UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
______________________________
FORM 20-F
______________________________
| REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
OR
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended June 30 , 2023
OR
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
OR
| SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
Date of event requiring this shell company report
For the transition period from ______ to ______
Commission file number 001-37626
______________________________
MESOBLAST LIMITED
(Exact name of Registrant as specified in its charter)
______________________________
N/A
(Translation of Registrant’s name into English)
AUSTRALIA
(Jurisdiction of incorporation or organization)
Telephone: +61 (3) 9639 6036
(Address of principal executive offices)
Chief Executive Officer
Telephone: +61 (3) 9639 6036 ; Fax: +61 (3) 9639 6030
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
______________________________
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
o Yes x No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
o Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
x Yes o No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
x Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | o | Accelerated filer | x | Non-accelerated filer | o | ||||||||||||
| Emerging growth company | |||||||||||||||||
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.x
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP | o | Accounting Standards Board | x | Other | o | ||||||||||||
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 o Item 18 o
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
o Yes x No
Table of Contents
| 6.F | |||||||||||
| 10.J | |||||||||||
INTRODUCTION AND USE OF CERTAIN TERMS
Mesoblast Limited and its consolidated subsidiaries publish consolidated financial statements expressed in U.S. dollars, unless otherwise indicated. This Annual Report on Form 20-F is presented in U.S. dollars, unless otherwise indicated. Our consolidated financial statements found in Item 18 of this Annual Report on Form 20-F are prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board and Australian equivalents to International Financial Reporting Standards as issued by the Australian Accounting Standards Board.
Except where the context requires otherwise and for purposes of this Form 20-F only:
•“ADSs” refers to our American depositary shares, each of which represents ordinary shares, and “ADRs” refers to the American depositary receipts that evidence our ADSs.
•“Mesoblast,” “we,” “us” or “our” refer to Mesoblast Limited and its subsidiaries.
•“A$” or “Australian dollar” refers to the legal currency of Australia.
•“AIFRS” refers to the Australian equivalents to International Financial Reporting Standards as issued by the Australian Accounting Standards Board, or AASB.
•“CHF” refers to the legal currency of Switzerland.
•“FDA” refers to the United States Food and Drug Administration.
•“GBP” refers to the legal currency of the United Kingdom.
•“IFRS” refers to the International Financial Reporting Standards as issued by the International Accounting Standards Board, or IASB.
•“S$” or “SGD” or “Singapore dollar” refers to the legal currency of Singapore.
•“U.S. GAAP” refers to the Generally Accepted Accounting Principles in the United States.
•“US$” or “U.S. dollars” refers to the legal currency of the United States.
•“U.S.” or “United States” refers to the United States of America.
•“€” or “Euro” refers to the legal currency of the European Union.
Australian Disclosure Requirements
Our ordinary shares are primarily quoted on the Australian Securities Exchange (“ASX”) in addition to our listing of our ADSs on The Nasdaq Global Select Market. As part of our ASX listing, we are required to comply with various disclosure requirements as set out under the Australian Corporations Act 2001 and the ASX Listing Rules. Information furnished under the sub-heading “Australian Disclosure Requirements” is intended to comply with ASX listing and Corporations Act 2001 disclosure requirements and is not intended to fulfill information required by this Annual Report on Form 20-F.
3
FORWARD-LOOKING STATEMENTS
This Form 20-F includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on our current expectations, assumptions, estimates and projections about the Company, our industry, economic conditions in the markets in which we operate, and certain other matters. These statements include, among other things, the discussions of our business strategy and expectations concerning our market position, future operations, margins, profitability, liquidity and capital resources. These statements are subject to known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “target,” “likely,” “will,” “would,” “could,” “should”, “may”, “goal,” “objective” and similar expressions or phrases identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and future events and financial trends that we believe may affect our financial condition, results of operation, business strategy and financial needs. Forward- looking statements include, but are not limited to, statements about:
•the initiation, timing, progress and results of our preclinical and clinical studies, and our research and development programs;
•our ability to advance product candidates into, enroll and successfully complete, clinical studies, including multi-national clinical trials;
•our ability to advance our manufacturing capabilities;
•the timing or likelihood of regulatory filings and approvals, manufacturing activities and product marketing activities, if any;
•our ability to take advantage of the potential benefits of the 21st Century Cures Act;
•the impact that any future pandemic could have on business operations;
•the commercialization of our product candidates, if approved;
•regulatory or public perceptions and market acceptance surrounding the use of cell based therapies;
•the potential for our product candidates, if any are approved, to be withdrawn from the market due to patient adverse events or deaths;
•the potential benefits of strategic collaboration agreements and our ability to enter into and maintain established strategic collaborations;
•our ability to establish and maintain intellectual property on our product candidates and our ability to successfully defend these in cases of alleged infringement;
•the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology;
•our ability to obtain additional financing;
•estimates of our expenses, future revenues, capital requirements and our needs for additional financing;
•our financial performance;
•developments relating to our competitors and our industry;
•the pricing and reimbursement of our product candidates, if approved; and
•other risks and uncertainties, including those listed under the caption “Risk Factors”.
You should read this Form 20-F and the documents that we refer to herein thoroughly with the understanding that our actual future results may be materially different from and/or worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements. Other sections of this Form 20-F include additional factors which could adversely impact our business and financial performance. Moreover, we operate in an evolving environment. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
4
This Form 20-F also contains third-party data relating to the biopharmaceutical market that includes projections based on a number of assumptions. The biopharmaceutical market may not grow at the rates projected by market data, or at all. The failure of this market to grow at the projected rates may have a material adverse effect on our business and the market price of our ordinary shares and ADSs. Furthermore, if any one or more of the assumptions underlying the market data turns out to be incorrect, actual results may differ from the projections based on these assumptions. You should not place undue reliance on these forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. The forward-looking statements made in this Form 20-F relate only to events or information as of the date on which the statements are made in this Form 20-F. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
5
PART I
Item 1. Identity of Directors, Senior Management and Advisers
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
Item 3. Key Information
3.A [Reserved]
3.B Capitalization and Indebtedness
Not applicable.
3.C Reasons for the offer and use of proceeds
Not applicable.
3.D Risk Factors
You should carefully consider the risks described below and all other information contained in this Annual Report on Form 20-F before making an investment decision. If any of the following risks actually occur, our business, financial condition and results of operations could be materially and adversely affected. In that event, the trading price of our ordinary shares and ADSs could decline, and you may lose part or all of your investment. This Annual Report on Form 20-F also contains forward-looking information that involves risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of many factors, including the risks described below and elsewhere in this Annual Report on Form 20-F.
Risks Related to Our Financial Position and Capital Requirements
We have incurred operating losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future. We may never achieve or sustain profitability.
We are a clinical-stage biotechnology company and we have not yet generated significant revenues. We have incurred net losses during most of our fiscal periods since our inception. Our net loss for the year ended June 30, 2023 was $81.9 million. As of June 30, 2023, we have an accumulated deficit of $820.8 million since our inception. We do not know whether or when we will become profitable. Our losses have resulted principally from costs incurred in clinical development and manufacturing activities.
We anticipate that our expenses will increase as we move toward commercialization, including the scaling up of our manufacturing activities and our establishment of infrastructure and logistics necessary to support potential product launches. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. To achieve and maintain profitability, we must successfully develop our product candidates, obtain regulatory approval, and manufacture, market and sell those products for which we obtain regulatory approval. If we obtain regulatory approval to market a product candidate, our future revenue will depend upon the size of any markets in which our product candidates may receive approval, and our ability to achieve and maintain sufficient market acceptance, pricing, reimbursement from third-party payors, and adequate market share for our product candidates in those markets. We may not succeed in these activities, and we may never generate revenue from product sales that is significant enough to achieve profitability. Our failure to become or remain profitable would depress our market value and could impair our ability to raise capital, expand our business, discover or develop other product candidates or continue our operations. A decline in the value of our company could cause you to lose part or all of your investment.
6
We have never generated revenue from product sales and may never be profitable.
Our ability to generate revenue and achieve profitability depends on our ability, either alone or with strategic collaboration partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, our product candidates. We do not currently generate revenues from product sales (other than licensing revenue from sales of TEMCELL® HS. Inj. (“TEMCELL”), a registered trademark of JCR Pharmaceuticals Co., Ltd. (“JCR”), by JCR in Japan, and royalty revenue from net sales of Alofisel® a registered trademark of TiGenix NV (“TiGenix”), previously known as Cx601, an adipose-derived mesenchymal stromal cell product developed by TiGenix, now a wholly owned subsidiary of Takeda Pharmaceutical Company Limited (“Takeda”) and approved for marketing in the EU), and we may never generate product sales. Our ability to generate future revenues from product sales depends heavily on our success in a number of areas, including:
•completing research, preclinical and clinical development of our product candidates;
•seeking and obtaining regulatory and marketing approvals for product candidates for which we complete clinical studies;
•establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products and services to support clinical development and the market demand for our product candidates, if approved;
•launching and commercializing product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing commercial and distribution capabilities necessary to effectively seek and maintain market access and ensure compliance with legal and regulatory requirements relating to interactions with healthcare providers, healthcare organizations and government agencies;
•obtaining market acceptance of our product candidates as viable treatment options;
•addressing competing technological and market developments;
•obtaining and sustaining an adequate level of reimbursement from payors;
•identifying and validating new cell therapy product candidates;
•negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter;
•maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets, know-how and trademarks;
•attracting, hiring and retaining qualified personnel; and
•implementing additional internal systems and infrastructure, as needed.
Even if one or more of the product candidates that we develop is approved for commercial sale, we anticipate incurring significant costs associated with commercializing and distributing any approved product candidate. Our expenses could increase beyond expectations if we are required by the United States Food and Drug Administration (“FDA”), the European Medicines Agency (“EMA”), or other regulatory agencies, to perform clinical and other studies in addition to those that we currently anticipate. We may not become profitable and may need to obtain additional funding to continue operations.
We require substantial additional financing to achieve our goals, and our failure to obtain this necessary capital or establish and maintain strategic partnerships to provide funding support for our development programs could force us to delay, limit, reduce or terminate our product development or commercialization efforts.
Our operations have consumed substantial amounts of cash since inception. As of June 30, 2023, our cash and cash equivalents were $71.3 million. We expect to continue to incur significant expenses and increase our cumulative operating losses for the foreseeable future in connection with our planned research, development and product commercialization efforts. In addition, we will require additional financing to achieve our goals and our failure to do so could adversely affect our commercialization efforts. We anticipate that our expenses will increase if and as we:
•continue the research and clinical development of our product candidates, including MPC-150-IM (Class II-IV Chronic Heart Failure (“CHF”)), MPC-06-ID (Chronic Low Back Pain (“CLBP”)), remestemcel-L and MPC-300-IV (inflammatory conditions) product candidates;
•seek to identify, assess, acquire, and/or develop other and combination product candidates and technologies;
7
•seek regulatory and marketing approvals in multiple jurisdictions for our product candidates that successfully complete clinical studies and identify and apply for regulatory designations to facilitate development and ultimate commercialization of our products;
•establish and maintain collaborations and strategic partnerships with third parties for the development and commercialization of our product candidates, or otherwise build and maintain a sales, marketing and distribution infrastructure and/or external logistics to commercialize any products for which we may obtain marketing approval;
•further develop and implement our proprietary manufacturing processes in both planar technology and our bioreactor programs and expand our manufacturing capabilities and resources for commercial production;
•seek coverage and reimbursement from third-party payors, including government and private payors for future products;
•make milestone or other payments under our agreements pursuant to which we have licensed or acquired rights to intellectual property and technology;
•seek to maintain, protect and expand our intellectual property portfolio;
•seek to attract and retain skilled personnel; and
•develop the compliance and other infrastructure necessary to support product commercialization and distribution.
If we were to experience any delays or encounter issues with any of the above, including clinical holds, failed studies, inconclusive or complex results, safety or efficacy issues, or other regulatory challenges that require longer follow-up of existing studies, additional studies, or additional supportive studies in order to pursue marketing approval, it could further increase the costs associated with the above. Further, the net operating losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a shareholder or as a holder of the ADSs. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through strategic collaborations or partnerships, or marketing, distribution or licensing arrangements with third parties, we may be required to do so at an earlier stage than would otherwise be ideal and/or may have to limit valuable rights to our intellectual property, technologies, product candidates or future revenue streams, or grant licenses or other rights on terms that are not favorable to us. Furthermore, any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates.
We have continued our focus on maintaining tight control of net cash usage for operating activities, which was $63.3 million for the year ended June 30, 2023. As of June 30, 2023, we held total cash reserves of $71.3 million. We are implementing various cost containment and deferment strategies, including the reprioritization of projects and operational streamlining to manage net operating cash usage. In August 2023, the FDA provided a complete response to our BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data. In line with our overall commercial strategy to progress to adult populations, we intend to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. In conjunction with implementing cost containment and deferment strategies, additional inflows from royalty monetization, capital markets, strategic partnerships or product specific financing will be required to meet our projected expenditure consistent with our business strategy over at least the next 12 months. As a result of these matters, there is material uncertainty related to events or conditions that may cast significant doubt (or raise substantial doubt as contemplated by Public Company Accounting Oversight Board (“PCAOB”) standards) on our ability to continue as a going concern and, therefore, that we may be unable to realize our assets and discharge our liabilities in the normal course of business. Our consolidated financial statements do not include any adjustments that may result from the outcome of this uncertainty. If we are unable to obtain adequate funding or partnerships beyond the 12-month period we may not be able to continue as a going concern, and our shareholders and holders of the ADSs may lose some or all of their investment in Mesoblast. See Note 1(i) of our accompanying financial statements.
8
The terms of our loan facilities with funds associated with Oaktree Capital Management, L.P. (“Oaktree”) and NovaQuest Capital Management, L.L.C. (“NovaQuest”) could restrict our operations, particularly our ability to respond to changes in our business or to take specified actions.
On November 19, 2021, we entered into a loan agreement and guaranty with Oaktree, for a $90.0 million, five-year senior debt facility. We drew the first tranche of $60.0 million at closing. We will seek to extend our option to draw the additional $30.0 million tranche beyond September 30, 2023, subject to us achieving certain milestones. On June 29, 2018, we entered into a loan and security agreement with NovaQuest for a $40.0 million non-dilutive, eight-year term credit facility, repayable from net sales of our allogeneic product candidate remestemcel-L in pediatric patients with steroid-refractory acute graft versus host disease (“SR-aGVHD”), in the United States and other geographies excluding Asia. We drew the first tranche of $30.0 million on closing. Our loan facilities with Oaktree and NovaQuest contain a number of covenants that impose operating restrictions on us, which may restrict our ability to respond to changes in our business or take specified actions. Under the terms of our Oaktree agreement the minimum unrestricted cash balance we need to maintain is $35.0 million. Our ability to comply with the various covenants under the agreements may be affected by events beyond our control, and we may not be able to continue to meet the covenants. Upon the occurrence of an event of default, Oaktree or NovaQuest could elect to declare all amounts outstanding under the loan facility to be immediately due and payable and terminate all commitments to extend further credit. If Oaktree or NovaQuest accelerates the repayment, we may not have sufficient funds to repay our existing debt. If we were unable to repay the owed amounts, Oaktree or NovaQuest could proceed against the collateral granted to it to secure such indebtedness. We have pledged substantially all of our assets as collateral under the loan facility with Oaktree, and a portion of our assets relating to the SR-aGVHD product candidate as collateral under the loan facility with NovaQuest.
We are subject to risks associated with currency fluctuations, and changes in foreign currency exchange rates could impact our results of operations.
Historically, a substantial portion of our operating expenses has been denominated in U.S. dollars and our main currency requirements are U.S. dollars, Australian dollars and Singapore dollars. Approximately 67% of our cash and cash equivalents as of June 30, 2023 were denominated in U.S. dollars and 33% were denominated in Australian dollars. Because we have multiple functional currencies across different jurisdictions, changes in the exchange rate between these currencies and the foreign currencies of the transactions recorded in our accounts could materially impact our reported results of operations and distort period-to-period comparisons. For example, a portion of our research and clinical trials are undertaken in Australia. As such, payment will be made in Australian dollar currency, and may exceed the budgeted expenditure if there are adverse currency fluctuations against the U.S. dollar.
More specifically, if we decide to convert our Australian dollars into U.S. dollars for any business purpose, appreciation of the U.S. dollar against the Australian dollar would have a negative effect on the U.S. dollar amount available to us. Appreciation or depreciation in the value of the Australian dollar relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. As a result of such foreign currency fluctuations, it could be more difficult to detect underlying trends in our business and results of operations.
Unfavorable global economic or political conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. A global financial crisis or a global or regional political disruption could cause extreme volatility in the capital and credit markets. A severe or prolonged economic downturn or political disruption could result in a variety of risks to our business, including weakened demand for our product candidates, if approved, and our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy or political disruption could also strain our manufacturers or suppliers, possibly resulting in supply disruption. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the political or economic climate and financial market conditions could adversely impact our business.
Risks Related to Clinical Development and Regulatory Review and Approval of Our Product Candidates
Our product candidates are based on our novel mesenchymal lineage cell technology, which makes it difficult to accurately and reliably predict the time and cost of product development and subsequently obtaining regulatory
9
approval. At the moment, no industrially manufactured, non-hematopoietic, allogeneic cell products have been approved in the United States.
Other than with respect to sales of products by our licensees, we have not commercially marketed, distributed or sold any products. The success of our business depends on our ability to develop and commercialize our lead product candidates. We have concentrated our product research and development efforts on our mesenchymal lineage cell platform, a novel type of cell therapy. Our future success depends on the successful development of this therapeutic approach. There can be no assurance that any development problems we experience in the future related to our mesenchymal lineage cell platform will not cause significant delays or unanticipated costs, or that such development problems can be solved. We may also experience delays in developing sustainable, reproducible and scalable manufacturing processes or transferring these processes to collaborators, which may prevent us from completing our clinical studies or commercializing our products on a timely or profitable basis, if at all.
In addition, the clinical study requirements of the FDA, the EMA and other regulatory agencies and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of the potential product candidates. The regulatory approval process for novel product candidates such as ours can be more expensive and take longer to develop than for other, better known or extensively studied pharmaceutical or other product candidates. In addition, adverse developments in clinical trials of cell therapy products conducted by others may cause the FDA or other regulatory bodies to change the requirements for approval of any of our product candidates.
We may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory agencies.
We must conduct extensive testing of our product candidates to demonstrate their safety and efficacy, including both preclinical animal testing and evaluation in human clinical trials, before we can obtain regulatory approval to market and sell them. Conducting such testing is a lengthy, time-consuming, and expensive process and there is a high rate of failure.
Our current and completed preclinical and clinical results for our product candidates are not necessarily predictive of the results of our ongoing or future clinical trials. Promising results in preclinical studies of a product candidate may not be predictive of similar results in humans during clinical trials, and successful results from early human clinical trials of a product candidate may not be replicated in later and larger human clinical trials or in clinical trials for different indications. If the results of our or our collaborators’ ongoing or future clinical trials are negative or inconclusive with respect to the efficacy of our product candidates, or if these trials do not meet the clinical endpoints with statistical significance, or if there are safety concerns or adverse events associated with our product candidates, we or our collaborators may be prevented or delayed in obtaining marketing approval for our product candidates.
Even if ongoing or future clinical studies meet the clinical endpoints with statistical significance, the FDA or other regulatory agencies may still find the data insufficient to support marketing approval based on other factors.
We may encounter substantial delays in our clinical studies, including as a result of disruptive events beyond our control, including the COVID-19 or any future pandemic.
We cannot guarantee that any preclinical testing or clinical trials will be conducted as planned or completed on schedule, if at all. As a result, we may not achieve our expected clinical milestones. A failure can occur at any stage of testing. Events that may prevent successful or timely commencement, enrollment or completion of clinical development include:
•problems which may arise as a result of our transition of research and development programs from licensors or previous sponsors;
•delays in raising, or inability to raise, sufficient capital to fund the planned trials;
•delays by us or our collaborators in reaching a consensus with regulatory agencies on trial design;
•changes in trial design;
•inability to identify, recruit and train suitable clinical investigators;
•inability to add new clinical trial sites;
•delays in reaching agreement on acceptable terms for the performance of the trials with contract research organizations (“CROs”), and clinical trial sites;
10
•delays in obtaining required Institutional Review Board (“IRB”), approval at each clinical trial site;
•delays in recruiting suitable clinical sites and patients (i.e., subjects) to participate in clinical trials and delays in accruing medical events necessary to complete any events-driven trial;
•imposition of a clinical hold by regulatory agencies for any reason, including negative clinical results, safety concerns or as a result of an inspection of manufacturing or clinical operations or trial sites;
•failure by CROs, other third parties or us or our collaborators to adhere to clinical trial requirements;
•failure to perform in accordance with the FDA’s current Good Clinical Practices (“cGCP”), or applicable regulatory guidelines in other countries;
•delays in testing, validation, manufacturing and delivery of a product candidate to clinical trial sites;
•delays caused by patients not completing participation in a trial or not returning for post-treatment follow-up;
•delays caused by clinical trial sites not completing a trial;
•failure to demonstrate adequate efficacy;
•occurrence of serious adverse events in clinical trials that are associated with a product candidates and that are viewed to outweigh its potential benefits;
•changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; or
•disagreements between us and the FDA or other regulatory agencies regarding a clinical trial design, protocol amendments, or interpreting the data from our clinical trials.
In addition, our ongoing clinical trials may be affected by delays caused by disruptive events outside our control, such as delays in monitoring and data collection as a result of geopolitical instability, significant climate events and the COVID-19 pandemic, including due to prioritization of hospital resources, travel restrictions, and the inability to access sites for patient monitoring. In addition, some patients may be unable to comply with clinical trial protocols if quarantines or stay at home orders impede patient movement or interrupt health services.
Delays, including delays caused by the above factors, can be costly and could negatively affect our or our collaborators’ ability to complete clinical trials for our product candidates. If we or our collaborators are not able to successfully complete clinical trials or are not able to do so in a timely and cost-effective manner, we will not be able to obtain regulatory approval and/or will not be able to commercialize our product candidates and our commercial partnering opportunities will be harmed.
We may find it difficult to enroll patients in our clinical trials, which could delay or prevent development of our product candidates.
Identifying and qualifying patients to participate in clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends on the speed at which we can recruit patients to participate in testing our product candidates as well as completion of required follow-up periods. In general, if patients are unwilling to participate in our cell therapy trials because of negative publicity from adverse events in the biotechnology or cell therapy industries or for other reasons, including competitive clinical trials for similar patient populations, the timeline for recruiting patients, conducting trials and obtaining regulatory approval for our product candidates may be delayed. Additionally, we or our collaborators generally will have to run multi-site and potentially multi-national trials, which can be time consuming, expensive and require close coordination and supervision. If we have difficulty enrolling a sufficient number of patients or otherwise conducting clinical trials as planned, we or our collaborators may need to delay, limit or terminate ongoing or planned clinical trials, any of which would have an adverse effect on our business.
If there are delays in accumulating the required number of trial subjects or, in trials where clinical events are a primary endpoint, if the events needed to assess performance of our clinical candidates do not accrue at the anticipated rate, there may be delays in completing the trial. These delays could result in increased costs, delays in advancing development of our product candidates, including delays in testing the effectiveness, or even termination of the clinical trials altogether.
Patient enrollment and completion of clinical trials are affected by factors including:
•size of the patient population, particularly in orphan diseases;
11
•severity of the disease under investigation;
•design of the trial protocol;
•eligibility criteria for the particular trial;
•perceived risks and benefits of the product candidate being tested;
•proximity and availability of clinical trial sites for prospective patients;
•availability of competing therapies and clinical trials;
•efforts to facilitate timely enrollment in clinical trials;
•patient referral practices of physicians and level and effectiveness of study site recruitment efforts; and
•ability to monitor patients adequately during and after treatment.
Once enrolled, patients may choose to discontinue their participation at any time during the trial, for any reason. Participants also may be terminated from the study at the initiative of the investigator, for example if they experience serious adverse clinical events or do not follow the study directions. If we are unable to maintain an adequate number of patients in our clinical trials, we may be required to delay or terminate an ongoing clinical trial, which would have an adverse effect on our business.
We may conduct multinational clinical trials, which present additional and unique risks.
We plan to seek initial marketing approval for our product candidates in the United States and in select non-U.S. jurisdictions such as Europe, Japan and Canada. Conducting trials on a multinational basis requires collaboration with foreign medical institutions and healthcare providers. Our ability to successfully initiate, enroll and complete a clinical trial in multiple countries is subject to numerous risks unique to conducting business internationally, including:
•difficulty in establishing or managing relationships with physicians, sites and CROs;
•standards within different jurisdictions for conducting clinical trials and recruiting patients;
•our ability to effectively interface with non-US regulatory authorities;
•our inability to identify or reach acceptable agreements with qualified local consultants, physicians and partners;
•the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatments, and anti-corruption/anti-bribery laws;
•differing genotypes, average body weights and other patient profiles within and across countries from our donor profile may impact the optimal dosing or may otherwise impact the results of our clinical trials; and
•the COVID-19 pandemic limiting our ability to commence and conduct studies, including recruiting patients.
The complexity of conducting multinational clinical trials could negatively affect our or our collaborators’ ability to complete trials as intended which could have an adverse effect on our business.
Serious adverse events or other safety risks could require us to abandon development and preclude, delay or limit approval of our product candidates, or limit the scope of any approved indication or market acceptance.
Participants in clinical trials of our investigational cell therapy products may experience adverse reactions or other undesirable side effects. While some of these can be anticipated, others may be unexpected. We cannot predict the frequency, duration, or severity of adverse reactions or undesirable side effects that may occur during clinical investigation of our product candidates. If any of our product candidates, prior to or after any approval for commercial sale, cause serious adverse events or are associated with other safety risks, a number of potentially significant negative consequences could result, including:
•regulatory authorities may suspend (e.g., through a clinical hold) or terminate clinical trials;
•regulatory authorities may deny regulatory approval of our product candidates;
•regulators may restrict the indications or patient populations for which a product candidate is approved;
12
•regulatory authorities may require certain labeling statements, such as warnings or contraindications or limitations on the indications for use, and/or impose restrictions on distribution in the form of a risk evaluation and mitigation strategy (“REMS”), in connection with approval, if any;
•regulatory authorities may withdraw their approval, require more onerous labeling statements or impose a more restrictive REMS than any product that is approved;
•we may be required to change the way the product is administered or conduct additional clinical trials;
•patient recruitment into our clinical trials may suffer;
•our relationships with our collaborators may suffer;
•we could be required to provide compensation to subjects for their injuries, e.g., if we are sued and found to be liable or if required by the laws of the relevant jurisdiction or by the policies of the clinical site; or
•our reputation may suffer.
There can be no assurance that adverse events associated with our product candidates will not be observed, in such settings where no prior adverse events have occurred. As is typical in clinical development, we have a program of ongoing toxicology studies in animals for our clinical-stage product candidates and cannot provide assurance that the findings from such studies or any ongoing or future clinical trials will not adversely affect our clinical development activities.
We may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to participants or if preliminary data demonstrate that our product candidates are unlikely to receive regulatory approval or unlikely to be successfully commercialized. In addition, regulatory agencies, IRBs or data safety monitoring boards may at any time recommend the temporary or permanent discontinuation of our clinical trials or request that we cease using investigators in the clinical trials if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements, or that they present an unacceptable safety risk to participants. If we elect or are forced to suspend or terminate a clinical trial for any of our product candidates, the commercial prospects for that product as well as our other product candidates may be harmed and our ability to generate product revenue from these product candidates may be delayed or eliminated. Furthermore, any of these events could prevent us or our collaborators from achieving or maintaining market acceptance of the affected product and could substantially increase the costs of commercializing our product candidates and impair our ability to generate revenue from the commercialization of these product candidates either by us or by our collaborators.
Several of our product candidates are being evaluated for the treatment of patients who are extremely ill, and patient deaths that occur in our clinical trials could negatively impact our business even if they are not shown to be related to our product candidates.
We are developing MPC-150-IM, which will focus on patients with heart failure with reduced ejection fraction associated with ischemic and/or diabetic etiology, and remestemcel-L, which will focus on SR-aGVHD. We have also been developing remestemcel-L in COVID-19 infected patients with moderate to severe acute respiratory distress syndrome (“ARDS”) on ventilator support. The patients who receive our product candidates are very ill due to their underlying diseases.
Generally, patients remain at high risk following their treatment with our product candidates and may more easily acquire infections or other common complications during the treatment period, which can be serious and life threatening. As a result, it is likely that we will observe severe adverse outcomes in patients during our Phase 3 and other trials for these product candidates, including patient death. If a significant number of study subject deaths were to occur, regardless of whether such deaths are attributable to our product candidates, our ability to obtain regulatory approval for the applicable product candidate may be adversely impacted and our business could be materially harmed. Should studies of a candidate product result in regulatory approval, any association with a significant number of study subject deaths could limit the commercial potential of an approved product candidate, or negatively impact the medical community’s willingness to use our product with patients.
13
The requirements to obtain regulatory approval of the FDA and regulators in other jurisdictions can be costly, time-consuming, and unpredictable. If we or our collaborators are unable to obtain timely regulatory approval for our product candidates, our business may be substantially harmed.
The regulatory approval process is expensive and the time and resources required to obtain approval from the FDA or other regulatory authorities in other jurisdictions to sell any product candidate is uncertain and approval may take years. Whether regulatory approval will be granted is unpredictable and depends upon numerous factors, including the discretion of the regulatory authorities. For example, governing legislation, approval policies, regulations, regulatory policies, or the type and amount of preclinical and clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. It is possible that none of our existing or future product candidates will ever obtain regulatory approval, even if we expend substantial time and resources seeking such approval.
Further, regulatory requirements governing cell therapy products in particular have changed and may continue to change in the future. For example, in December 2016, the 21st Century Cures Act (“Cures Act”) was signed into law in the United States. This law is designed to advance medical innovation, and includes a number of provisions that may impact our product development programs. For example, the Cures Act establishes a new “regenerative medicine advanced therapy” designation (“RMAT”), and creates a pathway for increased interaction with FDA for the development of products which obtain designations. Although the FDA issued guidance documents in 2019, it remains unclear how and when the FDA will fully implement all deliverables under the Cures Act.
Any regulatory review committees and advisory groups and any contemplated new guidelines may lengthen the regulatory review process, require us to perform additional studies, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of our product candidates or lead to significant post-approval limitations or restrictions. As we advance our product candidates, we will be required to consult with these regulatory and advisory groups, and comply with applicable guidelines. If we fail to do so, we may be required to delay or discontinue development of our product candidates. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a product candidate to market could decrease our ability to generate sufficient revenue to maintain our business.
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
•we may be unable to successfully complete our ongoing and future clinical trials of product candidates;
•we may be unable to demonstrate to the satisfaction of the FDA or other regulatory authorities that a product candidate is safe, pure, and potent for any or all of a product candidate’s proposed indications;
•we may be unable to demonstrate that a product candidate’s benefits outweigh the risk associated with the product candidate;
•the FDA or other regulatory authorities may disagree with the design or implementation of our clinical trials;
•the results of clinical trials may not meet the level of statistical significance required by the FDA or other regulatory authorities for approval;
•the FDA or other regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials;
•a decision by the FDA, other regulatory authorities or us to suspend or terminate a clinical trial at any time;
•the data collected from clinical trials of our product candidates may be inconclusive or may not be sufficient to support the submission of a Biologics License Application (“BLA”), or other submission or to obtain regulatory approval in the United States or elsewhere;
•our third party manufacturers of supplies needed for manufacturing product candidates may fail to satisfy FDA or other regulatory requirements and may not pass inspections that may be required by FDA or other regulatory authorities;
•the failure to comply with applicable regulatory requirements following approval of any of our product candidates may result in the refusal by the FDA or similar foreign regulatory agency to approve a pending BLA or supplement to a BLA submitted by us for other indications or new product candidates; and
•the approval policies or regulations of the FDA or other regulatory authorities outside of the United States may significantly change in a manner rendering our clinical data insufficient for approval.
14
We or our collaborators may gain regulatory approval for any of our product candidates in some but not all of the territories available and any future approvals may be for some but not all of the target indications, limiting their commercial potential. Regulatory requirements and timing of product approvals vary from country to country and some jurisdictions may require additional testing beyond what is required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other countries or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval.
Our drug candidates may not benefit from an expedited approval path for cellular medicines designated as Regenerative Medicine Advanced Therapies (RMATs) under the 21st Century Cures Act.
On December 21, 2017, the FDA granted RMAT designation for our novel MPC therapy in the treatment of heart failure patients with left ventricular systolic dysfunction and left ventricular assist devices. The FDA granted RMAT designation for our novel MPC therapy in the treatment of chronic lower back pain due to degenerative disc disease. While the Cures Act offers several potential benefits to drugs designated as RMATs, including eligibility for increased agency support and advice during development, priority review on filing, a potential pathway for accelerated or full approval based on surrogate or intermediate endpoints, and the potential to use patient registry data and other sources of real world evidence for post approval confirmatory studies, there is no assurance that any of these potential benefits will either apply to any or all of our drug candidates or, if applicable, accelerate marketing approval. RMAT designation does not change the evidentiary standards of safety and effectiveness needed for marketing approval.
Furthermore, there is no certainty as to whether any of our product candidates that have not yet received RMAT designation under the Cures Act will receive such designation under the Cures Act. Designation as an RMAT is within the discretion of the FDA. Accordingly, even if we believe one of our products or product candidates meets the criteria for RMAT designation, the FDA may disagree. Additionally, for any product candidate that receives RMAT designation, we may not experience a faster development, review or approval process compared to conventional FDA procedures. The FDA may withdraw RMAT designation if it believes that the product no longer meets the qualifying criteria for designation.
Even if we obtain regulatory approval for our product candidates, our products will be subject to ongoing regulatory scrutiny.
Any of our product candidates that are approved in the United States or in other jurisdictions will continue to be subject to ongoing regulatory requirements relating to the quality, identity, strength, purity, safety, efficacy, testing, manufacturing, marketing, advertising, promotion, distribution, sale, storage, packaging, pricing, import or export, record-keeping and submission of safety and other post-market information for all approved product candidates. In the United States, this includes both federal and state requirements. In particular, as a condition of approval of a BLA, the FDA may require a REMS, to ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals and elements to assure safe use (“ETASU”). ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. Moreover, regulatory approval may require substantial post-approval (Phase 4) testing and surveillance to monitor the drug’s safety or efficacy. Delays in the REMS approval process could result in delays in the BLA approval process. In addition, as part of the REMS, the FDA could require significant restrictions, such as restrictions on the prescription, distribution and patient use of the product, which could significantly impact our ability to effectively commercialize our product candidates, and dramatically reduce their market potential thereby adversely impacting our business, results of operations and financial condition. Post-approval study requirements could add additional burdens, and failure to timely complete such studies, or adverse findings from those studies, could adversely affect our ability to continue marketing the product.
Any failure to comply with ongoing regulatory requirements, as well as post-approval discovery of previously unknown problems, including adverse events of unanticipated severity or frequency, or with manufacturing operations or processes, may significantly and adversely affect our ability to generate revenue from our product candidates, and may result in, among other things:
•restrictions on the marketing or manufacturing of the product candidates, withdrawal of the product candidates from the market, or voluntary or mandatory product recalls;
•suspension or withdrawal of regulatory approval;
•costly regulatory inspections;
15
•fines, warning letters, or holds on clinical trials;
•refusal by the FDA to approve pending applications or supplements to approved applications filed by us or our collaborators, or suspension or revocation of BLAs;
•restrictions on our operations;
•product seizure or detention, or refusal to permit the import or export of products; or
•injunctions or the imposition of civil or criminal penalties by FDA or other regulatory bodies.
If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our business and our operating results will be adversely affected.
The FDA’s policies, or that of the applicable regulatory bodies in other jurisdictions, may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we or our collaborators are not able to maintain regulatory compliance, are slow or unable to adopt new requirements or policies, or effect changes to existing requirements, we or our collaborators may no longer be able to lawfully market our product, and we may not achieve or sustain profitability, which would adversely affect our business.
Ethical and other concerns surrounding the use of embryonic stem cell-based therapy may negatively affect regulatory approval or public perception of our non-embryonic stem cell product candidates, which could reduce demand for our products or depress our share price.
The use of embryonic stem cells (“ESCs”), for research and therapy has been the subject of considerable public debate, with many people voicing ethical, legal and social concerns related to their collection and use. Our cells are not ESCs, which have been the predominant focus of this public debate and concern in the United States and elsewhere. However, the distinction between ESCs and non-ESCs, such as our mesenchymal lineage cells, may be misunderstood by the public. Negative public attitudes toward cell therapy and publicity and harm from cell therapy usage clinically by others could also result in greater governmental regulation of cell therapies, which could harm our business. The improper use of cells could give rise to ethical and social commentary adverse to us, which could harm the market demand for new products and depress the price of our ordinary shares and ADSs. Ongoing lack of understanding of the difference between ESCs and non-ESCs could negatively impact the public’s perception of our company and product candidates and could negatively impact us.
Additional government-imposed restrictions on, or concerns regarding possible government regulation of, the use of cell therapies in research, development and commercialization could also cause an adverse effect on us by harming our ability to establish important partnerships or collaborations, delaying or preventing the development of certain product candidates, and causing a decrease in the price of our ordinary shares and ADSs, or by otherwise making it more difficult for us to raise additional capital. For example, concerns regarding such possible regulation could impact our ability to attract collaborators and investors. Also, existing and potential government regulation of cell therapies may lead researchers to leave the field of cell therapy research altogether in order to assure that their careers will not be impeded by restrictions on their work. This may make it difficult for us to find and retain qualified scientific personnel.
Orphan drug designation may not ensure that we will benefit from market exclusivity in a particular market, and if we fail to obtain or maintain orphan drug designation or other regulatory exclusivity for some of our product candidates, our competitive position would be harmed.
A product candidate that receives orphan drug designation can benefit from potential commercial benefits following approval. Under the Orphan Drug Act, the FDA may designate a product candidate as an orphan drug if it is intended to treat a rare disease or condition, defined as affecting (1) a patient population of fewer than 200,000 in the United States, (2) a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States, or (3) an “orphan subset” of a patient population greater than 200,000 in the United States. In the European Union (“EU”), the EMA’s Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than 10,000 persons in the EU. Currently, this designation provides market exclusivity in the U.S. and the EU for seven years and ten years, respectively, if a product is the first such product approved for such orphan indication. This market exclusivity does not,
16
however, pertain to indications other than those for which the drug was specifically designated in the approval, nor does it prevent other types of drugs from receiving orphan designations or approvals in these same indications. Further, even after an orphan drug is approved, the FDA can subsequently approve a drug with similar chemical structure for the same condition if the FDA concludes that the new drug is clinically superior to the orphan product or a market shortage occurs. In the EU, orphan exclusivity may be reduced to six years if the drug no longer satisfies the original designation criteria or can be lost altogether if the marketing authorization holder consents to a second orphan drug application or cannot supply enough drug, or when a second applicant demonstrates its drug is “clinically superior” to the original orphan drug.
Our remestemcel-L product candidate has received orphan drug designation for the treatment of aGVHD by the FDA and EMA, and our CHF product candidate, rexlemestrocel-L has received orphan drug designation from the FDA for prevention of post-implantation mucosal bleeding in end-stage CHF patients who require a left ventricular assist device (“LVAD”). If we seek orphan drug designations for other product candidates in other indications, we may fail to receive such orphan drug designations and, even if we succeed, such orphan drug designations may fail to result in or maintain orphan drug exclusivity upon approval, which would harm our competitive position.
We may face competition from biosimilars due to changes in the regulatory environment.
In the United States, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biological products that are demonstrated to be “highly similar”, or biosimilar, to or “interchangeable” with an FDA-approved innovator (original) biological product. This pathway could allow competitors to reference data from innovator biological products already approved after 12 years from the time of approval. For several years the annual budget requests of President Obama’s administration included proposals to cut this 12-year period of exclusivity down to seven years. Those proposals were not adopted by Congress. Under President Biden’s administration, it is unclear if a similar change will be pursued in the future. In Europe, the European Commission has granted marketing authorizations for several biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued over the past few years. In Europe, a competitor may reference data from biological products already approved, but will not be able to get on the market until ten years after the time of approval. This 10-year period will be extended to 11 years if, during the first eight of those 10 years, the marketing authorization holder obtains an approval for one or more new therapeutic indications that bring significant clinical benefits compared with existing therapies. In addition, companies may be developing biosimilars in other countries that could compete with our products. If competitors are able to obtain marketing approval for biosimilars referencing our products, our products may become subject to competition from such biosimilars causing the price for our products and our potential market share to suffer, resulting in lower product sales.
Our BLA submission for pediatric SR-aGVHD may not be approved and even if it is approved, we will continue to be closely regulated by FDA.
As a biological product, our allogeneic cellular medicine, remestemcel-L, for the treatment of children with SR-aGVHD, requires regulatory approval from the FDA before it may legally be distributed in U.S. commerce. In particular, remestemcel-L will require FDA approval of a BLA under Section 351 of the Public Health Service Act to be commercialized.
We have received Fast Track designation from the FDA for remestemcel-L in pediatrics with SR-aGVHD. Fast Track designation may provide for a more streamlined development or approval process but it does not change the standards for approval and may be rescinded by the FDA if the product no longer meets the qualifying criteria. A biologic product that receives Fast Track designation can be eligible for regulatory benefits, including rolling BLA review. Rolling review of a BLA enables individual modules of the application to be submitted to and reviewed by the FDA on an ongoing basis, rather than waiting for all sections of a BLA to be completed before submission. Note that there is no benefit of Fast Track in relation to the review time for the resubmitted BLA.
Remestemcel-L was accepted for Priority Review by the FDA with an action date of September 30, 2020, under the Prescription Drug User Fee Act (“PDUFA”). In August 2020, the Oncologic Drugs Advisory Committee (“ODAC”) of the FDA voted in favor that available data from a single-arm Phase 3 trial and evidence from additional studies support the efficacy of remestemcel-L in pediatric patients with SR-aGVHD. Although the FDA considers the recommendation of the panel, the final decision regarding the approval of the product is made solely by the FDA, and the recommendations by the panel are non-binding. On September 30, 2020, the FDA issued a Complete Response Letter to our BLA for remestemcel-L for the treatment of pediatric SR-aGVHD. Despite the overwhelming ODAC vote, the FDA recommended that we conduct at least one additional randomized, controlled study in adults and/or children to provide further evidence of the effectiveness of remestemcel-L for SR-aGVHD.
17
On January 31, 2023, we resubmitted the BLA and on August 1, 2023, the FDA issued a Complete Response Letter in relation to the resubmitted BLA and requires more data to support marketing approval, including potency assay or clinical data. The FDA acknowledged in the resubmission review that changes implemented appeared to improve assay performance relative to the original version of the assay used in the pediatric Phase 3 trial. In line with our overall commercial strategy to progress to adult populations, we intend to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. In connection with its review of the BLA, the FDA conducted a pre-license inspection of the manufacturing process of remestemcel-L which did not result in the issuance of a Form 483 and there were no observed concerns.
