UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
For the fiscal year ended December 31 , 2023
For the transition period from _____ to _____
Commission File Number 000-23186
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | ||||
(Address of principal executive offices)
(919 ) 859-1302
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Securities registered pursuant to Section 12(g) of the Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
x | Accelerated filer | o | ||||||||||||
| Non-accelerated filer | o | Smaller reporting company | ||||||||||||
| Emerging growth company | ||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. x
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The registrant estimates that the aggregate market value of the Common Stock on June 30, 2023 (based upon the closing price shown on the Nasdaq Global Select Market on June 30, 2023) held by non-affiliates was $1,322,900,797 .
The number of shares of Common Stock, par value $0.01, of the registrant outstanding as of February 23, 2024 was 206,149,929 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement to be filed in connection with the solicitation of proxies for its 2024 annual meeting of stockholders are incorporated by reference into Items 10, 11, 12, 13, and 14 under Part III hereof.
TABLE OF CONTENTS
| Page No. | |||||
i
When used in this report, unless otherwise indicated, “we,” “our,” “us,” the “Company,” and “BioCryst” refer to BioCryst Pharmaceuticals, Inc. and, where appropriate, its subsidiaries.
Cautionary Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K (this “report”) includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are subject to the “safe harbor” created in Section 21E. In particular, statements about our expectations, beliefs, plans, objectives or assumptions of future events or performance are contained or incorporated by reference in this report. All statements other than statements of historical facts contained herein are forward-looking statements. These forward-looking statements can generally be identified by the use of words such as “may,” “will,” “intends,” “plans,” “believes,” “anticipates,” “expects,” “estimates,” “predicts,” “potential,” the negative of these words or similar expressions. Statements that describe our future plans, strategies, intentions, expectations, objectives, goals or prospects are also forward-looking statements. Discussions containing these forward-looking statements are principally contained in the “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this report, as well as any amendments we make to those sections in filings with the Securities and Exchange Commission (“SEC”). These forward-looking statements include, but are not limited to, statements about:
•the preclinical development, clinical development, commercialization, or post-marketing studies of our products and product candidates, including ORLADEYO® (berotralstat), BCX10013, and early-stage discovery programs (including BCX17725, avoralstat, and our complement inhibitors), and our plans regarding the same;
•the potential for out-licensing of late-stage development and commercialization of BCX10013;
•our discovery and commercialization of best-in-class and first-in-class medicines;
•the timing and success of our commercialization of ORLADEYO in the United States and elsewhere and expectations regarding the commercial market for ORLADEYO;
•additional regulatory approvals, or milestones, royalties or profit from sales of our products by us or our partners;
•the implementation of our business model, strategic plans for our business, products, product candidates and technology;
•our ability to establish and maintain collaborations or out-license rights to our products and product candidates;
•plans, programs, progress and potential success of our collaborations, including with Torii Pharmaceutical Co., Ltd. (“Torii”) for ORLADEYO in Japan and Shionogi & Co., Ltd. (“Shionogi”) and Green Cross Corporation (“Green Cross”) for peramivir in their territories;
•our and our subsidiary guarantors’ ability to satisfy obligations under the Pharmakon Loan Agreement (as defined below) and to comply with the covenants as set forth in the agreements governing our debt obligations;
•the scope of protection we are able to establish and maintain for intellectual property rights covering our products, product candidates, and technology;
•our ability to operate our business without infringing the intellectual property rights of others;
•estimates of our revenues, expenses, capital requirements, annual cash utilization, and our needs for additional capital or financing;
•the timing or likelihood of regulatory filings or regulatory agreements, deferrals, approvals, and other decisions;
•our ability to manage our liquidity needs to fund our operations or repay our recourse debt obligations;
•our financial performance;
1
•statements and projections regarding financial goals, including timing for achieving profitability or positive cash flow;
•our ability to remediate any material weakness in our internal control over financial reporting; and
•competitive companies, technologies, and our industry.
We have based any forward-looking statements on our current expectations about future events or performance. While we believe these expectations are reasonable, forward-looking statements are inherently subject to known and unknown risks and uncertainties, many of which are beyond our control. Actual results may differ materially from those suggested or implied by these forward-looking statements for various reasons, including those discussed in this report under the heading “Risk Factors” in Part I, Item 1A, some of which are summarized in the “Risk Factor Summary” below. Any forward-looking statement is subject to these and other risks, uncertainties, and assumptions relating to our operations, results of operations, industry, and future growth. Given these risks and uncertainties, you are cautioned not to place undue reliance on our forward-looking statements. The forward-looking statements included in this report are made only as of the date hereof. We do not undertake, and specifically decline, any obligation to update any of these statements or to publicly announce the results of any revisions to any forward-looking statements to reflect future events or developments, except as may be required by U.S. federal securities laws.
Risk Factor Summary
An investment in the Company involves risks. You should carefully read this entire report and consider the uncertainties and risks discussed in the “Risk Factors” section in Part I, Item 1A of this report, which may adversely affect our business, financial condition, or results of operations, along with the other information included in our other filings with the SEC, before making an investment decision in the Company. A summary of the principal factors that make an investment in the Company speculative or risky is set forth below.
•We have incurred losses since our inception and may never be profitable.
•We may need to raise additional capital in the future. If we are unable to raise capital if and when needed, we may need to adjust our operations.
•Our success depends upon our ability to manage our product candidate pipeline, advance our product candidates through the various stages of development, especially through the clinical trial process, and to receive and maintain regulatory approvals for the commercial sale of our product candidates. The development process and related regulatory processes are complex and uncertain, may be lengthy and expensive, and require, among other things, an indication that our products and product candidates are safe and effective. For example, applicable regulatory agencies could refuse to approve, or impose restrictions or warnings on, our product candidates, require us to conduct additional studies or adopt study designs that differ from our planned development strategies, suspend or terminate our clinical trials, withdraw approval for our products, or take other actions that could materially impact the cost, timing, and success of our planned development and commercialization strategies.
•We rely heavily upon third parties, including development partners, contractors, contract research organizations, and third-party suppliers, manufacturers, and distributors, for many important stages of our product candidate development and in the commercialization of certain of our products and product candidates. Our failure to establish and maintain these relationships, the failure of any such third party to perform its obligations under agreements with us, or the failure of such a relationship to meet our expectations could have a material adverse impact on our business, financial condition, and results of operations.
•If we fail to obtain additional financing or acceptable partnership arrangements if and when needed, we may be unable to complete the development and commercialization of our products and product candidates or continue operations.
•If the FDA or comparable foreign regulatory authorities approve generic versions of any of our products that receive marketing approval, the sales of our products could be adversely affected.
2
•The commercial viability of any approved product could be compromised if the product is less effective than expected, causes undesirable side effects that either were not previously identified or were worse than expected, or fails to achieve market acceptance by physicians, patients, third-party payors, health authorities, and others.
•There can be no assurance that our or our partners’ commercialization efforts, methods, and strategies for our products or technologies will succeed, and our future revenue generation is uncertain.
•We have expanded, and may continue expanding, our development and regulatory capabilities and are implementing sales, marketing, and distribution capabilities, and as a result, we may encounter difficulties managing our growth, which could disrupt our operations.
•We face intense competition, and if we are unable to compete effectively, the demand for our products may be reduced. In addition, developments by others may render our products, product candidates, or technologies obsolete or noncompetitive.
•We are subject to various laws and regulations related to our products and product candidates, and if we or our employees, consultants, or partners do not comply with these laws and regulations, we could face substantial penalties and our reputation could be harmed. In addition, we and our partners may be subject to new legislation, regulatory proposals, and healthcare payor initiatives that may increase our costs of compliance and adversely affect our or our partners’ ability to market our products or develop our product candidates.
•If we fail to adequately protect or enforce our intellectual property rights, the value of those rights would diminish. Legal proceedings to protect or enforce our patents, the patents of our partners, or our other intellectual property rights could be expensive, time consuming, and unsuccessful. If we fail to secure the rights to patents of others, this could adversely affect our business.
•We face an inherent risk of liability in the event that the use or misuse of our products or product candidates results in personal injury or death, and our product liability insurance coverage may be insufficient.
•If we fail to reach milestones or to make annual minimum payments or otherwise breach our obligations under our license agreements, our licensors may terminate our agreements with them and/or seek additional remedies.
•The Pharmakon Loan Agreement contains conditions and restrictions that limit our flexibility in operating our business. We may be required to make a prepayment or repay our outstanding indebtedness under the Pharmakon Loan Agreement earlier than we expect if a prepayment event or an event of default occurs, including a material adverse change with respect to us, which could have a material adverse effect on our business.
•International expansion of our business exposes us to business, legal, regulatory, political, operational, financial, and economic risks. For example, our actual or perceived failure to comply with European governmental laws and regulations and other obligations related to privacy, data protection, and information security could harm our business. In addition, the United Kingdom’s withdrawal from the European Union could result in increased regulatory and legal complexity, which may make it more difficult for us to do business in Europe and impose additional challenges in securing regulatory approval of our product candidates in Europe.
•If our facilities incur damage or power is lost for a significant length of time, our business will suffer.
•Cyber incidents and related disruptions in our or our third-party vendors’ information technology systems could adversely affect our business.
•Our business, operations, clinical development or commercialization plans and timelines, and access to capital could be adversely affected by unpredictable and unstable market and economic conditions.
•If we fail to retain our existing key personnel or fail to attract and retain additional key personnel, the development of our product candidates, the commercialization of our products, and the related expansion of our business will be delayed or stopped.
•Future acquisitions, strategic investments, partnerships, alliances, or divestitures could fail to meet our expectations and/or adversely affect our operating results and financial condition.
3
•Our existing principal stockholders hold a substantial amount of our common stock and may be able to influence significant corporate decisions, which may conflict with the interests of other stockholders.
•Our stock price has been, and is likely to continue to be, highly volatile, which could cause the value of an investment in our common stock to decline significantly.
•We have identified a material weakness in our internal control over financial reporting. This material weakness could divert management’s attention and adversely affect our ability to produce accurate and timely financial statements, which may adversely affect investor confidence in us and our financial reporting, adversely affect our business and operating results and may negatively impact the trading price of our common stock.
•Natural disasters, epidemic or pandemic disease outbreaks, trade wars, armed conflicts, political unrest, or other events could disrupt our business or operations or those of our development partners, manufacturers, regulators, or third parties with whom we conduct business now or in the future.
•We are subject to legal proceedings, which could harm our reputation or result in other losses or unexpected expenditure of time and resources.
4
PART I
ITEM 1. BUSINESS
Our Business
We are a global biotechnology company with a deep commitment to improving the lives of people living with complement-mediated and other rare diseases. We leverage our expertise in structure-guided drug design with the goal of developing first-in-class or best-in-class oral small-molecule and protein therapeutics to target difficult-to-treat rare diseases. Structure-guided drug design is a drug discovery approach by which we design synthetic compounds from detailed structural knowledge of the active sites of targets associated with particular diseases. We use X-ray crystallography, computer modeling of molecular structures and advanced chemistry techniques to focus on the three-dimensional molecular structure and active site characteristics of the protein targets that control cellular biology. Our goal generally is to design a compound that will fit in the active site of a protein target and thereby prevent its activity.
In addition to these discovery and development efforts, our business strategy includes the efficient commercialization of these drugs in the United States and certain other regions upon regulatory approval. By focusing on rare disease markets, we believe that we can more effectively control the costs of, and our strategic allocation of financial resources toward, post-approval commercialization. Molecules from our discovery efforts that are commercially available or that are in active development are summarized in the table below and are discussed in further detail under “Products and Product Candidates” in this “Part I—Item 1—Business” section of this report.
| Drug/Drug Candidate | Drug Class | Therapeutic Area(s) | Phase | Rights* | ||||||||||||||||||||||
ORLADEYO® (berotralstat) | Oral Serine Protease Inhibitor Targeting Kallikrein (once-daily treatment) | Hereditary angioedema | Approved (United States and multiple global markets) | BioCryst (worldwide)** | ||||||||||||||||||||||
| Oral Serine Protease Inhibitor Targeting Kallikrein (once-daily treatment for patients who are 2 to <12 years of age) | Hereditary angioedema | Phase 3 | BioCryst (worldwide)** | |||||||||||||||||||||||
| BCX10013 | Oral Factor D Inhibitor | Complement-mediated diseases | Phase 1 | BioCryst (worldwide) | ||||||||||||||||||||||
RAPIVAB® (peramivir injection) | Intravenous Neuraminidase Inhibitor | Acute uncomplicated Influenza | Approved (United States, Australia & Canada) | BioCryst (worldwide, except Japan, Taiwan, Korea and Israel) | ||||||||||||||||||||||
RAPIACTA® (peramivir injection) | Intravenous Neuraminidase Inhibitor | Uncomplicated seasonal Influenza | Approved (Japan & Taiwan) | Shionogi & Co., Ltd. (Japan & Taiwan) | ||||||||||||||||||||||
PERAMIFLU® (peramivir injection) | Intravenous Neuraminidase Inhibitor | Uncomplicated seasonal Influenza | Approved (Korea) | Green Cross Corporation (Korea) | ||||||||||||||||||||||
* See “Business—Collaborations and In-License Relationships” for a description of our relationships with the third parties identified in this column.
** We share rights in Japan with Torii Pharmaceutical Co., Ltd.
5
In addition to the molecules referenced in the table above, we are pursuing medicines directed at other targets across the classical, lectin and alternative pathways of the complement system, as well as inhibitors designed to treat patients with Netherton syndrome and diabetic macular edema. See “Business—Products and Product Candidates” below for additional details.
Business Strategy
Our business strategy is twofold: to serve patients and to create stockholder value both by focusing our discovery and development efforts on potential first-in-class or best-in-class oral small-molecule and protein therapeutics to target difficult-to-treat rare diseases and by efficiently commercializing these drugs in the United States and certain other regions upon regulatory approval. By focusing on rare disease markets, we believe that we can more effectively control the costs of, and our strategic allocation of financial resources toward, post-approval commercialization.
We select disease targets and product candidates in which an oral small molecule or protein therapeutic has the potential to be best-in-class or would be the first to market. We strive to advance our product candidate portfolio from discovery to commercial markets efficiently by utilizing a small group of talented and highly-skilled employees working in conjunction with strategic outsource partners. We are unique in our approach to treat difficult-to-treat rare diseases with orally-administered small molecules and with protein therapeutics, each identified by utilizing crystallography and structure-guided drug design. The principal elements of our strategy are:
•Focusing on High Value-Added Structure-Guided Drug Design Technologies. We utilize structure-guided drug design in order to most efficiently develop new therapeutic candidates. Structure-guided drug design is a process by which we design a product candidate through detailed structural analysis of the protein target, which the product candidate must inhibit in order to stop the progression of the disease or disorder. We believe that structure-guided drug design is a powerful tool for the efficient development of small-molecule and protein therapeutic product candidates that have the potential to be safe and effective. Our structure-guided drug design technologies typically allow us to design and synthesize multiple product candidates that inhibit the same protein target, with the goal of establishing broad intellectual property protection and formulating compounds with competitive advantages.
•Selecting Inhibitors that are Promising Product Candidates. We start by selecting disease targets with well-understood biology and characteristics that fit with our ability to utilize structure-guided drug design capabilities to build potent and specific inhibitors. Next, we narrow our selection of these product candidates based on product characteristics, such as initial indications of safety and biologic activity on the target.
•Developing our Product Candidates Efficiently. An important element of our business strategy is to progress our product candidates efficiently through the development process. In order to accomplish this, we typically strive for disease targets with a defined clinical and regulatory pathway for approval or diseases where high unmet needs allow for frequent interactions with regulators. In addition, as we determine such relationships to be appropriate or desirable, we control certain fixed costs and overhead by outsourcing with strategic partners and contractors or entering into license agreements with third parties. By outsourcing certain aspects of our operations, we are able to control overhead costs and focus financial resources directly where they provide the most benefit and reduce our business risk.
•Commercializing our Product Candidates Globally. A core part of our strategy is to commercialize our rare disease products globally. We have established commercial teams in the United States and other global markets for the commercialization of ORLADEYO, and we are continuing to build the structure and expertise to commercialize our products in additional markets where we believe we can do this efficiently and effectively. We have also established relationships with licensing, distribution and other partners in certain markets, including Japan, the pan-Latin America region, and parts of Europe and Asia, and will continue to seek such relationships where we determine this to be an effective approach.
Products and Product Candidates
ORLADEYO (Berotralstat)
ORLADEYO is an oral, once-daily therapy discovered and developed by us for the prevention of hereditary angioedema (“HAE”) attacks. HAE is a rare, severely debilitating and potentially fatal genetic condition with an estimated
6
prevalence of between 1 in 33,000 to 1 in 67,000 people. HAE symptoms include recurrent episodes of edema in various locations, including the hands, feet, face, genitalia and airway. Airway swelling is particularly dangerous and can lead to death by asphyxiation. In addition, patients often have bouts of severe abdominal pain, nausea and vomiting caused by swelling in the intestinal wall. By inhibiting plasma kallikrein, ORLADEYO suppresses bradykinin production. Bradykinin is the mediator of acute swelling attacks in HAE patients.
ORLADEYO was approved by the U.S. Food and Drug Administration (“FDA”) in December 2020 for prophylaxis to prevent attacks of HAE in adults and pediatric patients 12 years and older. Our specialty pharmacy provider for ORLADEYO in the United States began shipping ORLADEYO to patients with a prescription in the United States in December 2020. Through EMPOWER Patient Services, administered by our specialty pharmacy provider, we aim to streamline access to therapy by providing each HAE patient and their healthcare provider with a single point of contact for access to ORLADEYO. A dedicated care coordinator supports access for each patient with comprehensive financial support tools and reimbursement support.
In 2021, we announced that ORLADEYO received approvals in the European Union (the “EU”), Japan, the United Arab Emirates (“UAE”) and the United Kingdom, and in 2022, we announced that ORLADEYO received approvals in Canada, Switzerland, Saudi Arabia, and Israel.
Under our agreement with Torii, our collaborative partner for the commercialization of ORLADEYO in Japan, we are entitled to receive tiered royalty payments, ranging from 20% to 80% of net sales of ORLADEYO in Japan during each calendar year. See “Collaborations and In-License Relationships” below for a description of our relationship with Torii.
On January 23, 2023, we announced that the Company entered into a collaboration with Swixx BioPharma AG (“Swixx”) to commercialize ORLADEYO in Central and Eastern Europe (“CEE”). Under the terms of the agreement, Swixx will be responsible for commercializing ORLADEYO in 15 markets within CEE.
On January 26, 2023, we announced the enrollment of the first patient in the pivotal APeX-P trial evaluating ORLADEYO in pediatric HAE patients who are 2 to <12 years of age.
On February 21, 2023, we announced that the Canadian Agency for Drugs and Technologies in Health Canadian Drug Expert Committee issued a final draft positive recommendation for ORLADEYO to be reimbursed for the routine prevention of HAE attacks in adults and pediatric patients 12 years of age and older.
On February 24, 2023, we announced that new data from the APeX-S and APeX-2 clinical trials, which evaluated ORLADEYO for the prophylactic treatment of HAE, demonstrated sustained reductions in attack rates and improvement in quality of life among patients living with HAE, highlighting its profile as a well-tolerated, effective and convenient prophylactic HAE therapeutic option. We also announced additional analyses from new real-world data that further demonstrate a meaningful reduction in attack rates experienced by patients on ORLADEYO, in addition to findings from a survey that underscore a significant disease and treatment burden among pediatric HAE patients, as reported by their caregivers.
On April 27, 2023, we announced that new data from the APeX-S clinical trial, which evaluated ORLADEYO for the prophylactic treatment of HAE, showed sustained reduction in disease burden for patients across multiple subgroups through 96 weeks of treatment.
On May 22, 2023, we announced that the Public Health Institute of Chile granted marketing authorization for ORLADEYO for the prophylaxis of HAE in patients 12 years of age or older. We have an exclusive collaboration with Pint Pharma GmbH (“Pint Pharma”) to register and promote ORLADEYO in the pan-Latin America region. Under the terms of the agreement, Pint Pharma is responsible for obtaining and maintaining all marketing authorizations and for commercializing ORLADEYO in the region.
On July 19, 2023, we announced that we entered into a collaboration with Er-Kim Pharmaceuticals to commercialize ORLADEYO in Turkey.
On November 2, 2023, we announced that Austria approved the reimbursement of ORLADEYO for the targeted prophylaxis of HAE in patients 12 years of age or older.
7
On November 3, 2023, we announced that we expect to submit a U.S. supplemental new drug application for the pediatric use of ORLADEYO in 2025. The ongoing APeX-P clinical trial is assessing an oral granule formulation of ORLADEYO in pediatric HAE patients who are 2 to <12 years of age.
On November 10, 2023, we announced new analyses of real-world use of ORLADEYO leading to a reduction in monthly attack rates in patients with HAE who have normal C1-inhibitor level and function. We also announced a new post-hoc analysis from the APeX-S clinical trial that showed a sustained reduction in HAE attacks compared to patients’ self-reported baseline attack rates.
On November 21, 2023, we announced that the Spanish Ministry for Health granted marketing authorization for ORLADEYO for the routine prevention of recurrent HAE attacks in HAE patients 12 years and older.
On November 29, 2023, we announced that the National Administration of Drugs, Foods, and Medical Devices in Argentina granted approval for ORLADEYO for the prophylaxis of HAE attacks in adults and pediatric patients 12 years of age or older.
On December 19, 2023, we announced that data from the open-label extension of the APeX-2 trial of ORLADEYO for the prophylactic treatment of HAE in patients 12 years and older have been published online by the Journal of Allergy and Clinical Immunology: In Practice (JACI: In Practice). The authors concluded that ORLADEYO was generally well tolerated, provided rapid and sustained reductions in HAE attacks and improved quality of life over the study duration of 96 weeks.
On February 19, 2024, we announced that the Italian Medicines Agency finalized reimbursement and recommended ORLADEYO for the routine prevention of recurrent attacks of HAE in eligible patients 12 years and older.
On February 23, 2024, we announced new analyses of real-world use of ORLADEYO that showed patients who initiated ORLADEYO experienced rapid, substantial and sustained reductions in attack rates through 18 months of treatment regardless of the severity of their disease, their history of prior prophylaxis or their C1-inhibitor level and function.
On each of December 7, 2020 and November 19, 2021, we entered into a Purchase and Sale Agreement with RPI 2019 Intermediate Finance Trust (“RPI”), pursuant to which we sold to RPI the right to receive certain royalty payments from the Company (the “RPI Royalty Purchase Agreements”). On November 19, 2021, we also entered into a Purchase and Sale Agreement (the “OMERS Royalty Purchase Agreement” and, collectively with the RPI Royalty Purchase Agreements, the “Royalty Purchase Agreements”) with OCM IP Healthcare Holdings Limited, an affiliate of OMERS Capital Markets (“OMERS”), pursuant to which we sold to OMERS the right to receive certain royalty payments from the Company. The transactions contemplated under the Royalty Purchase Agreements are referred to herein as the “Royalty Sales.” See “Note 8—Royalty Financing Obligations—ORLADEYO and Factor D Inhibitors” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report for additional information about our obligations under the Royalty Purchase Agreements.
We have built out our U.S. commercial infrastructure to support the launch and continued commercialization of ORLADEYO in the United States and are continuing to build our commercial infrastructure to support launches in other markets. Based on proprietary analyses of HAE prevalence and market research studies with HAE patients, physicians, and payors in the United States and Europe, and three full years of commercialization experience with ORLADEYO in the United States from 2021 through 2023, we anticipate the global commercial market for ORLADEYO has the potential to reach a global peak of $1 billion in annual net ORLADEYO revenues. We expect approximately 80 percent of our revenue at peak to come from the United States. Based on three full years of commercialization experience with ORLADEYO, we believe there is a seasonal impact to our business in the first quarter of each year due to typical first quarter requirements from payors for prescription reauthorization of specialty products, like ORLADEYO, that can temporarily move patients from paid drug to free product. These expectations are subject to numerous risks and uncertainties that may cause our actual results, performance, or achievements to be materially different. There can be no assurance that our commercialization methods and strategies will succeed, or that the market for ORLADEYO will develop in line with our current expectations. See “Risk Factors—Risks Relating to Our Business—Risks Relating to Drug Development and Commercialization—There can be no assurance that our or our partners’ commercialization efforts, methods, and strategies for our products or technologies will succeed, and our future revenue generation is uncertain” in Part I, Item 1A of this report for further discussion of these risks.
8
Complement-Mediated Diseases
The goal of our overall complement program is to advance several first-in-class and/or best-in-class compounds across multiple pathways in the complement system to treat many complement-mediated diseases. The complement system is part of the body’s natural immune system and is responsible for helping the body eliminate microbes (including viral and bacterial infections) and damaged cells. It is comprised of proteins that are primarily produced in the liver and circulate in the blood. Once activated, the complement system stimulates inflammation, phagocytosis and cell lysis. Excessive or uncontrolled activation of the complement system can cause severe, and potentially fatal, immune and inflammatory disorders. The complement system comprises biological cascades of amplifying enzyme cleavages involving more than 30 proteins and protein fragments, and may be activated through three pathways: the classical pathway (initiated by antibody-antigen complexes), the lectin pathway (initiated by lectin binding) and the alternative pathway (initiated by microbial surfaces). The alternative pathway also provides a critical amplification loop for all three pathways, regardless of the initiating mechanism. Several rare diseases are known to be mediated by dysregulation of the complement system.
BCX10013
BCX10013 is a potential once-daily, oral Factor D inhibitor, which targets the alternative pathway of complement. Factor D is an essential enzyme in the alternative pathway, thus making Factor D an attractive target to address complement-mediated diseases. On January 9, 2023, we announced that initial data from ongoing phase 1 single ascending dose and multiple ascending dose trials in healthy volunteers showed rapid, sustained and >97 percent suppression of the alternative pathway of the complement system 24 hours following a single 110 mg dose, and that BCX10013 had been safe and generally well-tolerated at all doses studied to date. On February 21, 2023, we announced that dose-related observations in an ongoing BCX10013 nonclinical study would delay the clinical program.
On August 3, 2023, we announced that we began opening clinical trial sites for a dose-ranging trial in patients with paroxysmal nocturnal hemoglobinuria (“PNH”) and expected to begin patient enrollment (in countries without other approved therapies) by the end of the year. On October 26, 2023, we announced the enrollment of the first patient in a proof-of-concept clinical trial evaluating BCX10013, and on November 3, 2023, we announced that we expect to report data from our ongoing proof-of-concept trial in 2024. On November 3, 2023, we also presented data at our R&D Day from the recently completed 160 mg cohort of our multiple ascending dose healthy volunteer trial, which highlights the strength and duration of alternative pathway suppression achieved at this dose level, supporting once-daily clinical dosing.
On January 5, 2024, we announced that, if our ongoing proof-of-concept trial produces best-in-class data, we plan to out-license late-stage development and commercialization of BCX10013 to a partner that can drive the speed and breadth of investment required to accelerate BCX10013 for patients across multiple complement-mediated diseases and maximize the commercial potential of the program.
Under the RPI Royalty Purchase Agreements, RPI will be entitled to receive tiered, sales-based royalties on net product sales, if any, of BCX10013, as well as tiered, profit share amounts from certain other permitted sales in certain markets. See “Note 8—Royalty Financing Obligations—ORLADEYO and Factor D Inhibitors” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report for additional information about our obligations under the RPI Royalty Purchase Agreements.
Oral C5 Inhibitor
On November 3, 2023, we announced that we are developing an oral C5 inhibitor that could be the first targeted oral therapy with competitive efficacy to currently-approved injected and infused anti-C5 therapies, such as eculizumab and ravulizumab. A drug with this profile could enable patients with generalized myasthenia gravis (“gMG”) to switch from infused therapy and address their disease earlier in the treatment paradigm. gMG is a chronic autoimmune, neuromuscular disease that causes muscle weakness that worsens after periods of activity.
Bifunctional Complement Inhibitor
On November 3, 2023, we announced that we are developing a bifunctional complement inhibitor anti-C2 monoclonal antibody that also inhibits the alternative pathway. This investigational candidate could be a first-in-class combined inhibitor of the classical, lectin and alternative pathways of the complement system to treat complex renal complement-mediated diseases like Immunoglobulin A Nephropathy (“IgAN”) and lupus nephritis, which are influenced by multiple complement pathways.
9
Oral C2 Inhibitor
On November 3, 2023, we announced that we are developing a classical and lectin pathway complement inhibitor to treat autoimmune hemolytic anemias, including cold agglutinin disease (“CAD”) and warm autoimmune hemolytic anemia (“wAIHA”). The limited approved options for treating diseases like CAD and wAIHA are injectable or infused. An oral C2 inhibitor developed by us could be first-in-class and allow patients to switch from infused therapy and address their disease earlier in the treatment paradigm. Inhibiting C2 could decrease red cell destruction (hemolysis) in autoimmune hemolytic anemias by blocking the classical and lectin pathways.
Netherton Syndrome
On November 3, 2023, we announced that we are developing BCX17725, a potent and selective investigational fusion protein KLK5 inhibitor designed to provide best-in-class, potentially disease-modifying treatment for people with Netherton syndrome. Netherton syndrome is a rare, lifelong genetic disorder that often presents in neonates or infancy. The disease is caused by the deficiency of a natural inhibitor (SPINK5) of KLK5, a serine protease responsible for regulating skin shedding. Patients may have red, scaly and inflamed skin and susceptibility to recurrent immune reactions. Netherton syndrome can be life-threatening, especially during infancy when patients are vulnerable to dehydration and recurrent infections. Currently, there is no approved treatment for Netherton syndrome.
Avoralstat
On November 3, 2023, we announced that we entered into a license agreement (the “Clearside Agreement”) with Clearside Biomedical, Inc. (“Clearside”), enabling us to develop our investigational plasma kallikrein inhibitor, avoralstat, with Clearside’s SCS Microinjector® to deliver avoralstat to the back of the eye through the suprachoroidal space to treat patients with diabetic macular edema (“DME”). DME is the most common cause of vision loss in individuals with diabetes, and at least one-third of patients have persistent DME despite anti-vascular endothelial growth factor therapies, which are administered via monthly injection. Avoralstat, which was previously studied in an oral formulation in a phase 3 trial in patients with HAE, has high potency and low solubility, two characteristics we believe are important to achieving potential efficacy with reduced dosing frequency in the eye for DME patients.
Under the Clearside Agreement, Clearside received a $5.0 million upfront license fee from us. Clearside is eligible to receive up to an additional $30.0 million in clinical and regulatory milestone payments, and up to a total of $47.5 million in three post-approval sales-based milestone payments as annual global net sales progress to $2.0 billion. We will pay Clearside tiered mid-single digit royalties on annual global net product sales, at three tiers, including a top tier of >$1.5 billion.
Peramivir Injection (RAPIVAB, RAPIACTA, PERAMIFLU)
RAPIVAB (peramivir injection) was developed under a $234.8 million contract from the Biomedical Advanced Research and Development Authority within the U.S. Department of Health and Human Services (“BARDA/HHS”). In January 2010, our partner, Shionogi, received the first approval for peramivir injection and launched it in Japan under the commercial name RAPIACTA. It is approved in Japan for the treatment of adults, children, and infants with uncomplicated seasonal influenza and those patients at high-risk for complications associated with influenza. In August 2010, our partner, Green Cross, received marketing and manufacturing approval from the Korean Food & Drug Administration under the commercial name PERAMIFLU to treat patients with influenza A & B viruses, including pandemic H1N1 and avian influenza. See “Collaborations and In-License Relationships” below for a discussion of these licensing arrangements.
Peramivir was also approved in the United States in 2014, Taiwan in 2016, Canada in 2017, and Australia in 2018. A Supplemental New Drug Application (“sNDA”) was approved in the United States in February 2021, extending RAPIVAB’s availability for the treatment of acute uncomplicated influenza to pediatric patients six months and older. Prior to this approval, peramivir had been indicated in the United States for pediatric patients two years and older. In the United States, peramivir is indicated for the treatment of acute uncomplicated influenza in patients who have been symptomatic for no more than two days.
In September 2018, the U.S. Department of Health and Human Services (“HHS”) awarded us a $34.7 million contract for the procurement of up to 50,000 doses of RAPIVAB over a five-year period to supply the Strategic National Stockpile for use in a public health emergency. We initially delivered 20,000 doses of RAPIVAB under this contract in 2019 for a total price of approximately $13.9 million. We further delivered 20,000 and 9,980 doses of RAPIVAB in 2022
10
and 2021, respectively, and recorded revenue of $13.9 million and $6.9 million for the years ending December 31, 2022 and 2021, respectively. As of December 31, 2022, we had delivered a total of 49,980 RAPIVAB doses of the 50,000 RAPIVAB doses available under the contract, effectively completing the contract with HHS.
Collaborations and In-License Relationships
ORLADEYO
Torii Pharmaceutical Co., Ltd. (“Torii”)
On November 5, 2019, we entered into a Commercialization and License Agreement with Torii (the “Original Torii Agreement”), granting Torii the exclusive right to commercialize ORLADEYO for the prevention of HAE attacks in Japan. Under the Original Torii Agreement, we received an upfront, non-refundable payment of $22.0 million. We received an additional milestone payment of $15.0 million in the second quarter of 2021 upon receipt from the Japanese National Health Insurance System (“NHI”) of a reimbursement price approval for ORLADEYO. On November 30, 2023, we entered into an Amended and Restated Commercialization and License Agreement with Torii (as amended, the “Torii Agreement”).
Under the Torii Agreement, we are entitled to receive tiered royalty payments, ranging from 20% to 80% of annual net sales of ORLADEYO in Japan during each calendar year. We are now responsible for all commercial promotion activities to support ORLADEYO sales in Japan, and Torii will be responsible for HAE disease awareness activities in Japan. We will receive a 20% royalty on annual Japanese sales below a prespecified threshold and an 80% royalty on annual Japanese sales above the prespecified threshold.
Torii’s updated royalty payment obligations commenced upon November 30, 2023 and expire upon the later of (i) the tenth anniversary of the date of first commercial sale of ORLADEYO in Japan, (ii) the expiration of our patents covering ORLADEYO, and (iii) the expiration of regulatory exclusivity for ORLADEYO in Japan.
Other Collaborations for ORLADEYO
We have entered into a number of collaborations with commercial partners to help support the global launch of ORLADEYO. In 2021, we entered into supply and distribution agreements with Neopharm Ltd. (“Neopharm”) and NewBridge Pharmaceuticals (“NewBridge”) to support commercialization efforts in Israel and the UAE, respectively. Under the terms of these agreements, Neopharm will have the exclusive rights to commercialize ORLADEYO in Israel and the Palestinian Authority, and NewBridge will support commercialization efforts in the UAE, as well as the Gulf Cooperation Council and Iraq. On June 9, 2022, we announced that we have entered into an exclusive collaboration with Pint Pharma to register and promote ORLADEYO in the pan-Latin America region. Under the terms of the agreement, Pint Pharma will be responsible for obtaining and maintaining all marketing authorizations and for commercializing ORLADEYO in the region. On January 23, 2023, we announced that we have entered into a collaboration with Swixx to commercialize ORLADEYO in CEE. Under the terms of the agreement, Swixx will be responsible for commercializing ORLADEYO in 15 markets within CEE. On July 19, 2023, we announced that we have entered into a collaboration with Er-Kim Pharmaceuticals to commercialize ORLADEYO in Turkey.
Peramivir Injection (RAPIVAB, RAPIACTA, PERAMIFLU)
Shionogi & Co., Ltd. (“Shionogi”)
In February 2007, we entered into a License, Development and Commercialization Agreement (as amended, supplemented or otherwise modified, the “Shionogi Agreement”), an exclusive license agreement with Shionogi to develop and commercialize peramivir in Japan for the treatment of seasonal and potentially life-threatening human influenza. In October 2008, we and Shionogi amended the Shionogi Agreement to expand the territory covered by the agreement to include Taiwan. The Shionogi Agreement provided for an upfront payment in exchange for the rights to injectable formulations of peramivir in Japan, development milestone payments (which have all been paid), commercial milestone payments, and royalty payments on product sales of peramivir, in accordance with the terms of the Shionogi Agreement.
Generally, all payments under the Shionogi Agreement are non-refundable and non-creditable, but they are subject to audit. Shionogi is responsible for all development, regulatory, and marketing costs in Japan. The term of the Shionogi Agreement is from February 28, 2007 until terminated. Either party may terminate the Shionogi Agreement in the event of
11
an uncured breach. Shionogi has the right of termination without cause. In the event of termination, all license and rights granted to Shionogi shall terminate and shall revert back to us. We developed peramivir under a license from the University of Alabama Birmingham (“UAB”) and have paid sublicense payments to UAB on the upfront payments and will owe sublicense payments on any future event payments and/or royalties received by us from Shionogi.
Shionogi Royalty Financing and Non-Recourse Notes Payable
On March 9, 2011, we completed a $30.0 million financing transaction to monetize certain future royalty and milestone payments under the Shionogi Agreement. Pursuant to the transaction, JPR Royalty Sub LLC, a wholly-owned subsidiary of the Company (“Royalty Sub”), issued $30.0 million in aggregate principal amount of its PhaRMA Senior Secured 14.0% Notes due 2020 (the “PhaRMA Notes”) in a private placement to institutional investors. The PhaRMA Notes were issued under an indenture, dated as of March 9, 2011 (the “Indenture”), by and between Royalty Sub and U.S. Bank National Association, as Trustee. We received net proceeds of $22.7 million from this transaction.
Principal and interest on the PhaRMA Notes are payable from, and are secured by the rights to royalty and milestone payments under the Shionogi Agreement, which were transferred by us to Royalty Sub in 2011. Royalty Sub’s obligations to pay principal and interest on the PhaRMA Notes are obligations solely of Royalty Sub and are without recourse to any other person, including the Company, except to the extent of our pledge of our equity interests in Royalty Sub in support of the PhaRMA Notes.
In September 2014, Royalty Sub was unable to pay the full amount of interest payable in September 2013 by the next succeeding payment date for the PhaRMA Notes, which was September 1, 2014. This inability constituted an event of default under the terms of the Indenture. As of December 31, 2023, the PhaRMA Notes remained in default. We wrote off the balance due under the PhaRMA Notes to other income as a debt extinguishment as of December 31, 2021. See “Note 8—Royalty Financing Obligations—RAPIACTA—Non-Recourse Notes Payable—Debt Extinguishment” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report for additional information about the write-off.
Green Cross Corporation (“Green Cross”)
In June 2006, we entered into an agreement with Green Cross to develop and commercialize peramivir in Korea. Under the terms of the agreement, Green Cross is responsible for all development, regulatory, and commercialization costs in Korea and we are entitled to share in profits resulting from the sale of peramivir in Korea, including the sale of peramivir to the Korean government for stockpiling purposes. Furthermore, Green Cross will pay us a premium over its cost to supply peramivir for development and any future marketing of peramivir products in Korea. Both parties have the right to terminate the agreement in the event of an uncured material breach. In the event of termination, all rights, data, materials, products, and other information would be transferred to us.
Additional Collaborations
We have previously entered into contracts with the U.S. Government, including the procurement contract with HHS for up to 50,000 doses of RAPIVAB over a five-year period to supply the Strategic National Stockpile for use in a public health emergency and contracts with the National Institute of Allergy and Infectious Diseases within the HHS (“NIAID/HHS”) and BARDA/HHS for the development of galidesivir, as more fully discussed in “Note 15—Collaborative and Other Relationships” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report. As of December 31, 2022, we had delivered a total of 49,980 RAPIVAB doses of the 50,000 RAPIVAB doses available under the procurement contract, effectively completing the contract with HHS, and all of our government funding for galidesivir has expired.
We also have non-material license agreements with certain third parties, such as Albert Einstein College of Medicine of Yeshiva University (“AECOM”), Industrial Research, Ltd. (“IRL”), and the University of Alabama at Birmingham (“UAB”), which require that we make certain payments related to development of the product candidates covered by these agreements, net sales on any resulting product made by us, and annual license fees. We licensed a series of potent inhibitors of purine nucleoside phosphorylase (“PNP”) from AECOM and IRL, as well as an exclusive worldwide license of galidesivir for any antiviral use, and we have agreements with UAB for influenza neuraminidase and complement inhibitors. There is currently no activity between us and UAB on these agreements, but when we license this technology, such as in the case of the Shionogi and Green Cross agreements, or commercialize products related to these programs, we will owe sublicense fees or royalties on amounts received.
12
As discussed above under “Products and Product Candidates” and in “Note 15—Collaborative and Other Relationships” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report, we entered into the Clearside Agreement to develop our investigational plasma kallikrein inhibitor, avoralstat, with Clearside’s SCS Microinjector® to deliver avoralstat to the back of the eye through the suprachoroidal space to treat patients with DME.
Patents and Proprietary Information
Our success will depend in part on our ability to obtain and enforce patent protection for our products, methods, processes, and other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and in other countries. We own or have rights to certain proprietary information, proprietary technology, issued and allowed patents and patent applications which relate to compounds we are developing. We actively seek, when appropriate, protection for our products, proprietary technology, and proprietary information by means of U.S. and foreign patents, trademarks, and contractual arrangements. In addition, we rely upon trade secrets and contractual arrangements to protect certain of our proprietary information, proprietary technology, and products and product candidates.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position for our technology will depend on our success in obtaining effective patent claims and enforcing those claims once granted. We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Our issued patents and those that may issue in the future, or those licensed to us, may be challenged, invalidated, rendered unenforceable or circumvented, which could limit our ability to stop competitors from marketing related products or the length of term of patent protection that we may have for our products. In addition, the rights granted under any issued patents may not provide us with competitive advantages against competitors with similar compounds or technology. Furthermore, our competitors may independently develop similar technologies or duplicate any technology developed by us in a manner that does not infringe our patents or other intellectual property. Because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before any of our product candidates or those developed by our partners can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
As of December 31, 2023, we have been issued approximately 39 U.S. patents that expire between 2027 and 2039 and that relate to our kallikrein inhibitor compounds, neuraminidase inhibitor compounds, broad-spectrum antiviral (“BSAV”) compounds, PNP inhibitor compounds, and complement-mediated disease program compounds. We have licensed a number of compounds protected by certain composition of matter patents from AECOM and IRL, totaling two additional U.S. patents that expire between 2024 and 2029. Additionally, we have approximately 28 Patent Cooperation Treaty or U.S. patent applications pending related to kallikrein inhibitor compounds, neuraminidase inhibitor compounds, BSAV compounds, PNP inhibitor compounds, KLK5 program compounds, and complement-mediated disease program compounds. Our pending applications may not result in issued patents, our patents may not cover the products of interest or may not be enforceable in all, or any, jurisdictions and our patents may not provide us with sufficient protection against competitive products or otherwise be commercially viable. After expiration of composition of matter patents for our products and product candidates, we may rely on data exclusivity, or in some cases, method of use patents. The enforceability of these patents varies from jurisdiction to jurisdiction and may not be allowed or enforceable in some territories where we may seek approval. We may not have the funds to continue patent prosecution or to defend all of our existing patents in our current patent estate and may selectively abandon patents or patent families worldwide or in certain territories.
Our success is also dependent upon the skills, knowledge and experience of our scientific and technical personnel, none of which is patentable. To help protect our rights, we require all employees, consultants, advisors and partners to enter into confidentiality agreements, which prohibit the disclosure of confidential information to anyone outside of BioCryst and, where possible, require disclosure and assignment to us of their ideas, developments, discoveries, and inventions. These agreements may not provide adequate protection for our trade secrets, know-how, or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information.
Competition
The pharmaceutical and biotechnology industries are intensely competitive. Many companies, including biotechnology, chemical and pharmaceutical companies, are actively engaged in activities similar to ours, including research, development, and commercialization of drugs for the treatment of rare medical conditions. Many of these
13
companies have substantially greater financial and other resources, larger research and development staffs, and more extensive commercial and manufacturing organizations than we do. In addition, many have considerable experience in preclinical testing, clinical trials, and other regulatory approval procedures. In addition, there are also academic institutions, governmental agencies and other research organizations who conduct research in areas in which we are working.
We expect to encounter significant competition for any of the pharmaceutical products we plan to develop. Companies that successfully complete clinical trials, obtain required regulatory approvals, and commence commercial marketing and sales of their products may achieve a significant competitive advantage. Our commercial potential could also be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than products that we may develop. Any of these competitive factors may impact our decisions with respect to our products, product candidates and early-stage discovery programs. See “Risk Factors—Risks Relating to Our Business—Risks Relating to Competing in our Industry” in Part I, Item 1A of this report for further discussion of these risks.
