UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___ to ___
Commission File Number
(Exact name of registrant as specified in its charter)
| ||
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification No.) |
|
|
|
| ||
(Address of principal executive offices) |
| (Zip Code) |
(
(Registrant’s telephone number, including area code)
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading symbol(s) |
| Name of each exchange on which registered |
|
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large, accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large, accelerated filer,” “accelerated filer” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated Filer ☐ |
|
|
| Accelerated Filer ☐ | ||
Smaller reporting company | ||||||
|
|
|
|
|
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes
Shares of Common Stock outstanding as of February 8, 2024:
iBio, Inc.
TABLE OF CONTENTS
3 | ||
|
| |
3 | ||
Management’s Discussion and Analysis of Financial Condition and Results of Operations | 44 | |
57 | ||
57 | ||
|
|
|
57 | ||
|
| |
57 | ||
57 | ||
61 | ||
62 | ||
|
| |
63 | ||
2
PART I - FINANCIAL INFORMATION
Item 1. Consolidated Financial Statements (Unaudited).
iBio, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
(In thousands, except share and per share amounts)
December 31, | June 30, | |||||
2023 | 2023 | |||||
(Unaudited) | (See Note 2) | |||||
Assets | ||||||
Current assets: | ||||||
Cash and cash equivalents | $ | | $ | | ||
Restricted cash | | | ||||
Subscription receivable | — | | ||||
Prepaid expenses and other current assets |
| |
| | ||
Current assets held for sale | | | ||||
Total Current Assets |
| |
| | ||
|
|
| ||||
Restricted cash | | | ||||
Promissory note receivable and accrued interest | | | ||||
Finance lease right-of-use assets, net of accumulated amortization |
| |
| | ||
Operating lease right-of-use asset | | | ||||
Fixed assets, net of accumulated depreciation |
| |
| | ||
Intangible assets, net of accumulated amortization | | | ||||
Security deposits | | | ||||
Total Assets | $ | | $ | | ||
|
|
|
| |||
Liabilities and Stockholders' Equity |
|
|
|
| ||
Current liabilities: |
|
|
|
| ||
Accounts payable | $ | | $ | | ||
Accrued expenses |
| |
| | ||
Finance lease obligations - current portion | | | ||||
Operating lease obligation - current portion | | | ||||
Equipment financing payable - current portion | | | ||||
Insurance premium financing payable | | — | ||||
Term note payable - net of deferred financing costs | | | ||||
Current liabilities related to assets held for sale |
| |
| | ||
Total Current Liabilities |
| |
| | ||
|
|
|
| |||
Finance lease obligations - net of current portion | | | ||||
Operating lease obligation - net of current portion | | | ||||
Equipment financing payable - net of current portion | | | ||||
Accrued expenses - noncurrent | — | | ||||
|
|
|
| |||
Total Liabilities |
| |
| | ||
|
|
|
| |||
Stockholders' Equity |
|
|
|
| ||
Series 2022 Convertible Preferred Stock - $ |
|
| ||||
Common stock - $ |
| |
| | ||
Additional paid-in capital |
| |
| | ||
Accumulated deficit | ( | ( | ||||
Total Stockholders’ Equity |
| |
| | ||
Total Liabilities and Stockholders' Equity | $ | | $ | | ||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
3
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited; in thousands, except per share amounts)
|
| Three Months Ended | Six Months Ended | |||||||||
| December 31, | December 31, | ||||||||||
|
| 2023 |
| 2022 | 2023 |
| 2022 | |||||
| ||||||||||||
Revenues | $ | — | $ | — | $ | | $ | — | ||||
|
|
|
|
|
|
| ||||||
Operating expenses: |
|
|
|
|
|
| ||||||
Research and development |
| |
| |
| |
| | ||||
General and administrative |
| |
| |
| |
| | ||||
Total operating expenses |
| |
| |
| |
| | ||||
|
|
|
|
|
|
|
|
| ||||
Operating loss |
| ( |
| ( |
| ( |
| ( | ||||
|
|
|
|
|
| |||||||
Other income (expense): |
|
|
|
|
|
| ||||||
Interest expense | ( | ( | ( | ( | ||||||||
Interest income |
| | | | | |||||||
Total other income |
| |
| |
| |
| | ||||
|
|
|
|
|
|
| ||||||
Net loss from continuing operations | ( | ( | ( | ( | ||||||||
Loss from discontinued operations | ( | ( | ( | ( | ||||||||
|
|
|
|
|
|
| ||||||
Net loss | $ | ( | $ | ( | $ | ( | $ | ( | ||||
Comprehensive loss: |
|
|
|
|
|
| ||||||
Consolidated net loss | $ | ( | $ | ( | $ | ( | $ | ( | ||||
Other comprehensive loss - unrealized gain on debt securities | — | | — | | ||||||||
|
|
|
|
|
|
|
|
| ||||
Comprehensive loss | $ | ( | $ | ( | $ | ( | $ | ( | ||||
|
|
|
|
|
|
|
|
| ||||
Loss per common share attributable to iBio, Inc. stockholders - basic and diluted - continuing operations | ( | ( | ( | ( | ||||||||
Loss per common share attributable to iBio, Inc. stockholders - basic and diluted - discontinued operations | ( | ( | ( | ( | ||||||||
Loss per common share attributable to iBio, Inc. stockholders - basic and diluted - total | ( | ( | ( | ( | ||||||||
|
|
|
|
|
|
|
|
| ||||
Weighted-average common shares outstanding - basic and diluted |
| |
| |
| |
| | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
4
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Stockholders’ Equity
(Unaudited; in thousands)
Three and Six Months Ended December 31, 2023
| ||||||||||||||||||||
| Additional | |||||||||||||||||||
| Preferred Stock | Common Stock | Paid-In | Accumulated | ||||||||||||||||
|
| Shares |
| Amount |
| Shares |
| Amount |
| Capital |
| Deficit |
| Total | ||||||
Balance as of July 1, 2023 | — | $ | — | | $ | | $ | | $ | ( | $ | | ||||||||
Capital raise | — | — | | | | — | | |||||||||||||
Costs to raise capital | — | — | | | ( | — | ( | |||||||||||||
Vesting of RSUs | — | — | | — | — | — | — | |||||||||||||
Share-based compensation | — | — | — | — | | — | | |||||||||||||
Net loss | — | — | — |
| — |
| — |
| ( |
| ( | |||||||||
Balance as of September 30, 2023 | — | $ | — | | $ | | $ | | $ | ( | $ | | ||||||||
Capital raise | — | — | | | | — |
| | ||||||||||||
Cost to raise capital | — | — | — | — | ( | — | ( | |||||||||||||
Payment for fractional shares after reverse stock split | — | — | ( | — | ( | — | ( | |||||||||||||
Vesting of RSUs | — | — | | — | — | — | — | |||||||||||||
Share-based compensation | — | — | — | — | | — |
| | ||||||||||||
Net loss | — | — | — | — | — | ( | ( | |||||||||||||
Balance as of December 31, 2023 | — | $ | — | | $ | | $ | | $ | ( | $ | | ||||||||
Three and Six Months Ended December 31, 2022
| Accumulated | ||||||||||||||||||||||
| Additional | Other | |||||||||||||||||||||
| Preferred Stock | Common Stock | Paid-In | Comprehensive | Accumulated | ||||||||||||||||||
|
| Shares |
| Amount |
| Shares |
| Amount |
| Capital |
| Loss |
| Deficit |
| Total | |||||||
Balance as of July 1, 2022 | | $ | — | | $ | | $ | | $ | ( | $ | ( | $ | | |||||||||
Capital raise | — | — | | — | | — | — | | |||||||||||||||
Conversion of preferred stock to common stock | ( | — | — | — | — | — | — | — | |||||||||||||||
Common stock issued - RubrYc transaction | — | — | | — | | — | — | | |||||||||||||||
Vesting of RSUs | — | — | * | — | — | — | — | — | |||||||||||||||
Share-based compensation |
| — |
| — | — |
|
| — |
|
| |
|
| — |
|
| — |
|
| | |||
Unrealized gain on available-for-sale debt securities | — | — | — | — | — | ( | — | ( | |||||||||||||||
Net loss | — | — | — |
| — |
| — |
| — |
| ( |
| ( | ||||||||||
Balance as of September 30, 2022 |
| — |
| $ | — | |
| $ | |
| $ | |
| $ | ( |
| $ | ( |
| $ | | ||
Capital raise | — | — | |
| |
| |
| — |
| — |
| | ||||||||||
Cost to raise capital | — | — | — | — | ( | — | — | ( | |||||||||||||||
Payment for fractional shares after reverse stock split | — | — | * |
| — |
| ( |
| — |
| — |
| ( | ||||||||||
Vesting of RSUs | — | — | * |
| — |
| — |
| — |
| — |
| - | ||||||||||
Share-based compensation | — | — | — | — | | — | — | | |||||||||||||||
Unrealized loss on debt securities | — | — | — | — | — | | — | | |||||||||||||||
Net loss | — | — | — |
| — |
| — |
| — |
| ( |
| ( | ||||||||||
Balance as of December 31, 2022 | — | $ | — | | $ | | $ | | $ | ( | $ | ( | $ | | |||||||||
* Represents amount less than 0.5 thousand. | |||||||||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
5
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(Unaudited; in thousands)
|
| Six Months Ended | ||||
| December 31, | |||||
|
| 2023 |
| 2022 | ||
| ||||||
Cash flows from operating activities: | ||||||
Consolidated net loss | $ | ( | $ | ( | ||
Adjustments to reconcile consolidated net loss to net cash used in operating activities: |
|
| ||||
Share-based compensation |
| |
| | ||
Amortization of intangible assets |
| |
| | ||
Amortization of finance lease right-of-use assets | | | ||||
Amortization of operating lease right-of-use assets | | | ||||
Depreciation of fixed assets |
| |
| | ||
Gain on sale of fixed assets | ( | | ||||
Accrued interest receivable on promissory note receivable | ( | ( | ||||
Amortization of premiums on debt securities | — | | ||||
Amortization of deferred financing costs | | | ||||
Inventory reserve | — | | ||||
Impairment of fixed assets | | | ||||
Accrued payment in kind on Term Loan | ( | |||||
Impairment of intangible assets | — | | ||||
Gain on disposition of finance lease ROU assets | — | ( | ||||
Changes in operating assets and liabilities: |
|
| ||||
Accounts receivable - trade |
| — |
| | ||
Inventory |
| — |
| ( | ||
Prepaid expenses and other current assets |
| |
| | ||
Prepaid expenses - noncurrent | — | | ||||
Security deposit |
| — |
| ( | ||
Accounts payable |
| |
| | ||
Accrued expenses |
| ( |
| | ||
Accrued expenses - noncurrent | ( | | ||||
Operating lease obligations |
| ( |
| | ||
Contract liabilities |
| — |
| ( | ||
Net cash used in operating activities |
| ( |
| ( | ||
|
|
| ||||
Cash flows from investing activities: |
|
| ||||
Redemption of debt securities | — | | ||||
Purchases of fixed assets |
| — | ( | |||
Sales proceeds for fixed assets | | | ||||
Payment for RubrYc asset acquisition | — | ( | ||||
|
|
|
|
| ||
Net cash provided by (used in) investing activities |
| |
| ( | ||
|
| |||||
Cash flows from financing activities: |
|
| ||||
Proceeds from sales of common stock | | | ||||
Cost to acquire capital |
| ( | | |||
Payments for fractional shares after reverse stock split | ( | ( | ||||
Subscription receivable | | | ||||
Proceeds from equipment financing loan | — | | ||||
Payment of equipment financing loan | ( | ( | ||||
Payment of term note payable | ( | ( | ||||
Costs to attain term note | — | ( | ||||
Payment of finance lease obligation |
| ( | ( | |||
Net cash provided by (used in) financing activities |
| |
| ( | ||
|
|
| ||||
Net decrease in cash, cash equivalents and restricted cash |
| ( |
| ( | ||
Cash, cash equivalents and restricted cash - beginning |
| |
| | ||
Cash, cash equivalents and restricted cash - end | $ | | $ | | ||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
6
iBio, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(Unaudited; in thousands)
| Six Months Ended | |||||
December 31, | ||||||
|
| 2023 |
| 2022 | ||
Schedule of non-cash activities: |
|
| ||||
Fixed assets included in accounts payable in prior period, paid in current period | $ | | $ | | ||
Increase in finance lease right-of-use assets for new leases | $ | | $ | | ||
Increase in finance lease obligation for new leases | $ | | $ | | ||
RubrYc asset acquisition by issuance of common stock | $ | | $ | | ||
Insurance premium financing | $ | | $ | | ||
Costs to raise capital paid directly from gross proceeds | $ | | $ | | ||
Costs to raise capital included in accrued expenses | $ | | $ | | ||
Unpaid fixed assets included in accounts payable | $ | | $ | | ||
Unrealized (gain) loss on available-for-sale debt securities | $ | | $ | ( | ||
Supplemental cash flow information: |
|
| ||||
Cash paid during the period for interest | $ | | $ | | ||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
7
iBio, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Nature of Business
iBio, Inc. (the "Company" or “iBio”) is a preclinical stage biotechnology company that leverages the power of Artificial Intelligence (“AI”) for the development of precision antibodies. The Company’s proprietary technology stack is designed to minimize downstream development risks by employing AI-guided epitope-steering and monoclonal antibody (“mAb’) optimization.
In September 2022, the Company made a strategic pivot by acquiring substantially all of the assets of RubrYc Therapeutics, Inc. ("RubrYc"). This acquisition commenced the Company’s transition to an AI-enabled biotech company and led to the divestiture of its Contract Development and Manufacturing Organization (“CDMO”) business. This strategic decision allowed the Company to focus resources on the development of AI-powered precision antibodies, positioning iBio at the forefront of this exciting field.
One of the key features of the Company’s technology stack is the patented epitope-steering AI-engine. This advanced technology allows the Company to target specific regions of proteins with precision enabling the creation of antibodies highly specific to therapeutically relevant regions within large target proteins, potentially improving their efficacy and safety profile. Another integral part of the Company’s technology stack is the machine learning (“ML”) based antibody-optimizing StableHu™ technology. When integrated with the Company's mammalian display technology, StableHu has demonstrated its ability to expedite the Lead Optimization process. This integration not only potentially reduces downstream risks but also streamlines the overall development process, making it faster, more efficient, and cost-effective. As a result, optimization can be achieved in less than four weeks.
The Company also developed the EngageTx™ platform, which provides an optimized next-generation CD3 T-cell engager antibody panel. This panel is characterized by a wide spectrum of potencies, Non-Human Primate (“NHP”) cross-reactivity, enhanced humanness of the antibodies, and a maintained tumor cell killing capacity, all while reducing cytokine release. These attributes are meticulously designed to fine-tune the efficacy, safety, and tolerability of the Company’s antibody products. By incorporating EngageTx into the Company’s own development initiatives, the Company’s internal pre-clinical pipeline reaps the benefits of the same cutting-edge technology extended to its potential partners.
The Company recently announced the expansion of its AI-powered technology stack with the launch of ShieldTx™, a patent-pending antibody masking technology designed to enable specific, highly targeted antibody delivery to diseased tissue without harming healthy tissue. By adding ShieldTx to the Company’s technology stack, iBio uniquely integrates antibody engineering and masking in one accelerated process to potentially overcome the challenges of complex targets, safety, and developability in next-generation antibody discovery and development.
iBio’s scientific team, composed of experienced AI/ML scientists and biopharmaceutical scientists, located side-by-side in its San Diego laboratory, possess the skills and capabilities to rapidly advance antibodies in-house from concept to in vivo proof-of-concept (“POC’). This multidisciplinary expertise allows the Company to quickly translate scientific discoveries into potential therapeutic applications.
Artificial Intelligence in Antibody Discovery and Development
The potential of AI in antibody discovery is immense and is being increasingly recognized in the biopharmaceutical industry. The mAbs market has seen impressive growth in recent years, with mAbs increasingly the top-selling drugs in the United States. This success has driven the industry to seek innovative methods for refining and improving their antibody pipelines. AI and deep learning, which have already revolutionized small molecule drug design, are now making significant strides in the development and optimization of antibodies.
The Company is leveraging its AI-powered technology stack to enhance the success rate of identifying antibodies for challenging target proteins, expedite the process of antibody optimization, improve developability, and engineer finely calibrated bi-specifics. By continually refining the Company’s AI algorithms, incorporating new data sources, and developing robust experimental validation processes, iBio is paving the way for groundbreaking advancements in antibody design and drug discovery.
Strategy
The Company is a pioneering biotechnology company at the intersection of AI and biologics, committed to reshaping the landscape of discovery. The Company’s core mission is to harness the potential of AI and machine learning to unveil elusive biologics that stand out and have evaded other scientists. Through the Company’s innovative platform, it champions a culture of innovation by identifying novel targets, forging strategic collaborations to enhance efficiency, diversify pipelines, with the goal of accelerating preclinical processes.
8
Additionally, the Company’s groundbreaking EngageTx™ technology enables the Company to target bi-specific molecules. With the ability to navigate sequence diversity and promote Human-Cyno cross reactivity while mitigating cytokine release, the Company’s goal is to enhance agility and bolster preclinical safety assessments.
The Company’s strategic approach to fulfilling its mission is outlined as follows:
| ● | Elevate Epitope Discovery: The Company believes it leads the field with its patented AI-engine uncovering "hard to develop" molecules. The Company’s unparalleled epitope engine stands out by allowing the ability to target select regions of a protein, potentially removing the lengthy trial and error out of mAb discovery. This capability is expected to improve probability of success while at the same time, reduces costs commonly caused by having an iterative process. The Company’s epitope engine is engineered to match its target, refined for stability and optimized for water solubility, allowing the Company to identify new drug candidates that have failed or have been abandoned due to their complexity. |
| ● | Capital Efficient Business Approach: The Company’s strategic business approach is structured around the following pillars of value creation: |
| o | Strategic Partnerships: The Company is leveraging its platform and pipeline by forming strategic partnerships. The Company’s aim is to become the preferred partner for major pharmaceutical and biotechnology companies seeking rapid and cost-effective integration of complex molecules into their portfolios, de-risking their early-stage pre-clinical work. Additionally, a rich array of fast follower molecules within the Company’s pre-clinical pipeline holds the potential to drive substantial partnerships, opening doors to innovative projects. By tapping into the Company’s platform, infrastructure, and expertise, partners have the potential to streamline timelines, reduce costs tied to biologic drug discovery applications and cell line process development, and expedite preclinical programs with efficiency. |
| o | Tech Licensing in Diverse Therapeutic Areas: In pursuit of adding value, the Company is exploring partnerships in diverse therapeutic domains such as CNS or vaccines. The Company’s intention is to license the AI tech stack, extending its benefits to the Company’s partners and amplifying its biological impact and insights. This strategic approach enables the Company to capitalize on the value of its meticulously curated data while empowering collaborations and innovations, while at the same time allowing the Company to focus on both the platform and its core therapeutic area, oncology. |
| o | Developing and Advancing the Company’s In-house Programs Cost Effectively: Clinical advancement is crucial for drug discovery. The Company is actively looking for opportunities to progress its internal pre-clinical programs, with a focal point on oncology, steadily reinforcing its pre-clinical pipeline. |
| ● | Unwavering Investment in Advancing the Platform: The Company maintains an unwavering commitment to invest in its platform, continually unlocking the potential of biology through AI and machine learning – the pinnacle of being on the forefront of machine learning advancing algorithms and models in order to improve its predictive power and reduce the time it takes to find a viable molecule. |
In essence, the Company is sculpting a future where cutting-edge AI-driven biotechnology propels the discovery of intricate biologics, fostering partnerships, accelerating innovation, and propelling the advancement of science.
AI Drug Discovery Platform
Overview
The Company’s platform comprises five key components, each playing a crucial role in the discovery and optimization of precision antibodies.
The first layer, epitope engineering, leverages the patented AI-engine to target specific regions of proteins, allowing the Company to engineer antibodies with high specificity and efficacy. The second layer involves the proprietary antibody library, which is built on clinically validated frameworks and offers a rich diversity of human antibodies. The third layer of the technology stack is the antibody optimizing StableHu AI technology, coupled with mammalian display technology. Next, the Company uses its EngageTx T-cell engager platform to create bispecific antibodies. Finally, antibodies are transformed into conditionally activated antibodies by ShieldTx, the Company’s antibody masking technology. Each layer of the tech stack is designed to work synergistically, enabling the Company to rapidly advance antibodies from concept to in vivo proof-of-concept (POC).
