UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
OR | |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended | |
OR | |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period from to | |
OR | |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report:
Commission file number:
(Exact name of Registrant as specified in its charter)
(Jurisdiction of incorporation)
Province of Santa Fe,
(Address of principal executive offices)
Head of Investor Relations
Province of Santa Fe,
Phone:
Email:
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Copies to:
Matthew S. Poulter, Esq.
Linklaters LLP
Phone: (
Fax: (212) 903-9100
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of Each Class |
| Trading Symbol |
| Name of each exchange on which registered |
|
|
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
Ordinary Shares
Indicate the number of outstanding shares of each of the issuer’s classes of capital stock or common stock as of the close of business covered by the annual report.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
☐ Yes ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
☐ Yes ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
☒
Indicate by check mark whether the registrant has submitted every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ | Non-accelerated filer ☐ | Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b).
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
US GAAP ☐ | Other ☐ | |
by the International Accounting Standards Board ☒ |
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow.
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes
Table of Contents
INTRODUCTORY NOTE AND PRESENTATION OF FINANCIAL AND OTHER INFORMATION | 1 | |
2 | ||
3 | ||
3 | ||
4 | ||
4 | ||
4 | ||
4 | ||
4 | ||
4 | ||
4 | ||
4 | ||
4 | ||
4 | ||
42 | ||
42 | ||
48 | ||
68 | ||
69 | ||
70 | ||
70 | ||
70 | ||
77 | ||
81 | ||
81 | ||
81 | ||
81 | ||
81 | ||
83 | ||
84 | ||
86 | ||
86 | ||
Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation | 87 | |
87 | ||
87 | ||
89 | ||
91 | ||
92 | ||
92 | ||
92 | ||
92 | ||
92 | ||
93 | ||
93 | ||
93 | ||
93 | ||
93 | ||
93 | ||
93 | ||
93 | ||
96 | ||
97 | ||
100 | ||
i
104 | ||
104 | ||
104 | ||
104 | ||
104 | ||
104 | ||
104 | ||
104 | ||
104 | ||
104 | ||
105 | ||
105 | ||
105 | ||
105 | ||
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS | 105 | |
105 | ||
105 | ||
Management’s Annual Report on Internal Control Over Financial Reporting | 105 | |
106 | ||
106 | ||
106 | ||
106 | ||
106 | ||
106 | ||
107 | ||
Purchases of Equity Securities by the Issuer and Affiliated Purchasers | 107 | |
107 | ||
107 | ||
108 | ||
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | 108 | |
108 | ||
108 | ||
108 | ||
108 | ||
109 | ||
ii
PART I
INTRODUCTORY NOTE AND PRESENTATION OF FINANCIAL AND OTHER INFORMATION
Introductory Note
Unless the context otherwise requires, “we,” “us,” “our,” “the Company,” “BIOX,” “Bioceres” and “Bioceres Crop Solutions” refers to Bioceres Crop Solutions Corp. and its subsidiaries.
Financial statement information
The consolidated statements of comprehensive income data for Bioceres for the years ended June 30, 2023, 2022 and 2021 and the consolidated statements of financial position data as of June 30, 2023, 2022 and 2021 are derived from our audited consolidated financial statements appearing elsewhere in this report. They have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standard Board (the “IASB”).
Our presentation currency is U.S. dollars.
We have applied the following standards and amendments for the first time for our annual reporting period commencing July 1, 2022:
| ● | Annual Improvements to IFRS Standards 2018–2020; |
| ● | Amendments to IAS 16 - Property, Plant and Equipment: Proceeds before intended use; |
| ● | Amendments to IFRS 3 - Reference to the Conceptual Framework; and |
| ● | Amendments to IAS 37 - Onerous Contracts – Cost of Fulfilling a Contract. |
The adoption of these amendments did not have a material impact on us. For more information see Note 3 of our audited consolidated financial statements beginning on page F-1 of this annual report on Form 20-F.
1
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
We make forward-looking statements in this report that are subject to risks and uncertainties. These forward-looking statements include information about possible or assumed future results of our business, financial condition, results of operations, liquidity, anticipated growth strategies, anticipated trends in our industry, our potential growth opportunities, plans and objectives. In some cases, you can identify forward-looking statements by terminology such as “believe,” “may,” “might,” “will,” “consider,” “estimate,” “continue,” “anticipate,” “intend,” “target,” “project,” “contemplate,” “should,” “plan,” “expect,” “predict,” “potential,” or the negative of these terms or other similar terms or expressions. The statements we make regarding the following matters are forward-looking by their nature:
| ● | the impact of armed conflict in Israel and Gaza, in addition to the Ukraine, and any possible escalation of such conflicts or contagion to neighboring countries or regions; |
| ● | our beliefs with respect to the agricultural industry and related markets, including production levels, consolidation trends, and market opportunity; |
| ● | our plans and expectations regarding growth in our business, expansion to new markets, diversification of our product portfolio and strategic acquisitions and partnerships; |
| ● | our ability to integrate new products, technologies, and employee teams after any strategic acquisition; |
| ● | our ability to develop, commercialize and register biotechnology products and crop productivity technologies; |
| ● | our ability to maintain our joint venture agreements with our current partners; |
| ● | our ability to maintain a competitive position in our markets; |
| ● | our ability to protect our intellectual property; |
| ● | the success of the HB4 technology that we license and that remains subject to receipt of regulatory approval in certain jurisdictions other than Argentina; |
| ● | our or our collaborators’ ability to develop commercial products that incorporate our licensed seed traits and complete the regulatory approval process for such products; |
| ● | our expectations regarding the commercial value of our key products in yield and abiotic stress and biotic stress; |
| ● | our expectations regarding regulatory approval of products developed or licensed by us, our joint ventures and third-party collaborators; |
| ● | our ability to adapt to continuous technological change in our industry; |
| ● | our ability to effectively manage sales and marketing teams and distribution networks; |
| ● | our expectations regarding revenues and sales, including potential growth in sales, changes to sales mix and expectations regarding seasonality and the impact of weather-related conditions; |
| ● | our plans and expectations with regarding to manufacturing and production; |
| ● | our expectations that products containing our licensed seed traits will be commercialized and we will earn royalties from the sales of such products; |
| ● | our expectations to accelerate the Microstar ramp up, our leading brand in micro-granulated fertilizers; |
2
| ● | our expectations regarding the future growth of the global agricultural, agricultural biotechnology, biological-based chemical and agro-industrial biotechnology markets; |
| ● | our ability to develop and exploit a proprietary channel for the sale of our licensed biotechnology products; |
| ● | our compliance with laws and regulations that impact our business and changes to such laws and regulations; |
| ● | our ability to assemble, store, integrate and analyze significant amounts of public and proprietary data; |
| ● | our ability to maintain our licensing arrangements for the products that we commercialize; |
| ● | our plans and expectations relating to our debt agreements; |
| ● | our ability to influence customer perception of biological agricultural products; |
| ● | our beliefs regarding our environmental, social, and governance leadership; |
| ● | our belief regarding our access to capital resources through equity offerings, debt financings, strategic collaborations or other means; |
| ● | our expectations with respect to our future expenditures, available cash and other financial and operating results; |
| ● | our anticipated impact of certain accounting pronouncements; |
| ● | our expectations regarding market risk, including interest rate changes, foreign currency fluctuations and commodity price changes; |
| ● | our ability to respond to health epidemics and other outbreaks, such as COVID-19, including responses by governmental bodies or regulators; |
| ● | the outcome of any known and unknown litigation and regulatory proceedings; and |
| ● | various other factors, including without limitation those described under “Item 3. Key Information – D. Risk Factors.” |
The preceding list is not intended to be an exhaustive list of all of our forward-looking statements. The forward-looking statements are based on our beliefs, assumptions and expectations of future performance, taking into account the information currently available to us. These statements are only predictions based upon our current expectations and projections about future events. There are important factors that could cause our actual results, levels of activity, performance or achievements to differ materially from the results, levels of activity, performance or achievements expressed or implied by the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be achieved or will occur. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this report or to conform these statements to actual results or to changes in our expectations.
ITEM 1.IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
| A. | Directors and Senior Management |
Not applicable.
3
| B. | Advisors |
Not applicable.
| C. | Auditors |
Not applicable.
ITEM 2.OFFER STATISTICS AND EXPECTED TIMETABLE
A. | Offer Statistics |
Not applicable.
B. | Method and Expected Timetable |
Not applicable.
ITEM 3.KEY INFORMATION
A. | [RESERVED] |
B. | Capitalization and Indebtedness |
Not applicable.
C. | Reasons for the Offer and Use of Proceeds |
Not applicable.
D. | Risk Factors |
The following risk factors apply to the business and operations of Bioceres Crop Solutions. The occurrence of one or more of the events or circumstances described in these risk factors, alone or in combination with other events or circumstances, may have a material adverse effect on the business, cash flows, financial condition and results of operations of Bioceres Crop Solutions. You should carefully consider the following risk factors in addition to the other information included in this report, including matters addressed in the section entitled “Cautionary Note Regarding Forward-Looking Statements.” We may face additional risks and uncertainties that are not presently known to us, or that we currently deem immaterial, which may also impair our business or financial condition. The following discussion should be read in conjunction with the financial statements and notes to the financial statements included herein.
Summary Risk Factors
You should carefully consider all of the information in this annual report before making an investment in our ordinary shares. Below please find a summary of the principal risks and uncertainties we face, organized under relevant headings. These risks are discussed more fully under “Item 3. Key Information—3.D. Risk Factors.”
Risks Related to our Business and Strategy include:
We are subject to the following risks in respect of our business and our strategy:
| ● | difficulties implementing our inorganic growth strategy and integrating the operations of the recently acquired and future businesses; |
| ● | we may not realize the benefits of future businesses or products we acquire or strategic alliances we form; |
4
| ● | acquisitions may not be successful in achieving intended benefits, cost savings and synergies and may disrupt current operations; |
| ● | developing marketable or commercial technologies; |
| ● | obtaining timely necessary regulatory approvals for the business and the commercialization of our products currently under development; |
| ● | our HB4 Seed business is dependent on the success of a technology that we license; |
| ● | limited number of prospective collaborators in our market; |
| ● | for some of our technologies, we are dependent on intellectual property rights licensed to us by Bioceres Group PLC, together with its direct and indirect subsidiaries, as applicable (“Bioceres PLC”), which we do not control, to generate revenue; |
| ● | our joint venture agreements or any partnerships that we may enter into in the future may not be successful, and we or our collaborators may fail to perform our respective contractual obligations, which could result in disputes; |
| ● | difficulties in collecting payments or royalties; |
| ● | we may not have sufficient cash flow to pay interest and principal in respect of the Notes due 2026; |
| ● | ability to attract and retain qualified scientific and business personnel; |
| ● | we may be unable to prevent our competitors from benefiting from the expertise of our former employees; |
| ● | global economic conditions, including as a result of the armed conflict in Israel and Gaza and the war in the Ukraine; |
| ● | ultimate impact on our results and operations of global pandemic, such as COVID-19 pandemic, and measures adopted by the governments of the countries in which we operate in response to global pandemics; |
| ● | sales and operating results may fluctuate significantly as a result of the seasonal nature of crops and factors beyond our control; |
| ● | our results of operations from our crop productivity products may vary significantly from period to period due to circumstances beyond our control; |
| ● | certain estimates of market opportunity included in this report are based on assumptions that are inherently uncertain and subject to risks and uncertainties; |
| ● | resistance to genetically modified (“GM”) crops may negatively affect our public image and reduce sales of seeds or other products containing our licensed seed traits; |
| ● | competition in crop productivity products is intense and requires continuous technological development; |
| ● | changes in laws and regulations may materially increase our costs of operation, decrease our operating revenue and disrupt our business; |
| ● | our indebtedness could adversely affect our financial condition; |
| ● | price increases and shortages of raw materials; |
| ● | exposure of the company and its customers to market risks from commodity prices volatility; |
| ● | we may be required to pay uninsured substantial damages as a result of product liability claims; |
5
| ● | various health and environmental risks associated with our use, handling and disposal of potentially toxic materials; |
| ● | requirements of being a public company may strain our resources and distract our management; |
| ● | obtaining required future financing on favorable terms to avoid delays, reductions or the termination of some of our activities; |
| ● | scrutiny under the Convention on Biological Diversity Treaty; |
| ● | failure to accurately forecast and manage inventory; |
| ● | IT disruptions or failures in our operating system can affect our reputation and business; |
| ● | computer system failures, cyber-attacks or a deficiency in our cyber-security; |
| ● | labor union disputes may arise; |
| ● | reliance on third parties to grow our seeds; |
| ● | non-compliance with anti-corruption and anti-money laundering laws can subject us to criminal and civil liability; |
| ● | recently introduced economic substance legislation of the Cayman Islands may adversely impact us or our operations; |
| ● | taxation in the Cayman Islands; and |
| ● | our “foreign private issuer” status under the rules and regulations of the United States Securities and Exchange Commission (the “SEC”). |
Risks Related to our Intellectual Property
We are subject to the following risks in respect of our intellectual property rights:
| ● | our agreements may not adequately prevent disclosure of proprietary information; |
| ● | protection of our intellectual property rights throughout the world; |
| ● | changes in Argentine and U.S. patent law could diminish the value of patents and impair our ability to protect our product candidates; |
| ● | potential litigation relating to third party intellectual property rights infringement; |
| ● | technological developments or challenges by competitors; and |
| ● | requirement to pay royalties to employees who develop inventions commercialized by us. |
Risks Related to Operating in Latin America
We are subject to the following risks in respect of our operations in Latin America:
| ● | adverse economic or political conditions, including considerable economic uncertainty; |
| ● | significant government influence over the economies of the countries in which we operate; |
| ● | fluctuations in currency exchange rates; |
6
| ● | currency of a hyperinflationary economy requires that we apply inflationary adjustments to our Argentine subsidiaries’ financial statements; |
| ● | inflation in Argentina and government intervention controls; |
| ● | limited access of the Argentine government and the private sector to the international capital markets; |
| ● | increase in export and import duties and controls; |
| ● | decline in the global prices of Latin America’s main commodity exports; |
| ● | Latin America continues to face considerable economic uncertainty; |
| ● | special protections for employees in the private sector; |
| ● | taxation relating to disposition or sale of our ordinary shares in Argentina; |
| ● | taxation relating to the payment of the In-Kind Consideration under the Rizobacter Call Option in Argentina; |
| ● | exchange controls and restrictions limit access to the FX Market (as defined below) to make payments and distributions from our Argentine subsidiaries; |
| ● | mandatory repatriation of export receivables; and |
| ● | changes in Argentine tax laws. |
Risks Relating to our Ordinary Shares Include:
Our shareholders are subject to the following risks:
| ● | reduction of liquidity due to our share repurchase program; |
| ● | compliance with listing standards of Nasdaq cannot be assured; |
| ● | the market price of our ordinary shares may be adversely affected if there are sales of a substantial number of our ordinary shares; |
| ● | dilution to shareholders will result from conversion of the Secured Convertible Guaranteed Notes due 2026; |
| ● | our status as a “controlled company” as defined by Nasdaq rules; |
| ● | fluctuation in the price of our securities; |
| ● | decrease in the price and trading volume of our ordinary shares due to publicity and market recommendations; |
| ● | difficulty faced by public shareholders in protecting their interests through the U.S. Federal courts; |
| ● | increased costs as we ceased to be an “emerging growth company”; |
| ● | less protection for our shareholders due to our status as a foreign private issuer; |
| ● | history of no cash dividends payments on our ordinary shares; and |
| ● | dilution to our shareholders due to issuance of additional securities. |
7
Risks Related to our Business and Strategy
We may face difficulties implementing our inorganic growth strategy, including in respect of integrating the operations of the business we have acquired or expect to acquire in the future.
From time to time, we may acquire businesses, assets, or securities of companies that we believe will provide a strategic fit with our business, such as the merger (the “Pro Farm Merger”) with Pro Farm Group, Inc. (formerly Marronne Bio Innovations Inc.) (“Pro Farm”) and the acquisition of a majority interest in Rizobacter which is held by RASA Holding, on October 19, 2016 (the “Rizobacter Acquisition”). We integrate acquired businesses with our existing operations, our overall internal control over financial reporting processes, and our financial, operations, and information systems. If the financial performance of our business, as supplemented by the assets and businesses acquired, does not meet our expectations, our results of operations may fail to meet market expectations and we may face difficulties to service our debt obligations. We may not effectively assimilate the business or product offerings of acquired companies into our business or within the anticipated costs or timeframes, retain key customers and suppliers or key employees of acquired businesses, or successfully implement our business plan for the combined business. In addition, our final determinations and appraisals of the estimated fair value of assets acquired and liabilities assumed in our acquisitions may vary materially from earlier estimates and we may fail to realize fully anticipated cost savings, growth opportunities or other potential synergies. We cannot assure that the fair value of acquired businesses or investments will remain constant.
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We have acquired, and we may in the future acquire, other companies, employee teams, or technologies to further complement or expand our product portfolio, enhance our technical capabilities, obtain personnel, or otherwise offer growth opportunities. In addition, we plan to selectively partner, in-license or acquire key enabling technologies and businesses across our value chain that we believe will keep us on the cutting edge of our industry. We may not be able to identify appropriate targets or make acquisitions under satisfactory conditions, in particular, satisfactory price conditions. In addition, we may be unable to obtain financing for these acquisitions under other purposes in the context of existing operations. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure that, following any such acquisition, we will achieve the expected synergies to justify the transaction, which could have a material adverse effect on our business, financial conditions, earnings and prospects.
Acquisitions, such as the Pro Farm Merger and Rizobacter Acquisition, may not be successful in achieving their intended benefits, cost savings and synergies and may disrupt current operations.
One component of our growth strategy historically has been acquisitions. These involve various inherent risks and the benefits, cost savings and synergies sought that may not be realized.
The integration process of any newly acquired company may be complex, costly and time-consuming. The potential difficulties of integrating the operations of an acquired business and realizing our expectations for an acquisition, including the benefits that may be realized, include, among other things:
| ● | failure of the business to perform as planned following the acquisition or achieve anticipated revenue or profitability targets; |
| ● | delays, unexpected costs or difficulties in completing the integration of acquired companies or assets; |
| ● | higher than expected costs, lower than expected cost savings or synergies and/or a need to allocate resources to manage unexpected operating difficulties; |
| ● | difficulties assimilating the operations and personnel of acquired companies into our operations; |
| ● | diversion of the attention and resources of management or other disruptions to current operations; |
| ● | the impact on our or an acquired business’ internal controls and compliance with legal requirements; |
8
| ● | ineffective or inadequate controls, procedures, or policies at the acquired company; |
| ● | multiple product lines or service offerings, as a result of our acquisitions, that are offered, priced, and supported differently; |
| ● | adverse effects on our existing business relationships with business partners and customers as a result of the acquisition; |
| ● | potential write-offs of acquired assets and potential financial and credit risks associated with acquired customers; |
| ● | inability to maintain relationships with key customers, suppliers, and partners of the acquired business; |
| ● | lack of experience in new markets, products, or technologies; |
| ● | diversion of management’s attention from other business concerns; |
| ● | use of resources that are needed in other parts of our business; |
| ● | retaining key customers, suppliers and key personnel; |
| ● | retaining and obtaining required regulatory approvals, licenses and permits; |
| ● | operating risks inherent in the acquired business and our business; |
| ● | lower than anticipated demand for product offerings by us or our licensees; and |
| ● | unanticipated issues, expenses and liabilities, including those arising from existing contractual obligations or litigation matters. |
Our failure to successfully complete the integration of any acquired business and any adverse consequences associated with future acquisition activities, could have an adverse effect on our business, financial condition and operating results.
Completed acquisitions may result in additional goodwill and/or an increase in other intangible assets on our balance sheet. We are required annually, or as facts and circumstances exist, to assess goodwill and other intangible assets to determine if impairment has occurred. If the testing performed indicates that impairment has occurred, we are required to record a non-cash impairment charge for the difference between the carrying value of the goodwill or other intangible assets and the implied fair value of the goodwill or the fair value of other intangible assets in the period the determination is made. We cannot accurately predict the amount and timing of any potential future impairment of assets. Should the value of goodwill or other intangible assets become impaired, there could be a material adverse effect on our financial condition and results of operations.
Certain of the Rizobacter shares are subject to a judicial injunction and, if decided unfavorably to us, we may be required to surrender part of our interest in Rizobacter thereby reducing our equity interest in Rizobacter.
Concurrently with the closing of business combination, the Rizobacter Call Option was exercised and we acquired 80.00% of Rizobacter’s capital stock, which we hold through our subsidiary RASA Holding. Of our total interest in Rizobacter, 29% of the shares we hold are subject to an injunction that affects 44% of the total share capital of Rizobacter. In addition, the injunction also requires that 30% of the dividends distributed on such shares be paid into a judicially created escrow account. The injunction relates to litigation among historical shareholders of Rizobacter arising from a disputed transfer of shares that occurred in 1995. Although the Argentine Supreme Court ruled against certain of the litigating historical shareholders, such shareholders subsequently pursued other legal recourse—including the injunction and non-innovative measure (medida de no innovar)—to further dispute the original transfer of shares. The non-innovative measure was overturned by an Argentine court of appeals on April 17, 2018.
We acquired our controlling stake in Rizobacter subject to the injunction and associated ongoing litigation. Should the court rule against the free transferability of the affected shares, we will be obligated to return certain shares, thereby reducing our equity interest in Rizobacter. Given the Argentine Supreme Court’s finding that the 1995 share transfer was valid, it is likely or probable that Bioceres PLC may be obligated to pay a contingent purchase price of US$17.3 million.
9
We may not be successful in developing marketable or commercial technologies, as our product development cycle is lengthy and uncertain, and we may never generate revenues or earn royalties on the sale of our products currently in development.
Our success depends in part on our ability to identify and develop high-value crop productivity commercial products, which is an expensive, complex, prolonged, and uncertain process. Through our technology sourcing and product development collaborations, we commit substantial efforts and other resources to accomplish this. Our process of developing and commercializing technologies involves several phases and can take several years from discovery to commercialization of a product. On average, it takes between five and twelve years to develop a product for our crop productivity products. Some development initiatives may never result in a commercially viable product.
As of the date of this report, many of our products have been commercialized by Rizobacter, Bioceres Semillas and Pro Farm. There can be no assurance that our future crop productivity technologies will be viable for commercial use, or that we will be able to generate revenues from those technologies, in a significant manner or at all. If our products are unsuccessful in achieving their desired effect or otherwise fail to be commercialized, we will not receive revenues from our customers or royalty payments from the commercialization the technologies we develop or license, which could materially and adversely affect our business, financial condition, results of operations and growth strategy.
Our products may be unsuccessful or fail to achieve commercialization for any of the following reasons:
| ● | our products may not be effective in the target crops or may have adverse effects on consumers; |
| ● | our licensed products may not have the desired effect on the relevant crop sought by our end-market; |
| ● | we or our joint ventures or collaborators may be unable to obtain the requisite regulatory approvals for our products; |
| ● | our competitors may develop and launch competing or more effective products; |
| ● | our products may be viewed as too expensive by food companies or growers as compared to competitive products; |
| ● | our products may no longer be desirable as consumer preferences, which are unpredictable and can vary greatly, may change quickly; |
| ● | a market may not exist for seeds containing our licensed seed traits or biological treatments or such products may not be commercially successful; |
| ● | our products may be difficult to produce on a large scale or will not be economical to grow; |
| ● | we or the customers to whom we sell our products may be unable to fully develop or commercialize our products in a timely manner or at all; |
| ● | we may be unable to patent and/or obtain breeders’ rights or any other intellectual property rights on our technologies in the necessary jurisdictions; |
| ● | even if we obtain patent and/or breeders’ rights or any other intellectual property rights on our technologies, such rights may be later challenged by competitors or other parties; |
| ● | even if we obtain patent and/or breeders’ rights or any other intellectual property rights in respect of our technologies, competitors may design competing products that do not infringe these intellectual property rights; and |
| ● | third parties may develop superior or equivalent products. |
Further, we intend to continue to invest in R&D including additional and expanded field testing to validate potential products in real world conditions. Because of the long product development cycle and the complexities and uncertainties associated with biotech technologies, there can be no assurance that we will ever generate significant revenues from the technologies or products that we are currently developing without significant delay, without the incurrence of unanticipated costs or at all.
10
Our business and the commercialization of our products currently in development are subject to various government regulations and we or our collaborators may be unable to obtain, or may face delays in obtaining, necessary regulatory approvals. In addition, if we are unable to comply with applicable regulatory regimes, we may not be able to sell our products in certain jurisdictions.
We are subject to approvals, registrations and regulations relating to different jurisdictions according to the countries in which we operate, which includes, among others, the (i) Argentine regulations relating to biotechnology, food safety and registration of seeds, (ii) the regulations imposed by the U.S. Environmental Protection Agency (“EPA”), among other governmental authorities, and (iii) European Union Registration, Evaluation, Authorization, and Restriction of Chemicals regulation.
Further, our business is generally subject to two types of regulations: (i) those that apply to our operations and (ii) those that apply to products containing or based on our technology. We are responsible for applying for and maintaining the regulatory approvals necessary for our operations, particularly those covering our field trials, bio-safety evaluations and feed and food tests. Under the terms of our joint venture agreements, we and our joint venture partners are jointly responsible for obtaining and maintaining the regulatory approvals necessary for the commercialization of products that contain our licensed seed traits and other technologies in the various relevant markets. Regulatory and legislative requirements affect the development, production and sale of our products, including the testing, commercialization and planting of seeds containing our biotechnology licensed seed traits. Failure to receive such approvals or non-compliance with the applicable regulatory regime could adversely impact our operations and business strategy. Additionally, we may face difficulties in obtaining regulatory approvals in jurisdictions in which we have not previously operated or in which we have limited experience.
Regulatory regimes in some of our key target markets may be more onerous and enforced by several entities. For example, in Argentina, the Argentine government’s regulation of agricultural biotechnology is enacted and enforced primarily by three agencies; (i) the Argentine National Advisory Commission on Agricultural Biotechnology (Comisión Nacional Asesora de Biotecnología Agropecuaria), which regulates activity related to biosafety; (ii) the National Food Safety and Quality Service (Servicio Nacional de Sanidad y Calidad Agroalimentaria) which regulates activity related to food and feed safety; and (iii) the National Seed Institute (Instituto Nacional de Semillas) which controls the registries for both entities and products that are under its jurisdiction, among other functions. Additionally, the National Market Regulator (Dirección Nacional de Mercados) must conduct an economic evaluation.
In the United States, the field testing, manufacture, sale and use of crop protection, plant health and plant nutrition products are extensively regulated by the EPA and other state, local and foreign governmental authorities. As we introduce new formulations of and applications for our products, we may need to seek EPA approval prior to commercial sale. For any such approval, the EPA may require us to fulfill certain conditions within a specified period of time following initial approval. Although the EPA has in place a registration procedure for biopesticides there can be no assurance that all of our products or product extensions will be eligible for this streamlined procedure or that additional requirements will not be mandated by the EPA that could make the procedure more time consuming and costly for our future products. Furthermore, in most of our key target markets, including the United States, regulatory approvals must be received prior to the importation and commercialization of genetically modified products.
When products containing our licensed seed traits or other technology reach large-scale field trials, bio-safety evaluations and commercial approval stages, if we, our joint ventures or other collaborators are unable to obtain the requisite regulatory approvals or if there is a delay in obtaining such approvals (for example, as a result of negative market perception, consumer groups’ legal actions against genetically modified organisms (“GMO”), heightened regulatory standards or unfamiliarity with the applicable regulatory regime), such products may not be commercialized, which would negatively impact our business and results of operations.
Our HB4 Seed business is dependent predominantly on the success of a technology that we license.
Many of our biotechnology licensed seed products currently under development incorporate HB4 technology, a yield improvement technology. We expect that the sale of biotech seeds that contain HB4 technology, our HB4 Seed business, will comprise an increasing portion of our future revenues. As a result, our future growth and financial performance will be influenced by our ability to commercialize our licensed HB4 technology, and if this effort is unsuccessful our business could be materially and adversely affected.
We also depend on our continued exclusive use of the HB4 technology pursuant to the terms of licensing agreements with Bioceres S.A., the National Scientific and Technical Research Council of Argentina (Consejo Nacional de Investigaciones Científicas y Técnicas) and the National University of the Litoral. Bioceres S.A. holds an exclusive license for HB4, which terminates on the expiration date of the last of the HB4 patents in 2040, unless terminated before such date in accordance with its terms. If this licensing agreement is declared unenforceable or invalid, we could lose access to one of our principal technologies and could become involved in costly or time-consuming legal disputes.
11
There are a limited number of prospective collaborators in the markets in which we operate.
Our Research and Development (“R&D”) and commercialization activities are costly, time-intensive and require significant infrastructure and resources. Therefore, our business strategy involves entering into collaboration and joint venture arrangements with global agricultural firms to leverage their resources, know-how and distribution channels and into collaborations with research institutions and governmental agencies to facilitate our low-cost approach to R&D. The crop productivity market is highly consolidated and dominated by a relatively small number of large companies. Additionally, there are a limited number of researchers and research institutions focused on the technologies that we seek to develop and competition for entry into collaboration arrangements with them can be challenging. Due to the small number of companies in our markets and the small number of potential collaborators, there are limited opportunities for us to pursue additional joint ventures and collaborations with new partners and collaborators. If we may cease to be attractive to prospective collaborators if our technology platform or track record is not perceived to be sufficiently developed or successful or if, in the case of prospective joint venture partners, such prospective partners view us as a competitor and choose not to collaborate with us. In addition, if we fail to develop or maintain our relationships with any of our existing collaborators, we could lose our opportunity to work with that collaborator and could be subject to reputational damage that could negatively impact our relationships with other collaborators in what is a relatively small industry community. If we are unable to enter into new joint venture agreements or collaborations, we may face higher development costs than initially anticipated, greater difficulties in achieving commercialization, challenges in expanding our portfolio of technologies and distribution networks and commercial products, or other adverse impacts, which could have a material adverse effect on our business prospects.
The licenses that Bioceres PLC grants to us related to HB4 wheat are currently exclusive with respect to certain territories and/or crops. We are seeking to expand these licenses and we may be limited in our ability to use the licensed technology in other territories in the future, either independently or with another partner, if we fail to extend the licenses to additional crops and territories. However, all current and short-term commercial activities are covered by our licenses. In addition, such licensed technology may be rendered obsolete.
The licenses Bioceres PLC granted to us are exclusive with respect to HB4 wheat technology in Argentina, Brazil, Paraguay and Uruguay. Pursuant to the terms of the license we may be limited to use the licensed technology in other countries either independently or with another partner or third party, while we seek to expand these licenses, as needed. The restrictions imposed by the terms of these licenses may limit our flexibility to commercialize HB4 wheat technology and expand our business in such territories and could adversely affect our business, results of operations and prospects.
In addition, the technology that Bioceres PLC licenses to us may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenues from the commercialization of our licensed seed traits and technology.
Our joint venture agreements or any partnerships that we may enter into in the future may not be successful, or we may fail to perform our respective contractual obligations, which could adversely affect our ability to develop and commercialize our product candidates, as well as result in disputes with our collaborators.
We may seek partnerships or joint venture arrangements with third parties for the development or commercialization of our product candidates depending on the merits of retaining commercialization rights for ourselves as compared to entering into partnerships or joint venture arrangements. We will face, to the extent that we decide to enter into partnerships or joint venture agreements, significant competition in seeking appropriate partners. Moreover, partnerships or joint venture arrangements are complex and time-consuming to negotiate document implement and maintain. We may not be successful in our efforts to establish and implement partnerships, joint ventures, or other alternative arrangements and such partnerships, joint ventures, or arrangements may not be successful. Furthermore, the terms of any partnerships, joint ventures, or other arrangements that we may establish may not be favorable to us.
Pursuant to our joint venture agreements, other agreements with our joint venture partners and collaboration arrangements, we are required to provide R&D services over a particular period of time and meet other contractual obligations. If we fail to perform our obligations under these agreements, our collaborators’ obligations to us may be reduced and, in other cases, our collaborators may seek to dissolve the corresponding joint venture or terminate their agreements with us and, as a result, our anticipated revenues may decrease. In addition, the failure of any of our collaborators to perform their contractual obligations, due to financial hardship, disagreement under the relevant agreement or for any other reason, may hinder our research collaboration, development and commercialization activities, increase our costs and materially and adversely affect our results of operations. Because some of our intellectual property has been licensed to various joint ventures for use in several different fields, the interests of each of our partners in these joint ventures may not always be aligned. As a result, it is possible that potential disputes may arise between us and our partners.
12
Additionally, a licensee, collaborator or third party may use our intellectual property without our permission, dispute our ownership of certain intellectual property rights or argue that our intellectual property does not cover the joint venture’s marketed product. We seek to address these concerns in our contractual agreements; however, we may not have contractual arrangements with the party in question and/or such provisions may not be effective. If these provisions prove to be ineffective, we may not be able to achieve our objectives of generating significant revenues from crop productivity products sales and royalties from our seed technologies. Furthermore, regardless of any resort to legal action, a dispute with an end-customer, a licensee or collaborator over intellectual property rights may damage our relationship with that licensee or collaborator and may also harm our reputation in the industry.
Further, our ability to generate value from our joint ventures and research collaborations will depend on, among other things, our ability to work cooperatively with our collaborators for the discovery, development and commercialization of our technology and products and we may be unable to do so. We cannot be sure that this structure will be successful in commercializing our products. Furthermore, the agreements governing our partnership and collaborations are complex and cover a range of future activities. Such a dispute may also negatively affect our relationship with one or more of our other collaborators and may hinder our ability to enter into future collaboration agreements. Any of these occurrences could negatively impact our business and results of operations.
The success of our R&D partnerships or joint venture arrangements will depend heavily on the efforts and activities of our partners. Our joint venture arrangements may present financial, managerial, and operational challenges, including potential disputes, liabilities, or contingencies and may involve risks not otherwise present when operating independently including:
| ● | partners may have business interests, goals or cultures that are or become inconsistent with our business interests, goals or culture; |
| ● | partners may have significant discretion in determining the efforts and resources that they will apply to partnerships or joint ventures; |
| ● | partners may not pursue development and commercialization of our potential products or may elect not to continue or renew development or commercialization programs based on trial results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other external factors, such as business combination that diverts resources or creates competing priorities; |
| ● | partners may delay trials, provide insufficient funding for a trial program, stop a trial, abandon a product candidate, repeat or conduct new trials or require a new formulation of a product candidate for testing; |
| ● | partners could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates; |
| ● | a partner with marketing manufacturing and distribution rights to one or more products may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities; |
| ● | we could grant exclusive rights to our partners that would prevent us from collaborating with others; |
| ● | partners may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property rights or proprietary information or expose us to potential liability; |
| ● | we may incur liabilities or losses as a result of an action taken by the joint venture or our joint venture partners; |
| ● | disputes may arise between us and a partner that causes the delay or termination of the research development or commercialization of our current or future products or that results in costly litigation or arbitration that diverts management attention and resources; |
| ● | our joint venture partners may act contrary to our instructions, requests, policies or objectives, which could reduce our return on investment, harm our reputation or restrict our ability to run our business; |
13
| ● | partnerships may be terminated, and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable current or future products; |
| ● | partners may own or co-own intellectual property covering our products that results from our partnering with them and in such cases, we would not have the exclusive right to develop or commercialize such intellectual property; and |
| ● | a partner’s sales and marketing activities or other operations may not comply with applicable laws resulting in civil or criminal proceedings. |
The risks described above or the failure to continue any joint venture or joint development arrangement or to resolve disagreements with our current or future joint venture partners could materially and adversely affect our ability to transact the business that is the subject of such joint venture, which would in turn negatively affect our financial condition and results of operations.
We may experience difficulties in collecting payments or royalties to which we believe we are entitled.
We sell certain of our products to distributors through Rizobacter, Bioceres Semillas and Pro Farm. We also often license the use of certain technology to collaborators and licensees who use or will use the intellectual property to develop and commercialize seeds with improved seed traits. Additionally, we may be entitled under applicable intellectual property laws in the countries in which we operate to the payment of royalties from end users who subsequently multiply and use our seed technology. In each case, we may not actually receive the payments or royalties to which we are entitled, due to failure or refusal of the responsible parties to pay the amounts due. Failure to receive amounts owed to us could have an adverse impact on our business.
In the case of royalty payments from licensees, we rely on the good faith of the licensees to report to us the sales they earn from these products and to accurately calculate the royalties, to which we are entitled, processes that may involve complicated and difficult calculations. Under existing agreements, we have the right to inspect the inventory and accounts of multipliers of our seeds and licensees of our technologies; however, we must also rely on the good faith of end users to accurately report to us the multiplication of our seeds and remit royalty payments due in respect of the same, which may be respected to varying degrees in different jurisdictions given the absence of contractual privity and prevailing market practice.
We may face difficulty servicing our indebtedness, including the Notes due 2026.
Our ability to make scheduled payments of the principal of, to pay interest on, or to refinance our indebtedness depends on our future performance, which is subject to many factors, including economic, financial, competitive and other, beyond our control. We cannot assure you that our business will generate sufficient cash flow from operations or that future borrowings will be available to us in an amount sufficient to enable us to pay our indebtedness or to fund our other liquidity needs.
We may elect to make interest payments under the Secured Convertible Guaranteed Notes due 2026 in kind, which will be capitalized, but if the Secured Convertible Guaranteed Notes due 2026 are not converted into ordinary shares, we will be required to pay the principal thereof in cash. Our ability to refinance the Secured Convertible Guaranteed Notes due 2026, will depend on the financial markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations and limit our flexibility in planning for and reacting to changes in our business. Holders of the Secured Convertible Guaranteed Notes due 2026 have the right to require us to repurchase their Secured Convertible Guaranteed Notes due 2026 for cash upon the occurrence of a change of control. We cannot assure you that we will have sufficient financial resources, or will be able to arrange financing, to pay the price in cash with respect to any Secured Convertible Guaranteed Notes due 2026 surrendered by holders for repurchase upon a change of control. In addition, restrictions under our then existing credit facilities or other indebtedness, if any, may not allow us to repurchase the Secured Convertible Guaranteed Notes due 2026 upon a change of control. Our failure to repurchase the Secured Convertible Guaranteed Notes due 2026 upon a change of control when required would result in an event of default with respect to the Secured Convertible Guaranteed Notes due 2026 which could, in turn, constitute a default under the terms of our other indebtedness, if any. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repurchase the Secured Convertible Guaranteed Notes due 2026 and the Noteholders may therefore enforce their security interest over the collateral pledged to them.
14
We depend on our key personnel and research collaborators, and we may be adversely affected if we are unable to attract and retain qualified scientific and business personnel.
Our business is dependent on our ability to recruit and maintain highly skilled and educated individuals through direct employment or collaboration arrangements, with expertise in a range of disciplines, including biology, chemistry, plant genetics, agronomy, mathematics programming and other specializations which are relevant to our business. Our ability to recruit such a work force depends in part on our ability to maintain our market leadership in agricultural biotech industry in countries in which we operate. Maintaining our ability to attract highly skilled workers and leading scientific institutions depends in part on our ability to maintain a strong technology platform and state-of-the-art facilities, as well as our ability to consistently and successfully commercialize our technology. There can be no assurance that we will be able to maintain leading scientific capabilities or continue to successfully maintain advanced technology in the market.
Our success is also dependent to a significant degree upon the technical skills and continued service of certain members of our management team, in particular those of our CEO, Dr. Federico Trucco. Dr. Trucco has occupied several positions at Bioceres since 2005 and has vast experience and knowledge of our business, strategy and technologies. Furthermore, he has developed and maintained strong relationships with our original shareholders. The cessation of Dr. Trucco’s employment for any reason could have a material and negative impact on us. In addition, the number of qualified and highly educated personnel in Argentina, where most of our operations are located, is limited and competition for the services of such persons may be intense.
Our inability to secure, retain or find replacements for key management and technical personnel could adversely affect our business and could have a material adverse effect on our business, operating results, financial condition and growth prospects.
We do not enter into non-compete agreements with all of our employees, and therefore we may be unable to prevent our competitors from benefiting from the expertise of our former employees.
We do not enter into non-compete agreements with all of our employees, which prevents us from limiting our key employees from joining our competitors or competing directly against us. As a result, we may be unable to prevent our competitors from benefiting from the expertise of such employees. Direct competition by a former employee could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
Adverse changes in global economic conditions, including as a result of the armed conflict in Israel and Palestine and the war in Ukraine, may negatively affect our industry, business, and results of operations.
Our industry is affected by changes in economic conditions outside our control, which can result in a general decrease in product demand from our customers. Such economic developments, like inflationary pressures in the U.S. and elsewhere, the China trade wars, the armed conflict in Israel and Palestine and the war in Ukraine may affect our business in a number of ways. Reduced demand may drive us and our competitors to offer products at promotional prices, which would have a negative impact on our profitability. In addition, the tightening of credit in financial markets may adversely affect the ability of our customers and suppliers to obtain financing for significant purchases and operations and could result in a decrease in, or cancellation of, orders for our products. If demand for our products slows down or decreases, we will not be able to maintain our revenue and we may run the risk of failing to satisfy the financial and other restrictive covenants to which we are subject under our existing indebtedness. Reduced revenue as a result of decreased demand may also reduce our planned growth and otherwise hinder our ability to improve our performance in connection with our long-term strategy.
15
In addition, through Pro Farm, we maintain an indirectly wholly owned subsidiary in Russia, Pro Farm Russia, LLC, and own a 12% minority interest in a third-party manufacturing plant in Vyborg, Russia. While our revenues derived from sales to customers in Ukraine and Russia are currently immaterial, products for Pro Farm, our wholly owned subsidiary, are partially sourced by suppliers from the manufacturing plant in Russia. We may face risks associated with maintaining our subsidiary in Russia and minority ownership of the manufacturing plant, or with any international operations in Russia, including risks associated with our compliance with evolving international sanctions and potential reputational harm as a result of our operations in Russia. Further, Russia’s president Vladimir Putin has approved decrees which allow the government to seize assets owned by foreign businesses and has taken steps to invalidate intellectual property protections for U.S.-based companies operating in Russia. In addition, should the conflict escalate or be prolonged, supply chain, trade routes and agricultural markets could be adversely affected, which, in turn, could materially, adversely affect our business operations and financial performance. While we have policies and procedures in place designed to ensure compliance with applicable sanctions and trade restrictions, our employees, contractors, and agents may take actions in violation of such policies and applicable law, and we could be held ultimately responsible. If we are held responsible for a violation of U.S. sanctions laws, we may be subject to various penalties, any of which could have a material adverse effect on our business, financial condition or results of operations.
Further, our earnings may be adversely affected by fluctuations in the price of certain commodities, such as grains, milk, meat, biofuels and biomaterials. If commodity prices are negatively impacted, the value of our products could be directly and negatively impacted. Additionally, growers’ incomes have historically been negatively affected by commodity prices. As a result, fluctuations in commodity prices could have an impact on growers’ purchasing decisions and negatively affect their ability and decisions to purchase our seeds or products that incorporate our proprietary technology. We cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
The spread of global pandemics, such as the COVID-19 pandemic, and government efforts to control the effect and spread of such pandemics have had and will have a disruptive effect on different aspects of our business.
The spread of global pandemics, such as the COVID-19 pandemic, impacted the world economy and the jurisdictions in which we conduct our business. Jurisdictions in which we conduct our business have imposed mandates and/or regulations or implemented measures to counter the spread of the COVID-19 virus to control the impact of the pandemic on public health and their respective economies.
These control measures collectively have changed over the course of the pandemic and may be reinstated if another pandemic breaks out. Moreover, the emergence of variants of the COVID virus, caused by mutations, led to a resurgence in infections and prompted renewed uncertainty. We have been affected in a number of ways, such as the way in which we operate our headquarters operations, interact with our scientists and their activities, and plan for and carry out clinical trials, all of which have experienced some short-term disruption and may suffer long-term changes in the way we will do business. Actions such as government lock downs slowed or, in some cases, temporarily stopped research and development activities and clinical trials. Various safety protocols for personal interactions may hamper research and development activities. Since we are mostly focused on the activities related to research and development, we have not experienced the larger adverse economics of a slowed economy. The financial effect will be that our development expenses may increase and we may have to obtain additional capital funding. Any required additional equity funding will be dilutive to the equity of our investors and debt financing may have restrictive covenants that could adversely affect our business plans and operational objectives. Any further funding that we may need may not be available or even if available it may not be on terms that are acceptable to us.
While it is not possible to predict the future impact of global pandemics and the effect of the measures adopted by governments, global pandemics have in the past and in the future may significantly affect global economic conditions. Furthermore, global pandemics and governments’ future responses to global pandemics may affect our financial position and results of operations. We monitor the impact of pandemics, such as the COVID-19 pandemic, on us. However, the impact of such pandemics on our business, results of operations, and financial position remains highly uncertain.
Our crop productivity business is highly seasonal and affected by factors beyond our control, which may cause our sales and operating results to fluctuate significantly.
The sale of our products is dependent upon planting and harvest seasons and are expected to result in highly seasonal patterns and substantial fluctuations in quarterly sales and profitability. As we increase the variety of products sold within our current markets, and expand into new markets in different geographies, the seasonality of our business may change. Therefore, our business may be more seasonal or experience seasonality in different periods than anticipated.
16
Weather conditions and natural disasters, such as heavy rains, hail, floods, freezing conditions, windstorms, drought or fire, affect decisions by our distributors, direct customers and end users about the types and amounts of products to use and the timing of harvesting and planting. As a result, our operating results may vary substantially from year to year and quarter to quarter beyond the regular seasonal patterns’ predictions.
Other factors may also contribute to the unpredictability of our operating results, including the size and timing of significant distributor transactions, the delay or deferral of use of our commercial technology or products and the fiscal or quarterly budget cycles of our direct customers, distributors, licensees and end users. Customers may purchase large quantities of our products in a particular quarter to store and use over long periods of time or time their purchases to manage their inventories, which may cause significant fluctuations in our operating results for a particular quarter or year.
Our results of operations from our crop productivity products may vary significantly from period to period due to circumstances beyond our control, including as a result of climate change.
The crop productivity market is affected by various factors that make their operations relatively unpredictable from period to period. The development of our products may be adversely affected by circumstances beyond our control. For our crop productivity products, factors beyond our control include weather and climatic change, such as droughts or heat stress, or other factors we are unable to identify. For example, if there were a prolonged or permanent disruption to the electricity, climate control or water supply operating systems in our greenhouses or laboratories, the plants on which we are testing our licensed seed traits and the samples we store in freezers, both of which are essential to our development activities, would be severely damaged or destroyed, adversely affecting our development activities and thereby our business and results of operations. We have experienced crop failures in the past for various reasons, which have required us to re-start field trials and delays in achieving expected results.
The crop productivity market is also vulnerable to crop disease and to pests, which may vary in severity and effect, depending on the stage of production at the time of infection or infestation, the type of treatment applied, climatic conditions and the risks associated with ongoing global climate change. The costs to control disease and other infestations vary depending on the severity of the damage and the extent of the plantings affected. Moreover, there can be no assurance that available technologies to control such infestations will continue to be effective. These infestations can also increase costs, decrease revenues and lead to additional charges to earnings, which may have a material adverse effect on our business, financial position and results of operations.
Any development or product failure we may experience or any inability to economically source necessary materials could result in increased cost of development of our crop productivity products, which may negatively impact our business and results of operations.
Certain estimates of market opportunity included in this report are based on assumptions that are inherently uncertain and subject to risks and uncertainties that could have a material adverse effect on our business, operating results, and financial condition.
The information regarding our market opportunities, including for HB4 soy and HB4 wheat, has been prepared by management and our assumptions underlying our statements about these market opportunities are inherently uncertain and are subject to significant business, economic, regulatory and competitive risks and other uncertainties that could cause actual results to differ materially from those set forth in management’s estimates. No independent third party has compiled, examined, or performed any procedures with respect to our potential market opportunities, nor has any third party expressed any opinion or any other form of assurance on the information or its achievability by us, and no independent third party has assumed responsibility for, or claimed any association with, the information we have included herein regarding such potential market opportunities. The information regarding market opportunities is not fact and should not be relied upon as being indicative of future results. For example, we extrapolated from publicly available data for the past ten years to estimate soy and wheat production area in order to in turn estimate the size of HB4 soy and HB4 wheat-related market opportunities. Changes in economic, climate, regulatory and other factors could significantly reduce the target area and our market opportunity. We also made assumptions regarding the number of bags of soybeans and wheat seeds needed to plant one hectare as well as other information, which in each case may prove to be materially incorrect. Furthermore, we may not be able to take advantage of these market opportunities even if they are available. Our failure to take advantage of market opportunities or to correctly size our market opportunity could have a material adverse effect on our ability to take advantage of our investments in licensed technologies, including for HB4 soy and HB4 wheat, and therefore on our business, operating results and financial condition.
17
Consumer and government resistance to GM crops may negatively affect our public image and reduce sales of seeds or other products containing our licensed seed traits. In addition, Bioceres S.A., from whom we license HB4 technology, is party to a legal proceeding in Argentina, which if decided unfavorably would adversely affect our ability to sell HB4 products in Argentina.
We develop biotech seeds, including GM seeds and the successful commercialization of our products depends, in part, on public acceptance of genetically engineered agricultural products. Some consumers may reject foods made from GM seeds and production of certain GM crops is prohibited in certain countries. Any increase in negative perceptions of GM crops, or more restrictive government regulations in response thereto, could have a negative effect on our business and may delay or impair the development and commercialization of some of our products.
The commercial success of our products may be adversely affected by claims that biotechnology plant products are unsafe for consumption or use, pose risks of damage to the environment, or create legal, social and ethical dilemmas.
The high public profile of biotechnology in food production and food products and public attitudes about the safety and environmental hazards of, and ethical concerns over, genetic research and biotechnology plant products could negatively affect our public image and results of operations.
The prohibition of the production of certain GM crops in certain countries and the current resistance from consumer groups to GM crops not only limits our access to such markets but also has the potential of spreading to and influencing the acceptance of products developed through biotechnology in other regions of the world and may also influence regulators in other countries to limit or ban production of GM crops, which could limit the commercial opportunities to exploit biotechnology. For example, in the United States, no product may be labelled as “organic” if it contains any GMO. Additionally, some states in the United States are considering, and one state has passed a law relating to, mandatory labelling of GMO foods, which may carry a negative connotation for consumers and which could make it difficult and expensive for companies to use ingredients from GM crops and distribute products in compliance with the labelling requirements, each of which could in turn have an adverse impact on the sale of our licensed GM seeds. In Argentina, a class action suit has been filed against Bioceres S.A. (from whom we license HB4 technology), certain biotechnology companies and the national government. The class action plaintiffs request that GMO foods be mandatorily labelled as such and that measures be taken to protect land use, among others. As we license HB4 technology from Bioceres S.A, if such action were to be decided unfavorably, we would no longer be able to sell products that contain HB4 technology in Argentina, which would have a significant negative impact on our revenue. As of the date of this report, the plaintiffs’ request for injunctions against GMO approvals were rejected by the Federal Court of Appeals and an injunction appealed before the Argentine Supreme Court was also rejected.
GM crops are grown principally in the United States, Brazil and Argentina, where there are fewer restrictions on the production of GM crops. If these or other countries where GM crops are grown or where we engage in business activities enact laws or regulations that ban the production of such crops or make regulations more stringent, we could experience a longer product development cycle for our products and may be forced to abandon projects related to certain crops or geographies, both of which would negatively affect our business and results of operations. Public attitudes towards ownership of genetic material and potential changes to laws regulating such ownership could weaken our intellectual property rights with respect to our genetic material and discourage R&D partners from supporting, developing or commercializing our products and technologies. Furthermore, any future labeling requirements could heighten these concerns and make consumers less likely to purchase food products containing gene-edited ingredients.
Competition in crop productivity products, including biologicals, is intense and requires continuous technological development.
We currently face significant direct and indirect competition in the markets in which we operate. The markets for crop productivity products, including biologicals, are intensely competitive and rapidly changing. Many companies engage in the development these products, and speed in commercializing a new product can be a significant competitive advantage.
As an example, some of our competitors engage in research associated with discovery and therefore have R&D budgets that are more significant than our own R&D budget and that cover more activities than those in which we engage. In addition, former collaborators, by virtue of having had access to our proprietary technology, may utilize know-how acquired while employed by us for their own development efforts.
18
In most segments, the number of products available to end-consumers is steadily increasing as new products are introduced. We may be unable to compete successfully against our current and future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for our products and technologies. In addition, many of our competitors have substantially greater financial, marketing, sales, distribution and technical resources than us and some of our competitors have more experience in R&D, regulatory matters, manufacturing and marketing. We anticipate increased competition in the future as new companies enter the market and new technologies become available. Programs to improve genetics and crop protection chemicals are generally concentrated within a relatively small number of large companies, while non-genetic approaches are underway with broader set of companies. Mergers and acquisitions in the plant science, specialty food ingredient and agricultural biotechnology seed and chemical industries may result in even more resources being concentrated among a smaller number of our competitors.
The expiration of patents covering existing products reduces the barriers to entry for competitors. Our ability to compete effectively and to achieve commercial success depends, in part, on our ability to (i) control manufacturing and marketing costs, (ii) effectively price and market our products, (iii) successfully develop an effective marketing program and an efficient supply chain, (iv) develop new products with properties attractive to food manufacturers or growers, and (v) commercialize our products quickly without incurring major regulatory costs. We may not be successful in achieving these factors and any such failure may adversely affect our business, results of operations and financial condition.
Changes in laws and regulations to which we are subject, or to which we may become subject in the future, may materially increase our costs of operation, decrease our operating revenues and disrupt our business.
Laws and regulatory standards and procedures that impact our business are continuously changing. Responding to these changes and meeting existing and new requirements may be costly and burdensome. Changes in laws and regulations may occur that could:
| ● | impair or eliminate our ability to source technology and develop our products, including validating our products through field trials and passing biosafety evaluations; |
| ● | increase our compliance and other costs of doing business through increases in the cost to protect our owned and licensed intellectual property, including know-how, trade secrets and regulatory data, or increases in the cost to obtain the necessary regulatory approvals to commercialize and market the products we develop directly or jointly; |
| ● | require significant product redesign or redevelopment; |
| ● | render our licensed seed traits and technology and products that incorporate them less profitable or less attractive compared to competing products; |
| ● | reduce the amount of revenues generated from licenses or other royalties; |
| ● | restrict or increase the costs of making payments and distributions; |
| ● | increase our export and import duties and costs or intensify controls and restrictions on our imports; and |
| ● | discourage us and other collaborators from offering, and end-markets from purchasing, products that incorporate our licensed seed traits and technology. |
Any of these events could have a material adverse effect on our business, results of operations and financial condition. As of the date hereof, we believe that we substantially comply with regulations in Argentina and other countries in which we operate relating to GM crops. However, if these regulations change, our validation trials and compliance efforts may become costly and burdensome.
Any changes in regulation in countries where GM crops are grown or exported into could result in our collaborators, other third parties or us being unable or unwilling to develop, commercialize or sell products that incorporate our licensed seed traits or technology. In addition, we rely on various forms of intellectual property rights protection. Legislation and precedents relating to intellectual property rights in the key markets where we seek protection, such as the United States, Brazil and Argentina, is evolving and changes in laws could affect our ability to obtain or maintain intellectual property rights protection for our products. Any changes to these existing laws and regulations may materially increase our costs, decrease our revenues and disrupt our business.
19
Our indebtedness could adversely affect our financial condition.
As of June 30, 2023, our total indebtedness was US$243.5 million, of which US$107.6 million matures in the fiscal year ending June 30, 2024. We may incur additional indebtedness in the future. Our indebtedness could have important adverse consequences, including:
| ● | limiting our ability to obtain additional financing to fund future working capital, capital expenditures, acquisitions or other general corporate requirements; |
| ● | requiring a substantial portion of our cash flows to be dedicated to debt service payments instead of other purposes, thereby reducing the amount of cash flows available for working capital, capital expenditures, acquisitions and other general corporate purposes; |
| ● | increasing our vulnerability to general adverse economic and industry conditions; |
| ● | limiting our flexibility in planning for and reacting to changes in the industry in which we compete; |
| ● | placing us at a disadvantage compared to other, less leveraged competitors; and |
| ● | increasing the cost of borrowing. |
The occurrence of any of the above may negatively impact our business and results of operations. If any of our indebtedness gets accelerated as a result of our failure to meet certain covenants, the risks described above could intensify. See “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Indebtedness.”
In addition, our Argentine subsidiaries are temporarily subject to certain restrictions on payments of foreign indebtedness through the foreign exchange market (the “FX Market”). Therefore, our Argentine subsidiaries may be impeded from making payments under foreign indebtedness or the costs of performing such payments could be substantially higher, all of which could have an adverse effect on our business and results of operations. See “—Argentine exchange controls and restrictions limit access to the FX Market to make payments and distributions from our Argentine subsidiaries and receive the proceeds of any sale of our assets in Argentina.”
Price increases and shortages of raw materials could adversely affect our results of operations.
Our results of operations may be affected by the availability and pricing of raw materials, principally materials needed to design our technologies, such as raw glycerin. Factors such as changes in the global or regional levels of supply and demand, weather conditions, seasonal fluctuations, shortages or interruptions, changes in global climates and government regulations could substantially impact the price of raw materials. These and other factors could also cause plant shutdowns, reductions in capacity, delays and increased costs with associated manufacturers. To the extent we are unable to pass on increases in raw materials and energy prices to our customers, a substantial increase in raw material prices or a continued interruption in supply could have a material adverse effect on our business, financial condition and results of operations.
The overall agricultural industry is susceptible to commodity price volatility and, we, along with our food manufacturing customers and grower customers, are exposed to market risks from changes in commodity prices.
Volatility in the prices of certain commodity products could result in higher overall costs along the agricultural supply chain, which may negatively affect our ability to commercialize our products. We are susceptible to cost volatility in the agricultural industry as a result of factors beyond our control, such as general economic conditions, seasonal fluctuations, weather conditions, demand, food safety concerns, product recalls and government regulations. Further, we may not be able to anticipate or react to such volatility in a timely manner, which could cause our operating results to deteriorate.
20
We may be required to pay substantial damages as a result of uninsured product liability claims.
Product liability claims are a commercial risk for our business, particularly as we are involved in the sale of commercial technology and the supply of biotechnological products, some of which may be shown in the future to be harmful to humans and the environment. We may be held liable if any product we develop is found unsuitable during marketing, sale or consumption. We do not currently have insurance coverage for such claims. Courts have levied substantial damages in the United States and elsewhere against a number of companies in the agriculture industry in past years based upon claims for injuries allegedly caused by the use of their products. There is a possibility that a product liability case could be filed against us in Argentina, as there are precedents of cases relating to the use of pesticides, for example. However, in Argentina, damages relating to these cases may be substantial albeit potentially smaller than those typically awarded in the United States.
In addition, we may face product liability and similar claims involving cross-pollination of crops, which recently has affected other companies in our industry operating in the United States, and cross-contamination of GMO and non-GMO ingredients. Product liability claims against us, our joint ventures or third-party licensees selling products that contain our licensed seed traits or technology or allegations of product liability relating to seeds or other products containing seed traits or technology developed by us could damage our reputation, harm our relationships with our collaborators and other business counterparties and materially and adversely affect our business, results of operations, financial condition and prospects.
Our operations are subject to various health and environmental risks associated with our use, handling, storage and disposal of potentially toxic materials.
We are subject to numerous federal, state, local and foreign environmental, health and safety laws and regulations, including those governing laboratory procedures, the handling, use, storage, treatment, manufacture and disposal of hazardous materials and wastes, discharge of pollutants into the environment and human health and safety matters. As part of our technology sourcing and product development activities, we develop GMOs by inserting new genes into the genomes of certain plants and bacteria. Though we introduce these genes in order to improve plant traits, we cannot always predict the effect that these genes may have on the organism. In some cases, the genes may render the organism poisonous or toxic, or they may cause the organism to develop other dangerous characteristics that could harm the organism’s surrounding environment. Furthermore, there is a risk that, when testing GMOs, the seeds or strains of these organisms may escape the laboratory, greenhouse, industrial facility or field in which they are being tested and contaminate nearby areas. Poisonous or toxic organisms may therefore be inadvertently introduced into the environment or possibly enter the food production system, harming the people and animals who come in contact with them. Our crop protection products, which include Rizoderma, adjutants, therapies, herbicides, fungicides and insecticides, among others, bear similar risks in the development stage.
We cannot eliminate the risk of injuries or damages caused by the contamination or discharge of these materials. If these risks were to materialize, we could be subject to fines, liability, reputational harm or otherwise adverse effects on our business. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, or may otherwise be required to remedy the contamination, and our liability may exceed any insurance coverage and our total assets. Furthermore, compliance with environmental, health and safety laws and regulations may be expensive and may impair our R&D efforts. If we fail to comply with these requirements, we could incur substantial costs and liabilities, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict the impact on our business of new or amended environmental, health and safety laws or regulations or any changes in the way existing and future laws and regulations are interpreted and enforced. These current or future laws and regulations may impair our research, development or production efforts.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business.
We are required to comply with various regulatory and reporting requirements, including those required by the SEC. Complying with these reporting and regulatory requirements will be time consuming, resulting in increased costs to us or other adverse consequences.
21
As a public company, we are subject to the reporting requirements of the Exchange Act and the requirements of the Sarbanes-Oxley Act. These requirements may place a strain on our systems and resources. The Exchange Act requires that we file annual and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting. To maintain and improve the effectiveness of our disclosure controls and procedures, we will need to commit significant resources, hire additional staff and provide additional management oversight. We expect to implement additional procedures and processes for the purpose of addressing the applicable standards and requirements for public companies. These activities may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
On June 30, 2023, we ceased to be an “emerging growth company,” as defined in the JOBS Act, and we may no longer take advantage of certain temporary exemptions from various reporting requirements including, but not limited to, an exemption from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and the rules and regulations of the SEC thereunder. Therefore, we expect to incur additional expenses and devote increased management effort toward ensuring compliance with the additional reporting requirements that apply as we have ceased to be an “emerging growth company.” We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs.
We may require additional financing in the future and may not be able to obtain such financing on favorable terms, if at all, which could force us to delay, reduce or terminate some of our activities.
The process of developing and commercializing products is expensive, lengthy and risky and we expect to continue investing in our R&D services to identify new potential products for development. We may require additional capital to fund our technology sourcing and product development projects and to provide working capital to fund other aspects of our business, including changes in our business strategy or the occurrence of unanticipated events other strategic opportunities.
We may seek to issue additional equity securities, which could result in dilution to our existing shareholders and could adversely affect the market price of our ordinary shares. Alternatively, we may seek to raise additional debt financing, which could subject us to restrictive covenants that limit our operating flexibility and require us to comply with certain financial ratios. However, we may not be able to raise sufficient additional funds on terms that are favorable to us, if at all. If we fail to raise the funds we require, our ability to fund our operations, take advantage of strategic opportunities, develop and commercialize products or technologies, or otherwise respond to competitive pressures could be significantly limited. In such an event, we may be forced to delay or terminate our development initiatives or the commercialization of our technology and products, curtail operations or grant licenses to our technology on terms that are not favorable to us. In addition, we may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees and other costs. If adequate funds are not available and on commercially reasonable terms, we may not be able to successfully execute our business strategy or continue our business.
Development and commercialization of our products may incur scrutiny under the Convention on Biological Diversity Treaty.
The Convention on Biological Diversity Treaty is an international treaty that was adopted at the Earth Summit in Rio de Janeiro, Brazil in 1992. The treaty provides that if a company uses genetic resources, such as an indigenous plant, from a participating country to develop a product, then such company must obtain the prior informed consent of the participating country and owes fair and equitable compensation to the participating country. Although the United States is not a participating country, most countries where we currently obtain or may obtain genetic resources in the future, including Argentina, have ratified the treaty and are currently participants in the Convention on Biological Diversity Treaty. We may fall under scrutiny of the Convention on Biological Diversity Treaty with respect to the development or commercialization of any of our products derived from genetic resources originating from any of the countries that are participants in the Convention on Biological Diversity Treaty. There can be no assurance that the government of a participating country will not assert that it is entitled to fair and equitable compensation from us. Such compensation, if demanded, may make commercialization of some of our products impracticable.
22
Our failure to accurately forecast and manage seed and biological inventory could result in an unexpected shortfall or surplus of products which could harm our seed and biological business.
We are required to produce inventories of certain of our products (mainly seeds and biologicals) and we monitor our inventory levels based on our own projections of future demand. Because of the significant time it takes to produce commercial quantities of seeds, production decisions must be made well in advance of sales. An inaccurate forecast of demand for any seed variety can result in the unavailability of seeds in high demand. Such unavailability may depress sales volumes and adversely affect customer relationships. Conversely, an inaccurate forecast could also result in an over-supply of seeds which may increase costs, negatively impact cash flow, reduce the quality of inventory and ultimately result in inventory write-offs, which could have a material adverse effect on our business, results of operations and financial condition.
Disruption to our IT and operating system could adversely affect our reputation and have a material adverse effect on our business and results of operations.
Disruption or failure of our IT system due to technical reasons, natural disaster or other unanticipated catastrophic events, including power interruptions, storms, fires, floods, earthquakes, terrorist attacks or armed conflicts could significantly impair our ability to deliver data related to our projects to our collaborators on schedule and materially and adversely affect our relationships with our collaborators, therefore, negatively affect our business and our results of operations. If our existing or future IT system does not function properly, or if the IT system proves incompatible with our new technologies, we could experience interruptions in data transmissions and slow response times, preventing us from completing routine research and business activities. Furthermore, we can provide no assurance that our current IT system is fully protected against third-party intrusions, viruses, hacker attacks, information or data theft or other similar threats.
Our business and operations would suffer in the event of computer system failures, cyber-attacks or a deficiency in our cyber-security.
Despite the continuous implementation of security measures, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from computer viruses, malware, natural disasters, terrorism war telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber-intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number intensity and sophistication of attempted attacks and intrusions from around the world have increased. In addition to the extraction of sensitive information, such attacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, phishing attacks social engineering and other means to affect service reliability. If any such event were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur material legal claims and liability, damage to our reputation, the further development of our product candidates could be delayed, which could adversely affect our business, financial condition, and operating results, and could negatively impact our share price.
Labor unions can request, and have requested, the unionization of some of our employees.
In December 2016 and March 2017, the Argentine Trade Union of Truck Drivers (Sindicato de Choferes de Camiones) (the “SCC”) and the Argentine Union of Rural Workers and Stevedores (Unión Argentina de Trabajadores Rurales y Estibadores) (the “UATRE”), respectively requested the unionization of some employees of Rizobacter. With respect to the former, the SCC requested to unionize employees involved in logistics and operation of forklifts. UATRE requested to unionize workers engaged in the handling and storage of grain related to our seed treatment process undertaken seasonally. After negotiations, both SCC and UATRE came to an agreement with Rizobacter wherein Rizobacter agreed to hire companies to carry out the operations covered by each union. Each company agreed to indemnify Rizobacter in relation to any subsequent claims by the workers registered with the SCC or the UATRE, as the case may be, without direct cause to Rizobacter.
If new union disputes arise, they may be time consuming and distracting to management. The occurrence of a union dispute could have a material and adverse effect on our costs and business, results of operations and financial condition.
23
We rely on third parties to grow our seeds. If these parties do not grow our seeds at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our commercialization efforts could be delayed and our business could be negatively impacted.
We rely on affiliated and unaffiliated growers to grow the majority of our proprietary seed and to sell it to us at negotiated prices each year. Our current reliance on third parties upon others for the production of our seeds may adversely affect our ability to commercialize any products on a timely and competitive basis. If our growers decline to a significant degree to plant the acreage on which we rely, and if we cannot find other growers to plant the lost acreage, our inventory of seed could be insufficient to satisfy the needs of our customers. Furthermore, growers may refuse to grow our seeds for any reason, including deterioration in our business relationship or the existence of more favorable terms with other companies. For example, if a particular crop is paying a materially higher price than has been paid in the past, growers may decide to not grow our seeds in favor of receiving a higher return from an alternative crop planted on the same acreage. If third-party growers decline to grow our seeds or if they are unable to grow our seeds at acceptable quality levels, our business, results of operations and financial condition could materially decline.
We are subject to anti-corruption and anti-money laundering laws with respect to both our domestic and international operations, and noncompliance with such laws can subject us to criminal and civil liability and harm our business.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C.§201, the U.S. Travel Act, the USA PATRIOT Act, Argentine Law No. 27,401, as amended, and possibly other anti-bribery and anti-money laundering laws in countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit us and our collaborators from authorizing, offering, or directly or indirectly providing improper payments or benefits to recipients in the public or private sector. We or our collaborators may have direct and indirect interactions with government agencies and state-affiliated entities and universities in the course of our business. We may also have certain matters come before public international organizations such as the United Nations. We use third-party collaborators, joint venture and strategic partners, law firms, and other representatives for regulatory compliance, patent registration, lobbying, deregulation advocacy, field testing, and other purposes in a variety of countries, including those that are known to present a high corruption risk such as India, China, and Latin American countries. We can be held liable for the corrupt or other illegal activities of these third-party collaborators, our employees, representatives, contractors, partners, and agents, even if we do not explicitly authorize such activities. In addition, although we have implemented policies and procedures to ensure compliance with anti-corruption and related laws, there can be no assurance that all of our employees, representatives, contractors, partners, or agents will comply with these laws at all times. Noncompliance with these laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other or injunctions, suspension and debarment from contracting with certain governments or other persons, the loss of export licenses, reputational harm, adverse media coverage, and other negative consequences. If any subpoenas or investigations are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations, and financial condition could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees. Enforcement actions and sanctions could further harm our business, results of operations, and financial condition.
Recently introduced economic substance legislation of the Cayman Islands may adversely impact us or our operations.
The Cayman Islands, together with several other non-E.U. jurisdictions, have recently introduced legislation aimed at addressing concerns raised by the Council of the E.U. as to offshore structures engaged in certain activities, which attract profits without real economic activity. With effect from January 1, 2019, the International Tax Cooperation (Economic Substance) Act, as revised, came into force in the Cayman Islands introducing certain economic substance requirements for in-scope Cayman Islands entities, which are engaged in certain “relevant activities”. As we are a Cayman Islands company, our compliance obligations include filing annual notifications, which need to state whether we are carrying out any relevant activities and whether we are claiming an exemption from the obligations to meet the economic substance tests to the extent required under the Substance Act (the “substance test”). If we are carrying out such relevant activities, or we are claiming such an exemption, we are further required to file annually a report as to whether we have satisfied the substance test or the basis on which we are claiming such exemption. As it is a new regime, it is anticipated that the Substance Act will evolve and be subject to further clarification and amendments. We may need to allocate additional resources to keep updated with these developments and may have to make changes to our operations in order to comply with all requirements under the Substance Act. Failure to satisfy these requirements may subject us to penalties under the Substance Act.
24
We may become subject to taxation in the Cayman Islands which would negatively affect our results.
We are a Cayman Islands exempted company. Exempted companies are Cayman Islands companies conducting business mainly outside the Cayman Islands and, as such, are exempted from complying with certain provisions of the Companies Act. As an exempted company, we have applied for and received a tax exemption undertaking from the Cayman Islands government that, in accordance with Section 6 of the Tax Concessions Act (As Revised) of the Cayman Islands, for a period of 20 years from the date of the undertaking, no law which is enacted in the Cayman Islands imposing any tax to be levied on profits, income, gains or appreciations will apply to us or our operations and, in addition, that no tax to be levied on profits, income, gains or appreciations or which is in the nature of estate duty or inheritance tax will be payable (i) on or in respect of the shares, debentures or other obligations we have or (ii) by way of the withholding in whole or in part of a payment of dividend or other distribution of income or capital by us to our members or a payment of principal or interest or other sums due under a debenture or other obligation we have. If we were to become subject to taxation in the Cayman Islands, our financial condition and results of operations could be materially and adversely affected. See the section titled “Material Cayman Islands Income Tax Considerations.”
As a “foreign private issuer” under the rules and regulations of the SEC, we are permitted to, and will, file less or different information with the SEC than a company incorporated in the United States or otherwise subject to these rules, and will follow certain home country corporate governance practices in lieu of certain Nasdaq requirements applicable to U.S. issuers.
We are considered a “foreign private issuer” under the Exchange Act and we are therefore exempt from certain rules under the Exchange Act, including the proxy rules, which impose certain disclosure and procedural requirements for proxy solicitations for U.S. and other issuers. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or within the same time frames as U.S. companies with securities registered under the Exchange Act. We currently prepare our financial statements in accordance with IFRS. We will not be required to file financial statements prepared in accordance with or reconciled to the accounting principles generally accepted in the United States of America (“U.S. GAAP”) so long as our financial statements are prepared in accordance with IFRS as issued by the International Accounting Standards Board. We are not required to comply with Regulation FD, which imposes restrictions on the selective disclosure of material information to shareholders. In addition, our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our securities.
In addition, as a “foreign private issuer” whose ordinary shares are listed on the Nasdaq Stock Market LLC (“Nasdaq”), we are permitted to, and will, follow certain home country corporate governance practices in lieu of certain Nasdaq Global Market requirements. A foreign private issuer must disclose in its Annual Reports filed with the Securities and Exchange Commission, or the SEC, each Nasdaq requirement with which it does not comply followed by a description of its applicable home country practice. As an exempted company incorporated in the Cayman Islands and listed on the Nasdaq, we currently intend to follow our home country practice with respect to the composition of our board of directors and nominations committee and executive sessions. Unlike the requirements of the Nasdaq, the corporate governance practice and requirements in the Cayman Islands do not require us to have a majority of our board of directors to be independent; do not require us to establish a nominations committee; and do not require us to hold regular executive sessions where only independent directors shall be present. Such Cayman Islands home country practices may afford less protection to holders of our ordinary shares.
We could lose our status as a “foreign private issuer” under current SEC rules and regulations if more than 50% of our outstanding voting securities become directly or indirectly held of record by U.S. holders and one of the following is true: (i) the majority of our directors or executive officers are U.S. citizens or residents; (ii) more than 50% of our assets are located in the United States; or (iii) our business is administered principally in the United States. If we lose our status as a foreign private issuer in the future, we will no longer be exempt from the rules described above and, among other things, will be required to file periodic reports and annual and quarterly financial statements as if it we were a company incorporated in the United States. If this were to happen, we would likely incur substantial costs in fulfilling these additional regulatory requirements and members of our management would likely have to divert time and resources from other responsibilities to ensuring these additional regulatory requirements are fulfilled.
25
Risks Related to our Intellectual Property
Agreements with our collaborators and third parties may not adequately prevent disclosure of trade secrets, know-how and other proprietary information, which could materially adversely affect our technology and harm our business.
We rely on a combination of intellectual property rights laws and other agreements with our collaborators and third parties to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not prevent disclosure, infringement or misappropriation of our confidential information. Our confidentiality and nondisclosure agreements or covenants may not be enforceable under applicable law and, even if they are enforceable, may be breached, and we may not have adequate remedies for such a breach that would effectively prevent the dissemination of our confidential information or direct competition with us by a joint venture partner. We also have limited control over the protection of trade secrets used by our collaborators and could lose future trade secret protection if any unauthorized disclosure of such information occurs. Enforcement of any claim that a party illegally disclosed confidential information or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, others may independently discover our trade secrets and proprietary information, by reverse engineering or otherwise, and in such cases, we would not be able to enjoin such parties. Laws regarding trade secret rights in certain markets where we operate may afford little or no protection of our trade secrets. If any of our trade secrets were to be disclosed to or independently developed by a competitor, or if we otherwise were to lose protection for our trade secrets or proprietary know-how, the value of this information may be greatly reduced and our business and competitive position could be harmed. Moreover, our collaborators may allege that we have disclosed their trade secrets or confidential information.
We may not be able to adequately protect our intellectual property rights throughout the world.
Our commercial success depends in part on our ability to obtain intellectual property protection and/or trade secrets protection for the technologies we develop and use. Competitors may use our technologies in jurisdictions where we have not obtained intellectual property protection to develop their own products, and we may be unable to prevent such competitors from importing those infringing products into territories where we have intellectual property protection, but enforcement is not as strong due to the exhaustion of rights. These products may compete with our product candidates and our licensed patents and other intellectual property rights may not be effective or sufficient to prevent them from competing in those jurisdictions. In addition, competitors could use our licensed patent disclosures and/or reverse engineer our trade-secret-protected products in order to produce competing products. Moreover, growers or others in the chain of commerce may raise legal challenges against our intellectual property rights or may infringe upon our intellectual property rights, including through means that may be difficult to prevent or detect.
On March 6, 2022, Russia issued a decree permitting the use of certain patents without compensation or license for inventions, industrial designs, and utility models originating in unfriendly countries, which include the United States. Currently, we have eight patents issued by the Russian Federation, which following the March 6, 2022 decree may no longer be afforded any intellectual rights protections. If third party individuals, including the majority owners of the third-party manufacturing plant in Vyborg, Russia, utilize our patents and create versions of our products we would likely have limited to no recourse and would not receive any compensation for any unauthorized use.
In Argentina, legislation in respect of breeders’ rights includes a concept of a “farmer’s privilege,” which allows growers to use seeds obtained from their own harvests to be replanted on their own farm. According to the National Seed Institute of Argentina (Instituto Nacional de Semillas), the reserves of seeds kept for personal use has grown significantly in recent years, which may increase the likelihood that growers illegally claiming the privilege may use GM seeds into the market without paying royalties owed to us. During recent years, certain sectors of the Argentine agricultural industry have been requesting that the Argentine plant variety protection (“PVP”) Law No. 20,247 be amended.
The legal systems of certain countries, including China, where we have sublicensed patent applications, have not historically favored the enforcement of patents or other intellectual property rights, which could hinder us from preventing the infringement of our licensed patents or other intellectual property rights and result in substantial risks to us. Proceedings to enforce our licensed patent rights in the United States or foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our licensed patents at risk of being invalidated or interpreted narrowly and our sublicensed patent applications at risk of not issuing and could provoke third parties to assert patent infringement or other claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license from third parties.
26
Changes in U.S. and Argentine patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotech companies, our success is heavily dependent on intellectual property, including patents. Obtaining and enforcing biotech patents involves technological and legal complexity, and is costly, time consuming, and inherently uncertain.
The patent position of biotechnology and biochemical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Furthermore, our patents, and those patents for which we have license rights, may be challenged, narrowed, invalidated or circumvented. It is also not possible to patent and protect all knowledge and know-how associated with our products, so there may be areas that are not protected such as certain formulations and manufacturing processes. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
The Argentine Patent Office (Instituto Nacional de Propiedad Intelectual) issued Regulation 283/15 with new guidelines for examining biotech inventions. These guidelines seriously restrict the patentability of several categories of inventions in the agricultural field. This restriction is already being followed in the practice of the Argentine Patent Office.
In September 2016, the Argentine Patent Office issued Argentine Regulation 56/16, under which the Argentine Patent Office will deem that any patent application whose examination had not begun by October 15, 2016 satisfies the substantive requirements of patentability (novelty, non-obviousness and industrial application); provided that a patent has been granted abroad for the same invention by a foreign patent office carrying out substantive examination in a country whose patent law has the same substantive requirements as Argentine law. This can result in prosecution times that are substantially shorter, and similar to those of the fastest jurisdictions. However, the patent office has applied this regulation to biotech cases only when the granted equivalent is directed to matter that is not affected by the guidelines. Furthermore, the claims of the granted equivalent must be amended so as to comply with the requirements of Regulation 283/15.
In addition, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. In a recent ruling (in re “Monsanto Technology LLC c/ Instituto Nacional de la Propiedad Industrial s/ Denegatoria de Patente”, case number CCF 8044/2007), Tribunal III of the Civil and Commercial Federal Court of Appeals of the City of Buenos Aires confirmed, by revoking a decision of a lower court, the rejection of a biotechnological patent application by the Argentine Patent Office, with the understanding that the invention should be protected as a PVP and not under a patent (the patent application was for a recombinant DNA molecule and a cell transformed by such molecule). Lack of inventive activity and non-patentable matters are also mentioned as grounds in this precedent. The Court of Appeal’s decision was appealed to the court of last resort, but the Argentine Supreme Court refused to resolve the case based on the grounds that the issue was moot as the statutory term of the patent under consideration in the case had already expired. The decision of the Court of Appeals has, therefore, become a negative precedent that may adversely affect patentability of the technologies we develop in the future. Depending on decisions by the Argentine and U.S. Congresses, the federal courts in each country, the U.S. Patent and Trademark Office and the Argentine Patent Office, as well as the relevant authorities in other countries in which we hold licensed patents, the laws and regulations governing patents could change in unpredictable ways that may weaken or undermine our ability to obtain new patents or to enforce our existing licensed patents and patents we might obtain in the future.
If we or one of our collaborators or licensees are sued for infringing the intellectual property rights of a third party, such litigation could be costly and time consuming and could prevent us or our collaborators or licensees from developing or commercializing products that incorporate our technology.
Our ability to generate significant revenues from our products depends on our joint ventures’ and licensees’ ability to develop, market and sell products and utilize our proprietary technologies without infringing the intellectual property rights and other rights of any third parties.
27
As the agricultural biotech industry continues to develop, we, our collaborators or licensees may become party to, or threatened with, litigation or other adverse proceedings regarding intellectual property or proprietary rights in our technology, processes, developed seed traits or seed treatments. Third parties may assert claims based on existing or future intellectual property rights and the outcome of any proceedings is subject to uncertainties that cannot be adequately quantified in advance. Any litigation proceedings could be costly and time consuming. A negative outcome from an intellectual property infringement suit could result in liability for monetary damages, require us to indemnify our licensees for damages arising from warranties we have made in respect of the intellectual property rights we have licensed, which claims might not be subject to a cap, or treble damages and attorneys’ fees if we are found to have willfully infringed a patent. There is also no guarantee that we, our collaborators or licensees would be able to obtain a license under such infringed intellectual property rights on commercially reasonable terms or at all. A finding of infringement could prevent us, our collaborators or our licensees from developing, marketing or selling a product or force us to cease some or all of our business operations. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel may be diverted as a result of these proceedings, which could have a material adverse effect on our operations. In some cases, our agreements with our collaborators or licensees might oblige us to pay for the enforcement of our licensed intellectual property rights, even though our collaborators or licensees may be responsible for commercializing the potentially infringing products. Claims that we have misappropriated the confidential information or trade secrets of third parties could similarly have a negative impact on our business.
The value of our intellectual property could diminish due to technological developments or challenges by competitors, making our products less competitive.
Our intellectual property rights are important to the operation of our business and the commercialization of our crop productivity products. We rely on a combination of licensed patents, PVP, trademarks, trade secret laws, confidentiality provisions and licensing arrangements to establish and protect our intellectual property. However, the importance of technology development and intellectual property protection in the crop productivity industry increases the risk that technological advances by others could render our products less competitive. Our business could be negatively affected by any of the following:
| ● | our issued licensed patents, PVP certificates and trademark registrations may be successfully challenged by our competitors; |
| ● | we may be unable to obtain intellectual property licenses that are necessary or useful to our business on favorable terms, or at all; |
| ● | new technology that is independently developed by others may supersede our technology and make our products less desirable or costlier in the marketplace; |
| ● | competitors may design around our licensed patent and/or PVP protections; and |
| ● | competitors may reverse engineer our trade secret technologies. |
We may be required to pay royalties, or other additional compensation, to employees who develop inventions that have been or will be commercialized by us, even if the rights to such inventions have been assigned to us, exclusive licenses have been granted to us or the employees have waived their rights to royalties.
Under Argentine Patent and Utility Models Law No. 24,481 and Argentine Labor Law No. 20,744, which provide the legal framework related to ownership of inventions developed during an employer-employee relationship, the employer is awarded ownership of inventions when the employee was hired for the purpose of engaging in inventive discovery or when such invention otherwise derives from the knowledge acquired by virtue of the employee’s working for the employer. Depending on the nature and the scope of an employee’s contribution to an invention and the nature of his or her hiring, he or she may be entitled to additional compensation by the employer; however, the employer will still retain ownership rights on the conditions mentioned above. If an employee was hired for a purpose other than to engage in inventive discovery and he or she creates an invention that is not related to the employer’s processes, methods or business, the employee shall be the owner of the invention.
28
A significant portion of our employees in Argentina are dedicated to activities that may be considered inventive. As a result, a significant portion of our employees execute confidentiality and ownership rights agreements upon commencement of employment whereby they agree to classify all work undertaken by them as engagement in inventive discovery, which grants us all ownership rights in inventions created while such employees are employed by us. If these assignments or exclusive licenses were deemed invalid or unenforceable, we could be required to pay royalties to our employees who have invented intellectual property that we have commercialized, which in turn may have a material adverse effect on our results of operations. In addition, if these assignments or exclusive licenses were deemed invalid or unenforceable, it is possible that our employees could assign or license to third parties their rights in any inventions created while employed by us. This could have a material adverse effect on our results of operations.
Risks Related to Operating in Latin America
A major portion of our operations and/or development activities are based in Latin America. As of June 30, 2023, approximately 75% of our consolidated sales of goods and services were attributable to our Latin American operations. In particular, fluctuations in the economy of Latin America and actions adopted by the governments of Latin American countries have had and may continue to have a significant impact on our subsidiaries operating in those countries.
Latin America has experienced, and may continue to experience, adverse economic or political conditions, including considerable economic uncertainty that may impact our business, financial condition and results of operations.
A major portion of our operations and/or development activities are based in Latin America. Accordingly, our financial condition and results of operations are substantially dependent on economic conditions in Latin America.
Latin American countries have historically experienced uneven periods of economic growth, recessions, periods of high inflation and economic instability. Recently, the economic growth rates of the economies of many Latin American countries have slowed and some have entered recessions. Additionally, economic and political developments in Latin America, including future economic changes or crises (such as inflation, currency devaluation or recession), government deadlock, political instability, civil strife, changes in laws and regulations, restrictions on the repatriation of dividends or profits, expropriation or nationalization of property, restrictions on currency convertibility, volatility of the foreign exchange market and exchange controls could impact our operations and/or the market value of the ordinary shares and have a material adverse effect on our business, financial condition and results of operations.
Further, in Argentina, the fluctuation in currency exchange rates, the gap between the official and alternative exchange rates, and high inflation, along with the measures attempting to control inflation adopted by the Argentine government, caused a deepening recession (the GDP decreased 4.9% in the second quarter of 2023, 1.7% in 2019 and 6.2% in 2018 and), increasing unemployment and the failure of medium and small companies. In 2020, the Latin American general macroeconomic conditions were worsened as a result of the COVID-19 pandemic (see “—The spread of COVID-19 and government efforts to control the effect and spread of the COVID-19 virus have had and will have a disruptive effect on different aspects of our business”). In Argentina, the stay-home orders and businesses restrictions, along with the global recession contributed to deepening the recession further. According to INDEC, during the second quarter of 2020 the GDP decreased 19.1% interannual (what represents the highest decrease of the GDP in Argentine history) with a peak of 16.2% in the second quarter, and as of July 2020 the economic activity decreased 13.2% interannual. All these circumstances also provoked a significant increase in poverty, which, according to INDEC, as of December 2021 affected approximately 37.3% of the population. In order to contain the further deterioration of the currency exchange rate, the Argentine Central Bank has been selling its reserves, which decreased to US$24.9 billion as of October 20, 2023. In addition, the Argentine government financed all economic assistance in connection with the COVID-19 pandemic with a heavy issuance of currency, which is also contributing to inflation, demand for U.S. dollars and devaluation of the Argentine Peso.
On October 23, 2023, the general Argentina presidential elections took place. The actual Minister of Economy, Sergio Massa, obtained 36.68% of the votes, while the new coalition La Libertad Avanza, led by Javier Milei, and Juntos por el Cambio received 29.98% and 23.83% of the votes, respectively. Consequently, a the second round between Sergio Massa and Javier Milei will be held on November 19, 2023.
We cannot predict the result of the ballotage, nor to determine the measures that may be adopted by the winners of the elections and their impact, which could have a material adverse effect on the Argentine economy and could affect our financial condition and results of our operations. Further, we cannot assure that the programs, policies and regulations currently in place will continue to be applicable in the future, nor their impact on our business.
29
In Brazil, the economy has experienced significant volatility in recent decades, characterized by periods of low or negative growth, high and variable levels of inflation and currency devaluation. Brazil’s GDP decreased 3.3% in 2016, increased 1.3% in 2017, increased 1.8% in 2018, increased 1.2% in 2019, decreased 3.9% in 2020, increased 4.6% in 2021 and increased 2.9% in 2022. Historically, Brazil’s political situation has influenced the performance of the Brazilian economy, and political crisis have affected the confidence of investors and the general public. As a result, Brazil continues to face uncertainty related to the future developments in policies and whether and when such policies and regulations may be implemented.
If the Latin American governments do not adopt reforms required to address structural changes, then the current economic conditions may be worsened, which could have an adverse effect on our financial condition and results of operations.
Latin American governments have exercised, and continue to exercise, significant influence over the economies of the countries in which we operate, which could adversely affect our business, financial condition, results of operations and prospects.
Historically, governments in Latin America have frequently intervened in the economies of their respective countries and have occasionally made significant changes in policy and regulations. Governmental actions to control inflation and other policies and regulations have often involved, among others, price controls, currency devaluations, capital controls and tariffs. Our business, financial condition, results of operations and prospects may be adversely affected by the changes in government policies or regulations of Latin American governments, including:
| ● | exchange rates and exchange control policies; |
| ● | tariff and inflation control policies; |
| ● | price control policies; |
| ● | liquidity of domestic capital and lending markets; |
| ● | tax policies, royalty and tax increases and retroactive tax claims; and |
| ● | other political, diplomatic, social and economic developments in or affecting the countries in which we operate. |
In addition, between 2006 and 2012, the Argentine government conducted expropriations including mainly utilities or companies in strategic sectors of public interest, like Aguas y Saneaminentos S.A. in 2006, Aerolíneas Argentinas S.A. in 2008 and YPF S.A. in 2012. In an unprecedented case in 2020, the government unsuccessfully attempted the expropriation of a company fully involved in the private sector with no public interest, like Vicentín S.A., which is the fourth largest grain exporter in Argentina, and, in addition, was undergoing a restructuring under reorganization proceedings. However, the government desisted due to generalized massive spontaneous protests and opposition from opposing political parties and the legal and financial community. In September 2014, the Argentine Congress enacted Law No. 26,991 which amends the Supply Law No. 20,680 (Ley de Abastecimiento) that enables the Argentine government to intervene in certain markets when it considers that any party to such market is trying to impose prices or supply restrictions in such market. The law grants broad powers to the relevant enforcing agency including to order the sale, production, distribution and/or delivery of basic needs goods throughout the country in case of a shortage of supply.
