UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark one)
ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number:
(Exact name of registrant as specified in its charter)
Ontario |
| |
(State or other jurisdiction of | (I.R.S. Employer |
(Address of principal executive offices)
(
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading |
| Name of each exchange |
Common Shares, without par value |
| GBNHF |
| ***(1) |
(1) On February 26, 2024, the common shares (“Common Shares”) of Greenbrook TMS Inc. (the “Company”) were suspended from trading on the Nasdaq Capital Market of the Nasdaq Stock Market LLC (“Nasdaq”). On March 22, 2024, the Common Shares began trading on the OTCQB Market, operated by OTC Markets Group Inc (the “OTCQB Market”) under the symbol “GBNHF”. On April 1, 2024, the Company filed a Form 25 with the Securities and Exchange Commission (the “SEC”) to complete the delisting of the Common Shares from the Nasdaq, with the delisting becoming effective April 11, 2024. The deregistration of the Common Shares under Section 12(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), will become effective 90 days after the filing of the Form 25, or such shorter period as the SEC may determine.
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☐ | Accelerated filer | ☐ | ||
☒ | Smaller reporting company | ||||
Emerging growth |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the other registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b).
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes
The aggregate market value of the voting stock and nonvoting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked prices of such common equity, as of June 30, 2023, was $
As of December 31, 2023, the registrant had
PCAOB: 0
EXPLANATORY NOTE
On April 16, 2024, the audit committee of the Board of Directors (the “Audit Committee”) of Greenbrook TMS Inc, (the “Company”) concluded, after consultation with the Company’s management and its independent auditors, that the Company’s previously issued (i) audited annual financial statements for the year ended December 31, 2022 (“Fiscal 2022”) and (ii) quarterly condensed interim consolidated financial statements for the quarter ended September 30, 2023, prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Statements (“IFRS”) contained errors and therefore should no longer be relied upon.
After discussion of these errors with the Company’s independent registered public accounting firm, KPMG LLP, the Company filed a Current Report on Form 8-K under Item 4.02(a) on April 19, 2024, disclosing the finding of these errors and stating that the Company’s previously issued financial statements for Fiscal 2022, as well as the Company’s quarterly condensed interim consolidated financial statements for the quarter ended September 30, 2023, should no longer be relied upon.
The foregoing errors were the result of inappropriate application of variable consideration methodologies in the recognition of revenue and the Company not having effectively designed and maintained controls over the effective preparation, review, and approval of its adjustment to variable consideration.
For the audited annual financial statement for Fiscal 2022, the errors resulted in overstatement of revenue and understatement of loss in the amount of $2.3 million for Fiscal 2022 and overstatement of accounts receivable in the amount of $6.6 million as at December 31, 2022. There is also an understatement of total shareholders’ deficit in the amount of $4.3 million as at December 31, 2021, and for similar amounts for the periods prior to December 31, 2021.
The quarterly condensed interim consolidated financial statements for the quarter ended September 30, 2023 (“Fiscal Q3 2023”), contained cumulative misstatements year to date for the nine months ended September 30, 2023, of an overstatement of revenue and understatement of loss in the amount of $1.9 million and overstatement of accounts receivable of $8.5 million.
This Annual Report contains annual audited financial statements prepared in accordance with U.S. GAAP which reflect the correction of the foregoing errors in Fiscal 2022. As required under Canadian securities laws as a result of the Company’s change in financial reporting framework, the Company also intends to re-file its quarterly financial statements, prepared in accordance with U.S. GAAP, for the first, second and third quarters of 2023, which the Company plans to file on a Current Report on Form 8-K on or about the date of filing the Annual Report. These re-filed quarterly financial statements will reflect the correction of the foregoing errors in Fiscal Q3 2023.
- i -
TABLE OF CONTENTS
- ii -
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Some of the information contained in this Annual Report on Form 10-K (the “Annual Report”) filed by Greenbrook TMS Inc. (the “Company” or “Greenbrook”), including our expectations regarding the continued roll-out of the Spravato® Program and Medication Management (each as defined herein) at additional mental health service centers (the “Treatment Centers”) and our potential to enhance profit margins and diversify total revenue, the impact of the Restructuring Plan (as defined herein) on our business, our expansion opportunities, our expectations regarding our liquidity and available financing and continued compliance with the Credit Agreement (as defined herein) and our other outstanding debt obligations, our expectations regarding the Klein Matters (as defined herein), and our expectations of future results, performance, achievements, prospects or opportunities, constitutes “forward-looking information” within the meaning of applicable securities laws in Canada and “forward-looking statements” within the meaning of the United States Private Securities Litigation Reform Act of 1995 (collectively, “forward-looking statements”). This information is based on management’s reasonable assumptions and beliefs in light of the information currently available to us and is current as of the date of this Annual Report. Actual results and the timing of events may differ materially from those anticipated in the forward-looking information contained in this Annual Report as a result of various factors.
Particularly, forward-looking statements include information regarding our expectations of future results, performance, achievements, prospects or opportunities or the markets in which we operate. In some cases, forward-looking statements can be identified by the use of forward-looking terminology such as “plans”, “targets”, “expects” or “does not expect”, “is expected”, “an opportunity exists”, “budget”, “scheduled”, “estimates”, “outlook”, “forecasts”, “projection”, “prospects”, “strategy”, “intends”, “anticipates”, “does not anticipate”, “believes”, or variations of such words and phrases or statements that certain actions, events or results “may”, “should”, “could”, “would”, “might”, “will”, “will be taken”, “occur” or “be achieved”. In addition, any statements that refer to expectations, intentions, projections or other characterizations of future events or circumstances contain forward-looking information. Forward-looking statements are not facts but instead represent management’s expectations, estimates and projections regarding future events or circumstances.
Forward-looking statements are based on number of opinions, assumptions and estimates that, while considered reasonable by the Company as of the date of this Annual Report, are subject to known and unknown risks, uncertainties, assumptions and other factors that could cause our actual results, level of activity, performance or achievements or future events or developments to differ materially from those expressed or implied by the forward-looking statements, including, without limitation: macroeconomic factors such as inflation and recessionary conditions, substantial doubt regarding the Company’s ability to continue as a going concern due to recurring losses from operations; inability to increase cash flow and/or raise sufficient capital to support the Company’s operating activities and fund its cash obligations, repay indebtedness and satisfy the Company’s working capital needs and debt obligations; prolonged decline in the price of the Company’s common shares (the “Common Shares”) reducing the Company’s ability to raise capital; inability to satisfy debt covenants under the Credit Agreement and the potential acceleration of indebtedness; risks related to the resolution of the Company’s ongoing litigation with Benjamin Klein and compliance with the terms of their settlement agreement; risks related to our ability to continue to negotiate amendments to the Credit Agreement to prevent a default; risks relating to our ability to deliver and execute on our previously announced Restructuring Plan and the possible failure to complete the Restructuring Plan on terms acceptable to the Company or its suppliers (including Neuronetics, Inc. (“Neuronetics”)), or at all; risks relating to maintaining an active, liquid and orderly trading market for Common Shares as a result of our delisting from trading on the Nasdaq Capital Market of the Nasdaq Stock Market LLC (“Nasdaq”); risks relating to the Company’s ability to realize expected cost-savings and other anticipated benefits from the Restructuring Plan; risks related to the Company’s negative cash flows, liquidity and its ability to secure additional financing; increases in indebtedness levels causing a reduction in financial flexibility; inability to achieve or sustain profitability in the future; inability to secure additional financing to fund losses from operations and satisfy our debt obligations; risks relating to strategic alternatives, including restructuring or refinancing of our debt, seeking additional debt or equity capital, reducing or delaying our business activities and strategic initiatives, or selling assets, other strategic transactions and/or other measures, including obtaining bankruptcy protection, and the terms, value and timing of any transaction resulting from that process; claims made by or against the Company, including the Klein Matters, which may be resolved unfavorably to us; risks relating to our dependence on Neuronetics as our exclusive supplier of TMS Devices (as defined herein); risks and uncertainties relating to the restatement of our financial statements for Fiscal 2022 and Fiscal Q3 2023, including any potential litigation and/or regulatory proceedings as well as any adverse effect on investor confidence and our reputation; as well as the factors discussed in the “Risk Factors” section of this Annual Report. These factors are not intended to represent a complete list of the factors that could affect us or our ability to achieve the anticipated benefits from the acquisition of Check Five LLC (doing business as “Success TMS”), the Credit Agreement and the Restructuring Plan; however, these factors should be considered carefully.
The purpose of forward-looking statements is to provide the reader with a description of management’s current expectations regarding the Company’s financial performance and may not be appropriate for other purposes; readers should not place undue reliance on them. To the extent any forward-looking statements in this Annual Report constitutes future-oriented financial information or financial outlook, within the meaning of applicable securities laws, such information is being provided to demonstrate the potential of the Company and readers are cautioned that this information may not be appropriate for any other purpose. Future-oriented financial information and financial outlook, as with forward-looking statements generally, are based on current assumptions and are subject to risks, uncertainties and other factors. Furthermore, unless otherwise stated, the forward-looking statements contained in this Annual Report are made as of the date of this Annual Report and we have no intention and undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable securities law. The forward-looking statements contained in this Annual Report are expressly qualified by this cautionary statement.
- 2 -
PART I
ITEM 1.BUSINESS
Overview of Greenbrook
Through our Treatment Centers, we are a leading provider of TMS therapy in the United States for the treatment of Major Depressive Disorder (“MDD”) and other mental health disorders. Our predecessor, TMS US (as defined herein), was established in 2011 to take advantage of the opportunity created through the paradigm-shifting technology of TMS, a non-invasive therapy for the treatment of MDD cleared by the U.S. Food and Drug Administration (“FDA”). In 2018, our Treatment Centers began offering treatment for obsessive-compulsive disorder (“OCD”). Our business model takes advantage of the opportunity for a new, differentiated service channel for the delivery of innovative treatments – a patient-focused, centers-based service model to make treatment easily accessible to all patients while maintaining a high standard of care. We have identified the following key opportunity drivers for our business:
| ● | the safety and efficacy of TMS as a treatment option for patients suffering from MDD and OCD; |
| ● | the growing societal awareness and acceptance of depression as a treatable disease and a corresponding reduction in stigma surrounding depression, seeking treatment and mental health issues generally; |
| ● | the growing acceptance, but under-adoption, of TMS; |
| ● | the poor alignment of TMS treatment with traditional practices of psychiatry which created an opportunity for a new, differentiated service channel; |
| ● | the fragmented competitive landscape for TMS treatment (“Treatment”) which provides an opportunity for consolidation; and |
| ● | the track record of success by the management team in multi-location, center-based healthcare service companies. |
Beginning in 2021, we commenced our roll-out of Spravato® (esketamine nasal spray) therapy in our Treatment Centers to treat treatment-resistant depression in adults and depressive symptoms in adults with MDD with acute suicidal ideation or behavior. We have since grown to offer Spravato® at 82 Treatment Centers within our operating network as of the date of this Annual Report.
In late 2023, we commenced the facilitation of medication management (“Medication Management”) at select Treatment Centers across our footprint, building on our long-term business plan of utilizing our Treatment Centers as platforms for the delivery of innovative treatments to patients suffering from MDD and other mental health disorders. We believe that becoming a more comprehensive mental health care provider will allow us to provide greater access to those suffering from MDD and other mental health disorders.
In addition, we have also entered into the Research Collaboration Agreement (as defined below) to explore delivery models for investigational COMP360 psilocybin treatment (“COMP360”). We believe we are on the forefront of innovative treatment delivery using our experience and nationwide presence to offer a platform for scaling new treatments that solve issues with awareness, geographic convenience and fiscal viability so patients can receive and benefit from the latest therapeutics.
After opening our first Treatment Center in 2011 in Tysons Corner in Northern Virginia, we have grown to control and operate a network of outpatient mental health service centers that specialize in Treatment across the United States. We offer Treatment Centers in convenient locations to provide easy access to patients and clinicians. As of the date of this Annual Report, the Company owned and operated 130 Treatment Centers in the Commonwealths of Massachusetts, Virginia and Pennsylvania and the States of Maryland, North Carolina, Missouri, Illinois, Ohio, Texas, Connecticut, Florida, South Carolina, Michigan, Alaska, Oregon, California, New Jersey, and Nevada. In connection with the Restructuring Plan, the Company decreased its operating footprint by closing a total of 53 Treatment Centers, bringing the total number of Treatment Center locations to 130 (from 183), spanning 17 management regions. In addition to increased cost efficiencies, the Company believes that a more condensed operating footprint is optimal in light of the Company’s shift towards a more comprehensive mental health care model, including an increased focus on Spravato® therapy. For more information on our Treatment Centers, see Item 2 “Properties” below.
- 3 -
Our regional model seeks to develop leading positions in key markets and to leverage operational efficiencies by combining smaller local Treatment Centers within a region under a single shared regional management infrastructure. Management regions typically cover a specific metropolitan area that meets a requisite base population threshold. The management region is typically defined by a manageable geographic area which facilitates the use of regional staff working across the various Treatment Center locations within the management region and creates a marketing capture area that allows for efficiencies in advertising costs. Management regions often have similar economic characteristics and are not necessarily defined by state lines, other geographic borders, or differentiating methods of services delivery, but rather are defined by a functional management area.
Strengths and Investment Highlights
We believe that the following describes the key strengths and investment highlights of Greenbrook and our business:
| ● | TMS is a New Paradigm and a Clinically Effective Approach to Treating Depression: TMS is an FDA-cleared approach to treating depression that has been demonstrated to be clinically effective and for which reimbursement is available in all 50 states and from all major insurance providers. |
| ● | Experienced Executive Management Team and Independent Board: We have a highly experienced management team and clinical leadership with a track record of building center-based healthcare services businesses. Furthermore, our clinical leadership team are pioneers in the field of TMS therapy. Additionally, our Company’s board of directors (the “Board”) has extensive collective experience in the industry, capital markets and corporate governance. |
| ● | Leading Market Position: Our Treatment delivery model and recent acquisition of Success TMS has made us a leading provider of TMS therapy and Spravato® in the United States with over 1,450,000 Treatments to over 43,000 patients since our inception. |
| ● | Regional Operating Model: We believe our regional operating model, centralized business infrastructure, systems infrastructure and centralized buying power enables efficient delivery of Treatment, which provides a significant competitive advantage. |
| ● | Potential for Future TMS Indications: Multiple clinical studies and research projects, including under our Research Collaboration Agreement, are underway for label expansion of TMS therapy into additional mental health and/or neurological indications. Our established footprint and service delivery model is well-positioned to lead the delivery of new indications which can be rapidly incorporated into our existing Treatment Center network with minimal additional investment required. |
| ● | Potential for Delivery of Innovative Treatment Modalities: The initial successes of our Spravato® program in Fiscal 2022 and Fiscal 2023 (each as defined below) demonstrate our ability to leverage our Treatment Center as platforms for the delivery of innovative treatments. We believe that we are well positioned to replicate similar rollouts of new treatment options in the future, and we are continuously evaluating opportunities to collaborate with developers of new therapeutic solutions that may benefit patients suffering from MDD and other mental health disorders, including investigational COMP360 psilocybin treatment for which we have signed a Research Collaboration Agreement (as defined below). |
| ● | Comprehensive Mental Health Provider: In the third quarter of Fiscal 2023, we commenced our Medication Management Program (as defined below) and in the first quarter of 2024 we launched a talk therapy pilot program. We believe that expanding our continuum of care and becoming a more comprehensive mental health care provider will allow us to provide greater access and quality of care to those suffering from MDD and other mental health disorders. |
- 4 -
General Corporate Information
Greenbrook TMS Inc. was incorporated in Ontario, Canada, under the Business Corporations Act (Ontario) (the “OBCA”) on February 9, 2018, as a wholly owned subsidiary of our predecessor parent company, TMS NeuroHealth Inc. (now TMS NeuroHealth Centers Inc. (“TMS US”)), a corporation incorporated in 2011 under the laws of the State of Delaware. On March 29, 2018, the Company and TMS US completed a corporate reorganization pursuant to which all of the holders of common stock of TMS US exchanged their holdings of common stock of TMS US for our Common Shares, resulting in TMS US becoming a wholly owned subsidiary of Greenbrook. On September 28, 2018, in anticipation of our initial public offering in Canada (the “Canadian IPO”), Greenbrook filed articles of amendment to increase the minimum and maximum size of the Board, to remove the transfer restrictions on the Common Shares and to remove certain other private company restrictions. On October 3, 2018, we completed the Canadian IPO and the Common Shares commenced trading on the Toronto Stock Exchange (“TSX”) under the symbol “GTMS”.
On March 12, 2021, the Common Shares were certified for listing on the Nasdaq and on March 16, 2021, the Common Shares commenced trading on the Nasdaq under the symbol “GBNH”.
On February 27, 2023, the Company announced that it had applied and received approval for a voluntary delisting of the Common Shares from the TSX, which became effective following the close of markets on March 13, 2023.
On February 22, 2024, the Company received the final delisting notice from the Listing Qualifications Department of the Nasdaq due to the continued failure to satisfy the Nasdaq Requirements (as defined herein). Accordingly, the Company’s Common Shares were suspended from trading on Nasdaq beginning on February 26, 2024. On April 1, 2024, the Company filed its own Form 25 with the SEC, which completed the process for delisting its Common Shares from Nasdaq when the Form 25 became effective on April 11, 2024.
Following the suspension of trading of the Common Shares on Nasdaq, the Common Shares began trading on the OTC Pink Market, operated by OTC Markets Group Inc. (the “OTC Pink Market”) under the symbol “GBNHF”. On March 22, 2024, the Common Shares began trading on the OTCQB Market, operated by OTC Markets Group Inc (the “OTCQB Market”) under the symbol “GBNHF”.
- 5 -
The following chart identifies our material subsidiaries, their governing jurisdictions and the percentage of their voting securities which are beneficially owned, or controlled or directed, directly or indirectly, by Greenbrook:

| (1) | As at December 31, 2023, our Treatment Center network consisted of 130 Treatment Center locations spanning 17 management regions in the Commonwealths of Massachusetts, Virginia and Pennsylvania and the States of Maryland, North Carolina, Missouri, Illinois, Ohio, Texas, Connecticut, Florida, South Carolina, Michigan, Alaska, Oregon, California, New Jersey, and Nevada. For more information on our properties and Treatment Centers, see Item 2 “Properties” below. |
Recent Developments in Our Business
Business Developments
Research Collaboration Agreement
On December 29, 2023, the Company entered into a research collaboration agreement (the “Research Collaboration Agreement”) with Compass Pathways plc, a corporation registered in England and Wales to explore delivery models for investigational COMP360 upon regulatory approval by the U.S. Food and Drug Administration. The collaboration will research and investigate models for the delivery of scalable, commercial COMP360, within healthcare systems, assuming FDA approval. Under the Research Collaboration Agreement, the Company has the opportunity to earn up to $3,000,000 in incentives upon hitting various mutually agreed upon milestones. As of the date of this Annual Report, the Company has received $1,300,000 of the $3,000,000 payable under the Research Collaboration Agreement.
- 6 -
Restructuring Plan
On March 6, 2023, the Company announced that it is embarking on a comprehensive restructuring plan (the “Restructuring Plan”) that aims to strengthen the Company by leveraging its scale to further reduce complexity, streamlining its operating model and driving operational efficiencies to achieve profitability.
As part of this Restructuring Plan, the Company has decreased its operating footprint by closing a total of 53 Treatment Centers as of the date of this Annual Report, allowing management to focus on the remaining 130 Treatment Centers. The remaining Treatment Centers will continue clinical Transcranial Magnetic Stimulation (“TMS”) offerings and a select and growing number of Treatment Centers will continue offering Spravato® therapy.
The Restructuring Plan is intended to fortify the Company’s path to achieve sustainable profitability and long-term growth. Together, these reductions to the Company’s footprint, headcount and operating expenses have resulted in cost savings of between $22 million and $25 million on an annualized run-rate basis. For Fiscal 2023, the Company incurred approximately $23 million of Restructuring Plan related savings. As of the date of this Annual Report, the Restructuring Plan is substantially complete, but the Company expects to achieve additional operating expense savings from the final components of the Restructuring Plan in the first half of Fiscal 2024 (as defined below).
TMS Device Supply Arrangement with Neuronetics
In January 2023, the Company and Neuronetics jointly announced an expanded commercial partnership through year end 2028. Under the amended and restated master sales agreement between the Company and Neuronetics, dated as of January 17, 2023 (as amended by an amending agreement dated March 16, 2023, the “Neuronetics Agreement”), Neuronetics has become the Company’s exclusive supplier of TMS devices, an FDA-regulated medical device specifically manufactured to transmit the magnetic pulses required to stimulate the cortical areas in the brain to effectively treat MDD and other mental health disorders (each, a “TMS Device”). Over time, Neuronetics’ NeuroStar TMS Devices will replace competitive TMS Devices at the Company’s Treatment Centers. Under the Neuronetics Agreement, the parties are working together to grow, through co-branding and co-marketing programs, enhanced patient and clinician awareness, improved patient access to care, and collaboration on product development and publications. The Neuronetics Agreement also contains minimum purchase commitments, and all treatment session purchases will convert to a “per-click” consumable model.
On March 31, 2023, the Company and Neuronetics agreed to convert the Company’s outstanding account balance payable to Neuronetics for the supply of TMS Devices and treatment sessions to the Company from Neuronetics in the amount of approximately $5.7 million (the outstanding balance as of December 7, 2022), together with Neuronetics’ out-of-pocket transaction costs, into a $6.0 million secured promissory note (the “Neuronetics Note”). See “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness and Capital Raising—Neuronetics Note and Warrants”.
Our Medication Management Program
During the third quarter of Fiscal 2023, the Company commenced a pilot to roll-out our facilitation of Medication Management to select Treatment Centers across our footprint, building on our long-term business plan of utilizing our Treatment Centers as platforms for the delivery of innovative treatments to patients suffering from MDD and other mental health disorders (the “Medication Management Program”). Although Medication Management is a lower margin business, we believe this program will allow us to reach patients earlier in their treatment journey, develop an internal patient pipeline for TMS and Spravato®, while also further optimizing marketing costs. We also believe that becoming a more comprehensive mental health care provider will allow us to provide greater access to those suffering from MDD and other mental health disorders. As at the date of this Annual Report, we had a total of nine Treatment Centers offering Medication Management.
Strategic Opportunities
In addition to the Restructuring Plan, the Research Collaboration Agreement, and the Neuronetics Agreement, the Company is also selectively seeking strategic partnerships, acquisitions and merger opportunities and is in various stages of negotiations and due diligence in respect of certain potential merger, acquisition, investment or other commercial partnership opportunities. There can be no assurance that any of these negotiations will result in a merger, acquisition, investment or other commercial partnership or, if they do, what the final terms or timing of such transactions or arrangements would be. The Company expects to continue current negotiations and discussions and actively pursue other strategic opportunities.
- 7 -
Talk Therapy
During the first quarter of Fiscal 2024, the Company commenced a pilot to roll-out talk therapy at select Treatment Centers across our footprint. Consistent with Medication Management, we believe this program will allow us to reach patients earlier in their treatment journey, develop an internal patient pipeline for TMS and Spravato®, while also further optimizing marketing costs. We believe that expanding our continuum of care and becoming a more comprehensive mental health care provider will allow us to provide greater access and quality of care to those suffering from MDD and other mental health disorders. As at the date of this Annual Report, we offer talk therapy at Treatment Centers in Florida and Missouri.
Debt Financings
Term Loans
From February 2023 through March 2024, the Company entered into various amendments to that certain Credit Agreement, dated as of July 14, 2022 (as amended, restated, amended and restated, supplemented, extended or otherwise modified from time to time), by and among the Company, certain of its subsidiaries party thereto as guarantors, and affiliates of Madryn Asset Management, LP (“Madryn”) (the “Credit Agreement”), pursuant to which Madryn and its affiliates have extended eighteen additional tranches of term loans to the Company in an aggregate principal amount of approximately $41.2 million (collectively, the “Additional Term Loans”). After giving effect to the Original Term Loans (as defined below) as well as the Additional Term Loans, the aggregate principal amount outstanding under the Credit Agreement as of the date of this Annual Report is approximately $96 million (collectively, the “Madryn Loans”). The terms and conditions of the Additional Term Loans are consistent with the terms and conditions of the Original Term Loans in all material respects. We entered into the Additional Terms Loans in part to remain in compliance with certain covenants in the Credit Agreement and in order to satisfy our near-term cash requirements necessary to operate our business.
The Madryn Loans provide Madryn with the option to convert up to approximately $7.4 million of the outstanding principal amount of the Madryn Loans into Common Shares (the “Madryn Conversion Instruments”) at a price per share equal to $1.90, subject to customary anti-dilution adjustments (the “Madryn Conversion Price”).
In addition, from December 2022 through March 2024, the Company and Madryn have agreed on a number of occasions to amend the Credit Agreement to temporarily waive the Company’s covenant to maintain minimum liquidity of $3,000,000 (“Minimum Liquidity Covenant”) in order to avoid a breach as a result of the Company’s non-compliance. The most recent amendment to the Minimum Liquidity Covenant, executed on March 28, 2024, extends the reduced Minimum Liquidity Covenant requirement of $300,000 to April 30, 2024, at which time (unless further amended or waived), the Minimum Liquidity Covenant requirement will revert to $3,000,000. We anticipate needing to obtain a further amendment to (or waiver of) the Minimum Liquidity Covenant in order to extend the application of the reduced liquidity requirement of $300,000 beyond April 30, 2024.
For more information on the Credit Agreement see “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness and Capital Raising—Madryn Credit Agreement”.
Note Purchase Agreement
On August 15, 2023, the Company entered into a note purchase agreement (as amended, restated, amended and restated, supplemented or otherwise modified from time to time, the “Note Purchase Agreement”), pursuant to which the Company may issue subordinated convertible promissory notes (“Subordinated Convertible Notes”) from time to time. Between August 2023 and October 2023, the Company issued certain Subordinated Convertible Notes to Madryn, Greybrook Health Inc. (“Greybrook Health”) and certain other investors, in an aggregate principal amount equal to $6,945,000. On August 28, 2023, the Company exchanged notes in an aggregate principal amount of $2,750,000 that had been previously issued to insiders for an equal amount of Subordinated Convertible Notes pursuant to the terms of the Note Purchase Agreement. As of the date of this Annual Report, there is approximately $9.7 million aggregate principal amount of Subordinated Convertible Notes issued and outstanding.
For more information on the Credit Agreement see “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness and Capital Raising—Subordinated Convertible Notes”.
- 8 -
Equity Financings
February 2024 Public Offering
On February 26, 2024, the Company completed a registered direct offering of 2,828,249 Common Shares (the “February 2024 Public Offering”) at a price of US$0.20 per Common Share, for gross proceeds of approximately US$565,649 before deducting legal fees and other offering expenses payable the Company. The net proceeds of the February 2024 Public Offering were used for working capital and general corporate purposes.
Alumni Purchase Agreement
On July 13, 2023, the Company entered into a purchase agreement (the “Alumni Purchase Agreement”) with Alumni Capital LP (“Alumni”). The Alumni Purchase Agreement provides equity line financing for sales from time to time of up to $4,458,156 of Common Shares. The Common Shares were issuable from time to time (the “Purchase Shares”) in connection with the delivery of purchase notices delivered by the Company to Alumni, at variable prices set forth therein, in accordance with the terms of the Alumni Purchase Agreement.
The Alumni Purchase Agreement expired on December 31, 2023. Prior to expiration, we issued an aggregate of 1,761,538 Purchase Shares for aggregate proceeds to the Company of $481,437. The Company also issued an additional 212,293 Common Shares to Alumni in exchange for Alumni entering into the Alumni Purchase Agreement.
2023 Private Placement
On March 23, 2023, the Company completed a non-brokered private placement of Common Shares (the “2023 Private Placement”) pursuant to private placement exemptions and/or Regulation S under the U.S. Securities Act of 1933, as amended (the “U.S. Securities Act”). Pursuant to the 2023 Private Placement, an aggregate of 11,363,635 Common Shares were issued at a price of $0.55 per Common Share, for aggregate gross proceeds to the Company of approximately $6.25 million. The 2023 Private Placement included investments by Madryn, together with certain of the Company’s other major shareholders, including Greybrook Health and affiliates of Masters (as defined below). The Company used the net proceeds from the 2023 Private Placement to fund the Restructuring Plan and for working capital and general corporate purposes.
Stock Exchange Matters
Nasdaq Delisting
On May 15, 2023, the Company received notification from Nasdaq that the Company is not in compliance with the minimum bid price requirement for continued listing on Nasdaq set forth in Nasdaq Listing Rule 5550(a)(2), which requires Nasdaq listed issuers to maintain a minimum bid price of at least $1 per share (the “Minimum Bid Requirement”), as the bid price of the Common Shares on Nasdaq closed below $1.00 for 30 consecutive trading days from March 31 to May 12, 2023.
Additionally, on May 16, 2023, the Company received a separate notification from Nasdaq that it was not in compliance with the minimum market value of listed securities requirement set forth in Nasdaq Listing Rule 5550(b)(2), which requires Nasdaq listed issuers to maintain a market value of listed securities of at least $35 million (the “MVLS Requirement”), as the market value of the Common Shares was below $35 million for 30 consecutive trading days from April 3 to May 15, 2023.
On November 17, 2023, the Company announced that it received a letter from Nasdaq that stated that Nasdaq determined to delist the Company’s Common Shares due to non-compliance with the Nasdaq listing rules relating to the Minimum Bid Requirement and the MVLS Requirement. The Company appealed such determination and presented its plan to regain compliance with these requirements at a delisting hearing that took place on February 13, 2024.
- 9 -
On February 22, 2024, the Company received the final delisting notice from the Listing Qualifications Department of the Nasdaq, in which Nasdaq determined to delist the Common Shares due to the Company’s continued non-compliance with the Minimum Bid Requirement and the MVLS Requirement, and found that the Company was otherwise unable to meet any of the alternative equity requirements of Nasdaq Listing Rule 5550(b) (collectively, the “Nasdaq Requirements”). As per the procedures set forth in the final delisting notice, the Company’s Common Shares were suspended from trading on Nasdaq on February 26, 2024. The Company filed its own Form 25 with the SEC on April 1, 2024, which completed the process for delisting its Common Shares from Nasdaq when the Form 25 became effective on April 11, 2024.
Following the suspension of trading of the Common Shares on Nasdaq, the Common Shares began trading on the OTC Pink Market, under the symbol “GBNHF”. On March 22, 2024, the Common Shares began trading on the OTCQB Market, under the symbol “GBNHF”.
Toronto Stock Exchange Delisting
On February 27, 2023, the Company announced that it had applied and received approval for a voluntary delisting (the “TSX Delisting”) of the Common Shares from the TSX. The TSX Delisting was made effective following the close of markets on March 13, 2023.
Corporate and Other Developments
Director and Officer Appointments
On March 4, 2024, the Company announced that it appointed Mr. Andrew Crish as the Company’s full-time Chief Operating Officer effective immediately.
On January 29, 2024, the Company announced that it appointed Mr. Peter Willett, as the Company’s full-time Chief Financial Officer, effective immediately. Mr. Willett previously served as the Company’s Interim Chief Financial Officer and replaces the Company’s previous Chief Financial Officer, Erns Loubser, who stepped down on October 20, 2023.
On January 23, 2024, the Company announced that it appointed Ms. Juliana Elstad to the Board as an independent director, effective immediately. Ms. Elstad has also been appointed to the Company’s Governance and Nominating Committee.
On October 31, 2023, the Company announced that it appointed Ms. Surindra Mann to the Board as an independent director, filling the vacancy created by the resignation of Dr. Adrienne Graves, Ph.D.
On June 23, 2023, the Company announced the resignation of Mr. Benjamin Klein from the Board after Mr. Klein received more “withheld” votes than votes “for” at the Company’s annual meeting of shareholders held on June 20, 2023.
Klein Settlement
On November 21, 2023, the Company announced it had entered into a settlement agreement (the “Klein Settlement Agreement”) with the plaintiff regarding the Klein Note Action (as defined below). Under the terms of the Klein Settlement Agreement, the Company agreed to make payments to the plaintiff in the total amount of approximately $2.2 million, structured as an initial immediate payment of $250,000, weekly payments of $75,000 thereafter up to and until the May 1, 2024, maturity date of the promissory note, upon which the balance owing will be due. In exchange for entry into the Klein Settlement Agreement, the plaintiff dismissed, with prejudice, the Klein Note Action on November 27, 2023 and both parties provided a mutual release of claims.
On November 20, 2023, the court stayed the separate complaint concerning alleged disputes arising out of the purchase agreement for the acquisition of Success TMS and other related matters (the “Purchase Agreement Claims” and, collectively with the Klein Note Action, the “Klein Matters”) until May 13, 2024. See Item 3 “Legal Proceedings” of this Annual Report for further information regarding the Klein Matters.
- 10 -
Acquisition of Success TMS
On July 14, 2022, we, through our wholly-owned U.S. subsidiary, TMS US, completed the acquisition of all of the issued and outstanding equity interests in Success TMS from its parent company, Success Behavioral Holdings LLC (the “Success TMS Acquisition”) pursuant to a Membership Interest Purchase Agreement dated as of May 15, 2022 (the “Purchase Agreement”) by and among the Company, Success TMS and its direct and indirect owners, including Success Behavioral Holdings LLC, Theragroup LLC, The Bereke Trust U/T/A Dated 2/10/03, Batya Klein and Benjamin Klein (collectively, the “Seller Parties”).
As consideration for the purchase of Success TMS, the Seller Parties received, in the aggregate, 8,725,995 Common Shares, and an additional 2,908,665 Common Shares have been held back and deposited with an escrow agent (the “Escrowed Shares”), to be released to Mr. Klein or the Company, as applicable, upon satisfaction of customary working capital and certain other adjustments, including to satisfy any indemnity claims against the Seller Parties. Although we do not believe Mr. Klein is currently entitled to the release of any of the Escrowed Shares, Mr. Klein has disputed this and we may be required to release some or all of the Escrowed Shares, or otherwise settle the dispute, in the future. The purchase price consideration was determined based on the pro forma revenue contribution of the two companies and was fixed at an amount equal to approximately 40% of the total issued and outstanding Common Shares on a post-acquisition basis and subject to adjustments, as described above.
As contemplated by the Purchase Agreement, the Company also entered into an investor rights agreement with Benjamin Klein, dated as of July 14, 2022 (the “Klein Investor Rights Agreement”), which provides Benjamin Klein with a right to nominate a single representative to the Board for so long as the Seller Parties own at least 5% of the issued and outstanding Common Shares, subject to certain conditions, including applicable securities laws and stock exchange requirements. In accordance with the terms of the Klein Investor Rights Agreement, Mr. Klein was appointed to the Board as the board nominee, effective on July 14, 2022, immediately following completion of the Success TMS Acquisition. In connection with the Success TMS Acquisition, Mr. Klein was also appointed the Chief Operating Officer of the Company, whereby Mr. Klein served in that role from July 14, 2022, until his departure on May 4, 2023. Mr. Klein also resigned from the Board effective June 23, 2023.
Industry Overview
Depression – Disease Overview
MDD is a mood disorder characterized by depressed mood and/or a loss of interest or pleasure from activities. Other common signs and symptoms that define the condition include feelings of worthlessness or guilt, sleep disturbance, changes in appetite or weight, psychomotor slowing or agitation, fatigue, concentration difficulties, and recurrent thoughts of death or suicide (Source: American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, fifth edition, or DSM-5).
MDD is often a recurrent disease and follows a fluctuating course over an individual’s lifetime, with alternating periods of remission and relapse. Experiencing one episode of MDD places the individual at an estimated 50% risk of experiencing an additional episode of MDD in the future. Approximately 80% of individuals who have experienced two episodes of MDD will experience an additional episode in the future (Sources: American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, fourth edition; Interpersonal Factors in the Origin and Course of Affective Disorders, 1996; American Journal of Psychiatry, 1992 Aug; 149(8)).
A clinical diagnosis of MDD is determined by conducting a clinical exam and interview to establish if the patient is experiencing the combination of symptoms as defined in the DSM-5. The severity of a patient’s symptom profile is typically measured using a standardized rating scale. These scales can be derived from a patient-driven, self-reported questionnaire, such as the Patient Health Questionnaire-9 (“PHQ-9”), or from an observer-dependent and interview-based scale, such as the Hamilton Depression Rating Scale (“HAMD”). These rating scales, among other diagnostic criteria, can be used to grade a patient’s MDD symptoms on a continuum from mild to severe. In addition to the depression symptoms and their negative impact on quality of life, MDD is also commonly associated with a number of serious co-morbidities, including other mental health disorders, with an estimated 65.8% of patients with recurrent MDD suffering from accompanying psychiatric conditions or substance abuse disorders (Source: American Journal of Psychiatry, 1997 Dec; 154(2)). MDD patients also have a substantially increased risk of committing suicide and increased risk for other conditions, such as heart disease (Sources: Acta Psychiatrica Scandinavica, 2008 Mar, 117(3); Frontiers in Psychiatry, 2016 Jul; 33(7)). The common and most widely accepted clinical measurement threshold for determining whether there has been a positive response to treatment of MDD is a significant decrease in depressive symptoms as measured using a standardized ratings scale from certain baseline scores. Where a patient demonstrates few or no symptoms at all, the patient is commonly referred to as being “in remission”. The return of symptoms is commonly referred to as a “relapse”.
- 11 -
As with many psychiatric disorders, the direct causes of MDD and underlying pathophysiology remain to be fully elucidated. However, a variety of interrelated factors are known to likely be involved, including (i) the physical and neurochemical status of the brain, including specific brain regions, (ii) hormonal changes, (iii) genetics, (iv) acute life events, (v) chronic stress, (vi) childhood exposure to adversity, and (vii) a myriad of other environmental factors. A signaling network in the brain that is known to function in the regulation of mood and is believed to play a significant role in the occurrence of MDD, is a circuit that includes the prefrontal cortex, the anterior cingulate cortex and the limbic brain structures (Source: CMAJ, 2009 Feb; 180(3)). This network, and all other networks and signaling structures in the brain, are created by connections between neurons, the individual nerve cells in the brain. A neuron is a specialized cell-type that responds to both chemical and electrical signals and is connected to other neurons through specialized cell-to-cell connections known as synapses. The release of chemical messengers, or neurotransmitters, in the brain occurs across these synapses and results in changes in the electrical properties of the receiving neuron and the further propagation of the signal in the brain to form neuronal circuits.
This communication process between two neurons across individual synapses or between different regions of the brain is ordinarily regulated by feedback mechanisms that result in the decreased release of neurotransmitter signals through a process known as reabsorption or reuptake, in which the neurons actively reabsorb the neurotransmitters back into the cell once adequate signaling has occurred. In people with MDD, however, this complex system of neuronal communication is impaired and does not function properly. In MDD, a number of causes may underlie this impaired signaling. For example, specialized neurotransmitter receptors may be either oversensitive or insensitive to a specific neurotransmitter, causing their response to its release to be either excessive or inadequate, or the signal might also be weakened if the originating cell produces too little of a neurotransmitter or if the reuptake process is too active and reabsorbs too much of the neurotransmitter to allow for proper signaling.
It is now widely accepted in neuroscience that improper regulation of one or more of the three major neurotransmitters, serotonin, norepinephrine and dopamine, plays a role in the emergence of depression. This understanding has been essential to the development of psychiatric drugs and the treatment of depression based on targeting chemically based mechanisms underlying mood regulation. In contrast to chemically based treatment, TMS therapy is a newer treatment paradigm that instead uses a targeted, circuit-based approach that relies on the ability of electrical mechanisms to help restore and augment neurotransmitter signaling to help re-establish proper function to neuronal pathways to treat depression.
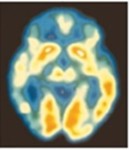
The images below illustrate brain activity as measured by positron emission tomography or PET imaging for patients suffering from MDD as compared to normal functioning brain activity (blue and green represents decreased brain activity) (Source: Mayo Foundation):
Depressed | Not Depressed |
|
|
Prevalence and Societal Cost
The World Health Organization (the “WHO”) now ranks MDD as the single largest contributor to global disability and a major contributor to the occurrence of suicide worldwide (Source: Depression and Other Common Mental Disorders – Global Health Estimates (WHO 2017)). A study published in the Journal of Clinical Psychiatry estimated the economic burden of the disease at approximately $210 billion annually in the United States alone, including outpatient and inpatient medical costs, pharmacy costs, suicide related costs and workplace costs (Source: Journal of Clinical Psychiatry, 2015 Feb; 76(2)). A study published in Psychological Medicine reported that the global point prevalence of MDD is approximately 4.7% (Source: Psychological Medicine, 2013 Mar; 43(3)) and the WHO estimates that there are approximately 300 million people in the world struggling with depression (Source: Depression and Other Common Mental Disorders – Global Health Estimates (WHO 2017)).
- 12 -
Traditional Treatment Options
In the United States, an initial diagnosis of MDD in adult patients is typically determined by the patient’s primary care physician. Upon diagnosis, the most common form of treatment for MDD is the prescribing of an initial course of antidepressant medication, which may or may not be accompanied by psychotherapy. The physician would typically discuss a number of different treatment options with the patient and then design a treatment plan tailored to the patient’s specific symptoms, personal preferences and the psychiatric services available in proximity to the patient’s home or workplace.
The most commonly prescribed antidepressant medications are selective serotonin reuptake inhibitors (“SSRIs”). SSRIs primarily act to affect the levels and activity of serotonin in the brain and attempt to combat depression by blocking or inhibiting the reuptake of this particular neurotransmitter, thereby increasing the levels of available serotonin to promote proper signaling. Different classes of antidepressant medications also work on different combinations of underlying neurotransmitters. For example, serotonin norepinephrine reuptake inhibitors (“SNRIs”) work by blocking the reuptake of both serotonin and norepinephrine. Other medications may have more diverse effects on all three major neurotransmitters. During the initial treatment period, patients commonly suffer from negative side effects that may offset any benefits in symptoms experienced and result in discontinuing treatment. Therefore, it is common for a patient and their primary care physician to experiment with different antidepressant drugs and drug combinations before determining a medication regimen for the patient that provides both adequate symptom relief and is tolerable from a side effect perspective.
Depression-focused psychotherapy, or “talk therapy”, is also commonly recommended as a treatment option for patients suffering from MDD. Psychotherapy is generally implemented as part of a treatment plan in conjunction with the use of antidepressant medication. Two of the most well studied and commonly available psychotherapy techniques for MDD are cognitive behavioral therapy and interpersonal psychotherapy, both of which are interactive therapies conducted between a trained professional and the patient.
If initial treatment approaches do not sufficiently relieve a patient’s symptoms, a primary care physician will often refer the patient to a psychiatrist trained in psychopharmacology. There are a substantial number of drugs and drug combinations that a psychiatrist may consider as second line therapies for MDD after an initial treatment has failed. For example, a psychiatrist may recommend combining two or more antidepressant medications, which is referred to as “combination therapy”, or using a second medication such as an atypical antipsychotic drug that is not an antidepressant along with the initial antidepressant medication to potentially augment the efficacy of such antidepressant, which is referred to as “augmentation”.
Other, later-stage treatment options, such as electroconvulsive therapy (“ECT”) and vagus nerve stimulation (“VNS”), are associated with greater medical risk, and are usually only considered for patients with severe cases of MDD. ECT is a hospital-based, inpatient treatment approach that is typically reserved for patients exhibiting the most severe MDD symptoms and is implemented most commonly in patients that are experiencing catatonia, psychosis, or acute suicidality that necessitate inpatient hospitalization. ECT involves the direct application of high voltage electrical current to the surface of the head and must be administered under anesthesia. VNS is the most invasive treatment option currently approved by the FDA for MDD and is usually only considered for patients who have proven to be severely treatment resistant. VNS involves the surgical implantation of a stimulating electrode that is wrapped around the vagus nerve, which travels through the neck near the carotid artery, and a pulse generator that is separately implanted under the skin near the patient’s collarbone. The pulse generator sends electrical impulses to the electrode with the aim of stimulating the regions of the brain known to be directly associated with the regulation of mood.
Limitations of Traditional Treatment Options
Although a large number of antidepressant drugs have been approved and can be efficacious in subsets of patients in relieving depression symptoms, drug therapy has two primary limitations as it relates to the treatment of MDD:
| 1. | Limited efficacy, which can decline with each successive cycle of medication, with respect to both the same or different class(es) of antidepressant drugs; and |
| 2. | Treatment-emergent negative side effects and toxicities causing poor patient treatment adherence or discontinuation of treatment therapy altogether. |
- 13 -
The limitations of drug therapy in MDD were well demonstrated in the Sequenced Treatment Alternatives to Relieve Depression Study (the “STAR*D Study”) conducted by the U.S. National Institute of Mental Health that enrolled 4,041 adult patients (aged 18-75) suffering from MDD at 41 clinical sites in order to examine the outcome of a sequenced series of antidepressant medication attempts that replicated current views on best practices. In the STAR*D Study, the results of which were published in 2006, only approximately 49% of patients responded to their first course of medication, and 28% of patients achieved remission in their first course of medication. Only approximately 21% of patients achieved remission in their second course of medication.
Many patients taking antidepressant medications experience negative and/or intolerable side effects to treatment that contribute to a delay or failure in attaining an effective or optimal antidepressant dose, poor patient treatment adherence or discontinuation of treatment altogether. Furthermore, the likelihood of achieving remission is limited and such likelihood declines with each successive medication attempt (Source: STAR*D Study). Antidepressant medication therapy for the treatment of MDD is often administered along with a recommendation for a depression-focused psychotherapy. While these treatment options have demonstrated efficacy in some clinical studies, they are also associated with limitations in practice. For instance, the experience level of the therapist may significantly affect the treatment outcome and access to such therapy can be limited for many patients.
The other treatments that offer patients alternatives where drugs and psychotherapy have failed, ECT and VNS, can have significant disadvantages when compared to TMS. ECT typically requires general anesthesia and must be administered in a controlled hospital setting with direct access to emergency resuscitation equipment. ECT is typically administered three times per week for up to 12 treatments, with some patients requiring as many as 20 treatments. Some patients experience a rapid return of symptoms after a course of ECT, requiring ongoing maintenance sessions to sustain benefit. The two most common side-effects ECT patients may experience are confusion and memory loss, each of which can occur immediately following a treatment session. Other side effects may include nausea, headache, jaw pain, muscle ache, hypertension and hypotension and life-threatening complications including adverse reactions to anesthesia, arrhythmias, ischemia or prolonged seizures. (Source: Psychiatric Clinics of North America, 2016 Sep; 39(3)). Despite the risks and potential side-effects of ECT, VNS is actually considered the most invasive treatment option currently approved by the FDA for MDD patients who have proven to be severely treatment resistant. The surgical implantation of the VNS device (both stimulating electrode and pulse generator) introduces risks including infection or local damage to the recurrent laryngeal nerve, which may lead to permanent voice alteration. Other significant potential adverse events associated with VNS include risk of developing cardiac arrhythmias and the need for repeated invasive procedures required to replace the pulse generator battery (Source: Psychiatry (Edgmont), 2006 May; 3(5)). VNS has a delayed onset of action, requiring up to a year to realize its full potential. Lastly, complications and delays relating to reimbursement for the implantation and ongoing monitoring of the VNS device results in limiting access to the procedure for many patients.
TMS as a Safe and Effective Treatment Alternative
Overview of TMS
TMS is differentiated from traditional drug therapy approaches for the treatment of MDD and represents a different paradigm for the treatment of depression. TMS uses a pulsed magnetic field to induce electrical currents in neural tissue designed to stimulate specific areas of the brain associated with mood. The target for stimulation and activation in TMS to treat MDD is the prefrontal cortex, which serves as a starting point to regulate the neuronal circuitry connected to this region of the brain. This stimulation triggers a cascading electro-chemical effect that can pass along the neuronal circuit and reach into the deeper structures of the brain that also serve to regulate mood. This process can change the level of excitability and neuronal connections among these structures in a manner that improves the overall activity of the neuronal circuit which is believed to underlie the improvement in MDD symptoms in responsive patients (Source: Frontiers in Human NeuroScience, 2013 Feb; 7(37)).
TMS is most commonly performed as an office-based procedure using an FDA-cleared medical device specifically designed to deliver the magnetic pulses necessary to stimulate the neurons. A course of treatment typically requires treatment sessions five times per week, conducted over a four- to six-week period that can last from 19 to 45 minutes per session. Post-TMS treatment, patients can immediately return to their normal routine, including driving home or to the workplace.
- 14 -
TMS is considered to be an appropriate alternative and a potentially life-changing treatment for a patient suffering from MDD who has failed to achieve satisfactory improvement from prior antidepressant medications and psychotherapy in the current MDD episode. One of the main advantages of TMS therapy is that it has few side effects, with the most common side effect being short-lasting mild pain or discomfort around the treatment site which typically only lasts during the first week of treatment. Other adverse reactions such as jaw and face pain, muscle pain, spasm or twitching, and neck pain were reported as mild or moderate and were also resolved shortly after treatment, as well as seizures in certain patients. The less severe side effects associated with TMS therapy make it an attractive option for patients, particularly when compared with more aggressive treatment options, such as ECT, which may have significant and relatively severe side effects which may include nausea, headache, jaw pain, muscle ache, hypertension and hypotension and life-threatening complications including adverse reactions to anesthesia, arrhythmias, ischemia or prolonged seizures. The side effect profile of TMS therapy also compares favorably to VNS, which is considered to be the most invasive therapy option approved by the FDA for MDD patients who have proven to be severely treatment resistant. VNS is associated with surgical related risks, such as infection or local damage to the recurrent laryngeal nerve, which may lead to permanent voice alteration.
Safety and Efficacy of TMS Therapy for MDD
TMS is an FDA-cleared, safe and effective neurostimulation therapy for the treatment of patients suffering from MDD. The safety and efficacy of TMS therapy has been demonstrated through two large, sham-controlled trials. For real world outcomes, a clinical trial was conducted on patients who failed to achieve satisfactory improvement from antidepressant medication treatment, demonstrated that approximately 58% of patients responded positively to TMS therapy, and approximately 37% of patients achieved remission of their MDD symptoms (Source: Depression and Anxiety, July 2012; 29(7)). Another analysis of patients in a multi-site, naturalistic observational clinical trial who consented to 12 months of follow-up showed a response rate of approximately 62% and a remission rate of 42% at six weeks, and a response rate of 68% and a remission rate of 45% at 12 months (Source: Journal of Clinical Psychiatry, 2014 Dec; 75(12)). This is contrasted with the results from the STAR*D Study on MDD drug treatment outcomes where only approximately 28% of patients achieved remission in their first round of drug treatment. For patients failing the first-line drug therapy and undergoing a second round of drug treatment, approximately 21% of patients achieved remission in their second medication attempt (Source: STAR*D Study). In addition to the higher efficacy rates, as measured by remission of MDD symptoms versus medication therapy, the discontinuation rate in the sham-controlled TMS clinical studies were approximately 5% (Source: Journal of Clinical Psychiatry, 2008 Feb; 69(2)), which is a marked contrast to the single medication treatment in the STAR*D Study in which the adverse events discontinuation rate increased from 9% to 41% as additional alternative monotherapy treatment attempts were administered (Source: STAR*D Study). These study results demonstrate TMS to be better tolerated by patients than medication therapy, with the most common side effect being transient pain or discomfort around the treatment site, with a minimal risk of seizures.
Sources:
(1) STAR*D Study.
(2) Journal of Clinical Psychiatry, 2014 Dec; 75(12).
(3) Journal of Clinical Psychiatry, 2004 Apr; 65(4); Biol Psychiatry, 2004 Feb; 55(3).
TMS Delivery Systems
TMS treatments are delivered using TMS Devices, federally regulated medical devices specifically manufactured to transmit the magnetic pulses required to stimulate the cortical areas in the brain to effectively treat MDD. Pursuant to the Neuronetics Agreement, Neuronetics is now the exclusive supplier of TMS Devices to the Company. For more information, see “Our TMS Business Model—Relationships with our TMS Device Suppliers and Cost Model” below.
Key Benefits of TMS Therapy
| ● | Effective treatment option – In a clinical study, TMS demonstrated a response rate of approximately 62% and a remission rate of approximately 42% (Source: Journal of Clinical Psychiatry, 2014 Dec; 75(12)). |
| ● | Positive patient experience with convenient treatments – TMS is a short office-based procedure administered in an office setting, allowing for convenient patient access. Patients can immediately return to their normal routine following each treatment session, including driving home or to the workplace. |
| ● | Non-invasive and non-sedative procedure – In contrast to other second-line treatment alternatives, TMS therapy requires no anesthesia and no hospitalization. |
- 15 -
| ● | Well-tolerated treatment option with no major side effects – TMS is generally well-tolerated with minor side effects experienced in a small subset of patients. The most common side effect is mild and temporary scalp discomfort. TMS is also associated with a minimal increase in the risk of seizures experienced in a small subset of patients. In contrast, drug therapy is often associated with side effects such as blurred vision, anxiety, weight gain, constipation, nausea and insomnia, and is less tolerated by patients as evidenced by the 42% rate of treatment discontinuance for patients that had received three separate medication trials in the STAR*D Study. |
| ● | Compelling value proposition for insurance companies and reimbursed by all major insurance carriers in the United States – We believe that a broader adoption of TMS therapy is likely to significantly reduce costs to the U.S. healthcare system and broader economy due to the well documented economic burden of depression and related co-morbidities. Broader access could also help to address an underserved patient population with limited treatment alternatives based on poor access to traditional psychiatric treatments. Inpatient medical costs, pharmacy costs, suicide-related costs and workplace costs can be significantly reduced by providing access to care in the form of TMS therapy. The direct cost of a TMS treatment course in the United States generally ranges from approximately $6,500 to $10,000. TMS is equally compelling to the United States insurance providers (or other payors in the healthcare system) given that the costs are comparable to or, in many cases, less expensive than the ongoing cost of combination drug therapies and psychotherapy treatments typically associated with patients that are determined to be suitable candidates for TMS therapy. TMS therapy is now covered by all major commercial insurance carriers and Medicare, representing approximately 300 million covered lives in the United States. |
Market Opportunity for the Delivery of TMS
Based on U.S. Census Bureau data and the 2017 National Survey on Drug Use and Health, management estimates that approximately 17.3 million adults in the United States suffer from MDD annually. Of these people, we estimate that approximately 7.4 million of these individuals actively seek treatment and, based on applying data from the STAR*D Study, approximately 5.3 million of these patients are likely to have failed to achieve remission of their MDD from a course of antidepressant drug therapy.
Based on these figures and expected provider revenues for a standard course of TMS treatment generally ranging from $6,500 to $10,000, we believe that there exists a significant potential market for TMS treatment in the United States.