The FDA reviews a BLA to determine, among other things, whether a product is safe, pure and potent and the facility in which it is manufactured, processed, packed, or held meets standards designed to assure the product’s continued safety, purity and potency. During the course of review of our BLA, the FDA may request or require additional preclinical, clinical, chemistry and manufacturing, controls (or CMC), or other data and information. The development and provision of these data and information may be time consuming and expensive. Our failure to comply, or the failure of our contract manufacturers to satisfy, applicable FDA CMC requirements could result in a delay or failure to obtain approval of our BLA. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in our submission and may request additional testing or information. The testing and approval process requires substantial time, effort and financial resources, and may take several years to complete. In addition, the FDA or other regulatory agencies may find the data from our clinical studies insufficient to support marketing approval. For example, our Phase 3 study for remestemcel-L for the treatment of pediatric SR-aGVHD, which met the primary clinical endpoint with statistical significance, was conducted as a single-arm study due to the seriousness of the condition, the rapid clinical deterioration of affected patients, the mounting literature suggesting a meaningful treatment effect, and the position in the medical community that a randomized controlled trial was neither feasible nor ethical in this patient population. While we have provided the FDA with comparator outcomes from control subjects, it is possible that the FDA may not find the data sufficient for approval. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
It is possible that we will have to participate in other Advisory Committee proceedings for other of our product candidates. FDA Advisory Committees are convened to conduct public hearings on matters of importance that come before the FDA, to review the issues involved, and to provide advice and recommendations to the FDA. New product candidates may be referred for review by Advisory Committees whether the FDA has identified issues or concerns in respect of such candidates or not. Advisory Committee input and recommendations may be used at the discretion of the FDA. Advisory Committee proceedings are in part conducted publicly. While the recommendations made by Advisory Committees in respect of marketing applications for any product are not dispositive, such determinations and recommendations are often influential, and may be made available publicly and to the advantage of our competitors. In addition, it is possible that safety findings and recommendations as well as other concerns and considerations raised by Advisory Committee members, who constitute a multi-disciplinary group of experts (including representatives and/or advocates from the consumer sector), may impact the FDA’s review of our product candidate submissions or labeling unfavorably. Furthermore, commentary from Advisory Committee proceedings can figure into future product and other litigation.
Even if we receive regulatory approval for our remestemcel-L product, such approval may entail limitations on the indicated uses for which such product may be marketed and/or require post-marketing testing and surveillance to monitor safety or efficacy of our product. The FDA may limit further marketing of our product based on the results of post-marketing studies, if compliance with pre- and post-marketing regulatory standards is not maintained, or if problems occur after our product reaches the marketplace such as later discovery of previously unknown problems or concerns with our product, including adverse events of unanticipated severity or frequency, or with our manufacturing processes.
Risks Related to Collaborators
We rely on third parties to conduct our nonclinical and clinical studies and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates in a timely and cost-effective manner or at all, and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third-party entities, including CROs, academic institutions, hospitals and other third-party collaborators, to monitor, support, conduct and/or oversee preclinical and clinical studies of our current and future product candidates. We rely on these parties for execution of our nonclinical and clinical studies, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is
18
conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities. If we or any of these third-parties fail to comply with the applicable protocol, legal, regulatory, and scientific standards, the clinical data generated in our clinical studies may be deemed unreliable and the FDA, EMA or comparable foreign regulatory authorities may require us to perform additional clinical studies before approving our marketing applications.
If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative parties or do so on commercially reasonable terms. In addition, these parties are not our employees, and except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our on-going nonclinical and clinical programs. If third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our protocols, regulatory requirements, or for other reasons, our clinical studies may be extended, delayed, or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. Third parties may also generate higher costs than anticipated. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
Switching or adding additional third parties involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with these third parties, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition, and prospects.
Our existing product development and/or commercialization arrangements, and any that we may enter into in the future, may not be successful, which could adversely affect our ability to develop and commercialize our product candidates.
We are a party to, and continue to seek additional, collaboration arrangements with biopharmaceutical companies for the development and/or commercialization of our current and future product candidates. We may enter into new arrangements on a selective basis depending on the merits of retaining certain development and commercialization rights for ourselves as compared to entering into selective collaboration arrangements with leading pharmaceutical or biotechnology companies for each product candidate, both in the United States and internationally. To the extent that we decide to enter into collaboration agreements, we will face significant competition in seeking appropriate collaborators. Any failure to meet our clinical milestones with respect to an unpartnered product candidate would make finding a collaborator more difficult. Moreover, collaboration arrangements are complex, costly and time consuming to negotiate, document and implement, and we cannot guarantee that we can successfully maintain such relationships or that the terms of such arrangements will be favorable to us. If we fail to establish and implement collaboration or other alternative arrangements, the value of our business and operating results will be adversely affected.
We may not be successful in our efforts to establish, implement and maintain collaborations or other alternative arrangements if we choose to enter into such arrangements. The terms of any collaboration or other arrangements that we may establish may not be favorable to us. The management of collaborations may take significant time and resources that distract our management from other matters.
Our ability to successfully collaborate with any existing or future collaborators may be impaired by multiple factors including:
•a collaborator may shift its priorities and resources away from our programs due to a change in business strategies, or a merger, acquisition, sale or downsizing of its company or business unit;
•a collaborator may cease development in therapeutic areas which are the subject of our strategic alliances;
•a collaborator may change the success criteria for a particular program or product candidate thereby delaying or ceasing development of such program or candidate;
•a significant delay in initiation of certain development activities by a collaborator will also delay payments tied to such activities, thereby impacting our ability to fund our own activities;
•a collaborator could develop a product that competes, either directly or indirectly, with our current or future products, if any;
19
•a collaborator with commercialization obligations may not commit sufficient financial or human resources to the marketing, distribution or sale of a product;
•a collaborator with manufacturing responsibilities may encounter regulatory, resource or quality issues and be unable to meet demand requirements;
•a collaborator may exercise its rights under the agreement to terminate our collaboration;
•a dispute may arise between us and a collaborator concerning the research or development of a product candidate or commercialization of a product resulting in a delay in milestones, royalty payments or termination of a program and possibly resulting in costly litigation or arbitration which may divert management attention and resources;
•the results of our clinical trials may not match our collaborators’ expectations, even if statistically significant;
•a collaborator may not adequately protect or enforce the intellectual property rights associated with a product or product candidate; and
•a collaborator may use our proprietary information or intellectual property in such a way as to invite litigation from a third party.
Any such activities by our current or future collaborators could adversely affect us financially and could harm our business reputation.
Risks Related to Our Manufacturing and Supply Chain
We have no experience manufacturing our product candidates at a commercial scale. We may not be able to manufacture our product candidates in quantities sufficient for development and commercialization if our product candidates are approved, or for any future commercial demand for our product candidates.
We have manufactured clinical and commercial quantities of our mesenchymal lineage cell product candidates in manufacturing facilities owned by Lonza Walkersville, Inc. and Lonza Bioscience Singapore Pte. Ltd. (collectively referred to as “Lonza”). Earlier this year, FDA conducted a pre-license inspection of the manufacturing process of remestemcel-L which did not result in the issuance of a Form 483 and there were no observed concerns.
The production of any biopharmaceutical, particularly cell-based therapies, involves complex processes and protocols. We cannot provide assurance that such production efforts will enable us to manufacture our product candidates in the quantities and with the quality needed and in a timely manner for clinical trials, regulatory approval(s), and/or any resulting commercialization.
If we are unable to do so, our clinical trials and commercialization efforts, if any, may not proceed in a timely fashion and our business will be adversely affected. If any of our product candidates are approved for commercialization and marketing, we may be required to manufacture the product in large quantities to meet demand. Producing product in commercial quantities requires developing and adhering to complex manufacturing processes that are different from the manufacture of a product in smaller quantities for clinical trials, including adherence to additional and more demanding regulatory standards. Although we believe that we have developed processes and protocols that will enable us to consistently manufacture commercial-scale quantities of product, we cannot provide assurance that such processes and protocols will enable us to manufacture our product candidates in quantities that may be required for commercialization of the product with yields and at costs that will be commercially attractive. If we are unable to establish or maintain commercial manufacture of the product or are unable to do so at costs that we currently anticipate, our business will be adversely affected.
We are focusing on the introduction of novel manufacturing approaches with the potential to result in efficiency and yield improvements to our current process. Certain of these novel approaches include modifying the media used in cell production. Another approach includes the development of 3-dimensional (“3D”) bioreactor-based production for mesenchymal lineage cells. There is no guarantee that we will successfully complete either of these processes or meet all applicable regulatory requirements. This may be due to multiple factors, including the failure to produce sufficient quantities and the inability to produce cells that are equivalent in physical and therapeutic properties as compared to the products produced using our current manufacturing processes. In the event our transition to these improved manufacturing processes is unsuccessful, we may not be able to produce certain of our products in a cost-efficient manner and our business may be adversely affected.
20
Global events may adversely impact the manufacturing and commercialization of remestemcel-L, and other product candidates.
On October 17, 2019, we announced that we had entered into a manufacturing service agreement with Lonza Bioscience Singapore Pte. Ltd. for the supply of commercial product for the potential approval and launch of remestemcel-L. We currently also manufacture our other product candidates with Lonza Singapore.
Due to the effects of the COVID-19 pandemic, and recent geopolitical instability, countries in which we have operations, including Singapore, have experienced some challenges in the ability of our suppliers and contractors to source, supply or acquire raw materials or components needed for our manufacturing process and supply chain. As a result, the manufacturing and commercialization of remestemcel-L and other product candidates could be adversely affected if those impacts and impacts from other disruptive events such as significant climate events, are experienced, with potential for increased costs.
We rely on contract manufacturers to supply and manufacture our product candidates. Our business could be harmed if Lonza fails to provide us with sufficient quantities of these product candidates or fails to do so at acceptable quality levels or prices.
We do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture our mesenchymal lineage cell product candidates for use in the conduct of our clinical trials, and we currently lack the internal resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. As a result, we currently depend on Lonza to manufacture our mesenchymal lineage cell product candidates. Relying on Lonza to manufacture our mesenchymal lineage cell product candidates entails risks, and Lonza may:
•cease or reduce production or deliveries, raise prices or renegotiate terms;
•be unable to meet any product specifications and quality requirements consistently;
•delay or be unable to procure or expand sufficient manufacturing capacity, which may harm our reputation or frustrate our customers;
•not have the capacity sufficient to support the scale-up of manufacturing for our product candidates;
•have manufacturing and product quality issues related to scale-up of manufacturing;
•experience costs and validation of new equipment facilities requirement for scale-up that it will pass on to us;
•fail to comply with cGMP and similar international standards;
•lose its manufacturing facility in Singapore, stored inventory or laboratory facilities through fire or other causes, or other loss of materials necessary to manufacture our product candidates;
•experience disruptions to its operations by conditions unrelated to our business or operations, including the bankruptcy or interruptions of its suppliers;
•experience carrier disruptions or increased costs that it will pass on to us;
•fail to secure adequate supplies of essential ingredients in our manufacturing process;
•experience failure of third parties involved in the transportation, storage or distribution of our products, including the failure to deliver supplies it uses for the manufacture of our product candidates under specified storage conditions and in a timely manner;
•terminate agreements with us; and
•appropriate or misuse our trade secrets and other proprietary information.
Any of these events could lead to delays in the development of our product candidates, including delays in our clinical trials, or failure to obtain regulatory approval for our product candidates, or it could impact our ability to successfully commercialize our current product candidates or any future products. Some of these events could be the basis for FDA or other regulatory action, including injunction, recall, seizure or total or partial suspension of production.
In addition, the lead time needed to establish a relationship with a new manufacturer can be lengthy and expensive, and we may experience delays in meeting demand in the event we must switch to a new manufacturer. We are expanding our manufacturing collaborations in order to meet future demand and to provide back-up manufacturing options, which also involves risk and requires significant time and resources. Our future collaborators may need to expand their facilities
21
or alter the facilities to meet future demand and changes in regulations. These activities may lead to delays, interruptions to supply, or may prove to be more costly than anticipated. Any problems in our manufacturing process could have a material adverse effect on our business, results of operations and financial condition.
We may not be able to manufacture or commercialize our product candidates in a profitable manner.
We intend to implement a business model under which we control the manufacture and supply of our product candidates, including but not exclusively, through our product suppliers, including Lonza. We and the suppliers of our product candidates, including Lonza, have no experience manufacturing our product candidates at commercial scale. Accordingly, there can be no assurance as to whether we and our suppliers will be able to scale-up the manufacturing processes and implement technological improvements in a manner that will allow the manufacture of our product candidates in a cost effective manner. Our or our collaborators’ inability to sell our product candidates at a price that exceeds our cost of manufacture by an amount that is profitable for us will have a material adverse result on the results of our operations and our financial condition.
Collaborators’ ability to identify, test and verify new donor tissue in order to create new master cell banks involves many risks.
The initial stage of manufacturing involves obtaining mesenchymal lineage cell-containing bone marrow from donors, for which we currently rely on our suppliers. Mesenchymal lineage cells are isolated from each donor’s bone marrow and expanded to create a master cell bank. Each individual master cell bank comes from a single donor. A single master cell bank can source many production runs, which in turn can produce up to thousands of doses of a given product, depending on the dose level. The process of identifying new donor tissue, testing and verifying its validity in order to create new master cell banks and validating such cell bank with the FDA and other regulatory agencies is time consuming, costly and prone to the many risks involved with creating living cell products. There could be consistency or quality control issues with any new master cell bank. Although we believe we and our collaborators have the necessary know-how and processes to enable us to create master cell banks with consistent quality and within the timeframe necessary to meet projected demand and we have begun doing so, we cannot be certain that we or our collaborators will be able to successfully do so, and any failure or delays in creating new master cell banks may have a material adverse impact on our business, results of operations, financial conditions and growth prospects and could result in our inability to continue operations.
We and our collaborators depend on a limited number of suppliers for our product candidates’ materials, equipment or supplies and components required to manufacture our product candidates. The loss of these suppliers, or their failure to provide quality supplies on a timely basis, could cause delays in our current and future capacity and adversely affect our business.
We and our collaborators depend on a limited number of suppliers for the materials, equipment and components required to manufacture our product candidates, as well as various “devices” or “carriers” for some of our programs (e.g., the catheter for use with MPC-150-IM, and the hyaluronic acid used for chronic lower back pain). The main consumable used in our manufacturing process is our media, which currently is sourced from fetal bovine serum (“FBS”). This material comes from limited sources, and as a result is expensive. Consequently, we or our collaborators may not be able to obtain sufficient quantities of our product candidates or other critical materials equipment and components in the future, at affordable prices or at all. A delay or interruption by our suppliers may also harm our business, and operating results. In addition, the lead time needed to establish a relationship with a new supplier can be lengthy, and we or our collaborators may experience delays in meeting demand in the event we must switch to a new supplier. The time and effort to qualify for and, in some cases, obtain regulatory approval for a new supplier could result in additional costs, diversion of resources or reduced manufacturing yields, any of which would negatively impact our operating results. Our and our collaborators’ dependence on single-source suppliers exposes us to numerous risks, including the following:
•our or our collaborators’ suppliers may cease or reduce production or deliveries, raise prices or renegotiate terms;
•our or our collaborators’ suppliers may not be able to source materials, equipment or supplies and components required to manufacture our product candidates as a result of the after effects of the COVID-19 pandemic or geopolitical and/or economic instability adversely or the impact of climate events affecting the supply chain;
•we or our collaborators may be unable to locate suitable replacement suppliers on acceptable terms or on a timely basis, or at all; and
22
•delays caused by supply issues may harm our reputation, frustrate our customers and cause them to turn to our competitors for future needs.
We and our collaborators and Lonza are subject to significant regulation with respect to manufacturing our product candidates. The Lonza manufacturing facilities on which we rely may not continue to meet regulatory requirements or may not be able to meet supply demands.
All entities involved in the preparation of therapeutics for clinical studies or commercial sale, including our existing manufacturers, including Lonza, are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical studies must be manufactured in accordance with current international Good Manufacturing Practice and other international regulatory requirements. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of our product candidates. We, our collaborators, or suppliers must supply all necessary documentation in support of a BLA on a timely basis and must adhere to current Good Laboratory Practice and current Good Manufacturing Practice regulations enforced by the FDA and other regulatory agencies through their facilities inspection program. Lonza and other suppliers have never produced a commercially approved cellular therapeutic product and therefore have not yet obtained the requisite regulatory authority approvals to do so.
Before we can begin commercial manufacture of our products for sale in the United States, we must obtain FDA regulatory approval for the product, in addition to the approval of the processes and quality systems associated with the manufacturing of such product, which requires a successful FDA inspection of the facility handling the manufacturing of our product, including Lonza’s manufacturing facilities. The novel nature of our product candidates creates significant challenges in regards to manufacturing. For example, the U.S. federal and state governments and other jurisdictions impose restrictions on the acquisition and use of tissue, including those incorporated in federal Good Tissue Practice regulations. We may not be able to identify or develop sources for the cells necessary for our product candidates that comply with these laws and regulations.
In addition, the regulatory authorities may, at any time before or after product approval, audit or inspect a manufacturing facility involved with the preparation of our product candidates or raw materials or the associated quality systems for compliance with the regulations applicable to the activities being conducted. Earlier this year, FDA conducted a pre-license inspection of the manufacturing process of remestemcel-L which did not result in the issuance of a Form 483 and there were no observed concerns. Although we oversee each contract manufacturer involved in the production of our product candidates, we cannot control the manufacturing process of, and are dependent on, the contract manufacturer for compliance with the regulatory requirements. If the contract manufacturer is unable to comply with manufacturing regulations, we may be subject to fines, unanticipated compliance expenses, recall or seizure of any approved products, total or partial suspension of production and/or enforcement actions, including injunctions, and criminal or civil prosecution. These possible sanctions would adversely affect our business, results of operations and financial condition. If the manufacturer fails to maintain regulatory compliance, the FDA or other applicable regulatory authority can impose regulatory sanctions including, among other things, refusal to approve a pending application for a new drug product or biologic product, withdrawal of an approval, or suspension of production. As a result, our business, financial condition, and results of operations may be materially harmed.
We will rely on third parties to perform many necessary services for the commercialization of our product candidates, including services related to the distribution, storage and transportation of our products.
We will rely upon third parties for certain storage, distribution and other logistical services. In accordance with certain laws, regulations and specifications, our product candidates must be stored and transported at extremely low temperatures within a certain range. If these environmental conditions deviate, our product candidates’ remaining shelf-lives could be impaired or their efficacy and safety could become adversely affected, making them no longer suitable for use. If any of the third parties that we intend to rely upon in our storage, distribution and other logistical services process fail to comply with applicable laws and regulations, fail to meet expected deadlines, or otherwise do not carry out their contractual duties to us, or encounter physical damage or natural disaster at their facilities, our ability to deliver product to meet commercial demand may be significantly impaired. In addition, as our cellular therapies will constitute a new form of product, experience in commercial distribution of such therapies in the United States is extremely limited, and as such is subject to execution risk. While we intend to work closely with our selected distribution logistics providers to define appropriate parameters for their activities to ensure product remains intact throughout the process, there is no assurance that
23
such logistics providers will be able to maintain all requirements and handle and distribute our products in a manner that does not significantly impair them, which may impact our ability to satisfy commercial demand. Likewise, the after effects from the COVID-19 pandemic, geopolitical and economic instability, and climate events may adversely impact access to raw materials and distribution, storage and transportation of our products, and the cost of those activities.
Product recalls or inventory losses caused by unforeseen events may adversely affect our operating results and financial condition.
Our product candidates are manufactured, stored and distributed using technically complex processes requiring specialized facilities, highly specific raw materials and other production constraints. The complexity of these processes, as well as strict company and government standards for the manufacture, storage and distribution of our product candidates, subjects us to risks. For example, during the manufacturing process we have from time to time experienced several different types of issues that have led to a rejection of various batches. Historically, the most common reasons for batch rejections include major process deviations during the production of a specific batch and failure of manufactured product to meet one or more specifications. While product candidate batches released for the use in clinical trials or for commercialization undergo sample testing, some latent defects may only be identified following product release. In addition, process deviations or unanticipated effects of approved process changes may result in these product candidates not complying with stability requirements or specifications. The occurrence or suspected occurrence of production and distribution difficulties can lead to lost inventories, and in some cases product recalls, with consequential reputational damage and the risk of product liability. The investigation and remediation of any identified problems can cause production delays, substantial expense, lost sales and delays of new product launches. In the event our production efforts require a recall or result in an inventory loss, our operating results and financial condition may be adversely affected.
Risks Related to Commercialization of Our Product Candidates
Our future commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among physicians, patients and healthcare payors.
Even when product development is successful and regulatory approval has been obtained, our ability to generate significant revenue depends on the acceptance of our products by physicians, payors and patients. Many potential market participants have limited knowledge of, or experience with, cell therapy-based products, so gaining market acceptance and overcoming any safety or efficacy concerns may be more challenging than for more traditional therapies. Our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful. Such efforts to educate the marketplace may require more or different resources than are required by the conventional therapies marketed by our competitors. We cannot assure you that our products will achieve the expected market acceptance and revenue if and when they obtain the requisite regulatory approvals. Alternatively, even if we obtain regulatory approval, that approval may be for indications or patient populations that are not as broad as intended or desired or may require labeling that includes significant use or distribution restrictions or safety warnings. The market acceptance of each of our product candidates will depend on a number of factors, including:
•the efficacy and safety of the product candidate, as demonstrated in clinical trials;
•the clinical indications for which the product is approved, and the label approved by regulatory authorities for use with the product, including any warnings or contraindications that may be required on the label;
•acceptance by physicians, patients, and with pediatric indications by parents/caregivers of the product as a safe and effective treatment;
•the cost, safety and efficacy of treatment in relation to alternative treatments;
•the continued projected growth of markets for our various indications;
•relative convenience and ease of administration;
•the prevalence and severity of adverse side effects;
•the effectiveness of our, and our collaborators’ sales and marketing efforts; and
•sufficient third-party insurance and other payor (e.g., governmental) coverage and reimbursement.
Market acceptance is critical to our ability to generate significant revenue. Any product candidate, if approved and commercialized, may be accepted in only limited capacities or not at all. If any approved products are not accepted by the market to the extent that we expect, we may not be able to generate significant revenue and our business would suffer.
24
If, in the future, we are unable to establish our own commercial capabilities across sales, marketing and distribution, or enter into licensing or collaboration agreements for these purposes, we may not be successful in independently commercializing any future products.
We have limited sales, marketing or distribution infrastructure and experience. Commercializing our product candidates, if such product candidates obtain regulatory approval, would require significant sales, distribution and marketing capabilities. Where and when appropriate, we may elect to utilize contract sales forces or distribution collaborators to assist in the commercialization of our product candidates. If we enter into arrangements with third parties to perform sales, marketing and distribution/price reporting services for our product candidates, the resulting revenue or the profitability from this revenue to us may be lower than if we had sold, marketed and distributed that product ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute any future products or may be unable to do so on terms that are favorable to us. We may have little control over such third parties, and any of these third parties may fail to devote the necessary resources and attention to sell, market and distribute our current or any future products effectively.
To the extent we are unable to engage third parties to assist us with these functions, we will have to invest significant amounts of financial and management resources, some of which will need to be committed prior to any confirmation that any of our proprietary product candidates will be approved. For any future products for which we decide to perform sales, marketing and distribution functions ourselves, we could face a number of additional risks, including:
•our inability to recruit and retain adequate numbers of effective sales and marketing personnel or to develop alternative sales channels;
•the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future products;
•the inability of account teams to obtain formulary acceptance for our products, allowing for reimbursement and hence patient access;
•the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with multiple products; and
•unforeseen costs and expenses associated with creating and maintaining an independent sales and marketing organization.
We face substantial competition, which may result in others discovering, developing or commercializing products before, or more successfully, than we do.
The biopharmaceutical industry is highly competitive and subject to rapid change. The industry continues to expand and evolve as an increasing number of competitors and potential competitors enter the market. Many of our potential competitors have significantly greater development, financial, manufacturing, marketing, technical and human resources than we do. Large pharmaceutical companies, in particular, have extensive experience in conducting clinical trials, obtaining regulatory approvals, manufacturing pharmaceutical and biologic products and commercializing such therapies. Recent and potential future merger and acquisition activity in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel compounds that could make our product candidates obsolete. As a result of all of these factors, our competitors may succeed in obtaining patent protection and/or FDA approval or discovering, developing and commercializing our product candidates or competitors to our product candidates before we do. Specialized, smaller or early-stage companies may also prove to be significant competitors, particularly those with a focus and expertise in cell therapies. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. If we are not able to compete effectively against potential competitors, our business will not grow and our financial condition and results of operations will suffer.
Our marketed products may be used by physicians for indications that are not approved by the FDA. If the FDA finds that we marketed our products in a manner that promoted off-label use, we may be subject to civil or criminal penalties.
Under the Federal Food, Drug and Cosmetic Act (“FDCA”), and other laws and regulations, if any of our product candidates are approved by the FDA, we would be prohibited from promoting our products for off-label uses. This means, for example, that we would not be able to make claims about the use of our marketed products outside of their approved indications, and we would not be able to proactively discuss or provide information on off-label uses of such products, with
25
very specific and limited exceptions. The FDA does not, however, prohibit physicians from prescribing products for off-label uses in the practice of medicine. Should the FDA determine that our activities constituted the promotion of off-label use, the FDA could issue a warning or untitled letter or, through the Department of Justice, bring an action for seizure or injunction, and could seek to impose fines and penalties on us and our executives. In addition, failure to follow FDA rules and guidelines relating to promotion and advertising can result in, among other things, the FDA’s refusal to approve a product, the suspension or withdrawal of an approved product from the market, product recalls, fines, disgorgement of money, operating restrictions, injunctions or criminal prosecutions, and also may figure into civil litigation against us.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Affordable Care Act, was passed. The Affordable Care Act is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and the health insurance industry, impose new taxes and fees on the healthcare industry and impose additional health policy reforms. There have been a number of judicial and congressional challenges to certain aspects of the Affordable Care Act. We can provide no assurance that laws such as the Affordable Care Act, as currently enacted or as amended in the future, will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
Currently, the outcome of potential reforms and changes to government negotiation/regulation to healthcare costs are unknown. If changes in policy limit reimbursements that we are able to receive through federal programs, it could negatively impact reimbursement levels from those payors and private payors, and our business, revenues or profitability could be adversely affected.
If we or our collaborators fail to obtain and sustain an adequate level of reimbursement for our products by third-party payors, sales and profitability would be adversely affected.
Our and our collaborators’ ability to commercialize any products successfully will depend, in part, on the extent to which coverage and reimbursement for our products and related treatments will be available from government healthcare programs, private health insurers, managed care plans, and other organizations. Additionally, even if there is a commercially viable market, if the level of third-party reimbursement is below our expectations, our revenue and profitability could be materially and adversely affected.
Third-party payors, such as government programs, including Medicare or Medicaid in the United States, or private healthcare insurers, carefully review and increasingly question the coverage of, and challenge the prices charged for medical products and services, and many third-party payors limit or delay coverage of or reimbursement for newly approved healthcare products. Reimbursement rates from private health insurance companies vary depending on the company, the insurance plan and other factors, including the third-party payor’s determination that use of a product is:
•a covered benefit under its health plan;
•safe, effective and medically necessary;
•appropriate for the specific patient;
•cost-effective; and
•neither experimental nor investigational.
A current trend in the U.S. healthcare industry as well as in other countries around the world is toward cost containment. Large public and private payors, managed care organizations, group purchasing organizations and similar organizations are exerting increasing influence on decisions regarding the use of, and reimbursement levels for, particular treatments. In particular, third-party payors may limit the covered indications. Cost-control initiatives could decrease the price we might establish for any product, which could result in product revenue and profitability being lower than anticipated.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or other regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that a drug will be paid for in all cases or at a rate that
26
covers our costs, including research, development, manufacture, sale and distribution expenses. Interim reimbursement levels for new drugs, if applicable, may also be insufficient to cover our and any collaborator’s costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments and treatment codes for other services. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Furthermore, reimbursement systems in international markets vary significantly by country and by region, and reimbursement approvals must be obtained on a country-by-country basis. Our existing or future collaborators, if any, may elect to reduce the price of our products in order to increase the likelihood of obtaining reimbursement approvals which could adversely affect our revenues and profits. In many countries, including for example in Japan, products cannot be commercially launched until reimbursement is approved. Further, the post-approval price negotiation process in some countries can exceed 12 months. In addition, pricing and reimbursement decisions in certain countries can be affected by decisions taken in other countries, which can lead to mandatory price reductions and/or additional reimbursement restrictions across a number of other countries, which may thereby adversely affect our sales and profitability. In the event that countries impose prices which are not sufficient to allow us or our collaborators to generate a profit, our collaborators may refuse to launch the product in such countries or withdraw the product from the market, which would adversely affect sales and profitability.
Due to the novel nature of our cell therapy and the potential for our product candidates to offer therapeutic benefit in a single administration, we face uncertainty related to pricing and reimbursement for these product candidates.
Our target patient populations for some of our product candidates may be relatively small, and as a result, the pricing and reimbursement of our product candidates, if approved, must be adequate to support commercial infrastructure. If we are unable to obtain adequate levels of reimbursement, our ability to successfully market and sell our product candidates will be adversely affected. Due to the novel nature of our cell therapy technology, the manner and level at which reimbursement is provided for services related to our product candidates (e.g., for administration of our product to patients) is uncertain. Inadequate reimbursement for such services may lead to physician resistance and adversely affect our ability to market or sell our products. Further, if the results of our clinical trials and related cost benefit analyses do not clearly demonstrate the efficacy or overall value of our product candidates in a manner that is meaningful to prescribers and payors, our pricing and reimbursement may be adversely affected.
Price controls may be imposed in foreign markets, which may adversely affect our future profitability.
In some countries, particularly EU member states, Japan, Australia and Canada, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we or our collaborators may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business, revenues or profitability could be adversely affected.
If the market opportunities for our product candidates are smaller than we believe they are, our revenues may be adversely affected and our business may suffer. Because the target patient populations of certain of our product candidates are small, we must be able to successfully identify physicians with access to appropriate patients and achieve a significant market share to maintain profitability and growth.
Our projections of the number of people with diseases targeted by our product candidates are based on estimates. These estimates may prove to be incorrect and new studies may change the estimated incidence or prevalence of these diseases. In addition, physicians who we believe have access to patients in need of our products may in fact not often treat the diseases targeted by our product candidates, and may not be amenable to use of our product. Further, the number of
27
patients in the United States, Europe and elsewhere may turn out to be lower than expected, may not be otherwise amenable to treatment with our products, or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business.
We are exposed to risks related to our licensees and our international operations, and failure to manage these risks may adversely affect our operating results and financial condition.
We and our subsidiaries operate out of Australia, the United States, Singapore, the United Kingdom and Switzerland. We have licensees, with rights to commercialize products based on our MSC technology, including JCR in Japan. Our primary manufacturing collaborator, Lonza, serves us primarily out of their facilities in Singapore, and through contractual relationships with third parties, has access to storage facilities in the U.S., Europe, Australia and Singapore. As a result, a significant portion of our operations are conducted by and/or rely on entities outside the markets in which certain of our trials take place, our suppliers are sourced, our product candidates are developed, and, if any such product candidates obtain regulatory approval, our products may be sold. Accordingly, we import a substantial number of products and/or materials into such markets. We may be denied access to our customers, suppliers or other collaborators or denied the ability to ship products from any of these sites as a result of a closing of the borders of the countries in which we operate, or in which these operations are located, due to economic, legislative, political, health or military conditions in such countries. If any of our product candidates are approved for commercialization, we may enter into agreements with third parties to market them on a worldwide basis or in more limited geographical regions. We expect that we will be subject to additional risks related to entering into international business relationships, including:
•unexpected changes in tariffs, trade barriers and regulatory requirements;
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•logistics and regulations associated with shipping cell samples and other perishable items, including infrastructure conditions and transportation delays;
•potential import and export issues and other trade barriers and restrictions with the U.S. Customs and Border Protection and similar bodies in other jurisdictions;
•compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•workforce uncertainty in countries where labor unrest is more common than in the United States;
•reduced protection for intellectual property rights in some countries and practical difficulties of enforcing intellectual property and contract rights abroad;
•changes in diplomatic and trade relationships, including new tariffs, trade protection measures, import or export licensing requirements, trade embargoes and other trade barriers;
•tariffs imposed by the U.S. on goods from other countries, including the recently implemented tariffs and additional tariff that have been proposed by the U.S. government on various imports from China and the EU and by the governments of these jurisdictions on certain U.S. goods, and any other possible tariffs that may be imposed on products such as ours, the scope and duration of which, if implemented, remains uncertain;
•deterioration of political relations, for example between Russia and other nations, and between the U.K. and members of the EU, which could have a material adverse effect on our sales and operations in these countries;
•changes in social, political and economic conditions or in laws, regulations and policies governing foreign trade, manufacturing, development and investment both domestically as well as in the other countries and jurisdictions into which we sell our products;
•fluctuations in currency exchange rates and the related effect on our results of operations;
•increased financial accounting and reporting burdens and complexities;
•potential increases on tariffs or restrictions on trade generally;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
•business interruptions resulting from geopolitical actions, including war (such as Russia’s invasion of Ukraine) and terrorism, or natural disasters including earthquakes, typhoons, floods and fires.
28
Use of animal-derived materials could harm our product development and commercialization efforts.
Some of the manufacturing materials and/or components that we use in, and which are critical to, implementation of our technology involve the use of animal-derived products, including FBS. Suppliers or regulatory changes may limit or restrict the availability of such materials for clinical and commercial use. While FBS is commonly used in the production of various marketed biopharmaceuticals, the suppliers of FBS that meet our strict quality standards are limited in number and region. As such, to the extent that any such suppliers or regions face an interruption in supply (for example, if there is a new occurrence of so-called “mad cow disease”), it may lead to a restricted supply of the serum currently required for our product manufacturing processes. Any restrictions on these materials would impose a potential competitive disadvantage for our products or prevent our ability to manufacture our cell products. The FDA has issued regulations for controls over bovine material in animal feed. These regulations do not appear to affect our ability to purchase the manufacturing materials we currently use. However, the FDA may propose new regulations that could affect our operations. Our inability to develop or obtain alternative compounds would harm our product development and commercialization efforts. There are certain limitations in the supply of certain animal-derived materials, which may lead to delays in our ability to complete clinical trials or eventually to meet the anticipated market demand for our cell products.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the human clinical use of our product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product design, testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection and other acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
•decreased demand for our products, even if such products are approved;
•injury to our reputation;
•withdrawal of clinical trial participants;
•costs to defend the related litigations;
•a diversion of management’s time and our resources;
•substantial monetary awards to trial participants or patients;
•product recalls, withdrawals, or labeling, marketing or promotional restrictions;
•increased cost of liability insurance;
•loss of revenue;
•the inability to commercialize our product candidates; and
•a decline in our ordinary share price.
Failure to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. Additionally, our insurance policies have various exclusions, and we may be subject to a product liability claim for which we have no coverage or reduced coverage. Any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Risks Related to Our Intellectual Property
We may not be able to protect our proprietary technology in the marketplace.
Our success will depend, in part, on our ability to obtain patents, protect our trade secrets and operate without infringing on the proprietary rights of others. We rely upon a combination of patents, trade secret protection, and
29
confidentiality agreements to protect the intellectual property of our product candidates. Patents might not be issued or granted with respect to our patent applications that are currently pending, and issued or granted patents might later be found to be invalid or unenforceable, be interpreted in a manner that does not adequately protect our current product or any future products, or fail to otherwise provide us with any competitive advantage. As such, we do not know the degree of future protection that we will have on our proprietary products and technology, if any, and a failure to obtain adequate intellectual property protection with respect to our product candidates and proprietary technology could have a material adverse impact on our business.
Filing, prosecuting and defending patents throughout the world would be prohibitively expensive, so our policy is to patent technology in jurisdictions with significant or otherwise relevant commercial opportunities or activities. However, patent protection may not be available for some of the products or technology we are developing. If we must spend significant time and money protecting or enforcing our patents, designing around patents held by others or licensing, potentially for large fees, patents or other proprietary rights held by others, our business, results of operations and financial condition may be harmed.
The patent positions of biopharmaceutical products are complex and uncertain.
The scope and extent of patent protection for our product candidates are particularly uncertain. To date, our principal product candidates have been based on specific subpopulations of known and naturally occurring adult stem cells. We anticipate that the products we develop in the future will continue to include or be based on the same or other naturally occurring stem cells or derivatives or products thereof. Although we have sought and expect to continue to seek patent protection for our product candidates, their methods of use and methods of manufacture, any or all of them may not be subject to effective patent protection. Publication of information related to our product candidates by us or others may prevent us from obtaining or enforcing patents relating to these products and product candidates. Furthermore, others may independently develop similar products, may duplicate our products, or may design around our patent rights. In addition, any of our issued patents may be declared invalid. If we fail to adequately protect our intellectual property, we may face competition from companies who attempt to create a generic product to compete with our product candidates. We may also face competition from companies who develop a substantially similar product to our other product candidates that may not be covered by any of our patents.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the U.S. can be less extensive than those in the U.S. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S., or from selling or importing products made using our inventions in and into the U.S. or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the U.S. These products may compete with our current or future products, if any, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We maintain certain of our proprietary know-how and technological advances as trade secrets, especially where we do not believe patent protection is appropriate or obtainable, including, but not exclusively, with respect to certain aspects of the manufacturing of our products. However, trade secrets are difficult to protect. We take a number of measures to protect our trade secrets including, limiting disclosure, physical security and confidentiality and non-disclosure agreements.
30
We enter into confidentiality agreements with our employees, consultants, outside scientific collaborators, contract manufacturing partners, sponsored researchers and other advisors and third parties to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights. Failure to obtain or maintain trade secret protection, or failure to adequately protect our intellectual property could enable competitors to develop generic products or use our proprietary information to develop other products that compete with our products or cause additional, material adverse effects upon our business, results of operations and financial condition.
We may be forced to litigate to enforce or defend our intellectual property rights, and/or the intellectual property rights of our licensors.
We may be forced to litigate to enforce or defend our intellectual property rights against infringement by competitors, and to protect our trade secrets against unauthorized use. In so doing, we may place our intellectual property at risk of being invalidated, unenforceable, or limited or narrowed in scope and may no longer be used to prevent the manufacture and sale of competitive product. Further, an adverse result in any litigation or other proceedings before government agencies such as the United States Patent and Trademark Office (“USPTO”), may place pending applications at risk of non-issuance. Further, interference proceedings, derivation proceedings, entitlement proceedings, ex parte reexamination, inter partes reexamination, inter partes review, post-grant review, and opposition proceedings provoked by third parties or brought by the USPTO or any foreign patent authority may be used to challenge inventorship, ownership, claim scope, or validity of our patent applications. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential and proprietary information could be compromised by disclosure during this type of litigation.
Intellectual property disputes could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and/or management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our ADSs and ordinary shares. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of litigation proceedings more effectively than we can because of their greater financial resources and personnel. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to conduct our clinical trials, continue our internal research programs, in-license needed technology or enter into strategic collaborations that would help us bring our product candidates to market. As a result, uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
U.S. patent reform legislation and court decisions could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued U.S. patents.
Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Under the current patent laws, a third party that files a patent application in the USPTO before us for a particular invention could therefore be awarded a patent covering such invention even if we had made that invention before it was made by such third party. This requires us to be cognizant of the time from invention to filing of a patent application.
The current US legislation allows third party submissions of prior art to the USPTO during patent prosecution and additional procedures for attacking the validity of a patent through USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because a lower evidentiary standard applies in USPTO proceedings compared to the evidentiary standards applied in United States federal courts in actions seeking to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if challenged in a district court action. Accordingly, a third party may attempt to use available USPTO procedures to invalidate our patent claims that
31
would not otherwise have been invalidated if first challenged by the third party in a district court action. These post-grant review (PGR) proceedings, which are similar to European “opposition” proceedings and provide third-party petitioners with the ability to challenge the validity of a patent on more expansive grounds than those permitted in other USTPO proceedings, allow for validity to be examined by the USPTO based not only on prior art patents and publications, but also on prior invalidating public use and sales, the presence of non-statutory subject matter in the patent claims and inadequate written description or lack of enablement. Discovery for PGR proceedings is accordingly likely to be expansive given that the issues addressed in PGR are more comprehensive than those addressed in other USPTO proceedings.
As compared to intellectual property-reliant companies generally, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. These rulings have created uncertainty with respect to the validity and enforceability of patents, even once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
If third parties claim that intellectual property used by us infringes upon their intellectual property, commercialization of our product candidates and our operating profits could be adversely affected.
There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biopharmaceutical industry. We may, from time to time, be notified of claims that we are infringing upon patents, trademarks, copyrights, or other intellectual property rights owned by third parties, and we cannot provide assurances that other companies will not, in the future, pursue such infringement claims against us or any third-party proprietary technologies we have licensed. Any such claims could also be expensive and time consuming to defend and divert management’s attention and resources, and could delay or prevent us from commercializing our product candidates. Our competitive position could suffer as a result. Although we have reviewed certain third-party patents and patent filings that we believe may be relevant to our product candidates, we have not conducted a freedom-to-operate search or analysis for our product candidates, and we may not be aware of patents or pending or future patent applications that, if issued, would block us from commercializing our product candidates. Thus, we cannot guarantee that our product candidates, or our commercialization thereof, do not and will not infringe any third party’s intellectual property.
If we do not obtain patent term extension in the United States under the Hatch-Waxman Act and in foreign countries under similar legislation, thereby potentially extending the term of our marketing exclusivity of our product candidates, our business may be materially harmed.
Depending on the timing, duration and specifics of FDA marketing approval of our product candidates, if any, one of the U.S. patents covering each of such approved product(s) or the use thereof may be eligible for up to five years of patent term restoration under the Hatch-Waxman Act. The Hatch-Waxman Act allows a maximum of one patent to be extended per FDA approved product. Patent term extension also may be available in certain foreign countries upon regulatory approval of our product candidates, including by the EMA in the EU or the PMDA in Japan. Nevertheless, we may not be granted patent term extension either in the United States or in any foreign country because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the term of extension, as well as the scope of patent protection during any such extension, afforded by the governmental authority could be less than we request. In addition, if a patent we wish to extend is owned by another party and licensed to us, we may need to obtain approval and cooperation from our licensor to request the extension.
If we are unable to obtain patent term extension or restoration, or the term of any such extension is less than we request, the period before we might face generic or follow-on competition could be shortened and we may not be able to stop our competitors from launching competing products following our patent expiration, and our revenue could be reduced, possibly materially.
32
Risks Related to Our Business and Industry
If we fail to attract and keep senior management and key scientific, commercial, regulatory affairs and other personnel, we may be unable to successfully develop our product candidates, conduct our clinical trials and commercialize our product candidates.
We are highly dependent on members of our executive management, particularly Dr. Silviu Itescu, our Chief Executive Officer. Dr. Itescu was an early pioneer in the study and clinical development of cell therapeutics and is globally recognized in the field of regenerative medicine. The loss of the services of Dr. Itescu or any other member of the executive management team could impede the achievement of our research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing, regulatory affairs, sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions.
Our employees, principal investigators, consultants and collaboration partners may engage in misconduct or other improper activities, including noncompliance with laws and regulatory standards and requirements and insider trading.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include failures to comply with FDA regulations, to provide accurate information to the FDA, to comply with manufacturing standards we have established, to comply with federal and state healthcare fraud and abuse laws and regulations, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements (including arrangements with healthcare providers, opinion leaders, research institutions, distributors and payors) in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of activity relating to pricing, discounting, marketing and promotion, sales commissions, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation, or, given we are a listed company in Australia and the United States, breach of insider trading or other securities laws and regulations. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
We may acquire other companies or assets which could divert our management’s attention, result in additional dilution to our shareholders and otherwise disrupt our operations and harm our operating results.
We have in the past and may in the future seek to acquire businesses, products or technologies that we believe could complement or expand our product offerings, enhance our technical capabilities or otherwise offer growth opportunities. For example, we acquired MSC assets from Osiris Therapeutics, Inc. in 2013. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated. If we acquire additional businesses, we may not be able to integrate the acquired personnel, operations and technologies successfully, or effectively manage the combined business following the acquisition. We also may not achieve the anticipated benefits from the acquired business due to a number of factors, including:
•incurrence of acquisition-related costs;
•diversion of management’s attention from other business concerns;
•unanticipated costs or liabilities associated with the acquisition;
•harm to our existing business relationships with collaborators as a result of the acquisition;
•harm to our brand and reputation;
•the potential loss of key employees;
•use of resources that are needed in other parts of our business; and
33
•use of substantial portions of our available cash to consummate the acquisition.
In the future, if our acquisitions do not yield expected returns, we may be required to take charges to our operating results arising from the impairment assessment process. Acquisitions may also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our operating results. In addition, if an acquired business fails to meet our expectations, our business, results of operations and financial condition may be adversely affected.
