UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
OR
For the fiscal year ended
OR
For the transition period from to .
OR
Date of event requiring this shell company report
Commission
file number:
(Exact name of Registrant as specified in its charter)
The
(Jurisdiction of incorporation or organization)
+49
(3641) 508 180
(Address of principal executive offices)
Chief Financial Officer
Tel:
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Copies to:
Sophia Hudson
Kirkland & Ellis LLP
601 Lexington Avenue
New York, NY 10022
Phone: +1 (212) 446-4750
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
The
number of outstanding ordinary shares as of December 31, 2023 was
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes
☐
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes
☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically, if any, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer”, “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer ☐ | Non-accelerated Filer ☐ | ||
| Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
| † | The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012. |
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
☐ U.S. GAAP
☒
☐ Other
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes
☐ No
InflaRx N.V.
Table of Contents
i
ii
iii
Unless otherwise indicated or the context otherwise requires, all references in this Annual Report on Form 20-F, or this Annual Report, to “InflaRx N.V.,” “InflaRx,” the “Company,” “we,” “our,” “ours,” “us” or similar terms refer to InflaRx N.V. and its subsidiaries.
Presentation of Financial Statements
We report in Euros under International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or the IASB. We made rounding adjustments to some of the figures included in this Annual Report. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that preceded them.
In this Annual Report, unless otherwise indicated, translations from U.S. dollars to Euros (and vice versa) relating to payments made on or before December 31, 2023, were made at the rate in effect at the time of the relevant payment.
The terms “$” or “dollar” refer to U.S. dollars, and the terms “€” or “Euro” refer to the currency introduced at the start of the third stage of European economic and monetary union pursuant to the treaty establishing the European Community, as amended.
Industry and Other Data
We obtained the industry, statistical and market data in this Annual Report from our own internal estimates and research as well as from industry and general publications and research, surveys and studies conducted by third parties. All of the market data used in this Annual Report involves a number of assumptions and limitations. While we believe that the information from these industry publications, surveys and studies is reliable, the industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of important factors, including those described in the section titled “ITEM 3. KEY INFORMATION — C. Risk factors.” These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
Trademarks
InflaRx®, GOHIBIC® and Vilwaysi® are our trademarks. The trademarks, trade names and service marks appearing in this Annual Report are property of their respective owners. Solely for convenience, the trademarks and trade names in this Annual Report are referred to without the symbols ® and ™, but such references should not be construed as any indication that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
FORWARD-LOOKING STATEMENTS
This Annual Report contains forward-looking statements that involve substantial risks and uncertainties. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions intended to identify statements about the future. These statements speak only as of the date of this Annual Report and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements include, without limitation, statements about the following:
| ● | our ability to successfully commercialize and the receptiveness of GOHIBIC (vilobelimab) as a treatment for COVID-19 patients by U.S. hospitals, our ability to positively influence treatment recommendations by medical/healthcare institutes, guideline bodies and other third-party organizations; |
| ● | our expectations regarding the size of the patient populations for, market opportunity for, coverage and reimbursement for, estimated returns and return accruals for, and clinical utility of GOHIBIC (vilobelimab) in its approved or authorized indication or for vilobelimab and any other product candidates, under an Emergency Use Authorization, or the EUA, and in the future if approved for commercial use in the United States or elsewhere; |
iv
| ● | our ability to successfully implement The InflaRx Commitment Program, the success of our future clinical trials for vilobelimab’s treatment of COVID-19 and other debilitating or life-threatening inflammatory indications, including pyoderma gangrenosum, and any other product candidates, including INF904, and whether such clinical results will reflect results seen in previously conducted pre-clinical studies and clinical trials; |
| ● | the timing, progress and results of preclinical studies and clinical trials of vilobelimab, INF904 and any other product candidates, including for the development of vilobelimab in several indications, including to treat PG, and statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, the costs of such trials and our research and development programs generally; |
| ● | our interactions with and the receptiveness and approval by regulators regarding the results of clinical trials and potential regulatory approval or authorization pathways including related to our Marketing Authorization Application, or MAA, submission for vilobelimab and our biologics license application submission, or BLA, for GOHIBIC (vilobelimab); the timing and outcome of any discussions or submission of filings for regulatory approval or authorization of vilobelimab, INF904 or any other product candidate, and the timing of and our ability to obtain and maintain full regulatory approval or the EUA, of vilobelimab or GOHIBIC (vilobelimab) for any indication; our ability to leverage our proprietary anti-C5a and anti-C5aR technologies to discover and develop therapies to treat complement-mediated autoimmune and inflammatory diseases; |
| ● | our ability to protect, maintain and enforce our intellectual property protection for vilobelimab, INF904 and any other product candidates, and the scope of such protection; |
| ● | whether the U.S. Food and Drug Administration, or the FDA, European Medicines Agency, or the EMA, or any comparable foreign regulatory authority will accept or agree with the number, design, size, conduct or implementation of our clinical trials, including any proposed primary or secondary endpoints for such trials; |
| ● | the success of our future clinical trials for vilobelimab, INF904 and any other product candidates and whether such clinical results will reflect results seen in previously conducted preclinical studies and clinical trials; |
| ● | our expectations regarding the size of the patient populations for, the market opportunity for, the medical need for and clinical utility of vilobelimab, INF904 or any other product candidates, if approved or authorized for commercial use; |
| ● | our manufacturing capabilities and strategy, including the scalability and cost of our manufacturing methods and processes and the optimization of our manufacturing methods and processes, and our ability to continue to rely on our existing third-party manufacturers and our ability to engage additional third-party manufacturers for our planned future clinical trials and for commercial supply of vilobelimab and for the finished product GOHIBIC (vilobelimab); |
| ● | our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional financing; |
| ● | our expectations regarding the scope of any approved indication for vilobelimab; |
| ● | our ability to defend against liability claims resulting from the testing of our product candidates in the clinic or, if, approved or authorized, any commercial sales; |
| ● | if any of our product candidates obtain regulatory approval or authorization, our ability to comply with and satisfy ongoing drug regulatory obligations and continued regulatory overview; |
| ● | our ability to comply with enacted and future legislation in seeking marketing approval or authorization and commercialization; |
| ● | our future growth and ability to compete, which depends on our retaining key personnel and recruiting additional qualified personnel; and |
| ● | our competitive position and the development of and projections relating to our competitors in the development of C5a and C5aR inhibitors and other therapeutic products being developed in similar medical conditions in which vilobelimab, INF904 or any other of our product candidates is being developed or our industry. |
v
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. You should refer to the “ITEM 3. KEY INFORMATION: — 3. Risk factors.” section of this Annual Report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. You should, however, review the factors and risks and other information we describe in the reports we will file from time to time with the Securities and Exchange Commission, or the SEC, after the date of this Annual Report.
ENFORCEMENT OF JUDGMENTS
We are a public company with limited liability (naamloze vennootschap) incorporated under the laws of the Netherlands and our headquarters is located in Germany. Substantially all of our assets are located outside the United States. The majority of our executive officers and directors reside outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the federal securities laws of the United States.
The United States and the Netherlands currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent jurisdiction in the Netherlands.
This court will have discretion to attach such weight to the judgment rendered by the relevant U.S. court as it deems appropriate. Under current practice, the courts of the Netherlands may be expected to render a judgment in accordance with the judgment of the relevant foreign court, provided that such judgment (i) is a final judgment and has been rendered by a court which has established its jurisdiction vis-à-vis the relevant Dutch companies or Dutch company, as the case may be, on the basis of internationally accepted grounds of jurisdiction, (ii) has not been rendered in violation with the principles of proper procedure (behoorlijke rechtspleging), (iii) is not contrary to the public policy of the Netherlands, and (iv) is not incompatible with (a) a prior judgment of a Dutch court rendered in a dispute between the same parties, or (b) a prior judgment of a foreign court rendered in a dispute between the same parties, concerning the same subject matter and based on the same cause of action, provided that such prior judgment is recognizable in the Netherlands. Dutch courts may deny the recognition and enforcement of punitive damages or other awards. Moreover, a Dutch court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages. Enforcement and recognition of judgments of U.S. courts in the Netherlands are solely governed by the provisions of the Dutch Civil Procedure Code. If no leave to enforce is granted, claimants must litigate the claim again before a Dutch competent court.
Dutch civil procedure differs substantially from U.S. civil procedure in a number of respects. Insofar as the production of evidence is concerned, U.S. law and the laws of several other jurisdictions based on common law provide for pre-trial discovery, a process by which parties to the proceedings may prior to trial compel the production of documents by adverse or third parties and the deposition of witnesses. Evidence obtained in this manner may be decisive in the outcome of any proceeding. No such pre-trial discovery process exists under Dutch law.
The United States and Germany currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, in civil and commercial matters. Consequently, a final judgment for payment or declaratory judgments given by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in Germany. German courts may deny the recognition and enforcement of a judgment rendered by a U.S. court if they consider the U.S. court not to be competent or the decision to be in violation of German public policy principles. For example, judgments awarding punitive damages are generally not enforceable in Germany. A German court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages.
In addition, actions brought in a German court against us, our directors, our senior management and the experts named herein to enforce liabilities based on U.S. federal securities laws may be subject to certain restrictions. In particular, German courts generally do not award punitive damages. Litigation in Germany is also subject to rules of procedure that differ from U.S. rules, including with respect to the taking and admissibility of evidence, the conduct of the proceedings and the allocation of costs. German procedural law does not provide for pre-trial discovery of documents, nor does Germany support pre-trial discovery of documents under the 1970 Hague Evidence Convention. Proceedings in Germany would have to be conducted in the German language and all documents submitted to the court would, in principle, have to be translated into German. For these reasons, it may be difficult for a U.S. investor to bring an original action in a German court predicated upon the civil liability provisions of U.S. federal securities laws against us, our directors, our senior management and the experts named in this Annual Report.
vi
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
| A. | Directors and senior management |
Not applicable.
| B. | Advisers |
Not applicable.
| C. | Auditors |
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
| A. | Offer statistics |
Not applicable.
| B. | Method and expected timetable |
Not applicable.
ITEM 3. KEY INFORMATION
| A. | Capitalization and indebtedness |
Not applicable.
| B. | Reasons for the offer and use of proceeds |
Not applicable.
-1-
| C. | Risk factors |
You should carefully consider the risks and uncertainties described below and the other information in this Annual Report before making an investment in our ordinary shares. Our business, financial condition or results of operations could be materially and adversely affected if any of these risks occurs, and as a result, the market price of our ordinary shares could decline, and you could lose all or part of your investment. This Annual Report also contains forward-looking statements that involve risks and uncertainties. See “Forward-Looking Statements.” Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain factors.
Risk factor summary
The following is a summary of the principal risks that could adversely affect our business, operations and financial results. This summary does not address all of the risks that we face. For a more complete discussion of the material risks facing our business, see further below.
Risks related to our financial position and need for additional capital
| ● | Risk of never achieving or maintaining profitability and of investors losing their entire investment Risk related to obtaining additional funding and risk of delay, reduction or elimination of product discovery and development programs or commercialization efforts |
| ● | Risks related to limited operating history and history of commercializing pharmaceutical products Risk related to grants funded by the German federal government |
Risks related to the discovery, development, manufacturing and commercialization of our product candidates
| ● | Risk related to the discovery, development and commercialization of our product candidates |
| ● | Risk related to our dependence on the success of our product candidates, including our lead product candidate, vilobelimab |
| ● | Risk related to regulatory oversight over GOHIBIC (vilobelimab) for which we received the EUA, which may lead to a withdrawal or revocation of the granted the EUA |
| ● | Risk related to possible occurrence of clinical failure at any stage of clinical development |
| ● | Risk of failing to maintain compliance with FDA requirements and/or remain in alignment with FDA feedback, which may prevent or delay the development, marketing or manufacturing of vilobelimab for the treatment of critically ill COVID-19 patients and, potentially, of vilobelimab in ulcerative PG |
| ● | Risk of incurring additional significant expenses in connection with ongoing regulatory obligations and continued regulatory review of GOHIBIC (vilobelimab) and any other product candidates for which we receive approval or the EUA |
| ● | Risk of incurring additional costs or experience delays in completing, or ultimately being unable to complete, the development and commercialization of product candidates, if clinical trials of our product candidates fail to satisfactorily demonstrate safety and efficacy to the FDA and other regulators |
| ● | Risk if our product candidates cause or are perceived to cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any |
-2-
Risks related to our dependence on third parties
| ● | Risk of reliance on third parties to conduct our clinical trials |
| ● | Risk of dependence on third-party manufacturers and suppliers and maintaining key manufacturing relationships |
| ● | Risk related to the process of manufacturing biologics, such as vilobelimab, that is extremely susceptible to product loss |
| ● | Risk regarding the manufacturing process due to product risk and quality controls Risk that, if our third-party manufacturers are unable to increase the scale of their production of our product candidates and increase their product yield, our manufacturing costs may increase and product commercialization may be delayed |
| ● | Risk that, if we are unable to establish collaborations on commercially reasonable terms, we may have to alter our development and commercialization plans |
Risks related to our intellectual property
| ● | Risks related to our dependence on our ability to obtain, maintain, protect, defend and enforce patent, trade secret and other intellectual property protection |
| ● | Risks related to our patents covering our proprietary anti-C5a and anti C5aR technologies that may be subject to challenge, narrowing, circumvention and invalidation by third parties |
| ● | Risks related to uncertainty that we were the first to make the anti-C5a and anti-C5aR technologies claimed in our patents or patent applications or that we were the first to file for patent protection |
| ● | Risks related to patent application process and obtaining patents for which we have applied |
Risks related to employee matters and managing growth
| ● | Risk of having a limited number of employees to manage and operate our business |
| ● | Risk of depending heavily on certain of our executive officers and directors |
| ● | Risk of disruption to our business as a result of managing growth in business operations and number of personnel |
| ● | Risk of liability to our business by improper activities of our employees and third-party contractors |
Risks related to our ordinary shares and our status as a public company
| ● | Risks rated to the trading price of our ordinary shares, that has been and may in the future be highly volatile |
| ● | Risk associated with being a foreign private issuer and not being subject to U.S. proxy rules, following home country governance practices rather than the Nasdaq listing requirements | |
| Risk related to us not anticipating paying any cash dividends on our share capital in the foreseeable future |
General risk factors
| ● | Risk of business impact resulting out of financial markets, changes to political and regulatory policies and economic conditions generally |
| ● | Risk of legal, regulatory or market measures to address environmental objectives |
| ● | Risks of dilution to shareholders through raising capital, risk of restriction and/or relinquishment to rights to technologies and product candidates |
| ● | Risk of facing substantial competition |
| ● | Risk of product liability lawsuits |
| ● | Risks of damage and disruption to our business through cyber-attacks and failure of telecommunication and information technology equipment |
-3-
Risks related to our financial position and need for additional capital
We have a history of significant operating losses and expect to incur significant and increasing losses for the foreseeable future; we may never achieve or maintain profitability and investors may lose their entire investment
We incurred net losses of €42.7 million, €29.5 million and €45.6 million for the years ended December 31, 2023, 2022 and 2021, respectively. In addition, our accumulated deficit as of December 31, 2023, was €286.1 million.
We expect our net losses to increase as we advance vilobelimab and other product candidates into additional clinical trials, as well as larger and later-stage clinical trials. Further, we expect our net losses to increase as we advance the implementation of commercialization of GOHIBIC (vilobelimab) in the United States under the EUA while investing in start-up commercialization and marketing activities. To date, we have not commercialized any products other than for GOHIBIC (vilobelimab) for the treatment of severe COVID-19 or generated any meaningful revenues from the sale of products other than limited initial sales of GOHIBIC (vilobelimab) and absent the realization of sufficient revenues from product sales, we may never attain profitability. We have devoted substantially all of our financial resources and efforts to research and development, including preclinical studies, clinical trials and manufacturing development. Our net losses may fluctuate significantly from quarter to quarter and year to year. Net losses and negative cash flows have had, and will continue to have, an adverse effect on our shareholders’ equity and working capital.
We anticipate that our expenses might increase if and as we:
| ● | continue to develop and conduct clinical trials with respect to our lead product candidate, vilobelimab; |
| ● | continue research, preclinical and clinical development efforts for any future product candidates, including IFX002 and INF904; |
| ● | actively seek to identify additional research programs and additional product candidates; |
| ● | seek regulatory and marketing approvals for our product candidates that successfully complete clinical trials, if any; |
| ● | establish sales, marketing, distribution and other commercial infrastructure now and in the future to commercialize various products for which we may obtain marketing authorization or approval, if any; |
| ● | require the scale-up and validation of the manufacturing process and the manufacturing of larger quantities of product candidates for clinical development and, potentially, commercialization; |
| ● | collaborate with strategic partners to optimize the manufacturing process for vilobelimab, IFX002, INF904 and other pipeline products; |
| ● | maintain, expand and protect our intellectual property portfolio; |
| ● | hire and retain additional personnel, such as commercial, marketing, clinical, quality control and scientific personnel; and |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development as well as commercialization and help us comply with our obligations as a public company. |
Our ability to become and remain profitable depends on our ability to generate revenue. We do not expect to generate significant revenue unless and until we are, or any future collaborator is, able to obtain marketing authorization or approval for, and successfully commercialize, one or more of our product candidates. Successful commercialization will require achievement of key milestones, including completing clinical trials of vilobelimab and any other product candidates, obtaining marketing authorization or approval for these product candidates, manufacturing, marketing and selling those products for which we, or any of our future collaborators, may obtain marketing authorization or approval, satisfying any post-marketing requirements and obtaining reimbursement for our products from private insurance or government payors. Because of the uncertainties and risks associated with these activities, we are unable to accurately predict the timing and amount of revenues, and if or when we might achieve profitability. We and any future collaborators may never succeed in these activities and, even if we do, or any future collaborators do, we may never generate revenue that is large enough for us to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
-4-
We expect our financial condition and operating results to continue to fluctuate from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. In order to succeed, we will need to transition from a company with a research and development focus to a company capable of undertaking commercial activities. We may encounter unforeseen expenses, difficulties, complications and delays, and may not be successful in such a transition.
Our failure to become and remain profitable could depress the market price of our ordinary shares and could impair our ability to raise capital, pay dividends, expand our business, diversify our product offerings or continue our operations. If we continue to suffer losses as we have in the past, investors may not receive any return on their investment and may lose their entire investment.
We will need substantial additional funding, and if we are unable to raise capital when needed, we could be forced to delay, reduce or discontinue our product discovery and development programs or commercialization efforts
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. For example, for the years ended December 31, 2023 and December 31, 2022, we used €37.8 million and €33.7 million, respectively, in net cash for our operating activities, most of which were related to research and development activities. We expect our expenses to increase in connection with our ongoing activities, particularly as we initiate new clinical trials of, initiate new research and preclinical development efforts for, establish robust manufacturing processes for, establish comprehensive commercialization and marketing processes and seek marketing authorization or approval for, our current product candidates or any future product candidates, including those that we may acquire. In particular, we will incur significant expenses as we conduct our planned clinical trial program and initiate new research and preclinical development efforts. In addition, after obtaining the EUA for GOHIBIC (vilobelimab) in the United States and if we obtain marketing approval for any of our product candidates, we may incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution to the extent that such sales, marketing, manufacturing and distribution are not the responsibility of a future collaborator. Furthermore, we expect to incur significant additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we may be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
We plan to use our cash on hand primarily to fund our planned clinical trial programs, to initiate new research and preclinical development efforts, to establish commercial scale manufacturing processes and for working capital and other general corporate purposes. We will be required to expend significant funds in order to advance the development of vilobelimab in later stages of clinical development, as well as other product candidates we may seek to develop, including IFX002 and INF904. We are also evaluating vilobelimab for a number of additional indications. Any future development activities for our pipeline product candidates will depend heavily on the clinical as well as commercialization and marketing success of vilobelimab in any indication.
Our existing cash and cash equivalents will not be sufficient to fund all the efforts that we plan to undertake or to fund the completion of development of any of our product candidates. Accordingly, we will be required to obtain further funding through public or private equity offerings, debt financings, royalty-based financings, collaborations and licensing arrangements or other sources. Currently, we do not have any committed external source of funds. Adequate additional financing may not be available to us on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts and on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of vilobelimab or any of our other product candidates or potentially discontinue operations altogether. Our failure to raise capital as and when needed could have a negative impact on our financial condition and our ability to pursue our business strategy.
-5-
We believe that our existing cash, cash equivalents and marketable securities will enable us to fund our operating expenses and capital expenditure requirements under our current business plan for at least the next 24 months. Changing circumstances, some of which may be beyond our control, could cause us to consume capital significantly faster than we anticipate, and we may need to seek additional funds sooner than planned. Our future funding requirements, both short-term and long-term, will depend on many factors, including:
| ● | the scope, progress, timing, costs and results of clinical trials of, and research and preclinical development efforts for, our current and future product candidates, particularly for vilobelimab; |
| ● | the number of future product candidates and indications that we pursue and their development requirements; |
| ● | the outcome, timing and costs of seeking regulatory approvals; |
| ● | the costs of preparation for and implementation of commercialization and commercialization activities for any of our product candidates that receive marketing authorization approval to the extent such costs are not the responsibility of any future collaborators, including the costs and timing of establishing product sales, marketing, distribution and commercial-scale manufacturing capabilities; |
| ● | the effect of competing technological and market developments; |
| ● | subject to receipt of marketing authorization or approval, revenue, if any, received from commercial sales of our current and future product candidates; |
| ● | our ability to enter into, and the terms and timing of, any collaborations, licensing or other arrangements; |
| ● | our headcount growth and associated costs as we expand our research, development, manufacturing, regulatory and commercial activities; |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights including enforcing and defending intellectual property related claims; and |
| ● | the costs of operating as a public company. |
We have a limited operating history and history of commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability
We commenced operations in 2008. Our operations to date have been limited to establishing our Company, raising capital, developing our proprietary anti-C5a and anti-C5aR technologies, identifying and testing potential product candidates and conducting clinical trials of our lead product candidate, vilobelimab and establishing a commercial scale manufacturing process for vilobelimab. In April 2023, we received the EUA for vilobelimab for the treatment of certain critically ill COVID-19 patients by the FDA. We have just started to arrange for third parties to manufacture and distribute our product at small commercial scale on our behalf. However, we have not yet demonstrated an ability to obtain full marketing approvals or conduct sales and marketing activities at large scale as necessary for successful product commercialization. Also, we are still in early stages of clinical development with our other product candidates. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development, especially clinical-stage biopharmaceutical companies such as ours. Any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history or a history of successfully developing and commercializing pharmaceutical products.
We may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors in achieving our business objectives. We will eventually need to transition from a company with a predominant development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We expect our financial condition and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
We may be subject to risks in relation to financial grants received by the German federal government, which may result in the partial repayment of such grants
The clinical Phase III study of vilobelimab in critically ill COVID-19 patients and certain manufacturing development related activities of our product candidate vilobelimab were partly funded by the German federal government through a grant awarded to us in October 2021. The grant was structured as a reimbursement of 80% of certain pre-specified expenses related to the clinical development and manufacturing of vilobelimab. The grant period ended on June 30, 2023, as planned. In total, throughout the duration of the grant period and up to the date hereof, we received a total amount of €33.3 million. We might be subject to future audits by financial oversight authorities of the German federal government or European regulatory bodies and a failure to pass these audits in case the authorities identify non-adherence to all grant conditions could potentially lead to a retroactive revocation of parts of the funds awarded by the grant.
-6-
In addition, the German federal government has, in the case of a special public interest, a non-exclusive and transferable right to use intellectual property generated as part of the funded work. Contracts with third parties relating to the exploitation of the results of the funded work must be disclosed to the agency managing the grant on behalf of the German federal government and any such contracts with parties outside of the European Union require the prior consent of the German federal government to the extent they deviate from a commercial exploitation plan previously approved by the German federal government. Additionally, we may be required to grant third parties licenses to use such results under certain conditions. In certain scenarios, including if we come under the decisive influence of foreign investors, the funded results are exclusively or predominantly used outside Germany without the prior consent of the German federal government or if we are in breach of our obligations under the grant, the grant funding, including funding already received, can be revoked.
Exchange rate fluctuations may materially affect our results of operations and financial condition
Potential future expense and revenue may be incurred or derived from outside the European Union, particularly the United States. As a result, our business and our share price may be affected by fluctuations in foreign exchange rates between the euro and other currencies, particularly the U.S. dollar, which may also have a significant impact on our reported results of operations and cash flows from period to period. We currently do not have any exchange rate hedging arrangements in place.
We may face the risk of substantial write-downs of inventory due to the excess or obsolescence of our inventory relating to GOHIBIC (vilobelimab) due to expiration prior to sale or unsuitability for alternative use
Excess purchase of raw materials and production of products or the commitments to purchase or produce such items may result in high inventory levels that might not be commercially viable and could lead to the necessity to partially or fully write-down these items. Excess and obsolescence risks for our inventory could arise from factors such as shelf-life expiration, overstocking, or events like supply chain disruptions, manufacturing mistakes, raw material flaws which can result in errors, wastage, or delays and more. Whether such obsolescence risks materialize, ultimately depends on market demand/penetration and medical need for our products, regulatory factors such as approvals or approval withdrawals by regulatory agencies in the territories in which we intend to commercialize our product, the competitive landscape of the markets in which we operate, including the development of comparable products by our competitors as well as our ability to command prices at which we can be competitive and our own plans regarding alternative use of unfinished or finished products in our inventory for alternative purposes, such as clinical trials in additional indications. Furthermore, changes in currency exchange rates can impact the cost of imported goods and affect inventory valuations. In valuing our inventory, we make assumptions regarding these factors and update these assumptions when applicable.
1. Risks related to the discovery, development, manufacturing and commercialization of our product candidates
We are at a clinical development stage in our development efforts, our approach of targeting C5a or C5aR inhibition is novel and we may not be able to successfully develop and commercialize any product candidates
Vilobelimab is a novel therapeutic antibody and its potential therapeutic benefit is unproven, and C5a or C5aR inhibition to treat complement-mediated autoimmune and inflammatory diseases has only been partly validated. We have not yet succeeded and may never succeed in demonstrating efficacy and safety for vilobelimab in pivotal clinical trials or in obtaining marketing approval thereafter for severe COVID-19 or any other indication. The aforementioned continues to be true, although GOHIBIC(vilobelimab) has been granted the EUA by the FDA for the treatment of coronavirus disease 19, or COVID-19, in hospitalized adults when initiated within 48 hours of receiving invasive mechanical ventilation, or IMV, or extracorporeal membrane oxygenation ECMO. If we are unsuccessful in our development efforts, we may not be able to advance the development of our product candidates, commercialize products, raise capital, expand our business, or continue our operations.
-7-
We depend on the success of our product candidates, including our lead product candidate, vilobelimab, and if we are unable to obtain approval for and commercialize our product candidates for one or more indications in a timely manner, our business will be materially harmed
Our success depends on our ability to timely complete clinical trials and obtain marketing authorization or approval for, and then successfully commercialize, our product candidates, including our lead product candidate, vilobelimab, for one or more indications. Our product candidates will require additional clinical development, preclinical and manufacturing development activities, marketing approval from government regulators, commercial manufacturing, substantial investment, and significant marketing efforts before we generate any revenue from product sales. We are not permitted to market or promote any product candidates in a jurisdiction before receiving marketing authorization or approval from the relevant regulatory authority, including the FDA for marketing in the United States and EMA for marketing in Europe, and we may never receive such marketing approvals or marketing authorizations beyond the EUA for GOHIBIC (vilobelimab) granted by the FDA in April 2023. The success of our product candidates will depend on numerous factors, including:
| ● | raising additional funds, or entering into collaborations, necessary to complete the clinical development of and to commercialize of our product candidates; |
| ● | successful and timely completion of our ongoing clinical trials; |
| ● | initiation of successful patient enrollment and completion of additional clinical trials on a timely basis; |
| ● | efficacy, safety and tolerability profiles that are satisfactory to the FDA, EMA or any comparable foreign regulatory authority for marketing approval; |
| ● | timely receipt of marketing authorizations or approvals for our product candidates from applicable regulatory authorities; |
| ● | the extent of any required post-marketing approval commitments to applicable regulatory authorities; |
| ● | the maintenance of existing, or the establishment of new, supply arrangements with third-party drug product suppliers and manufacturers; |
| ● | the maintenance of existing, or the establishment of new, scaled production arrangements with third-party manufacturers to obtain finished products that are appropriately packaged for sale; |
| ● | obtaining and maintaining patent protection, trade secret protection and regulatory exclusivity, in the United States and elsewhere; |
| ● | protection of our rights in our intellectual property portfolio, including our licensed intellectual property; |
| ● | successful launch of commercial sales following any marketing authorization or approval; |
| ● | a continued acceptable safety profile following any marketing authorization or approval; |
| ● | commercial acceptance by patients, the medical community and third-party payors; |
| ● | inclusion of approved or, such as GOHIBIC (vilobelimab), authorized products in guidelines by medical bodies, including but not limited to National Institute of Health, or NIH, guidelines; |
| ● | inclusion of approved or, such as GOHIBIC (vilobelimab), authorized products on hospitals formulary; and |
| ● | our ability to compete with other therapies. |
Additionally, we cannot be sure that we can obtain necessary regulatory authorizations or approvals on a timely basis, if at all, for any of the products we are developing or manufacturing or that we can maintain necessary regulatory authorizations or approvals for our existing products, and all of the following could have a material adverse effect on our business:
| ● | significant delays in obtaining or failing to obtain required authorizations approvals; |
| ● | loss of, or changes to, previously obtained authorizations or approvals; |
| ● | failure to comply with existing or future regulatory requirements and; |
| ● | changes to manufacturing processes or manufacturing process standards following authorization or approval, or changing interpretations of these factors. |
Many of such factors remain outside of our control, and if we are unable to achieve one or more of the objectives set forth above, our business will be materially harmed.
-8-
GOHIBIC (vilobelimab) for which we received the EUA is subject to continuing regulatory oversight, which might lead to a withdrawal or revocation of the granted EUA, if the FDA considers the underlying requirements or conditions for the EUA not to be given anymore. We may incur significant losses including loss of reputation, if we the EUA for GOHIBIC (vilobelimab) is withdrawn or revoked by the FDA.
GOHIBIC (vilobelimab) for which we received the EUA for the treatment of COVID-19 in certain hospitalized adult patients, pursuant to Section 564 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. §360bbb-3) is subject to continuing regulatory oversight, including the determination and verification of underlying requirements such as a public health emergency, or a significant potential for a public health emergency, that affects, or has a significant potential to affect, national security or the health and security of United States citizens living abroad, and that involves the virus that causes COVID-19. The granted EUA will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic is terminated under Section 564(b)(2) of the Act or the EUA is revoked under Section 564(g) of the Act. If the conditions or requirements for granting the EUA cease to be apparent in the future, or if reported drug safety events lead to a new situation in determining the overall pharmacovigilance of vilobelimab, the FDA may suspend, withdraw or revoke the granted EUA. Any of these events could have a material adverse effect on our business, financial condition, results of operations and prospects.
Clinical failure may occur at any stage of clinical development, and the results of our clinical trials may not support our proposed indications for our product candidates
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the results of later clinical trials will replicate the results of prior clinical trials and preclinical testing. Moreover, success in clinical trials in a particular indication does not ensure that a product candidate will be successful in other indications, even for the same underlying disease. A number of companies in the pharmaceutical industry, including biotechnology companies, have suffered significant setbacks in clinical trials, even after promising results in earlier preclinical studies or clinical trials or successful later-stage trials in other related indications, including in the context of controlling complement activation through C5 and C5a or C5aR inhibition. For example, while others in our industry have attempted to develop C5a-specific antibodies, there is no approved therapy inhibiting C5a. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway and safety or efficacy observations made in clinical trials, including previously unreported adverse events as well as lack of efficacy and patient benefit as reported by clinical trial investigators. In particular, development of antibodies that target C5a rather than C5 to control complement activation is comparatively novel, and there is no approved therapy specifically targeting C5a. As a result, inhibition of C5a rather than C5, which blocks signaling to the two receptors C5aR and C5L2, may have unforeseen consequences or negative results that may lead to clinical failure or withdrawal in later stages of our product candidate development. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical and initial clinical trials for a variety of reasons, including differences in patient populations, changes in trial protocols and complexities of larger, multi-center trials among others. We may fail to complete clinical trials and/or to meet predetermined endpoints in the clinical trials, which may cause us to abandon a product candidate or an indication and may delay development of any other product candidates. Any delay in, or termination of, our clinical trials will delay the submission of the Biologics License Application, or BLA, or the EUA to the FDA, the MAA to the EMA or other similar applications with other relevant foreign regulatory authorities and, ultimately, our ability to commercialize any of our product candidates and generate revenue.
Failure to maintain compliance with FDA requirements and/or remain in alignment with FDA feedback may prevent or delay the development, marketing or manufacturing of vilobelimab for the treatment of critically ill COVID-19 patients and, potentially, of vilobelimab in ulcerative PG
Our manufacturing and laboratory facilities are periodically subject to inspection by the FDA and other governmental agencies to ensure they meet production and quality requirements. Operations at these facilities could be interrupted or halted if the FDA or another governmental agency deems the findings of such inspections unsatisfactory. Further, failure to comply with FDA or other regulatory requirements regarding the development, marketing, promotion, manufacturing and distribution of vilobelimab could result in fines, unanticipated compliance expenditures, recall or seizures of our products, total or partial suspension of production or distribution, restrictions on labeling and promotion, termination of ongoing research, disqualification of data for submission to regulatory authorities, enforcement actions, injunctions and criminal prosecution. If we do not meet applicable regulatory or quality standards, our products may be subject to recall, and under certain circumstances, we may be required to notify applicable regulatory authorities about a recall.
-9-
GOHIBIC (vilobelimab) and any other product candidates for which we receive approval or the EUA are subject to continuing regulatory oversight, and we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense. We may be subject to penalties if we fail to comply with regulatory requirements.
GOHIBIC (vilobelimab) and any other product candidates for which we received or might receive approval or the EUA are subject to continuing regulatory oversight, including the review of promotional materials and additional safety information, and the applicable regulatory authority may still impose significant restrictions on the indicated uses or marketing of our product or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable federal and state laws. In other countries, advertising and promotional material may be subject to similar rules. If we fail to comply with applicable regulatory requirements following authorization or approval of any of our product candidates, a regulatory agency may:
| ● | issue a warning letter asserting that we are in violation of the law; |
| ● | seek an injunction or impose civil or criminal penalties or monetary fines; |
| ● | suspend or withdraw the granted EUA or regulatory approval or revoke a license; |
| ● | suspend any ongoing clinical studies; |
| ● | seize product; or |
| ● | refuse to allow us to enter into supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize any authorized or approved products and generate revenues.
Any of these events could prevent us from achieving or maintaining market acceptance of any products we may identify and develop and could have a material adverse effect on our business, financial condition, results of operations and prospects.
If clinical trials of our product candidates fail to satisfactorily demonstrate safety and efficacy to the FDA and other regulators, we, or any future collaborators, may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of these product candidates
We, and any future collaborators, are not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining marketing approval or the EUA from the FDA. Foreign regulatory authorities, such as the EMA, impose similar requirements in their respective markets. We, and any future collaborators, must complete extensive preclinical development and clinical trials to demonstrate the safety and efficacy of our product candidates in humans before we will be able to obtain these approvals.
The clinical development of our product candidates is susceptible to the risk of failure inherent at any stage of product development. It is possible that even if one or more of our product candidates has a beneficial effect, that effect will not be detected during clinical evaluation as a result of one or more of a variety of factors, including the size, duration, design, measurements, conduct or analysis of our clinical trials. In addition, many of our product candidates are in early stages of development or clinical testing. As a result, it may be years before any of our product candidates receives regulatory approval, if at all, and additional clinical trials may fail to demonstrate safety, efficacy or tolerability for our targeted indications.
-10-
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or any future collaborators and impair our ability to generate revenue from product sales, regulatory and commercialization milestones and royalties. Moreover, if we or any future collaborators are required to conduct additional clinical trials or other testing of our product candidates beyond the trials and testing that we or they contemplate, if we or they are unable to successfully complete clinical trials of our product candidates or other testing or the results of these trials or tests are unfavorable, uncertain or are only modestly favorable, or there are unacceptable safety concerns associated with our product candidates, we or any future collaborators may:
| ● | incur additional unplanned costs, including costs relating to additional required clinical trials or preclinical testing; |
| ● | be delayed in obtaining marketing approval for vilobelimab or any of our other product candidates; |
| ● | not obtain marketing approval at all or the withdrawal or revocation of the EUA by the FDA; |
| ● | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| ● | obtain approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings; |
| ● | be subject to additional post-marketing testing or other requirements; or |
| ● | be required to remove the product from the market after obtaining marketing approval or the EUA. |
Our failure to successfully complete clinical trials of our product candidates and to demonstrate the efficacy and safety necessary to obtain regulatory approval to market any of our product candidates would significantly harm our business.
Our product candidates may cause or be perceived to cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay, denial or withdrawal of regulatory approval by the FDA or comparable foreign regulatory authorities. Results of our clinical trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics. In addition, many of the patients that we enrolled in our clinical trials of vilobelimab suffer from serious pre-existing disorders. While such disorders may lead to serious adverse events, or SAEs, during trial periods that may be found to be unrelated to vilobelimab, such events may create a negative safety perception and adversely impact market acceptance of vilobelimab following any approval.
If unacceptable side effects arise in the development of our product candidates, we, the FDA or comparable foreign regulatory authorities, the Institutional Review Boards, or IRBs, or independent ethics committees at the institutions in which our studies are conducted or elsewhere, or the Data Safety Monitoring Board, or DSMB, could suspend or terminate our clinical trials or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Side effects, whether treatment-related or not, could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We expect to have to train medical personnel using our product candidates to understand the side effect profiles for our clinical trials and upon any commercialization of any of our product candidates. Inadequate training in recognizing or managing the potential side effects of our product candidates could result in patient injury or death. Any of these occurrences may harm our business, financial condition and prospects significantly.
Moreover, clinical trials of our product candidates are conducted in carefully defined sets of patients who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials, or those of any future collaborator, may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify undesirable side effects. If, following approval of a product candidate, we, or others, discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, any of the following adverse events could occur:
| ● | regulatory authorities may withdraw their authorization or approval of the product or seize the product; |
| ● | we, or any future collaborators, may need to recall the product, or be required to change the way the product is administered or conduct additional clinical trials; |
-11-
| ● | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular product; |
| ● | we may be subject to fines, injunctions or the imposition of civil or criminal penalties; |
| ● | regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication; |
| ● | we, or any future collaborators, may be required to create a Medication Guide outlining the risks of the previously unidentified side effects for distribution to patients; |
| ● | we, or any future collaborators, may be required to implement a REMS that imposes distribution and use restrictions or to conduct post-market studies or clinical trials; |
| ● | we, or any future collaborators, could be sued and held liable for harm caused to patients; |
| ● | the product may become less competitive; and |
| ● | our reputation may suffer. |
Any of these events could harm our business and operations and could negatively impact our share price.
Our most advanced product candidates are either chimeric or humanized antibody proteins that could cause an immune response in patients, resulting in the creation of harmful or neutralizing antibodies against these therapeutic proteins
In addition to the safety, efficacy, manufacturing and regulatory hurdles faced by our product candidates, the administration of proteins such as monoclonal antibodies that are chimeric or humanized, including our product candidates vilobelimab and IFX002, respectively, can cause an immune response, resulting in the creation of antibodies against the therapeutic protein. These anti-drug antibodies, or ADAs, can have no effect or can neutralize the effectiveness of the protein or require that higher doses be used to obtain a therapeutic effect. Whether ADAs will be created and how they react can often not be predicted from preclinical or even clinical studies, and their detection or appearance is often delayed. As a result, neutralizing antibodies may be detected at a later date or upon longer exposure of patients with our product candidates, such as following more chronic administration in longer lasting clinical trials. In some cases, detection of such neutralizing antibodies can even occur after pivotal clinical trials have been completed. Therefore, there can be no assurance that neutralizing antibodies will not be detected in future clinical trials or at a later date upon longer exposure (including after commercialization). If ADAs reduce or neutralize the effectiveness of our product candidates, the continued clinical development or receipt of marketing approval for any of our product candidates could be delayed or prevented and, even if any of our product candidates is approved, their commercial success could be limited, any of which would impair our ability to generate revenue and continue operations. Low levels of ADAs were detected in previously completed clinical studies.
Even if we complete the necessary preclinical studies and clinical trials for vilobelimab and any other product candidates, the marketing approval process including the EUA process is expensive, time consuming and uncertain and may prevent us or any future collaborators from obtaining approvals for the commercialization of some or all of our product candidates. As a result, we cannot predict when or if, and in which territories, we, or any future collaborators, will obtain marketing authorization or approval to commercialize a product candidate
The research, testing, manufacturing, labeling, approval, selling, marketing, promotion and distribution of products are subject to extensive regulation by the FDA and comparable foreign regulatory authorities. We, and any future collaborators, are not permitted to market our product candidates in the United States or in other countries until we, or they, receive approval of a BLA or the EUA from the FDA or marketing approval from applicable regulatory authorities outside the United States. Our product candidates are in various stages of development and are subject to the risks of failure inherent in drug development. We have not submitted an application for or received marketing approval for any product candidate in the United States or in any other jurisdiction. We have limited experience in conducting and managing the clinical trials necessary to obtain marketing approvals, including FDA approval of a BLA or EUA. Further, there is no prior history of regulatory approval for product candidates targeting C5a inhibition.
-12-
The process of obtaining marketing authorizations or approvals, both in the United States and elsewhere is lengthy, expensive and uncertain. It may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Securing marketing approval, including the EUA, requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing marketing approval, including the EUA, also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. The FDA or other regulatory authorities may determine that our product candidates are not safe and effective, only moderately effective or have undesirable or unintended side effects, toxicities or other characteristics that preclude our obtaining marketing approval or prevent or limit commercial use. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a drug candidate’s clinical development and may vary among jurisdictions. Any marketing approval, including the EUA, we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable. For example, the EUA for the GOHIBIC (vilobelimab) has been granted for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. The FDA, EMA or any comparable foreign regulatory authorities may delay, limit or deny approval of vilobelimab for many reasons, including:
| ● | we may not be able to demonstrate that vilobelimab is safe and effective as a treatment for our targeted indications to the satisfaction of the FDA, the EMA or comparable foreign regulatory agencies; |
| ● | the FDA, EMA or comparable foreign regulatory authorities may require additional clinical trials or non-clinical studies of vilobelimab in addition to those already performed or planned, either before approval or as a post-approval commitment, which would increase our costs and prolong our development time for vilobelimab; |
| ● | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA, EMA or comparable foreign regulatory authorities to obtain marketing approval; |
| ● | the FDA, EMA or comparable foreign regulatory authorities may disagree with the number, design, size, conduct or implementation of our clinical trials, including designated clinical endpoints; |
| ● | the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval; |
| ● | the contract research organizations, or CROs, that we retain to conduct clinical trials may take actions outside of our control that materially adversely impact our clinical trials; |
| ● | the FDA, EMA or comparable foreign regulatory authorities may not find the data from preclinical studies and clinical trials sufficient to demonstrate that the clinical and other benefits of vilobelimab and any other product candidates outweigh its safety risks; |
| ● | the FDA, EMA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies and clinical trials; |
| ● | the FDA, EMA or comparable foreign regulatory authorities may not accept data generated at clinical trial sites, including for non-compliance with cGCP; |
| ● | if our BLA, when submitted, is reviewed by an advisory committee, the FDA may have difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| ● | the FDA, EMA or comparable foreign regulatory authorities may require development of a risk evaluation and mitigation strategy, or REMS, as a condition of approval; |
| ● | the FDA, EMA or comparable foreign regulatory authorities may identify deficiencies in the manufacturing processes or facilities of our third-party manufacturers, including non-compliance with current Good Manufacturing Practices, or cGMP; or |
| ● | the FDA, EMA or comparable foreign regulatory authorities may change their respective approval policies or adopt new regulations. |
-13-
Of the large number of drugs in development in the biopharmaceutical industry, only a small percentage result in the submission of a BLA to the FDA and even fewer are approved for commercialization. Furthermore, even if we do receive regulatory approval to market vilobelimab, any such approval may be subject to limitations on the indicated uses or patient populations for which we may market the product. Accordingly, even if we are able to obtain the requisite financing to continue to fund our development programs, we cannot assure you that vilobelimab and/or any other product candidates will be successfully developed or commercialized.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or other regulatory authorities. The FDA or other regulatory authorities may conclude that a principal investigator, potentially including because of a financial relationship with us, has a conflict of interest that has affected interpretation of the study. The FDA or other regulatory authorities may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or other regulatory authorities, as the case may be, and may ultimately lead to the denial of marketing authorization or approval of one or more of our product candidates.
Any delay in obtaining or failure to obtain required approvals could negatively impact our ability or that of any future collaborators to generate revenue from the particular product candidate, which likely would result in significant harm to our financial position and adversely impact our share price.
We depend on enrollment of patients in our clinical studies for our product candidates. If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected
We will also be required to identify and enroll a sufficient number of patients with PG, and potentially other indications, for our planned or ongoing clinical trial of vilobelimab in these indications. Some of these are rare disease indications or indication with a relatively small patient population. Trial participant enrollment could be limited in future trials given that many potential participants may be ineligible because they are already undergoing treatment with approved medications or are participating in other clinical trials.
Patient enrollment is affected by other factors, including:
| ● | severity of the disease under investigation; |
| ● | design of the clinical trial protocol; |
| ● | size and nature of the patient population; |
| ● | eligibility criteria for the trial in question; |
| ● | perceived risks and benefits of the product candidate under trial; |
| ● | perceived safety and tolerability of the product candidate; |
| ● | proximity and availability of clinical trial sites for prospective patients; |
| ● | availability of competing therapies and clinical trials; |
| ● | clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including standard-of-care and any new drugs that may be approved for the indications we are investigating; |
| ● | efforts to facilitate timely enrollment in clinical trials; |
| ● | patient referral practices of physicians; and |
| ● | our ability to monitor patients adequately during and after treatment. |
Further, there are only a limited number of specialist physicians who treat patients with these diseases and major clinical centers are concentrated in a few geographic regions. We also may encounter difficulties in identifying and enrolling such patients with a stage of disease appropriate for our ongoing or future clinical trials. In addition, the process of finding and diagnosing patients may prove costly. Our inability to enroll a sufficient number of patients for any of our clinical trials, if any, would result in significant delays or may require us to abandon one or more clinical trials.
We have experienced slower recruitment than anticipated in the clinical trials of vilobelimab in severe COVID-19, PG and cSCC, because of other compounds in clinical development for the same patient population, low disease prevalence, difficulties in diagnosis or due to restrictions at clinical trial sites in light of the COVID-19 pandemic. Further delays in the completion of any clinical trials will increase our costs, slow down our product candidate development and delay or potentially jeopardize our ability to commence marketing and generate revenue. In addition, we may not be able to initiate or continue clinical trials required by the FDA, EMA or other foreign regulatory agencies for vilobelimab or any of our other product candidates that we pursue if we are unable to locate and enroll a sufficient number of eligible patients to participate in these clinical trials.
-14-
Even if one of our product candidates receives marketing approval, including the EUA, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success, in which case we may not generate significant revenues or become profitable
Even if our other product candidates are approved by the appropriate regulatory authorities for marketing and sale or receives the EUA, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. As a general proposition, physicians are often reluctant to switch their patients from existing therapies, such as for the treatment of cSCC, even when new and potentially more effective or convenient treatments enter the market. Further, patients often acclimate to existing therapies and do not want to switch therapies unless their physicians recommend doing so or they are required to do so due to lack of reimbursement for existing therapies. Further, we may face a lack of acceptance by the physician community of the efficacy of targeting C5a to inhibit terminal complement activation compared to targeting C5, which is well established in clinical practice (such as eculizumab). In addition, vilobelimab may not be accepted by physicians or patients if we cannot demonstrate, or if vilobelimab is perceived as not having, strong duration of effect, including compared to existing treatments. The duration of effect of vilobelimab has only been studied prospectively for durations less than the expected duration of any pivotal Phase III clinical trials. It is possible that the effects seen in shorter term clinical trials will not be replicated at later time points or in larger clinical trials. Further, even if we are able to demonstrate our product candidates’ safety and efficacy to the FDA and other regulators, safety concerns in the medical community may hinder market acceptance.
Efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources, including management time and financial resources, and may not be successful. If any of our product candidates is approved but does not achieve an adequate level of market acceptance, we may not generate significant revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| ● | the efficacy and safety of the product; |
| ● | the potential advantages of the product compared to competitive therapies, notwithstanding success in meeting or exceeding clinical trial endpoints; |
| ● | the prevalence and severity of any side effects; |
| ● | whether the product is designated under physician treatment guidelines as a first-, second- or third-line therapy; |
| ● | our ability, or the ability of any future collaborators, to offer the product for sale at competitive prices; |
| ● | the product’s convenience and ease of administration compared to alternative treatments; |
| ● | the willingness of the target patient population to try, and of physicians to prescribe, the product; |
| ● | limitations or warnings, including distribution or use restrictions contained in the product’s approved labeling; |
| ● | the strength of sales, marketing and distribution support; |
| ● | changes in the standard of care for the targeted indications for the product; and |
| ● | availability and amount of coverage and reimbursement from government payors, managed care plans and other third-party payors. |
The failure of any of our product candidates, if authorized or approved, to find market acceptance would harm our business and could require us to seek additional financing.
-15-
Even if we, or any future collaborators, are able to commercialize any product candidate that we, or they, develop, the product may become subject to unfavorable pricing regulations or third-party payor coverage and reimbursement policies, any of which could harm our business
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Therefore, our ability, and the ability of any future collaborators, to commercialize any of our product candidates will depend in part on the extent to which coverage and reimbursement for these products and related treatments will be available from third-party payors including government health administration authorities and public or private health coverage insurers. Third-party payors decide which medications they will cover and establish reimbursement levels. We cannot be certain that reimbursement will be available for vilobelimab or any of our product candidates. Also, we cannot be certain that less fulsome reimbursement policies will not reduce the demand for, or the price we can charge for, our products, if approved. The insurance coverage and reimbursement status of newly approved products for orphan diseases is particularly uncertain and failure to obtain or maintain adequate coverage and reimbursement for vilobelimab or any other product candidates could limit our ability to generate revenue.
If coverage and reimbursement are not available, or reimbursement is available only to limited levels, we, or any future collaborators, may not be able to successfully commercialize our product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us, or any future collaborators, to establish or maintain pricing sufficient to realize a sufficient return on our or their investments. In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors and coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved drugs. Marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. Some countries require approval of the sales price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we, or any future collaborators, might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay commercial launch of the product, possibly for lengthy time periods, which may negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability or the ability of any future collaborators to recoup our or their investment in one or more product candidates, even if our product candidates obtain marketing approval.
The healthcare industry is acutely focused on cost containment, both in the United States and elsewhere. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability or that of any future collaborators to sell our product candidates profitably. These payors may not view our products, if any, as cost-effective, and coverage and reimbursement may not be available to our customers, or those of any future collaborators, or may not be sufficient to allow our products, if any, to be marketed on a competitive basis. Cost-control initiatives or other policy measures by government authorities could cause us, or any future collaborators, to decrease the price we, or they, might establish for products, which could result in lower than anticipated product revenues. If the prices for our products, if any, decrease or if governmental and other third-party payors do not provide coverage or adequate reimbursement, our prospects for revenue and profitability will suffer.
There may also be delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the indications for which the drug is authorized or approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Reimbursement rates may vary, by way of example, according to the use of the product and the clinical setting in which it is used. Reimbursement rates may also be based on reimbursement levels already set for lower cost drugs or may be incorporated into existing payments for other services.
In addition, increasingly, third-party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging the prices charged. We cannot be sure that reimbursement coverage will be available for any product candidate that we, or any future collaborator, commercialize and, if available, that the reimbursement rates will be adequate. Further, the net reimbursement for drug products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. An inability to promptly obtain coverage and adequate payment rates from both government-funded and private payors for any of our product candidates for which we, or any future collaborator, obtain marketing authorization or approval could significantly harm our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
-16-
If we are unable to develop our sales, marketing and distribution capability on our own or through collaborations with marketing partners, we will not be successful in commercializing our product candidates
We have limited marketing, sales or distribution capabilities and experience within our organization. If any of our product candidates is authorized or approved, we either have to establish a sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize any such candidate, or to outsource this function to a third party. These activities were initiated in 2023 regarding the FDA’s EUA for GOHIBIC (vilobelimab) in the United States. Either of these options would be expensive and time consuming. Some or all of these costs may be incurred in advance of any approval of our product candidates, including our lead candidate vilobelimab. In addition, we may not be able to hire a sales force in the United States, Europe or other target market that is sufficient in size or has adequate expertise in the medical markets that we intend to target. These risks may be particularly pronounced due to our focus on severe COVID-19, as well as additional focus on PG, each of which are disease areas with relatively small patient populations. Any failure or delay in the development of our or third parties’ internal sales, marketing and distribution capabilities would adversely impact the commercialization of vilobelimab and other future product candidates.
With respect to our existing and future product candidates, we may choose to collaborate with third parties that have direct sales forces and established distribution systems, either to augment or to serve as an alternative to our own sales force and distribution systems. Our product revenue may be lower than if we directly marketed or sold any approved products. In addition, any revenue we receive will depend in whole or in part upon the efforts of these third parties, which may not be successful and are generally not within our control. If we are unable to enter into these arrangements on acceptable terms or at all, we may not be able to successfully commercialize any authorized or approved products. If we are not successful in commercializing any authorized or approved products, our future product revenue will suffer and we may incur significant additional losses.
We may be subject to risks in relation to shelf-life expiration of drug product for GOHIBIC (vilobelimab), including but not limited to substantial write-offs in the balance sheet
Our finished drug product for GOHIBIC (vilobelimab) for use of commercial purposes under the granted the EUA in the United States is subject to limited shelf-life periods. If we fail to use such finished drug product for the intended use prior to its shelf-life expiration, such drug product will have to be destroyed accordingly, which might cause additional and substantial costs and expense as well as which might lead to substantial write-offs of destroyed drug product.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success
We have limited financial and managerial resources, and therefore we intend to focus on developing product candidates for specific indications that we identify as most likely to succeed, in terms of both their potential for marketing authorization, approval and commercialization. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that may prove to have greater commercial potential.
Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable product candidates. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to the product candidate.
-17-
Clinical development involves a lengthy and expensive process, with an uncertain outcome. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of vilobelimab or any other product candidate we may develop
The risk of failure for vilobelimab and any other product candidates we may develop such as INF904 is high. It is impossible to predict when or if vilobelimab will prove to be effective and safe in humans or will receive full regulatory approval for the treatment of severe COVID-19 or PG indication, or other indications. Additionally, before regulatory authorities grant marketing approval or the EUA for vilobelimab, for any future indications, or any future product candidate that we seek to develop, we will be required to complete our ongoing extensive clinical trials to demonstrate safety and efficacy in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is inherently uncertain as to outcome. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their drugs.
We may experience numerous unforeseen events during or as a result of the regulatory authorization and/or approval process that could delay or prevent our ability to receive marketing approval or the EUA from regulators or commercialize vilobelimab or any future product candidate, including:
| ● | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| ● | clinical trials of our product candidates may produce negative or inconclusive results, including failure to demonstrate statistical significance, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon drug development programs; |
| ● | our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators, ethics committees or institutional review boards to suspend or terminate the trials; |
| ● | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; and |
| ● | regulators, ethics committees or institutional review boards may require that we or our investigators suspend or terminate clinical development for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks. |
We could also encounter delays if a clinical trial is suspended or terminated by us, by an overseeing ethics committee, by the institutional review boards of the institutions in which such trials are being conducted, by the data safety monitoring board for such trial or by the FDA or other regulatory authorities. Such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate drug revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence drug sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval.
-18-
Our product development costs will further increase if we experience delays in testing or marketing approvals. Significant clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring drugs to market before we do and impair our ability to successfully commercialize our product candidates. We are evaluating applications for orphan drug or breakthrough therapy designation for vilobelimab in various indications, but we may be unable to obtain any such designation or to maintain the benefits associated with orphan drug status, including market exclusivity, even if that designation is granted
We are evaluating applications for orphan drug or breakthrough therapy designation for vilobelimab in some indications, and we may seek orphan drug designation for other preclinical product candidates in our pipeline or that we may develop. In the United States and other countries, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and user-fee waivers. After the FDA or other foreign regulatory agency grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the FDA review and approval process. Breakthrough therapy designation is a process designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint. Breakthrough therapy designation may make us eligible for intensive guidance by the FDA on an efficient drug development program and organizational commitment involving senior FDA managers, among others. Although we are evaluating applications for orphan drug or breakthrough therapy designation in some indications, there can be no assurance that we will obtain such designations. Moreover, obtaining orphan drug or breakthrough therapy designation for one indication does not mean we will be able to obtain such designation for another indication.
If a product that has orphan drug designation from the FDA subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications, including a BLA, to market the same drug for the same indication for seven years, except in limited circumstances such as if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Similarly, the FDA can subsequently approve a drug with the same active moiety for the same condition during the exclusivity period if the FDA concludes that the later drug is clinically superior, meaning the later drug is safer, more effective, or makes a major contribution to patient care. Even if we were to obtain orphan drug designation for vilobelimab from the FDA, we may not be the first to obtain marketing approval for any particular orphan indication due to the uncertainties associated with developing pharmaceutical products, and thus approval of vilobelimab could be blocked for seven years if another company obtains approval and orphan drug exclusivity for the same drug and same condition before us. If we do obtain exclusive marketing rights in the United States, they may be limited if we seek approval for an indication broader than the orphan designated indication and may be lost if the FDA later determines that the request for designation was materially defective or if we are unable to assure sufficient quantities of the product to meet the needs of the relevant patients. Further, exclusivity may not effectively protect the product from competition because different drugs with different active moieties can be approved for the same condition, the same drugs can be approved for different indications and might then be used off-label in our approved indication, and different drugs for the same condition may already be approved and commercially available.
Even if we obtain FDA authorization or approval of vilobelimab or any of our other product candidates, we may never obtain authorization or approval or commercialize our products outside of the United States
In order to market any approved products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding clinical trial design, safety and efficacy. If approved by the relevant governmental authorities, we expect to market vilobelimab for the treatment of COVID-19 and other indications in Europe and jurisdictions outside the United States, in part due to the relatively larger patient population that exists in Europe as compared to that in the United States. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. Approval procedures vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approvals could result in significant delays, difficulties and costs for us and may require additional preclinical studies or clinical trials, which would be costly and time consuming and could delay or prevent introduction of vilobelimab or any of our other product candidates in those countries.
-19-
In addition, we expect to be subject to a variety of risks related to operating in other countries if we obtain the necessary approvals, including:
| ● | differing regulatory requirements in countries outside the United States; |
| ● | the potential for so-called parallel importing (i.e., when a local seller, faced with high or higher local prices, opts to import goods from a foreign market (with low or lower prices) rather than buying them locally); |
| ● | unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements; |
| ● | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| ● | foreign reimbursement, pricing and insurance regimes; |
| ● | compliance with tax, employment, immigration and labor laws for employees living or traveling outside the United States; |
| ● | foreign taxes, including withholding of payroll taxes; |
| ● | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| ● | difficulties staffing and managing foreign operations; |
| ● | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| ● | potential liability under the Foreign Corrupt Practices Act of 1977 or comparable foreign regulations; |
| ● | challenges enforcing our contractual and intellectual property rights, especially in countries that do not protect intellectual property rights to the same extent as in the United States; |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities as well as supply chain disruptions outside the United States; and |
| ● | business interruptions, including as a results of geopolitical uncertainty and instability (including related to the Russia-Ukraine conflict). |
If we or our partners fail to comply with regulatory requirements or to obtain and maintain required approvals, our target market will be reduced, including if we are unable to market vilobelimab in Europe or elsewhere, and our ability to realize the full market potential of our product candidates will be harmed.
We are subject to extensive government regulation and the failure to comply with these regulations may have a material adverse effect on our operations and business
Both before and after approval of any product, we and our suppliers, contract manufacturers and clinical investigators are subject to extensive regulation by governmental authorities in the United States and other countries, covering, among other things, testing, manufacturing, quality control, clinical trials, post-marketing studies, labeling, advertising, promotion, distribution, import and export, governmental pricing, price reporting and rebate requirements. Failure to comply with applicable requirements could result in one or more of the following actions: warning letters; unanticipated expenditures; delays in approval or refusal to approve a product candidate; product recall or seizure; interruption of manufacturing or clinical trials; operating or marketing restrictions; injunctions; criminal prosecution and civil or criminal penalties including fines and other monetary penalties; adverse publicity; and disruptions to our business. Further, government investigations into potential violations of these laws would require us to expend considerable resources and face adverse publicity and the potential disruption of our business even if we are ultimately found not to have committed a violation.
Obtaining FDA, EMA or other regulatory agency authorization or approval of our product candidates requires substantial time, effort and financial resources and may be subject to both expected and unforeseen delays, and there can be no assurance that any approval will be granted on any of our product candidates on a timely basis, if at all. The FDA, EMA or other regulatory agencies may decide that our data are insufficient for authorization or approval of our product candidates and require additional preclinical, clinical or other studies or additional work related to chemistry, manufacturing and controls, or CMC. If we are required to conduct additional trials or to conduct other testing of our product candidates beyond that which we contemplate for regulatory approval, if we are unable to complete successfully our clinical trials or other testing or if the results of these and other trials or tests fail to demonstrate efficacy or raise safety concerns, we may face substantial additional expenses, be delayed in obtaining marketing approval for our product candidates or may never obtain marketing approval.
-20-
We are also required to comply with extensive governmental regulatory requirements after a product has received marketing authorization or approval. Governing regulatory authorities may require post-marketing studies that may negatively impact the commercial viability of a product. Once on the market, a product may become associated with previously undetected adverse effects and/or may develop manufacturing difficulties. As a result of any of these or other problems, a product’s regulatory approval could be withdrawn, which could harm our business and operating results.
Our current and future relationships with third-party payors, health care professionals and customers in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, physician payment transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to significant penalties
Healthcare providers, physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our current and future arrangements with health care professionals, third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including the federal Anti-Kickback Statute and the federal civil False Claims Act, that may constrain the business or financial arrangements and relationships through which we conduct clinical research, sell, market and distribute any drugs for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation in the United States and other jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include the following:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. Further, several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act; |
| ● | federal civil and criminal false claims laws, including the federal civil False Claims Act (that can be enforced through civil whistleblower or qui tam actions), and the civil monetary penalties law, which impose criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| ● | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
-21-
| ● | the Physician Payments Sunshine Act, created under Section 6002 of Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the Affordable Care Act, and its implementing regulations, which requires specified manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services, or CMS, information related to payments or other “transfers of value” made to physicians, which is defined to include doctors, dentists, optometrists, podiatrists and chiropractors, and teaching hospitals and applicable manufacturers to report annually to CMS ownership and investment interests held by physicians and their immediate family members by the 90th day of each calendar year. All such reported information is publicly available; and |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices, including our relationships with physicians and other healthcare providers, some of whom may recommend, purchase or prescribe vilobelimab, if approved, may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations.
If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including damages, fines, disgorgement, individual imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
Enacted and future legislation may increase the difficulty and cost for us to obtain marketing authorization or approval of and commercialize vilobelimab and affect the prices we may obtain
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of vilobelimab, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing authorization or approval.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives such as the Affordable Care Act in 2010, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. In the coming years, additional legislative and regulatory changes could be made to governmental health programs that could significantly impact pharmaceutical companies and the success of our drug candidate.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. These changes included aggregate reductions to Medicare payments to providers of 2% per fiscal year effective April 1, 2013, and, due to subsequent legislative amendments to the statute, will stay in effect through 2025, unless additional congressional action is taken. The American Taxpayer Relief Act of 2012, further reduced, among other things, Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our drugs, if approved, and, accordingly, our financial operations.
-22-
Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our drugs.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of vilobelimab, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent drug labeling and post-marketing testing and other requirements.
Even if we, or any future collaborators, obtain marketing approvals or the EUA for our product candidates, the terms of approvals and ongoing regulation of our products may limit how we manufacture and market our products, which could impair our ability to generate revenue
Once marketing approval or the EUA has been granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation. We, and any future collaborators, must therefore comply with requirements concerning advertising and promotion for any of our product candidates for which we or they obtain marketing approval. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved labeling. Thus, we and any future collaborators will not be able to promote any products we develop for indications or uses for which they are not approved.
In addition, manufacturers of authorized and approved products and those manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to cGMPs, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, our contract manufacturers, any future collaborators and their contract manufacturers could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with cGMPs.
Accordingly, assuming we, or any future collaborators, receive marketing approval for one or more of our product candidates, we, and any future collaborators, and our and their contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control.
The manufacturing and distribution of GOHIBIC (vilobelimab) is subject to a number of risks that could harm our reputation, business, financial condition and operating results.
The manufacturing processes of GOHIBIC(vilobelimab) is complex. We may encounter manufacturing difficulties, including difficulties related to product storage and shelf-life. Such difficulties could result from the complexities of manufacturing product batches at a larger scale, equipment failure, availability of excipients and other product components/ingredients (including related to choice and quality of raw materials), analytical testing technology and product instability. Specifically, insufficient product stability or shelf-life of GOHIBIC (vilobelimab) or its components could materially limit or delay our or our collaborators’ ability to distribute and commercialize GOHIBIC(vilobelimab) at the current price or at all. Further, if GOHIBIC(vilobelimab) becomes subject to a product recall, including as the result of manufacturing errors, design/labeling defects or other deficiencies, our reputation would be adversely affected.
GOHIBIC (vilobelimab) is a “cold-chain product” that must be shipped and stored at cold temperatures. We could lose supply of GOHIBIC (vilobelimab) due to distribution difficulties, including generally related supply chain management (e.g., shelf-life expiration) and specifically related to shipping and storing GOHBIC(vilobelimab) at cold temperatures. If so, we could incur additional manufacturing costs in order to supply required quantities to U.S. hospitals under the EUA.
Any such manufacturing and distribution difficulties may harm our reputation, business, financial condition and operating results.
