which is payable in kind (“PIK”) for the first three years , and payable 3 % in cash interest and 7 % PIK interest thereafter until maturity, unless otherwise elected by Lifecore to pay a greater proportion in cash. The Long-term note receivable is secured by a Pledge and Security agreement (“security agreement”) whereby Alcon is granted first priority security interest in certain collateral, including but not limited to equipment, fixtures, real property and intellectual property. The security agreement is in effect until the payment in full of the term loan facility.
Due to Lifecore's significant financial difficulties at the time the loan was originated, Alcon concluded the financial assets were originated credit-impaired. The lifetime ECL was analyzed at inception and utilized in calculating the credit-adjusted effective interest rate with no impact on the carrying value of the financial assets or effective interest rate of 10 %. In addition, as of December 31, 2023, Alcon assessed there was no lifetime ECL due to the assessment of the collateral under the security agreement.
Level 3 financial instruments measured at fair value on a recurring basis
Financial assets
Long-term financial investments measured at FVOCI | Financial investments measured at FVPL | ||||||||||||||||
| ($ millions) | 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Balance as of January 1 | |||||||||||||||||
| Additions | |||||||||||||||||
| (Losses) recognized in Consolidated Statement of Comprehensive Income | ( | ( | |||||||||||||||
| (Losses)/gains in Consolidated Income Statement | ( | ||||||||||||||||
| Amortization | ( | ||||||||||||||||
| Settlement | ( | ( | ( | ||||||||||||||
| Balance as of December 31 | |||||||||||||||||
If the pricing parameters for the Level 3 inputs were to change for Long-term financial investments measured at FVOCI and Financial investments measurement at FVPL by 10 15
Financial liabilities
Contingent consideration liabilities | |||||||||||
| ($ millions) | 2023 | 2022 | |||||||||
| Balance as of January 1 | ( | ( | |||||||||
| Accretion for passage of time | ( | ( | |||||||||
| Adjustments for changes in assumptions | |||||||||||
| Balance as of December 31 | ( | ( | |||||||||
Changes in contingent consideration liabilities in the current year include fair value adjustments for changes in assumptions of $17 million, primarily due to revised expectations for timing of settlement and probability of success for development and commercial milestones. As of December 31, 2023, the probability of success for various development and commercial milestones ranges from 3 % to 55 % and the maximum remaining potential payments related to contingent consideration from business combinations is $395 million, plus other amounts calculated as a percentage of commercial sales in cases where there is not a specified maximum contractual payment amount. The estimation of probability typically
F-47
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
depends on factors such as technical milestones or market performance and is adjusted for the probability of payment. If material, probable payments are appropriately discounted to reflect the impact of time.
Changes in contingent consideration liabilities in the prior year included fair value adjustments for changes in assumptions of $23 million, primarily due to revised expectations for achievement and timing of settlement for development and commercial milestones. As of December 31, 2022, the probability of success for various development and commercial milestones ranged from 55 % to 57 % and the maximum remaining potential payments related to contingent consideration from business combinations was $395 million, plus other amounts calculated as a percentage of commercial sales in cases where there is not a specified maximum contractual payment amount.
Contingent consideration liabilities are reported in “Provisions & other non-current liabilities" based on the projected timing of settlement which is estimated to range from 2029 through 2035 for contingent consideration obligations as of December 31, 2023.
For the determination of the fair value of a contingent consideration various unobservable inputs are used. A change in these inputs might result in a significantly higher or lower fair value measurement. The inputs used are, among others, the probability of success, sales forecast and assumptions regarding the discount rate, timing and different scenarios of triggering events. The significance and usage of these inputs to each contingent consideration may vary due to differences in the timing and triggering events for payments or in the nature of the asset related to the contingent consideration.
As the most significant Level 3 input, if the probability of success were to change by 10 17
Derivatives
As of December 31, 2023, the net value of unsettled positions for derivative forward contracts and swaps was $8 million, including $2 million of unrealized gains in Other current assets and $10 million of unrealized losses in Current financial debts. As of December 31, 2022, the net value of unsettled positions for derivative forward contracts and swaps was $2 million, including $8 million of unrealized gains in Other current assets and $10 million of unrealized losses in Current financial debts. There are master agreements with several banking counterparties for derivatives financial instruments; however, there were no derivative financial instruments meeting the offsetting criteria under IFRS as of December 31, 2023 or December 31, 2022.
Nature and extent of risks arising from financial instruments
Market risk
Alcon is exposed to market risk, primarily related to foreign currency exchange rates, interest rates and the market value of investments of liquid funds. Alcon actively monitors and seeks to reduce, where it deems it appropriate to do so, fluctuations in these exposures. It is Alcon policy and practice to enter into a variety of derivative financial instruments to manage the volatility of these exposures and to enhance the yield on the investment of liquid funds. Alcon does not enter into any financial transactions containing a risk that cannot be quantified at the time the transaction is concluded. In addition, Alcon does not sell short assets it does not have, or does not know it will have, in the future. Alcon only sells existing assets or enters into transactions and future transactions (in the case of anticipatory hedges) that it confidently expects it will have in the future, based on past experience. In the case of liquid funds, Alcon may write call options on assets it has, or write put options on positions it wants to acquire and has the liquidity to acquire. Alcon expects that any loss in value for these instruments generally would be offset by increases in the value of the underlying transactions.
Foreign currency exchange rate risk
Alcon uses the US Dollar as its reporting currency and is therefore exposed to foreign currency exchange movements, primarily in Euros, Japanese Yen, Chinese Renminbi, Canadian Dollars, Korean Won, Swiss Francs, Russian Rubles and emerging market currencies. Fluctuations in the exchange rate between the US Dollar and other currencies can have a significant effect on both Alcon’s results of operations, including reported sales and earnings, as well as on the reported value of Alcon's assets, liabilities and cash flows. This, in turn, may significantly affect the comparability of period-to-period results of operations.
Alcon manages its global currency exposure by engaging in hedging transactions where management deems appropriate (forward contracts and swaps). Specifically, Alcon enters into various contracts that reflect the changes in the value of foreign currency exchange rates to preserve the value of assets. Refer to Note 2 for information regarding the hyperinflationary economies in which Alcon operates.
F-48
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Interest rate risk
Alcon's exposure to cash flow interest rate risks arises from the portion of financial debts at variable rates. Alcon may enter into interest rate swap agreements, in which it exchanges periodic payments based on a notional amount and agreed-upon fixed and variable rate interests. If the interest rates for financial debts at variable rates had been higher / lower by 1 % in 2023, the income before taxes would have been lower / higher by $2 million from the impacts of interest expense based on the change in the interest rate. As of December 31, 2023, 97 % of Alcon's financial debt is at fixed interest rates materially reducing future exposure to cash flow interest rate risk.
Commodity price risk
Alcon's exposure to commodity price risk arises from inflation and supply chain challenges related to anticipated purchases of certain commodities used as raw materials by Alcon's businesses. A change in those prices may alter the gross margin of a specific business, but generally not by more than 10 % of the gross margin and thus below Alcon's risk management tolerance levels. Alcon primarily manages inflationary pressures through pricing actions and productivity initiatives. Based on historical and anticipated price fluctuations, Alcon does not enter into significant forward and option contracts to manage fluctuations in prices of anticipated purchases.
Credit risk
Credit risks arise from the possibility that customers may not be able to settle their obligations as agreed. To manage this risk, Alcon periodically assesses credit risk, assigns individual credit limits, and takes actions to mitigate credit risk where appropriate. Refer to Note 13 for more information.
No customer accounted for 10% or more of Alcon's net sales in 2023, 2022 or 2021.
Credit risk also arises from originated credit-impaired financial assets (Long-term note receivable and other financial assets at amortized cost). The maximum exposure to credit risk is reflected in the carrying value of the assets, which amounted to $162 million as of December 31, 2023, including a non-current portion of $161 million in "Long-term note receivable and other financial assets measured at amortized cost" in Financial assets and a current portion of $1 million in "Other receivables, security deposits and current assets" in Other current assets. As of December 31, 2023, the credit risk exposure is fully mitigated by the collateral, with an estimated amount of approximately $375 million, in accordance with the terms of the security agreement. In addition, Alcon performs an ongoing credit evaluation of Lifecore’s financial condition, monitors payment performance and assesses current economic conditions, as well as reasonable and supportable forecasts of future economic conditions, that may affect collectability of the outstanding financial assets.