We and our collaborators must comply with environmental laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We and our collaborators are subject to various federal, state and local environmental laws, rules and regulations, including those relating to the discharge of materials into the air, water and ground, the manufacture, storage, handling, use, transportation and disposal of hazardous and biological materials, and the health and safety of employees with respect to laboratory activities required for the development of products and technologies. In the event of contamination or injury, or failure to comply with environmental, occupational health and safety and export control laws and regulations, it could cause an interruption of our commercialization efforts, research and development efforts, or business operations, and we could be held liable for any resulting damages and any such liability could exceed our assets and resources.
We work with outside scientists and their institutions in developing product candidates. These scientists may have other commitments or conflicts of interest, which could limit our access to their expertise and harm our ability to leverage our discovery platform.
We work with scientific advisors and collaborators at academic research institutions in connection with our product development. These scientific advisors serve as our link to the specific pools of trial participants we are targeting in that these advisors may:
•identify individuals as potential candidates for study;
•obtain their consent to participate in our research;
•perform medical examinations and gather medical histories;
•conduct the initial analysis of suitability of the individuals to participate in our research based on the foregoing; and
•collect data and biological samples from trial participants periodically in accordance with our study protocols.
These scientists and collaborators are not our employees, rather they serve as either independent contractors or the primary investigators under research collaboration agreements that we have with their sponsoring academic or research institution. Such scientists and collaborators may have other commitments that would limit their availability to us. Although our scientific advisors generally agree not to do competing work, if an actual or potential conflict of interest between their work for us and their work for another entity arises, we may lose their services. It is also possible that some of our valuable proprietary knowledge may become publicly known through these scientific advisors if they breach their confidentiality agreements with us, which would cause competitive harm to our business.
If our ability to use cumulative carry forward net operating losses is or becomes subject to certain limitations or if certain tax incentive credits from which we may benefit expire or no longer apply to us, our business, results of operations and financial condition may be adversely affected.
We are an Australian company subject to taxation in Australia and other jurisdictions. As of June 30, 2023, our cumulative operating losses have a total potential tax benefit of $201.7 million at local tax rates (excluding other temporary differences). These losses may be available for use once we are in a tax profitable position. These losses were incurred in different jurisdictions and can only be offset against profits earned in the relevant jurisdictions. Tax losses are able to be carried forward at their nominal amount indefinitely in Australia and in Singapore, and for up to 20 years in the U.S. as long as certain conditions are met; however, new tax reform legislation in the United States allows for indefinite carryforward of any net operating loss arising in a tax year ending after December 31, 2018, subject to certain conditions. In order to use these tax losses, it is necessary to satisfy certain tests and, as a result, we cannot assure you that the tax losses will be available to offset profits if and when we earn them. Utilization of our net operating loss and research and development credit carryforwards in the U.S. may be subject to substantial annual limitation due to ownership change limitations that could occur in the future generally provided by Section 382 of the Internal Revenue Code of 1986, as amended. In addition, U.S. tax reform introduced a limitation on the amount of net operating losses arising in taxable years
34
beginning after December 31, 2017, that a corporation may deduct in a single tax year equal to the lesser of the available net operating loss carryover or 80 percent of a taxpayer’s pre-net operating loss deduction taxable income. With respect to carryforward net operating losses in the U.S. that are subject to the 20-year carry-forward limit, our carry forward net operating losses first start to expire in 2032.
In addition, we may be eligible for certain research and development tax incentive refundable credits in Australia that may increase our available cash flow. The Australian federal government's Research and Development Tax Incentive grant is available for eligible research and development purposes based on the filing of an annual application. The Australian government may in the future decide to modify the requirements of, reduce the amounts of the research and development tax incentive credits available under, or discontinue its research and development tax incentive program. For instance, the Australian government undertook a review of its Research and Development Tax Incentive program in the May 2020 Federal budget and in October 2020 introduced new legislation for the refundable tax offset applicable to eligible companies for income tax years commencing from July 1, 2021. One of the legislation changes made was to allow a tax offset for companies with an aggregated turnover of A$20.0 million or more. For companies with an aggregated turnover of A$20.0 million or more, the rate of the tax offset is the company’s corporate tax rate plus a rate between 8.5% and 16.5% depending on the proportion of research and development expenditures in relation to total expenditures. For companies with an aggregated turnover below A$20.0 million, the rate of the refundable research and development tax offset was set as at 18.5% above the company’s tax rate. If the Research and Development Tax program incentives are revoked or modified, or if we are no longer eligible for such incentives due to other circumstances, our business, results of operations and financial condition may be adversely affected.
For the year ended June 30, 2023, we recognized research and development tax incentive income of $3.5 million from the Research and Development Tax Incentive program relating to a claim for eligible expenditure under the incentive scheme for the years ended June 30, 2023, 2022 and 2021. During the year ended June 30, 2023, management concluded its assessment of qualifying activities and we recognized the relevant income for the years ended June 30, 2023, 2022 and 2021. No income was recognized in the years ended June 30, 2022 and 2021 as management were yet to confirm if our research and development activities were eligible under the incentive scheme and therefore had not applied for a tax offset. There can be no assurances that we will benefit from these incentives in the future if our activities are not eligible under the incentive scheme or that the tax incentive credit programs will not be revoked or modified in any way in the future.
Taxing authorities could reallocate our taxable income within our subsidiaries, which could increase our consolidated tax liability.
We conduct operations in multiple tax jurisdictions and the tax laws of those jurisdictions generally require that the transfer pricing between affiliated companies in different jurisdictions be the same as those between unrelated companies dealing at arms’ length, and that such prices are supported by contemporaneous documentation. While we believe that we operate in compliance with applicable transfer pricing laws and intend to continue to do so, our transfer pricing procedures are not binding on applicable tax authorities. If tax authorities in any of these countries were to successfully challenge our transfer pricing as not reflecting arms’ length transactions, they could require us to adjust our transfer pricing and thereby reallocate our income to reflect these revised transfer pricing, which could result in a higher tax liability to us, and possibly interest and penalties, and could adversely affect our business, results of operations and financial condition.
The pharmaceutical industry is highly regulated and pharmaceutical companies are subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act.
Healthcare fraud and abuse regulations are complex and can be subject to varying interpretations as to whether or not a statute has been violated. The laws that may affect our ability to operate include:
•the federal Anti-Kickback Statute which prohibits, among other things, the knowing and willful payment of remuneration to induce or reward patient referrals, prescribing or recommendation of products, or the generation of business involving any item or service which may be payable by the federal health care programs (e.g., drugs, supplies, or health care services for Medicare or Medicaid patients);
•the federal False Claims Act which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment for government funds (e.g., payment from Medicare or Medicaid) or knowingly making, using, or causing to be made or used a false record or statement, material to a false or fraudulent claim for government funds;
•the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act, and its implementing regulations,
35
imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HIPAA imposes civil and criminal liability for the wrongful access or disclosure of protected health information;
•the federal Physician Payments Sunshine Act, created under Section 6002 of the Patient Protection and Affordable Care Act (“ACA”), as amended, requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, those physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members;
•the FDCA, which, among other things, regulates the testing, development, approval, manufacture, promotion and distribution of drugs, devices and biologics. The FDCA prohibits manufacturers from selling or distributing “adulterated” or “misbranded” products. A drug product may be deemed misbranded if, among other things, (i) the product labeling is false or misleading, fails to contain requisite information or does not bear adequate directions for use; (ii) the product is manufactured at an unregistered facility; or (iii) the product lacks the requisite FDA clearance or approval;
•the U.S. Foreign Corrupt Practices Act (“FCPA”), which prohibits corrupt payments, gifts or transfers of value to non-U.S. officials; and
•non-U.S. and U.S. state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
Any failure to comply with these laws, or the regulations adopted thereunder, could result in administrative, civil, and/or criminal penalties, and could result in a material adverse effect on our reputation, business, results of operations and financial condition.
The federal fraud and abuse laws have been interpreted to apply to arrangements between pharmaceutical manufacturers and a variety of health care professionals and healthcare organizations. Although the federal Anti-Kickback Statute has several statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, all elements of the potentially applicable exemption or safe harbor must be met in order for the arrangement to be protected, and prosecutors have interpreted the federal healthcare fraud statutes to attack a wide range of conduct by pharmaceutical companies. In addition, most states have statutes or regulations similar to the federal anti-kickback and federal false claims laws, which apply to items and services covered by Medicaid and other state programs, or, in several states, apply regardless of the payor. Administrative, civil and criminal sanctions may be imposed under these federal and state laws.
Further, the ACA, among other things, amended the intent standard under the Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA makes clear that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim under the federal False Claims Act. Any violations of these laws, or any action against us for violation of these laws, even if we successfully defend against it, could result in a material adverse effect on our reputation, business, results of operations and financial condition.
A failure to adequately protect private health information could result in severe harm to our reputation and subject us to significant liabilities, each of which could have a material adverse effect on our business.
Throughout the clinical trial process, we may obtain the private health information of our trial subjects. There are a number of state, federal and international laws protecting the privacy and security of health information and personal data. As part of the American Recovery and Reinvestment Act 2009 (“ARRA”), Congress amended the privacy and security provisions of HIPAA. HIPAA imposes limitations on the use and disclosure of an individual’s healthcare information by healthcare providers conducting certain electronic transactions, healthcare clearinghouses, and health insurance plans, collectively referred to as covered entities. The HIPAA amendments also impose compliance obligations and corresponding penalties for non-compliance on certain individuals and entities that provide services to or perform certain functions on behalf of healthcare providers and other covered entities involving the use or disclosure of individually identifiable health information, collectively referred to as business associates. ARRA also made significant increases in the penalties for improper use or disclosure of an individual’s health information under HIPAA and extended enforcement
36
authority to state attorneys general. The amendments also create notification requirements to federal regulators, and in some cases local and national media, for individuals whose health information has been inappropriately accessed or disclosed. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with certain encryption or other standards developed by the U.S. Department of Health and Human Services, or HHS. Most states have laws requiring notification of affected individuals and state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms to ensure ongoing protection of personal information. Activities outside of the U.S. implicate local and national data protection standards, impose additional compliance requirements and generate additional risks of enforcement for non-compliance. The EU’s General Data Protection Regulation, Canada’s Personal Information Protection and Electronic Documents Act and other data protection, privacy and similar national, state/provincial and local laws and regulations may also restrict the access, use and disclosure of patient health information abroad. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws, to protect against security breaches and hackers or to alleviate problems caused by such breaches, and the failure to so comply may lead to fines or penalties.
Our operations are subject to anti-corruption laws, including Australian bribery laws, the United Kingdom Bribery Act, and the FCPA and other anti-corruption laws that apply in countries where we do business.
Anti-corruption laws generally prohibit us and our employees and intermediaries from bribing, being bribed or making other prohibited payments to government officials or other persons to obtain or retain business or gain some other business advantage. Although we believe that we have adequate policies and enforcement mechanisms to ensure legal and regulatory compliance with the FCPA, the U.K. Bribery Act 2010 and other similar regulations, we participate in collaborations and relationships with third parties, and it is possible that any of our employees, subcontractors, agents or partners may violate any such legal and regulatory requirements, which may expose us to criminal or civil enforcement actions, including penalties and suspension or disqualification from U.S. federal procurement contracting. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted.
There is no assurance that we will be completely effective in ensuring our compliance with all applicable anti-corruption laws or other laws including trade related laws. If we are not in compliance with these laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, and legal expenses, which could have an adverse impact on our business, financial condition, results of operations and liquidity. Likewise, any investigation of any potential violations of these laws by respective government bodies could also have an adverse impact on our reputation, our business, results of operations and financial condition.
We may lose our foreign private issuer status, which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur additional legal, accounting and other expenses.
In order to maintain our current status as a foreign private issuer, either (1) a majority of our ordinary shares must be either directly or indirectly owned of record by non-residents of the United States or (2) (a) a majority of our executive officers or directors must not be U.S. citizens or residents, (b) more than 50 percent of our assets cannot be located in the U.S. and (c) our business must be administered principally outside the U.S. If we lost this status, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC rules and Nasdaq listing standards. Further, we would be required to comply with U.S. GAAP, as opposed to IFRS, in the preparation and issuance of our financial statements for historical and current periods. The regulatory and compliance costs to us under U.S. securities laws if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be higher than the cost we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would increase our legal and financial compliance costs.
If we fail to maintain proper internal controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.
Section 404(a) of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) requires that our management assess and report annually on the effectiveness of our internal controls over financial reporting and identify any material weaknesses in our internal controls over financial reporting. In order to maintain and improve the effectiveness of our
37
disclosure controls and procedures and internal control over financial reporting, we have expended, and anticipate that we will continue to expend, significant resources, including accounting-related costs and significant management oversight.
If either we are unable to conclude that we have effective internal controls over financial reporting or our independent auditors are unwilling or unable to provide us with an unqualified report on the effectiveness of our internal controls over financial reporting as required by Section 404(b) of the Sarbanes-Oxley Act, investors may lose confidence in our operating results, the price of the ADSs could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, we may not be able to remain listed on Nasdaq Global Select Market (“Nasdaq”).
We have incurred and will continue to incur significant increased costs as a result of operating as a company whose ADSs are publicly traded in the United States, and our management will continue to be required to devote substantial time to compliance initiatives.
As a company whose ADSs are publicly traded in the United States, we have incurred and will continue to incur significant legal, accounting, insurance and other expenses. The Sarbanes-Oxley Act, Dodd-Frank Wall Street Reform and Consumer Protection Act and related rules implemented by the SEC and Nasdaq, have imposed various requirements on public companies including requiring establishment and maintenance of effective disclosure and financial controls. Our management and other personnel will need to continue to devote a substantial amount of time to these compliance initiatives, and we will need to add additional personnel and build our internal compliance infrastructure. Moreover, these rules and regulations have increased and will continue to increase our legal and financial compliance costs and will make some activities more time-consuming and costly. These laws and regulations could also make it more difficult and expensive for us to attract and retain qualified persons to serve on our board of directors, our board committees or as our senior management. Furthermore, if we are unable to satisfy our obligations as a public company, we could be subject to delisting of the ADSs, fines, sanctions and other regulatory action and potentially regulatory investigations and enforcement and/or civil litigation.
We have never declared or paid dividends on our ordinary shares, and we do not anticipate paying dividends in the foreseeable future. Therefore, you must rely on price-appreciation of our ordinary shares or ADSs for a return on your investment.
We have never declared or paid cash dividends on our ordinary shares. For the foreseeable future, we currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to compliance with applicable laws and covenants under the loan facilities with Oaktree and NovaQuest or other current or future credit facilities, which may restrict or limit our ability to pay dividends, and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. We do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. As a result, a return on your investment in our ordinary shares or ADSs will likely only occur if our ordinary share or ADS price appreciates. There is no guarantee that our ordinary shares or ADSs will appreciate in value in the future.
Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares or ADSs.
We are incorporated in Australia and are subject to the takeover laws of Australia. Among other things, we are subject to the Australian Corporations Act 2001 (the “Corporations Act”). Subject to a range of exceptions, the Corporations Act prohibits the acquisition of a direct or indirect interest in our issued voting shares if the acquisition of that interest will lead to a person’s voting power in us increasing to more than 20%, or increasing from a starting point that is above 20% and below 90%. Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares. This may have the ancillary effect of entrenching our board of directors and may deprive or limit our shareholders’ opportunity to sell their ordinary shares or ADSs and may further restrict the ability of our shareholders to obtain a premium from such transactions.
38
Significant disruptions of information technology systems, data security breaches or unauthorized disclosure of sensitive data could adversely affect our business by exposing us to liability and affect our business and reputation.
The Company is increasingly dependent on critical, complex, and interdependent information technology systems (IT systems), including cloud-based software and external servers, some of which are managed or hosted by third parties, to support business processes as well as internal and external communications. The information and data processed and stored in our IT systems, and those of our research collaborators, CROs, contract manufacturers, suppliers, distributors, or other third parties for which we depend to operate our business, may be vulnerable to cybersecurity breaches from unauthorized activity by our employees, contractors or malware, hacking, business email compromise, phishing or other cyberattacks directed by other parties. Such breaches can result in loss, damage, denial-of-service, unauthorized access or misappropriation and may pose a risk that sensitive data, including our intellectual property, trade secrets or personal information of our employees, patients, customers or other business partners may be exposed to unauthorized persons or to the public. In addition, our increased reliance on personnel working from home may negatively impact productivity, or disrupt, delay, or otherwise adversely impact our business. The increase in working remotely could increase our cybersecurity risk, create data accessibility concerns, and make us more susceptible to communication disruptions, any of which could adversely impact our business operations or delay necessary interactions with local and federal regulators, manufacturing sites, clinical trial sites, and other third parties.
The rapidly moving nature of technology and the increasing sophistication of cybersecurity threats, may mean our measures to prevent, respond to and minimize such risks may be ineffective. If a material incident or interruption were to occur, it could result in a disruption of our development programs and future commercial operations, including due to a loss, corruption or unauthorized disclosure of our proprietary or sensitive information. Additionally, the costs to the company to investigate and mitigate cybersecurity incidents could be significant. Any disruption, security breach, or action by the company, its employees, or contractors that might be inconsistent with the rapidly evolving data privacy and security laws and regulations applicable within Australia and the United States and elsewhere where we conduct business, could result in; enforcement actions by both countries state and federal governments or foreign governments, liability or sanctions under data privacy laws including healthcare laws such as the Privacy Act or HIPAA that protect certain types of sensitive information, regulatory penalties, other legal proceedings such as but not limited to private litigation, the incurrence of significant remediation costs, disruptions to our development programs, business operations and collaborations, diversion of management efforts and damage to our reputation which could harm our business and operations.
Risks Related to Our Trading Markets
The market price and trading volume of our ordinary shares and ADSs may be volatile and may be affected by economic conditions beyond our control. Such volatility may lead to securities litigation.
The market price of our ordinary shares and ADSs may be highly volatile and subject to wide fluctuations. In addition, the trading volume of our ordinary shares and ADSs may fluctuate and cause significant price variations to occur. We cannot assure you that the market price of our ordinary shares and ADSs will not fluctuate or significantly decline in the future.
Some specific factors that could negatively affect the price of our ordinary shares and ADSs or result in fluctuations in their price and trading volume include:
•results of clinical trials of our product candidates;
•results of clinical trials of our competitors’ products;
•regulatory actions with respect to our products or our competitors’ products;
•actual or anticipated fluctuations in our quarterly operating results or those of our competitors;
•publication of research reports by securities analysts about us or our competitors in the industry;
•our failure or the failure of our competitors to meet analysts’ projections or guidance that we or our competitors may give to the market;
•fluctuations of exchange rates between the U.S. dollar and the Australian dollar;
•additions to or departures of our key management personnel;
•issuances by us of debt or equity securities;
39
•litigation or investigations involving our company, including: shareholder litigation; investigations or audits by regulators into the operations of our company; or proceedings initiated by our competitors or clients;
•strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy;
•the passage of legislation or other regulatory developments affecting us or our industry;
•fluctuations in the valuation of companies perceived by investors to be comparable to us;
•changes in trading volume of ADSs on the Nasdaq and of our ordinary shares on the ASX;
•sales or perceived potential sales of the ADSs or ordinary shares by us, our directors, senior management or our shareholders in the future;
•short selling or other market manipulation activities;
•announcement or expectation of additional financing efforts;
•terrorist acts, acts of war or periods of widespread civil unrest (such as Russia’s invasion of Ukraine);
•natural disasters, the impact of climate change and other calamities;
•changes in market conditions for biopharmaceutical companies; and
•conditions in the U.S. or Australian financial markets or changes in general economic conditions.
In the past, following periods of volatility in the market price of a company’s securities, shareholders often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of senior management, require significant expenditure for defense costs, and, if adversely determined, could have a material adverse effect on our results of operations and financial condition.
A class action proceeding in the Federal Court of Australia was served on the Company in May 2022 by the law firm William Roberts Lawyers on behalf of persons who, between February 22, 2018, and December 17, 2020, acquired an interest in Mesoblast shares, American Depository Receipts, and/or related equity swap arrangements. In June 2022, the firm Phi Finney McDonald commenced a second shareholder class action against the Company in the Federal Court of Australia asserting similar claims arising during the same period. Like the class action lawsuit from October 2020 filed in the U.S. Federal District Court for the Southern District of New York (which had court approval for settlement in August 2022), the Australian class actions relate to the Complete Response Letter released by the FDA in relation to our GvHD product candidate; they also relate to certain representations made by the Company in relation to our COVID-19 product candidate and the decline in the market price of our ordinary shares in December 2020. The Australian class actions have been consolidated into one lawsuit. The Company will continue to vigorously defend against the proceedings. The Company cannot provide any assurance as to the possible outcome or cost to us from the lawsuit, particularly as it is at an early stage, nor how long it may take to resolve such lawsuit. Thus, the Company has not accrued any amounts in connection with such legal proceedings.
The dual listing of our ordinary shares and the ADSs may adversely affect the liquidity and value of these securities.
Our ADSs are listed on the Nasdaq and our ordinary shares are listed on the ASX. We cannot predict the effect of this dual listing on the value of our ordinary shares and ADSs. However, the dual listing of our ordinary shares and ADSs may dilute the liquidity of these securities in one or both markets and may adversely affect the development of an active trading market for the ADSs in the United States. The price of the ADSs could also be adversely affected by trading in our ordinary shares on the ASX, and vice versa.
If securities or industry analysts do not publish research reports about our business, or if they issue an adverse opinion about our business, the market price and trading volume of our ordinary shares and/or ADSs could decline.
The trading market for our ordinary shares and ADSs could be influenced by the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts may discontinue research on our company, to the extent such coverage currently exists, or in other cases, may never publish research on our company. If too few securities or industry analysts commence coverage of our company, the trading price for our ordinary shares and ADSs would likely be negatively impacted. If one or more of the analysts who cover us downgrade our ordinary shares or ADSs or publish inaccurate or unfavorable research about our business, the market price of our ADSs would likely decline. If one
40
or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our ordinary shares and/or ADSs could decrease, which might cause our price and trading volume to decline.
Risks Related to Ownership of Our ADSs
An active trading market for the ADSs may not develop in the United States.
Our ADSs are listed in the United States on the Nasdaq under the symbol “MESO.” However, we cannot assure you that an active public market in the United States for the ADSs will develop on that exchange, or if developed, that this market will be sustained.
We currently report our financial results under IFRS, which differs in certain significant respect from U.S. GAAP.
Currently we report our financial statements under IFRS. There have been and there may in the future be certain significant differences between IFRS and U.S. GAAP, including differences related to revenue recognition, intangible assets, share-based compensation expense, income tax and earnings per share. As a result, our financial information and reported earnings for historical or future periods could be significantly different if they were prepared in accordance with U.S. GAAP. In addition, we do not intend to provide a reconciliation between IFRS and U.S. GAAP unless it is required under applicable law. As a result, you may not be able to meaningfully compare our financial statements under IFRS with those companies that prepare financial statements under U.S. GAAP.
As a foreign private issuer, we are permitted and expect to follow certain home country corporate governance practices in lieu of certain Nasdaq requirements applicable to domestic issuers and we are permitted to file less information with the Securities and Exchange Commission than a company that is not a foreign private issuer. This may afford less protection to holders of our ADSs.
As a “foreign private issuer”, as defined in Rule 405 under the Securities Exchange Act of 1933, as amended (the “Securities Act”), whose ADSs will be listed on the Nasdaq, we will be permitted to, and plan to, follow certain home country corporate governance practices in lieu of certain Nasdaq requirements. For example, we may follow home country practice with regard to certain corporate governance requirements, such as the composition of the board of directors and quorum requirements applicable to shareholders’ meetings. This difference may result in a board that is more difficult to remove and less shareholder approvals required generally. In addition, we may follow home country practice instead of the Nasdaq Global Select Market requirement to hold executive sessions and to obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions or private placements of securities. The above differences may result in less shareholder oversight and requisite approvals for certain acquisition or financing related decisions. Further, we may follow home country practice instead of the Nasdaq Global Select Market requirement to obtain shareholder approval prior to the establishment or amendment of certain share option, purchase or other compensation plans. This difference may result in less shareholder oversight and requisite approvals for certain company compensation related decisions. A foreign private issuer must disclose in its annual reports filed with the Securities and Exchange Commission, or SEC, and the Nasdaq Global Select Market, the requirements with which it does not comply followed by a description of its applicable home country practice. The Australian home country practices described above may afford less protection to holders of the ADSs than that provided under the Nasdaq Global Select Market rules.
Further, as a foreign private issuer, we are exempt from certain rules under the “Exchange Act”, that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as a company that files as a domestic issuer whose securities are registered under the Exchange Act, nor are we generally required to comply with the SEC’s Regulation FD, which restricts the selective disclosure of material non-public information. Accordingly, the information may not be disseminated in as timely a manner, or there may be less information publicly available concerning us generally than there is for a company that files as a domestic issuer.
41
ADS holders may be subject to additional risks related to holding ADSs rather than ordinary shares.
ADS holders do not hold ordinary shares directly and, as such, are subject to, among others, the following additional risks.
•As an ADS holder (and not the holder of ordinary shares underlying your ADSs), we will not treat you as one of our shareholders and you will not be able to exercise shareholder rights, except through the American depositary receipt, or ADR, depositary as permitted by the deposit agreement.
•Distributions on the ordinary shares represented by your ADSs will be paid to the ADR depositary, and before the ADR depositary makes a distribution to you on behalf of your ADSs, any withholding taxes that must be paid will be deducted. Additionally, if the exchange rate fluctuates during a time when the ADR depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution.
•We and the ADR depositary may amend or terminate the deposit agreement without the ADS holders’ consent in a manner that could prejudice ADS holders.
ADS holders must act through the ADR depositary to exercise your voting rights and, as a result, you may be unable to exercise your voting rights on a timely basis.
As a holder of ADSs (and not the ordinary shares underlying your ADSs), we will not treat you as one of our shareholders, and you will not be able to exercise shareholder rights. The ADR depositary will be the holder of the ordinary shares underlying your ADSs, and ADS holders will be able to exercise voting rights with respect to the ordinary shares represented by the ADSs only in accordance with the deposit agreement relating to the ADSs. There are practical limitations on the ability of ADS holders to exercise their voting rights due to the additional procedural steps involved in communicating with these holders. For example, holders of our ordinary shares will receive notice of shareholders’ meetings by mail or email and will be able to exercise their voting rights by either attending the shareholders meeting in person or voting by proxy. ADS holders, by comparison, will not receive notice directly from us. Instead, in accordance with the deposit agreement, we will provide notice to the ADR depositary of any such shareholders meeting and details concerning the matters to be voted upon. As soon as practicable after receiving notice from us of any such meeting, the ADR depositary will mail to holders of ADSs the notice of the meeting and a statement as to the manner in which voting instructions may be given by ADS holders. To exercise their voting rights, ADS holders must then instruct the ADR depositary as to voting the ordinary shares represented by their ADSs. Due to these procedural steps involving the ADR depositary, the process for exercising voting rights may take longer for ADS holders than for holders of ordinary shares. The ordinary shares represented by ADSs for which the ADR depositary fails to receive timely voting instructions will not be voted. Under Australian law and our Constitution, any resolution to be considered at a meeting of the shareholders shall be decided on a show of hands unless a poll is demanded by the shareholders at or before the declaration of the result of the show of hands. Under voting by a show of hands, multiple “yes” votes by ADS holders will only count as one “yes” vote and will be negated by a single “no” vote, unless a poll is demanded.
If we are or become classified as a passive foreign investment company, our U.S. securityholders may suffer adverse tax consequences.
Based upon an analysis of our income and assets for the taxable year ended June 30, 2023, we do not believe we were a passive foreign investment company (a "PFIC") for our most recent tax year. In general, if at least 75% of our gross income for any taxable year consists of passive income or at least 50% of the average quarterly value of assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, then we will be classified as a PFIC for U.S. federal income tax purposes. Passive income for this purpose generally includes dividends, interest, certain royalties and rents, and gains from commodities and securities transactions. Passive assets for this purpose generally includes assets held for the production of passive income. Accordingly, passive assets generally include any cash, cash equivalents and cash invested in short-term, interest bearing, debt instruments or bank deposits that are readily convertible into cash. Since PFIC status depends upon the composition of our income and assets and the market value of our assets from time to time, and since the determination of PFIC status must be made annually at the end of each taxable year, there can be no assurance that we will not be considered a PFIC for any future taxable year. Investors should be aware that our gross income for purposes of the PFIC income test depends on the receipt of active revenue, and there can be no assurances that such active revenue will continue, or that we will receive other gross income that is not considered passive for purposes of the PFIC income test. If we were a PFIC for any taxable year during a U.S. investor’s holding period for the ordinary shares or ADSs, we would ordinarily continue to be treated as a PFIC for each subsequent year during which the U.S. investor owned the ordinary shares or ADSs. If we were treated as a PFIC, U.S. investors would be subject to special punitive tax rules with respect to any "excess distribution" received from us and any gain realized
42
from a sale or other disposition (including a pledge) of the ordinary shares or ADSs unless a U.S. investor made a timely "qualified electing fund" or "mark-to-market" election. For a more detailed discussion of the U.S. tax consequences to U.S. investors if we were classified as a PFIC, see Item 10.E- "Taxation — Certain Material U.S. Federal Income Tax Considerations to U.S. Holders — Passive Foreign Investment Company".
Changes in foreign currency exchange rates could impact amounts you receive as a result of any dividend or distribution we declare on our ordinary shares.
Any significant change in the value of the Australian dollar may impact amounts you receive in U.S. dollars as a result of any dividend or distribution we declare on our ordinary shares as a holder of our ADSs. More specifically, any dividends that we pay on our ordinary shares will be in Australian dollars. The depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses, including any such fees or expenses incurred to convert any such Australian dollars into U.S. dollars. You will receive these distributions in U.S. dollars in proportion to the number of our ordinary shares your ADSs represent. Depreciation of the U.S. dollar against the Australian dollar would have a negative effect on any such distribution payable to you.
You may not receive distributions on our ordinary shares represented by the ADSs or any value for such distribution if it is illegal or impractical to make them available to holders of ADSs.
While we do not anticipate paying any dividends on our ordinary shares in the foreseeable future, if such a dividend is declared, the depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of our ordinary shares your ADSs represent. However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to take any other action to permit the distribution of the ADSs, ordinary shares, rights or anything else to holders of the ADSs. This means that you may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical to make them available to you. These restrictions may have a material adverse effect on the value of your ADSs.
You may be subject to limitations on transfers of your ADSs.
ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
U.S. investors may have difficulty enforcing civil liabilities against our company, our directors or members of our senior management.
Several of our officers and directors are non-residents of the United States, and a substantial portion of the assets of such persons are located outside the U.S. As a result, it may be impossible to serve process on such persons in the United States or to enforce judgments obtained in U.S. courts against them based on civil liability provisions of the securities laws of the U.S. Even if you are successful in bringing such an action, there is doubt as to whether Australian courts would enforce certain civil liabilities under U.S. securities laws in original actions or judgments of U.S. courts based upon these civil liability provisions. In addition, awards of punitive damages in actions brought in the U.S. or elsewhere may be unenforceable in Australia or elsewhere outside the U.S. An award for monetary damages under the U.S. securities laws would be considered punitive if it does not seek to compensate the claimant for loss or damage suffered and is intended to punish the defendant. The enforceability of any judgment in Australia will depend on the particular facts of the case as well as the laws and treaties in effect at the time. The U.S. and Australia do not currently have a treaty or statute providing for recognition and enforcement of the judgments of the other country (other than arbitration awards) in civil and commercial matters. As a result, our public shareholders and holders of the ADSs may have more difficulty in protecting their interests through actions against us, our management, our directors than would shareholders of a corporation incorporated in a jurisdiction in the United States.
43
Item 4. Information on the Company
4.A History and Development of Mesoblast
Mesoblast Limited
Mesoblast Limited was incorporated on June 8, 2004 as a public company in Australia under the Corporations Act 2001 with an indefinite duration. On December 16, 2004 we became listed on the Australian Securities Exchange (the “ASX”). On November 13, 2015, we became listed on the Nasdaq Global Select Market (“Nasdaq”) and from this date we have been dual-listed in Australia and the United States. Our registered office is located at the following address:
Mesoblast Ltd
Level 38
55 Collins Street
Melbourne VIC 3000
Australia
Telephone: +61 3 9639 6036
Web: www.mesoblast.com
Our agent for service of process in the United States is Mesoblast Inc., 505 Fifth Avenue, Level 3, New York, NY 10017. All information we file with the SEC is available through the SEC's Electronic Data Gathering, Analysis and Retrieval system, which may be accessed through the SEC's website at www.sec.gov.
For a list of our significant subsidiaries, see Exhibit 8.1 to this Annual Report.
Important Corporate Developments
Fiscal year 2023 to date of annual report
| August 2023 | United States Food & Drug Administration (“FDA”) provided a complete response to the Biologics License Application (“BLA”) resubmission for remestemcel-L for the treatment of pediatric steroid-refractory acute graft versus host disease (“SR-aGVHD”) and requires more data to support marketing approval, including potency assay or clinical data. Mesoblast intends to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. This adult study is in line with our overall commercial strategy, which envisioned a sequenced progression from pediatric to adult SR-aGVHD indications. As part of its review FDA completed the Pre-License Inspection ("PLI") of the manufacturing facility, did not issue any Form 483, and found no objectionable conditions. In addition, FDA acknowledged in the resubmission review that changes implemented appear to improve assay performance relative to the original version of the assay used in the pediatric Phase 3 trial. | ||||
| June 2023 | Announced the appointment of Dr. Philip Krause, a member of the Board of Directors, to a formal strategic advisory role. | ||||
| April 2023 | Completed a global private placement primarily to existing major shareholders raising approximately US$40.0 million, net of transaction costs. Proceeds will be used to fund launch and commercialization of the company’s lead product, remestemcel-L, subject to approval by FDA, ongoing manufacturing of commercial remestemcel-L product, and initiation of patient enrollment in the confirmatory Phase 3 clinical trial of rexlemestrocel-L for CLBP. | ||||
| March 2023 | As part of the ongoing review of the BLA for remestemcel-L in the treatment of children with SR-aGVHD, the FDA scheduled a PLI of Mesoblast’s cell therapy manufacturing operations at Lonza Bioscience in Singapore. | ||||
| February 2023 | The DREAM-HF Phase 3 trial results were published in the premier peer-reviewed journal for cardiovascular medicine, the Journal of the American College of Cardiology (“JACC”). The results of the randomized, double-blind, controlled study in 537 patients showed that Mesoblast’s mesenchymal precursor cell therapy (“MPCs”; rexlemestrocel-L) strengthened heart function at 12 months, as measured by left ventricular ejection fraction (“LVEF”) and decreased cardiovascular death, myocardial infarction (“MI”) or stroke in patients with chronic heart failure due to reduced ejection fraction (“HFrEF”) over a mean follow-up of 30 months. | ||||
44
Two studies on the remestemcel-L for the treatment of children with SR-aGVHD were selected by peer review to be presented at the 2023 Tandem Meetings of the American Society for Transplantation and Cellular Therapy ("ASTCT") and the Center for Blood and Marrow Transplant Research ("CIBMTR"). | |||||
The FDA Office of Tissues and Advanced Therapies ("OTAT") had granted Regenerative Medicine Advanced Therapy ("RMAT") designation for rexlemestrocel-L in the treatment of CLBP associated with disc degeneration, in combination with hyaluronic acid (HA) as delivery agent for injection into the lumbar disc. | |||||
| January 2023 | Resubmitted to the FDA the BLA for approval of remestemcel-L in the treatment of children with SR-aGVHD. | ||||
| December 2022 | Announced that funds managed by Oaktree Capital Management, L.P ("Oaktree") extended to Mesoblast the availability of up to an additional $30.0 million of its $90.0 million five-year facility subject to achieving certain milestones on or before September 30, 2023. | ||||
| November 2022 | Announced top-line long-term survival results for remestemcel-L from a four-year observational cohort survival study performed by the CIBMTR on 51 evaluable children with SR-aGVHD who were enrolled in Mesoblast’s phase 3 clinical trial (MSB-GVHD001) of remestemcel-L across 20 centers in the US. The results showed durable survival through 4 years of follow-up. | ||||
| October 2022 | Submission to the FDA substantial new information on clinical and potency assay items identified in the Complete Response Letter ("CRL") received from FDA in September 2020 to the BLA for remestemcel-L in the treatment of children with SR-aGVHD. The new information submitted to the Investigational New Drug ("IND") file for remestemcel-L in the treatment of children with SR-aGVHD represents a major milestone in the Company’s complete response to the FDA. | ||||
| August 2022 | Announced the appointment of Ms. Jane Bell to the board of directors of the Company as a non-executive director. | ||||
| Completed a US$45.0 million (A$65.0 million) financing in a global private placement predominantly to major shareholders of the Company. The proceeds from the placement will facilitate activities for launch and commercialization for remestemcel-L, in the treatment of children with SR-aGVHD for which Mesoblast seeks FDA approval under a planned resubmission of its BLA; and commencement of a second Phase 3 clinical trial of rexlemestrocel-L to confirm reduction in chronic low back pain associated with degenerative disc disease. | |||||
| July 2022 | Announced that analysis from the DREAM-HF Phase 3 trial showed that patients with chronic heart failure and reduced ejection fraction (“HFrEF”) treated with rexlemestrocel-L demonstrated greater improvement in the pre-specified analysis of left ventricular ejection fraction at 12 months relative to controls. Improvement in LVEF was most pronounced in the setting of inflammation and preceded long-term reduction in the 3-point MACE of cardiovascular death, non-fatal heart attack or stroke. | ||||
Environmental, Social and Governance (“ESG”) Statement
Introduction: Our Approach to Sustainability
We consider the greatest contribution Mesoblast makes to sustainability is its purpose in seeking to provide access to treatment for patients suffering a range of hitherto unmet medical needs including cardiac diseases, immune-mediated and inflammatory conditions, oncology and haematology diseases, and spine orthopaedic disorders, subject to regulatory approval. This has not only a potentially high social and financial value, but in terms of adding value in the way it operates, the Company prizes and develops its people as key assets, while its environmental footprint is light. Together with a strong ethical and governance framework, this puts the Company on a sound footing for delivering on its purpose in the medium to long term.
Our commitment to sustainability is instilled through Mesoblast’s five key corporate values which articulate who we are and what we stand for. Mesoblast values reflect our commitment to our customers, our colleagues, and the patients we
45
serve. Integrity is at our core, while accountability to our commitments, collective teamwork, a pursuit of excellence, and outside the-box thinking and innovation surround our every business decision. Mesoblast personnel are expected to practice these values each and every day.
Integrity - We act with integrity in all of our dealings, with the best interest of patients, care givers and our people as our guide. What we do we do with conviction. Accountability - We hold ourselves and each other responsible and ensure that our words and actions support Mesoblast’s vision and values Teamwork - We believe in what we can achieve collectively and have an appreciation of our shared and unique ability to collaborate with our people and our partners, while focused on our patients and their families. Excellence - We engage in continual learning so that we, as individuals and as an organization, can reach our highest potential. Innovation - We are focused on the bold pursuit of developing and delivering novel treatments to improve patient outcomes through cutting edge science. |  | ||||
Acknowledging that sustainability is an overarching concept that can be applied to all areas of business finance, operations and impact, for the purposes of this Statement, we specifically focus on key environmental, social and governance (“ESG”) matters. When assessing and reporting our ESG initiatives and performance, we take into account:
•Mesoblast’s size and stage in its growth cycle: it is a small development-stage biotechnology company with fewer than 100 employees, limited manufacturing and currently no commercialized product. This means that some reporting topics will be less relevant for us and our stakeholders until we grow our product portfolio and operations; and
•Appropriate sustainability standards: for example, the Sustainability Accounting Standards Board’s (“SASB”) Biotechnology & Pharmaceuticals Sustainability Accounting Standard, the Global Reporting Initiative’s (“GRI”) Universal Standards, and the Biopharma Investor ESG Communications Guidance 4.0 are relevant.
We identified the following material ESG topics based on an assessment of their impact on the business and our understanding of their importance to stakeholders:
1.Corporate Governance
2.Business Ethics, Integrity, and Compliance
3.Risk Management
4.Human Capital Management
5.Product Quality and Patient Safety
6.Supply Chain Management
7.Access to Healthcare
8.Environmental Impacts
These are dealt with in turn below.
1.Corporate Governance
Mesoblast is committed to implementing and achieving an effective corporate governance framework to ensure that the Company is managed effectively, honestly and ethically. More information on our corporate governance practices is set out in Mesoblast’s Corporate Governance Statement, available at www.mesoblast.com. The Company references and reports against ASX Corporate Governance Council’s (Council) Corporate Governance Principles and Recommendations.
46
Mesoblast’s Board of Directors (“the Board”) provides oversight of the Company’s ESG-related risks and opportunities on a regular basis at Board meetings, and in particular focus through its two committees:
•Nomination and Remuneration Committee (“NRC”)
•Audit and Risk Committee (“ARC”)
The NRC assists the Board in the discharge of its responsibilities, and in particular to ensure that there is an environment where the Board can carry out effective and responsible decision making and oversight, including on ESG matters such as fair remuneration and health & safety. Since June 2022, all members of the Board are members of the NRC reflecting the importance the Board places on ESG.
In addition to its main financial reporting responsibilities, the ARC is tasked with overseeing the effective operation of Mesoblast’s risk management framework, in which certain ESG matters are considered.
Management is responsible for assessing and managing ESG-related risks and opportunities within the board approved control framework, and for reporting progress against goals and targets to the Board.
2.Business Ethics, Integrity, and Compliance
We are committed to the highest standards of ethical conduct and transparency in the way we deal with our patients, employees, strategic partners, and other important stakeholders. We comply with all national and local laws and regulations applying to our Company. Zero cases of material non-compliance occurred in FY23.
Mesoblast has established a Code of Business Conduct & Ethics (“Code”) to promote honest and ethical conduct, comprehensive disclosures of business dealings, compliance with government laws and regulations, and a positive work environment. All Mesoblast personnel, including Directors, officers, employees, contractors, and consultants, are expected to comply with the principles set out in the Code. The Code covers the following topics:
•Our Values
•Ethical business practices
•Safe workplace and respectful workplace conduct
•Fair competition
•Conflicts of interest
•Social media use
•Confidentiality and protection of assets
•Quality assurance
•Price reporting
•Financial reporting
•Securities trading
•Ethical research
•Interactions with the patient community
•Ensuring product quality and patient safety
•Interactions with healthcare professionals
•Ethical marketing and advertising
•Compliance with laws and regulations
The Code also states that it is against Mesoblast policy for personnel to use illegal drugs or be under the influence of or impaired by alcohol or drugs while on company property or performing company work.
No issues of Code non-compliance have been brought forward to the Board in FY23.
47
Mesoblast has an Anti-Bribery and Anti-Corruption Policy and complies with global and regional laws preventing corrupt business practices and bribery, including the U.S. Foreign Corrupt Practices Act and the United Kingdom Bribery Act.
We have a Disclosure of Complaints and Concerns Policy which addresses, among other things, breaches under the Company’s Code, Anti-Bribery and Anti-Corruption Policy, or other Company policies. Under the Disclosure of Complaints and Concerns Policy, Mesoblast personnel are entitled to robust employment protections if they report concerns and suspected violations covered under the policy. Personnel can report to Compliance, Legal, the Audit and Risk Committee, or other officers or senior managers, and may do so anonymously. Further, Mesoblast’s Fair Treatment Policy requires personnel to report workplace harassment and prohibits retaliation of any kind against anyone who does so in good faith. During FY23, Mesoblast received and, in compliance with the Fair Treatment Policy, promptly investigated and resolved a small number of reports related to workplace conduct. The Company is satisfied that it adhered to its policies.
In addition, Mesoblast has an ‘Ethics Hotline’ that is managed by a third-party, where our personnel may make a report anonymously, 24 hours a day, seven days a week. There have been no whistle-blower reports to this hotline in the reporting period.
