UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended December 31 , 2023
or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the transition period from ___________ to ___________
Commission File Number: 001-38593
(Exact name of Registrant as specified in its charter)
State or Other Jurisdiction of Incorporation or Organization | I.R.S. Employer Identification No. | |||||||
| Not applicable | ||||||||
Address of Principal Executive Offices | Zip Code | |||||||
| +506-2434-2400 | ||||||||||||||
| Registrant’s Telephone Number, Including Area Code | ||||||||||||||
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of Each Exchange on Which Registered | ||||||||||||
The | ||||||||||||||
Securities registered pursuant to Section 12(g) of the Act:
| None | ||
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company”, and "emerging growth company" in Rule 12b-2 of the Exchange Act. (Check one)
☒ | Accelerated filer | ☐ | |||||||||||||||
| Non-accelerated filer | ☐ | Smaller reporting company | |||||||||||||||
| Emerging growth company | |||||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☒ No ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by an of the registrant’s executive officers during the relevant recovery period pursuant to (§240.10D-1(b)). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2). Yes ☐ No ☒
The aggregate market value of the common stock held by non-affiliates of the registrant on June 30, 2023 was approximately $1,342,497,565 . Shares of the registrant’s common stock held by each executive officer, director and holder of 10% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This calculation does not reflect a determination that certain persons are affiliates of the registrant for any other purpose. The registrant has no non-voting equity.
As of March 1, 2024, the number of the registrant’s common shares outstanding was 27,205,003 .
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy statement relating to its 2024 annual meeting of shareholders (the “2024 Proxy Statement”) are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. The 2024 Proxy Statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
TABLE OF CONTENTS
| Page | ||||||||
Item 1A. | Risk Factors | |||||||
i
EXPLANATORY NOTE
In this report, unless the context indicates otherwise, the terms “Establishment Labs,” “Company,” “we”, “us” and “our” refer to Establishment Labs Holdings Inc., a British Virgin Islands entity, and its consolidated subsidiaries.
We own, or have rights to, trademarks and trade names that we use in connection with the operation of our business, including Establishment Labs and our logo as well as other brands such as Motiva Implants, SilkSurface/SmoothSilk, VelvetSurface, ProgressiveGel, TrueMonobloc, BluSeal, Divina, Ergonomix, Ergomonix2, Ergonomix2 Diamond, Mia Femtech, MotivaImagine and Zen, among others. Other trademarks and trade names appearing in this report are the property of their respective owners. Solely for your convenience, some of the trademarks and trade names referred to in this report are listed without the ® and TM symbols, but we will assert, to the fullest extent under applicable law, our rights to our trademarks and trade names.
WEBSITE REFERENCES
In this Annual Report on Form 10-K, we make references to our website at establishmentlabs.com. References to our website through this Form 10-K are provided for convenience only and the content on our website does not constitute a part of, and shall not be deemed incorporated by reference into, this Annual Report on Form 10-K.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended or the Exchange Act. You can find many (but not all) of these statements by looking for words such as “approximates,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “would,” “may” or other similar expressions in this report. Any statements that refer to projections of our future financial or operating performance, our liquidity and anticipated cash plans, anticipated trends in our business, our goals, strategies, focus and plans, and other characterizations of future events or circumstances, including statements expressing general optimism about future operating results, are forward-looking statements.
We claim the protection of the safe harbor contained in the Private Securities Litigation Reform Act of 1995. We caution investors that any forward-looking statements presented in this report, or that we may make orally or in writing from time to time, are expressions of our beliefs and expectations based on currently available information at the time such statements are made. Such statements are based on assumptions, and the actual outcome will be affected by known and unknown risks, trends, uncertainties and factors that are beyond our control. Although we believe that our assumptions are reasonable, they are not guarantees of future performance. As a result, our actual future results may differ from our expectations, and those differences may be material.
Factors that could cause or contribute to these differences include, among others, those risks and uncertainties discussed below under “Summary Risk Factors” and under Part I, Item 1A. “Risk Factors,” as such risk factors may be amended, updated or superseded from time to time by our subsequent filings with the Securities and Exchange Commission. The risks and uncertainties included herein are not exhaustive, and additional factors could adversely affect our business and financial performance. We operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for us to predict all such risk factors, nor can we assess the impact of all such risk factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
We are not undertaking any obligation to update any forward-looking statements. Accordingly, investors should use caution in relying on past forward-looking statements, which speak only as of the date they are made.
SUMMARY RISK FACTORS
The following is a summary of certain key risk factors for investors in our securities. You should read this summary together with the more detailed description of risks and uncertainties discussed below under Item 1A. “Risk Factors” before investing in the Company.
•Unfavorable global economic conditions, including slower growth or recession, inflation or decreases in consumer spending power or confidence, could adversely affect our business, financial condition or results of operations.
•We expect to incur losses for the foreseeable future, and our ability to achieve and maintain profitability depends on the commercial success of our Motiva Implants.
1
•If our available cash resources and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements, we may seek to sell equity or convertible debt securities, enter into a credit facility or another form of third-party funding, or seek other debt financing.
•The clinical trial process is lengthy and expensive with uncertain outcomes, and often requires the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. In addition, safety issues or other challenges may arise during the conduct of a trial. Delays or failures in our clinical trials will prevent us from commercializing any modified or new products and will adversely affect our business, operating results and prospects.
•If the FDA or similar regulatory authority does not approve our products or requires additional clinical trials or preclinical data before any approval or if any approval of our products includes additional restrictions on the label, or requires a characterization of our products, including the description of the product surface (e.g. smooth, texture, other) that differs from ours and/or other regulatory authorities, our business, financial condition, results of operations and growth prospects could be materially adversely affected.
•Pandemics, epidemics, or other public health crises may adversely affect our business and financial results in the future, as was the case with the COVID-19 pandemic in recent years.
•In certain large markets, we engage in direct sales efforts. We may fail to maintain and develop our direct sales force, and our revenues and financial outcomes could suffer as a result. Furthermore, our direct sales personnel may not effectively sell our products.
•If we are unable to educate clinicians on the safe, effective and appropriate use of our products and designed surgeries, we may experience unsatisfactory patient outcomes, negative publicity and increased claims of product liability and may be unable to achieve our expected growth.
•We have a limited operating history in the United States and may face difficulties encountered by companies early in their commercialization in competitive and rapidly evolving markets.
•Our success depends, in part, on our ability to continue to enhance our existing products and services and develop or commercialize new products and services that respond to customer needs and preferences, which we expect will require us to incur significant expenses.
•Our business depends on maintaining our brand and ongoing customer demand for our products and services, and a significant reduction in sentiment or demand could affect our results of operations.
•If we fail to compete effectively against our competitors, many of whom have greater resources than we have, our revenues and results of operations may be negatively affected.
•Any disruption at our existing facilities could adversely affect our business and operating results.
•The medical technology industry is complex and intensely regulated at the federal, state, and local levels and government authorities may determine that we have failed to comply with applicable laws or regulations.
•We rely on a single-source, third-party supplier for medical-grade long-term implantable silicone, which is the primary raw material used in our Motiva Implants. If this supplier were to increase prices for this raw material over time or experience interruptions in its ability to supply us with this raw material, our business, financial condition and results of operations could be adversely affected.
•We have significant exposure to the economic and political situations in emerging market countries, and developments in these countries could materially impact our financial results, or our business more generally.
•Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance by financial institutions or transactional counterparties, could adversely affect our liquidity and financial performance.
•Our results of operations could be affected by fluctuations in currency rates.
•Negative publicity concerning our products or our competitors’ products, including due to product defects, recalls and any resulting litigation, could harm our reputation and reduce demand for silicone breast implants, either of which could adversely impact our financial results and/or share price.
2
•Recent news coverage has called into question the long-term safety of breast implants and reports of breast implant-associated anaplastic large cell lymphoma linked to our competitors’ products which have led to regulatory actions regarding macrotextured devices in several countries and the worldwide recall of one of our competitor’s macrotextured implants and tissue expanders. These events and reports of other forms of cancer, including squamous cell carcinoma and various lymphomas, from breast implant products may lead to a reduction in the demand for silicone breast implants and could adversely affect our business.
•The medical device industry is characterized by patent litigation and we could become subject to litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages or prevent us from marketing our existing or future products.
3
PART I
ITEM 1. BUSINESS
Overview
We are a medical technology company focused on improving patient safety and aesthetic outcomes, initially in the breast aesthetics and reconstruction market. We initially incorporated in Costa Rica in 2004 and subsequently reorganized under a parent holding company in the British Virgin Islands in 2013.
Our line of silicone gel-filled breast implants, branded as Motiva Implants, is the centerpiece of our medical technology platform. Our post-market surveillance data (which was not generated in connection with a United States Food and Drug Administration, or FDA, pre-market approval, or PMA, study collected at defined follow-ups, but was patient or practitioner reported) and published third-party registries and data indicate that Motiva Implants have low rates of adverse events (including rupture, capsular contracture, and safety related reoperations) that we believe compare favorably with those of our competitors. We believe the proprietary technologies that differentiate our Motiva Implants enable improved safety and aesthetic outcomes and drive our revenue growth. We have developed other complementary products and services, which are aimed at further enhancing patient outcomes.
Since launching Motiva Implants in October 2010, the majority of our revenue has been generated from sales of our Motiva Implants. To date, our Motiva Implants are registered to be sold in 86 countries, including, most recently, in China. We currently sell our products via exclusive distributors or our direct sales force and have introduced five generations of Motiva Implants. We currently commercially sell five product families: (i) Round and Ergonomix Round, (ii) Ergonomix Oval, (iii) Anatomical TrueFixation, (iv) Ergonomix2 Round and Ergonomix2 Diamond and (v) Flora Tissue Expander. Our products incorporate first of-its-kind safety features including: (i) SmoothSilk / SilkSurface (an optimized biocompatible advanced smooth surface that is designed to reduce capsular contracture), (ii) Qid RFID technology (a non-invasive, readable serial number that enables product identification and enhances safety and patient peace of mind), (iii) BluSeal visual barrier layer (a proprietary indicator that allows for verification of complete barrier layer presence) and (iv) TrueMonobloc gel-shell-patch configuration (a highly durable, easy-to-insert performance shell, gel and patch system that allows for smaller incisions and smaller scars).
Recent Developments
In January 2024, we announced the commercial launch of Motiva Implants in China and the completion of the first procedure with the Motiva Flora SmoothSilk Tissue Expander in the United States.
We also entered into a securities purchase agreement, pursuant to which we sold an aggregate of two million common shares and pre-funded warrants for gross proceeds of approximately $50 million. See Note 15 “Subsequent Events” for additional information.
We are in the process of expanding our manufacturing facilities and corporate offices in the Coyol Free Zone, or CFZ, in Costa Rica. Construction of the cold shell structure of the Sulàyöm Innovation Campus was initially funded by the Coyol Free Zone in 2021 until we exercised our option to purchase the title of the land and cold shell building for approximately $12.6 million in 2022. In July 2023, we announced the grand opening of the first phase of the Sulàyöm Innovation Campus, which includes approximately 100,000 square feet of facility space intended to increase our manufacturing capacity by approximately 730,000 units per year. We estimate a total of $51.7 million in costs for this initial phase of our expansion project, of which the majority has been incurred to date. Additional phases of the project may be executed, at our option, to further expand manufacturing capacity at the new facility. We expect to commence manufacturing from the new facility in 2024. See Note 3 “Balance Sheet Accounts” for additional information.
In November 2023, we received National Medical Products Administration, or NMPA, approval in China for Motiva Implants, 510(k) clearance from the FDA for the Motiva Flora SmoothSilk Tissue Expander in the United States, and CE mark approval under the European Medical Device Regulation for the Motiva Injector, the Motiva Inflatable Balloon and the Motiva Channel Dissector.
In addition, in October 2023, we completed and announced the results of the two-year 100-patient clinical study for Mia Femtech, our patented technologies that can increase breast shape by 1 to 2 cups in a 15-minute procedure without the need for general anesthesia. The single-center, Institutional Review Board approved study began in December 2020 and involved participation of fifteen board-certified plastic surgeons from Costa Rica, Sweden, England, Brazil, Austria, Italy, Belgium, and the United States. We have launched Mia Femtech globally
4
through partnerships with clinics in Japan, Spain, Switzerland, Sweden, Germany, France, Costa Rica, Turkey and the Middle East. In October 2023, we also launched, in select geographies, Zen - the newest generation of our passive RFID technology that is now non-ferromagnetic. Zen is available with Motiva Ergonomix2 Round implants in the Joy program.
In April 2023, we issued 1,165,000 common shares in an underwritten public offering for net proceeds of approximately $84.6 million.
In February 2023, we submitted the final module of our clinical trial for Motiva Implants in the United States to the FDA. We received FDA approval to start our clinical trial in 2018. By August 2019, we had completed all surgeries in the aesthetic cohorts and implemented a bifurcated regulatory strategy for data submission. In April 2022, we released preliminary results for the primary augmentation cohort and, by June 2022, we completed enrollment and surgeries for the primary reconstruction cohort. By September 2022, we had completed the three-year follow-up for the aesthetic cohort.
In January 2023, we announced a partnership with Seishin Plastic and Aesthetic Surgery Clinic in Japan for Mia Femtech. Previously, we obtained regulatory approval from the Pharmaceuticals and Medical Devices Agency, as well as reimbursement for post-mastectomy reconstruction under the Japanese National Health System, for Motiva Implants and the Motiva Flora tissue expander in November 2022.
In April 2022, we entered into a credit agreement, or the Credit Agreement, for term loans to the Company in an aggregate principal amount of up to $225 million, with Oaktree Fund Administration, LLC, as administrative agent. The first and second tranche were advanced in the amount of $150 million and $25 million in April and December 2022, respectively. A portion of the proceeds from the first tranche was used to repay in full and terminate the $65 million in aggregate principal amount outstanding under the Company’s previous credit agreement with Madryn Health Partners, LP, or the Madryn Credit Agreement, and the $6.5 million early repayment penalty. In February 2024, we amended the Credit Agreement, modifying the access conditions, commitment termination dates and interest rates for the two remaining available tranches. See Note 5 “Debt” and Note 15 “Subsequent Events” for additional information.
We are focused on investing in manufacturing capacity, marketing, customer service, and sales force in multiple geographies to promote the use of our Motiva Implants. This expansion may result in short-term losses as we grow our organization and invest in research, clinical trials, and other commercialization efforts.
Our Market
Breast Augmentation
Breast augmentation surgery is one of the leading aesthetic surgical procedures by number of procedures globally. Approximately 2.2 million breast augmentations were performed worldwide in 2022, according to International Society of Aesthetic Plastic Surgery, or ISAPS. The following table lists the top markets by country for total breast augmentations in 2022 according to ISAPS.
5
| Total Breast Augmentation Procedures | |||||||||||
Rank(1) | Country | Procedures | Percentage of World-Wide Total | ||||||||
| 1 | United States | 255,200 | 11.7% | ||||||||
| 2 | Brazil | 243,923 | 11.2% | ||||||||
| 3 | Mexico | 103,524 | 4.8% | ||||||||
| 4 | Argentina | 77,710 | 3.6% | ||||||||
| 5 | Germany | 76,658 | 3.5% | ||||||||
| 6 | Colombia | 63,204 | 2.9% | ||||||||
| 7 | Turkey | 55,254 | 2.5% | ||||||||
| 8 | Italy | 42,058 | 1.9% | ||||||||
| 9 | Spain | 39,519 | 1.8% | ||||||||
| 10 | India | 29,986 | 1.4% | ||||||||
(1) Rankings are based solely on those countries from which a sufficient survey response was received and data was considered to be representative. | |||||||||||
Breast Reconstruction
The American Society of Plastic Surgeons noted in their Procedural Statistics Release that 151,641 breast reconstructions were performed in 2022 in the United States. According to Fairfield Market Research 2023 report, the global breast reconstruction market is expected to grow at a CAGR of 7.2% from 2023 to 2030. The market, currently valued at $0.6 billion in 2022, is projected to exceed $1 billion by 2030. This growth is expected to be driven by factors like technological advancements, increased awareness, and a rising number of breast cancer cases worldwide.
Traditional Breast Implants and Their Limitations
Despite the global demand for breast augmentation procedures, there has been relatively little innovation since the 1990s. In 1992, due to emerging safety concerns, the FDA placed a moratorium on sales of silicone breast implants in the United States, which was lifted in 2006. This, combined with the ongoing FDA requirement for a PMA approval of all marketed breast implants, has discouraged breast implant innovation over the past 30 years. Many of the legacy breast implant options have relatively high complication rates, and we believe many do not mimic natural breast tissue.
10-year primary augmentations
The table below reports key adverse event information from published data from their 10-year prospective Core clinical trials conducted by the only three companies currently approved to market silicone breast implants in the United States.
| Sientra 10-Year | Allergan 10-Year | Mentor 10-Year | ||||||||||||||||||
| Number of Patients | N=1,116 Patients | N=455 Patients | N=552 Patients | |||||||||||||||||
Rupture(1) | 8.5% | 9.3% | 24.2% | |||||||||||||||||
| Capsular Contracture | 12.9% | 18.9% | 12.1% | |||||||||||||||||
| Reoperation | 24.0% | 36.1% | 25.5% | |||||||||||||||||
| Kaplan-Meier risk rates were the primary method of analysis for the above data. This table represents the final data from the primary cohort of the same study referenced in the above five- and six-year PMA studies conducted by our competitors. This 10-year data for Sientra, Allergan and Mentor were released in 2018, 2018, and 2015, respectively. | ||||||||||||||||||||
| (1) The rupture rates represent the MRI cohort only for each respective study, which consisted of 571 patients for Sientra, 158 patients for Allergan and 202 patients for Mentor. | ||||||||||||||||||||
6
We believe that the improved appearance, more natural feel and patient safety profile of our Motiva Implants provides a strong competitive advantage that will help us to both capture market share and achieve higher patient conversion rates by addressing the primary concerns described by patients who choose not to pursue breast augmentation surgery.
Our Competitive Strengths
▪Patient-centric innovative implant technologies. We have developed our Motiva Implants by enhancing and creating novel product components for our implants, and then combining these components into products that deliver improved aesthetic outcomes, increased patient satisfaction and favorable safety profiles.
▪Extensive suite of complementary products and services. Our product portfolio includes innovative devices and tools. We believe our designed surgical procedures, such as MotivaHybrid, Motiva MinimalScar and Mia Femtech, will address key unmet needs for both the physician and the patient.
▪Proprietary internal manufacturing processes and capabilities. We manufacture our silicone products in state-of-the-art manufacturing facilities in Costa Rica. In these facilities, we utilize our novel 3D imprinted molding method to create proprietary surface features that, in combination with other proprietary materials and methods, differentiate our products from those of our competitors. We believe our modern facilities, focus on product quality and deep technological expertise have helped us establish and maintain a brand of consistency, quality and safety.
▪Dynamic worldwide sales platform. We sell our products both through exclusive arrangements with leading local distributors who have strong local surgeon relationships and our direct sales force in key markets such as Brazil and primary markets in Europe. Using this market-specific approach, we have built an effective and efficient worldwide sales platform.
▪Proven management team with expansive industry experience. We have a highly experienced management team that is comprised of leaders from the medical aesthetic market.
Our Growth Strategy
Our goal is to be the global leader in aesthetic surgical implant technology, including breast implants, while improving patient safety through product innovation. The key elements of our strategy include:
▪Expand revenues in existing markets. We believe we can continue to grow market share in our existing markets due to the favorable safety profile and improved aesthetic outcomes of our Motiva Implants.
▪Launch Motiva Implants in additional markets outside the United States. We expect that continued geographic expansion will be a key driver of growth in the near term. In recent years, we started sales through distributors in Australia, Israel, Peru, Russia, Saudi Arabia, Taiwan, Thailand and South Korea, as well as starting direct sales in Brazil, the second largest market for breast augmentation procedures. Expansion into China commenced in January 2024.
▪Obtain FDA approval and enter the U.S. market. We are conducting our IDE clinical trial in the United States, with the goal of obtaining FDA PMA and commercializing our Motiva Implants in the United States. The Motiva IDE clinical trial enrolled 827 patients at 32 centers in the U.S., Germany, and Sweden. All enrollment surgeries in the IDE U.S. clinical trial were completed by June 2022. In February 2023, we submitted the final module of our clinical trial for Motiva Implants in the United States to the FDA. In April 2023, we presented the results of the clinical trial for 451 primary augmentation patients enrolled in the study through the three-year follow-up visit. We are awaiting FDA PMA approval of our Motiva Implants. We received 510(k) clearance for the Motiva Flora SmoothSilk Tissue Expander in October 2023 and announced the completion of the first commercial procedure in January 2024.
▪Optimize patient conversion through sales and marketing programs. We employ a multi-faceted marketing strategy that includes social media engagement, conference presence, online advertising and patient and physician education. This approach enables us to engage with and educate patients on the Motiva brand and the benefits of our products, as well as increase clinical efficiency for our physician collaborators. In the future, we expect our social media and online patient and physician education to have important strategic synergies with our designed surgeries, which are promoted globally.
7
▪Seek out and pursue strategic acquisitions. We intend to seek out other innovative products, services and procedures that satisfy unmet needs in the aesthetics space and complement our existing product portfolio as we believe this can be additive to future revenue growth. We have purchased distributor networks in strategic markets and may acquire other third-party sales organizations in the future. While we have no specific acquisitions or planned licensing agreements currently ongoing, we may engage in these, or other strategic transactions, with the goal of augmenting our existing product portfolio and global footprint.
▪Continue a high level of engagement with key opinion leaders. We promote Motiva Implants, in part, via an extensive and robust calendar of physician education events led by key opinion leaders in the field of aesthetic and reconstructive surgery. In 2023 and 2022, we conducted 192 and 201 events, respectively, through our medical educational platform. We also collaborate actively with respected and influential key opinion leader surgeons to identify and develop new clinical applications for our existing products, as well as new product and strategic opportunities.
8
Our Products and Technologies
The key characteristics of our primary products are described in the table below:
| Product | Motiva Round | Motiva Ergonomix | Motiva Ergonomix2 | Motiva Flora Tissue Expander | ||||||||||||||||||||||
 |  |  |  | |||||||||||||||||||||||
| Description | Round soft silicone-gel filled breast implants | Gravity sensitive round soft silicone-gel-filled breast implants | Gravity sensitive soft silicone-gel-filled breast implants with improved mechanical properties | Breast tissue expander, used to gradually expand a patient’s breast tissue prior to the placement of a long-term breast implant | ||||||||||||||||||||||
Product Catalog | Available in 160 round catalogs, including four projection heights | Available in 160 round catalogs, including four projection heights | Available in more than 160 round catalogs, including four projection heights; Available in 60 catalogs for Diamond implants | Available in 15 catalogs, with three different heights | ||||||||||||||||||||||
| Key Features | •SilkSurface/SmoothSilk shell surface •ProgressiveGel PLUS Silicone gel fill •TrueMonobloc construction •BluSeal shell barrier layer •Qid Safety Technology RFID microtransponder | ▪SilkSurface/SmoothSilk shell surface ▪ProgressiveGel Ultima, Silicone gel fill ▪TrueMonobloc construction ▪BluSeal shell barrier ▪Qid Safety Technology RFID microtransponder ▪Ergonomy and more natural look | ▪SilkSurface/SmoothSilk shell surface •ProgressiveGel Ultima, Silicone gel fill ▪TrueMonobloc+ construction ▪BluSeal+ shell barrier •Qid Safety Technology RFID microtransponder ▪Motiva SuperSilicones | ▪SilkSurface/SmoothSilk shell surface ▪Anatomical design ▪Compatible with MRI and CT scans ▪Injection site located with RF technology, using the Motiva Port Locator ▪Orientation line observable on X-Ray ▪Fixation suture tabs | ||||||||||||||||||||||
| Sales Territories | 86 countries | |||||||||||||||||||||||||
Motiva Implants
The Motiva breast implants are a Class III Medical Device indicated for breast augmentation and breast reconstruction, including revision surgeries to correct or improve the result of a previous breast implant surgery. We launched Motiva Implants commercially in October 2010, and to date we have sold approximately 3.3 million units in various countries outside the United States. Motiva Implants incorporate several proprietary features that we believe contribute to their favorable safety profile, natural appearance and feel. Our latest generation of Motiva Implants utilizes our proprietary Gravity Sensitive Ergonomix design, with a round base implant that responds to gravity by shifting its maximum point of projection, offering the more “natural” projection of a shaped implant without the malposition and rotation issues frequently associated with shaped implants. Furthermore, our fill material with the ProgressiveGel platform of silicone gel rheologies consists of highly purified biocompatible gels with specific visco-elastic properties that we believe enables Motiva Implants to respond to the patient’s motion in ways that more closely mimic the appearance, feel and movement of natural breast tissue. Our catalog includes over 1,000 product variations, with round, oval and anatomical shapes, two different surfaces, SmoothSilk and
9
VelvetSurface, and volumes ranging from 95cc to 1060cc, resulting in a wider range of options than those offered by our major competitors.
Ergonomix2 incorporates the latest innovations, including our most advanced ultra-high purity chemistries for enhanced device safety mechanical properties and improved patient ergonomics. Ergonomix2 also features our patented SmoothSilk surface technology, which is the basis of Motiva Implants’ low inflammatory characteristics that have contributed to the lowest capsular contracture rates in the industry. Ergonomix2 was CE marked in December 2020 and labeled for use in both aesthetic and reconstruction procedures.
A study published in February 2023 by the British Association of Plastic, Reconstructive and Aesthetic Surgeons of breast reconstruction procedures using Ergonomix from January 2017 to January 2022 for 156 patients concluded that Ergonomix implants showcase a unique set of technologies that provide good results with a low complication rate.
Shell Surface: SilkSurface/SmoothSilk
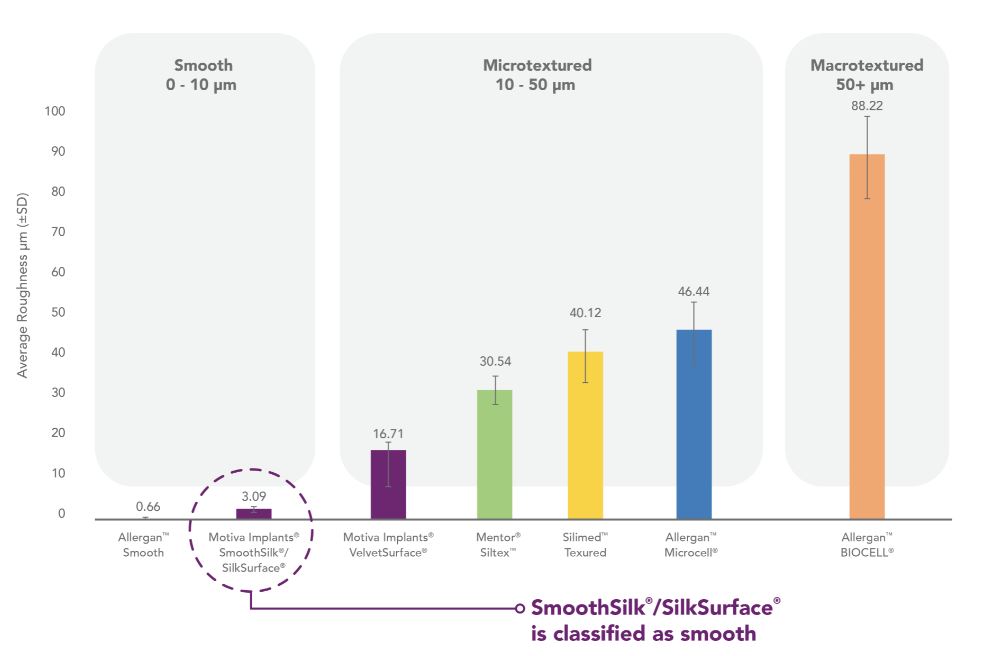
The surface topography of the breast implant shell surface varies between commercially available breast implants. Our SmoothSilk surface was designed to improve biocompatibility and to provide for the same surface topography around the entire implant for the benefit of the patients. The International Standard Organization, or ISO, through the new April 2018 standard (ISO 14607:2018), created a classification of implant surface textures according to roughness. This standard includes an objective method of defining the difference between smooth, micro and macro surfaces based on surface roughness average. The topology of SilkSurface/SmoothSilk is categorized in the smooth category, having a low roughness value of approximately 3.09 microns with thousands of contact features per square centimeter, which is significantly lower than the higher limit of the smooth surface clarification defined by ISO (< 10 microns). The Company and the FDA are in discussions on the product’s labeling, including surface description terminology.
Our retrospective implant data shows that Motiva Implants have a lower rate of capsular contracture and seromas when compared to published data from competitors. We believe that these results are due in large part to the proprietary surface of our Motiva Implants. Our proprietary shell surfaces are smoother and have more regular surface features than those of our primary competitors based on several studies using methods such as scanning electron microscopy, profilometry testing and statistical parameters comparisons.
An abstract presented in 2017 by researchers at Montana State University showed less accumulation of both bacteria and biofilm on SmoothSilk in vitro when compared to smoother and textured surfaces. Biofilm formed on implant surfaces increases the risk of bacteria accumulation and capsule formation.
In December 2018, we commissioned an independent report from the French reference laboratory Laboratoire National de Metrologie et d’Essais, or LNE, on the surface characteristics of our Motiva Implants. Based upon its testing, LNE concluded that the SmoothSilk shell surface in the Motiva Implants is a smooth surface as defined by ISO 14607:2018 categorization.
A 2021 published study in Nature Biomedical Engineering led by Professor Robert Langer, Institute Professor at the Massachusetts Institute of Technology (MIT) at David H. Koch Institute for Integrative Cancer Research, concluded that the SmoothSilk surface can largely suppress the foreign body response and fibrosis provoking the least amount of inflammation in comparison with the other commercially available surfaces. A larger percentage of macrophages in the cell mix indicates an inflammatory response, which is an early stage in capsule formation. We believe the more moderate inflammatory response observed on SmoothSilk is responsible for improved biocompatibility and lower complication profile.
In November 2022, another publication related to the SmoothSilk surface was published in the Journal of Engineering Tribology by researchers from the College of Engineering at UC, Santa Barbara. The extent of the surface roughness as examined for the silicone implant shells for SmoothSilk resulted in the lowest friction coefficient and fewest wear debris particles in the size range favored by the macrophages. This highlights the optimal ranges of the SmoothSilk design to reduce frictional shared stress and wear debris during the tribological interactions.
10
The graph below shows how our surface features compares with those of our competitors.
ProgressiveGel Family
The proprietary silicone chemistries that comprise our ProgressiveGel family allow for a high degree of cohesiveness and strength but add characteristics such as softness and high ductility that enable movement dynamics more like that of natural breast tissue. We believe that the cohesive properties reduce the likelihood of silicone gel leakage in the event of a rupture in the shell. The strength of the gel is believed to contribute to a reduced frequency of gel fracture, a condition which leads to deformed implant shape and stress on the implant’s shell. While other manufacturers have claimed a “high strength” gel, ours combines a notably high elasticity (the ability to stretch without permanent deformation) with low viscosity, both of which are designed to reduce the susceptibility of the implants to rupture while improving their tactile feel and movement dynamics. Additionally, the improved adhesion of the gel to the shell structure avoids the appearance of separation spots, an aesthetic defect commonly seen in competitor products.
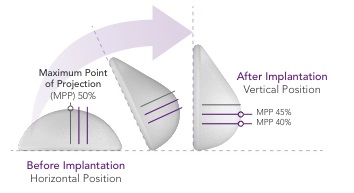
In addition to the anticipated safety advantages, our ProgressiveGel family provides for movement characteristics that resemble natural breast tissue. Our later generation Ergonomix products further mimic natural tissue, with a maximum point of projection that shifts lower to create a natural human breast shape when a patient is standing. This allows our Motiva Implants to provide the more natural aesthetics of “shaped” or “teardrop” implants without the risk of associated drawbacks such as breast deformation from rotation and unnaturally hard tactile feel. The images below illustrate the implants’ ability to change shape depending on the patient’s positioning.
11
 |  | ||||
TrueMonobloc
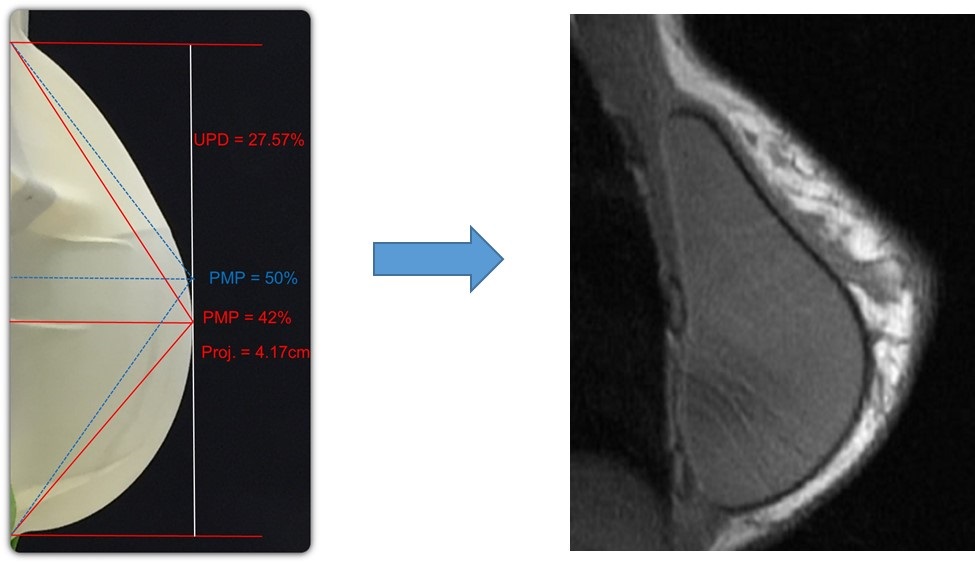
Our TrueMonobloc technology, which is incorporated into all generations of Motiva Implants currently sold, combines proprietary chemistry with our proprietary manufacturing techniques to create a shell, gel and other components that are tightly bound to one another. This results in an implant that is more homogeneously elastic and resistant to separation of the gel from the shell, addressing one type of implant failure that can lead to shell ruptures and silicone leaks. This also enables Motiva Implants to be stretched and squeezed to a more significant degree, which we believe currently enables breast augmentation through incision sizes smaller than one inch, compared with the published industry norm of approximately two inches. A surgical technique that we have developed, which we call Minimally Invasive Aesthetics, or Mia Femtech, utilizes our next-generation Ergonomix2 Diamond implant to take advantage of these physical properties to enable a less-invasive procedure for the patient. The following image shows that TrueMonobloc enables significant manipulation of a Motiva Implant without separation of gel from shell.

RFID Technology
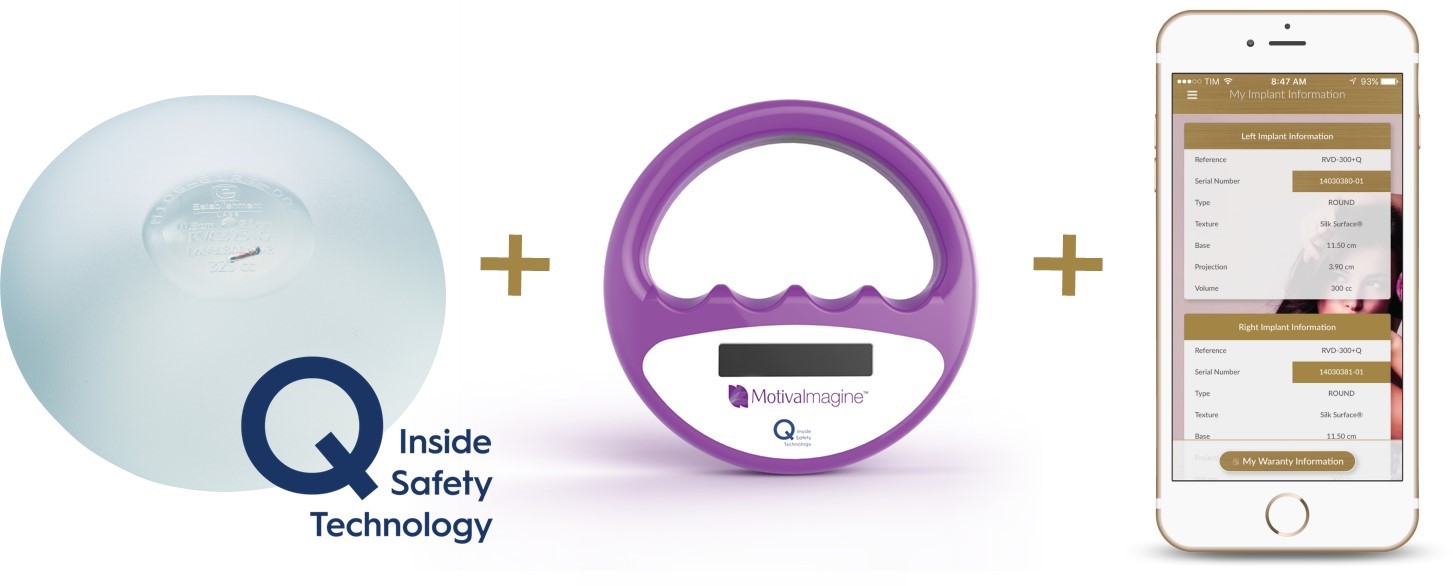
We offer a Radio-Frequency Identification Device microtransponder (also referred to as Qid) that is placed in the filling gel as an optional feature for all implant styles. This microtransponder provides each device with a unique electronic serial number for traceability purposes.
The microtransponder contains only a unique 15-digit code that identifies the product and does not contain any patient information. This microtransponder can be read with a simple pass from our non-invasive and inexpensive reading device, the Qid Safety Technology Reader, and the serial number corresponds with related information in our MotivaImagine database such as implant type, size and other characteristics. Patients can create a secure account, register the products and include applicable patient information either through the MotivaImagine application or our website, to access their implant information. The MotivaImagine application and Motiva Implants website also allow the patient to access the implant warranty information. This traceability is intended to give patients comfort that any future recalls can be positively identified as applying, or not applying, to that patient’s particular implant. This addresses a key concern that often discourages women who are otherwise interested in implants from making the choice to move forward with the surgery. Motiva Implants are currently the only breast
12
implants on the international market with Qid Safety Technology; however, we believe there is an opportunity to sell these microtransponders to other medical device companies in the space.
Each implant’s unique electronic serial number is encoded into the RFID circuitry as part of a three-point authentication system: the microtransponder, the reader and the database. This authentication system prevents unauthorized access to any personal information of the patient and is compliant with FDA regulations.
In October 2023, we announced Zen, which is part of our next generation Zensor RFID platform. The new Zen technology has all the previous benefits of Qid, but is now entirely non-ferromagnetic. Motiva Ergonomix2 Diamond implants used in the Mia Femtech system feature Zen, and are available in select geographies with Motiva Ergonomix2 Round Implants in the Joy program. We believe the Zensor platform has the potential to add additional capabilities in future generations, such as the measurement of temperature and other parameters. Zen with temperature sensing is currently in human trials as part of an IRB-approved study.
BluSeal
The Motiva Implant shell is constructed of successive layers of silicone elastomer and a low diffusion barrier layer. The key function of the low diffusion barrier layer is to prevent diffusion of low molecular weight siloxane species from the implant to the tissues. This barrier layer embeds our BluSeal indicator technology, which is a key feature used during the manufacturing process to verify that the barrier is present in a uniform way around the entire shell. It is also used as a visual quality control and safety measure to minimize potential gel diffusion. This patented manufacturing innovation is intended to highlight any imperfections in the barrier layer coverage with a distinct color. Our BluSeal indicator technology also provides the plastic surgeon with the ability to verify whether the barrier layer has coverage defects or other imperfections before implantation that might lead to post-
13
implantation shell rupture or gel bleed. We believe this is another safety innovation that contributes to our substantially lower reported implant rupture rates as compared to reports for our primary competitors.
Motiva Flora Tissue Expander
The Motiva Flora Tissue Expander is used in breast reconstruction surgery for temporary implantation (less than six months) to gradually expand the breast tissue prior to the placement of a long-term breast implant. After implantation, the device is periodically filled with saline solution via an injection port to increase its volume to stretch the skin and create a pocket for breast implant placement. The injection port is dome-shaped and includes an RFID coil, which can be accurately located utilizing the port locator. The Motiva Flora Tissue Expander is the first MRI Conditional expander and is the only tissue expander in the market with an integrated RFID port with no magnets, allowing for use of the expander safely alongside MRI (1.5 and 3 Tesla) scanning. The Motiva Flora received CE mark in June 2020 and has been registered in 57 countries. The Motiva Flora also includes the SmoothSilk surface, which provides biocompatibility benefits described above. Our catalog includes 15 variations, including three different heights, and a range of volumes from 260 to 995 cc. The Motiva Flora received 510(k) clearance from the FDA in October 2023.