Expansion of Market Opportunity Through New Indications
TMS therapy gently modulates brain activity allowing for targeting interventions to restore normal function without the need for anesthesia, invasive procedures, or systemic medications. Beyond MDD, there has been a strong interest in finding methods of treating other neuropsychiatric conditions.
For example, in 2018, the FDA provided clearance for the use of a TMS Device in the treatment of OCD. This was followed in 2020 with the announcement that the FDA provided clearance for the use of a TMS Device in smoking cessation treatment.
TMS Device manufacturers are actively exploring utility in other medical conditions such as bipolar disorder, multiple sclerosis related fatigue, alcohol dependence, post-stroke rehabilitation, and opioid dependence. Our established footprint and proven service delivery model for TMS therapy makes us well-positioned to lead the delivery of treatment for any new indications if and when such treatments are approved by the FDA and eligible for reimbursement by insurance carriers. Management believes that the treatment of new indications can be rapidly incorporated into our Treatment Center network with minimal incremental investment required.
- 16 -
Our Role as a Leading Provider of TMS Therapy
Despite the magnitude of the market opportunity, based on the proven safety and efficacy of TMS, and the fact that TMS is generally accepted by psychiatrists and neurologists as an effective treatment for patients suffering from MDD, the number of TMS procedures performed annually in the United States remains low relative to the addressable market. Key factors contributing to this discrepancy include a historical lack of insurance reimbursement for TMS, the social stigma attached to publicly seeking treatment for depression, the lack of awareness of TMS among both the general population and physicians as a viable treatment alternative for depression, and the overall poor alignment of TMS treatment with the traditional practice of psychiatry. Furthermore, the almost-daily nature of TMS treatment is one of the key challenges facing our patients in successfully completing their TMS treatment protocol and is generally a major obstacle to access to TMS therapy. Our Treatment Center network is purpose-built in order to address this challenge through the implementation of multiple convenient locations within a given region and operating hours that allow our patients to easily and effectively incorporate TMS into their daily schedules (see “—Our Business Model—Our Focus on the Patient Experience” below).
There are some significant challenges involved in incorporating TMS into the existing model for the practice of psychiatric medicine or the provision of mental health treatment more generally. A typical psychiatrist office is simply not conducive to high patient throughput using device-oriented therapy such as TMS and psychiatrists have generally been slow to deviate from the more standard practices of talk therapy and the administration of antidepressant drugs.
Our business model was developed to overcome all of these challenges and to take advantage of the opportunity for a new, differentiated service channel – a patient-focused, customer service model to make TMS therapy easily accessible to all patients while maintaining a high standard of patient care.
Our TMS Business Model
A Regional Approach to Center-Based Delivery of Care
Our regional model seeks to develop leading positions in key markets and to leverage operational efficiencies by combining smaller local Treatment Centers within a region under a single shared regional management infrastructure. Management regions typically cover a specific metropolitan area that meets a requisite base population threshold. The management region is typically defined by a manageable geographic area which facilitates the use of regional staff working across the various Treatment Center locations within the management region and creates a marketing capture area that allows for efficiencies in advertising costs.
Our scale and density within selected geographies provides valuable and mutually beneficial long-term relationships with key payors, local clinicians and behavioral health groups. Our regional operations team is responsible for managing local clinicians, non-clinical staff and referral relationships to provide for a patient-centric, customer service model, which makes TMS easily accessible to patients.
We provide centralized support to management regions through corporate training programs, standardized policies and procedures, systems and business infrastructure support as well as the sharing of best practices among the clinicians and support staff across our regional networks. Centralized services include professional marketing management, call center support, centralized patient scheduling, legal and finance support and centralized medical billing services.
Our Patient-Focused Treatment Model
Our patient-focused, customer service model makes TMS therapy easily accessible to all patients through three core business processes supported by a centralized, scalable business infrastructure: (1) Patient Inquiry; (2) Patient Conversion; and (3) Treatment Delivery. Each of these core business processes are further described below:
Patient Inquiry
The patient inquiry process consists of utilizing several marketing channels in order to drive patient and clinician awareness of TMS and the Greenbrook brand. Direct to consumer marketing strategies (such as radio, web and digital) are combined with a regional account management sales team that develops relationships with local clinicians, clinician groups, primary care providers and behavioral health groups. We offer clinician groups the opportunity to offer access to TMS by referring their patients to one of our local Treatment Centers or in partnership as an extension of their own practice. With our focus on the provision of TMS and not on the provision of general psychiatric services, we generally do not compete with our referral network.
- 17 -
We further support regional and corporate marketing strategies with local community events and sponsorships. Patient inquiries are directed to our technology-enabled call center, which gives call center administrators the ability to schedule patients centrally to a nearby local Treatment Center for a free patient consultation to educate patients on the benefits of TMS, as further described below.
Patient Conversion
The process begins with our team of experienced consultants that operate regionally to conduct an initial free consultation at a Treatment Center of the patient’s choice. The consultant introduces and explains TMS therapy to the patient and answers any initial questions the patient may have in order to determine whether TMS therapy might be an appropriate treatment option for the patient. Following this consultation process, the consultant assesses reimbursement support available to patients interested in proceeding with treatment, subject to the pre-assessment examination by a clinician (which may be a psychiatrist, physician, nurse practitioner or physician assistant). As part of the reimbursement support, an initial benefits review is conducted by the consultant to provisionally determine whether the patient will be covered by insurance for the TMS treatment as well as the extent of any out-of-pocket costs to be incurred by the patient in respect thereof. If a patient desires to proceed with treatment, the consultant or an administrative staff member will schedule a pre-assessment appointment with an on-site clinician to review the patient’s medical history and ultimately decide whether TMS is a clinically appropriate treatment option for the patient.
Treatment Delivery
If it is determined by the clinician during the pre-assessment that TMS is clinically appropriate for the particular patient, and assuming the patient wishes to proceed with such treatment (which can occasionally be dependent on the outcome of the insurance eligibility investigation conducted by the billing and reimbursement support team), a course of TMS treatment is scheduled at a Treatment Center of the patient’s choosing. The location selected is typically one that is most convenient relative to the patient’s home or workplace.
A treatment course typically consists of 36 individual treatment sessions, including an initial and repeated motor threshold determination to determine the TMS intensity level necessary to evoke a peripheral motor response, thereby optimizing the treatment protocol based on each individual patient. Treatment is then conducted by a trained and certified TMS technician and supervised by a clinician. A course of treatment typically requires treatment sessions that last from 19 to 38 minutes per session, five times per week, conducted over a four- to six-week period. Post-TMS treatment, patients can immediately return to their normal routine, including driving home or to the workplace.
We are not involved in the practice of medicine and do not interfere with, or exercise control over, the professional medical judgment of the clinicians involved in the provision of medical services at our Treatment Centers. Rather, we are involved in the operation and administration of the medical practices operating at our Treatment Centers in order to facilitate the successful delivery of TMS therapy.
Centralized, Scalable Business Infrastructure
Our three core business processes are supported by a technology-enabled, corporate business infrastructure including information technology, medical billing, human resources, branding, training, regulatory and finance. Our custom end-to-end integrated systems are designed to enable a seamless process from the first patient interaction, through the patient treatment process until payment is received with the assistance of our centralized billing support team.
Our Focus on the Patient Experience
The almost-daily nature of TMS treatment is one of the key challenges facing our patients in successfully completing their TMS treatment protocol and is generally a major obstacle to access TMS therapy. Our Treatment Center network is purpose-built in order to address this challenge through the implementation of multiple convenient locations within a given region and operating hours that allow our patients to easily and effectively incorporate TMS into their daily schedules.
Our regional model provides a seamless patient experience. The consultation with a TMS technician and the initial clinician visit can be attended at any of our regional locations. Our highly trained technician team (under the supervision of a clinician) provides the treatment at a local Treatment Center selected by the patient, which is typically in close proximity to the patient’s home or workplace.
- 18 -
We aim to embed our Treatment Centers within class A office space that provides a comfortable and discrete experience for our patients, in an effort to counteract the stigma often associated with a mental health facility or a mental health in-patient clinic. Our standardized center design and color pallets provide for a relaxing and welcoming treatment environment that is designed to not feel like a hospital or medical office. Our highly trained technician team and experienced clinical leadership are focused on delivering the highest standard of patient care while also delivering a pleasant and welcoming patient experience. Our TMS Devices are located in comfortable private treatment rooms where patients can relax while receiving treatment. The daily nature of the treatment allows our technicians to develop a relationship with many of our patients and we believe this relationship can be a supportive factor in the success of the treatment and a positive improvement in many of our patient’s lives.
Regional Development Strategy
As discussed above, our regional model seeks to develop leading positions in key markets and to leverage operational efficiencies by combining smaller local Treatment Centers within a region under a single shared regional management infrastructure.
A new regional build-out is typically associated with a metropolitan area that meets a requisite base population threshold. The management region is typically defined by a manageable geographic area, which facilitates the use of regional staff working across the various Treatment Center locations within the management region, and which resides within a marketing capture area that allows for efficiencies in advertising costs. Management regions are not strictly defined by state lines or other geographic borders, but rather a functional management area.
In order to maximize cost efficiencies, we focus on developing our clinic networks within metropolitan areas that can support multiple centers. Treatment Centers are placed in sub-populations within the metropolitan area that provides local treatment access to patients in order to alleviate the time and effort associated with the almost-daily nature of the treatment course. Convenient, local patient access is essential to attract patients suffering from MDD, as depression is a condition associated with low motivation, a lack of energy, and typically a reluctance to take action and travel to a medical facility. In establishing our regional footprint, we carefully evaluate elements such as traffic patterns, highway access and major regional employers to optimize accessibility and to ensure that the addition of new Treatment Centers incrementally adds to the addressable patient population within the management region. We also sometimes engage in discussions with TMS Device manufacturers as part of our assessment of a potential region. Our current development focus for existing regions is on incrementally increasing the patient volume within the management region rather than assessing an individual Treatment Center location in isolation. As a result, we will from time to time establish a Treatment Center that may, over the short term, negatively impact the patient volume at another nearby Treatment Center, but which adds incremental patient access and volume to the region as a whole in an economically beneficial manner.
Management regions are evaluated, assessed and ultimately selected for development through careful consideration of the following core factors, among others:
| ● | population density and demographics; |
| ● | state legislation as it relates to the practice of medicine; |
| ● | local insurance reimbursement rates and coverage criteria; |
| ● | commercial real estate rates; |
| ● | availability of high-quality clinical partners and regional staff; |
| ● | awareness of TMS (which is typically greater in areas where TMS therapy is incorporated into local university research programs); and |
| ● | regional marketing overlaps which generates cost synergies. |
Once we are comfortable with the profitability of treatment delivery based on the factors highlighted above, we establish an initial single Treatment Center as the regional hub for the management region, typically in partnership with an anchor physician partner. Additional Treatment Centers are then added based on capacity utilization and required patient coverage areas which is monitored based on referral data and patient inquiry activity. Subsequent Treatment Centers share allocations of regional and corporate overhead costs.
- 19 -
Relationships with our TMS Device Suppliers and Cost Model
Our business model is focused on providing a differentiated service channel – a patient-focused, customer service-oriented model that strives to make TMS therapy easily accessible to as many patients as possible. We aim to make best-in-class TMS technology available to our patients and clinicians throughout our Treatment Center network. We do not own the intellectual property associated with any TMS Device. Instead, we cultivate and foster relationships with TMS Device manufacturers.
There are currently eight FDA-cleared TMS Devices available in the United States, including NeuroStar Advanced Therapy Systems, BrainsWay Deep TMS, Magstim, MagVita TMS Therapy, Cloud TMS, Nexstim Plc and Apollo TMS. Neuronetics has a market leading position in respect of TMS Devices and was the first TMS Device to receive FDA clearance in 2008.
In January 2023, the Company entered into the Neuronetics Agreement pursuant to which Neuronetics has become the Company’s exclusive supplier of TMS Devices. As of December 31, 2023, we had 260 TMS Devices throughout our Treatment Center network.
Our TMS Devices are typically leased from Neuronetics, with an option to purchase the device at the end of the lease term. As of December 31, 2023, we had 45 leased TMS Devices and 215 owned TMS Devices. The cost structure in respect of a particular TMS Device is generally dependent on the specific pricing model for each device manufacturer. Our blended TMS Device cost represented 15% of gross revenue in Fiscal 2023.
Insurance Reimbursement for TMS Therapy
Revenue represents net patient fees received (or receivable), for TMS services and is billed on a per treatment basis by our centralized billing team. TMS provides a highly compelling value proposition to payors and is fully validated with reimbursement in all 50 states and from all major insurance providers (including Medicare), representing approximately 300 million covered lives, with over 97% of our patients having commercial insurance, Medicare or other non-Medicare government-based coverage for TMS. Approximately 72% of these patients are covered by commercial insurance plans while 28% are covered by Medicare or other non-Medicare government-based programs.
TMS therapy is billed under three Current Procedural Terminology codes:
| ● | 90867 – Therapeutic repetitive transcranial magnetic stimulation treatment; initial, including cortical mapping, motor threshold determination, delivery and management; |
| ● | 90868 – Subsequent delivery and management, per session; and |
| ● | 90869 – Subsequent motor threshold re-determination with delivery and management. |
A course of TMS typically consists of 36 treatments, with each treatment billed separately, with some patients returning for additional treatments when clinically appropriate. Insurance carriers typically reimburse 36 sessions per course of treatment. A typical course of treatment will consist of an initial cortical mapping and motor threshold determination and treatment (90867), various daily treatment sessions (90868), and a subsequent motor threshold re-determination and treatment (90869).
The Centers for Medicare & Medicaid Services (“CMS”) has not established a national coverage determination or centralized fee schedule for TMS. Instead, CMS leaves pricing discretion to the various Medicare Administrative Contractors (“MACs”). Commercial payors also individually exercise discretion over pricing and may establish a base fee schedule for TMS or negotiate a specific reimbursement rate with an individual Treatment provider. Commercial payors are not bound by any CMS coverage policies or pay rates and have the option to tailor their individual payment policies.
Our Spravato® Program
During January 2021, we implemented the Spravato® program at select Treatment Centers to enable our affiliated clinicians to provide Spravato® (esketamine nasal spray) therapy to their patients (the “Spravato® Program”). Spravato® is a nasal spray marketed by Janssen Pharmaceuticals, Inc. which is currently approved by the FDA for use, in conjunction with an oral antidepressant, to treat treatment-resistant depression in adults and depressive symptoms in adults with MDD with acute suicidal ideation or behavior. The active ingredient in Spravato® is esketamine, which is the s-enantiomer of ketamine.
- 20 -
In its initial phase beginning in January 2021, the Spravato® Program began as a pilot program meant to provide us with the opportunity to assess the value of making this treatment option more widely available to patients at our Treatment Centers. The factors that were assessed in making this determination included: (i) clinical outcomes, as determined by Greenbrook affiliated clinicians using validated rating scales collected from patients on a weekly basis as part of routine clinical care; (ii) confirmation that the subjective patient experience is compatible with the clinical care model at the Company’s Treatment Centers; (iii) validation of payor reimbursement; and (iv) confirmation that operational requirements for delivery of the therapy can be met with our current infrastructure.
The roll-out of our Spravato® Program at select Treatment Centers continued through Fiscal 2023, building on our long-term business plan of utilizing our Treatment Centers as platforms for the delivery of innovative treatments to patients suffering from MDD and other mental health disorders. As of December 31, 2023, 70 of our Treatment Centers currently offer Spravato® which included our first Spravato® “buy & bill” program. This “buy & bill” program will allow us to further enhance our access to patients in specific markets that require this program offering as compared to our current “administer and observe” programs.
Efficacy and Safety of Spravato®
The efficacy of Spravato® for treatment resistant depression was evaluated in one short-term (four-week) (Am J Psychiatry. 2019 Jun 1;176(6)) and one long-term clinical trial (JAMA Psychiatry. 2019 Sep 1;76(9)). Efficacy for depressive symptoms in patients with major depressive disorder with acute suicidal ideation or behavior was evaluated in two short-term (four-week) trials (J Clin Psychiatry, 2020 May 12; 81(3); Int J Neuropsychopharmacol, 2021 Jan 20; 24(1)). The three short-term studies were randomized trials, were patients received Spravato® or a placebo nasal spray. The primary measure of efficacy in each of the short-term studies was the change from baseline on a scale used to assess the severity of depressive symptoms. Spravato® demonstrated a favorable statistically significant effect compared to placebo on the severity of depression in one of the three short-term studies. The pre-specified statistical tests for demonstrating effectiveness was not met in the two other short-term trials. In the longer-term maintenance-of-effect trial, patients in stable remission or with stable response who continued treatment with Spravato® plus an oral antidepressant experienced a statistically significantly longer time to relapse of depressive symptoms than patients on placebo nasal spray plus an oral antidepressant.
The most common side effects experienced by patients treated with Spravato® in the clinical trials were disassociation, dizziness, nausea, sedation, vertigo, decreased feeling or sensitivity (hypoesthesia), anxiety, lethargy, increased blood pressure, vomiting and intoxication.
The FDA-approved label for Spravato® contains a boxed warning that cautions that patients are at risk for sedation and difficulty with attention, judgment and thinking (dissociation), abuse and misuse, and suicidal thoughts and behaviors after administration of the drug.
Administration of Spravato® Therapy
Due to the risk of serious adverse outcomes resulting from sedation and dissociation caused by Spravato® administration, and the potential for abuse and misuse of the drug, it is only available through a restricted distribution system, under a Risk Evaluation and Mitigation Strategy (“REMS”). The REMS includes several components, including requiring both prescribing clinicians and patients to sign a patient enrollment form that states that the patient understands that they should make arrangements to safely leave the health care setting to return home and that the patient should not drive or use heavy machinery for the remainder of the day on which they received treatment. In addition, Spravato® must be dispensed with a patient medication guide that outlines the drug’s uses and risks. All of the Treatment Centers at which we offer Spravato® are REMS-certified.
Patients for whom Spravato® is medically indicated will self-administer the esketamine nasal spray under the supervision of a Greenbrook affiliated clinician. The clinician will instruct the patient on how to operate the nasal spray device and staff will observe the patient during and after each use of the nasal spray device. In addition, because of the risk of sedation and dissociation, patients must be monitored by a Greenbrook affiliated clinician for at least two hours after receiving their Spravato® dose.
- 21 -
Our Revenue and Profit Model
Average revenue per treatment was $220 in Fiscal 2023 and is dependent on various factors including timing of collections, payor mix, treatment modality mix and ruling reimbursement rates from commercial insurance plans, Medicare or other non-Medicare government-based programs. Depending on a patient’s specific insurance plan, secondary insurance plan (if any) and our enrollment status with the insurance provider, the patient may be responsible for a co-pay, coinsurance or deductible out-of-pocket cost. Approximately 4% of our payments received represent patients’ out-of-pocket cost, with the remainder paid directly to us by the applicable insurance provider.
Direct center and patient care costs, including device costs (as outlined in “—Relationships with our TMS Device Suppliers and Cost Model” above), the cost of clinical and non-clinical staff, the cost of the space lease for the Treatment Center and other day-to-day running costs, represent approximately 55% of gross revenue in an established Treatment Center. We currently target an average margin of 45% after direct center and patient care costs with the current actual margin at 27% as of December 31, 2023. Although the cost reductions associated with the Restructuring Plan have been substantially executed, we expect our new operating structure will continue to allow us to rationalize costs, while further reducing business complexity, streamline our operating model and drive operational efficiencies. As we add Treatment Center density in our respective management regions, the contribution margin after direct center and patient care costs from the various Treatment Centers scale into the single shared regional management infrastructure to ultimately target a regional operating income margin of 25% in a mature region with an actual blended margin currently at -3.7% as of December 31, 2023.
Competition
The market for TMS and esketamine nasal spray services is becoming increasingly competitive. We compete principally on the basis of our reputation and brand, the location of our centers, the quality of our services and the reputation of our affiliated clinicians. In the markets in which we are operating, or anticipate operating in the future, competition predominantly consists of individual psychiatrists that can offer TMS therapy and/or esketamine nasal spray therapy directly to their patients. We also face competition from a limited number of multi-location psychiatric practices or behavioral health groups that offer TMS therapy and/or esketamine nasal spray therapy as part of their overall practice, as well as a few other specialist TMS providers and esketamine nasal spray therapy or intravenous ketamine providers.
We also face indirect competition from pharmaceutical and other companies that develop competitive products, such as anti-depressant medications, with certain competitive advantages such as widespread market acceptance, ease of patient use and well-established reimbursement. Our commercial opportunity could be reduced or eliminated if these competitors develop and commercialize anti-depressant medications or other treatments that are safer or more effective than TMS or esketamine nasal spray therapy. At any time, these and other potential market entrants may develop treatment alternatives that may render our products uncompetitive or less competitive. We are also subject to competition from providers of invasive neuromodulation therapies such as ECT and VNS.
Regulation
Overview
The healthcare industry is subject to numerous laws, regulations and rules including, among others, those related to government healthcare program participation requirements, various licensure and accreditation standards, reimbursement for patient services, health information privacy and security rules, and government healthcare program fraud and abuse provisions. Providers that are found to have violated any of these laws and/or regulations may be excluded from participating in government healthcare programs, subjected to loss or limitation of licenses to operate, subjected to significant fines or penalties and/or required to repay amounts received from the government for previously billed patient services.
The Federal Anti-Kickback Statute and Stark Law
The federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)) (the “Anti-Kickback Statute”) is a criminal statute that prohibits healthcare providers and others from directly or indirectly soliciting, receiving, offering or paying any remuneration, in cash or in kind, as an inducement or reward for using, referring, ordering, recommending or arranging for referrals or orders of services or other items paid in whole or in part by a government healthcare program. The Anti-Kickback Statute may be found to have been violated if at least one purpose of the remuneration is to induce or reward referrals. An individual is not required to have actual knowledge or specific intent to commit a violation of the Anti-Kickback Statute to be found guilty of violating the law.
- 22 -
The Office of Inspector General of the United States Department of Health and Human Services has issued safe harbor regulations that protect certain types of common arrangements from prosecution or sanction under the Anti-Kickback Statute. Other types of arrangements may be protected under statutory exceptions. According to the Office of Inspector General, arrangements that comply with a safe harbor are immune from prosecution under the Anti-Kickback Statute. All the conditions of a safe harbor must be met for it to apply; substantial compliance is not sufficient. The fact that conduct or a business arrangement does not fall within a safe harbor does not automatically render the conduct or business arrangement illegal under the Anti-Kickback Statute. However, conduct and business arrangements falling outside the safe harbors may lead to increased scrutiny by government enforcement authorities.
Where the Anti-Kickback Statute has been violated, the government may proceed criminally or civilly. If the government proceeds criminally, a violation of the Anti-Kickback Statute is a felony that is punishable by up to ten years imprisonment, a fine, and mandatory exclusion from participation in all federal health care programs. If the government proceeds civilly, it may impose civil monetary penalties per violation, among other penalties. In addition, a claim that includes items or services resulting from a violation of the Anti-Kickback Statute constitutes a false claim for purposes of the federal False Claims Act (“FCA”).
Although the Company believes that our financial arrangements with physicians and other referral sources comply with current law and available interpretative guidance, as a practical matter it is not always possible to structure our arrangements so as to fall squarely within an available Anti-Kickback Statute safe harbor. Where that is the case, compliance with the Anti-Kickback Statute is evaluated on a case-by-case basis. We cannot guarantee that applicable regulatory authorities will not assert and/or determine that these financial arrangements violate the Anti-Kickback Statute or other applicable laws, including state anti-kickback laws.
In addition to the Anti-Kickback Statute, the federal Physician Self-Referral Law, also known as the Stark Law, prohibits physicians from referring Medicare and Medicaid patients to healthcare entities with which they or any of their immediate family members have a financial relationship for the furnishing of any “designated health services,” unless certain exceptions apply. The Stark Law is a strict liability statute, meaning that no intent is required to violate the law, and even a technical violation may lead to significant penalties. A violation of the Stark Law, including schemes to circumvent the Stark Law, may result in a denial of Medicare or Medicaid payment, required refunds to the Medicare or Medicaid programs and/or the imposition of civil monetary penalties for each claim knowingly submitted in violation of the Stark Law. A violation of the Stark Law may also result in liability under the FCA. There are ownership and compensation arrangement exceptions for many customary financial arrangements between physicians and entities, including the employment exception, personal service arrangements exception, lease exception and certain recruitment exceptions, among others. The Company believes that the TMS therapy services furnished by the physician practices with which the Company contracts do not implicate the Stark Law because they do not constitute “designated health services.” However, it is possible that the federal government could designate TMS therapy services or additional service lines offered by the Company as “designated health services” in the future, which might require the Company to restructure its arrangements with physicians.
These laws and regulations are complex, and, in many cases, we do not have the benefit of regulatory or judicial interpretation. It is possible that different interpretations or enforcement of these laws and regulations could subject our current or past practices to allegations of impropriety or illegality or could require us to make changes in our arrangements relating to facilities, equipment, personnel, services, capital expenditure programs and operating expenses. It is also possible that these laws and regulations are revised in such a way as to require the Company to change its business practices, which could have a material adverse effect on our business, operations and prospects. A determination that we have violated one or more of these laws, or a public announcement that we are being investigated for possible violations of one or more of these laws, could have a material adverse effect on our business, financial condition or results of operations. In addition, we cannot predict whether other federal or state legislation or regulations will be adopted, what form such legislation or regulations may take or what their impact on us may be.
If we or our contracted physician practices are deemed to have failed to comply with the Anti-Kickback Statute, the Stark Law or other applicable laws and regulations, we could be subjected to liabilities, including criminal penalties, civil penalties, exclusion of us and/or one or more of our contracted physician practices from participation in the government healthcare programs, or substantial overpayment refunds. The imposition of such penalties could have a material adverse effect on our business, financial condition or results of operations.
- 23 -
Federal False Claims Act and Other Fraud and Abuse Provisions
The FCA provides the government a tool to pursue healthcare providers for submitting false claims or requests for payment for healthcare items or services. Under the FCA, the government may fine any person or entity that, among other things, knowingly submits, or causes the submission of, false or fraudulent claims for payment to the federal government or knowingly and improperly avoids or decreases an obligation to pay money to the federal government. The federal government has widely used the FCA to prosecute Medicare and other federal health care program fraud, such as billing for services not provided or not supported by appropriate documentation, submitting false cost reports, and providing care that is not medically necessary or that is substandard in quality. Claims for services or items rendered in violation of the Anti-Kickback Statute or the Stark Law are also a basis for liability under the FCA. The FCA is also implicated by the knowing failure to report and return an identified overpayment to the Medicare or Medicaid programs within 60 days of identifying the overpayment or by the date a corresponding cost report is due, whichever is later.
Violations of the FCA are punishable by significant monetary penalties for each fraudulent claim plus three times the amount of damages sustained by the government. In addition, under the qui tam, or whistleblower, provisions of the FCA, private parties may bring actions under the FCA on behalf of the federal government. These private parties, known as relators, are entitled to share in any amounts recovered by the government, and, as a result, whistleblower lawsuits have increased significantly in recent years. Even if federal enforcement authorities decide not to pursue a case brought by a relator, the relator may in certain circumstances continue to pursue the case on its own. Many states have similar false claims statutes that impose liability for the types of acts prohibited by the FCA or that otherwise prohibit the submission of false or fraudulent claims to the state government or Medicaid program. Any FCA action brought against us, even if we successfully defend against it, could result in significant legal expenses and divert our management’s attention from the operation of our business, which could have a material adverse effect on our business, operations and prospects.
In addition to the FCA, the federal government may use several criminal laws, such as the federal mail fraud, wire fraud or healthcare fraud statutes, to prosecute the submission of false or fraudulent claims for payment to the federal government.
Most states have also adopted generally applicable insurance fraud statutes and regulations that prohibit healthcare providers from submitting inaccurate, incorrect or misleading claims to private insurance companies. Management believes that, working with our contracted physician practices, we have implemented safeguards and procedures to complete claim forms and requests for payment in an accurate manner and to operate in compliance with applicable laws. However, the possibility of billing or other errors can never be completely eliminated, and we cannot guarantee that the federal government, a state government, or a qui tam relator, upon audit or review, would not take the position that billing or other errors, should they occur, are violations of the FCA.
HIPAA Administrative Simplification and Privacy and Security Requirements
The administrative simplification provisions of the Health Insurance Portability and Accountability Act (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), require the use of uniform electronic data transmission standards for healthcare claims and payment transactions submitted or received electronically. These provisions are intended to encourage electronic commerce in the healthcare industry. HIPAA, HITECH, and their respective implementing regulations also established federal rules relating to the privacy and security of individually identifiable protected health information (“PHI”). The HIPAA privacy regulations govern the use and disclosure of PHI and the rights of patients to be informed about and control how such PHI is used and disclosed. The HIPAA security regulations require healthcare providers to implement administrative, physical and technical safeguards to protect the confidentiality, integrity and availability of electronic PHI. Concerns regarding compliance with the HIPAA privacy and security regulations have been an area of increased focus and enforcement by regulators in the Department of Health and Human Services Office for Civil Rights. Violations of HIPAA can result in both criminal and civil fines and penalties.
Among other things, HITECH strengthened certain HIPAA rules regarding the use and disclosure of PHI, extended certain HIPAA provisions to business associates and created security breach notification requirements, including notifications to the individuals affected by the breach, the Department of Health and Human Services, and in certain cases, the media. HITECH has also increased maximum civil and criminal penalties for violations of HIPAA. Management believes that we have been in material compliance with the HIPAA regulations and have developed our policies and procedures to ensure ongoing compliance, although we cannot guarantee that our contracted physician practices will not be subject to fines or penalties as a result of erroneous disclosures, security incidents or breaches, each of which could have a material adverse effect on our business, financial condition or results of operations.
- 24 -
Corporate Practice of Medicine and Fee-Splitting
There are states in which we operate that have laws that prohibit business entities, such as our Company, from directly practicing medicine, employing physicians to practice medicine and/or exercising control over medical decisions by physicians (known generally as the prohibition on corporate practice of medicine). In addition, various state laws also prohibit entities from engaging in certain financial arrangements, such as splitting or sharing a physician’s professional fees. These laws are intended to avoid interference with or undue influence of a physician’s professional judgment. The laws of some other states do not prohibit non-physician entities from employing physicians to practice medicine but may retain a ban on some types of fee-splitting arrangements.
Corporate practice of medicine and fee splitting laws vary from state to state and are not always consistent among states. In some states these prohibitions are set forth in a statute or regulation, while in other states the prohibition is a matter of judicial or regulatory interpretation. Decisions and activities beyond those directly related to the delivery of healthcare, such as scheduling, contracting, setting rates and the hiring and management of non-clinical personnel, may also implicate the restrictions on the corporate practice of medicine in many states.
The consequences of violating the corporate practice of medicine laws vary by state and may result in physicians being subject to disciplinary action, as well as the forfeiture of revenues from payors for services rendered. For lay entities, violations may also bring both civil and, in more extreme cases, criminal liability for engaging in medical practice without a license. Some of the relevant laws, regulations and agency interpretations in states with corporate practice of medicine restrictions have been subject to limited judicial and regulatory interpretation. In limited cases, courts have required management services companies to divest or reorganize structures deemed to violate corporate practice restrictions. Moreover, these state laws are subject to change.
While we believe that we are in substantial compliance with state laws prohibiting the corporate practice of medicine and fee-splitting, other parties may assert that, despite the way we are structured, we could be engaged in the corporate practice of medicine and/or unlawful fee-splitting. In this event, failure to comply could lead to adverse judicial or administrative action against us and/or our contracted physician practices providers, overpayment demands, civil or criminal penalties, receipt of cease and desist orders from state regulators, loss of provider licenses, and/or the need to make changes to the terms of engagement of our contracted physician practices that interfere with our business, each of which could have a material adverse impact on our business, results of operations and financial condition.
Employees
As of December 31, 2023, we had 489 employees, of which approximately 391 were employed as regional personnel at our Treatment Centers or as part of the regional management infrastructure, and 98 were employed or contracted as corporate personnel to support our centralized business infrastructure. Of our 489 employees and contractors, 42 are located in Canada, and 447 are located in the United States.
Information Systems
We focus on creating optimal workflows and processes by investing and continuously improving our technology-enabled centralized business infrastructure. Our custom integration of various best-in-class software applications includes call center, customer relationship and lead management, medical billing, electronic health records, financial reporting and analysis and human resources systems. This creates an end-to-end integrated platform, which enables a seamless process from the first patient interaction, through the patient treatment process until payment is received. We will continue to optimize our information systems to create standardized policies, procedures and cost efficiencies.
Intellectual Property
As of the date of this Annual Report, we own the Greenbrook service mark for psychiatric and neurological consultation and treatment services in the United States. We do not own the intellectual property associated with any TMS Device.
- 25 -
Internet Availability of Company Information
The SEC maintains an Internet site (http://www.sec.gov) that makes available reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The Company’s website can be found at www.greenbrooktms.com. The information on our website is not incorporated by reference into this Annual Report and should not be considered a part of this Annual Report, and the reference to our website in this Annual Report is an inactive textual reference only.
ITEM 1A.RISK FACTORS
The following factors could materially adversely affect us and should be considered when deciding whether to make an investment in us and our securities, including the Common Shares. Other risks and uncertainties that we do not presently consider to be material, or of which we are not presently aware, may become important factors that affect our future financial condition or results of operations. The occurrence of any of the risks discussed below could materially adversely affect our business, prospects, financial condition, results of operations or cash flows, and consequently, the trading price of our securities, could be materially and adversely affected. In all these cases, the trading price of the Common Shares and the market value of our other securities, as applicable, could decline, and prospective investors could lose all or part of their investment.
Summary of Risk Factors
We are providing the following summary of risk factors contained in the Annual Report to enhance the readability and accessibility of our risk factor disclosures. We encourage you to review the full risk factors in their entirety for additional information regarding the material risks that could adversely affect our business, prospects, financial condition, operating results, or credit ratings, which could cause the trading price of our Common Shares to decline. These risks and uncertainties include, but are not limited to, the following:
Business and Operational Risk Factors
| ● | There is substantial doubt about our ability to continue as a going concern due to recurring losses from operations, accumulated deficit and insufficient cash resources to meet our business objectives. |
| ● | Inability to satisfy our obligations under the Credit Agreement, the Neuronetics Agreement, the Klein Settlement Agreement, Note Purchase Agreement, and the Neuronetics Note, and the potential acceleration of indebtedness, which would force us into bankruptcy. |
| ● | We rely exclusively on Neuronetics as the supplier of the TMS Devices that we utilize under the Neuronetics Agreement. The loss of this relationship, or any negative impacts on Neuronetics’ ability to manufacture such machines, would severely harm our business. |
| ● | We identified a material weakness in our internal controls which led to errors in our financial statements and as such, the Company has concluded that such previously issued financial statements can no longer be relied upon. There can be no assurance that these weaknesses will be fully remediated or that they will not continue to impact our financial disclosure. |
| ● | Our level of indebtedness may increase and reduce our financial flexibility. |
| ● | Our ability to manage our operations at our current size and successfully execute on our business strategies is subject to significant risks and uncertainties. |
| ● | Our future expansion into new geographic regions may present increased risks due to lower awareness of our brand, or TMS therapy and Spravato®, our unfamiliarity with those regions and other factors. |
| ● | Our strategy to grow our business through the expansion of the Spravato® Program is subject to significant risks. |
| ● | Failure to timely or accurately bill for services could have a negative impact on our revenue, bad debt expense and cash flow. |
| ● | Our ability to generate revenue depends in large part on our ability to attract new patients and if we fail to attract new patients, we may not be able to increase revenues. |
| ● | We may be unable to execute successfully on our acquisitions and other business initiatives or do so within the intended timeframes, which could have an adverse effect on our financial condition, results of operations and business prospects. |
| ● | Our ability to manage our operations at our current size and successfully execute on our business strategies is subject to numerous risks and uncertainties. |
- 26 -
Legal and Regulatory Risk Factors
| ● | An unfavorable outcome of the Purchase Agreement Claims could result in a material adverse effect to the Company’s financial position. |
| ● | Regulatory and compliance requirements associated with our billing and collections system could have a material adverse effect on our revenues, cash flows and operating results. |
| ● | We may engage in litigation with our clinical partners and contractors and there are claims made against us from time to time that can result in litigation that could distract management from our business activities and result in significant liability or damage to us. |
| ● | We may become subject to professional malpractice liability, which could be costly and negatively impact our business. |
| ● | Our management services arrangements with physician practices and those practices’ professional services agreements with contracted or employed psychiatrists must be structured in compliance with state laws relating to the practice of medicine, including, without limitation, fee-splitting prohibitions. |
| ● | An inability to maintain effective internal controls over financial reporting could increase the risk of an error in our financial statements and/or call into question the reliability of our financial statements. |
Industry Risk Factors
| ● | Our ability to obtain TMS Devices and Spravato® from our suppliers on a timely basis at competitive costs could suffer as a result of events that adversely affect our suppliers or cause disruptions in their businesses. |
| ● | If commercial payor plans are subject to restriction in plan designs or the average rates that commercial payors pay us decline significantly, or if there are changes in Medicare or other non-Medicare government-based programs or payment rates, such occurrences would have a material adverse effect on our revenues, earnings and cash flows. |
| ● | We may incur increased costs if third-party payors impose additional requirements related to the provision of services at our Treatment Centers. |
| ● | If there is a reduction in reimbursement rates by higher-paying commercial insurance providers, our revenues, earnings and cash flows would be substantially reduced. |
| ● | We experience competition from other Treatment providers, providers of esketamine nasal spray therapy, hospitals and pharmaceutical and other companies, and this competition could adversely affect our business and revenue. |
Risks Related to Our Common Shares
| ● | Our Common Shares were delisted from Nasdaq and are currently traded on the OTCQB Market, which involves additional risks compared to being listed on a national securities exchange. Our ability to sell equity securities and the liquidity of the Common Shares publicly or privately will be adversely affected if we are unable to transfer the Company’s listing to another stock exchange. |
| ● | Trading in our securities is highly speculative, and we may be required to file for bankruptcy protection in the future. |
| ● | There are unexercised options, warrants, convertible notes and conversion instruments outstanding, and which may be issued from time to time. If these are exercised or converted, an investor’s interest in Common Shares will be substantially diluted. |
| ● | A decline in the price of the Common Shares could affect our ability to raise further working capital and adversely impact our ability to continue operations. |
| ● | Benjamin Klein, Madryn, and Greybrook Health continue to have significant influence over us, including control over decisions that require the approval of shareholders, which could limit your ability to influence the outcome of matters submitted to shareholders for a vote. |
General Risk Factors
| ● | Our business is labor intensive and could be adversely affected if we are unable to maintain satisfactory relations with our employees or the occurrence of union attempts to organize our employees. |
| ● | A material disruption in or security breach affecting our information technology systems could significantly affect our business and lead to reduced sales, growth prospects and reputational damage. |
| ● | Many of our business functions are centralized at our head office locations. Disruptions to the operations at these locations could have an adverse effect on our business. |
| ● | We are a Canadian company and shareholder protections differ from shareholder protections in the United States and elsewhere. |
| ● | Certain adverse tax consequences may result from the treatment of the Company as a U.S. domestic corporation for U.S. federal income tax purposes. |
| ● | Non-U.S. shareholders could be subject to materially adverse U.S. federal income tax consequences if the Company is classified as a United States real property holding corporation. |
| ● | We are subject to insurance-related risks. |
- 27 -
Business and Operational Risk Factors
There is substantial doubt about our ability to continue as a going concern due to recurring losses from operations, accumulated deficit and insufficient cash resources to meet our business objectives.
Based on recurring losses from operations and negative cash flows from operations for the financial year ended December 31, 2023, as well as current cash and liquidity projections, we have concluded that there is substantial doubt about our ability to continue as a going concern. Furthermore, as discussed in Note 2(a) within the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023, included in this Annual Report, we have generated operating losses since inception, and we had negative cash flow from operating activities for the year ended December 31, 2023, which together raises substantial doubt about our ability to continue as a going concern.
The Company has experienced significant cash constraints and ended Fiscal 2023 with a cash balance of $3.3 million. As of April 19, 2024, we had cash on hand of approximately $0.3 million. We believe that we have sufficient capital to meet our future operating expenses, capital expenditures and debt service requirements for at least the next one month. Our near-term cash requirements include repayment of the Klein Note (as defined herein) on May 1, 2024 in accordance with the terms of our settlement of the Klein Note Action (see Item 3 “Legal Proceedings”) as well as the remaining amount payable of $1,200,000 (as of the date of this Annual Report) in respect of the TMS Device Settlement (as defined in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness and Capital Raising—Other Indebtedness”). Accordingly, we will be required to obtain additional financing in the near to medium term in order to satisfy our cash requirements, which may include additional financing under our Credit Agreement, securities offerings or other financing sources that may become available to us. If any such additional financing is not available to us, or is not available on satisfactory terms, our ability to operate our business or respond to competitive pressures would be curtailed and we may need to delay, limit or eliminate growth plans or operations or other elements of our growth strategies. There can be no assurance that these sources of financing will be available when needed or that these financings will be on terms at least as favorable to the Company as they have been in the past, or available to the Company at all.
To the extent that we continue to have negative cash flow in future periods, we will need to allocate a portion of the net proceeds received from additional financings to fund such negative cash flow and to ensure that we remain in compliance with the Minimum Liquidity Covenant under our Credit Agreement (see Item 1 “Business—Recent Developments in Our Business—Debt Financings”). In addition, we can provide no assurances that we will be able to satisfy payment obligations on the Company’s outstanding indebtedness (including, without limitation, the Credit Agreement, the Neuronetics Note, the Subordinated Convertible Notes, the TMS Device Settlement, the Klein Note and the Klein Settlement Agreement).
Even if we are able to obtain additional financing, there can be no assurance that we will achieve profitability or sustain profitability. Our failure to achieve or maintain profitability could negatively impact the value of our securities, including the Common Shares, and there can be no assurance that we will be able to raise the additional funding that we may require in order to carry out our business objectives and growth strategies.
The issuance of additional Common Shares under any equity financing may have a dilutive effect on the interests of shareholders. The number of Common Shares that we are authorized to issue is unlimited. We may, in our sole discretion, subject to applicable law and the rules of applicable stock exchanges, issue additional Common Shares from time to time (including pursuant to any equity-based compensation plans or upon exercise of outstanding warrants or convertible instruments), and the interests of our shareholders may be diluted as a result.
Inability to satisfy our obligations under the Credit Agreement, the Neuronetics Agreement, the Klein Note, the Klein Settlement Agreement, the TMS Device Settlement, Note Purchase Agreement, and the Neuronetics Note, and the potential acceleration of indebtedness, which would force us into bankruptcy.
During Fiscal 2023 and up until the date of the filing of this Annual Report, the Company received multiple waivers from Madryn with respect to the Company’s non-compliance with the Minimum Liquidity Covenant, including most recently on March 28, 2024, which extended our ability to rely on a reduced Minimum Liquidity Covenant of $300,000 (compared to $3,000,000 in the Credit Agreement) until April 30, 2024. We can provide no assurance that we will remain in compliance in with the Minimum Liquidity Covenant in the future, or that Madryn will continue to extend our ability to rely on a reduced covenant amount.
- 28 -
The Company may not have the financial resources to satisfy the Minimum Liquidity Covenant under the Credit Agreement in the future or satisfy our payment obligations under the Credit Agreement, the Neuronetics Agreement, the Klein Note, the Klein Settlement Agreement, the TMS Device Settlement, the Note Purchase Agreement, or the Neuronetics Note. Our obligations under the Credit Agreement and the Neuronetics Note are secured by first priority liens on substantially all assets of the Company and certain of its subsidiaries, subject to customary exceptions. In the event either Madryn or Neuronetics elects to accelerate the indebtedness upon the occurrence of a default and foreclose on the collateral, the Company will likely be required to file for bankruptcy protection and our assets will likely be liquidated. Our equity holders would likely not receive any recovery in a bankruptcy scenario, in which case you will likely receive no recovery for your Common Shares.
Pursuant to the Neuronetics Agreement, the Company must also obtain consent from Neuronetics in connection with any debt financing that causes indebtedness outstanding under the Credit Agreement to exceed $115 million (the “Debt Cap”). As of April 15, 2024, the amount of indebtedness outstanding under the Credit Agreement is $96 million. In order to continue to satisfy its working capital needs in the future, the Company expects to need to incur additional indebtedness under the Credit Agreement that would exceed this figure. There can be no assurance that Neuronetics will continue to provide consent to raise or waive the Debt Cap. If the Debt Cap cannot be raised on terms favorable to the Company or at all, then the Company may have insufficient cash to continue operations.
We rely exclusively on Neuronetics as the supplier of the TMS Devices that we utilize under the Neuronetics Agreement. The loss of this relationship, or any negative impacts on Neuronetics’ ability to manufacture such machines, would severely harm our business.
Pursuant to the Neuronetics Agreement, Neuronetics is now the exclusive supplier of TMS Devices to the Company. While we currently still use TMS Devices from other suppliers, over time, we expect that in the near future Neuronetics’ NeuroStar TMS Devices will replace competitive TMS Devices at the Company’s Treatment Centers. Any failure by Neuronetics to fulfill its obligations under the Neuronetics Agreement (or any such failure by the Company that would result in termination of the Neuronetics Agreement), or any adverse change in the financial health of Neuronetics that would impact their ability to perform their obligations under the Neuronetics Agreement, could have a material adverse effect on our revenue and operating results and operating cash flows.
Further, in the event of supply shortages and interruptions, long lead times, and act-of-God events such as global pandemics, weather related catastrophes, or conflict, any of which could disrupt the operations of Neuronetics, we may be unable to identify or contract with new suppliers or producers in the event of a disruption to our supply and we could experience a material adverse effect on our revenue, operating results, and ability to operate our business.
The Neuronetics Agreement contains minimum purchase commitments, and we could be subject to shortfall payments if we do not purchase enough treatments during each year.
Under the Neuronetics Agreement, we must purchase a minimum number of treatment sessions equal to the total number of TMS treatments we perform each year (see Item 1 “Business—TMS Device Supply Arrangement with Neuronetics”). If we do not purchase a sufficient number of treatments in 2024, we may be required to pay Neuronetics for the new and existing shortfalls in our outstanding balance. These amounts, if they become payable and are not credited or further restructured, may be material and could have an adverse impact on our liquidity and financial condition. The failure to timely repay the remainder of the outstanding balance will constitute an event of default under the Neuronetics Agreement and the Neuronetics Note, which would require us to issue the Neuronetics Warrants (as described above in Item 1 “Business—TMS Device Supply Arrangement with Neuronetics”), which would have the potential to result in substantial dilution and depending on the trading price of our Common Shares as of the date of such default, could result in a change of control of the Company. In addition, default under the Neuronetics Note could trigger a default under the Credit Agreement and, unless such default was waived by Madryn, would likely lead us to file for bankruptcy protection.
- 29 -
We identified a material weakness in our control over effective preparation, review, and approval of our impairment analysis and adjustment to variable consideration in Fiscal 2022 and Fiscal 2023 respectively. This material weakness led to errors in our financial statements and as such, the Company has concluded that such previously issued financial statements can no longer be relied upon. There can be no assurance that these weaknesses will be fully remediated or that they will not continue to impact our financial disclosure.
We are responsible for establishing and maintaining adequate internal controls over financial reporting, which is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”). Because of our inherent limitations and the fact that we are a relatively new public company and are implementing new financial control and management systems, internal controls over financial reporting may not prevent or detect all misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. A failure to prevent or detect errors or misstatements may result in a decline in the market price of our securities, including the Common Shares, and harm our ability to raise capital in the future..
In connection with the audit of our consolidated financial statements from Fiscal 2023 that were prepared in accordance with U.S. GAAP and audited in accordance with the standards of the Public Company Accounting Oversight Board (United States), our management identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses in respect of Fiscal 2023 related to approval of our impairment analysis and adjustment to variable considerations in Fiscal 2022 and Fiscal 2023, respectively.
On April 16, 2024, the Audit Committee, in consultation management and the Company’s independent auditors, determined that the material weakness regarding variable consideration led to certain errors in our audited annual financial statements for Fiscal 2022 and quarterly condensed interim consolidated financial statements for Fiscal Q3 2023, each of which required restatement. Given these restatements, the Audit Committee also determined that these financial statements could no longer be relied upon. For more information, see the Explanatory Note following the cover page of this Annual Report. As a result of these restatements, we have incurred unanticipated costs for accounting and legal fees in connection with these restatements, and we have also become subject to a number of additional risks and uncertainties, including potential litigation and/or regulatory proceedings as well as any adverse effect on investor confidence and our reputation.
We intend to implement a remediation plan to address these material weaknesses see Item 9A “Material Changes in Internal Controls Over Financing Reporting in Fiscal 2022” and “Material Changes in Internal Controls Over Financing Reporting in Fiscal 2023” below. We intend to take all measures necessary to address and cure the underlying causes of the material weaknesses. However, once implemented, our remediation plan may not prove to be successful in remediating the material weaknesses and we do not guarantee that we will not suffer additional material weaknesses and/or significant deficiencies in the future. If additional material weaknesses in our internal controls are identified, we could be subject to regulatory scrutiny and a loss of public confidence, which could harm our business and cause a decline in the price of our securities, including the Common Shares. In addition, if we do not maintain adequate financial and management personnel, processes and controls, we may not be able to accurately report our financial performance on a timely basis, which could cause a decline in the market price of our securities, including the Common Shares, and harm our ability to raise capital.
We do not expect that our disclosure controls and procedures and internal controls over financial reporting will prevent all error or fraud. A control system, no matter how well-designed and implemented, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Due to the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues within an organization are detected. The inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of simple errors or mistakes. Controls can also be circumvented by individual acts of certain persons, by collusion of two or more people or by management override of the controls. Due to the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected in a timely manner or at all. If we cannot provide reliable financial reports or prevent fraud, our reputation and operating results could be materially adversely affected, which could also cause investors to lose confidence in our reported financial information, which in turn could result in a reduction in the trading price of our securities, including the Common Shares.
- 30 -
We may face litigation and other risks as a result of the restatement and material weakness in our internal control over financial reporting.
We identified a material weakness in our internal control over financial reporting that led to a restatement of our audited annual financial statements for Fiscal 2022 and Fiscal Q3 2023. As a result of the restatement and other matters raised or that may in the future be raised by the SEC, we face potential for litigation or other disputes which may include, among others, claims invoking the federal and state securities laws, contractual claims or other claims arising from the restatement and the material weakness in our internal control over financial reporting and the preparation of our financial statements. As of the date of this Annual Report, we have no knowledge of any such litigation or dispute. However, we can provide no assurance that such litigation or dispute will not arise in the future. Any such litigation or dispute, whether successful or not, could adversely affect our business, financial condition, and results of operations.
We are an “emerging growth company” and a “smaller reporting company” and as such, our independent registered accounting firm will not be required to attest to the effectiveness of internal controls over financial reporting. Furthermore, given the nature of our business, the testing of our internal controls over financial reporting is only expected to become more time consuming and expensive.
We are subject to the requirements of the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley”). Section 404 of Sarbanes-Oxley (“Section 404”) requires companies subject to the reporting requirements of the U.S. securities laws to complete a comprehensive evaluation of our internal controls over financial reporting. To comply with this statute, we will be required to document and test our internal control procedures and our management will be required to assess and issue a report concerning our internal controls over financial reporting. Pursuant to the Jumpstart Our Business Startups Act (“JOBS Act”), we are classified as an “emerging growth company”. Under the JOBS Act, emerging growth companies are exempt from certain reporting requirements, including the independent auditor attestation requirements of Section 404(b) of Sarbanes-Oxley. Under this exemption, our independent auditor will not be required to attest to and report on management’s assessment of our internal control over financial reporting during a transition period of up to five years from our initial registration with the SEC. We will need to prepare for compliance with Section 404(b) by strengthening, assessing and testing our system of internal controls to provide the basis for our report. However, the continuous process of strengthening our internal controls and complying with Section 404(b) is complicated and time-consuming. Furthermore, we believe that our business will grow in the United States, in which case our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of our testing, our management may identify additional material weaknesses or significant deficiencies, which may not be remedied in a timely manner to meet the deadline imposed by Sarbanes-Oxley. If our management cannot favorably assess the effectiveness of our internal controls over financial reporting, or our independent registered public accounting firm identifies additional material weaknesses in our internal controls, investor confidence in our financial results may weaken, and the market price of our securities may suffer.
Our level of indebtedness may increase and reduce our financial flexibility.
We are currently primarily indebted under the Credit Agreement, the Neuronetics Note and the Note Purchase Agreement, and we may incur additional indebtedness in the future. In addition, we have substantial liabilities under our rental leases and device leases, as well as other payables including the Klein Note, the Klein Settlement Agreement and the TMS Device Settlement. As of December 31, 2023, we had $90.6 million of long-term debt and $8.7 million of short-term debt. See Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” below. We are exposed to changes in interest rates on our cash, bank and shareholder indebtedness and long-term debt. Debt issued at variable rates exposes us to cash flow interest rate risk. Debt issued at fixed rates exposes us to fair value interest rate risk. Our borrowings, current and future, will require interest payments and need to be repaid or refinanced, could require us to divert funds identified for other purposes to debt service and could create additional cash demands or impair our liquidity position and add financial risk for us.
The terms of the Credit Agreement require the Company to satisfy various affirmative and negative covenants and to meet certain financial tests, including but not limited to, financial covenants that require the Company to (i) generate consolidated revenues for any 4 consecutive quarter period, measured quarterly for the 4 consecutive quarter period prior to the end of each quarter, in amounts that represent a specified percentage of the Company’s projected consolidated revenues for such measurement periods, assuming modest growth over time; and (ii) maintain minimum liquidity of $300,000 until April 30, 2024 and $3.0 million thereafter, tested on a daily basis. In addition, the Credit Agreement contains affirmative and negative covenants that limit, among other things, the Company’s ability to incur additional indebtedness, incur certain liens, declare certain dividends and engage in certain types of transactions. The Credit Agreement also contains affirmative covenants that require the Company to deliver, within 90 days of each fiscal year end, audited financial statements for such fiscal year, accompanied by a report and opinion of an independent certified public accountant which is not subject to any “going concern” or like qualification or exception or any qualification or exception as to the scope of such audit. See “Risks Relating to our Business – Inability to satisfy debt covenants under the Credit Agreement and the potential acceleration of indebtedness, which would force us into bankruptcy” for further information.