As significant portion of our assets are located in Argentina, we are subject to political uncertainties, including expropriation or nationalization of our business or assets, or subject to renegotiation or annulment of existing contracts and other similar risks. In the future, intervention in the economy by the Argentine government may continue or increase, the occurrence of which may adversely affect Argentina’s economy and, in turn, our business, results of operations and financial condition. We cannot assure investors that these or other measures that may be adopted by the Argentine government in the future, such as nationalizations, forced renegotiations or modifications of existing contracts, new tax policies, price fixing, regulations and reforms affecting foreign trade and investments, will not have a material adverse effect on the Argentine economy and, consequently, will not adversely affect our business, results of operations and financial condition.
30
Our business, results of operations and financial condition may be adversely affected by fluctuations in currency exchange rates relative to the U.S. dollar in the countries in which we operate.
The majority of our operations are based in Latin America and, accordingly, a significant portion of our costs are incurred in local currencies, while our revenues are primarily denominated in or influenced by U.S. dollars. Currencies in Argentina and Brazil have fluctuated significantly against the U.S. dollar in the past. Accordingly, fluctuations in exchange rates relative to the U.S. dollar could impair the comparability of our results from period to period and have a material adverse effect on our results of operations and financial condition.
The Argentine Peso depreciated 70.7% against the U.S. dollar in 2022, 20.0% in 2021, 40.5% in 2020, 58% in 2019, 102.1% in 2018 and 17.4% in 2017 based on the official exchange rates published by the Argentine Central Bank. In the past years, the Argentine government has imposed restrictions on the purchase of foreign currency. These measures gave rise to an unofficial market where the U.S. dollar trades at a different market value than the official Argentine Peso – U.S. Dollar exchange rate. The rigid exchange controls and transfer restrictions substantially limit the ability to obtain foreign currency or make certain payments or distributions out of Argentina. See “—Argentine exchange controls and restrictions limit access to the FX Market to make payments and distributions from our Argentine subsidiaries and receive the proceeds of any sale of our assets in Argentina”.
The Brazilian real has historically suffered frequent fluctuations. As a consequence of inflationary pressures, in the past, the Brazilian government has implemented various economic plans and adopted a number of exchange rate policies, including sudden devaluations, periodic mini-devaluations during which the frequency of adjustments has ranged from daily to monthly, floating exchange rate systems, exchange controls and dual exchange rate markets. Formally the value of the real against foreign currencies is determined under a free-floating exchange rate regime, but in fact the Brazilian government is currently intervening in the market, through currency swaps and trading in the spot market, among other measures, every time the currency exchange rate is above or below the levels that the Brazilian government considers appropriate, taking into account, inflation, growth, the performance of the real against the U.S dollar in comparison with other currencies and other economic factors. Periodically, there are significant fluctuations in the value of the Brazilian real against the U.S. dollar. In 2022, the Brazilian real appreciated 7.3% against the U.S. dollar. In 2021, the Brazilian real depreciated 9.1% against the U.S. dollar. In 2020, the Brazilian real depreciated 28.9% against the U.S. dollar. In 2019, 2018 and 2017 the Brazilian real depreciated 4.02%, 17.1% and 1.5%, respectively, against the U.S. dollar.
Our consolidated financial information included herein is presented in U.S. dollars. Fluctuations in exchange rates relative to the U.S. dollar could impair the comparability of our results from period to period and could have a material adverse effect on our results of operations and financial condition.
We are required to apply inflationary adjustments and tax indexation procedures in our Argentine subsidiaries, which could adversely affect our financial statements, results of operations and financial condition.
Pursuant to the International Accounting Standard 29, Financial Reporting in Hyperinflationary Economies (“IAS 29”), the financial statements of entities whose functional currency is that of a hyperinflationary economy must be adjusted for the effects of changes in a suitable general price index. IAS 29 does not prescribe when hyperinflation arises but includes several characteristics of hyperinflation. The IASB does not identify specific hyperinflationary jurisdictions. However, in June 2018, the International Practices Task Force of the Centre for Quality, which monitors “highly inflationary countries” categorized Argentina as a country with projected three-year cumulative inflation rate greater than 100%. Additionally, some of the other qualitative factors of IAS 29 were present, providing prima facie evidence that the Argentine economy is hyperinflationary for purposes of IAS 29. Therefore, Argentine companies using IFRS as adopted by the IASB are required to apply IAS 29 to their financial statements for periods ending on and after July 1, 2018.
Beginning with the period ending on December 31, 2018, Bioceres’ Argentine subsidiaries which have Argentine Peso as its functional currency began preparing financial statements in compliance with IFRS, adopting IAS 29. Effective July 1, 2022 our main subsidiaries changed their functional currency from the Argentine Peso to the United States Dollars as a result of changes in events and conditions relevant to their business operations. The effect of the functional currency change was recorded prospectively in accordance with IAS 21. As a result, from July 1, 2022 there are no longer significant effects of inflation adjustments in our financial statements. See “Item 5. Operating and Financial Review and Prospects.”
31
From a tax perspective, in December 2019, the Argentine Government promulgated Law 27,541. It provided that the tax rate reduction established by Law 27,430 (reduction of the income tax rate from 35% to 30% for fiscal periods beginning on January 1, 2018 until December 31, 2019, and 25% for fiscal periods beginning on or after January 1, 2020, inclusive) be suspended until the fiscal years beginning on or after January 1, 2021. Thus, the tax rate of 30% was maintained. Law 27,541 also provided that, for the first and second financial years starting on or after January 1, 2019, one-sixth of the inflation adjustment (provided by Law 27.420) will be computed in the fiscal year of the adjustment calculation and the remaining five-sixths in equal parts in the five tax periods immediately following.
On June 16, 2021, the Argentine Government enacted Law No. 27,630 which amended the Argentine Income Tax Law and replaced the fixed rate paid by Argentine companies on their corporate income from 30% to a progressive rate ranging from 25% to 35%. Depending on net incomes, companies will now have to pay a fixed amount and a progressive rate over the surplus of the minimum base rate in their category.
The reform also extends the 7% withholding tax on dividends for tax years beginning 1 January 2021 and thereafter. According to applicable law and if no additional tax reform is passed, for fiscal years beginning on or after January 1, 2021, 100% of the tax inflation adjustment (negative of positive) would be allocated by fiscal year.
We cannot predict the future impact that the application of IAS 29 and the eventual application of the tax indexation procedure and related adjustments will have on our Argentine subsidiaries’ financial statements or the effects on our business, results of operations and financial condition. Also, we cannot predict when the application of IAS 29 and the tax indexation will no longer be mandatory.
Economic and political developments in Argentina, including inflation and government controls may adversely affect the economy and our financial condition and results of operations.
Presidential and federal congressional elections in Argentina were held in October 2023, and the second round to determine the president will be held in November 2023, and their impact on the future economic and political environment is uncertain. No assurances can be made as to the policies that may be implemented by a new Argentine administration, or that political developments in Argentina, will not adversely affect the Argentine economy or our business, financial condition or results of operations. In addition, we cannot assure you that future economic, regulatory, social and political developments in Argentina will not impair our business, financial condition or results of operations, or cause the market value of our shares to decline.
In recent years, Argentina has confronted inflationary pressures, including the depreciation of the Argentine Peso, evidenced by significantly higher fuel, energy, and food prices, among other factors. Further, the Argentine government increased its direct intervention in the economy, including through the implementation of regulation of market conditions, expropriations or nationalizations and price controls. Therefore, the inflation rate in Argentina and the government’s intervention in the economy may adversely affect our business.
Further, according to data published by the Argentine National Institute of Statistics and Census (Instituto Nacional de Estadística y Censos) (“INDEC”), the Consumer Price Index (“CPI”) increased 80.2% from January through August 2023, 94.8% in 2022, 50.9% in 2021, 36.1% in 2020, 53.8% in 2019, 47.6% in 2018 and 24.8% in 2017. If inflation remains high or continues to rise towards a hyperinflation, Argentina’s economy will continue to be negatively impacted, and our results of operations could be materially adversely affected.
Limited access of the Argentine government and the private sector to the international capital markets could adversely affect our financial condition.
During recent years, the Argentine government and provinces have defaulted in the payment of their debt, which has limited their access as well as that of private companies to the international financial markets, or it has substantially increased their financing costs. Further, the COVID-19 pandemic has negatively impacted Argentina’s economy and deepened its recession which resulted in the need of restructuring of its sovereign debt.
Currently, the Argentine government has entered into facility agreements with the International Monetary Fund (the “IMF”) relating to an amount of approximately US$36 billion. Further, on August 4, 2023, Argentina’s government and Qatar signed a loan agreement in the amount of US$775 million in order to help Argentina repay US$1.411 million owed to the IMF regarding previous loan facilities. In addition, on August 23, 2023, the Argentine Minister of Economy announced agreements with the World Bank and the Inter-American Development Bank by which Argentina will be granted a financing totaling US$1,310 million. The funds will be destined to social, economic and infrastructure policies.
32
If the Argentine government defaults again on the payment of its sovereign debt or the measures adopted and to be adopted by the Argentine government to reduce the fiscal deficit, control inflation and stabilize the foreign exchange market are not effective, Argentina’s ability to obtain international or multilateral private financing or direct foreign investment may be limited, which may in turn impair its ability to implement reforms and public policies to foster economic growth, impair the ability of private sector entities to access the international capital markets or make the terms of such financing much less favorable that those accessible by companies in other countries in the region and may accelerate the depreciation of the Peso, foster inflation and deepen the economic crisis and recession. Lack of access to international or domestic financial markets or increase in the costs of such financing could affect the projected capital expenditures for our operations in Argentina, which, in turn, may have an adverse effect on our financial condition or the results of our operations.
An increase in export and import duties and controls may have an adverse impact on our business.
Since 2002, the Argentine government has imposed duties on the exports of various primary and manufactured products. Most such duties were suspended or subject to progressive reduction after the Macri administration took office in December 2015. However, in September 2018, general export duties were re-imposed and the progressive reduction of export duties on soybean products stopped. For example, a general additional export duty has been imposed on all exports of goods, levied on the lower of 12% of the good’s FOB value or AR$3 or AR$4 per U.S. dollar, depending on the goods.
On December 4, 2018, the Argentine Congress approved the budget bill for 2019 through Law No. 27,467, which amended the Customs Code to allow for duties to be applied to the exportation of services. In addition, the Argentine government was allowed to impose export duties of up to 30% until December 31, 2020. However, in case of services and goods that were not subject to export duties before September 2, 2018, the maximum rate was 12%. On January 2, 2019, the Argentine government issued Decree No. 1201/2018, which established an export duty on export of services at a rate of 12% with a maximum limit of AR$4 per each U.S. dollar of the amount arising from the invoice or equivalent document. On December 28, 2019, Decree No. 99/2019 extended the application of duties on export of services through December 31, 2021 with a rate of 5% without limit.
On December 21, 2019, the Argentine Congress enacted Law No. 27,541 (Ley de Solidaridad Social y Reactivación Productiva en el Marco de la Emergencia Pública, or the “Solidarity Law”), that established new maximum export duties of 33% for soybean, 15% for goods that were not subject to the payment of export duties as of September 2, 2018, 5% for agroindustry products of regional economies defined by the Argentine government, and 5% for industry goods and services.
As of February 29, 2020, the following crop exports were subject to the following export duties: soybean and soy products 30%, corn, wheat, barley, sunflower and sorghum 12%, corn and wheat flour 9% and sunflower oil 12%.
In October 2020, the Argentine government adopted a series of measures seeking to promote production, exports and the liquidation of U.S. dollars from exports in the FX Market, including the temporary reduction of export duties for soybean and soybean derived products through Decree No. 790/2020. Further, pursuant to Decree 790/2020, a temporary reduction scheme of export duties for soybean products and by-products was established, which increased on a monthly basis to return to the initial level as from January 2021.
In October 2022, Argentina has also imposed controls to the import of goods and services. In respect of import of goods, Argentine companies currently have limited access to the FX Market to acquire foreign currency in order to make payments abroad, provided that certain requirements are met, including the registration of the import transaction under the importation payment follow-up (Seguimiento de pagos de importaciones).
Different requirements apply to goods with customs entry registration, including, among others, that: (i) there is proof of the customs registry of the entry into the country of the goods that originated the payment to be canceled; (ii) the importer has a copy of the commercial invoice issued abroad in the name of the company who made the purchase abroad; and (iii) the documentation presented allows the importer to establish the expiration date of the foreign obligation. Goods with pending customs entry registration must comply with other requirements, including, among others: (i) documentation determining the existence of a purchase of goods abroad; (ii) a sworn statement submission demonstrating the registration of the customs entry of the goods within the term that corresponds according to the type of good to be imported; (iii) provision of elements that guarantee the reasonableness of the amounts to be paid considering the importing activity of the client in recent years and the business plans presented by the importer; and (iv) no delays in the regularization of payments with pending customs entry registration made as of September 2, 2019.
33
Recently, the BCRA strengthened the requirements for importers that intend to access to the FX Market by requesting an approved declaration submitted in the Argentine System of Imports (“SIRA”) from local authorities. If the SIRA declaration is approved, the local authorities will inform the importer the term in which the access to the FX Market will be granted. Such date usually ranges from 60 to 270 calendar days. Foreign exchange regulations provide for certain alternatives that would allow the Argentine importer to access the FX Market before the date stated in the SIRA. These alternatives may involve financing granted by a financial institution. The declaration of the SIRA will be analyzed by the AFIP, which will assess each individual situation based on information available in the AFIP’s records and the financial and economic capacity of the importer through the Economic Financial Capacity System (Sistema de Capacidad Económica Financiera) (the “CEF”).
In respect of import of services, there are currently restrictions imposed to Argentine companies looking to access the FX Market such as the limitation on the payment of imports of services. Prior approval by the BCRA is required to access the FX Market for prepayments and payment of services to foreign-related parties.
As of October 13, 2022, access to the FX Market for payments of services rendered by non-residents requires a declaration made through the System of Imports of the Argentine Republic and Payments of Services Abroad (“SIRASE”), approved by the Secretary of Commerce.
The purpose of the SIRASE is to determine whether importers willing to make payments of services abroad have complied with tax obligations and are capable of carrying out certain financial transactions. Once the SIRASE declaration is submitted, the AFIP will review the information provided and confirm the importer’s compliance regarding tax return filings and its financial economic capacity through the CEF regime.
On April 20, 2023, through Communication “A” 7746, BCRA modified foreign exchange regulations regarding foreign currency outflows. This amendment had a strong impact, mainly in the access to the FX Market, in the payment of services rendered by non-Argentine residents. BCRA’s prior approval must be requested to access the FX Market prior to 60 calendar days from the date of approval of the SIRASE declaration for research, legal and accounting, advertising, and engineering services, among others. Such prior approval will not be required, for example, if using own funds in foreign currency. Also, payment to related parties abroad for freight and transportation services must be made 90 calendar days after the date that the service has been rendered.
We cannot make assurances or predictions that there will not be further increases in the export duties or that other new export duties, taxes, quotas or restrictions will not be imposed. The imposition of new export duties, taxes, quotas or restrictions or a significant increase in existing export duties or the application of export quotas or the imposition of regimes that aim to restrict or control imports and exports could adversely affect our financial condition or results of operations.
A decline in the global prices of Latin America’s main commodity exports could have an adverse effect on Latin America’s economic growth.
A decline in the prices of the commodities that Latin America countries export, such as wheat and soy, could have an adverse effect on the region’s economic growth. High commodity prices have contributed significantly to the increase in Latin American exports as well as governmental revenues from export taxes. However, relying on the export of certain commodities has made the Latin American economy more vulnerable to fluctuations in the prices of commodities. A decline in global commodity prices negatively impacts ability of Latin American countries to service their sovereign debt as a result of reduced government revenue and, available foreign, which may result in recessionary or inflationary pressures. Either of these results would adversely impact the prospects of economic growth in Latin America and, therefore, our financial condition and results of operations. Further, we may not be able to anticipate or react to such volatility in a timely manner, which could cause our operating results to deteriorate.
The Argentine government may order special protections for employees in the private sector.
Due to high levels of inflation, the Argentine government could order salary increases for employees in the private sector, as occurred in the past. Notwithstanding the above, no mandatory salary increase has been ordered by the Argentine government since January 2020.
As per the current context, the prohibition on terminations and duplication of severance are not in force. A company is entitled to dismiss, without cause, employees at its convenience, as long as severance is paid in duly manner and time. However, considering the current high levels of inflation and devaluation, we cannot assure that the government may create special measures against further salary depreciation.
34
Furthermore, during 2023, salary increases in the private sector have been more regular than previously reported. However, there have been claims by employees that the increase in salary were not following inflation rates.
In addition, the presidential elections held on October 23, 2023, and the results of the second round of the presidential election, which will be held on November 19, 2023, may have an impact on the economic, labor and salary measures that may be adopted, such as a possible labor reform.
The disposition or sale of our ordinary shares may be subject to taxation in Argentina.
Under the Argentine Income Tax Law, gains realized from the indirect sale or disposal of assets located in Argentina, including shares or other equity participations in Argentine companies by an entity or individual not resident in Argentina (“Non-Argentine Resident”) are taxable under certain conditions, as if a direct sale took place (the “Tax on Indirect Sales”).
The Argentine Income Tax Law establishes presumption of income from Argentine source on the sale or disposition by Non-Argentine Residents of shares and participations (or rights to receive such shares or participations) in foreign entities whose underlying assets are fully or partially located in Argentina, as long as the following conditions are met:
| ● | At least thirty percent of the value of the shares, participations or rights of the foreign entity, at the time of sale or in any of the 12 previous months, derives from assets that the entity owns directly or indirectly in Argentina. For this purpose, such Argentine assets or rights will be valued at their fair market value and will include, among others, shares or other forms of ownership, control or participation in the profits of a company incorporated in Argentina; and |
The securities or rights of the foreign entity being sold or disposed represent, at least, ten percent of the equity of that entity, at the time of their disposal or in any of the 12 previous months. For purposes of this calculation, ownership of related entities, spouses and other relatives must be considered jointly.
| ● | The relevant shares and participations in the foreign entity have been acquired on or after January 1, 2018. |
In case the Tax on Indirect Sales applies, the Argentine source gain on which the Tax on Indirect Sales will be calculated is a proportion to the value of the Argentine assets held by the foreign entity with respect to the total value of the securities or rights being transferred.
The Tax on Indirect Sales is levied at a 15% rate on the net capital gain (gross sale price less acquisition cost), or at a 13.5% effective rate on the gross sale price, at the option of the seller. However, such rate may be reduced if a Treaty to Avoid Double Taxation applies to the seller. If the Tax on Indirect Sales becomes applicable and the tax is applied on a net basis, pursuant to current income tax rules, the acquisition costs may be adjusted by inflation to calculate the net capital gain.
The mechanism to comply with the payment of the Tax on the Indirect Sales depends on the tax residency of the buyer: (i) if the buyer or acquirer is an Argentine corporate entity (in general, entities organized or incorporated under Argentine law, certain traders and intermediaries, local branches of foreign entities, sole proprietorships and individuals carrying on certain commercial activities in Argentina) (“Argentine Entity”) or an Argentine resident individual (“Argentine Individual”), by the Argentine Entity or Argentine Individual who will act as withholding agent, (ii) if the buyer is not an Argentine Entity or and Argentine Individual, the tax has to be paid by the seller or transferor directly (through an international wire transfer or through its legal representative in Argentina, if any). There are specific rules if the non-Argentine resident is a resident in a non-cooperative jurisdiction or the funds invested proceed from a non-cooperative jurisdiction as defined in the Income Tax Law.
Argentine Income Tax Law sets forth that the Tax on Indirect Sales does not apply to transfers made within the same economic group. Pursuant to the Regulatory Decree of the Argentine Income Tax Law, transfers within the same economic group would take place when the seller or sellers participate or participates, directly or indirectly, in at least 80% of the transferee’s capital, or vice versa, or when one or more entities participate, directly or indirectly, in at least 80% of the transferor’s and transferee’s capital, and such participations have been held for at least two years prior to the transfer.
35
The payment of the In-Kind Consideration under the Rizobacter Call Option may be subject to taxation in Argentina.
Our ordinary shares delivered to grantors in connection with the payment of the In-Kind Consideration under the Rizobacter Call Option would be subject to the Tax on Indirect Sales at our level, levied on the gain resulting from the difference between our ordinary shares’ tax basis (acquisition cost, equal to the redemption price of such shares) and the value received by us in connection with the payment of the In-Kind Consideration. Any resulting Tax on Indirect Sales would be calculated on the proportion of the value that the Argentine assets represent with respect to the total value of our ordinary shares delivered and should be paid to the Argentine tax authorities by our legal representative in Argentina. However, to the extent that the value received by us in connection with the delivery of our ordinary shares and satisfaction of the In-Kind Consideration equals the redemption price of such shares, and as a result equals the acquisition costs, there would be no tax basis for the Tax on Indirect Sales.
Argentine exchange controls and restrictions limit access to the FX Market to make payments and distributions from our Argentine subsidiaries and receive the proceeds of any sale of our assets in Argentina.
The Argentine government has imposed strict exchange controls and transfer restrictions, substantially limiting the ability of legal entities and individuals to obtain foreign currency or make certain payments or distributions out of Argentina. See “—Item 10. Additional Information—D. Exchange Controls.”
On September 15, 2020, Communication “A” 7106 of the Argentine Central Bank restricted the access to the FX Market for the payment of principal under foreign financial debt with third parties (other than with international or multilateral credit organizations) in excess of US$1,000,000 per month in the aggregate with maturities between October 15, 2020 and March 31, 2021 (the “Temporal Scope”) to an amount equal to up to 40% of the amount originally due; and provided that the remaining unpaid principal balance is refinanced through a new foreign financial debt with an average life of at least two years (the “Refinancing Plan”).
On February 25, 2021, the Argentine Central Bank issued Communication “A” 7230, which modified certain aspects of Communication “A” 7106 and extended the Temporal Scope through December 31, 2021. The main provisions of Communication “A” 7230 included: (i) the increase in the amount allowed for access to the FX Market without filing a Refinancing Plan from US$1,000,000 to US$2,000,000; and (ii) expressly excluded from the obligation to furnish a Refinancing Plan with respect to debt refinancing closed during 2020 which allowed achieving the parameters established by Communication “A” 7106.
On April 12, 2022, the Argentine Central Bank issued Communication “A” 7490, which extended the Temporal Scope through December 31, 2022. For further information, see “—Item 10. Additional Information—D. Exchange Controls.” On April 4, 2023, the Argentine Central Bank issued Communication “A” 7746, which extended the Temporal Scope through December 31, 2023. For further information, see “—Item 10. Additional Information—D. Exchange Controls.”
In response to the reinstatement of the foreign exchange restrictions, an unofficial U.S. dollar trading market developed in which the Peso-U.S. dollar exchange rate differs substantially from the official Peso-U.S. dollar exchange rate, and the use of blue-chip swaps expanded which, are much more expensive than acquiring foreign currency through FX Market at the official exchange rate. Exchange controls have generally affected the level of international reserves deposited with the Argentine Central Bank, which decreased to US$38.3 billion as of September 23, 2022. See “—Our business, results of operations and financial condition may be adversely affected by fluctuations in currency exchange rates relative to the U.S. dollar in the countries in which we operate.”
All these measures could lead to political and social tensions and undermine the Argentine government’s public finances, as has occurred in the past, which could adversely affect Argentina’s economy and prospects for economic growth, and could adversely affect our business and results of operations.
In addition, we may be impaired from receiving payments and transfers from our Argentine subsidiaries or such payments and transfers could be subject to substantial additional costs which, in either case, could adversely affect our business and results of operations.
Foreign exchange restrictions have reinstated the mandatory repatriation of export receivables.
Since September 2, 2019, the Argentine government has reinstated the mandatory repatriation into Argentina and the conversion into Pesos through the FX Market of the proceeds from the collections of foreign currency outside of Argentina by Argentine residents in connection with exports of goods within certain specific dates. See “—Item 10. Additional Information—D. Exchange Controls.”
36
Regardless of the applicable maximum terms described above, upon collection of the export receivables, the proceeds thereof are subject to the mandatory repatriation within the five consecutive days computed from the date of payment or collection.
In addition, the Argentine government also reinstated the mandatory repatriation into Argentina and the conversion into Pesos through the FX Market of the receivables for export of services within five consecutive days computed from the date they are received.
The reinstatement of the repatriation of export of goods and services receivables and the additional restrictions imposed on the access to the FX Market could have a material adverse effect on our business, results of operations and financial condition.
Changes in Argentine tax laws may adversely affect the results of our operations, financial condition and cash flows.
On December 29, 2017, the Argentine government enacted the Tax Reform, which reduced the corporate income tax rate from 35% to 30% for fiscal years beginning on or after January 1, 2018 and 25% for fiscal years beginning on or after January 1, 2020. Additionally, the distribution of dividends is subject to income tax withholding of 7% tax rate if the distributed profits derive from the fiscal years beginning on or after January 1, 2018 or 13% tax rate if the distributed profits derive from fiscal years beginning on or after January 1, 2020. The 13% domestic rate may be reduced by application of tax treaties signed by Argentina. However, on December 23, 2019, the Solidarity Law suspended the reduction of the corporate tax rate for Argentine Entities to 25% (with dividends being taxed at 13%) and established that 30% rate (with dividends being taxed at 7%) remains applicable until fiscal years initiated until January 1, 2021. Consequently, the effectiveness of the 25% and 13% tax rates has been delayed until tax years commencing after January 1, 2021.
On June 16, 2021, the Argentine Government enacted Law No. 27,630 which amended the Argentine Income Tax Law and replaced the fixed rate paid by Argentine companies on their corporate income from a fixed 30% to a progressive rate ranging from 25% to 35%. Depending on net incomes, companies will now have to pay a fixed amount and a progressive rate over the surplus of the minimum base rate in their category. Such income amounts will be adjusted once a year starting January 1, 2022. To that effect, the annual variation of the CPI stipulated by the INDEC will be computed.
The applicable rate over dividends paid to shareholders, whether they are individuals or undivided estates residing in Argentina or non-Argentine residents, will continue to be 7% in all cases, regardless of the tax rate paid by the company at the corporate level.
The Solidarity Law also introduced amendments to the excise tax on certain goods, tax on debits and credits in local bank accounts and social security rules. It also established a new tax on certain purchases of foreign currency by Argentine residents, a new tax debt settlement plan for certain taxpayers, and established new rates on exports of goods and services.
Argentine companies are required to pay the personal assets tax corresponding to Argentine resident individuals, foreign individuals and foreign entities for holding equity interests in such companies as of December 31 of each year. The applicable tax rate until 2018 was 0.25% and the tax is levied on the equity stated in the latest financial statements.
Under the Solidarity Law, the tax rate applicable to shares or participations in the capital of companies governed by the Argentine General Companies Act was increased from 0.25% to 0.50% of the pro-rata equity value.
On December 1, 2022, Law No. 27,701 introduced certain changes to the Income Tax Law related to the tax inflation adjustment. Taxpayers that determine a positive tax inflation adjustment (situation which entails a higher tax liability) in the first and second fiscal year starting on or after January 1, 2022, may compute one third of the resulting amount of such adjustment in that fiscal period and the remaining two thirds, in equal parts, in the following two fiscal periods. The deferral will only be applicable to taxpayers whose investment in the purchase, construction, manufacture, processing or definitive import of fixed assets, except automobiles, during each of the two fiscal periods immediately following the computation of the first third of the period in question, is equal to or exceeds AR$30,000 million.
Non-Argentine residents are subject to Argentine personal assets tax for holding shares and other equity participations in Argentine companies as of December 31 of each year at a rate of 0.50%, which is levied on the proportional net worth value (valor patrimonial proporcional) of the shares arising from the last balance sheet. Argentine companies are obliged to pay the tax on behalf of their Non-Argentine resident shareholders, partners or owners and are entitled to seek reimbursement from them.
37
We cannot assure that the Argentine government or any of its political divisions will not adopt additional changes and reforms in tax matters, nor that these reforms and those that may be adopted in the future will not adversely affect our business, results of operations or financial condition; or our Non-Argentine Residents affiliates or subsidiaries in connection with their holding of shares and equity in Argentine companies.
Risks Related to Our Ordinary Shares
Our share repurchase program may reduce liquidity.
On May 6, 2020, our board of directors approved a program to repurchase our own securities. As described in Item 10 of this report, we may repurchase up to US$10,000,000 to enhance the allocation of capital to certain equity-finance obligations (the “Buy-Back Program”). As of June 30, 2023, we have acquired 667,578 ordinary shares under the Buy-Back Program. Future purchases, if any, would be funded from our cash balances and could reduce our financial liquidity and indirectly add to our indebtedness, as well as reduce liquidity of our ordinary shares.
There can be no assurance that we will be able to comply with the continued listing standards of the Nasdaq Global Select Market.
Our ordinary shares are currently listed on Nasdaq. Our continued eligibility for listing may depend on, among other things, the amount of “public float” (equity held by non-affiliates). If Nasdaq delists our ordinary shares from trading on its exchange for failure to meet the listing standards, our shareholders could face significant material adverse consequences including:
| ● | a limited availability of market quotations for our ordinary shares; |
| ● | reduced liquidity and in ability to sell our ordinary shares; |
| ● | a determination that our ordinary shares are a “penny stock” which will require brokers trading in our ordinary shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our ordinary shares; |
| ● | a limited amount of news and analyst coverage; and |
| ● | a decreased ability to issue additional ordinary shares or obtain additional financing in the future. |
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Because our ordinary shares are listed on Nasdaq or another national securities exchange, they are covered securities. If our securities were no longer listed on Nasdaq, they would not be covered securities and we would be subject to regulation in each state in which we offer securities.
Sales of a substantial number of our ordinary shares in the public market by our shareholders could adversely affect the market price of our ordinary shares.
Sales of a substantial number of our ordinary shares in the public market by our shareholders, or the perception that those sales might occur, could depress the market price of our ordinary shares and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that such sales may have on the prevailing market price of our ordinary shares.
Conversion of the Notes would increase the number of ordinary shares and result in dilution to shareholders.
On August 5, 2022, we issued an aggregate principal amount of US$55.0 million secured guaranteed convertible notes (the “Secured Convertible Guaranteed Notes due 2026”, and together with the Secured Guaranteed Notes due 2026, the “Notes”) pursuant to a note purchase agreement (the “JRL NPA”) among us, Jasper Lake Ventures One LLC, Redwood Enhanced Income Corp, Liminality Partners LP, the holders from time to time party thereto and Wilmington Savings Fund Society, FSB, as collateral agent.
38
The Secured Convertible Guaranteed Notes due 2026 are convertible into our ordinary shares. The issuance of a substantial number of additional ordinary shares upon conversion of the Secured Convertible Guaranteed Notes due 2026 will result in dilution to the then existing holders of our ordinary shares and will increase the number of ordinary in the public market. Sales of a substantial number of our ordinary shares in the public market could adversely affect the market price of our ordinary shares.
We are a “controlled company” as defined in the Nasdaq rules and, as a result, qualify for exemptions from certain corporate governance requirements.
Bioceres PLC controls, directly or indirectly, a majority of the voting power of our outstanding shares. Under Nasdaq rules, a listed company of which more than 50.0% of the voting power for the appointment of directors is held by any person or group of persons acting together is a “controlled company” and may elect not to comply with certain Nasdaq corporate governance requirements, including the requirement (i) that a majority of the board of directors consist of independent directors, as defined under the Nasdaq rules and (ii) to have a compensation committee and a nominating and governance committee, although we have established a compensation committee and a nominating and governance committee, the majority of which consists of independent directors. We have decided to be treated as a “controlled company” and, even though three members of our board of directors and a majority of the members of our compensation committee and our nominating and governance committee (that we chose to establish) consists of independent directors, you may not have the same protections afforded to shareholders of companies that are subject to all of the Nasdaq corporate governance requirements.
The price of our ordinary shares may fluctuate, and you may not be able to resell our ordinary shares at or above the price you paid.
Fluctuations in the price of our ordinary shares could contribute to the loss of all or part of your investment. If an active market for our ordinary shares develops and continues, the trading price of our ordinary shares could be volatile and subject to wide fluctuations in response to various factors, some of which are beyond our control. Any of the factors listed below could have a material adverse effect on your investment in our ordinary shares and our ordinary shares may trade at prices significantly below the price you paid for them. In such circumstances, the trading price of our ordinary shares may not recover and may experience a further decline.
Factors affecting the trading price of our ordinary shares may include, among others:
| ● | actual or anticipated fluctuations in our interim financial results or the interim financial results of companies perceived to be similar to us; |
| ● | our public float relative to the total number of ordinary shares that are issued and outstanding; |
| ● | announcements of technological innovations, new products or services or new commercial relationships by us or our competitors; |
| ● | disruption to our operations; |
| ● | announcements concerning our competitors or the pest management industry in general; |
| ● | our entry into, modification of or termination of key license, research and development or collaborative agreements; |
| ● | changes in the market’s expectations about our operating results; |
| ● | the public’s reaction to our press releases, our other public announcements and our filings with the SEC; |
| ● | speculation in the press or in the investment community; |
| ● | success of competitors; |
| ● | the operating results failing to meet the expectation of securities analysts or investors in a particular period; |
| ● | changes in financial estimates and recommendations by securities analysts concerning our ordinary shares or the market in general; |
39
| ● | operating and stock price performance of other companies that investors deem comparable to us; |
| ● | our ability to market new and enhanced products on a timely basis; |
| ● | changes in laws and regulations affecting our business; |
| ● | commencement of, or involvement in, litigation involving us; |
| ● | changes in our capital structure, such as future issuances of ordinary shares or the incurrence of additional debt; |
| ● | the volume of our ordinary shares available for public sale; |
| ● | any major change in our board of directors or management; |
| ● | sales of substantial amounts of our ordinary shares by our directors, officers or significant shareholders or the perception that such sales could occur; and |
| ● | general economic and political conditions such as recessions, interest rates, fuel prices, international currency fluctuations and acts of war or terrorism. |
Broad market and industry factors may materially harm the market price of our ordinary shares irrespective of our operating performance. The stock market in general and Nasdaq have experienced price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the particular companies affected. The trading prices and valuations of these stocks, and of our ordinary shares, may not be predictable. A loss of investor confidence in the market for the stocks of other companies which investors perceive to be similar to us could depress our share price regardless of our business, prospects, financial conditions or results of operations. A decline in the market price of our ordinary shares also could adversely affect our ability to issue additional ordinary shares and our ability to obtain additional financing in the future.
In the past, securities class action litigation has often been initiated against companies following periods of volatility in their stock price. This type of litigation could result in substantial costs and divert our management’s attention and resources and could also require us to make substantial payments to satisfy judgments or to settle litigation.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business, or our market, or if they change their recommendations regarding our ordinary shares adversely, then the price and trading volume of our ordinary shares could decline.
The trading market for our ordinary shares will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market, or our competitors. Securities and industry analysts do not currently, and may never, publish research on us. If no securities or industry analysts commence coverage of us, our ordinary share price and trading volume would likely be negatively impacted. If any of the analysts who may cover our operations change their recommendation regarding our ordinary shares adversely, or provide more favorable relative recommendations about our competitors, the price of our ordinary shares would likely decline. If any analyst who may cover our operations were to cease coverage or fail to regularly publish reports on it, we could lose visibility in the financial markets, which could cause our ordinary share price or trading volume to decline.
We are incorporated under the laws of the Cayman Islands. Accordingly, you may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. Federal courts may be limited.
The Company is an exempted company incorporated under the laws of the Cayman Islands and a majority of our officers and directors are residents of jurisdictions outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon our directors or executive officers, or enforce judgments obtained in the United States courts against our directors or officers.
40
Our corporate affairs are governed by our amended and restated memorandum and articles of association (the “Articles”), the Cayman Islands Companies Act (As Revised) (as the same may be supplemented or amended from time to time) or the common law of the Cayman Islands. We are also subject to the federal securities laws of the United States. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, the decisions of whose courts are of persuasive authority, but are not binding on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are different from statutes or judicial precedent in some jurisdictions in the United States, and certain states, such as Delaware, may have more fully developed and judicially interpreted bodies of corporate law. In particular, the Cayman Islands has a different body of securities laws as compared to the United States. In addition, Cayman Islands companies may not have standing to initiate a shareholders derivative action in a Federal court of the United States.
We have been advised by our Cayman Islands legal counsel that the courts of the Cayman Islands are unlikely (i) to recognize or enforce against us judgments of courts of the United States predicated upon the civil liability provisions of the federal securities laws of the United States or any state; and (ii) in original actions brought in the Cayman Islands, to impose liabilities against us predicated upon the civil liability provisions of the federal securities laws of the United States or any state, so far as the liabilities imposed by those provisions are penal in nature. In those circumstances, although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will recognize and enforce a foreign money judgment of a foreign court of competent jurisdiction without retrial on the merits based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given provided certain conditions are met. For a foreign judgment to be enforced in the Cayman Islands, such judgment must be final and conclusive and for a liquidated sum, and must not be in respect of taxes or a fine or penalty, inconsistent with a Cayman Islands judgment in respect of the same matter, impeachable on the grounds of fraud or obtained in a manner, and or be of a kind the enforcement of which is, contrary to natural justice or the public policy of the Cayman Islands (awards of punitive or multiple damages may well be held to be contrary to public policy). A Cayman Islands court may stay enforcement proceedings if concurrent proceedings are being brought elsewhere.
As a result of all of the above, public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors or controlling shareholders than they would as public shareholders of a United States company.
We expect to incur increased costs as a result of being a public company, particularly as we have ceased to qualify as an “emerging growth company”.
As a public company, we expect to incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the SEC and Nasdaq Global Select Market, impose various requirements on the corporate governance practices of public companies. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, in the assessment of the emerging growth company’s internal control over financial reporting. The JOBS Act also permits an emerging growth company to delay adopting new or revised accounting standards until such time as those standards apply to private companies.
As we have ceased to be an “emerging growth company,” we have expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 and the other rules and regulations of the SEC. Operating as a public company also makes it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. In addition, we expect to incur additional costs associated with our public company reporting requirements. It may also be more difficult for us to find qualified persons to serve on our board of directors or as executive officers.
41
As a foreign private issuer, we are exempt from certain disclosure requirements under the Exchange Act, which may afford less protection to our shareholders than they would enjoy if we were a domestic U.S. company.
As a foreign private issuer, we are exempt from, among other things, the rules prescribing the furnishing and content of proxy statements under the Exchange Act. In addition, our executive officers, directors and principal shareholders are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the Exchange Act. We are also not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as domestic U.S. companies with securities registered under the Exchange Act. As a result, our shareholders may be afforded less protection than they would under the Exchange Act rules applicable to domestic U.S. companies.
Historically, we have not paid any dividends.
We have not paid any cash dividends on our ordinary shares to date. The payment of cash dividends on our ordinary shares in the future will be dependent upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that our board of directors and shareholders may deem relevant. Our board of directors does not anticipate declaring any dividends on our ordinary shares in the foreseeable future. As a result, any gain you will realize on our ordinary shares will result solely from the appreciation of such shares.
We may issue additional securities in the future, which may result in dilution to our shareholders.
We are not restricted from issuing additional ordinary shares or securities convertible into or exchangeable for ordinary shares. Because we may need to raise additional capital in the future to operate and/or expand our business, we may conduct additional equity offerings. To the extent our outstanding options are exercised, or we conduct additional equity offerings, additional ordinary shares will be issued, which may result in dilution to our shareholders. Sales of a substantial number of our ordinary shares in the public market could adversely affect the market price of our ordinary shares.
ITEM 4.INFORMATION ON THE COMPANY
A.History and Development of the Company
General Overview
We are a leading company in the development and commercialization of productivity solutions designed to regenerate agricultural ecosystems while making crops more resilient to climate change. We have a unique biotech platform with high-impact, licensed and patented technologies for seeds and microbial ag-inputs, as well as biological and next generation conventional crop nutrition and protection solutions. Along with our HB4® – drought tolerant seed technology, we also bring digital solutions to support growers’ decisions and provide end-to-end traceability for production outputs.
As of June 30, 2023, we owned or licensed more than 1,600 brands and more than 550 registered products and, either exclusively or non-exclusively, over 750 patents and patent applications. Our product portfolio includes (i) biological crop nutrition products such as seed inoculants, biofertilizers or biostimulants, (ii) a full range of biocontrol products including bionematicides, biofungicides and bioinsecticides, (iii) next generation crop nutrition and crop protection products such as microbeaded fertilizers and adjuvants, and (iv) seed germplasm, traits and ready-to-use seed pack solutions.
We are a global company, and our agricultural inputs are marketed across more than 45 countries, primarily in South America, the United States and Europe. Our total revenue and Adjusted EBITDA for the year ended June 30, 2023 totaled US$420.1 million and US$81.2 million, respectively. Adjusted EBITDA is a non-IFRS financial measure. See “Item 5. Operating and Financial Review and Prospects—A. Operating Results—Non-IFRS Financial Measures” and “Item 3. Key Information—D. Risk Factors—Risks Related to Our Business” for information regarding our use of Adjusted EBITDA and a reconciliation of net profit to Adjusted EBITDA.
We sell our products through our international subsidiaries and a sales and marketing team of more than 200 people. We enjoy exceptional access to the end-users (farmers) as a result of: (i) our strategic alliances and partnerships with global agriculture leaders and our joint ventures; (ii) the shareholders of Bioceres PLC, which is the majority holder of Bioceres S.A. (the “Parent”), who collectively control significant agricultural land in South America; and (iii) our longstanding relationships with more than 1,500 dealers and distributors. Our customers include global blue-chip companies and industry leaders, large distributors, co-ops and dealers, as well as large farmers and individual growers.
42
Our leading infrastructure, the success of our unique technology platform, and a commanding presence in key markets have made us a flagship agricultural solutions provider, as well as the natural partner for global conglomerates.
Our History
Bioceres Crop Solutions Corp is a Cayman Islands exempted company incorporated on November 14, 2017.
Our principal executive offices are located at Ocampo 210 bis, Predio CCT, Rosario, Province of Santa Fe, Argentina. Our telephone number at that address is +54 341 486-1100. The address of our registered office in the Cayman Islands is P.O. Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. Our agent for service of process in the United States is Cogency Global Inc., located at 122 E. 42th Street, 18th Floor, New York, NY 100168. Our website is www.biocerescrops.com and the SEC also maintains a website at http://www.sec.gov which contains reports and other information regarding registrants that file electronically with the SEC. The information on our website is not incorporated by reference into this annual report.
We have developed our business by establishing joint ventures and collaborations, in addition to implementing an acquisitive growth strategy. Our joint ventures with industry leaders, such as Florimond Desprez, De Sangosse and Arcadia Biosciences, and non-joint venture collaborations with companies such as Syngenta, Corteva and Momentive, have allowed us to bring our products to market in an efficient and cost-effective manner. In addition, we have acquired and created multiple companies. For more details, see “Item 4. Information on the Company —B. Business Overview— Joint Ventures and Key Collaborations” and “Item 4. Information on the Company —A. History and Development of the Company — Significant Transactions.”
The timeline below illustrates the establishment of key joint ventures and our main acquisitions, which are discussed below.
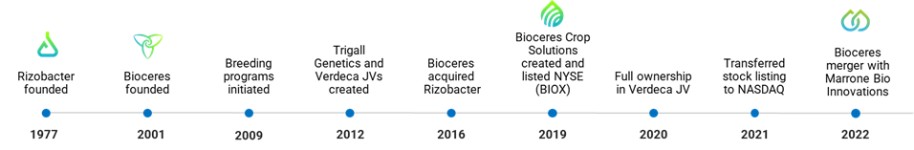
The Parent was founded in 2001 by a group of farmers and agronomists in Argentina that partnered to address the demand for higher crop productivity in a sustainable and environmentally conscious way.
In October 2016, we acquired a controlling stake in Rizobacter S.A., a global leader in biological products and a pioneer in liquid inoculants, high-tech adjuvants, and specialty fertilizers, with more than 46 years history of excellence.
On March 14, 2019, Union Acquisition Corp. (“Union” or “UAC”) completed a business combination pursuant to a share exchange agreement, dated as of November 8, 2018 (as amended, the “Exchange Agreement”), by and among UAC and Bioceres, Inc., a company incorporated under the laws of Delaware, which converted into Bioceres LLC on February 28, 2019. Upon the consummation of the business combination, Union changed its name to Bioceres Crop Solutions Corp, and our ordinary shares and public warrants started trading on the NYSE American.
On July 27, 2020, we successfully and cost-effectively removed all 24.2 million outstanding warrants from our capital structure, with 90% of holders electing to tender their warrants and 99% of these holders choosing to receive our shares in exchange. Following completion of the offer to exchange, we implemented the amendment to the existing warrant agreement and redeemed the remaining warrants, thereby retiring all our warrants.
On November 12, 2020, we acquired from Arcadia Biosciences the remaining ownership interest in Verdeca LLC, aiming to accelerate the breeding and the go-to market strategies. As part of this transaction, we gained full access and control of Verdeca’s soybean library of genome modified materials used to develop new quality and productivity, as well as exclusive rights to all Arcadia technologies that are applicable to soybean. In addition, through this transaction, we obtained rights to Arcadia’s quality wheat traits and the related Good Wheat™ brand for Latin America and other GLA non-core assets.
On April 26, 2021, we voluntarily transferred our stock exchange listing from the NYSE American to The Nasdaq Global Select Market, in order to raise our visibility as an ag-tech company focused on sustainable solutions.
43
On July 12, 2022, we announced the closing of the Pro Farm Merger in which Pro Farm merged into us pursuant to the Merger Agreement. Pro Farm is a leading pure-play company in the agricultural biologicals space, with a track record of revenue growth and margin expansion. The Pro Farm Merger combined our expertise in bionutrition and seed care products with Pro Farm’s expertise in developing biological crop protection and plant health solutions, creating a global leader in the development and commercialization of sustainable agricultural solutions.
Since our founding, we believe that we have built one of the leading fully-integrated biotechnology platforms of its kind to source, validate, develop and commercialize agricultural productivity technologies that regenerate agricultural ecosystems while making crops more resilient to climate change. Together with our subsidiaries, we have a diverse customer base, comprehensive product portfolio and expanded geographic reach across a wide range of crops well positioned to serve this significant market opportunity.
Competitive Strengths
Our diversified platform generates revenues through multiple technologies, customers, distribution channels and end-markets, providing us with a profitable growth trajectory. Our key competitive strengths include:
Pioneering high impact technology platform with strong IP
Our greatest competitive strength lies in the uniqueness and innovative nature of our portfolio of technologies, designed to address the large market opportunity that stems from the need to reduce agriculture’s impact on climate change and biodiversity loss.
We are pioneers in the agriculture biotechnology industry as the first and only company in the world to develop a drought-tolerance technology for soybean and wheat cropping systems. We are also the first non-governmental Latin America-based entity with an approved biotech event in a major global crop.
We are leaders in the development and commercialization of high impact, patented products that support regenerative agriculture, one of the most dynamic segments of agriculture, with one of the broadest and most innovative portfolio and pipeline for biologicals. The merger with Pro Farm combined our expertise in bionutrition and seed care products with Pro Farm’s leadership in the development of biological crop protection and plant health solutions, positioning us to serve all major agriculture input categories, conventional, organic and regenerative, with low environmental impact solutions and with our now large commercial footprint in the Americas and Europe.
Protecting our technologies and products under intellectual property rights allows us to offer the agricultural value chain unique and highly differentiated products and technologies, improving our competitiveness in the market. We believe that our patent and trademark portfolio is among the most competitive globally. As of June 30, 2023, we have identified and sought patent protection in our capacity as either title holder or licensee, or as exclusive or non-exclusive licensee, for over 750 patents or patent applications. Through our subsidiaries Rizobacter and Pro Farm, we have over 1,150 trademarks and over 300 applications globally.
Strong R&D engine to sustain long-term organic growth
Our robust R&D engine, in synergy with our subsidiaries, serves as the driving force behind our sustained, long-term organic growth.
Our strategy for acquiring technology and products relies on strategic partnerships, collaborative ventures, and enduring relationships with research institutions and scientists. Our R&D pipeline is dedicated to creating biological products that not only improve crop performance but also promote environmental sustainability. In integrating Bioceres’ legacy R&D platform with capabilities from Pro Farm, we formed what we believe is a world-class research and development team fully focused on upgrading our portfolio of commercially available technologies. We are enhancing the efficiency of adjuvants and micro-beaded fertilizers through their integration with biological inputs. Our Integrated Seed projects develop integrated products for soybean and wheat that comprise several technologies and are targeted to specific geographic regions.
With over 100 active projects, we are advancing biotechnology traits, biofungicides, bioinsecticides, bioherbicides, bio-stimulants, and inoculants, as well as developing soybean and wheat varieties. All of these endeavors exemplify our commitment to achieving sustainable organic growth.
44
Capital-Efficient, Risk Mitigated Development Model
Development and regulatory approval for our products and technologies requires a highly evolved and complicated process that can take more than a decade. Furthermore, capital allocation requirements can be onerous due to the expensive discovery activities usually associated with life sciences research and strict requirements for regulatory approvals.
We believe that we have created a highly competitive and capital efficient, independent platform for developing such products and technologies. We consider Bioceres PLC, through the Parent, to be the premier partner for advanced validation of promising research leads developed by research institutions in Argentina, most of which do not have the necessary capabilities for this purpose. During the technology sourcing process, Bioceres PLC designs and develops an efficient open architecture model that allows the scientific community (public institutions, other companies, entrepreneurs, etc.) to interact with it actively and productively.
Upon technology validation, we enter into joint ventures, partnerships and collaborative agreements with industry participants that agree on the merits of a new technology and pursue the business opportunity jointly with us. Partnering with others in this stage of the R&D process allows us to reduce our capital exposure while retaining a controlling interest in the product or technology under development. By co-funding projects, we not only reduce our financial burden and risk from product development activities, but we also enhance our capacity to develop multiple products.
As of June 2023, our ecosystem had more than 25 collaborations and joint initiatives with various players on a global scale. Our collaboration network includes projects with both public institutions at national and international levels as well as with other companies in the private sector. Two of our most valuable products (HB4 and Rizoderma) were incubated through such collaborations.
We followed this concept when we acquired Pro Farm, which we believe possesses a cost-effective R&D platform. We expect this will enable us to bring new bio-pesticides to market at less than 5% of the development cost for a conventional crop protection chemical, and in one-third of the time or less.
Established Relationships with Key Industry Players Leading to Early and Broad Adoption of Technologies and Products
We possess a competitive advantage in commercializing our products, leveraging our strong industry relationships to bring our products to the market faster than our competitors.
We have a well-established high quality brand perception among customers, that positions us as a key reference for farmers who recognize the quality of our brands and value of our product offering. This reputation was built over our history of more than 40 years. We also have unique access to producer/end users through our parent company. Bioceres PLC and the Parent (our largest shareholder as of June 30, 2023) are jointly owned by more than 400 shareholders, amongst which are some of the largest farm operators, processors, distributors and commercial participants in the Latin American agricultural sector. Bioceres PLC’s and Parent’s shareholder structures also include founding members of Argentine Association of Producers in Direct Seeding (Asociación Argentina de Productores en Siembra Directa) and leading members of Argentine Association of Regional Consortia of Agricultural Experimentation (Asociación Argentina de Consorcios Regionales de Experimentación Agrícola) – early adopters and leaders in the sustainable agriculture movement. These unique relationships not only allow us to quickly bring our products to market and integrate our technologies into the broad market by creating a proprietary distribution and commercialization channel, but also provide us with a highly desired early-stage testing platform that allows us to receive direct market feedback in the testing process in order to vet and facilitate faster market penetration.
We have also become a leading choice for partnerships with global agriculture conglomerates, as evidenced by the number of development and commercial agreements. These partnerships are the result of our long-standing relationships with key distributors and major global players, which have played a key role in expanding our presence into new markets.
Highly Accomplished and Disciplined Management Team with a Unique Blend of Technical and Commercial Experience
Our management team brings a wealth of executive experience, scientific and technical knowledge, and commercial acumen to the table, setting us apart in the industry. We leverage the experience and know-how of our management to efficiently source and develop our technologies and products, pursue strategic alliances and acquisitions, and to execute our business objectives. Their combined capabilities ensure that that we remain at the forefront of innovation while strategically aligning our business goals and delivering consistently strong financial results.
45
Significant Transactions
Binding Memorandum of Understanding with Moolec Science SA
On October 15, 2023, we entered into a binding memorandum of understanding (the “MoU”) with one of our affiliates, Moolec Science SA (“Moolec”). Pursuant to the MOU, we will enter into a note purchase agreement (the “Note Purchase Agreement”) and a HB4 soy supply agreement (the “HB4 Soy Supply Agreement”) with Moolec. Under the terms of the HB4 Soy Supply Agreement, we will supply to Moolec an amount of HB4 soy equivalent to US$9 million under our firm commitment. In exchange, Moolec Science will issue to us convertible notes in an aggregate principal amount equal to the value of the HB4 soy delivered by us. Further, Moolec has the option to request additional delivers of an amount of HB4 soy equivalent to US$9 million and will issue additional notes in connection with this option. HB4 soy delivered pursuant to the HB4 Soy Supply Agreement will be priced at the VWAP of the Chicago Board of Trade price for soybeans on the date that delivery is made to Moolec plus a 20% premium.
The notes will mature 36 months after they are issued by Moolec and will include a “payment-in-kind” feature. If the trading price of Moolec’s ordinary shares exceeds the strike price of US$6.00 per ordinary share for 10 trading days, we have the option to exercise the early conversion option pursuant to which the principal amount outstanding under the notes may be converted into ordinary shares of Moolec at the strike price. At maturity, Moolec has the option to convert the principal amount outstanding under the note into ordinary shares. In connection with our early conversion option and Moolec’s optional conversion at maturity, Moolec may deliver ordinary shares, cash, or a combination of cash and ordinary shares. The signing of the definitive Note Purchase Agreement and HB4 Soy Supply Agreement is contingent on Moolec having received an appraisal report from an independent auditor in respect of the value of the HB4 soy to be contributed by us to Moolec pursuant to the HB4 Soy Supply Agreement.
Key Agreements
Corteva Agreement
On July 18, 2023, we entered into an exclusive agreement with Corteva Agriscience (“Corteva”) to advance the availability of biological solutions in Europe. Under the agreement, we will work jointly to accelerate the regulatory processes required to bring a cutting-edge bioinsecticide developed by Pro Farm, to the European market.
The product is an extremely viable biological insecticide that can be as effective as conventional insecticides and is a good fit for mainstream agriculture, with target crops including corn and other cereals, as well as sunflower and rape seeds. Once registrations are secured, Corteva will be the exclusive distributor in Europe, through their Seed Applied Technologies team and will also treat its Pioneer® brand seed products with the technology.
In addition, Corteva Agriscience will continue to commercialize Pro Farm’s Lumidapt™, a growth nutrition seed treatment for crops.
Syngenta Exclusive Global Distribution Agreement
On September 12, 2022, we entered into a 10-year agreement with Syngenta, pursuant to which Syngenta will be the exclusive global distributor of certain Rizobacter biological solutions for seed care applications. Products included within the scope of the agreement are the nitrogen-fixing Rhizobia seed treatment solutions (inoculants), and other biological seed and soil treatment solutions currently in Rizobacter’s portfolio or pipeline. The products in the agreement will be sold under the trademarks owned by us or our affiliates, or any other trademark approved by us.
Pro Farm’s biological solutions are not included within the scope of the current agreement. We have also retained global rights for use of products included in the agreement on HB4® crops and, in the United States, Syngenta rights are non-exclusive for upstream applications.
The exclusive commercial collaboration is global, except for Argentina where both parties will continue to work under the existing framework. Implementation has been staggered, commencing in January 2023 for territories in the first phase, and in January 2024 for territories in the second phase, and subject to regulatory clearances.
The agreement establishes a global joint R&D program to accelerate the development and registration of our pipeline products and new solutions for seed treatment. Funding for the R&D platform will be shared, with Syngenta contributing 70% of the investment.
46
In consideration for the rights granted to Syngenta under the distribution agreement and the R&D collaboration, Syngenta made an upfront payment of US$50 million to us, on October 6th, 2022. Additionally, for the duration of the agreement, we will receive between 50% to 30% of the profits generated by sales conducted by Syngenta, depending on the geography and the year. The agreement sets global minimum targets for profits to be received by us, that amount to a total of US$230 million for the life of the agreement. If we fail to receive the minimum profit targets set for any rolling two calendar year period, we will have the option to terminate Syngenta’s exclusivity. Syngenta may opt to retain exclusivity by compensating for the shortfall in cash or other economic consideration. Syngenta will cover all operating expenses incurred in connection with the marketing and sale in the exclusive territory. Our subsidiary Rizobacter will act as the exclusive supplier to Syngenta for products under the agreement.
Significant Acquisitions
Pro Farm Merger
On July 12, 2022, we announced the closing of the Pro Farm Merger pursuant to which Pro Farm merged into us and became our wholly owned subsidiary. Each share of Pro Farm common stock was exchanged for our ordinary shares at a fixed exchange ratio of 0.088.
In 2019, Marrone Bio Innovations (the predecessor of Pro Farm) completed two strategic acquisitions: (i) the acquisition of Pro Farm Technologies OÜ, a Finnish entity with subsidiaries in Delaware, Brazil, Uruguay, Finland, Estonia and Russia, and (ii) the Jet-Ag and Jet-Oxide product families. Pro Farm’s business and Jet-Ag and Jet-Oxide products were integrated into Pro Farm’s operations and contributed to revenue growth and margin expansion.
As a result of the Pro Farm Merger, Pro Farm gained access to the key row crop markets of Europe and the Commonwealth of Independent States and the merger facilitated its expansion in South America, including Brazil, Uruguay and Argentina.
Rizobacter Acquisition
On October 19, 2016, RASA Holding, our wholly owned subsidiary incorporated in Delaware, acquired 20,004,000 shares of Rizobacter, an Argentine company located in Pergamino, Province of Buenos Aires, representing 50.01% of the outstanding capital stock (the “Rizobacter Acquisition”). The total purchase price was US$57.3 million. In addition, a contingent payment of US$17.3 million may be payable by Bioceres PLC, through Parent, to certain of the selling shareholders of Rizobacter subject to an injunction and related ongoing litigation. The Rizobacter Acquisition was approved by the Argentine Antitrust Commission (Comisión Nacional de Defensa de la Competencia or CNDC) on August 25, 2017. See “Item 3. Key Information —D. Risk Factors—Risks Related to our Business and Strategy—Certain of the Rizobacter shares are subject to a judicial injunction and, if decided unfavorably to us, we may be required to surrender part of our interest in Rizobacter thereby reducing our equity interest in Rizobacter” and “Item 8. Financial Information—A. Consolidated Statements and other Financial Information—Legal Proceedings—An injunction in connection with the Rizobacter Acquisition may require us to surrender part of our interest in Rizobacter.”
On October 22, 2018, RASA Holding, entered into the Rizobacter Call Option Agreement. On March 14, 2019, we exercised the Rizobacter Call Option to acquire an additional 29.99% of the capital stock of Rizobacter, thereby becoming owners of 80% of the capital stock of Rizobacter.
With a 46-year history, Rizobacter has developed a leading global position in biological products and a leading ag-input channel for high-value products. Prior to the Rizobacter Acquisition, we developed a partnership relationship with Rizobacter through a jointly owned company, Bioceres Crops S.A. (formerly Semya), a product development initiative focused on identifying customized seed treatments for our Integrated Seed products. The Rizobacter Acquisition combined Rizobacter’s experience in microbials with our pre-existing pipeline of germplasm and trait assets. This combination has provided us with a unique position with respect to biological assets for key row crops. Rizobacter also provided a unique platform that facilitates the commercial launch of new products and the continued development of our pipeline of innovations.
Verdeca Acquisition
In February 2012, we signed a joint venture agreement with Arcadia Biosciences. The resulting joint venture, Verdeca, in which we held a 50% equity interest, is engaged in the development and deregulation of soybean traits.
47
Our joint venture agreement provided for each of the partners the right to license or sublicense their trait technologies to Verdeca for use in soybeans. Accordingly, Bioceres PLC has agreed to grant us an exclusive, worldwide, sublicensable license for HB4 technologies for use in soybeans. The main product in the Verdeca pipeline is HB4 trait for soybeans.
On November 12, 2020, we acquired from Arcadia Biosciences the remaining ownership interest in Verdeca. Please see “Item 5 Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Consideration of payment of acquisitions,” for more details.
B.Business Overview
Our Operational and Organizational Structure
Our principal executive offices are located in Rosario, Argentina. We own and operate manufacturing and distribution facilities in Pergamino, Argentina; Bangor, Michigan; and Londrina, Brazil. We also have a minority ownership in a third-party manufacturing facility in Vyborg, Russia. We are a light fixed asset company, well invested and with spare capacity to continue to expand internationally. See “Item 4. Information on the Company—D. Property, Plant and Equipment” for more details on our manufacturing assets and capabilities.
Our research and development facilities are in Pergamino, Argentina; Davis, California; and Helsinki, Finland. Additionally, we have sales offices or subsidiaries in 11 countries including: Argentina, Brazil, Bolivia, Colombia, Estonia, France, Paraguay, Russia, South Africa, United States and Uruguay. See “Item 4. Information on the Company—C. Organizational Structure” for our organizational chart and list of subsidiaries, affiliates and joint ventures as of June 30, 2023.
As of June 30, 2023, we had 790 full-time employees globally. See “Item 6 Directors, Senior Management and Employees—D. Employees” for more information on number of employees by role and location.
Our Segments and Key Products
We divide our business into the following three segments: crop protection, seed and integrated products, and crop nutrition.
48
The following table sets forth the key products, growth drivers, revenue and gross profit, and selected commercial partners for each of our segments. The segments are described in further detail below.
Total Revenue and | ||||||||
Gross Margin | Selected | |||||||
Business | (Year Ended | Commercial | ||||||
Segment |
| Key Products |
| Growth Drivers |
| June 30, 2023) |
| Partners |
Crop Protection |
| Adjuvants Pest control molecules |
| Penetration of bioprotection portfolio into mainstream row crop agriculture |
| US$205.8 million |
| Corteva |
Adjuvant expansion in Brazil and Paraguay | DOW | |||||||
Expansion of manufacturing capabilities | Zschimmer & Schwarz | |||||||
New registration approvals | ||||||||
Seed & Integrated Products |
| Seed treatment packs |
| Commercialization of HB4 technology |
| US$56.9 million 46% Gross Margin |
| Florimond Desprez |
Seed traits | Corteva | |||||||
Seed germoplasm | Syngenta | |||||||
Crop Nutrition |
| Micro-bead fertilizers |
| International expansion of bionutrition sales |
| US$157.3 million 57% Gross Margin |
| De Sangosse Syngenta |
Inoculants | Valent BioSciences | |||||||
Biofertilizers | Corteva Biowish | |||||||
Crop health products and biostimulants |
Crop Protection
Our key crop protection products include high-tech adjuvants and a full range of pest control molecules.
Adjuvants
Adjuvants are high-tech molecules used in tank mixes to facilitate the application and effectiveness of active ingredients, such as herbicides, insecticides and fungicides, leading to a better spaying performance, reduced usage rates and lower residue levels. We distribute Silwet, a well-known high-end silicone-based adjuvant, and we are currently developing biological-based adjuvants in partnership with other companies.
49
Pest Control Molecules
Biopesticides are pesticides derived from natural materials such as plants, minerals, fungi or bacteria. We develop and market a portfolio of biopesticides capable of reducing the harm of insect pests and plant diseases during the lifecycle of crops, including biological fungicides, insecticides, nematicides and soil fumigants. These microbial pesticides are high-performance tools for growers which have the added benefit of being nontoxic to workers, end consumers and the environment, including pollinators and other beneficial organisms.
Seed and Integrated Products
The key products of this segment include seed traits, germplasm and seed treatment packs for healthier and higher yielding crops.
Seed Traits
Our seed trait effort is primarily focused on improving plant yields by increasing plant tolerance to abiotic stress, such as drought or soil salinity. This effort is also enhanced by a secondary focus on crop protection and quality traits. We gain access to these technologies through Bioceres PLC’s collaboration with the original developers of the technologies or by co-developing new events with our partners. Our HB4 technology helps increase yields by an average of 10% to 20% for soybean and wheat crops under various growing seasons and conditions, including sporadic drought episodes. HB4 does not adversely impact yields in optimal growing conditions, which is a distinct and important factor compared to other stress tolerance technologies. Additionally, through collaborations, we are expanding the use of the HB4 traits into crops that are not part of our core business but will benefit from the technology.
Other Traits
Biotech traits are positioned as core assets in our product development efforts. Our soybean trait portfolio includes new crop protection platforms for weeds and insects that address basic farmers needs. However, our main focus is traits related to abiotic stress tolerance and nutrient use efficiency, that will help achieve our goal to an agriculture with less environmental impact. For wheat, in addition to our the HB4 technology, we have developed several traits oriented to consumers’ demand, focused on reducing gluten content, resistant starch varieties with lower glycemic index and flour with increased shelf-life. We also have started R&D projects to develop transgenic/genome edited traits to confer resistance to the main diseases that affect wheat production and limit yield potential, as well as nutrient used efficiency traits.
Germplasms
We currently have our own breeding programs for soybean and wheat and have a pipeline to create elite germplasm and use them as a channel for our own technologies as well as meet demand for non-GMO crops and other technologies from strategic partners that complement our portfolio. Our soybean breeding program, operated through Verdeca, delivers varieties that are registered or are in the process of being registered in Argentina, Uruguay, Paraguay, Bolivia and South Africa. Our wheat breeding program is operated through our joint venture, Trigall Genetics, through which we co-develop conventional and HB4 varieties with Florimond Desprez, a global leader in wheat genetics. For both crops we are now expanding the target markets, starting to develop germplasm adapted to important regions, like the United States, Australia, and Brazil.
Seed Treatment Packs
Seed treatment packs comprise high value-added products, produced and commercialized by our subsidiary Rizobacter in partnership with Syngenta Seedcare, including our flagship soybean proprietary inoculants and biofungicides. We also offer certain customized variations for peanuts, beans and chickpeas. In addition, we are pursuing the development of next-generation biologicals, particularly for seed treatments tailored for specific germplasms, seed traits and environment combinations.
Crop Nutrition
Our main crop nutrition products include inoculants, biofertilizers, micro-beaded fertilizers and crop health products.
50
Inoculants
Inoculants are biological fertilizers, broadly using nitrogen-fixing bacteria, which promote growth of leguminous crops such as soybeans and alfalfa. We hold a leadership position in sales of soybean inoculants, with approximately 23% global market share as of June 30, 2023. We developed the next generation of inoculants, “Ultra High Concentrate” (UHC) and Extended Shelf-Life products, for professional seed treatment.
Biofertilizers
We develop and commercialize biofertilizers for foliar or seed application based on organic acids and biopolymers that are combined with nutrients in a unique organic molecular complex that allows plants to absorb various beneficial elements in a novel and effective way. Additionally, we are developing new biofertilizers, such as plant-growth promoting rhizobia bacteria, for wheat, corn, chickpeas and peas.
Micro-beaded Fertilizers
We produce and commercialize fertilizers based on formulated micro-beads. As these fertilizers can be applied next to the seed at planting, they require a lower application dose than conventional fertilizers, resulting in logistical efficiencies and environmental benefits, minimizing contamination through water runoff into lakes and streams. Approximately 20 to 30 kilograms of our microgranular fertilizers can replace 80 kilograms of a basic fertilizer, achieving similar crop productivity and quality. Currently, our production is focused on Microstar PZ, CMB, PZ Bio and CMB Bio providing nitrogen, phosphorus, sulfur and zinc to different crops by allowing immediate nutrient availability and uptake by plant seedlings. CMB Bio is the first micro-beaded fertilizer formulated with four different bacteria.
Crop Health Products and Biostimulants
These products are derived from plant extracts or microorganisms with the aim to enhance nutrition efficiency, abiotic stress tolerance and/or crop quality traits for crops. Biostimulants are substances, microorganisms, or mixtures thereof, that, when applied to seeds, plants, the rizosphere, soil or other growth media, act to support a plant’s natural processes independently of the products nutrient content, including improving nutrient availability, uptake or use efficiency, tolerance to abiotic stress and consequent growth development, quality, or yield. Our current portfolio is mainly focused on three insignia products in this space: Emergen, which optimizes conditions for plant nutrition and resistance to abiotic stress by providing additional nutrients to the plant; Pacesetter, which is a bio-based plant health product that improves chlorophyll production and increases yield; and UBP 110, a complex micronutrient fertilizer that enhances conditions which lead to increased yield and improved crop quality.
Our Business Model
Our business model covers the entire product life cycle: technology sourcing, product development, production, and market access.
In-House R&D and Technology Sourcing
The integration of Pro Farm has led to a significant transformation in our approach to innovation. Previously, our primary focus was on sourcing technology externally. However, with the integration of Pro Farm, our emphasis has shifted towards strengthening our internal R&D capabilities. Internally, we have developed a dynamic R&D team equipped with state-of-the-art laboratories and pilot-scale facilities. We now have specialized teams in entomology and nematology for the study and development of products. Our bioprocess department has been strengthened with the capability to establish optimal parameters for the production of active metabolites and conduct larger-scale fermentation processes. The chemistry department is focused on identifying active ingredients and understanding their behavior. Our formulation team explores innovative technologies to develop effective and stable formulations. Additionally, our plant science department contributes expertise in various agriculture-related areas. This team utilizes proprietary technologies to isolate and assess naturally occurring microorganisms and plant extracts, driving the development of innovative biological-based products.
Simultaneously, we engage in collaborations with leading academic and independent research institutions, startups, and third-party companies during the initial stages of technology development. This open-architecture approach enables us to identify promising technologies and establish strategic partnerships, expediting the development of market-ready innovations.
51
Our current strategy strikes a balance between internal innovation and external collaboration. The Pro Farm integration has significantly elevated the importance of R&D within our innovation framework. We believe that this strategic shift positions us as a leader in biotechnology, enabling us to offer a diverse portfolio of high-quality products that cater to the evolving demands of our customers and the market.
Product Development
We are continually working on product development, both internally and in collaboration with other companies. In selecting partners, we look for internationally recognized entities that can provide complementary funding, technology, sourcing, product development capabilities, intellectual property and market access.
Production and Market Access
The production and market access stage of our business model focuses on leveraging our proprietary sales channels and partnering with third parties to access and establish multiple pathways to markets and maximize market reach. Once a technology obtains the required regulatory approvals, we, our joint ventures, or our strategic partners commercialize products that employ such technology and sell them to global end-users. We also complement our direct sales efforts by licensing our technologies to other companies for inclusion in their products or production systems. This complementary approach seeks to widen the presence of our technologies in the global agriculture market and increase our revenues.
In-House R&D, Technology Sourcing and Product Development Timeline and Process
Our process for creating innovative technologies and product development encompasses various phases: discovery, proof of concept, early development, advanced development, pre-launch, and product launch. This process involves the development and integration of technologies into commercially viable products. The duration of this process varies based on the complexity of the technology and the crop type. Additionally, the timeline can influence the uncertainty of successful product development outcomes. For instance, during technology sourcing and product development, a technology may not meet the required performance criteria to advance to later stages. Competitive landscape changes can also impact the development of certain technologies.
We have strategically integrated our in-house R&D capabilities into this process, improving our ability to innovate. This incorporation of in-house R&D, significantly bolstered through our merger with Pro Farm, strengthens our capacity to navigate each phase effectively, ensuring the delivery of high-quality biological products that meet market demands.
The chart below illustrates an estimated timeline for the development of biotechnology traits (GM and Non-GM) and biological-based products, based on the phases described above:
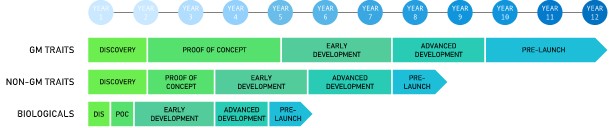
The depicted durations of each phase are based on our estimates and experience. The phases may overlap during the product development cycle and the total development time for a particular product may be longer or shorter than the duration presented above, depending on a range of factors including the type of crop and trait involved and the resources available or devoted to the development of the product. For example, although the process for developing seed traits or biological seed treatments is relatively similar, the two differ significantly in terms of development timelines. Obtaining regulatory approval for GM seeds is a far more comprehensive and lengthy process than for biological seed treatments.
Discovery: This initial project phase typically involves the identification and characterization of potential variants (genes or microorganisms) with product potential. Key activities encompass (i) project planning, (ii) screenings, and (3) isolation of potential candidates. In our experience, the discovery phase typically lasts 18 months, although it may range from as little as six months for microbial solutions to as many as 36 months for plant GM traits.
52
Proof of Concept: The proof of concept phase is aimed at validating the data gathered during the discovery stage and initiating the development of a product from selected variants. Key activities include (i) efficacy validation under controlled conditions (in-vitro and in-vivo), (ii) identification and characterization of selected variants, (iii) selection of candidate variants based on efficacy and characterization, and (iv) candidate improvement (mutagenesis or transgenesis methods). In our experience, the PoC phase typically lasts 36 months, although it may range from as little as six months for a microbial solution to as many as five years for plant GM traits.
Early Development: In this phase, project objectives become more defined, pinpointing the specific conditions a final product must meet, such as application rates and pre-inoculation days. Primary activities encompass (i) bioprocess development for variant production, (ii) formulation prototype development, and (iii) initial efficacy testing under field conditions.
At the end of the early development phase and before initiating the most financially demanding stages of product development, we tend to identify strategic partners for our technologies. For collaborations involving multiple technologies within a pipeline, we often create new entities or joint ventures with our strategic partners. Examples of such collaborations are Trigall Genetics and Synertech, dedicated to wheat technologies and micro beaded fertilizers, respectively.
Advanced Development: The advanced development phase signifies that there is substantial evidence of efficacy for the selected variant, allowing for a more precise estimation of the project’s completion date. Key activities include (i) optimization of bioprocesses for variant production, (ii) final formulation, and (iii) extensive field testing to determine the mode of use. The advanced development phase typically lasts about 24 months, with some projects requiring substantial regulatory data taking from three to five years.
Pre-Launch: Activities in this phase primarily focus on finalizing the necessary details for product launch, such as data for biological production, securing registrations and regulatory approvals, and seed increases. Key activities comprise (i) transferring bioprocesses and formulations to production and (ii) managing registries and label extensions. The pre-launch phase may last up to 24 months.
Product Launch: In general, we, our joint ventures and/or our technology licensees carry out the launch and commercialization of the technology, which is the last phase of the technology sourcing and product development process. When we commercialize technology through collaboration partners or licensees, pursuant to the respective agreements, a successful product launch triggers royalty payments, which are generally calculated as a percentage of the net sales generated by the technology and captured upon commercialization. Typically, in this phase, revenues at launch are limited by our ability to make products available, especially when dealing with seed products that need multiple seasons of multiplication before they can satisfy demand.
Sales and Marketing
Our business model is based on a multi-channel sales structure of (i) direct sales to distributors and end-users via our proprietary sales channels, (ii) B2B commercial agreements, and (iii) licensing of commercial technology to third parties.
Proprietary Channels
Rizobacter
Rizobacter commercializes its products through more than 700 distributors and retailers around the world, including Argentina, Brazil, Paraguay, Uruguay, Bolivia, Colombia, USA, Canada, Mexico, Europe (Austria, France, Germany, Italy, Russia, Ukraine, among others) and Sub-Saharan Africa (South Africa, Kenya, Zambia, Ghana, among others).
In addition to distribution network sales, Rizobacter directly caters to other businesses, particularly large end-users, which include growers or seed companies that use Rizobacter products in professional seed treatment products or for other needs.
Bioceres Semillas
Bioceres Semillas is our proprietary commercial channel for seeds, including leading wheat and soybean varieties, selling to a number of distributors and end-users under the Bioceres Semillas brand. This proprietary channel also serves as a competitive driver for third-party non-exclusive licensees of our technology seeking a rapid path to market.
53
HB4 Program
One of the channels through which we sell our HB4 soy and wheat seeds is through our identity preserved HB4 Program. The system requires having contracts with growers committed to maintaining the identity of the crop under a full seed production offtake agreement. Under these agreements, we contribute HB4 integrated seeds and other goods to growers for a pre-agreed price (based on prevailing market prices), which are later deducted from the service fees paid to growers at the time of harvest for the seed multiplication services they have provided to us.
Pro Farm
Pro Farm products are commercialized in the United States, primarily through its internal sales force, focused on managing distributor relationships and creating grower demand for its products. Pro Farm has a dedicated team of employees who provide technical service support to both customers and sales representatives on the use of their products in integrated pest management (“IPM”) and crop production programs, both for conventional and organic growers. Pro Farm’s sales force covers all major regions in the United States, with an emphasis on high-value specialty crops (fruits, nuts and vegetables). Crop protection product lines are sold through leading agricultural distributors, such as Albaugh, Aligned Ag, Helena Chemical, Nutrien Ag, Simplot and Wilbur Ellis. These are the same distribution partners that most major agrichemical companies use for delivering solutions to growers across the country.
B2B Commercial Agreements
We have commercial agreements with leading industry players with a market presence outside of our core geographic areas, or core crop expertise, for the exclusive or non-exclusive distribution of some of our products. See “Item 4. Information on the Company — B. Business Overview — Joint Ventures and Key Collaborations” for more details on these agreements.
Licensing to Third Parties
We also rely on third-party channels for the commercialization of our proprietary technologies and licenses, either directly or through our joint venture companies, among participants in the biotech seed and agro-industrial market. We license such technologies in each case for incorporation into non-proprietary products and subsequent sale to end-customers. Subsequent sales of products incorporating our technologies generate royalty income. See “Item 4. Information on the Company — B. Business Overview — Joint Ventures and Key Collaborations” for more details on licensing agreements.
Joint Ventures and Key Collaborations
We conduct some of our business through joint ventures and key collaborations. We participate in joint ventures to develop certain technologies and to maintain a diversified product pipeline. When a joint venture successfully develops a product, we integrate such product into our commercial offering and/or license the technology to third-party channels. We engage in non-joint venture collaborations to develop a single or otherwise limited product opportunity. We generate revenue from our non-joint venture collaborations primarily by licensing our technology for inclusion in end-products, or for the use of our technologies in industrial processes. Finally, we have relationships with third parties who have product development capabilities and/or market presence outside of our core geographies or crops, to whom we license our technologies. For our corporate chart, see “Item 4. Information on the Company—C. Organizational Structure.”
Joint Ventures and Unconsolidated Entities
Trigall Genetics S.A.
In December 2013, we formed a joint venture with the French company Florimond Desprez. The resulting joint venture, Trigall Genetics, in which we have a 50% equity interest, is engaged in the development of conventional wheat varieties and the development and deregulation of GM wheat varieties in Latin America. The first biotech trait is our HB4 technology.
Trigall genetics sells conventional wheat varieties through licenses with Bioceres Semillas, Grupo Don Mario (“GDM Seeds”), Asociación de Cooperativas Argentinas (“ACA”), among others.
54
In January 2023, Trigall Genetics entered into a joint venture with S&W Seed Company, Trigall Australia PTY, to advance wheat breeding activities in Australia. Trigall Genetics owns 80% of the newly created company. The program will encompass both conventional and transgenic varieties, including our drought-tolerance HB4 technology, that can contribute to improving yields in a region whose productivity has been increasingly affected by drought and soil salinization.
Synertech Industrias S.A.
Synertech, acquired as part of the Rizobacter Acquisition in 2016, was formed by Rizobacter in partnership with De Sangosse for the production and commercialization of micro-beaded fertilizers. Rizobacter, together with De Sangosse, operates the production plant for Synertech in Pergamino with an annual production capacity of 50,000 tons of microbeaded fertilizers.
Alfalfa Technologies S.R.L.
In December 2020, Bioceres Semillas and Produsem SA acquired a local start-up focused on developing biotech traits for alfalfa and other pastures. The joint venture is engaged in the development and deregulation of conventional and GM alfalfa varieties initially for Latin America market. The joint venture pipeline includes several technologies, of which HB4 and herbicides tolerance are the most advanced ones.
Moolec Science SA
On March 16, 2021, we acquired a 6% ownership interest in Moolec Science Ltd., a molecular farming company pursuing a hybrid concept between plant and cell-based technologies for the production of animal-free food solutions. In consideration for the acquisition, the license to use and commercialize GLA/ARA safflower patents was transferred to Moolec.
On June 14, 2022, Moolec entered into a business combination agreement by and among LightJump Acquisition Corporation, Moolec, and Moolec Acquisition, Inc., a Delaware corporation, providing for, among other things, LightJump and Moolec to become subsidiaries of Moolec Holdco and all stockholders of LightJump and Moolec to become shareholders of Moolec Holdco (the “Moolec Business Combination”). On December 28, 2022, we contributed all of our ownership in Moolec Science Limited to Moolec in exchange of 1,560,000 ordinary shares of Moolec. As of June 30, 2023, our total ownership in Moolec reached 1,860,000 ordinary shares, which represented 4.95% ownership interest. The Moolec Business Combination closed on December 30, 2022 and, as a result, Moolec’s ordinary shares began to be traded on Nasdaq.
On October 15, 2023, we entered into an MoU with Moolec. See “Item 4. Information on the Company—Significant Transactions—Binding Memorandum of Understanding with Moolec Science SA.”
Non-Joint Venture Collaborations
We engage in strategic non-joint venture collaborations for product development and commercialization with leading industry players, and with academic entities and internationally recognized research institutions with whom we collaborate in pre-competitive technology sourcing and early-stage research.
Rizobacter Non-Joint Venture Collaborations
Rizobacter has a longstanding strategic alliance with Syngenta, one of the leading global companies in the research, development, marketing and sales of seed treatment products and solutions. Syngenta placed their Seed Care Institute in Rizobacter’s principal facility located in Pergamino for the research, development and marketing of Syngenta’s seed treatment products and solutions in Argentina, including Maxim Integral, Maxim Evolution, Suren Plus, Rizopack® 420 Hc, Ekey Top, Funcion Pack and Cruiser Pack. We also purchase from Syngenta seed treatment products including Maxim XL, Maxim Integral, Maxim Evolution, Suren Plus, Compinche, Compinche SX, Tenacius, Tenacius SX, among others. The synergy of the product portfolio produced by both companies allows us to combine the most advanced technologies with a highly efficient service for agriculture producers around the world. On September 16, 2022, we announced an expansion of the collaboration through which Syngenta becomes the exclusive distributor of our biological seed treatment solutions globally, except in Argentina, see “Item 4. Information on the Company—A. History and Development of the Company—Significant Transactions—Syngenta Agreement.”
55
Rizobacter also engages in a strategic partnership with Momentive for the exclusive distribution and commercialization of Momentive’s well-known silicone spray adjuvant, Silwet, in Argentina, Brazil, Bolivia, Uruguay, Paraguay, and non-exclusively, in the United States. Our strategic relationship with Momentive has allowed us to jointly develop the latest generation of agricultural adjuvants and to learn about technologies that add efficiency to agricultural applications.
Additionally, Rizobacter has a strategic alliance with De Sangosse Group for the exclusive distribution in Argentina of their molluscicide line, and an agreement for De Sangosse to distribute Rizobacter RIZOLIQ and SIGNUM products in certain European countries.
Rizobacter also has distribution agreements with Microbial Biological Fertilizer International PYD LTD for the distribution of several products within Rizobacter’s portfolio in South Africa, with Brett Young for distribution in the U.S., and with BIOWISH for the provision of bascillus for the formulation of the biological support added to the chemical fertilizer “Microstar”.
Rizobacter also executed significant agreements for the provision of formulation services and production with several companies, among which are Syngenta, Corteva, UPL, FMC, and Summit Agro.
Pro Farm Non-Joint Venture Collaborations
Pro Farm has exclusive legacy international agreements with two major business partners: Syngenta for the distribution of biofungicides for specialty crop markets in Europe, the Middle East and Africa, and Corteva for distribution of UBP family of bionutrient products for the European market (foliar and seed treatments for row crops) and in Brazil (seed treatments for row crops). As of July 2023, we also entered into an exclusive distribution agreement with Corteva to advance the availability of a bioinsecticide developed by Pro Farm in Europe. See “Item 4. Information on the Company—A. History and Development of the Company—Significant Transactions” for more details on the agreement with Corteva.
Pro Farm also has a distribution agreement with PGG Wrightson Seed in Uruguay related to Pro Farm foliar and seed treatment product sales in the region, and has a number of distribution agreements with Agristar, Nufarm, UPL Limited, Jocanima/Great Havest, Elephant Vert, Kenya Biologics, Hoptri, Lidorr, AMC/Agrimatco, Disagro, Kyung Nong Corporation and Vive Crop Protection.
HB4 Non-Joint Venture Collaborations
We license our HB4 technology to third parties that have breeding and product development capabilities or a market presence outside of our core geographic areas and with whom we have developed and maintain strong relationships.
Through Verdeca, we entered into a series of agreements with GDM Seeds and TMG for the licensing of our biotechnology related to soy for breeding and/or sales in Argentina, Brazil, Paraguay, Uruguay and United States.
We also have HB4 soy R&D license agreements with Criadero Santa Rosa and INTA in Argentina, FT Sementes and Integrado Sementes in Brazil, BAUP and INBIO in Paraguay, and Prograin and Sollio in Canada, and Southern Hemisphere Seeds in South Africa
Trigall licenses wheat varieties to a number of companies including ADP Uruguay, GDM, Biosemenis, ACA and Bioceres Semillas. These are “transactional” relationships, where there are no preferred partners and licenses are re-evaluated every year.
Further under Trigall, HB4 wheat has R&D agreements with EMBRAPA and OR Sementes in Brazil, and GDM Argentina. We have a material transfer agreement for HB4 trait introgression with BUCK in Argentina, Stellenbosch in South Africa and ANAPO and SEMEXA in Bolivia.
In addition to the licensing agreements listed above, we have two main breeding collaborations — with GDM to develop HB4 soy varieties for specific regions in the United States, for maturity groups II and below, and with TMG for the development of HB4 and non-HB4 varieties for any geographic territory.
Customers and Contracts
Below is a description of our principal customers and contracts across our main segments.
56
Seed and Integrated Products
In the year ended June 30, 2023, we sold our seed and integrated products to customers in Argentina, Uruguay, France, Austria, among other countries. Our top five customers in our seed and integrated products segment represented approximately 17% of our total revenue in this segment for the twelve-month period ended June 30, 2023.
Crop Protection
In the year ended June 30, 2023, sales from our crop protection segment were made to customers in Argentina, United States, Brazil, Paraguay, Uruguay, among others. Our top five customers in our crop protection segment represented approximately 24% of our total revenue in this segment for the year ended June 30, 2023.
Crop Nutrition
In the year ended June 30, 2023, sales from our crop nutrition segment were made in Argentina, Brazil, Uruguay, Paraguay, Bolivia, United States, Canada, Europe, South Africa, as well as other international markets. Our top five customers in our crop nutrition segment represented approximately 37% of our total revenue in this segment for the year ended June 30, 2023.
Industry Overview
The Market Opportunity
Agriculture and the way in which we produce food are at the center of a major global challenge —increasing food production in an affordable way, while simultaneously not only reducing but reversing its impact on climate change and biodiversity loss.
There is a pressing need for the “sustainable intensification” of global agriculture in which yields can be increased without any adverse environmental impact and without the cultivation of more land. This challenge presents a massive opportunity to be addressed through additional technological innovation — sustainable approaches that integrate climate-adaptive biotechnology, the expanded use of biological products and the integration of new data, monitoring and precision agriculture tools.
Climate smart food production
Climate change is disrupting weather patterns and leading to more frequent and extreme events such as droughts, floods, and unseasonal temperatures, all of which have negative effects on food production. The latest Intergovernmental Panel on Climate Change Report indicates with high confidence that “Climate change, including increases in frequency and intensity of extremes, has adversely impacted food security and terrestrial ecosystems, as well as contributed to the desertification and land degradation in many regions”. As global food demand continues to increase, climate change will have a negative impact on crop yields and nutritional value of the food produced, decreasing agricultural outputs. Most studies agree that the impact of climate change on crop yields will be negative from the 2030s onwards, and nearly half of those predict yield reductions greater than 10% beyond 2050. There is an opportunity, and a need, to climate proof food production by improving crop resiliency to climate change, developing crops that can tolerate abiotic stress such as drought.
Reversing climate change and biodiversity loss
Agriculture is a significant contributor to climate change, with total agri-food system emissions in 2020 corresponding to 31% of total anthropogenic emissions, according to the latest estimates of the Food and Agriculture Organization. However, agriculture is also the main industry that utilizes and relies upon valuable soil microorganisms, often referred to as the soil microbiome, that are instrumental to capture atmospheric carbon in significant amounts. Therefore agriculture, particularly those agricultural practices that are biologically-based, can play a key role in mitigating climate change.
Soil conservation practices such as conservation tillage, organic production, cover cropping and crop rotations, practices that are central to the creation of Rizobacter over 40 years ago and to the now-growing regenerative agriculture movement, can drastically increase the amount of carbon stored in soils. Using higher-yielding crops or varieties, or more climate resilient crops, and maximizing the yield potential of crops can also increase soil carbon.
57
Addressing consumer demand for sustainable and affordable foods
Consumer attitudes and behavior are increasingly shifting towards more sustainable lifestyles, with the expectation that brands also “do good”. These preferences are redefining the entire food system all the way through to raw material origination.
Multiple studies have shown that consumers are more likely to choose products with an environmental or environmental, social and governance (“ESG”) related claim. For example, according to McKinsey and NielsenIQ’s reports, products making ESG claims outperformed those without such claims in 68% of surveyed product categories.
Younger generations are more likely to consider sustainability outcomes in their consumption decisions. A 2021 Forbes report, “The State of Consumer Spending: Gen Z Shoppers Demand Sustainable Retail,” finds that 62% of Generation Z prefers to buy from sustainable brands, a similar percentage to Millennials. Additionally, 54% of Generation Z stated that they are willing to spend an incremental 10% or more on sustainable products, with 50% of Millennials reporting the same. This contrasts with 34% of Generation X and 23% of Baby Boomers. The trend indicates that the desire and willingness to spend on sustainability is increasing with each new generation.
Traceability of products from “farm to fork” is becoming an increasingly important factor for consumers. Whole chain traceability can reduce information asymmetry, rebuilding public confidence in the entire food chain while delivering quality, safe, and nutritious foods. A US Food and Drug Administration (the “FDA”) regulation requires participants in the food value chain to maintain end-to-end traceability records to be made available upon request during a food-borne outbreak or food recall investigation. The regulation applies categories that are estimated to account for approximately 20% to 30% of food consumed in the United States.
Increased Regulation
Globally, the international regulatory landscape continues to place greater restrictions on the use of synthetic pesticides and other chemical crop inputs. For example, a new Sustainable Use of Pesticides Regulation, currently pending in the European Commission, mandates a 50% reduction in use of chemical pesticides by 2030 in all member states. To meet this mandate, the EU relies heavily on the expedited approval on new biologically-based pesticides and their increased adoption, via research and education about the use of biologicals and direct subsidies to growers, under the Common Agricultural Policy, to promote more biologically-based IPM strategies.
Similarly, following years of litigation and under a December 2022 court order, the United States Environmental Protection Agency has agreed to aggressive timelines to complete Endangered Species Act assessments for several, commonly-used chemical insecticides, herbicides, fungicides and nematicides. Under these reviews, EPA has recently proposed restrictions, or is expected to propose restrictions, on many chemical pesticides that currently hold a significant presence in the US market. In conjunction with an increasingly restrictive landscape for chemical pesticides, and other chemical crop inputs, the global public policy landscape continues to move towards the expedited approval of reduced risk biological crop inputs and their increased adoption, with South American countries at the forefront.
Sustainability-Driven Solutions
A variety of promising technologies exist with the potential to substantially modify the way in which we produce food, manage scarce resources, mitigate climate change and ensure the safety of our food supply chains. These technologies include the advancement of biotech crops to improve resilience, the increased utilization of microorganisms in replacement of chemical inputs, adoption of soil preserving cropping techniques, and the use of technologies such as blockchain to provide traceability and enhance the safety of the entire food system, farm to fork. We are at the forefront of this transformation of how we produce food, feed and fiber.
58
Biotechnology
Biotech crops have been around for almost 25 years and are planted on approximately 190 million hectares annually, according to the International Service for the Acquisition of Agri-biotech Applications (the “ISAAA”). The commercial adoption by farmers of transgenic crops has been one of the most rapid cases of technology diffusion in the history of agriculture. Historically, biotech traits have focused on herbicide tolerance and insect resistance. However, biotechnology can deliver much more substantial agronomic, environmental, health, economic and social benefits, including increased productivity, conservation of biodiversity, improved nutrition, and mitigation of negative impacts of climate change. Our HB4 technology, which has now been approved in multiple countries for food and feed uses and for cultivation, is the first in the world that confers tolerance to climate changes in soybeans and wheat by allowing these crops to tolerate drought and soil salinity conditions that are a significant threat to the world’s food supply.
According to ISAAA’s last report, corn and soybeans represented most of the seed biotechnology market in 2019, making up approximately 80% of the global biotech seed market. The United States, Brazil and Argentina were the top planters of biotech seeds, with approximately 150 million hectares under production of biotech crops. As of 2019, the adoption of GM varieties is over 74% for soybean, over 31% for corn and over 79% for cotton.
Until the HB4 wheat approval in Argentina, wheat had historically been an orphan crop in the sphere of biotechnology, despite being planted in 200 million hectares globally, the largest of any crop. Argentina is Latin America’s largest wheat producer, accounting for approximately 70% of wheat produced in the region. Today, drought tolerant HB4 wheat is the only genetically modified wheat approved and on a commercial path to market anywhere in the world, which represents a major milestone in wheat’s global value chain.
Biologicals
Biological agricultural products, or biologicals, include bioprotection products, biofertilizers (bionutrition) and biostimulants, and occupy a unique space in agriculture as technologies that offer proven economic and environmental benefits to the consumer, the grower and the distributor. For consumers, biologicals are part of the trend toward accessible, affordable, high-quality food produced in an environmentally sustainable manner. For growers and distributors, biologicals offer alternative solutions that not only enhance crop quality but also protect natural resources and reduce carbon footprint.
Once considered a niche market, biologicals are currently the fastest-growing segment in the agricultural input market with double-digit growth industrywide, as compared with low-single-digit growth for conventional products. The market for agricultural biologicals, estimated to be approximately US$12.4 billion for 2022, still only represents about 3-5% of the total agricultural inputs market for the same year, according to data from Duham Trimmer.
The main drivers for this strong growth performance are: regulatory policies that either encourage the adoption of bio-based products or restrict the use of chemical products; the need for products that improve soil health and nutrient utilization, yield and crop quality; insects, plant disease and weed resistance towards chemical products; need for greater convenience and flexibility in growing practices; and a need for greater R&D and innovation to meet the ever increasing food security demands and environmental regulations.
To meet these objectives, an increasing number of growers use IPM programs to produce crops by the most economical means, and with the lowest possible risk to people, property, and the environment. Biological agricultural products and crop cultivating techniques such as crop rotation and low-or-no tillage are among the most commonly used IPM practices. Adoption of biologically based crop inputs are increasingly becoming a central component of the IPM toolbox for farmers. There is growing recognition that IPM, particularly biologically-based IPM, is an important ecosystem service that contributes to climate mitigation and biodiversity—a critical ecosystem service provided by agriculture. Recent disruptions in supply chains that significantly impacted grower access to commonly used synthetic crop inputs have resulted in new interest in integrating biologicals into existing IPM strategies that often relied solely on chemical inputs.
59
Our Product Portfolio Promotes Climate-Smart Outcomes
Our vast range of products not only help farmers and food companies meet growing demand for more sustainable agricultural practices, but they also preserve, and oftentimes help restore or improve the natural environment for future generations by promoting soil and soil microbiome health, enhancing on-farm carbon sequestration, safeguarding biodiversity, ensuring farmworker safety and optimizing the use of increasingly distressed and diminishing natural resources.
We develop science-driven solutions that preserve biodiversity and promote climate-smart outcomes:
| ● | Soy and wheat crops tolerant to adverse weather conditions: we develop crops that are resilient to adverse weather conditions associated with climate change such as drought and heat via the adoption of our HB4-traited drought tolerant crops and biologically-based seed treatment packages that help plants better weather yield and quality-robbing environmental stresses. |
| ● | Complete portfolio of biological solutions: we enhance sequestration of GHGs in agriculture by promoting crop health and yields with effective environmentally beneficial crop protection tools, such as Rizonema, Rizoderma and MBI-306, and with microbial seed inoculants that enhance plant sustaining atmospheric nitrogen fixation, while also reducing the use rates of synthetic fertilizers – all of which reduce the negative impacts associated with runoff or soil leaching of persistent chemical compounds. |
| ● | Next generation solutions that reduce input application rates: we promote efficient resource use with products such as ajuvants and Microstar PZ. Adjuvants are high-tech molecules used to increase the effectiveness of spray applications of crop inputs and, therefore, reduce the rate of application of both biological and chemical pesticides and other agricultural inputs. Microstar PZ is a low application rate micro-beaded starter fertilizer that provides newly planted seeds necessary nutrients, like nitrogen, phosphorus, sulphur and zinc, precisely at the time of planting. Microstar’s novel micro-beaded formulation enhances nutrient uptake efficiency at a critical point in a crops growth cycle that reduces the need for additional fertilizer applications later in the crop growth cycle —which also reduces negative air and water quality issues associated with traditional fertilizers. |
HB4 Technology
HB4 technology significantly increases yields under drought conditions and positively impacts the environment through more efficient use of water resources and the reduction of GHG emissions.
Biotech traits like HB4 can only be launched after a time-consuming breeding process to develop varieties that combine the technology with locally adapted, elite germplasm. We have been developing varieties that specifically target areas identified for maximum trait performance.
Our key markets for HB4 wheat in the Southern Cone comprise approximately nine million hectares of wheat planted per year, including Argentina, Latin America’s largest wheat producer and the world’s first country to adopt HB4 drought tolerance technology, and Brazil, the second largest producer in South America and the country with the greatest potential to increase the planting area by an additional two to three million hectares. Another key market for HB4 wheat is Australia, with approximately twelve million hectares planted with the crop every year and high incidence of water stress events and salinity on soil.
For HB4 soy, our key markets include Argentina, Brazil and the United States, representing approximately 75% of the world’s total soybean acreage.
In order to estimate the size of opportunity, we obtained the official wheat and soybean production statistics in each of the aforementioned countries between 2015 and 2019/2020. We used the average yield data to estimate the yield benefit that HB4 could have produced. We then used the average price of soybean and wheat for the ten-year period to calculate the expected monetary benefit.
60
Subject to the conventional non-binary biotechnology solutions pricing scheme, where 75% of the value creation remains on-farm and 25% goes off-farm, the maximizing of the price times quantity equation will define the supply and demand equilibrium for the technology. We expect the target area to find higher correlation in the more drought prone areas, highlighted in darker blue in the heat maps below:

At the time of filing, HB4 soy is approved for cultivation and commercialization in the United States, Canada, Brazil, Argentina, and Paraguay, which together represent over 90% of the global soybean trade. The commercialization of HB4 soybeans for cultivation in Argentina was cleared once China’s approval for food and feed uses was obtained in April 2022. HB4 soy is also approved for food and feed uses in South Africa (September 2022) and for food uses in Indonesia (September 2023). Regulatory submissions of HB4 soy are currently under review in Bolivia, Uruguay, the European Union, India, Malaysia, Thailand, Australia and New Zealand.
HB4 wheat is approved for cultivation and commercialization in Argentina, Brazil and Paraguay. Additional key food and feed approvals obtained include Colombia (February 2022), Australia and New Zealand (May 2022), United States (June 2022), Nigeria (July 2022), South Africa (September 2022), and Indonesia (March 2023). Submissions for cultivation have been presented in Bolivia, Uruguay and United States, and for food and feed uses in Chile and Thailand. Additional submissions for both HB4 soybean and wheat will continue to be pursued as we advance to new territories.
The HB4 Identity Preserved Program: Our Systemic Approach to Empower Growers and Consumers Alike
The standard commercial method for transgenic traits is based on trait licensing to multiple breeding companies. These companies commercialize their varieties with the trait through their own channels.
We follow this model, but we have also created an innovative approach called “Generation HB4” or “HB4 Program”. We have initiated the HB4 program to multiply HB4 material and at the same time to evaluate product performance, determine product positioning, and conduct field days to showcase the HB4 technology to different stakeholders in the agriculture value chain.
Through the HB4 Program, producers grow HB4 seeds under an identity preserved model, following environmentally friendly farming practices that include crop rotation, no till, and the use of carbon footprint minimizing inputs. We contribute the HB4 seeds as well as additional inputs from our current portfolio, including micro-beaded fertilizers that reduce by one quarter the use of conventional fertilizers, nitrogen fixing inoculants that act as biological fertilizers and high-tech adjuvants that reduce the need for chemicals. In addition to mitigating production losses during drought periods, HB4 technology also facilitates double cropping, which seasonally rotates soy and wheat, an environmentally friendly farming system that is otherwise limited by water availability. When combined with soil regenerative practices, such as no-till farming, HB4 double cropping system captures more carbon than conventional growing practices. Higher crop yields with HB4 also reduce the need to expand agriculture’s land use footprint, which often entails deforestation, while aiding the rehabilitation of fragile agricultural land back to its historically productive status.
61
The identity-preserved production system requires having contracts with growers committed to maintaining the identity of the crop under a full seed production offtake agreement. Under these agreements, we contribute HB4 integrated seeds and other goods to growers for a pre-agreed price (based on prevailing market prices), which are later deducted from the service fees paid to growers at the time of harvest for the seed multiplication services they have provided to us. The grain produced by HB4 Program participants then enter a grading and selection process, where grain that meets specific quality standards is saved as seed inventory for the next growing season and the rest of the grain is sold as commodity dry products (grain). Our program includes a contract with processors who agree to process the qualifying grain and channel it to a selected market. Managing the production system logistics as well as the contractual requirements with farmers, processors and seed treatment plants requires that we have dedicated team. In the fiscal year ended June 30, 2023, the HB4 program covered approximately 70,000 hectares, including wheat and soy.
Digital β-platform
The HB4 Program employs robust, closed growing systems that are combined with a high level of traceability via state-of-the-art digital farming technologies and strong stewardship to ensure environmentally friendly farming practices. Growers participating in this program have access to satellite-based crop imaging, weather monitoring and crop scouting digital application platforms. The purpose of these platforms, particularly the crop imaging application, is to provide a tool to keep track of performance differences between fields planted under the HB4 program protocols versus a control group of neighboring fields that are grown under typical commercial protocols that do not include the HB4 technology package. These platforms facilitate the recording of field activities and equipment data integration and help refine our products’ positioning based on field performance.
The use of digital platforms allows us to provide traceability of HB4 crops in the identity-preserved production system, from farm to fork, and to produce specific ESG reports that provide end users with solid, traceable ESG seed and grain scoring information. We measure carbon and water footprint and generate a blended ecotoxicological score that combines Cornell University’s environmental impact quotients with the RIPEST dose-dependent pesticide risk approach. These scores consider factors such as dermal toxicity, toxicity to birds, bees, fish and beneficial arthropods, soil half-life, surface loss potential, plant surface half-life, systemicity, and leaching potential, among other factors affecting farm workers, the environment and consumers.
We believe this report will set a new industry standard. The environmental impact of exposure to different active ingredients, the carbon intensity of production processes and the water footprint of agricultural ecosystems are all key elements in designing and promoting a 21st century regenerative agriculture.
Trends and Performance by Segment
We have a top tier complementary portfolio of products addressing demand in a global market which is estimated to be worth more than US$120 billion by 2029.
Crop Protection: Adjuvants
According to Woodstone Research & Consulting, the global adjuvants market is valued at US$3.0 billion in 2023 and is projected to reach US$4.0 billion by 2029.
Segment growth is driven by the need to maintain crop protection, while improving the efficiency and effectiveness of agrochemicals usage, given an increased awareness of the negative implications of excessive usage of agrochemicals in the environment. Growth in the segment also comes from government initiatives to promote eco-friendly agricultural products, the expansion of precision agriculture, and the development of eco-friendly and biodegradable adjuvants.
By crop type, the cereals and grains segment are projected to be the fastest-growing segment in the agricultural adjuvants market during the forecast period. By product type, the herbicides segment is estimated to witness the fastest growth in the agricultural adjuvants market, in terms of value.
North America, followed by Europe, is the largest market for agricultural adjuvants at present, and it is expected to account for a significant proportion of the global market in the future. The government policies adopted by European countries toward sustainable agricultural practices support consumption of adjuvants, which is a key factor driving the growth in the region. However, the Brazilian market is expected to see the largest growth rate, as adoption of improved crop protection technologies increases.
62
Crop Protection: Biocontrol
The global biocontrol market is estimated at US$5.2 billion for 2022, and is projected to reach US$11.9 billion by 2029, recording an estimated CAGR of 12.6% for the forecast period, according to Woodstone Research & Consulting. Europe and the United States are the two largest markets for these products, representing 40% and 26%, respectively. Brazil and China follow, with a 6% share each.
As a result of the Pro Farm Merger, we have greatly expanded our portfolio and pipeline of biological products – with foliar, soil and seed applications – that protect both row and specialty crops against a broad range of pests.
The increasing demand for biological products can be attributed to the rise in sustainable organic farming practices and more biologically based integrated pest management strategies, as well as the need to address pest resistance, and novel invasive species. In recent years, supply chain disruptions that have restricted the availability of synthetic chemistry products and fertilizers have spurred greater grower interest in and adoption of biological products, particularly microbial-based products. Climate change has a considerable global impact on agricultural productivity and crop susceptibility to pests, making crops more vulnerable to various diseases and abiotic stresses, which significantly reduce crop yields. All of these factors have increased farmers adoption of biologically based and sustainable crop protection solutions, increasing market demand for biocontrol products.
Crop Nutrition: Biostimulants
Biostimulants are substances, microorganisms, or mixtures thereof, that, when applied to seeds, plants, the rizosphere, soil or other growth media, act to support a plant’s natural processes independently of the products nutrient content, including improving nutrient availability, uptake or use efficiency, tolerance to abiotic stress and consequent growth development, quality, or yield.
Biostimulants are among the most rapidly growing categories in agricultural inputs. The market was valued at US$2.5 billion in 2022 and is projected to grow at an estimated CAGR of 11.9% to reach US$5.5 billion by 2029. The largest markets for biostimulants are Europe, North America and China. As in other biologicals, growth is mainly attributed to the increasing impact of climate change, soil degradation and abiotic stress to crops globally.
Crop Nutrition: Biofertilizers
The biofertilizers market was valued at US$2.6 billion in 2021 and is expected to reach US$3.7 billion by 2026, growing at an estimated CAGR of 13.1% during the forecast period. These novel crop nutrition products encourage better uptake of vital nutrients that allow the plant to withstand stresses and increase output.
The acquisition of Pro Farm, with its large portfolio of biofertilizers and growing customer base, has allowed us to expand our product offering in this rapidly expanding market, adding to our existing leading position in the global inoculants market.
The nitrogen-fixing seed inoculants market is projected to increase from US$1.1 billion in 2022 to US$1.7 billion in 2028, according to Markets and Markets, recording an estimated CAGR of 7.5% in value. In South America, countries like Brazil and Argentina are projected to continue to offer high-growth prospects for seed inoculants use in coming years given their leadership position in global soybean production. Currently, the adoption of seed inoculants is very low in other major markets such as India and China, but interest in these products is growing on a global scale and we expect to play a major role in developing these markets — particularly through our recently signed global collaboration with Syngenta.
Decades of cultivation practices using synthetic pesticides and fertilizers have led to a significant negative impact on groundwater reserves and on soil health and fertility – ultimately harming crop productivity. These factors have led to an increase in the adoption of sustainable farming. This trend is still in early stages, but the number of product approvals and investment in bio-ingredients is growing rapidly to support this transformation and is expected to lead to intense market competition in the future. Among biological products, biofertilizers and biostimulants are gaining significant traction as they enhance the phosphorus and zinc absorption properties of plants as well as their resistance to pathogen attacks.
63
Crop Nutrition: Specialty Fertilizers
Specialty fertilizers are a category of fertilizers designed to meet specific nutritional needs of crops, soil conditions, or growth stages more precisely than standard or conventional fertilizers. They are formulated to provide targeted and balanced nutrition, which can lead to improved crop yields, quality, and resource efficiency. Increasing awareness about farm resource management, affordability of farm inputs, and a focus on agricultural profitability have helped drive the rapid expansion of specialty fertilizers usage, and these currently account for nearly 17% share of the global market for plant nutrition products.
The global market for specialty fertilizers is estimated at US$34.0 billion in 2023 and expected to reach US$42.5 billion by 2029, growing at an estimated CAGR of 3.8% for the period. Demand for specialty fertilizers has been increasing as an effective alternative to conventional fertilizers, due to their lower impact on the environment.
Seed and Integrated Products: Biotech traits
Biotechnology tools are seeing a significant increase in demand across agricultural applications. These include tissue culture and micropropagation, marker-assisted selection or molecular breeding, genetically modified crops and genetic engineering, molecular diagnostic technologies, and conventional plant breeding.
A rise in the adoption of GM crops globally is expected to drive this market. This is because genetic modification allows the production of feed and food crops with enhanced characteristics, such as high nutritional value, high yield, enhanced food-processing qualities, and resistance to diseases and insects.
The global seed market size was estimated at US$67 billion in 2022 and is projected to reach US$86 billion by 2028, growing at an estimated CAGR of 6.6%. The genetically modified seed market is estimated to account for the largest share in the seeds market. The global genetically modified seeds market size was estimated at approximately US$32 billion in the year 2022, and is projected to reach US$48 billion by 2028, growing at an estimated CAGR of 7%. This increase in the market size of GM seeds underlines the increasing significance and need for agricultural biotechnology.
Competition
The market for agricultural biotechnology products is characterized by intense commercial and technological change and we face significant direct and indirect competition in each of our business segments.
The crop productivity sector is highly competitive and includes large companies, such as Bayer, BASF, Corteva, Syngenta AG, UPL Limited and FMC Corporation and Sumitomo Corporation. Other companies, including bio-specialized biopesticide businesses such as BioSAfe Systems, Certis Biologicals, Gowan, Novozymes and Valent Biosciences may prove to be significant competitors in the biological pest management market.
Rizobacter remains a leader in the Argentine adjuvants market with a 29% market share, differentiating itself in a competitive market by introducing new, quality products year after year. The Brazilian market is an attractive market for adjuvants, given the growth in the chemical crop protection market and the strong adoption of improved technologies for crops, and we are addressing this opportunity through a B2B and channel coverage strategy. North America, followed by Europe, are the largest markets for agricultural adjuvants at present, and they are expected to account for a significant proportion of the markets for Rizobacter products in coming years.
In respect of inoculants, Rizobacter is the global leader with an estimated global market share of 23%, followed by other key competitors including Novozymes and Becker Underwood. We expect to continue to expand internationally through our recent agreement with Syngenta. See “Item 4. Information on the Company—A. History and Development of the Company—Significant Transactions—Syngenta Agreement.”
Within the fertilizer market in Argentina, Rizobacter’s focus is the phosphorous market. In this market, Rizobacter participates with a specialty product that is used at substantially lower doses than competitor’s products, with a competitive advantage in terms of storage and logistics. In Argentina, we are leaders in this niche followed by Recuperar, FYO, Red Surcos, among others. In Brazil, farmers are overcoming cultural barriers to adopt new technologies, benefitting from our micro beaded fertilizers which provide logistics and storage advantages specially for a country with large geographic extensions such as Brazil. We also expect a potential synergy with biological products and new integrated launches in the Brazilian market.
64
The market for bionutrition and crop health products is very fragmented and diverse, as it has lower regulatory barriers when compared with crop protection products. It includes larger players like Valagro (acquired by Syngenta Crop Protection), UPL Limited and Acadian Seaplants.
Competition in the seed and integrated products sector extends to each of the components that we provide for our integrated products. We face competition for our biotech traits from different companies. Companies such as Bayer, BASF, Corteva and Syngenta own packages of traits that focus on herbicide tolerance, which could indirectly compete with the components of HB4. Other companies such as Evogene Ltd., INARI and DBNBC also engage in trait discovery and licensing technology to customers. Genome Editing is another area that is showing a significant increase in the number of companies and may develop competition to our trait packages. There are several companies offering services for editing and R&D collaborations, however, while promising, there are no large-scale technologies commercially available.
We also face competition for our proprietary germplasm from other companies that focus on developing proprietary germplasm in open platforms. In soybeans, the South American market is dominated by local companies such as GDM Seeds in Argentina and Brazil and TMG in Brazil, though several others have market share in specific regions. Stine Seeds, a dominant company in the United States, is setting bases to commercialize soybeans in Latin America. These companies, despite being competitors in the germplasm space, offer great opportunity to collaborate and act as licensor to our traits. See “Item 4. Information on the Company—B. Business Overview—Joint Ventures and Key Collaborations—HB4 Non-Joint Venture Collaborations.” In wheat, traditional European companies such as Limagrain, KWS and RAGT compete in South and North America along with the previously mentioned local players. The multinational companies that develop biotech traits mentioned above complete the landscape of competitors.
Our Growth Strategy
Our long-term growth strategy is based on an open-architecture approach to technology origination, identifying and accessing promising technologies from third parties, as well as forming strategic and capital-efficient partnerships that leverage each party’s strategic strengths and capabilities to bring innovations more quickly. Our near-term growth strategy includes the following:
Continue to Lead Development and Commercialization of New Agricultural Biotechnology Products in Existing and New Markets
We intend to build upon our diverse portfolio of crop productivity solutions by consolidating our position in biological assets, including microbial, seed trait and germplasm assets, and continuing to pursue an integrated approach in the development of superior yielding products. We intend to expand upon our direct reach to customers by offering additional high demand technologies, such as digital farming solutions, which we believe will facilitate the adoption and subsequent sales of our products as well as achieve efficiencies to create additional value opportunities.
Scale-Up Production of Rizobacter and Pro Farm Products to Accelerate Penetration in Crop Nutrition and Protection Markets
We have invested significant capital in the future development of specialty fertilizers and operate a micro-beaded fertilizer facility in Pergamino, Argentina. The facility began operations in January 2017 and increasingly supplies high-demand specialty fertilizers in Argentina and neighboring countries. We are in the process of expanding capacity in our biological manufacturing plant in Pergamino.
We have a well invested asset base in Brazil, which includes a brand-new adjuvant production facility to sustain the execution of our international expansion plan, in addition to product registrations in place.
With the acquisition of Pro Farm, we incorporated two packaging and manufacturing facilities, one in Bangor, Michigan, and the other a third-party manufacturer in Vyborg, Russia, in which we hold a minority ownership interest. At our Bangor plant, we have made investments which aim to increase production capacity and reduce production costs.
Commercialization of HB4 Seed Products to Drive Penetration of Seed Market
HB4 wheat and HB4 soy seeds integrate the uniqueness of HB4 stress tolerance into locally adapted germplasms, customized with a seed treatment solution prescribed for specific environments. We believe that the product differentiation provided by our unique and varied technologies increase the value of our products for HB4 Seed customers and will drive significant growth. In the medium-term, we expect royalties from HB4 licenses to represent a significant component of our revenue, as this landmark technology is more broadly adopted through strategic partnerships and third-party channels.
65
Expand our International Business by Accelerating Registration and Sales of Products Through Multiple Subsidiaries
We are a global leader in the biological market for agriculture inputs and have used this position to establish subsidiaries in Brazil, Paraguay, Bolivia, Uruguay, the United States, South Africa, Colombia and France. We believe we can use our international footprint and sales force to continue to define our key brands by bringing our broader portfolio of crop productivity solutions to these markets, leveraged with our recent Pro Farm acquisition, which provided us with new commercial channels in the United States and Europe. We expect international growth to be driven initially by continued growth in our biological business, as well as by incorporating high-value adjuvants and crop nutrition solutions.
Pursue Strategic Collaborations and Acquisitions in Key Markets
We intend to continue working with our collaboration partners to bring our products to customers in key markets. We also plan to continue pursuing acquisitions and in-licensing opportunities seeking global expansion and to gain access to validated and important later-stage products and technologies that we believe to be a strategic fit for our business.
Intellectual Property
Our success depends mostly on our ability to obtain and maintain intellectual property protection for our products and technologies, defend and enforce our intellectual property rights (in particular, our patent rights, plant varieties, trademarks and trade secrets), preserve the confidentiality of our intellectual property and operate without infringing valid and enforceable intellectual property rights of others.
We seek to protect our proprietary products, technology, and trade secrets, in part, by entering into confidential disclosure agreements with our employees, consultants and potential and actual third-party collaborators. By protecting our proprietary technologies, we can offer our customers and partners unique products unavailable from our competitors, while also preventing our competitors from using technologies that we have developed or licensed exclusively or non-exclusively, from other parties.
The main countries in which we seek patent protection are the United States, Canada, Brazil, Argentina, Bolivia, Paraguay, Uruguay, Mexico, South Africa, Australia, India, China, Ukraine, Russia and certain other countries in Europe.
As of the date of this report, we, in our capacity as sublicensee (either as exclusive or non-exclusive licensee), have over 750 patents and patent applications. In some instances, our patents and our sublicenses are limited in terms of duration, geography and/or field of use.
Our licensed patents for the HB4 soy and HB4 wheat expire in 2039 and 2040, respectively, for the Coxc5-1 family in 2027 and for the HB10 family in 2030. We are the exclusive sublicensees and non-exclusive licensees of the following patents: rGRF3 technology patents which expire in 2033; NUE technology patents which expire in 2026; WUE technology patents which expire in 2029; herbicide resistant patents which expire in 2028; LXR®, a delayed senescence technology which expires in 2028; ZFP® technology, which expires in 2033; RS (Hexapolid and Tetraploid) technology which expires in 2033; RG (Reduced gluten) technology which expires in 2036; OX technology which expires in 2037; PlantArcBio technology which expires in 2042; and UHC technology ultra-highly concentrated bacterial liquid soybean inoculant technology, which expires in 2040.
During 2019 and 2020, Bioceres PLC filed patent applications covering the HB4 soy event which expires in 2039 and the HB4 wheat event which expires in 2040, respectively, within the main countries where we will launch our products once regulatory approvals are received.
Our other patents include patents around our products Regalia, Grandevo, Venerate, Emergen, Majestene, Stargus, Zelto, Pacesetter, Zequanox, Ennoble, Haven and the UBP bionutrient portfolio.
Crop Protection: the crop protection segment boasts a diverse product range, including fungicides Regalia® (Reynoutria sachalinensis) Rizoderma® and Stargus® (Bacillus amyloliquefaciens F727), insecticides Grandevo® (Chromobacterium subtsugae sp. nov.) and Venerate® (Burkholderia sp. A369), nematicides Majestene® and Zelto® (Burkholderia sp. A369), and sun and heat stress protector Haven® (stearyl alcohol). The segment, also featuring biocides, molluscicides, fumigants, and bioherbicides, holds over 450 patents.
Crop Health: Focusing on nurturing crop development, this segment spotlights Pacesetter®, a growth regulator that stimulates root development and boosts fungicides like Regalia® (Reynoutria sachalinensis). The segment holds over 20 patents.
66
Crop Nutrition: we deliver innovative crop nutrition solutions, spanning both foliar treatments and seed treatments. Standout products include Foramin® and Foramin ST®, Emergen®, Rizofos®and UBP 110® for foliar applications, as well as Rizoliq®, UHC® Inoculants, Lumibio Optima® and Ympact® for seed treatments. Our excellence in this area is reflected in the over 30 patents we have registered.
We seek additional protection of our seed and germplasm intellectual property through PVP certificates, which preserve a variety owner’s exclusive rights to sell, reproduce, import, and export a plant variety and our seed. The duration of PVP protection varies among jurisdictions and is 20 years from the time of issue in the United States and 20 and 15 years from the time of issue in Argentina and Brazil, respectively. As of the date of this report, we do not have PVP certificates in the United States. In addition, in Argentina, we have received, as owner and/or as licensee, registrations with the National Cultivar Registry (Registro Nacional de Cultivares) (“RNC”) for 16 wheat varieties, 19 soybean varieties, two sunflower hybrids, three corn hybrids, and two Amaranthus varieties, all of which are authorized for our marketing in Argentina. We are currently seeking registration for four soybean varieties and three wheat varieties at the RNC. We have also received, as owner and/or as licensee registrations with the Argentinian National Registry of Cultivar Ownership (Registro Nacional de la Propiedad de Cultivares) (“RNPC”) for eleven soybean varieties and 13 wheat varieties at the RNPC. We have also received the registration for 13 soybean varieties and 6 wheat varieties in Uruguay, two soybean variety in South Africa, three soybean varieties in Paraguay and two soybean varieties in Bolivia. We are currently seeking registration for one soybean variety in South Africa.
We seek to protect our non-patent intellectual property, such as know-how and regulatory data, through contracts and confidentiality agreements. Know-how generated by the activities of our companies is protected by specific services agreements or employment agreements. Employment agreements include undertakings regarding confidentiality and assignment of inventions and discoveries. Our regulatory data is protected by standard confidentiality and data protection mechanisms. We have over 1,150 trademarks and over 300 trademark applications worldwide.
We will continue to file and prosecute patents, PVP certificate and trademark applications in the United States and foreign jurisdictions, and maintain trade secrets, consistent with our business plan, to protect our intellectual property rights.
Government Regulation
We are subject to agriculture, health and environmental regulations in the countries in which we operate or in countries where final products containing our technologies (e.g., grains containing biotech traits) will be consumed. We must obtain and comply with various permits and licenses from government authorities and municipalities in the jurisdictions in which we operate before we can test and ultimately commercialize our products/technologies.
For all of our products, particularly our pesticidal products and HB4-traited seeds, compliance with the regulatory requirements of each country is one of our highest priorities. These regulations are multilayered and touch all steps in the research, development, approval and commercialization of our products. Regulators typically require extensive product performance, product characterization and quality control and product safety data for each product. On the regulations related exclusively to genetically modified organisms, we continue to conduct field tests under special permits in countries where our technologies are still not fully approved, while we continue to seek approvals, always complying with the local regulations.
The laws and regulations we are subject to will continue to evolve as there are advances in biotechnology and our other businesses. Our regulatory affairs team is responsible for generating the data necessary to secure product approvals and to oversee product stewardship programs to ensure our products are used safely, effectively and fully in compliance with all relevant regulations.
In Brazil, CTNBio, is the local regulatory agency for biosafety. Both HB4 soy and wheat have obtained approval from CTNBio for their consumption and cultivation. We further obtained regulatory approvals for consumption of the HB4 technology from many regulatory agencies around the world, including from the FDA which is one of the most important approvals. We continue to seek approvals on a global scale, always complying with the local regulations. See “Item 4. Information on the Company—B. Business Overview—Industry Overview—Sustainability-Driven Solutions—The HB4 technology” for the complete list of approvals and submissions to date.
67
In the United States, the EPA regulates the bio-based pest management products under the Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”), the Federal Food, Drug and Cosmetics Act (“FFDCA”) and the Food Quality Protection Act (“FQPA”). In addition, some of the plant health products are regulated as fertilizers, auxiliary plant substances, soil amendments and/or beneficial substances in each of the 50 states. In addition to EPA approval, we are required to obtain regulatory approval from the appropriate state regulatory authority in individual states and foreign regulatory authorities before we can market or sell any pest management product in those jurisdictions. We also generally pursue organic certification for our product portfolio, including USDA National Organic Program, Organic Materials Review Institute, EcoCert and ControlUnion.
Around the globe, the regulatory process for biostimulants and bionutrients (biofertilizers) is significantly accelerated compared to that for biopesticides. In the United States, if plant health products are not used to control pests or do not act as plant (growth) regulators, they currently fall outside the legal scope of FIFRA, FFDCA and FQPA and, therefore, we do not need to submit applications for EPA registrations for such products. However, we must still submit state registrations for some of our products. Products containing microbes of foreign origin may also need to be “deregulated” (or determined not to be a plant pest) under the Plant Protection Act by the USDA Animaland Plant Health Inspection Service prior to use in field trials or for large scale release.
Insurance
We maintain customary insurance policies that we consider to be in line with market practice and adequate for our business. Our principal insurance policies are personal injury (as mandated by Argentine labor law), Worker’s compensation, all operational risk, civil liability, fire, theft, cars, transport, credit, work injury risk, cyber liability, cargo cyber link, cargo STP, ERISA bond, domestic property, boiler & machinery, general liability, business auto, foreign package, umbrella, and bond insurance entered into in connection with grants received. We maintain product liability insurance coverage in respect of Pro Farm Group’s products portfolio which is covered by our general liability insurance policy.
Legal Proceedings
From time to time, we may become party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business. We are not currently involved in any legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition, or results of operations, other than the proceeding described in “Item 8. Financial Information—A. Consolidated Statements and other Financial Information—Legal Proceedings— An injunction in connection with the Rizobacter Acquisition may require us to surrender part of our interest in Rizobacter.” As of the date of this report, we are involved in one material legal proceeding, as referenced above, and we do not face any claims of possible intellectual property infringement. We may become involved in material legal proceedings in the future as part of the ordinary course of our business.
C.Organizational Structure
The following diagram depicts our current organizational structure:
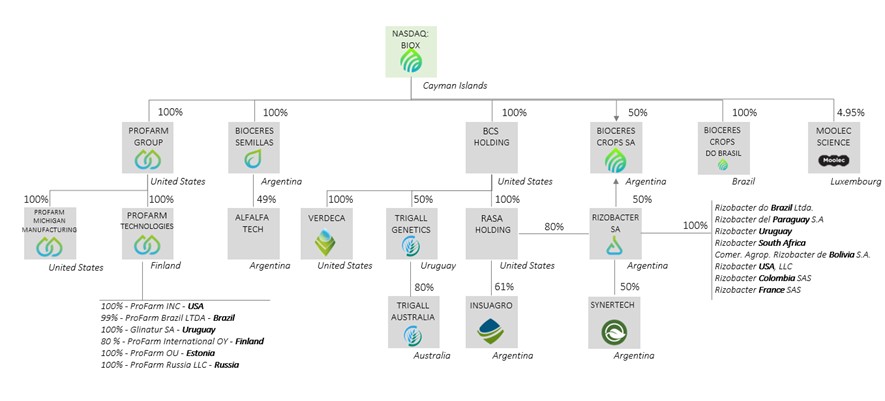
68
The following table identifies our main subsidiaries and joint ventures as of June 30, 2023:
Country of | Ownership | Voting |
| ||||
Name |
| Incorporation |
| Interest |
| Interest |
|
BCS Holding Inc. |
| USA |
| 100 | % | 100 | % |
Bioceres Crops do Brasil Ltda. |
| Brazil |
| 100 | % | 100 | % |
Bioceres Crops S.A. (1) |
| Argentina |
| 50 | % | 50 | % |
Bioceres Semillas S.A.U. |
| Argentina |
| 100 | % | 100 | % |
Alfalfa Tehcnologies S.R.L. (2) |
| Argentina |
| 49 | % | 49 | % |
Comer. Agrop. Rizobacter de Bolivia S.A. (1) |
| Bolivia |
| 80 | % | 80 | % |
Glinatur S.A. |
| Uruguay |
| 100 | % | 100 | % |
Indrasa S.A. (1) (2) |
| Argentina |
| 35 | % | 35 | % |
Insumos Agroquímicos S.A. |
| Argentina |
| 61 | % | 61 | % |
Pro Farm Group, Inc. |
| USA |
| 100 | % | 100 | % |
Pro Farm International, OÜ |
| Finland |
| 80 | % | 80 | % |
Pro Farm Michigan Manufacturing LLC |
| USA |
| 100 | % | 100 | % |
Pro Farm Russia, LLC |
| Russia |
| 100 | % | 100 | % |
Pro Farm Technologies Comércio de Insumo Agrícolas do Brasil Ltda. |
| Brazil |
| 99 | % | 99 | % |
Pro Farm Technologies, OÜ |
| Finland |
| 100 | % | 100 | % |
Pro Farm, Inc. |
| USA |
| 100 | % | 100 | % |
Rasa Holding LLC |
| USA |
| 100 | % | 100 | % |
Rizobacter Argentina S.A. |
| Argentina |
| 80 | % | 80 | % |
Rizobacter Colombia SAS (1) | Colombia |
| 80 | % | 80 | % | |
Rizobacter del Paraguay S.A. (1) | Paraguay | 80 | % | 80 | % | ||
Rizobacter do Brasil Ltda. (1) | Brazil | 80 | % | 80 | % | ||
Rizobacter France SAS (1) | France | 80 | % | 80 | % | ||
Rizobacter South Africa (1) | South Africa | 80 | % | 80 | % | ||
Rizobacter Uruguay (1) | Uruguay | 80 | % | 80 | % | ||
Rizobacter USA, LLC (1) | United States | 80 | % | 80 | % | ||
Synertech Industrias S.A. (1) (2) | Argentina | 40 | % | 40 | % | ||
Trigall Genetics S.A. (2) | Uruguay | 50 | % | 50 | % | ||
Verdeca LLC | USA | 100 | % | 100 | % |
Notes:—
| (1) | Calculated considering the indirect interests held through Rizobacter. The indirect equity interest participation included in this table is 80% of the direct equity interest participation that Rasa Holding LLC owns in each entity. |
| (2) | Joint ventures and unconsolidated entities. |
D.Property, Plant and Equipment
Our main manufacturing and distribution facilities are located in Pergamino, Buenos Aires province, Argentina. Our manufacturing facilities include (i) the adjuvant formulation plant with 2.1 million-gallons in annual production capacity, (ii) the biological production plant with an annual capacity of 1 million-gallons, (iii) the insecticides and fungicides formulation plant with an annual production capacity of 1 million gallons, (iv) the micro-beaded fertilizer facility with an annual production capacity of 50,000, and (v) over 375,000 square feet of warehouse space for packaging and logistics. We test and conduct trial runs of our key technologies at our main field station located in Pergamino, Argentina, which also has processing capabilities for foundation seed.
In Londrina, Paraná state, Brazil, where our main subsidiary is located, we recently completed the construction of a new high-tech adjuvant facility plant with an annual capacity of approximately 2.6 million-gallon.
As a result of closing of the Pro Farm Merger, we incorporated a 11,400 square-foot manufacturing facility in Bangor, Michigan, where we ferment and formulate most of Pro Farm’s biopesticides, with an annual capacity of up to 600,000 gallons, and a formulation plant of insecticides and fungicides with a production capacity of up to 800,000 gallons per year. We test and conduct trial runs of our key technologies at our Laboratory and Research Centers in Helsinki, Finland and Davis, California, USA.
69
ITEM 4A.UNRESOLVED STAFF COMMENTS
None.
ITEM 5.OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following discussion of our financial condition and results of operations should be read in conjunction with our audited consolidated financial statements for the years ended June 30, 2023, 2022 and 2021, and the notes thereto, included elsewhere in this report.
The following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those discussed in the forward-looking statements as a result of various factors, including those set forth in “Cautionary Note Regarding Forward-Looking Statements” and “Item 3. Key Information—D. Risk Factors.”
A.Operating Results
Factors affecting our results of operations
Our results of operations have been influenced and will continue to be influenced by the following factors:
Market demand for our products and services
Our sales and profitability are influenced by the demand for our crop productivity products in the particular markets in which we operate. Demand for our seed and integrated products, crop nutrition products and crop protection products are affected by the purchase decisions of our distributors and customers, which are typically driven by fluctuation in agricultural commodities prices, crop profitability and planting decisions, as well as externalities such as general market conditions, grower production decisions and new technology adoption, commodity prices, operating costs and weather conditions.
Seasonality and weather conditions
Our revenues fluctuate depending on the timing of orders from our distributors and customers and on prevailing market prices, which influence the purchase decisions of growers, the end users of our seed and integrated products, crop protection products and crop nutrition products. Given the cyclicality of crop planting and harvesting, as well as growing seasons, which vary from year to year, our business is highly seasonal. This results in substantial fluctuations in quarterly sales and profitability. Our portfolio is highly oriented to crop planting. Generally, our sales are concentrated in the third and fourth quarters of each calendar year, when demand for our seed and integrated products, crop protection products and crop nutrition products increases as growers begin planting their summer crops in South America, while winter crop season occurs in the Northern hemisphere. With our seed and integrated products business, we contract with growers and seed suppliers based upon our anticipated market demand. Generally, in our seed and integrated products business we stock the seed during the harvest season and ship from inventory throughout the year, with the objective of selling most of the inventory from the current year’s harvest before the next year’s, with our crop protection and our crop nutrition business following a similar cycle to the seed cycle. Milestone, royalty, and license revenues are also likely to fluctuate from period to period given the seasonality of agriculture and time required to progress from one milestone to the next.
Our seed and integrated products, crop protection and crop nutrition businesses are also affected by unpredictable weather conditions such as heavy rains, hail, floods, freezing conditions, windstorms, drought or fire, as well as other hazardous situations beyond our control, which may cause our sales and operating results to fluctuate significantly. In addition, disruptions that cause delays by growers in harvesting or planting can result in the movement of orders to a future quarter, which also causes fluctuations in our quarterly operating results. Finally, some of our customers and distributors order in bulk only one or two times a year, which may further cause our seed product revenues to fluctuate from period to period.
70
Fluctuations in commodity prices
Our results of operations, particularly the demand and price for our seed and integrated products, crop production products and crop nutrition products, are affected by global agricultural commodities prices, such as grains, biofuels and biomaterials. Global prices of agricultural commodities vary in accordance with domestic and export market prices, which are primarily affected by the local and global demand for, and supply of, those commodities. Prices for agricultural commodities are also significantly influenced by speculative actions and by currency exchange rates, volatility in credit markets and fluctuation in consumer and business confidence. In addition, prices for agricultural commodities are affected by governmental programs and policies regarding agriculture, as well as general trade, fiscal and exchange control policies. Extrinsic factors, such as drought, floods, general weather conditions, disease and natural disasters may also affect agricultural commodities prices. Demand for agricultural commodities, such as wheat and soybeans, both for human consumption and as cattle feed, has generally increased with worldwide economic growth and prosperity.
Macroeconomic conditions in Latin America
We generate a significant portion of our revenue from production in emerging markets. Therefore, our operating results and financial condition are directly impacted by macroeconomic and fiscal developments, including fluctuations in currency exchange rates, inflation and interest rate fluctuations, in those markets. The emerging markets where we conduct our business (including mainly Argentina and Brazil) remain subject to such fluctuations. See “Item 3. Key Information—D. Risk Factors—Risks Related to Operating in Latin America— Inflation in Argentina and government controls may adversely affect the economy and our financial condition and results of operations.”
From July 1, 2022 our main Argentine subsidiaries have changed their functional currency from the Argentine Peso to United States Dollars as a result of changes in events and conditions relevant to their business operations. However, a significant portion of our operating costs in Argentina are denominated in Argentine Pesos and most of our operating costs in Brazil are denominated in Brazilian Reais. For each of our subsidiaries’ statements of income, foreign currency transactions are translated into the local currency, as such subsidiaries’ functional currency, using the exchange rates prevailing as of the dates of the relevant specific transactions. Exchange differences resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in the consolidated statement of income (“statement of income”) under “finance income” or “finance costs,” as applicable. Our Consolidated Financial Statements are presented in U.S. dollars, and foreign exchange differences that arise in the translation process are disclosed in the consolidated statement of comprehensive income.
As of July 1, 2018, the Argentine economy qualifies as a hyperinflationary economy according to the guidelines of the IAS 29, since the total cumulative inflation in Argentina in the 36 months prior to July 1, 2018, as measured by the wholesale price index published by the INDEC, exceeded 100%. Accordingly, IAS 29 guidance is applicable to our financial statements for periods ending after July 1, 2018 for those subsidiaries that use the Argentine Peso as functional currency. IAS 29 requires the financial information of an entity whose functional currency is a currency of a hyperinflationary economy to be adjusted by applying a general price index and expressed in the measuring unit at the end of the reporting period and then such financial information to be translated into the presentation currency at the prevailing exchange rate. See Note 2 to our consolidated financial statements included elsewhere in this report. See “Item 3. Key Information—D. Risk Factors—Risks Related to Operating in Latin America—We are required to apply inflationary adjustments and tax indexation procedures in our Argentine subsidiaries, which could adversely affect our financial statements, results of operations and financial condition.”
Effective July 1, 2022 our main subsidiaries changed their functional currency from the Argentine Peso to the United States Dollars as a result of changes in events and conditions relevant to their business operations. The effect of the functional currency change was recorded prospectively in accordance with IAS 21. As a result, from July 1, 2022 there are no longer significant effects of inflation adjustments in our financial statements.
Effects of export taxes on our products
Since 2002, the Argentine government has imposed duties on the exports of various primary and manufactured products. Most such duties were suspended or subject to progressive reduction after the Macri administration took office in December 2015. However, in September 2018, general export duties were re-imposed and the progressive reduction of export duties on soybean products stopped. For example, a general additional export duty has been imposed on all exports of goods, levied on the lower of 12% of the good’s FOB value or AR$3 or AR$4 per U.S. dollar, depending on the goods.
71
On December 4, 2018, the Argentine Congress approved the budget bill for 2019 through Law No. 27,467, which amended the Customs Code to allow for duties to be applied to the exportation of services. In addition, the Argentine government was allowed to impose export duties of up to 30% until December 31, 2020. However, in case of services and goods that were not subject to export duties before September 2, 2018, the maximum rate was 12%. On January 2, 2019, the Argentine government issued Decree No. 1201/2018, which established an export duty on export of services at a rate of 12% with a maximum limit of AR$4 per each U.S. dollar of the amount arising from the invoice or equivalent document. On December 28, 2019, Decree No. 99/2019 extended the application of duties on export of services until December 31, 2021 with a rate of 5% without limit.
On December 21, 2019, the Argentine Congress enacted the Solidarity Law, that established new maximum export duties of 33% for soybean, 15% for goods that were not subject to the payment of export duties as of September 2, 2018, 5% for agroindustry products of regional economies defined by the Argentine government, and 5% for industry goods and services.
As of February 29, 2020, the following crop exports were subject to the following export duties: soybean and soy products 30%, corn, wheat, barley, sunflower and sorghum 12%, corn and wheat flour 9% and sunflower oil 12%.
In October 2020, the Argentine government adopted a series of measures seeking to promote production, exports and the liquidation of U.S. dollars from exports in the FX Market, including the temporary reduction of export duties for soybean and soybean derived products through Decree No. 790/2020. Further, pursuant to Decree 790/2020, a temporary reduction scheme of export duties for soybean products and by-products was established, which increased on a monthly basis to return to the initial level as from January 2021.
In October 2022, Argentina has also imposed certain controls to the import of goods and services. In respect of imports of goods, Argentine companies currently have limited access to the FX Market to acquire foreign currency in order to make payments abroad, provided that certain requirements are met, including the registration of the import transaction under the importation payment follow-up (Seguimiento de pagos de importaciones).
Different requirements apply to goods with customs entry registration, including, among others, that: (i) there is proof of the customs registry of the entry into the country of the goods that originated the payment to be canceled; (ii) the importer has a copy of the commercial invoice issued abroad in the name of the company who made the purchase abroad; and (iii) the documentation presented allows the importer to establish the expiration date of the foreign obligation. Goods with pending customs entry registration must comply with other requirements, including, among others: (i) documentation determining the existence of a purchase of goods abroad; (ii) a sworn statement submission demonstrating the registration of the customs entry of the goods within the term that corresponds according to the type of good to be imported; (iii) provision of elements that guarantee the reasonableness of the amounts to be paid considering the importing activity of the client in recent years and the business plans presented by the importer; and (iv) no delays in the regularization of payments with pending customs entry registration made as of September 2, 2019.
Recently, the BCRA strengthened the requirements for importers that intend to access to the FX Market by requesting an approved declaration submitted in the Argentine System of Imports (“SIRA”) from local authorities. If the SIRA declaration is approved, the local authorities will inform the importer the term in which the access to the FX Market will be granted. Such date usually ranges from 60 to 270 calendar days. Foreign exchange regulations provide for certain alternatives that would allow the Argentine importer to access the FX Market before the date stated in the SIRA. These alternatives may involve financing granted by a financial institution. The declaration of the SIRA will be analyzed by the AFIP, which will assess each individual situation based on information available in the AFIP’s records and the financial and economic capacity of the importer through the CEF.
Is respect of import of services, there are currently restrictions imposed to Argentine companies looking to access the FX Market such as the limitation on the payment of imports of services. Prior approval by the BCRA is required to access the FX Market for prepayments and payment of services to foreign-related parties.
As of October 13, 2022, access to the FX Market for payments of services rendered by non-residents requires a declaration made through the SIRASE, approved by the Secretary of Commerce.
The purpose of the SIRASE is to determine whether importers willing to make payments of services abroad have complied with tax obligations and are capable of carrying out certain financial transactions. Once the SIRASE declaration is submitted, the AFIP will review the information provided and confirm the importer’s compliance regarding tax return filings and its financial economic capacity through the CEF regime.
72
On April 20, 2023, through Communication “A” 7746, BCRA modified foreign exchange regulations regarding foreign currency outflows. This amendment had a strong impact, mainly in the access to the FX Market, in the payment of services rendered by non-Argentine residents. BCRA’s prior approval must be requested to access the FX Market prior to 60 calendar days from the date of approval of the SIRASE declaration for research, legal and accounting, advertising, and engineering services, among others. Such prior approval will not be required, for example, if using own funds in foreign currency. Also, payment to related parties abroad for freight and transportation services must be made 90 calendar days after the date that the service has been rendered.
Stages of development of our products
Our results of operations will vary depending on the stage of development of our products and technologies. Some of our products are currently in the early stages of development and our historical operating results are not indicative of the operating results we expect to experience in later stages of product development. As we are able to advance such technologies and products through the development and regulatory phases to commercial launch, we expect our revenues and cash flows to increase.
As our seed and integrated products, crop protection and crop nutrition businesses continue to develop internationally, we expect to experience continued increases in sales of micro-bead fertilizers, inoculants, biofertilizers and biostimulants from our crop nutrition solutions, biopesticides, adjuvants and other crop protection solutions and seed and integrated products, including the HB4 technologies. We expect to continue to generate license fees from payments that we receive from third parties pursuant to license agreements, as well as royalty fees from distributors and growers who save harvested seeds that contain our technology and then use the seeds in subsequent harvests. We also expect to generate additional revenues from distribution fees that our joint venture partners pay to our proprietary distribution channels for selling seed and integrated products that incorporate our technologies.
Our costs are impacted by the stage of development of our products and technologies, requiring, for example, expenditures in the research, development, and regulatory phases of a product without corresponding flows of revenue until the time of commercial launch. Product development expenses may fluctuate from period to period and may also increase if we choose to accelerate certain product development programs or if we elect to take a greater role in the regulatory and commercialization process with respect to one or more of our crop productivity products in development incorporating our crop productivity technologies.
Regulatory environment
Our results of operations will vary depending on the speed in which we are able to obtain regulatory approvals for our products and the cost and expense associated with gaining such approvals. The degree of regulation to which we are subject varies by activity and country. Our ability to sell our technologies and products depends on our obtaining and maintaining necessary authorizations, permits and regulatory approvals in the markets in which we operate.
Results of operations
We have based the following discussion on our consolidated financial statements included elsewhere in this report. You should read it along with these financial statements, and it is qualified in its entirety by reference to them.
73
Comparison of the years ended June 30, 2023 and 2022
The table below illustrates our results of operations for the years ended June 30, 2023 and 2022.
| For the years ended June 30, | |||
2023 |
| 2022(3) | ||
(in millions of US$) | ||||
Revenues from contracts with customersTotal revenue and initial recognition and changes in the fair value of biological assets at the point of harvest | 420.1 | 334.8 | ||
Cost of sales |
| (235.5) |
| (208.4) |
Gross Profit |
| 184.6 |
| 126.4 |
Research and development expenses |
| (15.3) |
| (6.9) |
Selling, general and administrative expenses |
| (113.0) |
| (77.5) |
Share of profit of joint ventures and associates |
| 1.2 |
| 1.1 |
Changes in the net realizable value of agricultural products after harvest and other income or expenses, net |
| (3.3) |
| (3.3) |
Operating profit |
| 54.2 |
| 39.9 |
Net financial cost |
| (35.1) |
| (25.8) |
Profit before income tax |
| 19.1 |
| 14.1 |
Income tax |
| 1.1 |
| (18.0) |
Profit (Loss) |
| 20.2 |
| (3.9) |
Other comprehensive income |
| (0.8) |
| 35.2 |
Total comprehensive profit(1) |
| 19.3 |
| 31.3 |
Non-IFRS measures(2) |
|
| ||
Adjusted EBITDA (unaudited) |
| 81.2 |
| 51.5 |
Notes:—
(1)Includes (i) exchange differences on translation of foreign operations from joint ventures, (ii) exchange differences on translation of foreign operations, (iii) revaluation of property, plant and equipment, net of tax from joint ventures and (iv) revaluation of property, plant and equipment, net of tax.
(2)Adjusted EBITDA is a non-IFRS measures. For a complete presentation of the reconciliation, see the section entitled “—Non-IFRS Financial Measures.”
(3)Our results of operations for the year ended June 30, 2022 do not include the results of operations of Pro Farm, as the Pro Farm Merger closed on July 12, 2022.
Revenues from contracts with customers and initial recognition and changes in the fair value of biological assets at the point of harvest
Total revenue increased by 25%, totaling US$420.1 million for the year ended June 30, 2023, compared to our total revenue for the year ended June 30, 2022. Pro Farm revenue totaled US$14.1 million for the year ended June 30, 2023. Top line expansion was achieved despite a strong historical comparison for the year ended June 30, 2022, and a number of external factors that challenged growth such as weather conditions in two of Bioceres’ key markets – a drought of historical magnitude in Argentina – and negative industry dynamics in the U.S. and Brazil.
Crop Protection. Total revenue increased by US$39.4 million, to US$205.8 million for the year ended June 30, 2023, from US$174.4 million for the year ended June 30, 2022. Pro Farm’s crop protection revenue for the year ended June 30, 2023 was US$32.5 million. Therefore, the Crop Protection segment remained almost flat, as it faced the greatest headwinds in terms of weather and high channel inventories in several geographies.
Crop Nutrition. Total revenue increased by US$48.2 million, to US$157.3 million for the year ended June 30, 2023, compared to US$109.1 million for the year ended June 30, 2022. Considering Pro Farm’s crop nutrition revenue for the year ended June 30, 2023 was US$9.8 million, crop nutrition saw the largest year-on-year increase, driven by growth in biostimulants, inoculants and micro-beaded fertilizer.
Seed and Integrated Products. Total revenue increased by US$5.4 million, to US$56.7 million for the year ended June 30, 2023, compared to US$51.3 million for the year ended June 30, 2022. Seed and integrated products saw expansion in HB4 sales as well as seed treatment packs.
74
Gross profit
Total gross profit increased by US$57.9 million, to US$184.6 million for the year ended June 30, 2023, from US$126.4 million for the year ended June 30, 2022. Leading gross profit contributing products included positive results from the agreement with Syngenta of $34.1 million and Pro Farm products gross profit contribution of US$24.9 million.
Segment performance was mainly driven by positive results from the agreement with Syngenta on global distribution for inoculants, as well as growth in micro-beaded fertilizers sales. Considering Pro Farm crop protection gross profit for the year ended June 30, 2023 was of US$16.8 million, crop protection gross profit contribution was almost flat, in line with sales performance. Despite higher sales, gross profit contribution from seed & integrated Products decreased by 13%, heavily impacted by product mix.
Research and development expenses
Research and development expenses include ongoing efforts to maintain and continuously update our existing product portfolio. Research and development expenses increased by US$8.4 million, to US$15.3 million for the year ended June 30, 2023 from US$6.9 million for the year ended June 30, 2022, primarily as a result of additional expenses with R&D at Pro Farm, which totaled of US$7.2 million.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by US$35.5 million, to US$113.0 million for the year ended June 30, 2023 from US$77.5 million for the year ended June 30, 2022 mainly as a result of additional selling, general and administrative expenses from Pro Farm totaling US$26.8 million.
When excluding depreciation and amortization, transaction expenses and share-based incentives, selling, general and administrative expenses for the year ended June 30, 2023 was US$94.4 million, an increase of US$28.8 million compared to US$65.6 million for the year ended June 30, 2022, of wich US$20.1 was due to Pro Farm’s selling, general and administrative expenses. The remaining US$8.7 million was mainly due to an increase in employee benefits and social security expenses, professional fees, expenses, taxes, among others.
Share of profit of joint ventures and associates
The profit resulting from our share in the profit of joint ventures and associates remained almost flat in US$1.2 million, as operational results of our joint ventures were similar to those relating to the year ended June 30, 2022.
Changes in the net realizable value of agricultural products after harvest and other income or expenses, net
Changes in the net realizable value of agricultural products after harvest and other income or expenses, net Other net results expenses maintained flat in US$0.3 million, to US$3.03 million for the year ended June 30, 2023 from US$3.3 million for the year ended June 30, 2022.and 2022. These results are presented together as they are explained due to the changes in fair value of agricultural products and its commercialization.
Finance results
Finance costs: finance costs increased by US$5.9 million, to US$23.8 million for the year ended June 30, 2023 from US$17.9 million for the year ended June 30, 2022 driven by a higher debt position compared with to the year ended June 30, 2022. Interest expenses does not include a gain from translation effects on Argentine Peso denominated loans.
Other finance results: the charge of other finance results increased by US$3.4 million to US$11.3 million for the year ended June 30, 2023, compared to a loss of US$7.9 million for the year ended June 30, 2022, mainly as a result of changes in fair value of financial assets or liabilities and other financial results.
75
Income tax
Income tax gain amounted to US$1.1 million for the year ended June 30, 2023, compared to an expense of US$18.0 million for the year ended June 30, 2022, primarily due to greater profitability in low taxation jurisdictions and lower tax profitability in operational subsidiaries.
Profit (Loss) for the year
As a result of the foregoing, loss for the year ended June 30, 2023 amounted to US$20.2 million compared to a loss of US$3.9 million for the year ended June 30, 2022.
Other comprehensive income (loss)
Other comprehensive loss amounted to US$0.8 million for the year ended June 30, 2023 compared to a comprehensive loss of US$35.2 million for the year ended June 30, 2022, primarily as a result of US$39.8 million reduction of the benefits of foreign differences on translation of foreign operations due to the change of functional currency in our main Argentine operational subsidiaries, which was offset by a decrease of the loss in revaluation of property, plant and equipment of US$3.8 million.
Total comprehensive income
As a result of the foregoing, we recorded a total comprehensive income of US$19.3 million for the year ended June 30, 2023 compared to a total comprehensive loss of US$31.3 million for the year ended June 30, 2022.
Comparison of the years ended June 30, 2022 and 2021
Please refer to our Annual Report on Form 20-F for the fiscal year ended June 30, 2022.
Non-IFRS Financial Measures
We supplement the use of IFRS financial measures in this report with non-IFRS financial measures, including Adjusted EBITDA.
These non-IFRS measures should not be considered in isolation or as a substitute for measures of performance prepared in accordance with IFRS and may be different from non-IFRS measures used by other companies. In addition, these non-IFRS measures are not based on any comprehensive set of accounting rules or principles. Non-IFRS measures have limitations in that they do not reflect all of the amounts associated with our results of operations as determined in accordance with IFRS. These non-IFRS financial measure should only be used to evaluate our results of operations in conjunction with the most comparable IFRS financial measures.
Adjusted EBITDA
We define Adjusted EBITDA as profit/(loss) exclusive of financial income/(costs), income tax benefit/(expense), depreciation, amortization, share-based compensation, inventory purchase allocation and one-time transactional expenses.
We believe that Adjusted EBITDA provide useful supplemental information to investors about us and our results. Adjusted EBITDA is among the measures used by our management team to evaluate our financial and operating performance and make day-to-day financial and operating decisions. In addition, Adjusted EBITDA, similarly titled measures are frequently used by our competitors, rating agencies, securities analysts, investors and other parties to evaluate companies in our industry. We also believe that Adjusted EBITDA is helpful to investors because they provide additional information about trends in our core operating performance prior to considering the impact of capital structure, depreciation, amortization and taxation on our results. Adjusted EBITDA should not be considered in isolation or as a substitute for other measures of financial performance reported in accordance with IFRS. Adjusted EBITDA has limitations as an analytical tool, including:
| ● | Adjusted EBITDA does not reflect changes in, including cash requirements for, our working capital needs or contractual commitments; |
| ● | Adjusted EBITDA does not reflect our financial expenses, or the cash requirements to service interest or principal payments on our indebtedness, or interest income or other financial income; |
76
| ● | Adjusted EBITDA does not reflect our income tax expense or the cash requirements to pay our income taxes; |
| ● | although depreciation and amortization are non-cash charges, the assets being depreciated or amortized often will need to be replaced in the future, and Adjusted EBITDA does not reflect any cash requirements for the replacements; |
| ● | although share-based compensation is a non-cash charge, Adjusted EBITDA does not consider the potentially dilutive impact of share-based compensation; and |
| ● | other companies may calculate Adjusted EBITDA, and similarly titled measures differently, limiting its usefulness as a comparative measure. |
We compensate for the inherent limitations associated with using Adjusted EBITDA through disclosure of these limitations, presentation of our consolidated financial statements in accordance with IFRS and reconciliation of Adjusted EBITDA to the most directly comparable IFRS measure, income/(loss) for the period or year.
The table below provides a reconciliation of our income or loss for the period/year to Adjusted EBITDA:
Year Ended June 30, | ||||
| 2023 |
| 2022 | |
(in millions of US$) | ||||
Reconciliation of Net Loss to Adjusted EBITDA: |
|
|
|
|
Profit (Loss) |
| 20.2 |
| (3.9) |
Income tax |
| (1.1) |
| 18.0 |
Financial results |
| 35.1 |
| 25.8 |
Depreciation of property, plant and equipment |
| 8.4 |
| 5.0 |
Amortization of intangible assets |
| 11.0 |
| 4.2 |
Share-based incentive and stock options |
| 3.4 |
| 1.4 |
Transactional expenses |
| 4.2 |
| 1.0 |
Adjusted EBITDA (unaudited) |
| 81.2 |
| 51.5 |
B.Liquidity and Capital Resources
Overview
Since our inception, we have funded our operations primarily with sales of our products, borrowings, including the issuance of notes and corporate bonds, and equity contributions from our shareholders. Our principal use of cash is to fund our operations, investments in intangible assets, expenditures in property, plant and equipment, working capital requirements and repayment of debt obligations.
As of June 30, 2023, our total indebtedness was US$243.5 million, of which approximately 56% consisted of long-term obligations. Cash and cash equivalents, short-term deposits and other short-term investments represented approximately 56 % of the current portion of debt. As of June 30, 2023, our cash and cash equivalents amounted to US$48.1 million, and we held other current financial assets that amounted to US$12.1 million.
We believe that our existing cash and cash equivalents, cash inflows from revenue and borrowings (including refinancing debt and credit lines) will be adequate to meet our anticipated cash needs for the next 12 months.
Our ability to generate cash is subject to our performance, general economic conditions, industry trends and other factors. To the extent that our existing cash and cash equivalents are insufficient to fund our future activities and requirements, we may need to raise additional funds through public or private equity or debt financing. If we issue equity securities in order to raise additional funds, substantial dilution to existing shareholders may occur. If we raise cash through the issuance of indebtedness, we may be subject to additional contractual restrictions on our business. We cannot assure the investor that we would be able to raise additional funds on favorable terms or at all.
77
Cash flows
Set forth below is a comparative discussion of our cash flows, which includes cash flows from discontinued operations.
Statement of Cash Flows
The tables below illustrate our statement of cash flows for the periods indicated:
Year Ended June 30, | ||||
| 2023 |
| 2022 | |
(in millions of US$) | ||||
Statement of cash flow |
|
|
| |
Net cash flows used in (generated by) operating activities | 2.6 |
| (17.5) | |
Net cash flows generated by investing activities | (25.7) |
| 2.9 | |
Net cash flows generated by financing activities | 33.0 |
| 14.8 | |
Net increase (decrease) in cash and cash equivalents | 9.8 | 0.2 | ||
Inflation effects on cash and cash equivalents | (0.1) | (9.6) | ||
Effect of exchange rate changes on cash and equivalents | 4.9 | 6.8 | ||
We believe that funds from operations, the availability of liquid financial assets and our access to external borrowing through the financial markets will be sufficient to satisfy our working capital needs, to finance our planned capital spending program and to service our debt in the future twelve months and to address short-term changes in business conditions.
Net cash flows generated by (used in) operating activities
Cash generated by operating activities for the year ended June 30, 2023 amounted to US$2.6 million. Our loss of US$20.2 million, non-cash positive adjustments relating primarily to financial results of US$35.1 million, transactional expenses and share-based incentives of US$7.6 million, changes in the net realizable value of agricultural products after harvest of US$4.4 million and depreciation and amortization charges of US$19.3 million were offset by working capital outflows of US$83.8 million.
Cash used in operating activities for the year ended June 30, 2022 amounted to US$17.5 million. Our loss of US$3.91 million non-cash positive adjustments for income tax expense of US$18.0 million, non-cash positive adjustments relating primarily to financial results of US$25.8 million, depreciation and amortization charges of US$9.2 million were offset by other negative non-cash adjustments of US$4.3 million and working capital outflows of US$62.3 million.
Net cash flows generated by (used in) investing activities
Cash used in investing activities for the year ended June 30, 2023 amounted to US$25.7 million and was primarily attributable to net investments in property, plant and equipment of US$11.2 million and purchases and capitalized development expenditures relating to intangible assets of US$11.2 million and net investment in financial assets of US$7.7 million which was partially offset by cash acquired in the Pro Farm Merger.
Cash generated by investing activities for the year ended June 30, 2022 amounted to US$2.9 million and was primarily attributable to net proceeds from financial assets of US$10.3 million partially offset by net investments in property, plant and equipment of US$1.4 million and purchases and capitalized development expenditures relating to intangible assets of US$5.5 million.
Net cash flows generated by financing activities
Cash provided by financing activities for the year ended June 30, 2023 amounted to US$33.0 million and consisted mainly of net proceeds from borrowings of US$40.3 million, partially offset by leased assets payments and the purchase of own shares for US$3.0 million and U$S3.9 million, respectively.
Cash provided by financing activities for the year ended June 30, 2022 amounted to US$14.8 million and consisted mainly of net proceeds from borrowings of US$16.8 million, partially offset by leased assets payments and the consideration of the non-controlling interest acquired for US$1.0 million and U$S0.7 million, respectively.
78
Indebtedness
As of June 30, 2023, our total outstanding borrowings, including interest accrued, were US$168.3 million, which consists of US$107.6 million of current borrowings, including US$61.3 million of the short-term portion of long-term loans, US$35.5 million in corporate bonds, US$7.3 million in trust debt securities and US$3.5 million parent companies and related parties to the Parent, US$135.9 million of non-current corporate bonds of US$50.0 million and borrowings of US$10.7 million. In addition, as of June 30, 2023, we had US$75.2 million non-current debt from the Notes due 2026.
As of June 30, 2023, our borrowings denominated in US dollars totaled US$143.2 million, which bear a fixed weighted average interest rate of 2.12%. Our borrowings denominated in Argentine Pesos totaled US$14.4 million that bear a fixed weighted average interest rate of 87.11%. Our borrowings denominated in Brazilian reais totaled US$10.7 million and bear a fixed weighted average interest rate of 12.39%.
As of June 30, 2023, of our total outstanding borrowings, US$168.3 million was unsecured.
Issuance of Notes
See “Item 10. Additional Information—C. Material Contracts— Issuance of Notes due 2026.”
Bioceres LLC Loan
On May 7, 2019, we entered into an agreement with Bioceres LLC for the restructuring of US$15 million of our outstanding intercompany loans into a facility with a 5-year maturity. Cash interest is payable quarterly at a 9.875% rate. As of June 30, 2023, the principal outstanding debt amounted to US$3.5 million.
Public Corporate Bonds
Below is a summary of public corporate bonds outstanding as of June 30, 2023:
Sector |
| US$million |
| Rate |
| Maturity |
IV |
| 17.0 |
| 0.00 | % | August 18, 2023 |
V– Class B |
| 20.8 |
| 5.50 | % | March 5, 2024 |
VI – Class B |
| 3.4 |
| 5.25 | % | September 7, 2024 |
VII |
| 20.0 |
| 1.49 | % | December 28, 2024 |
VIII – Class A | 21.5 | 1.5 | % | February 10, 2025 | ||
VIII – Class B |
| 5.0 |
| 3.98 | % | February 10, 2026 |
Series IV
On August 13, 2020, we issued US$17.0 million Series IV corporate bonds in Argentina. The Series IV bonds mature on August 18, 2023 and pay an annual nominal interest rate of 0.0%.
Series V
On March 4, 2021, we issued US$26.0 million Series V corporate bonds in Argentina. The bonds were issued in two tranches: the Class A bonds matured in March 2022 and paid an annual nominal interest rate of 0.98% and the Class B bonds mature in March 2024 and pay an annual nominal interest rate of 5.5%.
Series VI
On August 31, 2021, we issued US$16.1 million Series VI corporate bonds in Argentina. The bonds were issued in two tranches: the Class A bonds matured in March 2023 and paid an annual nominal interest rate of 3.75% and the Class B bonds mature in September 2024 and pay an annual nominal interest rate of 5.25%.
79
Series VII
On December 23, 2021, we issued US$20.0 million Series VII corporate bonds in Argentina. The Series VII bonds mature in December 2024 and pay an annual nominal interest rate of 1.49%.
Series VII
On February 8, 2023, we issued US$26.5 million Series VIII corporate bonds in Argentina. The bonds were issued in two tranches: the Class A bonds mature in February 2025 and pay an annual nominal interest rate of 1.5% and the Class B bonds mature in February 2026 and pay an annual nominal interest rate of 3.98%.
Consideration of payment of acquisitions
Bioceres Crops S.A.
In June 2019, we signed a share purchase agreement with Bioceres S.A. for 50% of ownership in Bioceres Crops. In consideration for the share purchase agreement, we will pay installments equivalent to 50% of the royalties that Bioceres Crops Solutions will accrue from Bioceres Crops, up to a total amount of US$0.7 million.
Verdeca and other intangibles assets
On November 12, 2020, we acquired from Arcadia Biosciences the remaining ownership interest in Verdeca. As part of this transaction, we gained full access and control of Verdeca’s vetted soybean library of gene-edited materials used to develop new quality and productivity, as well as exclusive rights to all Arcadia technologies that are applicable to soybean. In addition, through this transaction, we have also acquired rights to Arcadia’s quality wheat traits and the related Good Wheat™ brand for Latin America. In consideration for the acquisition, we paid Arcadia US$5 million in cash and US$15 million in equity consisting of 1,875,000 of our ordinary shares, as well as US$1 million in reimbursement costs associated with the transaction. In addition, we will pay Arcadia (i) US$2 million upon obtaining Chinese import clearance for HB4 soy or achieving penetration of this technology in a minimum number of planted hectares, and (ii) royalty payments equivalent to (a) 6% of the net HB4 soy technology revenues realized by Verdeca, capped at US$10 million aggregate amount, and (b) 25% of the net wheat technology revenues resulting from the in-licensed materials. On April 22, 2022, Verdeca obtained the Chinese import clearance for HB4 and we have already paid US$2 million for the contingent payment mentioned earlier.
Insuagro
On April 9, 2021, as part of the reorganization process of our crop protection business segment, we acquired a controlling interest in Insuagro, an Argentine public company listed on Bolsas y Mercados Argentinos S.A. (“BYMA”). The interest acquired is represented by a total of 11,022,000 shares, distributed as follows: (i) 2,749,390 ordinary, registered shares of AR$0.10 nominal value each and five votes per share, denominated Class A; and (ii) 8,272,610 ordinary, registered shares of Pesos 0.10 nominal value each and one vote per share, denominated Class B, jointly representing 50.1% of equity interest and 55.05% of voting interest.
The consideration for the acquisition was US$0.282 per share, totaling an amount of US$3.1 million (the “Fixed Price”). At closing, we paid US$0.2 million, and the rest is payable in three installment due August 31, 2022, 2023 and 2024 for an amount of US$0.9 million, US$0.9 million and US$1.2 million, respectively. The amount payable will accrue an interest annual rate of 5.5%. Furthermore, the Fixed Price may be increased up to 3.5x Adjusted EBITDA (as defined in the share exchange agreement) per share to be measured in each annual reporting period. As we reached a controlling interest in Insuagro and pursuant to the Argentine Capital Market Law and the National Securities Commission rules, we submitted a mandatory public offer of acquisition (“OPA”, which is the anacronym in Spanish) of the Class B ordinary shares listed at BYMA. As a result of the OPA, we acquired 2,467,990 ordinary shares Class B, increasing our shareholding in Insuagro to 61.32% of the equity interest and 61.22% of the voting rights.
On August 31, 2022 and based on the Adjusted EBITDA (as defined in the share exchange agreement) measured for the year ended June 30, 2022, the fixed price was increased by US$0.965 per share and was fully paid in the year ended June 30, 2023.
80
C.Research and Development, Patents and Licenses, etc.
For a discussion of our research and development policy, see “Item 4. Information on the Company-B. Business Overview- In-House R&D, Technology Sourcing and Product Development Timeline and Process”. For a discussion of patent and licenses, see “Item 4. Information on the Company-B. Business Overview-Intellectual Property.”
D.Trend Information
For a discussion of trend information, see “—A. Operating Results—Factors affecting our results of operations.”
E.Critical Accounting Estimates
The preparation of our consolidated financial statements and related disclosures in accordance with IFRS requires our management to make certain judgments, estimates and assumptions regarding the future. Estimates and judgments are continually evaluated based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. In the future, actual experience may differ from these judgments, estimates and assumptions. The judgments, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed in our audited consolidated financial statements included elsewhere in this report.
In order to provide an understanding of the manner in which our management forms its judgments about future events, including the variables underlying our judgments, estimates and assumptions, we summarize our critical accounting policies in Note 5 to our audited consolidated financial statements included elsewhere in this report.
ITEM 6.DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A.Directors and Senior Management
Directors
As of June 30, 2023, the following persons (with ages as of the date of this report) are the directors and executive officers of Bioceres Crop Solutions.
Our Executive Officers and Directors
Name |
| Age | Position |
| Initial Appointment | |
Federico Trucco, Ph.D. |
| 46 | Chief Executive Officer and Executive Director |
| February 27, 2019 | |
Enrique Lopez Lecube |
| 41 | Chief Financial Officer and Executive Director |
| February 27, 2019 | |
Gloria Montaron Estrada |
| 52 | Non-Executive Director |
| March 14, 2019 | |
Natalia Zang |
| 47 | Non-Executive Director |
| February 27, 2019 | |
Ari Freisinger |
| 38 | Non-Executive Director |
| February 27, 2019 | |
Yogesh Mago |
| 42 | Non-Executive Director |
| July 12, 2022 | |
Keith McGovern |
| 58 | Non-Executive Director |
| July 12, 2022 |
81
Biographical information for each member of our board of directors and senior management is set forth below.
Federico Trucco. Federico Trucco, Ph.D., has served as our chief executive officer since June 2011 and was appointed as a member of Bioceres S.A.’s board of directors in December 2014 and President of Bioceres LLC (formerly Bioceres Inc.) in February 2012. He previously served in various positions at INDEAR including as general manager from 2009 to 2011, director of product development from 2008 to 2009 and research team leader of the Amaranth project from 2005 to 2009. Dr. Trucco also serves as president of Rasa Holding LLC, director and president of BCS Holding Inc., manager of Verdeca, director of Trigall, director of Rizobacter Argentina, director of Bioceres Semillas, vice president of Bioceres Crops S.A. (formerly SEMYA), director of Synertech Industrias S.A., director of Heritas S.A. and director of Moolec Science Ltd. Dr. Trucco received a Ph.D. in crop sciences and a CBA from the University of Illinois, a Master of Science degree in plant pathology and weed science from the Colorado State University and a bachelor’s degree in biochemistry from the Louisiana State University. In 2018, Federico was recognized with the Konex Award for business innovation for 2008-18, one of Argentina’s most prestigious leadership prizes. He was also recognized with the EY Entrepreneur of the Year Award for Argentina in 2019. Mr. Trucco was appointed President of the Argentine Chamber of Biotechnology in 2020.
Enrique López Lecube. Enrique López Lecube has served as our chief financial officer since November 2017. Mr. Lopez Lecube serves as director of Bioceres Semillas and Bioceres Crops S.A. (formerly SEMYA). He has served as Manager for M&A and Corporate Finance at Lartirigoyen & Cia S.A. – a Glencore subsidiary – since 2016, where he has led transactions in the food, chemical and service industries and worked in the finance department to oversee 10 group entities. Previously, he worked as head trader from 2010 to 2016 at Lartirigoyen & Cia S.A. From 2008 to 2010, he worked at Cargill focusing on grain and oilseed. Mr. López Lecube received an MBA from Kellogg of School of Management of Northwestern University and a degree as an industrial engineer from ITBA (Instituto Tecnológico de Buenos Aires).
Gloria Montaron Estrada. Gloria Montaron Estrada is the Legal and Intellectual Property Director of Bioceres Group PLC and manager of Bioceres LLC, our controlling shareholder. She has served as our non-executive director since March 2019. She previously served as an attorney at Marval, O’Farrell & Mairal in Buenos Aires for 16 years, where her practice as centered around intellectual property, corporate and banking matters. Ms. Montaron also serves as a director of Bioceres LLC (formerly Bioceres Inc.), BCS Holding Inc., Bioceres Tech Services LLC, AG Biomolecules LLC, BF Farming Technologies Ltd. and SF500 Trust. She received an LLM in intellectual property from the University of Palermo, Buenos Aires, a degree in Law from the University of Buenos Aires, Argentina, and she also graduated as Intellectual Property Agent in 2013. Ms. Montaron is an active international lecturer in law congresses and seminars on her field of practice, and she also teaches at various universities in the area of intellectual property. Bioceres’ legal team, led by Ms. Montaron, won the award of “Deal of the Year – Private Equity” in 2019. Ms. Montaron was included at the GC Powerlist of Legal500 in Argentina. Ms. Montaron is a member of the Bar Association of the City of Buenos Aires.
Natalia Zang.Ms. Zang serves as one of our non-executive directors. Ms. Zang is a business leader with more than 25 years of experience in private equity and corporate finance in Latin America, Europe and Australia. Ms. Zang serves as our non-executive director and chair of our audit committee. She is currently advisor of Jazzya Investments, CFO of Overture Life and member of the Investment Committee of MBV Fund. Previously, Ms. Zang held C-level positions in several industries, including retail and real estate. In late 2015, Ms. Zang joined President Macri’s administration in Argentina, initially as Undersecretary for the Chief of Staff and then as Secretary (General Coordinator of the G20). Ms. Zang is a sought-after lecturer on business and women’s leadership issues, and she is active in mentoring programs for students and women entrepreneurs. Ms. Zang received a master’s degree in finance from the Universidad del CEMA and a bachelor’s degree in Business Administration from the Universidad Torcuato Di Tella.
Ari Freisinger. Ari Freisinger has served as our non-executive director since March 2019. He was a Principal at Highfields Capital from 2010-2018. Prior to that, he served as an investment banker at Barclays Capital in the mergers & acquisitions group. Mr. Freisinger received a master’s degree in economics and social history from the University of Oxford and a bachelor’s degree in economics from Columbia University. He currently serves on the board of the Jewish Family Service of Metrowest, a philanthropic organization in the greater Boston area.
Yogesh Mago. Yogesh Mago has served as our non-executive director since July 12, 2022. He has been a senior advisor for Ospraie Ag Science LLC, following the closing of the Pro Farm Merger one of our shareholders, since October 2016 and has over 15 years of experience in investing across a variety of industries globally, including agriculture, travel, consumer, transportation, industrials and real estate. Mr. Mago is the president and co-founder of Operation Water Inc., a nonprofit organization that aims to deliver sustainable access to clean water in impoverished countries through the development of scalable infrastructure projects. In addition, he is on the Advisory Board of Girl Rising, the nonprofit organization behind the worldwide social action campaign for girls’ education and empowerment. Mr. Mago has a Bachelor’s degree in Finance and International Business from New York University.
82
Keith McGovern. Mr. McGovern has over 30 years of experience in the agriculture industry, specializing in leading commercial potato farming and potato processing operations. He is President of R.D. Offutt Farms, a division of R.D. Offutt Company, one of the largest farming and food processing concerns in the United States. Mr. McGovern joined R.D. Offutt Company in August 1988. Mr. McGovern serves on the Management Committee of Lamb-Weston/RDO Frozen, a joint venture frozen potato product processing plant. He also serves on the Board of Alliance for Potato Research and Education, on the Management Committee for Columbia River Technologies, a whey processor in partnership with Tillamook and Fonterra, and the Management Committee for Simplot RDO, a frozen vegetable plant in Pasco, Washington. Mr. McGovern is a graduate of Embry Riddle Aeronautical University with a degree in Aeronautical Science and is still an active pilot.
B.Compensation
Remuneration of Directors and Senior Management
The aggregate compensation, including benefits in kind, accrued or paid to our senior management with respect to the year ended June 30, 2023, for services in all capacities was US$1.42 million. In addition, in the year ended June 30, 2023, we granted our senior management options to purchase US$1.2 million of our ordinary shares.
Annual Bonuses
The Annual Bonus is an annual cash incentive bonus awarded to certain employees and not contemplated in the Equity Compensation Plans, to tie a portion of their compensation to financial and operational objectives achievable within the applicable fiscal year according to a target. The objectives and other terms and conditions of the annual cash bonuses for all its employees are determined every year.
Equity Compensation Plans
2023 Omnibus Equity Incentive Plan
On May 12, 2023, our board of directors approved the 2023 Omnibus Equity Incentive Plan (the “Plan”), to attract and retain the best available personnel, provide additional incentives to employees, directors and consultants and to promote the success of our business. Under the Plan, the maximum aggregate number of shares that may be issued pursuant to all awards is 1,700,000 ordinary shares. The Plan allows us to establish the terms and conditions of the equity awards granted thereunder.
Our board of directors or our Compensation Committee shall determine the manner and timing of vesting, exercise and expiration of options granted under the Plan. Option periods shall not exceed ten years, unless the option period (other than in the case of an incentive share option) would expire at a time when trading in the Shares is prohibited by our securities trading policy or a self-imposed “blackout period,” in which case options period shall be extended automatically until the 30th day following the expiration of such prohibition (so long as such extension shall not violate Section 409A of the Internal Revenue Code of 1986). We may accelerate the vesting and/or exercisability of any option, which acceleration shall not affect any other terms and conditions of such option.
Amount set aside for pension, retirement or similar benefits
The total amounts set aside or accrued by us to provide pension, retirement or similar benefits was US$186,713 for the fiscal year ended June 30, 2023.
Director Compensation
Our board of directors has established a compensation program for non-executive directors, which consists of an annual retainer, board fees in accordance with their attendance at board meetings and committee fees for their service as members of a committee. We also reimburse our independent directors for reasonable out-of-pocket expenses incurred in connection with the performance of their duties as directors, including, without limitation, travel expenses in connection with their attendance in-person at board and committee meetings. Executive Directors do not receive any compensation for their services as directors.
83
C.Board Practices
Our Articles provide that the board of directors must comprise at least one member. Our board currently consists of seven directors. Each of our directors will have a term that expires at our annual general meeting in 2023, or until their respective successors are duly appointed and qualified, or until their earlier resignation, removal or death. None of our directors have service contracts with us or any of our subsidiaries providing for benefits upon termination of employment.
Audit Committee
As of the date of this report, our audit committee consists of Natalia Zang, Yogesh Mago and Ari Freisinger, with Natalia Zang serving as the chair of the audit committee. Each of Yogesh Mago, Ari Freisinger and Natalia Zang meets the applicable audit committee independence standards. Ari Freisinger qualifies as an “audit committee financial expert,” as such term is defined in applicable SEC rules.
Our audit committee will, among other matters, oversee (i) our financial reporting, auditing and internal control activities; (ii) the integrity and audits of our financial statements; (iii) our compliance with legal and regulatory requirements; (iv) the qualifications and independence of our independent auditors; (v) the performance of our internal audit function and independent auditors; and (vi) our overall risk exposure and management. Duties of the audit committee will also include the following:
| ● | annually review and assess the adequacy of the audit committee charter and the performance of the audit committee; |
| ● | be responsible for recommending the appointment, retention and termination of our independent auditors and determine the compensation of the independent auditors; |
| ● | review the plans and results of the audit engagement with the independent auditors; |
| ● | evaluate the qualifications, performance and independence of the independent auditors; |
| ● | have sole authority to approve in advance all audit and non-audit services by the independent auditors, the scope and terms thereof and the fees therefor; |
| ● | review the adequacy of our internal accounting controls; and |
| ● | meet at least quarterly with our executive officers, internal audit staff and independent auditors in separate executive sessions. |
Our board of directors adopted a written charter for the audit committee, which is available free of charge on Bioceres Crop Solutions’ corporate website at biocerescrops.com. The information on our website is not part of this report.
Compensation Committee
As of the date of this report, our compensation committee consists of Ari Freisinger, Enrique López Lecube and Yogesh Mago, with Ari Freisinger serving as the chair of the compensation committee. As a result of our status as a “controlled company” under the rules of Nasdaq, we were not required to have a compensation committee. However, we have established a compensation committee, the majority of which consists of independent directors.
The compensation committee has the sole authority to retain and terminate any compensation consultant, to assist in the evaluation of employee compensation and to approve consultants’ fees and the other terms and conditions of consultants’ retention. The compensation committee will also, among other matters:
| ● | assist the board of directors in developing and evaluating potential candidates for executive officer positions and oversee the development of executive succession plans; |
| ● | administer, review and make recommendations to our board of directors regarding our compensation plans; |
84
| ● | annually review and approve our corporate goals and objectives with respect to compensation for executive officers and, at least annually, evaluate each executive officer’s performance in light of such goals and objectives to set his or her annual compensation, including salary, bonus and any equity and non-equity incentive compensation, subject to approval by our board of directors; |
| ● | provide oversight of management’s decisions regarding the performance, evaluation and compensation of other officers; and |
| ● | review our incentive compensation arrangements to confirm that incentive pay does not encourage unnecessary risk-taking and review and discuss, at least annually, the relationship between risk management policies and practices, business strategy and executive officer compensation. |
Nominating and Governance Committee
Our nominating and governance committee consists of Gloria Montaron Estrada, Keith McGovern and Natalia Zang, with Gloria Montaron Estrada serving as the chair of the nominating and governance committee. As a result of our status as a “controlled company” under the rules of Nasdaq, we were not required to have a nominating and governance committee. However, we have established a nominating and governance committee, the majority of which consists of independent directors.
The nominating and governance committee will, among other matters:
| ● | evaluate the independence of directors and nominate individuals to serve as directors; |
| ● | review the committee structure of our board of directors and recommend directors to serve as members or chairs of each committee of our board of directors; |
| ● | review and recommend committee slates annually and recommend additional committee members to fill vacancies as needed; |
| ● | develop and recommend to our board of directors a set of corporate governance guidelines applicable to us and, at least annually, review such guidelines and recommend changes to our board of directors for approval as necessary; and |
| ● | oversee the annual self-evaluation of our board of directors. |
Code of Ethics
We have adopted a Code of Ethics applicable to our directors, executive officers and employees and to the board members, employees, and officers of our controlled companies. The Code of Ethics codifies the business and ethical principles that govern all aspects of our business. A copy of the Code of Ethics will be filed with the SEC and will be provided without charge upon written request to us in writing at investorelations@biocerescrops.com. We intend to disclose any amendments to or waivers of certain provisions of our Code of Ethics in a Current Report on Form 6-K (as required) or on our website.
Nasdaq Corporate Governance Exemptions
We are a “controlled company” under Nasdaq rules. Controlled companies under Nasdaq rules are companies of which more than 50% of the voting power for the appointment of directors is held by an individual, a group or another company. Bioceres PLC owns more than 50% of our ordinary shares and, as a result, under the Nasdaq’s current listing standards, we qualify for and intend to rely on the controlled company exception under the corporate governance rules of the Nasdaq. As a controlled company, we are not required to have (i) a majority of “independent directors” on our board of directors or (ii) a compensation committee and a nominating and governance committee, although we have established a compensation committee and a nominating and governance committee, the majority of which consists of independent directors. The controlled company exception does not modify the independence requirements for the audit committee, and we have complied with the requirements of Nasdaq rules, which require that our audit committee be composed of at least three members, all of whom are independent directors.
85
D.Employees
The table below shows our employees by role and location, as of the dates indicated, and does not include employees of our research collaborators or joint venture partners.
As of June 30, |
| ||||||
| 2023 |
| 2022 |
| 2021 |
| |
Management, administrative and manufacturing |
| 462 |
| 359 | 283 | ||
Sales |
| 222 |
| 179 | 177 | ||
Argentina |
| 114 |
| 96 | 104 | ||
Brazil |
| 51 |
| 55 | 54 | ||
Rest of Latin America |
| 16 |
| 17 | 15 | ||
North America, Europe and Asia |
| 41 |
| 11 | 4 | ||
Research and development services |
| 106 |
| 27 | 37 | ||
Total |
| 790 |
| 565 | 497 | ||
Our management, administrative and research and development services employees are located mainly in Argentina.
E.Share Ownership
The table below sets forth information regarding the beneficial ownership of our ordinary shares as of September 30, 2023, by our directors and senior management and major shareholders. For the purposes of this table, a person is deemed to have “beneficial ownership” of any shares on a given date in which such person has the right to acquire within the 60 days after such date. For the purpose of computing the percentage of outstanding shares held by each person, or group of persons, named below on a given date, any security which such person or persons have the right to acquire within 60 days after such date is deemed to be outstanding, but is not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. Except as otherwise indicated, the holders listed below have sole voting and investment power with respect to all shares beneficially owned by them. They have the same voting rights as all other holders of ordinary shares.
Beneficial owners |
| Number of shares |
| % owned (3) |
|
Federico Trucco |
| 413,674 |
| * | |
Enrique López Lecube |
| 270,590 |
| * | |
Gloria Montaron Estrada |
| 164,425 |
| * | |
Natalia Zang |
| — |
| * | |
Ari Freisinger |
| 62,483 |
| * | |
Yogesh Mago |
| 55,399 |
| * | |
Keith McGovern |
| — |
| * | |
5% Shareholders |
|
|
| ||
Bioceres LLC(2) |
| 31,560,863 |
| 50.2 | % |
Solel Partners LP |
| 4,579,364 |
| 7.3 | % |
Notes:—
*Less than 1%.
| (1) | Includes shares held by Union Acquisition Associates, LLC, an entity controlled by Mr. Bransfield. |
| (2) | Represents shares and the shared power to vote 3,196,917 ordinary shares pursuant to Rizobacter Shareholders’ Agreement, dated as of March 5, 2019, by and between Bioceres LLC, Pedro Enrique Mac Mullen, Maria Marta Mac Mullen and International Property Services Corp., held by Bioceres LLC, a limited liability company incorporated under the laws of Delaware, with its registered office at 1209 Orange Street, Wilmington 19801-1120, County of New Castle. Bioceres LLC is wholly owned by Bioceres S.A., a company organized under the laws of Argentina with its registered office at Ocampo 2010bis, Predio CCT, Rosario, Argentina. As a result, Bioceres PLC may be deemed to be the ultimate beneficial owner of shares of common stock held by Bioceres LLC. 8.15% of Bioceres PLC’s equity interest is owned by Garruchos S.A. and 54.9% of Bioceres S.A.’s equity interest owned by Bioceres PLC. |
| (3) | 62,796,774 ordinary shares outstanding as of June 30, 2023. |
86
For more information on our shares and share options owned by individual Directors and by individual members of our Executive Committee see “Item 6. Directors, Senior Management and Employees—B. Compensation—Bioceres Crop Solutions Stock Option Plan.”
F.Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
Not applicable.
ITEM 7.MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A.Major Shareholders
See “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
Changes in Ownership
We were informed of the following significant changes in the percentage ownership held by our major shareholders during the past three years:
| ● | On February 14, 2023, Solel Partners LP filed a Schedule 13G reporting reporting beneficial ownership of 4,579,364 ordinary shares, representing 7.3% of our share capital. |
| ● | On January 25, 2023, Ardsley Advisory Partners LP, Ardsley Advisory Partners GP LLC, Ardsley Partners I GP LLC, Philip J. Hempleman and Ardsley Partners Renewable Energy Fund, L.P. filed a Schedule 13G. Ardsley Advisory Partners LP, Ardsley Advisory Partners GP LLC, Ardsley Partners I GP LLC and Philip J. Hempleman reported beneficial ownership of 1,854,000 ordinary shares, representing 3.0% of our share capital. Ardsley Partners Renewable Energy Fund, L.P. reported beneficial ownership of 1,845,000, representing 2.9% of our share capital. |
| ● | On November 18, 2022, DRACO Capital Investment Management Company S.A. and Draco I Event Opportunity Segregated Portfolio filed a Schedule 13G/A reporting beneficial ownership of 215,000 ordinary shares, representing 0.3% of our share capital. |
| ● | On November 17, 2022, Bioceres S.A., Bioceres LLC and THEO I SCSp filed a Schedule 13D/A reporting beneficial ownership of 31,560,683 ordinary shares, representing 50.2% of our share capital. |
| ● | On August 4, 2022, DRACO Capital Investment Management Company S.A. and Draco I Event Opportunity Segregated Portfolio filed a Schedule 13G/A reporting beneficial ownership of 8,376,900 ordinary shares, representing 13.5% of our share capital. |
| ● | On July 25, 2022, Bioceres LLC, Bioceres S.A. and THEO I SCSp filed a Schedule 13D/A reporting beneficial ownership of 23,554,111 ordinary shares, representing 37.8% of our share capital. |
| ● | On July 15, 2022, Ospraie Ag Science LLC, Ospraie Management, LLC, Ospraie Holding I, LP, Ospraie Management, Inc., OAS MM, LLC and Dwight Anderson filed a Schedule 13G reporting beneficial ownership of 6,233,590 ordinary shares, representing 10.0% of our share capital. |
| ● | On April 5, 2022, Bioceres LLC, Bioceres S.A. and THEO I SCSp filed a Schedule 13D/A reporting a beneficial ownership of 23,487,020 ordinary shares, representing 51.2% of our share capital. |
| ● | On February 14, 2022, DRACO Capital Investment Management Company S.A. and Draco I Event Opportunity Segregated Portfolio filed a Schedule 13G/A reporting a beneficial ownership of 8,158,878 ordinary shares, representing 19.9% or our share capital. |
87
| ● | On October 15, 2021, Bioceres LLC, Bioceres S.A. and THEO I SCSp filed a Schedule 13D/A reporting a beneficial ownership of 23,572,333 ordinary shares, representing 57.4% of our share capital. |
| ● | On October 4, 2021, DRACO Capital Investment Management Company S.A. and 5D+ Draco I Latam Income Plus Segregated Portfolio filed a Schedule 13G/A reporting a beneficial ownership of 8,051,973 ordinary shares, representing 19.6% of our share capital. This 13G/A was also filed by Biotech Investment Holding Ltd. which ceased to be the beneficial owner of more than five percent of our ordinary shares. |
| ● | On August 6, 2021, Bioceres LLC and Bioceres S.A. filed a Schedule 13D/A reporting a beneficial ownership of 23,572,333 ordinary shares, representing 57.4% of our share capital, and 57.4% of our share capital, respectively. |
| ● | On August 5, 2021, DRACO Capital Investment Management Company S.A., 5D+ Draco I Latam Income Plus Segregated Portfolio and Biotech Investment Holding Ltd. filed a Schedule 13D/A reporting a beneficial ownership of 7,991,226 ordinary shares, representing 19.4% of our share capital. |
| ● | On July 2, 2021, filed a Schedule 13D/A DRACO Capital Investment Management Company S.A., 5D+ Draco I Latam Income Plus Segregated Portfolio and Biotech Investment Holding Ltd. filed a Schedule 13D/A reporting a beneficial ownership of 8,940,635 ordinary shares, representing 21.8% of our share capital. |
| ● | On February 16, 2021, 5D+ Capital Investment Management Company S.A., 5D+Draco I Latam Income Plus Segregated Portfolio and Biotech Investment Holding Ltd. filed a Schedule 13G/A reporting a beneficial ownership of 7,276,652 ordinary shares, representing 18.0% of our share capital. |
| ● | On February 16, 2021, Biotech International Investment Holding Ltd., The Electricas Trust and Merco Trustees (BVI) Limited filed a Schedule 13G/A reporting that they ceased to be the beneficial owners of more than five percent of our share capital. |
| ● | On November 25, 2020, Bioceres LLC and Bioceres S.A. filed a Schedule 13D/A reporting beneficial ownership of 22,604,544 ordinary shares, representing 56.1% % of our share capital. |
| ● | On August 28, 2020, Bioceres LLC and Bioceres S.A. filed a Schedule 13D/A reporting beneficial ownership of 24,644,721 ordinary shares, representing 63.7% of our share capital. |
| ● | On July 2, 2020, Bioceres LLC and Bioceres S.A. filed a Schedule 13D/A reporting beneficial ownership of 23,745,721 ordinary shares, representing 65.7% of our share capital. |
| ● | On July 2, 2020, 5D+ Capital Investment Adviser Company S.A. and Draco I Latam Corporate Income Plus Segregated Portfolio filed a Schedule 13G reporting beneficial ownership of 7,151,515 ordinary shares, representing 19.8% of our share capital. |
| ● | On May 8, 2020, 5D+ Capital Investment Adviser Company S.A. and Draco I Latam Corporate Income Plus Segregated Portfolio filed a Schedule 13G reporting beneficial ownership of 1,818,182 ordinary shares, representing 5.0% of our share capital. |
Difference in Voting Rights
All of our ordinary shares have the same voting rights and none of major shareholder has different voting rights.
Securities Held in the Host Country
As of June 30, 2023, we had 62,796,774 ordinary shares issued and outstanding, of which 42,871,580 ordinary shares, or 68%, were held by our 105 shareholders in the United States.
88
Arrangements for Change in Control
We are not aware of any arrangements that may, when in force, result in a change in control.
B.Related Party Transactions
Relationship with Bioceres PLC
As of the date of this report, Bioceres PLC owns approximately 45.2% of our ordinary shares. In addition, pursuant to the Rizobacter Shareholders’ Agreement, dated as of March 5, 2019, by and between Bioceres LLC (which is controlled by Bioceres PLC), Pedro Enrique Mac Mullen, Maria Marta Mac Mullen and International Property Services Corp., who own approximately 5.1 % of our ordinary shares, respectively, have agreed to vote in agreement with Bioceres LLC.
Policy Concerning Related Party Transactions
Our board of directors has adopted a written policy (the “related person transaction approval policy”), for the review of any transaction, arrangement or relationship in which it is a participant, if the amount involved exceeds US$120,000 and one of our executive officers, directors, director nominees or beneficial holders of more than 5% of our total equity (or their immediate family members), each of whom we refer to as a related person, has a direct or indirect material interest.
A copy of the related person transaction approval policy is available on our website.
Binding Memorandum of Understanding with Moolec Science SA
See “Item 4. Information on the Company—Recent Developments— Binding Memorandum of Understanding with Moolec Science SA.”
R&D Services Agreements
In connection with R&D services, Bioceres Semillas and Rizobacter typically enter into agreements with INDEAR.
Rizobacter Term. One-year term from the date of effectiveness. Once the initial one-year term expires, the parties can agree to extend the term of the Agreement for one year and can do so indefinitely.
Bioceres Semillas Term. One-year term from the date of effectiveness. Once the initial one-year term expires, the parties can agree to extend the term of the original contract for another two years and can do so indefinitely.
Rizobacter Compensation. In return for the services provided by INDEAR during the one-year term ended June 30, 2023, Rizobacter Argentina S.A. did not accrue any amount.
Bioceres Semillas Compensation. In return for the services provided by INDEAR during the one-year term ended June 30, 2023, Bioceres Semillas accrued an amount of US$2.5 million.
Intellectual Property. Under each contract, INDEAR’s counterparties will gain ownership over all intellectual property rights that arise from services rendered. The intellectual property rights include, but are not limited to, patents, trademarks, plant variety rights, trade secrets, and any intellectual property rights that arise out of the R&D services.
Confidentiality. The parties have agreed to keep the terms of the contract confidential, subject to certain exceptions, during the term of the contract and for a period of five years after effectiveness and/or termination of the contract.
Indemnification. INDEAR has agreed to indemnify each of the counterparties for any liability that arises out of the performance of R&D services.
89
Field Station Lease Agreement
INDEAR entered into a field station lease agreement with Rizobacter, pursuant to which Rizobacter Argentina S.A. leases its field station located in Pergamino.
Term. Three-year term from the date of effectiveness. Once the initial term expires, the parties can agree to extend the term of the agreement for one year and can do so indefinitely.
Compensation. INDEAR shall pay an annual amount of US$54,000.
Indemnification. INDEAR has agreed to indemnify Rizobacter Argentina S.A. for any liability that arises during the term of the Lease Agreement.
Storage Agreement
Synertech Industrias S.A. entered into a deposit and storage services agreement with Rizobacter (the “Deposit Agreement”), pursuant to which Rizobacter stores certain Synertech products on July 1, 2022.
Term. Two-year term from the date of effectiveness. Once the initial term expires, the parties can agree the extension of the term.
Compensation. In return for the deposit and storage services provided by Rizobacter, Synertech Industrias S.A must pay a monthly amount of US$6,500.
Indemnification. Rizobacter has agreed to indemnify Synertech Industrias S.A. for any liability that arises during the term of the Deposit Agreement related to the storage of the products.
License Agreements
Trigall Genetics S.A.
License agreement dated December 19, 2013, between BCS Holding Inc and Trigall Genetics S.A. (the “Trigall License Agreement”), pursuant to which BCS Holding Inc. granted to Trigall Genetics S.A. the exclusive sub-license of HB4 technology in wheat for research and commercial use of wheat in Argentina, Paraguay, Brazil and Uruguay.
Term. The Trigall License Agreement will remain in full force and effect until the date of dissolution of Trigall Genetics S.A.
Reservation of Rights. BCS Holding Inc. reserved the rights to research, develop, make or use (but not to sell, offer to sell or otherwise commercially exploit) HB4 technology solely for research purposes, during the term of the Trigall License Agreement within the defined territory of Argentina, Paraguay, Brazil and Uruguay.
Service Agreements to Joint Ventures
Trigall Genetics S.A., FD Admiral SAS, BCS Holding Inc. and INDEAR.
The service agreement dated December 19, 2013, by and among Trigall Genetics S.A., FD Admiral SAS and its affiliate Florimond Desprez Veuve & Fils SAS, BCS Holding Inc., Bioceres S.A. and INDEAR (the “Trigall Service Agreement”), pursuant to which research and development services are provided by BCS Holding Inc. and Florimond Desprez Veuve & Fils SAS through its affiliates.
Term. The Trigall Service Agreement will remain in full force and effect until the date of the dissolution of Trigall Genetics S.A.
Compensation. Trigall Genetics S.A. will pay to BCS Holding Inc. and Florimond Desprez Veuve & Fils SAS for the services provided. The services and the budget for such services is approved, every calendar year, by the board of directors of Trigall in advance.
90
Intellectual Property. All rights, titles and interest in or to the results and/or the intellectual property related thereto will vest in and be owned by Trigall Genetics S.A. All rights, title and interest in or to the intellectual property furnished to Trigall Genetics S.A. by BCS Holding Inc. or its affiliates and Florimond Desprez Veuve & Fils SAS or its affiliates in the framework of the Trigall Service Agreement remains owned by each party.
Synertech Industrias S.A. and Rizobacter.
Service Agreement dated June 30, 2016, by and between Synertech Industrias S.A. and Rizobacter (the “Synertech Services Agreement”), detailing the services regarding the production, management services and maintenance of the industrial facility.
Term. Nine-year term from date of effectiveness of the Synertech Services Agreement and its extensions. Once the term expires, the parties can agree the extension of the term.
Compensation. Synertech Industrias S.A. must pay an amount of US$25,764 on monthly basis.
Other Related Party Transactions
The following are certain other transactions with our directors, executive officers and shareholders.
Collaboration Agreement by and between Rizobacter and Espartina S.A. controlled by Marcelo Carrique, President of Rizobacter
Collaboration agreement by and between Espartina S.A. and Rizobacter (the “Collaboration Agreement”), pursuant to which Espartina S.A. performs services related to planting and harvesting of multiple crops for the 2021-2022 and 2022-2023 seasons and Rizobacter provides agricultural supplies at a price on arms’ length terms, that is, at the price offered to third parties under the same conditions. The parties to the Collaboration Agreement will distribute the profits according to their contributions.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. These agreements provide that the director or officer will be indemnified by us to the fullest extent permitted by law against liability and against all expenses reasonably incurred or paid by him or her in connection with any claim, action, suit or proceeding which he or she becomes involved as a party or otherwise by virtue of his or her being or having been such a director or officer of and against amounts paid or incurred by him or her in the resolution thereof. The agreements are subject to certain exceptions, including, among other exceptions, that no indemnification will be provided to any director or officer against any liability to us or our shareholders (i) by reason of actual fraud, dishonesty, actual fraudulent conduct, or gross negligence on the part of the director or officer; (ii) by reason of payment made under an insurance policy or any third party that has no recourse against the indemnitee director or officer; or (iii) if contrary to applicable law.
Secured Convertible Guaranteed Notes due 2026 and Secured Convertible Guaranteed Notes due 2026 Registration Rights Agreement
Funds affiliated with Mr. Ari Freisinger, a member of our board of directors, purchased US$9 million Secured Convertible Guaranteed Notes, see “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources— Issuance of Notes”, “Item 10. Additional Information—C. Material Contracts—Issuance of Notes due 2026—Secured Guaranteed Notes due 2026” and “Item 10. Additional Information—C. Material Contracts—Issuance of Notes—Secured Convertible Guaranteed Notes due 2026 Registration Rights Agreement.”
Shareholders Agreement
See “Item 10. Additional Information—C. Material Contracts—Sponsors Shareholders’ Agreement.”
C.Interests of Experts and Counsel
Not applicable.
91
ITEM 8.FINANCIAL INFORMATION
A.Consolidated Statements and Other Financial Information
Financial Statements
Our financial statements are set forth under “Item 18. Financial Statements.” The financial statements are attached to this Annual Report on Form 20-F. The audit report of Price Waterhouse & Co. S.R.L., independent registered public accounting firm, is included herein immediately preceding the consolidated financial statements.
Export Sales
As of June 30, 2023, 4% of our revenue are from export sales. Our total export revenues for the year ended June 30, 2023 totaled US$18.2 million.
Legal Proceedings
From time to time, we may become party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business. We are not currently involved in any legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition or results of operations, other than the proceeding described below. As of the date of this report, we do not face any claims of possible intellectual property infringement. We may become involved in material legal proceedings in the future as part of the ordinary course of our business.
An injunction in connection with the Rizobacter Acquisition may require us to surrender part of our interest in Rizobacter
Concurrently with the closing of business combination, the Rizobacter Call Option was exercised and we acquired 80.00% of Rizobacter’s capital stock, which we hold through our subsidiary RASA Holding. Of our total interest in Rizobacter, 29% of the shares we hold are subject to an injunction that affects 44% of the total share capital of Rizobacter. In addition, the injunction also requires that 30% of the dividends distributed on such shares be paid into a judicially created escrow account. The injunction relates to litigation among historical shareholders of Rizobacter arising from a disputed transfer of shares that occurred in 1995. Although the Argentine Supreme Court ruled against certain of the litigating historical shareholders, such shareholders subsequently pursued other legal recourse — including the injunction and non-innovative measure (medida de no innovar) — to further dispute the original transfer of shares. The non-innovative measure was overturned by an Argentine court of appeals on April 17, 2018.
Should the court rule against the free transferability of the affected shares, we would be obligated to return certain shares, thereby reducing our equity interest in Rizobacter.
Dividend Policy
We currently intend to retain any earnings for use in our business and do not intend, as of the date of this report, to pay cash dividends on our ordinary shares for the foreseeable future. Dividends, if any, on our outstanding ordinary shares will be proposed by our board of directors and subject to the approval of our shareholders. Even if our shareholders decide to distribute dividends, the form, frequency and amount of such dividends will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that our board of directors and shareholders may deem relevant. Currently, due to contractual restrictions, we are not entitled to distribute dividends to our shareholders.
B.Significant Changes
No significant changes have occurred since June 30, 2023.
ITEM 9.THE OFFER AND LISTING
A.Offer and Listing Details
Not applicable.
92
B.Plan of Distribution
Not applicable.
C.Markets
The Nasdaq Global Select Market.
D.Selling Shareholders
Not applicable.
E.Dilution
Not applicable.
F.Expenses of the Issue
Not applicable.
ITEM 10.ADDITIONAL INFORMATION
A.Share Capital
As of June 30, 2023, we had (i) 100,000,000 ordinary shares of a US$0.0001 par value authorized, (ii) 62,796,774 ordinary shares issued and outstanding, (iii) 1,000,000 preference shares of a US$0.0001 par value authorized, (iv) no preference shares issued and outstanding, (v) 4,042,869 ordinary shares reserved for our equity compensation plans. Of the total issued shares, we have repurchased 2,194,032, shares of our own.
Holders of the ordinary shares are entitled to one vote for each ordinary share.
B.Memorandum and Articles of Association
Registration Number
Our registration number with the Cayman Islands Registrar of Companies is 329214.
Objects
Our Articles state that the objects for which we are established are unrestricted and we shall have full power and authority to carry out any object not prohibited by the laws of the Cayman Islands.
Directors
Our Articles do not restrict our directors’ power to vote in respect of any contract or transaction in which they are interested (provided that the nature of the interest of any director in any such contract or transaction shall be disclosed by them at or prior to its consideration and any vote thereon), vote on compensation to themselves or any other members of their body in the absence of an independent quorum or exercise borrowing powers. There is no mandatory retirement age for our directors and our directors are not required to own our securities in order to serve as directors.
Ordinary Shares
Holders of ordinary shares are entitled to receive ratable dividends when and if declared by our board of directors out of funds legally available therefore, subject to any rights of any outstanding series of preferred shares.
93
Upon our winding up, liquidation and dissolution and after payment in full of all amounts required to be paid to creditors and to the holders of preferred shares having liquidation preferences, if any, holders of ordinary shares will be entitled to receive pro rata our remaining assets available for distribution.
The rights, powers and privileges of holders of our ordinary shares are subject to those of holders of any shares of our preferred shares or any other series or class of shares we may authorize and issue in the future.
Our ordinary shares are not subject to any sinking fund. All of our issued shares are fully paid up and none of our shareholders are liable for further capital calls. There are no provisions in the Articles that discriminate against any existing or prospective holder of our ordinary shares as a result of such shareholder owning a substantial number of shares.
Preferred Shares
Our Articles provide that preferred shares may be issued from time to time in one or more series. Our board of directors will be authorized to fix the voting rights, if any, designations, powers, preferences, the relative, participating, optional or other special rights and any qualifications, limitations and restrictions thereof, applicable to the shares of each series. Our board of directors will be able, without shareholder approval, to issue preferred shares with voting and other rights that could adversely affect the voting power and other rights of the holders of the ordinary shares and could have anti-takeover effects. The ability of the board to issue preferred shares without shareholder approval could have the effect of delaying, deferring or preventing a change of control of us or the removal of existing management. We have no preferred shares issued and outstanding at the date hereof. Although we do not currently intend to issue any preferred shares, we cannot assure you that we will not do so in the future.
Share Buy-Back Program
On May 6, 2020, our board of directors approved a program to repurchase its own securities for an aggregate of up to US$10,000,000 to enhance the allocation of capital to certain equity-finance obligations. As of June 30, 2023, we have acquired 667,578 shares under the Buy-Back Program.
Voting and Appointment of Directors
Voting Power
Except as otherwise required by law or as otherwise provided in any certificate of designation for any series of preferred shares, the holders of our ordinary shares will possess all voting power for the appointment of our directors and all other matters requiring shareholder action and will at all times vote together as one class on all matters submitted to a vote of our shareholders. Holders of our ordinary shares will be entitled to one vote for each share held of record on all matters on which shareholders are entitled to vote generally, including the appointment or removal of directors. Holders of our ordinary shares will not have cumulative voting rights in the appointment of directors.
Pre-emptive or Other Rights
There will be no sinking fund or redemption provisions applicable to our ordinary shares.
Appointment of Directors
Our board consists of eight directors. Each of our directors will have a term that expires at our annual general meeting in 2023, or until their respective successors are duly appointed and qualified, or until their earlier resignation, removal or death. There will be no cumulative voting with respect to the appointment of directors, with the result that directors will be appointed by a majority of the votes cast at our annual general meeting.
94
General Meetings
At least five clear days’ notice are required to be given of any general meeting, which notice shall specify the place, the day and the hour of the meeting and the general nature of the business to be conducted at the general meeting. No business will be transacted at any general meeting unless a quorum is present. The holders of a majority of the shares being individuals present in person or by proxy or if a corporation or other non-natural person by its duly authorized representative or proxy shall be a quorum. If a quorum is not present within half an hour from the time appointed for the meeting to commence or if during such a meeting a quorum ceases to be present, the meeting, if convened upon a shareholders’ request, will be dissolved and in any other case it shall stand adjourned to the same day in the next week at the same time and/or place or to such other day, time and/or place as the directors may determine, and if at the adjourned meeting a quorum is not present within half an hour from the time appointed for the meeting to commence, the shareholders present will be a quorum.
Annual General Meetings
Any annual general meeting will be held at such time and place as the directors shall appoint and if no other time and place is prescribed by them, it shall be held at the Registered Office on the second Wednesday in October of each year at ten o’clock in the morning. At these meetings the report of the directors (if any) shall be presented. Shareholders seeking to bring business before the annual general meeting or to nominate candidates for appointment as directors at the annual general meeting must deliver notice to our principal executive offices not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the scheduled date of the annual general meeting.
Extraordinary General Meetings
All general meetings other than annual general meetings are extraordinary general meetings. Shareholders holding not less than 10% in par value of the issued ordinary shares with voting rights can request, and the directors shall convene, extraordinary general meetings. Such shareholders’ request must state the objects of the meeting and must be signed by the requesting shareholders and deposited at the Registered Office.
If there are no directors as at the date of the deposit of the shareholders’ request or if the directors do not within twenty-one days from the date of such request duly proceed to convene a general meeting to be held within a further twenty-one days, the requesting shareholders, or any of them representing more than 50% of the total voting rights of all of the requesting shareholders, may themselves convene a general meeting, but any meeting so convened shall be held no later than the day which falls three months after the expiration of the said twenty-one day period.
Our Articles do not contain any provisions that would have an effect of delaying, deferring or preventing a change in control of our Company. Our Articles provide we shall have the power to merge or consolidate with one or more other constituent companies (as defined in the Cayman Islands Companies Act (As Revised)) upon such terms as our directors may determine and (to the extent required by the Cayman Islands Companies Act (As Revised)) with the approval of a Special Resolution.
Our Articles do not contain any provisions governing the ownership threshold above which shareholder ownership must be disclosed.
Our Articles are not significantly different from the requirements of the Cayman Islands Companies Act (As Revised) and the conditions imposed by our Articles governing changes in capital are not more stringent than what is required by the Cayman Islands Companies Act (As Revised).
Amendment of Governing Documents
As permitted by the Companies Act, our amended and restated memorandum and articles of association may only be amended with a special resolution of our shareholders.
Rights of Non-Resident or Foreign Shareholders
There are no limitations imposed by our Articles of Association on the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. In addition, there are no provisions in the Articles of Association governing the ownership threshold above which shareholder ownership must be disclosed.
95
C.Material Contracts
Issuance of Notes due 2026
Secured Guaranteed Notes due 2026
On March 6, 2020, we issued US$42.5 million aggregate principal amount of secured guaranteed convertible notes due 2023 (the “Secured Convertible Guaranteed Notes due 2023”) pursuant to a note purchase agreement among us, the purchasers party thereto and Solel Partners LP, as collateral agent (the “2020 Note Purchase Agreement”). The Secured Convertible Guaranteed Notes due 2023 were guaranteed by certain of our subsidiaries and secured by: (i) an intercompany loan pledge agreement; (ii) a share pledge granted by RASA Holding LLC of 41.3% of its shares in Rizobacter Argentina S.A., representing 33% of the capital stock of Rizobacter Argentina S.A. and (iii) an account pledge agreement and fiduciary assignment agreement granted by Rizobacter do Brasil Ltda.
Pursuant to the 2020 Note Purchase Agreement, the holders of the Secured Convertible Guaranteed Notes due 2023 requested the conversion of approximately 75% of the outstanding Secured Convertible Guaranteed Notes due 2023 into our ordinary shares. Accordingly, in March 2022 we issued 4,617,281 ordinary shares to holders of the Secured Convertible Guaranteed Notes due 2023.
Pursuant to the 2020 Note Purchase Agreement and a side-letter dated August 5, 2022: (i) the balance of Secured Convertible Guaranteed Notes due 2023 were converted into 1,526,454 of our ordinary shares, (ii) we repurchased such shares for US$24.0 million and (iii) we financed such repurchase with the proceeds from the issuance of an aggregate principal amount of US$24.0 million Secured Guaranteed Notes due 2026 pursuant to a Note Purchase Agreement, by and among us, Solel-Bioceres SPV, L.P., the holders from time to time party thereto and Wilmington Savings Fund Society, FSB as collateral agent. The Secured Guaranteed Notes due 2026 are secured by substantially all of the assets located in the United States of the Pro Farm. In addition, the Secured Guaranteed Notes due 2026 are guaranteed by BCS Holding Inc., Bioceres Crops do Brasil Ltda., Bioceres Crops S.A., Bioceres Semillas S.A.U., Verdeca LLC, Rasa Holding LLC, Rizobacter Argentina S.A., Rizobacter del Paraguay S.A., Rizobacter do Brasil Ltda., Rizobacter South Africa, Rizobacter Uruguay, Rizobacter USA, LLC, Glinatur S.A., Pro Farm, Pro Farm Michigan Manufacturing LLC, Pro Farm Technologies Comércio de Insumo Agrícolas do Brasil Ltda. and Pro Farm, Inc. (the “Guarantors”).
The Secured Guaranteed Notes due 2026 mature 48 months after the issue date and bear interest at 9.0% from the issue date through 24 months after the issue date, 13.0% from 25 through 36 months after the issue date and 14.0% from 37 through 48 months after the issue date. Interest is payable semi-annually. The Secured Guaranteed Notes due 2026 have no conversion rights into our ordinary shares.
Secured Convertible Guaranteed Notes due 2026
On August 5, 2022, we issued an aggregate principal amount of US$55.0 million secured convertible guaranteed notes due 2026 (the “Secured Convertible Guaranteed Notes due 2026”) pursuant to a note purchase agreement among us, Jasper Lake Ventures One LLC, Redwood Enhanced Income Corp, Liminality Partners LP, the holders from time to time party thereto and Wilmington Savings Fund Society, FSB, as collateral agent. The Secured Convertible Guaranteed Notes due 2026 are secured by substantially all of the assets located in the United States of Pro Farm. In addition, the Secured Convertible Guaranteed Notes due 2026 are guaranteed by the Guarantors.
The Secured Convertible Guaranteed Notes due 2026 mature 48 months after the issue date, unless converted earlier, and bear interest at 5% in cash plus 4% in kind per year, payable quarterly.
Secured Convertible Guaranteed Notes due 2026 Registration Rights Agreement
On August 5, 2020, we and the initial purchasers under the JRL NPA entered into a Registration Rights Agreement, by means of which we and the JRL NPA initial purchasers agreed to enter into the agreement to provide the JRL NPA initial purchasers with certain registration rights with respect to our ordinary shares issuable upon conversion of the Secured Convertible Guaranteed Notes due 2026.
96
Sponsors Shareholders’ Agreement
In connection with the consummation of the business combination, we, Bioceres LLC and UAC’s initial shareholders prior to Union’s initial public offering and their affiliates entered into the agreement, pursuant to which, among other things, the initial shareholders will have the right to nominate one director to our board of directors following the consummation of the business combination at each of our annual general meetings, provided, that, they do not hold less than a certain percentage of our shares. Bioceres LLC will agree to vote for such director nominee proposed by the initial shareholders.
D.Exchange Controls
Within the framework of the uncertain economic situation faced by the Argentine Republic, and for the purpose of “(…) strengthening the ordinary operation of the economy, contribute to a sound administration of the exchange market, reduce the volatility of financial variables, and contain the impact of any fluctuations of financial cash flows on real economy” on September 1, 2019, Argentina’s Executive Branch issued Decree No. 609/2019 whereby it was provided, among other measures, that Argentine residents must repatriate export proceeds into Argentina, and delegates to the Argentine Central Bank the power to regulate the access to the FX Market for the purchase of foreign currency with Pesos and its transfer abroad.
Since the Export Controls Decree, the BCRA has periodically issued several regulations to regulate the inflow and outflow of funds through the FX Market, which are all unified under the consolidated text of foreign exchange available at the BCRA’s website: http://www.bcra.gob.ar/Pdfs/Texord/t-excbio.pdf. The most recent and comprehensive communications issued by BCRA are Communications “A” (6844, 7272, 7422, 7516, and 7746).
Current foreign exchange controls in Argentina affects the ability of both Argentine and non-Argentine residents to access the FX Market at the official exchange rate to acquire and/or transfer foreign currency to and from Argentina. They affect all industries and cover a wide variety of matters, including, among others, trading activities, imports and exports of goods and services, financial indebtedness, payment of profits and dividends, and repatriation of investments of non-Argentine residents.
As a general rule, transfers of foreign currency to and from Argentina must be made through the FX Market and are subject to compliance with certain requirements and conditions established by the regulations issued by the Argentina Central Bank from time to time.
In this regard, the Argentine Central Bank is empowered to establish the cases in which access to the Foreign Exchange Market will require its prior authorization, the granting of which is discretionary and, in practice, rarely occurs.
Pursuant to the foreign exchange regulations currently in force, among others:
| (1) | The prior authorization of the Argentine Central Bank is required for the access to the FX Market for the purchase of foreign currency, for the following purposes, among others: |
| ● | For portfolio investment purposes for more than US$200 per calendar month by individuals, subject to certain additional requirements and restrictions, including the filing of an affidavit by which the client states that he or she has not sold and commits not to sell securities for U.S. dollars in the preceding and following 180 consecutive days – the term will only be 90 days if the securities are issued under Argentine law; |
| ● | For portfolio investment purposes by legal entities, local governments, funds, trusts and foreign legal entities; |
| ● | In excess of US$100 by non-Argentine resident individuals; provided that such individual may only access the FX Market to purchase up to US$100 to the extent that during the immediately preceding 90 consecutive days it sold an equal or higher amount of foreign currency through FX Market; |
97
| ● | For the payment of profits and dividends out of Argentina, except up to an amount equal to 30% of the value of all new capital contributions of foreign direct investments made to the Argentine resident since such date to the extent that (i) the proceeds of those capital contributions have been repatriated into Argentina and converted into Pesos through FX Market and they have been capitalized, (ii) the registration of such capitalization has been requested before the Public Registry of Commerce and (iii) ) access to the FX Market for the payment of profits and dividends must happen in a term of no less than 30 calendar days as of the conversion into Pesos through the FX Market; |
| ● | For the pre-payment of principal and interest on foreign financial indebtedness with an anticipation of more than three business days in advance to the scheduled maturity dates, except under certain limited exceptions; |
| ● | For the repayment of principal maturing on or before the Temporal Scope under external financial indebtedness with non-related parties and foreign-currency-denominated securities issued in the local market (including, in both cases, the indebtedness of financial institutions for own operations and excluding borrowings granted or guaranteed by international organizations and official credit agencies) unless the Refinancing Plan is furnished with the Argentine Central in order to have access to the FX Market; |
| ● | The Refinancing Plan shall comply, at least, with the following requirements: |
| i. | 60% of the outstanding principal amount under the relevant financings with maturities falling during the Temporal Scope should be refinanced with a new financing or debt restructuring with an average life of not less than two years; and |
| ii. | the remaining 40% of the outstanding principal amount under the relevant financings with maturities falling during the Temporal Scope can be repaid under the original terms. |
These restrictions do not apply to the repayment of principal for amounts not exceeding US$ 2 million per calendar month in all financial institutions. In addition, access to the FX Market for the repayment of principal amounts in excess of 40% of the outstanding principal amounts under the relevant financing will be granted in certain cases provided by the foreign exchange regulations, such as the issuance of Export Increase Certificates (for the amount provided by said certificates”) or the repatriation and exchange into Pesos of new securities issuances or external financial debts.
| ● | The Refinancing Plan must be filed at least 30 calendar days before the maturity date of the relevant financings to be refinanced. |
The foreign exchange regulations establish that the Refinancing Plan should not be furnished in the following cases, among others:
| iii. | financial indebtedness originated as of January 1, 2020 and whose proceeds have been repatriated and exchanged into Pesos through the FX Market; |
| iv. | indebtedness originated as of January 1, 2020 and that constitute refinancing to reach the parameters established before; and |
| v. | the remaining portion of maturities already refinanced to the extent that the refinancing reached the parameters established before including hold-outs of refinanced debt. |
| ● | Subject to certain exemptions, until December 31, 2023, for the payment of principal under financial indebtedness to related parties; |
| ● | For the pre-payment of indebtedness for the import of goods and services; |
| ● | Until December 31, 2023, the prior consent from the Argentine Central Bank shall be required to have access to the FX Market to make payments of imports of goods or advance payments of imports of goods or repayment of the principal amount of debts originated in the import of goods, except if certain conditions or exceptions are met; |
98
| ● | For the payment of services to foreign related parties except for expenses payable for their normal operation. |
In addition to all the foregoing, access to the FX Market for the purchase of foreign currency is subject to the filing by the client of an affidavit declaring that (a) on the day it requests access to the foreign exchange market and in the previous 180 calendar days, and committing to refrain from executing them in the subsequent 180 calendar days (only regarding securities transactions executed as of April 21, 2023): (i) it has not entered into sales in Argentina of securities with settlement in foreign currency; (ii) it has not exchanged securities issued by residents for external assets; (iii) it has not transferred securities to foreign depositaries; (iv) it has not acquired in Argentina securities issued by non-residents with settlement in Pesos; (v) it has not acquired Argentine depositary receipts of foreign shares (“CEDEARS”); (vi) it has not acquired securities representing private debt issued in foreign jurisdictions; (vii) it has not delivered funds in local currency or other local assets (except funds in foreign currency deposited in local financial institutions) to any human or legal person, resident or non-resident, related or not, receiving as prior or subsequent consideration, directly or indirectly, by itself or through a related, controlled or controlling entity, foreign assets, crypto-assets or securities deposited abroad. This does not include transactions involving securities issued under Argentine law, in which the term remains 90 calendar days; (b) all its holdings of foreign currency in Argentina are deposited at local bank accounts; (c) does not hold liquid foreign assets (i.e. holdings of foreign currency) outside Argentina or CEDEARS in excess of US$100,000; (d) commits to settle through the FX Market, within the five business days from settlement, all amounts received outside Argentina from the payment of loans granted to third parties, withdraw or expiration of term deposits or sale of any assets, when the asset was acquired, the term deposit made or the loan granted, after May 28, 2020; and (e) stating that as of the date on which access to the FX Market is requested, and in the 180 preceding days, no funds in local currency or other liquid local assets were delivered in Argentina to any individual or legal entity exercising a ‘direct control’ relationship or to a legal entity of the same economic group, excluding transactions directly associated with the acquisition of goods and services in the regular course of business. If the client has delivered local currency or any local asset (issued by an Argentine resident) to the direct controlling shareholder or to a legal entity of the same economic group, client may still comply with the abovementioned obligation if it submits an affidavit of the direct controlling shareholder and the legeal entities of the same economic group, stating that they have not carried out any Restricted Operation in the previous 180 calendar days, and undertake not to carry out any such transactions in the subsequent 180 calendar days.
(2) | The access to the FX Market for the purchase of foreign currency for any of the payments described above is subject to the compliance with the foreign indebtedness information regime before the Argentine Central Bank. |
(3) | The proceeds of the disbursements of foreign financial loans incurred since September 1, 2019 must be transferred into Argentina and converted into Pesos (the “Repatriation”) in order for the Argentine resident debtor to have access to the FX Market for the payment of principal and interests under such foreign financial loan on their scheduled maturity. Foreign currency proceeds disbursed by non-Argentine residents as of September 1, 2019, must be transferred to Argentina and sold for Pesos in the FX Market. As such, a prior BCRA approval is not required for Argentine residents. In addition, the transaction must have been declared under the Reporting Foreign Assets and Liabilities Regime. See “—Reporting of Foreign Assets and Liabilities Regime.” |
Prepayments of principal and interests within more than three business days prior to the scheduled maturity require prior approval by the BCRA, subject to certain exceptions.
Further, prior BCRA approval is currently required to access the FX Market to pay the principal of any foreign financial indebtedness involving related parties, subject to certain exceptions. The BCRA approval has been granted and extended until December 31, 2023. However, it may be further extended in the future. Likewise, as from Communication “A” 4776, BCRA’s prior approval will be required to access the FX Market until such date, for the payment of interest on commercial debts for imports and/or financial indebtedness between related partiesAccess to the FX Market is prohibited for the purchase of foreign currency for payment of local debts and other obligations incurred in foreign currency between Argentine residents since September 1, 2019.
(4) | Proceeds from exports of goods (the consideration (“contravalor”) in foreign currency) must be transferred to Argentina and sold for Pesos in the FX Market at the official exchange rate within a term ranging from 15 to 180 calendar days depending on the goods exported and the relationship with the importer. Regardless of the applicable term, upon collection of the export, the proceeds thereof must be repatriated no later than five business days from the date of collection. |
Amounts collected in foreign currency for insurance claims related to the exported goods must also be repatriated and settled in Pesos in the FX Market, up to the amount of the insured exported goods.
99
The exporter must appoint a financial entity to track each export transaction. The obligation of repatriation and settlement of foreign currency through the FX Market corresponding to a shipping permit will be considered satisfied when the entity designated for tracking purposes certifies that repatriation and settlement has taken place.
(5) | The collections of foreign currency by Argentine residents out of Argentina for the export of services are subject to mandatory Repatriation within the 5 consecutive days computed from the date of payment or collection. |
E.Taxation
The following is a summary of the material Cayman Islands and U.S. tax consequences of acquiring, owning and disposing of securities.
Material Cayman Islands Tax Considerations
The following is a discussion on certain Cayman Islands income tax consequences of an investment in our securities. The discussion is a general summary of present law, which is subject to prospective and retroactive change. It is not intended as tax advice, does not consider any investor’s particular circumstances, and does not consider tax consequences other than those arising under Cayman Islands law.
Payments of dividends and capital in respect of our securities will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of the securities, nor will gains derived from the disposal of the securities be subject to Cayman Islands income or corporate tax. The Cayman Islands currently has no income, corporate or capital gains tax and no estate duty, inheritance tax or gift tax.
No stamp duty is payable in respect of the issue of our securities or on an instrument of transfer in respect of our securities.
The Government of the Cayman Islands will not, under existing legislation, impose any income, corporate or capital gains tax, estate duty, inheritance tax, gift tax or withholding tax upon the Company or its shareholders. The Cayman Islands is not party to a double tax treaty with any country that is applicable to any payments made to or by the Company.
The Company has applied for, and received, an undertaking from the Financial Secretary of the Cayman Islands that, in accordance with section 6 of the Tax Concessions Act (As Revised) of the Cayman Islands, for a period of 20 years from the date of the undertaking, no law which is enacted in the Cayman Islands imposing any tax to be levied on profits, income, gains or appreciations shall apply to the Company or its operations and, in addition, that no tax to be levied on profits, income, gains or appreciations or which is in the nature of estate duty or inheritance tax shall be payable (i) on or in respect of the shares, debentures or other obligations of the Company or (ii) by way of the withholding in whole or in part of a payment of dividend or other distribution of income or capital by the Company to or its shareholders or a payment of principal or interest or other sums due under a debenture or other obligation of the Company.
Material U.S. Federal Income Tax Considerations
General
The following is a summary of certain U.S. federal income tax consequences for U.S. Holders and Non-U.S. Holders (each, as defined below) of ordinary shares of the Company that hold the ordinary shares as capital assets. The discussion does not cover all aspects of U.S. federal income taxation that may be relevant to, or the actual tax effect that any of the matters described herein will have on, the ownership or disposition of ordinary shares by particular investors (including consequences under the alternative minimum tax or net investment income tax), and does not address state, local, non-U.S. or other tax laws.
This summary also does not address tax considerations applicable to investors that own (directly, indirectly or by attribution) 5 per cent. or more of the ordinary shares of the Company by vote or value, nor does this summary discuss all of the tax considerations that may be relevant to certain types of investors subject to special treatment under the U.S. federal income tax laws, such as:
| ● | financial institutions, |
| ● | insurance companies, |
100
| ● | individual retirement accounts and other tax-deferred accounts, |
| ● | tax-exempt organizations, |
| ● | dealers in securities or currencies, |
| ● | investors that will hold the ordinary shares as part of straddles, hedging transactions or conversion transactions for U.S. federal income tax purposes, |
| ● | persons that have ceased to be U.S. citizens or lawful permanent residents of the United States or are U.S. expatriates, |
| ● | investors holding the ordinary shares in connection with a trade or business conducted outside of the United States, |
| ● | U.S. Holders whose functional currency is not the U.S. dollar, |
| ● | Non-U.S. Holders holding the ordinary shares in connection with a trade or business conducted within the United States, or |
| ● | Non-U.S. Holders who are individuals present in the United States for 183 days or more in the taxable year at disposition. |
As used herein, the term “U.S. Holder” means a beneficial owner of ordinary shares that is, for U.S. federal income tax purposes, (i) an individual citizen or resident of the United States, (ii) a corporation created or organized under the laws of the United States, any state thereof or the District of Columbia, (iii) an estate the income of which is subject to U.S. federal income tax without regard to its source or (iv) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or the trust has validly elected to be treated as a domestic trust for U.S. federal income tax purposes.
As used herein, the term “Non-U.S. Holder” means a beneficial owner of ordinary shares that is, for U.S. federal income tax purposes, (i) a nonresident alien, (ii) a foreign corporation, or (iii) an estate or trust that in either case is not subject to U.S. federal income tax on a net income basis on income or gain from ordinary shares.
The U.S. federal income tax treatment of a partner in an entity or arrangement treated as a partnership for U.S. federal income tax purposes that holds ordinary shares will depend on the status of the partner and the activities of the partnership. Investors that are entities or arrangements treated as partnerships for U.S. federal income tax purposes should consult their tax advisers concerning the U.S. federal income tax consequences to them and their partners of the acquisition, ownership and disposition of ordinary shares by the partnership.
This summary is based on the tax laws of the United States, including the Internal Revenue Code of 1986, as amended, its legislative history, existing and proposed regulations thereunder, published rulings and court decisions, all as of the date hereof and all subject to change at any time, possibly with retroactive effect.
THE SUMMARY OF U.S. FEDERAL INCOME TAX CONSEQUENCES SET OUT BELOW IS FOR GENERAL INFORMATION ONLY. INVESTORS SHOULD CONSULT THEIR TAX ADVISERS AS TO THE PARTICULAR TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING, AND DISPOSING OF THE ORDINARY SHARES, INCLUDING THE APPLICABILITY AND EFFECT OF STATE, LOCAL, NON-U.S. AND OTHER TAX LAWS AND POSSIBLE CHANGES IN TAX LAW.
101
U.S. Holders
Dividends
Subject to the PFIC rules discussed below, distributions paid by the Company out of current or accumulated earnings and profits (as determined for U.S. federal income tax purposes) generally will be taxable to a U.S. Holder as dividend income, and will not be eligible for the dividends received deduction allowed to corporations. Distributions in excess of current and accumulated earnings and profits will be treated as a nontaxable return of capital to the extent of the U.S. Holder’s basis in the ordinary shares, and thereafter as capital gain. However, the Company cannot provide any assurance that it will maintain calculations of its earnings and profits in accordance with U.S. federal income tax accounting principles. U.S. Holders should therefore assume that any distribution by the Company with respect to ordinary shares may be reported as ordinary dividend income. U.S. Holders should consult their own tax advisers with respect to the appropriate U.S. federal income tax treatment of any distribution received or treated as received from the Company.
Dividends paid by the Company generally will be taxable to a non-corporate U.S. Holder at the reduced rate normally applicable to long-term capital gains, provided that (i) the ordinary shares are readily tradable on an established securities market in the United States; (ii) certain holding period requirements are satisfied; and (3) the Company is not classified as a PFIC for its taxable year during which the dividend is paid or its immediately preceding taxable year. See “—Passive Foreign Investment Company Considerations” below.
Sale or Other Disposition
Subject to the PFIC rules discussed below, upon a sale or other disposition of ordinary shares (including as a result of receipt of cash pursuant to the exercise of a warrant), a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes equal to the difference, if any, between the amount realized on the sale or other disposition and the U.S. Holder’s adjusted tax basis in the ordinary shares. This capital gain or loss will be long-term capital gain or loss if the U.S. Holder’s holding period in the ordinary shares exceeds one year. Any gain or loss generally will be U.S. source. The deductibility of capital losses is subject to limitations.
See “—Passive Foreign Investment Company Considerations” below for a discussion of more adverse rules that will apply to a sale or other disposition of ordinary shares if the Company is or has been a PFIC for U.S. federal income tax purposes.
Passive Foreign Investment Company Considerations
In general, a non-U.S. corporation will be a Passive Foreign Investment Company (“PFIC”) in any taxable year in which, after taking into account the income and assets of the corporation and certain subsidiaries pursuant to applicable “look-through rules,” either (i) at least 75 per cent. of its gross income is “passive income” or (ii) at least 50 per cent. of the average value of its assets is attributable to assets which produce passive income or are held for the production of passive income. Passive income generally includes interest, dividends, rents, royalties and gains from commodities transaction). Goodwill is treated as an active asset under the PFIC rules to the extent attributable to activities that produce active income. Cash is a passive asset.
The Company does not believe that it was a PFIC for its most recent taxable year and does not expect to be a PFIC for the current taxable year. However, a company’s PFIC status is an annual factual determination that can be made only after the end of each taxable year and the Company’s PFIC status for any taxable year will depend on the composition of its income and assets and the value of its assets from time to time (which may be determined, in part, by reference to the market price of its Shares, which could be volatile) and the manner in which it operates its projects. Furthermore, the Company owns, and may continue to own, minority stakes in entities or joint ventures. Any entities or joint ventures in which the Company owns a less than 25% minority stake will generally be treated as a passive asset for purposes of the PFIC rules. Accordingly, no assurance can be given that the Company will not be a PFIC for any taxable year. If the Company is a PFIC for any taxable year and any entity in which it owns or is deemed to own equity interests is also a PFIC (any such entity, a “Lower-tier PFIC”), a U.S. Holder will be deemed to own a proportionate amount (by value) of the shares of each such Lower-tier PFIC and will be subject to U.S. federal income tax according to the rules described in the next paragraph on (i) certain distributions by a Lower-tier PFIC, and (ii) dispositions of shares of Lower-tier PFICs, in each case as if such U.S. Holder held such shares directly, even though such U.S. Holder did not receive any proceeds of those distributions or dispositions.
102
If the Company is a PFIC in any year during which a U.S. Holder owns ordinary shares, and the U.S. Holder has not made a mark-to-market or qualified electing fund election (if available), the U.S. Holder generally will be subject to special rules with respect to (i) any “excess distribution” (generally, any distributions treated as received by the U.S. Holder on the ordinary shares in a taxable year that are greater than 125 per cent. of the average annual distributions treated as received by the U.S. Holder in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for the ordinary shares and (ii) any gain realized on the sale or other disposition of ordinary shares. Under these rules (a) the excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period, (b) the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which the Company is a PFIC will be taxed as ordinary income, and (c) the amount allocated to each of the other taxable years will be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year and an interest charge for the deemed deferral benefit will be imposed with respect to the resulting tax attributable to each such other taxable year. If the Company is a PFIC for any taxable year during which a U.S. Holder holds the Shares, the Company would generally continue to be treated as a PFIC with respect to such U.S. Holder for all succeeding years during which such holder owns the Shares even if the Company ceases to meet the threshold requirements for PFIC status. Dividends paid by the Company in any year for which it is a PFIC or any year following such year will not be eligible for the reduced rate of tax described above under “Dividends.”
A U.S. Holder who has made a qualified electing fund (“QEF”) election (on or before the due date of the U.S. Holder’s tax return for the first taxable year to which the QEF applies) or a mark-to-market election with respect to the ordinary shares will not be subject to the PFIC regime if the Company ceases to be a PFIC. If the Company becomes a PFIC in future years, any prior QEF or mark-to-market election made by a U.S. Holder would still be valid, and new elections would not have to be made. However, a mark-to-market election cannot be made for any Lower-tier PFICs. U.S. Holders should consult their tax advisers regarding the application of the PFIC rules to their indirect ownership of shares in any Lower-tier PFICs.
If the Company were a PFIC for a prior taxable year during the U.S. Holder’s holding period, but ceases to be a PFIC for a subsequent taxable year, a U.S. Holder may make an election (a “deemed sale election”) to be treated for U.S. federal income tax purposes as having sold its ordinary shares, as applicable, on the last day of the last taxable year of the Company during which it was a PFIC. A U.S. Holder that makes a deemed sale election will cease to be treated as owning stock in a PFIC. However, gain recognized by a U.S. Holder as a result of making the deemed sale election will be subject to the rules described above.
A U.S. Holder who owns, or who is treated as owning, PFIC stock during any taxable year for which the Company is classified as a PFIC may be required to file Internal Revenue Service (“IRS”) Form 8621. Prospective purchasers should consult their tax advisers regarding the requirement to file IRS Form 8621 and the potential application of the PFIC regime.
Foreign Financial Asset Reporting
U.S. taxpayers that own certain foreign financial assets, including equity of foreign entities, with an aggregate value in excess of US$50,000 at the end of the taxable year or US$75,000 at any time during the taxable year (or, for certain individuals living outside the United States and married individuals filing joint returns, certain higher thresholds) may be required to file an IRS Form 8938 with respect to such assets with their tax returns. The ordinary shares are expected to constitute foreign financial assets subject to these requirements unless the ordinary shares are held in an account at a financial institution (in which case the account may be reportable if maintained by a foreign financial institution). U.S. Holders should consult their tax advisers regarding the application of the rules relating to foreign financial asset reporting.
Non-U.S. Holders
Subject to the discussion below under “—Backup Withholding and Information Reporting,” any proceeds of a sale or other disposition of the ordinary shares, as well as dividends and other proceeds with respect to the ordinary shares, are not subject to U.S. federal income tax, including withholding taxes, if paid to a Non-U.S. Holder.
Backup Withholding and Information Reporting
Payments of dividends and other proceeds with respect to ordinary shares by a U.S. paying agent or other U.S. intermediary will be reported to the IRS and to a holder as may be required under applicable U.S. Treasury Regulations. Backup withholding may apply to these payments if the holder fails to provide an accurate taxpayer identification number or certification of exempt status or fails to comply with applicable certification requirements. Certain holders are not subject to backup withholding (including corporations and Non-U.S. Holders who provide certification of their exempt status). Holders should consult their tax advisers as to their qualification for exemption from backup withholding and the procedure for obtaining an exemption.
103
F.Dividends and Paying Agents
Not applicable.
G.Statement by Experts
Not applicable.
H.Documents on Display
We are subject to the information requirements of the Exchange Act, except that as a foreign issuer, we will not be subject to the proxy rules or the short-swing profit disclosure rules of the Exchange Act. In accordance with these statutory requirements, we will file or furnish reports and other information with the SEC, which you may inspect and copy at the Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
I.Subsidiary Information
See “Item 4. Information on the Company—C. Organizational Structure” and Exhibit 8.1.
J.Annual Report to Security Holders.
If we are required to provide an annual report to security holders in response to the requirements of Form 6-K, we will submit the annual report to security holders in electronic format in accordance with the EDGAR Filer Manual.
ITEM 11.QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our board of directors has overall responsibility for establishing and monitoring our risk management objectives and policies. While it retains ultimate responsibility for risk management, it has delegated the day-to-day monitoring to the executive management team to design and operate processes that ensure effective implementation of the risk management objectives and policies, and management periodically to the board of directors. Our audit committee will also review the risk management policies and processes, and report findings to the board of directors. The overall objective of the board of directors is to set policies that seek to reduce risk as far as possible without unduly affecting our competitiveness and flexibility.
The risks and methods for managing the risks are reviewed regularly, in order to reflect changes in market conditions and our activities. Through training, management standards and procedures, we aim to develop a disciplined and constructive control environment in which all our employees understand their roles and obligations.
For further information on our market risks, please see Note 15 to our audited consolidated financial statements included elsewhere in this report.
ITEM 12.DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A.Debt Securities
Not applicable.
B.Warrants and Rights
Not applicable.
C.Other Securities
Not applicable.
104
D.American Depositary Shares
Not applicable.
PART II
ITEM 13.DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
A.Defaults
Not applicable.
B.Arrears and Delinquencies
Not applicable.
ITEM 14.MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Not applicable.
ITEM 15.CONTROLS AND PROCEDURES
A.Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of June 30, 2023. Based on this evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were effective as of June 30, 2023, the end of the period covered by this report, at a reasonable assurance level and, accordingly, provide reasonable assurance that the information required to be disclosed by us in reports filed under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our chief executive officer and chief financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives.
B.Management’s Annual Report on Internal Control Over Financial Reporting
Management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act as a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS as adopted by the IASB and includes those policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transaction and dispositions of the assets of the company; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS as adopted by the IASB, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
105
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting (as defined in Rules 13(a)-13(f) and 15(d)-15(f) under the U.S. Securities Exchange Act of 1934) as of June 30, 2023. In making this assessment, it used the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on their assessment, subject to the exclusion below, management concluded that, as of June 30, 2023, our internal control over financial reporting is effective based on those criteria.
Management has excluded Pro Farm from its assessment of internal control over financial reporting as of June 30, 2023, because it was acquired by the Company in the year ended June 30, 2023. Pro Farm is a wholly-owned subsidiary whose total assets and total revenues excluded from management’s assessment of internal control over financial reporting represent 9% and 10%, respectively, of the Company’s consolidated financial statement amounts as of and for the year ended June 30, 3023.
The effectiveness of the Company’s internal control over financial reporting as of June 30, 2023 has been audited by Price Waterhouse & Co. S.R.L. (“PwC”), an independent registered public accounting firm, as stated in their report which appears herein.
C.Attestation Report of the Registered Public Accounting Firm
The effectiveness of the Company’s internal control over financial reporting as of June 30, 2023 has been audited by PwC, an independent registered public accounting firm, as stated in their report which appears herein.
D.Changes in Internal Control Over Financial Reporting
During the period covered by this annual report, there have not been any changes in our internal control over financial reporting that have materially affected or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16.Reserved
A.Audit Committee Financial Expert
Our board of directors has determined that Ari Freisinger is an “audit committee financial expert” as defined by the SEC item 16A of Form 20-F. All members of the audit committee are independent directors as defined in the Nasdaq Global Select Market corporate governance standards and Rule 10A-3 under the Exchange Act.
B.Code of Ethics
We have adopted a Code of Ethics applicable to our directors, executive officers and employees and to all board members, employees and officers of our controlled companies. The Code of Ethics codifies the business and ethical principles that govern all aspects of our business. A copy of the Code of Ethics will be filed with the SEC and will be provided without charge upon written request to Bioceres Crop Solutions in writing at investorelations@biocerescrops.com. Bioceres Crop Solutions intends to disclose any amendments to or waivers of certain provisions of our Code of Ethics in a Current Report on Form 6-K (as required) or on our website.
C.Principal Accountant Fees and Services
PwC have acted as our principal accountants for the years ended June 30, 2023 and 2022. The following is a summary of fees paid or to be paid to PwC for services rendered for each of the last two fiscal years.
Audit Fees
Audit fees consist of fees billed for standard audit work that needs to be performed each year in order to issue opinions on our consolidated financial statements and for services that are normally provided by PwC in connection with regulatory filings. The aggregate fees billed by PwC for professional services rendered for the audit of our annual consolidated financial statements and review of the financial information included in our required filings with the SEC for the year ended June 30, 2023 and 2022 totaled US$0.5 and US$0.4 million, respectively.
106
Audit-Related Fees
Audit-related fees billed consist of fees for services such as auditing of non-recurring transactions, reviews of semi-annual financial results, consents and comfort letters and any other audit services required for SEC or other regulatory filings. PwC did not bill us for audit-related services for the years ended June 30, 2023. The aggregate fees billed by PwC for professional services rendered for audit-related services for the year ended June 30, 2022 totaled US$0.1 million.
Tax Fees
Tax services relate to the aggregated fees for services on tax compliance. The aggregate fees by PwC for tax fees for the year ended June 30, 2023 and 2022 amounted to less than US$0.1 million each.
All Other Fees
We did not pay PwC for other services for the years ended June 30, 2023 and 2022.
Pre-approval Policies
Our audit committee approves all auditing services and permitted non-audit services performed for us by our independent auditor in advance of an engagement. All auditing services and permitted non-audit services (including the fees and terms thereof) to be performed for us by our independent auditor must be approved by the audit committee in advance, subject to the de minimis exceptions for non-audit services described in Section 10A(i)(1)(B) of the Exchange Act which are approved by the committee prior to the completion of the audit.
D.Exemptions from the Listing Standards for Audit Committees
No exemptions from the listing standards for our audit committee.
E.Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
F.Change in Registrant’s Certifying Accountant
Not applicable.
G.Corporate Governance
We are exempt from certain corporate governance requirements of the Nasdaq Global Select Market by virtue of being a “foreign private issuer.” Although our foreign private issuer status exempts us from most of the Nasdaq Global Select Market’s corporate governance requirements, we intend to voluntarily comply with these requirements, except those from which we would be exempt by virtue of being a “controlled company.” Bioceres PLC, directly or indirectly, a majority of the voting power of our issued and outstanding ordinary shares and we are a controlled company within the meaning of the Nasdaq Global Select Market corporate governance standards, entitled to certain limited corporate governance exemptions. Under these Nasdaq Global Select Market standards, a controlled company may elect not to comply with certain Nasdaq Global Select Market corporate governance requirements, including the requirements that:
| ● | a majority of the board of directors consist of independent directors; |
| ● | the nominating and governance committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; |
| ● | the compensation committee be composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities; and |
107
| ● | there be an annual performance evaluation of the nominating and corporate governance and compensation committees. |
As a controlled company, we utilize certain of these exemptions, including the exemption from the requirement to have a compensation committee that is entirely composed of independent directors.
As a result of the foregoing exemptions, we can cease voluntary compliance at any time, and you may not have the same protections afforded to shareholders of companies that are subject to all of the Nasdaq Global Select Market corporate governance requirements.
H.Mine Safety Disclosure
Not applicable.
I.Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
J.Insider Trading Policies
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16J will be applicable to the Company from the fiscal year ending June 30, 2024.
K.Cybersecurity.
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16K will be applicable to the Company from the fiscal year ending June 30, 2024.
Part III
ITEM 17.FINANCIAL STATEMENTS
We have responded to Item 18 in lieu of this item.
ITEM 18.FINANCIAL STATEMENTS
Financial Statements are filed as part of this report, see page F-1.
108
ITEM 19.EXHIBITS
1.1* |
| |
1.2* | ||
2.2* | ||
2.4* | ||
2.5* | ||
4.1* | ||
4.2* | Form of Indemnification Agreement by and between Bioceres Crop Solutions Corp. and its directors | |
4.3* | ||
8.1 | ||
12.1 | Rule 13a-14(a)/15d-14(a) -– Section 302 - Certification of Chief Executive Officer | |
12.2 | Rule 13a-14(a)/15d-14(a) -– Section 302 - Certification of Chief Financial Officer | |
13.1 | 18 U.S.C. SECTION 1350 - Section 906 - Certification of Chief Executive Officer | |
13.2 | 18 U.S.C. SECTION 1350 - Section 906 - Certification of Chief Financial Officer | |
15.1 | ||
101.INS | Inline XBRL Instance Document | |
101.SCH | Inline XBRL Taxonomy Extension Schema Document | |
101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |
101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | |
101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |
104 | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
*Previously filed.
109
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this registration statement on its behalf.
Date: November 14, 2023 | ||
BIOCERES CROP SOLUTIONS CORP. | ||
By: | /s/ Federico Trucco | |
Name: Federico Trucco | ||
Title: Chief Executive Officer | ||
110