14

Mia Femtech System for Minimally Invasive Aesthetics
In April 2023, we launched Mia Femtech — a patient centric procedure designed to allow breast augmentation to be performed under local anesthesia rather than general anesthesia, through smaller incisions, with faster recovery times and a resulting reduction in surgical complications. The Mia Femtech system includes the specially -designed Ergonomix2 Diamond implant, which received CE mark in December 2020, and its proprietary tools, including the Motiva Inflatable Balloon and the Motiva Injector.
In December 2020, we received a CE mark for our Motiva Ergonomix2 Diamond breast implant, the implant used in the Mia Femtech procedure. In early 2021, we completed enrollment in our one hundred patient Mia® Femtech case series in Costa Rica. The IRB approach study began in December 2020 and one year follow up was completed in early 2022 and the two-year follow up started in December 2022 and was presented in October 2023. The study, which had a two-year follow-up compliance rate of 90%, found no reports of capsular contracture (Baker Grade III/IV), ruptures (suspected or confirmed), bleeding, hematoma, or seroma requiring intervention in the study. Based on our market research, we believe Mia Femtech will continue to attract new consumers and expand the market for breast aesthetic procedures.
This premium and personalized breast harmonization experience is currently available at partner clinics in Japan, Spain, Switzerland, Sweden, Germany, France, and Costa Rica. We have also partnered with our distributors in Turkey and the Middle East to begin opening sites in those regions in 2024.
15

Our Clinical Data
13-Year Safety Post-Market Surveillance Data
Dating from the commercial launch of Motiva Implants in October 2010 through December 2023, we have sold approximately 3.3 million breast implants in various countries outside the United States and Canada. We maintain a Quality Management System database to track and report complaints received from patients or physicians. From October 2010 through December 2023, a total of 4,457 complaints have been reported, investigated and processed, representing approximately 0.1% of the total Motiva Implants sold through December 2023. There were no reported cases of double capsule formation or breast-implant associated anaplastic large-cell lymphoma, or BIA- ALCL, in this data set, and there were 48 cases of early seroma and 4 case of late seroma. The table below shows the rates of rupture, capsular contracture and reoperation for adverse events of our Motiva Implants from the data gathered through December 2023. In contrast to the above competitor data, our data is self-reported rather than collected at mandatory follow-ups and was generated solely for our post-market surveillance instead of in connection with an FDA PMA study. All of these patients were located outside the United States.
| Motiva Implants | ||||||||
| Number of Implants Sold | N= 3,277,844 Implants(1) | |||||||
| Rupture | < 0.1% | |||||||
| Capsular Contracture | < 0.1% | |||||||
| Reoperation for Adverse Events | < 0.1% | |||||||
| Reoperation (All Causes) | N/A(2) | |||||||
| (1) Data is internally tracked on an individual implant basis rather than by patient. | ||||||||
| (2) Complaint database does not capture reoperations for reasons not related to safety. | ||||||||
Independent Clinical Experience
An independent study by Sforza et al., published in the peer-reviewed Aesthetic Surgery Journal in 2017, conducted at a single center, the Hospital Group Ltd.’s Dolan Park Clinic, or Dolan Park, in Bromsgrove, England,
16
between April 2013 and April 2016, reported 5,813 consecutive cases of breast augmentation with Motiva Implants. This independent study was commissioned by Dolan Park’s medical director, Dr. Sforza, who is also a member of our medical advisory board and receives compensation from us in such capacity. The study, conducted by a group of 16 plastic surgeons at Dolan Park, reported overall rates of complication and reoperation of 0.76% over an interval of three years. Beginning in March of 2014, we started supplying our products to Dolan Park under a series of long-term supply agreements with Dolan Park’s affiliated companies. The last supply agreement expired in July of 2019. There were no serious adverse events and no cases of implant rupture for device failure, capsular contracture (Baker III/IV) in primary cases, double capsules, or late seromas. The authors presented consistent real-world data and believe that their free, three-year aftercare system is a strong method for patient retention and follow-up by eliminating any financial limitations for patients to return for follow-up consultations if any issues occur. Anecdotally, the same group of surgeons utilizing the same aftercare system for the last seven years reported substantially different results utilizing other types of silicone breast implants (i.e., non-Motiva Implants). The overall revision rate for this group from 2010 to 2013 utilizing a different, macro-textured, FDA approved implant (N > 10,000) was 8.43%, which is more than 10 times higher than the rate for Motiva Implants reported in this analysis.
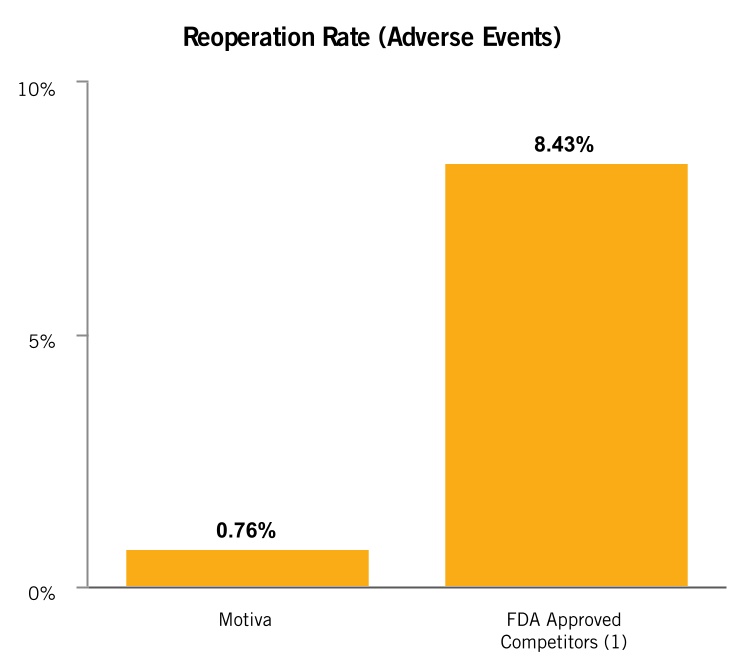
(1) Names of FDA approved competitors have not been published.
Study to Support a PMA
We are conducting a prospective IDE clinical trial in the United States on our Motiva Round and Motiva Ergonomix Round product families. Our IDE submission was approved by the FDA on March 20, 2018 to perform a single open-label, prospective, multi-center trial, with follow-up visit data reported annually and at the time of filing. We will continue to monitor patients for ten years post-implantation. The primary endpoints of the trial are safety, effectiveness and patient satisfaction. In general, our trial design and patient enrollment are consistent with prior PMA studies conducted by Allergan, Mentor, and Sientra. In August 2019, we announced that we were implementing a bifurcated regulatory strategy in the United States, which is designed to allow us to initiate the rolling submission of data to the FDA from the primary augmentation and revision augmentation cohorts, and then subsequently supplement our PMA with data from the reconstruction cohorts. All the enrollment procedures and the three-year study subject follow-up have been completed in the aesthetics cohort, which includes primary augmentation and revision augmentation, with total enrollment of 451 and 265 subjects, respectively. In the fourth quarter of 2021, we initiated a modular PMA submission process with the FDA and submitted the first of four expected modules. In April 2022, the Company released preliminary results of the two-year patient follow-up data for the primary augmentation cohort of its IDE clinical trial. The second module was submitted in May 2022. In June 2022, full enrollment of the IDE clinical trial was complete, and all surgeries in the primary reconstruction cohort were performed. In August 2022, the third module was submitted to the FDA. The final fourth module was submitted to the FDA in February 2023.
17
In May 2023, we presented the results of the Motiva US IDE Study of data for 451 primary augmentation patients enrolled in the study through the three-year follow-up visit. The three-year, by-patient, Kaplan-Meier risk rates of first occurrence of complications for patients (95% confidence interval) in the primary augmentation cohort were as follows:
Primary Augmentation | 3-year (N=451), 95% CI | |||||||
Capsular contracture (Baker Grade III/IV) | 0.5% | |||||||
Rupture, suspected or confirmed; MRI cohort(1) | 0.6% | |||||||
Breast pain | 0.7% | |||||||
Infection | 0.9% | |||||||
Implant removal, with or without replacement | 1.6% | |||||||
Any reoperation(2) | 6.1% | |||||||
Any complication(3) | 8.4% | |||||||
| Kaplan-Meier risk rates were the primary method of analysis for the above data. This table represents preliminary data available as of April 2023 and does not necessarily reflect final clinical results nor demonstrate the investigational device’s safety and effectiveness for the United States trial. | ||||||||
(1) MRI cohort N=176 (2) Any surgery on the breast or chest area, device or non-device related, including size change (3) Any device or non-device related event, including reoperation | ||||||||
Sales and Marketing
We primarily derive revenue from sales of our Motiva Implants from two types of customers: (1) medical device distributors and (2) direct sales to physicians, hospitals, and clinics. Our products are commercially available in 86 countries through exclusive distributors, except in Brazil, Argentina and several European countries where we sell through our direct sales force. As of December 31, 2023, our sales organization included 133 employees and contractors. All of these sales personnel are supported through a suite of tools, including marketing and training materials, mobile smartphone applications, and access to a robust schedule of physician education events. We also pay significant attention to helping our distributors maintain positive relationships with surgeons and clinics in their respective regions, and to positioning our product in the marketplace as a premium product with consequent premium pricing.
We demonstrate our confidence in Motiva Implants with the Motiva Always Confident Warranty, which offers patients a free replacement for any Motiva Implant that ruptures, for the life of the product. We also replace any implant which is replaced due to capsular contracture of Baker Grade III or IV severity at any time in the first 10 years post-implantation. We also offer an extended warranty at additional cost outside the JOY program, which provides financial assistance of up to $2,500 to cover surgical costs resulting from rupture or capsular contracture.
We employ a multi-faceted marketing strategy that includes social media engagement, conferences, advertisements and education.
Intellectual Property
Our success depends at least in part upon our ability to protect our core technology and intellectual property. To accomplish this, we rely on a combination of intellectual property rights, including patents, trade secrets and trademarks, as well as customary contractual protections.
We have assembled a broad portfolio of intellectual property related to our medical device and aesthetics products. We believe this intellectual property, combined with proprietary manufacturing processes and the regulatory approvals we have successfully obtained outside of the United States, provides us with a strong market position. As of December 31, 2023, we own or have rights to 30 issued, two allowed and 14 pending patents in the United States related to various aspects of our Motiva Implants (such as implant barrier layers, surface texture technology, minimally invasive implant delivery systems, and our Qid Safety Technology radio frequency identification devices). In addition, we own or have rights to 126 issued, one allowed and 93 pending foreign
18
applications and one pending Patent Cooperation Treaty, or PCT, applications. Our owned and licensed patents are expected to expire at various times between February 2025 and February 2039. Our owned and licensed pending applications, if granted, likely would expire between September 2033 and December 2043.
In addition to pursuing patents on our products, we have taken steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners, and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. In addition, we intend to expand our international operations, and effective patent, copyright, trademark and trade secret protection may not be available or may be limited in foreign countries.
In general, the medical device industry is characterized by the existence of a large number of patents and frequent allegations and related litigation regarding patent and other intellectual property rights. Third parties, including our competitor companies, may assert patent, copyright, trademark and other intellectual property rights against us, our partners or our customers. Our standard license and other agreements may obligate us to indemnify our partners and customers against such claims. We could incur substantial costs and divert the attention of our management and technical personnel in defending against any such claims. Successful claims of infringement by a third party could prevent us from selling or distributing certain products or performing certain services, require us to expend time and resources to develop non-infringing products, or force us to pay substantial damages, including treble damages if we are found to have willfully infringed patents-royalties or other fees. We cannot assure you that we do not currently infringe, or that we will not in the future infringe, upon any third-party patents or other proprietary rights.
Research and Development
Our goal is to continue to improve our existing products, as well as develop new products and new surgical techniques. We have a highly experienced team and deep customer and key opinion leader relationships. We also have sophisticated internal prototyping and testing equipment.
We have and will continue to work with several institutions in our effort to advance implant technology, and generate additional scientific data to support the improved safety outcomes associated with our products, including:
▪Massachusetts Institute of Technology
▪Medical University of Innsbruck
▪Department of Bioengineering at Rice University
▪John Hopkins Department of Biomedical Engineering
▪Plastic and Reconstructive Research Center at the University of Manchester
▪Center for Biofilm Engineering of Montana State University
▪College of Engineering at UC Santa Barbara
▪The Chair of Plastic Surgery at the School of Medicine and Psychology of Sapienza University of Rome
We have incurred, and expect to continue to incur, significant R&D expenses. Our R&D expenses increased $6.1 million, or 30.0%, to $26.4 million for the year ended December 31, 2023, compared to $20.3 million for the year ended December 31, 2022. Our R&D expenses consist of costs associated with our clinical and post-approval studies, regulatory activity and product development, including the development of Motiva Implants and other current and future aesthetic and reconstruction surgical devices on our product platform.
Implantable RFID Microtransponder Platform
The RFID technology platform that we use in the Qid feature of our Motiva Implants is independently cleared as a system via the FDA’s 510(k) pathway. We are developing more sophisticated functionality using this technology platform. We believe our RFID technology will be an attractive platform for a variety of other applications, including unique device identification for other types of implantable medical devices, functional implantable biosensors, and diagnostic monitoring. Future specific indications include detection of device life cycles (e.g., flexion/contraction cycles for artificial hip and knee joints) and monitoring of analytes such as circulating tumor cells and blood chemistry components. Some of these applications we may choose to develop and commercialize
19
internally, while others may be more appropriately commercialized via partnerships with other medical device companies. In October 2023, we launched Zen, the next, now non-ferromagnetic, generation of passive RFID technology, in select geographies with Motiva Ergonomix2 Round Implants in the Joy program.
We control all the activities of the development and manufacturing of our Qid Safety Technology RFID transponders. This allows us for adapting to specific needs or new developments in our field.
Manufacturing and Suppliers
Facilities
We manufacture our products in ISO-13485-certified manufacturing facilities located in the Coyol Free Zone office park in Costa Rica, a park populated by a number of international medical device companies and granted tax-advantaged status by the government of Costa Rica. Our largest manufacturing facility opened at the end of 2016 and we began shipping manufactured product from this facility in March 2017. This facility has approximately 28,000 square feet of office space and production areas which are capable of producing over 400,000 implants a year, with state-of-the-art support systems for sustaining production, including an ice-bank system for cooling the controlled air in the clean room and support areas, water-lubricated air compressors for eliminating the presence of oil particulates, heat recovery systems for energy saving, and an energy micro-grid comprised of solar panels and energy-storage batteries. These energy efficient systems generate up to 80% of the total energy consumption of the building, which received LEED Gold Certification by the U.S. Green Building Council in August 2017. Our initial facility was established in 2009 and has about 3,000 square feet of production areas, capable of producing over 100,000 implants a year.
We continue to look for ways to improve manufacturing processes and facility organization to increase capacity in these two current facilities. We completed an internal assessment and identified the potential additional manufacturing capacity of approximately 250,000 implants per year, which we added during fiscal 2021, thereby increasing the efficiencies in our process flow.
In July 2017, both facilities received the MDSAP regulatory certification. MDSAP was established by a coalition of international medical device regulatory authorities including Australia’s TGA, Brazil’s ANVISA, Health Canada, Japan’s MHLW and PMDA and the U.S. FDA. The goal of MDSAP is to allow a single regulatory audit of a medical device manufacturer’s Quality Management System to satisfy the needs of the participating regulatory jurisdictions. This program enables manufacturers to contract with an authorized third-party auditing organization, in our case the British Standards Institute, to conduct a single audit to satisfy the relevant regulatory requirements of the participating regulatory authorities including the FDA, which recognizes MDSAP audit reports as a substitute for FDA Establishment Inspection Reports.
In May 2019, both of our facilities in Coyol Free Zone received the Carbon Neutral certification from the Costa Rican Ministry of Environment, Energy, and Telecommunications, based on the implementation of efficiency-aimed actions such as the reduction of energy consumption through the acquisition of more efficient equipment; the combined use of solar panels, ice banks, and battery storage units; and the avoidance of fossil fuels for our operations.
We are also subject to periodic inspections and audits by various international regulatory and notified bodies, and we believe our past performance in these audits reflects the strength of our quality system and manufacturing controls. We consider this to be a key element of our risk management and business continuity strategies and a competitive advantage as we have full control of the product lifecycle. Our in-house manufacturing team undergoes well defined training programs throughout their period of employment. We believe our manufacturing experience, know-how, and process-related trade secrets are also a competitive advantage.
We are in the process of expanding our manufacturing facilities and corporate offices in the Coyol Free Zone, or CFZ, in Costa Rica. Construction of the cold shell structure of the Sulàyöm Innovation Campus was initially funded by the Coyol Free Zone in 2021 until we exercised our option to purchase the title of the land and cold shell building for approximately $12.6 million in 2022. In July 2023, we announced the grand opening of the first phase of the Sulàyöm Innovation Campus, which includes approximately 100,000 square feet of facility space to increase our manufacturing capacity by approximately 730,000 units per year. We currently expect to commence manufacturing from the new facility in fiscal 2024. See Note 3 “Balance Sheet Accounts” for additional information regarding this construction project.
20
Process
We produce our shell surfaces using a novel 3D negative imprinting molding technique that allows much more precise control over feature size, a uniform distribution of features on the surface, no particles creation, and less unit-to-unit variation. Our primary competitors utilize the “salt-loss” technique or “polyurethane foam imprint” technique. The “salt-loss” technique blows crystals of salt or sugar onto the uncured silicone shell in order to produce surface texture and the “polyurethane foam imprint” technique uses a foreign material to press against the last uncured silicone layer to produce surface features. We believe our 3D negative imprinting technique is more efficient and consistent than the techniques used by our competitors because the application of our advanced smooth surface is integrated with the molding process, rather than requiring a separate, subsequent process step.
Suppliers
We source manufacturing inputs from a number of outside suppliers. In particular, we obtain NuSil brand medical-grade silicone from Avantor (previously NuSil Technology LLC), which is a sole-source supplier of such products to the majority of the silicone breast implant industry. In May 2022, we entered into a master supply agreement with Avantor, which provides for specified prices per unit of each relevant component, has an initial term ending on December 31, 2026 and automatically renews for successive terms of one year each for up to five successive renewal terms. The agreement superseded and replaced the previous master supply agreement originally executed in 2016, as amended, that expired on May 13, 2022.
Other critical materials are the silicone patches and other silicone components used for the assembly of our breast implants. All these components are also made with NuSil medical-grade silicone and manufactured by specialized silicone contract manufacturing suppliers. All component suppliers undergo strict quality inspections to ensure these can meet our quality standard. Other important components are the primary packaging polycarbonate trays, the Tyvek sealing lids and packaging. All these components are also critical to maintain integrity of the product throughout its shelf-life and all these suppliers must be qualified and materials must be validated prior to being approved for manufacturing activities. Most suppliers are evaluated annually, and we carry second source supplier activities to ensure business continuity and quality and costs improvement.
Competition
The market for silicone breast implants is relatively concentrated, within Allergan Aesthetics, a division of AbbVie, and Mentor Worldwide LLC, a division of Johnson & Johnson. In the United States, Sientra, Inc. is the only other company with an approved silicone implant product. Internationally, the market is more fragmented, with GC Aesthetics plc, Silimed, Inc., Groupe Sebbin SAS, Hans Biomed Crop., Polytech Health & Aesthetics, and Arion Laboratories.
Our major competitors in the silicone breast implant marketplace are either publicly traded companies or divisions or subsidiaries of publicly traded companies with significantly more market share and resources than we have. These companies have greater financial resources for sales, marketing and product development, broader established relationships with health care providers and third-party payers, and larger and more established distribution networks. In some instances, our competitors also offer products that include features that we do not currently offer in all geographies. Our competitors also have regulatory approval to market and sell their products in countries where we currently do not, notably the United States. In addition, our competitors may offer pricing programs with discounts across their non-breast aesthetic product portfolios.
We also face potential future competition from a number of companies, medical researchers and existing medical device companies that may be pursuing new implant technologies. These include non-implant breast augmentation through injections of autologous adipose tissue, new material technologies such as synthetic fillers, and new methods of enhancing and reconstructing the breast.
We believe the primary competitive factors in our current and future markets include:
▪safety and outcomes data generated in clinical studies;
▪regulatory approvals;
▪technological characteristics of products;
▪complementary platforms of non-implant products, such as facial fillers and fat grafting technologies;
▪product price;
▪customer service; and
21
▪support by key opinion leaders.
Federal Food, Drug, and Cosmetic Act
Breast implants are regulated as medical devices in the United States, and are subject to the Federal Food, Drug, and Cosmetic Act, or FDC Act, as implemented and enforced by the FDA. The FDA administers requirements covering the design, development, testing (non-clinical and clinical research), safety, effectiveness, manufacturing, labeling, packaging, promotion, advertising, distribution, recordkeeping, import/export and postmarket surveillance of medical devices in order to ensure that devices distributed in the U.S. are safe and effective for their intended uses and otherwise meet the requirements of the FDC Act. The FDA also collects user fees for certain medical device submissions and annual fees for medical device establishments.
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) premarket notification, authorization of a de novo application, or approval of a PMA. Under the FDC Act, medical devices are classified as Class I (lowest risk), II (moderate risk), or III (highest risk), with each successive class reflecting a greater extent of manufacturer and regulatory control needed to ensure device safety and effectiveness. Class I devices are those for which safety and effectiveness can be assured by adherence to the FDA’s General Controls for medical devices, which include compliance with the applicable portions of the Quality System Regulation (QSR), facility registration and product listing, reporting of adverse medical events, and truthful and non-misleading labeling, advertising, and promotional materials. Class II devices are subject to the FDA’s General Controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents. Class III devices are those deemed by the FDA to pose the greatest risks, such as life sustaining, life supporting, or some implantable devices, or devices that have a new intended use or employ advanced technology that is not substantially equivalent to that of a legally marketed device. These must comply with all requirements under the FDC Act, including specific requirements and limitations pursuant to the order issued by FDA subsequent to PMA approval. While our instruments are cleared as class II devices, breast implants are currently classified as Class III devices requiring an approved PMA for commercial distribution.
510(k) Clearance Pathway
Manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDC Act requesting permission to commercially distribute the device (generally known as 510(k) clearance). To obtain 510(k) clearance, the submitted 510(k) notice must demonstrate that the proposed device is “substantially equivalent” to a predicate device already on the market; a predicate device is a legally marketed device that is not subject to PMA approval. The FDA’s 510(k) clearance process usually takes from six to twelve months, but it can take longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence. Over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products.
If the FDA agrees that the device is substantially equivalent to a legally marketed predicate device, it will grant 510(k) clearance to commercially market the device. If the FDA determines that the device is “not substantially equivalent” to a previously cleared device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process, which is a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) clearance or, depending on the modification, PMA approval. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k) or a PMA in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. If that happens, the FDA can require the manufacturer to cease marketing and/or request the recall of the modified device until 510(k) clearance or PMA approval is obtained. Also, in these circumstances, the manufacturer may be subject to significant regulatory fines or penalties.
22
In October 2023, the FDA granted 510(k) clearance for the Motiva Flora SmoothSilk Tissue Expander.
Premarket Approval Application Pathway
Class III devices generally require approval of a PMA before they can be marketed. The process of obtaining PMA approval is much more costly, lengthy, and uncertain than the 510(k) process. In a PMA, the manufacturer must demonstrate that the device is safe and effective, and the application must be supported by extensive data, including data from preclinical studies and human clinical trials. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities, and controls used for manufacturing, and proposed labeling. Following receipt of a PMA, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDC Act to complete its review, although in practice, the review often takes significantly longer, and can take up to several years. An advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device, which FDA may or may not accept. In addition, the FDA will conduct a pre-approval inspection of the applicant’s manufacturing facility or facilities to ensure compliance with the QSR.
The FDA will approve the new class III device for commercial distribution if it determines that the data and information in the PMA constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). A PMA may include post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution, and collection of long-term follow-up data from patients in the clinical study that supported the PMA or requirements to conduct additional clinical studies post-approval. The FDA may condition PMA approval on some form of post-market surveillance when deemed necessary to protect the public health or to provide additional safety and effectiveness data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Failure to comply with the conditions of approval can result in material adverse enforcement action, including withdrawal of the approval.
Related to our devices specifically, the FDA’s guidance document “Saline, Silicone Gel, and Alternative Breast Implants” currently recommends that a core study, which can be a single, open label, multi-center study, be conducted with ten years or more of prospective patient follow-up. To date, PMAs for silicone breast implants have been submitted for approval to the FDA with a minimum of three years of premarket core study data. Additionally, the FDA will not approve the PMA until it conducts a pre-approval inspection of our manufacturing facility and determines that it is in compliance with good manufacturing practices, as set forth in the FDA’s Quality System Regulation or QSR. The PMA review and approval process generally takes from one to three years but may take longer. The FDA’s guidance document “Saline, Silicone Gel, and Alternative Breast Implants” also states that manufacturers seeking approval of breast implants will be subject to post-approval requirements, which may include, but are not limited to, long-term follow-up of the core clinical study patients, conduct new enrollment post-approval studies, participation in a patient registry or other studies, training programs for physicians and surgeons, and periodic reporting requirements.
Certain changes to an approved device, such as changes in manufacturing facilities, methods, or quality control procedures, or changes in the design performance specifications, which affect the safety or effectiveness of the device, require submission of a PMA supplement. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel. Certain other changes to an approved device require the submission of a new PMA, such as when the design change causes a different intended use, mode of operation, and technical basis of operation, or when the design change is so significant that a new generation of the device will be developed, and the data that were submitted with the original PMA are not applicable to demonstrate a reasonable assurance of safety and effectiveness with respect to the change.
Clinical Trials
Clinical trials are almost always required to support a PMA and are sometimes required to support a 510(k) submission. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s IDE regulations, including regulations related to informed consent, Institutional Review Board, or IRB, review and approval, Good Clinical Practices, or GCPs, and labeling of investigational devices. Our clinical study sites are additionally subject to possible inspection by the FDA.
23
If the device presents a “significant risk,” to human health, as defined by the FDA, the device sponsor must submit an IDE application to the FDA, which must be approved prior to commencing human clinical trials. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies the company that the investigation may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE for which modification is required, it may permit a clinical trial to proceed under a conditional approval.
In addition, the study must be approved by, and conducted under the oversight of, an IRB for each clinical site. The IRB is responsible for the initial and continuing review of the IDE study, and may pose additional requirements for the conduct of the study. If an IDE application is approved by the FDA and one or more IRBs, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements. Acceptance of an IDE application for review does not guarantee that the FDA will allow the study to begin and, if it does, the FDA may or may not determine that the data derived from the trials support the safety and effectiveness of the device or warrant the continuation of clinical trials. An IDE supplement must be submitted to, and approved by, the FDA before a sponsor or investigator may make a change to the investigational plan that may affect its scientific soundness, study plan, or the rights, safety or welfare of human subjects.
During a study, the sponsor is required to comply with the applicable FDA requirements, including, for example, trial monitoring, selecting clinical investigators and providing them with the investigational plan, ensuring IRB review, adverse event reporting, record-keeping and prohibitions on the promotion of investigational devices or on making safety or effectiveness claims for them. The clinical investigators are also subject to FDA regulations and must obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of the investigational device, and comply with all reporting and recordkeeping requirements. Additionally, after a trial begins, we, the FDA or the IRB could suspend or terminate it at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.
Post-Market Regulation
After a device is cleared or approved for marketing, numerous and extensive regulatory requirements continue to apply. These include:
•establishment registration and device listing with the FDA;
•QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process;
•labeling regulations and FDA prohibitions against the promotion of ‘‘off-label’’ uses of cleared or approved products, as well as other requirements related to promotional activities;
•clearance or approval of product modifications to cleared or approved devices, where warranted;
•medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur;
•correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDC Act that may present a risk to health;
•the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
•post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device; and
24
•any post-market restrictions or conditions imposed by the FDA on a specific device.
Our manufacturing facilities, as well as those of certain of our suppliers, will be subject to periodic and for-cause inspections by the FDA to verify compliance with the QSR and other regulatory requirements. The QSR, which covers the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. These requirements impose certain procedural and documentation requirements upon us and our third-party manufacturers related to the methods used in and the facilities and controls used for designing, manufacturing, packaging, labeling and storing medical devices. As a manufacturer, we are subject to periodic scheduled or unscheduled inspections by the FDA. Following these inspections, the FDA may assert noncompliance with QSR requirements on a Form 483, which is a report of observations from an inspection, or by way of “untitled letters” or “warning letters” that could cause us or any third-party manufacturers to modify certain activities. A Form 483 notice, if issued at the conclusion of an FDA inspection, can list conditions the FDA investigators believe may have violated QSR or other FDA requirements. Our failure to maintain compliance with the QSR requirements could result in the shut-down of, or restrictions on, our manufacturing operations and the recall or seizure of our products, which would have a material adverse effect on our business. The discovery of previously unknown problems with any of our products could result in restrictions, including the removal of the device from the market or voluntary or mandatory device recalls.
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that we failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, which may result in sanctions including (but not limited to) Warning Letters, fines, injunctions, consent decrees and civil penalties, customer notifications or repair, replacement, refunds, recall, detention or seizure of our products, operating restrictions, partial suspension or total shutdown of production, refusal to grant export approval, or criminal prosecution.
The FDA and comparable foreign regulatory authorities closely regulate the post-approval marketing and promotion of medical devices, including standards and regulations for direct-to-consumer advertising, communications about unapproved uses, industry- sponsored scientific and educational activities and promotional activities involving the internet. Devices may be marketed only for the approved or cleared indications and in accordance with the provisions of the approved or cleared label.
HIPAA and Other Privacy Laws
We are subject to various laws governing the privacy and security of health information and other personally identifiable information. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, established comprehensive U.S. federal protection for the privacy and security of protected health information. HIPAA standards apply to “Covered Entities,” which are health plans, health care clearing houses, and certain health care providers which conduct certain health care transactions electronically, and to “Business Associates,” persons or entities that perform a function or provide specified services on behalf of a Covered Entity that involves the creation, use, maintenance or transmission of protected health information. Both Covered Entities and Business Associates must have in place administrative, physical, and technical standards to guard against the misuse of protected health information. Some of the institutions and physicians from which we obtain biological specimens that we use in our research and validation work are Covered Entities and must obtain proper authorization from their patients for the subsequent use of those samples and associated clinical information. We may perform future activities that may implicate HIPAA, such as providing clinical laboratory testing services or entering into specific kinds of relationships with a Covered Entity or a Business Associate of a Covered Entity.
Additionally, the Health Information Technology for Economic and Clinical Health Act, enacted as part of the American Recovery and Reinvestment Act of 2009 amended HIPAA by increasing the civil and criminal penalties that may be imposed against Covered Entities, their Business Associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions.
Our activities must also comply with other applicable privacy laws, including the EU General Data Protection Regulation, including as implemented in the UK, or collectively, the GDPR. There are also additional national, state, and provincial privacy laws that impose restrictions on the access, use, and disclosure of personal information, including data that is not protected health information, or are otherwise more stringent than HIPAA. All of these laws may impact our business. If we fail to comply with these privacy laws, or if significant changes in the
25
laws restrict our ability to obtain tissue samples and associated patient information, this could significantly impact our business and our future business plans.
Fraud and Abuse Laws
As participants in national and local health care programs, we may be subject to anti-fraud and abuse laws in various countries. Many of these anti-fraud laws are broad in scope and impose significant penalties for violation. Prohibitions under some of these laws include:
▪the submission of false claims or false information to government programs;
▪deceptive or fraudulent conduct;
▪excessive or unnecessary services, services that do not meet medical necessity, or services at excessive prices; and
▪prohibitions in defrauding private sector health insurers.
Numerous national and local agencies enforce the anti-fraud and abuse laws. In addition, private insurers may also bring private actions. In some circumstances, private whistleblowers are authorized to bring fraud suits on behalf of the government against providers and are entitled to receive a portion of any final recovery. In addition, we, and our partners, may be subject to foreign laws and regulations and other compliance requirements, including, without limitation, anti-kickback laws, false claims laws and other fraud and abuse laws.
In particular, if our products are approved in the United States and become eligible for federal government reimbursement, our business activities could be subject to scrutiny and enforcement under one or more U.S. federal or state health care fraud and abuse laws and regulations, including:
•The federal Anti-Kickback Law, which prohibits, among other things, knowingly or willingly offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any health care items or service for which payment may be made, in whole or in part, by federal healthcare programs such as Medicare and Medicaid.
•The federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government.
•The fraud provisions of the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which impose criminal liability for knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors, and prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false fictitious or fraudulent statement or entry, in connection with the delivery of or payment for healthcare benefits, items or services.
•Analogous state and local laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require medical technology companies to comply with the device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; and state laws and local ordinances that require identification or licensing of sales representatives.
If we or our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil, criminal and administrative penalties, damages, fines, exclusion from participation in government health care programs, additional reporting and government oversight, and the curtailment or restructuring of our operations. As we expand the geographic market for our products, we may be subject to similar national or local laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to health care professionals. To reduce the risks associated with these various laws and governmental regulations, we have implemented a compliance plan. Although compliance programs can mitigate the risk of investigation and
26
prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable global privacy, security and fraud laws may prove costly.
Transparency Laws
We are subject to transparency requirements (also known as “sunshine laws”) in France, including obligations to report payments or transfers of value to, and the nature of the agreements we sign with, a broad class of French healthcare professionals and organizations. If our products are approved in the United States and become eligible for federal government reimbursement, we will also become subject to the Physician Payment Sunshine Act and its amendments and implementing regulations, which require annual reporting of payments and transfers of value to physicians, teaching hospitals and other “covered recipients”, along with physician ownership information. Various states have also implemented regulations prohibiting certain financial interactions with health care professionals or mandating public disclosure of such financial interactions. We may incur significant costs to comply with such laws and regulations now or in the future.
Coverage and Reimbursement
Significant uncertainty exists regarding the coverage and reimbursement status of products approved or cleared by the FDA and other government authorities. In the United States, sales of any products for which we may receive regulatory approval or clearance for commercial sale will depend in significant part on the availability and adequacy of coverage and reimbursement from third-party payors. Third-party payors include federal and state government authorities, managed care providers, private health insurers and other organizations. Decisions regarding the extent of coverage and amount of reimbursement to be provided are made on a plan-by-plan basis. One third-party payor’s decision to cover a product does not ensure that other payors will also provide coverage for the product. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the reimbursement rate that the payor will pay for the product. Third-party payors may limit coverage to specific products on an approved list which might not include all of the FDA approved or cleared products for a particular indication. Moreover, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Third-party payors are increasingly challenging the prices charged for, examining the medical necessity of, and assessing the cost-effectiveness of medical products and services, in addition to their safety and efficacy. If third-party payors do not consider a product to be cost-effective compared to other available products, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit. A decision by a third-party payor not to cover a product could reduce physician ordering and patient demand for the product.
The marketability of any products for which we receive market authorization for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Health Reform
In the United States and some foreign jurisdictions, there has been significant interest in implementing cost-containment programs to limit the growth of government-paid healthcare costs, including price controls and restrictions on reimbursement. Because private payers often follow Medicare and Medicaid coverage policy and payment limitations in setting their own reimbursement rates, any reduction in reimbursement that results from federal legislation or regulation may result in a similar reduction in payments from private payers. We expect to experience pricing pressures in connection with the sale of our products due to the trend toward managed healthcare, the increasing influence of health maintenance organizations, and additional legislative and regulatory measures.
Such legislative changes in the United States include the Patient Protection and Affordable Care Act (PPACA), which intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and
27
health insurance industries, and impose additional health policy reforms. We expect that additional federal, state, and foreign healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal, state, and foreign governments will pay for healthcare products and services, which could result in limited coverage and reimbursement and reduced demand for our products, once approved, or additional pricing pressures.
International Medical Device Regulations
International marketing of medical devices is subject to foreign government regulations, which vary substantially from country to country.
As of May 2021, medical device products that are marketed in the European Union must comply with the requirements of the Medical Device Regulation, or the MDR. The MDR essentially operates in the same way as the Medical Device Directive (described below) to ensure a harmonized approach in the European Union to ensuring the safety and performance of medical devices, and failure to comply with the MDR could affect our ability to market and sell our products in the European Union member states. The European Commission is the legislative body responsible for regulation on the safely of medical devices, including Regulation (EU) 2017/745. Following the transition time, the manufacturers selling medical products in the EU and the European Economic Area, or EEA, will need to comply with the new regulatory framework. All our legacy devices, as well as our Quality Management System, already obtained the certification under the MDR. The EU includes most of the major countries in Europe, while other countries, such as Norway, are part of the EEA and European Free Trade Area, or EFTA, respectively, and have voluntarily adopted laws and regulations that mirror those of the EU with respect to medical devices. The EU regulation addresses regulation of the design, manufacture, labeling, clinical studies and post-market vigilance for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear the CE conformity marking, indicating that the device conforms to the General Safety and Performance Requirements, or GSPRs, and, accordingly, can be marketed throughout the EU and EEA. In December 2022, the MDR published two Commission Implementing Regulations, which expanded its authority over products without intended medical purpose, and provided more specific risk management and safety requirements, including conformity assessments and clinical evaluations. In March 2023, the MDR published an additional regulation that provided a staggered extension of the transitional period based on the risk classification of medical devices.
Prior to May 2021, medical device products that were marketed in the European Union were required to comply with the requirements of Medical Device Directive, or the MDD, as implemented in the national legislation of the European Union member states. The MDD, as implemented, provided for a regulatory regime with respect to the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices to ensure that medical devices marketed in the European Union are safe and effective for their intended uses. Medical devices that complied with the MDD, as implemented, are entitled to bear a Conformité Européenne, or CE, marking evidencing such compliance and may be marketed in the European Union. Failure to comply with these domestic and international regulatory requirements could affect our ability to market and sell our products in these countries.
Outside of the EU, regulatory pathways for the marketing of medical devices vary greatly from country to country. In many countries, local regulatory agencies conduct an independent review of medical devices prior to granting marketing approval. For example, in China, approval by the National Medical Products Administration, or NMPA, must be obtained prior to marketing a medical device. In Brazil, the inspections and approvals of products and facilities carried out by the ANVISA and InMetro agencies are required prior to marketing a Class 3a medical device like our Motiva Implants. We received regulatory clearance in Brazil in March 2017 and launched our Motiva Implants commercially in July 2017. In November 2022, Motiva Implants and the Motiva Flora tissue expander have been approved for use in Japan by the Pharmaceuticals and Medical Devices Agency (PMDA). These products have also received reimbursement for post-mastectomy reconstruction under the Japanese National Health System. In November 2023,Motiva Implants received National Medical Products Administration (NMPA) approval in China. The process in such countries may be lengthy and require the expenditure of significant resources, including the conduct of clinical trials. In other countries, the regulatory pathway may be shorter or less costly. The timeline for the introduction of new medical devices is heavily impacted by these various regulations on a country-by-country basis, which may become longer and more costly over time.
28
Anti-Corruption Laws
We are subject to applicable anti-corruption laws, such as the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act, and similar anti-corruption laws in the countries in which we distribute our products. Anti-corruption laws generally prohibit offering, promising, giving, or authorizing others to provide anything of value, either directly or indirectly, to a government official or private party in order to influence official action or otherwise gain an unfair business advantage, such as to obtain or retain business. The Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, prohibits any U.S. individual or U.S.-controlled business from paying, offering or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring us to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. We have implemented policies, procedures, and internal controls that are designed to comply with these laws and regulations.
Human Capital
As of December 31, 2023, we had 908 employees. None of our employees are represented by a labor union or covered by collective bargaining agreements except for employees in Brazil and Argentina.
The human capital measures and objectives we focus on in managing our business include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of share-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
We believe that our future success largely depends upon our continued ability to attract and retain highly qualified management and technical personnel. Talent management is critical to our ability to execute on our long-term growth strategy. To facilitate talent attraction and retention, we strive to make our company a safe and rewarding workplace, with opportunities for our employees to grow and develop in their careers, supported by strong compensation and benefits, and by programs that build connections among our employees. We continue to be committed to an inclusive culture which values equity, opportunity, and respect. In support of our inclusive culture, we offer competitive compensation and benefits, including stock awards and strive to recruit a diverse talent pool across all levels of the organization.
Environment and Corporate Sustainability
Our manufacturing processes currently require the controlled use of potentially harmful chemicals, including highly flammable solvents and we are subject to inspections and other regulatory requirements, including Costa Rican regulations regarding environmental protection and hazardous and controlled substance controls, among others. Environmental laws and regulations are complex, change frequently and have tended to become more stringent over time. We have incurred, and may continue to incur, significant expenditures to ensure we are in compliance with these laws and regulations. We would be subject to significant penalties for failure to comply with these laws and regulations. For more information, please refer to Section 1A “Risk Factors”.