All Mesoblast personnel are required to acknowledge the Code and other key policies and are required to participate in annual compliance training.
The Company has a process in place to inform the Board or a committee of the Board of any material breaches of the Code, the Anti-Bribery and Anti-Corruption Policy, and material incidents reported under the Disclosure of Complaints and Concerns Policy.
A copy of the Code and other key policies can be found at www.mesoblast.com.
3.Risk Management
The Board is responsible for satisfying itself annually, or more frequently as required, that management has developed and implemented an effective system of risk management and internal control. Management is responsible for ensuring there are adequate policies in relation to risk management, compliance, and internal control systems. The ARC monitors Mesoblast’s risk management by overseeing management’s actions in the evaluation, management, monitoring, and reporting of material operational, financial, compliance, strategic, and certain ESG risks.
Mesoblast’s risk management group is part of the Operating Committee and is headed by the Chief Operating Officer. This group is responsible for designing, implementing, monitoring, and reporting on Mesoblast’s management of material business risks and the effectiveness of Mesoblast’s risk management and internal control system. ESG risks have been incorporated into and are considered as part of Mesoblast’s risk management system. The Operating Committee regularly reviews Mesoblast’s risks across its business and operations, and Mesoblast’s material business risks and risk management framework are reviewed at least annually by the ARC.
As part of the process of continual improvement, we introduced a standardized tool to assess our portfolio and corporate risk.
4.Human Capital Management
4.1Diversity and Inclusion
Mesoblast has a Diversity Policy which encompasses differences in ethnicity, gender, language, age, sexual orientation, religion, socioeconomic status, physical and mental ability, thinking styles, experience, and education. We believe that the wide array of perspectives that results from such diversity promotes innovation and business success. Being diverse makes us more creative, flexible, and productive. Mesoblast’s policy is to engage the most appropriate and relevant partner organizations, consultants, experts, and personnel. This includes recruiting people who are well-qualified for their position and those who as aligned to Mesoblast’s five values and will embrace the Mesoblast culture and work ethic.
In order to meet and comply with our Diversity Policy, Mesoblast employs the following principles:
•Mesoblast seeks and encourages diversity in current and potential employees;
48
•Mesoblast promotes equal employment opportunities based on capability, performance and potential for growth and progression;
•Recruitment, professional development, succession management, promotion, and remuneration decisions are all based on performance and capability aligned to the specific job role, salary ranges, and a pre-set criteria prior to the activities to ensure any biases are reduced;
•Mesoblast seeks to build a safe working environment by recognizing and taking action against inappropriate workplace behavior, including bullying, discrimination, harassment, victimization, and vilification;
•Mesoblast promotes flexible work practices where possible and reasonable in the circumstances, to meet the differing needs of our employees; and
•Mesoblast ensures appropriate policies and procedures exist that encourage diversity and meet legislative requirements.
Line management is supported to manage diversity to ensure that employees are treated fairly and objectively. We have clear reporting procedures for any type of discrimination or harassment, combined with follow-up procedures to prevent future incidents.
The Board, through the NRC, is responsible for overseeing our Diversity Policy. Mesoblast’s Head of Human Resources, with the support of the Chief Executive Officer and the Executive Team, is responsible for implementing the Diversity Policy.
The Board, through the NRC, is responsible for approving and reviewing measurable objectives for achieving gender diversity in the workplace. Mesoblast has set the following measurable objectives:
i)Increase the number of women on the Board as vacancies arise and circumstances permit;
ii)Increase the number of women who hold senior executive positions as vacancies arise and circumstances permit; and
iii)Ensure the opportunity exists for equal gender participation in all levels of professional development programs.
During FY23, one female was appointed to the Board and one resigned, the senior management team remained stable. All Mesoblast employees were provided access to the same development programs. A copy of the Company’s Diversity Policy can be found at www.mesoblast.com.
Table – Gender diversity statistics*
| Gender | FY23 Senior Executives** | FY23 Total Workforce | FY22 Senior Executives** | FY22 Total Workforce | ||||||||||||||||||||||
| Male | 6 | 39 | 6 | 37 | ||||||||||||||||||||||
| Female | 3 | 44 | 3 | 40 | ||||||||||||||||||||||
| Other | — | — | — | — | ||||||||||||||||||||||
| % Female | 33 | % | 53 | % | 33 | % | 52 | % | ||||||||||||||||||
*Based on number of active employees as at June 30. Excludes contractors and consultants.
**A senior executive position is one held by an executive who reports directly to the Chief Executive.
Every employee, consultant and service provider has the right to work with Mesoblast in an environment that is safe, and free from intimidation, harassment, and abuse. Mesoblast prohibits harassment for any reason, including veteran status, uniform service member status, or any other protected class under federal, state, or local law. Inappropriate behavior, including verbal or physical conduct by any individual that harasses another, disrupts another’s work performance, or creates an intimidating, offensive, abusive, or hostile workplace, is not tolerated. In addition, we will not tolerate comments, jokes, or materials, including emails, which others might consider offensive. All Mesoblast personnel are required to complete mandatory training on an annual basis to recognize and deal with inappropriate behavior in our workplaces, including the New York City Commission on Human Rights – Accredited Program: Confronting Sexual
49
Harassment; Tools & Strategies to Create a Harassment Free Workplace and Mesoblast’s Fair Treatment policy. There were no cases of harassment were reported in FY23 or in FY22.
4.2Health and Safety
Mesoblast provides a workplace that is clean and safe for all associates and one that complies with health and safety laws. As an organization whose activities are predominantly office and laboratory based, Mesoblast chooses to track its safety record using total recordable incident frequency rate (“TRIFR”) i.e., number of recorded injuries for each one million hours worked. No incidents were recorded for FY23. In FY23, the Environment Health and Safety Management System and supporting policies were developed and aligned for each jurisdiction in preparation for company wide training . Mesoblast continued to implement hybrid/flexible working arrangements and the employee assistance program was made available across all sites.
4.3Recruitment, Development and Retention
Mesoblast operates at the forefront of a highly specialized industry and we recognize that our talented people are key to developing our cell therapy technology.
Our policies and procedures follow equal employment opportunities principles for fair treatment, including diversity and compensation. Our employees are given equal access to job opportunities and promotions based on capability, performance and potential for growth and progression as part of our retention program.
Mesoblast’s recruitment process enables our line managers to prepare a job description that outlines accountabilities and selection criteria that emphasize the skills, knowledge and experience. Job criteria and interview guides are prepared for each role advertised to ensure consistency across all the interviews. Jobs are advertised through multiple channels based on the specialization of the job role. All job roles are published on the Mesoblast intranet site providing transparency to all employees within the company and an equal opportunity to apply. Job descriptions are prepared in a way that enables employees to consider lateral moves based on competence rather than expertise in years of service.
In FY23, the voluntary turnover rate was approximately 11% with 42% male and 58% females departing the Company. Exit interviews are conducted with all departing employees and trends are monitored so that actions to minimize the turnover can be taken. Mesoblast employed eight females and six males for the replacement roles. While acting and higher duty opportunities were minimal during this period, job profiles were prepared to enable existing employees to consider lateral moves based on competence rather than years of service, where appropriately credentialed.
We provide opportunities for all colleagues to participate in professional training and education so they can enhance their skill sets and career. During FY23 all employees were given the opportunity to participate in a development program that is linked to the annual Performance Management System.
During the reporting period, Mesoblast continued with an online performance management program and integrated an online professional development program that links the recording of participation in professional development aligned to job role. The online performance management program enables employees to track their performance and receive regular feedback from their manager. The formal annual review process assesses the individual employee’s performance against objectives and quantifiable criteria that are aligned to the Mesoblast business plan, reducing the risk of bias. All employees below the executive level participated in this program during the period.
5.Product Quality and Safety
5.1Scientific Research and Innovation
Over the past decade there has been a surge of interest internationally in the cutting-edge science of cellular medicines and their use in treating a wide range of diseases.
Mesoblast is a clinical stage biotechnology company and works in close collaborative associations with leading cell therapy research centers, as well as having our own in-house R&D laboratories and specialists. We ensure rigorous scientific investigations are performed with well characterized cell populations in order to understand mechanisms of action for each potential medical application. We undertake extensive pre-clinical translational studies to guide subsequent clinical trials.
50
5.2Use of Stem Cells
Mesoblast’s novel allogeneic product candidates are based on rare (approximately 1:100,000 in bone marrow) mesenchymal lineage cells that respond to tissue damage, secreting mediators that promote tissue repair and modulate immune responses.
Mesenchymal lineage cells are collected from the bone marrow of healthy adult donors, and proprietary processes are utilized to expand them to a uniform, well characterized, and highly reproducible cell population. This enables manufacturing at industrial scale for commercial purposes. Mesoblast’s cells can be administered to patients without the need for donor-recipient matching or recipient immune suppression.
The distinction between embryonic stem cells (“ESCs”) and non-ESCs, such as our mesenchymal lineage cells, can be easily misunderstood by the public and has the potential to create negative public attitudes toward cell therapy. As Mesoblast’s cells are not ESCs, we minimize the risk of being exposed to ethical, legal, or social concerns that have arisen in relation to the collection and use of ESCs.
5.3Use of Animal in Research
Mesoblast is committed to the welfare and humane treatment of animals and only undertakes development studies in animal models where required by applicable regulatory bodies. These studies are undertaken by expert third-party providers who are specialists in the management of animals and their welfare.
Mesoblast’s approach to product development is to ensure rigorous scientific investigations are performed with well-characterized cell populations in order to understand mechanisms of action for each potential indication. Extensive preclinical translational studies guide clinical trials that are structured to meet stringent safety and efficacy criteria set by international regulatory agencies.
In the United States where the majority of our clinical development takes place, all of our product candidates are regulated as biological products by the Center for Biologics Evaluation and Research (“CBER”) in the FDA. Biological products are subject to federal regulation under the Federal Food, Drug, and Cosmetic Act (“FDCA”), the Public Health Service (“PHS”) Act, and other federal, state, local and foreign statutes and regulations. Both the FDCA and the PHS Act, as applicable, and their corresponding regulations govern, among other things, the testing, manufacturing, safety, efficacy, labeling, packaging, storage, record keeping, distribution, import, export, reporting, advertising and other promotional practices involving drugs and biological products.
The process required by the FDA before a biological product may be marketed in the U.S. generally involves years of studies and many complex steps. The first of these is completion of nonclinical laboratory studies, meaning in vivo and in vitro experiments in which an investigational product is studied prospectively in a test system under laboratory conditions to determine its safety, must be conducted according to Good Laboratory Practice (“GL”) regulations, as well as, in the case of nonclinical laboratory studies involving animal test systems, in accordance with applicable requirements for the humane use of laboratory animals and other applicable regulations.
Some of the manufacturing materials and/or components that we use in, and which are critical to, implementation of our technology involve the use of animal-derived products. Our media is sourced from fetal bovine serum (“FBS”), and is the main consumable used in our manufacturing process.
While FBS is commonly used in the production of various marketed biopharmaceuticals, our suppliers of FBS must meet our strict quality standards are thus limited in number and region.
5.4Product Quality
The Company has a Quality Management Department with appropriate controls in place for monitoring and compliance of clinical and non-clinical studies as well as manufacturing operations. Our quality assurance processes align with the widely accepted quality standards from the ICH Guidelines created by The International Conference on Harmonization of Technical Requirements for Pharmaceuticals for Human Use (“ICH”) as well as FDA Regulations. All Mesoblast personnel are responsible for the identification and prompt reporting of all actual or potential adverse events or product quality complaints. This may include any reported problem with a finished product, its packaging, inappropriate healthcare professional use, or unintended patient reaction. We have a regulatory obligation to report all adverse events and
51
product complaints, with serious adverse events requiring reporting within 24 hours of receiving notification. The Company provides personnel with regular training in relation to our obligations and responsibilities.
5.5Clinical Trials and Patient Safety
Mesoblast works with healthcare professionals, academic organizations, and contract research organizations (“CRO”) to perform company-sponsored pre-clinical and clinical research. The Company also provides financial support or drug product for independent third-party studies such as Investigator Initiated Trials (IITs) via grant requests. All studies must be scientifically valid and likely to generate data that will be relevant to a defined product development or other clinical and/or business need. These research initiatives are never used as a way to induce a healthcare professional or healthcare organization to use, recommend, or purchase Mesoblast products, or to encourage off-label use of marketed products.
Each potential study subject/study subject legal guardian is provided with an Informed Consent Form (“ICF”) by the clinical trial site study team. The ICF contains information that must be provided to each possible study candidate, such as an explanation of the purpose of the research, possible risks/benefits as well as statements describing the confidentiality of information collected, how the information may be used and who may view this information. Each potential study subject/legal guardian is given time to read the ICF and to ask questions about anything they don’t understand. In addition, the ICF provides the Primary Investigator’s (“PI”) and Independent Review Board’s (“IRB”) contact information to the subject to ask questions and/or report any study related concerns. Once all questions are answered, signatures are obtained to record consent. Mesoblast, as the Sponsor, together with the CRO, monitors the sites for any protocol deviations throughout the course of the study. If and when protocol deviations are identified, we will work with the CRO and site(s) to address them as quickly as possible. Study subject safety is front and foremost in our conduct of all our clinical studies. Between our Therapeutic Area Heads, Quality Assurance (“QA”), and Safety and Clinical Operations, we monitor the conduct of our clinical trials extremely thoroughly and work to protect the well-being of the study subjects as well as the integrity of the trial.
Company exploration of innovative therapies, including research projects, database reviews, and pre-clinical and clinical trials, are designed to first and foremost protect the rights and safety of study subjects and to maintain the integrity of research data. We do this by complying with all regulatory standards regarding research programs and encouraging all involved persons to report any deviations, including inaccurate reporting of study data, inappropriate use of study funds or pharmaceutical product, falsification of study reports, or failure to obtain Independent Review Board or other required approval prior to conducting a study. This process includes all clinical trial investigators attesting that they’ve read and understood the contents of the clinical trial protocol and agree to conduct the trial in compliance with the protocol, good clinical practice and applicable regulatory requirements.
6.Supply Chain Management
Mesoblast has an established vendor assurance program through which suppliers are audited for purposes of being qualified and added to an approved suppliers list. All approved suppliers are audited on a routine basis. Our Supplier Management procedure describes the detailed process for qualifying and managing suppliers which includes quality agreements, supply agreements, due diligence activities, and audits.
6.1Manufacturing Safe Products
Given the current scale of our operations, elements of our business including manufacturing are outsourced to third-party providers. Mesoblast has established a strategic alliance with Lonza, a global leader in biopharmaceutical manufacturing. We monitor Lonza and other third-party providers through our vendor assurance program. In addition, all entities involved in the preparation of therapeutics for clinical studies or commercial sale, including Lonza, are subject to extensive external regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical studies must be manufactured in accordance with current international Good Manufacturing Practice (“GMP”) and other international regulatory requirements. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale.
Mesoblast, our collaborators, and our suppliers as appropriate must supply all necessary documentation in support of any application for product approval and must adhere to current GLP and current GMP regulations enforced by the FDA and other regulators through their facilities inspection program. Before we can begin commercial manufacture of our
52
products for sale in the United States, we must obtain FDA regulatory approval for the product. In addition, the processes and quality systems associated with the manufacturing of such product must also be approved, which requires a successful FDA inspection of the manufacturing facilities, including Lonza’s manufacturing facilities.
In addition, regulators may at any time audit or inspect a manufacturing facility involved with the preparation of our product candidates, raw materials, or the associated quality systems. Although we cannot control the manufacturing process of, and are dependent on, the contract manufacturer for compliance with the regulatory requirements, through our vendor assurance program, we monitor the performance and undertake an annual audit of each contract manufacturer involved in the production of our product candidates. In addition, Lonza is monitored through an established governance structure with multiple feedback loops to ensure compliance to established contracts, specifications, and policies. In addition to having staff onsite and personnel in the plant to oversee ongoing activities, the organizations review numerous manufacturing and quality metrics to ensure consistent product manufacture.
6.2Bone Marrow
The initial stage of manufacturing involves obtaining mesenchymal lineage cell-containing bone marrow from healthy consenting donors. The process of identifying new donor tissue, testing and verifying its validity in order to create new cell banks is tightly regulated and validated with the FDA and other regulators. For example, U.S. federal and state governments and other jurisdictions impose restrictions on the acquisition and use of tissue, including those incorporated in federal Good Tissue Practice regulations. Our manufacturing partner Lonza also has a dedicated U.S. facility for bone marrow acquisition. Lonza maintains all documents and records generated during the lifecycle of donor screening and bone marrow aspiration in a donor-specific file under its site quality system.
6.3Storage and Distribution
Storage and distribution of our product candidates are contracted to CSM on Demand, ICS AmerisourceBergen, CryoSite, and CryoPort Solutions who are experts in innovative storage and/or distribution solutions for pharmaceutical manufacturers. Performance is monitored through established contractual agreements, and the interactions of our joint project teams, as well as through regular supplier audits and qualifications.
7.Access to Healthcare
Mesoblast does not currently have a product approved and commercialized. In August 2023, FDA provided a complete response to a resubmission of the Biologics License Application for our lead product candidate remestemcel-L for the treatment of children with steroid-refractory graft versus host disease. We are currently engaging with the FDA to agree on the regulatory pathway for approval and if successful, this product would constitute the Company’s first commercialized product. We acknowledge and support the social importance of providing access to healthcare across all geographic regions regardless of socio-economic status and recognize this is frequently regarded as one of the top ESG topics for the Biopharma sector. Despite our current size, financial status, and stage of clinical development, we have in place elements that reflect this important social topic.
7.1Expanded Access Programs
Under a compassionate use protocol in the US, Mesoblast has continued to make remestemcel-L available to children as ‘salvage therapy’ where all other treatment avenues have been exhausted and the risk of mortality is high. More than 250 children have had access to remestemcel-L under these circumstances, provided by us at no cost.
In 2020, an Expanded Access Protocol (“EAP”) was initiated in the US for compassionate use of remestemcel-L in the treatment of COVID-19 infected children with cardiovascular and other complications of MIS-C (multisystem inflammatory syndrome in children). MIS-C is a life-threatening complication of COVID-19 in otherwise healthy children and adolescents that includes massive simultaneous inflammation of multiple critical organs and their vasculature. Mesoblast has provided treatment at no charge to three children under this EAP.
7.2Product Pricing
In the United States, Federal and state government agencies may purchase Mesoblast products and provide reimbursement on those products via the state and federal healthcare programs, such as Medicare and Medicaid, once Mesoblast’s product receives regulatory approval and is able to be commercialized. Various federal laws and/or
53
government contracting requirements give some of these purchasers and reimbursors the right to discounted prices and/or rebates on Company products. Depending on the requirements that apply to the pricing terms the Company is reporting, our prices should reflect any reductions, rebates, up-front payments, coupons, goods in kind, free or reduced-price services, grants, price concessions, or other benefits offered to induce a sale may be considered pricing terms. Mesoblast is committed to accurately taking these items into account.
8.Environment
Mesoblast is committed to protecting the world in which we live and work, and we aim to minimize our impact on the wider environment and its component parts. Currently, Mesoblast’s direct physical footprint is limited to office and laboratory space for our employee base of less than 100, so our direct, physical environmental impact is currently limited. Nonetheless, Mesoblast has begun initiatives to improve our impact such as sourcing our electricity from green energy providers and introducing office waste recycling programs. In addition, as noted above, many of our employees and consultants are dispersed and are infrequently in our office spaces.
We are also driving initiatives to minimize the inputs and outputs to our manufacturing processes through our investment in research and development that focuses on the scaling of technologies and minimizing waste. We are developing a 3D bioreactor process to expand our cell product which will replace our current 2D process involving plates. This will reduce the amount of plastic and biohazardous waste that will be generated by our manufacturing processes.
As mentioned above, we rely on third-party providers for important elements of our business. We and our partners must comply with environmental laws and regulations, including those relating to the discharge of materials into the air, water and ground, the manufacture, storage, handling, use, transportation and disposal of hazardous and biological materials, and the health, wellbeing and safety of employees with respect to laboratory activities required for the development of products and technologies.
4.B Business Overview
Mesoblast has developed a range of late-stage product candidates derived from our first and second generation proprietary mesenchymal lineage cell therapy technology platforms.
Remestemcel-L is our first-generation mesenchymal lineage stromal cell (“MSC”) product platform and is in late stage development for treatment of systemic inflammatory diseases including:
•Steroid refractory acute graft versus host disease (SR-aGVHD);
•Acute respiratory distress syndrome (ARDS); and
•Biologic refractory inflammatory bowel disease.
Rexlemestrocel-L is our second generation mesenchymal lineage precursor cell product platform and is in late stage development for treatment of:
•Chronic heart failure (CHF); and
•Chronic low back pain (CLBP) due to degenerative disc disease.
Both platforms have life cycle management strategies with promising emerging pipelines.
The Company’s proprietary manufacturing processes yield industrial-scale, cryopreserved, off-the-shelf, cellular medicines. These cell therapies, with defined pharmaceutical release criteria, are planned to be readily available to patients worldwide upon receiving marketing authorizations.
Mesoblast’s immuno-selected, culture expanded cellular medicines are based on mesenchymal precursor cells (“MPCs”) and their progeny, MSCs. These are rare cells (approximately 1:100,000 in bone marrow) found around blood vessels that are central to blood vessel maintenance, repair and regeneration. These cells have a unique immunological profile with immunomodulatory effects that reduce inflammation allowing healing and repair. This mechanism of action enables the targeting of multiple disease pathways across a wide spectrum of complex diseases with significant unmet medical needs.
54
Mesenchymal lineage cells are collected from the bone marrow of healthy adult donors and proprietary processes are utilized to expand them to a uniform, well characterized, and highly reproducible cell population. This enables manufacturing at industrial scale for commercial purposes. Another key feature of Mesoblast’s cells is they can be administered to patients without the need for donor–recipient matching or recipient immune suppression.
Mesoblast’s approach to product development is to ensure rigorous scientific investigations are performed with well-characterized cell populations in order to understand mechanisms of action for each potential indication. Extensive preclinical translational studies guide clinical trials that are structured to meet stringent safety and efficacy criteria set by international regulatory agencies. All trials are conducted under the continuing review of independent Data Safety Monitoring Boards comprised of independent medical experts and statisticians. These safeguards are intended to ensure the integrity and reproducibility of results, and to ensure that outcomes observed are scientifically reliable.
Allogeneic, Off-the-Shelf, Commercially Scalable Products
Our technology platform enables development of a diverse range of products derived from the mesenchymal cell lineage in adult tissues. MPCs constitute the earliest known cell type in the mesenchymal lineage in-vivo.
MPCs can be isolated using monoclonal antibodies and culture-expanded using methods that enable efficient expansion without differentiation. MSCs are defined biologically in culture following density gradient separation from other tissue cell types and following culture by plastic adherence. MSCs presumably represent culture-expanded in-vitro progeny of the undifferentiated MPCs present in-vivo. The functional characteristics of each cell type enable product development for specific indications.
Our proprietary mesenchymal lineage cell-based products have distinct biological characteristics enabling their use for allogeneic purposes.
Immune Privilege: Mesenchymal lineage cells are immune privileged, in that they do not express specific cell surface co-stimulatory molecules that initiate immune allogeneic responses.
Expansion: We have developed proprietary methods that enable the large-scale expansion of our cells while maintaining their ability to produce the key biomolecules associated with tissue health and repair. This allows us to produce a cellular product intended to demonstrate consistent and well-defined characterization and activity.
Products Commercialized by Licensees
Two allogeneic mesenchymal stromal cell (MSC) products developed and commercialized by Mesoblast licensees have been approved in Japan and Europe, with both licensees the first to receive full regulatory approval for an allogeneic cellular medicine in these major markets.
Mesoblast’s licensee in Japan, JCR Pharmaceuticals Co. Ltd. (“JCR”), is marketing its MSC-based product in Japan for the treatment of aGVHD in children and adults. TEMCELL® HS Inj. (“TEMCELL”) was the first allogeneic cellular medicine to receive full regulatory approval in Japan. Mesoblast receives royalty income on sales of TEMCELL® in Japan.
In 2017, Mesoblast granted TiGenix S.A.U (“TiGenix”), now a wholly owned subsidiary of Takeda Pharmaceutical Co. Ltd. (“Takeda”), exclusive access to certain of its patents to support global commercialization of Alofisel®, the first allogeneic MSC therapy to receive central marketing authorization approval from the European Commission. Mesoblast receives royalty income on Takeda’s worldwide sales of Alofisel® in the local treatment of perianal fistulae.
55
Mesoblast Product Candidates
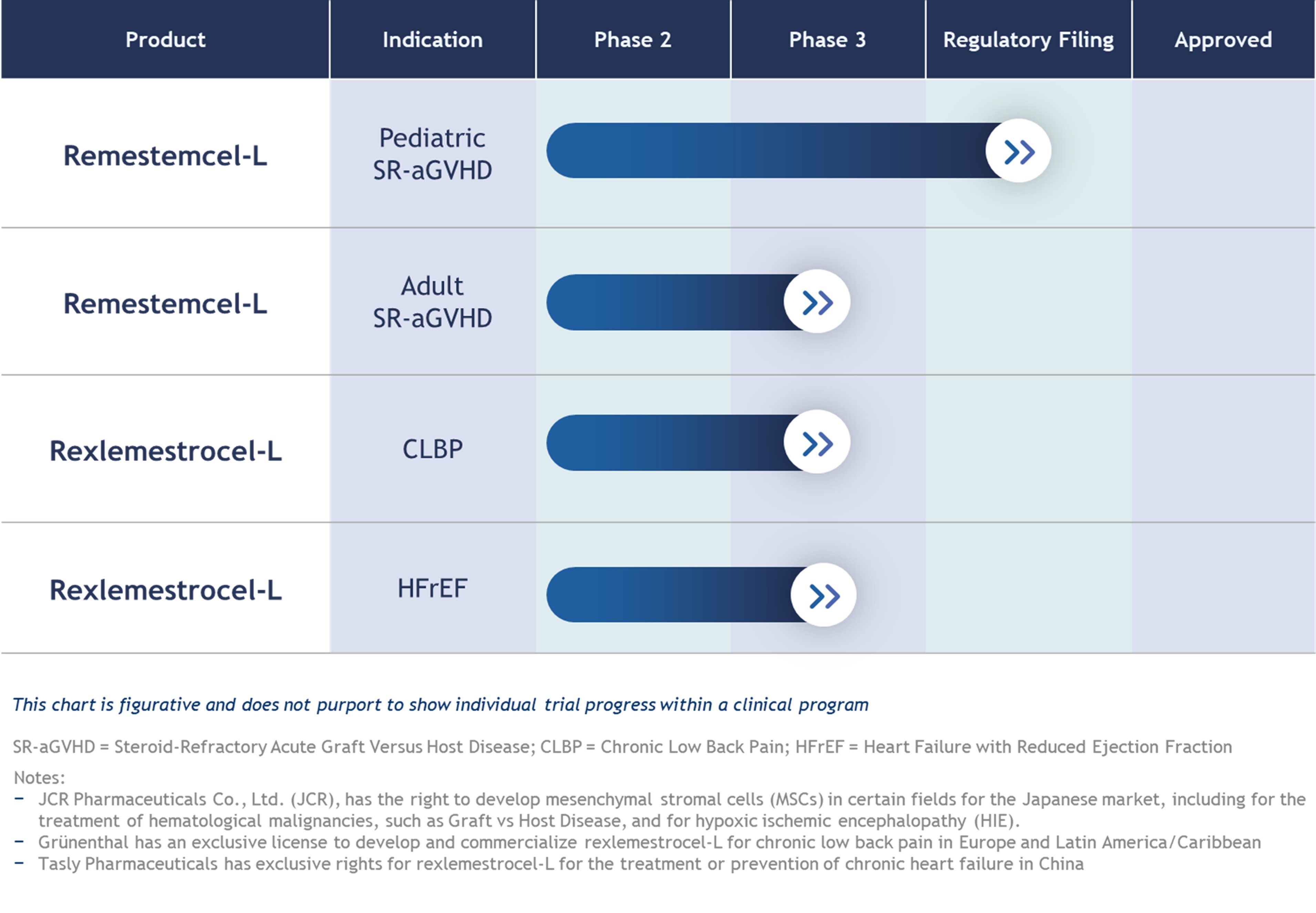
Remestemcel-L for the Treatment of Steroid Refractory Acute Graft Versus Host Disease
Overview
Remestemcel-L is an intravenously delivered product candidate for the treatment of steroid-refractory acute graft versus host disease, or SR-aGVHD, following an allogeneic bone marrow transplant (“BMT”).
In a bone marrow transplant, donor cells can attack the recipient, causing a-GVHD. The donor T-cell mediated inflammatory response involves secretion of TNF-alpha and IFN-gamma, resulting in activation of pro-inflammatory T-cells and tissue damage in the skin, gut and liver, which can be fatal.
Remestemcel-L is suggested to have immunomodulatory properties to counteract the cytokine storm that is implicated in various inflammatory conditions. The mechanism of action is thought to involve down-regulating the production of pro-inflammatory cytokines, increasing production of anti-inflammatory cytokines, and enabling recruitment of naturally occurring anti-inflammatory cells to involved tissues.
This life-threatening disease occurs in approximately 50% of patients who receive an allogeneic BMT. Over 30,000 patients worldwide undergo an allogeneic BMT annually, primarily during treatment for blood cancers, and these numbers are increasing. In patients with the most severe form of SR-aGVHD (Grade C/D or III/IV) mortality can be as high as 90% despite optimal best available therapy. There are currently no FDA-approved treatments in the United States for children under 12 with SR-aGVHD.
Current Status and Anticipated Milestones
Mesoblast submitted its completed BLA to the FDA for remestemcel-L in January 2020. The BLA was subsequently accepted for priority review by the FDA on March 30, 2020, with a Prescription Drug User Fee Act (“PDUFA”) action date set for September 30, 2020. In August 2020, the FDA’s Oncologic Drugs Advisory Committee
56
(“ODAC”) voted overwhelmingly in favor (nine to one(1)) that the available data support the efficacy of remestemcel-L in pediatric patients with SR-aGVHD. FDA issued a CRL on September 30, 2020, noting deficiencies related to clinical and Chemistry, Manufacturing and Controls (“CMC”) data.
Mesoblast has worked to address the issues noted in the Complete Response Letter, through multiple interactions with FDA for guidance. Mesoblast provided these new data to FDA to address all CMC outstanding items as required in January 2023. In March, 2023, the FDA accepted the BLA resubmission considering the resubmission to be a complete response and set a PDUFA goal date of August 2, 2023. In August 2023, the FDA provided a CRL to the BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD requiring more data to support marketing approval, including potency assay or clinical data.
There are currently no FDA-approved treatments in the US for children under 12 with SR-aGVHD and only one FDA-approved treatment in the US for other SR-aGVHD patients.
As part of the BLA review, FDA completed the PLI of the manufacturing facility, did not issue any Form 483, and found no objectionable conditions. In addition, FDA acknowledged in the resubmission review that changes implemented appear to improve assay performance relative to the original version of the assay used in the pediatric Phase 3 trial.
Mesoblast intends to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. This adult study is in line with our overall commercial strategy, which envisioned a sequenced progression from pediatric to adult SR-aGVHD indications. Adults comprise 80% of the SR-aGVHD market. Mesoblast will initially meet with the FDA to seek alignment on the trial design for the adult study at a Type A meeting scheduled in mid-September 2023.
We believe the U.S. adult and pediatric SR-aGVHD market requires a small, targeted commercial footprint. The target call point for SR-aGVHD will primarily be physicians in hematology/oncology who perform hematopoietic stem cell transplants. In the U.S., there are approximately 80 centers that perform pediatric transplants, with 50% of all transplants occurring at approximately 15 centers. Similarly, there are approximately 110 centers that perform adult transplants with half of those transplants occurring at approximately 20 centers.
The Company has put in place a lifecycle extension strategy to generate evidence-based clinical outcomes to maximize the value of remestemcel-L in other pediatric and adult rare diseases that do not require large distribution channels. In addition, we plan to expand investigator-initiated clinical trials for chronic GVHD and other indications that are currently underway or planned for the near future.
(1)This vote includes a change to the original vote by one of the ODAC panel members after electronic voting closed.
Remestemcel-L for Moderate to Severe Acute Respiratory Distress Syndrome due to COVID-19 Infection
Overview
COVID-19 ARDS results from a severe inflammatory reaction, referred to as a cytokine storm, to infection from the SARS CoV-2 virus. This cytokine storm can cause significant damage to the lungs and other organs and ARDS remains a major cause of mortality for COVID-19 patients who are immunocompromised, unvaccinated, or with comorbidities, as well as those with seasonal influenza and other pathogens.
The extensive safety data of remestemcel-L and its anti-inflammatory effects in acute GVHD is a compelling rationale for evaluating remestemcel-L in COVID-19 ARDS. Following intravenous delivery of remestemcel-L, the cells migrate to the areas of inflammation particularly in the lungs resulting in the potential for remestemcel-L to tame the cytokine storm in ARDS.
The clinical protocol evaluating remestemcel-L in patients in the Phase 2/3 trial was based on results from patients treated with remestemcel-L under an emergency IND/EAP compassionate use at Mount Sinai Hospital in New York. Twelve patients with moderate to severe COVID ARDS on mechanical ventilation were given 2 infusions within one week. Nine of the 12 patients (75%) were successfully taken off the ventilator and discharged from hospital within a median of 10 days. These pilot study results were published during the year in the peer-reviewed journal Cytotherapy.
The Phase 2/3 placebo-controlled trial, initiated in 2020, randomized 1:1 to either standard of care alone or standard of care plus two doses of remestemcel-L 2 million cells/kg 3-5 days apart in ventilator-dependent patients with moderate/severe ARDS due to COVID-19. The trial was halted in December 2020 after the Data Safety Monitoring Board
57
(DSMB) performed a third interim analysis on the trial’s first 180 patients, noting that the trial was not likely to meet the 30-day mortality reduction endpoint at the planned 300 patient enrolment. The trial was powered to achieve a primary endpoint of 43% reduction in mortality at 30 days for treatment with remestemcel-L on top of maximal care. The DSMB recommended that the trial complete with the enrolled 222 patients, and that all be followed-up as planned.
Current Status and Anticipated Milestones
Mesoblast has entered into a non-binding Memorandum of Understanding (MOU) with Vanderbilt University Medical Center, which coordinates and works closely with clinical investigators at over 40 sites across the United States focused on studying ARDS and other critical illnesses. The MOU proposes a collaboration toward the design and execution of a second COVID-19 trial for remestemcel-L; to jointly develop a trial protocol and seek FDA approval for the trial, the results from which Mesoblast may use to support regulatory filings (such as seeking Emergency Use Authorization from FDA); and to negotiate a written, cooperative agreement and proceeding with the trial upon receipt of FDA approval.
Remestemcel-L for Inflammatory Bowel Disease (IBD) – Ulcerative Colitis (UC) and Crohn’s Colitis
Overview
According to recent estimates, more than three million people (1.3%) in the United States alone have inflammatory bowel disease, with more than 33,000 new cases of Crohn’s disease and 38,000 new cases of ulcerative colitis diagnosed every year. Despite recent advances, approximately 30% of patients are primarily unresponsive to anti-TNFα agents and even among responders, up to 10% will lose their response to the drug every year. Up to 80% of patients with medically refractory Crohn’s disease eventually require surgical treatment of their disease, which can have a devastating impact on quality of life.
Current Status
A small investigator-initiated randomized, controlled study of remestemcel-L delivered by an endoscope directly to the areas of inflammation and tissue injury with medically refractory Crohn’s disease and ulcerative colitis commenced at Cleveland Clinic. The study is the first in humans using local cell delivery in the gut and will enable Mesoblast to compare clinical outcomes using this delivery method with results from an ongoing randomized, placebo-controlled trial in patients with biologic-refractory Crohn’s disease where remestemcel-L was administered intravenously. Results from the first patient cohort in the randomized, controlled study of remestemcel-L by direct endoscopic delivery to areas of inflammation in patients with medically refractory Crohn’s colitis were published in the peer-reviewed journal British Journal of Surgery.
Strategically, Mesoblast views UC and Crohn’s colitis as a potentially important label extension for remestemcel-L given the gastrointestinal involvement common to acute graft versus host disease and inflammatory bowel disease. Gastrointestinal damage is the major driver of aGVHD mortality and is linked to systemic inflammation in aGVHD. Biomarkers that predict high mortality in aGVHD, such as blood levels of soluble suppression of tumorigenicity 2 (ST2) have shown to be significantly reduced in patients treated with remestemcel-L. ST2 has also been shown to be associated with active IBD (UC & Crohn’s).
Rexlemestrocel-L for Chronic Low Back Pain (CLBP) associated with Degenerative Disc Disease (DDD)
Overview
Rexlemestrocel-L (MPC-06-ID) for CLBP consists of a unit dose of 6 million MPCs administered by syringe directly into a damaged disc.
In CLBP, damage to the disc is the result of a combination of factors related to aging, genetics, and micro-injuries, which compromises the disc’s capacity to act as a fluid-filled cushion between vertebrae and to provide anatomical stability. Damage to the disc also results in an inflammatory response with ingrowth of nerves which results in chronic pain. This combination of anatomic instability and nerve ingrowth results in CLBP and functional disability.
With respect to mechanisms of action in CLBP, extensive pre-clinical studies have established that MLCs have anti-inflammatory effects and secrete multiple paracrine factors that stimulate new proteoglycan and collagen synthesis by chondrocytes in vitro and by resident cells in the nucleus and annulus in vivo.
58
It is estimated that over 7 million people in the U.S. alone suffer from CLBP associated with DDD, of which 3.2 million patients have moderate disease. This market is projected to have annual growth rate similar to that of the US population annual growth rate. After failure of conservative measures (medication, injections, physical therapy etc.), there is a need for non-opioid treatments that are effective over a sustained period of time. When disc degeneration has progressed to a point that pain and loss of function can no longer be managed by conservative means, major invasive surgery such as spinal fusion is the most commonly offered option.
All non-surgical therapies for progressive, severe and debilitating pain due to degenerating intervertebral discs treat the symptoms of the disease. However, they do not address the underlying cause of the disease. Surgical intervention is not always successful in addressing the patient’s pain and functional deficit. It has been estimated that the incidence of failed back surgery is as high as 50% for standard procedures and may increase for more complex surgeries. Total costs of low back pain are estimated to be between $100.0 billion and $200.0 billion annually with two thirds attributed to patients’ decreased wages and productivity.
As a result, we believe that the most significant unmet need and commercial opportunity in the treatment of CLBP is a therapy that has the ability to impact the chronic pain and disability associated with the condition.
Current Status and Anticipated Milestones
The Phase 3 clinical trial for CLBP completed enrollment in March 2018 with 404 patients enrolled across 48 centers in the United States and Australia randomized 1:1:1 to receive either 6 million MPCs with hyaluronic acid (MPC+HA), 6 million MPCs without hyaluronic acid (MPC) or saline control. Although the trial's composite outcomes of pain reduction together with functional responses to treatment were not met by either MPC group; the MPC+HA treatment group achieved substantial and durable reductions in pain compared to control through 24 months across the entire evaluable study population (n=391) compared with saline controls. Greatest pain reduction was observed in the pre-specified population with CLBP of shorter duration than the study median of 68 months (n=194) and subjects using opioids at baseline (n=168) with the MPC+HA group having substantially greater reduction at all time points (1, 3, 6, 12, 18 and 24 months) compared with saline controls. There was no appreciable difference in the safety of MPC groups compared to saline control over the 24-month period of follow-up in the entire study population. In subjects using opioids at baseline, the MPC+HA demonstrated a reduction in the average opioid dose over 24 months, while saline control subjects had essentially no change.
Mesoblast has received feedback from FDA on the Phase 3 program for CLBP and plans to conduct an additional US Phase 3 trial which may support submissions for potential approval in both the US and EU. Following review of the completed Phase 3 trial data, FDA agreed with Mesoblast’s proposal for pain reduction at 12 months as the primary endpoint of the next trial, with functional improvement and reduction in opioid use as secondary endpoints.
In February 2023, FDA granted Regenerative Medicine Advanced Therapy ("RMAT") designation for rexlemestrocel-L in the treatment of CLBP associated with disc degeneration, in combination with HA as delivery agent for injection into the lumbar disc. RMAT designations aim to expedite the development of regenerative medicine therapies intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition where preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for the disease or condition. An RMAT designation for rexlemestrocel-L provides all the benefits of Breakthrough and Fast Track designations, including rolling review and eligibility for priority review on filing of a BLA.
Rexlemestrocel-L for Chronic Heart Failure
Overview
Mesoblast is developing rexlemestrocel-L to fill the treatment gap for chronic heart failure (CHF). Patients with CHF continue to represent high unmet medical need despite recent advances in new therapeutic agents for chronic heart failure. The American Heart Association (AHA) estimated in 2017 that prevalence is expected to grow 46% by 2030 in the U.S., affecting more than 8 million Americans. CHF causes severe economic, social, and personal costs. In the U.S., it is estimated that CHF results in direct costs of $60.2 billion annually when identified as a primary diagnosis and $115.0 billion as part of a disease milieu. Mesoblast believes that targeting high-risk chronic patients with the highest unmet clinical needs provides the company with the most efficient path to market.
Rexlemestrocel-L for HFrEF consists of 150 million MPCs administered by direct cardiac. MPCs release a range of factors when triggered by specific receptor-ligand interactions within damaged tissue. Based on preclinical data, we
59
believe that the factors released from the MPCs induce functional cardiac recovery by simultaneous activation of multiple pathways, including induction of endogenous vascular network formation, reduction in harmful inflammation, reduction in cardiac fibrosis, and reversal of endothelial dysfunction through activation of intrinsic tissue precursors.
CHF is classified in relation to the severity of the symptoms experienced by the patient. The most commonly used classification system for functional severity of heart failure, established by the NYHA, is:
•Class I (mild): patients experience none or very mild symptoms with ordinary physical activity
•Class II (mild/moderate): patients experience fatigue and shortness of breath during moderate physical activity
•Class III (moderate/severe): patients experience shortness of breath during even light physical activity
•Class IV or end-stage (severe): patients are exhausted even at rest
Risk for recurrent heart failure-related hospitalizations, occurrence of non-fatal myocardial infarction (MI, heart attack) or non-fatal stroke, or death increases progressively with increases in left ventricular volumes, reduction in left ventricular ejection fraction (LVEF), and progression in NYHA functional class. Approximately 50% of all CHF patients have heart failure with reduced ejection fraction (HFrEF) defined as LVEF <40%, and are at considerable risk of repeated hospitalizations and death despite maximal drug therapy.
Program for Class II/III CHF patients
A multicenter, double-blinded, 1:1 randomized, sham-procedure-controlled Phase 3 study of remestemcel-L was completed across North America with 565 NYHA Class II/III patients at high risk of repeated heart failure hospitalizations or a terminal cardiac event (cardiac death, LVAD placement, heart transplant or insertion of an artificial heart). The enrollment criteria for this trial included a prior decompensated heart failure event (e.g. hospitalization) within the previous nine months and/or very high level of NT-proBNP, a protein used in diagnosis and screening of CHF. These inclusion criteria were designed for enrichment in patients with substantial left ventricular contractile abnormality, advanced CHF due to left ventricular systolic dysfunction and higher risk of recurrent decompensated heart failure hospitalizations and TCEs. This target patient population was shown to respond effectively to treatment with rexlemestrocel-L in our previous Phase 2 trial.
Topline results from the 537 patients who met the criteria which allowed for treatment to occur on a 1:1 randomization basis between rexlemestrocel-L and sham control were announced in December 2021. Over a mean 30 months of follow-up, patients with advanced chronic heart failure who received a single endomyocardial treatment with rexlemestrocel-L on top of maximal therapies had 60% reduction in incidence of heart attacks or strokes and 60% reduction in death from cardiac causes when treated at an earlier stage in the progressive disease process. Despite significant reduction in the pre-specified endpoint of cardiac death, there was no reduction in study primary end point of recurrent non-fatal decompensated heart failure events, which was the trial’s primary endpoint.