BIOCERES CROP SOLUTIONS CORP.
Consolidated financial statements as of and for the years ended
June 30, 2023, 2022 and 2021.
INDEX TO FINANCIAL STATEMENTS
BIOCERES CROP SOLUTIONS CORP. – AUDITED FINANCIAL STATEMENTS
F-1
Report of Independent Registered Public Accounting Firm
To the board of directors and shareholders of Bioceres Crop Solutions Corp.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated statements of financial position of Bioceres Crop Solutions Corp. and its subsidiaries (the “Company”) as of June 30, 2023, 2022 and 2021, and the related consolidated statements of comprehensive income, of changes in equity and of cash flows for each of the three years in the period ended June 30, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of June 30, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2023, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2023 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under item 15B. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As described in Management’s Annual Report on Internal Control over Financial Reporting, management has excluded Pro Farm Group, Inc. from its assessment of internal control over financial reporting as of June 30, 2023 because it was acquired by the Company in a purchase business combination during 2023. We have also excluded Pro Farm Group, Inc. from our audit of internal control over financial reporting. Pro Farm Group, Inc. is a wholly-owned subsidiary whose total assets and total revenues excluded from management’s assessment and our audit of internal control over financial reporting represent 9% and 10%, respectively, of the related consolidated financial statement amounts as of and for the year ended June 30, 2023.
F-2
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Impairment Tests on Goodwill (Pro Farm Group, Inc. and Rizobacter S.A. Cash Generating Units) and Intangible Assets Not Yet Available for Use
As described in Notes 4.6, 7.8 and 7.9 to the consolidated financial statements, the Company’s goodwill associated with the Pro Farm Group, Inc. and Rizobacter S.A. cash generating units (“CGU”) and intangible assets not yet available for use amounted to $76.1 million, $28.0 million and $27.4 million, respectively, as of June 30, 2023. Impairment tests on goodwill and intangible assets not yet available for use are undertaken annually at the end of the reporting period. Where the carrying value of an asset exceeds its recoverable amount (i.e. the higher of value in use and fair value less costs to sell), the asset is written down to its recoverable amount. In each case, management determined the recoverable amount based on value in use calculations prepared by management. The determination of the recoverable amount of the CGU’s and intangible assets not yet available for use includes significant and numerous judgments and assumptions that are subject to various risks and uncertainties. The principal assumptions used in management’s models consisted of (i) budgeted market shares of joint ventures and other customers, (ii) budgeted product prices, (iii) growth rates used to extrapolate future cash flow projections to the terminal period and (iv) discount rates.
The principal considerations for our determination that performing procedures relating to impairment tests on goodwill (Pro Farm Group, Inc. and Rizobacter S.A. cash generating units) and intangible assets not yet available for use is a critical audit matter are (i) the significant judgment by management when developing the recoverable amount of the CGUs and intangible assets not yet available for use; (ii) a high degree of auditor judgment, subjectivity and effort in performing procedures and evaluating management’s significant assumptions used in the models related to the (i) budgeted market shares of joint ventures and other customers, (ii) budgeted product prices, (iii) growth rates used to extrapolate future cash flow projections to the terminal period and (iv) discount rates; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
F-3
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s impairment tests on goodwill and intangible assets not yet available for use, including controls over the determination of the recoverable amounts for the CGUs and intangible assets not yet available for use. These procedures also included, among others (i) testing management’s process for developing the recoverable amounts of CGUs and intangible assets not yet available for use; (ii) evaluating the appropriateness of the models used; (iii) testing the completeness and accuracy of underlying data used in the models; (iv) evaluating the reasonableness of the significant assumptions used by management in the models related to budgeted market shares of joint ventures and other customers, budgeted product prices, growth rates used to extrapolate future cash flow projections to the terminal period and discount rates. Evaluating management’s assumptions used in the models related to budgeted market shares of joint ventures and other customers, budgeted product prices, growth rates used to extrapolate future cash flow projections to the terminal period and discount rates involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the CGUs and the Company’s business; (ii) the consistency with external market and industry data; and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in the evaluation of the Company’s discounted cash flow model and the discount rate assumptions.
Acquisition of Pro Farm Group, Inc. – Valuation of Intangible Assets
As described in Notes 4.10 and 6 to the consolidated financial statements, on July 12, 2022, the Company completed the acquisition of Pro Farm Group, Inc. for net consideration of $156.4 million, of which approximately $79.1 million was allocated to identifiable intangible assets. To value intangible assets acquired from a business combination, valuation techniques generally accepted in the market were applied, based mainly on the revenue approach (such as excess earnings, relief from royalty, and with or without), considering the characteristics of the assets to be valued and available information to estimate their acquisition date fair value. Application of these valuation techniques requires management to develop several assumptions related to future cash flows, such as revenue growth rates, discount rates and royalty rates.
The principal considerations for our determination that performing procedures relating to acquisition of Pro Farm Group, Inc. – valuation of intangible assets is a critical audit matter are (i) the significant judgment by management when developing the fair value estimate of intangible assets acquired; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to revenue growth rates, discount rates and royalty rates; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the acquisition accounting, including controls over management’s valuation of intangible assets acquired. These procedures also included, among others (i) reading the purchase agreement; (ii) testing management’s future cash flows used to estimate the fair value of identifiable intangible assets, which included evaluating the reasonableness of significant assumptions used by management related to revenue growth, discount rates and royalty rates. Evaluating management’s assumptions related to revenue growth rates involved considering (i) the current and past performance of the Pro Farm Group, Inc and (ii) whether the assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the appropriateness of the excess earnings and relief from royalty methods and (ii) the reasonableness of the revenue growth, discount rate and royalty rate assumption.
F-4
Change in Functional Currency
As described in Note 2a to the consolidated financial statements, from July 1, 2022 the main Argentinian subsidiaries of the Group have changed their functional currency from Argentine Pesos to United States Dollars as a result of changes in events and conditions relevant to their business operations. These include a macroeconomic context with high inflation and depreciation of the Argentine peso, and inorganic growth at the close of the fiscal year ended June 30, 2022, which led to a global unification of management and commercial strategy whereby integration of the businesses was done by business units, regardless of the legal entities.
The principal considerations for our determination that performing procedures relating to change in functional currency is a critical audit matter are that there was significant judgment by management to determine the functional currency that most faithfully represents the economic effects of the underlying transactions, events and conditions, which in turn led to a high degree of auditor judgment, subjectivity and audit effort to address this matter due to its pervasive nature and impact on the consolidated financial statements of the Company.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the year end financial reporting process and disclosures. These procedures also included, among others, obtaining and evaluating the reasonableness of management’s assessment of primary and other indicators and evaluating the sufficiency of the Company’s disclosures in the consolidated financial statements regarding the facts and circumstances that led to a change in its functional currency.
/s/
/s/Guillermo Miguel Bosio | |
Guillermo Miguel Bosio | |
Partner | |
November 14, 2023 |
We have served as the Company’s auditor since 2018.
F-5
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
As of June 30, 2023, 2022 and 2021
(Amounts in US$)
| Notes |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
ASSETS |
|
|
|
|
|
|
|
|
CURRENT ASSETS |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
| 7.1 |
| |
| |
| |
Other financial assets |
| 7.2 |
| |
| |
| |
Trade receivables |
| 7.3 |
| |
| |
| |
Other receivables |
| 7.4 |
| |
| |
| |
Income and minimum presumed recoverable income taxes |
| |
| |
| | ||
Inventories |
| 7.5 |
| |
| |
| |
Biological assets | 7.6 | | | | ||||
Total current assets |
| |
| |
| | ||
NON-CURRENT ASSETS |
|
|
| |||||
Other financial assets |
| 7.2 |
| |
| |
| |
Trade receivables | 7.3 | — | | | ||||
Other receivables |
| 7.4 |
| |
| |
| |
Income and minimum presumed recoverable income taxes |
|
| |
| |
| | |
Deferred tax assets | 9 |
| |
| |
| | |
Investments in joint ventures and associates |
| 13 |
| |
| |
| |
Investment properties | 7.10 | | — | — | ||||
Property, plant and equipment |
| 7.7 |
| |
| |
| |
Intangible assets |
| 7.8 |
| |
| |
| |
Goodwill |
| 7.9 |
| |
| |
| |
Right of use asset | 16 | | | | ||||
Total non-current assets |
|
|
| |
| |
| |
Total assets |
|
|
| |
| |
| |
The accompanying Notes are an integral part of these Consolidated financial statements. Related parties’ balances and transactions are disclosed in Note 17.
F-6
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
As of June 30, 2023, 2022 and 2021
(Amounts in US$)
| Notes |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
LIABILITIES | ||||||||
CURRENT LIABILITIES |
|
|
|
|
|
|
|
|
Trade and other payables |
| 7.11 |
| |
| |
| |
Borrowings |
| 7.12 |
| |
| |
| |
Employee benefits and social security |
| 7.14 |
| |
| |
| |
Deferred revenue and advances from customers |
| 7.15 |
| |
| |
| |
Income tax payable |
| |
| |
| | ||
Consideration for acquisition | | | — | |||||
Lease liabilities | 16 | | | | ||||
Total current liabilities |
| |
| |
| | ||
NON-CURRENT LIABILITIES |
|
|
| |||||
Borrowings |
| 7.12 |
| |
| |
| |
Deferred revenue and advances from customers | 7.15 | | — | — | ||||
Government grants |
|
| — |
| — |
| | |
Joint ventures and associates |
| 13 |
| |
| |
| |
Deferred tax liabilities | 9 |
| |
| |
| | |
Provisions |
| 7.16 |
| |
| |
| |
Consideration for acquisition | | | | |||||
Secured notes | 7.13 | | | | ||||
Lease liabilities | 16 | | | | ||||
Total non-current liabilities |
| |
| |
| | ||
Total liabilities |
|
|
| |
| |
| |
|
| |||||||
EQUITY |
|
|
|
|
|
|
| |
Equity attributable to owners of the parent |
|
|
| |
| |
| |
Non-controlling interest |
|
|
| |
| |
| |
Total equity |
|
|
| |
| |
| |
Total equity and liabilities |
|
|
| |
| |
| |
The accompanying Notes are an integral part of these Consolidated financial statements. Related parties’ balances and transactions are disclosed in Note 17.
F-7
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
For the years ended June 30, 2023, 2022 and 2021
(Amounts in US$)
| Notes |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Revenues from contracts with customers |
| 8.1 |
| |
| |
| |
Government grants | — | — | | |||||
Initial recognition and changes in the fair value of biological assets at the point of harvest |
|
| |
| |
| | |
Changes in the net realizable value of agricultural products after harvest | ( | ( | — | |||||
Total |
| | | | ||||
Cost of sales |
| 8.2 |
| ( |
| ( |
| ( |
Research and development expenses |
| 8.3 |
| ( |
| ( |
| ( |
Selling, general and administrative expenses |
| 8.4 |
| ( |
| ( |
| ( |
Share of profit or loss of joint ventures and associates |
| 13 |
| |
| |
| |
Other income or expenses, net | 8.5 |
| |
| ( |
| ( | |
Operating profit |
| |
| |
| | ||
Financial cost |
| 8.6 |
| ( |
| ( |
| ( |
Other financial results | 8.6 | ( | ( | ( | ||||
Profit before income tax |
| |
| |
| | ||
Income tax | 9 |
| |
| ( |
| ( | |
Profit (Loss) for the year |
| |
| ( |
| ( | ||
Profit (loss) for the year attributable to: | ||||||||
Equity holders of the parent | | ( | ( | |||||
Non-controlling interests | | | | |||||
| ( | ( | ||||||
Profit (Loss) per share |
|
|
|
|
|
| ||
Basic profit (loss) attributable to ordinary equity holders of the parent |
| 10 |
| |
| ( |
| ( |
Diluted profit (loss) attributable to ordinary equity holders of the parent | 10 | | ( | ( |
The accompanying Notes are an integral part of these consolidated financial statements. Related parties’ balances and transactions are disclosed in Note 17.
F-8
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
For the years ended June 30,2023, 2022 and 2021
(Amounts in US$)
Profit (Loss) for the year |
| |
| ( |
| ( |
Other comprehensive (loss) income |
| ( |
| |
| |
Items that may be subsequently reclassified to profit and loss |
| |
| |
| |
Foreign exchange differences on translation of foreign operations from joint ventures |
| ( |
| | | |
Foreign exchange differences on translation of foreign operations |
| |
| | | |
Items that will not be subsequently reclassified to loss and profit |
| ( |
| ( |
| ( |
Revaluation of property, plant and equipment, net of tax, of joint ventures and associates 1 |
| ( |
| ( |
| ( |
Revaluation of property, plant and equipment, net of tax 2 |
| ( |
| ( |
| ( |
Total comprehensive profit |
| |
| |
| |
Total comprehensive profit attributable to: | ||||||
Equity holders of the parent | | | | |||
Non-controlling interests | | | | |||
| | | |
(1) |
(2) |
The accompanying Notes are an integral part of these Consolidated financial statements. Related parties’ balances and transactions are disclosed in Note 17.
F-9
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
For the years ended June 30, 2023, 2022 and 2021
(Amounts in US$)
Attributable to the equity holders of the parent | ||||||||||||||||||||||||||
Changes in | Stock | Foreign | Revaluation of | Equity / (deficit) | ||||||||||||||||||||||
non- | Own shares | options and | currency | PP&E and effect | attributable to | |||||||||||||||||||||
Issued | Share | controlling | trading | share based | Convertible | Cost of own | Retained | translation | of tax rate | owners of the | Non-controlling | |||||||||||||||
Description |
| capital |
| premium |
| interests |
| premium |
| incentives |
| instruments |
| shares held |
| deficit |
| reserve |
| change |
| parent |
| Interests |
| Total equity |
06/30/2020 | |
| | — |
| — | |
| | ( | ( |
| ( |
| |
| |
| |
| | |||||
Capitalization of warrants | | | — | ( | — | — | — | — | — | — | | — | | |||||||||||||
Shares issued (Note 6) | | | — | — | — | — | — | — | — | — | | — | | |||||||||||||
Share-based incentives (Note 19) | | | — | — | | — | — | — | — | — | | — | | |||||||||||||
Purchase of own shares (Note 11) | — | — | — | — | — | — | ( | — | — | — | ( | — | ( | |||||||||||||
Non-controlling interest for business combination (Note 6) | — | — | — | — | — | — | — | — | — | — | — | | | |||||||||||||
(Loss) profit for the year | — | — | — | — | — | — | — | ( | — | — | ( | | ( | |||||||||||||
Other comprehensive income or (loss) | — | — | — | — | — | — | — | — | | ( | | | | |||||||||||||
06/30/2021 | | | — | ( | | | ( | ( | ( | | | | | |||||||||||||
Share-based incentives (Note 19) | | | — | — | | — | — | — | — | — | | — | | |||||||||||||
Capitalization of convertible notes (Note 7.13) | | | — | — | — | ( | — | — | — | — | | — | | |||||||||||||
Changes in non-controlling interests | — | — | ( | — | — | — | — | — | — | — | ( | ( | ( | |||||||||||||
(Loss) Profit or the year | — | — | — | — | — | — | — | ( | — | — | ( | | ( | |||||||||||||
Other comprehensive income or (loss) | — | — | — | — | — | — | — | — | | ( | | | | |||||||||||||
06/30/2022 | | | ( | ( | | | ( | ( | | | | | | |||||||||||||
Share-based incentives | | | — | | | — | — | — | — | — | | — | | |||||||||||||
Business combination (Note 6) | | | — | — | | — | — | — | — | — | | — | | |||||||||||||
Capitalization of convertible notes (Note 7.13) | | | — | — | — | — | — | — | — | — | | — | | |||||||||||||
Purchase of own shares (Note 11) | — | — | — | — | — | — | ( | — | — | — | ( | — | ( | |||||||||||||
Issuance of convertible notes (Note 7.13) | — | — | — | — | — | | — | — | — | — | | — | | |||||||||||||
Distribution of dividends by subsidiary | — | — | — | — | — | — | — | — | — | — | — | ( | ( | |||||||||||||
(Loss) profit for the year | — | — | — | — | — | — | — | | — | — | | | | |||||||||||||
Other comprehensive income (loss) | — | — | — | — | — | — | — | — | | ( | ( | | ( | |||||||||||||
06/30/2023 | | | ( | ( | | | ( | ( | | ( | | | | |||||||||||||
The accompanying Notes are an integral part of these consolidated financial statements. Related parties’ balances and transactions are disclosed in Note 17.
F-10
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the years ended June 30, 2023, 2022 and 2021
(Amounts in US$)
| Notes |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
OPERATING ACTIVITIES |
|
|
|
|
|
|
| |
Profit (loss) for the year |
|
|
| | ( | ( | ||
|
| |||||||
Adjustments to reconcile profit to net cash flows |
|
|
|
|
| |||
Income tax |
| 9 |
| ( | | | ||
Financial results |
|
|
| | | | ||
Depreciation of property, plant and equipment |
| 7.7 |
| | | | ||
Amortization of intangible assets |
| 7.8 |
| | | | ||
Depreciation of leased assets | 16 | | | | ||||
Transactional expenses |
|
| | | | |||
Share-based incentive and stock options |
|
| | | | |||
Share of profit or loss of joint ventures and associates |
| 13 |
| ( | ( | ( | ||
Loss of participation in joint ventures and associates | 13 | | — | — | ||||
Provisions for contingencies |
| | | | ||||
Allowance for impairment of trade debtors |
|
| | | | |||
Allowance for obsolescence |
|
| | | | |||
Initial recognition and changes in the fair value of biological assets |
|
| ( | ( | ( | |||
Changes in the net realizable value of agricultural products after harvest | | | — | |||||
Gain or loss on sale of equipment and intangible assets |
|
|
| ( | ( | | ||
|
|
| ||||||
Working capital adjustments |
|
|
|
|
| |||
Trade receivables |
|
|
| ( | ( | | ||
Other receivables |
|
|
| ( | ( | ( | ||
Income and minimum presumed income taxes payable |
|
|
| ( | | | ||
Inventories and biological assets |
|
|
| ( | ( | ( | ||
Trade and other payables |
|
|
| ( | | ( | ||
Employee benefits and social security |
|
|
| | | ( | ||
Deferred revenue and advances from customers |
|
| | ( | | |||
Income taxes paid |
|
|
| ( | ( | ( | ||
Government grants |
|
|
| — | ( | ( | ||
Interest collected | | | | |||||
Inflation effects on working capital adjustments |
|
|
| | ( | ( | ||
Net cash flows generated (used) by operating activities |
|
|
| | ( | ( |
The accompanying Notes are an integral part of these consolidated financial statements. Related parties’ balances and transactions are disclosed in Note 17.
F-11
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the years ended June 30, 2023, 2022 and 2021
(Amounts in US$)
| Notes |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
INVESTMENT ACTIVITIES |
|
|
|
|
|
|
|
|
Proceeds from sale of property, plant and equipment |
| |
| |
| | ||
Investment in joint ventures and associates |
|
| — |
| — |
| ( | |
Net cash received from business combination | 6 | | — | | ||||
Net loans granted to shareholders and other related parties | — | ( | — | |||||
Proceeds from financial assets | | | | |||||
Investment in financial assets |
|
| ( |
| ( |
| ( | |
Purchase of property, plant and equipment | 7.7 | ( | ( | ( | ||||
Capitalized development expenditures | 7.8 |
| ( |
| ( |
| ( | |
Purchase of intangible assets |
| 7.8 |
| ( |
| ( |
| ( |
Net cash flows (used) generated by investing activities |
| ( | | ( | ||||
FINANCING ACTIVITIES |
|
|
|
|
|
| ||
Proceeds from borrowings |
| |
| |
| | ||
Repayment of borrowings and financed payments |
| ( |
| ( |
| ( | ||
Interest payments | ( | ( | ( | |||||
Decrease in bank overdrafts and other short-term borrowings |
| — |
| ( | ( | |||
Other financial proceeds or payments, net |
| ( |
| ( | ( | |||
Acquisition of non-controlling interest in subsidiaries | — | ( | — | |||||
Purchase of own shares | ( | — | ( | |||||
Leased assets payments | 16 | ( | ( | ( | ||||
Warrants tender offer payments | — | — | ( | |||||
Cash dividend distributed by subsidiary | ( | — | — | |||||
Net cash flows generated by financing activities |
| |
| | | |||
Net increase in cash and cash equivalents |
| |
| |
| ( | ||
| ||||||||
Inflation effects on cash and cash equivalents | ( | ( | ( | |||||
|
|
| ||||||
Cash and cash equivalents as of beginning of the year |
| 7.1 |
| |
| |
| |
Effect of exchange rate changes on cash and equivalents |
| |
| |
| | ||
Cash and cash equivalents as of the end of the year |
| 7.1 |
| |
| |
| |
The accompanying Notes are an integral part of these Consolidated financial statements. Related parties’ balances and transactions are disclosed in Note 17.
F-12