-23-
Governments, including those outside the United States, tend to impose strict price controls, which may adversely affect our revenues, if any
In many countries, such as countries of the European Union, the pricing of prescription pharmaceuticals is subject to varying price control mechanisms, often as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we, or any future collaborators, may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed. Additional price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates, and we believe the increasing emphasis on cost-containment initiatives in the European Union has and will continue to put pressure on the pricing and usage of our product candidates. As a result, given the relatively smaller target markets for severe COVID-19 and PG, any reduced reimbursement for such product candidates may be insufficient for us to generate commercially reasonable revenue and profits and would adversely affect our financial condition and results of operations.
Any of our product candidates for which we, or any future collaborators, obtain marketing approval or the EUA in the future could be subject to post-marketing restrictions or withdrawal from the market and we, or any future collaborators, may be subject to substantial penalties if we, or they, fail to comply with regulatory requirements or if we, or they, experience unanticipated problems with our products following approval or the EUA
Any of our product candidates for which we, or any future collaborators, obtain marketing approval or the EUA, as well as the manufacturing processes, post-approval studies and measures, labeling, advertising and promotional activities for such product, among other things, will be subject to ongoing requirements of and review by the FDA, the EMA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval or the EUA of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, including the requirement to implement a REMS.
The FDA, the EMA and other regulatory authorities may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of a product. The FDA and other agencies, including the Department of Justice, closely regulate and monitor the post-approval marketing and promotion of products to ensure that they are manufactured, marketed and distributed only for the authorized or approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we, or any future collaborators, do not market any of our product candidates for which we, or they, receive marketing approval for only their approved indications, we, or they, may be subject to warnings or enforcement action for off-label marketing. Violation of the FDCA and other statutes relating to the promotion and advertising of prescription drugs may lead to investigations or allegations of violations of federal and state health care fraud and abuse laws and state consumer protection laws, including the False Claims Act.
In addition, later discovery of previously unknown adverse events or other problems with our products or their manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| ● | restrictions on the manufacturing of such products; |
| ● | restrictions on the labeling or marketing of such products; |
| ● | restrictions on product distribution or use; |
| ● | requirements to conduct post-marketing studies or clinical trials; |
| ● | warning letters or untitled letters; |
-24-
| ● | withdrawal of the products from the market; |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; |
| ● | recall of products; |
| ● | restrictions on coverage by third-party payors; |
| ● | fines, restitution or disgorgement of profits or revenues; |
| ● | suspension or withdrawal of marketing approvals, including the EUA; |
| ● | refusal to permit the import or export of products; |
| ● | product seizure; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
Our ability to successfully commercialize and generate revenue from sales of GOHIBIC (vilobelimab) is subject to a number of risks that could harm our business, financial condition and operating results.
Our ability to successfully commercialize GOHIBIC (vilobelimab) is subject to a number of risks that could impact our business, financial condition and operating results. Specifically, our ability to generate revenue from sales of GOHIBIC (vilobelimab) is uncertain, including due to the market opportunity for, and interest and perception in, GOHIBIC (vilobelimab). In particular, given fluctuations in the number of patients developing severe symptoms from COVID-19 infections, the size of the addressable patient population and, thus, the market opportunity for GOHIBIC (vilobelimab) is uncertain and may shrink over time. In addition, since GOHIBIC (vilobelimab) has the EUA, but not the FDA approval, sales of GOHIBIC (vilobelimab) depend on whether healthcare providers at U.S. hospitals are interested in and receptive to providing GOHIBIC (vilobelimab) as a treatment for COVID-19. Specifically, if GOHIBIC (vilobelimab) is not included in the treatment guidelines issued by medical institutions and other third-party medical/healthcare organizations, such as the NIH, or if such institutions and organizations do not recommend GOHIBIC (vilobelimab), hospitals may not be willing to make GOHIBIC (vilobelimab) available for treatment of patients. For example, the NIH guidelines stipulate that there is insufficient evidence to recommend either for or against the use of GOHIBIC (vilobelimab) for the treatment of critically ill COVID-19 patients. This neutral NIH guideline has negatively affected the commercial adoption of GOHIBIC (vilobelimab) as many healthcare providers, particularly hospitals, rely on NIH treatment guidelines when deciding to include prescription drugs to their formularies allowing for the placement of product orders by hospital staff. Ultimately, if we are unable to successfully commercialize and generate revenue from sales of GOHIBIC (vilobelimab), our business, financial condition and operating results could be adversely affected.
2. Risks related to our dependence on third parties
We rely on third parties to conduct our clinical trials. If they do not perform satisfactorily, our business could be harmed
We do not independently conduct clinical trials of any of our product candidates. We rely on third parties, such as CROs, clinical data management organizations, third-party consultants, medical institutions and clinical investigators, to conduct these clinical trials and expect to rely on these third-parties to conduct clinical trials of any other product candidate that we develop. Any of these third parties may terminate their engagements with us under certain circumstances. We may not be able to enter into alternative arrangements or do so on commercially reasonable terms. In addition, there is a natural transition period when a new CRO begins work. As a result, delays would likely occur, which could negatively impact our ability to meet our expected clinical development timelines and harm our business, financial condition and prospects.
-25-
Further, although our reliance on these third parties for clinical development activities limits our control over these activities, we remain responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal and regulatory requirements and scientific standards. For example, notwithstanding the obligations of a CRO for a trial of one of our product candidates, we remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA, the EMA and potentially other regulatory agencies of different countries require us to comply with requirements, commonly referred to as current Good Clinical Practices, or cGCP, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. The FDA and regulatory agencies inside the European Union and other regulatory agencies enforce these cGCP regulations through periodic inspections of trial sponsors, principal investigators, clinical trial sites and IRBs. If we or our third-party contractors fail to comply with applicable cGCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or other regulatory agencies may require us to perform additional clinical trials before approving our product candidates, which would delay the marketing approval process. We cannot be certain that, upon inspection, the FDA or other regulatory agencies will determine that any of our clinical trials comply with cGCP. We are also required to register clinical trials and post the results of completed clinical trials on a government-sponsored database, such as ClinicalTrials.gov in the United States, within certain timeframes. The same requirement applies to clinical trials outside the United States, such as EudraCT.ema.europa.eu in Europe. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, the third parties conducting clinical trials on our behalf are not our employees, and except for remedies available to us under our agreements with such contractors, we cannot control whether or not they devote sufficient time, skill and resources to our ongoing development programs. These contractors may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug development activities, which could impede their ability to devote appropriate time to our clinical programs. If these third parties, including clinical investigators, do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates. If that occurs, we will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates. In such an event, our financial results and the commercial prospects for any product candidates that we seek to develop could be harmed, our costs could increase and our ability to generate revenues could be delayed, impaired or foreclosed.
Our reliance on foreign third-party manufacturers and suppliers increases our risk of obtaining adequate, timely and cost-effective product candidates and products
Foreign manufacturing is subject to a number of risks, including political and economic disruptions, the imposition of tariffs, quotas and other import or export controls, changes in governmental policies and geopolitical uncertainty and instability, which have created market volatility. In particular, we rely on third-party manufacturer located in China and elsewhere for supply of vilobelimab. We outsource all manufacturing of our product candidates and products to third parties while conducting certain quality control tests in-house. The supply chain and manufacturing in China may, also as a result of the current global pandemic as well as the global political situation, significantly impact our operations.
We engage a third-party manufacturer located in China for the clinical supply of the final drug product formulation of vilobelimab. There is no assurance that we would be able to timely secure needed alternative supply arrangements on satisfactory terms, or at all, if needed. Our reliance on our manufacturer and our failure to secure alternative supply arrangements as needed could have a material adverse effect on our ability to complete the development of our product candidates or, to commercialize them, if approved. There may be difficulties in scaling up to commercial quantities or optimization of processes and formulation of vilobelimab and the costs of manufacturing could be prohibitive.
Even if we were able to establish and maintain arrangements with other third-party manufacturers, reliance on third-party manufacturers generally entails additional risks beyond our control, including:
| ● | reliance on third parties for manufacturing process development, regulatory compliance and quality assurance; |
| ● | reliance on third parties for storage and safeguarding of substantially all inventory; |
| ● | costs and validation of new equipment and facilities required for additional scale-up or optimization of processes; |
| ● | failure to comply with cGMP and similar foreign standards; |
| ● | limitations on supply availability resulting from capacity and scheduling constraints of third parties; |
| ● | lack of qualified backup suppliers for those components that are purchased from a sole or single source supplier; |
| ● | closures and restrictions on critical facilities resulting from public health crises; |
-26-
| ● | the ability to freely import clinical trial material and potentially marketing material manufactured at our third-party manufacturer in China into the countries in which the clinical trials are being conducted or product potentially to be sold; |
| ● | the possible breach of manufacturing agreements by third parties because of factors beyond our control; and |
| ● | the possible termination or non-renewal of the manufacturing agreements by the third party, at a time that is costly or inconvenient to us, and our ability to obtain alternative supply. |
If we do not maintain our key manufacturing relationships, we may fail to find replacement manufacturers or develop our own manufacturing capabilities, which could delay or impair our ability to obtain regulatory approval for our products. If we do find replacement manufacturers, we may not be able to enter into agreements with them on terms and conditions favorable to us and there could be a substantial delay before new facilities could be qualified and registered with the FDA and other foreign regulatory authorities. In addition, a change of the manufacturing facility contains inherent risks and is generally viewed as a major change in the manufacturing process such that comparability studies have to be conducted to assure comparability between the before established manufacturing process and the newly established manufacturing process potentially causing delays in the drug product supply or, in case of a non-comparability of the manufactured drug product, warrant further additional pre-clinical and or clinical studies with such non-comparable drug product which may also be imposed by any regulatory agency upon review of the comparability data. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
The process of manufacturing biologics, such as vilobelimab, is extremely susceptible to product loss
The process of manufacturing our products is complex, highly regulated and subject to several other risks. The process of manufacturing biologics, such as vilobelimab, is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, vendor or operator error, inconsistency in yields, variability in product characteristics and difficulties in scaling the production process. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. Further, our product candidates that have been produced and are stored for later use may degrade, become contaminated or suffer other quality defects, which may cause the affected product candidates to no longer be suitable for their intended use in clinical trials or other development activities. If the defective product candidates cannot be replaced in a timely fashion, we may incur significant delays in our development programs that could adversely affect the value of such product candidates and, thus, adversely affect our business, financial condition, results of operations and prospects.
Our manufacturing process is subject to quality control risks and related regulatory requirements
We participate in the manufacturing process with crucial quality control testing within our own laboratories, and we hold the manufacturer license for, and therefore oversee, the overall manufacturing process, and we are responsible for ensuring that this part of our business also operates according to cGMP standards. Additionally, we hold an importing license. We therefore employ key personnel within the manufacturing process, such as a head of quality assurance, a head of manufacturing and a qualified person.
Thus, our laboratories and our quality control system and related documentation and personnel, are also subject to frequent governmental inspections to assure adherence to cGMP guidelines and to maintain our manufacturing and importing license. Related to these activities, there are risks which could negatively impact our ability to meet our expected clinical development timelines and harm our business, financial condition and prospects, including:
| ● | a loss of key personnel within the manufacturing activities could result in significant delays in the manufacturing and release testing of our drug candidate and replacement of such personnel could be time consuming and be associated with additional costs for us; |
| ● | mistakes or misconduct within the release testing could result in false results which could result in both, the wrongfully rejection of a manufactured drug product from being released or the wrongfully acceptance of a dysfunctional drug product, causing data and trial results achieved with such drug product being false and potentially wrongly interpreted; and |
| ● | an inadequate cGMP compliance could result in a potential temporary or permanent loss of the manufacturing or importing license resulting from an inspection of regulatory agencies. |
-27-
Our third-party manufacturers, or we, may not be able to comply with the cGMP regulatory requirements applicable to vilobelimab and biologics, including applicable provisions of the FDA’s drug cGMP regulations, device cGMP requirements embodied in the Quality System Regulation, or QSR, or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, seizures or voluntary recalls of product candidates, operating restrictions and criminal prosecutions, any of which could significantly affect supplies of our product candidates. In addition, our third-party manufacturers and suppliers and we are subject to FDA and other local regulatory authority inspection from time to time. Failure by our third-party manufacturers and suppliers or us to pass such inspections and otherwise satisfactorily complete the FDA approval regimen with respect to our product candidate may result in regulatory actions such as the issuance of FDA Form 483 notices of observations, warning letters or injunctions or the loss of operating licenses.
In addition, we and our third-party manufacturers and suppliers are subject to numerous environmental, health and safety laws and regulations, including those governing the handling, use, storage, treatment and disposal of waste products, and failure to comply with such laws and regulations could result in significant costs associated with civil or criminal fines and penalties for such third parties. Based on the severity of the regulatory action, our clinical or commercial supply of drug and packaging and other services could be interrupted or limited, which could have a material adverse effect on our business, including our clinical research activities and our ability to develop our product candidates and market our products following approval, if any.
If any third-party manufacturer of our product candidates is unable to increase the scale of its production of our product candidates, and/or increase the product yield of its manufacturing, then our costs to manufacture the product may increase and commercialization may be delayed
In order to produce sufficient quantities to meet the demand for clinical trials and, if approved, subsequent commercialization of vilobelimab or any of our other product candidates in our pipeline or that we may develop, our third-party manufacturers will be required to increase their production and optimize their manufacturing processes while maintaining the quality of the product. The transition to larger scale production could prove difficult or costly. Further, any claims in our manufacturing process as a result of scaling up or optimization of the manufacturing, supply and fill process may result in the need to obtain regulatory approvals. If our third-party manufacturers are not able to optimize manufacturing process to increase the product yield for our product candidates or are unable to produce increased amounts of our product candidates while maintaining the quality of the product, then we may not be able to meet the demands of clinical trials or market demands, which could decrease our ability to generate profits. Difficulty in achieving commercial scale-up production or production optimization or the need for additional regulatory approvals as a result could have a material adverse impact on our business and results of operations.
We expect to seek to establish collaborations and, if we are not able to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans
We expect to seek one or more collaborators for the development and commercialization of one or more of our product candidates. Likely collaborators may include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. In addition, if we obtain marketing authorization or approval for product candidates from foreign regulatory authorities, we may enter into strategic relationships with international biotechnology or pharmaceutical companies for the commercialization of such product candidates outside of the United States.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the potential differentiation of our product candidate from competing product candidates, design or results of clinical trials, the likelihood of approval by the FDA, the EMA or comparable foreign regulatory authorities and the regulatory pathway for any such approval, the potential market for the product candidate, the costs and complexities of manufacturing and delivering the product to patients and the potential of competing products. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available for collaboration and whether such a collaboration could be more attractive than the one with us for our product candidate. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
-28-
Collaborations are complex and time-consuming to negotiate and document. Further, there have been a significant number of recent business combinations among large pharmaceutical companies that may have resulted in a reduced number of potential future collaborators. Any collaboration agreements that we enter into in the future may contain restrictions on our ability to enter into potential collaborations or to otherwise develop specified product candidates. We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all.
If we are unable to do so, we may have to curtail the development of the product candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense.
If we enter into collaborations with third parties for the development and commercialization of our product candidates, our prospects with respect to those product candidates will depend in significant part on the success of those collaborations
We expect to maintain existing collaborations and enter into additional collaborations for the development and commercialization of certain of our product candidates and in certain geographies. We may have limited control over the amount and timing of resources that our collaborators will dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on any future collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. In addition, any future collaborators may have the right to abandon research or development projects and terminate applicable agreements, including funding obligations, prior to or upon the expiration of the agreed upon terms.
Collaborations involving our product candidates pose a number of risks, including the following:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| ● | collaborators may not perform their obligations as expected; |
| ● | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs, based on clinical trial results, changes in the collaborators’ strategic focus or available funding or external factors, such as an acquisition, that divert resources or create competing priorities; |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates; |
| ● | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| ● | disagreements with collaborators, including disagreements over proprietary rights, including trade secrets and other intellectual property, contract interpretation, or the preferred course of research and development might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would be time-consuming and expensive; |
| ● | collaborators may not properly prosecute, maintain, defend or enforce our intellectual property rights or may use our proprietary information or other intellectual property in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or expose us to potential litigation; |
| ● | collaborators may infringe, misappropriate or otherwise violate the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| ● | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates; and |
| ● | collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If any future collaborator of ours is involved in a business combination, it could decide to delay, diminish or terminate the development or commercialization of any product candidate licensed to it by us. |
-29-
Changes in funding or disruptions at the FDA and other governmental agencies caused by funding shortages or global health concerns could hinder their ability to perform normal business functions on which the operation of our business may rely, which could negatively impact our business
The ability of the FDA to review and clear or approve new product candidates and products can be affected by a variety of factors, including:
| ● | government budget and funding levels, and statutory, regulatory and policy changes; |
| ● | the FDA’s ability to hire and retain key personnel and accept the payment of user fees; and |
| ● | federal government shutdowns and other events that may otherwise affect the FDA’s ability to perform routine functions. |
Average review times at the agency have fluctuated in recent years as a result.
Further, if a prolonged government shutdown occurs, or if global health concerns continue to prevent or delay the FDA or other regulatory authorities from conducting, at all or in a timely manner, their regular inspections, reviews or other regulatory activities, the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions could be significantly impacted, which could have a material adverse effect on our business.
3. Risks related to our intellectual property
Our success depends on our ability to obtain, maintain, protect, defend and enforce patent, trade secret and other intellectual property protection
Our success depends on our ability to obtain, maintain, protect, defend and enforce patent, trade secret and other intellectual property protection in the United States and other countries with respect to vilobelimab and other proprietary product candidates. If we do not adequately protect, maintain, defend and enforce our intellectual property rights, competitors may be able to erode, negate or preempt any competitive advantage we may have, which could adversely affect our business and ability to achieve profitability. To seek to protect our proprietary position, we file patent applications in the United States and in certain other countries related to our novel product candidates and their potential use in different medical indications that are important to our business. The patent application and approval process is expensive and time-consuming and we may not be able to file and prosecute all necessary or desirable patent applications and obtain and maintain issued patents at a reasonable cost or in a timely manner.
If the scope of the patent protection we obtain is not sufficiently broad, we may not be able to prevent others from developing and commercializing technology and products similar or identical to ours. The degree of patent protection we require to successfully compete in the market may be unavailable or severely limited in some cases and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Although we enter into non-disclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, contractors, prospective business collaborators, clinical investigators and other third parties, any of these parties could breach the agreements and disclose such output before a patent application is filed, which could jeopardize our ability to seek and obtain patent protection. In addition, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions.
-30-
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights may be uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or product candidates or which effectively prevent others from commercializing competitive technologies and product candidates. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. For example, there can be no assurance that our issued patents contain and pending patent applications will contain, when granted, claims of sufficient breadth to cover all antibodies alleged to be a biosimilar of our product candidates. Furthermore, there can be no assurance that our issued patents will not be challenged at the United States Patent and Trademark Office, or USPTO, or foreign patent offices or in court proceedings, and if any such challenge were successful, the scope of our issued patent claims could be limited so as to not cover antibodies alleged to be a biosimilar of our product candidates. In addition, changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. In addition, the laws of other countries may not protect our rights to the same extent or in the same manner as the laws of the United States. For example, patent laws in various jurisdictions, including significant commercial markets, such as Europe, restrict the patentability of methods of treatment of the human body more than patent laws in the United States.
Some of our future patents and patent applications and other intellectual property may be co-owned with third parties. If we are unable to obtain an exclusive license to any such third-party co-owners’ interest in such patents or patent applications or other intellectual property, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In addition, we would need the cooperation of any such co-owners of our patents in order to enforce such patents against third parties, and such cooperation may not be provided to us. Furthermore, we, or any future partners, collaborators, or licensees, may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, we may miss potential opportunities to strengthen our patent position. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Our patents covering our proprietary anti-C5a and anti C5aR technologies may be subject to challenge, narrowing, circumvention and invalidation by third parties
Any of our patents may be challenged, narrowed, circumvented, or invalidated by third parties. The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, and our patents may be challenged in the courts or patent offices in the United States and elsewhere. We may be subject to a third-party pre-issuance submission of prior art to the USPTO or become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review, or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, enforceability or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Moreover, we may have to participate in interference proceedings declared by the USPTO to determine priority of invention or in post-grant challenge proceedings, such as oppositions in a foreign patent office, that challenge priority of invention or other features of patentability. Such challenges may result in loss of patent rights, loss of exclusivity, or in patent claims being narrowed, invalidated, or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and product candidates. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
In addition, our competitors and other third parties may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner. For example, a third party may develop a competitive therapy that provides benefits similar to vilobelimab or other product candidates but that uses a technology that falls outside the scope of our patent protection. Our competitors may also seek approval to market generic versions of any approved products and in connection with seeking such approval may claim that our patents are invalid, unenforceable or not infringed. In these circumstances, we may need to defend or assert our patents, or both, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid or unenforceable, or that our competitors are competing in a non-infringing manner. Thus, even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives. If the patent protection provided by the patents and patent applications we hold or pursue with respect to our product candidates is not sufficiently broad to impede such competition, our ability to successfully commercialize our product candidates could be negatively affected, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
-31-
We cannot be sure that we were the first to make the anti-C5a and anti-C5aR technologies claimed in our patents or patent applications or that we were the first to file for patent protection
Assuming the other requirements for patentability are met, currently, the first to file a patent application is generally entitled to the patent. However, prior to March 16, 2013, in the United States, the first to invent was entitled to the patent. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. Similarly, we cannot be certain that parties from whom we may license or purchase patent rights were the first to make relevant claimed inventions, or were the first to file for patent protection for them. If third parties have filed patent applications on inventions claimed in our patents or applications on or before March 15, 2013, an interference proceeding in the United States can be initiated by such third parties to determine who was the first to invent the subject matter covered our patent applications. If third parties have filed such applications after March 15, 2013, a derivation proceeding in the United States can be initiated by such third parties to determine whether our invention was derived from theirs.
The patent application process is subject to numerous risks and there can be no assurance that we will be successful in obtaining patents for which we have applied
Pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until such patent is issued. The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that we or any of our future development partners will be successful in protecting our product candidates by obtaining and defending patents. These risks and uncertainties include the following:
| ● | the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case; |
| ● | the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance; |
| ● | patent applications may not result in any patents being issued; |
| ● | patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked, circumvented, narrowed, found to be unenforceable or otherwise may not provide any competitive advantage; |
| ● | our competitors, many of whom have substantially greater resources and many of whom have made significant investments in competing technologies, may seek or may have already obtained patents that will limit, interfere with or eliminate our ability to make, use, and sell our potential product candidates; |
| ● | there may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns; and |
| ● | countries other than the United States may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop and market competing product candidates. |
Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
-32-
It is difficult and costly to protect our intellectual property and our proprietary anti-C5a and anti-C5aR technologies, and we may not be able to ensure their protection
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection for the composition, use and structure of our product candidates, the methods used to manufacture them, the related therapeutic targets and associated methods of treatment as well as on successfully defending these patents against potential third-party challenges. Our ability to protect our product candidates from unauthorized making, using, selling, offering to sell or importing by third parties is dependent on the extent to which we have rights under valid and enforceable patents that cover these activities.
The ultimate determination by the USPTO or by a court or other trier of fact in the United States, or any corresponding foreign patent offices or courts or other triers of fact, on whether a claim meets all requirements of patentability cannot be assured. Although our C5a and C5aR inhibitor portfolio consists of six families of patents and patent applications that we own directed to C5a and C5aR inhibitors and related methods of use, we cannot predict the breadth of claims that may be allowed or enforced in our patents or patent applications, in our future licensed patents or patent applications or in third-party patents.
We cannot provide assurances that any of our patent applications will be found to be patentable, including over our own prior art patents, publications or other disclosures. Furthermore, given the differences in patent laws in the United States, Europe and other foreign countries, for example, the availability of grace periods for filing patent applications and what can be considered as prior art, we cannot make any assurances as to the scope of any claims that may issue from our pending and future patent applications in the United States or in other jurisdictions. Similarly, we cannot make any assurances as to the scope of any claims that may survive a proceeding initiated by a third party challenging the patentability, validity or enforceability of our patents and patent applications in the United States or in other jurisdictions. Any such challenge, if successful, could limit patent protection for our product candidates and/or materially harm our business.
The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| ● | we may not be able to generate sufficient data to support patent applications that protect the entire breadth of developments in one or more of our programs; |
| ● | it is possible that one or more of our pending patent applications will not become an issued patent or, if issued, that the patent(s) will be insufficient to protect our technology or products, provide us with a basis for commercially viable products or provide us with any competitive advantages; |
| ● | if our pending patent applications issue as patents, they may be challenged by third parties as not infringed, invalid or unenforceable under United States or foreign laws; or |
| ● | if issued, the patents under which we hold rights may not be valid or enforceable. |
In addition, to the extent that we are unable to obtain and maintain patent protection for one of our product candidates or in the event that such patent protection expires, it may no longer be cost-effective to extend our portfolio by pursuing additional development of a product or product candidate for follow-on indications. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Obtaining and maintaining patent protection of our anti-C5a and anti-C5aR technologies depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. We may enter into certain license agreements where we will not have the ability to maintain or prosecute patents in the portfolio and must therefore rely on third parties to take such actions and comply with certain requirements. Failure by us or our future or any existing licensors to maintain protection of our patent portfolio could have a material adverse effect on our business, financial condition, results of operations and prospects.
-33-
In addition, it is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, or requests for patent term adjustments. If we fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced, eliminated, invalid and/or unenforceable. If any of our present or future partners, collaborators, licensees, or licensors, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form, preparation, prosecution, or enforcement of our patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents. Any of these outcomes could impair our ability to prevent competition from third parties, which may have a material adverse effect on our business, financial condition, results of operations and prospects.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time and if we do not obtain protection under the Drug Price Competition and Patent Term Restoration Act of 1984, or Hatch-Waxman Amendments, and similar non-U.S. legislation for extending the term of patents covering each of our product candidates, our business may be materially harmed.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is generally twenty years after it is filed. Various extensions may be available, however, the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with adequate and continuing patent protection sufficient to exclude others from commercializing products similar to our product candidates.
Depending upon the timing, duration and conditions of the FDA marketing approval of our product candidates, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments and similar legislation in the EU and other jurisdictions. The Hatch-Waxman Amendments permit a patent term extension of up to five years for a patent covering an approved product as compensation for effective patent term lost during product development and the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. In Europe, a maximum of five and a half years of supplementary protection can be achieved for an active ingredient or combinations of active ingredients of a medicinal product protected by a basic patent, if a valid marketing authorization exists (which must be the first authorization to place the product on the market as a medicinal product) and if the product has not already been the subject of supplementary protection. However, we may not receive an extension if we fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the extension could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for that product will be shortened and our competitors may obtain approval to market competing products sooner. As a result, our revenue from applicable products could be reduced and could have a material adverse effect on our business, financial condition, results of operations and prospects.
Others may claim an ownership interest in our intellectual property and proprietary anti-C5a and anti-C5aR technologies which could expose us to litigation and have a significant adverse effect on our prospects
A third party may claim an ownership interest in one or more of our, or our future or any existing licensors’, patents or other proprietary or other intellectual property rights. A third party could bring legal actions against us and seek monetary damages and/or enjoin clinical testing, manufacturing and marketing of the affected product or products. While we are presently unaware of any material claims or assertions by third parties with respect to our patents or other intellectual property, we cannot guarantee that a third party will not assert a claim or an interest in any of such patents or other intellectual property. If we become involved in any litigation, it could consume a substantial portion of our resources, and could cause a significant diversion of effort by our technical and management personnel. If any of these actions are successful, in addition to any potential liability for damages, we could be required to obtain a license to continue to manufacture or market the affected product, in which case we may be required, for example, to pay substantial royalties or grant cross-licenses to our patents. We cannot, however, assure you that any such license will be available on acceptable terms, if at all. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations as a result of claims of patent infringement or other violations of other intellectual property rights. Further, the outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance, including the demeanor and credibility of witnesses and the identity of any adverse party. This is especially true in intellectual property cases that may turn on the testimony of experts as to technical facts upon which experts may reasonably disagree. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
-34-
If we are sued for infringing, misappropriating, or otherwise violating intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing our product candidates
Our commercial success depends, in part, on our ability to develop, manufacture, market and sell our product candidates without infringing, misappropriating, or otherwise violating the proprietary or any other intellectual property rights of third parties. Third parties may have U.S. and non-U.S. issued patents and pending patent applications relating to compounds, methods of manufacturing compounds and/or methods of use for the treatment of the disease indications for which we are developing our product candidates that may cover our product candidates or approach to complement inhibition. If any third-party patents or patent applications are found to cover our product candidates or their methods of use or manufacture, or our approach to complement inhibition, we may not be free to manufacture or market our product candidates as planned without obtaining a license, which may not be available on commercially reasonable terms or at all.
There is a substantial amount of intellectual property litigation in the biotechnology and pharmaceutical industries, and we may become party to, or threatened with, litigation or other adversarial proceedings regarding intellectual property rights with respect to our product candidates, including interference and post-grant proceedings before the USPTO. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the composition, use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be pending patent applications which may later result in issued patents that our product candidates may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Accordingly, third parties may assert infringement claims against us based on intellectual property rights that exist now or arise in the future. The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance. The pharmaceutical and biotechnology industries have produced a significant number of patents, and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use or manufacture. The scope of protection afforded by a patent is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our product candidates, products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could significantly harm our business and operating results. In addition, we may not have sufficient resources to bring these actions to a successful conclusion.
If we are found to infringe, misappropriate, or otherwise violate a third party’s intellectual property right, we could be forced, including by court order, to cease developing, manufacturing or commercializing the infringing product candidate or product. Alternatively, we may be required to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or commercializing the infringing product candidate or product. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us; alternatively or additionally, it could include terms that impede or destroy our ability to compete successfully in the commercial marketplace. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could harm our business. Claims that we have misappropriated the trade secrets or other confidential information of any third parties could have a similar negative impact on our business. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
-35-
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property and proprietary anti-C5a and anti-C5aR technologies
Many of our current and former employees and our licensors’ current and former employees, including our senior management, were previously employed at universities or at other biotechnology or pharmaceutical companies, including some which may be competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such third party. Litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms or at all. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while we typically require our employees, consultants and contractors who are involved in the development of intellectual property for us within the scope of such employees’, consultants’ and contractors’ employment or other engagement by us to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, or such agreements may be breached or alleged to be ineffective, which may result in claims by or against us related to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may lose exclusivity to certain of our intellectual property rights to the German federal government
We hold all of our intellectual property through our wholly owned subsidiary InflaRx GmbH in Germany. In the event of a national epidemic or pandemic, the German federal government, and the Federal Ministry of Health and other authorities have the right to order the use of our owned and in-licensed patents in the interest of the public welfare or the security of the Federal Republic of Germany. The German federal government may issue such an order with respect to our owned and in-licensed patents and we may lose exclusivity with respect to the technologies covered by such patents.
Additionally, the research resulting in certain of our patents and technology, including patents and technology relating to our clinical development in severe COVID-19, was funded in part by the German federal government. Results of such government funded research projects must, subject to certain conditions, be made available free of charge for academic research and teaching in Germany and must be published in bi-annual interim reports and a final report following completion of the funded work. Information relating to intellectual property generated, commercial expectations, scientific chances of success, next steps and certain additional information must be disclosed to the German government and to third parties for academic research and teaching upon request under a written confidentiality agreement. The German federal government additionally has, in the case of a special public interest, a non-exclusive and transferable right to use intellectual property generated as part of the funded work.
-36-
Certain of our employees and directors are subject to German law, including as it relates to the ownership of, and compensation for, inventions
A number of our personnel, including some of our directors, work in Germany and may be subject to German employment law. Inventions that may be the subject of a patent or of protection as a utility model as well as technical improvement proposals for other technical innovations that may not be the subject of a patent or of protection as a utility model made by such employees are subject to the provisions of the German Act on Employees’ Inventions (Gesetz über Arbeitnehmererfindungen), which regulates the ownership of, and compensation for, inventions made by employees. We face the risk that disputes may occur between us and our current or past employees pertaining to the sufficiency of compensation paid by us, allocation of rights to inventions under the German Act on Employee’s Inventions or alleged non-adherence to the provisions of this act, any of which may be costly to resolve and take up our management’s time and efforts whether we prevail or fail in such dispute. In addition, under the German Act on Employees’ Inventions, certain employees retain rights to patents they invented or co-invented and disclosed to us prior to October 1, 2009. While we believe that all of our current and past German employee inventors have subsequently assigned to us their interest in patents and inventions they invented or co-invented, there can be no assurance that all such assignments are fully effective. Even if we lawfully own all inventions of our employee inventors who are subject to the German Act on Employees’ Inventions, we are required under German law to reasonably compensate such employees for the use of the patents.
If any of our current or past employees obtain or retain ownership of any inventions or other intellectual property rights that we believe we own, we may lose valuable intellectual property rights and may be required to obtain and maintain licenses from such employees to such inventions or intellectual property rights, which may not be available on commercially reasonable terms or at all, or may be non-exclusive. If we are unable to obtain and maintain a license to any such employee’s interest in such inventions or intellectual property rights, we may need to cease the development, manufacture, and commercialization of one or more of the product candidates we may develop. In addition, any loss of exclusivity of our intellectual property rights could limit our ability to stop others from using or commercializing similar or identical technology and products. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may not be able to enforce our intellectual property rights throughout the world
Filing, prosecuting, maintaining, enforcing and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. The requirements for patentability may differ in certain countries, particularly in developing countries; thus, even in countries where we do pursue patent protection, there can be no assurance that any patents will issue with claims that cover our product candidates.
Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in the United States and foreign intellectual property laws. Additionally, laws of some countries outside of the United States and Europe do not afford intellectual property protection to the same extent as the laws of the United States and Europe. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, including India, China and other countries, do not favor the enforcement of patents and other intellectual property rights. This could make it difficult for us to stop the infringement of our patents or the misappropriation or other violations of our other intellectual property rights. For example, many countries outside the United States have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in certain countries outside the United States and Europe. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop and market their own products and, further, may export otherwise infringing products to jurisdictions where we have patent protection, if our ability to enforce our patents to stop infringing activities is inadequate. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Agreements under which we may be granted a license to any patent rights may not give us sufficient rights to permit us to pursue enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents (or control of enforcement or defense) of such patent rights in all relevant jurisdictions as requirements may vary.
Proceedings to enforce our patent rights in the United States or foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and resources from other aspects of our business. Moreover, such proceedings could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Furthermore, while we intend to seek to protect our intellectual property rights in major markets for our product candidates, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our product candidates. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
-37-
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful
Our competitors and others may infringe, misappropriate or otherwise violate our patents or other intellectual property rights. To counter infringement or unauthorized use, we may be required to file infringement or other claims, which can be expensive and time consuming and divert the time and attention of our management and scientific personnel.
Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents, in addition to counterclaims asserting that our patents are invalid or unenforceable, or both. In any patent infringement proceeding, there is a risk that a court will decide that a patent of ours is invalid or unenforceable, in whole or in part, and that we do not have the right to stop the other party from using the invention at issue. There is also a risk that, even if the validity of such patents is upheld, the court will construe the patent’s claims narrowly or decide that we do not have the right to stop the other party from using the invention at issue on the grounds that our patent claims do not cover the invention. An adverse outcome in a litigation or proceeding involving one or more of our patents could limit our ability to assert those patents against those parties or other competitors and may curtail or preclude our ability to exclude third parties from developing, making and selling similar or competitive products. Even if we establish infringement, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could adversely affect the price of our ordinary shares. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are concluded. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings. Any such litigation could have a material adverse effect on our business, financial condition, results of operations and prospects.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our marks of interest and our business may be adversely affected
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. During trademark registration proceedings, we may receive rejections that we are unable to overcome, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected.
If we fail to comply with our obligations under any future or other intellectual property licenses with third parties, we could lose license rights that are important to our business
We may be reliant upon licenses to certain patent rights and proprietary anti-C5a and anti-C5aR technologies and other intellectual property from third parties that are important or necessary to the development of our product candidates and the manufacture and other commercialization of our products. These and other licenses may not provide exclusive rights to use such intellectual property and technology in all relevant fields of use and in all territories in which we may wish to develop, manufacture or commercialize our technology and products in the future. As a result, we may not be able to prevent competitors from developing, manufacturing and commercializing competitive products in territories included in all of our licenses. Our licensors may have sublicensed patents and other intellectual property owned by a third party, or relied on third-party consultants or collaborators or funds from third parties that have an ownership or other right, title or interest in or to such in-licensed intellectual property, such that our licensors are not the sole and exclusive owners of the patents and other intellectual property we in-license. This could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
-38-
In addition, agreements under which we may license patent rights may not give us control over patent filings prosecution or maintenance, so that we may not be able to control which claims or arguments are presented and may not be able to secure, maintain, or successfully enforce and defend necessary or desirable patent protection from those patent rights. We cannot be certain that patent filing prosecution and maintenance activities by our licensors will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents. Even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our future or any existing licensors, and cannot guarantee that we would receive it and on what terms. We cannot be certain that our future licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in any licensed patents. If we cannot obtain patent protection or enforce existing or future patents against third parties, it could have a material adverse effect on our business, financial condition, results of operations and prospects.
Further, agreements under which we may license technology or any other intellectual property to or from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant technology or any other intellectual property, or increase what we believe to be our financial or other obligations under the relevant agreement. Moreover, if disputes over technology or other intellectual property that we may license prevent or impair our ability to maintain our licensing arrangements on commercially acceptable terms, we may be unable to successfully develop manufacture and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations and prospects.
Disputes may arise regarding intellectual property subject to a licensing agreement, including:
| ● | the scope of rights that may be granted under license agreements and other interpretation-related issues; |
| ● | the extent to which our technology and processes infringe on intellectual property rights of the licensor that is not subject to the licensing agreement; |
| ● | the sublicensing of patent and other rights under current and any future collaborative development relationships; |
| ● | our diligence obligations under any license agreement and what activities satisfy such obligations; |
| ● | the inventorship and ownership of inventions and know-how and other intellectual property resulting from the joint creation or use of intellectual property by our license counterparties and us and our partners; and |
| ● | the priority of invention of patented technology. |
In spite of our best efforts, our license counterparties might conclude that we have materially breached our license agreements and might therefore terminate the license agreements, which may remove our ability to develop manufacture- and commercialize the product candidates and technology covered by these license agreements. If any in-licenses are terminated, competitors may be able to seek regulatory approval of, and to market, products identical to ours. It is possible that we may be unable to obtain any additional licenses that we require at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to redesign our product candidates, technology, or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis. If we are unable to do so, we may be unable to develop, manufacture or commercialize the affected product candidates, which could harm our competitive position, business, financial conditions, results of operations and prospects.
-39-
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be negatively impacted and our business would be harmed
In addition to the protection afforded by patents, we also rely on trade secret protection for certain aspects of our intellectual property. However, trade secrets are difficult to protect. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, consultants, independent contractors, advisors, contract manufacturers, suppliers and other third parties. We also enter into confidentiality and invention or patent assignment agreements with employees and certain consultants and independent contractors. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. Further, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed or otherwise obtained by a competitor or other third party, it could have a material adverse effect on our business, financial condition, results of operations and prospects.
| 4. | Risks related to employee matters and managing growth |
We only have a limited number of employees to manage and operate our business
As of December 31, 2023, we had 66 full-time or part-time employees. Our focus on the development and commercialization of vilobelimab requires us to optimize cash utilization and to manage and operate our business with limited personnel. We cannot assure you that we will be able to hire additional employees and/or retain adequate staffing levels to develop and commercialize vilobelimab or run our operations or to accomplish all the objectives that we otherwise would seek to accomplish.
We depend heavily on our executive officers and directors, and the loss of their services would materially harm our business
Our success depends, and will likely continue to depend, upon our ability to hire and retain the services of our current executive officers, directors, principal consultants and others. We are highly dependent on the management, development, clinical, financial and business development expertise of Niels Riedemann, our Chief Executive Officer, Renfeng Guo, our Chief Scientific Officer, Thomas Taapken, our Chief Financial Officer, and, since July 2023, Camilla Chong, our Chief Medical Officer. Our ability to compete in the biotechnology and pharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel.
Our industry has experienced a high rate of turnover of management personnel in recent years. Any of our personnel may terminate their employment at will. If we lose one or more of our executive officers or other key employees, our ability to implement our business strategy successfully could be seriously harmed. Furthermore, replacing executive officers or other key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain marketing approval of and commercialize products successfully.
Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key employees on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions.
We rely on consultants and advisors, including scientific, strategic, regulatory and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by other entities and may have commitments under consulting or advisory contracts with those entities that may limit their availability to us. If we are unable to continue to attract and retain highly qualified personnel, our ability to develop and commercialize our product candidates will be limited.
-40-
We expect to expand our organization, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations
We expect to expand scope of our operations, particularly in the areas of clinical development and regulatory affairs. To manage such growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Our management may need to devote a significant amount of its attention to managing these growth activities. Moreover, our expected growth could require us to relocate to a different geographic area of the country. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion or relocation of our operations, retain key employees, or identify, recruit, attract and train retain human capital. Competition for qualified, motivated, and highly-skilled executives, professionals and other key personnel in biotechnology and pharmaceuticals industries is significant. Our inability to manage the expansion or relocation of our operations effectively may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could also require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If we are unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate revenues could be reduced and we may not be able to implement our business strategy, including the successful development and commercialization of our product candidates.
Our employees, independent contractors, consultants, collaborators and CROs may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation
We are exposed to the risk that our employees, independent contractors, consultants, collaborators and CROs may engage in fraudulent conduct or other illegal activity. Misconduct by those parties could include intentional, reckless or negligent conduct or disclosure of unauthorized activities to us that violates: (i) the FDA regulations or similar regulations of comparable non-U.S. regulatory authorities, including those laws requiring the reporting of true, complete and accurate information to such authorities, (ii) manufacturing and clinical trial conduct standards, (iii) federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable non-U.S. regulatory authorities, and (iv) laws that require the reporting of financial information or data accurately. Activities subject to these laws also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could have a material adverse effect on our ability to operate our business and our results of operations.
| 5. | Risks related to our ordinary shares and our status as a public company |
The trading price of our ordinary shares has been and may in the future be highly volatile, which could result in substantial losses for holders of our ordinary shares, and a decline in our share price and invite securities litigation against our company or our management
Our share price has been and is likely to be highly volatile in the future. The stock market in general and the market for smaller pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. You should consider an investment in our ordinary shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The market price for our ordinary shares may be influenced by many factors, including:
| ● | the timing, enrollment and results of clinical trials of vilobelimab and any other product candidates; |
| ● | regulatory actions with respect to vilobelimab, our other product candidates or our competitors’ products and product candidates; |
| ● | the success of the commercialization of GOHIBIC (vilobelimab); |
-41-
| ● | the success of existing or new competitive products or technologies; |
| ● | any delay in our development or regulatory filings for vilobelimab or any future product candidate and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings, including the FDA’s issuance of a “refusal to file” letter or a request for additional information; |
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
| ● | commencement or termination of collaborations for our development programs; |
| ● | failure or discontinuation of any of our development programs; |
| ● | results of clinical trials of product candidates of our competitors; |
| ● | regulatory or legal developments in the United States and other countries; |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| ● | the recruitment or departure of key personnel; |
| ● | the level of expenses related to any of our product candidates or clinical development programs; |
| ● | the results of our efforts to develop additional product candidates or products; |
| ● | actual or anticipated changes in estimates as to financial results or development timelines; |
| ● | announcement or expectation of additional financing efforts; |
| ● | sales of our ordinary shares by us, our insiders or other shareholders; |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; |
| ● | changes in estimates or recommendations by securities analysts, if any, that cover our shares; |
| ● | changes in the structure of healthcare payment systems; |
| ● | market conditions in the pharmaceutical and biotechnology industries; |
| ● | general economic, industry, market and political conditions; and |
| ● | the other factors described in this ‘ITEM 3. KEY INFORMATION — 3. Risk factors’ section. |
In the past, securities class action litigation has often been brought against a company and its management following a decline in the market price of its securities. This risk is especially relevant for biopharmaceutical companies, which have experienced significant stock price volatility in recent years. Such litigation, if instituted against us, could cause us or members of our management to incur substantial costs and divert management’s attention and resources from our business.
Future sales, or the possibility of future sales, of a substantial number of our ordinary shares could adversely affect the price of the shares and dilute shareholders
Future sales of a substantial number of our ordinary shares, or the perception that such sales will occur, could cause a decline in the market price of our ordinary shares. If we or our existing shareholders sell substantial amounts of ordinary shares in the public market, or the market perceives that such sales may occur, the market price of our ordinary shares and our ability to raise capital through an issue of equity securities in the future at attractive terms or at all could be adversely affected.
We have broad discretion in the use of our cash on hand and may invest or spend it in a way with which you do not agree and in ways that may not yield a return on your investment
As of December 31, 2023, we had €12.8 million in cash and cash equivalents and €85.6 million in marketable securities. Our management will have broad discretion in the use of such cash and could spend it in ways that do not improve our results of operations or enhance the value of our ordinary shares. You will not have the opportunity to influence our decisions on how to use our cash on hand. The failure by our management to apply these funds effectively could result in financial losses that could harm our business, cause the price of our ordinary shares to decline and delay the development of our product candidates. Pending its use, we may invest our cash on hand in a manner that does not produce income or that loses value.
-42-
We are a foreign private issuer and, as a result, we are not subject to U.S. proxy rules and are subject to Exchange Act reporting obligations that, to some extent, are more lenient and less frequent than those of a U.S. domestic public company
We will report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S. company with foreign private issuer status. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act, (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. In addition, foreign private issuers are not required to file their Annual Report on Form 20-F until four months after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their Annual Report on Form 10-K within 75 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
As a foreign private issuer and as permitted by the listing requirements of Nasdaq, we follow certain home country governance practices rather than the corporate governance requirements of Nasdaq
We are a foreign private issuer. As a result, in accordance with the listing requirements of Nasdaq we rely on home country governance requirements and certain exemptions thereunder rather than relying on the corporate governance requirements of Nasdaq. In accordance with Dutch law and generally accepted business practices, our Articles of Association do not provide quorum requirements generally applicable to general meetings of shareholders. To this extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. Although we must provide shareholders with an agenda and other relevant documents for the general meeting of shareholders, Dutch law does not have a regulatory regime for the solicitation of proxies and the solicitation of proxies is not a generally accepted business practice in the Netherlands; thus, our practice will vary from the requirement of Nasdaq Listing Rule 5620(b). As permitted by the listing requirements of Nasdaq, we have also opted out of the requirements of Nasdaq Listing Rule 5605(d), which requires, among other things, an issuer to have a compensation committee that consists entirely of independent directors and makes determinations regarding the independence of any compensation consultants, Nasdaq Listing Rule 5605(e), which requires independent director oversight of director nominations, and Nasdaq Listing Rule 5605(b)(2), which requires an issuer to have a majority of independent directors on its board. In addition, we have opted out of shareholder approval requirements, as included in the Nasdaq Listing Rules, for the issuance of securities in connection with certain events such as the acquisition of shares or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, a change of control of us and certain private placements. To this extent, our practice varies from the requirements of Nasdaq Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. Accordingly, you may not have the same protections afforded to shareholders of companies that are subject to these Nasdaq requirements.
We do not anticipate paying any cash dividends on our share capital in the foreseeable future. Accordingly, shareholders must rely on capital appreciation, if any, for any return on their investment
We have never declared nor paid cash dividends on our share capital. We plan to retain all of our future earnings, if any, to finance the operation, development and growth of our business. In addition, the terms of any future debt or credit agreements and any restrictions imposed by applicable law may preclude us from paying dividends. As a result, capital appreciation, if any, of our ordinary shares will be your sole source of gain for the foreseeable future. Investors seeking cash dividends should not purchase our ordinary shares.
See “ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS —1. Major shareholders.” elsewhere in this Annual Report for more information regarding the ownership of our outstanding ordinary shares by our executive officers, directors and principal shareholders and their affiliates.
-43-
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline
The trading market for our ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over such analysts. There can be no assurance that analysts will cover us or provide favorable coverage going forward. Securities or industry analysts may elect not to continue to provide research coverage of our ordinary shares, and such lack of research coverage may negatively impact the market price of our ordinary shares. In the event we do have analyst coverage, if one or more analysts downgrade our ordinary shares, change their opinion of our ordinary shares or publish inaccurate or unfavorable research about our business, our share price would likely decline. In addition, if one or more analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Our ability to use our net operating loss carry forwards and other tax attributes may be limited
Our ability to utilize our NOLs, or NOLs, is limited, and may be limited further, under Section 8c of the German Corporation Income Tax Act (Körperschaftsteuergesetz), or KStG, and Section 10a of the German Trade Tax Act (Gewerbesteuergesetz), or GewStG. These limitations apply if a qualified ownership change, as defined by Section 8c KStG, occurs and no exemption is applicable. Generally, a qualified ownership change occurs if more than 50% of the share capital or the voting rights are directly or indirectly transferred to a shareholder or a group of shareholders within a period of five years. A qualified ownership change may also occur in case of a transaction comparable to a transfer of shares or voting rights or in case of an increase in capital leading to a respective change in the shareholding. In the case of such a qualified ownership change tax loss carry forwards expire in full. To the extent that the hidden reserves (stille Reserven) taxable in Germany exceed the tax loss carry forward, they may be further utilized despite a qualified ownership change. In case of a qualified ownership change within a group, tax loss carry forwards will be preserved if certain conditions are satisfied. Alternatively, tax loss carry forwards may be retained upon application under certain conditions, to the extent that the corporation has exclusively maintained the same business operations since its establishment or at least since the beginning of the third year prior to qualified ownership change (fortführungsgebundener Verlustvortrag). If the aforementioned application is made and, after the qualified change of ownership, this business operation is discontinued, the most recently determined tax loss carry forward (fortführungsgebundener Verlustvortrag) would be lost.
An appeal has been filed by the fiscal court of Hamburg dated August 29, 2017 – 2 K 245/17 with regard to Section 8c, paragraph 1, sentence 2 KStG (in its superseded version, now: Section 8c paragraph 1 sentence 1 KStG) that is, the forfeiture of all tax loss carryforwards in case more than 50% of shares/voting rights will be assigned to a new shareholder. The appeal is still pending. It is unclear when the Federal Constitutional Court will decide this case. According to statements in German legal literature, there are good reasons to believe that the Federal Constitutional Court may come to the conclusion that Section 8, paragraph 1, sentence 2 KStG (in its superseded version) is not in line with the German constitution.
As of December 31, 2023, we had NOL carry forwards for German corporate tax purposes of €196.0 million and for trade tax purposes €164 million available. Future changes in share ownership may also trigger an ownership change and, consequently, a Section 8c KStG, or a Section 10a GewStG limitation. Any limitation may result in the expiration of the complete tax operating loss carry forwards before they can be utilized. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carry forwards to reduce German income tax may be subject to limitations, which could potentially result in increased future cash tax liability to us.
As of December 31, 2023, our U.S. subsidiary, InflaRx Pharmaceuticals, Inc., had €12.96 million or $14.3 million of NOLs for U.S. federal income tax purposes. Transfers or issuances of our equity may impair or reduce the ability of InflaRx Pharmaceuticals, Inc. to utilize U.S. federal net operating loss carryforwards and certain other tax attributes in the future. Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, contains rules that limit the ability of a company that undergoes an “ownership change” to utilize its net operating loss and tax credit carry forwards and certain built-in losses recognized in years after the ownership change. An “ownership change” is generally defined as an increase in ownership of a corporation’s stock by more than 50 percentage points over a rolling three-year period by stockholders that own (directly, indirectly or constructively) 5% or more of the stock of a corporation at any time during the relevant rolling three-year period. If an ownership change occurs, Section 382 imposes an annual limitation on the use of pre-ownership change NOLs, credits and certain other tax attributes to offset taxable income earned after the ownership change. The annual limitation is generally equal to the product of the applicable long-term tax-exempt rate in effect for the month in which the ownership change occurs and the value of the company’s stock immediately before the ownership change (subject to some adjustments). For example, this annual limitation may be adjusted to reflect any unused annual limitation for prior years and certain recognized (or treated as recognized) built-in gains and losses for the year. In addition, Section 383 generally limits the amount of tax liability in any post-ownership change year that can be reduced by pre-ownership change tax credit carryforwards or capital loss carryforwards. No assurance can be given that prior transactions have not resulted in an ownership change for purposes of Section 382 of the Code or that future transactions will not result in an ownership change. Even if a subsequent transaction does not result in an ownership change, it may materially increase the likelihood that we will undergo an ownership change in the future. Sales of our common shares by stockholders, whose interests may differ from our interests, may increase the likelihood that we or one of our subsidiaries undergoes an ownership change. If we or our subsidiaries have or were to undergo an ownership change, it could result in increased future tax liability to us.
-44-
We may become taxable in a jurisdiction other than Germany and this may increase the aggregate tax burden on us
Since incorporation we intend to have, on a continuous basis, our place of effective management in Germany. We will therefore be a tax resident of Germany under German national tax law. By reason of our incorporation under Dutch law, we are also deemed tax resident in the Netherlands under Dutch tax law. However, based on our current management structure and current tax laws of the United States, Germany and the Netherlands, as well as applicable income tax treaties, and current interpretations thereof, we should be tax resident solely in Germany for the purposes of the convention between the Federal Republic of Germany and the Netherlands for the avoidance of double taxation with respect to taxes on income of 2012, or the German-Dutch tax treaty.
Our sole tax residency in Germany for purposes of the German-Dutch tax treaty is subject to the application of the provisions on tax residency as stipulated in the German-Dutch tax treaty as amended from time to time. The Multilateral Convention to Implement Tax Treaty Related Measures to Prevent Base Erosion and Profit Shifting or the MLI, Germany and the Netherlands entered into, among other countries, should not, as of this date, affect the German-Dutch tax treaty’s rules regarding tax residency.
The applicable tax laws, tax treaties or interpretations thereof may change, including the MLI choices and reservation. Furthermore, whether we have our place of effective management in Germany and are as such solely tax resident in Germany is largely a question of fact and degree based on all the circumstances, rather than a question of law, which facts and degree may also change. Changes to applicable laws or interpretations thereof and changes to applicable facts and circumstances (for example, a change of board members or the place where board meetings take place), or changes to applicable tax treaties, including a change to the application of the MLI may result in us becoming a tax resident of a jurisdiction other than Germany. As a consequence, our overall effective income tax rate and income tax expense could materially increase, which could have a material adverse effect on our business, results of operations, financial condition and prospects, which could cause our share price and trading volume to decline.
We believe it is likely that we were a PFIC for U.S. federal income tax purposes in 2021, 2022 and 2023, and we may be a PFIC in one or more future taxable years. U.S. shareholders may be subject to adverse U.S. federal income tax consequences in 2023 and in any future taxable year in which we are a PFIC.
We believe it is likely that we are a PFIC for U.S. federal income tax purposes in 2021, 2022 and 2023 and we may be a PFIC in one or more future taxable years. In addition, we may, now or in the future directly or indirectly, hold equity interests in other PFICs. Under the Code, we will be a PFIC for any taxable year in which, after the application of certain look-through rules with respect to subsidiaries, either (i) 75% or more of our gross income consists of passive income or (ii) 50% or more of the average quarterly value of our assets consists of assets that produce, or are held for the production of, passive income. Passive income includes, among other things, dividends, interest, certain non-active rents and royalties, and capital gains. It is possible that we will be a PFIC in any future taxable year because, among other things, (i) we own a substantial amount of passive assets, including cash and securities that may give rise to passive income, (ii) the valuation of our assets that generate non-passive income for PFIC purposes, including our intangible assets, is uncertain and may vary substantially over time and (iii) the composition of our income may vary substantially over time.
If we are a PFIC for any taxable year during which a U.S. investor holds ordinary shares, we would continue to be treated as a PFIC with respect to that U.S. investor for all succeeding years during which the U.S. investor holds ordinary shares, even if we ceased to meet the threshold requirements for PFIC status, unless certain exceptions apply. Such a U.S. investor may be subject to adverse U.S. federal income tax consequences, including (i) the treatment of all or a portion of any gain on disposition as ordinary income, (ii) the application of a deferred interest charge on such gain and the receipt of certain dividends and (iii) compliance with certain reporting requirements.
For further discussion, see “ITEM 10. ADDITIONAL INFORMATION —5. Taxation — Material U.S. Federal Income Tax Considerations for U.S. Holders of Ordinary Shares.”
-45-
We may lose our foreign private issuer status which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses
We are a foreign private issuer and therefore we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to U.S. domestic issuers. If in the future we are not a foreign private issuer as of the last day of the second fiscal quarter in any fiscal year, we would be required to comply with all of the periodic disclosure, current reporting requirements and proxy solicitation rules of the Exchange Act applicable to U.S. domestic issuers. In order to maintain our current status as a foreign private issuer, either (a) a majority of our ordinary shares must be either directly or indirectly owned of record by non-residents of the United States or (b)(i) a majority of our directors and executive officers may not be United States citizens or residents, (ii) more than 50% of our assets cannot be located in the United States and (iii) our business must be administered principally outside the United States. If we were to lose this status, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC and stock exchange rules. The regulatory and compliance costs to us if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be significantly higher than the costs we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would increase our legal and financial compliance costs and would make some activities highly time consuming and costly. These rules and regulations could also make it more difficult for us to attract and retain qualified directors.
If we ever pay dividends, we may need to withhold tax on such dividends payable to holders of our shares in both Germany and the Netherlands
We do not intend to pay any dividends to holders of our shares. However, if we do pay dividends, we may need to withhold tax on such dividends both in Germany and the Netherlands. As an entity incorporated under Dutch law any dividends distributed by us are subject to Dutch dividend withholding tax on the basis of Dutch domestic law. However, on the basis of the double tax treaty between Germany and the Netherlands, the Netherlands will be restricted from imposing dividend withholding tax if we continue to be a tax resident of Germany and our place of effective management is in Germany. However, Dutch dividend withholding tax is still required to be withheld from dividends if and when paid to Dutch resident holders of our shares (and non-Dutch resident holders of our shares that have a permanent establishment in the Netherlands to which their shareholding is attributable). As a result, upon a payment (or deemed payment) of dividends, we will be required to identify our shareholders in order to assess whether there are Dutch residents (or non-Dutch residents with a permanent establishment to which the shares are attributable) in respect of which Dutch dividend tax has to be withheld. Such identification may not always be possible in practice. If the identity of our shareholders cannot be determined, withholding of both German and Dutch dividend tax from such dividend may occur, upon a payment of dividends.
Furthermore, the withholding tax restriction referred to above is based on the current choices and reservation made by Germany under the MLI. If Germany changes its MLI choices and reservation, we may not be entitled to any benefits of the double tax treaty between Germany and the Netherlands, including the withholding tax restriction, as long as Germany and the Netherlands do not reach an agreement on our tax residency for purposes of the double tax treaty between Germany and the Netherlands, except to the extent and in such manner as may be agreed upon by the authorities. As a result, any dividends distributed by us during the period no such agreement has been reached between Germany and the Netherlands, may be subject to withholding tax both in Germany and the Netherlands.
In addition, a proposed law is pending before the Dutch parliament, namely the Emergency Act Conditional Exit Dividend Tax (Spoedwet conditionele eindafrekening dividendbelasting) which would, if enacted, impose, possibly with retroactive effect, a dividend withholding (exit) tax on certain deemed distributions if we cease to be a Dutch tax resident and become a tax resident of a jurisdiction that is not a member of the EU or the EEA, when such jurisdiction does not satisfy certain conditions. In some cases, we would have a right to recover the amount of tax from our shareholders when such shareholder is not entitled to an exemption.
-46-
Dividends distributed on our shares to certain related entities in low-taxed or non-cooperative jurisdictions might in the future become subject to an additional Dutch withholding tax on dividends, as of January 1, 2024
We have no plans to pay regular dividends on our ordinary shares. However, if we do pay dividends, under current Dutch tax law, dividends paid by us to holders of our shares could become subject to Dutch dividend withholding tax at a rate of 15% under the Dutch Dividend Withholding Tax Act (Wet op de dividendbelasting 1965), unless a domestic or treaty exemption or reduction applies; see “ITEM 10. ADDITIONAL INFORMATION — 5. Taxation — Material Dutch Tax Considerations.” As of January 1, 2024, a Dutch conditional withholding tax will be imposed on dividends paid to related entities in jurisdictions that have a corporate income tax rate below 9% (low-tax jurisdiction) or jurisdictions that are included on the EU’s blacklist of non-cooperative jurisdictions (non-cooperative jurisdictions for tax purposes). In addition, the conditional withholding tax on dividends may also apply in situations where artificial structures are put in place with the main purpose or one of the main purposes to avoid the conditional withholding tax or in the event of a hybrid mismatch. The conditional withholding tax will be imposed at the highest Dutch corporate income tax rate in effect at the time of the distribution (currently 25.8%). The conditional withholding tax on dividends will be reduced, but not below zero, by any regular Dutch dividend withholding tax withheld in respect of the same dividend payment. As such, based on the currently applicable rates, the overall effective tax rate of withholding the regular dividend withholding tax and conditional withholding tax will not exceed the highest corporate income tax rate in effect at the time of the distribution (currently 25.8%). As of January 1, 2024, the withholding tax rate on dividends paid to shareholders that are (A) entities related (gelieerd) to us and (B)(i) established in a low-taxing state or non-cooperative jurisdiction for tax purposes, (ii) a hybrid entity or reverse hybrid entity or (iii) interposed to avoid tax otherwise due by another entity, may rise from 15% to the highest corporate tax rate (currently 25.8%).
We are a Dutch public company with limited liability. The rights of our shareholders are different from the rights of shareholders in companies governed by the laws of U.S. jurisdictions and may not protect investors in a similar fashion afforded by incorporation in a U.S. jurisdiction
We are a public company with limited liability (naamloze vennootschap) organized under the laws of the Netherlands. Our corporate affairs are governed by our Articles of Association and by the laws governing companies incorporated in the Netherlands. However, there can be no assurance that Dutch law will not change in the future or that it will serve to protect investors in a similar fashion afforded under corporate law principles in the United States, which could adversely affect the rights of investors.
The rights of shareholders and the responsibilities of directors may be different from the rights and obligations of shareholders and board members in companies governed by U.S. law. In the performance of its duties, our executive officers and board of directors are required by Dutch law to consider the interests of our company, its shareholders, its employees and other stakeholders, in all cases with due observation of the principles of reasonableness and fairness. It is possible that some of these parties will have interests that are different from, or in addition to, your interests as a shareholder.
Provisions of our Articles of Association or Dutch corporate law might deter acquisition bids for us that might be considered favorable and prevent, delay or frustrate any attempt to replace or remove the members of our board of directors
Under Dutch law, various protective measures are possible and permissible within the boundaries set by Dutch law and Dutch case law. Our governance arrangements include several provisions that may have the effect of making a takeover of our company more difficult or less attractive. In this respect, our general meeting of shareholders granted the right to an independent foundation under Dutch law, or protective foundation, to acquire preferred shares pursuant to a call option agreement, or the call option agreement, entered into between us and such foundation. This call option under the call option agreement shall be continuous in nature and can be exercised repeatedly on multiple occasions.
-47-
If the protective foundation exercises the call option pursuant to the call option agreement, an amount of preferred shares up to 100% of our issued capital held by others than the protective foundation, minus one share, will be issued to the protective foundation. These preferred shares will be issued to the protective foundation under the obligation to pay up to 25% of their nominal value upon issuance. In order for the protective foundation to finance the issue price in relation to the preferred shares, the protective foundation is expected to enter into a finance arrangement with a bank. As an alternative to securing financing with a bank, subject to applicable restrictions under Dutch law, the call option agreement provides that the protective foundation may request us to provide, or cause our subsidiaries to provide, sufficient funding to the protective foundation to enable it to satisfy the payment obligation (or part thereof) in cash and/or to charge an amount equal to the payment obligation (or part thereof) against our profits and/or reserves in satisfaction of such payment obligation.
The protective foundation’s articles of association provide that it will promote and protect the interests of the company, the business connected with the company and the company’s stakeholders from time to time, and repressing possible influences which could threaten the strategy, continuity, independence and/or identity of the company or the business connected with it, to such an extent that this could be considered to be damaging to the aforementioned interests. These influences may include a third party acquiring a significant percentage of our ordinary shares, the announcement of an unsolicited public offer for our ordinary shares, shareholder activism, other concentration of control over our ordinary shares or any other form of undue pressure on us to alter our strategic policies. The protective foundation shall be structured to operate independently of us.
If the protective foundation were to exercise its call option, the preferred shares to be issued pursuant thereto would be issued against the obligation to pay up to 25% of their nominal value. The voting rights of our shares are based on nominal value and, as we expect our ordinary shares to trade substantially in excess of nominal value, preferred shares issued at 25% of their nominal value can carry significant voting power for a substantially reduced price compared to the price of our ordinary shares and thus can be used as a defensive measure. These preferred shares will have both a liquidation and dividend preference over our ordinary shares and will accrue cash dividends at a pre-determined rate. The protective foundation would be expected to require us to cancel its preferred shares once the perceived threat to the company and its stakeholders has been removed or sufficiently mitigated or neutralized. However, subject to the same limitations described above, the protective foundation would continue to have the right to exercise the call option in the future in response to a new threat to the interests of us, our business and our stakeholders from time to time.
In addition, certain provisions of our Articles of Association may make it more difficult for a third party to acquire control of us or effect a change in our board of directors. These provisions include: a provision that our directors are appointed on the basis of a binding nomination prepared by our board of directors which can only be overruled by a two-thirds majority of votes cast representing more than 50% of our issued share capital; a provision that our directors may only be removed by the general meeting of shareholders by a two-thirds majority of votes cast representing more than 50% of our issued share capital (unless the removal is proposed by the board in which case a simple majority of the votes can be sufficient); and a requirement that certain matters, including an amendment of our Articles of Association, may only be brought to our shareholders for a vote upon a proposal by our board of directors.
We are not obligated to and do not comply with all the best practice provisions of the DCGC. This may affect your rights as a shareholder
We are a Dutch public company with limited liability (naamloze vennootschap), and we are subject to the DCGC. The DCGC contains both principles and best practice provisions that regulate relations between the board of directors and the shareholders (such as the general meeting of shareholders). The DCGC is based on a “comply or explain” principle. Accordingly, companies are required to disclose in their annual reports, filed in the Netherlands, whether they comply with the provisions of the DCGC. If they do not comply with those provisions (for example, because of a conflicting Nasdaq requirement), the company is required to give the reasons for such non-compliance.