Liquidity risk
F-49
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
18. Provisions and other non-current liabilities
The below table provides details related to Provisions and other non-current liabilities as of December 31, 2023 and 2022:
| ($ millions) | Note | 2023 | 2022 | |||||||||||
| Accrued liability for employee benefits: | ||||||||||||||
| Defined benefit pension plans | 22 | |||||||||||||
| Other post-employment benefits | 22 | |||||||||||||
| Other long-term employee benefits and deferred compensation | ||||||||||||||
| Provisions for litigation and other legal matters | ||||||||||||||
| Contingent consideration | 17 | |||||||||||||
| Other non-current liabilities | ||||||||||||||
| Total provisions and other non-current liabilities | ||||||||||||||
Alcon believes that its total provisions are adequate based upon currently available information. However, given the inherent difficulties in estimating liabilities in this area, Alcon may incur additional costs beyond the amounts provided. Management believes that such additional amounts, if any, would not be material to Alcon's financial condition but could be material to the results of operations or cash flows in a given period.
Provisions for litigation and other legal matters
Alcon has established provisions for certain litigation and other legal matters, where a potential cash outflow is probable and a reliable estimate can be made of the amount of the outflow. These provisions represent the current best estimate of the total financial effect for these matters. Potential cash outflows reflected in a provision may be fully or partially offset by insurance in certain circumstances.
Alcon has not established provisions for potential damage awards for certain additional legal claims if Alcon currently believes that a payment is either not probable or cannot be reliably estimated. A number of other legal matters are in such early stages or the issues presented are such that Alcon has not made any provisions since it cannot currently estimate either a potential outcome or the amount of any potential losses. For these reasons, among others, Alcon generally is unable to make a reliable estimate of possible loss with respect to such cases. It is therefore not practicable to provide information about the potential financial impact of those cases.
There might also be cases for which Alcon was able to make a reliable estimate of the possible loss or the range of possible loss, but Alcon believes that publication of such information on a case-by-case basis would prejudice Alcon's position in ongoing legal proceedings or in any related settlement discussions. Accordingly, in such cases, information would be disclosed with respect to the nature of the contingency, but no disclosure is provided as to an estimate of the possible loss or range of possible loss.
Note 25 contains additional information on contingencies.
Summary of significant legal proceedings
A number of Alcon companies are, and will likely continue to be, subject to various legal proceedings and investigations that arise from time to time, including proceedings regarding product liability, sales and marketing practices, commercial disputes, employment, wrongful discharge, antitrust, securities, health and safety, environmental, tax, international trade, privacy, intellectual property, including under the Hatch-Waxman Act, and anti-bribery matters such as those under the Foreign Corrupt Practices Act of 1977 ("FCPA"), as amended.
As a result, Alcon may become subject to substantial liabilities that may not be covered by insurance and could affect Alcon's business, financial position and reputation. While Alcon does not believe that any of these legal proceedings will have a material adverse effect on its financial position, litigation is inherently unpredictable and large judgments sometimes occur. As a consequence, Alcon may in the future incur judgments or enter into settlements of claims that could have a material adverse effect on its results of operations or cash flow. The following is a summary as of February 27, 2024 of significant legal proceedings to which Alcon or its subsidiaries were or are currently a party.
F-50
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
JJSVI patent dispute
On June 23, 2020, Johnson & Johnson Surgical Vision, Inc. ("JJSVI"), acting through its subsidiaries, filed a patent infringement action in the US District Court in Delaware alleging that the manufacture, use, sale, offer for sale, and/or importation of Alcon’s LenSx Laser System willfully infringes, directly and/or indirectly, one or more claims of 12 US patents. JJSVI subsequently amended its complaint to include copyright infringement claims relating to, among other things, source code used in the LenSx Laser System as well as additional claims of patent infringement. Also beginning on June 23, 2020, JJSVI filed claims in Mannheim, Germany, alleging that Alcon directly infringes certain European patents through its manufacture and sale of LenSx. In these cases, JJSVI sought monetary and injunctive relief. Alcon defended all of these cases vigorously and asserted various patent infringement and invalidity claims against JJSVI in Europe and the US. Prior to the trial on the copyright claims in the Delaware action set for February 2023, the parties entered into a confidential settlement agreement to resolve all of the pending legal proceedings described above. As part of that resolution, the parties exchanged cross-licenses of certain intellectual property and other mutually agreed covenants and releases, and Alcon made a one-time payment to JJSVI of $199
Asia / Russia investigation
In 2017 and 2018, Alcon and Novartis Group companies, as well as certain present and former executives and associates of Alcon and Novartis, received document requests and subpoenas from the US Department of Justice (“DoJ”) and the SEC requesting information concerning Alcon accounting, internal controls and business practices in Asia and Russia, including revenue recognition for surgical equipment and related products and services and relationships with third party distributors, both before and after Alcon became part of the Novartis Group. The investigations by the DoJ and the SEC have concluded. On June 25, 2020, Alcon entered into a three-year Deferred Prosecution Agreement ("DPA") with the DoJ regarding a charge that Alcon Pte Ltd. conspired to falsify financial books and records in violation of the US FCPA. The charge relates to payments made by a former distributor to health care providers in Vietnam between 2007 and 2014. Alcon agreed to pay the DoJ a penalty of $8.925 million, for which Novartis has indemnified Alcon. The DPA expired on June 25, 2023. On December 21, 2023, in accordance with the DPA, the DoJ filed a request with the US District Court for the District of New Jersey to dismiss the criminal charge against Alcon Pte Ltd. with prejudice. On December 21, 2023, the Court issued an order dismissing the charge against Alcon Pte Ltd. with prejudice. This case is now concluded.
Hoya patent dispute
On December 11, 2020, Hoya Corporation and one of its affiliates (collectively, "Hoya") filed suit against Alcon in the US District Court for the Northern District of Texas alleging that Alcon's UltraSert Pre-Loaded Delivery System infringes six of Hoya's US patents. The court denied in part Alcon’s motion to dismiss Hoya’s complaint on September 20, 2021. On December 13, 2023, the court denied Hoya’s motion for summary judgment with respect to Alcon’s defense that Hoya derived the subject matter of the asserted patents from certain Alcon products. On January 11, 2024, the court granted Alcon’s motion for summary judgment of non-infringement with respect to three of the six asserted patents and certain claims of the other three asserted patents, and also granted Alcon’s motion for summary judgment with respect to Hoya’s claim that Alcon’s alleged infringement was willful. This matter was fully and finally resolved prior to the trial scheduled to begin on February 20, 2024.
Hatch-Waxman patent litigation
From time to time, Alcon is a party to certain patent infringement proceedings in the US in connection with Notices of Paragraph IV Certification under the Hatch-Waxman Act received from third-party generic manufacturers respecting their applications for generic versions of certain products sold by or on behalf of Alcon, including Simbrinza, Pataday, Rhopressa and Rocklatan, or other similar suits.
During the third quarter of 2022, Alcon received a Paragraph IV Certification Letter under the Hatch-Waxman Act notifying Alcon that a generic drug company filed an application with the FDA seeking pre-patent expiry approval to sell a generic version of Simbrinza (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2%. In October 2022, Alcon filed a patent infringement lawsuit in the US District Court for the District of Delaware against that generic drug company. The lawsuit, which asserts two patents, automatically stays FDA approval of the generic drug application for up to 30 months from receipt of the Paragraph IV Certification Letter (or earlier if the court renders a decision adverse to Alcon). The court has entered a schedule that sets trial for October 2024. Alcon intends to defend its patents in this case vigorously.
On January 31, 2022, prior to Alcon's acquisition of Aerie, Aerie received three Paragraph IV Certification Letters under the Hatch-Waxman Act notifying Aerie that three generic drug companies had filed applications to the FDA seeking pre-patent expiry approval to sell generic versions of Rhopressa and/or Rocklatan. On March 14, 2022, Aerie filed patent infringement
F-51
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
lawsuits in the US District Court for the District of New Jersey against those generic drug companies. These lawsuits automatically stay FDA approval of the generic drug applications for up to 30 months from receipt of the respective Paragraph IV Certification Letters (or earlier if a court renders a decision adverse to Alcon). The lawsuits have been consolidated into a single case with a trial scheduled for January 2025. Alcon has resolved its patent infringement claims against one of the three generic defendants. Alcon continues to vigorously pursue its claims against the remaining two defendants.