In order to compete successfully, we must develop proprietary positions in patented drugs for therapeutic markets that have not been satisfactorily addressed by conventional research strategies and, in the process, expand our expertise in structure-based drug design. Our product candidates, even if successfully tested and developed, may not be adopted by physicians over other products and may not offer economically feasible alternatives to other therapies.
HAE
HAE is an autosomal dominant disease characterized by painful, unpredictable, recurrent attacks of inflammation affecting the hands, feet, face, abdomen, urogenital tract, and the larynx. The inflammation can be disfiguring, debilitating, or in the case of laryngeal attacks, life-threatening. Prevalence for HAE is uncertain but is estimated to be approximately 1 case per 33,000 to 67,000 persons without known differences among ethnic groups and is caused by deficient (Type I) or dysfunctional (Type II) levels of C1-Inhibitor (“C1-INH”), a naturally occurring molecule that is known to inhibit kallikrein, bradykinin, and other serine proteases in the blood. If left untreated, HAE can result in a mortality rate as high as 50% primarily due to upper airway obstruction. There are several licensed therapies for HAE, including the following:
•C1-INH replacement therapy is available as an acute therapy (Berinert®) and as a prophylactic therapy (Haegarda® and Cinryze®). These therapies are dosed subcutaneously and intravenously. Recombinant C1-INH (Ruconest®) is also available as an acute therapy.
•Kallikrein Inhibitors — Kalbitor® (ecallantide) is a specific recombinant plasma kallikrein inhibitor that is dosed subcutaneously by healthcare providers to treat acute HAE attacks. Takhzyro® (lanadelumab-flyo) is a monoclonal antibody approved for prophylaxis of HAE attacks and can be self-administered as a subcutaneous injection.
•Bradykinin receptor antagonist — Firazyr® (icatibant) and generic icatibant are indicated for the treatment of acute attacks and are administered by subcutaneous administration.
•Other medications — Prophylactic administration of synthetic attenuated androgens (generically available as danazol or stanozolol) has been utilized to reduce the frequency or severity of attacks. However, long-term use of danazol or stanozolol may result in liver damage, virilization and arterial hypertension. Six-month liver function tests, annual lipid profiles, and biennial hepatic ultrasound are recommended for patients on chronic androgen therapy.
14
We are aware of a number of HAE therapies in clinical development that, if approved, may compete with ORLADEYO. These include:
| Company | Asset | Mechanism of Action | Route of Administration | Trial Phase | Role in Therapy | |||||||||||||||||||||||||||
| KalVista | Sebetralstat | Kallikrein inhibitor | Oral | III | Acute | |||||||||||||||||||||||||||
| Pharvaris | PHA121 (PHVS416/PHVS719) | B2 receptor antagonist | Oral | II/III | Acute and Prophylaxis | |||||||||||||||||||||||||||
| Attune | ATN-249 | Kallikrein inhibitor | Oral | I/Potentially Inactive | ||||||||||||||||||||||||||||
| CSL | Garadacimab | Anti-factor XII mAb | Subcutaneous | Filed | Prophylaxis | |||||||||||||||||||||||||||
| Ionis | Donidalorsen | Prekallikrien Antisense | Subcutaneous | III | Prophylaxis | |||||||||||||||||||||||||||
| Astria | STAR-0215 | Kallikrein inhibitor | Subcutaneous | Ib/II | Prophylaxis | |||||||||||||||||||||||||||
| ADARx | ADX-324 | siRNA | Subcutaneous | I | Prophylaxis | |||||||||||||||||||||||||||
| Intellia | NTLA-2002 | Gene Editing | IV | I/II | Curative | |||||||||||||||||||||||||||
| BioMarin | BMN-331 | Gene Therapy | IV | I/II | Curative | |||||||||||||||||||||||||||
Complement-Mediated Diseases
Several rare diseases are known to be mediated by defects of the complement system, including, but not limited to, PNH, atypical hemolytic uremic syndrome (“aHUS”), complement 3 glomerulopathy (“C3G”), IgAN, and myasthenia gravis. Alexion’s (part of AstraZeneca Rare Disease) Soliris® (eculizumab) is a C5 inhibitor approved for PNH, aHUS, myasthenia gravis, and neuromyelitis optica spectrum disorder. Soliris had global sales of over $3.1 billion in 2023. Alexion also received FDA approval for Ultomiris® (ravulizumab), a longer-acting C5 inhibitor, as a treatment for PNH in late 2018, aHUS in late 2019, and myasthenia gravis in 2022. Global sales for Ultomiris were over $2.6 billion in 2023. Apellis Pharmaceuticals, Inc.’s Empaveli® is a C3 inhibitor approved for PNH in the United States and Europe in 2021.
We are aware of a number of complement pathway-based products in development, which include:
| Company | Asset | Mechanism of Action | Route of Administration | Trial Phase | ||||||||||||||||||||||
| Apellis | Empaveli® (pegcetacoplan) | C3 Inhibitor | Subcutaneous | Approved | ||||||||||||||||||||||
| Akari | Nomacopan | C5 Inhibitor | Subcutaneous | III | ||||||||||||||||||||||
| Roche | Crovalimab (RG6107) | C5 Inhibitor | IV / Subcutaneous | Filed (approved outside U.S.) | ||||||||||||||||||||||
| Regeneron | Pozelimab | C5 Inhibitor | IV / Subcutaneous | III | ||||||||||||||||||||||
| Omeros | Narsoplimab OMS906 | MASP-2 Inhibitor MASP-3 Inhibitor | IV / Subcutaneous Subcutaneous | BLA I | ||||||||||||||||||||||
| AstraZeneca | Danicopan (ALXN2040) | Factor D Inhibitor | Oral | Filed (approved outside U.S.) | ||||||||||||||||||||||
| Gefurulimab (ALXN1720) | C5 Inhibitor | Subcutaneous | III | |||||||||||||||||||||||
| Vemircopan (ALXN2050) | Factor D Inhibitor | Oral | II | |||||||||||||||||||||||
| Novartis | Fabhalta® (Iptacopan) | Factor B Inhibitor | Oral | Approved | ||||||||||||||||||||||
Amgen | Tavneos® (avacopan) | C5aR Inhibitor | Oral | Approved | ||||||||||||||||||||||
| Ra / UCB | Zilbrysq® (Zilucoplan) | C5 Inhibitor | Subcutaneous | Approved | ||||||||||||||||||||||
| Alnylam | Cemdisiran | C5 Inhibitor | Subcutaneous | II | ||||||||||||||||||||||
15
Amgen (Phase 3), Samsung (marketed in Europe and Japan as EPYSQLI®), and Isu Abxis are also in clinical trials developing biosimilars of eculizumab.
Certain diseases that are mediated by defects of the complement system, such as IgAN, may also have pathology that is mediated by other mechanisms. Products that are not inhibitors of the complement system, such as Tarpeyo (DR-budesonide) for IgAN, may change the treatment landscape and future competitive environment for our products.
Antivirals
The pharmaceutical market for products that prevent or treat influenza is very competitive. Key competitive factors for RAPIVAB (peramivir injection) include, among others, efficacy, ease of use, safety, price and cost-effectiveness, storage and handling requirements, and reimbursement. A number of products are currently available in the United States and/or other countries, including Japan, for the prevention or treatment of influenza, including seasonal flu vaccines, F. Hoffmann-La Roche Ltd.’s (“Roche”) TAMIFLU® (oseltamivir), generic oseltamivir, GlaxoSmithKline plc’s RELENZA®, Genentech and Shionogi’s XOFLUZA® and Daiichi Sankyo Co., Ltd.’s (“Daiichi”) INAVIR®. In addition, FUJIFILM Corporation’s favipiravir, a polymerase inhibitor, is approved in Japan.
Various government entities throughout the world are offering incentives, grants, and contracts to encourage additional investment into preventative and therapeutic agents against influenza, which may have the effect of further increasing the number of our competitors and/or providing advantages to certain competitors.
Netherton Syndrome
Netherton syndrome is a rare, lifelong genetic disorder that often presents in neonates or infancy. The disease is caused by the deficiency of a natural inhibitor (SPINK5) of KLK5, a serine protease responsible for regulating skin shedding. While there is currently no approved treatment for Netherton syndrome, we are aware of a number of therapies in development for treatment that, if approved, may compete with BCX17725. In particular, Quoin Pharmaceuticals Ltd.’s QRX-003 currently in Phase III and Daiichi’s DS-2325a currently in Phase Ib/II are in clinical trials for the treatment of Netherton syndrome.
Diabetic Macular Edema
Pursuant to the Clearside Agreement, we are developing our investigational plasma kallikrein inhibitor, avoralstat, with Clearside’s SCS Microinjector® to deliver avoralstat to the back of the eye through the suprachoroidal space to treat patients with DME. There are several approved anti-vascular endothelial growth factor (“anti-VEGF”) therapies available for the treatment of DME, including Roche’s VABYSMO® (faricimab-svoa) and Regeneron Pharmaceuticals, Inc.’s EYLEA® (aflibercept). In addition, we are aware of a number of products in development that would offer alternatives to anti-VEGF therapies, which could affect the competitive environment for our products. In particular, Rezolute Inc.’s oral plasma kallikrein inhibitor RZ-402 is currently in phase II for diabetic macular edema.
Research and Development
We initiated our research and development activities in 1986. We have assembled a scientific research staff with expertise in a broad base of advanced research technologies, including protein biochemistry, X-ray crystallography, chemistry, and pharmacology. Our research facilities, located in Birmingham, Alabama, include protein biochemistry and organic synthesis laboratories, testing facilities, X-ray crystallography, computer and graphics equipment and facilities to make product candidates on a small scale for early-stage clinical trials.
Government Regulation
Our business is subject to extensive regulation by the FDA and foreign governments. These regulations include, among other things, regulations for the development, testing, manufacture, safety, efficacy, record-keeping, labeling, storage, approval, advertising, promotion, sale and distribution of pharmaceutical products. The regulatory review and approval process is lengthy, expensive, and uncertain. Before obtaining regulatory approvals for the commercial sale of any products, we or our partners must demonstrate that our product candidates are safe and effective for use in humans. The approval process takes many years, substantial expenses may be incurred, and significant time may be devoted to clinical development. Further, the duration of the approval process may be exacerbated by global health concerns or other considerations that could prevent regulatory authorities from conducting their inspections, reviews, or other regulatory
16
activities that could significantly impact the ability of such authorities to timely review and process our regulatory submissions.
Even if the FDA or foreign regulatory authorities approve a product candidate, the approval may limit the indicated uses for the product candidate, impose other restrictions on the product candidate, and/or may require post-approval studies that could impair the commercial viability of the product candidate. Even upon any approval to market our potential products, whether in the United States or internationally, we will continue to be subject to extensive regulatory requirements. These requirements are wide ranging and govern, among other things:
•adverse drug experience reporting regulations;
•product promotion;
•product manufacturing, including good manufacturing practice requirements; and
•product changes or modifications.
These government regulations are a significant factor in the production and marketing of any pharmaceutical products that we develop. Failure to comply with applicable FDA and other regulatory requirements at any stage during the regulatory process may subject us to sanctions, including:
•delays;
•warning or untitled letters;
•fines;
•product recalls or seizures;
•injunctions;
•penalties;
•refusal of the FDA or any foreign regulatory authority to review pending market approval applications or supplements to approval applications;
•total or partial suspension of production;
•civil penalties;
•withdrawals of previously approved marketing applications; and
•criminal prosecutions.
The policies of the FDA and foreign regulatory authorities may change, and additional government regulations may be enacted that could prevent or delay regulatory approval of our product candidates or approval of new indications for our existing products. We cannot predict the likelihood, nature, or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
FDA Regulation
Before testing potential product candidates in humans, we carry out laboratory and animal studies to determine safety and biological activity. After completing preclinical trials, we must file an investigational new drug application (“IND”), including a proposal to begin clinical trials, with the FDA. Thirty days after filing an IND, a phase 1 human clinical trial can start, unless the FDA places a hold on the trial.
Clinical trials to support a new drug application (“NDA”) are typically conducted in three sequential phases, but the phases may overlap.
Phase 1 — During phase 1, which involves the initial introduction of the drug into healthy volunteers, the drug is tested to assess metabolism, pharmacokinetic, and pharmacological actions and safety, including side effects associated with increasing doses.
Phase 2 — Phase 2 usually involves trials in a limited patient population to: (1) assess the efficacy of the drug in specific, targeted indications; (2) assess dosage tolerance and optimal dosage; and (3) identify possible adverse effects and safety risks.
Phase 3 — If a compound is found to be potentially effective and to have an acceptable safety profile in phase 2 evaluations, phase 3 clinical trials, also sometimes called pivotal studies, major studies, or advanced clinical trials, are typically undertaken to further demonstrate clinical efficacy and to further test for safety within an expanded patient population at geographically dispersed clinical trial sites.
17
Initiation and completion of the clinical trial phases are dependent upon many factors, including things that are beyond our control. For example, the clinical trials cannot begin at a particular site until that site receives approval from its Institutional Review Board (“IRB”), which reviews the protocol and related documents. This process can take several weeks to several months. In addition, clinical trials are dependent on patient enrollment, but the rate at which patients enroll in a study depends on:
•willingness of investigators to participate in a study;
•ability of clinical sites to obtain approval from their IRB;
•the availability of existing or other experimental drugs for the disease we intend to treat;
•the willingness of patients to participate; and
•the availability of patients meeting the eligibility criteria.
Delays in planned patient enrollment may result in increased expense and longer development timelines. A sponsor may choose, but is not required, to conduct a foreign clinical study under an IND. When a foreign clinical study is conducted under an IND, all IND requirements must be met unless waived. When the foreign clinical study is not conducted under an IND, the sponsor must ensure that the study complies with certain FDA regulatory requirements in order to use the study as support for an IND or application for marketing approval or licensure. Good clinical practice standards are required for clinical studies regardless of the location of the study.
In general, the FDA requires that at least two adequate and well-controlled clinical trials be conducted. After successful completion of the required clinical testing, generally an NDA is submitted. Upon receipt of the NDA, the FDA will review the application for completeness. Within 60 days, the FDA will determine if the application is sufficiently complete to warrant full review and will consider the application “filed” at that time. Also upon receipt of the application, the FDA will assign a review priority to the application. Priority review applications are usually reviewed within 6 months; standard review applications are usually reviewed within 10 months. The FDA may refer NDAs for new molecular entities to an appropriate advisory committee for review and evaluation in regard to providing a recommendation as to whether the application should be approved. The FDA is not bound to follow the recommendation of an advisory committee.
Following the review of the application, which may include requests for additional information from the sponsor and results from inspections of manufacturing and clinical sites, the FDA will issue an “action letter” on the application. The action letter will either be an “approval letter,” in which case the product may be lawfully marketed in the United States, or a “complete response letter.” A complete response letter will state that the FDA cannot approve the NDA in its present form and, usually, will describe all of the specific deficiencies that the FDA has identified in the application. The complete response letter, when possible, will include the FDA’s recommended actions to place the application in a condition for approval. Deficiencies can be minor (e.g., labeling changes) or major (e.g., requiring additional clinical trials). A complete response letter may also be issued before the FDA conducts the required facility inspection and/or reviews labeling, leaving the possibility that additional deficiencies in the original NDA could be subsequently cited. An applicant receiving a complete response letter is permitted to resubmit the NDA addressing the identified deficiencies (in which case a new two- or six-month review cycle will begin), or withdraw the NDA. The FDA may consider a failure to take action within one year of a complete response letter to be a request to withdraw, unless the applicant has requested an extension of time in which to resubmit the NDA. If the FDA approves an NDA, the marketing of the product will be limited to the particular disease states and conditions of use that are described in the product label. The FDA strictly regulates marketing, labeling, advertising, and promotion of products that are placed on the market. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including investigation by federal and state authorities.
Post-Approval
Approved drugs that are manufactured or distributed in the United States pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion, and reporting of adverse experiences with the product. For example, advertising and promotion are subject to stringent FDA rules and oversight, and as an NDA holder, we may be held responsible for any advertising and promotion that is not in compliance with the rules and regulations. In particular, the claims in all promotional materials and activities must be consistent with the FDA approvals for approved products and must be appropriately substantiated and fairly balanced with information on the safety risks and limitations of the products. We are also required to engage in appropriate truthful, non-misleading, and non-promotional scientific exchange concerning our products.
18
After approval, most changes to the approved product, such as adding new indications or other labeling claims and some manufacturing and supplier changes, are subject to prior FDA review and approval. The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-marketing testing, including phase 4 clinical trials, and surveillance programs to further assess and monitor the product’s safety and effectiveness after commercialization.
We and all of our contract manufacturers are also required to comply with the applicable FDA current Good Manufacturing Practice (“cGMP”) regulations during clinical development and to ensure that the product can be consistently manufactured to meet the specifications submitted in an NDA. The cGMP regulations include requirements relating to product quality, as well as the corresponding maintenance of records and documentation. Manufacturing facilities must be approved by the FDA before they can be used to manufacture our products. Based on an inspection, the FDA determines whether manufacturing facilities are in compliance with applicable regulations. Manufacturing facilities in non-United States countries that are utilized to manufacture drugs for distribution into the United States are also subject to inspection by the FDA. Additionally, failure to comply with local regulatory requirements could affect production and availability of product in relevant markets.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States. Orphan drug designation, if sought, must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first NDA applicant with FDA orphan drug designation for a particular active ingredient to receive FDA approval of the designated drug for the disease indication for which it has such designation is entitled to a seven-year exclusive marketing period (“orphan drug exclusivity”) in the United States for that product, for that indication. During the seven-year period, the FDA may not finally approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if the license holder cannot supply sufficient quantities of the product. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition, provided that the sponsor has conducted appropriate clinical trials required for approval. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee for the orphan indication.
The FDA’s interpretation of the scope of orphan drug exclusivity may change. In light of recent litigation and FDA announcements, the scope of orphan drug exclusivity and other issues relating to the FDA’s implementation of the Orphan Drug Act with respect to both previously approved and future products continues to evolve and may be the subject of further litigation or legislative action.
Fast Track Designation
Under the Fast Track program, the sponsor of an IND may request the FDA to designate the drug candidate as a Fast Track drug if it is intended to treat a serious or life-threatening condition and data demonstrate its potential to fulfill an unmet medical need. The FDA must determine if the drug candidate qualifies for Fast Track designation within 60 days of receipt of the sponsor’s request. Once the FDA designates a drug as a Fast Track candidate, it is required to facilitate the development and expedite the review of that drug by providing more frequent communication with, and guidance to, the sponsor. The key benefits of Fast Track Designation are the eligibility for priority review, rolling review (submission of portions of an application before the complete marketing application is submitted), and accelerated approval, if relevant criteria are met.
In addition to other benefits, such as greater interactions with the FDA, the FDA may initiate review of sections of a Fast Track drug’s NDA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s review period as specified under the Prescription Drug User Fee Act for filing and reviewing an application does not begin until the last section of the NDA has been submitted. Additionally, the Fast Track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
19
Breakthrough Therapy Designation
In addition, the Food and Drug Administration Safety and Innovation Act of 2012 (“FDASIA”) established the Breakthrough Therapy designation. A sponsor may seek FDA designation of its product candidate as a breakthrough therapy if the drug is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. If a drug is designated as breakthrough therapy, the FDA will provide more intensive guidance on the drug development program and expedite its review.
Abbreviated New Drug Applications for Generic Drugs
In 1984, with passage of the Drug Price Competition and Patent Term Restoration Act of 1984 (commonly referred to as the “Hatch-Waxman Amendments”) amending the Federal Food, Drug, and Cosmetic Act (“FDCA”), Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application (“ANDA”) to the agency. Upon approval of an ANDA, the FDA indicates that the generic product is “therapeutically equivalent” to the drug product previously approved under an NDA, known as the reference listed drug (“RLD”), and it assigns a therapeutic equivalence rating to the approved generic drug in its publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” also referred to as the “Orange Book. Physicians and pharmacists consider the therapeutic equivalence rating to mean that a generic drug is fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, the FDA’s designation of a therapeutic equivalence rating often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or patient.
Under the Hatch-Waxman Amendments, the FDA may not approve an ANDA until any applicable period of nonpatent exclusivity for the RLD has expired. The FDCA provides a period of five years of data exclusivity for NDAs containing a new chemical entity. In cases where such exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification (discussed further below), in which case the applicant may submit its application four years following the original product approval. The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or indication.
Hatch-Waxman Patent Certification and the 30 Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s product or a method of using the product. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval.
A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicate that it is not seeking approval of a patented method of use, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
505(b)(2) New Drug Applications
As an alternative path to FDA approval for modifications to formulations or uses of products previously approved by the FDA pursuant to an NDA, an applicant may submit an NDA under Section 505(b)(2) of the FDCA. Section
20
505(b)(2) was enacted as part of the Hatch-Waxman Amendments and permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by, or for, the applicant, and for which the applicant has not obtained a right of reference. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous findings of safety and effectiveness is scientifically and legally appropriate, it may eliminate the need to conduct certain preclinical studies or clinical trials of the new product. The FDA may also require companies to perform additional bridging studies or measurements, including clinical trials, to support the change from the previously approved reference drug. The FDA may then approve the new drug candidate for all, or some, of the label indications for which the reference drug has been approved, as well as for any new indication sought by the 505(b)(2) applicant.
To the extent that a Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. As a result, approval of a 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003, an NDA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the drug product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. With the enactment of FDASIA, sponsors must also submit pediatric study plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric study or studies the applicant plans to conduct, including study objectives and design, any deferral or waiver requests, and other information required by regulation. The applicant, the FDA, and the FDA’s internal review committee must then review the information submitted, consult with each other, and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent and orphan exclusivity. This six-month exclusivity may be granted if an NDA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot accept or approve another application.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Amendments. Those Amendments permit a patent restoration of up to five years for patent term lost during product development and the FDA regulatory review. The restoration period granted is typically one-half the time between the effective date of an IND and the submission date of an NDA, plus the time between the submission date of an NDA and ultimate approval. Patent term restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the product’s approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. The U.S. Patent and Trademark Office reviews and approves the application for any patent term extension or restoration in consultation with the FDA.
21
Foreign Regulation
In addition to regulations in the United States, we are subject to a variety of foreign regulatory requirements governing human clinical trials and marketing approval, commercial sales, and distribution of drugs. The foreign regulatory approval process includes all of the risks associated with FDA approval set forth above, as well as additional country-specific regulations. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. Some countries, such as certain countries in Latin America and in the Middle East, have review processes and data requirements similar to those of the European Union, and, in some cases, can rely on prior marketing approval from U.S. or EU regulatory authorities. The regulatory process in these countries may include manufacturing/testing facility inspections, testing of drug product upon importation and other domestic requirements. Certain Asian countries may require local clinical-trial data for bridging purposes as part of the drug registration process in addition to global clinical trials, which can add to overall drug development and registration timelines. In most of the Asian markets, registration timelines depend on marketing approval in the United States or the European Union.
The requirements governing the conduct of clinical trials, product licensing, pricing, and reimbursement vary greatly from country to country, some of which are discussed below, and may also include post-approval commitments.
European Union
The various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. Pursuant to the Clinical Trials Directive 2001/20/EC, as amended (the “Clinical Trials Directive”), a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, approval must be obtained from the national competent authority of each EU member state in which a clinical trial is planned to be conducted. A clinical trial application (“CTA”) is submitted, which must be supported by an investigational medicinal product dossier and further supporting information prescribed by the Clinical Trials Directive and other applicable guidance documents, including, but not limited to, the clinical trial protocol. Further, a clinical trial may only be started after an independent ethics committee has issued a favorable opinion on the CTA in that country.
In April 2014, the European Union adopted a new Clinical Trials Regulation (EU) No 536/2014 (the “Regulation”), which replaced the Clinical Trials Directive. The Regulation came into effect on January 31, 2022 with a three-year transition period in which clinical trial sponsors will be able to choose among different submission pathways. The Regulation, which is directly applicable in all EU member states, aims to simplify and streamline the approval of clinical trials in the European Union. For instance, the Regulation provides for a streamlined application procedure via a single entry point and strictly defined deadlines for the assessment of clinical trial applications.
Manufacturing and import into the European Union of investigational medicinal products for use in clinical trials is subject to the holding of appropriate authorizations and must be carried out in accordance with cGMP.
Under EU regulatory systems, we may submit marketing authorizations either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all EU member states. Under the centralized procedure, a single marketing authorization application is submitted to the Committee for Medicinal Products for Human Use of the European Medicines Agency (“EMA”), which then makes a recommendation to the European Commission (“EC”). The EC makes the final determination on whether to approve the application. The decentralized procedure provides for mutual recognition of national approval decisions, and the holder of a national marketing authorization may submit an application to the remaining member states. The decentralized procedure is only available for pharmaceutical products not falling within the mandatory scope of the centralized procedure.
United Kingdom
The United Kingdom formally left the European Union on January 31, 2020 (“Brexit”), and EU laws now only apply to the United Kingdom in respect of Northern Ireland as laid out in the Protocol on Ireland and Northern Ireland. The European Union and the United Kingdom have agreed on a trade and cooperation agreement (“TCA”) which includes provisions affecting the life sciences sector (including on customs and tariffs). There are some specific provisions concerning pharmaceuticals, including the mutual recognition of GMP and issued GMP documents. The TCA does not, however, contain wholesale mutual recognition of U.K. and EU pharmaceutical regulations and protect standards.
22
The government of the United Kingdom has enacted the Medicines and Medical Devices Act 2021. The purpose of the act is to enable the existing regulatory frameworks in relation to human medicines and clinical trials of human medicines, among others, to be updated. The powers under the act may only be exercised in relation to specified matters and must safeguard public health. The Medicines and Medical Devices Act 2021 supplements the United Kingdom Medical Devices Regulations 2002, which are based on the EU Medical Devices Directive as amended to reflect the United Kingdom’s post-Brexit regulatory regime. Core aspects of the new regime are planned to come into force on July 1, 2025, with strengthened post-market surveillance proposals to be introduced in advance of such time.
Japan
Under the Japanese regulatory system administered by the Japanese Pharmaceuticals and Medical Devices Agency (“PMDA”), pre-marketing approval and clinical studies are required for all pharmaceutical products. To obtain manufacturing/marketing approval, we must submit an application for approval to the Ministry of Health, Labor and Welfare (“MHLW”) with results of nonclinical and clinical studies to show the quality, efficacy, and safety of a new drug. A data compliance review, good Clinical Practices on-site inspection, cGMP audit, and detailed data review are undertaken by the PMDA. The application is then discussed by the committees of the Pharmaceutical Affairs and Food Sanitation Council (“PAFSC”). Based on the results of these reviews, the final decision on approval is made by the MHLW. In Japan, the National Health Insurance system maintains a Drug Price List specifying which pharmaceutical products are eligible for reimbursement, and the MHLW sets the prices of the products on this list. The price will be determined within 60 to 90 days following approval unless the applicant disagrees, which may result in extended pricing negotiations. The government generally introduces price cut rounds every other year and also mandates price decreases for specific products. New products judged innovative or useful, that are indicated for pediatric use, or that target orphan or small population diseases, however, may be eligible for a pricing premium. The Japanese government has also promoted the use of generics, where available.
Fraud and Abuse and Related Regulatory Laws
We are subject to various federal and state laws pertaining to healthcare “fraud and abuse,” including both federal and state anti-kickback and false claims laws. Outside of the United States, we may be subject to analogous foreign laws and regulations in the various jurisdictions in which we operate. These laws and regulations apply to our or our partners’ operations, sales and marketing practices, price reporting, and relationships with physicians and other customers and third-party payors. Anti-kickback laws generally prohibit a manufacturer from soliciting, offering, receiving, or paying any remuneration to generate business, including the purchase or prescription of a particular drug. Although the specific provisions of these laws vary, their scope is generally broad and there may be no regulations, guidance or court decisions that clarify how the laws apply to particular industry practices. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented, for payment to third party payors (including Medicare and Medicaid) claims for reimbursement or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services.
In addition, we are subject to the federal physician sunshine act and certain similar physician payment and drug pricing transparency legislation in various states. The sunshine provisions apply to manufacturers with products reimbursed under certain government programs and require those manufacturers to disclose annually to the federal government certain payments made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians (as defined above) and their immediate family members. State laws may also require disclosure of pharmaceutical pricing information and marketing expenditures.
Violations of the physician sunshine act and similar legislation or the fraud and abuse laws may be punishable by civil or criminal sanctions, including fines and civil monetary penalties, and future exclusion from participation in government healthcare programs.
Reimbursement and Healthcare Reform
In both the United States and other countries, sales and reimbursement of any approved products will depend, in part, on the extent to which the costs of such products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly challenging the prices charged for medical products and services and imposing controls to manage costs. The containment of healthcare costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls,
23
restrictions on reimbursement, and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could limit our net revenue and results. In addition, there is significant uncertainty regarding the reimbursement status of newly approved healthcare products.
Adequate coverage and reimbursement in the United States and other countries is critical to the commercial success of approved products. Recently in the United States, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed, among other things, to reform government program reimbursement methodologies. In addition, individual states in the United States have been increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. Third-party payors are increasingly challenging the prices charged for medical products and services and, in some cases, imposing restrictions on the coverage of particular drugs. Many third-party payors negotiate the price of medical services and products and develop formularies that establish pricing and reimbursement levels. Exclusion of a product from a formulary can lead to its sharply reduced usage in the third-party payor’s patient population. The process for obtaining coverage can be lengthy and costly, and it could take several months before a particular payor initially reviews a product and makes a decision with respect to coverage. For example, third-party payors may require cost-benefit analysis data in order to demonstrate the cost-effectiveness of a particular product.
Outside the United States, ensuring adequate coverage and payment for drug products can have challenges. Pricing of prescription pharmaceuticals is subject to governmental control in many countries. Pricing negotiations with governmental authorities can extend well beyond the receipt of regulatory marketing approval for a product and may require us to conduct an active comparator clinical trial to demonstrate the relative effectiveness of our therapeutic candidates or products to other available therapies to support our pricing, which could be expensive and result in delays in our commercialization efforts. Third-party payors are challenging the prices charged for medical products and services, and many third-party payors limit reimbursement for newly-approved healthcare products. Recent budgetary pressures in many EU countries are also causing governments to consider or implement various cost-containment measures, including reference price grouping, price freezes, increased price cuts, and rebates. If budget pressures continue, governments may implement additional cost-containment measures. Cost-control initiatives could decrease the price we might establish for products that we may develop or sell, which would result in lower product revenues or royalties payable to us. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products.
The Patient Protection and Affordable Care Act (“PPACA”) made extensive changes to the delivery of healthcare in the United States. The PPACA included numerous provisions that affect pharmaceutical companies, some of which became effective immediately and others of which have taken effect over the past several years. For example, the PPACA expanded healthcare coverage to the uninsured through private health insurance reforms and an expansion of Medicaid. The PPACA also imposed substantial costs on pharmaceutical manufacturers, such as an increase in liability for rebates paid to Medicaid, new drug discounts that must be offered to certain enrollees in the Medicare prescription drug benefit, an annual fee imposed on all manufacturers of brand prescription drugs in the United States, and an expansion of an existing program requiring pharmaceutical discounts to certain types of hospitals and federally subsidized clinics. The PPACA also contains cost containment measures that could reduce reimbursement levels for healthcare items and services generally, including pharmaceuticals. It also required reporting and public disclosure of payments and other transfers of value provided by pharmaceutical companies to physicians and teaching hospitals.
In August 2022, the Inflation Reduction Act (“IRA”) was enacted and includes provisions requiring that (1) beginning in 2026, mandatory price setting be introduced in Medicare for certain drugs paid for under Parts B and D, whereby manufacturers must accept a price established by the government or face penalties on all U.S. sales (starting with 10 drugs in 2026, adding 15 in 2027 and 2028, and adding 20 in 2029 and subsequent years, such that by 2031 approximately 100 drugs could be subject to such set prices); (2) starting in 2024, Medicare Part D be redesigned to cap beneficiary out-of-pocket costs and, beginning January 1, 2025, federal reinsurance be reduced in the catastrophic phase (resulting in a shift and increase of such costs to Part D plans and manufacturers, including by requiring manufacturer discounts on certain drugs); and (3) beginning October 1, 2022, manufacturers owe rebates on drugs reimbursed under Medicare Part D if price increases outpace inflation, and beginning January 1, 2023, manufacturers owe rebates on drugs
24
reimbursed under Medicare Part B if price increases outpace inflation. Although the IRA has passed, the environment remains dynamic, and the presidential administration and Congress are continuing to consider drug pricing reforms. Other potential policies cover a wide range of areas, including allowing the importation of drugs from other countries; increasing transparency in drug pricing; and using third-party value assessments to determine drug prices.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare could result in decreased net revenues from our pharmaceutical products and decreased potential returns from our development efforts. In addition, pharmaceutical and device manufacturers are also required to report and disclose certain payments and transfers of value to, and investment interests held by, physicians and their immediate family members during the preceding calendar year. Failure to submit required information may result in civil monetary penalties for payments, transfers of value, or ownership or investment interests not reported in an annual submission. Compliance with the federal and state laws is difficult and time consuming, and companies that do not comply with these laws can face severe civil penalties.
In addition, there have been a number of other legislative and regulatory proposals aimed at changing the pharmaceutical industry. For example, legislation has been enacted in certain states and at a federal level that requires development of an electronic pedigree to track and trace each prescription drug at the saleable unit level through the distribution system. Compliance with these electronic pedigree requirements may increase our operational expenses and impose significant administrative burdens.
Data Privacy and Security Laws
Pharmaceutical companies may be subject to U.S. federal and state health information privacy, security, data breach notification, and consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act) which may govern the collection, use, disclosure, and protection of health-related and other personal data. State laws may be more stringent, broader in scope, or offer greater individual rights with respect to protected health information (“PHI”), than the federal Health Insurance Portability and Accountability Act of 1996, as amended, and its implementing regulations, which are collectively referred to as HIPAA, and state laws may differ from each other, which may complicate compliance efforts. Entities that are found to be in violation of HIPAA or that enter into a resolution agreement with the HHS to settle actual or potential allegations of HIPAA noncompliance may be subject to significant civil, criminal, and administrative fines and penalties and/or additional reporting and oversight obligations.
Many state laws govern the privacy of personal data in specified circumstances. For example, in California the California Consumer Privacy Act of 2018, as amended by the California Privacy Rights Act of 2020 (together, “CCPA”) establishes a privacy framework for covered businesses by creating an expanded definition of personal data, establishing data privacy rights for consumers in the State of California, imposing special rules on the collection of consumer data from minors, and creating a potentially severe statutory damages framework for violations of the CCPA and for businesses that fail to implement reasonable security procedures and practices to prevent data breaches. While clinical trial data and information governed by HIPAA are currently exempt from the CCPA, other personal data may be covered. Several other states, such as Virginia, Colorado and Utah, have also enacted comprehensive privacy laws, and it is possible that additional states will follow suit.
Outside the United States, an increasing number of laws and regulations may govern data privacy and security. For example, EU member states, the United Kingdom, Switzerland, and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations. In the European Economic Area (“EEA”), the collection and use of personal data, including clinical trial data, is governed by the provisions of the General Data Protection Regulation (“GDPR”). The GDPR, together with national legislation, regulations, and guidelines of the states in the EEA, the United Kingdom, and Switzerland governing the processing of personal data, impose strict obligations and restrictions on the ability to collect, analyze, and transfer personal data, including health data from clinical trials and adverse event reporting. The GDPR also imposes additional special protections for “special category data,” which includes health, biometric and genetic information of data subjects located in the EEA. Further, the GDPR provides a broad right for EU member states to create supplemental national laws, for example relating to the processing of health, genetic and biometric data, which could further limit our ability to use and share such data or could cause our costs to increase and harm our business and financial condition. The GDPR and similar national legislation grant individuals the opportunity to object to the processing of their personal data, allow them to request deletion of personal data in certain circumstances, and provide the individual with an express right to seek legal remedies in the event the individual believes his or her rights have been violated.
25
Further, the GDPR and similar legislation, such as the United Kingdom GDPR and Switzerland’s Federal Data Protection Act, impose strict rules on the transfer of personal data out of the EEA, the United Kingdom, Switzerland, and other countries to the United States or other regions that have not been deemed to offer “adequate” privacy protections. These obligations and regulations also concern security breach notifications, security and confidentiality of the personal data, and imposition of substantial potential fines for breaches of the data protection obligations. Local data protection authorities may interpret the GDPR and national laws differently and impose additional requirements, which add to the complexity of processing personal data in or from the EEA, the United Kingdom, or Switzerland. Guidance on implementation and compliance practices are often updated or otherwise revised.
The EU Clinical Trials Regulation also imposes new obligations to make publicly available certain information generated from clinical trials. Only very limited information is exempted from disclosure, i.e. commercially confidential information (which is construed increasingly narrowly) and protected personal data. It may be possible for others to use this data (for example, competitors who may use this data in their own research and development programs) once this data is in the public domain.
We are also subject to the supervision of local data protection authorities in those jurisdictions where we undertake clinical trials. We depend on a number of third parties in relation to the provision of our services, a number of which process personal data of EU individuals on our behalf. With each such provider we are required to enter into contractual arrangements under which they are contractually obligated to only process personal data according to our instructions, and conduct diligence to ensure that they have sufficient technical and organizational security measures in place.
We are also subject to evolving European privacy laws on electronic marketing and cookies. For example, the European Union is in the process of replacing the e-Privacy Directive (2002/58/EC) with a new set of rules taking the form of a regulation that will be directly implemented in the laws of each EU member state. While this e-Privacy Regulation was originally intended to be adopted on May 25, 2018, it is still going through the European legislative process and the timing of its adoption remains unclear.
Anti-Corruption Laws
We are also subject to the U.S. Foreign Corrupt Practices Act (“FCPA”), which prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party, or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. Similar laws exist in other countries, such as the United Kingdom or in EU member states, that restrict improper payments to public and private parties. Many countries have laws prohibiting these types of payments within the respective country. In addition to these anti-corruption laws, we are subject to import and export control laws, tariffs, trade barriers, economic sanctions, and regulatory limitations on our ability to operate in certain foreign markets.
Corporate Compliance
In order to ensure compliance with applicable laws and regulations, our Chief Financial Officer, Chief Legal Officer, and Chief People Officer oversee compliance training, education, auditing, and monitoring; enforce disciplinary guidelines for any infractions of our corporate polices; implement new policies and procedures; respond to any detected issues; and undertake corrective action procedures. Our controls address compliance with laws and regulations that govern public pharmaceutical companies, including, but not limited to, the following: federal and state law, such as the Sarbanes-Oxley Act of 2002 and the FCPA; Nasdaq listing requirements; the regulations of the Financial Industry Regulatory Authority, the SEC, the FDA, and HHS; and applicable laws and regulations administered by foreign regulatory authorities, including those of the European Union, the United Kingdom, and Japan. Our standard operating procedures are designed to provide a framework for corporate governance in accordance with ethical standards and best legal practices.
Human Capital Resources
As of January 31, 2024, we had approximately 536 employees, of whom approximately 197 employees were engaged in the research and development function of our operations, which we defined this year to include any employee included in research and development expenses for financial reporting purposes. Our research and development staff, many of whom hold Ph.D. or M.D. degrees, have diversified experience in biochemistry, pharmacology, X-ray crystallography,
26
synthetic organic chemistry, computational chemistry, medicinal chemistry, clinical development, quality assurance, and regulatory affairs.
We believe that our ability to successfully execute on our strategic initiatives is highly dependent upon our ability to recruit, retain, and reward our employees. We engage in targeted recruitment strategies to fill highly skilled positions. Our employees enjoy competitive salaries and benefits, as well as equity participation. Our compensation philosophy is designed to provide an appealing, competitive, and rewarding compensation program that encourages high personal and company performance, strong cultural and ethical behavior, and incentives aligned with stockholder interests.
We are committed to providing a workplace that protects the health and well-being of our employees. All employees are required to abide by our Code of Conduct and Compliance Plan and health and safety parameters and to contribute to a positive, inclusive, and friendly company culture. Where we believe such arrangements can be effective, we have implemented flexible working arrangements, including work from home arrangements, for our employees. We consider our relations with our employees to be satisfactory.
Corporate Information
We are a Delaware corporation originally founded in 1986. Our corporate headquarters is located at 4505 Emperor Blvd., Suite 200, Durham, North Carolina 27703, and our corporate telephone number is (919) 859-1302. For more information about us, please visit our website at www.biocryst.com. The information on our website is not incorporated into this report.
Financial Information
For information related to our revenues, profits, net loss and total assets, in addition to other financial information, please refer to the Consolidated Financial Statements and Notes to Consolidated Financial Statements contained in Part II, Item 8 of this report. Financial information about revenues derived from countries outside the United States is included in Note 2 to the Consolidated Financial Statements contained in this report.
Available Information
Our website address is www.biocryst.com. We make available, free of charge, on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. We also make available at our website copies of our audit committee charter, compensation committee charter, corporate governance and nominating committee charter and our Code of Conduct, which applies to all our employees as well as the members of our Board of Directors. Any amendment to, or waiver from, our Code of Conduct will be posted on our website.
27
ITEM 1A. RISK FACTORS
An investment in our stock involves risks. You should carefully read this entire report and consider the following uncertainties and risks, which may adversely affect our business, financial condition or results of operations, along with all of the other information included in our other filings with the SEC, before making an investment decision regarding our common stock.
Risks Relating to Our Business
Financial and Liquidity Risks
We have incurred losses since our inception and may never be profitable.
Since our inception, we have not achieved sustained profitability. Our expectations as to when we may achieve profitability may change based upon our ability to execute our commercialization goals and operational initiatives and whether or not the assumptions underlying our projected revenues and expenses are correct. Our beliefs and projections regarding the attainment of our financial goals may differ from actual results based on market factors like competition, patient and physician acceptance of our products, reimbursement levels, or on our ability to execute our operational and budget plans, including management’s ability to properly forecast our capital allocation needs. To achieve and maintain profitability, we, or our collaborative partners, must successfully manufacture and develop products and product candidates, receive regulatory approvals, and successfully commercialize our products and/or enter into profitable commercialization arrangements with other parties. Even if we are able to successfully commercialize our existing products, or to develop or otherwise acquire new commercially viable products, certain obligations we have to third parties, including, without limitation, our obligation to pay RPI and OMERS, as applicable, royalties on certain revenues from ORLADEYO and BCX10013 under the Royalty Purchase Agreements, may reduce the profitability of such products.
Because of the numerous risks and uncertainties associated with developing our product candidates, launching new products, and their potential for commercialization, we are unable to predict the extent of any future losses. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and sustain profitability on our anticipated timeline, or at all, the market value of our common stock will likely decline.
We may need to raise additional capital in the future. If we are unable to raise capital if and when needed, we may need to adjust our operations.
We have sustained operating losses for the majority of our corporate history and expect that our total 2024 expenses will exceed our total 2024 revenues. We expect to continue to incur operating losses and negative cash flows unless and until revenues reach a level sufficient to support ongoing operations.
Even if we are able to achieve profitability, in order to continue future operations, progress our drug discovery and development programs, and commercialize our current products and product candidates, we may be required to raise additional capital in the future. In addition to seeking strategic partnerships and transactions, we may access the equity or debt markets, incur additional borrowings, pursue royalty or other monetization transactions, or seek other sources of funding to meet liquidity needs at any time, including to take advantage of attractive opportunities in the capital markets. Additional funding, whether through additional sales of securities, additional borrowings, royalty or other monetization transactions, collaborative arrangements with partners, or from other sources, may not be available if or when needed or in a form or on terms acceptable to us. The issuance of preferred or common stock or convertible securities, with terms and prices significantly more favorable than those of our currently outstanding common stock, could have the effect of diluting or adversely affecting the holdings or rights of our existing stockholders. Additional borrowings may subject us to more restrictive covenants than are currently applicable to us under the Pharmakon Loan Agreement (as defined below). In addition, collaborative arrangements may require us to transfer certain material rights to our corporate partners. Insufficient funds or lack of an acceptable partnership may require us to delay, scale-back or eliminate certain of our research and development programs. See “Risks Relating to Our Business—Risks Relating to Drug Development and Commercialization—If we fail to obtain additional financing or acceptable partnership arrangements if and when needed, we may be unable to complete the development and commercialization of our products and product candidates or continue operations” in this section for further discussion of the capital requirements for our development and commercialization efforts.
