UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________________
FORM 10-K
____________________________________________________
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended: December 31 , 2023
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
Commission file number 001-38118
____________________________________________________
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) | ||||
(Zip Code) | |||||
Registrant’s telephone number, including area code: (858 ) 450‑4222
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
The | ||||||||||||||
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such report(s), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b‑2 of the Exchange Acts.
| Large accelerated filer | o | Accelerated filer | o | |||||||||||
| x | Smaller reporting company | |||||||||||||
| Emerging growth company | ||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Act). Yes o No x
The aggregate market value of the registrant’s common stock, $0.0001 par value, held by non-affiliates of the registrant as of the last business day of the registrant’s most recently completed second fiscal quarter was approximately $85,237,006 (based on the closing price of the registrant’s common stock on June 30, 2023 of $2.60 per share).
The number of shares outstanding of the registrant’s common stock, $0.0001 par value per share as of February 23, 2024 was 34,623,443 .
DOCUMENTS INCORPORATED BY REFERENCE
The registrant intends to file a definitive proxy statement pursuant to Regulation 14A within 120 days after the end of the fiscal year ended December 31, 2023. Portions of such proxy statement are incorporated by reference into Part III of this Form 10‑K.
Auditor Firm ID: | Auditor Name: | Auditor Location: | |||||||||
i
DERMTECH, INC
ANNUAL REPORT ON FORM 10‑K
YEAR ENDED DECEMBER 31, 2023
TABLE OF CONTENTS
| Page No. | ||||||||
ii
Special Note Regarding Forward-Looking Statements
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Forward-looking statements are statements other than historical facts and relate to future events or circumstances or our future performance, and they are based on our current assumptions, expectations and beliefs concerning future developments and their potential effect on our business. Words such as, but not limited to “anticipate,” “aim,” “believe,” “contemplate,” “continue,” “could,” “design,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “seek,” “should,” “strategy,” “target,” “will,” “would,” and similar expressions or variations thereof are intended to identify forward-looking statements, but are not deemed to represent an all-inclusive means of identifying forward-looking statements as denoted in this report. These statements include, among other things, statements regarding:
•our ability to attain profitability;
•our estimates regarding our future performance, including without limitation estimates of potential future revenues;
•our ability to maintain commercial reimbursement for our tests;
•our ability to efficiently bill for and collect revenue resulting from our tests;
•our anticipated need and ability to raise additional capital when and as needed and on acceptable terms to fund and continue our planned operations, commercialize our products, and expand our operations;
•our ability to market and sell our tests to physicians and other clinical practitioners;
•our ability to continue to develop our existing test and develop and commercialize additional novel tests;
•our dependence on third parties for the manufacture of our products;
•our ability to meet market demand for our current and planned future tests;
•our reliance on our sole laboratory facility and the harm that may result if this facility became damaged or inoperable;
•our ability to retain the requisite licenses and accreditations for our sole laboratory, including our current Joint Commission (“JCO”) accreditation;
•our ability to compete with our competitors and their competing products;
•the importance of our executive management team;
•our ability to retain and recruit key personnel;
•our dependence on third parties for the supply of our laboratory substances, equipment and other materials;
•the potential for us to incur substantial costs resulting from product liability lawsuits against us and the potential for these lawsuits to cause us to suspend sales of our products;
•the possibility that a third party may claim we have infringed or misappropriated our intellectual property rights and that we may incur substantial costs and be required to devote substantial time defending against these claims;
•the potential consequences of our expanding our operations internationally;
•our ability to continue to comply with applicable privacy laws and protect confidential information from breaches;
•how changes in federal health care policy and applicable regulations, including the potential additional regulation of LDTs, could increase our costs, decrease our revenues, impact sales of and reimbursement for our tests and otherwise materially and adversely affect our business;
•our ability to continue to comply with federal and local laws and regulations concerning our business and operations and the consequences resulting from our failure to comply with such laws;
•the possibility that we may be required to conduct additional clinical studies or trials for our tests and the consequences resulting from the delay in obtaining necessary regulatory approvals;
•the harm resulting from the potential loss, suspension, or other restriction on one or more of our licenses, permits, certifications or accreditations, or the imposition of a fine or penalty on us under federal, state, or foreign laws;
1
•our ability to maintain and our intellectual property protections;
•how recent and potential future changes in tax policy could negatively impact our business and financial condition;
•how recent and potential future changes in healthcare policy could negatively impact our business and financial condition;
•our ability to maintain Nasdaq listing; and
•our ability to manage the increased expenses and administrative burdens as a public company.
Although forward-looking statements in this report reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the heading “Risk Factors” below, as well as those discussed elsewhere in this report. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. We file reports with the Securities and Exchange Commission (the “SEC”), and our electronic filings with the SEC (including our quarterly reports on Form 10-Q and current reports on Form 8-K, and any amendments to these reports) are available free of charge on the SEC’s website at http://www.sec.gov.
We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this report, except as required by law. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this report, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition and results of operations. We qualify all of our forward-looking statements by this special note.
We own registered or unregistered trademark rights to DermTech™, DermTech Melanoma Test™, DermTech Stratum™, Smart Sticker™, Luminate™ and our company name and logo among others. Any other service marks, trademarks and trade names appearing in this report are the property of their respective owners. We do not use the ® or ™ symbol in each instance in which one of our trademarks appears in this report, but this should not be construed as any indication that we will not assert our rights thereto to the fullest extent under applicable law.
2
PART I
Item 1. Business
Unless specifically noted otherwise, as used throughout this Business section, “we,” “our,” “us,” or the “Company” refers to the business, operations and financial results of DermTech Operations prior to, and the Company and its subsidiaries subsequent to, the completion of the Business Combination as the context requires. “Constellation” refers to the Company prior to the completion of the Business Combination.
Business Overview
We are a molecular diagnostic company developing and marketing novel non-invasive genomics tests to aid in the diagnosis and management of melanoma. Our technology enhances evaluation of lesions suspicious for melanoma using non-invasive sample collection and detecting genomic markers associated with melanoma to identify higher risk lesions or rule out melanoma with a 99% or higher negative predictive value ("NPV") (Gerami et al. J Am Acad Dermatol. 2017; Skelsey et al. SKIN. 2021). Our scalable genomics assays have been designed to work with our adhesive patch, the DermTech Smart StickerTM (the “Smart Sticker”), which is used to non-invasively collect skin tissue samples for analysis.
We are addressing unmet needs in the clinical evaluation pathway of pigmented skin lesions, such as moles or dark colored skin spots. Our DermTech Melanoma Test (the “DMT”) facilitates the clinical assessment of pigmented skin lesions for melanoma. We initially focused on marketing the DMT to a large group of dermatology clinicians and are currently prioritizing billable samples in geographies where we have payor coverage versus overall volume growth as one factor to potentially increase average selling price. The application of our Smart Sticker to collect samples non-invasively may allow us to eventually market the DMT more broadly to primary care physicians, beyond integrated primary care networks. We process our tests in our high complexity molecular laboratory that is certified under the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”), accredited by the Joint Commission, formerly the Joint Commission on Accreditation of Healthcare Organizations (“JCO”), and licensed by the State of California as well as other states requiring out-of-state licensure, including New York. We also provide laboratory services to several pharmaceutical companies that access our technology on a contract basis typically related to ongoing clinical trials or other studies to advance therapeutics.

3
Our Business
We are a molecular diagnostic company developing and marketing novel non-invasive genomics tests to aid in the evaluation and management of pigmented lesions suspicious for melanoma. Our platform may change the diagnostic paradigm in dermatology from one that is subjective, invasive, less accurate and higher-cost, to one that is objective, non-invasive, more accurate and lower-cost. We currently offer the DMT, which is an innovative, non-invasive way to enhance evaluation of pigmented lesions suspicious for melanoma with a demonstrated negative predictive value of 99% or higher. Our scalable genomics platform has been designed to work with our Smart Sticker, which provides an easy and non-invasive way to collect a skin sample, in contrast to the existing standard of care of using a scalpel to biopsy suspicious lesions. We also provide our research services and technology platform on a contract basis to pharmaceutical companies that may use the technology to support their clinical trials. Pharmaceutical companies use our Smart Sticker to test for the existence of genomic targets of various skin diseases and measure the response of drugs under development. We process our test in our CLIA-certified commercial laboratory, which is also JCO accredited and licensed by the California Department of Public Health as well as other states that require out-of-state licensure. As described below, our technology platform is easy to use and integrates seamlessly into the current clinical diagnostic pathway by providing (i) simple and rapid tissue collection and shipping via standard express mail, (ii) sample processing via quantitative polymerase chain reaction (“qPCR”), DNA sequencing or assays and (iii) results are reported to a physician within 5 business days from receipt of the sample in the laboratory.
Dermatology is one of the largest medical markets in the United States. The skin cancer segment has over 15 million surgical diagnostic procedures performed each year, with an average annual spend of $8.1 billion, according to the American Academy of Dermatology (“AAD”). Current dermatologic diagnosis is primarily based on subjective visual assessments and subsequent surgical diagnostic procedures. This legacy paradigm is prone to error and results in a substantial number of unnecessary and invasive surgical procedures. Our platform provides a non-invasive alternative that minimizes patient discomfort, scarring, and risk of infection. Further, because our testing results utilize genomic analysis, we provide more accurate, objective diagnostic information than the currently prevailing diagnostic procedures. As described below, a study published in JAMA Dermatology demonstrated that the foundational assay of the DMT lowers the cost to diagnose melanoma while providing a more accurate and less invasive alternative to current methods used to evaluate lesions suspicious for melanoma (Hornberger and Siegel. JAMA Dermatol. 2018), providing a test that assesses genomic atypia for key genomic markers, and that has been demonstrated to rule-out melanoma with a 99% or higher negative predictive value.
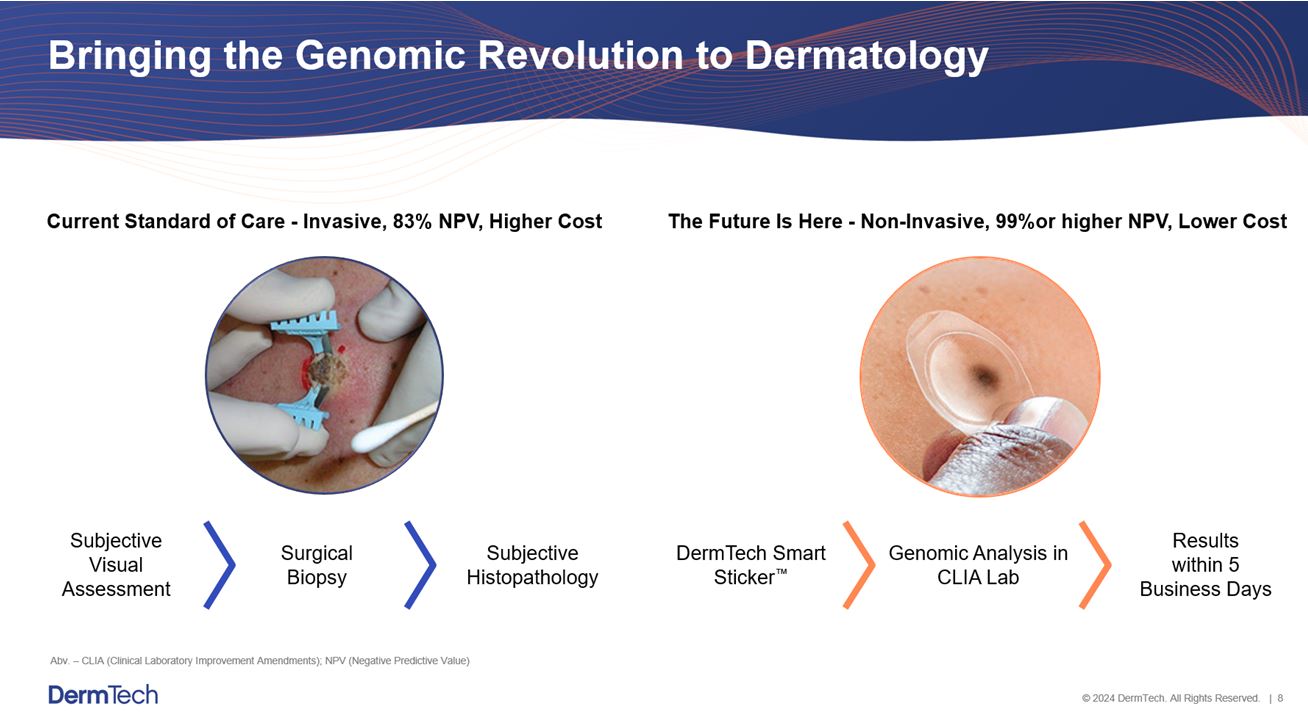
4
The healthcare market is saturated with genomic tests that are primarily marketed to pathology and oncology specialists. We are the first company to offer a non-invasive genomic test to the clinical dermatology market. We believe our technology platform may allow us to eventually expand the base of clinicians (e.g. primary care clinicians) that can incorporate our test to enhance the practice of precision dermatology. As healthcare delivery diverges to support more convenient delivery models, such as pharmacy-based/retail clinics and telemedicine, we believe our platform may facilitate the migration of dermatologic care to these alternative models. We also believe our platform may allow for expanded consumer-based sample collection shipped directly to our laboratory, positively impacting the ease of use and convenience of providing dermatologic care.
We originally marketed the DMT under the name Pigmented Lesion Assay or PLA. The PLA assessed pigmented skin lesions, moles or dark skin spots to rule out melanoma. In particular, the PLA detected expression of the LINC00518 (“LINC”) and preferentially expressed antigen in melanoma (“PRAME”) genes using a nucleic acid amplification process called reverse transcription-polymerase chain reaction (“RT-PCR”). We introduced an add-on assay to PLA in 2021, which was designed to identify the presence of mutations in TERT gene promoter region using DNA sequencing (Jackson et al. SKIN. 2020). We then branded the PLA and TERT add-on-assay as the DMT. Positive results for LINC, PRAME or TERT correlate with a lesion at higher risk for melanoma. If none of the biomarkers are detected, there is a 99% or higher probability (or negative predictive value) that the tested mole is not melanoma.
Effective March 1, 2024, the DMT will only include the foundational PLA assay and will no longer include the TERT add-on test, meaning the DMT will test for LINC and PRAME biomarkers, as TERT has limited impact on decision‑making when evaluating pigmented lesions for melanoma. Removal of TERT does not impact the negative predictive value of the test. Multiple studies, including the Company’s recently completed Trust 2 Study, demonstrated the foundational PLA's NPV to be 99% or higher. A high NPV for a rule-out test provides assurance to clinicians and patients that a suspicious pigmented lesion which tests negative is unlikely to be a melanoma. Each reference to DMT herein below refers to the foundational PLA assay only.
In March 2019, Medicare’s Molecular Diagnostic Services program, administered by Palmetto GBA (“MolDX”), which performs technology assessments for genomic tests, issued a favorable Local Coverage Determination (“LCD”) draft (the “Draft LCD”) for the DMT. In October 2019, the American Medical Association (“AMA”) provided us with a Current Procedural Technology Proprietary Laboratory Analysis code of 0089U for the DMT (the “PLA Code”). Pricing of $760 for the PLA Code was published on December 24, 2019 as part of the Centers for Medicare and Medicaid Services (“CMS”) Clinical Laboratory Fee Schedule (“CLFS”) for 2020, which has been confirmed for 2023. The Medicare final LCD (the “Final LCD”) first made available on December 26, 2019 expanded the coverage from the Draft LCD from one test per date of service to two tests per date of service for a certain percentage of patients, and is indicated only for use on pigmented lesion for which a diagnosis of melanoma is being considered. Also, the test may only be ordered by clinicians who have knowledge, experience and skill to evaluate pigmented lesions and perform biopsies. The test is covered for use as a source of information on whether or not to perform a biopsy. Our local Medicare Administrative Contractor, Noridian Healthcare Solutions, LLC (“Noridian”) relies upon MolDX for technology assessments of genomic-based tests and has adopted the Final LCD issued by MolDX. Noridian has issued its own LCD announcing coverage of the DMT. Even though the effective date of Noridian’s LCD is June 7, 2020, Noridian began reimbursing us for the DMT as of February 10, 2020.
Of the approximate 4 million surgical biopsies performed each year on pigmented skin lesions, over 90% are negative for melanoma and represent avoidable surgical procedures. The DMT improves the assessment of pigmented lesions by reducing the number of surgical biopsies required to diagnose melanoma by five to tenfold (from about 25:1 to about 2.5-5.0:1). In addition, the DMT improves the positive predictive value (“PPV”) approximately five-fold (from 3-4% with the current surgical techniques to 18.7% with DMT). (Gerami et al. J Am Acad Dermatol. 2017; Skelsey et al. SKIN. 2021; Brouha et al SKIN. 2021).
5
The performance of the DMT is supported by numerous investigational studies, which enrolled an aggregate of over 9,000 patients and yielded more than 20 peer-reviewed publications in top-rated medical dermatology journals. A study published in JAMA Dermatology directed to the DMT demonstrated that incorporation of the test in practice could significantly lower the cost to diagnose melanoma while providing a more accurate assessment and less invasive alternative to current methods. The current AAD melanoma guidelines indicate that non-invasive gene expression testing can be used as a part of the initial clinical assessment for pigmented lesions. In January 2021, the National Comprehensive Cancer Network® (“NCCN”) updated their NCCN Clinical Practice Guidelines in Oncology (“NCCN Guidelines®”) for cutaneous melanoma under “Common Follow-up Recommendations for All Patients” as Category 2A, which states the use of pre-diagnostic noninvasive genomic patch testing may be helpful to guide biopsy decisions for cutaneous melanoma. The NCCN reaffirmed their recommendation in February 2024 (version 1.2024) that “for melanocytic neoplasms that are clinically/dermoscopically suspicious for melanoma, pre-diagnostic noninvasive patch testing may also be helpful to guide biopsy decisions” for cutaneous melanoma. The NCCN’s recommendation indicated that there is uniform consensus that the intervention is appropriate. Also, recommendations and/or commentary regarding the DMT have been published by the National Society for Cutaneous Medicine (“NSCM”), the Melanoma Research Alliance (“MRA”), and UpToDate. Additionally, an independent panel of melanoma experts has produced consensus recommendations for use of the DMT. We believe the DMT can be used as an alternative for the majority of these surgical biopsy procedures.
We market our DMT directly to dermatology clinicians in the United States with a team of approximately 55 sales representatives. With coverage of the DMT by Medicare, Veterans Affairs, TRICARE, the second largest lab benefit manager in the United States, and coverage by seven of the ten largest insurers associated with the Blue Cross Blue Shield Association (“Blues plans”), more than 130 million patients have reimbursable access to the DMT. Based on the momentum of the coverage wins in 2023, as well as continued publication of clinical data, we believe the DMT is being reviewed for coverage by additional large commercial payors. We believe we will achieve successful coverage outcomes from these efforts over the next 12 to 24 months, although no assurances can be given that any reimbursement coverage approvals will be obtained, including in the event we are unable to continue as a going concern.
We process the DMT through our commercial laboratory located in San Diego, California, which is licensed by the State of California and all states requiring out-of-state licensure, including New York, which is generally considered to have a rigorous licensing process for clinical diagnostic laboratories. We believe we can scale our current laboratory facility to process over 1,000,000 DMT tests per year with additional headcount and equipment.
We believe the total annual United States market opportunity for the DMT may exceed $2.5 billion, and that the select annual worldwide market consisting of Australia, Europe, and Canada exceeds $750 million. We currently offer the DMT primarily in the United States.
We also provide contract research services using our non-invasive molecular skin analysis platform to pharmaceutical companies to facilitate the development of new therapies (including biologics) in dermatology and cancer. These companies may use our platform and services using genomic and other biomarkers to assess treatment response, monitor side effects, identify potential drug targets, and identify likely responders to the therapy under development. We have completed and have ongoing contract research services with large pharmaceutical companies. We have supported programs across the spectrum of pharmaceutical development stages from Phase 1 through Phase 3. We believe that some of these collaborations may lead to a complementary or companion diagnostic product for the pharmaceutical partner’s therapeutic candidate, if it reaches the commercial market. We have booked over $3.0 million of orders pursuant to research contracts in 2023, and many of these contracts are multi-year in length.
We have also progressed other skin cancer tests to various stages of development including for non-melanoma skin cancers (basal cell and squamous cell cancers). In the United States, approximately 12 million surgical biopsies are performed each year to diagnose approximately 5.4 million non-melanoma skin cancers. Many of the initial surgical procedures for these skin cancers are performed on cosmetically sensitive areas of the body, such as the face, neck and chest, creating significant demand for a non-invasive alternative. We believe the total market opportunity for our non-melanoma skin cancer products may exceed $3 billion in the United States and $1 billion in select world-wide markets.
We have also conducted some initial research and development regarding tests to facilitate the assessment of inflammatory skin diseases, such as atopic dermatitis and psoriasis, which may aid in the appropriate diagnosis and treatment of these conditions. The prevalence of atopic dermatitis in the United States is reported to be approximately 12% in children and 7.0% in adults with approximately 6.6 million patients having moderate-to-severe disease. The prevalence of psoriasis in the United States is approximately 2.2% with approximately 1.3 million patients having moderate to severe disease.
6
Our non-invasive specimen collection platform maximizes collection of relevant tissue with minimal patient discomfort using adhesive patches. We have developed significant intellectual property and know-how around the use of adhesives for non-invasive handling of this type of tissue sample (e.g., storage/transportation). We have developed a proprietary process that allows us to extract genomic material from the patches with sufficient quality and quantity to perform downstream analyses, including gene expression, DNA mutation and transcriptomic analyses. We believe our technology can be used to develop non-invasive tests to help inform on the clinical management for a variety of skin diseases and conditions. For example, we could attempt to develop tests to help diagnose non-melanoma skin cancer, to measure UV-induced DNA damage in healthy appearing skin, or to assess the skin microbiome. Future development efforts may also permit the introduction of our sample collection technology to facilitate evaluation of various non-dermatologic conditions where the skin can serve as a surrogate target organ.
Our Competitive Advantages
The DermTech Melanoma Test enhances pigmented lesion evaluation by differentiating high risk moles that should undergo biopsy from those that can be monitored over time. The DMT is used to assess pigmented lesions by detecting melanoma-associated genomic markers with an NPV demonstrated to be 99% or higher where the target genes are not detected. If one or both of the target genes are detected, there is a higher risk that tested lesions may be melanoma (e.g., melanoma in situ or stage 1a). In our clinical studies, the DMT has an observed sensitivity of 91-97% and a specificity of 69-91% in differentiating these early-stage melanomas from non-melanomas using expert consensus histopathologic diagnosis as the reference standard. With an NPV of 99% or higher, there is a high probability that a lesion that tests negative for melanoma is, in fact, not melanoma. We completed a long-term follow-up study directed to the DMT that also confirmed the 99% NPV by reevaluating and retesting lesions that tested negative 12 to 24 months prior to each subject’s enrollment in the study. Additionally, we completed another study ("TRUST Study"), that demonstrated that the DMT increases the PPV for melanoma diagnosis by approximately fivefold, from 3-4% for the current pathway to 18.7% for the DMT (Skelsey et al. SKIN. 2021). We have also completed our “TRUST 2 Study” as a long-term follow-up investigation to further validate the clinical utility of our test. The TRUST 2 study, which was initiated in 2021, analyzed over 20,000 patients tested with the DMT in a real-world clinical setting. Follow-up evaluations occurred for more than 5,000 tested lesions, with median and mean follow-up durations of 348 days and 337 days, respectively. Follow-up evaluations included pathology diagnoses for lesions that were biopsied and visual re-examination for lesions that were monitored rather than biopsied, in which the lesion was classified as either stable/unchanged or changed in a manner concerning for melanoma. Study results demonstrated an NPV of 99.7% for the DMT. In addition, DMT has demonstrated an approximate tenfold reduction in surgical procedures, relative to the current visual assessment and histopathology standard of care. Such a reduction can result in significant cost savings for the health care system and could reduce patient morbidity as compared to other diagnostic approaches.
7
| The DMT | Visual Assessment & Pathology (Current Standard) | ||||||||||
| Mechanism | Tumor Biology | Pattern Recognition | |||||||||
| Surgical Procedure Required | No | Yes | |||||||||
| Platform Technology | Yes | N/A | |||||||||
| Multiple Dermatologic Indications | Yes | Yes | |||||||||
| Physician Payment | Yes | Yes | |||||||||
| Simple Practice Integration | Yes | N/A | |||||||||
| Ease of Use | Yes | N/A | |||||||||
Number Needed to Biopsy(1) | 2.7 | >25 | |||||||||
Number Needed to Excise(2) | 1.6 | 5.2 | |||||||||
| Better Performance | |||||||||||
NPV(3) | ≥99% | >81-89% | |||||||||
PPV(4) | 18.7% | 4% | |||||||||
Sensitivity(5) | 91% | 65-84% | |||||||||
| Cost | $760 | (6) | $1,196 - $1,396 | ||||||||
| Capital Equipment | No | No | |||||||||
Table 1. The data summarized above compares the DMT with other techniques and the existing standard of care for assessing pigmented lesions suspicious for melanoma (Gerami et al. J Am Acad Dermatol. 2017; Jackson et al. SKIN. 2021).
Footnotes to Table 1:
(1)Number of surgical biopsies required to diagnose one melanoma.
(2)Number of wide excision surgical procedures per melanoma diagnosed.
(3)NPV measures the probability that a negative result is truly negative.
(4)PPV measures the probability that a positive result is truly positive.
(5)Sensitivity measures the proportion of actual positives that are correctly identified as such.
(6)Figure represents a projected United States reimbursed price, though this price has not yet been negotiated with all United States payors. Pricing of $760 for the PLA Code was published on December 24, 2019 as part of the CMS Laboratory Fee Schedule for 2020 and confirmed for 2024. The Medicare Final Coverage Decision was made available on December 26, 2019 and the DMT became eligible for Medicare reimbursement on February 10, 2020.
Our technology platform has the potential to transform dermatologic practice. We are the first and only company to offer non-invasive genomic testing to clinicians that practice dermatology. Current dermatologic practice is based on subjective visual assessments of cellular change that are prone to inaccuracy and lead to invasive surgical procedures that drive unnecessary costs. Our technology platform seeks to transform this paradigm by detecting changes at the genome level where cancer begins. Our product provides non-invasive, objective, and more accurate information, thereby broadening the base of clinicians that can provide dermatologic care while also improving the performance of specialists.
Ease of use. Our non-invasive Smart Sticker sample collection procedure can be performed in less than five minutes. All the necessary items, including Smart Stickers, instructions, a marking pen for outlining the lesion, and a preaddressed and prepaid return shipping label, are contained in our kit. With a clinician's order of the test, the collection procedure can also be performed at the patient’s home with clinician guidance, and where clinically appropriate.
8
Simple integration into clinical practice. Our Smart Sticker may replace the scalpel traditionally used in the initial clinical assessment of melanoma. Unlike other technologies, our platform does not require the installation and maintenance of capital equipment. Nursing support, documentation, specimen processing, and requisition are substantially similar to current practice. These advantages are critical in a busy clinical practice where clinicians see patients every five to seven minutes.
Strong intellectual property protection. We have thirteen issued United States patents, one of which is broadly directed to the use of an adhesive tape to collect skin samples for analysis of nucleic acids. In addition, we have been awarded patents directed to unique gene expression profiles and classifiers used to identify melanoma, two of which will not expire until 2029, and a third that will not expire until 2030. We also have a patent covering methods for analyzing skin samples collected using adhesive tapes, expiring in 2029. Additional efforts to further expand our patent portfolio are ongoing and a number of provisional and non-provisional patent applications have been filed. We have also developed unique know-how and proprietary processes that allow us to extract sufficient quantities of low-quality genomic material from adhesive patch samples suitable for analysis.
Our Strategy
Our goal is to become the global leader in non-invasive genomics testing for dermatologic conditions. We believe our robust intellectual property portfolio, platform technology, first-to-market advantage, and groundbreaking research will facilitate the achievement of this goal. Specifically, we will focus on the following objectives:
Build a specialized sales force to introduce our products to dermatologists. We have established a direct specialty sales force. We will recruit experienced sales representatives as needed, primarily those from the dermatology sector who have existing physician relationships. We believe we could also leverage this sales force by establishing additional distribution relationships with laboratory companies that do business with clinical dermatologists or sell molecular tests.
Secure broad reimbursement coverage for our assays. We have targeted regional and national commercial payors, as well as national government payors, to secure favorable reimbursement coverage for our test. The DMT has completed the necessary analytical validity, clinical validity, clinical utility and health economic studies that payors require of molecular tests. The cost to fully adjudicate a pigmented lesion suspicious for melanoma ranges from $1,196 to $1,396 in the United States. We believe the DMT could lead to cost savings of greater than $650 million per year in aggregate savings, based on approximately 4 million surgical biopsies performed per year to rule out melanoma if the DMT became the standard of care in the United States.
In addition to our demonstrated clinical validity, clinical utility is the most important attribute of a test for establishing coverage policies with payors because it demonstrates how frequently physicians adhere to the recommendation of the test and the resulting improvement in clinical outcomes. In 2020, we completed and published our largest clinical utility study of the DMT based on real-world commercial usage. This study involved 3,418 cases, corroborates earlier utility studies and demonstrates that clinicians adhere to the recommendation of the DMT more than 98% of the time. The DMT significantly reduces surgical procedures and improves the diagnostic pathway for pigmented lesion assessment. Lesions clinically suspicious for melanoma had negative test results in over 90% of cases, which led to an approximately 90% reduction in surgical biopsies in our 2020 study (Brouha et al. J Drugs Dermatol. 2020). In January of 2021, we published additional registry study data highlighting that use of the DMT increased PPV from approximately 4% to 18.7% (Brouha et al. SKIN. 2021). We believe our body of clinical evidence and utility data will lead to securing coverage policies from the major commercial payors over the next 12 to 24 months, although no assurances can be given that any reimbursement coverage approvals will be obtained, including in the event we are unable to continue as a going concern.
We have secured several contracts with major preferred provider networks and national governmental payers. We also received a favorable policy from the second largest lab benefit manager in the United States. We have submitted clinical and technology assessment packages to several lab benefit managers and third-party review organizations that provides consultative services for payors. We engage with national commercial payors throughout the year to evaluate coverage of the DMT.
9
Integrate our products into the standard of care. We conduct rigorous clinical research and publish the results in peer-reviewed journals. Overall, our research has yielded over 20 publications. The DMT’s performance is supported by over ten investigational studies, which enrolled an aggregate of over 9,000 patients. Our research is frequently highlighted at clinical meetings and has several times been accepted for peer-reviewed late-breaking presentations at major medical society meetings.
The AAD melanoma guidelines have indicated that non-invasive gene expression testing can be used as a part of the initial clinical assessment for pigmented lesions. In January 2021, NCCN recommended under "Common Follow-up Recommendations for All Patients" the use of pre-diagnostic noninvasive genomic patch testing may be helpful to guide biopsy decisions for cutaneous melanoma. In February 2024 (version 1.2024), NCCN reaffirmed its recommendation that "for melanocytic neoplasms that are clinically/dermoscopically suspicious for melanoma, pre-diagnostic noninvasive patch testing may also be helpful to guide biopsy decisions" for cutaneous melanoma. In addition, an independent panel of melanoma experts produced consensus recommendations for use of the DMT, which were published in 2019.
We have established an extensive group of advisors of Key Opinion Leaders (“KOLs”) in dermatology, including multiple former presidents of the AAD. These KOLs speak extensively about our technology platform and the DMT at various clinical and research meetings. In addition, these KOLs participate in our clinical studies and publish findings in peer-reviewed journals.
Establish alternate care delivery channels. We continue to expand our efforts in alternate care delivery channels including integrated primary care networks, reference labs and community primary care clinics.
These channels can help to democratize access to high quality dermatologic care, alleviate certain dermatologist capacity limitations and the related long lead times for dermatology specialist appointment availability. These channels can also improve the delivery of care to patients with ease of use, improved commute and wait times, and reduced patient fear that can accompany referrals to dermatology specialists.
Establish distribution partnerships for primary care. A substantial portion of dermatologic care is practiced in primary care. We plan to eventually access the primary care market more broadly by potentially establishing distribution relationships with companies that focus on these physicians. An ideal partner would have several hundred total sales professionals who access the primary care market with experience selling genomic diagnostic products. We have an agreement with Sonora Quest Laboratories ("Sonora Quest") that allows Sonora Quest to be the exclusive laboratory in Arizona and to offer the DMT to its vast network of healthcare providers.
Expand our marketing of research services to pharmaceutical companies. Our platform is used by several pharmaceutical companies to facilitate development of new targeted therapeutics in dermatology. Our Smart Sticker helps identify biomarkers, the assessment of which may inform treatment responses, track side effects, and identify patients that respond to therapy. We have branded our research service offering as DermTech Stratum and are offering additional services and capabilities. Our collaborations with pharmaceutical partners may result in the introduction of complementary or companion diagnostic products for the partners’ therapeutic candidates that reach the commercial market.
10
Market Opportunity – Skin Cancer
Melanoma is one of the fastest growing cancers and the subject of significant attention in the medical community. The incidence of melanoma has doubled from 1982 to 2011. While there has been a 32% decline in cancer deaths overall since 1991, melanoma is one of three cancers facing increasing death rates. According to a study from the Mayo Clinic, the incidence of melanoma increased eightfold among women under 40 and fourfold among men under 40 from 1970 to 2009.
Melanoma is one of the deadliest forms of skin cancer. On average, melanoma causes approximately one death in the United States each hour of every day of the year. The American Cancer Society projects that approximately 7,990 people will die from melanoma in 2023. If diagnosed and removed early, when confined to the outermost skin layer and deemed to be “in situ” (Stage 0), patients are expected to have a survival rate of almost 100%. Invasive melanomas that are thin and extend into the uppermost regions of the second skin layer (Stage 1) have cure rates greater than 90%. However, once the cancer advances into the deeper layers of skin, the risk of it spreading to other parts of the body, or metastasis, and death increases. The table below depicts the survival rate of melanoma based on the stage of the cancer at initial diagnosis.
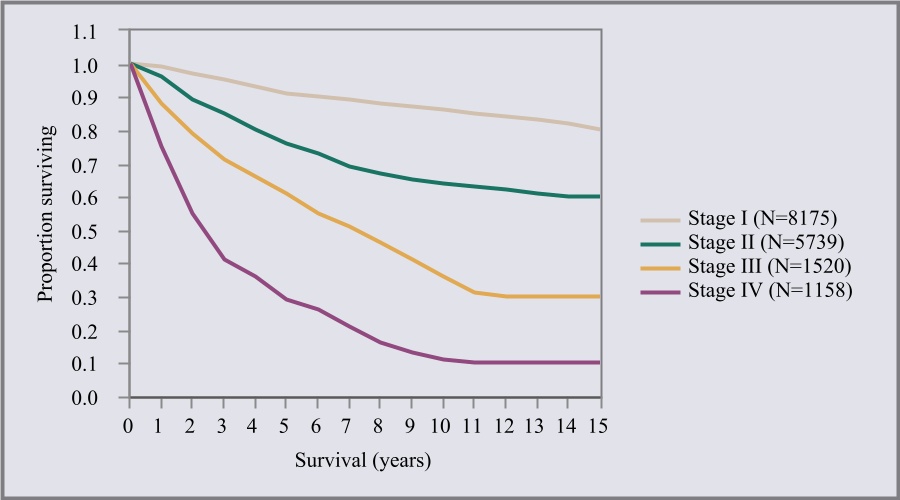
From Balch CM, Buzaid AC, Soong S-j et al: Final Version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. Journal of Clinical Oncology, August 2001.
Per the Skin Cancer Foundation, an estimated 186,680 cases of melanoma will be diagnosed in the U.S. in 2023. Of those, it is estimated that approximately half will be in situ (noninvasive), or confined to the epidermis (the top layer of skin), and the other half will be invasive, penetrating the epidermis into the skin’s second layer (the dermis). On average, 25 surgical biopsies are performed per early-stage melanoma diagnosed, creating a total market opportunity of approximately 4.0 million surgical procedures per year. Outside the United States, the incidence of melanoma is highest in Western Europe, Australia, and Canada. We estimate that these select worldwide markets perform over 1.5 million surgical biopsies annually to diagnose approximately 75,000 melanomas, creating additional market opportunity that we believe exceeds $750 million annually.
Over 5.4 million non-melanoma skin cancers (basal cell and squamous cell carcinomas) are diagnosed in the United States annually. The number of surgical biopsies needed to diagnose one non-melanoma skin cancer is approximately 2.5-3.0 among dermatologists and can be considerably higher when diagnosed by other clinicians such as nurse practitioners and primary care physicians. While these cancers are not as deadly as melanoma, they commonly occur on the face, head, neck, and other cosmetically sensitive areas, creating an important unmet medical need for a non-invasive alternative, and a potential market opportunity for non-melanoma sink cancer is approximately $3.0 billion annually in the United States based on the approximately 10-12 million surgical biopsies performed to diagnose basal and squamous cell skin cancers.
Limitations of Current Melanoma Diagnostic Pathway
The estimated prevalence of pigmented lesions (moles) ranges from 2% to 8% in fair-skinned persons.
11
Pigmented lesions may be classified as clinically atypical by meeting one or more of the American Cancer Society’s ABCDE criteria, which includes Asymmetric, irregular Border, variegated or dark Color, Diameter greater than 6 mm, or Evolving mole. Atypical pigmented lesions are at risk for harboring melanoma. A meta-analysis of case-control studies found that the relative risk of melanoma is 1.45 in patients with one atypical mole vs. those with none, and this risk increases to 6.36 in those patients with five atypical moles. Management of atypical pigmented lesions involves ruling out melanoma via a visual assessment followed by surgical biopsy and histopathology. Ideally, when melanomas are identified, they are found at the earliest stages (melanoma in situ or stage 1a) when a high cure rate is possible by wide excision. Since a biopsy only partially removes a lesion for histopathologic analysis, early-stage melanomas diagnosed histopathologically from biopsy material are treated with follow-up wide excision procedures (generally with 0.5-1.0 cm margins).
While the purpose of the visual assessment or surgical biopsy is to rule out melanoma, the subpar results of this diagnostic pathway leads to a low NPV for early-stage disease (Table 2 below). This is related to the low specificity of the visual assessment (3-10%), which results in a high number of biopsies on benign atypical nevi. During histopathologic assessment, a small number of melanomas must be identified from this large pool of biopsied atypical nevi. However, there is significant overlap in the histopathologic diagnostic criteria between atypical nevi and early-stage melanoma, invariably leading to false negative diagnoses and a relatively low sensitivity (65-84%). Elmore et al. BMJ (2017) 357:j2183, concluded that the diagnosis of early stage melanoma was not accurate after finding that 35% of slide interpretations for melanoma in situ or stage 1a melanomas by 187 pathologists received a false negative diagnosis as benign. With the prevalence of early-stage melanoma in biopsied lesions at approximately 5%, the negative predictive value ranges from 75-89%.
Welch and colleagues’ most recent N Engl J Med article (2021, 384:72-79) points out that melanoma diagnoses have increased more than 6-fold over the last 40 years. The authors attribute this increase to more frequent enhanced screening, lower pathological thresholds to label the morphologic changes as cancer, and heightened clinical awareness to biopsy pigmented lesions. However, the article fails to address the limitations of the current care standard for evaluating pigmented lesions, which relies primarily on visual atypia to guide biopsy decisions. About 4 million pigmented lesions are biopsied each year in the U.S. alone to diagnose fewer than 200,000 cutaneous melanomas (about 25 biopsies to detect 1 melanoma based on Anderson et al. JAMA Dermatol. (2018) 154(5):569-573). Using non-invasive assessment of genomic atypia offered by the DMT rather than visual atypia alone to guide pigmented lesion biopsy decisions reduces avoidable biopsies while missing fewer melanomas. Precision genomics is currently used in other areas of oncology and has changed the paradigm of treatment. Integrating use of the DMT and precision genomics to enhance early detection non-invasively into standard practice rather than performing fewer skin examinations appears to be a superior solution to the conundrum highlighted by Welch and colleagues.
According to several published papers, the real NPV of the visual assessment or surgical biopsy pathway is likely 80% to 85%. In a study by Malvehy et al., BJD (2014) 171:1099, 206 in situ and stage 1a (thickness less than 0.75 mm) melanomas were diagnosed with a sensitivity of 81% and a specificity of 10%. The prevalence of early melanoma in the study was about 10%, yielding an NPV of 83%. In addition, the current pathway using visual atypia to guide biopsy decisions suffers from a low PPV of approximately 4% for melanoma diagnosis. The addition of the DMT to the visual assessment by clinicians increased the PPV for a melanoma diagnosis by approximately five-fold to 18.7%.
| Current Pathway | DMT | ||||||||||
| Type | Surgical biopsy/ histopathology | Non-invasive gene expression | |||||||||
| NPV | 83% | 99% | |||||||||
| Probability of Mel Diagnosis | 4% | 18.7% | |||||||||
| Number Needed to Biopsy | 25 | 2.7 | |||||||||
| Number Needed to Excise | 5.2 | 1.6 | |||||||||
| Cost per Lesion Tested | $1,196 - $1,396 | $760 | |||||||||
Table 2. Data summarized above compares the key performance metrics of the DMT versus the current pathway (visual assessment and surgical biopsy/histopathology) for managing pigmented skin lesions.