- 31 -
As of the date of this Annual Report, we are in compliance with the Minimum Liquidity Covenant; however, on April 15, 2024, we obtained a waiver to the requirement under the Credit Agreement for the financial reporting covenants under the Credit Agreement. See Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness and Capital Raising—Madryn Credit Agreement” below. We can provide no assurance that we will remain in compliance with these covenants in the future or that we can receive a waiver for any future non-compliance. A failure by us to comply with the covenants specified in the Credit Agreement could result in an event of default under the agreement and trigger events of default under the Neuronetics Note and Note Purchase Agreement. An event of default under the Credit Agreement would give Madryn the right to terminate their commitments to provide additional loans under the Credit Agreement. The lenders under each of the Credit Agreement, the Neuronetics Note and the Note Purchase Agreement would have the ability to declare all amounts outstanding, together with accrued and unpaid interest and fees, to be immediately due and payable. If such debt were to be accelerated, we may not have sufficient cash or be able to borrow sufficient funds to refinance the debt or sell sufficient assets to repay the debt, which could materially and adversely affect our business, financial condition and results of operations.
Diverting funds identified for other purposes for debt service may adversely affect our business and growth prospects. If we cannot generate sufficient cash flow from operations to service our debt, we may need to refinance our debt, dispose of assets, reduce or delay expenditures or issue equity to obtain necessary funds. We do not know whether we would be able to take any of these actions on a timely basis, on terms satisfactory to us, or at all.
Our level of indebtedness could affect our operations in several ways, including the following:
| ● | a significant portion of our cash flows could be used to service our indebtedness; |
| ● | the covenants contained in the agreements governing our outstanding indebtedness may limit our ability to borrow additional funds, dispose of assets, pay dividends and make certain investments; |
| ● | our debt covenants may also affect our flexibility in planning for, and reacting to, changes in the economy and in our industry; |
| ● | a high level of debt would increase our vulnerability to general adverse economic and industry conditions; |
| ● | a high level of debt may place us at a competitive disadvantage compared to our competitors that are less leveraged and therefore may be able to take advantage of opportunities that our indebtedness would prevent us from pursuing; and |
| ● | a high level of debt may impair our ability to obtain additional financing in the future for working capital, capital expenditures, debt service requirements, acquisitions or other purposes. |
In addition to our debt service obligations, our operations require material expenditures on a continuing basis. For the year ended December 31, 2023, our net cash used in operating activities was $24.8 million. See Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Analysis of Cash Flows” below. Our ability to make scheduled debt payments, to refinance our obligations with respect to our indebtedness and to fund capital and non-capital expenditures necessary to maintain the condition of our operating assets and properties, as well as to provide capacity for the growth of our business, depends on our financial and operating performance. General economic conditions and financial, business and other factors affect our operations and our future performance. Many of these factors are beyond our control. We may not be able to generate sufficient cash flows to pay the interest on our debt, and future working capital, borrowings or equity financing may not be available to pay or refinance such debt.
Our ability to manage our operations at our current size and successfully execute on our business strategies is subject to significant risks and uncertainties.
The continued success of our growth initiatives depends on, among other things, our ability to scale our network of Treatment Centers and management regions within the United States, our ability to expand our Spravato® Program and our Medication Management Program, as well as factors which are beyond our control, including general economic conditions and consumer perceptions of TMS treatment and the other treatments we offer. A key component of our business strategy is the development and scaling of Treatment Centers in order to increase our revenues. Risks associated with developing and scaling Treatment Centers include:
| ● | finding appropriate clinical partners and clinicians with whom to partner in new management regions; |
- 32 -
| ● | finding, hiring, training and retaining high quality regional management teams; |
| ● | negotiating and establishing relationships with local commercial insurance carriers and/or obtaining state and federal certification for participation in the Medicare and other non-Medicare government-based programs; |
| ● | increasing awareness of TMS as a treatment option for MDD, OCD, smoking cessation, and other potential future indications; |
| ● | expanding our Spravato and Medication Management programs; |
| ● | identifying desirable locations and markets to open new Treatment Centers, which may be difficult and costly; |
| ● | negotiating acceptable lease terms, including favorable levels of tenant improvement allowances; |
| ● | successfully integrating new Treatment Centers into our existing control structure and operations, including our information technology systems; and |
| ● | addressing competitive, marketing and other challenges encountered in connection with expansion into new geographic areas and markets. |
To the extent that we open new Treatment Centers in regions where we already have existing Treatment Centers, we may experience reduced revenues at those existing locations.
There is no guarantee that newly opened Treatment Centers will be received as well as, or achieve profitability levels comparable to, our existing locations within our estimated time periods, or at all. In addition, our current expansion plans are only estimates, and the actual number of Treatment Centers that we open, the timeline on which we do so and the actual number of suitable locations for our new Treatment Centers could differ significantly from these estimates. If we fail to execute on our growth initiatives, face delays in executing our growth initiatives or fail to fully realize the benefits expected to result from these initiatives, our results of operations and our ability to remain competitive may be materially adversely impacted, and the price of our Common Shares could decline. Our results to date are not an indication of future results, and there can be no assurance that our business strategies will generate increased revenues or improve operating margins even if we are able to successfully implement our growth strategies.
As we move forward, we expect our growth to bring new challenges and complexities that we have not faced before. Among other difficulties that we may encounter, this growth could place a strain on our existing infrastructure, information technology systems, real estate requirements and employee base and may make it more difficult for us to adequately forecast expenditures. Our budgeting will become more complex, and we may also place increased burdens on our suppliers. The increased demands that our growth plans will place on our infrastructure and our management team may cause us to operate our business less efficiently, which could cause deterioration in our performance. Our growth may make it otherwise difficult for us to respond quickly to changing trends, preferences and other factors. This could result in deterioration of key affiliated clinician relationships, excess or deficient equipment, loss of market share and decreased revenues. We cannot anticipate all of the demands that our expanding operations will impose on our business, and our failure to appropriately address these demands could have an adverse effect on us.
In addition, we believe that an important contributor to our success has been our corporate culture, which we believe fosters innovation, teamwork and personalized customer service. As we continue to grow, we must effectively integrate, develop and motivate a growing number of new employees. As a result, we may find it difficult to maintain our corporate culture, which could limit our ability to innovate and operate effectively. Any failure to preserve our culture could also negatively affect our ability to retain and recruit personnel, continue to perform at current levels or execute on our growth strategies.
- 33 -
Our future expansion into new geographic regions may present increased risks due to lower awareness of our brand, or TMS therapy and Spravato®, our unfamiliarity with those regions and other factors.
Our long-term future growth depends, in part, on our expansion efforts into new geographic regions in the United States. As a primary component of our growth strategy, we intend to undertake a targeted expansion into new regions of the United States where we have little or no operating experience or brand awareness. While we have significant experience and awareness across many regions in the United States, we have significantly lower patient awareness outside of these regions and our operating experience with respect to our existing management regions may not be relevant or necessarily translate into similar results broadly in our target markets in the United States. In addition, any new markets that we enter in the future may have different competitive conditions and/or less familiarity with our brand or TMS therapy or Spravato® as treatment options. As a result, new Treatment Centers in these markets may be less successful than centers in our existing management regions. Accordingly, we cannot guarantee that we will be able to penetrate or successfully operate in any market outside of our current management regions. In order to build greater awareness surrounding Greenbrook, TMS therapy and Spravato® in these new markets, we will need to make greater investments in Treatment Center openings, clinician reach-out, and advertising, with no guarantee of success, which could negatively impact the profitability of our operations in those markets.
We may also find it more difficult in these new markets to hire, motivate and retain qualified employees and technicians with familiarity of the TMS Devices used by us as well as familiarity with administration of Spravato®. In addition, labor costs may be higher and new locations could have higher construction and occupancy costs. Entering into new regions may also present challenges, as we may have limited experience with the different regulatory regimes, insurance environments and market practices in these new regions as compared to those in our current management regions. These regulations and market practices could subject us to significant additional expense or impact our ability to achieve compliance. In connection with any future expansion efforts outside of our existing management regions, we would expect to encounter many obstacles that we do not currently face in our current regions, including differences in regulatory environments and market practices, and difficulties in keeping abreast of market, business and technical developments. Each of these factors may have an adverse impact on our revenues or profitability in those markets and could in turn adversely impact our revenues and results of operations. If we do not successfully execute our plans to enter new markets within in the broader United States, our business, financial condition and results of operations may be materially adversely affected.
Our strategy to grow our business through the expansion of the Spravato® Program is subject to significant risks.
In the first quarter of Fiscal 2021, we commenced offering Spravato® (esketamine nasal spray) at select Treatment Centers to treat adults with treatment-resistant depression and depressive symptoms in adults with MDD with suicidal thoughts or actions. In Fiscal 2022, we commenced our Medication Management Program at select Treatment Centers. As of the date of this Annual Report, the roll-out of our Spravato® Program and Medication Management program at select Treatment Centers has continued and we are also building on our long-term business plan of utilizing our Treatment Centers as platforms for the delivery of innovative treatments to patients suffering from MDD and other mental health disorders. We may, however, be unsuccessful in growing our business through the expansion of the Spravato® Program into our existing Treatment Center infrastructure.
The factors which may prevent us from successfully expanding the Spravato® and Medication Management programs include, among other things, (1) we may be unable to retain a sufficient number of clinicians/non-medical personnel to offer the service to our patients, including as a result of a general lack of qualified clinicians/non-medical personnel, (2) we may be unable to attract a sufficient number of patients willing to use Spravato® or Medication Management due to lack of awareness or otherwise, (3) we may be unable to implement the Spravato® or Medication Management programs into our existing Treatment Center network on a timely basis (e.g., there may be insufficient space in our existing Treatment Centers, and we may be unable to successfully implement systems or process changes in order to accommodate the Spravato® or Medication Management program), (4) we may not generate revenues from the expanded roll-out of the Spravato® and Medication Management progams that meets our expectations, (5) we may be unable to negotiate attractive reimbursement for Spravato® services from third-party payors, and (6) we are currently reliant on Janssen Pharmaceuticals, Inc. as the marketer of Spravato®.
In addition, the integration of the Spravato® Program or expansion of our existing facilities to accommodate the Spravato® Program may result in unforeseen operating difficulties and may require significant financial and managerial resources that would otherwise be used for our current operations. Any inability to manage growth related to the Spravato® Program could have a material adverse effect on our business, financial condition and results of operations.
- 34 -
Failure to timely or accurately bill for services could have a negative impact on our revenue, bad debt expense and cash flow.
Billing for healthcare services is an important and complex aspect of our business. If there are defects in the billing system, we may experience difficulties in our ability to successfully bill and collect for services rendered, including a delay in collections, a reduction in the amounts collected, increased risk of retractions from and refunds to commercial and government payors, an increase in uncollectible accounts receivable and noncompliance with reimbursement regulations, any or all of which could have a material adverse effect on our revenues, cash flows and operating results.
We bill numerous and varied payors, such as Medicare, non-Medicare government insurance plans, commercial payors and self-pay patients. These different payors typically have different billing requirements that must be satisfied prior to receiving payment for services rendered. Reimbursement is typically conditioned on our documenting medical necessity, the appropriate level of service and correctly applying diagnosis codes. Incorrect or incomplete documentation and billing information could result in non-payment for services rendered.
Additional factors that could complicate our ability to timely or accurately bill payors include:
| ● | disputes between payors as to which party is responsible for payment; |
| ● | failure of information systems and processes to submit and collect claims in a timely manner; |
| ● | variation in coverage for similar services among various payors; |
| ● | our reliance on third parties, whom we do not control, to provide billing services; |
| ● | the difficulty of adherence to specific compliance requirements and other procedures mandated by various payors; |
| ● | failure to obtain proper provider credentialing and documentation in order to bill various payors; and |
| ● | failure to collect patient balances due to economic conditions or other unknown reasons. |
To the extent that the complexity associated with billing for healthcare services we provide causes delays in our cash collections, we may experience increased carrying costs associated with the aging of our accounts receivable, as well as increased potential for bad debt expense.
Our ability to generate revenue depends in large part on our ability to attract new patients and if we fail to attract new patients, we may not be able to increase revenues.
Our success depends, in part, on our ability to attract new patients, particularly, in accordance with our growth strategy, within new management regions in the United States in markets in which we have limited or no Treatment Centers or brand awareness. In order to expand our patient base in these new markets as well as our existing markets, we depend, for a substantial portion of the services we perform, on patient referrals from unaffiliated clinicians. In addition, if a significant number of clinicians and other third parties were to discontinue or significantly reduce the rate at which they refer patients to us, our treatment volume could materially decrease, which would reduce our revenue and operating margins, which could have a material adverse effect on our business and financial condition.
We may be unable to execute successfully on our acquisitions and other business initiatives or do so within the intended timeframes, which could have an adverse effect on our financial condition, results of operations and business prospects.
Our growth through the successful acquisition and integration of complementary businesses is a critical component of our corporate strategy. For example, in Fiscal 2022, we completed the Success TMS Acquisition (as defined below), which added 47 Treatment Centers across the United States. In the future, we may continue to pursue acquisitions that complement our existing business, represent a strong strategic fit and are consistent with our overall business strategy and disciplined financial management. These activities create risks such as:
| ● | the need to integrate and manage the acquired business; |
- 35 -
| ● | additional demands on our resources, systems, procedures and controls; |
| ● | disruption of our ongoing business; |
| ● | diversion of management’s attention from other business concerns; |
| ● | inability to achieve the anticipated synergies and profitability from Treatment Centers we acquire; and |
| ● | litigation related to such acquisitions or other business combinations. |
Such acquisitions or other business collaborations may involve significant commitments of financial and other resources of our Company. We may have ongoing obligations and/or additional contingent consideration payable in connection with our acquisitions. Any such ongoing obligations and contingent consideration that may be payable in connection with future acquisitions may require substantial cash expenditures by us or may result in equity dilution in connection with the issuance by us of additional Common Shares.
Such acquisitions may also involve adjustments to the overall size and operation of our business, which may include closing Treatment Centers from recent acquisitions. For example, in connection with our Restructuring Plan, we have closed certain less profitable Treatment Centers, including one Treatment Center that had been acquired from Success TMS. Such closures may not be successful in improving our profitability, and the resources committed to such activities will not be available to us for other purposes. In addition, while we conduct due diligence prior to consummating an acquisition or business collaboration, such diligence may not identify all material issues associated with such transactions.
In addition, we may from time to time be involved in litigation in connection with acquisitions and other business combinations. For example, on May 24, 2023, in connection with the acquisition of Success TMS, the Seller Parties (as defined herein) filed the Delaware Complaint (as defined herein), concerning the Purchase Agreement Claims (see Item 3 “Legal Proceedings—Purchase Agreement Claims”). This litigation remains ongoing, but is currently stayed, and has required substantial time and expense to resolve, and we can provide no assurance regarding the timing or terms of its ultimate resolution. We can also provide no assurance that other litigation may arise in the future. Any such other litigation may require substantial time and expense to resolve, and the results of such litigation may be materially adverse to the Company.
The senior management of the Company and the Board have from time to time considered, and may consider in the future, various transactions in the context of its long-term business plan, including mergers, acquisitions, divestitures, alliances, joint ventures, investments or other strategic transactions. We may experience challenges or difficulties identifying and negotiating transactions with suitable new acquisition candidates that are available for purchase at reasonable prices. Moreover, if we are unable to access capital markets on acceptable terms or at all, we may not be able to consummate acquisitions, or may have to do so on the basis of a less than optimal capital structure.
Even if we are able to identify and enter into agreements with such candidates, we may be unable to consummate any such acquisition on suitable terms, in a timely manner or at all, and we may not realize the anticipated benefits of such transactions. Our ability to realize the anticipated benefits of acquired businesses will depend, in part, on our ability to successfully and efficiently integrate acquired businesses and operations with our own. The integration of acquired operations with our existing business may be complex, costly and time-consuming, and may result in additional demands on our resources, systems, procedures and controls, disruption of our ongoing business, and diversion of management’s attention from other business concerns. Although we cannot be certain of the degree and scope of operational and integration problems that may arise, the difficulties and risks associated with the integration of acquired businesses may include, among others:
| ● | the increased scope and complexity of our operations; |
| ● | coordinating geographically separate organizations, operations, relationships and facilities; |
| ● | integrating (i) personnel with diverse business backgrounds, corporate cultures and management philosophies, and (ii) the standards, policies and compensation structures, as well as the complex systems, technology, networks and other assets, of the businesses; |
| ● | retention of key employees; |
- 36 -
| ● | the possibility that we may have failed to discover obligations of acquired businesses or risks associated with those businesses during our due diligence investigations as part of the acquisition for which we, as a successor owner, may be responsible or subject to; and |
| ● | provisions in contracts with third parties that may limit flexibility to take certain actions. |
Our inability to (i) take advantage of growth opportunities for our business, (ii) address risks associated with acquisitions or business collaborations, or (iii) accomplish the integration of acquired businesses smoothly, successfully or within our budgetary expectations and anticipated timetables, may negatively affect our operating results and financial condition.
We may be unable to successfully integrate acquired businesses or do so within the intended timeframes, which could have an adverse effect on our financial condition, results of operations and business prospects.
Our ability to realize the anticipated benefits of acquired businesses will depend, in part, on our ability to successfully and efficiently integrate acquired businesses and operations with our own. The integration of acquired operations with our existing business may be complex, costly and time-consuming, and may result in additional demands on our resources, systems, procedures and controls, disruption of our ongoing business, and diversion of management’s attention from other business concerns. Although we cannot be certain of the degree and scope of operational and integration problems that may arise, the difficulties and risks associated with the integration of acquired businesses may include, among others:
| ● | the increased scope and complexity of our operations; |
| ● | coordinating geographically separate organizations, operations, relationships and facilities; |
| ● | integrating (i) personnel with diverse business backgrounds, corporate cultures and management philosophies, and (ii) the standards, policies and compensation structures, as well as the complex systems, technology, networks and other assets, of the businesses; |
| ● | retention of key employees; |
| ● | the possibility that we may have failed to discover obligations of acquired businesses or risks associated with those businesses during our due diligence investigations as part of the acquisition for which we, as a successor owner, may be responsible or subject to; and |
| ● | provisions in contracts with third parties that may limit flexibility to take certain actions. |
As a result of these difficulties and risks, we may not accomplish the integration of acquired businesses smoothly, successfully or within our budgetary expectations and anticipated timetables, which may result in a failure to realize some or all of the anticipated benefits of our acquisitions.
In addition, we may have ongoing obligations and/or additional contingent consideration payable in connection with our acquisitions. Any such ongoing obligations and contingent consideration that may be payable in connection with future acquisitions may require substantial cash expenditures by us or may result in equity dilution in connection with the issuance by us of additional Common Shares.
Our ability to manage our operations at our current size and successfully execute on our business strategies is subject to numerous risks and uncertainties.
The continued success of our growth initiatives depends on, among other things, our ability to scale our network of Treatment Centers and management regions within the United States, our ability to expand our Spravato® Program and our Medication Management Program, as well as factors which are beyond our control, including general economic conditions and consumer perceptions of TMS treatment and other treatments we offer. If we fail to execute on our growth initiatives, face delays in executing our growth initiatives or fail to fully realize the benefits expected to result from these initiatives, our results of operations and our ability to remain competitive may be materially adversely impacted, and the price of our Common Shares could decline. Our results to date are not an indication of future results, and there can be no assurance that our business strategies will generate increased revenues or improve operating margins even if we are able to successfully implement our growth strategies.
- 37 -
As we move forward, we expect our growth to bring new challenges and complexities that we have not faced before. Among other difficulties that we may encounter, this growth could place a strain on our existing infrastructure, information technology systems, real estate requirements and employee base and may make it more difficult for us to adequately forecast expenditures. Our budgeting will become more complex, and we may also place increased burdens on our suppliers. The increased demands that our growth plans will place on our infrastructure and our management team may cause us to operate our business less efficiently, which could cause deterioration in our performance. Our growth may make it otherwise difficult for us to respond quickly to changing trends, preferences and other factors. This could result in deterioration of key affiliated clinician relationships, excess or deficient equipment, loss of market share and decreased revenues. We cannot anticipate all of the demands that our expanding operations will impose on our business, and our failure to appropriately address these demands could have an adverse effect on us.
In addition, we believe that an important contributor to our success has been our corporate culture, which we believe fosters innovation, teamwork and personalized customer service. As we continue to grow, we must effectively integrate, develop and motivate a growing number of new employees. As a result, we may find it difficult to maintain our corporate culture, which could limit our ability to innovate and operate effectively. Any failure to preserve our culture could also negatively affect our ability to retain and recruit personnel, continue to perform at current levels or execute on our growth strategies.
We do not independently own all of our Treatment Centers and are accordingly subject to risks associated with leasing space and equipment, as well as subject to a number of long-term non-cancelable leases with substantial lease payments. Any failure to make these lease payments when due, or the inability to extend, renew or continue to lease space and equipment in key locations, would likely harm our business, profitability and results of operations.
We do not own any real estate. Instead, we lease all of our retail Treatment Center locations, as well as our head office and U.S. corporate headquarters. Accordingly, we are subject to all of the risks associated with leasing, occupying and making tenant improvements to real property, including adverse demographic and competitive changes affecting the location of the property, changes in availability of and contractual terms for leasable space, credit risk in relation to tenant improvement allowances from landlords and potential liability for environmental conditions or personal injury claims.
We currently do not independently own all of our Treatment Centers, and healthcare laws and regulations in the United States may impact our ability to operate or own our Treatment Centers in the future, thereby necessitating the use of partnerships and other management services frameworks. Consequently, we may be required to deal with diverse operating or ownership frameworks. In addition, from time to time, we may decide to use cash to restructure our arrangements with fellow owners, managers or operators of certain of our Treatment Centers.
The success of any Treatment Center depends substantially upon its location. There can be no assurance that our current Treatment Center locations will continue to be desirable in the future, or that we will be able to secure new desirable locations in the future on favorable terms or at all. Treatment Center locations, patient conversion and revenues may be adversely affected by, among other things, social and economic conditions in a particular area, competition from nearby Treatment Centers, out-of-pocket treatment costs, changes in stigma relating to mental health issues, and changing lifestyle choices of patients in a particular market. If we cannot obtain desirable locations at reasonable costs, our cost structure will increase, and our revenues will be adversely affected.
Our existing Treatment Centers are leased from third parties, with typical lease commitments ranging from “month-to-month” to seven years. Some of our lease agreements also have additional renewal options. However, there can be no assurances that we will be able to extend, renew or continue to lease our existing Treatment Center locations, or identify and secure alternative suitable locations. In addition to fixed minimum lease payments, most of our leases provide for additional rental payments, including payment of common area maintenance charges, real property insurance, real estate taxes and other charges. Many of our lease agreements have defined escalating rent provisions over the initial term and any extensions. Increases in our occupancy costs and difficulty in identifying economically suitable new Treatment Center locations could have significant negative consequences, which include:
| ● | requiring that a greater portion of our available cash be applied to pay our rental obligations, thus reducing cash available for other purposes and reducing our profitability; |
| ● | increasing our vulnerability to general adverse economic and industry conditions; and |
| ● | limiting our flexibility in planning for, or reacting to changes in, our business. |
- 38 -
We depend on cash flow from operations to pay our lease expenses and to fulfill our other cash needs. If our business does not generate sufficient cash flow from operating activities to fund these expenses and sufficient funds are not otherwise available to us, we may not be able to service our lease expenses, grow our business, respond to competitive challenges or fund our other liquidity and capital needs, which could harm our business. Additional sites that we lease may be subject to long-term non-cancelable leases if we are unable to negotiate shorter terms. If an existing or future location is not profitable, and we decide to close it (as we have done connection with the Restructuring Plan), we may nonetheless be committed to perform our obligations under the applicable lease including, among other things, paying the base rent for the balance of the lease term. In addition, if we are not able to enter into new leases or renew existing leases on terms acceptable to us, this could have an adverse effect on our results of operations and profitability.
Our new Treatment Centers may not be profitable initially, or at all, and may adversely impact our business.
Our new Treatment Centers may experience an initial ramp-up period during which they generate revenues below the levels we would otherwise expect. This is in part due to the time it takes to build a patient base in a new market, higher fixed costs relating to increased labor needs, other start-up inefficiencies that are typical of new locations and cash build-out costs of new Treatment Centers that may be higher than our target cash build-out costs, development costs, additional features, and budgets. It may also be difficult for us to attract a patient base, or otherwise overcome the higher costs associated with new locations. New locations may not have results similar to existing locations or may not be profitable. If new Treatment Centers remain unprofitable for a prolonged period of time, we may decide to close these Treatment Centers (as we have done in connection with the Restructuring Plan), which could have a negative impact on our business and results of operations.
Legal and Regulatory Risk Factors
An unfavorable outcome of the Purchase Agreement Claims could result in a material adverse effect to the Company’s financial position.
On May 24, 2023, in connection with the acquisition of Success TMS, the Seller Parties (as defined herein) filed a complaint in the Superior Court of the State of Delaware against the Company, TMS US and certain executive officers of the Company, and subsequently filed a first amended complaint on August 31, 2023 (the “Delaware Complaint”), concerning the Purchase Agreement Claims arising out of the acquisition of Success TMS. The Purchase Agreement Claims allege contractual fraud, indemnification for breach of certain representations and warranties of the Company contained in the Success Purchase Agreement (as defined herein), other breaches of the Success Purchase Agreement and a registration rights agreement, and breach of the implied covenant of good faith and fair dealing. The Delaware Complaint seeks damages in an amount to be determined at trial, which are alleged to exceed $1 million. On October 2, 2023, the Company and the other defendants moved to dismiss the Purchase Agreement Claims. On November 20, 2023, the court stayed the Purchase Agreement Claims until May 13, 2024.
If we are unable to resolve the Purchase Agreement Claims, they could result in a judgement that may have a material adverse effect on the Company. Even if we are successful in resolving this litigation in the Company’s favor, this and any other potential litigation can require a substantial number of resources as well as redirect management attention, as well as create a negative perception of the Company. Any decision resulting from any such litigation that is unfavorable to the Company, and any additional litigation brought by Benjamin Klein or anyone else, could have a material adverse effect on the Company’s financial position. See Item 3 “Legal Proceedings—Purchase Agreement Claims” below.
Regulatory and compliance requirements associated with our billing and collections system could have a material adverse effect on our revenues, cash flows and operating results.
We accept payments using a variety of methods, including credit cards and debit cards. For existing and future payment methods we offer to our customers, we may become subject to additional regulations and compliance requirements, as well as fraudulent activities. For certain payment methods, including credit and debit cards, we pay interchange and other fees, which may increase over time, raising our operating costs and lowering profitability. We rely on third party service providers for payment processing services, including the processing of credit and debit cards. Our business may be negatively affected if these third-party service providers become unwilling or unable to provide these services to us. We are also subject to payment card association operating rules, including data security and management rules, certification requirements and rules governing electronic funds transfers and if we fail to comply with these rules or requirements, or if our data security systems are breached or compromised, we may be liable for card issuing banks’ costs, subject to fines and higher transaction fees and/or lose our ability to accept credit and debit card payments from our patients and process electronic funds transfers or facilitate other types of payments, and our business and operating results may be adversely affected.
- 39 -
We may engage in litigation with our clinical partners and contractors and there are claims made against us from time to time that can result in litigation that could distract management from our business activities and result in significant liability or damage to us.
The nature of our relationships with our clinical partners and contractors may give rise to litigation or disputes. In the ordinary course of our business, we are the subject of complaints or litigation. We may also engage in future litigation to enforce the terms of our agreements and compliance with our brand standards as determined necessary to protect our brand, the consistency of our services and the consumer experience. Engaging in such litigation may be costly and time-consuming and may distract management and materially adversely affect our relationships with our clinical partners and contractors or potential clinical partners and contractors and our ability to attract new clinical partners and contractors. Any negative outcome of these or any other claims could materially adversely affect our results of operations, as well as our ability to increase our number of clinical partners and contractors and may damage our reputation and brand and our ability to expand into new regions.
We increasingly face the risk of litigation and other claims against us. Litigation and other claims may arise in the ordinary course of our business and include employee and patient claims, commercial disputes, landlord-tenant disputes, intellectual property issues, product-oriented allegations and personal injury claims. These claims can raise complex factual and legal issues that are subject to risks and uncertainties and could require significant management time. Most of our equipment is manufactured and supplied by third party suppliers and some of these products may expose us to various claims, including class action claims relating to medical devices subject to a product recall or liability claim. Litigation and other claims against us could result in unexpected expenses and liabilities, which could materially adversely affect our operations and our reputation.
Although we maintain liability insurance to mitigate potential claims, we cannot be certain that our coverage will be adequate for liabilities actually incurred or that insurance will continue to be available on economically reasonable terms or at all.
We may become subject to professional malpractice liability, which could be costly and negatively impact our business.
The clinicians contracted or employed by us or our contracted practices could be subject to malpractice claims from time to time. Where required by law, we structure our relationships with the practices under our management services agreements in a manner that we believe does not constitute the practice of medicine by us or subject us to professional malpractice claims for acts or omissions of clinicians employed by the contracted practices. Nevertheless, claims, suits or complaints relating to services provided by the clinicians contracted or employed by us or our contracted practices may arise. In addition, we may be subject to professional liability claims, including, without limitation, for improper use or malfunction of our TMS Devices, improper administration of Spravato® or the misconduct of our technicians. We may not be able to maintain adequate liability insurance to protect us against those claims at acceptable costs or at all. Any claim made against us that is not fully covered by insurance could be costly to defend, result in a substantial damage award against us and divert the attention of our management from our operations, all of which could have an adverse effect on our financial performance. In addition, successful claims against us may adversely affect our business or reputation.
The effect of the uncertainty relating to potential future changes to U.S. healthcare laws may increase our and our clinical partners’ and contractors’ healthcare costs, limit the ability of patients to obtain health insurance, increase patients’ share of healthcare costs and negatively impact our financial results.
The Biden Administration and the U.S. Congress are considering a number of legislative and regulatory proposals that could, if passed into law, impact the healthcare system, the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act of 2010 (collectively, the “ACA”), and/or the Medicare and Medicaid programs. Congress may take up legislation to increase or decrease the number of individuals covered by the Medicare or Medicaid programs, reduce prescription drug costs, increase price transparency for consumers, restrict the sale of certain classes of drugs, and reform medication management practices, among others. While not all of the potential legislation, if enacted, would affect our business directly, many of these legislative proposals could impact some or many of our business arrangements directly or indirectly. In addition, regulatory agencies have separately implemented price transparency rules for hospitals and insurers which, while not impacting our business directly, could change the way we interact with these entities. Given that legislative and regulatory change is still evolving, we cannot predict with any certainty the outcome of any future legislation or regulation. However, we believe that many of the legislative items noted above enjoy bipartisan support.
- 40 -
The environment regarding the provisions of the ACA has somewhat stabilized, but specific outcomes are difficult to predict. Certain provisions of the ACA continue to be challenged in the courts and Congress continues to consider modifications to the ACA. Any judicial determinations or legislation related to the ACA could have a material impact on our ability to conduct business and our operations.
Because of our U.S. operations, we could be adversely affected by violations of federal Anti-Kickback Statute and/or other fraud and abuse laws. If our arrangements with physicians were found to violate the law, we could suffer consequences that would have a material adverse effect on our revenues, earnings, cash flows and reputation.
The Anti-Kickback Statute and other fraud and abuse laws and regulations, both at the federal and state level, generally prohibit parties from giving remuneration to a physician or other person in a position to refer or generate business for a health care provider, such as our contracted physician practices, with the intent to induce or reward such referrals. Notwithstanding our strict policies and procedures designed to ensure no violation of such laws, financial relationships within the Company organization involving physicians and other potential referral sources, including amounts paid under our management services agreements, distributions made to referring physicians who are also equity holders in our contracted physician practices and all other financial arrangements involving the Company may result in violations of such laws. We have sought to structure our arrangements to satisfy federal Anti-Kickback Statute safe harbor requirements, but they remain susceptible to government scrutiny. If we were found to violate the law, we could suffer consequences, including fines, penalties, repayment obligations, criminal liability and exclusion for participation in federal health care programs, that would have a material adverse effect on our revenues, earnings, cash flows and reputation.
Our management services arrangements with physician practices and those practices’ professional services agreements with contracted or employed psychiatrists must be structured in compliance with state laws relating to the practice of medicine, including, without limitation, fee-splitting prohibitions.
The laws in certain states in which we operate prohibit us from owning physician practices, exercising control over the clinical judgment of physicians, and/or engaging in certain financial arrangements, such as splitting professional fees with physicians. These laws vary by state and are enforced by state courts and regulatory authorities, each with broad discretion, and often with limited precedent as to how challenges under these laws may be decided. One component of our business involves entering into management services agreements with physician practices whereby we provide management, administrative, technical and other non-medical services to the physician practices in relation to TMS therapy and other behavioral health therapy services in exchange for a service fee. We structure our relationships with these physician practices in a manner that we believe keeps us from engaging in the practice of medicine or exercising control over any physician’s medical judgment. However, there can be no assurance that our present arrangements with physicians providing medical services and medical supervision at our contracted physician practices will not be challenged in the future for violating these state laws and, if challenged, that they will not be found to violate applicable laws. Any such ruling against us could subject us to potential damages, injunctions and/or civil and criminal penalties or require us to restructure our arrangements in a way that would affect the non-medical control or quality of our services or change the amounts that we receive from the operation of our contracted physician practices, which could have a material adverse effect on our business.
The regulatory framework in which we operate is constantly evolving.
Healthcare laws and regulations are constantly evolving and could change significantly in the future. We closely monitor these developments and will modify our operations from time to time as the regulatory environment requires. There can be no assurances, however, that we will always be able to adapt our operations to address new laws or regulations or that new laws or regulations will not adversely affect our business. In addition, although we believe that we are operating in material compliance with applicable federal and state laws and regulations, neither our current or anticipated business operations nor the operations of our contracted physician practices have been the subject of judicial or regulatory interpretation. We cannot assure investors that a review of our business by regulatory authorities or courts will not result in a determination that could materially adversely affect our operations or that the healthcare regulatory environment will not change in a way that materially restricts our operations. Furthermore, governments, government agencies and industry self-regulatory bodies in the United States may, from time to time, adopt statutes, regulations and rulings that directly or indirectly affect the activities of the Company. These statutes, regulations and/or rulings could adversely impact our ability to execute our business strategy and generate revenues as planned.
- 41 -
Complying with U.S. federal and state regulations is an expensive and time-consuming process, and any failure to comply could result in penalties or repayments.
We are directly, and indirectly through our contracted physician practices, subject to extensive regulation by both the U.S. federal government and the state governments of those states in which our contracted physician practices operate, including:
| ● | the FCA; |
| ● | U.S. federal and state anti-kickback and self-referral prohibitions; |
| ● | U.S. federal and state billing and claims submission laws and regulations; |
| ● | HIPAA and HITECH and comparable state laws; and |
| ● | state laws that prohibit the practice of medicine by non-physicians and prohibit fee-splitting arrangements involving physicians. |
If our operations are found to be in violation of any of the laws and regulations to which we or our contracted physician practices are subject, we may be subject to penalties associated with the violation, including civil and criminal penalties, damages, fines, exclusion and the curtailment of our operations. Any penalties, damages, fines, exclusion or curtailment of our operations, individually or in the aggregate, could adversely affect our ability to operate our business and our financial results. The risks of our being found in violation of these laws and regulations is increased by the view that many of these laws and regulations are complex, have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these laws or regulations, even if we successfully defend against it, could result in significant legal expenses and divert our management’s attention from the operation of our business, which could have a material adverse effect on our business, operations and prospects.
Furthermore, the Medicare and Medicaid reimbursement rules impose extensive requirements upon healthcare providers that furnish services to Medicare and/or Medicaid beneficiaries, including our contracted physician practices. Moreover, additional laws and regulations potentially affecting healthcare providers participating in the Medicare and Medicaid programs continue to be promulgated that may impact us in the future. From time to time, in the ordinary course of business, we may conduct internal compliance reviews on behalf of our contracted physician practices, the results of which may involve the identification of errors in the manner in which our contracted physician practices submit claims to the Medicare or Medicaid program. Our contracted physician practices may also be subject to periodic audits by insurance companies, including those associated with the Medicare or Medicaid program. These reviews may result in the identification of errors in the manner in which we or our contracted physician practices bill such insurance programs for services, which may result in the receipt of incorrect payments from the insurance companies, including the Medicare program, that our contracted physician practices are required to repay. Under U.S. law, the failure to report and return Medicare overpayments can lead to liability under the FCA and associated penalties, including exclusion from Medicare or Medicaid and other federal health care programs. In addition, private payors may on occasion amend their coverage policies in a way that may impact our operations.
As part of our ongoing compliance efforts with these regulatory requirements, we periodically conduct reviews of our contracted physician practices’ past operations to assess our compliance with such requirements. If and when such reviews demonstrate that there may be a repayment obligation due to the failure to comply with certain regulatory requirements, the Company remedies the deficiency and returns and refunds any Medicare overpayments within the required time periods.
It may be difficult for United States investors to effect service of process or enforcement of actions against us or certain of our directors and officers under U.S. federal securities laws.
We are incorporated under the laws of the Province of Ontario, Canada. A number of our directors and officers reside in Canada. Because certain of our assets and these persons are located outside the United States, it will be difficult for United States investors to effect service of process in the United States upon us or our directors or officers, or to realize in the United States upon judgments of United States courts predicated upon civil liabilities under the U.S. Securities Exchange Act of 1934, as amended (the “U.S. Exchange Act”) or other United States laws. It may also be difficult to have a judgment rendered in a U.S. court recognized or enforced against us in Canada.
- 42 -
Industry Risk Factors
Our ability to obtain TMS Devices and Spravato® from our suppliers on a timely basis at competitive costs could suffer as a result of events that adversely affect our suppliers or cause disruptions in their businesses.
TMS treatments are delivered through TMS Devices. Esketmine nasal spray treatments require Spravato® to be obtained by Janssen Pharmaceuticals, Inc. or through specialty pharmacies, which are at times, restricted to those approved by the respective payors. We and our suppliers of TMS Devices and Spravato® may be affected by, among other things, increases in labor and fuel costs, labor disputes and disruptions, regulatory changes, political or economic instability or civil unrest, including terrorist activities, military and domestic disturbances and conflicts, natural disasters, pandemics, trade restrictions, tariffs, currency exchange rates, transport capacity and costs and other factors relating to trade. These factors are beyond our control, may adversely affect us and our suppliers or cause disruptions to their and our businesses and may impact their ability to supply us with TMS Devices or Spravato®. Consequently, our ability to provide TMS and Spravato® treatments to our patients on acceptable terms and within acceptable timelines may be impacted, which could have a material adverse effect on our profitability and results of operations. See Item 1A “Risk Factors—Business and Operational Risk Factors—We rely exclusively on Neuronetics as the supplier of the TMS Devices that we utilize under the Neuronetics Agreement. The loss of this relationship, or any negative impacts on Neuronetics’ ability to manufacture such machines, would severely harm our business.”
Our inability to attract key managerial and other non-medical personnel such as qualified behavioral health technicians may adversely impact our ability to carry out our business operations and strategies as planned.
We are highly dependent on qualified managerial personnel. Our anticipated growth will require additional expertise and the addition of new qualified personnel. In recent years, we have experienced shortages of qualified managerial and non-medical personnel due to labor shortages in the United States. In addition, there is intense competition for qualified personnel in the non-clinical healthcare management services space. Therefore, we may not be able to attract and retain the qualified personnel necessary for the development of our business. The loss of the services of existing personnel, as well as the failure to recruit additional key managerial personnel in a timely manner, could harm our business development and expansion plans as well as our ability to manage day-to-day operations, attract collaboration partners, attract and retain other employees and generate revenues, which could materially adversely affect our business, prospects, financial condition, results of operations or cash flows.
If commercial payor plans are subject to restriction in plan designs or the average rates that commercial payors pay us decline significantly, or if there are changes in Medicare or other non-Medicare government-based programs or payment rates, such occurrences would have a material adverse effect on our revenues, earnings and cash flows.
There is no guarantee that commercial, Medicare or other non-Medicare government payment rates will remain at existing levels as they could be subject to material decreases in the future. More than 97% of the patients that receive Spravato®, Medication Management, or TMS treatment at our Treatment Centers are covered, to a certain extent, by commercial payors, Medicare or other non-Medicare government programs. If we experience downward pressure on reimbursement conditions in the market due to employers shifting to less expensive options for medical services or as a result of consolidations among commercial payors, or if state governments and other governmental organizations face increasing budgetary pressure, resulting in decreases in the average reimbursement rates for TMS therapy and/or denial of reimbursement coverage for Spravato® or Medication Management, such occurrences would have a material adverse effect on our revenues, earnings and cash flows.
We may incur increased costs if third-party payors impose additional requirements related to the provision of services at our Treatment Centers.
Commercial payors, Medicare and other non-Medicare government programs set requirements that must be met for services to be deemed reimbursable. The imposition of additional requirements related to the provision of TMS and/or esketamine nasal spray therapy by commercial insurance plans, Medicare and other non-Medicare government insurance plans that increase the cost or complexity of furnishing these therapies to patients may result in increased costs. For example, certain commercial payors are increasing the levels of clinician supervision that must be provided to patients receiving TMS therapy, thereby restraining our ability to provide patient care when these increased levels of clinician supervision are not available and/or resulting in the incurrence of additional clinician compensation costs for ensuring the requisite level of supervision as a result of these increased requirements. The imposition of such requirements and any additional requirements by third-party payors may impact our revenues and costs, which could materially adversely affect our business, prospects, financial condition, results of operations or cash flows.
- 43 -
If there is a reduction in reimbursement rates by higher-paying commercial insurance providers, our revenues, earnings and cash flows would be substantially reduced.
Our revenue levels are affected by the percentage of our patients with higher-paying commercial insurance coverage. A patient’s insurance coverage may change for a number of reasons, including changes in the patient’s or a family member’s employment status. If there is a significant change in our payor mix, resulting in a reduction in the number of patients with higher-paying commercial insurance plans declining, our revenues, earnings and cash flows could be substantially reduced.
There are significant risks associated with estimating the amount of revenues and related refund liabilities that we recognize, and if our estimates of revenues and related refund liabilities are materially inaccurate, it could impact the timing and the amount of our revenues recognition or have a material adverse effect on our business, results of operations, financial condition and cash flows.
There are significant risks associated with estimating the amount of service revenues and related refund liabilities that we recognize in a reporting period. The billing and collection process is complex due to ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage and other payor issues, such as ensuring appropriate documentation. Determining applicable primary and secondary coverage for our patients, together with the changes in patient coverage that occur each month, requires complex, resource-intensive processes. Errors in determining the correct coordination of benefits may result in refunds to payors. Revenues associated with Medicare and non-Medicare government insurance plans are also subject to estimation risk related to the amounts not paid by the primary government payor that will ultimately be collectible from other government programs paying secondary coverage, the patient’s commercial health plan secondary coverage or the patient. Collections, refunds and payor retractions typically continue to occur for up to three years and longer after services are provided. If our estimates of services revenue and related refund liabilities are materially inaccurate, it could impact the timing and the amount of our revenues recognized and have a material adverse impact on our business, results of operations, financial condition and cash flows.
If our Treatment Centers lose a significant number of clinicians, our financial results could be adversely affected.
Against a backdrop of significant mental health and addiction issues in the United States and an increase in suicide rates, there is an unprecedented demand for clinicians. At times, there has been a shortage of qualified clinicians in some of the regional markets in which we serve. In addition, competition in recruiting clinicians may make it difficult for our contracted psychiatric practices to maintain adequate levels of clinicians. If a significant number of clinicians terminate their relationships with our practices and those practices are unable to recruit sufficient qualified clinicians to fulfill their obligations under our agreements with them, our ability to maximize the use of our Treatment Centers and our financial results could be materially adversely affected. Neither we, nor our practices, maintain insurance on the lives of any affiliated clinicians.
We are dependent on the timely credentialing of our affiliated clinicians.
We are responsible for credentialing our existing and new clinicians with all third-party payors (including commercial insurance plans, Medicare and other non-Medicare government insurance), and all of our clinicians need to be credentialed in order to administer TMS therapy, Medication Management, and Spravato® at our Treatment Centers. This credentialing process is completed by us, or by a contracted third party, and requires the submission of a substantial amount of documentation necessary to satisfy third-party payors that our clinicians are qualified to perform services intended to be covered by insurance. The amount of time required to complete credentialing varies substantially between payor and region and is largely out of our control. Any delay in completing credentialing will result in a delay in clinicians seeing patients and a concomitant delay in generating revenue. Any failure of our clinicians to maintain credentials and licenses could result in delays in our ability to deliver care to patients, and therefore adversely affect our reputation and our business.
Technological change in our industry or novel drug treatments for MDD or OCD could reduce the demand for our services or require us to incur significant cost to incorporate new technology into our centers.
Advances in technology or the development of novel drug treatments for MDD or OCD may reduce the demand for our services or result in significant cost to incorporate the new technology into our Treatment Centers. If we are unable to effectively respond to technological advancement, our treatment volumes could decline, which could have a material adverse effect on our revenues, earnings and cash flows.
- 44 -
We experience competition from other Treatment providers, providers of esketamine nasal spray therapy, hospitals and pharmaceutical and other companies, and this competition could adversely affect our business and revenue.
The market for TMS therapy, Medication Management, and esketamine nasal spray services is becoming increasingly competitive. We compete principally on the basis of our reputation and brand, the location of our centers, the quality of our services and the reputation of our affiliated clinicians. In the markets in which we are operating, or anticipate operating in the future, competition predominantly consists of individual clinicians that can offer TMS therapy, Medication Management, and/or esketamine nasal spray therapy directly to their patients. We also face competition from a limited number of multi-location psychiatric practices or behavioral health groups that offer TMS therapy, Medication Management, and/or esketamine nasal spray therapy as part of their overall practice, as well as a few other specialist Treatment providers and esketamine nasal spray therapy or intravenous ketamine providers.
We also face indirect competition from pharmaceutical and other companies that develop competitive products, such as anti-depressant medications, with certain competitive advantages such as widespread market acceptance, ease of patient use and well-established reimbursement. Our commercial opportunity could be reduced or eliminated if these competitors develop and commercialize anti-depressant medications or other treatments that are safer or more effective than TMS or esketamine nasal spray therapy. At any time, these and other potential market entrants may develop treatment alternatives that may render our products uncompetitive or less competitive. We are also subject to competition from providers of invasive neuromodulation therapies such as electroconvulsive therapy and vagus nerve stimulation.
Many of our competitors are, and many of our potential competitors may be, larger and have access to greater financial, marketing and other resources. Therefore, these competitors may be able to devote greater resources to the marketing and sale of their products or adopt more aggressive pricing policies than we can. As a result, we may lose market share, which could reduce our revenues and adversely affect our results of operations.
Our competitors may seek to emulate facets of our business strategy, which could result in a reduction of any competitive advantage that we might possess. As a result, our current and future competitors, especially those with greater financial, marketing or other resources, may be able to duplicate or improve upon some or all of the elements of our business strategy that we believe are important in differentiating our patients’ treatment experience. If our competitors were to duplicate or improve upon some or all of the elements of our business strategy, our competitive position and our business could suffer. There can be no assurances that we will continue to be able to compete successfully against existing or future competitors. If we are unable to successfully compete, our business and financial condition would be adversely affected.
Risks Related to Our Common Shares
Our Common Shares were delisted from trading on Nasdaq and are currently quoted on the OTCQB Market, which involves additional risks compared to being listed on a national securities exchange. Our ability to sell equity securities and the liquidity of the Common Shares publicly or privately will be adversely affected if we are unable to transfer the Company’s listing to another stock exchange.
On February 22, 2024, the Company received written notice from Nasdaq notifying the Company that, as a result of its inability to maintain compliance with the Nasdaq Requirements, the staff of Nasdaq had determined that the Company’s Common Shares would be delisted from Nasdaq. Trading of the Company’s securities was suspended at the opening of business on February 26, 2024, following which our Common Shares were quoted on the OTC Pink Market under the symbol “GBNHF”. On March 22, 2024, our Common Shares became quoted on OTCQB Market under the symbol “GBNHF”. The Company filed its own Form 25 with the SEC on April 1, 2024, which completed the process for delisting its Common Shares from Nasdaq when the Form 25 became effective on April 11, 2024.
Although our Common Shares are quoted on OTCQB Market, the delisting of our Common Shares on Nasdaq limits the public resale market for our Common Shares. The lack of an active, liquid trading market for our Common Shares could have material adverse effects on our business, financial condition and results of operations due to, among other things:
| ● | impairing the ability of holders of our Common Shares to sell their shares at the time they wish to sell them or at a price that they consider reasonable; |
| ● | reducing the trading liquidity and fair market value of the shares of our Common Shares; |
- 45 -
| ● | decreasing the number of institutional and other investors willing to hold or acquire our Common Shares or enter into strategic transactions, coverage by securities analysts, market making activity and information available concerning trading prices and volume, as well as fewer broker-dealers willing to execute trades in our Common Shares, thereby further restricting our ability to obtain equity financing; and |
| ● | the price of our Common Shares could be more likely to be affected by broad market fluctuations, general market conditions, fluctuations in our operating results, changes in the markets’ perception of our business, and announcements made by us, our competitors and parties with whom we have business relationships. |
Trading in our securities is highly speculative, and we may be required to file for bankruptcy protection in the future.
Trading in our Common Shares is highly speculative and poses substantial risks to investors. The market price of the Common Shares may be subject to significant fluctuations. Some of the factors that may cause the market price of the Common Shares to fluctuate include:
| ● | volatility in the market price and trading volume of comparable companies; |
| ● | actual or anticipated changes or fluctuations in our operating results or in the expectation of market analysts; |
| ● | adverse market reaction to any indebtedness we may incur or securities we may issue in the future; |
| ● | short sales, hedging and other derivative transactions in the Common Shares; |
| ● | litigation or regulatory action against us; |
| ● | investors’ general perception of us and the public’s reaction to our press releases, our other public announcements and our filings with Canadian securities regulators and the SEC, including our financial statements; |
| ● | publication of research reports or news stories about us, our competitors or our industry |
| ● | positive or negative recommendations or withdrawal of research coverage by securities analysts; |
| ● | changes in general political, economic, industry and market conditions and trends; |
| ● | sales of Common Shares by existing shareholders; |
| ● | recruitment or departure of key personnel; |
| ● | significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving us or our competitors; and |
| ● | the other risk factors described in this Item 1A, “Risk Factors”. |
These factors, as well as other related factors, may cause decreases in asset values that are deemed to be other than temporary, which may result in impairment losses. As well, certain institutional investors may base their investment decisions on consideration of our environmental, governance and social practices and performance against such institutions’ respective investment guidelines and criteria, and failure to satisfy such criteria may result in limited or no investment in the Common Shares by those institutions, which could materially adversely affect the trading price of the Common Shares.
- 46 -
There is no guarantee that continuing fluctuations in price and volume will not occur or that an investment in our Common Shares will earn any positive return in the short or long term, and we have encountered substantial cash shortages that have required amendments and waivers, as well as additional rounds of financing, including debt financings under the Credit Agreement and additional equity financing from public offerings and private placements to significant shareholders (including Greybrook Health) and management, and led to us adopting the Restructuring Plan. A purchase of Common Shares involves a high degree of risk and should be undertaken only by investors whose financial resources are sufficient to enable them to assume such risks, who have no need for immediate liquidity in their investment and who have the financial capacity to absorb a loss of some or all of their investment. Trading prices for our Common Shares may bear little or no relationship to the actual recovery, if any, by holders of our securities in any bankruptcy proceeding and our shareholders will likely not receive any recovery at all in a bankruptcy scenario. Our operations and ability to develop and execute our business plan, our financial condition, our liquidity and our continuation as a going concern, are subject to risks and uncertainties. These risks include, but are not limited to, the following:
| ● | our ability to achieve the anticipated cost-savings from the Restructuring Plan; |
| ● | our ability to meet our cash requirements for ongoing operations and to satisfy our outstanding debt obligations; |
| ● | our ability to achieve a favorable outcome in the Purchase Agreement Claims; |
| ● | our ability to maintain our current relationships with or attract new clinicians, patients, employees, and other third parties; |
| ● | our ability to maintain contracts that are critical to our operations, including the Neuronetics Agreement, and maintain our relationship with Neuronetics; |
| ● | our ability to attract, motivate and retain key employees; and |
| ● | the actions and decisions our creditors (including Madryn, Neuronetics, and Benjamin Klein) and other third parties including, but not limited to, our landlords and vendors who have interests that may be inconsistent with our plans. |
These risks and uncertainties, together with the other risk factors described in these “Risk Factors”, could affect our business and operations in various ways. If any of these risks materialize, our business may be materially and adversely affected, and we may not be able to have sufficient resources to continue to operate our business and we may be required to file for bankruptcy protection. Our shareholders may not receive any recovery at all in a bankruptcy scenario.
There are unexercised options, warrants, convertible notes and conversion instruments outstanding and which may be issued from time to time. If these are exercised or converted, an investor’s interest in Common Shares will be substantially diluted.
The number of Common Shares that the Company is authorized to issue is unlimited. The Company may, in its sole discretion, issue additional Common Shares from time to time subject to the rules of any applicable stock exchange on which the Common Shares are then listed and applicable securities law. The issuance of any additional Common Shares, including upon exercise of options, convertible instruments or warrants, may have a dilutive effect on the interests of holders of Common Shares.
If all of the Company’s options that were issued and outstanding as of April 8, 2024 (including options that are not yet exercisable) were to be exercised, we would be required to issue up to an additional 1,704,500 Common Shares, or approximately 3.7% of the issued and outstanding Common Shares as of April 8, 2024. In addition, Madryn Conversion Instruments are convertible at Madryn’s option into up to an additional 3,910,604 Common Shares, or approximately 8.6% of the issued and outstanding Common Shares as of April 8, 2024. Additionally, the Subordinated Convertible Notes are convertible into $9.7 million of Common Shares at the Subordinated Convertible Note Conversion Price. See Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness and Capital Raising—Subordinated Convertible Notes”. The maximum number of Common Shares that can be issued under the Subordinated Convertible Notes is 200,000,000 Common Shares. If the entirety of the Subordinated Convertible Notes were converted the Subordinated Convertible Note Conversion Price (calculated as of April 8, 2024), the Company would be required to issue an additional 135,115,950 Common Shares equal to 296.3% of the issued and outstanding Common Shares. Finally, if the Company defaults in respect of its obligations under the Neuronetics Note, Greenbrook will be required to issue the Neuronetics Warrants in an amount equal to (i) 200% of the unpaid amount of any delinquent amount or payment due and payable under the Neuronetics Note, together with all outstanding and unpaid accrued interest, fees, charges, and costs, divided by (ii) the exercise price of the Neuronetics Warrants, which will represent a 20% discount to the 30-day volume-weighted average closing price of the Common Shares quoted on the applicable over-the-counter market prior to the date of issuance.
- 47 -
See Item 1 “Business—Recent Developments in Our Business—TMS Device Supply Arrangement with Neuronetics” and Note 10(a) within the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023, in this Annual Report.
These issuances of additional Common Shares, to the extent they occur, would decrease the proportionate ownership and voting power of all shareholders. This dilution could cause the price of the Common Shares to decline. In addition, our shareholders could suffer dilution in the net book value per Common Share.