The DCGC applies to all Dutch companies listed on a government-recognized stock exchange, whether in the Netherlands or elsewhere, including Nasdaq. We do not comply with all the best practice provisions of the DCGC. This may affect your rights as a shareholder and you may not have the same level of protection as a shareholder in a Dutch company that fully complies with the DCGC.
-48-
Claims of U.S. civil liabilities may not be enforceable against us
We are incorporated under the laws of the Netherlands, and our headquarters are located in Germany. Substantially all of our assets are located outside the United States. The majority of our directors and executive officers reside outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them or us in U.S. courts, including judgements predicated upon the civil liability provisions of the federal securities laws of the United States.
There is no treaty between the United States and the Netherlands for the mutual recognition and enforcement of judgements (other than arbitration awards) in civil and commercial matters. Therefore, a final judgement for the payment of money rendered by any federal or state court in the United States based on civil liability, whether or not predicated solely upon the U.S. federal securities laws, would not be enforceable in the Netherlands unless the underlying claim is relitigated before a Dutch court of competent jurisdiction. Under current practice, however, a Dutch court will generally, subject to compliance with certain procedural requirements, grant the same judgement without a review of the merits of the underlying claim if such judgement (i) is a final judgement and has been rendered by a court which has established its jurisdiction vis-à-vis the relevant Dutch companies or Dutch company, as the case may be, on the basis of internationally accepted grounds of jurisdiction, (ii) has not been rendered in violation of principles of proper procedure (behoorlijke rechtspleging), (iii) is not contrary to the public policy of the Netherlands, and (iv) is not incompatible with (a) a prior judgement of a Netherlands court rendered in a dispute between the same parties, or (b) a prior judgement of a foreign court rendered in a dispute between the same parties, concerning the same subject matter and based on the same cause of action, provided that such prior judgement is capable of being recognized in the Netherlands. Dutch courts may deny the recognition and enforcement of punitive damages or other awards.
Moreover, a Dutch court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages. Enforcement and recognition of judgements of U.S. courts in the Netherlands are solely governed by the provisions of the Dutch Code of Civil Procedure. Based on the foregoing, there can be no assurance that U.S. investors will be able to enforce any judgements obtained in U.S. courts in civil and commercial matters, including judgements under the U.S. federal securities.
The United States and Germany do not have a treaty providing for the reciprocal recognition and enforcement of judgements in civil and commercial matters. Consequently, a final judgement for payment or declaratory judgements given by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in Germany. German courts may deny the recognition and enforcement of a judgement rendered by a U.S. court if they consider the U.S. court not to be competent or the decision to be in violation of German public policy principles. For example, judgements awarding punitive damages are generally not enforceable in Germany. A German court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages.
In addition, actions brought in a German court against us, our directors, our executive officers and the experts named herein to enforce liabilities based on U.S. federal securities laws may be subject to certain restrictions. In particular, German courts generally do not award punitive damages. Litigation in Germany is also subject to rules of procedure that differ from the U.S. rules, including with respect to the taking and admissibility of evidence, the conduct of the proceedings and the allocation of costs. German procedural law does not provide for pre-trial discovery of documents, nor does Germany support pre-trial discovery of documents under the 1970 Hague Evidence Convention. Proceedings in Germany would have to be conducted in the German language and all documents submitted to the court would, in principle, have to be translated into German. For these reasons, it may be difficult for a U.S. investor to bring an original action in a German court predicated upon the civil liability provisions of the U.S. federal securities laws against us, our directors, our executive officers and the experts named in this Annual Report.
Based on the lack of a treaty as described above, U.S. investors may not be able to enforce against us or directors, executive officers or certain experts named herein who are residents of or possessing assets in the Netherlands, Germany, or other countries other than the United States any judgements obtained in U.S. courts in civil and commercial matters, including judgements under the U.S. federal securities laws.
-49-
| 6. | General Risk Factors |
General economic, political and social conditions.
Our business and results of operations may be adversely affected by disruptions in the financial markets, changes to political and regulatory policies and economic conditions generally
General economic, political and social conditions affect the United States, Europe and other global markets and our business. In particular, U.S., European and other global markets, as well as our access to financing, may be affected by factors, including economic growth or its sustainability, persistent inflation, supply chain disruptions, employment levels, work stoppages, labor shortages and labor disputes, labor costs, wage stagnation, energy prices, oil, gas and fuel prices, fluctuations or other significant changes in both debt and equity capital markets and currencies, liquidity of the global financial markets, the growth of global trade and commerce, trade policies, the availability and cost of capital and credit (including as a result of increased interest rates) and investor sentiment and confidence. Additionally, global markets may be adversely affected by the current or anticipated impact of cyber incidents or campaigns, military conflict, including the Russia-Ukraine conflict as well as the Hamas-Israel conflict and rising tensions between China and Taiwan and the relationship between China and the United States, or other geopolitical uncertainty and instability. The ongoing spread of variants of infectious diseases, such as the COVID-19 virus, may interrupt, or delay, our clinical trial activities, regulatory reviews, manufacturing activities and supply chain. The COVID-19 outbreak delayed enrollment in our clinical trials due to prioritization of hospital resources towards the outbreak or other factors, and some patients may be unwilling to enroll in our trials or be unable to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services, which would delay our ability to conduct clinical trials or release clinical trial results and could delay our ability to obtain regulatory approvals and commercialize our product candidates. Any sudden or prolonged market downturn in the United States or elsewhere could adversely affect our business, results of operations and financial condition, including capital and liquidity levels.
Legal, regulatory or market measures to address environmental and other objectives may negatively affect our business or operations
Regulatory and legislative bodies in the United States, Europe and elsewhere continue to focus on environmental, social and governance, or ESG, matters including increasing attention on relating to climate change, greenhouse gas emissions, carbon taxes, emissions trading schemes, sustainable manufacturing, human rights and equity matters, and disclosure regarding the foregoing, many of which policies may be ambiguous, inconsistent, dynamic or conflicting. We expect to experience increased restrictions, compliance costs, legal costs and expenses related to such new or changing legal or regulatory requirements. Moreover, compliance with any such legal or regulatory requirements would require us to devote substantial time and attention to these matters. In addition, we may still be subject to penalties or potential litigation if such laws and regulations are interpreted or applied in a manner inconsistent with our practices. Additionally, we are subject to increased attention from the media, stockholders, activists and other stakeholders on climate change, social and sustainability matters, which could negatively affect our reputation or investor confidence.
We may not be able to maintain sufficient insurance to cover us for potential litigation or other risks
We may not be able to maintain sufficient insurance on commercially reasonable terms or with adequate coverage levels against potential liabilities we may face in connection with potential claims, which could have a material adverse effect on our business. We may face a risk of loss from a variety of claims, including related to securities, antitrust, contracts, cybersecurity, fraud and various other potential claims, whether or not such claims are valid. Insurance and other safeguards might only partially reimburse us for our losses, if at all, and if a claim is successful and exceeds or is not covered by our insurance policies, we may be required to pay a substantial amount in respect of such successful claim. Certain losses of a catastrophic nature, such as losses arising as a result of wars, earthquakes, typhoons, terrorist attacks or other similar events, may be uninsurable or may only be insurable at rates that are so high that maintaining coverage would cause an adverse impact on our business, our investment funds and their investments. In general, losses related to terrorism are becoming harder and more expensive to insure against. Some insurers are excluding terrorism coverage from their all-risk policies. In some cases, insurers are offering significantly limited coverage against terrorist acts for additional premiums, which can greatly increase the total cost of casualty insurance for a property. As a result, we, our products and their investments may not be insured against terrorism or certain other catastrophic losses.
-50-
Raising additional capital may cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates
We expect our expenses may increase in connection with expansion of operations. To the extent that we raise additional capital through the issuance of ordinary shares, convertible securities or other equity securities, your ownership interest may be diluted, and the terms of these securities could include liquidation or other preferences and anti-dilution protections that could adversely affect your rights as an ordinary shareholder. In addition, debt financing, if available, may result in fixed payment obligations and may involve agreements that include restrictive covenants that limit our ability to take specific actions, such as incurring additional debt, making capital expenditures, creating liens, redeeming shares or declaring dividends, that could adversely impact our ability to conduct our business. In addition, securing financing could require a substantial amount of time and attention from our management and may divert a disproportionate amount of their attention away from day-to-day activities, which may adversely affect our management’s ability to oversee the development of our product candidates.
If we raise additional funds through collaborations or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do, and reducing or eliminating our commercial opportunity
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we, or any future collaborators, may develop. Our competitors also may obtain the FDA or other marketing approval for their products before we, or any future collaborators, are able to obtain approval for ours, which could result in our competitors establishing a strong market position before we, or any future collaborators, are able to enter the market.
Many of our existing and potential future competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining marketing approvals and marketing approved products than we do, and may be able to reduce the price at which they sell their products. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly if acquired by, or through collaborative arrangements with, large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the development of our product candidates.
The development and commercialization of new products is highly competitive. We expect that we, and any future collaborators, will face significant competition from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide with respect to any of our product candidates that we, or any future collaborators, may seek to develop or commercialize in the future. For example, other pharmaceutical companies may commence development efforts for product candidates targeting the same indications as vilobelimab, including PG and severe COVID-19 or any other indications we may target. For a detailed analysis of the competitive environment in which we operate, see “ITEM 4. INFORMATION ON THE COMPANY — 2. Business Overview — Competition.”
-51-
If any product liability lawsuits are successfully brought against us or any of our collaboration partners, we may incur substantial liabilities and may be required to limit commercialization of our product candidates
We face an inherent risk of product liability lawsuits related to the testing of our product candidates in seriously ill patients and will face an even greater risk if our product candidates are approved by regulatory authorities and introduced commercially. Product liability claims may be brought against us or our partners by participants enrolled in our clinical trials, patients, health care providers or others using, administering or selling any of our future approved products. If we cannot successfully defend ourselves against any such claims, we may incur substantial liabilities.
If any of our product candidates are approved for commercial sale, we will be highly dependent upon consumer perceptions of us and the safety and quality of our products. We could be adversely affected if we are subject to negative publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies.
Although we maintain product liability insurance coverage, this insurance may not fully cover potential liabilities that we may incur. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. We will need to increase our insurance coverage if we commercialize any product that receives marketing approval. In addition, insurance coverage is becoming increasingly expensive and difficult to obtain. If we are unable to maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, it could prevent or inhibit the development and commercial production and sale of our product candidates, which could harm our business, financial condition, results of operations and prospects.
We may be unsuccessful in evaluating material risks involved in future acquisitions
We may, in the future, acquire companies, products and/or platforms that are complementary to our operational and customer needs. As part of the process, we may conduct business, legal and financial due diligence to identify and evaluate material risks involved in any particular transaction. Despite these efforts, we may be unsuccessful in ascertaining or evaluating all such risks. As a result, the intended advantages of any given acquisition may not be realized. If we fail to identify certain material risks from one or more acquisitions we may be exposed to significant costs and our business could be negatively impacted.
Cyber incidents or other failures in IT systems could result in information theft, data corruption and significant disruption of our business operations
We utilize information technology, or IT, systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate cyber-attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication, both internal and those provided by third-party service provider. These threats pose a risk to the security of our systems and networks, the confidentiality and the availability and integrity of our data. The wars in Europe and in the Middle East, as well as the tensions between China and Taiwan may also result in heightened cybersecurity risk across our networks and platforms. We have implemented processes, procedures and internal control to mitigate cybersecurity risks and cyber intrusions and rely on industry accepted security measures and technology to securely maintain confidential and proprietary information maintained on our information systems. However, these measures, as well as our increased awareness of the nature and extent of a risk of a cyber-incident, do not guarantee that a cyber-incident will not occur or that our financial results, operations or confidential information will not be negatively impacted by such an incident, especially because cyber threats change frequently or are not recognized until launched and because cyber incidents can originate from a wide variety of sources. Similarly, there can be no assurance that our collaborators, CROs, third-party logistics providers, distributors and other contractors and consultants will be successful in protecting our clinical and other data that is stored on their systems.
-52-
If a cyber incident were to occur and cause interruptions in our operations or any destruction or loss, corruption or unavailability of data, it could result in loss or misappropriation of confidential information, including trade secrets, other intellectual property or financial information, and a material disruption of our development programs and business operations, any of which could lead to significant delays or setbacks in our research and other further development and commercialization of our product candidates. For example, the loss of clinical trial data from completed, ongoing or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Finally, there has been significant evolution and developments in the use of artificial intelligence technologies, such as artificial generative chatbots. We cannot fully determine the impact or cybersecurity risk of such evolving technology to our business at this time.
Any such cyber incident or destruction or loss of data could have a material adverse effect on our business and prospects. In addition, we may suffer reputational harm or face litigation or adverse regulatory action as a result of cyber incidents, including cyber-attacks or other data security breaches, and may incur significant additional expense to implement further data protection measures.
The legal and regulatory environment related to data privacy is becoming stricter, which could result in additional costs or changes to the manner in which we handle personal information, and a failure to comply with such laws or regulations, or to otherwise protect personal data in our possession or control, could result in fines, litigation, or other penalties as well as reputational damage
We are subject to laws, regulations, and contractual obligations related to privacy, data protection, information security, including (i) the EU General Data Protection Regulation, which came into effect on May 25, 2018, and which provides for greater penalties for noncompliance than previous European data protection laws, with potential fines of up to the greater of €20 million or 4% of total annual worldwide turnover and (ii) the California Consumer Privacy Act, which came into effect on January 1, 2020, and provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation.
As privacy, data protection and information security laws evolve and are implemented, interpreted and applied, our compliance costs may increase, particularly in the context of ensuring that adequate data protection and data transfer mechanisms are in place. Additionally, compliance with such obligations and regulations could significantly impact our current and planned privacy and information security practices, our collection, use, sharing, retention and safeguarding of personal data, and our current and planned business activities and operations. A failure to comply with such obligations or regulations could result in fines, litigation, or other penalties and adversely impact our reputation.
If our internal controls over financial reporting fail to be effective, such failure could result in material misstatements in our financial statements, cause investors to lose confidence in our reported financial and other public information and have a negative effect on the trading price of our ordinary shares
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. Section 404 of the Sarbanes-Oxley Act of 2002 requires management of public companies to develop and implement internal controls over financial reporting and evaluate the effectiveness thereof. If we fail to design and operate effective internal controls, it could result in material misstatements in our financial statements, impair our ability to raise revenue, result in the loss of investor confidence in the reliability of our financial statements and subject us to regulatory scrutiny and sanctions, which in turn could harm the market value of our ordinary shares.
Banking system risk
We regularly maintain cash balances at third-party financial institutions in excess of the FDIC or other comparable foreign country (i.e., Germany) deposit insurance limits. If any banks or financial institutions at which we maintain cash balances enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our existing cash, cash equivalents and investments, or to draw on our existing lines of credit, may be threatened and could have a material adverse effect on our business and financial condition.
-53-
ITEM 4. INFORMATION ON THE COMPANY
| A. | History and development of the company |
We are a biopharmaceutical company pioneering anti-inflammatory therapeutics by targeting the complement system. We do this by applying our proprietary technologies to discover, develop and commercialize first-in-class, highly potent and specific inhibitors of the complement activation factor known as C5a and its receptor C5aR. The complement system is an integral part of the innate immune system and protects the body, for example by recognizing and removing bacteria, viruses, and other infectious agents, collectively referred to as pathogens. Terminal complement activation, to which the cleavage of C5 by C5-convertases is also referred to, leads to the release of C5a, which acts through its receptor C5aR. In addition, terminal complement activation, i.e., the cleavage of C5a from C5, can also be achieved directly through the extrinsic pathway by naturally occurring enzymes present throughout the body but not considered part of the complement system. With our therapeutic product candidates, we target C5a and its receptor C5aR to selectively inhibit the powerful inflammatory response observed in a wide variety of autoimmune and other inflammatory diseases elicited through C5a/C5aR activation.
Our lead product candidate, vilobelimab, is a novel intravenously delivered first-in-class anti-C5a monoclonal antibody that selectively binds to free C5a and has demonstrated disease-modifying clinical activity and tolerability in multiple clinical settings. We are and have been developing vilobelimab in a wide array of complement-mediated diseases with significant unmet medical need. These include pyoderma gangrenosum, or PG, a chronic inflammatory skin disorder for which we started a Phase III study with the first patient treated in November 2023, the treatment of critically ill COVID-19 patients, for which we concluded a Phase III study in March 2022 and subsequently were granted the EUA by the FDA in April 2023.
Up to November 2023, we were also developing vilobelimab in cutaneous squamous cell carcinoma, or cSCC, in which we were running a Phase II clinical study. We have also previously conducted Phase II studies with vilobelimab in other diseases, including hidradenitis suppurativa, or HS, a chronic debilitating systemic inflammatory skin disease and ANCA-associated vasculitis, or AAV, a rare and life-threatening autoimmune disease.
We are also developing INF904, an orally administered, small-molecule inhibitor of C5aR, for which we have completed Phase I study in healthy volunteers and plan to initiate Phase II clinical studies in the future. INF904 is a promising product candidate for being developed in several disease areas of inflammation, where orally available therapeutics are not available or do not meet the medical need. INF904 development will initially be targeted at chronic spontaneous urticaria, or CSU, and HS.
We are also developing IFX002, a life-cycle management product for vilobelimab, which is currently in advanced pre-clinical stage.
Our legal and commercial name is InflaRx N.V and we were incorporated under the laws of the Netherlands on June 6, 2017, and our headquarters, as registered with the local court of Jena, is located in Jena, Germany. InflaRx was founded in 2007 as InflaRx GmbH by Professor Niels Riedemann and Professor Renfeng Guo in Jena, Germany. Our agent for service of process in the United States is InflaRx Pharmaceuticals, Inc. located at 600 South Wagner Road, Ann Arbor, Michigan 48103. Our principal executive offices and laboratories are located in Winzerlaer Str. 2, 07745 Jena, Germany, telephone: (+49) 3641 508 180. We have additional offices in Planegg-Martinsried (Munich), Germany and in Ann Arbor, Michigan, United States, where we also have laboratories.
We employ a total of 66 employees, 22 of whom have M.D. or Ph.D. degrees. Our management team has extensive experience in the field of complement research, clinical research and the biopharmaceutical industry. Both our Chief Executive Officer and founder, Professor (Dr.) Niels Riedemann, and our Chief Scientific Officer and founder, Professor Renfeng Guo, have over 20 years of complement research experience, having published extensively on the role and function of C5a and its receptors. Our Chief Financial Officer, Dr. Thomas Taapken, has served in executive positions and boards for various private and public European biotechnology companies over the last 19 years and has over 25 years total experience in managerial roles in the biopharmaceutical and venture capital industries. Our Chief Medical Officer, Dr. Camilla Chong, has 25 years of experience in the global pharmaceutical industry in the areas of clinical development, medical affairs, clinical operations, regulatory and pharmacovigilance as well as the launch of new medicines.
-54-
The SEC maintains a website that contains reports and other information about issuers, like us, that file Electronically with the SEC. The address of that website is www.sec.gov. Our website can be found at www.inflarx.de. The information on our website is not incorporated by reference into this Annual Report, and you should not consider information contained on our website to be a part of this Annual Report.
| B. | Business overview |
Overview
C5a is a central mediator of the complement system and therefore a critical component of the innate immune system. The most prominent role of the complement system is to help the body defend itself against invading microorganisms through several mechanisms, including the rapid creation of an inflammatory environment and the production of factors that directly kill pathogens and recruit immune cells to sites of infection. Activation of the complement system ultimately results in the generation of C5a by cleavage from C5. C5a creates an inflammatory environment by attracting and strongly activating neutrophils as well as by causing many different cell types to generate pro-inflammatory molecules. Such inflammation normally benefits the body by helping to fight infection, but excessive or uncontrolled generation of C5a, as it occurs in certain diseases, can cause severe damage to the body’s own tissue, thereby contributing to the pathophysiology of many autoimmune and inflammatory diseases.
While the mode of action of C5a in inflammation has been intensely researched and confirmed, developing a highly specific antibody with the ability to fully block C5a while preserving a critical innate defense mechanism, the formation of the Membrane Attack Complex, or MAC, has been challenging. As such, there are currently no approved drugs that specifically target C5a. We identified antibodies, including our lead product candidate vilobelimab, that potently and selectively bind to a conformational epitope that is formed by C5a upon the cleavage of C5a from C5 that completely blocks C5a without compromising important upstream functions of the complement system, as well as MAC formation.
Unlike its ligand C5a, C5aR can also be pharmacologically inhibited by small molecules. It is generally believed that blockade of C5a using antibodies offers a fast, complete, and safe way to control C5a-induced inflammation. The advantage of a small molecule inhibitor to C5aR is that it can be administered orally, thereby offering broad, long-term ease of administration to patients, especially for the treatment of chronic diseases.
Through our in-house drug discovery efforts, we identified a potent inhibitor of the C5a receptor, INF904, which we believe is a promising candidate for development. We are currently developing INF904, an oral, low molecular weight drug candidate that targets the C5aR receptor. We plan on targeting complement-mediated, chronic auto-immune and inflammatory conditions where an oral small molecule is needed for patients.
Given the different advantages of blocking C5a and C5aR, we believe that the development of both, C5a and C5aR blocking agents is possible and potentially helpful to address a broader range of C5a/C5aR-molecular signaling axis-associated diseases.
Based on the broad anti-inflammatory properties, we are currently developing our lead anti-C5a antibody and our low molecular weight compound INF904 in several diseases. An overview can be found in the pipeline description below.

-55-
Vilobelimab for the treatment of PG
We are developing vilobelimab for the treatment of PG. PG is a rare, chronic inflammatory form of neutrophilic dermatosis characterized by accumulation of neutrophils in the affected skin areas. Vilobelimab was granted orphan drug designation for the treatment of PG by both the FDA in the United States and the EMA in Europe as well as fast track designation by the FDA. After a series of interactions with the FDA on the results of our successfully conducted Phase II clinical study and our plans for the further development towards a potential BLA submission, in 2023 we announced the start of a Phase III study with vilobelimab in ulcerative PG. In November 2023, we announced the enrollment of the first patient in the study.
Vilobelimab for the treatment of severe COVID-19
In April 2023, we received an EUA from FDA for GOHIBIC (vilobelimab) for the treatment of critically ill, invasively mechanically ventilated COVID-19 patients. The EUA is supported by the previously announced results of the multicenter Phase III PANAMO trial, which demonstrated a relative reduction in 28-day all-cause mortality by 23.9%. Subsequently, in June 2023, we began the commercialization of GOHIBIC (vilobelimab) in the United States under the EUA. Our MAA application for SARS-CoV-2 induced septic acute respiratory distress syndrome, or ARDS, receiving IMV or ECMO for vilobelimab is under regulatory review by the European Committee for Medicinal Products for Human Use, or CHMP, under the centralized procedure, which applies to all 27 member states of the EU. To support our development, we had received a total of €33.3 million as grant to us awarded by the German Federal Government for the period of October 2021 to June 2023.
Vilobelimab for the treatment of hidradenitis suppurativa, or HS
We have been developing vilobelimab for the treatment of HS. After having failed to meet our primary endpoint in an international Phase IIb study in 2019, in a post-hoc analysis of the study data we showed multiple signals of efficacy for the vilobelimab high dose group compared to the placebo group, demonstrating significant reductions in all combined inflammatory lesions, reductions of draining tunnels, or dTs, and reductions of the IHS4 score. Subsequently, we had several interactions with the FDA with the goal of agreeing on the possible design of a pivotal Phase III program for vilobelimab for the treatment of HS. Following the advice received in a Type A meeting with the FDA, in the fourth quarter of 2021, we submitted a full clinical trial protocol for the planned clinical Phase III trial of vilobelimab in HS and in January 2022 we initiated a randomized, double-blind, placebo-controlled, multi-center pivotal Phase III study to determine efficacy and safety of vilobelimab in patients with moderate to severe HS and active dTs with a modified primary clinical endpoint called m-HiSCR. Subsequently, the FDA provided conflicting advice to us, which was subsequently corrected, but in the meantime, we had halted the Phase III clinical program and are currently evaluating next steps regarding the development of vilobelimab in HS. Based on the logistical and financial effort necessary to successfully complete such a Phase III development program, we are assessing various future possible options to further this development, including collaborations with pharmaceutical partners.
Vilobelimab for the treatment of ANCA-associated vasculitis (AAV)
We have also been developing vilobelimab for the treatment of AAV. Our clinical development strategy for vilobelimab in AAV first focused on acutely ill AAV patients, where we believe vilobelimab has the potential to successfully induce remission and reduce or eliminate the need for high-dose corticosteroid, or HDCS, therapy and providing an improved safety profile. We also intend to focus on speed of induction of remission and reducing the rate of renal replacement and kidney dysfunction. After the successful completion of two Phase II studies in 2021, we are currently evaluating next steps regarding the development of vilobelimab in AAV. Based on the logistical and financial effort necessary to successfully complete a pivotal Phase III development program, we are currently assessing possible options to further this development including collaborations with pharmaceutical partners.
Anti-Ca5R inhibitor INF904
To expand the breadth of our anti-C5a/C5aR technologies, we are also developing INF904, a product candidate that targets the C5aR receptor. In INF904, we discovered a small molecule C5aR inhibitor that in pre-clinical studies has shown potential for superior characteristics to the only approved C5aR inhibitor, avacopan. INF904 has provided higher plasma exposure in animals, including non-human primates, and improved inhibitory activity in a hamster neutropenia model compared to avacopan. Furthermore, in contrast to avacopan, in vitro experiments showed INF904 has substantially less inhibition of the cytochrome P450 enzymes 3A4/5 (CYP3A4/5). INF904 demonstrated potential for anti-inflammatory therapeutic effects in several preclinical disease models. In January 2024, we announced the positive results of a single and multiple ascending dose study with INF904 in healthy volunteers. We plan to initiate Phase II clinical studies in the future. INF904 is a promising product candidate for being developed in several disease areas of inflammation, where orally available therapeutics are not available or a medical need exists despite availability of other therapies.
-56-
INF904 for the treatment of CSU
We also intend to pursue development of INF904 for the treatment of CSU. CSU is a debilitating and unpredictable skin disease characterized by intensely itchy hives / wheals and angioedema. The burden of this chronic disease is high and impacts sleep, mental health, quality of life and productivity due to absences from school and work. CSU is estimated to affect around 40 million people worldwide. CSU patients have been reported to show elevated C5a levels, a major activator of mast cells and basophils which are thought to be significant contributors to CSU pathogenesis. In addition, studies suggest that complement activation (including C5a) in CSU can lead to histamine release. Current treatments are limited, and a significant unmet need exists in a sizable proportion of patients.
INF904 for the treatment of HS
We also intend to pursue development of INF904 for the treatment of HS. HS is a chronic, recurrent, debilitating neutrophil-driven inflammatory disease that can persist for years and tremendously impacts quality of life; it is characterized by abscesses, nodules and draining tunnels which can flare and cause scarring. INF904 inhibits the known C5a-induced effects on neutrophil activation and tissue accumulation of immune cells, including generation of tissue damaging mechanisms (enzyme release and oxidative radical formation) as well as induction of NETosis – mechanisms thought to be involved in HS progression and draining tunnel formation. Clinical evidence with existing C5a/C5aR products also supports that blocking this pathway reduces lesion counts. Patients’ responses to treatment with approved anti-TNF-alpha or anti-IL17 drugs are known to wane over time in a significant number of cases; and treatment with new mechanisms are needed for these patients.
Anti-C5a antibody IFX002
We are also developing IFX002 for the treatment of chronic inflammatory diseases. IFX002 is a highly potent anti- C5a antibody, which binds to the same domain of the C5a protein as vilobelimab, but which has a higher humanization grade and altered pharmacokinetic properties compared to vilobelimab. IFX002 is currently in preclinical development. We consider IFX002 to be a life-cycle management product to vilobelimab, given the long remaining patent life of IFX002.
Vilobelimab for the treatment of cutaneous squamous cell carcinoma (cSCC)
We have been developing vilobelimab for the treatment of PD-1 / PD-L1 inhibitor resistant / refractory locally advanced or metastatic cSCC. We have been recruiting patients in two independent arms, vilobelimab as monotherapy and in combination with pembrolizumab. The main objectives of the trial were to assess the safety and antitumor activity of vilobelimab monotherapy and to determine the maximum tolerated or recommended dose, safety and antitumor activity in the combination arm in order to evaluate and establish the safety of vilobelimab in cSCC patients. The initial treatment responses of the study were encouraging. However, in view of the recent emergence of new alternative treatments for cSCC and the recommendation by our U.S. and international clinical experts to change course and to study additional patients with a higher dose of vilobelimab as monotherapy in a larger cohort, we have decided to cease the development in cSCC for the time being to prioritize our efforts and to reallocate our resources towards the development of our orally available C5aR inhibitor INF904. Nonetheless, patients who are currently in treatment will be treated for up to 24 months according to the protocol. However, we will not enroll any new patients in the study and we will close clinical sites in which no patients are being treated. Our decision to wind down this clinical study does not preclude us from considering the development of vilobelimab or INF904 in this or similar oncological indications in the future.
For more information on our technology or our development programs please refer to the detailed information included herein below.
Our technology
C5a is a central mediator of the complement system and therefore a critical component of the innate immune system. The most prominent role of the complement system is to help the body defend itself against invading microorganisms through several mechanisms, including the rapid creation of an inflammatory environment and the production of factors that directly kill pathogens and recruit immune cells to sites of infection. Activation of the complement system ultimately results in the generation of C5a by cleavage from C5. C5a creates an inflammatory environment by attracting and strongly activating neutrophils as well as by causing many different cell types to generate pro-inflammatory molecules.
The complement system and role of the C5a/C5aR axis as critical component in the immune system and the need for control
The complement cascade consists of approximately 30 interacting proteins and forms a critical component of the innate immune system. This system protects the body, for example by recognizing and removing bacteria, viruses and other infectious agents, collectively referred to as pathogens. Activation of the complement system leads to a series of enzyme-like reactions that produce factors that both directly kill pathogens and recruit immune cells to sites of infection. This activation can be triggered via three major pathways: the classical pathway, the mannose binding lectin, or MBL, pathway and the alternative pathway. Activation of any pathway will lead to the cleavage of C3 and formation of C5-convertases. Terminal complement activation, which is also referred to as cleavage of C5, can be achieved by these C5 convertases. In addition, terminal complement activation can also be achieved directly through the extrinsic pathway by naturally occurring enzymes present throughout the body but not considered part of the complement system.
-57-
Cleavage of C5 results in the generation of C5a and C5b, two molecules with distinct biological activities. C5a is a strong inflammatory amplifier that exerts its biological functions by binding to two different receptors, C5aR and C5L2. C5b on the other hand assembles with C6, C7, C8 and many C9 molecules to form the MAC, an important intrinsic defense mechanism that causes the membranes of microorganisms to become permeable, leading to their disintegration, or lysis.

Overview of critical functions of the complement system
The complement system serves many crucial functions within the innate immune response, such as:
| ● | Rapid creation of an inflammatory environment. Production of pro-inflammatory molecules, such as C5a, optimizes the conditions under which enzymatic and other processes can act against microorganisms. These inflammatory conditions include the onset of a fever or release of aggressive enzymes and oxygen radicals by neutrophils. |
| ● | Lysis of microorganisms through formation of the MAC. A rapid, first-line defense mechanism resulting in the formation of pores in the cell membranes of invading microorganisms, leading to their disintegration. |
| ● | Bridge to the adaptive immune system. This function is promoted by an activation product of C3, called C3b, which tags particles and makes them visible and more easily processed by immune stimulatory cells. Such cells then present these particles to B-cells, which in turn generate antibodies against the particles, leading to targeted elimination. This mechanism takes a few weeks to take full effect. |
| ● | Clearance of dead cell particles. The complement system also serves various other purposes, including the clearance of dead cell particles from the body. This function is especially important because uncleared cell particles are believed to potentially induce generation of antibodies against normal cells and tissues, leading to autoimmune inflammatory responses and diseases. |
Need for control
Complement activation is a double-edged sword: the fast acting and relatively non-specific functions of pro-inflammatory responses driven by C5a and the lysis of microorganisms through MAC formation are usually very tightly controlled. However, inappropriate activation of the system can quickly turn it from a beneficial defense system into an uncontrolled inflammatory response. C5a’s uncontrolled activity in certain disease states can generate an inflammatory environment within the body that results in tissue damage and promotes pro-inflammatory T-cell autoimmune responses. The resulting tissue damage is believed to critically contribute to the disease progression of many acute as well as chronic inflammatory and autoimmune diseases, particularly during flare-up phases. Examples of this include Lupus disease, inflammatory bowel disease and neutrophil-driven diseases.
Despite the MAC’s role as a rapid, first-line defense mechanism, MAC formation can also result in damage to our body’s cells in some diseases. Normally, the body’s cells and tissues are protected from MAC-mediated lysis through surface inhibitors that prevent MAC formation. However, in paroxysmal nocturnal hemoglobinurea, or PNH, the patients’ cells lack the ability to hold MAC inhibitors on their cell surface, resulting in extreme susceptibility to MAC-related cell lysis. In addition, patients with diseases involving the kidney endothelial cells, such as atypical hemolytic uremic syndrome and certain forms of glomerulonephritis, also often appear to be burdened by MAC-related damage. Blockade of MAC formation in these very rare diseases can be lifesaving.
-58-
While blockade of MAC formation can be beneficial in certain circumstances, substantially blocking MAC formation can also result in susceptibility to life-threatening infections. For example, patients dosed with drugs that block MAC formation, such as with the marketed antibody eculizumab, must be immunized against meningococcal disease, which also carries the risk of side effects. Therefore, it is desirable to leave MAC formation intact when blocking complement-mediated damage in the broad variety of diseases in which an uncontrolled inflammatory response, and especially C5a, has been described as key driver of the damage.
We believe that C5a is a key inflammatory mediator driving tissue damage in many inflammatory diseases and thus represents a very meaningful drug target with large therapeutic potential. Therefore, we have conducted substantial research since our inception to generate highly specific antibodies targeting only C5a while leaving MAC formation intact, to deliver an ideal therapeutic approach for this attractive target.
Mechanisms of C5 activation
C5 can be produced by many cells, including epithelial cells of various organs, T-cells and other immune competent cells. Terminal C5 activation does not require activation of the three complement pathways and related formation of C5-convertases. Other enzymes can also directly cleave and activate C5, such that functionally active C5a can be generated in the complete absence of other complement components. For example, in the absence of other complement factors in the cell culture, lung epithelial cells can generate C5 upon stimulation, and lung macrophages can cleave and activate C5, leading to generation of C5a. This example illustrates that C5 can be activated and C5a can be generated independently from the complement pathways.
We further demonstrated that direct enzymatic cleavage of C5 occurs uninhibited in the presence of eculizumab, a known C5 inhibitor that binds to the MG-7 domain of C5 and hinders the C5 convertases from engaging and binding to C5. This research suggests that direct enzymatic cleavage of C5a from C5 works through a mechanism that is not blocked by C5 inhibitors such as eculizumab. Our studies further demonstrate that patients sufficiently dosed with eculizumab may still display elevated plasma C5a levels, implying that C5 inhibitors like eculizumab are not capable of fully blocking and controlling the C5a signaling pathway. Therefore, in diseases in which it plays a key promoting role, we believe targeting C5a directly may yield a meaningful therapeutic benefit.
C5a and its role in disease and inflammation
C5a is a small, 74-amino acid-spanning protein whose biochemical and immunological properties have been well documented in the scientific literature. C5a creates an inflammatory environment by attracting and strongly activating neutrophils as well as by causing many different cell types to generate pro-inflammatory and inflammation-related molecules. While this can help the body to respond strongly and rapidly to infections by optimizing the defense environment, uncontrolled C5a generation can induce damage to the body’s tissues in a broad variety of diseases. As a result, we believe that controlling and limiting C5a generation in the body may prevent the negative effects of an over-activated C5a immune response.
C5a quickly interacts with at least two independent receptors—C5aR and C5L2 (sometimes referred to as C5aR2). C5aR and C5L2 serve as a large signaling pool for effects elicited by C5a. C5aR has been well characterized as a signaling receptor that can be strongly upregulated in almost any cell across a variety of disease settings. Although less understood, C5L2 has also been shown to promote inflammation and negatively affect outcomes in various experimental disease settings by promoting the adverse effects, or AEs, elicited by uncontrolled C5a. Importantly, various other complement activation products (e.g., C3a, C3a-desArg and C4a) have been shown to bind to C5L2 and elicit effects different from those elicited by C5a. Thus, blocking specifically C5a as achieved by use of vilobelimab will eliminate only C5a mediated effects.
Role of C5a in neutrophil-driven inflammatory diseases
In the inflammatory response, C5a is an accelerator or “booster” of inflammation. This role of C5a extends to a broad variety of responses, including the following mechanisms:
| ● | C5a boosts the generation of many different cytokines such as IL-8, IL-6, IL17, TNF-alpha and others in a variety of cell types as well as within the bloodstream; |
| ● | C5a induces a complex change in the cell-signaling cascade of immune-competent cells that leads to an altered and often intensified signal transduction of other known signaling stimuli, such as the toll-like receptor signaling; |
| ● | C5a affects T-cell responses and causes a pro-inflammatory response, leading to the generation of further pro-inflammatory cytokines; and |
| ● | C5a is capable of inducing adhesion molecule expression on the surfaces of blood vessels, leading to neutrophil adherence to the internal vessel wall and migration through the vessel to the site of infection. |
-59-
When C5a binds to its receptors on neutrophils, they are strongly activated and move to the source of damage or infection, through a process referred to as chemotaxis, generating oxygen radicals and activated enzymes both believed to be major contributors to cellular and tissue damage in the body. In addition, C5a has been suggested to induce neutrophil extracellular trap, or NET, formation and a process in which neutrophils undergo a certain form of cell death while forming NETs called Netosis, which is believed to cause additional inflammation and damage in the tissue. Given this central function, C5a is a powerful tool that, when inappropriately activated, is capable of promoting damage to the body, ultimately leading to organ dysfunction and failure.
Neutrophil activation is assessed by observing the upregulation of the neutrophil surface marker CD11b (an established method to demonstrate neutrophil activation). In studies conducted in 2013 and 2014 as part of an investigative project in collaboration with an investigator from the University of Athens, we found that CD11b, as a marker for neutrophil activation, was greatly enhanced in fresh human whole blood from healthy volunteers when either recombinant human C5a was added or when plasma from hidradenitis suppurativa patients was added. Vilobelimab, our highly specific anti-C5a antibody, completely inhibited neutrophil activation resulting from the addition of the HS plasma, suggesting that C5a may be the key mediator in plasma from patients affected by this disease, leading to neutrophil activation.

Flow cytometry assay in fresh human whole blood demonstrating CD11b increase on blood neutrophils as marker of neutrophil activation: recombinant human C5a strongly activates human neutrophils in whole blood (huPP-ctr + 20 nM rhC5a), which can be fully blocked by addition of vilobelimab (“IFX-1” in the above graph) (huPP-ctr + 20 nM rhC5a + 20 nM vilobelimab) (open white bars). Plasma from two different HS patients (pat088 and pat092) also activates human neutrophils in whole blood and this effect can be fully blocked by the addition of vilobelimab (middle and darker grey bars) thus implying that C5a in HS patient plasma is the key neutrophil activating factor.
Various chronic inflammatory and autoimmune diseases in humans are characterized by flare-up phases during which substantial tissue damage occurs. Given C5a’s numerous inflammatory promoting functions, blocking it in chronic inflammatory diseases may have a positive effect on T-cell function, overall control of the inflammatory status of the disease and a strong anti-inflammatory effect on neutrophils, which may reduce tissue damage during the flare-up phases. Multiple international research groups have demonstrated in various inflammatory animal models that blocking the C5a/C5aR signaling axis leads to reduced inflammation, improved organ performance and favorable outcomes on clinical endpoints, including improved mortality rate, disease severity or damage scores.
C5a also has been described as a potential disturbing factor for a balanced T-cell response by down-regulating regulatory T-cells and promoting pro-inflammatory T-cell responses. Research published in 2013 in Nature Immunology and the Journal of Experimental Medicine demonstrated that blocking the C5a/C5aR signaling axis in mice restored regulatory T-cell function, inhibiting the progression of induced autoimmune diseases. Therefore, C5a is a potential drug target for the treatment of autoimmune and chronic inflammatory diseases associated with T-cell imbalance.
Role of C5a in cancer growth and metastatic disease
Different cancer cells have been found to generate their own C5a when cultured in vitro in the absence of any other complement factors or intact complement pathways. This result is possible because cancer cells produce C5, together with enzymes to directly cleave C5, thereby generating functionally active C5a. Recent research suggests that C5a contributes to cancer growth and metastatic disease, with multiple mechanisms proposed in the literature to explain this phenomenon. C5a appears to be associated with the recruitment and activation of myeloid-derived suppressor cells, or MDSCs, in tumors. Activating MDSCs suppresses the important T-cell-mediated mechanisms that usually inhibit tumor growth. Recently published findings in Cancer Cell in 2018 confirmed this mode of action that has been suggested in earlier published work. In addition, C5a generates a microenvironment favorable for tumor growth by increasing angiogenesis and enhancing the expression of the checkpoint molecule PDL1, as well as other mediators that enable tumor growth. These and other existing data may explain why combined therapy of anti-PD-1/PD-L1 and C5a blockade has been shown to effectively reduce tumor growth and metastasis in a pre-clinical mouse model.
-60-
Role of C5aR as potential target for therapeutic intervention
Two C5a receptors, C5aR (also known as C5aR1 or CD88) and C5aR2 (also known as C5L2 or GPR77), mediate the biological activities of C5a. Activation of C5aR has broadly acknowledged proinflammatory effects, while the role of activation of C5aR2 remains less well understood and recent scientific work has suggested a potential regulatory role on C5aR1 activation. In animal models of sepsis, anti-C5a treatment ameliorated the development of inflammatory responses and improved survival. In addition, experimental evidence suggests that blockade of C5aR signaling similarly improves survival in animals with sepsis. Finally, C5aR antagonists have shown excellent therapeutic effects in numerous models of inflammatory diseases involving complement activation.
Unlike its ligand C5a, C5aR can be pharmacologically inhibited by small molecules. In October 2021, avacopan, an oral C5aR antagonist, received market approval in the United States as an adjunctive treatment in adults for severe active ANCA-associated vasculitis (specifically MPA and GPA) in combination with standard therapy including glucocorticoids.
It is generally believed that blockade of C5a using antibodies offers a fast, complete, and safe way to control C5a-induced inflammation. The advantage of a low molecular weight compound inhibitor to C5aR is that it can be administered orally, thereby offering broad, long-term ease of administration to patients.
Our proprietary anti-C5a/C5aR technologies and product candidates
Our anti-C5a technology
Despite C5a’s well-characterized role in promoting inflammation and related tissue and organ damage in different diseases, no marketed drug targeting C5a exists. Based on more than 17 years of research in this field, we believe the challenge in targeting C5a is to fully block the biological functions of C5a in its natural environment and leave MAC formation intact. We believe our proprietary anti-C5a technology enables us to overcome this challenge through our discovery of a novel epitope, or binding site, on C5a. We believe this conformational epitope is formed only after the cleavage of C5a from the C5 molecule, suggesting that the three-dimensional structure of C5a changes upon release from C5, creating new epitopes that are only present on the free C5a molecule. This permits binding to free C5a only after it is cleaved from C5 and thus allows blocking of C5a while keeping MAC formation intact. We believe that this represents a breakthrough in the field of terminal complement C5a inhibition and that this may be particularly valuable when treating diseases that are driven by C5a, such as PG and severe COVID-19, cSCC, HS, AAV and others.
We identified antibodies, including our lead product candidate vilobelimab, that potently and selectively bind to a conformational epitope that is formed by C5a upon the cleavage of C5a from C5 to completely block C5a without compromising important upstream functions of the complement system, as well as MAC formation. We intend to discover and develop treatments leveraging our proprietary anti-C5a technology to address a wide array of complement-mediated diseases with significant unmet needs.

A conformational epitope on the surface of the C5a molecule allows for generation of highly specific blocking antibodies directed against C5a.
-61-
Our anti-C5a monoclonal antibodies are designed to have the following properties:
| ● | Complete immunological blockade and inhibition of C5a-induced effects: The human body has an abundant capacity to generate C5a, and induce inflammatory effects through its two receptors, C5aR and C5L2. Therefore, our anti-C5a antibodies are designed to: |
| o | generate complete immunological blockade of the C5a molecule to achieve potent and effective treatments. Antibodies or inhibitors lacking this quality may leave a “signaling gap” for C5a, which, in a disease setting, will likely be sufficient to allow for strong pro-inflammatory effects. This signaling gap would limit the ability to silence the C5a/C5aR and C5a/C5L2 signaling axis to achieve the desired therapeutic effect; and |
| o | bind with high affinity to C5a to counteract the molecule’s rapid interactions with its two receptors, C5aR and C5L2, which are abundantly present on the vast majority of cell types in the human body and that can be upregulated in various disease settings. |
| ● | Limited effect on MAC formation: C5 blocking molecules that inhibit MAC formation in the blood increase the risk of life-threatening infections caused by encapsulated bacteria such as meningococci. Therefore, leaving MAC formation intact may offer a significant advantage in C5a driven diseases. |
We believe that all these features are necessary for any drug targeting C5a, in order to achieve clinically meaningful pharmacological performance for the treatment of C5a-driven diseases such as PG, severe COVID-19, cSCC or others. Furthermore, we believe that C5a-driven diseases may not be effectively targeted with complement inhibitory approaches that do not specifically and fully block C5a. These approaches such as blocking the complement pathway-driven cleavage of C5 or inhibiting the complement pathways upstream of C5, are characterized by two fundamental shortcomings set forth below.
| ● | Inability to fully block C5a without targeting it directly: C5a can be generated through C5 activation by various enzymes in the complete absence of the complement pathways. For example, blocking the complement C5-convertase-driven cleavage with the C5 inhibitor eculizumab cannot block direct enzymatic C5 activation and C5a generation in an experimental setting. This may explain why elevated C5a levels remain measurable in patients effectively dosed with eculizumab. Therefore, non-specific approaches that do not bind and inhibit C5a directly may fail to fully block its effects. |
| ● | Lack of control over C5a’s signaling ability: C5a receptors are abundantly present on the majority of cells in humans and can be strongly and rapidly upregulated in certain disease states. As such, even with low levels of C5a, the receptors create a large “signaling sink” providing an abundant ability for even small amounts of C5a to transmit a signal. Therefore, a fully blocking targeted C5a approach is warranted in order to achieve full control over C5a-induced signaling events that may be especially important in highly acute inflammatory settings. |
Vilobelimab as first-in-class anti-C5a monoclonal antibody
Our lead product candidate, vilobelimab, is an intravenously delivered monoclonal anti-C5a antibody. It is based on our proprietary anti-C5a technology and was the first C5a monoclonal antibody to enter clinical development. Vilobelimab is differentiated by its ability to:
| ● | fully inhibit C5a-induced signaling and derived biological functions, as evidenced by its ability to completely prevent C5a-induced neutrophil activation in human whole blood; and |
| ● | leave MAC formation intact, as evidenced by testing the intact complement pathway driven MAC formation on red blood cells, leading to the lysis of these cells. |
-62-
We completed one placebo-controlled, single-center Phase I study of vilobelimab in healthy volunteers and completed two double-blind, placebo-controlled, multi-center Phase IIa studies in two acute care indications, early septic organ dysfunction and complex cardiac surgery. We also completed a Phase IIa and a Phase IIb clinical study in HS, two Phase II studies in AAV, a Phase IIa study in PG and a Phase II/III clinical study in critically ill, mechanically ventilated COVID-19 patients.
In all completed studies, vilobelimab was observed to be well tolerated. The placebo-controlled, multi-center Phase IIa studies in the two acute care indications demonstrated that vilobelimab blocked C5a with high statistical significance (p-values < 0.001) and that MAC formation, as demonstrated by a CH50 assay (as described below), in the groups treated with vilobelimab was not influenced, with mean CH50 values for treatment groups and control groups within the normal range.
To determine whether data is statistically significant, we use a “p-value,” which represents the probability that random chance could explain the results. The FDA utilizes the reported statistical measures when evaluating the results of a clinical trial, including statistical significance as measured by p-value as an evidentiary standard of efficacy, to evaluate the reported evidence of a product candidate’s safety and efficacy. If not otherwise specified, we used a conventional 5% or lower p-value (p < 0.05) to define statistical significance for the clinical trials and studies and data presented in this Annual Report.
Based on our clinical trials completed to date as well as the results from an EpiScreen ex vivo immunogenicity T-cell response assay, we believe that vilobelimab carries a low risk of provoking an immune response following administration. The immunogenicity assay used peripheral blood mononuclear cells from 21 donors and tested how many donors’ cells showed a CD4+ T-cell response following introduction of vilobelimab ex vivo. A response rate of over 10% (or more than three out of 21) means the applicable protein is considered to be high risk for immunogenicity, while a response rate of less than 10% means the protein is considered to be low risk. The results of the assay for vilobelimab showed that zero out of the 21 donors had a T-cell response rate, as compared to a control arm (using the A33 antibody), which showed a 30% response rate. In addition, based on an ADA detection assay conducted in connection with our Phase IIb clinical trial in HS, 10% of patients had ADAs at any time during the study. Only one participant the presence of ADAs was associated with any specific AE pattern indicating symptoms possibly related to the presence or emergence of ADAs leading to an immune reaction.
We are currently evaluating vilobelimab in various disease indications. In all ongoing and completed clinical studies so far, we have never observed effects that would raise doubts on the established safety of vilobelimab as a therapeutic drug candidate.
We will also continue to assess the potential for development of vilobelimab in other disease settings where we believe an anti-C5a antibody could be successfully developed into a marketed therapy.
Development of small molecule inhibitors of C5aR
Two C5a receptors, C5aR (also known as C5aR1 or CD88) and C5aR2 (also known as C5L2 or GPR77), mediate the biological activities of C5a. Activation of C5aR has broadly acknowledged proinflammatory roles, while activation of C5aR2 remains less well understood and recent scientific work has suggested a potential regulatory role on C5aR activation. C5aR is a G-protein-coupled-receptor expressed primarily by granulocytes, and, especially under disease conditions, broadly in various tissues and other immune cell types, which mediates the pathophysiological effects of C5a.
In animal models of sepsis, anti-C5a treatment ameliorated the development of inflammatory responses and improved survival. In addition, experimental evidence suggests that blockade of C5aR signaling similarly improves survival in animals with sepsis. Unlike its ligand C5a, C5aR can also be pharmacologically inhibited by low molecular weight compounds.
-63-
Low molecular weight C5aR antagonists have shown excellent therapeutic effects in numerous in vitro and in vivo animal models of inflammatory diseases involving complement activation. The advantage of a low molecular weight inhibitor of C5aR is that it can be administered orally, thereby offering broad, long-term ease of administration to patients, especially for the treatment of chronic diseases. Through proper clinical investigation of these small molecule C5aR antagonists in diseases induced by the activation of C5a/C5aR axis, the safety and efficacy of these agents can be established.
Avacopan is the first oral C5aR antagonist, which has market approval in the United States as an adjunctive treatment in adults for severe active ANCA-associated vasculitis (specifically MPA and GPA) in combination with standard therapy, including glucocorticoids.
Through our in-house drug discovery efforts, we identified a potent inhibitor of the C5a receptor, INF904, which we believe is a promising candidate for development. We are currently developing INF904, an oral, low molecular weight drug candidate that targets the C5aR receptor. We plan on targeting complement-mediated, chronic auto-immune and inflammatory conditions where an oral small molecule is needed for patients.
Given the different advantages of blocking C5a and C5aR, we believe that the development of both C5a and C5aR blocking agents is possible and potentially helpful to address a broader range of C5a/C5aR-molecular signaling axis-associated diseases.
Our preclinical and clinical development programs
Vilobelimab for the treatment of PG
We are developing vilobelimab for the treatment of PG. PG is a rare, chronic inflammatory form of neutrophilic dermatosis characterized by accumulation of neutrophils in the affected skin areas. The exact pathophysiology is not fully understood, but it is postulated that inflammatory cytokine production as well as neutrophil activation and dysfunction contribute to a sterile inflammation in the skin. PG often presents as painful pustule or papule, mainly on the lower extremities, which can rapidly progress to an extremely painful enlarging ulcer. Associated symptoms include fever, malaise, weight loss and myalgia. PG usually has a devastating effect on a patient’s life due to the severe pain and induction of significant movement impairment depending on lesions’ location. The exact prevalence of PG is not yet known but is estimated that up to 51,000 patients in the United States and Europe are affected by this disease.
There are 4 disease types recognized: ulcerative (the classical variant, which is the focus of our development), bullous (atypical), pustular, and vegetative (superficial, granulomatous). The ulcerative variant is the most frequent and typical form of PG, with lesions predominantly on the lower extremities.
There are currently no drugs approved for the treatment of PG in the US or in Europe. The only locally approved treatment is adalimumab, which has been approved in Japan but in no other country. There is no established standard of care based on controlled studies in PG. However, due to the high medical need associated with the disease, certain drugs are used in medical practice as treatment attempts for affected patients. These include certain orally administered drugs such as immunosuppressants, including cyclosporine or corticosteroids which are sometimes also used concomitantly, as well as topically applied drugs such as tacrolimus and others. Lastly intravenously administered TNF-alpha inhibitors such as infliximab or adalimumab or other biological drugs are also used as treatment attempt, despite the fact that no formal regulatory approvals are in place.
In February 2019, we initiated an open label, multi-center Phase IIa exploratory study enrolling 19 patients with moderate to severe PG in Canada, the United States and Poland. The objectives of this study were to evaluate the safety and efficacy of vilobelimab in this patient population in three different doses and to determine the appropriate dose for the future development of vilobelimab in registrational Phase III studies for the treatment of PG.
-64-
In April 2021, the study reached its enrollment target with 19 patients. In October 2021, we announced preliminary results from the study. In the third dosing cohort at 2400mg biweekly, six of the seven patients achieved clinical remission with a PGA score of ≤ 1, which reflected a closure of the target ulcer. All patients in the third dosing cohort had elevated C5a levels at baseline that were continuously suppressed after initiation of treatment with vilobelimab.
From all three dose cohorts in the study, two patients had related SAEs that were reported: one patient experienced an erysipelas leading to hospitalization (judged as non-drug related by sponsor), another developed a rash due to a delayed hypersensitivity reaction and withdrew from study. No dose-related AEs were found. Overall, the observed AE profile was in line with the underlying disease.
Final results from all patients were presented at the American Academy of Dermatology Association, AAD, Annual Meeting in March 2022 in an oral late-breaker session by Afsaneh Alavi, MD, Associate Professor of Dermatology, Mayo Clinic. The reported final results showed a dose-dependent effect in the highest dose cohort of 2400 mg, confirming the preliminary results with six out of seven patients showing a clinical remission (Physician Global Assessment, or PGA, score ≤ 1) and closure of the target ulcer in this dose cohort. The seventh patient showed a slight improvement (PGA score 4) with a decrease of the target ulcer area of over 50%. During the follow-up period, ulcers remained closed two months after treatment completion in all but one patient, and a sustained suppression of C5a was observed for up to 20 days after the last dosing.
With these results, vilobelimab was granted orphan drug designation for the treatment of PG by both the FDA in the United States and the EMA in Europe as well as fast-track designation be the FDA. Furthermore, we announced that we had a productive end-of-phase II meeting with the FDA related to our plans for a Phase III development program in PG in June 2022. In January 2023, we announced details related to the design of our planned Phase III study with vilobelimab in ulcerative PG.
The Phase III study is designed to enroll patients in the United States, Europe and selected countries in other regions. The study design is based on detailed feedback and recommendations from the FDA Division of Dermatology and Dentistry and was developed in close collaboration with the Company´s advisors from the United States, Europe and other regions. The multi-national, randomized, double-blind, placebo-controlled Phase III trial has two arms: vilobelimab (2,400mg every other week) plus a low dose of corticosteroids and placebo plus the same low dose of corticosteroids. In both arms, corticosteroid treatment will be initiated on day one and will be tapered off within the first eight weeks of the treatment period. The primary endpoint of the study will be complete closure of the target ulcer at any time up to 26 weeks after initiation of treatment. Treatment will be discontinued for patients whose disease progresses or fails to improve at defined time points during the study. The study has an adaptive trial design with an interim analysis blinded for the sponsor and investigators (but unblinded for the independent data safety monitoring committee), which is planned upon enrollment of approximately 30 patients, divided equally between the two arms of the study. The interim analysis with a set of predefined rules will take into account the then-observed difference in complete target ulcer closure between the two arms and will then determine whether the trial sample size will be adapted or whether the trial should be stopped due to futility. The enrollment period is projected to last at least two years, and its overall period will depend on the total trial size after sample size adaptation.
In November 2023, we announced the enrollment of the first patient in the trail.
Vilobelimab for the treatment of critically ill, invasively mechanically ventilated COVID-19 patients
Severe COVID-19 is characterized by severe lung inflammation and activation of coagulation, frequently requiring mechanical ventilation while the patient is in the intensive care unit. Mortality and morbidity rates are high among critically ill, invasively mechanically ventilated patients with COVID-19, despite the established broad use of corticosteroids and other anti-inflammatory agents. Poor disease outcomes have been associated with activation of the complement system, specifically the C5a/C5aR molecular signaling axis. Experimental studies in other viral lung diseases have shown that C5a is a potent anaphylatoxin, attracting neutrophils and monocytes to the site of infection that causes tissue damage, endothelialitis, and micro- thrombosis. Mouse studies also showed that blockade of the C5a/C5aR1 molecular signaling axis limits the infiltration of myeloid cells in damaged organs and prevents excessive lung inflammation and endothelialitis.
-65-
Despite wide-spread use of vaccines against SARS-CoV-2 and improvement in disease management, including use of immune modulators like anti-IL6 antibodies or JAK inhibitors, glucocorticosteroids and anti-coagulant therapy during the recent COVID-19 pandemic, mortality rates of critically ill, intubated and mechanically ventilated patients have remained at levels over 50%. With 450 to 3,800 COVID-19 related fatalities per week in the United States throughout 2023, according to the Centers of Disease Control and Prevention, or CDC, (https://covid.cdc.gov/covid-data-tracker/#trends_weeklydeaths_select_00 ) this indicates the still prevailing medical need for effective therapies for the treatment of these patients.
During the COVID-19 pandemic of 2020-2023, it became clear that effective therapies for the treatment of severely or critically ill COVID-19 patients were not available or had not tested for this indication. In an effort to provide a contribution to this medical emergency and based on our existing pre-clinical research on the role of C5a in viral-induced pneumonia, we decided to initiate a clinical development program with vilobelimab in critically ill COVID-19 patients with severely progressed pneumonia.
Clinical development
In March 2020, we initiated a randomized open label multi-center trial Phase II/III clinical development program with vilobelimab in severe COVID-19 patients with severely progressed pneumonia. In the Phase II part of the study, we evaluated vilobelimab treatment plus best supportive care compared to best supportive care alone for up to 28 days. Relative change (%) from baseline to day 5 in oxygenation index (defined as PaO2/FiO2 ratio) was assessed as the primary endpoint along with additional clinical parameters until day 28. In the study, patients were randomized to two treatment arms, either Arm A, best supportive care and vilobelimab or Arm B, best supportive care alone. The primary endpoint was the relative percentage change from baseline to day 5 in the oxygenation index (PaO2 / FiO2).
On June 17, 2020, we announced results from the Phase II part of the study. A total of 30 patients were randomized in the trial, and 15 patients were treated in each arm: vilobelimab plus best supportive care or best supportive care alone. Over a treatment period of 28 days, patients in the vilobelimab arm received a maximum of seven doses of 800 mg vilobelimab intravenously on separate days. At randomization, 18 patients were intubated (60%), and 12 patients (40%) had other oxygen supply. A higher number of patients with two or more comorbidities associated with increased COVID-19 mortality were reported in the vilobelimab treatment group compared to best supportive care group. Relative change in the oxygenation index at day 5 showed no differences between treatment groups. However, vilobelimab treatment was associated with a lower 28-day all-cause mortality when compared to the best supportive care group, along with trends in disease improvement, as evidenced by fewer patients experiencing renal impairment assessed by estimated glomerular filtration rates, more patients showing reversal of blood lymphocytopenia and a greater lowering of lactate dehydrogenase concentrations. In vilobelimab-treated patients, pulmonary embolisms reported as SAEs occurred less compared to the best supportive care arm. Also, a temporary increase of D-dimer levels, as potential expression of induction of blood clot lysis, was detected in the first days after initiation of vilobelimab treatment. Twenty-eight-day all-cause mortality in the vilobelimab treatment group was 13% (2 out of 15) versus 27% (4 out of 15) in the control group. In the best supportive care group, four patients died of COVID-19-induced multi-organ failure, and three of them had pulmonary embolisms reported as a SAE. In the vilobelimab arm, one patient died after an acute ventilator tube complication (leakage) and one patient with a history of severe chronic obstructive pulmonary disease died of pulmonary failure.
SAE rates were comparable between groups, but the rate of pulmonary embolisms reported as SAEs was substantially lower in the vilobelimab treatment group. Upon review of the safety data, the independent data safety monitoring board recommended continuation of the trial into the Phase III part.
In the Phase III part of the study, from September 2020 to October 2021, we enrolled 369 mechanically ventilated patients with COVID-19 across sites in the European Union, South America and other regions. Patients were randomized 1:1 to receive either vilobelimab or placebo; most patients received standard of care (97% glucocorticosteroids, 98% anti-thrombotic agents). The primary endpoint is 28-day all-cause mortality; key secondary endpoints include assessment of organ support and disease improvement.
-66-
In March 2022, we announced that the Phase III part of the Phase II/III PANAMO study with mechanically ventilated COVID-19 patients was successfully completed and showed a relative reduction in 28-day all-cause mortality of 23.9% (p = 0.094). At the recommendation of regulatory authorities during the course of the trial, we changed the statistical analysis method for the primary endpoint. The original protocol specified a non-stratified Cox regression analysis, and the final statistical analysis plan specified a site-stratified analysis intended to account for the site stratification of patients at randomization. The original protocol specified analysis would have resulted in a p-value of 0.027 (statistically significant), whereas the site-stratified Cox regression led to a p-value of 0.094 (not statistically significant). Additionally, pre-specified logistic regression analyses of the 28-day mortality resulted in p-values of <0.05 for three out of the four pre-specified analyses. Furthermore, a pre-specified analysis of patients from Western European countries (n=209) showed a relative reduction in 28-day all-cause mortality of 43% (vilobelimab 21.2% versus placebo 37.2%, hazard ratio: 0.5, p=0.014), suggesting an improvement in mortality in line with the reported Phase II data of the PANAMO Phase II/III study.
Regulatory activities
In September 2022, we announced the submission for EUA following encouraging interactions with the FDA at a Type B meeting held in summer 2022. Additionally, we were granted fast track designation from the FDA for vilobelimab for the treatment of critically ill, intubated, mechanically ventilated COVID-19 patients.
In April 2023, the FDA issued the EUA for GOHIBIC (vilobelimab) for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO.
In July 2023, we submitted a Marketing Authorization Application (MAA) for SARS-CoV-2 induced septic ARDS receiving IMV or ECMO to the EMA. In August 2023, the EMA validated the MAA. This means that the application is now under regulatory review by the CHMP under the centralized procedure, which applies to all 27 member states of the European Union.
To achieve full commercial scale and successfully reach the full market potential of the product in the future, we also aspire to obtain full market approval for Gohibic (vilobelimab). We are therefore planning the submission of the BLA for full approval of Gohibic (vilobelimab) in our COVID-19 indication and potentially, in the future, in similar indications that may apply to other virally induced acute respiratory distress conditions. In October 2023, in furtherance of our continued efforts to obtain a BLA, we had an encouraging Type C meeting with the FDA. In that meeting, the FDA indicated their willingness to collaborate with us in identifying a development pathway towards a BLA for a broader ARDS label. To achieve this, we would need to conduct an additional well-controlled and adequately powered study in a broader ARDS setting that demonstrates the safety and efficacy of vilobelimab. During the meeting, we discussed different options for such a trial, including potential trial designs, patient population and trial power aspects.
We are actively evaluating and working towards next steps to enable such a trial in a broader ARDS setting and are currently exploring different funding options, including government grants as well as collaborations with third parties.
Commercialization
We intend to seek full marketing authorization in major markets, including the United States and Europe. For this we might hire experts in sales and marketing and build the necessary commercial and logistical infrastructure internally and/or with the potential assistance of external service providers. In parallel, we also intend to seek partners to support our commercialization, such as partnerships in select regions and potentially building commercial infrastructure in other regions if EUA is granted.
In June 2023, we began the commercialization of GOHIBIC (vilobelimab) in the United States for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. We entered into agreements with certain subsidiaries of Cencora Inc., or Cencora (formerly known as AmerisourceBergen Corp.) to act as our U.S. distributor and to make GOHIBIC (vilobelimab) available for order by U.S. hospital customers under the EUA. Cencora provides cold storage, cold-chain distribution services, inventory management and secondary labeling/packaging, among other services. To support our commercial efforts, we have hired and are continuing to hire U.S. experts with relevant experience in the commercialization of medical products in the hospital market, including in the areas of sales, sales operations, marketing, market access, distribution, medical affairs and others. In addition, we are expanding the necessary infrastructure, including IT systems, supply chain, financial reporting systems and inventory management systems both, internally and with the assistance of external service providers.
-67-
As we expand our commercial efforts, we continue to enhance our commercial strategic plan, further expanding our sales force and medical affairs teams, preparing relevant promotional and medical education materials to target healthcare providers and other stakeholders, refining our medical affairs strategy to increase awareness of the EUA among the medical community, and initiating our sales efforts.
In July 2023 and subsequently in October 2023, the NIH published and subsequently updated their guidelines for the treatment of COVID-19 patients. The NIH guidelines stipulate that there is insufficient evidence to recommend either for or against the use of vilobelimab for the treatment of critically ill COVID-19 patients. This neutral NIH guideline recommendation has negatively affected the commercial adoption of GOHIBIC (vilobelimab) as many healthcare providers, particularly hospitals, rely on NIH treatment guidelines when drafting their formularies and placing new orders. We believe that the NIH analysis does not take into account positive factors that the FDA considered in granting the EUA, and we are working to provide as much scientific evidence as possible to the NIH and others in the medical community with the goal of reaching concurrence of the NIH guideline committee position with the detailed published FDA review and thus reconsidering their recommendation for the treatment of critically ill COVID-19 patients with Gohibic (vilobelimab).