Working towards a more sustainable future by reducing our environmental footprint is important to us. In July 2019, we have been certified as Carbon Neutral by the Costa Rican Ministry of Environment, Energy, and Telecommunications, or MINAE. Additional information on our environmental efforts can be found in the 2022 Annual Review, which is available at establishmentlabs.com/wp-content/uploads/2023/07/ESTA-Annual-review-2022-1.pdf.
We are defined by our commitment to women’s health and well-being. We believe offering innovative options that empower women in their breast health and wellness journey is the right path to building a new industry. Sustainability is key to our existence and future. As a global medical technology company, we seek to create positive and long-term social, environmental, and economic impact with our products, experiences, activities, and corporate efforts. Accordingly, our global sustainability commitment leads us to increase our contribution toward long-term sustainability. Our sustainability framework lays the foundation to ensure this commitment is present in everything we do. It describes our pillars, statements, priorities, and guidelines that enable our journey toward
29
sustainability. All these efforts are founded on our four material categories: women’s health and well-being, environment, people, and governance.
Additional information on our governance, women’s empowerment initiatives and sustainability efforts can be found at establishmentlabs.com/our-impact/esg-documents/.
ITEM 1A. RISK FACTORS
Investing in our common shares involves a high degree of risk. We operate in a rapidly changing economic and competitive environment that presents numerous risks, many of which are driven by factors that we cannot control or predict. The following risk factors describe circumstances or events that could have a negative effect on our business, financial condition or operating results. You should consider the following risks carefully, together with all the other information in this Annual Report on Form 10-K, including our consolidated financial statements and notes thereto, before you invest in our common shares. If any of the following risks occur, our business, financial condition, or operating results, could be adversely affected. As a result, the trading price of our common shares could decline, and you could lose part or all of your investment. Additional risks and uncertainties not currently known to us or that we currently believe are not material could also impair our business, financial condition or operating results.
Risks Related to the Development and Commercialization of Our Products
We have a limited operating history in the United States and may face difficulties encountered by companies early in their commercialization in competitive and rapidly evolving markets.
Our Motiva Implants have been marketed solely in countries outside of the United States since October 2010, and as such, we have a limited operating history upon which to evaluate our business and forecast our future net sales and operating results. In assessing our business prospects, you should consider the various risks and difficulties frequently encountered by companies early in their commercialization in competitive markets, particularly companies that develop and sell medical devices. These risks include our ability to:
•implement and execute our business strategy;
•expand and improve the productivity of our direct sales force, distributors and marketing programs to grow sales of our products;
•increase awareness of our brands and build loyalty among plastic surgeons and patients;
•manage expanding operations;
•respond effectively to competitive pressures and developments;
•enhance our existing products and develop new products;
•maintain and obtain regulatory clearance or approval of our existing products and commercialize new products;
•respond to changing regulations associated with medical devices across all geographies;
•perform clinical trials with respect to our existing products and any new products;
•attract, retain and motivate qualified personnel in various areas of our business; and
•obtain and maintain coverage and adequate levels of reimbursement for our products.
Due to our limited operating history in the United States, we may not have the institutional knowledge or experience to be able to effectively address these and other risks that we may face. In addition, we may not be able to develop insights into trends that could emerge and negatively affect our business and may fail to respond effectively to those trends. As a result of these or other risks, we may not be able to execute key components of our business strategy, and our business, financial condition and operating results may suffer.
Our success depends, in part, on our ability to continue to enhance our existing products and services and develop or commercialize new products and services that respond to customer needs and preferences, which we expect will require us to incur significant expenses.
In recent years, we have incurred significant costs in connection with the development of Motiva Implants, the Mia Femtech technology, and other products and services. We expect our research and development expenses to increase significantly as we continue with our IDE clinical trial in the United States. We have been incurring significant expenses to expand our sales and marketing organization to support sales of Motiva Implants,
30
including but not limited to a direct sales force in Brazil and several European countries, as well as in preparation of the future commercialization in the United States if we receive FDA approval.
We may not be able to compete effectively with our competitors, and ultimately satisfy the needs and preferences of our customers, unless we can continue to enhance existing products and develop or acquire new innovative products and services. Product development requires the investment of significant financial, technological and other resources. Product improvements and new product introductions also require significant planning, design, development and testing at the product and manufacturing process levels. We may not be able to timely or effectively develop product improvements or new products and services. Likewise, we may not be able to acquire new products on terms that are acceptable to us, or at all. Furthermore, in most countries, we need to obtain regulatory approval in order to market and sell our products, which may limit our ability to act quickly in scaling commercialization in those countries, including the United States. Our competitors’ new products may beat our products to market, be more effective or safer or have new features, obtain better market acceptance or render our products and services obsolete. Any new or modified products and services that we develop may not receive regulatory clearance or approval, or achieve market acceptance or otherwise generate any meaningful sales or profits for us.
The clinical trial process is lengthy and expensive with uncertain outcomes, and often requires the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. In addition, safety issues or other challenges may arise during the conduct of a trial. Delays or failures in our clinical trials will prevent us from commercializing any modified or new products and will adversely affect our business, operating results and prospects.
We have obtained a CE Mark for Motiva Implants and certain of our other products and are therefore authorized to sell in the EU. We have received regulatory approval for Motiva Implants and the Motiva Flora tissue expander in Japan by the PMDA. Additionally, the FDA has granted 501(k) clearance for the Motiva Flora tissue expander in the United States. However, in order to market in other regions or jurisdictions, such as the Asia Pacific region, we must obtain separate regulatory approvals. Neither we, nor any future collaboration partner, can commercialize Motiva Implants in the United States without first obtaining regulatory approval for the product from the FDA.
Before obtaining regulatory approval for the sale of a planned product, we may be required to conduct extensive preclinical and clinical studies to demonstrate the safety and effectiveness of our planned products in human patients. Clinical studies can be expensive, difficult to design and implement, can take many years to complete, and are uncertain as to outcome. A failure of one or more of our clinical studies could occur at any stage of testing. The approval procedures vary among countries and can involve additional clinical testing, and the time required to obtain approvals may differ from that required to obtain the CE Mark, PMDA approval, FDA or other regulatory approval.
Investigators for our clinical trials and other health care providers may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. We are required to collect and provide financial disclosure notifications or certifications for our clinical investigators to the FDA. If the FDA concludes that a financial relationship between us and a clinical investigator has created a conflict of interest or otherwise affected interpretation of the trial, the FDA may question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of marketing approval of our current and future product candidates.
In the United States, FDA guidance on silicone breast implants mandates approval via the PMA process. Extensive preclinical and clinical testing is required to support the PMA and at least one well-controlled clinical trial is required for approval. In connection with the initiation of a clinical study for our Motiva Implants, we filed an IDE application in 2017, which was approved in March 2018. In August 2019, we completed all patient surgeries for the IDE aesthetic cohorts, which include primary augmentation and revision. In June 2022, we completed the enrollment of all subjects in the remaining reconstruction cohort, and all surgeries were completed. Our ongoing U.S. IDE trial may be stopped for unforeseen safety issues or may not be successful in meeting its endpoints, in which case our U.S. regulatory pathway would require subsequent additional clinical trials.
Additionally, we will be required to commit to significant and costly post-approval requirements, which will include follow-up of our clinical trial patients for up to ten years, creation of a patient registry or large post approval study, and/or other studies, and implementation of training programs for physicians. We may be unable to fund, enroll, or complete such trials in a timely fashion, or at all, and we may have an insufficient number of enrolled patients
31
follow up as instructed. The results of clinical studies may not be favorable enough to support marketing approval in the United States, or may raise other questions (pertaining, for example, to product safety or effectiveness) that jeopardizes our current approvals for sale in other territories. We must also demonstrate that our manufacturing facilities, processes and controls are adequate to support FDA approval and that our clinical investigators complied with good clinical practices in the conduct of the clinical trial for our Motiva Implants.
In general, numerous unforeseen events during, or as a result of, preclinical and clinical studies could occur, which would delay or prevent our ability to receive regulatory approval or commercialize Motiva Implants or any of our planned products, including the following:
•clinical studies may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical studies or abandon product development programs;
•the number of patients required for clinical studies may be larger than we anticipate, enrollment in these clinical studies may be insufficient or slower than we anticipate, or patients may drop out of these clinical studies at a higher rate than we anticipate;
•the cost of clinical studies may be greater than we anticipate;
•third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
•we might suspend or terminate clinical studies of our planned products for various reasons, including a finding that our planned products have unanticipated serious side effects or other unexpected characteristics, or that the study subjects are being exposed to unacceptable health risks;
•regulators may not approve our proposed clinical development plans;
•regulators or independent institutional review boards, or IRBs, may not authorize us or our investigators to commence a clinical study or conduct a clinical study at a prospective study site;
•regulators or IRBs may require that we, or our investigators, suspend or terminate clinical studies for various reasons, including noncompliance with regulatory requirements;
•regulators may determine that the clinical data submitted to support our request for approval is unreliable or incomplete as a result of any number of factors, including potential financial bias associated with equity holdings in the Company by study investigators, or significant payments by the Company to study investigators for consulting work, which may result in regulators requesting further data analysis or other confirmatory studies to be performed, or determining the data does not support regulatory approval;
•regulators in countries where Motiva Implants are currently marketed may require that we suspend commercial distribution if there is noncompliance with regulatory requirements or safety concerns;
•regulators in countries where Motiva Implants are currently marketed may suspend commercial distribution of silicone breast implants due to safety or other concerns generally applicable to the product category;
•the supply or quality of our planned products or other materials necessary to conduct clinical studies of our planned products may be insufficient or inadequate; and/or
•the enactment of new regulatory requirements in Europe under the new Medical Device Regulation may make approval times longer and standards more difficult to pass.
If we, or any future collaboration partner, are required to conduct additional clinical trials or other testing of Motiva Implants or any planned products, those clinical studies or other testing may not be successfully completed. Additionally, if the results of these studies or tests are not positive, or if they raise safety concerns, we may:
•be delayed in obtaining marketing approvals for Motiva Implants or our planned products;
•not obtain marketing approval at all;
•obtain approval for indications that are not as broad as intended;
•have a product removed from the market after obtaining marketing approval;
•be subject to additional post-marketing testing requirements; and/or
•be subject to restrictions on how the product is distributed or used.
FDA regulatory approval in the United States is not a guarantee upon successful completion of preclinical and clinical studies, and the filing and approval process itself is expensive and may take several years. The FDA also has substantial discretion in the approval process. Despite the time and expense exerted, failure may occur at any
32
stage, and we could encounter problems that cause us to abandon or repeat clinical studies, including our ongoing IDE clinical trial that commenced in April 2018 and for which we have submitted all four modules to the FDA, and we not obtain FDA approval on the timeline we anticipate or at all. The FDA can delay, limit, or deny approval of a product candidate for many reasons, including, but not limited, to:
•a product candidate may not be deemed to be safe and effective;
•FDA officials may not find the data from clinical and preclinical studies sufficient;
•the FDA may not approve our manufacturing or our third-party suppliers’ processes or facilities;
•the FDA may consider clinical studies inadequate and the data inadequate if, among other things, appropriate steps have not been taken in the design, conduct, reporting and analysis of the studies to minimize bias; or
•the FDA may change its approval policies or adopt new regulations.
Even if we obtain regulatory approvals or clearances in a jurisdiction, our products may be removed from the market due to a variety of factors, including adverse events, recalls, suspension of regulatory clearance to sell, or other factors. For example, during the summer of 2016 while we were transitioning from one notified body to another, our CE Mark for Motiva Implants was temporarily not in force. We expect that the initial U.S. approval will be subject to a lengthy and expensive follow-up period, during which we must monitor patients enrolled in clinical studies and collect data on their safety outcomes. Even if FDA approval is obtained, the FDA has authority to impose post-market approval conditions, which can include (i) restrictions on sale, distribution, or use, (ii) continuing evaluation of the device’s safety and effectiveness, (iii) additional warning/hazard labeling requirements, (iv) significant record management, (v) periodic reporting requirements, and (vi) any other requirements the FDA determines necessary to provide reasonable assurance of the device’s safety and effectiveness. Completion of the IDE follow-up study, in a manner which results in data sufficient to maintain FDA approval, is subject to multiple risks, many of which are outside of our control. These include, but are not limited to, our ability to fund the ongoing study from our operations or via additional fundraising; study participants’ willingness and ability to return for follow-up study visits; and maintenance of a suitable study database over a long period of time. Even if completed and appropriately evaluated, the study follow-up may reveal safety or other issues that impact the approved labeling or may result in withdrawal of Motiva Implants from the marketplace in the United States or elsewhere.
Although we launched Motiva Implants commercially in October 2010 and have sold approximately 3.3 million units to date in various countries outside the United States, we do not have as much post-market surveillance data as our competitors and may not have clearly identified all possible or actual risks of our products. Furthermore, if our clinical trials do not produce patient data that compares favorably with breast implants that are already on the market, physicians and patients may opt to not use our products, and our business would suffer.
Our product development costs will also increase if we experience delays to our clinical trials or approvals. We do not know whether any clinical studies will begin as planned, will need to be restructured, or will be completed on schedule, or at all.
Significant clinical study delays could allow our competitors to bring products to market before we do, which would impair our ability to commercialize our planned products and harm our business and results of operations.
Moreover, clinical studies or manufacturing processes conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more international regulatory authorities does not ensure approval by regulatory authorities in other countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. An international regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain international regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and, even if we file, we may not receive necessary approvals to commercialize our products in any market. If Motiva Implants, or our future products, fail to demonstrate safety and efficacy in further clinical studies that may be required for U.S. approval, or do not gain regulatory approval, our business and results of operations will be harmed.
33
If the FDA or similar regulatory authority does not approve our products or requires additional clinical trials or preclinical data before any approval or if any approval of our products includes additional restrictions on the label, or requires a characterization of our products, including the description of the product surface (e.g. smooth, texture, other) that differs from ours and/or other regulatory authorities, our business, financial condition, results of operations and growth prospects could be materially adversely affected.
It is possible that the FDA or similar regulatory authorities may not consider the results of our clinical trials to be sufficient for approval of Motiva Implants for our desired indications for use. Guidance issued by the FDA in 2006 suggests that a single well-controlled study is required for approval of a new silicone breast implant. The FDA may nonetheless require that we conduct additional clinical studies, possibly using a different clinical study design.
Moreover, even if the FDA or other regulatory authorities approve the marketing of Motiva Implants and our other products, the approvals may include additional restrictions on the label or require a characterization of our products that differ from ours and/or other regulatory authorities and result in additional descriptions or other information on the label. Any of these events could make Motiva Implants or our other products less attractive to physicians and patients compared to other approved products, which could limit potential sales of Motiva Implants or our other products.
If we fail to obtain FDA or other regulatory approval of Motiva Implants or our other products, or if the approval is narrower than or otherwise differs from what we seek, it could impair our ability to realize value from those products, and therefore may have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Changes in funding or disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new or modified products from being developed, approved or commercialized in a timely manner or at all, or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, the ability to hire and retain key personnel, the ability to accept the payment of user fees, statutory, regulatory and policy changes and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, during various times in the recent past, the U.S. government has shut down and certain regulatory agencies, such as the FDA and the SEC, had to furlough critical employees and stop critical activities. Separately, in response to the COVID-19 pandemic, the FDA postponed most inspections of foreign manufacturing facilities and routine surveillance inspections of domestic manufacturing facilities in 2020. If a prolonged government shutdown occurs, or if other events, including global health concerns, prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews or other regulatory activities in a timely manner, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future federal government shutdowns or delays in annual appropriations could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Any future distribution or commercialization agreements we may enter into with respect to our current or planned products may place the development of these products outside our control, or may otherwise be on terms unfavorable to us.
We may enter into additional distribution or commercialization agreements with third parties with respect to our current or planned products, for commercialization in or outside the United States. Our likely collaborators for any distribution, marketing, licensing or other collaboration arrangements include large and mid-size medical device and diagnostic companies, regional and national medical device and diagnostic companies, and distribution or group purchasing organizations. We will have limited control over the amount and timing of resources that our
34
collaborators dedicate to the development or commercialization of our planned products. Our ability to generate revenue from these arrangements will depend in part on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable planned products. Collaborators may own or co-own intellectual property covering our products that results from our collaboration with them. In such cases, we would not have the exclusive right to commercialize such intellectual property.
Any termination or disruption of collaborations could result in delays in the development of planned products, increases in our costs to develop the planned products or the termination of development of a planned product.
If we are unable to educate clinicians on the safe, effective and appropriate use of our products and designed surgeries, we may experience unsatisfactory patient outcomes, negative publicity and increased claims of product liability and may be unable to achieve our expected growth.
We make extensive physician medical education resources available to clinicians in an effort to ensure that they have access to current treatment methodologies, are aware of the advantages and risks of our Motiva Implants and other products and are educated regarding the safe and appropriate use of our products. It is critical to the success of our business to broadly educate clinicians who use or desire to use our products to provide them with adequate instructions in the appropriate use of our products and designed surgeries. Certain of our products require the use of specialized techniques which may not be covered in medical school curricula and/or product-specific knowledge. For example, metal implant such as screws or artificial joints, produce an artifact when magnetic resonance imaging, or MRI, is used to image the area in which the object resides. Our Qid Safety Technology microtransponder embedded in certain Motiva Implants contains metal and causes an artifact that can affect breast cancer screening using MRI, and this artifact is not present in other imaging modalities such as breast ultrasound and film or digital mammography. It is important that we educate physicians and patients on the risks associated with MRI artifacts and how to mitigate them if they choose to utilize Motiva Implants that contain a Qid microtransponder. Failure to provide adequate training and education could result in, among other things, unsatisfactory patient outcomes, patient injury, negative publicity or increased product liability claims or lawsuits against the company, any of which could have a material and adverse effect on our business and reputation. Claims against the company may occur even if such claims are without merit and/or no product defect is present, due to improper surgical technique, inappropriate use of our products, or other lack of awareness regarding the safe and effective use of our products. If we fail to educate physicians and patients about any of these factors, they may make decisions or conclusions regarding Motiva Implants without full knowledge of the risks and benefits or may view our Motiva Implants negatively.
As part of our effort to educate and train plastic surgeons through our medical educational platform, we completed 192 and 201 medical training sessions worldwide during 2023 and 2022, respectively. If we are unable to offer, or if we experience a delay in offering, medical training sessions, we may experience reduced or slower than expected adoption of our products. Although we offer virtual training sessions through our medical educational platform, any limited ability to provide in-person programs to surgeons may reduce the effectiveness of, and interest in, our medical education efforts.
Commercial success of Motiva Implants in the United States or elsewhere depends on our ability to accurately forecast customer demand and manufacture sufficient quantities of product in the implant sizes that patients and physicians request, and to manage inventory effectively and the failure to do so could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Manufacturing of silicone breast implants requires costly capital equipment and a highly skilled workforce. There is a significant lead time to build and certify a new manufacturing facility. Until 2017, we had one manufacturing facility in Costa Rica, and we experienced inventory shortages from time-to-time that impaired our ability to meet market demand. In March 2017, our second manufacturing facility, also located in Costa Rica, became operational, and we received certification under the multi-country MDSAP protocol and began shipping saleable product. Although we believe our current facilities give us adequate manufacturing capacity to meet current demand, we have, in the past, been unable to fill all incoming orders. We began construction of the third facility in Costa Rica during the third quarter of 2021. In July 2023, we announced the grand opening of the first phase of the Sulàyöm Innovation Campus. We currently expect to commence manufacturing from the new facility in fiscal 2024. If this expansion is not completed in a timely manner, or if we are not able to get required regulatory
35
approvals for the new facility, our ability to fill incoming orders may be adversely impacted. In addition, if we obtain FDA approval, we will likely need to obtain additional manufacturing capacity prior to any commercialization of our Motiva Implants in the United States. If demand increases faster than we expect, or if we are unable to produce the quantity of goods that we expect with our current facilities, we may not be able to grow revenue at an optimal rate. There may be other negative effects from supply shortages, including loss of our reputation in the marketplace and a negative impact on our relationships with our distributors.
On the other hand, if demand for our products declines, or if market supply surpasses demand, we may not be able to reduce manufacturing expenses or overhead costs proportionately. We have invested significantly in our manufacturing capacity in order to vertically integrate our business. If an increase in supply outpaces the increase in market demand, or if demand decreases, the resulting oversupply could adversely impact our sales and result in the underutilization of our manufacturing capacity, higher inventory carrying costs and associated working capital, changes in revenue mix, and/or price erosion, any of which would lower our margins and adversely impact our financial results.
Risks Related to Our Business, Industry and Operations
We expect to incur losses for the foreseeable future, and our ability to achieve and maintain profitability depends on the commercial success of our Motiva Implants.
We have incurred losses to date and expect to continue to incur losses for the foreseeable future. Sales of our Motiva Implants accounted for approximately 95% and 98% of our revenues for each of the years ended December 31, 2023 and December 31, 2022, and we expect our revenues to continue to be driven primarily by sales of these products. In order to achieve and sustain profitability, our revenues from these products will need to grow beyond the levels we have achieved in the past. If physicians and/or patients do not perceive our products to be competitive in features and safety when compared to other products in the market, or if demand for our Motiva Implants or for breast implants in general decreases, we may fail to achieve sales levels that provide for future profitability.
Our ability to successfully market Motiva Implants and our other current and future offerings depends on numerous factors, including but not limited to:
•the outcomes of current and future clinical studies of Motiva Implants to demonstrate our products’ value in improving safety outcomes and/or patient satisfaction;
•acceptance of Motiva Implants as safe and effective by patients, caregivers and the medical community;
•an acceptable safety profile of Motiva Implants in the global market;
•whether key thought leaders in the medical community accept that such clinical studies are sufficiently meaningful to influence their or their patients’ choices of product;
•maintenance of our existing regulatory approvals and expansion of the geographies in which we have regulatory approvals;
•designing commercially viable processes at a scale sufficient to meet anticipated demand at an adequate cost of manufacturing, and that are compliant with ISO 13485 Quality Management System requirements and/or good manufacturing practice, or GMP, requirements, as set forth in the FDA’s Quality System Regulation, Brazilian and other international regulations;
•our success in educating physicians and patients about the benefits, administration and use of Motiva Implants;
•the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments;
•the willingness of patients to pay out-of-pocket for breast augmentation and reconstruction procedures in the absence of coverage and reimbursement for such procedures;
•the success of our internal sales and marketing organization and the sales forces of our distributors; and
•continued demand for breast augmentation and reconstruction procedures using silicone implants, which may be adversely affected by events involving either our products or those of our competitors, including FDA warnings to patients regarding Breast Implant-Associated Anaplastic Large Cell Lymphoma, or BIA-ALCL and other lymphomas or cancers, including squamous cell carcinoma.
Some of these factors are beyond our control. If we are unable to continue to commercialize Motiva Implants and our other products, or unable to obtain a partner to commercialize them, we may not be able to produce any
36
incremental revenues related to Motiva Implants and our other products. This would result in an adverse effect on our business, financial condition, results of operations and growth prospects.
Unfavorable global economic conditions, including slower growth or recession, inflation or decreases in consumer spending power or confidence, could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets, such as slower growth or recession, continued inflation or decreases in consumer spending or confidence. A severe or prolonged economic downturn could result in a variety of risks to our business, including general economic pressure on our customers’ patients. Elective aesthetic procedures, including breast augmentation, are typically not covered by insurance and are less of a priority than other items for those patients that have lost their jobs, are furloughed, have reduced work hours or have to allocate their cash to other priorities. As a result, adverse changes in the global economy, including as a result of current inflationary pressures, higher interest rates, geopolitical conflicts, including the Russia-Ukraine war and the Hamas-Israel conflict, or macroeconomic fallout from a potential U.S. government default on its debt, may cause consumers to reassess their spending choices and reduce demand for elective aesthetic procedures, which could have an adverse effect on our net sales and profitability. A weak or declining global economy could also strain our manufacturers or suppliers, possibly resulting in supply disruption, or cause our customers or distributors to delay making payments for our products. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the economic climate and financial market conditions could adversely affect our business.
We have incurred net operating losses in the past and expect to incur net operating losses for the foreseeable future.
We have incurred net operating losses since our inception, and we continue to incur significant research and development and general and administrative expenses related to our operations. We do not expect to be profitable in 2024, and in future years we expect to incur significant research and development expenses related to, among other things, the IDE clinical study of Motiva Implants in the United States. Investment in medical device product development, particularly clinical studies, is highly speculative. It entails substantial upfront capital expenditures and significant risk that any potential planned product will fail to demonstrate adequate accuracy or clinical utility. We may not be profitable for some time. As of December 31, 2023, we had an accumulated deficit of $360.1 million.
We expect that our future financial results will depend primarily on our success in launching, selling and supporting Motiva Implants and other products that are part of our product platform. This will require us to be successful in a range of activities, including manufacturing, marketing, and selling Motiva Implants. We may not succeed in these activities and may never generate revenue that is sufficient to be profitable in the future. Even if we are profitable, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to achieve sustained profitability would depress the value of our company and could impair our ability to raise capital, expand our business, diversify our planned products, market our current and planned products, or continue our operations.
If our available cash resources and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements, we may seek to sell equity or convertible debt securities, enter into a credit facility or another form of third-party funding, or seek other debt financing.
We may need additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all, which would force us to delay, reduce or suspend our planned development and commercialization efforts. Raising additional capital may subject us to unfavorable terms, cause dilution to our existing shareholders, restrict our operations, or require us to relinquish rights to our products and technologies.
Our operations have consumed substantial amounts of cash since our inception, and we expect to incur significant expenses in connection with our planned research, development and product commercialization efforts. We believe that our available cash and cash from operations will be sufficient to satisfy our liquidity requirements for at least the next 12 months. If our available cash resources and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements, we may seek to sell equity or convertible debt securities, enter into a credit facility or another form of third-party funding, or seek other debt financing. However, we are subject to restrictive covenants under the Credit Agreement which restrict our ability to incur additional debt. Any failure to raise the funds necessary to support our operations or liquidity requirements may force us to delay, reduce or suspend our planned clinical trials, research and development programs, or other commercialization efforts.
37
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a shareholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic collaborations or partnership, or marketing, distribution or licensing arrangements with third parties, we may be required to do so at an earlier stage than would otherwise be ideal and/or may have to limit valuable rights to our intellectual property, technologies, products, or future revenue streams, or grant licenses or other rights on terms that are not favorable to us. Furthermore, any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our products.
Our business depends on maintaining our brand and ongoing customer demand for our products and services, and a significant reduction in sentiment or demand could affect our results of operations.
Our success depends on the reputation of our brands, which depends on factors such as the safety and quality of our products, our communication activities, including marketing and education efforts, and our management of our customer experience. Maintaining, promoting and positioning our brands is important to expanding our customer base. This will depend largely on the success of our education and marketing efforts and our ability to provide a consistent, high-quality customer experience.
We may need to make substantial investments in the areas of education and marketing in order to maintain and enhance our brands. Ineffective marketing, negative publicity, significant discounts by our competitors, product defects and related liability litigation, failure to obtain regulatory clearance for our products, counterfeit products, unfair labor practices and failure to protect the intellectual property rights in our brands are some of the potential threats to the strength of our business. To protect our brands’ status, we may need to make substantial expenditures to mitigate the impact of such threats.
We believe that maintaining and enhancing our brands in the countries in which we currently sell our products, and in new countries where we have limited brand recognition, is important to expanding our customer base. If we are unable to maintain or enhance the strength of our brands in the countries in which we currently sell our products and in new countries, then our growth strategy could be adversely affected.
If we fail to compete effectively against our competitors, many of whom have greater resources than we have, our revenues and results of operations may be negatively affected.
Alternatives exist for Motiva Implants and for our other products, and we will likely face competition with respect to any planned products that we may seek to develop or commercialize in the future from major pharmaceutical companies, specialty pharmaceutical companies, medical device companies and biotechnology companies worldwide. There are several large pharmaceutical and biotechnology companies that currently market silicone breast implants. We also face competition from manufacturers of saline-filled breast implants, and we see emerging competition from non-implant breast augmentation techniques such as hyaluronic acid injection and novel fat grafting methodologies. Any of these may present competitive barriers to Motiva Implants.
Our leading competitors are large, multi-national companies with significant resources and capabilities. Three of these companies, Sientra, Inc., Mentor Worldwide LLC (a division of Johnson & Johnson), and Allergan plc (recently acquired by AbbVie Inc.), have conducted large prospective clinical studies that started in the United States in 2002, 2000 and 1998, respectively, and they use this data extensively to promote their products. This can put us at a disadvantage when promoting our products to physicians and patients, even outside the United States. In addition, the significant financial and staff resources and brand recognition that our competitors possess mean they may be able to compete with us regardless of the differentiating features of our products. If we are not successful in capturing market share, even outside the United States, or if physicians or patients do not perceive our products to be safer or more favorable, our revenues and/or our operating margins may be significantly impaired.
In addition, manufacturers of competitive products may reduce prices for their competing products in an effort to gain or retain market share and undermine the value proposition that Motiva Implants might otherwise be able to offer to customers. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish
38
collaborative arrangements for research, development, manufacturing and commercialization. These competitors may develop new technologies that are superior to our products or replace silicone.
Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties may compete with us in recruiting and retaining qualified technical and management personnel, establishing clinical study sites and patient registrations for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs.
Pricing pressure from customers and our competitors may impact our ability to sell our products at prices necessary to support our current business strategies and future expansion.
The industry environment for silicone implants and complementary products in certain international markets is price sensitive. In these markets, or in the United States if we are successful in obtaining the required regulatory approval to sell in the U.S. market, our competitors may adopt aggressive pricing strategies to intensify the competitive pricing pressure for breast implants. If we are not successful in educating customers or third-party payers on the differentiation of our Motiva Implants as compared to our competitors’ products, customers may choose our competitors’ products. Additionally, as more competitors introduce products that compete with ours, we may face additional pricing pressure that would adversely impact our future results.
We expect to increase the size of our organization in certain jurisdictions and functions; as a result, we may encounter difficulties in managing our growth, which could disrupt our operations and/or increase our net losses.
As of December 31, 2023, we had 908 employees. Unless it is necessary for us to make reductions to our workforce as a cost management strategy, over the next several years, we expect to experience growth in the number of our employees and the scope of our operations, principally in the areas of manufacturing and sales and marketing, and particularly as we prepare our operations in the anticipation of obtaining approval from the FDA to commercialize our Motiva Implants in the United States. We also intend to continue to improve our operational, financial and management controls, reporting systems and procedures, which may require additional personnel. Such growth could place a strain on our administrative and operational infrastructure, and/or our managerial abilities, and we may not be able to make improvements to our management information and control systems in an efficient or timely manner. We may discover deficiencies in existing systems and controls.
Many of these employees will be in countries outside of our corporate headquarters, which adds additional complexity. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. We may not be able to effectively manage these activities. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Future growth would impose significant added responsibilities on members of management, including:
•identifying, recruiting, maintaining, motivating and integrating additional employees with the expertise and experience we will require, in multiple countries;
•managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties;
•managing additional relationships with various distributors, suppliers, and other third parties;
•improving our managerial, development, operational and finance reporting systems and procedures; and
•expanding our facilities.
Our failure to accomplish any of these tasks could prevent us from growing successfully. Any inability to manage growth could delay the execution of our business plans or disrupt our operations. We may also be exposed or subject to additional unforeseen or undisclosed liabilities as well as increased levels of indebtedness.
In certain large markets, we engage in direct sales efforts. We may fail to maintain and develop our direct sales force, and our revenues and financial outcomes could suffer as a result. Furthermore, our direct sales personnel may not effectively sell our products.
We have established a direct sales force for our business in Brazil, and we have implemented a direct sales strategy in several European countries. We have hired and will need to retain and motivate a significant number of sales and marketing personnel in order to support our anticipated growth in these countries. There is significant competition for quality personnel experienced in such activities, including from companies with greater financial resources than ours. If we are not successful in our efforts to continue recruiting, retaining, and motivating such
39
personnel, we may not be able to increase our revenues, or we may increase our expenses in greater measure than our revenues, negatively impacting our operating results.
We are also working on creating a direct sales structure and strategy in certain markets. We are working to put in place the correct legal and business structures to comply with taxation and operational requirements. These structures may not ultimately be implemented or, if implemented, be successful or effective and may not be able to increase our revenues or improve our gross margins. In addition, our expenses or tax related costs may increase in greater measure than our revenues, negatively impacting our operating results.
We may be subject to substantial warranty or product liability claims or other litigation in the ordinary course of business that may adversely affect our business, financial condition and operating results.
We face an inherent risk of product liability exposure related to the sale of Motiva Implants and any planned products in clinical studies. The marketing, sale and use of Motiva Implants and our planned products could lead to the filing of product liability claims against us if someone alleges that our products failed to perform as designed or caused significant adverse events in patients. We may also be subject to liability for a misunderstanding of, or inappropriate reliance upon, the information we provide. If we cannot successfully defend ourselves against claims that Motiva Implants or our planned products caused injuries, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
•decreased demand for any planned products we may develop;
•injury to our reputation and significant negative media attention;
•withdrawal of patients from clinical studies or cancellation of studies;
•significant costs to defend the related litigation and distraction to our management team;
•substantial monetary awards to plaintiffs;
•loss of revenue; and
•the inability to commercialize any products that we may develop.
We currently hold $25 million in product liability insurance coverage, which may not be adequate to cover all liabilities we may incur. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Counterfeit products may be represented as ours, which could compete with our genuine products and may also expose us to risks associated with adverse events and product liability.
We routinely see counterfeit versions of our major competitor’s branded products in the marketplace, and we are aware of potential counterfeiting of our Motiva Implants. This is particularly common in emerging markets, where sensitivity to price is higher and regulatory enforcement is under-resourced. These counterfeit products are typically manufactured with significantly lower quality than the products they are claimed to be, and in some cases may be manufactured with silicones that are not medical grade. They may expose patients to significant adverse event risks, and there is a risk that certain adverse events with counterfeit products may be attributed to our genuine products. This could reduce demand for our products, result in negative publicity, or otherwise impact our business and the price of our shares.
Negative publicity concerning our products or our competitors’ products, including due to product defects, recalls and any resulting litigation, could harm our reputation and reduce demand for silicone breast implants, either of which could adversely impact our financial results and/or share price.
The silicone breast implant industry has been the focus of significant regulatory and media scrutiny. Silicone breast implants were removed from the U.S. marketplace for a period in the 1990s and 2000s related to safety concerns. Certain patient advocacy groups exist to publicize real and perceived health risks associated with silicone breast implants and plastic surgery generally. Recently, some breast implant patients have begun to self-identify and report various symptoms that they believe are related to their breast implants; they refer to these symptoms as Breast Implant Illness, or BII, but BII is not an official medical diagnosis. Additionally, the activities of legislative bodies, regulatory agencies, physician organizations, and other groups may lead to publicity around the real and perceived risks to patients from silicone implants. The responses of potential patients, physicians, the news media, legislative and regulatory bodies and others to information about complications or alleged complications of our products or our competitors’ products, or products liability litigation against us or our competitors, could materially reduce market acceptance and patient demand for our products, or could, even in the absence of a change in demand, negatively impact our business and reputation and negatively impact our
40
financial condition, results of operations or the market price of our common shares. In addition, activity of this type could result in an increase in the number or size of product liability claims, which would adversely affect our business, financial results, and/or the price of our shares.
Recent news coverage has called into question the long-term safety of breast implants and reports of breast implant-associated anaplastic large cell lymphoma linked to our competitors’ products which have led to regulatory actions regarding macrotextured devices in several countries and the worldwide recall of one of our competitor’s macrotextured implants and tissue expanders. These events and reports of other forms of cancer, including squamous cell carcinoma and various lymphomas, from breast implant products may lead to a reduction in the demand for silicone breast implants and could adversely affect our business.
Women with breast implants have reported higher rates, as compared to the general population, of breast implant-associated anaplastic large cell lymphoma, or BIA-ALCL, an uncommon type of cancer affecting cells of the immune system. In January 2011, the FDA indicated that there was a possible association between certain saline and silicone gel-filled breast implants and higher rates of BIA-ALCL, with the causal links neither yet understood nor confirmed. In March 2015, France’s National Cancer Institute, or NCI, noted that there is a clearly established link between anaplastic large cell lymphoma and certain breast implants, which is referred to as breast implant-associated ALCL, or BIA-ALCL. The NCI noted in that report that most of the reported cases occurred in women with textured implants.
In August 2017, the FDA updated its advisory on BIA-ALCL and subsequently requested all breast implant manufacturers to revise their physician and patient labeling with the most current information. The August 2017 update described BIA-ALCL as “rare” and stated “we have strengthened our understanding of this condition and concur with the World Health Organization designation of BIA-ALCL as a rare T-cell lymphoma that can develop following breast implants. The exact number of cases remains difficult to determine due to significant limitations in world-wide reporting and lack of global implant sales data. At this time, most data suggest that BIA-ALCL occurs more frequently following implantation of breast implants with textured surfaces rather than those with smooth surfaces.” The FDA noted it does not recommend prophylactic breast implant removal in a patient without symptoms or other abnormalities.
In March 2018, the FDA further updated its advisory on BIA-ALCL stating “we are reporting that we are aware of 414 total cases of BIA-ALCL. Additionally, studies reported in medical literature estimate that the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000.” The FDA noted that the update did not change the agency’s recommendation and that choosing to obtain a breast implant is a personal decision that patients and providers should make with the most complete information available. In the fourth quarter of 2018, following the non-renewal of its textured breast implant CE Mark licenses in Europe, Allergan plc suspended sales of textured breast implants in Europe and withdrew its remaining textured breast implants on the market within Europe.
On February 6, 2019, the FDA further reported that as of September 2018, the agency had received a total of 660 medical device reports regarding BIA-ALCL cases since 2010. Of the 660 reports, the FDA’s analysis suggested that there were 457 unique cases of BIA-ALCL, including nine patient deaths. Additionally, on February 12, 2019, Health Canada confirmed that as of January 1, 2019, it had received reports of 22 confirmed and 22 suspected Canadian cases of BIA-ALCL and that it would be updating its safety review of BIA-ALCL in Spring 2019. In April 2019, the Agence Nationale de Securite du Medicament et des Produits de Sante, or ANSM, the regulatory authority in France, announced that 59 cases of BIA-ALCL had been reported in France since 2011 and banned several types of macrotextured and polyurethane implants linked to BIA-ALCL. Between February and September 2019, authorities from Australia, Colombia, Canada, South Korea and Singapore announced similar bans.
In July 2019, the FDA requested that Allergan plc recall its Biocell® textured implants in the U.S. market and Allergan subsequently announced the global recall of its Biocell® textured breast implants and tissue expanders. In the announcement, the FDA noted that it had reviewed 573 unique cases globally of BIA-ALCL, including 33 patient deaths, of which 12 of the 13 known deaths were attributed to Biocell® implants. The FDA further noted that it will continue to monitor the incidence of BIA-ALCL across other textured and smooth breast implants and tissue expanders as well as other devices intended for use in the breast. The FDA subsequently identified the recall as a Class I recall in September 2019 and stated that use of the recalled devices may cause serious injuries and death. As the BIA-ALCL risk continues to become more highly publicized, this could have a significant negative impact on demand for breast implants globally, including our Motiva Implants.
41
In August 2020, the FDA updated its analysis of medical device reports of breast implant illness and breast implant associated lymphoma. In this update, the FDA updated the table on the agency’s BIA-ALCL webpage to include a total of 733 unique cases and 36 patient deaths globally as of January 5, 2020, which reflect an increase of 160 new cases and 3 deaths since the early-July 2019 update.
In September 2020, the FDA released finalized guidance on breast implant labeling recommendations, including the addition of a boxed warning, a patient decision checklist, material and device descriptions, implant rupture screening recommendations and a patient device card. In October 2021, the FDA took several additional actions to strengthen breast implant risk communication, including restricting the sale and distribution of breast implants to only health care providers and facilities that provide information to patients using the patient decision checklist. The FDA also approved new labeling for all legally marketed breast implants that includes a boxed warning, a patient decision checklist, updated silicone gel-filled breast implant rupture screening recommendations, a device description with a list of specific materials, and a patient device card.
In September 2022, the FDA informed the public about reports of cancers, including squamous cell carcinoma, or SCC, and various lymphomas, in the scar tissue (capsule) that forms around breast implants different from the lymphomas described in previous FDA communications as BIA-ALCL. In March 2023, the FDA provided updated information about SCC, noting it has received 24 reports of SCC related to breast implants, but that this this does not necessarily represent cancer incidence because of potential underreporting or duplicated reports. The FDA noted that, while the agency believes the occurrences of SCC or various lymphomas in the capsule around the breast implant to be rare, health care providers and people who have or are considering breast implants should be aware that cases have been reported to the FDA and in the literature.