28
Our liquidity needs will largely be determined by the success of operations in regard to the commercialization of our products, particularly ORLADEYO, the progression of our product candidates in the future, and our ability to execute our budget plans. Our current plans for managing our liquidity needs primarily include controlling the timing and spending on our research and development programs and commercializing our approved products. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” in Part II, Item 7 of this report for additional information about our liquidity needs, capital requirements, potential funding alternatives, and adequacy of available funds.
There can be no assurance that any of our plans will be successful or that additional capital will be available to us on reasonable terms, or at all, if needed. If we are unable to obtain sufficient additional capital if and when needed, we may be forced to adjust or curtail our operations; delay, reduce, or stop ongoing clinical trials or commercialization efforts; cease operations altogether; or file for bankruptcy.
Risks Relating to Drug Development and Commercialization
Our success depends upon our ability to manage our product candidate pipeline, advance our product candidates through the various stages of development, especially through the clinical trial process, and to receive regulatory approvals for the commercial sale of our product candidates.
The success of our business depends upon our ability to manage our product candidate pipeline, including through expanding the pipeline, as appropriate, through our internal identification and discovery of product candidates or otherwise in-licensing or acquiring products or product candidates and integrating them into our business effectively and efficiently; advancing our product candidates through the various stages of development; and receiving regulatory approvals for the commercial sale of our product candidates. Identifying, selecting, and in-licensing or acquiring products or product candidates requires substantial expense and technical and financial expertise, and if we are unable to effectively manage our pipeline or integrate viable products or product candidates into our business on acceptable terms, or at all, our business and drug development efforts could suffer.
To receive the regulatory approvals necessary for the commercial sale of our product candidates, we or our partners must demonstrate through preclinical studies and clinical trials that each product candidate is safe and effective. The development process and related regulatory process are complex and uncertain. The preclinical and clinical development of our product candidates is susceptible to the risk of failure inherent at any stage of drug development, including failure to demonstrate efficacy and safety, failure to demonstrate adequate benefit-risk balance, failure to achieve a commercially attractive and competitive product label, failure to achieve approval in commercially attractive indications, the occurrence of adverse events that are severe or medically or commercially unacceptable, our or our partners’ failure to comply with trial protocols, applicable regulatory requirements, or industry standards, or a determination by the FDA or any comparable foreign regulatory authority that a product candidate may not continue development or be approved in accordance with our development plans or at all. We cannot guarantee that any preclinical studies and clinical trials will be conducted as planned or completed on schedule, if at all, or that the results of such trials will be sufficient to support regulatory approval for our product candidates.
Progression of our product candidates through the clinical development process is dependent upon our trials indicating that our product candidates have adequate safety and efficacy in the patients being treated by achieving pre-determined safety and efficacy endpoints according to the clinical trial protocols, as well as an adequate benefit-risk profile. Failure to achieve any of these endpoints or to show adequate benefit-risk profile in any of our programs, including our complement program (inclusive of BCX10013) and our other rare disease product candidates (including the additional therapies in our pipeline described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Overview—Recent Developments” in Part II, Item 7 of this report), could result in delays in or modifications to our trials or require the performance of additional unplanned trials. For example, dose-related observations in an ongoing BCX10013 nonclinical study reported in 2023 delayed the clinical program. If any of our product candidates is associated with adverse events or undesirable side effects or has properties that are unexpected, we may need to abandon development or limit development of that product candidate to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a benefit-risk perspective. Product candidates that initially show promise in clinical or preclinical testing could later be found to be associated with or to cause undesirable or unexpected side effects that could result in substantial modifications or delays in the development plans for our product candidates, significant unexpected costs, or the termination of programs, such as we experienced with BCX9930 in 2022 prior to discontinuing its development later that year.
29
In addition, the development plans for our product candidates, including our clinical trials (inclusive of BCX10013), may not be adequately designed or executed, which could negatively affect the outcome and analysis of study results. Because of the cost and duration of clinical trials, we have decided in the past, and may in the future decide, to discontinue development of product candidates for various reasons, including, but not limited to, that they are unlikely to show favorable results in clinical trials, unlikely to help advance a product to the point of a meaningful collaboration, or unlikely to have reasonable commercial potential.
Undesirable or inconclusive data in our preclinical studies and clinical trials or side effects in humans could result in the FDA or foreign regulatory authorities (including, e.g., the EMA, the MHLW or the United Kingdom’s Medicines and Healthcare products Regulatory Agency (“MHRA”)) refusing to approve a product candidate for any targeted indications or imposing restrictions or warnings that could impact development or the ultimate commercial viability of a product candidate. In addition, the FDA or foreign regulatory authorities may determine that study data from our product candidates necessitates additional studies or study designs which differ from our planned development strategy, and such regulatory authorities may also require patient monitoring and testing or may implement restrictions or other conditions on our development activities, any of which could materially impact the cost and timing of our planned development strategy. We, our partners, the FDA, or foreign regulatory authorities have previously, and may again in the future, pause enrollment in, suspend, or terminate clinical trials at any time if we or they believe the trial participants face unacceptable health risks.
Our ability to complete the clinical development process successfully is dependent upon many factors, including, but not limited to:
•our or our partners’ ability to secure suitable clinical sites and investigators and to enroll and maintain an adequate number of patients on a timely basis or at all;
•patients that enroll in a clinical trial may not comply with the clinical trial protocols or maintain contact with investigators to provide complete data during and after treatment;
•our product candidates may not prove to be either safe or effective for our targeted indications, or at all, or may produce unfavorable or inconclusive results;
•we or our partners may decide, or be required by regulatory authorities, to pause enrollment in, suspend, or terminate clinical research for various reasons, including a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics of the product candidate, noncompliance with regulatory requirements or their standards of conduct, or findings of undesirable effects caused by a chemically or mechanistically similar product or product candidate;
•regulatory authorities may disagree with our or our partners’ clinical trial protocols or our or their interpretation of data from preclinical studies and clinical trials;
•clinical protocols or study procedures may not be adequately designed or followed by the investigators;
•formulation improvements may not work as expected, which could negatively impact commercial demand for our product candidates;
•regulatory authorities may fail to approve or subsequently find fault with the manufacturing processes or facilities of third-party manufacturers with which we or our partners enter into agreements for clinical and commercial supplies;
•the supply or quantity of raw materials or manufactured product candidates or other materials necessary to conduct development activities may be insufficient, inadequate, or unavailable at an acceptable cost, and we or our partners may experience interruptions in supply;
•our or our partners’ development plans may be delayed or changed as a result of changes in development strategy, the impact of new or different regulations, requirements, and guidelines, or other unexpected events or conditions;
•the cost of preclinical studies and clinical trials may be greater than we anticipate;
•we or our third-party contractors, including those manufacturing our product candidates or components or ingredients thereof, or conducting clinical trials or laboratory testing on our or our partners’ behalf, may fail to comply with regulatory requirements and industry standards or meet contractual obligations in a timely manner or at all; and
•the impact of any global health pandemic, such as COVID-19, on one or more of the foregoing factors.
Clinical trials are lengthy and expensive. Many of the factors listed above could result in increased clinical development costs or longer clinical development times for any of our programs. We and our partners incur substantial expense for, and devote significant time to, preclinical testing and clinical trials, yet we cannot be certain that the tests and trials will ever result in the commercial sale of a product. Even if we or our partners successfully complete clinical trials for our product candidates, we or our partners might not file the required regulatory submissions in a timely manner or may not
30
receive regulatory approval for the product candidates, which in either case would adversely impact or preclude our ability to generate any revenues from product sales or licensing arrangements. In addition, any product candidate, if approved, may be subject to restrictions on labeling, marketing, distribution, prescribing, and use, which could adversely impact the sales of such product.
If our development collaborations with third parties, such as our development partners, contractors and contract research organizations, fail, the development of our product candidates will be delayed or stopped.
We rely heavily upon third parties for many important stages of our product candidate development, including, but not limited to:
•discovery of natural proteins that cause or enable biological reactions necessary for the progression of the disease or disorder;
•execution of certain pharmacology preclinical studies and late-stage development for our compounds and product candidates;
•management of our phase 1, 2 and 3 clinical trials, including medical monitoring, laboratory testing, and data management;
•execution of toxicology studies that may be required to obtain approval for our product candidates;
•formulation improvement strategies and methods;
•manufacturing the starting materials and drug substance required to formulate our products and the product candidates to be used in our clinical trials, toxicology studies and any potential commercial product; and
•management of certain regulatory interactions outside of the United States.
Our failure to engage in successful collaborations at any one of these stages would greatly impact our business. If we do not license protein targets or inhibitors from academic institutions or from other biotechnology companies on acceptable terms, or at all, our drug development efforts could suffer. Similarly, if the contract research organizations or third-party contractors that conduct our initial or late-stage clinical trials, conduct our toxicology or other studies, manufacture our starting materials, drug substance and product candidates, provide laboratory testing or other services (including clinical operation services) in connection with our clinical trials, provide medical writing services, or assist with our regulatory function breach their obligations to us, perform their services inconsistent with industry standards, or fail to comply with regulatory requirements, this would delay or prevent both the development of our product candidates and the availability of any potential commercial product.
If we lose our relationship with any one or more of these parties, we could experience a significant delay in both identifying another comparable provider and then contracting for its services. We may be unable to retain an alternative provider on reasonable terms, if at all. Even if we locate an alternative provider, it is likely that this provider may need additional time to respond to our needs and may not provide the same type or level of service as the original provider. In addition, any provider that we retain will be subject to applicable FDA current Good Laboratory Practices, cGMP, and current Good Clinical Practices, and comparable foreign standards. We do not have control over compliance with these regulations by these providers. Consequently, if these practices and standards are not adhered to by these providers, the development and commercialization of our product candidates could be delayed. If any of the foregoing risks is realized, our business, financial condition and results of operations could be materially adversely affected.
If we fail to obtain additional financing or acceptable partnership arrangements if and when needed, we may be unable to complete the development and commercialization of our products and product candidates or continue operations.
As our programs advance, our costs could increase. Our current and planned discovery, development, approval, and commercialization efforts may require significant capital. Our expenses, revenues and cash utilization rate could vary significantly depending on many factors, including: our ability to effectively manage our product candidate pipeline; our ability to obtain regulatory approvals for our product candidates; our ability to maintain regulatory approvals for, successfully commercialize, and achieve market acceptance of our products, including ORLADEYO; our ability to raise additional capital if needed; our ability to secure partnerships with third parties for our product candidates when deemed advisable (such as for the potential out-licensing of the late-stage development and commercialization of BCX10013); the amount of funding we receive from partnerships with third parties for the development and commercialization of our products and product candidates; the commercial success of our products achieved by our partners; the progress and results of our current and proposed clinical trials for our product candidates; and the progress made in the manufacture of our lead products and the progression of our other programs.
31
In order to continue future operations, progress our drug discovery and development programs, and commercialize our current products and product candidates, we may be required to raise additional capital. Our ability to raise additional capital as and when needed, or at all, may be limited and may greatly depend upon our success in commercializing and achieving market acceptance of ORLADEYO and the success of our current drug development programs, including the progress, timeline and ultimate outcome of the development programs (including, but not limited to, formulation progress, long-term human safety studies, clinical trial investigations, and carcinogenicity, drug-drug interaction, toxicity, or other required studies) for our complement program for diseases of the complement system and other rare disease product candidates (including the additional therapies in our pipeline described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Overview—Recent Developments” in Part II, Item 7 of this report), as well as any post-approval studies for our products. In addition, constriction and volatility in the equity and debt markets, including as a result of the impacts of inflation, increased interest rates, disruption or instability in the banking industry, geopolitical instability, or public health emergencies such as the COVID-19 pandemic, may restrict our future flexibility to raise capital if and when such needs arise. See “Risks Relating to Our Business—Financial and Liquidity Risks—We may need to raise additional capital in the future. If we are unable to raise capital if and when needed, we may need to adjust our operations” in this section and “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” in Part II, Item 7 of this report for additional information about our liquidity risks and capital requirements.
Furthermore, we have exposure to many different industries, financing partners and counterparties, including commercial banks, investment banks and partners (which include investors, licensing partners, distribution partners, and others), which may be unstable or may become unstable in the current economic and political environment, including as a result of the impacts of inflation, increased interest rates, disruption or instability in the banking industry, a potential U.S. Government shutdown, the upcoming presidential election in the United States, geopolitical instability (including the Ukraine-Russia and Israel-Hamas conflicts or rising tensions between China and Taiwan), or public health emergencies such as the COVID-19 pandemic. Any such instability may impact these parties’ ability to fulfill contractual obligations to us, or it might limit or place burdensome conditions upon future transactions with us. Also, it is possible that suppliers may be negatively impacted. Any such unfavorable outcomes in our current programs or unfavorable economic conditions have in the past and could again place severe downward pressure on the price of our common stock and may decrease opportunities to raise capital in the capital or credit markets, and further could reduce the return available on invested corporate cash, which, if severe and sustained, could have a material and adverse impact on our results of operations and cash flows and limit our ability to continue development and commercialization of our products and product candidates.
If we or our partners do not obtain regulatory approvals for our product candidates or maintain regulatory approvals for our products, we or our partners will not be able to commercialize and sell these products and potential products, which would significantly harm our business because we will receive no revenue.
We or our partners must obtain regulatory approvals before marketing or selling our products. If the FDA or a comparable foreign regulatory authority delays or denies regulatory approval of one of our product candidates, or revokes approval of a previously approved product, we would be unable to market or sell the product in the applicable jurisdiction and would not receive revenue from sales or licensing arrangements related thereto, which could have a material and adverse impact on our business.
The process of preparing for and obtaining regulatory approval in any jurisdiction may be lengthy and expensive, and approval is never certain. Because of the risks and uncertainties inherent to the development process, our product candidates could take a significantly longer time to gain regulatory approval than we expect or may never gain approval. As discussed under “Risk Factors—Risks Relating to Our Business—Risks Relating to Drug Development and Commercialization—Our success depends upon our ability to manage our product candidate pipeline, advance our product candidates through the various stages of development, especially through the clinical trial process, and to receive regulatory approvals for the commercial sale of our product candidates,” we and our partners have experienced, and may again in the future experience, any number of unfavorable outcomes during or as a result of preclinical studies and clinical trials that could delay or prevent regulatory approval of our product candidates, or negatively impact our management’s credibility, our value and our operating results.
Even if the FDA or foreign regulatory authorities approve a product candidate, the approval may limit the indicated uses for a product candidate, impose other restrictions on the product candidate, and/or may require post-approval studies that could impair the commercial viability of a product candidate. Even upon any approval to market our potential products, whether in the United States or internationally, we will continue to be subject to extensive regulatory requirements, as discussed under “Risk Factors—Risks Relating to Our Business—Legal and Regulatory Risks—We are
32
subject to various laws and regulations related to our products and product candidates, and if we or our partners do not comply with these laws and regulations, we could face substantial penalties.”
Our failure to comply with existing or future regulatory requirements for regulatory approval, or our loss of, or changes to, previously obtained approvals, could impair our ability to generate any revenues from product sales or licensing arrangements, which could have a material adverse effect on our business, financial condition, and results of operations.
We focus on rare diseases, which may create additional risks and challenges, including that the target patient populations of our products and product candidates may be small.
Because we focus on developing drugs as treatments for rare diseases, we may seek orphan drug, breakthrough therapy or fast track designations for our product candidates in the United States or the equivalent designations elsewhere in the world. Often, regulatory authorities have broad discretion in determining whether or not to grant such designations. We cannot guarantee that our product candidates will receive orphan drug status from the FDA or equivalent designations from other regulatory authorities. Even with an orphan drug designation for our current and potential future product candidates, we may not be the first to obtain marketing approval for any particular orphan indication due to the uncertainties associated with developing pharmaceutical products. Further, even if we obtain orphan drug exclusivity for an existing or future product candidate, that exclusivity may not effectively protect the product from competition. See “Business—Government Regulation—FDA Regulation—Orphan Drugs” in Part I, Item 1 of this report.
We also cannot guarantee that we will receive breakthrough therapy, fast track, or equivalent designations, which provide certain potential benefits such as more frequent meetings with the applicable regulatory authorities to discuss development plans, intensive guidance on efficient drug development programs, and potential eligibility for rolling review or priority review. Even if we are successful in obtaining any such designations for our product candidates, such designations may not lead to faster development or regulatory review or approval and do not increase the likelihood that our product candidates will receive marketing approval. We may not be able to obtain or maintain these designations for our product candidates that receive them, and our competitors may obtain these designations for their product candidates, which could impact our ability to develop and commercialize our products and product candidates or compete with such competitors, which may adversely impact our business, financial condition or results of operations.
Given the small number of patients who have the diseases that we are targeting, it is critical to our ability to grow and become profitable that we continue to successfully identify patients with these rare diseases. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our products and product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, surveys of clinics, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for each of our products and product candidates may be limited or may not be amenable to treatment with our products and product candidates, and new patients may become increasingly difficult to identify or access. Further, even if we obtain significant market share for our products and product candidates, because the potential target populations are small, we may never become or remain profitable nor generate sufficient revenue growth to sustain our business.
If the FDA or comparable foreign regulatory authorities approve generic versions of any of our products that receive marketing approval, or such authorities do not grant our products appropriate periods of data or market exclusivity before approving generic versions of our products, the sales of our products could be adversely affected.
Once an NDA is approved, the drug covered thereby becomes a “reference-listed drug” in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluations.” Manufacturers may seek marketing approval of generic versions of reference-listed drugs through submission of abbreviated new drug applications, or ANDAs, in the United States, as described in “Business—Government Regulation—FDA Regulation—Abbreviated New Drug Applications for Generic Drugs” in Part I, Item 1 of this report. Generic drugs may be significantly less costly to bring to market than the reference-listed drug and companies that produce generic drugs are generally able to offer them at lower prices. Thus, following the introduction of a generic drug, a significant percentage of the sales of any branded product or reference-listed drug is typically lost to the generic drug.
The FDA may not approve an ANDA for a generic drug until any applicable period of non-patent exclusivity for the reference-listed drug has expired, as described in “Business—Government Regulation—FDA Regulation—Abbreviated
33
New Drug Applications for Generic Drugs” in Part I, Item 1 of this report, but such exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the nonclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness. Manufacturers may seek to launch generic drugs following the expiration of the marketing exclusivity period, even if we still have patent protection for such drugs. Competition that our products or product candidates may face from generic drugs could materially and adversely impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on the investments we have made in those product candidates. Our future revenues, profitability and cash flows could also be materially and adversely affected and our ability to obtain a return on the investments we have made in those product candidates may be substantially limited if our products or, if and when approved, product candidates, are not afforded the appropriate periods of non-patent exclusivity.
The commercial viability of any approved product could be compromised if the product is less effective than expected, causes undesirable side effects that either were not previously identified or were worse than expected, or fails to achieve market acceptance within the medical community.
If, after obtaining regulatory approval of a product, we or others discover that the product is less effective than previously believed or causes undesirable side effects that either were not previously identified or were worse than expected, any of the following adverse events could occur:
•regulatory authorities may withdraw their approval of, or impose marketing or manufacturing restrictions on, the product, or require us or our partners to create a medication guide outlining the risks of unidentified side effects for distribution to patients;
•we or our partners may be required to recall the product, change the way the product is administered, conduct additional clinical trials, or be subject to civil or criminal penalties; and
•the product may become less competitive and our reputation may suffer.
Even after receiving regulatory approval, any product could fail to gain sufficient, or any, market acceptance by physicians, patients, third-party payors, health authorities and others in the medical community. For example, physicians are often reluctant to switch their patients from existing therapies even when new and potentially more effective or convenient treatments enter the market. Further, patients often acclimate to the therapy that they are currently taking and do not want to switch unless their physicians recommend switching products or they are required to switch therapies due to lack of reimbursement for existing therapies. If an approved product does not achieve an adequate level of market acceptance, it may not generate significant revenues. The occurrence of any of the foregoing could have a material and adverse impact on our business.
If we fail to successfully commercialize or establish collaborative relationships to commercialize certain of our products and product candidates, or if any partner terminates or fails to perform its obligations under agreements with us, potential revenues from commercialization of our products and product candidates could be reduced, delayed or eliminated.
Our business strategy includes increasing the asset value of our product and product candidate portfolio. We believe this is best achieved by retaining full product rights or through collaborative arrangements with third parties as appropriate. As needed, potential third-party relationships could relate to preclinical development, clinical development, regulatory approval, marketing, sales, and distribution of our products and product candidates.
Currently, we have established collaborative relationships, including with Torii for the commercialization of ORLADEYO in Japan, with third-party distributors for ORLADEYO in certain other markets, and with each of Shionogi and Green Cross for the development and commercialization of peramivir. The process of establishing and implementing collaborative relationships is difficult, time-consuming and involves significant uncertainty, including:
•we or our partners may seek to renegotiate or terminate our relationships due to unsatisfactory commercial, regulatory or clinical results, including post-approval clinical commitments, a change in business strategy, a change of control or other reasons;
•our contracts for collaborative arrangements may expire;
•the possibility that expiration or termination of collaborative relationships, such as those with certain of our distribution partners, may trigger repurchase obligations of the Company for unsold product held by our partners;
•our partners may choose to pursue alternative technologies, including those of our competitors;
34
•we have had in the past, and in the future may have, disputes with a partner that could lead to litigation or arbitration, which could result in substantial costs and divert the attention of our management;
•we do not have day-to-day control over the activities of our partners and have limited control over their decisions;
•our ability to generate future event payments and royalties from our partners depends upon their abilities to establish the safety and efficacy of our product candidates, obtain regulatory approvals and achieve market acceptance of products developed from our product candidates;
•we or our partners may fail to properly initiate, maintain or defend our intellectual property rights, where applicable, or a party may utilize our proprietary information in such a way as to invite litigation that could jeopardize or potentially invalidate our proprietary information or expose us to potential liability;
•we or our partners may not devote sufficient capital or resources toward our products and product candidates; and
•we or our partners may not comply with applicable government regulatory requirements.
If we or our partners fail to fulfill our responsibilities in a timely manner, or at all, our development and commercialization efforts related to that collaboration could be reduced, delayed or terminated, or it may be necessary for us to assume responsibility for activities that would otherwise have been the responsibility of our partner. If we are unable to establish and maintain collaborative relationships on acceptable terms, we may have to delay or discontinue further development or commercialization of one or more of our products or product candidates, undertake commercialization activities at our own expense or find alternative sources of funding. Any delay in the development or commercialization of our products and product candidates would severely affect our business, because if our product candidates do not progress through the development process or reach the market in a timely manner, or at all, or if our products do not achieve market success, we may not receive any revenues from product sales or licensing arrangements.
The results of our partnership with Torii may not meet our current expectations.
We have a partnership agreement with Torii for ORLADEYO in Japan. Under the Torii Agreement, we are responsible for all field promotional activities with respect to ORLADEYO in Japan, which we conduct through our Japanese subsidiary, BioCryst Japan K.K. Furthermore, we remain responsible for regulatory activities with respect to ORLADEYO in Japan, and we use third parties to satisfy those regulatory responsibilities and certain other obligations in Japan. If any party fails to meet its obligations, the commercial success of ORLADEYO in Japan and the economic benefit expected could be negatively impacted.
There can be no assurance that our or our partners’ commercialization efforts, methods, and strategies for our products or technologies will succeed, and our future revenue generation is uncertain.
There can be no assurance that our or our partners’ commercialization efforts, methods and strategies will succeed. We may be unable to establish or sufficiently increase our sales, marketing and distribution capabilities for products we currently, or plan to, commercialize. Our ability to receive revenue from products we or our partners commercialize is subject to several risks, including:
•we or our partners may fail to complete clinical trials successfully, or satisfy post-marketing commitments, sufficient to obtain and maintain regulatory agency marketing approval;
•many competitors are more experienced and have significantly more resources, and their products could reach the market faster, be more cost effective or have a better efficacy or tolerability profile than our products and product candidates;
•we may fail to employ a comprehensive and effective intellectual property strategy, which could result in decreased commercial value of our Company, our products and product candidates, or royalties associated with such products (e.g., the loss of the peramivir patent in Korea, which may result in a reduced royalty from Green Cross);
•we may fail to employ a comprehensive and effective regulatory strategy, which could result in a delay or failure in commercialization of our products;
•our and our partners’ ability to successfully commercialize our products is affected by the competitive landscape, which cannot be fully known at this time;
•revenue from product sales depends on our ability to obtain and maintain favorable pricing;
•reimbursement is constantly changing, which could greatly affect usage of our products;
•future revenue from product sales will depend on our ability to successfully complete clinical studies, obtain regulatory approvals, and manufacture, market, distribute and commercialize our approved drugs; and
35
•the impact of public health emergencies or the outbreak of disease, such as the COVID-19 pandemic, on us or our partners.
In addition, future revenue from sales of ORLADEYO is subject to uncertainties and will depend on several factors, including the success of our and our partners’ commercialization efforts in the United States and elsewhere, the number of new patients switching to ORLADEYO, patient retention and demand, the number of physicians prescribing ORLADEYO, the rate of monthly prescriptions, reimbursement from third-party and government payors, the number of patients receiving free product, the conversion of patients from our clinical trials and early access programs to commercial customers, our pricing strategy, and market trends.
Even if we are able to successfully commercialize our existing products, or to develop new commercially viable products, certain obligations we have to third parties, including, without limitation, our obligations to pay royalties on certain revenues from ORLADEYO and BCX10013 under the Royalty Purchase Agreements, may reduce the profitability of such products.
We have expanded, and may continue expanding, our development and regulatory capabilities and are implementing sales, marketing and distribution capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We have experienced, and may continue to experience, significant growth in the number of our employees and the scope of our operations in the United States and internationally, particularly in the areas of drug development, regulatory affairs, sales, marketing, and distribution. To manage our growth, we must continue to implement and improve our managerial, operational and financial systems and processes, expand our facilities and continue to recruit and train qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such growth, we may not be able to effectively manage the expansion of our operations, implement appropriate systems and processes in a timely manner or at all, or recruit, train, and retain qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. In addition, if a commercial launch for any product or product candidate for which we recruit a commercial team and establish marketing capabilities in any region is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We depend on third-party vendors in the manufacture and distribution of our products, product candidates and the materials for our products and product candidates. If we cannot rely on existing third-party vendors, we will be required to incur significant costs and potential delays in finding new third-party vendors, which could adversely impact the development and commercialization timeframes for our products and product candidates.
We depend on third-party vendors, including third-party manufacturers, distributors, and specialty pharmacies, in the manufacture and distribution of our products, product candidates, and the materials for our products and product candidates. Often, especially in the early development and commercialization process, we have only one or limited sources for a particular product or service, such as manufacturing and/or distribution. We depend on these third-party vendors to perform their obligations in a timely manner and in accordance with applicable governmental regulations. Our third-party vendors, particularly our third-party manufacturers and distributors, each of which may be the only vendor we have engaged for a particular product, product candidate, or service or in a particular region, may encounter difficulties with meeting our requirements, including, but not limited to, problems involving, as applicable:
•insufficient resources being devoted in the manner necessary to satisfy our requirements within expected timeframes;
•inconsistent production yields;
•product liability claims or recalls of commercial product;
•difficulties in scaling production to commercial and validation sizes;
•interruption of the delivery of materials required for the manufacturing process;
•failure to distribute commercial supplies of our products to commercial vendors or end users in a timely manner;
•scheduling of plant time with other vendors or unexpected equipment failure;
•potential catastrophes that could strike their facilities or have an effect on infrastructure;
•potential impurities in our drug substance or products that could affect availability of product for our clinical trials or future commercialization;
36
•poor quality control and assurance or inadequate process controls;
•failure to provide us with accurate or timely information regarding inventories, the number of patients who are using our products, or serious adverse events and/or product complaints regarding our products;
•inability of third parties to satisfy their financial obligations to us or to others;
•potential breach of the manufacturing or distribution agreement by the third party;
•possible termination or nonrenewal of a critical agreement by the third party at a time that is costly or inconvenient to us; and
•lack of compliance or cooperation with regulations and specifications or requests set forth by the FDA or other foreign regulatory agencies or local customs, particularly associated with ORLADEYO, BCX10013, peramivir and our early-stage compounds.
Many additional factors could cause production or distribution interruptions with the manufacture and distribution of any of our products and product candidates, including human error, natural disasters, pandemics, labor disputes or shortages, acts of terrorism or war, equipment malfunctions, raw material shortages or supply chain issues. If our commercial distribution partners are not able to satisfy our requirements within the expected timeframe, or are unable to provide us with accurate or timely information and data, including with respect to inventories and sales, serious adverse events, and/or product complaints, our business, including our commercialization efforts for and sales of ORLADEYO, may be at risk. In addition, if specialty pharmacy services, including our third-party call center services, which provide patient support and financial services, prescription intake and distribution, reimbursement adjudication, and ongoing compliance support, are not effectively managed, the continuance of our commercialization efforts for and sales of ORLADEYO may be delayed or compromised.
In addition, our contract manufacturers may not be able to manufacture the materials required for our products or product candidates at a cost or in quantities necessary to make them commercially viable. Our raw materials, drug substances, products, and product candidates are manufactured by a limited group of suppliers, including some at a single facility. If any of these suppliers were unable to produce these items, this could significantly impact our supply of products and product candidate material for further preclinical testing and clinical trials. Our third-party manufacturers also may not meet our manufacturing requirements. Furthermore, changes in the manufacturing process or procedures, including a change in the location where the drug is manufactured or a change of a third-party manufacturer, may require prior review and approval in accordance with the FDA’s cGMP and comparable foreign requirements. This review may be costly and time-consuming and could delay or prevent the launch of a product. The FDA or foreign regulatory authorities may at any time implement new standards, or change their interpretation and enforcement of existing standards, for manufacture, packaging or testing of products. If we or our contract manufacturers are unable to comply, we or they may be subject to regulatory action, civil actions or penalties, any of which could be costly to us and could result in a delay or shortage of product.
If we are unable to maintain current third-party relationships, or enter into new agreements with additional third parties on commercially reasonable terms, or at all, or if there is poor manufacturing or distribution performance or failure to comply with any regulatory agency on the part of any of our third-party vendors, we may not be able to complete development of, obtain timely approval of, or commercialize our products and product candidates.
Commercialization of our products by us and our partners is subject to the potential commercialization risks described herein and numerous additional risks. Any potential revenue benefits to us, including in the form of milestone payments, royalties or other consideration are highly speculative.
Commercial success of our products is uncertain and is subject to all the risks and uncertainties disclosed in our other risk factors relating to drug development and commercialization. In addition, commercialization of our products is subject to further risks and may be negatively impacted by a number of factors, including, but not limited to, the following:
•our products may not prove to be adequately safe and effective for market approval in markets other than the markets in which they are currently approved;
•necessary funding for post-marketing commitments and further development of our products may not be available timely, at all, or in sufficient amounts;
•advances in competing products could substantially replace potential demand for our products;
•government and third-party payors may not provide sufficient coverage or reimbursement, which would negatively impact the demand for our products;
37
•we may not be able to supply commercial material to our partners and our partners may not be able to maintain or establish sufficient and acceptable commercial manufacturing, either directly or through third-party manufacturers;
•the commercial demand and acceptance for our products by healthcare providers and by patients may not be sufficient to result in substantial product revenues to us or to our partners and may result in little to no revenue, milestone payments, or royalties to us;
•effectiveness of marketing and commercialization efforts for our products by us or our partners;
•market satisfaction with existing alternative therapies;
•perceived efficacy relative to other available therapies;
•disease prevalence;
•cost of treatment;
•our pricing and reimbursement strategy may not be effective;
•new legislative or regulatory proposals may influence our pricing and reimbursement strategy, which could impact product revenues;
•pricing and availability of imports or alternative products;
•marketing and sales activities of competitors;
•shifts in the medical community to new treatment paradigms or standards of care; and
•relative convenience and ease of administration.
Risks Relating to Competing in Our Industry
We face intense competition, and if we are unable to compete effectively, the demand for our products may be reduced.
The biotechnology and pharmaceutical industries are highly competitive and subject to rapid and substantial technological change. There are many companies seeking to develop products for the same indications that we currently target. Our competitors in the United States and elsewhere are numerous and include, among others, major multinational pharmaceutical and chemical companies and specialized biotechnology firms. Most of these competitors have greater resources than we do, including greater financial resources, larger research and development staffs and more experienced manufacturing, marketing, and sales organizations. In addition, most of our competitors have greater experience than we do in conducting clinical trials and obtaining FDA and other regulatory approvals. Accordingly, our competitors may succeed in obtaining FDA or other regulatory approvals of product candidates more rapidly than we do for products that compete with our products. Companies that complete clinical trials, obtain required regulatory approvals, and commence commercial sale of their drugs before we do may achieve a significant competitive advantage, including patent and FDA exclusivity rights that would delay our ability to market products. We face, and will continue to face, competition in the commercialization of our products, licensing of potential product candidates for desirable disease targets, licensing of desirable product candidates, and development and marketing of our product candidates from academic institutions, government agencies, research institutions and biotechnology and pharmaceutical companies. Competition may also arise from, among other things:
•other drug development technologies;
•methods of preventing or reducing the incidence of disease, including vaccines; and
•new small molecule or other classes of therapeutic agents.
Developments by others may render our products, product candidates, or technologies obsolete or noncompetitive.
We received FDA approval of ORLADEYO, an oral, once-daily therapy for the prevention of HAE attacks in adults and pediatric patients aged 12 years and older, in December 2020. We subsequently received regulatory approvals for ORLADEYO in multiple markets. In addition, we are performing research on or developing products for the treatment of several other rare diseases, including diseases of the complement system. We expect to encounter significant competition for our pharmaceutical products and product candidates. Companies that complete clinical trials, obtain required funding or government support, obtain required regulatory approvals and commence commercial sales or stockpiling orders of their products before their competitors may achieve a significant competitive advantage. In addition, various government entities throughout the world may offer incentives, grants and contracts to encourage additional investment into certain preventative and therapeutic agents, which may have the effect of further increasing the number of our competitors and/or providing advantages to certain competitors. See “Business—Competition” in Part I, Item 1 of this report for further discussion of our competitors, competitive products or programs, and the competitive conditions in these and other therapeutic areas.
38
If one or more of our competitors’ products or programs, including potential competitors not currently identified, are successful, the market for our products may be reduced or eliminated.
Compared to us, many of our competitors and potential competitors have substantially greater:
•capital resources;
•research and development resources, including personnel and technology;
•regulatory experience;
•preclinical study and clinical testing experience;
•manufacturing, marketing, and sales experience; and
•production facilities.
Any of these competitive factors could impede our funding efforts, render our products, product candidates, or technologies noncompetitive or eliminate or reduce demand for our products and product candidates.
Legal and Regulatory Risks
We are subject to various laws and regulations related to our products and product candidates, and if we or our partners do not comply with these laws and regulations, we could face substantial penalties.
Our and our partners’ activities related to approved products or, following their regulatory approval (if applicable), any of our product candidates under development, such as BCX10013, are subject to regulatory and law enforcement authorities in the United States (including the FDA, the Federal Trade Commission, the Department of Justice (“DOJ”), and state and local governments) and their foreign equivalents (including the EMA, MHLW, MHRA, and others).
We are responsible for reporting adverse drug experiences, have responsibility for certain post-approval studies, and may have responsibilities and costs related to a recall or withdrawal of our products from sale in the jurisdictions in which they are approved. We may also incur liability associated with product manufacturing contracted by us or in support of any of our partners. We are required to maintain records and provide data and reports to regulatory agencies related to our products (e.g. risk evaluation and mitigation strategies, track and trace requirements, and adverse events), and we may incur certain promotional regulatory and government pricing risks, all of which could have a material adverse impact on our operations and financial condition. Similar responsibilities would apply upon regulatory approval of any of our other product candidates currently under development.
In addition, we are subject to the federal physician sunshine act and certain similar physician payment and drug pricing transparency legislation in various states. We are also subject to various federal and state laws pertaining to healthcare “fraud and abuse,” including both federal and state anti-kickback and false claims laws. Outside of the United States, we may be subject to analogous foreign laws and regulations in the various jurisdictions in which we operate. These laws and regulations apply to our and our partners’ operations, sales and marketing practices, price reporting, and relationships with physicians and other customers and third-party payors. Although we seek to comply with these statutes, it is possible that our practices, or those of our partners, might be challenged under healthcare fraud and abuse, anti-kickback, false claims or similar laws. Violations of the physician sunshine act and similar legislation or the fraud and abuse laws may be punishable by civil or criminal sanctions, including fines and civil monetary penalties, and future exclusion from participation in government healthcare programs.
The principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to certain regulatory authorities, including the FDA and comparable foreign regulatory authorities. Consequently, the FDA or other regulatory authority may conclude that a financial relationship between us and a principal investigator creates a conflict of interest or otherwise affects interpretation of the study. In the event of a conflict of interest with respect to a study, the integrity of the data generated at the applicable clinical trial site may be questioned or the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or other regulatory authority, as the case may be, and may ultimately lead to the denial of marketing approval of one or more of our product candidates.
The FDA and foreign regulatory authorities may also impose post-approval commitments on us for approved products, which we may not complete successfully or on time for any number of reasons, including, but not limited to, lack of funds to complete the studies and insufficient interest by appropriate sites, investigators or study subjects. We are
39
currently subject to certain post-approval commitments. If we fail to comply with post-approval legal and regulatory requirements, we could be subject to penalties, and our products could be subject to continual recordkeeping and reporting requirements, review and periodic inspections by the FDA and other regulatory bodies. Regulatory approval of a product may be subject to limitations on the indicated uses for which the product may be marketed or to the other restrictive conditions of approval that limit our ability to promote, sell or distribute a product. Furthermore, the approval of our products and any other future product candidates may be subject to requirements for costly post-approval testing and surveillance to monitor their safety or efficacy or certain post-approval labeling, packaging and storage requirements.
Advertising and promotion are subject to stringent oversight from the FDA and foreign regulators, and as an NDA holder, we may be held responsible for any advertising and promotion that is not in compliance with applicable rules and regulations. Applicable regulatory authorities, competitors, and other third parties may take the position that we are not in compliance with such regulations. In addition to medical education efforts, we may offer patient support services to assist patients receiving treatment with our commercially approved products which have increasingly become the focus of government investigation.
Adverse event information concerning approved products must be reviewed, and as an NDA holder, we are required to make expedited and periodic adverse event reports to the FDA and other regulatory authorities. In addition, the research, manufacturing, distribution, sale and promotion of products are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services (“CMS”), other divisions of HHS, the DOJ and individual U.S. Attorney offices within the DOJ, state and local governments, and foreign equivalents of the foregoing. All of these activities are also potentially subject to healthcare false claims and fraud and abuse laws, as well as consumer protection and unfair competition laws.
If our operations with respect to our products that are subject to healthcare laws and regulations are found to be in violation of any of the healthcare fraud and abuse laws described above or in “Business—Government Regulation” in Part I, Item 1 of this report or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, debarment or exclusion from participating in government-funded healthcare programs such as Medicare or Medicaid, and the curtailment or restructuring of our operations. Any penalties, damages, fines, debarment, exclusion, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with all applicable fraud and abuse laws may be costly.
Our employees, consultants and partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are subject to the risk of fraud or other misconduct by our employees, consultants and partners, including intentional or unintentional failures to comply with FDA regulations or similar regulations of comparable other regulatory authorities, provide accurate information to the FDA or comparable other regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable other regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee and consultant misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee and consultant misconduct, whether intentional, reckless, negligent, or unintentional, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
40
We and our partners may be subject to new legislation, regulatory proposals and healthcare payor initiatives that may increase our costs of compliance and adversely affect our or our partners’ ability to market our products or develop our product candidates.
We are subject to new legislation, regulatory, and healthcare payor initiatives, including the PPACA, which made extensive changes to the delivery of healthcare in the United States, as discussed in “Business—Government Regulation” in Part I, Item 1 of this report. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare could result in decreased net revenues from our pharmaceutical products and decrease potential returns from our development efforts. In addition, pharmaceutical and device manufacturers are also required to report and disclose certain payments and transfers of value to, and investment interests held by, physicians and their immediate family members during the preceding calendar year. Failure to submit required information may result in civil monetary penalties for payments, transfers of value, or ownership or investment interests not reported in an annual submission. Compliance with the PPACA and state laws with similar provisions is difficult and time consuming, and companies that do not comply with these state laws face civil penalties. Because of the breadth of these laws and the narrowness of the applicable safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Such a challenge could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
In addition, there have been a number of other legislative and regulatory proposals aimed at changing the pharmaceutical industry. For example, legislation has been enacted in certain states and at a federal level that requires development of an electronic pedigree to track and trace each prescription drug at the saleable unit level through the distribution system. Compliance with these electronic pedigree requirements may increase our operational expenses and impose significant administrative burdens. In addition, our compliance may be deemed insufficient and we could face a material adverse effect on our business, financial condition, results of operations and growth prospects. As a result of these and other new proposals, we may determine to change our current manner of operation, provide additional benefits or change our contract arrangements, any of which could have a material adverse effect on our business, financial condition and results of operations.
Adequate coverage and reimbursement in the United States and other markets is critical to the commercial success of our approved products. Recently in the United States, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. For example, the IRA implements a number of drug pricing measures intended to lower the cost of prescription drugs and related healthcare reforms, including limits on price increases and subjecting an escalating number of drugs to annual price negotiations with CMS. The IRA includes several provisions that will impact our business to varying degrees, including provisions that reduce the out-of-pocket spending cap for Medicare Part D beneficiaries to $2,000 starting in 2025; impose new manufacturer financial liability on all drugs in Medicare Part D; allow the U.S. Government to negotiate Medicare Part B and Part D pricing for certain high-cost drugs and biologics without generic or biosimilar competition; require companies to pay rebates to Medicare for drug prices that increase faster than inflation; and delay the rebate rule that would require pass through of pharmacy benefit manager rebates to beneficiaries. Further, under the IRA, orphan drugs are exempted from the Medicare drug price negotiation program, but only if they have one orphan designation and for which the only approved indication or indications are for that disease or condition. If a product receives multiple rare disease designations or has multiple approved indications for more than one disease or condition, it may not qualify for the orphan drug exemption.
We cannot be sure whether additional legislation or rulemaking related to the IRA will be issued or enacted, how insurance pharmacy benefit managers and other insurance providers that manage benefits for Medicare recipients will react to the IRA, or what impact, if any, such changes will have on the insurance coverage and profitability of our products or any of our product candidates, if approved for commercial use, in the future. The effect of the IRA on our business and the healthcare industry in general is not yet known. The IRA or other government efforts to reduce the price of prescription drugs or to limit the amount that governments pay for healthcare products and services could result in additional pricing pressure and have a significant impact on our business.
In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. Third-party payors are increasingly challenging the prices charged for medical products and services and, in some cases, imposing restrictions on the coverage of particular drugs. Many third-party payors negotiate the price of medical services and products and develop formularies which establish pricing and reimbursement levels. Exclusion of a product from a formulary can lead to its sharply reduced usage in the third-party payor’s patient population. The process for obtaining coverage can be lengthy and costly, and we expect that it could take several months before a particular payor
41
initially reviews a product and makes a decision with respect to coverage. For example, third-party payors may require cost-benefit analysis data from us in order to demonstrate the cost-effectiveness of our products or any other product we might bring to market. For any individual third-party payor, we may not be able to provide data sufficient to gain reimbursement on a similar or preferred basis to competitive products, or at all, which may have a material adverse effect on our business, financial condition and results of operations.
We may be subject to data privacy and security risks, and our actual or perceived failure to comply with regulations and other legal obligations related to privacy and data protection could harm our business.
We may be subject to legal obligations at the federal, state, and local level related to privacy and data protection, as described in “Business—Government Regulation—Data Privacy and Security Laws” in Part I, Item 1 of this report. Compliance with stringent and evolving U.S. data protection laws and regulations could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use, and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. For example, we may be subject to the CCPA, which gives California residents expanded rights to access and require deletion of their personal data, opt out of certain personal data sharing, and receive detailed information about how their personal data is used. The CCPA provides for civil penalties of up to $7,500 per violation and allows private litigants affected by certain data breaches to recover significant statutory damages. Although the CCPA exempts some data processed in the context of clinical trials, the CCPA may increase compliance costs and potential liability with respect to other personal data we may maintain about California residents.
We also may be subject to the GDPR in the EEA and similar legislation in the United Kingdom and Switzerland. See “Business—Government Regulation—Data Privacy and Security Laws” in Part I, Item 1 of this report and “Risks Relating to Our Business—Risks Relating to International Operations—Our actual or perceived failure to comply with European governmental laws and regulations and other legal obligations related to privacy, data protection and information security could harm our business” in this section for additional discussion of privacy laws and regulations. Failure to comply with these laws and regulations could result in government enforcement actions, private litigation, or harm to our reputation and our business.