Litigation and other legal matters provision movements
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| January 1 | |||||||||||||||||
| Additions to provisions | |||||||||||||||||
| Cash payments | ( | ( | ( | ||||||||||||||
| Releases of provisions | ( | ( | |||||||||||||||
| December 31 | |||||||||||||||||
| Less current portion | ( | ( | ( | ||||||||||||||
| Non-current provisions for litigation and other legal matters at December 31 | |||||||||||||||||
Alcon believes that its total provisions for litigation and other legal matters are adequate based upon currently available information. However, given the inherent difficulties in estimating liabilities, additional liabilities and costs may be incurred beyond the amounts provided.
19. Provisions and other current liabilities
The following table provides details related to Provisions and other current liabilities as of December 31, 2023 and 2022:
| ($ millions) | Note | 2023 | 2022 | ||||||||
| Accruals for compensation and benefits including social security | |||||||||||
| Accruals for deductions from revenue | |||||||||||
| Deferred income | |||||||||||
| Taxes other than income taxes | |||||||||||
| Restructuring provisions | |||||||||||
| Accrued expenses for goods and services received but not invoiced | |||||||||||
| Accruals for royalties | |||||||||||
| Provisions for litigation and other legal matters | 18 | ||||||||||
| Accrued equity-based payments | |||||||||||
| Accrued interest on financial debts | |||||||||||
| Other payables | |||||||||||
| Total provisions and other current liabilities | |||||||||||
Provisions and accruals are based upon management's best estimate and adjusted for actual experience. Such adjustments to the historical estimates have not been material.
F-52
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Accruals for deductions from revenue
The following table shows the movement of accruals for deductions from revenue:
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| January 1 | |||||||||||||||||
| Additions | |||||||||||||||||
| Impact of business combination | |||||||||||||||||
| Payments/utilizations | ( | ( | ( | ||||||||||||||
| Changes in offset against gross trade receivables | ( | ( | ( | ||||||||||||||
| Currency translation effects | ( | ( | ( | ||||||||||||||
| December 31 | |||||||||||||||||
Restructuring provisions
The following table shows the movement of restructuring provisions:
| ($ millions) | 2023 | 2022 | 2021 | |||||||||||
| January 1 | ||||||||||||||
| Additions | ||||||||||||||
| Cash payments | ( | ( | ( | |||||||||||
| Releases | ( | |||||||||||||
| December 31 | ||||||||||||||
20. Consolidated Statement of Cash Flows - additional details
The Consolidated Statement of Cash Flows was prepared in accordance with IAS 7, Statement of Cash Flows. The below tables provide additional detail supporting select line items in the Consolidated Statement of Cash Flows.
20.1 Depreciation, amortization, impairments and fair value adjustments
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| Property, plant & equipment | |||||||||||||||||
| Right-of-use assets | |||||||||||||||||
| Intangible assets | |||||||||||||||||
| Financial assets | ( | ||||||||||||||||
| Other non-current assets | ( | ( | |||||||||||||||
| Total | |||||||||||||||||
F-53
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
20.2 Change in net current assets and other operating cash flow items
| ($ millions) | 2023 | 2022 | 2021 | ||||||||||||||
| (Increase) in inventories | ( | ( | ( | ||||||||||||||
| (Increase) in trade receivables | ( | ( | ( | ||||||||||||||
| (Decrease)/increase in trade payables | ( | ( | |||||||||||||||
| Net change in other operating assets | ( | ( | ( | ||||||||||||||
| Net change in other operating liabilities | ( | ||||||||||||||||
| Total | ( | ( | ( | ||||||||||||||
20.3 Reconciliation of assets and liabilities arising from financing activities
| Financial Liabilities | ||||||||||||||||||||||||||
| ($ millions) | Non-current financial debts | Current financial debts | Non-current lease liabilities | Current lease liabilities | ||||||||||||||||||||||
| January 1, 2023 | ||||||||||||||||||||||||||
| Repayment of current portion of non-current financial debts | — | ( | ||||||||||||||||||||||||
| Proceeds from current financial debts | — | |||||||||||||||||||||||||
| Proceeds from non-current financial debts, net of issuance costs | — | |||||||||||||||||||||||||
| Additions to leases | ||||||||||||||||||||||||||
| Other changes in current financial debts | — | |||||||||||||||||||||||||
| Amortization of discounts on financial debts | — | |||||||||||||||||||||||||
| Payments of lease liabilities, net | — | ( | ||||||||||||||||||||||||
| Interest payments for amounts included in lease liabilities classified as cash flows from operating activities | — | ( | ||||||||||||||||||||||||
| Changes in fair values and other non-cash changes, net | ( | ( | ||||||||||||||||||||||||
| Currency translation effects | ( | |||||||||||||||||||||||||
| Reclassification from non-current to current | ( | |||||||||||||||||||||||||
| December 31, 2023 | ||||||||||||||||||||||||||
F-54
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
| Financial Liabilities | ||||||||||||||||||||||||||
| ($ millions) | Non-current financial debts | Current financial debts | Non-current lease liabilities | Current lease liabilities | ||||||||||||||||||||||
| January 1, 2022 | ||||||||||||||||||||||||||
| Proceeds from non-current financial debts, net of issuance costs | — | |||||||||||||||||||||||||
| Repayment of non-current financial debts | ( | — | ||||||||||||||||||||||||
| Proceeds from 2022 Bridge Loan Facility, net of issuance costs | — | |||||||||||||||||||||||||
| Repayment of 2022 Bridge Loan Facility | — | ( | ||||||||||||||||||||||||
| Impact from business combination | — | |||||||||||||||||||||||||
| Repayment of financial debts assumed in acquisition of business | — | ( | ||||||||||||||||||||||||
| Additions to leases | ||||||||||||||||||||||||||
| Impact of asset acquisitions | ||||||||||||||||||||||||||
| Other changes in current financial debts | — | ( | ||||||||||||||||||||||||
| Amortization of discounts on financial debts | — | |||||||||||||||||||||||||
| Payments of lease liabilities, net | — | ( | ||||||||||||||||||||||||
| Interest payments for amounts included in lease liabilities classified as cash flows from operating activities | — | ( | ||||||||||||||||||||||||
| Changes in fair values and other non-cash changes, net | ( | |||||||||||||||||||||||||
| Currency translation effects | ( | ( | ( | ( | ||||||||||||||||||||||
| Reclassification from non-current to current | ( | ( | ||||||||||||||||||||||||
| December 31, 2022 | ||||||||||||||||||||||||||
20.4 Additional disclosure of non-cash investing and financing activities
| ($ millions) | 2023 | 2022 | 2021 | |||||||||||||||||
| Treasury stock issued for settlement of equity-based compensation plan, net of withholding taxes | ||||||||||||||||||||
| Non-cash additions of right-of-use assets in exchange for a lease liability | ||||||||||||||||||||
| Non-cash additions of property, plant & equipment | ||||||||||||||||||||
| Non-cash additions of intangible assets | ||||||||||||||||||||
F-55
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
21. Acquisitions
21.1 Acquisition of business
Fair value of assets and liabilities arising from acquisition of business
There were no acquisitions of businesses in 2023 or 2021. During 2022, cash paid for the acquisition of a business, net of cash acquired, was $666 million for Aerie Pharmaceuticals, Inc. described below.
Vision care - Acquisition of Aerie Pharmaceuticals, Inc.
On November 21, 2022, Alcon acquired 100 % of the outstanding shares and equity of Aerie, a pharmaceutical company focused on the discovery, development, manufacturing and commercialization of first-in-class ophthalmic therapies. The acquisition includes with the business among other assets, two commercial pharmaceutical ophthalmic eye drop products, Rocklatan and Rhopressa, as well as AR-15512, a Phase 3 product candidate for dry eye disease, and a pipeline of several ophthalmic pharmaceutical product candidates. This transaction helps bolster Alcon’s presence in the ocular health space with its portfolio of commercial products and development pipeline within the Vision Care reportable segment. Pursuant to the terms of the Agreement and Plan of Merger, Alcon paid $15.25 per share to acquire all outstanding shares of Aerie. The total purchase consideration amounted to $744 million and total cash paid for the net identifiable assets recognized, net of cash acquired, was $666 million.