Governmental funding
In October 2021, we announced that we received a grant of up to €43.7 million from the German Ministry of Education and Research and the German Ministry of Health to support our development of vilobelimab for the treatment of severe COVID-19 patients. Due to subsequent changes in our research and development plan and fewer costs projected within the timeframe of the grant, we were notified that the amount available to us is €41.4 million. The grant is structured as a reimbursement of 80% of certain pre-specified expenses related to the clinical development and manufacturing of vilobelimab. The grant period ended on June 30, 2023. In total, during the duration of the grant period through December 31, 2023, we received €33.3 million to support our activities regarding the development of vilobelimab as a new therapeutic agent for the treatment of critically ill COVID-19 patients and for the establishment of a commercial scale manufacturing process to ensure the ability of being able to provide such treatment to the broader population.
Vilobelimab for the treatment of cSCC
Cutaneous squamous cell carcinoma, or cSCC, is the second most common skin cancer. The incidence of cSCC increases with increasing sun exposure and age and individuals with fair skin and hair are more often affected. Approximately 200,000 to 400,000 cases of cSCC per year are being reported in the United States reaching up to estimates as high as 1 million per year. Estimates in Europe vary by geographic location from approximately 30 cases in 100,000 people per year in northern Europe to approximately 10 cases in 100,000 people in southern Europe. The incidence of cSCC is increasing around the world. However, advanced and metastatic forms of cSCC are rare. While treatment response rates of advanced and metastatic forms of cSCC with programmed cell death protein-1, or PD-1, / programmed death ligand-1, or PD-L1, inhibitors is believed to be in the range of 50%, patients frequently relapse, and resistant / refractory patients typically have a very poor prognosis.
The potential for local recurrence or metastasis of cSCC varies with the pathologic variant and localization of the primary lesion, and the risk for metastasis in cSCC is approximately 2-5%. Advanced cSCC 10-year survival rates are less than 20% with regional lymph node involvement and less than 10% with distant metastases. Patients with distant metastases have median survival times of less than 2 years.
We are also developing vilobelimab for the treatment of PD-1 / PD-L1 inhibitor resistant / refractory, locally advanced or metastatic cSCC. cSCC is the second most common skin cancer. The incidence of cSCC increases with cumulative sun exposure and age, and individuals with fair skin and hair are more often affected. The potential for local recurrence or metastasis of cSCC varies with the pathologic variant and localization of the primary lesion, and the risk for metastasis in cSCC is approximately 2%-5%. Advanced cSCC 10-year survival rates are less than 20% with regional lymph node involvement and less than 10% with distant metastases.
-68-
In the study, which was initiated in April 2021, we recruited patients in two independent arms, vilobelimab alone (Arm A) and vilobelimab in combination with pembrolizumab (Arm B). The main objectives of the trial are to assess the safety and antitumor activity of vilobelimab monotherapy, to determine the maximum tolerated or recommended dose in combination with pembrolizumab, as well as to evaluate the safety and antitumor activity in the combination treatment arm in cSCC patients.
As of the date hereof, 10 patients were enrolled in Arm A, in which they received a run-in dose of 800 mg of vilobelimab on days 1, 4, 8 and 15, followed by a dose of 1,600 mg vilobelimab every two weeks starting on day 22. An interim analysis in Arm A of the 10 patients was conducted in July 2023 and the treatment responses in Arm A were evaluated. The interim efficacy analysis showed that one patient had a complete response (CR) and one patient continued with stable disease according to the protocol and as per “Response Evaluation Criteria In Solid Tumors” (RECIST). The patient with the CR is still on treatment.
After five weeks of treatment with the first three patients in Arm A of the study, a safety assessment was successfully completed, and enrollment in Arm B was also opened.
In Arm B, as of the date hereof, three patients have been treated in the first dosing cohort of the study (400 mg intravenous infusions of vilobelimab on days 1, 4, 8 and 15 and 800 mg from day 22 and every two weeks thereafter, in addition to 400 mg of pembrolizumab on day 8 and every six weeks thereafter). Six patients were treated in the next higher (second) dose cohort (600 mg intravenous infusions of vilobelimab on days 1, 4, 8 and 15 and 1,200 mg from day 22 and every two weeks thereafter, in addition to 400 mg of pembrolizumab on day 8 and every six weeks thereafter). In the third dosing cohort, six patients were treated at the highest planned dose per protocol (800 mg intravenous infusions of vilobelimab on days 1, 4, 8 and 15 and 1,600 mg from day 22 and every two weeks thereafter, in addition to 400 mg of pembrolizumab on day 8 and every six weeks thereafter). Each dose escalation was done per recommendation and after review of the safety data by an independent Steering Committee comprised of external clinical advisors. In total, as of the date hereof, 15 patients were enrolled in Arm B (3+6+6 in the three dosing cohorts). Before proceeding with the second stage of the study in Arm B, the interim efficacy data as assessed in recent discussions with our panel of experts showed one patient with partial response from the second cohort, and one patient with partial response from the third cohort, who are still in treatment.
The treatment responses in the single dose Arm A and the initially observed results in the combination Arm B of the study were encouraging. However, in view of the recent emergence of new alternative treatments for cSCC and the recommendation by our U.S. and international experts to change course and to study additional patients with a higher dose of vilobelimab as monotherapy in a larger cohort, we have decided to cease the development in cSCC for the time being. While we remain interested in further understanding of the potential monotherapeutic effect of vilobelimab in this oncology indication, further research would require substantial resources and significantly extend the timeline of the ongoing clinical program. Therefore, in November 2023, we decided to prioritize our efforts and to reallocate our resources towards the development of our orally available C5aR inhibitor INF904.
Patients who are currently in treatment will be treated for up to 24 months according to the protocol. However, we will not enroll any new patients in the study and we will close clinical sites in which no patients are being treated. Our decision to wind down this clinical study does not preclude us from considering the development of vilobelimab or INF904 in this or similar oncological indications in the future.
Vilobelimab for the treatment of HS
HS is a chronic debilitating systemic skin disease that results in painful inflammation of the hair follicles, most notably in the armpit, groin and genital regions. The clinical hallmarks of this disease include very painful inflammatory nodules, boils or abscesses that typically open and release odorous inflammatory fluids. In the more chronic form of the disease, patients experience dTs (previously referred to as draining fistulas or sinus tracts), which ultimately lead to scarring and related functional disability in certain areas. HS patients suffer primarily from pain and significant discomfort resulting from the constant formation of pus, often requiring the use of bandages and diapers, resulting in social isolation. HS severely adversely affects patients’ quality of life. HS typically presents in the second and third decade of a patient’s life and often develops into a life-long debilitating chronic disease.
The target patient population for vilobelimab is HS patients displaying a moderate to severe form of the disease. In the United States, we estimate that moderate to severe HS has a prevalence of up to 200,000 patients, although recent publications suggest a higher prevalence. In Europe, the number of affected patients is also believed to be greater, with higher prevalence and incidence of HS in countries with warmer climates.
-69-
The accepted (but not approved) standard of care for HS patients includes topical, oral or intravenous antibiotic treatment, as well as surgery, which often provide only temporary symptomatic relief. HS is recognized as a systemic autoimmune disease, for which there are numerous suggested etiological factors, including genetics. Neutrophils are believed to play a potential disease-promoting role as well as certain cytokines and mediators commonly found in autoimmune diseases such as TNF-alpha, IL-17, IL-1 and others. C5a promotes inflammatory mediators and is a strong activator of neutrophils, which was the basis for our investigation of our C5a blocking drug candidate vilobelimab in patients with HS. We established that patients suffering from HS show proof of significant systemic complement activation with elevated plasma concentrations of C5a and other markers.
The only approved drug in the United States and in Europe to treat HS is adalimumab, an inhibitor of tumor necrosis factor-alpha, or TNF-alpha. Although it provides clinical benefit to a portion of moderate to severe HS patients, approximately 50% or more of the patients did not respond to adalimumab treatment. Therefore, a high unmet medical need among HS patients still persists.
The Hidradenitis Suppurativa Clinical Response, or HiSCR, score has been developed to assess the effectiveness of treatments for HS in clinical trials. Patients are defined as HiSCR responders when a ≥ 50% reduction in inflammatory lesion count, including abscesses and inflammatory nodules, or AN, is observed. At the same time, no increase in abscesses or dTs when compared with baseline shall be observed. The HiSCR is the primary endpoint that was used to support regulatory approval by the FDA and EMA of adalimumab for the treatment of HS patients.
In contrast to the dichotomous nature of the HiSCR, the IHS4 score was developed to score severity and track treatment response in a continuous manner as an alternative to HiSCR. However, the IHS4 score has not yet been utilized as primary endpoint in late-stage clinical studies in HS. This composite score weights the most fluctuating inflammatory nodules with one point, abscesses with two points and dTs with four points.
We have been developing vilobelimab for the treatment of HS. Initially we evaluated vilobelimab in a Phase IIa, single center open-label study in 12 patients with severe HS, who had partly failed to respond to prior treatment attempts. Results from the trial demonstrated a HiSCR response in 75% of patients at the end of eight weeks of treatment and in 83% of patients at the end of the 12-week trial observation period, demonstrating initial clinical evidence of the product candidate’s disease-modifying effect. The results also demonstrated that vilobelimab administration was well tolerated, with no drug-related adverse events detected and no infusion-related, allergic or anaphylactic reactions were observed.
Subsequently, we completed a larger multi-center, international Phase IIb study (SHINE) to determine the efficacy and safety of vilobelimab in moderate to severe HS patients. The trial was a randomized, double-blind and placebo-controlled, multi-center study with five dose groups, including one placebo group. After a placebo-controlled double-blind period of 16 weeks, each patient received vilobelimab open label for additional 28 weeks to assess long-term efficacy and safety. The main objective of the study was to evaluate a dose response signal assessed by the HiSCR score at week 16 as the primary endpoint.
In June 2019, we announced the top-line results of the international SHINE Phase IIb study, in which we failed to meet our primary endpoint utilizing HiSCR at week 16. The randomized, double-blind, placebo-controlled, multi-center study enrolled a total of 179 patients in four active dose arms and a placebo arm at over 40 sites in 9 countries in North America and Europe. While the highest dose (1200mg every two weeks) led to a 45.5% reduction in HiSCR, the placebo response amounted to 47.1% on the HiSCR endpoint. No difference could be detected in treatment-emergent AEs between placebo and vilobelimab treatment groups.
In a subsequent post-hoc analysis published in July 2019, we showed multiple signals of efficacy for the vilobelimab high dose group compared to the placebo group, demonstrating significant reductions in all combined inflammatory lesions, reductions of dT counts and reductions of the IHS4 score. For example, at week 16, a statistically significant reduction of dT count relative to baseline in the high dose vilobelimab group when compared to placebo could be observed (mean: -63.3% vs. -18.0%; p=0.0359; all patients with at least one dT at baseline).
-70-
In 2021 we submitted a Special Protocol Assessment, or SPA, to the FDA for the Phase III HS program for vilobelimab HS, suggesting IHS4 as the primary efficacy endpoint, which was subsequently declined by the FDA. The FDA agreed to the dosing regimen in the protocol but did not agree with the assessment of the primary endpoint using IHS4. We later held a Type A meeting with the FDA to align on the Phase III study design and a proposed new primary endpoint instead of IHS4. The discussion focused on reaching consensus on the overall study population and the primary endpoint measure. In September 2021, we announced the outcome of this meeting in which the FDA was supportive of the proposed pivotal study program focusing on patients with active dTs. The FDA also supported a new primary efficacy endpoint that would include measuring the reduction of all three inflammatory lesions associated with HS - inflammatory nodules, abscesses and dTs, called m-HiSCR (modified HiSCR). A m-HISCR responder is defined as, relative to baseline, at least a 50% reduction of ANdT count and at least a 50% reduction of dT count. The FDA provided advice on how to implement, name and validate the meaningfulness of the m-HiSCR for the intended patient population, especially since a reduction in dT count is not adequately captured by the HiSCR. Following the advice received in the Type A meeting, we submitted a full clinical trial protocol for the planned clinical Phase III trial of vilobelimab in HS patients with actively draining disease to the FDA. Upon submission of study protocol for review, we received no comments from FDA within the 30-day and 60-day review periods.
In January 2022, we initiated a randomized, double-blind, placebo-controlled, multi-center pivotal Phase III study to determine efficacy and safety of vilobelimab in patients with moderate to severe HS and active dTs with the m-HiSCR as primary endpoint. In February 2022, we paused the study after we receive an advice letter from the FDA that stated that the FDA recommended using the HiSCR as the primary endpoint in the Phase III trial. The FDA advice was provided nearly three months after our protocol submission and contrasted with the FDA advice provided to us in the Type A meeting held previously. However, the FDA did not issue a clinical hold. In March 2022, the FDA corrected its advice to us. In the corrected advice letter, the FDA stated that, contrary to its February 2022 advice letter, the FDA no longer recommended using the HiSCR as the primary endpoint for the chosen patient population, but gave recommendations related to implementation of the m-HiSCR endpoint. Subsequently, we halted the Phase III clinical program and are currently evaluating next steps regarding the development of vilobelimab in HS.
In February 2022, we also held a virtual research and development event in which we disclosed a post-hoc analysis of the m-HiSCR on the Phase IIb SHINE data (as shown below).
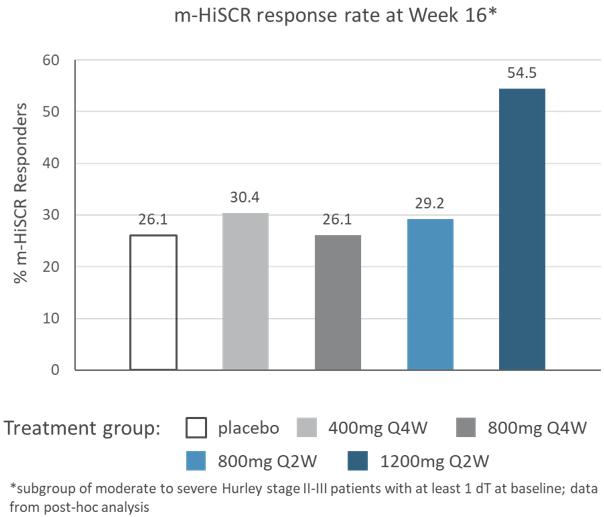
The data is consistent with the fact that in the Phase IIb SHINE study, significant reduction of dT count is only achieved within the high dose group.
Based on the logistical and financial effort necessary to successfully continue and complete a Phase III development program for vilobelimab in HS, we are currently evaluating options to further this development in the future, including with a potential pharmaceutical partner.
-71-
Vilobelimab for the treatment of anti-neutrophil cytoplasm antibody (ANCA) associated vasculitis
ANCA associated vasculitis, or AAV is a rare, life-threatening autoimmune disease with a relapsing nature, characterized by necrotizing vasculitis, an inflammation of blood vessels. The disease is characterized by life-threatening flare phases affecting the kidney function and other organs leading to organ dysfunction and failure, a potentially fatal outcome unless treated appropriately. AAV predominantly affects small vessels associated with anti-neutrophil cytoplasmic antibodies, or ANCA. It comprises three disease entities: GPA, or granulomatosis with polyangiitis (known as Wegener’s Granulomatosis); MPA, or microscopic polyangiitis; and EGPA, or eosinophilic granulomatosis with polyangiitis (known as Churg-Strauss syndrome).
AAV is designated as an orphan disease and affects approximately 40,000 and 75,000 patients in the United States and Europe, respectively. In addition, AAV has a reported incidence of 4,000 and 7,500 new patients per year in the United States and Europe, respectively.
Because of the life-threatening character of this disease, it is crucial to induce remission rapidly when a flare presents. The treatment to induce remission differs from maintenance therapy. The current treatment regimen to induce remission uses a combination of High Dose Corticosteroids, or HDCS, together with either rituximab or cyclophosphamide. In addition, avacopan has recently been approved and may be added to these therapies. The long lasting HDCS therapy is associated with significant side effects and additional life-threatening risks for the patients.
The disease promoting role of C5a for AAV is well established. A priming effect of C5a for neutrophils appears to be the essential factor leading to neutrophil-related damage of the endothelial cells in the vessels. In addition, patients with acute AAV disease have significantly elevated complement activation parameters in their plasma when compared to AAV patients in remission. In an experimental AAV disease model in mice, it was shown that while C5aR deficiency leads to reduction in disease activity, C6 deficiency does not lead to such improvement, suggesting that MAC formation might not play a major role in this disease. However, additional research is warranted to confirm this conclusion.
We have also been developing vilobelimab for the treatment of AAV. Our clinical development strategy for vilobelimab in AAV first focused on acutely ill AAV patients, where we believe vilobelimab has the potential to successfully induce remission and reduce or eliminate the need for high-dose corticosteroid, or HDCS, therapy and providing an improved safety profile. We also intended to focus on speed of induction of remission and reducing the rate of renal replacement and kidney dysfunction.
In May 2021, we announced topline results from a randomized, triple blind, placebo-controlled Phase II clinical study with vilobelimab to evaluate the efficacy and safety in patients with moderate to severe AAV, in which 19 patients were enrolled at centers in the United States. Patients in all three groups received the standard of care dosing therapy consisting of rituximab or cyclophosphamide and were randomized to either receive a low dose of vilobelimab in combination with a standard dose of glucocorticoids, a high dose of vilobelimab in combination with a standard dose of glucocorticoids or placebo in combination with a standard dose of glucocorticoids. The primary endpoint of the study was the number and percentage of subjects who experience at least one treatment-emergent AE per treatment group at week 24. The key secondary endpoint of the study is a 50% reduction in Birmingham Vasculitis Activity Score, or BVAS, at week 16, a well-established endpoint that has been used in the previous AAV studies, along with clinical remission. Overall, vilobelimab was safe and well tolerated, as observed treatment-emergent AEs were reflective of the disease and SOC treatment. The proportion of patients achieving a clinical response was defined as a 50% reduction in BVAS at week 16 (and no worsening in any body system) compared to baseline, and clinical remission was defined as BVAS=0. Although the sample size of the trial was small and it is difficult to interpret results not powered to show statistical significance, patients across all three treatment groups demonstrated a strong response at week 16, and more patients treated with SOC plus vilobelimab had clinical remissions at various timepoints throughout the study compared to SOC plus placebo.
In November 2021, we announced topline results from a randomized, double-blind, placebo-controlled Phase II clinical study with vilobelimab to evaluate efficacy and safety in patients with moderate to severe AAV, in which 54 patients were enrolled at centers in Europe. The primary endpoint of the study was a 50% reduction in BVAS at week 16. Secondary efficacy endpoints being analyzed include clinical remission, evaluation of the Vasculitis Damage Index, or VDI, reduction of glucocorticoid toxicity index, or GTI, several relevant biomarkers like glomerular filtration rate, and patient reported outcomes. The study was conducted in two parts. In part 1, patients were randomized to receive either vilobelimab plus a reduced dose of glucocorticoids, or placebo plus a standard dose of glucocorticoids. In part 2 of the study, patients were randomized to receive either vilobelimab plus placebo, glucocorticoids or placebo plus a standard dose of glucocorticoids. Patients in both arms received standard of care immunosuppressive therapy, consisting of rituximab or cyclophosphamide. The study achieved its principal objective, demonstrating comparable clinical response of vilobelimab to standard of care, while significantly reducing the need for glucocorticoid (GC) treatment in this life-threatening indication. Clinical response as well as remission were achieved in comparably high rates in all three arms: clinical response at week 16 was observed in 16 out of 18 (88.9%) evaluable patients in the group receiving vilobelimab alone; in 22 out of 23 (95.7%) patients receiving SDGC; and in 10 out of 13 (76.9%) patients in the vilobelimab + RDGC group. The GTI composite score at week 16 was substantially lowered in the vilobelimab only group (mean value of 0.8) when compared to the SDGC group (mean value of 44.9) and the vilobelimab + RDGC group (mean value of 26.1). Assessment of the VDI at week 16 suggested comparable values between groups with the vilobelimab only group showing the lowest value: vilobelimab only group (1.0), SDGC group (1.5) and vilobelimab + RDGC group (1.9). eGFR, a secondary endpoint of the study, demonstrated no medically meaningful changes in all three arms. The vilobelimab only group had the lowest number of reported treatment-emergent AEs, as well as related treatment-emergent AEs.
-72-
We are currently evaluating next steps regarding the development of vilobelimab in AAV and are considering discussing the data from both the U.S. and EU studies with regulatory authorities before determining next steps. Based on the logistical and financial effort necessary to successfully complete a pivotal Phase III development program, we are currently assessing different options to further this development, including with a potential pharmaceutical partner.
Additional clinical and pre-clinical development for vilobelimab
Beyond PG, severe COVID-19, cSCC, HS and AAV, the indications we described in the above sections, we keep exploring the possibility to advance the clinical development of vilobelimab in additional inflammatory and chronic complement-mediated autoimmune disease indications for which a good pre-clinical or clinical proof of concept exists and where C5a has been demonstrated as a critical disease promoting factor or where similar mechanisms, such as neutrophil-driven systemic diseases affecting the skin and or other organs, are identified.
INF904 as orally administered low molecular weight molecule for inhibition of C5aR
Inhibition of the C5a/C5aR axis provides strong anti-inflammatory effects in a variety of diseases. Blockade of C5a using highly specific antibodies, such as vilobelimab, may offer a fast, effective, and safe way to control C5a-induced inflammation. In addition to this approach, inhibition of C5aR by oral small molecules may provide the ease of administration required for effective long-term treatment for more chronic inflammatory diseases. To expand the breadth of our anti-C5a/C5aR technologies, we are also developing INF904, an oral, small molecule drug candidate that targets the C5aR receptor. C5aR, a G-protein-coupled-receptor expressed primarily by granulocytes, mediates the pathophysiological effects of C5a. In INF904, we discovered a small molecule C5aR inhibitor that in pre-clinical studies has shown potential for superior characteristics to the only approved C5aR inhibitor, avacopan. INF904 has provided higher plasma exposure in animals, including non-human primates, and improved inhibitory activity in a hamster neutropenia model compared to avacopan. Furthermore, in contrast to avacopan, in vitro experiments showed INF904 has substantially less inhibition of the cytochrome P450 3A4/5 (CYP3A4/5) enzymes, which play an important role in the metabolism of a variety of drugs, including glucocorticoids. No obvious toxicological findings, even in the highest dose groups tested in required GLP toxicity analyses, were identified. INF904 demonstrated potential for anti-inflammatory therapeutic effects in several preclinical disease models.
All IND-enabling studies, including certain GLP-toxicological studies, have been completed, and we conducted a Phase I single and multiple ascending dose clinical study from November 2022 to January 2024.
In September 2023, we announced the topline results from the single ascending dose, or SAD part of a randomized, double-blind, placebo-controlled Phase I trial with INF904.
The SAD part of the Phase I first-in-human trial enrolled 62 healthy volunteers within six different dosing groups from 3 mg to 240 mg who were randomly assigned to receive INF904 or a placebo. Different drug concentrations were tested for the 60 mg dosing group. The main objectives were to assess safety and tolerability of single ascending doses under fasting conditions. Secondary endpoints included several pharmacokinetic, or PK, parameters, and the effect of INF904 on C5a-induced neutrophil activation in blood samples from treated volunteers ex vivo also was explored.
The results show that INF904 was well tolerated in treated patients and resulted in no safety signals of concern in single doses ranging from 3 mg to 240 mg. The overall percentage of adverse events (AEs) was lower in the INF904 treated patients compared to the placebo group, and no serious or severe AEs were observed at any dosing level. No related AEs were reported in conjunction with INF904 dosing.
-73-
Analysis of INF904 PK in subject plasma samples revealed sustained exposure to INF904 with six hours to maximum concentration, or tmax. INF904 plasma levels were dose proportional for systemic exposure (AUClast) and nearly dose proportional for maximum concentration (Cmax) over the dose range used in the study. With the 30 mg dose, INF904 reached a Cmax of 289 ng/ml with an AUClast of 5197 h.ng/ml, which are approximately 3-fold and 10-fold, respectively, higher than the published Phase I data from the only marketed comparator, avacopan.
Single doses of 30 mg or higher of INF904 achieved ≥90% blocking of C5a induced up-regulation of the activation marker CD11b on neutrophils in plasma samples from subjects ex vivo at 24 hours post dosing. This inhibition was achieved when 12.6 nM recombinant C5a was added as stimulus in this assay, a C5a concentration which can be observed in patients with severe inflammatory conditions such as the immuno-dermatological disease, hidradenitis suppurativa, or during life-threatening inflammation (e.g., in critically ill COVID-19 patients or septic patients). Thus, INF904 inhibition of C5a-induced neutrophil activation in human plasma achieved the set goal for effective C5aR control at disease relevant C5a levels.
In January 2024, we announced topline results from the multiple ascending dose, or MAD, part a randomized, double-blind, placebo-controlled Phase I trial for INF904. The PK and pharmacodynamic, or PD parameters confirm the favorable data we observed during the SAD part of the study, which provides support for the best-in-class potential of INF904. INF904 was well tolerated and there were no adverse safety events of concern after repeated dosing in participants over the entire tested dose range.
In the MAD part of the randomized, double-blind, placebo-controlled Phase I trial, 24 participants received multiple doses of INF904 for 14 days of either 30 mg once per day, or QD, 30 mg twice per day, or BID, or 90 mg BID. The study’s primary objective was to evaluate the safety and tolerability of repeated dosing. Several PK parameters were analyzed as secondary endpoints, and the effect of the dosing scheme on C5a-induced neutrophil activation in blood samples from the participants was also explored in an ex vivo assay.
The safety analysis of INF904 in the MAD part of the Phase I study demonstrated that it was well tolerated in participants over the entire dose range and resulted in no safety signals of concern. The overall percentage of AEs in INF904 treated participants was 77.8%, which was lower than the 83.3% observed in the placebo group. There were no serious or severe AEs observed at any dosing level.
Analysis of the PK profile showed that potential target AUC0-12h, Cmax, and trough values were achieved rapidly within 14 days of 30 mg BID dosing. INF904 exposure further increased proportionally with dosing up to 90 mg BID. These results were demonstrated even when participants ingested the drug in a fasted state, suggesting that food is not required to achieve potentially therapeutic drug levels.
Analysis of the PD profile showed that the blocking activity of C5a-induced neutrophil activation by INF904 reached equal to or above 90% over the 14-day dosing period for all tested doses in an ex vivo challenge assay where physiological and disease- relevant levels of C5a were added to blood samples provided by the trial participants.
In parallel, we have progressed with the development of a commercially viable formulation of INF904 which we plan to introduce into Phase II development towards the end of 2024.
We are currently conducting additional required pre-clinical studies, including long-term chronic toxicology studies, to enable longer-term dosing of INF904 for chronic inflammatory diseases. We currently plan to initiate a short-term dosing Phase II study towards the end of 2024, followed by a longer-term dosing Phase II study in 2025. We initially plan to develop INF904 for the treatment of two initial immuno-dermatology indications: HS and CSU.
-74-
IFX002 as follow-on anti-C5a monoclonal antibody product candidate
To expand the breadth of our anti-C5a technologies, we are also developing IFX002 for the treatment of chronic inflammatory indications. IFX002 is an advancement of the anti-C5a technology. It is a highly potent anti-C5a antibody with a higher humanization grade and altered pharmacokinetic properties and is currently in pre-clinical development.
IFX002 is an injectable product candidate with a prolonged blood plasma half-life than vilobelimab, making it potentially more amenable for the treatment of chronic inflammatory indications with less severe flares or closer to the onset of the disease. IFX002 shares the same mechanism of action as vilobelimab in its potential to block C5a with high specificity but is designed for a dosing regimen that may be more suitable for chronic therapy. Furthermore, IFX002 binds to the same epitope of free C5a as vilobelimab with comparable selectivity. The pre-clinical development of IFX002 was partly supported by a grant from the German government. IFX002 will keep the performance relevant properties to fully block C5a-induced biological effects while leaving MAC formation intact. We believe that IFX002 holds the potential to treat various chronic inflammatory diseases and could benefit from a dosing regimen more suitable for chronic therapy. We also consider IFX002 to be a life-cycle management product to vilobelimab, given the long remaining patent life of IFX002.
Pipeline
We intend to leverage our expertise within the complement field as well as our proprietary technologies to sustain our lead in the anti-C5a/anti-C5aR space by developing a diverse pipeline focused on complement-mediated autoimmune and inflammatory diseases with high unmet need. Rights to our proprietary anti-C5a/anti-C5aR technologies are currently expected to extend at least up to 2041 on the basis of our latest patents granted.
The figure below summarizes key information about and the development status of our current pipeline of product candidates:

Our strategy
Our goal is to maintain and further advance our leadership position within the anti-C5a/anti-C5aR complement space, delivering first-in-class autoimmune and anti-inflammatory therapies to market. To achieve this goal, we expect to execute the strategies set forth below.
| ● | Advance vilobelimab in PG. Based on the positively concluded open label Phase IIa study, we are conducting a Phase III pivotal clinical program after having received advice related to the clinical trial design from the FDA. |
-75-
| ● | Proceed with clinical development of INF904. We have conducted a single and multiple ascending dose study of our C5aR antagonist INF904 and currently are conducting additional required non-clinical studies to proceed to Phase II trials in patients. |
Continue to optimize the manufacturing process for vilobelimab. We established a fully validated manufacturing process for vilobelimab with an established and reputable CDMO with the goal of fulfilling the quality criteria to gain regulatory approval for such process. We are establishing the final manufacturing of the finished pharmaceutical product (i.e., “fill and finish”) in Germany and are considering the transfer of the drug substance manufacturing process from China to Germany or other countries.
Assess development options for vilobelimab in HS, cSCC and AAV. Following our decision to halt these development programs due to the resources required to conduct these on our own, we are currently evaluating options regarding the development of vilobelimab in HS, cSCC and AAV. Based on the logistical and financial effort necessary to successfully complete pivotal Phase III development programs in each of these indications, such options include potential collaborations with a pharmaceutical partner.
| ● | Pursue the further development of IFX002 to get prepared for potential clinical development. We are developing IFX002 as an injectable with a longer half-life than vilobelimab, making it suitable for chronic inflammatory indications with less severe flares or closer to the onset of disease. Based on a patent lifetime potentially beyond 2040, we see this project as life-cycle management for vilobelimab and are conducting pre-clinical development work to get closer to the possible start of clinical development. |
| ● | Solidify and continue to expand the breadth of our leadership position in the anti-C5a/anti-C5aR space by leveraging the full potential of our proprietary technologies and expertise in complement and inflammation research. We intend to continue to discover and develop treatments that have the potential to address a broad spectrum of complement-mediated or immune response mediated indications with significant unmet need, either internally or in collaboration with a partner. To accomplish this, we continue to supplement our research and development activities with our discovery unit in Ann Arbor, Michigan and we are further building out our intellectual property portfolio and our business development capabilities. |
Commercialize GOHIBIC (vilobelimab) under the granted EUA either independently or in collaboration with pharmaceutical partners. We are commercializing Gohibic (vilobelimab) for severe COVID-19 in the United States independently, and assessing the options to commercialize the drug in Europe independently or in collaboration with potential partners. We have employed a targeted commercial infrastructure to promote access to vilobelimab through centers-of-excellence that treat patients suffering from the disease in these core markets in US. Outside of the United States and Europe, we may pursue the approval and commercialization of vilobelimab for severe COVID-19 either independently or in collaboration with others. For other indications, we intend to develop and commercialize vilobelimab either independently or through collaborations with other parties.
Advance vilobelimab to market approval for severe COVID-19: continue the approval process of MAA by the EMA and for a full BLA submission to the FDA.
| ● | Explore the possibility to expand the applications of vilobelimab into related diseases. If we gain regulatory approval in the United States or in Europe for the use of vilobelimab in severe COVID-19, we intend to explore the possibility of expanding the label into other critical care indications for which we have generated pre-clinical data in the past. Most notably, we may consider additional studies, potentially in collaboration with government agencies or commercial partners, to expand the label into a product for virally induced ARDS. |
Our intellectual property
We aim to protect our product candidates and other commercially important proprietary anti-C5a and C5aR technologies by seeking and maintaining U.S. and foreign patents that are intended to cover our product candidates and compositions, and their methods of use, the methods used to manufacture them, the related therapeutic targets and associated methods of treatment and any other inventions that are commercially important to our business. We also rely on trade secrets and know-how and other intellectual property rights to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Furthermore, we aim to protect our trademarks, service marks, and trade names by seeking and maintaining U.S. and foreign trademark registrations. Our success will depend significantly on our ability to obtain and maintain such patent and other proprietary protection, defend and enforce our patents, preserve the confidentiality of our trade secrets and operate our business without infringing, misappropriating or otherwise violating any patents or other intellectual property, including any proprietary rights of third parties. See the section titled “ITEM 3. KEY INFORMATION — 3. Risk factors—Risks related to our intellectual property” for additional information.
-76-
As of December 31, 2023, we owned ten issued U.S. patents and five pending U.S. non-provisional patent applications, 34 issued foreign patents, including four granted European patents validated in 88 countries and two granted Eurasian patent validated in 16 countries, as well as 84 foreign patent applications, including eight European patent applications and three Eurasian patent applications covering C5a and C5aR inhibitors and associated methods of use.
Our patent portfolio relating to vilobelimab, IFX002 and INF904, as of December 31, 2023, is summarized below.
As of December 31, 2023, we owned four issued U.S. patents covering the composition of matter of antibodies that block C5a and their use in blocking C5a-induced biological effects in patients with diseases that involve acute or chronic inflammation, which would include in their scope HS and AAV. In addition, we owned 20 issued foreign patents, including two granted European patents validated in 74 countries and one Eurasian patent validated in nine countries, two pending foreign patent applications, including one pending European patent application, covering the composition of matter of antibodies that block C5a and their use in the treatment of various diseases that involve acute or chronic inflammation, which would include in their scope HS and AAV, and, depending on the jurisdiction of the applicable patent, specifically cover the use of such antibodies in treating diseases such as ischemia and reperfusion related injuries, acute lung injury and pneumonia.
The issued U.S. and foreign patents are expected to expire in 2030, excluding any additional term for patent term adjustments or patent term extensions. If granted, the pending U.S. and foreign patents applications would be expected to expire in 2030, excluding any additional term for patent term adjustments or patent term extensions.
As of December 31, 2023, we owned two granted U.S. patents and four granted foreign patents, including one European patent validated in three countries and one foreign patent application covering the use of certain binding moieties, such as antibodies, that inhibit C5a for the treatment of viral pneumonia.
If issued, the U.S. and foreign patents are expected to expire in 2035, excluding any additional term for patent term adjustments or patent term extension.
As of December 31, 2023, we owned three granted U.S. patents, one pending U.S. non-provisional patent application, eight granted foreign patents, including one European patent validated in 12 countries and one granted Eurasian patent validated in eight countries, 25 pending foreign patent applications, including three pending European patent applications covering the use of an inhibitor of C5a activity, for example, vilobelimab, for treating HS and other cutaneous, neutrophilic inflammatory diseases.
The issued U.S. and foreign patents are expected to expire in 2038, excluding any additional term for patent term adjustments or patent term extensions.
As of December 31, 2023, we owned one U.S. patent application and 16 foreign patent applications including an European patent application covering an improved C5a specific antibody.
If issued, the U.S. and foreign patents are expected to expire in 2041, excluding any additional term for patent term adjustments or patent term extensions.
As of December 31, 2023, we owned one pending U.S. non-provisional patent application and 16 foreign patent applications including one European patent application covering the use of inhibitor of C5a activity, for example vilobelimab, for treating Corona viral diseases.
If issued, the U.S. and foreign patents based on the application under the PCT are expected to expire in 2040, excluding any additional term for patent term adjustments or patent term extensions.
As of December 31, 2023, we owned one granted U.S. patent, one pending U.S. non-provisional patent application, and 18 foreign patent applications including one European patent application covering inhibitors of C5aR.
-77-
The issued U.S. and foreign patents are expected to expire in 2040, excluding any additional term for patent term adjustments or patent term extensions.
As of December 31, 2023, we owned one U.S. patent application and one international patent application covering the use of an inhibitor of C5a activity and standard of care inhibitors in the treatment of infectious pneumonia and infectious ARDS.
If issued, the U.S. and foreign patents are expected to expire in 2043, excluding any additional term for patent term adjustments or patent term extensions.
As of December 31, 2023, we owned two European patent applications covering the use of inhibitors of C5aR for the treatment of various diseases.
If issued, the U.S. and foreign patents are expected to expire in 2043, excluding any additional term for patent term adjustments or patent term extensions.
As of December 31, 2023, we owned trademark registrations for two trademarks, “GOHIBIC” and “Vilwaysi” in the United States and 29 foreign countries, including 23 European countries and 7 foreign trademark applications for goods and services in the field of pharmaceutical products, among others.
As of December 31, 2023, we owned two trademark registrations for “InflaRx” in the United States for goods and services in the field of pharmaceutical preparations for the treatment of inflammatory, inflammatory-related, oncological and neurological diseases. Outside the United States, as of December 31, 2023, we owned trademark registrations for “InflaRx” in 30 countries.
UPC
Twenty-four member states of the twenty-seven member EU states, with the exception of Poland, Spain and Croatia have acceded to the Agreement on a Unified Patent Court, or UPCA, which provides for a Unitary Patent, or UP, and led to the establishment of a Unified Patent Court, UPC. The UPCA has entered into force on June, 1 2023. Seventeen out of the twenty-four member states have fully ratified the UPCA when it entered into force and participate in the UPCA from the start. The remaining seven EU member states are expected to join within the next years. Since the start of the UPCA, a conventional European patent can be enforced centrally at the UPC but may also face central revocation proceedings in the UPC, unless it is opted-out of the jurisdiction of the new court system.
The UPC has exclusive competence in respect of civil litigation on matters relating to European patents, Unitary Patents, supplementary protection certificates issued for a product covered by such patents and European patent applications (for details see Article 32 of the UPCA). Therefore, the UPC is not only competent to hear cases concerning UPs but also cases concerning traditional European patent applications and patents (hereinafter “European patent(s)”), unless opted-out. The decision of the UPC in one country is binding in all other states participating in the UPCA. The decision, however, does not have any legally binding effect in EU member states not participating in the UPCA as well as in EPC contracting states which are not EU member states (e.g. Great Britain and Switzerland) and, therefore, are not eligible to participate in the new system.
It is generally expected that the UPC will be patent holder friendly both when it comes to assessment of validity of granted patents and infringement and that it may be beneficial for a patent holder to enforce and defend its patent at the UPC rather than at the EPO and/or national nullity court or civil court. Presently, however, there is no case law of the UPC that would allow a prediction of how the UPC may apply the patentability criteria when deciding on a central revocation action or a revocation counterclaim or how it will construe a claim in a central infringement proceeding. In view of these uncertainties, which are immanent to any new system on one hand, and the well-established practice of oppositions at the EPO as well as national invalidity and infringement proceedings on the other, we consider it a cautious and recommendable approach to opt-out all European patent applications and granted European patents from the UPC and to follow the case law of the UPC to determine the pros-and-cons of the UPC system. In case that the UPC appears to come to more favorable decision on validity and/or infringement than the EPO and national courts, it should be reconsidered to opt-in for one or more European patent or patent applications that have been previously opted out.
-78-
Our collaboration agreements
Co-development agreement with Staidson (Beijing) BioPharmaceuticals Co., Ltd. (as successor to Beijing Defengrei Biotechnology Co. Ltd (BDB))
In December 2015, we entered into a co-development agreement, or the Co-Development Agreement, with Beijing Defengrei Biotechnology Co. Ltd., or BDB, for the use of the vilobelimab manufacturing cell line in BDB’s development of drug candidates for sale in China. Pursuant to the agreement, we granted BDB an exclusive, non-transferable license to use the vilobelimab cell line and related intellectual property solely to develop and commercialize in China BDB’s drug candidates BDB-001 and BDB-002, as well as molecules that bind or interact with certain specified targets, or target-binding molecules.
Pursuant to the agreement, we are entitled to receive mid-single-digit percentage royalties on net sales of BDB’s products containing BDB-001 or BDB-002. We retain the right to develop and manufacture vilobelimab and IFX002 in China solely for the purpose of commercializing products outside of China and to use the vilobelimab cell line and IFX002 cell line in China for non-commercial purposes. To the extent that we are granted regulatory approval outside of China for commercialization of a product using vilobelimab or IFX002 for an indication, and BDB does not pursue regulatory approval for BDB-001 or BDB-002 in the same or a substantially similar indication in China, by providing written notice to BDB, we may elect to pursue regulatory approval to commercialize such products in the relevant indication in China. Should we exercise such right, we would be required to pay BDB mid-single-digit percentage royalties on our net sales of such products.
Pursuant to the Co-Development Agreement, BDB has the right to use the vilobelimab cell line to manufacture an anti-C5a antibody, namely BDB-001. BDB-001 may only be commercialized in China (PRC) by BDB, and InflaRx is not directly involved in the BDB-001 development, which remains the sole responsibility of BDB. Pursuant to the Co-Development Agreement, InflaRx owns all global commercial rights outside China to any and all discoveries derived from the development of BDB-001. To support BDB’s development of BDB-001, in 2020, InflaRx allowed BDB to conduct clinical studies with BDB-001 in Spain, India, Indonesia and Bangladesh. However, InflaRx remains the sole owner of all commercial rights to BDB-001 outside of China, including in countries in which BDB is conducting clinical trials. BDB has no rights to seek marketing authorization or to commercially exploit BDB-001 outside of China. Vilobelimab is not the product being tested in clinical trials by BDB in China. Rather, it is BDB’s own antibody called BDB-001.
In addition, we reserved the right to commercialize products containing BDB-001 and BDB-002 outside of China in indications for which we elect not to commercialize vilobelimab or IFX002. To the extent that we exercise this right, we would be required to pay BDB low single-digit percentage royalties on our net sales of such products.
BDB must notify us without undue delay of tests it conducts on target-binding molecules. If any such test results in binding or interaction with targets in a satisfactory manner to both BDB and us, BDB must notify us of such results and may, within a six-month period following such notice, exercise an option to commence commercializing the successfully tested target-binding molecules in China. To the extent that BDB exercises such option, BDB would be required to pay us low single-digit percentage royalties on net sales of products containing such target-binding molecules. BDB also grants us the right to exploit any target-binding molecules outside of China or, to the extent that BDB does not pursue regulatory approval in the same or a substantially similar indication, in China. To the extent that we exercise such rights, we would be required to pay BDB low to mid single-digit percentage royalties on our net sales of such products.
In November 2021, we entered into a second addendum to the Co-Development Agreement with BDB and Staidson (Beijing) BioPharmaceuticals Co., Ltd., or Staidson. Under the second addendum, BDB, being a wholly-owned affiliate of Staidson, assigned the Co-Development Agreement to Staidson together with all rights and obligations thereunder.
-79-
In December 2022, we amended our existing co-development agreement with Staidson to support Staidson in its regulatory approval efforts for its proprietary drug candidate BDB-001 for the treatment of COVID-19 in China. Pursuant to the amendment, we will receive increased royalties of 10% on net sales of BDB-001 for the treatment of COVID-19 in China. We granted Staidson an exclusive license for use in China to certain of our clinical, manufacturing and regulatory data regarding vilobelimab in order to support and facilitate the regulatory filing for BDB-001 for the treatment of severely ill COVID-19 patients with the Chinese National Medical Products Administration, or NMPA. Under the existing Co-Development Agreement, BDB-001 is being developed by Staidson for the treatment of severe COVID-19 and other inflammatory diseases in China. The agreement continues to be in force unless earlier terminated. The agreement may be terminated upon the mutual agreement of the parties, or by one party upon a breach by the other party that is not cured within 30 days after receiving notice of such breach. In addition, either party may terminate the agreement if the other party challenges the terminating party’s ownership of any intellectual property licensed to the non-terminating party under the agreement or undergoes certain bankruptcy or insolvency events.
Concomitantly to amending the Co-Development Agreement, we also entered into a share purchase agreement with Staidson Hong Kong Investment Company Limited, an affiliate of Staidson and a limited liability company organized under the law of Hong Kong, pursuant to which Staidson Hong Kong Investment Company Limited purchased ordinary shares from us for an aggregate amount of $2.5 million (€2.3 million) at a price of $5.00 per share, resulting in the sale of 500,000 shares. The share purchase agreement also includes an option pursuant to which Staidson Hong Kong Investment Company Limited may purchase additional ordinary shares, at our discretion, for an aggregate amount of an additional $7.5million. The option for such subsequent purchase will expire on the twelve-month anniversary of Staidson receiving regulatory approval for BDB-001 in China. Such subsequent investment would be made at the greater of $5.00 per share or at a 20% premium to the weighted average share price over the 15 trading days prior to the closing date of such subsequent investment.
Clinical trial collaboration and supply agreement with Merck & Co., Inc.
On March 20, 2020, we entered into a clinical trial collaboration and supply agreement with Merck & Co., Inc. (known as MSD outside the United States and Canada) to evaluate the combination of vilobelimab and Merck’s anti-PD-1 therapy, KEYTRUDA®1 (pembrolizumab) in patients with cSCC. Under the terms of the agreement, we conduct a Phase II clinical study with two vilobelimab arms, including one with pembrolizumab. In November 2023, we announced the development stop of vilobelimab in cSCC to prioritize other programs. Patients who are currently still in treatment will be treated for up to 24 months according to the protocol; however, no new patients will be enrolled in the study and clinical sites in which no patients are currently being treated will be closed down.
Sales and marketing
In June 2023, we began the commercialization of GOHIBIC (vilobelimab) in the United States for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. In connection with the start of the commercialization, we entered into agreements with certain subsidiaries of Cencora to act as the Group’s U.S. distributor and make GOHIBIC(vilobelimab) available for order by U.S. hospital customers under the EUA. Cencora provides cold storage, cold-chain distribution services, inventory management and secondary labeling/packaging, among other services.
To support our commercial efforts, we have hired and are continuing to hire U.S. experts with relevant experience in the commercialization of medical products in the hospital market, including in the areas of sales, sales operations, marketing, market access, distribution, medical affairs and others. In addition, we are expanding the necessary infrastructure, including IT systems, supply chain, financial reporting systems and inventory management systems both, internally and with the assistance of external service providers.
As we expand our commercial efforts, we continue to enhance our commercial strategic plan, further expanding our sales force and medical affairs teams, preparing relevant promotional and medical education materials to target healthcare providers and other stakeholders, refining our medical affairs strategy to increase awareness of the EUA among the medical community, and furthering our sales efforts.
| 1 | KEYTRUDA® is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. |
-80-
In 2023, we recognized revenues in the amount of €63,089 from product sales for the first time since our inception. Revenues reported are sales to end customers (hospitals) in the United States.
During the twelve months ended December 31, 2023, the Group incurred €4.0 million of sales and marketing expenses. These expenses are mainly composed of €1.0 million in personnel costs and €1.9 million in external services for distribution of Gohibic. The Group started with its commercialization activities when the EUA was granted in April 2023. Prior to that, no sales and marketing expenses had been incurred.
We also intend to independently pursue the commercialization of vilobelimab for PG in the United States and Europe, if and when approved by the applicable regulators, by employing a targeted commercial infrastructure to promote access to vilobelimab through centers-of-excellence that treat PG in these core markets. We believe that such an organization will be able to address the community of physicians who are key specialists in treating the patient populations for which vilobelimab and any other product candidates are being developed. The responsibilities of the organization would include developing educational initiatives with respect to approved products and establishing relationships with key specialists in PG and any other relevant fields of medicine. The option to collaborate with a larger organization with an established commercial infrastructure will also be evaluated.
We might also consider pursuing the commercialization of vilobelimab in other indications or commercialization of our other development products independently. However, we are also considering potential partnerships with larger companies that have a more established infrastructure and greater financial resources in different areas, including in sales and marketing.
Manufacturing
We do not currently own or operate manufacturing facilities for the production of clinical or commercial quantities of our product candidates. We intend to rely on existing third-party contract manufacturers to produce our products and intend to recruit additional personnel with experience to manage the third-party contract manufacturers producing our product candidates and other product candidates or products that we may develop in the future. In addition, we engaged additional third-party manufacturers in Germany, the United States and other countries for activities related to potential sales, e.g. packaging and labeling of any of our approved products in the United States and elsewhere. We hold a manufacturing and importing license and participate in the drug product release procedure for vilobelimab by running a key immunological release assay in-house, allowing us to release only drug product batches that demonstrate the necessary, pre-specified high biological blocking activity. Thus, we are responsible for overseeing the entire manufacturing process and we release final fill-finished drug product with our qualified person.
Competition
The biopharmaceutical industry is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technologies, knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
Competition in Pyoderma Gangrenosum
There are currently no drugs approved for the treatment of PG in major markets. The only locally approved treatment is adalimumab, which has been approved in Japan but in no other country on the basis of a small, locally conducted clinical trial. However, due to the high medical need associated with the disease, certain drugs are used in regular medical practice as treatment attempts for affected patients. These include certain orally administered drugs such as immunosuppressants, including cyclosporine or corticosteroids or antibiotics such as dapsone. In addition, topically applied tacrolimus is used in certain cases. Lastly intravenously administered TNF-alpha inhibitors such as infliximab or adalimumab or other biological drugs are also used, despite the fact that no formal regulatory approvals are in place.
-81-
As of the date hereof, to our knowledge, other treatments in active clinical trials include:
| ● | Orally administered baricitinib, a janus kinase-1 and janus kinase-2, or JAK1/JAK2, inhibitor is currently being investigated in a proof-of-concept, open label Phase II study |
| ● | Intravenously injected spesolimab, an interleukin-36 receptor, or IL-36R, monoclonal antibody is currently being investigated in a Phase II open label single-arm study |
| ● | Orally administered deucravacitinib, a janus kinase-2, or JAK2, inhibitor, is currently being investigated in a Phase I single arm with 10 patients |
| ● | Subcutaneously administered canakinumab, an anti-interleukin-1 beta, or IL-1 beta, monoclonal antibody in a 24 patients clinical Phase II trial of a subtype of PG, a pyogenic sterile arthritis pyoderma gangrenosum and acne (PAPA) syndrome |
Furthermore, the following developments have been terminated, completed or abandoned and have not advanced to registrational Phase III trials in recent years or failed in previous clinical trials:
| ● | Subcutaneously administered gevokizumab, an anti-interleukin-1 beta, or IL-1 beta, monoclonal antibody in three clinical Phase III trials enrolling 16, 15 and nine patients and in a Phase II study enrolling eight patients |
| ● | Intravenously administered bermekimab, an anti-interleukin-1 alpha, or IL-1 alpha, monoclonal antibody in a clinical Phase II study enrolling 10 patients |
| ● | Subcutaneously administered ixekizumab, an anti-interleukin-17 alpha, or IL-17 alpha, monoclonal antibody in a clinical Phase II trial after enrolling four patients |
| ● | Orally administered etrasimod, (APD334), a selective sphingosine-1-phosphate, or S1P-1, receptor modulator in a clinical Phase II trial enrolling two patients |
| ● | Subcutaneously administered ustekinumab, a monoclonal antibody targeting the shared p40 subunit of interleukin-12, or IL-12 and interleukin-23, or IL-23, described as being successfully treated in a patient on chronic immunosuppressive therapy in a single patient case-report study |
| ● | Subcutaneously administered secukinumab, an anti-interleukin-17 alpha, or IL-17 alpha, monoclonal antibody, described as being successfully treated after failure of other systemic therapies in a single patient case-report study |
If approved for the treatment of PG, vilobelimab would potentially face competition from currently used therapies, such as glucocorticoids, cyclosporin or other immunosuppressive therapies like adalimumab, infliximab or others.
Competition in the treatment of critically ill, invasively mechanically ventilated COVID-19 patients
If approved for treatment of critically ill, invasively mechanically ventilated COVID-19 patients, vilobelimab would face competition from currently used or approved therapeutics such as corticosteroids, the interleukin-1, or IL-1, inhibitor anakinra, IL-6 inhibitors such as tocilizumab, JAK-inhibitors such as baricitinib and anti-thrombotic therapy. Given the high medical need for effective treatments as a consequence of the COVID-19 pandemic, many different therapeutic entities and targets have or are still being assessed for the treatment of this patient population. While the performance of clinical trials in a particular patient population is the prerequisite to be able to gain regulatory approval for the treatment of that particular patient population, several clinical trials have been conducted in other patient populations (e.g., hospitalized patients as opposed to our targeted sub-group of critically ill, invasively mechanically ventilated patients) and results of these trials have partly been extrapolated into our targeted population. None of these treatments have received EUA, and we are of the opinion that in order to gain full BLA approval, these treatments will need to show clinical efficacy in randomized controlled clinical trials. Therefore, we believe that competition will mainly be faced by products developed for the intended use population.
-82-
Treatments currently or previously under investigation or completed for severe COVID-19, including for the treatment of critically ill, mechanically ventilated patients, include:
| ● | Orally administered sabizabulin, a microtubule disruptor, completed a Phase III study in 204 hospitalized COVID-19 patients at high risk of developing ARDS or death, demonstrating a 55% reduction in mortality at day 60. On November 9, 2022 the FDA Pulmonary-Allergy Drugs Advisory Committee voted 8-5 that the known and potential benefits of sabizabulin do not outweigh the known and potential risks of sabizabulin. On March 2, 2023 the sponsor of the trial, Veru Pharmaceuticals Inc. announced that the FDA declined to grant EUA for sabizabulin. |
| ● | Intravenously administered nangibotide, a synthetic peptide and first-in-class triggering receptor in myeloid cell-1, or TREM-1, inhibitor in a randomized controlled Phase II trial in COVID-19 ICU patients. Results from this study showed a 43% relative reduction in 28-day all-cause mortality in the analyzed patient population. |
| ● | Intravenously administered eculizumab, a monoclonal antibody inhibitor of C5, has been in an open label Phase II trial in patients with COVID-19 infection receiving Continuous-Positive-Airway-Pressure, or CPAP, ventilator support. Only 10 patients were treated with eculizumab in this non-randomized study and compared to 52 patients. Eculizumab was safe and well tolerated but did not show a major effect on reduction of mortality in this relatively small group of patients. No additional studies with eculizumab in this patient population have been reported. |
| ● | Intravenously administered ravulizumab, a monoclonal antibody inhibitor of C5, in a Phase II study in patients with COVID-19 severe pneumonia, acute lung injury, or ARDS. This study has been stopped after an interim analysis and no results are available. |
| ● | Intravenously administered avdoralimab (IPH5401), an anti-C5aR antibody, in an investigator initiated, double-blind, randomized Phase II study versus placebo in patients with COVID-19 severe pneumonia. 208 patients were enrolled, and the program was stopped in July 2021 after the trial did not reach the primary endpoints. |
| ● | Intravenously administered asunercept, a CD95-Fc fusion protein, specifically binding to and efficiently blocking CD95L, in a double-blind, randomized Phase II study in 438 patients suffering from severe COVID-19, which has been completed in October 2021 showing efficacy on certain outcome measures. While no full dataset has been published, asunercept tried further developed in a Phase III study with 636 patients. On August 18, 2023 the study has been terminated due to the lack of patients with only 34 patients recruited. |
| ● | Intravenously administered AMY-101, a cyclic peptide targeting complement factor C3, in a Phase II clinical trial to assess safety and efficacy in patients with ARDS, due to COVID-19 infection did not meet its primary efficacy endpoint. |
| ● | Intravenously administered APL-9, a protein specifically targeting C3, a central protein in the complement system of the immune system, in a Phase I/II study for the treatment of severe COVID-19 patients. After an interim analysis by an independent data monitoring committee, no meaningful reduction in the overall mortality rate in patients treated with APL-9 could be observed and thus the study was terminated early. |
Competition in cSCC
If approved in programmed death-1, or PD-1 and programmed death-ligand-1, or PD-L1 inhibitor, resistant / refractory, locally advanced or metastatic cSCC, vilobelimab would face competition from currently used therapeutics such as epidermal growth factor receptor, or EGFR, inhibitors such as cetuximab, chemotherapeutic agents such as cisplatin, doxorubicin, taxane, gemcitabine, methotrexate and 5-fluorouracil, or 5-FU, as well as topically applied products such as imiquimod or tirbanibulin, even if some of these treatment are not all approved for use in this indication.
-83-
In addition, two PD-1 inhibitors are FDA approved to treat locally advanced or metastatic cSCC. Pembrolizumab, a monoclonal antibody targeting PD-1 is indicated for recurrent or metastatic cSCC that is not curable by surgery or radiation as well as cemiplimab, a monoclonal antibody targeting PD-1, which is indicated for metastatic cSCC or locally advanced cSCC for those patients that are not candidates for curative surgery or radiation.
Other treatments currently under investigation include:
| ● | Intravenously administered cosibelimab, a monoclonal antibody targeting PD-L1, in a completed registration-enabling trial, showing an overall response rate, or ORR, of 54.8%, submitted for BLA to the FDA in January 2023, accepted by FDA in March 2023. In December 2023 FDA issued a Complete Response Letter due to the third party CMO finding. Despite of that the drug is expected to be approved in 2024 |
| ● | Intravenously administered avelumab, a monoclonal antibody targeting PD-L1, in combination with radical radiation therapy, in a Phase II study for the treatment of unresectable cSCC |
| ● | Intravenously administered cetuximab, a monoclonal antibody targeting EGFR, in combination with avelumab, a monoclonal antibody targeting PD-L1, in a Phase II randomized trial in advanced cSCC |
| ● | Intravenously administered nivolumab, a monoclonal antibody targeting PD-1 in two Phase II trials, as monotherapy and in combination with ipilimumab, a monoclonal antibody targeting cytotoxic T-lymphocyte associated protein 4, or CTLA-4, in a Phase II study |
| ● | Orally administered cobimetinib, a small molecule inhibitor of the mitogen-activated protein kinase, or MEK, in combination with atezulizumab, a monoclonal antibody targeting PD-L1, in a Phase II study in advanced rare tumors, including metastatic cSCC |
| ● | Intravenously administered oncolytic vaccine using Maraba virus vector expressing melanoma antigen A3, or MAGE-A3, or MG1MA3, as monotherapy and as booster after intramuscular priming with adenovirus vaccine with transgenic MAGE-A3 insertion, or AdMA3, in a Phase I/II trial in patients with incurable advanced/metastatic MAGE-A3-expressing solid tumors, including cSCC |
| ● | Intratumorally injected oncolytic viral vector talimogene laherparepvec in combination with panitumumab a monoclonal antibody targeting EGFR (and also in combination with other antibodies targeting PD-1 or PD-L1) in several Phase I and Phase II studies for the treatment of refractory and/or advanced cSCC |
| ● | Intravenously administered nanrilkefusp alfa, an IL-15 superagonist as monotherapy or in combination with pembrolizumab in a Phase I multi-center open-label Phase I/Ib study to evaluate the safety and preliminary efficacy of SO-C101 in relapsed/refractory, advanced/metastatic cSCC |
| ● | Intravenously infused ASP-1929 photoimmune therapy either as monotherapy or in combination with pembrolizumab in two Phase II studies for the treatment of primary or recurrent locoregional cSCC. The trial has been discontinued due to the lack of efficacy in October 2023 |
| ● | Intratumorally injected exosomes (CDK-002 or exoSTING) in an exploratory Phase I/II study, including several solid tumors (such as cSCC) |
Competition in HS
Until 2023 the only approved and marketed systemically administered product to treat moderate to severe HS patients in the United States and Europe was adalimumab, an inhibitor of tumor necrosis factor-alpha, or TNF-alpha. In 2023, the Phase 3 results were published for two additional drugs, with the following outcomes::
| ● | Subcutaneously administered secukinumab, an IL-17 alpha monoclonal antibody has been submitted and approved |
| ● | Subcutaneously administered bimekizumab, a monoclonal antibody blocking IL-17A/F, has completed Phase III studies and is currently in the process of registration |
-84-
If we would develop and receive approval for vilobelimab in HS, we would face competition from currently approved therapeutics such as adalimumab, secukinumab and bimekizumab (if approved), from topical therapies, including clindamycin, resorcinol and others, from intralesionally applied corticosteroids, from orally administered antibiotics such as tetracycline, clindamycin, rifampicin, metronidazole, cephalosporin, dapsone and others. In addition, a range of surgical procedures, laser and radiotherapy procedures are being investigated and used for the treatment of HS. Finally, we could face competition from additional product candidates currently under development that might receive approvals for HS before us.
Several additional systemically administered product candidates have previously or are currently being investigated and developed to treat HS with varying mechanisms of action:
| ● | Subcutaneously administered SAR442970, an anti-TNF-OX40L nanobody, is being investigated in a Phase 2 clinical trial in 84 moderate to severe HS patients |
| ● | Orally administered SAR444656, a low molecular weight IRAK4 degrader, is being investigated in a Phase 2 clinical trial in 99 moderate to severe HS patients |
| ● | Subcutaneously administered amlitelimab (SAR445229), a fully human OX40L binding antibody, is being investigated in a Phase 2 clinical trial in 84 moderate to severe HS patients |
| ● | Orally administered tofacitinib, an IFN signaling blocker, is being investigated in Phase II study in 47 patients with Down Syndrome to evaluate the efficacy of the drug in treatment of various skin diseases, including HS |
| ● | Orally administered povorcitinib (INCB 54707), a low molecular weight JAK-1 inhibitor, is being investigated in two Phase III clinical studies in 1,560 moderate to severe HS patients |
| ● | Orally administered avacopan, a low molecular weight C5aR inhibitor, completed a Phase II study in 435 moderate to severe HS patients in 2021 |
| ● | Subcutaneously administered bermekimab, a monoclonal antibody targeting IL1-alpha, completed three Phase II clinical studies in a total of 337 patients with moderate to severe HS |
| ● | Subcutaneously administered izokibep, a selective inhibitor of IL-17A, is currently being investigated in a Phase IIb study with 180 patients and in the Phase III with 250 patients |
| ● | Subcutaneously administered sonelokimab (ALX 0761, M1095), a trivalent nanobody comprised of monovalent camelid-derived nanobodies specific to human interleukin IL-17A, IL-17F and human serum albumin VHHs, completed a 234 patient Phase II clinical trial in patients with active, moderate to severe HS |
| ● | Subcutaneously administered lutikizumab, a monoclonal antibody targeting IL-1alpha/beta is currently being investigated in 200 patient Phase II study in moderate to severe HS patients who failed Anti-TNF Therapy as well as patients naïve to biologic therapy |
| ● | Subcutaneously administered iscalimab (CFZ-533), a nondepleting anti-CD40 antibody, is being tested in a 200 patient Phase II exploratory study in patients with moderate to severe HS in parallel with other experimental therapies, including LYS006, MAS825, remibrutinib (LOU064) and ianalumab (VAY736) |
| ● | Orally administered LYS006, a selective inhibitor of leukotriene A4 hydrolase, or LTA4H, is being tested in a 200 patient Phase II exploratory study in patients with moderate to severe HS in parallel with other experimental therapies, including iscalimab (CFZ-533), MAS825, remibrutinib (LOU064) and ianalumab (VAY736) |
| ● | Subcutaneously administered MAS825, a T-cell immunoglobulin and mucin domain 3, or TIM-3, inhibitor is being tested in a 200 patient Phase II exploratory study in patients with moderate to severe HS in parallel with other experimental therapies, including iscalimab (CFZ-533), LYS006, remibrutinib (LOU064) and ianalumab (VAY736) |
-85-
| ● | Orally administered remibrutinib (LOU064), a low molecular weight Burton tyrosine kinase, or BTK, inhibitor, is being tested in a 200 patient Phase II exploratory study in patients with moderate to severe HS in parallel with other experimental therapies, including iscalimab (CFZ-533), LYS066, MAS825 and ianalumab (VAY736) |
| ● | Subcunaeously administered ianalumab (VAY736), a fully human anti-BAFF-R monoclonal antibody, is being tested in a 200 patient Phase II exploratory study in patients with moderate to severe HS in parallel with other experimental therapies, including iscalimab (CFZ-533), LYS 066, MAS825 and remibrutinib (LOU064) |
| ● | Subcutaneously administered eltrekibart (LY3041658), a monoclonal antibody targeting and neutralizing several human chemokines of the CXC family containing the ELR peptidic motiv, is currently being evaluated in a clinical Phase II trial with 350 patients suffering from moderate to severe HS |
| ● | Orally administered upadacitinib, a Janus kinase inhibitor, is completed a Phase II clinical study with 68 patients study to investigate the treatment effect in moderate to severe HS |
| ● | Intravenously administered spesolimab, an interleukin-36, or IL-36, receptor (IL1RL2/IL1RAP) targeted antibody, is currently being developed for patients with HS and is in Phase II clinical testing in 52 patients |
| ● | Subcutaneously and intravenously administered imsidolimab (ANB019), an antibody that inhibits the function of the interleukin-36-receptor, or IL-36R, completed a Phase II clinical trial in 149 patients in order to explore the immune response to imsidolimab in subjects with HS |
| ● | Topically administered ruxolitinib, a low molecular weights JAK1 and JAK2 inhibitor, formulated as 1.5% cream is currently being investigated in Phase II with 93 patients with HS |
| ● | Orally administered PTM-001, an experimental drug development candidate with undisclosed mode of action is currently in a Phase II clinical study in 50 patients with moderate to severe HS |
| ● | Three different orally administered novel kinase inhibitors, were tested in a 194 patient Phase II exploratory study in patients with moderate to severe HS in parallel. Reported results in 2022 indicate that differences between placebo and tested dose regimens of experimental therapies PF 06826647 and PF 06650833 were not statistically significant, while PF 06700841 showed superiority to placebo |
| ● | Orally administered orismilast, a phosphodiesterase-IV, or PDE-IV, inhibitor is currently being investigated in a clinical trial with 24 patients to assess the efficacy and safety of oral administration of orismilast for treatment of mild, moderate, or severe HS in adults |
| ● | intravenously administered brodalumab, a monoclonal antibody targeting the IL-17 receptor, or IL17R, completed a Phase II clinical study for the treatment of moderate HS |
We consider the following product candidates that were under clinical investigation not being a competitive threat for the time being or at all:
| ● | Orally administered RIST4721, an IL-8B receptor antagonist, has been tested in a Phase II clinical study in 33 patients with HS, the study has been terminated due to safety findings |
| ● | Subcutaneously administered risankizumab, a monoclonal antibody targeting interleukin-23A, or IL-23A, was investigated in a clinical Phase II trial in 243 patients (which was completed in 2021) -differences between placebo and tested dose regimens of risankizumabwere not statistically significant |
| ● | Intravenously and subcutaneously administered guselkumab, a monoclonal antibody targeting IL-23, completed a Phase II clinical trial in 184 moderate to severe HS patients (which was completed in 2020) – differences between placebo and tested dose regimens of guselkumab were not statistically significant |
-86-
| ● | Intravenously administered anakinra, an IL-1 receptor antagonist, has been investigated in three Phase II trials in TNF-alpha treatment refractory patients (which was completed in 2017) |
| ● | Orally administered apremilast, a phosphodiesterase-IV, or PDE-IV, has been investigated in a Phase II trial in 20 moderate to severe HS patients (which was completed in 2017) |
| ● | Subcutaneously administered CJM112, a monoclonal antibody targeting IL-17A and IL-17A/F has completed a Phase II study in 66 patients suffering from moderate to severe, chronic HS (which was completed in 2016 and did not reach the primary outcome) |
| ● | Subcutaneously administered MEDI8968, an investigational monoclonal antibody drug candidate selective against IL-1R, has been investigated in a Phase II clinical trial in 221 moderate to severe HS patients (which was competed in 2014 and did not meet its primary outcome) |
| ● | Orally administered zunsemetinib (ATI-450), a mitogen-activated protein, or MAP, kinase-activated protein kinase 2, or MAPKAPK2, or MK2, inhibitor, is currently investigated in a Phase Iia clinical study with 95 patients study to investigate the treatment effect in moderate to severe HS. The study did not meet its primary endpoint |
| ● | Subcutaneously administered ustekinumab, a monoclonal antibody targeting the shared p40 subunit of IL-12 and IL-23 completed a Phase II clinical trial in 20 moderate to severe HS patients (which was completed in 2014) |
Competition in ANCA associated vasculitis
If approved for the treatment of AAV, vilobelimab would potentially face competition from currently used therapies, including the low molecular weight C5aR-inhibitor avacopan (FDA approved for this indication in October 2021), corticosteroids, azathioprine, methotrexate, cyclosporin, mycophenolate mofetil and rituximab. The current standard of care to induce remission in acutely ill AAV patients is done through a combination of either rituximab or azathioprine with HDCS. Rituximab is approved and marketed for this indication and label extension studies are ongoing. Therapies to maintain remission include low dose corticosteroids, methotrexate, mycophenolate mofetil and rituximab. Mepolizumab, a monoclonal antibody targeting interleuline-5, or IL-5, is also FDA approved to treat a type of AAV in adults called EGPA.
| ● | Intravenously administered KYV-01, a fully human anti-CD19 CAR T-cell therapy, is investigated in a Phase I study in 24 patients with a number of autoimmune diseases, including AAV |
| ● | Intravenously administered Obinutuzumab, an anti-CD20 monoclonal antibody, is investigated in a 30 patients Phase II study of PR3-Patients with AAV |
| ● | Intravenously administered MT 2990, a monoclonal antibody targeting interleukin-33, in a 10 patient Phase I study of patients with AAV |
| ● | Intravenously administered benralizumab a monoclonal antibody targeting interleukin-5, or IL-5 receptor or mepolizumab a monoclonal antibody targeting IL-5, in a 140 patient Phase III clinical study within a type of AAV, EGPA |
| ● | Intravenously administered abatacept, a monoclonal antibody targeting CTLA-4, in a Phase III clinical study in 66 patients with relapsing, non-severe, EGPA |
| ● | Intravenously administered depemokimab in a Phase III clinical trial with 160 patients with relapsing or refractory EGPA |
| ● | Orally administered NS-229, a JAK-1 inhibitor, is in a Phase II clinical trial with 45 patients with EGPA |
-87-
Competition to INF904 by oral C5aR inhibitors
We completed a single and multiple ascending dose Phase I program with our oral C5aR inhibitor, INF904 in the second half in January 2024. This program may prove the assumed favorable PK and safety profile and ease of administration required for effective long-term treatment for chronic inflammatory diseases. The development of low molecular weight drug candidates through the different stages of clinical and nonclinical development is a time-consuming and cost-intensive process. As of the date hereof, and to our knowledge, avacopan is the only currently approved oral C5aR inhibitor for the treatment of AAV. If we ever and until we reach the market approval stage, we might have encountered a variety of competing products and might potentially not be second to avacopan on the market. Even if marketed, INF904 might face future competition from other oral small molecules.