A decline in the price of the Common Shares could affect our ability to raise further working capital and adversely impact our ability to continue operations.
A prolonged decline in the price of the Common Shares could result in a reduction in the liquidity of the Common Shares and a reduction in our ability to raise capital. Because a portion of our operations has been and may be financed through the sale of equity securities, a decline in the price of the Common Shares could be detrimental to our liquidity and our operations. Such reductions may force us to reallocate funds from other planned uses and may have a significant negative effect on our business plan and operations, including our ability to implement the planned expansion of our Spravato® and Medication Management programs or continue current operations. If our stock price declines, we can offer no assurance that we will be able to raise additional capital on acceptable terms or generate funds from operations sufficient to meet our obligations. If we are unable to raise sufficient capital in the future, we may not have the resources to continue our normal operations.
Benjamin Klein, Madryn, and Greybrook Health continue to have significant influence over us, including control over decisions that require the approval of shareholders, which could limit your ability to influence the outcome of matters submitted to shareholders for a vote.
As of April 8, 2024, Benjamin Klein, a seller of Success TMS, which we acquired in July 2022 beneficially owns, controls or directs, directly and indirectly, approximately 19.1% of our issued and outstanding Common Shares. Madryn, our largest lender under the Credit Agreement, beneficially owns directly and indirectly, approximately 63.4% of the issued and outstanding Common Shares. Greybrook Health beneficially owns, controls or directs, directly and indirectly, approximately 54.4% of the issued and outstanding Common Shares. As long as Mr. Klein, Madryn, and Greybrook Health own or control a significant number of the outstanding Common Shares, they will have the ability to exercise substantial control over all corporate actions requiring shareholder approval, irrespective of how our other shareholders may vote, including the election and removal of directors and the size of our Board, any amendments to our articles of incorporation (“Articles”), or the approval of any merger, acquisition or other significant corporate transaction, including a sale of all or substantially all of our assets.
In addition to Mr. Klein’s large share ownership position, he also has a board nomination right as set forth in the Klein Investor Rights Agreement. This right allows him to nominate one qualified nominee to represent Mr. Klein’s interest on the Board. As of the date of this Annual Report Mr. Klein’s board nomination right has not been elected for 2024. Greybrook Health also have a board nomination right so long as they retain an ownership position of 5% or greater in the Company pursuant to the investor rights agreement, dated June 14, 2021, by and among Greybrook Health and certain other investors (the “2021 Investor Rights Agreement”).
Future sales of our securities by existing shareholders or by us could cause the market price for the Common Shares to decline.
Sales of a substantial number of the Common Shares in the public market could occur at any time, including by any of the Company’s major shareholders, directors or officers. These sales, or the market perception that the holders of a large number of Common Shares intend to sell their Common Shares, could significantly reduce the market price of the Common Shares. We cannot predict the effect, if any, that future public sales of Common Shares will have on the market price of the Common Shares. If the market price of the Common Shares was to drop as a result, this might impede our ability to raise additional capital and might cause remaining shareholders to lose all or part of their investment.
The Company has filed a prospectus supplement under its Form F-3 to register the 2,353,347 Common Shares that the Company sold to certain investors in a private placement that took place in June 2021 (the “June 2021 Private Placement”). This prospectus supplement was filed in connection with the resale registration rights agreement dated June 14, 2021 (“June 2021 RRA”), among the Company and the participants in the June 2021 Private Placement. The June 2021 RRA requires the Company to maintain an effective registration statement which could force the Company to file a registration statement following the filing of this Annual Report. The Company’s filing of a registration statement may trigger “piggyback registration rights” for other holders of non-registered Common Shares and could lead to further sales of the Common Shares in the public market.
- 48 -
Additionally, the Common Shares issuable upon the exercise of outstanding options, warrants, convertible notes, and conversion instruments will, to the extent permitted by any applicable vesting requirements, lock-up restrictions, and restrictions under applicable securities laws, also become eligible for sale in the public market. We are also obligated to register the resale of (1) up to 11,634,660 Common Shares that were issued to the Success TMS sellers (including Mr. Klein) in July 2022, which includes the 2,908,665 Escrowed Shares, to be released to Mr. Klein or the Company, as applicable, upon satisfaction of customary working capital and certain other adjustments, including to satisfy any indemnity claims against the Success TMS sellers; (2) up to 3,910,604 Common Shares issuable upon conversion of the Madryn Conversion Instruments; (3) up to 11,363,635 Common Shares that were issued in connection with the non-brokered private placement completed in March 2023; and (4) up to 200,000,000 Common Shares which represent the maximum conversion amount available under the Subordinated Convertible Notes, in each case, once the conditions for satisfying such registration rights are satisfied. We cannot predict the size of future sales of any such Common Shares or the effect, if any, that future sales of any such Common Shares will have on the market price of the Common Shares.
Future offerings of debt securities, which would rank senior to the Common Shares upon our bankruptcy or liquidation, and future offerings of equity securities that may be senior to the Common Shares for the purposes of dividend and liquidating distributions, may adversely affect the market price of the Common Shares.
In the future, we may attempt to increase our capital resources by making offerings of debt securities or additional offerings of equity securities. Upon bankruptcy or liquidation, holders of our debt securities and lenders with respect to other borrowings will receive a distribution of our available assets prior to the holders of Common Shares. Any offerings of convertible securities or additional equity offerings may dilute the holdings of our existing shareholders or reduce the market price of the Common Shares, or both. Our decision to issue securities in any future offering will depend on market conditions and other factors beyond our control. As a result, we cannot predict or estimate the amount, timing or nature of our future offerings, and holders of Common Shares bear the risk of our future offerings reducing the market price of the Common Shares and diluting their ownership interest in the Company.
Any issuance of preferred shares could make it difficult for another company to acquire us or could otherwise adversely affect holders of the Common Shares, which could depress the price of the Common Shares.
Our Board has the authority to issue preferred shares and to determine the preferences, limitations and relative rights of preferred shares and to fix the number of shares constituting any series and the designation of such series, without any further vote or action by our shareholders. Our preferred shares may be issued with liquidation, dividend and other rights superior to the rights of the Common Shares. The potential issuance of preferred shares may delay or prevent a change in control of us, discourage bids for the Common Shares at a premium over the market price and adversely affect the market price and other rights of the holders of Common Shares.
We incur significantly increased costs and devote substantial management time as a result of operating as a U.S. public company.
As a U.S. public company, we incur significant legal, accounting and other expenses that we did not incur as a private company or as a Canadian public company. For example, we are subject to the reporting requirements of the U.S. Exchange Act, and are required to comply with the applicable requirements of Sarbanes-Oxley and the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules and regulations subsequently implemented by the SEC and including the establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Compliance with these requirements has increased our legal and financial compliance costs and make some activities more time consuming and costly. In addition, we expect that management and other personnel will need to divert attention from operational and other business matters to devote substantial time to these public company requirements. In particular, when the Company loses “emerging growth company” status, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404, which involve annual assessments of a company’s internal controls over financial reporting. We may need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge and may need to establish an internal audit function. We cannot predict or estimate the amount of additional costs we may incur as a result of Section 404 compliance or the timing of such costs.
- 49 -
We do not expect to pay any cash dividends for the foreseeable future.
We currently expect to retain all available funds and future earnings, if any, for use in the operation and growth of our business and do not anticipate paying any cash dividends for the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board, subject to compliance with applicable law and any contractual provisions, including under any existing or future agreements for indebtedness we may incur, that restrict or limit our ability to pay dividends, and will depend upon, among other factors, our results of operations, financial condition, earnings, capital requirements and other factors that our Board deems relevant. Accordingly, realization of a gain on your investment will depend on the appreciation of the price of the Common Shares, which may never occur. Investors seeking cash dividends in the foreseeable future should not invest in Common Shares.
General Risk Factors
Our business is labor intensive and could be adversely affected if we are unable to maintain satisfactory relations with our employees or the occurrence of union attempts to organize our employees.
Our business is labor intensive, and our results are subject to variations in labor-related costs, productivity and the number of pending or potential claims against us related to labor and employment practices. If political efforts at the national and local level result in actions or proposals that increase the likelihood of increased labor costs, or if labor and employment claims, including the filing of class action lawsuits, or work stoppages, trend upwards, our operating costs could correspondingly increase and our employee relations, productivity, earnings and cash flows could be adversely affected.
None of our employees are currently subject to collective bargaining agreements. As we continue to grow and enter different management regions, unions may attempt to organize all or part of our employee base at certain Treatment Centers or within certain management regions. Responding to such organization attempts may distract management and employees and may have a negative financial impact on individual locations, or on our business as a whole.
The maintenance of a productive and efficient labor environment and, in the event of unionization of these employees, the successful negotiation of a collective bargaining agreement, cannot be assured. Protracted and extensive work stoppages or labor disruptions, such as strikes or lockouts, could have a material adverse effect on our business, financial condition and results of operations.
A significant portion of our employees are subject to federal or state laws governing such matters as minimum wage, working conditions and overtime. Changes in these laws in the markets in which we operate, particularly increases to minimum wage, could cause our operating expenses to increase. A significant increase in labor costs could have an adverse effect on our business, financial condition and results of operations.
We are subject to federal and state laws that govern our employment practices, including minimum wage and overtime payment. Failure to comply with labor and employment laws and regulations could subject us to legal liability and costs, including fines or penalties, as well as reputational damage that could harm our business.
We are subject to federal, state and local laws and regulations relating to the terms of employment, hiring, hours worked, wage and hour requirements, compensation, termination and treatment of employees. These laws and regulations cover financial compensation (including wage and hour standards), benefits (including insurance and 401(k) plans), discrimination, workplace safety and health, benefits, and workers’ compensation. For example, the Fair Labor Standards Act establishes a national minimum wage and guarantees overtime paid at “time-and-a-half” for employees in certain jobs. These laws can vary significantly among states, can be highly technical and costs and expenses related to these requirements may represent a significant operating expense that we may not be able to offset.
Any failure to comply with these laws, including even a minor infraction, could expose us to civil and, in some cases, criminal liability, including fines and penalties. Further, government or employee claims that we have violated any of these laws, even if ultimately disproven, could result in increased expense and management distraction, as well as have an adverse reputational impact on us and have a material adverse effect on our business.
- 50 -
A material disruption in or security breach affecting our information technology systems could significantly affect our business and lead to reduced sales, growth prospects and reputational damage.
The protection of patient, employee and company data is critical to us. We rely extensively on our computer systems to track treatment and patient data, manage our supply chain, record and process transactions, collect and summarize data and manage our business. While our systems are designed to operate without interruption, we may experience interruptions to the availability of our computer systems from time to time. The failure of our computer systems to operate effectively, keep pace with our growing capacity requirements, smoothly transition to upgraded or replacement systems or integrate with new systems could adversely affect our business. In addition, our computer systems face ongoing threats from damage or interruption from power outages, computer and telecommunications failures, computer viruses, cyber-attacks, phishing attacks, denial-of-service attacks, social engineering attacks, ransomware attacks, security breaches, catastrophic events such as fires, floods, earthquakes, tornadoes, hurricanes, acts of war or terrorism, public health emergencies and usage errors by our employees. If our computer systems are damaged or cease to function properly, we may have to make a significant investment to fix or replace them, and we may suffer loss of critical data, compromise to the integrity or confidentiality of patient and employee information in our systems or networks, disruption to the systems or networks of third parties on which we rely, and interruptions or delays in our operations. A lack of relevant and reliable information that enables management to effectively manage our business could preclude us from optimizing our overall performance. Any actual or potential theft, loss, corruption, exposure, fraudulent, unauthorized or accidental use or misuse of data, whether by third parties or as a result of employee malfeasance or otherwise, non-compliance with our contractual or legal obligations regarding such data could have a material adverse effect on our business and results of operations, including significant remediation and other costs, fines, litigation and regulatory actions against us by governments, various regulatory organizations or exchanges, or affected individuals, in addition to significant reputational harm, decreased patient confidence and/or financial loss, and it may not be possible to recover losses suffered from such incidents under our insurance policies.
Experienced computer programmers and hackers, or even internal users, may be able to penetrate or create system disruptions or cause shutdowns of our network security or that of third-party companies with which we have contracted to provide services. We generally collect and store confidential medical information regarding our patients and any compromise of such information could subject us to patient or government litigation, which would harm our reputation and could adversely affect our business and growth. Moreover, we could incur significant expenses or disruptions of our operations in connection with system failures or data breaches. An increasing number of websites, including several large internet companies, have recently disclosed breaches of their security, some of which have involved sophisticated and highly targeted attacks on portions of their sites. Because the techniques used to obtain unauthorized access, disable or degrade services or sabotage systems, change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures. In addition, sophisticated hardware and operating system software and applications that we buy or license from third parties may contain defects in design or manufacture, including “bugs” and other problems that could unexpectedly interfere with the security and operation of the systems. The costs to us to eliminate or alleviate security problems, viruses and bugs could be significant, and efforts to address these problems could result in interruptions, delays or cessation of service that may impede our revenues or other critical functions.
In addition, many jurisdictions in which we operate have adopted breach of privacy and data security laws or regulations that require notification to consumers if the security of their personal information is breached, among other requirements. Governmental focus on data security may lead to additional legislative action, and the increased emphasis on information security may lead patients to request that we take additional measures to enhance security or restrict the manner in which we collect and use patient information. As a result, we may have to modify our business systems and practices with the goal of further improving data security, which would result in increased expenditures and operating complexity. Any compromise of our security or accidental loss or theft of patient data in our possession could result in a violation of applicable privacy and other laws, significant legal and financial exposure and damage to our reputation, which could adversely impact our business, results of operations and the price of our securities, including the Common Shares.
Recently, data security breaches suffered by well-known companies and institutions have attracted a substantial amount of media attention, prompting new foreign, federal and state laws and legislative proposals addressing data privacy and security, as well as increased data protection obligations imposed on merchants by credit card issuers. As a result, we may become subject to more extensive requirements to protect the patient information that we process in connection with the payment for Treatment, resulting in increased compliance costs.
- 51 -
In addition, a portion of our corporate and head office operational functions are carried out by employees working from home or otherwise remotely. Remote working by employees, increased use of Wi-Fi and use of office equipment off-premises has become necessary for the foreseeable future and may make our business more vulnerable to cybersecurity breach attempts, create data accessibility concerns, and make us more susceptible to communication disruptions, any of which could have a material adverse effect on our business and results of operations. There is no guarantee that our disaster recovery procedures will be adequate in these circumstances. Remote work may also result in increases in phishing and social engineering attacks.
Many of our business functions are centralized at our head office locations. Disruptions to the operations at these locations could have an adverse effect on our business.
Our head office is located in Toronto, Ontario, and our U.S. corporate headquarters is located in Tysons Corner, Virginia. We have centralized a large number of business functions at these locations, including Treatment Center design, patient support, marketing and management. Most of our senior management, our primary data center and critical resources dedicated to financial and administrative functions, are located at our head office and U.S. corporate headquarters. If we were required to shut down either one of these sites for any reason, including fire, earthquake or other natural disaster, civil disruption, or pandemic, our management and our operations staff would need to find an alternative location, causing significant disruption and expense to our business and operations.
A number of our employees, including those working from our head office and U.S. corporate headquarters, work remotely. Employing a remote work environment could affect employee productivity, including due to a lower level of employee oversight, health conditions or illnesses, disruptions due to caregiving or childcare obligations or slower or unreliable internet access. If our productivity is impacted as a result of the transition, we may incur additional costs to address such issues and our financial condition and results may be adversely impacted.
We recognize the need to enhance our disaster recovery, business continuity and document retention plans that would allow us to be operational despite unforeseen events impacting our head office or U.S. corporate headquarters and intend to do so in the future. Without disaster recovery, business continuity and document retention plans, if we encounter difficulties or disasters at our head office or corporate headquarters, our critical systems, operations and information may not be restored in a timely manner, or at all, and may adversely impact our business operations.
We are a Canadian company and shareholder protections differ from shareholder protections in the United States and elsewhere.
We are organized under the laws of Ontario, Canada and, accordingly, are governed by the OBCA. The OBCA differs in certain material respects from laws generally applicable to United States corporations and shareholders, including the provisions relating to interested directors, mergers and similar arrangements, takeovers, shareholders’ suits, indemnification of directors and inspection of corporation records.
Our financial statements are prepared according to U.S. GAAP and are no longer prepared under IFRS, and any changes in accounting standards and subjective assumptions, estimates and judgments by management related to complex accounting matters as a result of application of US GAAP instead of IFRS could significantly affect our reported financial results or financial condition.
Historical filings of our consolidated interim period and full year financial statements were previously prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”) and may not be comparable to financial statements prepared according to U.S. GAAP. Although generally similar in principle, U.S. GAAP includes specific disclosure requirements that are not explicitly required in IFRS. Therefore, disclosures provided under IFRS and U.S. GAAP may differ depending on the nature of the risks and uncertainties associated with the underlying transaction.
- 52 -
In addition, U.S. GAAP and related accounting pronouncements, implementation guidelines and interpretations are highly complex and involve many subjective assumptions, estimates and judgments, including with regard to a wide range of matters that are relevant to our business, including revenue recognition, business combinations, impairment of goodwill and intangible assets, right-of-use assets, prepaid expenses and other assets, income taxes and litigation. Changes in these rules or their interpretation or changes in underlying assumptions, estimates or judgments could significantly change our reported financial performance or financial condition in accordance with generally accepted accounting principles.
Our by-laws designate the Superior Court of Justice of the Province of Ontario, Canada, as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our shareholders, which could limit our shareholders’ ability to choose a favorable judicial forum for disputes with us or our directors or officers under U.S. securities laws.
Article 12 of our by-laws provides that, subject to our consent in writing to the selection of an alternative forum, the Superior Court of Justice of the Province of Ontario, Canada shall, to the fullest extent permitted by law, be the sole and exclusive forum for (i) any derivative action or proceeding brought on behalf of the Company; (ii) any action or proceeding asserting a claim of a fiduciary duty owed by any director, officer or other employee of the Company to the Company; (iii) any action or proceeding asserting a claim arising pursuant to any provision of the OBCA or the Articles or the by-laws of the Company (as either may be amended from time to time); or (iv) any action or proceeding asserting a claim otherwise related to the “affairs”, as defined in the OBCA, of the Company. Under the terms of our by-laws, any investor purchasing any interest in our Common Shares shall be deemed to have notice of and consented to the foregoing forum selection provisions.
The foregoing forum selection provisions would apply to all actions described above, which may include actions that arise under the U.S. Securities Act or the U.S. Exchange Act. However, Section 27 of the U.S. Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the U.S. Exchange Act or the rules and regulations thereunder, and Section 22 of the U.S. Securities Act provides for concurrent U.S. federal and state court jurisdictions over actions under the U.S. Securities Act and the rules and regulations thereunder, subject to a limited exception for certain “covered class actions” as defined in Section 16 of the U.S. Securities Act and interpreted by U.S. courts. Accordingly, there is uncertainty whether a U.S. court would enforce our forum selection clause in a lawsuit that alleges violation of the U.S. Exchange Act and/or the U.S. Securities Act, and investors cannot waive compliance with U.S. federal securities laws and the rules and regulations thereunder.
As a result of the foregoing uncertainty, the forum selection provision in our by-laws may result in increased costs to a shareholder that seeks to bring a claim under the U.S. Exchange Act and/or the U.S. Securities Act and otherwise limit any such shareholder’s ability to bring such a claim in a judicial forum that it finds favorable for disputes with us or our directors or officers, which may discourage such lawsuits against us and our directors or officers under U.S. securities laws.
Certain adverse tax consequences may result from the treatment of the Company as a U.S. domestic corporation for U.S. federal income tax purposes.
Although the Company is organized as a corporation under the OBCA, the Company takes the position that it is treated as a U.S. domestic corporation for all U.S. federal income tax purposes under Section 7874 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”). As a result, the Company generally is subject to tax on its worldwide income by both Canada and the United States. This treatment is expected to continue indefinitely, which could have a material adverse effect on our financial condition and results of operations.
We do not currently anticipate paying dividends on the Common Shares. If we pay dividends on the Common Shares, any dividends received by shareholders that are not residents of Canada for purposes of the Income Tax Act (Canada) (the “Tax Act”) generally will be subject to Canadian withholding tax at the rate of 25%, except as reduced by an applicable income tax treaty. A shareholder that is a U.S. taxpayer may not be permitted to claim a U.S. foreign tax credit for any such Canadian withholding tax, unless the shareholder has sufficient non-U.S.-source income from other sources and certain other conditions are met.
Dividends received by non-U.S. shareholders generally will be subject to U.S. federal withholding tax at the rate of 30%, except as reduced by an applicable income tax treaty. Shareholders that are residents of Canada for purposes of the Tax Act will not be permitted to claim a Canadian foreign tax credit for any such U.S. withholding tax. A shareholder who is neither a U.S. taxpayer nor a resident of Canada for purposes of the Tax Act generally will be subject to both U.S. withholding tax and Canadian withholding tax. Shareholders subject to both U.S. and Canadian withholding tax are urged to consult their own tax advisers regarding the availability of reduced withholding under an applicable income tax treaty.
- 53 -
Prospective investors are urged to consult their own tax advisers regarding the U.S. tax treatment of the Company and the tax consequences of owning Common Shares in light of their particular circumstances.
Non-U.S. shareholders could be subject to materially adverse U.S. federal income tax consequences if the Company is classified as a United States real property holding corporation.
Generally, a corporation is a “United States real property holding corporation” for U.S. federal income tax purposes if the fair market value of its “United States real property interests” equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. The determination of whether a corporation is a United States real property holding corporation is made from time to time and depends on the relative fair market values of its assets. The Company has made no determination as to whether it is currently treated as a United States real property holding corporation. If the Company is a United States real property holding corporation, then any gain realized by a non-U.S. shareholder upon the sale or other disposition of the Common Shares (regardless of the percentage of Common Shares owned) generally would be taxed on a net income basis at the graduated rates applicable to U.S. persons, by reason of the treatment of such Common Shares as United States real property interests for U.S. federal income tax purposes. In addition, the non-U.S. shareholder generally would be required to file a U.S. federal income tax return for the taxable year in which such gain were recognized. Moreover, a purchaser of Common Shares from a non-U.S. shareholder generally would be required to withhold and remit to the Internal Revenue Service an amount equal to 15% of the amount realized (generally, the purchase price paid to the non-U.S. shareholder) upon the disposition.
Prior to our expected delisting from Nasdaq, non-U.S. shareholders may have qualified for an exception from the adverse U.S. federal income tax consequences described in the preceding paragraph, although no assurance can be provided in this regard. Under the exception, a United States real property interest does not include stock of a U.S. domestic corporation held by a non-U.S. person, provided that at any time during the shorter of the five-year period preceding the disposition or the non-U.S. person’s holding period for the stock (i) the non-U.S. person beneficially owns, directly, indirectly, and constructively, 5% or less of such stock, and (ii) such class of stock is treated as “regularly traded on an established securities market” (as provided in applicable Treasury Regulations). Based on our expected delisting from Nasdaq, our Common Shares may fail to qualify as regularly traded on an established securities market within the meaning of the foregoing exception, and therefore the exception may not be available to non-U.S. shareholders. Non-U.S. shareholder are urged to consult their tax advisers regarding the possible adverse U.S. federal income tax consequences to them of the treatment of the Company as a United States real property holding corporation.
Macroeconomic conditions may adversely affect our business, including inflation and potential recessionary conditions.
Demand for our services may be impacted by weak economic conditions, unfavorable interest rates, unfavorable foreign exchange rates, inflation, stagflation, recession, equity market volatility, or other negative economic factors in the United States. Inflation has the potential to adversely affect our liquidity, business, financial condition and results of operations by increasing our overall cost structure. The existence of inflation in the economy has resulted in, and may continue to result in, higher interest rates and capital costs, supply shortages, increased costs of labor, components, manufacturing and shipping, as well as weakening exchange rates and other similar effects. Accordingly, inflation may have a negative impact on our future results of operations, which may be materially adverse. Further, as recessionary conditions develop, our suppliers and other third-party partners may suffer their own financial and economic challenges and as a result they may demand pricing accommodations, delay payment, or become insolvent, which could harm our ability to meet our patients’ demands or collect revenue or otherwise could harm our business. Similarly, disruptions in financial and/or credit markets may impact our ability to manage normal commercial relationships with our patients, suppliers and creditors and might cause us to not be able to continue to access preferred sources of liquidity when we would like, and our borrowing costs could increase. These adverse macroeconomic conditions may also negatively impact patient spending ability, which in turn may negatively impact our revenues. Thus, if general macroeconomic conditions deteriorate, our business and financial results could be materially and adversely affected.
- 54 -
Public health crises and a future outbreak of a highly infectious or contagious disease, pandemic or epidemic, could have an adverse effect on our business and future growth opportunities.
The COVID-19 pandemic had an adverse impact on our business and financial performance, including a decline in patient visits/treatments and new patient treatments, as well as disruptions or delays to our supply chain. In the future, any local, regional, national or international outbreak of a contagious disease, including, but not limited to, the emergence of new COVID-19 variants, Middle East Respiratory Syndrome, Severe Acute Respiratory Syndrome, H1N1 influenza virus, avian flu or any other similar illness, or a fear of any of the foregoing, could adversely impact us by causing operating delays and supply chain disruptions, including the availability of key TMS Device components, Spravato® and other critical supplies, labor shortages and shutdowns (including as a result of government regulation and prevention measures). It is unknown how we may be affected if such an epidemic persists for an extended period of time, and we may not be able to mitigate the impacts of such an event. A widespread health crisis could adversely affect the global economy, resulting in an economic downturn that could impact demand for the services we provide.
We are subject to insurance-related risks.
We maintain director and officer insurance, liability insurance, business interruption and property insurance and our insurance coverage includes deductibles, self-insured retentions, limits of liability and similar provisions. There is no guarantee, however, that our insurance coverage will be sufficient, or that insurance proceeds will be paid to us on a timely basis. In addition, there are types of losses we may incur but against which we cannot be insured or which we believe are not economically reasonable to insure, such as losses due to acts of war or certain natural disasters. If we incur these losses and they are material, our business, operating results and financial condition may be adversely affected. Also, certain material events may result in sizable losses for the insurance industry and materially adversely impact the availability of adequate insurance coverage or result in significant premium increases. Accordingly, we may elect to self-insure, accept higher deductibles, or reduce the amount of coverage in response to such market changes.
Natural disasters and unusual weather could adversely affect our operations and financial results.
Extreme weather conditions, as a result of climate change or otherwise, in the areas in which our Treatment Centers are located, could adversely affect our business. For example, frequent or unusually heavy snowfall, ice storms, rainstorms or other extreme weather conditions over a prolonged period could make it difficult for our patients to travel to our Treatment Centers and thereby reduce the number of treatments we provide. Reduced treatments from extreme or prolonged unseasonable weather conditions could adversely affect our business.
In addition, natural disasters such as hurricanes, tornadoes and earthquakes, or a combination of these or other factors, could severely damage or destroy one or more of our Treatment Centers located in the affected areas, thereby disrupting our business operations.
Furthermore, a significant portion of our business functions operate out of our head office in Toronto, Ontario, and our U.S. corporate headquarters in Tysons Corner, Virginia. As a result, our business is also vulnerable to disruptions due to local weather, economics and other factors in these regions.
We may upgrade or replace certain core information technology systems which could disrupt our operations and adversely affect our financial results.
The implementation of new information technology systems may cause delays or disruptions or may be used improperly, either of which might negatively impact our business, prospects, financial condition and results of operations.
The risks associated with information technology systems changes, as well as any failure of such systems to operate effectively, could adversely impact human capital management and the promptness and accuracy of our transaction processing and financial accounting and reporting capabilities. Internal controls over financial reporting, the efficiency of our operations and our ability to properly forecast earnings and cash requirements may be adversely affected, and we may be required to make significant additional capital expenditures to remediate any such failures or problems.
- 55 -
We believe that other companies have experienced significant delays and cost overruns in implementing similar system changes, and we may encounter problems as well. Our planned investments in maintenance capital expenditures and infrastructure are forward-looking information and are based on opinions, estimates and assumptions that may prove incorrect. Additionally, unforeseen costs in developing infrastructure and other information technology improvements may adversely impact our business operations. We may not be able to successfully implement these new systems or, if implemented, we may still face unexpected disruptions in the future. Any resulting delays or disruptions could harm our business, prospects, financial condition and results of operations.
If securities or industry analysts cease to publish research or publish inaccurate or unfavorable research about us or our business, our trading price and our trading volume could decline.
The trading market for the Common Shares depends in part on the research and reports that industry or securities analysts publish about us or our business. If we obtain industry or securities analyst coverage and if one or more of the analysts who cover us downgrade the Common Shares, the trading price of the Common Shares may decline. If one or more of the analysts cease coverage of our Company or fails to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause the Common Share price or trading volume to decline. Moreover, if our results of operations do not meet the expectations of the investor community, or one or more of the analysts who cover our Company publishes inaccurate or unfavorable research about our business, the trading price of the Common Shares may decline.
ITEM 1B.UNRESOLVED STAFF COMMENTS
None.
ITEM 1C.CYBERSECURITY
Risk management and strategy
Cybersecurity and information privacy are important parts of our overall approach to risk management. We have documented cybersecurity policies and procedures to identify, assess and manage risks from cybersecurity threats. We believe our cybersecurity policies and procedures are reasonably designed to materially protect the security of both our data and the data in our custody. To protect our information systems, we employ various security tools supporting protection, detection and response capabilities, including anti-malware and security applications, and periodic risk analyses. We maintain a cybersecurity incident response plan to help ensure a timely, consistent response to actual or attempted cybersecurity incidents impacting the Company.
We may engage third parties from time to time, including consultants and auditors to conduct assessments of information systems. We have policies and processes in place to govern third-party access and the risks associated with such access.
Our systems face cybersecurity risks, and we have in the past experienced threats to and breaches of our data and systems. However, to date, these incidents have not had a material impact on our business, results of operations or financial conditions. There can be no assurance that we will not experience any material cybersecurity threats or incidents in the future. See Item 1A “Risk Factors—A material disruption in or security breach affecting our information technology systems could significantly affect our business and lead to reduced sales, growth prospects and reputational damage”.
Governance
Cybersecurity at our Company is led by our Chief Information Security Officer (“CISO”) and our Compliance and Privacy Officer and overseen by our Board. One of the key functions of our Board is informed oversight of our risk management process, including risks from cybersecurity threats. Our CISO and our Compliance and Privacy Officer provide periodic reports to the Board regarding our cybersecurity risks and activities, including any recent cybersecurity incidents and related responses, cybersecurity systems testing and activities of third parties. The current CISO has over twenty years of experience in information technology and security, and his background includes technical experience, strategy and architecture focused roles, cyber and threat experience, and various leadership roles.
- 56 -
ITEM 2.PROPERTIES
We do not own any real estate. Instead, we lease all of our retail Treatment Center locations, as well as our head office and U.S. corporate headquarters. Our existing Treatment Centers are leased from third parties, with typical lease commitments ranging from “month-to-month” to seven years. The entirety of the Company’s revenue is generated through treatment delivered at the Treatment Centers.
As at December 31, 2023, our Treatment Center network consisted of 130 Treatment Center locations spanning 17 management regions in the Commonwealths of Massachusetts, Virginia and Pennsylvania and the States of Maryland, North Carolina, Missouri, Illinois, Ohio, Texas, Connecticut, Florida, South Carolina, Michigan, Alaska, Oregon, California, New Jersey, and Nevada.
Our head and registered office is located at 890 Yonge Street, 7th Floor, Toronto, Ontario, Canada, M4W 3P4 and our telephone number is 416-915-9100. Our United States corporate headquarters is located at 8401 Greensboro Drive, Suite 425, Tysons Corner, Virginia, United States, 22102. We have designated TMS US as our agent for service of process in the United States and its address is 8401 Greensboro Drive, Suite 425, Tysons Corner, Virginia, USA, 22102.
ITEM 3.LEGAL PROCEEDINGS
Klein Note Action
As previously disclosed, on July 14, 2022, we, through our wholly-owned U.S. subsidiary, TMS US, completed the Success TMS Acquisition pursuant to the Success Purchase Agreement by and among the Company, Success TMS and its direct and the Seller Parties. “Item 1 Business—Recent Developments in our Business—Acquisition of Success TMS”.
In addition, on July 14, 2022, in connection with the Success TMS Acquisition, we assumed the obligation of Success TMS to repay a promissory note totaling $2.1 million to a lender associated with Benjamin Klein, who is a significant shareholder and former director of the Company (the “Klein Note”). Mr. Klein was also the Chief Operating Officer of Greenbrook from July 2022 until his termination by the Company effective May 4, 2023.
On April 25, 2023, Batya Klein, as trustee of the Marital Trust created by Kenneth S. Klein Revocable Trust U/A/D 10/20/80 (the “Klein Plaintiff”), filed a complaint against Success TMS in the Superior Court of New Jersey, Law Division (Bergen County) alleging a single claim for breach of contract of the Klein Note (the “Klein Note Action”). Specifically, the complaint alleged that there was an event of default under the Klein Note and demanded acceleration of the indebtedness due thereunder. The Company moved to dismiss the Klein Note Action on the basis that there was no event of default and the demand for acceleration was defective, and that the New Jersey court lacked jurisdiction to hear the matter. On August 18, 2023, the New Jersey court denied the motion to dismiss, ruling that it had jurisdiction to hear the matter and that, assuming the truth of the allegations in the complaint, the Klein Plaintiff had the right to seek legal remedy for the alleged default.
On November 21, 2023, the Company announced it entered into the Klein Settlement Agreement. Under the terms of the Klein Settlement Agreement, the Company agreed to make payments to the Klein Plaintiff in the total amount of approximately $2.2 million, structured as an initial immediate payment of $250,000, weekly payments of $75,000 thereafter up to and until the May 1, 2024 maturity date of the Klein Note, upon which the balance owing will be due. In exchange for entry into the Klein Settlement Agreement, the Klein Plaintiff dismissed, with prejudice the Klein Note Action on November 27, 2023, and both parties provided a mutual release of claims.
Purchase Agreement Claims
As previously disclosed, on May 24, 2023, the Seller Parties filed a complaint in the Superior Court of the State of Delaware against the Company, TMS US and certain executive officers of the Company, and subsequently filed the Delaware Complaint on August 31, 2023, concerning the Purchase Agreement Claims. The Purchase Agreement Claims allege contractual fraud, indemnification for breach of certain representations and warranties of the Company contained in the Success Purchase Agreement, other breaches of the Success Purchase Agreement and a registration rights agreement, and breach of the implied covenant of good faith and fair dealing. The Delaware Complaint seeks damages in an amount to be determined at trial, which are alleged to exceed $1 million. On October 2, 2023, the Company and the other defendants moved to dismiss the Purchase Agreement Claims.
On November 20, 2023, the court stayed the Purchase Agreement Claims until May 13, 2024.
- 57 -
One of the claims at issue in the Purchase Agreement Claims includes alleged breach of a Membership Interest Purchase Agreement entered into between the Company and the Seller Parties for failure by the Company to release the Escrowed Shares. Although we do not believe Mr. Klein is currently entitled to the release of any of the Escrowed Shares, Mr. Klein has disputed this and we may be required to release some or all of the Escrowed Shares, or otherwise settle the dispute, in the future.
Additional Legal Proceedings
In the normal course of business, the Company may become a defendant in certain employment claims and other litigation. The Company records a liability when it is probable that a loss has been incurred and the amount is reasonably estimable. The Company is not involved in any legal proceedings other than such matters described above and routine litigation arising in the normal course of business, which the Company does not believe will have a material adverse effect on the Company’s business, financial condition or results of operations.
ITEM 4.MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5.MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our Common Shares are currently quoted on OTCQB Market under the symbol “GBNHF”.
Holders of Record
As of March 29, 2024, there were approximately 59 shareholders of record of our Common Shares, which included Cede & Co., a nominee for Depository Trust Company, and CDS & Co., a nominee for The Canadian Depository for Securities Ltd. Common Shares that are held by financial institutions as nominees for beneficial owners are deposited into participant accounts at either Depository Trust Company or The Canadian Depository for Securities Ltd and are considered to be held of record by Cede & Co. or CDS & Co., each such depository representing one shareholder of record.
Dividend Policy
We have not paid any cash dividends or distributions on any class of our securities, and we have no current plans to pay dividends.
Exchange and Foreign Ownership Controls
We are not aware of any Canadian federal or provincial laws, decrees, or regulations that restrict the export or import of capital, including foreign exchange controls, or that affect the remittance of dividends, interest, or other payments to non-Canadian holders of the Common Shares. There are no limitations under the laws of Canada or by the charter or our other constituent documents on ownership of our voting shares by non-Canadians, except the Investment Canada Act which may require review and approval by the Minister of Innovation (Canada) of certain acquisitions of control of us by non-Canadians. The threshold for acquisitions of control is generally defined as being one-third or more of our voting shares, provided certain financial thresholds are also exceeded. If the investment is potentially injurious to national security, it may be subject to review under the Investment Canada Act notwithstanding the percentage interest acquired or amount of the investment. “Non-Canadian” generally means an individual who is not a Canadian citizen, or a corporation, partnership, trust, or joint venture that is ultimately controlled by non-Canadians.
- 58 -
Certain Canadian Federal Income Tax Considerations for U.S. Residents
The following general summary fairly describes the principal Canadian federal income tax consequences applicable to a holder who is a beneficial owner of our Common Shares and who, at all relevant times, (i) is not, and is not deemed to be, resident in Canada for purposes of the Tax Act and any applicable income tax treaty or convention, (ii) is resident in the United States for purposes of the Treaty (as defined below) and is fully entitled to all benefits of the Treaty, (iii) deals at arm’s length and is not affiliated with us for purposes of the Tax Act, and (iv) holds our Common Shares as capital property for purposes of the Tax Act and does not use or hold, and is not deemed to use or hold, our Common Shares in the course of carrying on, or otherwise in connection with, a business in Canada (a “U.S. Resident Holder”). Special rules, which are not discussed in this summary, may apply to a U.S. Resident Holder that is an insurer carrying on an insurance business in Canada and elsewhere or an “authorized foreign bank” (as defined in the Tax Act).
This summary is based upon the current provisions of the Tax Act, the regulations thereunder (the “Regulations”), the current publicly announced administrative policies and assessing practices of the Canada Revenue Agency and the Canada-United States Tax Convention as amended by the Protocols thereto (the “Treaty”). This summary also takes into account the amendments to Tax Act and the Regulations publicly announced by the Minister of Finance (Canada) prior to the date hereof (the “Tax Proposals”) and assumes that all such Tax Proposals will be enacted in their present form. However, no assurances can be given that the Tax Proposals will be enacted in the form proposed, or at all. This summary is not exhaustive of all possible Canadian federal income tax consequences applicable to a holder of our Common Shares and, except for the foregoing, this summary does not take into account or anticipate any changes in law, whether by legislative, administrative or judicial decision or action, nor does it take into account provincial, territorial or foreign income tax legislation or considerations, which may differ from the Canadian federal income tax consequences described herein.
This summary is of a general nature only and is not intended to be, and should not be construed to be, legal, business or tax advice to any particular holder or prospective holder of our Common Shares, and no opinion or representation with respect to the tax consequences to any holder or prospective holder of our Common Shares is made. Accordingly, holders and prospective holders of our Common Shares should consult their own tax advisors with respect to the income tax consequences of purchasing, owning and disposing of our Common Shares in their particular circumstances.
Currency
For the purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of our Common Shares (including dividends, adjusted cost base and proceeds of disposition) must be expressed in Canadian dollars based on the rate quoted by the Bank of Canada for the applicable day or such other rate of exchange that is acceptable to the Canada Revenue Agency.
Dividends
Dividends paid or credited, or deemed to be paid or credited, on our Common Shares to a U.S. Resident Holder will be subject to Canadian withholding tax. Under the Tax Act, the rate of Canadian withholding tax is 25% of the gross amount of such dividends, which rate is subject to reduction under the Treaty. Under the Treaty, the rate of Canadian withholding tax on dividends paid or credited, or deemed to be paid or credited, on shares of a corporation resident in Canada (such as our Company) to a U.S. Resident Holder is reduced to 15% or, in the case of a U.S. Resident Holder that is a corporation that owns at least 10% of the voting shares of the corporation paying the dividend, the rate is reduced to 5%. U.S. Resident Holders who may be eligible for a reduced rate of withholding tax on dividends pursuant to the Treaty should consult with their own tax advisors with respect to taking all appropriate steps in this regard.
- 59 -
Disposition of Common Shares
A U.S. Resident Holder generally will not be subject to tax under the Tax Act in respect of a disposition or deemed disposition of a Common Share unless the Common Share constitutes “taxable Canadian property” (as defined in the Tax Act) of the U.S. Resident Holder at the time of disposition and the U.S. Resident Holder is not entitled to relief under the Treaty. Generally, a Common Share will not constitute “taxable Canadian property” of a U.S. Resident Holder at a particular time provided that the Common Share is listed on a “designated stock exchange” (as defined in the Tax Act, which currently includes the TSX and the Nasdaq) at that time, unless, at any time during the 60-month period that ends at that time, both: (i) one or any combination of (a) the U.S. Resident Holder, (b) persons with whom the U.S. Resident Holder does not deal at arm’s length for purposes of the Tax Act and (c) partnerships in which the U.S. Resident Holder or a person described in (b) holds a membership interest (directly or indirectly through one or more partnerships), own 25% or more of the issued shares of any class or series of our Company, and (ii) more than 50% of the fair market value of the Common Share was derived directly or indirectly from one or any combination of: (a) real or immovable property situated in Canada, (b) “timber resource properties” (as defined in the Tax Act), (c) ”Canadian resource properties” (as defined in the Tax Act) or (d) options in respect of, or interests in, or for civil law rights in, any of the foregoing, whether or not the property exists. If the Common Shares are not listed on any designated stock exchange, the Common Shares would generally be “taxable Canadian property” of a U.S. Resident Holder at any time if, at any particular time during the 60-month period that ends at that time, the condition described in (ii) above is met. Notwithstanding the foregoing, in certain circumstances set out in the Tax Act, a Common Share may be deemed to be “taxable Canadian property”.
In the case of a U.S. Resident Holder for whom our Common Shares constitute “taxable Canadian property”, no Canadian taxes will generally be payable on a capital gain realized on the disposition or deemed disposition of such shares by reason of the Treaty, unless the value of such shares is derived principally from “real property situated in Canada” for purposes of the Treaty at the time of the disposition. U.S. Resident Holders for whom Common Shares may constitute “taxable Canadian property” should consult their own tax advisor as to the Canadian federal income tax consequences of the disposition, including potential compliance requirements and withholding under section 116 of the Tax Act.
Securities Authorized for Issuance under Equity Compensation Plans
Information about our equity compensation plan is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Recent Sales of Unregistered Securities
For the three months ended December 31, 2023, the Company issued an aggregate amount of approximately $3.4 million aggregate principal amount of Subordinated Convertible Notes, see “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness and Capital Raising—Subordinated Convertible Notes”. The Subordinated Convertible Notes were issued to certain significant shareholders and accredited investors and in each case the Company relied upon Section 4(a)(2) of the Securities Act to complete the private placements. The proceeds of these sales have been used by the Company for general corporate and working capital purposes.
Repurchases of Equity Securities
The Company did not repurchase any of its Common Shares during the three months ended December 31, 2023.
ITEM 6.[RESERVED]
ITEM 7.MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following management’s discussion and analysis (“MD&A”) provides information concerning the financial condition and results of operations of the Company and should be read in conjunction with our audited consolidated financial statements as at and for the fiscal years ended December 31, 2023 and 2022, in each case, together with the notes thereto. The financial information contained in this MD&A is derived from the financial statements prepared in accordance with generally accepted accounting principles in the United States (“US GAAP”) as issued by the Financial Accounting Standards Board (“FASB”).
- 60 -
We have restated our previously issued consolidated financial statements for Fiscal 2022 contained in this Annual Report. In addition, we intend to restate the quarterly condensed interim consolidated financial statements for Fiscal 2023 and 2022. Refer to the “Explanatory Note” preceding Item 1, Business, for background on the restatement, the fiscal periods impacted, and other information.
Basis of Presentation
As of June 30, 2023, the Company lost its “foreign private issuer” status because a majority of the Common Shares were held in the United States and the Company does not meet the additional requirements under the “business contacts” test. As a result, beginning on January 1, 2024, the Company is required to follow SEC reporting standards of a U.S. domestic issuer, convert its financial statements to U.S. GAAP and will no longer be able to rely on foreign private issuer exemptions from U.S. proxy rules and Section 16 insider reporting.
Our audited consolidated financial statements have been prepared in accordance with US GAAP as a result of the above. Our fiscal year is the 12-month period ending December 31.
All references to “Fiscal 2024”,”Fiscal 2023”, and “Fiscal 2022” are to the fiscal years ending December 31, 2024, December 31, 2023 and December 31, 2022, respectively. All references to “Q4 2022” are to our fiscal quarter for the three-month period ended December 31, 2022.
Amounts stated in this MD&A are in United States dollars, unless otherwise indicated.
Key Highlights and Recent Developments
During Fiscal 2023, our commitment to the execution of the Restructuring Plan has established a path forward for the Company to achieve sustainable profitability and long-term growth. The reduction in headcount, rationalization of marketing spend, extinguishment of lease liabilities and a reduction of other recurring corporate, general and administrative expenses has effectively removed $23 million in annualized costs from the Company as compared to Q4 2022, achieving our targeted range of $22 to $25 million in cost reductions. We remain optimistic about our future as we execute on the remaining components of the Restructuring Plan in early Fiscal 2024.
Revenue in Fiscal 2023 increased by 10% to a record $73.8 million, as compared to Fiscal 2022 (Fiscal 2022: $66.8 million), despite the closure of 53 Treatment Centers in connection with the Restructuring Plan (approximately 29% of active Treatment Centers as at the end of Fiscal 2022). Furthermore, we believe Fiscal 2023 revenue proved resilient at approximately 90% of the annualized total quarterly revenue achieved in Q4 2022 (with Q4 2023 generating above 95% of Q4 2022 revenue), despite these closures and a significantly reduced marketing spend (marketing spend in Fiscal 2023 represented 12% of annualized marketing spend in Q4 2022).
Although the cost reductions associated with the Restructuring Plan have been substantially executed, we expect our new operating structure will continue to allow us to rationalize costs, while further reducing business complexity, streamline our operating model and drive operational efficiencies. We believe that these structural changes, paired with continued support from our significant shareholders, Madryn and Neuronetics, will improve our near-term cash flows and ultimately lead to profitability in the future. Treatment volumes in Fiscal 2023 were 343,790, a 10% increase compared to Fiscal 2022 (Fiscal 2022: 312,940), and new patient starts increased in Fiscal 2023 by 12% to 10,401, as compared to Fiscal 2022 (Fiscal 2022: 9,253), despite the closure of 53 Treatment Centers in connection with the Restructuring Plan as compared to Fiscal 2022.
We believe that mental health remains a key focus in the United States, and the unmet demand for Treatment remains at an all-time high, with our network of Treatment Centers well-positioned to serve this unmet demand. We believe our business fundamentals are stronger than ever with the growth of the Spravato® Program, the opportunity to increase marketing investment in our streamlined business, the introduction of Medication Management (see “Key Highlights and Recent Developments—Medication Management Program” below) and potential future treatment opportunities.
- 61 -
Restructuring Plan
As intended, the Restructuring Plan established a path forward for the Company to achieve sustainable profitability and long-term growth. As of the end of Fiscal 2023, we have realized approximately $23 million in annualized recurring cost reductions through a significant reduction in headcount, optimizing marketing spend through key learnings from our Q4 2022 marketing efforts as well as a reduction in recurring corporate, general and administrative expenses. As of the date of this Annual Report, the Restructuring Plan is substantially complete, but the Company expects to achieve additional operating expense savings from the final components of the Restructuring Plan in the first half of Fiscal 2024. We expect to be within the disclosed estimated range of restructuring and related charges of $1 million to $2 million upon completion of the Restructuring Plan, of which approximately $1.0 million were recorded in Fiscal 2023 with the balance to be recorded in Fiscal 2024. See “Item 1 Business – Recent Developments in our Business – Restructuring Plan”.
Spravato® Program
The roll-out of our Spravato® Program at select Treatment Centers continued throughout Fiscal 2023, building on our long-term business plan of utilizing our Treatment Centers as platforms for the delivery of innovative treatments to patients suffering from MDD and other mental health disorders. We also rolled-out our first Spravato® “buy & bill” program which will allow us to further enhance our access to patients in specific markets that require this program offering as compared to our current “administer and observe” programs. As at the date of this Annual Report, we had a total 81 Treatment Centers offering Spravato®.
Medication Management Program
During Fiscal 2023, the Company commenced a pilot to roll-out our facilitation of Medication Management to select Treatment Centers across our footprint, building on our long-term business plan of utilizing our Treatment Centers as platforms for the delivery of innovative treatments to patients suffering from MDD and other mental health disorders. Although Medication Management is a lower margin business, we believe this program will allow us to reach patients earlier in their treatment journey, develop an internal patient pipeline for TMS and Spravato®, while also further optimizing marketing costs. We also believe that becoming a more comprehensive mental health care provider will allow us to provide greater access to those suffering from MDD and other mental health disorders. As at the date of this Annual Report, we had a total of nine Treatment Centers offering Medication Management.
Talk Therapy Program
During the first quarter of Fiscal 2024, the Company commenced a pilot to roll-out talk therapy at select Treatment Centers across our footprint. Consistent with Medication Management, we believe this program will allow us to reach patients earlier in their treatment journey, develop an internal patient pipeline for TMS and Spravato®, while also further optimizing marketing costs. We believe that expanding our continuum of care and becoming a more comprehensive mental health care provider will allow us to provide greater access and quality of care to those suffering from MDD and other mental health disorders. As at the date of this Annual Report, we offer talk therapy at Treatment Centers in Florida and Missouri.
Debt Financings and Equity Financings
For more information on our recent debt and equity financings and see “Liquidity and Capital Resources – Indebtedness and Capital Raising” below.
Stock Exchange Matters
On February 23, 2024, the Company received the final delisting notice from the Listing Qualifications Department of the Nasdaq. As per the procedures set forth in the final delisting notice, the Common Shares were suspended from trading on Nasdaq on February 26, 2024. The Company filed its own Form 25 with the SEC on April 1, 2024, which completed the process for delisting its Common Shares from Nasdaq when the Form 25 became effective on April 11, 2024. Following the suspension of trading of the Common Shares on Nasdaq, the Common Shares began trading on the OTC Pink Market, under the symbol “GBNHF”. On March 22, 2024, the Common Shares became quoted on the OTCQB Market, under the symbol “GBNHF”. See “Item 1 Business—Recent Developments in our Business—Stock Exchange Matters”.
- 62 -
Director and Officer Appointments
During Fiscal 2023 and in early Fiscal 2024, the Company has had a number of appointments and resignations of directors and officers. See “Item 1 Business—Recent Developments in our Business—Corporate and Other Developments—Director Appointments”.
Factors Affecting Our Performance
We believe that our performance and future success depend on a number of factors that present significant opportunities for us. These factors are also subject to a number of inherent risks and challenges, some of which are discussed below. See also “Item 1A—Risk Factors”.
Number of Treatment Centers
Following the completion of the Restructuring Plan, we believe we will continue to have a meaningful opportunity to selectively increase the number of our Treatment Centers and the number of Treatment Centers offering Spravato® and Medication Management. The opening and success of new Treatment Centers or offering ancillary products in those Treatment Centers is subject to numerous factors, including our ability to locate the appropriate space, finance the operations, build relationships with clinicians, negotiate suitable lease terms and local payor arrangements, and other factors, some of which are beyond our control. At the same time, we have selectively closed certain Treatment Centers to maximize our profitability, which may temporarily reduce our number of active Treatment Centers from quarter to quarter as we continue to aim for overall expansion of the business.
Capital Management
Our objective is to maintain a capital structure that supports our long-term business strategy, maintain creditor and customer confidence, and maximize shareholder value. Our primary uses of capital are to finance operations, finance new center start-up costs, increase non-cash working capital and capital expenditures, as well as to service debt obligations. We have experienced losses since inception and, although we secured $55 million pursuant to the Original Term Loan (as defined below), $48.9 million pursuant to the Note Financings (as defined below), $6.25 million pursuant to the 2023 Private Placement (as defined below), $0.5 million pursuant to the Alumni Purchase Agreement, and $0.6 million pursuant to the February 2024 Public Offering, we expect that we will require additional financing to fund our operating activities and to repay our debt obligations and satisfy our cash requirements, including our near-term obligations under the Klein Note and the TMS Device Settlement. We have historically been able to obtain financing from Madryn, supportive shareholders and other sources when required; however, there can be no assurance that we will continue to receive financing support from Madryn and our shareholders in the future. In addition, we are constrained in our ability to raise capital as a result of the suspension of trading of our Common Shares by Nasdaq. See Item 1 “Business—Recent Developments in Our Business—Stock Exchange Matters”. See also “Liquidity and Capital Resources” below and “Risk Factors” in Item 1A of this Annual Report.
Industry and Reimbursement Trends
Our revenue is impacted by changes to United States healthcare laws, our clinical partners’ and contractors’ healthcare costs, the ability to secure favorable pricing structures with device manufacturers and payors’ reimbursement criteria and associated rates. In addition, the geographic distribution of our Treatment Centers can impact our revenues per Treatment because reimbursement rates vary from state to state. See “Risk Factors—Legal and Regulatory Risk Factors” and “Risk Factors—Industry Risk Factors” in Item 1A of this Annual Report.
Technology
Our revenues are affected by the availability of, and reimbursement for, new TMS indications, new technology or other novel treatment modalities (including Spravato®) and our ability to incorporate the new technology into our Treatment Centers.
Components of Our Results of Operations
Segments
We evaluate our business and report our results based on organizational units used by management to monitor performance and make operating decisions on the basis of one operating and reportable segment: Outpatient Mental Health Service Centers. We currently measure this reportable operating segment’s performance based on total revenues and entity-wide regional operating income.
- 63 -
Total Revenue
Total revenue consists of service revenue attributable to the performance of treatments. In circumstances where the net patient fees have not yet been received, the amount of revenue recognized is estimated based on an expected value approach. Due to the nature of the industry and complexity of our revenue arrangements, where price lists are subject to the discretion of payors, variable consideration exists that may result in price concessions and constraints to the transaction price for the services rendered.