12
This low NPV for the current pathway is accompanied by a high number of unnecessary surgical procedures, again driven by the poor specificity of the visual assessment. The number of surgical biopsies needed to identify one melanoma averages 25 and ranges from eight to greater than 30 depending on the clinical setting. Further, the histopathologic review of biopsied lesions is extremely limited with 2% or less of the lesion sectioned and evaluated, leaving doubt as to what may be occurring in the rest of the lesion. Consequently, lesions that have cellular atypia and positive margins are often clinically managed conservatively and subjected to full excisions with margins. However, only 0.2% to less than 1.0% of lesions with atypia and positive margins that undergo excision are diagnostically upgraded, most commonly to a higher level of atypia and rarely to melanoma in situ, and such excisions can be considered unnecessary. Approximately 5.2 excisions with margins are performed per melanoma identified, emphasizing how the current pathway of surgical biopsy and limited histopathology assessment leads to more complex and invasive excisions.
Our Products
DermTech Melanoma Test
The DMT non-invasively detects genomic markers associated with melanoma to provide a rule-out with high NPV and identify higher risk lesions suspicious for melanoma. The performance of the DMT is supported by over ten investigational studies, which enrolled over 9,000 patients and yielded over 20 peer-reviewed publications in top rated medical dermatology journals. Key studies and manuscripts are summarized in Table 3 below. The DMT is based on a platform technology for non-invasive genomic testing of the skin, which allows the molecular analysis of samples collected from adhesive patches. In contrast to the current visual assessment pathway, the DMT has a very high NPV (99% or higher) and high sensitivity (91%), ensuring a very low probability of missing melanoma (Jackson et al. SKIN. 2020).
The NPV of the DMT is supported by a 12-month follow-up study of 734 patients, which demonstrated that no melanomas were missed in the 12-month period following initial testing. In the third quarter of 2019 we initiated the TRUST study, which further examined long-term follow up of lesions previously tested negative by the DMT, and incorporated repeat testing of the previously tested lesion. This study more definitively confirmed the high NPV of the DMT in a real-world clinical setting, and we announced those topline results in December 2020. We have also completed our TRUST 2 Study, initiated in 2021, as a long-term follow-up investigation to further validate the clinical utility of our test and we announced those topline results in January 2024. The TRUST 2 study analyzed over 20,000 patients tested with the DMT in a real-world clinical setting. Study results demonstrated an NPV of 99.7% for the DMT.
In the first TRUST study, of the lesions evaluated by means of repeat testing with the DMT (n=302), none were found to have clinically obvious melanoma upon the subject’s return to the clinic, confirming the results of the initial chart review. Eighty-nine percent of these lesions were negative on repeat testing with the DMT and 11.2% were positive. Positive lesions were biopsied and subjected to a single read histopathologic review. One percent of lesions (n=3) that tested positive on repeat testing were diagnosed as Stage 0, in situ. Photographic review of the three Stage 0 cases identified changes in clinical appearance since the initial test. The pathology reports from the remaining biopsied lesions indicated a variety of non-melanoma diagnoses, including compound nevi with mild to moderate atypia. Given the early stage (in situ) of the melanomas detected on repeat testing, and length of time from the initial test (an average of 15 months), it is difficult to determine whether these melanomas evolved after the initial test or were present at the time of the initial test. In any case, the finding of three melanomas in a cohort of 302 lesions subjected to repeat testing further confirms an NPV of the DMT of at least 99.0% and is consistent with the results from the full long-term follow‑up cohort. These results exemplify how the DMT repeat testing of lesions that may have evolved over time after the initial negative DMT test has potential to identify early-stage melanoma and benefit patients. TRUST study findings corroborating the DMT’s high NPV were complemented by most recent registry data on the DMT’s high PPV (Brouha et al., SKIN, January 2021, 5(1):13-18). This data show that 316 lesions clinically suspicious for melanoma that were biopsied based on guidance offered by genomic atypia (positive DMT results rather than visual atypia alone) were enriched approximately five-fold for histopathologic features of melanoma.
During the second quarter of 2021, we announced the launch of the optional add-on test for TERT (then known as PLAplus) available to those ordering the DMT, which delivered objective and actionable information to guide clinical management decisions for skin lesions suspicious of melanoma. This add-on test combined TERT promoter DNA driver mutation analyses. TERT promoter driver mutations are associated with histopathologic features of aggressiveness and poor survival in melanoma. The combined tests elevated the sensitivity from 91% to 97% and maintained a negative predictive value of >99% (Jackson et al. SKIN. 2020). As noted above, the TERT add-on test will be discontinued as of March 1, 2024.
13
| Study | Status | Size (n) | Publication | |||||||||||||||||
| Analytical Validation | Complete | 125 | Yao Z et al. Analytical characteristics of a noninvasive gene expression assay for pigmented skin lesions. Assay Drug Dev Technol. 2016;14(6):355-363. | |||||||||||||||||
| Clinical Validation-Pathology | Complete | 555 | Gerami P et al. Development and validation of a noninvasive 2-gene molecular assay for cutaneous melanoma. J Am Acad Dermatol. 2017;76(1):114-120.e2. | |||||||||||||||||
| Clinical Validation-Driver Mutations | Complete | 626 | Ferris L et al. Noninvasive analysis of high-risk driver mutations and gene expression profiles in primary cutaneous melanoma. J Invest Dermatol. 2019; 139(5):1127-1134. | |||||||||||||||||
| Clinical Utility | Complete | 45 Derms | Ferris L et al. Utility of a noninvasive 2-gene molecular assay for cutaneous melanoma and effect on the decision to biopsy. JAMA Dermatol. 2017;153(7):675-680. | |||||||||||||||||
| Real-World Clinical Utility | Complete | 381 | Ferris L et al. Real-world performance and utility of a noninvasive gene expression assay to evaluate melanoma risk in pigmented lesions. Melanoma Res. 2018; 28(5):478-482. | |||||||||||||||||
| Real-World Clinical Utility | Complete | 1535 | Skelsey et al, Non-Invasive Detection of Genomic Atypia Increases Real-World NPV and PPV of the Melanoma Diagnostic Pathway and Reduces Biopsy Burden. SKIN. 2021 Sep; 5(5). | |||||||||||||||||
| 1-Year Follow Up | Complete | 734 | Ferris L et al. Impact on clinical practice of a non-invasive gene expression melanoma rule-out test: 12-month follow-up of negative test results and utility data from a large US registry study. Dermatology Online Journal. 2019; 25(5). | |||||||||||||||||
| Real-World Utility Registry | Complete | 1575 | Ferris L et al. Impact on clinical practice of a non-invasive gene expression melanoma rule-out test: 12-month follow-up of negative test results and utility data from a large US registry study. Dermatology Online Journal. 2019; 25(5). | |||||||||||||||||
| Real-World Utility Registry | Complete | 3418 | Brouha B et al. Real-world utility of a non-invasive gene expression test to rule out primary cutaneous melanoma: a large US registry study. J Drugs Dermatol. 2020; 19(3). Brouha B et al. Genomic atypia to enrich melanoma positivity in biopsied lesions: gene expression and pathology findings from a large U.S. registry study. SKIN 2021; 5(1):13-18. | |||||||||||||||||
| Adhesive Patch Validation | Complete | N/A | Yao Z et al. An adhesive patch-based skin biopsy device for molecular diagnostics and skin microbiome studies. J Drugs Dermatol. 2017; 16(10):611-618. | |||||||||||||||||
| Association With Severe Atypia | Complete | 103 | Jackson S et al. Risk Stratification of Severely Dysplastic Nevi by Non-Invasively Obtained Gene Expression and Mutation Analyses. SKIN. 2020 March; 4(2). | |||||||||||||||||
| Recommendations that Support DMT Use | Complete | N/A | Berman B et al. Appropriate use criteria for the integration of diagnostic and prognostic gene expression profile assays into the management of cutaneous malignant melanoma: an expert panel consensus-based modified Delphi process assessment. SKIN The Journal of Cutaneous Medicine. 2019; 3(5):291-306. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Cutaneous Melanoma. Version 1.2023 page ME-11 Swetter SM et al. Melanoma: Clinical features and diagnosis. UpToDate. Waltham, MA. September 11 2020 | |||||||||||||||||
14
| TERT Validation | Complete | 103 | Jackson Cullison, S. R., Jansen, B., Yao, Z., & Ferris, L. K. (2020). Risk Stratification of Severely Dysplastic Nevi by Non-Invasively Obtained Gene Expression and Mutation Analyses. SKIN The Journal of Cutaneous Medicine, 4(2), 124–129 | |||||||||||||||||
| Health Economics | Complete | 319 | Hornberger J, Siegel D. Clinical and economic implications of a noninvasive molecular pathology assay for early detection of melanoma. JAMA Dermatol. 2018;154(9):1-8. | |||||||||||||||||
| Genome Screen | Complete | 202 | Wachsman W et al., Noninvasive genomic detection of melanoma. British Journal of Dermatology. 2011; 164:797-806. | |||||||||||||||||
Table 3. Summarizes key clinical studies and publications supporting the DMT. Additional publications can be found on our Company website.
Smart Sticker
We are the inventor and owner of the intellectual property for the Smart Sticker collection kit (pictured below). We have contracted with a Food and Drug Administration (“FDA”) registered supplier to produce our kit under applicable quality systems requirements, and we control the exclusive distribution rights for the kit. Our Smart Sticker allows for the collection of skin samples with minimal patient discomfort. A single kit contains all of the necessary components to complete the sample collection for our analysis, including the Smart Stickers, instructions for use, a marking pen for lesion outlining, and a pre-addressed and prepaid return shipping pack. The unique properties of the Smart Sticker maximize the collection of informative cellular material for the DMT. The sample collection in the clinician’s office usually takes less than five minutes.


Telemedicine Option for the DermTech Melanoma Test
Telemedicine is typically considered to be the provision of health-related services via electronic information and telecommunication technologies. Telemedicine is sometimes used interchangeably with telehealth, but some organizations define telemedicine in a more limited sense to describe remote clinical services, such as diagnosis, treatment and monitoring. Telemedicine enables patient and clinician interaction when rural settings, lack of transport, lack of mobility, decreased funding, lack of staff or other limitations hinders or restricts in-office care.
For patients who cannot easily attend an in-office visit, we offer a remote telemedicine collection solution that enable dermatology clinicians to conduct a virtual office visit with a patient to collect a skin sample.
15
Using the remote telemedicine collection option, a clinician can choose to assess the patient’s skin and suspicious lesion(s) via a telemedicine appointment they arrange with their patient and, if indicated, submit a patient-specific order to DermTech for the DMT. In this case, a Smart Sticker Collection Kit is then mailed to the patient directly. During a follow-up telemedicine appointment, a clinician instructs and supervises the patient to collect their sample with the easy-to-use DermTech Smart Sticker. The patient then returns the collected sample(s) back to DermTech via a pre-labeled shipping envelope for analysis. Test results are made available to the ordering clinician within a few days. The telemedicine market is relatively new and unproven, especially within dermatology, and it is uncertain whether the telemedicine options for the DMT will achieve and sustain high levels of demand, consumer acceptance and market adoption.
In May 2020, SKIN, the official journal of the National Society for Cutaneous Medicine, published proof-of-concept data demonstrating that patients are able to reliably perform remote self-sampling of concerning moles using the DMT under clinical supervision via telemedicine, enabling actionable molecular testing for accurate melanoma detection. As part of the Institutional Review Board (“IRB”) approved pilot study, 258 eligible melanoma survivors were contacted, and of the 211 who expressed interest in the DMT, there were seven cases of self-identified concerning lesions, which were confirmed by a clinician to be suspicious of melanoma. These patients then conducted sample collections using DermTech’s Smart Sticker at home under the supervision of a clinician via telemedicine. Results from the study showed that skin samples collected by patients enabled successful DMT testing to objectively rule out melanoma in all of the cases evaluated. These findings are in line with sample collection results by licensed providers.
Clinical Research Services
Research on the genomic basis of diseases has increased significantly over the last decade. Genomic analysis can facilitate drug development by identifying drug targets and stratifying patients into groups that will maximize drug response. Genomic analysis is part of the effort to personalize medical therapy to patients’ individual needs. Consequently, tools to facilitate this type of research are in high demand.
We offer a suite of services to facilitate clinical research using our technology platform. We have developed a proprietary process that allows us to extract genomic and protein material from our Smart Sticker with sufficient quality and quantity to perform gene expression, DNA mutation analysis, broad scale proteomics, and transcriptomic analyses. In addition, our platform may be utilized to assess the microbiome of the skin with improved performance to some existing methods. We market these service capabilities to pharmaceutical companies developing drug products in dermatology. In addition, we can develop custom gene assays to support development for these pharmaceutical partners. We have completed and have ongoing business relationships with large pharmaceutical companies to facilitate their development of new targeted therapeutics in dermatology. Our technology platform has been deployed in Phase 1 through Phase 3 clinical programs. These efforts may also lead to the development of complementary and companion diagnostic products.
Leveraging Our Platform for Other Indications
We believe our Smart Sticker specimen collection platform is applicable to numerous other indications in dermatology. While we are currently focused on melanoma products, we may expand the use of our platform in the future to include products to diagnose or support the diagnosis of non-melanoma skin cancers as well as a variety of inflammatory skin conditions. We have identified gene expression profiles for other conditions, such as psoriasis, atopic dermatitis, and aging of the skin. Should we determine that there are viable market opportunities for these biomarker profiles, we may consider further developing these tests as commercial products. Alternatively, we may seek development partners or licensing opportunities as warranted. This also applies to the development of complementary and companion diagnostics for the pharmaceutical partners’ drug product candidates, should they reach the commercial market. In addition, because the processing of samples is the same regardless of the disease indication, our development activities could leverage our existing laboratory operations.
In the first half of 2023, we had been developing a direct to consumer ("DTC") skin cancer DNA risk assessment test (Luminate) to assess genomic changes that develop in UV damaged skin and are linked to a higher risk for developing certain carcinomas. More specifically, the product would comprise of a laboratory developed test that is ordered with physician oversight via a telemedicine platform. The test is designed to detect DNA mutations in key genes associated with a risk for developing skin cancers like basal cell carcinoma, squamous cell carcinoma, and precancerous actinic keratosis. Consumers would learn of the DNA mutation detected and the overall relative risk for the conditions linked to those mutations, which could allow them to be better informed and educated about their skin health and various options to prevent further UV damage or reparative treatments in partnership with their healthcare provider. The skin samples would be collected at-home by the consumer using a non-invasive adhesive patch collection kit. We suspended the development of the Luminate test in June 2023 as we focused on prioritizing growth opportunities for the DMT.
16
•Non-Melanoma Skin Cancer Diagnostic Products: We have identified differentially expressed genes that may allow the identification of basal cell carcinoma and we were working (and may in the future work) to identify differentially expressed genes to identify squamous cell carcinoma. Nearly 5.4 million basal and squamous cell carcinoma skin cancers are diagnosed each year making skin cancer the most common of all types of cancer leading to a potentially large commercial opportunity.
•Cutaneous T Cell Lymphoma: We also were exploring (and may in the future explore) the use of our platform technology to develop products to support the clinical management of non-melanoma skin cancer and Cutaneous T-cell lymphoma (“CTCL”) or in inflammatory and microbiome indications. As with Luminate, these activities have also been suspended as we focus or prioritizing activities that directly support DMT. CTCL is a rare type of skin cancer in which T-cells become immunologically active and attack the skin. CTCL results in rash-like skin redness, slightly raised or scaly round patches on the skin, and, sometimes, skin tumors. These features can resemble much more common inflammatory skin conditions. Several types of cutaneous T-cell lymphoma exist including mycosis fungoides and Sezary syndrome. Mycosis fungoides is the most common form of CTCL while Sezary syndrome is less common but causes skin redness across larger areas of the body. The definitive diagnosis of CTCL is often challenging because of its nonspecific clinical and pathologic features, which requires integration of clinical, histopathologic, immunophenotyping, and molecular data by the treating physician.
•Inflammatory Indications: Atopic dermatitis and psoriasis are chronic inflammatory skin diseases that affect millions of people and are characterized by both local and systemic inflammation. We have investigated gene expression profiles in the skin of atopic dermatitis and psoriasis. Responses to biologic therapy used in moderate to severe forms of these diseases can be variable and may wane over time. For example, only 30-40% of patients have a robust response to either anti-TNF alpha drugs used in psoriasis or the Th2 targeting (anti-IL-4R or anti-IL-13) drugs used in atopic dermatitis. The low response rate of these drugs creates an unmet need for drug companion and complementary diagnostic products that identify responders to a specific therapy and that monitor responses over time. Atopic dermatitis and psoriasis are largely characterized by significant epidermal inflammation that can be used to assess the benefit of interventional therapies. Due to their existing aberrant and damaged skin barrier, patients are unlikely to consent to repeated surgical biopsy procedures for the purposes of assessing therapy response. Our non-invasive genomics platform is therefore advantageous for these types of conditions because it specifically samples tissue from the epidermis.
•Microbiome Indications: The study of bacterial microbes that inhabit the skin and their relationship to health and disease has been the subject of intense investigation over the last several years. Numerous products are under development that seek to alter the composition and populations of these microbes for therapeutic purposes. We have demonstrated in studies that our platform can be used to assess the genomics of skin microbes and that the quantity of microbial genomic material are superior to the swab-based methods currently in use. In addition, our platform (which simultaneously and non-invasively collects skin host and microbiome samples) has the potential to separate and assess microbial populations at different depth levels in the epidermis. Given the growing interest in this area, we may look to develop products for this market in the future.
Sales and Marketing
The vast majority of molecular diagnostic tests are used by pathology and oncology practitioners. These markets are quickly becoming saturated with products and services. We believe that we have a unique opportunity as the first company to market a novel non-invasive molecular diagnostic test to dermatologists and other clinical practitioners of dermatology. We believe there are fewer barriers to adoption in this customer base than in other medical markets because our product fits within the current diagnostic and reimbursement pathway for various skin conditions.
We have established a sales team with extensive backgrounds in selling dermatology or molecular diagnostic products. Our Chief Commercial Officer is a proven commercial leader with over 20 years of experience driving growth through the development and commercialization of multiple novel molecular diagnostics.
There are approximately 13,000 healthcare professionals specializing in dermatology in the United States. We segment these practices into three categories: primarily cosmetic practices (10-15%), mixed medical and cosmetic practices (50-75%), and medical only practices (15-25%). We focus much of our effort on practices that deliver medical dermatology services. We have initially focused our selling activity on these accounts, which typically have a shorter adoption cycle.
17
Our sales and marketing focus includes community dermatology practices, multi-site group practices and integrated dermatology networks. Multi-site group practices and large integrated dermatology networks make up approximately 25% and 15%, respectively, of the remaining dermatology market. We are actively working to integrate the DMT in large dermatology networks in order to penetrate this market opportunity.
Dermatology is also practiced in primary care. We may target the primary care market by establishing distribution relationships with companies that focus on these physicians. These potential partners should have 400-600 sales professionals in the aggregate who access the primary care market, and ideally have experience offering a diagnostic or genomics product. We have an agreement with Sonora Quest that allows them to be the exclusive reference laboratory in Arizona to offer the DMT to its vast network of healthcare providers.
Our marketing is focused on a mix of professional targeted campaigns including in person physician education, dermatology symposia, publication distribution and, peer to peer education. We participate as an exhibitor and sponsor at key dermatology conferences. We often submit scientific abstracts for presentation at the conferences we attend. Our KOLs speak on our behalf at various medical conferences, present data from our clinical studies, and chair continuing medical education courses on genomics in dermatology, which include our products.
These efforts extend to supporting our policy coverage review process with payors. Our KOL group includes multiple former AAD presidents and numerous melanoma, skin cancer and inflammatory disease experts.
We continuously expand and improve on the validation of our tests by conducting additional clinical trials, and we publish the results of our scientific and clinical work in peer-reviewed medical journals. Through these efforts, we elevated our positioning in the AAD guidelines, obtained a recommendation from NCCN, and consensus group recommendations.
We plan to engage in the marketing of our product in other countries outside the United States only after we have established the United States market. We may focus our efforts in regions that have a high incidence of melanoma and skin cancers such as Australia and Western Europe. We may seek distribution partners in these select countries for the sales and marketing of our tests. While we have demonstrated that the stability of the skin samples collected with our Smart Sticker sampling device is suitable for shipping from countries outside the United States, we may establish clinical laboratories or laboratory partnerships in some of these countries.
Reimbursement Strategy
On January 1, 2020, the AMA released a Proprietary Laboratory Analysis code, (0089U), for the DMT. This code uniquely identifies the DMT and enables us to bill commercial and government payors when our test is ordered by a clinician.
On February 10, 2020, Medicare Administrative Contractor Palmetto GBA/MolDx issued an LCD for the Pigmented Lesion Assay (L38051), now referred to as the DMT. On June 10, 2020, Noridian, which is our Medicare Administrative Contractor, harmonized its LCD with MolDx, in effect making our test nationally covered and available for all Medicare and Medicare Advantage enrollees. The published reimbursement for our PLA code, 0089U, is $760 and was included in the 2020, 2021, 2022, 2023 and 2024 CLFS.
We have developed internal reimbursement capabilities, including claims submittal, follow-up and appeals functions to bill and collect reimbursement for services provided. We are currently out of network with many commercial payors and our initial claims are commonly denied. In situations where payment is denied, we work through the claims appeals process to secure payment for services performed. The appeals process can require several cycles and can culminate in an independent committee review for blocks of claims. We are not routinely successful in winning appealed claims.
To improve our allowed claim rate and payment, we are seeking contractual relationships and reimbursement coverage policy decisions from commercial payors. Reimbursement coverage decisions for clinical tests are primarily supported by clinical utility studies, increases in the patient experience and inclusion in guidelines.
We have secured several contracts with major preferred provider networks. We have submitted clinical and technology assessment packages to several lab benefit managers and third-party review organizations that provides consultative services to payors, and several national commercial payors that have the DMT currently under review.
18
Competition
The molecular diagnostics market is highly competitive. We compete with a number of manufacturers and distributors of molecular diagnostic tests as well as new and traditional medical devices and other technologies that are used to assist physicians with the assessment of pigmented lesions and the diagnosis of skin cancer. We are currently the only company to offer a non-invasive genomics test to clinical dermatology professionals. However, LEO Pharma A/S, a large Danish pharmaceutical company, and Mindera Corporation, a small early-stage start-up, are also working on minimally invasive genomic tests for the assessment of skin diseases. Castle Biosciences, Inc. launched gene expression assays as CLIA laboratory tests for surgical biopsy tissue specimens. Castle Biosciences, Inc. also markets a product to determine metastatic potential in later stage melanoma by utilizing surgical tissue samples.
There are several companies that market or are developing medical devices and imaging tools to detect melanoma. In general, medical devices have capital equipment costs and maintenance requirements, do not integrate well into clinical practice, and do not have clear mechanisms to provide physician payment. Strata Sciences, Inc. owns the rights to Melafind, an FDA-approved device that utilizes varying wavelengths of light to capture lesion images at different depths and conducts an algorithmic image analysis to determine the degree of lesion disorganization and the need for biopsy. The clinical trials of this device demonstrated marginal improvement in the assessment of pigmented lesions, and the device has not been adopted in the United States largely due to its specificity of less than 10%. SciBase AB is marketing an epidermal electrical impedance spectrometer to assess pigmented lesions, which received FDA approval in 2018. Verisante Technology, Inc. has received regulatory approval in Europe and Australia to market a device that uses real-time Raman spectroscopy to assess changes in the chemical composition of skin tissue. Welch-Allen, Inc. and various others manufacture dermatoscopes, which provide magnified views of a pigmented lesion during diagnosis. Caliber I.D. and others offer confocal microscopy solutions for enhanced imaging of pigmented skin lesions. DermaSensor, Inc. has an FDA-approved point-and-click handheld device that is marketed solely to primary care physicians for evaluation of suspicious lesions.
Research and Development
We have expertise in the development of gene expression profiles and other genomic analyses for the evaluation of dermatologic disease. In addition, we have developed know-how related to the collection of skin samples using adhesive patches. We have also developed expertise in statistical programs and algorithms that are used to process genomic data.
Our product development process involves several stages. The first stage involves a genome-wide screen for differential gene expression or screens for differences in mutations, methylation patterns, micro-RNAs and other factors. In the case of gene expression, differentially expressed genes are then narrowed down to specific gene sets that categorize disease states. These genes sets are then validated by comparison to clinical reference standards to produce a clinical product. We have developed substantial expertise in designing and conducting clinical validation and utility studies.
We have identified additional gene targets that may further improve the performance of the DMT. The qPCR assays for these genes may be added to our platform in the future if their performance is validated in additional clinical studies.
Intellectual Property
We have developed a comprehensive portfolio of intellectual property, currently comprised of 13 issued U.S. utility or design patents, 24 pending U.S. utility or design patent applications, three pending U.S. provisional patent applications, five pending U.S. design patent applications, five issued foreign patents, 45 pending foreign patent applications, and one Patent Cooperation Treaty (“PCT”) application.
The portfolio includes patents or patent applications directed to different aspects of our assays, a sample collection system using adhesive tapes, methods for automated scanning and cutting of cells from skin collection kits, telemedicine methods, methods of detecting nucleic acid expression, methods of quantifying a mutation burden, and methods of diagnosing or treating various skin conditions including melanoma and non-melanoma skin cancers, cutaneous T cell lymphoma, UV damage and various autoimmune disorders or inflammatory skin conditions.
Our entire intellectual property portfolio not only includes utility and design patents and patent applications, but also trademarks, design patents, trade secrets and proprietary know-how. We believe our intellectual property adequately protects our products and technology, and may prevent others from commercializing products or laboratory methods substantially similar to ours.
19
Laboratory Operations
Our CLIA laboratory located in San Diego, California, occupies approximately 13,000 square feet and is divided into an accession area, pre-qPCR-laboratory and post-qPCR-laboratory area as per CLIA standards. Access to all areas is controlled and requires gowning. The laboratory employs commercial state-of-the-art equipment including high-throughput qPCR machines. We use a laboratory information system to track all of our samples. We employ clinical laboratory scientists holding appropriate state licenses to perform all testing.
The DermTech Melanoma Test is comprised of two assays. The optional TERT add-on assay will be discontinued in March of 2024. The DMT utilizes qPCR techniques that requires the extraction and purification of genomic material from the skin adhered to our Smart Stickers. This extraction process is extremely challenging, and we have developed a proprietary method involving customized reagents and tools to provide suitable material yields reliably. In general, the process involves three main steps:
•Nucleic acid extraction using our proprietary process to maximize the yields and quantity of RNA from the cells on the Smart Sticker;
The gene expression process involves:
•reverse transcription, which converts the RNA into complementary DNA; and
•expression level quantification, using qPCR to determine the expression levels of the target genes in our expression profile.
After testing is complete, a laboratory report is prepared and reviewed by one of our California-licensed and American Board of Medical Genetics and Genomics-certified Laboratory Directors. This report is made available to the ordering physician by fax, via an internet portal, or via an electronic medical record system while adhering to requirements of the Health Insurance Portability and Accountability Act (“HIPAA”). The reports are generated in industry-standard PDF format that allows for high-definition figures to be reproduced clearly.
We continuously work to automate various steps in our end-to-end test processes. Much of this automation will come from purchasing and qualifying off-the-shelf and customized laboratory equipment such as liquid handlers and pipetting robots. We have developed a laser-cutting robot to automate the cutting of the lesion area circumscribed on the Smart Sticker by the clinician. We expect these automation efforts to improve assay throughput by reducing processing time compared to manual processing, reducing the need for direct labor, and improving quality by reducing the potential for human error.
Third-Party Suppliers and Manufacturers
We are the owner of intellectual property for the Smart Sticker with our logo and have contracted with an FDA-registered supplier to produce our specimen collection kits. We believe this kit is considered a Class I medical device and is exempt from FDA premarket notification requirements. This product is manufactured according to the FDA’s applicable quality system manufacturing requirements. Our FDA-registered supplier conducts the assembly and labeling of this kit. Most of our suppliers are high-quality medical component and finished-product suppliers accustomed to working on high volume disposable FDA-regulated products. Our product has a shelf life tested to three years that allows us to build inventory to mitigate against disruptions.
We currently have a sole source provider for the adhesive used in our Smart Sticker. We are actively working to identify second source suppliers for this component. We currently have sufficient adhesive supplies and inventory to meet our plans and objectives.
Governmental Regulation
The services that we provide are regulated by federal, state and foreign governmental authorities. Failure to comply with the applicable laws and regulations can subject us to repayment of amounts previously paid to us, significant civil and criminal penalties, loss of licensure, certification, or accreditation, or exclusion from government health care programs.
We believe our Smart Sticker, as a specimen collection device, is a Class I medical device that is exempt from obtaining premarket approval or clearance from the FDA. The FDA could declare our Smart Sticker a Class II or III device or as non-exempt. This would require us to submit an application for premarket clearance or approval, which may require us to develop additional clinical data to support premarket clearance or approval of the specimen collection product that could come at substantial expense and could disrupt our current business or affect our results of operations.
20
Our qPCR gene expression assay is a laboratory developed test (“LDT”) that is currently regulated under CLIA. Although the FDA has asserted that it has authority to regulate LDTs, it has generally exercised enforcement discretion and is not otherwise regulating most tests developed and performed within a single, high-complexity, CLIA-certified laboratory. We have commercialized our test as an LDT and will process all tests in our single CLIA-certified central laboratory. We may at some time in the future seek FDA clearance or approval for our qPCR gene expression assay. We believe the data we have collected in the development of our LDT will support any FDA medical device clearance or approval process, but cannot guarantee that the FDA will find these data sufficient to support clearance or approval as a medical device under the applicable FDA regulations. This may require us to collect additional clinical data, which could come at substantial expense and could affect our results of operations.
CLIA and State Regulation of Laboratories
Clinical laboratories must hold certain federal, state, and local licenses, certifications, and permits to conduct business. Laboratories that perform testing on human specimens for the purpose of providing information for the diagnosis, prevention, or treatment of disease are subject to CLIA. CLIA requires such laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality, and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification is also a prerequisite to be eligible to bill state and federal health care programs, as well as many private insurers, for laboratory testing services.
Standards for testing under CLIA vary based on the test and level of test complexity. Laboratories performing high complexity testing must comply with more stringent requirements than laboratories performing waived or moderate complexity testing. In addition, CLIA requires each certified laboratory to enroll in an approved proficiency-testing program if it performs testing in any category for which proficiency testing is required. Such laboratories must periodically test specimens received from an outside proficiency testing organization and then must submit the results back to that organization for evaluation. A laboratory that fails to achieve a passing score on a proficiency test may lose its right to perform testing in the category at issue. Further, failure to comply with other proficiency testing regulations, such as the prohibition on referral of a proficiency- testing specimen to another laboratory for analysis, can result in revocation of the referring laboratory’s CLIA certification.
We have a current CLIA certificate of accreditation from the CMS to perform high-complexity testing and a state license issued by California’s Department of Public Health, Laboratory Field Services. To renew our CLIA certificate, we are subject to survey and inspection every two years. We hold certificates of accreditation from the College of American Pathologists (“CAP”) and JCO, which have each been granted deeming authority by CMS. CAP and JCO are independent, non-governmental organizations that accredit laboratories nationwide on a voluntary basis. Our laboratory must comply with all CLIA requirements as well as with any additional requirements imposed by an accrediting organization. As a condition of CLIA certification, we are subject to surveys and inspections by our designated accrediting organization every other year, in addition to being subject to unannounced inspections. In mid-February 2024, we received a notification from CAP that our accreditation with CAP would not be renewed on its renewal date, and thus would terminate as of April 6, 2024. The notice did not provide a specific reason for this decision. We designated JCO as our primary accrediting organization, effective February 29, 2024.
We are also subject to certain state regulations. We hold a laboratory permit from the State of California, as well as certain states that require an out-of-sate laboratory doing business in its state to be licensed. One such state is New York, which has state licensure standards that are more stringent than CLIA and its implementing regulations.
Our laboratory is licensed by the appropriate state agencies in the states in which we do business, if such licensure is required. If a laboratory is out of compliance with state laws or regulations governing licensed laboratories, penalties for violation vary from state to state but may include suspension, limitation, revocation or annulment of the license, assessment of financial penalties or fines, or imprisonment. We believe that we are in material compliance with all applicable licensing laws and regulations.
We may become aware from time to time of other states that require out-of-state laboratories to obtain licensure to accept specimens from patients within the state, and other states may impose such requirements in the future. If we identify any other state with such requirements, or if we are contacted by any other state advising us of such requirements, we intend to follow all instructions from the state regulators regarding compliance with such requirements.
21
The FDA
The tests and testing services that we offer may be considered medical devices. Pursuant to its authority under the Federal Food, Drug, and Cosmetic Act (“FDCA”), the FDA has jurisdiction over medical devices, which are defined to include, among other things, in vitro diagnostic products, or IVDs, used for clinical purposes. The laws and regulations governing the marketing of IVDs are evolving, are extremely complex, and in many instances, there are no significant regulatory or judicial interpretations of these laws and regulations. The FDA regulates, among other things, the research, testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, marketing and promotion, and sales and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the import and export of medical devices. Many of the instruments, reagents, kits or other consumable products used within our laboratory are regulated as medical devices and therefore must comply with FDA quality system regulations and certain other device requirements. We have policies and procedures in place to ensure that we source such materials from suppliers that are in compliance with any applicable medical device regulatory requirements.
As noted above, we believe our DMT test falls within the definition of an LDT and we market it as an LDT. LDTs are a subset of IVDs that are intended for clinical use and are designed, manufactured and used within a single laboratory. Although the FDA has asserted that it has the authority to regulate LDTs that are validated by the developing laboratory and performed only by that laboratory, it has generally exercised enforcement discretion by not otherwise regulating most tests developed and performed within a single high-complexity CLIA-certified laboratory. However, in October 2023, the FDA issued a proposed rule aimed at regulating LDTs under the current medical device framework and proposing to phase out its existing enforcement discretion policy for this category of diagnostic tests; the public comment period ended in early December 2023. The proposal envisions that the LDT enforcement policy phase-out process would occur in gradual stages over a total period of four years, with premarket approval applications for high-risk tests to be submitted by the 3.5-year mark, although more details are expected to be provided with the upcoming final rule. The likelihood of the FDA finalizing the proposed rule in April 2024 (as currently projected), as well as potential litigation challenging the agency’s authority to take such action, is uncertain at this time. Affected stakeholders continue to press for a comprehensive legislative solution to create a harmonized paradigm for oversight of LDTs by both the FDA and CMS, instead of implementation of the proposed FDA administrative action, which may be disruptive to the industry and to patient access to certain diagnostic tests.
Separately, federal legislators have been working with stakeholders for several years on a possible bill to reform the regulation of in vitro clinical tests including LDTs. For example, as drafted and re-introduced for consideration by the current Congress, the Verifying Accurate, Leading-edge IVCT Development (VALID Act) would codify into law the term “in vitro clinical test” (“IVCT”) to create new medical product category separate from medical devices that includes products currently regulated as IVDs as well as LDTs. The VALID Act would also create a new system for labs and hospitals to use to submit their tests electronically to the FDA for approval, which is aimed at reducing the amount of time it takes for the agency to approve such tests, and establish a new program to expedite the development of diagnostic tests that can be used to address a current unmet need for patients.
It is unclear whether the VALID Act would be passed in Congress in its current form or if it or similar legislation would be signed into law by the President. Until the FDA finalizes LDT through the ongoing notice-and-comment rulemaking process, or the VALID Act or other legislation is passed reforming the federal government’s regulation of LDTs, it is unknown how the FDA may regulate our tests or what testing and data may be required to support any required clearance or approval.
If Congress enacts comprehensive legislation to regulate in vitro diagnostics or if the FDA finalizes its proposal to enforce medical device requirements for LDTs, or alternatively, if the FDA disagrees with our assessment that our tests fall within the definition of an LDT, we will be subject to increased regulatory burdens such as registration and listing, medical device reporting and quality control requirements. Any legislation or formal FDA regulatory framework affecting LDTs is also likely to have premarket submission requirements prohibiting commercialization without FDA authorization, controls regarding modification to the tests that may require further FDA submissions, as well as other increased regulatory burdens that will likely our ability to continue marketing our tests and to develop and introduce new tests in the future. The process would likely be costly and time-consuming. In addition, if premarket review is required for some or all of our tests, the FDA could require that we stop selling our products pending clearance or approval and conduct clinical testing prior to making submissions to the FDA to obtain premarket clearance or approval. The FDA could also require that we label our tests as investigational or limit the labeling claims we are permitted to make. We cannot assure that the DMT, or any new tests that we may develop or new uses for our products that we develop will be cleared or approved by the FDA in a timely or cost-effective manner, if at all. Even if such tests are cleared or approved, the products may not be cleared or approved for all of our proposed or desired indications. Any of these events could significantly limit the market for that product and may adversely affect our results of operations.
22
We believe that the Smart Sticker we provide for collection and transport of skin samples from a healthcare provider (or in our telemedicine option, from a patient at home with supervised collection) to our clinical laboratory is a Class I medical device subject to FDA regulations, although currently exempt from premarket review by the FDA. It is manufactured by a third party on our behalf. Class I products like our specimen collection kit are required to meet FDA’s general controls for in vitro diagnostic products, including that they be manufactured in compliance with applicable requirements of the Quality System Regulation for medical devices, adhere to device labeling requirements, and be listed with FDA upon commercial distribution, among other regulatory controls.
HIPAA and Other Privacy and Data Security Laws
HIPAA, which established for the first time comprehensive federal protection for the privacy and security of health information, was expanded and strengthened by Subtitle D of the Health Information Technology for Economic and Clinical Health Act (“HITECH”) provisions of the American Recovery and Reinvestment Act of 2009. HIPAA, as amended by HITECH, applies to health plans, healthcare clearing houses, and healthcare providers that conduct certain healthcare transactions electronically, called “Covered Entities,”, as well as individuals or entities that perform services to Covered Entities involving the use, or disclosure of, individually identifiable health information or "PHI" under HIPAA. Such service providers are called "Business Associates." Under HIPAA, as amended by HITECH, the United States Department of Health and Human Services (“HHS”) has issued regulations to protect the privacy and security of PHI (“HIPAA Regulations”). HIPAA also regulates and standardizes the codes, formats and identifiers used in certain healthcare transactions and standardization of identifiers for health plans and providers, for example insurance billing. Any non-compliance with HIPAA and HITECH and related penalties, could adversely impact our business.
HIPAA’s privacy regulations protect medical records and other PHI by limiting its use and release, giving individuals a variety of rights with respect to their PHI, including the right to access their medical records and limiting most disclosures of PHI to the minimum amount necessary to accomplish an intended purpose.
HIPAA’s security rule requires the implementation of administrative, physical, and technical safeguards and the adoption of written security policies and procedures to ensure the confidentiality, integrity and availability of PHI stored electronically. HIPAA also requires Covered Entities and Business Associates to enter into business associate agreements with certain required provisions documenting assurances of compliance with applicable HIPAA regulations by the Business Associate.
HIPAA Regulations also require breach notification. Covered Entities must report breaches of PHI that have not been encrypted or otherwise secured in accordance with guidance from the Secretary of HHS (the “Secretary”). Required breach notices must be made as soon as is reasonably practicable, but no later than sixty days following discovery of the breach. Reports must be made to affected individuals and to the Secretary and in some cases, they must be reported through local and national media, depending on the size of the breach. Large-scale breaches affecting 500 or more individuals must be immediately reported to the Secretary and are then publicly posted on an HHS website. To avoid penalties for failure to comply with breach notification provisions, we must ensure that breaches of unsecured PHI are promptly detected and reported within the company, so that we can make all required notifications on a timely basis. However, even if we make required reports on a timely basis, we may still be subject to penalties for the underlying breach and at risk of significant penalties and reputational harm if we experience a large-scale data breach.
We are currently subject to the HIPAA Regulations as a Covered Entity and maintain an active compliance program. We are subject to audit by HHS, and we may be investigated in connection with a privacy or data security complaint or reported breach. HHS may also initiate a compliance review focusing on all or any part of our HIPAA compliance program. Significant civil and criminal fines and other penalties may be imposed for violating HIPAA directly, and in connection with acts or omissions of any agents, including a downstream Business Associate, as determined according to the federal common law of agency. Civil penalties are adjusted for inflation on an annual basis and can exceed one million dollars per year for failure to comply with a HIPAA requirement. A single breach incident can violate multiple requirements. Additionally, a person who knowingly obtains or discloses PHI in violation of HIPAA may face criminal penalties, including fines and imprisonment, which increase if the wrongful conduct involves false pretenses or the intent to sell, transfer or use PHI for commercial advantage, personal gain or malicious harm. Covered entities are also subject to enforcement by state Attorneys General who were given authority to enforce HIPAA.