The combination of the three pre-specified outcomes of cardiac death, heart attack or stroke into a single composite outcome - called the three-point major adverse cardiovascular event (MACE) is a well-established endpoint used by the FDA to determine cardiovascular risk. Rexlemestrocel-L reduced this three-point MACE by 30% compared to controls across the population of 537 patients. In the NYHA class II subgroup of 206 patients, rexlemestrocel-L reduced the three-point MACE by 55% compared to controls.
DREAM-HF Phase 3 trial results were published in the premier peer-reviewed journal for cardiovascular medicine, the Journal of the American College of Cardiology (JACC) in February 2023.
Program in End Stage Heart Failure Patients Requiring Mechanical Support
Rexlemestrocel-L is also being evaluated in patients with end-stage HFrEF implanted with a left ventricular assist device (“LVAD”).
A Phase 2 trial was conducted by a multi-center team of researchers within the United States National Institutes of Health (“NIH”)-funded Cardiothoracic Surgical Trials Network (“CTSN”), led by Icahn School of Medicine at Mount Sinai, New York. The National Institute of Neurological Disorders and Stroke, and the Canadian Institutes for Health Research also supported this trial. Results of this Phase 2 trial were released in November 2018. The trial was a
60
prospective, multi-center, double-blind, placebo controlled, 2:1 randomized (MPC to placebo), single-dose cohort trial to evaluate the safety and efficacy of injecting a dose of 150 million MPCs into the native myocardium of LVAD recipients. Patients with advanced CHF, implanted with an FDA-approved LVAD as bridge-to-transplant or destination therapy, were eligible to participate in the trial. All patients were followed until 12 months post randomization.
In this Phase 2 trial, the trial did not show a significant difference in the ability for patients to tolerate a wean for a period of 60 minutes. However, in relation to the clinically meaningful endpoint of reduction in major GI bleeding episodes and related hospitalizations, a single injection of rexlemestrocel-L administered directly into the heart resulted in a 76% reduction in major GI bleeding events and in a 65% reduction in associated hospitalizations. This suggests that rexlemestrocel-L reversed endothelial dysfunction which is responsible for the abnormal vasculature in the GI tract and severe bleeding in LVAD patients.
Current Status and Anticipated Milestones
Recently Mesoblast reported that treatment with rexlemestrocel-L resulted in greater improvement in the pre-specified analysis of left ventricular ejection fraction (LVEF) at 12 months relative to controls after a single intervention in the Phase 3 trial in NYHA class II/III chronic heart failure. Improvement in LVEF was most pronounced in the setting of inflammation and preceded long-term reduction in the 3-point MACE of cardiovascular death, non-fatal heart attack or stroke. Effects on LVEF and MACE outcomes were even more pronounced in 301 HFrEF patients with high baseline levels of inflammation as measured by hsCRP. LVEF improvement at 12 months may be an appropriate early surrogate endpoint for long-term reduction in MACE.
Results from three randomized controlled trials in class II/III HFrEF and in end-stage HFrEF with LVADs support the idea of a common MOA by which rexlemestrocel-L reverses inflammation-related endothelial dysfunction and reduces adverse clinical outcomes across the spectrum of HFrEF patients.
Rexlemestrocel-L has regenerative medicine advanced therapy (RMAT) designation from the FDA for treatment of chronic heart failure with left ventricular systolic dysfunction in patients with an LVAD. Mesoblast now intends to meet with FDA under the RMAT framework to discuss the totality of the data and the evidence of a common rexlemestrocel-L MOA across the broader HFrEF spectrum.
Complementary Technologies
In addition to having the most mature and diverse allogeneic cell therapy product pipeline and technology platform in the field of cellular medicines, we have strategically targeted the acquisition of rights to technologies that are complementary to and synergistic with our mesenchymal lineage cell technology platform. The aim of this activity is to maintain our technology leadership position in the regenerative medicine space, while simultaneously expanding our targeted disease applications and managing the life-cycle of our current lead programs.
Our complementary technologies and additional product candidates include other types of mesenchymal lineage cells, cell surface modification technologies, pay-loading technology and protein and gene technologies.
Manufacturing and Supply Chain
Our manufacturing strategy for our cellular product candidates focuses on the following important factors:
(i)ability for product delineation to protect pricing and partner markets by creating distinct products using discrete manufacturing processes, culture conditions, formulations, routes of administration, and/or dose regimens;
(ii)establishing proprietary commercial scale-up and supply to meet increasing demand;
(iii)implementing efficiencies and yield improvement measures to reduce cost-of-goods;
(iv)maintaining regulatory compliance with best practices; and
(v)establishing and maintaining multiple manufacturing sites for product supply risk mitigation.
61
The cell therapy manufacturing and distribution process generally involves five major steps.
•Procure bone marrow—acquire bone marrow from healthy adults with specific FDA-defined criteria, which is accompanied by significant laboratory testing to establish the usability of the donated tissues.
•Create master cell banks—isolate MLCs from the donated bone marrow and perform a preliminary expansion to create master cell banks. Each individual master cell bank comes from a single donor.
•Expand to therapeutic quantities—expand master cell banks to produce therapeutic quantities, a process that can yield thousands of doses per master cell bank, with the ultimate number depending on the dose for the respective product candidate being produced.
•Formulate, package and cryopreserve.
•Distribute—our cellular products are cryopreserved at the manufacturer and shipped to storage sites in the U.S. and other jurisdictions via cryoshippers. Those distribution centers then re-package and send the products on to treatment centers in cryoshippers. Treatment centers will either move the products into their own freezers or receive the cryoshipper in “real time” and the product stays in the cryoshipper until thawed for patient use within a well-defined window. We intend to continue utilizing this approach in the future.
To date our product candidates have been manufactured in two-dimensional, or 2D, planar, 10-layer cell factories, using media containing fetal bovine serum, or FBS.
The relatively small patient numbers and orphan drug designation for remestemcel-L lead us to believe that 2D manufacturing will be adequate to meet demand for this product candidate if fully approved. We also believe that 2D manufacturing process and facilities are commercially feasible for Phase 3 trial supply and the initial launch of MPC-06-ID for CLBP.
However, to build up commercial supply for certain of our product candidates long-term, we are developing novel manufacturing processes using three-dimensional, or 3D, bioreactors with greater capacity to improve efficiency and yields, with resulting lower-cost of goods. We intend to evaluate products produced in 3D bioreactors in pre-clinical and potentially clinical studies, which may serve as FDA required comparability studies to 2D if successful.
We are also focusing on the introduction of FBS-free media which has the potential to result in efficiency and yield improvements to the current 2D process. We intend to conduct comparability studies to illustrate that products produced with this media are equivalent to those produced using FBS based media. While we remain confident in our ability to deliver successful outcomes from each of these activities, any unexpected issues or challenges faced in doing so could delay our programs or prevent us from continuing our programs.
Our manufacturing activities to date have met stringent criteria set by international regulatory agencies, including the FDA. By using well-characterized cell populations, our manufacturing processes promote reproducibility and batch-to-batch consistency for our allogeneic cell product candidates. We have developed robust quality assurance procedures and lot release assays to support this reproducibility and consistency.
Intellectual Property
We have a large patent portfolio of issued and pending claims covering compositions of matter, uses for our mesenchymal lineage cell-based technologies and other proprietary regenerative product candidates and technologies, as well as for elements of our manufacturing processes. As of July 2023, the patent portfolio comprises approximately 1,035 patents and patent applications across 54 patent families, with protection extending through to at least 2042 in all major markets.
One of our major objectives is to continue to protect and expand our extensive estate of patent rights and trade secrets, which we believe enables us to deliver commercial advantages and long-term protection for our product candidates based on our proprietary technologies, and support our corporate strategy to target large, mature and emerging healthcare markets for our exploratory therapeutic product candidates.
More specifically, our patent estate includes issued patent and patent applications in major markets, including, but not limited to, the United States, Europe, Japan and China. The patents that we have obtained, and continue to apply for,
62
cover mesenchymal lineage cell technologies and product candidates derived from these technologies, irrespective of the tissue source, including bone marrow, adipose, placenta, umbilical cord and dental pulp.
These patents cover, among other technology areas, a variety of MLCs (including MPCs and MSCs), and the use of MLC for expansion of hematopoietic stem cells, or HSCs. Among the indication-specific issued or pending patents covering product candidates derived from our mesenchymal lineage cells are those which are directed to our lead product candidates: aGVHD, ARDS, CLBP, CHF and chronic inflammatory conditions such as RA. We also have issued and pending patents covering other pipeline indications, including diabetic kidney disease, inflammatory bowel disease (e.g., Crohn’s disease), neurologic diseases, eye diseases and additional orthopedic diseases. In addition, we have in-licensed patents covering complementary technologies, such as other types of mesenchymal lineage cells, cell surface modification technologies, pay-loading technology and protein and gene technologies, as part of our strategy to expand our targeted disease applications and manage the life-cycle of our current lead programs.
Our patent portfolio also includes issued and pending coverage of proprietary manufacturing processes that are being used with our current two-dimensional manufacturing platform as well as the 3D bioreactor manufacturing processes currently under development. These cell manufacturing patents cover isolation, expansion, purification, scale up, culture conditions, aggregates minimization, cryopreservation, release testing and potency assays. In addition, we maintain as a trade secret, among other things, our proprietary FBS-free media used in our 3D bioreactor manufacturing processes.
We maintain trade secrets covering a significant body of know-how and proprietary information relating to our core product candidates and technologies. We protect our confidential know-how and trade secrets in a number of ways, including requiring all employees and third parties that have access to our confidential information to sign non-disclosure agreements, limiting access to confidential information on a need-to-know basis, maintaining our confidential information on secure computers, and providing our contract manufacturers with certain key ingredients for our manufacturing process.
In addition, in many major jurisdictions there are other means that may be available to us by which we would be able to extend the period during which we have commercial exclusivity for our product candidates, which include, but are not limited to the exclusive right to reference our data, orphan drug exclusivity and patent term extensions.
As part of our strategy, we seek patent protection for our product candidates and technologies in major jurisdictions including the United States, Europe, Japan, China, and Australia and file independent and/or counterpart patents and patent applications in other jurisdictions globally that we deem appropriate under the circumstances, including India, Canada, Hong Kong, Israel, Korea and Singapore. As of July 2023, our patent portfolio includes the following patents and patent applications in the following major jurisdictions: 53 granted U.S. patents and 41 pending U.S. patent applications; 57 granted Japanese patents and 34 pending Japanese patent applications; 33 granted Chinese patents and 28 pending Chinese patent applications; 42 granted European patents and 32 pending European patent applications; and 50 granted Australian patents and 28 pending Australian patent applications.
Our policy is to patent the technology, inventions and improvements that we consider important to the development of our business, only in those cases in which we believe that the costs of obtaining patent protection is justified by the commercial potential of the technology and associated product candidates, and typically only in those jurisdictions that we believe present significant commercial opportunities to us. In those cases where we choose neither to seek patent protection nor protect the inventions as trade secrets, we may publish the inventions so that it defensively becomes prior art in order for us to secure a freedom to operate position and to prevent third parties from patenting the invention.
We also seek to protect as trade secrets our proprietary and confidential know-how and technologies that are either not patentable or where we deem it inadvisable to seek patent protection. To this end, we generally require all third parties with whom we share confidential information and our employees, consultants and advisors to enter into confidentiality agreements prohibiting the disclosure of confidential information. These agreements with our employees and consultants engaged in the development of our technologies require disclosure and assignment to us of the ideas, developments, discoveries and inventions, and associated intellectual property rights, important to our business. Additionally, these confidentiality agreements, among others, require that our employees, consultants and advisors do not bring to us, or use without proper authorization, any third party’s proprietary technology.
63
License and Collaboration Agreements
All of our revenue relates to upfront, royalty and milestone payments recognized under the license and collaboration agreements below. For further information on the categorical revenue breakdown during the last three fiscal years, see “Item 18. Financial Statements – Note 3”.
Grünenthal arrangement
In September 2019, Mesoblast entered into a strategic partnership with Grünenthal GmbH (Grünenthal) to develop and commercialize MPC-06-ID, the Company’s Phase 3 allogeneic cell therapy candidate for the treatment of chronic low back pain due to degenerative disc disease in patients who have exhausted conservative treatment options. The agreement was amended by the parties in June 2021. Under the partnership, Grünenthal will have exclusive commercialization rights to MPC-06-ID for Europe and Latin America. Mesoblast may receive up to $112.5 million in upfront and milestone payments prior to product launch, inclusive of $17.5 million already received, if certain clinical and regulatory milestones are satisfied and reimbursement targets are achieved. Cumulative milestone payments could exceed $1.0 billion depending on the final outcome of Phase 3 studies and patient adoption. Mesoblast will also receive tiered double-digit royalties on product sales. There cannot be any assurance as to the total amount of future milestone and royalty payments that Mesoblast will receive nor when they will be received.
JCR Pharmaceuticals Co., Ltd.—Hematological Malignancies and Hepatocytes Collaboration in Japan
In October 2013, we acquired all of Osiris Therapeutics, Inc.’s business and assets related to culture expanded MSCs. These assets included assumption of a collaboration agreement with JCR (“JCR Agreement”), which will continue in existence until the later of 15 years from the first commercial sale of any product covered by the agreement and expiration of the last Osiris patent covering any such product. JCR is a research and development oriented pharmaceutical company in Japan. Under the JCR Agreement we assumed from Osiris, JCR has the right to develop our MSCs in two fields for the Japanese market: exclusive in conjunction with the treatment of hematological malignancies by the use of HSCs derived from peripheral blood, cord blood or bone marrow, or the First JCR Field; and non-exclusive for developing assays that use liver cells for non-clinical drug screening and evaluation, or the Second JCR Field. Under the JCR Agreement, JCR obtained rights in Japan to our MSCs, for the treatment of aGVHD. JCR also has a right of first negotiation to obtain rights to commercialize MSC-based products for additional orphan designations in Japan. We retain all rights to those products outside of Japan.
JCR received full approval in September 2015 for its MSC-based product for the treatment of children and adults with aGVHD, TEMCELL. TEMCELL is the first culture-expanded allogeneic cell therapy product to be approved in Japan. It was launched in Japan in February 2016.
Under the JCR Agreement, JCR is responsible for all development and manufacturing costs including sales and marketing expenses. With respect to the First JCR Field, we have received all sales milestone payments, a total of $3.0 million. Ongoing we are entitled to escalating double-digit royalties in the twenties. These royalties are subject to possible renegotiation downward in the event of competition from non-infringing products in Japan. With respect to the Second JCR Field, we are entitled to a double digit profit share in the fifties.
Intellectual property is licensed both ways under the JCR Agreement, with JCR receiving exclusive and non-exclusive rights as described above from us and granting us non-exclusive, royalty-free rights (excluding in the First JCR Field and Second JCR Field in Japan) under the intellectual property arising out of JCR’s development or commercialization of MSC-based products licensed in Japan.
JCR has the right to terminate the JCR Agreement for any reason, and we have a limited right to terminate the JCR Agreement, including a right to terminate in the event of an uncured material breach by JCR. In the event of a termination of the JCR Agreement other than for our breach, JCR must provide us with its owned product registrations and technical data related to MSC-based products licensed in Japan and all licenses of our intellectual property rights will revert to us.
We have expanded our partnership with JCR in Japan for two new indications: for wound healing in patients with EB in October 2018, and for neonatal HIE, a condition suffered by newborns who lack sufficient blood supply and oxygen to the brain, in June 2019. We will receive royalties on TEMCELL product sales for EB and HIE, if and when such indications receive marketing approval in Japan.
64
We have the right to use all safety and efficacy data generated by JCR in Japan to support our development and commercialization plans for our MSC product candidate remestemcel-L in the United States and other major healthcare markets, including for GVHD, EB and HIE.
Lonza—Manufacturing Collaboration
In September 2011, we entered into a manufacturing services agreement, or MSA, with Lonza Walkersville, Inc. and Lonza Bioscience Singapore Pte. Ltd., collectively referred to as Lonza, a global leader in biopharmaceutical manufacturing. Under the MSA, we pay Lonza on a fee for service basis to provide us with manufacturing process development capabilities for our product candidates, including formulation development, establishment and maintenance of master cell banks, records preparation, process validation, manufacturing and other services.
We have agreed to order a certain percentage of our clinical requirements and commercial requirements for MPC products from Lonza. Lonza has agreed not to manufacture or supply commercially biosimilar versions of any of our product candidates to any third party, during the term of the MSA, subject to our meeting certain thresholds for sales of our products.
We can trigger a process requiring Lonza to construct a purpose-built manufacturing facility exclusively for our product candidates. In return if we exercise this option, we will purchase agreed quantities of our product candidates from this facility. We also have a right to buy out this manufacturing facility at a pre-agreed price two years after the facility receives regulatory approval.
The MSA will expire on the three-year anniversary of the date of the first commercial sale of product supplied under the MSA, unless it is terminated earlier. We have the option of extending the MSA for an additional 10 years, followed by the option to extend for successive three-year periods subject to Lonza’s reasonable consent. We may terminate the MSA with two years prior written notice, and Lonza may terminate with five years prior written notice. The MSA may also terminate for other reasons, including if the manufacture or development of a product is suspended or abandoned due to the results of clinical trials or guidance from a regulatory authority. In the event we request that Lonza construct the manufacturing facility described above, neither we nor Lonza may terminate before the third anniversary of the date the facility receives regulatory approval to manufacture our product candidates, except in certain limited circumstances. Upon expiration or termination of the MSA, we have the right to require Lonza to transfer certain technologies and lease the Singapore facility or the portion of such facility where our product candidates are manufactured, subject to good faith negotiations.
We currently rely, and expect to continue to rely, on Lonza for the manufacture of our product candidates for preclinical and clinical testing, as well as for commercial manufacture of our product candidates if marketing approval is obtained.
In October 2019, we entered into an agreement with Lonza for commercial manufacture of remestemcel-L for pediatric SR-aGVHD. This agreement will facilitate inventory build ahead of the planned US market launch of remestemcel-L and commercial supply to meet Mesoblast’s long-term market projections. The agreement provides for Lonza to expand its Singapore cGMP facilities if required to meet long-term growth and capacity needs for the product. Additionally, it anticipates introduction of new technologies and process improvements which are expected to result in significant increases in yields and efficiencies.
Singapore Economic Development Board (EDB)—Singapore Operations
In 2014, the Economic Development Board of Singapore, or EDB, granted us certain financial incentives tied to revenues generated by our Singapore operations, among other things. The incentive for manufacturing activities is for a 15-year period (broken into five-year increments). We will be eligible for this incentive if we meet certain investment or activity thresholds in Singapore, including employment levels, amounts of business or manufacturing related expenses.
For example, in order to obtain full financial benefits from the EDB for our manufacturing-related incentives, we must manufacture at least 50% of the global volume of our first three commercial products in Singapore (subject to certain exceptions), and we would be required to construct and operate a manufacturing facility in Singapore, and hire and maintain a specified number of professionals (including supply chain personnel) in connection with the operation of that facility. The activities under our MSA with Lonza could be used to fulfill all or part of the requirements to obtain the EDB financial incentives.
65
Central Adelaide Local Health Network Incorporated—Mesenchymal Precursor Cell Intellectual Property
In October 2004, we, through our wholly-owned subsidiary, Angioblast Systems Inc., now Mesoblast, Inc., acquired certain intellectual property relating to our MPCs, or Medvet IP, pursuant to an Intellectual Property Assignment Deed, or IP Deed, with Medvet Science Pty Ltd, or Medvet. Medvet’s rights under the IP Deed were transferred to Central Adelaide Local Health Network Incorporated, or CALHNI, in November 2011. In connection with our use of the Medvet IP, we are obligated to pay CALHNI, as successor in interest to Medvet, (i) certain aggregated milestone payments of up to $2.2 million and single-digit royalties on net sales of products covered by the Medvet IP, for cardiac muscle and blood vessel applications and bone and cartilage regeneration and repair applications, subject to minimum annual royalties beginning in the first year of commercial sale of those products and (ii) and single-digit royalties on net sales of the specified products for applications outside the specified fields. Additionally, we are obligated to pay CALHNI a double-digit percentage in the teens of any revenue that we receive in exchange for a grant of a sublicense to the Medvet IP in the specified fields. Under the IP Deed, we also granted to Medvet a non-exclusive, royalty-free license to the Medvet IP for non-commercial, internal research and academic research.
Pursuant to the IP Deed, we were assigned the rights in three U.S. patents or patent applications (including all substitutions, continuations, continuations-in-part, divisional, supplementary protection certificates, renewals, all letters patent granted thereon, and all reissues, reexaminations, extensions, confirmations, revalidations, registrations and patents of addition and foreign equivalents thereof) and all future intellectual property rights, including improvements, that might arise from research conducted at CALHNI related to MPCs and methods of isolating, culturing and expanding MPCs and their use in any therapeutic area. We also acquired all related materials, information and know-how.
Osiris Acquisition—Continuing Obligations
In October 2013, we and Osiris entered into a purchase agreement, as amended, or the Osiris Purchase Agreement, under which we acquired all of Osiris’ business and assets related to culture expanded MSCs. Pursuant to the Osiris Purchase Agreement, we also agreed to make certain milestone and royalty payments to Osiris pertaining to remestemcel-L for the treatment of aGVHD and Crohn’s disease. Each milestone payment is for a fixed dollar amount and may be paid in cash or our ordinary shares or ADSs, at our option. The maximum amount of future milestone payments we may be required to make to Osiris is $40.0 million. Any ordinary shares or ADSs we issue as consideration for a milestone payment will be subject to a contractual one year holding period, which may be waived in our discretion. In the event that the price of our ordinary shares or ADSs decreases between the issue date and the expiration of any applicable holding period, we will be required to make an additional payment to Osiris equal to the reduction in the share price multiplied by the amount of issued shares under that milestone payment. This additional payment can be made either wholly in cash or 50% in cash and 50% in our ordinary shares, in our discretion. We have also agreed to pay varying earnout amounts as a percentage of annual net sales of acquired products, ranging from low single-digit to 10% of annual sales in excess of $750.0 million. These royalty payments will cease after the earlier of a ten year commercial sales period and the first sale of a relevant competing product. The first royalty payments were made in 2016.
Tasly Pharmaceutical Group — Cardiovascular Alliance for China
In July 2018, we entered into a Development and Commercialization Agreement with Tasly.
The Development and Commercialization Agreement provides Tasly with exclusive rights to develop, manufacture and commercialize REVASCOR in China for the treatment or prevention of CHF and MPC-25-IC for the treatment or prevention of AMI. Tasly will fund all development, manufacturing and commercialization activities in China for REVASCOR and MPC-25-IC. On closing, we received a $20.0 million upfront technology access fee. Further, we will receive $25.0 million upon product regulatory approvals in China. Mesoblast will receive double-digit escalating royalties on net product sales. Mesoblast is eligible to receive six escalating milestone payments upon the product candidates reaching certain sales thresholds in China.
Tasly can terminate the Development and Commercialization Agreement with a specified amount of notice, on the later of (a) third anniversary of the agreement coming into effect and (b) receipt of marketing approval in China for each of REVASCOR or MPC-25-IC. Mesoblast has termination rights with respect to certain patent challenges by Tasly and if certain competing activities are undertaken by Tasly. Either party may terminate the agreement on material breach of the agreement if such breach is not cured within the specified cure period or if certain events related to bankruptcy of the other party occur.
66
TiGenix NV – patent license for treatment of fistulae
In December 2017, we entered into a Patent License Agreement with TiGenix, now a wholly owned subsidiary of Takeda, which granted Takeda exclusive access to certain of our patents to support global commercialization of the adipose-derived MSC product Alofisel®, previously known as Cx601, a product candidate of Takeda, for the local treatment of fistulae. The agreement includes the right for Takeda to grant sub-licenses to affiliates and third parties.
As part of the agreement, we received $5.9 million (€5.0 million) before withholding tax as a non-refundable upfront payment and a further payment of $5.9 million (€5.0 million) before withholding tax 12 months after the patent license agreement date. We are entitled to further payments of up to €10.0 million when Takeda reaches certain product regulatory milestones. Additionally, we receive single digit royalties on net sales of Alofisel®.
The agreement will continue in full force in each country (other than the United States) until the date upon which the last issued claim of any licensed patent covering Alofisel® expires in such country (currently expected to be 2029) or, with respect to the United States, until the later of (i) the date upon which the last issued claim of any licensed patent covering Alofisel® in the United States expires (currently expected to be around 2031) or (ii) the expiration of the regulatory exclusivity period in the United States with an agreed maximum term.
Either we or Takeda may terminate the agreement for any material breach that is not cured within 90 days after notice thereof. We also have the right to terminate the agreement, with a written notice in the event that Takeda file a petition in bankruptcy or insolvency or Takeda makes an assignment of substantially all of its assets for the benefit of its creditors.
Takeda have the right to terminate their obligation to pay royalties for net sales in a specific country if it is of the opinion that there is no issued claim of any licensed patent covering Alofisel® in such country, subject to referral of the matter to the joint oversight/cooperation committee established under the agreement if we disagree.
Competition
The biotechnology and pharmaceutical industries are highly competitive and are characterized by rapidly advancing technologies and a strong emphasis on proprietary products. Any product candidates that we and our collaborators successfully develop and commercialize will compete with existing products and new products that may become available in the future.
A number of our potential competitors, particularly large biopharmaceutical companies, have significantly greater financial resources and general expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Our market has been characterized by significant consolidation by pharmaceutical and biotechnology companies, which is likely to result in even more resources being concentrated among a smaller number of our potential competitors.
Government Regulation
We are developing cellular therapy product candidates. These products are subject to extensive legislation. Governmental authorities around the world, including the FDA, are charged with the administration and enforcement of numerous laws and regulations that impact all aspects of the development, production, importing, testing, approval, labeling, promotion, advertising, and sale of products such as ours. Such governmental authorities are also charged with administering what is often a lengthy and technical review and approval process before candidate therapies such as ours may be marketed for any use. Authorization or approval for marketing must generally be obtained from the local health authorities in each country in which the product is to be sold. Approval and authorization procedures may differ from country to country, as may the requirements for maintaining approvals. It is typical however for these procedures to require evidence of rigorous testing and documentation regarding the candidate therapy, which may include significant non-clinical and clinical evaluations. Extensive controls and requirements apply to the non-clinical and clinical development of our therapeutic candidates. Those requirements and their enforcement and implementation by local regulatory authorities around the world significantly impact whether a product candidate can be developed into a marketable product, and notably impact the cost, resources and timing for any such development. Changes in regulatory requirements and differences in requirements from country to country may also increase the costs of bringing new technologies such as ours to market and maintaining approvals, if obtained.
67
To obtain marketing approval of a new product, an extensive dossier of evidence establishing the safety, efficacy and quality of the product must be submitted for review by regulatory authorities. Dossier form and substance, while often similar may have notable differences in different countries. Submission of an application to regulators does not guarantee approval to market that product, despite the fact that criteria for approval in many countries may be quite similar. Some regulatory authorities may require additional data and analyses, and may have standards that apply that are more stringent than others for review of the submitted dossier and content. Additionally, the review process, risk tolerance, and openness to new technologies may vary from country to country.
Obtaining marketing approval can take several months to several years, depending on the country, the quality of the data, the efficiencies and procedures of the reviewing regulatory authority and their familiarity with the product technology. Some countries, like the US, may have accelerated approval processes for certain categories of products, for example products which represent a breakthrough in the field, or which meet certain thresholds and have obtained certain designations of particular interest. Nevertheless, ultimate availability to patients may be affected, even post approval, by requirements in some countries to negotiate selling prices and reimbursement terms with government regulators or other payors.
Maintaining marketing approval may require the conduct of additional post-approval studies in some situations, and the continued capture, monitoring and assessment of safety and other information about the product, as well as adherence to requirements to ensure the purity and integrity of manufactured product. The process for obtaining and maintaining regulatory authorizations and approvals to market our products and the subsequent compliance with appropriate federal, state, local and foreign laws and regulations require the expenditure of substantial time and the commitment of significant financial and other resources, and we may not be able to obtain the required regulatory approvals.
Product Development Process
All of our product candidates are regulated as biological products by the Center for Biologics Evaluation and Research in the FDA. In the United States, biological products are subject to federal regulation under the Federal Food, Drug, and Cosmetic Act (“FDCA”), the Public Health Service (“PHS”) Act, and other federal, state, local and foreign statutes and regulations. Both the FDCA and the PHS Act, as applicable, and their corresponding regulations govern, among other things, the testing, manufacturing, safety, efficacy, labeling, packaging, storage, record keeping, distribution, import, export, reporting, advertising and other promotional practices involving drugs and biological products. Before clinical testing of a new drug or biological product may commence, the sponsor of the clinical study must submit an application for investigational new drug (“IND”) application to FDA, which must include, among other information, the proposed clinical study protocol(s). To obtain marketing authorization once clinical testing has concluded, a BLA must be submitted for FDA approval.
The process required by the FDA before a biological product may be marketed in the U.S. generally involves the following:
•completion of nonclinical laboratory studies, meaning in vivo and in vitro experiments in which an investigational product is studied prospectively in a test system under laboratory conditions to determine its safety, must be conducted according to cGLP (good laboratory practice) regulations, as well as, in the case of nonclinical laboratory studies involving animal test systems, in accordance with applicable requirements for the humane use of laboratory animals and other applicable regulations;
•submission to the FDA of an application for an IND, which must become effective before human clinical studies may begin;
•performance of adequate and well-controlled human clinical studies according to the FDA’s cGCPs (good clinical practices) and all other applicable regulatory requirements for the protection of human research subjects and their health information, to establish the safety, purity and potency of the proposed product for its intended use and to ensure the product has an appropriate risk-benefit profile;
•development and demonstration of a manufacturing process that can produce product of consistent and adequate quality;
•submission to the FDA of a BLA for marketing approval demonstrating the quality, safety, and efficacy of the product which must be supported by substantial evidence from adequate and well-controlled clinical investigations as well as demonstration of mode of action through non-clinical studies, evidence
68
to support appropriate manufacturing capabilities and controls, and evidence of the stability of the product in the form it is intended to be provided;
•negotiation with FDA of proposed product labeling (and determination of appropriate risk mitigation strategies and programs, if any required), as well as participation in any required advisory committee proceedings;
•satisfactory completion of an FDA inspection of all manufacturing, testing and distribution facilities where the product is produced, tested or stored and distributed, to assess compliance with cGMP (good manufacturing practices) to assure that the facilities, methods and controls for production are adequate to preserve the product’s identity, strength, purity and potency;
•potential FDA inspection of nonclinical facilities and likely inspection of select clinical study sites that generated the data in support of the BLA; and
•FDA review and approval of the BLA.
Human testing of a biological product candidate is preceded by preclinical testing, including nonclinical laboratory studies in which the product candidate is studied prospectively in a test system under laboratory conditions to determine its safety. A test system may include any animal, plant, microorganism, or subparts thereof to which the test or control article is administered or added for study.
The clinical study sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA places the clinical study covered by the IND on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical study can begin. The FDA may also impose clinical holds on a product candidate at any time during clinical studies due to safety concerns or non-compliance. If the FDA imposes a clinical hold, studies may not recommence unless FDA removes the clinical hold and then only under terms authorized by the FDA. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical studies to begin, or that, once begun, issues will not arise that suspend or terminate such studies.
Clinical studies involve the administration of the product candidate to subjects under the supervision of qualified independent investigators, generally physicians or other qualified scientists and medical personnel who are not employed by or under the study sponsor’s control. Clinical studies are conducted under protocols detailing, among other things, the objectives of the clinical study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical study will be stopped if certain adverse events, or AEs, should occur. Each new protocol and certain amendments to the protocol must be submitted to the FDA. Clinical studies must be conducted in accordance with the FDA’s cGCP regulations and guidance, and monitored to ensure compliance with applicable regulatory requirements. These include the requirement that written informed consent is obtained from all subjects who participate in the study. Further, each clinical study must be reviewed and approved by an independent Institutional Review Board, or IRB, at or servicing each institution at which the clinical study will be conducted. An IRB is charged with protecting the welfare and rights of study participants and considers such items as whether the risks to individuals participating in the clinical studies are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the form and content of the informed consent document that must be signed by each clinical study subject or his or her legal representative and must monitor the clinical study until completed. Throughout the study, certain information about certain serious adverse events must be reported to the IRB, in some cases on an expedited basis, and to FDA (as well as to regulators in other countries in which studies of the product are also being conducted).
Human clinical studies are typically conducted in three sequential phases that may in some cases overlap or be combined:
•Phase 1. The product candidate is initially introduced into a small number of human subjects. In the case of cellular therapy products, the initial human testing is conducted in patients with the disease or condition targeted by the biological product candidate. Phase 1 studies are intended to determine the metabolism and pharmacologic actions (including adverse reactions), the side effects associated with increasing doses, immunogenicity, and, if possible, to gain early evidence of effectiveness. The information obtained in Phase 1 should be sufficient to permit the design of well-controlled, scientifically valid Phase 2 studies.
69
•Phase 2. Controlled clinical studies are conducted in a larger number of human subjects to evaluate the effectiveness of the drug for a particular indication or indications in patients with the disease or condition under study. Phase 2 studies are intended to assess side effects and risks, and to examine exposure–response relationships, and to further explore pharmacologic actions and immunogenicity associated with the drug. These studies also provide helpful information for the design of phase 3 studies.
•Phase 3. Assuming preliminary evidence suggesting effectiveness has been obtained in phase 2 (generally considered to be “proof of concept”), controlled studies are conducted in a larger group of subjects to gather additional information about effectiveness and safety in order to evaluate the overall benefit-risk relationship of the drug and to provide an adequate basis for physician labeling.
Post-approval clinical studies, sometimes referred to as Phase 4 clinical studies, may be conducted after initial marketing approval. In some cases, FDA may require a Phase 4 study to be performed as a condition of product approval. Sponsors also can voluntarily conduct Phase 4 studies to gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow-up or in select populations. FDA regulations extend to all phases of clinical development and apply to sponsors and investigators of clinical studies. FDA oversight includes inspection of the sites and investigators involved in conducting the studies.
Concurrent with clinical studies, companies usually complete additional animal studies, and must also develop additional information about the physical characteristics of the biological product as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements.
To help reduce the risk of the introduction of adventitious agents with use of biological products, the PHS Act emphasizes the importance of manufacturing control for products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things; the sponsor must develop methods for testing the identity, purity and potency of the final biological product. All such testing and controls requires the application of significant human and financial resources.
Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
After the completion of clinical studies of a product candidate, FDA approval of a BLA must be obtained before commercial marketing of the biological product. The BLA must include results of product development, laboratory and animal studies, human studies, information on the manufacture and composition of the product, proposed labeling and other relevant information. In addition, under the Pediatric Research Equity Act (“PREA”), a BLA or supplement to a BLA must contain data to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any biological product for an indication for which orphan designation has been granted. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the BLA for filing and, even if filed, that any approval will be granted on a timely basis, if at all.
Under the Prescription Drug User Fee Act (“PDUFA”), as amended, each BLA must be accompanied by a substantial user fee. PDUFA also imposes an annual product fee for biologics and an annual establishment fee on facilities used to manufacture prescription biologics. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business.
Additionally, an application fee is not assessed on BLAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
Within 60 days following submission of the application, the FDA reviews the BLA submitted to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to file any marketing application that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review of the BLA. The FDA reviews the application to determine, among other things, whether the proposed product is safe and effective, for its intended use, and has an acceptable purity profile, and whether the product is being manufactured
70
in accordance with cGMP to assure and preserve the product’s identity, safety, potency and purity. The FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. During the product approval process, the FDA also will determine whether a Risk Evaluation and Mitigation Strategy, or REMS, is necessary to assure the safe use of the product. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the FDA will not approve the application without a REMS, if required.
Before approving a BLA, the FDA will typically inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure that the clinical studies were conducted in compliance with IND study and cGCP requirements. To assure cGMP and cGCP compliance, an applicant must incur significant expenditure of time, money and effort in the areas of training, record keeping, production, and quality control.
Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the BLA does not satisfy its regulatory criteria for approval and deny approval. Data obtained from clinical studies are not always conclusive and the FDA may interpret data differently than we interpret the same data. If the agency decides not to approve the marketing application, it will issue a complete response letter describing specific deficiencies in the application identified by the FDA. Additionally, the complete response letter may recommend actions that the applicant might take to place the application in a condition for approval. Such recommended actions could include the conduct of additional studies. If a complete response letter is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. The FDA may impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval. In addition, the FDA may require post-approval clinical studies, to further assess a product’s safety and effectiveness, and testing and surveillance programs to monitor the safety of approved products that have been commercialized.
One of the performance goals agreed to by the FDA under the PDUFA is to complete its review of 90% of standard BLAs within 10 months from filing and 90% of priority BLAs within six months from filing, whereupon a review decision is to be made. The FDA does not always meet its PDUFA goal dates and its review goals are subject to change from time to time. The review process and the PDUFA goal date may be extended by three months if the FDA requests or the application sponsor otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA goal date.
Post-Approval Requirements
Maintaining substantial compliance with applicable federal, state, and local statutes and regulations requires the expenditure of substantial time and the commitment of substantial human and financial resources. Rigorous and extensive FDA regulation of biological products continues after approval, particularly with respect to cGMP. We will rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of any products that we may commercialize. Manufacturers of our products are required to comply with applicable requirements in the cGMP regulations, including quality control and quality assurance and maintenance of records and documentation.
Other post-approval requirements applicable to drug and biological products include reporting post marketing surveillance to continuously monitor the safety of the approved product. This is done through the collection of spontaneous reports of adverse events and side effects, the assessment of safety signals, if any, and prescription event monitoring, among other methods. FDA maintains a system of postmarketing surveillance because all possible side effects of a new drug may not be evident in preapproval studies, which involve only several hundred to several thousand patients. Through postmarketing surveillance and risk assessment programs, FDA and sponsors seek to identify adverse events that did not appear during the drug approval process. In addition, FDA monitors adverse events such as adverse reactions and poisonings. FDA may use this information for a variety of purposes to identify safety signals not previously identified with
71
the product, to update drug labeling, and, on rare occasions, to reevaluate the approval or marketing decision with respect to a product.
In addition, post-approval regulatory requirements include reporting of cGMP deviations that may affect the identity, potency, purity and overall safety of a distributed product, record-keeping requirements, and complying with electronic record and signature requirements. After a BLA is approved, the product also may be subject to official lot release. As part of the manufacturing process, the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release by the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot. The FDA also may perform certain confirmatory tests on lots of some products before releasing the lots for distribution by the manufacturer. In addition, the FDA conducts laboratory research related to the regulatory standards on the safety, purity, potency, and effectiveness of drug and biological products. The FDA will also conduct routine scheduled and unannounced inspections of drug production and control facilities and processes, using field investigators and analysts, to assure ongoing safety and effectiveness of approved marketed products. Inspections may be made in conjunction with regulators from other jurisdictions and in certain cases, inspection findings and observations may be made public or may impair our ability to use the inspected facility, or to continue to produce and market a product.
We also must comply with the FDA’s advertising and promotion requirements, such as those related to direct- to-consumer advertising, the prohibition on promoting products for uses or in patient populations that are not described in the product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities, and promotional activities involving the internet and notably, social media. In addition, discovery of previously unknown problems or the failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant or manufacturer to administrative or judicial civil or criminal sanctions and adverse publicity. Sanctions authorized under FDA’s legal authorities could include refusal to approve pending applications, withdrawal of an approval, clinical hold, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, mandated corrective advertising or communications with doctors, debarment, restitution, disgorgement of profits, or civil or criminal penalties.
Violations of the FDCA may serve as a basis for the refusal of, or exclusion from, government contracts, including federal reimbursement programs, as well as other adverse consequences including lawsuits and actions by state attorneys general. Any agency or judicial enforcement action could have a material adverse effect on us. Drug and biological product manufacturers and other entities involved in the manufacture and distribution of approved drug or biological products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA, including withdrawal of the product from the market. In addition, changes to a manufacturing process or facility generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
U.S. Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of the FDA approval of the use of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a new drug application, or NDA, or BLA plus the time between the submission date of an NDA or BLA and the approval of that application. Only one patent applicable to an approved product can be extended and the application for the extension must be submitted prior to the expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
72
Under the Hatch-Waxman Amendments, a drug product containing a new chemical entity as its active ingredient is entitled to five years of market exclusivity, and a product for which the sponsor is required to generate new clinical data is entitled to three years of market exclusivity. A drug or biological product can also obtain pediatric market exclusivity in the U.S. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which runs from the end of other exclusivity protection or patent term, may be granted based on the voluntary completion of a pediatric study in accordance with an FDA-issued “Written Request” for such a study.
The Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic.
A new biologic is granted 12 years of exclusivity from the time of first licensure during which a biosimilar may not be launched.
Government Regulation Outside of the U.S.
European Union Regulation
In addition to regulations in the U.S., we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical studies and any commercial sales and distribution of our products. In particular, we view the EU and Japan as important jurisdictions for our business.
For purposes of developing our products, we must obtain the requisite approvals from regulatory authorities in each country prior to the commencement of clinical studies or marketing of the product in those countries. Certain countries outside of the U.S. have a similar process that requires the submission of a clinical study application much like the IND prior to the commencement of human clinical studies. In the EU, for example, a clinical trial application (“CTA”), must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical study development may proceed.
The EU has two main procedures for obtaining marketing authorizations in the EU Member States: a centralized procedure or national authorization procedure, under the latter of which one can seek to go through the mutual recognition procedure or the decentralized procedure. All biotechnology products are assessed through the centralized procedure.
Under the centralized authorization procedure, sponsors submit a single marketing-authorization application to the EMA. This allows the marketing-authorization holder to market the product and make it available to patients and healthcare professionals throughout the EU on the basis of a single marketing authorization. EMA's Committee for Medicinal products for Human Use (“CHMP”) carries out a scientific assessment of the application and give a recommendation on whether the medicine should be marketed or not. Once granted by the EMA, the centralized marketing authorization is valid in all EU Member States as well as in the European Economic Area countries Iceland, Liechtenstein and Norway. The centralized procedure is mandatory for biotechnology products.
Any product candidates we seek to commercialize in the EU are subject to review and approval by the European Medicines Authority (“EMA”). Submissions for marketing authorization to the EMA must be received and validated by that body which appoints a Rapporteur and Co-Rapporteur to review it. The entire review process must be completed within 210 days, with a “clock-stop” at day 120 to allow the submitting company to respond to questions set forth in the Rapporteur and Co-Rapporteur’s assessment report. Once the company responds in full, the clock for review re-starts on day 121. If further clarification is needed, the EMA may request an Oral Explanation on day 180, and the company submitting the application must appear before the CHMP to provide the requested information. On day 210, the CHMP will vote to recommend for or against the approval of the application. The final decision of EMA for marketing authorization following a positive CHMP recommendation is typically made within 60 days, with a draft decision within 15 days of the CHMP recommendation.
73
After Marketing Authorizations have been granted, the company must submit periodic safety reports to the EMA (if approval was granted under the Centralized Procedure) or to the National Health Authorities (if approval was granted under the DCP or the MRP). In addition, pharmacovigilance measures must be implemented and monitored to ensure appropriate adverse event collection, evaluation and expedited reporting, as well as timely updates to any applicable risk management plans. For some medications, post approval studies may be required to complement available data with additional data to evaluate long term effects or to gather additional efficacy data.
European marketing authorizations have an initial duration of five years. After this time, the marketing authorization may be renewed by the competent authority on the basis of re-evaluation of the risk/benefit balance. Any marketing authorization which is not followed within three years of its granting by the actual placing on the market of the corresponding medicinal product ceases to be valid.
United Kingdom (post BREXIT)
Marketing Authorization in the United Kingdom no longer falls under the EMA centralized process, and requires compliance to local laws and regulations, with a separate application required either concurrently or sequentially with the centralized procedure.