The most advanced orally available C5aR inhibitor in active development to our knowledge is ACT-1014-6470. In a completed Phase I clinical trial, the company developing the product reported positive data on safety in both, healthy subjects and patients with renal impairment. However, neither the targeted indication for Phase II development of ACT-1014-6470 nor the status of development has yet been disclosed publicly.
Furthermore, there are and have been several product candidates in pre-clinical and early clinical development. These include low molecular weight compounds, cyclic peptides and other classes of drug candidates. To our knowledge, none of these drug candidates, with exception of avacopan, was ever successfully tested in Phase III registration trials or has been under review at any regulatory agency. At least 10-15 different C5aR-inhibitors have been mentioned to be in different stages of pre-clinical and early clinical development, but there have been no updates on their respective development progress in recent years, therefore we assume most of these development programs were meanwhile paused or terminated.
Competition from therapeutics agents in the field of terminal complement inhibition
There are several clinical or commercial stage companies focusing on the inhibition of C5aR with biological molecules, including monoclonal antibodies.
The C5a/C5aR1 signaling pathway plays an essential role in various inflammatory diseases. It has been discovered that C5a can be generated not only by conventional complement activation pathways (classical, lectin, alternative) through C5a convertases, but also by a direct enzymatic cleavage (enzymatic pathway) by various enzymes (e.g., thrombin and plasmin). It has been reported that C5a generation via the enzymatic pathway is not affected by the upstream complement blockers, including C5 blockers like eculizumab. As such, controlling and fully blocking C5a induced signaling in humans therefore warrants a targeted approach by directly blocking either C5a or C5aR1.
There are currently several C5a and C5aR inhibitors in different stages of active clinical development:
| ● | Avdoralimab (IPH5401), an anti-C5aR1 antibody is being investigated in different inflammatory diseases. A Phase II clinical trial evaluating the safety and efficacy of avdoralimab in COVID-19 patients with severe pneumonia, did not meet its primary endpoints in all three cohorts of the trial. The compound is not in active development as of today. A Phase II study in patients with bullous pemphigoid, or BP, is ongoing, |
| ● | STSA-1002, an anti-C5a humanized antibody is currently in Phase Ib/II clinical trials in patients with ARDS. | |
| ● | AON-D21, an anti-C5a L-aptamer is currently in Phase IIa clinical development for critically ill, intubated patients with pneumonia. In a single-ascending dose study in healthy volunteers, AON-D21 was shown to be safe and well tolerated. A multiple-ascending dose study was completed in mid 2022. |
| ● | MOR210, an anti-C5aR antibody, is a novel human antibody directed against C5aR1. MOR210 was investigated as a treatment for relapsed or refractory advanced solid tumors in a Phase I trial. |
| ● | VIS954, an anti-C5aR antibody is investigated in the Phase I SAD study in healthy adult participants. |
-88-
More broadly, in the terminal complement space, there are currently several approved drugs, including eculizumab and ravulizumab for the treatment of Paroxysmal nocturnal hemoglobinuria, or PNH, and atypical hemolytic uremic syndrome, or aHUS, zilicoplan for the treatment of generalized myasthenia gravis, or gMG, Avacincaptad pego for the treatment of geographical athropy, or GA. In addition, there are several development programs to develop C5 inhibitors for other indications, including by Hoffmanm-La Roche AG, Akari Therapeutics Plc., Alnylam Pharmaceuticals, Inc., Regeneron Pharmaceuticals, Inc. and Novartis AG.
Beyond C5 and C5a, there are clinical stage companies targeting complement inhibition upstream from C5, such as C3, factor D and components of the lectin pathway. These approaches will likely also result in a lowering of C5a generation in blood. Companies in this area include Apellis Pharmaceuticals, Inc., UCB S.A., AstraZeneca plc and Omeros Corporation, among others. Furthermore, there are numerous additional companies developing pre-clinical drug candidates that target terminal complement factors and their receptors.
Summary
The key competitive factors affecting the success of our product candidates, if approved or authorized, are likely to be their efficacy, safety, dosing convenience, price and degree of market acceptance, as well as our or our partners marketing capabilities, the level of competition and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, even if our product candidates are approved for marketing and sale, they may fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community, including if physicians are reluctant to switch their patients from existing therapies (such as adalimumab for the treatment of HS). See “ITEM 3. KEY INFORMATION — 3. Risk factors — Risks related to the discovery, development and commercialization of our product candidates—Even if one of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success, in which case we may not generate significant revenues or become profitable.”
Government regulation and product approval
Government authorities in all major pharmaceutical markets extensively regulate, among other things, the research, development, testing, manufacture, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing and import and export of pharmaceutical products such as those we are developing. Although our initial focus will be on the United States and Europe, we intend to develop and seek marketing approval for our products also in other countries and territories, such as Canada or Japan, and for markets that follow the leading authorities, such as Brazil or South Korea. The processes for obtaining regulatory approvals in the United States, Europe and other countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
FDA approval process
All of our current product candidates are subject to regulation in the United States by the FDA either as biological products, or biologics, or as new chemical entities, or NCEs. The FDA subjects biologics and NCEs to extensive pre- and post-market regulation. The Public Health Service Act, or PHSA, the Federal Food, Drug, and Cosmetic Act and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of biologics and NCEs. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending BLAs or NDAs, withdrawal of approvals, clinical holds, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines or civil or criminal penalties.
The PHSA emphasizes the importance of manufacturing control for products whose attributes cannot be precisely defined. The PHSA also provides authority to the FDA to immediately suspend licenses in situations where there exists a danger to public health, to prepare or procure products in the event of shortages and critical public health needs, and to authorize the creation and enforcement of regulations to prevent the introduction or spread of communicable diseases in the United States and between states.
-89-
BLA and NDA application and approval
The process required by the FDA before a new biologic or NCE may be marketed in the United States is long, expensive, and inherently uncertain. Biologics and NCE development in the United States typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND (which must become effective before clinical testing may commence) and adequate and well-controlled clinical trials to establish the safety, purity and potency (safety and effectiveness) of the biologic or NCE for each indication for which FDA approval is sought. Developing the data to satisfy FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product or disease.
Preclinical studies include laboratory evaluation of the purity and stability of the manufactured drug substance or active pharmaceutical ingredient and the formulated drug or drug product, as well as in vitro and animal studies to assess the safety and activity of the drug candidate for initial testing in humans and to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations. The results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical trials, among other things, are submitted to the FDA as part of an IND. Some long-term preclinical testing, such as animal tests of reproductive adverse events and carcinogenicity, may continue after the IND is submitted.
An IND must become effective before United States clinical trials may begin. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug or biologic to healthy volunteers or patients with the condition under investigation, all under the supervision of a qualified investigator. Clinical trials must be conducted (i) in compliance with federal regulations, (ii) in compliance with good clinical practice, or GCP, which is an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors, and (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with requirements or presents an unacceptable risk to the clinical trial subjects. The study protocol and informed consent information for subjects in clinical trials must also be submitted to an institutional review board (IRB) for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions. The study sponsor may also suspend a clinical trial at any time on various grounds, including a determination that the subjects or patients are being exposed to an unacceptable health risk.
Clinical trials to support BLAs or NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap or be combined. In Phase I, the drug candidate is initially introduced into healthy human subjects or patients and is tested to assess its pharmacokinetic, or PK, properties, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, such as cancer treatments, initial human testing has to be conducted in the intended patient population. Phase II usually involves trials in a limited and well-specified patient population to determine the effectiveness of the biologic for a particular indication, dosage tolerance and optimum dosage, and to identify common AEs and potential safety risks. If a drug candidate demonstrates evidence of effectiveness and an acceptable safety profile in Phase II evaluations, Phase III trials are undertaken to obtain additional information about clinical efficacy and safety in a larger number of patients, representing the future intended use population, typically at geographically dispersed clinical trial sites. These Phase III clinical trials are intended to establish data sufficient to demonstrate substantial evidence of the efficacy and safety of the product to permit the FDA to evaluate the overall benefit-risk relationship of the biologic or NCE and to provide adequate information for the labeling of the drug. Trials conducted outside of the United States under similar, GCP-compliant conditions in accordance with local applicable laws may also be acceptable to the FDA in support of product licensing.
-90-
Sponsors of clinical trials for investigational drugs must publicly disclose certain clinical trial information, including detailed trial design and trial results in public government databases. These requirements are subject to specific timelines and apply to most controlled clinical trials of FDA-regulated products.
After completion of the required clinical testing, a BLA (for a biologic) or a NDA (for a NCE) is prepared and submitted to the FDA. FDA review and approval of the BLA or NDA is required before marketing of the product may begin in the United States. The BLA or NDA must include the results of all preclinical, clinical, and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls, and must demonstrate the safety and efficacy of the product based on these results. The BLA or NDA must also contain extensive manufacturing information. The cost of preparing and submitting a BLA or NDA is substantial. Under federal law, the submission of most BLAs or NDAs is additionally subject to a substantial application user fee, as well as an annual program user fee, which may total several million dollars and are typically increased annually.
The FDA has 60 days from its receipt of a BLA or NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of BLAs and NDAs. Most such applications for standard review drug candidates are reviewed within 10 months from the date the application is accepted for filing. Although the FDA often meets its user fee performance goals, it can extend these timelines if necessary, and its review may not occur on a timely basis. The FDA usually refers applications for novel drugs, or drugs which present difficult questions of safety or efficacy, to an advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation, and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it frequently follows such recommendations. Before approving a BLA or NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless it verifies that compliance with cGMP standards is satisfactory and the BLA or NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the BLA or NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the BLA or NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. The FDA approval is never guaranteed, and the FDA may refuse to approve a BLA or NDA if applicable regulatory criteria are not satisfied.
Under the PHSA, the FDA may approve a BLA or NDA if it determines that the product is safe, pure and potent and the facility where the product will be manufactured meets standards designed to ensure that it continues to be safe, pure, and potent. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. The approval for a drug may be significantly more limited than requested in the application, including limitations on the specific diseases and dosages or the indications for use, which could restrict the commercial value of the product. The FDA may also require that certain contraindications, warnings, or precautions be included in the product labeling. In addition, as a condition of BLA or NDA approval, the FDA may require a risk evaluation and mitigation strategy, or REMS, to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS or use of a companion diagnostic with a drug can materially affect the potential market and profitability of the drug. Moreover, product approval may require, as a condition of approval, substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
-91-
After a BLA or NDA is approved, the product may also be subject to official lot release. As part of the manufacturing process, the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official lot release by the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot. The FDA may also perform certain confirmatory tests on lots of some products, such as viral vaccines, before releasing the lots for distribution by the manufacturer. In addition, the FDA conducts laboratory research related to the regulatory standards on the safety, purity, potency, and effectiveness of products. After approval of drugs, manufacturers must address any safety issues that arise, are subject to recalls or a halt in manufacturing, and are subject to periodic inspection.
Emergency use authorization
The FDA can facilitate the availability and use of medical countermeasures needed during public health emergencies via EUA. When The United States Secretary of Health and Human Services, or HHS Secretary, declares that an EUA is appropriate, FDA may authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat or prevent serious or life-threatening diseases or conditions caused by chemical, biological, radiological or nuclear threat agents. For example, in January 2020, the HHS Secretary determined that a public health emergency, or PHE, existed (and has subsequently extended the declaration on numerous occasions,) that had a significant potential to affect national security or the health and security of U.S. citizens due to the emergence and spread of COVID-19. Based on this determination, the HHS Secretary also declared that circumstances existed justifying EUA of certain medical products. The EUA declaration is distinct from the PHE declaration. As long as the applicable EUA declaration and determination remains in effect, an EUA may remain in effect beyond the duration of the PHE declaration if all other statutory conditions are met. Even if the EUA declaration remains in effect, the FDA may revoke the EUA at any time if certain circumstances or criteria are met. We have been granted the EUA for GOHIBIC (vilobelimab), for the treatment of COVID-19 in hospitalized adults when initiated within 48hours of receiving IMV or ECMO.
Adverse event reporting an cGMP compliance
Adverse event reporting and submission of periodic reports are required following FDA approval of a BLA or NDA. The FDA also may require post-marketing testing, known as Phase IV testing, REMS and surveillance to monitor the effects of an approved product, or may place conditions on an approval that could restrict the distribution or use of the product. In addition, manufacture, packaging, labeling, storage and distribution procedures must continue to conform to current cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals, request product recalls or impose marketing restrictions through labeling changes or product removals if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Orphan drug designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to biologics or NCEs intended to treat a rare disease or condition—generally a disease or condition that affects fewer than 200,000 individuals annually in the United States. Orphan drug designation must be requested before submitting a BLA or NDA. After the FDA grants orphan drug designation, the generic identity of the drug candidate and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not necessarily convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first BLA or NDA applicant to receive FDA approval for a particular product to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the United States for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition or, a drug that is otherwise the same drug which may be clinically superior. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the BLA or NDA application user fee.
-92-
Fast track designation
Fast track is a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need. The purpose is to get important new drugs to the patient earlier. Fast track addresses a broad range of serious conditions. Determining whether a condition is serious is a matter of judgment, but generally is based on whether the drug will have an impact on such factors as survival, day-to-day functioning, or the likelihood that the condition, if left untreated, will progress from a less severe condition to a more serious one. Filling an unmet medical need is defined as providing a therapy where none exists or providing a therapy which may be potentially better than available therapy. Any drug being developed to treat or prevent a condition with no current therapy is directed at an unmet need. If there are available therapies, a fast track drug must show some advantage over available therapy, such as: showing superior effectiveness, effect on serious outcomes or improved effect on serious outcomes; avoiding serious side effects of an available therapy; improving the diagnosis of a serious condition where early diagnosis results in an improved outcome; decreasing a clinical significant toxicity of an available therapy that is common and causes discontinuation of treatment or ability to address an emerging or anticipated public health need. A drug that receives fast track designation is eligible for some or all of the following: more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of appropriate data needed to support drug approval; more frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers; eligibility for Accelerated Approval and Priority Review, if relevant criteria are met; Rolling Review, which means that a drug company can submit completed sections of its BLA or NDA for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA. Fast track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and make a decision within sixty days based on whether the drug fills an unmet medical need in a serious condition. Once a drug receives fast track designation, early and frequent communication between the FDA and a drug company is encouraged throughout the entire drug development and review process. The frequency of communication assures that questions and issues are resolved quickly, often leading to earlier drug approval and access by patients.
We have been granted orphan drug status and fast track designation for the PG indication in the United States for vilobelimab. We have also been granted fast track designation for the COVID-19 indication in the United States. Depending on the outcome and available data of vilobelimab studies in the other indications, we may apply for orphan drug status in the United States.
Accelerated approval
The FDA instituted its accelerated approval program to allow for earlier approval of drugs that treat serious conditions, and fill an unmet medical need based on a surrogate endpoint. A surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. The use of a surrogate endpoint can considerably shorten the time required prior to receiving FDA approval. Drug companies are still required to conduct studies to confirm the anticipated clinical benefit. These studies are known as Phase IV confirmatory trials. If the confirmatory trial shows that the drug actually provides a clinical benefit, then the FDA grants traditional approval for the drug. If the confirmatory trial does not show that the drug provides clinical benefit, FDA has regulatory procedures in place that could lead to removing the drug from the market.
Priority review
In 1992, under the Prescription Drug User Act, or PDUFA, FDA agreed to specific goals for improving the drug review time and created a two-tiered system of review times – standard review and priority review. A priority review designation means FDA’s goal is to take action on an application within six months (compared to 10 months under standard review). A priority review designation will direct overall attention and resources to the evaluation of applications for drugs that, if approved, would be significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions when compared to standard applications. Significant improvement may be demonstrated by the following examples: evidence of increased effectiveness in treatment, prevention, or diagnosis of condition; elimination or substantial reduction of a treatment-limiting drug reaction; documented enhancement of patient compliance that is expected to lead to an improvement in serious outcomes; or evidence of safety and effectiveness in a new subpopulation. FDA decides on the review designation for every application. However, an applicant may expressly request priority review. It does not affect the length of the clinical trial period. FDA informs the applicant of a priority review designation within 60 days of the receipt of the BLA or NDA. Designation of a drug as “Priority” does not alter the scientific/medical standard for approval or the quality of evidence necessary.
-93-
SPA process
SPA is a process in which companies may ask to meet with FDA to reach agreement on the design and size of certain clinical trials, clinical studies, or animal studies to determine if they adequately address scientific and regulatory requirements for a study that could support marketing approval. An SPA agreement indicates concurrence by FDA with the adequacy and acceptability of specific critical elements of overall protocol design (e.g., entry criteria, dose selection, endpoints and planned analyses) for a study intended to support a future marketing application. These elements are critical to ensuring that the trial conducted under the protocol can be considered an adequate and well-controlled study that can support marketing approval. Feedback on these issues provides the benefit of certainty of adequacy in planning a late-phase development strategy. However, an SPA agreement does not indicate FDA’s concurrence on every protocol detail. The existence of an SPA agreement does not guarantee that FDA will file (accept) a BLA or NDA or that the results will be adequate to support approval.
Other healthcare laws and compliance requirements
In the United States, our activities are potentially subject to regulation by federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health and Human Services (for example, the Office of Inspector General), the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments.
EU approval process
The EMA is a decentralized scientific agency of the European Union. It coordinates the evaluation and monitoring of centrally-authorized medicinal products. It is responsible for the scientific evaluation of applications for EU marketing authorizations, as well as the development of technical guidance and the provision of scientific advice to sponsors. The EMA decentralizes its scientific assessment of medicines by working through a network of about 4,500 experts throughout the European Union, nominated by the member states. The EMA draws on resources of over 40 National Competent Authorities, or the NCAs, of EU member states. The Paul Ehrlich Institute, or PEI, is one of the NCAs for Germany, and regulates, among others, antibody products.
The process regarding approval of medicinal products in the European Union follows roughly the same lines as in the United States and generally involves satisfactorily completing each of the following:
| ● | preclinical laboratory tests, animal studies and formulation studies all performed in accordance with the applicable EU Good Laboratory Practice regulations; |
| ● | submission to the relevant national authorities of a clinical trial application or CTA for each trial in humans, which must be approved before the trial may begin in each country where patient enrollment is planned; |
| ● | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication; |
| ● | submission to the relevant competent authorities of a Marketing Authorization Application or MAA, which includes the data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product in clinical development and proposed labelling; |
| ● | satisfactory completion of an inspection by the relevant national authorities of the manufacturing facility or facilities, including those of third parties, at which the product is produced to assess compliance with strictly enforced cGMP; |
| ● | potential audits of the non-clinical and clinical trial sites that generated the data in support of the MAA; and |
| ● | review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product. |
-94-
Preclinical studies
Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animal studies, in order to assess the quality and potential safety and efficacy of the product. The conduct of the preclinical tests and formulation of the compounds for testing must comply with the relevant international, EU and national legislation, regulations and guidelines. The results of the preclinical tests, together with relevant manufacturing information and analytical data, are submitted as part of the CTA.
Clinical trial approval
The Clinical Trials Regulation (EU) No 536/2014 repealed the Clinical Trials Directive 2001/20/EC, on January 31, 2022 and applies to all new clinical trials from January 31, 2023. All ongoing trials must be transitioned to the new processes by 31 January 2025. The Clinical Trials Regulation harmonizes the processes and supervision of clinical trials throughout the EU. Sponsors submit one application via the Clinical Trials Information System, or CTIS, for approval to run a clinical trial in several European countries. The evaluation, authorization and supervision of clinical trials are the responsibilities of EU member states and European Economic Area, or EEA, countries. The CTIS application must be supported by an investigational medicinal product dossier, or IMPD, the study protocol and further supporting information prescribed by the Clinical Trials Regulation and other applicable guidance documents. The CTIS process has predetermined timelines which may vary depending on the phase of the study. The process includes timelines for one or more rounds of questions to be answered or requests to be met by the regulatory authority.
Regulation (EU) No 536/2014, includes transparency requirements (the proactive publication of clinical trial data in the EU database) with associated rules on the timing of the publication of submitted documents at the time of, during and after completion of the clinical trial. The transparency requirement can apply to documents included in the clinical trial application, or CTA, dossier provided in CTIS, and all the clinical trial information submitted during the trial life cycle, with the exception of the quality related documents, financial arrangements and some supervision related information.
Manufacturing and import into the EU of investigational medicinal products is subject to the holding of appropriate authorizations and must be carried out in accordance with cGMP.
Marketing authorization application
Authorization to market a product in the EU member states proceeds under one of four procedures: a centralized authorization procedure, a mutual recognition procedure, a decentralized procedure or a national procedure. Since our products by their virtue of being antibody-based biologics or NCEs, fall under the centralized procedure, only this procedure will be described here.
Centralized authorization procedure
Certain drugs, including medicinal products developed by means of biotechnological processes, must be approved via the centralized authorization procedure for marketing authorization. A successful application under the centralized authorization procedure results in a marketing authorization from the European Commission, which is automatically valid in all EU member states as well as in the other EEA, member states (namely Norway, Iceland and Liechtenstein). The EMA and the European Commission administer the centralized authorization procedure.
Under the centralized authorization procedure, the CHMP serves as the scientific committee that renders opinions about the safety, efficacy and quality of human products on behalf of the EMA. The CHMP is composed of experts nominated by each member state’s national drug authority, with one of them appointed to act as Rapporteur for the co-ordination of the evaluation with the possible assistance of a further member of the committee acting as a Co-Rapporteur. After approval, the Rapporteur(s) continue to monitor the product throughout its life-cycle. The CHMP is required to issue an opinion within 210 days of receipt of a valid application, though the clock is stopped when it is necessary to ask the applicant for clarification or further supporting data. The process is complex and involves extensive consultation with the regulatory authorities of member states and a number of experts. Once the procedure is completed, a European Public Assessment Report, or EPAR, is produced. If the CHMP concludes that the quality, safety and efficacy of the medicinal product is sufficiently proven, it adopts a positive opinion. The CHMP’s opinion is sent to the European Commission, which uses the opinion as the basis for its decision whether or not to grant a marketing authorization. If the opinion is negative, information is given as to the grounds on which this conclusion was reached.
-95-
Policy 0070, which is intended to promote transparency of EMA decision making and clinical data, governs how EMA collects, reviews and publishes clinical data submitted by applicants and Marketing Authorization Holders, or MAHs, through the centralized procedure.
After a drug has been authorized and launched, it is a condition of maintaining the marketing authorization that all aspects relating to its quality, safety and efficacy must be kept under review. Sanctions may be imposed for failure to adhere to the conditions of the marketing authorization. In extreme cases, the authorization may be revoked, resulting in withdrawal of the product from sale.
Accelerated assessment procedure
When an application is submitted for a marketing authorization in respect of a drug for human use which is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation, the applicant may request an accelerated assessment procedure pursuant to Article 14(9) of Regulation (EC) 726/2004. Under the accelerated assessment procedure, the CHMP is required to issue an opinion within 150 days of receipt of a valid application, subject to clock stops. We believe that some of the disease indications in which our product candidates are currently being or may be developed in the future qualify for this provision, and we will take advantage of this provision as appropriate.
Conditional approval
As per Article 14(7) of Regulation (EC) 726/2004, a medicine that would fulfill an unmet medical need may, if its immediate availability is in the interest of public health, be granted a conditional marketing authorization on the basis of less complete clinical data than are normally required, subject to specific obligations being imposed on the authorization holder. These specific obligations are to be reviewed annually by the EMA. The list of these obligations shall be made publicly accessible. Such an authorization shall be valid for one year, on a renewable basis.
Period of authorization and renewals
A marketing authorization is initially valid for five years and may then be renewed on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder shall provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variants introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization shall be valid for an unlimited period, unless the Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the EU market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization shall cease to be valid (the so-called sunset clause).
Orphan drug designation
Regulation (EC) 141/2000 states that a drug shall be designated as an orphan drug if its sponsor can establish:
| ● | that it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the European Union when the application is made, or that it is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the European Union and that without incentives it is unlikely that the marketing of the drug in the European Union would generate sufficient return to justify the necessary investment; and |
| ● | that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the European Union or, if such method exists, the drug will be of significant benefit to those affected by that condition. |
-96-
Regulation (EC) 847/2000 sets out criteria for the designation of orphan drugs. An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. Marketing authorization for an orphan drug leads to a 10-year period of market exclusivity, which means that no similar medicinal product can be authorized in the same indication. This period may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan drug designation, for example because the product is sufficiently profitable not to justify continued market exclusivity. In addition, derogation from market exclusivity may be granted on an individual basis in very selected cases, such as consent from the marketing authorization holder, inability to supply sufficient quantities of the product or demonstration of “clinically relevant superiority” by a similar medicinal product. Medicinal products designated as orphan drugs pursuant to Regulation (EC) 141/2000 are eligible for incentives made available by the European Union and by the member states to support research into, and the development and availability of, orphan drugs.
If the MAA of a medicinal product designated as orphan drug pursuant to Regulation (EC) 141/2000 includes the results of all studies conducted in compliance with an agreed PIP, and a corresponding statement is subsequently included in the marketing authorization granted, the 10-year period of market exclusivity will be extended to 12 years.
We have been granted orphan drug status for the PG indication in the European Union for vilobelimab. Depending on the outcome and available data of vilobelimab studies in the other indications, we may also apply for orphan drug status in Europe for these indications.
Regulatory data protection
Without prejudice to the law on the protection of industrial and commercial property, marketing authorizations for new medicinal products benefit from an 8+2+1 year period of regulatory protection.
This regime consists of a regulatory data protection period of eight years plus a concurrent market exclusivity of 10 years plus an additional market exclusivity of one further year if, during the first eight years of those 10 years, the marketing approval holder obtains an approval for one or more new therapeutic indications which, during the scientific evaluation prior to their approval, are determined to bring a significant clinical benefit in comparison with existing therapies. Under the current rules, a third party may reference the preclinical and clinical data of the reference product beginning eight years after first approval, but the third party may market a generic version of the reference product after only 10 (or 11) years have lapsed.
Other international regulations
In addition to regulations in the United States and Europe, a variety of foreign regulations govern clinical trials, commercial sales, and distribution of pharmaceutical products. The approval process varies from country to country and the time to approval may be longer or shorter than that required for FDA or European Commission approval.
Pharmaceutical coverage, pricing and reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of products approved by the FDA and other government authorities. Sales of our products will depend, in part, on the extent to which third-party payors, including government health programs in the United States such as Medicare and Medicaid, commercial private and public health insurers and managed care organizations, provide coverage and establish adequate reimbursement levels for, such products. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product once coverage is approved. Third-party payors are increasingly challenging the prices charged, examining the medical necessity, and reviewing the cost-effectiveness of medical products and services and imposing controls to manage costs. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the approved products for a particular indication.
-97-
In order to secure coverage and reimbursement for any pharmaceutical product approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Nonetheless, product candidates may not be considered medically necessary or cost effective. Additionally, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product. Third-party reimbursement may not be sufficient to maintain price levels high enough to realize an appropriate return on investment in product development.
The containment of healthcare costs also has become a priority of federal, state and foreign governments and the prices of drugs have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which a company or its collaborators receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Outside the United States, ensuring adequate coverage and payment for our products will face challenges. Pricing of prescription pharmaceuticals is subject to governmental control in many countries. Pricing negotiations with governmental authorities can extend well beyond the receipt of regulatory marketing approval for a product and may require us to conduct a clinical trial that compares the cost effectiveness of our products or products to other available therapies. The conduct of such clinical trials could be expensive and result in delays in our commercialization efforts.
In the European Union, pricing and reimbursement schemes to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug product to currently available therapies. EU member states may also require approval of a specific price for a drug product or, instead, may adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other EU member states allow companies to fix their own prices for drug products but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the market entry of new pharmaceutical products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements.
C. Organizational structure
InflaRx N.V. has two direct wholly-owned subsidiaries, InflaRx GmbH and InflaRx Pharmaceuticals, Inc., that are each listed in Exhibit 8.1 filed herewith.
D. Property, plant and equipment
Our headquarters are in Jena, Germany, where we occupy approximately 8,000 square feet of office and laboratory space under a lease that expires in December 2025. In addition, we occupy approximately 13,700 square feet of office space in Planegg-Martinsried (near Munich), Germany under a lease that expires in May 2027. Furthermore, we have leased office and laboratory space in Ann Arbor, Michigan, United States under a lease that expires in April 2026.
-98-
ITEM 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
| A. | Operating results |
You should read the following discussion and analysis of our financial condition and results of operations together with the information in our Consolidated Financial Statements and the notes thereto.
The following discussion is based on our financial information prepared in accordance with IFRS as issued by the IASB, which may differ in material respects from generally accepted accounting principles in the United States and other jurisdictions. The following discussion includes forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those described under “ITEM 3. KEY INFORMATION — 3. ‘Risk factors’ and Forward-Looking Statements.”
For more information regarding our consolidated results, segment results, and liquidity and capital resources for the year ended December 31, 2022 as compared to the year ended December 31, 2021, refer to “Item 5. Operating and Financial Review and Prospects” in the Company’s 2022 Annual Report on Form 20-F, which information is incorporated herein by reference.
Overview
We are a biotechnology company pioneering anti-inflammatory therapeutics focused on applying our proprietary anti-C5a and C5aR technologies to discover, develop and commercialize first-in-class, potent and specific inhibitors of the complement activation factor known as C5a and its receptor C5aR. C5a is a powerful inflammatory mediator involved in the progression of a wide variety of autoimmune and other inflammatory diseases. Our lead product candidate, vilobelimab, is a novel intravenously delivered first-in-class anti-C5a monoclonal antibody that selectively binds to free C5a and has demonstrated disease-modifying clinical activity and tolerability in multiple clinical settings. In April 2023, we received the EUA from the FDA for GOHIBIC (vilobelimab) for the treatment of critically ill, invasively mechanically ventilated COVID-19 patients. Subsequently, in June 2023, we began the commercialization of GOHIBIC (vilobelimab) in the United States. We are also developing vilobelimab for the treatment of pyoderma gangrenosum, or PG, a chronic inflammatory skin disorder for which we are currently conducting a Phase III study. Beyond PG, we have been developing vilobelimab to generate proof of concept data in a wide array of complement-mediated diseases with significant unmet medical needs. For this purpose, we have previously conducted Phase II studies with vilobelimab in HS, a chronic debilitating systemic inflammatory skin disease, in ANCA-associated vasculitis, or AAV, a rare and life-threatening autoimmune disease and in cSCC, where we have recently decided to cease the development for the time being, since further clinical development would require substantial resources and significantly extend the timeline of the ongoing clinical program. We decided to prioritize our efforts and to reallocate our resources towards the development of our orally available C5aR inhibitor INF904, an oral small-molecule drug candidate that targets the C5aR receptor. We plan on targeting complement-mediated, chronic autoimmune and inflammatory conditions for which an oral small molecule is the preferred route of administration for patients. We have recently completed a Phase I single ascending dose, or SAD, and multiple ascending dose, or MAD, study in healthy volunteers in which we were able to confirm the favorable safety profile and the superior pharmacokinetic and pharmacodynamic profile of INF904. We are currently conducting additional required pre-clinical studies, including long-term chronic toxicology studies, to enable longer-term dosing of INF904 for chronic inflammatory diseases. We are also developing IFX002, a life-cycle management product for vilobelimab.
Since our inception in December 2007, we have devoted substantially all our resources to establishing our company, raising capital, developing our proprietary anti-C5a/C5aR technologies, identifying and testing potential product candidates and conducting clinical trials of our lead product candidate, vilobelimab and additional product candidates IFX002 and INF904. To date, we have only generated minimal product revenue and so far have primarily financed our operations through the issuance of securities in public offerings and private placements and through other income from various grants, including a grant awarded by the German federal government for certain research and development activities during the period from October 2021 to June 2023. As of December 31, 2023, we had cash and cash equivalents of €12.8 million and €85.6 million in marketable securities. In addition, as of December 31, 2023, we had received a total of €33.3 million to support the development of our COVID-19 clinical development as part of a grant awarded to us for the period of October 2021 to June 2023.
-99-
On July 8, 2020, we filed a Form F-3 registration statement with the SEC with respect to the offer and sale of securities of the Company, or 2020-Shelf Registration Statement. We also filed a prospectus supplement with the SEC relating to an at-the-market program providing for the sales of our stock over time of up to $50.0 million of our ordinary shares pursuant to a Sales Agreement with SVB Leerink LLC. During the fiscal year 2023, we issued 3,235,723 ordinary shares under its at-the-market program resulting in €14.4 million or $15.7 million in net proceeds. As of December 31, 2023, and throughout the term of the at-the-market program, we have issued an aggregate total of 5,803,931 ordinary shares, resulting in a total of €26.2 million in net proceeds to us. The term of the at-the-market program expired on July 8, 2023.
Through an underwritten public offering in April 2023, we sold and issued an aggregate of 10,823,529 ordinary shares, at a price of $4.25 per share and have a nominal value of €0.12 per share, of which 1,411,764 were sold pursuant to the exercise of an overallotment option by the underwriters. Net proceeds of this offering amounted to €38.7 million, after accounting for underwriting discounts and other offering expenses.
On June 30, 2023, we filed the 2023-Registration Statement under which no ordinary shares or active offering programs were issued by the Company in the 2023 fiscal year. The aggregate initial offering price of the securities will not exceed $250 million.
During the fiscal year 2023, we issued and registered a total of 120,257 ordinary shares resulting from the exercise of stock option rights by former employees. All stock option rights had been granted under the 2017 Long-Term Incentive Plan. 14,930 stock options thereof were already exercised in December 2022 and 105,327 were exercised during fiscal year 2023. The ordinary shares have a nominal value of €0.12 per share. 98,754 ordinary shares were sold at a price of $1.85 per share, and 21,503 ordinary shares were sold at a price of $3.35 per share.
As of December 31, 2023, we had an accumulated deficit of €286.1 million. We have incurred significant net operating losses in every year since our inception and expect to continue to incur comparable or increasing net operating losses for the foreseeable future. Our net losses may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses may increase significantly if, and as we:
| ● | expand our commercial efforts for GOHIBIC (vilobelimab) in the United States, by enhancing our commercial strategic plan, further expanding our sales force and medical affairs teams, preparing relevant promotional and medical education materials to target healthcare providers and other stakeholders, refining our medical affairs strategy to increase awareness of the EUA among the medical community, and expand our sales and marketing efforts; |
| ● | continue to pursue regulatory activities for vilobelimab in the United States and Europe, including severe COVID-19, PG and potentially various other indications; |
| ● | continue to manufacture vilobelimab while adhering to applicable regulatory standards to serve clinical and commercial needs; |
| ● | continue to advance vilobelimab through clinical development, including in PG and potentially other indications; |
| ● | evaluate and continue additional Phase II clinical development of INF904; |
| ● | initiate and continue research programs and development activities, including development of IFX002; |
| ● | actively seek to identify additional research programs and additional product candidates; |
| ● | maintain, expand and protect our intellectual property portfolio; |
| ● | hire and retain personnel, such as for research and development, regulatory affairs, business development and commercial operations, manufacturing and supply chain management, and others; and |
| ● | incur additional costs with operating as a public company, including expanding our operational, administrative, finance and management teams. |
-100-
| B. | Financial operations overview |
| 1. | Revenue |
In June 2023, we began the commercialization of GOHIBIC (vilobelimab) in the United States. In connection with the start of the commercialization, the Group entered into agreements with certain subsidiaries of Cencora Inc. (formerly known as AmerisourceBergen Corp.) to act as the Group’s U.S. distributor and make GOHIBIC (vilobelimab) available for order by U.S. hospital customers. Cencora provides cold storage, cold-chain distribution services, inventory management and secondary labeling/packaging, among other services.
In 2023, we realized revenues, totaling €63 thousand, from the product sales for the first time since its inception. Revenues reported are sales to end customers (hospitals). Sales to distributors do not constitute completion of the earnings process and, thus, do not result in the recognition of revenue for the Company under IFRS 15.
| 2. | Cost of Sales |
Cost of sales recognized during the twelve months ended December 31, 2023, of €0.5 million, are related to GOHIBIC (vilobelimab) revenues in the United States and to write-downs of inventory.
Costs of sales for products sold in these periods do not include costs of materials, as the associated costs of these materials were incurred in prior periods, before the FDA granted the EUA for GOHIBIC (vilobelimab) in April 2023. These materials were recorded as ‘research and development expenses’ in the periods they were incurred.
The cost of sales during the twelve months ended December 31, 2023 mainly consists of write-downs of inventories that will expire prior to their expected sale.
| 3. | Other income |
In October 2021, we announced that we had been awarded a grant of up to €43.7 million from the German Ministry of Education and Research and the German Ministry of Health to support certain research and development activities of vilobelimab for the treatment of severe COVID-19 patients. Due to subsequent changes in our research and development plan and fewer costs projected within the timeframe of the grant, we were notified that the maximum total amount available to us was reduced to €41.4 million. The grant was structured as a reimbursement of 80% of certain pre-specified expenses related to the clinical development and manufacturing of vilobelimab. The grant was awarded for qualifying activities during the period from October 1, 2021 to June 30, 2023.During the duration of the grant period, as of December 31, 2023, we received a total of €33.3 million to support our activities regarding the development of vilobelimab as a new therapeutic agent for the treatment of critically ill COVID-19 patients and for the establishment of a commercial scale manufacturing process to ensure the ability of being able to provide such treatment to the broader population.
| 4. | Sales and marketing expenses |
Sales and marketing expenses have consisted principally of:
| ● | external services for distribution of GOHIBIC to build the necessary commercial and logistical infrastructure, including external sales professionals; |
| ● | marketing activities; |
| ● | employee-related expenses, including salaries, benefits and stock-based compensation expense based upon employees’ role within the organization; and |
| ● | professional services fees in conjunction to enable making GOHIBIC available in the U.S. |
Total expense for sales and marketing amount to €4.0 million. These expenses are mainly comprised of €1.0 million in personnel costs, €1.0 million in legal and consulting costs and €1.9 million in external services for distribution of GOHIBIC. The Group started with its commercialization activities when the EUA was granted in April 2023. Prior to that, no sales and marketing expenses had been incurred.
-101-
| 5. | Research and development expenses |
Research and development expenses have consisted principally of:
| ● | expenses incurred under agreements with CROs, contract manufacturing organizations, or CDMOs, consultants and independent contractors that conduct research and development, preclinical and clinical activities on our behalf; |
| ● | employee-related expenses, including salaries, benefits and stock-based compensation expense based upon employees’ role within the organization; and |
| ● | professional fees for lawyers related to the protection and maintenance of our intellectual property. |
Our total research and development expenses in 2023 were higher than our expenses in 2022 and higher than our expenses in 2021. Costs are expected to increase in 2024 (as compared to 2023) as we advance the Phase III development of vilobelimab in PG and initiate the Phase II development of INF904. The increase of research and development expenses in 2024 and future periods is expected to primarily relate to the following key programs and activities:
Vilobelimab.
We expect our expenses associated with vilobelimab will increase in 2024 compared to 2023, as we progress to conduct the Phase III the clinical study in PG. In addition, we are incurring and expect to further incur expenses in conjunction with the preparation and filing of full market authorizations for vilobelimab in the United States, Europe and elsewhere. We might also potentially consider development of vilobelimab in additional indications. In addition, we are also incurring expenses related to the manufacturing of clinical trial material and the completion of activities towards the final establishment of commercial scale production.
| ● | INF904. We are also developing INF904, a product candidate that targets the C5aR receptor. We expect to incur additional costs by advancing the clinical and non-clinical development of INF904. Specifically, we expect to incur expenses by developing a new formulation, conducting long-term toxicological studies in several animal species and initiation Phase II clinical trials. We plan to study INF904 in complement-mediated, chronic autoimmune and inflammatory conditions where an oral low molecular weight compound might have advantages or is needed for patients and where oral delivery is the medically preferred route of administration. |
| ● | IFX002. We are also developing IFX002 for the treatment of chronic inflammatory indications. IFX002 is a highly potent anti-complement C5a antibody with a higher humanization grade and altered pharmacokinetic properties compared to vilobelimab and is currently in pre-clinical development. Expenses for this program mainly consist of salaries, costs for preclinical testing conducted by CROs and costs to produce preclinical material. |
| ● | Other development programs. Our other research and development expenses relate to our preclinical studies of other product candidates and discovery activities, expenses for which mainly consist of salaries, costs for production of preclinical compounds and costs paid to CROs. |
In 2023 and 2022, we incurred €41.0 million and €37.5 million of research and development expenses, respectively. Our research and development expenses may vary substantially from period to period based on the timing of our research and development activities, including due to timing of clinical trial initiation and potential enrollment.
We expense research and development costs as incurred. We recognize costs for certain development activities, such as preclinical studies and clinical trials, based on an evaluation of the progress to completion of specific tasks. We use information provided to us by our vendors such as patient enrollment or clinical site activations for services received and efforts expended. Research and development activities are central to our business model.
-102-
The successful development of our product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing and estimated costs of the efforts that will be necessary to complete the development of, or the period, if any, in which material net cash inflows may commence from, any of our product candidates. This is due to numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
| ● | clinical trials or our product candidates producing negative or inconclusive results, including failure to demonstrate statistical significance; |
| ● | the scope, rate of progress, results and cost of our clinical trials, nonclinical testing, and other related activities; |
| ● | delays in reaching, or failing to reach, agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites or prospective CROs, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | the cost of manufacturing clinical supplies and establishing commercial supplies of our product candidates and any products that we may develop; |
| ● | third-party contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| ● | the number and characteristics of product candidates that we pursue; |
| ● | undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or institutional review boards to suspend or terminate the trials; |
| ● | potential additional safety monitoring or other studies requested by regulatory agencies; |
| ● | the cost, timing, and outcomes of regulatory approvals; |
| ● | the number of trials required for approval; |
| ● | the duration of patient follow-up; |
| ● | the cost and timing of establishing sales, marketing, and distribution capabilities; and |
| ● | the terms and timing of any collaborative, licensing and other arrangements that we may establish, including any milestone and royalty payments thereunder. |
A change in the outcome of any of these variables with respect to the development of vilobelimab, IFX002 or any other product candidate that we may develop could mean a significant change in the costs and timing associated with the development of such product candidate.
| 6. | General and administrative expenses |
Our general and administrative expenses consist principally of:
| ● | employee-related expenses, including salaries, benefits and stock-based compensation expense based upon employees’ role within the organization; |
| ● | insurance expenses including directors´ and officers´ liability insurance premiums; |
| ● | professional fees for auditors and consulting expenses not related to research and development activities; |
| ● | professional fees for lawyers not related to the filing, prosecution, protection and maintenance of our intellectual property; and |
| ● | cost of facilities, travel, communication and office expenses. |
We expect that our general and administrative expenses will increase in the future as our business expands and we incur additional costs associated with operating as a public company. These public company-related costs relate primarily to additional personnel, additional legal fees, audit fees, directors’ and officers’ liability insurance premiums and costs associated with investor relations.
-103-
| 7. | Results of operations |
The Group is exposed to the exchange rate between Euros and U.S. dollars. Due to the Company’s various registered offerings of ordinary shares in U.S. dollars, the Group holds significant cash, cash equivalents and marketable securities in U.S. dollars. This could have a material impact on our operating results.
The numbers below have been derived from our consolidated financial statements included elsewhere herein. The discussion below should be read along with these consolidated financial statements, and it is qualified in its entirety by reference to them.
Comparison of the years ended December 31, 2023 and 2022
| 2023 | 2022 | Change | ||||||||||
| (in €) | ||||||||||||
| Revenues | 63,089 | — | 63,089 | |||||||||
| Cost of Sales | (532,262 | ) | — | (532,262 | ) | |||||||
| Gross profit | (469,173 | ) | — | (469,173 | ) | |||||||
| Sales and marketing expenses | (4,001,299 | ) | — | (4,001,299 | ) | |||||||
| Research and development expenses | (41,024,131 | ) | (37,526,090 | ) | (3,498,041 | ) | ||||||
| General and administrative expenses | (12,628,756 | ) | (14,869,564 | ) | 2,240,808 | |||||||
| Other income and expenses (net) | 13,215,264 | 20,157,788 | (6,942,524 | ) | ||||||||
| Loss before interest and income taxes | (44,908,096 | ) | (32,237,866 | ) | (12,670,230 | ) | ||||||
| Net financial result | 2,240,566 | 2,753,255 | (512,689 | ) | ||||||||
| Loss before tax | (42,667,529 | ) | (29,484,611 | ) | (13,182,918 | ) | ||||||
| Income tax expense | — | — | — | |||||||||
| Loss for the period | (42,667,529 | ) | (29,484,611 | ) | (13,182,918 | ) | ||||||
| Exchange differences on translating operations in foreign currency | 125,085 | 4,206,810 | (4,081,725 | ) | ||||||||
| Total comprehensive loss | (42,542,444 | ) | (25,277,801 | ) | (17,264,643 | ) | ||||||
Revenues
| 2023 | 2022 | Change | ||||||||||
| (in €) | ||||||||||||
| Revenues | 63,089 | — | 63,089 | |||||||||
| Total | 63,089 | — | 63,089 | |||||||||
In June 2023, we began the commercialization of GOHOBIC (vilobelimab) in the United States. In connection with the start of the commercialization, we entered into agreements with certain subsidiaries of Cencora Inc. (formerly known as AmerisourceBergen Corp.) to act as the Group’s U.S. distributor and make GOHIBIC (vilobelimab) available for order by U.S. hospital customers. Cencora provides cold storage, cold-chain distribution services, inventory management and secondary labeling/packaging, among other services.
In 2023, we recognized revenues from the product sales for the first time since our inception. Revenues reported are sales to end customers (hospitals). Sales to distributors do not constitute the completion of a performance obligation towards a customer earnings process and, thus, do not result in the recognition of revenue for the Company under IFRS 15.
-104-
Cost of Sales
| 2023 | 2022 | Change | ||||||||||
| (in €) | ||||||||||||
| Cost of Sales | 532,262 | — | 532,262 | |||||||||
| Total | 532,262 | — | 532,262 | |||||||||
Cost of sales recognized during the twelve months ended December 31, 2023 are related to GOHBIC (vilobelimab) revenues in the United States. Costs of sales for products sold in these periods do not include costs of materials, as the associated costs of these materials were incurred in prior periods, before granting of the EUA for GOHIBIC (vilobelimab). The costs of manufacturing of these materials were recorded as research and development expenses in the period they were incurred.
The cost of sales during the twelve months ended December 31, 2023 mainly consists of write-downs of inventories that will expire prior to their expected sale. Early product batches subsequently capitalized in inventory were produced with material which had been manufactured in previous years.
Sales and marketing expenses
| 2023 | 2022 | Change | ||||||||||
| (in €) | ||||||||||||
| Third-party expenses | 1,851,158 | — | 1,851,158 | |||||||||
| Personnel expenses | 1,040,587 | — | 1,040,587 | |||||||||
| Legal and consulting fees | 1,054,971 | — | 1,054,971 | |||||||||
| Other expenses | 54,581 | — | 54,581 | |||||||||
| Total sales and marketing expenses | 4,001,299 | — | 4,001,299 | |||||||||
During the twelve months ended December 31, 2023 we incurred €4.0 million of sales and marketing expenses. These expenses are mainly composed of €1.0 million in personnel costs, €1.0 million in legal and consulting costs and €1.9 external services, for distribution of GOHIBIC.
Research and development expenses
| 2023 | 2022 | Change | ||||||||||
| (in €) | ||||||||||||
| Third-party expenses | 31,802,983 | 28,543,503 | 3,259,480 | |||||||||
| Personnel expenses | 6,776,853 | 6,957,866 | (181,013 | ) | ||||||||
| Other expenses | 2,444,295 | 2,024,721 | 419,574 | |||||||||
| Total | 41,024,131 | 37,526,090 | 3,498,042 | |||||||||
Research and development expenses increased by €3.5 million for the year ended December 31, 2023 compared to the corresponding costs for the year ended December 31, 2022 primarily due to higher third party material and manufacturing (CDMO) costs and from clinical trials, which increased by €1.9 million.
-105-
General and administrative expenses
| 2023 | 2022 | Change | ||||||||||
| (in €) | ||||||||||||
| Personnel expenses | 5,392,905 | 7,125,798 | (1,732,893 | ) | ||||||||
| Legal, consulting and audit fees | 3,239,809 | 3,104,624 | 135,185 | |||||||||
| Other expenses | 3,996,042 | 4,639,142 | (643,100 | ) | ||||||||
| Total | 12,628,756 | 14,869,564 | (2,240,809 | ) | ||||||||
General and administrative expenses decreased by €2.2 million to €12.6 million for the year ended December 31, 2023, from €14.9 million for the year ended December 31, 2022. This decrease is partially attributable to a €1.7 million decrease in personnel expenses, driven by a decrease in expenses from share-based compensation. The decrease of other expenses by €0.6 million is primarily attributable to lower D&O insurance cost.
Net financial result
| 2023 | 2022 | Change | ||||||||||
| (in €) | ||||||||||||
| Interest income | 3,804,827 | 608,679 | 3,196,148 | |||||||||
| Interest expenses | (16,538 | ) | (23,303 | ) | 6,765 | |||||||
| Interest on lease liabilities | (19,090 | ) | (21,947 | ) | 2,857 | |||||||
| Net interest result | 3,769,199 | 563,429 | 3,205,770 | |||||||||
| Foreign exchange income | 5,529,389 | 6,924,697 | (1,395,308 | ) | ||||||||
| Foreign exchange expense | (7,371,261 | ) | (4,482,399 | ) | (2,888,862 | ) | ||||||
| Net foreign exchange result | (1,841,872 | ) | 2,442,298 | (4,284,170 | ) | |||||||
| Other financial result | 313,240 | (252,471 | ) | 565,711 | ||||||||
| Net financial result | 2,240,566 | 2,753,256 | 512,690 | |||||||||
Net financial result decreased by €0.5 million to €2.2 million for the year ended December 31, 2023 compared to €2.7 million for the year ended December 31, 2022. This overall net decrease is mainly attributable to a decrease of €4.3 million in foreign exchange result and €3.2 million higher interest income from marketable securities compared to the year ended December 31, 2022.
-106-
Comparison of the years ended December 31, 2022 and 2021
| 2022 | 2021 | Change | ||||||||||
| (in €) | ||||||||||||
| Research and development expenses | (37,526,090 | ) | (35,697,935 | ) | (1,828,155 | ) | ||||||
| General and administrative expenses | (14,869,564 | ) | (11,984,722 | ) | (2,884,842 | ) | ||||||
| Other income and expenses (net) | 20,157,788 | 47,840 | 20,109,948 | |||||||||
| Loss before interest and income taxes | (32,237,866 | ) | (47,634,816 | ) | 15,396,950 | |||||||
| Net financial result | 2,753,255 | 2,004,757 | 748,498 | |||||||||
| Loss before tax | (29,484,611 | ) | (45,630,059 | ) | 16,145,448 | |||||||
| Income tax expense | — | — | — | |||||||||
| Loss for the period | (29,484,611 | ) | (45,630,059 | ) | 16,145,448 | |||||||
| Exchange differences on translating operations in foreign currency | 4,206,810 | 6,777,061 | (2,570,251 | ) | ||||||||
| Total comprehensive loss | (25,277,801 | ) | (38,852,998 | ) | 13,575,197 | |||||||
Research and development expenses
| 2022 | 2021 | Change | ||||||||||
| (in €) | ||||||||||||
| Third-party expenses | 28,543,503 | 28,247,081 | 296,422 | |||||||||
| Personnel expenses | 6,957,866 | 5,941,813 | 1,016,053 | |||||||||
| Other expenses | 2,024,721 | 1,509,041 | 515,680 | |||||||||
| Total | 37,526,090 | 35,697,935 | 1,828,155 | |||||||||
Research and development expenses increased by €1.8 million in the year ended December 31, 2022 compared to the year ended December 31, 2021. This increase is mainly attributable to a €1.0 million increase in employee-related costs, mainly caused by a €0.9 million increase in expenses from share-based compensation.
General and administrative expenses
| 2022 | 2021 | Change | ||||||||||
| (in €) | ||||||||||||
| Personnel expenses | 7,125,798 | 6,500,680 | 625,118 | |||||||||
| Legal, consulting and audit fees | 3,104,624 | 2,065,423 | 1,039,201 | |||||||||
| Other expenses | 4,639,142 | 3,418,619 | 1,220,523 | |||||||||
| Total | 14,869,564 | 11,984,722 | 2,884,842 | |||||||||
General and administrative expenses increased by €2.9 million to €14.9 million for the year ended December 31, 2022, from €12.0 million for the year ended December 31, 2021. This increase is partially attributable to a €0.9 million increase in expenses from share-based compensation. Legal, consulting and audit fees and other expenses increased by €1.0 million to €3.1 million for the year ended December 31, 2022, mainly due to higher consulting and legal costs, incurred in enhancing our internal control environment as we are complying with the auditor attestation requirement of Section 404(b) of the Sarbanes–Oxley Act of 2002 for the first time. The increase of other expenses by €1.2 million is primarily due to higher D&O insurance cost.
-107-
Net financial result
| 2022 | 2021 | Change | ||||||||||
| (in €) | ||||||||||||
| Interest income | 608,679 | 109,391 | 499,288 | |||||||||
| Interest expenses | (23,303 | ) | (10,714 | ) | (12,589 | ) | ||||||
| Interest on lease liabilities | (21,947 | ) | (14,055 | ) | (7,892 | ) | ||||||
| Net interest result | 563,429 | 84,622 | 478,807 | |||||||||
| Foreign exchange income | 6,924,697 | 5,569,836 | 1,354,862 | |||||||||
| Foreign exchange expense | (4,482,399 | ) | (3,605,701 | ) | (876,699 | ) | ||||||
| Net foreign exchange result | 2,442,298 | 1,964,135 | 478,163 | ) | ||||||||
| Other financial result | (252,471 | ) | (44,000 | ) | (208,471 | ) | ||||||
| Net financial result | 2,753,256 | 2,004,757 | 748,498 | |||||||||
Net financial result increased by €0.7 million in the year ended December 31, 2022 compared to the year ended December 31, 2021. This overall net increase is mainly attributable to a net increase of €0.5 million in foreign exchange income and expense and €0.5 million in higher interest income from marketable securities compared to the year ended December 31, 2021.
| C. | Liquidity and capital resources |
| 1. | Overview on cash requirements and sources of liquidity |
Since inception, we have incurred significant operating losses due to our research and development activities and G&A costs. For the years ended December 31, 2023 and 2022, we incurred net losses of €42.7 million and €29.5 million, respectively. Our primary uses of cash are for working capital, operating leases and general corporate purposes.
Our primary sources of funds are proceeds from the sale of our shares including our initial public offering and follow-on offerings. Additionally, in 2021, we were awarded a grant from the German federal government under which we received €33.3 million between October 2021 and June 2023. The grant period ended on June 30, 2023. Historically, we have been able to fund our capital needs with cash from equity financing through placement of shares.
Through an underwritten public offering in April 2023, we sold and issued an aggregate of 10,823,529 ordinary shares, of which 1,411,764 were sold pursuant to the exercise of an overallotment option by the underwriters. The ordinary shares were sold at a price of $4.25 per share and have a nominal value of €0.12 per share. Proceeds of this offering after deducting €2.5 million ($2.8 million) in underwriting discounts amounted to €39.1 million ($43.2 million). Other offering expenses amounted to €0.4 million, resulting in a total of €38.7 million in net proceeds from this offering.
On June 30, 2023, we filed a Form F-3 (2023-Registration Statement) with the SEC with respect to the offer and sale of securities of the Company, which became effective on July 11, 2023. The aggregate initial offering price of the securities that the Company may offer and sell under this prospectus will not exceed $250 million. No ordinary shares were issued by the Company under the 2023-Registration Statement within the fiscal year 2023 and no active offering programs have been launched.
Our working capital did not include any indebtedness in 2023 or in 2022.
-108-
Our cash and cash equivalents amounted to €12.8 million as of December 31, 2023 (2022: €16.3 million). We also held marketable securities valued at €85.6 million (2022: €67.2 million) as of December 31, 2022. Our cash and cash equivalents primarily consist of cash denominated in U.S. dollars and euros in bank deposit accounts. Our marketable securities consist of quoted debt securities issued by financial institutions with investment grade credit ratings (BBB+ to AAA). Our cash is deposited at banks with equally high credit ratings as assessed by international rating agencies.
We expect to finance our future operations and working capital needs in the near future predominantly with our cash and cash equivalents and proceeds from the sale of our marketable securities.
| 2. | Cash flows - Comparison of the years ended December 31, 2023 and 2022 |
The table below summarizes our consolidated statement of cash flows for the years ended December 31, 2023 and 2022:
| 2023 | 2022 | |||||||
| (in €) | ||||||||
| Net cash used in operating activities | (37,812,966 | ) | (33,742,817 | ) | ||||
| Net cash (used in)/from investing activities | (17,696,616 | ) | 19,358,095 | |||||
| Net cash from financing activities | 52,986,269 | 1,937,459 | ||||||
| Cash and cash equivalents at the beginning of the period | 16,265,355 | 26,249,995 | ||||||
| Exchange (losses)/gains on cash and cash equivalents | (974,099 | ) | 2,462,622 | |||||
| Cash and cash equivalents at the end of the period | 12,767,943 | 16,265,355 | ||||||
Net cash used in operating activities
The use of cash in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital.
Net cash used in operating activities increased to €37.8 million in the year ended December 31, 2023, from €33.8 million in the year ended December 31, 2022, mainly due to lower income recognized from the German federal government grant, significant production of inventory as well as higher expenditures from marketing and sales activities for GOHIBIC(vilobelimab), which were recorded for the first time due to start of the commercialization of this product in 2023.
Net cash used in investing activities
Net cash used in investing activities during the year ended December 31, 2023 amounted to €17.7 million due to net higher purchases than proceeds of sales of marketable securities. During the previous year ending on December 31, 2022, €19.4 million of cash was generated from the sale of marketable securities.
Net cash from financing activities
Net cash generated from financing activities increased to €53.0 million in the year ended December 31, 2023 from €1.9 million in the year ended December 31, 2022 primarily due to the share issuances through the underwritten public offering of April 2023 and through utilization of the at-the-market facility prior to its expiration on July 8, 2023.
-109-
| 3. | Contractual obligations and commitments |
The table below sets forth our operating expenses and capital expenditures from contractual obligations as of December 31, 2023.
| Payments due by Period | ||||||||||||||||||||
| Total | Less than 1 year | Between 1 and 3 Years | Between 3 and 5 Years | More than 5 years | ||||||||||||||||
| (in €) | ||||||||||||||||||||
| Unavoidable contractual (CRO, CDMO) commitments and other contractual obligations under operating contracts or services: | 18,233,216 | 13,926,289 | 4,265,136 | 41,791 | — | |||||||||||||||
| Contractual lease obligations (incl. capitalized leases) | 1,158,363 | 397,942 | 760,421 | — | — | |||||||||||||||
| Total | 19,391,579 | 14,324,231 | 5,025,557 | 41,791 | — | |||||||||||||||
We enter into contracts with CROs and clinical sites for the conduct of clinical trials, professional consultants for expert advice and other vendors like CDMOs for clinical supply manufacturing or other services in the normal course of business. These contracts can usually be terminated with 30 to 180 days notice. In addition to this minimum duration, these contracts require full payment for services already commenced. In the table above, the amounts for unavoidable contractual obligations assumes that the contracts were terminated on December 31, 2023 and would then continue to run for approximately 30 to 180 days.
Contractual lease obligations
Contractual lease obligations mainly consist of payments pursuant to non-cancellable lease agreements relating to our leases of office space. The lease term of our premises in Jena, Germany expires in December 2025. The lease term of our premises in Planegg-Martinsried, Germany expires in May 2027. The lease term of our premises in Ann Arbor, Michigan, United States expires in April 2026.
Funding requirements for future capital expenditure
We believe that our existing cash and cash equivalents and financial assets will enable us to fund our operating expenses and capital expenditure requirements under our current business plan for at least the next 24 months.
We anticipate that our expenses will increase in the next years in connection with our ongoing activities. In particular, we anticipate that we might expand our sales and marketing efforts for GOHIBIC(vilobelimab) in the United States, we might advance our Phase III clinical development program with vilobelimab in PG, pursue the further clinical development for INF904 and will advance preparation of necessary submission documents for additional regulatory submissions to the EMA and for a full BLA submission to the FDA beyond the received the EUA as granted by the FDA in April 2023 for GOHIBIC (vilobelimab). We will also explore clinical development of vilobelimab in several other indications. We also plan to continue clinical development of INF904 and to initiate Phase II clinical trials once we selected the appropriate indications. We also plan to continue preclinical development of IFX002. We plan to initiate new research and preclinical development efforts. If clinical data is supportive, we may seek marketing approval for any product candidates that we successfully develop. Additionally, we will validate our manufacturing process for vilobelimab to be able to apply for marketing authorization and to be able to provide commercial grade product. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to establishing sales, marketing, distribution and other commercial infrastructure to commercialize such products. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce, or eliminate our research and development programs or future commercialization efforts.
-110-
Until such time, if ever, that we can generate substantial meaningful product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, royalty-based financings, future collaborations, strategic alliances, licensing arrangements and government grants. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the interest of our current shareholders will be diluted, and the terms of these securities may include voting or other rights that adversely affect your rights as an ordinary shareholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends. If we raise funds through additional collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. Money received through government grants may require us to provide our product, if approved by regulatory authorities, at unfavorable conditions in such jurisdictions.
| D. | Research and development, patents and licenses, etc. |
See “ITEM 4. INFORMATION ON THE COMPANY — 2. Business overview — Our intellectual property.”
| E. | Trend information |
Other than as disclosed elsewhere in this annual report on Form 20-F, we are not aware of any trends, uncertainties, demands, commitments, or events for the year ended December 31, 2023 that are reasonably likely to have a material adverse effect on our net revenue, income, profitability, liquidity, or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial condition (see “ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS”).
| F. | Critical accounting estimates |
Our consolidated financial statements are prepared in conformity with IFRS, as issued by the IASB. In preparing our consolidated financial statements, we make judgements, estimates and assumptions about the application of our accounting policies which affect the reported amounts of assets, liabilities, revenue and expenses. Our critical accounting judgements and sources of estimation uncertainty are described in Note B.2. to our consolidated financial statements, which are included elsewhere in this Annual Report.