23
Even when HIPAA does not apply, according to the United States Federal Trade Commission (“FTC”), failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards. The FTC and states' Attorneys General have also brought enforcement actions and prosecuted some data breach cases as unfair and/or deceptive acts or practices under the FTC Act and comparable state laws. In addition to the federal privacy regulatory regime, there are a number of state laws regarding the privacy and security of health information and personal data that are applicable to clinical laboratories. The compliance requirements of these laws, including additional breach reporting requirements, and the penalties for violation vary widely and new privacy and security laws in this area are evolving. For example, several states, such as California, have implemented comprehensive privacy laws and regulations. The California Confidentiality of Medical Information Act (“CMIA”) imposes restrictive requirements regulating the use and disclosure of personally identifiable medical information. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. California’s patient privacy laws, for example, provide for penalties of up to $250,000 and permit injured parties to sue for damages. In addition to CMIA, California also enacted the California Consumer Privacy Act of 2018 (“CCPA”), which became effective January 1, 2020. The CCPA, among other things, creates data privacy obligations for covered businesses, and provides privacy rights for California residents, including the right to opt out of certain disclosures of their information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches. Although the CCPA includes limited exceptions, including for PHI maintained by a Covered Entity or Business Associate under HIPAA and medical information maintained by healthcare providers under the CMIA, it may regulate or impact our processing of personal information. Further, the California Privacy Rights Act (CPRA) went into effect on January 1, 2023, amending the CCPA. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive personal information. It also created a new California Privacy Protection Agency authorized to issue substantive regulations, which may result in increased privacy and information security enforcement in California. The CPRA also makes the personal information of our California-based employees subject to the CCPA, as amended by the CPRA, to the personal information of our California-based employees. In addition to California, more U.S. states are enacting similar consumer privacy legislation, increasing compliance complexity and increasing risks of failures to comply. As of 2023, comprehensive privacy laws were in effect in Virginia, Colorado, Connecticut, and Utah. Additional consumer privacy laws have also been enacted in Delaware, Indiana, Iowa, Montana, New Jersey, Oregon, Tennessee, and Texas, which laws will take effect over the next three years.
Many states, such as Massachusetts, have also implemented genetic testing and privacy laws imposing specific patient consent requirements and requirements for protecting test results. The interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify.
The applicability and requirements of these laws and penalties for violations vary widely. We believe that we have taken the steps required of us to comply with applicable health information, consumer privacy and security statutes and regulations in all jurisdictions, both state and federal. However, we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal laws regarding privacy or security, could result in civil and/or criminal penalties and could have a material adverse effect on our business, financial condition, results of operation and cash flows.
Federal and State Self-Referral Prohibitions
We are subject to the federal self-referral prohibitions, commonly known as the Stark Law or the Physician Self-Referral Law, and to similar state restrictions such as California’s Physician Ownership and Referral Act, commonly known as PORA. Together these restrictions generally prohibit us from billing the Medicare or Medicaid program or any patient or commercial payor for a test when the physician ordering the test, or any member of such physician’s immediate family, has an investment interest in or compensation arrangement with us, unless the arrangement meets an exception to the prohibition.
24
Both the Stark Law and PORA contain an exception for compensation paid to a physician for personal services rendered by the physician, provided that certain conditions are satisfied. We have compensation arrangements with a number of physicians for personal services, such as speaking engagements. We have structured these arrangements with terms intended to comply with the requirements of the personal services exception to the Stark Law and PORA. However, we cannot be certain that regulators would find these arrangements to be in compliance with the Stark Law, PORA or similar state laws.
These prohibitions apply regardless of the reasons for the financial relationship and the referral. No finding of intent to violate the Stark Law is required to commit a violation. In addition, knowing violations of the Stark Law may also serve as the basis for liability under the Federal False Claims Act.
Sanctions for a violation of the Stark Law include the following:
•denial of payment for the services provided in violation of the prohibition;
•refunds of amounts collected by an entity in violation of the Stark Law;
•a substantial civil penalty for each service arising out of the prohibited referral;
•exclusion from federal health care programs, including the Medicare and Medicaid programs; and
•substantial civil penalties for parties entering into a scheme to circumvent the Stark Law’s prohibition.
Further, a violation of PORA is a misdemeanor and could result in civil penalties and criminal fines. Finally, other states have self-referral restrictions with which we have to comply that differ from those imposed by federal and California law. While we have attempted to comply with the Stark Law, PORA and similar laws of other states, it is possible that some of our financial arrangements with physicians could be subject to regulatory scrutiny at some point in the future, and we cannot provide an assurance that we will be found to be in compliance with these laws following any such regulatory review.
Federal and State Fraud and Abuse Laws
Because of the significant federal funding involved in Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws to eliminate fraud and abuse in federal health care programs. Our business is subject to compliance with these laws.
Anti-Kickback Statutes
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, recommending or arranging for a good or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid.
The definition of “remuneration” has been broadly interpreted to include anything of value, including, for example, gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payment of cash, and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of services covered by the federal health care programs, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment, and possible exclusion from Medicare, Medicaid and other federal health care programs. In addition, kickback allegations can give rise to violations of the federal False Claims Act, as discussed in more detail below.
Recognizing that the Anti- Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Office of Inspector General (“OIG”) for the United States Department of Health and Human Services to issue a series of regulations known as “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, immunize the parties to the transaction or arrangement from prosecution under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, transactions and business arrangements that do not fully satisfy an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as OIG.
25
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of recipients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs. In addition, in October 2018, the Eliminating Kickbacks in Recovery Act of 2018 (“EKRA”) was enacted as part of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (“SUPPORT Act”). EKRA is an all-payor anti-kickback law that makes it a criminal offense to pay any remuneration to induce referrals to, or in exchange for, patients using the services of a recovery home, a substance use clinical treatment facility, or laboratory. Although it appears that EKRA was intended to reach patient brokering and similar arrangements to induce patronage of substance use recovery and treatment, the language in EKRA is broadly written. Further, certain of EKRA’s exceptions are inconsistent with the Anti-Kickback Statute safe harbor regulations. Significantly, EKRA permits the U.S. Department of Justice to issue regulations clarifying EKRA’s exceptions or adding additional exceptions, but such regulations have not yet been issued. Further, there is no agency guidance to indicate how and to what extent it will be applied and enforced. We cannot assure you that our relationships with physicians, sales representatives, hospitals, customers, or any other party will not be subject to scrutiny or will survive regulatory challenge under such laws. If imposed for any reason, sanctions under the EKRA could have a negative effect on our business.
Federal False Claims Act
Another development affecting the healthcare industry is the increased use of the federal False Claims Act, and in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has violated the False Claims Act and to share in any monetary recovery.
In addition, various states have enacted false claims law analogous to the False Claims Act. Some of these state laws apply where a claim is submitted to any commercial payor and not merely a federal healthcare program, and some authorize private citizens to bring suit on the state’s behalf.
When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus per-claim civil penalties, as set by statute and attorney’s fees. The civil penalty amounts are adjusted annually for inflation. Violation of the False Claims Act also can result in exclusion from government health care programs.
While we are unaware of any pending False Claims Act investigations or litigation, we are unable to predict whether we will be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly affect our financial performance.
Physician Sunshine Laws
The federal Physician Payments Sunshine Act imposes reporting requirements on manufacturers of certain devices, drugs and biologics for certain payments and transfers of value by them (and in some cases their distributors) to physicians, teaching hospitals and certain advanced non-physician health care practitioners, as well as ownership and investment interests held by physicians and their immediate family members. The reporting program (known as the Open Payments program) is administered by CMS and applies to manufacturers when their products become eligible for reimbursement under a federal healthcare program such as Medicare or Medicaid. We believe we are exempt from these reporting requirements as we manufacture our own LDTs solely for use and by or within our own laboratory. We may become subject to such reporting requirements under the terms of current CMS regulations, however, if enacted federal legislation renders our tests regulated by FDA, or if FDA finalizes its recently initiated notice-and-comment rulemaking to exercise authority over LDTs as medical devices or otherwise requires us to obtain premarket clearance or approval for one or more of our tests.
Corporate Practice of Medicine
Numerous states prohibit business corporations, such as us, from practicing medicine and employing or engaging physicians to practice medicine, generally referred to as the prohibition against the corporate practice of medicine. The prohibition against the corporate practice of medicine is designed to prevent interference in the medical decision-making process by anyone who is not a licensed physician. For example, California’s Medical Board has indicated that determining the appropriate diagnostic tests for a particular condition and taking responsibility for the ultimate overall care of a patient, including providing treatment options available to the patient, constitutes the unlicensed practice of medicine if performed by an unlicensed person. Violation of these corporate practice of medicine laws may result in civil or criminal fines, as well as sanctions imposed against the business corporation and/or the professional through licensure proceedings.
26
Civil Monetary Penalties Law
The federal Civil Monetary Penalties Law (the “CMP Law”), prohibits, among other things, (1) the offering or transfer of remuneration to a Medicare or state health care program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies; (2) employing or contracting with an individual or entity that the provider knows or should know is excluded from participation in a federal health care program; (3) billing for services requested by an unlicensed physician or an excluded provider; and (4) billing for medically unnecessary services. The penalties for violating the CMP Law include exclusion, substantial fines, and payment of up to three times the amount billed, depending on the nature of the offense.
Reimbursement and Billing
Reimbursement and billing for diagnostic services is highly complex. Laboratories must bill various payors, such as commercial insurers, including managed care organizations (“MCO”), as well as state and federal health care programs, such as Medicare and Medicaid, and each may have different billing requirements. Additionally, the audit requirements with which laboratories must comply to ensure compliance with applicable laws and regulations, as well as internal compliance policies and procedures, add further complexity to the billing process.
In April 2014, Congress passed the Protecting Access to Medicare Act of 2014 (“PAMA”), which included substantial changes to the way in which clinical laboratory services are paid under Medicare. Under PAMA, certain clinical laboratories are required to report to CMS commercial payor payment rates and volumes for their tests. Laboratories that fail to report the required payment information may be subject to substantial civil monetary penalties. Further, under PAMA, Medicare reimbursement for diagnostic tests are based on the weighted- median of the payments made by commercial payors for these tests, rendering commercial payor payment levels even more significant. As a result, future Medicare payments may fluctuate more often and become subject to the willingness of commercial payors to recognize the value of diagnostic tests generally and any given test individually.
Since December 2019, Congress has passed a series of laws to modify PAMA’s statutory requirements related to the data reporting period and phase-in of payment reductions under the CLFS for CDLTs that are not ADLTs. Most recently, the Further Continuing Appropriations and Other Extensions Act of 2024 (Pub.L. 118-22, enacted on November 16, 2023) further delayed the reporting requirement as well as the application of the 15% phase-in reduction. Under these statutory provisions, the next data reporting period for CDLTs that are not ADLTs will be January 1, 2025 through March 31, 2025, and will be based on the most recent data collection period of January 1, 2019 through June 30, 2019. After this data reporting period, the three-year data reporting cycle for these tests will resume (e.g., 2028, 2031, etc.).
The same series of laws modified the phase-in of payment reductions resulting from private payor rate implementation so that a 0.0% reduction limit was applied for calendar years 2021 through 2023, as compared to the payment amounts for a test the preceding year. The Further Continuing Appropriations and Other Extensions Act of 2024 further applied a 0.0% reduction limit for calendar year 2024. Consequently, payment may not be reduced by more than 15% per year for calendar years 2025 through 2027 as compared to the payment amounts established for a test the prior year.
We cannot predict whether or when these or other recently enacted healthcare initiatives will be implemented at the federal or state level or how any such legislation or regulation may affect us. For instance, the changes to reimbursement amounts paid by Medicare for tests such as ours based on the procedure set forth in PAMA could limit the prices we would be able to charge or the amount of available reimbursement for our tests, which would reduce our revenue. Additionally, these healthcare policy changes could be amended or additional healthcare initiatives could be implemented in the future.
Other Laws Applicable to Our Business
In some cases, we are prohibited from conducting certain tests without a certification of patient consent by the clinician ordering the test.
27
In addition, we are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste, and radioactive materials. For example, the United States Occupational Safety and Health Administration (“OSHA”) has established extensive requirements relating specifically to workplace safety for healthcare employers in the United States. This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, such as HIV and hepatitis B and C, including preventing or minimizing any exposure through needle stick injuries. For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the United States Department of Transportation, the United States Public Health Service, the United States Postal Service, and the International Air Transport Association. We generally use third-party vendors to dispose of regulated medical waste, hazardous waste, and radioactive materials and contractually requires them to comply with applicable laws and regulations.
Advertising of Laboratory Services or LDTs
Our physician-directed advertising for the DMT, the Smart Sticker and our laboratory services, as well as our direct-to-consumer advertising and social media presence, are subject to federal truth-in-advertising laws enforced by the FTC as well as comparable state consumer protection laws. Under the Federal Trade Commission Act, the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
Foreign Corrupt Practices Act
In general, the Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”) prohibits offering to pay, paying, promising to pay, or authorizing the payment of money or anything of value to a foreign official in order to influence any act or decision of the foreign official in his or her official capacity or to secure any other improper advantage in order to obtain or retain business for or with, or in order to direct business to, any person. The prohibitions apply not only to payments made to “any foreign official,” but also those made to “any foreign political party or official thereof,” to “any candidate for foreign political office” or to any person, while knowing that all or a portion of the payment will be offered, given, or promised to anyone in any of the foregoing categories. “Foreign officials” under the FCPA include officers or employees of a department, agency, or instrumentality of a foreign government. The term “instrumentality” is broad and can include state-owned or state-controlled entities. Importantly, United States authorities deem most healthcare professionals and other employees of foreign hospitals, clinics, research facilities and medical schools in countries with public healthcare and/or public education systems to be “foreign officials” under the FCPA. When we interact with foreign healthcare professionals and researchers in testing and marketing our products abroad, we must have policies and procedures in place sufficient to prevent us and agents acting on our behalf from providing any bribe, gift or gratuity, including excessive or lavish meals, travel or entertainment in connection with marketing our products and services or securing required permits and approvals. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring us to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. We have a policy entitled “Anti-Bribery and Anti-Corruption” that seeks to fully comply with the FCPA.
Foreign Regulations
When we market our tests outside of the United States, we will be subject to foreign regulatory requirements governing laboratory licensure, human clinical testing, use of tissue, privacy and data security, and marketing approval for our tests. These requirements vary by jurisdiction, differ from those in the United States, and may require us to implement additional compliance measures or perform additional pre-clinical or clinical testing. In the European Union, we may be subject to newly enacted legislation that imposes requirements and restrictions on medical devices and in vitro diagnostics.
In addition, we will also be subject to the E.U. General Data Protection Regulation (the “GDPR”) that significantly regulates the possession, use, and disclosure of personal information. In many countries outside of the United States, coverage, pricing, and reimbursement approvals are also required. We are also required to maintain accurate information and control over sales and distributors’ activities that may fall within the purview of the FCPA, our books and records provisions, and our anti-bribery provisions.
28
Employees
As of December 31, 2023, we had 208 employees, 206 of whom were full-time employees, including 14 engaged in research and development, 11 in clinical operations, 68 in general and administrative, 34 in laboratory operations, and 81 in sales and marketing. In January 2024, our board of directors approved a reduction in force of approximately 30 employees to continue to align our resources with our previously announced strategic prioritization for the DMT, further streamline operations and reduce overall operating expenses. We also engage consultants in various areas. None of our employees are represented by a labor union and we believe that our relationships with our employees and contractors are good.
Corporate and Other Information
We incorporated in the British Virgin Islands in 2015 and domesticated in the state of Delaware in 2019. DermTech Operations was incorporated in California in 1995 and reincorporated in the state of Delaware on May 15, 2014. Our commercial laboratory and our corporate office are located at 12340 El Camino Real, San Diego, CA 92130. Our telephone number is (858) 450-4222 and our website address is www.dermtech.com. We regularly post copies of our press releases as well as other information about us on our website. Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports, are available to you free of charge through the Investor Relations section of our website as soon as reasonably practicable after such materials have been electronically files with, or furnished to, the SEC. The SEC maintains an internet site (http://www.sec/gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The information contained on, or that can be accessed through, our website is not a part of this report, and our reference to the address for our website is intended to be an inactive textual reference only.
29
Item 1A. Risk Factors.
The Company is in a market environment that cannot be predicted and that involves significant risks, many of which are beyond our control. Before making a decision to invest in, hold or sell our common stock, stockholders and potential stockholders should carefully consider the risks and uncertainties described below, in addition to the other information contained in this report, as well as the other information we file with the SEC. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that case, the value of our common stock could decline and stockholders may lose all or part of their investment. Furthermore, additional risks and uncertainties of which we are currently unaware, or which we currently consider to be immaterial, could have a material adverse effect on our business, financial condition or results of operations.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in the section entitled “Risk Factors,” that represent challenges that we face in connection with the successful implementation of our strategy and growth of our business. The occurrence of one or more of the events or circumstances described in the section entitled “Risk Factors,” alone or in combination with other events or circumstances, may have an adverse effect on our business, financial condition, results of operations, and prospects. Such risks include, but are not limited to:
Risks Relating to Our Financial Condition and Capital Requirements
•We are a company with a history of net losses; we expect to incur net losses in the future and may never achieve profitability.
•We have a limited operating history and we expect a number of factors to cause our operating results to fluctuate on a quarterly and annual basis, which may make our future performance difficult to predict.
•Our commercial success could be compromised if customers do not pay our invoices or if commercial payors, including managed care organizations and Medicare, do not provide coverage and reimbursement, breach, rescind, or modify their contracts or reimbursement policies, reimburse at a low rate, or delay payments for the DMT and our planned tests.
•There is substantial doubt about our ability to continue as a going concern. If we are unable to raise additional capital when needed or on acceptable terms, we may be forced to delay, reduce and/or eliminate one or more of our business initiatives or liquidate (which could result in our stockholders not receiving full value, or receiving no value, for their investment) or, if we are successful in raising additional capital, it may be on terms that are highly dilutive to existing stockholders.
•If clinicians, including dermatologists, decide not to order the DMT or our future tests, we may be unable to generate sufficient revenue to sustain our business.
•We expect to continue to incur significant expenses to develop and market our existing and planned tests, which could make it difficult for us to achieve and sustain profitability.
•We may not be able to generate sufficient revenue from the commercialization of the DMT to achieve or sustain profitability.
•If we are unable to successfully execute our marketing strategy for the DMT and are unable to gain acceptance in the market, we may be unable to generate sufficient revenue to sustain our business.
•The telemedicine market is immature and unpredictable, and if it does not develop, if it develops more slowly than we expect, if it encounters negative publicity or if limitations on reimbursement or difficulties in obtaining regulatory approvals impede our ability to adopt telemedicine, the growth of our business will be harmed.
•If we cannot enhance our tests to keep pace with rapid advances in technology, medicine and science, our operating results and competitive position could be harmed.
•We rely on a limited number of suppliers and, in some cases, a single supplier, for certain of our laboratory substances, equipment and other materials, and any delays or difficulties securing these materials could disrupt our laboratory operations and materially harm our business.
•The DMT employs a novel diagnostic platform and may never be widely accepted by its intended markets.
•If the DMT does not perform as expected, as a result of human error or otherwise, it could have a material adverse effect on our operating results, reputation, and business.
30
•If our sole laboratory facility becomes damaged or inoperable, or we are required to vacate the facility, our ability to sell and provide molecular tests and pursue our research and development (“R&D”) efforts may be jeopardized.
•If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenues or achieve and sustain profitability.
•If we cannot support demand for the DMT, including successfully managing the evolution of our technology and manufacturing platforms, our business could suffer.
•If we were to be sued for product or professional liability, we could face substantial liabilities that exceed our resources.
•We may acquire other businesses, form joint ventures, or make investments in other companies or technologies that could harm our operating results, dilute our stockholders’ ownership, increase our debt, or cause us to incur significant expense.
•International expansion of our business would expose us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
•Intrusions into our computer systems could compromise confidential information and our ability to continue operations.
•We rely on FedEx Corporation (“FedEx”) and United Parcel Service, Inc. (“UPS”) to distribute our Smart Sticker to customers and transport specimens back to our laboratory facility, and any damage to their facilities, labor strike or inability to deliver our products could have a material and adverse effect on our results of operations and business.
Regulatory Risks Related to Our Business
•Changes in health care law and policy may have a material and adverse effect on our financial condition, results of operations, and cash flows.
•Billing for the DMT is complex, and we must dedicate substantial time and resources to the billing process to be paid for the DMT; long payment cycles and changes in claims processing practices of Medicare, Medicaid, and/or other commercial payors, or other payment delays, could hurt our cash flows and increase our need for working capital.
•Our business could be harmed by the loss, suspension, or other restriction on a license, certification, or accreditation (including the termination of our CAP accreditation scheduled for April 6, 2024), or by the imposition of a fine or penalties, under CLIA, its implementing regulations, or other state, federal, and foreign laws and regulations affecting licensure or certification, or by future changes in these laws or regulations.
•If the FDA were to begin requiring approval or clearance of the DMT and our planned future tests, or our proprietary Smart Sticker, we could incur substantial costs and time delays associated with meeting the requirements.
•If we were to be required by the FDA to conduct additional clinical studies or trials before continuing to offer tests that we have developed or may develop as LDTs those studies or trials could lead to delays or failure to obtain necessary regulatory clearance or approval, which could cause significant delays in commercializing any future products and harm our ability to achieve profitability.
•We are subject to numerous federal, local and foreign laws and regulations; complying with laws pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties and a material adverse effect to our business and operations.
Intellectual Property Risks Related to Our Business
•If we are unable to maintain intellectual property protection, our competitive position could be harmed.
Risks Related to Our Securities
•Future issuances of equity securities may dilute the interests of our security holders and reduce the price of our securities.
31
Risks Relating to Our Financial Condition and Capital Requirements
We are a company with a history of net losses; we expect to incur net losses in the future and may never achieve profitability.
We have historically incurred substantial net losses in each year since our inception, including net losses of $100.9 million for the twelve months ended December 31, 2023. As of December 31, 2023, we had an accumulated deficit of $423.9 million.
We expect our losses to continue as a result of costs relating to ongoing R&D and for increased sales and marketing costs for existing products. These losses have had, and will continue to have, an adverse effect on our working capital, total assets, and stockholders’ equity. Because of the numerous risks and uncertainties associated with our commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations, and cash flows.
We have a limited operating history and we expect a number of factors to cause our operating results to fluctuate on a quarterly and annual basis, which may make our future performance difficult to predict.
We are an emerging molecular diagnostics company with a limited operating history. Our operations to date have been primarily focused on developing and marketing our technology. We have not obtained regulatory approvals from the FDA for any of our existing and planned tests as we operate a clinical laboratory under the CLIA guidelines and believe the DMT is an LDT that is not currently being regulated by the FDA. Consequently, if regulatory approval is determined to be necessary or if Congress enacts legislation that alters the regulatory framework for LDTs, any predictions made about our future success or viability may not be as accurate as they could be if we had a longer operating history or more commercialized products. Our financial condition and operating results have varied significantly in the past and will continue to fluctuate from quarter-to-quarter or year-to-year due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include other factors described elsewhere in this report and also include:
•our ability to obtain additional funding to further develop and market our existing products and tests;
•the market adoption and demand for our existing and potential future tests;
•inflationary pressures, such as those the global market is currently experiencing, which have and may increase costs for materials, supplies, and services;
•natural disasters; political and economic instability, including wars, terrorism and political unrest, such as conflicts in the Ukraine and the Middle East; outbreak of disease; boycotts; and other business restrictions;
•the existence of favorable or unfavorable clinical guidelines for our existing and planned tests;
•the reimbursement of our existing or planned tests by Medicare and commercial payors;
•our ability to obtain and maintain any necessary regulatory approval for any of our existing and planned tests in the United States and foreign jurisdictions, if required;
•potential side effects of our existing and planned tests that could delay or prevent commercialization, limit the use of our existing and planned tests, or cause any of our commercialized tests to be taken off the market;
•our dependence on third-party suppliers and manufacturers, to supply or manufacture our specimen collection products;
•our ability to establish or maintain collaboration, licensing, or other arrangements;
•our ability to maintain and grow an effective sales and marketing infrastructure, either through the expansion of our commercial infrastructure or through strategic collaborations;
•competition from existing and planned tests or new tests that may emerge;
•the ability of patients or healthcare providers to obtain coverage of or sufficient reimbursement for our existing and planned tests;
•our ability to leverage our proprietary technology platform to discover and develop additional test candidates;
•our ability to successfully obtain, maintain, defend, and enforce intellectual property rights important to our business;
32
•our ability to attract and retain key personnel to manage our business effectively;
•our ability to build our finance infrastructure and improve our accounting systems and controls;
•potential product liability claims;
•potential liabilities associated with hazardous materials; and
•our ability to obtain and maintain adequate insurance policies.
Accordingly, the results of any quarterly or annual periods should not be relied upon as indications of future operating performance.
We expect to continue to incur increased costs as a result of operating as a public company and our management will be required to devote substantial time to compliance initiatives and corporate governance practices.
As a public company, we incur and expect to continue to incur additional significant legal, accounting and other expenses in relation to our status as a public reporting company. We may need to hire additional accounting, finance and other personnel in connection with our continuing efforts to comply with the requirements of being a public company, and our management and other personnel will need to continue to devote a substantial amount of time towards maintaining compliance with these requirements. In addition, the Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC and The Nasdaq Stock Market LLC have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”), we will be required to furnish a report by our management on our internal controls over financial reporting. However, while we remain a “smaller reporting company” and a non-accelerated filer, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we have been and will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting.
Our commercial success could be compromised if customers do not pay our invoices or if commercial payors, including managed care organizations and Medicare, do not provide coverage and reimbursement, breach, rescind, or modify their contracts or reimbursement policies, reimburse at a low rate, or delay payments for the DMT and our planned future tests.
Clinicians, including dermatologists, may not order the DMT or our planned tests unless commercial payors, such as managed care organizations and government health care programs (e.g., Medicare and Medicaid), pay a substantial portion of the test price. Coverage and reimbursement by a commercial payor may depend on a number of factors, including a payor’s determination that tests using our technologies are:
•not experimental or investigational;
•medically necessary;
•appropriate for the specific patient;
•cost-effective;
•deemed to require prior authorization;
•supported by peer-reviewed publications; and
•included in clinical practice guidelines.
33
Uncertainty surrounds commercial payor reimbursement of any test incorporating new technology, including tests developed using our technologies. Technology assessments of new medical tests conducted by research centers and other entities may be disseminated to interested parties for informational purposes. Commercial payors may use such technology assessments as grounds to deny coverage for a test or procedure. Technology assessments can include evaluation of clinical utility studies, which define how a test is used in a particular clinical setting or situation.
Because each payor generally determines for its own enrollees or insured patients whether to cover or otherwise establish a policy to reimburse the DMT, seeking payor approvals is a time-consuming and costly process. We cannot be certain that coverage for the DMT and our planned future tests will be provided in the future by additional commercial payors or that existing policy decisions or reimbursement levels will remain in place or be fulfilled under existing terms and provisions. In addition, the coding procedure used by all commercial payors with respect to establishing payment rates for various procedures, including the DMT, is complex, does not currently adapt well to the genetic tests we perform and may not enable coverage or adequate reimbursement rates for the DMT. If we cannot obtain or maintain coverage and reimbursement from commercial payors and federal health care programs such as Medicare and Medicaid for the DMT, or for new tests or test enhancements that we may develop in the future, our ability to generate revenues could be limited, which may have a material adverse effect on our financial condition, results of operations, and cash flows. Measures have been undertaken to reduce payment rates for and decrease utilization of the clinical laboratory testing generally, including PAMA, which has resulted in reduced rates on the CLFS. These reductions may also impact the DMT and tests we develop in the future. Because of the cost-trimming trends, commercial payors that cover and provide reimbursement for the DMT and our planned tests may suspend, revoke, or discontinue coverage at any time, or may reduce the reimbursement rates payable to us. Any such action could have a negative impact on our revenues, which may have a material adverse effect on our financial condition, results of operations, and cash flows. Additionally, if we are not able to obtain sufficient clinical information in support of the DMT, commercial payors could designate the DMT as experimental or investigational and decline to cover and reimburse the DMT because of this designation. As a result of these factors, obtaining approvals from commercial payors to cover the DMT and establishing adequate reimbursement levels is an unpredictable, challenging, time-consuming, and costly process, and we may never be successful. Further, we have experienced in the past, and will likely experience in the future, delays and interruptions in the receipt of payments from commercial payors due to missing documentation, changes in practices and/or other issues, which has in the past delayed and could again cause delay in recognizing our revenue.
Additionally, we are currently considered a “non-contracted provider” or “out of network” by most commercial payors because we have not entered into a specific contract to provide tests to their insured patients at specified rates of reimbursement. We also may be considered now or later to be designated as an “out of network” lab by private commercial payors, who may deny our claims in whole or in part as a result. If we were to become a contracted provider with one or more payors in the future, the amount of overall reimbursement we receive would likely decrease because we could be reimbursed less money per test performed at a contracted rate than at a non-contracted rate, which could have a negative impact on our revenues. Further, we pursue payment of patient co-payments, co-insurance and deductibles, but we typically do not collect substantial payments from patients and therefore experience overall loss to revenue as a result.
34
There is substantial doubt about our ability to continue as a going concern. We will need to raise additional capital, which may not be available on acceptable terms, if at all, to fund our existing operations, commercialize our products, and expand our operations. If we are unable to raise additional capital when and as needed, we may be required to further curtail our operations, liquidate or otherwise dispose of assets, wind-down or cease operations entirely. In these circumstances, investors may not receive full value, or any value, for their investment.
There is substantial doubt regarding our ability to continue as a going concern. As of December 31, 2023, our cash and cash equivalents totaled approximately $36.7 million and short-term marketable securities totaled $19.1 million. Based on our current business operations, we believe our current cash and cash equivalents will not be sufficient to meet our anticipated cash requirements for at least the next twelve months after the date the financial statements are issued. Based on the foregoing, we have concluded that substantial doubt exists about our ability to continue as a going concern. Based on our current operating plan, we believe that our existing cash and cash equivalents will be sufficient to fund our operating expenses and capital expenditure requirements only into the first quarter of 2025 but not to exceed 12 months from the date of issuance of the financial statements included in this Annual Report on Form 10-K. Our financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above. If we are unable to obtain additional funding on acceptable terms when and as needed, we may be forced to delay or reduce the scope of our commercial and sales activities, extend payment terms with suppliers, liquidate assets where possible at a potentially lower amount than as recorded in our financial statements, further curtail planned operations or cease operations entirely and wind down our business. Any of these could materially and adversely affect the Company's liquidity, financial condition and business prospects and, as a result, our stockholders may not receive full value, or may receive no value, for their investment. In light of our existing cash and cash equivalents and our current obligations, such a liquidation or disposition process may occur subject to bankruptcy protections, which may further reduce the value that we may receive for our assets.
During the year ended December 31, 2023, we raised aggregate gross proceeds of approximately $0.3 million, and net proceeds to the Company of approximately $0.1 million, in connection with our existing at the market offering. Our ability to utilize the $74.7 million of capacity remaining under our at the market offering sales agreement is limited by our compliance with the baby shelf rules (as defined below). As of the filing of this Annual Report on Form 10-K, our public float is less than $75 million, and under SEC regulations, for so long as our public float remains less than $75 million, the amount we can raise through primary public offerings of securities in any twelve-month period using shelf registration statements subject to Instruction I.B.6. to Form S-3 is limited to an aggregate of one-third of our public float, which is referred to as the “baby shelf rules.” As of February 23, 2024, our public float was approximately $43.2 million, based on 34,008,061 shares of outstanding common stock held by non-affiliates and at a price of $1.27 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on February 23, 2024.
We anticipate that we will need to raise additional capital through equity offerings, debt financings, collaborations, or licensing arrangements in the future in order to satisfy our anticipated liquidity requirements. We may also consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities, or for other reasons, including to:
•increase our efforts to drive market adoption of the DMT and address competitive developments;
•fund research and development activities and efforts of commercializing future products;
•acquire, license, or invest in technologies;
•acquire or invest in complementary businesses or assets; and
•finance capital expenditures and general and administrative expenses.
Our present and future funding requirements will depend on many factors, including:
•our revenue growth rate and ability to generate cash flows from operating activities;
•our sales and marketing and R&D activities;
•effects of competing technological and market developments;
•costs of and potential delays in product development;
•changes in regulatory oversight applicable to the DMT; and
•timing of and costs related to future international expansion.
35
There can be no assurances that we will be able to secure such additional financing, if at all, or on terms that are satisfactory to us, and that it will be sufficient to meet our needs. In the event that we are able to secure additional financing, the various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, convertible debt or securities convertible into equity our stockholders may experience substantial dilution and the terms of these new securities could provide for rights, preferences, or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences, and privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our platform technologies or products, or grant licenses on terms that are not favorable to us. Additional equity or debt financing might not be available on reasonable terms, if at all. We will also need to raise additional capital to expand our business to meet our long-term business objectives. Additional financing may be from the sale of equity or convertible or other debt securities in a public or private offering, from a credit facility or strategic partnership coupled with an investment in us, or a combination of both. If we are unable to raise additional funds when and as needed or on acceptable terms, our business, prospects, results of operations and potentially the price of our common stock will be adversely affected.
For further discussion of our liquidity requirements as they relate to our long-term plans, see the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations-Liquidity and Capital Resources.”
Our cash, cash equivalents and short-term marketable securities are subject to economic risk.
The Company invests its cash, cash equivalents and short-term marketable securities in domestic bank deposits, money market funds, U.S. Government debt securities, corporate debt, and certificates of deposit. Certain types of these investments are subject to general credit, liquidity, market and interest rate risks. In the event these risks caused a decline in value of any of the Company’s investments, it could adversely affect the Company’s financial condition.
We maintain cash deposits in excess of federally insured limits. Adverse developments affecting financial institutions, including bank failures, could adversely affect our liquidity and financial performance.
We maintain our cash, cash equivalents, and marketable securities with high quality, accredited financial institutions. However, some of these accounts exceed the Federal Deposit Insurance Corporation, or FDIC, insurance limit of $250,000 and, while we believe the Company is not exposed to significant credit risk due to the financial strength of these depository institutions or investments, if any such depositary institution fails to return our deposits, or if a depository institution is subject to other adverse conditions in the financial or credit markets, this could further impact access to our invested cash or cash equivalents and could adversely impact our operating liquidity and financial performance. Further, the failure or collapse of one or more of these depository institutions or default on these investments could materially adversely affect our ability to recover these assets and/or materially harm our financial condition.
If clinicians, including dermatologists, decide not to order the DermTech Melanoma Test, or our future tests, we may be unable to generate sufficient revenue to sustain our business to achieve or sustain profitability.
To generate demand for the DMT and our planned tests, we will need to educate dermatologists and other health care professionals on the clinical utility, benefits, and value of the tests we provide through published papers, presentations at scientific conferences, educational programs, and one-on-one education sessions by members of our sales force. In addition, we need to assure dermatologists of their ability to obtain and maintain adequate reimbursement coverage from commercial payors for office visits during which the specimens for the DMT are collected. Medical professionals are influenced by standard-setting bodies that influence and/or dictate the standard of care. If we are not successful in changing current guidelines from legacy standards to new molecular-based approaches our market adoption will suffer. If we cannot convince medical practitioners to order the DMT and our planned tests, we will likely be unable to create demand in sufficient volume for us to achieve profitability or meet our anticipated revenue projections.
36
We expect to continue to incur significant expenses to develop and market our existing and planned tests, which could make it difficult for us to achieve and sustain profitability.
In recent years, we have incurred significant costs in connection with the development of our existing and planned tests. For the twelve months ended December 31, 2023, our R&D expenses were $15.2 million, our sales and marketing expenses were $45.0 million and our general and administrative expenses were $43.8 million. For the twelve months ended December 31, 2022, our R&D expenses were $24.1 million, our sales and marketing expenses were $58.7 million and our general and administrative expenses were $36.1 million. Expenses may increase for the foreseeable future as we conduct studies of our existing tests, grow our sales and marketing organization, and drive adoption of and reimbursement for the DMT. As a result, we need to generate significant revenues in order to achieve profitability.
We may not be able to generate sufficient revenue from the commercialization of the DermTech Melanoma Test.
We launched the DMT, without the add-on test for TERT, during the first half of 2016 and the DMT with the add-on test for TERT in the second quarter of 2021. The optional TERT add-on assay will be discontinued in March of 2024. We believe that our commercialization success is dependent upon our ability to significantly increase the number of customers who are using the DMT. In addition, demand for the DMT may not increase as quickly as planned and we may be unable to increase our revenue levels as expected. We are currently not profitable. Even if we succeed in increasing adoption of the DMT by dermatologists, in maintaining and creating relationships with our existing and new customers, we may not be able to generate sufficient revenue to achieve or sustain profitability.
If we are unable to successfully execute our marketing strategy for the DermTech Melanoma Test and are unable to gain acceptance in the market, we may be unable to generate sufficient revenue to sustain our business.
Although we believe that the DMT represents a promising commercial opportunity, the DMT may never gain significant acceptance in the marketplace and therefore may never generate substantial revenue or profits for us. We will need to establish a market for the DMT and build that market through clinician education, awareness programs, and the publication of clinical trial results. Gaining acceptance in medical communities requires publication in leading peer-reviewed journals of results from studies using the DMT and/or our planned future tests. The process of publication in leading medical journals is subject to a peer-review process and peer-reviewers may not consider the results of our studies sufficiently novel or worthy of publication. Failure to have our studies published in peer-reviewed journals would limit the adoption of the DMT and our planned tests.
Our ability to successfully market our tests will depend on numerous factors, including:
•conducting clinical utility studies of such tests in collaboration with key thought leaders to demonstrate their use and value in important medical decisions such as treatment selection;
•the success of our sales force;
•whether health care providers believe such tests provide clinical utility;
•whether the medical community accepts that such tests are sufficiently sensitive and specific to be meaningful in patient care and treatment decisions; and
•whether health insurers, government health care programs, and other commercial payors will cover and pay for such tests and, if so, whether they will adequately reimburse us.
Failure to achieve widespread market acceptance of the DMT and our planned future tests would materially harm our business, financial condition, and results of operations.
37
The telemedicine market is immature and unpredictable, and if it does not develop, if it develops more slowly than we expect, if it encounters negative publicity or if limitations on reimbursement or difficulties in obtaining regulatory approvals impede our ability to utilize a telemedicine channel, the growth of our business will be harmed.
The DMT, can be ordered via telemedicine channels given the sample collection can be achieved at-home using the Smart Sticker Collection Kit. The telemedicine channel consists of clinicians use of third-party telemedicine technologies to assess their patients. However, it is uncertain whether these solutions, or telemedicine generally, will achieve and sustain high levels of demand, consumer acceptance and market adoption. Our success will depend to a substantial extent on the willingness of clinicians and their patients to use a telemedicine solution, as well as on our ability to demonstrate the value of a telemedicine solution to commercial payors and other purchasers of healthcare for beneficiaries. To the extent the COVID-19 pandemic continues to subside, and patient access to clinician offices for in-person testing improves, the demand for a telemedicine channel could be adversely affected. Negative publicity concerning use of a telemedicine solution or the telemedicine market as a whole could limit market acceptance. If clinicians or their patients do not believe that a telemedicine channel can provide accurate evaluation of suspicious lesions and testing using the DMT, as our clinical studies have already demonstrated, or if clinicians or their patients are not willing to utilize the clinician-supervised remote collection process due to technological limitations or otherwise then an adoption of a telemedicine solution to access the DMT may be slow to develop, or may not develop at all. Changes by state professional licensing boards to the standards of care or other requirements governing the practice of telemedicine, including any such requirements from federal regulatory bodies, could impact the growth or even adoption of a telemedicine solution. Additionally, reimbursement from governmental and commercial payors may not be available or may be too limited for physician services or laboratory testing ordered through a telemedicine channel. Similarly, individual and healthcare industry concerns or negative publicity regarding patient confidentiality and privacy in the context of telemedicine could limit market acceptance for telemedicine. If any of these events occur, it could have a material adverse effect on our business, financial condition or results of operations.
If we cannot enhance our tests to keep pace with rapid advances in technology, medicine and science, our operating results and competitive position could be harmed.
In recent years, there have been numerous advances in technologies relating to the molecular diagnosis for cancer and other medical conditions. Several new cancer drugs have been approved, including several for melanoma, and a number of new drugs in clinical development may increase patient survival time. There have also been advances in methods used to identify patients likely to benefit from these drugs based on analysis of biomarkers. We must continuously enhance our existing tests to keep pace with evolving standards of care. The DMT could become obsolete unless we continually innovate and expand them to demonstrate benefit in the diagnosis, monitoring, or prognosis of patients with cancer and other dermatologic conditions. If we cannot adequately demonstrate the applicability of the DMT to new diagnostic and treatment developments, sales of the DMT could decline, which would have a material adverse effect on our business, financial condition, results of operations and cash flows.