In estimating this variable consideration, we consider various factors including, but not limited to, the following:
| ● | commercial payors and the administrators of federally funded healthcare programs exercise discretion over pricing and may establish a base fee schedule for our services (which is subject to change prior to final settlement) or negotiate a specific reimbursement rate with an individual provider; |
| ● | average of previous net service fees received by the applicable payor and fees received by other patients for similar services; |
| ● | management’s best estimate, leveraging industry knowledge and expectations of third-party payors’ fee schedules; |
| ● | factors that would influence the contractual rate and the related benefit coverage, such as obtaining pre-authorization of services and determining whether the procedure is medically necessary; |
| ● | probability of failure in obtaining timely proper provider credentialing (including re-credentialling) and documentation, in order to bill various payors which may result in enhanced price concessions; and |
| ● | variation in coverage for similar services among various payors and various payor benefit plans. |
We update the estimated transaction price (including updating our assessment of whether an estimate of variable consideration is constrained) to represent faithfully the circumstances present at the end of the reporting period and the changes in circumstances during the reporting period in which such variances become known.
Third-party payors include federal and state agencies (under the Medicare programs), managed care health plans and commercial insurance companies. Variable consideration also exists in the form of settlements with these third-party payors as a result of retroactive adjustments due to audits and reviews. We apply constraint to the transaction price, such that net revenues are recorded only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in the future.
Entity-Wide Regional Operating Income (Loss) and Direct Center and Regional Costs
Regional operating income presents regional operating income on an entity-wide basis and is calculated as total revenue less direct center and regional costs. Direct center and regional costs consist of direct center and patient care costs, regional employee compensation, regional marketing expenses, and depreciation. These costs encapsulate all costs (other than incentive compensation such as share-based compensation granted to senior regional employees) associated with the center and regional management infrastructure, including the cost of the delivery of treatments to patients and the cost of our regional patient acquisition strategy.
Center Development Costs
Center development costs represent direct expenses associated with developing new Treatment Centers, including small furnishings and fittings, wiring and electrical and, in some cases, the cost of minor space alterations.
Corporate Employee Compensation
Corporate employee compensation represents compensation incurred to manage the centralized business infrastructure of the Company, including annual base salary, annual cash bonuses and other non-equity incentives.
- 64 -
Corporate Marketing Expenses
Corporate marketing expenses represent costs incurred that impact the Company on an overall basis including investments in website functionality and brand management activities.
Other Corporate, General and Administrative Expenses
Other corporate, general and administrative expenses represent expenses related to the corporate infrastructure required to support our ongoing business including insurance costs, professional and legal costs and costs incurred related to our corporate offices. Costs related to the Restructuring Plan and the Amendment Fee (as defined below) are also included within other corporate, general and administrative expenses.
Financing Costs
Financing costs represent accounting, legal and professional fees incurred as part of significant transactions, including the Success TMS Acquisition in Fiscal 2022. See “—Factors Affecting the Comparability of Our Results—Acquisition of Success TMS” below.
Share-Based Compensation
Share-based compensation represents stock options, restricted share units and performance share units granted as consideration in exchange for employee and similar services to align personnel performance with the Company’s long-term goals.
Amortization
Amortization relates to the reduction in useful life of the Company’s intangible assets.
Interest
Interest expense relates to interest incurred on loans and lease liabilities. Interest income relates to income realized as a result of investing excess funds into investment accounts.
Loss (Gain) on Extinguishment of Loans
Loss (gain) on extinguishment of loans represents costs related to the exchange of the Insider Notes for Subordinated Convertible Notes (each as defined below) for all Insider Notes held by individuals who are not shareholders. It also represents costs related to the extinguishment of the Oxford Credit Facility (as defined below), as well as the extinguishment of a loan previously held by Success TMS (as defined below) incurred during Fiscal 2022. See “Liquidity and Capital Resources—Indebtedness and Capital Raising” below.
Loss on Settlements
Loss on settlements represents costs related to legal settlements reached. This includes the loss incurred on the settlement and mutual release agreement with a TMS Device manufacturer for the termination of TMS Device contracts with such manufacturer, as required by the Neuronetics Agreement (as defined below). In accordance with the terms of the settlement, the Company recognized an amount payable of $6,600,000, due in equal instalments over 44 weeks beginning in August 2023 (of which we have $1,200,000 outstanding as of the date of this Annual Report). Pursuant to the terms of the mutual release, in the event of default, interest will accrue at a rate of 6% per annum on any unpaid portion. Loss on settlements also includes the loss incurred on the settlement of the Klein Note. In accordance with the terms of the settlement, the Company will make payment in total of $2.2 million, structured as an initial immediate payment of $250,000, weekly payments of $75,000 thereafter up to and until the May 1, 2024 maturity date of the promissory note, upon which the balance owing will be due (of which we have $328,169 outstanding as of the date of this Annual Report).
- 65 -
Impairment Loss
Impairment loss represents the impairment of assets as part of our annual impairment of goodwill and long-lived assets. Goodwill impairment charge is recognized to the extent that the carrying value exceeds the fair value of the Company’s reporting unit. Long-lived asset impairment charge is recognized to the extent that the carrying value exceeds the fair value of the respective asset. The Company recognized impairment losses in both Fiscal 2022 and Fiscal 2023 relating to the Restructuring Plan. See Note 7(b) and Note 7(c) and Note 8(b) within Company’s audited consolidated financial statements as at and for the year ended December 31, 2023.
Adjusted EBITDA
Adjusted EBITDA is a non-GAAP measure that deducts from EBITDA share-based compensation expenses and certain other expenses that represent one-time costs or costs that otherwise do not reflect our underlying business performance. See “Reconciliation of Non-GAAP Measures” below.
Factors Affecting the Comparability of Our Results
Acquisition of Success TMS
On July 14, 2022, we, through our wholly owned U.S. subsidiary, TMS US, completed the Success TMS Acquisition pursuant to the Purchase Agreement by and the Seller Parties. The purchase price consideration was determined based on the pro forma revenue contribution of the two companies and was fixed at an amount equal to approximately 40% of the total issued and outstanding Common Shares on a post-acquisition basis and subject to adjustments. See “Item 1 Business—Recent Developments in our Business—Corporate and Other Developments—Acquisition of Success TMS”.
Regional Development Activity
Our regional model seeks to develop leading positions in key markets, and to leverage operational efficiencies by combining smaller local Treatment Centers within a region under a single shared regional management infrastructure. Part of our core strategy is to continue to develop new Treatment Centers within our existing regions as well as in new management regions, in each case, organically or through acquisitions of existing centers or businesses, which may affect comparability of results. Although we are currently focusing on a more condensed footprint due to the execution of our Restructuring Plan, our long-term growth strategy remains. See “Item 1 Business—Our TMS Business Model—Regional Development Activity”.
Seasonality
Typically, we experience seasonal factors in the first quarter of each fiscal year that result in reduced revenues in those quarters as compared to the other three quarters of the year. These seasonal factors include cold weather and the reset of deductibles during the first part of the year. We also typically experience a slowdown in new patient starts during the third quarter of each fiscal year as a result of summer holidays.
- 66 -
Results of Operations
Summary Financial Information
The following table summarizes our results of operations for the periods indicated. The selected consolidated financial information set out below has been derived from our audited consolidated financial statements, and should be read in conjunction with those financial statements and the related notes thereto. In addition, we note that the results presented below may not be indicative of our results in future periods as a result of the recently announced Restructuring Plan (as described above).
(audited) ($) |
| Fiscal 2023 |
| Fiscal 2022 |
Total revenue(1) | 73,786,778 | 66,825,959 | ||
Direct center and patient care costs |
| 53,765,678 |
| 42,137,465 |
Regional employee compensation |
| 18,106,033 |
| 16,651,595 |
Regional marketing expenses |
| 1,944,745 |
| 10,807,453 |
Depreciation |
| 2,703,186 |
| 3,510,611 |
Total direct center and regional costs |
| 76,519,642 |
| 73,107,124 |
Regional operating loss |
| (2,732,864) |
| (6,281,165) |
Center development costs |
| 525,782 |
| 660,356 |
Corporate employee compensation |
| 15,941,141 |
| 16,185,688 |
Corporate marketing expenses |
| 170,837 |
| 628,145 |
Financing costs |
| 678,347 |
| 1,265,225 |
Other corporate, general and administrative expenses |
| 12,769,567 |
| 7,445,166 |
Share-based compensation |
| 726,679 |
| 347,787 |
Amortization |
| 66,192 |
| 1,358,212 |
Interest expense |
| 12,048,071 |
| 5,979,829 |
Interest income |
| (231) |
| (12,250) |
Loss on extinguishment of loan |
| 14,274 |
| 2,331,917 |
Loss on settlements |
| 3,295,904 |
| — |
Impairment loss |
| 285,390 |
| 45,834,688 |
Loss before income taxes |
| (49,254,817) |
| (88,305,928) |
Income tax expense |
| — |
| — |
Loss for the year and comprehensive loss |
| (49,254,817) |
| (88,305,928) |
Income (loss) attributable to non-controlling interest |
| (340,755) |
| (634,812) |
Loss attributable to the common shareholders of Greenbrook |
| (48,914,062) |
| (87,671,116) |
Net loss per share (basic and diluted) |
| (1.25) |
| (3.77) |
Note:
(1) | Revenue for Fiscal 2022 has been restated to correct for errors in the period. For more information, see the Explanatory Note following the cover page of this Annual Report. |
- 67 -
Selected Financial Position Data
The following table provides selected financial position data as at the dates indicated:
| As at December 31, |
| As at December 31, | |
(audited) ($) | 2023 | 2022 | ||
Cash and restricted cash |
| 4,323,708 |
| 2,623,957 |
Current assets (excluding cash) (1) |
| 14,973,336 |
| 12,493,479 |
Total assets |
| 51,417,615 |
| 71,139,994 |
Current liabilities |
| 32,654,425 |
| 35,366,419 |
Non-current liabilities |
| 119,710,980 |
| 94,885,417 |
Total liabilities |
| 152,365,405 |
| 130,251,836 |
Non-controlling interests (1) |
| (2,911,581) |
| (2,777,127) |
Shareholders’ equity (deficit) (1) |
| (98,036,209) |
| (56,334,715) |
Note:
(1) | Current assets (excluding cash), non-controlling interest and shareholder’ equity (deficit) as at December 31, 2022 has been restated to correct for errors in the period. For more information, see the Explanatory Note following the cover page of this Annual Report. |
For further information regarding our liquidity and financial position, see “Liquidity and Capital Resources” below. See also Item 1A, “Risk Factors—Business and Operational Risk Factors—We have incurred losses in the past and may be unable to achieve or sustain profitability in the future and may not be able to secure additional financing to fund losses if we fail to achieve or maintain profitability.” in this Annual Report.
Selected Operating Data
The following table provides selected operating data as at the dates indicated. As described above, as of the date of this Annual Report, the Company has reduced its operating footprint to 130 Treatment Centers in connection with the Restructuring Plan. See “Cautionary Note Regarding Forward-Looking Information”.
| As at December 31, |
| As at December 31, | |||
(unaudited) | 2023 | 2022 | ||||
Number of active Treatment Centers(1) |
| 130 |
| 183 | ||
Number of Treatment Centers-in-development(2) |
| — |
| — | ||
Total Treatment Centers |
| 130 |
| 183 | ||
Number of management regions |
| 17 |
| 18 | ||
Number of TMS Devices installed |
| 260 |
| 345 | ||
Number of regional personnel |
| 391 |
| 495 | ||
Number of shared-services / corporate personnel(3) |
| 98 |
| 134 | ||
Number of TMS treatment providers(4) |
| 205 |
| 225 | ||
Number of consultations performed(5) |
| 34,124 |
| 27,831 | ||
Number of patient starts(5) |
| 10,401 |
| 9,253 | ||
Number of TMS treatments performed(5) |
| 343,790 |
| 312,940 | ||
Average revenue per TMS treatment(5) | $ | 215 | $ | 214 | ||
Notes:
| (1) | Active Treatment Centers represent Treatment Centers that have performed billable TMS services during the applicable period. |
| (2) | Treatment Centers-in-development represents Treatment Centers that have committed to a space lease agreement and the development process is substantially complete. |
| (3) | Shared-services / corporate personnel is disclosed on a full-time equivalent basis. The Company utilizes part-time staff and consultants as a means of managing costs. |
| (4) | Represents clinician partners that are involved in the provision of TMS therapy services from our Treatment Centers. |
| (5) | Figure calculated for the applicable year ended December 31. |
- 68 -
Comparison of Results for the Year Ended December 31, 2023 to the Year Ended December 31, 2022
The following section provides an overview of our financial performance during Fiscal 2023 compared to Fiscal 2022.
Total Revenue
Consolidated revenue increased to $73.8 million in Fiscal 2023, a 10% increase compared to Fiscal 2022 (Fiscal 2022: $66.8 million). This was predominately due to the Success TMS Acquisition which was included in our results for only a portion of Fiscal 2022, partially offset by reduced revenues from the implementation of the Restructuring Plan described above.
New patient starts increased to 10,401 in Fiscal 2023, a 12% increase compared to Fiscal 2022 (Fiscal 2022: 9,253). Treatment volumes in Fiscal 2023 were 343,790, a 10% increase compared to Fiscal 2022 (Fiscal 2022: 312,940). Consultations performed were 34,124 in Fiscal 2023, a 23% increase compared to Fiscal 2022 (Fiscal 2022: 27,831). The increases are predominately due to the Success TMS Acquisition which was included in our results for only a portion of Fiscal 2022, partially offset by our smaller footprint resulting from implementation of the Restructuring Plan.
Average revenue per treatment remained relatively consistent at $215 in Fiscal 2023 (Fiscal 2022: $214). The change in average revenue per Treatment was primarily attributable to changes in payor mix, treatment modalities and the geographical distribution of revenue (including as a result of the Success TMS Acquisition).
Entity-Wide Regional Operating Loss and Direct Center and Regional Costs
Direct center and regional costs increased by 5% to $76.5 million during Fiscal 2023 (Fiscal 2022: $73.1 million). The increase is attributable to the Success TMS Acquisition which was included in our results for only a portion of Fiscal 2022, partially offset by cost savings in relation to the execution of the Restructuring Plan and the reduction in marketing spend described above.
Entity-wide regional operating loss decreased by 56% to $2.7 million during Fiscal 2023 (Fiscal 2022: $6.3 million). The decrease in entity-wide regional operating loss was primarily due to cost savings achieved by the reduction in head count, reduction of marketing spend and a reduction in other direct center expenses resulting from the execution of the Restructuring Plan.
Center Development Costs
Center development costs decreased by 20% to $0.5 million during Fiscal 2023 (Fiscal 2022: $0.7 million). The decrease is predominantly due to a reduction in the number of Treatment Centers developed during 2023 as compared to 2022, partially offset by the minimal incremental costs associated with establishing the Spravato® Program at additional Treatment Centers during 2023.
Corporate Employee Compensation
Corporate employee compensation incurred to manage the centralized business infrastructure of the Company decreased by 2% to $15.9 million during Fiscal 2023 (Fiscal 2022: $16.2 million). This decrease is predominately due to the execution of the Restructuring Plan, partially offset by increases in employee compensation resulting from the Success TMS Acquisition which was completed on July 14, 2022.
Corporate Marketing Expenses
Corporate marketing expenses decreased by 73% to $0.2 million during Fiscal 2023 (Fiscal 2022: $0.6 million). The decrease was primarily due to managing spend as a result of the execution of the Restructuring Plan in addition to limiting expenditures due to liquidity constraints which we are actively seeking to resolve (see “Factors Affecting Our Performance—Capital Management” above).
Financing Costs
Financing costs decreased by 46% to $0.7 million during Fiscal 2023 (Fiscal 2022: $1.3 million). The decrease was due to reduced accounting, legal and professional fees incurred in Fiscal 2023 as compared to Fiscal 2022.
- 69 -
Other Corporate, General and Administrative Expenses
Other corporate, general and administrative expenses increased by 72% to $12.8 million during Fiscal 2023 (Fiscal 2022: $7.4 million). The increase in Fiscal 2023 is predominantly due to one-time expenses incurred related to the execution of the Restructuring Plan, professional and legal fees related to the Klein Matters, and financing initiatives-related expenses, partially offset cost savings realized by the Restructuring Plan and the reduction of one-time costs including Success TMS related professional and legal fees, and Success TMS related integration expenses.
We believe we will be able to continue to achieve near-term operational synergies through the remaining components of the Restructuring Plan, resulting in a significant reduction in other corporate, general and administrative expenses (see “Cautionary Note Regarding Forward-Looking Information” and “Key Highlights and Recent Developments—Restructuring Plan”).
Share-Based Compensation
Share-based compensation increased by 109% to $0.7 million during Fiscal 2023 (Fiscal 2022: $0.3 million), predominantly due to the timing and fair value of stock options granted to key personnel to ensure retention and long-term alignment with the goals of the Company.
Amortization
Amortization decreased by 95% to $0.1 million during Fiscal 2023 (Fiscal 2022: $1.4 million) primarily as a result of the impairment recorded in Q4 2022 relating to goodwill and intangible assets (see “—Impairment Loss” below).
Interest
Interest expense increased by 101% to $12.0 million during Fiscal 2023 (Fiscal 2022: $6.0 million). The increase in interest expense is primarily due to the debt financings completed during Fiscal 2023.
Interest income was $231 in Fiscal 2023 (Fiscal 2022: $12,250). The changes were due to a change in the amount of excess funds invested.
Loss on Extinguishment of Loan
Loss on extinguishment of loans decreased by 99% to $0.01 million during Fiscal 2023 (Fiscal 2022: $2.3 million). The gain on extinguishment of loans in Fiscal 2023 relates to the exchange of Insider Notes for Subordinated Convertible Notes. The loss on extinguishment of loans in Fiscal 2022 is due to the extinguishment of the Oxford Credit Facility, as well as extinguishment of a loan previously held by Success TMS.
Loss on Settlements
Loss on settlements was $3.3 million in Fiscal 2023 as compared to nil in Fiscal 2022. The loss relates to the portion of the TMS Device Settlement recorded as a loss in Fiscal 2023, as well as the settlement of the Klein Note through the Klein Settlement Agreement.
Impairment Loss
Impairment loss was $0.3 million in Fiscal 2023 as compared to $45.8 million in Fiscal 2022. Impairment in Fiscal 2023 was due to impairment on operating right-of-use assets resulting from the execution of the Restructuring Plan. Impairment in Fiscal 2022 was due to impairment on goodwill and intangible assets, which was necessitated by the Restructuring Plan and current market conditions.
- 70 -
Loss for the Period and Comprehensive Loss and Loss for the Period Attributable to the Common Shareholders of Greenbrook
The loss for the year and comprehensive loss decreased by 44% to $49.3 million during Fiscal 2023 (Fiscal 2022: $88.3 million). The increase in Fiscal 2023 is due to the increase in interest expense arising from the debt financings, increase in the loss on settlements, increase in share-based compensation expense, increase in costs and depreciation as a result of the completion of the Success TMS Acquisition, as well as one-time costs, partially offset by the increase in regional operating income and cost savings associated with the execution of the Restructuring Plan. See “Interest Expense, Entity-Wide Regional Operating Income (Loss)”, “Direct Center and Regional Costs”, “Share-Based Compensation”, “Loss (Gain) on Extinguishment of Loans” and “Loss on Settlements” above, “Adjusted EBITDA and One-time Expenses” below and “Key Highlights and Recent Developments—Restructuring Plan” and “Factors Affecting the Comparability of Our Results—Acquisition of Success TMS” above.
The loss attributable to the common shareholders of Greenbrook decreased by 44% to $48.9 million during Fiscal 2023 (Fiscal 2022: $87.7 million). This was predominantly due to the factors described above impacting net losses.
Adjusted EBITDA and One-Time Expenses
The Adjusted EBITDA loss position decreased by 6% to $24.6 million during Fiscal 2023 (Fiscal 2022: $26.1 million) (see “Cautionary Note Regarding Non-US GAAP Measures and Industry Metrics” above). The decrease in the Adjusted EBITDA loss position in Fiscal 2023 as compared to Fiscal 2022 is primarily attributable to a reduction in the EBITDA loss position, the adjustment in Q3 2023 for loss on device contract termination (see “Components of Our Results of Operations” above) as well as a decrease in share-based compensation, offset by in the absence of an adjustment for the loss on extinguishment of loans. One-time costs that occurred in Fiscal 2023 related to restructuring costs incurred as we executed on our Restructuring Plan (see “Key Highlights and Recent Developments—Restructuring Plan”), the Amendment Fee, financing initiatives-related expenses, Success TMS related integration expenses, professional and legal fees related to the Klein Matters and the Neuronetics Note, the loss on device contract termination and the loss (gain) on extinguishment of loans, while one-time costs that occurred in Fiscal 2022 included professional and legal fees related to the Success TMS Acquisition, Success TMS integration and related expenses and the loss (gain) on extinguishment of loans. See “Reconciliation of Non-GAAP Measures” below.
EBITDA and Adjusted EBITDA
The table below illustrates our EBITDA and Adjusted EBITDA for the periods presented:
(unaudited) ($) |
| Fiscal 2023 |
| Fiscal 2022 |
EBITDA |
| (34,096,844) |
| (76,834,714) |
Adjusted EBITDA |
| (24,611,122) |
| (26,109,667) |
For a definition of EBITDA and Adjusted EBITDA and a quantitative reconciliation of EBITDA and Adjusted EBITDA to loss attributable to the common shareholders of Greenbrook, see “—Reconciliation of Non-GAAP Measures” immediately below.
Reconciliation of Non- GAAP Measures
This MD&A makes reference to certain non-GAAP measures. These measures are not recognized measures under US GAAP, do not have a standardized meaning prescribed by US GAAP and, therefore, may not be comparable to similar measures presented by other companies. Rather, these measures are provided as additional information to complement those US GAAP measures by providing further understanding of our results of operations from management’s perspective. Accordingly, these measures are not intended to represent, and should not be considered as alternatives to, loss attributable to the common shareholders of Greenbrook or other performance measures derived in accordance with US GAAP as measures of operating performance or operating cash flows or as a measure of liquidity. In addition to our results determined in accordance with US GAAP, we use non-GAAP measures including, “EBITDA” and “Adjusted EBITDA” (each as defined below). These non-GAAP measures are used to provide investors with supplemental measures of our operating performance and thus highlight trends in our core business that may not otherwise be apparent when relying solely on US GAAP measures. We also believe that securities analysts, investors and other interested parties frequently use non-GAAP measures in the evaluation of issuers. However, we caution you that “Adjusted EBITDA” may be defined by us differently than by other companies. Our management also uses non-GAAP measures to facilitate operating performance comparisons from period to period, to prepare annual operating budgets and forecasts and to determine components of management compensation.
- 71 -
We define our non-GAAP measures as follows:
“EBITDA” is a non-GAAP measure that is defined as net income (loss) before amortization, depreciation, interest expenses, interest income and income taxes. The US GAAP measurement most directly comparable to EBITDA is loss attributable to common shareholders of Greenbrook.
“Adjusted EBITDA” is a non-GAAP measure that is defined as EBITDA as adjusted for share-based compensation expenses (comprising share-based compensation), one-time expenses and other expenses that do not relate to our underlying business performance. We believe Adjusted EBITDA is a meaningful financial metric as it measures the ability of our Treatment Centers to generate earnings while eliminating the impact of one-time expenses, share-based compensation expenses that do not have an impact on the operating performance of our existing Treatment Center network or otherwise reflect our underlying business performance. The US GAAP measurement most directly comparable to Adjusted EBITDA is loss attributable to common shareholders of Greenbrook.
As a result of the execution of the Restructuring Plan, management has shifted from measuring performance on a region-by-region basis through Same-Region Sales Growth to Adjusted EBITDA as the Company’s primary non-GAAP measure.
The table below illustrates a reconciliation of loss attributable to the common shareholders of Greenbrook to EBITDA and Adjusted EBITDA for Fiscal 2023 and Fiscal 2022:
(unaudited) ($) |
| Fiscal 2023 |
| Fiscal 2022 |
Loss attributable to the common shareholders of Greenbrook | (48,914,062) | (87,671,116) | ||
Add the impact of: |
|
|
|
|
Interest expense |
| 12,048,071 |
| 5,979,829 |
Amortization |
| 66,192 |
| 1,358,212 |
Depreciation |
| 2,703,186 |
| 3,510,611 |
Less the impact of: |
|
|
|
|
Interest income |
| (231) |
| (12,250) |
EBITDA |
| (34,096,844) |
| (76,834,714) |
Add the impact of: |
|
|
|
|
Share-based compensation |
| 726,679 |
| 347,787 |
Add impact of: |
|
|
|
|
Restructuring and related costs |
| 998,574 |
| — |
Debt financing fees |
| 678,347 |
| — |
Klein Matters related professional and legal fees |
| 716,267 |
| — |
Neuronetics Note legal fees |
| 496,407 |
| — |
Credit Facility Amendment Fee |
| 1,000,000 |
| — |
Loss on settlements(1) |
| 3,295,904 |
| 3,240,251 |
Loss on extinguishment of loan(2) |
| 14,274 |
| 2,331,917 |
US GAAP transition costs |
| 322,949 |
| — |
Impairment loss(3) |
| 285,390 |
| 45,834,688 |
Success TMS Acquisition related professional fees |
| — |
| 1,265,225 |
Success TMS related integration and related expenses |
| 150,000 |
| 945,430 |
Exploratory Financing / Expansion investment cost |
| 800,931 |
| — |
Adjusted EBITDA |
| (24,611,122) |
| (26,109,667) |
Note:
| (1) | Loss on settlements represents the loss incurred on legal settlements. This includes the portion of the TMS Device Settlement and the settlement of the Klein Note through the Klein Settlement Agreement that were each recorded as a loss in Fiscal 2023. Due to their nature, losses on settlements are each considered a one-time cost and, accordingly, have been excluded from Adjusted EBITDA. In addition, losses on settlements are excluded from Adjusted EBITDA because they do not reflect the Company’s underlying business performance. |
| (2) | Loss on extinguishment of loan relates to the extinguishment of the Oxford Credit Facility in 2022, as well as the extinguishment of a loan previously held by Success TMS in 2023. Due to their nature, these are each considered one-time costs and, accordingly, have been excluded from Adjusted EBITDA. In addition, losses on extinguishment of loans are excluded from Adjusted EBITDA because they do not reflect the Company’s underlying business performance. |
- 72 -
| (3) | Impairment losses for Fiscal 2022 and Fiscal 2023 relate to the Restructuring Plan. Impairment losses are excluded from Adjusted EBITDA because they do not reflect the Company’s underlying business performance. See Note 7(b), Note 7(c) and Note 8(b) within the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023. |
Reconciliation of Accounts Receivable
A quantitative reconciliation of accounts receivable in respect of Fiscal 2023 and Fiscal 2022 is provided below, which includes a quantification of the adjustment to variable consideration estimate resulting from the additional price concessions which were deemed necessary:
(unaudited) ($) |
| Fiscal 2023 |
| Fiscal 2022 | ||
Opening accounts receivable balance |
| 7,348,846 |
| 6,726,417 | ||
Accounts receivable acquired as part of the Success TMS Acquisition |
| — |
| 3,728,255 | ||
Revenue recognized based on expected value |
| 83,221,303 |
| 73,631,665 | ||
Adjustment to variable consideration estimate |
| (9,434,525) |
| (6,805,706) | ||
Payments received |
| (73,565,781) |
| (69,931,785) | ||
Ending accounts receivable balance at the period end date | $ | 7,569,843 | $ | 7,348,846 | ||
Accounts Receivable
Accounts receivable increased by 3% to $7.6 million as at the end of Fiscal 2023 as compared to $7.3 million as at the end of Fiscal 2022. The increase in Fiscal 2023 as compared to Fiscal 2022 is primarily due to the timing of cash collection activity from payors. We also continue to collect on services rendered in excess of 24 months from the date such services were rendered.
Liquidity and Capital Resources
Overview
Since inception, we have financed our operations primarily from equity offerings, debt financings and revenue generated from our Treatment Centers. Our primary uses of capital are to finance operations, finance new Treatment Center development costs, increase non-cash working capital and fund investments in our centralized business infrastructure. Our objectives when managing capital are to ensure that we will continue to have enough liquidity to provide services to our customers and provide returns to our shareholders. We have also used capital to finance acquisitions and may continue to do so in the future. Cash is held primarily in U.S. dollars.
As part of our annual budgeting process and on an ongoing basis, we evaluate our estimated annual cash requirements to fund planned expansion activities and working capital requirements of existing operations. Based on this, in addition to historical cash flow, the debt financings and equity offerings completed in Fiscal 2023 and the early part of Fiscal 2024 (see “—Indebtedness and Capital Raising”) and considering our anticipated cash flows from regional operations and our holdings of cash, we believe that we have sufficient capital to meet our future operating expenses, capital expenditures and debt service requirements for approximately the next month as of the date of this Annual Report. However, we will need to raise additional funding in the near term, including to repay or refinance the Klein Note which is due and payable in May 2024 as well as additional funds to satisfy our near-term obligations under the TMS Device Settlement, in addition to our day-to-day operating expenses. However, our ability to fund operating expenses, capital expenditures and future debt service requirements will depend on, among other things, our ability to source external funding, our future operating performance, which will be affected by our ability to meet our debt obligations and remain in compliance with debt covenants, our ability to increase the Debt Cap set out in the Neuronetics Agreement, the outcome of Klein Matters and general economic, financial and other factors, including factors beyond our control such as inflation and recessionary conditions. See “Cautionary Note Regarding Forward-Looking Information”, Risk Factors in Item 1A, “Factors Affecting our Performance” and as well as further details on our indebtedness and capital raising below under “—Indebtedness and Capital Raising”.
- 73 -
Analysis of Cash Flows
The following table presents our cash flows for each of the periods presented:
($) |
| Fiscal 2023 |
| Fiscal 2022 |
Net cash used in operating activities |
| (35,912,722) |
| (24,181,935) |
Net cash generated from financing activities |
| 37,628,312 |
| 14,951,121 |
Net cash used in/ generated from investing activities |
| (15,839) |
| 155,092 |
Increase (decrease) in cash |
| 1,699,751 |
| (9,075,722) |
Cash Flows used in Operating Activities
For Fiscal 2023, cash flows used in operating activities (which includes the full cost of developing new Treatment Centers) totaled $35.9 million, as compared to $24.2 million in Fiscal 2022. The decrease in cash flows used in operating activities is primarily due to the change in non-cash working capital, offset by better regional performance and cost reductions related to the implementation of our Restructuring Plan. See “Key Highlights and Recent Developments—Restructuring Plan”.
Cash Flows generated from Financing Activities
For Fiscal 2023, cash flows generated from financing activities amounted to $37.6 million as compared to $15.0 million generated in Fiscal 2022. This change is primarily driven by the net proceeds from the 2023 Private Placement, the Alumni Purchase Agreement and the debt financing received in Fiscal 2023, as compared to the net loans advanced to the Company in relation to the Credit Agreement in Fiscal 2022.
Cash Flows generated from (used in) Investing Activities
For Fiscal 2023, cash flows generated from investing activities totaled $0.02 million as compared to cash flows used in investing activities of $0.16 million in Fiscal 2022, due to the purchase of property, plant and equipment in Fiscal 2023, as compared to the Success TMS Acquisition, net of cash acquired in Fiscal 2022, offset by the acquisition of subsidiary non-controlling interest.
Indebtedness and Capital Raising
Madryn Credit Agreement
Initial Agreement and Funding
On July 14, 2022 (the “Original Closing Date”), the Company entered into the Credit Agreement which provided the Company with a $55 million term loan (the “Original Term Loans”), which was funded on the Original Closing Date. In addition, the Credit Agreement permits the Company to draw up to an additional $20 million in a single draw at any time on or prior to December 31, 2024, for purposes of funding future mergers and acquisition activity. Prior to March 31, 2023, all amounts borrowed under the Credit Agreement bore interest at a rate equal to the three-month LIBOR rate plus 9.0%, subject to a minimum three-month LIBOR floor of 1.5%. Commencing March 31, 2023, as a result of an amendment to the Credit Agreement entered into between the parties on February 21, 2023, all advances under the Credit Agreement accrue interest at a rate equal to 9.0% plus the 3-month Term Secured Overnight Financing Rate (“SOFR”) (subject to a floor of 1.5%) plus 0.10%. The Credit Agreement matures over 63 months and provides for four years of interest-only payments. The initial principal balance of $55,000,000 is due in five equal 3 month installments beginning on September 30, 2026. The Company has granted a lien on, and security interest over all assets of the Company in connection with the performance and prompt payment of all obligations of the Company under the Credit Agreement.
- 74 -
The terms of the Credit Agreement require the Company to satisfy various affirmative and negative covenants and to meet certain financial tests, including but not limited to, financial covenants that require the Company to (i) generate consolidated revenues for each 12-month period (on a pro forma basis giving effect to the Success TMS Acquisition), measured quarterly (commencing with the 12 months ended September 30, 2022), in amounts that (A) for the first, second and third measurement periods, are in line with the historical trailing performance of the Company and Success TMS (taken together as a combined company) but substitute one, two and three quarters, respectively, of historical combined revenues with a portion of the projected combined revenues for those quarters; and (B) for measurement periods thereafter, represent a percentage of the Company’s projected consolidated revenues for such periods, assuming modest growth over time, and is capped at the minimum revenue requirement for the 12 months ending on June 30, 2027, and (ii) maintain the Minimum Liquidity Covenant tested on a daily basis, which covenant has been amended from time to time (as described under “—Credit Facility Amendments and Subsequent Funding” below). In addition, the Credit Agreement contains affirmative and negative covenants that limit, among other things, the Company’s ability to incur additional indebtedness, incur certain liens, declare certain dividends and engage in certain types of transactions. As of December 31, 2023, the Company was in compliance with the Minimum Liquidity Covenant. The Credit Agreement also contains affirmative covenants that require the Company to deliver, within 90 days of each fiscal year end, audited financial statements for such fiscal year (the “Financial Statements”), accompanied by a report and opinion of an independent certified public accountant (the “Report”), which is not subject to any “going concern” or like qualification or exception or any qualification or exception as to the scope of such audit. On March 28, 2024, we obtained a waiver to the requirement that the Report not be subject to any “going concern” qualification for Fiscal 2023. On March 29, 2024, we obtained a sixteen (16)-day extension to the March 30, 2024 deadline to deliver the Financial Statements and the Report for Fiscal 2023. On April 15, 2024, we received an additional one (1)-day extension to deliver the Financial Statement and the Report for Fiscal 2023 and on April 16, 2024, we received an additional extension until April 26, 2024 to deliver the Financial Statement and the Report for Fiscal 2023. In addition, the Credit Agreement contains affirmative and negative covenants that limit, among other things, the Company’s ability to incur additional indebtedness outside of what is permitted under the Credit Agreement, create certain liens on assets, declare dividends and engage in certain types of transactions.
On the Original Closing Date, in accordance with the terms of the Credit Agreement, Greenbrook issued conversion instruments to Madryn and certain of its affiliated entities that provide the holders thereof with the option to convert up to $5 million of the outstanding principal amount of the Original Term Loans into Common Shares at the Madryn Conversion Price.
On July 14, 2022, the Company used approximately $15.4 million of the proceeds from the Credit Agreement to repay in full the outstanding balance owing under the Oxford Credit Facility, as well as prepayment fees and legal fees incurred, and terminated the Oxford Credit Facility. Concurrently, the Company used approximately $15.1 million of the proceeds from the Credit Agreement to repay various loans previously held by Success TMS. The Company subsequently used $2.9 million to normalize vendor payment aging resulting from cash management practices prior to strengthening our balance sheet and made a $2.3 million cash investment due to a lag in working capital from the continued development of Treatment Center locations. After financing fees and closing costs of $3.1 million, the remaining balance of the proceeds in respect of the Credit Agreement totaled $16.2 million as of July 14, 2022.
The Credit Agreement contains a number of events of default including, without limitation, (a) non-payment of principal when due; (b) failure to pay interest, fees or repayment premiums within 3 business days after the same becomes due; (c) failure to pay other amounts under the Credit Agreement and Investment Documents (as defined in the Credit Agreement) within 5 business days after the same becomes due; (d) failure to comply with the covenants in the Credit Agreement (subject to specified grace periods); (e) cross-default to indebtedness in an aggregate principal amount in excess of $1,000,000; (f) the initiation of insolvency proceedings; (g) the Company or any of its Subsidiaries (as defined in the Credit Agreement) becomes unable to, admits in writing its inability to, or fails to pay, its debts as due; (h) the occurrence of specified events which may have a Material Adverse Effect (as defined in the Credit Agreement), as further described in Section 9.01(l) of the Credit Agreement; and (i) entry into a final judgment against the Company (or any subsidiary) for the payment of money in an aggregate amount exceeding $1,000,000 or any non-monetary final judgment that could reasonably be expected to have a Material Adverse Effect (as defined in the Credit Agreement) and, in either case, enforcement proceedings are commenced by a creditor or there is a period of 30 consecutive days during which a stay of enforcement is not in effect.
Credit Facility Amendments and Subsequent Funding
From February 2023 through March 2024, the Company entered into various amendments to the Credit Agreement, pursuant to which Madryn and its affiliates have extended eighteen Additional Term Loans to the Company in an aggregate principal amount of $41.2 million. The Madryn Loans outstanding under the Credit Agreement as of the date of this Annual Report is approximately $96 million. The terms and conditions of the Additional Term Loans are consistent with the terms and conditions of the Original Term Loans in all material respects. We entered into the Additional Terms Loans in part to remain in compliance with certain covenants in the Credit Agreement and in order to satisfy our near-term cash requirements necessary to operate our business.
- 75 -
The Company has provided Madryn the Madryn Conversion Instruments in connection with the Madryn Loans which provide Madryn with the option to convert up to approximately $7.4 million of the outstanding principal amount of the Madryn Loans into Common Shares at the Madryn Conversion Price. As of the date of this Annual Report, full conversion of the Madryn Conversion Instruments at the Madryn Conversion Price would, result in the issuance of up to approximately 3.9 million Common Shares, representing approximately 8.6% of the issued and outstanding Common Shares as at the date of this Annual Report. Madryn has received customary registration rights for the Common Shares issuable pursuant to the Madryn Conversion Instruments.
In addition, from December 2022 through March 2024, the Company and Madryn have agreed on a number of occasions to amend the Credit Agreement to temporarily waive the Company’s Minimum Liquidity Covenant in order to avoid a breach as a result of the Company’s non-compliance. The most recent amendment to the Minimum Liquidity Covenant, executed on March 28, 2024, extends the reduced Minimum Liquidity Covenant requirement of $300,000 to April 30, 2024, at which time (unless further amended or waived), the Minimum Liquidity Covenant requirement will revert to $3,000,000. We anticipate needing to obtain a further amendment to (or waiver of) the Minimum Liquidity Covenant in order to extend the application of the reduced liquidity requirement of $300,000 beyond April 30, 2024.
In connection with the Additional Term Loans, we have paid Madryn an amendment fee of $1.0 million that was paid-in-kind by adding the Amendment Fee to the outstanding principal balance of the Madryn Loans (the “Amendment Fee”). As of the date of the Annual Report we have also paid a total of approximately $1.0 million in additional amendment related fees in cash. On August 1, 2023, we amended the Credit Agreement to convert the June 30, 2023, cash interest payment into paid-in-kind interest. On September 29, 2023, we amended the Credit Agreement to defer the payment of the cash interest payment due September 30, 2023, to October 15, 2023. On October 12, 2023, we further amended the Credit Agreement to defer the payment of the cash interest payment due September 30, 2023, to November 15, 2023.
Subordinated Convertible Notes
On August 15, 2023, the Company entered into a Note Purchase Agreement, pursuant to which the Company may issue Subordinated Convertible Notes from time to time. On August 28, 2023, the Company exchanged notes in an aggregate principal amount of $2.8 million that had been previously issued to insiders for an equal amount of Subordinated Convertible Notes pursuant to the terms of the Note Purchase Agreement. In addition, between August 2023 and October 2023, the Company issued certain Subordinated Convertible Notes to Madryn, Greybrook Health and certain other investors, in an aggregate principal amount equal to $6.9 million. As of the date of this Annual Report, there is approximately $9.7 million aggregate principal amount of Subordinated Convertible Notes issued and outstanding.
All Subordinated Convertible Notes bear interest at a rate consistent with the Credit Agreement and mature on the earlier of March 31, 2028, in the event of a change of control, acceleration of other indebtedness, or six months following repayment or refinancing of all Madryn Loans under the Company’s credit facility with Madryn.
The Subordinated Convertible Notes provide holders the option to convert any amount up to the outstanding principal amount plus accrued interest into Common Shares at any time at the election of the holders thereof or on a mandatory basis by all noteholders at the request of Madryn. The Subordinated Convertible Notes are convertible into such number of Common Shares equal to the principal amount of Subordinated Convertible Notes to be converted (plus accrued and unpaid interest thereon) divided by a conversion price equal to the lesser of (1) 85% of the closing price per Common Share on Nasdaq or any other market as of the closing date for such notes, as adjusted from time to time for certain dilutive transactions, and (2) (i) 85% of the 30-day volume weighted average trading price of the Common Shares on an applicable trading market (which includes the OTCQB) prior to conversion, or (ii) if the Common Shares are not listed on an applicable trading market at the time of conversion, a per share price equal to 85% of the fair market value per Common Share as of such date; provided that, in any event, the conversion price shall not be lower than $0.078 (the “Subordinated Convertible Note Conversion Price”). As of the date of this Annual Report, the applicable Subordinated Convertible Note Conversion Price is the floor price of $0.078. Under the Note Purchase Agreement, the maximum number of Common Shares that can be issued under the Subordinated Convertible Notes is 200,000,000 Common Shares.
In connection with the Subordinated Convertible Notes, all noteholders also received customary resale, demand and “piggy-back” registration rights with respect to the Common Shares issuable upon conversion pursuant to a registration rights agreement, by and among the Company and the noteholders.
- 76 -
Insider Notes
The Company previously entered into a note purchase agreement, dated as of February 3, 2023, with certain significant shareholders (including Madryn and Greybrook Health) and certain members of management of the Company (the “Noteholders”), pursuant to which the Company issued unsecured notes in the aggregate principal amount of $1.75 million on February 3, 2023 and February 28, 2023 (the “February 2023 Notes”).
The Company also previously entered into a note purchase agreement, dated as of August 1, 2023, with Greybrook Health, pursuant to which the Company issued an unsecured subordinated note in an aggregate principal amount of $1.0 million to Greybrook Health (the “August 2023 Note”). In connection with the entry into such note purchase agreement, the Company concurrently entered into (i) an amendment to the Credit Agreement and (ii) a consent agreement in respect of the Neuronetics Note, in each case, permitting the incurrence of indebtedness under such note purchase agreement.
The Company subsequently exchanged the total par value of the principal for the February 2023 Notes and August 2023 Note (collectively, the “Insider Notes”) for the Subordinated Convertible Notes.
As of the date of this Annual Report, the aggregate proceeds of the Additional Term Loans, the Insider Notes (which were exchanged in full, and extinguished by the issuance of, the Subordinated Convertible Notes issued to the Noteholders on August 28, 2023) and the Subordinated Convertible Notes (collectively the “Note Financings”) is equal to approximately $48.9 million.
In connection with the issuance of the Insider Notes, the Company issued a combined total of 385,870 common share purchase warrants to Greybrook Health (the “Greybrook Warrants”). 135,870 of the Greybrook Warrants were issued alongside the February 2023 Notes and are exercisable for one Common Share at an exercise price of $1.84, subject to customary anti-dilution adjustments. 250,000 of the Greybrook Warrants were issued alongside the August 2023 Note and are exercisable for one Common Share at an exercise price equal to (a) if the Common Shares are listed on Nasdaq or any other trading market at the time of exercise, 85.0% of the volume-weighted average trading price of the Common Shares on Nasdaq (or, if not listed on Nasdaq, then such other trading market on which the Common Shares are principally traded, based upon daily share volume) for the five trading days immediately preceding the exercise date, or (b) if the Common Shares are not listed on any trading market at the time of exercise, a per share price based on fair market value, as determined by the Board, in each case subject to customary anti-dilution adjustments. The Greybrook Warrants will expire five years from the applicable date of issuance.
Neuronetics Note and Warrants
In January 2023, the Company and Neuronetics jointly announced an expanded commercial partnership through year end 2028. Under the Neuronetics Agreement, Neuronetics is the exclusive supplier of TMS Devices to the Company. Over time, Neuronetics’ NeuroStar TMS Devices will replace competitive TMS Devices at the Company’s Treatment Centers. The Neuronetics Agreement also contains minimum purchase commitments, and all treatment session purchases will convert to a “per-click” consumable model.
On March 31, 2023, the Company and Neuronetics agreed to convert the Company’s outstanding account balance payable to Neuronetics in the amount of approximately $5.9 million, together with Neuronetics’ out-of-pocket financing costs, into secured debt in the aggregate principal amount of the $6.0 million Neuronetics Note. All amounts borrowed under the Neuronetics Note will bear interest at a rate equal to the sum of (a) the floating interest rate of daily secured overnight financing rate as administered by the Federal Reserve Bank of New York on its website, plus (b) 7.65%. The Neuronetics Note matures on March 31, 2027. Pursuant to the terms of the Neuronetics Note, upon the occurrence of an event of default under the Neuronetics Note, Greenbrook will be required to issue the Neuronetics Warrants (defined below). Additionally, under the Neuronetics Agreement, the Company is required to pay all costs to relocate the TMS Devices supplied by Neuronetics from the Treatment Centers that are closed in connection with the Restructuring Plan and install such TMS Devices in the Company’s Treatment Centers that remain open. In connection with the entry into the Neuronetics Note, the Company concurrently entered into an amendment to the Credit Agreement in order to permit the Company to incur the indebtedness under the Neuronetics Note and the lien securing such obligations.
- 77 -
Pursuant to the terms of the Neuronetics Note, upon the occurrence of an event of default under the Neuronetics Note, the Company will be required to issue common share purchase warrants (the “Neuronetics Warrants”) to Neuronetics equal to (i) 200% of the unpaid amount of any delinquent amount or payment due and payable under the Neuronetics Note, together with all outstanding and unpaid accrued interest, fees, charges and costs, divided by (ii) the exercise price of the Neuronetics Warrants, which will represent a 20% discount to the 30-day volume-weighted average closing price of the Common Shares traded on Nasdaq prior to the date of issuance (subject to any limitations required by Nasdaq). The events of default under the Neuronetics Note include, without limitation, (a) failure of the Company to make any payment of principal or interest due under the Neuronetics Note within three business days after the payment becomes due; (b) failure of the Company to pay another amount (including late charge or collection costs), within five business days after written request from Neuronetics (c) failure of the Company to timely pay any amount owed under the Neuronetics Agreement; (d) failure of the Company to comply with the covenants in the Neuronetics Note (subject to specified grace periods); (e) cross-default to the Credit Agreement or any other indebtedness in an aggregate principal amount in excess of $1,000,000; and (f) entry into any judgment, order or award for payment against the Company or any Subsidiary (as defined in the Neuronetics Note), in each case, in excess of $1,000,000 which continues unsatisfied or unstayed for (i) 30 days after entry or (ii) if earlier, the date on which any lien attaches in respect of such judgment or order.
On May 25, 2023, the Neuronetics Agreement was amended to include additional out-of-pocket expenses, totaling $0.25 million, incurred by Neuronetics in connection with the negotiation, preparation and delivery of the Neuronetics Note. In addition, Neuronetics has agreed to waive the fee for TMS Device relocations. As at December 31, 2023, the aggregate principal amount remaining on the Neuronetics Note is $5.2 million. The Company has granted a lien on, and security interest in, substantially all of its assets in favor of Neuronetics, as security for the obligations under the Neuronetics Note, and Madryn as security for the obligations under the Credit Agreement. The liens and security interests granted to Neuronetics and Madryn are pari passu (equal priority) pursuant to the terms of an intercreditor agreement, dated as of March 31, 2023, by and among Neuronetics, Madryn and the Company.
February 2024 Public Offering
On February 26, 2024, the Company completed the February 2024 Public Offering for an issuance of 2,828,249 Common Shares at a price of $0.20 per Common Share, for gross proceeds of approximately $565,649 before deducting legal fees and other offering expenses payable the Company. The net proceeds of the February 2024 Public Offering were used for working capital and general corporate purposes.
2023 Private Placement
On March 23, 2023, the Company completed the 2023 Private Placement. An aggregate of 11,363,635 Common Shares were issued at a price of $0.55 per Common Share, for aggregate gross proceeds to the Company of approximately $6.25 million. The 2023 Private Placement included investments by Madryn, together with certain of the Company’s other major shareholders, including Greybrook Health and affiliates of Masters. The Company used the net proceeds from the 2023 Private Placement to fund the Restructuring Plan and for working capital and general corporate purposes.
In connection with the 2023 Private Placement, Greybrook Health, Madryn and Masters each received customary resale, demand and “piggy-back” registration rights pursuant to a registration rights agreement entered into among the parties on closing of the 2023 Private Placement.
Alumni Purchase Agreement
On July 13, 2023, the Company entered into the Alumni Purchase Agreement which provided equity line financing for sales from time to time of up to $4,458,156 of Common Shares. The Purchase Shares in connection with the delivery of purchase notices delivered by the Company to Alumni, at variable prices set forth therein, in accordance with the terms of the Alumni Purchase Agreement.
The Alumni Purchase Agreement expired on December 31, 2023. Prior to expiration, we issued an aggregate of 1,761,538 Purchase Shares for aggregate proceeds to the Company of $481,437. The Company also issued an additional 212,293 Common Shares to Alumni in exchange for Alumni entering into the Alumni Purchase Agreement.
- 78 -
Oxford Credit Facility; Oxford Warrants
On December 31, 2020, the Company entered into a credit and security agreement (the “Oxford Credit Agreement”) in respect of a $30 million credit facility (“Oxford Credit Facility”) with Oxford Finance LLC (“Oxford”). In connection with entering into the Credit Agreement on July 14, 2022, the Company repaid in full the outstanding balance owing under the Oxford Credit Facility and terminated the Oxford Credit Agreement.
As consideration for providing the Oxford Credit Facility, we issued 51,307 common share purchase warrants (the “Oxford Warrants”), each exercisable for one Common Share at an exercise price of C$11.20 per Common Share, to Oxford. To date, none of the Oxford Warrants have been exercised. The Oxford Warrants will expire on December 31, 2025.
Other Indebtedness
During the period ended September 30, 2022, the Company assumed loans as part of the Success TMS Acquisition from three separate financing companies for the purchase of TMS Devices. These TMS Device loans bear an average interest rate of 9.3% with average monthly blended interest and capital payments of $1,538 and mature during the years ending December 31, 2023 to December 31, 2025. There are no covenants associated with these loans.
During Fiscal 2023, the Company repaid TMS Device loans totaling $0.2 million (Fiscal 2022: $0.1 million).
During Fiscal 2022, the Company assumed two promissory notes totaling $0.2 million, bearing interest of 5% per annum with a maturity date of December 31, 2025. In addition, on July 14, 2022, in connection with the Success TMS Acquisition, the Company assumed the obligation of the Klein Note. The Klein Note totals $2.1 million, bears interest at a rate of 10% per annum and matures on May 1, 2024.
During Fiscal 2023, the Company paid $0.2 million towards these three promissory notes (collectively, including the Klein Note, the “Success TMS Notes”) (Fiscal 2022: $0.2 million).
On November 21, 2023, the Company announced it had entered into the Klein Settlement Agreement with the plaintiff regarding the Klein Note Action. Under the terms of the Klein Settlement Agreement, the Company agreed to make payments to the plaintiff in the total amount of approximately $2.2 million, structured as an initial immediate payment of $250,000, weekly payments of $75,000 thereafter up to and until the May 1, 2024, maturity date of the promissory note, upon which the balance owing will be due. In exchange for entry into the settlement agreement, the plaintiff dismissed, with prejudice, the Klein Note Action on November 27, 2023, and both parties provided a mutual release of claims.
In connection with a settlement and mutual release agreement with a device manufacturer for the termination of TMS Device contracts (the “TMS Device Settlement”), as required by the Neuronetics Agreement, the Company had an amount payable of $6,600,000, due in equal instalments over 44 weeks beginning in August 2023 (of which we have $1,200,000 outstanding as of the date of this Annual Report).
Critical Accounting Estimates
Our audited consolidated financial statements have been prepared in accordance with US GAAP. The preparation of our financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. We base our estimates on historical experience and on various other assumptions that we believe are reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. While our significant accounting policies are more fully described in the notes to our audited consolidated financial statements, we believe that the following accounting policies and estimates are critical to our business operations and understanding our financial results.
The following are the key judgments and sources of estimation uncertainty that we believe could have the most significant impact on the amounts recognized in our audited consolidated financial statements.
Revenue Recognition
Due to the nature of the industry and complexity of our revenue arrangements, where price lists are subject to the discretion of payors, variable consideration exists that may result in price concessions and constraints to the transaction price for the services rendered.
- 79 -
In estimating this variable consideration, we use significant judgement and considers various factors including, but not limited to, the following:
| ● | commercial payors and the administrators of federally-funded healthcare programs exercise discretion over pricing and may establish a base fee schedule for TMS (which is subject to change prior to final settlement) or negotiate a specific reimbursement rate with an individual Treatment provider; |
| ● | average of previous net service fees received by the applicable payor and fees received by other patients for similar services; |
| ● | management’s best estimate, leveraging industry knowledge and expectations of third-party payors’ fee schedules; |
| ● | factors that would influence the contractual rate and the related benefit coverage, such as obtaining pre-authorization of services and determining whether the procedure is medically necessary; |
| ● | probability of failure in obtaining timely proper provider credentialing (including re-credentialling) and documentation, in order to bill various payors which may result in enhanced price concessions; and |
| ● | variation in coverage for similar services among various payors and various payor benefit plans. |
We update the estimated transaction price (including updating its assessment of whether an estimate of variable consideration is constrained) to represent faithfully the circumstances present at the end of the reporting period and the changes in circumstances during the reporting period in which such variances become known.
The above factors are not related to the creditworthiness of the large medical insurance companies and government-backed health plans encompassing the significant majority of our payors. The payors (large insurers and government agencies) have the ability and intent to pay, but price lists for our services are subject to the discretion of payors. As a result, the adjustment to reduce the transaction price and constrain the variable consideration is a price concession and not indicative of credit risk on the payors (i.e. not a bad debt expense).
Business Combinations
We account for business combinations using the acquisition accounting method. The total purchase price is allocated to the assets acquired and liabilities assumed based on fair values as at the date of acquisition. Goodwill as at the date of acquisition is measured as the excess of the aggregate of the consideration transferred and the amount of any non-controlling interests in the acquired company over the net of the acquisition date fair values of the identifiable assets acquired and the liabilities assumed. Any non-controlling interest in the acquired company are measured at the non-controlling interests’ proportionate share of the identifiable assets and liabilities of the acquired business.