-111-
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
| A. | Directors and senior management |
Board of Directors and senior management team
The following table presents information about our Board of Directors and senior management as of the date of this Annual Report.
| Name | Position | Age | Initial year of appointment at InflaRx GmbH, InflaRx N.V. or InflaRx Pharmaceuticals Inc. (as applicable) | ||||||
| Niels Riedemann | Executive Director and Chief Executive Officer | 52 | 2007 | ||||||
| Renfeng Guo | Executive Director and Chief Scientific Officer | 53 | 2007 | ||||||
| Thomas Taapken | Chief Financial Officer | 58 | 2020 | ||||||
| Camilla Chong | Chief Medical Officer | 58 | 2023 (from July) | ||||||
| Derval O’Carroll | Senior Vice President, Global Head of Regulatory Affairs and Compliance | 58 | 2023 (from April) | ||||||
| Nicolas Fulpius | Non-Executive Director and Chairman of the Board | 50 | 2007 | ||||||
| Richard Brudnick | Non-Executive Director | 67 | 2019 | ||||||
| Mark Kubler | Non-Executive Director | 49 | 2015 | ||||||
| Anthony Gibney | Non-Executive Director | 53 | 2021 | ||||||
| Hege Hellstrom | Non-Executive Director | 58 | 2023 (from April) | ||||||
The terms for which Mark Kubler and Anthony Gibney have been appointed to the Board of Directors, will expire in 2024 and the terms for which Richard Brudnick, Nicolas Fulpius, Renfeng Guo, Niels Riedemann and Hege Hellstrom have been appointed to the Board of Directors will expire in 2026.
Ms. Derval O’Carroll was promoted to SVP in April 2023 and effectively became part of our senior management team through her promotion. Ms. Camilla Chong joined the Company as Chief Medical Officer effective July 2023.
Unless otherwise indicated, the current business address for our directors, senior management and key employees is InflaRx N.V., Winzerlaer Strasse 2, 07745 Jena, Germany.
The following is a brief summary of the business experience of our directors, senior management and key employees. Each director’s tenure reflects such director’s tenure on InflaRx GmbH’s board.
Non-executive directors
Nicolas Fulpius, Chairman. One of the co-founders of InflaRx, Nicolas Fulpius has served as Chairman of the Board since its inception in 2007. Long active in the venture capital field between the United States and Europe, for the Lombard Odier Immunology fund, for Ultreia Capital and as Partner at Affentranger Associates, Nicolas has become an entrepreneur at heart: he created, developed and helped finance several companies in the Biotech, cleantech and ICT field. Recently, Mr. Fulpius was - among others - CEO of Veltigroup, CDO of Swisscom and member of the Swisscom Ventures investment committee. In 2020, Nicolas Fulpius co-founded the Ansam Group one of the leading ICT services company in Switzerland for which he is acting as CEO and Chairman. Nicolas Fulpius holds an MBA from the University of St. Gallen, Switzerland, and a Masters in Science in Engineering from Stanford University, USA.
-112-
Richard Brudnick. Richard Brudnick currently serves as Chief Business Officer for Prime Medicine, Inc., a leader in the field of gene editing. Prior to joining Prime Medicine, Mr. Brudnick was Chief Business Officer and Head of Strategy for Codiak BioSciences, a leader in the field of exosome therapeutics. Before Codiak, Mr. Brudnick was Executive Vice President of Business Development and Alliance Management at Bioverativ, Inc., a company he helped found in 2016. Until Bioverativ’s acquisition by Sanofi in March 2018, Mr. Brudnick led business development efforts to build a significant pipeline in rare blood disorders, including an acquisition, a multi-product collaboration and additional scientific collaborations and licenses. Mr. Brudnick joined Bioverativ at its spin-off from Biogen where, over the course of nearly 15 years, he initiated, led and completed transactions that led to several of the company’s marketed products and late-stage pipeline, including Tecfidera, Spinraza, Leqembi and its biosimilars joint venture with Samsung. Mr. Brudnick also was CEO of a regional pharmaceutical distribution business, which he sold to a strategic buyer; co-founded two companies; and was a strategy consultant at Bain & Company.
Mark Kubler. Mr. Kubler has served as a director on our board since 2015. Mr. Kubler has been a partner with the GIG Ltd., a venture capital advisory firm with offices in Switzerland and Malta, since 2012. He previously served on the boards of WWM AG and Jobydu AG, each based in Switzerland. Mr. Kubler was a managing director and corporate secretary of a private equity holding company from 2003 to 2010. Before 2003, he held various roles in international investment banks and boutiques. Mr. Kubler has a master’s degree in business and economics, as well as a master’s degree in law from the University of St. Gallen, in Switzerland.
Anthony Gibney was formerly the Chief Business and Strategy Officer at Iveric Bio, overseeing the business development and corporate strategy for the retina-focused, biotechnology company, through the closing of the sale of Iveric to Astellas for $5.9 billion in 2023. Prior to Iveric, Mr. Gibney was the CFO and CBO at FogPharma, driving the business development and finance functions of the company. Mr. Gibney served as the Chief Business Officer of Achillion Pharmaceuticals, Inc., where he was responsible for corporate and portfolio strategy, business development and corporate communications and led the successful sale of Achillion to Alexion in 2020. Before Achillion, Tony Gibney was a life sciences-focused investment banker for 24 years. From 2009 through 2017, he served as a managing director and co-head of the biotechnology investment team for Leerink Partners LLC, where he was a senior leader of Leerink’s biopharmaceutical investment banking franchise. From 1999 to 2009, he worked as a managing director at Merrill Lynch Inc. and executed a variety of significant financing and M&A transactions for various biotechnology companies. From 1993 to 1999, Mr. Gibney was an investment banker at Lehman Brothers in the firm’s Healthcare Investment Banking Group. He graduated with distinction from Yale University in 1993 with a B.A. in History and Economics.
Hege Hellstrom is currently Chief Commercial Officer in Advicenne, a French pharmaceutical company specializing in the development of innovative treatments in Nephrology. She is a non-executive board member of Vivesto AB since 2019 and Camurus AB since 2020, both public Swedish companies and she is also a member of the Audit Committee in both companies. She is the founder and managing director of Belnor BV, an investment and consulting company. Mrs. Hellstrom has more than 30 years’ experience in sales, marketing, strategy development, commercialization, partner alliances and executive management. From 2013 to 2018, she worked as President Europe, Middle East, North Africa and Russia in Sobi, a Swedish biopharmaceutical company where she led several launches in rare diseases such as haemophilia and metabolic diseases. Before Sobi, she worked in Genzyme for 11 years in roles ranging from General manager in Benelux to head of Renal and Endocrine business in Europe, LATAM and JAPAC. When Genzyme was acquired by Sanofi she continued as Global Vice-president of Cardiovascular products in Sanofi. Before Genzyme she worked in Baxter Healthcare for 13 years. Mrs. Hellstrom holds a B.Sc., Biomedical Laboratory Scientist from Oslo Metropolitan University, Norway.
Executive directors
Niels Riedemann, Chief Executive Officer and Founder. Professor Riedemann is one of our co-founders and has served as our Chief Executive Officer since our inception in 2007. Prof. Riedemann has over 15 years of experience in the biotech industry and drug development as well as over 20 years of experience in complement immunology research. He founded InflaRx in 2007 and has served as Chief Executive Officer since inception of the company. He has been instrumental in and led numerous private and public financing rounds of the company and has been the responsible lead for its Nasdaq IPO in 2017. He is named inventor on several internationally granted core patents of InflaRx. As physician he has been appointed Vice Director (Leitender Oberarzt) of Intensive Care Medicine, and he has led a 50-bed University ICU unit for over 6 years at Friedrich Schiller University, Jena, Germany until 2015. Before that, he received his board certification as General Surgeon upon completion of his surgical fellowship at MHH (Hannover Medical School, Germany) in 2007 where he also received his habilitation (equivalent to Ph.D.) and where he still holds an Adjunct Professorship (APL Professor). He spent three years as postdoctoral research fellow at the University of Michigan, USA until 2003. He received his medical training at Albert Ludwig University (ALU), Freiburg, Germany, and Stanford University, USA and graduated as Dr. med. (equivalent to M.D.) from ALU in 1998. His research has been awarded with several national and international awards. He has received extensive extra-mural funding and published over 60 peer reviewed scientific publications in highly ranked journals. He has served as a member on a Board of Directors and a Scientific Advisory Board of two large scientific governmental funded programs. He currently serves as Co-Chair of the Health Politics working group of Bio-Deutschland and he serves as member of the board of trustees for the German Sepsis Foundation.
-113-
Renfeng Guo, Chief Scientific Officer and Founder. Prof. Renfeng Guo co-founded InflaRx in 2007. Since its inception, he has headed scientific development at InflaRx as the full-time CSO. Prof. Guo leverages his expertise in antibody research and inflammation, bringing together a highly effectual research team for drug development to build a focused pipeline based on cutting-edge technology. His early research led to the discovery of InflaRx’s leading drug, vilobelimab. He continues to be the driving force for the development of other pipeline drugs as well as a key inventor for InflaRx’s intellectual property portfolio. Prof. Guo received his M.D. degree from Norman Bethune Medical School in China and conducted post-doctoral research in the laboratory of Prof. Peter Ward at the University of Michigan, Ann Arbor. After stints as a junior and senior faculty member beginning in 2001 at the University of Michigan, he is currently an Adjunct Research Associate Professor. Prof. Guo has over 80 high-impact, peer reviewed publications in the fields of cancer, infectious disease, and inflammation research.
Senior management
Thomas Taapken, Chief Financial Officer. Dr. Taapken joined lnflaRx as CFO in 2020. He has over 25 years of experience in senior management positions within the life sciences sector and as a venture investor. He has previously held positions as CFO of Medigene AG (publicly listed in Germany), as CEO and CFO of Epigenomics AG (publicly listed in Germany), where he led the company’s efforts in gaining regulatory approval for the company’s lead product with the FDA and oversaw its subsequent introduction into the US market, and as CFO at Biotie Therapies (publicly listed in Finland, now Acorda Therapeutics) and its predecessor companies. Before that he was a venture investor for seven years with Deutsche Venture Capital (DVC) and Burrill & Co. in the US. Dr. Taapken started his career at Hoechst AG (now Sanofi). He holds a Ph.D. in organic chemistry from the Technical University of Berlin and also studied economics, chemistry and physics at the University of Göttingen. Dr. Taapken is a Board member of Scibase AB since 2017, he is Chairman of the Board at lmcyse SA since 2019 and Board member at memo therapeutics AG since 2021 until the end of 2023.
Camilla Chong, Chief Medical Officer. Dr. Camilla Chong joined as Chief Medical Officer, or CMO, in July 2023. She is a medical doctor with 25 years of experience in the global pharmaceutical industry. She has successfully led teams in clinical development, medical affairs, clinical operations, regulatory and pharmacovigilance. Her extensive experience also includes the development and launch of new medicines across different geographies in senior leadership roles at Kyowa Kirin, GlaxoSmithKline, Pfizer and Teva. Dr. Chong received her medical degree from the Royal Free Hospital School of Medicine, University College London, UK. She holds a Diploma in Pharmaceutical Medicine and was a member of the Faculty of Pharmaceutical Medicine (MFPM).
Derval O’Carroll, Senior Vice President Regulatory Affairs and Quality Assurance. Ms. O’Carroll joined InflaRx as VP, Head of Regulatory Affairs in 2022. She has over 30 years of experience in Regulatory Affairs with the last 19 years in senior management positions contributing strategically to global product development and commercialization activities. She has previously held positions as VP, Global Regulatory and Quality at rare disease company Amryt Pharmaceuticals for five years, Senior Director of Regulatory Affairs at rare disease company Travere for two years and Managing Consultant at Regulatory Consultancy, Real Regulatory for 11 years, where she worked on numerous drug development programs for international clients. She gained prior experience in a number of roles which included drug, device and IVD products. Ms. O’Carroll has a proven track record of leading global registration activities for innovative new products and is experienced in guiding teams through the FDA and EMA regulatory agency interactions for pre-authorization, authorization and complex post marketing commitments. She holds an M.Sc in Biochemistry and an M.B.A from University College, Dublin.
-114-
| B. | Compensation |
Compensation of directors and senior management
The aggregate compensation, including benefits in kind, accrued or paid to our senior management, or Senior Management, with respect to the year ended December 31, 2023, for services in all capacities amounted to €5,291,128. In 2023, we granted options to purchase 1,263,500 ordinary shares to our board of directors, or Board of Directors, and our Senior Management.
The aggregate compensation, including benefits in kind, accrued or paid to our Non-executive Directors with respect to the year ended December 31, 2023, for services in all capacities amounted to €606,677. In 2023 we granted options to purchase 157,500 ordinary shares to our Non-executive Directors.
We refer to Note C.10. Share based payments of Directors, Senior Management and Non-executive Directors.
| Stock options outstanding as of January 1, 2023 | Stock options granted in 2023 | Stock options exercised in 2023 | Stock options outstanding as of December 31, 2023 | weighted average exercise price in € | weighted average remaining contractual life | |||||||||||||||||||
| Stock options not exercised 2023 | ||||||||||||||||||||||||
| Senior Management including executive directors | ||||||||||||||||||||||||
| Prof. Niels C. Riedemann, CEO | 2,289,714 | 548,000 | 2,837,714 | 1.75 | 6.24 | |||||||||||||||||||
| Prof. Renfeng Guo, CSO | 1,814,197 | 430,500 | 2,244,697 | 1.82 | 6.39 | |||||||||||||||||||
| Thomas Taapken, CFO | 397,002 | 110,000 | 507,002 | 1.88 | 7,24 | |||||||||||||||||||
| Camilla Chong, CMO | 0 | 150,000 | 150,000 | 3.60 | 9.52 | |||||||||||||||||||
| Derval O’Carroll, Senior Vice President, Global Head of Regulatory Affairs and Compliance | 20,000 | 25,000 | 45,000 | 2.22 | 9.00 | |||||||||||||||||||
| 4,520,913 | 1,263,500 | 5,784,413 | ||||||||||||||||||||||
| Non-executive Directors | ||||||||||||||||||||||||
| Nicolas Fulpius, Chairman, and Member of the Audit Committee | 119,065 | 45,000 | 164,065 | 1.85 | 6.82 | |||||||||||||||||||
| Anthony Gibney, Chairman of the Audit Committee | 58,085 | 30,000 | 88,085 | 1.88 | 7.50 | |||||||||||||||||||
| Richard Brudnick, Member of the Audit Committee | 74,850 | 30,000 | 104,850 | 1.85 | 7.24 | |||||||||||||||||||
| Mark Kübler, Member of the Audit Committee | 98,172 | 30,000 | 128,172 | 1.73 | 6.0 | |||||||||||||||||||
| Hege Hellstrom (from April 2023) | - | 22,500 | 22,500 | 3.87 | 9.4 | |||||||||||||||||||
| 4,871,085 | 1.398,500 | 6,292,085 | ||||||||||||||||||||||
We established a policy in respect of the remuneration of our directors in accordance with Dutch law. Such policy addresses the following topics: the fixed and variable components of the remuneration (if any), remuneration in the form of shares and severance payments. The policy for the Board of Directors was adopted and approved by the general meeting of shareholders prior to the consummation of our initial public offering. The Board of Directors determines the remuneration of the directors in accordance with the compensation policy, with the understanding that executive directors will not participate in the decision-making process regarding the determination of the compensation of executive directors. Compensation schemes in the form of shares or rights to shares must be submitted by the Board of Directors to the general meeting for its approval. Any such proposal must set out at least the maximum number of shares or rights to shares to be granted to the directors and the criteria for granting or amendment.
As of December 31, 2023, we have no amounts set aside or accrued to provide pension, retirement or similar benefits to our senior managers or directors.
-115-
Clawback policy
We have implemented a robust clawback policy to ensure accountability and alignment of executive compensation with long-term sustainable financial performance. Under this policy, if our financial statements are restated due to material noncompliance with financial reporting standards or as a result of fraudulent activities, the Board of Directors has the right to recover any incentive-based compensation that was awarded to executive officers in excess of what they would have received under the restated financial results. The clawback provisions apply to performance-based bonuses, equity-based compensation, and any other compensation that is tied to achieving certain financial or non-financial targets. The policy is designed to discourage excessive risk-taking or dishonest behavior that could harm the long-term interests of the company and its shareholders. The clawback policy specifies the procedures, timelines, and criteria for determining when a clawback may be initiated, as well as the process for calculating and recovering the amounts. The Board of Directors, in consultation with the Compensation Committee, will review each individual case and make determinations based on the specific circumstances.
The clawback policy is disclosed in our Corporate Governance Principles and is subject to oversight by the Compensation Committee and the Board of Directors. We believe that the implementation of this policy reflects our commitment to maintaining strong corporate governance practices and enhancing shareholder value.
Management and director service agreements
We entered into management services agreements with each of our executive management team members, including our two executive directors that became effective upon the consummation of our initial public offering or at the time these managers joined the Company. The management services agreements contain a termination notice period for us and the executive directors appointed as such by a general meeting of shareholders. All of the management services agreements provide that the manager or executive director, as the case might be, may be terminated in the event of an urgent cause (dringende reden) without advance notice. In the event that an executive director no longer serves as an executive director but remains employed in his role as an executive employee of the Company, the executive director will not be entitled to any contractual severance or termination payments. Rather, we will enter into an employment agreement with the executive director, which may include substantially similar compensation terms as provided under the management services agreements. The management services agreements contain post-termination restrictive covenants, including perpetual confidentiality, and post-termination non-competition and non-solicitation covenants.
In addition, we entered into letter agreements with each of our non-executive directors which became effective upon the consummation of our initial public offering or at the time these directors were appointed to our board by a general meeting of shareholders. The letter agreements may be terminated, without advance notice, if the non-executive director is removed from the Board of Directors, resigns from the Board of Directors or such director’s term of office on the Board of Directors expires without his reappointment as a non-executive director. Additionally, each letter agreement provides for compensation, including an annual cash fee, an annual equity grant, an annual fee for membership on a committee of the Board of Directors, and an annual fee for acting as a chairperson of a committee of the Board of Directors. Also, the letter agreements contain a perpetual confidentiality covenant.
Share based compensation plans
2016 Plan
Under the Stock Option Plan 2016 Terms and Conditions, or the 2016 Plan, we granted rights to subscribe for our ordinary shares to directors, senior management and key employees.
All outstanding option awards under the 2016 Plan automatically vested upon closing of our initial public offering.
In conjunction with the corporate reorganization undertaken prior to our initial public offering, all outstanding awards granted under the 2016 Plan or otherwise converted into awards exercisable for ordinary shares of InflaRx N.V. will be governed by the terms of the 2016 Plan.
-116-
2017 Plan
In conjunction with the closing of our initial public offering, we established a new omnibus plan, the 2017 Long Term Incentive Plan, or the 2017 Plan, with the purpose of advancing the interests of our shareholders by enhancing our ability to attract, retain and motivate individuals who are expected to make important contributions to us. The 2017 Plan governs issuances of equity incentive awards from and after the closing of our initial public offering. The initial maximum number of ordinary shares available for issuance under equity incentive awards granted pursuant to the 2017 Plan initially equaled 2,341,097 ordinary shares. On January 1, 2021 and on January 1 of each calendar year thereafter, an additional number of shares equal to 4% of the total outstanding ordinary shares on December 31 of the immediately preceding year (or any lower number of shares as determined by the Board of Directors) will become available for issuance under equity incentive awards granted pursuant to the 2017 Plan.
The annual general meeting on July 16, 2020, approved an amendment to the 2017 Plan with effect from January 1, 2021:
| ● | increasing the maximum annual number of ordinary shares in the Company’s capital available for issuance under the 2017 Plan, starting on January 1, 2021, to 4% (from 3%) of the Company’s outstanding ordinary shares (determined as of December 31 of the immediately preceding year); and |
| ● | removing certain restrictions from the 2017 Plan, which will allow the committee administering the 2017 Plan and the Board to (i) lower the exercise price per share of any options and/or share appreciation rights issued under the 2017 Plan or take any other action treated as a ‘repricing’ of an award and (ii) cancel any option and/or share appreciation rights in exchange for cash or another award granted under the 2017 Plan, in either case, without prior approval of the Company’s shareholders. |
Plan Administration. The 2017 Plan is administered by a long-term incentive, or LTI, committee appointed by the Board of Directors, which consists of not less than three directors.
Eligibility. Equity incentive awards may be granted to our employees, non-employee directors, consultants or other advisors, as well as holders of equity compensation awards granted by a company that may be acquired by us in the future.
Awards. Equity incentive awards under the 2017 Plan may be granted in the form of stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards or other share-based awards. Stock options and stock appreciation rights will have an exercise price determined by the plan committee but that is no less than fair market value of the underlying ordinary shares on the date of grant.
Vesting. The vesting conditions for grants under the equity incentive awards under the 2017 Plan will be set forth in the applicable award documentation. However, subject to the acceleration provisions under certain circumstances described below, awards (other than replacement awards) may not vest in full prior to the first anniversary of the grant date, with the exception that up to 5% of the shares available for issuance under the 2017 Plan may provide for alternative vesting conditions.
Termination of Service and Change in Control. In the event of a participant’s termination of employment, the plan committee may, in its discretion, determine the extent to which an equity incentive award may be exercised, settled, vested, paid or forfeited. In the event of a change in control of the company (as defined in the 2017 Plan), any then successor or surviving corporation may continue outstanding awards, or convert or substitute such awards for award or right with respect to the stock of the successor or surviving corporation, in which case, if a participant is terminated by the successor or surviving corporation without “cause” or for “good reason” (in each case, as defined in the 2017 Plan) within 24 months following the change in control, all equity incentive awards held by the participant will immediately vest. If any outstanding awards are not continued or converted following a change in control of the company, then such awards will immediately vest, and options and stock appreciation rights will become fully exercisable. In connection with a change of control, the plan committee may, in its discretion, take a number of other actions, including accelerating the vesting of any equity incentive award or terminating or cancelling any equity incentive award for cash payment.
-117-
Insurance and indemnification
Our current and future directors (and such other officer or employee as designated by the Board of Directors) have the benefit of indemnification provisions in our Articles of Association. These provisions give the indemnified persons the right to recover from us amounts, including litigation expenses, and any damages they are ordered to pay, in relation to acts or omissions in the performance of their duties. However, there is no entitlement to indemnification for acts or omissions which are considered to constitute malice, gross negligence, intentional recklessness and/or serious culpability attributable to such indemnified person. In addition, upon the closing of our initial public offering, we entered into agreements with our directors and executive officers to indemnify them against expenses and liabilities to the fullest extent permitted by law. These agreements also provide, subject to certain exceptions, for indemnification for related expenses, including attorneys’ fees, judgments, penalties, fines and settlement amounts incurred by any of these individuals in any action or proceeding. In addition to such indemnification, we provide our directors with directors’ and officers’ liability insurance.
Insofar as indemnification of liabilities arising under the Securities Act may be permitted to directors or persons controlling us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Compliance with Nasdaq listing requirements
We are a foreign private issuer. As a result, in accordance with Nasdaq listing requirements, we comply with certain home country governance requirements rather than complying with certain Nasdaq corporate governance requirements. In accordance with Dutch law and generally accepted business practices, our Articles of Association do not provide quorum requirements generally applicable to general meetings of shareholders in the United States. To this extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. Although we must provide shareholders with an agenda and other relevant documents for the general meeting of shareholders, Dutch law does not have a regulatory regime for the solicitation of proxies and the solicitation of proxies is not a generally accepted business practice in the Netherlands, and thus our practice will vary from the requirement of Nasdaq Listing Rule 5620(b). As permitted by the listing requirements of Nasdaq, we also opted out of the requirements of Nasdaq Listing Rule 5605(d), which requires an issuer to have a compensation committee that, among other things, consists entirely of independent directors and makes determinations regarding the independence of any compensation consultants, Nasdaq Listing Rule 5605(e), which requires an issuer to have independent director oversight of director nominations, and Nasdaq Listing Rule 5605(b)(2), which requires an issuer to have a majority of independent directors on its board. In addition, we opted out of shareholder approval requirements for the issuance of securities in connection with certain events such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees and certain private placements. To this extent, our practice varies from the requirements of Nasdaq Listing Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. For an overview of our corporate governance principles, see “ITEM 10. ADDITIONAL INFORMATION — 2. Memorandum and articles of association.”
| C. | Board practices |
Board of Directors
The Board of Directors was composed of six members until the annual general meeting held on April 26, 2023, and seven members from such date throughout the remaining period under review, two of whom are executive directors. Our executive directors and the chairman of our board shall initially serve for four-year terms and our other non-executive directors shall initially serve for three-year terms, in each case until the earlier of their successors being duly appointed, their resignation or their removal. After these terms, our directors may be nominated for re-appointment for such terms as may be deemed appropriate by the Board of Directors. For the years of the directors’ initial appointment and term expiration dates, see ‘A. Directors and senior management.’
-118-
Nasdaq’s board diversity rule
Nasdaq’s Board Diversity Rule, which was approved by the SEC on August 6, 2021, is a disclosure standard designed to encourage minimum board diversity for companies and provide stakeholders with consistent, comparable disclosures concerning a company’s current board composition. Nasdaq’s Board Diversity Rule requires companies listed on Nasdaq to publicly disclose board-level diversity statistics using a standardized template.
Board Diversity Matrix (As of March 20, 2024) To be completed by Foreign Issuers (with principal executive offices outside of the U.S.) and Foreign Private Issuers | ||||
| Country of Principal Executive Offices | Germany | |||
| Foreign Private Issuer | Yes | |||
| Disclosure Prohibited Under Home Country Law | No | |||
| Total Number of Directors | 7 | |||
| Female | Male | Non-Binary | Did Not Disclose Gender | |
| Part I: Gender Identity | ||||
| Directors | 1 | 6 | 0 | 0 |
| Part II: Demographic Background | ||||
| Underrepresented Individual in Home Country Jurisdiction | 1 | |||
| LGBTQ+ | 0 | |||
| Did Not Disclose Demographic Background | 6 | |||
The Board of Directors adopted a Diversity Policy in December 2021 as amended to become a Diversity & Inclusion Policy in October 2023, which is published on the Company’s website. This policy sets out diversity and inclusion aspects including our targets relating to diversity in the composition of the Board of Directors. We believe that diversity encompasses acceptance and respect, recognizing that each individual is unique. We are committed to supporting, valuing and leveraging diversity throughout the Company and in the composition of the Board of Directors.
Board committees
Audit committee
The audit committee currently consists of Mr. Richard Brudnick, Mr. Nicolas Fulpius, Mr. Anthony Gibney, and Mr. Mark Kuebler. Mr. Anthony Gibney is the audit committee’s chair. The audit committee assists the Board of Directors in overseeing our accounting and financial reporting processes and the audits of our financial statements. In addition, the audit committee is directly responsible for the recommendation for appointment, compensation, retention and oversight of the work of our independent registered public accounting firm. The Board of Directors has determined that each member of the audit committee satisfies the “independence” requirements set forth in Rule 10A-3 under the Exchange Act and each qualifies as an “audit committee financial expert,” as such term is defined in the rules of the SEC. The audit committee is governed by a charter that complies with applicable Nasdaq rules, which charter has been posted on our website.
The audit committee’s responsibilities include:
| ● | recommending the appointment of the independent auditor to the general meeting of shareholders; |
| ● | the appointment, compensation, retention and oversight of any accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit services; |
| ● | pre-approving the audit services and non-audit services to be provided by our independent auditor before the auditor is engaged to render such services; |
| ● | evaluating the independent auditor’s qualifications, performance and independence, and presenting its conclusions to the full supervisory board on at least an annual basis; |
| ● | reviewing and discussing with the Board of Directors and the independent auditor the audit plan as well as our annual audited financial statements and quarterly financial statements prior to the filing of the respective annual and quarterly reports; |
-119-
| ● | overseeing the effectiveness and integrity of the internal audit function, ensuring its independence, objectivity, and adherence to established policies and procedures; |
| ● | reviewing our compliance with laws and regulations, including major legal and regulatory initiatives and also reviewing any major litigation or investigations against us that may have a material impact on our financial statements; |
| ● | reviewing internal audit results, including the effectiveness of the design and operation of our internal controls; |
| ● | reviewing the operation of and our compliance with our code of ethics; |
| ● | reviewing the operation of our compliance with our investment policy regulating all cash investment decisions regarding the investment of available cash amounts and the maintenance of the cash investment portfolio of all Company’s entities including all currency exchange transactions; |
| ● | reviewing the operation of our risk management system; and |
| ● | approving or ratifying any related person transaction (as defined in our related person transaction policy) in accordance with our related person transaction policy and reviewing potential conflicts of interest involving our directors. |
The audit committee meets as often as one or more members of the audit committee deem necessary, but in any event meets at least quarterly. The audit committee meets at least once per year with our independent accountant without our executive directors being present.
Compensation committee
The compensation committee consists of Mr. Richard Brudnick, Mr. Nicolas Fulpius and Mr. Mark Kubler. Mr. Nicolas Fulpius is the compensation committee’s chair. The compensation committee assists the Board of Directors in determining compensation for the directors. The committee recommends to the Board of Directors for determination the compensation of each of our directors. Under SEC and Nasdaq rules, there are heightened independence standards for members of the compensation committee, including a prohibition against the receipt of any compensation from us other than standard director fees. As permitted by the listing requirements of Nasdaq, we opted out of Nasdaq Listing Rule 5605(d), which requires that a compensation committee consist entirely of independent directors. The compensation committee is governed by a charter that has been posted on our website.
The compensation committee’s responsibilities include:
| ● | identifying, reviewing and approving corporate goals and objectives relevant to compensation of our executive officers and directors; |
| ● | analyzing the possible outcomes of the variable remuneration components and how they may affect the remuneration of our executive officers; |
| ● | determining any long-term incentive component of each executive officer’s compensation in line with the compensation policy and reviewing our executive officer compensation and benefits policies generally; |
| ● | preparing periodic compensation reports for the Board of Directors; |
| ● | reviewing and assessing risks arising from our employee compensation policies and practices and whether any such risks are reasonably likely to have a material adverse effect on us; and |
| ● | retaining or obtaining advice from a compensation consultant, legal counsel or other advisor as the compensation committee deems necessary or appropriate to carry out its responsibilities. |
-120-
Nomination and corporate governance committee
The nomination and corporate governance committee consists of Mr. Nicolas Fulpius, Ms. Hege Hellstrom and Mr. Mark Kubler. Mr. Nicolas Fulpius is the nomination and corporate governance committee’s chair. The nomination and corporate governance committee assists the Board of Directors in identifying individuals qualified to become members of the Board of Directors consistent with criteria established by the Board of Directors and in developing our corporate governance principles. As permitted by the listing requirements of Nasdaq, we opted out of Nasdaq Listing Rule 5605(e), which requires independent director oversight of director nominations. The nominating and corporate governance committee is governed by a charter that has been posted on our website.
The nomination and corporate governance committee’s responsibilities include:
| ● | preparing and reviewing selection criteria and appointment procedures for the Board of Directors; |
| ● | reviewing the size and composition of the Board of Directors by taking into account any diversity and inclusion criteria and submitting proposals for the composition profile of the Board of Directors; |
| ● | leading the Board of Directors in self-evaluation to determine whether it and its committees are functioning effectively; |
| ● | preparing and reviewing a plan for succession of directors by taking into account any diversity and inclusion criteria; and |
| ● | submitting proposals for the appointment or reappointment of directors. |
| D. | Employees |
As of December 31, 2023, we had 66 employees, including 22 with M.D. or Ph.D. degrees. As of December 31, 2022, we had 48 employees, including 18 with M.D. or Ph.D. degrees. As of December 31, 2021, we had 59 employees, including 18 with M.D. or Ph.D. degrees.
| E. | Share ownership |
See “ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS —1. Major shareholders.”
| F. | Disclosure of a registrant’s action to recover erroneously awarded compensation |
As of December 31, 2023, we did not have an accounting restatement that required recovery of erroneously awarded incentive-based compensation pursuant to our Clawback Policy. As of as of December 31, 2023, there were no outstanding balances of erroneously awarded incentive-based compensation to be recovered from the application of the policy to a prior restatement. Our Clawback Policy is included as Exhibit 97.1 to this Annual Report.
-121-
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
| A. | Major shareholders |
The following table presents information relating to the beneficial ownership of our ordinary shares as of December 31, 2023:
| ● | each person, or group of affiliated persons, known by us to own beneficially 5% or more of our outstanding ordinary shares (as of the date of such shareholder’s Schedule 13G filing for InflaRx N.V. with the SEC); |
| ● | each of our directors and senior management; and |
| ● | all directors and senior management as a group. |
The number of ordinary shares beneficially owned by each entity, person or director is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any ordinary shares over which the individual has sole or shared voting power or investment power or to receive the economic benefit of ownership of the shares, as well as any ordinary shares that the individual has the right to acquire within 60 days of December 31, 2023 through the exercise of any option, warrant or other right. The percentage of shares beneficially owned is computed on the basis of 58,883,272 ordinary shares outstanding as of December 31, 2023. Ordinary shares that a person has the right to acquire within 60 days of December 31, 2023 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and senior management as a group. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all ordinary shares held by that person. All shareholders have similar voting rights. As of December 31, 2023, 16,818,972 ordinary shares, representing approximately 28.6% of our issued and outstanding ordinary shares, were held by 20 U.S. record holders.
This table is based upon information supplied by our named Senior Management, directors, and principal shareholder, and Schedules 13D and 13G filed with the SEC. The percentage of outstanding ordinary shares is computed on the basis of 58,883,272 ordinary shares outstanding as of December 31, 2023. Ordinary shares that a person has the right to acquire within 60 days of December 31, 2023 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person.
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o InflaRx N.V., Winzerlaer Str. 2, 07745 Jena, Germany.
| Ordinary
Shares Beneficially Owned | ||||||||
| Number | Percent of | |||||||
| 5% Shareholders | ||||||||
| Entities affiliated with Suvretta Capital Management LLC(1) | 5,733,910 | 9.7 | % | |||||
| Directors and senior management | ||||||||
| Niels Riedemann(2) | 3,851,622 | 6.1 | % | |||||
| Renfeng Guo(3) | 3,951,841 | 6.3 | % | |||||
| Thomas Taapken(4) | 455,502 | * | ||||||
| Camilla Chong, officer from July 2023 | 0 | 0 | % | |||||
| Derval O’Carroll(5) | 25,000 | * | ||||||
| Nicolas Fulpius(6) | 631,986 | 1.1 | % | |||||
| Richard Brudnick(7) | 154,850 | * | ||||||
| Mark Kubler(8) | 1,088,187 | 1.8 | % | |||||
| Anthony Gibney(9) | 98,085 | * | ||||||
| Hege Hellstrom, director from April 2023 | 0 | 0 | % | |||||
| All directors and senior management as a group (10 persons) | 10,257,073 | 14.8 | % | |||||
| * | Indicates beneficial ownership of less than 1% of the total outstanding ordinary shares. |
| (1) | As per filing on Schedule 13G as of April 12, 2023, Aaron Cowen has beneficial ownership by virtue of his role as a control person of Suvretta Capital Management LLC. The address of Suvretta Capital Management LLC is 540 Madison Avenue, 7th Floor, New York, New York 10022. |
-122-
| (2) | Consists of (a) 1,068,908 ordinary shares, (b) 404,040 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2016 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on November 18, 2031, (c) 126,005 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the Series B financing at an exercise price of €0.0012 per share, (d) 689,253 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on December 13, 2025, (e) 5,409 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on November 20, 2026, (f) 350,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $5.14 per share), which shall expire on January 4, 2031, (g) 112,007 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $2.99 per share), which shall expire on July 1, 2031, (h) 548,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.13 per share), which shall expire on January 11, 2032, and (i) 548,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
| (3) | Consists of (a) 1,762,144 ordinary shares, (b) 336,672 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2016 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on November 18, 2031, (c) 623,610 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on December 13, 2025, (d) 5,409 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on November 20, 2026, (e) 275,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $5.14 per share), which shall expire on January 4, 2031, (f) 88,006 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $2.99 per share), which shall expire on July 1, 2031, (g) 430,500 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.13 per share), which shall expire on January 11, 2032, and (h) 430,500 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
| (4) | Consists of (a) 3,500 ordinary shares, (b) 150,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.83 per share), which shall expire on September 17, 2028, (c) 50,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $5.14 per share), which shall expire on January 4, 2031, (d) 32,002 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $2.99 per share), which shall expire on July 1, 2031, (e) 110,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.13 per share), which shall expire on January 11, 2032, and (f) 110,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
| (5) | Consists of 25,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
| (6) | Consists of (a) 467,921 ordinary shares, (b) 34,464 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on December 13, 2025, (c) 30,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $5.14 per share), which shall expire on January 4, 2031, (d) 9,601 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $2.99 per share), which shall expire on July 1, 2031, (e) 45,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.13 per share), which shall expire on January 11, 2032, and (f) 45,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
-123-
| (7) | Consists of (a) 50,000 ordinary shares, (b) 18,450 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on February 4, 2027, (c) 20,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $5.14 per share), which shall expire on January 4, 2031, (d) 6,400 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $2.99 per share), which shall expire on July 1, 2031, (e) 30,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.13 per share), which shall expire on January 11, 2032, and (f) 30,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
| (8) | Consists of (a) 960,015 ordinary shares, (b) 7,308 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the Series B financing at an exercise price of €0.0012 per share, (c) 34,464 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $3.35 per share), which shall expire on December 13, 2025, (d) 20,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $5.14 per share), which shall expire on January 4, 2031, (e) 6,400 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $2.99 per share), which shall expire on July 1, 2031, (f) 30,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.13 per share), which shall expire on January 11, 2032, and (g) 30,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
| (9) | Consists of (a) 10,000 ordinary shares, (b) 11,667 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $22.75 per share), which shall expire on February 7, 2026, (c) 16,418 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $2.99) per share, which shall expire on July 1, 2031, (d) 30,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $1.86 per share (after re-pricing on April 13, 2022 from $4.13 per share), which shall expire on January 11, 2032, and (e) 30,000 ordinary shares that may be acquired pursuant to the exercise of options which were issued pursuant to the 2017 Plan at an exercise price of $2.37 per share, which shall expire on January 24, 2033. |
-124-
| 1. | Significant changes in ownership by major shareholders |
On July 8, 2020, we filed the 2020-Shelf Registration Statement. We also filed with the SEC a prospectus supplement relating to an at-the-market program providing for the sales of our stock over time of up to $50.0 million of our ordinary shares pursuant to a Sales Agreement with SVB Leerink LLC.
As of December 31, 2023, we had issued a total of 5,803,931 ordinary shares throughout the term of the at-the-market program, resulting in a total of €26.2 million in net proceeds to us, of which 3,235,723 ordinary shares, resulting in €14.4 million in net proceeds to us were issued during fiscal year 2023. The term of the at-the-market program expired on July 8, 2023.
In April 2023 we sold and issued an aggregate of 10,823,529 ordinary shares in an underwritten public offering, of which 1,411,764 were sold pursuant to exercise by the underwriters of an overallotment option. The ordinary shares were sold at a price of $4.25 per share and have a nominal value of €0.12 per share. Proceeds of this offering after deducting underwriting discounts amounted to €39.1 million.
On June 30, 2023, we filed a Form F-3, or the 2023-Registration Statement, with the SEC with respect to the offer and sale of securities of the Company, which became effective on July 11, 2023. The aggregate initial offering price of the securities that the Company may offer and sell under this prospectus will not exceed $250 million. No ordinary shares were issued by the Company under the 2023-Registration Statement within the fiscal year 2023 and no active offering programs have been launched.
During the fiscal year 2023, we issued and registered a total of 120,257 ordinary shares resulting from the exercise of stock option rights by former employees. All stock option rights had been granted under the 2017 Plan. 14,930 stock options thereof were already exercised in December 2022 and 105,327 were exercised during fiscal year 2023. The ordinary shares have a nominal value of €0.12 per share. 98,754 ordinary shares were sold at a price of $1.85 per share, and 21,503 ordinary shares were sold at a price of $3.35 per share.
Suvretta Capital Management LLC and affiliated entities filed a Schedule 13G as of December 31, 2023, stating a shareholding of 5,733,910 shares resulting in a holding of 9.7% in the Company.
| B. | Related party transactions |
The following is a description of related party transactions we have entered since January 1, 2023 with any of our officers, directors and the holders of more than 5% of our ordinary shares:
| 1. | Indemnification agreements |
We entered into indemnification agreements with our directors and senior management. The indemnification agreements and our Articles of Association require us to indemnify our directors to the fullest extent permitted by law. See “ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES — 2. Compensation — Insurance and indemnification” for a description of these indemnification agreements.
| 2. | Agreements with Staidson and its affiliates |
On December 21, 2022, InflaRx GmbH and Staidson (Beijing) BioPharmaceuticals Co., Ltd., or Staidson, entered into a third addendum to the Co-Development Agreement, dated as of December 28, 2015 (Co-Development Addendum). Pursuant to the terms of the Co-Development Addendum, InflaRx GmbH will receive royalties of 10% on net sales of BDB-001 (as defined in the Co-Development Addendum) for COVID-19 in China. InflaRx GmbH has granted Staidson an exclusive license for use in China to certain of InflaRx GmbH’s clinical, manufacturing and regulatory documentation regarding vilobelimab in order to support and facilitate the regulatory filing for BDB-001 for the treatment of severely ill COVID-19 patients with the Chinese National Medical Products Administration.
In connection with the Co-Development Addendum, on December 21, 2022, the Company and Staidson Hong Kong Investment Company Ltd. entered into the Purchase Agreement. Pursuant to the Purchase Agreement, the Company sold 500,000 ordinary shares with a nominal value of €0.12 per share, to Staidson Hong Kong Investment Company Ltd. at a price of $5.00 per share, and at an aggregate purchase price of $2,500,000. Under the terms of the Purchase Agreement, at the Company’s option, Staidson Hong Kong Investment Company Ltd. may purchase additional shares for an aggregate purchase price of $7,500,000, which is subject to certain conditions. ordinary shares.
| 3. | Interests of experts and counsel |
Not applicable.
-125-
ITEM 8. FINANCIAL INFORMATION
| A. | Consolidated statements and other financial information |
| 1. | Financial statements |
See “ITEM 18. FINANCIAL STATEMENTS,” which contains our audited financial statements prepared in accordance with IFRS-IASB.
| 2. | Legal proceedings |
From time to time we are involved in legal proceedings that arise in the ordinary course of business. We believe that the outcome of these proceedings, if determined adversely, will not have a material adverse effect on our financial position. During the period covered by the audited and approved financial statements contained herein, we have not been a party to or paid any damages in connection with litigation that has had a material adverse effect on our financial position. Any future litigation may result in substantial costs and be a distraction to management and our employees. No assurance can be given that future litigation will not have a material adverse effect on our financial position. For an additional discussion of certain risks associated with legal proceedings, see “ITEM 3. KEY INFORMATION — 3. Risk factors.”
| 3. | Dividends and dividend policy |
We have never paid or declared any cash dividends on our ordinary shares, and we do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business. Under Dutch law, we may only pay dividends to the extent our shareholders’ equity (eigen vermogen) exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law or by our Articles of Association. Subject to such restrictions, any future determination to pay dividends will be at the discretion of the Board of Directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors the Board of Directors deems relevant.
| B. | Significant changes |
A discussion of the significant changes in our business can be found under “ITEM 4. INFORMATION ON THE COMPANY — 1. History and development of the company.”
-126-
ITEM 9. THE OFFER AND LISTING
| A. | Offering and listing details |
Not applicable.
| B. | Plan of distribution |
Not applicable.
| C. | Markets |
Our ordinary shares began trading on the Nasdaq Global Select Market under the symbol “IFRX” on November 8, 2017.
| D. | Selling shareholders |
Not applicable.
| E. | Dilution |
Not applicable.
| F. | Expenses of the issue |
Not applicable.
-127-
ITEM 10. ADDITIONAL INFORMATION
| A. | Share capital |
Not applicable.
| B. | Memorandum and articles of association |
Our shareholders adopted the Articles of Association included as Exhibit 1.1. to the Annual Report on Form 20-F for the year ended December 31, 2022 filed with the SEC on March 22, 2023.
We incorporate by reference into this Annual Report the description of our Articles of Association contained in our F-3 registration statement (File No. 333-273058) originally filed with the SEC on June 30, 2023, and effective as of July 11, 2023, as amended. Such description sets forth a summary of certain provisions of our Articles of Association as currently in effect.
The Company’s Articles of Association in effect for the period under review was adopted by the annual general meeting on August 25, 2021 and is available on our website.
| C. | Material contracts |
Except as otherwise disclosed in this Annual Report (including the Exhibits), we are not currently, and have not been in the last two years, party to any material contract, other than contracts entered into in the ordinary course of business.
| D. | Exchange controls |
Not applicable.
| E. | Taxation |
The following summary contains a description of certain U.S. federal income, Dutch and German tax consequences of ownership and disposition of our ordinary shares. the summary is based upon the tax laws of the United States, the Netherlands and Germany, and regulations thereunder as of the date hereof, which are subject to change.
-128-
Material
U.S. Federal income Tax Considerations for
U.S. Holders of ORDINARY Shares
The following is a description of the material U.S. federal income tax consequences to the U.S. Holders, as defined below, of owning and disposing of ordinary shares. It does not set forth all tax considerations that may be relevant to a particular person’s decision to hold the ordinary shares.
This section applies only to a U.S. Holder that holds ordinary shares as capital assets for U.S. federal income tax purposes. In addition, it does not set forth all of the U.S. federal income tax consequences that may be relevant in light of the U.S. Holder’s particular circumstances, including alternative minimum tax consequences, the potential application of the provisions of the Code known as the Medicare contribution tax and tax consequences applicable to U.S. Holders subject to special rules, such as:
| ● | certain financial institutions; |
| ● | dealers or traders in securities who use a mark-to-market method of tax accounting; |
| ● | persons holding ordinary shares as part of a hedging transaction, straddle, wash sale, conversion transaction or other integrated transaction or persons entering into a constructive sale with respect to the ordinary shares; |
| ● | persons whose functional currency for U.S. federal income tax purposes is not the U.S. dollar; |
| ● | entities classified as partnerships for U.S. federal income tax purposes or other pass-through entities; |
| ● | tax-exempt entities, including an “individual retirement account” or “Roth IRA”; |
| ● | persons that own or are deemed to own 10% or more of our shares (by vote or value); |
| ● | persons that acquire our shares directly or indirectly in connection with the performance of services; |
| ● | persons who are subject to Section 451(b) of the Code; or |
| ● | persons holding ordinary shares in connection with a trade or business conducted outside of the United States. |
If an entity that is classified as a partnership for U.S. federal income tax purposes holds ordinary shares, the U.S. federal income tax treatment of a partner will depend on the status of the partner and the activities of the partnership. Partnerships holding ordinary shares and partners in such partnerships should consult their tax advisers as to the particular U.S. federal income tax consequences of owning and disposing of the ordinary shares.
This section is based on the Code, administrative pronouncements, judicial decisions, final, temporary and proposed Treasury regulations, and the income tax treaty between Germany and the United States and the income tax treaty between the Netherlands and the United States (as applicable and as the context requires the “Treaty”) all as of the date hereof, any of which is subject to change or differing interpretations, possibly with retroactive effect. No assurance can be given that the IRS will agree with the views expressed in this discussion, or that a court will not sustain any challenge by the IRS in the event of litigation. We have not obtained, nor do we intend to obtain, a ruling from the IRS with respect to the statements made and the conclusions reached in the following summary.
A “U.S. Holder” is a holder who, for U.S. federal income tax purposes, is a beneficial owner of ordinary shares, who is eligible for the benefits of the Treaty and who is:
| ● | a citizen or individual resident of the United States; |
| ● | a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia; |
-129-
| ● | an estate or trust the income of which is subject to U.S. federal income taxation regardless of its source: or |
| ● | a trust, if a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons are authorized to control all substantial decisions of the trust. |
U.S. Holders should consult their tax advisers concerning the U.S. federal, state, local and non-U.S. tax consequences of owning and disposing of ordinary shares in their particular circumstances. In particular, because our group includes a U.S. subsidiary, InflaRx Pharmaceuticals, Inc., and therefore under current law our subsidiary InflaRx GmbH is treated as a controlled foreign corporation (regardless of whether we are or are not treated as a controlled foreign corporation), any U.S. Holder that owns or is deemed to own 10% or more of our shares (by vote or value) is urged to consult its tax advisor regarding the potential application of the “Subpart F income” and “global intangible low-taxed income” rules to an investment in our ordinary shares.
| 1. | Taxation of distributions |
As discussed above under “ITEM 8. FINANCIAL INFORMATION — 1. Consolidated statements and other financial information — 1.3 Dividends and dividend policy,” we do not currently expect to make distributions on our ordinary shares. In the event that we do make distributions of cash or other property, subject to the PFIC rules described below, distributions paid on ordinary shares, other than certain pro rata distributions of ordinary shares, will be treated as dividends to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). For so long as we are treated as a PFIC with respect to a U.S. Holder (or were treated as a PFIC with the respect to the U.S. Holder in the preceding taxable year), dividends paid to certain non-corporate U.S. Holders will not be eligible for taxation as “qualified dividend income.” To the extent we are not treated as a PFIC with respect to a U.S. Holder and were not treated as a PFIC with the respect to the U.S. Holder in the preceding taxable year (if for example in future years we cease to meet the threshold requirements for PFIC status and the U.S. Holder initially acquires our ordinary shares in a year in which we are not treated as a PFIC and we are not so treated thereafter or we were a PFIC with respect to a U.S. Holder for a year during which a U.S. Holder holds ordinary shares but the U.S. Holder makes a valid deemed sale or deemed dividend election under the applicable Treasury regulations with respect to its ordinary shares), for so long as our ordinary shares are listed on Nasdaq or another established securities market in the United States or we are eligible for benefits under the Treaty, dividends paid to such a U.S. Holder that is not a corporation would generally be eligible for taxation as “qualified dividend income” if certain other requirements are met, which is generally taxable at rates not in excess of the long-term capital gain rate applicable to such U.S. Holders. The amount of a dividend will include any amounts withheld by us in respect of German or Dutch income taxes. Subject to the PFIC rules described below, the amount of the dividend will be treated as foreign-source dividend income to U.S. Holders and will not be eligible for the dividends-received deduction available to U.S. corporations under the Code and dividends will be included in a U.S. Holder’s income on the date of the U.S. Holder’s receipt of the dividend. The amount of any dividend income paid in euros will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of actual or constructive receipt, regardless of whether the payment is in fact converted into U.S. dollars at that time. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt. Generally, gain or loss, if any, resulting from currency exchange fluctuations during the period from the date the dividend is paid to the date such payment is converted into U.S. dollars will be treated as U.S. source ordinary income or loss.
Subject to applicable limitations, German or Dutch income taxes withheld from dividends on ordinary shares at a rate not exceeding the rate provided by the Treaty will be eligible for credit against the U.S. Holder’s U.S. federal income tax liability. German or Dutch taxes withheld in excess of the rate applicable under the Treaty will not be eligible for credit against a U.S. Holder’s federal income tax liability. The rules governing foreign tax credits are complex and U.S. Holders should consult their tax advisers regarding the creditability of foreign taxes in their particular circumstances. In lieu of claiming a foreign tax credit, U.S. Holders may deduct foreign taxes, including any German or Dutch income tax, in computing their taxable income, subject to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year. See “ITEM 3. KEY INFORMATION — 3. Risk factors — Risks related to our ordinary shares and our status as a public company if we ever pay dividends, we may need to withhold tax on such dividends payable to holders of our shares in both Germany and the Netherlands.”
| 2. | Sale or other disposition of ordinary shares |
Subject to the PFIC rules described below, gain or loss realized on the sale or other disposition of ordinary shares will be capital gain or loss, and will be long-term capital gain or loss if the U.S. Holder held the ordinary shares for more than one year. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the ordinary shares disposed of and the amount realized on the disposition, in each case as determined in U.S. dollars.
-130-
| 3. | PFIC rules |
We believe it is likely that we were a PFIC for U.S. federal income tax purposes in 2021, 2022 and 2023, and we may be a PFIC in one or more future taxable years. In addition, we may, now or in the future directly or indirectly, hold equity interests in other PFICs (any such PFIC, a “Lower-tier PFIC”). Under the Code, generally a non-U.S. corporation will be a PFIC for any taxable year in which, after the application of certain look-through rules with respect to subsidiaries, either (i) 75% or more of our gross income consists of passive income or (ii) 50% or more of the average quarterly value of our assets consists of assets that produce, or are held for the production of, “passive income.” For purposes of the above calculations, we will be treated as if we hold our proportionate share of the assets of, and receive directly our proportionate share of the income of, any other corporation in which we directly or indirectly own at least 25%, by value, of the shares of such corporation. Passive income includes, among other things, dividends, interest, certain non-active rents and royalties, and capital gains. It is also possible that we will be a PFIC in any future taxable year because, among other things, (i) we currently own a substantial amount of passive assets, including cash and securities that may give rise to passive income, (ii) the valuation of our assets that generate non-passive income for PFIC purposes, including our intangible assets, is uncertain and may vary substantially over time, and (iii) the composition of our income may vary substantially over time. Accordingly, there can be no assurance that we will not be a PFIC for any taxable year. If we are a PFIC for any year during which a U.S. Holder holds ordinary shares, we would continue to be treated as a PFIC with respect to that U.S. Holder for all succeeding years during which the U.S. Holder holds ordinary shares, even if we ceased to meet the threshold requirements for PFIC status, unless under certain circumstances the U.S. Holder makes a valid deemed sale or deemed dividend election under the applicable Treasury regulations with respect to its ordinary shares.
Under attribution rules, assuming we are a PFIC, U.S. Holders will be deemed to own their proportionate shares of any Lower-tier PFICs and will be subject to U.S. federal income tax according to the rules described in the following paragraphs on (i) certain distributions by a Lower-tier PFIC and (ii) a disposition of shares of a Lower-tier PFIC, in each case as if the U.S. Holder held such shares directly, even if the U.S. Holder has not received the proceeds of those distributions or dispositions.
If we were a PFIC for any taxable year during which a U.S. Holder held ordinary shares (assuming such U.S. Holder has not made a timely mark-to-market election, as described below), gain recognized by a U.S. Holder on a sale or other disposition (including certain pledges) of the ordinary shares, or an indirect disposition of shares of a Lower-tier PFIC, would be allocated ratably over the U.S. Holder’s holding period for the ordinary shares. The amounts allocated to the taxable year of the sale or other disposition and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge would be imposed on the amount allocated to that taxable year. Further, to the extent that any distribution received by a U.S. Holder on its ordinary shares (or a distribution by a Lower-tier PFIC to its shareholder that is deemed to be received by a U.S. Holder) exceeds 125% of the average of the annual distributions on the ordinary shares received during the preceding three years or the U.S. Holder’s holding period, whichever is shorter, that distribution would be subject to taxation in the same manner as gain, described immediately above.
A U.S. Holder can avoid certain of the adverse rules described above by making a mark-to-market election with respect to its ordinary shares, provided that the ordinary shares are “marketable.” Ordinary shares will be marketable if they are “regularly traded” on a “qualified exchange” or other market within the meaning of applicable Treasury regulations. Our ordinary shares will be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of the ordinary shares is traded on a qualified exchange on at least 15 days during each calendar quarter. Nasdaq, on which the ordinary shares are currently listed, is a qualified exchange for this purpose. If a U.S. Holder makes the mark-to-market election, it will recognize as ordinary income any excess of the fair market value of the ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. Holder makes the election, the U.S. Holder’s tax basis in the ordinary shares will be adjusted to reflect the income or loss amounts recognized. Any gain recognized on the sale or other disposition of ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). U.S. Holders should consult their tax advisers regarding the availability and advisability of making a mark-to-market election in their particular circumstances.
In addition, in order to avoid the application of the foregoing rules, a United States person that owns stock in a PFIC for U.S. federal income tax purposes may make an election to treat the PFIC and each PFIC in which the PFIC holds equity interests as a qualified electing fund (any such election, a QEF Election) with respect to each such PFIC if the PFIC provides the information necessary for such election(s) to be made. In order to make such an election, a United States person would be required to make the QEF Election for each PFIC by attaching a separate properly completed IRS Form 8621 for each PFIC to the United States person’s timely filed U.S. federal income tax return generally for the first taxable year that the entity is treated as a PFIC with respect to the United States person. A U.S. Holder generally may make a separate election to defer payment of taxes on the undistributed income inclusion under the QEF rules, but if deferred, any such taxes are subject to an interest charge.
-131-
If a United States person makes a QEF Election with respect to a PFIC, the United States person will be currently taxable on its pro rata share of the PFIC’s ordinary earnings and net capital gain (at ordinary income and capital gain rates, respectively) for each taxable year that the entity is classified as a PFIC and will not be required to include such amounts in income when actually distributed by the PFIC. If a U.S. Holder makes a QEF Election with respect to us, any distributions paid by us out of our earnings and profits that were previously included in the U.S. Holder’s income under the QEF Election will not be taxable to the U.S. Holder. A U.S. Holder will increase its tax basis in its ordinary shares by an amount equal to any income included under the QEF Election and will decrease its tax basis by any amount distributed, if any, on the ordinary shares that is not included in its income. In addition, a U.S. Holder will recognize capital gain or loss on the disposition of ordinary shares in an amount equal to the difference between the amount realized and its adjusted tax basis in the ordinary shares. U.S. Holders should note that if they make QEF Elections with respect to us and Lower-tier PFICs, if any, they may be required to pay U.S. federal income tax with respect to their ordinary shares for any taxable year significantly in excess of any cash distributions, if any, received on the shares for such taxable year. U.S. Holders should consult their tax advisers regarding making QEF Elections in their particular circumstances.
In addition, if we were a PFIC or, with respect to a particular U.S. Holder, were treated as a PFIC for the taxable year in which we paid a dividend or for the prior taxable year, the preferential dividend rates with respect to dividends paid to certain non-corporate U.S. Holders would not apply.
If a U.S. Holder owns ordinary shares during any year in which we are a PFIC, the U.S. Holder must file annual reports, containing such information as the U.S. Treasury may require on IRS Form 8621 (or any successor form) with respect to us, with the U.S. Holder’s federal income tax return for that year, unless otherwise specified in the instructions with respect to such form.
The U.S. federal income tax rules relating to PFICs are very complex. U.S. Holders are strongly urged to consult their tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of our ordinary shares, the consequences to them of an investment in a PFIC (and any Lower-tier PFICs), any elections available with respect to our ordinary shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of ordinary shares of a PFIC.
The IRS has finalized Treasury Regulations that address various issues related to determining whether a foreign corporation is a PFIC and whether a U.S. shareholder holds PFIC stock and released proposed Treasury Regulations that address various issues related to determining whether a foreign corporation is a PFIC. These Treasury Regulations and proposed Treasury Regulations (if finalized) may affect whether we are a PFIC in any future year. You should consult your tax adviser regarding the effect, if any, these Treasury Regulations may have, or such proposed Treasury Regulations would have, on the determination of our PFIC status.
| 4. | Information reporting and backup withholding |
Payments of dividends and sales proceeds that are made within the United States or through certain U.S.-related financial intermediaries are subject to information reporting, and may be subject to backup withholding, unless (i) the U.S. Holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding.
The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle it to a refund, provided that the required information is timely furnished to the IRS.
| 5. | Information reporting with respect to foreign financial assets |
Certain U.S. Holders who are individuals and certain entities may be required to report information relating to an interest in our ordinary shares, subject to certain exceptions (including an exception for ordinary shares held in accounts maintained by certain U.S. financial institutions). U.S. Holders should consult their tax advisers regarding whether or not they are obligated to report information relating to their ownership and disposition of the ordinary shares.
-132-
| 6. | Material Dutch tax considerations |
General
The following is a general summary of certain material Dutch tax consequences of the acquisition, holding and disposal of our ordinary shares. This summary does not purport to describe all possible tax considerations or consequences that may be relevant to a holder or prospective holder of ordinary shares and does not purport to deal with the tax consequences applicable to all categories of investors, some of which (such as trusts or similar arrangements) may be subject to special rules. In view of its general nature, this general summary should be treated with corresponding caution. To the extent this summary relates to legal conclusions under current Netherlands tax law, and subject to the qualifications it contains, it represents the opinion of NautaDutilh N.V., our special Dutch counsel. Holders or prospective holders of shares should consult with their own tax advisors with regard to the tax consequences of investing in the shares in their particular circumstances. The discussion below is included for general information purposes only.
For the purposes of this discussion, it is assumed that we are a tax resident of Germany under German national tax laws since we intended to have, from our incorporation and on a continuous basis, our place of effective management in Germany. See Risk Factor “We may become taxable in a jurisdiction other than Germany and this may increase the aggregate tax burden on us.”
Please note that this summary does not describe the Dutch tax considerations for:
| ● | holders of our ordinary shares if such holders, and in the case of individuals, his or her partner or certain of their relatives by blood or marriage in the direct line (including foster children), have a substantial interest (aanmerkelijk belang) or deemed substantial interest (fictief aanmerkelijk belang) in the Company under the Dutch Income Tax Act 2001 (Wet inkomstenbelasting 2001). Generally speaking, a holder of securities in a company is considered to hold a substantial interest in such company, if such holder alone or, in the case of individuals, together with his or her partner (as defined in the Dutch Income Tax Act 2001), directly or indirectly, holds (i) an interest of 5% or more of the total issued and outstanding capital of that company or of 5% or more of the issued and outstanding capital of a certain class of shares of that company; or (ii) rights to acquire, directly or indirectly, such interest; or (iii) certain profit sharing rights in that company that relate to 5% or more of the company’s annual profits and/or to 5% or more of the company’s liquidation proceeds. A deemed substantial interest may arise if a substantial interest (or part thereof) in a company has been disposed of, or is deemed to have been disposed of, on a non-recognition basis; |
| ● | holders of our ordinary shares if the shares held by such holders qualify or qualified as a participation (deelneming) for purposes of the Dutch Corporate Income Tax Act 1969 (Wet op de vennootschapsbelasting 1969). Generally, a taxpayer’s shareholding of 5% or more in a company’s nominal paid-up share capital (or, in certain cases, in voting rights) qualifies as participation. A holder may also have a participation if such holder does not have a shareholding of 5% or more but a related entity (statutorily defined term) has a participation or if the company in which the shares are held is a related entity (statutorily defined term); |
| ● | holders of shares who are individuals for whom the shares or any benefit derived from the shares are a remuneration or deemed to be a remuneration for (employment) activities or services performed by such holders or certain individuals related to such holders, whether within or outside an employment relation, that provides the holder, economically speaking, with certain benefits that have a relation to the relevant work activities or services (as defined in the Dutch Income Tax Act 2001); and |
| ● | pension funds, investment institutions (fiscale beleggingsinstellingen), exempt investment institutions (vrijgestelde beleggingsinstellingen) (as defined in the Dutch Corporate Income Tax Act 1969) and other entities that are, in whole or in part, not subject to or exempt from corporate income tax in the Netherlands, as well as entities that are exempt from corporate income tax in their country of residence, such country of residence being another state of the European Union, Norway, Liechtenstein, Iceland or any other state with which the Netherlands have agreed to exchange information in line with international standards. |
-133-
Except as otherwise indicated, this summary only addresses Dutch national tax legislation and published regulations, whereby the Netherlands and Dutch law means the part of the Kingdom of the Netherlands located in Europe and its law respectively, as in effect on the date hereof and as interpreted in published case law (of the Dutch Supreme Court (Hoge Raad der Nederlanden)) until this date, without prejudice to any amendment introduced (or to become effective) at a later date and/or implemented with or without retroactive effect. The applicable tax laws or interpretations thereof may change, or the relevant facts and circumstances may change, and such changes may affect the contents of this section, which will not be updated to reflect any such changes.
This discussion is for general information purposes and is not tax advice or a complete description of all Dutch tax consequences relating to the acquisition, holding and disposal of our shares. Holders or prospective holders of our shares should consult their own tax advisor regarding the tax consequences relating to the acquisition, holding and disposal of our shares in light of their particular circumstances.
Dividend withholding tax
We are incorporated under the laws of the Netherlands, and therefore a Dutch tax resident for Dutch domestic tax law purposes, including the Dutch Dividend Withholding Tax Act 1969. As such, we are required to withhold Dutch dividend withholding tax at a rate of 15% from dividends distributed by us (which withholding tax will not be borne by us but will be withheld by us from the gross dividends paid on the shares). We are however also treated as a German tax resident for German domestic tax law purposes, since our place of effective management is located in Germany. As long as we continue to have our place of effective management in Germany, and not in the Netherlands, under the convention between the Federal Republic of Germany and the Netherlands for the avoidance of double taxation with respect to taxes on income of 2012, we will be considered to be exclusively tax resident in Germany. Consequently, the Netherlands will be restricted to impose Dutch dividend withholding tax on dividends distributed by us (we will not be required to withhold Dutch dividend withholding tax). This exemption from withholding does not apply to dividends distributed by us to a holder of our ordinary shares who is resident or deemed to be resident in the Netherlands for Dutch income tax purposes or Dutch corporation tax purposes or to a holder of our ordinary shares that is neither resident nor deemed to be resident of the Netherlands if the ordinary shares are attributable to a Dutch permanent establishment of such non-resident holder, in which events the following applies. See Risk Factor “If we ever pay dividends, we may need to withhold tax on such dividends payable to holders of our shares in both Germany and the Netherlands.”
-134-
Dividends distributed by us to individuals and corporate legal entities who are resident or deemed to be resident in the Netherlands for Dutch tax purposes (“Dutch Resident Individuals” and “Dutch Resident Entities” as the case may be) or to holders of our ordinary shares that are neither resident nor deemed to be resident of the Netherlands if the ordinary shares are attributable to a Dutch permanent establishment of such non-resident holder are subject to Dutch dividend withholding tax at a rate of 15%.