We rely on a limited number of suppliers and, in some cases, a single supplier, for certain of our laboratory substances, equipment and other materials, and any delays or difficulties securing these materials could disrupt our laboratory operations and materially harm our business.
We rely on a limited number of suppliers for certain of our laboratory substances, including reagents, as well as for the sequencers and various other equipment and materials we use in our laboratory operations. In particular, we rely on Thermo Fisher and VWR for supplies and Adhesive Research for our adhesive tape material. We do not have long-term supply agreements with any of our suppliers and, as a result, they could cease supplying these materials and equipment to us at any time due to an inability to reach agreement with us on supply terms, disruptions in their operations (including as a result of infectious disease outbreaks), a determination to pursue other activities or lines of business, or for other reasons, or they could fail to provide us with sufficient quantities of materials that meet our specifications. Transitioning to a new supplier or locating a temporary substitute, if any are available, would be time-consuming and expensive, could result in interruptions in or otherwise affect the performance specifications of our laboratory operations, or could require that we revalidate the DMT. In addition, the use of equipment or materials provided by a replacement supplier could require us to alter our laboratory operations and procedures as well as our research and development activities. Moreover, we believe there are currently only a few manufacturers that are capable of supplying and servicing some of the equipment and other materials necessary for our laboratory operations, including sequencers and various associated reagents. As a result, replacement equipment and materials that meet our quality control and performance requirements may not be available on reasonable terms, in a timely manner or at all. If we encounter delays or difficulties securing, reconfiguring or revalidating the equipment, reagents and other materials we require for the DMT, our operations could be materially disrupted and our business, financial condition, results of operations, and reputation could be adversely affected. As we introduce any new test, we may experience supply issues as we ramp test volume.
38
The DMT employs a novel diagnostic platform and may never be widely accepted by its intended markets.
Our future success depends on our ability to successfully commercialize the DermTech Melanoma Test. The scientific discoveries that form the basis of our proprietary technology platform and the DMT are relatively new. We are not aware of any other genomic tests such as ours and there can be no assurance that clinicians will be willing to use them. If we do not successfully develop and commercialize the DMT based upon our technological approach, we may not become profitable and the value of our common stock may decline.
The novel nature of our existing and planned tests also means that fewer people are trained in or experienced with products of this type, which may make it difficult to find, hire, and retain capable personnel for research, development, and clinical laboratory positions.
Further, our focus solely on genomic tests, as opposed to multiple, more proven technologies for patient diagnosis, increases the risks associated with the ownership of our common stock. If we do not achieve market acceptance for the DMT, we may be required to change the scope and direction of our product development activities. In that case, we may not be able to identify and implement successfully an alternative product development strategy.
If the DMT does not perform as expected, as a result of human error or otherwise, it could have a material adverse effect on our operating results, reputation, and business.
Our success depends on the market’s confidence that we can provide reliable, high-quality diagnostic results. There is no guarantee that any accuracy we have demonstrated to date will continue, particularly as the number of tests using our assays increases and if the number of different tests that we develop and commercialize expands. We believe that our customers are likely to be particularly sensitive to test defects and errors. As a result, the failure or perceived failure of our current tests to perform as expected could significantly impair our reputation and the public image of our tests or could have a material adverse effect on our business, financial condition, results of operations and cash flows.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
As part of our strategy, we expect to increase our number of employees as our business grows. This future growth could create strain on our organizational, administrative, and operational infrastructure, including laboratory operations, quality control, customer service, and sales and marketing. Our ability to manage our growth properly will require us to continue to improve our operational, financial, and management controls, as well as our reporting systems and procedures. If our current infrastructure is unable to handle our growth, we may need to further expand our infrastructure and staff and implement new reporting systems. The time and resources required to implement such expansion and systems could adversely affect our operations. Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, and integrate additional employees. Our future financial performance and our ability to commercialize our products and to compete effectively will depend, in part, on our ability to manage this potential future growth effectively, without compromising quality.
If our sole laboratory facility becomes damaged or inoperable, or we are required to vacate the facility, our ability to sell and provide molecular tests and pursue our R&D efforts may be jeopardized.
We do not have any clinical reference laboratory facilities outside of our new facility in San Diego, California. Our facilities and equipment could be harmed or rendered inoperable by natural or man-made disasters, including fire, earthquake, flooding, pandemics or other disease outbreaks and power outages, which may render it difficult or impossible for us to perform our diagnostic test for some period of time. The inability to perform the DMT or the backlog of tests that could develop if our facility is inoperable for even a short period of time, may result in the loss of customers or harm to our reputation or relationships with scientific or clinical collaborators, and we may be unable to regain those customers or repair our reputation in the future. Furthermore, our facilities and the equipment we use to perform our R&D work could be costly and time-consuming to repair or replace.
The San Diego area has recently experienced serious fires, floods and power outages, and is considered to lie in an area with earthquake and fire risk. If our sole laboratory facility is destroyed or otherwise rendered inoperable, we may have difficulty replacing or rebuilding this facility and there can be no assurance we could do so in a timely manner, on terms favorable to us or at all.
39
Additionally, a key component of our operations and R&D process involves using biological samples as the basis for the development of our diagnostic tests. In some cases, these samples are difficult to obtain. If the parts of our laboratory facility where we store these biological samples were damaged or compromised, our ability to pursue our R&D projects, as well as our reputation, could be jeopardized. We carry insurance for damage to our property and the disruption of our business, but this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
Further, if our CLIA-certified laboratory became inoperable or was destroyed, we may not be able to license or transfer our technology to another facility with the necessary state licensure and CLIA certification under which the DMT could be performed. For more information refer to the risk factor below under the heading “Our business could be harmed by the loss, suspension, or other restriction on a license, certification, or accreditation, or by the imposition of a fine or penalties, under CLIA, its implementing regulations, or other state, federal, and foreign laws and regulations affecting licensure or certification, or by future changes in these laws or regulations.” Even if we find a facility with such qualifications to perform the DMT, it may not be available to us on commercially reasonable terms. In addition, the use of a third-party laboratory to perform the DMT could affect their classification as LDTs and require us to seek FDA market authorization for the test prior to the completion of such a transfer.
If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenues or achieve and sustain profitability.
Our principal competition comes from mainstream clinical diagnostic methods, used by dermatologists for many years, which focus on visual tumor tissue analysis. It may be difficult to change the methods or behavior of dermatologists to incorporate the DMT and Smart Sticker into their practices in conjunction with, or instead of, tissue biopsies and analysis. In addition, companies offering capital equipment and kits or reagents to local dermatologists represent another source of potential competition. These tests are used directly by dermatologists, which can facilitate adoption. We plan to focus our marketing and sales efforts on medical dermatologists rather than pathologists.
We also face competition from companies that offer device products or are conducting research to develop device products for analysis of pigmented lesions. In particular, MELA Sciences, Inc., used to market its MelaFind® device to dermatologists, but we believe they no longer actively market this product. Scibase AB and Verisante Technology, Inc. have devices under development and may market their medical products directly to dermatologists if and when they obtain FDA approval. In addition to these companies, our competitors also include other device companies selling photographic technologies, whole body photography services, dermatoscopes, or confocal microscopy, such as Fotofinder, Molemate, Canfield Scientific, MedX, and Caliber I.D. Many of these groups, in addition to operating R&D laboratories, are selling equipment and devices. DermaSensor, Inc. has an FDA-approved point-and-click handheld device that is marketed solely to primary care physicians for evaluation of suspicious lesions.
In addition to these device companies, Castle Biosciences, Inc. offers an expression test for melanoma that is used on surgical biopsy specimens. Castle Biosciences, Inc. could also try and market their test as a biopsy aid at the point-of-care. Genomic testing is a relatively new area of science, especially in dermatology and we cannot predict what tests others will develop that may compete with or provide results similar or superior to the results we are able to achieve with the tests we develop. There are a number of companies that are focused on the oncology diagnostic market and expression tests including Exact Sciences Corporation, Veracyte, Inc., Guardant Health and others.
Additionally, projects related to cancer diagnostics and particularly genomics have received increased government funding, both in the United States and internationally. As more information regarding cancer genomics becomes available to the public, we anticipate that more products aimed at analyzing pigmented lesions and identifying melanoma may be developed and that these products may compete with ours. In addition, competitors may develop their own versions of our current or planned tests in countries where we did not apply for patents or where our patents have not issued or have expired and may compete with us in those countries, including encouraging the use of their test by clinicians or patients in other countries. In addition, one or more competitors may seek to invalidate or render unenforceable any of our patents in a court of competent jurisdiction or at the United States Patent and Trademark Office (“USPTO”). If any such proceeding were to be successful and result in the invalidation or unenforceability of one or more patents in our intellectual property portfolio, we may be unable to prevent unlicensed third-party competition in the marketplace with respect to our current and planned future tests.
40
Some of our present and potential competitors have widespread brand recognition and substantially greater financial and technical resources and development, production, and marketing capabilities than we do. Others may develop lower-priced, less complex tests that payors and dermatologists could view as functionally equivalent to our current or planned tests, which could force us to lower the list price of our tests and impact our operating margins and ability to achieve and maintain profitability. In addition, technological innovations that result in the creation of enhanced diagnostic tools that are more sensitive or specific than ours may enable other clinical laboratories, hospitals, clinicians, or medical providers to provide specialized diagnostic tests similar to ours in a more patient-friendly, efficient, or cost-effective manner than is currently possible. If we cannot compete successfully against current or future competitors, we may be unable to increase or create market acceptance and sales of our current or planned tests, which could prevent us from increasing or sustaining our revenues or achieving or sustaining profitability.
Our competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards, or customer requirements. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and distribution strategies and as new companies enter the market with new technologies and distribution strategies. We may not be able to compete effectively against these organizations. Our ability to compete successfully and to increase our market share is dependent upon our reputation for providing responsive, professional, and high-quality products and services and achieving strong customer satisfaction. Increased competition in the future could adversely affect our revenue, revenue growth rate, if any, margins and market share.
If we are unable to identify collaborators willing to work with us to conduct clinical utility studies, or the results of those studies do not demonstrate that a test provides clinically meaningful information and value, commercial adoption of the DMT may be slow, which would negatively impact our business.
We believe clinical utility studies will show how the DermTech Melanoma Test changes the decision-making of the dermatologist when making a surgical biopsy decision, particularly to avoid performing a surgical biopsy when the test is negative. Clinical utility studies also show the impact of the test results on patient care and management. Clinical utility studies are typically performed with collaborating dermatologists at medical centers and hospitals, analogous to a clinical trial, and generally result in peer-reviewed publications.
We are currently conducting a variety of clinical trials for the DermTech Melanoma Test and other non-melanoma tests with investigators at multiple sites in the U.S. We will need to conduct additional studies for these tests, as well as other tests we may offer in the future, to drive test adoption in the marketplace and reimbursement. Should we not be able to perform these studies, or should their results not provide clinically meaningful data and value for clinicians, including dermatologists and oncologists, adoption of our existing and planned tests could be impaired and we may not be able to obtain reimbursement for them.
We are undergoing a management transition.
We have recently experienced turnover in our executive team, with the addition of a new CEO and CCO, and the departure of our COO. Such a management transition subjects us to a number of risks, including risks pertaining to coordination of responsibilities and tasks, creation of new management systems and processes, differences in management style, effects on corporate culture, and the need for transfer of historical knowledge. In addition, our operations will be adversely affected if our management does not work together harmoniously, efficiently allocate responsibilities between themselves, or implement and abide by effective controls.
The loss of key members of our executive management team could adversely affect our business.
Our success in implementing our business strategy depends largely on the skills, experience, and performance of key members of our executive management team and others in key management positions, including Bret Christensen, the Company’s Chief Executive Officer, as well as their collective efforts. As a result of the difficulty in locating qualified new management, the loss or incapacity of existing members of our executive management team could adversely affect our operations. If we were to lose one or more of these key employees, we could experience difficulties in finding qualified successors, competing effectively, developing our technologies, and implementing our business strategy. Each member of our executive management team has an employment agreement; however, the existence of an employment agreement does not guarantee retention of the members of our executive management team and we may not be able to retain those individuals for the duration of or beyond the end of their respective terms. We do not maintain “key person” life insurance on any of our employees.
41
In addition, we rely on collaborators, consultants, and advisors, including scientific, clinical and payor advisors, to assist us in formulating our commercialization strategy. Our collaborators, consultants, and advisors are generally employed by employers other than us and may have commitments under agreements with other entities that may limit their availability to us.
The loss of a key employee, the failure of a key employee to perform in his or her current position, or our inability to attract and retain skilled employees could result in our inability to continue to grow our business or to implement our business strategy.
Most of our management has limited experience in operating a public company.
Most of our management team has limited experience in the management of a publicly traded company. Our management team may not successfully or effectively manage operating as a public company that is subject to significant regulatory oversight and reporting obligations under federal securities laws. Our limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage in that it is likely that an increasing amount of our time may be devoted to these activities which will result in less time being devoted to the management and growth of the Company. It is possible that we will be required to expand our employee base and hire additional employees to support our operations as a public company, which will increase our operating costs in future periods.
There is a scarcity of experienced professionals in our industry. If we are not able to retain and recruit personnel with the requisite technical skills, we may be unable to successfully execute our business strategy.
The specialized nature of our industry results in an inherent scarcity of experienced personnel in the field. Our future success depends upon our ability to attract and retain highly skilled personnel, including scientific, technical, laboratory, sales, marketing, business, regulatory, and administrative personnel necessary to support our anticipated growth, develop our business, and perform certain contractual obligations. Given the scarcity of professionals with the scientific knowledge that we require and the competition for qualified personnel among life science businesses, we may not succeed in attracting or retaining the personnel we require to continue and grow our operations.
Our inability to attract, hire, and retain a sufficient number of qualified sales professionals would hamper our ability to launch and increase demand for the DMT.
To succeed in selling the DMT, we must expand our sales force in the United States and/or internationally by recruiting sales representatives with extensive experience in dermatology and close relationships with medical dermatologists, dermatopathologists, and other hospital personnel. To achieve our marketing and sales goals, we will need to substantially build our sales and commercial infrastructure, with which to date we have had little experience. Sales professionals with the necessary technical and business qualifications are in high demand, and there is a risk that we may be unable to attract, hire, and retain the number of sales professionals with the right qualifications, scientific backgrounds, and relationships with decision-makers and potential customers needed to achieve our sales goals. We expect to face competition from other companies in our industry, some of whom are much larger than us and who can pay greater compensation and benefits than we can, in seeking to attract and retain qualified sales and marketing employees. If we are unable to hire and retain qualified sales and marketing personnel, our business will suffer.
We may encounter manufacturing problems or delays that could result in lost revenue.
The Smart Stickers specimen collection kits we distribute are produced by a third-party supplier. This contractor assembles several components, including the key adhesive patch trifold, into a finished product, then labels, stores, and ships this finished product to us. The adhesive tape subcomponent of the Smart Sticker is provided by a single-source third party. This tape is assembled into the individual Smart Stickers by another third-party supplier.
42
We believe we have arranged for adequate manufacturing capacity for the Smart Sticker through our third-party manufacturer. If demand for the DMT and our planned future tests increases significantly, we will need to either expand manufacturing capabilities through our existing third-party manufacturers or outsource to other manufacturers. If our third-party or other manufacturers engaged by us fail to manufacture and deliver the Smart Sticker or certain reagents in a timely manner for any reason, including as a result of supply chain failures, or they are unable to fulfil our orders due to regulatory non-compliance or other quality-related issues, our relationships with our customers could be seriously harmed. We cannot assure you that manufacturing or quality control problems will not arise as we attempt to increase the production of the Smart Sticker or that we can increase our manufacturing capabilities and maintain quality control in a timely manner or at commercially reasonable costs. If we cannot have the Smart Sticker manufactured consistently on a timely basis because of these or other factors, it could have a significant negative impact on our ability to perform tests and generate revenues.
If we cannot support demand for the DMT and our planned future tests, including successfully managing the evolution of our technology and manufacturing platforms, our business could suffer.
As the DMT volume grows, we will need to increase the DMT testing capacity, implement automation, increase our scale and related processing, customer service, billing, collection, and systems process improvements, and expand our internal quality assurance program and technology to support testing on a larger scale. We will also need additional technicians, certified laboratory scientists, and other scientific and technical personnel to process these additional tests. Any increases in scale, related improvements and quality assurance may not be successfully implemented and appropriate personnel may not be available. Failure to implement necessary procedures or to hire the necessary personnel could result in a higher cost of processing or an inability to meet market demand. We cannot assure you that we will be able to perform tests on a timely basis at a level consistent with demand, that our efforts to scale our commercial operations will not negatively affect the quality of the DMT results or that we will respond successfully to the growing complexity of the DMT testing operations. If we encounter difficulty meeting market demand or quality standards for the DMT, our reputation could be harmed and our future prospects and business could suffer, which may have a material adverse effect on our financial condition, results of operations, and cash flows.
If we were to be sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale, and use of the DMT could lead to the filing of product liability claims against us if someone alleges that our tests failed to perform as designed. We may also be subject to liability for errors in the test results we provide to clinicians or for a misunderstanding of, or inappropriate reliance upon, the information we provide. A product liability or professional liability claim could result in substantial damages and be costly and time-consuming for us to defend.
Although we believe that our existing product and professional liability insurance is adequate, our insurance may not fully protect us from the financial impact of defending against product liability or professional liability claims. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could damage our reputation, result in the recall of tests, or cause current partners to terminate existing agreements and potential partners to seek other partners, any of which could impact our results of operations.
If we use biological and hazardous materials in a manner that causes injury or violates laws or regulations, we could be liable for damages or subject to enforcement actions.
Our activities currently require the controlled use of potentially harmful biological and hazardous materials and chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, federal, state, and local laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations may become significant and could have a material adverse effect on our financial condition, results of operations and cash flows. In the event of an accident or if we otherwise fail to comply with applicable regulations, we could lose our permits or approvals or be held liable for damages or penalized with fines.
43
We may acquire other businesses, form joint ventures, or make investments in other companies or technologies that could harm our operating results, dilute our stockholders’ ownership, increase our debt, or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of businesses and assets. We also may pursue strategic alliances and joint ventures that leverage our core technology and industry experience to expand our offerings or distribution. We have no experience with acquiring other companies and limited experience with forming strategic alliances and joint ventures. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could have a material adverse effect on our financial condition, results of operations and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could have a material negative effect on our results of operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, technology license, strategic alliance, or joint venture.
To finance any acquisitions or joint ventures, we may choose to issue shares of our common stock as consideration, which would dilute the ownership of our stockholders. If the price of our common stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
International expansion of our business would expose us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
Our business strategy contemplates possible international expansion, including partnering with academic and commercial testing laboratories, and introducing the DermTech Melanoma Test outside the United States and exporting the Smart Sticker. Doing business internationally involves a number of risks, including:
•multiple, conflicting, and changing laws and regulations such as tax laws, export and import restrictions, privacy, data security and data transfer laws, employment laws, intellectual property laws, regulatory requirements, and other governmental approvals, permits and licenses;
•failure by us or our distributors to obtain regulatory approvals for the sale or use of the DMT and our planned future tests in various countries, if required;
•difficulties in managing foreign operations;
•complexities associated with managing government payor systems, multiple payor-reimbursement regimes, or self-pay systems;
•logistics and regulations associated with shipping blood samples, including infrastructure conditions and transportation delays;
•limits on our ability to penetrate international markets if the DMT and our planned future diagnostic tests cannot be processed by an appropriately qualified local laboratory;
•financial risks, such as longer payment cycles, difficulty enforcing contracts and collecting accounts receivable and exposure to foreign currency exchange rate fluctuations;
•reduced protection for intellectual property rights, or lack of them in certain jurisdictions, forcing more reliance on any trade secrets we may have, if such protection is available;
•natural or man-made disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions; and
•failure to comply with the Foreign Corrupt Practices Act, including its books and records provisions and its anti-bribery provisions, by maintaining accurate information and control over sales activities and distributors’ activities, as well as similar foreign anti-bribery and anti-corruption laws that may become applicable to our business.
Any of these risks, if encountered, could significantly harm our future international expansion and operations and, consequently, have a material adverse effect on our financial condition, results of operations, and cash flows.
44
Cybersecurity incidents, cyberattacks and other information technology failures could result in loss of data, compromise of confidential information and adverse effects to our business, financial results and our ability to continue operations.
Despite the implementation of security measures, our information technology and systems, including the Internet and related systems, may be damaged, disrupted or shut down due to cybersecurity attacks that are material and adverse to our business and operations, which are often carried out by experienced programmers or hackers, which may be able to penetrate our security. Cyberattacks include deployment of harmful malware and key loggers, ransomware, a denial-of-service attack, a malicious website and the use of other means to affect the confidentiality, integrity and availability of our technology systems and data. Cyberattacks may also be due to employee error or malfeasance, power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and our system redundancy and other disaster recovery planning may be ineffective or inadequate in preventing or responding to any of these circumstances. Techniques used in cybersecurity attacks to obtain unauthorized access, disable or sabotage information technology systems are evolving rapidly and cybersecurity events are becoming more prevalent. Any compromise of our information technology systems could result in the unauthorized access to, or acquisition or publication of our confidential business or proprietary information, patient, supplier or employee data, or other personal data or trade secrets information that are material and adverse to our business and operations and could expose us to legal claims or proceedings, liability under privacy or other laws, disruption of our operations and damage to our reputation, which could divert our management’s attention from the operation of our business and materially and adversely affect our business, revenues, and competitive position. In addition, our liability insurance may not be sufficient in type or amount to cover us against claims related to security incidents, cyberattacks, and other related cybersecurity incidents. The cost and operational consequences of implementing further data protection measures, either as a response to specific cybersecurity incidents or as a result of evolving risks, could be material. In addition, our inability to use or access our information systems at critical points in time could adversely affect the timely and efficient operation of our business.
If the security measures with respect to our telemedicine solution or the telemedicine platforms of third-party vendors that offer one or more of our tests fail or are breached, it could result in unauthorized persons accessing sensitive customer or patient data (including PHI), a loss of or damage to our data, an inability to access data sources, or process data or provide our services to our customers in a manner material to our business. In addition to risks affecting our own systems, we could also be negatively impacted by a security incident impacting a third party’s network and affecting us, such as our third-party vendors and service providers. In the event that these third parties do not adequately safeguard our data, cybersecurity incidents could result and negatively impact our business, operations and financial results. Such failures or breaches of our or our third-party vendors’ security measures, or our or our third-party vendors’ inability to effectively resolve such failures or breaches in a timely manner, could damage our reputation in a manner material to our business, adversely affect customer or investor confidence in us, and reduce the demand for our services from existing and potential customers. In addition, we could face litigation, damages for contract breach, monetary penalties, or regulatory actions for violation of applicable laws or regulations, and incur significant costs for remedial measures to prevent future occurrences and mitigate past violations that materially and adversely affect our business.
We may have to comply with laws governing the use and disclosure of genetic testing information.
Many states have adopted laws governing genetic testing and the use and disclosure of genetic test results. These laws impose specific testing consent requirements and patient authorization requirements for the use and disclosure of test results, and some impose limits on the retention and secondary use of patient samples. Many of these laws are vaguely written and some are overly broad. We must analyze and ensure compliance with the genetic testing laws in the jurisdictions from which we obtain samples and may be required to expend significant capital and other resources to ensure ongoing compliance. Our failure to comply could interfere with our ability to operate and/or lead to sanctions, fines, or other regulatory actions as well as civil claims.
45
We depend on our information technology and telecommunications systems, and any failure of these systems could harm our business.
We depend on information technology and telecommunications systems for significant aspects of our operations. In addition, our third-party billing and collections provider depends upon telecommunications and data systems provided by outside vendors and information we provide on a regular basis. These information technology and telecommunications systems support a variety of functions, including test processing, sample tracking, quality control, customer service and support, billing and reimbursement, R&D activities, and our general and administrative activities. Information technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts, and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses, and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our information technology and telecommunications systems, failures or significant downtime of our information technology or telecommunications systems, or those used by our third-party service providers could prevent us from processing tests, providing test results to oncologists, pathologists, billing payors, processing reimbursement appeals, handling patient or clinician inquiries, conducting R&D activities, and managing the administrative aspects of our business such that our business is materially and adversely harmed. Any disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could have a material effect on our business, financial condition, results of operations and cash flows.
We rely on FedEx and UPS for the distribution of our Smart Stickers to customers and to transport specimens back to our laboratory facility and, if FedEx or UPS incurs any damage to their facilities, labor strike or inability to deliver our products as needed, it could have a material and adverse effect on our results of operations and business.
We rely on FedEx and UPS for the distribution of our Smart Stickers to customers, as well as to transport patient specimens back to our laboratory facility for processing. The FedEx or UPS facilities involved in such distribution may be harmed or rendered inoperable by natural or man-made disasters, including earthquakes, power outages, communications failure, infectious disease outbreaks, severe weather, or terrorism. Any material destruction to their facilities could adversely affect the ability of FedEx or UPS to meet the needs of our customers. In addition, a disruption or slowdown in the operations of FedEx or UPS, including as a result of damage to the facilities of FedEx or UPS or a strike by FedEx or UPS employees, could cause delays in our ability to fulfill customer orders and may cause orders to be cancelled, lost, or delivered late, our shipments to be returned, or receipt of shipments to be refused, any of which could adversely affect our business and our results of operations. If our shipping costs were to increase as a result of an increase by FedEx or UPS or as a result of obtaining a new third-party logistics company and if we are unable to pass on these higher costs to our customers, it could have a material adverse effect on our results of operations and business, financial condition, results of operations and cash flows.
We may fail to achieve the expected cost savings and related benefits from our Restructuring Plans.
On June 26, 2023, our board of directors approved certain restructuring actions (the "2023 Restructuring Plan”) intended to prioritize the significant growth opportunities for the DMT, streamline operations, suspend pipeline programs and significantly reduce overall operating expenses. The 2023 Restructuring Plan primarily relates to sales, marketing and G&A functions and resulted in a workforce reduction of approximately 15% of the Company’s workforce. As part of the 2023 Restructuring Plan, the Company incurred one-time charges of $2.1 million in the second quarter of 2023. The one-time charges consist primarily of severance payments, employee benefits, and stock-based compensation for the acceleration of share-based awards. On January 29, 2024, our board of directors approved certain restructuring actions (the “2024 Restructuring Plan” and, together with the 2023 Restructuring Plan, the “Restructuring Plans”) to continue to align the Company’s resources with its previously announced strategic prioritization for the DMT. The restructuring includes operating expense reductions and a reduction in force. The 2024 Restructuring Plan primarily affected operations, but impacted the entire organization, and resulted in a workforce reduction of approximately 30 employees, or approximately 15% of the Company's workforce. The Company expects to achieve approximately $40 million in total operating expense reductions compared to fiscal 2022, from the aggregate of the Restructuring Plans.
The Company estimates that it will incur aggregate pre-tax charges of approximately $1.3 million in connection with the 2024 Restructuring Plan, primarily consisting of severance payments, employee benefits, outplacement services and related costs. The Company expects that the 2024 Restructuring Plan will be complete by the end of March 2024 and that these one-time charges will be incurred in the first quarter of 2024.
46
There is no guarantee that the Restructuring Plans will achieve their intended benefits. For example, the Company's cost restructuring efforts may not result in the anticipated savings or other economic benefits, or could result in total costs and expenses that are greater than expected. In addition, the Company may not be able to effectively realize all the cost savings anticipated by the Restructuring Plans and may incur termination and other costs not previously contemplated, which could be material. The Restructuring Plans may cause disruption to the Company's business operations. For example, the Restructuring Plans resulted in the loss of a number of long-term employees, which could result in the loss of institutional knowledge and expertise and the reallocation and combination of certain roles and responsibilities across the organization, all of which could adversely affect the Company's operations. In addition, the Restructuring Plans could negatively impact the Company's ability to attract, integrate, retain, and motivate key employees.
Inflation may adversely affect us by materially increasing our costs.
Recently, inflation has increased throughout the U.S. economy. Inflation can adversely affect us by materially increasing the costs of our suppliers, manufacturers, and other costs of doing business. We may experience material increases in the prices of labor. In an inflationary environment, cost increases may materially outpace our expectations, causing us to use our cash and other liquid assets faster than forecasted. If this happens, we may need to raise additional capital to fund our operations, which may not be available in sufficient amounts or on reasonable terms, if at all, sooner than expected.
Political uncertainty may have an adverse impact on our operating performance and results of operations.
General political uncertainty may have an adverse impact on our operating performance and results of operations. In particular, the United States continues to experience significant political events that cast uncertainty on global financial and economic markets, especially in light of the upcoming presidential election. It is presently unclear exactly what actions the new administration in the United States will implement, and if implemented, how these actions may impact the pharmaceutical and diagnostics industries in the United States. Any actions taken by a new U.S. administration may have a negative impact on the United States economies and on our business, financial conditions, and results of operations.
Regulatory Risks Related to Our Business
Changes in health care law and policy may have a material and adverse effect on our financial condition, results of operations, and cash flows.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the “ACA”), became law. This law substantially changed the way health care is financed by both governmental and commercial payors, and continues to significantly impact our industry. Future changes or additions to the ACA, the Medicare and Medicaid programs, and changes stemming from other health care reform measures, especially with regard to health care access, financing or other legislation in individual states, could have a material adverse effect on the health care industry in the U.S. The uncertainty around the future of the ACA, and in particular the impact to reimbursement levels and the number of insured individuals, may lead to delay in the purchasing decisions of our customers, which may in turn negatively impact our product sales. Further, if reimbursement levels are inadequate, our business and results of operations could be adversely affected.
In April 2014, Congress passed PAMA, which included substantial changes to the way in which clinical laboratory services are be paid under Medicare Clinical Laboratory Fee Schedule. Under PAMA, certain clinical laboratories are required to periodically report to CMS private payor payment rates and volumes for their tests, and laboratories that fail to report the required payment information may be subject to substantial civil monetary penalties. Medicare reimbursement for clinical laboratory diagnostic tests is based on the weighted-median of the payments made by private payors for these tests, rendering private payor payment levels even more significant than in the past. As a result, future Medicare payments may fluctuate more often and become subject to the willingness of private payors to recognize the value of diagnostic tests generally and any given test individually. The impact of this payment system on rates for our tests, including any current or future tests we may develop, is uncertain.
Additionally, state legislatures have increasingly passed legislation and implemented regulations designed to control the cost of health care services, including clinical laboratory and pathology services. States may pursue a variety of strategies to control spending growth, including but not limited to promoting competition, reducing prices through regulation, imposing spending targets and promoting payment reform. These cost containment strategies may result in less favorable reimbursement rates and in some cases could negatively impact our ability to change or expand our operations in certain states.
47
Further, the impact on our business of the expansion of the federal and state governments’ role in the U.S. health care industry generally, including the social, governmental and other pressures to reduce health care costs while expanding individual benefits, is uncertain. We expect that there will continue to be proposals by legislators and regulators at both the federal and state levels, as well as by commercial payors to reduce costs while expanding individual health care benefits. Certain of these changes could impose additional limitations on the prices we will be able to charge for the DMT or the amounts of reimbursement available for the DMT from governmental or commercial payors. Any future changes to legal or regulatory requirements or new cost containment initiatives could have a materially adverse effect on our business, financial condition, results of operation, and cash flows.
Our business could be adversely impacted by our failure or the failure of clinicians to comply with the ICD-10-CM Code Set.
Compliance with ICD-10-CM is required for all claims with dates of service on or after October 1, 2015. We believe we have fully implemented ICD-10-CM. However, our failure to effectively implement and apply the new code set could adversely impact our business. In addition, if clinicians fail to provide appropriate codes for desired tests, we may not be reimbursed for tests we perform.
Billing for the DMT is complex, and we must dedicate substantial time and resources to the billing process to be paid for the DMT; long payment cycles and changes in claims processing practices of Medicare, Medicaid, and/or other commercial payors, or other payment delays, could hurt our cash flows and increase our need for working capital.
Billing for clinical laboratory testing services is complex, time-consuming, and expensive. Depending on the billing arrangement and applicable law, we will bill various payors, including Medicare, Medicaid, and commercial payors, all of which have different billing requirements. As required by law or contract, we routinely bill patients for co-payments, co-insurance, and deductible amounts owed. We may also face increased risks in our collection efforts, including potential write-offs of doubtful accounts, long collection cycles, and failure by third parties to properly process payment of claims in a timely manner that could adversely affect our business, results of operations, and financial condition. Several factors make the billing practice complex, including:
•differences between the list price for the DMT and the reimbursement rates of payors;
•compliance with complex federal regulations related to Medicare billing;
•disputes among payors as to which party is responsible for payment and resistance by patients to cover any substantial amount of the payment;
•differences in coverage among payors and effect of patient co-payments, co-insurance, or deductibles;
•differences in information and billing requirements among payors;
•incorrect or missing billing information; and
•the resources required to manage the billing and claims appeals process.
Additionally, our billing activities require us to implement compliance procedures and oversight, train and monitor our employees, challenge coverage and payment denials, assist patients in appealing claims, and undertake internal audits to evaluate compliance with applicable laws and regulations as well as internal compliance policies and procedures. Payors also conduct external audits to evaluate payments and may seek refunds depending on the audit results, which adds further complexity to the billing process.
Failure to comply with these billing requirements may result in non-payment, refunds, exclusion from government healthcare programs, and civil or criminal liabilities, any of which may have a material adverse effect on our revenues and earnings. These billing complexities and the related uncertainties in obtaining reimbursement could negatively affect our cash flow and our ability to achieve profitability.
48
Our business could be harmed by the loss, suspension, or other restriction on a license, certification, or accreditation, or by the imposition of a fine or penalties, under CLIA, its implementing regulations, or other state, federal, and foreign laws and regulations affecting licensure or certification, or by future changes in these laws or regulations.
The diagnostic testing industry is subject to extensive laws and regulations, many of which have not been interpreted by the courts. CLIA requires virtually all laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality, and proficiency testing requirements intended to ensure that testing services are accurate, reliable, and timely. Our clinical laboratory must be certified under CLIA in order for us to perform testing on human specimens for diagnostic purposes. CLIA certification is also a prerequisite to be eligible to bill state and federal health care programs as well as commercial payors. Further, many commercial payors require CLIA certification as well as accreditation from specific accrediting organizations as a condition to contracting with clinical laboratories to cover their tests.
We have a current CLIA certificate of accreditation from the CMS to perform high-complexity testing and a state license issued by California’s Department of Public Health, Laboratory Field Services. To renew our CLIA certificate, we are subject to survey and inspection every two years. We hold certificates of accreditation from CAP and JCO, which have each been granted deeming authority by CMS. CAP and JCO are independent, non-governmental organization that accredit laboratories nationwide on a voluntary basis. Our laboratory must comply with all CLIA requirements as well as with any additional requirements imposed by an accrediting organization. As a condition of CLIA certification, we are subject to surveys and inspections by our designated accrediting organization every other year, in addition to being subject to unannounced inspections. In mid-February 2024, we received a notification from CAP that our accreditation with CAP would not be renewed on its renewal date, and thus would terminate as of April 6, 2024. The notice did not provide a specific reason for this decision. We designated JCO as our primary accrediting organization, effective February 29, 2024.
Sanctions for failure to comply with accreditation organization or CLIA requirements may include suspension, revocation, or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as the imposition of significant fines or criminal penalties. In addition, we are subject to regulation under state laws and regulations governing laboratory licensure. Two states, one of which is New York, have enacted state licensure laws that are more stringent than CLIA.
Failure to maintain CLIA certification, accreditation organization certification, or required state licenses could have a material adverse effect on the sales of the DMT and the results of our operations. As noted above, CAP elected to not renew and thus terminate our accreditation status effective April 6, 2024. If JCO were to terminate its accreditation with us for any reason, we may not again be able to attain CAP accreditation or accreditation from any other organization or obtain CAP accreditation or accreditation from another organization on a timely basis. To retain our CLIA certification, we must have at least one certificate of accreditation from an accrediting organization or, in the absence of a certificate of accreditation, obtain a CLIA certificate of compliance from the California Department of Public Health. If we were to lose our CLIA certification or California laboratory license, whether as a result of a revocation, suspension, or limitation, we would no longer be able to offer the DMT or any other testing, which would limit our revenues and harm our business or cause us to cease operations entirely. If we were to lose our license in any other state where we are required to hold a license, we would not be able to test specimens from those states. In addition, state and foreign requirements for laboratory certification may be costly or difficult to meet and could affect our ability to receive specimens from certain states or foreign countries. We receive specimens from all 50 U.S. states and certain provinces in Canada. Some states maintain independent licensure, registration, or certification procedures that apply to out-of-state laboratories with which we must maintain compliance in order to receive and test samples from those states. Maintaining compliance with the myriad state and foreign requirements is time consuming and resource intensive and failure to maintain compliance could result in sanctions.
Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our failure to renew a CLIA certificate, accreditation organization certificate, a state or foreign license, or accreditation, could have a material adverse effect on our business, financial condition, results of operations and cash flows. If the CLIA certificate of our laboratory is revoked, that could also impact our licensure or certification in the states or in foreign jurisdictions.
49
If the FDA were to begin requiring approval or clearance of the DMT and our planned future tests, or our proprietary specimen collection kit, we could incur substantial costs and time delays associated with meeting requirements for premarket clearance or approval.
The laws and regulations governing the marketing of diagnostic products are evolving, extremely complex and in many instances, there are no significant regulatory or judicial interpretations of these laws and regulations. Pursuant to its authority under the federal Food, Drug, and Cosmetic Act (“FDCA”), the FDA has jurisdiction over medical devices, including in vitro diagnostics and, therefore, our clinical laboratory tests; however, we believe our laboratory tests qualify as LDTs, which are currently subject to the FDA’s enforcement discretion. Among other things, pursuant to the FDCA and its implementing regulations, the FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, marketing and promotion, and sales and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. Although the FDA has asserted that it has authority to regulate the development and use of LDTs, such as our and many other laboratories’ tests, as medical devices, it has generally exercised enforcement discretion and has not otherwise enforced device regulatory requirements for most LDTs, which are designed, manufactured and performed within a single high-complexity CLIA-certified laboratory.
We believe that our tests, as utilized in our clinical laboratory. meet the requirements for classification as LDTs at this time. As a result, we believe that we are not required to obtain regulatory clearances or approvals from the FDA for our LDTs. However, in October 2023, the FDA issued a proposed rule aimed at regulating LDTs under the current medical device framework and proposing to phase out its existing enforcement discretion policy for this category of diagnostic tests. The agency’s proposal envisions that the LDT enforcement policy phase-out process would occur in gradual stages over a total period of four years, with premarket approval applications for high-risk tests to be submitted by the 3.5-year mark, although more details are expected to be provided with the upcoming final rule. The likelihood of the FDA finalizing the proposed rule in April 2024 (as currently projected), as well as potential litigation challenging the agency’s authority to take such action, is uncertain at this time. Affected stakeholders continue to press for a comprehensive legislative solution to create a harmonized paradigm for oversight of LDTs by both the FDA and CMS, instead of implementation of the proposed FDA administrative action, which may be disruptive to the industry and to patient access to certain diagnostic tests.
In addition, we believe the Smart Sticker we provide for collection and transport of skin samples from a health care provider (or in our available telemedicine option, from the patient directly) to our clinical laboratory is considered a Class I medical device subject to the FDA’s general device controls but exempt from premarket review. However, the FDA could assert the Smart Sticker is non-exempt or is a Class II or III device, which would subject it to premarket clearance or approval requirements, which could be time-consuming and expensive. While we believe that we are currently in material compliance with applicable laws and regulations, we cannot assure you that the FDA, or other regulatory agencies, would agree with our determinations. Any determination by the government that we have violated the FDCA or any FDA regulations, or a public announcement that we are being investigated for possible violations of these laws or regulations, could adversely affect our business, prospects, results of operations, or financial condition.
Even though we presently commercialize the DMT as an LDT, the DMT may in the future become subject to more onerous regulation by the FDA. For example, the FDA may disagree with our assessment that our test falls within the definition of an LDT and seek to regulate it as a medical device, or the proposed rule to regulate LDTs as medical devices may be finalized and implemented by the agency.
Separately, members of Congress have been working for the past several years on legislation to create an LDT and IVD regulatory framework that would be separate and distinct from the existing medical device regulatory framework. For example, as drafted and re-introduced for consideration by the current Congress, the Verifying Accurate, Leading-edge IVCT Development Act, or VALID Act, would codify into law the term “in vitro clinical test,” or IVCT, to create a new medical product category separate from medical device that includes all products currently regulated as IVDs as well as LDTs. The VALID Act would also create a new system for labs and hospitals to use to submit their tests electronically to the FDA for approval, which is aimed at reducing the amount of time it takes for the agency to approve such tests, and establish a new program to expedite the development of diagnostic tests that can be used to address a current unmet need for patients.
It is unclear whether the VALID Act would be passed by Congress in its current form or signed into law by President Biden. Until the FDA finalizes LDT regulations through the ongoing notice-and-comment rulemaking process, or the VALID Act or other legislation is passed reforming the federal government’s regulation of LDTs, it is unknown how the FDA may regulate our tests in the future and what testing and data may be required to support any required clearance or approval.