We do not produce the types of rough textured implants that have been involved in these reports. To date, no cases of BIA-ALCL or SCC have been reported in patients with Motiva Implants. Furthermore, there have been no reported cases of BIA-ALCL in patients with smooth implants with no history of previously having a textured device. Future clinical studies or clinical experience may indicate that breast implants expose potentially genetically predisposed patients to greater risks of BIA-ALCL, which may reduce demand for silicone implants generally, expose us to product liability claims, as well as to class actions and other lawsuits. These impacts may occur in the absence of any specific linkage with our products. Moreover, if cases of BIA-ALCL, SCC, or other complications are discovered in the future and/or are reported in patients with Motiva Implants, we could be subject to mandatory product recalls, suspension or withdrawal of our regulatory licensure for sale in one or more countries, and significant legal liability. Any of these may have an adverse effect on our business or operating results, or a negative impact on our share price.
The loss of members of our executive management team or other employees, or other turnover in our management team, could adversely affect our business.
Our success in implementing our business strategy depends largely on the skills, experience and performance of members of our executive management team and other key employees, including Juan José Chacón Quirós, our Chief Executive Officer, Roberto de Mezerville, our Chief Technology Officer, Rajbir Denhoy, our Chief Financial Officer, and Ross Mansbach, our General Counsel and Chief Human Resources Officer. The collective efforts of each of these persons, and others working with them as a team, are critical as we continue to develop our tests and technologies and pursue our research and development and sales programs. In addition, we have experienced significant changes in our executive leadership in recent years, including in our General Counsel and Chief Operating Officer positions. As a result of the difficulty in locating qualified new management and other key employees, the loss or incapacity of existing members of our executive management team could adversely affect our operations. If we were to lose one or more key employees, we could experience difficulties in finding qualified successors, competing effectively, developing our technologies and implementing our business strategy. In addition, changes to strategic or operating goals, which can often times occur with the appointment of new executives and directors, can create uncertainty, may negatively impact our ability to execute quickly and effectively, and may ultimately be unsuccessful. Executive leadership transition periods are often difficult as the new executives gain detailed knowledge of our operations, and friction can result from changes in strategy and management style. Management turnover inherently causes some loss of institutional knowledge, which can negatively affect strategy and execution. We do not have “key person” life insurance on our senior executives, and the loss of any of the key team members would have a negative impact to our business and financial results. In addition, the job market in Costa Rica and other locations in which we operate has recently become more
42
competitive and we are competing for talent with major multinational corporations which have significantly more resources than us, and we may find new difficulties in retaining our most talented employees.
In addition, we rely on collaborators, consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our collaborators, consultants and advisors are generally employed by employers other than us and may have commitments under agreements with other entities that may limit their availability to us.
We have made multiple acquisitions in the past, and in the future we may acquire other businesses, form joint ventures or make investments in other companies or technologies. If we are not successful in integrating these businesses, as well as identifying and controlling risks associated with the past operations of these businesses, we may incur significant costs, receive penalties or other sanctions from various regulatory agencies and/or incur significant diversions of management time and attention.
We believe our business growth will be enhanced if we continually seek opportunities to enhance and broaden our product offerings. As part of our business strategy, we may pursue acquisitions or licenses of assets, or acquisitions of businesses. We also may pursue strategic alliances and joint ventures that leverage our core technology and industry experience to expand our product offerings or sales and distribution resources. We have acquired companies and/or assets and licensed assets in a variety of countries, including Brazil and several European countries.
We may do more of these types of transactions in the future and may also form strategic alliances and joint ventures. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could have an adverse effect on our financial condition, results of operations and cash flows. Integration of an acquired company may also disrupt ongoing operations and require management resources that would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could have a negative effect on our results of operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, license, strategic alliance or joint venture. To finance such a transaction, we may choose to issue common shares as consideration, which would dilute the ownership of our shareholders. If the price of our common shares is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our shares as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
We do not know whether we will be able to successfully integrate any acquired business, product or technology. The success of any given acquisition may depend on our ability to retain any key employees related thereto, and we may not be successful at retaining or integrating such key personnel. Integrating any business, product or technology we acquire could be expensive and time-consuming, disrupt our ongoing business, impact our liquidity, and/or distract our management. If we are unable to integrate any acquired businesses, products or technologies effectively, our business may suffer. Whether as a result of unsuccessful integration, unanticipated costs, including those associated with assumed liabilities and indemnification obligations, negative accounting impact, or other factors, we may not realize the economic benefits we anticipate from acquisitions. In addition, any amortization or charges resulting from the costs of acquisitions could increase our expenses.
We have significant exposure to the economic and political situations in emerging market countries, and developments in these countries could materially impact our financial results, or our business more generally.
Many of the countries in which our products are sold are emerging markets. Our global growth strategy contemplates the expansion of our existing sales activities in Latin America, Europe, the Middle East, and Asia-Pacific region as well as North America. Our exposure to emerging markets has increased in recent years, as have the number and importance of our distributor arrangements. Economic and political developments in Brazil and other emerging markets, including economic crises, currency inflation, or political instability, have had in the past, and may have in the future, a material adverse effect on our financial condition and results of operations. Moreover, as these markets continue to grow, competitors may seek to enter these markets and existing market participants will likely try to aggressively protect or increase their market shares. Increased competition may result
43
in price reductions, reduced margins and our inability to gain or hold market share, which could have an adverse effect on our financial condition and results of operations.
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance by financial institutions or transactional counterparties, could adversely affect our liquidity and financial performance.
Bank failures, events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, on March 10, 2023, Silicon Valley Bank was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation, or FDIC, as receiver. Similarly, on March 12, 2023, Signature Bank Corp. and Silvergate Capital Corp. were each swept into receivership. We do not maintain balances with, and are not a borrower under or party to any credit agreement, material letter of credit or any other such instruments with, any financial institution currently in receivership. However, we regularly maintain cash balances at third-party financial institutions in excess of the FDIC standard insurance limit, with balances concentrated at a small number of financial institutions. The failure of a bank, or other adverse conditions in the financial or credit markets impacting financial institutions at which we maintain balances, or which we do business with, could adversely impact our liquidity and financial performance. There can be no assurance that our deposits in excess of the FDIC or other comparable insurance limits will be backstopped by the U.S. or any applicable foreign government in the future or that any bank or financial institution with which we do business will be able to obtain needed liquidity from other banks, government institutions or by acquisition in the event of a future failure or liquidity crisis. In addition, if any of our partners or parties with whom we conduct business are unable to access funds due to the status of their financial institution, such parties’ ability to pay their obligations to us or to enter into new commercial arrangements requiring additional payments to us could be adversely affected.
Investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all.
Our results of operations could be affected by fluctuations in currency rates.
We present our results of operations in U.S. dollars, which is our reporting currency. However, as of December 31, 2023, the majority of our revenues are denominated in currencies other than the U.S. dollar - primarily the euro, the Brazilian real, and the British pound. As of December 31, 2023, the majority of our expenses are denominated in U.S. dollars or in Costa Rican colones, which are linked to the U.S. dollar. In the future, we expect to have significant revenues and expenses denominated in these non-U.S. currencies. As such, unfavorable fluctuations in currency exchange rates have had, and in the future could continue to have, an adverse effect on our results of operations.
Because our consolidated financial statements are presented in U.S. dollars, we must translate revenues, expenses and income, as well as assets and liabilities, into U.S. dollars at exchange rates in effect during or at the end of each reporting period. Therefore, changes in the value of the U.S. dollar in relation to the British pound, the euro, Costa Rican colones and the Brazilian real will affect our revenue, cost of goods, and operating expenses as well as the value of balance sheet items originally denominated in other currencies. These changes would cause our growth in consolidated earnings stated in U.S. dollars to be higher or lower than our growth in local currency when compared against other periods. For example, the weakening of the euro for the majority of fiscal 2022 had a negative effect on our European revenue. We do not currently engage in currency hedging arrangements to protect us from fluctuations in the exchange rates of the euro and other currencies in relation to the U.S. dollar (and/or from inflation of such currencies), and we are exposed to material adverse effects from such movements. We cannot predict any future trends in rates of inflation or exchange rates of other currencies against the U.S. dollar, and there can be no assurance that any contractual provisions will offset their impact, or that any future currency hedging activities will be successful.
Continued international expansion of our business will expose us to business, regulatory, political, operational, financial and economic risks associated with doing business internationally.
Our products are commercially available in 86 countries, and we operate subsidiaries in the United States, Costa Rica, Brazil, and several European countries. Our business strategy contemplates continued international
44
expansion, including partnering with medical device distributors, and introducing Motiva Implants and other planned products outside the United States. The sale and shipment of our products internationally, as well as the purchase of components from international sources, subjects us to potential trade, import and export, and customs regulations and laws.
Compliance with these regulations and laws is costly and exposes us to penalties for non-compliance. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export or import privileges, seizure of shipments, restrictions on certain business activities and exclusion or debarment from government contracting. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our shipping, marketing and sales activities.
In addition, several of the countries in which we sell our products or conduct our operations are, to some degree, subject to political, economic or social instability. Doing business in Costa Rica and other countries outside the United States involves a number of other risks, including:
•compliance with the free zone regime regulations under which the manufacturing sites operate;
•different regulatory requirements for device approvals in international markets;
•multiple, conflicting and changing laws and regulations such as tariffs and tax laws, export and import restrictions, employment laws, environmental laws, regulatory requirements and other governmental approvals, permits and licenses;
•potential failure by us or our distributors to obtain and/or maintain regulatory approvals for the sale or use of our products in various countries;
•difficulties in managing global operations;
•logistics and regulations associated with shipping products, including infrastructure conditions and transportation delays;
•limits on our ability to penetrate international markets if our distributors do not execute successfully;
•governmental price controls, differing reimbursement regimes and other market regulations;
•financial risks, such as longer payment cycles, difficulty enforcing contracts and collecting accounts receivable, and exposure to currency exchange rate fluctuations;
•reduced protection for intellectual property rights, or lack of them in certain jurisdictions, forcing more reliance on our trade secrets, if available;
•economic weakness, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions;
•the British exit from the EU, including with respect to its effect on the value of the British pound relative to other currencies;
•failure to comply with the Foreign Corrupt Practices Act, including its books and records provisions and its anti-bribery provisions, by maintaining accurate information and control over sales activities and distributors’ activities;
•failure to comply with restrictions on the ability of companies to do business in foreign countries, including restrictions on foreign ownership of telecommunications providers imposed by the U.S. Office of Foreign Assets Control;
•failure to comply with evolving reporting expectations on environmental, social and governance issues;
•unexpected changes in tariffs, trade barriers and regulatory requirements;
•compliance with tax, employment, immigration and labor laws;
•taxes, including withholding of payroll taxes;
•currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country;
•workforce uncertainty in countries where labor unrest is more common than in the United States;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
•business and shipping interruptions resulting from natural or other disasters including earthquakes, volcanic activity, hurricanes, floods and fires.
45
Any of these risks, if encountered, could harm our future international expansion and operations and, consequently, have an adverse effect on our financial condition, results of operations and cash flows.
Any disruption at our existing facilities could adversely affect our business and operating results.
Our headquarters are located in Costa Rica, and all of our main manufacturing activities are conducted in two ISO-13485 and GMP compliant manufacturing facilities in Costa Rica through Establishment Labs, S.A. A third facility in Costa Rica is under construction and is currently expected to commence manufacturing in fiscal 2024. Despite our efforts to maintain and safeguard our manufacturing facilities, including acquiring insurance and adopting maintenance and health and safety protocols, vandalism, terrorism or a natural or other climate-related disaster, such as earthquake, volcanic activity, fire or flood, could damage or destroy our inventory of finished goods, cause substantial delays in our operations and manufacturing, result in the loss of key information and cause us to incur additional expenses. Our insurance may not cover our losses in any particular case. In addition, regardless of the level of insurance coverage, damage to our facilities may have an adverse effect on our business, financial condition and results of operations.
Fluctuations in insurance costs and availability, and future insurance requirements could adversely affect our profitability or our risk management profile.
We hold a number of insurance policies, including product liability insurance, directors’ and officers’ liability insurance, general liability insurance, property insurance and workers’ compensation insurance. If the costs of maintaining adequate insurance coverage increase significantly in the future, our operating results could be adversely affected. Likewise, if any of our current insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers. If we operate our business without insurance, we could be responsible for paying claims or judgments against us that would have otherwise been covered by insurance, which would adversely affect our results of operations or financial condition.
Risks Related to Manufacturing and Other Third-Party Relationships
Our operations involve hazardous materials and we and third parties with whom we contract must comply with environmental laws and regulations, which can be expensive and restrict how we do business, and could expose us to liability if our use of such hazardous materials causes injury.
Our manufacturing processes currently require the controlled use of potentially harmful chemicals, including highly flammable solvents. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. These are particularly stringent in California, where Avantor, one of our key suppliers, is located. The cost of compliance with these laws and regulations may become significant and could have an adverse effect on our financial condition, results of operations and cash flows. In the event of an accident or if we otherwise fail to comply with applicable regulations, we could lose our permits or approvals or be held liable for damages or penalized with fines.
We rely on third parties to conduct certain components of our clinical studies, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such studies, which could interfere with or delay our ability to obtain regulatory approval or commercialize our products.
We rely on third parties, such as contract research organizations, or CROs, clinical data management organizations, medical institutions and clinical investigators, to perform various functions for our clinical trials. These service providers may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. We are required to collect and provide financial disclosure notifications or certifications for our clinical investigators to the FDA. If the FDA concludes that a financial relationship between us and a clinical investigator has created a conflict of interest or otherwise affected interpretation of the trial, the FDA may question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized.
Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. We remain responsible for ensuring that each of our clinical studies is conducted in accordance with the general investigational plan and protocols for the study. Moreover, the
46
International Council for Harmonization, or ICH, and the FDA require us to comply with standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical studies to ensure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of patients in clinical studies are protected. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical studies in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our planned products and will not be able to, or may be delayed in our efforts to, successfully commercialize our planned products.
We rely on a single-source, third-party supplier for medical-grade long-term implantable silicone, which is the primary raw material used in our Motiva Implants. If this supplier were to increase prices for this raw material over time or experience interruptions in its ability to supply us with this raw material, our business, financial condition and results of operations could be adversely affected.
We rely on Avantor Inc. (formerly NuSil Technology, LLC), or Avantor, as the sole supplier of medical-grade silicone used in our Motiva Implants as well as other products that we manufacture under contract to other customers. To our knowledge, Avantor is the only supplier of such raw materials with the appropriate filings with the FDA and other regulatory bodies to enable the manufacturing of products with our requirements. Avantor supplies our major competitors with raw material as well, and at least two of these are larger-volume customers of Avantor than we are.
If Avantor becomes unable or unwilling to supply sufficient quantities of medical-grade silicone of the specifications required for our products, we may not be able to replace this supply source quickly, or at all. Similarly, they may become unable or unwilling to manufacture our needed raw materials in compliance with regulatory requirements, or their manufacturing facilities may not be able to maintain compliance with regulatory requirements. Any replacement supplier would have to be qualified with the relevant regulatory authorities, which is an expensive and time-consuming process during which we may experience an interruption in our manufacturing operations. We may also be unsuccessful in negotiating favorable terms with such a supplier. Any of these contingencies would likely affect the financial results of our operations and may have a negative impact on our share price. In particular, if we are not able to establish a replacement vendor for our medical-grade silicone, we would be unable to manufacture our Motiva Implants as well as other products that we manufacture under contract to other customers until such time as a replacement vendor is identified, which would likely significantly affect the financial results of our operations and have a significantly negative impact on our share price.
In addition, our relationship with Avantor involves other risks, including but not limited to the following:
•it may not be able, or willing, to manufacture silicone raw materials with our agreed-upon specifications;
•it may not be able, or willing, to manufacture our needed raw materials in compliance with regulatory requirements, or our its manufacturing facilities may not be able to maintain compliance with regulatory requirements;
•it may not be able to supply sufficient quantities of each raw material quickly enough for us to respond to rapid increases in demand;
•it may unintentionally convey information to our competitors that is helpful in understanding our proprietary compositions and other trade secrets of our manufacturing processes;
•we may be subject to price fluctuations if we fail to meet certain minimum order requirements, or if our existing contract expires or is renegotiated;
•it may lose access to critical services and components, resulting in interruption in manufacture or shipment of medical-grade silicone;
•its facilities may be affected by earthquakes, wildfires, mud slides or other natural disasters, which could delay or impede production of our raw materials;
•we may be required to obtain regulatory approvals related to any change in our supply chain;
•Avantor may wish to discontinue supply of products to us due to its existing relationships with our competitors;
•Avantor may stop supply and claim ownership of intellectual property on materials associated with future products;
47
•Avantor or its parent entity may encounter financial or other hardships unrelated to our demand for products, which could negatively impact their ability to fulfill our orders and support our regulatory approvals; and
•disputes may arise over the terms of the Master Supply Agreement, by and between the Company and Avantor, dated May 13, 2022.
Various factors outside our direct control, including the reliance on single-source suppliers, may adversely affect manufacturing and supply of our Motiva Implants and other products.
We currently manufacture Motiva Implants at our facilities in the Coyol Free Zone, Alajuela, Costa Rica, under the multi-country MDSAP protocol. Our Qid Safety Technology microtransponders are manufactured by contract manufacturers with final testing and packaging at a manufacturing supplier facility in Regensburg, Germany, with additional inspection of the units at our facilities in Coyol, Costa Rica, prior to approval for inclusion in Motiva Implants. If demand for our current products and our planned products increases more rapidly than we anticipate, or if we secure regulatory approval to commercialize our products in additional geographies, we will need to either expand our manufacturing capabilities or outsource to other manufacturers. The manufacture of these products in compliance with ISO standards and the FDA’s regulations requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of medical device products often encounter difficulties in production, including difficulties with production costs and yields, quality control, quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced FDA requirements, other federal and state regulatory requirements, and foreign regulations.
We currently purchase components for the Qid Safety Technology microtransponders under purchase orders and do not have long-term contracts with most of the suppliers of the materials included in these products. We rely on Avantor as the sole supplier of medical-grade silicone used in our Motiva Implants as well as other products that we manufacture under contract to other customers. See the risk factor above titled “We rely on a single-source, third-party supplier for medical-grade silicone, which is the primary raw material used in these products. If this supplier were to increase prices for these raw materials over time or experience interruptions in their ability to supply us with this raw material, our business, financial condition and results of operations could be adversely affected.” In addition, the suppliers of certain packaging components and the surgical tools that we sell with Motiva Implants, including the cannulas, retractors, and insertion sleeves, are all purchased by us from single-source suppliers.
If our single-source and other suppliers were to delay or stop producing our components, or if the prices they charge us were to increase significantly, or if they elected not to sell to us at all or on commercially reasonable terms, we would need to identify and initiate relationships with alternative suppliers, if possible. We could experience delays in manufacturing our products or the interruption of the availability of Motiva Implants or our other products for sale, while finding another acceptable supplier, which would impact our business, financial condition and results of operations. Even if such alternative suppliers are available on commercially reasonable terms, the changes could also result in increased costs associated with qualifying the new materials and in increased operating costs. Further, any prolonged disruption in a supplier’s operations could have a significant negative impact on our ability to manufacture and deliver products in a timely manner and as a result, our business, financial condition and results of operations could be adversely affected.
The manufacturing, sterilization and distribution of our Motiva Implants and other products are technically challenging. Changes that our suppliers may make, or additional requirements from regulatory agencies, are outside of our direct control and can have an impact on our processes, on quality, and on the successful delivery of products to our customers. Mistakes and mishandling are not uncommon and can affect supply and delivery. Some of these risks include:
•failure to complete sterilization on time or in compliance with the required regulatory standards;
•transportation and import and export risk, particularly given the global nature of our supply and distribution chains;
•delays in analytical results or failure of analytical techniques that we depend on for quality control and release of products;
•natural or other disasters, labor disputes, financial distress, lack of raw material supply, issues with facilities and equipment or other forms of disruption to business operations affecting our manufacturer or its suppliers;
48
•latent defects that may become apparent after products have been released and that may result in a recall of such products;
•contamination of our raw materials or manufactured products; and
•inclusion of vendors of raw materials not in compliance with ISO-13485 requirements.
As referenced above in this risk factor, some of the components used in our Motiva Implants and our other products are currently single-sourced, and substitutes for these components might not be obtained easily or may require substantial redesign or manufacturing modifications related to our specifications or due to regulatory requirements. Any significant problem experienced by one of our single-source suppliers may result in a delay or interruption in the supply of components or products to us because the number of third-party manufacturers with the necessary manufacturing and regulatory expertise and facilities is limited and certification of a new supplier may be complex and time consuming. Any delay or interruption would likely lead to a delay or interruption in our manufacturing or distribution operations and/or adversely affect our ability to sell Motiva Implants. The inclusion of substitute components or products must meet our specifications and could require us to qualify the new supplier with the appropriate regulatory authorities. The added time and cost to arrange for alternative suppliers could have a material adverse effect on our business. New manufacturers of any current or planned product would be required to qualify under applicable regulatory requirements and would need to have sufficient rights under applicable intellectual property laws to the design and method of manufacturing the planned product. Obtaining the necessary FDA or international approvals or other qualifications under applicable regulatory requirements and ensuring non-infringement of third-party intellectual property rights could result in a significant interruption of supply and could require the new manufacturer to bear significant additional costs that may be passed on to us.
A substantial proportion of our sales are through exclusive distributors, and we do not have direct control over the efforts these distributors may use to sell our products. If our relationships with these third-party distributors deteriorate, or if these third-party distributors fail to sell our products or engage in activities that harm our reputation, or fail to adhere to medical device regulations, our financial results may be negatively affected.
Historically, our sales model has been to sell primarily through distributors rather than through our own sales force, with the notable exception of Brazil and several European countries where we are selling directly, but, in the future, we may utilize a hybrid sales model that includes both distributors and a direct sales effort. We believe that our reliance on distributors improves the economics of our business, as we do not carry the high fixed costs of a direct sales force in many of the countries in which our Motiva Implants are sold. If we are unable to maintain or enter into such distribution arrangements on acceptable terms, or at all, we may not be able to successfully commercialize our products in certain countries. Furthermore, distributors can choose the level of effort that they apply to selling our products relative to others in their portfolio. The selection, training, and compensation of a distributors’ sales personnel are within their control rather than our own and may vary significantly in quality from distributor to distributor.
In addition, although our contract terms require our distributors to comply with all applicable laws regarding the sale of our products, including anti-competition, anti-money laundering, and sanctions laws, we may not be able to ensure proper compliance. If our distributors fail to effectively market and sell our products in full compliance with applicable laws, our results of operations and business may suffer.
Risks Related to Public Health Crises
Pandemics, epidemics, or other public health crises may adversely affect our business and financial results in the future, as was the case with the COVID-19 pandemic in recent years.
As was the case with the COVID-19 pandemic, which resulted in a material disruption of our operations in fiscal 2020 and to a lesser degree in fiscal 2021, we are subject to risks associated with pandemics, epidemics or other public health crises. The full extent to which any pandemic, epidemic, or public health crisis may, directly or indirectly, impact our business, results of operations and financial condition, including our sales, expenses, supply chain integrity, manufacturing capability, research and development activities, and employee-related compensation, is highly uncertain and will depend on future developments that are also highly uncertain and cannot be predicted with reasonable accuracy at this time, including, without limitation:
•the contagiousness or virulence of the virus, disease or other condition giving rise to the pandemic, epidemic or public health crisis;
49
•the scope and length of any governmental or other restrictions implemented to reduce the spread of virus, disease or other condition giving rise to the pandemic, epidemic or public health crisis or other actions required or recommended to contain or treat infected individuals;
•the deferral of procedures using our products or other adverse impact on patients’ willingness to undergo procedures in which our products could be used during or following any pandemic, epidemic or other public health crisis;
•volatility in the global capital markets, impacting access to and cost of capital;
•disruptions in the manufacture and distribution of our products and in our supply chain;
•delays in clinical trials;
•disruptions or restrictions on the ability of many of our employees, and of third parties on which we rely, to work effectively, including “stay-at-home” orders and similar government actions;
•temporary closures of our facilities and of the facilities of our customers and suppliers; and
•other direct and indirect economic impacts, both domestically and abroad, of a pandemic, epidemic, or public health crisis as a result of any or all of the foregoing, including actions taken by local, state, national and international governmental agencies, whether such impact affects customers, suppliers, or markets generally.
Risks Related to Intellectual Property and Data Security
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected, harming our business and competitive position.
In addition to our patented technology and products, we rely upon confidential proprietary information, including trade secrets, unpatented know-how, technology and other proprietary information, to develop and maintain our competitive position. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in the market. We seek to protect our confidential proprietary information, in part, by confidentiality agreements with our employees and our collaborators and consultants. We also have agreements with our employees and selected consultants that obligate them to assign their inventions to us. These agreements are designed to protect our proprietary information, however, we cannot be certain that our trade secrets and other confidential information will not be disclosed or that competitors will not otherwise gain access to our trade secrets, or that technology relevant to our business will not be independently developed by a person that is not a party to such an agreement. Furthermore, if the employees, consultants or collaborators that are parties to these agreements breach or violate the terms of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could be disclosed, misappropriated or otherwise become known or be independently discovered by our competitors. In addition, intellectual property laws in foreign countries may not protect trade secrets and confidential information to the same extent as the laws of the United States. If we are unable to prevent disclosure of the intellectual property related to our technologies to third parties, we may not be able to establish or maintain a competitive advantage in our market, which would harm our ability to protect our rights and have an adverse effect on our business.
If we are not able to obtain and maintain intellectual property protection for our products and technologies, or if the scope of our patents is not sufficiently broad, we may not be able to effectively maintain our market leading technology position.
Our success depends in large part on our ability to obtain and maintain patent and other intellectual property protection in the United States and in other countries with respect to our proprietary technology and products.
The patent position of medical device and diagnostic companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unresolved. In recent years, patent rights have been the subject of significant litigation. As a result, the issuance, scope, validity, enforceability and commercial value of the patent rights we rely on are highly uncertain. Pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of the patents we rely on or narrow the scope of our patent protection. The laws of other countries may not protect our rights to the same extent as the laws of the U.S. Publications of discoveries in the scientific literature often lag behind the actual
50
discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we or were the first to file for patent protection of such inventions.
Even if the patent applications we rely on issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and the patents we rely on may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop or prevent us from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new planned products, patents protecting such products might expire before or shortly after such products are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours or otherwise provide us with a competitive advantage.
We may not be able to protect or enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our planned products throughout the world may be prohibitively expensive to us. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as in the United States. These products may compete with our products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from competing. Many companies have encountered significant problems in protecting and defending intellectual property rights in international jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We may become involved in legal proceedings to protect or enforce our intellectual property rights, which could be expensive, time consuming, or unsuccessful.
Competitors may infringe or otherwise violate the patents we rely on, or our other intellectual property rights. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims that we assert against perceived infringers could also provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property rights. In addition, in an infringement proceeding, a court may decide that a patent we are asserting is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that the patents we are asserting do not cover the technology in question. An adverse result in any litigation proceeding could put one or more patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Interference or derivation proceedings provoked by third parties or brought by the U.S. Patent and Trademark Office, or USPTO, or any other patent authority may be necessary to determine the priority of inventions or other matters of inventorship with respect to patents and patent applications. We may become involved in proceedings, including oppositions, interferences, derivation proceedings, inter partes reviews, patent nullification proceedings, or re-examinations, challenging our patent rights or the patent rights of others, and the outcome of any such proceedings are highly uncertain. An adverse determination in any such proceeding could reduce the scope of, or invalidate, important patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Our business also could be harmed if a prevailing party does not offer us a license on commercially reasonable terms, if any license is offered at all. Litigation or other proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may also become involved in disputes with others regarding the ownership of intellectual property rights. If we are unable to resolve these disputes, we could lose valuable intellectual property rights.
51
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical or management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our common shares. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. Uncertainties resulting from the initiation and continuation of intellectual property litigation or other proceedings could have an adverse effect on our ability to compete in the marketplace.
The medical device industry is characterized by patent litigation and we could become subject to litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages or prevent us from marketing our existing or future products.
Patent litigation is prevalent in the medical device and diagnostic sectors. Our commercial success depends in part upon our ability and that of our distributors, contract manufacturers, and suppliers to manufacture, market and sell our planned products, and to use our proprietary technologies without infringing, misappropriating or otherwise violating the proprietary rights or intellectual property of third parties. We may become party to, or be threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology. Third parties may assert infringement claims against us based on existing or future intellectual property rights. If we are found to infringe a third-party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. We may also elect to enter into such a license in order to settle pending or threatened litigation. However, we may not be able to obtain any required license on commercially reasonable terms, or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us and could require us to pay significant royalties and other fees. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our planned products in commercially important territories, or force us to cease some of our business operations, which could harm our business. Many of our employees were previously employed at, and many of our current advisors and consultants are employed by, universities or other biotechnology, medical device or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, advisors and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we, or these employees, have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. These and other claims that we have misappropriated the confidential information or trade secrets of third parties can have a similar negative impact on our business to the infringement claims discussed above.
Even if we are successful in defending against intellectual property claims, litigation or other legal proceedings relating to such claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common shares. Such litigation or proceedings could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of litigation or other intellectual property related proceedings could have a material adverse effect on our ability to compete in the marketplace.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents or applications will be due to be paid by us to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents or applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In many cases, an inadvertent lapse can be cured
52
by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to use our technologies and this circumstance would have a material adverse effect on our business.
If we fail to comply with our obligations in our intellectual property agreements, we could lose intellectual property rights that are important to our business.
We are a party, and expect to become party in the future, to certain intellectual property agreements that impose various diligence, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with these obligations, any licensor may have the right to terminate such agreements, in which event we may not be able to develop and market any product that is covered by such agreements. Termination of such agreements, or reduction or elimination of our rights under such agreements, may result in our having to negotiate new or reinstated arrangements on less favorable terms, or our not having sufficient intellectual property rights to operate our business. The occurrence of such events could harm our business and financial condition.
The risks described elsewhere in this Annual Report on Form 10-K pertaining to our intellectual property rights also apply to any intellectual property rights that we may license, and any failure by us or any future licensor to obtain, maintain, defend and enforce these rights could have a material adverse effect on our business.
Our information systems, or those used by third parties which we rely on, may fail, be impacted by cybersecurity incidents, suffer other security breaches or be vulnerable to other forms of attack or damage.
The operation of our business depends on our information systems and, in some cases, the information systems used by third parties. We rely on our information systems to, among other things, effectively manage sales and marketing data, accounting and financial functions, inventory management, product development tasks, clinical data, customer service and technical support functions. Despite the implementation of security measures, our information systems, or those used by third parties which we rely on, are vulnerable to a variety of cybersecurity incidents and cybersecurity threats, as well as other forms of attack or damage, including damage or interruption from earthquakes, fires, floods and other natural disasters, terrorist attacks, power losses, computer system or data network failures, security breaches, data corruption, and cyberattacks. Cybersecurity incidents can include, but are not limited to, computer viruses, computer denial-of-service attacks, phishing attacks, ransomware attacks, worms, and other malicious software programs or other attacks, covert introduction of malware to computers and networks, social engineering or impersonation of authorized users, and efforts to discover and exploit any design flaws, bugs, security vulnerabilities, or security weaknesses, as well as intentional or unintentional acts by employees or other insiders with access privileges, intentional acts of vandalism by third parties and sabotage.
Attacks upon information systems are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, information systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience cybersecurity incidents or other security breaches that may remain undetected for an extended period. Even if identified, we may be unable to adequately investigate or remediate cybersecurity incidents or threats due to attackers increasingly using tools and techniques that are designed to circumvent controls, to avoid detection, and to remove or obfuscate forensic evidence.
While we have not experienced any material cybersecurity incident or other system failure or security breach to our knowledge to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations, leading to increased costs, product shortages or lost revenues. To the extent that any cybersecurity incident, security breach or other attack or damage to our information systems were to result in a loss or misuse of proprietary or confidential information, intellectual property, or sensitive or personal information, we could incur liability and the further development and commercialization of our current and future products could be delayed. For example, the loss of data from completed, ongoing or future studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. In addition, any such cybersecurity incident, security breach,
53
or other attack or damage could harm our reputation, erode customer confidence in the effectiveness of our security measures, and negatively impact our ability to attract new customers.
Our failure to adequately protect personal information in compliance with evolving legal requirements could harm our business.
In the ordinary course of our business, we collect and store sensitive data, including legally protected patient health information, credit card information and personally identifiable information. We collect this kind of information on our customers for purposes of servicing potential warranty claims and for post-marketing safety vigilance. These data protection and privacy-related laws and regulations are evolving and may result in ever-increasing regulatory and public scrutiny and escalating levels of enforcement and sanctions.
There are a number of state, federal and international laws protecting the privacy and security of health information and personal data. As part of the American Recovery and Reinvestment Act 2009, or ARRA, Congress amended the privacy and security provisions of the Health Insurance Portability and Accountability Act, or HIPAA. HIPAA imposes limitations on the use and disclosure of an individual’s protected health information by certain health care providers, health care clearinghouses, and health insurance plans, collectively referred to as covered entities, that involve the creation, use, maintenance or transmission of protected health information. The HIPAA amendments also impose compliance obligations and corresponding penalties for non-compliance on individuals and entities that perform a function or provide specified services to health care providers and other covered entities, collectively referred to as business associates. Most recently, on December 10, 2020, HHS issued a Notice of Proposed Rulemaking, which if finalized, would make changes to some of HIPAA’s regulatory requirements, which would impact us, to the extent we are a business associate. ARRA also made significant increases in the penalties for improper use or disclosure of an individual’s protected health information under HIPAA and extended enforcement authority to state attorneys general. The amendments also create notification requirements for individuals whose protected health information has been inappropriately accessed or disclosed, notification requirements to federal regulators and in some cases, notification to local and national media. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with encryption or other standards developed by the U.S. Department of Health and Human Services, or HHS. Most states have laws requiring notification of affected individuals and state regulators in the event of a breach of personal information, which is a broader class of information than the protected health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms to ensure ongoing protection of personal information.
In addition, even when HIPAA does not apply, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the FTCA, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Medical data is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule.
Many foreign countries and governmental bodies, including the EU, Canada, Australia and other relevant jurisdictions, have laws and regulations concerning the collection and use of personal or sensitive data obtained from their residents or by businesses operating within their jurisdiction. For example, the European Commission recently adopted the General Data Protection Regulation, including as implemented in the UK, or the GDPR, effective on May 25, 2018, that supersedes current EU data protection legislation, imposes more stringent EU data protection requirements and provides for greater penalties for noncompliance. The GDPR regulates the processing of personal data and places certain obligations on the processing of such personal data including ensuring the lawfulness of processing personal data (including obtaining valid consent of the individuals to whom the personal data relates, where applicable), the processing details disclosed to the individuals, the adequacy, relevance and necessity of the personal data collected, the retention of personal data collected, the sharing of personal data with third parties, the transfer of personal data out of the European Economic Area/UK to third countries including the US, contracting requirements (such as with clinical trial sites and vendors), the use of personal data in accordance with individual rights, the security of personal data and security breach/incident notifications. Non-compliance with the GDPR can trigger steep fines for the most serious breaches of up to €20 million or 4% of total worldwide annual revenues, whichever is higher. Given the breadth and depth of changes in data protection obligations, meeting the GDPR’s requirements requires time, resources and a review of the technology and systems currently in use against the GDPR’s requirements.
54
We may be at risk of enforcement actions taken by certain EU data protection authorities while we continue to build our business practices to ensure that all transfers of personal data to us from the European Economic Area, including the EU, United Kingdom and Switzerland, are conducted in compliance with all applicable regulatory obligations, the guidance of data protection authorities and evolving best practices. We may find it necessary to establish systems to maintain personal data originating from the EU in the European Economic Area, which may involve substantial expense and may cause us to need to divert resources from other aspects of our business, all of which may adversely affect our business.
Our failure to comply with applicable laws and regulations, or to protect such data, could result in enforcement actions against us, including fines, imprisonment of company officials and public censure, claims for damages by end-customers and other affected individuals, damage to our reputation and loss of goodwill, any of which could harm on our operations, financial performance, and business. Evolving and changing definitions of personal data and personal information, within the European Union, the United States, and elsewhere, may limit or inhibit our ability to operate or expand our business, including limiting strategic partnerships that may involve the sharing of data. Moreover, if the relevant laws and regulations change, or are interpreted and applied in a manner that is inconsistent with our data practices or the operation of our products, we may need to expend resources in order to change our business operations, data practices, or the manner in which our products operate. Even the perception of privacy concerns, whether or not valid, may harm our reputation and inhibit adoption of our products.
There is the risk that the limits we obtained for our cyber liability insurance may not cover the total loss experienced in the event of a data security incident, including the financial loss, legal costs, and business and reputational harm, particularly if there is an interruption to our systems. Additionally, there is the risk of a data privacy or security incident by an employee, which may expose us to liability. If personal information of our customers or employees is misappropriated, our reputation with our customers and employees may be injured resulting in loss of business and/or morale, and we may incur costs to remediate possible injury to our customers and employees or be required to pay fines or take other action with respect to judicial or regulatory actions arising out of such incidents.
Risks Related to Regulatory and Political Environment
The regulatory approval process is expensive, time consuming and uncertain. Our future success depends on our ability to develop, receive regulatory clearance or approval for, and introduce new products that will be accepted by the market in a timely manner. There is no guarantee that the FDA will authorize the commercialization of Motiva Implants or our planned products on a timely basis, if at all, and failure to obtain necessary clearances or approvals would adversely affect our ability to grow our business.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of medical devices are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, where regulations differ from country to country. Our products are registered to be sold in 86 countries. In the United States, we have received clearance from the FDA to sell our Intraoperative Sizers and Flora Tissue Expanders, but we are not permitted to market other planned products until we receive the requisite FDA approval or clearance.
The two primary types of FDA marketing authorization in the United States applicable to a device are a premarket approval (by filing a PMA) or a premarket notification clearance (by filing a 510(k) notice). Breast implants are currently classified as Class III Medical Devices requiring an approved PMA for commercial distribution. Certain of our other products or modifications to those products are currently eligible for 510(k) clearance or approval of a PMA supplement. We have not received marketing approval for Motiva Implants in the United States. We have received 501(k) clearance for the Intraoperative Sizers and Flora Tissue Expanders, which are now commercially available in the United States.
Obtaining approval for sale of a medical device from the FDA can be a lengthy, expensive and uncertain process. The PMA approval process generally takes from one to three years and the 510(k) clearance process usually takes from three to twelve months, although each could take longer.
Prior to receiving approval to commercialize any of our planned products in the United States or abroad, we may be required to demonstrate with substantial evidence from preclinical and well-controlled clinical studies, and to the satisfaction of the FDA or other regulatory authorities abroad, that such planned products are safe and effective for their intended uses. We may not successfully complete required clinical trials, or they may yield
55
results which are different than anticipated. Results from preclinical studies and clinical studies can be interpreted in different ways. Even if we believe the preclinical or clinical data for our planned products are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities. Administering any of our planned products to humans may produce undesirable side effects, which could interrupt, delay or cause suspension of clinical studies of our planned products and result in the FDA or other regulatory authorities denying approval of our planned products for any or all targeted indications.
Regulatory approval from the FDA is not guaranteed, and the approval process is expensive and may take several years, particularly where a PMA is required. The FDA also has substantial discretion in the approval process. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon or repeat clinical studies or perform additional preclinical studies and clinical studies. The number of preclinical studies and clinical studies that will be required for FDA approval varies depending on the planned product, the indication that the planned product is designed to address and the regulations applicable to any particular planned product. The FDA can delay, limit or deny approval of a planned product for many reasons, including, but not limited to, the following:
▪a planned product or one or more of its features may not be deemed safe or effective;
▪FDA officials may not find the data from preclinical studies and clinical studies sufficient;
▪the FDA might not approve our manufacturing or our third-party supplier’s processes or facilities; or
▪the FDA may change its approval policies or adopt new regulations.
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions that may prevent or delay approval or clearance of our products under development or impact our ability to modify our currently approved or cleared products on a timely basis. The FDA could also reclassify some or all of our products that are currently classified as Class II to Class III requiring additional controls, clinical studies and submission and approval of a PMA for us to market and sell those products. Any change in the FDA’s approval policies or new or amended regulations governing the clearance and approval processes could increase the costs of obtaining approval/clearance of a product or result in delays in, or failure to receive or maintain clearance or approval for our products.
If Motiva Implants or any planned products fail to demonstrate safety and effectiveness in preclinical and clinical studies, if there is a change in the FDA’s approval policies or new or amended regulations governing the clearance and approval process for our products, or if our products do not gain regulatory approval or clearance, our business and results of operations will be harmed.
Once commercialized, modifications to our marketed products may require new 510(k) clearances or approval of PMA supplements, or equivalent steps in other countries, or may require us to cease marketing or recall the modified products until certification, clearances or regulatory approvals are obtained.