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Index
| 1. | General information. |
| 2. | Accounting standards and basis of preparation. |
| 3. | New standards, amendments and interpretations issued by the IASB. |
| 4. | Summary of significant accounting policies. |
| 5. | Critical accounting judgments and estimates. |
| 6. | Acquisitions and other significant transactions. |
| 7. | Information about components of consolidated statement of financial position. |
| 8. | Information about components of consolidated statement of comprehensive income. |
| 9. | Taxation. |
| 10. | Earnings per share. |
| 11. | Information about components of equity. |
| 12. | Cash flow information. |
| 13. | Joint ventures and associates. |
| 14. | Segment information. |
| 15. | Financial instruments – Risk management. |
| 16. | Leases. |
| 17. | Shareholders and other related parties’ balances and transactions. |
| 18. | Key management personnel compensation. |
| 19. | Share-based payments. |
| 20. | Contingencies, commitments and restrictions on the distribution of profits. |
| 21. | Impact of COVID-19. |
| 22. | Events occurring after the reporting period. |
F-13

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
1. GENERAL INFORMATION
Bioceres Crop Solutions Corp. (NASDAQ: BIOX) is a leader in the development and commercialization of productivity solutions designed to regenerate agricultural ecosystems while making crops more resilient to climate change. To do this, Bioceres’ products create economic incentives for farmers and other stakeholders to adopt environmentally friendly production practices. Bioceres has a unique biotech platform with high impact, patented technologies for seeds and microbial ag inputs, as well as next generation crop nutrition and protection solutions.
Bioceres is a global company with an extensive geographic footprint. The Group’s agricultural inputs are marketed across more than
Unless the context otherwise requires, “we”, “us”, “our”, “Bioceres”, “BIOX”, “the Group”, and “Bioceres Crop Solutions” will refer to Bioceres Crop Solutions Corp. and its subsidiaries.
2. ACCOUNTING STANDARDS AND BASIS OF PREPARATION
Statement of compliance with IFRS as issued by IASB
The Consolidated financial statements of the Group have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by International Accounting Standard Board (“IASB”) following the accounting policies as set forth and summarized in Note 4. All IFRS issued by the IASB, effective at the time of preparing these Consolidated financial statements have been applied.
Authorization for the issue of the Consolidated financial statements
These Consolidated financial statements of the Group as of and for the years ended June 30, 2023, 2022 and 2021 have been authorized by the Board of Directors of Bioceres Crop Solutions on September 29, 2023.
Basis of measurement
The consolidated financial statements of the Group have been prepared using:
| ● | Going concern basis of accounting, considering the conclusion of the assessment made by the Group’s Management about the ability of the Group and its subsidiaries to continue as a going concern, in accordance with the requirements of paragraph 25 of IAS 1, “Presentation of Financial Statements”. |
| ● | Accrual basis of accounting (except for cash flows information). Under this basis of accounting, the effects of transactions and other events are recognized as they occur, even when there are no cash flows. |
Functional currency and presentation currency
a) | Functional currency |
Items included in the financial statements of each of the Group’s entities are measured using the currency of the primary economic market in which the entity operates (i.e., “the functional currency”).
From July 1, 2022 the main Argentinian subsidiaries of the Group have changed their functional currency from Argentine Pesos to United States Dollars as a result of changes in events and conditions relevant to their business operations. These include a macroeconomic context with high inflation and depreciation of the Argentine peso, and inorganic growth at the close of the fiscal year ended June 30, 2022, which led to a global unification of management and commercial strategy whereby integration of the businesses was done by business units, regardless of the legal entities.
F-14