Despite our efforts, our personnel or third parties on whom we rely may fail to comply with such data privacy and security obligations, which could negatively impact our business operations. If we or the third parties on which we rely fail, or are perceived to have failed, to address or comply with applicable data privacy and security obligations, we could face significant consequences, including, but not limited to, regulatory investigations or actions; litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; and other adverse business consequences.
If, because of our use of hazardous materials, we violate any environmental controls or regulations that apply to such materials, we may incur substantial costs and expenses in our remediation efforts.
Our research and development involves the controlled use of hazardous materials, chemicals and various radioactive compounds. We are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and some waste products. Accidental contamination or injury from these materials could occur. In the event of an accident, we could be liable for any damages that result, and any liabilities could exceed our resources. Compliance with environmental laws and regulations or a violation of such environmental laws and regulations could require us to incur substantial unexpected costs, which would materially and adversely affect our results of operations.
Intellectual Property Risks
If we fail to adequately protect or enforce our intellectual property rights, the value of those rights would diminish, and if we fail to secure the rights to patents of others, it could adversely affect our business.
Our success will depend in part on our ability and the abilities of our partners to obtain, protect and enforce viable intellectual property rights including, but not limited to, trade name, trademark and patent protection for our Company and its products, methods, processes and other technologies we may license or develop, to preserve our trade secrets, and to operate without infringing the proprietary rights of third parties both domestically and abroad. The patent position of biotechnology and pharmaceutical companies is generally highly uncertain, involves complex legal and factual questions and has recently been the subject of much litigation. Neither the United States Patent and Trademark Office (“USPTO”), the Patent Cooperation Treaty offices, nor the courts of the United States and other jurisdictions have consistent policies nor predictable rulings regarding the breadth of claims allowed or the degree of protection afforded under many
42
biotechnology and pharmaceutical patents. Further, we may not have worldwide patent protection for all of our product candidates and our intellectual property rights may not be legally protected or enforceable in all countries throughout the world. In some jurisdictions, some of our product candidates in certain programs, including our HAE program, may have short or no composition of matter patent life and we may therefore rely on orphan drug exclusivity or data exclusivity. There can be no assurance that we will obtain orphan drug exclusivity or data exclusivity in every jurisdiction. Further, in some jurisdictions, we may rely on formulation patents or method of use patents. Both the ability to achieve issuance and the enforcement of formulation and method of use patents can be highly uncertain and can vary from jurisdiction to jurisdiction, and such patents may therefore not adequately prevent competitors and potential infringers in some jurisdictions. The validity, scope, enforceability and commercial value of the rights protected by such patents, therefore, is highly uncertain.
We also rely on trade secrets to protect technology in cases when we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborators and advisors, our ability to receive patent protection or protect our proprietary information may be imperiled.
We may be involved in legal proceedings to protect or enforce our patents, the patents of our partners or our other intellectual property rights, which could be expensive, time consuming and unsuccessful.
Competitors may infringe or otherwise violate our patents, the patents of our licensors or our other intellectual property rights. To counter infringement or unauthorized use, we may be required to file legal claims, which can be expensive, time-consuming, and unsuccessful. An adverse result in any legal proceeding could put one or more of our patents at risk. Our success depends in part on avoiding the infringement of other parties’ patents and other intellectual property rights as well as avoiding the breach of any licenses relating to our technologies and products. In the United States, patent applications filed in recent years are confidential for 18 months, while older applications are not published until the patent issues. As a result, avoiding patent infringement may be difficult and we may inadvertently infringe third-party patents or proprietary rights. These third parties could bring claims against us, our partners or our licensors that even if resolved in our favor, could cause us to incur substantial expenses and, if resolved against us, could additionally cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, our partners or our licensors, we or they could be forced to stop or delay research, development, manufacturing or sales of any infringing product in the country or countries covered by the patent we infringe, unless we can obtain a license from the patent holder. Such a license may not be available on acceptable terms, or at all, particularly if the third party is developing or marketing a product competitive with the infringing product. Even if we, our partners or our licensors were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property.
If we or our partners are unable or fail to adequately initiate, protect, defend or enforce our intellectual property rights in any area of commercial interest or in any part of the world where we wish to seek regulatory approval for our products, methods, processes and other technologies, the value of our products and product candidates to produce revenue would diminish. Additionally, if our products, methods, processes, and other technologies or our commercial use of such products, processes, and other technologies, including, but not limited to, any trade name, trademark or commercial strategy infringe the proprietary rights of other parties, we could incur substantial costs. The USPTO and the patent offices of other jurisdictions have issued to us a number of patents for our various inventions, and we have in-licensed several patents from various institutions. We have filed additional patent applications and provisional patent applications with the USPTO. We have filed a number of corresponding foreign patent applications and intend to file additional foreign and U.S. patent applications, as appropriate. We have also filed certain trademark and trade name applications worldwide. We cannot assure you as to:
•the degree and range of protection any patents will afford against competitors with similar products;
•if and when patents will issue;
•if patents do issue, we cannot be sure that we will be able to adequately defend such patents and whether or not we will be able to adequately enforce such patents; or
•whether or not others will obtain patents claiming aspects similar to those covered by our patent applications.
If the USPTO or other foreign patent office upholds patents issued to others or if the USPTO grants patent applications filed by others, we may have to:
•obtain licenses or redesign our products or processes to avoid infringement;
•stop using the subject matter claimed in those patents; or
43
•pay damages.
We may initiate, or others may bring against us, litigation or administrative proceedings related to intellectual property rights, including proceedings before the USPTO or other foreign patent office. Any judgment adverse to us in any litigation or other proceeding arising in connection with a patent or patent application could materially and adversely affect our business, financial condition and results of operations. In addition, the costs of any litigation or administrative proceeding may be substantial whether or not we are successful.
Our success is also dependent upon the skills, knowledge and experience, none of which is patentable, of our scientific and technical personnel. To help protect our rights, we require all employees, consultants, advisors and partners to enter into confidentiality agreements that prohibit the disclosure of confidential information to anyone outside of our Company and require disclosure and assignment to us of their ideas, developments, discoveries and inventions. These agreements may not provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information, and if any of our proprietary information is disclosed, our business will suffer because our revenues depend upon our ability to license or commercialize our products and product candidates and any such events would significantly impair the value of such products and product candidates.
We have diversified our pipeline to include the development of protein therapeutics, which may create additional risks and challenges.
We have diversified our pipeline beyond small-molecule medicines to develop protein therapeutics. The development of protein therapeutics may create additional risks and challenges, including, among others:
•patent protection for protein therapeutics may be narrower in scope than for our small-molecule medicines, and our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our protein therapeutic candidates or prevent others from designing around our claims;
•formulation issues with our protein therapeutic candidates may require redevelopment of the formulation, which may be time-consuming or unsuccessful;
•the patent applications that we own or in-license may fail to result in issued patents with claims that cover our protein therapeutic candidates in the United States or in other countries;
•our competitors may be able to more easily develop and seek patent protection on similar protein therapeutic candidates; and
•orally-administered drugs are often less expensive and present a reduced treatment burden as compared to protein therapeutics and therefore would have competitive advantages if they were developed and shown to be safe and effective for the indication that our protein therapeutics product candidates are targeting.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biotechnology and pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology and pharmaceutical industries involves both technological and legal complexity. Therefore, obtaining and enforcing such patents is costly, time consuming, and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. Additionally, there have been recent proposals for additional changes to the patent laws of the United States and other countries that, if adopted, could impact our ability to obtain patent protection for our proprietary technology or our ability to enforce our proprietary technology. Depending on future actions by the U.S. Congress, the U.S. courts, the USPTO and the relevant law-making bodies in other countries, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We employ certain individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Our efforts to vet our employees, consultants, and independent contractors and prevent their use of the proprietary information or know-how of others in
44
their work for us may not be successful, and we may in the future be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and distract management and other employees.
Product Liability Risks
We face an inherent risk of liability in the event that the use or misuse of our products or product candidates results in personal injury or death, and our product liability insurance coverage may be insufficient.
If the use or misuse of any products we sell, or a partner sells, harms people, we may be subject to costly and damaging product liability claims brought against us by consumers, healthcare providers, pharmaceutical companies, third-party payors or others. The use of our product candidates in clinical trials, including post-marketing clinical studies, could also expose us to product liability claims. We cannot predict all of the possible harms or side effects that may result from the use of our products or the testing of product candidates, and therefore, the amount of insurance coverage we currently have may not be adequate to cover all liabilities or defense costs we might incur. A product liability claim or series of claims brought against us could give rise to a substantial liability that could exceed our resources. Even if claims are not successful, the costs of defending such claims and potential adverse publicity could be harmful to our business.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and face even greater risks upon commercialization by us of our products or product candidates. We have product liability insurance covering our clinical trials. Clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance or increase our existing coverage at a reasonable cost to protect us against losses that could have a material adverse effect on our business. An individual may bring a product liability claim against us if one of our products or product candidates causes, or is claimed to have caused, an injury or is found to be unsuitable for consumer use. Any product liability claim brought against us, with or without merit, could result in:
•liabilities that substantially exceed our product liability insurance, which we would then be required to pay from other sources, if available;
•an increase of our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable terms, or at all;
•withdrawal of clinical trial volunteers or patients;
•damage to our reputation and the reputation of our products, resulting in lower sales;
•regulatory investigations that could require costly recalls or product modifications;
•litigation costs; and
•the diversion of management’s attention from managing our business.
Risks Relating to Contractual Arrangements
We face risks related to our government-funded programs and are subject to various U.S. Government contract requirements, which may create a disadvantage and additional risks to us.
We had contracts with BARDA/HHS and NIAID/HHS for the development of galidesivir as a treatment for diseases caused by RNA pathogens, including Marburg virus disease, Yellow Fever and Ebola virus disease. In contracting with these government agencies, we became subject to various U.S. Government contract requirements, including general clauses for a cost-reimbursement research and development contract, which may limit our reimbursement. While all government funding for galidesivir expired in 2022, we still face risks related to our U.S. Government contracts.
U.S. Government contracts typically contain a number of extraordinary provisions that would not typically be found in commercial contracts and which may create a disadvantage and additional risks to us as compared to competitors that do not rely on U.S. Government contracts. These risks include the ability of the U.S. Government to unilaterally:
•terminate or reduce the scope of our contract with or without cause;
•interpret relevant regulations (federal acquisition regulation clauses);
•require performance under circumstances which may not be favorable to us;
45
•require an in-process review where the U.S. Government will review the project and its options under the contract;
•control the timing and amount of funding, which impacts the development progress of our programs; and
•audit and object to our contract-related costs and fees, including allocated indirect costs.
Upon termination or expiration of a contract, the U.S. Government may dispute wind-down and termination costs and may question prior expenses under the contract and deny payment of those expenses. Should we choose to challenge the U.S. Government for denying certain payments under a contract, such a challenge could subject us to substantial additional expenses which we may or may not recover.
In addition, as a U.S. Government contractor, we are required to comply with applicable laws, regulations and standards relating to our accounting practices and are subject to periodic audits and reviews, including a final financial audit. As part of any such audit or review, the U.S. Government may review the adequacy of, and our compliance with, our internal control systems and policies, including those relating to our purchasing, property, estimating, compensation and management information systems. Audits under the BARDA/HHS and NIAID/HHS galidesivir contracts may occur at the election of the U.S. Government and have been concluded through fiscal 2019; all subsequent fiscal years are still open and auditable. Based on the results of its audits, the U.S. Government may adjust our contract-related costs and fees, including allocated indirect costs. This adjustment could impact the amount of revenues reported on a historic basis. In addition, in the event BARDA/HHS or NIAID/HHS determines that certain costs and fees were unallowable or determines that the allocated indirect cost rate was higher than the actual indirect cost rate, BARDA/HHS or NIAID/HHS would be entitled to recoup any overpayment from us as a result. In addition, if an audit or review uncovers any improper or illegal activity, we may be subject to civil and criminal penalties and administrative sanctions, including forfeiture of profits, suspension of payments, fines and suspension or prohibition from doing business with the U.S. Government. We could also suffer serious harm to our reputation if allegations of impropriety were made against us. In addition, under U.S. Government purchasing regulations, some of our costs may not be reimbursable or allowed under our contracts. Further, as a U.S. Government contractor, we are subject to an increased risk of investigations, criminal prosecution, civil fraud, whistleblower lawsuits and other legal actions and liabilities as compared to private sector commercial companies.
If we fail to reach milestones or to make annual minimum payments or otherwise breach our obligations under our license agreements, our licensors may terminate our agreements with them and/or seek additional remedies.
If we are unable or fail to meet payment obligations, performance milestones relating to the timing of regulatory filings, product supply obligations, post-approval commitments, or development and commercial diligence obligations; are unable or fail to make milestone payments or material data use payments in accordance with applicable provisions; or fail to pay the minimum annual payments under any of our in-licenses relating to our products or product candidates, our licensors may terminate the applicable license and/or seek other available remedies. As a result, our development of the respective product candidate or commercialization of the product would cease.
Because continuing events of default exist under the PhaRMA Notes, the holders of the PhaRMA Notes may be able to foreclose on the collateral securing the PhaRMA Notes and our equity interest in Royalty Sub. As a result, we may not realize the benefit of future royalty payments, if any, that might otherwise accrue to us following repayment of the PhaRMA Notes and we could otherwise be adversely affected.
In March 2011, JPR Royalty Sub LLC, our wholly-owned subsidiary (“Royalty Sub”), issued $30.0 million in aggregate principal amount of PhaRMA Senior Secured 14% Notes due on December 1, 2020 (the “PhaRMA Notes”). The PhaRMA Notes are secured principally by (i) certain royalty and milestone payments under our agreement with Shionogi (the “Shionogi Agreement”), pursuant to which Shionogi licensed from us the rights to market peramivir in Japan and Taiwan and (ii) the pledge by us of our equity interest in Royalty Sub. Since September 1, 2014, payments from Shionogi have been insufficient for Royalty Sub to service its obligations under the PhaRMA Notes, resulting in a continuing event of default with respect to the PhaRMA Notes since that time. In addition, the PhaRMA Notes had a final legal maturity date of December 1, 2020, at which time the outstanding principal amount of the PhaRMA Notes of $30.0 million, together with accrued and unpaid interest of $20.6 million, was due in full. The failure by Royalty Sub to repay these amounts at the maturity date constituted an additional event of default under the PhaRMA Notes. As Royalty Sub has been unable to service its obligations under the PhaRMA Notes and continuing events of default exist under the PhaRMA Notes, the holders of the PhaRMA Notes may be able to foreclose on the collateral securing the PhaRMA Notes and our equity interest in Royalty Sub and may exercise other remedies available to them under the indenture or other documents related to the PhaRMA Notes. In such event, we may not realize the benefit of future royalty payments, if any, that might
46
otherwise accrue to us following repayment of the PhaRMA Notes, we may incur legal costs, and we might otherwise be adversely affected.
We cannot predict whether holders of PhaRMA Notes will seek to pursue any remedies as a result of the continuing events of default with respect to the PhaRMA Notes. The PhaRMA Notes are the obligation of Royalty Sub. Due to the non-recourse nature of the PhaRMA Notes, in the event of any potential foreclosure, we believe the primary impact to us would be the loss of future royalty payments, if any, from Shionogi and the legal costs associated with retiring the PhaRMA Notes. As a result, we do not currently expect the continuing events of default on the PhaRMA Notes to have a significant impact on our future results of operations or cash flows. However, we cannot assure you that this will be the case or that we will not otherwise be adversely affected as a result of the continuing events of default under the PhaRMA Notes or the failure by Royalty Sub to repay the PhaRMA Notes at maturity.
We wrote off the balance due under the PhaRMA Notes to other income as a debt extinguishment as of December 31, 2021. See “Note 8—Royalty Financing Obligations—RAPIACTA—Non-Recourse Notes Payable—Debt Extinguishment” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report for additional information about the write-off.
We have incurred significant indebtedness, which could adversely affect our business. Additionally, the Pharmakon Loan Agreement contains conditions and restrictions that limit our flexibility in operating our business. We may be required to make a prepayment or repay our outstanding indebtedness earlier than we expect if a prepayment event or an event of default occurs, including a material adverse change with respect to us, which could have a material adverse effect on our business.
On April 17, 2023, we entered into the $450.0 million Pharmakon Loan Agreement (the “Pharmakon Loan Agreement”) with BioPharma Credit Investments V (Master) LP and BPCR Limited Partnership, as lenders, and BioPharma Credit PLC, as collateral agent for the lenders, and closed on an initial term loan thereunder in the principal amount of $300.0 million. As of December 31, 2023, we had an outstanding principal balance under the Pharmakon Loan Agreement of $313.7 million, inclusive of the Pharmakon PIK Interest Payments (as defined in “Note 9—Debt—Pharmakon Loan Agreement” in the Notes to the Consolidated Financial Statements included in Part II, Item 8 of this report). Under the Pharmakon Loan Agreement, we will be required to pay to Pharmakon, for the account of the lenders, a prepayment premium or a make-whole premium, as applicable, plus certain fees or expenses set forth in the Pharmakon Loan Agreement in the event that we prepay, or are required to prepay, voluntarily or pursuant to a mandatory prepayment obligation under the Pharmakon Loan Agreement (e.g., upon a change of control of the Company and specified other events, subject to certain exceptions), all or part of the then-outstanding term loans under the Pharmakon Loan Agreement, in each case, subject to certain exceptions set forth in the Pharmakon Loan Agreement.
Our indebtedness could have important consequences to our stockholders. For example, it:
•increases our vulnerability to adverse general economic or industry conditions;
•limits our flexibility in planning for, or reacting to, changes in our business or the industry in which we operate;
•makes us more vulnerable to increases in interest rates, as borrowings under the Pharmakon Loan Agreement accrue interest at variable, uncapped rates, such that increases in interest rates will increase the associated interest payments that we are required to make on outstanding borrowings;
•requires us to dedicate a portion of our cash flow from operations to interest payments, limiting the availability of cash for other purposes;
•limits our ability to obtain additional financing or refinancing in the future for working capital or other purposes; and
•places us at a competitive disadvantage compared to our competitors that have less indebtedness.
Furthermore, the Pharmakon Loan Agreement contains various covenants that limit our ability to engage in specified types of transactions. Subject to certain exceptions, these covenants limit our ability to, among other things, dispose of assets; engage in certain mergers, acquisitions, and similar transactions; incur additional indebtedness; grant liens; make investments; pay dividends or make distributions or certain other restricted payments in respect of equity; prepay other indebtedness; enter into restrictive agreements; undertake fundamental changes; or amend certain material contracts.
47
The covenants contained in the Pharmakon Loan Agreement could cause us to be unable to pursue business opportunities that we or our stockholders may consider beneficial without the lenders’ permission or without repaying all outstanding obligations under the Pharmakon Loan Agreement.
A breach of any of these covenants could result in an event of default under the Pharmakon Loan Agreement. An event of default will also occur if, among other things, we fail to pay amounts due under the Pharmakon Loan Agreement, we fail to repay certain other indebtedness having an aggregate principal amount in excess of a threshold amount, an insolvency event occurs with respect to us, judgments for the payment of money in excess of a threshold amount are entered into against us, a material adverse change in our business, assets, properties, liabilities, or condition occurs, or a material impairment of our ability to perform our obligations under the Pharmakon Loan Agreement occurs, certain negative regulatory events occur, including without limitation certain withdrawal events with respect to ORLADEYO, or we fail to make required payments under our Royalty Purchase Agreements. In the case of a continuing event of default under the Pharmakon Loan Agreement, the lenders under the Pharmakon Loan Agreement could elect to declare all amounts outstanding to be immediately due and payable, proceed against the collateral in which we granted to the lenders a security interest, or otherwise exercise the rights of a secured creditor. Amounts outstanding under the Pharmakon Loan Agreement are secured by a security interest in, subject to certain exceptions, substantially all of our assets. Because substantially all of our assets are pledged to secure the Pharmakon Loan Agreement obligations, our ability to incur additional secured indebtedness or to sell or dispose of assets to raise capital may be impaired, which could have an adverse effect on our financial flexibility.
Risks Relating to International Operations
International expansion of our business exposes us to business, legal, regulatory, political, operational, financial, and economic risks.
Our business strategy includes international expansion, including the commercialization of products outside of the United States. In addition, we currently conduct clinical studies and regulatory activities and have hired, and expect to continue hiring, employees outside of the United States. Doing business internationally involves a number of risks, including, but not limited to:
•multiple, conflicting, and changing laws and regulations such as privacy and data regulations, transparency regulations, tax laws, export and import restrictions, employment laws, regulatory requirements, and other governmental approvals, permits, and licenses;
•introduction of new health authority requirements and/or changes in health authority expectations;
•failure by us or our partners to obtain and maintain regulatory approvals for the use of our products in various countries;
•complexities and difficulties in obtaining and maintaining protection for, and enforcing, our intellectual property;
•difficulties in staffing and managing foreign operations;
•complexities associated with managing multiple payor reimbursement regimes, government payors, or patient self-pay systems;
•limits on our ability to penetrate international markets;
•financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products, and exposure to foreign currency exchange rate fluctuations, which have been increasingly prevalent alongside a fluctuating U.S. dollar;
•natural disasters and political and economic instability, including wars (e.g., the Ukraine-Russia and Israel-Hamas conflicts), terrorism, political unrest, results of certain elections and votes, actual or threatened public health emergencies and outbreak of disease (e.g., the COVID-19 pandemic), boycotts, adoption or expansion of government trade restrictions, and other business restrictions;
•certain expenses including, among others, expenses for travel, translation, and insurance;
•regulatory and compliance risks that relate to maintaining accurate information and control over commercial operations and activities that may fall within the purview of the FCPA, including its books and records provisions or anti-bribery provisions, or the U.K. Bribery Act and similar foreign laws and regulations; and
•regulatory and compliance risks relating to doing business with any entity that is subject to sanctions administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury.
Any of these factors could significantly harm our international expansion of operations and adversely affect our business and results of operations.
48
Additionally, in some countries, such as Japan and the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control and access. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we or our partners may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be materially harmed.
Foreign currency exchange rate fluctuations could have an adverse impact on our results of operations, financial position, and cash flows.
We conduct operations in many countries outside of the United States involving transactions in a variety of currencies other than the U.S. dollar. These transactions include, without limitation, commercial sales, contract manufacturing, and clinical trial activities. Although most of our revenues and expenses are denominated in U.S. dollars, our commercial sales in Europe are primarily denominated in Euros and British Pounds. We also have foreign currency exposure to fluctuations in other foreign currencies, such as the Swiss Franc, Danish Krone, Swedish Krona, Norwegian Krone, Japanese Yen and Canadian Dollar. Changes in the value of these currencies relative to the U.S. dollar may impact our consolidated operating results, including our revenues and expenses, causing fluctuations in our operating results from period to period and/or resulting in foreign currency transaction losses that adversely impact our results of operations, financial position, and cash flows. As we continue to expand our operations internationally, our exposure to foreign currency transaction gains or losses may become more significant. See “Quantitative and Qualitative Disclosures about Market Risk—Foreign Currency Risk” in Part II, Item 7A of this report for additional information about our foreign currency risk.
Our actual or perceived failure to comply with European governmental laws and regulations and other legal obligations related to privacy, data protection and information security could harm our business.
Outside the United States, an increasing number of laws and regulations may govern data privacy and security. EU member states, the United Kingdom, Switzerland and other countries have adopted data protection laws and regulations, which impose significant compliance obligations. These laws include the GDPR and similar national legislation within the EEA, the United Kingdom GDPR, Switzerland’s Federal Data Protection Act, the EU Clinical Trials Regulation, and the e-Privacy Directive (2002/58/EC), and are discussed in more detail in “Business—Government Regulation—Data Privacy and Security Laws” in Part I, Item 1 of this report. Failure to comply with the requirements of these laws may result in significant fines. For example, the GDPR or related national data protection laws, which may deviate from the GDPR, may result in significant fines of up to 4% of global revenues, or €20.0 million, whichever is greater.
In addition to such fines, failure to comply with the requirements of the GDPR or similar national legislation may result in temporary or definitive bans on data processing and other corrective actions and subject us to litigation and/or adverse publicity, which could have material adverse effects on our reputation and business. As a result of the implementation of the GDPR, we are required to put in place additional mechanisms to ensure compliance with the data protection rules. For example, the GDPR requires us to make more detailed disclosures to data subjects, requires disclosure of the legal basis on which we can process personal data, makes it harder for us to obtain valid consent for processing, requires the appointment of a data protection officer where sensitive personal data (i.e., health data) is processed on a large scale, introduces mandatory data breach notification throughout the European Union, imposes additional obligations on us when we are contracting with service providers and requires us to adopt appropriate privacy governance including policies, procedures, training and data audits. We depend on a number of third parties in relation to the provision of our services, a number of which process personal data of EU individuals on our behalf. With each such provider, we are required to enter into contractual arrangements under which they are contractually obligated to only process personal data according to our instructions, and conduct diligence to ensure that they have sufficient technical and organizational security measures in place.
Compliance with evolving laws regarding the transfer of personal data to the United States and other countries also requires increased resources and may result in increased exposure to regulatory actions, fines and penalties, the inability to transfer data and work with partners, vendors and other third parties, and injunctions against our processing or transferring of personal data necessary to operate our business. We are also subject to evolving European privacy laws on electronic marketing and cookies. The European Union is in the process of replacing the e-Privacy Directive (2002/58/EC) with a new set of rules taking the form of a regulation that will be directly implemented in the laws of each EU member state. While this e-Privacy Regulation was originally intended to be adopted on May 25, 2018, it is still going through the European legislative process and the timing of its adoption remains unclear.
49
Compliance with the requirements imposed by the GDPR and other such laws can be time-consuming, expensive and difficult, and may increase our cost of doing business or require us to change our business practices, and despite our efforts we may not be successful in achieving compliance if our personnel, collaborators, partners or vendors do not comply with applicable data protection obligations. Despite our efforts, our personnel or third parties on whom we rely may fail to comply with such obligations, which could negatively impact our business operations. If we or the third parties on which we rely fail, or are perceived to have failed, to address or comply with applicable data privacy and security obligations, we could face significant consequences, including but not limited to: regulatory investigations or actions; litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; and other adverse business consequences.
The United Kingdom’s decision to withdraw from the European Union could result in increased regulatory and legal complexity, which may make it more difficult for us to do business in Europe and impose additional challenges in securing regulatory approval of our product candidates in Europe.
The United Kingdom’s exit from the European Union, or Brexit, has caused political and economic uncertainty, including in the regulatory framework applicable to our operations and product candidates, and this uncertainty may persist for years. Brexit could, among other outcomes, disrupt the free movement of goods, services and people between the United Kingdom and the European Union, and result in increased legal and regulatory complexities, as well as potential higher costs of conducting business in Europe. The long-term effects of Brexit will depend in part on how the current and future trade agreements between the United Kingdom and the European Union take effect in practice. Changes in U.K. or EU regulations may cause disruption or delays in granting clinical trial authorization or opinions for marketing authorization, disruption of importation and export of active substance and other components of new drug formulations, and disruption of the supply chain for clinical trial product and final authorized formulations.
The cumulative effects of the disruption to the regulatory framework may add considerably to the development lead time to marketing authorization and commercialization of products in the European Union and/or the United Kingdom. It is possible that there will be increased regulatory complexities, which can disrupt the timing of our clinical trials and regulatory approvals. In addition, changes in, and legal uncertainty with regard to, national and international laws and regulations may present difficulties for our clinical and regulatory strategy. Any delay in obtaining, or an inability to obtain, any marketing approvals, as a result of Brexit or otherwise, would prevent us from commercializing our product candidates in the United Kingdom and/or the European Union and restrict our ability to generate revenues and achieve and sustain profitability.
In addition, as a result of Brexit, other European countries may seek to conduct referenda with respect to their continuing membership with the European Union. Given these possibilities and others we may not anticipate, as well as the absence of comparable precedent, it is unclear what financial, regulatory and legal implications the withdrawal of the United Kingdom from the European Union will have and how such withdrawal will affect us, and the full extent to which our business could be adversely affected.
Risks Relating to Technology
If our facilities, or the facilities of our third-party vendors, incur damage or power is lost for a significant length of time, our business will suffer.
We and our third-party vendors store commercial product, clinical and stability samples, and manufacturing data at our facilities that could be damaged if the facilities incur physical damage or in the event of an extended power failure. We have backup power systems in addition to backup generators to maintain power to all critical functions, but any loss of these products or samples could result in significant delays in our commercialization or drug development process.
In addition, we store most of our preclinical and clinical data at our facilities. While duplicate copies of most clinical data are secured off-site, and a significant portion of our data is included in regular backups of our systems, we could lose important data if our facilities incur damage, or if our vendor data systems fail, suffer damage or are destroyed. Any significant degradation or failure of our computer systems could cause us to inaccurately calculate or lose our data. Loss of data could result in significant delays in our drug development process, and any system failure could harm our business and operations.
50
Cyber incidents and related disruptions in our or our third-party vendors’ information technology systems could adversely affect our business.
We are increasingly dependent on information technology systems to operate our business. In addition, the FDA and comparable foreign regulatory authorities regulate, among other things, the record keeping and storage of data pertaining to potential pharmaceutical products. Like other companies in our industry, our information technology systems and infrastructure (as well as those of our third-party providers) and our lab equipment and operations technology may be vulnerable to cyber incidents, intrusions, and other similar activities that threaten the confidentiality, integrity, and availability of our information. These threats come from a variety of sources, including by computer hackers, foreign governments, foreign companies, or competitors, or may be breached by employee error, malfeasance or other disruption. These threats are prevalent, continue to rise, and are becoming increasingly difficult to detect. Recently, there have been reports of disruptions in billing and data systems in healthcare (e.g., the cybersecurity incident affecting Change Healthcare). Such cybersecurity events which materially disrupt the healthcare system upon which our business relies could adversely affect our business if such disruption is widespread and continues for an extended period of time.
Cyber incidents could also include the use of artificial intelligence (“AI”) and machine learning to launch more automated, targeted and coordinated attacks on targets. Cyber incidents may lead to operational outages, loss of intellectual property due to industrial espionage, malware, and financial or data attacks via social engineering. These risks have increased as we have experienced significant growth in the number of our employees and the scope of our operations and as virtual and remote working have become more widely used, and sensitive data is accessed by employees working in less secure, home-based environments. A breakdown, invasion, corruption, destruction, or interruption of information technology systems could negatively impact operations. If our systems are damaged, fail to function properly or otherwise become unavailable, we may incur substantial costs to repair or replace them, and we may experience loss of critical data and interruptions or delays in our ability to perform critical functions, which could adversely affect our business, financial condition or results of operations.
Similarly, the increasing use of AI and machine learning technology in the biopharmaceutical industry presents new risks and challenges. The use of AI-based software may lead to the inadvertent release of confidential or proprietary information, which may adversely impact our ability to realize the benefit of our intellectual property, cause us to incur liabilities as the result of any breaches of confidentiality or impact our ability to comply with data security and privacy laws. Further, as the regulatory framework for these technologies evolves, it is possible that new laws and regulations will be adopted, or that existing laws and regulations may be interpreted in ways that would affect our business, including as a result of the cost to comply with such laws or regulations. In addition, we rely on third-party service providers and technologies to operate significant information technology systems and business infrastructure, and we currently use these providers to perform business critical information technology and business services. Supply-chain attacks have increased in frequency and severity, and we cannot guarantee that third parties’ infrastructure in our supply chain or our third-party partners’ supply chains have not been compromised.
We have experienced cybersecurity threats and incidents, which to date have not had a material impact on our reputation, business, financial condition, or operations; however; there is no assurance that such impacts will not be material in the future.
Any compromise of our data security could also result in a violation of applicable privacy and other laws, significant legal, regulatory, and financial exposure, damage to our reputation, loss or misuse of the information and a loss of confidence in our data security measures, which could harm our business. Loss or misuse of our intellectual property, clinical trial data, or commercially sensitive data could adversely impact our business. While we have implemented security measures designed to protect against security incidents and a significant portion of our data is included in regular backups of our systems, there can be no assurance that our efforts to protect our data and information technology systems will prevent breakdowns or breaches in our systems, or those of third parties with which we do business, and any such events could adversely affect our business.
Other Operational Risks
Health epidemics or pandemics could materially adversely affect our business, operations, clinical development or commercialization plans and timelines, or that of third parties with whom we conduct business, including without limitation our development partners, manufacturers, CROs, and others, as well as the regulatory and government agencies with whom we work.
51
A health epidemic or pandemic, such as the COVID-19 pandemic, and related government orders or evolving business policies and procedures, could cause disruptions to our business, operations, and clinical development or commercialization plans and timelines, as well as the business and operations of third parties with whom we conduct business.
If our operations or those of third parties with whom we conduct business, such as development partners, manufacturers, CROs and others, are impaired or curtailed as a result of such events, the development and commercialization of our products and product candidates could be stopped or delayed, or the costs of such development and commercialization activities could increase, any of which could have a material adverse impact on our business. For example, our suppliers or other vendors may be unable to meet their obligations to us or perform their services as expected. In such circumstances, we may not be able to enter into arrangements with alternative suppliers or vendors or do so on commercially reasonable terms or in a timely manner. Such delays could adversely impact our ability to meet our desired clinical development and any commercialization timelines.
In addition, our clinical trials were affected by the COVID-19 pandemic and we may experience similar delays or interruptions due to COVID-19 or other health epidemics or pandemics in the future, which could adversely impact our clinical trial operations. Health epidemics or pandemics could also affect the operations of regulators and other health and governmental authorities, which could result in delays of reviews and approvals, inspections, or other regulatory activities, including as we continue to expand internationally and bring ORLADEYO to additional global markets.
The global impact of a health epidemic or pandemic such as COVID-19 could also materially affect global economies and financial markets, which could reduce our ability to access the equity or debt capital markets or obtain other sources of capital if needed, which could negatively affect our liquidity. In addition, a recession or market correction could materially affect our business and the value of our common stock. Health epidemics or pandemics could also have the effect of heightening many of the other risks described in this report.
Our business, operations, clinical development or commercialization plans and timelines, and access to capital could be adversely affected by unpredictable and unstable market and economic conditions.
Our business, operations, clinical development or commercialization plans and timelines, and access to capital could be adversely affected by unpredictable and unstable market and economic conditions, including as a result of inflation, increased interest rates, disruption or instability in the banking industry, foreign exchange rate fluctuations, a potential U.S. Government shutdown, instability in connection with the upcoming presidential election in the United States, geopolitical instability (such as the Ukraine-Russia and Israel-Hamas conflicts or rising tensions between China and Taiwan), or public health emergencies, such as the COVID-19 pandemic. The magnitude, duration and long-term effect of each of these factors, as well as the effects of actions taken by governments to address them, are unknown at this time, but they could result in further significant disruption of the global economy and financial markets. Our business may be adversely affected by any related economic downturn, volatile geopolitical and business environment, or continued market instability.
Unstable market and economic conditions could materially affect our ability to access the equity or debt capital markets or obtain other sources of capital if needed in the future, which could negatively affect our liquidity. In addition, a recession or market correction could materially affect our business and the value of our common stock.
Market and economic conditions continue to evolve, with the ultimate impacts being uncertain and subject to change. These effects could be material, and we will continue to monitor the economic climate closely. We do not yet know the full extent and magnitude of the impacts that these developments will have on our business, on the healthcare system, or on the global economy. In addition, unstable market conditions could have the effect of heightening many of the other risks described in this report.
Insurance coverage is increasingly more costly and difficult to obtain or maintain.
While we currently have insurance for our business, property, directors and officers, and our products, insurance is increasingly more costly and narrower in scope, and we may be required to assume more risk in the future. If we are subject to claims or suffer a loss or damage in excess of our insurance coverage, we will be required to bear any loss in excess of our insurance limits. If we are subject to claims or suffer a loss or damage that is outside of our insurance coverage, we may incur significant uninsured costs associated with loss or damage that could have an adverse effect on our operations and financial position. Furthermore, any claims made on our insurance policies may impact our ability to obtain or maintain insurance coverage at reasonable costs or at all.
52
If we fail to retain our existing key personnel or fail to attract and retain additional key personnel, the development of our product candidates, the commercialization of our products, and the related expansion of our business will be delayed or stopped.
We are highly dependent upon our senior management and scientific team, the unexpected loss of whose services might impede the achievement of our development and commercial objectives. Competition for key personnel with the experience that we require is intense and is expected to continue to increase. Our inability to attract and retain the required number of skilled and experienced management, commercial, operational and scientific personnel would harm our business because we rely upon these personnel for many critical functions of our business.
If our risk management committee and other compliance methods are not effective, our business, financial condition and operating results may be adversely affected.
Our ability to identify, manage and respond to the various risks related to our business is largely dependent on our established and maintained compliance, risk, audit and reporting systems and procedures. The Board of Directors has ultimate responsibility for risk oversight of the Company and carries out this duty through its committees. The Board of Directors may delegate oversight authority with respect to certain issues in a committee’s applicable areas of expertise. At the Company level, our senior management team similarly monitors risk through the risk management committee and other sub-committees focused on specific areas of risk (e.g., cybersecurity, quality assurance). Membership of the risk management committee consists primarily of key department heads who are asked to bring to such committee relevant items for discussion that they or their teams have identified at the numerous sub-committees these individuals chair or attend. The risk management committee, along with the other sub-committees in the Company, identifies key risks and mitigation strategies which are reported directly to our senior management, the Audit Committee and to the full Board of Directors on a regular basis.
If our policies, procedures, and compliance systems, including our risk management committee, are not effective, or if we are not successful in monitoring or evaluating the risks to which we are or may be exposed, our business, reputation, financial condition and operating results could be materially adversely affected. We cannot provide assurance that our policies and procedures will always be effective, or that our management or the risk management committee would be able to identify any such ineffectiveness. If our compliance and risk management strategies are not effective, our business, financial condition and operating results may be adversely affected.
Future acquisitions, strategic investments, partnerships, alliances, or divestitures could be difficult to identify and integrate, divert the attention of management, disrupt our business, dilute stockholder value, materially change the risk profile of the Company and could fail to meet our expectations, any of which could adversely affect our operating results and financial condition.
We may in the future seek to acquire or invest in businesses, products or technologies that we believe could complement or expand our portfolio or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating and pursuing businesses or products. In addition, we may not be able to find and identify desirable acquisition targets or be successful in entering into an agreement with any particular target or consummating any such agreement. Even if we do consummate an acquisition, in connection therewith we may be required to issue equity (thereby diluting our current stockholders) or debt, we may not be able to integrate successfully the acquired personnel, operations and technologies, or effectively manage the combined business following the acquisition, or the acquired business could otherwise fail to meet our expectations, which, in each case, could have a material adverse effect on our business projections, financial condition, results of operations and prospects.
In addition, we may divest or license all or a portion of certain business or product categories, which could cause a decline in revenue or profitability and may make our financial results more volatile. We may be unable to complete any such divestiture or license on terms favorable to us, within the expected timeframes, or at all. We may have continued financial exposure to divested or licensed businesses following the completion of any such transaction, including increased costs due to potential litigation, contingent liabilities and indemnification of the buyer or licensee related to, among other things, lawsuits, regulatory matters or tax liabilities. Such divestitures or licenses may also divert management’s attention from our core businesses and lead to potential issues with employees, customers or suppliers.
53
Our business and operations could be negatively affected if we become subject to stockholder activism or hostile bids, which could cause us to incur significant expense, hinder execution of our business strategy and impact our stock price.
Stockholder activism, which takes many forms and arises in a variety of situations, has been increasingly prevalent. Stock price declines may also increase our vulnerability to unsolicited approaches. If we become the subject of certain forms of stockholder activism, such as proxy contests or hostile bids, the attention of our management and our Board of Directors may be diverted from execution of our strategy. Such stockholder activism could give rise to perceived uncertainties as to our future strategy, adversely affect our relationships with business partners and make it more difficult to attract and retain qualified personnel. Also, we may incur substantial costs, including significant legal fees and other expenses, related to activist stockholder matters. Our stock price could be subject to significant fluctuation or otherwise be adversely affected by the events, risks and uncertainties of any stockholder activism.
Risks Relating to Investing in Our Common Stock
Our existing principal stockholders hold a substantial amount of our common stock and may be able to influence significant corporate decisions, which may conflict with the interest of other stockholders.
Some of our stockholders own greater than 5% of our outstanding common stock. Our top ten stockholders own approximately 45% of our common stock and can individually, and as a group, influence our operations based upon their concentrated ownership and may also be able to influence the outcome of matters requiring approval of the stockholders, including the election of our directors and other corporate actions.
Our stock price has been, and is likely to continue to be, highly volatile, which could cause the value of an investment in our common stock to decline significantly.
The market prices for securities of biotechnology companies in general have been highly volatile and may continue to be highly volatile in the future. Moreover, our stock price has fluctuated frequently, and these fluctuations are often not related to our financial results. For the twelve months ended December 31, 2023, the 52-week range of the market price of our stock was from $4.83 to $12.08 per share. The following factors, in addition to other risk factors described in this section, may have, and in some cases have had, a significant impact on the market price of our common stock:
•announcements of technological innovations or new products by us or our competitors;
•developments or disputes concerning patents or proprietary rights;
•additional dilution through sales of our common stock or other derivative securities;
•status of new or existing licensing or collaborative agreements and government contracts;
•announcements relating to the status of our programs;
•us or our partners achieving or failing to achieve development milestones;
•publicity regarding actual or potential medical results relating to products under development by us or our competitors;
•publicity regarding certain public health concerns for which we are or may be developing treatments;
•regulatory developments in both the United States and foreign countries;
•public concern as to the safety of pharmaceutical products;
•actual or anticipated fluctuations in our operating results;
•changes in financial estimates or recommendations by securities analysts and the comparison of such estimates to our actual results;
•changes in our public guidance;
•changes in the structure of healthcare payment systems, including developments in price control legislation;
•announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, capital commitments or other monetization transactions;
•additions or departures of key personnel or members of our Board of Directors;
•purchases or sales of substantial amounts of our stock by existing stockholders, including officers or directors;
•economic and other external factors or other disasters or crises; and
•period-to-period fluctuations in our financial results.
This volatility could cause the value of an investment in our common stock to decline significantly. In addition, companies that have experienced volatility in the market price of their stock in the past have been subject to securities class action litigation. Securities litigation, and any other type of litigation, brought against us could result in substantial costs
54
and divert our management’s attention from other business concerns, which could seriously harm our business and adversely affect our results of operations.
We have identified a material weakness in our internal control over financial reporting. This material weakness could divert management’s attention and adversely affect our ability to produce accurate and timely financial statements, which may adversely affect investor confidence in us and our financial reporting, adversely affect our business and operating results and may negatively impact the trading price of our common stock.
Our management has identified a material weakness in our internal control over financial reporting, as described in “Controls and Procedures” in Part II, Item 9A of this report. As further described in that section, we are implementing measures, and will continue to implement measures, to remediate the material weakness identified by management and to improve our internal control over financial reporting such that these controls are designed, implemented, and operating effectively. This material weakness will not be considered remediated until the applicable remediated controls are operating for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively. Remediating the material weakness could take longer than expected and divert management’s attention away from other areas of the business.
A material weakness, as defined in Rule 12b-2 under the Exchange Act, is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Although we believe the financial statements included in this report fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented in conformity with U.S. GAAP, any failure to maintain effective internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If our internal control over financial reporting is not effective, investors may lose confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities.
Future sales and issuances of securities may dilute the ownership interests of our current stockholders and cause our stock price to decline.
Future sales of our common stock by us or our current stockholders into the public market could cause the market price of our stock to fall. As of December 31, 2023, there were 205,770,667 shares of our common stock outstanding. We may from time to time issue securities in relation to a license arrangement, collaboration, merger or acquisition. We may also sell, for our own account, shares of common stock or other equity securities, from time to time at prices and on terms to be determined at the time of sale.
As of December 31, 2023, there were 41,096,637 stock options and restricted stock units outstanding and 3,375,511 shares available for issuance under our Amended and Restated Stock Incentive Plan, 6,442,678 stock options and restricted stock units outstanding and 1,650,708 shares available for issuance under our Amended and Restated Inducement Equity Incentive Plan, and 5,454,386 shares available for issuance under our Amended and Restated Employee Stock Purchase Plan. In addition, we could also make equity grants outside of our Amended and Restated Stock Incentive Plan or Amended and Restated Inducement Equity Incentive Plan. The shares underlying existing stock options, restricted stock units and possible future stock options, stock appreciation rights, restricted stock units and stock awards have been, or will be, registered pursuant to registration statements on Form S-8.