Modifications to any of our products once they are commercialized may require new regulatory approvals or clearances, including 510(k) clearances or approval of PMA supplements, or require us to recall or cease marketing the modified systems until these clearances or approvals are obtained. The FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new approval, supplement or clearance. A manufacturer may determine that a modification could not affect safety or efficacy and does not represent a major change in its intended use, so that no new clearance or approval is necessary. However, the FDA can review a manufacturer’s decision and may disagree. The FDA may also on its own initiative determine that a new clearance or approval of a PMA supplement is required. We may make modifications in the future that we believe do not or will not require additional clearances or approvals. If the FDA disagrees and requires new clearances or approvals for the modifications, we may be required to recall and to stop marketing our products as modified, which could require us to redesign our products and/or seek new marketing authorizations and harm our operating results. In these circumstances, we may be subject to significant enforcement actions.
For example, if a manufacturer determines that a modification to a PMA approved device could affect its safety or effectiveness or would constitute a major change in its intended use, then the manufacturer must file for a new a new PMA or approval of a PMA supplement. Where we determine that modifications to our products require a new PMA approval, we may not be able to obtain those additional approvals for the modifications or additional indications in a timely manner, or at all. Obtaining new approvals can be a time-consuming process, and delays in
56
obtaining required future approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
For those products sold in the EEA, we must notify our EU notified body if significant changes are made to the products or if there are substantial changes to our quality assurance systems affecting those products. Obtaining certification can be a time-consuming process, and delays in obtaining required future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Even if we receive regulatory approval for a planned product, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and subject us to penalties if we fail to comply with applicable regulatory requirements.
When a regulatory approval is obtained, the approved product and its manufacturer are subject to continual review by the FDA or non-U.S. regulatory authorities. Our regulatory approval for Motiva Implants, as well as any regulatory approval that we receive for future modifications to Motiva Implants or for any planned products may be subject to limitations on the indicated uses for which the product may be marketed. Future approvals may contain requirements for potentially costly post-marketing follow-up studies to monitor the safety and effectiveness of the approved product in real-world post-market use. In addition, we are subject to extensive and ongoing regulatory requirements by the FDA and other regulatory authorities with regard to the labeling, packaging, adverse event quarterly reporting, device tracking, device retrieval studies, storage, advertising, promotion and recordkeeping for our products. We are also required to comply with regulations regarding the manufacture of Motiva Implants, which include requirements related to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Further, regulatory authorities must inspect these manufacturing facilities and determine that they are in compliance with FDA good manufacturing practice requirements as set forth in the Quality System Regulation, or QSR, before the products can be approved. These facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with the QSR and associated regulations. Failure to comply with FDA and other applicable U.S. and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including the following:
•Warning Letters;
•civil or criminal penalties and fines;
•injunctions;
•suspension or withdrawal of regulatory approval;
•suspension of any ongoing clinical studies;
•voluntary or mandatory product recalls and publicity requirements;
•refusal to accept or approve applications for marketing approval of new devices or supplements to approved applications filed by us;
•restrictions on operations, including costly new manufacturing requirements; or
•seizure or detention of our products or import bans.
Our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.
Our products are subject to medical device reporting regulations, which require us to report to the FDA any information that reasonably suggests one of our products may have caused or contributed to a death or serious injury, or one of our products malfunctioned and, if the malfunction were to recur, this device or a similar device that we market would be likely to cause or contribute to a death or serious injury. Our obligation to report under the medical device reporting regulations is triggered on the date on which we become aware of information that reasonably suggests a reportable adverse event occurred. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event, it is an adverse event that is unexpected or if the product characteristic that caused the adverse event is removed in time from our products. If
57
we fail to comply with our medical device reporting obligations, the FDA could issue warning letters or untitled letters, take administrative actions, commence criminal prosecution, impose civil monetary penalties, demand or initiate a product recall, seize our products, or delay the clearance of our future products.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new clearances or approvals for the device before we may market or distribute the corrected device. Seeking such clearances or approvals may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties or civil or criminal fines, which may harm our reputation and have a material adverse effect on our business.
We are subject to extensive and dynamic medical device regulation, and oversight in the United States and other countries. If we fail to obtain or maintain necessary regulatory approvals for our products, or if approvals or clearances for future products are delayed or not issued, it will negatively affect our business, financial condition and results of operations.
Our products, marketing, sales and development activities and manufacturing processes are subject to extensive and rigorous regulation by various regulatory agencies and governing bodies. Under the US Food, Drug and Cosmetic Act (FDC Act), medical devices must receive FDA clearance or approval or an exemption from such clearance or approval before they can be commercially marketed in the U.S. Additionally, such devices are subject to wide-ranging regulations including, among other things: product design, development, manufacture and release; laboratory and clinical testing, labeling, packaging, storage, and distribution; product safety and effectiveness; record-keeping; product promotion and advertising; post-marketing surveillance; post-market approval studies, where applicable; and import and export rules. In the EU, we are required to comply with applicable medical device directives (including the Medical Devices Directive and the European Medical Device Regulation) and obtain CE Mark certification in order to market medical devices. In addition, exported devices are subject to the regulatory requirements of each country to which the device is exported.
The FDA or other regulators could delay, limit, or deny clearance or approval of a device for many reasons, including:
•our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory entity or notified body that our devices and any accessories are substantially equivalent to a legally marketed predicate device or safe or effective for their proposed intended uses;
•disagreement of the FDA with the design or implementation of any clinical trials or the interpretation of data from preclinical studies or clinical trials;
•serious and unexpected adverse device effects experienced by participants in our clinical trials;
•insufficiency of the data from preclinical studies or clinical trials to support clearance or approval;
•our inability to demonstrate that the clinical and other benefits of the device outweigh the risks;
•failure of our manufacturing process or facilities to meet applicable requirements; and
•the potential for approval policies or regulations of the FDA or applicable foreign regulatory bodies to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval.
Many countries require that product approvals be renewed or recertified on a regular basis, generally every four to five years. The renewal or recertification process requires that we evaluate any device changes and any new regulations or standards relevant to the device and conduct appropriate testing to document continued compliance. Where renewal or recertification applications are required, they may need to be renewed and/or approved in order to continue selling our products in those countries. There can be no assurance that we will receive the required approvals for new products or modifications to existing products on a timely basis or that any approval will not be subsequently withdrawn or conditioned upon extensive post-market study requirements.
The European Union regulatory bodies finalized a new Medical Device Regulation (MDR) in 2017, which replaced the existing directives and provided three years for transition and compliance. After a one-year delay, the MDR became effective on May 26, 2021. The MDR changes several aspects of the existing regulatory framework, such as updating clinical data requirements and introducing new ones, such as Unique Device Identification, or UDI.
58
We and the Notified Bodies who will oversee compliance to the new MDR face uncertainties as the MDR is rolled out and enforced by the Commission and EEA Competent Authorities, creating risks in several areas, including the CE Marking process and data transparency, in the upcoming years. Additionally, the U.K.’s withdrawal from the EU and the end of the mutual recognition and related trade facilitating effects for medical devices between the EU and Switzerland in May 2021 have added certain costs and complexities to the shipment and sales of our products in those countries.
Regulations regarding the development, manufacture and sale of medical devices are evolving and subject to future change. We cannot predict what impact, if any, those changes might have on our business. Failure to comply with regulatory requirements could have a material adverse effect on our business, financial condition and results of operations. Later discovery of previously unknown problems with a product or manufacturer could result in fines, delays or suspensions of regulatory clearances or approvals, seizures or recalls of products, physician advisories or other field actions, operating restrictions and/or criminal prosecution. We may also initiate field actions as a result of a failure to strictly comply with our internal quality policies. The failure to receive product approval clearance on a timely basis, suspensions of regulatory clearances or approvals, seizures or recalls of products, physician advisories or other field actions, or the withdrawal of product approval or clearance by regulatory authorities could have a material adverse effect on our business, financial condition or results of operations.
Our products, such as Motiva Implants, may in the future be subject to product recalls that could harm our reputation, business and financial results.
Medical devices can experience performance problems in the field that require review and possible corrective action. The occurrence of component failures, manufacturing errors, design defects or labeling inadequacies affecting a medical device could lead to a government-mandated or voluntary recall by the device manufacturer, in particular when such deficiencies may endanger health. The FDA requires that certain classifications of recalls be reported to the agency within 10 working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA. We may initiate voluntary recalls involving Motiva Implants or other planned devices in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. Product recalls may divert management attention and financial resources, expose us to product liability or other claims, harm our reputation with customers and adversely impact our business, financial condition and results of operations.
The medical technology industry is complex and intensely regulated at the federal, state, and local levels and government authorities may determine that we have failed to comply with applicable laws or regulations.
As a company which manufactures and distributes medical devices and technologies, we are subject to numerous regional, national and local laws and regulations. There are significant costs involved in complying with these laws and regulations. Moreover, if we are found to have violated any applicable laws or regulations, we could be subject to civil and/or criminal damages, fines, sanctions or penalties, including exclusion from participation in governmental healthcare programs. We may also be required to change our method of operations. These consequences could be the result of current conduct or even conduct that occurred a number of years ago. We also could incur significant costs merely if we become the subject of an investigation or legal proceeding alleging a violation of these laws and regulations. We cannot predict whether any government authority will determine that we are not operating in accordance with law, or whether the laws will change in the future and impact our business.
Under some circumstances, government investigations can also be initiated by private individuals under whistleblower provisions which may be incentivized by the possibility for private recoveries. Responding to inquiries and enforcement activities can be costly and disruptive to our business operations, even when the allegations are without merit. We also may be subject to other financial sanctions or be required to modify our operations. Any of these actions could have a material adverse effect on our business, financial condition and results of operations.
If we obtain approval for our products, we may be subject to enforcement action if we engage in improper marketing or promotion of Motiva Implants or our other products.
We have obtained 501(k) clearance to sell Intraoperative Sizers and SmoothSilk Tissue Expanders in the United States. We are not permitted to promote or market other investigational products. After approval, our promotional
59
materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of unapproved, or off-label, use. Surgeons may use our products off-label, as the FDA does not restrict or regulate a surgeon’s choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine, or criminal penalties. It is also possible that other federal, state, or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an off-label use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products could be impaired. In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention, result in substantial damage awards against us, and harm our reputation.
Our relationship with customers and third party payors will be subject to applicable anti-kickback, fraud and abuse, and other health care laws and regulations, which could expose us to criminal sanctions, civil penalties, damages, reputational harm and diminished profits and future earnings.
Our future arrangements with third-party payors and health care providers may expose us to broadly applicable fraud and abuse and other health care laws and regulations that may constrain the business or the financial relationships and engagement we enter into to market, sell, promote and distribute our products for which we obtain clearance in the U.S. Efforts to ensure that our business arrangements with these third parties will comply with applicable health care laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other health care laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. The requirements and restrictions under these broadly applicable laws and regulations are described in the section on Fraud and Abuse Laws in Item 1. Business.
Health care reform measures could hinder or prevent our planned products’ commercial success.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of planned or future products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain clearance or approval for, manufacture, market or distribute our products. For example, In January of 2024, FDA issued a final rule to replace the QSR with the adoption of ISO 13485. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require: additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping.
In the United States, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the health care system in ways that could affect our future revenue and future profitability and the future revenue and future profitability of our potential customers. Federal and state lawmakers regularly propose and, at times, enact legislation that could result in significant changes to the health care system, some of which are intended to contain or reduce the costs of medical products and services.
60
For example, one of the most significant health care reform measures in decades, the Patient Protection and Affordable Care Act, or PPACA, was enacted in 2010. The PPACA contains a number of provisions, including those governing enrollment in federal health care programs, reimbursement changes and fraud and abuse measures, all of which will impact existing government health care programs and will result in the development of new programs. There have been judicial and Congressional challenges to certain aspects of the PPACA. Furthermore, the Tax Cuts and Jobs Act passed in December of 2017 included a provision repealing the PPACA’s individual mandate penalty, the tax-based shared responsibility payment on certain individuals who fail to maintain qualifying health coverage for all or part of a year. Congress may consider other legislation to repeal or replace elements of the PPACA on a provision-by-provision basis. We cannot assure you that the PPACA, as currently enacted or as amended in the future, will not adversely affect our business and financial results and we cannot predict how future federal or state legislative or administrative changes relating to health care reform will affect our business.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. For example, the Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals for spending reductions to Congress. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, which triggered the legislation’s automatic reduction to several government programs, including aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. Sequestration is currently set at 2% and will increase to 2.25% for the first half of fiscal year 2030, to 3% for the second half of fiscal year 2030, and to 4% for the remainder of the sequestration period that lasts through the first six months of fiscal year 2031.
We cannot predict the impact that ongoing health care reform will have on our business, and there is uncertainty as to what healthcare programs and regulations may be implemented or changed at the federal and/or state level in the United States, and as to the effect of any future legislation or regulation. However, it is possible that such initiatives could have an adverse effect on our ability to obtain approval and/or successfully commercialize products in the United States in the future. For example, any changes that reduce, or impede the ability to obtain, reimbursement for the type of products we intend to commercialize in the United States (or our products more specifically, if approved) or reduce medical procedure volumes could adversely affect our business plan to introduce our products in the United States.
There likely will continue to be legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care. We cannot predict the initiatives that may be adopted in the future or their full impact. The continuing efforts of the government, insurance companies, managed care organizations and other payers of health care services to contain or reduce costs of health care may adversely affect:
▪our ability to set a price that we believe is fair for our products;
▪our ability to generate revenue and achieve or maintain profitability; and
▪the availability of capital.
The coverage and reimbursement status of newly-approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue.
Market acceptance and sales of any one or more of our product candidates will depend on reimbursement policies and may be affected by future healthcare reform measures in the United States and in foreign jurisdictions. Government authorities and third-party payers, such as private health insurers and health maintenance organizations, decide which products they will cover and establish payment levels. We cannot be certain that reimbursement will be available for any of our products or product candidates. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for, our products. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize any product candidates that we develop.
The pricing, coverage and reimbursement of our product candidates must be adequate to support our commercial infrastructure. Our per-patient prices must be sufficient to recover our development and manufacturing costs and potentially achieve profitability. However, sales of any product depend, in part, on the extent to which such product will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations, and the level of reimbursement for such product by third-party payors. Significant uncertainty exists as to the coverage and reimbursement status of any newly approved product. Decisions regarding the extent of coverage and amount of
61
reimbursement to be provided are made on a plan-by-plan basis. One third-party payor’s decision to cover a product does not ensure that other payors will also provide coverage for the product. As a result, we do not have assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
Further, third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost effectiveness of medical products and product candidates. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit or delay sales of any of our future products. A decision by a third-party payor not to cover a product could reduce patient demand for any of our products.
The political situation in the United States can affect the ability of our company to conduct business in certain areas or countries if new trade conditions are imposed or enforced by the U.S. government.
There could be negative consequences to our company’s revenue if the U.S. government unexpectedly changes its trade policies towards determined geographies or countries. These policy changes can include such things as trade barriers, which serve to limit or prevent international trade. The U.S. government may request additional funds or tariffs in exchange for the right to export items into the country. Tariffs or quotas may be used to protect domestic producers from foreign competition. Changes may include the modification or withdrawal of free trade agreements already in place. This also can have a large effect on the profits of our company because it either cuts revenues as a result of a tax on imports/exports or restricts the amount of revenues that can be earned.
The global economic instability caused by the ongoing military conflicts in Ukraine and Israel may adversely affect our business in Europe and the Middle East.
The conflicts in Ukraine and Israel have resulted in worldwide geopolitical and macroeconomic uncertainty and have caused, and will likely continue to cause, disruption and instability in affected markets. It is not possible at this time to predict the ultimate consequences of the conflicts or the responses of governments or others to the conflicts, which could include, among other things, additional sanctions, greater regional instability or expansion of the conflict, embargoes, geopolitical shifts, litigation, and impacts on macroeconomic conditions, commodities, currency exchange rates, supply chains, and financial markets, which could adversely impact our business and results of operations.
Risks Related to Taxation
Tax authorities may disagree with our positions and conclusions regarding certain tax positions, resulting in unanticipated costs, taxes or non-realization of expected benefits.
A tax authority may disagree with tax positions that we have taken, which could result in increased tax liabilities. For example, the U.S. Internal Revenue Service or another tax authority could challenge the amounts paid between our affiliated companies pursuant to our intercompany arrangements and transfer pricing policies. A tax authority may take the position that material income tax liabilities, interest and penalties are payable by us, in which case, we expect that we might contest such assessment. Contesting such an assessment may be lengthy and costly and if we were unsuccessful in disputing the assessment, the implications could increase our anticipated effective tax rate, where applicable. In addition, we may be subject to additional tax liabilities, which could materially and adversely affect our business, financial condition and results of operations. The application, interpretation and enforcement of value-added tax, or VAT, and other taxes and related regulations applicable to medical device companies is complex and evolving.
We are a multinational organization faced with increasingly complex tax issues in many jurisdictions, and changes in tax laws or their application to the operation of our business could adversely impact our operating results and our business.
We conduct operations in multiple jurisdictions, and we are subject to certain taxes, including income, sales and use, employment, value added and other taxes, in the United States and other jurisdictions in which we do business. A change in the tax laws in the jurisdictions in which we do business, including an increase in tax rates or an adverse change in the treatment of an item of income or expense, possibly with retroactive effect, could result in a material increase in the amount of taxes we incur.
Our determination of our tax liability is subject to review by applicable U.S. and foreign tax authorities. Any adverse outcome of such a review could harm our operating results and financial condition. The determination of our worldwide provision for income taxes and other tax liabilities requires significant judgment and, in the ordinary
62
course of business, there are many transactions and calculations where the ultimate tax determination is complex and uncertain. Moreover, as a multinational business, we have subsidiaries that engage in many intercompany transactions in a variety of tax jurisdictions where the ultimate tax determination is complex and uncertain. The taxing authorities of the jurisdictions in which we operate may challenge our methodologies, which could impact our financial position and operating results. Historically, we have allocated some of our employees’ and contractors’ time across multiple business entities in the international jurisdictions in which we operate. If it were determined that we had misclassified our employees’ or contractors’ employment status or certain of our expenditures under local laws, we may be subjected to penalties or be required to pay withholding taxes, extend employee benefits, provide compensation for unpaid overtime, or otherwise incur substantially greater expenses with respect to such employees and contractors. Any of the foregoing circumstances could have a material adverse impact on our operating results and financial condition.
In addition, the European Union Economic and Financial Affairs Council has in recent years released a list of non-cooperative jurisdictions for tax purposes. The stated aim of the list is to promote good governance worldwide in order to maximize efforts to promote fair tax competition and address harmful tax practices. In February 2023, Costa Rica was added to this list, which now includes 16 jurisdictions. According to the European Union Economic and Financial Affairs Council, Costa Rica was added to the list because it has not fulfilled a commitment to abolish or amend harmful aspects of its foreign source income exemption regime. In October 2023, the EU Council announced that three jurisdictions have been removed from the list of non-cooperative tax jurisdiction, one of which was Costa Rica. This follows the reforms made to the Costa Rica’s Income Tax Law, amending aspects of the foreign-source income exemption regime. The reforms include a clarification to the scope of the territoriality principle and introduction of a new taxation regime for foreign-source passive income.
We are periodically reviewed and audited by tax authorities with respect to income and non-income taxes. Tax authorities may disagree with certain positions we have taken, and we may have exposure to additional income and non-income tax liabilities which could have an adverse effect on our operating results and financial condition. Such authorities could impose additional taxes, interest and penalties, claim that various withholding requirements apply to us or our subsidiaries or assert that benefits of tax treaties are not available to us or our subsidiaries. In addition, our future effective tax rates could be favorably or unfavorably affected by changes in tax rates, changes in the valuation of our deferred tax assets or liabilities, the effectiveness of our tax planning strategies, or changes in tax laws or their interpretation. Such changes could have an adverse impact on our financial condition.
As a result of these and other factors, the ultimate amount of tax obligations owed may differ from the amounts recorded in our financial statements and any such difference may harm our operating results in future periods in which we change our estimates of our tax obligations or in which the ultimate tax outcome is determined.
Our ability to use net operating losses to offset future taxable income and certain other tax attributes may be subject to certain limitations.
Federal and California laws impose restrictions on the utilization of net operating loss carryforwards and research and development credit carryforwards in the event of a change in ownership of the Company, which constitutes an “ownership change” as defined by Internal Revenue Code Sections 382 and 383. Generally, an ownership change occurs if the percentage of the value of the shares that are owned by one or more direct or indirect “five percent shareholders” increases by more than 50% over their lowest ownership percentage at any time during the applicable testing period. If we have experienced an “ownership change” at any time since our formation, we may already be subject to limitations on our ability to utilize our existing net operating losses and other tax attributes. We have not experienced an ownership change in the past that would materially impact the availability of its net operating losses and tax credits. Nevertheless, future changes in our share ownership, which may be outside of our control, may trigger an “ownership change” and, consequently, Section 382 and 383 limitations. We have not completed a Section 382 and 383 analysis to determine if an ownership change has occurred. Until such analysis is completed, we cannot be sure that the full amount of the existing net operating loss carryforwards will be available to us, even if we do generate taxable income before their expiration. In addition, under the newly enacted U.S. federal income tax law, federal net operating losses incurred in 2018 and in future years may be carried forward indefinitely, but the deductibility of such federal net operating losses is limited.
U.S. holders of our common shares may suffer adverse tax consequences if we are characterized as a passive foreign investment company.
A non-U.S. corporation will be classified as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes, in any taxable year in which either (1) at least 75% of its gross income is passive income; or
63
(2) at least 50% of the average quarterly value of its total gross assets is attributable to assets that produce “passive income” or are held for the production of passive income. Based on the project composition of our income and valuation of our assets, we do not believe we were a PFIC in 2023 and 2022, and we do not expect to become one in the future. However, because our PFIC status is subject to a number of uncertainties, neither we nor our tax advisors can provide any assurances regarding our PFIC status. If we are a PFIC for any taxable year during which a U.S. holder holds our common shares, the U.S. holder may be subject to adverse tax consequences. U.S. investors should consult their advisors regarding the application of these rules and the availability of any potential elections.
If a United States person is treated as owning at least 10% of our common shares, such holder may be subject to adverse U.S. federal income tax consequences.
If a United States person is treated as owning (directly, indirectly, or constructively) at least 10% of the value or voting power of our ordinary shares, such person may be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group (if any). We may become a controlled foreign corporation. In addition, because our group includes one or more U.S. subsidiaries, certain of our non-U.S. subsidiaries could be treated as controlled foreign corporations (regardless of whether or not we are treated as a controlled foreign corporation). A U.S. shareholder of a controlled foreign corporation may be required to report annually and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income,” and investments in U.S. property by controlled foreign corporations, regardless of whether we make any distributions. An individual that is a U.S. shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a U.S. shareholder that is a U.S. corporation. Failure to comply with these reporting obligations may subject a U.S. shareholder to significant monetary penalties and may prevent the statute of limitations with respect to such shareholder’s U.S. federal income tax return for the year for which reporting was due from starting. We cannot provide any assurances that we will assist investors in determining whether we or any of our non-U.S. subsidiaries is treated as a controlled foreign corporation or whether any investor is treated as a U.S. shareholder with respect to any such controlled foreign corporation or furnish to any U.S. shareholders information that may be necessary to comply with the aforementioned reporting and tax paying obligations. A U.S. investor should consult its advisors regarding the potential application of these rules to an investment in our common shares.
Discontinuation of preferential tax treatments we currently enjoy or other unfavorable changes in tax law could result in additional compliance obligations and costs.
Discontinuation of preferential tax treatments we currently enjoy or other unfavorable changes in tax law could result in additional compliance obligations and costs. We are currently the beneficiary of a tax holiday in Costa Rica pursuant to which we are subject to a tax at a 0% rate. The tax holiday is effective through December 31, 2030, and it may be extended if certain additional requirements are satisfied. However, there can be no assurance that we will continue to qualify for or receive such favorable tax treatment after the expiration date. If we fail to maintain such favorable tax treatment, we may be subject to tax in Costa Rica at a significantly higher rate.
Risks Related to Ownership of Our Securities
Our share price may be volatile, and purchasers of our securities could incur substantial losses.
The price at which our common shares trade may be volatile. The securities markets in general, and the market for biotechnology and medical device companies in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. Additionally, the lack of an active market may impair the value of our common shares, or your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. Although our common shares are listed on Nasdaq, if we fail to satisfy the continued listing standards, we could be delisted, which would negatively impact the price of our common shares. The market price for our shares may be influenced by many factors, including the following:
•our ability to successfully commercialize, and realize revenues from sales of, Motiva Implants;
▪the success of competitive products or technologies;
▪results of clinical studies of Motiva Implants or planned products or those of our competitors;
•regulatory or legal developments in the United States and other countries, especially changes in laws or regulations applicable to our products;
•introductions and announcements of new products by us, our commercialization partners, or our competitors, and the timing of these introductions or announcements;
64
•actions taken by regulatory agencies with respect to our products, clinical studies, manufacturing processes or sales and marketing terms;
•variations in our financial results or those of companies that are perceived to be similar to us;
•the success of our efforts to acquire or in-license additional products or planned products;
•developments concerning our collaborations, including but not limited to those with our sources of manufacturing supply and our commercialization partners;
•developments concerning our ability to bring our manufacturing processes to scale in a cost-effective manner;
•announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
•developments or disputes concerning patents or other proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our products;
•our ability or inability to raise additional capital and the terms on which we raise it;
•the recruitment or departure of key personnel;
•changes in the structure of health care payment systems;
•negative shifts in the economy effecting the number of aesthetic breast procedures;
•market conditions in the pharmaceutical and biotechnology sectors;
•actual or anticipated changes in earnings estimates or changes in securities analyst recommendations regarding our common shares, other comparable companies or our industry generally;
•trading volume of our common shares;
•sales of our common shares by us or our shareholders;
•short selling activities;
•the impact of pandemics, epidemics or other public health crises;
•general economic, industry and market conditions; and
•the other risks described in this “Risk Factors” section.
These broad market and industry factors may harm the market price of our common shares, regardless of our operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could adversely affect our business, financial condition, results of operations and growth prospects.
We identified a material weakness in our internal control over financial reporting as of December 31, 2023, 2022, and 2021, and may identify additional material weaknesses in the future that may cause us to fail to meet our reporting obligations or result in material misstatements of our consolidated financial statements. If we fail to establish and maintain effective control over financial reporting, our ability to accurately and timely report our financial results could be adversely affected.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. generally accepted accounting principles. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Prior to the completion of our IPO, we were a private company with limited accounting and compliance personnel and other resources to address our internal control over financial reporting.
On December 31, 2021, it was determined that our primary user access controls (i.e. provisioning, de-provisioning, and quarterly user access review) to ensure appropriate segregation of duties that would adequately restrict user and privileged access to the financially relevant systems and data to appropriate Company personnel were not operating effectively. These user access control deficiencies resulted in a lack of segregation of duties with respect to certain user roles. Automated process-level controls and manual controls that are dependent upon the information derived from such financially relevant systems were also determined to be ineffective as a result of such deficiency. During the fourth quarter of 2023, we completed our testing of the operating effectiveness of the
65
implemented controls and found them to be effective. As a result, we have concluded the material weakness identified as of December 31, 2021 has been remediated as of December, 31 2023.
It was determined that as of December 31, 2023, our primary change management controls for direct database changes were not designed and implemented effectively for specific localities. Other Information Technology General Controls, automated process-level controls, and manual controls that are dependent upon the information derived from such financially relevant systems were also determined to be ineffective as a result of such deficiency. We have made significant progress remediating change management controls as of December 31, 2023. We improved policies and procedures and designed and documented more effective change management monitoring controls, through the use of systematic audit logging, that addresses the relevant risks in order to remediate the identified material weakness. The material weakness persists and will not be considered remediated until the applicable controls operate for a sufficient period of time, and management has concluded, through testing, that the control objective is achieved, and the controls are operating effectively. We expect that the remediation of this material weakness will be completed during 2024.
We also expect that we will need to continue to improve existing, and implement new operational and financial systems, procedures and controls to manage our business effectively. Any delay in the implementation of, or disruption in the transition to, new or enhanced systems, procedures or controls, may cause our operations to suffer and we may be unable to conclude that our internal control over financial reporting is effective and to obtain an unqualified report on internal controls from our auditors as required under Section 404 of the Sarbanes-Oxley Act. If additional material weaknesses or significant deficiencies in our internal control are discovered or occur in the future, it may materially adversely affect our ability to report our financial condition and results of operations in a timely and accurate manner and impact investor confidence in our Company.
The actions we have taken are subject to continued review, supported by confirmation and testing by management as well as audit committee oversight. The identification and remediation of additional material weaknesses in the future, could adversely affect our ability to report financial information, including our filing of quarterly or annual reports with the SEC on a timely and accurate basis and prohibit us from producing timely and accurate consolidated financial statements, which may adversely affect our share price and we may be unable to maintain compliance with Nasdaq listing requirements.
Risks Related to Being a British Virgin Islands Company
Rights of shareholders under British Virgin Islands law differ from those under U.S. law, and, accordingly, you may have fewer protections as a shareholder.
Our corporate affairs are governed by our amended and restated memorandum and articles of association, the BVI Act, and the common law of the British Virgin Islands. The rights of shareholders to take legal action against our directors, actions by minority shareholders and the fiduciary responsibilities of our directors under British Virgin Islands law are to a large extent governed by the common law of the British Virgin Islands and by the BVI Act. The common law of the British Virgin Islands is derived in part from comparatively limited judicial precedent in the British Virgin Islands as well as from English common law, which has persuasive, but not binding, authority on a court in the British Virgin Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under British Virgin Islands law are not as clearly established as they would be under statutes or judicial precedents in some jurisdictions in the United States. In particular, the British Virgin Islands has a less developed body of securities laws as compared to the United States, and some states (such as Delaware) have more fully developed and judicially interpreted bodies of corporate law. As a result of the foregoing, holders of our ordinary shares may have more difficulty in protecting their interests through actions against our management, directors or major shareholders than they would as shareholders of a U.S. company.
British Virgin Islands companies may not be able to initiate shareholder derivative actions, thereby depriving shareholders of one avenue to protect their interests.
British Virgin Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the United States. The circumstances in which any such action may be brought, and the procedures and defenses that may be available in respect of any such action, may result in the rights of shareholders of a British Virgin Islands company being more limited than those of shareholders of a company organized in the United States. Accordingly, shareholders may have fewer alternatives available to them if they believe that corporate wrongdoing has occurred. The British Virgin Islands courts are also unlikely to recognize or enforce judgments of courts in the United States based on certain liability provisions of U.S. securities law, or to impose liabilities based on certain liability provisions of the U.S. securities laws that are penal in nature, in original actions brought in the
66
British Virgin Islands. There is no statutory recognition in the British Virgin Islands of judgments obtained in the United States, although the courts of the British Virgin Islands will generally recognize and enforce the non-penal judgment of a non-U.S. court of competent jurisdiction without retrial on the merits. This means that even if shareholders were to sue us successfully, they may not be able to recover anything to make up for the losses suffered.
British Virgin Islands law differs from the laws in effect in the United States, and U.S. investors may have difficulty enforcing civil liabilities against us, our directors or members of senior management.
Under our amended and restated memorandum and articles of association, we may indemnify and hold our directors harmless against all claims and suits brought against them, subject to limited exceptions. Furthermore, to the extent allowed by law, the rights and obligations among or between us, any of our current or former directors, officers and employees and any current or former shareholder will be governed exclusively by the laws of the British Virgin Islands and subject to the jurisdiction of the British Virgin Islands courts, unless those rights or obligations do not relate to or arise out of their capacities as such. Although there is doubt as to whether U.S. courts would enforce these provisions in an action brought in the United States, under U.S. securities laws, these provisions could make judgments obtained outside of the British Virgin Islands more difficult to enforce against our assets in the British Virgin Islands or jurisdictions that would apply British Virgin Islands law.
The laws of the British Virgin Islands provide limited protection for minority shareholders, so minority shareholders will have limited or no recourse if they are dissatisfied with the conduct of our affairs.
Under the laws of the British Virgin Islands, there is limited statutory law for the protection of minority shareholders other than the provisions of the BVI Act dealing with shareholder remedies, as summarized under “Description of Share Capital-Shareholders’ Rights Under British Virgin Islands Law Generally.” One protection under statutory law is that shareholders may bring an action to enforce the constituent documents of a British Virgin Islands company and are entitled to have the affairs of the Company conducted in accordance with the BVI Act and the amended and restated memorandum and articles of association of the Company. As such, if those who control the Company have disregarded the requirements of the BVI Act or the provisions of our amended and restated memorandum and articles of association, then the courts will likely grant relief. Generally, the areas in which the courts will intervene are the following: (i) an act complained of which is illegal; (ii) acts that constitute oppression, unfair discrimination or unfair prejudice against the minority where the wrongdoers control the Company; (iii) acts that infringe on the personal rights of the shareholders, such as the right to vote; and (iv) acts where we have not complied with provisions requiring approval of a special or extraordinary majority of shareholders, which are more limited than the rights afforded to minority shareholders under the laws of many states in the United States.
Provisions in our amended and restated memorandum and articles of association and under British Virgin Islands law could make an acquisition of us more difficult and may prevent attempts by our shareholders to replace or remove our current management.
Provisions in our amended and restated memorandum and articles of association may discourage, delay or prevent a merger, acquisition or other change in control of us that shareholders may consider favorable, including transactions in which shareholders might otherwise receive a premium for their shares. These provisions could also limit the price that investors might be willing to pay in the future for our common shares, thereby depressing the market price of our common shares. In addition, these provisions may frustrate or prevent any attempts by our shareholders to replace or remove our current management by making it more difficult for shareholders to replace members of our Board of Directors. Because our Board of Directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our shareholders to replace current members of our management team. Among others, these provisions include the following:
•while we are commencing a phased-in process to declassify our Board of Directors, our Board of Directors is divided into three classes with staggered three-year terms and will not be fully declassified until our 2026 annual meeting of shareholders, which may delay or prevent a change of our management or a change in control;
•our Board of Directors has the right to elect directors to fill a vacancy created by the expansion of our Board of Directors or the resignation, death or removal of a director, which will prevent shareholders from being able to fill vacancies on our Board of Directors;
•our shareholders are not able to act by written consent, and, as a result, a holder, or holders, controlling a majority of our shares are not able to take certain actions other than at annual shareholders’ meetings or special shareholders’ meetings;
67
•our amended and restated memorandum and articles of association do not allow cumulative voting in the election of directors, which limits the ability of minority shareholders to elect director candidates;
•our shareholders are required to provide advance notice and additional disclosures in order to nominate individuals for election to our Board of Directors or to propose matters that can be acted upon at a shareholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of our company; and
•our Board of Directors is able to issue, without shareholder approval, preferred shares with voting or other rights or preferences that could impede the success of any attempt to acquire us.
Moreover, because we are incorporated in the British Virgin Islands, we are governed by the provisions of BVI Business Companies Act, 2004, as amended, or the BVI Act, which provide for different shareholder rights than a Delaware corporation. See, for example, the risk factor titled “Rights of shareholders under British Virgin Islands law differ from those under U.S. law, and, accordingly, you may have fewer protections as a shareholder.”
68
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
The Audit Committee oversees the cybersecurity program and receives reports from management regarding the Company's cybersecurity policies and procedures on an annual basis. The Audit Committee also receives reports from management on any cybersecurity incidents that may occur.
As part of our cybersecurity program, our IT management reports to the Company's Chief Operating Officer and Chief Executive Officer and is responsible for assessing and managing cybersecurity risks and developing and implementing our cybersecurity program. Our current Head of Global IT has more than 15 years of experience in technology and process improvements, which includes the execution of digital business strategies within highly regulated industries. Our IT management has obtained relevant experience in various sectors such as technology firms, financial institutions, and consulting firms and have actively engaged in continuous learning and professional development initiatives to stay updated on evolving cybersecurity threats and trends. This includes participating in industry conferences, workshops, training programs, and becoming members of professional cybersecurity organizations.
Our cybersecurity program is designed to identify, assess, and mitigate risks from cybersecurity threats, and includes the following elements:
•a risk assessment process to identify and assess cybersecurity risks;
•a risk mitigation strategy to address cybersecurity risks;
•an incident response plan to identify, respond to, mitigate and remediate cybersecurity incidents;
•an awareness and training program to educate employees about cybersecurity risks;
•a procedure to procure information technology services, including cloud computing and data storage, from third-party providers with sufficient cybersecurity provisions, and to monitor their cybersecurity process on an ongoing basis; and
•periodic testing and evaluation by external parties we engage to assess the effectiveness of the cybersecurity intrusion protections and make recommendations to improve the security of our information systems.
As a result, we have implemented a multi-layered cybersecurity program that includes measures to protect our information systems, including firewalls, network access controls, intrusion detection systems, phishing campaigns, security awareness training and monitoring, network operation center, security operation center, and data encryption, and to monitor our information systems to detect potential cybersecurity incidents, including through the use of automated detection software. Through these processes and the other processes described above, including our incident response plan, our management is informed about cybersecurity threats and incidents affecting us.
Our company has implemented robust processes to assess, identify, and manage material cybersecurity risks effectively. These processes are an integral component of our overall risk management system, ensuring that cybersecurity concerns are comprehensively addressed within our broader risk management framework. Risk assessment, identification, and management processes are seamlessly integrated into our overall risk management system. The processes for assessing, identifying, and managing cybersecurity risks are aligned with our strategic objectives and business goals. We foster cross-functional collaboration and communication to facilitate the integration of cybersecurity risk management into various business functions and processes.
We also review our cybersecurity practices, their effectiveness, and the cybersecurity practices of the third-parties we rely on, on an ongoing basis and make changes as necessary to address new risks. However, we cannot guarantee that our efforts will be successful in preventing all cybersecurity incidents.
We are not aware of any risks from cybersecurity threats, including as a result of previous cybersecurity incidents, that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations or financial condition. We can give no assurance that we have detected or protected against all cybersecurity threats or incidents. We are subject to a variety of cybersecurity risks, which could have a material adverse effect on our business, financial condition, and results of operations. See the “Risks Related to
69
Intellectual Property and Data Security” section of Item 1A. Risk Factors for additional discussion on risks affecting our information systems.
ITEM 2. PROPERTIES
Our principal executive offices are located in Alajuela, Costa Rica, where we occupy approximately 36,000 square feet of office, laboratory and manufacturing space. I
We are in the process of expanding our manufacturing facilities and corporate offices in the Coyol Free Zone in Costa Rica. Construction began in 2021 and we exercised the option to purchase the land and cold shell building in 2022. The project includes approximately 170,000 square feet of facility space. In July 2023, we announced the grand opening of the first phase of the Sulàyöm Innovation Campus. We currently expect to commence manufacturing from the new facility in fiscal 2024.
We also have office or warehouse space in Wommelgem, Belgium; Sao Paulo and Rio de Janeiro, Brazil; Stockholm, Sweden; Barcelona, Spain; Rome, Italy; Addison, Texas, USA; Santa Barbara, CA, USA; London, England; Haar, Germany, Cavaillon, France and Buenos Aires, Argentina pursuant to a variety of leases that expire in 2024 through 2029.
ITEM 3. LEGAL PROCEEDINGS
We are and may become from time to time a party to various claims and lawsuits arising in the ordinary course of business, but we are not a party to any material legal proceeding required to be disclosed under Item 103 of Regulation S-K.
ITEM 4. MINE SAFETY DISCLOSURES
None.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common shares have been traded on the Nasdaq Capital Market under the symbol “ESTA” since our initial public offering on July 23, 2018. Prior to this time, there was no public market for our common shares.
Holders
There were 31 shareholders of record of our common shares as of March 1, 2024. Certain shares are held in “street” name and, accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number.
Sales of Unregistered Securities
None.
Dividends
We have not paid any cash dividends on our common shares since inception and do not anticipate paying cash dividends in the foreseeable future.
Purchases of Equity Securities by the Issuer or Affiliated Purchasers
There were no repurchases of shares of common shares made during the three months ended December 31, 2023.
Stock Performance Graph
The graph set forth below compares the cumulative total stockholder return on our common stock from December 31, 2018 through 2023, with the cumulative total return of (a) the NASDAQ Health Care Index and (b) the NASDAQ Composite Index, over the same period. This graph assumes an investment of $100 on December 31, 2018 in each of our common stock, the NASDAQ Health Care Index and the NASDAQ Composite Index and assumes the reinvestment of dividends, if any.
70
The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock. Information used in the graph was obtained from the Nasdaq Stock Market LLC, a financial data provider and a source believed to be reliable. The Nasdaq Stock Market LLC is not responsible for any errors or omissions in such information.
This performance graph shall not be deemed filed for purposes of Section 18 of the Exchange Act or otherwise subject to liabilities under that section and shall not be deemed to be incorporated by reference into any filing of the registrant. under the Securities Act, except as shall be expressly set forth by specific reference in such filing.