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
For the years ended June 30, 2022 and 2021, Argentinian subsidiaries had applied IAS 29. “Financial reporting in hyperinflationary economies” requires that the financial statements of an entity whose functional currency is the currency of a hyperinflationary economy, whether these are based on the historical cost method or the current cost method, be stated in terms of the measuring unit current at the closing date of the reporting period. For such purpose, the inflation produced since the acquisition date or the revaluation date, as applicable, must be computed in non-monetary items. The standard details a series of factors to be considered for concluding whether an economy is hyperinflationary, including, but not limited to, a cumulative inflation rate over a three-year period that approaches or exceeds 100%. The accumulated inflation in three years, as of June 30, 2018, was over 100%. It was for this reason that, in accordance with IAS 29, the Argentine economy was considered as hyperinflationary since July 1, 2018. Consequently, the Group has applied IAS 29 to these financial statements.
In an inflationary period, any entity that maintains an excess of monetary assets over monetary liabilities, will lose purchasing power, and any entity that maintains an excess of monetary liabilities over monetary assets, will gain purchasing power, provided that such items are not subject to an adjustment mechanism.
Briefly, the restatement mechanism of IAS 29 establishes that monetary assets and liabilities will not be restated because they are already expressed in a current unit of measurement at the end of the reporting period. Assets and liabilities subject to adjustments based on specific agreements, will be adjusted according to those agreements. Non-monetary items measured at their current values at the end of the reporting period, such as the net realizable value or others, do not need to be restated. The remaining non-monetary assets and liabilities will be restated according to a general price index. The loss or gain for the net monetary position will be included in the net result of the reporting period, listed in a separate line item.
The effect of the functional currency change was recorded prospectively as of July 1, 2022, in accordance with IAS 21. As a result, from July 1, 2022 there are no longer effects of inflation adjustments for the above-mentioned subsidiaries.
b) | Presentation currency |
The consolidated financial statements of the Group are presented in US dollars.
c) | Foreign currency |
Transactions entered into by Group entities in a currency other than their functional currency are recorded at the relevant exchange rates as of the date upon which such transactions occur. Foreign currency monetary assets and liabilities are translated at the prevailing exchanges rates as of the final day of each reporting period. Exchange differences arising from the retranslation of unsettled monetary assets and liabilities are recognized immediately in profit or loss, except for foreign currency borrowings qualifying as a hedge of a net investment in a foreign operation for which exchange differences are recognized in other comprehensive income and accumulated in the foreign exchange reserve along with the exchange differences arising from the retranslation of the foreign operation. Upon the disposal of a foreign operation, the cumulative exchange differences recognized in the foreign exchange reserve relating to such operation up to the date of disposal are transferred to the consolidated statement of profit or loss and other comprehensive income as part of the gain or loss recognized upon such disposal.
Subsidiaries
Where the Group holds a controlling interest in an entity, such entity is classified as a subsidiary. The Group exercises control over such an entity if all three of the following elements are present: (i) the Group has the power to direct or cause the direction of the management and policies of the entity; (ii) the Group is exposed to the variable returns of such entity; and (iii) the Group has power to affect the variability of such returns. Control is reassessed whenever facts and circumstances indicate that there may be a change in any of these elements of control.
F-15

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
De-facto control exists in situations where the Group has the practical ability to direct the relevant activities of an entity without holding the majority of the voting rights. In determining whether de facto control exists, the Group considers all relevant facts and circumstances, including:
| - | The relative share of the Group’s voting rights with respect both the size and dispersion of other parties who hold voting rights; |
| - | Substantive potential voting rights held by the Group and by other parties; |
| - | Other contractual arrangements; and |
| - | Historic patterns in voting attendance. |
The subsidiaries of the Group, all of which have been included in the consolidated financial statements of the Group, are as follows:
The Group holds a majority share of the voting rights in all of its subsidiaries.
Country of | |||||||||||||
incorporation | |||||||||||||
and principal | |||||||||||||
place of | % Equity interest | ||||||||||||
Name |
| Principal activities |
| business |
| Ref |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
RASA Holding, LLC |
| Investment in subsidiaries |
| USA |
|
|
| | % | | % | | % |
Rizobacter Argentina S.A. |
| Microbiology Business |
| Argentina |
|
|
| | % | | % | | % |
Rizobacter do Brasil Ltda. |
| Microbiology Business |
| Brazil |
| a |
| | % | | % | | % |
Rizobacter del Paraguay S.A. |
| Microbiology Business |
| Paraguay |
| a |
| | % | | % | | % |
Rizobacter Uruguay |
| Microbiology Business |
| Uruguay |
| a |
| | % | | % | | % |
Rizobacter South Africa |
| Microbiology Business |
| South Africa |
| a |
| | % | | % | | % |
Comer. Agrop. Rizobacter de Bolivia S.A. |
| Microbiology Business |
| Bolivia |
| a |
| | % | | % | | % |
Rizobacter USA, LLC |
| Microbiology Business |
| USA |
| a |
| | % | | % | | % |
Rizobacter Colombia SAS |
| Microbiology Business |
| Colombia |
| a |
| | % | | % | | % |
Rizobacter France SAS |
| Microbiology Business |
| France |
| a |
| | % | | % | | % |
Bioceres Crops S.A. |
| Corporate related expenses |
| Argentina |
|
|
| | % | | % | | % |
BCS Holding, LLC | Investment in subsidiaries | USA |
| | % | | % | | % | ||||
Bioceres Semillas S.A.U. | Seeds and Traits | Argentina |
| | % | | % | | % | ||||
Verdeca, LLC | Seeds and Traits | USA |
| | % | | % | | % | ||||
Insumos Agroquímicos S.A. | Selling of agricultural inputs | Argentina |
| | % | | % | | % | ||||
Bioceres Crops Do Brasil Ltda. | Seeds and Traits | Brazil |
| | % | | % | — | |||||
Pro Farm Group, Inc. | Microbiology Business | United States | b | | % | — | — | ||||||
Pro Farm International, OÜ | Microbiology Business | Finland | b | | % | — | — | ||||||
Pro Farm Michigan Manufacturing, LLC | Microbiology Business | United States | b | | % | — | — | ||||||
Pro Farm Russia, LLC | Microbiology Business | Russia | b | | % | — | — | ||||||
Pro Farm Technologies Comércio de Insumo Agrícolas do Brasil Ltda. | Microbiology Business | Brazil | b | | % | — | — | ||||||
Glinatur S.A. | Microbiology Business | Uruguay | b | | % | ||||||||
Pro Farm Technologies, OÜ | Microbiology Business | Finland | b | | % | — | — | ||||||
| a) | Indirect interests held through Rizobacter. The indirect equity interest participation included in this table was the |
b) | On July 12, 2022 we acquired a controlling interest in Pro Farm Group Inc. (“Pro Farm”). See Note 6. |
F-16

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Special purpose and structured entities (“SPE”)
A structured entity is an entity that has been designed so that voting or similar rights are not the dominant factor in deciding who controls the entity and the relevant activities are directed by means of contractual arrangements. In these cases, we consider the purpose and design of the SPE, including a consideration of the risks the SPE was expected to be exposed to, the risks it was designed to pass on to the parties involved with the SPE and whether we are exposed to some or all of those risks or potential returns. One then considers which activities have a significant impact on the SPE’s returns and determines which parties have an ability to direct each of those activities.
The Group controls an SPE when is exposed, or has rights, to variable returns from its involvement with the SPE and has the ability to affect those returns through its power over the SPE.
3. NEW STANDARDS, AMENDMENTS AND INTERPRETATIONS ISSUED BY THE IASB
a)The following new standards became applicable for the current reporting period and adopted by the Group.
Annual Improvements to IFRS Standards 2018–2020
The following improvements were finalized in May 2020:
| ● | IFRS 9 Financial Instruments – clarifies which fees should be included in the 10% test for derecognition of financial liabilities. |
| ● | IFRS 16 Leases – amendment of illustrative example 13 to remove the illustration of payments from the lessor relating to leasehold improvements, to remove any confusion about the treatment of lease incentives. |
| ● | IFRS 1 First-time Adoption of International Financial Reporting Standards – allows entities that have measured their assets and liabilities at carrying amounts recorded in their parent’s books to also measure any cumulative translation differences using the amounts reported by the parent. This amendment will also apply to associates and joint ventures that have taken the same IFRS 1 exemption. |
| ● | IAS 41 Agriculture – removal of the requirement for entities to exclude cash flows for taxation when measuring fair value under IAS 41. This amendment is intended to align with the requirement in the standard to discount cash flows on a post-tax basis. |
The new standard is effective for financial years beginning on or after January 1, 2022.
These amendments did not have a material impact on the Group.
Amendments to IAS 16 - Property, Plant and Equipment: Proceeds before intended use.
The amendment to IAS 16 Property, Plant and Equipment (PP&E) prohibits an entity from deducting from the cost of an item of PP&E any proceeds received from selling items produced while the entity is preparing the asset for its intended use. It also clarifies that an entity is ‘testing whether the asset is functioning properly’ when it assesses the technical and physical performance of the asset. The financial performance of the asset is not relevant to this assessment.
Entities must disclose separately the amounts of proceeds and costs relating to items produced that are not an output of the entity’s ordinary activities.
The amendments are effective for annual periods beginning on or after January 1, 2022.
These amendments did not have any material impact on the Group.
F-17

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Amendments to IFRS 3 - Reference to the Conceptual Framework.
Minor amendments were made to IFRS 3 Business Combinations to update the references to the Conceptual Framework for Financial Reporting and add an exception for the recognition of liabilities and contingent liabilities within the scope of IAS 37 Provisions, Contingent Liabilities and Contingent Assets and Interpretation 21 Levies. The amendments also confirm that contingent assets should not be recognized at the acquisition date.
The amendments are effective for financial years beginning on or after January 1, 2022.
These amendments did not have any material impact on the Group.
Amendments to IAS 37 - Onerous Contracts - Cost of Fulfilling a Contract.
The amendment to IAS 37 clarifies that the direct costs of fulfilling a contract include both the incremental costs of fulfilling the contract and an allocation of other costs directly related to fulfilling contracts. Before recognizing a separate provision for an onerous contract, the entity recognizes any impairment loss that has occurred on assets used in fulfilling the contract.
The amendments are effective for financial years beginning on or after January 1, 2022.
These amendments did not have any material impact on the Group.
b) The following new standards are not yet adopted by the Group.
Amendments to IFRS 16- Lease Liability in a Sale and Leaseback.
The amendment requires a seller-lessee to subsequently measure lease liabilities arising from a leaseback in a way that it does not recognize any amount of the gain or loss that relates to the right of use it retains. The new requirements do not prevent a seller-lessee from recognizing in profit or loss any gain or loss relating to the partial or full termination of lease.
These amendments are not expected to have material impact on the Group.
The amendments are effective for financial years beginning on or after January 1, 2024. Earlier application is permitted.
Amendments to IAS1 – Non- current liabilities with covenants.
The amendments modify the requirements introduced by Classification of Liabilities as Current or Non-current on how an entity classifies debt and other financial liabilities as current or non-current in particular circumstances. Only covenants with which an entity is required to comply on or before the reporting date affect the classification of a liability as current or non-current. In addition, an entity must disclose information in the notes that enables users of financial statements to understand the risk that non-current liabilities with covenants could become repayable within twelve months.
These amendments are not expected to have material impact on the Group.
The amendments apply retrospectively for annual reporting periods beginning on or after 1 January 2024, with early application permitted.
Amendment to IAS 12 –Deferred tax related to assets and liabilities arising from a single transaction.
The amendments introduce an exception to the initial recognition exemption in IAS 12. Applying this exception, an entity does not apply the initial recognition exemption for transactions that give rise to equal taxable and deductible temporary differences.
F-18

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The amendments apply to transactions that occur on or after the beginning of the earliest comparative period presented.
The amendments also apply to taxable and deductible temporary differences associated with right-of-use assets and lease liabilities, and decommissioning obligations and corresponding amounts recognized as assets at the beginning of the earliest comparative period presented.
The amendments are effective for annual reporting periods beginning on or after 1 January 2023. Early application of the amendments is permitted.
These amendments are not expected to have material impact on the Group.
Amendments to IAS 7- Statement of Cash Flows & to IFRS 7- Financial Instruments: Disclosures.
The amendments introduce new disclosure requirements in IFRS Standards to enhance the transparency and, thus, the usefulness of the information provided by entities about supplier finance arrangements.
The disclosure requirements in the amendments enhance the current requirements and are intended to assist users of financial statements in understanding the effects of supplier finance arrangements on an entity’s liabilities, cash flows and exposure to liquidity risk.
The amendments are effective for annual reporting periods beginning on or after 1 January 2024. Early application of the amendments is permitted.
These amendments are not expected to have material impact on the Group.
International Tax Reform—Pillar Two Model Rules (Amendments to IAS 12)
The amendments give companies temporary relief from accounting for deferred taxes arising from the Organization for Economic Co-operation and Development’s (OECD) international tax reform.
The amendments will introduce (i) a temporary exception to the accounting for deferred taxes arising from jurisdictions implementing the global tax rules. This will help to ensure consistency in the financial statements while easing into the implementation of the rules; (ii) and targeted disclosure requirements to help investors better understand a company’s exposure to income taxes arising from the reform, particularly before legislation implementing the rules is in effect.
Companies can benefit from the temporary exception immediately but are required to provide the disclosures to investors for annual reporting periods beginning on or after 1 January 2023.
These amendments are not expected to have material impact on the Group.
Amendments to IAS 1 and IFRS Practice Statement 2- Disclosure of Accounting Policies
An entity is now required to disclose its material accounting policy information instead of its significant accounting policies. The amendments clarify that accounting policy information may be material because of its nature, even if the related amounts are immaterial.
The amendments are applied prospectively and are effective for annual periods beginning on or after January 1, 2023. Earlier application is permitted.
These amendments are not expected to have material impact on the Group.
F-19