50
Whether as a result of new legislative authority or following formal notice-and-comment rulemaking, if the FDA begins to enforce new regulatory requirements for LDTs, or if the FDA disagrees with our assessment that the DMT is an LDT, the DMT could for the first time be subject to a variety of regulatory requirements, including registration and listing, medical device reporting, and quality control. We also could be required to obtain premarket clearance or approval for our existing test and any new tests we are developing or may develop, which may force us to cease marketing the DMT until we obtain the required clearance or approval. The premarket review process for diagnostic products can be lengthy, expensive, time-consuming, and unpredictable. Further, obtaining premarket clearance or approval may involve, among other things, successfully completing clinical trials. Clinical trials require significant time and cash resources and are subject to a high degree of risk, including risks of experiencing delays, failing to complete the trial or obtaining unexpected or negative results. If we are required to obtain premarket clearance or approval and/or conduct premarket clinical trials, our development costs could significantly increase, our introduction of any new tests we may develop may be delayed, and sales of our existing test could be interrupted or stopped. Any of these outcomes could reduce our revenue or increase our costs and materially adversely affect our business, prospects, results of operations, or financial condition. Moreover, any cleared or approved labeling claims may not be consistent with our current claims or adequate to support continued adoption of and reimbursement for the DMT. For instance, if we are required by the FDA to label the DMT as investigational, or if labeling claims the FDA allows us to make are limited, order levels may decline and reimbursement may be adversely affected. As a result, we could experience significantly increased development costs and a delay in generating additional revenue from our existing test or from tests we may develop. Until the FDA finalizes its regulatory position regarding LDTs, or federal legislation is passed concerning regulation of LDTs, it is unknown how the FDA may regulate the DMT in the future and what testing and data may be required to support any required clearance or approval as a medical device or an “in vitro clinical test” (as that category is being defined in the as-introduced VALID Act).
The requirement of premarket review could negatively affect our business until such review is completed and regulatory clearance or approval is obtained. The FDA could require that we stop selling the DMT pending premarket clearance or approval. The regulatory authorization process may involve, among other things, successfully completing additional clinical trials and making a premarket submission, such as a 510(k) notification, a premarket approval application (“PMA”), or a de novo device classification request to the FDA. If the FDA requires any form of premarket review, the DMT may not be cleared or approved on a timely basis, if at all. We may also decide voluntarily to pursue FDA premarket review and authorization of the DMT if we determine that doing so would be appropriate.
Additionally, should future regulatory actions affect any of the reagents we obtain from suppliers and use in conducting the DMT, our business could be adversely affected in the form of increased costs of testing or delays, limits, or prohibitions on the purchase of reagents necessary to perform DMT testing. While we qualify all materials used in our products in accordance with the regulations and guidelines of CLIA, the FDA could promulgate regulations or issue guidance for industry that may impact our ability to purchase materials necessary for the performance of the DMT. If any of the reagents we obtain from suppliers and use in the DMT are affected by future regulatory actions, our business could be adversely affected, including by increasing the cost of testing or delaying, limiting, or prohibiting the purchase of reagents necessary to perform testing with our products.
Failure to comply with any applicable FDA requirements could trigger a range of enforcement actions by the FDA, including but not limited to warning letters, civil monetary penalties, injunctions, criminal prosecution, recall or seizure, operating restrictions, partial suspension or total shutdown of operations and denial of or challenges to applications for clearance or approval, as well as significant adverse publicity.
51
If we were to be required by the FDA to conduct additional clinical studies before continuing to offer tests that we have developed or may develop as LDTs, those studies could lead to delays or failure to obtain necessary regulatory clearance or approval, which could cause significant delays in commercializing any future products and harm our ability to achieve profitability.
If the FDA decides to require that we obtain 510(k) clearance, premarket approvals pursuant to a PMA, or any other type of premarket authorization in order for us to commercialize our current DMT or our planned future tests, whether as a result of new legislative authority or following finalization and implementation of the October 2023 proposed rule or based on its determination that any of those tests does not meet the definition of an LDT, we may be required to conduct additional clinical testing before submitting a regulatory submission for commercial marketing authorization. In addition, as part of our long-term strategy we may plan to seek FDA clearance or approval for certain genomic tests in order to permit them to be offered by other clinical laboratories in addition to our own; however, we would need to conduct additional clinical validation activities on the DMT before we could submit an application for FDA approval or clearance. Clinical trials to support marketing authorization from the FDA must be conducted in compliance with various regulatory requirements, including investigational device exemption regulations and good clinical practices, or else the FDA may take certain enforcement actions or reject the data. We believe it would likely take two years or more to conduct the clinical studies necessary to obtain approval from the FDA to commercially launch the DMT and our planned future tests outside of our clinical laboratory.
Even if clinical trials are completed as planned, we cannot be certain that their results would be able to support the DMT claims or that the FDA or foreign authorities will agree with our conclusions regarding the results of our clinical trials. Success in early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior clinical trials and studies. If we are required to conduct clinical trials to support a premarket submission to the FDA, whether using prospectively acquired samples or archival samples, delays in the commencement or completion of clinical testing could significantly increase the development costs for the DMT or our planned future tests and delay commercialization. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites and the eligibility criteria for the clinical trial. Moreover, the clinical trial process may fail to demonstrate that the DMT and our planned future tests are effective for the proposed indications for use, which could cause us to abandon a test candidate and may delay development of other tests.
We may find it necessary to engage contract research organizations to perform data collection and analysis and other aspects of our clinical trials, which would increase the cost and complexity of our trials. We would also depend on clinical investigators, medical institutions, and contract research organizations to perform the trials properly. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, or if the quality, completeness, or accuracy of the data or results they obtain is compromised due to the failure to adhere to our clinical protocols or applicable regulations or for other reasons, our clinical trials may have to be extended, delayed or terminated. Many of these factors would be beyond our control. We may not be able to enter into replacement arrangements without undue delays or considerable expenditures. If there are delays in clinical testing or human research approvals as a result of the failure to perform by third parties, our R&D costs would increase, and we may not be able to obtain regulatory clearance or approval for the DMT or our planned future tests, if needed. In addition, we may not be able to establish or maintain relationships with these third parties on favorable terms, if at all. Each of these outcomes would harm our ability to market the DMT outside of the LDT context or to achieve profitability.
We are subject to numerous federal, local and foreign laws and regulations; complying with laws pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties and a material adverse effect to our business and operations.
Our operations are subject to extensive federal, state, local and foreign laws and regulations, all of which are subject to change. These laws and regulations currently include, among other things:
•CLIA, which requires that laboratories obtain certification from the federal government, and state licensure laws;
•FDA laws and regulations, including but not limited to requirements for offering LDTs;
52
•HIPAA, which imposes comprehensive federal standards with respect to the privacy and security of PHI, and requirements for the use of certain standardized electronic transactions; amendments to HIPAA under HITECH, which strengthened and expanded HIPAA privacy and security compliance requirements, increased penalties for violators, extended enforcement authority to state attorneys general and imposed requirements for breach notification;
•state laws regulating genetic testing and protecting the privacy of genetic test results, as well as state laws protecting the privacy and security of health information and personal data and mandating reporting of breaches to affected individuals and state regulators;
•the federal Anti-Kickback Statute, which prohibits knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for, or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program;
•the Eliminating Kickbacks in Recovery Act, which is an all-payor anti-kickback law that makes it a criminal offense to pay any remuneration to induce referrals to, or in exchange for, patients using the services of a recovery home, a substance use clinical treatment facility, or laboratory;
•the federal False Claims Act, which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government;
•the CMP Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or Medicaid beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or Medicaid, unless an exception applies;
•other federal and state fraud and abuse laws, such as anti-kickback laws, prohibitions on self-referral, and false claims acts, which may extend to services reimbursable by any commercial payor, including private insurers;
•PAMA, which requires applicable laboratories to report commercial payor data in a timely and accurate manner every three years (and in some cases annually);
•state laws that impose reporting and other compliance-related requirements; and
•similar foreign laws and regulations that apply to us in the countries in which we operate.
As a clinical laboratory, our business practices may face heightened scrutiny from government enforcement agencies such as the Department of Justice, the OIG and CMS. The OIG has issued fraud alerts in recent years that identify certain arrangements between clinical laboratories and referring physicians as implicating the Anti-Kickback Statute. The OIG has stated that it is particularly concerned about these types of arrangements because the choice of laboratory, as well as the decision to order laboratory tests, typically are made or strongly influenced by the physician, with little or no input from the patient. Moreover, the provision of payments or other items of value by a clinical laboratory to a referral source could be prohibited under the federal self-referral prohibition, commonly known as the Stark Law or the Physician Self-Referral Law, unless the arrangement meets all criteria of an applicable exception. The government has actively enforced these laws against clinical laboratories in recent years.
These laws and regulations are complex and are subject to interpretation by the courts and by government agencies. Our failure to comply could lead to significant civil or criminal penalties, exclusion from participation in state and federal health care programs, individual imprisonment, disgorgement of profits, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law, curtailment or restructuring of our operations, or prohibitions or restrictions on our laboratories’ ability to provide or receive payment for our services, any of which could adversely affect our ability to operate our business and pursue our strategy. We believe that we are in material compliance with all statutory and regulatory requirements, but there is a risk that one or more government agencies could take a contrary position, or that a private party could file suit under the qui tam provisions of the federal False Claims Act or a similar state law. Such occurrences, regardless of their outcome, could damage our reputation and adversely affect important business relationships with third parties, including managed care organizations, and other private commercial payors.
53
The growth of our business and our future expansion outside of the United States may increase the potential of violating similar foreign laws or our internal policies and procedures. The risk of us being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Any of the foregoing consequences could seriously harm our business and our financial results.
We must comply with complex and overlapping laws protecting the privacy and security of health information and personal data.
The privacy regulations regulate the use and disclosure of PHI by health plans, health care clearing houses, and health care providers engaging in certain electronic transactions (“Covered Entities”). They also set forth certain rights that an individual has with respect to his or her PHI maintained by Covered Entities, including the right to access or amend certain records containing PHI, request an accounting of PHI disclosures, or to request restrictions on the use or disclosure of PHI. The HIPAA security regulations establish administrative, physical, and technical standards for maintaining the confidentiality, integrity and availability of PHI in electronic form. These standards apply to Covered Entities and also to “business associates” or third parties providing services involving the use or disclosure of PHI. The HIPAA privacy and security regulations establish a uniform federal “floor” and do not supersede state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing PHI and other personal information, for example, the California Consumer Privacy Act (“CCPA”), which became effective January 1, 2020. The California Privacy Rights Act (“CPRA”), which amends the CCPA, went into effect in January of 2023 and creates new regulatory authority in California with enforcement powers, resulting in additional compliance costs and expenses, and additional potential harm and liability for failure to comply. Other states are enacting similar comprehensive privacy laws, for example Virginia, Colorado, Connecticut, and Utah all enacted new data privacy laws that took effect throughout 2023 that have similarities to the CCPA and CPRA, but also have significant differences, creating compliance challenges across different jurisdictions. While there is an exception for protected health information that is subject to HIPAA in the state consumer privacy laws, these laws may impact some business operations if we are covered under such laws. We are a “business” for purposes of the CCPA, and unlike other state privacy laws, the CCPA regulates personal information collected in a business to business and in human resources contexts related to our California employees. As a result, we are required to comply with both HIPAA privacy regulations and varying state privacy and security laws.
Moreover, HIPAA as amended by HITECH, established certain health information security breach notification requirements. In the event of a breach of unsecured PHI, a Covered Entity must notify each individual whose PHI is breached, federal regulators and in some cases, must publicize the breach in local or national media. Breaches affecting 500 individuals or more are publicized by federal regulators who publicly identify via website posting the breaching entity, the circumstances of the breach and the number of individuals affected.
These laws contain significant fines and other penalties for wrongful use or disclosure of PHI. Given the complexity of HIPAA and HITECH and their overlap with state privacy and security laws, and the fact that these laws are rapidly evolving and are subject to changing and potentially conflicting interpretation, our ability to comply with HIPAA, HITECH and state privacy requirements is uncertain and the costs of compliance are significant. Moreover, these laws and their interpretations may become more stringent or inclusive over time. For example, increasing concerns about health information privacy have recently prompted the federal government to issue guidance taking a newly expansive view of the scope of the laws and regulations that they enforce. Further adding to the complexity is that our operations are evolving and the requirements of these laws will apply differently depending on such things as whether or not we bill electronically for our services, or provide services involving the use or disclosure of PHI and incur compliance obligations as a business associate. The costs of complying with any changes to HIPAA, HITECH and state privacy and security restrictions may have a negative impact on our operations. Noncompliance could subject us to criminal penalties, civil sanctions and significant monetary penalties as well as consumer initiated litigation and reputational damage.
54
We also are required to collect and maintain personal information about our employees, and we collect information about customers as part of some of our marketing programs, as well as receive and transfer certain payment information using a third-party billing vendor, to accept payments from our customers, including credit card information. Most states have adopted laws requiring notification of affected individuals and state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms to ensure ongoing protection of personal information. In addition, many U.S. states are enacting consumer privacy statutes to enhance protections for personal data and to provide residents with more choices concerning their data collected by businesses, increasing compliance complexity and increasing risks of failures to comply. Activities outside of the United States implicate local and national data protection standards, impose additional compliance requirements, and generate additional risks of enforcement for non-compliance. The collection and use of such information may be subject to contractual obligations as well. If the security and information systems that we or our outsourced third-party providers use to store or process such information are compromised or if we, or such third parties, otherwise fail to comply with these laws, regulations, and contractual obligations, we could face litigation and the imposition of penalties that could adversely affect our financial performance.
We must comply with all applicable privacy and data security laws in order to operate our business and may be required to expend significant capital and other resources to ensure ongoing compliance, to protect against security breaches and hackers or to alleviate problems caused by such breaches. Breaches of health information and/or personal data may be extremely expensive to remediate, may prompt federal or state investigation, fines, civil and/or criminal sanctions and significant reputational damage.
Our services present the potential for embezzlement, identity theft or other similar illegal behavior by our employees, consultants, service providers or commercial partners.
Our operations involve the use and disclosure of personal and business information that could be used to impersonate third parties or otherwise gain access to their data or funds. If any of our employees, consultants, service providers or commercial partners takes, converts or misuses these funds or data, we could be liable for any resulting damages, which could harm our financial condition and damage our business reputation.
Clinical research is heavily regulated and failure to comply with human subject protection regulations may disrupt our research program leading to significant expense, regulatory enforcement, private lawsuits, and reputational damage.
Clinical research is subject to federal, state, and, for studies conducted outside of the United States, international regulation. At the federal level, HHS imposes regulations for the protection of human subjects and requirements such as initial and ongoing institutional review board review, informed consent requirements, adverse event reporting and other protections to minimize the risk and maximize the benefit to research participants. Clinical studies done under an investigational device exemption for purposes of an anticipated FDA premarket submission are subject to a separate layer of human subject protection regulations. Many states also impose human subject protection laws that mirror or in some cases exceed federal requirements and, in some states, the violation of federal human subject protection regulations constitutes a violation of state law. HIPAA and other privacy laws also regulate the use and disclosure of PHI in connection with research activities. Research conducted overseas is subject to a variety of national protections such as ethics committee review, informed consent and adverse event reporting, as well as laws regulating the use, disclosure and cross-border transfer of personal data. The costs of compliance with these laws may be significant and compliance with regulatory requirements may result in delay. Noncompliance may disrupt our research and result in data that is unacceptable to regulatory authorities, data lock, or other sanctions that may significantly disrupt our operations.
55
We could be adversely affected by alleged violations of the Federal Trade Commission Act or other truth-in-advertising and consumer protection laws.
Our advertising for laboratory services and tests is subject to federal truth-in-advertising laws enforced by the FTC as well as comparable state consumer protection laws. Under the Federal Trade Commission Act, the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution. Our direct-to-consumer advertising and social media presence, as well as our physician-directed advertising, are subject to these federal and state truth-in-advertising laws. Any actual or perceived non-compliance with those laws could lead to an investigation by the FTC or a comparable state agency, or could lead to allegations of misleading advertising by private plaintiffs. Any such action against us would disrupt our business operations, cause damage to our reputation, and result in material adverse effects on our business, financial condition, results of operation, and cash flows.
Medical product manufacturers’ use of social media platforms presents new risks.
We believe that our customer base and potential patient populations are active on social media and we have begun engaging through those platforms to elevate our national marketing presence. Social media practices in the diagnostic, pharmaceutical, biotechnology and medical device industries are evolving, which creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media platforms to comment on the effectiveness of, or adverse experiences with, one of our products, which could result in reporting obligations or the need for us to conduct an investigation. In addition, there is a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us or our products on any social networking website. If any of these events were to occur or we otherwise fail to comply with any applicable regulations, we could incur liability, face restrictive regulatory actions or incur other harm to our business.
Intellectual Property Risks Related to Our Business
Our collaborators may assert ownership or commercial rights to inventions we develop from our use of the biological materials which they provide to us, or otherwise arising from the collaboration.
We collaborate with several institutions, clinicians, and researchers in scientific matters. Also, we rely on numerous third parties to provide us with adhesive patch samples and biological materials that we use to develop tests. If we cannot successfully negotiate sufficient ownership, licensing, and/or commercial rights to any inventions that result from our use of a third-party collaborator’s materials, or if disputes arise with respect to the intellectual property developed with the use of a collaborator’s samples, or data developed in a collaborator’s study, our ability to capitalize on the market potential of these inventions or developments may be limited or precluded altogether.
If we are unable to maintain intellectual property protection, our competitive position could be harmed.
Our ability to protect our discoveries and technologies affects our ability to compete and to achieve profitability. Currently, we rely on a combination of U.S. and foreign patents and patent applications, copyrights, trademarks and trademark applications, confidentiality or non-disclosure agreements, material transfer agreements, licenses, consulting agreements, work-for-hire agreements, and invention assignment agreements to protect our intellectual property rights. We also maintain certain company know-how, trade secrets, and technological innovations designed to provide us with a competitive advantage in the marketplace as trade secrets. As of February 29, 2024, we own 13 issued U.S. utility or design patents, 24 pending U.S. utility or design patent applications, three pending U.S. provisional patent applications, five pending U.S. design patent applications, five issued foreign patents, 45 pending foreign patent applications, and one PCT application. While we intend to pursue additional patent applications, it is possible that our pending patent applications and any future applications may not result in issued patents. Even if patents are issued, third parties may independently develop similar or competing technology that avoids our patents. Further, we cannot be certain that the steps we have taken will prevent the misappropriation of our trade secrets and other confidential information as well as the misuse of our patents and other intellectual property, particularly in foreign countries where we have not filed for patent protection.
56
From time-to-time the U.S. Supreme Court, other federal courts, or the USPTO, may change the standards of patentability, and any such changes could have a negative impact on our business. For instance, in 2008, the Court of Appeals for the Federal Circuit issued a decision that methods or processes cannot be patented unless they are tied to a machine or involve a physical transformation. The U.S. Supreme Court later reversed that decision in Bilski v. Kappos, 561 U.S. 593 (2010), finding that the “machine-or-transformation” test is not the only test for determining patent eligibility. The Court, however, declined to specify how and when processes are patentable. In 2012, in the case Mayo Collaborative Services v. Prometheus Laboratories, Inc., 566 U.S. 55 (2012), the U.S. Supreme Court reversed the Federal Circuit’s application of Bilski and invalidated a patent focused on a diagnostic process because the patent claim embodied a law of nature.
In 2013, in Association for Molecular Pathology v. Myriad Genetics, the U.S. Supreme Court unanimously ruled that, “[a] naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated,” thereby invalidating Myriad Genetics’ patents on the BRCA1 and BRCA2 breast cancer genes. However, the U.S. Supreme Court also held that manipulation of a gene to create something not found in nature, such as a strand of synthetically-produced complementary DNA (“cDNA”) could still be eligible for patent protection. The U.S. Supreme Court noted that method patents, which concern technical procedures for carrying out a certain process, are not affected by the ruling.
More recently, the Federal Circuit has ruled on several patent cases—such as Univ. of Utah Research Found. v. Ambry Genetics Corp., 774 F.3d 755 (Fed. Cir. 2014), Ariosa Diagnostics, Inc. v. Sequenom, Inc., 788 F.3d 1371 (Fed. Cir. 2015), Genetic Tech. Ltd. v. Merial LLC, 818 F.3d 1369 (Fed. Cir. 2016), and Cleveland Clinic Found. v. True Health Diagnostics, 859 F.3d 1352 (Fed. Cir. 2017)—that some diagnostic method claims are patent ineligible. These decisions have narrowed the scope of patent protection available in certain circumstances or weakened the rights of patent owners in certain situations. Some aspects of our technology involve processes that may be subject to this evolving standard and we cannot guarantee that any of our pending process claims will be patentable as a result of such evolving standards. In addition, this combination of decisions has created uncertainty as to the value of certain issued patents, in particular patents in the molecular biology analysis and diagnostic space. Moreover, there is additional uncertainty around the evolving standard in light of the USPTO Revised Patent Subject Matter Eligibility Guidance issued in Jan. 2019.
It should also be noted that in 2010, the Secretary’s Advisory Committee on Genetics, Health and Society voted to approve a report entitled “Gene Patents and Licensing Practices and Their Impact on Patient Access to Genetic Tests.” That report defines “patent claims on genes” broadly to include claims to isolated nucleic acid molecules as well as methods of detecting particular sequences or mutations. The report also contains six recommendations, including the creation of an exemption from liability for infringement of patent claims on genes for anyone making, using, ordering, offering for sale, or selling a test developed under the patent for patient care purposes, or for anyone using the patent-protected genes in the pursuit of research. The report also recommended that HHS should explore, identify, and implement mechanisms that will encourage more voluntary adherence to current guidelines that promote nonexclusive in-licensing of diagnostic genetic and genomic technologies. It is unclear whether HHS will act upon these recommendations, or if the recommendations would result in a change in law or process that could negatively impact our patent portfolio or future R&D. If acted upon, implementation of such provisions could have a material negative impact on our business.
We may face intellectual property infringement claims that could be time-consuming and costly to defend, and could result in the loss of significant rights, the implementation of an injunction, and the assessment of treble damages.
From time-to-time we may face intellectual property infringement or misappropriation claims from third parties. Some of these claims may lead to litigation. The outcome of any such litigation can never be guaranteed, and an adverse outcome could affect us negatively. For example, were a third party to succeed on an infringement claim against us, we may be required to pay substantial damages, including treble damages if such infringement were found to be willful. In addition, we could face an injunction barring us from conducting the allegedly infringing activity, including an order preventing us from offering the DMT and future planned tests in the marketplace. The outcome of the litigation could require us to enter into a license agreement which may not be pursuant to acceptable or commercially reasonable or practical terms or which may not be available at all.
It is also possible that an adverse finding of infringement against us may require us to dedicate substantial resources and time in developing non-infringing alternatives, which may or may not be possible. In the case of diagnostic tests, we would also need to include non-infringing technologies, which would require us to re-validate the test. Any such re-validation, in addition to being costly and time-consuming, may be unsuccessful. Finally, we may initiate claims to assert or defend our own intellectual property against third parties. Any intellectual property litigation, irrespective of whether we are the plaintiff or the defendant, and regardless of the outcome, is expensive and time-consuming, and could divert and distract our management’s attention from our business and negatively affect our operating results or financial condition.
57
Tax Risks Related to Our Business
Our ability to use our net operating losses to offset future taxable income may be subject to certain limitations.
Our U.S federal net operating loss (“NOL”), carryforwards, may be unavailable to offset future taxable income because of restrictions under U.S. tax law. Our NOLs generated in tax years ending on or prior to December 31, 2017 are only permitted to be carried forward for 20 taxable years under applicable U.S. federal tax law, and therefore could expire unused. Under tax legislation commonly referred to as the Tax Cuts and Jobs Act (“TCJA”), as modified by the Coronavirus Aid, Relief, and Economic Security Act (“CARES”) Act, our U.S. federal NOLs generated in tax years ending after December 31, 2017 may be carried forward indefinitely and NOLs arising in taxable years beginning after December 31, 2017 and before January 1, 2021 may be carried back to each of the five taxable years preceding the tax year of such loss, but NOLs arising in taxable years beginning after December 31, 2020 may not be carried back. In addition, under the TCJA, as modified by the CARES Act, for taxable years beginning after December 31, 2020, the deductibility of federal NOLs generated in taxable years beginning after December 31, 2017 is limited to 80% of current year taxable income. Certain states do not conform to the TCJA, as modified by the CARES Act (depending on the applicable jurisdiction).
In addition, under Section 382 of the Internal Revenue Code of 1986, as amended (the “IRC”), a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize tax attribute carryforwards to offset future taxable income. Our existing NOL and R&D tax credit carryforwards may be subject to limitations arising from previous ownership changes, and if we underwent an ownership change in connection with or after the Business Combination, our ability to utilize NOLs could be further limited by Section 382 of the IRC. Future changes in our stock ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the IRC. There is also a risk that due to regulatory changes, such as suspensions on the use of NOLs, or other unforeseen reasons, our existing and any future NOLs could expire or otherwise be unavailable to offset future income tax liabilities. We have not conducted a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since inception due to the significant complexity and cost associated with such a study. In addition, we have not performed an R&D tax credit study to confirm the accuracy of applicable carryforwards and completion of such a study may reduce carryforward available to offset future taxable income.
Changes in tax laws or in their implementation or interpretation may adversely affect our business and financial condition.
In recent years, numerous legislative, judicial, and administrative changes have been made in the provisions of federal and state income tax laws applicable to holders of our common stock. In particular, the comprehensive tax reform legislation enacted in December 2017 pursuant to the TCJA, among other things, contains significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, limitation on the deductibility of interest expense to 30% of adjusted earnings (except for certain small businesses), limitation of the deduction for NOLs generated in taxable years beginning after December 31, 2017 to 80% of current year taxable income, elimination of NOL carrybacks, one time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, reduction or elimination of U.S. tax on foreign earnings (subject to certain important exceptions), immediate deductions for certain new investments instead of deductions for depreciation expense over time, eliminating the option to deduct research and development expenditures currently and requiring corporations to capitalize and amortize them over five years, and modifying or repealing many business deductions and credits. The CARES Act modifies certain provisions of the TCJA. Under the CARES Act, NOLs arising in taxable years beginning after December 31, 2017 and before January 1, 2021 may be carried back to each of the five taxable years preceding the tax year of such loss, but NOLs arising in taxable years beginning after December 31, 2020 may not be carried back. In addition, the CARES Act eliminates the limitation on the deduction of NOLs to 80% of current year taxable income for taxable years beginning before January 1, 2021, and increases the amount of interest expense that may be deducted to 50% of adjusted taxable income for taxable years beginning in 2019 or 2020. Furthermore, President Biden signed the Inflation Reduction Act of 2022 (the “IRA”) into law in August 2022, which contained certain tax measures, including an excise tax of 1% on certain corporate stock buy-backs. Regulatory guidance under the TCJA an the IRA is and continues to be forthcoming, and such regulatory guidance could adversely affect our business and financial condition. In addition, the impact of such regulatory guidance on holders of our common stock is also uncertain and could be adverse. It is uncertain if and to what extent various states will conform to the TCJA, the IRA, and such regulatory guidance. You are urged to consult with your legal and tax advisors with respect to such legislation and the potential tax consequences of investing in our common stock.
58
Risks Related to Our Securities
There is no assurance that we will continue satisfying the listing requirements of the Nasdaq Capital Market.
Our common stock is listed on the Nasdaq Capital Market. To maintain our listing we are required to satisfy continued listing requirements. There can be no assurance we will continue satisfying such continued listing requirements, which include that the closing bid price of our common stock be at least $1 per share, that we have at least 300 round lot holders and at least 500,000 publicly held shares, that the market value of our publicly held securities be at least $1 million, and that we meet one of these standards: stockholders’ equity of at least $2.5 million; market value of listed securities of at least $35 million; or net income from continuing operations of $500,000 in the most recently completed fiscal year or in two of the three most recently completed fiscal years. The delisting of our common stock for whatever reason could, among other things, substantially impair our ability to raise additional capital; result in the loss of interest from institutional investors, the loss of confidence in our company by investors and employees, and in fewer financing, strategic and business development opportunities; and result in potential breaches of agreements under which we made representations or covenants relating to our compliance with applicable listing requirements. Claims related to any such breaches, with or without merit, could result in costly litigation, significant liabilities and diversion of our management’s time and attention and could have a material adverse effect on our financial condition, business and results of operations. In addition, the delisting of our common stock for whatever reason may materially impair our stockholders’ ability to buy and sell shares of our common stock and could have an adverse effect on the market price of, and the efficiency of the trading market for, our common stock.
Future issuances of equity securities may dilute the interests of our security holders and reduce the price of our securities.
Any future issuance of our equity securities could dilute the interests of our then existing security holders and could substantially decrease the trading price of our securities. We may issue equity or equity-linked securities for a number of reasons, including to finance our operations and business strategy, to adjust our ratio of debt to equity, to satisfy our obligations upon the exercise of then-outstanding options or other equity-linked securities, if any, or for other reasons. We currently have registered up to $299.7 million of common stock, preferred stock, warrants, senior debt, subordinated debt, rights or units under an effective universal shelf registration statement. Our ability to utilize our effective universal shelf registration statement, including the $74.7 million of capacity remaining under the 2022 Sales Agreement is limited by our compliance with the baby shelf rules. As of the filing of this Annual Report on Form 10-K, our public float is less than $75 million, and under SEC regulations, for so long as our public float remains less than $75 million, the amount we can raise through primary public offerings of securities in any twelve-month period using shelf registration statements subject to the baby shelf rules is limited to an aggregate of one-third of our public float. As of February 23, 2024, our public float was approximately $43.2 million, based on 34,008,061 shares of outstanding common stock held by non-affiliates and at a price of $1.27 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on February 23, 2024. Sales of substantial amounts of shares of our common stock or other securities under our current universal shelf registration statement or otherwise could lower the market price of our common stock and impair our ability to raise capital.
We may amend the terms of our publicly traded warrants currently trading on the Pink Market under the ticker symbol “DMTKW,” or the publicly traded warrants, in a manner that may be adverse to holders with the approval by the holders of a majority of the then outstanding publicly traded warrants, and as a result, the exercise price of the publicly traded warrants could be increased, the exercise period could be shortened and the number of shares purchasable upon exercise of a publicly traded warrant could be decreased, all without your approval.
Our publicly traded warrants are subject to the Warrant Agreement, dated June 19, 2017, by and between the Company and Continental Stock Transfer & Trust Company (the “Warrant Agreement”). The Warrant Agreement provides that the terms of the publicly traded warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of a majority of the then outstanding publicly traded warrants to make any change that adversely affects the interests of the registered holders. Accordingly, we may amend the terms of the publicly traded warrants in a manner adverse to a holder if holders of a majority of the then outstanding publicly traded warrants approve of such amendment. Although our ability to amend the terms of the publicly traded warrants with the consent of a majority of the then outstanding publicly traded warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the publicly traded warrants, shorten the exercise period or decrease the number of shares of common stock purchasable upon exercise of the publicly traded warrants.
59
We may redeem your unexpired publicly traded warrants prior to their exercise at a time that is disadvantageous to you, thereby making your publicly traded warrants worthless.
We have the ability to redeem our outstanding publicly traded warrants at any time prior to their expiration, at a price of $0.01 per warrant, provided that the last reported sales price of our common stock equals or exceeds $36.00 per share for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date we give notice of redemption. To the extent that the publicly traded warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. Redemption of the outstanding publicly traded warrants could force you (i) to exercise your publicly traded warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so, (ii) to sell your publicly traded warrants at the then-current market price when you might otherwise wish to hold your publicly traded warrants or (iii) to accept the nominal redemption price which, at the time the outstanding publicly traded warrants are called for redemption, is likely to be substantially less than the market value of your publicly traded warrants.
Because we have no current plans to pay cash dividends on our shares for the foreseeable future, you may not receive any return on investment unless you sell your shares for a price greater than that which you paid for it.
We may retain future earnings, if any, for future operations, expansion and debt repayment and have no current plans to pay any cash dividends for the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of our board of directors and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that our board of directors may deem relevant. In addition, our ability to pay dividends may be limited by covenants of any existing and future outstanding indebtedness we or our subsidiaries incur. As a result, you may not receive any return on an investment in our shares unless you sell your shares of the Company for a price greater than that which you paid for them.
If securities or industry analysts cease publishing research or reports about us, our business, or our market, or if they change their recommendations regarding our securities adversely, the price and trading volume of our securities could decline.
The trading market for our securities will be influenced by the research and reports that industry or securities analysts may publish about us, our business, market or competitors. If any of the analysts who cover us change their recommendation regarding our shares of common stock adversely (including by reducing their price target with respect to our common stock, which we have experienced), or provide more favorable relative recommendations about our competitors, the price of our shares of common stock may decline. If any analyst who may cover us were to cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our share price or trading volume to decline.
Provisions of our charter documents or Delaware law could delay or prevent an acquisition of us, even if the acquisition would be beneficial to our stockholders, and could make it more difficult for you to change our management.
Provisions in our Amended and Restated Certificate of Incorporation and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control that our stockholders may consider favorable, including transactions in which our stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors. These provisions include:
•a classified board of directors so that not all directors are elected at one time;
•a prohibition on stockholder action through written consent;
•no cumulative voting in the election of directors;
•the exclusive right of our board of directors to elect a director to fill a vacancy however created, whether by the expansion of our board of directors, the resignation, death or removal of a director, or otherwise;
•a requirement that special meetings of our stockholders be called only by our board of directors, the chairman of our board of directors, the chief executive officer or, in the absence of a chief executive officer, the president;
•an advance notice requirement for stockholder proposals and nominations;
•the authority of our board of directors to issue preferred stock with such terms as our board of directors may determine; and
60
•a requirement of approval of at least 75% of all outstanding shares of our capital stock entitled to vote to amend any bylaws by stockholder action, or to amend specific provisions of our certificate of incorporation.
In addition, Delaware law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person who, together with his, her or its affiliates, owns or within the last three years has owned 15% or more of the company’s voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Delaware law may discourage, delay or prevent a change in control of the Company.
In addition, our Amended and Restated Certificate of Incorporation, to the fullest extent permitted by law, provides that the Court of Chancery of the State of Delaware will be the exclusive forum (the “Delaware Chancery forum provision”), for: any derivative action or proceeding brought on our behalf; any action or proceeding asserting a breach of fiduciary duty owed to us, our stockholders, or any of our current or former directors, officers or other employees; any action or proceeding asserting a claim against us or any of our current or former directors, officers or other employees, arising out of or pursuant to the Delaware General Corporation Law, our Amended and Restated Certificate of Incorporation, or our bylaws; any action or proceeding to interpret, apply, enforce or determine the validity of our Amended and Restated Certificate of Incorporation or our Bylaws; any action or proceeding as to which the Delaware General Corporation Law confers jurisdiction to the Court of Chancery of the State of Delaware; or any action asserting a claim against us that is governed by the internal affairs doctrine. This exclusive forum provision does not apply to suits brought to enforce a duty or liability created by the Securities Act, the Exchange Act, or any claim for which the federal courts have exclusive jurisdiction.
The Delaware Chancery forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, or other employees, which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the exclusive forum provisions contained in our Amended and Restated Certificate of Incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations and financial condition.
Further, on March 18, 2020, the Delaware Supreme Court ruled that provisions of a Delaware corporation’s certificate of incorporation that designate a federal forum for securities claims brought pursuant to the Securities Act, or federal forum provisions, are valid and enforceable under Delaware law (the “March 2020 Ruling”). Consistent with the March 2020 Ruling, on April 12, 2020, our board of directors approved a Certificate of Amendment to our Amended and Restated Certificate of Incorporation (the “2020 Certificate of Amendment”), which was approved by our stockholders at our 2020 annual meeting of stockholders on May 26, 2020. We filed the 2020 Certificate of Amendment with the Delaware Secretary of State on May 27, 2020. The 2020 Certificate of Amendment added a federal forum provision to our Amended and Restated Certificate of Incorporation, which now provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall, to the fullest extent permitted by law, be the sole and exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Various U.S. Supreme Court cases offer support for the argument that federal forum provisions do not violate federal policy. However, the March 2020 Ruling applies only to claims brought in Delaware state courts, and it is not binding on any other state court or the federal courts. Therefore, we are unable to predict whether a state court in any other state or a federal court would enforce a federal forum provision such as the one set forth in the 2020 Certificate of Amendment.
We adopted the 2020 Certificate of Amendment to reduce the costs and inefficiencies to the Company that would result from a Securities Act claim being litigated in both state and federal courts, which was permissible under our Amended and Restated Certificate of Incorporation before the 2020 Certificate of Amendment was adopted. Such simultaneous state and federal litigation could also result in inconsistent judgments and rulings, and the adoption of the 2020 Certificate of Amendment could reduce this risk. However, the federal forum provision set forth in the 2020 Certificate of Amendment may discourage Securities Act claims or limit a stockholder’s ability to submit claims in a judicial forum that the stockholder finds favorable, and may result in additional costs for a stockholder seeking to bring such a claim.
Provisions in our charter and other provisions of Delaware law could limit the price that investors are willing to pay in the future for shares of our common stock.
61
The price of our common stock has been and may continue to be volatile and may fluctuate substantially.
The stock market in general and, especially in recent history, the market for life sciences companies in particular, have experienced extreme volatility that has often been unrelated to companies’ operating performance. During the 12-month period ended December 31, 2023, the closing prices of our common stock as reported on the Nasdaq Capital Market were in the range of $1.18 to $6.20 per share. In addition, the stock market in general has recently experienced relatively large price and volume fluctuations in response to the macroeconomic environment, and geopolitical concerns. The market price for our common stock may be influenced by many factors, including:
•the results of our efforts to commercialize the DMT;
•commencement or termination of any collaboration or licensing arrangement;
•disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technology;
•announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures and capital commitments;
•additions or departures of key scientific or management personnel;
•variations in our financial results or those of companies that are perceived to be similar to us;
•results of clinical trials of product candidates of our competitors;
•general economic and market conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors, including changes in market valuations of similar companies;
•regulatory or legal developments related to our business or operations in the United States and other countries;
•changes in the structure of healthcare payment systems;
•delay or failure to obtain coverage policy decisions from commercial payors;
•conditions or trends in the life sciences industry;
•actual or anticipated changes in earnings estimates, guidance or recommendations by securities analysts;
•announcement or expectation of additional financing efforts;
•sales of common stock by us or our stockholders in the future, as well as the overall trading volume of our common stock; and
•other factors described in this “Risk Factors” section.
In the past, following periods of volatility in companies’ stock prices, securities class-action litigation has often been instituted against such companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business and financial condition.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity
We recognize the critical importance of maintaining the trust and confidence of customers, patients, business partners and employees toward our business and are committed to protecting the confidentiality, integrity and availability of our business operations and systems. Our board of directors is actively involved in oversight of our risk management activities, and cybersecurity represents an important element of our overall approach to risk management. Our cybersecurity policies, standards, processes and practices are based on recognized frameworks established by the National Institute of Standards and Technology, or NIST and other applicable industry standards. In general, we seek to address cybersecurity risks through a comprehensive, cross-functional approach that is focused on preserving the confidentiality, security and availability of the information that we collect and store by identifying, preventing and mitigating cybersecurity threats and effectively responding to cybersecurity incidents when they occur.
62
Cybersecurity Risk Management and Strategy; Effect of Risk
We face risks related to cybersecurity such as unauthorized access, cybersecurity attacks and other security incidents, including as perpetrated by hackers and unintentional damage or disruption to hardware and software systems, loss of data, and misappropriation of confidential information. To identify and assess material risks from cybersecurity threats, we maintain a comprehensive cybersecurity program to ensure our systems are effective and prepared for information security risks, including regular oversight of our programs for security monitoring for internal and external threats to ensure the confidentiality and integrity of our information assets. We consider risks from cybersecurity threats alongside other company risks as part of our overall risk assessment process. We employ a range of tools and services, including regular network and endpoint monitoring, audits, vulnerability assessments, penetration testing, threat modeling and tabletop exercises to inform our risk identification and assessment. As discussed in more detail under “Cybersecurity Governance” below, our audit committee of our board of directors provides oversight of our cybersecurity risk management and strategy processes, which are led by our Information Security Officer, General Counsel, Chief Compliance Officer and Chief Information Officer.