EU Exclusivity Periods
To obtain regulatory approval of an investigational biological product under EU regulatory systems, we must submit a marketing authorization application. The application used to file the BLA in the U.S. is similar to that required in the EU, with the exception of, among other things, country-specific document requirements. The EU also provides opportunities for market exclusivity. For example, in the EU, upon receiving marketing authorization, new chemical entities generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic product can be marketed until the expiration of the market exclusivity. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical entity, and products may not qualify for data exclusivity. Products receiving orphan designation in the EU can receive 10 years of market exclusivity, during which time no similar medicinal product for the same indication may be placed on the market. An orphan product can also obtain an additional two years of market exclusivity in the EU for pediatric studies. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications.
The criteria for designating an “orphan medicinal product” in the EU are similar in principle to those in the U.S. Under Article 3 of Regulation (EC) 141/2000, a medicinal product may be designated as orphan if (1) it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; (2) either (a) such condition affects no more than five in 10,000 persons in the EU when the application is made, or (b) the product, without the benefits derived from orphan status, would not generate sufficient return in the EU to justify investment; and (3) there exists no satisfactory method of diagnosis, prevention or treatment of such condition authorized for marketing in the EU, or if such a method exists, the product will be of significant benefit to those affected by the condition, as defined in Regulation (EC) 847/2000. Orphan medicinal products are eligible for financial incentives such as reduction of fees or fee waivers and are, upon grant of a marketing authorization, entitled to 10 years of market exclusivity for the approved therapeutic indication. The application for orphan drug designation must be submitted before the application for marketing authorization. The applicant will receive a fee reduction for the marketing authorization application if the orphan drug designation has been granted, but not if the designation is still pending at the time the marketing authorization is submitted. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
The 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Additionally, marketing authorization may be granted to a similar product for the same indication at any time if:
•the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior;
•the applicant consents to a second orphan medicinal product application; or
•the applicant cannot supply enough orphan medicinal product.
74
In addition to law and regulation specific to drug development, we note that new data protection regulations that have gone into effect in Europe are likely to have a significant impact on our activities, personnel, and may have an impact on our ability to timely complete clinical trials and effectively develop and commercialize our product candidates. The General Data Protection Regulation (the “GDPR”) was approved and adopted by the EU Parliament in April 2016 and went into effect on May 25, 2018. Unlike a Directive, the GDPR does not require any enabling legislation to be passed by any government. The GDPR not only applies to organizations located within the EU but may also apply to organizations located outside of the EU if they offer goods or services to, or monitor the behavior of, EU data subjects or if they process the personal data of subjects residing in the European Union. The implications of this regulation are therefore far reaching and may impose significant burdens on the Company and its processes and systems. Additionally, the UK government has implemented a Data Protection Bill, which also went into effect on May 25, 2018, that substantially implements the GDPR. For other countries outside of the EU, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical studies, product licensing, coverage, pricing and reimbursement vary from country to country. In all cases, again, the clinical studies are conducted in accordance with cGCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we obtain regulatory approval. In the U.S. and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend, in part, on the availability of coverage and adequate reimbursement from third-party payors. Third-party payors include government programs such as Medicare or Medicaid, managed care plans, private health insurers, and other organizations. These third-party payors may deny coverage or reimbursement for a product or therapy in whole or in part if they determine that the product or therapy was not medically appropriate or necessary or if another less expensive potential alternative exists. Third-party payors may attempt to control costs by limiting coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drug products for a particular indication, and by limiting the amount of reimbursement for particular procedures or drug treatments. In addition, in the United States, participation in government health programs such as Medicare and Medicaid are subject to complex rules and controls relating to price reporting and calculation of prices to ensure that pricing provided to government entities for periodic reporting purposes is aligned and compliant with numerous complex statutory requirements and the lowest possible price is the one used by government programs. The infrastructure and/or external resources necessary to ensure continued compliance with these requirements is extensive and manufacturers are subject to audit both by the Centers for Medicare and Medicaid Services and by State Medicaid authorities.
The cost of pharmaceuticals and devices continues to generate substantial governmental and third-party payor interest. We expect that the pharmaceutical industry will experience pricing pressures due to the trend toward managed healthcare, the increasing influence of managed care organizations and additional legislative proposals. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain the FDA approvals. More recently in the US and for certain high-cost rare disease drugs, payors have negotiated a provision that requires manufactures to refund the cost of the treatment if patients discontinue the drug for clinical reasons. Our product candidates may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Some third-party payors also require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. While we cannot predict whether any proposed cost-containment measures will be adopted or otherwise implemented in the future, these requirements or any announcement or adoption of such proposals could have a material adverse effect on our ability to obtain adequate prices for our product candidates and to operate profitably.
In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings (or mandatory price decreases) on specific products and therapies. There can be no
75
assurance that our products will be considered medically reasonable and necessary for a specific indication, that our products will be considered cost-effective by third-party payors, that coverage or an adequate level of reimbursement will be available or that the third-party payors reimbursement policies will not adversely affect our ability to sell our product profitably.
Healthcare Reform
In the U.S. and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs. In the U.S., the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The Medicare Modernization Act expanded Medicare coverage for drug purchases by the elderly by establishing Medicare Part D and introduced a new reimbursement methodology based on average sales prices for physician administered drugs under Medicare Part B. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class under the new Medicare Part D program. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and reimbursement rate that we receive for any of our approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates.
Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Affordable Care Act (“ACA”), a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers and impose additional health policy reforms. We expect that the rebates, discounts, taxes and other costs resulting from the ACA over time will have a negative effect on our expenses and profitability in the future. Furthermore, expanded government investigative authority and increased disclosure obligations may increase the cost of compliance with new regulations and programs.
The current presidential administration and Congress are also expected to continue recent attempts to make changes to the current health care laws and regulations. The impact of those changes on us and potential effect on the pharmaceutical industry as a whole is currently unknown. But, any changes to the health care laws or regulations, especially to Medicare drug reimbursement, are likely to have an impact on our results of operations and may have a material adverse effect on our results of operations. We cannot predict what other health care programs and regulations will ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the United States may have on our business.
It is possible that healthcare reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product, and could seriously harm our future revenue. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors, and formulary restrictions among private payors including the largest pharmacy benefit managers have increased over recent months, especially as regards to new and high cost market entrants. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products.
In addition, different pricing and reimbursement schemes exist in other countries. In the European Community, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may be marketed only once a reimbursement price has been agreed upon. Some of these countries may require, as condition of obtaining reimbursement or pricing approval, the completion of clinical trials that compare the cost- effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross- border imports from low-priced markets exert a commercial pressure on pricing within a country.
76
Other Healthcare Laws and Compliance Requirements
In the U.S., the research, manufacturing, distribution, sale and promotion of drug products, including biologics, and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, divisions of the U.S. Department of Health and Human Services, including the Office of Inspector General and the Centers for Medicare and Medicaid Services, the U.S. Department of Justice, state Attorneys General, and other state and local government agencies. For example, sales, marketing and scientific/educational grant programs must comply with fraud and abuse laws such as the federal Anti-Kickback Statute, as amended, the federal False Claims Act, as amended, and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
The federal Anti-Kickback Statute prohibits any person, including a prescription drug manufacturer (or a party acting on its behalf), from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce or reward either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. The term “remuneration” has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payments, ownership interests and providing anything at less than its fair market value. Even the award of grant moneys, or the provision of in kind support, publicity and even authorship, in certain cases, may be deemed to be “remuneration.” Although there are a number of statutory exceptions and regulatory safe harbors protecting certain business arrangements from prosecution, the exception and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from federal Anti-Kickback Statute liability. The reach of the Anti-Kickback Statute was broadened by the ACA, so that the government need no longer prove, for purposes of establishing intent under the federal Anti-Kickback Statute, that a person or entity had actual knowledge of the statute or specific intent to violate it. In addition, the ACA provides that a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act (discussed below). Additionally, many states have adopted laws similar to the federal Anti-Kickback Statute, and some of these state prohibitions apply to the referral of patients for healthcare items or services reimbursed by any third-party payor, including private payors. In at least some cases, these state laws do not contain safe harbors.
The federal False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government and share in any recovery. In recent years, the number of suits brought by private individuals has increased dramatically. In addition, various states have enacted false claims laws analogous to the False Claims Act. Many of these state laws apply where a claim is submitted to any third-party payor and not merely a federal healthcare program. There are many potential bases for liability under the False Claims Act. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The False Claims Act has been used to assert liability on the basis of inadequate care, kickbacks and other improper referrals, improperly reported government pricing metrics such as Best Price or Average Manufacturer Price, improper use of Medicare numbers when detailing the provider of services, improper promotion of off-label uses (i.e., uses not expressly approved by FDA in a drug’s label), and allegations as to misrepresentations with respect to the services rendered.
Substantial resources have been allocated by both the Department of Justice and the Federal Bureau of Investigation, among other branches of the US government to identify and investigate possible health care fraud activities. Recent investigations include those relating to allegedly egregious price increases by manufacturers and alleged fraud involving co-pay arrangements supported by sponsors. As new theories of liability arise, there is a corresponding cost of doing business in order to maintain compliance.
Our future activities relating to the reporting of discount and rebate information and other information affecting federal, provincial, state and third party reimbursement of our products, and the sale and marketing of our products and our service arrangements or data purchases, among other activities, may be subject to scrutiny under these laws. We are unable to predict whether we would be subject to actions under the False Claims Act or a similar state law, or the impact of such
77
actions. However, the cost of defending such claims, as well as any sanctions imposed, could adversely affect our financial performance. Also, the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), created several new federal crimes including healthcare fraud and false statements relating to healthcare matters. The healthcare fraud provision of HIPAA prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors. The false statements provision prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
In addition, we may be subject to, or our marketing activities may be limited by, data privacy and security regulation by both the federal government and the states in which we conduct our business. For example, HIPAA and its implementing regulations established uniform federal standards for certain “covered entities” (healthcare providers, health plans and healthcare clearinghouses) governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of protected health information. The American Recovery and Reinvestment Act of 2009, commonly referred to as the economic stimulus package, included expansion of HIPAA’s privacy and security standards called the Health Information Technology for Economic and Clinical Health Act (“HITECH”), which became effective on February 17, 2010. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates”—independent contractors or agents of covered entities that create, receive, maintain, or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions.
There are also an increasing number of state “sunshine” laws that require manufacturers to make reports to states on pricing and marketing information, as well as regarding payments to healthcare professionals. Several states have enacted legislation requiring pharmaceutical companies to, among other things, establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit certain other sales and marketing practices. State laws are not harmonized and contain different reporting requirements and restrictions which must be noted and adhered to. We currently do not report under these state laws, but will be required to do if we are successful in obtaining marketing authorization for our products. We will need to develop the infrastructure or rely on third party contractors to assist us in our compliance with these laws, and failure to comply may result in financial and other penalties and consequences. In addition, beginning in 2013, a similar “sunshine” federal requirement began requiring manufacturers to track and report to the federal government certain payments and other transfers of value made to certain covered recipients, including physicians and other healthcare professionals, and teaching hospitals. In addition to payments, reporting may encompass requirements to report on ownership or investment interests held by physicians and their immediate family members. The efforts and resources needed to track and report payments go well beyond our affiliates operating in the United States, as reporting is required also for payments made by affiliated entities in many cases to US covered recipients. In other jurisdictions (eg, Australia, Japan and Europe) similar “sunshine-like” laws have also been adopted, which may require disclosure of certain payment and other information to covered recipients. Extensive administration and systems, including to aggregate and categorize spend, are necessary in order to enable compliant and timely reporting under these requirements. The US federal government began disclosing the reported information on a publicly available website in 2014. These laws may affect our development, sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. If we fail to track and report as required by these laws or otherwise fail to comply with these laws, we could be subject to the penalty and sanctions of the pertinent state and federal authorities.
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the federal and state laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including criminal and significant civil monetary penalties, damages, fines, imprisonment, exclusion from participation in government healthcare programs, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of premarketing product approvals, private qui tam actions brought by individual whistleblowers in the name of the government or refusal to allow us to enter into supply contracts, including government contracts, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. To the extent that any of our products are sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-approval requirements, including safety surveillance, anti-fraud and abuse laws, implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
78
Australian Disclosure Requirements
Business Strategies and Prospects for Future Years
We are focused on the following core strategic imperatives:
•continue to innovate and optimize our disruptive technology platform for cell-based therapeutics;
•develop a portfolio of clinically distinct products;
•focus on bringing late-stage products to market and portfolio prioritization;
•enabling manufacturing scale-up to meet demands of the portfolio;
•leverage talent base to continue to establish a culture of shared leadership and accountability;
•focus on strategic partnerships;
•focus on prudent cash management; and
•continue to strengthen our substantial and robust intellectual property estate.
Dividends
No dividends were paid during the course of the fiscal year ended June 30, 2023. There are no dividends or distributions recommended or declared for payment to members, but not yet paid, during the year.
4.C Organizational Structure
See “Item 4. Information on the Company – 4.B Business Overview – Overview”, “Item 18. Financial Statements – Note 12” and Exhibit 8.1 to this Annual Report.
4.D Property, Plants and Equipment
We lease approximately 11,150 square feet of office space in Melbourne, Australia, where our headquarters are located. We pay approximately A$1,000,000 per year for this lease, which expires in April 2026. We have sub-leased approximately 5,400 square feet of this space and we receive approximately A$340,000 per year for this sub-lease, which expires in April 2026. We also lease approximately 15,600 square feet in New York City, where significant development and commercial activities are conducted. We pay approximately $995,000 per year for this lease, which expires in September 2024. We also lease laboratory and office space in Singapore. We pay approximately S$267,000 per year for this lease, which expires in September 2025. We also lease laboratory space in Texas and pay approximately $309,000 per year for this lease, which expires in December 2026. All of our manufacturing operations are currently located at Lonza’s manufacturing facilities. See “Item 4.B Business Overview – Manufacturing and Supply Chain.”
Item 4A. Unresolved Staff Comments
Not applicable.
Item 5. Operating and Financial Review and Prospects
5.A Operating Results
This operating and financial review should be read together with our consolidated financial statements in this Annual Report, which have been prepared in accordance with IFRS as published by the IASB.
Financial Overview
We have incurred significant losses since our inception. We have incurred net losses during most of our fiscal periods since our inception. As at June 30, 2023, we had an accumulated deficit of $820.8 million. Our net loss for the year ended June 30, 2023 was $81.9 million.
We anticipate that we may continue to incur significant losses for the foreseeable future. There can be no assurance that we will ever achieve or maintain profitability.
79
We expect our future capital requirements will continue as we:
•continue the research and clinical development of our product candidates;
•initiate and advance our product candidates into larger clinical studies;
•seek to identify, assess, acquire, and/or develop other product candidates and technologies;
•seek regulatory and marketing approvals in multiple jurisdictions for our product candidates that successfully complete clinical studies;
•establish collaborations with third parties for the development and commercialization of our product candidates, or otherwise build and maintain a sales, marketing, and distribution infrastructure to commercialize any products for which we may obtain marketing approval;
•further develop and implement our proprietary manufacturing processes and expand our manufacturing capabilities and resources for commercial production;
•seek coverage and reimbursement from third-party payors, including government and private payors for future products;
•make interest payments, principal repayments and other charges on our debt financing arrangements;
•make milestone or other payments under our agreements pursuant to which we have licensed or acquired rights to intellectual property and technology;
•seek to maintain, protect, and expand our intellectual property portfolio; and
•seek to attract and retain skilled personnel.
We expect our research and development and management and administration expenses to remain relatively consistent over the next 12 months. Subject to us achieving successful regulatory approval, we expect an increase in our total expenses driven by an increase in our product manufacturing and selling, general and administrative expenses as we move towards commercialization. Therefore, we will need additional capital to fund our operations, which we may raise through equity offerings, debt financings, other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. We do not know when, or if, we will generate revenues from our product sales significant enough to generate profits. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one or more of our cell-based product candidates. For further discussion on our ability to continue as a going concern, see Note 1(i) in our accompanying financial statements.
Commercialization and Milestone Revenue. Commercialization and milestone revenue relates to upfront, royalty and milestone payments recognized under development and commercialization agreements; milestone payments, the receipt of which is dependent on certain clinical, regulatory or commercial milestones; as well as royalties on product sales of licensed products, if and when such product sales occur; and revenue from the supply of products. Payment is generally due on standard terms of 30 to 60 days.
Amounts received prior to satisfying the revenue recognition criteria are recorded as deferred consideration in our consolidated balance sheet, depending on the nature of the arrangement. Amounts expected to be recognized as revenue within the 12 months following the consolidated balance sheet date are classified within current liabilities. Amounts not expected to be recognized as revenue within the 12 months following the consolidated balance sheet date are classified within non-current liabilities.
In the year ended June 30, 2023, we recognized $7.1 million in commercialization revenue relating to royalty income earned on sales of TEMCELL® Hs. Inj., a registered trademark of JCR Pharmaceuticals Co. Ltd. (“TEMCELL”), in Japan by our licensee, JCR Pharmaceuticals Co. Ltd. (“JCR”), compared with $8.7 million for the year ended June 30, 2022. Also in the years ended June 30, 2023 and 2022, we recognized $0.4 million and $0.3 million, respectively, in commercialization revenue from royalty income earned on sales of Alofisel® in Europe. These amounts were recorded in revenue as there are no further performance obligations required in regard to these items.
In the year ended June 30, 2022, we recognized $1.2 million in milestone revenue in relation to our patent license agreement with Takeda Pharmaceutical Company Limited (“Takeda”) entered into in December 2017. This $1.2 million amount was recognized with regards to the €1.0 million regulatory milestone payment from Takeda given Takeda received approval to manufacture and market Alofisel® (darvadstrocel) in Japan for the treatment of complex perianal fistulas in
80
patients with non-active or mildly active luminal Crohn’s Disease. This amount was recorded in revenue as there are no further performance obligations required regarding this item. There was no milestone revenue recognized in relation to this agreement with Takeda in the year ended June 30, 2023.
Research and Development. Research and development expenditure is recognized as an expense as incurred.
Our research and development expenses consist primarily of:
•third party costs comprising all external expenditure on our research and development programs such as fees paid to Contract Research Organizations (“CROs”) and on our pre-commercial activities, such as research pertaining to market access and pricing, brand marketing and initiation of trade and distribution contracts. Third party costs also comprise fees paid to consultants who perform research on our behalf and under our direction, rent and utility costs for our research and development facilities, and database analysis fees;
•third party costs under license and/or sub-license arrangements for the research and development, license, manufacture and/or commercialization of products and/or product candidates, such as payments for options to acquire rights to products and product candidates as well as contingent obligations under the agreements;
•product support costs consisting primarily of salaries and related overhead expenses for personnel in research and development and pre-commercial functions (for example wages, salaries and associated on costs such as superannuation, share-based incentives and payroll taxes, plus travel costs and recruitment fees for new hires);
•intellectual property support costs comprising payments to our patent attorneys to progress patent applications and all costs of renewing our granted patents; and
•amortization of currently marketed products on a straight-line basis over the life of the asset.
Our research and development expenses are not charged to specific products or programs, since the number of clinical and preclinical product candidates or development projects tends to vary from period to period and since internal resources are utilized across multiple products and programs over any given period of time. As a result, our management does not maintain and evaluate research and development costs by product or program. Acquired in-process research and development is capitalized as an asset and is not amortized but is subject to impairment review during the development phase. Upon completion of its development, the acquired in-process research and development amortization will commence.
Manufacturing Commercialization. Manufacturing commercialization expenditure is recognized as an expense as incurred. Our manufacturing commercialization expenses consist primarily of:
•salaries and related overhead expenses including share-based incentives for personnel in manufacturing functions;
•fees paid to our contract manufacturing organizations, which perform process development on our behalf and under our direction;
•costs related to laboratory supplies used in our manufacturing development efforts; and
•provision for the carrying value of pre-launch inventory costs on the consolidated balance sheet.
Management and Administration. Management and administration expenses consist primarily of salaries and related costs including share-based incentives for directors and employees in corporate and administrative functions, including the executives of those areas. Other significant management and administration expenses include legal and professional services, rent and depreciation of leasehold improvements, insurance and information technology services.
Fair Value Remeasurement of Contingent Consideration. Remeasurement of contingent consideration pertains to the acquisition of assets from Osiris Therapeutics, Inc. (“Osiris”). The fair value remeasurement of contingent consideration is recognized as a net result of changes to the key assumptions of the contingent consideration valuation such as developmental timelines, market growth, probability of success and payment, market penetration, product pricing and the increase in valuation as the time period shortens between the valuation date and the potential settlement dates of contingent consideration. As the net result of changes to the key assumptions and the time period shortening, we recognized a net remeasurement gain of $8.8 million and $0.9 million for the years ended June 30, 2023 and 2022, respectively.
81
Fair Value Movement of Warrants. Remeasurement of warrants pertain to the warrants granted to Oaktree in relation to the closing and amendment of the loan agreement with Oaktree. The fair value movement of warrants is recognized when there is a change in the valuation assumptions such as share price, risk-free interest rates and volatility. In the years ended June 30, 2023 and 2022, we recognized a remeasurement loss of $2.2 million and a remeasurement gain of $5.9 million, respectively.
Other Operating Income and Expenses. Other operating income and expenses primarily comprise foreign exchange gains and losses.
Tax incentives comprise payments from the Australian government’s Innovation Australia Research and Development Tax Incentive program for research and development activities conducted in relation to our qualifying research that meets the regulatory criteria. The research and development tax incentive credit is available for our research and development activities in Australia. Eligible companies can receive a refundable tax offset for a percentage of their research and development spending.
We recorded income of $3.5 million in research and development tax incentive income for the year ended June 30, 2023. Within this $3.5 million, $1.2 million pertains to the year ended June 30, 2023, $1.1 million pertains to the year ended June 30, 2022 and $1.2 million pertains to the year ended June 30, 2021. During the year ended June 30, 2023, management concluded its assessment of qualifying activities and we recognized the relevant income for the years ended June 30, 2023, 2022 and 2021. No income was recognized in the year ended June 30, 2022 as management were yet to confirm if our research and development activities were eligible under the incentive scheme.
Foreign exchange gains and losses relate to unrealized foreign exchange gains and losses on our foreign currency amounts in our Australian based entity, whose functional currency is the A$, and foreign currency amounts in our Switzerland and Singapore based entities, whose functional currencies are the US$, plus realized gains and losses on any foreign currency payments to our suppliers due to movements in exchange rates. We recognized a foreign exchange loss of $0.2 million in the year ended June 30, 2023 and a loss of $0.5 million in the year ended June 30, 2022.
Interest Income. Interest income is accrued on a time basis by reference to the principal outstanding and at the effective interest rate applicable.
Finance Costs. Finance costs primarily consists of remeasurement of borrowing arrangements, interest expense in relation to finance lease charges, accrued interest expense and interest expense in relation to the amortization of transaction costs and other charges associated with the borrowings as represented in our consolidated balance sheet using the effective interest rate method over the period of initial recognition through maturity.
Remeasurement of borrowing arrangements recognized primarily pertaining to our loan and security agreements with NovaQuest Capital Management, L.L.C. (“NovaQuest”) and Oaktree. Remeasurement of borrowing arrangements is recognized when there is a modification of the borrowing arrangement with no significant change to the contractual cash flows of the borrowings at the remeasurement date or when there is a revision in the estimated future cash flows which is recorded as an adjustment of the carrying amount of the financial liability. The carrying amount is recalculated by computing the present value of the revised estimated future cash flows at the financial instrument’s original effective interest rate.
In the years ended June 30, 2023 and 2022, we recognized remeasurement gains of $0.9 million and $0.5 million in relation to our existing credit facility with NovaQuest, respectively. In the years ended June 30, 2023 and 2022, we recognized a remeasurement loss of $1.6 million and a minimal gain in relation to our existing credit facility with Oaktree, respectively. Within this $1.6 million loss, $1.0 million relates to the remeasurement due to additional warrants being issued to Oaktree as a result of the first amendment to the loan agreement and $0.6 million relates to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facility. In the years ended June 30, 2023 and 2022, we recognized $Nil and a remeasurement loss of $0.9 million in relation to our extinguished senior debt facility, respectively.
Income Tax Benefit/Expense. Income tax benefit/expense consists of net changes in deferred tax assets and liabilities recognized on the balance sheet during the period. We recognized a non-cash income tax benefit of $0.2 million in the year ended June 30, 2023 and $0.2 million in the year ended June 30, 2022.
82
Results of Operations
Comparison of Our Results for the Year ended June 30, 2023 with the Year ended June 30, 2022
The following table summarizes our results of operations for the years ended June 30, 2023 and 2022, together with the changes in those items in dollars and as a percentage.
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands except per share information) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Consolidated Income Statement Data: | ||||||||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||||||
| Commercialization revenue | $ | 7,501 | $ | 9,039 | (1,538) | (17 | %) | |||||||||||||||||||
| Milestone revenue | — | 1,172 | (1,172) | (100 | %) | |||||||||||||||||||||
| Total revenue | 7,501 | 10,211 | (2,710) | (27 | %) | |||||||||||||||||||||
| Research & development | (27,189) | (32,815) | 5,626 | (17 | %) | |||||||||||||||||||||
| Manufacturing commercialization | (27,733) | (30,757) | 3,024 | (10 | %) | |||||||||||||||||||||
| Management and administration | (25,374) | (27,210) | 1,836 | (7 | %) | |||||||||||||||||||||
| Fair value remeasurement of contingent consideration | 8,771 | 913 | 7,858 | NM | ||||||||||||||||||||||
| Fair value movement of warrants | (2,205) | 5,896 | (8,101) | (137 | %) | |||||||||||||||||||||
| Other operating income and expenses | 4,250 | (536) | 4,786 | NM | ||||||||||||||||||||||
| Finance costs | (20,122) | (17,288) | (2,834) | 16 | % | |||||||||||||||||||||
| Loss before income tax | (82,101) | (91,586) | 9,485 | (10 | %) | |||||||||||||||||||||
| Income tax benefit | 212 | 239 | (27) | (11 | %) | |||||||||||||||||||||
| Loss attributable to the owners of Mesoblast Limited | $ | (81,889) | $ | (91,347) | 9,458 | (10 | %) | |||||||||||||||||||
| Losses per share from continuing operations attributable to the ordinary equity holders: | Cents | Cents | Cents | % Change | ||||||||||||||||||||||
| Basic - losses per share | (11.08) | (14.08) | 3.00 | (21 | %) | |||||||||||||||||||||
| Diluted - losses per share | (11.08) | (14.08) | 3.00 | (21 | %) | |||||||||||||||||||||
* NM = not meaningful.
Revenue
Revenues were $7.5 million for the year ended June 30, 2023, compared with $10.2 million for the year ended June 30, 2022, a decrease of $2.7 million. The following table shows the movement within revenue for the years ended June 30, 2023 and 2022, together with the changes in those items.
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||||||
| Commercialization revenue | 7,501 | 9,039 | (1,538) | (17 | %) | |||||||||||||||||||||
| Milestone revenue | — | 1,172 | (1,172) | (100 | %) | |||||||||||||||||||||
| Revenue | $ | 7,501 | $ | 10,211 | (2,710) | (27 | %) | |||||||||||||||||||
* NM = not meaningful.
Commercialization revenue from royalty income earned on sales of TEMCELL in Japan and Alofisel® in Europe decreased by $1.5 million for the year ended June 30, 2023. Royalty income on sales of TEMCELL in Japan by our licensee JCR were $7.1 million in the year ended June 30, 2023 compared to $8.7 million in the year ended June 30, 2022, a decrease of $1.6 million. Of this $1.6 million decrease, $1.0 million was due to foreign exchange rate changes as the
83
Japanese Yen depreciated against the U.S. dollar. Royalty income on sales of Alofisel® in Europe by our licensee Takeda increased by $0.1 million in the year ended June 30, 2023 compared with the year ended June 30, 2022.
We recognized $1.2 million in milestone revenue during the year ended June 30, 2022 in relation to our patent license agreement with Takeda. This $1.2 million was recognized with regards to the €1.0 million regulatory milestone payment from Takeda given Takeda received approval to manufacture and market Alofisel® (darvadstrocel) in Japan for the treatment of complex perianal fistulas in patients with non-active or mildly active luminal Crohn’s Disease. No milestone revenue was recognized in the year ended June 30, 2023.
Research and development
Research and development expenses were $27.2 million for the year ended June 30, 2023, compared with $32.8 million for the year ended June 30, 2022, a decrease of $5.6 million. The $5.6 million decrease in research and development expenses is due to a decrease in third party costs and product support costs.
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Research and development: | ||||||||||||||||||||||||||
| Third party costs | 8,398 | 10,626 | (2,228) | (21 | %) | |||||||||||||||||||||
| Product support costs | 14,107 | 17,942 | (3,835) | (21 | %) | |||||||||||||||||||||
| Intellectual property support costs | 3,222 | 2,785 | 437 | 16 | % | |||||||||||||||||||||
| Amortization of current marketed products | 1,462 | 1,462 | — | — | % | |||||||||||||||||||||
| Research and development | $ | 27,189 | $ | 32,815 | (5,626) | (17 | %) | |||||||||||||||||||
Third party costs, which consist of all external expenditure on our research and development programs, decreased by $2.2 million in the year ended June 30, 2023 compared with the year ended June 30, 2022.
This $2.2 million decrease was due to a reduction in our third party costs for our Phase 3 clinical trials for the treatment of MPC-06-ID (CLBP), MPC-150-IM (CHF) and ARDS in COVID-19 patients as activities and costs decline over time after enrollment was completed and these trials transitioned into the patient monitoring and data analysis stage. The decrease of these costs were primarily due to higher activities in relation to patient monitoring during follow up visits and higher data analysis being performed in year ended June 30, 2022 compared with the year ended June 30, 2023. In the year ended June 30, 2023, we also incurred costs of $1.4 million associated with our pre-commercial activities as we prepared for the potential launch of remestemcel-L in the United States.
Product support costs, which consist primarily of salaries and related overhead expenses for personnel in research and development and pre-commercial functions, have decreased by $3.8 million, for the year ended June 30, 2023 compared with the year ended June 30, 2022. Within this $3.8 million decrease, $4.0 million relates to a decrease in product support costs for research and development functions and $0.2 million relates to an increase in product support costs for pre-commercial functions.
The $4.0 million decrease in product support costs for personnel in research and development functions is primarily due to a decrease of $1.9 million in share-based payment expenses, $1.0 million in short-term incentives and $0.6 million in consulting expenses for the year ended June 30, 2023 compared with the year ended June 30, 2022. There was also a decrease of $0.7 million across salaries and associated costs as full time equivalents decreased by 1.2 (3%) from 44.9 for the year ended June 30, 2022 to 43.7 for the year ended June 30, 2023. These decreases were offset by an increase of $0.2 million in travel expenses for the year ended June 30, 2023 compared with the year ended June 30, 2022.
The $0.2 million increase in product support costs for personnel in pre-commercial functions is primarily due to an increase of $0.3 million in consulting expenses for the year ended June 30, 2023 compared with the year ended June 30, 2022. This increase was offset with a $0.1 million decrease across salaries and associated costs as full time equivalents decreased from 0.3 for the year ended June 30, 2022 to nil for the year ended June 30, 2023.
Also included in research and development expenses are intellectual property support costs, which consist of payments to our patent attorneys to progress patent applications and costs of renewing our granted patents. These costs
84
have increased by $0.4 million in the year ended June 30, 2023 compared with the year ended June 30, 2022 due to increased activities across our MSC- based patent portfolio.
Manufacturing commercialization
Manufacturing commercialization expenses were $27.7 million for the year ended June 30, 2023, compared with $30.7 million for the year ended June 30, 2022, a decrease of $3.0 million. This decrease is primarily due to a decrease in platform technology costs.
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Manufacturing commercialization: | ||||||||||||||||||||||||||
| Platform technology | 25,964 | 29,146 | (3,182) | (11 | %) | |||||||||||||||||||||
| Manufacturing support costs | 1,769 | 1,611 | 158 | 10 | % | |||||||||||||||||||||
| Manufacturing commercialization | $ | 27,733 | $ | 30,757 | (3,024) | (10 | %) | |||||||||||||||||||
Platform technology costs decreased by $3.2 million for the year ended June 30, 2023 compared with year ended June 30, 2022. These costs consist of fees paid for potency assay work that has supported the SR-aGVHD BLA resubmission as well as fees paid to our contract manufacturing organizations for process development of our proprietary technology that facilitates the increase in yields necessary for the long-term commercial supply of our product candidates and next generation manufacturing processes to reduce labor, drive down cost of goods and improve manufacturing efficiencies in our MPC and MSC based products. The decrease of these costs was primarily due to higher MSC development activities during the year ended June 30, 2022 as compared to the year ended June 30, 2023.
Manufacturing support costs, which consist primarily of salaries and related overhead expenses for personnel in manufacturing commercialization functions increased by $0.2 million for the year ended June 30, 2023 compared with the year ended June 30, 2022 primarily due to an increase of $0.6 million across salaries and associated costs as full time equivalents increased by 1.4 (23%) from 6.1 for the year ended June 30, 2022 to 7.5 for the year ended June 30, 2023. This increase was offset by a decrease of $0.4 million in share-based payment expenses for the year ended June 30, 2023 compared with the year ended June 30, 2022.
Management and administration
Management and administration expenses were $25.4 million for the year ended June 30, 2023, compared with $27.2 million for the year ended June 30, 2022, a decrease of $1.8 million. This decrease was primarily due to a decrease in legal and professional fees.
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Management and administration: | ||||||||||||||||||||||||||
| Labor and associated expenses | 9,854 | 9,747 | 107 | 1 | % | |||||||||||||||||||||
| Corporate overheads | 12,501 | 12,294 | 207 | 2 | % | |||||||||||||||||||||
| Legal and professional fees | 3,019 | 5,169 | (2,150) | (42 | %) | |||||||||||||||||||||
| Management and administration | $ | 25,374 | $ | 27,210 | (1,836) | (7 | %) | |||||||||||||||||||
Labor and associated expenses increased by $0.1 million from $9.7 million for the year ended June 30, 2022 to $9.8 million for the year ended June 30, 2023. This $0.1 million increase is primarily due to an increase of $0.5 million in share-based payment expenses and $0.5 million in consulting expenses. There was also an increase in overall cost of salaries and associated expenses by $0.3 million in the year ended June 30, 2023, compared with the year ended June 30, 2022 due to full time equivalents increasing by 1.1 (5%) from 23.4 for the year ended June 30, 2022 to 24.5 for the year ended June 30, 2023. These increases were offset by a decrease of $0.4 million in short-term incentives, $0.1 million in recruitment and $0.2 million in labor and associated expenses due to a one-off adjustment. Labor and associated expenses also experienced favorable exchange rate fluctuations of $0.5 million in the year ended June 30, 2023 compared with the year ended
85
June 30, 2022, as the A$ weakened against the US$ given the majority of management and administration expenses are incurred in A$ by our headquarter office located in Australia.
Corporate overhead expenses increased by $0.2 million from $12.3 million for the year ended June 30, 2022 to $12.5 million for the year ended June 30, 2023 primarily due to an increase in travel expenses.
Legal and professional fees decreased by $2.1 million from $5.1 million for the year ended June 30, 2022 to $3.0 million for the year ended June 30, 2023 due to a one-off adjustment in legal expenses during the year ended June 30, 2023 and increased professional fees associated with one-off corporate activities incurred during the year ended June 30, 2022.
Fair value remeasurement of contingent consideration
Fair value remeasurement of contingent consideration was a $8.8 million gain for the year ended June 30, 2023 compared with a $0.9 million gain for the year ended June 30, 2022. The $8.8 million gain for the year ended June 30, 2023 was due to the remeasurement of contingent consideration pertaining to the acquisition of assets from Osiris. This gain was a net result of changing the key assumptions of the contingent consideration valuation such as probability of payment, development timelines and the increase in valuation as the time period shortens between the valuation date and the potential settlement dates of contingent consideration, including the impact from the complete response from the FDA on our BLA for remestemcel-L for the treatment of pediatric SR-aGVHD in August 2023. The assumptions relating to development timelines have been updated to reflect current expectations as a result of the complete response.
The $0.9 million gain for the year ended June 30, 2022 was due to the remeasurement of contingent consideration pertaining to the acquisition of assets from Osiris. This gain was a net result of changing the key assumptions of the contingent consideration valuation such as development timelines, market growth and the increase in valuation as the time period shortens between the valuation date and the potential settlement dates of contingent consideration.
With respect to future milestone payments, contingent consideration will be payable in cash or shares at our discretion. With respect to commercialization, product royalties will be payable in cash which will be funded from royalties received from net sales.
Fair value movement of warrants
Fair value movement of warrants was a $2.2 million loss for the year ended June 30, 2023 compared with a $5.9 million gain for the year ended June 30, 2022. This $2.2 million loss for the year ended June 30, 2023 is a net result of changes to the key valuation inputs of the warrants such as the share price, risk-free interest rates and volatility.
Other operating income and expenses
In relation to other operating income and expenses, we recognized an income of $4.3 million for the year ended June 30, 2023, compared with a loss of $0.5 million for the year ended June 30, 2022, an increase in income of $4.8 million. The following table shows movements within other operating income and expenses for the year ended June 30, 2023 and 2022, together with the changes in those items:
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Other operating income and expenses: | ||||||||||||||||||||||||||
| Research and development tax incentive income | (3,506) | — | (3,506) | NM | ||||||||||||||||||||||
| Interest income | (831) | (3) | (827) | NM | ||||||||||||||||||||||
| Foreign exchange losses/(gains) (net) | 163 | 536 | (374) | (70 | %) | |||||||||||||||||||||
| Derecognition of right of use asset | (76) | — | (76) | NM | ||||||||||||||||||||||
| Foreign withholding tax | — | 3 | (3) | (100 | %) | |||||||||||||||||||||
| Other operating (income) and expenses | $ | (4,250) | $ | 536 | (4,786) | NM | ||||||||||||||||||||
* NM = not meaningful.
We recorded an income of $3.5 million in research and development tax incentive income for the year ended June 30, 2023 compared with $Nil for the year ended June 30, 2022. No income was recognized for the year ended June
86
30, 2022 as management were yet to confirm if our research and development activities were eligible under the incentive scheme.
Within the $3.5 million research and development tax incentive income recorded during the year ended June 30, 2023, $1.2 million pertains to the year ended June 30, 2023, $1.1 million pertains to the year ended June 30, 2022 and $1.2 million pertains to the year ended June 30, 2021.
The $0.8 million increase in interest income for the year ended June 30, 2023 compared with the year ended June 30, 2022 was primarily driven by higher interest rates on A$ cash deposits in the year ended June 30, 2023, when compared to the year ended June 30, 2022.
We are subject to foreign exchange gains and losses on foreign currency cash balances, creditors and debtors. In the year ended June 30, 2023, we recognized a foreign exchange loss of $0.2 million, primarily due to movements in exchange rates on US$ liabilities held in Mesoblast Limited, whose functional currency is the A$, as the A$ depreciated against the US$. In the year ended June 30, 2022, we recognized a foreign exchange loss of $0.5 million.
In the year ended June 30, 2023, we recognized an income of $0.1 million for the derecognition of right of use asset. There was no derecognition of right of use asset in the year ended June 30, 2022.
Finance costs
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Finance costs: | ||||||||||||||||||||||||||
| Remeasurement of borrowing arrangements | 678 | 382 | 296 | 77 | % | |||||||||||||||||||||
| Interest expense | 19,444 | 16,906 | 2,538 | 15 | % | |||||||||||||||||||||
| Finance costs | $ | 20,122 | $ | 17,288 | 2,834 | 16 | % | |||||||||||||||||||
In the year ended June 30, 2023, we recognized an overall loss of $0.7 million for remeasurement of borrowing arrangements in relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facilities with NovaQuest and Oaktree, an increase in losses of $0.3 million as compared with a $0.4 million loss for the year ended June 30, 2022.
Within the $0.7 million loss in the year ended June 30, 2023, in relation to our existing credit facility with NovaQuest, we recognized a $0.9 million gain for remeasurement of borrowing arrangements in relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows as a net result of changes to the key assumption in development timelines, an increase in gains of $0.4 million as compared with a $0.5 million gain recognized for the year ended June 30, 2022.
Also within the $0.7 million loss in the year ended June 30, 2023, in relation to our existing credit facility with Oaktree, we recognized a $1.6 million loss for remeasurement of borrowing arrangements of which $1.0 million relates to the remeasurement due to additional warrants being issued to Oaktree as a result of the first amendment to the loan agreement and $0.6 million relates to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows, an increase in losses of $1.6 million as compared with a minimal gain for the the year ended June 30, 2022.
In the year ended June 30, 2022, we also recognized a loss of $0.9 million for remeasurement of borrowing arrangements in relation to our extinguished senior debt facility of which $1.3 million related to prepaying the outstanding balance and extinguishing the loan. This loss was offset by a $0.4 million gain to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows. No remeasurement of borrowing arrangements was recognized in the year ended June 30, 2023.
Interest expense increased by $2.5 million from $16.9 million for the year ended June 30, 2022 to $19.4 million for the year ended June 30, 2023.
87
In the year ended June 30, 2023, in relation to our loan and security agreement with Oaktree, we recognized $9.2 million of interest expense, compared with $5.2 million for the year ended June 30, 2022. This increase of $4.0 million was primarily due to the loan commencing in November 2021 resulting in interest expenses accruing for a reduced portion during the year ended June 30, 2022. Within the $9.2 million recognized in the year ended June 30, 2023, $6.0 million was recognized with regards to interest expense payable on the loan balance within the year of which $4.9 million was paid and $1.1 million was added to the outstanding loan balance and shall accrue further interest. A further $3.2 million of interest expense was recognized with regard to the amortization of transaction costs incurred on the outstanding loan principal for the year ended June 30, 2023 using the effective interest rate method over the period of initial recognition through maturity.
In the year ended June 30, 2023, in relation to our loan and security agreement with NovaQuest, we recognized $9.1 million of interest expense, an increase of $1.3 million as compared with $7.8 million for the year ended June 30, 2022. Interest expense relating to the NovaQuest loan is accrued on the loan principal balance and all interest payments will be deferred until after the first commercial sale of our allogeneic product candidate remestemcel-L for the treatment of pediatric patients with SR-aGVHD in the United States and other geographies excluding Asia (“pediatric SR-aGVHD”).
Within the $16.9 million interest expense in the year ended June 30, 2022, we recognized $3.3 million of interest expense in relation to our extinguished senior debt facility. There was no interest expense recognized in the year ended June 30, 2023 in relation to the extinguished senior debt facility.
In line with IFRS 16 Leases, we also recognized interest expenses of $0.5 million and $0.6 million in relation to lease charges for the year ended June 30, 2023 and 2022, respectively.
In the year ended June 30, 2023, we recognized $0.6 million of interest charges primarily in relation to manufacturing payments. There was no interest expense recognized in the year ended June 30, 2022 in relation to manufacturing payments.
Loss after income tax
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Loss before income tax | (82,101) | (91,586) | 9,485 | (10 | %) | |||||||||||||||||||||
| Income tax benefit | 212 | 239 | (27) | (11 | %) | |||||||||||||||||||||
| Loss after income tax | $ | (81,889) | $ | (91,347) | 9,458 | (10 | %) | |||||||||||||||||||
Loss before income tax was $82.1 million for the year ended June 30, 2023 compared with $91.6 million for the year ended June 30, 2022, a decrease in the loss by $9.5 million. This decrease is the net effect of the changes in revenues and expenses that have been discussed above.
A non-cash income tax benefit of $0.2 million was recognized in the year ended June 30, 2023, in relation to the net change in deferred tax assets and liabilities recognized on the consolidated balance sheet during the period.
A non-cash income tax benefit of $0.2 million was recognized in the year ended June 30, 2022 in relation to the net change in deferred tax assets and liabilities recognized on the consolidated balance sheet during the period.
Comparison of Our Results for the Year ended June 30, 2022 with the Year ended June 30, 2021
For results of operations for the years ended June 30, 2022 and 2021, together with the changes in those items in dollars and as a percentage and the related discussions on these results, refer to Results of Operations within “Item 5.A Operating Results” in our Annual Report on Form 20-F for the year ended June 30, 2022, filed with the SEC on August 31, 2022.