If some or all of such shares are sold or otherwise issued into the public market over a short period of time, our current stockholders’ ownership interests may be diluted and the value of all publicly traded shares is likely to decline, as the market may not be able to absorb those shares at then-current market prices. Additionally, such sales and issuances may make it more difficult for us to sell equity securities or equity-related securities in the future at a time and price that our management deems acceptable, or at all.
We have anti-takeover provisions in our corporate charter documents that may result in outcomes with which you do not agree.
Our Board of Directors has the authority to issue up to 5,000,000 shares of undesignated preferred stock and to determine the rights, preferences, privileges and restrictions of those shares without further vote or action by our stockholders. The rights of the holders of any preferred stock that may be issued in the future may adversely affect the
55
rights of the holders of common stock. The issuance of preferred stock could make it more difficult for third parties to acquire a majority of our outstanding voting stock.
In addition, our Certificate of Incorporation provides for staggered terms for the members of the Board of Directors and supermajority approval of the removal of any member of the Board of Directors and prevents our stockholders from acting by written consent. Our Certificate of Incorporation also requires supermajority approval of any amendment of these provisions. These provisions and other provisions of our Amended and Restated By-Laws and of Delaware law applicable to us could delay or make more difficult a merger, tender offer or proxy contest involving us.
We have never paid dividends on our common stock and do not anticipate doing so in the foreseeable future.
We have never paid cash dividends on our stock. We currently intend to retain all future earnings, if any, for use in the operation of our business. Accordingly, we do not anticipate paying cash dividends on our common stock in the foreseeable future.
Our Amended and Restated By-Laws provide that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for certain litigation that may be initiated by our stockholders, which may limit a stockholder’s ability to obtain a favorable judicial forum for such disputes with us or our directors, officers or employees.
Our Amended and Restated By-Laws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, stockholders, employees or agents to us or our stockholders, (iii) any action asserting a claim against us or any of our directors, officers, stockholders, employees or agents arising out of or relating to any provision of the General Corporation Law of Delaware or our Certificate of Incorporation or Amended and Restated By-Laws, or (iv) any action against us or any of our directors, officers, stockholders, employees or agents governed by the internal affairs doctrine of the State of Delaware. This exclusive forum provision does not apply to establish the Delaware Court of Chancery as the forum for actions or proceedings brought to enforce a duty or liability created by the Securities Act or the Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction.
This exclusive forum provision may limit a stockholder’s ability to choose its preferred judicial forum for disputes with us or our directors, officers, employees or agents, which may discourage the filing of lawsuits with respect to such claims. If a court were to find this exclusive forum provision to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in another jurisdiction, which could adversely affect our business and financial condition.
General Risk Factors
Natural disasters, epidemic or pandemic disease outbreaks, trade wars, armed conflicts, political unrest or other events could disrupt our business or operations or those of our development partners, manufacturers, regulators or other third parties with whom we conduct business now or in the future.
A wide variety of events beyond our control, such as natural disasters (including as a result of climate change), epidemic or pandemic disease outbreaks (such as the COVID-19 pandemic), trade wars, armed conflict, political unrest, government shutdowns, instability in connection with the upcoming presidential election in the United States, or other events could disrupt our business or operations or those of our development partners, manufacturers, regulatory authorities, or other third parties with whom we conduct business. These events may cause businesses and government agencies to be shut down, supply chains or trade to be interrupted, slowed, or rendered inoperable, and individuals to become ill, quarantined, or otherwise unable to work and/or travel due to health reasons or governmental restrictions. If our operations or those of third parties with whom we conduct business are impaired or curtailed as a result of these events, the development and commercialization of our products and product candidates could be impaired or halted, which could have a material adverse impact on our business. See, for example, “Risk Factors—Risks Relating to Our Business—Other Operational Risks—Our business, operations, clinical development or commercialization plans and timelines, and access to capital could be adversely affected by unpredictable and unstable market and economic conditions.” In addition, other events, such as the Ukraine-Russia and Israel-Hamas conflicts, or rising tensions between China and Taiwan, could adversely impact our business. For example, the conflicts could lead to sanctions, embargoes, supply shortages, regional instability, geopolitical shifts, cyber-attacks, other retaliatory actions, and adverse effects on macroeconomic conditions,
56
currency exchange rates, and financial markets, which could adversely impact our operations and financial results, as well as those of third parties with whom we conduct business.
We are subject to legal proceedings, which could harm our reputation or result in other losses or unexpected expenditure of time and resources.
From time to time, we may be involved in disputes, including, without limitation, disputes with our employees, collaborative partners, and third-party vendors. We may be called upon to initiate legal proceedings or to defend ourselves in such legal proceedings relating to our relationships with these parties, our decisions and actions or omissions with respect thereto, and our business. In addition, if our stock price is volatile, we may become involved in securities class action lawsuits in the future. Due to the inherent uncertainties in legal proceedings, we cannot accurately predict the ultimate outcome of any such proceeding. An unfavorable outcome in any such proceeding could have an adverse impact on our business, financial condition and results of operations. Any current or future dispute resolution or legal proceeding, regardless of the merits of any such proceeding, could harm our reputation and result in substantial costs and a diversion of management’s attention and resources that are needed to successfully run our business.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
We maintain a cybersecurity program that is reasonably designed to assess, identify, and manage risks from cybersecurity threats that may result in material adverse effects on the confidentiality, integrity, and availability of our information systems.
Governance
Board of Directors
Our Board of Directors, directly and through its committees, oversees the Company’s risk management function. The Board of Directors has delegated the primary responsibility to oversee cybersecurity matters to the Audit Committee. The Audit Committee reviews the measures implemented by the Company to identify and mitigate data protection and cybersecurity risks. As part of such reviews, the Audit Committee regularly receives reports and presentations from members of our Cybersecurity Steering Committee as appropriate, with a minimum frequency of once per year. These reports and presentations address a wide range of topics including recent developments, status of ongoing and planned cybersecurity initiatives and strategies, evolving standards, vulnerability assessments, third-party and independent reviews, the threat environment, security spend, technological trends and information security considerations arising with respect to the Company’s peers and third parties. The Audit Committee reports to the Board of Directors on data protection and cybersecurity matters. We have protocols by which certain cybersecurity incidents are escalated within the Company and, where appropriate, reported to the Audit Committee, as well as ongoing updates regarding any such incident until it has been addressed.
Management
At the management level, the Chief Financial Officer and Chief Legal Officer attend meetings of the Company’s Cybersecurity Steering Committee (discussed further below) to receive reports on ongoing cybersecurity matters. This ensures that management is involved in an ongoing dialogue regarding the Company’s material risks from cybersecurity threats. In addition, members of the Cybersecurity Steering Committee provide updates on the Company’s cybersecurity control and risk posture and the status of ongoing and planned cybersecurity initiatives and strategies to the Company’s senior management team on an annual basis.
Cybersecurity Steering Committee
The Company has implemented a broad spectrum cross-functional approach to assessing, identifying, and managing risks from cybersecurity threats. Our Cybersecurity Steering Committee has broad oversight of the Company’s cybersecurity risk management processes. The Cybersecurity Steering Committee is composed of the Company’s Chief Financial Officer, Chief Legal Officer, Senior Vice President, Information Technology, senior cybersecurity professionals,
57
members of the finance and legal departments, and other individuals invited as appropriate on an ad hoc basis. On at least a quarterly basis, the Cybersecurity Steering Committee meets to discuss recent cybersecurity events or threats, status of ongoing and planned cybersecurity initiatives and strategies, external cybersecurity trends, and risk management measures implemented by the Company to identify and mitigate data protection and cybersecurity risks, among other topics. In addition to the scheduled meetings, the Cybersecurity Steering Committee is informed of potentially material cybersecurity events as they arise.
Within the Cybersecurity Steering Committee, our virtual Chief Information Security Officer (vCISO) and our Senior Manager, Security Engineering are primarily responsible for assessing, monitoring, and managing our cybersecurity risks. Our vCISO is a seasoned cyber consultant providing CISO-level advisory services to the Company and reports to the Senior Vice President, Information Technology, who is directly managed by the Chief Financial Officer. He has held CISO positions in several Fortune-500 companies across multiple industry sectors, has worked in information security for over 23 years, is a Certified Information Systems Security Professional (CISSP), and has extensive experience with multiple commercial and government security frameworks. He leads the Company’s information security program and sets the strategic direction for, and establishes and governs the structure of, the program.
Our Senior Manager, Security Engineering is managed by the Company’s Executive Director, IT Infrastructure & Operations, who directly reports to the Senior Vice President, Information Technology. He has over 38 years of experience in information security and data privacy and has CISSP and Cisco Certified Network Associate (CCNA) certifications. He implements and oversees processes for the regular monitoring of our information systems and detection of cybersecurity vulnerabilities.
The Cybersecurity Steering Committee also works closely with members of the legal department to oversee compliance with legal and regulatory security requirements. In addition, the Cybersecurity Steering Committee has implemented controls and procedures that provide for the prompt escalation of certain cybersecurity incidents so that decisions regarding the public disclosure and reporting of such incidents can be made by management in a timely manner.
Risk Management and Strategy
Cybersecurity Program
The Company’s cybersecurity program leverages the National Institute of Standards and Technology (NIST) Cybersecurity Framework (CSF) for governance and program management and refers to the Center for Internet Security (CIS) guidelines when reviewing the Company’s security controls posture. The Company uses certain advanced security measures, regular system audits, third party monitoring tools, and ongoing intelligence gathering on the latest developments in cybersecurity to identify, assess, and manage potential vulnerabilities and risks. In addition, the Company engages third parties to assist with assessing, identifying and managing material risks from cybersecurity threats. Once the relevant material risks have been identified, the Company implements controls and processes to help manage these risks, including conducting tabletop exercises to simulate response to a cybersecurity incident, regular testing (e.g., penetration tests, vulnerability scanning) and control gap analyses and assessments designed to confirm appropriate security controls are in place and are maintaining functionality in accordance with the established policies.
We also employ systems and processes designed to oversee, identify, and reduce the potential impact of cybersecurity threats associated with any third-party vendor, service provider or customer or otherwise implicating the third-party technology and systems we use.
Our cybersecurity program is integrated into the Company’s overall risk management framework to help identify, assess, educate, and manage the Company’s cybersecurity risk. Our Board of Directors and the Audit Committee, in its role assisting the Board of Directors in its oversight of the Company’s risk management function, consider cybersecurity threat risks alongside other Company risks as part of our overall risk assessment.
Incident Response
The Company has adopted a technology incident response plan (IRP) applicable to all Company employees and contractors, which sets forth the process for responding to and documenting data and information technology-related incidents such as security breaches, system failures, data loss, and service interruption. The IRP provides a standardized framework for investigating, containing, documenting and mitigating cybersecurity incidents, including reporting findings
58
and keeping senior management and other key stakeholders informed and involved as appropriate. The Company’s employees are required to review the IRP and undergo additional cybersecurity training on a regular basis.
Material Cybersecurity Risk, Threats & Incidents
As detailed elsewhere in this report, we rely on information technology systems and third-party providers to operate our business. Despite ongoing efforts to continually improve our and our third-party providers’ ability to protect against cyber incidents, our networks and infrastructure may be vulnerable to cyberattacks or intrusions, which could result in a violation of applicable privacy and other laws, significant legal and financial exposure, damage to our reputation, loss or misuse of the information or a loss of confidence in our data security measures, among other consequences. While we have not experienced any material cybersecurity threats or incidents, there can be no guarantee that we will not be the subject of future successful attacks, threats, or incidents. See “Risk Factors—Risks Relating to Our Business—Risks Relating to Technology—Cyber incidents and related disruptions in our or our third-party vendors’ information technology systems could adversely affect our business” in Part I, Item IA of this report for additional information on cybersecurity risks we face, which should be read together with the foregoing information.
ITEM 2. PROPERTIES
We lease property in both Durham, North Carolina and Birmingham, Alabama. Our headquarters, including our clinical and regulatory operations, are based in Durham, while our principal research facility is located in Birmingham. We currently lease approximately 24,500 square feet in Durham through leases expiring June 30, 2024 and August 31, 2025, and we lease approximately 49,000 square feet in Birmingham through July 31, 2030, with options for additional extensions. We also contract for smaller offices in a number of other countries. We believe that our facilities are adequate for our current and planned future operations.
ITEM 3. LEGAL PROCEEDINGS
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
59
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock trades on the Nasdaq Global Select Market under the symbol BCRX.
Holders
As of February 23, 2024, there were approximately 152 holders of record of our common stock.
Dividends
We have never paid cash dividends and do not anticipate paying cash dividends in the foreseeable future.
Stock Performance Graph
This performance graph is not “soliciting material,” is not deemed filed with the SEC and is not to be incorporated by reference in any filing by us under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. The stock price performance shown on the graph is not necessarily indicative of future price performance.
PERFORMANCE GRAPH FOR BIOCRYST
Indexed Comparison Since 2018
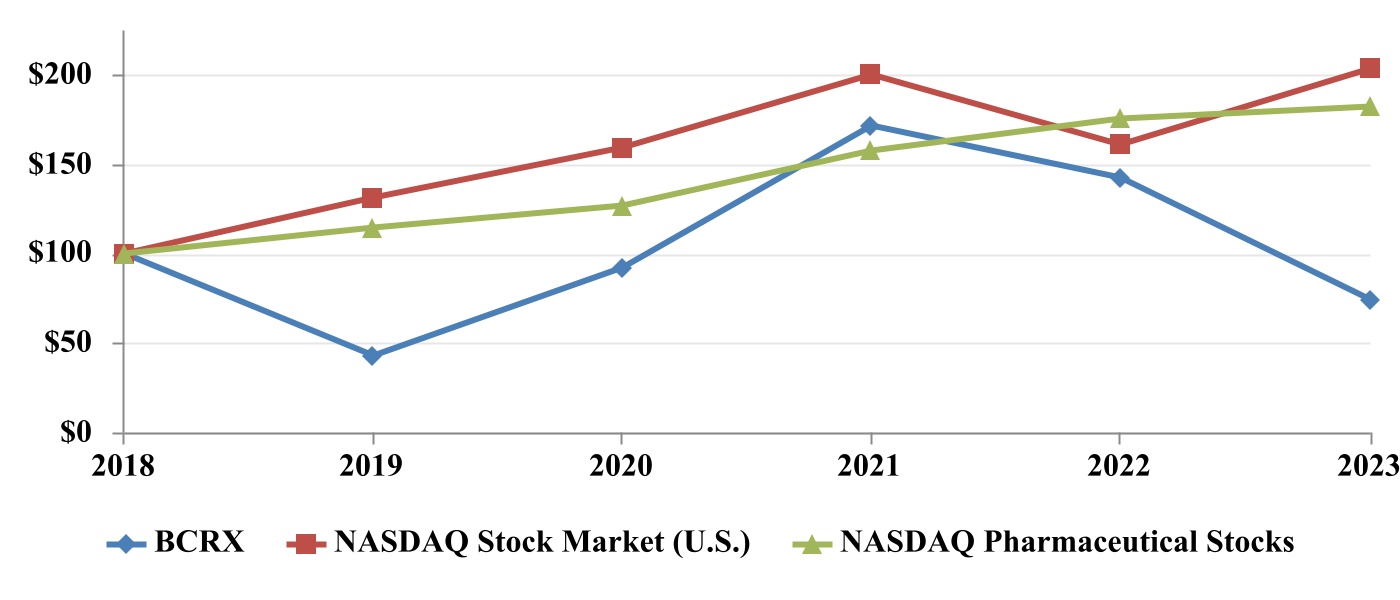
60
Beginning Investment at 12/31/18 | Investment at 12/31/19 | Investment at 12/31/20 | Investment at 12/31/21 | Investment at 12/31/22 | Investment at 12/31/23 | ||||||||||||||||||||||||||||||
| BioCryst Pharmaceuticals, Inc. | $ | 100.00 | $ | 42.75 | $ | 92.32 | $ | 171.62 | $ | 142.26 | $ | 74.23 | |||||||||||||||||||||||
| Nasdaq Stock Market (United States) | 100.00 | 131.17 | 159.07 | 200.26 | 160.75 | 203.23 | |||||||||||||||||||||||||||||
| Nasdaq Pharmaceutical Stocks | 100.00 | 114.51 | 126.56 | 157.42 | 175.29 | 182.08 | |||||||||||||||||||||||||||||
The above graph measures the change in a $100 investment in our common stock based on its closing price of $8.07 on December 31, 2018 and its year-end closing price thereafter. Our relative performance is then compared with the CRSP Total Return Indexes for the Nasdaq Stock Market (United States) and Nasdaq Pharmaceutical Stocks.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
There were no repurchases of our common stock during the fourth quarter of 2023.
ITEM 6. RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis (“MD&A”) is intended to help the reader understand our results of operations and financial condition. MD&A is provided as a supplement to, and should be read in conjunction with, our audited financial statements and the accompanying notes to the financial statements and other disclosures included in this report (including the “Cautionary Note Regarding Forward-Looking Statements” at the beginning of this report and the “Risk Factors” section in Part I, Item 1A of this report).
Overview
We are a global biotechnology company with a deep commitment to improving the lives of people living with complement-mediated and other rare diseases. We leverage our expertise in structure-guided drug design with the goal of developing first-in-class or best-in-class oral small-molecule and protein therapeutics to target difficult-to-treat rare diseases. In addition to these discovery and development efforts, our business strategy includes the efficient commercialization of these drugs in the United States and certain other regions upon regulatory approval. By focusing on rare disease markets, we believe that we can more effectively control the costs of, and our strategic allocation of financial resources toward, post-approval commercialization.
Products and Product Candidates
ORLADEYO® (berotralstat). ORLADEYO is an oral, once-daily therapy discovered and developed by us for the prevention of hereditary angioedema (“HAE”) attacks. ORLADEYO is approved in the United States and multiple global markets for the prevention of HAE attacks in adults and pediatric patients 12 years and older.
We have built out our U.S. commercial infrastructure to support the launch and continued commercialization of ORLADEYO in the United States and are continuing to build our commercial infrastructure to support launches in other markets. Based on proprietary analyses of HAE prevalence and market research studies with HAE patients, physicians, and payors in the United States and Europe, and three full years of commercialization experience with ORLADEYO, we anticipate the global commercial market for ORLADEYO has the potential to reach a global peak of $1 billion in annual
61
net ORLADEYO revenues. We expect approximately 80 percent of our revenue at peak to come from the United States. These expectations are subject to numerous risks and uncertainties that may cause our actual results, performance, or achievements to be materially different. There can be no assurance that our commercialization methods and strategies will succeed, or that the market for ORLADEYO will develop in line with our current expectations. See “Risk Factors—Risks Relating to Our Business—Risks Relating to Drug Development and Commercialization—There can be no assurance that our or our partners’ commercialization efforts, methods, and strategies for our products or technologies will succeed, and our future revenue generation is uncertain” in Part I, Item 1A of this report for further discussion of these risks.
Revenue from sales of ORLADEYO in 2023, which was our third full year of ORLADEYO sales, is discussed under “Results of Operations” in this MD&A. Revenue from sales of ORLADEYO in future periods is subject to uncertainties and will depend on several factors, including the success of our and our partners’ commercialization efforts in the United States and elsewhere, the number of new patients switching to ORLADEYO, patient retention and demand, the number of physicians prescribing ORLADEYO, the rate of monthly prescriptions, reimbursement from third-party and government payors, the number of patients receiving free product, the conversion of patients from our clinical trials and early access programs to commercial customers, our pricing strategy, and market trends. We are continuing to monitor and analyze this data as we continue to commercialize ORLADEYO.
Complement Program. The goal of our overall complement program is to advance several first-in-class and/or best-in-class compounds across multiple pathways in the complement system to treat many complement-mediated diseases. We are pursuing oral medicines and protein therapeutics directed at targets across the classical, lectin, and terminal pathways of the complement system. For more information on these therapies, see “Recent Developments” below in this MD&A.
RAPIVAB®/RAPIACTA®/PERAMIFLU® (peramivir injection). RAPIVAB (peramivir injection) is approved in the United States for the treatment of acute uncomplicated influenza for patients six months and older. Peramivir injection is also approved in Canada (RAPIVAB), Australia (RAPIVAB), Japan (RAPIACTA), Taiwan (RAPIACTA), and Korea (PERAMIFLU).
Netherton Syndrome. BCX17725 is a potent and selective investigational fusion protein KLK5 inhibitor designed to provide best-in-class, potentially disease-modifying treatment for people with Netherton syndrome. Netherton syndrome is a rare, lifelong genetic disorder that often presents in neonates or infancy. The disease is caused by the deficiency of a natural inhibitor (SPINK5) of KLK5, a serine protease responsible for regulating skin shedding. Patients may have red, scaly and inflamed skin and susceptibility to recurrent immune reactions. Netherton syndrome can be life-threatening, especially during infancy when patients are vulnerable to dehydration and recurrent infections. Currently, there is no approved treatment for Netherton syndrome.
Avoralstat. We are developing our investigational plasma kallikrein inhibitor, avoralstat, with Clearside Biomedical, Inc.’s (“Clearside”) Microinjector® to deliver avoralstat to the back of the eye through the suprachoroidal space to treat patients with diabetic macular edema (“DME”). DME is the most common cause of vision loss in individuals with diabetes, and at least one-third of patients have persistent DME despite anti-vascular endothelial growth factor therapies, which are administered via monthly injection. Avoralstat, which was previously studied in an oral formulation in a phase 3 trial in patients with HAE, has high potency and low solubility, two characteristics we believe are important to achieving potential efficacy with reduced dosing frequency in the eye for DME patients.
Revenues and Expenses
Our revenues are difficult to predict and depend on several factors, including those discussed in the “Risk Factors” section in Part I, Item 1A of this report. For example, our revenues depend, in part, on regulatory approval decisions for our products and product candidates, the effectiveness of our and our collaborative partners’ commercialization efforts, market acceptance of our products, particularly ORLADEYO, and the resources dedicated to our products and product candidates by us and our collaborative partners, as well as entering into or modifying licensing agreements for our product candidates. Furthermore, revenues related to our collaborative development activities are dependent upon the progress toward, and the achievement of, developmental milestones by us or our collaborative partners.
Our operating expenses are also difficult to predict and depend on several factors, including research and development expenses, drug manufacturing, clinical research activities, the ongoing requirements of our development programs, the costs of commercialization, the availability of capital and direction from regulatory agencies, which are difficult to predict, and the factors discussed in the “Risk Factors” section in Part I, Item 1A of this report. Management may be able to control the timing and level of research and development and selling, general and administrative expenses,
62
but many of these expenditures will occur irrespective of our actions due to contractually committed activities and/or payments.
As a result of these factors, we believe that period-to-period comparisons are not necessarily meaningful, and you should not rely on them as an indication of future performance. Due to the foregoing factors, it is possible that our operating results will be below the expectations of market analysts and investors. In such event, the prevailing market price of our common stock could be materially adversely affected.
Recent Developments
ORLADEYO (berotralstat)
On November 2, 2023, we announced that Austria approved the reimbursement of ORLADEYO for the targeted prophylaxis of HAE in patients 12 years of age or older.
On November 3, 2023, we announced that we expect to submit a U.S. supplemental new drug application for the pediatric use of ORLADEYO in 2025. The ongoing APeX-P clinical trial is assessing an oral granule formulation of ORLADEYO in pediatric HAE patients who are 2 to <12 years of age.
On November 10, 2023, we announced new analyses of real-world use of ORLADEYO leading to a reduction in monthly attack rates in patients with HAE who have normal C1-inhibitor level and function. We also announced a new post-hoc analysis from the APeX-S clinical trial that showed a sustained reduction in HAE attacks compared to patients’ self-reported baseline attack rates.
On November 21, 2023, we announced that the Spanish Ministry for Health granted marketing authorization for ORLADEYO for the routine prevention of recurrent HAE attacks in HAE patients 12 years and older.
On November 29, 2023, we announced that the National Administration of Drugs, Foods, and Medical Devices in Argentina granted approval for ORLADEYO for the prophylaxis of HAE attacks in adults and pediatric patients 12 years of age or older.
On December 19, 2023, we announced that data from the open-label extension of the APeX-2 trial of ORLADEYO for the prophylactic treatment of HAE in patients 12 years and older have been published online by the Journal of Allergy and Clinical Immunology: In Practice (JACI: In Practice). The authors concluded that ORLADEYO was generally well tolerated, provided rapid and sustained reductions in HAE attacks and improved quality of life over the study duration of 96 weeks.
On February 19, 2024, we announced that the Italian Medicines Agency finalized reimbursement and recommended ORLADEYO for the routine prevention of recurrent attacks of HAE in eligible patients 12 years and older.
On February 23, 2024, we announced new analyses of real-world use of ORLADEYO that showed patients who initiated ORLADEYO experienced rapid, substantial and sustained reductions in attack rates through 18 months of treatment regardless of the severity of their disease, their history of prior prophylaxis or their C1-inhibitor level and function.
Complement-Mediated Diseases
BCX10013
On October 26, 2023, we announced the enrollment of the first patient in a proof-of-concept clinical trial evaluating BCX10013. The goal of this proof-of-concept trial is to understand the preliminary efficacy and safety profile of once-daily dosing with BCX10013. On November 3, 2023, we announced that we expect to report data from our ongoing proof-of-concept trial in 2024.
On November 3, 2023, we presented data at our R&D Day from the recently completed 160 mg cohort of our multiple ascending dose healthy volunteer trial, which highlights the strength and duration of alternative pathway suppression achieved at this dose level, supporting once-daily clinical dosing.
63
On January 5, 2024, we announced that, if our ongoing proof-of-concept trial produces best-in-class data, we plan to out-license late-stage development and commercialization of BCX10013 to a partner that can drive the speed and breadth of investment required to accelerate BCX10013 for patients across multiple complement-mediated diseases and maximize the commercial potential of the program.
Oral C5 Inhibitor
On November 3, 2023, we announced that we are developing an oral C5 inhibitor that could be the first targeted oral therapy with competitive efficacy to currently approved injected and infused anti-C5 therapies, such as eculizumab and ravulizumab. A drug with this profile could enable patients with generalized myasthenia gravis (“gMG”) to switch from infused therapy and address their disease earlier in the treatment paradigm. gMG is a chronic autoimmune, neuromuscular disease that causes muscle weakness that worsens after periods of activity.
Bifunctional Complement Inhibitor
On November 3, 2023, we announced that we are developing a bifunctional complement inhibitor anti-C2 monoclonal antibody that also inhibits the alternative pathway. This investigational candidate could be a first-in-class combined inhibitor of the classical, lectin and alternative pathways of the complement system to treat complex renal complement-mediated diseases like Immunoglobulin A Nephropathy and lupus nephritis, which are influenced by multiple complement pathways.
Oral C2 Inhibitor
On November 3, 2023, we announced that we are developing a classical and lectin pathway complement inhibitor to treat autoimmune hemolytic anemias, including cold agglutinin disease (“CAD”) and warm autoimmune hemolytic anemia (“wAIHA”). The limited approved options for treating diseases like CAD and wAIHA are injectable or infused. An oral C2 inhibitor developed by us could be first-in-class and allow patients to switch from infused therapy and address their disease earlier in the treatment paradigm. Inhibiting C2 could decrease red cell destruction (hemolysis) in autoimmune hemolytic anemias by blocking the classical and lectin pathways.
Netherton Syndrome
On November 3, 2023, we announced that we are developing BCX17725, a potent and selective investigational fusion protein KLK5 inhibitor designed to provide best-in-class, potentially disease-modifying treatment for people with Netherton syndrome.
Avoralstat
On November 3, 2023, we announced that we entered into a license agreement (the “Clearside Agreement”) with Clearside, enabling us to develop our investigational plasma kallikrein inhibitor, avoralstat, with Clearside’s SCS Microinjector® to deliver avoralstat to the back of the eye through the suprachoroidal space to treat patients with DME.
Under the Clearside Agreement, Clearside received a $5.0 million upfront license fee from us, which has been recognized in research and development expenses during the year ended December 31, 2023. Clearside is eligible to receive up to an additional $30.0 million in clinical and regulatory milestone payments, and up to a total of $47.5 million in three post-approval sales-based milestone payments as annual global net sales progress to $2.0 billion. We will pay Clearside tiered mid-single digit royalties on annual global net product sales, at three tiers, including a top tier of >$1.5 billion.
Results of Operations
The discussion below presents a summary of our results of operations for fiscal years 2023 and 2022. See Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations” of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on February 27, 2023, for a summary of our results of operations for the fiscal year ended December 31, 2021.
Year Ended December 31, 2023 Compared to 2022
For the year ended December 31, 2023, total revenues were $331.4 million as compared to $270.8 million for the year ended December 31, 2022. The increase was primarily due to ORLADEYO net revenue, including royalties, of
64
$326.0 million, an increase of $74.4 million. The increase in ORLADEYO net revenue was partially offset by a decrease in other net revenues of $13.8 million, primarily due to RAPIVAB stockpiling sales to the U.S. Department of Health and Human Services (“HHS”) in the year ended December 31, 2022 that did not occur in 2023.
Cost of product sales for the years ended December 31, 2023 and 2022 was $4.5 million and $6.4 million, respectively. The decrease in cost of product sales for the year ended December 31, 2023 was primarily associated with the peramivir product sales to our partners and the RAPIVAB stockpiling sales to HHS during the year ended December 31, 2022 that did not occur in 2023, partially offset by an increase in ORLADEYO sales in the current year period.
The following table summarizes our research and development expenses for the periods indicated (amounts are in thousands). Certain prior period amounts have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the total research and development expenses.
| Years Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Research and development expenses by program: | |||||||||||
| Factor D Program | $ | 94,517 | $ | 174,234 | |||||||
| Berotralstat | 42,835 | 32,637 | |||||||||
| FOP | 1,076 | 24,042 | |||||||||
| Peramivir | 1,098 | 916 | |||||||||
| Galidesivir | 414 | 1,157 | |||||||||
| Other research, preclinical and development costs | 76,626 | 20,311 | |||||||||
| Total research and development expenses | $ | 216,566 | $ | 253,297 | |||||||
Research and development expenses decreased to $216.6 million for the year ended December 31, 2023 from $253.3 million for the year ended December 31, 2022. The decrease in Factor D program expenses was primarily due to the discontinuation of the BCX9930 program, which was announced in December 2022, partially offset by increased spending on BCX10013 due to an increase in the allocation of indirect costs as the program moved into the clinical trials. The decrease in fibrodysplasia ossificans progressiva (“FOP”) expenses was primarily due to the discontinuation of the BCX9250 program, which was announced in November 2022. These reductions were partially offset by increased spending on berotralstat due to ORLADEYO label expansion and life cycle investments, such as our ongoing ORLADEYO pediatric trial, and increased spending on other research, preclinical and development costs. Other research, preclinical and development costs increased due to increased spending on BCX17725 for manufacturing costs and investigational new drug application-enabling activities, an upfront payment of $5.0 million to Clearside under the Clearside Agreement, and increased spending related to our other early-stage discovery programs.
Research and development expenses include all direct and indirect expenses and are allocated to specific programs at the point of development of a lead product candidate. Direct expenses are charged directly to the program to which they relate, and indirect expenses are allocated based upon internal direct labor hours dedicated to each respective program. Direct expenses consist of compensation for research and development personnel and costs of outside parties to conduct laboratory studies, develop manufacturing processes, manufacture the product candidates, and conduct and manage clinical trials, as well as other costs related to our clinical and preclinical studies. Additionally, direct expenses consist of those costs necessary to discontinue and close out a development program, including termination fees and other commitments. Indirect research and development expenses consist of lab supplies and services, facility expenses, depreciation of development equipment and other overhead of our research and development efforts. Research and development expenses vary according to the number of programs in clinical development and the stage of development of our clinical programs. Later stage clinical programs tend to cost more than earlier stage programs due to the longer length of time of the clinical trials and the higher number of patients enrolled in these clinical trials.
Selling, general and administrative expenses for the year ended December 31, 2023 were $213.9 million compared to $159.4 million in the year ended December 31, 2022. The increase was primarily related to increased headcount and increased investment in order to expand and enhance the U.S. commercial team and expand and support international operations.
65
Interest expense for the year ended December 31, 2023 was $108.2 million compared to $99.1 million for the year ended December 31, 2022. The increase in interest expense was primarily associated with the interest accrued on the larger Tranche A Loan of $300.0 million under the Pharmakon Loan Agreement (as defined below).
Interest expense for the year ended December 31, 2023 included $70.4 million of non-cash interest expense due to the amortization of interest associated with the royalty financing obligations, $28.0 million of interest expense, including the amortization of the deferred financing associated with the borrowings under the Pharmakon Loan Agreement, and $9.5 million of interest expense, net of deferred financing amortization, associated with the borrowings under the Athyrium Credit Agreement (as defined below). Interest expense for the year ended December 31, 2022 consisted of $76.5 million of non-cash interest expense due to the amortization of interest associated with the royalty financing obligations and $22.5 million of interest expense, net of deferred financing amortization, associated with the borrowings under the Athyrium Credit Agreement.
For the year ended December 31, 2023, other expense was comprised primarily of a loss on extinguishment of debt of $29.0 million on the repayment of the term loans under the Athyrium Credit Agreement and net foreign currency losses of $1.0 million, partially offset by interest income of $15.8 million. For the year ended December 31, 2022, other income was comprised of interest income of $5.1 million, partially offset by net foreign currency losses of $2.0 million.
For the year ended December 31, 2023, we incurred a tax expense of $0.3 million as compared to tax expense of $2.7 million for the year ended December 31, 2022. The tax expense for 2023 was primarily the result of amendments to Section 174 of the Internal Revenue Code of 1986, as amended (“IRC”), which no longer permits an immediate deduction for research and development expenditures in the tax year that such costs are incurred. Instead, these IRC Section 174 research and development costs are capitalized and amortized over either a five or fifteen year period, depending on the location of the activities performed. The new amortization period begins with the midpoint of any taxable year that IRC Section 174 research and development costs are first incurred, regardless of whether the expenditures were made prior to or after July 1, and runs until the midpoint of year five for activities conducted in the United States or year fifteen for research and development activities conducted outside of the United States.
Liquidity and Capital Resources
Our operations have principally been funded through public offerings and private placements of equity securities; our credit facilities; revenues from ORLADEYO; royalty financing transactions; and cash from collaborative and other research and development agreements, including U.S. Government contracts. In addition to the above, we have previously received funding from other sources, including other collaborative and other research and development agreements, government grants, equipment lease financing, facility leases, research grants, and interest income on our investments.
On April 17, 2023, we entered into a $450.0 million Loan Agreement (the “Pharmakon Loan Agreement”) with BioPharma Credit Investments V (Master) LP and BPCR Limited Partnership, as lenders, and BioPharma Credit PLC, as collateral agent for the lenders. The Pharmakon Loan Agreement provides for an initial term loan in the principal amount of $300.0 million (the “Tranche A Loan”), which was funded on April 17, 2023. We utilized the proceeds from the Tranche A Loan to repay the approximate $241.8 million of outstanding indebtedness under the then-existing credit facility with Athyrium Opportunities III Co-Invest 1 LP (the “Athyrium Credit Agreement”) and to pay transaction costs and fees, and we used the remaining net proceeds of approximately $25.8 million for other general corporate purposes. The Pharmakon Loan Agreement also provides for three additional term loan tranches in principal amounts of $50.0 million each, which we may request, at our option, on or prior to September 30, 2024. The maturity date of the Pharmakon Loan Agreement is April 17, 2028. See “Note 9—Debt—Pharmakon Loan Agreement” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report for additional information about our obligations under the Pharmakon Loan Agreement.
In 2020 and 2021, we entered into the Royalty Purchase Agreements (as defined in “Note 8—Royalty Financing Obligations—ORLADEYO and Factor D Inhibitors” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report) with RPI 2019 Intermediate Finance Trust (“RPI”) and OCM IP Healthcare Holdings Limited, an affiliate of OMERS Capital Markets (“OMERS”). Under the Royalty Purchase Agreements, RPI and OMERS are entitled to receive tiered, sales-based royalties on net product sales of ORLADEYO in the United States and certain key European markets (collectively, the “Key Territories”), and other markets where we sell ORLADEYO directly or through distributors. In addition, RPI and OMERS are entitled to receive a tiered revenue share on amounts generally received by us on account of ORLADEYO sublicense revenue or net sales by licensees outside of the Key Territories. Our required payments to OMERS commenced with the calendar quarter beginning October 1, 2023. See “Note 8—Royalty Financing Obligations—
66
ORLADEYO and Factor D Inhibitors” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report for additional information about these financing transactions.
As of December 31, 2023, we had net working capital of $346.0 million, a decrease of approximately $65.0 million from $411.0 million at December 31, 2022. The decrease in working capital was primarily the result of normal operating expenses associated with the development of our product candidates and commercialization of ORLADEYO, partially offset by net proceeds of $25.8 million received following the funding of the Tranche A Loan of $300.0 million under the Pharmakon Loan Agreement. Our principal sources of liquidity at December 31, 2023 were approximately $110.6 million in cash and cash equivalents and approximately $278.3 million in investments considered available-for-sale.
We intend to contain costs and cash flow requirements by closely managing our third-party costs and headcount, leasing scientific equipment and facilities, contracting with other parties to conduct certain research and development projects, and using consultants. In addition, we reduced the size of our research and development organization and postponed previously planned capital expenditures at our Discovery Center in Alabama, which we believe will help accelerate our path to profitability.
We expect to incur additional expenses, potentially resulting in significant losses, as we continue to pursue our research and development activities, commercialize ORLADEYO, and hire additional personnel. We may incur additional expenses related to the filing, prosecution, maintenance, defense, and enforcement of patent and other intellectual property claims and additional regulatory costs as our clinical programs advance through later stages of development or as regulatory exclusivity for our products expires. The objective of our investment policy is to ensure the safety and preservation of invested funds, as well as to maintain liquidity sufficient to meet cash flow requirements. We place our excess cash with high credit quality financial institutions, commercial companies, and government agencies in order to limit the amount of our credit exposure. We have not realized any significant losses on our investments.
In the future, we may finance our needs principally from the following:
•our existing capital resources and interest earned on that capital;
•revenues from product sales;
•payments under current or future collaborative and licensing agreements with corporate partners;
•lease, royalty, or loan financing; and
•public or private equity and/or debt financing.
Our current and planned clinical trials, plus the related development, manufacturing, regulatory approval process requirements, and additional personnel resources and testing required for the continuing development of our product candidates and the commercialization of our products will consume significant capital resources and could increase our expenses.
Our expenses, revenues and cash utilization rate could vary significantly depending on many factors, including the development progress of our collaborative agreements for our product candidates, the amount of funding or assistance, if any, we receive from new partnerships with third parties for the development and/or commercialization of our products and product candidates, the progress and results of our current and proposed clinical trials for our most advanced product candidates, the progress made in the manufacturing of our lead product candidates, the success of our commercialization efforts for, and market acceptance of, our products, and the overall progression of our other programs.
Based on our expectations for revenue and operating expenses, we believe our financial resources will be sufficient to fund our operations for at least the next 12 months, and we have no immediate intentions to access the capital markets or draw down additional debt available to us. However, we have sustained operating losses for the majority of our corporate history and expect that our total 2024 expenses will exceed our total 2024 revenues. We expect to continue to incur operating losses and negative cash flows until revenues reach a level sufficient to support ongoing operations. Our liquidity needs will be largely determined by the success of operations in regard to the successful commercialization of our products and the future progression of our product candidates. We regularly evaluate other opportunities to fund future operations, including: (1) out-licensing rights to certain of our products or product candidates, pursuant to which we would receive cash milestone payments; (2) raising additional capital through equity or debt financings or from other sources, including royalty or other monetization transactions; (3) obtaining additional product candidate regulatory approvals, which would generate revenue, milestone payments and cash flow; (4) reducing spending on one or more research and development programs, including by discontinuing development; (5) restructuring operations to change our overhead structure; and/or (6) securing U.S. Government funding of our programs, including obtaining procurement contracts. We may, in the future,
67
issue securities, including common stock, preferred stock, depositary shares, purchase contracts, warrants, debt securities, and units, through private placement transactions or registered public offerings. Our future liquidity needs, and our ability to address those needs, will largely be determined by the success of our products and product candidates; the timing, scope, and magnitude of our research and development and commercial expenses; and key developments and regulatory events and our decisions in the future.
Our long-term capital requirements and the adequacy of our available funds will depend upon many factors, including:
•market acceptance of approved products and successful commercialization of such products by either us or our partners;
•our ability to receive reimbursement and stockpiling procurement contracts;
•the progress and magnitude of our research, drug discovery and development programs;
•changes in existing collaborative relationships;
•our ability to establish additional collaborative relationships with academic institutions, biotechnology or pharmaceutical companies and governmental agencies or other third parties;
•the extent to which our partners will share in the costs associated with the development of our programs or run the development programs themselves;
•our ability to negotiate favorable development and marketing strategic alliances for certain products and product candidates;
•any decision to build or expand internal development and commercial capabilities;
•the scope and results of preclinical studies and clinical trials to identify and develop product candidates;
•our ability to engage sites and enroll subjects in our clinical trials;
•the scope of manufacturing of our products to support our commercial operations and of our product candidates to support our preclinical research and clinical trials;
•increases in personnel and related costs to support the development and commercialization of our products and product candidates;
•the scope of manufacturing of our drug substance and product candidates required for future new drug application (“NDA”) filings;
•competitive and technological advances;
•the time and costs involved in obtaining regulatory approvals;
•post-approval commitments for ORLADEYO, RAPIVAB, and other products that receive regulatory approval; and
•the costs involved in all aspects of intellectual property strategy and protection, including the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing patent claims.
We may, in the future, be required to raise additional capital to complete the development and commercialization of our products and product candidates, and we may seek to raise capital in the future, including to take advantage of favorable opportunities in the capital markets. Additional funding may not be available when needed or in the form or on terms acceptable to us. Our future working capital requirements, including the need for additional working capital, will largely be determined by the advancement of our portfolio of product candidates and the commercialization of ORLADEYO. More specifically, our working capital requirements will be dependent on the number, magnitude, scope and timing of our development programs; regulatory approval of our product candidates; obtaining funding from collaborative partners; the cost, timing and outcome of regulatory reviews, regulatory investigations, and changes in regulatory requirements; the costs of obtaining patent protection for our product candidates; the timing and terms of business development activities; the rate of technological advances relevant to our operations; the efficiency of manufacturing processes developed on our behalf by third parties; the timing, scope and magnitude of commercial spending; and the level of required administrative support for our daily operations. See “Risk Factors—Risks Relating to Our Business—Financial and Liquidity Risks” and “Risk Factors—Risks Relating to Our Business—Risks Relating to Drug Development and Commercialization—If we fail to obtain additional financing or acceptable partnership arrangements if and when needed, we may be unable to complete the development and commercialization of our products and product candidates or continue operations” in Part I, Item 1A of this report for further discussion of the risks related to obtaining additional capital.
The Pharmakon Loan Agreement contains representations and warranties and affirmative and negative covenants customary for financings of this type, as well as customary events of default. Certain of the customary negative covenants limit the ability of the Company and certain of its subsidiaries to, among other things, dispose of assets; engage in mergers, acquisitions, and similar transactions; incur additional indebtedness; grant liens; make investments; pay dividends or make distributions or certain other restricted payments in respect of equity; prepay other indebtedness; enter into restrictive
68
agreements; undertake fundamental changes; or amend certain material contracts, among other customary covenants, in each case subject to certain exceptions. These covenants could cause us to be unable to pursue business opportunities that we or our stockholders may consider beneficial without the lenders’ permission or without repaying all obligations outstanding under the Pharmakon Loan Agreement. A breach of any of these covenants could result in an event of default under the Pharmakon Loan Agreement. As of December 31, 2023, we were in compliance with the negative covenants under the Pharmakon Loan Agreement.
Critical Accounting Estimates
We have established various accounting policies that govern the application of U.S. GAAP, which were utilized in the preparation of our consolidated financial statements. Certain accounting policies involve significant judgments and assumptions by management that have a material impact on the carrying value of certain assets and liabilities. Management considers such accounting policies to be critical accounting policies. The judgments and assumptions used by management are based on historical experience and other factors, which are believed to be reasonable under the circumstances. Because of the nature of the judgments and assumptions made by management, actual results could differ from these judgments and estimates, which could have a material impact on the carrying values of assets and liabilities and the results of operations.
While our significant accounting policies are more fully described in “Note 1—Significant Accounting Policies and Concentrations of Risk” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this report, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our financial statements.