Best estimates and assumptions are used in the purchase price allocation process to accurately value assets acquired and liabilities assumed at the business combination date. These estimates and assumptions are inherently uncertain and subject to refinement. As a result, during the measurement period, which may be up to one year from the business combination date, the Company may record adjustments to the assets acquired and liabilities assumed, with the corresponding offset to goodwill. On conclusion of the measurement period or final determination of the values of the assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded in the consolidated statement of net loss and comprehensive loss in the period in which the adjustments were determined.
Any deferred and contingent consideration is measured at fair value at the date of acquisition. During the measurement period, which may be up to one year from the business combination date and on conclusion of the measurement period, if an obligation to pay contingent consideration that meets the definition of a financial instrument is classified as equity, then it is not remeasured and the settlement is accounted for within equity. Otherwise, other contingent consideration is remeasured at fair value at each reporting date and subsequent changes in the fair value of the contingent consideration is recognized as part of the consolidated statement of net loss and comprehensive loss in the period in which the adjustments were determined.
- 80 -
Accounts Receivable
We consider a default to be a change in circumstances that results in the payor no longer having the ability and intent to pay. In these circumstances, a write-off of the related accounts receivable balance to bad debt would be recognized.
In estimating the collectability of its accounts receivable, we consider macroeconomic factors in assessing accounts receivable. Such factors would need to be significant to affect the ability and intent of our payors given their size and stature. No such factors were identified as at December 31, 2023, or the comparative period, and therefore no bad debt was recognized.
Impairment of non-financial assets
The Company assesses, at each reporting date, whether there is an indication that a non-financial asset, including goodwill and intangible assets, may be impaired. If any indication exists, the Company estimates the recoverable amount. The recoverable amount of an asset is the higher of its fair value, less costs to sell, and its value in use.
Fair value less costs to sell is the amount obtainable from the sale of an asset in an arm’s length transaction between knowledgeable, willing parties, less the costs of disposal. Costs of disposal are incremental costs directly attributable to the disposal of an asset and related income tax expense.
In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
If the carrying amount of an asset exceeds its recoverable amount, an impairment charge is recognized immediately in the consolidated statements of net loss and comprehensive loss by the amount by which the carrying amount of the asset exceeds the recoverable amount. Where an impairment loss subsequently reverses, the carrying amount of the asset (except goodwill) is increased to the lesser of the revised estimate of the recoverable amount, and the carrying amount that would have been recorded had no impairment loss been recognized previously.
Goodwill acquired in business combinations is allocated to reporting units (or groups of reporting units) that are expected to benefit from the synergies of the combination. The determination of reporting units and the level at which goodwill is monitored requires judgment by management. Goodwill is tested annually for impairment and as required when impairment indicators exist, by comparing the carrying value of the reporting units against the recoverable amount.
Impairment loss represents the impairment of assets as part of our annual impairment testing of goodwill and long-lived assets. A goodwill impairment charge is recognized to the extent that the carrying value exceeds the fair value of the Company’s reporting unit. As at December 31, 2022, we recorded impairment charges of $22.1 million on goodwill, which reduced our goodwill balance to nil as at December 31, 2022, and this amount remains nil as at December 31, 2023. See Note 7(b) within the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023. As at December 31, 2022, the Company performed its annual assessment of the long-lived assets allocated to each of the Company’s asset groups and determined that all asset groups met an overall indication of impairment and conducted the test for recoverability. The Company determined that as part of the long-lived asset impairment, the property, plant and equipment and right-of-use assets held by these asset groups had fair values in excess of the carrying values. However, the intangible assets held by certain asset groups had a recoverable amount of nil and were fully impaired. As a result, impairment loss of $23.8 million was recorded in the consolidated statement of net loss and comprehensive loss in Fiscal 2022. As at December 31, 2023, the Company performed its annual assessment of the long-lived assets allocated to each of the Company’s asset groups and determined that all asset groups met an overall indication of impairment and conducted the test for recoverability. The Company determined that the property, plant and equipment, right-of-use assets and intangible assets held by these asset groups had fair values in excess of the carrying values at December 31, 2023 and therefore no impairment loss was recorded in Fiscal 2023 relating to long-lived asset groups. See Note 7(c) within the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023. On December 31, 2023, we conducted impairment testing for our right-of-use assets resulting from the execution of the Restructuring Plan, resulting in an impairment loss of $0.3 million being recorded for Fiscal 2023. See Note 8(b) within the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023. As of December 31, 2023, the Company’s goodwill and intangible assets total $0.6 million.
- 81 -
Changes in Significant Accounting Policies
Other than as described herein, there are no recent accounting pronouncements that are applicable to the Company or that are expected to have a significant impact on the Company.
ITEM 7B.QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
Not Applicable.
ITEM 8.FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this item are included beginning on page F-1 of this Annual Report on Form 10-K. See Item 15, “Exhibits, Financial Statement Schedules.”
ITEM 9.CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
- 82 -
ITEM 9A.CONTROLS AND PROCEDURES
Disclosure Controls & Procedures
Management is responsible for establishing and maintaining a system of disclosure controls and procedures to provide reasonable assurance that information required to be disclosed by the Company in its annual filings, interim filings or other reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods specified in the securities legislation. Management is also responsible for the information relating to be disclosed by the Company is recorded, processed, summarized and reported to senior management, including the Chief Executive Officer (“CEO”) and the Chief Financial Officer (“CFO”), on a timely basis so that appropriate decisions can be made regarding public disclosure, including to ensure that information required to be disclosed by the Company in reports that the Company files or submits under the U.S. Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC.
Management, under the oversight of the CEO and CFO, has evaluated the design and effectiveness of the Company’s disclosure controls and procedures as of December 31, 2023. Based on this evaluation, the CEO and the CFO concluded that, as of December 31, 2023, the Company’s disclosure controls and procedures (as defined in National Instrument 52-109 – Certification of Disclosure in Issuers’ Annual and Interim Filings and in Rule 13a-15(e) and Rule 15d-15(e) under the U.S. Exchange Act) were ineffective due to the material weaknesses in internal controls described below.
The Company’s disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives, and the CEO and CFO do not expect that the disclosure controls and procedures will prevent all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
Management’s Annual Report on Internal Controls Over Financial Reporting
Management is also responsible for establishing and maintaining adequate internal controls over financial reporting (“ICFR”) which is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial reports for external purposes in accordance with US GAAP. In designing such controls, it should be recognized that, due to inherent limitations, any controls, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives and may not prevent or detect misstatements. Additionally, management is required to use judgment in evaluating controls and procedures.
An evaluation of the design and effectiveness of the Company’s internal controls over financial reporting was carried out by management, under the supervision of the CEO and CFO. In making this evaluation, the CEO and CFO used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) Internal Control – Integrated Framework (2013).
Based on this evaluation, the CEO and CFO has concluded that, as of December 31, 2023, the Company’s internal controls over financial reporting were not effective due to the material weaknesses in internal controls described below.
Material Changes in Internal Controls Over Financial Reporting in Fiscal 2022
In connection with the audit of our annual consolidated financial statements for Fiscal 2022 that were prepared in accordance with International Financial Reporting Standards (“IFRS”), and audited in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), our management identified a material weakness in our internal control over financial reporting as of December 31, 2022. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. Due to the forecasting complexities that arose from an evolving operating environment including the announcement of the Restructuring Plan and significant financing transactions throughout the first quarter of Fiscal 2023 and into April 2023, our management identified a material weakness related to the Company not having effectively designed and maintained controls over the effective preparation, review and approval of its impairment analysis.
This control deficiency, which was not pervasive in nature and was isolated in impact, resulted in a material misstatement to the Company’s financial statements identified through the audit, which was corrected by management. The identified error resulted in certain adjustments to the amounts or disclosures isolated to goodwill, intangible assets and impairment loss. This error was corrected prior to the release of the annual consolidated financial statements for the Fiscal 2022.
- 83 -
In light of the aforementioned material weakness, the Company retained an external valuation firm to assess and analyze impairment in Fiscal 2023. In addition, the company retained an accounting advisory firm to assess and analyze the impact of the conversion to US GAAP from IFRS, including its impact on impairment for both Fiscal 2022 and Fiscal 2023. However, due to the timing and complexities that arose as part of the conversion to US GAAP from IFRS, which delayed the impairment analysis for Fiscal 2023 into March 2024, the material weakness was not remediated by December 31, 2023.
As a result of the changes to goodwill and intangible assets from the conversion to US GAAP from IFRS, the balance of goodwill and intangible assets as at December 31, 2023 and December 31, 2022 is nil and $0.6 million respectively. Given the immaterial nature of the remaining balance as at December 31, 2023, no further remediation in respect of the amounts disclosed as at December 31, 2023 is necessary. Nevertheless, management will prospectively perform its impairment analysis in October of each year to ensure its completion prior to year-end in order to avoid future material weaknesses relating to impairment testing.
Material Changes in Internal Controls Over Financial Reporting in Fiscal 2023
In connection with the audit of our annual consolidated financial statements for Fiscal 2023 that were prepared in accordance with US GAAP, and audited in accordance with the standards of the PCAOB our management identified a material weakness in our internal control over financial reporting as of December 31, 2023. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. Due to the complexities and estimation uncertainty that inherently exist in the recognition of revenue, our management identified a material weakness related to the Company not having effectively designed and maintained controls over the effective preparation, review and approval of its adjustment to variable consideration.
This control deficiency, which was not pervasive in nature and was isolated in impact, resulted in a material misstatement to the Company’s financial statements in Fiscal 2022 identified through the audit, which was corrected by management prior to the release of the annual consolidated financial statements for Fiscal 2023 and Fiscal 2022 that are included in this Annual Report. The Company concluded following the discovery of this error that the previously issued financial statements for Fiscal 2022 could no longer be relied upon as the identified error resulted in certain adjustments to the amounts or disclosures isolated to revenue, retained earnings and accounts receivable in Fiscal 2022 and Fiscal 2023. For more information, see the Explanatory Note following the cover page of this Annual Report.
We intend to implement a remediation plan that involves enhancing our current controls surrounding the adjustment to variable consideration, and the expected credit loss model by which it is calculated, by more rigorously testing the inputs into the expected credit loss model.
The CEO and CFO do not expect that the internal controls over financial reporting will prevent all misstatements. The design of a system of internal controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that the design will success in achieving the stated goals under all potential future conditions. Nevertheless, management has designed and implemented controls to mitigate this risk to the extent practicable.
Notwithstanding the material weakness described above, management has concluded that the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023 that are included in this Annual Report present fairly, in all material respects, the Company’s financial position, results of operations, changes in equity and cash flows in accordance with US GAAP.
As an emerging growth company, we are not required to provide, and this Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting.
ITEM 9B.OTHER INFORMATION
None of our directors or executive officers
ITEM 9C.DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
- 84 -
PART II
ITEM 10.DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors
Set out below is information with respect to our directors. Our directors are elected by our shareholders at each annual general meeting.
Name |
| Age |
| Independent |
| Board Committee |
|
Brian P. Burke | 68 | Yes | Compensation Committee; Governance and Nominating Committee | ||||
Colleen Campbell | 66 | Yes | Audit Committee | ||||
Sasha Cucuz(1) | 46 | No | None | ||||
Juliana Elstad | 47 | Yes | Governance and Nominating Committee | ||||
Bill Leonard(2) | 59 | No | None | ||||
Surindra Mann | 57 | Yes | Audit Committee; Compensation Committee | ||||
Frank Tworecke | 77 | Yes | Audit Committee; Compensation Committee; Governance and Nominating Committee | ||||
Elias Vamvakas | 65 | Yes | None |
| (1) | Mr. Cucuz is the Executive Chairman of the Company and is considered a non-independent director because of his role in the Company’s management team. For more information on our independence standard see “—Corporate Governance—Composition and Independence of the Board” below. |
| (2) | Mr. Leonard is President and Chief Executive Officer of the Company is considered a non-independent director because of his role in the Company’s management team. For more information on our independence standard see “—Corporate Governance—Composition and Independence of the Board” below. |
Brian P. Burke is currently the Executive Director of the Professional Women’s Hockey League Players Association after having previously served as president of the Pittsburgh Penguins of the National Hockey League, from 2021 until 2023. Prior to joining the Pittsburgh Penguins, Mr. Burke was a studio analyst at Rogers Sportsnet, a Canadian television sports network, from May 2018 to February 2021. Following graduation from Harvard Law School in 1981, Mr. Burke practiced corporate and securities law, with a focus on professional athletes and teams. Mr. Burke has been the president and/or general manager of several hockey organizations, including the Calgary Flames, Toronto Maple Leafs, Anaheim Ducks, Vancouver Canucks and the Hartford Whalers during the period from 1992 to 2018. Mr. Burke previously served as a member of the boards of directors of the Sports Lawyers Association, Canuck Place Children’s Hospice Foundation and Rugby Canada. Mr. Burke is also a member, and served on the selection committee of, the Hockey Hall of Fame. Mr. Burke received a Juris Doctor from Harvard Law School and a bachelor’s degree in history from Providence College.
Colleen Campbell retired as vice-chair of Bank of Montreal (“BMO”) Capital Markets in January 2023 after a 44 year career in investment banking. Ms. Campbell served as global head of BMO’s debt capital markets group until 2023 working with corporate, infrastructure and government clients on bond underwriting. Colleen was also a member of the firm’s management committee. In 2012 Colleen was appointed as vice chair of the firm as well as chair of the Investment Committee for the Merchant Bank Real Estate Private Equity Fund, chair of the Investment Committee of the BMO Real Estate Fund and co-chair of the Investment Bank’s Diversity and Inclusion Steering Committee. Ms. Campbell holds an Honors Business Administration degree from Richard Ivey School of Business.
- 85 -
Sasha Cucuz is currently the chief executive officer of Greybrook Securities, a Toronto-based corporate finance and investment banking firm, a position he has held since 2005. As the chief executive officer of Greybrook Securities, Mr. Cucuz is responsible for co-managing the firm’s operation and investment strategy. Together with his partners, Mr. Cucuz has played a significant role in growing Greybrook Securities’ real estate investment portfolio to include over 100 multi-family and residential development projects, representing over $30 billion worth of estimated completion value. Mr. Cucuz also serves as the Co-chair of Greybrook Securities’ Investment and Project Advisory Committees where he is part of the team responsible for approving new acquisitions and overseeing existing limited partnerships. As the former chief executive officer of Greybrook Health, Mr. Cucuz has been involved in several key transactions throughout the Greybrook Health portfolio including the acquisition of MacuHealth, LLC and Bruder Healthcare Inc. and financings for portfolio companies including TearLab Inc. In addition to being a member of the Board of Greenbrook, Mr. Cucuz is also a Director of Greysprings Apartments and Delos Canada. In January 2022, Mr. Cucuz was appointed to the advisory board of Northland Properties, Canada’s largest privately-owned hospitality company which owns well-established brands such as Moxie’s restaurants, Sandman Hotels and the National Hockey League’s Dallas Stars. Charitably, Mr. Cucuz serves on the boards of the Greybrook Foundation and the Blu Genes Foundation. With over 15 years of experience on multiple boards combined with Mr. Cucuz’s commitment, collaborative nature, risk management and strategic vision, Mr. Cucuz offers a strong skill set and extensive industry insight vital for effective directorship. Mr. Cucuz track record on various boards showcases leadership, strategic acumen, and problem-solving abilities, enables him to provide valuable guidance aligned with Greenbrook’s objectives. Furthermore, Mr. Cucuz’s deep familiarity with Greenbrook as a company fosters informed decision making and contributing to its ongoing prosperity. Mr. Cucuz holds a Bachelor of Arts degree in economics from York University.
Juliana Elstad is currently the president and CEO of Vibrato Medical, a medical device company developing a novel non-invasive technology for treatment of Chronic Limb Threatening Ischemia and Peripheral Arterial Disease. Prior to Vibrato, Ms. Elstad served as CEO of Impleo Medical, a medtech company recognized as one of the world’s Top 50 most innovative startups in 2018; founder of Elstad Medical, advisory firm to medtech CEOs and boards; EVP of business development at Intelect Medical (acquired by Boston Scientific); and led business development efforts at Medtronic, Inc. as well as coordinated strategic planning, performed market analyses for new therapies, and led cross-functional committees. Ms. Elstad is currently a member of the board of directors of AdvaMed, the world’s largest medtech trade association, where she provides strategic oversight to the organization and prioritization of its policy goals.
Bill Leonard is currently the President and Chief Executive Officer of the Company and its predecessor, TMS NeuroHealth Centers Inc., a position he has held since 2011. For more than 20 years, Mr. Leonard has provided operational and strategic leadership in the development of medical devices, pharmaceuticals and healthcare services. Mr. Leonard previously served as president of Leonard Consulting LLC from 2008 to 2011, and president of the Bio-Pharmaceutical Division of Euclid Vision Corporation from November 2007 to December 2010 where he developed FDA strategy for an ophthalmic drop that was successfully approved to undergo clinical trials. Mr. Leonard also served as president of the Refractive Surgery Division of TLC Vision Corporation (“TLC”) from July 2004 to March 2007, where he piloted a comprehensive business strategy and leadership generating over $200 million in revenue with 900 employees and a client base of 13,000 eye care professionals. Mr. Leonard holds a business administration degree from Towson University.
Surindra Mann is the vice-president, global finance, at STAAR Surgical, a Medical Device company located in California, a position she has held since 2017. Prior to her role at STAAR Surgical, Ms. Mann held multiple positions at Abbott Labs leading all aspects of finance during her more than 15-year tenure. Ms. Mann currently serves on the board of directors of Beyond Blindness where she also served as a member of its finance, governance and audit committees. Ms. Mann received a Bachelor of Commerce from the University of Toronto and is a Chartered Professional Accountant (CMA, Ontario, Canada).
Frank Tworecke has more than 35 years of experience in leading major retail and apparel companies. Prior to his retirement in December 2012, Mr. Tworecke acted as group president of Sportswear of Warnaco Group Inc. from May 2004 to December 2011 where he was responsible for the global business units of Calvin Klein Jeans®, Calvin Klein® Underwear and Chaps® sportswear. Prior to this role, Mr. Tworecke served as the president of Cignal Division at Merry-Go-Round Enterprises, Inc., president and chief executive officer of Bon-Ton Stores Inc. and chief operating officer of Jos. A. Bank Clothiers. Mr. Tworecke also served in senior management positions with other apparel companies including MGR Inc., Federated Department Stores, John Wanamaker’s and Lord & Taylor. Mr. Tworecke also served on the boards of directors of Cherokee Inc., Hampshire Group Limited and Sinai Hospital of Baltimore and now serves as a member of the board of directors of Advisors of Grafton Fraser Inc. Mr. Tworecke holds a Bachelor of Science degree from Cornell University and a Master of Business Administration degree from Syracuse University. Mr. Tworecke was also a member of the Business Advisory Council of the Department of Applied Economics and Management at Cornell University.
- 86 -
Elias Vamvakas is the Chairman of the Board, a position he has held since February 2018. Mr. Vamvakas is currently the founder, chairman and chief executive officer of Greybrook Capital Inc., a private equity firm focused on healthcare and real estate, a position he has held since 2007, and the Chairman of The Caldwell Partners International Inc., a position he has held since July 2019. Mr. Vamvakas was previously the chairman of TearLab Corporation, a position he held until July 2020. Prior to founding Greybrook Capital, Mr. Vamvakas co-founded TLC where he served as president and chief executive officer from 1994 to 2004. During this period, Mr. Vamvakas built TLC into the largest eye care service provider organization in North America with revenues of more than $300 million and TLC’s stock appreciating well over $1 billion. Mr. Vamvakas holds a Bachelor of Science degree from the University of Toronto.
Former Directors
During Fiscal 2023, the following individuals also served on the Board, Adrienne Graves, Robert Higgins, Benjamin Klein, and Adele C. Olivia. Mr. Higgins and Ms. Olivia did not stand for re-election at the Company’s annual meeting of shareholders held on June 20, 2023 (the “2023 Annual Meeting”) and Mr. Klein resigned as director after receiving more “withheld” votes than “for” at the 2023 Annual Meeting. Ms. Graves was re-elected at the 2023 Annual Meeting but subsequently resigned on October 31, 2023.
Ms. Olivia, Mr. Higgins, and Ms. Graves were all considered independent directors during Fiscal 2023 while serving as directors. Mr. Klein was considered a non-independent director as a result of the fact that he was the Company’s Chief Operating Officer.
Executive Officers
Set out below is information with respect to our executive officers. Our executive officers serve for an indefinite term, subject to the terms of their employment agreements (if any).
Name |
| Age |
| Position |
|
Andrew Crish | 47 | Chief Operating Officer | |||
Sasha Cucuz | 46 | Chairman | |||
Geoffrey Grammer | 53 | Chief Medical Officer | |||
Bill Leonard | 59 | President and Chief Executive Officer | |||
Peter Willett | 34 | Chief Financial Officer |
Andrew Crish Mr. Crish has a diverse work experience spanning over two decades. Prior to joining Greenbrook, Andrew served as the Vice President of Operations at M2 Orthopedics since December 2021. Prior to this, he worked as the Vice President of Operations at National Veterinary Associates from November 2018 to November 2021. Before joining National Veterinary Associates, Andrew held various roles at DaVita Kidney Care, including Division Vice President; Division Vice President of Hospital Service; Group Director of Hospital Services; and Regional Operations Director. Andrew’s tenure at DaVita Kidney Care lasted from September 2013 to November 2018. Andrew’s earlier experience includes working at GE Healthcare as a Business Operations Manager, Director of Service, and Quality Assurance Leader from April 2007 to December 2012. Andrew also worked as a Consultant at BearingPoint, Inc from October 2006 to October 2007. Before transitioning into the corporate sector, Andrew served in the US Navy from May 2000 to October 2006 as a Surface Warfare Officer, Facilities Manager, and Instructor. Andrew completed his Bachelor of Science degree in Economics at the United States Naval Academy from 1996 to 2000. Andrew then pursued further education and obtained his MBA from Northwestern University - Kellogg School of Management, specializing in Marketing, Finance, Management & Strategy, from 2008 to 2011.
- 87 -
Sasha Cucuz – see “—Directors” above.
Geoffrey Grammer, M.D., (U.S. Army, Ret.), serves as Chief Medical Officer of Greenbrook, where he sets and implements clinical policies, protocols, and training for all of our Treatment Centers. A highly decorated military physician who holds both a Bronze Star and the Legion of Merit Award, Dr. Grammer served in a broad range of clinical and organizational leadership positions in the Army, including two deployments to Iraq, first as Medical Director for the 785th Combat Stress Control Company and later as a psychiatrist at the Combat Support Hospital at Contingency Operating Base Speicher. He also deployed to Afghanistan as a psychiatrist at the Combat Support Hospital in Bagram. In addition to those deployments, Dr. Grammer served as Chief of Inpatient Psychiatric Services at Walter Reed National Military Medical Center, where he launched one of the nation’s first TMS therapy programs. A globally respected researcher and thought leader, Dr. Grammer also served as Department Chief of Research at the National Intrepid Center of Excellence, a Department of Defense organization specializing in treatment-resistant psychological health and traumatic brain injury conditions in active duty service members, and later as National Director for the Defense and Veterans Brain Injury Center. He is published in numerous peer-reviewed journals and has authored several book chapters. Dr. Grammer graduated from Virginia Polytechnic Institute with a Bachelor of Science degree in Biology and earned his Doctor of Medicine from the Uniformed Services University in Bethesda, Maryland. He holds board certifications in Psychiatry and Geriatric Psychiatry.
Bill Leonard – see “—Directors” above.
Peter Willett is currently the Chief Financial Officer of the Company, prior to which he had been a key player on the Company’s finance team over the past seven years, serving most recently as its Interim Chief Financial Officer. Mr. Willett brings over 11 years of finance experience, filled with extensive knowledge about the financial and accounting matters that are unique to the mental health services industry. Mr. Willett has been instrumental in developing and implementing the financial strategies that have been successful in improving reporting functions and costs controls for the Company. Mr. Willett is a Chartered Professional Accountant and holds a Bachelor of Commerce degree from Queen’s University.
Corporate Governance
Composition and Independence of the Board
The Company’s Board is currently composed of eight directors, a majority (six) of whom meet the independence standards under the listing standards of Nasdaq and National Instrument 52-110 – Audit Committees. Each member of the Audit Committee, the Compensation Committee and the Governance and Nominating Committee (“GN Committee”) also meet such independence standards, and in the case of Audit Committee members, the additional independence requirements of Rule 10A-3 of the Exchange Act. Each year the Board reviews the composition of the Board, the Audit Committee, the Compensation Committee and the GN Committee and assesses whether a Board or Committee member is “independent”, including an assessment of any direct or indirect relationship between each Board member, on one hand, and the Company or any of its subsidiaries, or with management, on the other than, which, in the Board’s opinion, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Director Term Limits and Other Mechanisms of Board Renewal
Directors are to be elected at each annual meeting of our shareholders to hold office for a term expiring at the close of the next annual meeting, or until a successor is appointed or elected, and will be eligible for re-election at each annual meeting. Director nominees are nominated by the GN Committee, in each case for election by our shareholders as directors in accordance with the provisions of our constating documents and applicable corporate and securities laws. All nominees who are nominated by the GN Committee will be included in the proxy-related materials to be sent to shareholders prior to each annual meeting of our shareholders.
Our Board has not adopted director term limits or other automatic mechanisms of board renewal. The Company believes such limits and automatic mechanisms would discount the value of experience and unnecessarily deprive the Company of the contribution by directors who have developed a deep knowledge of the Company over time. Rather than adopting formal term limits, mandatory age-related retirement policies and other mechanisms of board renewal, the GN Committee will seek to maintain the composition of our Board in a way that provides, in the judgment of our Board, the best mix of skills and experience to provide for our overall stewardship. Our GN Committee is also expected to conduct a process for the assessment of our Board, each committee and each director regarding his, her or its effectiveness and performance, and to report evaluation results to our Board.
- 88 -
Board Committees
Our Board has now established three committees: the Audit Committee, the GN Committee and the Compensation Committee. All members of the Audit Committee, the GN Committee and the Compensation Committee are persons determined by our Board to be independent directors in accordance with applicable securities and stock exchange rules.
Audit Committee
The Board has a separately designated standing Audit Committee established for the purpose of overseeing the accounting and financial reporting processes of the Company and audits of the financial statements of the Company in accordance with Section 3(a)(58)(A) of the Exchange Act. Our Audit Committee consists of three (3) directors, each of whom are persons determined by our Board to be both independent directors and financially literate or sophisticated, each within the meaning of applicable U.S. and Canadian securities laws and/or stock exchange rules. Our Audit Committee is currently comprised of:
| ● | Colleen Campbell (Chair); |
| ● | Surindra Mann; and |
| ● | Frank Tworecke. |
Each of our Audit Committee members has an understanding of the accounting principles used to prepare financial statements and varied experience as to the general application of such accounting principles, as well as an understanding of the internal controls and procedures necessary for financial reporting. Each of Ms. Campbell, Ms. Mann and Mr. Tworecke is independent under Rule 10A-3 under the Exchange Act. The Company is currently registered under Section 12(b) of the Exchange Act; however, on April 1, 2024, the Company filed a Form 25 with the SEC to complete the delisting of the Common Shares from the Nasdaq on or about April 1, 2024, with the delisting becoming effective 10 days after such filing. The deregistration of the Common Shares under Section 12(b) of the Exchange Act will become effective 90 days after the filing of the Form 25, or such shorter period as the SEC may determine. Following deregistration under Section 12(b) of the Exchange Act, the Company will cease to be subject to the requirements of Rule 10A-3 under the Exchange Act.
Our Board has adopted a written charter for the Audit Committee that sets out the purpose, composition, authority and responsibility of our Audit Committee, consistent with applicable U.S. and Canadian securities laws and/or stock exchange rules. A copy of the charter of our Audit Committee has been posted on our website at www.greenbrooktms.com. The Audit Committee assists our Board in discharging its oversight of:
| ● | the quality and integrity of our financial statements and related information; |
| ● | the independence, qualifications and appointment of our external auditor; |
| ● | our disclosure controls and procedures, internal control over financial reporting and management’s responsibility for assessing and reporting on the effectiveness of such controls; |
| ● | our risk management processes; |
| ● | monitoring and periodically reviewing our whistleblower policy; and |
| ● | transactions with our related parties. |
Our Audit Committee has access to all of our books, records, facilities and personnel and may request any information about us as it may deem appropriate. It also has the authority, in its sole discretion and at our expense, to retain and set the compensation of outside legal, accounting or other advisors as necessary to assist in the performance of its duties and responsibilities. Our Audit Committee also has direct communication channels with the Chief Financial Officer and our external auditors to discuss and review such issues as our Audit Committee may deem appropriate.
- 89 -
As of the date of this Annual Report, the Audit Committee is comprised of Surindra Mann, Frank Tworecke and chair Colleen Campbell,
The Board has determined that Colleen Campbell, an independent director, is an “audit committee financial expert” within the meaning of the rules of the SEC.
Governance and Nominating Committee
Our GN Committee is comprised of three (3) directors, each of whom has been determined by our Board to be an independent director, and is charged with reviewing, overseeing and evaluating our corporate governance and nominating policies. Our GN Committee is currently comprised of:
| ● | Frank Tworecke; |
| ● | Brian P. Burke;and |
| ● | Juliana Elstad. |
No member of our GN Committee is an officer of Greenbrook, and as such, our Board believes that the GN Committee is able to conduct its activities in an objective manner.
Our Board believes that the members of the GN Committee individually and collectively possess the requisite knowledge, skill and experience in governance matters, including human resource management and general business leadership, to fulfill the GN Committee’s mandate. All members of the GN Committee have substantial knowledge and experience as current and former senior executives of large and complex organizations.
Our Board has adopted a written charter for the GN Committee that sets forth the purpose, composition, authority and responsibility of our GN Committee. A copy of the written charter of the GN Committee has been posted on our website at www.greenbrooktms.com. Our GN Committee’s purpose is to assist our Board in:
| ● | the recruitment, development and retention of our senior executives; |
| ● | maintaining talent management and succession planning systems and processes relating to our senior management; |
| ● | developing our corporate governance guidelines and principles and providing us with governance leadership; |
| ● | identifying and overseeing the recruitment of candidates qualified to be nominated as members of our Board; |
| ● | reviewing the structure, composition and mandate of Board committees; and |
| ● | evaluating the performance and effectiveness of our Board and of our Board committees. |
Our GN Committee is responsible for establishing and implementing procedures to evaluate the performance and effectiveness of our Board, committees of our Board and the contributions of individual Board members. Our GN Committee also takes reasonable steps to evaluate and assess, on an annual basis, directors’ performance and effectiveness of our Board, committees of our Board, individual Board members, our chair and committee chairs. The assessment addresses, among other things, individual director independence, individual director and overall Board skills, and individual director financial literacy. Our Board receives and considers the recommendations from our GN Committee regarding the results of the evaluation of the performance and effectiveness of our Board, committees of our Board, individual Board members, our chair and committee chairs. In identifying new candidates for our Board, the GN Committee will consider what competencies and skills our Board, as a whole, should possess and assess what competencies and skills each existing director possesses, considering our Board as a group, and the personality and other qualities of each director, as these may ultimately determine the boardroom dynamic. Our GN Committee is also responsible for orientation and continuing education programs for our directors.
- 90 -
Compensation Committee
Our Compensation Committee is comprised of three (3) directors, each of whom has been determined by our Board to be an independent director, and is charged with reviewing, overseeing and evaluating our compensation policies. Our Compensation Committee is currently comprised of:
| ● | Brian P. Burke (Chair); |
| ● | Frank Tworecke; and |
| ● | Surindra Mann. |
No member of our Compensation Committee is an officer of Greenbrook, and as such, our Board believes that the Compensation Committee is able to conduct its activities in an objective manner.
Our Board believes that the members of the Compensation Committee individually and collectively possess the requisite knowledge, skill and experience in compensation matters, including human resource management, executive compensation matters and general business leadership, to fulfill the Compensation Committee’s mandate. All members of the Compensation Committee have substantial knowledge and experience as current and former senior executives of large and complex organizations.
Our Board has adopted a written charter for the Compensation Committee that sets forth the purpose, composition, authority and responsibility of our Compensation Committee. A copy of the charter has been posted on our website at www.greenbrooktms.com. Our Compensation Committee’s purpose is to assist our Board in:
| ● | the appointment, performance, evaluation and compensation of our senior executives |
| ● | developing a compensation structure for our senior executives including salaries, annual and long-term incentive plans including plans involving share issuances and other share-based awards; |
| ● | establishing policies and procedures designed to identify and mitigate risks associated with our compensation policies and practices; |
| ● | assessing the compensation of our directors; and |
| ● | developing benefit, retirement and savings plans. |
Legal Proceedings
No director or executive officer is, to the knowledge of the Company as at the date of the Annual Report, or has been, within 10 years before the date of this Annual Report, a director, chief executive officer or chief financial officer of any company (including the Company) that: (i) was subject to a cease trade order, an order similar to a cease trade order or an order that denied the relevant company access to any exemption under Canadian securities legislation that was in effect for a period of more than 30 consecutive days, (ii) was subject to cease trade order, an order similar to a cease trade order or an order that denied the relevant company access to any exemption under Canadian securities legislation that was in effect for a period of more than 30 consecutive days that was issued after the proposed director ceased to be a director, chief executive officer or chief financial officer and which resulted from an event that occurred while that person was acting in the capacity as director, chief executive officer or chief financial officer, (iii) while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets, (iv) become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromised with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the proposed director, or (v) has been involved in any criminal convictions or proceedings, order or judgment or decree limiting the person from engaging in any type of business or securities, nor found by a court or the SEC to have violated a United States federal or state securities law nor found by a court or the Commodity Futures Trading Commission to have violated any United States federal commodities law.
- 91 -
No director or executive officer of the Company has been subject, to the knowledge of the Company, to (i) any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority, or (ii) any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable securityholder in deciding whether to vote for a proposed director.
Code of Ethics
We have adopted a Code of Conduct which is applicable to all directors, officers and employees. All amendments to the Code of Conduct, and all waivers of the Code of Conduct with respect to any of the officers covered by it, will be promptly posted on our website and provided in print to any shareholder who requests them. A copy of our Code of Conduct is located on our website at www.greenbrooktms.com under the heading “Investor Relations—Governance”.
Insider Trading Policy
The Company has an insider trading policy which governs the purchase, sale and other dispositions of our securities by our directors, officers and other employees. This policy promotes compliance with applicable securities laws and regulations, including those that prohibit insider trading. A copy of our Insider Trading Policy is filed as Exhibit 19.1 to this Annual Report and is located on our website at www.greenbrooktms.com under the heading “Investor Relations—Governance”.
ITEM 11.EXECUTIVE COMPENSATION.
Compensation Overview
The compensation of our executive officers includes two major elements: (i) base salary, and (ii) a discretionary annual bonus. Additionally, from time to time, the Board has granted awards to executives who have taken on additional responsibilities and/or as a way to periodically recognize executives who have consistently performed at an exceptional level. These awards are in the form of Options, RSUs and/or PSUs (each as defined and described further below) granted under the Greenbrook TMS Inc. Amended and Restated Omnibus Equity Incentive Plan (the “Equity Incentive Plan”), which assist the Company in retaining key employees who have the potential to add value to the Company over the longer term. Perquisites and benefits are not a significant element of compensation of our executive officers.
Named Executive Officers
Our executive leadership team includes our Chief Executive Officer, Mr. Bill Leonard, our Chief Financial Officer, Mr. Peter Willett, our Chief Medical Officer, Dr. Geoffrey Grammer and our former Chief Financial Officer and Treasurer, Mr. Erns Loubser (each a “Named Executive Officer” or “NEO”).
Summary Compensation Table
The following table details the compensation information for the fiscal years ended December 31, 2023 and December 31, 2022 of the Company for each of our NEOs, as determined pursuant to the SEC’s disclosure requirements for executive compensation in Item 402 of Regulation S-K. All amounts presented are as recorded in U.S. dollars.
|
|
| Bonus |
| Option |
| All Other |
| |||||||||
Name and Principal Position | Year | Salary | (6) | Awards (1) | Compensation | Total | |||||||||||
Bill Leonard Chief Executive Officer and President |
| 2023 | $ | 475,000 |
| — | $ | 66,000 | $ | 15,094 | (2) | $ | 556,094 | ||||
| 2022 | $ | 468,750 |
| — |
| — |
| — | $ | 468,750 | ||||||
Peter Willett(4)(5) Chief Financial Officer |
| 2023 | $ | 251,767 |
| — | $ | 49,500 |
| — | $ | 301,267 | |||||
| 2022 | $ | 172,555 | $ | 20,000 |
| — |
| — | $ | 192,555 | ||||||
Geoffrey Grammer Chief Medical Officer |
| 2023 | $ | 300,000 |
| — | $ | 50,700 | $ | 10,500 | $ | 361,200 | |||||
| 2022 | $ | 293,750 |
| — |
| — | $ | 10,281 | (3) | $ | 304,031 | |||||
Erns Loubser(4)(5) Chief Financial Officer (Former) |
| 2023 | $ | 283,234 |
| — | $ | 66,000 |
| — | $ | 349,234 | |||||
| 2022 | $ | 315,000 |
| — |
| — |
| — | $ | 315,000 | ||||||
| (1) | The amounts listed in this column represent the grant date fair value computed in accordance with Financial Accounting Standards Board ASC 718 of 100,000 Options granted to Mr. Leonard on May 15, 2023, 75,000 Options granted to Mr. Willett on May 15, 2023, 75,000 Options granted to Dr. Grammer on May 15, 2023 and 5,000 Options granted to Dr. Grammer on November 8, 2023. See Note 16 within the Company’s audited consolidated financial statements as at and for the year ended December 31, 2023 for a discussion of the relevant assumptions used in calculating value pursuant to ASC 718. |
- 92 -
| (2) | Mr. Leonard received $15,094 in employer 401(k) matching contributions during Fiscal Year 2023. |
| (3) | Dr. Grammer received $10,500 and $10,281 in employer 401(k) matching contributions during Fiscal Years 2023 and 2022, respectively. |
| (4) | Mr. Loubser voluntarily left the Company effective as of October 20th, 2023 and Mr. Willett was appointed Interim Chief Financial Officer. On January 29, 2024, Mr. Willett was appointed Chief Financial Officer. |
| (5) | Mr. Loubser and Mr. Willett were paid in Canadian Dollars. The exchange rate used for Fiscal Year 2023 was the average annual rate of C$1.00 = $0.7409. The exchange rate used for Fiscal Year 2022 was the average annual rate of C$1.00 = $0.7685. |
| (6) | Amounts reflect annual bonuses that were paid to NEOs in respect of Fiscal 2022. Annual bonuses have not been determined for Fiscal 2023. |
Narrative Disclosure to Summary Compensation Table
Employment and Termination Agreements
Mr. Leonard is party to an employment agreement with TMS NeuroHealth Centers Services, LLC, a subsidiary of the Company (“TMS Services US”). Under the employment agreement, Mr. Leonard is entitled to receive a base salary and is eligible to earn a discretionary annual bonus with the target bonus not to exceed $350,000. In connection with Mr. Leonard’s termination by TMS Services US on a without cause basis or his resignation with good reason, Mr. Leonard is entitled to his accrued base salary and paid time off through the termination date, a pro-rated annual bonus payment based on his service up to the termination date, and subject to Mr. Leonard’s execution of a general release of claims in favor of TMS Services US and its affiliates, continued payment of his base salary for a period of 18 months following the termination date. If Mr. Leonard was terminated on a without cause basis or resigned with good reason on December 31, 2023, he would have been entitled to his accrued base salary and paid time off through the termination date, an annual bonus earned for 2023 and a severance payment equal to US$712,500. Mr. Leonard would not have received any incremental payments or benefits in respect of his awards outstanding under the Equity Incentive Plan in connection with such termination events. Mr. Leonard is subject to a customary confidentiality covenant and certain restrictive covenants that will continue to apply following the termination of his employment, including non-competition and non-solicitation provisions which are in effect during Mr. Leonard’s employment and for eighteen months thereafter. Mr. Leonard’s employment agreement is governed by the laws of the state of Delaware.
Mr. Loubser voluntarily left the Company effective as of October 19th, 2023. Mr. Loubser was not entitled to receive any severance payments, incremental payments or benefits in respect of his awards outstanding under the Equity Incentive Plan in connection with his departure.
The other NEOs have not entered into an offer letter, employment agreement, restrictive covenant agreement or other written employment arrangement with the Company or any of its subsidiaries. Further, no NEOs (except with respect to Mr. Leonard as described above) are entitled to any payments or benefits at, following, or in connection with the resignation, retirement or other termination, or a change in control of the Company or any of its subsidiaries, or a change in the executive’s responsibilities following such change in control, whether pursuant to a written or unwritten contract, agreement, plan or other arrangement.
Base Salary
Base salary is provided as a fixed source of compensation for our executive officers. Base salaries are determined on an individual basis taking into account the scope of the executive officer’s responsibilities and their prior experience. Base salaries are reviewed annually by the Board and may be increased based on the executive officer’s success in meeting or exceeding individual objectives, as well as to maintain market competitiveness. In addition, base salaries can be adjusted as warranted throughout the year to reflect promotions or other changes in the scope or breadth of an executive officer’s role or responsibilities.
Discretionary Annual Bonus
The Board believes that its ability to exercise discretion and judgment is critical to ensuring that annual bonuses reflect the assessment of risk in the decisions and actions taken by our executive team and consider unexpected circumstances or events that have occurred during the year. In determining annual bonus amounts, the Board reviews each NEO’s performance over the year, including how their decisions and actions align with the Company’s long-term strategy and how they considered the risks associated with such decisions, along with the Company’s performance over the year. The discretionary annual bonus, if any, typically represents less than 50% of an NEO’s total compensation. No NEOs have a contractual right to a bonus in respect of Fiscal Year 2023. Any discretionary annual bonuses for each NEO for Fiscal Year 2023 have not yet been determined.
- 93 -
Equity Incentive Plan
In 2018, the Company established its a stock option plan, which was amended and restated by our Board on May 24, 2019 (and approved by our shareholders on June 28, 2019) (as amended and restated, the “Stock Option Plan”). In 2021, the Stock Option Plan was further amended and restated by our Board on May 6, 2021 (and approved by our shareholders on June 14, 2021) and is now referred to as the Equity Incentive Plan. The Equity Incentive Plan, as amended and restated on May 6, 2021, applies to all stock options (“Options”), restricted share units (“RSUs”) and performance share units (“PSUs” and, collectively with the Options and RSUs, the “Awards”) granted on or after May 6, 2021. No changes were made to the Equity Incentive Plan in Fiscal Year 2023. The Equity Incentive Plan provides eligible participants with compensation opportunities that enhance our ability to attract and retain our executive officers, other key employees, directors and consultants and ensure that their interests are aligned with the success of the Company and its affiliates. The material features of the Awards are summarized below.
Stock Options
The terms and conditions of grants of Options, including the quantity, grant date, exercise price, vesting conditions and other applicable terms and conditions, will be set out in the participant’s grant agreement. The exercise price for Options will be determined by our Board, which may not be less than the fair market value of a Common Share (the “Fair Market Value”) on the date the Option is granted. Options will vest in accordance with the vesting schedule established on the grant date, which historically has been as to one-third on each of the first three anniversaries of the grant date.
Options must be exercised within a period fixed by our Board that may not exceed ten years from the date of grant, provided that if the expiry date falls during a blackout period, the expiry date will be automatically extended until ten business days after the end of the blackout period. The Equity Incentive Plan also provides for earlier expiration of Options upon the occurrence of certain events, including the termination of a participant’s employment, as described further below.
In order to facilitate the payment of the exercise price of the Options, the Equity Incentive Plan contains a cashless exercise feature and a net settlement feature. Additionally, vested Options can be exercised by payment in full of the applicable exercise price in cash or by certified check, bank draft or money order payable to the Company or by such other means as might be specified from time to time by the Board.
RSUs and PSUs
The terms and conditions of grants of RSUs and PSUs, including the quantity, type of award, grant date, vesting conditions, vesting periods, settlement date and other applicable terms and conditions, will be set out in the participant’s grant agreement.
In the case of PSUs, the performance-related vesting conditions may include financial or operational performance of the Company, total shareholder return (either absolute or relative or both), individual performance criteria or other criteria as determined by our Board, which will be measured over a specified period, generally until the end of the third calendar year from the date of the grant.
Subject to the achievement of the applicable vesting and performance-related (if applicable) conditions, on the settlement date of an RSU or PSU, the Company will either, in its sole discretion (i) issue from treasury the number of Common Shares covered by the RSUs or PSUs and related Dividend Share Units (as defined below), or (ii) deliver to the participant an amount in cash (net of applicable withholding taxes) equal to the number of Common Shares covered by the RSUs or PSUs and related Dividend Share Units multiplied by the Fair Market Value as at the settlement date, or (iii) a combination of (i) and (ii). Notwithstanding the ability for the Company to settle vested RSUs (and related Dividend Share Units) in Common Shares pursuant to subsection (i) or through a cash payment pursuant to subsection (ii) above, vested RSUs held by directors who are Canadian taxpayers will be settled in Common Shares pursuant to subsection (i); provided that, the Company may, in its sole discretion, permit such a director to elect to receive a cash payment pursuant to subsection (ii).
- 94 -
Dividend Share Units
When dividends (other than stock dividends) are paid on Common Shares, additional share units (“Dividend Share Units”) will be automatically credited to each participant who holds RSUs or PSUs on the record date for such dividends. The number of Dividend Share Units to be credited to a participant is equal to the aggregate number of RSUs and PSUs held by the participant on the relevant record date multiplied by the amount of the dividend paid by the Company on each Common Share, and then divided by the Fair Market Value of the Common Shares on the dividend payment date. Dividend Share Units shall be in the form of RSUs or PSUs, as applicable. Dividend Share Units credited to a participant will be subject to the same vesting conditions applicable to the related RSUs or PSUs.
Cessation of Employment or Services
Unless otherwise determined by our Board or if a participant’s employment agreement or consulting agreement or arrangement expressly provides more favorable rights with respect to the Awards, in the event of a cessation of employment or services, the following rights apply.
In the event a participant ceases to be an employee, director or consultant of the Company or a designated affiliate, all outstanding Awards granted to the participant under the Equity Incentive Plan that are unvested on the cessation date will be forfeited. All outstanding Options that have vested as of the cessation date will be exercisable as follows, after which time such vested Options will automatically terminate: (i) if the participant ceases to be an employee, director or an individual consultant by reason of death or disability, the participant’s Options must be exercised within nine (9) months of the date of death or disability; (ii) if the participant ceases to be an employee, director or consultant (whether an individual or entity) by reason of termination without cause, the participant’s Options must be exercised within three (3) months of the cessation date; (iii) if the participant ceases to be an employee, director or consultant (whether an individual or entity) by reason of voluntary resignation or termination, the participant’s Options must be exercised within thirty (30) days of the cessation date; and (iv) if the participant ceases to be an employee, director or consultant (whether an individual or entity) by reason of termination for cause, the participant’s Options will automatically terminate on the cessation date and may no longer be exercised. In no event may Options be exercised later than the applicable expiry date of the Options, after which time all remaining Options will terminate. Any vested RSUs or PSUs held by the participant will be settled as soon as practicable following the cessation date.
In the event a participant ceases to be an employee, director or consultant of the Company or a designated affiliate due to a termination for cause, all Awards (whether vested or unvested) will be forfeited on the cessation date.
Retirement Benefits
The TMS NeuroHealth Services US sponsors a tax-qualified retirement savings plan (the “401(k) Plan”) for its U.S. employees. The TMS NeuroHealth Services US provides an employer safe harbor matching contribution equal to 100% of a participant’s salary deferrals under the 401(k) Plan up to the first 1% of plan eligible compensation, plus 50% of a participant’s salary deferrals under the 401(k) Plan up to the next 5% of plan eligible compensation, subject to the limits imposed by the U.S. Internal Revenue Service. Mr. Leonard participates in the 401(k)Plan and received $15,094 in employer matching contributions for fiscal 2023. Dr. Grammer participates in the 401(k) Plan and received $10,281 in employer matching contributions for Fiscal Year 2022 and $10,500 in employer matching contributions for Fiscal Year 2023.
Other than the 401(k) Plan, the Company does not have any retirement or pension plans that provide for payments of benefits at, following or in connection with, retirement or provide for retirement or deferred compensation plans for the NEOs or directors.
Other Benefits
The Company provides certain executive officers with disability, health and dental insurance programs on a comparable basis as other employees of the Company. These benefits are offered in a manner consistent with local market practice.
The Company does not offer significant perquisites as part of its compensation program.
- 95 -
Clawback Policy
On November 8, 2023, the Board approved an enhanced clawback policy (the “Clawback Policy”) covering the recoupment of compensation in the case of a financial restatement by the Company or misconduct by an executive officer. The Clawback Policy went into effect on December 1, 2023, and is filed as an exhibit to this Annual Report. The Clawback Policy covers all requirements under Section 10D of the Exchange Act and the new SEC regulation promulgated thereunder, as well as the applicable listing standards implementing these requirements, and continues to cover recoupment of compensation for all covered employees in the case of misconduct.
Outstanding Equity Awards at Fiscal Year-End
The following table sets out information on the outstanding equity awards held by each of our NEOs as of December 31, 2023.
Option | |||||||||||||
Awards | Stock Awards | ||||||||||||
|
|
|
|
|
| Equity | |||||||
incentive plan | |||||||||||||
awards: | |||||||||||||
market or | |||||||||||||
payout value | |||||||||||||
Number of | Number of | of unearned | |||||||||||
Securities | Securities | Equity incentive plan | shares, units | ||||||||||
Underlying | Underlying | awards: number of | or other | ||||||||||
Unexercised | Unexercised | unearned shares, units | rights that | ||||||||||
Options (#) | Options (#) | Option | Option | or other rights that | have not | ||||||||
Name | Exercisable | Unexercisable | Exercise Price (1) | Expiration Date | have not vested (#) |
| vested ($) | ||||||
Bill Leonard(2) |
| 50,000 |
| 50,000 | (3) | $ | 0.75 |
| May 15, 2033 |
| — |
| 1,047 |
| 10,000 |
| — | $ | 10.13 |
| February 3, 2030 |
| — |
| — | ||
Peter Willett(4) |
| 37,500 |
| 37,500 | (5) | $ | 0.75 | May 15, 2033 |
| — |
| — | |
| 6,667 |
| 3,333 | (6) | $ | 15.45 | February 17, 2031 |
| — |
| — | ||
| 4,000 |
| — | $ | 10.13 | February 3, 2030 |
| — |
| — | |||
| 5,000 |
| — | $ | 12.44 | March 27, 2029 |
| — |
| — | |||
| 3,000 |
| — | $ | 10.00 | October 3, 2028 |
| — |
| — | |||
| 2,000 |
| — | $ | 7.50 | March 31, 2028 |
| — |
| — | |||
| 3,000 |
| — | $ | 5.00 | March 31, 2027 |
| — |
| — | |||
Geoffrey Grammer(7) |
| 2,500 |
| 2,500 | (8) | $ | 0.2625 | November 8, 2033 |
| — |
| — | |
| 37,500 |
| 37,500 | (9) | $ | 0.75 | May 15, 2033 |
| — |
| — | ||
| 8,000 |
| 4,000 | (10) | $ | 15.45 | February 17, 2031 |
| — |
| — | ||
| 20,000 |
| — | $ | 10.13 | February 3, 2030 |
| — |
| — | |||
| 2,000 |
| — | $ | 5.00 | March 31, 2027 |
| — |
| — | |||
| 5,000 |
| — | $ | 5.00 | March 31, 2026 |
| — |
| — | |||
| 40,000 |
| — | $ | 5.00 | March 31, 2025 |
| — |
| — | |||
Erns Loubser(11) |
| 50,000 |
| 50,000 | (3) | $ | 0.75 | May 15, 2033 |
| — |
| — | |
| 6,667 |
| 3,333 | (12) | $ | 15.45 | February 17, 2031 |
| — |
| — | ||
| 15,000 |
| — | $ | 10.13 | February 3, 2030 |
| — |
| — | |||
| 135,000 |
| — | $ | 5.00 | March 31, 2027 |
| — |
| — | |||
| 5,000 |
| — | $ | 5.00 | March 31, 2026 |
| — |
| — | |||
| 10,000 |
| — | $ | 5.00 | March 31, 2025 |
| — |
| — | |||
| (1) | The Option Exercise Price is reported in U.S. dollars. Of the outstanding Options reported in the table above, the exercise price of the Options granted on February 3, 2020 and February 17, 2021 were C$13.40 and C$20.43, respectively, which has been converted to U.S. dollars based on the exchange rate on December 29, 2023 (the last business day of Fiscal Year 2023) of C$1.00 = US$1.32. All other outstanding Options reported in the table above were granted with an exercise price denominated in U.S. dollars. |
| (2) | Mr. Leonard was granted 10,000 Options on February 3, 2020, which are fully vested; and 100,000 Options on May 15, 2023, of which 50,000 are vested, and, in each case, are subject to the terms of the Equity Incentive Plan. |
| (3) | Of the remaining 50,000 Options, 25,000 will vest on each of May 15, 2024 and May 15, 2025. |
- 96 -
| (4) | Mr. Willett was granted 3,000 Options on March 31, 2017, which are fully vested; 2,000 Options on March 31, 2018, which are fully vested; 3,000 Options on October 3, 2018, which are fully vested; 5,000 Options on March 27, 2019, which are fully vested; 4,000 Options on February 3, 2020, which are fully vested; 10,000 Options on February 17, 2021, of which 6,667 are vested; and 75,000 Options on May 15, 2023, of which 37,500 are vested, and, in each case, are subject to the terms of the Equity Incentive Plan. |
| (5) | Of the remaining 37,500 Options, 18,750 will vest on each of May 15, 2024 and May 15, 2025. |
| (6) | The remaining 3,333 Options vested on February 17, 2024. |
| (7) | Dr. Grammer was granted 40,000 Options on March 31, 2015, which are fully vested; 5,000 Options on March 31, 2016, which are fully vested; 2,000 Options on March 31, 2017, which are fully vested; 20,000 Options on February 3, 2020, which are fully vested; 12,000 Options on February 17, 2021, which are fully vested; 75,000 Options on May 15, 2023, of which 37,500 are vested; and 5,000 Options on November 8, 2023, of which 2,500 are vested, and, in each case, are subject to the terms of the Equity Incentive Plan. |
| (8) | Of the remaining 2,500 Options, 1,250 will vest on each of November 8, 2024 and November 8, 2025. |
| (9) | Of the remaining 37,500 Options, 18,750 will vest on each of May 15, 2024 and May 15, 2025. |
| (10) | The remaining 4,000 Options vested on February 17, 2024. |
| (11) | Mr. Loubser was granted 10,000 Options on March 31, 2015, which are fully vested; 5,000 Options on March 31, 2016, which are fully vested; 135,000 Options on March 31, 2017, which are fully vested; 15,000 Options on February 3, 2020, which are fully vested; 10,000 Options on February 17, 2021, of which 6,667 are vested; and 100,000 Options on May 15, 2023, of which 50,000 are vested, and, in each case, are subject to the terms of the Equity Incentive Plan. |
| (12) | The remaining 3,333 Options vested on February 17, 2024.108$(193) $785$(101,146) $(40) $2,000 $1,500 $(60,000) $1,00 |
Director Compensation
Overview
The following discussion describes the significant elements of the compensation program for members of the Board and its committees. The compensation of our directors is designed to attract and retain committed and qualified directors and to align their compensation with the long-term interests of the holders of our Common Shares. Directors who are employees of the Company (each, an “Excluded Director”) are not entitled to receive any compensation for his or her service as a member of our Board.