The preliminary PPA for the Aerie acquisition was not finalized as of the date the 2022 financial statements were issued as the fair values of the acquired assets and assumed liabilities were provisional pending final measurement of the purchase consideration. Alcon's consolidated financial statements as of December 31, 2022 reflected the allocation of the purchase price based on a preliminary fair value assessment of the assets acquired and liabilities assumed. The PPA was subsequently finalized during the third quarter of 2023 and resulted in the reversal of a tax reserve with a corresponding decrease in goodwill. The below table summarizes the final PPA for the Aerie business combination as of December 31, 2023.
| ($ millions) | Preliminary PPA | Measurement period adjustments | Final PPA | ||||||||||||||
| Property, plant and equipment | — | ||||||||||||||||
| Right-of-use assets | — | ||||||||||||||||
| Currently marketed products | — | ||||||||||||||||
| Acquired in-process research & development | — | ||||||||||||||||
| Deferred tax assets | — | ||||||||||||||||
| Inventories | — | ||||||||||||||||
| Trade receivables | — | ||||||||||||||||
| Short-term investments | — | ||||||||||||||||
| Cash and cash equivalents | — | ||||||||||||||||
| Other assets | — | ||||||||||||||||
| Lease liabilities | ( | — | ( | ||||||||||||||
| Deferred tax liabilities | ( | — | ( | ||||||||||||||
| Provisions and other non-current and current liabilities | ( | — | ( | ||||||||||||||
| Current income tax liabilities | ( | ( | |||||||||||||||
| Trade payables | ( | — | ( | ||||||||||||||
| Financial debts | ( | — | ( | ||||||||||||||
| Net identifiable assets acquired | |||||||||||||||||
| Goodwill | ( | ||||||||||||||||
| Total purchase consideration | |||||||||||||||||
| Acquired liquidity | ( | — | ( | ||||||||||||||
| Net assets recognized as a result of business combinations | — | ||||||||||||||||
F-56
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
Alcon retrospectively adjusted the provisional amounts that were recognized at acquisition date, resulting in Current income tax liabilities of $175 million and Goodwill of $8,926 million as of December 31, 2022.
The short-term investments were liquidated in 2022 subsequent to the acquisition.
Provisions and other non-current liabilities recognized at the Aerie acquisition date included a contingent liability related to uncertainty associated with potential contractual payment obligations tied to the assertion of certain third party patents in certain markets. During the third quarter of 2023, the contingent liability of $58 million was released and recognized in Other income following the resolution of the uncertainty.
The goodwill is attributable to assembled workforce and pharmaceutical research and development capabilities, including early stage compounds under development. The goodwill is not deductible for tax purposes.
Direct acquisition costs of $20 million were recognized in Other expense in the 2022 Consolidated Income Statement and were reported in operating cash flows in the 2022 Consolidated Statement of Cash Flows.
Post-acquisition net sales and net loss attributable to Aerie
For the period from the date of the Aerie acquisition, November 21, 2022, through December 31, 2022, the acquired business increased Alcon's 2022 net sales by $16 million and reduced Alcon's 2022 net income by $32 million.
Unaudited Alcon consolidated pro forma net sales and net income
If the Aerie acquisition had occurred on January 1, 2022, unaudited consolidated pro forma net sales and net income for the twelve months ended December 31, 2022 would have been approximately $8,776 million and $192 million, respectively. This pro forma information is presented for illustrative purposes only and may not be indicative of the results of operations that would have actually occurred. In addition, future results may vary significantly from the results reflected in the pro forma information. These estimated amounts have been calculated using Aerie's results of operation beginning January 1, 2022 and adjusting them for:
•alignment of the accounting policies between Alcon and Aerie;
•additional amortization that would have been charged assuming the fair value adjustments to inventories and intangible assets had been applied from January 1, 2022;
•add back of interest expense from Aerie's convertible senior notes to pro forma net income assuming senior notes would have been repaid on January 1, 2022;
•additional interest expense that would have been recorded assuming the Series 2032 Notes and Series 2052 Notes were issued on January 1, 2022 to the extent the proceeds were used to refinance the 2022 Bridge Loan Facility;
•exclusion of Aerie's pre-acquisition transaction costs; and
•tax effects of the above adjustments.
21.2 Acquisitions of assets
Acquisitions of assets in 2023 amounted to $2 million. During 2022, cash paid for acquisitions of assets, net of cash acquired, was $485 million, the most significant of which was $477 million paid for Ivantis, Inc., described below. There were no acquisitions of assets in 2021.
F-57
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The below table summarizes the PPA for asset acquisitions for the year ended December 31, 2022:
| ($ millions) | 2022 | |||||||||||||
| Currently marketed products | ||||||||||||||
| Acquired in-process research & development | ||||||||||||||
| Other intangible assets (including software) | ||||||||||||||
| Deferred tax assets | ||||||||||||||
| Trade receivables | ||||||||||||||
| Inventory | ||||||||||||||
| Cash and cash equivalents | ||||||||||||||
| Other assets | ||||||||||||||
| Trade payables and other liabilities | ( | |||||||||||||
| Net identifiable assets acquired | ||||||||||||||
| Acquired liquidity | ( | |||||||||||||
| Net assets recognized as a result of asset acquisitions | ||||||||||||||
Surgical - Acquisition of Ivantis, Inc.
On January 7, 2022, Alcon acquired 100 % of the outstanding shares and equity of Ivantis, Inc., a privately-held, US-based company and manufacturer of the Hydrus Microstent, a MIGS device designed to lower intraocular pressure for open-angle glaucoma patients. The acquisition expands Alcon’s surgical portfolio and is expected to help provide a platform for more growth in the glaucoma space. Pursuant to the terms and subject to the conditions of the Option Agreement and Plan of Merger, as amended, Alcon agreed to pay total upfront consideration of $479 million and additional amounts to be potentially paid upon achievement of a development milestone and commercial milestones calculated as a percentage of sales in excess of defined targets that expire in calendar year 2024.
The acquisition was accounted for as an asset acquisition rather than a business combination as substantially all of the fair value of the gross assets acquired is concentrated in the value of the Hydrus Microstent commercially marketed product intangible assets, being a group of identifiable assets. Consequently, a relative fair value approach was taken for allocating the consideration to the acquired assets and liabilities with no goodwill recognized.
22. Post-employment benefits for associates
Defined benefit plans
In addition to the legally required social security schemes, Alcon has sponsored numerous independent pension and other post-employment benefit plans. In most cases, these plans are externally funded in entities that are legally separate from Alcon. For certain subsidiaries, however, no independent plan assets exist for the pension and other post-employment benefit obligations of associates. In these cases the related unfunded liability is included in the Consolidated Balance Sheet. The value of the post-employment benefits promised under the pension and other post-employment benefit plans is represented by the defined benefit obligation ("DBO"), which is measured based on the projected unit credit method ("PUC"). Independent actuaries reappraise the DBOs of all major pension and other post-employment benefit plans annually. Plan assets are recognized at fair value.
The major pension and other post-employment benefit plans are based in Switzerland, the United States, Germany, and the United Kingdom. As of December 31, 2023, these plans represent 88 % of Alcon's total DBO and are independently sponsored by Alcon. Details of the plans in those significant countries are provided below.
The pension plans in Switzerland represent the most significant portion of Alcon's total pension DBO and total plan assets. The principal plan in Switzerland is funded and open for new joiners. For the Swiss pension plan, active insured members' benefits are partially linked to the contributions paid into the plan. Certain features of the Swiss pension plan required by law preclude the plan from being categorized as a defined contribution plan. These factors include a minimum interest guarantee on retirement savings accounts, a pre-determined factor for converting the accumulated savings account balance into a pension and embedded death and disability benefits. All benefits granted under a Swiss-based principal
F-58
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
pension plan are vested, and Swiss legislation prescribes that the employer has to contribute a fixed percentage of an associate's pay to an external pension trust. Additional employer contributions may be required whenever the foundation's statutory funding ratio falls below a certain level. The associate also contributes to the plan.
Alcon's Swiss pension obligation is set-up under an Alcon-sponsored arrangement affiliated with Copré La Collective de Prévoyance ("Copré") – a Swiss collective foundation. As a collective foundation, Copré is governed by its own board of trustees which is responsible for the foundation regulations and asset investment strategy for multiple entities participating in the collective foundation. Alcon maintains its own pension committee, consisting of representatives nominated by Alcon and the active insured associates. During the fourth quarter of 2021, Copré announced the rates to be used to convert participant balances to pension annuities for 2024 to 2026. This announcement resulted in a plan amendment with a benefit of $15
The United States pension plans represent the second largest component of Alcon's total pension DBO and total plan assets. The principal plan (Qualified Plan) is funded, whereas the plans providing additional benefits for executives (Defined Benefit Restoration Plan and Grandfathered Supplemental Executive Plan) are unfunded. Benefits in the Qualified Plan and Restoration Plan are frozen for all participants. Employer contributions are required for the Qualified Plan whenever the statutory funding ratio falls below a certain level. Furthermore, the United States other post-employment benefit plans (US OPEB plans) represent 98 % of the total DBO for other post-employment benefit plans. These benefits in the US primarily consist of post-employment healthcare which has been closed to new members since 2015. Effective January 1, 2021, the Alcon sponsored group health plan for current and future eligible retired participants age 65 and over utilizes a private Medicare marketplace while providing an annual notional contribution to a Health Reimbursement Account for each covered member and spouse. There is no statutory funding requirement for the US OPEB plans.