9
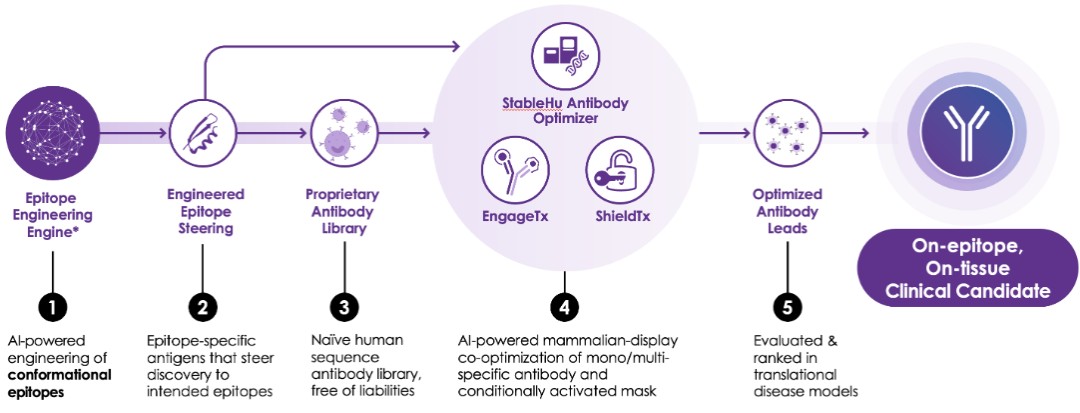
| ● | AI Epitope Steering Technology |
The Company’s epitope steering technology is designed to address these issues by guiding antibodies exclusively against the desired regions of the target protein. By focusing on these specific regions, the Company can overcome the limitations of traditional methods and significantly improve the efficiency and effectiveness of its antibody discovery process. The Company’s AI engine creates engineered epitopes, which are small embodiments of epitopes on the target protein. The engine is trained to match the epitope structure as closely as possible and refine the designs for greater stability and water solubility, which are critically important factors. The optimized engineered epitope is then used to identify antibodies from naïve or immunized libraries.
| ● | Naïve Human Antibody Library |
The fully human antibody library is built upon clinically validated, entirely human antibody frameworks. By leveraging public databases, the Company has extracted a diverse array of Complementarity-Determining Region (“CDR”) sequences. Subsequently, it has meticulously eliminated a range of sequence liabilities. Such careful curation process could potentially significantly reduce the development risk for antibodies identified from the Company’s library.
| ● | StableHuTM AI Antibody-Optimizing Technology |
The Company’s proprietary StableHu technology is instrumental in the optimization process. StableHu is an AI-powered tool designed to predict a library of antibodies with fully human CDR variants based on an input antibody. This input can range from an early, unoptimized molecule to an approved drug. The model has been trained utilizing a set of over 1 billion human antibodies, progressively masking known amino acids within CDRs until the algorithm could predict the correct human sequence.
While phage display libraries are often used in antibody optimization due to their vast diversity, they can increase developability risks such as low expression, instability, or aggregation of antibodies. Mammalian display libraries, on the other hand, offer significantly improved developability but reduced diversity due to the smaller library size they can handle. StableHu overcomes this limitation by utilizing a machine learning algorithm generating focused library diversity within the capacity of mammalian display.
Mammalian display is a technology that presents antibodies on the surface of mammalian cells, allowing for the direct screening and selection of antibodies in a mammalian cell environment. This approach is advantageous as antibodies that express well on the mammalian cells used in the display are more likely to express well in the production cell line. Moreover, single-cell sorting of antibody-displaying cells allows rapid selection of desired antibodies based on multiple dimensions, such as potency, selectivity, and cross-species selectivity.
10
When paired with mammalian display technology, StableHu enables antibody optimization with fewer iterative optimization steps, lower immunogenicity risk, and improved developability.
| ● | EngageTx CD3-Based T-Cell Engager Panel |
The Company has used antibodies from an epitope steering campaign as well as a first-generation T-cell engager as input and utilized its StableHu technology to identify a next-generation CD3 antibody panel. The sequence diversity generated by StableHu led to an antibody panel with a wide range of potencies, which allows the Company to pair the panel with a wide variety of tumor-targeting antibodies. Importantly, the Company was able to retain T-cell activation and tumor cell killing capacity with significantly reduced cytokine release. This reduction is believed to lower the risk of cytokine release syndrome. Additionally, the increased humanness of the predicted antibodies, thanks to the Company’s StableHu technology, has the ability to reduce the risk of immunogenicity.
Furthermore, the Company’s StableHu technology enabled it to engineer NHP cross-reactivity into EngageTx. This allows for advanced safety assessment in NHP ahead of clinical trials, providing another layer of safety assurance.
| ● | ShieldTx |
The Company has enhanced its proprietary technology with the introduction of ShieldTx, a patent-pending innovative antibody masking technique. ShieldTx leverages the Company's engineered epitope technology, which is utilized not only for the identification of antibodies against complex drug targets but also for concealing the antibodies' active sites. A significant hurdle in therapeutic antibody development is the expression of drug target on both healthy and diseased tissues, leading to adverse effects on non-targeted tissues. ShieldTx is designed to address this challenge by rendering antibodies inactive until they reach a specific environment unique to diseased tissues. Upon contact with this environment, the masking element is detached, activating the antibody. In the tumor microenvironment this is achieved by a highly expressed matrix metalloproteinase. This strategy aims to minimize or eliminate unintended effects on healthy tissues, thereby improving the safety profile and reducing the immunogenicity risks associated with bispecific antibodies.
Modalities
Epitope steering, a technology the Company is pioneering, has the potential to positively impact various areas of medicine. In the field of immuno-oncology, it can be used to develop antibodies targeting specific cancer antigens, potentially enhancing the efficacy of treatments like checkpoint inhibitors and CAR-T therapies.
The technology also holds promise in the realm of systemic secreted and cell-surface therapeutics. Epitope steering can be applied to the development of antibodies, circulating immune modulation factors, secreted enzymes, and transmembrane proteins. This could be particularly beneficial in treating diseases such as heart failure, infectious diseases, and rare genetic conditions. In the context of localized regenerative therapeutics, epitope steering could potentially be used to develop treatments that target specific damaged or diseased tissues. This approach could be particularly beneficial in the treatment of cardiovascular diseases. Intratumoral immuno-oncology is another area where epitope steering could make a significant impact. It could potentially be used to develop treatments that alter the tumor microenvironment to favor an immune response against tumors, potentially enhancing the efficacy of treatments that use immune-stimulatory proteins. The potential of epitope steering extends to cancer vaccine development as well. The ability to target specific epitopes could be beneficial in the development of vaccines, particularly those that aim to increase the number and antitumor activity of a patient's T cells. Finally, epitope steering could be used to develop treatments for a wide range of diseases, including those in the immune-oncology space, immunology, pain, and potentially in vaccine development. This is particularly relevant for complex and hard-to-drug protein structures.
Partnerships
As noted above, one of the three pillars of value creation that structures the Company’s strategic business approach are strategic partnerships. At the center of such pillar is the Company’s AI Discovery Platform. In June 2023, the Company entered into a research collaboration with the National Institute of Allergy and Infectious Diseases (“NIAID”), a component of the National Institutes of Health (“NIH”), to investigate the potential of the Company’s patented AI-driven epitope steering platform for the development of a vaccine for Lassa fever, a sometimes fatal viral disease endemic to parts of West Africa. During the first quarter FY 2024, the Company entered into a collaboration with a partner to license the use of the Company’s AI Discovery Platform to assist such partner with two targets of interest. During the second quarter FY 2024, the Company entered into a collaboration with a large pharmaceutical company to assist
11
such partner by using the Company’s patented AI-driven epitope steering platform to assist with one "hard to develop" molecule. The Company continues to seek out opportunities for future collaborations using the Company’s AI Discovery Platform.
Pipeline
The Company is currently in the process of building and advancing its pipeline. The focus of the Company’s pipeline is primarily on immuno-oncology, with one program also dedicated to the immunology space. By leveraging its technology stack, the pipeline is geared towards hard-to-drug targets and molecules offering differentiation. To mitigate target risk and capitalize on the learnings of competitors, the Company’s programs are primarily adopting a fast follower strategy. This approach allows the Company to focus on targets that have to some extent been validated and learn from the advancements of those ahead in the field.
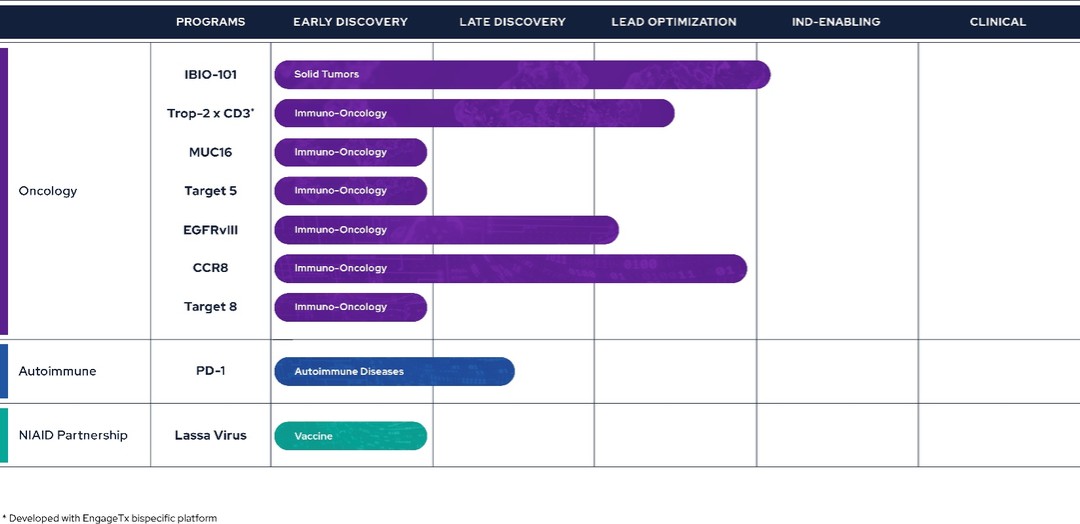
Therapeutics
Immuno-Oncology
IBIO-101
In August 2021, the Company signed a worldwide exclusive licensing agreement with RubrYc to develop and commercialize RTX-003 (now referred to as IBIO-101), an anti-CD25 mAb. In September 2022, the Company acquired exclusive ownership rights to IBIO-101. IBIO-101 is a second-generation anti-CD25 mAb that has demonstrated in preclinical models of disease the ability to bind and deplete immunosuppressive regulatory T (“Treg”) cells to inhibit the growth of solid tumors.
Targeting depletion of Treg cells to control tumors emerged as an area of interest in oncology over the past several years. Since Treg cells express interleukin-2 Rα (“IL-2Rα” or “CD25”), it was envisioned mAbs could be developed that bind CD25 and thereby trigger depletion by Natural Killer cells, resulting in stimulation of anti-tumor immunity.
Unfortunately, while first-generation mAbs successfully bound CD25+ cells, they also interfered with interleukin-2 [“IL-2”] signaling to T effector (“Teff”) cells to activate their cancer cell killing effects. The result was a failure of first-gen anti-CD25 mAbs as cancer immunotherapies, since their favorable anti-Treg effects were negated by their unfavorable impact on Teff cells.
In a humanized mouse disease model, IBIO-101, when used as a monotherapy, effectively demonstrated its mechanism of action by significantly enhancing the Treg/Teff ratio, resulting in the suppression of tumor growth. When paired with an anti-PD-1 checkpoint inhibitor in the same model, the combined treatment of IBIO-101 and anti-PD-1 exhibited superior tumor inhibition compared to either anti-PD-1 or IBIO-101 used independently.
12
The Company continues to advance its IL-2 sparing anti-CD25 antibody, IBIO-101, and intend moving the program from IND-enabling stage to an IND filing during the calendar year 2025, subject to funding availability.
TROP-2 x CD3 Bispecific
The Company has identified highly potent, fully human TROP-2 (Trophoblast Cell Surface Antigen 2) monoclonal antibodies, which have been formatted into bispecific TROP-2 x CD3 molecules using its T-cell engager antibody panel, EngageTx. TROP-2 is highly expressed in multiple solid tumors, including breast, lung, colorectal, and pancreatic cancers and is closely linked to metastasis and tumor growth. TROP-2 antibody drug conjugates have been developed to deliver toxic payloads to these cancer cells but could risk harming healthy cells and cause adverse effects. The Company’s bispecific approach has the potential to increase the therapeutic window, while promoting a robust and long-lasting anti-tumor response. Combining the bispecific TROP-2 approach with immunotherapies like checkpoint inhibitors can potentially lead to improved clinical outcomes.
Using EngageTx, the Company’s lead TROP-2 x CD3 bispecific antibody was engineered to potently kill tumor cells while limiting the release of cytokines, like Interferon Gamma (“IFNg’), Interleukin 2 (IL-2) and Tumor Necrosis Factor Alpha (“TNFa”), all of which have the potential to cause cytokine release syndrome. When compared to a bispecific molecule engineered with the Company’s TROP-2 binding arm and a first generation CD3 engager, SP34, its lead TROP-2 x CD3 bispecific antibody showed a markedly reduced cytokine release profile, potentially indicating a decreased risk for cytokine release syndrome.
When tested in a humanized mouse model of squamous cell carcinoma, the Company’s lead TROP-2 x CD3 bi-specific antibody demonstrated a significant 36 percent reduction in tumor size within just 14 days after tumor implantation, and after only a single dose.
MUC16
MUC16 is a well-known cancer target often overexpressed in several types of solid tumors, including ovarian, lung, and pancreatic cancers. Specifically, MUC16 is a large extracellular protein expressed on more than 80% of ovarian tumors. Tumor cells can evade immune attack by shedding or glycosylating MUC16, making it difficult for traditional antibody therapies to effectively target and destroy the cancer cells.
The Company’s patented epitope steering AI platform, its innovative approach to this challenge allows its new mAbs to bind to a specific region of MUC16 that is not shed or glycosylated, circumventing both tumor evasion mechanisms and potentially providing a powerful tool in the fight against cancer. During its immunization and screening campaign, the Company identified several hits that specifically bound to the non-shed region of MUC16 while no binding to the shed fragment of MUC16 was observed. During pre-clinical studies, the Company’s MUC16 molecule has demonstrated binding to MUC16 on OVCAR-3 ovarian cancer cells. After engineering the leading MUC16 molecule with a fully human framework, the MUC16 molecule retained potent binding to the engineered epitope and maintained binding to human OVCAR-3 ovarian cancer cells. The Company has utilized its EngageTx platform to engineer MUC16 x CD3 bispecific antibodies and has further optimized the molecules to be double-masked on the MUC16 and the CD3 binding arms of the antibody.
EGFRvIII
EGFRvIII is a specific variant of the EGFR protein, unique to tumor cells. Unlike the more common EGFR, EGFRvIII is not found in healthy cells, making it an attractive target for therapeutic interventions. This variant is most prominently associated with glioblastoma, a type of brain cancer and head and neck cancer but can also be present in certain cases of breast, lung, and ovarian cancers, among others. In the Company’s pursuit of innovative treatments, iBio is exploring antibody therapeutics that specifically target EGFRvIII, aiming to address these cancer types without affecting healthy cells.
Leveraging the Company’s patented AI-enabled epitope steering engine, it has specifically directed antibodies to target a unique epitope found exclusively on EGFRvIII, and not on the wildtype receptor, EGFR. Through this precision approach, iBio has designed tumor-specific molecules aimed at selectively targeting cancer cells while preserving healthy ones, potentially offering patients a more focused and safer therapeutic solution.
The Company’s hit molecules have demonstrated strong binding to the tumor-specific EGFRvIII protein without targeting the wildtype EGFR. Additionally, these molecules have effectively eliminated tumor cells, while sparing healthy ones, in in vitro cell killing tests. The Company’s lead anti-EGFRvIII antibody was specially engineered to enhance its ability to attack cancer cells and has proven effective in a mouse model for head and neck cancer. In preclinical studies, its anti-EGFRvIII antibody demonstrated a 43 percent reduction in tumor growth compared to untreated animals.
13
CCR8
GPCRs are one of the most successful therapeutic target classes, with approximately one-third of all approved drugs targeting these proteins. Compared to small molecule-based GPCR drugs, antibody-based GPCR therapeutics potentially offer several potential advantages, including superior selectivity, extended mechanisms of action, and longer half-life. However, GPCRs are intricate, multi-membrane spanning receptors, making clinically relevant regions difficult to identify and target.
The chemokine receptor CCR8 is a GPCR which is predominantly expressed on Tregs, which play a role in suppressing immune responses. In the context of cancer, Tregs can inhibit the body's natural immune response against tumor cells, promoting cancer progression. Anti-CCR8 antibodies are being explored as a therapeutic strategy to deplete these Tregs in the tumor environment. By targeting and reducing Tregs using anti-CCR8 antibodies, the hope is to enhance the body's immune response against cancer cells, offering a promising avenue for cancer treatment.
Aiming directly at CCR8 is believed to be a safer approach because it focuses on specific suppressive Treg cells in the tumor environment without affecting other immune cells and functions. It is important to make sure antibodies are fine-tuned to CCR8 and don't mistakenly target a similar receptor, CCR4. This is because CCR4 is found in many immune cells, and accidentally targeting it could potentially lead to unwanted side effects.
Using the Company’s unique AI-driven technology, it has successfully identified molecules targeting CCR8, addressing some of the hurdles often faced when creating therapies that target GPCR with antibodies. The Company’s specialized anti-CCR8 antibody has shown strong attachment to cells expressing CCR8 and effectively disrupted the CCR8 signaling process, resulting in the efficient elimination of Tregs derived from primary human immune cells. Notably, the Company’s CCR8-focused molecule did not attach to cells overproducing CCR4, highlighting its precision in targeting only CCR8.
The Company’s CCR8 antibody has proven effective in a mouse model for colon cancer. Preclinical studies show its anti-CCR8 molecule inhibited tumor growth and achieved a 22 percent reduction in tumor size compared to its pre-treatment dimensions. The Company has specifically engineered the anti-CCR8 molecule as a high Antibody-Dependent Cellular Cytotoxicity (ADCC) antibody to enhance its ability to attack cancer cells.
Autoimmune
PD-1 Agonist
Programmed cell death protein 1 (“PD-1”) is a pivotal player in the immune system, acting as a type of "off switch" that helps keep the cells from attacking other cells in the body. By agonizing or enhancing the signaling of PD-1, it is possible to temper the immune response, making it particularly valuable in the treatment of autoimmune diseases. In conditions where the immune system mistakenly wages war on the body's own cells, such as in autoimmune diabetes or lupus, therapies that target PD-1 can potentially reduce the severity of these autoimmune reactions. This approach offers a promising avenue for providing relief to patients suffering from these debilitating conditions. The figures below depict the mechanism of action of antagonistic and agonistic PD-1 antibodies.
iBio purchased the global rights to a partnership-ready PD-1 agonistic mAb intended to treat serious autoimmune disorders. While the goal in immuno-oncology is to remove immune tolerance towards cancer cells, in autoimmune diseases the opposite is the case, because autoimmune diseases can result from deficits in peripheral and/or central tolerance mechanisms which presents an opportunity for therapeutic intervention. Specifically, agonism or stimulation of inhibitory receptors like PD-1 or CTLA4, which mediate peripheral tolerance is a promising approach to treat autoimmune diseases. Unlike PD-1 antagonists used in immuno-oncology, PD-1 agonists are difficult to find. RubrYc used its AI Discovery Platform to discover PD-1. PD-1 is currently in the late-discovery stage, having undergone extensive screening and in vitro characterization, and the Company anticipates it will be advanced into in vivo models as IBIO-102, in the near future.
In preclinical studies, the Company’s PD-1 agonists have been evaluated using a primary T-cell assay. Its top-performing molecules showed a significant decrease in the proinflammatory cytokine IL-2 and reduced expression of the T-cell activation marker CD96. Both of these outcomes are indicative of the desired dampening of T-cell activation.
14
2. Basis of Presentation
Interim Consolidated Financial Statements
The accompanying unaudited condensed consolidated financial statements have been prepared from the books and records of the Company and include all normal and recurring adjustments which, in the opinion of management, are necessary for a fair presentation in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) for interim consolidated financial information and Rule 8-03 of Regulation S-X promulgated by the U.S. Securities and Exchange Commission (the “SEC”). Accordingly, these interim financial statements do not include all of the information and footnotes required for complete annual consolidated financial statements. Interim results are not necessarily indicative of the results that may be expected for the full year. Interim unaudited condensed consolidated financial statements should be read in conjunction with the audited financial statements and the notes thereto included in the Company’s Annual Report on Form 10-K for the prior year ended June 30, 2023, filed with the SEC on September 27, 2023 (the “Annual Report”), from which the accompanying condensed consolidated balance sheet dated June 30, 2023 was derived.
Principles of Consolidation
The condensed consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated as part of the consolidation.