ITEM 6. RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with the consolidated financial statements and related notes that are included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under the sections contained in this Annual Report on Form 10-K entitled Item 1A. “Risk Factors”; Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations”; and Item 7A. “Quantitative and Qualitative Disclosures about Market Risk”. See “Special Note Regarding Forward-Looking Statements” preceding Part I of this Annual Report on Form 10-K.
Overview
Our line of silicone gel-filled breast implants, branded as Motiva Implants, is the centerpiece of our medical technology platform. Our post-market surveillance data (which was not generated in connection with a United States Food and Drug Administration, or FDA, pre-market approval, or PMA, study collected at defined follow-ups, but was patient or practitioner reported) and published third-party registries and data indicate that Motiva Implants have low rates of adverse events (including rupture, capsular contracture, and safety related reoperations) that we believe compare favorably with those of our competitors. We believe the proprietary technologies that differentiate
71
our Motiva Implants enable improved safety and aesthetic outcomes and drive our revenue growth. We have developed other complementary products and services, which are aimed at further enhancing patient outcomes.
We have devoted a majority of our resources since inception to developing our Motiva Implants, which we began selling in October 2010. We have incurred net losses in each year since inception, and we have financed our operations primarily through equity financings and debt financings.
Financial Highlights
Our revenue for the years ended December 31, 2023 and 2022 was $165.2 million and $161.7 million, respectively, an increase of $3.5 million, or 2.2%. Net losses were $78.5 million for the year ended December 31, 2023 as compared to $75.2 million for the year ended December 31, 2022. As of December 31, 2023, we had an accumulated deficit of $360.1 million.
Our cash balance as of December 31, 2023 was $40.0 million.
Recent Developments
In January 2024, we announced the commercial launch of Motiva Implants in China and the completion of the first procedure with the Motiva Flora SmoothSilk Tissue Expander in the United States.
We also entered into a securities purchase agreement, pursuant to which we sold an aggregate of two million common shares and pre-funded warrants for gross proceeds of approximately $50 million. See Note 15 “Subsequent Events” for additional information.
We are in the process of expanding our manufacturing facilities and corporate offices in the Coyol Free Zone, or CFZ, in Costa Rica. Construction of the cold shell structure of the Sulàyöm Innovation Campus was initially funded by the Coyol Free Zone in 2021 until we exercised our option to purchase the title of the land and cold shell building for approximately $12.6 million in 2022. In July 2023, we announced the grand opening of the first phase of the Sulàyöm Innovation Campus, which includes approximately 100,000 square feet of facility space intended to increase our manufacturing capacity by approximately 730,000 units per year. We estimate a total of $51.7 million in costs for this initial phase of our expansion project, of which the majority has been incurred to date. Additional phases of the project may be executed, at our option, to further expand manufacturing capacity at the new facility. We expect to commence manufacturing from the new facility in 2024. See Note 3 “Balance Sheet Accounts” for additional information.
In November 2023, we received National Medical Products Administration, or NMPA, approval in China for Motiva Implants, 510(k) clearance from the FDA for the Motiva Flora SmoothSilk Tissue Expander in the United States, and CE mark approval under the European Medical Device Regulation for the Motiva Injector, the Motiva Inflatable Balloon and the Motiva Channel Dissector.
In addition, in October 2023, we completed and announced the results of the two-year 100-patient clinical study for Mia Femtech, our patented technologies that can increase breast shape by 1 to 2 cups in a 15-minute procedure without the need for general anesthesia. The single-center, Institutional Review Board approved study began in December 2020 and involved participation of fifteen board-certified plastic surgeons from Costa Rica, Sweden, England, Brazil, Austria, Italy, Belgium, and the United States. We have launched Mia Femtech globally through partnerships with clinics in Japan, Spain, Switzerland, Sweden, Germany, France, Costa Rica, Turkey and the Middle East. In October 2023, we also launched, in select geographies, Zen - the newest generation of our passive RFID technology that is now non-ferromagnetic. Zen is available with Motiva Ergonomix2 Round implants in the Joy program.
In April 2023, we issued 1,165,000 common shares in an underwritten public offering for net proceeds of approximately $84.6 million.
In February 2023, we submitted the final module of our clinical trial for Motiva Implants in the United States to the FDA. We received FDA approval to start our clinical trial in 2018. By August 2019, we had completed all surgeries in the aesthetic cohorts and implemented a bifurcated regulatory strategy for data submission. In April 2022, we released preliminary results for the primary augmentation cohort and, by June 2022, we completed enrollment and surgeries for the primary reconstruction cohort. By September 2022, we had completed the three-year follow-up for the aesthetic cohort.
In January 2023, we announced a partnership with Seishin Plastic and Aesthetic Surgery Clinic in Japan for Mia Femtech. Previously, we obtained regulatory approval from the Pharmaceuticals and Medical Devices Agency, as
72
well as reimbursement for post-mastectomy reconstruction under the Japanese National Health System, for Motiva Implants and the Motiva Flora tissue expander in November 2022.
In April 2022, we entered into a credit agreement, or the Credit Agreement, for term loans to the Company in an aggregate principal amount of up to $225 million, with Oaktree Fund Administration, LLC, as administrative agent. The first and second tranche were advanced in the amount of $150 million and $25 million in April and December 2022, respectively. A portion of the proceeds from the first tranche was used to repay in full and terminate the $65 million in aggregate principal amount outstanding under the Company’s previous credit agreement with Madryn Health Partners, LP, or the Madryn Credit Agreement, and the $6.5 million early repayment penalty. In February 2024, we amended the Credit Agreement, modifying the access conditions, commitment termination dates and interest rates for the two remaining available tranches. See Note 5 “Debt” and Note 15 “Subsequent Events” for additional information.
We are focused on investing in manufacturing capacity, marketing, customer service, and sales force in multiple geographies to promote the use of our Motiva Implants. This expansion may result in short-term losses as we grow our organization and invest in research, clinical trials, and other commercialization efforts.
Components of Results of Operations
Revenue
We commenced sales of our Motiva Implants in October 2010 and these products have historically accounted for the majority of our revenues. Sales of our Motiva breast implants accounted for over 95% of our revenues for the year ended December 31, 2023, and we expect our revenues to continue to be driven primarily by sales of these products. We primarily derive revenue from sales of our Motiva Implants to two types of customers: (1) medical distributors and (2) direct sales to physicians, hospitals, and clinics.
We recognize revenue related to the sales of products at the time of shipment, except for a portion of our direct sales revenue that is generated from the sale of consigned inventory maintained at physician, hospital, and clinic locations. For consignment sales, revenue is recognized at the time we are notified by the consignee that the product has been implanted. Our contracts with distributors do not typically contain right of return or price protection and have no post-delivery obligations.
We expect our revenue to increase as we enter new markets, expand awareness of our products in existing markets, and grow our distributor network and direct sales force. We also expect our revenue to fluctuate from quarter to quarter due to a variety of factors, including seasonal fluctuations in demand for Motiva Implants. We are also affected by foreign currency fluctuations.
Cost of Revenue and Gross Margin
Our implants are manufactured at our two facilities in Costa Rica. A third facility in Costa Rica is under construction and is currently expected to commence manufacturing in fiscal 2024. Cost of revenue is primarily the cost of silicone but also includes other raw materials, packaging, components, quality assurance, labor costs, as well as manufacturing and overhead expenses. Cost of revenue also includes depreciation expense for production equipment, and amortization of certain intangible assets.
We calculate gross margin as revenue less cost of revenue for a given period divided by revenue. Our gross margin may fluctuate from period to period depending, in part, on the efficiency and utilization of our manufacturing facilities, targeted pricing programs, and sales volume based on geography, customer and product type.
Operating Expenses
Sales, General and Administrative
Sales, general and administrative, or SG&A, expenses primarily consist of compensation, including salary, share-based compensation and employee benefits for our sales and marketing personnel, and for administrative personnel that support our general operations such as information technology, executive management, financial accounting, customer service, and human resources personnel. SG&A expenses also includes costs attributable to freight, marketing, sales support, travel, legal services, financial audit fees, insurance costs, and consulting services.
We expect our SG&A expenses to continue to increase in absolute dollars for the foreseeable future as our business grows and we continue to invest in our sales, marketing, medical education, training and general
73
administration resources to build our corporate infrastructure. However, we expect our SG&A expenses to decrease as a percentage of our revenue over the long term, although our SG&A expenses may fluctuate from period to period due to the timing of expenses related to our sales and marketing campaigns.
Research and Development
Our research and development, or R&D, activities primarily consist of engineering and research programs associated with our products under development, as well as R&D activities associated with our clinical development activities. Our R&D expenses primarily consist of compensation, including salary, share-based compensation and employee benefits for our R&D and clinical personnel. We also incur significant expenses for supplies, development prototypes, design and testing, clinical study costs and product regulatory and consulting expenses.
We expect our R&D expenses to remain elevated for the foreseeable future as we continue to advance our products under development, as well as initiate and prepare for additional clinical studies. We received an approval of an IDE from the FDA in March 2018 to initiate a clinical trial and enrolled the first patient in April 2018. In August 2019, we completed all patient surgeries for the IDE aesthetic cohorts, which include primary augmentation and revision augmentation, and have now completed the three-year study subject follow-up for the aesthetic cohort. In June 2022, full enrollment of the IDE clinical trial was complete, and all surgeries in the primary reconstruction cohort were performed. As of September 30, 2022, we also completed the three-year study subject follow-up for the aesthetic cohort. In the fourth quarter of 2021, we initiated a modular PMA submission process with the FDA and submitted the first of four modules. The second, third and fourth modules were submitted to the FDA in May 2022, August 2022 and February 2023, respectively. The IDE clinical trial is expected to cost between $30.0 million and $40.0 million over ten years. As of December 31, 2023, around $30 million has been spent on the trial. We also have other products under development for which we may be required to conduct clinical trials in future periods in order to receive regulatory approval to market these products.
Interest Expense
Interest expense consists primarily of cash and non-cash interest related to outstanding debt and amortization of debt discounts. As of December 31, 2023, we had $192.6 million in outstanding principal under our term loan, including interest accrued into the principal balance. See Note 5 “Debt” for additional information.
Other Expense, Net
Other expense, net primarily consists of foreign currency gains/losses and interest income.
Loss on Extinguishment of Debt
On April 26, 2022, we repaid in full the $65.0 million in aggregate principal amount outstanding under the Madryn Credit Agreement and the agreement was terminated. We recorded a loss on the extinguishment of debt in the amount of $19.0 million, which represents the difference between the carrying value of debt and the cash outflows to extinguish the debt including $6.5 million of the early repayment penalty.
Income Tax Expense
Income tax expense consists primarily of income taxes in foreign jurisdictions in which we conduct business. Due to our history of losses, with the exception of Belgium and JAMM Technologies, Inc., we maintain a full valuation allowance for deferred tax assets including net operating loss carry-forwards, R&D tax credits and other book versus tax differences.
Business Update Regarding Macroeconomic Conditions
•2023 Results: The first and second quarters of 2023 resulted in record quarterly revenue, primarily due to share gains in global markets, especially in our EMEA and in Asia-Pacific regions, and the launch of new products and technologies. The second half of 2023 showed a slowing in revenue growth, primarily in distributor markets, in our Asia-Pacific and, to a lesser degree, in Latin American regions, compared to the first half of 2023. We believe the slower growth is a result of an overall slowdown in demand for aesthetic procedures, primarily as a result of general uncertainty about macroeconomic and geopolitical conditions and seasonality. The overall increase in revenue year-to-date as compared to 2022 was driven by an increase in demand during the first half of the year, and our efforts to expand direct sales in multiple geographies.
•Outlook: Demand for our products is dependent on the relative strength of the global and regional medical device markets, which are sensitive to general macroeconomic conditions. The current global
74
macroeconomic environment remains complex, with elevated inflation and interest rates contributing to fear of potential recessionary conditions thus driving limitations on available discretionary spending in the markets we operate. These macroeconomic challenges, combined with geopolitical upheaval, have led to ongoing volatility within global markets. This negatively impacted demand for our products during the second half of 2023 and we expect for this to continue in 2024.
In response to the decrease in demand, we continue downsizing our global workforce, reducing operational expenditures, and effectively managing inventory levels. Our focus will be on investing in our primary growth initiatives, which include the launch of our product in the U.S., development of the Chinese market and promoting Mia Femtech.
For additional information on the various risks and other uncertain macroeconomic conditions on our business, financial condition and results of operations, please see Part I, Item 1A. “Risk Factors” of this report.
Business Update Regarding Russia-Ukraine and Hamas-Israel Conflicts
In February 2022, Russia invaded Ukraine and is still engaged in active armed conflict against the country. In October 2023, Hamas-led Palestinian militant groups started a military offensive against Israel. To date, the impact of these conflicts on our business operations and financial performance in Europe and the Middle East has not been and is not expected to be material. However, the full impact of these conflicts on our business operations and financial performance remains uncertain and will depend on future developments, including the severity and duration of the conflicts and their impact on regional and global economic conditions.
Consolidated Results of Operations
The following table sets forth our results of operations for the years presented, in dollars:
| 2023 | 2022 | ||||||||||
(in thousands) | |||||||||||
| Revenue | $ | 165,151 | $ | 161,700 | |||||||
| Cost of revenue | 58,174 | 55,105 | |||||||||
| Gross profit | 106,977 | 106,595 | |||||||||
| Operating expenses: | |||||||||||
| Sales, general and administrative | 145,575 | 125,984 | |||||||||
| Research and development | 26,428 | 20,269 | |||||||||
| Total operating expenses | 172,003 | 146,253 | |||||||||
| Loss from operations | (65,026) | (39,658) | |||||||||
| Interest expense | (15,393) | (11,760) | |||||||||
| Change in fair value of derivative instruments | — | 703 | |||||||||
| Loss on extinguishment of debt | — | (19,019) | |||||||||
| Other income (expense), net | 1,836 | (3,090) | |||||||||
| Loss before income taxes | (78,583) | (72,824) | |||||||||
| Provision for income taxes | 81 | (2,385) | |||||||||
| Net loss | $ | (78,502) | $ | (75,209) | |||||||
75
Comparison of the Year Ended December 31, 2023 and 2022
| 2023 | 2022 | ||||||||||
(in thousands) | |||||||||||
Revenue | $ | 165,151 | $ | 161,700 | |||||||
| Cost of revenue | 58,174 | 55,105 | |||||||||
| Gross profit | $ | 106,977 | $ | 106,595 | |||||||
| Gross margin | 64.8 | % | 65.9 | % | |||||||
Revenue
Revenue increased $3.5 million, or 2.2%, to $165.2 million for the year ended December 31, 2023, as compared to $161.7 million for the year ended December 31, 2022. The increase was primarily due to share gains in global markets and the launch of new products and technologies. The first half of 2023 resulted in record quarterly revenue, primarily due to strong demand in our EMEA and in Asia-Pacific regions. The third and fourth quarters of 2023 showed a slowing in revenue growth, primarily in distributor markets in our Asia-Pacific and, to a lesser degree, in Latin American regions, compared to the first half of 2023. We believe the slower growth is a result of an overall slowdown in demand for aesthetic procedures primarily as a result of general uncertainty about macroeconomic and geopolitical conditions and seasonality.
Cost of Revenue and Gross Margin
Cost of revenue increased $3.1 million, or 5.6%, to $58.2 million for the year ended December 31, 2023, compared to $55.1 million for the year ended December 31, 2022. The increase in cost of revenue is in line with the increase in revenue except as described below.
Gross margin decreased to 64.8% for the year ended December 31, 2023, compared to 65.9% for the year ended December 31, 2022, due to an increase in the reserve for inventory obsolescence and higher manufacturing costs, primarily driven by a higher cost of labor and overhead, due, in part, to the revaluation of the Costa Rica colón, partially offset by the positive impact of new product initiatives.
Operating Expenses
| 2023 | 2022 | ||||||||||
| (in thousands) | |||||||||||
| Operating expenses: | |||||||||||
| Sales, general and administrative | $ | 145,575 | $ | 125,984 | |||||||
| Research and development | 26,428 | 20,269 | |||||||||
| Total operating expenses | $ | 172,003 | $ | 146,253 | |||||||
Sales, General and Administrative Expense
SG&A expense increased $19.6 million, or 15.6%, to $145.6 million for the year ended December 31, 2023, compared to $126.0 million for the year ended December 31, 2022. The increase was primarily due to a $8.2 million increase in sales and marketing expenses, a $4.3 million increase in personnel and related costs due to increased headcount during the first three quarters of the year, a $3.0 million increase in freight associated with higher revenues, a $2.1 million increase in costs in facilities from our expanding operations, a $2.0 million increase in software implementation costs, a $0.9 million increase in consulting fees in part due to added costs for compliance with Section 404(b) of the Sarbanes-Oxley Act and a $0.3 million increase in depreciation and amortization costs, partially offset by a $1.8 million decrease in sales commissions. In the fourth quarter of 2023, we implemented measures targeting a decrease in operating expenses, including headcount reduction to lower global personnel costs.
76
Research and Development Expense
R&D expense increased $6.1 million, or 30.0%, to $26.4 million for the year ended December 31, 2023, compared to $20.3 million for the year ended December 31, 2022. The increase in R&D expense was primarily due to a $6.9 million increase in personnel cost, partially offset by a $0.6 million decrease in regulatory affairs costs and a $0.1 million decrease in expenditures related to our IDE clinical trial in the United States.
Interest Expense
Interest expense increased $3.6 million, or 30.5%, to $15.4 million for the year ended December 31, 2023, as compared to $11.8 million for the year ended December 31, 2022. The increase was primarily due to the new Credit Agreement entered into in April 2022 and the second tranche advanced pursuant to the Credit Agreement in December 2022.
Change in Fair Value of Derivative Instruments
Change in fair value of derivative instruments for the year ended December 31, 2022 resulted in a gain of $0.7 million due to changes in the fair value of Madryn derivatives embedded in the Madryn Credit Agreement we entered into in August 2017. The loan was repaid in June 2022.
Provision for Income Taxes
Provision for income taxes decreased $2.5 million to a benefit of $0.1 million for the year ended December 31, 2023, compared to a provision of $2.4 million for the year ended December 31, 2022. The change in income tax provision is mainly due to the release of a valuation allowance on deferred tax assets in Brazil. This was based on positive evidence that these assets will be realized in the near future.
Other Income (Expense), Net
Other income (expense), net, increased $4.0 million to a gain of $0.8 million for the year ended December 31, 2023, compared to a loss of $3.2 million for the year ended December 31, 2022. The increase was primarily due to the foreign currency fluctuations of the Brazilian real and the euro as compared to the U.S. dollar, resulting in a foreign currency transaction gain of $1.8 million, for the year ended December 31, 2023, compared to a loss of $3.0 million for the year ended December 31, 2022.
Loss on Extinguishment of Debt
Loss on extinguishment of debt was $19.0 million for the year ended December 31, 2022 due to the extinguishment of the Madryn Credit Agreement. The $19.0 million loss on the extinguishment of debt represents the difference between the carrying value of the debt under the Madryn Credit Agreement and the cash outflows to extinguish the debt, including $6.5 million of the early repayment penalty. There was no extinguishment of debt in 2023.
Comparison of the Year Ended December 31, 2022 and 2021
The discussion related to our results of operations and changes in financial condition for 2022 compared to 2021 is incorporated by reference to Part II, Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended December 31, 2022, which was filed with the SEC on March 1, 2023.
Liquidity and Capital Resources
As of December 31, 2023, we had an accumulated deficit of $360.1 million. Since our inception, we have generated losses and expect to continue to generate losses in the near term. We have financed our operations through a combination of equity financings and debt financings, and from cash generated from operations, primarily from the collection of accounts receivable resulting from sales. Our historical cash outflows have primarily been associated with cash used for operating activities such as expansion of our sales and marketing and distributor infrastructure, investing in inventory, R&D activities, asset acquisitions, capital improvements, including our new manufacturing facility and other working capital needs. As of December 31, 2023 and 2022, we had cash of $40.0 million and $66.4 million, respectively.
On April 27, 2023, we issued 1,100,000 common shares in an underwritten public offering, at a price to the public of $71.50 per share. The underwriters purchased the shares from the Company at a price of $67.21 per share and exercised the option to purchase additional 165,000 common shares, at the public offering price per share. Net
77
proceeds to us after deducting underwriting discounts and offering expenses were approximately $84.6 million. See Note 7 “Shareholders’ Equity (Deficit)” for additional information.
On January 9, 2024, we entered into a securities purchase agreement with select institutional accredited investors to sell, at price of $25.00 per share, 1,101,565 common shares and pre-funded warrants for purchase of 898,435 common shares. The pre-funded warrants may be exercisable immediately at a price of $0.001 per share. The aggregate gross proceeds from the offering, before deducting offering expenses, were approximately $50.0 million.
Our short-term liquidity requirements consist primarily of operating expenses and interest payments on the Credit Agreement. We believe that our available cash and cash from operations will be sufficient to satisfy our liquidity requirements for at least the next 12 months, including our contractual and other obligations summarized below under “Material Cash Requirements” section. Our long-term liquidity needs consist primarily of operating expenses, including expected increases in SG&A and R&D expenses related to our IDE clinical trial, regulatory compliance and product development and funds necessary to pay for the interest and principal payment on our Term Loans (as defined below). Our liquidity assumptions may prove to be incorrect, and we could utilize our available financial resources sooner than we currently expect.
Our future capital requirements will depend on many factors, including:
▪the degree and rate of market adoption of our products;
▪the cost and timing of our regulatory activities, especially the IDE clinical trial, and the timing of regulatory approval for our Motiva Implants in the United States;
▪the emergence of new competing technologies and products;
▪the costs of R&D activities we undertake to develop and expand our products;
▪the costs of commercialization activities, including sales, marketing and manufacturing;
▪the level of working capital required to support our growth; and
▪our need for additional personnel, information technology or other operating infrastructure to support our growth and operations as a public company.
We may need to raise additional capital to execute our business plan. In April 2023, we filed an automatic shelf registration statement, or Shelf Registration Statement, with the SEC that expires in April 2026, which will allow us to offer and sell our common shares, warrants, rights and units. We may use the Shelf Registration Statement or other capital sources, including other offerings of equity or debt securities or the credit markets, to satisfy future financing needs. If we are unable to raise additional capital when desired, or on terms acceptable to us, our business, results of operations, and financial condition would be adversely affected.
78
Cash Flows
The discussion related to our cash flows for 2022 is incorporated by reference to Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended December 31, 2022, which was filed with the SEC on March 1, 2023.
The following table sets forth the primary sources and uses of cash for each of the years presented below:
| 2023 | 2022 | ||||||||||
| (in thousands) | |||||||||||
| Net cash provided by (used in): | |||||||||||
| Operating activities | $ | (88,513) | $ | (52,166) | |||||||
| Investing activities | (24,547) | (34,791) | |||||||||
| Financing activities | 86,227 | 100,255 | |||||||||
| Effect of exchange rate changes on cash | 513 | (358) | |||||||||
| Net (decrease) increase in cash | $ | (26,320) | $ | 12,940 | |||||||
Net Cash Used in Operating Activities
Net cash used in operating activities of $88.5 million for the year ended December 31, 2023 was primarily comprised of a net loss of $78.5 million, changes in operating assets and liabilities of $34.1 million, $4.2 million of unrealized foreign currency gain and $3.6 million of interest capitalized for construction in progress, partially offset by $14.4 million of share-based compensation expense, $13.3 million of non-cash interest expense due to accretion of debt discounts, $4.2 million of non-cash depreciation and amortization expense, a $1.4 million change in provision for inventory obsolescence, a $1.2 million change in allowance for doubtful accounts and $0.7 million of non-cash amortization expense of right-to-use assets.
Net cash used in operating activities of $52.2 million for the year ended December 31, 2022 was primarily comprised of a net loss of $75.2 million, a $19.0 million loss on extinguishment of debt, $13.4 million of share-based compensation expense, $8.1 million of non-cash interest expense due to accretion of debt discounts and interest subsumed into the principal of the new Credit Agreement, $3.9 million of non-cash depreciation expense, $1.7 million of unrealized foreign currency loss, and a $1.6 million change in provision for inventory obsolescence, partially offset by a $1.9 million gain from write-off of liability and a $0.7 million change in fair value of derivatives, as well as changes in operating assets and liabilities of $21.5 million.
Net Cash Used in Investing Activities
Net cash used in investing activities of $24.5 million for the year ended December 31, 2023 primarily consisted of $15.3 million of cash paid for capital expenditures on construction in progress related to our new manufacturing facility in the Coyol Free Zone in Costa Rica, $7.9 million in purchases of property and equipment related to the new manufacturing facility and $1.3 million in purchases of intangibles.
Net cash used in investing activities of $34.8 million for the year ended December 31, 2022 primarily consisted of $29.9 million of cash paid for capital expenditures on construction in progress related to our new manufacturing facility in the Coyol Free Zone in Costa Rica, $2.9 million in purchases of property and equipment, $1.5 million of purchases of intangibles and $0.5 million of cash paid for past asset acquisition.
Net Cash Provided by Financing Activities
Net cash provided by financing activities of $86.2 million for the year ended December 31, 2023 primarily consisted of $84.5 million of proceeds received for the issuance of common shares, net of underwriters’ discount and issuance costs, from our public offering in April 2023 and $2.2 million in proceeds received for stock option exercises, partially offset by $0.5 million paid to satisfy tax withholding obligations upon the vesting of restricted stock.
Net cash provided by financing activities of $100.3 million for the year ended December 31, 2022 primarily consisted of $168.1 million of borrowings under the new Credit Agreement, net of discount and issuance costs,
79
and $3.9 million in proceeds received for stock option exercises, partially offset by $71.7 million used to repay borrowings under the Madryn Credit Agreement.
Material Cash Requirements
The following table provides a summary of our material cash requirements from known contractual and other obligations, including commitments for capital expenditures, as of December 31, 2023:
| 2024 | 2025 | 2026 | 2027 | 2028 | Thereafter | Total | ||||||||||||||||||||||||||||||||||||||
(in thousands) | ||||||||||||||||||||||||||||||||||||||||||||
Debt obligations - principal (1) | $ | — | $ | — | $ | — | $ | 196,399 | $ | — | $ | — | $ | 196,399 | ||||||||||||||||||||||||||||||
Debt obligations - Interest payments (1) | 14,142 | 17,921 | 17,921 | 5,696 | — | — | 55,680 | |||||||||||||||||||||||||||||||||||||
Future minimum lease payments (2) | 998 | 912 | 833 | 724 | 506 | 295 | 4,268 | |||||||||||||||||||||||||||||||||||||
License and software commitments (3) | 2,363 | 1,999 | 1,501 | 1,031 | 601 | — | 7,495 | |||||||||||||||||||||||||||||||||||||
Short-term borrowing (4) | 1,100 | — | — | — | — | — | 1,100 | |||||||||||||||||||||||||||||||||||||
| $ | 18,603 | $ | 20,832 | $ | 20,255 | $ | 203,850 | $ | 1,107 | $ | 295 | $ | 264,942 | |||||||||||||||||||||||||||||||
(1) Contractual obligations related to the Credit Agreement. The interest payments were projected as of December 31, 2023 assuming we will choose to PIK interest into principal though April 2024. See below under “Indebtedness” and Note 5 “Debt” for additional details. | ||||||||||||||||||||||||||||||||||||||||||||
(2) Contractual obligations related to the minimum lease payments and interest on our operating leases. See Note 6 “Leases” for additional details. | ||||||||||||||||||||||||||||||||||||||||||||
(3) Contractual obligations related to our current contracts for software solutions and support. | ||||||||||||||||||||||||||||||||||||||||||||
(4) Contractual obligations related to a short-term loan for our business insurance premiums. | ||||||||||||||||||||||||||||||||||||||||||||
In August 2021, we entered into a contract with the Zona Franca Coyol, S.A., or CFZ, to begin construction of a new manufacturing facility in Costa Rica. In 2022, we exercised our option to purchase the title of the land and cold shell building for approximately $12.6 million. We also have the option to buy an adjacent lot of land for approximately $2.8 million and engage CFZ to construct an additional manufacturing facility. In July 2023, we announced the grand opening of the first phase of the Sulàyöm Innovation Campus. We currently expect to commence manufacturing from the new facility in fiscal 2024.
In September 2023, the Company signed a lease for an office space in Irving, Texas that commenced on January 1, 2024 upon completion of tenant improvements. No right-of-use assets or lease liabilities have been recorded as of December 31, 2023. See Note 6.
Indebtedness
On April 26, 2022, or the Closing Date, we entered into the new Credit Agreement, pursuant to which the lenders agreed to make term loans to the Company in an aggregate principal amount of up to $225 million, which we collectively refer to as the Term Loans, with the first tranche of $150 million advanced on the Closing Date. Part of the first tranche was used to repay the outstanding principal and interest under the Madryn Credit Agreement in full, including the early repayment penalty of $6.5 million. In December 2022, $25 million was advanced under the second tranche. The Term Loans will mature on the 5-year anniversary of the Closing Date and accrue interest at a rate equal to 9% per annum. As of December 31, 2023, $192.6 million was outstanding under the Credit Agreement representing the initial principal of $150 million for the Tranche A Term Loan and $25 million for the Tranche B Term Loan and $17.6 million of interest accrued into the principal balance. In February 2024, we amended the Credit Agreement where funding milestones, commitment termination dates and interest rates for the two remaining available tranches were modified. See Note 5 and Note 15.
Critical Accounting Policies, Significant Judgments and Use of Estimates
This discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with the generally accepted accounting principles in the United States of America, or GAAP. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities, revenue and expenses, and the disclosure of contingent assets and liabilities. Our estimates are based on our historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities. Actual
80
results may differ from these estimates. We believe that the critical accounting policies discussed below are essential to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s estimates and judgments.
Revenue Recognition
The Company recognizes revenue related to sales of products to distributors or directly to customers in markets where it has regulatory approval, net of discounts and allowances. The Company recognizes revenue in accordance with Accounting Standards Codification, or ASC, 606, Revenue from Contracts with Customers. ASC 606 requires the Company to recognize revenue to depict the transfer of goods or services to a customer at an amount that reflects the consideration it expects to receive in exchange for those goods or services.
The Company recognizes revenue related to the sales of products to distributors at the time of shipment of the product, which represents the point in time when the distributor has taken ownership and assumed the risk of loss and the required revenue recognition criteria are satisfied. The Company’s distributors are obligated to pay within specified terms regardless of when, or if, they sell the products. The Company’s contracts with distributors typically do not contain right of return or price protection and have no post-delivery obligations.
The Company recognizes revenue when title to the product and risk of loss transfer to customers, provided there are no remaining performance obligations required of the Company or any written matters requiring customer acceptance. The Company allows for the return of product from direct customers in certain regions in limited instances within fifteen days after the original sale and records estimated sales returns as a reduction of sales in the same period revenue is recognized. Appropriate reserves are established for anticipated sales returns based on historical experience, recent gross sales and any notification of pending returns. Actual sales returns in any future period are inherently uncertain and thus may differ from the estimates. If actual sales returns differ significantly from the estimates, an adjustment to revenue in the current or subsequent period is recorded. As of December 31, 2023 an allowance of $0.3 million was recorded for product returns. As of December 31, 2022, the allowance for product returns was de minimis.
A portion of the Company’s revenue is generated from the sale of consigned inventory maintained at physician, hospital, and clinic locations. For these products, revenue is recognized at the time the Company is notified by the consignee that the product has been implanted, not when the consigned products are delivered to the consignee’s warehouse.
The Company has a limited warranty for the shelf life of breast implants, which is five years from the time of manufacture. Estimated warranty obligations are recorded at the time of sale. The Company also offers a warranty to patients in the event of rupture and a replacement program for capsular contracture events, provided certain registration requirements are met. Revenue for extended warranties is recognized ratably over the term of the agreement. To date, these warranty and program costs have been de minimis. The Company will continue to evaluate the warranty reserve policies for adequacy considering claims history.
Deferred revenue primarily consists of payments received in advance of meeting revenue recognition criteria. The Company has received payments from distributors to provide distribution exclusivity within a geographic area and recognizes deferred revenue on a ratable basis over the term of such contractual distribution relationship. Additionally, the Company has received payments from customers in direct markets prior to surgical implantation and recognizes deferred revenue at the time the Company is notified by the customer that the product has been implanted. For all arrangements, any revenue that has been deferred and is expected to be recognized beyond one year is classified as long-term deferred revenue and included in “Other liabilities, long-term” on the consolidated balance sheets.
Research and Development
Costs related to research and development, or R&D, activities are expensed as incurred. R&D costs primarily include personnel costs, materials, clinical expenses, regulatory expenses, product development, consulting services, and outside research activities, all of which are directly related to research and development activities.
The Company estimates IDE clinical trial expenses based on the services performed, pursuant to contracts with research institutions and clinical research organizations that conduct and manage clinical trials on its behalf. In accruing service fees, the Company estimates the time period over which services will be performed and the level of patient enrollment and activity expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, the Company will adjust the accrual accordingly.
81
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable is stated at invoice value less estimated allowances for returns and doubtful accounts. The Company continually monitors customer payments and maintains an allowance for estimated losses resulting from customers’ inability to make required payments. In evaluating the Company’s ability to collect outstanding receivable balances, the Company considers various factors including the age of the balance, the creditworthiness of the customer, which is assessed based on ongoing credit evaluations and payment history, and the customer’s current financial condition. In cases where there are circumstances that may impair a specific customer’s ability to meet its financial obligations, an allowance is recorded against amounts due, which reduces the net recognized receivable to the amount reasonably believed to be collectible.
Inventory and Cost of Revenue
Inventory is stated at the lower of cost to purchase or manufacture the inventory or the net realizable value of such inventory. Cost is determined using the standard cost method which approximates actual costs using the first-in, first-out basis. The Company regularly reviews inventory quantities considering actual losses, projected future demand, and remaining shelf life to record a provision for excess and slow-moving inventory.
Long-Lived Assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset might not be recoverable. When such an event occurs, management determines whether there has been impairment by comparing the anticipated undiscounted future net cash flows to the related asset group’s carrying value. If an asset is considered impaired, the asset is written down to fair value, which is determined based either on discounted cash flows or appraised value, depending on the nature of the asset. There were no impairment charges, or changes in estimated useful lives recorded during the years ended December 31, 2023 and 2022.
Income Taxes
The Company records income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s consolidated financial statements or income tax returns. In estimating future tax consequences, expected future events, enactments or changes in the tax law or rates are considered. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company operates in various tax jurisdictions and is subject to audit by various tax authorities.
The Company records uncertain tax positions based on a two-step process whereby (1) a determination is made as to whether it is more likely than not that the tax positions will be sustained based on the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold the Company recognizes the largest amount of tax benefit that is greater than 50% likely to be realized upon ultimate settlement with the related tax authority. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. Significant judgment is required in the identification of uncertain tax positions and in the estimation of penalties and interest on uncertain tax positions.
There were no material uncertain tax positions as of December 31, 2023 and 2022.
Foreign Currency
The financial statements of the Company’s foreign subsidiaries whose functional currencies are the local currencies are translated into U.S. dollars for consolidation as follows: assets and liabilities at the exchange rate as of the balance sheet date, stockholders’ equity at the historical rates of exchange, and income and expense amounts at the average exchange rate for the period. Translation adjustments resulting from the translation of the subsidiaries’ accounts are included in “Accumulated other comprehensive income” as equity in the consolidated balance sheet. Transactions denominated in currencies other than the applicable functional currency are converted to the functional currency at the exchange rate on the transaction date. At period end, monetary assets and liabilities are remeasured to the functional currency using exchange rates in effect at the balance sheet date. Non-monetary assets and liabilities are remeasured at historical exchange rates. Gains and losses resulting from foreign currency transactions are included within “Other income (expense), net” in the consolidated statement of operations. For the year ended December 31, 2023, foreign currency transaction gain amounted to $1.8 million as compared to a foreign currency transaction loss of $3.0 million for the year ended December 31, 2022.
82
Share-Based Compensation
The Company measures and recognizes compensation expense for all share-based awards in accordance with the provisions of ASC 718, Stock Compensation. Share-based awards granted include stock options, restricted stock units, or RSUs, and restricted stock awards, or RSAs. Share-based compensation expense for stock options and RSAs granted to employees is measured at the grant date based on the fair value of the awards and is recognized as an expense ratably on a straight-line basis over the requisite service period. The fair value of options to purchase shares granted to employees is estimated on the grant date using the Black-Scholes option valuation model.
The calculation of share-based compensation expense requires the Company to make assumptions and judgments about the variables used in the Black-Scholes model, including the expected term, expected volatility of the underlying common shares, risk-free interest rate and dividends.
See Note 9 “Share-Based Compensation” for additional information.
Recent Accounting Pronouncements
Please refer to Note 2 “Summary of Significant Accounting Policies” in the notes to the consolidated financial statements included in this Form 10-K for information on recent accounting pronouncements and the expected impact on our unaudited consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
We had cash of $40.0 million and $66.4 million as of December 31, 2023 and 2022, respectively. We manage our cash portfolio for operating and working capital purposes. Our cash balances are held in bank checking accounts, and we believe that we do not have any material exposure to changes in the fair value of our cash portfolio as a result of changes in interest rates.
Foreign Currency Exchange Risk
To date, the majority of our revenue has been denominated in U.S. dollars, Brazilian reals and euros. Some of our operating expenses are subject to fluctuations due to changes in foreign currency exchange rates, particularly changes in the euro and Brazilian real. Fluctuations in foreign currency exchange rates may cause us to recognize transaction gains and losses in our consolidated statements of operations. For the year ended December 31, 2023, foreign currency transaction gain amounted to $1.8 million primarily related to the remeasurement of transactions denominated in the U.S. dollar into the Brazilian real and the euro as part of the financial reporting consolidation process under GAAP. We have not engaged in any foreign currency hedging activities. As our international operations grow, we will continue to reassess our approach to managing the risks relating to fluctuations in foreign currency exchange rates. During the year ended December 31, 2023, the effect of an immediate 10% adverse change in foreign exchange rates on foreign-denominated accounts as of December 31, 2023 would have had an impact of approximately 1.9% on revenues and would have impacted our net loss by a commensurate amount.
Inflation Risk
We do not believe that inflation had a significant impact on our results of operations for any periods presented in our consolidated financial statements.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required to be filed pursuant to this Item 8 are appended to this report beginning on page F‑1. An index of those financial statements is included in Part IV, Item 15 below.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
83
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
As of December 31, 2023, the end of the period covered by this Annual Report on Form 10-K, our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act) are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to the Company’s management, including its principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Based on the evaluation of our disclosure controls and procedures, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of the end of the period covered by this annual report at the reasonable assurance level.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) of the Exchange Act. Our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2023 using the criteria established in “Internal Control—Integrated Framework” (2013), issued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Based on that assessment and due to the material weakness described below, our management has concluded that the Company’s internal control over financial reporting was not effective as of December 31, 2023.
Material Weaknesses in Internal Control and Plan for Remediation
It was determined that as of December 31, 2023, our primary change management controls for direct database changes were not designed and implemented effectively for specific localities. Other Information Technology General Controls, automated process-level controls, and manual controls that are dependent upon the information derived from such financially relevant systems were also determined to be ineffective as a result of such deficiency. We have made significant progress remediating change management controls as of December 31, 2023. We improved policies and procedures and designed and documented more effective change management monitoring controls, through the use of systematic audit logging, that addresses the relevant risks in order to remediate the identified material weakness. The material weakness persists and will not be considered remediated until the applicable controls operate for a sufficient period of time, and management has concluded, through testing, that the control objective is achieved, and the controls are operating effectively. We expect that the remediation of this material weakness will be completed during 2024.
Remediation of Previously Reported Material Weakness in Internal Control over Financial Reporting
On December 31, 2021, it was determined that our primary user access controls (i.e. provisioning, de-provisioning, and quarterly user access review) to ensure appropriate segregation of duties that would adequately restrict user and privileged access to the financially relevant systems and data to appropriate Company personnel were not operating effectively. These user access control deficiencies resulted in a lack of segregation of duties with respect to certain user roles. Automated process-level controls and manual controls that are dependent upon the information derived from such financially relevant systems were also determined to be ineffective as a result of such deficiency. We implemented a number of measures to address the material weakness identified as of December 31, 2021. We improved policies and procedures and designed and documented more effective controls that addressed the relevant risks in order to remediate the previously identified material weakness in addition to engaging both internal and external resources to review and remediate the deficient controls around sensitive access and segregation of duties, including identifying any specific areas within our information technology systems that require additional resources. During the fourth quarter of 2023, we completed our testing of the operating effectiveness of the implemented controls and found them to be effective. As a result we have concluded the material weakness identified as of December 31, 2021 has been remediated as of December, 31 2023.