The major pension arrangements in Germany are governed by the Occupational Pensions Act ("BetrAVG") and represent the third largest component of Alcon's total pension DBO and the fifth largest component of Alcon's total plan assets. The plans are partly funded by a Contractual Trust arrangement or direct insurances. The employer is responsible for contributing the premiums to the insurances and paying certain benefits when they fall due. All plans are closed for new entrants and the benefits are fully vested for all participants. For some participants the benefits are based on final salary and length of employment, and for others the benefit is earned each year based on the current salary in the year of service. Associates do not contribute towards the cost of the benefits.
The pension plan in the United Kingdom represents the fourth largest component of Alcon's total pension DBO and the third largest component of Alcon's total plan assets. The plan is closed with only former Alcon associates entitled to current or future benefits. The Alcon United Kingdom Pension Scheme is governed and administered by a board of trustees in accordance with its Trust Deed. United Kingdom legislation requires that pension schemes are funded prudently (i.e., to a level in excess of the "best estimate" expected cost of providing benefits). Funding is assessed on a triennial basis using assumptions agreed by the board of trustees and Alcon. The board of trustees is responsible for jointly agreeing with Alcon the level of contributions needed to eliminate any shortfall over a reasonable period of time, typically not exceeding 10 years. Under the governing documentation, if a surplus remains once liabilities have been settled it would be refunded to Alcon.
Alcon has certain pension plans with a surplus that are not recognized on the basis that future economic benefits are not available to the entity in the form of a reduction in future contributions or a cash refund.
F-59
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The following tables summarize the funded and unfunded DBO for pension and other post-employment benefit plans of Alcon associates at December 31, 2023 and 2022:
| Pension plans | Other post-employment benefit plans | ||||||||||||||||||||||
| ($ millions) | 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||
| Benefit obligation at January 1 | |||||||||||||||||||||||
| Current service cost | |||||||||||||||||||||||
| Interest cost | |||||||||||||||||||||||
| Past service costs and settlements | ( | ( | |||||||||||||||||||||
| Administrative expenses | |||||||||||||||||||||||
| Remeasurement losses/(gains) arising from changes in financial assumptions | ( | ( | |||||||||||||||||||||
| Remeasurement (gains) arising from changes in demographic assumptions | ( | ( | |||||||||||||||||||||
| Remeasurement losses/(gains) arising from experience-related changes | ( | ( | |||||||||||||||||||||
| Currency translation effects | ( | ||||||||||||||||||||||
| Benefit payments | ( | ( | ( | ( | |||||||||||||||||||
| Contributions of associates | |||||||||||||||||||||||
| Benefit obligation at December 31 | |||||||||||||||||||||||
| Fair value of plan assets at January 1 | |||||||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Return on plan assets excluding interest income | ( | ||||||||||||||||||||||
| Currency translation effects | ( | ||||||||||||||||||||||
| Employer contributions | |||||||||||||||||||||||
| Contributions of associates | |||||||||||||||||||||||
| Settlements | ( | ||||||||||||||||||||||
| Benefit payments | ( | ( | ( | ( | |||||||||||||||||||
| Fair value of plan assets at December 31 | |||||||||||||||||||||||
| Funded status | ( | ( | ( | ( | |||||||||||||||||||
| Limitation on recognition of fund surplus at January 1 | ( | ( | |||||||||||||||||||||
| Change in limitation on recognition of fund surplus (including exchange rate differences) | ( | ( | |||||||||||||||||||||
| Limitation on recognition of fund surplus at December 31 | ( | ( | |||||||||||||||||||||
| Net liability in the balance sheet at December 31 | ( | ( | ( | ( | |||||||||||||||||||
F-60
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The reconciliation of the net liability from January 1 to December 31 is as follows:
| Pension plans | Other post-employment benefit plans | ||||||||||||||||||||||
| ($ millions) | 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||
| Net liability at January 1 | ( | ( | ( | ( | |||||||||||||||||||
| Current service cost | ( | ( | ( | ( | |||||||||||||||||||
| Net interest expense | ( | ( | ( | ( | |||||||||||||||||||
| Administrative expenses | ( | ( | |||||||||||||||||||||
| Past service costs and settlements | |||||||||||||||||||||||
| Remeasurements | ( | ||||||||||||||||||||||
| Currency translation effects | ( | ||||||||||||||||||||||
| Employer contributions | |||||||||||||||||||||||
| Change in limitation on recognition of fund surplus | ( | ( | |||||||||||||||||||||
| Net liability at December 31 | ( | ( | ( | ( | |||||||||||||||||||
| Amounts recognized in the balance sheet | |||||||||||||||||||||||
| Prepaid benefit cost | |||||||||||||||||||||||
| Accrued benefit liability | ( | ( | ( | ( | |||||||||||||||||||
The following tables provide detail of the DBO for pension plans by geography and type of member and of plan assets based on the geographical locations in which they are held:
| 2023 | |||||||||||||||||||||||||||||||||||
| ($ millions) | Switzerland | United States | Germany | United Kingdom | Rest of the world | Total | |||||||||||||||||||||||||||||
| By type of member | |||||||||||||||||||||||||||||||||||
| Active | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
| Deferred pensioners | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Pensioners | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Benefit obligation at December 31 | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Thereof: unfunded plans | |||||||||||||||||||||||||||||||||||
| Thereof: unfunded portion of funded plans | |||||||||||||||||||||||||||||||||||
| Prepaid benefit costs and assets subject to limitation on recognition of fund surplus | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Fair value of plan assets at December 31 | |||||||||||||||||||||||||||||||||||
| Funded status | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
F-61
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
| 2022 | |||||||||||||||||||||||||||||||||||
| ($ millions) | Switzerland | United States | Germany | United Kingdom | Rest of the world | Total | |||||||||||||||||||||||||||||
| By type of member | |||||||||||||||||||||||||||||||||||
| Active | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
| Deferred pensioners | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Pensioners | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Benefit obligation at December 31 | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Thereof: unfunded plans | |||||||||||||||||||||||||||||||||||
| Thereof: unfunded portion of funded plans | |||||||||||||||||||||||||||||||||||
| Prepaid benefit costs and assets subject to limitation on recognition of fund surplus | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Fair value of plan assets at December 31 | |||||||||||||||||||||||||||||||||||
| Funded status | ( | ( | ( | ( | |||||||||||||||||||||||||||||||
The following table shows the principal weighted average actuarial assumptions used for calculating defined benefit plans and other post-employment benefits of Alcon associates:
| Pension plans | Other post-employment benefit plans | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Discount rate | % | % | % | % | |||||||||||||||||||
| Expected rate of pension increase | % | % | |||||||||||||||||||||
| Expected rate of salary increase | % | % | |||||||||||||||||||||
| Interest on savings account | % | % | |||||||||||||||||||||
| Current average life expectancy for a 65-year-old male (in years) | |||||||||||||||||||||||
| Current average life expectancy for a 65-year-old female (in years) | |||||||||||||||||||||||
The following table shows additional details related to the weighted average discount rates for the principal plan for each significant country:
| Pension plans | Other post-employment benefit plans | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Switzerland | % | % | |||||||||||||||||||||
| United States | % | % | % | % | |||||||||||||||||||
| Germany | % | % | |||||||||||||||||||||
| United Kingdom | % | % | |||||||||||||||||||||
Changes in the aforementioned actuarial assumptions can result in significant volatility in the accounting for pension plans and other post-employment benefit plans in the Consolidated Financial Statements. This can result in substantial changes in Alcon's other comprehensive income, non-current liabilities and prepaid pension assets.
The DBO is significantly impacted by assumptions related to the rate used to discount the actuarially determined post-employment benefit liability. This rate is based on yields of high-quality corporate bonds in the country of the plan. Decreasing corporate bond yields decrease the discount rate. A decrease in the discount rate results in an increase in the DBO and a decrease in the funded status.