Going Concern
The history of significant losses, the negative cash flow from operations, the limited cash resources on hand and the dependence by the Company on obtaining additional financing to fund its operations after the current cash resources are exhausted raise substantial doubt about the Company's ability to continue as a going concern. Management’s current financing and business plans have not mitigated such substantial doubt about the Company’s ability to continue as a going concern for at least 12 months from the date of filing this Quarterly Report on Form 10-Q for the quarterly period ended December 31, 2023. In an effort to mitigate the substantial doubt about continuing as a going concern and increase cash reserves, the Company has raised funds from time to time through equity offerings or other financing alternatives, reduced its work force by approximately
In July 2022, the Company initiated the selling of the CDMO assets and Facility, and since then has sold a substantial portion of the CDMO assets. (See Note 3 – Discontinued Operations for more information.)
Furthermore, on September 15, 2023, iBio CDMO LLC, or iBio CDMO, the Company’s subsidiary, entered into a purchase and sale agreement, dated as of September 15, 2023 (the “Purchase and Sale Agreement”), with Majestic Realty Co., a California corporation, (“Majestic Realty”), which sale if consummated would have allowed the Company to pay all outstanding amounts under the secured term loan with Woodforest National Bank (“Woodforest”) originally issued to iBio CDMO on November 1, 2021, in the amount of $
During the first quarter ended on September 30, 2023, the Company completed at-the-market offerings and sold
On December 7, 2023, the Company closed a public offering (the “2023 Offering”) pursuant to which it sold in the 2023 Offering, (i)
15
of
The Company’s cash, cash equivalents and restricted cash of approximately $
The accompanying consolidated financial statements do not include any adjustments related to the recoverability and classification of assets or the amounts and classification of liabilities that may result from the substantial doubt about the Company’s ability to continue as a going concern.
Reverse Stock Split
On September 22, 2022, the Company's Board of Directors (the “Board”) approved the implementation of a reverse stock split (the “Reverse Split”) at a ratio of one-for-twenty-five (:25) shares of the Company's Common Stock. The Reverse Split was effective as of October 7, 2022. All share and per share amounts of the Common Stock presented have been retroactively adjusted to reflect the Reverse Split. See Note 16 – Stockholders’ Equity for more information.
On November 27, 2023, the Company’s Board approved the implementation of a reverse stock split (the “2023 Reverse Split”) at a ratio of one-for-twenty (:20) shares of the Company's Common Stock. The 2023 Reverse Split was effective as of November 29, 2023. All share and per share amounts of the Common Stock presented have been retroactively adjusted to reflect the 2023 Reverse Split. See Note 16 – Stockholders’ Equity for more information.
3. Discontinued Operations
On November 3, 2022, the Company announced it was seeking to divest its subsidiary, iBio CDMO, in order to complete its transformation into an antibody drug discovery and development company. In conjunction with the divestment, the Company commenced a workforce reduction of approximately
Through the process of seeking to divest its contract development and manufacturing organization, on February 10, 2023, the Company, entered into an Auction Sale Agreement (the “Auction Sale Agreement”) with Holland Industrial Group, together with Federal Equipment Company and Capital Recovery Group LLC (collectively, the “Auctioneers”) for the sale at public auction of equipment and other tangible personal property (the “Equipment”) located at the Facility. The Auctioneer guaranteed an amount of gross proceeds from the sale of the Equipment of $
Additionally, the Company entered into a Purchase and Sale Agreement to sell the Facility to Majestic Realty for a purchase price of $
16
agreements relating to the operation or maintenance of the Land, Improvements or Personal Property which extend beyond the closing date (the “Contracts”); and (v) all iBio CDMO’s rights in intangible assets of any nature relating to any or all of the Land, the Improvements and the Personal Property (the “Intangibles”; and together with the Ground Lease, Improvements and Personal Property, collectively, the “Property”). On November 7, 2023, the Company received written notice from Majestic Realty of its election to terminate the Purchase and Sale Agreement, dated as of September 15, 2023, between Majestic Realty and iBio CDMO LLC, pursuant to which iBio CDMO had agreed to sell to Majestic Realty the Property. The Property continues to be listed for sale.
The Company incurred pre-tax charges of approximately $
The Company recorded an additional $
The results of iBio CDMO's operations are reported as discontinued operations for three and six months ended December 31, 2023 and for the three and six months ended December 31, 2022. In addition, those assets and liabilities associated with the discontinued operations of the CDMO that the Company intends to sell have been classified as “held for sale” on the condensed consolidated balance sheets at December 31, 2023 and as of June 30, 2023. The Company has chosen not to segregate the cash flows of iBio CDMO in the condensed consolidated statements of cash flows. Supplemental disclosures related to discontinued operations for the condensed consolidated statements of cash flows have been provided below. Unless noted otherwise, discussion in the Notes to the Condensed Consolidated Financial Statements refers to the Company's continuing operations.
The following tables present a reconciliation of the major financial lines constituting the results of operations for discontinued operations to the loss from discontinued operations presented separately in the condensed consolidated statements of operations and comprehensive loss (in thousands):
Three Months Ended | Three Months Ended | |||||
December 31, | December 31, | |||||
2023 | 2022 | |||||
Revenues | | $ | — | $ | | |
Cost of goods sold | — | | ||||
Gross profit | — | | ||||
Operating expenses: | ||||||
Research and development | — | | ||||
General and administrative | | | ||||
Fixed asset impairments | | | ||||
Inventory reserve | — | | ||||
Total operating expenses | | | ||||
Other expenses: | ||||||
Interest expense - term note payable | ( | ( | ||||
Total other expenses | | ( | ( | |||
Loss from discontinued operations | | $ | ( | $ | ( | |
17
Six Months Ended | Six Months Ended | |||||
December 31, | December 31, | |||||
2023 | 2022 | |||||
Revenues | | $ | — | $ | | |
Cost of goods sold | — | | ||||
Gross profit | — | | ||||
Operating expenses: | ||||||
Research and development | — | | ||||
General and administrative | | | ||||
Fixed asset impairments | | | ||||
Gain on sale of fixed assets | ( | — | ||||
Inventory reserve | — | | ||||
Total operating expenses | | | ||||
Other income (expenses): | ||||||
Interest expense - term note payable | ( | ( | ||||
Other | — | ( | ||||
Total other expenses | | ( | ( | |||
Loss from discontinued operations | | $ | ( | $ | ( | |
The following table presents net carrying values related to the major classes of assets that were classified as held for sale at December 31, 2023 and June 30, 2023 (in thousands):
December 31, | June 30, | |||||
2023 | 2023 | |||||
Current assets: | ||||||
Operating lease right-of-use assets | $ | | $ | | ||
Property and equipment, net | | | ||||
Total current assets | $ | | $ | | ||
Current liabilities: | ||||||
Operating lease obligation | $ | | $ | | ||
Total current liabilities | $ | | $ | | ||
The following table presents the supplemental disclosures related to discontinued operations for the condensed consolidated statements of cash flows (in thousands):
Six Months Ended | Six Months Ended | |||||
December 31, | December 31, | |||||
2023 | 2022 | |||||
Depreciation expense | | $ | — | $ | | |
Amortization of finance lease right-of-use assets | | | ||||
Purchase of fixed assets | — | | ||||
Fixed asset impairments | | | ||||
Inventory reserve | — | | ||||
Investing non-cash transactions: | ||||||
Fixed assets included in accounts payable in prior period, paid in current period | — | | ||||
Supplemental cash flow information: | ||||||
Cash paid during the period for interest | | | ||||
18
4. Summary of Significant Accounting Policies
The Company’s significant accounting policies are described in Note 4 of the Notes to Consolidated Financial Statements in the Annual Report on Form 10-K for the year ended June 30, 2023.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect reported amounts of assets and liabilities, disclosures of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. These estimates include liquidity assertions, the valuation of intellectual property and fixed assets held for sale, the incremental borrowing rate utilized in the finance and operating lease calculations, legal and contractual contingencies and share-based compensation. Although management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
Accounts Receivable
Accounts receivable are reported at their outstanding unpaid principal balances net of allowances for uncollectible accounts. The Company provides for allowances for uncollectible receivables based on its estimate of uncollectible amounts considering age, collection history, and other factors considered appropriate. Management’s policy is to write off accounts receivable against the allowance for doubtful accounts when a balance is determined to be uncollectible. At December 31, 2023 and June 30, 2023, the Company determined that an allowance for doubtful accounts was not needed. The Company had accounts receivable of $
Revenue Recognition
The Company accounts for its revenue recognition under Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers. Under this standard, the Company recognizes revenue when a customer obtains control of promised services or goods in an amount that reflects the consideration to which the Company expects to receive in exchange for those goods or services. In addition, the standard requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from customer contracts.
The Company applies the following steps when recognizing revenue from contracts with customers: (i) identify the contract, (ii) identify the performance obligations, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations and (v) recognize revenue when a performance obligation is satisfied. The Company only applies the five-step model to contracts when it is probable that the Company will collect the consideration the Company is entitled to in exchange for the goods or services the Company transfers to the customers. The Company analyzes its agreements to determine whether the elements can be separated and accounted for individually or as a single unit of accounting. Allocation of revenue to individual elements that qualify for separate accounting is based on the separate selling prices determined for each component, and total contract consideration is then allocated pro rata across the components of the arrangement. If separate selling prices are not available, the Company will use its best estimate of such selling prices, consistent with the overall pricing strategy and after consideration of relevant market factors.
If a loss on a contract is anticipated, such loss is recognized in its entirety when the loss becomes evident. When the current estimates of the amount of consideration that is expected to be received in exchange for transferring promised goods or services to the customer indicates a loss will be incurred, a provision for the entire loss on the contract is made. At December 31, 2023 and June 30, 2023, the Company had
The Company generates (or may generate in the future) contract revenue under the following types of contracts:
Fixed-Fee
Under a fixed-fee contract, the Company charges a fixed agreed upon amount for a deliverable. Fixed-fee contracts have fixed deliverables upon completion of the project. Typically, the Company recognizes revenue for fixed-fee contracts after projects are completed, delivery is made and title transfers to the customer, and collection is reasonably assured.
Revenue can be recognized either 1) over time or 2) at a point in time. Revenue reported in discontinued operation was recognized at a point in time for all periods presented.
Collaborations/Partnerships
The Company may enter into research and discovery collaborations with third parties that involve a joint operating activity, typically a research and/or development effort, where both parties are active participants in the activity and are exposed to the significant risks and rewards of the activity. The Company’s rights and obligations under its collaboration agreements vary and typically include milestone
19
payments, contingent upon the occurrence of certain future events linked to the success of the asset in development, as well as expense reimbursements from or payments to the collaboration partner.
The Company considers the nature and contractual terms of agreements and assesses whether an agreement involves a joint operating activity pursuant to which the Company is an active participant and is exposed to significant risks and rewards dependent on the commercial success of the activity as described under ASC 808, Collaborative Arrangements (“ASC 808”). For arrangements determined to be within the scope of ASC 808 where a collaborative partner is not a customer for certain research and development activities, the Company accounts for payments received for the reimbursement of research and development costs as a contra-expense in the period such expenses are incurred. If payments from the collaborative partner to the Company represent consideration from a customer in exchange for distinct goods and services provided, then the Company accounts for those payments within the scope of ASC 606, Revenue from Contracts with Customers (“ASC 606”).
Collaborative revenues generated typically include payment to the Company related to one or more of the following: non-refundable upfront license fees, development and commercial milestones, and partial or complete reimbursement of research and development costs.
For the six months ended December 31, 2023, revenue in the amount of $
Contract Assets
A contract asset is an entity’s right to payment for goods and services already transferred to a customer if that right to payment is conditional on something other than the passage of time. Generally, an entity will recognize a contract asset when it has fulfilled a contract obligation but must perform other obligations before being entitled to payment.
Contract assets consist primarily of the cost of project contract work performed by third parties for which the Company expects to recognize any related revenue at a later date, upon satisfaction of the contract obligations. At December 31, 2023 and June 30, 2023, contract assets were $
Contract Liabilities
A contract liability is an entity’s obligation to transfer goods or services to a customer at the earlier of (1) when the customer prepays consideration or (2) the time that the customer’s consideration is due for goods and services the entity will yet provide. Generally, an entity will recognize a contract liability when it receives a prepayment.
Contract liabilities consist primarily of consideration received, usually in the form of payment, on project work to be performed whereby the Company expects to recognize any related revenue at a later date, upon satisfaction of the contract obligations. At December 31, 2023, June 30, 2023 and June 30, 2022 contract liabilities were $
Leases
The Company accounts for leases under the guidance of ASC 842, Leases ("ASC 842"). The standard established a right-of-use (“ROU”) model requiring a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months and classified as either an operating or finance lease. The adoption of ASC 842 had a significant effect on the Company’s balance sheet, resulting in an increase in noncurrent assets and both current and noncurrent liabilities.
In accordance with ASC 842, at the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present and the classification of the lease including whether the contract involves the use of a distinct identified asset, whether the Company obtains the right to substantially all the economic benefit from the use of the asset, and whether the Company has the right to direct the use of the asset. Leases with a term greater than one year are recognized on the balance sheet as ROU assets, lease liabilities and, if applicable, long-term lease liabilities. The Company has elected not to recognize on the balance sheet leases with terms of one year or less under practical expedient in paragraph ASC 842-20-25-2. For contracts with lease and non-lease components, .
20
The lease liabilities and the corresponding ROU assets are recorded based on the present value of lease payments over the expected remaining lease term. The implicit rate within the Company’s existing finance (capital) lease was determinable and, therefore, used at the adoption date of ASC 842 to determine the present value of lease payments under the finance lease. The implicit rate within the Company’s operating lease was not determinable and, therefore, the Company used the incremental borrowing rate at the lease commencement date to determine the present value of lease payments. The determination of the Company’s incremental borrowing rate requires judgment. The Company will determine the incremental borrowing rate for each new lease using its estimated borrowing rate.
An option to extend the lease is considered in connection with determining the ROU asset and lease liability when it is reasonably certain the Company will exercise that option. An option to terminate is considered unless it is reasonably certain the Company will not exercise the option.
Cash, Cash Equivalents and Restricted Cash
The Company considers all highly-liquid instruments purchased with an original maturity of three months or less to be cash equivalents. Cash equivalents at December 31, 2023 and June 30, 2023 consisted of money market accounts. Restricted cash includes $
The following table summarizes the components of total cash, cash equivalents and restricted cash in the condensed consolidated statements of cash flows (in thousands):
December 31, | June 30, | |||||
2023 | 2023 | |||||
Cash and equivalents | | $ | | $ | | |
Collateral held for letter of credit - term note payable | | | ||||
Collateral held for letter of credit - San Diego lease | | | ||||
Collateral held for Company purchasing card | | | | |||
Total cash, cash equivalents and restricted cash | | $ | | $ | | |
The collateral held for the letters of credit for the San Diego lease and the Company purchasing card are classified as long-term on the condensed consolidated balance sheets at December 31, 2023 and June 30, 2023.
Investments in Debt Securities
Debt investments were classified as available-for-sale. Changes in fair value were recorded in other comprehensive income (loss). Fair value was calculated based on publicly available market information. Discounts and/or premiums paid when the debt securities were acquired are amortized to interest income over the terms of the debt securities. The Company held
Inventory
Inventory is stated at the lower of cost or net realizable value on the first-in, first-out basis. The Company held
Research and Development
The Company accounts for research and development costs in accordance with the Financial Accounting Standards Board (“FASB”) ASC 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and development costs are expensed when the contracted work has been performed or as milestone results have been achieved. Research and development expense was reported in continuing operations for the three and six months ended December 31. 2023.
21
Right-of-Use Assets
Assets held under the terms of finance (capital) leases are amortized on a straight-line basis over the terms of the leases or the economic lives of the assets. Obligations for future lease payments under finance (capital) leases are shown within liabilities and are analyzed between amounts falling due within and after one year. See Note 9 – Finance Lease ROU Assets and Note 14 – Finance Lease Obligations for additional information.
Fixed Assets
Fixed assets are stated at cost net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets ranging from to
The Company monitors fixed assets for impairment indicators throughout the year. When necessary, charges for impairments of long-lived assets are recorded for the amount by which the fair value is less than the carrying value of these assets. Changes in the Company’s business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
See Note 11 – Fixed Assets for additional information.
Intangible Assets
Identifiable intangible assets are comprised of definite life intangible assets and indefinite life intangible assets.
The Company accounts for definite life intangible assets at either their historical cost or allocated purchase price at asset acquisition and records amortization utilizing the straight-line method based upon their estimated useful lives. Intellectual property is amortized over . The Company reviews the carrying value of its definite life intangible assets for impairment whenever events or changes in business circumstances indicate the carrying amount of such assets may not be fully recoverable. The carrying value is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. An impairment loss is measured as the amount by which the carrying amount exceeds it fair value.
For indefinite life intangible assets, the Company performs an impairment test annually and whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable. The Company determines the fair value of the asset annually or when triggering events are present, based on discounted cash flows and records an impairment loss if book value exceeds fair value.
Evaluating for impairment requires judgment, including the estimation of future cash flows, future growth rates and profitability and the expected life over which cash flows will occur. Changes in the Company’s business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
See Note 12 – Intangible Assets for additional information.
Share-based Compensation
The Company recognizes the cost of all share-based payment transactions at fair value. Compensation cost, measured by the fair value of the equity instruments issued, adjusted for estimated forfeitures, is recognized in the financial statements as the respective awards are earned over the performance or service period. The Company uses historical data to estimate forfeiture rates.
The impact that share-based payment awards will have on the Company’s results of operations is a function of the number of shares awarded, the trading price of the Company’s stock at the date of grant or modification, the vesting schedule and forfeitures. Furthermore, the application of the Black-Scholes option pricing model employs weighted-average assumptions for expected volatility of the Company’s stock, expected term until exercise of the options, the risk-free interest rate, and dividends, if any, to determine fair value.
Expected volatility is based on historical volatility of the Common Stock; the expected term until exercise represents the weighted-average period of time that options granted are expected to be outstanding giving consideration to vesting schedules and the Company’s historical exercise patterns; and the risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods corresponding with the expected life of the option. The Company has not paid any dividends since its inception and does not anticipate paying any dividends for the foreseeable future, so the dividend yield is assumed to be
22
estimates forfeitures at each reporting period, rather than electing to record the impact of such forfeitures as they occur. See Note 18 – Share-Based Compensation for additional information.
Concentrations of Credit Risk
Cash
The Company maintains principally all cash balances in two financial institutions which, at times, may exceed the insured amounts. The exposure to the Company is solely dependent upon daily balances and the strength of the financial institutions. The Company has not incurred any losses on these accounts. At December 31, 2023 and June 30, 2023, amounts in excess of insured limits were approximately $
Revenue
During the three months ended December 31, 2023, the Company reported no revenue from continuing operations and discontinued operations. During the three months ended December 31, 2022, the Company reported
During the six months ended December 31, 2023, the Company reported license revenue from one research collaborator in continuing operations and no revenue in discontinued operations. During the six months ended December 31, 2022, the Company reported
Recently Issued Accounting Pronouncements
In June 2016, the FASB issued Accounting Standards Update (“ASU”) 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires an entity to assess impairment of its financial instruments based on its estimate of expected credit losses. Since the issuance of ASU 2016-13, the FASB released several amendments to improve and clarify the implementation guidance. In November 2019, the FASB issued ASU 2019-10, Financial Instruments - Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842): Effective Dates, which amended the effective date of the various topics. As the Company is a smaller reporting company, the provisions of ASU 2016-13 and the related amendments are effective for Fiscal years, and interim periods within those Fiscal years, beginning after December 15, 2022 (quarter ending September 30, 2023, for the Company). Entities are required to apply these changes through a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is effective. The adoption of ASU 2016-13 did not impact the Company’s condensed consolidated financial statements.
Management does not believe that any other recently issued, but not yet effective, accounting standard if currently adopted would have a material effect on the accompanying condensed consolidated financial statements. Most of the newer standards issued represent technical corrections to the accounting literature or application to specific industries which have no effect on the Company’s condensed consolidated financial statements.
5. Financial Instruments and Fair Value Measurement
The carrying values of cash and cash equivalents, restricted cash, accounts receivable, accounts payable and term note payable in the Company’s condensed consolidated balance sheets approximated their fair values as of December 31, 2023 and June 30, 2023 due to their short-term nature. The carrying value of the promissory note receivable, the term note payable and finance lease obligation approximated to fair value as of December 31, 2023 and June 30, 2023 as the interest rates related to the financial instruments approximated market.
The Company accounts for its investments in debt securities at fair value. The following provides a description of the three levels of inputs that may be used to measure fair value under the standard, the types of investments that fall under each category, and the valuation methodologies used to measure these investments at fair value:
| • | Level 1 – Inputs are based upon unadjusted quoted prices for identical instruments in active markets. |
| • | Level 2 – Inputs to the valuation include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in inactive markets, inputs other than quoted prices that are observable for the asset or liability, and inputs that are derived principally from or corroborated by observable market data by correlation or other means. If the asset or liability has a specified (contractual) term, the Level 2 input must be observable for substantially the full term of the asset or liability. All debt securities were valued using Level 2 inputs. |
| • | Level 3 – Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
23
The Company’s fixed assets and amortizable intangible assets are measured at fair value on a nonrecurring basis; that is, these assets are not measured at fair value on an ongoing basis, but are subject to fair value adjustments in certain circumstances, such as when there is evidence of impairment.