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Amendments to IAS 8-Definition of Accounting Estimates
These amendments help entities to distinguish between accounting policies and accounting estimates making a distinction between how an entity should present and disclose different types of accounting changes in its financial statements. Under the new definition, accounting estimates are “monetary amounts in financial statements that are subject to measurement uncertainty.”
The amendments are effective for annual periods beginning on or after January 1, 2023 and changes in accounting policies and changes in accounting estimates that occur on or after the start of that period. Earlier application is permitted.
These amendments are not expected to have material impact on the Group.
4. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
4.1. Cash and cash equivalents
For the purposes of the statements of financial position and statements of cash flows, cash and cash equivalents include cash on hand and in banks and short-term highly liquid investments. Investments can be readily convertible to known amounts of cash and they are subject to insignificant risk of changes in value. In the consolidated statements of financial position, bank overdrafts are included in borrowings within current liabilities.
4.2. Inventories
Inventories are recognized at cost initially and subsequently at the lower of cost and net realizable value. Cost comprises all costs of purchase and conversion as well as other costs incurred in bringing the inventories to their present location and condition.
Weighted average cost is used to determine the cost of ordinarily interchangeable items.
Estimates
The Group assesses the recoverability of inventories considering their sale price, whether the inventories are damaged and whether they have become obsolete in whole or in part.
Net realizable value is the sale price estimated to be attained in the ordinary course of business, less costs of completion and other selling expenses.
The Group sets up an allowance for obsolescence or slow-moving inventories in relation to finished and in-process products. The allowance for obsolescence or slow-moving inventories is recognized for finished products and in-process products based on an analysis by Management of the aging of inventory stocks.
4.3.Biological assets
Within current assets, growing crops are included as biological assets from the moment of sowing until the moment of harvest (approximately
Costs are capitalized as biological assets if, and only if, (a) it is probable that future economic benefits will flow to the entity, and (b) the cost can be measured reliably. The Group capitalizes costs such as: planting, harvesting, weeding, seedlings, irrigation, agrochemicals, fertilizers and a systematic allocation of fixed and variable production overheads that are directly attributable to the management of biological assets, among others.
F-20

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Biological assets, both at initial recognition and at each subsequent reporting date, are measured at fair value less costs to sell, except where fair value cannot be reliably measured. Cost approximates fair value when little biological transformation has taken place since the costs were originally incurred or the impact of biological transformation on price is not expected to be material.
Gains and losses that arise from measuring biological assets at fair value less costs to sell and measuring agricultural produce at the point of harvest at fair value less costs to sell are recognized in the statement of income in the period in which they arise in the line item “Initial recognition and changes in fair value of biological assets”.
From the harvest time, agricultural products are valued at net realizable value because there is a market asset, and the risk of non-sale is non-significant.
Generally, the estimation of the fair value of biological assets is based on models or inputs that are not observable in the market and the use of unobservable inputs is significant to the overall valuation of the assets. Unobservable inputs are determined based on the best information available. Key assumptions include future market prices, estimated yields at the point of harvest, estimated production cycle, future cash flows, future costs of harvesting and other costs, and estimated discount rate.
Market prices are generally determined by reference to observable data in the principal market for the agricultural produce. Harvesting costs and other costs are estimated based on historical and statistical data. Yields are estimated based on several factors, including the location of the farmland and soil type, environmental conditions, infrastructure and other restrictions and growth at the time of measurement. Yields are subject to a high degree of uncertainty and may be affected by several factors out of the Group’s control including but not limited to extreme or unusual weather conditions, plagues and other crop diseases, among other factors.
4.4. Business combinations
The Group applies the acquisition method to account for business combinations. The acquisition cost is measured as the aggregate of the consideration transferred for the acquisition of a subsidiary, which is measured at fair value at the acquisition date, and the amount of any non-controlling interest in such subsidiary. The Group recognizes any non-controlling interest in a subsidiary at the non-controlling interest’s proportionate share of the recognized amounts of subsidiary’s identifiable net assets. The acquisition related costs are expensed as incurred.
Any contingent consideration to be transferred by the Group is recognized at fair value at the acquisition date. The contingent consideration is classified as an asset or liability that is a financial instrument under IFRS 9 is measured at fair value through profit or loss.
Goodwill is initially measured at cost, which is the excess of the aggregate of the consideration transferred and the amount of the non-controlling interest and any previous interest carried over the net identifiable assets acquired, and liabilities assumed.
After initial recognition, goodwill is measured at cost less any accumulated impairment losses. For impairment testing, goodwill acquired in a business combination is, as of the acquisition date, allocated to each of the cash-generating units of the Group that is expected to benefit from the synergies of the combination, without considering whether other assets or liabilities of the subsidiary are allocated to those units.
Any impairment in the carrying value is recognized in the consolidated statement of comprehensive income. In the case of acquisitions in stages, prior to the write-off of the previously held equity interest in the subsidiary, said interest is re-measured at fair value as of the date of acquisition of control over the subsidiary. The result of the re-measurement at fair value is recognized in profit or loss.
F-21

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
When a seller in a business combination has contractually agreed to indemnify the Group for the result of a contingency or uncertainty related to the entirety or a portion of an asset or liability, the Group recognizes an indemnification asset. The indemnification asset is measured on the same basis as the indemnification item. At the end of each period, the Group measures the indemnification assets recognized at the acquisition date on the same basis as the indemnified liability, subject to any contractual limitation on the amount and, for an indemnification asset that is not periodically measured at fair value, based on Management’s assessment of the recoverability of the indemnification asset. The Group derecognizes the indemnification asset when it collects or sells it, or when it loses the right over it.
4.5. Business combination under common control
Common control of business combination is excluded from the scope of IFRS 3. There is no other specific guidance on this topic elsewhere in IFRS. Therefore, management needs to use judgement to develop an accounting policy that provides relevant and reliable information in accordance with IAS 8. Management accounting police choice for business combination under common control is “Predecessor value method”. A Predecessor value method involves accounting for the assets and liabilities of the acquired business using existing carrying values. Differences between the carrying value and the amount payable should be accounted as an equity contribution.
Management’s accounting policy choice is to use a prospective presentation method.
4.6. Impairment of non-financial assets (excluding inventories and deferred tax assets)
Impairment tests on goodwill and intangible assets not yet available for use are undertaken annually at the end of the reporting period. Other non-financial assets are subject to impairment tests whenever events or changes in circumstances indicate that their carrying amount may not be recoverable. Where the carrying value of an asset exceeds its recoverable amount (i.e. the higher of value in use and fair value less costs to sell), the asset is written down accordingly.
Where it is not possible to estimate the recoverable amount of an individual asset, the impairment test is carried out on the smallest group of assets to which it belongs for which there are separately identifiable cash flows (its Cash Generating Unit or CGU). Goodwill is allocated on initial recognition to each of the Group’s CGUs that are expected to benefit from a business combination that gives rise to the goodwill.
Impairment charges are included in profit or loss, except to the extent they reverse gains previously recognized in other comprehensive income. An impairment loss recognized for goodwill is not reversed.
Estimate
Impairment testing of goodwill and intangible assets not yet available for use requires the use of significant assumptions for the estimation of future cash flows and the determination of discount rates. The significant assumptions and the determination of discount rates for the impairment testing of goodwill are further explained in Note 7.9.
4.7. Joint arrangements
An associate is an entity over which the Group exerts significant influence. Significant influence is the power to participate in financial and operating policy decision-making at such entity, but it does not involve control or joint control over those policies.
The Group is a party to a joint arrangement when there is a contractual arrangement that confers joint control over the relevant activities of the arrangement to the Group and at least one other party. Joint control is assessed under the same principles as control over subsidiaries.
F-22

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The Group classifies its interests in joint arrangements as either:
| - | Joint ventures: where the group has rights to only the net assets of the joint arrangement. |
| - | Joint operations: where the group has both the rights to the assets and obligations for the liabilities of the joint arrangement. |
In assessing the classification of interests in joint arrangements, the Group considers:
| - | The structure of the joint arrangement; |
| - | The legal form of joint arrangements structured through a separate vehicle; |
| - | The contractual terms of the joint arrangement agreement; and |
| - | Any other facts and circumstances (including any other contractual arrangements). |
The Group accounts for its interests in joint ventures using the equity method, where the Group’s share of post-acquisition profits and losses and other comprehensive income is recognized in the Consolidated statement of profit and loss and other comprehensive income.
Losses in excess of the Group’s investment in the joint venture are recognized only to the extent that the Group has incurred legal or constructive obligations or made payments on behalf of the joint venture.
Profits and losses arising on transactions between the Group and its joint ventures are recognized only to the extent of unrelated investors’ interests in the joint venture. The Group’s share in a joint venture’s profits and losses resulting from a transaction is eliminated against the carrying amount of investment in the joint venture through the line “share of profit (or loss) of joint ventures” in the Consolidated statements of profit or loss and other comprehensive income.
Any premium paid for an investment in a joint venture above the fair value of the Group’s share of the identifiable assets, liabilities and contingent liabilities acquired is capitalized and included in the carrying amount of the investment in the joint venture. Where there is objective evidence that the investment in a joint venture has been impaired, the carrying amount of the investment is tested for impairment in the same way as other non-financial assets.
When the Group loses significant influence in an associate or joint control over a joint venture, it measures and recognizes any investment held at fair value. Any difference between the carrying amount of the associate or joint venture when losing significant influence or joint control and the fair value of the held investment and sale revenue are recognized in profit or loss.
The Group accounts for its interests in joint operations by recognizing its share of assets, liabilities, revenues and expenses in accordance with its contractually conferred rights and obligations.
For all joint arrangements structured in separate vehicles the Group must assess the substance of the joint arrangement in determining whether it is classified as a joint venture or joint operation. This assessment requires the Group to consider whether it has rights to the joint arrangement’s net assets (in which case it is classified as a joint venture), or rights to and obligations for specific assets, liabilities, expenses, and revenues (in which case it is classified as a joint operation).
Estimates
There is uncertainty regarding Management’s estimates of the Group’s ability to recover the carrying amounts of the investments in joint ventures, since such estimates depend on the joint ventures’ ability to generate sufficient funds to complete the development projects, the future outcome of the project deregulation process and the amounts and timing of the cash flows from projects, among other future events.
F-23

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Management assesses whether there are impairment indicators and, if any, it performs a recoverability analysis.
Management estimates of the recoverability of these investments represent the best estimate based on available evidence, the existing facts and circumstances, using reasonable and provable assumptions in the cash flow projections.
Therefore, the consolidated financial statements do not include adjustments that would be required if the Group were unable to recover the carrying amount of the above-mentioned assets by generating sufficient economic benefits in the future.
4.8. Property, plant and equipment
Property, plant and equipment items are initially recognized at cost. In addition to the purchase price, cost also includes costs directly attributable to such property, plant and equipment items. There are no unavoidable costs with respect to dismantling and removing items. The cost of property, plant and equipment items acquired in a business combination is their fair value at the acquisition date.
Depreciation is calculated using the straight-line method to allocate the property, plant or equipment items’ cost or revalued amounts, net of their residual values, over their estimated useful lives or, in the case of leasehold improvements and certain leased plant and equipment, the shorter lease term as follows:
Research instruments:
Office equipment:
Vehicles:
Computer equipment and software:
Fixture and fittings:
Machinery and equipment:
Buildings:
Useful lives and depreciation methods are reviewed every year as required by IAS 16.
Assets under items Land and Buildings, are accounted for at fair value arising from the last revaluation performed, applying the revaluation model indicated by IAS 16. Revaluations are performed on a regular basis, when there are signs that the book value differs significantly from that to be determined using the fair value at the end of the reporting year.
To obtain fair values, the existence of an active market is considered for the assets in their current status. For those assets for which an active market in their current status exists, the fair values were determined based on their market values. For the remaining cases, the market values of comparable new assets are analyzed, applying a discount based on the status and wear of each asset and considering the characteristics of each of the revalued assets (for example, improvements made, maintenance status, level of productivity, use, etc.
Estimates
The Group carries certain classes of property, plant and equipment under the revaluation model under IAS 16. The revaluation model requires that the Group carry property, plant and equipment at revalued amounts, being fair value at the date of revaluation less any subsequent accumulated depreciation and any subsequent accumulated impairment losses. IAS 16 requires that the Group carry out these revaluations with sufficient regularity so that the carrying amounts of its property, plant and equipment do not differ materially from that which would be determined using fair value at the end of a reporting period. The determination of fair value at the date of revaluation requires judgments, estimates and assumptions based on market conditions prevailing at the time of any such revaluation. Changes to any of the Group’s judgments, estimates or assumptions or to the market conditions subsequent to a revaluation will result in changes to the fair value of property, plant and equipment.
F-24

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The Group prepares the corresponding revaluations on a regular basis taking into account the work of independent appraisers. The Group uses different valuation techniques depending on the class of property being valued. Generally, the Group determines the fair value of its industrial buildings and warehouses based on a depreciated replacement cost approach. The Group determines the fair value of its land based on active market prices adjusted, if necessary, for differences in the nature, location or condition of the specific asset. If this information is not available, the Group may use alternative valuation methods, such as recent prices in less active markets.
Property valuation is a significant area of estimation uncertainty. Fair values are prepared regularly by Management, taking into account independent valuations. The determination of fair value for the different classes of property, plant and equipment is sensitive to the selection of various significant assumptions and estimates. Changes in those significant assumptions and estimates could materially affect the determination of the revalued amounts of property, plant and equipment. The Group utilizes historical experience, market information and other internal information to determine and/or review the appropriate revalued amounts.
The following are the most significant assumptions used in the preparation of the revalued amounts for its classes of property, plant and equipment:
a) Land: The Group generally uses the market price of a square meter of land for the same or similar location as the most significant assumption to determine the revalued amount. The Group typically uses comparable land sales in the same location to assess appropriateness of the value of its land.
b) Industrial buildings and warehouses: The Group generally determines the construction cost of a new asset and then the Group adjusts it for normal wear and tear. Construction prices may include, but are not limited to, construction materials, labor costs, installation and assembly costs, site preparation, professional fees and applicable taxes. Construction costs may differ significantly from year to year and are subject to macroeconomic changes in the economy where the Group operates, such as the impact of inflation and foreign exchange rates. The construction cost of its industrial buildings and warehouses is determined on a US dollar per constructed square meter basis, while the construction cost of its mills, facilities and grain storage facilities is determined by reference to their total capacity measured in tons milled or stored, respectively. A
Increases in the carrying amounts arising on revaluation of land and buildings are recognized, net of tax, in other comprehensive income and accumulated in reserves in shareholders’ equity. To the extent that the increase reverses a decrease previously recognized in profit or loss, the increase is first recognized in profit or loss. Decreases that reverse previous increases of the same asset are first recognized in other comprehensive income to the extent of the remaining surplus attributable to the asset; all other decreases are charged to profit or loss.
4.9. Leases
Leases are recognized as a right-of-use asset and corresponding liability at the date of which the leased asset is available for use by the Group. Each lease payment is allocated between the liability and finance cost. The finance cost is charged to profit or loss over the lease period so as to produce a constant periodic rate of interest on the remaining balance of the liability for each period. The right-of-use asset is depreciated over the shorter of the asset’s useful life and the lease term on a straight-line basis.
In determining the lease term, we consider all facts and circumstances that create an economic incentive to exercise an extension option, or not exercise a termination option. Extension options (or periods after termination options) are only included in the lease term if the lease is reasonably certain to be extended (or not terminated).
Short term leases are recognized on a straight-line basis as an expense in the income statement.
F-25

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
At initial recognition, the right-of-use asset is measured considering the value of the initial measurement of the lease liability; any lease payments made at or before the commencement date, less any lease incentives; and any initial direct costs incurred by the lessee. After initial recognition, the right-of-use assets are measured at cost, less any accumulated depreciation and/or impairment losses, and adjusted for any re-measurement of the lease liability. Depreciation of the right-of-use asset is calculated using the straight-line method over the estimated duration of the lease contract.
The lease liability is initially measured at the present value of the lease payments that are not paid at such date, including variable lease payments that depend on an index or rate, initially measured using the index or rate as of the commencement date; amounts expected to be payable by the lessee under residual value guarantees; the exercise price of a purchase option if the lessee is reasonably certain to exercise that option; payments of penalties for terminating the lease, if the lease term reflects the lessee exercising an option to terminate the lease; and fixed payments, less any lease incentives receivable. After the commencement date, we measure the lease liability by increasing the carrying amount to reflect interest on the lease liability; reducing the carrying amount to reflect lease payments made; and re-measuring the carrying amount to reflect any reassessment or lease modifications.
The above-mentioned inputs for the valuation of the right of use assets and lease liabilities including the determination of the contracts within the scope of the standard, the contract term ant interest rate used in the discounted cash flow involved a management’s estimations.
4.10. Intangible assets
a)Externally acquired intangible assets
Externally acquired intangible assets are initially recognized at acquisition date fair value (which is considered as their cost). After initial recognition, those assets are measured at cost less accumulated amortization and accumulated impairment losses.
Intangible assets acquired from third parties have an estimated useful life as follows (in years):
Software:
Trademarks and patents:
Certification ISO Standards:
Useful lives and amortization methods are reviewed every year as required by IAS 38.
Estimates
To value acquired intangible assets, valuation techniques generally accepted in the market are applied, based mainly on the revenue approach (such as excess earnings, relief from royalty, and with or without), considering the characteristics of the assets to be valued and available information to estimate their acquisition date fair value. Application of these valuation techniques requires the use of several assumptions related to future cash flows such as revenue grow, discount rate and royalty rate.
b)Internally generated intangible assets (development costs)
Expenditure on internally developed products is capitalized if it can be demonstrated that:
| - | It is technically feasible to develop the product for it to be sold; |
| - | Adequate resources are available to complete the development; |
| - | There is an intention to complete and sell the product; |
F-26

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
| - | The Group is able to sell the product; |
| - | Sale of the product will generate future economic benefits; and |
| - | Expenditure on the project can be measured reliably. |
Development expenditure not satisfying the above criteria and expenditure on the research phase of internal projects are recognized in the consolidated statement of profit or loss and other comprehensive income as incurred.
Capitalized development costs are amortized using the straight-line method over the periods the Group expects to benefit from selling the products developed.
Useful lives and amortization methods are reviewed every year as required by IAS 38.
The research and development process can be divided into several discrete steps or phases, which generally begin with discovery, validation and development and end with regulatory approval and commercial launch. The process for developing seed traits is relatively similar for both GM and non-GM traits. However, the two differ significantly in later phases of development. For example, obtaining regulatory approval for GM seeds is a far more comprehensive and lengthy process than for non-GM seeds. Although breeding programs and industrial biotechnology solutions may have shorter or simpler phases than those described below, the Group has used the industry consensus for seed-trait development phases to characterize its technology portfolios, which is generally divided into the following six phases:
i) Discovery: The first phase in the technology development process is the discovery or identification of candidate genes or genetic systems, metabolites, or microorganisms potentially capable of enhancing specified plant characteristics or enabling an agro-industrial biotech solution.
ii) Proof of concept: Upon successful validation of the technologies in model systems (in vitro or in vivo), promising technologies graduate from discovery and are advanced to the proof-of-concept phase. The goal of this phase is to validate a technology within the targeted organism before moving forward with technology escalation activities or extensive field validation.
iii) Early development: In this phase, field tests commenced in the proof-of-concept phase are expanded to evaluate various permutations of a technology in multiple geographies and growing cycles, as well as other characteristics in order to optimize the technology’s performance in the targeted organisms. The goal of the early development phase is to identify the best mode of use of a technology to define its performance concept.
iv) Advanced development and deregulation: In this phase, extensive field tests are used to demonstrate the effectiveness of the technology for its intended purpose. In the case of GM traits, the process of obtaining regulatory approvals from government authorities is also initiated during this phase, and tests are performed to evaluate the potential environmental impact of modified plants. For solutions involving microbial fermentation, industrial-scale runs are conducted.
v) Pre-launch: This phase involves finalizing the regulatory approval process and preparing for the launch and commercialization of the technology. The range of activities in this phase includes seed increases, pre-commercial production, and product and solution testing with selected customers. Usually, a more detailed marketing strategy and preparation of marketing materials occur during this phase.
vi) Product launch: In general, this phase, which is the last milestone of the research and development process, is carried out by the Group, the joint ventures and/or the Group’s technology licensees. When technology is commercialized through the joint ventures or technology licensees, a successful product launch will trigger royalty payments to the Group, which are generally calculated as a percentage of the net sales realized by the technology and captured upon commercialization.
F-27

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Demonstrability of technical feasibility generally occurs when the project reaches the “advanced development and deregulation” phase because at this stage success is considered to be probable.
c)Intangible assets acquired in a business combination
Intangible assets acquired in a business combination and recognized separately from goodwill are initially recognized at acquisition date fair value (which is considered as their cost). After initial recognition, those assets are measured at cost less accumulated amortization and accumulated impairment losses in the same manner as intangible assets acquired separately.
Intangible assets acquired in a business combination have an estimated useful life as follows (in years):
Product development:
Trademarks:
Customer loyalty:
Estimates
To value intangible assets acquired from a business combination, valuation techniques generally accepted in the market were applied, based mainly on the revenue approach (such as excess earnings, relief from royalty, and with or without), considering the characteristics of the assets to be valued and available information to estimate their acquisition date fair value. Application of these valuation techniques requires the use of several assumptions related to future cash flows such as revenue grow, discount rate and royalty rate.
4.11. Investment properties
Investment properties shall be measured initially at its cost. The cost of a purchased investment property comprises its purchase price and any directly attributable expenditure. Directly attributable expenditure includes, for example, professional fees for legal services, property transfer taxes and other transaction costs.
In the measurement after initial recognition, the Group has chosen the cost model for all investment property.
4.12. Financial assets and liabilities
The Group measures its financial assets and liabilities at initial recognition at fair value and subsequently at amortized cost using the effective interest method.
The Group has not irrevocably designated a financial asset or liability as measured at fair value through profit or loss to eliminate or significantly reduce a measurement or recognition inconsistency.
Financial assets or liabilities at fair value through profit or loss are measured at fair value through profit and loss due to the business model used in their negotiation and/or the contractual characteristics of their cash flows.
Estimates
The Group makes estimates of collectability of its recorded receivables. Management analyzes trade account receivables in accordance with conventional criteria, adjusting the amount through a charge of an allowance for bad debts upon recognition of the inability of third parties to afford their financial obligations to the Group. Management specifically analyzes the accounts receivable, the historical bad debts, solvency of customers, current economic trends and the changes to the payment conditions of customers to assess the adequate allowance for bad debts.
F-28

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Offsetting of financial assets with financial liabilities
Financial assets and liabilities are offset and presented for their net amount in the statements of financial position only when the Group has the right, legally enforceable, to compensate the recognized amounts and has the intention to liquidate for the net amount or to settle the asset and cancel the liability simultaneously.
4.13. Borrowings
The Group measures its borrowings at initial recognition at fair value and, subsequently, are measured at amortized cost using the effective interest rate method.
Borrowing costs, either generic or specific, attributable to the acquisition, construction or production of assets that necessarily take a substantial period of time to get ready for their intended use or sale (qualifying assets) are included in the cost of the assets until the moment that they are substantially ready for use or sale. Income earned on the temporary investments of funds generated in specific borrowings still pending use in the qualifying assets, are deducted from the total of financing costs potentially eligible for capitalization.
All other loan costs are recognized under financial costs, through profit and loss.
4.14. Convertible notes
The convertible notes were classified as compound instruments, a non-derivative financial instrument that contains both a liability and an equity component. The equity component was measured as the residual amount that results from deducting the fair value of the liability component from the initial carrying amount of the instrument. The fair value of the consideration of the liability component was measured first at the fair value of a similar liability (including any embedded non-equity derivative features, such as an issuer’s call option to redeem the bond early) that does not have any associated equity conversion option.
The Group considers that if the instrument meets the ‘fixed for fixed’ condition, as the strike price is pre-determined at inception and only varies over time, and it is therefore classified as equity. As regards to the mandatory conversion feature, as it is a contingent settlement provision, the Group decided to measure the liability component at initial recognition, based on its best estimate of the present value of the redemption amount and allocated the residual to the equity component.
4.15. Employee benefits
Employee benefits are expected to be settled wholly within 12 months after the end of the reporting period and are presented as current liabilities.
The accounting policies related to incentive payments based on shares are detailed in Note 4.21.
4.16. Provisions
The Group has recognized provisions for liabilities of uncertain timing or amount. The provision is measured at the best estimate of the expenditure required to settle the obligation at the end of the reporting period, discounted at a pre-tax rate reflecting current market assessments of the time value of money and risks specific to the liability.
F-29

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
4.17. Change in ownership interest in subsidiaries without change of control
Transactions with non-controlling interest that do not result in a loss of control are accounted for as equity transactions - ie., as transactions with the owners in their capacity as owners. The recorded value corresponds to the difference between the fair value of the consideration paid and/or received and the relevant share acquired and/or transferred of the carrying value of the net assets of the subsidiary.
4.18. Revenue recognition
Revenue is measured at fair value of consideration received or receivable.
Revenue from ordinary activities from contracts with customers is recognized and measured based on a five-step model, namely:
● Identification of the contract with the client. A contract is an agreement between two or more parties, which creates rights and obligations for the parties involved.
● Identification of performance obligations, issuing as such a commitment arising from the contract to transfer a good or service.
● Determination of the price of the transaction, in reference to the consideration for satisfying each performance obligation.
● Assignment of the transaction price between each of the performance obligations identified, based on the methods described in the standard.
● Revenue recognition when the performance obligations identified in contracts with customers are met, at any given time or over a period of time.
The Group’s commercial activities comprise the selling of manufactured products. These sales are measured at the fair value of the consideration received or receivable, net of returns and allowances, trade and other discounts, and sales taxes, as applicable.
Revenue is recognized when the full control has been transferred to the buyer, recovery of the consideration is probable, the associated costs and possible return of goods can be estimated reliably, and there is no continuing management involvement with the goods. Transfers of control vary depending on the individual terms of the contract of sale. Revenues are recognized when control of the products has transferred, being when the products are delivered to the customer, having this full discretion over the channel and price to sell the products, and there is no unfulfilled obligation that could affect the customer’s acceptance of the products.
When the outcome of a transaction involving the rendering of services can be estimated reliably, revenue associated with the transaction is recognized by reference to the stage of completion of the transaction at the end of the reporting period. The stage of completion for research and development services is generally determined on the basis of internal records of execution of the performed tasks of the respective work plan.
The recognition of revenue of usage-based royalties promised in exchange for a license of intellectual property is recognized at the later of when the performance obligation is satisfied and when the sales or usages occur.
Management estimates, and includes in the transaction price at contract inception, the amount of variable consideration to which it expects to be entitled. An entity includes some or all of an amount of variable consideration only to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
F-30

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
4.19. Government grants
Government grants are recognized where there is reasonable assurance that the grant will be received and all attached conditions will be complied with. When the grant relates to an expense item, it is recognized as income on a systematic basis over the periods that the related costs, for which it is intended to compensate, are expensed. When the grant relates to an asset, it is recognized as income in equal amounts over the expected useful life of the related asset. Management elected this accounting policy because the Group determined it better shows the financial effect of government grants in the Consolidated financial statements.
When the Group receives grants of non-monetary assets, the asset and the grant are recorded at nominal amounts and released to profit or loss over the expected useful life of the asset.
The difference between the money obtained under government loans at subsidized rates and the carrying amount of those loans is treated as a government grant, in accordance with IAS 20.
4.20. Current and deferred income tax
Deferred tax assets and liabilities are recognized where the carrying amount of an asset or liability in the Consolidated statement of financial position differs from its tax base, except for differences arising on:
| - | The initial recognition of goodwill; |
| - | The initial recognition of an asset or liability in a transaction which is not a business combination and at the time of the transaction affects neither accounting or taxable profit; and |
| - | Investments in subsidiaries and jointly controlled entities where the Group is able to control the timing of the reversal of the difference and it is probable that the difference will not reverse in the foreseeable future. |
Recognition of deferred tax assets is restricted to those instances where it is probable that taxable profit will be available against which the difference can be utilized.
The amount of the asset or liability is determined using tax rates that have been enacted or substantively enacted by the end of the reporting period and are expected to apply when the deferred tax liabilities / (assets) are settled / (recovered).
Deferred tax assets and liabilities are offset when the Group has a legally enforceable right to offset current tax assets and liabilities and the deferred tax assets and liabilities relate to taxes levied by the same tax authority on either:
| - | The same taxable entity within the Group, or |
| - | Different entities within the Group which intend either to settle current tax assets and liabilities on a net basis, or to realize the assets and settle the liabilities simultaneously, in each future period in which significant amounts of deferred tax assets or liabilities are expected to be settled or recovered. |
4.21. Share-based payments
Certain executives and directors of the Group were granted incentives in the form of shares and options to purchase Bioceres Crop Solutions shares as consideration for services.
The cost of these share-based transactions is determined based on their fair value at the date upon which such incentives are granted using a valuation model that is appropriate in the circumstances.
F-31

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
This cost is recognized as an expense together with an increase in equity throughout the period in which the service or performance conditions are satisfied (i.e., the vesting period). The accumulated expense recorded in connection with these transactions at the end of each year until the vesting date reflects the time elapsed between the vesting period and Management’s best estimate of the number of equity instruments that will vest. The charge to income/loss for the period represents the variation in the accumulated expense recorded between the beginning and the end of the year.
Non-market related service and performance conditions are not taken into account when determining the grant date fair value of the equity instruments, but the probability that the conditions are fulfilled is assessed as part of Management’s best estimate of the number of equity instruments that will vest. Market-related performance conditions are reflected in the grant date fair value. Any other conditions related to equity-settled share-based payment transactions but without a service requirement are considered as non-vesting conditions. Non-vesting conditions are reflected in the fair value of the equity instruments and are charged to income/loss immediately unless there are service and/or performance conditions as well.
No amount is recognized for transactions that will not vest because non-market related performance conditions and/or service conditions were not satisfied. When transactions include market-related conditions or non-vesting conditions, the transactions are considered to be vested, irrespective of whether a market-related condition or the non-vesting condition is satisfied, provided that all the other performance and/or service conditions are met.
When the terms and conditions of an equity-settled share-based payment transaction are modified, the minimum expense recognized is the grant date fair value, unmodified, provided that the original terms have been complied with. An additional expense, measured at the date of modification, is recognized for any modification that increases the total fair value of the share-based payment transaction, or is otherwise beneficial to the employee.
When the transaction is settled by the Bioceres Crop Solutions or by the counterparty, any remainder of the fair value is charged to income immediately.
The dilutive effect of current options is considered in the calculation of the diluted earnings per share.
Estimates
The estimate of the fair value of equity-settled share-based payment transactions requires a determination to be made of the most adequate option pricing model to apply depending on the terms and conditions of the arrangement. This estimate also requires a determination of those factors most appropriate to the pricing model, including the expected life of the option and the expected volatility of the share price upon the basis of which hypotheses are made. The Group measures the fair value of these transactions at the grant date applying the Black-Scholes formula adjusted to consider the possible dilutive effect of the future exercise of the share options granted on their estimated fair value at grant date, as established in paragraph B41 of IFRS 2.
5. CRITICAL ACCOUNTING JUDGMENTS AND ESTIMATES
The Group makes certain estimates and assumptions regarding the future. Estimates and judgments are continually evaluated based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. In the future, actual experience may differ from these estimates and assumptions. The estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are listed below.
Critical estimates
| - | Impairment testing of intangible assets not yet available for use (Note 4.7). |
| - | Impairment of goodwill (Notes 4.7). |
F-32

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
6. ACQUISITIONS AND OTHER SIGNIFICANT TRANSACTIONS
Pro Farm Group, Inc
On July 12, 2022, we announced the closing of the merger (the “Pro Farm Merger”) with Pro Farm Group, Inc. (formerly Marrone Bio Innovations Inc.), pursuant to the Agreement and Plan of Merger (the “Merger Agreement”) dated March 16, 2022, among us, BCS Merger Sub, Inc., a wholly owned subsidiary of Bioceres, and Pro Farm Group, Inc. Upon the closing of the Pro Farm Merger, Pro Farm Group, Inc. became a wholly owned subsidiary of Bioceres and each share of Pro Farm Group, Inc. common stock was exchanged for our ordinary shares at a fixed exchange ratio of
Pro Farm Group, Inc. leads the movement to environmentally sustainable farming practices through the discovery, development and sale of innovative biological products for crop protection, crop health and crop nutrition. The company’s commercial products are sold globally and supported by more than
The combined company has a diverse customer base, product portfolio and geographic reach across a wide range of crops, positioned to serve the massive market opportunity emerging from the bio-reduction and replacement of chemical ag inputs. The merger combines our expertise in bionutrition and seed care products with Pro Farm Group’s leadership in the development of biological crop protection and plant health solutions, creating a global leader in the development and commercialization of sustainable agricultural solutions.
The consideration of payment was measured at fair value, which was calculated as the sum of the acquisition-date fair values of the assets transferred, and the liabilities incurred.
Consideration of payment (amounts in thousands of dollars):
Shares issued |
| |
Assumed RSU & Stock options |
| |
Cash payment |
| |
Total consideration |
| |
F-33

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Assets acquired and liabilities assumed (amounts in thousands of dollars):
Net assets incorporated |
|
|
Cash and cash equivalents |
| |
Trade receivables |
| |
Other receivables |
| |
Inventories |
| |
Property, plant and equipment |
| |
Right of use assets, net |
| |
Intangible assets |
| |
Restricted cash |
| |
Other assets |
| |
Trade and other payables |
| ( |
Lease liabilities |
| ( |
Borrowings |
| ( |
Other liabilities |
| ( |
Revaluation of existing assets |
|
|
Property, plant and equipment |
| |
Intangible assets |
| |
Deferred tax |
| ( |
Total net assets identified |
| |
Goodwill |
| |
Total consideration |
| |
Goodwill is not expected to be deductible for tax purposes.
The amounts of revenue and net profit of the acquiree since the merger date included in the consolidated statement of comprehensive income for the year ended June 30, 2023, were $
The pro forma revenue and net profit of the combined entity for the year ended June 30, 2023 as though the date for the merger had been as of the beginning of the reporting period amount to $
Syngenta Seedcare Agreement
On September 12, 2022, we entered into a
F-34

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The exclusive commercial collaboration is applicable for all countries, except for Argentina where both parties will continue to work under the existing framework. It has an effective date at the beginning of the 2023 calendar year, but implementation will be staggered. Bioceres will continue distributing its products in South Africa, Bolivia, Chile, Colombia, Paraguay and Uruguay during the calendar year 2023. For those countries, implementation will be in calendar year 2024, subject to regulatory clearances.
In consideration for the rights granted under the agreements to use Bioceres intellectual property as it exists at closing date, Syngenta made an upfront payment of $
Concurrently with this Agreement, Rizobacter entered into a Supply Agreement with Syngenta, whereby it acts as the exclusive supplier of the products under the Agreement. The price of the products being sold under the Supply Agreement are set at fair market value. Syngenta establishes its own pricing policies and pays to Bioceres a royalty of an amount between
The Agreement sets global minimum targets for profits to be received by Bioceres that amount to a total of $
Additionally, the agreement establishes a joint R&D program to accelerate the development and registration of Bioceres’ pipeline products and new solutions for seed treatment, foliar and other applications, globally. Funding of thus R&D program will be shared, with Syngenta contributing up to
The Agreement sets out a “Clawback” clause, by which in case of certain breaches of the Agreement or events described therein, Bioceres shall pay a penalty to Syngenta for an amount up to $
Insumos Agroquímicos S.A. (“Insuagro”)
On April 9, 2021, as part of the reorganization process of the crop protection business segment, we acquired a controlling interest in Insuagro, an Argentine public company. The interest acquired is represented by a total of
F-35

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Acquiring control over Insuagro, we also acquired control over
The consideration for the acquisition was $
Fair value of the consideration of payment
Cash payment |
| |
Financed payment |
| |
Contingent payment |
| |
Total consideration |
| |
The consideration of payment was measured at fair value, which was calculated as the sum of the acquisition‑date fair values of the assets transferred, and the liabilities incurred. The fair values measured were based on discounting future cash flow using market discount rates. The difference between fair value and nominal value of consideration will be recognized as finance cost over the period the consideration will be paid.
Assets acquired, liabilities assumed, and non-controlling interest recognized.
Net assets incorporated |
|
|
Cash and cash equivalents |
| |
Other financial assets |
| |
Trade receivables |
| |
Other receivables |
| |
Income and minimum presumed income taxes recoverable |
| |
Inventory |
| |
Deferred tax assets |
| |
Property, plant and equipment |
| |
Intangible assets |
| |
Trade and other payables |
| ( |
Borrowings |
| ( |
Employee benefits and social security |
| ( |
Deferred revenues and advances from customers |
| ( |
Revaluation of existing assets |
|
|
Property, plant and equipment |
| |
Intangible assets |
| |
Deferred tax |
| ( |
Total net assets identified |
| |
Non-controlling interest |
| ( |
Goodwill |
| |
Total consideration |
| |
F-36

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Goodwill is not expected to be deductible for tax purposes.
Non-controlling interest was measured at the present ownership instruments’ proportionate share in the recognized amounts of the acquiree’s identifiable net assets.
The amounts of revenue and profit or loss of the acquiree since the acquisition date included in the consolidated statement of comprehensive income for year ended June 30, 2021, were $
As Insuagro is a public company listed in Bolsas y Mercados Argentinos S.A. (“BYMA”), and in accordance with the Capital Markets Law of Argentina, we did a mandatory public offer for the acquisition of the remaining Class B Shares for the minority shareholders who did not participate in the sale purchase agreement aforementioned. The total of shares subject to the offer and outstanding was
On August 2, 2021, the mandatory offer was completed, and we bought 2.467.990 shares. Consideration of payment was $
As of June 30, 2023, we owned
On August 31, 2022 and based on the adjusted EBITDA measured for the year ended June 30, 2022, the Fixed Price was increased up to $
Moolec Science Ltd
On March 16, 2021, we acquired a
On December 28, 2022, Bioceres has contributed all of its ownership in Moolec Science Limited to Moolec Science S.A. (“Moolec Science”) in exchange of
Verdeca and other intangibles assets
On November 12, 2020 we acquired from Arcadia the remaining ownership interest in Verdeca, a joint agreement formed by Bioceres and Arcadia in 2012 to develop second generation biotechnologies for soybean and to globally commercialize the HB4 Soy technology.
As part of the transaction, Bioceres has gained full access to and control of Verdeca´s vetted soybean library of gene-edited materials used to develop new quality and productivity traits for this crop, as well as exclusive rights to all Arcadia technologies that are applicable to soybean and in-licensing rights to Arcadia’s safflower and wheat traits and the related brands.
F-37

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The complementary portfolio of materials being licensed includes wheat varieties that produce flour with
In consideration for the acquisition of the above-mentioned rights and assets, Bioceres paid Arcadia at the closing of the transaction $
On April 22, 2022, Verdeca obtained the Chinese import clearance for HB4 and Bioceres has already paid the contingent payment mentioned before.
Following the transaction Bioceres agreed with Arcadia to make royalty payments equivalent to
7. INFORMATION ABOUT COMPONENTS OF CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
7.1. Cash and cash equivalents
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Cash at bank and on hand | | | | |||
Money market funds | — | | | |||
| |
| |
| |
7.2. Other financial assets
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current |
|
|
|
|
| |
Restricted short-term deposits |
| |
| | | |
US Treasury bills | | — | | |||
Mutual funds | | | — | |||
Other investments |
| |
| | | |
| |
| | | ||
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Non-current |
|
|
|
|
|
|
Shares of Bioceres S.A. |
| |
| |
| |
Other investments |
| |
| |
| |
| |
| |
| |
F-38

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
7.3. Trade receivables
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current | ||||||
Trade debtors | | | | |||
Allowance for impairment of trade debtors |
| ( |
| ( |
| ( |
Shareholders and other related parties (Note 17) |
| — |
| |
| — |
Allowance for credit notes to be issued |
| ( |
| ( |
| ( |
Trade debtors - Joint ventures and associates (Note 17) |
| |
| |
| |
Deferred checks |
| |
| |
| |
| |
| |
| | |
06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | ||
Non-current | ||||||
Trade debtors | — | | | |||
— | | |
The book value is reasonably approximate to the fair value given its short-term nature.
Variations in the allowance for uncollectible trade receivables are reported in Note 7.17.
7.4. Other receivables
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current |
|
|
|
|
|
|
Taxes |
| |
| |
| |
Receivables for PP&E sales |
| |
| |
| — |
Shareholders and other related parties (Note 17) |
| |
| |
| |
Other receivables - Parents companies and related parties to Parents (Note 17) |
| — |
| — |
| |
Other receivables - Joint ventures and associates (Note 17) |
| |
| |
| |
Prepayments to suppliers |
| |
| |
| |
Prepayments to suppliers - Shareholders and other related parties (Note 17) |
| — |
| — |
| |
Reimbursements over exports |
| |
| |
| |
Prepaid expenses and other receivables |
| |
| |
| |
Loans receivables |
| — |
| |
| |
Miscellaneous | | | | |||
| |
| |
| | |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Non-current |
|
|
|
|
|
|
Taxes |
| |
| |
| |
Reimbursements over exports |
| |
| |
| |
Loans receivables | | — | — | |||
Miscellaneous |
| |
| — |
| — |
| |
| |
| |
The book value of financial instruments in this note is reasonable.
F-39

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
7.5. Inventories
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Seeds |
| |
| |
| |
Resale products |
| |
| |
| |
Manufactured products | | | | |||
Goods in transit |
| |
| |
| |
Supplies |
| |
| |
| |
Agricultural products |
| |
| |
| |
Allowance for obsolescence | ( | ( | ( | |||
| |
| |
| | |
Net of agricultural products | | | |
The roll-forward of allowance for obsolescence is in Note 7.17. Inventories recognized as an expense during the years ended June 30, 2023, 2022 and 2021 amounted to $
7.6. Biological assets
Changes in Biological assets:
| Soybean |
| Corn |
| Wheat |
| Barley |
| Sunflower |
| Total | |
Beginning of the year |
| — |
| — |
| |
| |
| — |
| |
Initial recognition and changes in the fair value of biological assets at the point of harvest |
| |
| |
| |
| |
| |
| |
Costs incurred during the year |
| |
| |
| |
| |
| |
| |
Decrease due to harvest/disposals |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Year ended June 30, 2023 |
| — |
| — |
| |
| |
| — |
| |
| Soybean |
| Corn |
| Wheat |
| Barley |
| Sunflower |
| Total | |
Beginning of the year |
| |
| |
| |
| |
| |
| |
Initial recognition and changes in the fair value of biological assets at the point of harvest |
| |
| |
| |
| |
| |
| |
Costs incurred during the year |
| |
| |
| |
| |
| |
| |
Exchange differences |
| |
| |
| |
| |
| |
| |
Decrease due to harvest |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Year ended June 30, 2022 |
| — |
| — |
| |
| |
| — |
| |
F-40

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
| Soybean |
| Corn |
| Wheat |
| Barley |
| HB4 Soy |
| HB4 Wheat |
| Total | |
Beginning of the year |
| |
| |
| |
| |
| |
| |
| |
Initial recognition and changes in the fair value of biological assets at the point of harvest |
| |
| |
| |
| |
| |
| |
| |
Costs incurred during the year |
| |
| |
| |
| |
| |
| |
| |
Exchange differences |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Decrease due to harvest |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Year ended June 30, 2021 |
| |
| |
| |
| |
| — |
| |
| |
7.7. Property, plant and equipment
Property, plant and equipment as of June 30, 2023, 2022 and 2021, included the following:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Gross carrying amount |
| |
| |
| |
Accumulated depreciation |
| ( |
| ( |
| ( |
Net carrying amount |
| |
| |
| |
Net carrying amount for each class of assets is as follows:
Net carrying | Net carrying | Net carrying | ||||
amount | amount | amount | ||||
Class |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Office equipment | | | | |||
Vehicles | | | | |||
Equipment and computer software |
| |
| |
| |
Fixtures and fittings |
| |
| |
| |
Machinery and equipment |
| |
| |
| |
Land and buildings |
| |
| |
| |
Buildings in progress |
| |
| |
| |
Total |
| |
| |
| |
1.
Gross carrying amount | ||||||||||||||
Additions | ||||||||||||||
As of the | from | Foreign | ||||||||||||
beginning | business | currency | As of the end | |||||||||||
Class |
| of the year |
| Additions |
| combination |
| Disposals |
| Revaluation |
| translation |
| of the year |
Office equipment | | | — | ( | — | | | |||||||
Vehicles | | | — | ( | — | | | |||||||
Equipment and computer software | | | | ( | — | | | |||||||
Fixtures and fittings |
| |
| |
| |
| — |
| — |
| |
| |
Machinery and equipment |
| |
| |
| |
| ( |
| — |
| |
| |
Land and buildings |
| |
| |
| |
| — |
| ( |
| |
| |
Buildings in progress |
| |
| |
| |
| — |
| — |
| |
| |
Total |
| |
| |
| |
| ( |
| ( |
| |
| |
F-41

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
2.
Depreciation | ||||||||||||
Accumulated | ||||||||||||
as of the | Foreign | Accumulated | ||||||||||
beginning of | currency | as of the end | ||||||||||
Class |
| the year |
| Disposals |
| Of the year |
| translation |
| Revaluation |
| of the year |
Office equipment |
| |
| ( |
| |
| |
| — |
| |
Vehicles |
| |
| ( |
| |
| |
| — |
| |
Equipment and computer software |
| |
| ( |
| |
| |
| — |
| |
Fixtures and fittings |
| |
| — |
| |
| |
| — |
| |
Machinery and equipment |
| |
| ( |
| |
| |
| — |
| |
Land and buildings |
| |
| — |
| |
| |
| ( |
| |
Total |
| |
| ( |
| |
| |
| ( |
| |
3.
Gross carrying amount | ||||||||||||||
As of the | Foreign | |||||||||||||
beginning | currency | As of the end | ||||||||||||
Class |
| of the year |
| Additions |
| Transfers |
| Disposals |
| translation |
| Revaluation |
| of the year |
Office equipment | | | — | — | | — | | |||||||
Vehicles | | | | ( | | — | | |||||||
Equipment and computer software | | | — | ( | | — | | |||||||
Fixtures and fittings | |
| — | | ( |
| |
| — |
| | |||
Machinery and equipment |
| |
| |
| |
| ( |
| |
| — |
| |
Land and buildings |
| |
| — |
| |
| ( |
| |
| ( |
| |
Buildings in progress |
| |
| |
| ( |
| ( |
| |
| — |
| |
Total |
| |
| |
| — |
| ( |
| |
| ( |
| |
4.
Depreciation | ||||||||||||
Accumulated | ||||||||||||
as of the | Foreign | Accumulated | ||||||||||
beginning of |
| currency | as of the end | |||||||||
Class |
| the year |
| Disposals |
| Of the year |
| translation |
| Revaluation |
| of the year |
Office equipment | | — |
| |
| |
| — |
| | ||
Vehicles | | ( |
| |
| |
| — |
| | ||
Equipment and computer software | | ( |
| |
| |
| — |
| | ||
Fixtures and fittings | | — |
| |
| |
| — |
| | ||
Machinery and equipment |
| |
| ( |
| |
| |
| — |
| |
Land and buildings |
| |
| — |
| |
| |
| ( |
| |
Total |
| |
| ( |
| |
| |
| ( |
| |
F-42

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
5.
Gross carrying amount | ||||||||||||||||
Additions | ||||||||||||||||
As of the | from | Foreign | ||||||||||||||
beginning | business | currency | As of the | |||||||||||||
Class |
| of year |
| Additions |
| combination |
| Transfers |
| Disposals |
| translation |
| Revaluation |
| end of year |
Office equipment | | | | — | ( | | — | | ||||||||
Vehicles | | | | — | ( | | — | | ||||||||
Equipment and computer software | | | | — | — | | — | | ||||||||
Fixtures and fittings | | | — | | — | | — | | ||||||||
Machinery and equipment |
| |
| |
| — | — |
| ( |
| |
| — |
| | |
Land and buildings |
| |
| — |
| | |
| — |
| |
| ( |
| | |
Buildings in progress |
| |
| |
| — | ( |
| — |
| |
| — |
| | |
Total |
| |
| |
| | |
| ( |
| |
| ( |
| | |
6.
Depreciation | ||||||||||||
Accumulated | ||||||||||||
as of the | Foreign | Accumulated | ||||||||||
beginning of | Disposals/ | currency | as of the end | |||||||||
Class |
| year |
| Transfers |
| Of the year |
| translation |
| Revaluation |
| of year |
Office equipment | | ( | | | — | | ||||||
Vehicles | | ( | | | — | | ||||||
Equipment and computer software | | — | | | — | | ||||||
Fixtures and fittings |
| | — | | | — | | |||||
Machinery and equipment |
| |
| ( |
| |
| |
| — |
| |
Land and buildings |
| |
| — |
| |
| |
| ( |
| |
Total |
| |
| ( |
| |
| |
| ( |
| |
The depreciation charge is included in Notes 8.3 and 8.4.The Group has no commitments to purchase property, plant and equipment items.
A detail of restricted assets is provided in Note 20.
Revaluation of property, plant and equipment
The Group updates frequently their assessment of the fair value of its land and buildings taking into account the most recent independent valuations and market data. Valuations were performed as of June 30, 2023, 2022 and 2021. Management determined the property, plant and equipment’s value within a range of reasonable fair value estimates.
All resulting fair value estimates for properties are included in level 2 or 3 depending on the methodology used.
The following are the carrying amounts that would have been recognized if land and building were stated at cost.
Cost value | ||||||
Class of property |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Land and buildings |
| |
| |
| |
F-43

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
7.8. Intangible assets
Intangible assets as of June 30, 2023, 2022 and 2021 included the following:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Gross carrying amount |
| |
| |
| |
Accumulated amortization |
| ( |
| ( |
| ( |
Net carrying amount |
| |
| |
| |
Net carrying amount of each class of intangible assets is as follows:
Net carrying | Net carrying | Net carrying | ||||
amount | amount | amount | ||||
Class |
| 06/30/2023 (1) |
| 06/30/2022 |
| 06/30/2021 |
Seed and integrated products |
|
|
|
|
|
|
HB4 soy and breeding program |
| |
| |
| |
Integrated seed products |
| |
| |
| |
Crop nutrition |
|
|
|
| ||
Microbiological products |
| |
| |
| |
Other intangible assets |
|
|
|
| ||
Trademarks and patents |
| |
| |
| |
Software |
| |
| |
| |
Customer loyalty |
| |
| |
| |
RG/RS/OX Wheat | | | | |||
Total |
| |
| |
| |
| (1) | Includes $ |
1.
Gross carrying amount | ||||||||||
As of the | Additions from | Foreign | ||||||||
beginning of | business | currency | As of the end | |||||||
Class |
| the year |
| Additions |
| combination |
| translation |
| of the year |
Seed and integrated products |
|
|
|
|
| |||||
HB4 soy and breeding program | | | — | | ||||||
Integrated seed products | | — | — | | | |||||
Crop nutrition |
|
|
|
|
|
|
|
|
|
|
Microbiological products |
| |
| |
| |
| |
| |
Other intangible assets |
|
|
|
|
|
|
|
|
|
|
Trademarks and patents |
| |
| |
| |
| — |
| |
Software |
| |
| |
| — |
| |
| |
Customer loyalty |
| |
| — |
| |
| — |
| |
RG/RS/OX Wheat |
| |
| — |
| — |
| — |
| |
Total |
| |
| |
| |
| |
| |
F-44

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
2.
Amortization | ||||||||
Accumulated | ||||||||
as of | Accumulated | |||||||
beginning of | Foreign currency | as of the end | ||||||
Class |
| the year |
| Of the year |
| translation |
| of the year |
Seed and integrated products | ||||||||
HB4 soy and breeding program | | | — | | ||||
Integrated seed products | | | | | ||||
Crop nutrition |
|
|
|
| ||||
Microbiological products | | | — | | ||||
Other intangible assets |
|
|
|
| ||||
Trademarks and patents |
| |
| |
| — |
| |
Software |
| |
| |
| |
| |
Customer loyalty |
| |
| |
| — |
| |
Total |
| |
| |
| |
| |
3.
Gross carrying amount | ||||||||
As of the | Foreign | |||||||
beginning of | currency | As of the end | ||||||
Class |
| the year |
| Additions |
| translation |
| of the year |
Seed and integrated products |
|
|
|
| ||||
HB4 soy and breeding program | | | — | | ||||
Integrated seed products | | — | | | ||||
Crop nutrition |
|
|
|
|
|
|
| |
Microbiological products |
| |
| |
| |
| |
Other intangible assets |
|
|
|
|
|
|
| |
Trademarks and patents |
| |
| — |
| |
| |
Software |
| |
| |
| |
| |
Customer loyalty |
| |
| — |
| |
| |
RG/RS/OX Wheat |
| |
| — |
| — |
| |
Total |
| |
| |
| |
| |
F-45

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
4.
Amortization | ||||||||
Accumulated | ||||||||
as of | Accumulated | |||||||
beginning of | Foreign currency | as of the end | ||||||
Class |
| the year |
| Of the year |
| translation |
| of the year |
Seed and integrated products | ||||||||
HB4 soy and breeding program | — | | — | | ||||
Integrated seed products | — | | — | | ||||
Crop nutrition |
|
|
|
| ||||
Microbiological products | | | | | ||||
Other intangible assets |
|
|
|
| ||||
Trademarks and patents |
| |
| |
| |
| |
Software |
| |
| |
| |
| |
Customer loyalty |
| |
| |
| |
| |
Total |
| |
| |
| |
| |
5.
Gross carrying amount | ||||||||||||
Additions | ||||||||||||
As of the | from | Foreign | ||||||||||
beginning of | business | Transfers / | currency | As of the | ||||||||
Class |
| year |
| Additions |
| combination |
| Disposals |
| translation |
| end of year |
Seed and integrated products |
|
|
|
| ||||||||
HB4 soy and breeding program (1) | | | — | ( | — | | ||||||
Integrated seed products | | — | — | — | | | ||||||
Crop nutrition |
|
|
|
|
|
| ||||||
Microbiological products |
| |
| |
| — | ( |
| |
| | |
Other intangible assets |
|
|
|
|
|
|
|
|
|
|
| |
Trademarks and patents |
| |
| |
| | — |
| |
| | |
Software |
| |
| |
| — | ( |
| |
| | |
Customer loyalty | | — | | — | | | ||||||
GLA/ARA safflower | — | | — | ( | — | — | ||||||
RG/RS/OX Wheat |
| — |
| |
| — | — |
| — |
| | |
Total |
| |
| |
| | ( |
| |
| | |
(1) | Of the total additions, $ |
F-46

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
6.
Amortization | ||||||||
Accumulated | ||||||||
as of | Foreign | Accumulated | ||||||
beginning of | currency | as of the end of | ||||||
Class |
| the year |
| Of the year |
| translation |
| the year |
Crop nutrition |
|
|
|
|
| |||
Microbiological products |
| | | | | |||
Other intangible assets |
|
|
|
|
| |||
Trademarks and patents |
| | | | | |||
Software |
| |
| |
| |
| |
Customer loyalty |
| |
| |
| |
| |
Total |
| |
| |
| |
| |
The amortization charge is included in Notes 8.3 and 8.4.
There are no intangibles assets whose use has been restricted or which have been delivered as a guarantee. The Group has not assumed any commitments to acquire new intangibles.
Estimates
There is an inherent material uncertainty related to management’s estimation of the ability of the Group to recover the carrying amounts of internally generated intangible assets related to biotechnology projects because it is dependent upon Group`s ability to raise sufficient funds to complete the projects development, the future outcome of the regulatory process, and the timing and amount of the future cash flows generated by the projects, among other future events.
Management’s estimations about the demonstrability of the recognition criteria for these assets and the subsequent recoverability represent the best estimate that can be made based on all the available evidence, existing facts and circumstances and using reasonable and supportable assumptions in cash flow projections. Therefore, the Consolidated financial statements do not include any adjustments that would result if the Group were unable to recover the carrying amount of the above-mentioned assets through the generation of enough future economic benefits.
7.9. Goodwill
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Rizobacter Argentina S.A. |
| |
| |
| |
Bioceres Crops S.A. |
| |
| |
| |
Pro farm Group, Inc. (Note 6) | | — | — | |||
Insumos Agroquímicos S.A. | | | | |||
| |
| |
| |
The Group is required to test whether goodwill has suffered any impairment on an annual basis. The recoverable amount is determined based on value in use calculations. The use of this method requires the estimation of future cash flows and the determination of a discount rate in order to calculate the present value of the cash flows.
Rizobacter CGU. This CGU is composed of all revenues collected through Rizobacter from the production and sale of proprietary and third-party products, both in the domestic and international markets. Additionally, Rizobacter generates revenue from the formulation, fragmentation and resale of third-party products.
F-47

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Among the main groups of products are i) microbiological products (bio-inductors/inoculants, biological fertilizers and bio-controllers); ii) crop and seed protection (treatments, adjuvants, baits, stored grains and seed treatment); and iii) crop nutrition (fertilizers). Packs are generally a combination of a microbiological product (bio-inductors/inoculants) with a crop and seed protection product (treatments).
Bioceres Crops CGU. This CGU is composed of the expected revenues from the commercialization of intensive R&D products that previously were allocated on the equity participation.
Insuagro CGU. This CGU is composed of all revenues collected through Insuagro from the production and sale of proprietary and third-party products, both in the domestic markets.
Pro Farm Group Inc CGU. This CGU is composed of all revenues collected through Pro Farm from the production and sale of proprietary and third-party products, both in the domestic and international markets.
Management has made the estimates considering the cash flow projections projected by the management and third-party valuation reports on the assets, intangible assets and liabilities assumed. The key assumptions utilized are the following:
Key assumption | Management’s approach |
|---|---|
Discount rate | The discount rate used ranges was The weighted average cost of capital (“WACC”) rate has been estimated based on the market capital structure. For the cost of debt, the indebtedness cost of the CGUs was used. For the cost of equity, the discount rate is estimated based on the Capital Asset Pricing Model (CAPM). The value assigned is consistent with external sources of information. |
Budgeted market share of joint ventures and other customers | The projected revenue from the products and services of the CGUs has been estimated by the management based on market penetration data for comparable products and technologies and on future expectations of foreseen economic and market conditions. The value assigned is consistent with external sources of information. |
Budgeted product prices | The prices estimated in the revenue projections are based on current and projected market prices for the products and services of the CGUs. The value assigned is consistent with external sources of information. |
Growth rate used to extrapolate future cash flow projections to terminal period | The growth rate used to extrapolate the future cash flow projections to terminal period is The value assigned is consistent with external sources of information. |
Management believes that any reasonably possible change in any of these key assumptions would not cause the aggregate carrying amount of the CGU to exceed its recoverable amount.
F-48

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
7.10. Investment properties
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Investment properties |
| |
| — |
| — |
| |
| — |
| — |
On May 3, 2023, the Group acquired a property from a client as a collection of its commercial debt. To date, we have not determined its future use and therefore it was classified as investment property.
The book value of the investment property does not differ significantly from its fair value.
7.11. Trade and other payables
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current | ||||||
Trade creditors |
| |
| |
| |
Shareholders and other related parties (Note 17) |
| |
| |
| |
Trade creditors - Parent company (Note 17) |
| |
| |
| |
Trade creditors - Joint ventures and associates (Note 17) |
| |
| |
| |
Taxes |
| |
| |
| |
Miscellaneous |
| |
| |
| |
| |
| |
| |
The trade and other payables include debts with grain producers. These debts represent payment obligations contracted by purchase contracts, which give the producer the right to set the price at any time between the delivery date and a future date. Those debts that are not fixed at closing are valued at their fair value and debts with a price set by the producer at their amortized cost.
The book value of financial instruments in this note is reasonable.
7.12. Borrowings
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current | ||||||
Bank overdrafts |
| — |
| — |
| |
Bank borrowings |
| |
| |
| |
Corporate bonds |
| |
| |
| |
Trust debt securities |
| |
| |
| |
Net loans payables- Parents companies and related parties to Parent (Note 17) |
| |
| |
| |
Subordinated loan | — | — | | |||
|
| |
| |
| |
Non-current |
|
|
|
|
| |
Bank borrowings |
| |
| |
| |
Corporate bonds |
| |
| |
| |
Net loans payables- Parent companies and related parties to Parent (Note 17) |
| — |
| |
| |
| |
| |
| |
F-49

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The carrying value of some borrowings as of June 30, 2023 are measured at amortized cost differ from their fair value. The following fair values measured are based on discounted cash flows (Level 3) due to the use of unobservable inputs, including own credit risk.
06/30/2023 | 06/30/2022 | 06/30/2021 | ||||||||||
Amortized | Amortized | Amortized | ||||||||||
| cost |
| Fair value |
| cost |
| Fair value |
| cost |
| Fair value | |
Current | ||||||||||||
Bank borrowings |
| |
| |
| |
| |
| |
| |
Corporate Bonds |
| |
| |
| |
| |
| |
| |
|
|
|
| |||||||||
Non-current |
|
|
|
|
|
|
|
|
|
|
|
|
Bank borrowings |
| |
| |
| |
| |
| |
| |
Corporate Bonds |
| |
| |
| |
| |
| |
| |
7.13. Secured Notes
Secured Guaranteed Notes
On August 5, 2022, the
The Secured Guaranteed Notes due 2026 mature
The carrying value the Secured Guaranteed Notes as of June 30, 2023 are measured at amortized cost. Its fair value based on discounted cash flows, using a fair interest rate, would amount to $
Secured Convertible Guaranteed Notes
On August 8, 2022, we issued the Secured Guaranteed Convertible Notes for a total principal amount of $
At inception, the fair value of the liability component of the Secured Convertible Guaranteed Notes was measured using a discount rate of
The carrying value the Secured Convertible Guaranteed Notes as of June 30, 2023 are measured at amortized cost. Its fair value based on discounted cash flows, using a fair interest rate, would amount to $
Under the terms of the Secured Convertible Guaranteed Notes, the Group is in compliance with covenants.
F-50

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
7.14. Employee benefits and social security
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current | ||||||
Salaries, accrued incentives, vacations and social security |
| |
| |
| |
Key management personnel (Note 17) |
| |
| |
| |
| |
| |
| |
7.15. Deferred revenue and advances from customers
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current | ||||||
Advances from customers |
| |
| |
| |
Deferred revenue (Note 6) | | — | — | |||
| | | ||||
Non-current | ||||||
Advances from customers | | — | — | |||
Deferred revenue (Note 6) | | — | — | |||
| |
| — |
| — |
7.16. Provisions
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Provisions for contingencies |
| |
| |
| |
| |
| |
| |
The Group has recorded a provision for probable administrative, judicial and out-of-court proceedings that could arise in the ordinary course of business, based on a prudent criterion according to its professional advisors and on Management’s assessment of the best estimate of the amount of possible claims. These potential claims are not likely to have a material impact on the results of the Group’s operations, its cash flow or financial position.
Management considers that the objective evidence is not enough to determine the date of the eventual cash outflow due to a lack of experience in any similar cases. However, the provision was classified under current or non-current liabilities, applying the best prudent criterion based on Management’s estimates.
There are no expected reimbursements related to the provisions.
The roll forward of the provision is in Note 7.17.
In order to assess the need for provisions and disclosures in its consolidated financial statements, Management considers the following factors: (i) nature of the claim and potential level of damages in the jurisdiction in which the claim has been brought; (ii) the progress of the eventual case; (iii) the opinions or views of tax and legal advisers; (iv) experience in similar cases; and (v) any decision of the Group`s management as to how it will respond to the eventual claim.
F-51