We also identify our cybersecurity threat risks by comparing our processes to standards set by the NIST and Center for Internet Security (or “CIS”) as well as by engaging experts to attempt to infiltrate our information systems. To provide for the availability of critical data and systems, maintain regulatory compliance, manage our material risks from cybersecurity threats, and protect against and respond to cybersecurity incidents, we undertake the following activities:
•monitor emerging data protection laws and implement changes to our processes that are designed to comply with such laws;
•through our policies, practices and contracts (as applicable), require employees, as well as third parties that provide services on our behalf, to treat confidential information and data with care;
•employ technical safeguards that are designed to protect our information systems from cybersecurity threats, including firewalls, intrusion prevention and detection systems, anti-malware functionality and access controls, which are evaluated and improved through vulnerability assessments and cybersecurity threat intelligence;
•provide regular, mandatory training for our employees and contractors regarding cybersecurity threats as a means to equip them with effective tools to address cybersecurity threats, and to communicate our evolving information security policies, standards, processes and practices;
•conduct regular phishing email simulations for all employees and contractors with access to our email systems to enhance awareness and responsiveness to possible threats;
•conduct annual cybersecurity management and incident training for employees involved in our systems and processes that handle confidential information, personal data and other sensitive data;
•run annual tabletop exercises to simulate a response to a cybersecurity incident and use the findings to improve our processes and technologies;
•leverage incident handling frameworks to help us identify, protect, detect, respond and recover when there is an actual or potential cybersecurity incident;
•carry information security risk insurance that provides protection against the potential losses arising from a cybersecurity incident;
•use a third-party risk management platform to continually assess and manage risk, using CIS and NIST benchmarks, HIPAA security rules and guidance and overall information technology risk;
•use a third-party service providers to continuously monitor, perform vulnerability scanning, and penetration testing of our internal and/or external online presence, including wireless networks, cloud-based servers, business website, and electronic communications;
•utilize defined onboarding and monitoring procedures with third-party contractors, including risk- based surveys as part of the vendor management and selection process; and
•establish a cross-functional governance working group or committee led by the Information Security Officer to oversee and make recommendations as to data classification, management and access.
63
Our incident response plan coordinates the activities we take to prepare for, detect, respond to and recover from cybersecurity incidents, which include processes to triage, assess severity for, escalate, contain, investigate and remediate the incident, as well as to comply with potentially applicable legal obligations and mitigate damage to our business and reputation.
As part of the above processes, we regularly engage with consultants, auditors and other third parties, including annual review of our cybersecurity program to help identify areas for continued focus, improvement and compliance.
Our processes also address cybersecurity threat risks associated with our use of third-party service providers, including our suppliers and manufacturers or who have access to patient and employee data or our systems. In addition, cybersecurity considerations affect the selection and oversight of our third-party service providers. We perform diligence on third parties that have access to our systems, data or facilities that house such systems or data, and continually monitor cybersecurity threat risks identified through such diligence. Additionally, we generally require those third parties that could introduce significant cybersecurity risk to us to agree by contract to manage their cybersecurity risks in specified ways, and to agree to be subject to cybersecurity audits, which we conduct as appropriate.
We describe whether and how risks from identified cybersecurity threats could materially affect us, including our business strategy, results of operations, or financial condition, under the heading “Cybersecurity incidents, cyberattacks and other information technology failures could result in loss of data, compromise of confidential information, and adverse effects to our business, financial results and our ability to continue operations,” which disclosures are incorporated herein by reference. In the last three fiscal years, we have not experienced any material cybersecurity incidents.
Cybersecurity Governance; Management
Cybersecurity is an important part of our risk management processes and an area of focus for our board of directors and management. The audit committee of our board of directors is responsible for the oversight of risks from cybersecurity threats. In general, the audit committee oversees risk management activities designed and implemented by our management.
At least quarterly, our audit committee receives an update from management of our cybersecurity threat risk management and strategy processes covering topics such as data security posture, results from third-party assessments, progress towards pre-determined risk-mitigation-related goals, our incident response plan, and material cybersecurity threat risks or incidents and developments, as well as the steps management has taken to respond to such risks. In such sessions, our audit committee generally receives materials that include a cybersecurity scorecard / dashboard and other materials discussing current and emerging material cybersecurity threat risks, and describing our ability to mitigate those risks, as well as recent developments, evolving standards, technological developments and information security considerations arising with respect to our peers and third parties, and discusses such matters with our Information Security Officer. Our audit committee also will receive prompt and timely information regarding any cybersecurity incident that meets established reporting thresholds, as well as ongoing updates regarding any such incident until it has been addressed.
Members of our audit committee and board of directors are also encouraged to regularly engage in conversations with management on cybersecurity-related news events and discuss any updates to our cybersecurity risk management and strategy programs. Material cybersecurity threat risks are also considered during separate board meeting discussions of important matters like enterprise risk management, operational budgeting, business continuity planning, mergers and acquisitions, brand management, and other relevant matters.
Our cybersecurity risk management and strategy processes, which are discussed in greater detail above, are led by our Information Security Officer, Chief Information Officer, Chief Privacy Officer, Chief Financial Officer, Chief Compliance Officer and General Counsel. Such individuals have collectively over approximately 40 years of prior work experience in various roles involving managing information security, developing cybersecurity strategy, implementing effective information and cybersecurity programs, as well as several relevant degrees and certifications, including Certified Information Privacy Manager, and Certified Information Systems Security Professional. These management team members are informed about and monitor the prevention, mitigation, detection, and remediation of cybersecurity incidents through their management of, and participation in, the cybersecurity risk management and strategy processes described above, including the operation of our incident response plan. As discussed above, these management team members report to the audit committee of our board of directors about cybersecurity threat risks, among other cybersecurity related matters, on a quarterly basis and annually.
64
Item 2. Properties.
We currently occupy approximately 110,082 square feet of space in San Diego, California. See Note 5 in the Notes to Consolidated Financial Statements included in Part II, Item 8, “Consolidated Financial Statements and Supplementary Data” for further discussion surrounding our leased facilities.
We believe these facilities are adequate to meet our current and reasonably foreseeable requirements. We believe that we would be able to obtain additional space, if required, on commercially reasonable terms.
Item 3. Legal Proceedings.
From time to time, the Company may be subject to legal proceedings and claims arising in the ordinary course of business. Because legal proceedings are inherently uncertain, we are unable to predict the ultimate outcomes of these matters, but management does not believe that the outcome of any of these matters will have a material effect on the Company’s consolidated financial position, results of operations or cash flows. However, there can be no assurance as to the ultimate outcomes of these matters.
On October 16, 2023, a putative class action lawsuit titled Bagheri v. DermTech, Inc., et al., Case No. 23-cv-1885-DMS-JLB, was filed in the United States District Court for the Southern District of California against the Company and certain of its current and/or former officers (collectively, the “Defendants”). The complaint was filed on behalf of persons who purchased or otherwise acquired the Company’s publicly traded securities between May 3, 2022 and November 3, 2022 (collectively, the “Plaintiffs”). The Plaintiffs alleged in the complaint that the Defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 by making false and misleading statements regarding the Company’s business, operations, and prospects. The action includes claims for damages and an award of reasonable costs and attorneys’ fees and expert fees.
On December 5, 2023, another putative class action lawsuit titled Quarford v. DermTech, Inc. et al., Case No. 23-cv-2221-JES-DDL was filed in the United States District Court for the Southern District of California against the same Defendants and alleging same causes of action as the Bagheri lawsuit. The Quarford lawsuit expanded the class period to include as Plaintiffs persons who purchased or otherwise acquired the Company’s publicly traded securities from March 8, 2021 to November 3, 2022. On January 17, 2024, the Court consolidated the two actions, which is now titled In re DermTech, Inc. Securities Litigation, Case No. 3:23-cv-1885-DMS-JLB. The consolidated complaint is now due April 1, 2024 and Defendants' responses are due May 31, 2024. Given the early stage of this litigation, the probability of a particular outcome cannot be determined at this time. The Company intends to vigorously defend against all claims.
On December 15, 2023, Joseph Fleischman filed a shareholder derivative lawsuit titled Fleischman v. DermTech, Inc., et al., Case No. 23-cv-2289-DMS-JLB in the United District Court for Southern District of California against the Company’s current and/or former officers and directors (collectively, the “Defendants”) for breaches of their fiduciary duties as directors and/or officers of DermTech, unjust enrichment, gross mismanagement, abuse of control, waste of corporate assets, violations of Section 14(a) of the Securities Exchange Act of 1934, and contribution under Sections 10(b) and 21D of the Exchange Act. February 26, 2024, the Court granted the parties’ joint motion to stay the action pending a resolution of the Company's motion to dismiss the pending securities class action described above. Given the early stage of this litigation, the probability of a particular outcome cannot be determined at this time. The Company intends to vigorously defend against all claims.
Item 4. Mine Safety Disclosures.
Not applicable.
65
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information.
Our common stock is currently listed on the Nasdaq Capital Market under the symbol “DMTK.”
Holders of Our Common Stock
As of February 23, 2024, there were 34,623,443 shares of our common stock outstanding held by approximately 163 holders of record.
Recent Sales of Unregistered Securities
We did not sell any equity securities which were not registered under the Securities Act during the year ended December 31, 2023 that were not otherwise disclosed on our quarterly reports on Form 10-Q or our current reports on Form 8-K filed during the year ended December 31, 2023.
Item 6. [Reserved]
66
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following Management's Discussion and Analysis of Financial Condition and Results of Operations of DermTech, Inc. (together with its subsidiaries, “DermTech,” “we,” “us,” “our” or the “Company”) should be read in conjunction with the consolidated financial statements and the related notes thereto included elsewhere in this Annual Report on Form 10-K.
Overview
We are a molecular diagnostic company developing and marketing novel non-invasive genomics tests to aid in the diagnosis and management of melanoma. Our technology enhances evaluation of lesions suspicious for melanoma using non-invasive sample collection and detecting genomic markers associated with melanoma to identify higher risk lesions or to rule out melanoma with a 99% negative predictive value ("NPV") (Gerami et al. J Am Acad Dermatol. 2017; Skelsey et al. SKIN. 2021). Our scalable genomics assays have been designed to work with our adhesive patch, the DermTech Smart StickerTM (the “Smart Sticker”), which is used to non-invasively collect skin tissue samples for analysis.
We are addressing unmet needs in the clinical evaluation pathway of pigmented skin lesions, such as moles or dark colored skin spots. The DermTech Melanoma Test (“DMT”) facilitates the clinical assessment of pigmented skin lesions for melanoma. We initially focused on marketing the DMT to a large group of dermatology clinicians and are currently prioritizing billable samples in geographies where we have payor coverage versus overall volume growth as one factor to potentially increase average selling price. In connection with the restructuring completed in January, we reduced the number of our sales territories from approximately 60 territories to approximately 55 territories. The application of our Smart Sticker to collect samples non-invasively may allow us to eventually market the DMT to primary care physicians more broadly, beyond integrated primary care networks. We process our tests in our high complexity molecular laboratory that is Clinical Laboratory Improvement Amendments of 1988 (“CLIA”) certified, accredited by the Joint Commission, formerly the Joint Commission on Accreditation of Healthcare Organizations (“JCO”) and licensed by the State of California as well as other states requiring out-of-state licensure, including New York. We also provide laboratory services to several pharmaceutical companies that access our technology on a contract basis for their clinical trials or other studies to advance new drugs.
Based on publicly available estimates, approximately 4 million total skin biopsies are completed in the United States each year to diagnose approximately 190,000 melanomas, consisting of approximately 2 million biopsies covered by Medicare with a market value of approximately $1.5 billion per year and approximately 2 million biopsies that could potentially be reimbursed by commercial payors for an approximate value of $1 billion per year. In 2023, we increased covered lives for our test by approximately 45%. In the United States, approximately 12 million surgical biopsies are performed each year to diagnose approximately 5.4 million non-melanoma skin cancers. Many of the initial surgical procedures for these skin cancers are performed on cosmetically sensitive areas of the body, such as the face, neck and chest, creating significant demand for a non-invasive alternative. We believe the total market opportunity for our non-melanoma skin cancer products may exceed $3 billion in the United States and $1 billion in select world-wide markets.
Events, Trends and Uncertainties
The DMT without the additional test for the presence of telomerase reverse transcriptase gene driver mutations (“TERT”) (formerly known as PLAplus) became eligible for Medicare reimbursement on February 10, 2020. Each reference to the DMT in this paragraph refers only to the DMT without the add-on test for TERT. In late October 2019, the American Medical Association provided us with a Proprietary Laboratory Analyses Code (“PLA Code”). Pricing of $760 for the PLA Code was published on December 24, 2019 as part of the Clinical Laboratory Fee Schedule for 2020. The final Local Coverage Determination (“LCD”) expanded the coverage proposal in the draft LCD from one to two tests per date of service, and it allows clinicians to order the DMT if they have sufficient skill and experience to decide whether a pigmented lesion should be biopsied. Our local Medicare Administrative Contractor, Noridian, has issued its own LCD (“Noridian’s LCD”) announcing coverage of the DMT. Even though the effective date of Noridian’s LCD was June 7, 2020, Noridian began reimbursing us for the DMT as of February 10, 2020. With Medicare coverage granted, we have the opportunity to approach commercial payors, and, as a result, we believe that the DMT may generate significant revenues in the future. No LCD currently covers the optional add-on test for TERT available to those ordering the DMT.
67
Despite the grant of Medicare coverage for the DMT, uncertainty surrounds commercial payor reimbursement, including governmental and commercial payors, of any test incorporating new technology, including tests developed using our technologies. Because each payor generally determines for its own enrollees or insured patients whether to cover or otherwise establish a policy to reimburse our tests, seeking payor approvals is a time-consuming and costly process. Payors can change, and have changed, their policies and procedures regarding their coverage of or reimbursement for our tests, from time to time, even after we have received relevant payor approvals. We cannot be certain that coverage for our current tests and our planned tests will be provided in the future by additional commercial payors or that existing policy decisions or reimbursement levels will remain in place or be fulfilled under existing terms and provisions. If we cannot obtain or maintain coverage and reimbursement from private and governmental payors, such as Medicare and Medicaid, for our current tests, or new tests or test enhancements, our ability to generate revenues could be limited. This may have a material adverse effect on our business, financial condition, results of operation and cash flows.
Discontinuation of Optional TERT Add-On Assay
Effective March 1, 2024, the Company intends to discontinue the optional TERT promoter mutation add-on assay for the DMT. Multiple studies, including the Company’s recently completed Trust 2 Study, demonstrated the foundational DMT’s NPV to be 99% or higher. A high NPV for a rule-out test provides assurance to clinicians and patients that a suspicious pigmented lesion which tests negative is unlikely to be a melanoma. The TERT promoter mutation assay was offered as an optional add-on to the foundational gene expression assay for LINC00518 and PRAME based on initial validation data suggesting it conferred a modest increase in NPV. However, in the clinical setting, less than one-third of patient samples contain sufficient genomic material for TERT analysis. In the Trust 2 Study, the addition of TERT analysis decreased specificity slightly without providing a statistically significant increase in NPV.
Restructuring Plans
On June 26, 2023, the Company’s board of directors approved the 2023 Restructuring Plan, which included restructuring actions primarily related to sales, marketing and general and administrative functions and resulted in a workforce reduction of approximately 15% of the Company’s workforce.
On January 29, 2024, our board of directors approved a restructuring plan (the “2024 Restructuring Plan” and, together with the 2023 Restructuring Plan, the “Restructuring Plans”) to continue to align the Company’s resources with its previously announced strategic prioritization for the DMT. The 2024 Restructuring Plan includes operating expense reductions and a reduction in force. The 2024 Restructuring Plan will primarily affect operations, but impact the entire organization, and will result in a workforce reduction of approximately 30 employees, or approximately 15% of the Company's workforce. The Company expects to achieve approximately $40 million in total operating expense reductions compared to fiscal 2022, from the aggregate of the Restructuring Plans.
The Company estimates that it will incur aggregate pre-tax charges of approximately $1.3 million in connection with the 2024 Restructuring Plan, primarily consisting of severance payments, employee benefits, outplacement services and related costs. The Company expects that the 2024 Restructuring Plan will be complete by the end of March 2024 and that these one-time charges will be incurred in the first quarter of 2024.
Contract Revenue
Contract revenues with pharmaceutical companies relate to ongoing clinical trial contracts and new contracts. Contract revenue can be highly variable as it is dependent on the pharmaceutical customers’ clinical trial progress, which can be difficult to forecast due to variability of patient enrollment, drug safety and efficacy and other factors. Many of our historical contracts with third parties were structured to contain milestone billing payments, which typically are advance payments on work yet to be performed. These advanced payments are structured to help fund operations and are included in deferred revenue as the work has not yet been performed. These advance payments will remain in deferred revenue until we process the laboratory portion of the contracts allowing us to recognize the revenue.
Supply Chain and Inflationary Environment
Global supply chain disruptions and the higher inflationary environment have resulted in higher prices, which could impact our liquidity, business, financial condition and results of operations.
68
Financial Overview
Revenue
We generate revenue through laboratory services that are billed to Medicare, private medical insurance companies and pharmaceutical companies who order our laboratory services, which can include sample collection kits, test development, patient segmentation and stratification, genomic analysis, data analysis and reporting. Our revenue is generated from two revenue streams: test revenue and contract revenue. Test revenue can be highly variable as it is based on payments received by government and private insurance payors that are and are not under contract and can vary based on patient insurance coverage, deductibles and co-pays. As much of our test revenue is driven by the samples that are sent by physicians to our central lab for testing, a key performance measure for us is samples that are received and processed by our central lab successfully, also known as billable samples. We are currently prioritizing billable samples in geographies where we have payor coverage versus overall volume growth as one factor to potentially increase average selling price. Historically, less than one third of our total billable samples has been reimbursed. We continuously take measures with the goal of improving that proportion by leveraging our Medicare coverage and the over 130 million total covered lives for our test, as well as increasing our appeals and administrative process with payors’ claim processing and medical affairs departments.
We have a current CLIA certificate of accreditation from the CMS to perform high-complexity testing and a state license issued by California’s Department of Public Health, Laboratory Field Services. We hold certificates of accreditation from CAP and JCO. In mid-February 2024, we received a notification from CAP that our accreditation with CAP would not be renewed on its renewal date, and thus would terminate as of April 6, 2024. The notice did not provide a specific reason for this decision. We designated JCO as our primary accrediting organization, effective February 29, 2024.
Our laboratory services are ordered by customers on projects that may span over several years, which makes our contract revenue highly variable. Segments of these contracts may be increased, delayed or eliminated based on the success of our customers’ clinical trials or other factors.
Operating Expenses
Sales and Marketing Expenses
Sales and marketing expenses are primarily related to our specialty field sales force, reimbursement efforts, conference attendance, public relations, advertising and general marketing.
Research and Development Expenses
Our research and development ("R&D") expenses consist primarily of salaries and fringe benefits, clinical trials, consulting costs, facilities costs, laboratory costs, equipment expense and depreciation. Our R&D efforts are currently focused on optimizing the performance of the DMT. We also conduct clinical trials to validate the performance characteristics of our tests and to show medical cost benefit in support of our reimbursement efforts.
General and Administrative Expenses
Our general and administrative expenses consist of senior management compensation, consulting, legal, billing and collections, human resources, information technology, accounting, insurance and general business expenses.
Financing Activities
2020 At-The-Market Offering
On November 10, 2020, the Company entered into a sales agreement with Cowen and Company, LLC ("Cowen") relating to the sale of shares of the Company’s common stock from time to time with an aggregate offering price of up to $50.0 million (the "2020 Sales Agreement"). Through December 31, 2021, the Company issued an aggregate of 1,482,343 shares of common stock pursuant to the 2020 Sales Agreement at a weighted average purchase price of $30.05, net of $1.6 million in issuance costs resulting in net proceeds to the Company of approximately $42.9 million. During 2022, the Company did not issue or sell any shares of common stock pursuant to the 2020 Sales Agreement. During 2023, the Company issued an aggregate of 2,038,661 shares of common stock pursuant to the 2020 Sales Agreement at a weighted average purchase price of $2.68 resulting in aggregate gross proceeds of approximately $5.5 million, reduced by $0.3 million in issuance costs, resulting in net proceeds to the Company of approximately $5.2 million.
As of December 31, 2023, the 2020 Sales Agreement had been fully utilized, and no additional shares of common stock may be sold pursuant to the 2020 Sales Agreement.
69
2022 At-The-Market Offering
On August 8, 2022, the Company entered into a sales agreement with Cowen relating to the sale of shares of the Company’s common stock from time to time with an aggregate offering price of up to $75.0 million (the "2022 Sales Agreement"). The Company did not issue any shares of common stock pursuant to the 2022 Sales Agreement prior to the year ended December 31, 2022. During 2023, the Company issued an aggregate of 130,598 shares of common stock pursuant to the 2022 Sales Agreement at a weighted average purchase price of $2.49 resulting in aggregate gross proceeds of approximately $0.3 million, reduced by $0.2 million in issuance costs, resulting in net proceeds to the Company of approximately $0.1 million. As of December 31, 2023, $74.7 million is available pursuant to the Company's 2022 Sales Agreement. Our ability to utilize the $74.7 million of capacity remaining under the 2022 Sales Agreement is limited by our compliance with the baby shelf rules (as defined below). As of the filing of this Annual Report on Form 10-K, our public float is less than $75 million, and under SEC regulations, for so long as our public float remains less than $75 million, the amount we can raise through primary public offerings of securities in any twelve-month period using shelf registration statements subject to Instruction I.B.6. to Form S-3 is limited to an aggregate of one-third of our public float, which is referred to as the “baby shelf rules.” As of February 23, 2024, our public float was approximately $43.2 million, based on 34,008,061 shares of outstanding common stock held by non-affiliates and at a price of $1.27 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on February 23, 2024.
70
Results of Operations
Comparison of the Fiscal Years Ended December 31, 2023 and 2022
| (In thousands, except per share amounts, percentages and billable test revenue samples) | Year Ended December 31, | ||||||||||||||||||||||
| 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||
| Revenues: | |||||||||||||||||||||||
| Test revenue | $ | 14,384 | $ | 13,790 | $ | 594 | 4 | % | |||||||||||||||
| Contract revenue | 912 | 728 | 184 | 25 | % | ||||||||||||||||||
| Total revenues | 15,296 | 14,518 | 778 | 5 | % | ||||||||||||||||||
| Cost of revenues: | |||||||||||||||||||||||
| Cost of test revenue | 14,792 | 13,702 | 1,090 | 8 | % | ||||||||||||||||||
| Cost of contract revenue | 228 | 169 | 59 | 35 | % | ||||||||||||||||||
| Total cost of revenues | 15,020 | 13,871 | 1,149 | 8 | % | ||||||||||||||||||
Gross profit | 276 | 647 | (371) | (57) | % | ||||||||||||||||||
Gross profit as a percent of total revenue | 2 | % | 4 | % | |||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| Sales and marketing | 44,995 | 58,674 | (13,679) | (23) | % | ||||||||||||||||||
| Research and development | 15,239 | 24,052 | (8,813) | (37) | % | ||||||||||||||||||
| General and administrative | 43,781 | 36,086 | 7,695 | 21 | % | ||||||||||||||||||
| Total operating expenses | 104,015 | 118,812 | (14,797) | (12) | % | ||||||||||||||||||
| Loss from operations | (103,739) | (118,165) | 14,426 | (12) | % | ||||||||||||||||||
| Other income: | |||||||||||||||||||||||
| Interest income, net | 2,846 | 1,341 | 1,505 | * | |||||||||||||||||||
| Change in fair value of warrant liability | 5 | 141 | (136) | (96) | % | ||||||||||||||||||
| Total other income | 2,851 | 1,482 | 1,369 | 92 | % | ||||||||||||||||||
| Net loss | $ | (100,888) | $ | (116,683) | $ | 15,795 | (14) | % | |||||||||||||||
| Basic and diluted net loss per share | $ | (3.09) | $ | (3.88) | $ | 0.79 | (20) | % | |||||||||||||||
| Other Operating Data: | |||||||||||||||||||||||
| Billable test revenue samples | 66,540 | 68,230 | (1,690) | (2) | % | ||||||||||||||||||
* Absolute value percentage change greater than 100
Test Revenue
Test revenues grew $0.6 million or 4% to $14.4 million for fiscal year 2023 compared to $13.8 million for fiscal year 2022. The increase in test revenues was primarily driven by an increase in average selling price as we have prioritized billable sample volume with contracted payors, and lower revenue adjustments for tests run in prior periods.
Billable samples decreased to approximately 66,540 for fiscal year 2023 compared to approximately 68,230 for fiscal year 2022. Sample volume is dependent on two major factors: the number of clinicians who order a test in any given quarter and the number of tests ordered by each clinician during the period. The number of ordering clinicians and the utilization per clinician can vary based on a number of factors including the types of skin cancer conditions presented to clinicians, clinician reimbursement, office workflow, market awareness, clinician education and other factors. We are also currently prioritizing volume in geographies where we have payor coverage versus overall volume growth as one factor to potentially increase average selling price. The decrease in sample volume was due in part to our prioritizing reimbursed tests instead of total volume, and the overall reduction in the size of our sales force, seasonality in demand where fewer tests were ordered during the end of year holiday season and our cessation of testing pediatric patients and patients with certain Fitzpatrick skin types. We anticipate that the reduction in sales territories from our January restructuring may further reduce billable sample volumes in the first half of 2024.
71
Contract Revenue
Contract revenues with pharmaceutical companies increased $0.2 million to $0.9 million for fiscal year 2023, or 25%, compared to $0.7 million for fiscal year 2022. The increase is attributable to a higher number of kit shipments to our contract research customers. Contract revenues can be highly variable as it is dependent on the pharmaceutical customers’ clinical trial progress, which can be difficult to forecast due to variability of patient enrollment, drug safety and efficacy and other factors.
Cost of Revenue
Cost of revenues increased $1.1 million, or 8%, to $15.0 million for fiscal year 2023 compared to $13.9 million for fiscal year 2022. While billable sample volume decreased in 2023 relative to billable sample volume in 2022, cost of revenue increased during the current year primarily due to higher overhead costs pertaining to our new laboratory facility. As of December 31, 2023, a large portion of the costs of revenue are fixed, and these costs include the CLIA facility, quality assurance, management and supervision and equipment calibration and depreciation. The variable cost of revenue expenses incurred primarily relate to compensation-related costs for our laboratory scientists and technicians, laboratory supplies, shipping costs and Smart Sticker collection kits. We remain committed to continuing the improvements of our laboratory processes in order to become more cost efficient and productive.
Operating Expenses
Sales and Marketing
Sales and marketing expenses decreased $13.7 million, or 23%, to $45.0 million for fiscal year 2023 compared to $58.7 million for fiscal year 2022. The decrease was due to a reduction in employee compensation related cost from our 2023 Restructuring Plan, reduced spend around marketing activities, reduced consulting and conference costs.
Research and Development
R&D expenses decreased $8.8 million, or 37%, to $15.2 million for fiscal year 2023 compared to $24.1 million for fiscal year 2022. The decrease was due to reduced compensation costs from lower headcount, lower clinical study costs and lower lab supply spending.
General and Administrative
General and administrative expenses increased $7.7 million, or 21%, to $43.8 million for fiscal year 2023 compared to $36.1 million for fiscal year 2022. The increase was primarily due to $2.1 million in costs related to the 2023 Restructuring Plan, separation benefits of $3.6 million related to our former CEO, including $3.0 million of stock-based compensation for the acceleration of share-based awards, and higher overhead from our new facility.
Interest Income, net
Interest income, net for fiscal year 2023 was $2.8 million compared to interest income, net of $1.3 million for fiscal year 2022. Interest income, net consists primarily of interest earned on our short-term marketable securities.
Change in Fair Value of Warrant Liability
Change in fair value of warrant liability for fiscal year 2023 was a gain of $5,000 compared to a gain of $0.1 million for fiscal year 2022. The change in fair value of warrant liability is calculated by adjusting the value of the outstanding Private SPAC Warrants held by original holders to the current market value at each reporting period.
Liquidity and Capital Resources
We have never been profitable and have historically incurred substantial net losses, including net losses of $116.7 million for the twelve months ended December 31, 2022, and $100.9 million for the twelve months ended December 31, 2023. As of December 31, 2023, our accumulated deficit was $423.9 million, and for the twelve months ended December 31, 2023, we had negative operating cash flow of $77.0 million. At the end of 2020 and throughout 2021 and 2023, we raised approximately $50.3 million in gross proceeds facilitated through our at-the-market offerings. In addition, we completed an underwritten public offering in January 2021, which raised a total of $143.7 million in gross proceeds. We have historically financed operations through private placement and public equity offerings.
72
We expect our losses to continue as a result of costs relating to ongoing R&D expenses, general and administrative expenses and sales and marketing costs for existing products. These losses have had, and will continue to have, an adverse effect on our working capital. Because of the numerous risks and uncertainties associated with our commercialization and development efforts, we are unable to predict when we will become profitable, and we may never become profitable. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations and cash flows.
As of December 31, 2023, our cash and cash equivalents totaled approximately $36.7 million and short-term marketable securities totaled approximately $19.1 million. The Company's management has evaluated whether or not our cash and cash equivalents on hand would be sufficient to sustain projected operating activities through at least the next 12 months from the issuance of our consolidated financial statements for the year ended December 31, 2023 (this 12-month period from the date of issuance, the “Evaluation Period”) as required by Accounting Standards Codification (ASC) 205-40 Disclosure of Uncertainties About an Entity’s Ability to Continue as a Going Concern. This assessment must be made by management on a quarterly basis based on the facts and circumstances then in existence and available to or known by management and entails analyzing prospective operating budgets and forecasts for expectations of the Company’s cash needs and comparing those needs to the current cash and cash equivalent balances.
The Company's management expects that based on its currently planned business operations and the anticipated effects of the Restructuring Plans, currently available resources will not provide sufficient funds to meet its anticipated operating costs for at least the next 12 months from the Evaluation Period. Accordingly, the Company has concluded that substantial doubt exists about the Company's ability to continue as a going concern for a period of at least 12 months from the date of issuance of the consolidated financial statements for the year ended December 31, 2023. If we are unable to obtain additional funding on acceptable terms when and as needed, we may be forced to delay or reduce the scope of our commercial and sales activities, extend payment terms with suppliers, liquidate assets where possible at a potentially lower amount than as recorded in our financial statements, further curtail planned operations or cease operations entirely and wind down our business. Any of these could materially and adversely affect the Company's liquidity, financial condition and business prospects and, as a result, our stockholders may not receive full value, or may receive no value, for their investment. In light of our existing cash and cash equivalents and our current obligations, such a liquidation or disposition process may occur subject to bankruptcy protections, which may further reduce the value that we may receive for our assets.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above.
Even if we obtain enough capital to fund anticipated operating costs for at least the next 12 months, we expect to incur significant additional operating losses over at least the next several years. We anticipate that we will raise additional capital through equity offerings, debt financings, collaborations or licensing arrangements in order to support our planned operations and to continue developing and commercializing genomic tests. We may also consider raising additional capital in the future to expand our business and to pursue strategic investments. Our present and future funding requirements will depend on many factors, including:
•our revenue growth rate and ability to generate cash flows from operating activities;
•our ability to successfully execute and realize the intended benefits of the Restructuring Plans;
•our sales and marketing and R&D activities;
•effects of competing technological and market developments;
•costs of and potential delays in product development;
•changes in regulatory oversight applicable to our tests;
•timing of and costs related to future international expansion; and
•our ability to maintain the listing of our common stock on The Nasdaq Capital Market.
73
There can be no assurances that we will be able to secure such additional financing, if at all, or on terms that are satisfactory to us, and that it will be sufficient to meet our needs. In the event that we are able to secure additional financing, the various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, convertible debt or securities convertible into equity our stockholders may experience substantial dilution and the terms of these new securities could provide for rights, preferences, or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences, and privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our platform technologies or products, or grant licenses on terms that are not favorable to us. Additional equity or debt financing might not be available on reasonable terms, if at all. We will also need to raise additional capital to expand our business to meet our long-term business objectives. Additional financing may be from the sale of equity or convertible or other debt securities in a public or private offering, from a credit facility or strategic partnership coupled with an investment in us, or a combination of both.
Cash Flow Analysis
| (amounts in thousands) | Twelve Months Ended December 31, | ||||||||||
| 2023 | 2022 | ||||||||||
| Net cash used in operating activities | $ | (76,979) | $ | (95,260) | |||||||
| Net cash provided by/(used in) investing activities | 29,864 | (4,319) | |||||||||
| Net cash provided by financing activities | 6,079 | 917 | |||||||||
Fiscal Year Ended December 31, 2023
Net cash used in operating activities for the twelve months ended December 31, 2023 totaled $77.0 million primarily driven by the $100.9 million net loss offset partially by non-cash related items, including $18.6 million in stock-based compensation, $4.0 million in amortization of operating lease right-of-use assets, $1.8 million in depreciation, and $0.5 million in accretion of discounts, net of amortization of premiums on marketable securities. In addition, we had a net cash inflow of $11,000 through net changes in working capital balances driven primarily by cash inflows of $1.6 million due to the decrease in accounts receivable, $0.8 million from the decrease in inventory, $1.8 million through the decrease of prepaid expenses and other current assets offset by the cash outflows of $1.2 million from the decrease in accrued compensation, $1.8 million through the decrease in accounts payable, accrued liabilities and deferred revenue and $1.1 million from the decrease in operating lease liabilities.
Net cash provided by investing activities for the twelve months ended December 31, 2023 totaled $29.9 million, which related to the cash inflow from the sale and maturity of marketable securities of $65.2 million partially offset by cash outflows of $34.4 million from the purchase of marketable securities and $0.9 million from the purchase of equipment.
Net cash provided by financing activities for the twelve months ended December 31, 2023 totaled $6.1 million, which was driven primarily by $5.2 million in net proceeds raised through our at-the-market offerings from the 2020 Sales Agreement and the 2022 Sales Agreement and $0.9 million in proceeds from contributions to the employee stock purchase plan.
Off-Balance Sheet Arrangements
As of December 31, 2023 and 2022, we did not have any off-balance sheet arrangements, as such term is defined under Item 303 of Regulation S-K, that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Critical Accounting Policies and Significant Judgments and Estimates
The preparation of consolidated financial statements in conformity with U.S. generally accepted accounting principles (“U.S. GAAP”) requires that management make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the amounts of revenues and expenses reported during the period. On an ongoing basis, management evaluates these estimates and judgments, including but not limited to those related to test revenue, stock-based compensation, short-term marketable securities, accounts receivable, accrued bonus, warrant liability, right-of-use (“ROU”) assets and the realization of deferred tax assets. Actual results may differ from those estimates.
74
The SEC has defined a company’s critical accounting policies as the ones that are most important to the portrayal of the company’s financial condition and results of operations, and which require the company to make its most difficult and subjective judgments, often as a result of the need to make estimates of matters that are inherently uncertain. While our significant accounting policies are more fully described in Note 1 of our consolidated financial statements included in this report, we believe that the following accounting policies and judgments are most critical to aid in fully understanding and evaluating our reported financial results based upon the SEC’s defined criteria.
Revenue Recognition
Our revenue is generated from two revenue streams, contract revenue and test revenue. We account for revenue in accordance with Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of ASC 606 is that the Company recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. The ASC 606 revenue recognition model consists of the following five steps: (1) identify the contracts with a customer, (2) identify the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price to the performance obligations in the contract and (5) recognize revenue when (or as) the entity satisfies a performance obligation.
(a)Test Revenue
We generate revenues from the DMT we provide to healthcare clinicians throughout the United States to assist in a clinician’s diagnosis of melanoma. We provide prescribing clinicians with our Smart Sticker to perform non-invasive skin biopsies of clinically ambiguous pigmented skin lesions on patients.
Once the sample is collected by the healthcare clinician or the patient via the telemedicine solution, it is returned to our CLIA laboratory for analysis. The patient’s ribonucleic acid (“RNA”) and deoxyribonucleic acid (“DNA”) are extracted from the Smart Sticker and analyzed using gene expression technology to determine if the pigmented skin lesion contains certain genomic features indicative of melanoma. Upon completion of the gene expression analysis, a final report is drafted and provided to the clinician detailing the test results for the pigmented skin lesion indicating whether the sample collected is indicative of melanoma or not. A detailed historical analysis of payments made to us by private health insurance payors is used to estimate the expected receipt of funds for payment of billed amounts. These payments can vary widely from payor to payor and can be halted for routine audits or other reasons.
(b)Contract Revenue
Contract revenue is generated from the sale of laboratory services and Smart Stickers to third party companies through contract research agreements. Revenues are generated from providing gene expression services to facilitate the development of drugs designed to treat dermatologic conditions. The provision of gene expression services may include sample collection using our Smart Stickers, assay development for research partners, RNA extraction, isolation, expression, amplification and detection, including data analysis and reporting.
See Note 1(l) of our consolidated financial statements for a full discussion of our revenue recognition policy around test revenue and contract revenue.
Stock-Based Compensation
Compensation costs associated with stock option awards and other forms of equity compensation are measured at the grant-date fair value of the awards and recognized over the requisite service period of the awards on a ratable basis. We grant stock options to purchase common stock to employees with exercise prices equal to the fair market value of the underlying stock. The fair market value of stock options is based on the closing stock price on the grant date.
The fair value of each stock option award is estimated using the Black-Scholes-Merton valuation model. Such value is recognized as expense over the requisite service period using the ratable method. The expected term of options is based on the simplified method which defines the expected term as the average of the contractual term of the options and the weighted average vesting period for all option tranches. The expected volatility of stock options is based upon the historical volatility of a number of related publicly traded companies in similar stages of development as well as the volatility of our common stock. The risk-free interest rate is based on the average yield of U.S. Treasury securities with remaining terms similar to the expected term of the share-based awards. The assumed dividend yield was based on our expectation of not paying dividends in the foreseeable future.
75
We account for stock options to non-employees using the fair value approach. The fair value of these options is measured using the Black-Scholes-Merton option pricing model, reflecting the same assumptions applied to employee options, other than expected life, which is assumed to be the remaining contractual life of the award. Options that are granted to employees generally have a requisite service period of three to four years.
Restricted stock units (“RSUs”) are considered restricted stock. The fair market value of RSUs is based on the closing stock price on the grant date. We recognize stock-based compensation expense based on the fair value on a ratable basis over the requisite service periods of the awards. RSUs that are granted to employees have a requisite service period typically between two and four years.
Recent Accounting Pronouncements
See Note 1(u) of our consolidated financial statements for a discussion of the impact of new accounting pronouncements on our consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Our cash, cash equivalents, and short-term marketable securities are subject to economic risk which could affect our results of operations, financial condition and cash flows. We manage our exposure to this market risk through our regular operating and financing activities.
Interest Rate Risk
The primary objective of our investment activities is capital preservation to fund operations, while at the same time maximizing investment income without significantly increasing investment risk. To achieve these objectives, our investment policy allows for a portfolio of cash equivalents and investments in a variety of securities, including money market funds, U.S. government debt and corporate debt securities. Due to the short-term and conservative nature of our investments, we do not believe that we have a material exposure to interest rate risk. A 100 basis point change in interest rates would not have a significant impact on the total value of our portfolio.
76
Item 8. Consolidated Financial Statements and Supplementary Data
DERMTECH, INC.
Index to Consolidated Financial Statements
| Page | |||||
77
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
DermTech, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of DermTech, Inc. and subsidiaries (the Company) as of December 31, 2023 and 2022, the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for the years then ended, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has an accumulated deficit that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Measurement of test revenue
As discussed in Note 1 of the consolidated financial statements, the Company’s revenue is generated from two revenue streams: contract revenue and test revenue. The Company generates test revenue from its DermTech Melanoma Test it provides to healthcare clinicians. The transaction price is the amount of consideration that the Company expects to collect in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties. The consideration expected from an agreement with a customer may include fixed amounts, variable amounts, or both. The Company estimates the amount of variable consideration
78
using the expected value method, which represents the sum of probability-weighted amounts in a range of possible consideration amounts. When estimating the amount of variable consideration, the Company considers several factors, such as historical collections experience, patient insurance eligibility and payor reimbursement agreements. The Company recorded $14,384 thousand of test revenue for the year ended December 31, 2023.
We identified the evaluation of the measurement of test revenue as a critical audit matter. Evaluating the measurement of test revenue, specifically the estimate of revenue expected to be collected, involved complex auditor judgment.
The following are the primary procedures we performed to address this critical audit matter. For a sample of tests performed in the current period, we evaluated the claims included within the Company’s test revenue recognition model by comparing them to certain relevant documentation, including test requisition forms, test results, payor contracts, and proof of delivery to the physician. For a sample of payments received in the current period, we evaluated the payments included within the Company’s test revenue recognition model by agreeing them to payments received. We tested the accuracy of the Company’s test revenue recognition model, which includes the collection history used by management in assessing the current period revenue realization percentages, by payor. We developed an expectation of the estimated revenue realization percentages applied to certain payor groups, using actual collection history, to assess its impact on the Company’s measurement of test revenue. We further evaluated the estimate of test revenue expected to be collected by inquiring of individuals of the Company responsible for monitoring and tracking the status of collections and developing the estimate.
/s/ KPMG LLP
We have served as the Company’s auditor since 2016.
San Diego, California
February 29, 2024
79
DERMTECH, INC.