The Company initially marketed the CDMO business and during the second quarter of Fiscal 2023, changed its strategy to selling the stand-alone CDMO assets, including the Facility and Equipment. These assets were assessed for impairment and the analysis resulted in the expected future cash flows from the sale of the Facility and Equipment falling below its carrying value. The Company utilized a market approach, using independent third-party appraisals, including comparable assets, in addition to bids received from prospective buyers, to estimate the fair value of the Facility and Equipment. As a result, the carrying value of the Facility and Equipment was reduced to their estimated fair values of $
During the second quarter of Fiscal 2024, the Company made the decision to auction the Facility. The Company utilized a market approach, using an independent third-party appraisal, including comparable assets, to estimate the fair value of the Facility. An additional $
The following table shows the fair value of the Company's fixed assets included in Current Assets Held For Sale measured at fair value on a non-recurring basis as of December 31, 2023 (amounts in thousands):
December 31, 2023 | |||||||||||||||
Fair Value Hierarchy | |||||||||||||||
Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total Fair Value | Total Impairments | |||||||||||
Building in Bryan, Texas | $ | — | $ | — | $ | | $ | | $ | | |||||
During the second quarter of Fiscal 2023, the Company re-evaluated its business strategy and reviewed its product portfolio. After such review, the Company identified intellectual property, patent and licenses that would no longer be utilized and therefore were fully impaired (Level 3). See Note 12 – Intangible Assets for additional information.
6. Significant Transactions
Affiliates of Eastern Capital Limited
On November 1, 2021, the Company and its subsidiary, iBio CDMO LLC (“iBio CDMO”, and collectively with the Company, the “Purchaser”) entered into a series of agreements (the “Transaction”) with College Station Investors LLC (“College Station”), and Bryan Capital Investors LLC (“Bryan Capital” and, collectively with College Station, “Seller”), each affiliates of Eastern Capital Limited (“Eastern,” a former significant stockholder of the Company) described in more detail below whereby in exchange for a certain cash payment and a warrant the Company:
| (i) | acquired both the Facility where iBio CDMO at that time and currently conducts business and also the rights as the tenant in the Facility’s ground lease; |
| (ii) | acquired all of the equity owned by one of the affiliates of Eastern in the Company and iBio CDMO; and |
24
| (iii) | otherwise terminated all agreements between the Company and the affiliates of Eastern. |
iBio CDMO had held a sublease for the Facility through 2050, subject to extension until 2060 (the “Sublease”) until the purchase of the Facility by the Purchaser as described below.
The Purchase and Sale Agreement
On November 1, 2021, the Purchaser entered into a Purchase and Sale Agreement (the “PSA”) with the Seller pursuant to which: (i) the Seller sold to the Purchaser all of its rights, title and interest as the tenant in the Ground Lease Agreement (the “Ground Lease Agreement”) that it entered into with Texas A&M (the “Landlord’’) related to the land at which the Facility is located together with all improvements pertaining thereto (the “Ground Lease Property”), which previously had been the subject of the Sublease; (ii) the Seller sold to Purchaser all of its rights, title and interest to any tangible personal property owned by Seller and located on the Ground Lease Property including the Facility; (iii) the Seller sold to Purchaser all of its rights, title and interest to all licensed, permits and authorization for use of the Property; and (iv) College Station and iBio CDMO terminated the Sublease. The total purchase price for the Ground Lease Property, the termination of the Sublease and other agreements among the parties, and the equity described below was $
As discussed above, iBio CDMO is being accounted for as a discontinued operation. As such, the assets acquired and/or leased are now classified as assets held for sale on the December 31, 2023 and June 30, 2023 condensed consolidated balance sheets.
The Equity Purchase Agreement
The Company also entered into an Equity Purchase Agreement with Bryan Capital on November 1, 2021 (the “Equity Purchase Agreement”) pursuant to which the Company acquired for $
The Credit Agreement
In connection with the PSA, iBio CDMO entered into a Credit Agreement, dated November 1, 2021, with Woodforest pursuant to which Woodforest provided iBio CDMO a $
The Warrant
As part of the consideration for the purchase and sale of the rights set forth above, the Company issued to Bryan Capital a Warrant to purchase
RubrYc
On August 23, 2021, the Company entered into a series of agreements with RubrYc Therapeutics, Inc. (“RubrYc”) described in more detail below:
Collaboration and License Agreement
The Company entered into a collaboration and licensing agreement (the “RTX-003 License Agreement”) with RubrYc to further develop RubrYc’s immune-oncology antibodies in its RTX-003 (now referred to as IBIO-101) campaign. Under the terms of the agreement, the Company is solely responsible for worldwide research and development activities for development of the RTX-003 antibodies for use in pharmaceutical products in all fields. RubrYc was also entitled to receive royalties in the mid-single digits on net sales of RTX-003
25
antibodies, subject to adjustment under certain circumstances. The RTX-003 License Agreement was terminated when the Company acquired substantially all of the assets of RubrYc in September 2022.
Collaboration, Option and License Agreement
The Company entered into an agreement with RubrYc (the “Collaboration, Option and License Agreement”) to collaborate for up to
Stock Purchase Agreement
In connection with the entry into the Collaboration, Option and License Agreement and RTX-003 License Agreement, the Company also entered into a Stock Purchase Agreement (“Stock Purchase Agreement”) with RubrYc whereby the Company purchased a total of
The Company accounted for the agreements as an asset purchase and allocated the purchase price of $
Preferred stock | | $ | |
Intangible assets | | ||
Prepaid expenses | | | |
| $ | |
On September 16, 2022, the Company entered an Asset Purchase Agreement with RubrYc pursuant to which it acquired substantially all of the assets of RubrYc. The Company issued
Subsequently after the Company acquired substantially all of the assets of RubrYc in September 2022, RubrYc ceased its operations and completed bankruptcy proceedings in June 2023. The Company recorded an impairment of the investment in the amount of $
The Company accounted for the agreements as an asset purchase and allocated the purchase price of approximately $
Intangible assets | | $ | |
Fixed assets | | | |
| $ | |
In addition, the Company assumed
26
Former CEO Departure
Effective December 1, 2022, the Company and Mr. Thomas F. Isett, the former Chief Executive Officer (the “CEO”) and former Chairman of the Board, agreed for Mr. Isett to resign as a member of the Board and relinquish his duties, rights and obligations as the CEO of the Company.
Separation Agreement and General Release
In connection with Mr. Isett’s resignation, the Company entered into a separation agreement and general release with Mr. Isett effective December 1, 2022 (the “Agreement”). Pursuant to the Agreement, Mr. Isett resigned as CEO of the Company effective December 1, 2022, and remained an employee of the Company until December 31, 2022, on which date his employment with the Company terminated. Following Mr. Isett’s termination of employment with the Company, pursuant to the Agreement, Mr. Isett receives the severance benefits set forth in his employment agreement, as previously disclosed by the Company, including (i) an amount equal to his base salary in equal bi-monthly installments for (24) months; (ii) an amount equal to a pro rata share of his target bonus for the Fiscal 2023; (iii) an amount equal to the target bonus in equal bi-monthly installments for the (24) month severance period and (iv) provided that he elects continuation coverage for health insurance under the Consolidated Omnibus Budget Reconciliation Act of 1985 (“COBRA”), the Company will pay the full cost of this benefit for up to (18) months, or if he has not obtained alternative employer-provided health coverage by the end of the (18) month COBRA subsidy period, the Company will provide him with a lump-sum cash payment equal to
7. Promissory Note Receivable
On June 19, 2023, the Company was issued a promissory note (the “Note”) with Safi Biosolutions, Inc. (“Safi”) in the principal amount of $
For the three months ended December 31, 2023 and 2022, interest income amounted to $
8. Investments in Debt Securities
The Company did not hold any investments in debt securities at December 31, 2023 and June 30, 2023. Amortization of premiums paid on the debt securities amounted to $
9. Finance Lease ROU Assets
As discussed above, the Company assumed three equipment leases as part of the RubrYc asset acquisition. In addition, the Company leased a mobile office trailer which was classified as part of assets held for sale prior to its termination. The mobile office trailer lease was terminated in December 2022. See Note 14 – Finance Lease Obligations for more details of the terms of the leases.
27
The following table summarizes by category the gross carrying value and accumulated amortization of finance lease ROU (in thousands):
| December 31, |
| June 30, | |||
2023 | 2023 | |||||
ROU - Equipment | $ | | $ | | ||
Accumulated amortization |
| ( |
| ( | ||
Net finance lease ROU assets | $ | | $ | | ||
Amortization of finance lease ROU assets was approximately $
10. Operating Lease ROU Assets
San Diego, California
On September 10, 2021, the Company entered into a lease for approximately
Bryan, Texas
On November 1, 2021, as discussed above, iBio CDMO acquired the Facility and became the tenant under the Ground Lease Agreement upon which the Facility is located. Based on the terms of the lease payments, the Company recorded an operating lease ROU asset of $
11. Fixed Assets
The following table summarizes by category the gross carrying value and accumulated depreciation of fixed assets (in thousands):
| December 31, |
| June 30, | |||
2023 | 2023 | |||||
Building and improvements | $ | | $ | | ||
Machinery and equipment |
| |
| | ||
Office equipment and software | | | ||||
| | |||||
Accumulated depreciation | ( | ( | ||||
Net fixed assets | $ | | $ | | ||
Depreciation expense reported in continuing operations was approximately $
At December 31, 2023 and June 30, 2023 fixed assets held for sale in the amounts of $
The Company re-evaluated its business strategy and reviewed its product portfolio during Fiscal 2023 which resulted in an impairment charge of approximately $
28
12. Intangible Assets
On August 23, 2021, the Company entered into a series of agreements with RubrYc described in more detail above (see Note 6 – Significant Transactions) whereby in exchange for a $
On September 16, 2022, the Company entered into an Asset Purchase Agreement with RubrYc described in more detail above (see Note 6 – Significant Transactions) pursuant to which it acquired substantially all of the assets of RubrYc. The assets acquired include the patented AI drug discovery platform, all rights with no future milestone payments or royalty obligations, to IBIO-101, in addition to CCR8, EGFRvIII, MUC16, CD3, and one additional immuno-oncology candidate, plus a PD-1 agonist.
In January 2014, the Company entered into a license agreement with the University of Pittsburgh whereby the Company acquired exclusive worldwide rights to certain issued and pending patents covering specific candidate products for the treatment of fibrosis (the "Licensed Technology") which license agreement was amended in August 2016 and again in December 2020 and February 2022. The license agreement provided for payment by the Company of a license issue fee, annual license maintenance fees, reimbursement of prior patent costs incurred by the university, payment of a milestone payment upon regulatory approval for sale of a first product, and annual royalties on product sales. In addition, the Company has agreed to meet certain diligence milestones related to product development benchmarks. As part of its commitment to the diligence milestones, the Company successfully commenced production of a plant-made peptide comprising the Licensed Technology before March 31, 2014. The next milestone – filing an Investigational New Drug Application with the FDA or foreign equivalent covering the Licensed Technology ("IND") – initially was required to be met by December 1, 2015, and on November 2, 2020, was extended to be required to be met by December 31, 2021 and on February 8, 2022, was further extended to December 31, 2023. In addition, the amounts of the annual license maintenance fee and payment upon completion of various regulatory milestones were amended. On February 14, 2023, the Company provided notification to the University of Pittsburgh terminating the license agreement. Pursuant to the termination of the license agreement with the University of Pittsburgh, the Company’s financial obligations for the management of the patents under the license ceased on August 14, 2023, and transitioned back to the University of Pittsburgh. As a result of the termination of the license agreement, the Company recorded a full impairment of the related intangible asset associated with IBIO-100 in the amount of $
The Company re-evaluated its business strategy and reviewed its product portfolio during Fiscal 2023. After such review, the Company identified intellectual property, patent and licenses that would no longer be utilized and therefore were fully impaired. Accordingly, the Company recorded an impairment charge during Fiscal 2023 in general and administrative expenses of approximately $
The following table summarizes by category the gross carrying value and accumulated amortization of intangible assets (in thousands):
|
| December 31, |
| June 30, | ||
2023 | 2023 | |||||
Intellectual property – gross carrying value | $ | | $ | | ||
Intellectual property – accumulated amortization |
| ( |
| ( | ||
Total definite lived intangible assets, net of accumulated amortization | | | ||||
License - indefinite lived | | | ||||
Total net intangible assets | $ | | $ | | ||
Amortization expense was approximately $
See Note 4 - Summary of Significant Accounting Policies and Note 5 – Financial Instruments and Fair Value Measurement for more information.
29
13. Debt
The Credit Agreement
In connection with the PSA, iBio CDMO entered into a Credit Agreement, dated November 1, 2021, with Woodforest pursuant to which Woodforest provided iBio CDMO a $
On October 11, 2022, iBio CDMO and Woodforest amended the Credit Agreement to: (i) include a payment of $
In January 2023, the Company’s unrestricted cash decreased below the required $
On February 20, 2023, iBio CDMO entered into a third amendment to the Credit Agreement (the “Third Amendment”), which removed the added milestone specified in the Second Amendment, the failure of which would be an event of default. In addition, the Guaranty was amended to allow the Company until February 28, 2023, to account for the Fraunhofer Settlement Funds in determining whether the Company is in compliance with the Liquidity Covenant without being dependent upon a specified milestone. In addition, the Company agreed that each time it consummates an at-the-market issuance of Equity Interests (as defined within the Credit Agreement), no later than (5) days following such issuance of Equity Interests, it will (i) pay to Woodforest in immediately available cash funds, without setoff or counterclaim of any kind,
On March 24, 2023, iBio CDMO and Woodforest entered into a fourth amendment to the Credit Agreement (the “Fourth Amendment”), which within the Fourth Amendment Woodforest agreed to (i) reduce the percentage of any payment to Woodforest the Company is required to make from the proceeds of sales of its common stock under its at-the-market facility from
On May 10, 2023, iBio CDMO and Woodforest entered into a fifth amendment to the Credit Agreement (the “Fifth Amendment”), which within the Fifth Amendment Woodforest agreed to: (i) waive the Company’s obligation to deliver to Woodforest an executed copy of a Purchase Agreement for the sale of the Facility no later than April 14, 2023 and, (ii) release $
30
Woodforest. In addition, starting on the effective date of the Fifth Amendment, the interest on the Term Loan increased to
On September 18, 2023, iBio CDMO and Woodforest entered into a sixth amendment to the Credit Agreement (the “Sixth Amendment”), pursuant to which Woodforest agreed to modify the Maturity Date to the earlier of December 31, 2023, or the acceleration of maturity of the Term Loan pursuant to the Credit Agreement, provided that (i) iBio CDMO shall deliver an executed copy of a Purchase Agreement (as defined in the Credit Agreement) for the sale of the Facility within business day after entry into the Sixth Amendment, and (ii) if the Facility is not sold on or before December 1, 2023, iBio CDMO will pay a fee in the amount of $
On October 4, 2023, iBio CDMO and Woodforest entered into the seventh amendment to the Credit Agreement (the “Seventh Amendment”), which amendment among other things, permits the Company, in each case, so long as no Potential Default or Default (as such terms are defined in the Credit Agreement) to make the following withdrawals from the Reserve Funds Deposit Account (as defined in the Credit Agreement): (i) up to $
On December 22, 2023, iBio CDMO and Woodforest entered into the Eighth Amendment (the “Eighth Amendment”) to the Credit Agreement, which amendment among other things, amends the Credit Agreement to: (i) set the Maturity Date of the Term Loan to the earlier of (a) March 29, 2024, or (b) the acceleration of maturity of the Term Loan in accordance with the Credit Agreement; (ii) reduce the interest rate from
At December 31, 2023, the balance of the Term Loan was $
Equipment Financing
On October 12, 2022, the Company entered into an equipment financing master lease agreement and a lease supplement whereby $
31
Future minimum payments under the finance lease obligation are due as follows (in thousands):
Fiscal period ending on December 31: |
| Principal |
| Interest |
| Total | |||
2024 | $ | | $ | | $ | | |||
2025 |
| |
| |
| | |||
|
|
|
|
|
| ||||
Total minimum equipment financing payments |
| | $ | | $ | | |||
Less: current portion |
| ( |
|
|
|
| |||
Long-term portion of minimum equipment financing obligation | $ | |
|
|
|
| |||
Insurance Premium Financing
On October 30, 2023, the Company entered into an insurance premium financing agreement with FIRST Insurance Funding, a division of Lake Forest Bank & Trust Company, N.A., whereby approximately $
Future minimum payments under the insurance premium financing obligation are as follows (in thousands):
Fiscal period ending on December 31: |
| Principal |
| Interest |
| Total | |||
2024 | $ | | $ | | $ | | |||
14. Finance Lease Obligations
Sublease
As discussed above, until November 1, 2021, iBio CDMO leased the Facility as well as certain equipment from College Station under the Sublease. The Sublease was terminated on November 1, 2021, when iBio CDMO acquired the Facility and became the tenant under the ground lease for the property upon which the Facility is located. See Note 15 – Operating Lease Obligations for additional information related to the ground lease.
Equipment
As discussed above, the Company assumed
Mobile Office Trailer
Commencing April 1, 2021, the Company leased a mobile office trailer that was located at the Facility in Bryan, Texas, at a monthly rental of $
32
The following tables present the components of lease expense and supplemental balance sheet information related to the finance lease obligation (in thousands).
| Three Months Ended | Three Months Ended | ||||
December 31, | December 31, | |||||
2023 | 2022 | |||||
Finance lease cost: |
|
|
| |||
Amortization of ROU assets | $ | | $ | | ||
Interest on lease liabilities |
| |
| | ||
Total lease cost | $ | | $ | | ||
|
|
|
| |||
Other information: |
|
|
|
| ||
Cash paid for amounts included in the measurement lease liabilities: |
|
|
|
| ||
Operating cash flows from finance lease | $ | — | $ | — | ||
Financing cash flows from finance lease obligations | $ | | $ | | ||
| Six Months Ended | Six Months Ended | ||||
December 31, | December 31, | |||||
2023 | 2022 | |||||
Finance lease cost: |
|
|
| |||
Amortization of ROU assets | $ | | $ | | ||
Interest on lease liabilities |
| |
| | ||
Total lease cost | $ | | $ | | ||
|
|
|
| |||
Other information: |
|
|
|
| ||
Cash paid for amounts included in the measurement lease liabilities: |
|
|
|
| ||
Financing cash flows from finance lease obligations | $ | | $ | | ||
December 31, | June 30, | |||||||
2023 | 2023 | |||||||
Finance lease ROU assets | $ | | $ | | ||||
Finance lease obligation - current portion | $ | | $ | | ||||
Finance lease obligation - noncurrent portion | $ | | $ | | ||||
Weighted-average remaining lease term - finance lease |
| years |
| years | ||||
Weighted-average discount rate - finance lease obligation |
| | % |
| | % | ||
Future minimum payments under the finance lease obligation are as follows (in thousands):
Fiscal year ending on December 31: |
| Principal |
| Interest |
| Total | |||
2024 | $ | | $ | | $ | | |||
2025 | | | | ||||||
|
|
|
|
|
| ||||
Total minimum lease payments |
| | $ | | $ | | |||
Less: current portion |
| ( |
|
|
|
| |||
Long-term portion of minimum lease obligations | $ | |
|
|
|
| |||
15. Operating Lease Obligations
Texas Ground Lease
As discussed above, as part of the Transaction, iBio CDMO became the tenant under the Ground Lease Agreement for the Ground Lease Property until 2060 upon exercise of available extensions. The base rent payable under the Ground Lease Agreement, which was $
33
San Diego
On September 10, 2021, the Company entered into a lease for approximately
| ● | The length of term of the lease is |
| ● | The lease commencement date was estimated to be on or around January 1, 2022. |
| ● | The monthly rent for the first year of the lease is $ |
| ● | The lease provides for a base rent abatement for months through in the first year of the lease. |
| ● | The Landlord provided a tenant improvement allowance of $ |
| ● | The Company is responsible for other expenses such as electric, janitorial, etc. |
| ● | The Company opened an irrevocable letter of credit in the amount of $ |
As discussed above, the lease provides for scheduled increases in base rent and scheduled rent abatements. Rent expense is charged to operations using the straight-line method over the term of the lease which results in rent expense being charged to operations at inception of the lease in excess of required lease payments. This excess (formerly classified as deferred rent) is shown as a reduction of the operating lease ROU asset in the accompanying condensed consolidated balance sheets.
The following tables present the components of lease expense and supplemental balance sheet information related to the operating lease obligation (in thousands).