Director Fees
In consideration for serving on our Board, each director (other than Excluded Directors) was entitled to receive the following retainer fees for Fiscal Year 2023: $40,000 for serving as a member of the Board; an additional $50,000 for serving as the Chair of the Board; an additional $15,000 for serving as the Chair of the Audit Committee; an additional $10,000 for serving as the Chair of the Governance and Nominating Committee; and an additional $10,000 for serving as the Chair of the Compensation Committee. Directors were not entitled to receive any additional fees for attending a Board or Committee meeting or for serving as a member of a Committee.
All directors (including Excluded Directors) were entitled to be reimbursed for reasonable out-of-pocket expenses incurred by them in connection with their services as directors.
Deferred Share Unit Plan for Non-Employee Directors
On May 6, 2021, the Company adopted a deferred share unit plan for non-employee directors (the “DSU Plan”). Each director (other than Excluded Directors) is required to take at least 50% of their annual retainer (other than annual committee Chair retainers) in deferred share units (“DSUs”) and may elect to take additional amounts of their annual retainer in the form of DSUs. Discretionary DSUs may also be granted to non-employee directors under the DSU Plan. Each director wishing to make an election for additional DSUs will be required to elect no later than the end of the calendar year preceding the year in which such election is to apply (and, in the case of a new director, within 30 days after the director’s appointment).
- 97 -
A DSU is a unit, equivalent in value to a Common Share, credited by means of a bookkeeping entry in the books of the Company, to an account in the name of the director. If and when cash dividends are paid on Common Shares, additional DSUs will automatically be granted to each director who holds DSUs on the record date for such dividends. Following an eligible director ceasing to hold all positions with the Company, the director will receive a payment in cash at the fair market value of the Common Shares represented by his or her DSUs generally within ten days of the director’s elected redemption date. Each director’s elected redemption date will not be earlier than the date the director ceases to hold all positions with the Company and will not be later than December 31 of the year following the year in which the director ceases to hold all positions with the Company.
Director Option Grants
Prior to Fiscal Year 2021, in lieu of receiving a higher annual cash retainer, each director (other than Excluded Directors) was granted Options under the Equity Incentive Plan each year as follows: 10,000 Options were granted to the Chair of the Board; 5,000 Options were granted to each director; and an additional 1,000 Options were granted to each director who was also a Board committee chair. These Options generally vest in three instalments and will generally expire on the tenth anniversary of the grant date. For further details regarding the Equity Incentive Plan, see, “—Executive Compensation—Narrative Disclosure to Summary Compensation Table—Equity Compensation.”
No Options were granted to any non-employee directors in Fiscal Year 2021 and in subsequent fiscal years.
Director Compensation Table
The following table sets out the compensation that was earned by, paid to, or awarded to directors (other than Excluded Directors) during Fiscal Year 2023 under the compensation arrangements described above.
Name |
| Fees Earned(1) |
| Total(2) | ||
Brian P. Burke | $ | 90,000 | $ | 90,000 | ||
Colleen Campbell | $ | 95,000 | $ | 95,000 | ||
Sasha Cucuz | $ | 92,857 | $ | 92,857 | ||
Adrienne Graves | $ | 75,082 | $ | 75,082 | ||
Robert Higgins | $ | 37,802 | $ | 37,802 | ||
Benjamin Klein |
| — |
| — | ||
Surindra Mann | $ | 13,261 | $ | 13,261 | ||
Adele C. Oliva | $ | 37,802 | $ | 37,802 | ||
Frank Tworecke | $ | 81,658 | $ | 81,658 | ||
Elias Vamvakas | $ | 87,143 | $ | 87,143 | ||
| (1) | The amount reported in this column for each director represents the total amount of the director’s retainer fees earned during Fiscal Year 2023, including such portion of the retainer fees that the director elected to receive in DSUs. See the table below for the portion of the retainer fees that were paid in cash vs. DSUs. |
| (2) | Other than the DSUs that were granted in lieu of the cash retainer fees, there were no share-based awards, Option-based awards, non-equity incentive plan compensation, or any other compensation paid to the directors in Fiscal Year 2023. |
- 98 -
Form of Receipt of Director Fees
The following table identifies for each non-employee director the portion of the dollar amount included in the “Fees Earned” column in the Director Compensation Table that is received in cash, and the portion of such dollar amount that is received in DSUs. The number of DSUs awarded is equal to the dollar amount of such portion of the fees accruing each quarter which is required or elected to be received in DSUs, divided by the Fair Market Value at the designated date of grant of each quarter for which fees were earned. The value of the DSUs is calculated based on the Fair Market Value on the applicable date of grant.
Fees Earned | Paid in Cash | Number of DSUs | Value | ||||||||
Name | ($) | ($) | (#) | ($) | |||||||
Brian P. Burke | $ | 90,000 | $ | 10,000 |
| 210,632 | $ | 80,000 | |||
Colleen Campbell | $ | 95,000 | $ | 15,000 |
| 210,632 | $ | 80,000 | |||
Sasha Cucuz | $ | 92,857 |
| — |
| 253,209 | $ | 92,857 | |||
Adrienne Graves | $ | 75,082 | $ | 41,712 |
| 81,292 | $ | 33,370 | |||
Robert Higgins | $ | 37,802 | $ | 18,901 |
| 26,706 | $ | 18,901 | |||
Benjamin Klein |
| — |
| — |
| — |
| — | |||
Surindra Mann | $ | 13,261 | $ | 6,630 |
| 24,023 | $ | 6,630 | |||
Adele C. Oliva | $ | 37,802 |
| — |
| 53,420 | $ | 37,802 | |||
Frank Tworecke | $ | 81,658 | $ | 1,658 |
| 210,632 | $ | 80,000 | |||
Elias Vamvakas | $ | 87,143 | $ | 10,000 |
| 220,714 | $ | 87,143 | |||
Outstanding Option-Based Awards
The following table sets out information on the outstanding Options held by each of our directors (other than Excluded Directors) as of December 31, 2023. The Company has not issued any RSUs under the Equity Incentive Plan as of December 31, 2023.
| NUMBER OF | |
SECURITIES | ||
UNDERLYING | ||
UNEXERCISED | ||
NAME | OPTIONS | |
Brian P. Burke |
| 12,000 |
Colleen Campbell |
| 12,000 |
Sasha Cucuz |
| 10,000 |
Adrienne Graves |
| — |
Robert Higgins |
| — |
Benjamin Klein |
| — |
Surindra Mann |
| — |
Adele C. Olivia |
| 5,000 |
Frank Tworecke |
| 10,000 |
Elias Vamvakas |
| 20,000 |
- 99 -
ITEM 12.SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Security Ownership of Certain Beneficial Owners
The following table states the number of Common Shares beneficially owned by each person known to the Company who beneficially owns more than 5% of our Common Shares as of April 8, 2024. The persons listed below are deemed to be the beneficial owners of Common Shares underlying options, warrants, and conversion instruments that are exercisable within 60 days from the above date. The percentages shown below are based on an aggregate total of 45,602,260 outstanding Common Shares as of April 8, 2024, plus the number of Common Shares underlying options, warrants, and conversion instruments that are exercisable within 60 days for the indicated beneficial owner.
Security Ownership of Certain Beneficial Owners |
| ||||||
Title of Class |
| Name of Beneficial Owner |
| Amount |
| Percent of Class | |
Common Shares |
| Greybrook Health(1) |
| 45,962,922 |
| 54.4 | % |
Common Shares |
| Benjamin Klein(2) |
| 8,725,995 |
| 19.1 | % |
Common Shares |
| Madryn Asset Management, LP(3) |
| 67,966,548 |
| 63.4 | % |
Common Shares |
| Michael Masters(4) |
| 4,327,269 |
| 9.5 | % |
Common Shares |
| Adele C. Olivia(5) |
| 5,272,569 |
| 10.9 | % |
| (1) | Includes (i) 7,000,424 Common Shares directly held by Greybrook Health and its affiliate, Greybrook Realty, (ii) 38,576,628 Common Shares estimated to be issuable to Greybrook Health upon conversion of the Subordinated Convertible Notes by Greybrook Health and (iii) 385,870 Common Shares issuable to Greybrook Health upon exercise of the Greybrook Warrants. All 21,335,994 Common Shares beneficially owned by Greybrook Health share voting power with The Vamvakas Family Trust (2015) (the “Vamvakas Trust”). Greybrook Health’s address is 890 Yonge Street, 7th Floor Toronto, Ontario, Canada M4W 3P4. This disclosure reflects the Schedule 13D/A filed by Greybrook Health and the Vamvakas Trust on April 8, 2024. |
| (2) | Includes (i) 4,763,263 Common Shares beneficially owned by Benjamin Klein, The Bereke Trust ATA Dated 2/10/03, and Theragroup LLC, and (ii) 3,962,732 Common Shares beneficially owned solely by Benjamin Klein. This total amount does not include 2,908,665 Escrowed Shares which have been held back from the purchase price consideration in connection with the acquisition of Success TMS and deposited with an escrow agent, to be released to Benjamin Klein or the Company, as applicable, upon satisfaction of working capital and certain other adjustments, including to satisfy any indemnity claims against the vendors. Benjamin Klein’s address is 284 East Palisade Avenue Englewood, New Jersey 07631. This disclosure reflects the Schedule 13D filed by Benjamin Klein on July 27, 2022. |
| (3) | Includes (i) 3,910,605 Common Shares estimated to be issuable Madryn upon conversion of the Madryn Conversion Instruments, (ii) 57,692,308 Common Shares estimated to be issuable to Madryn upon conversion of the Subordinated Convertible Notes and (iii) 6,363,636 Common Shares issued to Madryn in connection with the 2023 Private Placement. Madryn Asset Management, LP holds shared voting power over all 45,962,922 Common Shares, Madryn Health Partners II, LP holds shared voting power over 4,162,959 Common Shares, Madryn Health Partners II (Cayman Master), LP holds shared voting power over 63,166,888 Common Shares, Madryn Health Advisors II, LP and Madryn Health Advisors GP II, LLC each hold shared voting power over 67,329,847, and Madryn Select Advisors, LP, Madryn Select Advisors GP, LLC, and Madryn Select Opportunities, LP each hold shared voting power over 636,701 Common Shares. Madryn Asset Management, LP’s address is 330 Madison Avenue Floor 33, New York, New York 10017. This disclosure reflects the Schedule 13D/A filed by Madryn on April 8, 2024. |
| (4) | Includes 4,327,269 Common Shares directly or indirectly held by Michael Masters, of which (i) 3,427,272 are beneficially owned by Masters Capital Management LLC managed funds and (ii) 899,997 are beneficially owned by each of MSS GB SPV LP, MSS GB SPV GP LLC and Special Situations, LLC (collectively, “Masters”). Masters exercises shared voting power over the Common Shares beneficially owned by each of them. Masters address is 3060 Peachtree Road, NW, Suite 1425 Atlanta, Georgia 30305. This disclosure reflects the Schedule 13D/A filed by MSS GB SPV LP on February 28, 2024. |
| (5) | Includes (i) 2,294,648 Common Shares which are beneficially owned by 1315 Capital II, L.P. and 1315 Capital Management II, LLC, (ii) 2,972,921 Common Shares estimated to be issuable to 1315 Capital II, L.P. upon conversion of the Subordinated Convertible Notes and (iii) 5,000 fully vested and exercisable options with each option exercisable for one Common Share, beneficially owned by Adele Olivia. Ms. Olivia as Managing Member and Founding Partner of 1315 Capital exercises shared voting power with 1315 Capital II, L.P. and 1315 Capital Management II, LLC over the Common Shares. 1315 Capital II, L.P.’s address is 2929 Walnut Street, Suite 1240 Philadelphia, Pennsylvania 19104. |
- 100 -
Security Ownership of Directors and Executive Officers
The following table states the number of Common Shares beneficially owned by each of our NEOs and directors as of April 8, 2024. The persons listed below are deemed to be the beneficial owners of Common Shares underlying options, warrants and/or conversion instruments that are exercisable within 60 days from the above date. The percentages shown below are based on an aggregate total of 45,602,260 outstanding Common Shares as of April 8, 2024, plus the number of Common Shares underlying options, warrants and/or conversion instruments that are exercisable within 60 days for the indicated beneficial owner.
As of April 8, 2024, our directors and executive officers, as a group, beneficially own, or control or direct, directly or indirectly, 2,239,777 Common Shares, representing approximately 4.8% of the issued and outstanding Common Shares, plus the number of Common Shares underlying options, warrants and/or conversion instruments that are exercisable within 60 days.
Directors |
| ||||
Name of Beneficial Owner |
| Amount |
| Percent of Class |
|
Brian P. Burke |
| * |
| * | |
Colleen Campbell |
| * |
| * | |
Sasha Cucuz |
| * |
| * | |
Julia Elstad |
| * |
| * | |
Bill Leonard(1) |
| 1,452,381 |
| 3.1 | % |
Surindra Mann |
| * |
| * | |
Frank Tworecke |
| * |
| * | |
Elias Vamvakas(2) |
| * |
| * | |
*Represents less than 1% beneficial ownership of Common Shares.
| (1) | Includes (i) 832,500 Common Shares, (ii) 60,000 fully vested and exercisable options, each representing one Common Share and (iii) 559,881 Common Shares estimated to be issuable to Mr. Leonard upon conversion of the Subordinated Convertible Notes. |
| (2) | Mr. Vamvakas, chairman of the Company, is the chairman and founder of Greybrook Capital, the parent company of Greybrook Health, and is a trustee of the Vamvakas Trust. Mr. Vamvakas disclaims beneficial ownership of the Common Shares directly or indirectly held by Greybrook Health and the Vamvakas Trust. See “—Security Ownership of Certain Beneficial Owners” above. |
Named Executive Officers |
| ||||
Name of Beneficial Owner |
| Amount |
| Percent of Class |
|
Geoffrey Grammer(1) |
| 608,896 |
| 1.3 | % |
Bill Leonard(2) |
| 1,452,381 |
| 3.1 | % |
Erns Loubser+ |
| * |
| * | |
Peter Willett |
| * |
| * | |
*Represents less than 1% beneficial ownership of Common Shares.
+Represents former officers of the Company.
| (1) | Includes (i) 119,000 fully vested and exercisable options and (ii) 489,896 Common Shares to be issuable to Mr. Grammer upon conversion of the Subordinated Convertible Notes. |
| (2) | See footnote 1 to the table in “—Security Ownership of Certain Beneficial Owners” above. |
- 101 -
Securities Authorized for Issuance under Equity Compensation Plans
The following table sets out information on the number of securities authorized for issuance under the Company’s equity compensation plans as of December 31, 2023.
| Number of |
|
| Number of securities | |||||
securities to be | remaining available | ||||||||
issued upon | Weighted-average | for future issuance | |||||||
exercise of | exercise price of | under equity | |||||||
outstanding | outstanding | compensation plans | |||||||
options, | options, | (excluding securities | |||||||
warrants and | warrants and | reflected in column | |||||||
Plan Category | rights | rights | (a)) | ||||||
Equity compensation plans approved by security holders | $ | 1,704,500 | $ | 3.11 | $ | 41,069,511 | |||
Equity compensation plans not approved by security holders |
| N/A |
| N/A |
| N/A | |||
Total | $ | 1,704,500 | $ | 3.11 | $ | 41,069,511 | |||
For more information on the Company’s Equity Incentive Plan see “Item 11 Executive Compensation— Narrative Disclosure to Summary Compensation Table—Equity Incentive Plan” above.
Share Information
The Company is authorized to issue an unlimited number of Common Shares and an unlimited number of preferred shares, issuable in series. As of April 8, 2024, there were 45,602,260 Common Shares, which includes 2,908,665 Escrowed Shares and nil preferred shares issued and outstanding. In addition, there were 764,667 stock options, 51,307 Oxford Warrants and 385,870 Greybrook Warrants, each representing a right to acquire one Common Share, issued and outstanding; the Madryn Conversion Instruments issued to Madryn and certain of its affiliates permit such holders to exchange such Madryn Conversion Instruments for up to an aggregate of 3,910,604 Common Shares; and 135,115,950 Common Shares currently issuable upon full conversion of the Subordinated Convertible Notes at their respective Reference Conversion Price. As of the date hereof, assuming exercise, exchange and conversion of all outstanding options, Oxford Warrants, Madryn Conversion Instruments, and the Subordinated Convertible Notes there would be 185,830,658 Common Shares issued and outstanding, on a fully diluted basis.
In addition, if the Neuronetics Note is in default, the Company will be required to issue the Neuronetics Warrants, which would have the potential to result in substantial dilution.
ITEM 13.CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
Related Party Transactions
For information on our independent directors see “Item 10 Directors Executive Officers and Corporate Governance—Directors”, “Item 10 Directors Executive Officers and Corporate Governance—Former Directors” and “Item 10 Directors Executive Officers and Corporate Governance—Corporate Governance—Composition and Independence of the Board.”
Greybrook Health
As at December 31, 2023, $0.005 million was included in accounts payable and accrued liabilities related to payables for Greybrook Health.
- 102 -
In connection with the February 2023 Notes, the Company issued promissory notes to Greybrook Health and certain other significant shareholders and management members of the Company. In conjunction with the issuance of the August 2023 Note to Greybrook Health, the Company granted Greybrook Health a conversion instrument for the August 2023 Note but this was extinguished upon Greybrook exchanging the August 2023 Note for Subordinated Convertible Notes. As additional consideration for the purchase of the Insider Notes by Greybrook Health, the Company issued the Greybrook Warrants to Greybrook Health. Greybrook Health also participated in the 2023 Private Placement, purchasing 2,272,727 Common Shares for approximately $1.25 million. See “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operation—Liquidity and Capital Resources—Indebtedness and Capital Raising—Insider Notes” above.
Madryn
On July 14, 2022, the Company entered into the Credit Agreement with Madryn and has since entered into amendments to the Credit Agreement, whereby Madryn and its affiliated entities extended the Additional Term Loans to the Company. During Fiscal 2023, the Company recognized $10.0 million in interest expense related to the Credit Agreement (Fiscal 2022: nil). See “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operation—Liquidity and Capital Resources—Indebtedness and Capital Raising—Madryn Credit Agreement” above.
Madryn also participated in the 2023 Private Placement, purchasing 6,363,636 Common Shares at an aggregate subscription price of approximately $3.5 million. See Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operation—Liquidity and Capital Resources—Indebtedness and Capital Raising—2023 Private Placement” above.
Benjamin Klein
During Fiscal 2023, the Company recognized $0.2 million in other corporate, general, and administrative expenses (Fiscal 2022: 0.04 million) related to Benjamin Klein. As at December 31, 2023, nil was included in accounts payable and accrued liabilities related to payables for Benjamin Klein and entities he owns.
On July 14, 2022, in connection with the Success TMS Acquisition, the Company assumed the obligation of the Klein Note to Benjamin Klein, who is a significant shareholder, officer and director of the Company. The Klein Note totals $2.1 million and bears interest at a rate of 10% per annum and matures on May 1, 2024. The carrying value of this Klein Note as at December 31, 2023 was nil (December 31, 2022 – $2.1 million). During Fiscal 2023, the Company paid $0.2 million towards this Klein Note (Fiscal 2022: $0.1 million). On November 20, 2023, the Company entered into the Klein Settlement Agreement. See “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operation—Liquidity and Capital Resources—Indebtedness and Capital Raising—Other Indebtedness”.
1315 Capital
1315 Capital purchased $212,396 aggregate principal amount of the February 2023 Notes which were exchanged on August 28, 2023, for Subordinated Convertible Notes. See “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operation—Liquidity and Capital Resources—Indebtedness and Capital Raising—Subordinated Convertible Notes”.
Masters
Masters participated in the 2023 Private Placement, purchasing 2,737,272 Common Shares at an aggregate subscription price of approximately $1.5 million. See “Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operation—Liquidity and Capital Resources—Indebtedness and Capital Raising—2023 Private Placement” above.
- 103 -
ITEM 14.PRINCIPAL ACCOUNTANT FEES AND SERVICES.
We incurred the following fees by our external auditor, KPMG LLP, (Vaughan, ON, Canada, Auditor Firm ID: 085) during the periods provided below.
| Year Ended |
| Year Ended | |||
December 31, 2023 | December 31, 2022 | |||||
Audit Fees(1) | $ | 803,108 | $ | 675,361 | ||
Audit-Related Fees(2) | $ | 144,882 | $ | 119,472 | ||
Tax Fees(3) | $ | 226,684 | $ | 171,655 | ||
All Other Fees | $ | — | $ | — | ||
Total Fees | $ | 1,174,674 | $ | 966,488 | ||
Notes:
| (1) | Consist of fees related to audits of annual financial statements, involvement with registration statements and other filings with various regulatory authorities, quarterly reviews of interim financial statements and consultations related to accounting matters impacting the consolidated financial statements. |
| (2) | Consists primarily of fees related to the US GAAP transition (Fiscal 2023) and Success TMS Acquisition (Fiscal 2022) |
| (3) | Consists of fees for tax consultation and compliance services, including indirect taxes. |
The written charter of the Audit Committee provides that the Audit Committee must pre-approve the retaining of the auditors for any audit or non-audit service. The pre-approval process involves management presenting the Audit Committee with a description of any proposed non-audit services. The Audit Committee considers the appropriateness of such services and whether the provision of those services would impact the auditor’s independence. The Audit Committee may delegate to one or more members the authority to pre-approve the retaining of the auditors for any non-audit service to the extent permitted by law, but pre-approval by such member or members so delegated must be presented to the full Audit Committee at its first scheduled meeting following such pre-approval. None of the services provided by the Company’s external auditors described above were approved pursuant to a waiver of pre-approval provisions under SEC rules (paragraph (c)(7)(i)(C) of Rule 2-01 of Regulation S-X).
- 104 -
PART III
ITEM 15.EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| (a) | Documents filed as part of this report. |
| 1. | Financial Statements. |
Page | |
F-4 | |
F-5 | |
F-6 | |
F-7 | |
F-8 |
| 2. | Financial Statement Schedules |
All schedules are omitted because they are not applicable, or the required information is shown in the Financial Statements or notes thereto.
| (b) | Exhibits |
The following exhibits are filed as part of, or incorporated by reference into, this report:
3.1 |
| ||
3.2 | |||
3.3 | |||
3.4 | |||
3.5 | |||
4.1* | |||
4.2 | |||
4.3 | |||
4.4 ˄ | |||
4.5 | |||
10.1 | |||
- 105 -
10.2 | |||
10.3 | |||
10.4 | |||
10.5 | |||
10.6 | |||
10.7* | |||
10.8* | |||
10.9 * | |||
10.10*Ù | |||
10.11* | |||
10.12 | |||
10.13 | |||
10.14 ˄ | |||
10.15 | |||
- 106 -
10.16 ˄ | |||
10.17*Ù | |||
10.18+ | |||
10.19* | |||
10.20* ˄+ | |||
10.21* ˄+ | |||
19.1* | |||
21.1* | |||
23.1* | |||
24.1 | |||
31.1* | Section 302 Certification under Sarbanes-Oxley Act of 2002 for CEO | ||
31.2* | Section 302 Certification under Sarbanes-Oxley Act of 2002 for CFO | ||
32.1* | Section 906 Certification under Sarbanes-Oxley Act of 2002 for CEO | ||
32.2* | Section 906 Certification under Sarbanes-Oxley Act of 2002 for CFO | ||
97.1* | |||
101.INS* | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | ||
101.SCH* | Inline XBRL Taxonomy Extension Schema Document | ||
101.CAL* | Inline XBRL Taxonomy Extension Calculation Linkbase Document | ||
101.DEF* | Inline XBRL Taxonomy Extension Definition Linkbase Document | ||
101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase Document | ||
101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase Document | ||
- 107 -
104.1 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |
˄ | Certain portions of this exhibit have been redacted pursuant to Item 601(b)(10) of Regulation S-K, including the omission of certain schedules to such exhibits. The Company agrees to furnish to the Securities and Exchange Commission a copy of any omitted portions of the exhibits or schedules thereto upon request. | |
+ | Indicates management contract or compensatory plan. | |
* | Filed electronically herewith. |
ITEM 16.FORM 10-K SUMMARY
None.
- 108 -
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on the 25th day of April 2024.
Greenbrook TMS Inc. | ||
By: | /s/ Bill Leonard | |
Name: Bill Leonard | ||
Title: President & Chief Executive Officer | ||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Bill Leonard and Peter Willett, and each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Signature |
| Title |
|---|---|---|
/s/ Bill Leonard | President, Chief Executive Officer and Director | |
Bill Leonard | ||
/s/ Peter Willett | Chief Financial Officer | |
Peter Willett | ||
/s/ Brian P. Burke | Director | |
Brian P. Burke | ||
/s/ Colleen Campbell | Director | |
Colleen Campbell | ||
/s/ Sasha Cucuz | Director | |
Sasha Cucuz | ||
/s/ Juliana Elstad | Director | |
Juliana Elstad | ||
/s/ Surindra Mann | Director | |
Surindra Mann | ||
/s/ Frank Tworecke | Director | |
Frank Tworecke | ||
/s/ Elias Vamvakas | Director | |
Elias Vamvakas |
- 109 -
Consolidated Financial Statements
(Expressed in U.S. dollars)
Greenbrook TMS Inc.
And Report of Independent Registered Public
Accounting Firm thereon
As of December 31, 2023 and December 31, 2022
and for the two years ended December 31, 2023
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Greenbrook TMS Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Greenbrook TMS Inc. (and subsidiaries) (the Company) as of December 31, 2023 and 2022, the related consolidated statements of comprehensive loss, changes in equity (deficit), and cash flows for each of the years in the two year period ended December 31, 2023 and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the two year period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2(a) to the consolidated financial statements, the Company has experienced losses since inception, has negative cash flows from operations, negative working capital, risks associated with future non-compliance with covenants on loans payable and the uncertainty related to outcome of the litigation, that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2(a). The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
F-2
/s/ KPMG LLP
Chartered Professional Accountants, Licensed Public Accountants
We have served as the Company’s auditor since 2017.
Vaughan, Canada
April 25, 2024
F-3
GREENBROOK TMS INC.
Consolidated Balance Sheets
(Expressed in U.S. dollars, unless otherwise stated)
December 31, | December 31, | |||||
| 2023 |
| 2022 | |||
Assets |
|
|
|
| ||
Current assets: |
|
|
|
| ||
Cash | $ | | $ | | ||
Restricted cash (note 13) | | | ||||
Accounts receivable, net (note 20(b)) |
| |
| | ||
Prepaid expenses and other |
| |
| | ||
Total current assets |
| |
| | ||
Property, plant and equipment (note 6) |
| |
| | ||
Intangible assets (note 7) |
| |
| | ||
Goodwill (notes 5 and 7) |
| — |
| — | ||
Finance right-of-use assets (note 8(a)) |
| |
| | ||
Operating right-of-use assets (note 8(b)) | | | ||||
Total assets | $ | | $ | | ||
Liabilities and Shareholders’ Deficit |
|
|
|
| ||
Current liabilities: |
|
|
|
| ||
Accounts payable and accrued liabilities (note 9) | $ | | $ | | ||
Current portion of loans payable (note 10(a)) |
| |
| | ||
Current portion of finance lease liabilities (note 8(a)) | | | ||||
Current portion of operating lease liabilities (note 8(b)) | | | ||||
Current portion of shareholder loan (note 11) |
| |
| | ||
Other payables (note 12) | | | ||||
Non-controlling interest loans (note 10(b)) |
| |
| | ||
Deferred and contingent consideration (note 13) |
| |
| | ||
Advance for research collaboration (note 14) |
| |
| — | ||
Total current liabilities |
| |
| | ||
Loans payable (note 10(a)) |
| |
| | ||
Finance lease liabilities (note 8(a)) | | | ||||
Operating lease liabilities (note 8(b)) | | | ||||
Shareholder loans (note 11) |
| |
| | ||
Total liabilities |
| |
| | ||
Shareholders’ deficit: |
|
|
|
| ||
Common shares (note 15) |
| |
| | ||
Contributed surplus (note 16) |
| |
| | ||
Deficit |
| ( |
| ( | ||
Total shareholders’ deficit excluding non-controlling interest |
| ( |
| ( | ||
Non-controlling interest (note 24) |
| ( |
| ( | ||
Total shareholders’ deficit |
| ( |
| ( | ||
Basis of presentation and going concern (note 2(a)) |
|
|
|
| ||
Contingencies (note 17) |
|
|
|
| ||
Subsequent events (notes 2(a) and 26) |
|
|
|
| ||
Related parties (note 22) | ||||||
Total liabilities and shareholders’ deficit | $ | | $ | | ||
See accompanying notes to consolidated financial statements.
F-4
GREENBROOK TMS INC.
Consolidated Statements of Comprehensive Loss
(Expressed in U.S. dollars, unless otherwise stated)
December 31, | December 31, | |||||
| 2023 |
| 2022 | |||
Revenue: |
|
|
|
| ||
Service revenue | $ | | $ | | ||
Expenses: |
|
|
| |||
Direct center and patient care costs |
| |
| | ||
Other regional and center support costs (note 25) |
| |
| | ||
Depreciation (notes 6 and 8(a)) |
| |
| | ||
| |
| | |||
Regional operating loss |
| ( |
| ( | ||
Center development costs |
| |
| | ||
Corporate, general and administrative expenses (note 25) |
| |
| | ||
Share-based compensation (note 16) |
| |
| | ||
Amortization (note 7(a)) |
| |
| | ||
Interest expense |
| |
| | ||
Interest income |
| ( |
| ( | ||
Loss on extinguishment of loans (note 10(a)(ii)) | | | ||||
Loss on settlements (note 12(d) and 12(e)) | | — | ||||
Impairment loss (note 7(b), note 7(c) and note 8(b)) | | | ||||
Loss before income taxes |
| ( |
| ( | ||
Income tax expense (note 19) |
| |
| | ||
Loss for the year and comprehensive loss | $ | ( | $ | ( | ||
Non-controlling interest (note 24) | ( | ( | ||||
Loss for the year and comprehensive loss attributable to Greenbrook | $ | ( | $ | ( | ||
Net loss per share (note 23): |
|
|
|
| ||
Basic | $ | ( | $ | ( | ||
Diluted | ( | ( | ||||
See accompanying notes to consolidated financial statements.
F-5
GREENBROOK TMS INC.
Consolidated Statements of Changes in Equity (Deficit)
(Expressed in U.S. dollars, unless otherwise stated)
Non- | |||||||||||||||||
|
|
| controlling | Total | |||||||||||||
Common shares | Contributed | interest | equity | ||||||||||||||
Year ended December 31, 2022 |
| Number |
| Amount |
| surplus |
| Deficit |
| (note 24) |
| (deficit) | |||||
Balance, December 31, 2021 |
| | $ | | $ | | $ | ( | $ | ( | $ | | |||||
Net comprehensive loss for the year |
| — |
| — |
| — |
| ( |
| ( |
| ( | |||||
Issuance of common shares (note 15) |
| |
| |
| — |
| — |
| — |
| | |||||
Share-based compensation (note 16) |
| — | — | | — | — | | ||||||||||
Distributions to non-controlling interest |
| — |
| — |
| — |
| — |
| ( |
| ( | |||||
Acquisition of subsidiary non-controlling interest (note 24) | — | — | — | ( | ( | ( | |||||||||||
Balance, December 31, 2022 |
| | $ | | $ | | $ | ( | $ | ( | $ | ( | |||||
|
|
|
|
|
|
|
| controlling |
| Total | |||||||
Common shares | Contributed | interest | equity | ||||||||||||||
Year ended December 31, 2023 |
| Number |
| Amount |
| surplus |
| Deficit |
| (note 24) |
| (deficit) | |||||
Balance, December 31, 2022 |
| | $ | | $ | | $ | ( | $ | ( | $ | ( | |||||
Net comprehensive loss for the year |
| — |
| — |
| — |
| ( |
| ( |
| ( | |||||
Issuance of common shares (note 15) |
| |
| |
| — |
| — |
| — |
| | |||||
Share-based compensation (note 16) |
| — |
| — |
| |
| — |
| — |
| | |||||
Issuance of lender warrants | — | — | | — | — | | |||||||||||
Gain on extinguishment of shareholder loan | — | — | | — | — | | |||||||||||
Acquisition of subsidiary non-controlling interest (note 24) |
| — |
| — |
| — |
| ( |
| |
| ( | |||||
Distribution to non-controlling interest | — | — | — | — | ( | ( | |||||||||||
Balance, December 31, 2023 |
| | $ | | $ | | $ | ( | $ | ( | $ | ( | |||||
See accompanying notes to consolidated financial statements.
F-6
GREENBROOK TMS INC.
Consolidated Statements of Cash Flows
(Expressed in U.S. dollars, unless otherwise stated)
December 31, | December 31, | |||||
| 2023 |
| 2022 | |||
Cash provided by (used in) |
|
|
| |||
Operating activities: |
|
|
|
| ||
Loss for the year | $ | ( | $ | ( | ||
Adjusted for: |
|
|
|
| ||
Amortization |
| |
| | ||
Depreciation |
| |
| | ||
Operating lease expense | | | ||||
Interest expense |
| |
| | ||
Interest income |
| ( |
| ( | ||
Share-based compensation |
| |
| | ||
Loss (gain) on extinguishment of loan |
| |
| | ||
Loss on settlements (note 12) | | — | ||||
Credit facility amendment fee (note 10(a)) | | — | ||||
Neuronetics Note non-cash financing costs (note 10(a)) | | — | ||||
Gain on lender warrants (note 12(a)) |
| ( |
| ( | ||
(Gain) loss on deferred share units (note 12(b)) | ( | | ||||
Gain on performance share units (note 12(c)) | ( | ( | ||||
Impairment loss (note 7) | |
| | |||
Change in non-cash operating working capital: |
| |||||
Accounts receivable |
| ( |
| | ||
Prepaid expenses and other |
| ( |
| | ||
Advance for research collaboration (note 14) | | — | ||||
Other payables | ( | — | ||||
Accounts payable and accrued liabilities |
| |
| | ||
Interest paid | ( | ( | ||||
Interest received | | | ||||
Payment of operating lease liabilities | ( | ( | ||||
( | ( | |||||
Financing activities: |
|
|
|
| ||
Net proceeds on issuance of common shares (note 15) |
| |
| — | ||
Finance costs incurred (note 10(a)) | ( | ( | ||||
Bank loans advanced | | | ||||
Bank loans repaid (note 10(a)) |
| ( |
| ( | ||
Promissory notes advanced (note 10(a) and note 11) | | — | ||||
Promissory notes repaid (note 10(a)) | ( | — | ||||
Principal repayment of finance lease liabilities |
| ( |
| ( | ||
Non-controlling interest loans advanced |
| |
| | ||
Non-controlling interest loans repaid |
| ( |
| — | ||
Distribution to non-controlling interest |
| ( |
| ( | ||
| |
| | |||
Investing activities: |
|
|
|
| ||
Acquisition, net of cash acquired (note 5) |
| — |
| | ||
Change in restricted cash | — | | ||||
Acquisition of subsidiary non-controlling interest (note 24) | ( | ( | ||||
Deferred and contingent consideration paid (note 13) | — | ( | ||||
Purchase of property, plant and equipment |
| ( |
| ( | ||
| ( |
| | |||
Increase (decrease) in cash |
| |
| ( | ||
Cash, beginning of the year |
| |
| | ||
Cash, end of the year | $ | | $ | | ||
See accompanying notes to consolidated financial statements.
F-7
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
1. | Reporting entity: |
Greenbrook TMS Inc. (the “Company”), an Ontario corporation along with its subsidiaries, controls and operates a network of outpatient mental health services centers that specialize in the provision of Transcranial Magnetic Stimulation (“TMS”) therapy, Spravato® (esketamine nasal spray) and other treatment modalities for the treatment of depression and related psychiatric services.
The Company’s head and registered office is located at 890 Yonge Street, 7th Floor, Toronto, Ontario, Canada, M4W 3P4. The Company’s United States corporate headquarters is located at 8401 Greensboro Drive, Suite 425, Tysons Corner, Virginia, USA, 22102.
2. | Basis of presentation: |
(a) | Going concern: |
These consolidated financial statements have been in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) and applicable rules and regulations of the Securities and Exchange Commission (“SEC”) and the basis of presentation outlined in note 2(b) on the assumption that the Company is a going concern and will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the normal course of business for the next 12 months.
The Company has experienced losses since inception and has negative cash flow from operating activities of $
On July 14, 2022, the Company entered into a credit agreement (the “Madryn Credit Agreement”), as amended, for a $
On March 23, 2023, the Company completed a non-brokered private placement (the “2023 Private Placement”), for aggregate gross proceeds to the Company of approximately $
On July 13, 2023, the Company entered into a purchase agreement (the “Alumni Purchase Agreement”) with Alumni Capital LP (“Alumni”). The Alumni Purchase Agreement provides equity line financing for sales from time to time of up to $
F-8
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
2. | Basis of presentation (continued): |
(a) | Going concern (continued): |
During the year ended December 31, 2023, the Company received an aggregate of $
The terms of the Madryn Credit Facility require the Company to satisfy various financial covenants including a minimum liquidity and minimum consolidated revenue amounts that became effective on July 14, 2022 and September 30, 2022, respectively. A failure to comply with these covenants, or failure to obtain a waiver for any non-compliance, would result in an event of default under the Madryn Credit Agreement and would allow Madryn to accelerate repayment of the debt, which could materially and adversely affect the business, results of operations and financial condition of the Company. On February 21, 2023, March 20, 2023, June 14, 2023, July 3, 2023, July 14, 2023, August 1, 2023, August 14,2023, September 15, 2023, September 29, 2023, October 12, 2023, November 15, 2023, December 14, 2023, January 19, 2024 , February 15, 2024, March 15, 2024 and March 29, 2024, the Company received waivers from Madryn with respect to the Company’s non-compliance with the minimum liquidity covenant which has been extended to April 30, 2024. In addition, the Company also received a waiver relating to the requirement to deliver financial statements within 90 days of each fiscal year end until April 26, 2024, and audited financial statements for such fiscal year, accompanied by a report and opinion of an independent certified public accountant which is not subject to any “going concern” or like qualification or exception or any qualification or exception as to the scope of such audit. As at December 31, 2023, the Company was in compliance with the financial covenants of the Madryn Credit Agreement, as amended.
On January 19, 2024, February 5, 2024, February 15, 2024, March 1, 2024, March 15, 2024, March 19,2024 and April 15,2024, the Company received an aggregate of $
On February, 22, 2024, the Company received the final delisting notice from the Listing Qualifications Department of the Nasdaq Stock Market LLC (“Nasdaq”) due to the continued failure to satisfy either the $1.00 minimum bid price listing requirement in Nasdaq Listing Rule 5550(a)(2) or the minimum stockholders’ equity requirements in Nasdaq Listing Rule 5550(b). Consequently, the trading of the Company’s common shares was suspended as of the open of trading on February 26, 2024. The Company determined that it was in the overall best interests of the Company not to appeal the decision. Subsequently, the Company’s shares have been quoted on OTC Markets.
F-9
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
2.Basis of presentation (continued):
(a) | Going concern (continued): |
Although the Company believes it will become cash flow positive in the future, the timing of this is uncertain given that the Company has historically not been able to meet its forecast, and is also dependent on the continued execution of the Restructuring Plan (as defined below) (see note 25), our ability to meet our debt obligations and remain in compliance with debt covenants and the outcome of the pending Delaware Complaint (as defined below) (see note 17). The Company will require additional financing in order to fund its operating and investing activities, including making timely payments to certain vendors, landlords, lenders (including shareholders) and similar other business partners. The delay in such payments may result in potential defaults under the terms of the agreements the Company has with various parties. As such, additional financing is required in order for the Company to repay its short-term obligations. The Company has historically been able to obtain financing from supportive shareholders, its lenders and other sources when required; however, the Company may not be able to access further equity or debt financing when needed. As such, there can be no assurance that the Company will be able to obtain additional liquidity when needed or under acceptable terms, if at all. If additional financing is not obtained, the Company may not be able to repay its short-term obligations and will need to obtain additional amendments from Madryn in order to remain compliant with the covenants or waivers from Madryn to
waive its rights to accelerate repayment of the debt; however, there can be no assurances that such amendments or waivers will be obtained, which may result in a requirement to file for bankruptcy protection.
The existence of the above-described conditions indicate substantial doubt as to the Company’s ability to continue as a going concern as of December 31, 2023.
These consolidated financial statements do not reflect adjustments that would be necessary if the going concern assumptions were not appropriate. If the going concern basis was not appropriate for these consolidated financial statements, then adjustments would be necessary to the carrying value of assets and liabilities, the reported expenses, and the consolidated balance sheet classification used, and these adjustments may be material.
(b) | Basis of measurement: |
These consolidated financial statements have been prepared on a historic cost basis except for financial instruments classified as fair value through profit or loss, which are stated at their fair value. Other measurement bases are described in the applicable notes.
Presentation of the consolidated balance sheet differentiates between current and non - current assets and liabilities. The consolidated statements of comprehensive loss are presented using the function classification of expense.
Regional operating income (loss) presents regional operating income (loss) on an entity-wide basis and is calculated as total service revenue less direct center and patient care costs, other regional and center support costs, and depreciation. These costs encapsulate all costs (other than incentive compensation such as share-based compensation granted to senior regional employees) associated with the center and regional management infrastructure, including the cost of the delivery of treatments to patients and the cost of the Company’s regional patient acquisition strategy.
F-10
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
2.Basis of presentation (continued):
(c) | Basis of consolidation: |
The Company consolidates entities in which it has a controlling financial interest based on either the variable interest entity (VIE) or voting interest model (VOE). The Company is required to first apply the VIE model to determine whether it holds a variable interest in an entity, and if so, whether the entity is a VIE. ASC 810, Consolidation ("ASC 810") defines the criteria for determining the existence of VIEs and provides guidance for consolidation.
An entity is considered to be a VIE if (i) the entity does not have enough equity to finance its own activities without additional support, (ii) the entity's at-risk equity holders lack the characteristics of a controlling financial interest, or (iii) the entity is structured with non-substantive voting rights. The primary beneficiary of a VIE is the party that has the power to direct the activities that most significantly impact the performance of the entity and the obligation to absorb losses or the right to receive benefits that could potentially be significant to the entity. The primary beneficiary is required to consolidate the VIE for financial reporting purposes. A VIE can have only one primary beneficiary but may not have a primary beneficiary if no party meets the criteria described above.
If the Company determines it does not hold a variable interest in a VIE, the Company applies the VOE model. To the extent the entity does not meet the definition of a VIE, the ASC 810 guidance for voting interest entities is applied. The usual condition for a controlling financial interest, and therefore consolidation by the Company, is ownership of a majority
voting interest of a corporation or a majority of kick-out rights for a limited partnership. The Company has determined that all its subsidiaries are VOEs primarily because it holds a majority voting interest in the entities.
All significant intercompany balances and transactions have been eliminated on consolidation.
(d) | Use of estimates and judgments: |
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting year. Actual results could differ from those estimates. As additional information becomes available or actual amounts are determinable, the recorded estimates are revised and reflected in operating results in the period in which they are determined.
Significant estimates in connection with these consolidated financial statements include the measurement and determination of the transaction price in the estimation of revenue and accounts receivable, estimated useful life of property, plant and equipment; estimated value and useful life of intangible assets; amounts recorded as accrued liabilities; amounts recorded as performance share units, convertible instruments, deferred income taxes provisions; goodwill; assessment of contingent consideration; inputs used in the valuation of warrants and stock options granted; and the estimate of lease terms.
Significant judgments in connection with these consolidated financial statements include assessment of control of subsidiaries; assessment of conditions relating to the Company’s ability to continue as a going concern; determination of functional currency; determination of the Company’s reporting units and asset groups; determination of whether a contract is or contains a lease; and determination of the incremental borrowing rate used to measure lease liabilities.
F-11
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
2.Basis of presentation (continued):
(e) | Functional and reporting currency: |
The functional and reporting currency of the Company and its subsidiaries is the U.S. dollar. Monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars at the rates of exchange prevailing at the consolidated balance sheet dates. Non-monetary assets and liabilities are translated at rates prevailing at the dates of acquisition. Expenses are translated at the average rate of exchange in effect during the month the transaction occurred.
(f) | Adoption of U.S. GAAP: |
Until fiscal 2022, the Company prepared and presented its financial statements under International Financial Reporting Standards (“IFRS”) issued by the International Accounting Standards Board.
As of June 30, 2023, the Company lost its “foreign private issuer” status as a majority of the common shares in the Company (“Common Shares”) were held in the United States and the Company does not meet the additional requirements under the “business contacts” test. As a result, beginning January 1, 2024, the Company is required to follow SEC reporting standards of a U.S. domestic issuer, convert its financial statements to generally accepted accounting principles in the United States (“U.S. GAAP”) and will no longer be able to rely on foreign private issuer exemptions from U.S. proxy rules and Section 16 insider reporting or foreign private issuer exemptions under Nasdaq listing rules (including in respect of shareholder approval requirements for certain dilutive transactions). As a result, the Company has presented the financial statements as of December 31, 2023 and December 31, 2022 and for the two years ended December 31, 2023, in accordance with U.S. GAAP. Therefore, the amounts previously reported as of and for the year ended December 31, 2022, have been presented in these consolidated financial statements in accordance with the US GAAP.
These consolidated financial statements also incorporate the necessary corrections relating to errors in the previously issued consolidated financial statements in accordance with IFRS. These errors were the result of inappropriate application of variable consideration methodologies which resulted in an overstatement of revenue and understatement of loss in the amount of $
3. | Significant accounting policies: |
(a) | Operating segments: |
Operating segments are reported in a manner consistent with the internal reporting provided to the chief operating decision maker of the Company. The chief operating decision maker, which is responsible for allocating resources and assessing performance of the operating segments, has been identified as the executive committee consisting of the President and Chief Executive Officer, and the Chief Financial Officer. As the chief operating decision maker evaluates performance using entity-wide metrics, the Company has
F-12
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3.Significant accounting policies (continued):
(b) | Business combinations: |
The Company accounts for business combinations using the acquisition method of accounting. The total purchase price is allocated to the identifiable assets acquired and liabilities assumed based on fair values as at the date of acquisition. Goodwill as at the date of acquisition is measured as the excess of the aggregate of the consideration transferred and the amount of any non-controlling interests in the acquired company over the net of the acquisition date fair values of the identifiable assets acquired and the liabilities assumed.
Goodwill is not subjected to be amortized. Any non-controlling interest in the acquired company are measured at fair value.
Best estimates and assumptions are used in the purchase price allocation process to accurately value assets acquired and liabilities assumed at the business combination date.
These estimates and assumptions are inherently uncertain and subject to refinement. As a result, during the measurement period, which may be up to one year from the business combination date or earlier if all the information that is necessary to complete the acquisition accounting is obtained, the Company may record adjustments to the assets acquired and liabilities assumed, with the corresponding offset to goodwill and non-controlling interest. On conclusion of the measurement period, any subsequent adjustments are recorded in the consolidated statements of comprehensive loss in the period in which the adjustments were determined.
Any deferred and contingent consideration is measured at fair value at the date of acquisition and included in the consideration transferred in the acquisition. During the measurement period and on conclusion of the measurement period, if an obligation to pay contingent consideration that meets the definition of a financial instrument is classified as equity, then it is not remeasured and the settlement is accounted for within equity. Otherwise, other contingent consideration is remeasured at fair value at each reporting date until the settlement and subsequent changes in the fair value of the contingent consideration is recognized as part of the consolidated statements of comprehensive loss in the period in which the fair value adjustments were determined.
F-13
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3.Significant accounting policies (continued):
(c) | Impairment of non-financial assets: |
The Company recognizes the excess of the purchase price over the fair value of identifiable net assets acquired as goodwill. The Company may perform the optional qualitative assessment annually, at each year-end, or more frequently if events or changes in circumstances indicate that the carrying value of goodwill may not be recoverable. If the optional qualitative assessment is not performed or if it is determined in the qualitative assessment that the fair value of a reporting unit is more likely than not below its carrying amount, then the Company performs a quantitative impairment test.
Finite-lived intangible assets are reviewed for impairment in conjunction with other long-lived assets. The Company’s long-lived assets, including finite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset or asset group may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of an asset or asset group to the future undiscounted cash flows expected to be generated by the asset or asset group. If such asset or asset group is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value.
The quantitative goodwill impairment test is performed by comparing the fair value of a reporting unit with its carrying amount. Any excess in the carrying value of a reporting unit including goodwill over its fair value is recognized as an impairment loss, limited to the total amount of goodwill allocated to that reporting unit. For purposes of goodwill impairment testing, the Company has
(d) | Cash and restricted cash: |
Cash includes cash on hand and cash held with financial institutions with an initial term of
(e) | Revenue recognition: |
Service fee revenue is recognized at a point in time upon the performance of services under contracts with customers and represents the consideration to which the Company expects to be entitled. Service fee revenue is determined based on net patient fees, which includes estimates for contractual allowances and discounts. Net patient fees are estimated using an expected value approach where management considers such variables as the average of previous net patient fees received by the applicable payor and fees received by other patients for similar services and management’s best estimate leveraging industry knowledge and expectations of third-party payors’ fee schedules. Third-party payors include federal and state agencies (under the Medicare programs), managed care health plans and commercial insurance companies.
A key determinant of ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”) is estimating the transaction price when variable consideration may arise. ASC 606 allows for the transaction price with variable consideration to be estimated using either the expected value method or the most-likely value method. The Company’s estimates are calculated using the expected value method when using the sum of probability-weighted amounts in a range of possible consideration amounts.
F-14
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3.Significant accounting policies (continued):
(e) | Revenue recognition (continued): |
Variable consideration also exists in the form of settlements with certain insurance companies, including Medicare, as a result of retroactive adjustments due to audits and reviews. The Company applies constraint to the transaction price, such that net revenues are recorded only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in the future. If actual amounts of consideration ultimately received differ from the Company’s estimates, the Company adjusts these estimates, which would affect net revenues in the period such variances become known.
Due to the nature of the industry and complexity of the Company’s revenue arrangements, where price lists are subject to the discretion of payors, variable consideration exists that may result in price concessions and constraints to the transaction price for the services rendered.
In estimating this variable consideration, the Company uses significant judgment and considers various factors including, but not limited to, the following:
| ● | commercial payors and the administrators of federally-funded healthcare programs exercise discretion over pricing and may establish a base fee schedule for TMS (which is subject to change prior to final settlement) or negotiate a specific reimbursement rate with an individual TMS provider; |
| ● | average of previous net service fees received by the applicable payor and fees received by other patients for similar services; |
| ● | management’s best estimate, leveraging industry knowledge and expectations of third-party payors’ fee schedules; |
| ● | factors that would influence the contractual rate and the related benefit coverage, such as obtaining pre-authorization of services and determining whether the procedure is medically necessary; |
| ● | probability of failure in obtaining timely proper provider credentialing (including re-credentialling) and documentation, in order to bill various payors which may result in enhanced price concessions; and |
| ● | variation in coverage for similar services among various payors and various payor benefit plans. |
The Company updates the estimated transaction price (including updating its assessment of whether an estimate of variable consideration is constrained) to represent faithfully the circumstances present at the end of the reporting period and the changes in circumstances during the reporting period in which such variances become known.
The above factors are not related to the creditworthiness of the large medical insurance companies and government-backed health plans encompassing the significant majority of the Company’s payors. The payors (large insurers and government agencies) have the ability and intent to pay, but price lists for the Company’s services are subject to the discretion of payors. As a result, the adjustment to reduce the transaction price and constrain the variable consideration is a price concession and not indicative of credit risk on the payors (i.e. not a bad debt expense).
F-15
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3.Significant accounting policies (continued):
(f) | Accounts receivable: |
Accounts receivable are non-interest bearing, unsecured obligations due from patients and third-party payors. The Company makes an implicit allowance for potentially uncollectible amounts to arrive at net receivables through its revenue recognition policy. In accordance with ASC Topic 326, Financial Instruments – Credit Losses (“ASC 326”), the Company evaluates the credit risk on accounts receivable and measures a loss allowance at an amount equal to the expected credit losses.
The methodology to arrive at net receivables is reviewed by management periodically. The balance of accounts receivable represents management’s estimate of the net realizable value of receivables after discounts and contractual adjustments.
The Company performs an estimation and review process of methodology and inputs periodically to identify instances on a timely basis where such estimation models need to be revised.
The Company considers a default to be a change in circumstances that results in the payor no longer having the ability and intent to pay. In these circumstances, the Company will recognize a write-off against the related accounts receivable balance and a corresponding bad debt expense.
In estimating the collectability of its accounts receivable, the Company considers macroeconomic factors in assessing accounts receivable. Such factors would need to be significant in order to affect the ability and intent of the Company’s payors given their size and stature. As at December 31, 2023, no such factors were identified and therefore
(g) | Concentration of Credit Risk and Significant Customers: |
Financial instruments that potentially subject the company to concentration of credit risk consist of cash, cash equivalents and accounts receivable. The Company is not exposed to any significant concentrations of credit risk from these financial instruments. The Company’s concentration of credit risk is limited by the diversity, geography and number of patients and payors.
The Company does not have any customers or payors that individually represented 10% or more of the Company's accounts receivable, net balance as of December 31, 2023 and December 31, 2022.
(h) | Property, plant and equipment: |
Property, plant and equipment are stated at cost, net of accumulated depreciation and accumulated impairment losses. Depreciation is computed on a straight-line basis over the estimated useful lives of the assets, unless stated otherwise, as follows:
Furniture and equipment | ||
Leasehold improvements | Lesser of | |
TMS devices |
Expenditures for maintenance and repairs are charged to operations as incurred.
F-16
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3.Significant accounting policies (continued):
(i) | Intangible assets: |
The Company classifies intangible assets, obtained through acquisitions, as definite lived assets. Intangible assets consist of covenants not to compete, management service agreements with a professional organization and software. These intangible assets are recorded at cost and are amortized over their estimated useful lives, as follows:
Covenants not to compete |
| |
Management service agreements | ||
Software |
(j) | Leases: |
At inception of a contract, the Company assesses whether that contract is, or contains, a lease in accordance with ASC Topic 842, Leases (“ASC 842”). A lease exists when a contract conveys to the customer the right to control the use of an identified asset for a period of time in exchange for consideration. To assess whether a contract conveys the right to control the use of an identified asset, the Company assesses whether (i) the contract involves the use of an identified asset, (ii) the Company has the right to obtain substantially all of the economic benefits from the use of the identified asset throughout the period of use, and (iii) the Company has the right to direct the use of the identified asset throughout the period of use. As such, the practical expedients are not applicable and have not been utilized.