The impact of decreasing interest rates on a plan's assets is more difficult to predict. A significant part of plan assets is invested in bonds. Bond values typically are inversely correlated to interest rates. Bond values usually increase when interest rates fall and may therefore partially offset the decrease in the funded status. Furthermore, pension assets also
F-62
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
include significant holdings of equity instruments. Share prices tend to rise when interest rates decrease and therefore often offset the negative impact of the increasing DBO on the funded status (although the correlation of interest rates with returns on equities is not as strong as with bonds, especially in the short term).
The assumption for the expected rate for pension increases significantly affects the DBO of most plans in Switzerland, Germany and the United Kingdom. While the average rate remained flat at 1.1 % in the current year, such pension increases generally decrease the funded status, although there is no strong correlation between the value of the plan assets and pension/inflation increases.
Assumptions regarding life expectancy significantly impact the DBO. While the life expectancy assumption remained flat in the current year, generally an increase in longevity increases the DBO. There is no offsetting impact from the plan assets, as no longevity bonds or swaps are held by the pension funds. Generational mortality tables are used where this data is available.
The following table shows the sensitivity of the defined benefit pension and other post-employment benefit obligations to the principal actuarial assumptions as of December 31, 2023:
| ($ millions) | (Decrease)/increase in 2023 year-end liability | ||||
| 25 basis point increase in discount rate | ( | ||||
| 25 basis point decrease in discount rate | |||||
| 1 year increase in life expectancy | |||||
| 25 basis point increase in rate of pension increase | |||||
25 basis point decrease in rate of pension increase(1) | ( | ||||
| 25 basis point increase of interest on savings account | |||||
| 25 basis point decrease of interest on savings account | ( | ||||
| 25 basis point increase in rate of salary increase | |||||
| 25 basis point decrease in rate of salary increase | ( | ||||
(1)Decrease in rate of pension increase is limited to zero.
The above sensitivity analyses are based on a change in an assumption while holding all other assumptions constant. In practice, this is unlikely to occur, and changes of the assumptions may be correlated. When calculating the sensitivity of the DBO to significant actuarial assumptions the same method (present value of the defined benefit obligation calculated with the PUC method at the end of the reporting period) has been applied as when calculating the net liability recognized in the Consolidated Balance Sheet.
The healthcare cost trend rate assumptions used for other post-employment benefits are as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Healthcare cost trend rate assumed for next year | % | % | % | ||||||||||||||
| Rate to which the cost trend rate is assumed to decline | % | % | % | ||||||||||||||
| Year that the rate reaches the ultimate trend rate | 2030 | 2030 | 2029 | ||||||||||||||
F-63
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
The following table shows the weighted average plan asset allocation of funded defined benefit pension plans at December 31, 2023, and 2022:
| Pension plans | |||||||||||||||||||||||
| (as a percentage) | Long-term target minimum | Long-term target maximum | 2023 | 2022 | |||||||||||||||||||
| Equity securities | |||||||||||||||||||||||
| Debt securities | |||||||||||||||||||||||
| Real estate | |||||||||||||||||||||||
| Alternative investments | |||||||||||||||||||||||
| Cash and other investments | |||||||||||||||||||||||
| Total | |||||||||||||||||||||||
Cash and most of the equity and debt securities have a quoted market price in an active market. Real estate and alternative investments, which include hedge fund and private equity investments, usually do not have a quoted market price.
The strategic allocation of assets of the different pension plans is determined with the objective of achieving an investment return that, together with employer contributions and contributions of associates (where applicable), is sufficient to manage the various funding risks of the plans. Based upon the market and economic environments, actual asset allocations may temporarily be permitted to deviate from policy targets.
The weighted average duration of the DBO is 12.4 years and 11.6 years as of December 31, 2023 and December 31, 2022, respectively.
Alcon's ordinary contribution to the various pension plans is based on the rules of each plan and its respective country. Additional contributions are made whenever required by local statute or law (i.e., usually when statutory funding levels fall below predetermined thresholds).
The following table summarizes expected employer contributions for one year and expected future benefit payments for ten years for pension and other post-employment benefit plans as of December 31, 2023:
| ($ millions) | Pension plans | Other post-employment benefit plans | |||||||||
| Employer contributions | |||||||||||
| 2024 (estimated) | |||||||||||
| Expected future benefit payments | |||||||||||
| 2024 | |||||||||||
| 2025 | |||||||||||
| 2026 | |||||||||||
| 2027 | |||||||||||
| 2028 | |||||||||||
| 2029-2033 | |||||||||||
Defined contribution plans
In many countries, associates are covered by defined contribution plans. Contributions charged to the 2023 Consolidated Income Statement for the defined contribution plans were $151 million (2022: $144 million; 2021: $133 million).
F-64
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
23. Equity-based compensation
For the year ended December 31, 2023, Alcon recorded equity-based compensation expense of $159 million (2022: $152 million, 2021: $151 million).
Liabilities from cash-settled equity-based compensation plans were $13 million as of December 31, 2023 ($12 million as of December 31, 2022).
At December 31, 2023, Alcon has various equity-based incentive plans, under which Alcon may grant awards in the form of restricted stock units ("RSUs"), performance-based restricted stock units ("PSUs"), restricted stock awards ("RSAs"), or any other form of award at the discretion of the Board. Certain associates in select countries may also participate in share ownership savings plans.
Summary of unvested share movements
The below table summarizes unvested share movements for all Alcon equity-based incentive plans for the years ended December 31, 2023 and 2022:
| 2023 | 2022 | ||||||||||||||||||||||||||||||||||
| Number of shares in thousands | Weighted average fair value at grant date in $ | Fair value at grant date in $ millions | Number of shares in thousands | Weighted average fair value at grant date in $ | Fair value at grant date in $ millions | ||||||||||||||||||||||||||||||
| Unvested shares at January 1 | |||||||||||||||||||||||||||||||||||
| Granted | |||||||||||||||||||||||||||||||||||
| Restricted awards | |||||||||||||||||||||||||||||||||||
| Performance awards | |||||||||||||||||||||||||||||||||||
| Vested | ( | ( | ( | ( | |||||||||||||||||||||||||||||||
| Forfeited | ( | ( | ( | ( | |||||||||||||||||||||||||||||||
| Unvested shares at December 31 | |||||||||||||||||||||||||||||||||||
The remaining weighted-average vesting period of unvested equity-based awards as of December 31, 2023 was 1.3 years.
Equity-based incentive plans
The below table summarizes the number of shares authorized under the plans as of December 31, 2023:
| (thousands) | Authorized shares | ||||
| Long-term Incentive Plan | |||||
Deferred Bonus Stock Plan(1) | |||||
| Swiss Employee Share Ownership Plan | |||||
| Other share savings plans | |||||
| Total | |||||
(1) No grants under the Deferred Bonus Stock Plan were made in 2023, 2022 or 2021.
Long-term Incentive Plan ("LTIP") - Restricted Stock Units and Restricted Stock Awards
Under Alcon's LTIP, certain associates may receive grants of RSUs and RSAs (together "Restricted awards"). The awards generally vest on the third anniversary of the award and are generally forfeited if the employment relationship with Alcon terminates prior to vesting. Recipients of RSU awards do not have any shareholder rights, such as voting or dividend rights, until the shares are delivered. Alcon associates receiving grants of RSAs are entitled to the dividends that may be declared and paid over the vesting period only if the associates vest in such award.
LTIP - Performance Stock Units
The Alcon CEO and certain senior-level associates participate in Alcon's long-term performance program. PSUs granted under the LTIP each convert to unrestricted Alcon Inc. shares at vesting, subject to the achievement of performance measures.
F-65
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
PSUs awarded to plan participants are granted at target incentive ranges from 35 % to 430 % of base compensation and vest over a three-year period. The payout between 0 % and 200 % of target is dependent upon four equally weighted performance metrics which are determined at the onset of the performance period by the Board. The metrics include compound annual growth rate of Net sales, compound annual growth rate of core earnings per share, market share of peers, and innovation. The Board and the Compensation Committee assess the performance against the defined measures, including input from the Innovation Committee for the innovation metric, and approve the final payout. PSUs granted under the performance plan do not carry voting rights, but do carry dividend equivalents that are paid in cash or Alcon Inc. shares at vesting, provided participants remain associates of Alcon.
Swiss Employee Share Ownership Plan and other share savings plans
Alcon associates in certain countries are encouraged to invest in share savings plans. Under the share savings plans, participants may elect to receive some of their wages or annual incentives in Alcon Inc. shares in lieu of cash. Subject to plan rules and limitations, as a reward for their participation in the share savings plans, at no additional cost to the participant, Alcon may partially match their investments in shares after a holding period of 3 years.