Three Months Ended December 31, | |||||
2023 | 2022 | ||||
Operating lease cost: | $ | | $ | | |
Total lease cost | $ | | $ | | |
|
|
| |||
Other information: |
|
|
|
| |
Cash paid for amounts included in the measurement lease liability: |
|
|
|
| |
Operating cash flows from operating lease | $ | | $ | | |
Operating cash flows from operating lease obligation | $ | | $ | | |
Six Months Ended December 31, | |||||
2023 | 2022 | ||||
Operating lease cost: | $ | | $ | | |
Total lease cost | $ | | $ | | |
|
| ||||
Other information: |
|
|
|
| |
Cash paid for amounts included in the measurement lease liability: |
|
|
|
| |
Operating cash flows from operating lease | $ | | $ | | |
Operating cash flows from operating lease obligation | $ | | $ | | |
34
Future minimum payments under the operating lease obligation are as follows (in thousands):
Fiscal year ending on December 31: |
| Principal |
| Imputed Interest |
| Total | |||
2024 | $ | | $ | | $ | | |||
2025 | | | | ||||||
2026 |
| |
| |
| | |||
2027 |
| |
| |
| | |||
2028 |
| |
| |
| | |||
Thereafter |
| |
| |
| | |||
|
|
|
|
|
| ||||
Total minimum lease payments |
| | $ | | $ | | |||
Less: current portion |
| ( |
|
|
|
| |||
Long-term portion of minimum lease obligation | $ | |
|
|
|
| |||
16. Stockholders’ Equity
Preferred Stock
The Company’s Board is authorized to issue, at any time, without further stockholder approval, up to
Series 2022 Convertible Preferred Stock (“Series 2022 Preferred”)
On May 9, 2022, the Board of the Company created the Series 2022 Preferred, par value $
The Company issued
iBio CMO Preferred Tracking Stock (“Preferred Tracking Stock”)
On February 23, 2017, the Company entered into an exchange agreement with Bryan Capital pursuant to which the Company acquired substantially all of the interest in iBio CDMO held by Bryan Capital and issued
On February 23, 2017, the Board of the Company created the Preferred Tracking Stock out of the Company’s
On November 1, 2021, the Company purchased the Preferred Tracking Stock held by Bryan Capital.
Common Stock
The number of authorized shares of the Common Stock is
Reverse Stock Splits
On June 30, 2022, the Company held a special meeting of its stockholders at which the stockholders approved a proposal to affect an amendment to the Company's certificate of incorporation, as amended, to implement a reverse stock split at a ratio of -for-twenty-five (1:25). On September 22, 2022, the Company's Board approved the implementation of the reverse stock split of the Common Stock. As a result of the reverse stock split, every twenty-five (25) shares of the Common Stock either issued and outstanding or held by the Company in its treasury immediately prior to the effective time was, automatically and without any action on the part of the respective
35
holders thereof, combined and converted into (1) share of the Common Stock. No fractional shares were issued in connection with the reverse stock split. Stockholders who otherwise were entitled to receive a fractional share in connection with the reverse stock split instead were eligible to receive a cash payment, which was not material in the aggregate, instead of shares. On October 7, 2022, the Company filed a Certificate of Amendment of its Certificate of Incorporation, as amended with the Secretary of State of Delaware effecting a -for-twenty-five (1:25) reverse stock split of the shares of the Common Stock, either issued or outstanding, effective October 7, 2022. The Common Stock began trading on a reverse split adjusted basis when the market opened Monday, October 10, 2022.
On November 27, 2023, the Company approved a proposal at the 2023 Annual Meeting of Stockholders (the “Annual Meeting”) to amend the Company’s Certificate of Incorporation to effect a reverse stock split of the Company’s Common Stock, par value $
Recent issuances of Common Stock include the following:
Cantor Fitzgerald Underwriting
On November 25, 2020, the Company entered into a Controlled Equity Offering SM Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. ("Cantor Fitzgerald") to sell shares of Common Stock, from time to time, through an “at-the-market offering” program having an aggregate offering price of up to $
In Fiscal year ended June 30, 2023, Cantor Fitzgerald sold as sales agent pursuant to the Sales Agreement
The Company is no longer eligible to sell securities pursuant to its registration statement on Form S-3, including pursuant to the Sales Agreement, from the date of the filing of the Annual Report on Form 10-K until March 1, 2024 due to its late filing of its Quarterly Report on Form 10-Q for the quarter ended December 31, 2022.
RubrYc Transaction
On September 19, 2022, the Company issued
Wainwright Underwriting
On December 6, 2022, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”). Pursuant to the Underwriting Agreement, the Company agreed to sell to Wainwright, in a firm commitment underwritten offering (the “2022 Offering”) (i)
Wainwright acted as the sole book-running manager for the 2022 Offering. The Company paid Wainwright an underwriting discount equal to
36
Underwriting Agreement, the Company has granted Wainwright a
The Company has also agreed to issue to Wainwright, as the representative of the underwriters, warrants (the “Representative’s Warrants”) to purchase a number of shares of Common Stock equal to
The Company received net proceeds of approximately $
Securities Purchase Agreement
On December 5, 2023, the Company entered into a securities purchase agreement with certain Purchasers identified on the signature pages of the Purchase Agreement, pursuant to which the Company agreed to issue and sell, in the 2023 Offering, (i)
The Company agreed to pay the Placement Agents an aggregate cash fee equal to
The Company received net proceeds of approximately $
Vesting of Restricted Stock Units “RSUs”
During the first quarter of Fiscal 2024, RSUs for
During the second quarter of Fiscal 2024, RSUs for
Warrants
Bryan Capital
As discussed above, the Company issued to Bryan Capital a Warrant to purchase
Wainwright
As discussed above, the Company issued various warrants with the following terms:
| ● | 2022 Pre-Funded Warrants – Immediately exercisable at an exercise price of $ |
| ● | Class A Warrants – Immediately exercisable at an exercise price of $ |
| ● | Class B Warrants – Immediately exercisable at an exercise price of $ |
| ● | Representative Warrants – Immediately exercisable at an exercise price of $ |
37
On August 4, 2023, the Company agreed to amend the exercise price with certain holders of the Series A Warrants and Series B Warrants that were acquired from the Company in the 2022 Offering that was completed in December 2022. Under the amended warrants, the Company agreed to amend existing Series A Warrants to purchase up to
Lincoln Park Stock Purchase Agreement
As discussed above, on August 4, 2023, the Company entered into a Purchase Agreement, with Lincoln Park, pursuant to which, under the terms and subject to the satisfaction of specified conditions set forth therein, the Company may sell to Lincoln Park up to $
Beginning on the Commencement Date and for a period of up to 24 months thereafter, under the terms and subject to the conditions of the Purchase Agreement, from time to time, at the Company’s discretion, it had the right, but not the obligation, to sell to Lincoln Park, and Lincoln Park was obligated to purchase, up to $
In addition, provided that the Company had directed Lincoln Park to purchase the maximum amount of shares that it is then able to sell to Lincoln Park in a Regular Purchase on a particular business day on which the closing price of the common stock on the NYSE American is equal to or greater than $
The Company controlled the timing and amount of any sales of Common Stock to Lincoln Park pursuant to the Purchase Agreement. Lincoln Park had no right to require the Company to sell any shares of Common Stock to Lincoln Park, but Lincoln Park was obligated to make purchases as the Company directs, subject to certain conditions.
As consideration for Lincoln Park’s commitment to purchase shares of Common Stock at the Company’s direction pursuant to the Purchase Agreement, the Company issued
38
if any, that it elects, in its sole discretion, to make from time to time from and after the Commencement Date, pursuant to the Purchase Agreement.
During the first quarter of Fiscal 2024, Lincoln Park purchased pursuant to the Purchase Agreement
A.G.P./Alliance Global Partners
On December 7, 2023, the Company, completed the 2023 Offering of (i)
Each share of Common Stock and Pre-Funded Warrant, as applicable, was sold together with
During the second quarter of Fiscal 2024,
During the third quarter of Fiscal 2024,
17. Earnings (Loss) Per Common Share
Basic earnings (loss) per common share is computed by dividing the net income (loss) allocated to common stockholders by the weighted-average number of shares of Common Stock outstanding during the period. For purposes of calculating diluted earnings (loss) per common share, the denominator includes both the weighted-average number of shares of Common Stock outstanding during the period and the number of common stock equivalents if the inclusion of such common stock equivalents is dilutive. Dilutive common stock equivalents potentially include stock options and warrants using the treasury stock method. The following table summarizes the components of the earnings (loss) per common share calculation (in thousands, except per share amounts):
39
Three Months Ended | Six Months Ended | ||||||||||||
December 31, | December 31, | ||||||||||||
|
| 2023 |
| 2022 | 2023 |
| 2022 | ||||||
Basic and diluted numerator: | |||||||||||||
| |||||||||||||
Net loss from continuing operations | $ | ( | $ | ( | $ | ( | $ | ( | |||||
Net loss from discontinued operations | $ | ( | $ | ( | $ | ( | $ | ( | |||||
Net loss - total | $ | ( | $ | ( | $ | ( | $ | ( | |||||
Basic and diluted denominator: | |||||||||||||
Weighted-average common shares outstanding |
| |
| |
| |
| | |||||
|
|
|
| ||||||||||
Per share amount - continuing operations | ( | ( | ( | ( | |||||||||
Per share amount - discontinued operations | ( | ( | ( | ( | |||||||||
Per share amount - total | ( | ( | ( | ( | |||||||||
In Fiscal 2024 and Fiscal 2023, the Company incurred net losses which cannot be diluted; therefore, basic and diluted loss per common share is the same. As of December 31, 2023 and 2022, shares issuable which could potentially dilute future earnings were as follows:
December 31, | ||||
|
| 2023 |
| 2022 |
| (in thousands) | |||
Stock options |
| |
| |
Restricted stock units |
| |
| |
Warrants | | | ||
Shares excluded from the calculation of diluted loss per share |
| |
| |
18. Share-Based Compensation
The following table summarizes the components of share-based compensation expense in the condensed consolidated statements of operations and comprehensive loss (in thousands):
|
| Three Months Ended | ||||
December 31, | ||||||
| 2023 |
| 2022 | |||
Research and development | $ | | $ | | ||
General and administrative |
| |
| | ||
Total | $ | | $ | | ||
|
| Six Months Ended | ||||
December 31, | ||||||
| 2023 |
| 2022 | |||
Research and development | $ | | $ | | ||
General and administrative |
| |
| | ||
Total | $ | | $ | | ||
In addition, share-based compensation expense included in loss from discontinued operations totaled approximately $
Stock Options
iBio, Inc. 2023 Omnibus Equity Incentive Plan (the “2023 Plan”)
On December 9, 2023, the Company adopted the 2023 Plan for employees, officers, directors and external service providers which is the successor to the 2020 Omnibus Equity Incentive Plan (the “2020 Plan”) and once approved became effective on January 1, 2024.
40
The maximum number of shares of Common Stock reserved and available for issuance under the 2023 Plan is
Vesting of service awards are determined by the Board and stated in the award agreements. In general, vesting occurs ratably on the anniversary of the grant date over the service period, generally or
As of December 31, 2023,
iBio, Inc. 2020 Omnibus Equity Incentive Plan (the “2020 Plan”)
On December 9, 2020, the Company adopted the 2020 Plan for employees, officers, directors and external service providers. The total number of shares of Common Stock reserved under the 2020 Plan is
Vesting of service awards are determined by the Board and stated in the award agreements. In general, vesting occurs ratably on the anniversary of the grant date over the service period, generally or
Under the 2020 Plan,
Stock options issued
During the first quarter of Fiscal year 2024, the Company granted stock option agreements to various employees to purchase
The Company estimated the fair value of options granted using the Black-Scholes option pricing model with the following assumptions:
|
|
| |
Weighted-average risk-free interest rate | | % | |
Dividend yield |
| | % |
Volatility |
| | % |
Expected term (in years) |
|
|
RSUs
41
19. Fraunhofer Settlement
On May 4, 2021, the Company and Fraunhofer USA, Inc. (“FhUSA”) entered into a Confidential Settlement Agreement and Mutual Release (the “Settlement Agreement”) to settle all claims and counterclaims in the litigation captioned iBio, Inc. v. Fraunhofer USA, Inc. (Case No. 10256-VCF) in Delaware Chancery Court (the “Lawsuit”). The Settlement Agreement, among other things, resolves the Company’s claims to ownership of certain plant-based technology developed by FhUSA from 2003 through 2014, and sets forth the terms of a license of intellectual property. The Lawsuit was commenced against FhUSA by the Company in March 2015 in the Court of Chancery of the State of Delaware and is described in more detail in the Company’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2020. The Settlement Agreement is not an admission of liability or fault of the parties.
The terms of the Settlement Agreement provided for cash payments to the Company of $
As of June 30, 2021, the Company held receivables related to the settlement in the amount of $
On March 17, 2023, the Company received a payment of $
The Company would recognize the $
All cash payments owed pursuant to the terms of the Settlement Agreement have been received as of December 31, 2023.
20. Income Taxes
The Company recorded
21. Commitments and Contingencies
CRO Agreement
On October 10, 2022, the Company entered into an agreement with a CRO for cell line development and master cell banking to produce iBio-101 in addition to process development and GMP manufacturing of iBio-101 drug substance and drug product to support GLP toxicology and Phase 1 clinical studies. The Company incurred costs of $
Inflation
Although the Company has not experienced any material adverse effects on its business due to increasing inflation, it has raised operating costs for many businesses and, in the future, could impact demand or pricing of manufacturing services, foreign exchange rates or employee wages. The Company is actively monitoring the effects these disruptions and increasing inflation could have on its operations.
42
22. Employee 401(k) Plan
Commencing January 1, 2018, the Company established the iBio, Inc. 401(k) Plan (the “Plan”). Eligible employees of the Company may participate in the Plan, whereby they may elect to make elective deferral contributions pursuant to a salary deduction agreement and receive matching contributions upon meeting age and length-of-service requirements. The Company will make a
23. Subsequent Events
Credit and Security Agreement
On January 16, 2024, the Company entered into a credit and security agreement (the “Credit and Security Agreement”) with Loeb Term Solutions LLC, an Illinois limited liability company (“Lender”), for a term loan or equipment line of credit loan (the “Loan”) pursuant to which the Company issued to Lender a term promissory note in the principal amount of $
The 2024 Term Note provides for monthly payments of principal and interest based on a
The Credit and Security Agreement provides that the Company may request that Lender make further loan advances to the Company subject to certain conditions, including that the Company is not otherwise in default under the Credit and Security Agreement and its obligations and liabilities to Lender do not exceed a borrowing base equal to the lesser of: (a)
The Company’s obligations to Lender under the 2024 Term Note and Credit Security Agreement are further secured by an validity guarantee, dated January 16, 2024 (the “Validity Guarantee”), executed by Dr. Martin Brenner and Felipe Duran in their individual capacity (the “Indemnitors’) for the benefit of Lender. The Validity Guarantee provides that the Indemnitors will indemnify the Lender from any loss or damage, including any actual, consequential or incidental loss or damage, suffered by Lender as a result of, or arising out of, among other things, any willful or intentional misrepresentation or gross negligence by the Company in connection with the Loan and any acts of fraud, conversion, misappropriation or misapplication of funds or proceeds of any Loeb Collateral by the Company or the Indemnitors.
The Credit and Security Agreement contains customary events of default. If an event of default occurs, the 2024 Term Note provides that regardless of whether the Lender elects to accelerate the maturity of the 2024 Term Note, the entire principal remaining unpaid hereunder shall thereafter bear interest at the rate equal to the Effective Rate plus
Vesting of RSUs
During the third quarter of Fiscal 2024, RSUs for
43
RSU Grant
During the third quarter of Fiscal 2024, the Compensation Committee of the Board of the Company approved a special equity award program pursuant to which it awarded to its employees an aggregate of
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following information should be read together with the consolidated financial statements and the notes thereto and other information included elsewhere in this Quarterly Report on Form 10-Q (this “Report”) and in our Annual Report on Form 10-K for the year ended June 30, 2023, as filed with the SEC on September 27, 2023 (the “Annual Report”). Unless the context requires otherwise, references in this Report to “iBio,” the “Company,” “we,” “us,” or “our” and similar terms mean iBio, Inc.
Forward-Looking Statements
This Report contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). For this purpose, any statements contained herein regarding our strategy, future operations, financial position, future revenues, projected costs and expenses, prospects, plans and objectives of management, other than statements of historical facts, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “may,” “plan,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such statements reflect our current views with respect to future events. Because these forward-looking statements involve known and unknown risks and uncertainties, actual results, performance or achievements could differ materially from those expressed or implied by these forward-looking statements for a number of important reasons, including those discussed in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Report, as well as in the section titled “Risk Factors” in our Annual Report. We cannot guarantee any future results, levels of activity, performance or achievements. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this Report as anticipated, believed, estimated or expected. The forward-looking statements contained in this Report represent our estimates as of the date of this Report (unless another date is indicated) and should not be relied upon as representing our expectations as of any other date. While we may elect to update these forward-looking statements, we specifically disclaim any obligation to do so unless otherwise required by securities laws.
44
Overview
iBio, Inc. (“iBio,” “we,” “us,” or “our”) is a pioneering biotechnology company at the intersection of AI and biologics, committed to reshaping the landscape of discovery. Our core mission is to harness the potential of AI and machine learning to unveil elusive biologics that stand out and have evaded other scientists. Through our innovative platform, we champion a culture of innovation by identifying novel targets, forging strategic collaborations to enhance efficiency, diversify pipelines, and with the goal of accelerating preclinical processes.
Additionally, our groundbreaking EngageTx™ technology enables us to target bi-specific molecules. With the ability to navigate sequence diversity and promote Human-Cyno cross reactivity while mitigating cytokine release, our goal is to enhance agility and bolster preclinical safety assessments.
Our strategic approach to fulfilling our mission is outlined as follows:
| ● | Elevate Epitope Discovery: We believe we lead the field with our patented AI-engine uncovering "hard to develop" molecules. Our unparalleled epitope engine stands out by allowing the ability to target select regions of a protein, potentially removing the lengthy trial and error out of mAb discovery. This capability is expected to improve probability of success while at the same time, reduces costs commonly caused by having an iterative process. Our epitope engine is engineered to match its target, refined for stability and optimized for water solubility, allowing us to identify new drug candidates that have failed or have been abandoned due to their complexity. |
| ● | Capital Efficient Business Approach: Our strategic business approach is structured around the following pillars of value creation: |
| o | Strategic Partnerships: We are leveraging our platform and pipeline by forming strategic partnerships. Our aim is to become the preferred partner for major pharmaceutical and biotechnology companies seeking rapid and cost-effective integration of complex molecules into their portfolios, de-risking their early-stage pre-clinical work. Additionally, a rich array of fast follower molecules within our pre-clinical pipeline holds the potential to drive substantial partnerships, opening doors to innovative projects. By tapping into our platform, infrastructure, and expertise, partners have the potential to streamline timelines, reduce costs tied to biologic drug discovery applications and cell line process development, and expedite preclinical programs with efficiency. |
| o | Tech Licensing in Diverse Therapeutic Areas: In pursuit of adding value, we are exploring partnerships in diverse therapeutic domains such as CNS or vaccines. Our intention is to license the AI tech stack, extending its benefits to our partners and amplifying its biological impact and insights. This strategic approach enables us to capitalize on the value of our meticulously curated data while empowering collaborations and innovations, while at the same time allowing us to focus on both the platform and our core therapeutic area, oncology. |
| o | Developing and Advancing Our In-house Programs Cost Effectively: Clinical advancement is crucial for drug discovery. We are actively looking for opportunities to progress our internal pre-clinical programs, with a focal point on oncology, steadily reinforcing our pre-clinical pipeline. |
| ● | Unwavering Investment in Advancing the Platform: We maintain an unwavering commitment to invest in our platform, continually unlocking the potential of biology through AI and machine learning – the pinnacle of being on the forefront of machine learning advancing algorithms and models in order to improve its predictive power and reduce the time it takes to find a viable molecule. |
In essence, we are sculpting a future where cutting-edge AI-driven biotechnology propels the discovery of intricate biologics, fostering partnerships, accelerating innovation, and propelling the advancement of science.
AI Drug Discovery Platform
Overview
Our platform comprises five key components, each playing a crucial role in the discovery and optimization of precision antibodies.
The first layer, epitope engineering, leverages the patented AI-engine to target specific regions of proteins, allowing us to engineer antibodies with high specificity and efficacy. The second layer involves the proprietary antibody library, which is built on clinically validated frameworks and offers a rich diversity of human antibodies. The third layer of the technology stack is the antibody optimizing
45
StableHu AI technology, coupled with mammalian display technology. Next, we use our EngageTx T-cell engager platform to create bispecific antibodies. And last, antibodies are transformed into conditionally activated antibodies by ShieldTx, our antibody masking technology. Each layer of the tech stack is designed to work synergistically, enabling us to rapidly advance antibodies from concept to in vivo proof-of-concept (POC).