84
Changes in Internal Control over Financial Reporting
Other than with respect to the remediation effort discussed above, there was no change in our internal control over financial reporting that occurred during the three months ended December 31, 2023 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls and Procedures
Our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving the desired control objectives. Our management recognizes that any control system, no matter how well designed and operated, is based upon certain judgments and assumptions and cannot provide absolute assurance that its objectives will be met. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs. Similarly, an evaluation of controls cannot provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected.
Attestation Report of the Independent Registered Public Accounting Firm
The effectiveness of our internal control over financial reporting as of December 31, 2023 has been audited by Marcum LLP, an independent registered public accounting firm, as stated in their report which is included below.
85
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
ON INTERNAL CONTROL OVER FINANCIAL REPORTING
To the Shareholders and Board of Directors of
Establishment Labs Holdings Inc.
Adverse Opinion on Internal Control over Financial Reporting
We have audited Establishment Labs Holdings Inc.’s (the "Company") internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, because of the effect of the material weakness described in the following paragraph on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
A material weakness is a control deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company's annual or interim financial statements will not be prevented or detected on a timely basis. The following material weakness have been identified and included in “Management's Annual Report on Internal Control Over Financial Reporting”:
It was determined that as of December 31, 2023, the Company's primary change management controls for direct database changes were not designed and implemented effectively for specific localities. Other Information Technology General Controls, automated process-level controls, and manual controls that are dependent upon the information derived from such financially relevant systems were also determined to be ineffective as a result of such deficiency.
This material weakness was considered in determining the nature, timing and extent of audit tests applied in our audit of the fiscal 2023 consolidated financial statements, and this report does not affect our report dated March 4, 2024 on those financial statements.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheets as of December 31, 2023 and the related consolidated statements of operations, comprehensive loss, shareholders’ (deficit) equity, and cash flows for the three years ended December 31, 2023 of the Company and our report dated March 4, 2024 expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company's management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying "Management Annual Report on Internal Control Over Financial Reporting". Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
86
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of the inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that degree of compliance with the policies or procedures may deteriorate.
/s/ Marcum LLP
Marcum LLP
Los Angeles, California
March 4, 2024
87
ITEM 9B. OTHER INFORMATION
Insider Trading Arrangements
During the three months ended December 31, 2023, no director or Section 16 officer of the Company adopted terminated
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Code of Business Conduct and Ethics
Our Board has adopted a Code of Business Conduct and Ethics that applies to all of our employees, officers and directors, including our chief executive officer, and other executive and senior financial officers. A copy of the code is posted under "Corporate Governance" in the Investors section of our website at https://establishmentlabs.com. If we make any substantive amendments to, or grant any waivers from, the Code of Business Conduct and Ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a current report on Form 8-K.
The remaining information required by this Item is incorporated by reference from the information in our Proxy Statement for our 2024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
ITEM 11. EXECUTIVE COMPENSATION
Incorporated by reference from the information in our Proxy Statement for our 2024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Incorporated by reference from the information in our Proxy Statement for our 2024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Incorporated by reference from the information in our Proxy Statement for our 2024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Incorporated by reference from the information in our Proxy Statement for our 2024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
PART IV
ITEM 15. EXHIBIT AND FINANCIAL STATEMENT SCHEDULES
The following statements are filed as part of this Annual Report on Form 10-K:
1. Financial Statements.
A listing of the Consolidated Financial Statements, related notes and Report of Independent Registered Public Accounting is set forth on page F-1 in this Annual Report on Form 10-K.
88
2. Financial Statement Schedules.
All schedules have been omitted since the required information is not present or is not present in amounts sufficient to require submission of a schedule, or because the information required is included in the financial statements or related notes.
3. Index to Exhibits.
| Exhibit Number | Description of Exhibit | Incorporation by Reference | ||||||||||||
| 3.1 | Incorporated by reference from Registrant’s Current Report on Form 8-K filed May 31, 2023 | |||||||||||||
| 4.1 | Incorporated by reference from Registrant’s Annual Report on Form 10-K filed March 16, 2019. | |||||||||||||
| 10.1 | Incorporated by reference from Registrant’s Current Report on Form 8-K filed December 10, 2021. | |||||||||||||
| 10.2+ | Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018. | |||||||||||||
| 10.3+ | Incorporated by reference from Registrant’s Form S-1/A filed July 9, 2018. | |||||||||||||
| 10.4+ | Incorporated by reference from Registrant’s Form S-1/A filed July 9, 2018. | |||||||||||||
| 10.5‡ | Incorporated by reference from Registrant’s Current Report on Form 8-K filed June 6, 2022. | |||||||||||||
| 10.6 | Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018. | |||||||||||||
| 10.7 | Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018. | |||||||||||||
| 10.8+ | Incorporated by reference from Registrant’s Current Report on Form 8-K filed December 28, 2018. | |||||||||||||
| 10.9 | Incorporated by reference from Registrant’s Current Report on Form 8-K filed June 26, 2019. | |||||||||||||
| 10.10 | Incorporated by reference from Registrant’s Current Report on Form 8-K filed April 28, 2022 | |||||||||||||
10.11 | Filed herewith. | |||||||||||||
10.12 | Incorporated by reference from Registrant’s Current Report on Form 8-K filed February 22, 2024 | |||||||||||||
10.13 | Incorporated by reference from Registrant’s Current Report on Form 8-K filed April 28, 2022 | |||||||||||||
10.14+ | Incorporated by reference from Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2022. | |||||||||||||
| Exhibit Number | Description of Exhibit | Incorporation by Reference | ||||||||||||
10.15+ | Incorporated by reference from Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2022. | |||||||||||||
10.16+ | Incorporated by reference from Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2022. | |||||||||||||
10.17+ | Incorporated by reference from Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2022. | |||||||||||||
10.18+ | Incorporated by reference from Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2022. | |||||||||||||
| 21.1 | Filed herewith. | |||||||||||||
| 23.1 | Filed herewith. | |||||||||||||
| 31.1 | Filed herewith. | |||||||||||||
| 31.2 | Filed herewith. | |||||||||||||
| 32.1* | Furnished herewith. | |||||||||||||
97.1 | Filed herewith. | |||||||||||||
| 101.INS | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | |||||||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document | |||||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |||||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |||||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |||||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |||||||||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |||||||||||||
‘+ Indicates management contract or compensatory plan or arrangement. ‡ Certain portions that constitute confidential information have been omitted in compliance with Item 601(b)(10) of Regulation S-K. * The certifications furnished in Exhibit 32.1 hereto is deemed to accompany this Annual Report on Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, except to the extent that the registrant specifically incorporates it by reference. | ||||||||||||||
ITEM 16. FORM 10-K SUMMARY
None.
| PAGE | |||||
Report of Independent Registered Public Accounting Firm (PCAOB ID: 688) | F-1 | ||||
| Consolidated Financial Statements | |||||
F-2 | |||||
F-3 | |||||
F-4 | |||||
F-5 | |||||
F-6 | |||||
F-8 | |||||
91
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Establishment Labs Holdings Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Establishment Labs Holdings Inc. (the “Company”) as of December 31, 2023 and 2022, the related consolidated statements of operations, comprehensive loss, shareholders’ (deficit) equity and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) ("PCAOB"), the Company's internal control over financial reporting as of December 31, 2023, based on the criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013 and our report dated March 4, 2024, expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting because of the existence of a material weakness.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Marcum LLP
Marcum LLP (688 )
We have served as the Company’s auditor since 2016.
March 4, 2024
F-1
ESTABLISHMENT LABS HOLDINGS INC.
Consolidated Balance Sheets
(In thousands, except share data)
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
Cash and cash equivalents | $ | $ | |||||||||
Accounts receivable, net of allowance for doubtful accounts of $ | |||||||||||
| Inventory, net | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Long-term assets: | |||||||||||
| Property and equipment, net of accumulated depreciation | |||||||||||
| Goodwill | |||||||||||
| Intangible assets, net of accumulated amortization | |||||||||||
| Right-of-use operating lease assets, net | |||||||||||
| Other non-current assets | |||||||||||
| Total assets | $ | $ | |||||||||
Liabilities and shareholders’ equity (deficit) | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued liabilities | |||||||||||
| Other liabilities, short-term | |||||||||||
| Total current liabilities | |||||||||||
| Long-term liabilities: | |||||||||||
| Note payable, Oaktree, net of debt discount and issuance costs | |||||||||||
| Operating lease liabilities, non-current | |||||||||||
| Other liabilities, long-term | |||||||||||
| Total liabilities | |||||||||||
Commitments and contingencies (Note 14) | |||||||||||
Shareholders’ equity (deficit): | |||||||||||
Common shares – zero par value, unlimited amount of shares authorized at December 31, 2023 and 2022; | |||||||||||
| Additional paid-in-capital | |||||||||||
Treasury shares, at cost, | ( | ( | |||||||||
| Accumulated deficit | ( | ( | |||||||||
| Accumulated other comprehensive income | |||||||||||
Total shareholders’ equity (deficit) | ( | ||||||||||
| Total liabilities and shareholders’ equity (deficit) | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-2
ESTABLISHMENT LABS HOLDINGS INC.
Consolidated Statements of Operations
(In thousands, except share and per share data)
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Revenue | $ | $ | $ | |||||||||||||||||
| Cost of revenue | ||||||||||||||||||||
| Gross profit | ||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Sales, general and administrative | ||||||||||||||||||||
| Research and development | ||||||||||||||||||||
| Total operating expenses | ||||||||||||||||||||
| Loss from operations | ( | ( | ( | |||||||||||||||||
| Interest income | ||||||||||||||||||||
| Interest expense | ( | ( | ( | |||||||||||||||||
| Change in fair value of derivative instruments | ||||||||||||||||||||
| Loss on extinguishment of debt | ( | |||||||||||||||||||
Other income (expense), net | ( | ( | ||||||||||||||||||
| Loss before income taxes | ( | ( | ( | |||||||||||||||||
Benefit (provision) for income taxes | ( | ( | ||||||||||||||||||
| Net loss | $ | ( | $ | ( | $ | ( | ||||||||||||||
| Basic and diluted net loss per share | $ | ( | $ | ( | $ | ( | ||||||||||||||
| Weighted average outstanding shares used for basic and diluted net loss per share | ||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
ESTABLISHMENT LABS HOLDINGS INC.
Consolidated Statements of Comprehensive Loss
(In thousands)
| Year Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Net loss | $ | ( | $ | ( | $ | ( | |||||||||||
| Other comprehensive income: | |||||||||||||||||
| Foreign currency translation gain (loss) | ( | ( | |||||||||||||||
| Other comprehensive income (loss) | ( | ( | |||||||||||||||
| Comprehensive loss | $ | ( | $ | ( | $ | ( | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
ESTABLISHMENT LABS HOLDINGS INC.
Consolidated Statements of Shareholders’ (Deficit) Equity
(In thousands, except share data)
| Common Shares | Treasury Shares | Additional Paid-In Capital | Accumulated Deficit | Accumulated Other Comprehensive Income | Total | |||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||
Balance at January 1, 2021 | $ | ( | $ | ( | $ | $ | ( | $ | $ | |||||||||||||||||||||||||||||||||||||||||
| Stock option exercises | — | — | ( | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Shares withheld to cover strike price upon cashless option exercise | ( | ( | — | — | — | — | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Shares withheld to cover income tax obligation upon vesting of restricted stock | ( | ( | — | — | ( | — | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Foreign currency translation gain (loss) | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2021 | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||||||||
| Warrant exercises | — | — | ( | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Stock option exercises | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Shares withheld to cover income tax obligation upon vesting of restricted stock | ( | — | — | — | ( | — | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Foreign currency translation gain (loss) | — | — | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2022 | ( | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||||||||||
Issuance of common stock, net of underwriters’ discount and issuance costs | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
Issuance of common shares, post-IPO | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
Issuance of common stock in lieu of cash compensation | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Stock option exercises | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Shares withheld to cover income tax obligation upon vesting of restricted stock | ( | — | ( | — | — | ( | — | — | ( | |||||||||||||||||||||||||||||||||||||||||
Foreign currency translation gain (loss) | — | — | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2023 | $ | ( | $ | ( | $ | $ | ( | $ | $ | |||||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
ESTABLISHMENT LABS HOLDINGS INC.
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Cash flows from operating activities: | ||||||||||||||||||||
| Net loss | $ | ( | $ | ( | $ | ( | ||||||||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||||||
| Depreciation and amortization | ||||||||||||||||||||
| Provision for doubtful accounts | ||||||||||||||||||||
| Provision for inventory obsolescence | ||||||||||||||||||||
Change in reserve for PP&E impairment | ( | |||||||||||||||||||
| Provision for deferred income taxes | ( | |||||||||||||||||||
| Share-based compensation | ||||||||||||||||||||
| Loss from disposal of property and equipment | ||||||||||||||||||||
Unrealized foreign currency (gain)/loss, net | ( | |||||||||||||||||||
| Loss on extinguishment of debt | ||||||||||||||||||||
| Amortization of right-to-use asset | ||||||||||||||||||||
| Gain from write-off of liability | ( | ( | ||||||||||||||||||
| Change in fair value of derivative instruments | ( | ( | ||||||||||||||||||
Stock compensation in lieu of cash fees | ||||||||||||||||||||
| Interest capitalized for construction in progress | ( | ( | ||||||||||||||||||
Non-cash interest expense and amortization of debt discount | ||||||||||||||||||||
| Changes in operating assets and liabilities: | ||||||||||||||||||||
| Accounts receivable | ( | ( | ( | |||||||||||||||||
| Inventory | ( | ( | ( | |||||||||||||||||
| Prepaid expenses and other current assets | ( | ( | ||||||||||||||||||
| Other assets | ( | |||||||||||||||||||
| Accounts payable | ||||||||||||||||||||
| Accrued liabilities | ( | |||||||||||||||||||
| Operating lease liabilities | ( | ( | ( | |||||||||||||||||
| Other liabilities | ||||||||||||||||||||
| Net cash used in operating activities | ( | ( | ( | |||||||||||||||||
| Cash flows from investing activities: | ||||||||||||||||||||
| Purchases of property and equipment | ( | ( | ( | |||||||||||||||||
| Cash used in asset acquisitions | ( | ( | ||||||||||||||||||
| Cost incurred for intangible assets | ( | ( | ( | |||||||||||||||||
| Capital expenditures on construction in progress | ( | ( | ( | |||||||||||||||||
| Net cash used in investing activities | ( | ( | ( | |||||||||||||||||
| Cash flows from financing activities: | ||||||||||||||||||||
| Issuance of common stock, net of underwriters’ discount and issuance costs | ||||||||||||||||||||
Borrowings under Oaktree credit agreement, net of debt discount and issuance costs | ||||||||||||||||||||
| Repayment of Madryn debt agreement | ( | |||||||||||||||||||
| Repayments on finance leases | ( | ( | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
ESTABLISHMENT LABS HOLDINGS INC.
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Proceeds from stock option exercises | ||||||||||||||||||||
| Tax payments related to shares withheld upon vesting of restricted stock | ( | ( | ( | |||||||||||||||||
| Net cash provided by financing activities | ||||||||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | ( | ( | ||||||||||||||||||
Net (decrease)/increase in cash and cash equivalents | ( | ( | ||||||||||||||||||
| Cash at beginning of period | ||||||||||||||||||||
Cash and cash equivalents at end of period | $ | $ | $ | |||||||||||||||||
| Supplemental disclosures: | ||||||||||||||||||||
| Cash paid for interest | $ | $ | $ | |||||||||||||||||
| Cash paid for income taxes | $ | $ | $ | |||||||||||||||||
| Supplemental disclosures of non-cash investing and financing activities: | ||||||||||||||||||||
| Unpaid balance for property and equipment | $ | $ | $ | |||||||||||||||||
Unpaid balance for intangible assets | $ | $ | $ | |||||||||||||||||
| Equity consideration in an asset acquisition | $ | $ | $ | |||||||||||||||||
| Consideration payable related to asset acquisition | $ | $ | $ | |||||||||||||||||
| Cashless option exercises | $ | $ | $ | |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
1. Formation and Business of the Company
Formation and Business of the Company
Establishment Labs Holdings Inc. and its wholly owned subsidiaries, or the Company, is a global company that manufactures and markets innovative medical devices for aesthetic and reconstructive plastic surgery. The Company was established in the British Virgin Islands on October 9, 2013, at which time Establishment Labs, S.A., the Costa Rican manufacturing company, was reincorporated as a wholly-owned subsidiary. As of December 31, 2023, the Company also has wholly-owned subsidiaries in the United States (JAMM Technologies, Inc. and Motiva USA LLC), Brazil (Establishment Labs Produtos para Saude Ltda), Belgium (European Distribution Center Motiva BV), France (Motiva Implants France SAS), Sweden (Motiva Nordica AB), Switzerland (JEN-Vault AG), the United Kingdom (Motiva Implants UK Limited), Italy (Motiva Italy S.R.L), Spain (Motiva Implants Spain, S.L.), Austria (Motiva Austria GmbH), Germany (Motiva Germany GmbH) and Argentina (Motiva Argentina S.R.L). Substantially all of the Company’s revenues are derived from the sale of silicone gel-filled breast implants, branded as Motiva Implants.
The main manufacturing activities are conducted at two manufacturing facilities in Costa Rica. The Company is in the process of finishing the construction for a third facility in Costa Rica and currently expects to commence manufacturing from the new facility in fiscal 2024. In 2010, the Company began operating under the Costa Rica free zone regime (Régimen de Zona Franca), which provides for reduced income tax and other tax obligations pursuant to an agreement with the Costa Rican authorities.
The Company’s products are approved for sale in Europe, the Middle East, Latin America, and Asia, and the Company’s Motiva Flora SmoothSilk Tissue Expander is also approved for sale in the United States. The Company sells its products internationally through a combination of distributors and direct sales to customers.
The Company is pursuing regulatory approval to commercialize its Motiva Implant products in the United States. The Company received approval for an investigational device exemption, or IDE, from the United States Food and Drug Administration, or FDA, in March 2018 to initiate a clinical trial in the United States for its Motiva Implants. In August 2019, the Company completed all patient surgeries for the IDE aesthetic cohorts, which include primary augmentation and revision. In 2021, the Company initiated a modular pre-market approval, or PMA, submission process with FDA and submitted the first of four modules. In April 2022, the Company released preliminary results of the two-year patient follow-up data for the primary augmentation cohort of its IDE clinical trial. The second module was submitted in May 2022. In June 2022, full enrollment of the IDE clinical trial was complete, and all surgeries in the primary reconstruction cohort were performed. In August 2022, the third module was submitted to the FDA. By June 30, 2022, the Company completed the three-year study subject follow-up for the aesthetic cohort. The final fourth module was submitted to the FDA in February 2023. The Company presented three-year patient follow-up data for the primary augmentation cohort of the IDE clinical trial at The Aesthetic Meeting in April 2023. In October 2023, the FDA granted 510(k) clearance for the Motiva Flora SmoothSilk Tissue Expander. In January 2024, the Company announced completion of the first commercial procedure of the Motiva Flora SmoothSilk Tissue Expander in the United States and the commercial launch of Motiva Implants in China.
2. Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
F-8
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
The consolidated financial statements include the Company’s accounts and those of its wholly owned subsidiaries as of December 31, 2023 as follows:
| Subsidiary | Incorporation/Acquisition Date | |||||||
| Establishment Labs, S.A. (Costa Rica) | January 18, 2004 | |||||||
| Motiva USA, LLC (USA) | February 20, 2014 | |||||||
| JAMM Technologies, Inc. (USA) | October 27, 2015 | |||||||
| Establishment Labs Produtos par Saude Ltda (Brazil) | January 4, 2016 | |||||||
| European Distribution Center Motiva BV (Belgium) | March 4, 2016 | |||||||
| Motiva Implants France SAS (France) | September 12, 2016 | |||||||
| JEN-Vault AG (Switzerland) | November 22, 2016 | |||||||
| Motiva Nordica AB (Sweden) | November 2, 2017 | |||||||
| Motiva Implants UK Limited (the United Kingdom) | July 31, 2018 | |||||||
| Motiva Italy S.R.L (Italy) | July 31, 2018 | |||||||
| Motiva Implants Spain, S.L. (Spain) | January 3, 2019 | |||||||
| Motiva Austria GmbH (Austria) | January 14, 2019 | |||||||
| Motiva Germany GmbH (Germany) | August 1, 2019 | |||||||
| Motiva Argentina S.R.L. (Argentina) | February 7, 2020 | |||||||
Segments
The chief operating decision maker for the Company is the Chief Executive Officer. The Chief Executive Officer reviews financial information presented on a consolidated basis, accompanied by information about revenue by geographic region, for purposes of allocating resources and evaluating financial performance. The Company has one business activity and there are no segment managers who are held accountable for operations, operating results or plans for levels or components below the consolidated unit level. Accordingly, the Company has determined that it has a single reportable and operating segment structure. The Company and its Chief Executive Officer evaluate performance based primarily on revenue in the geographic regions in which the Company operates.
Geographic Concentrations
For the years ended December 31, 2023, 2022 and 2021, Brazil accounted for 13.3 %, 16.6 % and 11.6 %, respectively, of consolidated revenue and no other individual country exceeded 10% of consolidated revenue, on a ship-to destination basis.
The majority of the Company’s consolidated total assets, including cash and tangible assets, is held in the United States. The Company’s long-lived assets, which primarily consist of property and equipment and intangible assets located in Costa Rica represented 80 % and 88 % of the total long-lived assets as of December 31, 2023 and 2022, respectively.
Use of Estimates
The preparation of financial statements in accordance with GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Significant accounting estimates and management judgments reflected in the consolidated financial statements
F-9
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
include items such as accounts receivable valuation and allowances, inventory valuation and allowances, valuation of acquired intangible assets, valuation of derivatives and valuation of deferred income tax assets, including tax valuation allowances. Estimates are based on historical experience, where applicable, and other assumptions believed to be reasonable by management. Actual results may differ from those estimates under different assumptions or conditions.
Concentration of Credit Risk and Other Risks and Uncertainties
Financial instruments that potentially subject the Company to a concentration of credit risk consist principally of cash and accounts receivable. The majority of the Company’s cash is held at two financial institutions in the United States. Balances in the Company’s cash accounts exceed the Federal Deposit Insurance Corporation, or FDIC, limit of $250,000. The Company has not experienced any losses to its deposits of cash.
Substantially all of the Company’s revenue has been derived from sales of its products in international markets, principally Europe, the Middle East, Latin America, and Asia. In the international markets in which the Company operates, the Company uses a combination of distributors and direct sales to customers. The Company performs ongoing credit evaluations of its distributors and customers, does not require collateral, and maintains allowances for potential credit losses on customer accounts when deemed necessary.
Substantially all of the Company’s revenues were derived from the sale of Motiva Implants. During the years ended December 31, 2023, 2022 and 2021, no customer accounted for more than 10% of the Company’s revenue. Two customers accounted for 12.7 % and 11.6 % of the Company’s trade accounts receivable balance as of December 31, 2023, respectively. One customer accounted for 11.5 % of the Company’s trade accounts receivable balance as of December 31, 2022.
The Company relies on Avantor, Inc. (formerly NuSil Technology, LLC) as the sole supplier of medical-grade silicone used in Motiva Implants. During the years ended December 31, 2023, 2022 and 2021, the Company had purchases of $53.7 million, or 68.4 % of total purchases, $32.1 million, or 38.2 % of total purchases, and $23.1 million or 51.4 % of total purchases, respectively, from Avantor Inc. As of December 31, 2023 and 2022, the Company had an outstanding balance owed to this vendor of $5.3 million and $5.6 million, respectively.
The Company’s financial condition and future results of operations involve a number of risks and uncertainties. Factors that could affect the Company’s future operating results and cause actual results to vary materially from expectations include, but are not limited to, unfavorable economic conditions, uncertainty of regulatory approval of the Company’s current and potential future products, uncertainty of market acceptance of the Company’s products, competition from substitute products and larger companies, securing and protecting proprietary technology, access to capital, strategic relationships and dependence on key individuals and sole source suppliers.
Cash and Cash Equivalents
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable is stated at invoice value less estimated allowances for returns and doubtful accounts. The Company continually monitors customer payments and maintains an allowance for estimated losses resulting from customers’ inability to make required payments. In evaluating the Company’s ability to collect outstanding receivable balances, the Company considers various factors including the age of the balance, the creditworthiness of the customer, which is assessed based on ongoing credit evaluations and payment history,
F-10
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
A roll-forward of the allowance for doubtful accounts is as follows:
| Year Ended December 31, | ||||||||||||||||||||
2023 | 2022 | 2021 | ||||||||||||||||||
(in thousands) | ||||||||||||||||||||
| Beginning balance | $ | $ | $ | |||||||||||||||||
| Provision for doubtful accounts | ||||||||||||||||||||
| Write-offs | ( | ( | ( | |||||||||||||||||
| Ending balance | $ | $ | $ | |||||||||||||||||
Inventory and Cost of Revenue
A roll-forward of the inventory reserve is as follows:
| Year Ended December 31, | ||||||||||||||||||||
2023 | 2022 | 2021 | ||||||||||||||||||
(in thousands) | ||||||||||||||||||||
| Beginning balance | $ | $ | $ | |||||||||||||||||
| Provision for inventory obsolescence | ||||||||||||||||||||
| Write-offs | ( | ( | ( | |||||||||||||||||
| Ending balance | $ | $ | $ | |||||||||||||||||
The Company recognizes the cost of inventory transferred to the customer in cost of revenue when revenue is recognized.
Leases
The Company determines if an arrangement is, or contains, a lease at the inception date of the contract. The Company has elected an expedient to account for each separate lease component and its associated non-lease components as a single lease component for the majority of its asset classes.
F-11
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
Shipping and Handling Costs
Revenue Recognition
The Company recognizes revenue related to sales of products to distributors or directly to customers in markets where it has regulatory approval, net of discounts and allowances. The Company recognizes revenue in accordance with Accounting Standards Codification, or ASC, Revenue from Contracts with Customers (Topic 606). ASC 606 requires the Company to recognize revenue to depict the transfer of goods or services to a customer at an amount that reflects the consideration it expects to receive in exchange for those goods or services.
The Company recognizes revenue related to the sales of products to distributors at the time of shipment of the product, which represents the point in time when the distributor has taken ownership and assumed the risk of loss, and the required revenue recognition criteria are satisfied since each product unit represents a single performance obligation. The Company’s distributors are obligated to pay within specified terms regardless of when, or if, they sell the products. The Company’s contracts with distributors typically do not contain right of return or price protection and have no post-delivery obligations.
The Company recognizes revenue when title to the product and risk of loss transfer to customers, provided there are no remaining performance obligations required of the Company or any written matters requiring customer acceptance. The Company allows for the return of product from direct customers in certain regions in limited instances within fifteen days after the original sale and records estimated sales returns as a reduction of sales in the same period revenue is recognized. Appropriate reserves are established for anticipated sales returns based on historical experience, recent gross sales and any notification of pending returns. Actual sales returns in any future period are inherently uncertain and thus may differ from the estimates. If actual sales returns differ significantly from the estimates, an adjustment to revenue in the current or subsequent period is recorded. As of December 31, 2023, an allowance of $0.3 million was recorded for product returns. As of December 31, 2022 and 2021, allowance for product returns was de minimis. Taxes collected from customers for remittance to governmental authorities are excluded from net sales.
A portion of the Company’s revenue is generated from the sale of consigned inventory maintained at physician, hospital, or clinic locations. For these products, revenue is recognized at the time the Company is notified by the consignee that the product has been implanted, not when the consigned products are delivered to the consignee’s warehouse.
Revenue was generated in these primary geographic markets:
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
(in thousands) | ||||||||||||||||||||
| EMEA (Europe / Middle East / Africa) | $ | $ | $ | |||||||||||||||||
| Latin America | ||||||||||||||||||||
| Asia-Pacific | ||||||||||||||||||||
| Other | ||||||||||||||||||||
Total revenue | $ | $ | $ | |||||||||||||||||
The Company has a limited warranty for the shelf life of breast implants, which is five years from the time of manufacture. Estimated warranty obligations are recorded at the time of sale. The Company also offers a warranty to patients in the event of rupture and a replacement program for capsular contracture events, provided
F-12
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
certain registration requirements are met. Revenue for extended warranties is recognized ratably over the term of the agreement. To date, these warranty and program costs have been de minimis. The Company will continue to evaluate the warranty reserve policies for adequacy considering claims history.
Deferred revenue primarily consists of payments received in advance of meeting revenue recognition criteria. The Company has received payments from distributors to provide distribution exclusivity within a geographic area and recognizes deferred revenue on a ratable basis over the term of such contractual distribution relationship. Additionally, the Company has received payments from customers in direct markets prior to surgical implantation and recognizes deferred revenue at the time the Company is notified by the customer that the product has been implanted. For all arrangements, any revenue that has been deferred and is expected to be recognized beyond one year is classified as long-term deferred revenue and included in “Other liabilities, long-term” on the consolidated balance sheets (see Note 3).
Research and Development
Costs related to research and development, or R&D, activities are expensed as incurred. R&D costs primarily include personnel costs, materials, clinical expenses, regulatory expenses, product development, consulting services, and outside research activities, all of which are directly related to research and development activities.
Selling, General and Administrative Expenses
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization.
Goodwill and Intangible Assets
The Company records the excess of the acquisition purchase price over the net fair value of the tangible and identifiable intangible assets acquired and liabilities assumed as goodwill. In accordance with ASC 350, Intangibles - Goodwill and Other, the Company tests goodwill for impairment annually during the fourth quarter of each year and whenever events or changes in circumstances indicate that the carrying value of the asset may not be recoverable. In connection with the annual impairment test for goodwill, the Company elected the option to perform a qualitative assessment to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying amount. If the Company determines that it was more likely than not that the fair value of the reporting unit is less than its carrying amount, then the impairment test is performed.
Consistent with the Company's assessment that it has only one reporting segment, the Company has determined that it has only one reporting unit and tests goodwill for impairment at the entity level using the two-step process required by ASC 350. In the first step, the Company compares the carrying amount of the reporting unit to the fair value of the enterprise. If the fair value of the enterprise exceeds the carrying value, goodwill is not considered impaired and no further testing is required. If the carrying value of the enterprise exceeds the fair value, goodwill is potentially impaired, and the second step of the impairment test must be performed. In the second step, the Company compares the implied fair value of the goodwill, as defined by ASC 350, to its carrying amount to
F-13
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
determine the impairment loss, if any.
The Company capitalizes certain costs related to intangible assets, such as patents, trademarks and software development costs. The Company follows the provisions of ASC 350-40, Internal Use Software for determining whether computer software is internal-use software and on accounting for the costs of computer software originally developed or obtained for internal use. The Company expenses all costs incurred during the preliminary project stage of software development and capitalizes the costs incurred during the application development stage. Costs incurred relating to upgrades and enhancements to the software are capitalized if it is determined that these upgrades or enhancements add additional functionality to the software. Costs incurred to improve and support products after they become available are charged to expense as incurred.
During the years ended December 31, 2023, 2022 and 2021, there has been no
Long-Lived Assets
Debt and Embedded Derivatives
The Company applies the accounting standards for derivatives and for distinguishing liabilities from equity when accounting for hybrid contracts. The Company accounts for convertible debt instruments when the Company has determined that the embedded conversion options should not be bifurcated from their host instruments in accordance with ASC 470-20 Debt with Conversion and Other Options (see Note 5).
Debt Issuance Costs and Debt Discounts
Costs incurred in connection with the issuance of new debt are capitalized. Capitalizable debt issuance costs paid to third parties and debt discounts, net of amortization, are recorded as a reduction to the long-term debt balance on the consolidated balance sheets. Amortization expense on capitalized debt issuance costs and debt discounts related to loans are calculated using the effective interest method over the term of the loan commitment and is recorded as interest expense in the consolidated statements of operations.
Income Taxes
The Company records income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s consolidated financial statements or income tax returns. In estimating future tax consequences, expected future events, enactments or changes in the tax law or rates are considered. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company operates in various tax jurisdictions and is subject to audits by various tax authorities.
F-14
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
The Company records uncertain tax positions based on a two-step process whereby (1) a determination is made as to whether it is more likely than not that the tax positions will be sustained based on the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold the Company recognizes the largest amount of tax benefit that is greater than 50% likely to be realized upon ultimate settlement with the related tax authority. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. Significant judgment is required in the identification of uncertain tax positions and in the estimation of penalties and interest on uncertain tax positions.
There were no material uncertain tax positions in the years ended December 31, 2023, 2022 and 2021.
Foreign Currency
Comprehensive Loss
Share-Based Compensation
The Company measures and recognizes compensation expense for all share-based awards in accordance with the provisions of ASC 718, Stock Compensation. Share-based awards granted include stock options, restricted stock units, or RSUs, and restricted stock awards, or RSAs. Share-based compensation expense for RSUs and RSAs granted is measured at the grant date based on the fair value of the awards and is recognized as an expense ratably on a straight-line basis over the requisite service period. The fair value of options to purchase shares is estimated on the grant date using the Black-Scholes option valuation model.
Net Income (Loss) Per Share
Reclassifications
Certain reclassifications have been made to prior year amounts to conform to the current year presentation. These reclassifications had no material impact on the Company’s financial position as of December 31, 2023 or results of operations for year ended December 31, 2023.
F-15
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
Recent Accounting Standards
Periodically, new accounting pronouncements are issued by the Financial Accounting Standards Board, or FASB, or other standard setting bodies and adopted by the Company as of the specified effective date. Unless otherwise discussed, the impact of recently issued standards that are not yet effective will not have a material impact on the Company’s consolidated financial statements upon adoption.
Recently Adopted Accounting Standards
In August 2020, the FASB issued Accounting Standards Update, or ASU, No. 2020-06, Debt with Conversion and other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40). The new guidance eliminates the beneficial conversion and cash conversion accounting models for convertible instruments. It also amends the accounting for certain contracts in an entity’s own equity that are currently accounted for as derivatives because of specific settlement provisions. In addition, the new guidance modifies how particular convertible instruments and certain contracts that may be settled in cash or shares impact the diluted EPS computation. The Company adopted ASU No. 2020-06 as of January 1, 2022 and the adoption did not have a material impact on its consolidated financial statements and related disclosures.
Recently Issued Accounting Standards
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280):Improvements to Reportable Segment Disclosures, which expands annual and interim disclosure requirements for reportable segments, especially significant segment expenses, and provides new disclosure requirements for entities with a single reportable segment. The new guidance is effective for our annual periods beginning January 1, 2024, and for interim periods beginning January 1, 2025. The Company is currently evaluating the potential impact of the updated requirements, but based on current understanding, does not expect a material impact on its consolidated financial statements and related disclosures.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topics 740): Improvements to Income Tax Disclosures, which enhances the disclosure requirements for income taxes, specifically related to the rate reconciliation and income taxes paid. The guidance is effective for annual periods beginning January 1, 2025, with early adoption permitted. The Company is currently evaluating the potential effect that the updated standard will have on the financial statement disclosures.
3. Balance Sheet Accounts
Inventory, Net
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
(in thousands) | ||||||||||||||
| Raw materials | $ | $ | ||||||||||||
| Work in process | ||||||||||||||
| Finished goods | ||||||||||||||
Total inventory, net | $ | $ | ||||||||||||
As of December 31, 2023 and 2022, $7.1 million and $2.0 million of inventory was on consignment, respectively.
F-16
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
Prepaid Expenses and Other Current Assets
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
(in thousands) | ||||||||||||||
| Prepaid insurance | $ | $ | ||||||||||||
| Prepaid construction in process | ||||||||||||||
| Prepaid services | ||||||||||||||
| Prepaid taxes | ||||||||||||||
| Prepaid assets | ||||||||||||||
| Prepaid raw materials and accessories | ||||||||||||||
| Prepaid U.S. clinical trial costs | ||||||||||||||
| Prepaid warranty and distribution rights | ||||||||||||||
| Prepaid software | ||||||||||||||
Other | ||||||||||||||
Total prepaid expenses | $ | $ | ||||||||||||
Property and Equipment, Net
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
(in thousands) | ||||||||||||||
| Machinery and equipment | $ | $ | ||||||||||||
| Building improvements | ||||||||||||||
| Furniture and fixtures | ||||||||||||||
| Building | ||||||||||||||
| Leasehold improvements | ||||||||||||||
| Land | ||||||||||||||
| Vehicles | ||||||||||||||
| Construction in process | ||||||||||||||
| Total | ||||||||||||||
| Less: Accumulated depreciation and amortization | ( | ( | ||||||||||||
Total property and equipment, net | $ | $ | ||||||||||||
For the years ended December 31, 2023, 2022 and 2021, depreciation and amortization expense related to property and equipment was $2.8 million, $2.5 million and $2.5 million, respectively.
In August 2021, the Company entered into a contract with the Zona Franca Coyol, S.A., or CFZ, to begin construction of a new manufacturing facility in Costa Rica. The costs for improvement of the land and construction of a cold shell building were being paid for by CFZ while the Company was paying for internal improvements and customization. In 2022, the Company exercised it’s option to purchase the title to the land and cold shell building for approximately $12.6 million. The Company has the option to buy an adjacent lot of land for approximately
F-17
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
$2.8 million and engage CFZ to construct an additional manufacturing facility. In July 2023, the Company announced the grand opening of the first phase of the Sulàyöm Innovation Campus. The Company currently expects to complete construction and commence manufacturing from the new facility in fiscal 2024.
Accrued Liabilities
Accrued liabilities consisted of the following:
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
(in thousands) | ||||||||||||||
| Performance bonus | $ | $ | ||||||||||||
| Payroll and related expenses | ||||||||||||||
| Bonus feature of stock option grants | ||||||||||||||
| Commissions | ||||||||||||||
| Professional and legal services | ||||||||||||||
| Warranty reserve | ||||||||||||||
| Taxes | ||||||||||||||
| Other | ||||||||||||||
Total accrued liabilities | $ | $ | ||||||||||||
Other Liabilities, Short-Term
Other liabilities, short-term consisted of the following:
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
(in thousands) | ||||||||||||||
| Deferred revenue | ||||||||||||||
Other Liabilities, Long-Term
Other liabilities, long-term consisted of the following:
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
(in thousands) | ||||||||||||||
| Deferred revenue | $ | $ | ||||||||||||
| Other | ( | |||||||||||||
Total other liabilties, long-term | $ | $ | ||||||||||||
F-18
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
4. Goodwill and Other Intangible Assets
Goodwill represents the excess of the purchase price over the fair value of the net tangible and identifiable intangible assets acquired in a business combination. Intangible assets resulting from the acquisitions of entities accounted for using the acquisition method of accounting are recorded at the estimated fair value of the assets acquired. Purchased intangibles include certain patents and license rights, 510(k) authorization by the FDA to sell a medical device and other intangible assets.
The Company’s goodwill and most intangibles at December 31, 2023 are the result of previous asset and business acquisitions. Finite-lived intangibles are amortized over their estimated useful lives based on expected future benefit.
In addition to the intangibles acquired, the Company capitalized certain patent and license rights as identified intangibles based on patent and license rights agreements entered into over the past several years. Additionally, the Company capitalized certain software development costs.
There were no changes in the carrying amount of goodwill during the year ended December 31, 2023:
Balance as of January 1, 2023 | Additions | Accumulated Impairment Losses | Balance as of December 31, 2023 | ||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| Goodwill | $ | $ | $ | $ | |||||||||||||||||||
The carrying amounts of these intangible assets other than goodwill as of December 31, 2023 were as follows:
| Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Estimated Useful Lives | ||||||||||||||||||||
| (in thousands) | (in years) | ||||||||||||||||||||||
| Patents and license rights | $ | $ | ( | $ | |||||||||||||||||||
| Customer relationships | ( | ||||||||||||||||||||||
| 510(k) authorization | ( | ||||||||||||||||||||||
| Developed technology | ( | ||||||||||||||||||||||
| Capitalized software development costs | ( | ||||||||||||||||||||||
| Other | ( | ||||||||||||||||||||||
Capitalized software development costs not yet amortized | — | ||||||||||||||||||||||
Patents and license rights not yet amortized | — | ||||||||||||||||||||||
Total intangibles other than goodwill | $ | $ | ( | $ | |||||||||||||||||||
F-19
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
The carrying amounts of intangible assets other than goodwill as of December 31, 2022 were as follows:
| Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Estimated Useful Lives | ||||||||||||||||||||
| (in thousands) | (in years) | ||||||||||||||||||||||
| Patents and license rights | $ | $ | ( | $ | |||||||||||||||||||
| Customer relationships | ( | ||||||||||||||||||||||
| 510(k) authorization | ( | ||||||||||||||||||||||
| Developed technology | ( | ||||||||||||||||||||||
| Capitalized software development costs | ( | ||||||||||||||||||||||
| Other | ( | ||||||||||||||||||||||
| Capitalized patents and license rights not yet amortized | — | ||||||||||||||||||||||
Total intangibles other than goodwill | $ | $ | ( | $ | |||||||||||||||||||
The amortization expense associated with intangible assets was $1.2
As of December 31, 2023, the amortization expense related to identifiable intangible assets, with definite useful lives, in future periods is expected to be as follows:
| Year Ending December 31, | (in thousands) | |||||||
2024 | $ | |||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| 2028 | ||||||||
| Thereafter | ||||||||
Total expected future amortization expense | $ | |||||||
The Company evaluates the recoverability of goodwill and indefinite-lived intangible assets annually and whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. As of December 31, 2023, there has been no impairment of goodwill or intangible assets based on the qualitative assessments performed by the Company. As of December 31, 2023, no triggering events have occurred which would indicate that the acquired intangible asset values may not be recoverable.