Revenue Recognition
Pursuant to Accounting Standards Codification (“ASC”) Topic 606, we recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve this core principle, Topic 606 includes provisions within a five step model that includes (i) identifying the contract with a customer, (ii) identifying the performance obligations in the contract, (iii) determining the transaction price, (iv) allocating the transaction price to the performance obligations, and (v) recognizing revenue when, or as, an entity satisfies a performance obligation.
At contract inception, we identify the goods or services promised within each contract, assess whether each promised good or service is distinct, and determine those that are performance obligations. We recognize as revenue the amount of the transaction price that is allocated to the respective performance obligation when the performance obligation is satisfied.
Product Sales, Net
Our principal sources of product sales are sales of ORLADEYO, which we began shipping to patients in December 2020, sales of peramivir to our licensing partners, and in prior years, sales of RAPIVAB to HHS under our historical procurement contract, which was completed in 2022. In the United States, we ship ORLADEYO directly to patients through a single specialty pharmacy, which is considered our customer. In the European Union, United Kingdom, Canada, Latin America, and elsewhere, we sell ORLADEYO to specialty distributors as well as hospitals and pharmacies, which collectively are considered our customers.
We recognize revenue for sales when our customers obtain control of the product, which generally occurs upon delivery. For ORLADEYO, we classify payments to our specialty pharmacy customer for certain services provided by our customer as selling, general and administrative expenses to the extent such services provided are determined to be distinct from the sale of our product.
Net revenue from sales of ORLADEYO is recorded at net selling price (transaction price), which includes estimates of variable consideration for which reserves are established for (i) estimated government rebates, such as Medicaid and Medicare Part D reimbursements, and estimated managed care rebates, (ii) estimated chargebacks, (iii) estimated costs of co-payment assistance programs and (iv) product returns. These reserves are based on the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable or as a current liability. Overall, these reserves reflect our best estimates of the amount of consideration to which we are entitled based on the terms of the applicable contract. The amount of variable consideration included in the transaction price may be constrained and is included in the
69
net sales price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period. Actual amounts of consideration ultimately received may differ from our estimates. If actual results in the future vary from estimates, we adjust these estimates, which would affect net product revenue and earnings in the period such variances become known.
Government and Managed Care Rebates. We contract with government agencies and managed care organizations or, collectively, third-party payors, so that ORLADEYO will be eligible for purchase by, or partial or full reimbursement from, such third-party payors. We estimate the rebates we will provide to third-party payors and deduct these estimated amounts from total gross product revenues at the time the revenues are recognized. These reserves are recorded in the same period in which the revenue is recognized, resulting in a reduction of product revenue and the establishment of a current liability. We estimate the rebates that we will provide to third-party payors based upon (i) our contracts with these third-party payors, (ii) the government mandated discounts applicable to government-funded programs, (iii) a range of possible outcomes that are probability-weighted for the estimated payor mix, and (iv) product distribution information obtained from our specialty pharmacy.
Chargebacks. Chargebacks are discounts that occur when certain contracted customers, pharmacy benefit managers, insurance companies, and government programs purchase directly from our specialty pharmacy. These customers purchase our products under contracts negotiated between them and our specialty pharmacy. The specialty pharmacy, in turn, charges back to us the difference between the price the specialty pharmacy paid and the negotiated price paid by the contracted customers, which may be higher or lower than the specialty pharmacy’s purchase price with us. We estimate chargebacks and adjust gross product revenues and accounts receivable based on the estimates at the time revenues are recognized.
Co-payment assistance and patient assistance programs. Patients who have commercial insurance and meet certain eligibility requirements may receive co-payment assistance. Based upon the terms of the program and co-payment assistance utilization reports received from the specialty pharmacy, we are able to estimate the co-payment assistance amounts, which are recorded in the same period in which the related revenue is recognized, resulting in a reduction of product revenue. We also offer a patient assistance program that provides free drug product, for a limited period of time, to allow a patient’s insurance coverage to be established. Based on patient assistance program utilization reports provided by the specialty pharmacy, we record gross revenue of the product provided and a full reduction of the revenue amount for the free drug discount.
Product returns. We do not provide contractual return rights to our customers, except in instances where the product is damaged or defective. Non-acceptance by the patient of shipped drug product by the specialty pharmacy is reflected as a reversal of sales in the period in which the sales were originally recorded. Reserves for estimated non-acceptances by patients are recorded as a reduction of revenue in the period that the related revenue is recognized, as well as a reduction to accounts receivable. Estimates of non-acceptance are based on quantitative information provided by the specialty pharmacy.
Collaborative and Other Revenues
We have collaboration and license agreements with a number of third parties as well as research and development agreements with certain government entities. Our primary sources of revenue from these collaborative and other research and development arrangements are license, service and royalty revenues.
Revenue from license fees, royalty payments, milestone payments, and research and development fees are recognized as revenue when the earnings process is complete and we have no further continuing performance obligations or we have completed the performance obligations under the terms of the agreement.
Arrangements that involve the delivery of more than one performance obligation are initially evaluated as to whether the intellectual property licenses granted by us represent distinct performance obligations. If they are determined to be distinct, the value of the intellectual property licenses would be recognized up-front while the research and development service fees would be recognized as the performance obligations are satisfied. For performance obligations based on services performed, we measure progress using an input method based on the effort we expend or costs we incur toward the satisfaction of the performance obligation in relation to the total estimated effort or costs. Variable consideration is assessed at each reporting period as to whether it is not subject to significant future reversal and, therefore, should be included in the transaction price at the inception of the contract. If a contract includes a fixed or minimum amount of research and development support, this also would be included in the transaction price. Changes to collaborations, such as
70
the extensions of the research term or increasing the number of targets or technology covered under an existing agreement, are assessed for whether they represent a modification or should be accounted for as a new contract. For contracts with multiple performance obligations, revenue is allocated to each performance obligation based on its relative standalone selling price. Standalone selling prices are based on observable prices at which we separately sell the products or services. If a standalone selling price is not directly observable, then we estimate the standalone selling price using either an adjusted market assessment approach or an expected cost plus margin approach, representing the amount that we believe the market is willing to pay for the product or service. Analyzing the arrangement to identify performance obligations requires the use of judgment, and each may be an obligation to deliver services, a right or license to use an asset, or another performance obligation.
Milestone payments are recognized as licensing revenue upon the achievement of specified milestones if (i) the milestone is substantive in nature and the achievement of the milestone was not probable at the inception of the agreement and (ii) we have a right to payment. Any milestone payments received prior to satisfying these revenue recognition criteria are recorded as deferred revenue.
Reimbursements received for direct out-of-pocket expenses related to research and development costs are recorded as revenue in the Consolidated Statements of Comprehensive Loss rather than as a reduction in expenses. Under our historical contracts with BARDA/HHS and NIAID/HHS, revenue was recognized as reimbursable direct and indirect costs were incurred.
Under certain of our license agreements, we receive royalty payments based upon our licensees’ net sales of covered products. Royalties are recognized at the later of when (i) the subsequent sale or usage occurs; or (ii) the performance obligation to which some or all of the sales-based or usage-based royalty has been satisfied.
Inventory
Our inventories primarily relate to ORLADEYO. Additionally, our inventory includes RAPIVAB and peramivir.
We value our inventories at the lower of cost or estimated net realizable value. We determine the cost of our inventories, which includes amounts related to materials, labor, manufacturing overhead and shipping and handling costs on a first-in, first-out (FIFO) basis. Raw materials and work-in-process include all inventory costs prior to packaging and labeling, including raw material, active product ingredient, and the drug product. Finished goods include packaged and labeled products.
Our inventories are subject to expiration dating. We regularly evaluate the carrying value of our inventories and provide valuation reserves for any estimated obsolete, short-dated or unmarketable inventories. In addition, we may experience spoilage of our raw materials and supplies. Our determination that a valuation reserve might be required, in addition to the quantification of such reserve, requires us to utilize significant judgment. During the year ended December 31, 2023, we evaluated our inventory levels and associated expiration dating relative to the latest sales forecasts for ORLADEYO, RAPIVAB, and peramivir and estimated those inventories at risk of obsolescence. Accordingly, we recorded an increase to the inventory valuation reserve of $0.4 million for a total reserve of $1.6 million as of December 31, 2023.
We expense costs related to the production of inventories as research and development expenses in the period incurred until such time it is believed that future economic benefit is expected to be recognized, which generally is upon receipt of regulatory approval. Upon regulatory approval, we capitalize subsequent costs related to the production of inventories.
Research and Development Expenses and Related Accruals
Research and development expenses include, among other items, personnel costs, including salaries and benefits, manufacturing costs, clinical, regulatory, and toxicology services performed by clinical research organizations (“CROs), materials and supplies, and overhead allocations consisting of various administrative and facilities related costs, as well as termination fees and other commitments associated with discontinued programs. Most of our manufacturing and clinical and preclinical studies are performed by third-party CROs. Our research and development costs are charged to expense
71
when incurred. Research and development expenses include all direct and indirect development costs related to the development of our portfolio of product candidates.
Additionally, we have license agreements with third parties, such as Albert Einstein College of Medicine of Yeshiva University, Industrial Research, Ltd., and the University of Alabama at Birmingham (“UAB”), which require fees related to sublicense agreements. We expense sublicense payments as incurred.
We group our research and development expenses into two major categories: direct external expenses and indirect expenses. Direct expenses consist of compensation for research and development personnel and costs of outside parties to conduct laboratory studies, develop manufacturing processes and manufacture the product candidate, conduct and manage clinical trials, as well as other costs related to our clinical and preclinical studies. Additionally, direct expenses consist of those costs necessary to discontinue and close out a development program, including termination fees and other commitments. These costs are accumulated and tracked by active program. Indirect expenses consist of lab supplies and services, facility expenses, depreciation of development equipment and other overhead of our research and development efforts. These costs apply to work on non-active product candidates and our discovery research efforts.
As part of the process of preparing our consolidated financial statements, we are required to estimate our accrued research and development expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel and third-party vendors to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. When evaluating the adequacy of accrued expenses, we consider facts and circumstances known to us at the time, which can include assumptions such as expected patient enrollment, site activation and estimated project duration. Examples of estimated accrued research and development expenses include (i) fees paid to CROs in connection with preclinical and toxicology studies and clinical trials; (ii) fees paid to investigative sites in connection with clinical trials; (iii) fees paid to contract manufacturers in connection with the production of our raw materials, drug substance, drug products, and product candidates; and (iv) professional fees.
The financial terms of our agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of milestones. We record liabilities under these contractual commitments when we determine an obligation has been incurred, regardless of the timing of the invoice. In expensing service fees, we estimate the time period over which services will be performed and the level of effort expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we will adjust the accrual accordingly. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of these costs, our actual expenses could differ from our estimates. As of December 31, 2023 and 2022, the carrying value of accrued expenses approximates their fair value due to their short-term settlement.
Stock-Based Compensation
All share-based payments, including grants of stock option awards and restricted stock unit awards, are recognized in our Consolidated Statements of Comprehensive Loss based on their fair values. Stock-based compensation cost is estimated at the grant date based on the fair value of the award and is recognized as expense on a straight-line basis over the requisite service period of the award. Determining the appropriate fair value model and the related assumptions for the model requires judgment, including estimating the life of an award, the stock price volatility, and the expected term. We utilize the Black-Scholes option-pricing model to value our stock option awards and recognize compensation expense on a straight-line basis over the vesting periods. We reduce stock-based compensation expense for estimated forfeitures. The estimation of share-based payment awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from our current estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. In addition, we have outstanding performance-based stock options and restricted stock units for which no compensation expense is recognized until “performance” is deemed to have occurred. Significant management judgment is also required in determining estimates of future stock price volatility and forfeitures to be used in the valuation of the options. Actual results, and future changes in estimates, may differ substantially from our current estimates.
72
Interest Expense and Royalty Financing Obligations
The royalty financing obligations are eligible to be repaid based on royalties from net sales of ORLADEYO and BCX10013. Interest expense is accrued using the effective interest rate method over the estimated period each of the related liabilities will be paid. This requires us to estimate the total amount of future royalty payments to be generated from product sales over the life of the agreement. We impute interest on the carrying value of each of the royalty financing obligations and record interest expense using an imputed effective interest rate. We reassess the expected royalty payments each reporting period and account for any changes through an adjustment to the effective interest rate on a prospective basis. The assumptions used in determining the expected repayment term of the debt and amortization period of the issuance costs requires that we make estimates that could impact the carrying value of each of the liabilities, as well as the periods over which associated issuance costs will be amortized. A significant increase or decrease in forecasted net sales could materially impact each of the liability balances, interest expense and the time periods for repayment.
Income Taxes
The liability method is used in our accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse.
We account for uncertain tax positions in accordance with U.S. GAAP. Significant management judgment is required in determining our provision for income taxes, deferred tax assets and liabilities and any valuation allowance recorded against net deferred tax assets. We have recorded a valuation allowance against substantially all potential tax assets, due to uncertainties in our ability to utilize deferred tax assets, primarily consisting of certain net operating losses carried forward, before they expire. The valuation allowance is based on estimates of taxable income in each of the jurisdictions in which we operate and the period over which our deferred tax assets will be recoverable.
Recent Accounting Pronouncements
“Note 1—Significant Accounting Policies and Concentrations of Risk” in the Notes to the Consolidated Financial Statements included in Part II, Item 8 of this report discusses accounting pronouncements recently issued or proposed but not yet required to be adopted.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Interest Rate Risk
We are subject to interest rate risk on our investment portfolio and borrowings under our Pharmakon Loan Agreement. The Tranche A Loan under the Pharmakon Loan Agreement accrues interest each quarter at a rate equal to the three-month Secured Overnight Financing Rate (“SOFR”), which is capped to be no less than 1.75%, plus 7.00% or, for each quarterly interest period in which a Pharmakon PIK Interest Payment (as defined in “Note 9—Debt—Pharmakon Loan Agreement” in the Notes to the Consolidated Financial Statements included in Part II, Item 8 of this report) is made, SOFR plus 7.25%. Accordingly, increases in interest rates will increase the associated interest payments that we are required to make on the Tranche A Loan. For the year ended December 31, 2023, interest was accrued at an effective rate of 13.30% on the $300.0 million borrowing under the Pharmakon Loan Agreement.
We invest in marketable securities in accordance with our investment policy. The primary objectives of our investment policy are to preserve capital, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any single issue, issuer or type of investment. We place our excess cash with high credit quality financial institutions, commercial companies, and government agencies in order to limit the amount of credit exposure. Some of the securities we invest in may have market risk. This means that a change in prevailing interest rates may cause the principal amount of the investment to fluctuate.
Our investment exposure to market risk for changes in interest rates relates to the increase or decrease in the amount of interest income we can earn on our portfolio, changes in the market value due to changes in interest rates and other market factors, as well as the increase or decrease in any realized gains and losses. Our investment portfolio includes only marketable securities and instruments with active secondary or resale markets to help ensure portfolio liquidity. A hypothetical 100 basis point increase or decrease in interest rates along the entire interest rate yield curve would not significantly affect the fair value of our interest sensitive financial instruments, including our borrowings, but may affect
73
our future earnings and cash flows. We generally have the ability to hold our fixed-income investments to maturity and, therefore, do not expect that our operating results, financial position or cash flows will be materially impacted due to a sudden change in interest rates. However, our future investment income may fall short of expectations due to changes in interest rates, or we may suffer losses in principal if forced to sell securities which have declined in market value due to changes in interest rates or other factors, such as changes in credit risk related to the securities’ issuers. To minimize this risk, we schedule our investments to have maturities that coincide with our expected cash flow needs, thus avoiding the need to redeem an investment prior to its maturity date. Accordingly, we do not believe that we have material exposure to interest rate risk arising from our investments. Generally, our investments are not collateralized. We have not realized any significant losses from our investments.
We do not use interest rate derivative instruments to manage exposure to interest rate changes. We ensure the safety and preservation of invested principal funds by limiting default risk, market risk and reinvestment risk. We reduce default risk by investing in investment grade securities.
Foreign Currency Risk
Most of our revenues and expenses are denominated in U.S. dollars. Our commercial sales in Europe are primarily denominated in Euros and the British Pound, and our royalties from Torii are in Japanese Yen. We also had other transactions denominated in foreign currencies during the year ended December 31, 2023, primarily related to operations in Europe, contract manufacturing and ex-U.S. clinical trial activities, and we expect to continue to do so. Our limited foreign currency exposure relative to our European operations is to fluctuations in the Euro, British Pound, Swiss Franc, Danish Krone, Swedish Krona, and Norwegian Krone. Additionally, we have operations in Canada and have foreign currency exposure relative to the Canadian Dollar.
We do not anticipate that foreign currency transaction gains or losses will be significant at our current level of operations. However, transaction gains or losses may become significant in the future as we continue to expand our operations internationally. We have not engaged in foreign currency hedging during 2023; however, we may do so in the future.
Inflation Risk
Inflation generally impacts us by potentially increasing our operating expenses, including cost of product sales, clinical trial costs and selling activities. We do not believe that inflation has had a material impact on our business or results of operations during the periods for which the consolidated financial statements are presented in this report. Significant adverse changes in inflation could negatively impact our future results of operations.
74
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
BIOCRYST PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except par value amounts)
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| ASSETS | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Restricted cash | |||||||||||
| Investments | |||||||||||
| Trade receivables | |||||||||||
| Inventory, net | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Property and equipment, net | |||||||||||
| Long-term investments | |||||||||||
| Other assets | |||||||||||
| Total assets | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued expenses | |||||||||||
| Deferred revenue | |||||||||||
| Operating lease liabilities | |||||||||||
| Finance lease liabilities | |||||||||||
| Royalty financing obligations | |||||||||||
| Total current liabilities | |||||||||||
| Operating lease liabilities | |||||||||||
| Finance lease liabilities | |||||||||||
| Royalty financing obligations | |||||||||||
| Secured term loan | |||||||||||
| Stockholders’ deficit: | |||||||||||
Preferred stock, $ | |||||||||||
Common stock, $ | |||||||||||
| Additional paid-in capital | |||||||||||
| Accumulated other comprehensive income | |||||||||||
| Accumulated deficit | ( | ( | |||||||||
| Total stockholders’ deficit | ( | ( | |||||||||
| Total liabilities and stockholders’ deficit | $ | $ | |||||||||
See accompanying notes to consolidated financial statements.
75
BIOCRYST PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands, except per share amounts)
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Revenues | $ | $ | $ | ||||||||||||||
| Expenses: | |||||||||||||||||
| Cost of product sales | |||||||||||||||||
| Research and development | |||||||||||||||||
| Selling, general and administrative | |||||||||||||||||
| Royalty | |||||||||||||||||
| Total operating expenses | |||||||||||||||||
| Loss from operations | ( | ( | ( | ||||||||||||||
| Interest and other income | |||||||||||||||||
| Interest expense | ( | ( | ( | ||||||||||||||
| (Loss) gain on extinguishment of debt | ( | ||||||||||||||||
| Foreign currency losses, net | ( | ( | ( | ||||||||||||||
| Loss before income taxes | ( | ( | ( | ||||||||||||||
| Income tax expense | |||||||||||||||||
| Net loss | $ | ( | $ | ( | $ | ( | |||||||||||
| Foreign currency translation adjustment | |||||||||||||||||
| Unrealized gain (loss) on available for sale investments | ( | ( | |||||||||||||||
| Net comprehensive loss | $ | ( | $ | ( | $ | ( | |||||||||||
| Basic and diluted net loss per common share | $ | ( | $ | ( | $ | ( | |||||||||||
| Weighted average shares outstanding | |||||||||||||||||
See accompanying notes to consolidated financial statements.
76
BIOCRYST PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Cash flows from operating activities: | |||||||||||||||||
| Net loss | $ | ( | $ | ( | $ | ( | |||||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Inventory obsolescence | |||||||||||||||||
| Stock-based compensation expense | |||||||||||||||||
| Non-cash interest expense on royalty financing obligations and secured term loan and amortization of debt issuance costs | |||||||||||||||||
| Amortization of premium/discount on investments | ( | ( | ( | ||||||||||||||
| Loss (gain) on extinguishment of debt | ( | ||||||||||||||||
| Loss on impairment | |||||||||||||||||
| Changes in operating assets and liabilities: | |||||||||||||||||
| Receivables | ( | ( | ( | ||||||||||||||
| Inventory | ( | ( | ( | ||||||||||||||
| Prepaid expenses and other assets | ( | ( | ( | ||||||||||||||
| Accounts payable and accrued expenses | ( | ( | |||||||||||||||
| Interest payable | |||||||||||||||||
| Deferred revenue | ( | ( | |||||||||||||||
| Net cash used in operating activities | ( | ( | ( | ||||||||||||||
| Cash flows from investing activities: | |||||||||||||||||
| Acquisitions of property and equipment | ( | ( | ( | ||||||||||||||
| Purchases of investments | ( | ( | ( | ||||||||||||||
| Sales and maturities of investments | |||||||||||||||||
| Net cash (used in) provided by investing activities | ( | ( | |||||||||||||||
| Cash flows from financing activities: | |||||||||||||||||
| Sale of common stock, net | |||||||||||||||||
| Net proceeds from common stock issued under stock-based compensation plans | |||||||||||||||||
| Net proceeds from common stock issued to directors in lieu of cash retainer | |||||||||||||||||
| Withholding taxes paid on stock-based awards | ( | ||||||||||||||||
| Net proceeds from secured term loans | |||||||||||||||||
| Repayment of Athyrium secured term loans principal | ( | ||||||||||||||||
| Prepayment and repayment fees on Athyrium secured term loans | ( | ||||||||||||||||
| Payment of debt issuance costs on Pharmakon Tranche A term loan | ( | ||||||||||||||||
| Principal payments on finance lease liabilities | ( | ||||||||||||||||
| Net proceeds from royalty financing liabilities | |||||||||||||||||
| Net cash provided by financing activities | |||||||||||||||||
| Effects of exchange rates on cash, cash equivalents and restricted cash | |||||||||||||||||
| (Decrease) increase in cash, cash equivalents and restricted cash | ( | ( | |||||||||||||||
| Cash, cash equivalents and restricted cash at beginning of year | |||||||||||||||||
| Cash, cash equivalents and restricted cash at end of year | $ | $ | $ | ||||||||||||||
| Supplemental cash flow disclosure: | |||||||||||||||||
| Cash paid for interest | $ | $ | $ | ||||||||||||||
| Cash paid for taxes | $ | $ | $ | ||||||||||||||
| Taxes withheld on stock-based awards included in accrued expenses | $ | $ | $ | ||||||||||||||
See accompanying notes to consolidated financial statements.
77
BIOCRYST PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
(In thousands)
| Common Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Income | Accumulated Deficit | Total Stockholders’ Deficit | |||||||||||||||||||||||||
| Balance at December 31, 2020 | $ | $ | $ | $ | ( | $ | ( | ||||||||||||||||||||||
| Net loss | — | — | — | ( | ( | ||||||||||||||||||||||||
| Other comprehensive income | — | — | — | ||||||||||||||||||||||||||
Exercise of stock options, | — | — | |||||||||||||||||||||||||||
Employee stock purchase plan sales, | — | — | |||||||||||||||||||||||||||
Issuance of common stock associated with royalty financing agreement, | — | — | |||||||||||||||||||||||||||
Issuance of shares to directors in lieu of cash retainer, | — | — | — | ||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | ||||||||||||||||||||||||||
| Balance at December 31, 2021 | $ | $ | $ | $ | ( | $ | ( | ||||||||||||||||||||||
| Net loss | — | — | — | ( | ( | ||||||||||||||||||||||||
| Other comprehensive loss | — | — | ( | — | ( | ||||||||||||||||||||||||
Exercise of stock options, | — | — | |||||||||||||||||||||||||||
Vesting of restricted stock units, | ( | — | — | — | |||||||||||||||||||||||||
Employee stock purchase plan sales, | — | — | |||||||||||||||||||||||||||
Exercise of warrants, | — | — | — | ||||||||||||||||||||||||||
Issuance of shares to directors in lieu of cash retainer, | — | — | — | ||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | ||||||||||||||||||||||||||
| Balance at December 31, 2022 | $ | $ | $ | $ | ( | $ | ( | ||||||||||||||||||||||
| Net loss | — | — | — | ( | ( | ||||||||||||||||||||||||
| Other comprehensive income | — | — | — | ||||||||||||||||||||||||||
Exercise of stock options, | — | — | |||||||||||||||||||||||||||
Vesting of restricted stock units, | ( | — | — | — | |||||||||||||||||||||||||
Shares withheld for taxes for vesting of restricted stock units, | ( | ( | — | — | ( | ||||||||||||||||||||||||
Employee stock purchase plan sales, | — | — | |||||||||||||||||||||||||||
Exercise of warrants, | ( | — | — | — | |||||||||||||||||||||||||
Issuance of shares to directors in lieu of cash retainer, | — | — | — | ||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | ||||||||||||||||||||||||||
| Balance at December 31, 2023 | $ | $ | $ | $ | ( | $ | ( | ||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
78
BIOCRYST PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands)
Note 1— Significant Accounting Policies and Concentrations of Risk
The Company
BioCryst Pharmaceuticals, Inc. (the “Company”) is a global biotechnology company with a deep commitment to improving the lives of people living with complement-mediated and other rare diseases. The Company leverages its expertise in structure-guided drug design with the goal of developing first-in-class or best-in-class oral small-molecule and protein therapeutics to target difficult-to-treat rare diseases. The Company was founded in 1986 and incorporated in Delaware in 1991, and its headquarters is located in Durham, North Carolina. The Company integrates the disciplines of biology, crystallography, medicinal chemistry and computer modeling to discover and develop small molecule and protein therapeutics through the process known as structure-guided drug design.
The Company’s marketed products include oral, once-daily ORLADEYO® for the prevention of hereditary angioedema (“HAE”) attacks and RAPIVAB® (peramivir injection) for the treatment of acute uncomplicated influenza in the United States. ORLADEYO received regulatory approval in the United States in December 2020. ORLADEYO has also received regulatory approvals in multiple global markets. The Company is commercializing ORLADEYO in each of these territories directly or through distributors, except in Japan where Torii Pharmaceutical Co., Ltd. (“Torii”), the Company’s collaborative partner, conducts certain commercialization activities with respect to ORLADEYO for the prevention of HAE attacks in exchange for certain royalty payments to the Company. In addition to its approval in the United States, peramivir injection has received regulatory approvals in Canada, Australia, Japan, Taiwan and Korea.
Based on the Company’s expectations for revenue and operating expenses, the Company believes its financial resources available at December 31, 2023 will be sufficient to fund its operations for at least the next 12 months. The Company has sustained operating losses for the majority of its corporate history and expects that its total 2024 expenses will exceed its total 2024 revenues. The Company expects to continue to incur operating losses and negative cash flows until revenues reach a level sufficient to support ongoing operations. The Company’s liquidity needs will be largely determined by the success of operations in regard to the successful commercialization of its products and the progression of its product candidates in the future. The Company regularly evaluates other opportunities to fund future operations, including: (1) out-licensing rights to certain of its products or product candidates, pursuant to which the Company would receive cash milestone payments; (2) raising additional capital through equity or debt financings or from other sources, including royalty or other monetization transactions; (3) obtaining additional product candidate regulatory approvals, which would generate revenue, milestone payments and cash flow; (4) reducing spending on one or more research and development programs, including by discontinuing development; (5) restructuring operations to change its overhead structure; and/or (6) securing U.S. Government funding of its programs, including obtaining procurement contracts. The Company may, in the future, issue securities, including common stock, preferred stock, depositary shares, purchase contracts, warrants, debt securities and units, through private placement transactions or registered public offerings. The Company’s future liquidity needs, and ability to address those needs, will largely be determined by the success of its products and product candidates; the timing, scope and magnitude of its research and development and commercial expenses; and key developments and regulatory events and its decisions in the future.
Basis of Presentation
The consolidated financial statements include the accounts of the Company and its subsidiaries. All intercompany transactions and balances among the consolidated entities have been eliminated from the consolidated financial statements.
The Company’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). Such consolidated financial statements reflect all adjustments that are, in management’s opinion, necessary to present fairly, in all material respects, the Company’s consolidated financial position, results of operations, and cash flows. There were no adjustments other than normal recurring adjustments. Certain prior year amounts have been reclassified to conform to the current year presentation.
The Company has made certain presentation changes relative to its revenue, which management considers fundamental to understanding the Company’s current business and financial performance related to its primary product, ORLADEYO, including expanded international sales of ORLADEYO, relative to the Company’s other sources of revenue. Accordingly, certain disaggregated revenue information has been provided in this Note 1 and “Note 2—Revenue” to these
79
consolidated financial statements. These presentation changes have been applied to prior year revenue amounts for consistency and comparability.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. Significant estimates in the Company’s consolidated financial statements have been made relative to the calculation of net product sales, the ORLADEYO and Factor D inhibitors royalty financing obligations, inventory reserves, certain accruals, primarily related to the Company’s research and development expenses, the valuation of stock options and the valuation allowance for deferred tax assets resulting from net operating losses. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from those estimates.
Revenue Recognition
The Company recorded the following revenues for the years ended December 31, 2023, 2022, and 2021 (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Product sales, net | |||||||||||||||||
| Collaborative and other revenues | |||||||||||||||||
| Total revenues | $ | $ | $ | ||||||||||||||
Pursuant to Accounting Standards Codification (“ASC”) Topic 606, the Company recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve this core principle, Topic 606 includes provisions within a five step model that includes (i) identifying the contract with a customer, (ii) identifying the performance obligations in the contract, (iii) determining the transaction price, (iv) allocating the transaction price to the performance obligations, and (v) recognizing revenue when, or as, an entity satisfies a performance obligation.
At contract inception, the Company identifies the goods or services promised within each contract, assesses whether each promised good or service is distinct and determines those that are performance obligations. The Company recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when the performance obligation is satisfied.
Product Sales, Net
The Company’s principal sources of product sales are sales of ORLADEYO, which the Company began shipping to patients in December 2020, sales of peramivir to the Company’s licensing partners, and in prior years, sales of RAPIVAB to the U.S. Department of Health and Human Services (“HHS”) under the Company’s historical procurement contract, which was completed in 2022. In the United States, the Company ships ORLADEYO directly to patients through a single specialty pharmacy, which is considered its customer. In the European Union, United Kingdom and elsewhere, the Company sells ORLADEYO to specialty distributors as well as hospitals and pharmacies, which collectively are considered its customers.
The Company recognizes revenue for sales when its customers obtain control of the product, which generally occurs upon delivery. For ORLADEYO, the Company classifies payments to its specialty pharmacy customer for certain services provided by its customer as selling, general and administrative expenses to the extent such services provided are determined to be distinct from the sale of ORLADEYO.
Net revenue from sales of ORLADEYO is recorded at net selling price (transaction price), which includes estimates of variable consideration for which reserves are established for (i) estimated government rebates, such as Medicaid and Medicare Part D reimbursements, and estimated managed care rebates, (ii) estimated chargebacks, (iii) estimated costs of co-payment assistance programs and (iv) product returns. These reserves are based on the amounts earned or to be claimed
80
on the related sales and are classified as reductions of accounts receivable or as a current liability. Overall, these reserves reflect the Company’s best estimates of the amount of consideration to which it is entitled based on the terms of the applicable contract. The amount of variable consideration included in the transaction price may be constrained and is included in the net sales price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period. Actual amounts of consideration ultimately received may differ from the Company's estimates. If actual results in the future vary from estimates, the Company adjusts these estimates, which would affect net product revenue and earnings in the period such variances become known.
Government and Managed Care Rebates. The Company contracts with government agencies and managed care organizations or, collectively, third-party payors, so that ORLADEYO will be eligible for purchase by, or partial or full reimbursement from, such third-party payors. The Company estimates the rebates it will provide to third-party payors and deducts these estimated amounts from total gross product revenues at the time the revenues are recognized. These reserves are recorded in the same period in which the revenue is recognized, resulting in a reduction of product revenue and the establishment of a current liability. The Company estimates the rebates that it will provide to third-party payors based upon (i) the Company's contracts with these third-party payors, (ii) the government mandated discounts applicable to government-funded programs, (iii) a range of possible outcomes that are probability-weighted for the estimated payor mix, and (iv) product distribution information obtained from the Company's specialty pharmacy.
Chargebacks. Chargebacks are discounts that occur when certain contracted customers, pharmacy benefit managers, insurance companies, and government programs purchase directly from the Company’s specialty pharmacy. These customers purchase the Company’s product under contracts negotiated between them and the Company’s specialty pharmacy. The specialty pharmacy, in turn, charges back to the Company the difference between the price the specialty pharmacy paid and the negotiated price paid by the contracted customers, which may be higher or lower than the specialty pharmacy’s purchase price from the Company. The Company estimates chargebacks and adjusts gross product revenues and accounts receivable based on the estimates at the time revenues are recognized.
Co-payment assistance and patient assistance programs. Patients who have commercial insurance and meet certain eligibility requirements may receive co-payment assistance. Based upon the terms of the program and co-payment assistance utilization reports received from the specialty pharmacy, the Company is able to estimate the co-payment assistance amounts, which are recorded in the same period in which the related revenue is recognized, resulting in a reduction of product revenue. The Company also offers a patient assistance program that provides free drug product, for a limited period of time, to allow a patient’s insurance coverage to be established. Based on patient assistance program utilization reports provided by the specialty pharmacy, the Company records gross revenue of the product provided and a full reduction of the revenue amount for the free drug discount.
Product returns. The Company does not provide contractual return rights to its customers, except in instances where the product is damaged or defective. Non-acceptance by the patient of shipped drug product by the specialty pharmacy is reflected as a reversal of sales in the period in which the sales were originally recorded. Reserves for estimated non-acceptances by patients are recorded as a reduction of revenue in the period that the related revenue is recognized, as well as a reduction to accounts receivable. Estimates of non-acceptance are based on quantitative information provided by the specialty pharmacy.
Collaborative and Other Revenues
The Company has collaboration and license agreements with a number of third parties, as well as research and development agreements with certain government entities. The Company’s primary sources of revenue from these collaborative and other research and development arrangements are license, service and royalty revenues.
Revenue from license fees, royalty payments, milestone payments, and research and development fees are recognized as revenue when the earnings process is complete and the Company has no further continuing performance obligations or the Company has completed the performance obligations under the terms of the agreement.
Arrangements that involve the delivery of more than one performance obligation are initially evaluated as to whether the intellectual property licenses granted by the Company represent distinct performance obligations. If they are determined to be distinct, the value of the intellectual property licenses would be recognized up front while the research and development service fees would be recognized as the performance obligations are satisfied. For performance obligations based on services performed, the Company measures progress using an input method based on the effort it expends or costs it incurs toward the satisfaction of the performance obligation in relation to the total estimated effort or costs. Variable
81
consideration is assessed at each reporting period as to whether it is not subject to significant future reversal and, therefore, should be included in the transaction price at the inception of the contract. If a contract includes a fixed or minimum amount of research and development support, this also would be included in the transaction price. Changes to collaborations, such as the extensions of the research term or increasing the number of targets or technology covered under an existing agreement, are assessed for whether they represent a modification or should be accounted for as a new contract. For contracts with multiple performance obligations, revenue is allocated to each performance obligation based on its relative standalone selling price. Standalone selling prices are based on observable prices at which the Company separately sells the products or services. If a standalone selling price is not directly observable, then the Company estimates the standalone selling price using either an adjusted market assessment approach or an expected cost plus margin approach, representing the amount that the Company believes the market is willing to pay for the product or service. Analyzing the arrangement to identify performance obligations requires the use of judgment, and each may be an obligation to deliver services, a right or license to use an asset, or another performance obligation.
Milestone payments are recognized as licensing revenue upon the achievement of specified milestones if (i) the milestone is substantive in nature and the achievement of the milestone was not probable at the inception of the agreement; and (ii) the Company has a right to payment. Any milestone payments received prior to satisfying these revenue recognition criteria are recorded as deferred revenue.
Reimbursements received for direct out-of-pocket expenses related to research and development costs are recorded as revenue in the Consolidated Statements of Comprehensive Loss rather than as a reduction in expenses. Under the Company’s historical contracts with the Biomedical Advanced Research and Development Authority within the HHS (“BARDA/HHS”) and the National Institute of Allergy and Infectious Diseases (“NIAID/HHS”), revenue was recognized as reimbursable direct and indirect costs are incurred.
Under certain of the Company’s license agreements, the Company receives royalty payments based upon its licensees’ net sales of covered products. Royalties are recognized at the later of when (i) the subsequent sale or usage occurs, or (ii) the performance obligation to which some or all of the sales-based or usage-based royalty has been satisfied.
Cash and Cash Equivalents
The Company generally considers cash equivalents to be all cash held in commercial checking accounts, money market accounts, or investments in debt instruments and certificates of deposit with maturities of three months or less at the time of purchase. The carrying value of cash and cash equivalents approximates fair value due to the short-term nature of these items.
Restricted Cash
Total restricted cash was $1,804 and $1,472 as of December 31, 2023 and 2022, respectively, and primarily consisted of $1,493 and $1,449 as of December 31, 2023 and 2022, respectively, for a letter of credit the Company is required to maintain associated with its Birmingham lease.
Investments
The Company invests in high credit quality investments in accordance with its investment policy, which is designed to minimize the possibility of loss. The objective of the Company’s investment policy is to ensure the safety and preservation of invested funds, as well as maintaining liquidity sufficient to meet cash flow requirements. The Company places its excess cash with high credit quality financial institutions, commercial companies, and government agencies in order to limit the amount of its credit exposure. In accordance with its policy, the Company is able to invest in marketable debt securities that may consist of U.S. Government and government agency securities, money market and mutual fund investments, certificates of deposits, municipal and corporate notes and bonds, and commercial paper, among others. The Company’s investment policy requires it to purchase high-quality marketable securities with a maximum individual maturity of three years and requires an average portfolio maturity of no more than 12 months. Some of the securities in which the Company invests may have market risk. This means that a change in prevailing interest rates may cause the principal amount of the investment to fluctuate. To minimize this risk, the Company schedules its investments with maturities that coincide with expected cash flow needs, thus avoiding the need to redeem an investment prior to its maturity date. Accordingly, the Company does not believe it has a material exposure to interest rate risk arising from its investments. Generally, the Company’s investments are not collateralized. The Company has not realized any significant losses from its investments.
82
The Company classifies all of its investments as available-for-sale. Available-for-sale investments are reported at fair value at each balance sheet date, and include any unrealized holding gains and losses in accumulated other comprehensive income, unless an unrealized loss is considered to be other than temporary, in which case the unrealized loss is charged to operations. The Company reviews its investments for other than temporary declines in fair value below cost basis at the end of each reporting period and whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Factors considered include whether a decline in fair value below the amortized cost basis is due to credit-related factors or non-credit-related factors, the financial condition and near-term prospects of the Company, and the Company's intent and ability to hold the investment to allow for an anticipated recovery in fair value. A credit-related impairment is recognized as an allowance on the balance sheet with a corresponding adjustment to earnings. Any impairment that is not credit-related is recognized in other comprehensive income, net of applicable taxes unless deemed other than temporary. Realized gains and losses are reflected in interest and other income in the Consolidated Statements of Comprehensive Loss and are determined using the specific identification method with transactions recorded on a settlement date basis. Investments with original maturities at date of purchase beyond three months and which mature at or less than 12 months from the balance sheet date are classified as current. Investments with a maturity beyond 12 months from the balance sheet date are classified as long-term.
Fair Value Measurements
Assets and liabilities recorded at fair value on a recurring basis on the Consolidated Balance Sheets are categorized based upon the level of judgment associated with the inputs used to measure their fair values. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs.
Items measured at fair value on a recurring basis include investments (Note 3). The carrying amounts reflected in the Consolidated Balance Sheets for cash and cash equivalents, trade receivables, prepaid expenses and other current assets, accounts payable and accrued expenses approximate their fair values due to the short-term nature of these assets and liabilities.
Trade Receivables
The majority of the Company’s trade receivables arise from product sales and primarily represent amounts due from its specialty pharmacy customer in the United States and other third-party distributors, hospitals and pharmacies in the European Union, United Kingdom and elsewhere and have standard payment terms that generally require payment within 30 to 90 days.
Receivables from collaborations are recorded for amounts due to the Company related to reimbursable research and development costs from the HHS (Note 15), and royalty receivables from the Company’s partners, including Shionogi, Green Cross, and Torii (Note 15).
Monthly invoices were submitted to the HHS related to reimbursable research and development costs. The Company was also entitled to monthly reimbursement of indirect costs based on rates stipulated in the underlying contract. The Company’s calculations of its indirect cost rates are subject to audit by the U.S. Government.
The Company does not adjust its receivables for the effects of a significant financing component at contract inception if it expects to collect the receivables in one year or less from the time of sale.
The Company provides reserves against trade receivables for estimated losses that may result from a customer's inability to pay. Receivables are evaluated to determine if any reserve or allowance should be recorded based on consideration of the current economic environment, expectations of future economic conditions, specific circumstances and the Company’s own historical collection experience. Amounts determined to be uncollectible are charged or written-off against the reserve.
83
Inventory
The Company’s inventories primarily relate to ORLADEYO. Additionally, the Company’s inventories include RAPIVAB and peramivir.
The Company values its inventories at the lower of cost or estimated net realizable value. The Company determines the cost of its inventories, which includes amounts related to materials, labor, manufacturing overhead, and shipping and handling costs on a first-in, first-out (FIFO) basis. Raw materials and work-in-process include all inventory costs prior to packaging and labelling, including raw material, active product ingredient, and the drug product. Finished goods include packaged and labelled products.
The Company’s inventories are subject to expiration dating. The Company regularly evaluates the carrying value of its inventories and provides valuation reserves for any estimated obsolete, short-dated or unmarketable inventories. In addition, the Company may experience spoilage of its raw materials and supplies. The Company’s determination that a valuation reserve might be required, in addition to the quantification of such reserve, requires it to utilize significant judgment.
The Company expenses costs related to the production of inventories as research and development expenses in the period incurred until such time it is believed that future economic benefit is expected to be recognized, which generally is reliant upon receipt of regulatory approval. Upon regulatory approval, the Company capitalizes subsequent costs related to the production of inventories.
Property and Equipment
Property and equipment are recorded at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Computer equipment is depreciated over a life of three years . Laboratory equipment, office equipment, and software are depreciated over a life of five years . Furniture and fixtures are depreciated over a life of seven years . Leasehold improvements are amortized over their estimated useful lives or the expected lease term, whichever is less.
The Company periodically reviews its property and equipment for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values. Property and equipment to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
Accrued Expenses
The Company enters into contractual agreements with third-party vendors who provide research and development, manufacturing, distribution, and other services in the ordinary course of business. Some of these contracts are subject to milestone-based invoicing, and services are completed over an extended period of time. The Company records liabilities under these contractual commitments when it determines an obligation has been incurred, regardless of the timing of the invoice. This process involves reviewing open contracts and purchase orders, communicating with applicable Company personnel to identify services that have been performed on its behalf and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced or otherwise notified of actual cost. The majority of service providers invoice the Company monthly in arrears for services performed. The Company makes estimates of accrued expenses as of each balance sheet date in its financial statements based on the facts and circumstances which can include assumptions such as expected patient enrollment, site activation and estimated project duration. The Company periodically confirms the accuracy of its estimates with the service providers and makes adjustments if necessary. Examples of estimated accrued expenses include (i) fees paid to clinical research organizations (“CROs”) in connection with preclinical and toxicology studies and clinical trials; (ii) fees paid to investigative sites in connection with clinical trials; (iii) fees paid to contract manufacturers in connection with the production of the Company’s raw materials, drug substance, drug products, and product candidates; and (iv) professional fees.
The Company bases its expenses related to clinical trials on its estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on the Company’s behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and
84
may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, the Company estimates the time period over which services will be performed and the level of effort expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, the Company will adjust the accrual accordingly. If the Company underestimates or overestimates the level of these costs, actual expenses could differ from such estimates. As of December 31, 2023 and December 31, 2022, the carrying value of accrued expenses approximates their fair value due to their short-term settlement.
Cost of Product Sales
Cost of product sales includes the cost of producing and distributing inventories that are related to product revenue during the respective period, including freight. In addition, shipping and handling costs for product shipments are recorded as incurred. Finally, cost of product sales may also include costs related to excess or obsolete inventory adjustment charges.