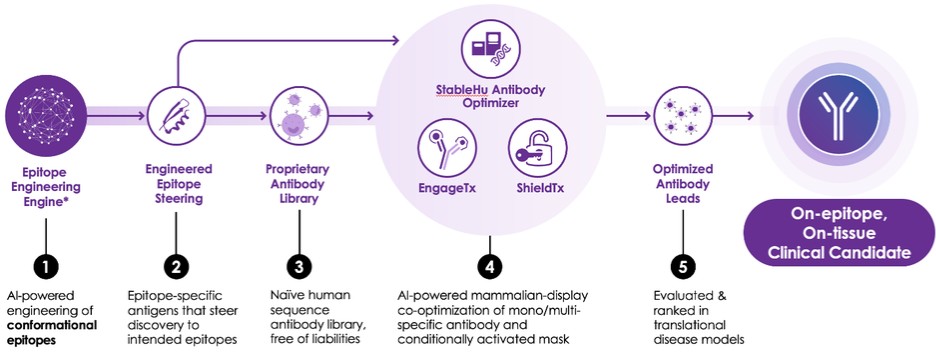
| ● | AI Epitope Steering Technology |
Our epitope steering technology is designed to address these issues by guiding antibodies exclusively against the desired regions of the target protein. By focusing on these specific regions, we can overcome the limitations of traditional methods and significantly improve the efficiency and effectiveness of our antibody discovery process. Our AI engine creates engineered epitopes, which are small embodiments of epitopes on the target protein. The engine is trained to match the epitope structure as closely as possible and refine the designs for greater stability and water solubility, which are critically important factors. The optimized engineered epitope is then used to identify antibodies from naïve or immunized libraries.
| ● | Naïve Human Antibody Library |
The fully human antibody library is built upon clinically validated, entirely human antibody frameworks. By leveraging public databases, we have extracted a diverse array of Complementarity-Determining Region (“CDR”) sequences. Subsequently, we have meticulously eliminated a range of sequence liabilities. Such careful curation process could potentially significantly reduce the development risk for antibodies identified from our library.
| ● | StableHuTM AI Antibody-Optimizing Technology |
Our proprietary StableHu technology is instrumental in the optimization process. StableHu is an AI-powered tool designed to predict a library of antibodies with fully human CDR variants based on an input antibody. This input can range from an early, unoptimized molecule to an approved drug. The model has been trained utilizing a set of over 1 billion human antibodies, progressively masking known amino acids within CDRs until the algorithm could predict the correct human sequence.
While phage display libraries are often used in antibody optimization due to their vast diversity, they can increase developability risks such as low expression, instability, or aggregation of antibodies. Mammalian display libraries, on the other hand, offer significantly improved developability but reduced diversity due to the smaller library size they can handle. StableHu overcomes this limitation by utilizing a machine learning algorithm generating focused library diversity within the capacity of mammalian display.
Mammalian display is a technology that presents antibodies on the surface of mammalian cells, allowing for the direct screening and selection of antibodies in a mammalian cell environment. This approach is advantageous as antibodies that express well on the mammalian cells used in the display are more likely to express well in the production cell line. Moreover, single-cell sorting of antibody-displaying cells allows rapid selection of desired antibodies based on multiple dimensions, such as potency, selectivity, and cross-species selectivity.
46
When paired with mammalian display technology, StableHu enables antibody optimization with fewer iterative optimization steps, lower immunogenicity risk, and improved developability.
| ● | EngageTx CD3-Based T-Cell Engager Panel |
We have used antibodies from an epitope steering campaign as well as a first-generation T-cell engager as input and utilized our StableHu technology to identify a next-generation CD3 antibody panel. The sequence diversity generated by StableHu led to an antibody panel with a wide range of potencies, which allows us to pair the panel with a wide variety of tumor-targeting antibodies. Importantly, we were able to retain T-cell activation and tumor cell killing capacity with significantly reduced cytokine release. This reduction is believed to lower the risk of cytokine release syndrome. Additionally, the increased humanness of the predicted antibodies, thanks to our StableHu technology, has the ability to reduce the risk of immunogenicity.
Furthermore, our StableHu technology enabled us to engineer NHP cross-reactivity into EngageTx. This allows for advanced safety assessment in NHP ahead of clinical trials, providing another layer of safety assurance.
| ● | ShieldTx |
We have enhanced our proprietary technology with the introduction of ShieldTx, patent-pending innovative antibody masking technique. ShieldTx leverages our engineered epitope technology, which is utilized not only for the identification of antibodies against complex drug targets but also for concealing the antibodies' active sites. A significant hurdle in therapeutic antibody development is the expression of drug target on both healthy and diseased tissues, leading to adverse effects on non-targeted tissues. ShieldTx is designed to address this challenge by rendering antibodies inactive until they reach a specific environment unique to diseased tissues. Upon contact with this environment, the masking element is detached, activating the antibody. In the tumor microenvironment this is achieved by a highly expressed matrix metalloproteinase. This strategy aims to minimize or eliminate unintended effects on healthy tissues, thereby improving the safety profile and reducing the immunogenicity risks associated with bispecific antibodies.
Modalities
Epitope steering, a technology we are pioneering, has the potential to positively impact various areas of medicine. In the field of immuno-oncology, it can be used to develop antibodies targeting specific cancer antigens, potentially enhancing the efficacy of treatments like checkpoint inhibitors and CAR-T therapies.
The technology also holds promise in the realm of systemic secreted and cell-surface therapeutics. Epitope steering can be applied to the development of antibodies, circulating immune modulation factors, secreted enzymes, and transmembrane proteins. This could be particularly beneficial in treating diseases such as heart failure, infectious diseases, and rare genetic conditions. In the context of localized regenerative therapeutics, epitope steering could potentially be used to develop treatments that target specific damaged or diseased tissues. This approach could be particularly beneficial in the treatment of cardiovascular diseases. Intratumoral immuno-oncology is another area where epitope steering could make a significant impact. It could potentially be used to develop treatments that alter the tumor microenvironment to favor an immune response against tumors, potentially enhancing the efficacy of treatments that use immune-stimulatory proteins. The potential of epitope steering extends to cancer vaccine development as well. The ability to target specific epitopes could be beneficial in the development of vaccines, particularly those that aim to increase the number and antitumor activity of a patient's T cells. Finally, epitope steering could be used to develop treatments for a wide range of diseases, including those in the immune-oncology space, immunology, pain, and potentially in vaccine development. This is particularly relevant for complex and hard-to-drug protein structures.
Partnerships
As noted above, one of the three pillars of value creation that structures of our strategic business approach are strategic partnerships. At the center of such pillar is our AI Discovery Platform. In June 2023, we entered into a research collaboration with the National Institute of Allergy and Infectious Diseases (“NIAID”), a component of the National Institutes of Health (“NIH”), to investigate the potential of our patented AI-driven epitope steering platform for the development of a vaccine for Lassa fever, a sometimes fatal viral disease endemic to parts of West Africa. During the first quarter FY 2024, we entered into a collaboration with a partner to license the use of our AI Discovery Platform to assist such partner with two targets of interest. During the second quarter FY 2024, we entered into a collaboration with a large pharmaceutical company to assist such partner by using our patented AI-driven epitope steering platform to
47
assist with one "hard to develop" molecule. We continues to seek out opportunities for future collaborations using our AI Discovery Platform.
Pipeline
We are currently in the process of building and advancing our pipeline. The focus of our pipeline is primarily on immuno-oncology, with one program also dedicated to the immunology space. By leveraging our technology stack, the pipeline is geared towards hard-to-drug targets and molecules offering differentiation. To mitigate target risk and capitalize on the learnings of competitors, our programs are primarily adopting a fast follower strategy. This approach allows us to focus on targets that have to some extent been validated and learn from the advancements of those ahead in the field.
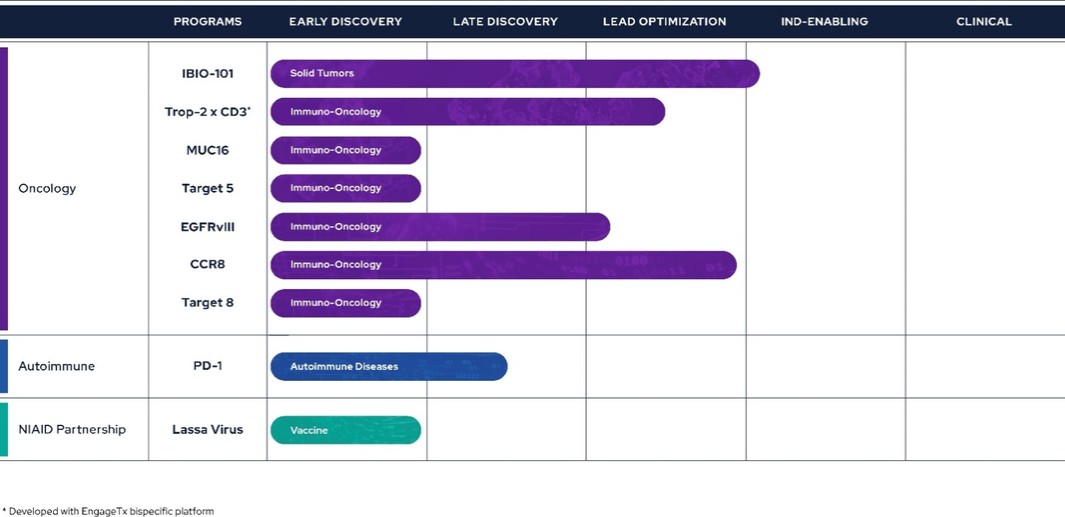
Therapeutics
Immuno-Oncology
IBIO-101
In August 2021, we signed a worldwide exclusive licensing agreement with RubrYc to develop and commercialize RTX-003 (now referred to as IBIO-101), an anti-CD25 monoclonal antibody (“mAb”). In September 2022, we acquired exclusive ownership rights to IBIO-101. IBIO-101 is a second-generation anti-CD25 mAb that has demonstrated in preclinical models of disease the ability to bind and deplete immunosuppressive regulatory T (“Treg”) cells to inhibit the growth of solid tumors.
Targeting depletion of Treg cells to control tumors emerged as an area of interest in oncology over the past several years. Since Treg cells express interleukin-2 Rα (“IL-2Rα” or “CD25”), it was envisioned mAbs could be developed that bind CD25 and thereby trigger depletion by Natural Killer cells, resulting in stimulation of anti-tumor immunity.
Unfortunately, while first-generation mAbs successfully bound CD25+ cells, they also interfered with interleukin-2 (“IL-2”) signaling to T effector (“Teff”) cells to activate their cancer cell killing effects. The result was a failure of first-gen anti-CD25 mAbs as cancer immunotherapies, since their favorable anti-Treg effects were negated by their unfavorable impact on Teff cells.
In a humanized mouse disease model, IBIO-101, when used as a monotherapy, effectively demonstrated its mechanism of action by significantly enhancing the Treg/Teff ratio, resulting in the suppression of tumor growth. When paired with an anti-PD-1 checkpoint inhibitor in the same model, the combined treatment of IBIO-101 and anti-PD-1 exhibited superior tumor inhibition compared to either anti-PD-1 or IBIO-101 used independently.
48
We continue to advance our IL-2 sparing anti-CD25 antibody, IBIO-101, and intend moving the program from IND-enabling stage to an IND filing during the calendar year 2025, subject to funding availability.
TROP-2 x CD3 Bispecific
We have identified highly potent, fully human TROP-2 (Trophoblast Cell Surface Antigen 2) monoclonal antibodies, which have been formatted into bispecific TROP-2 x CD3 molecules using our T-cell engager antibody panel, EngageTx. TROP-2 is highly expressed in multiple solid tumors, including breast, lung, colorectal, and pancreatic cancers and is closely linked to metastasis and tumor growth. TROP-2 antibody drug conjugates have been developed to deliver toxic payloads to these cancer cells but could risk harming healthy cells and cause adverse effects. Our bispecific approach has the potential to increase the therapeutic window, while promoting a robust and long-lasting anti-tumor response. Combining the bispecific TROP-2 approach with immunotherapies like checkpoint inhibitors can potentially lead to improved clinical outcomes.
Using EngageTx, our lead TROP-2 x CD3 bispecific antibody was engineered to potently kill tumor cells while limiting the release of cytokines, like Interferon Gamma (“IFNg”), Interleukin 2 (IL-2) and Tumor Necrosis Factor Alpha (“TNFa”), all of which have the potential to cause cytokine release syndrome. When compared to a bispecific molecule engineered with our TROP-2 binding arm and a first generation CD3 engager, SP34, our lead TROP-2 x CD3 bispecific antibody showed a markedly reduced cytokine release profile, potentially indicating a decreased risk for cytokine release syndrome.
When tested in a humanized mouse model of squamous cell carcinoma, our lead TROP-2 x CD3 bi-specific antibody demonstrated a significant 36 percent reduction in tumor size within just 14 days after tumor implantation, and after only a single dose.
MUC16
MUC16 is a well-known cancer target often overexpressed in several types of solid tumors, including ovarian, lung, and pancreatic cancers. Specifically, MUC16 is a large extracellular protein expressed on more than 80% of ovarian tumors. Tumor cells can evade immune attack by shedding or glycosylating MUC16, making it difficult for traditional antibody therapies to effectively target and destroy the cancer cells.
Using our patented epitope steering AI platform, our innovative approach to this challenge allows our new mAbs to bind to a specific region of MUC16 that is not shed or glycosylated, circumventing both tumor evasion mechanisms and potentially providing a powerful tool in the fight against cancer. During its immunization and screening campaign, we identified several hits that specifically bound to the non-shed region of MUC16 while no binding to the shed fragment of MUC16 was observed. During pre-clinical studies, our MUC16 molecule has demonstrated binding to MUC16 on OVCAR-3 ovarian cancer cells. After engineering the leading MUC16 molecule with a fully human framework, the MUC16 molecule retained potent binding to the engineered epitope and maintained binding to human OVCAR-3 ovarian cancer cells. We have utilized our EngageTX platform to engineer MUC x CD3 bispecific antibodies and have further optimized the molecules to be double-masked on the MUC16 and the CD3 binding arms of the antibody.
EGFRvIII
EGFRvIII is a specific variant of the EGFR protein, unique to tumor cells. Unlike the more common EGFR, EGFRvIII is not found in healthy cells, making it an attractive target for therapeutic interventions. This variant is most prominently associated with glioblastoma, a type of brain cancer and head and neck cancer but can also be present in certain cases of breast, lung, and ovarian cancers, among others. In our pursuit of innovative treatments, we are exploring antibody therapeutics that specifically target EGFRvIII, aiming to address these cancer types without affecting healthy cells.
Leveraging our patented AI-enabled epitope steering engine, we've specifically directed antibodies to target a unique epitope found exclusively on EGFRvIII, and not on the wildtype receptor, EGFR. Through this precision approach, we have designed tumor-specific molecules aimed at selectively targeting cancer cells while preserving healthy ones, potentially offering patients a more focused and safer therapeutic solution.
Our hit molecules have demonstrated strong binding to the tumor-specific EGFRvIII protein without targeting the wildtype EGFR. Additionally, these molecules have effectively eliminated tumor cells, while sparing healthy ones, in in vitro cell killing tests. Our lead anti-EGFRvIII antibody was specially engineered to enhance its ability to attack cancer cells and has proven effective in a mouse model for head and neck cancer. In preclinical studies, our anti-EGFRvIII antibody demonstrated a 43 percent reduction in tumor growth compared to untreated animals.
49
CCR8
GPCRs are one of the most successful therapeutic target classes, with approximately one-third of all approved drugs targeting these proteins. Compared to small molecule-based GPCR drugs, antibody-based GPCR therapeutics potentially offer several potential advantages, including superior selectivity, extended mechanisms of action, and longer half-life. However, GPCRs are intricate, multi-membrane spanning receptors, making clinically relevant regions difficult to identify and target.
The chemokine receptor CCR8 is a GPCR which is predominantly expressed on Tregs, which play a role in suppressing immune responses. In the context of cancer, Tregs can inhibit the body's natural immune response against tumor cells, promoting cancer progression. Anti-CCR8 antibodies are being explored as a therapeutic strategy to deplete these Tregs in the tumor environment. By targeting and reducing Tregs using anti-CCR8 antibodies, the hope is to enhance the body's immune response against cancer cells, offering a promising avenue for cancer treatment.
Aiming directly at CCR8 is believed to be a safer approach because it focuses on specific suppressive Treg cells in the tumor environment without affecting other immune cells and functions. It is important to make sure antibodies are fine-tuned to CCR8 and don't mistakenly target a similar receptor, CCR4. This is because CCR4 is found in many immune cells, and accidentally targeting it could potentially lead to unwanted side effects.
Using our unique AI-driven technology, we have successfully identified molecules targeting CCR8, addressing some of the hurdles often faced when creating therapies that target GPCR with antibodies. Our specialized anti-CCR8 antibody has shown strong attachment to cells expressing CCR8 and effectively disrupted the CCR8 signaling process, resulting in the efficient elimination of Tregs derived from primary human immune cells. Notably, our CCR8-focused molecule did not attach to cells overproducing CCR4, highlighting its precision in targeting only CCR8.
Our CCR8 antibody has proven effective in a mouse model for colon cancer. Preclinical studies show our anti-CCR8 molecule inhibited tumor growth and achieved a 22 percent reduction in tumor size compared to its pre-treatment dimensions. We have specifically engineered the anti-CCR8 molecule as a high Antibody-Dependent Cellular Cytotoxicity (ADCC) antibody to enhance its ability to attack cancer cells.
Autoimmune
PD-1 Agonist
Programmed cell death protein 1 (“PD-1”) is a pivotal player in the immune system, acting as a type of "off switch" that helps keep the cells from attacking other cells in the body. By agonizing or enhancing the signaling of PD-1, it is possible to temper the immune response, making it particularly valuable in the treatment of autoimmune diseases. In conditions where the immune system mistakenly wages war on the body's own cells, such as in autoimmune diabetes or lupus, therapies that target PD-1 can potentially reduce the severity of these autoimmune reactions. This approach offers a promising avenue for providing relief to patients suffering from these debilitating conditions. The figures below depict the mechanism of action of antagonistic and agonistic PD-1 antibodies.
iBio purchased the global rights to a partnership-ready PD-1 agonistic mAb intended to treat serious autoimmune disorders. While the goal in immuno-oncology is to remove immune tolerance towards cancer cells, in autoimmune diseases the opposite is the case, because autoimmune diseases can result from deficits in peripheral and/or central tolerance mechanisms which presents an opportunity for therapeutic intervention. Specifically, agonism or stimulation of inhibitory receptors like PD-1 or CTLA4, which mediate peripheral tolerance is a promising approach to treat autoimmune diseases. Unlike PD-1 antagonists used in immuno-oncology, PD-1 agonists are difficult to find. RubrYc used its AI Discovery Platform to discover PD-1. PD-1 is currently in the late-discovery stage, having undergone extensive screening and in vitro characterization, and we anticipate it will be advanced into in vivo models as IBIO-102, in the near future.
In preclinical studies, our PD-1 agonists have been evaluated using a primary T-cell assay. Our top-performing molecules showed a significant decrease in the proinflammatory cytokine IL-2 and reduced expression of the T-cell activation marker CD96. Both of these outcomes are indicative of the desired dampening of T-cell activation.
Recent Developments
On October 4, 2023, iBio CDMO and Woodforest National Bank (“Woodforest”) entered into the seventh amendment (the “Seventh Amendment”), to the Credit Agreement dated November 1, 2021 which amendment among other things, permits us, in each case, so
50
long as no Potential Default or Default (as such terms are defined in the Credit Agreement) to make the following withdrawals from the Reserve Funds Deposit Account (as defined in the Credit Agreement): (i) up to $1,000,000 on October 4, 2023 so long as we maintain a minimum balance of $2,000,000 until October 16, 2023, (ii) up to an additional $750,000 after October 16, 2023 so long as we maintain a minimum balance of $1,250,000 until November 13, 2023, and (iii) up to an additional $250,000 after November 13, 2023 so long as we maintain a minimum balance of $1,000,000 until Payment in Full (as defined in the Credit Agreement). On the earlier of (a) the closing of the Purchase Agreement, or (b) the Maturity Date (as defined in the Credit Agreement), we will pay Woodforest $20,000. In addition, on October 4, 2023, we, as guarantor, entered into the Fifth Amendment to the Guaranty, which amendment reduces the liquidity covenant that requires us to maintain a specified amount in unrestricted cash to $0.