The expression “dividends distributed” includes, among other things:
| ● | distributions in cash or in kind, deemed and constructive distributions and repayments of paid-in capital not recognized for Dutch dividend withholding tax purposes; |
| ● | liquidation proceeds, proceeds of redemption of shares, or proceeds of the repurchase of shares by us or one of our subsidiaries or other affiliated entities to the extent such proceeds exceed the average paid-in capital of those shares as recognized for purposes of Dutch dividend withholding tax, unless in case of a repurchase, a particular statutory exemption applies; |
| ● | an amount equal to the par value of shares issued or an increase of the par value of shares, to the extent that it does not appear that a contribution, recognized for purposes of Dutch dividend withholding tax, has been made or will be made; and |
| ● | partial repayment of the paid-in capital, recognized for purposes of Dutch dividend withholding tax, if and to the extent that we have net profits (zuivere winst), unless the holders of shares have resolved in advance at a general meeting to make such repayment and the par value of the shares concerned has been reduced by an equal amount by way of an amendment of our Articles of Association. |
Dutch Resident Individuals and Dutch Resident Entities can generally credit the Dutch dividend withholding tax against their income tax or corporate income tax liability. The same applies to holders of our ordinary shares that are neither resident nor deemed to be resident of the Netherlands if the shares are attributable to a Dutch permanent establishment of such non-resident holder.
-135-
Pursuant to legislation to counteract “dividend stripping,” a reduction, exemption, credit or refund of Dutch dividend withholding tax is denied if the recipient of the dividend is not the beneficial owner (uiteindelijk gerechtigde) as described in the Dutch Dividend Withholding Tax Act 1965 (Wet op de dividendbelasting 1965). This legislation generally targets situations in which a shareholder retains its economic interest in shares but reduces the withholding tax costs on dividends by a transaction with another party. It is not required for these rules to apply that the recipient of the dividends is aware that a dividend stripping transaction took place. The Dutch State Secretary for Finance takes the position that the definition of beneficial ownership introduced by this legislation will also apply in the context of a double taxation convention. As of January 1, 2024, more stringent rules apply to the setoff, exemption from, and reduction or refund of Dutch dividend withholding tax to address situations where a claim for setoff, exemption, reduction or refund may align with the letter of Dutch tax law or a double taxation convention but goes against the underlying intention or spirit of the dividend stripping rules, as perceived by the legislator. In addition, the burden of proof in cases related to dividend stripping and beneficial owner status has in certain circumstances been shifted from the tax inspector to the person making a claim for a setoff, reduction or refund of or exemption from Dutch dividend withholding tax. Furthermore, for shares traded on a regulated market, including the Ordinary Shares, it has been codified that the record date is used when determining the person who is entitled to the dividend.
Conditional withholding tax on dividends (as of January 1, 2024)
Furthermore, it cannot be excluded that dividends distributed by us to certain related entities which are not resident in the Netherlands for Dutch tax purposes will become subject to a Dutch conditional withholding tax in certain specific situations (see below), irrespectively of the fact that we have our place of effective management in Germany and, therefore, are a tax resident of Germany under German national tax laws. As of January 1, 2024, a Dutch conditional withholding tax will be imposed on dividends distributed by us to related entities (gelieerd) resident in certain listed jurisdictions or in case of abusive arrangements (all within the meaning of the Dutch Withholding Tax Act 2021; Wet bronbelasting 2021). The Dutch conditional withholding tax on dividends will be imposed at the highest Dutch corporate income tax rate in effect at the time of the distribution (2024: 25.8%). The Dutch conditional withholding tax on dividends will be reduced, but not below zero, by any regular Dutch dividend withholding tax withheld in respect of the same dividend distribution. As such, based on the currently applicable rates, the overall effective tax rate of withholding the regular Dutch dividend withholding tax (as described above) and the Dutch conditional withholding tax on dividends will not exceed the highest corporate income tax rate in effect at the time of the distribution (2024: 25.8%).
Taxes on income and capital gains
Dutch resident entities
Any benefit derived or deemed to be derived from the shares held by a Dutch Resident Entity, including any capital gains realized on the disposal thereof, will generally be subject to Dutch corporate income tax at a rate of 19% with respect to taxable profits up to €200,000 and 25.8% with respect to taxable profits in excess of that amount (rates and brackets for 2024).
Dutch resident individuals
If a holder of shares is a Dutch Resident Individual, any benefit derived or deemed to be derived from the ordinary shares is taxable at the progressive income tax rates (with a maximum of 49.5%, rate for 2024), if:
| i. | the ordinary shares are attributable to an enterprise from which the holder of such shares derives a share of the profit, whether as an entrepreneur (ondernemer) or as a person who has a co-entitlement to the net worth (medegerechtigd tot het vermogen) of such enterprise, without being a shareholder, as defined in the Dutch Income Tax Act 2001); or |
| ii. | the holder of the ordinary shares is considered to perform activities with respect to such shares that go beyond ordinary asset management (normaal, actief vermogensbeheer) or derives benefits from the shares that are taxable as benefits from other activities (resultaat uit overige werkzaamheden). |
-136-
Taxation of savings and investments
If the above-mentioned conditions (i) and (ii) do not apply to the Dutch Resident Individual, the ordinary shares will be subject to an annual Dutch income tax under the regime for savings and investments (inkomen uit sparen en beleggen). Taxation only occurs insofar the Dutch Resident Individual’s net investment assets for the year exceed a statutory threshold (heffingvrij vermogen). The net investment assets for the year are the fair market value of the investment assets less the fair market value of the liabilities on January 1 of the relevant calendar year (reference date; peildatum). Actual income or capital gains realized in respect of the ordinary shares are as such not subject to Dutch income tax.
The Dutch Resident Individual’s assets and liabilities taxed under this regime, including the ordinary shares, are allocated over the following three categories: (a) bank savings (banktegoeden), (b) other investments (overige bezittingen), including the ordinary shares, and (c) liabilities (schulden). The taxable benefit for the year (voordeel uit sparen en beleggen) is equal to the product of (x) the total deemed return divided by the sum of bank savings, other investments and liabilities and (y) the sum of bank savings, other investments and liabilities minus the statutory threshold, and is taxed at a flat rate of 36% (rate for 2024).
The deemed return applicable to other investments, including the ordinary shares, is set at 6.04% for the calendar year 2024. Transactions in the three-month period before and after January 1 of the relevant calendar year implemented to arbitrate between the deemed return percentages applicable to bank savings, other investments and liabilities will for this purpose be ignored if the holder of ordinary shares cannot sufficiently demonstrate that such transactions are implemented for other than tax reasons.
The current Dutch income tax regime for savings and investments was implemented in Dutch tax law following the decision of the Dutch Supreme Court (Hoge Raad) of December 24, 2021 (ECLI:NL:2021:1963) (the “Decision”). In the Decision, the Dutch Supreme Court ruled that the (old) system of taxation for savings and investments based on a deemed return may under specific circumstances contravene with Section 1 of the First Protocol to the European Convention on Human Rights in combination with Section 14 of the European Convention on Human Rights (the “EC-Human Rights”). A new court procedure is pending before the Dutch Supreme Court questioning whether the current tax system for savings and investments is in line with the Decision. On September 18, 2023 (ECLI:NL:PHR:2023:655) the Attorney General Wattel concluded that the new tax system is not in line with the Decision, except for the taxation of bank savings, as the system is, in short, still based on a deemed return rather than actual returns, and as a result, the regime contravenes with the EC-Human Rights. The decision of the Dutch Supreme Court is expected mid-2024. In addition, on September 8, 2023, the former cabinet published a law proposal for a new tax system for savings and investments on the basis of actual returns according to an asset accumulation system, the ‘Actual Return Box 3 Act’ (Wet werkelijk rendement box 3). The proposed system is expected to come into effect on January 1, 2027 at the earliest. However, it is up to the new cabinet to submit a final law proposal to the Dutch parliament.
Holders of ordinary shares are advised to consult their own tax advisor to ensure that the tax in respect of the ordinary shares is levied in accordance with the applicable Dutch tax rules at the relevant time.
Non-residents of the Netherlands
A holder of our ordinary shares that is neither a Dutch Resident Entity nor a Dutch Resident Individual will not be subject to Dutch taxes on income or capital gains in respect of any payment under the ordinary shares or in respect of any gain or loss realized on the disposal or deemed disposal of the ordinary shares, provided that:
| i. | such holder does not have an interest in an enterprise or a deemed enterprise (as defined in the Dutch Income Tax Act and the Dutch Corporate Income Tax Act 1969) which, in whole or in part, is either effectively managed in the Netherlands or is carried out through a permanent establishment, a deemed permanent establishment or a permanent representative in the Netherlands and to which enterprise or part of an enterprise the ordinary shares are attributable; and |
| ii. | in the event such holder is an individual, such holder does not carry out any activities in the Netherlands with respect to the ordinary shares that go beyond ordinary asset management (normaal, actief vermogensbeheer) and does not derive benefits from the ordinary shares that are taxable as benefits from other activities in the Netherlands (resultaat uit overige werkzaamheden). |
-137-
Gift and inheritance tax
Residents of the Netherlands
Gift or inheritance taxes will arise in the Netherlands with respect to a transfer of the ordinary shares by way of a gift by, or on the death of, a holder of our ordinary shares who is resident or deemed to be resident in the Netherlands at the time of the gift or such holder’s death.
Non-residents of the Netherlands
No Dutch gift or inheritance taxes will arise on the transfer of our ordinary shares by way of gift by, or on the death of, a holder of the ordinary shares who is neither resident nor deemed to be resident in the Netherlands, unless in the case of a gift of shares by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands, such individual dies within 180 days after the date of the gift, while being resident or deemed to be resident in the Netherlands.
For purposes of Dutch gift and inheritance taxes, amongst others, a person that holds the Dutch nationality will be deemed to be resident in the Netherlands if such person has been resident in the Netherlands at any time during the 10 years preceding the date of the gift or his/her death. Additionally, for purposes of Dutch gift tax, amongst others, a person not holding the Dutch nationality will be deemed to be resident in the Netherlands if such person has been resident in the Netherlands at any time during the 12 months preceding the date of the gift. Applicable tax treaties may override deemed residency.
Furthermore, for purposes of Netherlands gift and inheritance tax, a gift that is made under a condition precedent is deemed to have been made at the moment such condition precedent is satisfied. If the condition precedent is fulfilled after the death of the donor, the gift is deemed to be made upon the death of the donor.
Other taxes and duties
No Dutch value added tax and no Dutch registration tax, stamp duty or any other similar documentary tax or duty will be payable by a holder of our ordinary shares on any payment in consideration for the holding or disposal of the ordinary shares.
The following is a general summary of certain material Dutch tax consequences of the acquisition, holding and disposal of our ordinary shares. This summary does not purport to describe all possible tax considerations or consequences that may be relevant to a holder or prospective holder of ordinary shares and does not purport to deal with the tax consequences applicable to all categories of investors, some of which (such as trusts or similar arrangements) may be subject to special rules. In view of its general nature, this general summary should be treated with corresponding caution. To the extent this summary relates to legal conclusions under current Netherlands tax law, and subject to the qualifications it contains, it represents the opinion of NautaDutilh N.V., our special Dutch counsel. Holders or prospective holders of shares should consult with their own tax advisors with regard to the tax consequences of investing in the shares in their particular circumstances. The discussion below is included for general information purposes only.
For the purposes of this discussion, it is assumed that we are a tax resident of Germany under German national tax laws since we intended to have, from our incorporation and on a continuous basis, our place of effective management in Germany. See Risk Factor “We may become taxable in a jurisdiction other than Germany and this may increase the aggregate tax burden on us.”
Please note that this summary does not describe the Dutch tax considerations for:
| ● | holders of our ordinary shares if such holders, and in the case of individuals, his or her partner or certain of their relatives by blood or marriage in the direct line (including foster children), have a substantial interest (aanmerkelijk belang) or deemed substantial interest (fictief aanmerkelijk belang) in the Company under the Dutch Income Tax Act 2001 (Wet inkomstenbelasting 2001). Generally speaking, a holder of securities in a company is considered to hold a substantial interest in such company, if such holder alone or, in the case of individuals, together with his or her partner (as defined in the Dutch Income Tax Act 2001), directly or indirectly, holds (i) an interest of 5% or more of the total issued and outstanding capital of that company or of 5% or more of the issued and outstanding capital of a certain class of shares of that company; (ii) rights to acquire, directly or indirectly, such interest; or (iii) certain profit sharing rights in that company that relate to 5% or more of the company’s annual profits and/or to 5% or more of the company’s liquidation proceeds. A deemed substantial interest may arise if a substantial interest (or part thereof) in a company has been disposed of, or is deemed to have been disposed of, on a non-recognition basis; |
-138-
| ● | holders of our ordinary shares if the shares held by such holders qualify or qualified as a participation (deelneming) for purposes of the Dutch Corporate Income Tax Act 1969 (Wet op de vennootschapsbelasting 1969). Generally, a taxpayer’s shareholding of 5% or more in a company’s nominal paid-up share capital (or, in certain cases, in voting rights) qualifies as participation. A holder may also have a participation if such holder does not have a shareholding of 5% or more but a related entity (statutorily defined term) has a participation or if the company in which the shares are held is a related entity (statutorily defined term); |
| ● | holders of shares who are individuals for whom the shares or any benefit derived from the shares are a remuneration or deemed to be a remuneration for (employment) activities or services performed by such holders or certain individuals related to such holders, whether within or outside an employment relation, that provides the holder, economically speaking, with certain benefits that have a relation to the relevant work activities or services (as defined in the Dutch Income Tax Act 2001); and |
| ● | pension funds, investment institutions (fiscale beleggingsinstellingen), exempt investment institutions (vrijgestelde beleggingsinstellingen) (as defined in the Dutch Corporate Income Tax Act 1969) and other entities that are, in whole or in part, not subject to or exempt from corporate income tax in the Netherlands, as well as entities that are exempt from corporate income tax in their country of residence, such country of residence being another state of the European Union, Norway, Liechtenstein, Iceland or any other state with which the Netherlands have agreed to exchange information in line with international standards. |
Except as otherwise indicated, this summary only addresses Dutch national tax legislation and published regulations, whereby the Netherlands and Dutch law means the part of the Kingdom of the Netherlands located in Europe and its law respectively, as in effect on the date hereof and as interpreted in published case law (of the Dutch Supreme Court (Hoge Raad der Nederlanden)) until this date, without prejudice to any amendment introduced (or to become effective) at a later date and/or implemented with or without retroactive effect. The applicable tax laws or interpretations thereof may change, or the relevant facts and circumstances may change, and such changes may affect the contents of this section, which will not be updated to reflect any such changes.
This discussion is for general information purposes and is not tax advice or a complete description of all Dutch tax consequences relating to the acquisition, holding and disposal of our shares. Holders or prospective holders of our shares should consult their own tax advisor regarding the tax consequences relating to the acquisition, holding and disposal of our shares in light of their particular circumstances.
Dividend withholding tax
We are incorporated under the laws of the Netherlands, and therefore a Dutch tax resident for Dutch domestic tax law purposes, including the Dutch Dividend Withholding Tax Act 1969. As such, we are required to withhold Dutch dividend withholding tax at a rate of 15% from dividends distributed by us (which withholding tax will not be borne by us but will be withheld by us from the gross dividends paid on the shares). We are however also treated as a German tax resident for German domestic tax law purposes, since our place of effective management is located in Germany. As long as we continue to have our place of effective management in Germany, and not in the Netherlands, under the convention between the Federal Republic of Germany and the Netherlands for the avoidance of double taxation with respect to taxes on income of 2012, we will be considered to be exclusively tax resident in Germany. Consequently, the Netherlands will be restricted to impose Dutch dividend withholding tax on dividends distributed by us (we will not be required to withhold Dutch dividend withholding tax). This exemption from withholding does not apply to dividends distributed by us to a holder of our ordinary shares who is resident or deemed to be resident in the Netherlands for Dutch income tax purposes or Dutch corporation tax purposes or to a holder of our ordinary shares that is neither resident nor deemed to be resident of the Netherlands if the ordinary shares are attributable to a Dutch permanent establishment of such non-resident holder, in which events the following applies. See Risk Factor “If we ever pay dividends, we may need to withhold tax on such dividends payable to holders of our shares in both Germany and the Netherlands.”
-139-
Dividends distributed by us to individuals and corporate legal entities who are resident or deemed to be resident in the Netherlands for Dutch tax purposes (“Dutch Resident Individuals” and “Dutch Resident Entities” as the case may be) or to holders of our ordinary shares that are neither resident nor deemed to be resident of the Netherlands if the ordinary shares are attributable to a Dutch permanent establishment of such non-resident holder are subject to Dutch dividend withholding tax at a rate of 15%.
The expression “dividends distributed” includes, among other things:
| ● | distributions in cash or in kind, deemed and constructive distributions and repayments of paid-in capital not recognized for Dutch dividend withholding tax purposes; |
| ● | liquidation proceeds, proceeds of redemption of shares, or proceeds of the repurchase of shares by us or one of our subsidiaries or other affiliated entities to the extent such proceeds exceed the average paid-in capital of those shares as recognized for purposes of Dutch dividend withholding tax, unless in case of a repurchase, a particular statutory exemption applies; |
| ● | an amount equal to the par value of shares issued or an increase of the par value of shares, to the extent that it does not appear that a contribution, recognized for purposes of Dutch dividend withholding tax, has been made or will be made; and |
| ● | partial repayment of the paid-in capital, recognized for purposes of Dutch dividend withholding tax, if and to the extent that we have net profits (zuivere winst), unless the holders of shares have resolved in advance at a general meeting to make such repayment and the par value of the shares concerned has been reduced by an equal amount by way of an amendment of our Articles of Association. |
Dutch Resident Individuals and Dutch Resident Entities can generally credit the Dutch dividend withholding tax against their income tax or corporate income tax liability. The same applies to holders of our ordinary shares that are neither resident nor deemed to be resident of the Netherlands if the shares are attributable to a Dutch permanent establishment of such non-resident holder.
Pursuant to legislation to counteract “dividend stripping,” a reduction, exemption, credit or refund of Dutch dividend withholding tax is denied if the recipient of the dividend is not the beneficial owner (uiteindelijk gerechtigde) as described in the Dutch Dividend Withholding Tax Act 1965 (Wet op de dividendbelasting 1965). This legislation generally targets situations in which a shareholder retains its economic interest in shares but reduces the withholding tax costs on dividends by a transaction with another party. It is not required for these rules to apply that the recipient of the dividends is aware that a dividend stripping transaction took place. The Dutch State Secretary for Finance takes the position that the definition of beneficial ownership introduced by this legislation will also apply in the context of a double taxation convention.
Conditional withholding tax on dividends (as of January 1, 2024)
Furthermore, it cannot be excluded that dividends distributed by us to certain related entities which are not resident in the Netherlands for Dutch tax purposes will become subject to a Dutch conditional withholding tax in certain specific situations (see below), irrespectively of the fact that we have our place of effective management in Germany and, therefore, are a tax resident of Germany under German national tax laws. As of January 1, 2024, a Dutch conditional withholding tax will be imposed on dividends distributed by us to related entities (gelieerd) resident in certain listed jurisdictions or in case of abusive arrangements (all within the meaning of the Dutch Withholding Tax Act 2021; Wet bronbelasting 2021). The Dutch conditional withholding tax on dividends will be imposed at the highest Dutch corporate income tax rate in effect at the time of the distribution (2022: 25.8%). The Dutch conditional withholding tax on dividends will be reduced, but not below zero, by any regular Dutch dividend withholding tax withheld in respect of the same dividend distribution. As such, based on the currently applicable rates, the overall effective tax rate of withholding the regular Dutch dividend withholding tax (as described above) and the Dutch conditional withholding tax on dividends will not exceed the highest corporate income tax rate in effect at the time of the distribution (2022: 25.8%).
-140-
Taxes on income and capital gains
Dutch resident entities
Any benefit derived or deemed to be derived from the shares held by a Dutch Resident Entity, including any capital gains realized on the disposal thereof, will generally be subject to Dutch corporate income tax at a rate of 19% with respect to taxable profits up to €200,000 and 25.8% with respect to taxable profits in excess of that amount (rates and brackets for 2023).
Dutch resident individuals
If a holder of shares is a Dutch Resident Individual, any benefit derived or deemed to be derived from the ordinary shares is taxable at the progressive income tax rates (with a maximum of 49.5%, rate for 2023), if:
| i. | the ordinary shares are attributable to an enterprise from which the holder of such shares derives a share of the profit, whether as an entrepreneur (ondernemer) or as a person who has a co-entitlement to the net worth (medegerechtigd tot het vermogen) of such enterprise, without being a shareholder, as defined in the Dutch Income Tax Act 2001); or |
| ii. | the holder of the ordinary shares is considered to perform activities with respect to such shares that go beyond ordinary asset management (normaal, actief vermogensbeheer) or derives benefits from the shares that are taxable as benefits from other activities (resultaat uit overige werkzaamheden). |
Taxation of savings and investments
If the above-mentioned conditions (i) and (ii) do not apply to the Dutch Resident Individual, the ordinary shares will be subject to an annual Dutch income tax under the regime for savings and investments (inkomen uit sparen en beleggen). Taxation only occurs insofar the Dutch Resident Individual’s net investment assets for the year exceed a statutory threshold (heffingvrij vermogen). The net investment assets for the year are the fair market value of the investment assets less the fair market value of the liabilities on January 1 of the relevant calendar year (reference date; peildatum). The ordinary shares are included as investment assets. The taxable benefit for the year (voordeel uit sparen en beleggen) is taxed at a flat rate of 32% (rate for 2023). Actual income or capital gains realized in respect of the ordinary shares are as such not subject to Dutch income tax.
The taxable benefit for the year is calculated as follows:
| i. | The Dutch Resident Individual’s assets and liabilities taxed under this regime, including the ordinary shares, are allocated over the following three categories: (a) bank savings, (b) other investments, including the ordinary shares, and (c) liabilities. |
| ii. | The return (rendement) in respect of these assets and liabilities is calculated as follows (the return is at a minimum nihil): |
| ● | a deemed return on the fair market value of the actual amount of bank savings and cash on January 1 of the relevant calendar year; plus |
| ● | a deemed return on the fair market value of the actual amount of other investments, including the ordinary shares, on January 1 of the relevant calendar year; minus |
| ● | a deemed return on the sum of the fair market value of the actual amount of liabilities on January 1 of the relevant calendar year less the statutory threshold for liabilities (drempel). |
| iii. | The return percentage (%) (rendementspercentage) is calculated as follows: |
| ● | by dividing the return calculated under (ii) above by the net investment assets for the year of the Dutch Resident Individual; multiplied by 100. |
| iv. | The taxable base (grondslag sparen en beleggen) is calculated as follows: |
| ● | the net investment assets for the year of the Dutch Resident Individual; minus |
| ● | the applicable statutory threshold. |
| v. | The taxable benefit for the year is equal to the taxable base calculated under (iv) above multiplied by the return percentage calculated under (iii) above. |
-141-
At the date hereof, the deemed returns for the different investment categories mentioned under (ii) above have been temporarily set at: (a) 0.01%, (b) 5.69% and (c) 2.46%. The definitive percentages for the year 2023 will be published in the first months of 2024 and will have retroactive effect to January 1, 2023. Transactions in the three-month period before and after January 1 of the relevant calendar year implemented to arbitrate between the deemed return percentages applicable to bank savings, other investments and liabilities will for this purpose be ignored if the holder of ordinary shares cannot sufficiently demonstrate that such transactions are implemented for other than tax reasons.
Non-residents of the Netherlands
A holder of our ordinary shares that is neither a Dutch Resident Entity nor a Dutch Resident Individual will not be subject to Dutch taxes on income or capital gains in respect of any payment under the ordinary shares or in respect of any gain or loss realized on the disposal or deemed disposal of the ordinary shares, provided that:
| i. | such holder does not have an interest in an enterprise or a deemed enterprise (as defined in the Dutch Income Tax Act and the Dutch Corporate Income Tax Act 1969) which, in whole or in part, is either effectively managed in the Netherlands or is carried out through a permanent establishment, a deemed permanent establishment or a permanent representative in the Netherlands and to which enterprise or part of an enterprise the ordinary shares are attributable; and |
| ii. | in the event such holder is an individual, such holder does not carry out any activities in the Netherlands with respect to the ordinary shares that go beyond ordinary asset management (normaal, actief vermogensbeheer) and does not derive benefits from the ordinary shares that are taxable as benefits from other activities in the Netherlands (resultaat uit overige werkzaamheden). |
Gift and inheritance tax
Residents of the Netherlands
Gift or inheritance taxes will arise in the Netherlands with respect to a transfer of the ordinary shares by way of a gift by, or on the death of, a holder of our ordinary shares who is resident or deemed to be resident in the Netherlands at the time of the gift or such holder’s death.
Non-residents of the Netherlands
No Dutch gift or inheritance taxes will arise on the transfer of our ordinary shares by way of gift by, or on the death of, a holder of the ordinary shares who is neither resident nor deemed to be resident in the Netherlands, unless in the case of a gift of shares by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands, such individual dies within 180 days after the date of the gift, while being resident or deemed to be resident in the Netherlands.
For purposes of Dutch gift and inheritance taxes, amongst others, a person that holds the Dutch nationality will be deemed to be resident in the Netherlands if such person has been resident in the Netherlands at any time during the 10 years preceding the date of the gift or his/her death. Additionally, for purposes of Dutch gift tax, amongst others, a person not holding the Dutch nationality will be deemed to be resident in the Netherlands if such person has been resident in the Netherlands at any time during the 12 months preceding the date of the gift. Applicable tax treaties may override deemed residency.
Furthermore, for purposes of Netherlands gift and inheritance tax, a gift that is made under a condition precedent is deemed to have been made at the moment such condition precedent is satisfied. If the condition precedent is fulfilled after the death of the donor, the gift is deemed to be made upon the death of the donor.
Other taxes and duties
No Dutch value added tax and no Dutch registration tax, stamp duty or any other similar documentary tax or duty will be payable by a holder of our ordinary shares on any payment in consideration for the holding or disposal of the ordinary shares.
-142-
7. Material German tax Considerations
The following section is a description of the material German tax considerations that become relevant when purchasing, holding or transferring the Company’s shares. The Company has its sole place of management in Germany and, therefore, qualifies as a corporation subject to German unlimited corporate income taxation; however, because a company’s tax residency depends on future facts regarding the location in which the company is managed and controlled the German unlimited corporate income tax liability may change in the future. This section does not set forth all German tax aspects that may be relevant for shareholders. The section is based on the German tax law applicable as of the date of this document. It should be noted that the law may change following the date of this Annual Report and that such changes may have retroactive effect.
The material German tax principles of purchasing, owning and transferring of shares are set forth in the following. This section does not purport to be a comprehensive or complete analysis or listing of all potential tax effects of the purchase, ownership or disposition of shares and does not set forth all tax considerations that may be relevant to a particular person’s decision to acquire ordinary shares. All of the following is subject to change. Such changes could apply retroactively and could affect the consequences set forth below. This section does not refer to any foreign account tax compliance act (or FATCA) aspects.
Shareholders are advised to consult their own tax advisers with regard to the application of German tax law to their particular situations, in particular with respect to the procedure to be complied with to obtain a relief of withholding tax on dividends and on capital gains (Kapitalertragsteuer) and with respect to the influence of double tax treaty provisions, as well as any tax consequences arising under the laws of any state, local or other foreign jurisdiction. For German tax purposes, a shareholder may include an individual who or an entity that does not have the legal title to the shares, but to whom nevertheless the shares are attributed, based either on such individual or entity owning a beneficial interest in the shares or based on specific statutory provisions.
This section does not constitute particular tax advice. Potential purchasers of the Company’s shares are urged to consult their own tax advisers regarding the tax consequences of the purchase, ownership and disposition of shares in light of their particular circumstances.
Taxation of dividends
Withholding tax on dividends
Dividends distributed from a company to its shareholders are subject to withholding tax, subject to certain exemptions (for example, repayments of capital from the tax equity account (steuerliches Einlagekonto)), as further described. The withholding tax rate is 25% plus a 5.5% solidarity surcharge (Solidaritätszuschlag) thereon (for a total of 26.375%) of the gross dividend approved by the ordinary shareholders’ meeting. Withholding tax is to be withheld and passed on for the account of the shareholders by a domestic branch of a domestic or foreign credit or financial services institution (Kredit- und Finanzdienstleistungsinstitut), by the domestic securities trading company (inländisches Wertpapierhandelsunternehmen) or a domestic securities trading bank (inländische Wertpapierhandelsbank) which keeps and administers the shares and disburses or credits the dividends or disburses the dividends to a foreign agent, or by the securities custodian bank (Wertpapiersammelbank) to which the shares were entrusted for collective custody if the dividends are distributed to a foreign agent by such securities custodian bank (which is referred to as the “Dividend Paying Agent”). In case the shares are not held in collective deposit with a Dividend Paying Agent, the Company is responsible for withholding and remitting the tax to the competent tax office.
Such withholding tax is levied and withheld irrespective of whether and to what extent the dividend distribution is taxable at the level of the shareholder and whether the shareholder is a person residing in Germany or in a foreign country.
-143-
In the case of dividends distributed to a company within the meaning of Art. 2 of the amended EU Directive 2011/96/EU of the Council of November 30, 2011, or the EU Parent Subsidiary Directive, domiciled in another Member State of the European Union, an exemption from the withholding tax will be granted upon request if further prerequisites are satisfied (Freistellung im Steuerabzugsverfahren). This also applies to dividends distributed to a permanent establishment located in another Member State of the European Union of such a parent company or of a parent company tax resident in Germany if the participation in the Company is effectively connected with this permanent establishment. The key prerequisite for the application of the EU Parent Subsidiary Directive is that the shareholder has held a direct participation in the share capital of the Company of at least 10% for at least one year.
The withholding tax on distributions to other foreign resident shareholders is reduced in accordance with a double taxation treaty if Germany has concluded such double taxation treaty with the country of residence of the shareholder and if the shareholder does not hold his shares either as part of the assets of a permanent establishment or a fixed place of business in Germany or as business assets for which a permanent representative has been appointed in Germany. The reduction of the withholding tax is procedurally granted in such a manner that the difference between the total amount withheld, including the solidarity surcharge, and the tax liability determined on the basis of the tax rate set forth in the applicable double taxation treaty (15% unless further qualifications are met) is refunded by the German tax administration upon request (Federal Central Office for Taxes (Bundeszentralamt für Steuern), main office in Bonn-Beuel, An der Küppe 1, D-53225 Bonn).
In the case of dividends received by corporations whose statutory seat and effective place of management are not located in Germany and who are therefore not tax resident in Germany, two-fifths of the withholding tax deducted and remitted are refunded without the need to fulfill all prerequisites required for such refund under the EU Parent Subsidiary Directive or under a double taxation treaty or if no double taxation treaty has been concluded between Germany and the state of residence of the shareholder.
In order to receive a refund pursuant to a double taxation treaty or the aforementioned option for foreign corporations, the shareholder has to submit a completed form for refund (available at the Federal Central Office for Taxes (www.bzst.de) as well as at the German embassies and consulates) together with a withholding tax certificate (Kapitalertragsteuerbescheinigung) issued by the institution that withheld the tax.
The availability of an exemption from withholding tax in accordance with the EU Parent Subsidiary Directive or a double tax treaty and the aforementioned options for a refund of the withholding tax (with or without protection under a double taxation treaty) depends on whether certain additional prerequisites are fulfilled. The applicable withholding tax relief will only be granted if the preconditions of the German anti-avoidance rules (or “Directive Override” or “Treaty Override”), in particular Section 50d, paragraph 3 of the German Income Tax Act (Einkommensteuergesetz), are fulfilled.
The aforementioned reductions of (or exemptions from) withholding tax are further restricted if (i) the applicable double taxation treaty provides for a tax reduction resulting in an applicable tax rate of less than 15% and (ii) the shareholder is not a corporation that directly holds at least 10% in the equity capital of the Company and is subject to tax on its income and profits in its state of residence without being exempt. In this case, the reduction of (or exemption from) withholding tax is subject to the following three cumulative prerequisites: (i) the shareholder must qualify as beneficial owner of the shares in the Company for a minimum holding period of 45 consecutive days occurring within a period of 45 days prior and 45 days after the due date of the dividends, (ii) the shareholder has to bear at least 70% of the change in value risk related to the shares in the Company during the minimum holding period without being directly or indirectly hedged, and (iii) the shareholder must not be required to fully or largely compensate directly or indirectly the dividends to third parties. However, these further prerequisites do not apply if the shareholder has been the beneficial owner of the shares in the Company for at least one uninterrupted year upon receipt of the dividends. Furthermore, the special rules on the restriction of withholding tax credit do not apply to a shareholder whose overall dividend earnings within an assessment period do not exceed €20,000 or that has been the beneficial owner of the shares in the Company for at least one uninterrupted year upon receipt of the dividends.
For individual or corporate shareholders tax resident outside Germany not holding the shares through a permanent establishment (Betriebsstätte) in Germany or as business assets (Betriebsvermögen) for which a permanent representative (ständiger Vertreter) has been appointed in Germany, the remaining and paid withholding tax (if any) is final (i.e., not refundable) and settles the shareholder’s limited tax liability in Germany. For individual or corporate shareholders tax resident in Germany (for example, those shareholders whose residence, domicile, registered office or place of management is located in Germany) holding their shares as business assets, as well as for shareholders tax resident outside of Germany holding their shares through a permanent establishment in Germany or as business assets for which a permanent representative has been appointed in Germany, the withholding tax withheld (including solidarity surcharge) can be credited against the shareholder’s personal income tax or corporate income tax liability in Germany. Any withholding tax (including solidarity surcharge) in excess of such tax liability is refunded. For individual shareholders tax resident in Germany holding the Company’s shares as private assets, the withholding tax is a final tax (Abgeltungsteuer), subject to the exceptions described in the following section.
-144-
Pursuant to special rules on the restriction of withholding tax credit, the credit of withholding tax is subject to the following three cumulative prerequisites: (i) the shareholder must qualify as beneficial owner of the shares in the Company for a minimum holding period of 45 consecutive days occurring within a period of 45 days prior and 45 days after the due date of the dividends, (ii) the shareholder has to bear at least 70% of the change in value risk related to the shares in the Company during the minimum holding period without being directly or indirectly hedged, and (iii) the shareholder must not be required to fully or largely compensate directly or indirectly the dividends to third parties. Absent of the fulfillment of all of the three prerequisites, three-fifths of the withholding tax imposed on the dividends must not be credited against the shareholder’s (corporate) income tax liability, but may, upon application, be deducted from the shareholder’s tax base for the relevant assessment period. A shareholder that has received gross dividends without any deduction of withholding tax due to a tax exemption without qualifying for a full tax credit has to notify the competent local tax office accordingly and has to make a payment in the amount of the omitted withholding tax deduction.
Taxation of dividend income of shareholders tax resident in Germany holding the Company’s shares as private assets
For individual shareholders (individuals) resident in Germany holding the Company’s shares as private assets, dividends are subject to a flat rate tax which is satisfied by the withholding tax actually withheld (Abgeltungsteuer). Accordingly, dividend income will be taxed at a flat tax rate of 25% plus 5.5% solidarity surcharge thereon (in total 26.375%) and church tax (Kirchensteuer) in case the shareholder is subject to church tax because of his individual circumstances. An automatic procedure for deduction of church tax by way of withholding will apply to shareholders being subject to church tax unless the shareholder has filed a blocking notice (Sperrvermerk) with the German Federal Tax Office (details related to the computation of the concrete tax rate including church tax are to be discussed with the individual tax adviser of the relevant shareholder). Except for an annual lump sum savings allowance (Sparer-Pauschbetrag) of up to €1,000 (for individual filers) or up to €2,000 (for married couples and for partners in accordance with the registered partnership law (Gesetz über die Eingetragene Lebenspartnerschaft) filing jointly), private individual shareholders will not be entitled to deduct expenses incurred in connection with the capital investment from their dividend income.
The income tax owed for the dividend income is satisfied by the withholding tax withheld by the Dividend Paying Agent. However, if the flat tax results in a higher tax burden as opposed to the private shareholder’s individual tax rate, the private shareholder can opt for taxation at his individual personal income tax rate. In that case, the final withholding tax will be credited against the income tax. However, pursuant to the German tax authorities and a court ruling, private shareholders are nevertheless not entitled to deduct expenses incurred in connection with the capital investment from their income. The option can be exercised only for all capital income from capital investments received in the relevant assessment period uniformly and married couples as well as partners in accordance with the registered partnership law filing jointly may only jointly exercise the option.
Exceptions from the flat rate tax (satisfied by withholding at source) (Abgeltungsteuer) may apply—that is, only upon application—for shareholders who have a shareholding of at least 25% in a company and for shareholders who have a shareholding of at least 1% in the Company, work for that company in a professional capacity and have a material influence in the economic activity of aforementioned company. In such a case, the same rules apply as for sole proprietors holding the shares as business assets (see below “—Taxation of dividend income of shareholders tax resident in Germany holding the Company’s shares as business assets—(ii) Sole proprietors”).
-145-
Taxation of dividend income of shareholders tax resident in Germany holding the Company’s shares as business assets
If a shareholder holds the Company’s shares as business assets, the taxation of the dividend income depends on whether the respective shareholder is a corporation, a sole proprietor or a partnership.
(a) Corporations
Dividend income of corporate shareholders is exempt from corporate income tax, provided that the incorporated entity holds a direct participation of at least 10% in the share capital of a company at the beginning of the calendar year in which the dividends are paid. The acquisition of a participation of at least 10% in the course of a calendar year is deemed to have occurred at the beginning of such calendar year for the purpose of this rule. Participations in the share capital of the Company which a corporate shareholder holds through a partnership, including co-entrepreneurships (Mitunternehmerschaften), are attributable to such corporate shareholder only on a pro rata basis at the ratio of the interest share of the corporate shareholder in the assets of the relevant partnership. However, 5% of the tax exempt dividends are deemed to be non-deductible business expenses for tax purposes and therefore are subject to corporate income tax (plus solidarity surcharge) and trade tax; i.e. tax exemption of 95%. Business expenses incurred in connection with the dividends received are entirely tax deductible.
For trade tax purposes the entire dividend income is subject to trade tax (i.e. the tax exempt dividends must be added back when determining the trade taxable income), unless the corporation shareholder holds at least 15% of the Company’s registered share capital at the beginning of the relevant tax assessment period (Erhebungszeitraum). In case of an indirect participation via a partnership please refer to the section “Partnerships” below.
If the shareholding is below 10% in the share capital, dividends are taxable at the applicable corporate income tax rate of 15% plus 5.5% solidarity surcharge thereon and trade tax (the rate of which depends on the municipalities the corporate shareholder resides in).
Special regulations apply which abolish the 95% tax exemption, if the Company’s shares are held as trading portfolio assets in the meaning of Section 340e German commercial code (Handelsgesetzbuch) by (i) a credit institution (Kreditinstitut), (ii) a security institution (Wertpapierinstitut), (iii) a financial service institution (Finanzdienstleistungsinstitut), or (iv) a financial enterprise within the meaning of the German Banking Act (Kreditwesengesetz), in case more than 50% of the shares of such financial enterprise are held directly or indirectly by a credit institution, a security institution or a financial service institution, as well as by a life insurance company, a health insurance company or a pension fund in case the shares are attributable to the capital investments, resulting in fully taxable income.
(b) Sole proprietors
For sole proprietors (individuals) resident in Germany holding shares as business assets dividends are subject to the partial income rule (Teileinkünfteverfahren). Accordingly, only (i) 60% of the dividend income will be taxed at his/her individual personal income tax rate plus 5.5% solidarity surcharge thereon and church tax (if applicable) and (ii) 60% of the business expenses related to the dividend income are deductible for tax purposes. In addition, the dividend income is entirely subject to trade tax if the shares are held as business assets of a permanent establishment in Germany within the meaning of the GewStG, unless the shareholder holds at least 15% of the Company’s registered share capital at the beginning of the relevant assessment period. The trade tax levied will be eligible for credit against the shareholder’s personal income tax liability based on the applicable municipal trade tax rate and the individual tax situation of the shareholder.
(c) Partnerships
In case shares are held by a partnership, the partnership itself is not subject to corporate income tax or personal income tax (Unless the option according to section 1a of the German Corporate Income Tax Act was applied for that the partnership is taxed as a corporation). In this regard, corporate income tax or personal income tax (and church tax, if applicable) as well as solidarity surcharge are levied only at the level of the partner with respect to their relevant part of the profit and depending on their individual circumstances.
If the partner is a corporation, the dividend income will be subject to corporate income tax plus solidarity surcharge (see “—(i) Corporations”).
If the partner is a sole proprietor (individual), the dividend income will be subject to the partial income rule (see “—(ii) Sole proprietors”).
-146-
The dividend income is subject to trade tax at the level of the partnership (provided that the partnership is liable to trade tax), unless the partnership holds at least 15% of a company’s registered share capital at the beginning of the relevant assessment period, in which case the dividend income is exempt from trade tax. There are no clear statutory provisions concerning the taxation of dividends with regard to a corporate shareholder of the partnership. However, trade tax will be levied on 5% of the dividends to the extent they are attributable to the shares of such corporate partners to whom at least 10% of the shares of the Company are attributable on a look-through basis, since such portion of the dividends will be deemed to be non-deductible business expenses.
If a partner is an individual, depending on the applicable municipal trade tax rate and the individual tax situation, the trade tax paid at the level of the partnership is partly or entirely be credited against the partner’s personal income tax liability.
In case of a corporation being a partner, special regulations will apply with respect to trading portfolio assets of credit institutions, security institution, financial service institutions or financial enterprises within the meaning of the German Banking Act (Kreditwesengesetz) or life insurance companies, health insurance companies or pension funds (see “—(i) Corporations”).
Thus, the actual trade tax charge, if any, at the level of the partnership depends on the shareholding quota of the partnership and the nature of the partners (e.g. individual or corporation).
Taxation of dividend income of shareholders tax resident outside of Germany
For foreign individual or corporate shareholders tax resident outside of Germany not holding the shares through a permanent establishment in Germany or as business assets for which a permanent representative has been appointed in Germany, the deducted withholding tax (possibly reduced by way of a tax relief under a double tax treaty or domestic tax law, such as in connection with the EU Parent Subsidiary Directive) is final (that is, not refundable) and settles the shareholder’s limited tax liability in Germany, unless the shareholder is entitled to apply for a withholding tax refund or exemption.
In contrast, individual or corporate shareholders tax resident outside of Germany holding the Company’s shares through a permanent establishment in Germany or as business assets for which a permanent representative has been appointed in Germany are subject to the same rules as applicable (and described above) to shareholders resident in Germany holding the shares as business assets. The withholding tax withheld (including solidarity surcharge) is credited against the shareholder’s personal income tax or corporate income tax liability in Germany.
8. Taxation of capital gains
Withholding tax on capital gains
For individual shareholders (individuals) resident in Germany holding shares as private assets, capital gains realized on the disposal of shares are subject to final withholding tax. Accordingly, capital gains will be taxed at a flat tax rate of 25% plus 5.5% solidarity surcharge thereon (in total 26.375%) and church tax, in case the shareholder is subject to church tax because of his individual circumstances. An automatic procedure for deduction of church tax by way of withholding will apply to shareholders being subject to church tax unless the shareholder has filed a blocking notice (Sperrvermerk) with the German Federal Tax Office (details related to the computation of the concrete tax rate including church tax are to be discussed with the individual tax adviser of the relevant shareholder). The taxable capital gain is calculated by deducting the acquisition costs of the shares and the expenses directly related to the disposal from the proceeds of the disposal. Apart from that, except for an annual lump sum savings allowance (Sparer-Pauschbetrag) of up to €1,000 (for individual filers) or up to €2,000 (for married couples and for partners in accordance with the registered partnership law (Gesetz über die Eingetragene Lebenspartnerschaft) filing jointly), private individual shareholders will not be entitled to deduct expenses incurred in connection with the capital investment from their capital gain.
-147-
Taxation of capital gains realized by shareholders tax resident in Germany holding shares as private assets
For individual shareholders (individuals) resident in Germany holding shares as private assets, capital gains realized on the disposal of shares are subject to final withholding tax. Accordingly, capital gains will be taxed at a flat tax rate of 25% plus 5.5% solidarity surcharge thereon (in total 26.375%) and church tax, in case the shareholder is subject to church tax because of his individual circumstances. An automatic procedure for deduction of church tax by way of withholding will apply to shareholders being subject to church tax unless the shareholder has filed a blocking notice (Sperrvermerk) with the German Federal Tax Office (details related to the computation of the concrete tax rate including church tax are to be discussed with the individual tax adviser of the relevant shareholder). The taxable capital gain is calculated by deducting the acquisition costs of the shares and the expenses directly related to the disposal from the proceeds of the disposal. Apart from that, except for an annual lump sum savings allowance (Sparer- Pauschbetrag) of up to €801 (for individual filers) or up to €1,602 (for married couples and for partners in accordance with the registered partnership law (Gesetz über die Eingetragene Lebenspartnerschaft) filing jointly), private individual shareholders will not be entitled to deduct expenses incurred in connection with the capital investment from their capital gain.
In case the flat tax results in a higher tax burden as opposed to the private shareholder’s individual tax rate the private shareholder can opt for taxation at his individual personal income tax rate. In that case, the withholding tax (including solidarity surcharge) withheld will be credited against the income tax. However, pursuant to the German tax authorities the private shareholders are nevertheless not entitled to deduct expenses incurred in connection with the capital investment from their income. The option can be exercised only for all capital income from capital investments received in the relevant assessment period uniformly and married couples as well as for partners in accordance with the registered partnership law filing jointly may only jointly exercise the option.
Capital losses arising from the sale of the shares can only be offset against other capital gains resulting from the disposition of the shares or shares in other stock corporations during the same calendar year. Offsetting of overall losses with other income (such as business or rental income) and other capital income is not possible. Such losses are to be carried forward and to be offset against positive capital gains deriving from the sale of shares in stock corporations in future years.
The final withholding tax will not apply if the seller of the shares or in case of gratuitous transfer, its legal predecessor has held, directly or indirectly, at least 1% of the Company’s registered share capital at any time during the five years prior to the disposal. In that case capital gains are subject to the partial income rule. Accordingly, only (i) 60% of the capital gains will be taxed at his individual personal income tax rate plus 5.5% solidarity surcharge thereon and church tax (if applicable) and (ii) 60% of the business expenses related to the capital gains are deductible for tax purposes. The withholding tax withheld (including solidarity surcharge) will be credited against the shareholder’s personal income tax liability in Germany.
Taxation of capital gains realized by shareholders tax resident in Germany holding the Company’s shares as business assets
If a shareholder holds shares as business assets, the taxation of capital gains realized on the disposal of such shares depends on whether the respective shareholder is a corporation, a sole proprietor or a partnership:
(a) Corporations
Capital gains realized on the disposal of shares by a corporate shareholder are generally exempt from corporate income tax and trade tax. However, 5% of the tax exempt capital gains are deemed to be non-deductible business expenses for tax purposes and therefore are subject to corporate income tax (plus solidarity surcharge) and trade tax; i.e., tax exemption of 95%. Business expenses incurred in connection with the capital gains are entirely tax deductible.
Capital losses incurred upon the disposal of shares or other impairments of the share value are not tax deductible. A reduction of profit is also defined as any losses incurred in connection with a loan or security in the event the loan or the security is granted by a shareholder or by a related party thereto or by a third person with the right of recourse against the before mentioned persons and the shareholder holds directly or indirectly more than 25% of the company’s registered share capital.
Special regulations apply, if the shares are held as trading portfolio assets by a credit institution, a security institution, a financial service institution or a financial enterprise within the meaning of the German Banking Act (Kreditwesengesetz) as well as by a life insurance company, a health insurance company or a pension fund (see “—(i) Corporations”).
-148-
(b) Sole proprietors
If the shares are held by a sole proprietor, capital gains realized on the disposal of the shares are subject to the partial income rule. Accordingly, only (i) 60% of the capital gains will be taxed at his /her individual personal income tax rate plus 5.5% solidarity surcharge thereon and church tax (if applicable) and (ii) 60% of the business expenses related to the dividend income are deductible for tax purposes. In addition, 60% of the capital gains are subject to trade tax if the shares are held as business assets of a permanent establishment in Germany within the meaning of the GewStG. The trade tax levied, depending on the applicable municipal trade tax rate and the individual tax situation, is partly or entirely credited against the shareholder’s personal income tax liability.
(c) Partnerships
In case the shares are held by a partnership, the partnership itself is not subject to corporate income tax or personal income tax as well as solidarity surcharge (and church tax) since partnerships qualify as transparent for German tax purposes (Unless the option according to section 1a of the German Corporate Income Tax Act was applied for that the partnership is taxed as a corporation). In this regard, corporate income tax or personal income tax as well as solidarity surcharge (and church tax, if applicable) are levied only at the level of the partner with respect to their relevant part of the profit and depending on their individual circumstances.
If the partner is a corporation, the capital gains will be subject to corporate income tax plus solidarity surcharge (see “—(i) Corporations”). Trade tax will be levied additionally at the level of the partner insofar as the relevant profit of the partnership is not subject to trade tax at the level of the partnership. However, with respect to both corporate income and trade tax, the 95%-exemption rule as described above applies.
If the partner is a sole proprietor (individual), the capital gains are subject to the partial income rule (see “—(ii) Sole proprietors”).
In addition, if the partnership is liable to trade tax, 60% of the capital gains are subject to trade tax at the level of the partnership, to the extent the partners are individuals, and 5% of the capital gains are subject to trade tax, to the extent the partners are corporations. However, if a partner is an individual, depending on the applicable municipal trade tax rate and the individual tax situation, the trade tax paid at the level of the partnership is credited against the partner’s personal income tax liability.
With regard to corporate partners, special regulations apply if they are held as trading portfolio assets by credit institutions, a security institution, financial service institutions or financial enterprises within the meaning of the German Banking Act or life insurance companies, health insurance companies or pension funds, as described above.
(d) Taxation of capital gains realized by shareholders tax resident outside of Germany
Capital gains realized on the disposal of the shares by a shareholder tax resident outside of Germany are subject to German taxation provided that (i) the Company’s shares are held as business assets of a permanent establishment or as business assets for which a permanent representative has been appointed in Germany, or (ii) the shareholder or, in case of a gratuitous transfer, its legal predecessor has held, directly or indirectly at least 1% of the company’s shares capital at any time during a five years period prior to the disposal. In these cases, capital gains are generally subject to the same rules as described above for shareholders resident in Germany. However, it is unclear whether in case of a corporation being shareholder of the Company the 5% taxation (see “— Corporations— Taxation of capital gains realized by shareholders tax resident in Germany holding the Company’s shares as business assets”) applies or whether the capital gains are fully exempt from German tax.
However, except for the cases referred to in (i) above, some of the double tax treaties concluded with Germany provide for a full exemption from German taxation.
-149-
Inheritance and gift tax
The transfer of the Company’s shares to another person by way of succession or donation is subject to German inheritance and gift tax (Erbschaft-und Schenkungsteuer) if:
| i. | the descedent, the donor, the heir, the donee or any other beneficiary has his /her /its residence, domicile, registered office or place of management in Germany at the time of the transfer, or is a German citizen who has not stayed abroad for more than five consecutive years without having a residence in Germany; |
| ii. | (irrespective of the personal circumstances) the shares are held by the decedent or donor as business assets for which a permanent establishment in Germany is maintained or a permanent representative is appointed in Germany; or |
| iii. | (irrespective of the personal circumstances) at least 10% of the shares are held directly or indirectly by the decedent or person making the gift, himself or together with a related party in terms of Section 1 paragraph 2 Foreign Tax Act. |
Special regulations apply to qualified German citizens who maintain neither a residence nor their domicile in Germany but in a low tax jurisdiction and to former German citizens, also resulting in inheritance and gift tax. The few double tax treaties on inheritance and gift tax which Germany has entered into provide that German inheritance and gift tax is levied only in case of (i) and, with certain restrictions, in case of (ii).
Other taxes
No German capital transfer tax (Kapitalverkehrsteuer), value added tax (Umsatzsteuer), stamp duty (Stempelgebühr) or similar taxes are levied when acquiring, holding or transferring the Company’s shares. No value added tax will be levied unless the shareholder validly opts for it. Net wealth tax (Vermögensteuer) is currently not levied in Germany.
On January 22, 2013, the Council of the European Union approved the resolution of the ministers of finance from 11 EU member states (including Germany) to introduce Financial Transaction Tax, or FTT, within the framework of enhanced cooperation. On February 14, 2013, the European Commission accepted the proposal for a Council Directive implementing enhanced cooperation in the area of financial transaction tax. The plan focuses on levying a financial tax of 0.1% (0.01% for derivates) on the purchase and sale of financial instruments.
A joint statement issued by 10 of the 11 participating EU member states in October 2016 reaffirmed the intention to introduce FTT. However, at the moment not many details are available. Thus, it is not known to what extent the elements of the European Commission’s proposal outlined in the preceding paragraph will be followed in relation to the taxation of shares. The FTT proposal remains subject to negotiation between the participating Member States and is subject to political discussion. It may therefore be altered prior to the implementation, the timing of which remains unclear. The European Commission has committed to putting forward a proposal by January 1, 2024 and has published a working paper in June 2023. However, it is not expected that any proposal would be agreed on in the short term. Additional EU member states may decide to participate. Prospective holders of the shares are advised to seek their own professional advice in relation to FTT.
9. Dividends and paying agents
Not applicable.
10. Statement by experts
Not applicable.
11. Documents on display
We are subject to the informational requirements of the Exchange Act. Accordingly, we are required to file reports and other information with the SEC, including Annual Reports and reports on Form 6-K. The SEC maintains a website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
12. Subsidiary information
Not applicable.
-150-
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Market risk arises from our exposure to fluctuation in currency exchange rates. We are exposed to such market risks in the ordinary course of our business as our exposure to the U.S. dollar broadens from future expenses and revenues that may be derived from the United States. Currently, we do not have any exchange rate hedging arrangements in place.
We do not engage in activities involving other market price risks. For additional information on market risk, refer to Note D.12 ‘Risk’ within our audited financial statements and notes prepared in accordance with IFRS-IASB, included in “ITEM 18. FINANCIAL STATEMENTS.”
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
| A. | Debt securities |
Not applicable.
| B. | Warrants and rights |
Not applicable.
| C. | Other securities |
Not applicable.
| D. | American Depositary Shares |
Not applicable.
-151-
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
| A. | Defaults |
No matters to report.
| B. | Arrears and delinquencies |
No matters to report.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
| A. | Material modifications to instruments |
Not applicable.
| B. | Material modifications to rights |
Not applicable.
| C. | Withdrawal or substitution of assets |
Not applicable.
| D. | Change in trustees or paying agents |
Not applicable.
| E. | Use of proceeds |
Not applicable.
-152-
ITEM 15. CONTROLS AND PROCEDURES
| A. | Disclosure controls and procedures |
As of December 31, 2023, under the supervision of and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act). There are inherent limitations to the effectiveness of any disclosure controls and procedures system, including the possibility of human error and circumventing or overriding them. Even if effective, disclosure controls and procedures can provide only reasonable assurance of achieving their control objectives.
Based on such evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective to provide reasonable assurance that the information we are required to disclose in the reports we file or submit under the Exchange Act is (1) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (2) accumulated and communicated to our management to allow timely decisions regarding required disclosures.
| B. | Management’s annual report on internal control over financial reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) of the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based upon criteria established in Internal Control – Integrated Framework (2013) by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2023.
| C. | Attestation report of the registered public accounting firm |
EY GmbH & Co. KG Wirtschaftsprüfungsgesellschaft, or EY, an independent registered accounting firm, has issued an attestation report on the effectiveness of our internal control over financial reporting as of December 31, 2023, which expressed an unqualified opinion thereon, as stated in their report included herein. See “Reports of independent registered public accounting firm” on page F-2.
| D. | Changes in internal control over financial reporting |
There have been certain changes in our internal control over financial reporting during the period covered by this Annual Report, relating to the implementation of internal controls over inventory management and valuation following the manufacture of GOHIBIC (vilobelimab) for commercial sale under the EUA in the United States, which have materially affected our internal control over financing reporting.
ITEM 16. RESERVED
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
The Board of Directors has determined that each of Mr. Anthony Gibney, Mr. Nicolas Fulpius, Mr. Mark Kuebler and Mr. Richard Brudnick is an audit committee financial expert, as that term is defined by the SEC, and all four are independent for the purposes of SEC and Nasdaq rules relating to the independence of the audit committee.
ITEM 16B. CODE OF ETHICS
We adopted a code of ethics that applies to all of our employees, officers and directors and posted the full text of our code of ethics on the investor relations section of our website, www.inflarx.com. We intend to disclose future amendments to our code of ethics, or any waivers of such code, on our website or in public filings. The information on our website is not incorporated by reference into this Annual Report, and you should not consider information contained on our website to be a part of this Annual Report.
-153-
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
| A. | Audit fees |
The audit committee has adopted a policy that requires the pre-approval of all services performed for us by our independent registered public accounting firm. All audit-related services rendered by our independent registered public accounting firm were pre-approved by the audit committee and are compatible with maintaining the auditor’s independence.
Set forth below are the total fees billed (or expected to be billed), on a consolidated basis, by the independent registered public accounting firm or their affiliates for providing audit and other professional services in each of the last two years.
Audit fees in 2023 amounted to €1,009,952 and relate to audit services. These services were provided by our principal accountants, EY GmbH & Co. KG Wirtschaftsprüfungsgesellschaft (formerly Ernst & Young GmbH Wirtschaftsprüfungsgesellschaft) in connection with our annual audit, quarterly reviews and review of registration statements for the Company.
Audit fees in 2022 amounted to €988,541 and relate to audit services. These services were provided by our principal accountants, EY GmbH & Co. KG Wirtschaftsprüfungsgesellschaft (formerly Ernst & Young GmbH Wirtschaftsprüfungsgesellschaft) in connection with our annual audit, quarterly reviews and review of registration statements for the Company.
| B. | Audit-related fees |
None.
| C. | Tax fees |
None.
| D. | All other fees |
None.
| E. | Audit Committee’s pre-approval policies and procedures |
The audit committee is responsible for the appointment, replacement, compensation, evaluation and oversight of the work of the independent auditors. As part of this responsibility, the audit committee pre-approves all audit and non-audit services performed by the independent auditors in order to assure that they do not impair the auditor’s independence from the Company in accordance with the audit committee’s pre-approval policy.
| F. | Audit work performed by other than principal accountant if greater than 50% |
Not applicable.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
-154-
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
None.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G. CORPORATE GOVERNANCE
For a description of the significant ways in which our corporate governance practices differ from those required for U.S. companies listed on Nasdaq, see “ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES — 3. Board practices — Corporate governance practices.”
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
ITEM 16J. INSIDER TRADING POLICIES
Not applicable.
ITEM 16K. CYBERSECURITY RISK MANAGEMENT AND STRATEGY
Our Board of Directors is responsible for reviewing the Company’s cybersecurity risk management and control systems in relation to the financial reporting by the Company, including the Company’s cybersecurity strategy. Our Board of Directors has delegated periodic oversight of, as appropriate cybersecurity risk management to the Audit Committee, who reports to our Board of Directors.
Our IT department is responsible for targeted and regular monitoring of cybersecurity risks. They independently and continuously monitor cybersecurity risks and countermeasures to defend against such threats and, in the event of a cybersecurity threat or cybersecurity incident, inform Executive Management, our Audit Committee and our Board of Directors. In addition to the regular meetings between Executive Management and the individual risk owners mainly consisting out of the various departments’ heads, a comprehensive cybersecurity risk analysis for internal and external risks is carried out as appropriate.
The cybersecurity risks identified and evaluated by the IT department are included in an overall risk catalogue. The identified cybersecurity risks are recorded, described, documented and evaluated in the overall risk catalogue. Changes are also documented accordingly. According to the priority of the cybersecurity risks as result of the risk evaluation, risks are addressed by concrete actions and, if appropriate and possible, necessary countermeasures. In order to be able to react quickly and flexibly to cybersecurity risks, risk management is integrated into existing processes and reporting channels. Our risk management program considers cybersecurity risks alongside other company risks, and our enterprise risk professionals consult with company subject matter experts to gather information necessary to identify cybersecurity risks and evaluate their nature and severity, as well as identify mitigations and assess the impact of those mitigations on residual risk. We may engage third parties from time to time to conduct risk assessments.
-155-
The main cybersecurity risks we continuously monitor include threats and potential incidents resulting in the unavailability of central IT systems, the loss of critical business data, data theft, intellectual property theft, fraud, extortion, harm to employees and patients, violation of privacy laws and other litigation and legal risks, and risks to our reputation. The materialization of these cybersecurity threats may materially affect or may be reasonably likely to materially affect the Company, including its business strategy, results of operations, or financial condition. The unavailability of central IT systems for example, would result in an interruption or delay of any clinical development activities of the Company. The loss of critical business data such as the results of clinical study reports and underlying information, such as tables, listings and filings, could retrospectively destroy the work of several years of clinical development and respective cost. Data theft could, if critical, confidential or proprietary business data is concerned, result in a loss in strength of competition. Intellectual property theft can jeopardize our ability to generate revenue for our shareholders. Breach of privacy laws can put our employees and patients at risk of additional cybersecurity and personal risks, which could lead to a litigation and further costs for the Company. Cybersecurity risks may also result in our Company’s reputation being affected.
Executive Management, our IT department and employees are the foundation of an effective cybersecurity risk management. The Company implemented IT Security guidelines, which stipulate measures to securely handle personal data, the settings for a reasonably secure password for devices and software, the handling of mobile IT devices as well as the proper use of the internet and e-mail communication software. The Company also implemented two-factor-authentication to access and use a Company user account, including e-mail. All employees as well as all members of Executive Management are trained on IT security on a regular basis.
While we do not believe that our business strategy, results of operations or financial condition have been materially adversely affected by any cybersecurity incidents, we describe whether and how future incidents could have a material impact on our business strategy, results of operations or financial condition in “Item 3 C. Risk Factors—General risk factors—Cyber incidents or other failures in IT systems could result in information theft, data corruption and significant disruption of our business operations.” Additionally, although we have insurance coverage for cybersecurity events, there can be no assurance that we will be able to maintain our insurance coverage or it will be enough to cover the cost associated with one or more cybersecurity events. See “Item 3 C. Risk factors—General risk factors—We may not be able to maintain sufficient insurance to cover us for potential litigation or other risks.”
-156-
PART III
ITEM 17. FINANCIAL STATEMENTS
We responded to Item 18 in lieu of this item.
ITEM 18. FINANCIAL STATEMENTS
Financial statements are filed as part of this Annual Report, see pages F-1 to F-35 to this Annual Report.
ITEM 19. EXHIBITS
| * | Filed herewith. |
| + | Previously filed. |
| † | Confidential treatment granted as to portions of the exhibit. Confidential materials omitted and filed separately with the SEC. |
-157-
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on Form 20-F on its behalf.
| InflaRx N.V. | |||
| By: | /s/ Niels Riedemann | ||
| Name: | Niels Riedemann | ||
| Title: | Chief Executive Officer and Director | ||
Date: March 21, 2024
| By: | /s/ Thomas Taapken | ||
| Name: | Thomas Taapken | ||
| Title: | Chief Financial Officer | ||
Date: March 21, 2024
-158-
Index to Consolidated Financial Statements
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of InflaRx N.V.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of InflaRx N.V. and subsidiaries (the Company) as of December 31, 2023 and 2022, the related consolidated statements of operations and comprehensive loss, changes in shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 20, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing a separate opinion on the critical audit matters or on the accounts or disclosures to which they relate.
F-2
Recognition of clinical trial and contracted manufacturing expenses
| Description of the Matter | As discussed in Note B.3 to the consolidated financial statements, the Company recognizes research and development (R&D) expenses, which include costs for clinical trial and contracted manufacturing, charged by contract research organizations and contract manufacturing organizations (together, “clinical vendors”). The total clinical trial and contracted manufacturing expenses recognized in the year-ended December 31, 2023, amounted to €31.8 million and the related prepayments and accrued liabilities from R&D projects were €3.7 million and €4.4 million, respectively, as of December 31, 2023.
The Company’s determination of clinical trial and contracted manufacturing expenses involves estimating a percentage-of-completion, whereby the degree to which services have been rendered for the individual project activities contracted from the clinical vendors is assessed and estimated by management. While the Company’s estimates of clinical trial and contracted manufacturing expenses are primarily based on information received related to each study from its clinical vendors, the Company may need to make an estimate for costs incurred based on management judgment. Payments for these activities are based on the terms of the individual arrangements, which differ from the pattern of costs incurred.
Auditing clinical trial and contracted manufacturing expenses was challenging, due to the judgement and subjectivity involved in management’s assessment of the progress of clinical trial and contracted manufacturing expenses, relative to the costs incurred, to estimate the related accrued liabilities and prepayments from R&D projects, and the evaluation of the completeness and accuracy of the data used in the estimate. |
How We Addressed the Matter in Our Audit
|
We obtained an understanding, evaluated the design and tested the operating effectiveness of controls related to the Company’s estimation of clinical vendor costs for clinical trial and contracted manufacturing expenses. For example, we tested controls over management’s review of the estimated percentage-of-completion used in determining the amount of clinical trial and contracted manufacturing expenses and the related impacts to prepayments and accrued liabilities from R&D projects.
To assess the accounting for clinical trial and contracted manufacturing expenses, our audit procedures included, among others, testing the accuracy and completeness of the underlying data used in the percentage-of-completion estimates, by assessing the progress of the clinical trial activities through discussion with the Company’s R&D project managers, who oversee these activities and by reviewing progress reports, invoices, and other correspondence provided by the clinical vendors to the R&D project managers. We inspected the Company’s clinical vendor contracts, amendments, and pending change orders to assess whether the key financial and contractual terms align with the amounts recognized. We also performed analytical reviews of fluctuations in the percentage-of-completion by project throughout the period subject to audit. We compared invoices received from and cash disbursements made to clinical vendors prior to and following year-end and evaluated credit memos received from clinical vendors prior to and following year-end. |
F-3
Net realizable value of unfinished goods inventory
| Description of the Matter | At December 31, 2023, the Company’s net unfinished goods inventory balance was €10.6 million, all of which relates to its severe COVID-19 treatment Gohibic. As discussed in Notes B.2, B.3 and D.5 of the consolidated financial statements, in order to value inventory, including unfinished goods, at the lower of cost or net realizable value, the Company reviews its inventory for excess amounts or obsolescence, primarily using estimates of expected future sales, which are sensitive to significant inputs and assumptions, such as expected medical need and expected market penetration.