24. Related parties transactions
Executive officers
The following table summarizes compensation information for key management personnel:
| ($ millions) | 2023 | 2022 | 2021 | ||||||||
| Cash and other compensation | |||||||||||
| Post-employment benefits | |||||||||||
| Equity-based compensation | |||||||||||
| Total | |||||||||||
Investment in an associated company
F-66
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
25. Commitments and contingencies
Commitments
Research & development
Alcon has entered into long-term research agreements with various institutions which provide for potential milestone payments and other payments by Alcon that may be capitalized. As of December 31, 2023, the commitments to make payments under those agreements, and their estimated timing, were as follows:
| ($ millions) | 2023 | ||||
| 2024 | |||||
| 2025 | |||||
| 2026 | |||||
| 2027 | |||||
| 2028 | |||||
| Thereafter | |||||
| Total | |||||
Other
Alcon entered into various purchase commitments for services and materials as well as for equipment in the ordinary course of business. These commitments are generally entered into at current market prices and reflect normal business operations. For disclosure of Property, plant and equipment purchase commitments, see Note 8.
Contingencies
The Alcon companies have to observe the laws, government orders and regulations of the country in which they operate.
A number of Alcon companies are, and will likely continue to be, subject to various legal proceedings and investigations that arise from time to time, including proceedings regarding product liability, sales and marketing practices, commercial disputes, employment, wrongful discharge, antitrust, securities, health and safety, environmental, tax, international trade, privacy, intellectual property including under the Hatch-Waxman Act, and anti-bribery matters such as those under the FCPA, as amended. As a result, Alcon may become subject to substantial liabilities that may not be covered by insurance and could affect Alcon's business, financial position and reputation. While Alcon does not believe that any of these legal proceedings will have a material adverse effect on its financial position, litigation is inherently unpredictable and large judgments sometimes occur. As a consequence, Alcon may in the future incur judgments or enter into settlements of claims that could have a material adverse effect on its results of operations or cash flow.
Governments and regulatory authorities around the world have been stepping up their compliance and law enforcement activities in recent years in key areas, including marketing practices, pricing, corruption, trade restrictions, embargo legislation, insider trading, antitrust, cybersecurity and data privacy. Further, when one government or regulatory authority undertakes an investigation, it is not uncommon for other governments or regulators to undertake investigations regarding the same or similar matters. Responding to such investigations is costly and requires an increasing amount of management's time and attention. In addition, such investigations may affect Alcon's reputation, create a risk of potential exclusion from government reimbursement programs in the United States and other countries, and may lead to (or arise from) litigation. These factors have contributed to decisions by Alcon and other companies in the healthcare industry, when deemed in their interest, to enter into settlement agreements with governmental authorities around the world prior to any formal decision by the authorities or a court. Those government settlements have involved and may continue to involve, in current government investigations and proceedings, large cash payments, sometimes in the hundreds of millions of dollars or more, including the potential repayment of amounts allegedly obtained improperly and other penalties, including treble damages. In addition, settlements of government healthcare fraud cases often require companies to enter into corporate integrity agreements, which are intended to regulate company behavior for a period of years. Also, matters underlying governmental investigations and settlements may be the subject of separate private litigation.
While provisions have been made for probable losses, which management deems to be reasonable or appropriate, there are uncertainties connected with these estimates. Note 18 contains additional information on these matters.
Alcon is involved in legal proceedings concerning intellectual property rights. The inherent unpredictability of such proceedings means that there can be no assurances as to their ultimate outcome. A negative result in any such
F-67
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
proceeding could potentially adversely affect the ability of certain Alcon companies to sell their products, or require the payment of substantial damages or royalties.
Alcon's potential for environmental remediation liability is assessed based on a risk assessment and investigation of the various sites identified by Alcon as at risk for environmental remediation exposure. Alcon's future remediation expenses are affected by a number of uncertainties. These uncertainties include, but are not limited to, the method and extent of remediation, the percentage of material attributable to Alcon at the remediation sites relative to that attributable to other parties, and the financial capabilities of the other potentially responsible parties.
Alcon has no significant environmental liabilities as at December 31, 2023 and 2022 and has incurred no significant remediation costs for the years ended December 31, 2023, 2022 and 2021.
26. Subsequent events
On February 27, 2024, the Board approved the proposal to submit the 2023 financial statements of Alcon Inc. and these Consolidated Financial Statements for approval at the Annual General Meeting on May 8, 2024. Additionally on February 27, 2024, the Board proposed a dividend of CHF 0.24 per share to be approved at the same Annual General Meeting. If approved by the shareholders, the total dividend payments would amount to a maximum of approximately $137 million using the CHF/USD exchange rate as of February 21, 2024.
The Board has evaluated subsequent events as they relate to Alcon for potential recognition or disclosures from January 1, 2024 to the date of the approval of these Consolidated Financial Statements and has determined there are no additional subsequent events to be reported in these Consolidated Financial Statements.
F-68
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
27. Alcon subsidiaries and associated companies
The following table lists the subsidiaries of Alcon Inc. with Total assets or Net sales to third parties in excess of $5 million included in the Consolidated Financial Statements at and for the year ended December 31, 2023, respectively. The equity interest percentage shown in the table represents Alcon's share in voting rights in those entities. Unless otherwise stated, each entity has share capital consisting of equity held directly by the Company or another of its consolidated subsidiaries.
| Country of organization/Entity name | Place of business | Equity interest | ||||||
| Argentina | ||||||||
| Alcon Laboratorios Argentina S.A. | Buenos Aires | % | ||||||
| Australia | ||||||||
| Alcon Laboratories (Australia) Pty Ltd | Macquarie Park | % | ||||||
| Austria | ||||||||
| Alcon Ophthalmika GmbH | Wien | % | ||||||
| Belgium | ||||||||
| Alcon Laboratories Belgium BVBA | Puurs | % | ||||||
| Alcon N.V. | Mechelen | % | ||||||
| Brazil | ||||||||
| Alcon Brasil Cuidados com a Saúde Ltda. | São Paulo | % | ||||||
| Canada | ||||||||
| Alcon Canada Inc. | Mississauga, Ontario | % | ||||||
| Cayman Islands | ||||||||
| Aerie Pharmaceuticals Limited | Grand Cayman | % | ||||||
| Chile | ||||||||
| Alcon Laboratorios Chile Ltd. | Santiago de Chile | % | ||||||
| China | ||||||||
| Alcon (China) Ophthalmic Product Co., Ltd. | Beijing | % | ||||||
| Alcon Hong Kong Limited | Hong Kong | % | ||||||
| Colombia | ||||||||
| Laboratorios Alcon de Colombia S.A. | Santafé de Bogotá | % | ||||||
| Czech Republic | ||||||||
| Alcon Pharmaceuticals (Czech Republic) s.r.o. | Prague | % | ||||||
| Denmark | ||||||||
| Alcon Nordic A/S | Copenhagen | % | ||||||
| Ecuador | ||||||||
| AlconLab Ecuador S.A. | Quito | % | ||||||
| France | ||||||||
| Laboratoires Alcon S.A.S. | Rueil-Malmaison | % | ||||||
| Germany | ||||||||
| Alcon Deutschland GmbH | Freiburg im Breisgau | % | ||||||
| CIBA Vision GmbH | Grosswallstadt | % | ||||||
| WaveLight GmbH | Erlangen | % | ||||||
| Greece | ||||||||
| Alcon Laboratories Hellas- Single Member Commercial and Industrial S.A.C.I. | Maroussi, Athens | % | ||||||
| Hungary | ||||||||
| Alcon Hungary Pharmaceuticals Trading Limited Liability Company | Budapest | % | ||||||
| India | ||||||||
| Alcon Laboratories (India) Private Limited | Bangalore | % | ||||||
| Indonesia | ||||||||
| PT. CIBA Vision Batam | Batam | % | ||||||
| Ireland | ||||||||
| Alcon Laboratories Ireland Limited | Cork City | % | ||||||
| Aerie Pharmaceuticals Ireland Limited | Athlone | % | ||||||
| Italy | ||||||||
| Alcon Italia S.p.A. | Milano | % | ||||||
F-69
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
| Country of organization/Entity name | Place of business | Equity interest | ||||||
| Japan | ||||||||
| Alcon Japan Ltd. | Tokyo | % | ||||||
| Malaysia | ||||||||
| Alcon Laboratories (Malaysia) Sdn. Bhd. | Petaling Jaya | % | ||||||
| CIBA Vision Johor Sdn. Bhd. | Johor | % | ||||||
| Mexico | ||||||||
| Alcon Laboratorios, S.A. de C.V. | Ciudad de Mexico | % | ||||||