On November 27, 2023, we approved a proposal at the 2023 Annual Meeting of Stockholders (the “Annual Meeting”) to amend our Certificate of Incorporation to effect a reverse stock split of the Company’s Common Stock, par value $0.001 per share, at a ratio between one-for-five to one-for-twenty, with the ratio within such range to be determined at the discretion of our Board of Directors (the “Board”), without reducing the authorized number of shares of Common Stock. Following the Annual Meeting, the Board approved a final split ratio of one-for-twenty (1:20). Following such approval, on November 28, 2023, we filed an amendment to the Certificate of Incorporation with the Secretary of State of the State of Delaware to effect the reverse stock split, with an effective time of 12:01 a.m. Eastern Time on November 29, 2023 (the “2023 Reverse Stock Split”). As a result of the 1:20 Reverse Stock Split, each twenty (20) pre-split shares of Common Stock outstanding automatically combined into one (1) new share of Common Stock without any action on the part of the holders. No fractional shares were issued in connection with the 2023 Reverse Stock Split. In lieu of fractional shares, any person who would otherwise be entitled to a fractional share of Common Stock as a result of the reclassification and combination following the effective time of the 2023 Reverse Stock Split (after taking into account all fractional shares of Common Stock otherwise issuable to such holder) were entitled to receive a cash payment equal to the number of shares of the Common Stock held by such stockholder before the 2023 Reverse Stock Split that would otherwise have been exchanged for such fractional share interest multiplied by the average closing sales price of the Common Stock as reported on the NYSE American for the ten days preceding November 29, 2023.
On December 7, 2023, we issued and sold, in a public offering (i) 600,000 shares (the “Shares”) of the Company’s common stock, par value $0.001 per share (the “Common Stock”), (ii) 1,650,000 pre-funded warrants (the “Pre-Funded Warrants”) exercisable for an aggregate of 1,650,000 shares of Common Stock, (iii) 2,250,000 Series C common warrants (the “Series C Common Warrants”) exercisable for an aggregate of 2,250,000 shares of Common Stock, and (iv) 2,250,000 Series D common warrants (the “Series D Common Warrants,” and together with the Series C Common Warrants, the “Common Warrants”) exercisable for an aggregate of 2,250,000 shares of Common Stock.
A.G.P./Alliance Global Partners (“A.G.P.”) acted as the lead placement agent, and Brookline, as co-placement agent (A.G.P. and Brookline are referred to herein, collectively, as the “Placement Agents”). We agreed to pay the Placement Agents an aggregate cash fee equal to 5.5% of the gross proceeds received by us from the sale of the securities in the Offering. Pursuant to the Placement Agency Agreement, dated December 5, 2023, entered into by and between us and the Placement Agents, we also agreed to reimburse the Placement Agents for their accountable offering-related legal expenses in an amount up to $75,000 and pay a non-accountable expense allowance of up to $15,000. We received net proceeds of approximately $4 million after deducting commissions and other issuance costs.
On December 22, 2023, iBio CDMO and Woodforest entered into the Eighth Amendment (the “Eight Amendment”) to the Credit Agreement, which amendment among other things, amends the Credit Agreement to: (i) set the Maturity Date of the Term Loan to the earlier of (a) March 29, 2024, or (b) the acceleration of maturity of the Term Loan in accordance with the Credit Agreement; (ii) reduce the interest rate from 5.25% to 4.5% and increase the payment in kind from 3% to 4.5%; and (iii) permit us, so long as no Potential Default or Default (as such terms are defined in the Credit Agreement) exists to make a withdrawal from the Reserve Funds Deposit Account (as defined in the Credit Agreement) so long as we maintain a minimum balance of $900,000 until Payment in Full (as defined in the Credit Agreement). The Eighth Amendment provides that we will use its best efforts to consummate and close a sale of the Collateral (as defined in the Credit Agreement) on or before the Maturity Date. The amendment also increased the fees payable by us to Woodforest by $10,000. Accordingly, per the amendment, on the earlier of (a) the closing of the sale of the Collateral, or (b) the Maturity Date, we will pay Woodforest a fee in the amount of $155,000.
On January 16, 2024, we entered into a credit and security agreement (the “Credit and Security Agreement”) with Loeb Term Solutions LLC, an Illinois limited liability company (“Lender”), for a term loan or equipment line of credit loan (the “Loan”) pursuant to which we issued to Lender a term promissory note in the principal amount of $1,071,572 (the “2024 Term Note”) bearing interest at the Prime Rate, as quoted in the Wall Street Journal plus 8.5% (the “Effective Rate”), for proceeds of $1,027,455.23 after payment of $42,862.88 to Lender as an origination fee, $1,172.89 for appraisal costs, and $75.00 for bank wire fees. The 2024 Term Note provides for monthly payments of principal and interest based on a four-year amortization period, with a balloon payment of all principal, accrued interest and any other amounts due on the two year anniversary of the 2024 Term Note. The Credit and Security Agreement granted to Lender a security interest in substantially all of our assets other than any intellectual property related to any of our filed patents (the “Loeb
51
Collateral”) to secure our obligations under the 2024 Term Note. The 2024 Term Note is subject to a prepayment fee of: 4% of the principal amount being prepaid if the 2024 Term Note is prepaid during the first 12 months from its issuance, and 3% of the principal amount being prepaid if the Term Note is prepaid during the second 12 months from its issuance date. See Note 23 – Subsequent Event to the notes to financial statements for additional information regarding the Loan and Credit and Security Agreement.
Liquidity and Capital Resources
The history of significant losses, the negative cash flow from operations, the limited cash resources on hand and the dependence by us on obtaining additional financing to fund our operations after the current cash resources are exhausted raises substantial doubt about our ability to continue as a going concern. Our management concluded that the current financing and business plans have not mitigated such substantial doubt about the Company’s ability to continue as a going concern for at least 12 months from the date of filing this Quarterly Report on Form 10Q for the quarterly period ended December 31, 2023. Our auditors also included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended June 30, 2023 with respect to this uncertainty.
In an effort to mitigate the substantial doubt about continuing as a going concern and increase cash reserves, we raised funds through equity offerings or other financing alternatives including entering into an equipment line of credit, reduced our work force by approximately 60% (a reduction of approximately 69 positions) in November 2022, ceased operations of our 130,000 square foot cGMP facility located in Bryan, Texas (the “Facility”) thereby reducing annual spend on expenses by approximately 68% and generating cash savings of approximately 61% from first quarter Fiscal year 2023 compared to first quarter Fiscal year 2024. Additionally, we had executed a Purchase and Sale Agreement for the sale of the Facility, which sale if consummated would have allowed us to pay all outstanding amounts under the Term Loan. On November 7, 2023, we received written notice from Majestic Realty Co. (“Majestic Realty”) of its election to terminate the Purchase and Sale Agreement, dated as of September 15, 2023, between Majestic Realty and iBio CDMO LLC (“iBio CDMO”), pursuant to which iBio CDMO had agreed to sell to Majestic Realty the Property. Although the Facility has been listed for sale, we do not currently have a buyer for the Property at a price that will allow us to pay the Term Loan in full. If a sale of the Facility is not consummated prior to the March 29, 2024 maturity date of the Term Loan, we will not have sufficient funds to repay the Term Loan on its maturity date, which Term Loan has an outstanding balance of $12.7 million as of December 31, 2023. In addition, if a sale of the Facility is not consummated at a price at or above the outstanding balance under the Term Loan we do not expect to have sufficient funds to repay the Term Loan upon its maturity. There can be no assurance that we will be able to sell the Facility or that we will be able to sell the Facility for an amount that will allow us to repay all outstanding amounts under the Term Loan. (See Note 3 – Discontinued Operations for more information.)
During the first quarter ended on September 30, 2023, we completed at-the-market offerings and sold 170,989 shares of Common Stock which we received approximately $1.7 million. We also sold 181,141 shares of Common Stock under our purchase agreement entered into on August 4, 2023 (the “Purchase Agreement”), with Lincoln Park Capital Fund, LLC (“Lincoln Park”) and received approximately $1.2 million in proceeds. During the second quarter ended December 31, 2023, we sold an additional 21,457 shares to Lincoln Park under the Purchase Agreement for approximately $0.1 million. (See Note 16 – Stockholder’s Equity for more information.)
On December 7, 2023, we closed the 2023 Offering pursuant to which we sold in the 2023 Offering, (i) 600,000 Shares of our Common Stock, (ii) 1,650,000 Pre-Funded Warrants exercisable for an aggregate of 1,650,000 shares of Common Stock, (iii) 2,250,000 Series C Common Warrants exercisable for an aggregate of 2,250,000 shares of Common Stock, and (iv) 2,250,000 Series D Common Warrants exercisable for an aggregate of 2,250,000 shares of Common Stock. A.G.P. acted as lead placement agent, and Brookline acted as the Placement Agents for the 2023 Offering. We received approximately $4.5 million in gross proceeds from the 2023 Offering, including the exercise of all Pre-Funded Warrants and prior to deducting placement agent fees and other estimated offering expenses payable by us and excluding the net proceeds, if any, from the exercise of the Common Warrants. (See Note 16 – Stockholders’ Equity for more information.)
Our cash, cash equivalents and restricted cash was approximately $4.1 million as of December 31, 2023, which is inclusive of restricted cash of $1.3 million which was deposited in accordance with the Fourth Amendment with Woodforest, and is not anticipated to be sufficient to support operations beyond the third quarter of Fiscal 2024 unless we reduce our cash burn rate to cover operations further, sublease all or a portion of the San Diego Lease, sell the Facility for amounts above its term note payable, or raise additional capital. (See Note 13 – Debt and Note 23 – Subsequent Events for more information.) As of the filing of this Report, the Company’s cash balance is approximately $3.4 million, which is inclusive of approximately $1.1 million of restricted cash. Regardless of whether we are able to reduce our burn rate or sell or out-license certain assets or parts of the business, we will need to raise additional capital in order to fully execute our near and long-term business plans. It is our goal to implement one or more potential options described above to allow us to have a cash runway for at least 12 months from the date of the filing of this Report. However, there can be no assurance that we will be successful in implementing any of the options that we are evaluating.
52
Our liquidity and operations could also be impacted by our obligations under the Woodforest Credit Agreement. If a sale of the Facility is not consummated prior to the March 29, 2024 maturity date of the Term Loan at a favorable price, we will not have sufficient funds to repay the Term Loan on its maturity date.
Results of Operations - Comparison of the three months ended December 31, 2023 and 2022
Revenue
Revenue from the CDMO operations is now included in discontinued operations and not broken out separately on the financial statements. Our ongoing business is primarily focused on i) development of our pipeline for which we do not expect revenue and ii) on our AI-driven discovery platform. No revenue was recognized for the three months ended December 31, 2023.
Research and Development Expenses (“R&D”)
R&D expenses for the three months ended December 31, 2023 and 2022 were $1.5 million and $2.8 million, respectively, a reduction of approximately ($1.3) million. The decrease in R&D expenses is mainly due to certain tasks and assays being performed in-house which were previously outsourced, and a decrease in spending for consultants or outside services.
General and Administrative Expenses (“G&A”)
G&A expenses for the three months ended December 31, 2023 and 2022 were approximately $3.0 million and $7.8 million, respectively, a decrease of ($4.8) million. The decrease in expenses is primarily attributable to the reduction in personnel costs, an intangible impairment recorded in the second quarter of Fiscal 2023 that did not recur, and a decrease in spending for consultants or outside services.
Total Operating Expenses
Total operating expenses, consisting primarily of R&D and G&A expenses, for the three months ended December 31, 2023 were approximately $4.5 million, compared to approximately $10.6 million in Fiscal year 2023.
Discontinued Operations
On November 2, 2022, we announced our plans to divest our contract development and manufacturing organization (iBio CDMO, LLC) in order to complete its transformation into an AI-driven precision antibody discovery and development company. In conjunction with the divestment, we completed a workforce reduction of approximately 60% and discontinued CDMO operations. CDMO operations remain treated as a discontinued operation on our financial statements. Losses for Discontinued Operations for the three months ended December 31, 2023 were approximately ($3.7) million which consisted of an impairment of fixed assets ($3.1) million, interest related to the Term Note on the Facility ($0.3) million and costs to maintain the Facility ($0.3) million. Losses for Discontinued Operations for the three months ended December 31, 2022 were approximately ($23.0) million which consisted of impairments of fixed asset of approximately ($17.6) million, personnel costs including severance of ($2.9) million, inventory reserves of approximately ($0.8) million, and the remaining costs were operational.
Net Loss from Continuing Operations
Net loss from continuing operations for the three months ended December 31, 2023 was ($4.5) million, or ($2.42) per share. Net loss from continuing operations for the three months ended December 31, 2022 was approximately ($10.6) million, or ($21.54) per share.
Results of Operations - Comparison of the six months ended December 31, 2023 and 2022
Revenue
Revenue from the CDMO operations is now included in discontinued operations and not broken out separately on the financial statements. Revenue was otherwise immaterial. Our ongoing business is primarily focused on i) development of our pipeline for which we do not expect revenue and ii) on our AI-driven discovery platform. Revenue for the six months ended December 31, 2023 was related to a research licensing agreement utilizing our AI-driven discovery platform.
53
Research and Development Expenses (“R&D”)
Research and development expenses for the six months ended December 31, 2023 and 2022 were $3.1 million and $5.3 million, respectively, a decrease of approximately $2.2 million. The decrease in R&D expenses is mainly due to certain tasks and assays being performed in-house which were previously outsourced, and a decrease in spending for consultants or outside services.
General and Administrative Expenses (“G&A”)
General and administrative expenses for the six months ended December 31, 2023 and 2022 were approximately $6.5 million and $12.9 million, respectively, a decrease of $6.4 million. The decrease is primarily attributable to the reduction in personnel costs, an intangible impairment recorded in the second quarter of Fiscal 2023 that did not recur, and a decrease in spending for consultants or outside services.
Total Operating Expenses
Total operating expenses, consisting primarily of R&D and G&A expenses, for the six months ended December 31, 2023, were approximately $9.6 million, compared to approximately $18.2 million in the same period of 2022.
Discontinued Operations
On November 2, 2022, we announced our plans to divest its contract development and manufacturing organization (iBio CDMO, LLC) in order to complete its transformation into an AI-driven precision antibody discovery and development company. In conjunction with the divestment, we completed a workforce reduction of approximately 60% and discontinued CDMO operations. CDMO operations are now treated as a discontinued operation on our financial statements. Losses for Discontinued Operations for the six months ended December 31, 2023 were approximately ($4.4) million and consisted of an impairment of fixed assets ($3.1) million, interest related to the Term Note on the Facility ($0.7) million and costs to maintain the Facility ($0.6). Losses for Discontinued Operations for the six months ended December 31, 2022 were approximately ($33.6) million and consisted of an impairment of fixed assets ($17.6) million, consumables and inventory write-down of approximately ($4.9) million, personnel costs including severance of approximately ($5.8) million, interest related to the Term Note on the Facility approximately ($0.4) million and the remaining costs were operational.
Net Loss from Continuing Operations
Net loss from continuing operations for the six months ended December 31, 2023, was ($9.5) million, or ($6.24) per share. Net loss from continuing operations for the six months ended December 31, 2022, was approximately ($18.1) million or ($38.82) per share.
Uses of Cash and Funding Requirements
Net Cash Used in Operating Activities
Net cash used in operating activities was approximately ($10.0) million for the six months ended December 31, 2023. The use of cash was primarily attributable to funding our net loss for the period.
Net Cash Provided by Investing Activities
Net cash provided by investing activities of approximately $0.1 million for the six months ended December 31, 2023 was attributable to sale of fixed assets.
Net Cash Used in Financing Activities
Net cash used in financing activities during the six months ended December 31, 2023, was approximately $6.5 million and was mainly attributable to the proceeds from the sale of securities, offset by payments towards the term note made to Woodforest (see Note 13 – Debt for further details).
Funding Requirements
We have incurred significant losses and negative cash flows from operations since our spin-off from Integrated BioPharma in August 2008. As of December 31, 2023, our accumulated deficit was approximately ($302.9) million and we used approximately ($10.0) million of cash for operating activities during the six months ended December 31, 2023.
54
We plan to fund our future business operations using cash on hand, through proceeds realized in connection with the commercialization of our technologies, through proceeds from the possible sale of the CDMO entity or the Facility, through potential proceeds from the sale or out-licensing of assets, and through proceeds from the sale of additional equity or other securities. However, there can be no assurance that we will be successful in implementing these plans, many of which will take several years before we will realize proceeds. If we should default on the Credit Agreement and Woodforest does not waive the default, and if Woodforest makes a demand for the acceleration of all payments due to this default, it could result in all obligations that are guaranteed being due and payable immediately without further notice. We cannot be certain that such funding will be available on favorable terms or available at all. If we are unable to raise funds when required or on favorable terms, this assumption may no longer be operative, and we may have to: a) significantly delay, scale back, or discontinue the product application and/or commercialization of our proprietary technologies or restructure our Company including a further work force reduction; b) seek collaborators for our technology and product candidates on terms that are less favorable than might otherwise be available; c) relinquish or otherwise dispose of rights to technologies, product candidates, or products that we would otherwise seek to develop or commercialize; or d) possibly cease operations.
See Liquidity and Capital Resources above for further information.
Off-Balance Sheet Arrangements
As part of our ongoing business, we do not participate in transactions that generate relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities (“SPE”s), which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually limited purposes. As of December 31, 2023, we were not involved in any SPE transactions.
Critical Accounting Estimates
Our condensed consolidated financial statements are presented in accordance with U.S. GAAP, and all applicable U.S. GAAP accounting standards effective as of December 31, 2023, have been taken into consideration in preparing the condensed consolidated financial statements. The preparation of condensed consolidated financial statements requires estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. Some of those estimates are subjective and complex, and, consequently, actual results could differ from those estimates. We base our estimates, to the extent possible, on historical experience. Historical information is modified as appropriate based on current business factors and various assumptions that we believe are necessary to form a basis for making judgments about the carrying value of assets and liabilities. We evaluate our estimates on an ongoing basis and make changes when necessary. Actual results could differ from our estimates.
Critical accounting estimates are those estimates made in accordance with U.S. GAAP that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on our financial condition or results of operations. The following accounting estimate had a material impact on our results of operations for the three and six months ended December 31, 2023.
Impairment of Fixed Assets
We monitor fixed assets for impairment indicators throughout the year. When necessary, charges for impairments of long-lived assets are recorded for the amount by which the fair value is less than the carrying value of these assets. Changes in our business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although we base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
On November 3, 2022, we announced we are seeking to divest our contract development and manufacturing organization (iBio CDMO) in order to complete our transformation into an antibody discovery and development company. Through the process of seeking to divest its contract development and manufacturing organization, we continue to market for sale the Facility. The decision to divest triggered a quantitative impairment analysis at the end of the second quarter of Fiscal 2023 of our CDMO fixed assets, including the building in Bryan, Texas totaling $22.65 million and machinery and equipment totaling $13.4 million.
We utilized a market approach in the second quarter of Fiscal 2023, using independent third-party appraisals, including comparable assets, in addition to bids received from prospective buyers, to estimate the fair value of the Facility, the machinery and equipment. We recorded an impairment charge of $6.3 million for the Facility and $11.3 million for the machinery and equipment in the quarter ended December 31, 2022. The key assumption in the valuation analysis was the expected sale price of $21.1 million for the Facility and the associated machinery and equipment less approximate costs to sell of $2.7 million. In the first quarter of Fiscal 2024, we entered into an agreement for the sale of the Facility for $17.25 million, and an additional impairment of $0.3 million was recorded as of June 30, 2023 to reflect the agreed upon sales price of $17.25 million less estimated costs to sell. The CDMO Equipment was sold during Fiscal 2023.
55
On November 7, 2023, we received written notice terminating the agreement for the sale of the Facility. Upon receiving the termination notice, we reassessed the CDMO fixed assets for impairment which included obtaining appraisal values as of November 9, 2023. We utilized a market approach, using an independent third-party appraisal, including comparable assets, in addition to bids received from prospective buyers, to estimate the fair value of the Facility and concluded that fair value of the assets approximated their carrying value and no further impairment was required at that time.
During the second quarter of Fiscal 2024, we made the decision to auction the Facility. We utilized a market approach, using an independent third-party appraisal, including comparable assets, to estimate the fair value of the Facility. An additional $3.1 million fixed asset impairment was recorded the second quarter of Fiscal 2024 in discontinued operations to write down the carrying value of the Facility to its fair value. The key assumption in the valuation analysis is the expected sale price of $13.8 million for the Facility less approximate costs to sell of $0.7 million. We may have to record a further, potentially material, impairment to the fair value of the Facility if we do not realize a sale transaction for the expected sales price.
Impairment of Indefinite-Lived Intangible Assets
For indefinite life intangible assets, we perform an impairment test annually and whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable.
Evaluating for impairment requires judgment, including the estimation of future cash flows, future growth rates and profitability and the expected life over which cash flows will occur. Changes in our business strategy or adverse changes in market conditions could impact impairment analyses and require the recognition of an impairment charge. Although we base our estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ from these estimates.