Consolidated Balance Sheets
(in thousands, except share and per share data)
| December 31, 2023 | December 31, 2022 | ||||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Short-term marketable securities | |||||||||||
| Accounts receivable | |||||||||||
| Inventory | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Property and equipment, net | |||||||||||
| Operating lease right-of-use assets | |||||||||||
| Restricted cash | |||||||||||
| Other assets | |||||||||||
| Total assets | $ | $ | |||||||||
| Liabilities and Stockholders’ Equity | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued compensation | |||||||||||
| Accrued liabilities | |||||||||||
| Short-term deferred revenue | |||||||||||
| Current portion of operating lease liabilities | |||||||||||
| Current portion of finance lease obligations | |||||||||||
| Total current liabilities | |||||||||||
| Warrant liability | |||||||||||
| Long-term finance lease obligations, less current portion | |||||||||||
| Operating lease liabilities, long-term | |||||||||||
| Total liabilities | |||||||||||
| Stockholders’ equity: | |||||||||||
Common stock, $ | |||||||||||
| Additional paid-in capital | |||||||||||
| Accumulated other comprehensive income/(loss) | ( | ||||||||||
| Accumulated deficit | ( | ( | |||||||||
| Total stockholders’ equity | |||||||||||
| Total liabilities and stockholders’ equity | $ | $ | |||||||||
See accompanying notes to consolidated financial statements.
80
DERMTECH, INC.
Consolidated Statements of Operations
(in thousands, except share and per share data)
Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Revenues: | |||||||||||
| Test revenue | $ | $ | |||||||||
| Contract revenue | |||||||||||
| Total revenues | |||||||||||
| Cost of revenues: | |||||||||||
| Cost of test revenue | |||||||||||
| Cost of contract revenue | |||||||||||
| Total cost of revenues | |||||||||||
| Gross profit | |||||||||||
| Operating expenses: | |||||||||||
| Sales and marketing | |||||||||||
| Research and development | |||||||||||
| General and administrative | |||||||||||
| Total operating expenses | |||||||||||
| Loss from operations | ( | ( | |||||||||
| Other income: | |||||||||||
| Interest income, net | |||||||||||
| Change in fair value of warrant liability | |||||||||||
| Total other income | |||||||||||
| Net loss | $ | ( | $ | ( | |||||||
| Weighted average shares outstanding used in computing net loss per share, basic and diluted | |||||||||||
| Net loss per share of common stock outstanding, basic and diluted | $ | ( | $ | ( | |||||||
See accompanying notes to consolidated financial statements.
81
DERMTECH, INC.
Consolidated Statements of Comprehensive Loss
(in thousands)
Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Net loss | $ | ( | $ | ( | |||||||
| Unrealized net gain/(loss) on marketable securities and cash equivalents | ( | ||||||||||
| Comprehensive loss | $ | ( | $ | ( | |||||||
See accompanying notes to consolidated financial statements.
82
DERMTECH, INC.
Consolidated Statements of Stockholders’ Equity
(in thousands, except share and per share data)
| Common stock | Additional paid-in capital | Accumulated other comprehensive loss | Accumulated deficit | Total stockholders’ equity | |||||||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||||||||
Balance, December 31, 2021 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||
| Issuance of common stock from option exercises and RSU releases | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock from warrant exercises | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock from Employee Stock Purchase Plan | — | — | — | ||||||||||||||||||||||||||||||||
| Unrealized net loss on available-for-sale marketable securities and cash equivalents | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
Balance, December 31, 2022 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||
Issuance of common stock at a weighted average price of $ | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock from option exercises and RSU releases | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock from Employee Stock Purchase Plan | — | — | — | ||||||||||||||||||||||||||||||||
| Unrealized net gain on available-for-sale marketable securities and cash equivalents | — | — | — | — | |||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
Balance, December 31, 2023 | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
83
DERMTECH, INC.
Consolidated Statements of Cash Flows (in thousands)
Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Cash flows from operating activities: | |||||||||||
| Net loss | $ | ( | $ | ( | |||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
| Depreciation | |||||||||||
| Change in fair value of warrant liability | ( | ( | |||||||||
| Amortization of operating lease right-of-use assets | |||||||||||
| Stock-based compensation | |||||||||||
| (Accretion) Amortization of (discount) premium on marketable securities | ( | ||||||||||
| Loss on disposal of equipment | |||||||||||
| Changes in operating assets and liabilities: | |||||||||||
| Accounts receivable | ( | ||||||||||
| Inventory | ( | ||||||||||
| Prepaid expenses and other current assets | ( | ||||||||||
| Operating lease right-of-use assets and liabilities, net | ( | ( | |||||||||
| Accounts payable, accrued liabilities and deferred revenue | ( | ||||||||||
| Accrued compensation | ( | ||||||||||
| Net cash used in operating activities | ( | ( | |||||||||
| Cash flows from investing activities: | |||||||||||
| Purchases of marketable securities | ( | ( | |||||||||
| Maturities and sales of marketable securities | |||||||||||
| Purchases of property and equipment, net | ( | ( | |||||||||
| Net cash provided by/(used in) investing activities | ( | ||||||||||
| Cash flows from financing activities: | |||||||||||
| Proceeds from issuance of common stock in connection with at-the-market offering, net | |||||||||||
| Proceeds from exercise of common stock warrants | |||||||||||
| Proceeds from exercise of stock options | |||||||||||
| Proceeds from contributions to the employee stock purchase plan | |||||||||||
| Principal repayments of finance lease obligations | ( | ( | |||||||||
| Net cash provided by financing activities | |||||||||||
| Net decrease in cash, cash equivalents and restricted cash | ( | ( | |||||||||
| Cash, cash equivalents and restricted cash, beginning of period | |||||||||||
| Cash, cash equivalents and restricted cash, end of period | $ | $ | |||||||||
| Reconciliation of cash, cash equivalents and restricted cash, end of period: | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Restricted cash | |||||||||||
| Total cash, cash equivalents and restricted cash | $ | $ | |||||||||
| Supplemental cash flow information: | |||||||||||
| Cash paid for interest on finance lease obligations | $ | $ | |||||||||
| Supplemental disclosure of noncash investing and financing activities: | |||||||||||
| Purchases of property and equipment recorded in accounts payable | $ | $ | |||||||||
| Right-of-use assets obtained in exchange for lease obligations | $ | $ | |||||||||
| Property and equipment acquired under finance leases | $ | $ | |||||||||
| Change in unrealized net gains/(losses) on available-for-sale marketable securities | $ | $ | ( | ||||||||
See accompanying notes to consolidated financial statements.
84
DERMTECH, INC.
Notes to Consolidated Financial Statements
1. The Company and a Summary of its Significant Accounting Policies
(a) Nature of Operations
On August 29, 2019, DermTech, Inc., formerly known as Constellation Alpha Capital Corp, (the “Company”), and DermTech Operations, Inc., formerly known as DermTech, Inc., (“DermTech Operations”), consummated the transactions contemplated by the Agreement and Plan of Merger, dated as of May 29, 2019, by and among the Company, DT Merger Sub, Inc., a wholly owned subsidiary of the Company (“Merger Sub”), and DermTech Operations. The Company refers to this agreement, as amended by that certain First Amendment to Agreement and Plan of Merger dated as of August 1, 2019, as the Merger Agreement. Pursuant to the Merger Agreement, Merger Sub merged with and into DermTech Operations, with DermTech Operations surviving as a wholly-owned subsidiary of the Company. The Company refers to this transaction as the Business Combination. In connection with and two days prior to the completion of the Business Combination, the Company domesticated from the British Virgin Islands to Delaware. DermTech Operations changed its name from DermTech, Inc. to DermTech Operations, Inc. shortly before the completion of the Business Combination. On August 29, 2019, immediately following the completion of the Business Combination, the Company changed its name from Constellation Alpha Capital Corp. to DermTech, Inc., and then effected a one-for-two reverse stock split of its common stock (“Reverse Stock Split”).
The Company is a molecular diagnostic company developing and marketing its Clinical Laboratory Improvement Amendments of 1988 (“CLIA”) laboratory services including molecular pathology tests to facilitate the diagnosis of melanoma and management of skin cancer. The Company has developed a proprietary, non-invasive technique for sampling the surface layers of the skin using an adhesive patch called the DermTech Smart Sticker™ (the “Smart Sticker”) in order to collect individual biological information for commercial applications in the medical diagnostic field.
(b) Basis of Presentation
(c) Going Concern
At each reporting period, the Company evaluates whether there are conditions or events that raise substantial doubt about the Company’s ability to continue as a going concern within 12 months after the date that the financial statements are issued (this 12-month period from the date of issuance, the “Evaluation Period”). The Company’s evaluation is based on the facts and circumstances then in existence and available to or known by management and entails analyzing prospective operating budgets and forecasts for expectations of the Company’s cash needs and comparing those needs to the current cash and cash equivalent balances. The Company is required to make certain additional disclosures if it concludes substantial doubt exists and it is not alleviated by the Company’s plans or when its plans alleviate substantial doubt about the Company’s ability to continue as a going concern.
In accordance with Accounting Standards Codification ("ASC") 205-40, Going Concern, the Company evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about its ability to continue as a going concern within the Evaluation Period. This evaluation initially does not take into consideration the potential mitigating effect of management’s plans that have not been fully implemented as of the date the financial statements are issued. When substantial doubt exists under this methodology, management evaluates whether the mitigating effect of its plans sufficiently alleviates substantial doubt about the Company’s ability to continue as a going concern. The mitigating effect of management’s plans, however, is only considered if both (1) it is probable that the plans will be effectively implemented within one year after the date that the financial statements are issued, and (2) it is probable that the plans, when implemented, will mitigate the relevant conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued.
85
The Company has incurred operating losses since inception and has an accumulated deficit of $423.9 million as of December 31, 2023. As of December 31, 2023, cash and cash equivalents totaled approximately $36.7 million and short-term marketable securities totaled approximately $19.1 million. For the year ended December 31, 2023, The Company reported a net loss of $100.9 million and cash used in operating activities of $77.0 million. The Company's transition to profitable operations is dependent upon achieving a level of revenues adequate to support its cost structure. The timing and amount of the Company's actual expenditures will be based on many factors, including cash flows from operations and the potential growth of its business, and may vary from current estimates. The Company's management expects that based on its currently planned business operations and considering the restructuring activities (Note 5) implemented in June 2023, currently available resources will not provide sufficient funds to meet its anticipated operating costs within the Evaluation Period. The Company currently anticipates that it will need to raise additional capital, increase average selling prices and revenues and may need to further reduce operating costs following the Evaluation Period. Accordingly, the Company has concluded that substantial doubt exists about the Company's ability to continue as a going concern for a period of at least 12 months from the date of issuance of the consolidated financial statements for the year ended December 31, 2023. If we are unable to obtain additional funding on acceptable terms when and as needed, we may be forced to delay or reduce the scope of our commercial and sales activities, extend payment terms with suppliers, liquidate assets where possible at a potentially lower amount than as recorded in our financial statements, further curtail planned operations or cease operations entirely and wind down our business. Any of these could materially and adversely affect the Company's liquidity, financial condition and business prospects and, as a result, our stockholders may not receive full value, or may receive no value, for their investment. In light of our existing cash and cash equivalents and our current obligations, such a liquidation or disposition process may occur subject to bankruptcy protections, which may further reduce the value that we may receive for our assets.
(d) Use of Estimates
(e) Cash, Cash Equivalents and Restricted Cash
The Company considers all highly liquid investments with remaining maturities of three months or less when purchased to be cash equivalents. The Company maintains its cash balances at banks and financial institutions. The balances are insured up to the Federal Deposit Insurance Corporation legal limit. The Company maintains cash balances that may, at times, exceed this insured limit.
Restricted cash consists of cash deposited with a financial institution as collateral for the Company’s letters of credit for its facility leases. Restricted cash is classified as non-current based on the terms of the underlying lease arrangement.
(f) Marketable Securities
The Company considers securities with maturities of greater than 90 days at the time of purchase to be marketable securities. The Company has the ability, if necessary, to liquidate any of its cash equivalents and marketable securities to meet its liquidity needs in the next 12 months. Accordingly, such marketable securities are classified as current assets on the accompanying consolidated balance sheets even if they have contractual maturities greater than one year from the date of purchase. The Company’s marketable securities consist of U.S. Treasury and agency securities, commercial paper, and corporate debt securities. Marketable securities are recorded at fair value and unrealized gains and losses are recorded within accumulated other comprehensive loss. The estimated fair value of the marketable securities is determined based on quoted market prices or rates for similar instruments. The Company evaluates securities with unrealized losses to determine whether such losses, if any, are due to credit-related factors. The Company records an allowance for credit losses when unrealized losses are due to credit-related factors. Realized gains and losses are calculated using the specific identification method and recorded as interest income or expense. The Company does not generally intend to sell the investments and it is more likely than not that the Company will not be required to sell the investments before recovery of their amortized cost basis, which may occur at maturity.
86
(g) Property and Equipment, Net
Property and equipment is recorded at cost less accumulated depreciation. Maintenance and repairs are expensed as incurred, and improvements are capitalized. Property and equipment consists mainly of assets such as leasehold improvements, office, computer and laboratory equipment, including laboratory equipment acquired under finance lease arrangements. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, which range from to seven years . Leasehold improvements are depreciated over the shorter of the remaining term of the lease or the useful life of the asset. Amortization of assets that are recorded under finance leases is recorded in depreciation expense. When assets are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the consolidated balance sheet and any resulting gain or loss is reflected in the consolidated statements of operations in the period realized.
(h) Leases
The Company acts as lessee in its lease agreements, which include operating leases for corporate offices and finance leases for certain laboratory and office equipment.
In accordance with Accounting Standards Update (“ASU”) No. 2016-02, Leases (Topic 842), as adopted on January 1, 2021, the Company determines if an arrangement is a lease at inception. Finance leases are included in the consolidated balance sheets as property and equipment, net and finance lease obligations at the present value of the lease payments. Operating leases are included in the consolidated balance sheet as ROU assets and operating lease liabilities at the present value of the lease payments. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent an obligation to make lease payments based on the present value of lease payments over the lease term. Classification of lease liabilities as either current or non-current is based on the expected timing of payments due under the Company’s obligations.
As most of the Company’s leases do not provide an implicit interest rate, the Company uses its incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The incremental borrowing rate is the rate of interest that a lessee would have to pay to borrow on a collateralized basis over a similar term and at an amount equal to the lease payments in a similar economic environment. In order to determine the appropriate incremental borrowing rates, the Company has used a number of factors including the credit rating, and the lease term.
The ROU asset also consists of any lease incentives received. The lease terms used to calculate the ROU asset and related lease liability include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. “Reasonably certain” is assessed internally based on economic, industry, company, strategic and contractual factors. Operating lease expense and amortization of finance lease ROU assets are recognized on a straight-line basis over the lease term as an operating expense. Finance lease interest expense is recorded as interest income, net on the Company’s consolidated statements of operations.
The Company has taken advantage of certain practical expedients offered to registrants at adoption of ASC 842. The Company does not apply the recognition requirements of ASC 842 to short-term leases. Instead, those lease payments are recognized in profit or loss on a straight-line basis over the lease term. Further, as a practical expedient, all lease contracts are accounted for as one single lease component, as opposed to separating lease and non-lease components to allocate the consideration within a single lease contract.
(i) Research and Development
Costs incurred in connection with research and development (“R&D”) activities are expensed as incurred. R&D expenses consist of (i) employee-related expenses, including salaries, benefits, travel and stock-based compensation expense; and (ii) facilities and other expenses, which include direct and allocated expenses for rent and maintenance of facilities and laboratory and other supplies.
87
(j) Concentration of Credit Risk
(k) Income Taxes
The Company provides for federal and state income taxes on the asset and liability approach which requires deferred tax assets and liabilities to be recognized based on temporary differences between the consolidated financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the temporary differences are expected to reverse.
Deferred tax assets are reduced by a valuation allowance when, in management’s opinion, it is more likely than not that some portion or all of the deferred tax assets will not be realized. The Company considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. The Company’s valuation allowance is based on available evidence, including its current year and prior year operating losses, evaluation of positive and negative evidence with respect to certain specific deferred tax assets including evaluation sources of future taxable income to support the realization of the deferred tax assets. The Company has established a full valuation allowance on the deferred tax assets as of December 31, 2023.
Current and deferred tax assets and liabilities are recognized based on the tax positions taken or expected to be taken in the Company’s income tax returns. U.S. GAAP requires that the tax benefits of an uncertain tax position can only be recognized when it is more likely than not that the tax position will be sustained upon examination by the relevant taxing authority. Tax benefits related to tax positions that do not meet this criterion are not recognized in the consolidated financial statements, of which there are none.
(l) Revenue Recognition
The Company’s revenue is generated from two revenue streams: contract revenue and test revenue. The Company accounts for revenue in accordance with ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of ASC 606 is that the Company recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. The ASC 606 revenue recognition model consists of the following five steps: (1) identify the contracts with a customer, (2) identify the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price to the performance obligations in the contract and (5) recognize revenue when (or as) the entity satisfies a performance obligation.
The Company recognizes revenue from its test and contract services in accordance with the core principles and key aspects considered by the Company. These considerations are described in detail below, first for Test Revenue and then for Contract Revenue.
88
Test Revenue
The Company generates revenues from its DermTech Melanoma Test or “DMT” which may consist at the option of the ordering clinician of either (i) the DMT or (ii) the DMT with the TERT add-on test (which add-on test will be discontinued effective March 1, 2024), which assists a clinician’s diagnosis of melanoma in patients. The Company provides prescribing clinicians with its Smart Sticker to perform non-invasive skin biopsies of clinically ambiguous pigmented skin lesions on patients. The Company also offers clinicians a telemedicine solution where they can request the Smart Sticker collection kit be sent to the patient’s home for a clinician-guided remote collection on ambiguous pigmented skin lesions. A patient can also initiate the process by downloading the Company’s telemedicine app, DermTech Connect, which uses store-and-forward technology to allow the patient to take a picture of a suspicious lesion with their phone and have the picture reviewed by an independent clinician who is subscribing to the DermTech Connect platform to assess the suspicious lesion, and if medically necessary, order a DMT where a collection kit would be sent to the patient’s home. The DermTech Connect app and telemedicine service were initially beta tested in Florida and is currently available in most states where permitted by law and applicable standards of practice guidelines. Once the sample is collected by the patient via the telemedicine solution or by a healthcare clinician in person, it is returned to the Company’s CLIA laboratory for analysis. The patient’s ribonucleic acid (“RNA”) and deoxyribonucleic acid (“DNA”) are extracted from the Smart Sticker and analyzed using gene expression and sequencing technology to determine if the pigmented skin lesion contains certain genomic features indicative of melanoma. Upon completion of the gene expression analysis, a final report is drafted and provided to the clinician detailing the test results for the pigmented skin lesion indicating whether the sample collected is indicative of melanoma or not.
Contracts
The Company’s customer is the patient. However, the Company does not enter into a formal reimbursement agreement with a patient, as formal reimbursement agreements are more commonly established with insurance payors. Accordingly, the Company establishes an agreement with a patient in accordance with other customary business practices.
•Approval of an agreement is established by the use of the Company’s Smart Sticker on a patient by an ordering clinician, which is then sent to the Company’s central lab for testing.
•The Company is obligated to perform the Company’s laboratory services upon receipt of a sample from a clinician, and the patient and/or applicable payor are obligated to reimburse the Company for services rendered based on the patient’s insurance benefits.
•Payment terms are a function of a patient’s existing insurance benefits.
•Once the Company delivers a patient’s test result to the ordering physician, the Company is legally able to collect payment and bill an insurer and/or patient, depending on payor agreement status or patient insurance benefit status.
•The Company’s consideration is deemed to be variable, and the Company considers collection of such consideration to be probable to the extent that it is unconstrained.
Performance Obligations
A performance obligation is a promise in an agreement to transfer a distinct good or service (or a bundle of goods or services) to the customer. The customer is able to order a DMT, which is treated as a single performance obligation.
Transaction Price
The transaction price is the amount of consideration that the Company expects to collect in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration expected from an agreement with a customer may include fixed amounts, variable amounts, or both.
The consideration derived from the Company’s agreements is deemed to be variable, though the variability is not explicitly stated in any agreement. Rather, the implied variability is due to several factors, such as the amount of contractual adjustments, any patient co-payments, deductibles or patient compliance incentives, the existence of secondary payors and claim denials.
The Company estimates the amount of variable consideration using the expected value method, which represents the sum of probability-weighted amounts in a range of possible consideration amounts. When estimating the amount of variable consideration, the Company considers several factors, such as historical collections experience, patient insurance eligibility and payor reimbursement agreements.
89
The Company limits the amount of variable consideration included in the transaction price to the unconstrained portion of such consideration. In other words, the Company recognizes revenue up to the amount of variable consideration that is not subject to a significant reversal until additional information is obtained or the uncertainty associated with the additional payments or refunds is subsequently resolved. Differences between original estimates and subsequent revisions, including final settlements, represent changes in the estimate of variable consideration and are included in test revenue in the period in which such revisions are made.
The Company monitors its estimates of transaction price to depict conditions that exist at each reporting date. If the Company subsequently determines that it will collect more consideration than it originally estimated for an agreement with a patient, it will account for the change as an increase in the estimate of the transaction price (i.e., an upward revenue adjustment) in the period identified. Similarly, if the Company subsequently determines that the amount it expects to collect from a patient is less than it originally estimated, it will generally account for the change as a decrease in the estimate of the transaction price (i.e., a downward revenue adjustment) in the period identified.
When the Company does not have significant historical experience or that experience has limited predictive value, the constraint over estimates of variable consideration may result in no revenue being recognized upon delivery of a patient’s test result to the ordering physician, with recognition, generally occurring at the date of cash receipt.
The Company periodically updates its estimate of the variable consideration recognized for previously delivered performance obligations. These updates resulted in a decrease of $1.2 million and $1.8 million of revenue reported for the years ended December 31, 2023 and December 31, 2022, respectively. These amounts included (i) adjustments for actual collections versus estimated variable consideration as of the beginning of the reporting period and (ii) cash collections and the related recognition of revenue in the current period for tests delivered in prior periods due to the release of the constraint on variable consideration, offset by (iii) reductions in revenue for the accrual for reimbursement claims and settlements.
Allocate the Transaction Price
The entire transaction price is allocated entirely to the single performance obligation contained within the agreement with a patient.
Recognize Revenue
The Company’s single performance obligation is satisfied at a point in time, and that point in time is defined as the date a patient’s successful test result is delivered to the patient’s ordering physician. The Company considers this date to be the time at which the patient obtains control of the final results of the promised test service.
Contract Revenue
Contract revenue is generated from the sale of laboratory services and Smart Stickers to third-party companies through contract research agreements. Revenues are generated from providing gene expression tests to facilitate the development of drugs designed to treat dermatologic conditions. The provision of gene expression services may include sample collection using the Company’s Smart Sticker, assay development for research partners, RNA extraction, isolation, expression, amplification and detection, including data analysis and reporting.
Contracts
As part of the Company’s contract revenue, the Company has established agreements and work orders with the Company’s third-party partners that fall under the scope of ASC 606.
Performance Obligations
ASC 606 requires an entity to assess the goods or services promised in a contract and identify as a performance obligation each promise to transfer to the customer either a good or service (or a bundle of goods or services) that is distinct, or a series of distinct goods or services that are substantially the same and that have the same pattern of transfer to the customer. Based upon review of existing contracts, a majority of the Company’s contract revenue agreements contain three performance obligations:
(1)Smart Stickers
(2)RNA extractions and analysis
(3)Certain project management fees
90
Many of the Company’s contract revenue agreements contain promises such as start-up activities and quality system setup fees, which are activities that the Company performs to fulfill the agreement. These promises encompass the administrative tasks associated with beginning and initiating a new project or study with a third-party company. In accordance with ASC 606, an entity does not account for these activities as a promised good or service within the agreement nor evaluate whether they are a performance obligation.
Transaction Price
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer. The consideration promised in an agreement with a customer may include fixed amounts, variable amounts, or both.
The transaction prices of the Company’s performance obligations are listed in its agreements on a per unit basis and are fixed for adhesive sample collection kits and RNA extractions and analysis. The project management fees are assessed based on a monthly service fee which range within the agreements depending on certain factors which include length of the project and the amount of Smart Stickers or RNA extractions and analysis promised within the agreement. The fixed and variable rates are materially consistent within the Company’s agreements. Therefore, the Company utilizes the prices listed in its agreements as the transaction price for each performance obligation.
In determining the transaction price, ASC 606 requires an entity to adjust the promised amount of consideration for the effects of the time value of money if the agreement contains a significant financing component. The Company’s agreements state fixed transaction prices for each deliverable associated with the agreement and do not qualify for the significant financing component of ASC 606.
Allocate the Transaction Price
The Company’s contracts have a directly observable transaction price pertaining to each promised good or service. Those prices are consistent across agreements for Smart Stickers and RNA extractions and analysis, with the exception of the Company’s project management fees, which the Company’s believes encompass a sufficiently narrow range of prices that are dictated upon factors of each agreement previously discussed above. Therefore, the Company relies on those transaction prices as the basis to allocate the stand-alone selling prices to the performance obligations of the agreement.
Most of the Company’s agreements contain a discount that is allocated to items within the agreement, whether they are performance obligations or not. Those items that are not performance obligations (e.g., quality system setup and start up fees) have the associated discount allocated to the transaction prices of the performance obligations evenly.
Recognize Revenue
An entity should recognize revenue when (or as) it satisfies a performance obligation by transferring a promised good or service to a customer. A good or service is transferred when (or as) the customer obtains control of that good or service. The Smart Stickers are recognized at a point in time when shipped to the customer. The RNA extraction and analysis are recognized at a point in time when the extraction and analysis process is complete and the results are sent to the customer. The Company provides its project management service over the life of the agreement, providing equal benefit to the customer throughout the life of the project or study. Therefore, the revenue related to the Company’s project management fees is recognized straight-line over the life of the agreement.
(a) Disaggregation of Revenue
The following table presents the Company’s revenues disaggregated by revenue source during the years ended December 31, 2023 and 2022, respectively (in thousands):
Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Test Revenue | |||||||||||
| DermTech Melanoma Test | $ | $ | |||||||||
| Contract Revenue | |||||||||||
| Smart Stickers | |||||||||||
| RNA extractions | |||||||||||
| Project management fees | |||||||||||
| Total revenues | $ | $ | |||||||||
91
The following table sets forth the percentages of total revenue or accounts receivable for customers that represent 10% or more of the respective amounts for the periods shown:
| Total Revenue | Accounts Receivable | ||||||||||||||||||||||
Year Ended December 31, | As of December 31, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Test Revenue | |||||||||||||||||||||||
| Payor A | % | % | % | % | |||||||||||||||||||
| Payor B | * | * | * | % | |||||||||||||||||||
There were no other payors or customers that individually accounted for more than 10% of total revenue or accounts receivable for the periods shown in the table above.
(b) Deferred Revenue and Remaining Performance Obligations
The timing of revenue recognition, billings and cash collections results in billed accounts receivable and deferred revenue on the consolidated balance sheets.
In a majority of historical agreements that produced contract revenue, the Company received a substantial up-front payment and additional payments upon the achievement of various milestones over the life of the agreement. This results in deferred revenue and is relieved upon delivery of the applicable Smart Stickers or RNA extraction results. Changes in accounts receivable and deferred revenue were not materially impacted by any other factors.
The Company records a deferred revenue liability if a customer pays consideration before the Company transfers a good or service to the customer. Deferred revenue primarily represents upfront milestone payments, for which consideration is received prior to when goods/services are completed or delivered. Upfront fees that are estimated to be recognized as revenue more than one year from the date of collection are classified as long-term deferred revenue. Short-term deferred revenue was $0.2 million and $0.1 million as of December 31, 2023 and December 31, 2022, respectively. As of December 31, 2022 we reclassified $1.0 million of short-term deferred revenue to accrued liabilities for a customer refund obligation in connection with cancellation of future services.
Remaining performance obligations include deferred revenue and amounts the Company expects to receive for goods and services that have not yet been delivered or provided under existing agreements. For agreements that have an original duration of one year or less, the Company has elected the practical expedient applicable to such agreements and does not disclose the remaining performance obligations at the end of each reporting period.
(m) Accounts Receivable
Test Accounts Receivable
Due to the nature of the Company’s test revenue, it can take a significant amount of time to collect upon billed tests. The Company prepares an analysis on reimbursement collections and data obtained for each financial reporting period to determine the amount of receivables to be recorded relating to tests performed in the applicable period. The Company generally does not perform evaluations of customers’ financial condition and generally does not require collateral. Accounts receivable are written off when all efforts to collect the balance have been exhausted. Adjustments for implicit price concessions attributable to variable consideration are incorporated into the measurement of the accounts receivable balances. The Company recorded $2.5 million and $4.1 million of net test accounts receivable as of December 31, 2023 and 2022, respectively.
Contract Accounts Receivable
Contract accounts receivable are recorded at the net invoice value and are not interest bearing. The Company reserves specific receivables if collectability is no longer reasonably assured, and as of December 31, 2023, the Company did not maintain any reserves over contract receivables as they relate to large established credit worthy customers. The Company re-evaluates such reserves on a regular basis and adjusts its reserves as needed. Once a receivable is deemed to be uncollectible, such balance is charged against the reserve. The Company recorded $0.1 million and $0.1 million of contract accounts receivable as of December 31, 2023 and 2022, respectively.
92
Allowance for Credit Losses
The Company accrues an allowance for credit losses against our accounts receivable based on management’s current estimate of amounts that will not be collected. Management’s estimates are typically based on historical loss information adjusted for current conditions. The Company generally does not perform evaluations of customers’ financial condition and generally do not require collateral. Historically, the Company's credit losses have not been significant. The allowance for credit losses was zero
(n) Freight and Shipping Costs
(o) Comprehensive Loss
(p) Segment Reporting
(q) Net Loss Per Share
Outstanding anti-dilutive securities not included in diluted net loss per share (in thousands):
| Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Shares issuable upon exercise of common stock warrants | |||||||||||
| Shares issuable upon exercise of stock options | |||||||||||
| Shares issuable upon the release of restricted stock units | |||||||||||
(r) Stock-Based Compensation
Compensation costs associated with stock option awards and other forms of equity compensation are measured at the grant-date fair value of the awards and recognized over the requisite service period of the awards on a ratable basis. Forfeitures are accounted as they occur by reversing any share-based compensation expense related to awards that will not vest.
The Company grants stock options to purchase common stock to employees with exercise prices equal to the fair market value of the underlying stock. The fair market value of stock options is based on the closing stock price on the grant date.
93
The fair value of each stock option award is estimated using the Black-Scholes-Merton valuation model. Such value is recognized as expense over the requisite service period using the ratable method. The expected term of options is based on the simplified method which defines the expected term as the average of the contractual term of the options and the weighted average vesting period for all option tranches. The expected volatility of stock options is based upon the historical volatility of a number of related publicly traded companies in similar stages of development as well as the volatility of the Company’s common stock. The risk-free interest rate is based on the average yield of U.S. Treasury securities with remaining terms similar to the expected term of the share-based awards. The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future.
The Company accounts for stock options to non-employees using the fair value approach. The fair value of these options is measured using the Black-Scholes-Merton option pricing model, reflecting the same assumptions applied to employee options, other than expected life, which is assumed to be the remaining contractual life of the award. Options that are granted to employees generally have a requisite service period of to four years .
RSUs are considered restricted stock. The fair market value of RSUs is based on the closing stock price on the grant date. The Company recognizes stock-based compensation expense based on the fair value on a ratable basis over the requisite service periods of the awards. RSUs that are granted to employees have a requisite service period typically between and four years .
(s) Warrant Liability
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in Financial Accounting Standards Board (“FASB”) ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815-40. The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815-40, including whether the warrants are indexed to the Company’s own common stock, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding. Warrant liabilities are classified as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants classified as liabilities and are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a component of other income/(expense) in the consolidated statements of operations. The fair value of the warrants is estimated using a Black-Scholes-Merton valuation model. The Company will continue to adjust the liability for changes in fair value until the earlier of the exercise or expiration of its warrants. At that time, the portion of the warrant liability related to the Company’s warrants will be reclassified to additional paid-in capital.
(t) Fair Value Measurements
The Company measures certain financial assets and liabilities at fair value on a recurring basis. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the measurement date. The Company uses a three-tier fair value hierarchy to prioritize the inputs used in the Company’s fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions.
94
The following table provides a summary of the assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2023 and 2022 (in thousands):
Fair Value Measurements at Reporting Date
| December 31, 2023 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
| Assets: | |||||||||||||||||||||||
| Cash equivalents: | |||||||||||||||||||||||
| Money Market | $ | $ | $ | $ | |||||||||||||||||||
| U.S. government debt securities | |||||||||||||||||||||||
| Total cash equivalents | |||||||||||||||||||||||
| Marketable securities, available for sale: | |||||||||||||||||||||||
| Corporate debt securities | |||||||||||||||||||||||
| U.S. government debt securities | |||||||||||||||||||||||
| Total marketable securities, available for sale | |||||||||||||||||||||||
| Total assets measured at fair value on a recurring basis | $ | $ | $ | $ | |||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
| Assets: | |||||||||||||||||||||||
| Cash equivalents: | |||||||||||||||||||||||
| Money Market | $ | $ | $ | $ | |||||||||||||||||||
| Corporate debt securities | |||||||||||||||||||||||
| U.S. government debt securities | |||||||||||||||||||||||
| Total cash equivalents | |||||||||||||||||||||||
| Marketable securities, available for sale: | |||||||||||||||||||||||
| Corporate debt securities | |||||||||||||||||||||||
| Municipal debt securities | |||||||||||||||||||||||
| U.S. government debt securities | |||||||||||||||||||||||
| Total marketable securities, available for sale | |||||||||||||||||||||||
| Total assets measured at fair value on a recurring basis | $ | $ | $ | $ | |||||||||||||||||||
The Company’s marketable debt securities are classified as available-for-sale securities based on management's intentions and are at level 2 of the fair value hierarchy, as these investment securities are valued based upon quoted prices for identical or similar instruments in markets that are not active. The Company has classified marketable securities with original maturities of greater than one year as short-term investments based upon the Company’s ability to use all of those marketable securities to satisfy the liquidity needs of the Company’s current operations.
As of December 31, 2023 and 2022, the Company maintains letters of credit of $3.5 million and $3.5 million, respectively, related to its lease arrangements, which are secured by money market accounts in accordance with certain of our lease agreements.
The Company believes the carrying amount of cash and cash equivalents, accounts payable and accrued expenses approximate their estimated fair values due to the short-term nature of these accounts.
95
(u) Accounting Pronouncements Issued But Not Yet Effective
In June 2022, the FASB issued Accounting Standards Update (“ASU”) 2022-03, Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions (“ASU 2022-03“). Under the guidance of ASU 2022-03, a contractual restriction on the sale of an equity security is not considered in measuring the security’s fair value. ASU 2022-03 also requires certain disclosures for equity securities that are subject to contractual restrictions. For public business entities, the provisions of ASU 2022-03 are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. The Company is still evaluating the impact of this pronouncement on the consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures ("ASU 2023-09"). The amendments in ASU 2023-09 address investor requests for enhanced income tax information primarily through changes to the rate reconciliation and income taxes paid information. Early adoption is permitted. A public entity should apply the amendments in ASU 2023-09 prospectively to all annual periods beginning after December 15, 2024. The Company is currently evaluating the impact of this pronouncement on the consolidated financial statements.
The Company does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material impact on its consolidated financial statements or disclosures.
(v) Reclassifications
Certain prior year amounts have been reclassified to conform to the current year presentation in the consolidated financial statements and accompanying notes to the consolidated financial statements.
2. Balance Sheet Details
Short-Term Marketable Securities
The amortized cost, gross unrealized holding gains, gross unrealized holding losses, and fair value of debt securities classified as available-for-sale securities by major security type and class of security as of December 31, 2023 were as follows (in thousands):
| December 31, 2023 | |||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gain | Gross Unrealized Loss | Estimated Market Value | ||||||||||||||||||||
| Short-term marketable securities, available-for-sale: | |||||||||||||||||||||||
| Corporate debt securities | $ | $ | $ | ( | $ | ||||||||||||||||||
| U.S. government debt securities | ( | ||||||||||||||||||||||
| Total short-term marketable securities, available-for-sale | $ | $ | $ | ( | $ | ||||||||||||||||||
As of December 31, 2023, all debt securities with estimated market value of $19.1 million have contractual maturities of less than twelve months.
96
The amortized cost, gross unrealized holding gains, gross unrealized holding losses and fair value of debt securities classified as available-for-sale securities by major security type and class of security as of December 31, 2022 were as follows (in thousands):
| December 31, 2022 | |||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gain | Gross Unrealized Loss | Estimated Market Value | ||||||||||||||||||||
| Short-term marketable securities, available-for-sale: | |||||||||||||||||||||||
| Corporate debt securities | $ | $ | $ | ( | $ | ||||||||||||||||||
| Municipal debt securities | ( | ||||||||||||||||||||||
| U.S. government debt securities | ( | ||||||||||||||||||||||
| Total short-term marketable securities, available-for-sale | $ | $ | $ | ( | $ | ||||||||||||||||||
As of December 31, 2022, the estimated market value of debt securities with contractual maturities of less than twelve months was $40.2 million; the remaining debt securities that the Company held at that date had an estimated market value of $8.2 million and contractual maturities of up to 23 months.
The Company evaluates securities with unrealized losses to determine whether such losses, if any, are due to credit-related factors. It was determined that no credit losses existed as of December 31, 2023 or December 31, 2022, because the change in market value for those securities in an unrealized loss position has resulted from fluctuating interest rates rather than a deterioration of the credit worthiness of the issuers. Gross realized gains and losses on the Company’s debt securities for the years ended December 31, 2023 and 2022 were not significant.
The following tables present gross unrealized losses and fair values for those investments that were in an unrealized loss position as of December 31, 2023 and December 31, 2022, aggregated by investment category and the length of time that individual securities have been in a continuous loss position:
December 31, 2023 | |||||||||||||||||||||||||||||||||||
| Less Than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||||||||||||||
| Fair Value | Gross Unrealized Loss | Fair Value | Gross Unrealized Loss | Fair Value | Gross Unrealized Loss | ||||||||||||||||||||||||||||||
| Short-term marketable securities, available-for-sale: | |||||||||||||||||||||||||||||||||||
| Corporate debt securities | $ | $ | ( | $ | $ | $ | $ | ( | |||||||||||||||||||||||||||
| U.S. government debt securities | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Total short-term marketable securities, available-for-sale | $ | $ | ( | $ | $ | ( | $ | $ | ( | ||||||||||||||||||||||||||
December 31, 2022 | |||||||||||||||||||||||||||||||||||
| Less Than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||||||||||||||
| Fair Value | Gross Unrealized Loss | Fair Value | Gross Unrealized Loss | Fair Value | Gross Unrealized Loss | ||||||||||||||||||||||||||||||
| Short-term marketable securities, available-for-sale: | |||||||||||||||||||||||||||||||||||
| Corporate debt securities | $ | $ | ( | $ | $ | ( | $ | $ | ( | ||||||||||||||||||||||||||
| Municipal debt securities | ( | ( | |||||||||||||||||||||||||||||||||
| U.S. government debt securities | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Total short-term marketable securities, available-for-sale | $ | $ | ( | $ | $ | ( | $ | $ | ( | ||||||||||||||||||||||||||
97
Prepaid Expenses and Property and Equipment, Net
Consolidated balance sheet details are as follows (in thousands):
| December 31, 2023 | December 31, 2022 | ||||||||||
| Prepaid expenses and other current assets: | |||||||||||
| Prepaid expenses | $ | $ | |||||||||
| Other current assets | |||||||||||
| Total prepaid expenses and other current assets | $ | $ | |||||||||
| Property and equipment, gross: | |||||||||||
| Laboratory equipment | $ | $ | |||||||||
| Computer equipment | |||||||||||
| Furniture and fixtures | |||||||||||
| Leasehold improvements | |||||||||||
| Total property and equipment, gross | |||||||||||
| Less accumulated depreciation | ( | ( | |||||||||
| Total property and equipment, net | $ | $ | |||||||||
Depreciation expense for the years ended December 31, 2023 and 2022 was $1.8 million and $1.6 million, respectively.
Loss on disposal of property and equipment during the years ended December 31, 2023 and 2022 recorded in operating expenses was $18,000 and $324,000 , respectively.
Accrued Compensation and Accrued Liabilities
Consolidated balance sheet details are as follows (in thousands):
| December 31, 2023 | December 31, 2022 | ||||||||||
| Accrued compensation: | |||||||||||
| Accrued bonus and commissions | $ | $ | |||||||||
| Accrued salaries and wages | |||||||||||
| Total accrued compensation | $ | $ | |||||||||
| Accrued liabilities: | |||||||||||
| Accrued consulting services | $ | $ | |||||||||
| Customer refund liability | |||||||||||
| Restructuring liability | |||||||||||
| Other accrued expenses | |||||||||||
| Total accrued liabilities | $ | $ | |||||||||
3. Stockholders’ Equity
(a) Classes of Stock
The Company’s amended and restated certificate of incorporation authorizes it to issue 100,000,000 shares of common stock and 5,000,000 shares of preferred stock. Both classes of stock have a par value of $0.0001
On June 2, 2023, the Company filed an amendment to its Amended and Restated Certificate of Incorporation, as amended, with the Secretary of State of Delaware to increase the authorized number of shares of common stock of the Company from 50,000,000 to 100,000,000 shares (the “Amended Certificate”). The Amended Certificate was approved by the Company’s stockholders at the 2023 Annual Meeting.