| Netherlands | ||||||||
| Alcon Finance B.V. | Amsterdam | % | ||||||
| Alcon Nederland B.V. | Utrecht | % | ||||||
| New Zealand | ||||||||
| Alcon Laboratories (New Zealand) Ltd. | Remuera | % | ||||||
| Panama | ||||||||
| Alcon Centroamerica S.A. | Panama City | % | ||||||
| Peru | ||||||||
| Alcon Pharmaceutical del Peru S.A. | Lima | % | ||||||
| Philippines | ||||||||
| Alcon Laboratories (Philippines), Inc. | Pasig City | % | ||||||
| Poland | ||||||||
| Alcon Polska Sp. z o.o. | Warszawa | % | ||||||
| Portugal | ||||||||
| Alcon Portugal-Produtos e Equipamentos Oftalmológicos Lda. | Oeiras | % | ||||||
| Puerto Rico | ||||||||
| Alcon (Puerto Rico), Inc. | Cataño, PR | % | ||||||
| Romania | ||||||||
| Alcon Romania S.R.L. | Bucharest | % | ||||||
| Russian Federation | ||||||||
| Alcon Farmacevtika LLC | Moscow | % | ||||||
| Singapore | ||||||||
| Alcon Pte Ltd | Singapore | % | ||||||
| Alcon Singapore Manufacturing Pte Ltd | Singapore | % | ||||||
| CIBA Vision Asian Manufacturing and Logistics Pte Ltd. | Singapore | % | ||||||
| South Africa | ||||||||
| Alcon Laboratories (South Africa) (Pty) Ltd. | Midrand | % | ||||||
| South Korea | ||||||||
| Alcon Korea Ltd. | Seoul | % | ||||||
| Spain | ||||||||
| Alcon Healthcare S.A. | Barcelona | % | ||||||
| Switzerland | ||||||||
| Alcon Grieshaber AG | Schaffhausen | % | ||||||
| Alcon Management SA | Vernier | % | ||||||
| Alcon Pharmaceuticals Ltd. | Fribourg | % | ||||||
| Alcon Services AG | Fribourg | % | ||||||
| Alcon Switzerland SA | Zug | % | ||||||
| Thailand | ||||||||
| Alcon Laboratories (Thailand) Limited | Bangkok | % | ||||||
| Turkey | ||||||||
| Alcon Laboratuvarlari Ticaret A.S. | Istanbul | % | ||||||
| Ukraine | ||||||||
| Alcon Ukraine LLC | Kiev | % | ||||||
| United Kingdom | ||||||||
| Alcon Eye Care UK Limited | Frimley/Camberley | % | ||||||
| United States of America | ||||||||
| Aerie Distribution, Inc. | Fort Worth, TX | % | ||||||
F-70
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF ALCON INC. (Continued)
| Country of organization/Entity name | Place of business | Equity interest | ||||||
| Aerie Pharmaceuticals, Inc. | Fort Worth, TX | % | ||||||
| Alcon Finance Corporation | Fort Worth, TX | % | ||||||
| Alcon Laboratories, Inc. | Fort Worth, TX | % | ||||||
| Alcon RefractiveHorizons, LLC | Fort Worth, TX | % | ||||||
| Alcon Research, LLC | Fort Worth, TX | % | ||||||
| Alcon Vision, LLC | Fort Worth, TX | % | ||||||
| CIBA Vision, LLC | Fort Worth, TX | % | ||||||
| WaveLight, Inc. | Fort Worth, TX | % | ||||||
| Ivantis, Inc. | Fort Worth, TX | % | ||||||
| MDBackline, Inc. | Fort Worth, TX | % | ||||||
| PowerVision, Inc. | Fort Worth, TX | % | ||||||
| Tear Film Innovations, Inc. | Fort Worth, TX | % | ||||||
| TrueVision Systems, Inc. | Fort Worth, TX | % | ||||||
| Uruguay | ||||||||
| Alcon Laboratorios Uruguay S.A. | Montevideo | % | ||||||
There was one investment in an associated company as of December 31, 2023. Refer to Note 24 for additional information.
F-71
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Alcon Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Alcon Inc. and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of income, comprehensive income, changes in equity and cash flows for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Annual Report on Internal Control over Financial Reporting appearing under Item 15. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become
F-72
inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Goodwill and Alcon Brand Name Impairment Assessments
As described in Notes 2 and 9 to the consolidated financial statements, as of December 31, 2023 the Company had $8.9 billion of goodwill, as well as a $3.0 billion indefinite life intangible asset related to the Alcon brand name. An impairment assessment on goodwill and indefinite life intangible assets, which is performed over the groupings of cash generating units containing goodwill or the Alcon brand name, is performed at least annually. A cash generating unit to which goodwill has been allocated is considered impaired when its carrying amount, including the goodwill, exceeds its recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. An intangible asset other than goodwill is considered impaired when its balance sheet carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. Usually, management applies the fair value less costs of disposal method for its impairment assessment. In most cases, no direct or indirect observable market prices for identical or similar assets are available to measure the fair value less costs of disposal. Therefore, an estimate of fair value less costs of disposal is based on net present value techniques utilizing post-tax cash flows and discount rates. The estimates of the fair value less costs of disposal involve significant judgment by management and include assumptions with measurement uncertainty, such as the amount and timing of projected cash flows, long-term sales forecasts, terminal growth rate, discount rate, and, additionally for the Alcon brand name, royalty rate.
The principal considerations for our determination that performing procedures relating to the goodwill and Alcon brand name impairment assessments is a critical audit matter are the significant judgment by management when estimating the fair value less costs of disposal, which in turn led to a high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating, for (i) goodwill, management’s significant assumptions related to long-term sales forecasts and discount rate, and (ii) the Alcon brand name, management’s significant assumptions related to long-term sales forecasts, discount rate and royalty rate. In addition, the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s goodwill and the Alcon brand name impairment assessments, including controls over the estimation of the fair value less costs of disposal. These procedures also included, among others, testing management’s process for developing the fair value less costs of disposal estimates; evaluating the appropriateness of the estimates; testing the completeness and accuracy of underlying data used; and evaluating the significant assumptions used by management related to long-term sales forecasts, discount rates and royalty rate. Evaluating management’s assumptions related to long-term sales forecasts involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the business, (ii) the consistency with external market and industry data, and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in the evaluation of management's valuation method and the discount rate and royalty rate significant assumptions.
In-Process Research and Development Intangible Asset Impairment Assessments
As described in Notes 2 and 9 to the consolidated financial statements, as of December 31, 2023 the Company had $739 million of in-process research and development (IPR&D) intangible assets. IPR&D is evaluated for potential impairment on an annual basis or when facts and circumstances warrant. IPR&D is considered impaired when its carrying amount exceeds its estimated recoverable amount, which is defined as the higher of its fair value less costs of disposal and its value in use. Usually, management applies the fair value less costs of disposal method for its impairment assessments. Under this approach, fair value less costs of disposal is estimated using net present value techniques utilizing post-tax cash flows and discount rates as there are no direct or indirect observable prices in active markets for identical or similar assets. The estimates of fair value less costs of disposal involve significant judgment by management and include assumptions with measurement uncertainty, such as the amount and timing of projected cash flows, long-term sales forecasts, discount rate and the timing and probabilities of success.
The principal considerations for our determination that performing procedures relating to the IPR&D intangible asset impairment assessment is a critical audit matter are the significant judgment by management when estimating the fair
F-73
value less costs of disposal, which in turn led to a high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating management’s significant assumptions related to long-term sales forecasts, discount rates and probabilities of success. In addition, the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s IPR&D intangible asset impairment assessments, including controls over the estimation of the fair value less costs of disposal. These procedures also included, among others, testing management’s process for developing the fair value less costs of disposal estimates; evaluating the appropriateness of the estimates; testing the completeness and accuracy of underlying data used; and evaluating the significant assumptions used by management related to long-term sales forecasts, discount rates and probabilities of success. Evaluating management’s assumptions related to long-term sales forecasts and probabilities of success involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the business, and (ii) whether these assumptions were consistent with evidence obtained in other areas of the audit. Evaluating management’s assumption related to long-term sales forecasts also involved considering consistency with external market and industry data. Professionals with specialized skill and knowledge were used to assist in the evaluation of management's valuation method and the discount rates significant assumption.
/s/ PricewaterhouseCoopers LLP
Fort Worth, Texas
February 27, 2024
We have served as the Company’s auditor since 2019.
F-74