Research and Development Expenses
The Company’s research and development costs are expensed when incurred. Research and development expenses include all direct and indirect development costs related to the development of the Company’s portfolio of product candidates. Advance payments for goods or services that will be used or rendered for future research and development activities are deferred and capitalized. Such amounts are recognized as expense when the related goods are delivered or the related services are performed. Research and development expenses include, among other items, personnel costs, including salaries and benefits, manufacturing costs, clinical, regulatory, and toxicology services performed by CROs, materials and supplies, and overhead allocations consisting of various administrative and facilities related costs, as well as termination fees and other commitments associated with discontinued programs. Most of the Company’s manufacturing and clinical and preclinical studies are performed by third-party CROs. Costs for studies performed by CROs are accrued by the Company over the service periods specified in the contracts, and estimates are adjusted based upon the Company’s ongoing review of the level of services actually performed.
Additionally, the Company has license agreements with third parties, such as Albert Einstein College of Medicine of Yeshiva University, Industrial Research, Ltd., and the University of Alabama at Birmingham (“UAB”), which require fees related to sublicense agreements. The Company expenses sublicense payments as incurred.
The Company groups its research and development expenses into two major categories: direct expenses and indirect expenses. Direct expenses consist of compensation for research and development personnel and costs of outside parties to conduct laboratory studies, develop manufacturing processes and manufacture the product candidate, conduct and manage clinical trials, as well as other costs related to the Company’s clinical and preclinical studies. Additionally, direct expenses consist of those costs necessary to discontinue and close out a development program, including termination fees and other commitments. These costs are accumulated and tracked by active program. Indirect expenses consist of lab supplies and services, facility expenses, depreciation of development equipment and other overhead of the Company’s research and development efforts. These costs apply to work on non-active product candidates and the Company’s discovery research efforts.
Selling, General and Administrative Expenses
Selling, general and administrative expenses are primarily comprised of compensation and benefits associated with sales and marketing, finance, human resources, legal, information technology and other administrative personnel. Additionally, selling, general and administrative expenses are comprised of market research, marketing, advertising and legal expenses, including patent costs, licenses and other general and administrative costs.
Advertising costs related to ORLADEYO of $14,404 , $14,891 and $5,705 were expensed as incurred for the years ended December 31, 2023, 2022 and 2021 respectively.
All patent related costs are expensed to selling, general and administrative expenses when incurred as recoverability of such expenditures is uncertain.
85
Leases
The Company leases certain assets under operating and finance leases, which consist of real estate leases, laboratory equipment leases and office equipment leases as of December 31, 2023. The Company accounts for lease obligations in accordance with ASU 2016-02: Leases (Topic 842), which requires a lessee to recognize a right-of-use asset and a lease liability on its balance sheet for most leases.
Certain of the Company’s operating leases provide for renewal options, which can vary by lease. The right-of-use asset and lease liabilities on the Company’s Consolidated Balance Sheets represent payments over the lease term, which includes renewal options for certain real estate leases that the Company is likely to exercise. As part of the Company’s assessment of the lease term, the Company elected the hindsight practical expedient, which allows companies to use current knowledge and expectations when determining the likelihood to extend lease options. Certain operating leases include rent escalation provisions, which the Company recognizes as expense on a straight-line basis. Lease expense for leases with an initial term of twelve months or less was not material.
The discount rate used in the calculation of the Company’s right-of-use asset and lease liability was determined based on the stated rate within each contract when available, or the Company’s collateralized borrowing rate from lending institutions.
The Company has not made any residual value guarantees related to its leases; therefore, the Company has no corresponding liability recorded on its Consolidated Balance Sheets.
Stock-Based Compensation
All share-based payments, including grants of stock option awards and restricted stock unit awards, are recognized in the Company’s Consolidated Statements of Comprehensive Loss based on their fair values. Stock-based compensation cost is estimated at the grant date based on the fair value of the award and is recognized as expense on a straight-line basis over the requisite service period of the award. Determining the appropriate fair value model and the related assumptions for the model requires judgment, including estimating the life of an award, the stock price volatility, and the expected term. The Company utilizes the Black-Scholes option-pricing model to value its stock option awards and recognize compensation expense on a straight-line basis over the vesting periods. The Company reduces stock-based compensation expense for estimated forfeitures. The estimation of share-based payment awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from the Company’s current estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised. In addition, the Company has outstanding performance-based stock options and restricted stock units for which no compensation expense is recognized until “performance” is deemed to have occurred. Significant management judgment is also required in determining estimates of future stock price volatility to be used in the valuation of the options. Actual results, and future changes in estimates, may differ substantially from the Company’s current estimates.
Interest Expense and Deferred Financing Costs
Interest expense primarily relates to the PhaRMA Notes (Note 8), royalty financing obligations (Note 8), the secured term loan borrowings under the Athyrium Credit Agreement (Note 9), and during 2023, the term loan borrowings under the Pharmakon Loan Agreement (Note 9).
Interest Expense and Royalty Financing Obligations
The royalty financing obligations are eligible to be repaid based on royalties from net sales of ORLADEYO and BCX10013. Interest expense is accrued using the effective interest rate method over the estimated period each of the related liabilities will be paid. This requires the Company to estimate the total amount of future royalty payments to be generated from product sales over the life of the agreement. The Company imputes interest on the carrying value of each of the royalty financing obligations and records interest expense using an imputed effective interest rate. The Company
86
reassesses the expected royalty payments each reporting period and accounts for any changes through an adjustment to the effective interest rate on a prospective basis. The assumptions used in determining the expected repayment term of the debt and amortization period of the issuance costs require that the Company make estimates that could impact the carrying value of each of the liabilities, as well as the periods over which associated issuance costs will be amortized. A significant increase or decrease in forecasted net sales could materially impact each of the liability balances, interest expense and the time periods for repayment.
Income Taxes
The liability method is used in the Company’s accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse.
The Company accounts for uncertain tax positions in accordance with U.S. GAAP. Significant management judgment is required in determining the Company’s provision for income taxes, deferred tax assets and liabilities and any valuation allowance recorded against net deferred tax assets. The Company has recorded a valuation allowance against substantially all potential tax assets, due to uncertainties in its ability to utilize deferred tax assets, primarily consisting of certain net operating losses carried forward, before they expire. The valuation allowance is based on estimates of taxable income in each of the jurisdictions in which the Company operates and the period over which its deferred tax assets will be recoverable.
Beginning in fiscal year 2021, the Company began accruing for U.S. state taxes and foreign income taxes as a result of increased nexus in both U.S. state and foreign jurisdictions where historically the Company had no presence.
Foreign Currency
The functional currencies of the Company’s foreign subsidiaries primarily are the local currencies of the country in which the subsidiary operates. The Company’s asset and liability accounts are translated at the current exchange rate as of the balance sheet date. Revenue and expense accounts are translated at the average exchange rate over the period. Adjustments resulting from the translation of the financial statements of the Company’s foreign subsidiaries into U.S. dollars are accumulated as a separate component of stockholders’ equity within accumulated other comprehensive income. Gains or losses resulting from transactions denominated in foreign currencies are included in foreign currency losses, net, within the Consolidated Statement of Comprehensive Loss.
Net Loss Per Share
Basic net loss per share is based upon the weighted average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding during the period, after giving consideration to the dilutive effect of potentially dilutive common shares. The Company has generated a net loss in all periods presented, so the diluted net loss per share is equivalent to basic net loss per share for all periods presented herein because common equivalent shares from unexercised stock options, warrants and common shares expected to be issued under the Company’s equity compensation plans would be anti-dilutive. The Company excluded the following potential common shares, presented based on amounts outstanding as of December 31, 2023 and 2022, from the computation of diluted net loss per share attributable to common stockholders because including them would have had an anti-dilutive effect:
87
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Outstanding stock options | |||||||||||
| Unvested restricted stock unit awards | |||||||||||
| Warrants to purchase common stock | |||||||||||
| Total | |||||||||||
Accumulated Other Comprehensive Income
Accumulated other comprehensive income is comprised of cumulative foreign currency translation adjustments and unrealized gains and losses on available-for-sale investments and is disclosed as a separate component of stockholders’ equity. Realized gain and loss amounts on available-for-sale investments are reclassified from accumulated other comprehensive loss and recorded as interest and other income on the Consolidated Statements of Comprehensive Loss. There were no 1 were reclassified out of accumulated other comprehensive income.
Significant Customers and Other Risks
Significant Customers
The Company’s primary sources of revenue and cash flow are the sales of ORLADEYO in the United States and other global markets and, during the years ended December 31, 2022 and 2021, sales of RAPIVAB (peramivir injection) under the Company’s historical procurement contract with the Assistant Secretary for Preparedness and Response within HHS. Additionally, the Company previously received reimbursement of galidesivir (formerly BCX4430) development expenses earned under cost-plus-fixed-fee contracts with BARDA/HHS and NIAID/HHS during the years ended December 31, 2022 and 2021.
ORLADEYO is distributed through an arrangement with a single specialty pharmacy in the United States, which represents the substantial majority of the ORLADEYO net product sales. The specialty pharmacy subsequently sells ORLADEYO to its customers (pharmacy benefit managers, insurance companies, government programs and group purchasing organizations) and dispenses product to patients. The specialty pharmacy’s inability or unwillingness to continue these distribution activities could adversely impact the Company’s business, results of operations and financial condition.
The Company is distributing ORLADEYO in other global markets directly or through distributors, except in Japan where Torii, the Company’s collaborative partner, has the exclusive right to commercialize ORLADEYO.
The Company relied on BARDA/HHS and NIAID/HHS to reimburse predominantly all of the development costs for its galidesivir program and stockpiling sales of RAPIVAB to HHS. Accordingly, reimbursement of these expenses represented a significant portion of the Company’s collaborative and other research and development revenues. All government funding for galidesivir expired in 2022. Additionally, HHS was the primary customer for RAPIVAB, and it exercised the remaining options for the purchase of RAPIVAB under the procurement contract with the Company during 2022.
Further, the Company’s drug development activities are performed by a limited group of third-party vendors. If any of these vendors were unable to perform its services, this could significantly impact the Company’s ability to complete its drug development activities.
Risks from Third-Party Manufacturing and Distribution Concentration
The Company relies on a single source manufacturer for active pharmaceutical ingredient and finished drug product manufacturing of product candidates in development and on a single specialty pharmacy for distribution of approved drug product in the United States. Delays or disruption in the manufacture or distribution of any product could adversely impact the future procurement stockpiling of the Company’s commercial product, commercial revenue and product candidates.
88
Credit Risk
Cash equivalents and investments are financial instruments that potentially subject the Company to concentration of risk to the extent recorded on the Consolidated Balance Sheets. The Company deposits excess cash with major financial institutions in the United States. Balances may exceed the amount of insurance provided on such deposits. The Company believes it has established guidelines for investment of its excess cash relative to diversification and maturities that maintain safety and liquidity. To minimize the exposure due to adverse shifts in interest rates, the Company maintains a portfolio of investments with an average maturity of approximately 12 months or less.
The Company’s receivables from sales of ORLADEYO are primarily due from one customer, resulting in a concentration of credit risk. Sales of ORLADEYO from the Company to the specialty pharmacy only occur once an order of product has been received by the specialty pharmacy from one of its customers, which include pharmacy benefit managers, insurance companies, government programs and group purchasing organizations.
The majority of the Company’s receivables from collaborations are due from the U.S. Government, for which there is no assumed credit risk.
Recently Adopted Accounting Pronouncements
In December 2020, the FASB issued ASU No. 2019-12 (ASC Topic 740), Simplifying the Accounting for Income Taxes. ASU 2019-12 simplifies accounting for income taxes by removing certain exceptions to the general principles and clarifying existing guidance. This standard is effective for fiscal years, and interim periods within those years, beginning after December 15, 2020. The Company adopted ASU No. 2019-12 as of January 1, 2021. The adoption of this standard did not have a material impact to the Company’s financial position, results of operations or cash flows.
New Accounting Pronouncements Not Yet Adopted
The Company has reviewed new accounting pronouncements that were issued as of December 31, 2023 and does not believe that these pronouncements are either applicable to the Company, or that they will have a material impact on its financial position or results of operations.
Note 2— Revenue
The Company recorded the following revenues for the years ended December 31 (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| ORLADEYO: | |||||||||||||||||
| U.S. | $ | $ | $ | ||||||||||||||
| Outside of U.S. | |||||||||||||||||
| Total ORLADEYO | |||||||||||||||||
| Other revenues | |||||||||||||||||
| Total revenues | $ | $ | $ | ||||||||||||||
ORLADEYO revenues represent total revenues from product sales, collaborative revenues and royalties. Royalty revenue from sales of ORLADEYO in Japan by the Company’s collaborative partner, Torii, were $2,178 , $1,944 , and $690 for the years ended December 31, 2023, 2022, and 2021 respectively.
Other revenues primarily relate to the Company’s product sales and royalties for peramivir injection (RAPIVAB/RAPIACTA/PERAMIFLU), and for the years ended December 31, 2022 and 2021, galidesivir development contracts with BARDA/HHS and NIAID/HHS. Other revenues for the year ended December 31, 2021 also includes milestone revenue of $15,000 upon receipt from the Japanese National Health Insurance System (“NHI”) of a reimbursement price approval for ORLADEYO.
89
Note 3— Investments
Fair value is an exit price, representing the amount that would be received from the sale of an asset or paid to transfer a liability in an orderly transaction between market participants. Fair value is determined based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect certain market assumptions. As a basis for considering such assumptions, U.S. GAAP establishes a three-tier value hierarchy, which prioritizes the inputs used to develop the assumptions and for measuring fair value as follows: (Level 1) observable inputs such as quoted prices in active markets for identical assets; (Level 2) inputs other than the quoted prices in active markets that are observable either directly or indirectly; and (Level 3) unobservable inputs for which there is little or no market data, which requires the Company to develop its own assumptions. This hierarchy requires the Company to use observable market data, when available, and to minimize the use of unobservable inputs when determining fair value.
The Company’s financial instruments that are measured at fair value on a recurring basis consist of fixed income investments. These valuations are based on observable direct and indirect inputs, primarily quoted prices of similar, but not identical, instruments in active markets or quoted prices for identical or similar instruments in markets that are not active. These fair values are obtained from independent pricing services.
Assets measured at fair value on a recurring basis were as follows (in thousands):
| December 31, 2023 | |||||||||||||||||||||||
| Quoted Price in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total | ||||||||||||||||||||
| Assets: | |||||||||||||||||||||||
| Obligations of U.S. Government and its agencies | $ | $ | $ | $ | |||||||||||||||||||
| Certificates of deposit | |||||||||||||||||||||||
| Total assets | $ | $ | $ | $ | |||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||
| Quoted Price in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total | ||||||||||||||||||||
| Assets: | |||||||||||||||||||||||
| Obligations of U.S. Government and its agencies | $ | $ | $ | $ | |||||||||||||||||||
| Corporate debt securities | |||||||||||||||||||||||
| Certificates of deposit | |||||||||||||||||||||||
| Total assets | $ | $ | $ | $ | |||||||||||||||||||
There were no changes in valuation techniques during the years ended December 31, 2023 and 2022. There were no liabilities measured at fair value on a recurring basis as of December 31, 2023 and 2022.
As of December 31, 2023, the Company had 11 securities with a total estimated fair market value of $18,513 in an unrealized loss position. The Company believes the individual unrealized losses represent temporary declines primarily resulting from interest rate changes. The Company does not have intent to sell these investments, and it is more likely than not that the investments will be held until recovery of their amortized cost basis. As such, no allowance was recognized.
90
The following tables summarize the fair value of the Company’s investments by type (in thousands):
| December 31, 2023 | |||||||||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | ||||||||||||||||||||||||||
| Obligations of U.S. Government and its agencies | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||
| Certificates of deposit | ( | ||||||||||||||||||||||||||||
| Total investments | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Estimated Fair Value | ||||||||||||||||||||||||||
| Obligations of U.S. Government and its agencies | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||
| Corporate debt securities | ( | ||||||||||||||||||||||||||||
| Certificates of deposit | ( | ||||||||||||||||||||||||||||
| Total investments | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||
The following table summarizes the scheduled maturity for the Company’s investments at December 31, 2023 and 2022 (in thousands):
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Maturing in one year or less | $ | $ | |||||||||
| Maturing after one year through two years | |||||||||||
| Total investments | $ | $ | |||||||||
Note 4— Trade Receivables
Product Sales
Receivables from product sales are recorded for amounts due to the Company related to sales of ORLADEYO and RAPIVAB. At December 31, 2023 and 2022, receivables, net of reserves, related to sales of ORLADEYO were $54,149 and $41,508 , respectively. A returns reserve related to sales of ORLADEYO of $288 No reserve or allowance amounts were recorded as of December 31, 2022 related to sales of ORLADEYO. At December 31, 2023 and 2022, receivables related to sales of RAPIVAB were $505 and $823 , respectively. No
Collaborations
Receivables from collaborations were as follows (in thousands):
| December 31, 2023 | |||||||||||||||||
| Billed | Unbilled | Total | |||||||||||||||
| Royalty receivables from partners | $ | $ | $ | ||||||||||||||
| Total receivables from collaborators | $ | $ | $ | ||||||||||||||
91
| December 31, 2022 | |||||||||||||||||
| Billed | Unbilled | Total | |||||||||||||||
| U.S. Department of Health and Human Services, net | $ | $ | $ | ||||||||||||||
| Royalty receivables from partners | |||||||||||||||||
| Other collaborations | |||||||||||||||||
| Total receivables from collaborators | $ | $ | $ | ||||||||||||||
As of December 31, 2023 and 2022, the reserve related to royalties from collaborations was not material.
Note 5— Inventory
At December 31, 2023 and 2022, the Company’s inventory primarily related to ORLADEYO. Additionally, inventory included RAPIVAB and peramivir, which is manufactured for the Company’s partners.
The Company’s inventories consisted of the following (in thousands):
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Raw materials | $ | $ | |||||||||
| Work-in-process | |||||||||||
| Finished goods | |||||||||||
| Total inventory | $ | $ | |||||||||
| Reserves | ( | ( | |||||||||
| Total inventory, net | $ | $ | |||||||||
Note 6— Property and Equipment
Property and equipment consisted of the following (in thousands):
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Furniture and fixtures | $ | $ | |||||||||
| Office equipment | |||||||||||
| Software | |||||||||||
| Laboratory equipment | |||||||||||
| Leasehold improvements | |||||||||||
| Total property and equipment | $ | $ | |||||||||
| Less accumulated depreciation and amortization | ( | ( | |||||||||
| Property and equipment, net | $ | $ | |||||||||
Depreciation expense for the years ended December 31, 2023, 2022, and 2021 was $1,655 , $1,437 , and $777 , respectively. The Company recorded an impairment loss of $1,548 and contract termination fees of $440 related to the discontinuation of the Birmingham research facilities expansion, which has been recognized in research and development expenses during the year ended December 31, 2023. The Company did not
92
Note 7— Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Compensation and benefits | $ | $ | |||||||||
| Revenue-related reserves for discounts and allowances | |||||||||||
| Royalties payable | |||||||||||
| Development costs | |||||||||||
| Inventory | |||||||||||
| Other | |||||||||||
| Total accrued expenses | $ | $ | |||||||||
Note 8— Royalty Financing Obligations
ORLADEYO and Factor D Inhibitors
On December 7, 2020, the Company and RPI 2019 Intermediate Finance Trust (“RPI”) entered into a Purchase and Sale Agreement (the “2020 RPI Royalty Purchase Agreement”), pursuant to which the Company sold to RPI the right to receive certain royalty payments from the Company for a purchase price of $125,000 in cash (the “2020 RPI Royalty Sale”). Under the 2020 RPI Royalty Purchase Agreement, RPI is entitled to receive tiered, sales-based royalties on net product sales of ORLADEYO in the United States and certain key European markets (collectively, the “Key Territories”), and other markets where the Company sells ORLADEYO directly or through distributors (collectively, the “Direct Sales”) in an amount equal to: (i) 8.75 % of aggregate annual net sales of ORLADEYO for annual net sales up to $350,000 and (ii) 2.75 % of annual net sales for annual net sales between $350,000 and $550,000. No royalty payments are payable on annual Direct Sales over $550,000.
Under the 2020 RPI Royalty Purchase Agreement, RPI is also entitled to receive a tiered revenue share on ORLADEYO sublicense revenue or net sales by licensees outside of the Key Territories (the “Other Markets”) equal to: (i) 20 % of the proceeds received by the Company for upfront license fees and development milestones for ORLADEYO in the Other Markets; (ii) 20 % of proceeds received on annual net sales of up to $150,000 in the Other Markets; and (iii) 10 % of proceeds received by the Company on annual net sales between $150,000 and $230,000 in the Other Markets. No royalty payments are payable on annual net sales above $230,000 in the Other Markets.
On November 19, 2021, the Company and RPI entered into (i) a Purchase and Sale Agreement (the “2021 RPI Royalty Purchase Agreement” and together with the 2020 RPI Royalty Purchase Agreement, the “RPI Royalty Purchase Agreements”), pursuant to which the Company sold to RPI the right to receive certain royalty payments from the Company for a purchase price of $150,000 in cash, and (ii) a Purchase and Sale Agreement with OCM IP Healthcare Holdings Limited, an affiliate of OMERS Capital Markets (“OMERS”) (the “OMERS Royalty Purchase Agreement” and collectively with the RPI Royalty Purchase Agreements, the “Royalty Purchase Agreements”), pursuant to which the Company sold to OMERS the right to receive certain royalty payments from the Company for a purchase price of an additional $150,000 in cash.
Under the 2021 RPI Royalty Purchase Agreement, RPI is entitled to receive tiered, sales-based royalties on Direct Sales in an amount equal to: (i) 0.75 % of aggregate annual net sales of ORLADEYO for annual net sales up to $350,000 and (ii) 1.75 % of annual net sales of ORLADEYO for annual net sales between $350,000 and $550,000. No royalty payments are payable on Direct Sales over $550,000. RPI is also entitled to receive a tiered revenue share on ORLADEYO sublicense revenue or net sales by licensees in the Other Markets in an amount equal to (i) 3.0 % of proceeds received by the Company on annual net sales of up to $150,000 in the Other Markets and (ii) 2.0 % of proceeds received by the Company on annual net sales between $150,000 and $230,000 in the Other Markets. No royalty payments are payable on annual net sales above $230,000 in the Other Markets.
Under the 2021 RPI Royalty Purchase Agreement, RPI is also entitled to receive tiered, sales-based royalties on net product sales of BCX10013 in an amount equal to: (i) 3.0 % of worldwide aggregate annual net sales up to $1,500,000 and (ii) 2.0 % of worldwide aggregate annual net sales between $1,500,000 and $3,000,000. No royalty payments are payable
93
on annual net sales above $3,000,000 . RPI is also entitled to receive tiered profit share amounts of up to 3.0 % from certain other permitted sales in certain other markets.
The royalties payable under the 2021 RPI Royalty Purchase Agreement are in addition to the royalties payable to RPI under the 2020 RPI Royalty Purchase Agreement.
Under the OMERS Royalty Purchase Agreement, commencing with the calendar quarter beginning October 1, 2023, OMERS is entitled to receive tiered, sales-based royalties on Direct Sales in an amount equal to: (i) 7.5 % of aggregate annual net sales of ORLADEYO for annual net sales up to $350,000 and (ii) 6.0 % of annual net sales of ORLADEYO for annual net sales between $350,000 and $550,000 (with no royalty payments payable on annual Direct Sales over $550,000). For each calendar quarter beginning on or after January 1, 2024, OMERS is entitled to receive tiered, sales-based royalties on Direct Sales in an amount equal to: (i) 10.0 % of aggregate annual net sales of ORLADEYO for annual net sales up to $350,000 and (ii) 3.0 % of annual net sales of ORLADEYO for annual net sales between $350,000 and $550,000 (with no royalty payments payable on annual Direct Sales over $550,000).
Under the OMERS Royalty Purchase Agreement, OMERS is also entitled to receive a tiered revenue share on ORLADEYO sublicense revenue or net sales by licensees in the Other Markets in an amount equal to: (i) 20.0 % of the proceeds received by the Company for upfront license fees and development milestones for ORLADEYO in the Other Markets, (ii) 20.0 % of proceeds received by the Company on annual net sales of up to $150,000 in the Other Markets, and (iii) 10.0 % of proceeds received by the Company on annual net sales between $150,000 and $230,000 in the Other Markets. No royalty payments are payable on annual net sales above $230,000 in the Other Markets. OMERS is also entitled to receive profit share amounts of up to 10 % from certain other permitted sales in certain other markets.
Under the 2020 RPI Royalty Purchase Agreement, the Company is required to make royalty payments of amounts owed to RPI each calendar quarter following the first commercial sale of ORLADEYO in any country. Under the 2021 RPI Royalty Purchase Agreement, the Company is required to make payments to RPI in respect of net sales or sublicense revenue in each calendar quarter from and after October 1, 2021. Under the OMERS Royalty Purchase Agreement, the Company is required to make payments to OMERS in respect of net sales or sublicense revenue in each calendar quarter from and after October 1, 2023. OMERS will no longer be entitled to receive any payments on the date in which aggregate payments actually received by OMERS equals 155.0 % of the $150,000 purchase price.
The transactions contemplated by each of the Royalty Purchase Agreements are referred to herein as the “Royalty Sales.”
Under the Royalty Purchase Agreements, the Company has agreed to specified affirmative and negative covenants, including covenants regarding periodic reporting of information by the Company to RPI and OMERS, third-party audits of royalties paid under the Royalty Purchase Agreements, and restrictions on the ability of the Company or any of its subsidiaries to incur indebtedness other than certain royalty sales and as was permitted to be incurred under the terms of the Athyrium Credit Agreement (as defined in Note 9 herein) through its payoff and termination on April 17, 2023 or, subsequent to that date and the Pharmakon Loan Agreement (as defined in Note 9 herein), as applicable. See “Note 9—Debt” for further details on the Athyrium Credit Agreement and the Pharmakon Loan Agreement. The restrictions under the Royalty Purchase Agreements on the ability of the Company or any of its subsidiaries to incur indebtedness are eliminated after the achievement of certain specified milestones in the Royalty Purchase Agreements.
The cash consideration obtained pursuant to the Royalty Purchase Agreements is recorded in “Royalty financing obligations” on the Company’s Consolidated Balance Sheets. The fair value for the royalty financing obligations at the time of the transactions was based on the Company’s estimates of future royalties expected to be paid to the counterparty over the life of the arrangement. The Company subsequently records the obligations at their carrying value using the effective interest method. In order to amortize the royalty financing obligations, the Company utilizes the prospective method to estimate the future royalties to be paid by the Company to the counterparty over the life of the arrangement. Under the prospective method, a new effective interest rate is determined based on the revised estimate of remaining cash flows. The new rate is the discount rate that equates the present value of the revised estimate of remaining cash flows with the carrying amount of the debt, and it will be used to recognize interest expense for the remaining periods. The Company periodically assesses the amount and timing of expected royalty payments using a combination of internal projections and forecasts from external sources. The estimates of future net product sales (and resulting royalty payments) are based on key assumptions including population, penetration, probability of success, and sales price, among others. To the extent such payments are greater or less than the Company’s initial estimates or the timing of such payments is materially different than its original estimates, the Company will prospectively adjust the amortization of the royalty financing obligations and
94
the effective interest rate. On a quarterly basis, the Company assesses the projected royalty payments relative to the projected interest accretion for the next twelve months to determine if the royalty liability balance is reduced relative to the current outstanding liability, which would signify a repayment of the liability. In such case of excess payments relative to interest accretion for the next twelve months, the excess payments are considered to be a short-term liability and classified within current liabilities on the Company’s Consolidated Balance Sheets.
During the year ended December 31, 2023, the Company adjusted its forecasts related to its BCX10013 program and updated its ORLADEYO forecast based on actual results for the year ended December 31, 2023. The primary factors that impacted forecasts on the BCX10013 development program were development delays, reduced probability of success, reduced pricing assumptions and reduced market share assumptions. These adjustments impacted the amount and timing of expected royalties to be made under the Royalty Purchase Agreements. As a result, the effective interest rate related to the 2020 RPI Royalty Purchase Agreement decreased from 22.4 % to 22.3 %, the effective interest rate related to the 2021 RPI Royalty Purchase Agreement decreased from 13.1 % to 0.0 %, and the effective interest rate related to the OMERS Royalty Purchase Agreement decreased from 10.6 % to 10.0 %.
The following table shows the activity within the Royalty financing obligations account (in thousands) as well as the effective interest rate as of December 31, 2023:
| 2020 RPI Royalty Agreement | 2021 RPI Royalty Agreement | OMERS Royalty Agreement | Total | ||||||||||||||||||||
| Balance as of December 31, 2020 | $ | $ | — | $ | — | $ | |||||||||||||||||
| 2021 Royalty sale: Royalty financing obligations, net of issuance costs | — | ||||||||||||||||||||||
| Non-cash Interest expense on Royalty financing obligations | |||||||||||||||||||||||
| Royalty revenues paid and payable | ( | ( | — | ( | |||||||||||||||||||
| Balance as of December 31, 2021 | $ | $ | $ | $ | |||||||||||||||||||
| Deferred financing costs | — | ( | — | ( | |||||||||||||||||||
| Non-cash Interest expense on Royalty financing obligations | |||||||||||||||||||||||
| Royalty revenues paid and payable | ( | ( | — | ( | |||||||||||||||||||
| Balance as of December 31, 2022 | $ | $ | $ | $ | |||||||||||||||||||
| Non-cash Interest expense on Royalty financing obligations | |||||||||||||||||||||||
| Royalty revenues paid and payable | ( | ( | ( | ( | |||||||||||||||||||
| Balance as of December 31, 2023 | $ | $ | $ | $ | |||||||||||||||||||
| Effective interest rate | % | % | % | ||||||||||||||||||||
Deferred issuance costs pursuant to the Royalty financing obligations, which consist primarily of advisory and legal fees, totaled $8,532
Concurrent with entering into the 2021 RPI Royalty Purchase Agreement, the Company and RPI entered into a Common Stock Purchase Agreement (the “Common Stock Purchase Agreement”), pursuant to which the Company sold common stock to RPI for a premium of $4,269 . This premium has been deferred and is being amortized through interest expense using the effective interest method over the term of the applicable arrangement. See “Note 11—Stockholders’ Equity” for further details on the common stock sale premium.
95
RAPIACTA
Overview
On March 9, 2011, the Company completed a $30,000 financing transaction to monetize certain future royalty and milestone payments under the Company’s agreement (the “Shionogi Agreement”) with Shionogi & Co., Ltd. (“Shionogi”), pursuant to which Shionogi licensed from the Company the rights to market RAPIACTA in Japan and Taiwan. The Company received net proceeds of $22,691 from the transaction.
As part of the transaction, the Company entered into a purchase and sale agreement dated as of March 9, 2011 with JPR Royalty Sub LLC, a wholly-owned subsidiary of the Company (“Royalty Sub”), whereby the Company transferred to Royalty Sub, among other things, its rights to receive certain royalty and milestone payments from Shionogi arising under the Shionogi Agreement. Royalty payments are paid by Shionogi in Japanese Yen, and any milestone payments will be paid in U.S. dollars. The Company’s collaboration with Shionogi was not impacted as a result of this transaction.
Non-Recourse Notes Payable
On March 9, 2011, Royalty Sub completed a private placement to institutional investors of $30,000 in aggregate principal amount of its PhaRMA Senior Secured 14 % Notes due on December 1, 2020 (the “PhaRMA Notes”). The PhaRMA Notes were issued by Royalty Sub under an Indenture, dated as of March 9, 2011 (the “Indenture”), by and between Royalty Sub and U.S. Bank National Association, as Trustee. Principal and interest on the PhaRMA Notes are payable from, and are secured by, the rights to royalty and milestone payments under the Shionogi Agreement transferred by the Company to Royalty Sub. The PhaRMA Notes bear interest at 14 % per annum, payable annually in arrears on September 1st of each year. The Company remains entitled to receive any royalties and milestone payments related to sales of peramivir by Shionogi following repayment of the PhaRMA Notes. Royalty Sub’s obligations to pay principal and interest on the PhaRMA Notes are obligations solely of Royalty Sub and are without recourse to any other person, including the Company, except to the extent of the Company’s pledge of its equity interests in Royalty Sub in support of the PhaRMA Notes.
In September 2014, Royalty Sub was unable to pay the accrued interest obligation due September 3, 2013. Under the terms of the Indenture, Royalty Sub’s inability to pay the full amount of interest payable in September 2013 by the next succeeding payment date for the PhaRMA Notes, which was September 1, 2014, constituted an event of default. Due to the non-recourse nature of the PhaRMA Notes, in the event of any potential foreclosure, the primary impact to the Company would be the loss of future royalty payments, if any, from Shionogi and legal costs associated with retiring the PhaRMA Notes. The PhaRMA Notes had a final legal maturity date of December 1, 2020, at which time the outstanding principal amount of the PhaRMA Notes of $30,000 , together with accrued and unpaid interest of $20,614 , was due in full.
Non-Recourse Notes Payable – Debt Extinguishment
Note 9— Debt
Pharmakon Loan Agreement
On April 17, 2023, the Company entered into a $450,000 Loan Agreement (the “Pharmakon Loan Agreement”) with BioPharma Credit Investments V (Master) LP and BPCR Limited Partnership, as lenders, and BioPharma Credit PLC, as collateral agent for the lenders. Certain of the Company’s wholly-owned subsidiaries are guarantors to the Pharmakon Loan Agreement. The Pharmakon Loan Agreement provides for an initial term loan in the principal amount of $300,000 (the “Tranche A Loan”) funded on April 17, 2023 (the “Tranche A Closing Date”). The Company used a portion of the proceeds from the Tranche A Loan to repay the $241,787 of outstanding indebtedness (principal and interest due as of April 17, 2023) under the then-existing Athyrium Credit Agreement (defined below) and to pay associated transaction costs and fees, and used the remaining net proceeds of $25,805 for other general corporate purposes.
96
The Pharmakon Loan Agreement also provides for three additional term loan tranches, at the Company’s option, in principal amounts of $50,000
The Pharmakon Loan Agreement provides for quarterly interest-only payments until the Maturity Date, with the unpaid principal amount of the outstanding Pharmakon Term Loans due and payable on the Maturity Date. During the first 18 months following the Tranche A Closing Date, the Company has the option to make a portion of the applicable interest payment on the Tranche A Loan in-kind (a “Pharmakon PIK Interest Payment”) by capitalizing as principal up to 50 % of the amount of interest accrued on the Tranche A Loan during the applicable interest period. The Pharmakon Term Loans will bear interest at a rate equal to the three-month Secured Overnight Financing Rate (“SOFR”) rate, which shall be no less than 1.75 %, plus 7.00 %, per annum or, for each interest period in which a Pharmakon PIK Interest Payment is made, with respect to the Tranche A Loan, SOFR plus 7.25 %, per annum.
The Tranche A Loan accrued interest at an effective interest rate of 13.30 % for the year ended December 31, 2023.
The Company is required to make a mandatory prepayment of the Pharmakon Term Loans (i) upon the occurrence of a change of control and (ii) prior to any repayment of any convertible debt that the Company may issue in the future, subject to certain exceptions. The Company may make voluntary prepayments in whole or in part, in minimum $25,000 increments. Prepayments are subject to a prepayment premium equal to, (i) with respect to any prepayment made prior to the second anniversary of the applicable Pharmakon Term Loan borrowing date, the sum of (1) 3.00 % of the principal amount of the Pharmakon Term Loan being prepaid plus (2) the aggregate amount of all interest that would have accrued on the principal amount of the Pharmakon Term Loan being prepaid from the date of prepayment through and including the second anniversary of the date of the borrowing of such Pharmakon Term Loan; (ii) with respect to any prepayment made on or after the second anniversary and prior to the third anniversary of the applicable Pharmakon Term Loan borrowing date, 3.00 % of the principal amount of the Pharmakon Term Loan being prepaid; (iii) with respect to any prepayment made on or after the third anniversary and prior to the fourth anniversary of the applicable Pharmakon Term Loan, 2.00 % of the principal amount of the Pharmakon Term Loan being prepaid; and (iv) with respect to any prepayment made on or after the fourth anniversary of the applicable Pharmakon Term Loan borrowing date and before the Maturity Date, 1.00 % of the principal amount of the Pharmakon Term Loan being prepaid. In addition, upon the drawing of any Subsequent Tranche Loan, certain funding fees are required to be paid.
The Pharmakon Loan Agreement also contains representations and warranties and affirmative and negative covenants customary for financings of this type, as well as customary events of default. Certain of the customary negative covenants limit the ability of the Company and certain of its subsidiaries to, among other things, dispose of assets, engage in mergers, acquisitions, and similar transactions, incur additional indebtedness, grant liens, make investments, pay dividends or make distributions or certain other restricted payments in respect of equity, prepay other indebtedness, enter into restrictive agreements, undertake fundamental changes or amend certain material contracts, among other customary covenants, in each case subject to certain exceptions.
A failure to comply with the covenants in the Pharmakon Loan Agreement, or an occurrence of any other event of default, could permit the lenders under the Pharmakon Loan Agreement to declare the borrowings thereunder, together with accrued interest and fees, and any applicable prepayment premium, to be immediately due and payable.
The Company’s obligations under the Pharmakon Loan Agreement are secured by a security interest in, subject to certain exceptions, substantially all of the Company’s assets.
As of December 31, 2023, the Company had total borrowings of $300,000 under the Pharmakon Loan Agreement. Interest expense on the Tranche A Loan for the year ended December 31, 2023 totaled $27,326 . As allowable under the Pharmakon Loan Agreement, the Company has designated and accounted for 50 % of the quarterly interest payments for the year ended December 31, 2023 as a Pharmakon PIK Interest Payment and the amount of $13,663 has been added to the outstanding principal balance of the borrowing. The remaining 50 % of the quarterly interest payments of $13,663 have been paid at the end of each quarterly period. As of December 31, 2023, borrowings, including the Pharmakon PIK Interest Payments, totaled $313,663 . The fair value of the debt approximates its carrying value based on prevailing interest rates as of the balance sheet date and is considered as Level 2 in the fair value hierarchy.
Incurred debt fees and issuance costs associated with the Tranche A Loan under the Pharmakon Loan Agreement totaled $11,147 and have been deferred and are being amortized as interest expense on an effective interest rate method
97
over the remaining term of the Tranche A Loan. Deferred financing amortization of $715 was recognized for the year ended December 31, 2023.
Athyrium Credit Agreement
On December 7, 2020, the Company entered into a $200,000 Credit Agreement (the “Athyrium Credit Agreement”) with Athyrium Opportunities III Co-Invest 1 LP (“Athyrium”), as lender and as administrative agent for the lenders. Certain of the Company’s direct and indirect subsidiaries were guarantors to the Athyrium Credit Agreement. The Athyrium Credit Agreement provided for an initial term loan in the principal amount of $125,000 (the “Term A Loan”), which was received by the Company on December 7, 2020 and is recorded in “Secured term loan” on the Company’s balance sheet. The Company used a portion of the proceeds from the Term A Loan to repay $43,298 of outstanding indebtedness, including accrued interest, under its prior credit facility with MidCap Financial Trust.
The Athyrium Credit Agreement also provided for two additional term loans, at the Company’s option, in the respective principal amounts of $25,000 (the “Term B Loan”) and $50,000 (the “Term C Loan” and, collectively with the Term A Loan and the Term B Loan, the “Athyrium Term Loans”). Having achieved all required revenue-based milestones, the Company exercised its option to draw upon the additional funding available under the Athyrium Credit Agreement, borrowing the principal amounts of $25,000 under the Term B Loan and $50,000 under the Term C Loan. Both the Term B Loan and the Term C Loan were funded on July 29, 2022 in the aggregate principal amount of $75,000 . The Company incurred deferred debt fees and issuance costs associated with the Term B and Term C Loans of $3,428 . The Term B Loan and the Term C Loan were subject to all the provisions under the Athyrium Credit Agreement.
On November 19, 2021, the Company entered into an amendment to the Athyrium Credit Agreement to, among other things, (i) permit the Company to enter into the 2021 RPI Royalty Purchase Agreement, the OMERS Royalty Purchase Agreement, and the other definitive documentation related thereto and to perform its obligations thereunder; and (ii) require the Company to pay to Athyrium, for the account of the lenders, a make-whole premium plus certain fees set forth in the Athyrium Credit Agreement in the event that the Company prepaid or repaid, or was required to prepay or repay, voluntarily or pursuant to mandatory prepayment obligations under the Athyrium Credit Agreement (e.g., with the proceeds of certain asset sales, certain ORLADEYO out-licensing or royalty financing transactions (excluding the Royalty Sales), extraordinary receipts, debt issuances, or upon a change of control of the Company and specified other events, subject to certain exceptions), all of the then-outstanding Athyrium Term Loans, in each case, subject to certain exceptions set forth in the Athyrium Credit Agreement.
The Athyrium Credit Agreement provided for quarterly interest-only payments until the maturity date, with the unpaid principal amount of the outstanding Athyrium Term Loans due and payable on the maturity date. For each of the first eight full fiscal quarters following December 7, 2020, the Company had the option to make the applicable interest payment-in-kind (an “Athyrium PIK Interest Payment”) by capitalizing the entire amount of interest accrued during the applicable interest period with the unpaid original principal amount outstanding on the last day of such period. The Athyrium Term Loans accrued interest at a rate equal to the three-month LIBOR rate, which was no less than 1.75 % and no more than 3.50 % (“LIBOR”), plus 8.25 %, or for each interest period in which an Athyrium PIK Interest Payment was made, LIBOR plus 10.25 %. The quarter ended December 31, 2022 was the last period eligible for the Athyrium PIK Interest Payment designation.
The Athyrium Term Loans accrued interest at an effective interest rate of 13.71 % during the period in which the debt was outstanding for the year ended 2023 compared to 12.87 % for fiscal year 2022.
Quarterly interest payments under the Athyrium Credit Agreement for the year ended December 31, 2023 totaled $8,476 . Quarterly interest payments under the Athyrium Credit Agreement for the year ended December 31, 2022 totaled $23,387 and were designated and accounted for as Athyrium PIK Interest Payments and added to the outstanding principal balance of the borrowing. From the Athyrium Term Loans’ inception through December 31, 2022, the quarterly interest payments were designated and accounted for as Athyrium PIK Interest Payments and added to the outstanding principal balance of the borrowing. The quarter ended December 31, 2022 was the last period eligible for the Athyrium PIK Interest Payment designation. Deferred financing amortization of $1,069 and $916 , and $531 was recognized for the years ended December 31, 2023, 2022, and 2021 respectively.
On April 17, 2023, the outstanding principal of the Athyrium Term Loans, including the Athyrium PIK Interest Payments of $240,452 along with interest accrued of $1,335 for the first 17 days of the quarterly interest period ended June 30, 2023, was repaid with the funding received through the Pharmakon Loan Agreement.
98
In accordance with the Athyrium Credit Agreement, upon the prepayment or repayment of all or any of the Athyrium Term Loans, the Company was obligated to pay an exit fee in an amount equal to 2.00 % of the principal amount of the Athyrium Term Loans prepaid or repaid. In addition, each Athyrium Term Loan was subject to a 1.00 % commitment fee at its respective borrowing date. As a result, the Company incurred prepayment and final payment fees of $17,261 upon repayment of the Athyrium Term Loans. Additionally, unamortized deferred financing costs of $11,758 associated with the Athyrium Term Loans were written-off at the time of repayment. Collectively, the prepayment and final payment fees and unamortized deferred financing costs totaled $29,019 and are reflected as a one-time loss on extinguishment of debt on the Consolidated Statements of Comprehensive Loss for the year ended December 31, 2023.
Note 10— Lease Obligations
The Company leases certain assets under operating and finance leases, which consist of real estate leases, laboratory equipment leases and office equipment leases as of December 31, 2023. Renewal options for the Company’s leases range from 1 to 3 years in length and begin from 2024 through 2030.