98
(b) At-The Market Offering
On November 10, 2020, the Company entered into a sales agreement (the “2020 Sales Agreement”) with Cowen and Company, LLC (“Cowen”) relating to the sale of shares of the Company’s common stock from time to time with an aggregate offering price of up to $50.0 million. Through December 31, 2021, the Company issued an aggregate of 1,482,343 shares of common stock pursuant to the 2020 Sales Agreement at a weighted average purchase price of $30.05 , net of $1.6 million in issuance costs resulting in net proceeds to the Company of approximately $42.9 million. During 2022, the Company did not issue or sell any shares of common stock pursuant to the 2020 Sales Agreement.
During 2023, the Company issued an aggregate of 2,038,661 shares of common stock pursuant to the 2020 Sales Agreement at a weighted average purchase price of $2.68 resulting in aggregate gross proceeds of approximately $5.5 million, reduced by $0.3 million in issuance costs, resulting in net proceeds to the Company of approximately $5.2 million. As of December 31, 2023, the 2020 Sales Agreement has been fully utilized, and no additional shares of common stock may be sold pursuant to the 2020 Sales Agreement.
On August 8, 2022, the Company entered into a second sales agreement (the “2022 Sales Agreement”) with Cowen relating to the sale of shares of the Company’s common stock from time to time with an aggregate offering price of up to $75.0 million under a second at-the-market offering program. For the year ended December 31, 2022, the Company did not issue any shares pursuant to the 2022 Sales Agreement.
During the year ended December 31, 2023, the Company issued an aggregate of 130,598 shares of common stock pursuant to the 2022 Sales Agreement at a weighted average purchase price of $2.49 resulting in aggregate gross proceeds of approximately $0.3 million, reduced by $0.2 million in issuance costs, resulting in net proceeds to the Company of approximately $0.1 million. As of December 31, 2023, $74.7 million is available pursuant to the Company's 2022 Sales Agreement.
(c) Warrants
SPAC Warrants
The Company previously issued a total of 14,936,250 SPAC Warrants to purchase common stock in public offering and private placement offerings which were consummated on June 23, 2017. As part of the public offering, the Company issued 14,375,000 Public SPAC Warrants and as part of the private placement offering, the Company issued 561,250 Private SPAC Warrants. The SPAC Warrants have a five-year life from the date the Business Combination was consummated and every four SPAC Warrants entitle the holder to purchase one whole share of common stock at an exercise price of $23.00 per whole share.
The Private SPAC Warrants are identical to the Public SPAC Warrants, but they (i) are exercisable either for cash or on a cashless basis at the holder’s option, (ii) are not redeemable by the Company as long as such warrants are held by the initial purchasers or their affiliates and permitted transferees, and (iii) may be subject to the limitations on exercise as specified in the warrant agreement. As a result of these difference in features between the Public SPAC Warrants and Private SPAC Warrants, the Company concluded that the Private SPAC Warrants should be classified as a liability, if still held by the original Private SPAC Warrant holder, and marked to market each financial reporting period in the Company’s statement of operations.
In 2021, a total of 12,120,397 SPAC Warrants were exercised, resulting in the Company’s issuance of 3,030,092 shares of common stock and the receipt of $69.7 million in gross proceeds. Outstanding SPAC Warrants totaled 2,815,853 80,350
99
Placement Agent Warrants
In connection with several of DermTech Operations’ financings that took place between 2015 and 2018, DermTech Operations engaged a registered placement agent to assist in marketing and selling of common and preferred units. From 2015 to 2016, DermTech Operations issued 168,522 seven-year warrants to purchase one share of common stock each at an exercise price of $8.68 per share. From 2016 to 2018, DermTech Operations issued 72,658 seven-year warrants to purchase one share of common stock at an exercise price of $9.54 per share. In 2020, the Company issued 15,724 seven-year warrants to purchase one share of common stock at an exercise price of $9.54 per share in connection with the Company’s 2018 bridge note financing. Outstanding placement agent warrants totaled 570 and 4,510 as of December 31, 2023 and 2022, respectively.
Management Warrants
Warrants to purchase DermTech Operations common stock were issued to executive officers of DermTech Operations in lieu of issuing certain stock options (the “Management Warrants”). The Management Warrants were assumed by the Company in connection with the Business Combination. The Management Warrants have a ten year life and are exercisable for Company common stock at $1.08 per share. The Management Warrants vested monthly over a four-year period. In 2022, the Company issued 20,320 shares of common stock pursuant to the exercise of Management Warrants. Outstanding Management Warrants totaled zero
(d) Stock-Based Compensation Plans
Equity Incentive Plans
In May 2020, the Company adopted the 2020 Equity Incentive Plan (the “2020 Plan”), which replaced its Amended and Restated 2010 Stock Plan and provides for the granting of incentive and non-qualified stock options, restricted stock and stock-based awards to employees, directors and consultants providing services to the Company. Under the 2020 Plan, incentive and non-qualified stock options may be granted at not less than 100 % of the fair market value of the Company’s common stock on the date of grant. If an incentive stock option is granted to an individual who owns more than 10 % of the combined voting power of all classes of the Company’s capital stock, the exercise price may not be less than 110 % of the fair market value of the Company’s common stock on the date of grant and the term of the option may not be longer than five years .
The 2020 Plan authorizes the Company to issue up to 1,900,000 shares of the Company’s common stock pursuant to awards granted under the 2020 Plan, plus the number of shares underlying any stock option and other stock-based awards previously granted under the 2010 Plan that are forfeited, canceled, or terminated (other than by exercise) on or after May 26, 2020; provided that no more than 1,400,000 shares may be added to the 2020 Plan pursuant to such forfeitures, cancellations and terminations. In addition, the number of shares available for issuance under the 2020 Plan will automatically increase on the first day of each fiscal year beginning in fiscal year 2021 and ending on the second day of fiscal year 2025, by an amount equal to the smaller of (i) 3.5 % of the number of shares of common stock outstanding on such date and (ii) an amount determined by the administrator of the 2020 Plan (the “2020 Plan Evergreen Provision”. In May 2023, the 2020 Plan Evergreen Provision was amended to the smaller of (i) 5.0 % of the number of shares of common stock outstanding on such date and (ii) an amount determined by the administrator of the 2020 Plan. The 2020 Plan will expire on April 12, 2030 or an earlier date approved by a vote of the Company’s stockholders or board of directors. The contractual term of options granted under the 2020 Plan is not more than ten years . Vesting provisions vary based on the specific terms of the individual option awards. On January 1, 2023, the shares reserved for future grants under the 2020 Plan increased by 1,060,409 pursuant to the 2020 Plan Evergreen Provision.
In March 2022, the Company’s board of directors adopted the 2022 Inducement Equity Incentive Plan (as amended, the “Inducement Plan”), pursuant to which the Company reserved 950,000 shares of its common stock (subject to customary adjustments in the event of a change in capital structure of the Company) for the issuance of equity awards under the Inducement Plan. In September 2022, the Company amended and restated the Inducement Plan to reserve an additional 1,000,000 shares of its common stock. In May 2023, the Company amended and restated the Inducement Plan to reserve an additional 500,000 shares of its common stock. The Inducement Plan and its amendment were approved by the Company’s board of directors without stockholder approval pursuant to Rule 5635(c)(4) of the Nasdaq Listing Rules. The Inducement Plan may be used exclusively for grants of awards to individuals who were not previously employees or directors of the Company, or following a bona fide period of non-employment, as an inducement material to the individual’s entry into employment with the Company within the meaning of Rule 5635(c)(4) of the Nasdaq Listing Rules.
As of December 31, 2023, 1,094,034 shares remained available for future grant under the Company's equity incentive plans.
100
The following table summarizes stock option transactions for the years ended December 31, 2023 and 2022:
| Total options | Weighted average exercise price | Weighted average remaining contractual term (in years) | Aggregate intrinsic value (in thousands) | ||||||||||||||||||||
Outstanding at December 31, 2022 | $ | $ | |||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( | ||||||||||||||||||||||
| Forfeited | ( | ||||||||||||||||||||||
Outstanding at December 31, 2023 | $ | $ | |||||||||||||||||||||
Options vested and expected to vest as of December 31, 2023 | $ | $ | |||||||||||||||||||||
Options exercisable as of December 31, 2023 | $ | $ | |||||||||||||||||||||
The following table summarizes RSU transactions for the years ended December 31, 2023 and 2022:
| Total RSUs | Weighted average grant date fair value per share | ||||||||||
Outstanding at December 31, 2022 | $ | ||||||||||
| Granted | |||||||||||
| Released | ( | ||||||||||
| Forfeited | ( | ||||||||||
Outstanding at December 31, 2023 | |||||||||||
RSUs vested and expected to vest as of December 31, 2023 | $ | ||||||||||
RSUs vested, but not yet issued as of December 31, 2023 | $ | ||||||||||
Employee Stock Purchase Plan
In May 2020, the Company adopted the 2020 Employee Stock Purchase Plan (as amended, the "2020 ESPP"), which allows for full-time and certain part-time employees of the Company to purchase shares of common stock at a discount to fair market value. The 2020 ESPP authorizes the Company to issue up to 400,000 shares of the Company’s common stock. In addition, the number of shares available for issuance under the 2020 ESPP will automatically increase on the first day of each of the Company’s fiscal years beginning in 2021 and ending on the first day of 2030, in an amount equal to the lesser of (i) 300,000 shares, (ii) 1 % of the shares of Company common stock outstanding on the last day of the immediately preceding fiscal year, or (iii) such lesser number of shares as is determined by the Company's board of directors, subject to adjustment upon changes in capitalization of the Company.
The 2020 ESPP permits eligible employees to purchase discounted shares of our common stock at semi-annual intervals through periodic payroll deductions. Shares are purchased at a price equal to 85 % of the lower of: (i) the fair market value of the Company’s common stock on the first business day of an offering period or (ii) the fair market value of the Company’s common stock on the last business day of an offering period. A new offering period commences every year on approximately March 1 and September 1. At the end of each offering period, the accumulated contributions are used to purchase shares of the Company’s common stock. The last business day of each purchase period is referred to as the purchase date. Purchase dates are every six months on February 28 or February 29 and August 31.
In August 2022, the Company’s board of directors approved an amendment to the 2020 ESPP. Effective September 1, 2022, each offering period is -months and consists of four six-month purchase periods during which payroll deductions of the participants are accumulated under the 2020 ESPP. Prior to September 1, 2022, each offering period was six months .
101
On February 28, 2022 and August 31, 2022, the Company issued 47,339 and 100,749 shares of its common stock, respectively, pursuant to scheduled purchases under the 2020 ESPP. As of December 31, 2022, 698,930 shares of common stock were reserved for future issuance under the 2020 ESPP. On January 1, 2023, an additional 300,000 shares of common stock became available under the 2020 ESPP Plan Evergreen Provision. On February 28, 2023 and August 31, 2023, the Company issued 174,025 and 158,667 shares of its common stock, respectively, pursuant to scheduled purchases under the 2020 ESPP. As of December 31, 2023, 666,238 shares of common stock remained available for future grants under the 2020 ESPP.
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance consists of the following as of December 31, 2023 and 2022 (in thousands):
| December 31, 2023 | December 31, 2022 | ||||||||||
| Warrants to purchase common stock | |||||||||||
| SPAC Warrants to purchase common stock* | |||||||||||
| Stock options issued and outstanding | |||||||||||
| Restricted stock units issued and outstanding | |||||||||||
| Authorized for future equity grants | |||||||||||
| Authorized for future ESPP purchases | |||||||||||
| Total common stock reserved for future issuance | |||||||||||
(e) Stock-Based Compensation
Stock-based compensation expense for employee options, RSUs, the purchase rights issued under the 2020 ESPP, and consultant options was recorded in the consolidated statements of operations as follows (in thousands):
| Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Cost of revenue | $ | $ | |||||||||
| Sales and marketing | |||||||||||
| Research and development | |||||||||||
| General and administrative | |||||||||||
| Total stock-based compensation | $ | $ | |||||||||
The total compensation cost related to non-vested awards not yet recognized as of December 31, 2023 was $18.1 million, which is expected to be recognized over a weighted average term of 2.11 years.
Departure of Former Chief Executive Officer
Stock-based compensation expense for the year ended December 31, 2023 included accelerated expense $3.0 million in connection with the transition agreement dated March 1, 2023, between the Company and its former Chief Executive Officer, John Dobak, M.D. (the "Transition Agreement"). The accelerated expense is included within general and administrative expenses in the condensed consolidated statement of operations.
Dr. Dobak resigned from his position as Chief Executive Officer and member of the board of directors of the Company (the "Board") effective May 8, 2023 and agreed to serve as a consultant to the Company on an as needed basis until January 1, 2024. The terms of the Transition Agreement allow for continuing vesting of Dr. Dobak's equity awards through the end of the consulting period on January 1, 2024. At the termination of the consulting period, consistent with Dr. Dobak's change of control and severance plan, he immediately received an additional 10 months vesting of equity awards and the period to exercise his vested stock options was increased from 90 days to 12 months.
The Company assessed the consulting services under the Transition Agreement as nonsubstantive pursuant to ASC 718, Compensation – Stock Compensation (ASC 718) and recognized all stock-based compensation expense related to Dr. Dobak's equity awards vesting in connection with the Transition Agreement upon his resignation.
102
Valuation Assumptions
The Company estimates the fair value of our stock-based equity awards using the Black-Scholes valuation model. Assumptions used in the Black-Scholes valuation model were as follows:
Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Stock Options: | |||||||||||
| Assumed risk-free interest rate | |||||||||||
| Assumed volatility | |||||||||||
| Expected option term | |||||||||||
| Expected dividend yield | |||||||||||
| ESPP: | |||||||||||
| Assumed risk-free interest rate | |||||||||||
| Assumed volatility | |||||||||||
| Expected option term | |||||||||||
| Expected dividend yield | |||||||||||
4. Income Taxes
The Company has reported net losses since inception, and therefore, the minimum provision for state income taxes has been recorded. The following table provides a reconciliation between income taxes computed at the federal statutory rate of 21
Year ended December 31 | |||||||||||
| 2023 | 2022 | ||||||||||
| Income tax at statutory rate | % | % | |||||||||
| State tax, net of federal tax benefit | |||||||||||
| Permanent items | ( | ( | |||||||||
| Tax credits | |||||||||||
| Other | ( | ( | |||||||||
| Valuation allowance increase | ( | ( | |||||||||
| Income tax expense | % | % | |||||||||
103
Significant components of the Company’s deferred tax assets and liabilities from federal and state income taxes as of December 31, 2023 and 2022 are shown below (in thousands):
| December 31, 2023 | December 31, 2022 | ||||||||||
| Deferred tax assets: | |||||||||||
| Net operating loss | $ | $ | |||||||||
| Research and development credits | |||||||||||
| Stock based compensation | |||||||||||
| Operating lease liability | |||||||||||
| R&E expenditures | |||||||||||
| Deferred revenue | |||||||||||
| Accruals and other | |||||||||||
| Total deferred tax assets | |||||||||||
| Deferred tax liabilities: | |||||||||||
| Depreciation and amortization | ( | ( | |||||||||
| Operating lease right-of-use assets | ( | ( | |||||||||
| Total deferred tax liabilities | ( | ( | |||||||||
| Net deferred tax assets before valuation allowance | |||||||||||
| Less: valuation allowance | ( | ( | |||||||||
| Net deferred tax assets | $ | $ | |||||||||
The Company maintains a full valuation allowance against its net deferred tax assets as realization of such assets is not more likely than not.
As of December 31, 2023 and 2022, the Company had federal tax net operating loss (“NOL”) carryforwards of approximately $342.0 million and $267.0 million, respectively, as well as state tax NOL carryforwards as of December 31, 2023 and 2022, of approximately $257.4 million and $196.7 million, respectively. Federal NOL carryforwards began to expire during 2023 while the Company’s state NOL carryforwards will begin to expire during various years, dependent on the jurisdiction.
The Company also had federal research and development tax credit carryforwards as of December 31, 2023 and 2022 of approximately $2.6 million and $1.8 million, respectively, and state research and development tax credits of approximately $2.1 million and $1.6 million as of December 31, 2023 and 2022, respectively. The federal credit carryforwards began to expire during 2023 and the state credit carryforwards do not expire. The Company has not performed a formal study validating its federal and state R&D tax credits and upon preparation, such tax credit carryforwards could vary from what was originally claimed on applicable income tax returns.
The utilization of NOL and tax credit carryforwards to offset future taxable income may be subject to an annual limitation as a result of ownership changes that have occurred previously or may occur in the future. Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, ("IRC"), a corporation that undergoes an ownership change may be subject to limitations on its ability to utilize its pre-change NOLs and other tax attributes otherwise available to offset future taxable income and/or tax liability. An ownership change is defined as a cumulative change of 50%
The Company conducts intensive research and experimentation activities, generating research tax credits for federal and state purposes under IRC Section 41. The Company has not performed a formal study validating such credits claimed on its tax returns. Once a study is completed, the amount of R&D tax credits available could vary from what was originally claimed on the tax returns.
104
Due to generation of NOLs, since inception of the Company, U.S. federal and state returns are open to examination for all years, in which NOLs exist. The Company’s policy is to record any penalties or interest related to any unrecognized tax benefits as a component of the income tax provision. As of December 31, 2023 and 2022, the Company has
5. Leases, Commitments and Contingencies
Finance Leases
The Company leases certain laboratory equipment from various third parties, through equipment finance leases. These leases either include a bargain purchase option or the terms of the leases are at least 75 percent of the useful lives of the assets and are therefore classified as finance leases. These leases are capitalized in property and equipment, net on the accompanying consolidated balance sheets. Initial asset values and finance lease obligations are based on the present value of future minimum lease payments. Gross assets recorded under finance leases were $0.4 million and $0.4 million as of December 31, 2023 and 2022, respectively. Accumulated amortization associated with finance leases was $0.2 million and $0.2 million as of December 31, 2023 and 2022, respectively. Total finance lease interest expense was approximately $5,000 and $13,000 for the years ended December 31, 2023 and 2022, respectively, and is included within interest income, net on the consolidated statements of operations. Long-term finance lease obligations are as follows (in thousands):
| December 31, 2023 | December 31, 2022 | ||||||||||
| Gross finance lease obligations | $ | $ | |||||||||
| Less: imputed interest | ( | ( | |||||||||
| Present value of net minimum lease payments | |||||||||||
| Less: current portion of finance lease obligations | ( | ( | |||||||||
| Total long-term finance lease obligations | $ | $ | |||||||||
Operating Leases
Del Mar Heights Lease
On July 1, 2021, the Company entered into an Office Lease (the “Del Mar Lease”) with Kilroy Realty, L.P. (the “Landlord”), with respect to an aggregate of 95,997 rentable square feet consisting of the entire building located at 12340 El Camino Real, San Diego, California 92130 (the “Entire Premises”). The Entire Premises covered by the Lease will serve as the Company’s new principal office.
The Del Mar Lease provides for a tenant improvement allowance of $125.00 per rentable square foot of the Entire Premises for a total of $12.0 million that the Landlord would use to fund the installation and/or construction of certain improvements to the Entire Premises in four phases, with each phase pertaining to a specified portion of the Entire Premises. The initial term of the Del Mar Lease is ten years and six months beginning on the earlier to occur of (i) January 1, 2023 and (ii) the date that Landlord tenders possession of the Phase III Premises (as defined in the Del Mar Lease) to the Company following the substantial completion of the improvements to the Phase III Premises required by the Del Mar Lease. The Company has the option to extend the term of the Lease for two additional five-year periods, subject to the terms of the Del Mar Lease.
As the Landlord tenders possession of each portion of the Entire Premises for which the applicable improvements required by the Del Mar Lease are substantially complete, the Company will be obligated to make monthly payments of base rent with respect to such portion of the Entire Premises as set forth on Schedule 1 to the Del Mar Lease. In the event the Company exercises its option to extend the Del Mar Lease term, the Lease provides for monthly rent payments during the additional five-year periods at the then-current market rent as determined in accordance with the Del Mar Lease. In addition to rent, the Del Mar Lease requires the Company to pay additional rent amounts for taxes, insurance, maintenance and other expenses.
105
During the year ended December 31, 2021, the Company took initial possession of the first phase of what is expected to become its corporate headquarters, and the Company capitalized a right-of-use asset and related lease liability of $5.7 15.8
Del Mar Lease Amendments
During April 2022, the Company amended the Del Mar Lease through the execution of the First Amendment to Office Lease (the "First Amendment") and the Second Amendment to Office Lease (the "Second Amendment") (collectively, the "Del Mar Lease Amendments"). Pursuant to the First Amendment to the Del Mar Lease, the Company elected to utilize a one-time increase in an additional improvement allowance of $25.00 per rentable square foot, which increased the tenant improvement allowance by $2.4 million to $14.4 million, provided under the Del Mar Lease to make certain improvements to the Entire Premises. As a result, the Company will pay an increased monthly base rent to the Landlord, in order to repay costs relating to the additional design and construction. Pursuant to the Second Amendment, the Company elected to expand the Entire Premises to include 14,085 rentable square feet comprising the executive parking level (the “Expansion Premises”), which increased the tenant improvement allowance by $2.1 million to $16.5 million. The Landlord will tender possession of the Expansion Premises following substantial completion of improvements, pursuant to an agreed upon work letter and will run contemporaneously with the term of the Existing Premises. The Company intends to use the additional space for general office and laboratory use. As the Landlord tenders possession of the Expansion Premises, the Company is obligated to pay the Landlord increased monthly installments of base rent for the Expansion Premises. Upon inclusion of the Expansion Premises, the Company leased approximately 110,082 rentable square feet from the Landlord.
The Del Mar Lease Amendments were negotiated with a single commercial objective and are treated as a combined contract for accounting purposes. The Company evaluated the Del Mar Lease Amendments under ASC 842 and concluded that the Del Mar Lease Amendments would be accounted for as a single contract with the Del Mar Lease because the additional lease payments related to the tenant improvement allowance due to the Del Mar Lease Amendments were not commensurate with ROU asset granted to the Company. Accordingly, the Company remeasured the lease liability using the additional monthly rent payments and the incremental borrowing rate at the effective date of the modification of 6.50 %. The remeasurement for the modification resulted in an increase to the lease liability and the ROU asset of approximately $1.2
In November 2022, the lease for the remaining phases of the Company’s corporate headquarters commenced and the Company capitalized a ROU asset of $34.1 million and related lease liability of $32.1 million. The extension option periods were not considered in the determination of the ROU asset or the lease liability as the Company did not consider it reasonably certain that it would exercise such extension options.
In connection with the original lease agreement, in lieu of a cash security deposit, the Company’s bank issued a letter of credit on its behalf, which is secured by a deposit, of $3.0 million and is included in restricted cash on the consolidated balance sheet based on the term of the underlying lease. In April 2022, pursuant to the Second Amendment, the Company’s bank increased the letter of credit on its behalf by $0.5 million, totaling $3.5 million. As of December 31, 2023, none of the standby letter of credit amount has been used.
106
The components of lease expense for the years ended December 31, 2023 and 2022 were as follows (in thousands):
| Year ended December 31 | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Operating lease cost | ||||||||||||||
| Operating lease cost | $ | $ | ||||||||||||
Variable lease costs (1) | ||||||||||||||
Sublease income (2) | ( | |||||||||||||
| Total operating lease cost, net of sublease income | $ | $ | ||||||||||||
| Finance lease cost | ||||||||||||||
| Amortization of leased assets | $ | $ | ||||||||||||
| Interest on lease liabilities | ||||||||||||||
| Total finance lease cost | $ | $ | ||||||||||||
(1) Variable lease costs are primarily related to common area maintenance charges and property taxes.
(2) Sublease income is related to a sublease of 1,802 square feet of office and laboratory space related to the Del Mar Lease. The sublease will expire on September 30, 2024, and the parties have no option to extend the sublease.
Other information related to leases was as shown in the table below.
| Year ended December 31 | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||||||||
| Operating cash flows from operating leases | $ | $ | ||||||||||||
| Operating cash flows from finance leases | $ | $ | ||||||||||||
| Financing cash flows from finance leases | $ | $ | ||||||||||||
| Right-of-use assets obtained in exchange for lease obligations: | ||||||||||||||
| Operating leases | $ | $ | ||||||||||||
| Finance leases | $ | $ | ||||||||||||
| Weighted average remaining lease term in years: | ||||||||||||||
| Operating leases | ||||||||||||||
| Finance leases | ||||||||||||||
| Weighted average discount rate: | ||||||||||||||
| Operating leases | % | % | ||||||||||||
| Finance leases | % | % | ||||||||||||
107
The Company’s future minimum lease payments under operating and finance leases as of December 31, 2023 are as follows (in thousands):
| Operating Leases | Finance Leases | Total | |||||||||||||||
| Year ending December 31: | |||||||||||||||||
| 2024 | $ | $ | $ | ||||||||||||||
| 2025 | |||||||||||||||||
| 2026 | |||||||||||||||||
| 2027 | |||||||||||||||||
| 2028 | |||||||||||||||||
| Thereafter | |||||||||||||||||
| Total minimum lease payments | |||||||||||||||||
| Less: imputed interest | ( | ( | ( | ||||||||||||||
| Total lease obligation | |||||||||||||||||
| Less: current lease obligations | ( | ( | ( | ||||||||||||||
| Long-term lease obligations | $ | $ | $ | ||||||||||||||
2023 Restructuring Plan
On June 26, 2023, the Company's board of directors approved a restructuring plan (the “2023 Restructuring Plan”) to prioritize growth opportunities for the DMT, streamline operations, suspend pipeline programs, and significantly reduce overall operating expenses. The 2023 Restructuring Plan included a reduction of the Company’s workforce by approximately 15 %. The actions associated with the employee restructuring under the 2023 Restructuring Plan were substantially completed in the third quarter of 2023.
The Company incurred $2.1 million in restructuring charges in connection with the 2023 Restructuring Plan for the twelve months ended December 31, 2023, which consist of $1.8 million in charges related to severance payments and employee benefits and $0.3 million in charges related to stock-based compensation for the acceleration of share-based awards. Restructuring charges are included in general and administrative expenses in the consolidated statement of operations.
The restructuring liability as of December 31, 2023 was $10,000 and is included within accrued liabilities in the consolidated balance sheets.
Legal Proceedings
From time to time, the Company may be subject to legal proceedings and claims arising in the ordinary course of business. Because legal proceedings are inherently uncertain, we are unable to predict the ultimate outcome of these matters, management does not believe that the outcome of any of these matters will have a material effect on the Company’s consolidated financial position, results of operations or cash flows. However, there can be no assurance as to the ultimate outcome of these matters.
On October 16, 2023, a putative class action lawsuit titled Bagheri v. DermTech, Inc., et al., Case No. 23-cv-1885-DMS-JLB, was filed in the United States District Court for the Southern District of California against the Company and certain of its current and/or former officers (collectively, the “Defendants”). The complaint was filed on behalf of persons who purchased or otherwise acquired the Company’s publicly traded securities between May 3, 2022 and November 3, 2022 (collectively, the “Plaintiffs”). The Plaintiffs alleged in the complaint that the Defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 by making false and misleading statements regarding the Company’s business, operations, and prospects. The action includes claims for damages and an award of reasonable costs and attorneys’ fees and expert fees. On December 5, 2023, another putative class action lawsuit titled Quarford v. DermTech, Inc. et al., Case No. 23-cv-2221-JES-DDL was filed in the United States District Court for the Southern District of California against the same Defendants and alleging same causes of action as the Bagheri lawsuit. The Quarford lawsuit expanded the class period to include as Plaintiffs persons who purchased or otherwise acquired the Company’s publicly traded securities from March 8, 2021 to November 3, 2022. On January 17, 2024, the Court consolidated the two actions, which is now titled In re Dermtech, Inc. Securities Litigation, Case No. 3:23-cv-1885-DMS-JLB. The consolidated complaint is now due April 1, 2024 and Defendants' responses are due May 31, 2024. Given the early stage of this litigation, the probability of a particular outcome cannot be determined at this time. The Company intends to vigorously defend against all claims.
108
6. Retirement Plan
The Company has an IRC Section 401(k) retirement plan, covering all eligible employees. Under the terms of the 401(k) Plan, the Company may elect to match a discretionary percentage of contributions. The Company did not offer a contribution percentage match during 2023. Total matching contributions were $1.5 million for the year ended December 31, 2022.
7. Related Party Transactions
During 2023 and 2022, the Company engaged EVERSANA Life Science Services, LLC and its subsidiary Intouch Group, LLC (collectively, “EVERSANA”) to provide certain marketing services to the Company. Leana Wood, the spouse of Todd Wood, the Company’s former Chief Commercial Officer, is an employee of EVERSANA. Mr. Wood's last day of employment as Chief Commercial Officer of the Company was July 3, 2023. The Company incurred $0.9 million and $3.2 million in costs for the years ended December 31, 2023 and 2022, respectively. Amount due to EVERSANA were zero and $0.3 million as of December 31, 2023 and 2022, respectively.
8. Subsequent Events
On January 29, 2024, the Company's board of directors approved a restructuring plan (the “2024 Restructuring Plan”) to continue to align the Company’s resources with its previously announced strategic prioritization for the DMT in June 2023. The 2024 Restructuring Plan included a reduction of the Company’s workforce by approximately 15 %.
The Company estimates that it will incur aggregate pre-tax charges of approximately $1.3 million in connection with the 2024 Restructuring Plan, primarily consisting of severance payments, employee benefits, outplacement services and related costs. The actions associated with the employee restructuring under the 2024 Restructuring Plan are expected to be substantially complete in the first quarter of 2024.
109
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures.
As required by Rules 13a‑15(b) and 15d-15(b) under the Securities Exchange Act of 1934, as amended (the Exchange Act), our management, including our principal executive officer and principal financial officer, conducted an evaluation as of the end of the period covered by this report, of the effectiveness of our disclosure controls and procedures as defined in Rules 13a‑15(e) and 15d-15(e) under the Exchange Act.
Based on that evaluation, our principal executive officer and principal financial officer have concluded that these disclosure controls and procedures were effective as of December 31, 2023 to provide reasonable assurance that information required to be disclosed by us in reports that we file under the Exchange Act is recorded, processed, summarized, and reported, within the time periods specified in Securities and Exchange Commission rules and forms and that material information relating to the Company is accumulated and communicated to management, including our principal executive officer and our principal financial officer, as appropriate to allow timely decisions regarding required disclosures.
Management’s Annual Report on Internal Control over Financial Reporting.
Management of the Company is responsible for establishing and maintaining effective internal control over financial reporting as defined in Rule 13a‑15(f) under the Exchange Act. The Company’s internal control over financial reporting is designed to provide reasonable assurance to the Company’s management and board of directors regarding the preparation and fair presentation of published financial statements in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2023. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control—Integrated Framework (2013). Based on our assessment, we concluded that, as of December 31, 2023, our internal control over financial reporting was effective based on those criteria.
This Annual Report on Form 10-K does not include an attestation report on internal control over financial reporting issued by our independent registered accounting firm. Our auditors will not be required to formally opine on the effectiveness of our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002 until we are no longer a smaller reporting company.
Changes in Internal Control over Financial Reporting.
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by Rules 13a-15(d) and 15d-15(d) under the Exchange Act during the quarter ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions That Prevent Inspections.
Not applicable.
110
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2024 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2023.
Item 11. Executive Compensation.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2024 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2023.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2024 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2023.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2024 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2023.
Item 14. Principal Accountant Fees and Services.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2024 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2023.
111
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(a)The following documents are filed as part of this Form 10‑K:
(1)Financial Statements (see “Consolidated Financial Statements and Supplementary Data” at Item 8 and incorporated herein by reference).
(2)Financial Statement Schedules (Schedules to the Financial Statements have been omitted because the information required to be set forth therein is not applicable or is shown in the accompanying Financial Statements or notes thereto).
(3)Exhibits
| Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||||||||||||||||||||||
| 2.1 | S-4/A | 8/7/2019 | 333-232181 | |||||||||||||||||||||||||||||
| 2.2 | S-4/A | 8/2/2019 | 333-232181 | |||||||||||||||||||||||||||||
| 3.1 | 10-Q | 8/3/2023 | 001-38118 | |||||||||||||||||||||||||||||
| 3.2 | 10-K | 3/11/2020 | 001-38118 | |||||||||||||||||||||||||||||
| 4.1 | 10-Q | 8/5/2020 | 001-38118 | |||||||||||||||||||||||||||||
| 4.2 | S-1/A | 6/9/2017 | 333-218093 | |||||||||||||||||||||||||||||
| 4.3 | 8-K | 6/23/2017 | 001-38118 | |||||||||||||||||||||||||||||
| 4.4* | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 4.5 | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 4.6 | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 4.7 | S-1 | 5/4/2020 | 333-237991 | |||||||||||||||||||||||||||||
| 4.8 | S-1 | 5/4/2020 | 333-237991 | |||||||||||||||||||||||||||||
| 4.9 | S-1/A | 2/6/2020 | 333-235780 | |||||||||||||||||||||||||||||
| 4.10 | S-1 | 5/4/2020 | 333-237991 | |||||||||||||||||||||||||||||
| 4.11 | S-1 | 5/4/2020 | 333-237991 | |||||||||||||||||||||||||||||
| 4.12 | X | |||||||||||||||||||||||||||||||
| 10.1 | 8-K | 11/10/2020 | 001-38118 | |||||||||||||||||||||||||||||
112
| 10.2 | 8-K | 3/2/2020 | 001-38118 | |||||||||||||||||||||||||||||
| 10.3 | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
10.4* | S-4 | 6/18/2019 | 333-232181 | |||||||||||||||||||||||||||||
10.5* | S-4 | 6/18/2019 | 333-232181 | |||||||||||||||||||||||||||||
10.6* | S-4 | 6/18/2019 | 333-232181 | |||||||||||||||||||||||||||||
10.7* | S-4 | 6/18/2019 | 333-232181 | |||||||||||||||||||||||||||||
10.8* | 8-K | 9/17/2019 | 001-38118 | |||||||||||||||||||||||||||||
10.9* | 8-K | 3/24/2020 | 001-38118 | |||||||||||||||||||||||||||||
10.10* | 10-K | 3/5/2021 | 001-38118 | |||||||||||||||||||||||||||||
10.11* | 8-K | 06/05/23 | 001-38118 | |||||||||||||||||||||||||||||
10.12* | 8-K | 5/27/2020 | 001-38118 | |||||||||||||||||||||||||||||
10.13* | 8-K | 5/27/2020 | 001-38118 | |||||||||||||||||||||||||||||
10.14* | S-4/A | 8/7/2019 | 333-232181 | |||||||||||||||||||||||||||||
10.15* | S-1 | 1/3/2020 | 333-235780 | |||||||||||||||||||||||||||||
10.16* | S-1 | 1/3/2020 | 333-235780 | |||||||||||||||||||||||||||||
10.17* | 8-K | 1/21/2020 | 001-38118 | |||||||||||||||||||||||||||||
10.18* | 8-K | 1/21/2020 | 001-38118 | |||||||||||||||||||||||||||||
10.19* | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
10.20* | 10-K | 3/2/2023 | 001-38118 | |||||||||||||||||||||||||||||
113
10.21* | 8-K | 7/2/2021 | 001-38118 | |||||||||||||||||||||||||||||
10.22* | 8-K | 4/1/2021 | 001-38118 | |||||||||||||||||||||||||||||
10.23* | 10-Q | 5/13/2021 | 001-38118 | |||||||||||||||||||||||||||||
10.24* | 10-Q | 5/13/2021 | 001-38118 | |||||||||||||||||||||||||||||
| 10.25 | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 10.26 | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 10.27 | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 10.28 | 8-K | 9/5/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 10.29 | 8-K | 9/23/2019 | 001-38118 | |||||||||||||||||||||||||||||
| 10.30 | S-1/A | 2/6/2020 | 333-235780 | |||||||||||||||||||||||||||||
| 10.31 | 8-K | 7/7/2021 | 001-38118 | |||||||||||||||||||||||||||||
10.32* | 8-K | 05/09/2023 | 333-263484 | |||||||||||||||||||||||||||||
10.33* | S-8 | 3/11/2022 | 333-263484 | |||||||||||||||||||||||||||||
10.34* | S-8 | 3/11/2022 | 333-263484 | |||||||||||||||||||||||||||||
| 10.35 | 10-Q | 8/8/2022 | 001-38118 | |||||||||||||||||||||||||||||
| 10.36 | 10-Q | 8/8/2022 | 001-38118 | |||||||||||||||||||||||||||||
| 10.37 | 8-K | 11/14/2022 | 001-38118 | |||||||||||||||||||||||||||||
| 10.38 | 10-Q | 8/8/2022 | 001-38118 | |||||||||||||||||||||||||||||
114
| 10.39 | S-3 | 8/8/2022 | 333-266650 | |||||||||||||||||||||||||||||
10.40* | 10-Q | 11/3/2022 | 001-38118 | |||||||||||||||||||||||||||||
10.41* | 10-Q | 5/4/2023 | 001-38118 | |||||||||||||||||||||||||||||
10.42*^ | 8-K/A | 7/13/2023 | 001-38118 | |||||||||||||||||||||||||||||
10.43*^ | 8-K | 5/9/2023 | 001-38118 | |||||||||||||||||||||||||||||
10.44* | 8-K | 9/11/2023 | 001-38118 | |||||||||||||||||||||||||||||
10.45*^ | X | |||||||||||||||||||||||||||||||
| 21.1 | 10-K | 03/02/23 | 001-38118 | |||||||||||||||||||||||||||||
| 23.1 | X | |||||||||||||||||||||||||||||||
| 24.1 | X | |||||||||||||||||||||||||||||||
| 31.1 | X | |||||||||||||||||||||||||||||||
| 31.2 | X | |||||||||||||||||||||||||||||||
| 32.1** | X | |||||||||||||||||||||||||||||||
| 97.1 | 10-Q | 11/02/23 | 001-38118 | |||||||||||||||||||||||||||||
| 101.INS | Inline XBRL Instance Document | X | ||||||||||||||||||||||||||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | X | ||||||||||||||||||||||||||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||||||||||||||||||||||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | X | ||||||||||||||||||||||||||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | X | ||||||||||||||||||||||||||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||||||||||||||||||||||||||
| 104 | The cover page from the Company's Annual Report on Form 10-K for the year ended December 31, 2023 has been formatted in Inline XBRL | X | ||||||||||||||||||||||||||||||
* | Management contract or compensatory plan or arrangement. | |||||||||||||||||||||||||||||||
115
| ^ | Certain exhibits and schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company hereby undertakes to furnish supplementally a copy of any omitted exhibit or schedule upon request by the SEC. | |||||||||||||||||||||||||||||||
** | This certification is being furnished solely to accompany this report pursuant to 18 U.S.C. 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation by reference language in such filing. | |||||||||||||||||||||||||||||||
Item 16. Form 10-K Summary
None.
116
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| DERMTECH, INC. | ||||||||
Date: February 29, 2024 | By: | /s/ Bret Christensen | ||||||
| Bret Christensen | ||||||||
Chief Executive Officer (Principal Executive Officer) | ||||||||
POWER OF ATTORNEY AND SIGNATURES
We, the undersigned officers and directors of DermTech, Inc., hereby severally constitute and appoint Bret Christensen and Kevin Sun, and each of them singly (with full power to each of them to act alone), our true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution in each of them for her or him and in her or his name, place and stead, and in any and all capacities, to sign any and all amendments to this Annual Report on Form 10‑K, and generally to do all things in our names and on our behalf in such capacities to enable DermTech, Inc. to comply with the provisions of the Securities Exchange Act of 1934, as amended, and all the requirements of the Securities Exchange Commission.
Pursuant to the requirements of the Securities and Exchange Act of 1934 this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||||||||||||
| /s/ Bret Christensen | Chief Executive Officer and Director (Principal Executive Officer) | February 29, 2024 | ||||||||||||
| Bret Christensen | ||||||||||||||
| /s/ Kevin Sun | Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) | February 29, 2024 | ||||||||||||
| Kevin Sun | ||||||||||||||
| /s/ Cynthia Collins | Director | February 29, 2024 | ||||||||||||
| Cynthia Collins | ||||||||||||||
| /s/ Nathalie Gerschtein Keraudy | Director | February 29, 2024 | ||||||||||||
| Nathalie Gerschtein Keraudy | ||||||||||||||
| /s/ Matthew Posard | Director | February 29, 2024 | ||||||||||||
| Matthew Posard | ||||||||||||||
| /s/ Herm Rosenman | Director | February 29, 2024 | ||||||||||||
| Herm Rosenman | ||||||||||||||
| /s/Mark Capone | Director | February 29, 2024 | ||||||||||||
| Mark Capone | ||||||||||||||
| /s/Kirk Malloy | Director | February 29, 2024 | ||||||||||||
| Kirk Malloy | ||||||||||||||
117