The Company’s ROU (as defined below) assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset or asset group may not be recoverable. Recoverability of an ROU asset to be held and used is measured by a comparison of the carrying amount of an ROU asset to the future undiscounted cash flows expected to be generated by the ROU asset. If such ROU asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the ROU asset exceeds its fair value.
Lease liabilities represent the obligation to make lease payments and right-of-use ("ROU") assets represent the right to use the underlying asset during the lease term. Leases with a term greater than one year are recognized in the consolidated balance sheet as lease liabilities and ROU assets at the commencement date of the lease based on the present value of lease payments over the lease term. The Company has elected not to recognize on the balance sheet leases with terms of one year or less.
The Company classifies a lease as a finance lease when the lease meets any of the following criteria at the lease commencement date: (i) the lease transfers ownership of the underlying asset to the lessee by the end of the lease term, (ii) the lease grants the lessee an option to purchase the underlying asset that the lessee is reasonably certain to exercise, (iii) the lease term is for the major part of the remaining economic life of the underlying asset, (iv) the present value of the sum of the lease payments and any residual value guaranteed by the lessee that is not already reflected in the lease payments equals or exceeds substantially all of the fair value of the underlying asset or (v) the underlying asset is of such a specialized nature that it is expected to have no alternative use to the lessor at the end of the lease term. When none of the criteria noted above is met, the Company classifies the lease as an operating lease.
F-17
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3.Significant accounting policies (continued):
(j)Leases (continued):
For both finance and operating leases, the right-of-use asset is initially measured at cost, which comprises the initial amount of the lease liability adjusted for any lease payments made at or before the commencement date, plus any initial direct costs incurred and an estimate of costs to dismantle and remove the underlying asset or to restore the underlying asset or the site on which it is located, less any lease incentives received. The lease liability is initially measured at the present value of the lease payments that are due to be paid at the commencement date plus any initial direct costs incurred and an estimate of costs at lease commencement to dismantle and remove the underlying asset or to restore the underlying asset or the site on which it is located. The lease payments are discounted using the implicit interest rate in the lease. If the rate cannot be readily determined, the Company’s incremental borrowing rate is used.
For finance leases, the ROU asset is subsequently amortized on a straight-line basis, over the shorter of the lease term or the useful life of the ROU asset. The lease liability is accreted based on the effective interest method using the discount rate determined at the lease commencement. The lease liability is reduced by the payments made up to the subsequent measurement date.
For operating leases, the ROU asset is subsequently measured throughout the lease term at the carrying amount of the lease liability, plus initial direct costs, plus (minus) any prepaid (accrued) lease payments, less the unamortized balance of lease incentives received. The lease liability is subsequently measured at the present value of the remaining lease payments using the discount rate determined at the lease commencement.
The Company makes estimates when considering the length of the lease term, including considering facts and circumstances that can create an economic incentive to exercise an extension option. The Company makes certain qualitative and quantitative assumptions when deriving the value of the economic incentive. Periodically, the Company will reassess whether it is reasonably certain to exercise extension options and will account for any changes at the date of reassessment.
The Company makes judgments in determining whether a contract contains an identified asset and in determining whether or not the Company has the right to control the use of the underlying asset. The Company also makes judgments in determining the incremental borrowing rate used to measure its lease liability in respect of each lease contract. As there are currently no market participants of a similar size and scale as the Company, the incremental borrowing rate is reflective of the interest rate applied historically on borrowings or loans received.
F-18
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3.Significant accounting policies (continued):
(k) | Defined contribution pension plan: |
A defined contribution pension plan is a plan that provides pension benefits in return for services rendered, provides an individual account for each plan participant, and specifies how contributions to the individual’s account are to be determined rather than specifies the amount of benefits the individual is to receive. Under a defined contribution pension plan, the benefits a plan participant will receive depend solely on the amount contributed to the plan participant’s account by the employer or the employee, the returns earned on investments of those contributions, and the forfeitures of other plan participants' benefits that may be allocated to that plan participant's account.
(l) | Provisions: |
Provisions are recognized if it is probable that a liability has been incurred and the amount is reasonably estimable. Provisions are measured based on a reasonable estimate of the expenditure required to settle the obligation at the end of the reporting period.
(m) | Contingencies: |
Contingent liabilities are existing conditions, situations, or sets of circumstances involving uncertainty as to possible loss to an entity that will ultimately be resolved when one or more future events occur or fail to occur. The term loss is used for convenience to include many charges against income that are commonly referred to as expenses and others that are commonly referred to as losses.
A loss contingency is accrued if it is both probable and reasonably estimable. Loss contingencies are not recognized but are disclosed in the notes to the consolidated financial statements, including an estimate of their potential financial effect and uncertainties relating to the amount or timing of any outflow, unless there is a reasonable possibility of settlement. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company, with assistance from its legal counsel, evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
F-19
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3. | Significant accounting policies (continued): |
(n) | Financial instruments: |
The Company initially measures its financial liabilities at fair value. Financial assets such as trade receivables are classified as either held-for sale or held for investment. After initial measurement, financial assets (which include cash and accounts receivable) and liabilities (which include accounts payable and accrued liabilities, lease liabilities, loans payable, and non-controlling interest loans payable) are subsequently measured at amortized cost using the effective interest rate method, with any resulting premium, discount and/or finance costs from the face value being amortized to the consolidated statements of comprehensive loss.
Financial liabilities that are derivative in nature (which include other payables) are subsequently measured at fair value at each reporting date, with any gain or loss being recorded in the consolidated statements of comprehensive loss. Embedded derivatives are assessed for bifurcation. If bifurcation is required, the embedded derivative is initially measured and subsequently measured at fair value at each reporting date, with any gain or loss being recorded in the consolidated statements of comprehensive loss.
The Company recognizes loss allowances for expected credit losses on financial assets measured at amortized cost. Loss allowances for accounts receivable are always measured at an amount equal to the lifetime expected credit losses. A financial asset carried at amortized cost is considered to contain credit loss and may contain further credit deterioration if factors exist which indicate that one or more events have had a negative effect on the estimated future cash flows of that asset that can be estimated reliably. Individually significant financial assets are tested for credit-impairment on an individual basis.
An impairment loss in respect of a financial asset measured at amortized cost is calculated as the difference between its carrying amount and the present value of the estimated future cash flows discounted at the asset’s original effective interest rate.
Losses are recognized in the consolidated statements of comprehensive loss. When a subsequent event causes the amount of impairment loss to decrease, the decrease in impairment loss is reversed through the consolidated statements of comprehensive loss.
(o) | Fair value measurement: |
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, regardless of whether that price is directly observable or estimated using another valuation technique. In estimating the fair value of an asset or a liability, the Company takes into account the characteristics of the asset or liability if market participants would take those characteristics into account when pricing the asset or liability at the measurement date.
The Company categorizes its financial assets and liabilities measured at fair value into one of three different levels depending on the observability of the inputs used in the measurement.
| ● | Level 1 - This level includes assets and liabilities measured at fair value based on unadjusted quoted prices for identical assets and liabilities in active markets that are accessible at the measurement date. |
F-20
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3. | Significant accounting policies (continued): |
(o)Fair value measurement (continued):
| ● | Level 2 - This level includes valuations determined using directly or indirectly observable inputs other than quoted prices included within Level 1. Derivative instruments in this category are valued using models or other standard valuation techniques derived from observable market inputs. |
| ● | Level 3 - This level includes valuations based on inputs which are less observable, unavailable or where the observable data is not significant to the measurement of the instruments’ fair value. Fair value measurements are categorized in their entirety based on the lowest level input that is significant to entire measurement. |
(p) | Share capital: |
Common shares are classified as shareholders’ equity. Incremental financing costs directly attributable to the issue of common shares and share purchase options are recognized as a deduction from shareholders’ equity, net any of tax effects.
When share capital recognized as equity is repurchased, the amount of the consideration paid, including directly attributable costs, is recognized as a deduction from shareholders’ equity.
Dividends are discretionary and are recognized as distributions within equity upon approval by the Board.
(q) | Share-based compensation: |
| (i) | Options: |
The Company has adopted an omnibus equity incentive plan (the “Equity Incentive Plan”). The Equity Incentive Plan is open to employees, directors, officers and consultants of the Company and its affiliates. For employees, the value of equity-settled options is measured by reference to the fair value of the equity instrument on the date which they are granted. The fair value is recognized as an expense with a corresponding increase in contributed surplus over the vesting period. The Board has the discretion to establish the vesting period for stock options granted.
Non-market vesting conditions are taken into account by adjusting the number of equity instruments expected to vest at each reporting date so that, ultimately, the cumulative amount recognized over the vesting period is based on the number of options that eventually vest. Fair value is calculated using the Black-Scholes option pricing model, which requires the input of highly subjective assumptions, including the volatility of share prices, forfeiture rate and expected life and changes in subjective input assumptions that can materially affect the fair value estimate. The Company estimates the expected forfeiture rate of equity-settled share-based compensation based on historical experience and management’s expectations.
Consideration received upon the exercise of stock options is credited to share capital, at which time the related contributed surplus is transferred to shareholder’s funds (deficit).
F-21
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3. | Significant accounting policies (continued): |
(q) | Share-based compensation (continued): |
| (ii) | Performance share units and restricted share units: |
The Company may issue performance share units (“PSUs”) and restricted share units (“RSUs”) under the Equity Incentive Plan to employees, directors and consultants of the Company and its affiliates; however, non-employee directors of the Company are not entitled to receive grants of PSUs. Each tranche in an award of PSUs or RSUs is considered a separate award with its own grant date fair value. The Company, at its discretion, will determine at the time of grant if the applicable PSUs or RSUs, as the case may be, are to be cash-settled or equity-settled.
Non-market vesting conditions are taken into account by adjusting the number of equity instruments expected to vest at each reporting date so that, ultimately, the cumulative amount recognized over the vesting period is based on the number of PSUs or RSUs that eventually vest.
If cash-settled, the fair value of the grants of PSUs and RSUs are recorded in corporate, general and administrative expenses. The fair value is recognized as a liability in the consolidated balance sheet. The PSUs and RSUs are subsequently remeasured at the end of each reporting period and any changes are recognized as an expense in the consolidated statements of comprehensive loss until the award is settled.
If equity-settled, the fair value of the grants of PSUs and RSUs are recognized as an expense in the consolidated statements of comprehensive loss. The total amount to be expensed is determined by the fair value of the PSUs and RSUs granted. The total expense is recognized over the vesting period which is the period over which all of the service vesting conditions are satisfied.
| (iii) | Deferred share units: |
The Company has adopted a deferred share unit plan for its non-employee directors (the “DSU Plan”). Each tranche in an award of deferred share units (“DSUs”) is considered a separate award with its own grant date fair value. Grants of DSUs are recorded at fair value in corporate, general and administrative expenses. As DSUs are cash-settled, the fair value of a DSU is recognized as a liability in the consolidated balance sheets. The DSUs are subsequently remeasured at the end of each reporting period and any changes are recognized as an expense in the consolidated statements of comprehensive loss until the award is settled.
F-22
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
3. | Significant accounting policies (continued): |
(r) | Finance income and finance costs: |
Finance income comprises interest income on cash equivalents recognized in the consolidated statements of comprehensive loss as it accrues, using the effective interest method. Finance costs comprise interest expense on borrowings and lease liabilities that are recognized in the consolidated statements of comprehensive loss at the effective interest rate.
(s) | Income taxes: |
Income tax expense comprises current and deferred tax. Income tax expense (recovery) is recognized in the consolidated statements of comprehensive loss. Current income tax expense represents the amount of income taxes payable in respect of taxable profit (loss) for the reporting period based on tax law that is enacted at the reporting date and is adjusted for changes in estimates of tax expense recognized in prior periods. A current tax liability or asset is recognized for income taxes payable, or paid but recoverable, in respect of all years to date.
Deferred tax assets and liabilities are recognized for the deferred tax consequences attributable to differences between the consolidated financial statements’ carrying amounts of assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the periods in which those temporary differences are expected to be recovered or settled. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the consolidated statements of comprehensive loss in the year in which the change in enactment or substantive enactment occurs. A deferred tax asset is recognized for net operating losses, tax credits and deductible temporary differences, to the extent that it is more likely than not that future taxable income will be available to utilize such amounts. Deferred tax assets are reviewed at each reporting date and are adjusted to the extent that it is more likely than not that the related tax benefits will not be realized. Deferred tax assets and liabilities are offset when they relate to income taxes levied by the same tax authority and the Company intends to settle its current tax assets and liabilities on a net basis.
In determining the amount of current and deferred taxes, the Company takes into account the impact of uncertain tax positions and whether additional taxes, penalties and interest may be due. The Company believes that its tax liabilities for uncertain tax positions are adequate for all open tax years based on its assessment of many factors, including interpretations of tax law and prior experience. The assessment relies on estimates and assumptions and may involve a series of judgments about future events. New information may become available that causes the Company to change its judgment regarding the adequacy of existing tax liabilities; such changes to tax liabilities will impact tax expense in the period that such a determination is made.
(t) | Earnings (loss) per share: |
Basic earnings (loss) per common share (“EPS”) is calculated by dividing the net earnings (loss) available to common shareholders by the weighted average number of common shares outstanding during the year. Diluted EPS is calculated based on the weighted average of potentially dilutive incremental shares included in each interim period resulting in the year-to-date period.
F-23
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
4. | Recent accounting pronouncements: |
Recent accounting pronouncements adopted:
The SEC has issued the following amendments to the existing standards that became effective for periods beginning on or after January 1, 2023:
(i) | Accounting Standards Update 2023-07—Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. The amendments were adopted on January 1, 2023. |
The adoption of the amendments to the existing standards did not have a material impact on these consolidated financial statements.
The SEC has issued the following amendment to the existing standard that will become effective for periods beginning on or after January 1, 2024:
(i) | Accounting Standards Update 2023-09—Income Taxes (Topic 740): Improvements to Income Tax Disclosures. This standard introduces improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. |
5. | Business acquisition: |
On July 14, 2022, the Company, through its wholly-owned U.S. subsidiary, TMS NeuroHealth Centers Inc., completed the acquisition of all of the issued and outstanding equity interests in Check Five LLC, a Delaware limited liability company (doing business as “Success TMS”) (“Success TMS”) from its parent company, Success Behavioral Holdings LLC (the “Success TMS Acquisition”) pursuant to a Membership Interest Purchase Agreement dated as of May 15, 2022 (the “Purchase Agreement”), by and among the Company, Success TMS and its direct and indirect owners, including Success Behavioral Holdings, LLC, Theragroup LLC, The Bereke Trust U/T/A Dated 2/10/03, Batya Klein and Benjamin Klein (collectively, the “Seller Parties”).
As consideration for the purchase of Success TMS, the Seller Parties received, in the aggregate,
The purchase price consideration was determined based on the pro forma revenue contribution of the two companies and was fixed at an amount equal to approximately
The Success TMS Acquisition represented the addition of
F-24
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
5. | Business acquisition (continued): |
The Success TMS Acquisition has been accounted for using the acquisition method of accounting. The allocation of the purchase price consideration for the Success TMS Acquisition is comprised as follows:
Purchase consideration |
|
| |
Share issuance | $ | | |
Share issuance, held in escrow |
| | |
| | ||
Net assets acquired |
|
| |
Cash acquired |
| | |
Accounts receivable, net |
| | |
Prepaid expenses and other |
| | |
Property, plant and equipment |
| | |
Software |
| | |
Management services agreements | | ||
| |||
| |||
Accounts payable and accrued liabilities | ( | ||
Deferred grant income | ( | ||
Loans payable | ( | ||
Shareholder loan |
| ( | |
( | |||
| ( | ||
| | ||
Goodwill | $ | |
As part of the Success TMS Acquisition, the Company acquired
Goodwill is primarily attributable to the ability to expand the Company’s national footprint and the synergies expected to result from combining Success TMS’ operations with the Company, and is allocated to the Success TMS cash generating unit. Goodwill is deductible for tax purposes.
F-25
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
6. | Property, plant and equipment: |
|
|
| ||||||||||
Furniture and | Leasehold | |||||||||||
| equipment |
| improvements |
| TMS devices |
| Total | |||||
Cost |
|
|
|
|
|
|
|
| ||||
Balance, December 31, 2021 | $ | | $ | | $ | | $ | | ||||
Additions |
| — |
| |
| |
| | ||||
Additions through business combinations (note 5) | — | | | | ||||||||
Asset disposal | — |
| — |
| ( |
| ( | |||||
| ||||||||||||
Balance, December 31, 2022 | | | | | ||||||||
Additions |
| — |
| |
| |
| | ||||
Additions through business combinations (note 5) |
| — |
| — |
| — |
| — | ||||
Asset disposal | ( | — | ( | ( | ||||||||
Balance, December 31, 2023 | $ | — | $ | | $ | | $ | | ||||
Accumulated depreciation |
|
|
|
|
|
|
| |||||
Balance, December 31, 2021 | | | | | ||||||||
Depreciation |
| |
| |
| |
| | ||||
Asset disposal |
| — |
| — |
| ( |
| ( | ||||
Balance, December 31, 2022 |
| |
| |
| |
| | ||||
Depreciation |
| |
| |
| |
| | ||||
Asset disposal |
| ( |
| — |
| ( |
| ( | ||||
Balance, December 31, 2023 | $ | — | $ | | $ | | $ | | ||||
Net book value |
|
|
|
|
|
|
|
| ||||
Balance, December 31, 2022 | $ | | $ | | $ | | $ | | ||||
Balance, December 31, 2023 |
| — |
| |
| |
| | ||||
F-26
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
7. | Intangible assets and goodwill: |
(a) | Intangible assets: |
Management | Covenants not | |||||||||||
| services agreements |
| to compete |
| Software |
| Total | |||||
Cost |
|
|
|
|
|
|
|
| ||||
Balance, December 31, 2021 | $ | |
| $ | |
| $ | — |
| $ | | |
Additions through business combinations (note 5) | |
| — |
| |
| | |||||
Impairment loss (note 7(c)) | ( | ( | ( | ( | ||||||||
Balance, December 31, 2022 |
| |
| |
| |
| | ||||
Additions |
| — |
| — |
| — |
| — | ||||
Balance, December 31, 2023 | $ | | $ | | $ | | $ | | ||||
Accumulated amortization |
|
|
|
|
|
|
|
| ||||
Balance, December 31, 2021 | $ | | $ | | $ | — | $ | | ||||
Amortization | | | | | ||||||||
Balance, December 31, 2022 | | | | | ||||||||
Amortization |
| |
| |
| — |
| | ||||
Balance, December 31, 2023 | $ | | $ | | $ | | $ | | ||||
Net book value |
|
|
|
|
|
|
|
| ||||
Balance, December 31, 2022 | $ | | $ | | $ | — | $ | | ||||
Balance, December 31, 2023 |
| |
| |
| — |
| | ||||
As a part of the Success TMS Acquisition, the Company goodwill, the management service agreements and software intangible assets (see note 5).
F-27
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
7. | Intangible assets and goodwill (continued): |
(b) | Goodwill: |
| Total | ||
Cost |
|
| |
Balance, December 31, 2021 | $ | | |
Additions (note 5) |
| | |
Balance, December 31, 2022 |
| | |
Additions |
| | |
Balance, December 31, 2023 | $ | | |
Impairment |
|
| |
Balance, December 31, 2021 | $ | — | |
Impairment (note 7(c)) |
| ( | |
Balance, December 31, 2022 |
| ( | |
Impairment |
| — | |
Balance, December 31, 2023 | $ | ( | |
Net book value |
|
| |
Balance, December 31, 2022 | $ | — | |
Balance, December 31, 2023 |
| — | |
The Company has 4 reporting units, comprised of Achieve TMS East/Central, Achieve TMS West, Success TMS and the remaining Company operations. In accordance with ASC Topic 350, Intangible Assets and Goodwill (“ASC 350”), as the majority of the criteria for aggregation have been met, the Company’s 4 reporting units, all under the same operating segment, will be aggregated for the purpose of goodwill impairment testing. All goodwill is allocated to the aggregated reporting unit.
Annually, the reporting unit is assessed for impairment of the goodwill balance. The Company has adopted the income approach for the valuation of the reporting unit. The Company utilizes post tax amounts for the purpose of the goodwill impairment assessment. The recoverable amount of the reporting unit is estimated based on an assessment of discounted cash flows. The discounted cash flow for each reporting unit is determined by discounting five-year cash flow projections (cash flows beyond the five-year period are extrapolated using terminal growth rates). These projections reflect management’s expectations based on experience and future estimates of operating performance.
In measuring the recoverable amount for the reporting unit as at December 31, 2022, significant estimates include the average five-year budgeted revenue growth rate of
F-28
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
7. | Intangible assets and goodwill (continued): |
(b) | Goodwill (continued): |
An impairment charge is recognized to the extent that the carrying value exceeds the fair value. Impairment charges that have arisen as a result of the reviews performed total $
The Company did not perform the goodwill impairment assessment in 2023 as the carrying value of goodwill is
(c) | Impairment of long-lived assets: |
As at December 31, 2022, the Company performed its annual assessment of the long-lived assets allocated to each of the Company’s asset groups and determined that all asset groups met an overall indication of impairment and conducted the test for recoverability. The Company determined that the property, plant and equipment and right-of-use assets held by these asset groups had recoverable amounts in excess of the carrying values at December 31, 2022. The Company determined that the intangible assets held by
As at December 31, 2023, the Company performed its annual assessment of the long-lived assets allocated to each of the Company’s asset groups and determined that all asset groups met an overall indication of impairment and conducted the test for recoverability. The Company determined that the property, plant and equipment, right-of-use assets and intangible assets held by these asset groups had fair values in excess of the carrying values at December 31, 2023.
8. | Right-of-use assets and lease liabilities: |
The Company enters into lease agreements related to TMS devices and mental health treatment centers (“Treatment Centers”). These lease agreements range from
Right-of-use assets are initially measured at cost, which is comprised of the initial amount of the lease liability adjusted for any lease payments made at or before the commencement date, plus any initial direct costs incurred.
Lease liabilities have been measured by discounting future lease payments using a rate implicit in the lease or the Company’s incremental borrowing rate. The Company’s incremental borrowing rate during the year ended December 31, 2023 is
(a) | Finance leases: |
Finance leases include lease agreements relating to TMS devices.
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Finance right-of-use assets, beginning of the year | $ | | $ | | ||
Impact of lease additions, disposals and/or modifications |
| ( |
| | ||
Additions through business combinations (note 5) |
| — |
| | ||
Exercise of buy-out options into property, plant and equipment |
| ( |
| ( | ||
Depreciation on right-of-use assets |
| ( |
| ( | ||
Finance right-of-use assets, end of the year | $ | |
| |||
F-29
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
8. | Right-of-use assets and leases liabilities (continued): |
(a) | Finance leases (continued): |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Finance lease liabilities, beginning of the year | $ | | $ | | ||
Impact of lease additions, disposals and/or modifications |
| ( |
| | ||
Additions through business combinations (note 5) |
| — |
| | ||
Interest expense on lease liabilities |
| |
| | ||
Payments of lease liabilities |
| ( |
| ( | ||
Finance lease liabilities, end of the year | $ | |
| | ||
Less current portion of finance lease liabilities |
| |
| | ||
Long term portion of finance lease liabilities | $ | | $ | | ||
During the current year, certain device leases were amended from a fixed fee arrangement to a variable fee arrangement and the Company re-assessed the renewal options relating to certain device leases and concluded that it is not probable that the renewal options under those leases will be exercised, which resulted in a decrease in right-of-use assets and liabilities. Certain device leases were derecognized as a result of a settlement and mutual release with a device manufacturer. See note 10(a)(iii) and note 12(d).
(b) | Operating leases: |
Operating leases include lease agreements relating to Treatment Centers.
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Operating right-of-use assets, beginning of the year | $ | | $ | | ||
Impact of lease additions, disposals and/or modifications |
| ( |
| | ||
Additions through business combinations (note 5) |
| — |
| | ||
Impairment of right-of-use assets | ( | — | ||||
Right-of-use asset lease expense |
| ( |
| ( | ||
Operating right-of-use assets, end of the year |
| |
| | ||
F-30
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
8. | Right-of-use assets and leases liabilities (continued): |
(b) | Operating leases (continued): |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Operating lease liabilities, beginning of the year | $ | | $ | | ||
|
|
|
| |||
Impact of lease additions, disposals and/or modifications |
| ( |
| | ||
Additions through business combinations (note 5) |
| — |
| | ||
Lease liability expense |
| |
| | ||
Payments of lease liabilities |
| ( |
| ( | ||
Operating lease liabilities, end of the year |
| |
| | ||
Less current portion of operating lease liabilities |
| |
| | ||
Long term portion of operating lease liabilities | $ | | $ | | ||
During the current year, certain building leases were terminated as part of the execution of the Restructuring Plan (see note 25), which resulted in a decrease in right-of-use assets and liabilities.
Undiscounted cash flows for lease liabilities as at December 31, 2023 are as follows:
| Finance |
| Operating | |||
2024 | | | ||||
2025 | | | ||||
2026 | | | ||||
2027 |
| — | | |||
2028 |
| — | | |||
Thereafter |
| — | | |||
Total minimum lease payments |
| | | |||
Less discounted cash flows |
| | | |||
Present value of minimum lease payments | $ | | $ | | ||
9. | Accounts payable and accrued liabilities: |
The accounts payable and accrued liabilities are as follows:
December 31, | December 31, | |||||
| 2023 |
| 2022 | |||
Accounts payable | $ | | $ | | ||
Accrued liabilities |
| |
| | ||
Total | $ | | $ | | ||
F-31
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
10.Loans payable:
(a) | Bank loans: |
TMS | |||||||||||||||
device | Credit | Promissory |
| Neuronetics | |||||||||||
| loans (i) |
| Facility (ii) |
| notes (iii) |
| Note (iv) |
| Total | ||||||
Short term | $ | $ | | $ | | $ | — | $ | |||||||
Long term | | |
| — |
| ||||||||||
Total, net | $ | $ | | $ | | $ | — | $ | |||||||
Unamortized capitalized financing costs | — | — | — | ||||||||||||
Total, December 31, 2022 | $ | $ | $ | | $ | — | $ | ||||||||
Short term | $ | $ | $ | | $ | | $ | ||||||||
Long term | | | |||||||||||||
Total, net | $ | $ | $ | | $ | | $ | ||||||||
Unamortized capitalized financing costs | — | | — | ||||||||||||
Total, December 31, 2023 | $ | $ | $ | | $ | | $ | ||||||||
Undiscounted cash flows for bank loans as at December 31, 2023, inclusive of principal and interest are as follows:
TMS | |||||||||||||||
device | Credit | Promissory |
| Neuronetics | |||||||||||
| loans (i) |
| Facility (ii) |
| notes (iii) |
| Note (iv) |
| Total | ||||||
2024 | $ | | $ | | $ | — | $ | | $ | | |||||
2025 |
| |
| |
| | |
| | ||||||
2026 |
| — |
| |
| — | |
| | ||||||
2027 |
| — |
| |
| — | |
| | ||||||
2028 |
| — |
| — |
| | — |
| | ||||||
Thereafter |
| — |
| — |
| — | — |
| — | ||||||
Total Cash Payments | $ | | $ | | $ | | $ | | $ | | |||||
| (i) | TMS Device loans |
During the year ended December 31, 2022, the Company assumed loans as part of the Success TMS Acquisition from three separate financing companies for the purchase of TMS devices. These TMS device loans bear an average interest rate of
During the year ended December 31, 2023, the Company repaid TMS device loans totalling $
F-32
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
10.Loans payable (continued):
(ii) | Madryn Credit Facility |
On July 14, 2022, the Company entered into the Madryn Credit Agreement in respect of the Madryn Credit Facility. The Madryn Credit Facility provided the Company with a $
On February 1, February 21, March 20, March 24, August 1, September 15, October 19, November 2, November 15, December 5, December 14 and December 28, 2023, the Company entered into amendments to the Madryn Credit Facility, whereby Madryn extended
In addition, the Madryn Credit Facility was amended on February 21, 2023 to provide that, commencing March 31, 2023, all advances under the Madryn Credit Facility (including the New Loans) will cease to accrue interest using the LIBOR benchmark and instead will accrue interest at a rate equal to
The carrying amount of the Madryn Credit Facility as at December 31, 2023 is $
In accordance with the terms of the Madryn Credit Agreement, the Company has issued conversion instruments (each, a “Madryn Conversion Instrument”) to Madryn and certain of its affiliated entities that provide the holders thereof with the option to convert up to $
F-33
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
10.Loans payable (continued):
(ii)Madryn Credit Facility (continued)
The terms of the Madryn Credit Agreement require the Company to satisfy various affirmative and negative covenants and to meet certain financial tests, including but not limited to, consolidated minimum revenue and minimum liquidity covenants. In addition, the Madryn Credit Agreement contains affirmative and negative covenants that limit, among other things, the Company’s ability to incur additional indebtedness outside of what is permitted under the Madryn Credit Agreement, create certain liens on assets, declare dividends and engage in certain types of transactions. The Madryn Credit Agreement also includes customary events of default, including payment and covenant breaches, bankruptcy events and the occurrence of a change of control. The Madryn Credit Facility also requires the Company to deliver to Madryn annual audited financial statements that do not contain any going concern note, however, the Company has obtained waivers from Madryn with respect to such obligation for fiscal 2023.
On June 14, 2023, the Company received a waiver from Madryn under the Madryn Credit Agreement to temporarily reduce the Company’s minimum liquidity covenant until June 30, 2023. As consideration for the waiver, Madryn received an amendment fee in the amount of $
On February 21, 2023, March 20, 2023, June 14, 2023, July 3, 2023, July 14, 2023, August 1, 2023, August 14,2023, September 15, 2023, September 29, 2023, October 12, 2023, November 15, 2023 and December 14, 2023, the Company received waivers from Madryn with respect to the Company’s non-compliance with the minimum liquidity covenant. As at December 31, 2023, the Company was in compliance with the financial covenants under the Madryn Credit Agreement. In addition, the Company also received a waiver relating to the requirement to deliver financial statements within 90 days of each fiscal year end until April 26, 2024, and audited financial statements for such fiscal year, accompanied by a report and opinion of an independent certified public accountant which is not subject to any “going concern” or like qualification or exception or any qualification or exception as to the scope of such audit.
Pursuant to the 2023 Private Placement completed on March 23, 2023, Madryn is now also a major shareholder of the Company. See note 15.
(iii) | Promissory notes: |
On July 14, 2022, the Company assumed
On February 3, 2023, the Company issued additional promissory notes to certain officers of the Company, in the aggregate amount of $
F-34
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
10.Loans payable (continued):
(iii)Promissory notes (continued):
On August 15, August 28, September 1, September 25, September 26, September 27, September 29, October 3, October 12 and October 13, 2023, the Company issued subordinated convertible promissory notes (the “Subordinated Convertible Notes”) to Madryn, certain officers of the Company and various investors in an aggregate amount of $
In accordance with the terms of the Note Purchase Agreement, each holder of a Subordinated Convertible Note has the option to convert any amount up to the outstanding principal amount plus accrued interest into common shares of the Company at any time at the election of the holders of the Subordinated Convertible Notes or on a mandatory basis by all noteholders at the request of Madryn. The Subordinated Convertible Notes are convertible into common shares at a conversion price equal to the lesser of 85% of the closing price per common share on Nasdaq or any other market as of the closing date for such Subordinated Convertible Note, as adjusted from time to time,
In connection with the issuance of the Subordinated Convertible Notes, the Company concurrently entered into amendments to the Madryn Credit Agreement and the Neuronetics Note, pursuant to which the Company is permitted to incur the indebtedness under the Subordinated Convertible Notes.
Financing costs of $
The carrying value of all promissory notes referenced in note 10(a)(iii) as at December 31, 2023 is $
(iv)Neuronetics Note:
On March 31, 2023, the Company entered into an agreement with Neuronetics, Inc. (“Neuronetics”) to convert the Company’s outstanding account balance payable to Neuronetics of $
F-35
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
10.Loans payable (continued):
(iv)Neuronetics Note (continued):
Pursuant to the terms of the Neuronetics Note, in the event of default under the Neuronetics Note, the Company will be required to issue common share purchase warrants (the “Neuronetics Warrants”) to Neuronetics equal to (i)
In connection with the entry into the Neuronetics Note, the Company concurrently entered into an amendment to the Madryn Credit Agreement pursuant to which the Company is permitted to incur the indebtedness under the Neuronetics Note.
The carrying value of the Neuronetics Note as at December 31, 2023 is $
(b) | Non-controlling interest loans: |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Non-controlling interest loans | $ | | $ | | ||
The non-controlling interest holder partners of the Company, from time to time, provide additional capital contributions in the form of capital loans to the Company’s subsidiaries. These loans bear interest at a rate of
F-36
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
11.Shareholder loans:
(a) | Klein Note: |
On July 14, 2022, in connection with the Success TMS Acquisition, the Company assumed the obligation of Success TMS to repay a promissory note to Benjamin Klein, who is a significant shareholder, former officer and former director of the Company. The promissory note totals $
The carrying value of the shareholder loan as at December 31, 2023 is
(b) | February 2023 Notes, February 2023 Greybrook Note and August 2023 Greybrook Note: |
On February 3, 2023, the Company issued the February 2023 Notes to certain shareholders of the Company in an aggregate amount of $
On February 28, 2023, the Company issued a promissory note to Greybrook Health, who is a significant shareholder of the Company (the “February 2023 Greybrook Note”). The February 2023 Greybrook Note totals $
On February 28, 2023, the fair value of the February 2023 Greybrook Warrants (as defined below) at grant date was $
F-37
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
11. | Shareholder loans (continued): |
(b)February 2023 Notes, February 2023 Greybrook Note and August 2023 Greybrook Note (continued):
On August 1, 2023, the Company issued an additional promissory note to Greybrook Health (the “August 2023 Greybrook Note”). The August 2023 Greybrook Note totals $
On August 1, 2023, the fair value of the August 2023 Greybrook Warrants at grant date was $
Financing costs of $
The carrying value of the February 2023 Notes, the February 2023 Greybrook Note and the August 2023 Greybrook Note as at December 31, 2023 is
F-38
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
11. | Shareholder loans (continued): |
(c) | Subordinated Convertible Notes: |
On August 15, 2023, the Company issued Subordinated Convertible Notes to certain shareholders of the Company in an aggregate amount of $
In connection with the issuance of the Subordinated Convertible Notes, the Company concurrently entered into amendments to the Madryn Credit Agreement and the Neuronetics Note, pursuant to which the Company is permitted to incur the indebtedness under the Subordinated Convertible Notes.
The carrying value of the Subordinated Convertible Notes as at December 31, 2023 is $
Interest expense for the year ended December 31, 2023 was $
12. | Other payables: |
(a) | Lender warrants: |
| December 31, |
| December 31, | |||
| 2023 |
| 2022 | |||
Lender warrants | $ | — | $ | | ||
As consideration on entering into the Oxford Credit Facility which was terminated on July 14, 2022, the Company issued
As the exercise price is denoted in a different currency than the Company’s functional currency, the lender warrants are recorded at fair value as a financial liability in other payables on the consolidated balance sheets. As at December 31, 2023, the fair value of the lender warrants was
The change in fair value of the lender warrants during the year ended December 31, 2023 was a decrease of $
F-39
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
12. | Other payables (continued): |
(b) | Deferred share units: |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Deferred share units | $ | | $ | | ||
On May 6, 2021, the Company adopted the DSU Plan for non-employee directors (each, a “Non-Employee Director”). Each Non-Employee Director is required to take at least
Following a Non-Employee Director ceasing to hold all positions with the Company, the Non-Employee Director will receive a payment in cash at the fair market value of the common shares represented by the Non-Employee Director’s DSUs generally within
As the DSUs are cash-settled, the DSUs are recorded as cash-settled share-based payments and a financial liability has been recognized on the consolidated balance sheets. During the year ended December 31, 2023,
(c) | Performance share units: |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Performance share units | $ | | $ | | ||
On May 6, 2021, the Company’s Equity Incentive Plan was amended and restated to permit the Company to grant PSUs and RSUs, in addition to stock options. Under the Equity Incentive Plan, the Company pays equity instruments of the Company, or a cash payment equal to the fair market value thereof, as consideration in exchange for employee and similar services provided to the Company. The Equity Incentive Plan is open to employees, directors, officers and consultants of the Company and its affiliates; however, Non-Employee Directors are not entitled to receive grants of PSUs.
On August 5, 2021,
The Company finalized that
F-40
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
12. | Other payables (continued): |
(c) | Performance share units: |
As at December 31, 2023, the value of the financial liability attributable to the PSUs is $
As at December 31, 2023, the Company has
The change in fair value of the PSUs during the year ended December 31, 2023 was a decrease of $
(d) | Device contract termination: |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Device contract termination | $ | | $ | — | ||
On August 21, 2023, the Company entered into a settlement and mutual release agreement with a device manufacturer for the termination of TMS device contracts. In accordance with the terms of the settlement, the Company recognized an amount payable of $
(e) | Klein Note settlement payable: |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Klein Note settlement payable | $ | | $ | — | ||
On November 20, 2023, the Company entered into a settlement agreement with on the Klein Note. In accordance with the terms of the settlement, the Company will make payment in total of $
F-41
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
13. | Deferred and contingent consideration: |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Deferred and contingent consideration | $ | | $ | | ||
The deferred and contingent consideration payable balance related to the acquisition of Achieve TMS East, LLC and Achieve TMS Central, LLC (the “Achieve TMS East/Central Acquisition”) as at December 31, 2021 was $
As at December 31, 2023, the deferred and contingent consideration in relation to the of Achieve TMS East/Central Acquisition was $
14.Advance for research collaboration:
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Advance for research collaboration | $ | | $ | – | ||
On December 29, 2023, the Company entered into a
The research collaboration agreement outlines a payout to the Company of $
F-42
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
15. | Common shares: |
The Company is authorized to issue an unlimited number of common shares and an unlimited number of preferred shares, issuable in series. As at December 31, 2023 and December 31, 2022, there were
|
| Total | |||
Number | amount | ||||
December 31, 2021 |
| | $ | | |
Common share issuances: |
|
|
| ||
Success TMS Acquisition issuance | | | |||
December 31, 2022 |
| | $ | | |
Common share issuances: | |||||
Issuance of common shares – 2023 Private Placement | | | |||
Issuance of common shares – Alumni Purchase Agreement | | | |||
December 31, 2023 | | $ | | ||
The following common shares were issued during the year ended December 31, 2023:
| (a) | On March 23, 2023, the Company completed the 2023 Private Placement. Pursuant to the 2023 Private Placement, an aggregate of |
| (b) | On July 13, 2023, the Company entered into the Alumni Purchase Agreement with Alumni, pursuant to which Alumni has agreed to provide equity line financing for sales from time to time of up to $ |
In exchange for Alumni entering into the Alumni Purchase Agreement, the Company issued
The following common shares were issued during the year ended December 31, 2022:
(a) | On July 14, 2022, the Company completed the Success TMS Acquisition (see note 5). The Company issued as purchase consideration |
F-43
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
16. | Contributed surplus: |
Contributed surplus is comprised of share-based compensation and lender warrants.
| (a) | Share-based compensation - options |
Stock options granted under the Equity Incentive Plan are equity-settled. The fair value of the grant of the options is recognized as an expense in the consolidated statements of comprehensive loss. The total amount to be expensed is determined by the fair value of the options granted. The total expense is recognized over the vesting period which is the period over which all of the service vesting conditions are satisfied. The vesting period is determined at the discretion of the Board and has ranged from immediate vesting to over
The maximum number of common shares reserved for issuance, in the aggregate, under the Equity Incentive Plan is
As at December 31, 2023,
Weighted | |||||
Number of | average | ||||
| stock options |
| exercise price | ||
Outstanding as at December 31, 2021 |
| | $ | | |
Forfeited | ( | ( | |||
Outstanding as at December 31, 2022 | | $ | | ||
Granted | | | |||
Forfeited | ( | ( | |||
Outstanding as at December 31, 2023 | | $ | | ||
The weighted average contractual life of the outstanding options as at December 31, 2023 was
The total number of stock options exercisable as at December 31, 2023 was
During the year ended December 31, 2023, the Company recorded a total share-based options compensation expense of $
F-44
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
16. | Contributed surplus (continued): |
(a)Share-based compensation - options (continued)
The following stock options were granted during the year ended December 31, 2023:
| (i) | On May 15, 2023, |
| (ii) | On November 8, 2023, |
There were
As at December 31, 2023, the total compensation cost not yet recognized related to options granted is approximately $
(b) | Greybrook Warrants |
As consideration for the purchase of the February 2023 Greybrook Note, the Company issued
The fair value of the February 2023 Greybrook Warrants granted on February 28, 2023 was estimated to be $
As consideration for the purchase of the August 2023 Greybrook Note issued on August 1, 2023, the Company issued
The fair value of the August 2023 Greybrook Warrants granted on August 1, 2023 were valued at $
The weighted average contractual life of the Greybrook Warrants as at December 31, 2023 was
The total number of the February Greybrook Warrants exercisable as at December 31, 2023 was
The aggregate fair value of the Greybrook Warrants granted during the year ended December 31, 2018 was $
F-45
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
17. | Contingencies: |
The Company may be involved in certain legal matters arising from time to time in the normal course of business. The Company records provisions that reflect management’s best estimate of any potential liability relating to these matters.
On May 24, 2023, the Seller Parties filed a complaint in the Superior Court of the State of Delaware against the Company and certain executive officers of the Company, and subsequently filed a first amended complaint on August 31, 2023 (the “Delaware Complaint”), concerning alleged disputes arising out of the Success TMS Acquisition (the “Purchase Agreement Claims”). The Purchase Agreement Claims allege contractual fraud, indemnification for breach of certain representations and warranties of the Company contained in the Purchase Agreement, other breaches of the Purchase Agreement and a registration rights agreement, and breach of the implied covenant of good faith and fair dealing. The Delaware Complaint seeks damages in an amount to be determined at trial, which are alleged to exceed $
The Company believes that the resolution of the matter is not expected to have a material adverse effect on the Company’s financial position, results of operations or cash flows.
18. | Pensions: |
The Company has adopted a defined contribution pension plan for its employees whereby the Company matches contributions made by participating employees up to a maximum of
F-46
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
19. | Income taxes: |
(a) | Numerical reconciliation of income tax expense: |
As at December 31, 2023, the Company has approximately $
The Company’s provision for income taxes is reconciled as follows:
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Accounting net loss before income tax – Greenbrook TMS | $ | ( | $ | ( | ||
Accounting net loss before income tax - non-controlling interest | ( | ( | ||||
Accounting net loss before income tax | $ | ( | $ | ( | ||
Income tax provision at statutory rate | ||||||
(December 31, 2022 – | $ | ( | $ | ( | ||
Non-controlling interest | |
| | |||
Non-deductible expenses and other permanent differences | |
| | |||
Future rate differential | ( |
| | |||
Change in valuation allowance | |
| | |||
Income tax expense at effective rate | $ | — | $ | — | ||
(b) | Deferred tax asset/liability: |
The components of the deferred income tax assets and liabilities are as follows:
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Assets: | ||||||
Non-capital loss carry-forward | $ | 37,738,560 | $ | 21,835,486 | ||
Other, including share-based compensation | 6,332,421 | 8,228,183 | ||||
Intangible assets | 13,281,892 | 13,917,720 | ||||
Total deferred income tax assets | $ | 57,352,873 | $ | 43,981,389 | ||
Liabilities: | ||||||
Property, plant and equipment | $ | ( | $ | ( | ||
Total deferred income tax liabilities |
| ( |
| ( | ||
Net deferred income taxes | 56,551,634 | 43,069,703 | ||||
Valuation allowance |
| ( |
| ( | ||
Net deferred income taxes, net of valuation allowance | $ | | $ | | ||
F-47
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
20. | Risk management arising from financial instruments: |
In the normal course of business, the Company is exposed to risks related to financial instruments that can affect its operating performance. These risks, and the actions taken to manage them, are as follows:
| (a) | Fair value: |
The Company has Level 1 financial instruments which consists of cash, restricted cash, accounts receivable and accounts payable and accrued liabilities which approximate their fair value given their short-term nature. The Company also has lender warrants, DSUs and PSUs that are considered Level 2 financial instruments (see note 12). The Company has contingent consideration (note 5) that are considered Level 3 financial instruments.
The carrying value of the loans payable, shareholder loan and finance lease obligations approximates their fair value given the difference between the discount rates used to recognize the liabilities in the consolidated balance sheets and the market rates of interest is insignificant.
Financial instruments are classified into one of the following categories: financial assets or financial liabilities.
| (b) | Credit risk: |
Credit risk arises from the potential that a counterparty will fail to perform its obligations. The Company is exposed to credit risk from patients and third-party payors including federal and state agencies (under the Medicare programs), managed care health plans and commercial insurance companies. The Company’s exposure to credit risk is mitigated in large part due to the majority of the accounts receivable balance being receivable from large, creditworthy medical insurance companies and government-backed health plans.
The Company’s aging schedule in respect of its accounts receivable balance as at December 31, 2023 and December 31, 2022 is provided below:
| December 31, |
| December 31, | |||
Days since service delivered | 2023 | 2022 | ||||
0 - 90 | $ | | $ | | ||
91 - 180 |
| |
| | ||
181 - 270 |
| | | |||
270+ | | | ||||
Total accounts receivable | $ | | $ | | ||
Based on the Company’s industry, none of the accounts receivable in the table above are considered “past due”. Furthermore, the payors have the ability and intent to pay, but price lists for the Company’s services are subject to the discretion of payors. As such, the timing of collections is not linked to increased credit risk. The Company continues to collect on services rendered in excess of 24 months from the date such services were rendered.
| (c) | Liquidity risk: |
Liquidity risk is the risk that the Company may encounter difficulty in raising funds to meet its financial commitments or can only do so at excessive cost. The Company ensures there is sufficient liquidity to meet its short-term business requirements, taking into account its anticipated cash flows from operations, its holdings of cash and its ability to raise capital from existing or new investors and/or lenders (see note 2(a)).
F-48
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
20. | Risk management arising from financial instruments (continued): |
| (d) | Currency risk: |
Currency risk is the risk to the Company’s earnings that arises from fluctuations in foreign exchange rates and the degree of volatility of those rates. The Company has minimal exposure to currency risk as substantially all of the Company’s revenue, expenses, assets and liabilities are denominated in U.S. dollars. The Company pays certain vendors and payroll costs in Canadian dollars from time to time, but due to the limited size and nature of these payments it does not give rise to significant currency risk.
| (e) | Interest rate risk: |
Interest rate risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company is exposed to changes in interest rates on its cash and long-term debt. Certain loans payable and shareholder loans (see note 10 and note 11) bear interest at a rate equal to the 3-month Term SOFR plus
21. | Capital management: |
The Company’s objective is to maintain a capital structure that supports its long-term growth strategy, maintains creditor and customer confidence, and maximizes shareholder value.
The capital structure of the Company consists of its shareholders’ equity, including contributed surplus and deficit, as well as loans payable and shareholders loans.
The Company’s primary uses of capital are to finance operations, finance new center start-up costs, increase non-cash working capital, capital expenditures and finance service debt obligations. The Company’s objectives when managing capital are to ensure the Company will continue to have enough liquidity so it can provide its services to its customers and returns to its shareholders. The Company, as part of its annual budgeting process and on an ongoing basis, periodically evaluates its estimated cash requirements to fund working capital requirements of existing operations. Based on this and taking into account its anticipated cash flows from operations and its holdings of cash, the Company validates whether it has the sufficient capital or needs to obtain additional capital.
F-49
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
22. | Related party transactions: |
(a) | Compensation of key management personnel: |
The Company transacts with key individuals from management who have authority and responsibility to plan, direct and control the activities of the Company. Key management personnel are defined as the executive officers of the Company, including the President and Chief Executive Officer, the Chief Financial Officer, the former Chief Financial Officer, the former Chief Operating Officer, the Chief Marketing Officer and the Senior Vice President of Operations.
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Salaries and bonuses | $ | | $ | | ||
Share-based compensation |
| |
| | ||
Performance share units | ( | ( | ||||
Total | $ | | $ | | ||
(b) | Transactions with significant shareholder - Greybrook Health : |
As at December 31, 2023, $
During the year ended December 31, 2023, the Company recognized $
(c) | Loans from shareholder – Greybrook Health |
In connection with the February 2023 Notes, the February 2023 Greybrook Note and the August 2023 Greybrook Note, the Company received loans from and issued promissory notes to Greybrook Health, who is a significant shareholder of the Company. The February 2023 Notes, the February 2023 Greybrook Note and the August 2023 Greybrook Note total $
On August 15, 2023, the Company issued Subordinated Convertible Notes to Greybrook Health in an aggregate amount of $
During the year ended December 31, 2023, the Company recognized $
F-50
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
22. | Related party transactions (continued): |
(d) | Transactions with the significant shareholder, former officer and former director – Benjamin Klein |
During the year ended December 31, 2023, the Company recognized $
As at December 31, 2023,
(e) | Loan from significant shareholder, former officer and former director – Benjamin Klein |
On July 14, 2022, in connection with the Success TMS Acquisition, the Company assumed the obligation to repay the Klein Note to Benjamin Klein, who is a significant shareholder of the Company. The Klein Note totals $
During the year ended December 31, 2023, the Company recognized $
(f) | Loans from shareholders and officers |
The February 2023 Notes (not including Greybrook Health’s contribution) total $
During the year ended December 31, 2023, the Company recognized $
(g) | Loan from significant shareholder – Madryn |
On July 14, 2022, the Company entered into the Madryn Credit Agreement in respect of the Madryn Credit Facility, which was subsequently amended during the year ended December 31, 2023, for a total principal balance of $
On August 15, September 1 and October 12, 2023 the Company issued Subordinated Convertible Notes to Madryn in an aggregate amount of $
During the year ended December 31, 2023, the Company recognized $
F-51
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
23. | Basic and diluted loss per share: |
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Net loss attributable to the common shareholders of: |
|
|
|
| ||
Greenbrook TMS | ( | ( | ||||
Weighted average common shares outstanding: |
|
|
|
| ||
Basic and diluted |
| |
| | ||
Loss per share: |
|
|
|
| ||
Basic and diluted | ( | ( | ||||
For the year ended December 31, 2023, the effect of
24. | Non-controlling interest: |
As a result of operating agreements with non-wholly owned entities, the Company has control over these entities under U.S. GAAP, as the Company has power over all significant decisions made by these entities and thus
The following summarizes changes in the Company’s non-wholly owned entities during the reporting or comparative periods:
| (a) | On February 27, 2023, the Company acquired a portion of the non-controlling ownership interest in Greenbrook TMS Connecticut LLC for the release of liabilities and losses. As at December 31, 2023, the Company has an ownership interest of |
| (b) | On September 29, 2023, the Company acquired a portion of the non-controlling ownership interest in Greenbrook TMS Arlington LLC for $ |
| (c) | On August 1, 2022, the Company acquired a portion of the non-controlling ownership interest in TMS NeuroHealth Centers Rockville LLC for $ |
F-52
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
24. | Non-controlling interest (continued): |
The following table summarizes the aggregate financial information for the Company’s non-wholly owned entities as at December 31, 2023 and December 31, 2022:
| December 31, | December 31, | ||||
| 2023 |
| 2022 | |||
Cash | $ | | $ | | ||
Accounts receivable, net | |
| | |||
Prepaid expenses and other | |
| | |||
Property, plant and equipment | |
| | |||
| | |||||
| | |||||
Account payable and accrued liabilities | |
| | |||
| | |||||
| | |||||
Loans payable | |
| | |||
Shareholder's equity (deficit) attributable to the shareholders of Greenbrook TMS | ( |
| ( | |||
Shareholder's equity (deficit) attributable to non-controlling interest | ( |
| ( | |||
Distributions paid to non-controlling interest | ( |
| ( | |||
Partnership buyout | | ( | ||||
Historical subsidiary investment by non-controlling interest | |
| | |||
The following table summarizes the aggregate financial information for the above-noted entities for the years ended December 31, 2023 and December 31, 2022:
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Revenue | $ | | $ | | ||
Net (loss) income attributable to the shareholders of Greenbrook TMS |
| ( |
| ( | ||
Net (loss) income attributable to non-controlling interest |
| ( |
| ( | ||
F-53
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
25. | Expenses by nature: |
The components of the Company’s other regional and center support costs include the following:
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Salaries and bonuses | $ | | $ | | ||
Marketing expenses |
| | | |||
Total | $ | | $ | | ||
The components of the Company’s corporate, general and administrative expenses include the following:
| December 31, |
| December 31, | |||
2023 | 2022 | |||||
Salaries and bonuses | $ | | $ | | ||
Marketing expenses |
| |
| | ||
Professional and legal fees |
| |
| | ||
Computer supplies and software |
| |
| | ||
Financing and transaction costs |
| |
| | ||
Travel, meals and entertainment |
| |
| | ||
Restructuring expense | | — | ||||
Insurance | | | ||||
Credit facility amendment fee (note 10(a)) | | — | ||||
Other |
| |
| | ||
Total | $ | | $ | | ||
On March 6, 2023, the Company announced that it is embarking on a comprehensive restructuring plan (the "Restructuring Plan") that aims to strengthen the Company by leveraging its scale to further reduce complexity, streamlining its operating model and driving operational efficiencies to achieve profitability.
As part of this Restructuring Plan, the Company is decreasing its operating footprint and headcount and operating expenses. The remaining Treatment Centers will continue clinical TMS offerings and a select and growing number of Treatment Centers will continue offering Spravato (R) (esketamine nasal spray) therapy.
F-54
GREENBROOK TMS INC.
Notes to Consolidated Financial Statements (continued)
(Expressed in U.S. dollars, unless otherwise stated)
Years ended December 31, 2023 and December 31, 2022
26.Subsequent events:
(a) | Additional Loans under Madryn Credit Facility |
On January 19, February 5, February 15, March 1, March 15 March 29 and April 15, 2024 the Company entered into amendments to the Madryn Credit Facility, whereby Madryn and its affiliated entities extended
In addition, on March 29, 2024, the Company entered into an amendment to the Madryn Credit Facility to extend the period during which the Company’s minimum liquidity covenant is reduced from $
(b) | February 2024 Public Offering |
On February 26, 2024, the Company completed a registered direct offering of
(c) | Nasdaq Delisting |
On February, 22, 2024, the Company received the final delisting notice from the Listing Qualifications Department of the Nasdaq due to the continued failure to satisfy either the $
F-55