F-20
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
5. Debt
Oaktree Debt
On April 26, 2022, or the Closing Date, the Company entered into a Credit Agreement and Guaranty, or the Credit Agreement, together with certain of its subsidiaries party thereto as guarantors, the lenders from time to time party thereto, or the Lenders, and Oaktree Fund Administration, LLC, as administrative agent for the Lenders, or the Administrative Agent, pursuant to which the Lenders agreed to make term loans to the Company in an aggregate principal amount of up to $225 million, or collectively, the Term Loans.
Pursuant to the terms of the Credit Agreement, the Term Loans will be advanced in four tranches:
•The first tranche, or the Tranche A Term Loan, was advanced in the amount of $150 million on the Closing Date. A portion of the first tranche was used to repay the outstanding principal and interest under the Company’s credit agreement with Madryn Health Partners, LP, as administrative agent, and a syndicate of lenders in full, including the early repayment penalty of $6.5 million.
•The second tranche, or the Tranche B Term Loan, of $25 million was advanced in December 2022 at the Company’s election upon satisfaction of specified gross sales thresholds and subject to the other terms and conditions of the Credit Agreement.
•The third tranche, or the Tranche C Term Loan, of $25 million will be advanced at the Company’s election prior to March 31, 2024, subject to the Administrative Agent having received either (a) evidence that specified FDA approvals have been issued or (b) evidence that specified gross sales thresholds have been met, and subject to the other terms and conditions of the Credit Agreement.
•The fourth tranche, or the Tranche D Term Loan, of $25 million will be advanced at the Company’s election prior to December 31, 2024, subject to the Administrative Agent having received both (a) evidence that specified FDA approvals have been issued and (b) evidence that specified gross sales thresholds have been met, or the Tranche D Funding Milestone, and subject to the other terms and conditions of the Credit Agreement.
The Term Loans will mature on the 5-year anniversary of the Closing Date, or the Maturity Date. The Term Loans accrue interest at a rate equal to 9 % per annum or, at any time following the Tranche D Funding Milestone, 8.25 % per annum. Accrued interest is due and payable in cash on the last business day of March, June, September, and December of each year; provided, however, that prior to the second anniversary of the Closing Date, the Company may pay an amount of interest on the outstanding Term Loans corresponding to 600 basis points of the interest rate in kind, or PIK, on each applicable payment date, subject to prior written notice delivered to the Administrative Agent, which has been delivered. Each of the Term Loans will be subject to the original issue discount of 2 % of the principal amount thereof upon the drawing of each applicable tranche. Upon any payment or prepayment in full or in part of the Term Loans, whether voluntary or involuntary, the Company is required to pay an exit fee equal to 3 % of the principal amount of the Term Loan paid, or the Exit Fee.
The Company may elect to prepay all or any portion of the amounts owed prior to the Maturity Date, provided that the Company provides notice to the Administrative Agent, the amount is not less than $5 million, and the amount is accompanied by all accrued and unpaid interest thereon through the date of prepayment, plus the applicable yield protection premium and the applicable Exit Fee. Prepayments of the Term Loans prior to the second anniversary of the Closing Date will be accompanied by a yield protection premium equal to the sum of all interest that would have accrued through such second anniversary plus 4 % of the principal amount so prepaid. Prepayments of the Term Loans after the second anniversary will be accompanied by a yield protection premium equal to 4 % of the principal amount so prepaid if made prior to the third anniversary of the Closing Date, 2 % if made on or after the 3rd anniversary of the Closing Date but prior to the fourth anniversary of the Closing Date, and 0 % if made on or after the 4th anniversary of the Closing Date. If the Term Loans are accelerated following the occurrence of an event of default, the Company shall immediately pay to Lenders the sum of all obligations for principal, accrued interest, the applicable yield maintenance premium and the applicable Exit Fee.
Pursuant to the Credit Agreement, the obligations of the Company are guaranteed by its subsidiaries that are party thereto as guarantors. On the Closing Date, the Company and such subsidiaries entered into a U.S. Security Agreement in favor of the Administrative Agent on behalf of Lenders, or the U.S. Security Agreement. Pursuant to the U.S. Security Agreement, the Company and its subsidiaries party thereto granted the
F-21
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
Administrative Agent a security interest in substantially all of its personal property, rights and assets to secure the payment of all amounts owed to Lenders under the Credit Agreement.
The Credit Agreement contains customary affirmative and restrictive covenants and representations and warranties. The Company and its subsidiaries are bound by certain affirmative covenants setting forth actions that are required during the term of the Credit Agreement, including, without limitation, certain information delivery requirements, obligations to maintain certain insurance, and certain notice requirements. Additionally, the Company and its subsidiaries are bound by certain restrictive covenants setting forth actions that are not permitted to be taken during the term of the Credit Agreement without prior written consent, including, without limitation, incurring certain additional indebtedness, consummating certain mergers, acquisitions or other business combination transactions, or incurring any non- permitted lien or other encumbrance on the assets of the Company or any of its subsidiaries. The Credit Agreement also contains other customary provisions, such as confidentiality obligations and indemnification rights for the benefit of Lenders. The Credit Agreement contains financial covenants requiring (a) the Company to maintain minimum liquidity of at least $20 million from and after the Closing Date or $25 million from and after the funding of the Tranche B Term Loans, and (b) for each fiscal quarter until gross sales of the Company and its subsidiaries for any 12 -consecutive month period are no less than $200 million, minimum gross sales of the Company and its subsidiaries for each consecutive 12-month period ending on the last day of each fiscal quarter in excess of 50 % of specified target gross sales for such period. The Credit Agreement provides for a customary equity cure right in the event the Company fails to comply with the minimum gross sales covenant.
The effective interest rate under the Credit Agreement is 10.4 %, and the weighted average interest rate is 9.0 %. The Company elected to pay interest in kind on up to two-thirds of cash interest payments prior to the second anniversary of the Closing Date, resulting in a minimum initial cash interest rate of 3.00 %. The Company incurred $16.9 million and $9.4 million in interest expense in connection with the Credit Agreement during the years ended December 31, 2023 and 2022, respectively. No principal payments are due on the Term Loans until the final maturity date on April 26, 2027.
As of December 31, 2023, $192.6 million was outstanding under the Credit Agreement representing the initial principal of $150 million for the Tranche A Term Loan and $25 million for the Tranche B Term Loan and $17.6 million of interest accrued into the principal balance.
The Company recorded Oaktree debt on the consolidated balance sheets as follows:
| December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| (in thousands) | |||||||||||
| Principal | $ | $ | |||||||||
| Net unamortized debt discount and issuance costs | ( | ( | |||||||||
| Net carrying value of Oaktree debt | $ | $ | |||||||||
As of December 31, 2023, the Company was in compliance with all financial debt covenants.
Madryn Debt
In August 2017, the Company entered into the Madryn Credit Agreement with Madryn and a syndicate of lenders that was scheduled to mature on September 30, 2025. After an amendment in August 2020, the Madryn Credit Agreement provided for term loans in a maximum aggregate principal amount of $65.0 million.
In connection with the Madryn Credit Agreement, the Company and certain of its subsidiaries granted a security interest in substantially all of their respective assets, including, without limitation, intellectual property, and pledges of certain shares of the Company’s subsidiaries, subject to certain excluded collateral exceptions.
Borrowings under the Madryn Credit Agreement bore interest at a rate equal to 3-month LIBOR plus 8.0 % per annum provided that no default had occurred. The effective interest rate under the amended Madryn Credit
F-22
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
Agreement was 18.4 %, and the weighted average interest rate was approximately 10.6 % until extinguishment. The Company incurred $2.2 million and $6.9 million in interest expense in connection with Madryn Credit Agreement during the years ended December 31, 2022 and 2021, respectively. No principal payments were due on the term loans until the final maturity date on September 30, 2025.
The Company also determined that the Madryn Credit Agreement contained put options which were mandatory repayment provisions related to liquidity events or an event of default and a call option related to voluntary repayment option. The Company allocated a fair value of $15.1 million for these embedded derivatives as a debt discount on the original commitment date in August 2017. An additional $5.0 million and $1.6 million debt discount was recorded on respective borrowing dates when the Company met the required milestones and borrowed an additional $10.0 million in the fourth quarter of fiscal 2017 and $25.0 million in August 2019. The Company revalued the embedded derivatives as of each reporting period and recorded the change in the fair value in the consolidated statement of operations.
On April 26, 2022 the Company repaid in full the $65.0 million in aggregate principal amount outstanding under the Madryn Credit Agreement and the agreement was terminated. The Company recorded a loss on the extinguishment of debt in the amount of $19.0 million, which represents the difference between the carrying value of debt and the cash outflows to extinguish the debt including $6.5 million of the early repayment penalty.
As of December 31, 2023 and 2022, the Company did not have any Madryn debt recorded on the balance sheet.
6. Leases
The Company recognizes lease liabilities and ROU assets upon commencement for all material leases with a term greater than 12 months. The Company has elected an expedient not to recognize leases with a lease term of 12 months or less on the balance sheet. These short-term leases are expensed on a straight-line basis over the lease term.
ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. ROU assets and lease liabilities are recognized at commencement date of the lease based on the present value of lease payments over the lease term. When the rate implicit to the lease cannot be readily determined, the Company utilizes its incremental borrowing rate in determining the present value of the future lease payments. Lease liabilities are accreted each period and reduced for payments. The ROU asset also includes other adjustments, such as for the effects of escalating rents, rent abatement or initial lease costs. The lease term may include periods covered by options to extend or terminate the lease when it is reasonably certain that the Company will exercise a renewal option, or reasonably certain it will not exercise an early termination option. For operating leases, lease expense for minimum lease payments is recognized on a straight-line basis over the expected lease term. For finance leases, the ROU asset depreciates on a straight-line basis over the shorter of the lease term or useful life of the ROU asset and the lease liability accretes interest based on the interest method using the discount rate determined at lease commencement. The Company’s finance leases are not material.
The Company has operating leases for facilities and office spaces. Operating lease assets and the related lease liabilities are included within the ROU operating lease assets on the consolidated balance sheets. The determination of whether an arrangement is, or contains, a lease is performed at the inception of the arrangement. The Company has operating leases for certain facilities and office spaces to be used in its operations, with remaining lease terms ranging from monthly to 7
During the year ended December 31, 2023, the Company earned income from subleasing a warehouse facility for the remaining life of an existing master lease. The sublease agreement did not release the Company from its obligations under the master lease, and no modifications were made to the lease agreement. Income from the sublease is recognized on a straight-line basis over the term of the agreement.
F-23
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
The Company’s lease and sublease agreements do not contain any termination options, material residual value guarantees, material bargain purchase options or material restrictive covenants. The Company does not have any lease transactions with related parties.
Total lease cost includes the following components for the years ended December 31, 2023 and 2022:
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| (in thousands) | ||||||||||||||
| Operating lease expense cost | $ | $ | ||||||||||||
| Sublease income | $ | ( | $ | |||||||||||
| Total lease cost, net of sublease income | $ | $ | ||||||||||||
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
Supplemental balance sheet information | (in thousands) | |||||||||||||
| Operating lease right-of-use assets | $ | $ | ||||||||||||
| Operating lease liabilities - short-term | ||||||||||||||
| Operating lease liabilities - long-term | ||||||||||||||
| Total operating lease liabilities | $ | $ | ||||||||||||
Weighted-average remaining lease term (years) | ||||||||||||||
Operating leases | ||||||||||||||
Weighted-average discount rate (%) | ||||||||||||||
| Operating leases | % | % | ||||||||||||
F-24
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
| December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Cash paid for amounts included in the measurement of lease liabilities | (in thousands) | |||||||||||||
| Operating cash outflows from operating leases | $ | $ | ||||||||||||
| Operating cash inflows from subleases | $ | ( | $ | |||||||||||
| Operating cash outflows from operating leases, net of sublease income | $ | $ | ||||||||||||
| ROU assets obtained in exchange for new lease liabilities | ||||||||||||||
| Operating leases | $ | $ | ||||||||||||
Maturities of lease liabilities as of December 31, 2023 were as follows:
| Years Ending December 31, | Operating Leases | |||||||
| (in thousands) | ||||||||
2024 | $ | |||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| 2028 | ||||||||
| Thereafter | ||||||||
| Total future minimum lease payments | ||||||||
| Less: Amount of lease payments representing interest | ( | |||||||
| Present value of future minimum lease payments | $ | |||||||
The undiscounted future cash receipts from the Company’s sublease as of December 31, 2023 were as follows:
| Years Ending December 31, | Sublease | ||||||||||
| (in thousands) | |||||||||||
| 2024 | $ | ||||||||||
| 2025 | |||||||||||
| 2026 | |||||||||||
| 2027 | |||||||||||
| 2028 | |||||||||||
| Thereafter | |||||||||||
Total undiscounted future sublease cash receipts | $ | ||||||||||
In September 2023, the Company signed a lease for an office space in Irving, Texas that commenced on January 1, 2024 upon completion of tenant improvements. No 5.25 years with an option to renew, and the monthly rent will start at $21,800 and increase annually until it reaches $24,500 in the last year. The first three months of rent are waived.
F-25
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
7. Shareholders’ Equity (Deficit)
Under the Company’s Amended and Restated Memorandum of Association and Articles of Association, or Articles, the Company has authorized an unlimited number of common shares with no par value.
As of December 31, 2023 and 2022, 26,495,250 and 24,815,908 common shares, respectively, were issued and 26,087,180 and 24,407,838 common shares, respectively, were outstanding.
During the year ended December 31, 2023, the Company granted stock options and restricted stock to employees and contractors (see Note 9).
On April 27, 2023, the Company issued 1,100,000 common shares in an underwritten public offering, at a price to the public of $71.50 per share. The underwriters purchased the shares from the Company at a price of $67.21 per share and exercised the option to purchase additional 165,000 common shares, at the public offering price per share. Net proceeds to the Company after deducting underwriting discounts and offering expenses were approximately $84.6 million.
The Company had reserved common shares for future issuances at December 31:
| 2023 | 2022 | |||||||||||||
| Options to purchase common shares | ||||||||||||||
| Remaining shares available under the 2018 Equity Incentive Plan | ||||||||||||||
| Shares issuable on vesting of grants of RSUs | ||||||||||||||
| Remaining shares available under the 2018 ESPP | ||||||||||||||
Total reserved common shares for future issuances | ||||||||||||||
8. Warrants
In March 2017, the Company issued warrants for the purchase of 145,000 Class B ordinary shares to parties related to Rockport Ventures, with a fixed exercise price of $3.80 per share.
During the year ended December 31, 2022, warrants to purchase 5,000 shares were net exercised to obtain 4,703 shares. As of December 31, 2023 and 2022, no
9. Share-Based Compensation
In 2015, the Board of Directors approved and adopted the 2015 Equity Incentive Plan, or 2015 Plan. Pursuant to the 2015 Plan, the Company granted RSAs and stock options to members of the Board of Directors, employees and consultants.
In 2018, the Board of Directors terminated the 2015 Plan and approved the 2018 Equity Incentive Plan, or the 2018 Plan, with an initial reserve of 1,500,000 common shares. Under the 2018 Plan, the Company may grant stock options, equity appreciation rights, RSUs and RSAs. If an award granted under the 2018 Plan expires, terminates, is unexercised, or is forfeited, or if any shares are surrendered in connection with an incentive award, the shares subject to such award and the surrendered shares become available for further awards under the 2018 Plan.
Pursuant to the “evergreen” provision contained in the 2018 Plan, the number of common shares reserved for issuance under the 2018 Plan automatically increases on first day of each fiscal year, commencing on January 1, 2019, in an amount equal to the least of (1) 750,000 shares, (2) 4 % of the total number of the Company’s common shares outstanding on the last day of the preceding fiscal year, or (3) a number of common shares as may be determined by the Company’s Board of Directors prior to any such increase date. On each of January 1, 2019 through 2023 the number of common shares authorized for issuance increased automatically by 750,000 5,250,000 as of December 31, 2023.
F-26
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
During the periods presented, the Company recorded the following share-based compensation expense for stock options, RSUs and RSAs:
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Sales, general and administrative | $ | $ | $ | |||||||||||||||||
| Research and development | ||||||||||||||||||||
Total stock compensation expense | $ | $ | $ | |||||||||||||||||
Stock Options
| Number of Options Outstanding | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | |||||||||||||||||||||||
Balance at December 31, 2022 | $ | $ | ||||||||||||||||||||||||
Granted (weighted-average fair value of $ | ||||||||||||||||||||||||||
| Exercised | ( | |||||||||||||||||||||||||
| Forfeited/canceled | ( | |||||||||||||||||||||||||
Balance at December 31, 2023 | $ | $ | ||||||||||||||||||||||||
As of December 31, 2023, 931,798 options were vested and exercisable with weighted-average exercise price of $39.57 per share and a total aggregate intrinsic value of $4.0 million.
During the year ended December 31, 2023, 349,967 options were exercised at a weighted-average price of $19.06 per share. The intrinsic value of the options exercised during the years ended December 31, 2023, 2022, and 2021 was $5.1 million, $15.0 million, and $28.7 million, respectively. Upon the exercise of stock options, the Company issued new shares from its authorized shares.
At December 31, 2023, unrecognized compensation expense was $12.7 million related to stock options granted to employees and members of the Board of Directors and $1.0 million related to stock options granted to consultants. The weighted-average period over which such compensation expense will be recognized is 1.9 years.
Stock Options Granted to Employees
Share-based compensation expense for employees is based on the grant date fair value. The Company recognizes compensation expense for all share-based awards ratably on a straight-line basis over the requisite service period of the awards, which is generally the vesting term of four years . During the year ended December 31, 2023, 2022 and 2021, the Company recognized $8.7 million, $9.9 million and $8.2 million, respectively, of share-based compensation expense for stock options granted to employees.
The Company uses the Black-Scholes option valuation model to value options granted to employees and consultants, which requires the use of highly subjective assumptions to determine the fair value of share-based awards. The assumptions used in the Company’s option-pricing model represent management’s best estimates. These estimates are complex, involve a number of variables, uncertainties and assumptions and the application of management’s judgment. If factors change and different assumptions are used, the Company’s share-based
F-27
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
compensation expense could be materially different in the future. The assumptions and estimates that the Company uses in the Black-Scholes model are as follows:
▪Fair Value of Common Shares. The closing price of the Company’s publicly-traded common shares on the date of grant is used as the fair value of the shares. The Board of Directors intended all options granted to be exercisable at a price per share not less than the estimated per share fair value of the shares underlying those options on the date of grant.
▪Risk-Free Interest Rate. The Company bases the risk-free interest rate used in the Black-Scholes valuation model on the implied yield available on U.S. Treasury zero-coupon issues with a term equivalent to that of the term of the options for each option group on the measurement date.
▪Term. For employee stock options, the expected term represents the period that the Company’s share-based awards are expected to be outstanding. Because of the limitations on the sale or transfer of the Company’s shares during the period the Company was a privately held company, the Company does not believe its historical exercise pattern is indicative of the pattern it experiences as a publicly traded company. The Company consequently uses the Staff Accounting Bulletin 110, or SAB 110, simplified method to calculate the expected term of employee stock options, which is the average of the contractual term and vesting period. The Company plans to continue to use the SAB 110 simplified method until it has sufficient trading history as a publicly traded company. For consultant stock options, the term used is equal to the contractual term on the measurement date.
▪Volatility. The Company determines the price volatility based on the historical volatilities of industry peers as it does not have sufficient trading history for its shares. Industry peers consist of several public companies in the medical device industry with comparable characteristics, including revenue growth, operating model and working capital requirements. The Company intends to continue to consistently apply this process using the same or a similar peer group of public companies until a sufficient amount of historical information regarding the volatility of its own shares becomes available, or unless circumstances change such that the identified peer companies are no longer similar, in which case other suitable peer companies whose common share prices are publicly available would be utilized in the calculation. The volatility is calculated based on the term on the measurement date.
▪Dividend Yield. The expected dividend assumption is based on the Company’s current expectations about its anticipated dividend policy. The Company has no expectation that it will declare dividends on its common shares, and therefore has used an expected dividend yield of zero.
The fair value of stock options granted to employees was estimated using the following assumptions:
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Volatility | ||||||||||||||||||||
| Risk-free interest rate | ||||||||||||||||||||
| Term (in years) | ||||||||||||||||||||
| Dividend yield | ||||||||||||||||||||
Stock Options Granted to Non-Employees
Share-based compensation expense related to stock options granted to non-employees is recognized as the stock options are earned using an accelerated attribution method. The Company believes that the estimated fair value of the stock options is more readily measurable than the fair value of the services rendered. For the years ended December 31, 2023, 2022 and 2021, the Company recognized expense of $0.9 million, $0.4 million and $1.8 million, respectively, for stock options granted to consultants.
F-28
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
The fair value of stock options granted to consultants was estimated using the following assumptions during the following periods presented:
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Volatility | ||||||||||||||||||||
| Risk-free interest rate | ||||||||||||||||||||
| Term (in years) | ||||||||||||||||||||
| Dividend yield | ||||||||||||||||||||
Restricted Stock
Each vested RSU entitles the holder to be issued one common share. These awards vest according to a vesting schedule determined by the Board of Directors or the Compensation Committee of the Company’s Board of Directors, generally over a to four year period.
The following table represents RSU activity for fiscal 2023:
| Restricted Stock | Weighted- Average Grant Date Fair Value | |||||||||||||
Outstanding unvested at December 31, 2022 | $ | |||||||||||||
| Granted | ||||||||||||||
| Vested | ( | |||||||||||||
| Forfeited/canceled | ( | |||||||||||||
Outstanding unvested at December 31, 2023 | $ | |||||||||||||
The fair value of RSUs is the grant date market value of common shares. The Company recognizes share-based compensation expense related to RSUs using a straight-line method over the vesting term of the awards. The share-based compensation expense for RSUs that vested during the years ended December 31, 2023, 2022 and 2021 was $4.8 million, $3.0 million and $0.4 million, respectively, which was calculated based on the market value of the Company’s common shares on the applicable grant date.
F-29
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
10. Income Taxes
For the year ended December 31, loss before income tax consisted of the following:
2023 | 2022 | 2021 | |||||||||||||||
| (in thousands) | |||||||||||||||||
| Costa Rica operations | $ | ( | $ | ( | $ | ||||||||||||
| Non-Costa Rica operations | ( | ( | ( | ||||||||||||||
Total loss before income taxes | $ | ( | $ | ( | $ | ( | |||||||||||
For the year ended December 31, the income tax (benefit) provision consisted of the following:
2023 | 2022 | 2021 | |||||||||||||||
| (in thousands) | |||||||||||||||||
Current: | |||||||||||||||||
| Costa Rica | $ | $ | $ | ||||||||||||||
| Non-Costa Rica | |||||||||||||||||
| Total current | |||||||||||||||||
Deferred: | |||||||||||||||||
| Costa Rica | |||||||||||||||||
| Non-Costa Rica | ( | ||||||||||||||||
| Total deferred | ( | ||||||||||||||||
Total (benefit) provision for income taxes | $ | ( | $ | $ | |||||||||||||
F-30
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
The items accounting for the difference between income taxes computed at the Costa Rica statutory income tax rate and the income tax provision (benefit) consisted of the following for the year ended December 31:
2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||||||||||||||
| Tax benefit at Costa Rica statutory rate | $ | ( | % | $ | ( | % | $ | ( | % | ||||||||||||||||||||||||||
| Foreign tax rate differential | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Tax rate changes | ( | ||||||||||||||||||||||||||||||||||
| Return to provision adjustment | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Tax credits | ( | ( | |||||||||||||||||||||||||||||||||
| Change in valuation allowance | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Tax holiday adjustment (benefit) | ( | ( | ( | ||||||||||||||||||||||||||||||||
U.S. stock compensation | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Other | ( | ( | |||||||||||||||||||||||||||||||||
Total provision (benefit) for income taxes | $ | ( | % | $ | ( | % | $ | ( | % | ||||||||||||||||||||||||||
The Company's tax holiday benefit was related to the Company’s subsidiary in Costa Rica which enjoyed a zero zero percent tax holiday, as extended, is effective for a period of 12 years through the year 2030.
As of December 31, the components of the Company's deferred tax assets and liabilities are as follows:
2023 | 2022 | ||||||||||
| (in thousands) | |||||||||||
| Accruals and reserves | $ | $ | |||||||||
| Deferred revenue | |||||||||||
| Intangibles | |||||||||||
| Stock compensation | |||||||||||
| Net operating loss | |||||||||||
| R&D credits | |||||||||||
| Other | ( | ||||||||||
| Valuation allowance | ( | ( | |||||||||
| Total net deferred tax assets (included in “Other non-current assets”) | $ | $ | |||||||||
As of December 31, 2023, the Company assessed that it is more-likely-than-not that it will not realize its deferred tax assets based on the absence of sufficient positive objective evidence that it would generate sufficient taxable income in its U.S. tax jurisdiction (Motiva USA, LLC) to realize the deferred tax assets. The Company intends to continue maintaining a full valuation allowance on its deferred tax assets in this jurisdiction until there is sufficient evidence to support the reversal of all or some portion of these allowances. The Company released the valuation allowance on its deferred tax assets in Brazil believing there is enough positive evidence that the deferred tax assets in Brazil will be realized in the following years.
F-31
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
As of December 31, 2023, the Company had U.S. and California tax credit carryforwards of approximately $0.5 million in total. The federal research credits begin to expire in 2037. However, the California research credits can be carried forward indefinitely.
As of December 31, 2023, the Company had U.S. federal, state, U.K. and Brazil net operating losses of approximately $102.6 million, $10.2 million, $0.2 million and $10.9 million, respectively. The U.S. federal net operating losses of $3.3 million generated prior to 2018, and state net operating losses will begin to expire on December 31, 2030. The U.S. federal net operating losses generated in 2018 and future years will be carried forward indefinitely. Brazil net operating losses can be carried forward indefinitely.
The United States federal and California laws impose restrictions on the utilization of net operating loss carryforwards and R&D credit carryforwards in the event of a change in ownership of the Company, which constitutes an “ownership change” as defined by Internal Revenue Code Sections 382 and 383. Generally, an ownership change occurs if the percentage of the value of the shares that are owned by one or more direct or indirect “five percent shareholders” increases by more than 50% over their lowest ownership percentage at any time during the applicable testing period. If the Company has experienced an “ownership change” at any time since its formation, it would already be subject to limitations on its ability to utilize its existing net operating losses and other tax attributes. The Company did not experience an ownership change in the past that would materially impact the availability of its net operating losses and tax credits. Nevertheless, future changes in the Company’s share ownership, which may be outside of the Company’s control, may trigger an “ownership change” and, consequently, Section 382 and 383 limitations. The Company has not completed a Section 382 and 383 analysis to determine if an ownership change has occurred. Until such analysis is completed, the Company cannot be sure that the full amount of the existing net operating loss carryforwards will be available, even if the Company does generate taxable income before their expiration. In addition, under the newly enacted U.S. federal income tax law, federal net operating losses incurred in 2018 and in future years may be carried forward indefinitely, but the deductibility of such federal net operating losses is limited.
Discontinuation of preferential tax treatment the Company currently enjoys or other unfavorable changes in tax law could result in additional compliance obligations and costs. The Company is currently the beneficiary of a tax holiday in Costa Rica pursuant to which it is subject to a tax at a 0 % rate, with the exception of types pf income that is subject to 30 % income tax. The tax holiday is effective through December 31, 2030, and it may be extended if certain additional requirements are satisfied. However, there can be no assurance that the Company will continue to qualify for or receive such favorable tax treatment. If the Company fails to maintain such favorable tax treatment it may be subject to tax in Costa Rica at a significantly higher rate.
A tax authority may disagree with tax positions that the Company has taken, which could result in increased tax liabilities. For example, the U.S. Internal Revenue Service or another tax authority could challenge the amounts paid between the Company and its subsidiaries pursuant to the Company’s intercompany arrangements and transfer pricing policies. A tax authority may take the position that material income tax liabilities, interest and penalties are payable by the Company, in which case, the Company expects that it might contest such assessment. Contesting such an assessment may be lengthy and costly and, if the Company is unsuccessful in disputing the assessment, the implications could increase its anticipated effective tax rate, where applicable. In addition, the Company may be subject to additional tax liabilities, which could materially and adversely affect its business, financial condition and results of operations. The application, interpretation and enforcement of the value-added tax, or VAT, and other taxes and related regulations applicable to medical device companies are complex and evolving.
The Company conducts operations in multiple jurisdictions and is subject to certain taxes, including income, sales and use, employment, value added and other taxes, in the United States and other jurisdictions in which the Company does business. A change in the tax laws in the jurisdictions in which the Company does business, including an increase in tax rates or an adverse change in the treatment of an item of income or expense, possibly with retroactive effect, could result in a material increase in the amount of taxes incurred.
The Company’s determination of its tax liability is subject to review by applicable U.S. and foreign tax authorities. Any adverse outcome of such a review could harm the Company’s operating results and financial condition. The determination of the Company’s worldwide provision for income taxes and other tax liabilities requires significant judgment and, in the ordinary course of business, there are many transactions and calculations where the ultimate
F-32
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
tax determination is complex and uncertain. Moreover, as a multinational business, the Company has subsidiaries that engage in many intercompany transactions in a variety of tax jurisdictions where the ultimate tax determination is complex and uncertain. The taxing authorities of the jurisdictions in which the Company operates may challenge the Company’s methodologies, which could impact its financial position and operating results. Historically, the Company has allocated some of its employees’ and contractors’ time across multiple business entities in the international jurisdictions in which the Company operates. If the Company determined that it had misclassified the employees’ or contractors’ employment status or certain of its expenditures under local laws, the Company may be subjected to penalties or be required to pay withholding taxes for, extend employee benefits to, provide compensation for unpaid overtime to, or otherwise incur substantially greater expenses with respect to such employees and contractors. Any of the foregoing circumstances could have a material adverse impact on the Company’s operating results and financial condition.
The Company is periodically reviewed and audited by tax authorities with respect to income and non-income taxes. Tax authorities may disagree with certain positions the Company has taken, and the Company may have exposure to additional income and non-income tax liabilities which could have an adverse effect on the Company’s operating results and financial condition. Such authorities could impose additional taxes, interest and penalties, claim that various withholding requirements apply to the Company or its subsidiaries or assert that benefits of tax treaties are not available to the Company or its subsidiaries. In addition, the Company’s future effective tax rates could be favorably or unfavorably affected by changes in tax rates, changes in the valuation of the Company’s deferred tax assets or liabilities, the effectiveness of its tax planning strategies, or changes in tax laws or their interpretation. Such changes could have an adverse impact on the Company’s financial condition.
As a result of these and other factors, the ultimate amount of tax obligations may differ from the amounts recorded in the Company’s financial statements and any such difference may harm its operating results in future periods in which the Company changes the estimates of such tax obligations or in which the ultimate tax outcome is determined.
A non-U.S. corporation is classified as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes, in any taxable year in which either (1) at least 75% of its gross income is passive income; or (2) at least 50% of the average quarterly value of its total gross assets is attributable to assets that produce “passive income” or are held for the production of passive income. Based on the project composition of the Company’s income and valuation of its assets, the Company does not believe it is a PFIC and does not expect to be a PFIC for the current taxable year or to become one in the future. However, because the PFIC status is subject to a number of uncertainties, neither the Company nor its tax advisors can provide any assurances regarding the PFIC status. If the Company is a PFIC for any taxable year during which a U.S. holder holds the Company’s common shares, the U.S. holder may be subject to adverse tax consequences.
The Company records income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or income tax returns. In estimating future tax consequences, expected future events, enactments or changes in the tax law or rates are considered. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount expected to be realized.
Accounting for Uncertainty in Income Taxes
The Company records uncertain tax positions based on a two-step process whereby (1) a determination is made as to whether it is more likely than not that the tax positions will be sustained based on the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold the Company recognizes the largest amount of tax benefit that is greater than 50% likely to be realized upon ultimate settlement with the related tax authority. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. Significant judgment is required in the identification of uncertain tax positions and in the estimation of penalties and interest on uncertain tax positions.
The Company has adopted ASC 740-10 Accounting for Uncertainty in Income Taxes. ASC 740-10 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of uncertain tax positions taken or expected to be taken in the Company's income tax return, and provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition.
F-33
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
For the years ended December 31, 2023 and 2022 the Company has no material uncertain tax positions. The Company has R&D credits in the United States and has recorded reserves of $0.1 million which offsets R&D credit deferred tax assets. The Company does not expect any significant increases or decreases to its unrecognized tax benefits within the next 12 months. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense.
As of December 31, 2023, the Company is subject to taxation in Costa Rica, Belgium, France, Brazil, the United Kingdom, Sweden, Italy, Germany, Austria, Spain, Argentina, Switzerland and the United States and the Company’s fiscal tax years 2018 through 2023 are subject to examination by the tax authorities.
11. Net Loss Per Share
The following table summarizes the computation of basic and diluted net loss per share for the periods presented:
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||||||
| Numerator: | ||||||||||||||||||||
| Net loss | $ | ( | $ | ( | $ | ( | ||||||||||||||
| Denominator: | ||||||||||||||||||||
| Weighted average common shares used for basic and diluted earnings per share | ||||||||||||||||||||
| Net loss per share: | ||||||||||||||||||||
| Basic and diluted | $ | ( | $ | ( | $ | ( | ||||||||||||||
Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares outstanding for the period. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of shares and dilutive share equivalents outstanding for the period, determined using the treasury-share method and the as-if converted method, for convertible securities, if inclusion of these is dilutive.
If the Company reports a net loss, diluted net loss per share is the same as basic net loss per share for those periods because including the dilutive securities would be anti-dilutive.
The following potentially dilutive securities outstanding at the end of the periods presented have been excluded from the computation of diluted shares:
| Year Ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Options to purchase common shares | ||||||||||||||||||||
| Shares issuable on vesting of grants of RSUs | ||||||||||||||||||||
| Warrants to purchase common shares | ||||||||||||||||||||
Total potentially dilutive shares outstanding | ||||||||||||||||||||
12. Related Party Transactions
During the years ended December 31, 2023, 2022 and 2021, the Company recorded revenue of $1.2 million, $1.2 million and $1.4 million, respectively, for product sales to Herramientas Medicas, S.A., a distribution company owned by a family member of the Chief Executive Officer of the Company. Accounts receivable owed to the Company from this distribution company amounted to approximately $0.6 million and $0.4 million as of December 31, 2023 and 2022, respectively.
F-34
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
In 2016, the Company also entered into a separate agreement with Dr. Chacón Quirós, the brother of the Company’s Chief Executive Officer Juan José Chacón Quirós, to maintain his clinic in Costa Rica as a MotivaImagine Excellence Center and to host and train physicians in the use of the Company products in relevant procedures, among other services, in exchange for cash reimbursement of up to $4,500 per day that such services are rendered. In August 2022, the Company entered into a new agreement with Dr. Chacón Quirós, replacing the original agreement, to continue the training services in exchange for cash reimbursement of his hourly rate of $531 when such services are rendered. During the years ended December 31, 2023, 2022 and 2021, the Company paid Dr. Chacón Quirós approximately $0.4 million, $0.2 million and $0.4 million, respectively, for services rendered.
On December 12, 2022, the Company granted to Nicholas Lewin, a member of the board of directors, a stock option award for 7,829 options with a grant date fair value of $0.4 million as a compensation for consulting services he performs for the Company in addition to his services as a non-employee director. In addition, on May 28, 2023, the Company awarded Mr. Lewin a performance-based grant for 27,756 restricted stock units with a grant date fair value of $1.8 million as compensation for consulting services he performs for the Company.
On April 1, 2022, the Company entered into a consulting agreement with Lisa Gersh, who served on our Board of Directors until March 31, 2022. Pursuant to the consulting agreement, Ms. Gersh will perform consulting services as requested by the Company, with the expectation that she will advise our Board on elements of corporate leadership and governance. As payment for Ms. Gersh’s consulting services, the Company paid Ms. Gersh a consulting fee of $0.1
13. Employee Benefits
Short-term employee benefits, including vacation (paid absences) and year-end bonuses (also known as 13th month salary), are current liabilities included in accrued liabilities on the consolidated balance sheets and are calculated at the non-discounted amount that the Company expects to pay as a result of uncharged employee salaries or retentions.
Regarding employee termination benefits, Costa Rica labor laws establish the payment of benefits in case of death, retirement or termination without cause. This compensation is calculated according to time served in the Company and the corresponding salary in the last six months of employment and is equal to between 19.5 and 22 days’ salary for each year served, up to a maximum of 8 years.
Company policy recognizes termination benefits as expenses of the period during which the termination occurs, when the legal obligation is assumed due to the aforementioned events.
As of December 31, 2023, the Company has 44 employees in Brazil and 3 employees in Argentina who were represented by a labor union.
14. Commitments and Contingencies
Contingencies
Periodically, the Company may have certain contingent liabilities that arise in the ordinary course of business activities. The Company accrues a liability for such matters when it is probable that future expenditures will be made, and such expenditures can be reasonably estimated. As of December 31, 2023 and 2022, contingent liabilities were not material, individually or in the aggregate, to the Company's financial condition, results of operations or cash flows. However, any monetary liability or financial impact to the Company from these contingent liabilities could differ materially from the Company's expectations.
Indemnification
The Company enters into standard indemnification arrangements in the ordinary course of business. Pursuant to these arrangements, the Company indemnifies, holds harmless, and agrees to reimburse the indemnified parties for losses suffered or incurred by the indemnified party, in connection with any trade secret, copyright, patent or other intellectual property infringement claim by any third party with respect to the Company’s technology. The term of these indemnification agreements is generally perpetual. The maximum potential amount of future
F-35
ESTABLISHMENT LABS HOLDINGS, INC.
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2023, 2022 and 2021
payments the Company could be required to make under these agreements is not determinable because it involves claims that may be made against the Company in the future that have not yet been made.
The Company has entered into indemnification agreements with its directors and officers that may require the Company to indemnify its directors and officers against liabilities that may arise by reason of their status or service as directors or officers, other than liabilities arising from willful misconduct of the individual.
The Company has not incurred costs to defend lawsuits or settle claims related to these indemnification agreements. No liability associated with such indemnifications has been recorded to date.
15. Subsequent Events
On January 9, 2024. the Company entered into a securities purchase agreement with select institutional accredited investors to sell 1,101,565 common shares and pre-funded warrants to purchase 898,435 common shares at a price of $25.00 per share. The pre-funded warrants may be exercised immediately at a price of $0.001 per share. The aggregate gross proceeds from the offering, before deducting offering expenses, were approximately $50.0 million.
F-36
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
ESTABLISHMENT LABS HOLDINGS INC. | |||||||||||
| Dated: | March 4, 2024 | By: | /s/ Juan José Chacón Quirós | ||||||||
| Name: | Juan José Chacón Quirós | ||||||||||
| Title: | Chief Executive Officer and Director | ||||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||||||||||||||||||
/s/ Juan José Chacón Quirós | Chief Executive Officer and Director (Principal Executive Officer) | March 4, 2024 | ||||||||||||||||||
Juan José Chacón Quirós | ||||||||||||||||||||
/s/ Rajbir S. Denhoy | Chief Financial Officer (Principal Financial and Accounting Officer) (Authorized Representative in the United States) | March 4, 2024 | ||||||||||||||||||
| Rajbir S. Denhoy | ||||||||||||||||||||
| /s/ Nicholas Lewin | Chairman of the Board of Directors | March 4, 2024 | ||||||||||||||||||
| Nicholas Lewin | ||||||||||||||||||||
| /s/ Dennis Condon | Director | March 4, 2024 | ||||||||||||||||||
| Dennis Condon | ||||||||||||||||||||
| /s/ Ann Custin | Director | March 4, 2024 | ||||||||||||||||||
| Ann Custin | ||||||||||||||||||||
| /s/ Leslie Gillin | Director | March 4, 2024 | ||||||||||||||||||
| Leslie Gillin | ||||||||||||||||||||
| /s/ Edward Schutter | Director | March 4, 2024 | ||||||||||||||||||
| Edward Schutter | ||||||||||||||||||||
| /s/ Bryan Slotkin | Director | March 4, 2024 | ||||||||||||||||||
| Bryan Slotkin | ||||||||||||||||||||
F-37