Lease expense under operating and finance leases was as follows (in thousands):
| Years Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Operating lease expense | $ | $ | $ | |||||||||||||||||
| Finance lease expense: | ||||||||||||||||||||
| Amortization of right-of-use assets | $ | $ | ||||||||||||||||||
| Interest on lease liabilities | $ | $ | ||||||||||||||||||
| Total finance lease expense | $ | $ | $ | |||||||||||||||||
Other supplemental information related to leases was as follows:
| As of December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Weighted average remaining lease term: | |||||||||||
| Operating leases | |||||||||||
| Finance leases | |||||||||||
| Weighted average discount rate: | |||||||||||
| Operating leases | % | % | |||||||||
| Finance leases | % | % | |||||||||
99
The following table summarizes the presentation in the Consolidated Balance Sheets of the Company’s operating leases (in thousands):
| As of December 31, | |||||||||||||||||
| Balance Sheet Location | 2023 | 2022 | |||||||||||||||
| Operating lease assets: | |||||||||||||||||
| Operating lease assets, net | $ | $ | |||||||||||||||
| Operating lease liabilities: | |||||||||||||||||
| Current operating lease liabilities | Operating lease liabilities – current liabilities | $ | $ | ||||||||||||||
| Non-current operating lease liabilities | Operating lease liabilities – long-term liabilities | ||||||||||||||||
| Total operating lease liabilities | $ | $ | |||||||||||||||
The following table summarizes the presentation in the Consolidated Balance Sheets of the Company’s finance leases (in thousands):
| As of December 31, | |||||||||||||||||
| Balance Sheet Location | 2023 | 2022 | |||||||||||||||
| Finance lease assets: | |||||||||||||||||
| Finance lease assets, net | $ | $ | |||||||||||||||
| Finance lease liabilities: | |||||||||||||||||
| Current finance lease liabilities | Finance lease liabilities – current liabilities | $ | $ | ||||||||||||||
| Non-current finance lease liabilities | Finance lease liabilities – long-term liabilities | ||||||||||||||||
| Total finance lease liabilities | $ | $ | |||||||||||||||
Operating lease assets are recorded net of accumulated amortization of $4,794 and $3,268 as of December 31, 2023 and 2022, respectively. Finance lease assets are recorded net of accumulated amortization of $2,293 and $1,081 as of December 31, 2023 and 2022, respectively.
Maturities of lease liabilities as of December 31, 2023 are as follows (in thousands):
| Operating Leases | Finance Leases | |||||||||||||
| 2024 | $ | $ | ||||||||||||
| 2025 | ||||||||||||||
| 2026 | ||||||||||||||
| 2027 | ||||||||||||||
| 2028 | ||||||||||||||
| Thereafter | ||||||||||||||
| Total lease payments | ||||||||||||||
| Less imputed interest | ( | ( | ||||||||||||
| Total | $ | $ | ||||||||||||
100
Supplemental cash flow information related to leases was as follows (in thousands):
| Years Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||||||||||||||
| Operating cash flows for finance leases | $ | $ | $ | |||||||||||||||||
| Operating cash flows for operating leases | $ | $ | $ | |||||||||||||||||
| Financing cash flows for finance leases | $ | $ | $ | |||||||||||||||||
| Operating lease assets obtained in exchange for operating lease liabilities: | $ | $ | $ | |||||||||||||||||
| Finance lease assets obtained in exchange for finance lease liabilities: | $ | $ | $ | |||||||||||||||||
| Non-cash increase to operating lease assets due to remeasurement of operating lease liabilities: | $ | $ | $ | |||||||||||||||||
Note 11— Stockholders ’ Equity
Sales of Common Stock
On March 1, 2021, the Company filed an automatic shelf registration statement on Form S-3 with the SEC. This shelf registration statement became effective automatically upon filing and allows the Company to sell an indeterminate number of securities, including common stock, preferred stock, depositary shares, purchase contracts, warrants, debt securities, and units, from time to time at prices and on terms to be determined at the time of sale.
On November 19, 2021, concurrent with the Company entering into the 2021 RPI Royalty Purchase Agreement, the Company and RPI entered into the Common Stock Purchase Agreement, pursuant to which the Company issued 3,846 shares of the Company’s common stock to RPI for an aggregate purchase price of $50,000 , at a price of $13.00 per share, calculated based on the 20-day volume weighted average price. The $13.00 per share price represented a premium of $1.11 over the closing price of $11.89 of the Company’s common stock on November 19, 2021, the last trading day prior to the execution of the Common Stock Purchase Agreement. The premium of $4,269 paid by RPI on the purchase of the Company’s common stock has been deferred and is being amortized as a component of interest expense of the 2021 RPI royalty financing obligation.
On October 23, 2023, certain entities affiliated with Baker Bros. Advisors LP (the “Baker Entities”) net exercised the remaining balance of the pre-funded warrants held by such Baker Entities that were issued on November 21, 2019. Additionally, certain of the Baker Entities net exercised all of the pre-funded warrants that were issued on June 1, 2020. The exercises resulted in the issuance of 14,997 common shares. Following the exercises, there are no outstanding warrants.
Shares Reserved for Future Issuance of Common Stock
The Company had reserved shares of common stock for issuance as follows (in thousands):
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Shares reserved for exercises of outstanding stock options | |||||||||||
| Shares reserved for vesting of restricted stock units | |||||||||||
| Shares reserved for exercises of warrants | |||||||||||
| Shares reserved for future issuance under the Stock Incentive Plan | |||||||||||
| Shares reserved for future issuance under the Inducement Equity Incentive Plan | |||||||||||
| Shares reserved for future issuance under the Employee Stock Purchase Plan | |||||||||||
| Total shares reserved for future issuance | |||||||||||
101
Note 12— Stock-Based Compensation
As of December 31, 2023, the Company had three stock-based employee compensation plans: the Amended and Restated Stock Incentive Plan (“Incentive Plan”), the Amended and Restated Inducement Equity Incentive Plan (“Inducement Plan”) and the Amended and Restated Employee Stock Purchase Plan (“ESPP”). The Incentive Plan was most recently amended and restated on April 24, 2023 and approved by the Company’s stockholders on June 13, 2023. The Inducement Plan was most recently amended and restated by the Company’s Board of Directors on October 26, 2023. The ESPP was most recently amended and restated by the Company’s Board of Directors on July 7, 2023.
The Company recorded the following stock-based compensation expense (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Incentive Plan | $ | $ | $ | ||||||||||||||
| Inducement Plan | |||||||||||||||||
| ESPP | |||||||||||||||||
| Stock-based compensation expense | $ | $ | $ | ||||||||||||||
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Research and development | $ | $ | $ | ||||||||||||||
| Selling, general and administrative | |||||||||||||||||
| Total stock-based compensation expense | $ | $ | $ | ||||||||||||||
Stock Incentive Plan
The Company grants stock option awards, restricted stock and restricted stock units to its employees, directors, and consultants under the Incentive Plan. Under the Incentive Plan, stock option awards are granted with an exercise price equal to the market price of the Company’s common stock at the date of grant. Stock option awards and restricted stock units granted to employees generally vest 25 % each year until fully vested after four years .
In December 2014, the Company issued 1,250 performance-based stock options. These awards vest upon successful completion of specific development milestones. As of December 31, 2023, 85 % of these grants have vested.
In January 2022, the Company issued 221 performance-based restricted stock unit awards. 21 of the awards met the performance objectives in 2022 and became eligible for vesting at 50 % on the first anniversary of the grant date and 25 % on each of the second and third anniversaries of the grant date, until fully vested after three years . The Company recognized $34 and $158 of stock compensation expense related to these awards during the years ended December 31, 2023 and December 31, 2022, respectively.
Stock option awards and restricted stock unit awards granted to non-employee directors of the Company generally vest over one year . Stock option awards granted to new non-employee directors when they first join the Company’s Board of Directors generally vest, subject to the terms of the Incentive Plan, in 36 equal monthly installments over a three-year period measured from the grant date. All stock option awards have contractual terms of 10 years. Restricted stock unit awards granted to new non-employee directors when they first join the Company’s Board of Directors generally vest, subject to the terms of the Incentive Plan, in three equal annual installments beginning on the first anniversary of the grant date. The vesting and exercise provisions of all awards granted under the Incentive Plan are subject to acceleration in the event of certain stockholder-approved transactions, or upon the occurrence of a change in control as defined in the Incentive Plan.
The following table summarizes stock option activity under the Incentive Plan:
102
| Shares (in thousands) | Weighted Average Exercise Price per Share | Weighted Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||||||
| Outstanding at December 31, 2022 | $ | ||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( | $ | |||||||||||||||||||||
| Cancelled or Forfeited | ( | ||||||||||||||||||||||
| Outstanding at December 31, 2023 | $ | $ | |||||||||||||||||||||
| Exercisable at December 31, 2023 | $ | $ | |||||||||||||||||||||
| Vested and expected to vest at December 31, 2023 | $ | $ | |||||||||||||||||||||
The total intrinsic value of stock option awards exercised under the Incentive Plan was $21,150 and $25,484 during the years ended December 31, 2022 and 2021, respectively. The aggregate intrinsic value represents the total proceeds (calculated as the difference between the exercise price of the underlying stock options and the estimated fair value of the Company’s common stock on the date of exercise for those stock options that had exercise prices lower than the fair value of the Company’s common stock on the exercise date) received by all individuals who exercised stock option awards during the period.
The following table summarizes restricted stock unit activity under the Incentive Plan:
| Shares (in thousands) | Weighted Average Grant Date Fair Value | ||||||||||
| Unvested at December 31, 2022 | $ | ||||||||||
| Granted | |||||||||||
| Vested | ( | ||||||||||
| Forfeited | ( | ||||||||||
| Unvested at December 31, 2023 | $ | ||||||||||
For restricted stock unit awards granted under the Incentive Plan, the fair value of the awards is determined based on the market value of the Company’s shares on the grant date. The weighted average grant date fair value of these awards granted during 2023, 2022, and 2021 was $6.71 , $11.20 , and 11.36 , respectively. The fair value of the restricted stock unit awards is amortized to expense over the vesting periods using a straight-line expense attribution method.
As of December 31, 2023, total unrecognized compensation cost related to unvested restricted stock unit awards granted under the Incentive Plan was $40,981 , which is expected to be recognized over a weighted average period of 2.0 years.
Inducement Equity Incentive Plan
The Company has the ability to grant stock option and restricted stock unit awards to newly-hired employees as inducements material to each employee entering employment with the Company. Awards granted to newly hired employees generally vest 25 % each year until fully vested after four years and are subject to the terms and conditions of the Inducement Plan. Each stock option has a term of 10 years. The vesting and exercise provisions of all awards granted under the Inducement Plan are subject to acceleration in the event of certain stockholder-approved transactions, or upon the occurrence of a change in control as defined in the Inducement Plan.
The following table summarizes stock option activity under the Inducement Plan:
103
| Shares (in thousands) | Weighted Average Exercise Price per Share | Weighted Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||||||
| Outstanding at December 31, 2022 | $ | ||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( | $ | |||||||||||||||||||||
| Cancelled or Forfeited | ( | ||||||||||||||||||||||
| Outstanding at December 31, 2023 | $ | $ | |||||||||||||||||||||
| Exercisable at December 31, 2023 | $ | $ | |||||||||||||||||||||
| Vested and expected to vest at December 31, 2023 | $ | $ | |||||||||||||||||||||
The total intrinsic value of stock option awards exercised under the Inducement Plan was $3,710 and $6,700 during the years ended December 31, 2022 and 2021, respectively. The aggregate intrinsic value represents the total proceeds (calculated as the difference between the exercise price of the underlying stock options and the estimated fair value of the Company’s common stock on the date of exercise for those stock options that had exercise prices lower than the fair value of the Company’s common stock on the exercise date) received by all individuals who exercised stock option awards during the period.
The following table summarizes restricted stock unit activity under the Inducement Plan:
| Shares (in thousands) | Weighted Average Grant Date Fair Value | ||||||||||
| Unvested at December 31, 2022 | $ | ||||||||||
| Granted | |||||||||||
| Vested | ( | ||||||||||
| Forfeited | ( | ||||||||||
| Unvested at December 31, 2023 | $ | ||||||||||
For restricted stock unit awards granted under the Inducement Plan, the fair value of the awards is determined based on the market value of the Company’s shares on the grant date. The weighted average grant date fair value of these awards granted during 2023 and 2022 was $7.81 and 13.21 , respectively. The fair value of the restricted stock unit awards is amortized to expense over the vesting periods using a straight-line expense attribution method. No restricted stock unit awards were granted under the Inducement Plan during 2021.
As of December 31, 2023, total unrecognized compensation cost related to unvested restricted stock unit awards granted under the Inducement Plan was $7,044 , which is expected to be recognized over a weighted average period of 1.8 years.
Weighted Average Assumptions for Stock Option Awards Granted to Employees and Directors under the Incentive and Inducement Plans
For stock option awards granted under the Incentive Plan and the Inducement Plan, the fair value is estimated on the date of grant using a Black-Scholes option pricing model and the assumptions noted below. The fair value of the stock option awards is amortized to expense over the vesting periods using a straight-line expense attribution method.
Historically, the expected life was based on the average of the assumption that all outstanding stock option awards will be exercised at full vesting and the assumption that all outstanding stock option awards will be exercised at the midpoint of the current date (if already vested) or at full vesting (if not yet vested) and the full contractual term. Effective July 1, 2023, the expected life is based on the historical settlement of options by taking into account exercises and post-
104
vesting terminations and weighing them based on the number of options settled. This change in approach did not have a significant impact on the value of the stock option awards granted. The expected volatility represents the historical volatility on the Company’s publicly-traded common stock. The Company has assumed no expected dividend yield, as dividends have never been paid to stock or option holders and will not be paid for the foreseeable future. The weighted average risk-free interest rate is the implied yield currently available on zero-coupon government issues with a remaining term equal to the expected term.
Stock Incentive Plan
The following table summarizes the key assumptions used by the Company to value the stock option awards granted under the Incentive Plan during the years ended December 31, 2023, 2022, and 2021:
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Expected Life in Years | |||||||||||||||||
| Expected Volatility | % | % | % | ||||||||||||||
| Expected Dividend Yield | % | % | % | ||||||||||||||
| Risk-Free Interest Rate | % | % | % | ||||||||||||||
| Weighted average grant date fair value per share | $ | $ | $ | ||||||||||||||
The total fair value of the stock option awards vested under the Incentive Plan was $33,731 , $28,916 , and $20,510 during the years ended December 31, 2023, 2022, and 2021, respectively. As of December 31, 2023, total unrecognized compensation cost related to unvested stock option awards granted under the Incentive Plan was $75,164 , which is expected to be recognized over a weighted average period of 1.9 years.
Inducement Equity Incentive Plan
The following table summarizes the key assumptions used by the Company to value the stock option awards granted under the Inducement Plan during the years ended December 31, 2023, 2022, and 2021:
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Expected Life in Years | |||||||||||||||||
| Expected Volatility | % | % | % | ||||||||||||||
| Expected Dividend Yield | % | % | % | ||||||||||||||
| Risk-Free Interest Rate | % | % | % | ||||||||||||||
| Weighted average grant date fair value per share | $ | $ | $ | ||||||||||||||
The total fair value of the stock option awards vested under the Inducement Plan was $7,698 , $4,659 , and $2,885 during the years ended December 31, 2023, 2022, and 2021, respectively. As of December 31, 2023, total unrecognized compensation cost related to unvested stock option awards granted under the Inducement Plan was $16,216 , which is expected to be recognized over a weighted average period of 1.5 years.
Employee Stock Purchase Plan
The Company has reserved a total of 7,975 shares of common stock to be purchased under the ESPP, of which 5,454 shares remain available for purchase at December 31, 2023. Eligible employees may authorize up to 15 % of their salary to purchase common stock at the lower of 85 % of the beginning or 85 % of the ending price during six-month purchase intervals. No more than three thousand shares may be purchased by any one employee at each purchase date, and no employee may purchase stock having a fair market value at the commencement date of $25 or more in any one calendar year.
During the years ended December 31, 2023, 2022, and 2021, the Company issued 338 , 260 , and 321 shares of common stock under the ESPP, respectively, at a weighted average price per share of $7.68 , $11.03 , and $6.20 ,
105
respectively. Compensation expense for shares purchased under the ESPP related to the purchase discount and the “look-back” option were determined using a Black-Scholes option pricing model. The weighted average grant date fair values of shares granted under the ESPP during the years ended December 31, 2023, 2022, and 2021, were $3.82 , $6.02 , and $2.80 , respectively.
Note 13— Income Taxes
The components of loss before provision for income taxes were as follows (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Domestic | $ | ( | $ | ( | $ | ( | |||||||||||
| Foreign | ( | ( | ( | ||||||||||||||
| Loss before provision for income taxes | $ | ( | $ | ( | $ | ( | |||||||||||
The components of the (benefit) expense for income taxes were as follows (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Current expense (benefit) provision: | |||||||||||||||||
| U.S. Federal and state | $ | ( | $ | $ | |||||||||||||
| Foreign | |||||||||||||||||
| Total current expense provision | |||||||||||||||||
| Deferred expense (benefit) provision: | |||||||||||||||||
| U.S. Federal and state | ( | ( | |||||||||||||||
| Foreign | ( | ||||||||||||||||
| Total deferred expense provision | ( | ( | |||||||||||||||
| Total expense provision | $ | $ | $ | ||||||||||||||
The differences between the Company’s effective tax rate and the statutory tax rate in 2023, 2022, and 2021 were as follows (in thousands):
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
Income tax benefit at federal statutory rate ( | $ | ( | $ | ( | $ | ( | |||||||||||
| State and local income taxes net of federal tax benefit | ( | ( | ( | ||||||||||||||
| Permanent items | ( | ( | |||||||||||||||
| Expiration of attribute carryforwards | ( | ||||||||||||||||
| Research and development tax credits | ( | ( | ( | ||||||||||||||
| Foreign rate differential | |||||||||||||||||
| Other | ( | ||||||||||||||||
| Change in valuation allowance | |||||||||||||||||
| Income tax expense | $ | $ | $ | ||||||||||||||
The Company recognizes the impact of a tax position in its financial statements if it is more likely than not that the position will be sustained on audit based on the technical merits of the position. The Company has concluded that it has an uncertain tax position pertaining to its research and development and orphan drug credit carryforwards. The Company has established these credits based on information and calculations it believes are appropriate and the best estimate of the underlying credit. Any changes to the Company’s unrecognized tax benefits are offset by an adjustment to the valuation
106
allowance and there would be no impact on the Company’s financial statements. The Company does not expect its unrecognized tax benefits to change significantly over the next 12 months.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| 2023 | 2022 | ||||||||||
| Balance at January 1, | $ | $ | |||||||||
| Additions to current period tax positions | |||||||||||
| Reductions to prior period tax provisions | ( | ||||||||||
| Balance at December 31, | $ | $ | |||||||||
The Company’s ability to utilize the net operating loss and tax credit carryforwards in the future may be subject to substantial restrictions in the event of past or future ownership changes as defined in Section 382 of the IRC and similar state tax law.
Significant components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Deferred tax assets: | |||||||||||
| Net federal and state operating losses | $ | $ | |||||||||
| Research and development credits | |||||||||||
| Royalty income | |||||||||||
| Stock-based compensation | |||||||||||
| Capitalized R&D | |||||||||||
| Leasing obligations | |||||||||||
| Other | |||||||||||
| Total deferred tax assets | |||||||||||
| Deferred tax liabilities: | |||||||||||
| Fixed assets | ( | ( | |||||||||
| Right of use asset | ( | ( | |||||||||
| Total deferred tax liabilities | ( | ( | |||||||||
| Valuation allowance | ( | ( | |||||||||
| Net deferred tax assets (liabilities) | $ | $ | ( | ||||||||
The majority of the Company’s deferred tax assets relate to net operating loss and research and development carryforwards that can only be realized if the Company is profitable in future periods. It is uncertain whether the Company will realize any tax benefit related to these carryforwards. Accordingly, the Company has provided a valuation allowance against substantially all the net deferred tax assets due to uncertainties as to their ultimate realization. The valuation allowance will remain at the full amount of the deferred tax assets until it is more likely than not that the related tax benefits will be realized. The Company’s valuation allowance increased by $47,490 , $70,894 , and $47,954 in 2023, 2022, and 2021, respectively.
As of December 31, 2023, the Company had U.S. federal operating loss carryforwards of $432,328 , state operating loss carryforwards of $183,478 , foreign net operating losses of $65,416 , and U.S. research and development and orphan drug credit carryforwards of $102,752 , which will expire at various dates from 2024 through 2043. Federal losses, state losses, and research and development credit carryforwards began expiring in 2021. The foreign net operating losses have an indefinite carryforward period.
Tax years 2020-2023 remain open to examination by the major taxing jurisdictions to which the Company is subject. Additionally, years prior to 2020 are also open to examination to the extent of loss and credit carryforwards from those
107
years. The Company recognizes interest and penalties accrued related to unrecognized tax benefits as components of its income tax provision. However, there were no
As of December 31, 2023, the Company has minimal accumulated undistributed earnings generated by its foreign subsidiaries which have already been subject to local and U.S. tax (as part of the global intangible low-taxed income provisions). The Company intends to indefinitely reinvest these earnings, as well as future earnings from its foreign subsidiaries to fund its international operations. In addition, the Company expects future U.S. cash generation will be sufficient to meet future U.S. cash needs.
Note 14— Employee 401(k) Plan
In January 1991, the Company adopted an employee retirement plan (“401(k) Plan”) under Section 401(k) of the IRC covering all employees. Employee contributions may be made to the 401(k) Plan up to limits established by the Internal Revenue Service. Company matching contributions may be made at the discretion of the Board of Directors. The Company made matching contributions of $5,716 , $3,758 , and $2,834 in 2023, 2022, and 2021, respectively.
Note 15— Collaborative and Other Relationships
Government Collaborations
National Institute of Allergy and Infectious Diseases (“NIAID/HHS”)
In September 2013, NIAID/HHS contracted with the Company for the development of galidesivir as a treatment for Marburg virus disease and subsequently, Yellow Fever and Ebola virus disease. On September 15, 2021, the Company entered into an amendment to pay for certain additional costs, including additional manufacturing development costs and overhead, and to change the total value of the contract, as amended, to $47,315 from $45,931 . All options under the contract have been awarded.
In August 2020, NIAID/HHS awarded the Company a new contract, with potential aggregate funding of up to $43,908 if all contract options were exercised, to manufacture and evaluate the safety, efficacy and tolerability of galidesivir. NIAID/HHS made an initial award of $6,326 to the Company under this contract. Revenue related to this contract was recognized during the years ended December 31, 2022 and 2021.
Biomedical Advanced Research and Development Authority (“BARDA/HHS”)
In March 2015, BARDA/HHS awarded the Company a contract for the continued development of galidesivir as a potential treatment for diseases caused by RNA pathogens, including filoviruses. This BARDA/HHS contract included a base contract of $16,265 to support galidesivir drug manufacturing, as well as $22,855 in additional development options that could be exercised by the government, bringing the potential value of the contract to $39,120 . As of December 31, 2022, a total of $20,574 was awarded under exercised options within this contract. The most recent development option was completed as of December 31, 2022.
The contracts with NIAID/HHS and BARDA/HHS were cost-plus-fixed-fee contracts. That is, the Company was entitled to receive reimbursement for all costs incurred in accordance with the contract provisions that were related to the development of galidesivir plus a fixed fee, or profit. BARDA/HHS and NIAID/HHS made periodic assessments of progress, and the continuation of the contracts was based on the Company’s performance, the timeliness and quality of deliverables, and other factors. The government had rights under certain contract clauses to terminate these contracts. These contracts were terminable by the government at any time for breach or without cause. As of December 31, 2022, all of the Company’s government funding for galidesivir had expired.
U.S. Department of Health and Human Services (“HHS”)
In September 2018, HHS awarded the Company a $34,660 contract for the procurement of up to 50,000 doses of RAPIVAB (peramivir injection) over a five-year period. The Company initially delivered 20,000 doses of RAPIVAB under this contract in 2019 for a total price of approximately $13,864 . The Company further delivered 20,000 and 9,980 doses of RAPIVAB in 2022 and 2021, respectively, and recorded revenue of $13,864 and $6,918 for the years ending December 31, 2022 and 2021, respectively. As of December 31, 2022, the Company had delivered a total of 49,980
108
RAPIVAB doses of the 50,000 RAPIVAB doses available under the contract, effectively completing the contract with HHS.
ORLADEYO
Torii Pharmaceutical Co., Ltd. (“Torii”)
On November 5, 2019, the Company entered into a Commercialization and License Agreement with Torii (the “Original Torii Agreement”), granting Torii the exclusive right to commercialize ORLADEYO for the prevention of hereditary angioedema (“HAE”) attacks in Japan. Under the Original Torii Agreement, the Company received an upfront, non-refundable payment of $22,000 . The Company received an additional milestone payment of $15,000 in the second quarter of 2021 upon receipt from the Japanese NHI of a reimbursement price approval for ORLADEYO. In addition, the Company was entitled to receive tiered royalty payments, ranging from 20 % to 40 % of annual net sales of ORLADEYO in Japan during each calendar year. Torii’s royalty payment obligations were subject to customary reductions in certain circumstances, but could not be reduced by more than 50 % of the amount that otherwise would have been payable to the Company in the applicable calendar quarter.
The Company identified performance obligations under the Original Torii Agreement related to (i) the license to develop and commercialize ORLADEYO, (ii) regulatory approval support, and (iii) reimbursement pricing approval support. These were each determined to be distinct from the other performance obligations. The Company allocated the $22,000 upfront consideration to the identified performance obligations using estimation approaches to determine the standalone selling prices under ASC Topic 606. Specifically, in determining the value related to the license, a valuation approach utilizing risk adjusted discounted cash flow projections was used, and an expected cost plus margin approach was utilized for the other performance obligations.
On November 30, 2023, the Company entered into an Amended and Restated Commercialization and License Agreement with Torii (as amended, the “Torii Agreement”). Under the Torii Agreement, the Company is entitled to receive tiered royalty payments, ranging from 20 % to 80 % of annual net sales of ORLADEYO in Japan during each calendar year. The Company is now responsible for all commercial promotion activities to support ORLADEYO sales in Japan, and Torii is responsible for HAE disease awareness activities in Japan. The Company will receive a 20 % royalty on annual Japanese sales below a prespecified threshold and an 80 % royalty on annual Japanese sales above the prespecified threshold.
Torii’s updated royalty payment obligations commenced upon November 30, 2023 and expire upon the later of (i) the tenth anniversary of the date of first commercial sale of ORLADEYO in Japan, (ii) the expiration of the Company’s patents covering ORLADEYO, and (iii) the expiration of regulatory exclusivity for ORLADEYO in Japan.
The Company determined that the Torii Agreement represented a contract modification to be accounted for as if it were part of the Original Torii Agreement under ASC Topic 606. As the performance obligations under the Original Torii agreement had been fully satisfied, the Company was not required to adjust revenue previously recognized.
Peramivir Injection (RAPIVAB, RAPIACTA, PERAMIFLU)
Shionogi & Co., Ltd. (“Shionogi”)
In February 2007, the Company entered into an exclusive license agreement with Shionogi to develop and commercialize peramivir in Japan for the treatment of seasonal and potentially life-threatening human influenza. Under the terms of the agreement, Shionogi obtained rights to injectable formulations of peramivir in Japan. In October 2008, the Company and Shionogi amended the license agreement to expand the territory covered by the agreement to include Taiwan. Shionogi has commercially launched peramivir under the commercial name RAPIACTA in Japan and Taiwan. The Company developed peramivir under a license from UAB and will owe sublicense payments to UAB on any future milestone payments and/or royalties received by the Company from Shionogi.
Green Cross Corporation (“Green Cross”)
In June 2006, the Company entered into an agreement with Green Cross to develop and commercialize peramivir in Korea. Under the terms of the agreement, Green Cross is responsible for all development, regulatory, and commercialization costs in Korea and the Company is entitled to share in profits resulting from the sale of peramivir in Korea, including the sale of peramivir to the Korean government for stockpiling purposes. Furthermore, Green Cross will
109
pay the Company a premium over its cost to supply peramivir for development and any future marketing of peramivir products in Korea.
Other Collaborations
Clearside Biomedical, Inc. (“Clearside”)
On November 3, 2023, the Company announced that it entered into a license agreement (the “Clearside Agreement”) with Clearside, enabling the Company to develop its investigational plasma kallikrein inhibitor, avoralstat, with Clearside’s SCS Microinjector® to deliver avoralstat to the back of the eye through the suprachoroidal space to treat patients with diabetic macular edema.
Under the Clearside Agreement, Clearside received a $5,000 upfront license fee from the Company, which has been recognized in research and development expenses during the year ended December 31, 2023. Clearside is eligible to receive up to an additional $30,000 in clinical and regulatory milestone payments, and up to a total of $47,500 in three post-approval sales-based milestone payments as annual global net sales progress to $2,000,000 . The Company will pay Clearside tiered mid-single digit royalties on annual global net product sales, at three tiers, including a top tier of >$1,500,000 .
Note 16— Workforce Reduction
In January 2024, the Company announced a reduction of workforce. The majority of the impacted employees had a termination date in January 2024, with certain employees exiting later in 2024. The Company notified the impacted employees in January 2024.
The Company incurred costs related to employee severance, benefits, and related costs which were accounted for as ongoing terminations benefits under ASC Topic 712, Nonretirement Postemployment Benefits. As of December 31, 2023, it was considered probable that payment would be owed and the amount of payment was considered to be reasonably estimable, which resulted in the recognition of $3,380 of costs related to the workforce reduction during the year ended December 31, 2023, of which $3,026 has been recognized in research and development expenses and $354 has been recognized in selling, general and administrative expenses in the Consolidated Statement of Comprehensive Loss. As of December 31, 2023, none of the costs were paid and are included within accrued expenses on the Consolidated Balance Sheet. These costs are expected to be disbursed during the period of January 1, 2024 through December 31, 2024.
In addition, the employees impacted by the workforce reduction received an amount equal to the bonus amount the employee would have received through continued employment with the Company, which was considered a one-time termination benefit pursuant to ASC Topic 420, Exit or Disposal Costs. As a result, $1,264 will be recognized during the three months ended March 31, 2024, the period in which the communication occurred, of which $1,201 will be recognized in research and development expenses, and $63 will be recognized in selling, general and administrative expenses in the Consolidated Statement of Comprehensive Loss. This cost is expected to be disbursed during the period of January 1, 2024 through March 31, 2024.
The Company does not expect to incur any additional significant costs related to this workforce reduction.
Note 17— Subsequent Events
The Company considers events or transactions that occur after the balance sheet date but prior to the issuance of the consolidated financial statements to provide additional evidence for certain estimates or to identify matters that require additional disclosure. The Company has concluded that no subsequent events have occurred that require disclosure, except as described in “Note 16— Workforce Reduction”.
110
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of BioCryst Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BioCryst Pharmaceuticals, Inc. (the Company) as of December 31, 2023 and 2022, the related consolidated statements of comprehensive loss, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 27, 2024 expressed an adverse opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
111
Royalty Financing Obligations | |||||
| Description of the Matter | As described in Note 8 to the consolidated financial statements, the Company entered into Royalty Purchase Agreements (“RPA’s”) with third parties. Pursuant to the RPA’s, the Company received proceeds of approximately $425 million in exchange for the right to receive royalty payments based on future net revenues of the Company’s commercialized drug, ORLADEYO, and other drug candidates as specified in the agreements. The Company recorded the RPA’s as liability instruments (royalty financing obligations) on the balance sheet at their carrying value of $531.6 million as of December 31, 2023, and imputed interest expense associated with these liabilities using the effective interest rate method. The effective interest rate is calculated based on the rate that would enable the liability to be repaid in full over the anticipated life of each arrangement. The interest rates on these instruments may vary during the term of the agreement depending on a number of factors, including the level and timing of forecasted net revenues which affects the repayment timing and ultimate amount of repayment. In order to amortize the royalty financing obligations, the Company utilizes the prospective method to estimate the future royalties to be paid by the Company to the third parties over the life of the arrangements. Under the prospective method, a new effective interest rate is determined based on the revised estimate of remaining cash flows. The Company periodically assesses the amount and timing of expected royalty payments using a combination of internal projections and forecasts from external sources. Auditing the royalty financing obligations may be complex and highly judgmental due to the estimation uncertainty in determining the effective interest rates. The Company’s effective interest rate models include actual revenues recorded and royalties paid to-date, as well as revenue projections for which future royalties will be paid, which are sensitive to significant assumptions (including population, market penetration, probability of success, and sales price, among others) that are affected by expectations about future market conditions. | ||||
| How We Addressed the Matter in Our Audit | To evaluate the royalty financing obligations, our audit procedures included, among others, assessing the underlying data and assumptions used by the Company in its effective interest rate models. We compared the significant assumptions in the revenue projections to current industry, market and economic trends. We recalculated the current year interest expense based on the amortization schedules and estimates of royalties using the effective interest method and performed sensitivity analyses to evaluate the changes in the effective interest rates, and associated interest expense, that would result from changes in the assumptions. | ||||
112
Product Sales, net | |||||
| Description of the Matter | As discussed in Note 1, when recognizing product revenue, the Company makes an estimate of the net selling price (transaction price), which includes estimates of variable consideration. For the year ended December 31, 2023, the Company recorded net product sales of $324.7 million. Product sales are recorded net of adjustments for variable consideration including estimated government rebates, managed care rebates, chargebacks, costs of co-payment for assistance programs, and product returns at the time revenue is recorded. The Company’s estimates of variable consideration depend on the identification of key customer contract terms and conditions. The revenue recognition process can be complex and can involve judgment related to these estimates as well as to identify and assess the terms and conditions of customer agreements and related government regulations that could affect revenue recognition, as the Company’s revenue expands with new customers and new markets. | ||||
| How We Addressed the Matter in Our Audit | To test product sales, our audit procedures included, among others, tracing a sample of revenue transactions recognized during the year to source documentation. We also confirmed a sample of outstanding receivable balances directly with the Company’s customers. To test management’s estimates of variable consideration, we obtained management’s calculations for the respective estimates and performed one or more of the following procedures: tested the adjustments for variable consideration recorded for a sample of revenue transactions, tested management’s estimation process to assess whether the recorded reserve balances are within a reasonable range of estimate, assessed subsequent events, and tested credits issued throughout the year. | ||||
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 1993.
February 27, 2024
113
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of BioCryst Pharmaceuticals, Inc.
Opinion on Internal Control Over Financial Reporting
We have audited BioCryst Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, because of the effect of the material weakness described below on the achievement of the objectives of the control criteria, BioCryst Pharmaceuticals, Inc. (the Company) has not maintained effective internal control over financial reporting as of December 31, 2023, based on the COSO criteria.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weakness has been identified and included in management’s assessment:
The Company has experienced insufficient staffing resources in its accounting function, which restricts its ability to consistently execute review procedures over financial statement close processes.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the 2023 consolidated financial statements of the Company. This material weakness was considered in determining the nature, timing and extent of audit tests applied in our audit of the 2023 consolidated financial statements, and this report does not affect our report dated February 27, 2024, which expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
114
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
February 27, 2024
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain a set of disclosure controls and procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Our disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. We carried out an evaluation as required by paragraph (b) of Rule 13a-15 or Rule 15d-15 under the Exchange Act, under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) or Rule 15d-15(e) under the Exchange Act). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2023, our disclosure controls and procedures were not effective as of that date due to a material weakness in internal control over financial reporting, described below.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting and for the assessment of the effectiveness of internal control over financial reporting. As defined in Rule 13a-15(f) or Rule 15d-15(f) under the Exchange Act, internal control over financial reporting is a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the financial statements in accordance with U.S. GAAP. Internal control over financial reporting includes policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and the dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In connection with the preparation of our annual financial statements, management has undertaken an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) (the COSO Framework). Management’s assessment included an evaluation of the design of our internal control over financial reporting and testing of the operational effectiveness of those controls.
115
Based on this assessment, management has concluded that, as of December 31, 2023, our internal control over financial reporting was not effective due to the material weakness described below. In our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, we identified two material weaknesses in our disclosure controls. As noted below, we believe we have remediated one of those material weaknesses.
We have experienced insufficient staffing resources in our accounting function, which restricts our ability to consistently execute review procedures over financial statement close processes.
Ernst & Young LLP, the independent registered public accounting firm that audited our financial statements included in this report, has also issued an adverse report on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023.
Remediation of Material Weakness in Internal Control over Financial Reporting
We have remediated the material weakness identified in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023 concerning our ability to perform requisite reviews and approval of manual journal entries posted to the general ledger. In continuing to address the remaining material weakness we have engaged third party experts and undertaken actions in each of the steps identified below:
•Hiring, developing, and retaining additional personnel with appropriate accounting and internal controls expertise;
•Reviewing and updating (as appropriate) the organizational design of the controllership function;
•Reviewing and updating (as appropriate) our methodologies, policies, and procedures designed to ensure adequate internal control over financial reporting, including underlying information technology and business process controls; and
•Ongoing (as appropriate) training programs on relevant internal control over financial reporting matters.
This is a high priority for the Company. Management will continue to implement measures to remediate this material weakness, such that these controls are designed, implemented, and operating effectively.
Once implemented, these remediation activities are designed to strengthen our internal control over financial reporting and remediate the material weakness; however, remediation will not be confirmed until management has completed the requisite remediation activities and tested the design and operation of such controls.
Despite the existence of this material weakness, we believe the consolidated financial statements included in the period covered by this Annual Report on Form 10-K fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented in conformity with U.S. GAAP.
Changes in Internal Control over Financial Reporting
Other than described above, there have been no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
Director and Officer Trading Arrangements
During the three months ended December 31, 2023, the directors and officers of the Company adopted terminated
Name (Title) | Action | Termination Date | Type of Trading Arrangement | Duration of Trading Arrangement | Aggregate Number of Securities | |||||||||||||||||||||||||||
| Rule 10b5-1 trading arrangement | (1) | (1) | ||||||||||||||||||||||||||||||
116
| Rule 10b5-1 trading arrangement | (1) | (1) | ||||||||||||||||||||||||||||||
| Rule 10b5-1 trading arrangement | (2) | (2) | ||||||||||||||||||||||||||||||
| Rule 10b5-1 trading arrangement | (1) | (1) | ||||||||||||||||||||||||||||||
| Rule 10b5-1 trading arrangement | (2) | (2) | ||||||||||||||||||||||||||||||
(1) This trading plan was originally adopted on March 11, 2022, to cover tax withholding obligations, commissions and any fees related to the vesting of restricted stock units, with a scheduled expiration date of December 31, 2025. The aggregate number of securities that would have been sold under this plan is indeterminable because the number of shares that would have been sold to satisfy applicable tax withholding obligations upon vesting is unknown, as the number would vary based on the extent to which vesting conditions were satisfied and the market price of the Company’s common stock at the time of settlement. | ||||||||||||||||||||||||||||||||
(2) This trading plan was originally adopted on March 14, 2022, to cover tax withholding obligations, commissions and any fees related to the vesting of restricted stock units, with a scheduled expiration date of December 31, 2025. The aggregate number of securities that would have been sold under this plan is indeterminable because the number of shares that would have been sold to satisfy applicable tax withholding obligations upon vesting is unknown, as the number would vary based on the extent to which vesting conditions were satisfied and the market price of the Company’s common stock at the time of settlement. | ||||||||||||||||||||||||||||||||
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
117
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item is set forth under the captions “Items to be Voted upon — 1. Election of Directors,” “Executive Officers,” and “Corporate Governance” in our definitive Proxy Statement for the 2024 Annual Meeting of Stockholders and incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is set forth under the captions “Compensation Discussion and Analysis,” “Executive Compensation,” “2023 Director Compensation,” “Compensation Committee Interlocks and Insider Participation,” and “Compensation Committee Report” in our definitive Proxy Statement for the 2024 Annual Meeting of Stockholders and incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is set forth under the captions “Equity Compensation Plan Information” and “Security Ownership of Certain Beneficial Owners and Management” in our definitive Proxy Statement for the 2024 Annual Meeting of Stockholders and incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is set forth under the caption “Corporate Governance” in our definitive Proxy Statement for the 2024 Annual Meeting of Stockholders and incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Our independent registered public accounting firm is Ernst & Young LLP, Raleigh, NC, Auditor Firm ID: 42 .
The information required by this item is set forth under the caption “Items to be Voted upon — 2. Ratification of Appointment of Independent Registered Public Accountants” in our definitive Proxy Statement for the 2024 Annual Meeting of Stockholders and incorporated herein by reference.
118
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) Financial Statements
The following financial statements appear in Item 8 of this report:
| Page in Form 10-K | |||||
No financial statement schedules are included because the information is either provided in the consolidated financial statements or is not required under the related instructions or is inapplicable and such schedules therefore have been omitted.
(b) Exhibits
| Number | Description | ||||||||||
3.1 | |||||||||||
3.2 | |||||||||||
3.3 | |||||||||||
3.4 | |||||||||||
3.5 | |||||||||||
3.6 | |||||||||||
4.1 | |||||||||||
4.2 | |||||||||||
119
10.1& | |||||||||||
10.2& | |||||||||||
10.3& | |||||||||||
10.4& | |||||||||||
10.5& | |||||||||||
10.6& | |||||||||||
10.7& | |||||||||||
10.8& | |||||||||||
10.9& | |||||||||||
10.10& | |||||||||||
10.11& | |||||||||||
10.12& | |||||||||||
10.13& | |||||||||||
10.14& | |||||||||||
10.15& | |||||||||||
10.16& | |||||||||||
120
10.17& | |||||||||||
10.18& | |||||||||||
10.19& | |||||||||||
10.20& | |||||||||||
10.21& | |||||||||||
10.22& | |||||||||||
10.23& | |||||||||||
10.24& | |||||||||||
10.25& | |||||||||||
10.26& | |||||||||||
10.27& | |||||||||||
10.28& | |||||||||||
10.29& | |||||||||||
10.30& | |||||||||||
10.31& | |||||||||||
121
10.32& | |||||||||||
10.33& | |||||||||||
10.34& | |||||||||||
10.35& | |||||||||||
10.36& | |||||||||||
10.37& | |||||||||||
10.38† | |||||||||||
10.39† | |||||||||||
10.40 | |||||||||||
10.41 | |||||||||||
10.42†* | |||||||||||
10.43†* | |||||||||||
10.44†* | |||||||||||
10.45†* | |||||||||||
122
10.46†* | |||||||||||
(21) | |||||||||||
(23) | |||||||||||
(31.1) | |||||||||||
(31.2) | |||||||||||
(32.1) | |||||||||||
(32.2) | |||||||||||
(97) | |||||||||||
| (101) | Financial statements from the Annual Report on Form 10-K of BioCryst Pharmaceuticals, Inc. for the fiscal year ended December 31, 2023, formatted in Inline XBRL: (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Comprehensive Loss, (iii) Consolidated Statements of Cash Flows, (iv) Consolidated Statements of Stockholders’ Equity and (v) Notes to Consolidated Financial Statements. | ||||||||||
| (104) | Cover Page Interactive Data File – The cover page from this annual report on Form 10-K for the fiscal year ended December 31, 2023 is formatted in Inline XBRL (contained in Exhibit 101). | ||||||||||
| † | Certain identified information has been omitted pursuant to Item 601(b)(10) of Regulation S-K because it is both not material and would likely cause competitive harm to the Company if publicly disclosed. | ||||||||||
| * | Certain personally identifiable information has been omitted from this exhibit pursuant to Item 601(a)(6) of Regulation S-K. | ||||||||||
| & | Management contracts. | ||||||||||
| ( ) | Filed herewith. | ||||||||||
ITEM 16. FORM 10-K SUMMARY.
None.
123
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on February 27, 2024.
| BIOCRYST PHARMACEUTICALS, INC. | ||||||||
| By: | /s/ Jon P. Stonehouse | |||||||
| Jon P. Stonehouse | ||||||||
| Chief Executive Officer | ||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated on February 27, 2024:
| Signature | Title(s) | |||||||
| /s/ Jon P. Stonehouse | President, Chief Executive Officer and Director | |||||||
| Jon P. Stonehouse | (Principal Executive Officer) | |||||||
| /s/ Anthony J. Doyle | Chief Financial Officer | |||||||
| Anthony J. Doyle | (Principal Financial Officer and Interim Principal Accounting Officer) | |||||||
| /s/ George B. Abercrombie | Director | |||||||
| George B. Abercrombie | ||||||||
| /s/ Stephen J. Aselage | Director | |||||||
| Stephen J. Aselage | ||||||||
| /s/ Steven K. Galson | Director | |||||||
| Steven K. Galson, M.D. | ||||||||
| /s/ Theresa M. Heggie | Director | |||||||
| Theresa M. Heggie | ||||||||
| /s/ Nancy J. Hutson | Director | |||||||
| Nancy J. Hutson, Ph.D. | ||||||||
| /s/ Alan G. Levin | Director | |||||||
| Alan G. Levin | ||||||||
| /s/ Amy E. McKee | Director | |||||||
| Amy E. McKee, M.D. | ||||||||
| /s/ Vincent J. Milano | Director | |||||||
| Vincent J. Milano | ||||||||
| /s/ Machelle Sanders | Director | |||||||
| Machelle Sanders | ||||||||
124