We tested for impairment of the IBIO-101 therapeutic technology (or “IP”), classified as an indefinite-lived intangible asset, which had a carrying amount of $5 million at December 31, 2023. The key impairment trigger was the decline in the Common Stock price during the second quarter of Fiscal 2024. We engaged a third party to perform valuation assistance with estimating the fair value of IBIO-101 and preparing a market capitalization reconciliation. The Multi-Period Excess Earnings Method (“MPEEM”) under the income approach was utilized to value the indefinite-lived asset. The MPEEM determines the value of a specified asset by calculating the present value of future earnings attributed to the asset. Since IBIO-101 is currently in its pre-clinical development phase, a probability of success was applied to the cash flows to account for the probability of reaching each step of development. The MPEEM requires that charges for the use of other contributory assets be subtracted under the theory that the owner of the subject asset does not own the other contributory assets and would have to rent/lease them in order to earn the cash flows related to the subject asset.
The resulting probability of success adjusted “excess earnings” were discounted to the present value using a 14.5% discount rate, which was based on iBio’s weighted-average cost of capital. The sum of the discounted excess earnings and the present value of the tax benefit related to amortization of the IBIO-101 indefinite-lived intangible indicated that the fair value was $6.5 million as of the December 31, 2023, valuation date. Given that the carrying amount of the asset was $5 million at December 31, 2023, it was concluded that no impairment existed.
We will continue to monitor the value of the IP, since we believe it is at risk for impairment. The primary impairment indicators that may arise in the near future are (1) any sustained decline in our common stock market price and (2) FDA decisions on similar competing technologies that are applying for Phase 1 approval.
We continue to operate in a highly competitive environment, rising interest rates (and cost of capital) and experience liquidity challenges. Accordingly, we may have to adjust our cash flow projections and valuation assumptions in the near future to account for market trends and any changes to our research and development plans. Any such future adjustments may lead to material future impairments in the IP and other related assets.
Our remaining critical accounting estimates remain consistent with the information disclosed in the same section in our last annual report on Form 10-K for the year ended June 30, 2023.
In addition to the aforementioned critical accounting estimates, the following accounting policies and estimates have been highlighted as significant because changes to certain judgments and assumptions inherent in these policies could affect our consolidated financial statements:
| • | revenue recognition; |
| • | legal and contractual contingencies; |
| • | research and development expenses; and |
| • | share-based compensation expenses. |
56
We base our estimates, to the extent possible, on historical experience. Historical information is modified as appropriate based on current business factors and various assumptions that we believe are necessary to form a basis for making judgments about the carrying value of assets and liabilities. We evaluate our estimates on an ongoing basis and make changes when necessary. Actual results could differ from our estimates. See Note 4 – Summary of Significant Accounting Policies - for a complete discussion of our significant accounting policies and estimates.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
As a smaller reporting company, we are not required to provide the information required by this Item 3.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, under the direction of our Chief Executive Officer (our Principal Executive Officer) and Chief Financial Officer (our Principal Financial Officer) have evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15I and 15d-15(e) under the Exchange Act, as amended, as of December 31, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to our management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Our disclosure controls and procedures are also designed to ensure that such information is accumulated and communicated to management to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Based on our evaluation, our Principal Executive Officer and Principal Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2023.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act, during the quarter ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
We are not currently subject to any material legal proceedings. From time to time, we may be subject to various legal proceedings and claims that arise in the ordinary course of its business activities. Litigation, regardless of the outcome, could have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 1A. Risk Factors
Our business is subject to risks and events that, if they occur, could adversely affect our financial condition and results of operations and the trading price of our securities. The following information updates, and should be read in conjunction with, the information disclosed in Part I, Item 1A, “Risk Factors,” contained in the Annual Report. Except as described below, our risk factors as of the date of this Report have not changed materially from those described in “Part I, Item 1A. Risk Factors” of our Annual Report.
We are reviewing potential options to extend our cash runway. This review could impact our future operations and financial position.
We are currently evaluating a number of potential options to expand our cash runway, the implementation of which will impact our liquidity. In an effort to improve liquidity and our runway, we have placed our CDMO business and Facility on the market for sale, reduced our work force and ceased operations of our CDMO, thereby reducing annual spend on expenses by approximately 67% and
57
generating cash savings of approximately 64% from first quarter Fiscal year 2023 compared to the fourth quarter Fiscal year 2023. Potential options being considered to further increase liquidity include focusing product development on a select number of product candidates, the sale or out-licensing of certain product candidates, raising money from the capital markets, grant revenue or collaborations, or a combination thereof. However, we anticipate that our expenses will increase as we continue our research and development activities and conduct clinical trials.
Our cash, cash equivalents and restricted cash of $4.1 million as of December 31, 2023, is not anticipated to be sufficient to support our operations beyond the third quarter of Fiscal 2024 unless we reduce our burn rate further, sublease all or a portion of the San Diego Lease, sell the Facility for amounts above its term note payable, or raise additional capital. Regardless of whether we are able to reduce our burn rate or sell or out-license certain assets or parts of the business, we will need to raise additional capital in order to fully execute our near and long-term business plans. In fact, our current cash, cash equivalents and restricted cash as of December 31, 2023, is not anticipated to be sufficient to support operations through the third quarter of Fiscal 2024.
There can be no assurance that we would be able to sell the Facility or that if we are able to do so that we do so on favorable terms or that we will be able to do so before the maturity date of the Term Loan or that the exploration of potential options will result in any agreements or transactions, or that, if completed, any agreements or transactions will be successful or on attractive terms. If we determine to change our business strategy, our future business, prospects, financial position and operating results could be significantly different than those in historical periods or projected by our management. Because of the significant uncertainty regarding our future plans, we are not able to accurately predict the impact of a potential change in our business strategy and future funding requirements.
Our historical operating results indicate substantial doubt exists related to our ability to operate as a going concern.
We have incurred net losses and used significant cash in operating activities since inception, and we expect to continue to generate operating losses for the foreseeable future. As of December 31, 2023, we have an accumulated deficit of $302.9 million. As a result of our continued losses, our cash resources have not been sufficient to sustain our operations, and we have continued to depend on financing transactions to generate sufficient cash to stay in operation. We are currently incurring negative operating cash flows of approximately $1.6 million per month.
We held cash, cash equivalents and restricted cash of $4.1 million as of December 31, 2023. Based on current trends and activities, there is significant doubt that we can continue as a going concern beyond the third quarter of Fiscal 2024. We are currently evaluating a number of potential options to expand our cash runway, the implementation of which will impact our liquidity. Potential options being considered to increase liquidity include focusing product development on a select number of product candidates, the possible sale of the Facility, the sale or out-licensing of certain product candidates or parts of the business, raising money from capital markets, grant revenue or collaborations, or a combination thereof. Regardless of whether we are able to reduce our burn rate or sell or out-license certain assets or parts of the business, we will need to raise additional capital in order to fully execute our longer-term business plan. We believe based on input from expert advisors, that it is likely we will be able to implement one or more options that will extend our cash runway for 12 months or more from the date of the filing of this Report. However, there can be no assurance that we will be successful in implementing any of the options that we are evaluating.
Our condensed consolidated financial statements as of and for the year ended December 31, 2023 have been prepared under the assumption that we will continue as a going concern for the next 12 months. Our management concluded that our recurring losses from operations and the fact that we have not generated significant revenue or positive cash flows from operations raise substantial doubt about our ability to continue as a going concern for the next 12 months. In addition, the Term Loan with Woodforest, with an outstanding principal balance of which is $12,654,867 as of February 9, 2024, matures on March 29, 2024 and unless the Property is sold prior to the Term Loan maturity date at a price that will allow us to repay the outstanding amounts owed under the Term Loan in full we will not have sufficient funds to pay the Term Loan when due. In addition, if a sale of the Facility is not consummated at a price at or above the outstanding balance under the Term Loan we do not expect to have sufficient funds to repay the Term Loan upon its maturity. There can be no assurance that we will be able to sell the Facility or that we will be able to sell the Facility for an amount that will allow us to repay all outstanding amounts under the Term Loan. Our auditors also included an explanatory paragraph in its report on our financial statements as of and for the year ended June 30, 2023 with respect to this uncertainty. If we continue to experience operating losses, and we are not able to generate additional liquidity through a capital raise or other cash infusion, we might need to secure additional sources of funds, which may or may not be available to us. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to further scale back or discontinue the development of our product candidates or other research and development initiatives, restructure our Company including a further work force reduction, or initiate steps to cease operations or liquidate our assets.
Unless and until we are able to raise sufficient capital, for which there can be no assurance, our lack of cash will continue to constrain our business and subject us to significant risks, including the following. First, we may be unable to make the necessary investment in personnel, equipment or other resources to effectively pursue our business plan. Second, our suppliers, vendors and service providers could slow down or stop supplying components or services or could stop extending credit in connection with commercial transactions,
58
which could curtail our business. Third, we may be subject to lawsuits from claimants relating to past due balances, if we cannot work out or continue to renegotiate payment terms. There is no assurance that we will be able to successfully defend against such claims, and our creditors or claimants may seek to seize our assets or assert other judicial remedies. Ultimately, any or all of the above factors could lead to a possible reduction or suspension of our operations, ultimately forcing us to declare bankruptcy, reorganize or go out of business. Should this occur, the value of any investment in our securities could be adversely affected, and an investor would likely lose all or a significant portion of their investment.
We have incurred significant losses since our inception. We expect to incur losses during our next Fiscal year, we do not anticipate generating significant revenue for several years and may never achieve or maintain profitability.
Since our 2008 spinoff from Integrated BioPharma, we have incurred operating losses and negative cash flows from operations. Our comprehensive net loss was approximately ($8.2) million and ($33.6) million for the three months ended December 31, 2023 and 2022, respectively. Our comprehensive net loss was approximately ($14.0) million and ($51.7) million for the six months ended December 31, 2023 and 2022, respectively. Our comprehensive net loss was approximately ($64.8) million and ($50.5) million for the years ended June 30, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of approximately ($302.9) million.
To date, we have financed our operations primarily through the sale of common stock, preferred stock and warrants. We are devoting substantially all of our efforts to research and development, including the development and validation of our technologies, and the development of a proprietary therapeutic products against oncology. We have not completed development of or commercialized any vaccine or therapeutic product candidates. We expect to continue to incur significant expenses and may incur operating losses for at least the next year. We anticipate that our expenses and losses will increase substantially if we:
| ● | initiate clinical trials of our product candidates; |
| ● | continue the research and development of our product candidates; |
| ● | seek to discover or license in additional candidates; and |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development and manufacturing efforts. |
Our future profitability and cash flow in large part depends on our research and development programs, including our AI platform, and our ability to successfully develop, partner or commercialize our product candidates and to a lesser extent, which is not anticipated for several years. Our cash position is expected to limit the number of product candidates that we seek to develop. This will require us, alone or with our licensees and collaborators, to be successful in a range of challenging activities, including completing preclinical testing and clinical trials of our product candidates, obtaining regulatory approval for these product candidates and manufacturing, marketing and selling those products for which regulatory approval is obtained or establishing collaborations with parties willing and able to provide necessary capital or other value. We may never succeed in these activities. We may never generate revenues that are significant or large enough to achieve profitability.
All of our existing product candidates are in various stages of development and will require extensive additional clinical evaluation, regulatory review and approval, significant marketing efforts and substantial investment before they could provide us with any revenue. As a result, even if we successfully develop, achieve regulatory approval and commercialize our products, we may be unable to generate revenue for many years, if at all. We do not anticipate that we will generate revenue from product sales for at least several years, if at all. If we are unable to generate revenue from product sales, we will not become profitable, and we may be unable to continue our operations.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would diminish the value of our company and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
Changes in general economic conditions, geopolitical conditions, domestic and foreign trade policies, monetary policies and other factors beyond our control may adversely impact our business and operating results.
The uncertain financial markets, disruptions in supply chains, mobility restraints, and changing priorities as well as volatile asset values could impact our business in the future. We and our third-party contract manufacturers, contract research organizations, and any clinical sites that may conduct our clinical trials in the future may also face disruptions in procuring items that are essential to our research and development activities, including, for example, medical and laboratory supplies used in our clinical trials or preclinical studies, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the outbreak. These minor disruptions have had an immaterial effect on business, which we have been able to address with minimal impact to our business operations to date. Further, although we have not experienced any material adverse effects on our business due to
59
increasing inflation, it has raised operating costs for many businesses and, in the future, could impact demand or pricing manufacturing of our drug candidates or services providers, foreign exchange rates or employee wages. We are actively monitoring the effects these disruptions and increasing inflation could have on our operations.
Our operations and performance depend on global, regional and U.S. economic and geopolitical conditions. In addition, the global macroeconomic environment could be negatively affected by, among other things, pandemics or epidemics, instability in global economic markets, increased U.S. trade tariffs and trade disputes with other countries, instability in the global credit markets, supply chain weaknesses, instability in the geopolitical environment as a result of the withdrawal of the United Kingdom from the European Union, the Russian invasion of Ukraine, the war in the Middle East and other political tensions, and foreign governmental debt concerns. Such challenges have caused, and may continue to cause, uncertainty and instability in local economies and in global financial markets.
The above factors, including a number of other economic and geopolitical factors both in the U.S. and abroad, could ultimately have material adverse effects on our business, financial condition, results of operations or cash flows, including the following:
| ● | effects of significant changes in economic, monetary and fiscal policies in the U.S. and abroad including currency fluctuations, inflationary pressures and significant income tax changes; |
| ● | supply chain disruptions; |
| ● | a global or regional economic slowdown in any of our market segments; |
| ● | changes in government policies and regulations affecting the Company or its significant customers; |
| ● | industrial policies in various countries that favor domestic industries over multinationals or that restrict foreign companies altogether; |
| ● | new or stricter trade policies and tariffs enacted by countries, such as China, in response to changes in U.S. trade policies and tariffs; |
| ● | postponement of spending, in response to tighter credit, financial market volatility and other factors; |
| ● | rapid material escalation of the cost of regulatory compliance and litigation; |
| ● | difficulties protecting intellectual property; |
| ● | longer payment cycles; |
| ● | credit risks and other challenges in collecting accounts receivable; and |
| ● | the impact of each of the foregoing on outsourcing and procurement arrangements. |
If the Property is not sold prior to the March 29, 2024 Term Loan maturity date, iBio CDMO will not have sufficient funding to pay the Term Loan with Woodforest for which we are a guarantor.
Although we have listed the Property for sale, we do not currently have a buyer for the Property. On November 7, 2023, we received written notice from Majestic Realty of its election to terminate the Purchase and Sale Agreement, dated as of September 15, 2023, between Majestic Realty and iBio CDMO LLC, pursuant to which iBio CDMO had agreed to sell to Majestic Realty the Property. There can be no assurance that we will find another buyer for the Property or that the sale of the Property will be completed in a timely manner. If the Property is not sold prior to the March 29, 2024 maturity date of the Term Loan, we will not have sufficient funds to repay the Term Loan on its maturity date, the outstanding principal balance of which is $12,654,867 as of February 9, 2024. In addition, if a sale of the Facility is not consummated at a price at or above the outstanding balance under the Term Loan we do not expect to have sufficient funds to repay the Term Loan upon its maturity. Our failure to make such payments when due could result in our loss of the Facility. Any action to proceed against our assets would likely have a serious disruptive effect on our business operations, especially if the Facility or our other assets were foreclosed upon or our guarantee were enforced.
Failure to sell the Facility could negatively impact our stock price and our future business and financial results.
If we do not sell the Facility for any reason, our ongoing business may be materially and adversely affected and we would be subject to a number of risks, including experiencing negative reactions from the financial markets, and negative impacts on the trading price of our common stock, which could affect our ability to secure sufficient financing in the future on attractive terms (or at all). In addition, we could be subject to litigation related to any failure to complete the sale. In addition, if a sale of the Facility is not consummated at a price at or above the outstanding balance under the Term Loan we do not expect to have sufficient funds to repay the Term Loan upon its maturity.
The failure to comply with the terms of the Credit Agreement, as amended, could result in a default under the terms of the Credit Agreement, as amended, and, if uncured, it could potentially result in action against our pledged assets.
There is no assurance that we will generate sufficient revenue or raise sufficient capital to be able to make the required principal payment under the Term Loan that iBio CDMO entered into with Woodforest. The Term Loan with Woodforest is secured by (a) a leasehold
60
deed of trust on our Facility, and (b) a first lien on all assets of iBio CDMO including the Facility. We have also guaranteed the payment of all iBio CDMO’s obligations under the Credit Agreement. The Term Loan matures the earlier of March 29, 2024, or the acceleration of maturity of the Term Loan pursuant to the Credit Agreement. If we or iBio CDMO fail to comply with the terms of the Term Loan and/or the related agreements, including the affirmative and negative covenants contained therein, Woodforest National Bank could declare a default and if the default were to remain uncured, Woodforest National Bank would have the right to proceed against any or all of the collateral securing their Term Loan. Our failure to make such payments when due could result in our loss of the Facility. In addition, we have guaranteed the repayment of the Term Loan and could be responsible for such payment. Any action to proceed against our assets would likely have a serious disruptive effect on our business operations, especially if the Facility or our other assets were foreclosed upon.
The Credit Agreement, as amended, requires that we pay a significant amount of cash to the lender. Our ability to generate sufficient cash to make all required payments under the Credit Agreement, as amended, depends on many factors beyond our control.
Our ability to make payments on and to refinance the Term Loan, to fund planned capital expenditures and to maintain sufficient working capital depends on our ability to raise capital and generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. We cannot assure you that our business will generate sufficient cash flow from operations or from other sources in an amount sufficient to enable us to service our debt or to fund our other liquidity needs. To date, we have generated minimal revenue and have financed a significant portion our capital needs from sales of our equity and most recently the Term Loan. There can be no assurance that financing options will be available to us when needed to make payments under the Term Loan or if available, that they will be on favorable terms. If our cash flow and capital resources are insufficient to allow us to make payments due under the Term Loan, we may need to seek additional capital or restructure or refinance all or a portion of the Term Loan on or before the maturity thereof, any of which could have a material adverse effect on our business, financial condition or results of operations. Although we plan to explore potential longer-term financing options for our Facility, including, but not limited to, the possible sale of the Facility, we cannot assure you that we will be able to enter consummate the sale prior to the maturity date of the Term Loan or refinance the Term Loan on commercially reasonable terms or at all. If we are unable to generate sufficient cash flow to repay or refinance our debt on favorable terms, it could significantly adversely affect our financial condition. Our ability to restructure or refinance the Term Loan will depend on the condition of the capital markets and our financial condition. Any refinancing of the Term Loan could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. There can be no assurance that we will be able to obtain any financing when needed.
Covenant restrictions in the Credit Agreement, as amended, may limit our ability to operate our business.
The Credit Agreement contains, and our future indebtedness agreements may contain covenants that restrict our ability to finance future operations or capital needs or to engage in other business activities. The Credit Agreement, as amended, restricts our ability to:
| ● | incur, assume or guarantee additional Debt (as defined in the Credit Agreement); |
| ● | repurchase capital stock; |
| ● | make other restricted payments including, without limitation, paying dividends and making investments; |
| ● | sell or otherwise dispose of assets. |
As of the date of this filing, iBio is in compliance with this covenant in the Credit Agreement, as amended.
Item 5. Other Information
During the three months ended December 31, 2023, no director or officer of the Company
61
Item 6. Exhibits.
Exhibit No. |
| Description |
1.1 | ||
3.1 | ||
3.2 |
| |
3.3 | ||
3.4 | ||
3.5 | ||
3.6 | ||
3.7 | ||
3.8 | ||
3.9 | ||
3.10 | ||
4.1 | ||
4.2 | ||
4.3 | ||
4.4 | ||
10.5 | ||
10.6 | ||
10.7 | ||
10.8 | ||
10.9 | ||
10.10 | ||
10.11 | ||
10.12 |
62
31.1* |
| |
31.2* |
| |
32.1* |
| |
32.2* | ||
101.INS |
| Inline XBRL Instance* |
101.SCH |
| Inline XBRL Taxonomy Extension Schema* |
101.CAL |
| Inline XBRL Taxonomy Extension Calculation* |
101.DEF |
| Inline XBRL Taxonomy Extension Definition* |
101.LAB |
| Inline XBRL Taxonomy Extension Labeled* |
101.PRE |
| Inline XBRL Taxonomy Extension Presentation* |
104 | Cover page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| iBio, Inc. |
| (Registrant) |
|
|
Date: February 9, 2024 | /s/ Martin Brenner |
| Martin Brenner |
| Chief Executive Officer and Chief Scientific Officer |
| |
Date: February 9, 2024 | /s/ Felipe Duran |
| Felipe Duran |
| Chief Financial Officer |
| Principal Financial Officer and Principal Accounting Officer |
63