Auditing management’s estimate of the net realizable value of unfinished goods inventory, which is based, in part, on estimates of expected future sales was complex and highly judgmental, due to the Company having a limited sales history to consider. Additionally, these estimates rely, in part, on management’s assumptions about future events outside of the Company’s control, such as continuation of the emergency use authorization in the United States and granting of marketing authorization in the European Union. |
How We Addressed the Matter in Our Audit |
To test management’s estimate of the net realizable value of unfinished goods inventory, which is based, in part, on estimates of expected future sales, we performed audit procedures that included, among others, comparing inputs used in developing assumptions of medical need to data points observable from United States government COVID-19 data and the Company’s clinical trial results for Gohibic. We also compared inputs used in developing assumptions for market penetration, to studies on market share achievable in the pharmaceutical industry. We performed sensitivity analyses over the medical need and market penetration assumptions. We evaluated management’s comparison of unfinished goods inventory to the estimates of expected future sales, which included consideration of applicable inventory expiration dates. Additionally, we evaluated the reasonableness of management’s assessment of the probability that the emergency use authorization in the United States remains in place and that the marketing authorization in the European Union is granted, by reference to correspondence with the relevant regulatory authorities and inquiries of management in relation to the Company’s actions to achieve any required conditions for the authorizations. We also tested the clerical accuracy of the calculations underlying the Company’s estimates of expected future sales. |
/s/ EY GmbH & Co. KG Wirtschaftsprüfungsgesellschaft
We have served as the Company’s auditor since 2020.
Munich, Germany
March 20, 2024
F-4
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of InflaRx N.V.
Opinion on Internal Control Over Financial Reporting
We have audited InflaRx N.V. and subsidiaries’ internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, InflaRx N.V. and subsidiaries (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated statements of financial position of the Company as of December 31, 2023 and 2022, the related consolidated statements of operations and comprehensive loss, changes in shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2023, and the related notes and our report dated March 20, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statementsfor external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that: (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/
March 20, 2024
F-5
InflaRx N.V. and subsidiaries
Consolidated statements of operations and comprehensive loss for the years ended December 31, 2023, 2022 and 2021
| Note | 2023 | 2022 | 2021 | ||||||||||||
| (in €, except for share data) | |||||||||||||||
| Revenues | C.1. | ||||||||||||||
| Cost of sales | C.2. | ( | ) | ||||||||||||
| Gross profit | ( | ) | — | — | |||||||||||
| Sales and marketing expenses | C.3. | ( | ) | ||||||||||||
| Research and development expenses | C.4. | ( | ) | ( | ) | ( | ) | ||||||||
| General and administrative expenses | C.5. | ( | ) | ( | ) | ( | ) | ||||||||
| Other income | C.6. | ||||||||||||||
| Other expenses | ( | ) | ( | ) | ( | ) | |||||||||
| Operating result | ( | ) | ( | ) | ( | ) | |||||||||
| Finance income | C.8. | ||||||||||||||
| Finance expenses | C.8. | ( | ) | ( | ) | ( | ) | ||||||||
| Foreign exchange result | C.8. | ( | ) | ||||||||||||
| Other financial result | C.8. | ( | ) | ( | ) | ||||||||||
| Income taxes | — | — | — | ||||||||||||
| Loss for the period | ( | ) | ( | ) | ( | ) | |||||||||
| Other comprehensive income (loss) that may be reclassified to profit or loss in subsequent periods: | |||||||||||||||
| Exchange differences on translation of foreign currency | |||||||||||||||
| Total comprehensive loss | ( | ) | ( | ) | ( | ) | |||||||||
| Share information (based on loss for the period) | C.9. | ||||||||||||||
| Weighted average number of shares outstanding | |||||||||||||||
| ( | ) | ( | ) | ( | ) | ||||||||||
| Loss for the period | ( | ) | ( | ) | ( | ) | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
InflaRx N.V. and subsidiaries
Consolidated statements of financial position as of December 31, 2023 and 2022
| Note | December 31, 2023 | December 31, 2022 | |||||||||
| ASSETS | (in €) | ||||||||||
| Non-current assets | |||||||||||
| Property and equipment | D.1. | ||||||||||
| Right-of-use assets | D.2. | ||||||||||
| Intangible assets | D.3. | ||||||||||
| Other assets | D.6. | ||||||||||
| Financial assets | D.8. | ||||||||||
| Total non-current assets | |||||||||||
| Current assets | |||||||||||
| Inventories | D.5. | ||||||||||
| Current other assets | D.6. | ||||||||||
| Tax receivable | D.7. | ||||||||||
| Financial assets from government grants | D.8. | ||||||||||
| Other financial assets | D.8. | ||||||||||
| Cash and cash equivalents | D.9. | ||||||||||
| Total current assets | |||||||||||
| TOTAL ASSETS | |||||||||||
| EQUITY AND LIABILITIES | |||||||||||
| Equity | D.10. | ||||||||||
| Issued capital | D.10.(a). | ||||||||||
| Share premium | D.10.(a). | ||||||||||
| Other capital reserves | D.10.(a). | ||||||||||
| Accumulated deficit | D.10.(a). | ( | ) | ( | ) | ||||||
| Other components of equity | D.10.(a). | ||||||||||
| Total equity | |||||||||||
| Non-current liabilities | |||||||||||
| Lease liabilities | E.2. | ||||||||||
| Other liabilities | |||||||||||
| Total non-current liabilities | |||||||||||
| Current liabilities | |||||||||||
| Trade and other payables | D.11. | ||||||||||
| Liabilities from government grants | |||||||||||
| Lease liabilities | E.2. | ||||||||||
| Employee benefits | |||||||||||
| Other liabilities | D.11. | ||||||||||
| Total current liabilities | |||||||||||
| Total liabilities | |||||||||||
| TOTAL EQUITY AND LIABILITIES | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
InflaRx N.V. and subsidiaries
Consolidated statements of changes in shareholders’ equity for the years ended December 31, 2023, 2022 and 2021
| Note | Shares outstanding | Issued capital | Share premium | ||||||||||||
| (in €) | |||||||||||||||
| Balance as of January 1, 2021 | |||||||||||||||
| Loss for the period | — | — | |||||||||||||
| Exchange differences on translation of foreign currency | — | — | |||||||||||||
| Total comprehensive loss | — | — | |||||||||||||
| Issuance of ordinary shares | |||||||||||||||
| Transaction costs | — | ( | ) | ||||||||||||
| Equity-settled share-based payments | C.10. | — | |||||||||||||
| Share options exercised | |||||||||||||||
| Balance as of December 31, 2021 | |||||||||||||||
| Loss for the Period | — | — | |||||||||||||
| Exchange differences on translation of foreign currency | — | — | |||||||||||||
| Total comprehensive loss | — | — | |||||||||||||
| Issuance of ordinary shares | D.10.(a). | ||||||||||||||
| Transaction costs | — | ( | ) | ||||||||||||
| Equity-settled share-based payments | C.10. | ||||||||||||||
| Share options exercised | |||||||||||||||
| Balance as of December 31, 2022 | |||||||||||||||
| Loss for the Period | — | — | |||||||||||||
| Exchange differences on translation of foreign currency | — | — | |||||||||||||
| Total comprehensive loss | — | — | |||||||||||||
| Issuance of ordinary shares | D.10.(a). | ||||||||||||||
| Transaction costs | — | — | ( | ) | |||||||||||
| Equity-settled share-based payments | C.10. | ||||||||||||||
| Share options exercised | |||||||||||||||
| Balance as of December 31, 2023 | |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
InflaRx N.V. and subsidiaries
Consolidated statements of changes in shareholders’ equity for the years ended December 31, 2023, 2022 and 2021
| Note | Other capital reserves | Accumulated deficit | Other components of equity | Total equity | |||||||||||||||
| (in €) | |||||||||||||||||||
| Balance as of January 1, 2021 | ( | ) | ( | ) | |||||||||||||||
| Loss for the Period | — | ( | ) | ( | ) | ||||||||||||||
| Exchange differences on translation of foreign currency | — | ||||||||||||||||||
| Total comprehensive Loss | — | ( | ) | ( | ) | ||||||||||||||
| Issuance of ordinary shares | D.10. | ||||||||||||||||||
| Transaction costs | — | — | — | ( | ) | ||||||||||||||
| Equity-settled share-based payments | C.10. | ||||||||||||||||||
| Share options exercised | — | — | — | ||||||||||||||||
| Balance as of December 31, 2021 | ( | ) | |||||||||||||||||
| Loss for the period | — | ( | ) | — | ( | ) | |||||||||||||
| Exchange differences on translation of foreign currency | — | — | |||||||||||||||||
| Total comprehensive loss | — | ( | ) | ( | ) | ||||||||||||||
| Issuance of ordinary shares | D.10. | — | — | — | |||||||||||||||
| Transaction costs | — | — | — | ( | ) | ||||||||||||||
| Equity-settled share-based payments | C.10. | ||||||||||||||||||
| Share options exercised | — | — | — | ||||||||||||||||
| Balance as of December 31, 2022 | ( | ) | |||||||||||||||||
| Loss for the period | — | ( | ) | — | ( | ) | |||||||||||||
| Exchange differences on translation of foreign currency | — | — | |||||||||||||||||
| Total comprehensive loss | — | ( | ) | ( | ) | ||||||||||||||
| Issuance of ordinary shares | — | — | — | ||||||||||||||||
| Transaction costs | — | — | — | ( | ) | ||||||||||||||
| Equity-settled share-based payments | C.10. | — | — | ||||||||||||||||
| Share options exercised | C.10. | — | — | — | |||||||||||||||
| Balance as of December 31, 2023 | ( | ) | |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-9
InflaRx N.V. and subsidiaries
Consolidated statements of cash flows for the years ended December 31, 2023, 2022 and 2021
| Note | 2023 | 2022 | 2021 | ||||||||||||
| (in €) | |||||||||||||||
| Operating activities | |||||||||||||||
| Loss for the period | ( | ) | ( | ) | ( | ) | |||||||||
| Adjustments for: | |||||||||||||||
| Depreciation & amortization of property and equipment, right-of-use assets and intangible assets | |||||||||||||||
| Net finance income | C.8. | ( | ) | ( | ) | ( | ) | ||||||||
| Share-based payment expense | C.10. | ||||||||||||||
| Net foreign exchange differences | |||||||||||||||
| Changes in: | |||||||||||||||
| Financial assets from government grants | D.8. | ( | ) | ||||||||||||
| Other assets | ( | ) | ( | ) | |||||||||||
| Employee benefits | ( | ) | ( | ) | |||||||||||
| Other liabilities | |||||||||||||||
| Liabilities from government grants received | D.8. | ( | ) | ( | ) | ||||||||||
| Trade and other payables | D.11. | ( | ) | ||||||||||||
| Inventories | D.5. | ( | ) | ||||||||||||
| Interest received | |||||||||||||||
| Interest paid | ( | ) | ( | ) | ( | ) | |||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ( | ) | |||||||||
| Investing activities | |||||||||||||||
| Purchase of intangible assets and property and equipment | ( | ) | ( | ) | ( | ) | |||||||||
| Purchase of current and non-current financial assets | ( | ) | ( | ) | ( | ) | |||||||||
| Proceeds from the maturity of current financial assets | |||||||||||||||
| Net cash from/ (used in) investing activities | ( | ) | ( | ) | |||||||||||
| Financing activities | |||||||||||||||
| Proceeds from issuance of ordinary shares | D.10. | ||||||||||||||
| Transaction costs from issuance of ordinary shares | D.10. | ( | ) | ( | ) | ( | ) | ||||||||
| Proceeds from exercise of share options | C.10 | ||||||||||||||
| Repayment of lease liabilities | ( | ) | ( | ) | ( | ) | |||||||||
| Net cash from financing activities | |||||||||||||||
| Net decrease in cash and cash equivalents | ( | ) | ( | ) | ( | ) | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | ( | ) | |||||||||||||
| Cash and cash equivalents at beginning of period | |||||||||||||||
| Cash and cash equivalents at end of period | D.9. | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-10
InflaRx N.V. and subsidiaries
A. Notes to the consolidated financial statements
1. Corporate information
The consolidated financial statements of InflaRx N.V. and its subsidiaries (collectively, the “Group”) for the year ended December 31, 2023 were authorized for issue in accordance with a resolution of the Board of Directors on March 20, 2024. InflaRx N.V. (the “Company”) is a Dutch public company with limited liability (naamloze vennootschap) with its corporate seat in Amsterdam, The Netherlands, and is registered in the Commercial Register of The Netherlands Chamber of Commerce Business Register under CCI number 68904312. The Company’s registered office is at Winzerlaer Straße 2 in 07745 Jena, Germany. Since November 10, 2017, the Company’s ordinary shares have been listed on the Nasdaq Global Select Market under the symbol “IFRX”.
The Company and its subsidiaries, collectively, are a biotechnology group pioneering anti-inflammatory therapeutics by applying its proprietary anti-C5a and anti-C5aR technologies to discover, develop and commercialize highly potent and specific inhibitors of the complement activation factor known as C5a and its receptor C5aR.
Subsidiaries are all entities over which the Group has control. The Group controls an entity when the Group is exposed to, or has rights to, variable returns from its involvement with the entity and could affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is obtained by the Group. They are deconsolidated from the date control ceases. The acquisition method of accounting is used to account for business combinations by the Group. Intercompany transactions, balances and unrealized gains on transactions between Group companies are eliminated. Unrealized losses are also eliminated unless the transaction provides evidence of an impairment of the transferred asset.
The
Group’s subsidiaries as at December 31, 2023 are set out below.
| Place of business/ country of | Functional | Ownership
interest held by the Company | |||||||||||||
| Name | incorporation | currency | 2023 | 2022 | Principal activities | ||||||||||
| InflaRx GmbH | % | % | |||||||||||||
| InflaRx Pharmaceuticals, Inc. | % | % | |||||||||||||
B. Material accounting policies
1. Basis of preparation
The consolidated financial statements of the Group have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (herein “IFRS”).
The consolidated financial statements have been prepared on a historical cost basis. These consolidated financial statements of the Group comprise the Company and its wholly owned subsidiaries, InflaRx GmbH and InflaRx Pharmaceuticals, Inc. The consolidated financial statements are presented in Euro (€). The presentation currency of the Group is the Euro, as the functional currency of the largest operating company, InflaRx GmbH, continues to be the Euro. Effective January 1, 2023, the functional currency of InflaRx N.V. changed from the U.S. dollar to the Euro due to a change in the Company’s operational function and, in turn, a change in the primary currency of its underlying transactions. This change in functional currency has been accounted for prospectively. The functional currency of InflaRx Pharmaceuticals, Inc. is the U.S. dollar ($), as most of their income and expenses occurred in U.S. dollars in 2023. All financial information presented in Euro has been rounded to the nearest Euro, unless stated otherwise.
F-11
2. Summary of material accounting policies
This section describes material accounting policies adopted in the preparation of these consolidated financial statements. These policies have been consistently applied to all the years presented, unless otherwise stated.
(a) New and amended standards adopted by the Group
The following amendments were adopted effective January 1, 2023, and do not have a material impact on the consolidated financial statements of the Group:
| ● | IFRS 17 insurance contracts | |
| ● | Amendments to IAS 8 accounting policies, changes in accounting estimates and errors: definition of accounting estimates | |
| ● | Amendments to IAS 12 deferred tax related to assets and liabilities arising from a single transaction | |
| ● | Amendments to IAS 1 and IFRS practice statement 2 - disclosure of accounting policies |
Accounting policies for the following IFRS standards have been applied starting in Q2 2023 for the first time, as no transactions in the scope of these IFRS standards had been previously recognized.
| ● | IAS 2 inventories |
According to IAS 2, inventories are stated at the lower amount of their cost or at their net realizable value. Cost comprises direct material cost and, where applicable, direct labor costs and those overhead costs that have been incurred in bringing the inventories to their present location and condition. Cost is calculated using the weighted average cost method. Net realizable value represents the estimated selling price less all estimated costs of completion and costs to be incurred in marketing, selling and distribution. Recognizing inventories at net realizable value includes writing down inventories considered excess or obsolete.
| ● | IFRS 15 revenue from contracts with customers |
At present, the Company exclusively uses distributors to sell its products to end customers (e.g., hospitals). The end customers (e.g. hospitals) have been determined to be the customer in these sales arrangements. As such, payments received from the distributors are not contract liabilities but are rather “other liabilities” recognized in “other accrued liabilities.” Revenue is therefore recognized when a performance obligation has been satisfied through the transfer of a promised good or service to a customer, that is, when the customer obtains control of that asset and is measured considering estimated return liabilities and expected rebates or cash discounts.
(b) New standard not yet adopted
The following standards issued will be adopted in a future period, and the potential impact on the Group’s consolidated financial statements, if any, is being assessed:
| ● | Amendments to IFRS 16 leases: leases on sale and leaseback | |
| ● | Amendments to IAS 1 presentation of financial statements: classification of liabilities as current or non-current and non-current liabilities with covenants |
| ● | Amendments to IAS 21 effects of changes in foreign exchange rates: lack of exchangeability |
(c) Current and non-current classification
The Group presents assets and liabilities in the statement of financial position based on current/non-current classification.
Current assets include assets that are sold, consumed or realized as part of the normal operating cycle (operating cycle is assumed to be 12 months), or cash and cash equivalent unless restricted from being exchanged or used to settle a liability for at least 12 months after the reporting period. All other assets are classified as non-current.
Current liabilities, such as trade payables, lease liabilities or employee benefits with a term of up to 12 months, and payables for operating costs or social security charges, are part of the working capital used in the Company’s normal operating cycle. Such operating items are classified as current liabilities even if they are due to be settled more than 12 months after the reporting period. All other liabilities are classified as non-current.
F-12
(d) Foreign currency transactions
Transactions in a foreign currency are initially translated into the respective functional currency using the spot rate prevailing on the dates of the transaction. Monetary items which are not denominated in the functional currency are subsequently translated using the rate applicable at the end of the period. The resulting currency gains and losses are recognized directly in profit or loss.
On consolidation, the assets and liabilities of operations in a currency other than Euro (the presentation currency of the Company) are translated into Euros at the rate of exchange prevailing at the reporting date and their statements of operations are translated with monthly average exchange rates during the reporting period. The exchange differences arising on translation for consolidation are recognized in ‘other comprehensive income’ (OCI). On disposal of a foreign operation, the component of OCI relating to that particular foreign operation is reclassified to profit or loss. OCI is disclosed as ‘other components of equity’ in consolidated statements of financial position.
(e) Grants from government and similar bodies
The Group receives grants from government agencies and similar bodies for the active participation in specific research and development projects. The grants are recognized when there is reasonable assurance that the grant will be received and all grant conditions will be met. If grant funds are received prior to qualifying expenses being incurred or assets purchased or prior to all grant conditions have been met, such amounts are recorded as a liability in other liabilities. If the funds reimburse expenses, the liability is amortized into other operating income in the period in which the corresponding expenses are incurred (or, for expenses incurred prior to all grant conditions being met, in the period in which reasonable assurance that all grant conditions will be met is attained). If the funds reimburse purchased assets, the liability is reduced with a corresponding amount deducted from the asset’s carrying amount upon recording of the qualified asset. According to the terms of the grants, grantors generally have the right to audit qualifying expenses submitted by the Group up to five years after concluding the project sponsored by the government.
In
October 2021, the Group announced that it received a grant of up to €
(f) Notes to the cash flow statement, cash, and cash equivalents
The consolidated statements of cash flows have been prepared using the indirect method for cash flows from operating activities. The cash disclosed in the consolidated statements of cash flows is comprised of cash and cash equivalents. Cash comprises cash on hand and demand deposits. Cash equivalents are short-term bank deposits that are readily convertible to a known amount of cash and are not subject to a significant risk of changes in value with an original maturity of three months or less. Interest paid and received is included in the cash from operating activities.
(g) Research and development expenses
Research and development expenses comprise third party services, wages and salaries, cost of materials, intellectual property related expenses, depreciation and amortization of relevant equipment and intangibles as well as overhead. Research and development expenses mainly consist of costs for clinical trials and manufacturing of the Company’s clinical drug products; additionally, costs are incurred for pre-clinical activities as well as basic research activities.
Development expenses must be capitalized if the criteria of IAS 38 are met. In the periods presented, no development expenses were capitalized because management assessed that not all the recognition criteria of IAS 38 had been met. This assessment is due to the general uncertainties in drug development and the unpredictability of regulatory requirements. Therefore, research and development expenditures are expensed when incurred.
F-13
(h) Employee benefits
(i) Short-term employee benefits
Liabilities for wages and salaries and cash bonuses are measured at the amounts expected to be paid when the liabilities are settled. The liabilities are presented as employee benefits in the consolidated statements of financial position. A liability is recognized if the Group has a present legal or constructive obligation to pay such amount as a result of past service provided by the employee and if such obligation can be estimated reliably.
(ii) Share-based payment transactions
The grant-date fair value of equity-settled share-based payment arrangements granted to employees is generally recognized as an expense, with a corresponding increase in equity, over the vesting period of the awards. The amount recognized as an expense is adjusted to reflect the number of awards for which the related service conditions are expected to be met, including an estimate of forfeitures, such that the amount ultimately recognized is based on the number of awards that meet the related service conditions at the vesting date. For share-based payment awards with immediate vesting, the grant-date fair value of the share-based payment is measured to reflect such conditions and there is no gain or loss recognized for differences between expected and actual outcomes.
(i) Lease arrangements
The
Group leases various properties, laboratory and office equipment and cars. Rental contracts are typically made for fixed periods of
(i) Right-of-use assets
The Group recognizes right-of-use assets at the commencement date of the lease (i.e., the date the underlying asset is available for use). Right-of-use assets are measured at cost, less any accumulated depreciation and impairment losses, and adjusted for any re-measurement of lease liabilities. The cost of right-of-use assets includes the amount of lease liabilities recognized, initial direct costs incurred, and lease payments made at or before the commencement date less any lease incentives received. Unless the Group is reasonably certain to obtain ownership of the leased asset at the end of the lease term, the recognized right-of-use assets are depreciated on a straight-line basis over the shorter of its estimated useful life and the lease term. On December 31, 2023, the remaining useful lives of the Company’s right-of-use assets ranged between 3 and 41 months. Right-of-use assets are subject to impairment.
(ii) Lease liabilities
At the commencement date of the lease, the Group recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in-substance fixed payments) less any lease incentives receivable, variable lease payments which depend on an index or a rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option reasonably certain to be exercised by the Group and payments of penalties for terminating a lease, if the lease term reflects the Group exercising the option to terminate. The variable lease payments that do not depend on an index or a rate are recognized as expense in the period on which the event or condition that triggers the payment occurs.
In calculating the present value of lease payments, the Group uses the incremental borrowing rate at the lease commencement date, since the interest rate implicit in the lease is not readily determinable. After the commencement date, the amount of lease liabilities is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of lease liabilities is re-measured if there is a modification, a change in the lease term, a change in the in-substance fixed lease payments or a change in the assessment to purchase the underlying asset.
F-14
(iii) Short-term leases and leases of low-value assets
The Group applies the short-term lease recognition exemption to its short-term leases of equipment (i.e., those leases that have a lease term of 12 months or less from the commencement date and do not contain a purchase option). It also applies the lease of low-value assets recognition exemption to leases of office equipment that are considered of low value. Lease payments on short-term leases and leases of low-value assets are recognized as expense on a straight-line basis over the lease term.
(iv) Determining the lease term of contracts
After the commencement date, the Group reassesses the lease term if there is a significant event or change in circumstances that is within its control and affects its ability to exercise the option to renew.
The Group further determines the lease term as the non-cancellable term of the lease, together with any periods covered by an option to extend the lease if it is reasonably certain to be exercised, or any periods covered by an option to terminate the lease, if it is reasonably certain not to be exercised.
The leases which currently also result in the capitalization of a right of use asset, do not include any renewal options. For future lease contracts with potential renewal options the Company applies judgement in evaluating whether it is reasonably certain to exercise the option to renew. In doing so, management would consider all relevant factors that create an economic incentive for it to exercise the renewal.
(j) Interest income
Interest income is derived from interest-bearing financial assets, including cash equivalents. Interest income on cash and cash equivalents, financial assets at amortized cost calculated using the effective interest rate method is recognized in the consolidated statements of operations and comprehensive loss as part of finance income.
(k) Intangible assets
Intangible
assets mainly comprise purchased IT software. Intangible assets are initially measured at acquisition cost, including any directly attributable
costs of preparing the asset for its intended use less accumulated amortization and accumulated impairment losses, if any. Amortization
begins when an asset is available for use and amortization is calculated using the straight-line method to allocate cost over the estimated
useful lives. The useful lives of intangible assets are reviewed at each reporting date. Software is amortized over
(l) Property and equipment
Laboratory and office equipment are stated at historical cost less accumulated depreciation and accumulated impairment losses, if any. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
All repairs and maintenance are recognized in profit or loss during the financial period in which they are incurred, because they do not constitute a separate asset.
Depreciation on laboratory and office equipment is calculated using the straight-line method to allocate their cost over their estimated useful lives, as follows:
| ● | Laboratory equipment: | |
| ● | Office equipment: |
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at the end of each reporting period.
Gains and losses on disposals are determined by comparing the proceeds with the carrying amount and are recognized within ‘other income’ or ‘other expenses’ in the consolidated statements of operations and comprehensive loss.
F-15
(m) Impairment of assets
At each reporting date, the Group assesses whether there is an indication that an asset may be impaired. If there is any indication of impairment or if an annual impairment test is required, the Group estimates the recoverable amount of the asset. The recoverable amount of an asset is the higher of the asset’s fair value less costs of disposal and its value-in-use. It is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets, in which case it is determined at the level of the cash-generating unit. If the carrying amount of an asset exceeds its recoverable amount, the asset is impaired and written down to its recoverable amount. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
When there has been a change in the estimates used to determine the asset’s recoverable amount since the last impairment loss was recognized, any impairment loss previously recognized is reversed. The reversal may not exceed the carrying amount that would have been determined after amortization or depreciation had no impairment loss been recognized for the asset in prior periods. The amount of the reversal is recognized in profit or loss for the period.
There were no impairments or reversals of impairments in 2021, 2022 or 2023.
(n) Financial assets and liabilities (financial instruments)
(i) Definition
A financial instrument is any contract that gives rise to a financial asset of one entity and a financial liability or equity instrument of another entity. The Group’s financial assets include predominantly quoted fixed-interest debt securities. The financial liabilities comprise trade and other payables (incl. accrued liabilities from the R&D projects).
(ii) Criteria for the recognition and derecognition, initial measurement
In general purchases or sales of financial assets are recognized on the settlement date, i.e., the date that the Group renders or receives the counter performance (typically cash). The Group initially measures a financial asset at its fair value plus transaction costs.
The Group initially recognizes non-derivative financial liabilities on the date that they are originated at fair value net of directly attributable transaction costs. The Group derecognizes a financial liability when its contractual obligations are discharged, cancelled, or expire.
(iii) Subsequent measurement method
Considering the Group’s business model for managing the financial assets, with an objective to hold them in order to collect contractual cash flows, and their contractual cash flow characteristics, that are solely payments of principal and interest on the principal amount outstanding, the Group classifies the quoted debt securities with fixed interest rates as subsequently measured at amortized cost using the effective interest method (EIR). The financial assets are also subject to impairment.
The Group’s financial liabilities are classified as subsequently measured at amortized cost which is calculated by considering any discount or premium on acquisition and fees or costs that are an integral part of the EIR.
An analysis of the carrying amounts from the consolidated statements of financial position by measurement category is disclosed under ‘3.8 Financial assets and financial liabilities.’
F-16
(iv) Criteria for realization of income and expenses
Interest income is accrued using the relevant effective interest rate. Interest expense on liabilities, if any, is also accrued based on the effective interest rate.
Gains and losses on the disposal of financial instruments are recognized in full when all significant risks and rewards have been transferred. In the case of a partial transfer of risks and rewards, a distinction would be made as to whether control remains with the company or is transferred.
Impairment losses on financial assets are recognized in profit or loss. The Group recognizes an allowance for expected credit losses (ECLs) for the financial assets held, see Note ‘C.8. Net Financial Result’.
ECLs are based on the difference between the contractual cash flows due in accordance with the contract and all the cash flows that the Group expects to receive, discounted at an approximation of the original effective interest rate. ECLs are generally recognized in two stages. For credit exposures for which there has not been a significant increase in credit risk since initial recognition, ECLs are provided for credit losses that result from default events that are possible within the next 12-months (a 12-month ECL). For those credit exposures for which there has been a significant increase in credit risk since initial recognition, a loss allowance is required for credit losses expected over the remaining life of the exposure, irrespective of the timing of the default (a lifetime ECL). For the quoted debt securities with fixed interest rates, which have high credit ratings and no significant increases in credit risk since initial recognition, the Group determines the exposure to credit default using CDS pricing information (i.e., credit default swap values) published by credit agencies and recognizes a 12-month ECL.
(o) Fair Value Measurement
The Group does not measure any financial asset or liability at fair value. The carrying amount of all financial instruments approximates their fair value, with the exception of quoted debt securities for which fair values are disclosed (see Note ‘D.8. Financial assets and financial liabilities’).
When measuring the fair value of an asset or a liability, the Group would use observable market data as far as possible. Fair values are categorized into different levels in a fair value hierarchy based on the inputs used in the valuation techniques as follows:
| ● | Level 1, quoted prices in active markets for identical assets or liabilities. |
| ● | Level 2, inputs other than quoted prices included within Level 1 that are observable for the instrument, either directly (as prices) or indirectly (derived from prices). |
| ● | Level 3, inputs for instruments that are not based on observable market data (unobservable inputs). |
If the inputs used to measure the fair value of an asset or a liability fall into different levels of the fair value hierarchy, then the fair value measurement is categorized in its entirety in the same level of the fair value hierarchy as the lowest level input that is significant to the entire measurement.
The Group would recognize transfers between levels of the fair value hierarchy at the end of the reporting period during which the change has occurred.
(p) Income tax
Income taxes comprise current and deferred taxes. Current and deferred taxes are recognized in profit or loss except to the extent that they relate to items recognized directly in equity or in other comprehensive loss.
(i) Current income tax
Current income tax assets and liabilities are measured at the amount expected to be recovered from or paid to the taxation authorities. Expected tax payable or receivable on the taxable income or loss for the year, are calculated using tax rates enacted or substantively enacted at the reporting date, and any adjustment to tax payable in respect of previous years.
In the periods presented; the Group did not incur income tax expense. Taxes withheld by banks and remitted to tax authorities were reimbursed after filing of the annual tax declaration.
(ii) Deferred income tax
Deferred tax is recognized in respect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax is not recognized for temporary differences associated with assets and liabilities if the transaction which led to their initial recognition is a transaction that is not a business combination and that affects neither accounting nor tax profit or loss.
Deferred tax is measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the laws that have been enacted or substantively enacted by the reporting date.
Deferred tax assets arising from tax loss carryforwards are recognized only to the extent that the Group has sufficient taxable temporary differences or there is convincing evidence that sufficient future taxable profit will be available against which the unused tax losses can be utilized. As of December 31, 2023 and 2022, based on management’s judgment, it was not probable that taxable profit will be available against which the unused tax losses can be utilized; no deferred tax assets were therefore recognized in the consolidated statements of financial position.
F-17
3. Significant accounting judgements, estimates and assumptions
The preparation of the consolidated financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected. In preparing these consolidated financial statements, the critical judgments made by management in applying the Group’s accounting policies involve the following areas:
(a) Accounting for share-based payments
Additionally, the Company must estimate the number of equity instruments which will vest in future periods as awards may be forfeited prior to vesting due to an awardee’s failure to satisfy a performance condition, including due to employment termination. An assumption of the forfeiture rate is regularly made on the basis of historical information and adjusted to reflect future expectations. Revisions to the forfeiture rate could result in a cumulative effect of the change in estimate for current and prior periods to be recognized in the period of change.
(b) Measurement of third-party R&D clinical trial and contracted manufacturing expense
In measuring R&D expenses for the reporting period, the Company estimates the amount of expense to recognize and liability to accrue to the extent that invoices of the Company’s contract research organizations (“CROs”) and contract manufacturing organizations (“CDMOs”) are not yet received and exceed any prepayments made. The timing of the invoicing of project services by CROs follow contractual billing schedules and can occur several months prior to or following a reporting period. This estimation involves determining a percentage-of-completion whereby the degree to which services have been rendered for the individual project activities contracted from the CRO and CDMOs is assessed and estimated by in-house R&D project managers and reviewed by the controlling department. This percentage-of-completion is used to measure the amount of the unbilled project activities which have already been rendered by the reporting date and the associated R&D expense and liability to recognize as a result.
The percentage-of-completion estimates are based on the best information available at the time. However, additional information may become available in the future and management may adjust the estimate in such future periods. In this event, the Company may be required to record adjustments to research and development expenses in future periods when the actual level of activity becomes more certain. The Company considers resulting increases or decreases in expenses as changes in estimates and reflects such changes in research and development expenses in the period identified.
The
Company accrued €
F-18
(c) Realizability of Inventories
Inventories are valued at the lower of cost and net realizable value. Net realizable value comprises the estimated sales proceeds less the necessary expected costs up to the time of sale. For determining the net realizable value, at each reporting date, the Company estimates excess and obsolete inventory primarily using a model of expected future sales and using assumptions with significant estimation uncertainty such as expected medical need and expected market penetration.
Additionally, these estimates rely, in part, on management’s assumptions about future events outside of the Company’s control, such as continuation of the emergency use authorization in the United States and granting of marketing authorization in the European Union. In making these assumptions, management assesses the probability of these authorizations remaining in place or being granted, as applicable, by considering correspondence with the relevant regulatory authorities and the Company’s actions to achieve any required conditions for the authorizations.
Furthermore, the possible alternative uses for raw materials, unfinished and finished products is taken into consideration.
Management regularly assesses market and sales trends, market conditions, disease prevalence, competitive landscape, and regulatory environment to refine estimates for excess and obsolete inventory. Based on these assessments, and taking manufacturing lead-times into consideration, management takes operational decisions to order inventory based on inventory aging and the criteria mentioned above.
Inventory
write-downs for the year ended December 31, 2023 amounted to €
Assumptions included in the model of expected future demand may require revision in future periods which could result in changes to the estimate of excess and obsolete inventory and in inventory write-downs.
C. Consolidated statements of operations and comprehensive loss
1.
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Revenues | ||||||||||||
| Total | ||||||||||||
In June 2023, the Group began the commercialization of GOHIBIC (vilobelimab) in the United States. In connection with the start of the commercialization, the Group entered into agreements with certain subsidiaries of Cencora Inc. (“Cencora”) (formerly known as AmerisourceBergen Corp.) to act as the Group’s U.S. distributor and make GOHIBIC (vilobelimab) available for order by U.S. hospital customers. Cencora provides cold storage, cold-chain distribution services, inventory management and secondary labeling/packaging, among other services.
In 2023, the Company realized revenues from the product sales for the first time since its inception. Revenues reported are sales to end customers (hospitals). Sales to distributors do not constitute completion of a performance obligation towards a customer and, thus, do not result in the recognition of revenue for the Company under IFRS 15.
2.
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Cost of Sales | ||||||||||||
| Total | ||||||||||||
Cost of sales recognized during the twelve months ended December 31, 2023, are related to GOHIBIC (vilobelimab) revenues in the United States and to write-downs of inventory.
F-19
Costs of sales for products sold in these periods do not include costs of materials, as the associated costs of these materials were incurred in prior periods, before the U.S. Food and Drug Administration (the “FDA”) granted an Emergency Use Authorization (the “EUA”) for GOHIBIC (vilobelimab) in April 2023. These materials were recorded as ‘research and development expenses’ in the periods they were incurred.
The cost of sales during the twelve months ended December 31, 2023 mainly consists of write-downs of inventories that will expire prior to their expected sale.
3.
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Third-party expenses | ||||||||||||
| Employee benefits expenses | ||||||||||||
| of which equity-settled share-based payment expense | ||||||||||||
| Legal and consulting fees | ||||||||||||
| Other expenses | ||||||||||||
| Total sales and marketing expenses | ||||||||||||
During
the twelve months ended December 31, 2023 the Group incurred €
4. Research and development expenses
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Third-party services | ||||||||||||
| of which clinical material and related manufacturing services | ||||||||||||
| of which clinical, pre-clinical studies | ||||||||||||
| Employee benefits expenses | ||||||||||||
| of which equity-settled share-based payment expense | ||||||||||||
| Legal and consulting fees | ||||||||||||
| Other expenses | ||||||||||||
| Total | ||||||||||||
5. General and administrative expenses
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Employee benefits expenses | ||||||||||||
| of which equity-settled share-based payment expense | ||||||||||||
| Legal and consulting fees | ||||||||||||
| Insurance expenses | ||||||||||||
| Depreciation & amortization expense | ||||||||||||
| Compensation expense for non-executive directors | ||||||||||||
| Other expenses | ||||||||||||
| Total | ||||||||||||
F-20
6. Other income
Other
income was €
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Other income from government grants | ||||||||||||
| Further other income | ||||||||||||
| Total | ||||||||||||
7. Employee benefits expenses
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Wages and salaries | ||||||||||||
| Social security contributions (employer’s share) | ||||||||||||
| Equity-settled share-based payment expenses (see Note C.10. Share-based payments) | ||||||||||||
| Other | ||||||||||||
| Total | ||||||||||||
The number of employees as of December 31, 2023 increased to 66 (equivalent to 62.2 full time equivalents (FTEs)) from 48 employees (equivalent to 44.3 FTEs) at the end of 2022 and 59 employees (equivalent to 55.9 FTEs) at the end of 2021. These numbers are as of December 31 and do not constitute annual average numbers.
8.
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Interest income | ||||||||||||
| Interest expenses | ( | ) | ( | ) | ( | ) | ||||||
| Interest on lease liabilities | ( | ) | ( | ) | ( | ) | ||||||
| Financial result | ||||||||||||
| Foreign exchange income | ||||||||||||
| Foreign exchange expense | ( | ) | ( | ) | ( | ) | ||||||
| Foreign exchange result | ( | ) | ||||||||||
| Other financial result | ( | ) | ( | ) | ||||||||
| Net financial result | ||||||||||||
Net
financial result decreased by €
Foreign currency income and expenses arise from the translation of cash and cash equivalents, marketable securities and other financial assets and liabilities denominated in foreign currencies at the exchange rates prevailing at the balance sheet date. All resulting translation differences are recognized in the income statement. These gains and losses are caused by a change in exchange rates at the reporting dates and may not ultimately be realized.
F-21
9. Loss per share
Loss
per ordinary share is calculated by dividing the loss of the period by the weighted average number of ordinary shares outstanding during
the period. The weighted number of ordinary shares outstanding for the financial year 2023 was
As the Company is in a loss-making situation, the diluted loss per share is the same as basic loss per share, because the weighted average number of shares to be issued upon the exercise of the stock options, the only dilutive instruments issued, would produce an anti-dilutive effect. Refer to Note ‘C.10. for the balances of outstanding share options.’
10. Share-based payments
a) Equity-settled share-based payment arrangements
| 2023 Options | 2023 WAEP* | 2022 Options | 2022 WAEP* | |||||||||||||
| Outstanding at January 1 | € | € | ||||||||||||||
| Exercised during the year | ||||||||||||||||
| Outstanding at December 31 | € | € | ||||||||||||||
| Exercisable at December 31 | € | € | ||||||||||||||
| * |
The
exercise price for all options granted prior to 2016 outstanding at the end of the year was €
Under
the terms and conditions of the share option plan of 2016 (the “2016 Plan”), InflaRx GmbH granted rights to subscribe for
InflaRx GmbH’s common shares to directors, senior management, and key employees.
| 2023 Options | 2023 WAEP* | 2022 Options | 2022 WAEP* | |||||||||||||
| Outstanding at January 1 | $ | $ | ||||||||||||||
| Exercised during the year | ||||||||||||||||
| Outstanding at December 31 | $ | $ | ||||||||||||||
| Exercisable at December 31 | $ | $ | ||||||||||||||
| * |
The
weighted average remaining contractual life for the share options outstanding under the
F-22
| 2023 Options | 2023 WAEP* | 2022 Options | 2022 WAEP* | |||||||||||||
| Outstanding at January 1 | $ | $ | ||||||||||||||
| Granted during the year | $ | $ | ||||||||||||||
| Forfeited during the year | ( | ) | $ | ( | ) | $ | ||||||||||
| Exercised during the year | ( | ) | $ | ( | ) | $ | ||||||||||
| Outstanding at December 31 | $ | $ | ||||||||||||||
| Exercisable at December 31 | $ | $ | ||||||||||||||
| * |
The
weighted average remaining contractual life for the share options outstanding under the 2017 Plan as of December 31, 2023 was
All Options granted in 2023 vest over one year except for options granted on July 7, 2023 which partially vest over three years. Options granted before 2023 vest over a period of one, two or three years, depending on the grant, with 1/2 or 1/3, respectively, of the options vesting after the end of the 1st year from vesting start and the remaining options vesting quarterly in equal portions thereafter. Vesting of these unvested share options is subject to a service condition at the time of vesting, and no market or performance conditions are applicable.
The
weighted average fair value of options granted during 2023 was $
All shares issued for share options exercised during the year were recorded to the commercial register by December 31, 2023.
b) Measurement of fair values of share options granted under the 2017 Plan
The fair value of options granted under the 2017 Plan was determined using the Black-Scholes valuation model. As the Company’s ordinary shares are listed on the Nasdaq Global Select Market, the closing price of the ordinary shares at grant date was used.
| Share options granted | Options | Fair value option | FX rate as of grant date | Fair value option | Share price at grant date/ Exercise price | Expected volatility | Expected life (midpoint | Risk-free rate (interpolated, U.S. sovereign strips curve) | |||||||||||||||||||||||||
| 2021 | |||||||||||||||||||||||||||||||||
| January 4 | $ | € | $ | % | |||||||||||||||||||||||||||||
| January 4 | $ | € | $ | % | |||||||||||||||||||||||||||||
| July 2 | $ | € | $ | % | |||||||||||||||||||||||||||||
| July 2 | $ | € | $ | % | |||||||||||||||||||||||||||||
Of
the
| Share options granted | Options | Fair value per share option | FX rate as of grant date | Fair value per share option | Share price at grant date/ Exercise price | Expected volatility | Expected life (midpoint based) | Risk-free rate (interpolated, U.S. sovereign strips curve) | |||||||||||||||||||||||||||
| 2022 | |||||||||||||||||||||||||||||||||||
| January 12 | $ | € | $ | ||||||||||||||||||||||||||||||||
| January 12 | $ | € | $ | ||||||||||||||||||||||||||||||||
| Repricing, April 13 | $ | € | $ | ||||||||||||||||||||||||||||||||
| November 21 | $ | € | $ | ||||||||||||||||||||||||||||||||
F-23
Of
the
| Share options granted | Number | Fair value option | FX rate as of | Fair value option | Share Exercise | Expected volatility | Expected (midpoint based) | Risk-free rate (interpolated, U.S. sovereign strips curve) | |||||||||||||||||||||||||
| 2023 | |||||||||||||||||||||||||||||||||
| January 24 | $ | € | $ | % | |||||||||||||||||||||||||||||
| January 24 | $ | € | $ | % | |||||||||||||||||||||||||||||
| May 31 | $ | € | $ | % | |||||||||||||||||||||||||||||
| July 7 | $ | € | $ | % | |||||||||||||||||||||||||||||
| July 7 | $ | € | $ | % | |||||||||||||||||||||||||||||
| July 19 | $ | € | $ | % | |||||||||||||||||||||||||||||
| September 18 | $ | € | $ | % | |||||||||||||||||||||||||||||
Of
the
Expected dividends are for all share options listed above.
Share price volatility is calculated on the basis of annualized monthly volatility rate of the Company’s share price over the last five years preceding the valuation date.
The range of outcomes for the expected life of the instruments has been based on expectations on option holder behavior in the scenarios considered.
The dividend yield has no impact due to the anti-dilution clause as defined in the 2017 Plan.
D. Notes to the consolidated statements of financial position
1.
| Property and equipment | Advance payments | Total | ||||||||||
| Cost | (in €) | |||||||||||
| At January 1, 2022 | ||||||||||||
| Additions | ||||||||||||
| Exchange differences | ||||||||||||
| At December 31, 2022 | ||||||||||||
| Additions | ||||||||||||
| Disposals | ( | ) | ( | ) | ||||||||
| Exchange differences | ( | ) | ( | ) | ||||||||
| At December 31, 2023 | ||||||||||||
| Accumulated depreciation | ||||||||||||
| At January 1, 2022 | ( | ) | ( | ) | ||||||||
| Depreciation charge for the year | ( | ) | ( | ) | ||||||||
| Exchange differences | ( | ) | ( | ) | ||||||||
| At December 31, 2022 | ( | ) | ( | ) | ||||||||
| Depreciation charge for the year | ( | ) | ( | ) | ||||||||
| Disposals | ||||||||||||
| Exchange differences | ||||||||||||
| At December 31, 2023 | ( | ) | ( | ) | ||||||||
| Net book value | ||||||||||||
| At December 31, 2022 | ||||||||||||
| At December 31, 2023 | ||||||||||||
F-24
2.
| Buildings | Cars | Total | ||||||||||
| Cost | (in €) | |||||||||||
| At January 1, 2022 | ||||||||||||
| Additions | ||||||||||||
| Exchange differences | ||||||||||||
| At December 31, 2022 | ||||||||||||
| Additions | ||||||||||||
| Exchange differences | ( | ) | ( | ) | ||||||||
| At December 31, 2023 | ||||||||||||
| Accumulated depreciation | ||||||||||||
| At January 1, 2022 | ( | ) | ( | ) | ( | ) | ||||||
| Depreciation charge for the year | ( | ) | ( | ) | ( | ) | ||||||
| Exchange differences | ( | ) | ( | ) | ||||||||
| At December 31, 2022 | ( | ) | ( | ) | ( | ) | ||||||
| Depreciation charge for the year | ( | ) | ( | ) | ( | ) | ||||||
| Exchange differences | ||||||||||||
| At December 31, 2023 | ( | ) | ( | ) | ( | ) | ||||||
| Net book value | ||||||||||||
| At December 31, 2022 | ||||||||||||
| At December 31, 2023 | ||||||||||||
3.
| Purchased IT-software | Advances paid for software | Total | ||||||||||
| Cost | (in €) | |||||||||||
| At January 1, 2022 | ||||||||||||
| Additions | ||||||||||||
| Exchange differences | ||||||||||||
| At December 31, 2022 | ||||||||||||
| Additions | ||||||||||||
| Disposals | ( | ) | ( | ) | ||||||||
| Exchange differences | ( | ) | ( | ) | ||||||||
| At December 31, 2023 | ||||||||||||
| Accumulated amortization | ||||||||||||
| At January 1, 2022 | ( | ) | ( | ) | ||||||||
| Amortization charge for the year* | ( | ) | ( | ) | ||||||||
| Exchange differences | ( | ) | ( | ) | ||||||||
| At December 31, 2022 | ( | ) | ( | ) | ||||||||
| Amortization charge for the year | ( | ) | ( | ) | ||||||||
| Disposals | ||||||||||||
| Exchange differences | ||||||||||||
| At December 31, 2023 | ( | ) | ( | ) | ||||||||
| Net book value | ||||||||||||
| At December 31, 2022 | ||||||||||||
| At December 31, 2023 | ||||||||||||
Amortization
of intangible assets is included in the line items ‘research and development expenses’ (2023: €
F-25
4. Leases
Lease obligations consist of payments pursuant to non-cancellable lease agreements mainly relating to the Company’s leases of office space. The lease terms of the Company’s premises expire as follows: Jena, Germany in December 2025, Martinsried, Germany in May 2027 and Ann Arbor, Michigan, United States in April 2026.
| Lease liabilities | 2023 | 2022 | ||||||
| (in €) | ||||||||
| As of January 1 | ||||||||
| Additions | ||||||||
| Derecognition | ( | ) | ( | ) | ||||
| Payments | ( | ) | ( | ) | ||||
| Short-term liability for accrued interest expense | ( | ) | ||||||
| Foreign exchange difference | ( | ) | ||||||
| As of December 31 | ||||||||
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Depreciation expense of right-of-use assets (see Note E.2.) | ||||||||||||
| Interest expense on lease liabilities | ||||||||||||
| Rental expense from leases | ||||||||||||
| Thereof short-term leases (included in administrative expenses) | ||||||||||||
| Thereof leases of low-value assets (included in administrative expenses) | ||||||||||||
| Total amounts recognized in profit or loss | ||||||||||||
The
Group had total cash outflows for leases of €
5.
| 2023 | 2022 | 2021 | ||||||||||
| (in €) | ||||||||||||
| Raw material and supplies | ||||||||||||
| Unfinished goods | ||||||||||||
| Finished goods | ||||||||||||
| Total | ||||||||||||
The
Company’s inventory consists of materials relating to GOHIBIC (vilobelimab) primarily manufactured following the EUA for the US
market at the beginning of April 2023 (refer to Note A.2.) In the year ended December 31, 2023, inventory write-downs of €
F-26
| 6. |
| December 31, 2023 | December 31, 2022 | | ||||||
| (in €) | ||||||||
| Non-current other assets | — | — | ||||||
| Prepaid expenses | ||||||||
| Total | ||||||||
| Current other assets | ||||||||
| Prepayments on research & development projects | ||||||||
| Prepaid expenses | ||||||||
| Others | ||||||||
| Total | ||||||||
| Total other assets | ||||||||
Prepayments on research & development projects consists of prepayments on CRO and manufacturing contracts. Prepaid expenses mainly consist of prepaid insurance expenses.
| 7. | Income tax Income tax reconciliation |
| InflaRx Group | 2023 | 2022 | 2021 | |||||||||
| (in €) | ||||||||||||
| Loss for the period (accounting profit before income tax) | ( | ) | ( | ) | ( | ) | ||||||
| Tax rate | % | % | % | |||||||||
| Tax benefits at tax rate | ||||||||||||
| Temporary differences and tax losses for which no deferred tax asset was recognized | ( | ) | ( | ) | ( | ) | ||||||
| Non-recognition of tax effect on share-based payments | ( | ) | ( | ) | ( | ) | ||||||
| Non-deductible expenses for tax purposes | ( | ) | ( | ) | ( | ) | ||||||
| Other differences due to tax rate | ( | ) | ||||||||||
| Income tax | ||||||||||||
The tax rate applied above represents the weighted
average of the statutory tax rates in Germany and the United States. In Germany, InflaRx N.V. and its subsidiary InflaRx GmbH are subject
to corporate income tax (2023/2022/2021:
F-27
| a) | Tax losses carried forward |
The Group has total tax loss carryforwards of
€
As of December 31, 2023 the Group had €
| ● | Tax losses of InflaRx GmbH until December 31, 2017 (€ |
| ● | In addition, the Group still has tax loss carryforwards of $ |
As of December 31, 2023, 2022 and 2021, no deferred tax assets were recognized for the carryforward of unused tax losses.
| b) | Current income tax receivable |
Current income tax receivable includes tax claims
because of income tax withheld on interest income earned by the Group on the financial assets (2023: €
| 8. | Financial assets and financial liabilities |
| Financial assets and financial liabilities | December 31, 2023 | December 31, 2022 | ||||||
| (in €) | ||||||||
| Financial assets at amortized cost | ||||||||
| Non-current financial assets | ||||||||
| Financial assets from government grants | ||||||||
| Other current financial assets | ||||||||
| Financial liabilities at amortized cost | ||||||||
| Liabilities from government grants | ||||||||
| Trade and other payables | ||||||||
The fair value of current and non-current financial
assets amounted to €
The maturities of all securities held as of December
31, 2023 are between one and seventeen months (2022: between one and sixteen months); they bear nominal fixed interest in the range of
As of December 31, 2023, financial assets and
liabilities from government grants amount to € , due to the end of the grant period on June 30, 2023 (€
F-28
| 9. |
| December 31, 2023 | December 31, 2022 | |||||||
| (in €) | ||||||||
| Short-term deposits | — | — | ||||||
| Deposits held in U.S. dollars | ||||||||
| Deposits held in Euro | ||||||||
| Total | ||||||||
| Cash at banks | ||||||||
| Cash held in U.S. dollars | ||||||||
| Cash held in Euro | ||||||||
| Total | ||||||||
| Total cash and cash equivalents | ||||||||
| 10. | Equity |
| a) | Issued capital |
As of December 31, 2023, the issued capital of
the Company is divided into
On July 8, 2020, the Company filed a Form
F-3 (2020-Registration Statement) with the U.S. Securities and Exchange Commission (the “SEC”) with respect
to the offer and sale of securities of the Company. The Company also filed with the SEC a prospectus supplement relating
to an at-the-market program providing for the sale of up to $
As of December 31, 2022, the Company had issued
Through an underwritten public offering in April
2023, the Company sold and issued an aggregate of
In connection with amending the Co-Development
Agreement with Staidson (Beijing) BioPharmaceuticals Co., Ltd. (“Staidson”) on December 21, 2022, the Company entered into
a share purchase agreement with Staidson pursuant to which Staidson purchased ordinary shares of the Company for an aggregate amount of
$
During 2023, the Company issued a total of
F-29
| b) | Authorized capital |
According to the articles of association of the
Company, up to
In order to deter acquisition bids, the Company’s
general meeting of shareholders approved the right of an independent foundation under Dutch law, or protective foundation, to exercise
a call option pursuant to the call option agreement, upon which preferred shares will be issued by the Company to the protective foundation
of up to
These preferred shares will have both a liquidation and dividend preference over the Company’s ordinary shares and will accrue cash dividends at a pre-determined rate. The protective foundation would be expected to require the Company to cancel its preferred shares once the perceived threat to the Company and its stakeholders has been removed or sufficiently mitigated or neutralized. The Company believes that the call option does not represent a significant fair value based on a level 3 valuation since the preferred shares are restricted in use and can be cancelled by the Company.
For the year ended December 31, 2023, the Company
expensed €
| c) | Nature and purpose of equity reserves |
In addition to the issued capital, the Company discloses the following other reserves:
| ● | Share premium records the amounts paid in upon issuance of ordinary shares in excess of nominal value of € |
| ● | The other capital reserves include the expense resulting from the issue of share options. |
| ● | Accumulated deficit includes the losses of previous reporting periods. |
Other components of equity exclusively include currency reserves from the conversion of financial statements in foreign currencies.
| 11. |
| December 31, 2023 | December 31, 2022 | |||||||
| (in €) | ||||||||
| Accrued liabilities from R&D projects | ||||||||
| Accrued liabilities from commercial activities | ||||||||
| Accounts payable | ||||||||
| Other accrued liabilities and payables | ||||||||
| Total trade and other payables | ||||||||
Accrued liabilities from R&D projects include services from the Company’s ongoing projects that have not yet been invoiced to the Company as of the reporting date.
Accrued liabilities from commercial activities include services provided by commercial manufacturing partners that have not yet been invoiced to the Company as of the reporting date.
Other accrued liabilities and payables include
payments for GOHIBIC (vilobelimab) received from our distribution partner under the title distribution model, against which revenue will
be recognized at the time of final sale and delivery to hospital customers. These accrued liabilities payments amount to €
F-30
| 12. | Financial risk management |
| a) | Financial risk management objectives and policies |
The Group’s financial risks are predominantly controlled by central treasury activities under an investment policy approved by the Board of Directors on November 3, 2022, as revised on October, 27, 2023. Those treasury activities identify, evaluate and manage financial risks consistent with the Group’s operating needs. The Board of Directors provides policies for overall risk management, covering specific areas, such as foreign exchange risk and credit risk. The Company does not intend to use derivative financial instruments because the Group’s future risk exposures cannot be reliably forecasted (volume of business activity, liquidity needs, foreign exchange exposure).
Hedging is not applied as most of the business activity is intended to be executed in U.S. dollars and paid with the U.S. dollars funds raised in public offerings. The foreign exchange exposure from costs incurred in currencies other than Euro is deemed immaterial.
The Group’s principal financial assets comprise quoted debt securities with high credit ratings. Besides these financial assets, the Group has significant cash and cash equivalents. The Group’s principal financial liabilities comprise trade and other payables. The main purpose of these financial assets, cash/cash equivalents and liabilities are to finance the Group’s development activities.
| Exposure | Measurement | Risk Management | |
| Market risk | Achievement of a natural hedge in the future | ||
| Credit risk | Cash and cash equivalents, current and non-current |
Credit rating |
Diversification of bank deposits, Investment guidelines for debt investments |
| Liquidity | Rolling cash flow forecast |
| b) | Market risk |
Market risk is the risk that changes in market prices (e.g., due to foreign exchange rates) will affect the Group’s income, expenses or the value of its holdings of financial instruments. The objective of market risk management is to identify, manage and control market risk exposures within acceptable parameters.
Foreign exchange risk arises when commercial transactions or recognized assets or liabilities are denominated in a currency that is not an entity’s functional currency. The Group is exposed to transactional foreign currency risk to the extent that there is a mismatch between the currencies in which costs and purchases are denominated and the respective functional currencies of Group companies. The functional currencies of Group companies are primarily the Euro and U.S. dollars. The currencies in which these transactions and financial assets are primarily denominated are Euro and U.S. dollars. The Group is exposed to the exchange rate between the Euro and the U.S. dollars. Due to the Company’s various registered offerings of ordinary shares in U.S. dollars, the Group has significant cash and cash equivalents in U.S. dollars. Currently the Group does not hedge U.S. dollars but intends to achieve a natural hedge by contracting suppliers in U.S. dollars in the future. In 2023, the Group recognized significant foreign exchange gains and losses as the natural hedge is not yet achieved and the functional currency for InflaRx N.V. and InflaRx GmbH is Euro.
The Group is primarily exposed to changes in U.S. dollar to Euro exchange rates. The sensitivity of profit or loss to changes in the exchange rates arises mainly from U.S. dollar denominated financial instruments at InflaRx N.V. and InflaRx GmbH.
F-31
| Cash, cash equivalents and financial assets denominated in U.S. dollars, InflaRx N.V. and InflaRx GmbH | December 31, 2023 | December 31, 2022 * | ||||||
| (in €) | ||||||||
| Current and non current financial assets (securities and accrued interest) | ||||||||
| Cash and cash equivalents | ||||||||
| Total assets exposed to the risk | ||||||||
| Conversion rate Euro to U.S. dollars at reporting date 1/1.1050 | ||||||||
| * |
| Sensitivity analysis: | Conversion rate | Profit/(loss) | carrying amount | |||||||||
| (in €) | ||||||||||||
| Euro weakens against U.S. dollars | ||||||||||||
| Euro strengths against U.S. dollars | ( | ) | ||||||||||
Based on the exchange rate fluctuations from
the last three years, the Company expects that exchange rate fluctuations of the Euro to the U.S. dollar between
| c) | Credit risk |
Credit risk is the risk that a counterparty will not meet its obligations leading to a financial loss for the Company. The Company is exposed to credit risk mainly from its financing activities, including deposits with banks and financial institutions, foreign exchange transactions and other financial instruments.
Credit risk from balances with banks and financial institutions is managed by the Company in accordance with the Company’s investment policy. Investment of financial resources which are currently not used to fund R&D or G&A activities, are made only with counterparties within the credit limits approved by the investment policy. For investments in Euro or U.S. dollar debt securities, a BBB+ to AAA credit rating (Standard & Poors and Fitch ratings; or equivalent ratings by Moody’s and DBRS) is required. Complex financial products as well as other investments denominated in currencies other than Euros or U.S. dollars are not permitted by the investment policy. Counterparty credit limits and the investment policy are discussed with the Company’s Audit Committee on an annual basis and may be updated throughout the year subject to approval of the Company’s Audit Committee. The limits are set to minimize the concentration of risks and therefore mitigate financial loss through a counterparty’s potential failure to make payments.
The maximum exposure to counterparty credit risk
is €
| d) | Liquidity risk |
The Company monitors its risk of a shortage of funds in every quarterly forecast as well as on an ongoing basis. The Company disclosed the maturities of its principal liabilities under Note E ‘Commitments’. Prudent liquidity risk management involves maintaining sufficient cash and marketable securities and the availability of funding to meet obligations when due. The Group continually monitors its risk of a shortage of funds using short and mid-term liquidity planning. This takes into account of the expected cash flows from all activities. The management team performs regular reviews of the budget.
The Company has a history of significant operating losses. Management expects that the Company incurs significant and increasing losses for the foreseeable future; as the Company may not achieve or maintain profitability in the near future, it is dependent on capital contributions or other funding.
F-32
The Group raised significant funding from various registered offerings that it estimates will enable the Group to fund operating expenses and capital expenditure requirements for at least 24 months from December 31, 2023. The Group expects to require additional funding to continue to advance the development of product candidates. In the event regulatory approval is received and the Company implements a strategy to commercialize the products itself, the Group would require additional capital.
In 2023, as a result of the BMBF agreements (see
Notes C.6.) the Company received €
| Liquidity | December 31, 2023 | December 31, 2022 | ||||||
| (in €) | ||||||||
| Short-term deposits | ||||||||
| Cash at banks | ||||||||
| Marketable Securities (current and non-current) | ||||||||
| Other (non-current portion) | ||||||||
| Other (current) | ||||||||
| Total funds available | ||||||||
| 13. | Capital management |
The Group’s policy for capital management is to ensure that it maintains its liquidity in order to finance its operating activities, future business development and meet its liabilities when due. The Group manages its capital structure primarily through equity. The Group does not have any financial liabilities, other than trade and other payables or leasing liabilities.
No changes were made in the objectives, policies or processes for managing capital during the year.
| E. | Commitments |
| 1. | Operating contracts or services |
The Group enters into contracts in the normal course of business with CROs and clinical sites for the conduct of clinical trials, professional consultants for expert advice and other vendors for clinical supply manufacturing or other services. These contracts can usually be terminated with 30 to 180 days’ notice. In addition to this minimum duration, these contracts require full payment for services already rendered.
During 2023, the Group did not have any commitments to purchase property, plant and equipment or patents and trademarks (respectively in 2022).
| 2. | Lease obligations |
| Maturity analysis for capitalized leases in 2023 | Contractual minimum lease obligations | Effect of discounting | Lease liabilities | |||||||||
| (in €) | ||||||||||||
| Within one year | ||||||||||||
| After one year but not more than five years | ||||||||||||
| More than five years | ||||||||||||
| Total | ||||||||||||
F-33
| Maturity analysis for capitalized leases in 2022 | Contractual minimum lease obligations | Effect of discounting | Lease liabilities | |||||||||
| (in €) | ||||||||||||
| Within one year | ||||||||||||
| After one year but not more than five years | ||||||||||||
| More than five years | ||||||||||||
| Total | ||||||||||||
| Maturity analysis for all lease obligations in 2023 | Total | Low value leases | Short-term leases | Capitalized leases | ||||||||||||
| Within one year | ||||||||||||||||
| After one year but not more than five years | ||||||||||||||||
| More than five years | ||||||||||||||||
| Total | ||||||||||||||||
| Maturity analysis for all lease obligations in 2022 | Total | Low value leases | Short-term leases | Capitalized leases | ||||||||||||
| (in €) | ||||||||||||||||
| Within one year | ||||||||||||||||
| After one year but not more than five years | ||||||||||||||||
| More than five years | ||||||||||||||||
| Total | ||||||||||||||||
Anticipated future lease expenses were converted
with the exchange rate as of December 31, 2023,
The Group applies the ‘lease of low-value assets’ recognition exemptions. The Group also applied the ‘short-term lease’ exemption for leases with a maturity of less than 12 months.
| F. | Other information |
| 1. | Segment reporting |
The Group predominantly operates as a R&D focused biopharmaceutical company applying its proprietary anti-C5a and C5aR technologies towards the development of novel therapeutic products targeting diseases of high unmet medical need. However, since the EUA of its lead product vilobelimab for the treatment of severe COVID-19 patient in April 2023, it also has commercial activities around the sales and marketing of GOHIBIC (vilobelimab) in the U.S. The Group is not steered by segments. The Board of Directors is the chief operating decision maker. Management of resources and reporting to the decision maker is based on the Group as a whole.
All operational activities are conducted in Germany
and the United States. Revenues in the amount of $
| ● | December 31, 2023: € |
| ● | December 31, 2022: € |
None of the non-current assets are in the country where the Company is incorporated (the Netherlands).
F-34
| 2. | Related party transactions |
| Executive and Board compensation | 2023 | 2022 | 2021 | |||||||||
| (in €) | ||||||||||||
| Executive management | ||||||||||||
| Short-term employee benefits | ||||||||||||
| Share-based payments | ||||||||||||
| Sub-total | ||||||||||||
| Non-executive Board of Directors members | ||||||||||||
| Short-term employee benefits | ||||||||||||
| Share-based payments | ||||||||||||
| Sub-total | ||||||||||||
| Total compensation | ||||||||||||
Executive management comprises executive Directors of the Board of Directors and members of the Senior management of the Company.
The table above discloses short-term employee
benefits that were contractually agreed for the Board of Directors and executive management. As of December 31, 2023, €
Remuneration of the Group’s executive management comprises fixed and variable components and share-based payment awards. In addition, executive management receive supplementary benefits and allowances.
The Company entered into indemnification agreements with its directors and senior management. The indemnification agreements and the Company’s Articles of Association require the Company to indemnify its directors to the fullest extent permitted by law.
The Company’s current and future directors (and such other officer or employee as designated by the Board of Directors) have the benefit of indemnification provisions in the Articles of Association of InflaRx N.V. These provisions give the indemnified persons the right to recover from the Company amounts, including, but not limited to, litigation expenses, and any damages they are ordered to pay, in relation to acts or omissions in the performance of their duties. However, there is no entitlement to indemnification for acts or omissions which are considered to constitute malice, gross negligence, intentional recklessness and/or serious culpability attributable to such indemnified person. These agreements also provide, subject to certain exceptions, for indemnification for related expenses including, among others, attorneys’ fees, judgements, penalties, fines and settlement amounts incurred by any of these individuals in any action or proceeding. In addition to such indemnification, the Company provides its directors with directors’ and officers’ liability insurance.
| G. | Significant events after the reporting date |
None.
F-35