Certain Differences Between IFRS and U.S. GAAP
IFRS differs from U.S. GAAP in certain respects. Management has not assessed the materiality of differences between IFRS and U.S. GAAP. Our significant accounting policies are described in “Item 18 Financial Statements – Note 23”.
88
Quantitative and Qualitative Disclosure about Market Risk
The following sections provide quantitative information on our exposure to interest rate risk, share price risk, and foreign currency exchange risk. We make use of sensitivity analyses which are inherently limited in estimating actual losses in fair value that can occur from changes in market conditions. For further assessment on our market risks, see “Item 18. Financial Statements – Note 10(a).”
Foreign currency exchange risk
We have foreign currency amounts owing relating to clinical, regulatory and overhead activities and foreign currency deposits held primarily in our Australian based entity, whose functional currency is the A$. We also have foreign currency amounts in our Switzerland and Singapore based entities, whose functional currencies are the US$. These foreign currency balances give rise to a currency risk, which is the risk of the exchange rate moving, in either direction, and the impact it may have on our financial performance.
We manage the currency risk by evaluating levels to hold in each currency by assessing our future activities which will likely be incurred in those currencies which enables us to minimize foreign currency deposits held in each entity.
As of June 30, 2023, we held 67% of our cash in US$, and 33% in AU$. As of June 30, 2022, we held 97% of our cash in US$, and 3% in AU$.
Interest rate risk
Our main interest rate risk arises from the portion of our long-term borrowings with a floating interest rate, which exposes us to cash flow interest rate risk. As interest rates fluctuate, the amount of interest payable on financing where the interest rate is not fixed will also fluctuate. We can repay the loan facility at our discretion and we can also refinance if we are able to achieve terms suitable to us in the marketplace or from our existing lenders. In November 2021, we refinanced our variable interest rate loan with a fixed rate loan thereby eliminating our current exposure to interest rate risk on long-term borrowings. As at June 30, 2023, we do not hold any floating interest rate borrowings.
We are also exposed to interest rate risk that arises through movements in interest income we earn on our deposits. The interest income derived from these balances can fluctuate due to interest rate changes. This interest rate risk is managed by periodically reviewing interest rates available for suitable interest bearing accounts to ensure we earn interest at market rates. We ensure that sufficient funds are available, in at call accounts, to meet our working capital requirements.
Price risk
Price risk is the risk that future cash flows derived from financial instruments will be altered as a result of a market price movement, which is defined as movements other than foreign currency rates and interest rates. We are exposed to price risk which arises from long-term borrowings under our facility with NovaQuest, where the timing and amount of principal and interest payments is dependent on net sales of remestemcel-L for the treatment of pediatric SR-aGVHD. As net sales of remestemcel-L for the treatment of SR-aGVHD in pediatric patients in these territories increase/decrease, the timing and amount of principal and interest payments relating to this type of financing arrangement will also fluctuate, resulting in an adjustment to the carrying amount of the financial liability. The adjustment is recognized in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs and expenses in the period the revision is made.
We are also exposed to price risk on contingent consideration provision balances, as expected unit revenues are a significant unobservable input used in the level 3 fair value measurements.
We do not consider any exposure to price risk other than those already described above.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, other than the purchase commitments and contingent liabilities as mentioned below.
89
Contractual Obligations and Commitments
Contractual commitments:
Purchase commitments means an agreement to purchase goods or services that is enforceable and legally binding that specifies all significant terms, including: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and the approximate timing of the transaction. Purchase obligations are not recognized as liabilities at June 30, 2023.
In December 2019, we commenced production under our manufacturing service agreement with Lonza for the supply of commercial product for the potential approval and launch of remestemcel-L for the treatment of pediatric SR-aGVHD in the US market. This agreement contains lease and non-lease components. As of June 30, 2023, the agreement contains a minimum remaining financial commitment of the non-lease component of $16.8 million, payable until December 2024, which is cancellable in limited circumstances. We have accounted for the lease component within the agreement as a lease liability separately from the non-lease components. As of June 30, 2023, the lease component is $3.0 million on an undiscounted basis, as disclosed within the total contractual cash flows as lease liabilities. At our discretion, the minimum financial commitment under this manufacturing services agreement can be reduced by $12.2 million under certain conditions, with $1.1 million of this reduction relating to the lease component and $11.1 million relating to the non-lease component of the agreement.
We have agreements with third parties related to contract manufacturing and other goods and services. As of June 30, 2023, we had $9.1 million of non-cancellable purchase commitments related to raw materials, manufacturing agreements and other goods and services (excluding those with Lonza). This amount represents our minimum contractual obligations, including termination fees. Certain agreements provide for termination rights subject to termination fees. Under such agreement, we are contractually obligated to make certain payments, mainly, to reimburse them for their unrecoverable outlays incurred prior to cancellation.
We do not have any other purchase commitments as of June 30, 2023.
Lease commitment – as lessee:
We lease various offices under non-cancellable leases expiring within 1 to 5 years. The leases have varying terms, escalation clauses and renewal rights. On renewal, the terms of the leases are renegotiated. We subleased a portion of our office in Melbourne Australia under a non-cancellable lease expiring within 3 years. We also lease a manufacturing suite under a manufacturing services agreement with Lonza for the supply of commercial product for the potential approval and launch of remestemcel-L for the treatment of pediatric SR-aGVHD in the US market expiring within 2 years from June 30, 2023, which is cancellable in limited circumstances.
Contingent liabilities
We acquired certain intellectual property relating to our MPCs, or Medvet IP, pursuant to an Intellectual Property Assignment Deed, or IP Deed, with Medvet Science Pty Ltd, or Medvet. Medvet’s rights under the IP Deed were transferred to Central Adelaide Local Health Network Incorporated, or CALHNI, in November 2011. In connection with our use of the Medvet IP, on completion of certain milestones we will be obligated to pay CALHNI, as successor in interest to Medvet, (i) certain aggregated milestone payments of up to $2.2 million, and single-digit royalties on net sales of products covered by the Medvet IP, for cardiac muscle and blood vessel applications and bone and cartilage regeneration and repair applications, subject to minimum annual royalties beginning in the first year of commercial sale of those products and (ii) single-digit royalties on net sales of the specified products for applications outside the specified fields.
We have entered into a number of agreements with other third parties pertaining to intellectual property. Contingent liabilities may arise in the future if certain events or developments occur in relation to these agreements and as of June 30, 2023 we have assessed that the probability of outflows is remote.
Capital commitments
We did not have any commitments for future capital expenditure outstanding as of June 30, 2023.
90
Australian Disclosure Requirements
Significant Changes in the State of Affairs
There have been no significant changes within the state of our affairs during the year ended June 30, 2023 except as noted in the “Important Corporate Developments” section included in Item 4.A.
Likely Developments and Expected Results of Operations
In August 2023, the FDA provided a complete response to our BLA resubmission for remestemcel-L for the treatment of children with SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data. In line with our overall commercial strategy to progress to adult populations, we intend to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. We will initially meet with the FDA to seek alignment on the trial design for the adult study at a Type A meeting scheduled in mid-September 2023.
Other significant milestones are expected in the upcoming financial year in relation to our other lead product candidates, as detailed elsewhere in this report.
Environmental Regulations
Our operations are not subject to any significant environmental regulations under either Commonwealth of Australia or State/Territory legislation. We consider that adequate systems are in place to manage our obligations and are not aware of any breach of environmental requirements pertaining to us.
5.B Liquidity and Capital Resources
Sources of Liquidity
We have continued our focus on maintaining tight control of net cash usage for operating activities, which was $63.3 million for the year ended June 30, 2023. As of June 30, 2023, we held total cash reserves of $71.3 million. We are implementing various cost containment and deferment strategies, including the reprioritization of projects and operational streamlining to manage net operating cash usage.
In August 2023, the FDA provided a complete response to our BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data. In line with our overall commercial strategy to progress to adult populations, we intend to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. In conjunction with implementing cost containment and deferment strategies, additional inflows from royalty monetization, capital markets, strategic partnerships or product specific financing will be required to meet our projected expenditure consistent with our business strategy over at least the next 12 months. As a result of these matters, there is material uncertainty related to events or conditions that may cast significant doubt (or raise substantial doubt as contemplated by Public Company Accounting Oversight Board (“PCAOB”) standards) on our ability to continue as a going concern and, therefore, that we may be unable to realize our assets and discharge our liabilities in the normal course of business. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Our primary sources of liquidity have historically been equity raisings, upfront and milestone payments from strategic license agreements, and borrowings under our loan agreements. We also expect net sales to become a source of liquidity. While in the long-term we expect to be able to complete transactions, draw upon these facilities and achieve approval of our product candidates to provide liquidity as needed, there can be no assurance as to whether we will be successful or, if successful, what the terms or proceeds may be.
91
Cash flows
| Year ended June 30, | ||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||||
| Cash Flow Data: | ||||||||||||||||||||||||||
| Net cash (outflows) in operating activities | (63,269) | (65,782) | 2,513 | (4 | %) | |||||||||||||||||||||
| Net cash (outflows) in investing activities | (194) | (232) | 38 | (16 | %) | |||||||||||||||||||||
| Net cash inflows/(outflows) by financing activities | 74,502 | (9,870) | 84,372 | NM | ||||||||||||||||||||||
| Net increase in cash and cash equivalents | 11,039 | (75,884) | 86,923 | (115 | %) | |||||||||||||||||||||
Comparison of cash flows for the Year ended June 30, 2023 with the Year ended June 30, 2022
Net cash outflows in operating activities
Net cash outflows for operating activities were $63.3 million for the year ended June 30, 2023, compared with $65.8 million for the year ended June 30, 2022, a decrease of $2.5 million. The decrease of $2.5 million is due to a decrease in cash outflows of $3.1 million and a decrease in cash inflows of $0.6 million in the year ended June 30, 2023, compared with the year ended June 30, 2022.
The $0.6 million decrease of inflows comprised: inflows from royalty income earned on sales of TEMCELL in Japan and Alofisel® in Europe decreased by $2.5 million during the year ended June 30, 2023, compared with the year ended June 30, 2022; received $1.1 million of receipts for research and development tax incentive during the year ended June 30, 2023, compared to $Nil for the year ended June 30, 2022; and inflows from interest receipts increased by $0.8 million in the year ended June 30, 2023, compared with the year ended June 30, 2022.
Outflows for payments to suppliers and employees decreased by $3.1 million from $75.8 million for the year ended June 30, 2022 to $72.7 million for the year ended June 30, 2023, primarily due to a decrease in payments in relation to product manufacturing and operating costs and research and development costs.
Net cash outflows in investing activities
Net cash outflows for investing activities remained consistent in the year ended June 30, 2023, compared with the year ended June 30, 2022.
Net cash inflows/(outflows) in financing activities
Net cash inflows for financing activities increased by $84.4 million for the year ended June 30, 2023, compared with the year ended June 30, 2022. The increase of $84.4 million is due to an increase in cash inflows of $28.4 million and a decrease in cash outflows of $56.0 million in the year ended June 30, 2023 compared with the year ended June 30, 2022.
The $28.4 million increase in inflows comprised: $45.1 million of proceeds received in August 2022 and $43.5 million of proceeds received in April 2023 on completion of global private placement during the year ended June 30, 2023, compared with $Nil for the year ended June 30, 2022; received $0.2 million in receipts from employee share option exercises during the year ended June 30, 2022, compared with $Nil for the year ended June 30, 2023. In the year ended June 30, 2022, received a total of $60.0 million of receipts for gross proceeds drawn pursuant to a five-year credit facility with Oaktree during the year ended June 30, 2022 compared with $Nil for the year ended June 30, 2023.
The $56.0 million decrease in outflows comprised: payments of $2.6 million and $2.8 million for lease liabilities during the years ended June 30, 2023 and 2022, respectively; payments of $6.0 million and $6.1 million for interest and other costs of finance during the years ended June 30, 2023 and 2022, respectively; payments of $4.9 million and $0.2 million for capital raising costs in the years ended June 30, 2023 and 2022, respectively; payments of $0.5 million and $5.5 million for borrowings costs in the years ended June 30, 2023 and 2022, respectively; repayment of the outstanding balance of $55.4 million to extinguish our senior debt facility in the year ended June 30, 2022.
Operating Capital Requirements
We do not know when, or if, we will generate revenues from our product sales significant enough to generate profits. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval
92
of and commercialize more of our cell-based product candidates. We anticipate that we will continue to incur losses for the foreseeable future, and we expect the losses to increase as we continue the development of, and seek regulatory approvals for, our cell-based product candidates, and begin to commercialize any approved products either directly ourselves or through a collaborator or partner. We are subject to all of the risks inherent in the development of new cell-based products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. We anticipate that we will need substantial additional funding in connection with our continuing operations.
We expect that our research and development expenses and our management and administration expenses to remain relatively consistent over the next 12 months. Subject to us achieving successful regulatory approval we expect an increase in our total expenses driven by an increase in our product manufacturing and selling, general and administrative expenses as we move towards commercialization. Therefore, we will need additional capital to fund our operations, which we may raise through a combination of equity offerings, debt financings, other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements.
Additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates. If we raise additional funds through the issuance of additional debt or equity securities, it could result in dilution to our existing shareholders, increased fixed payment obligations and the existence of securities with rights that may be senior to those of our ordinary shares. If we incur further indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events could significantly harm our business, financial condition and prospects.
Borrowings
Oaktree arrangement
In November 2021, we entered into a $90.0 million five-year senior debt facility provided by funds associated with Oaktree. We drew the first tranche of $60.0 million on closing. We will seek to extend our option to draw the additional $30.0 million tranche beyond September 30, 2023, subject to us achieving certain milestones. The facility has a three-year interest only period, at a fixed rate of 9.75% per annum, after which time 40% of the principal amortizes over two years and a final payment is due no later than November 2026. The facility also allows us to make quarterly payments of interest at a rate of 8.0% per annum for the first two years, and the unpaid interest portion (1.75% per annum) will be added to the outstanding loan balance and currently accrues further interest at a fixed rate of 9.75% per annum.
On November 19, 2021, Oaktree were granted warrants to purchase 1,769,669 American Depositary Shares (“ADSs”) at $7.26 per ADS, a 15% premium to the 30-day VWAP. We determined that an obligation to issue the warrants arose from the time the debt facility was signed; consequently, a liability for the warrants was recognized in November 2021. The warrants were legally issued on January 11, 2022 and may be exercised within 7 years of issuance. On the issuance date of the Oaktree facility and the warrants, the warrants were initially measured at fair value and the Oaktree borrowing liability measured as the difference between the $60.0 million received from the Oaktree facility and the fair value of the warrants. In December 2022, we amended the terms of the loan agreement with Oaktree and in connection with the loan amendment, Oaktree was granted warrants to purchase 455,000 ADSs at $3.70 per ADS, a 15% premium to the 30-day VWAP. We determined that an obligation to issue the warrants arose from the time the first amendment to the loan agreement was signed; consequently, a liability for the warrants was recognized in December 2022. The warrants were legally issued on March 8, 2023 and may be exercised within 7 years of issuance.
In the year ended June 30, 2023, we recognized a loss of $1.6 million in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs. Within this $1.6 million loss, $1.0 million relates to the remeasurement due to additional warrants being issued to Oaktree as a result of the first amendment to the loan agreement and $0.6 million relates to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facility. In the year ended June 30, 2022, we recognized a minimal gain in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facility.
We have pledged substantially all of our assets as collateral under the loan facility with Oaktree.
93
NovaQuest arrangement
On June 29, 2018, we entered into an eight-year, $40.0 million loan and security agreement with NovaQuest before drawing the first tranche of $30.0 million of the principal in July 2018. The loan term includes an interest only period of approximately four years through until July 8, 2022, then a four-year amortization period through until maturity on July 8, 2026. All interest and principal payments will be deferred until after the first commercial sale of remestemcel-L for the treatment in pediatric patients with SR-aGVHD. Principal is repayable in equal quarterly instalments over the amortization period of the loan and is subject to the payment cap described below. The loan has a fixed interest rate of 15% per annum. If there are no net sales of remestemcel-L for pediatric SR-aGVHD, the loan is only repayable at maturity. We can elect to prepay all outstanding amounts owing at any time prior to maturity, subject to a prepayment charge, and may decide to do so if net sales of remestemcel-L for pediatric SR-aGVHD are significantly higher than current forecasts.
Following approval and first commercial sales, repayments commence based on a percentage of net sales and are limited by a payment cap which is equal to the principal due for the next 12 months, plus accumulated unpaid principal and accrued unpaid interest. During the four-year period commencing July 8, 2022, principal amortizes in equal quarterly instalments payable only after approval and first commercial sales. If in any quarterly period, 25% of net sales of remestemcel-L for pediatric SR-aGVHD exceed the annual payment cap, we will pay the payment cap and an additional portion of excess sales which will be used towards the prepayment amount in the event there is an early prepayment of the loan. If in any quarterly period 25% of net sales of remestemcel-L for pediatric SR-aGVHD is less than the annual payment cap, then the payment is limited to 25% of net sales of remestemcel-L for pediatric SR-aGVHD. Any unpaid interest will be added to the principal amounts owing and shall accrue further interest. At maturity date, any unpaid loan balances are repaid.
Because of this relationship of net sales and repayments, changes in our estimated net sales may trigger an adjustment of the carrying amount of the financial liability to reflect the revised estimated cash flows. The carrying amount is recalculated by computing the present value of the revised estimated future cash flows at the financial instrument’s original effective interest rate. The adjustment is recognized in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in the period the revision is made.
In the years ended June 30, 2023 and 2022, we recognized a gain of $0.9 million and $0.5 million, respectively, in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows as a net result of changes to the key assumptions in development timelines.
We recognize a liability as current based on repayments linked to estimates of sales of remestemcel-L. However, if sales of remestemcel-L are higher than estimated, actual repayments will exceed this amount, subject to the annual payment cap described above.
The carrying amount of the loan and security agreement with NovaQuest is subordinated to our fixed rate loan with our senior creditor, Oaktree. We have pledged a portion of our assets relating to the SR-aGVHD product candidate as collateral under the loan facility with NovaQuest.
Compliance with loan covenants
Our loan facilities with Oaktree and NovaQuest contain a number of covenants that impose operating restrictions on us, which may restrict our ability to respond to changes in our business or take specified actions. We have an operating objective to at all times maintain unrestricted cash reserves in excess of six months liquidity. The objective aligns with our loan and security agreement with Oaktree where we are currently obliged to maintain a minimum cash balance in the United States of $35.0 million.
We have complied with the financial and other restrictive covenants of our borrowing facilities during the years ended June 30, 2023 and 2022.
5.C Research and Development, Patents and Licenses
For a description of the amount spent during each of the last two fiscal years on company-sponsored research and development activities, as well as the components of research and development expenses, see “Item 5.A Operating Results – Results of Operations.”
94
For a description of our research and development process, see “Item 4.B Business Overview.”
5.D Trend Information
As a biotechnology company which primarily is still in the development stage, we are subject to costs of our clinical trials and other work necessary to support applications for regulatory approval of our product candidates. Health regulators have increased their focus on product safety. In addition, regulators have also increased their attention on whether or not a new product offers evidence of substantial treatment effect. These developments have led to requests for more clinical trial data, for the inclusion of a higher number of patients in clinical trials, and for more detailed analyses of the trials. In light of these developments, we expect these aspects of our research and development expenses may need to increase as we continue to fund our programs to the market. Notwithstanding this upward trend, our research and development expenses may still fluctuate from period to period due to varied rates of patient enrollment and the timing of our clinical trials as our existing trials are completed and new trials commence. We cannot predict with any degree of accuracy the outcome of our research or commercialization efforts.
5.E Critical Accounting Estimates
Not applicable. See “Item 18. Financial Statements.”
Item 6. Directors, Senior Management and Employees
6.A Directors and Senior Management Personnel
Details of Directors and Senior Management
Board of Directors
Joseph Swedish, MHA
Chairman of the Board of Directors
Experience and expertise
Joseph. R. Swedish has more than two decades of healthcare leadership experience as the CEO for major United States healthcare enterprises. Most recently, he has served as Executive Chairman, President and CEO of Anthem Inc., America’s leading health benefits provider. For 12 consecutive years, Modern Healthcare named Mr Swedish as one of the 100 Most Influential People in Healthcare, ranking in the top 20 of the health sector’s most senior level executives, high-level government administrators, elected officials, academics, and thought leaders for five consecutive years. Prior to joining Anthem, Mr. Swedish was CEO for several major integrated healthcare delivery systems, including Trinity Health and Colorado’s Centura Health. He has been a Mesoblast board member since June 2018, and also serves on the boards of IBM Corporation, CDW Corporation, and Centrexion Therapeutics. Mr. Swedish is a member of Duke University’s Fuqua School of Business Board of Visitors. Previously, he was Chairman of the Catholic Health Association. Mr. Swedish received a bachelor’s degree from the University of North Carolina and his master’s degree in health administration from Duke University.
Other current directorships of listed public companies
Non-Executive Director, IBM Corporation (since 2017)
Non-Executive Director, CDW Corporation (since 2015)
Former directorships of listed public companies within the last 3 years
None
William Burns, BA
Non-Executive Member of the Board of Directors
Experience and expertise
Mr. Burns has served on our Board of Directors since 2014 and was appointed Vice Chairman in 2016. He spent his entire management career at the Beecham Group and F. Hoffmann-La Roche Ltd. Mr Burns was Chief Executive Officer of Roche Pharmaceuticals from 2001 to 2009, when he joined the Board of Directors of F. Hoffmann-La Roche
95
Ltd. until he retired in 2014. He is the Chair of Molecular Partners, and has been a Non-Executive Director of Shire PLC, Chugai Pharmaceutical Co., Genentech, Crucell, and Chairman of Biotie Therapies Corp. from 2014 until its sale to Acorda Therapeutics Inc. in 2016. Mr Burns is also a member of the Oncology Advisory Board of the Universities of Cologne/Bonn in Germany. In 2014, he was appointed a trustee of the Institute of Cancer Research, London, and in 2016 a Governor of The Wellcome Trust in London, UK.
Other current directorships of listed public companies
Chair of Molecular Partners (since 2018)
Former directorships of listed public companies within the last 3 years
None
Philip Facchina
Non-Executive Member of the Board of Directors
Experience and expertise
Mr. Facchina brings more than 35 years of experience in corporate strategy, finance, and business development across several industries, including healthcare. Since 2018, Mr. Facchina has been Chief Strategy Officer at SurgCenter, overseeing the company’s strategic relationships, including its relationships with the broad US ambulatory surgical center (ASC) market and its constituents. Prior to SurgCenter, Mr. Facchina spent two decades in the public and private capital markets, where he directly managed public and private capital transactions of equity and debt, led M&A and special advisory processes including take-privates. From 2008 to 2017, Mr. Facchina served as a Partner, Co-Portfolio Manager and the Chief Operating Officer of Ramsey Asset Management, an institutional investment management firm, and from 1998 to 2008 Mr. Facchina led the technology, media, and communications and healthcare investment banking groups of FBR Capital Markets. Mr. Facchina currently serves as an independent director for ViON Corporation and MilltechFX, and is Advisor to the CEO of Johanna Foods Inc, where he chairs the Audit Committee. Previously, among other directorships and committee posts, Mr. Facchina served on the Board of Web.com (Nasdaq: WEB), where he led Corporate Governance.
Other current directorships of listed public companies
None
Former directorships of listed public companies within the last 3 years
None
Michael Spooner, BCom
Non-Executive Member of the Board of Directors
Experience and expertise
Mr. Spooner has served on the Board of Directors since 2004. During this period he has filled various roles including as Chairman from the date of the ASX public listing in 2004 until 2007, Chair of the Audit and Risk Committee as well as a member of the Remuneration Committee . Over the past several years Mr. Spooner has served on the board of directors in various capacities at several Australian and international biotechnology companies, including BiVacor Pty Ltd (2009-2013), Advanced Surgical Design & Manufacture Limited (2010-2011), Peplin, Inc. (2004-2009), Hawaii Biotech, Inc. (2010-2012), Hunter Immunology Limited (2007-2008), and Ventracor Limited (2001-2003). He has been the Chairman of Simavita Ltd since May 2016 and Chairman of MicrofluidX since February 2018. Prior to returning to Australia in 2001, he spent much of his career internationally where he served in various roles including as a partner to PA Consulting Group, a UK-based management consultancy, and a Principal Partner and Director of Consulting Services with PricewaterhouseCoopers (Coopers & Lybrand) in Hong Kong. In addition Mr Spooner has owned and operated several international companies providing services and has consulted to a number of American and Asian public companies.
Other current directorships of listed public companies
Former directorships of listed public companies within the last 3 years
Chairman, Simavita Ltd (2016 - 2021)
96
Shawn Cline Tomasello, BS, MBA
Non-Executive Member of the Board of Directors - resigned effective August 18, 2022
Experience and expertise
With more than 30 years’ experience in the pharmaceutical and biotech industries, Shawn Cline Tomasello has substantial commercial and transactional experience. Since 2015, Ms. Tomasello had been Chief Commercial Officer at leading immuno-oncology cell therapy company Kite Pharma, where she played a pivotal role in the company’s acquisition in 2017 by Gilead Sciences for $11.9 billion. Prior to this she served as Chief Commercial Officer at Pharmacyclics, Inc., which was acquired in 2015 by AbbVie, Inc. for $21 billion. Ms. Tomasello previously was President of the Americas, Hematology and Oncology at Celgene Corporation where she managed over $4 billion in product revenues, and was instrumental in various global expansion and acquisition strategies. She has also held key positions at Genentech, Pfizer Laboratories, Miles Pharmaceuticals and Procter & Gamble. Ms. Tomasello currently serves on the Board of Directors of Gamida Cell, Ltd., AlloVir, 4D Molecular Therapeutics and Cabaletta Bio. She previously served on the board of Principia Biopharma; acquired by Sanofi, Abeona Therapeutics (resigned), Clementia Pharmaceuticals, Inc. which was acquired by Ipsen, SA, Diplomat Specialty which was acquired by United Healthcare and Urogen Pharma. She received a MBA from Murray State University and a B.S. in Marketing from the University of Cincinnati. Her extensive experience in the pharmaceutical and biotech industries, particularly in the commercial and transactional fields, provides industry, leadership and management expertise.
Other current directorships of listed public companies
Director, Gamida Cell, Ltd. (since 2019)
Director, AlloVir (since 2022)
Director, 4D Molecular Therapeutics (since 2020)
Director, Cabaletta Bio (since 2023)
Former directorships of listed public companies within the last 3 years
Non-Executive Director, Diplomat Pharmacy, Inc. (2015 – 2020)
Director, Abeona Therapeutics, Inc. (2020)
Director, Principia Biopharma, Inc. which was acquired by Sanofi (2019-2020)
Director, UroGen Pharma (2018-2022)
Director, TCR Therapeutics Inc. which was acquired by Adaptimmune Therapeutics plc (2021-2023)
Philip Krause, MD
As of August 28, 2023 ceased Membership of the Nomination and Remuneration Committee.
Non-Executive Member of the Board of Directors
Experience and expertise
With over 30 years of experience at the Food and Drug Administration, Dr. Krause has a unique combination of scientific, regulatory, clinical, and public health experience. He is a physician with board certification in internal medicine and infectious diseases and a researcher with over 100 publications on topics spanning clinical evaluation of vaccines, viral pathogenesis and immunology, and biological product development. He is currently an independent consultant, providing strategic and regulatory advice related to biological product development. He recently served as deputy director of FDA’s Office of Vaccines Research and Review, where he led assessments of biological products for evaluation and licensure and helped to oversee the development and evaluation of all vaccines authorized and licensed in the US from 2011-2021. He graduated from Yale Medical School (MD), Florida State University (MBA) and the University of Illinois (BS and MS in Computer Science). Dr Krause has a strategic advisory consulting role with Mesoblast, providing advice on regulatory strategies.
Other current directorships of listed public companies
None
Former directorships of listed public companies within the last 3 years
None
97
Jane Bell – B.Ec, LLB, LLM (London)
Appointed as a Non-Executive Member of the Board of Directors on August 18, 2022
Experience and expertise
Ms. Bell is a banking and finance lawyer with 30 years experience in international law firms, financial services and corporate treasury operations focusing on international investment transactions in the United States, Canada, Australia and the United Kingdom. She has served as a non-executive Director in a diverse range of highly regulated sectors including delivery of healthcare, life sciences, medical research, and funds management. Ms. Bell currently serves as Deputy Chair of Monash Health and Chair of its Audit Committee, Australia’s largest and most diverse public health service delivering more than 3.46 million episodes of care across an extensive network of hospitals, rehabilitation, aged care, community health and mental health facilities. She is also a current non-executive Director of Amplia Therapeutics and Chair of its Audit and Risk Committee (ASX:ATX) as well as Jessie McPherson Private Hospital. From 2014 until 2021 she was a director of U Ethical, Australia’s first ethical funds manager with over $1.2B of funds under management, and a member of its Investment Committee. She is a former Chair of Melbourne Health and former director of Hudson Institute of Medical Research. In 2023, Ms Bell was appointed a Member of the Order of Australia (AM) for significant service to governance in the medical research, healthcare and not-for-profit sectors.
Other current directorships of listed public companies
Non-Executive Director, Amplia Therapeutics Limited (since 2021)
Former directorships of listed public companies within the last 3 years
None
Company Secretary
Niva Sivakumar – BCom, LLB
Joint Company Secretary
Experience and expertise
Ms. Sivakumar joined Mesoblast’s legal team in 2014 and is a member of the company’s Intellectual Property Committee. Previously, she was a senior associate in the corporate and commercial teams at major law firm, Dentons, and a senior lawyer at K&L Gates. Ms. Sivakumar has a Commerce/Law degree from the University of Melbourne.
Other current directorships of listed public companies
None
Former directorships of listed public companies within the last 3 years
None
Paul Hughes – BPharm, BBus (Banking & Finance)
Joint Company Secretary
Experience and expertise
Mr. Hughes began working with Mesoblast in February 2019 and has served as the Company’s Global Head of Corporate Communications since December 2020. He has an extensive background as an investment banker and corporate advisor for firms including Macquarie Bank and Commonwealth Bank of Australia. Mr. Hughes has a Bachelor of Pharmacy and Bachelor of Business (Banking & Finance) from Monash University, Melbourne.
Other current directorships of listed public companies
None
Former directorships of listed public companies within the last 3 years
None
98
Senior Management – Key Management Personnel
Silviu Itescu, MBBS (Hons), FRACP, FACP, FACRA
Chief Executive Officer (CEO)
Executive Member of the Board of Directors
Experience and expertise
Dr. Itescu has served on the Board of Directors since the Company's founding in 2004, was Executive Director from 2007, and became Chief Executive Officer and Managing Director in 2011. Prior to founding Mesoblast in 2004, Dr. Itescu established an international reputation as a physician scientist in the fields of stem cell biology, autoimmune diseases, organ transplantation, and heart failure. Dr Itescu has been a faculty member of Columbia University in New York, and the University of Melbourne and Monash University in Australia. In 2013, Dr Itescu received the inaugural Key Innovator Award from the Vatican’s Pontifical Council for Culture for his leadership in translational science and clinical medicine in relation to adult stem cell therapy. In 2011, Dr. Itescu was named BioSpectrum Asia Person of the Year. Dr. Itescu has consulted for various international pharmaceutical companies, has been an adviser to biotechnology and health care investor groups, and has served on the board of directors of several publicly listed life sciences companies.
Other current directorships of listed public companies
None
Former directorships of listed public companies within the last 3 years
None
Eric Rose, MD
Chief Medical Officer (CMO)
Executive Member of the Board of Directors
Experience and expertise
Dr. Rose is a highly respected physician scientist with focus on clinical investigation, drug discovery, biodefense, and health policy. As a world-renowned heart surgeon and scientist, Dr. Rose led the Columbia Presbyterian heart transplantation program from 1982 through 1992 and made history in 1984 when he performed the first successful pediatric heart transplant. From 1994 through 2007, he served as Chairman of Columbia University’s Department of Surgery and Surgeon-in-Chief of Columbia Presbyterian Medical Center in New York. During this time his leadership of the NIH supported program Randomized Evaluation of Mechanical Circulatory Support in Heart Failure (REMATCH) resulted in the first FDA approval of an implantable left ventricular assist device for long term circulatory support, spawning an entire new industry. From 2007-2011, Dr. Rose served on the National Biodefense Scientific Board which advises the United States Health and Human Services Secretary on biodefense, influenza, and emerging diseases. In 2007 he was appointed Chairman and CEO of SIGA Technologies where he oversaw development of the first antipoxviral drug approved in the United States, TPOXX for the treatment of smallpox. Dr. Rose played a key role in obtaining FDA approval of the drug in 2019, and he was responsible for securing contracts with BARDA under which the US Government has procured 1.7 million courses of TPOXX for more than US$1billion into the Strategic National Stockpile (SNS). Dr. Rose’s tenure on the ABIOMED board ended in December 2022 with the sale of the company to Johnson & Johnson for $17.7 billion.
Other current directorships of listed public companies
None
Former directorships of listed public companies within the last 3 years
Chairman, SIGA Technologies, Inc. (2017 - 2021)
Non-executive Director, ABIOMED, Inc. which was acquired by Johnson & Johnson. (2007 - 2012, 2014 - 2022)
Other Senior Management
Andrew Chaponnel, BCom, CAANZ
Chief Financial Officer (interim)
99
Mr. Chaponnel has around 25 years of experience in finance roles including 11 years with Mesoblast, initially as the Group Financial Controller (6 years), then as Head of Finance (3 years) and now as interim Chief Financial Officer for the past two years. As part of Mesoblast Group finance leadership he has been integral to the implementation and maintenance of our borrowing arrangements, various strategic partnerships, equity placements, the NASDAQ IPO and leads both ASX and NASDAQ financial reporting. Previously Mr. Chaponnel has held several roles including management roles in chartered accountancy, logistics, retail and a CFO role within construction before moving into Healthcare. He is a member of the Chartered Accountants of Australia & New Zealand.
Fred Grossman D.O. FAPA
Resigned effective January 30, 2022 but remained a consultant until January 31, 2023
Chief Medical Officer
Dr. Grossman joined Mesoblast in August 2019 and leads the Medical Affairs, Drug Safety Clinical Operations and Biostatistics teams. Dr Grossmann is a Board-Certified psychiatrist and Fellow of the American Psychiatric Association with over 30 years of experience in research, academia, and practice. He has held executive positions leading and building clinical development, medical affairs, and pharmacovigilance in large and small pharmaceutical companies including Eli Lilly, Johnson & Johnson, Bristol Myers Squibb, Sunovion, Glenmark, and NeuroRx. Dr. Grossman has developed and supported the launch of numerous blockbuster medications addressing significant unmet medical needs across multiple therapeutic areas including CNS, immunology, immuno-oncolology, respiratory, cardiovascular/metabolics, and virology. He has close relationships with thought leaders worldwide and has negotiated directly with the FDA and Global Health Authorities for approval of many drugs across therapeutic areas. He has numerous publications and presentations and has held several academic appointments.
Peter Howard, BSc, LLB (Hons)
General Counsel
Mr. Howard has served as our General Counsel and Corporate Executive since July 2011. As external counsel and partner at Australian law firm, Middletons (now, K&L Gates), Mr. Howard has been integrally involved with Mesoblast since its inception and public listing on the ASX in 2004. More generally, Mr. Howard has extensive experience with many biopharmaceutical firms and major research institutions, covering public listings, private financings, strategic, licensing, intellectual property and mergers and acquisition activities. He has done so in several roles, including as a partner at a major law firm, entrepreneur, director and senior executive.
Justin Horst, BS
Head of Manufacturing
Justin Horst has 18 years of experience in clinical cell therapy manufacturing and industry development. During the past eight years, he has been Mesoblast’s Deputy Head of Manufacturing, with accountability for chemistry, manufacturing and control of the manufacturing processes. Before joining Mesoblast, Mr. Horst was at Lonza Walkersville Inc. for 10 years, holding numerous senior level positions within the manufacturing, project management, and business development groups. At Lonza, he was instrumental in the establishment of the contract manufacturing business, and managed multiple manufacturing teams supporting numerous custom supply processes. Mr. Horst obtained his B.S. in Biology from Towson University in Maryland.
Dagmar Rosa-Bjorkeson, MS, MBA
Chief Operating Officer
Dagmar Rosa-Bjorkeson has more than 25 years of global experience in the pharmaceutical industry, including executive leadership in corporate and product strategy, market development and operational execution. She has led multiple successful product launches, including Gilenya® for multiple sclerosis and Elidel® for atopic eczema. During her 17 years at Novartis, Ms. Rosa-Bjorkeson was Vice President and Head of its Multiple Sclerosis Business Unit; Vice President, Business Development and Licensing in the United States; and Country Head and President for Novartis Sweden. More recently, she served as Executive Vice President and President, Biosimilars, at Baxalta, now a wholly owned subsidiary of Takeda Pharmaceutical Company. Ms. Rosa-Bjorkeson was also Executive Vice President and Chief Strategy and Development Officer at Mallinckrodt Pharmaceuticals. She holds an MBA in Marketing, an MS in Chemistry and a BS, Chemistry from the University of Texas.
100
Michael Schuster, MBA
Pharma Partnering
Mr. Schuster, who joined Mesoblast in 2004, leads the Group's partnering discussions. Previously he was the head of the Group's investor relations outreach program and was part of the founding executive team at both Mesoblast Limited and Angioblast Systems, Inc. Mr. Schuster was Executive Vice President of Global Therapeutic Programs from 2010 to 2013 and was the Director of Business Development and Vice President of Operations from 2004 to 2010. He holds an undergraduate degree in science from Tufts University, a Master’s degree in Immunology & Microbiology from New York Medical College, and an MBA from Fordham University in New York.
Paul Simmons, PhD
Scientific Advisor to the Chief Executive Officer
Dr. Simmons served as our Head of Research and New Product Development since 2011 and transitioned to Scientific Advisor to the Chief Executive Officer in the current year. He has nearly 30 years of experience in stem cell research, especially research in basic hematopoiesis and in precursor cells for the stromal system of the bone marrow, and served as President of the International Society of Stem Cell Research, or ISSCR, from 2006 to 2007. Prior to joining Mesoblast, Dr. Simmons held the C. Harold and Lorine G. Wallace Distinguished University Chair at the University of Texas Health from 2008 to 2011 and served as the inaugural Professor and Director of the Centre for Stem Cell Research at the Brown Foundation Institute of Molecular Medicine from 2006 to 2011. Dr. Simmons is, or has served as, an associate editor, a member of the editorial board, or a reviewer on multiple scientific and medical journals including Experimental Hematology, Cytotherapy and Stem Cell Research, Cell Stem Cell, Stem Reports, Science and Nature.
Geraldine Storton, BSc, MMS, MBA
Head of Regulatory Affairs and Quality Management
Ms. Storton is a seasoned pharmaceutical executive with more than 30 years’ experience across the full value chain of Pharmaceutical and Medical Device Research and Development, production and commercialization worldwide. She has an extensive background in regulatory affairs and quality, most recently as a consultant to cell therapy companies. Prior to this, Ms. Storton held executive roles at Hospira, and its predecessor companies in both regulatory affairs and quality, with a focus on major program management. As Vice President, Program Management, Quality, at Hospira headquarters in Chicago, she led a company-wide quality remediation program to improve compliance in manufacturing across 15 facilities worldwide. As Regional Director, Commercial Quality ANZ, Asia and Japan, Ms. Storton was responsible for quality oversight and management of all products sold in Asia Pacific countries. Her responsibilities included regulatory compliance, batch release, field actions, complaints management, change control, due diligence and new product launch. As director of global regulatory operations, Ms. Storton managed development and registration of new products and on-market management of the existing product portfolio for all Hospira’s products developed or manufactured within Asia Pacific for global distribution. She joined Mesoblast in December 2015.
There are no family relationships among any of our directors and senior management. The business address of each of our directors and senior management is Mesoblast Limited, Level 38, 55 Collins Street, Melbourne, VIC 3000, Australia.
The Mesoblast board of directors (“the Board”) presents the 2022/2023 Remuneration Report, which has been prepared in accordance with the relevant Corporations Act 2001 (“Corporations Act”) and accounting standards requirements.
The remuneration report sets out remuneration information for our company’s key management personnel (“KMP”) as defined in the International Accounting Standards 24 ‘Related Party Disclosures’ and the Australian Corporations Act 2001 for the financial year ended June 30, 2023.
(Start of the Remuneration Report for Australian Disclosure Requirements)
Introductory Comments from Bill Burns, Nomination and Remuneration Committee Chairman
Mesoblast is a late-stage development company. During the year there was a concerted focus on the Biologics License Application (“BLA”) submission and continued dialogue with the United States Food & Drug Administration ("FDA"), involving all programs in our late-stage clinical pipeline. With the recent FDA decision requesting further work
101
before achieving approval for the Company’s lead product candidate, the Company is now focused on the reduction of cash burn and conducting the required study for licensure by the FDA.
Within the backdrop of uncertain financial markets, throughout FY23 management continued to implement measures focused on financial discipline and accountability throughout the year, working on reducing operating expenditure. The net cash usage for operating activities in this fiscal year was reduced by a further 4%. In April 2023, the Company raised net proceeds of US$40.0 million, strengthening our balance sheet as we progressed activities for the potential launch and commercialization of remestemcel-L.
The Company’s market valuation has been hard hit by the recent FDA decision and associated company announcement on August 4, 2023, resulting in a Mesoblast share price lower than this time last year. Since that time, the Company is concentrating resources on investing in clinical and regulatory progression of remestemcel-L for steroid-refractory acute graft versus host disease (SR-aGVHD) and rexlemestrocel-L for chronic low back pain (CLBP). To support the Company's investment in these research and development programs and preserve cash, the Company is implementing a number of costs containment and deferment strategies including the following changes to remuneration:
•The CEO and CMO have volunteered to reduce their base salary by 30% for the next 12 months. In compensation for the reduction in cash pay, an option grant will be proposed which will increase their long-term equity-based at-risk pay, further aligning their interests to those of shareholders, subject to shareholder approval. The program will also be offered to our management team;
•Cash payment of the STI relating to the financial year ended June 30, 2023 has been deferred and is dependent on an FDA approval; and
•Non-executive directors have voluntarily deferred 100% of the cash payments for their director fees and agreed to receive 50% of their fees in long-term non-cash incentives, subject to shareholder approval. No cash payments will be made until we receive an FDA approval.
During the fiscal year 2023, a huge amount of time and effort has gone into addressing the outstanding Chemistry, Manufacturing, and Controls ("CMC") issues including work on potency assays. Alongside this, the team has been busy ensuring other key programs continue to progress which included important interactions and feedback from FDA on chronic heart failure and chronic low back pain programs. The latter is in a position to move into a second Phase 3 trial.
As a biotechnology company, we believe our proactive approach to cyber security and environment, social and corporate governance (ESG) is in our DNA. We are researchers and developers focused on making peoples’ lives better. We combine this active spirit with formal programs and are continuously working to make improvements for not only our employees, our stakeholders and continuity of business. Mesoblast’s programs for health, safety and well-being ensured all employees were able to continue to work remotely and hybrid. As a small late-stage biotechnology company our manufacturing and supply chain, is outsourced to third-party providers. We implement a rigorous due diligence process to ensure that our suppliers place an equal importance on their ESG obligations as what we do at Mesoblast.
The CEO and C-suite executives are evenly split between Australia and the United States and Mesoblast are finely balancing the remuneration packages for value and retention across our sites. Senior executive benchmarked salaries have remained the same for several years. Exercising restraint and responsible remuneration management was positively received by the investors who voted for the remuneration resolution at the 2022 AGM with an approval vote of 95%.