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
7.17. Changes in allowances and provisions
Additions | Currency | |||||||||||
from business | Uses and | conversion | ||||||||||
Item |
| 06/30/2022 |
| Additions |
| combination |
| reversals |
| difference |
| 06/30/2023 |
DEDUCTED FROM ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Allowance for impairment of trade debtors |
| ( |
| ( |
| — |
| |
| ( |
| ( |
Allowance for obsolescence |
| ( |
| ( |
| ( |
| |
| ( |
| ( |
|
|
|
|
|
|
|
|
|
|
|
| |
Total deducted from assets |
| ( |
| ( |
| ( |
| |
| ( |
| ( |
|
|
|
|
|
|
|
|
|
|
|
| |
INCLUDED IN LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Provisions for contingencies |
| ( |
| ( |
| ( |
| — |
| |
| ( |
|
|
|
|
|
|
|
|
|
| |||
Total included in liabilities |
| ( |
| ( |
| ( |
| — |
| |
| ( |
|
|
|
|
|
|
|
|
|
|
| ||
Total |
| ( |
| ( |
| ( |
| |
| ( |
| ( |
Currency | ||||||||||
Uses and | conversion | |||||||||
Item |
| 06/30/2021 |
| Additions |
| reversals |
| difference |
| 06/30/2022 |
DEDUCTED FROM ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Allowance for impairment of trade debtors |
| ( |
| ( |
| — |
| |
| ( |
Allowance for obsolescence |
| ( |
| ( |
| |
| |
| ( |
|
|
|
|
|
|
|
|
|
| |
Total deducted from assets |
| ( |
| ( |
| |
| |
| ( |
|
|
|
|
|
|
|
|
|
| |
INCLUDED IN LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Provisions for contingencies |
| ( |
| ( |
| — |
| |
| ( |
|
|
|
|
|
|
|
| |||
Total included in liabilities |
| ( |
| ( |
| — |
| |
| ( |
|
|
|
|
|
|
|
|
|
| |
Total |
| ( |
| ( |
| |
| |
| ( |
F-52

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Additions | Currency |
| ||||||||||
from business | Uses and | conversion | ||||||||||
Item |
| 06/30/2020 |
| Additions |
| combination |
| reversals |
| difference |
| 06/30/2021 |
DEDUCTED FROM ASSETS |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
| |
Allowance for impairment of trade debtors |
| ( |
| ( |
| ( |
| |
| ( |
| ( |
Allowance for impairment of related parties |
| ( |
| — |
| — |
| |
| |
| — |
Allowance for obsolescence |
| ( |
| ( |
| ( |
| |
| |
| ( |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deducted from assets |
| ( |
| ( |
| ( |
| |
| ( |
| ( |
|
|
|
|
|
|
|
|
|
|
|
|
|
INCLUDED IN LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provisions for contingencies |
| ( |
| ( |
| — |
| |
| |
| ( |
|
|
|
|
|
|
|
|
|
|
|
| |
Total included in liabilities |
| ( |
| ( |
| — |
| |
| |
| ( |
|
|
|
|
|
|
|
|
|
|
|
| |
Total |
| ( |
| ( |
| ( |
| |
| ( |
| ( |
8. INFORMATION ABOUT COMPONENTS OF CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
8.1. Revenue from contracts with customers
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Sale of goods and services |
| |
| |
| |
Royalties |
| |
| |
| |
Right of use licenses | | — | — | |||
| |
| |
| |
Transactions of sales of goods and services with joint ventures and with shareholders and other related parties are reported in Note 17.
8.2. Cost of sales
Item |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Inventories as of the beginning of the year |
| |
| |
| |
Business combination | | — | | |||
Purchases of the year |
| |
| |
| |
Production costs |
| |
| |
| |
Foreign currency translation |
| |
| |
| ( |
Subtotal |
| |
| |
| |
Inventories as of the end of the year (*) |
| ( |
| ( |
| ( |
Cost of sales |
| |
| |
| |
(*) Net of agricultural products.
F-53

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
8.3. R&D classified by nature
| Research |
| Research |
| Research | |
and | and | and | ||||
| development |
| development |
| development | |
| expenses |
| expenses |
| expenses | |
Item |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Amortization of intangible assets |
| |
| |
| |
Analysis and storage | | — | — | |||
Commissions and royalties |
| |
| |
| — |
Import and export expenses |
| |
| — |
| |
Depreciation of property, plant and equipment | | | | |||
Freight and haulage |
| |
| — |
| |
Employee benefits and social securities |
| |
| |
| |
Maintenance |
| |
| |
| |
Energy and fuel |
| |
| |
| |
Supplies and materials |
| |
| |
| |
Mobility and travel |
| |
| |
| |
Impairment of R&D projects | — | — | | |||
Share-based incentives |
| |
| |
| — |
Professional fees and outsourced services | | | | |||
Professional fees related parties |
| |
| |
| |
Office supplies | | | | |||
Information technology expenses | | | | |||
Insurance |
| |
| |
| |
Depreciation of leased assets | | | | |||
Miscellaneous |
| |
| |
| |
Total |
| |
| |
| |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
R&D capitalized (Note 7.8) |
| |
| |
| |
R&D profit and loss |
| |
| |
| |
Total |
| |
| |
| |
F-54

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
8.4. Expenses classified by nature and function
|
| Selling, general |
| |||
and | ||||||
administrative | Total | |||||
Item | Production costs | expenses | 06/30/2023 | |||
Amortization of intangible assets |
| |
| |
| |
Analysis and storage | | | | |||
Commissions and royalties |
| |
| |
| |
Import and export expenses |
| |
| |
| |
Depreciation of property, plant and equipment |
| |
| |
| |
Depreciation of leased assets |
| |
| |
| |
Impairment of receivables |
| — |
| |
| |
Freight and haulage |
| |
| |
| |
Employee benefits and social securities |
| |
| |
| |
Maintenance |
| |
| |
| |
Energy and fuel |
| |
| |
| |
Supplies and materials |
| |
| |
| |
Mobility and travel |
| |
| |
| |
Publicity and advertising |
| |
| |
| |
Contingencies |
| — |
| |
| |
Share-based incentives |
| — |
| |
| |
Professional fees and outsourced services |
| |
| |
| |
Professional fees related parties |
| — |
| |
| |
Office supplies and registrations fees |
| |
| |
| |
Insurance |
| |
| |
| |
Information technology expenses |
| |
| |
| |
Obsolescence |
| |
| |
| |
Taxes |
| |
| |
| |
Miscellaneous |
| |
| |
| |
Total |
| |
| |
| |
F-55

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
|
| Selling, |
| |||
|
| general and |
| |||
Production |
| administrative | Total | |||
Item | costs |
| expenses | 06/30/2022 | ||
Amortization of intangible assets |
| |
| |
| |
Commissions and royalties |
| |
| |
| |
Import and export expenses |
| |
| |
| |
Depreciation of property, plant and equipment |
| |
| |
| |
Depreciation of leased assets |
| |
| |
| |
Impairment of receivables |
| — |
| |
| |
Freight and haulage |
| |
| |
| |
Employee benefits and social securities |
| |
| |
| |
Maintenance |
| |
| |
| |
Energy and fuel |
| |
| |
| |
Supplies and materials |
| |
| |
| |
Mobility and travel |
| |
| |
| |
Publicity and advertising |
| — |
| |
| |
Contingencies |
| — |
| |
| |
Share-based incentives |
| — |
| |
| |
Professional fees and outsourced services |
| |
| |
| |
Professional fees related parties |
| — |
| |
| |
Office supplies and registrations fees |
| |
| |
| |
Insurance |
| |
| |
| |
Information technology expenses |
| |
| |
| |
Obsolescence |
| |
| — |
| |
Taxes |
| |
| |
| |
Miscellaneous |
| |
| |
| |
Total |
| |
| |
| |
F-56

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
|
| Selling, |
| |||
|
| general and |
| |||
Production |
| administrative | Total | |||
Item | costs |
| expenses | 06/30/2021 | ||
Amortization of intangible assets |
| — |
| |
| |
Analysis and storage |
| |
| |
| |
Commissions and royalties |
| |
| |
| |
Import and export expenses |
| |
| |
| |
Depreciation of property, plant and equipment |
| |
| |
| |
Depreciation of leased assets |
| |
| |
| |
Impairment of receivables |
| — |
| |
| |
Freight and haulage |
| |
| |
| |
Employee benefits and social securities |
| |
| |
| |
Maintenance |
| |
| |
| |
Energy and fuel | |
| |
| | |
Supplies and materials | |
| |
| | |
Mobility and travel |
| |
| |
| |
Publicity and advertising |
| — |
| |
| |
Contingencies |
| — |
| |
| |
Share-based incentives |
| — |
| |
| |
Professional fees and outsourced services |
| |
| |
| |
Professional fees related parties |
| — |
| |
| |
Office supplies and registrations fees |
| |
| |
| |
Insurance |
| |
| |
| |
Information technology expenses | |
| |
| | |
Obsolescence |
| |
| — |
| |
Taxes |
| |
| |
| |
Miscellaneous |
| |
| |
| |
Total |
| |
| |
| |
8.5. Other income or expenses, net
| 06/30/2023 | 06/30/2022 | 06/30/2021 | |||
Net result from commercialization of agricultural products |
| |
| ( |
| ( |
Reimbursements for exports |
| — |
| |
| |
Expenses recovery |
| |
| |
| |
Other income or expenses, net |
| |
| |
| |
| |
| ( |
| ( |
F-57

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
8.6. Finance results
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Financial costs |
| |||||
Interest expenses with the Parents (Note 17) |
| ( | ( | ( | ||
Interest expenses |
| ( | ( | ( | ||
Financial commissions |
| ( | ( | ( | ||
| ( | ( | ( | |||
Other financial results | ||||||
Exchange differences generated by assets |
| ( | | | ||
Exchange differences generated by liabilities |
| | ( | ( | ||
Changes in fair value of financial assets or liabilities and other financial results |
| ( | | ( | ||
Net gain of inflation effect on monetary items | | | | |||
( | ( | ( |
9. TAXATION
The balances of income tax and minimum presumed income tax recoverable and payable are as follows:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current assets |
|
|
|
|
|
|
Income tax |
| |
| |
| |
| |
| |
| | |
Non-current assets |
|
|
|
|
|
|
Income tax |
| |
| |
| |
Minimum presumed income tax |
| |
| |
| — |
| |
| |
| |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Liabilities |
|
|
|
|
|
|
Income tax |
| |
| |
| |
| |
| |
| |
The roll forward of net deferred tax as of June 30, 2023, 2022 and 2021 is as follows:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Beginning of the year deferred tax | ( | ( | ( | |||
Additions for business combination | ( | — | ( | |||
Charge for the year | | | ( | |||
Charge to OCI | | | ( | |||
Conversion difference | ( | ( | ( | |||
Total net deferred tax | ( | ( | ( |
F-58

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The roll forward of deferred tax assets and liabilities as of June 30, 2023, 2022 and 2021 are as follows:
Additions | Income | |||||||||||
Balance | for business | tax | Charge | Conversion | Balance | |||||||
Deferred tax assets |
| 06/30/2022 |
| combination |
| provision |
| to OCI |
| difference |
| 30/06/2023 |
Tax Loss-Carry Forward |
| |
| |
| |
| — |
| ( |
| |
Changes in fair value of financial assets or liabilities |
| |
| — |
| ( |
| — |
| ( |
| |
Trade receivables |
| |
| — |
| |
| — |
| |
| |
Allowances |
| |
| — |
| |
| — |
| ( |
| |
Royalties |
| |
| — |
| |
| — |
| ( |
| |
Others |
| |
| — |
| |
| — |
| ( |
| |
Total deferred tax assets |
| |
| |
| |
| — |
| ( |
| |
Additions | Income | |||||||||||
Balance | for business | tax | Charge | Conversion | Balance | |||||||
Deferred tax liabilities |
| 06/30/2022 |
| combination |
| provision |
| to OCI |
| difference |
| 30/06/2023 |
Intangible assets |
| ( |
| ( |
| |
| — |
| |
| ( |
Property, plant and equipment depreciation |
| ( |
| ( |
| |
| |
| ( |
| ( |
Inflation tax adjustment |
| ( |
| — |
| |
| — |
| |
| ( |
Inventories |
| ( |
| — |
| ( |
| — |
| — |
| ( |
Government grants |
| ( |
| — |
| |
| — |
| — |
| — |
Others financial assets |
| ( |
| — |
| ( |
| — |
| — |
| ( |
Right-of-use leased asset |
| ( |
| — |
| ( |
| — |
| — |
| ( |
Others |
| ( |
| — |
| |
| — |
| — |
| ( |
Total deferred tax liabilities |
| ( |
| ( |
| ( |
| |
| |
| ( |
|
| |||||||||||
Net deferred tax |
| ( |
| ( |
| |
| |
| ( |
| ( |
Transfer | ||||||||||||
from | ||||||||||||
Income | deferred | |||||||||||
Balance | tax | tax | Charge | Conversion | Balance | |||||||
Deferred tax assets |
| 06/30/2021 |
| provision |
| liabilities |
| to OCI |
| difference |
| 06/30/2022 |
Tax Loss-Carry Forward |
| |
| ( |
| — |
| — |
| |
| |
Changes in fair value of financial assets or liabilities |
| |
| |
| — |
| — |
| |
| |
Trade receivables |
| |
| ( |
| — |
| — |
| |
| |
Allowances |
| — |
| — |
| |
| — |
| — |
| |
Royalties |
| |
| ( |
| — |
| — |
| |
| |
Others |
| |
| |
| — |
| — |
| |
| |
Total deferred tax assets |
| |
| ( |
| |
| — |
| |
| |
F-59

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Transfer | ||||||||||||
|
| from |
|
|
| |||||||
| Income |
| deferred |
| ||||||||
Balance |
| tax | tax |
| Charge | Conversion | Balance | |||||
| 06/30/2021 |
| provision |
| assets |
| to OCI |
| difference |
| 06/30/2022 | |
Intangible assets |
| ( |
| ( |
| — |
| — |
| ( |
| ( |
Property, plant and equipment |
| ( |
| ( |
| — |
| |
| ( |
| ( |
Inflation tax adjustment |
| ( |
| |
| — |
| — |
| ( |
| ( |
Allowances |
| ( |
| |
| ( |
| — |
| |
| — |
Inventories |
| ( |
| |
| — |
| — |
| ( |
| ( |
Biological assets |
| ( |
| |
| — |
| — |
| ( |
| — |
Government grants |
| ( |
| |
| — |
| — |
| ( |
| ( |
Others financial assets |
| ( |
| ( |
| — |
| — |
| ( |
| ( |
Right-of-use leased asset |
| ( |
| ( |
| — |
| — |
| ( |
| ( |
Others |
| ( |
| ( |
| — |
| — |
| — |
| ( |
Total deferred tax liabilities |
| ( |
| |
| ( |
| |
| ( |
| ( |
|
| |||||||||||
Net deferred tax |
| ( |
| |
| — |
| |
| ( |
| ( |
Transfer | ||||||||||||||
from | ||||||||||||||
Additions | Income | deferred | ||||||||||||
Balance | for business | tax | tax | Charge | Conversion | Balance | ||||||||
Deferred tax assets |
| 06/30/2020 |
| combination |
| provision |
| liabilities |
| to OCI |
| difference |
| 06/30/2021 |
Tax Loss-Carry Forward |
| |
| — |
| |
| — |
| — |
| ( |
| |
Changes in fair value of financial assets or liabilities |
| |
| — |
| |
| — |
| — |
| ( |
| |
Trade receivables |
| |
| — |
| |
| — |
| — |
| ( |
| |
Royalties | | — | | — | — | | | |||||||
Right-of-use leased asset | | — | ( | | — | | — | |||||||
Others |
| |
| |
| ( |
| — |
| — |
| |
| |
Total deferred tax assets |
| |
| |
| |
| |
| — |
| |
| |
Transfer | ||||||||||||||
|
|
| from |
|
|
| ||||||||
| Additions |
| Income |
| deferred |
| ||||||||
Balance |
| for business | tax | tax |
| Charge | Conversion | Balance | ||||||
Deferred tax liabilities |
| 06/30/2020 |
| combination |
| provision |
| assets |
| to OCI |
| difference |
| 06/30/2021 |
Intangible assets |
| ( |
| ( |
| ( |
| — |
| — |
| ( |
| ( |
Property, plant and equipment |
| ( |
| ( |
| ( |
| — |
| ( |
| ( |
| ( |
Borrowings |
| ( |
| — |
| |
| — |
| — |
| ( |
| — |
Inflation tax adjustment |
| ( |
| |
| ( |
| — |
| — |
| ( |
| ( |
Allowances | ( | | ( | — | — | ( | ( | |||||||
Inventories | ( | ( | ( | — | — | ( | ( | |||||||
Biological assets | — | — | ( | — | — | — | ( | |||||||
Government grants | ( | — | | — | — | ( | ( | |||||||
Others financial assets | — | — | ( | — | — | | ( | |||||||
Right-of-use leased asset | — | — | — | ( | — | — | ( | |||||||
Others |
| ( |
| — |
| |
| — |
| — |
| ( |
| ( |
Total deferred tax liabilities |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
| ||||||||||||||
Net deferred tax |
| ( |
| ( |
| ( |
| — |
| ( |
| ( |
| ( |
F-60

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The following table provides a reconciliation of the statutory tax rate to the effective tax rate. As the operations of the Group’s Argentine subsidiaries are the most significant source of profit or loss before tax, the following reconciliation has been prepared using the Argentine statutory tax rate:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Earnings before income tax-rate | | | | |||
Income tax benefit (expense) by applying tax rate in force in the respective countries |
| |
| ( | ( | |
Share of profit or loss of subsidiaries, joint ventures and associates |
| |
| | | |
Stock options charge |
| ( |
| ( | ( | |
Rate change adjustment |
| — |
| — | ( | |
Non-deductible expenses |
| ( |
| ( | ( | |
Non-taxable gain | — | — | | |||
Foreign investment coverage |
| — | | | ||
Tax inflation adjustment | | | ( | |||
Result of inflation effect on monetary items and other finance results | ( | ( | ( | |||
Others | | ( | | |||
Income tax expenses |
| |
| ( | ( |
The Group did not recognize deferred income tax liabilities of $
Principal statutory taxes rates in the countries where the Group operates for all of the years presented are:
Income tax rate |
| ||||||
Tax jurisdiction |
| 2023 |
| 2022 |
| 2021 |
|
Argentina |
| % | % | | % | ||
Cayman Island |
| | % | | % | | % |
Paraguay | | % | | % | | % | |
Uruguay | | % | | % | | % | |
France | | % | | % | | % | |
Brazil | | % | | % | | % | |
United States of America |
| | % | | % | | % |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current tax expense |
| ( |
| ( |
| ( |
Deferred tax |
| | |
| ( | |
Total |
| |
| ( |
| ( |
F-61

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The charge for income tax charged directly to profit or loss and the amount and expiry date of carry forward tax losses as of June 30, 2023 are as follows:
Fiscal year |
| Tax-Loss Carry forward |
| Tax-Loss Carry forward |
| Prescription |
| Tax jurisdiction |
2018 |
| |
| |
| 2023 |
| Argentina |
2019 |
| |
| |
| 2024 |
| Argentina |
2020 |
| |
| |
| 2025 |
| Argentina |
2020 |
| |
| |
| 2040 |
| United States of America |
2021 | | | 2026 | Argentina | ||||
2021 |
| |
| |
| 2041 |
| United States of America |
2022 |
| |
| |
| 2027 |
| Argentina |
2022 |
| |
| |
| 2042 |
| United States of America |
2023 | | | 2028 | Argentina | ||||
2023 | | | 2043 | United States of America | ||||
2023 | | | 2028 | Brazil | ||||
Total |
| |
| |
|
|
The amount of tax losses for the fiscal year ended on June 30, 2023 is an estimate of the amount to be presented in the tax return.
The amount and expiry date of unused tax credits of Argentina minimum presumed income tax as of June 30, 2023 amounted to $
Estimates
There is an inherent material uncertainty related to management’s estimation of the ability of the Group to use the deferred tax assets (both carryforward of unused tax losses and deductible temporary differences) and the credit of minimum presumed income tax because their future utilization depends on the generation of enough future taxable income by the entities within the Group during the periods in which those temporary differences are deductible or when the unused tax losses can be used.
Based on the projections of future taxable income for the periods in which the deferred tax assets are deductible, the Group’s management estimates that, except for the part of deferred tax asset that were unrecognized, it is probable that the entities within the Group can utilize those deferred tax assets, which depends, among other factors, on the success of the current projects of agricultural biotechnology, the future market price of commodities and the market share of the entities within the Group.
The estimates of management about the demonstrability of the recognition criteria for these deferred tax assets and their subsequent recoverability represent the best estimate that can be made based on all the available evidence, existing facts and circumstances and the use of reasonable and supportable assumptions in the projections of future taxable income. Therefore, the Consolidated financial statements do not include adjustments that could result if the entities within the Group would not be able to recover the deferred tax assets through the generation of enough future taxable income.
F-62

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
10. EARNING PER SHARE
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Numerator |
|
|
|
|
|
|
Profit (Loss) for the year (basic EPS) |
| | ( | ( | ||
Profit (Loss) for the year (diluted EPS) |
| | ( | ( | ||
Denominator |
|
|
|
|
| |
Weighted average number of shares (basic EPS) |
| | | | ||
Weighted average number of shares (diluted EPS) |
| | | | ||
|
| |||||
Basic profit (loss) attributable to ordinary equity holders of the parent | | ( | ( | |||
Diluted profit (loss) attributable to ordinary equity holders of the parent |
| | ( | ( |
For the year ended June 30, 2023, diluted earnings per share was calculated by adjusting the weighted average number of shares outstanding to assume conversion of all dilutive potential shares. The Group had
The stock options were included in the diluted EPS calculation for the year ended June 30, 2023 only for the tranches in which the average market price of ordinary shares during the periods was higher than the assumed proceeds per option.
Convertible notes outstanding were not included in the diluted EPS calculations for the year ended June 30, 2023 because the interest (net of tax and other changes in income or expense) per ordinary share obtainable on conversion exceeds basic earnings per share.
11. INFORMATION ABOUT COMPONENTS OF EQUITY
Capital issued
As of June 30, 2023, we had, (i)
Holders of the ordinary shares are entitled to
Convertible notes
Convertibles notes were classified as compound instruments, a non-derivative financial instrument that contains both a liability and an equity component. The equity consideration was included in the “Convertible instruments” column. See Note 7.13.
Non-controlling interests
The subsidiaries whose non-controlling interest is significant as of June 30, 2023, 2022 and 2021 is:
Name |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Rizobacter Argentina S.A. | | % | | % | | % | |
Insumos Agroquímicos S.A. | | % | | % | | % |
Below is a detail of the summarized financial information of Rizobacter and Insuagro, prepared in accordance with IFRS, and modified due to fair value adjustments at the acquisition date and differences in accounting policies. The information is presented prior to eliminations between that subsidiary and other Group companies.
F-63

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Rizobacter
Summary financial statements:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current assets |
| |
| |
| |
Non-current assets |
| |
| |
| |
Total assets |
| |
| |
| |
|
|
| ||||
Current liabilities |
| |
| |
| |
Non-current liabilities |
| |
| |
| |
Total liabilities |
| |
| |
| |
|
| |||||
Equity attributable to controlling interest |
| |
| |
| |
Equity attributable to non-controlling interest |
| |
| |
| |
Total equity |
| |
| |
| |
|
| |||||
Total liabilities and equity |
| |
| |
| |
Summary statements of comprehensive income or loss
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Revenues |
| | | | ||
Initial recognition and changes in the fair value of biological assets at the point of harvest |
| ( | | | ||
Cost of sales | ( | ( | ( | |||
Gross margin |
| | | | ||
|
| |||||
Research and development expenses |
| ( | ( | ( | ||
Selling, general and administrative expenses |
| ( | ( | ( | ||
Share of profit or loss of joint ventures and associates |
| | | | ||
Other income |
| | | | ||
Operating profit |
| | | | ||
|
| |||||
Financial results |
| ( | ( | ( | ||
Profit before taxes |
| | | | ||
|
| |||||
Income tax expense |
| ( | ( | ( | ||
Result for the year |
| | | | ||
|
| |||||
Foreign exchange differences on translation of foreign operations |
| | | | ||
Revaluation of property, plant and equipment, net of tax |
| ( | ( | ( | ||
Total comprehensive result |
| | | |
There were no dividends paid to Rizobacter non-controlling interest (NCI) in the years ended June 30, 2023, 2022 and 2021.
F-64

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Insuagro
Summary financial statements:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Current assets |
| |
| |
| |
Non-current assets |
| |
| |
| |
Total assets |
| |
| |
| |
Current liabilities |
| |
| |
| |
Non-current liabilities |
| |
| |
| |
Total liabilities |
| |
| |
| |
Total equity |
| |
| |
| |
Total liabilities and equity |
| |
| |
| |
Summary statements of comprehensive income or loss
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Revenues | | | | |||
Cost of sales | ( | ( | ( | |||
Gross margin | | | | |||
|
|
| ||||
Selling, general and administrative expenses | ( | ( | ( | |||
Other income or expenses, net | | | | |||
Operating profit | | | | |||
|
|
| ||||
Financial results | ( | ( | ( | |||
Profit/(loss) before tax | | | ( | |||
Income tax | ( | ( | | |||
Profit/(loss) for the year | | | ( | |||
|
|
| ||||
Exchange differences on translation of foreign operations | — | | | |||
Revaluation of property, plant and equipment, net of tax | ( | — | — | |||
Total comprehensive result | | | |
F-65

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
12. CASH FLOW INFORMATION
Significant non-cash transactions related to investing and financing activities are as follows:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Investment activities | ||||||
Net assets acquisition by business combination (Note 6) | | — | | |||
Investment in-kind in other related parties (Note 17) | | | | |||
Settlement of receivables through PPE contribution | — | — | | |||
Acquisition of assets financed by debt | — | — | | |||
Acquisition of assets through issuance of capital |
| — | — | | ||
Capitalization of interest on buildings in progress | | — | — | |||
Financed sale of property, plant and equipment | — | | — | |||
Investment properties | | — | — | |||
Sale of equity investee (Note 13) | ( | — | — | |||
Non-monetary contributions in joint ventures and associates (Note 13) | — | | | |||
| | | | |||
06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | ||
Financing activities |
|
|
|
|
|
|
Capitalization of convertible notes (Note 7.13) |
| |
| |
| — |
Purchase of own shares (Note 7.13) | ( | — | — | |||
Consideration for acquisition | — | — | ( | |||
Acquisition of non-controlling interest in subsidiaries |
| — |
| |
| — |
| ( |
| |
| ( |
The Group has incorporated the assets and liabilities from Pro Farm Group Inc mentioned in Note 6.
F-66

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Disclosure of changes in liabilities arising from financing activities:
Financing activities | ||||||||
|
| Consideration |
| Convertible |
| |||
Borrowings | for acquisition | notes | Total | |||||
As of June 30, 2020 |
| |
| | |
| | |
Proceeds |
| |
| — | — |
| | |
Decrease bank overdraft and other short-term borrowings |
| ( | — | — | ( | |||
Payments |
| ( |
| — | — |
| ( | |
Financing for assets acquisitions | — | |
| | ||||
Debt incorporated by business combination | |
|
| | ||||
Interest payment |
| ( |
| — | — |
| ( | |
Exchange differences, currency translation differences and other financial results |
| ( |
| | |
| | |
As of June 30, 2021 |
| |
| | |
| | |
Financing activities | ||||||||
Consideration | Convertible | |||||||
| Borrowings |
| for acquisition |
| notes |
| Total | |
As of June 30, 2021 |
| | | | | |||
Proceeds |
| |
| — |
| — |
| |
Decrease bank overdraft and other short-term borrowings | ( |
| — |
| — |
| ( | |
Payments |
| ( |
| — |
| — |
| ( |
Financing for assets acquisitions | — |
| |
| — |
| | |
Conversion of Convertible Notes (Note 7.13) | — |
| — |
| ( |
| ( | |
Interest payment |
| ( |
| — |
| ( |
| ( |
Exchange differences, currency translation differences and other financial results |
| ( |
| |
| |
| |
As of June 30, 2022 |
| |
| |
| |
| |
Financing activities | ||||||||
Consideration | Convertible | |||||||
| Borrowings |
| for acquisition |
| notes |
| Total | |
As of June 30, 2022 | | | | | ||||
Proceeds |
| |
| — |
| |
| |
Payments |
| ( |
| ( |
| — |
| ( |
Conversion of Convertible Notes (Note 7.13) |
| — |
| — |
| ( |
| ( |
Interest payment |
| ( |
| — |
| ( |
| ( |
Exchange differences, currency translation differences and other financial results |
| |
| ( |
| |
| |
As of June 30, 2023 |
| |
| |
| |
| |
F-67

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
13. JOINT VENTURES AND ASSOCIATES
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Assets | ||||||
Synertech Industrias S.A. |
| |
| |
| |
Indrasa Biotecnología S.A. |
| — |
| |
| |
Alfalfa Technologies S.R.L. |
| |
| |
| |
Moolec Science Limited | — | | | |||
Moolec Science S.A. | | | — | |||
| |
| |
| |
On December 28, 2022, Bioceres has contributed all of its ownership in Moolec Science Limited to Moolec Science S.A. (“Moolec Science”) in exchange of
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Liabilities |
|
|
|
|
| |
Trigall Genetics S.A. |
| |
| |
| |
| |
| |
| |
Changes in joint ventures investments and affiliates:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
As of the beginning of the year |
| |
| | | |
Monetary contributions |
| — |
| — | | |
Non-monetary contributions (Note 12) |
| — |
| | | |
Revaluation of property, plant and equipment |
| ( |
| ( | ( | |
Share-based incentives | | | — | |||
Sale of equity investee - Indrasa Biotecnología S.A. | ( | — | — | |||
Foreign currency translation |
| ( | | | ||
Share of profit or loss |
| | | | ||
As of the end of the year |
| | | |
Share of profit or loss of joint ventures and affiliates:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Trigall Genetics S.A. |
| | | | ||
Synertech Industrias S.A. |
| | | | ||
Moolec Science Limited | — | ( | — | |||
Moolec Science S.A. |
| | — | — | ||
Indrasa Biotecnología S.A. |
| | | | ||
| | | |
There are no significant restrictions on the ability of the joint ventures and affiliates to transfer funds to the Group for cash dividends, or to repay loans or advances made by the Group, except for the Argentinian legal obligation to establish a legal reserve for
F-68

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Summarized financial information prepared in accordance with International Financial Reporting Standards (“IFRS”) in relation to the joint ventures is presented below:
Trigall Genetics (i) | ||||||
Summarized balance sheet |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Current assets | ||||||
Cash and cash equivalents |
| |
| |
| |
Other current assets |
| |
| |
| |
Total current assets |
| |
| |
| |
Non-current assets |
|
|
| |||
Intangible assests |
| |
| |
| |
Investments in joint ventures and associates | | — | — | |||
Total non-current assets |
| |
| |
| |
Current liabilities |
|
|
| |||
Other current liabilities |
| |
| |
| |
Total current liabilities |
| |
| |
| |
Non-current liabilities |
|
|
| |||
Financial liabilities | | | | |||
Other non- current liabilities |
| |
| |
| |
Total non-current liabilities |
| |
| |
| |
Net assets |
| |
| |
| |
Trigall Genetics (i) | ||||||
Summarized statements of comprehensive income |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Revenue |
| |
| |
| |
Finance income |
| — |
| — |
| |
Finance expense |
| ( |
| ( |
| ( |
Depreciation and amortization | ( | ( | — | |||
Profit of the year |
| |
| |
| |
Other comprehensive income |
| ( |
| — |
| — |
Total comprehensive income |
| |
| |
| |
Synertech | ||||||
Summarized balance sheet |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Current assets |
|
|
|
|
| |
Cash and cash equivalents |
| |
| |
| |
Other current assets |
| |
| |
| |
Total current assets |
| |
| |
| |
Non-current assets |
|
|
| |||
Property, plan and equipment |
| |
| |
| |
Other non- current assets |
| |
| — |
| |
Total non-current assets |
| |
| |
| |
Current liabilities |
|
|
|
|
| |
Financial liabilities |
| |
| |
| |
Other current liabilities |
| |
| |
| |
Total current liabilities |
| |
| |
| |
Non-current liabilities |
|
|
|
|
| |
Financial liabilities |
| — |
| |
| |
Other non- current liabilities |
| |
| |
| |
Total non-current liabilities |
| |
| |
| |
Net assets |
| |
| |
| |
F-69

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Synertech | ||||||
Summarized statements of comprehensive income |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Revenue |
| |
| |
| |
Finance income |
| |
| |
| |
Finance expense |
| ( |
| ( |
| ( |
Depreciation and amortization |
| ( |
| ( |
| ( |
(Loss)/Profit of the year |
| |
| ( |
| |
Other comprehensive (loss)/income |
| ( |
| ( |
| ( |
Total comprehensive (loss)/income |
| |
| ( |
| |
14. SEGMENT INFORMATION
The Group is organized into
Seed and integrated products
The seed and integrated products segment focuses mainly on the development and commercialization of seed technologies and products that increase yield per hectare, with a focus on the provision of seed technologies integrated with crop protection and crop nutrition products designed to control weeds, insects or diseases, to increase their quality characteristics, to improve nutritional value and other benefits. The segment focuses on the commercialization of integrated products that combine
Currently the segment generates revenue from ordinary activities through the sale of seeds, integrated product packs, royalties and licenses charged to third parties, among others.
Crop protection
The crop protection segment mainly includes the development, production and marketing of high-tech adjuvants and a full range of pest control molecules and biocontrol products. Adjuvants are used in mixtures to facilitate the application and effectiveness of active ingredients, such as insecticides, leading to better performance, reduced usage rates and lower residue levels Insecticides and fungicides are applied to control pests and significantly reduce disease during the germination period.
The segment currently generates revenue from ordinary activities through the sale of adjuvants, insecticides, fungicides and baits, among others.
Crop nutrition
The crop nutrition segment focuses mainly on the development, production and commercialization of inoculants that allow the biological fixation of nitrogen in the crops, and of fertilizers including biofertilizers and microgranulated fertilizers that optimize the productivity and yield of the crops.
Currently the segment generates income from ordinary activities through the sale of inoculants, bio-inductors, biological fertilizers and microgranulated fertilizers, among others.
The measurement principles for the Group’s segment reporting structure are based on the IFRS principles adopted in the Consolidated financial statements. Revenue generated by products and services exchanged between segments and entities within the Group are calculated based on market prices.
F-70

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The following tables present information with respect to the Group’s reporting segments:
| Seed and |
|
|
|
| ||||
integrated | Crop | Crop | |||||||
Year ended June 30, 2023 | products | protection | nutrition | Consolidated | |||||
Revenues from contracts with customers |
|
|
|
|
|
|
|
| |
Sale of goods and services |
| |
| |
| |
| | |
Royalties |
| |
| — |
| — |
| | |
Others |
|
|
|
|
|
|
| ||
Initial recognition and changes in the fair value of biological assets at the point of harvest |
| |
| |
| |
| | |
Changes in the net realizable value of agricultural products after harvest | ( | ( | ( | ( | |||||
Total |
| |
| |
| |
| | |
Cost of sales |
| ( |
| ( |
| ( |
| ( | |
Gross profit per segment |
| |
| |
| |
| | |
% Gross margin | | % | | % | | % | | % |
| Seed and |
|
|
|
| ||||
| integrated |
| Crop |
| Crop | ||||
Year ended June 30, 2022 |
| products | protection | nutrition | Consolidated | ||||
Revenues from contracts with customers |
|
|
|
|
|
|
|
| |
Sale of goods and services |
| |
| |
| |
| | |
Royalties |
| |
| — |
| — |
| | |
Others |
|
|
|
|
|
|
|
| |
Initial recognition and changes in the fair value of biological assets at the point of harvest |
| |
| |
| |
| | |
Changes in the net realizable value of agricultural products after harvest | ( | | | ( | |||||
Total |
| |
| |
| |
| | |
| |||||||||
Cost of sales |
| ( |
| ( |
| ( |
| ( | |
Gross profit per segment |
| |
| |
| |
| | |
% Gross margin | | % | | % | | % | | % |
| Seed and |
|
|
|
| ||||
| integrated |
| Crop |
| Crop | ||||
Year ended June 30, 2021 |
| products | protection | nutrition | Consolidated | ||||
Revenues from contracts with customers | |||||||||
Sale of goods and services |
| |
| |
| |
| | |
Royalties |
| |
| — |
| — |
| | |
Others |
|
|
|
|
|
|
|
| |
Government grants |
| |
| — |
| — |
| | |
Initial recognition and changes in the fair value of biological assets | |
| |
| |
| | ||
Total |
| |
| |
| |
| | |
Cost of sales |
| ( |
| ( |
| ( |
| ( | |
Gross margin per segment |
| |
| |
| |
| | |
% | | % | | % | | % | | % |
F-71

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Revenue by similar group of products or services
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Seed and integrated products |
| |
| |
| |
Seed Treatments Packs |
| |
| |
| |
Seed & Royalties Payments |
| |
| |
| |
HB4 Program |
| |
| |
| — |
|
|
| ||||
Crop protection |
| |
| |
| |
Adjuvants |
| |
| |
| |
Seed CP Products and Services |
| |
| |
| |
Bioprotection | | — | — | |||
Other CP Products and Services |
| |
| |
| |
|
|
| ||||
Crop nutrition |
| |
| |
| |
Inoculants & Biofertilizers |
| |
| |
| |
Micro-beaded Fertilizers | | | | |||
Biostimulants |
| |
| — |
| — |
|
|
| ||||
Total revenues | | | |
Geographical information
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Argentina |
| |
| |
| |
United States of America |
| |
| |
| |
Cayman Islands (Note 6) | | — | — | |||
Brazil |
| |
| |
| |
France |
| |
| |
| |
Uruguay |
| |
| |
| |
Estonia | | — | — | |||
Bolivia |
| |
| |
| |
Paraguay |
| |
| |
| |
South Africa |
| |
| |
| |
Rest of the world |
| |
| |
| |
Total revenues |
| |
| |
| |
F-72

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2022 | |
Non-current assets | ||||||
Argentina |
| |
| |
| |
Cayman Islands | | | | |||
United States |
| |
| |
| |
Paraguay |
| |
| |
| |
Brazil |
| |
| |
| |
Bolivia |
| |
| |
| |
South Africa |
| — |
| |
| |
Francia | | | | |||
Colombia | | | | |||
Uruguay | | | | |||
Finland | | — | — | |||
Total non-current assets |
| | | | ||
|
| |||||
Property, plant and equipment | | | | |||
Intangible assets | | | | |||
Goodwill | | | | |||
Total reportable assets | | | | |||
Total non-reportable assets | | | | |||
Total assets | | | |
15. FINANCIAL INSTRUMENTS – RISK MANAGEMENT
The Group is exposed to a variety of financial risks that arise from its activities and from its use of financial instruments. This Note provides information on the Group’s exposure to certain main risks, the Group’s objectives, policies and processes regarding the measurement and management of each risk.
The Group does not use derivative financial instruments to hedge any of the above risks.
General objectives, policies and processes
The Board of Directors has overall responsibility for establishing and monitoring the Group’s risk management objectives and policies and, whilst retaining ultimate responsibility for them, it has delegated the function to design and operate processes that ensure the effective implementation of the objectives and policies to the management that periodically reports to the Board of Directors on the evolution of the risk management activities and results. The overall objective of the Board of Directors is to set policies that seek to reduce risk as much as possible without unduly affecting the Group’s competitiveness and flexibility.
The Group’s risk management policy is established to identify and analyze the risks facing the Group, to set appropriate risk limits and controls and to monitor risks and adherence to limits. The risks and methods for managing the risks are reviewed regularly in order to reflect changes in market conditions and the Group’s activities. The Group, through training and management standards and procedures, aims to develop a disciplined and constructive control environment in which all the employees understand their roles and obligations.
The Group seeks to use suitable means of financing to minimize the Group’s capital costs and to manage and control the Group’s financial risks effectively. There have been no substantive changes in the Group’s exposure to financial instrument risks, its objectives, policies and processes for managing those risks or the methods used to measure them from previous periods unless otherwise stated in this Note.
The Group adopted a code of ethics applicable to its principal executive, financial and accounting officers and all employees.
F-73

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The principal risks and uncertainties facing the business, set out below, do not appear in any particular order of potential materiality or probability of occurrence.
Credit risk
Credit risk is the risk of financial loss to the Group if a customer or counterparty fails to meet its contractual obligations, which derives mainly from trade and other receivables, as well as from cash and deposits in financial institutions.
The credit risk to which the Group is exposed is mainly defined in the Group’s accounts receivable followed by cash and cash equivalents, with the logical importance of being able to satisfy the Group’s needs in the short term.
Trade and other receivables
Credit risk is the risk of financial loss to the Group if a customer or counterparty fails to meet its contractual obligations and derives mainly from trade receivables and other receivables generated by services and product sales. The Group is also exposed to political and economic risk events, which may cause nonpayment of local and foreign currency obligations to the Group owed by customers, partners, contractors and suppliers.
The Group sells its products to a diverse base of customers. Customers include multi-national and local agricultural companies, distributors, and farmers who purchase the Group’s products. Type and class of customers may differ depending on the Group’s business segments.
The Group’s management determines concentrations of credit risk by periodically monitoring the credit worthiness rating of existing customers and through a monthly review of the trade receivables’ aging analysis. In monitoring the customers’ credit risk, customers are grouped according to their credit characteristics.
The Group’s policy is to manage credit exposure to counterparties through a process of credit rating. The Group performs credit evaluations of existing and new customers, and every new customer is examined thoroughly regarding the quality of its credit before offering the customer transaction terms. The examination made by the Group includes outside credit rating information, if available. Additionally, and even if there is no independent outside rating, the Group assesses the credit quality of the customer taking into account its financial position, past experience, bank references and other factors. A credit limit is prescribed for each customer. These limits are examined periodically. Customers that do not meet the Group’s criteria for credit quality may do business with the Group on a prepayment basis or by furnishing collateral satisfactory to the Group. The Group may still seek collateral and guarantees as it may consider appropriate regardless the credit profile of any customer.
To cover trade receivables, the Group has a credit insurance for main subsidiaries, which periodically analyzes its customer portfolio.
The financial statements contain specific provisions for doubtful debts, which properly reflect, in Management’s estimate, the loss embedded in debts, the collection of which is doubtful. The maximum exposure to credit risk is represented by the carrying amount of each financial asset in the statement of financial position after deducting any impairment allowance.
F-74

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
On that basis, the loss allowance as of June 30, 2023 was determined as follows:
Gross carrying | ||||||
amount-trade | Expected Loss | Loss | ||||
| receivables |
| rate |
| allowance | |
Current |
| | | % | | |
More than 15 days past due | | | % | | ||
More than 30 days past due |
| |
| | % | |
More than 60 days past due | | | % | | ||
More than 90 days past due | | | % | | ||
More than 120 days past due | | | % | | ||
More than 180 days past due | | | % | | ||
More than 365 days past due | | | % | | ||
Total 06/30/2023 |
| |
| |
Cash and deposits in banks
The Group is exposed to counterparty credit risk on cash and cash equivalent balances. The Group holds cash on deposit with a number of financial institutions. The Group manages its credit risk exposure by limiting individual deposits to clearly defined limits. The Group only deposits with high quality banks and financial institutions.
The maximum exposure to credit risk is represented by the carrying amount of cash and cash equivalents in the statement of financial position.
Liquidity risk
Liquidity risk is the risk that the Group will encounter difficulty in meeting its financial obligations when they come due.
The Group’s approach to managing its liquidity risk is to manage the profile of debt maturities and funding sources, maintaining sufficient cash, and ensuring the availability of funding from an adequate amount of committed credit facilities. The Group’s ability to fund its existing and prospective debt requirements is managed by maintaining diversified funding sources with adequate committed funding lines from high quality lenders.
The cash flow forecast is determined at both an entity level and consolidated level. The forecasts are reviewed by the Board of Directors in advance, enabling the Group’s cash requirements to be anticipated. The Group examines the forecasts of its liquidity requirements in order to ascertain that there is sufficient cash for the operating needs, including the amounts required in order to settle financial liabilities.
The following table sets out the contractual maturities of financial liabilities:
Between one | ||||||
Up to 3 | 3 to 12 | and three | ||||
As of June 30, 2023 |
| months |
| months |
| years |
Trade and other payables |
| |
| |
| |
Borrowings | | | | |||
Convertible notes |
| — |
| — |
| |
Leasing liabilities |
| |
| |
| |
Total |
| |
| |
| |
F-75

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
|
|
| Between one | |||
Up to 3 | 3 to 12 | and three | ||||
As of June 30, 2022 | months | months | years | |||
Trade and other payables |
| |
| |
| — |
Borrowings |
| | | | ||
Convertible notes |
| — |
| — |
| |
Leasing liabilities | |
| |
| | |
Total |
| |
| |
| |
|
|
| Between one | |||
Up to 3 | 3 to 12 | and three | ||||
As of June 30, 2021 | months | months | years | |||
Trade and other payables |
| |
| |
| |
Borrowings |
| | | | ||
Convertible notes |
| — |
| — |
| |
Leasing liabilities | |
| |
| | |
Total |
| |
| |
| |
As of June 30, 2023, and 2022 the Group had no exposure to derivative liabilities.
Inventory price risk
The Group is exposed to the volatility of commodities prices in its inventory. For the purposes of ensuring supply, the Group enters into purchasing agreements, granting the producer the right to set the price at any time between the delivery date and a future date. The Group eventually covers risks on its financial position and on the results of a possible variation in commodities prices by selling the grains at the same time producers set prices in purchasing agreements.
As of June 30, 2023, the impact of a simultaneous movement of
Currency risk
Foreign currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rate. Currency on foreign exchange risk arises when the Group enters into transactions denominated in a currency other than its functional currency.
The table below sets forth our net exposure to currency risk as of June 30, 2023:
Net foreign currency position |
| 06/30/2023 |
Amount expressed in US$ |
| ( |
The main Argentinian subsidiaries of the Group have changed their functional currency from Argentine Pesos to US Dollar (See note 2).
Considering only this net currency exposure as of June 30, 2023 if an US Dollar revaluation or depreciation in relation to other foreign currencies with the remaining variables remaining constant, would have a positive or a negative impact on comprehensive income as a result of foreign exchange gains or losses. We estimate that a devaluation or an appreciation of the US Dollar other currencies of
F-76

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Interest rate risk
The Group’s financing costs may be affected by interest rate volatility. Borrowings under the Group’s interest rate management policy may be fixed or floating rate. The Group maintains adequate committed borrowing facilities and holds most of its financial assets primarily in cash or checks collected from customers that are readily convertible into known amounts of cash.
The Group’s interest rate risk arises from long-term borrowings. Borrowings issued at floating rates expose the Group to cash flow interest rate risk. Borrowings issued at fixed rates expose the Group to fair value interest rate risk. The Group has not entered into derivative contracts to hedge this exposure.
The Group’s debt composition is set out below.
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Carrying | Carrying | Carrying | ||||
amount | amount | amount | ||||
Fixed-rate instruments |
|
|
|
|
|
|
Current financial liabilities |
| ( |
| ( |
| ( |
Non-current financial liabilities |
| ( |
| ( |
| ( |
Variable-rate instruments |
|
|
| |||
Current financial liabilities |
| ( |
| ( |
| ( |
Non-current financial liabilities |
| — |
| ( |
| ( |
Holding all other variables constant, including levels of our external indebtedness, as of June 30, 2023 a
The Company does not use derivative financial instruments to hedge its interest rate risk exposure.
Capital risk
The Group’s objectives when managing capital are to safeguard the Group’s ability to continue as a going concern, in order to provide returns for shareholders and benefits for other stakeholders, and to maintain an optimal capital structure to reduce the cost of capital.
The Group manages its capital structure and makes adjustments to it in the light of changes in economic conditions and the risk characteristics of the underlying assets. In order to maintain or adjust the capital structure, the Group may adjust the amount of any dividends it could pay to shareholders, return capital to shareholders, issue new shares, or sell assets to reduce debt.
F-77

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Financial instruments by category
The following tables show additional information required under IFRS 7 on the financial assets and liabilities recorded as of June 30, 2023, and 2022.
Financial assets by category
|
| Mandatorily measured at fair value | ||||||||||
Amortized cost | through profit or loss | |||||||||||
Financial asset |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Cash and cash equivalents | | | | — | | | ||||||
Other financial assets |
| |
| |
| |
| |
| |
| |
Trade receivables |
| |
| |
| |
| — |
| — |
| — |
Other receivables (*) |
| |
| |
| |
| — |
| — |
| — |
Total |
| |
| |
| |
| |
| |
| |
(*) Advances expenses and tax balances are not included.
Financial liabilities by category
|
| Mandatorily measured at fair value | ||||||||||
Amortized cost | through profit or loss | |||||||||||
Financial liability |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Trade and other payables | | | | | — | — | ||||||
Borrowings | | | | — | — | — | ||||||
Secured notes |
| |
| |
| |
| — |
| — |
| — |
Lease liability |
| |
| |
| |
| — |
| — |
| — |
Consideration for acquisition of assets |
| |
| |
| |
| — |
| — |
| — |
Total |
| |
| |
| |
| |
| — |
| — |
Financial instruments measured at fair value
Fair value by hierarchy
According to the requirements of IFRS 7, the Group classifies each class of financial instrument valued at fair value into three levels, depending on the relevance of the judgment associated to the assumptions used for measuring the fair value.
Level 1 comprises financial assets and liabilities with fair values determined by reference to quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 comprises inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices);
Level 3 comprises financial instruments with inputs for estimating fair value that are not based on observable market data.
F-78

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
Measurement at fair value at 06/30/2023 |
| Level 1 |
| Level 2 |
| Level 3 |
Financial assets at fair value |
|
|
|
|
|
|
US Treasury bills |
| |
| — |
| — |
Mutual funds |
| |
| — |
| — |
Other investments |
| |
| — |
| — |
Financial liability at fair value |
|
|
|
|
|
|
Trade and other payables |
| — |
| |
| — |
Measurement at fair value at 06/30/2022 |
| Level 1 |
| Level 2 |
| Level 3 |
Financial assets at fair value |
|
|
|
|
|
|
Mutual funds |
| |
| — |
| — |
Other investments |
| |
| |
| — |
Measurement at fair value at 06/30/2021 |
| Level 1 |
| Level 2 |
| Level 3 |
Financial assets at fair value |
|
|
|
|
|
|
Mutual funds |
| |
| — |
| — |
US Treasury bills |
| |
| — |
| — |
Other investments | | | — |
Estimation of fair value
The fair value of marketable securities, mutual funds and US Treasury Bills is calculated using the market approach using quoted prices in active markets for identical assets. The quoted marked price used for financial assets held by the Group is the current bid price. These instruments are included in level 1.
The Group’s financial liabilities, which were not traded in an active market, were determined using valuation techniques that maximize the use of available market information, and thus rely as little as possible on specific estimates of the entity specific estimates. If all significant inputs required to fair value an instrument are observable, the instruments are included in level 2.
If one or more of the significant inputs is not based on observable market data, the instruments are included in level 3.
The Group’s policy is to recognize transfers between different categories of the fair value hierarchy at the time they occur or when there are changes in the circumstances that cause the transfer. There were no transfers between levels of the fair value hierarchy. There were no changes in economic or business circumstances affecting fair value.
Financial instruments not measured at fair value
The financial instruments not measured at fair value include cash and cash equivalents, trade accounts receivable, other accounts receivable, trade payables and other debts, borrowings, financed payments and convertible notes.
The carrying value of financial instruments not measured at fair value does not differ significantly from their fair value, except for borrowings (Note 7.12).
Management estimates that the carrying value of the financial instruments measured at amortized cost approximates their fair value.
F-79

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
16. LEASES
The right-of-use asset was initially measured at the amount of the lease liability plus initial direct costs incurred, adjusted by pre-payments made in relation to the lease. The right-of-use asset was measured at cost less accumulated depreciation and accumulated impairment.
The lease liability was initially measured at the present value of the lease payments payable over the lease term, discounted at the rate implicit in the lease if it can be readily determined. If that rate cannot be readily determined, the Group uses its incremental borrowing rate.
The information about the right-of-use and liabilities related with lease assets is as follows:
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Right-of-use leased asset |
|
| ||||
Book value at the beginning of the year |
| | | | ||
Additions of the year |
| | | | ||
Additions from business combination |
| | — | — | ||
Disposals | ( | — | — | |||
Exchange differences | | | | |||
Book value at the end of the year |
| | | | ||
|
| |||||
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Depreciation | ||||||
Book value at the beginning of the year | | | | |||
Depreciation of the year |
| | | | ||
Disposals |
| ( | — | — | ||
Exchange differences |
| | | | ||
Accumulated depreciation at the end of the year |
| | | | ||
Total |
| | | | ||
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Lease liability | ||||||
Book value at the beginning of the year |
| | | | ||
Additions of the year |
| | | | ||
Additions from business combination |
| | — | — | ||
Interest expenses, exchange differences and inflation effects |
| ( | | | ||
Payments of the year |
| ( | ( | ( | ||
Total |
| | | | ||
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Lease Liabilities | ||||||
Non-current |
| | | | ||
Current |
| | | | ||
Total |
| | | | ||
06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | ||
Machinery and equipment | | | | |||
Vehicles | | | | |||
Equipment and computer software | | | | |||
Land and buildings | | | | |||
| | |
| (1) | The incremental borrowing rate used was |
F-80

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
17. SHAREHOLDERS AND OTHER RELATED PARTIES BALANCES AND TRANSACTIONS
During the year ended June 30, 2023, and 2022, the transactions between the Group and related parties, and the related balances owed by and to them, are as follows:
Value of transactions for the year ended | ||||||||
Party |
| Transaction type |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Joint ventures and associates |
| Sales and services |
| |
| |
| |
Joint ventures and associates |
| Purchases of goods and services |
| ( |
| ( |
| ( |
Joint ventures and associates |
| Equity contributions |
| — |
| |
| |
Key management personnel |
| Salaries, social security benefits and other benefits |
| ( |
| ( |
| ( |
Key management personnel |
| Net loans cancelled |
| — |
| — |
| |
Key management personnel |
| Interest gain |
| — |
| — |
| |
Key management personnel | Sales and services | | — | — | ||||
Shareholders and other related parties |
| Sales of goods and services |
| |
| |
| |
Shareholders and other related parties |
| Purchases of goods and services |
| ( |
| ( |
| ( |
Shareholders and other related parties | In-kind contributions | | | | ||||
Shareholders and other related parties | Net loans granted/(cancelled) | — | | — | ||||
Shareholders and other related parties |
| Interest gain |
| |
| |
| — |
Parent company and related parties to Parent (Note 8.6) |
| Net loans cancelled |
| — |
| — |
| ( |
Parent company and related parties to Parent (Note 8.6) |
| Interest expenses |
| ( |
| ( |
| ( |
Total |
|
|
| ( |
| ( |
| ( |
Amounts receivable from related parties | ||||||||
Party |
| Transaction type |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Parent company and related parties to Parent |
| Other receivables |
| — |
| — |
| |
Shareholders and other related parties |
| Other receivables |
| — |
| |
| — |
Shareholders and other related parties |
| Other receivables |
| |
| |
| |
Joint ventures and associates |
| Trade debtors |
| |
| |
| |
Joint ventures and associates |
| Other receivables |
| |
| |
| |
Total |
| |
| |
| | ||
Amounts payable to related parties | ||||||||
Party |
| Transaction type |
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 |
Parent company and related parties to Parent | Trade creditors | ( | ( | ( | ||||
Parent company and related parties to Parent | Net loans payables | ( | ( | ( | ||||
Key management personnel |
| Salaries, social security benefits and other benefits |
| ( |
| ( |
| ( |
Shareholders and other related parties |
| Trade and other payables |
| ( |
| ( |
| ( |
Joint ventures and associates |
| Trade creditors |
| ( |
| ( |
| ( |
Total |
| ( |
| ( |
| ( | ||
18. KEY MANAGEMENT PERSONNEL COMPENSATION
Key management personnel are those persons having authority and responsibility for planning, directing, and controlling the activities of the Group.
The compensation of directors and other members of key management personnel, including social contributions and other benefits, were as follows for the year ended June 30, 2023, and 2022.
| 06/30/2023 |
| 06/30/2022 |
| 06/30/2021 | |
Salaries, social security and other benefits |
| |
| |
| |
Share-based incentives | | | | |||
Total |
| |
| |
| |
F-81

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The Company entered into indemnification agreements with each of its directors and executive officers. These agreements generally provide that the relevant director or officer will be indemnified by the Company to the fullest extent permitted by law against liability and against all expenses reasonably incurred or paid by him or her in connection with any claim, action, suit or proceeding which he or she becomes involved as a party or otherwise by virtue of his or her being or having been such a director or officer of the Company and against amounts paid or incurred by him or her in the settlement thereof.
The agreements are subject to certain exceptions, including that no indemnification will be provided to any director or officer against any liability to the Group or its shareholder (i) by reason of intentional fraudulent conduct, dishonesty, willful misconduct, or gross negligence on the part of the director or officer; or (ii) by reason of payment made under an insurance policy or any third party that has no recourse against the indemnitee director or officer.
The compensation of key executives is determined by the Compensation Committee based on the performance of individuals and market trends.
19. SHARE-BASED PAYMENT
2023 Omnibus Equity Incentive Plan
On May 12, 2023, the board of directors of the Company approved the 2023 Omnibus Equity Incentive Plan to attract and retain the best available personnel, provide additional incentives to employees, directors and consultants and to promote the success of our business.
Under the Plan, the maximum aggregate number of shares that may be issued pursuant to all awards is
Incentive payments based on options
- | Share option plan for directors and senior management: plan granted up to |
- | Share option plan for junior management: plan granted up to |
Options can be exercised for a period of up to
The fair value of the stock options at the grant date was estimated using the “Black-Scholes” model, considering the terms and conditions under which the options on shares were granted and adjusted to consider the possible dilutive effect of the future exercise of options.
Factor |
| Directors and Sr. Management |
| Junior Management | |||
Weighted average fair value of shares |
| $ | $ | | |||
Exercise price |
| $ | $ | | |||
Weighted average expected volatility (*) |
| % | | % | |||
Dividend rate |
| % | % | ||||
Weighted average risk-free interest rate |
| % | | % | |||
Weighted average expected life |
| | years | | years | ||
Weighted average fair value of stock options at measurement date |
| $ | $ | | |||
There are no market-related performance conditions or non-vesting conditions that should be considered for determining the fair value of options.
F-82

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The Group estimates that
2013 Stock Incentive Plan
As part of the merger described in Note 6, we have assumed the outstanding “2013 Stock Incentive Plan” from Pro Farm Group. On the merger date the total equity awards outstanding was converted consistent with the terms of the merger agreement into an aggregate of
The total converted options outstanding on the date of the merger was
| July 12, 2022 |
| ||
Exercise price | $ | |||
Expected life (years) |
| |||
Estimated volatility factor |
| % | ||
Risk-free interest rate |
| | % | |
Expected dividend yield |
| — | ||
The following table shows the weighted average amount and exercise price and the movements of the stock options during the years ended June 30, 2023, 2022 and 2021:
06/30/2023 | 06/30/2022 | 06/30/2021 | |||||||||||||
| Number of |
| Exercise |
| Number of |
| Exercise |
| Number of | Exercise | |||||
options | price | options | price | options | price | ||||||||||
At the beginning of the year |
| |
| $ | |
| |
| $ | |
| | $ | | |
Assumed for business combination |
| |
| $ | |
| — |
| — |
| — | — | |||
Granted during the year |
| — |
| — |
| |
| $ | |
| — |
| — | ||
Exercised during the year |
| ( |
| $ | |
| ( |
| $ | |
| ( | $ | | |
Effective at the end of the year |
| |
| $ | |
| |
| $ | |
| | $ | | |
Exercise price of options effective at the end of the period was calculated using weighted average.
Annual compensation - Bonus
Bonus in Cash is an annual cash incentive awarded up to an amount that is
Bonus in Kind is an annual in-kind incentive awarded in ordinary shares to certain executive officers, to tie a portion of their compensation to financial and operational objectives. Each year the Board of Directors will define the objectives upon approval of the annual budget.
The number of shares that can be awarded under each bonus will be determined by using a
F-83

NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of and for the years ended June 30, 2023, 2022 and 2021
(Amounts in US$, except otherwise indicated)
The total charge of share-based incentives plans recognized during the year ended June 30, 2023, 2022 and 2021 was $
20.CONTINGENCIES, COMMITMENTS AND RESTRICTIONS ON THE DISTRIBUTION OF PROFITS
The secured guaranteed notes and the convertibles notes referenced in Note 7.13 are secured by substantially all of the assets located in the United States of Pro Farm Group, Inc. and its U.S. subsidiaries and are guaranteed by BCS Holding Inc., Bioceres Crops do Brasil Ltda., Bioceres Crops S.A., Bioceres Semillas S.A.U., Verdeca LLC, Rasa Holding LLC, Rizobacter Argentina S.A., Rizobacter del Paraguay S.A., Rizobacter do Brasil Ltda., Rizobacter South Africa, Rizobacter Uruguay, Rizobacter USA, LLC, Pro Farm Group, Inc., Pro Farm Michigan Manufacturing LLC, Pro Farm, Inc., Pro Farm Technologies Comércio de Insumo Agrícolas do Brasil Ltda., Glinatur S.A. and Pro Farm OU.
21.IMPACT OF COVID-19
The Group’s operations, which involve agricultural production and commercialization activities, have been mostly exempted from the disruptions caused by covid-19. Consequently, our financial condition, liquidity position and results of operations have not been materially impacted as we have been allowed to continue with our operations.
22.EVENTS OCCURRING AFTER THE REPORTING PERIOD
Subsequent to June 30, 2023, there have been no other situations or circumstances that may require significant adjustments or further disclosure in these consolidated financial statements that were not mentioned above.
F-84