The focus to preserve cash resources for investment in research and development is consistent with the focus of executive remuneration, which continued to comply with the following board approved conditions:
•The long-term incentives (LTI) remain a major part of the executive KMP’s remuneration. It is subject to achievement of milestone conditions over three years, with vesting after 3 years if these are met. If milestones are met sooner up to 1/3 of the LTI may vest in any one year;
•The options only have value if they vest and then only if share price exceeds the exercise price at grant after they vest; and
•The short-term incentive (STI) for the CEO and senior executive opportunity remained unchanged.
In FY23, the remuneration mix is weighted towards long term performance to ensure the executive outcomes are aligned to those of the shareholders. The milestone LTI awards in the biotechnology sector typically have a higher risk profile than those in the broader market and may not materialize to levels anticipated at grant.
The executives only receive the value from any milestone options that vest if shareholders also realize increases in the share price over the same period. Our decision to maintain this framework in FY23 is also consistent with the stated preference of our investors for long term at risk pay that is aligned to increasing shareholder wealth.
In terms of outcomes, the Board assessed our company’s KPI performance for the financial year ended June 30, 2023 as achieving 60% of target. While this reflects achievement of several significant financial and operational goals,
102
when assessed in the context of our current cash position, the Board determined that performance did not meet a sufficient threshold for an STI payment. The STI payment for the financial year ended June 30, 2023 has been deferred and is dependent on an FDA approval.
Despite progress towards certain milestones in the year resulting in the vesting of a limited amount of LTI milestones for both the CEO and CMO, given the market reaction to the FDA decision, these and all other LTI grants remain well under water and have zero intrinsic value.
On balance, we believe the remuneration framework, including the absence of any fixed remuneration increases over several years, the Board's decision to defer any cash payment of the FY23 STI until FDA approval has been achieved, and the use of share options as an LTI vehicle has worked for shareholder alignment in terms of executive focus on what is of value and in terms of realized outcomes.
I invite you to read the remainder of the remuneration report and welcome your feedback.
Bill Burns
Nomination and Remuneration Committee Chairman
Key Management Personnel (KMP)
Key management personnel (KMP), defined as individuals who have authority and responsibility for planning, directing and controlling the activities of the company, directly or indirectly, and including all directors, are listed in Table 1.
Table 1 – Mesoblast KMP during FY2023, including to the Date of this Report
| Name | Position | Country | Portion of FY2023 year served as KMP | ||||||||
| Non-executive directors | |||||||||||
| Joseph Swedish | Independent Chairman, Board of Directors Member, Audit and Risk Committee Member, Nomination and Remuneration Committee | US | Full Year | ||||||||
| William Burns | Independent Vice Chair, Board of Directors Chair, Nomination and Remuneration Committee | Switzerland | Full Year | ||||||||
| Michael Spooner | Independent Non-executive Director Chair, Audit and Risk Committee Member, Nomination and Remuneration Committee | Singapore | Full Year | ||||||||
| Shawn Cline Tomasello | Independent Non-executive Director Member, Nomination and Remuneration Committee | US | Resigned effective August 18, 2022 | ||||||||
103
| Philip Facchina | Independent Non-executive Director Member, Audit and Risk Committee Member, Nomination and Remuneration Committee | US | Full Year | ||||||||
| Philip Krause | Independent Non-executive Director Member, Nomination and Remuneration Committee (ceased August 28, 2023) | US | Full Year. As of August 28, 2023 no longer considered an independent director and ceased Membership of the Nomination and Remuneration Committee. | ||||||||
| Jane Bell | Independent Non-executive Director (from August 18, 2022) Member, Nomination and Remuneration Committee (from August 24, 2022) Member, Audit and Risk Committee (from August 24, 2022) | Australia | Appointed effective August 18, 2022 | ||||||||
| Executive directors | |||||||||||
| Silviu Itescu | Chief Executive Officer Executive Director | Australia | Full Year | ||||||||
| Eric Rose | Chief Medical Officer Executive Director | US | Full Year | ||||||||
6.B Compensation
KMP Remuneration Governance
The Board is responsible for Mesoblast’s remuneration strategy and approach. The Nomination and Remuneration Committee advises the Board on remuneration and incentive policies and practices generally, and makes specific recommendations on remuneration packages and other terms of employment for executive Directors, other senior executives and non-executive Directors.
The Nomination and Remuneration Committee is wholly comprised of independent members. The board is satisfied that all members of the Nomination and Remuneration Committee during the reporting period are independent, including Michael Spooner despite his long-standing tenure on the board and brief role as an executive Chairman following the company’s incorporation.
The Nomination and Remuneration Committee is primarily responsible for making recommendations to the Board on:
•Board appointments
•Non-executive director fees
•Executive remuneration framework
104
•Remuneration for executive directors, namely the CEO, and other key executives
•Short-term and long-term incentive awards
•Share ownership plans
The Nomination and Remuneration Committee’s objective is to ensure remuneration policies are fair and competitive and have regard for industry benchmarks whilst being aligned with the objectives of our company.
The Committee receives proposals from the executive team, which it critically reviews. When appropriate the Nomination and Remuneration Committee will seek advice or recommendations from independent expert consultants, including benchmarking studies. Advice provided by consultants during the year did not constitute a ‘remuneration recommendation’ as a defined in section 9B of the Corporations Act and was received free from any undue influence by Key Management Personnel to whom the advice related.
Executive Remuneration Strategy
The Company’s remuneration strategy is designed to ensure Mesoblast can:
•Attract and retain experienced leaders and emerging experts in an innovative field and on a global basis
•Reward performance that will lead in the long term to improved patient outcomes and increased shareholder wealth.
Our team is small. Mesoblast has only 83 employees, 59% of whom are in the US, with the remainder in Australia, Singapore and Switzerland. Retaining these employees, who often are at the top of their respective fields, is imperative in ensuring Mesoblast can continue in a consistent manner to work towards what are difficult, complex and long-term goals.
Biopharmaceutical product development is a highly specialized and speculative undertaking and it involves a substantial degree of risk. To achieve and maintain long term profitability, companies must successfully develop product candidates, obtain regulatory approval, and manufacture, market and sell those products for which regulatory approval is obtained. If this occurs, revenues depend on the size of markets in which product candidates receive approval, the ability to achieve and maintain sufficient market acceptance, pricing, reimbursement from third-party payors, and adequate market share for our product candidates in those markets. Not all companies succeed in these activities, and not all companies generate revenue from product sales that is significant enough to achieve profitability.
To have a chance of success, it is imperative that executives
a)possess the specialized skills to understand the complex products being developed and the various regulatory requirements imposed across the globe
b)apply high degrees of discipline to ensure research and trials are undertaken safely and effectively, to a rigorous standard and schedule, within tight budget constraints
c)seek to deliver earlier, with lower costs, key, well-defined milestones critical to progressing Mesoblast technology
d)stay focused on the end goal of commercialization.
While it may be many years from initial research until milestones lead to profitable outcomes, this does not reduce the importance of the milestones themselves. Without the interim milestone steps on the way to therapy commercialization, the extensive safety and efficacy data required would not be sufficient and approval by global regulatory authorities would not be achievable. Time and costs are an important component in this process of research, testing and milestone achievement, as both have compounding effects on shareholder value.
To address the above, Mesoblast’s remuneration framework comprises:
-competitive fixed remuneration
-annual incentives payments contingent on intensive research, approvals and trials being undertaken on time and budget
105
-longer term milestone-based incentive payments
-payment delivered, in part, as options, which conserves cash, aligns with shareholder interests, and focuses executives on strategy, risk management, and execution that optimizes shareholder value.
Mesoblast generally sets cash-based STIs at a lower quantum than option-based LTIs to conserve cash flow, focus executives on value creation, and align executives with shareholders.
The current average tenure of our executive team of 9 years suggests that the framework works well to attract and retain appropriate executive leadership.
106
Executive Remuneration Framework
Further details on the Mesoblast Executive Remuneration Framework is provided in Table 2 – Executive Remuneration Framework.
Table 2 – Executive Remuneration Framework
| Fixed Pay | Performance-based Remuneration | ||||||||||||||||
| Short-term Incentives | Long-term Incentives | ||||||||||||||||
| Strategic Rationale | Attract and retain key personnel on a global basis via competitive remuneration. Comply with regional statutory and customary benefits (e.g., superannuation in Australia; medical insurance in the US.) | Focuses attention on key KPIs (in areas such as clinical, financial and partnering strategy, manufacturing, commercial, or organizational structure and development) under cost and time constraints that will lead to long-term improvement in patient outcomes and shareholder wealth. | Serves multi-pronged purpose: -Aligns remuneration outcomes with shareholder wealth creation. -Provides a framework for wealth creation by prioritizing key objectives that are critical for long-term profitability. -Rewards speed of achievement, that can have long term compounding effects -Retains employees via deferral -Provides value only if milestones accumulate for increases in share price, aligning with the shareholder experience. -Conserves cash. -Enables risk management via malus. | ||||||||||||||
| Process | Assessed annually on market relativities in relevant markets based on position accountabilities. The Nomination and Remuneration Committee makes specific recommendations to the board on remuneration packages for senior executives for approval. | Paid annually for performance against annual corporate and individual KPIs. The Nomination and Remuneration Committee sets the CEO’s KPIs. These are used to measure the company performance, which determines the pool available for other employees. Allocations from that pool for senior management are determined with reference to individual KPIs which have been set by the CEO. Resulting outcomes are approved by the Nomination and Remuneration Committee. | The Nomination and Remuneration Committee assesses vesting for the LTI milestones. | ||||||||||||||
| Eligibility | All employees | All employees hired on or before March 31, 2023 are eligible for consideration. Employees hired during the year are recognized on a pro-rata basis. | All eligible participants who are in positions to influence achievement of our long- term outcomes and, where required, for attraction and retention. | ||||||||||||||
107
| Quantum of opportunity | Set according to each position’s accountabilities, the incumbent’s experience and qualifications, and regional market relativities. | Set as a percentage of fixed pay. Quantum generally lower than LTI to conserve cash. Current CEO maximum STI: 50% of Fixed Remuneration. Current CMO maximum STI: 50% of Fixed Remuneration. | Set using a percentage of fixed pay as a guideline. Current CEO maximum LTI: approximately 200% of fixed remuneration. As disclosed in the FY20 AGM, the CEO grant was increased to bridge some of the gap with industry LTI practice while also decreasing the weighting of the CEO fixed remuneration and STI. Current CMO maximum LTI: 100% of fixed remuneration, excluding a sign on LTI granted The actual grant value for the CEO and CMO LTI may vary year on year from this proportion based on various factors being taken account including: -shareholder dilution -internal relativities -share price volatility While the value may fluctuate on a year-to-year basis, the guideline should stand on a long term basis. | ||||||||||||||
| Delivered as | Cash | Cash | Options over ordinary shares in Mesoblast Limited with a 7-year expiry date. Option exercise price will be based on the higher of the VWAP of the 5 ASX trading days up to board approval of the grant, and the last closing price of an ordinary share on the ASX at board approval. | ||||||||||||||
| Performance and service period | N/A | 1 year | Three years with provision for earlier vesting limited to one third per year to (a) encourage speed of achievement, and (b) defer material amounts for better governance and (c) encourage executive focus on achievements that have a longer term impact on shareholder value. | ||||||||||||||
108
| Discretion, malus and clawback | N/A | The board has the authority to use its discretion to amend individual outcomes “in year”, including down to zero, prior to any payment. | The board has ultimate discretion in determining vesting outcomes. Until options are exercised, the board may also apply discretion in situations where executives have behaved dishonestly or fraudulently to lapse options (unvested and vested). | ||||||||||||||
| Cessation of employment | No award will be made to employees who have ceased employment. | Unvested options are forfeited unless Board exercises discretion. Vested options can be retained subject to being exercised within a certain period specified in our Employee Share Option Rules (usually 60 days of cessation). | |||||||||||||||
| Hedging | The company’s share trading policy prohibits hedging via the company’s derivatives. | ||||||||||||||||
| Oversight | Individual outcomes are reviewed and approved first by the Nomination & Remuneration Committee and then the Board. | ||||||||||||||||
Remuneration Mix
FY23 target remuneration mix at maximum for the CEO and the CMO is described in Figure 1.
Figure 1 – Executive KMP Remuneration Mix.
The actual grant value year-on-year may vary from the target remuneration mix depending on factors such as:
•Dilution considerations
•Internal relativities
•Date of grant
109
Responses to frequent questions on the Mesoblast framework
The following table presents responses to common queries on the Mesoblast remuneration framework.
Table 3 – Executive Remuneration Framework
| Why do you use milestone performance measures for the STI and LTI? | Traditional financial metrics are not meaningful, nor can they be effectively used to accurately reflect the performance of our company. What creates lasting shareholder value are successful outcomes from research and development, entry into new collaborations and achievement of other planned and well considered corporate objectives. Success will only result in significant reward under the LTI if the market values our achievements. If it does, our share price increases. The LTI options become valuable. If not, the options have no intrinsic value. This combination of milestones and payment in options work in tandem for a sober, fair payment for performance aligned with shareholder returns. This is a standard biotechnology company practice. | ||||
| Why does some of the long term incentive award vest earlier than a three year period? | Within biotechnology, basing long term incentives on achievement of performance milestones is an established method for aligning pay with performance. The other factor that is critical is time. While we allow three years for milestones, earlier achievement is better, because if milestones are achieved earlier then less cash will have been used by the Group to support the program and associated overheads than if achieved at the end of 3 years. Therefore, we have configured the plan to allow for early vesting for early achievement, but only to a point. We still insist that even if all milestones are achieved early, some options remain unvested for 3 years, to ensure that, if given a choice with a limited budget, employees focus on those milestones most likely to deliver the most value over the longer term, as well as encouraging employee retention. We believe that this framework is innovative, and a great fit for the nature of our business. We acknowledge it does not look and feel like a typical ASX-listed company LTI, and therefore may not meet the standard guidelines applied by many, but we are not typical. We are open to considering alternatively designed incentives that address the value drivers of milestone achievement, time to achieve them, prioritization of milestones with most value potential given limited resourcing, and impact on longer term share price, but so far we have not found any quite as effective. | ||||
| What is Mesoblast’s position on diversity? | The Group values diversity and recognizes the benefits it can bring to the organization’s ability to achieve its goals. Diversity can lead to a competitive advantage through broadening the talent pool for recruitment of high quality employees, by encouraging innovation and improving a corporation’s professionalism and reputation. Accordingly, the Group is committed to promoting diversity within the organization and has adopted a formal policy outlining the Group’s diversity objectives. With respect to gender diversity, as at June 30, 2023, 53% of the Company’s employee base were female and 33% of the Company’s senior executives were female. The Board is conscious of the gender imbalance at board level (with only one of the six non-executive directors being female) and has an objective to increase this number as vacancies arise and circumstances permit. | ||||
110
| Why is there no STI deferral? | STI is not a heavily weighted part of the remuneration framework across the Company. There is sufficient remuneration deferred already, in the form of unvested options, that would be at risk in the event of poor conduct, mismanagement or reputational damage. | ||||
| Why is there no consideration of ENS (Environment and Sustainability) issues in the STI or LTI vesting considerations? | Mesoblast’s mission to bring to market innovative medicines comprised of naturally-occurring cellular materials to treat serious and life-threatening illnesses is fundamentally consistent with ENS principles, although there are relevant supply chain and carbon footprint considerations. At this stage, Mesoblast’s physical footprint is limited to office and laboratory space for its employee base of less than 100, so while management is actively engaged in reducing the Company’s carbon footprint, its ability to materially improve its ENS impact is currently limited. We will continue to consider having ENS-related remuneration milestones in the future, in particular if and when Mesoblast has its own manufacturing facilities and approved products. | ||||
Mesoblast performance during FY2023
Table 4 provides share price performance data and selected financial results.
Table 4 – Company share price performance and selected financial results over the last five years
| Currency | 2023 | 2022 | 2021 | 2020 | 2019 | |||||||||||||||||||||||||||||||||
| Share price (ASX:MSB) | ||||||||||||||||||||||||||||||||||||||
| – closing at June 30 | A$ | 1.14 | 0.61 | 1.98 | 3.25 | 1.48 | ||||||||||||||||||||||||||||||||
| – high for the year | A$ | 1.28 | 2.10 | 5.50 | 4.45 | 2.34 | ||||||||||||||||||||||||||||||||
| – low for the year | A$ | 0.68 | 0.61 | 1.72 | 1.02 | 1.04 | ||||||||||||||||||||||||||||||||
| Market capitalization at June 30 (millions) | A$ | 924 | 397 | 1,285 | 1,898 | 738 | ||||||||||||||||||||||||||||||||
| – increase/(decrease) – (millions) | A$ | 527 | (888) | (613) | 1,160 | 24 | ||||||||||||||||||||||||||||||||
| – increase/(decrease) – as % | 133% | (69%) | (32%) | 157% | 3% | |||||||||||||||||||||||||||||||||
| Revenue (millions) | US$ | 7.5 | 10.2 | 7.5 | 32.2 | 16.7 | ||||||||||||||||||||||||||||||||
| - increase/(decrease) - as % | (27%) | 37% | (77%) | 92% | (4%) | |||||||||||||||||||||||||||||||||
| Loss before income tax (millions) | US$ | 82.1 | 91.6 | 99.6 | 87.4 | 98.8 | ||||||||||||||||||||||||||||||||
| Net Assets (millions) | US$ | 501.8 | 497.0 | 581.4 | 549.3 | 481.1 | ||||||||||||||||||||||||||||||||
| Dividends paid | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Return of Capital to Shareholders | — | — | — | — | — | |||||||||||||||||||||||||||||||||
During FY23 Mesoblast completed the resubmission of the Biologics License Application (BLA) to FDA for remestemcel-L for the treatment of pediatric SR-aGVHD. In August 2023, the FDA provided a complete response to our BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data.
Mesoblast intends to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. Completing an adult study is in line with our overall commercial strategy, which envisioned a sequenced progression from pediatric to adult SR-aGVHD indications. As part of its review the FDA completed the Pre-License Inspection ("PLI") of the manufacturing facility, did not issue any Form 483, and found no objectionable conditions. In addition, FDA acknowledged in the resubmission review that changes implemented appear to improve assay performance relative to the original version of the assay used in the pediatric Phase 3 trial.
Mesoblast continued its focus on financial discipline and accountability throughout the year. After reducing net cash usage for operating activities by 35% in the financial year ended June 30, 2022 we continued to contain our spend, reducing net cash usage for operating activities by a further 4% in the financial year ended June 30, 2023. This has resulted
111
in a significant reduction in operating expenditure, effectively preserving capital and extending the Company’s cash runway.
In relation to funding, with support from its largest shareholders the Company raised $45.0 million and $40.0 million through private placements in August 2022 and April 2023, respectively. These raises strengthen our consolidated balance sheet as we undertake activities for the potential launch and commercialization of remestemcel-L.
In summary management executed on the following corporate achievements:
-We closed a $45 million private placement in August 2022 for which credit was attributed in the FY22 STI assessment, and a further $40 million was raised through a private placement in April 2023.
-Our senior debt facility was successfully refinanced to extend the availability of undrawn tranches to align with our regulatory timelines.
-During the year we appointed Jane Bell as a non-executive director and appointed Dr. Philip Krause, a member of the Board of Directors, to a formal strategic advisory role. Dr. Krause will advise the Group on its regulatory strategies related to broadening out the remestemcel-L platform, including the ground breaking treatment for SR-aGVHD, inflammatory bowel disease and inflammatory lung disease, and the second-generation platform rexlemestrocel-L, including the Phase 3 candidates for discogenic chronic lower back pain and inflammatory heart failure.
In relation to our product candidates, management executed on the following achievements:
-The DREAM-HF Phase 3 trial results were published in the premier peer-reviewed journal for cardiovascular medicine, the Journal of the American College of Cardiology (“JACC”). The results of the randomized, double-blind, controlled study in 537 patients showed that Mesoblast’s mesenchymal precursor cell therapy (“MPCs”; rexlemestrocel-L) strengthened heart function at 12 months, as measured by left ventricular ejection fraction (“LVEF”) and decreased cardiovascular death, myocardial infarction (“MI”) or stroke in patients with chronic heart failure due to reduced ejection fraction (“HFrEF”) over a mean follow-up of 30 months.
-Two studies on the remestemcel-L for the treatment of children with SR-aGVHD were selected by peer review to be presented at the 2023 Tandem Meetings of the American Society for Transplantation and Cellular Therapy ("ASTCT") and the Center for Blood and Marrow Transplant Research ("CIBMTR").
-The FDA Office of Tissues and Advanced Therapies ("OTAT") had granted Regenerative Medicine Advanced Therapy ("RMAT") designation for rexlemestrocel-L in the treatment of CLBP associated with disc degeneration, in combination with hyaluronic acid (HA) as delivery agent for injection into the lumbar disc.
-Resubmitted to the FDA the BLA for approval of remestemcel-L in the treatment of children with SR-aGVHD
-Announced top-line long-term survival results for remestemcel-L from a four-year observational cohort survival study performed by the CIBMTR on 51 evaluable children with SR-aGVHD who were enrolled in Mesoblast’s phase 3 clinical trial (MSB-GVHD001) of remestemcel-L across 20 centers in the US. The results showed durable survival through 4 years of follow-up.
-Announced that analysis from the DREAM-HF Phase 3 trial showed that patients with chronic heart failure and reduced ejection fraction (“HFrEF”) treated with rexlemestrocel-L demonstrated greater improvement in the pre-specified analysis of left ventricular ejection fraction at 12 months relative to controls. Improvement in LVEF was most pronounced in the setting of inflammation and preceded long-term reduction in the 3-point MACE of cardiovascular death, non-fatal heart attack or stroke.
Remuneration outcomes for the year ended June 30, 2023
STI
The CEO’s STI objectives and outcomes for FY23 are described in Table 5.
The Board assessed our company’s performance on these KPIs for the financial year ended June 30, 2023 as achieving 60% of targets. This reflects achievement of several significant operational goals. When assessed in the context
112
of our current cash position, the Board determined that performance did not meet a sufficient threshold for an STI payment in FY23. Subsequent to FY23, there has been a modification to the conditions of achievement of the STI outcome relating to the financial year ended June 30, 2023, which is now dependent on an FDA approval of remestemcel-L in the treatment of graft versus host disease for employees who remain employed with Mesoblast through to at least June 30, 2024.
| Table 5 - Performance against FY23 STI KPIs | |||||||||||||||||
| KPI Category/Performance Assessment | Maximum as % of total STI | Rating | Outcome as % of total STI | ||||||||||||||
| Execute on FDA approval of Remestemcel-L for the treatment of acute GVHD | |||||||||||||||||
Despite the FDA issuing a Complete Response Letter (CRL) to the Group's BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD requiring more data to support marketing approval, including potency assay or clinical data, progress was made during the year with the FDA completing a Pre-License Inspection of the manufacturing facility after which they did not issue any Form 483, and found no objectionable conditions. In addition, FDA acknowledged in the resubmission review that changes implemented appear to improve assay performance relative to the original version of the assay used in the pediatric Phase 3 trial. In line with the Group's overall commercial strategy to progress to adult populations, the Group intends to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. The Board acknowledges that the BLA resubmission timeline has not been achieved as planned and therefore the Board assessed that this objective was only partially achieved. | 45% | 44% | 20% | ||||||||||||||
| Execute on Financing Strategy | |||||||||||||||||
| In relation to Finance, there have been substantial achievements during the year. Our senior debt facility was successfully refinanced to extend the availability of undrawn tranches to September 30, 2023. We closed a $45 million private placement in August 2022 for which credit was attributed in the FY22 STI assessment, and a further $40 million was raised through a private placement in April 2023. The Board has decided this objective has been met. | 25% | 100% | 25% | ||||||||||||||
| Execute on Pipeline, Manufacturing Process Development and Organizational Structure & Development | |||||||||||||||||
| During the year we progressed the development of our pipeline assets, enhanced our manufacturing processes and appointed one of our existing non-executive directors, Dr Philip Krause, to a formal strategic advisory role. Despite this progress, because Dr Krause was already a director and a key element of the KPI was to appoint senior management and improve diversity, the Board has decided this objective has been partially met. | 30% | 50% | 15% | ||||||||||||||
LTI
Two conditions must be met for milestone options to vest.
•The milestone for that option must be met
•Achievement must be within the performance period
When LTI milestones are set it is not expected that all or any milestones will be achieved within the next 12 months. The LTI plan is designed to align the CEO objectives with creating long term shareholder value.
The vesting of the CEO’s LTI is based on meeting clinical and commercialization milestones, as well as completion of licensing or collaboration agreements to build shareholder value.
Details on the LTI options that could have vested based on both FY23 performance and prior year performance are summarized in Table 6a, along with the financial year in which those options will vest after milestones have been met.
113
Where an LTI milestone remains commercial in confidence it has been described in general terms. Many milestones also have an associated delivery window and/or budget which are taken into account when determining if it was achieved. Some clinical outcomes can be partially met depending on the quality and/or cost of results or extent of patient participation.
Table 6a – LTI Outcomes of CEO milestone-based grants
| Number of options granted/Date granted | Milestone | Portion of grant attributed to milestone | Status | FY in which the tranche will vest after factoring in time- based vesting conditions | |||||||||||||
| CEO | 2,325,000 Nov 2022(1) | •Commercial and clinical milestones in relation to the potential launch of remestemcel-L. •Financial and business development milestones in relation to remestemcel-L and rexlemestrocel-L platforms | 60% 40% | Pending Pending | Pending Pending | ||||||||||||
1,550,000 Nov 2021(2) | •Regulatory/Commercialisation progress with respect to our aGVHD program and clinical progress across the Company’s lead programs with specific allocation for each program milestone based on priority. •Completion of a significant licensing/collaboration agreement to build shareholder value and other confidential financing objectives. •Manufacturing milestones related to process development. | 40% 40% 20% | 33% Achieved 7% Pending Pending Pending | FY23 - Nil(6) FY24 - 100% Pending Pending Pending | |||||||||||||
114
1,200,000 Nov 2020(3) | •Clinical/Commercialisation milestones related to clinical and commercialization progress across the Company’s lead programs. •Completion of a significant licensing/collaboration agreement to build shareholder value and other confidential financing objectives. •Manufacturing milestones related to process development. | 40% 40% 20% | Achieved Pending Achieved | FY22- Nil(7) FY23- 55.6% FY24- 44.4% Pending FY22- Nil(7) FY23- 55.6% FY24- 44.4% | |||||||||||||
1,346,667(4) Nov 2019 | •Granting of a PDUFA date for remestemcel-L(5). •US FDA approval of remestemcel-L(5). | 50% 50% | Achieved during FY20 Pending | FY21- 66.7% FY22- 33.3% Pending | |||||||||||||
(1)This grant was approved by the Board on October 17, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(2)This grant was approved by the Board on September 8, 2021 and granted on November 29, 2021 after shareholder approval for the grant was received at the AGM.
(3)This grant was approved by the Board on July 16, 2020 and granted on November 24, 2020 after shareholder approval for the grant was received at the AGM.
(4)This grant was approved by the Board on July 20, 2019 and granted on November 27, 2019 after shareholder approval for the grant was received at the AGM. 538,667 of the options granted were not milestone based and have not been included in the above table. The 538,667 options were granted as a substitute for a reduction made to the FY19 short-term cash bonus to conserve cash.
(5)For the treatment of pediatric SR acute GVHD.
(6)Regardless of when the milestone was achieved, the milestone vesting date is determined as the date of Board approval. In this case Board approval was in July 2023.
(7)Regardless of when the milestone was achieved, the milestone vesting date is determined as the date of Board approval. In this case Board approval was in August 2022.
115
Table 6b – LTI Outcomes of CMO milestone-based grants
| Number of options granted/Date granted | Milestone | Portion of grant attributed to milestone | Status | FY in which the tranche will vest after factoring in time- based vesting conditions | |||||||||||||
| CMO | 900,000 Nov 2022(1) | •Milestones related to the regulatory progress of remestemcel-L for pediatric patients with SR-aGVHD with the United States Food and Drug Administration (FDA), including the resubmission of its Biologics License Application and its potential approval by the FDA. •Milestone related to the clinical progress of the Company’s lead products. | 67% 33% | 34% Achieved 33% Pending Pending | FY24 - 100% Pending Pending | ||||||||||||
(1)This grant was approved by the Board on October 17, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
116
Table 7 represents remuneration paid to each executive KMP during the year as required by Section 300A of the Corporations Act 2001.
Table 7 – Statutory remuneration paid to executive KMP
| Short-term benefits | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Base salary | Short-term cash bonus | Annual Leave/ Holiday Pay(1) | Non- monetary benefits | Health and Other Benefits (2) | Post-employment benefits Super- annuation | Long-term benefits Long service leave(1) | Share- based payments Options | Other Termi- nation benefits | Total Statutory Remuneration | % of performance-based remuneration | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name | Year | Currency | $ | $ | $ | $ | $ | $ | $ | $ | $ | $ | % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silviu Itescu | 2023 | A$ | 1,010,000 | — | 77,694 | — | — | 25,292 | 16,880 | 536,138 | — | 1,666,004 | 32 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silviu Itescu | 2022 | A$ | 1,010,000 | 303,000 | 46,616 | — | — | 23,568 | 16,880 | 569,314 | — | 1,969,378 | 44 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eric Rose | 2023 | A$ | 916,542 | — | 5,878 | — | 34,194 | — | — | 578,236 | — | 1,534,850 | 38 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eric Rose | 2022 | A$ | 354,377 | 106,313 | 27,273 | — | — | — | — | 71,560 | — | 559,523 | 32 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Executive Directors | 2023 | A$ | 1,926,542 | — | 83,572 | — | 34,194 | 25,292 | 16,880 | 1,114,374 | — | 3,200,854 | 35 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Executive Directors | 2022 | A$ | 1,364,377 | 409,313 | 73,889 | — | — | 23,568 | 16,880 | 640,874 | — | 2,528,901 | 42 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Executive Directors | 2023 | US$(3) | 1,292,710 | — | 56,077 | — | 22,944 | 16,971 | 11,326 | 747,745 | — | 2,147,773 | 35 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Executive Directors | 2022 | US$(3) | 986,581 | 295,974 | 53,430 | — | — | 17,042 | 12,206 | 463,416 | — | 1,828,649 | 42 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Josh Muntner | 2022 | A$ | 88,047 | — | 4,066(4) | — | 9,011 | — | — | (288,561) | — | (187,437) | 154 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Executive KMP | 2022 | A$ | 88,047 | — | 4,066 | — | 9,011 | — | — | (288,561) | — | (187,437) | 154 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total Executive KMP | 2022 | US$(3) | 63,667 | — | 2,940 | — | 6,516 | — | — | (208,659) | — | (135,536) | 154 | % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1)Annual leave and Long service leave reflect the movement in provision balances at June 30, 2023 compared with June 30, 2022.
(2)Includes health, dental, vision, life, long and short-term disability insurances.
(3)The A$ results have been determined by calculating the average rate of the exchange rates on the last trading day of each month during the period. A US$:A$ exchange rate of 1:0.6710 has been used for the year ended June 30, 2023 and 1:0.7231 for the year ended June 30, 2022.
(4)The comparative figure for Josh Muntner for June 30, 2022 has been updated, resulting in an decrease of A$46,730 in the Annual Leave/Holiday Pay.
Fixed remuneration
The CEO fixed remuneration has not changed since 2015. Eric Rose was appointed as our CMO in FY22, his monthly fixed remuneration did not change in FY23.
Non-Executive Director (“NED”) Remuneration
As at June 30, 2023 the Board comprised of six NEDs; one based in Australia, three in the United States, one in Singapore and one in Switzerland. These directors are global experts in the biopharmaceutical industry and capital markets, each with relevant experience in biotechnology and/or healthcare industries.
The NED fees (in Table 8) reflect responsibilities and work involved with directing a company of Mesoblast’s technological and geographical complexity, our financial position, regulatory and compliance context, and market practice in each director’s domicile. The fee levels and structures reflect what is necessary to recruit and retain directors with global experience in this industry. There have been no changes to NED fees from last year.
117
Table 8 – NED fees
(exclusive of superannuation where applicable for Australian directors)
| As at June 30, 2023 | ||||||||||||||||||||||||||
| Position | Currency | Board of Directors | Audit and Risk Committee | Nomination and Remuneration Committee | ||||||||||||||||||||||
| Chair | US$ | 250,000 | — | — | ||||||||||||||||||||||
| Chair | A$ | — | 20,000 | 20,000 | ||||||||||||||||||||||
| Vice Chair | A$ | 175,000 | — | — | ||||||||||||||||||||||
| Member | A$ | 128,250 | 10,000 | 10,000 | ||||||||||||||||||||||
The NEDs' fixed fees for their services are not to exceed a maximum fee pool of A$1,500,000, as approved by shareholders at the 2018 Annual General Meeting.
NEDs do not receive performance-related remuneration and are not provided with retirement benefits other than statutory superannuation. NEDs are reimbursed for costs directly related to conducting Mesoblast business. The key terms of NED service are documented in a letter of appointment to the Board.
Mesoblast grants options to NEDs, usually at the start of their tenure. Options in lieu of cash are typical in the biotechnology industry. These options vest one third each after one, two and three years. For our NEDs, options are only forfeited if the director engages in conduct that is adverse to the company or breach the terms of their engagement.
The grants enable Mesoblast to secure NEDs with global pharmaceutical experience cash-effectively. Governance is not compromised because no performance or service conditions apply. The majority of shareholders voted in favor of our NED LTI grants at the November 2022 and 2021 AGMs.
Further details on the number of options and exercise price can be found in section “Terms and conditions of share-based payment arrangements”.
118
Remuneration Details - NEDs
Details of the remuneration of our NEDs for the years ended June 30, 2023 and June 30, 2022 are in Table 9.
Table 9 – Director Fees
| Year | Currency | Base Salary | Super- annuation | Share-based payments Options | Total Statutory Remuneration | |||||||||||||||||||||||||||||||||
| Jane Bell | 2023 | A$ | 128,798 | 13,524 | 66,804 | 209,126 | ||||||||||||||||||||||||||||||||
Jane Bell(1) | 2022 | A$ | — | — | — | — | ||||||||||||||||||||||||||||||||
| William Burns | 2023 | A$ | 195,247 | — | 3,989 | 199,236 | ||||||||||||||||||||||||||||||||
| William Burns | 2022 | A$ | 185,000 | — | 19,525 | 204,525 | ||||||||||||||||||||||||||||||||
| Philip Facchina | 2023 | A$ | 148,497 | — | 55,120 | 203,617 | ||||||||||||||||||||||||||||||||
| Philip Facchina | 2022 | A$ | 137,417 | — | 124,921 | 262,338 | ||||||||||||||||||||||||||||||||
| Philip Krause | 2023 | A$ | 138,497 | — | 69,199 | 207,696 | ||||||||||||||||||||||||||||||||
| Philip Krause | 2022 | A$ | 34,873 | — | 7,638(4) | 42,511 | ||||||||||||||||||||||||||||||||
| Donal O'Dwyer | 2022 | A$ | 105,500 | 10,550 | 2,534 | 118,584 | ||||||||||||||||||||||||||||||||
Eric Rose(2) | 2023 | A$ | — | — | 3,989 | 3,989 | ||||||||||||||||||||||||||||||||
Eric Rose(2) | 2022 | A$ | 74,813 | — | 19,525 | 94,338 | ||||||||||||||||||||||||||||||||
| Michael Spooner | 2023 | A$ | 158,250 | — | — | 158,250 | ||||||||||||||||||||||||||||||||
| Michael Spooner | 2022 | A$ | 158,250 | 9,231 | 2,534 | 170,015 | ||||||||||||||||||||||||||||||||
| Joseph Swedish | 2023 | A$ | 372,276 | — | — | 372,276 | ||||||||||||||||||||||||||||||||
| Joseph Swedish | 2022 | A$ | 344,157 | — | 19,731 | 363,888 | ||||||||||||||||||||||||||||||||
| Shawn Tomasello | 2023 | A$ | 23,042 | — | — | 23,042 | ||||||||||||||||||||||||||||||||
| Shawn Tomasello | 2022 | A$ | 138,250 | — | 463 | 138,713 | ||||||||||||||||||||||||||||||||
| Total Non-Executive Directors | 2023 | A$ | 1,164,607 | 13,524 | 199,101 | 1,377,232 | ||||||||||||||||||||||||||||||||
| Total Non-Executive Directors | 2022 | A$ | 1,178,260 | 19,781 | 196,871 | 1,394,912 | ||||||||||||||||||||||||||||||||
Total Non-Executive Directors (3) | 2023 | US$ | 781,451 | 9,075 | 133,597 | 924,123 | ||||||||||||||||||||||||||||||||
Total Non-Executive Directors (3) | 2022 | US$ | 851,999 | 14,304 | 142,357 | 1,008,660 | ||||||||||||||||||||||||||||||||
(1)Jane Bell was appointed on August 18, 2022 and was paid $Nil director fees in the year ended June 30, 2022.
(2)Eric Rose has been a non-executive director of Mesoblast since 2013. On February 1, 2022, he was appointed as an executive director of Mesoblast and payments of director fees ceased at that time. Share-based payments reported as part of Eric’s director fees above relate to options granted during his appointment as a non-executive director.
(3)The A$ results have been determined by calculating the average rate of the exchange rates on the last trading day of each month during the period. A US$:A$ exchange rate of 1:0.6710 has been used for the year ended June 30, 2023 and 1:0.7231 for the year ended June 30, 2022.
(4)At June 30, 2022, an estimate of the expense relating to the option award on appointment for Philip Krause was omitted from the Share-based Payments Options. The comparative figure has been updated, resulting in an increase of A$7,638 in the Share-based Payments Options.
119
Terms and conditions of option grants and equity holdings
Details of options over ordinary shares provided as remuneration to each director and member of key management personnel for the years ended June 30, 2023 and June 30, 2022 are provided in the tables below.
Table 10 – The value of options granted, exercised and lapsed.
| Number of options granted | Remuneration consisting of options (1) | Values of options granted (2) A$ | Value of options exercised (3) A$ | Value of options lapsed (4) A$ | |||||||||||||||||||||||||
| For the year ended June 30, 2023 | |||||||||||||||||||||||||||||
Silviu Itescu(5) | 2,325,000 | 32 | % | 1,422,203 | — | — | |||||||||||||||||||||||
Eric Rose(5) | 2,150,000 | 38 | % | 1,315,155 | — | — | |||||||||||||||||||||||
Jane Bell(6) | 200,000 | 32 | % | 128,780 | — | — | |||||||||||||||||||||||
| William Burns | — | 2 | % | — | — | — | |||||||||||||||||||||||
| Philip Facchina | — | 27 | % | — | — | — | |||||||||||||||||||||||
Philip Krause(7) | 200,000 | 33 | % | 120,120 | — | — | |||||||||||||||||||||||
| Michael Spooner | — | — | — | — | — | ||||||||||||||||||||||||
| Joseph Swedish | — | — | — | — | — | ||||||||||||||||||||||||
| Shawn Tomasello | — | — | — | — | — | ||||||||||||||||||||||||
| For the year ended June 30, 2022 | |||||||||||||||||||||||||||||
| Silviu Itescu | 1,550,000 | 29 | % | 386,105 | — | — | |||||||||||||||||||||||
| Eric Rose | — | 14 | % | — | — | — | |||||||||||||||||||||||
| William Burns | — | 10 | % | — | — | — | |||||||||||||||||||||||
Philip Facchina(8) | 200,000 | 48 | % | 222,000 | — | — | |||||||||||||||||||||||
| Philip Krause | — | — | — | — | — | ||||||||||||||||||||||||
| Donal O’Dwyer | — | 2 | % | — | — | — | |||||||||||||||||||||||
| Michael Spooner | — | 1 | % | — | — | — | |||||||||||||||||||||||
| Shawn Tomasello | — | — | — | — | — | ||||||||||||||||||||||||
| Joseph Swedish | — | 5 | % | — | — | — | |||||||||||||||||||||||
| Josh Muntner | — | NM | — | — | 97,500 | ||||||||||||||||||||||||
(1)The percentage of the value of remuneration consisting of options, based on the value of options expensed during the year presented in accordance with IFRS 2 Share-based Payment. For details on the assumptions made for each grant, see information in note 17 Share-based payments within Item 18 Financial Statements of this report.
(2)The fair value at grant date of options that were granted during the year presented as part of remuneration, determined using Black-Scholes valuation model and in accordance with IFRS 2 Share-based Payment. The grant date is the date at which the entity and the employee agree to a share-based payment arrangement, being when the entity and the employee have a shared understanding of the terms and conditions of the arrangement.
(3)The intrinsic value at exercise date of options that were exercised during the year presented, having been granted as part of remuneration previously.
(4)The intrinsic value at lapse date of options that lapsed during the year.
(5)This grant was approved by the Board on October 17, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(6)This grant was approved by the Board on August 24, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(7)This grant was approved by the Board on May 23, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(8)This grant was approved by the Board on April 15, 2021 and granted on November 29, 2021 after shareholder approval for the grant was received at the AGM.
120
There have been no modifications to any terms and conditions of share-based payment transactions during the years ended June 30, 2023 and 2022.
Reconciliation of Options held by KMP
The table below shows a reconciliation of options over ordinary shares of Mesoblast Limited held by each KMP from the beginning to the end of FY23.
Table 11 – Reconciliation of options held by each KMP during FY23.
| Balance at July 1, 2022 | Granted as compensation during FY2023 | Vested during FY2023 | Exercised during FY2023 | Forfeited / Lapsed during FY2023 | Balance at June 30, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name | Grant Date | Vested | Unvested | Number | Number | % | Number | % | Number | % | Vested and exercisable | Unvested | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silviu Itescu | 23-Nov-22(1) | — | — | 2,325,000 | — | — | — | — | — | — | — | 2,325,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silviu Itescu | 29-Nov-21(2) | — | 1,550,000 | — | — | — | — | — | — | — | — | 1,550,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silviu Itescu | 24-Nov-20(3) | — | 1,200,000 | — | 720,000 | 60 | — | — | — | — | 720,000 | 480,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Silviu Itescu | 27-Nov-19(4) | 1,032,446 | 852,888 | — | 179,555 | 10 | — | — | — | — | 1,212,001 | 673,333 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eric Rose | 23-Nov-22(1) | — | — | 1,250,000 | — | — | — | — | — | — | — | 1,250,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eric Rose | 23-Nov-22(1) | — | — | 900,000 | — | — | — | — | — | — | — | 900,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eric Rose | 30-Nov-18 | 120,000 | — | — | — | — | — | — | — | — | 120,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eric Rose | 27-Nov-19 | 66,666 | 33,334 | — | 33,334 | 33 | — | — | — | — | 100,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jane Bell | 23-Nov-22(5) | — | — | 200,000 | — | — | — | — | — | — | — | 200,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| William Burns | 30-Nov-18 | 120,000 | — | — | — | — | — | — | — | — | 120,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| William Burns | 27-Nov-19 | 66,666 | 33,334 | — | 33,334 | 33 | — | — | — | — | 100,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Philip Facchina | 29-Nov-21(6) | 66,667 | 133,333 | — | 66,667 | 33 | — | — | — | — | 133,334 | 66,666 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Philip Krause | 23-Nov-22(7) | — | — | 200,000 | 66,667 | 33 | — | — | — | — | 66,667 | 133,333 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Michael Spooner | 30-Nov-18 | 100,000 | — | — | — | — | — | — | — | — | 100,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Joseph Swedish | 27-Nov-19 | 300,000 | — | — | — | — | — | — | — | — | 300,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Joseph Swedish | 30-Nov-18 | 200,000 | — | — | — | — | — | — | — | — | 200,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shawn Tomasello | 30-Nov-18 | 200,000 | — | — | — | — | — | — | — | — | 200,000 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1)This grant was approved by the Board on October 17, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(2)This grant was approved by the Board on September 8, 2021 and granted on November 29, 2021 after shareholder approval for the grant was received at the AGM.
(3)This grant was approved by the Board on July 16, 2020 and granted on November 24, 2020 after shareholder approval for the grant was received at the AGM.
(4)This grant was approved by the Board on July 20, 2019 and granted on November 27, 2019 after shareholder approval for the grant was received at the AGM.
(5)This grant was approved by the Board on August 24, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(6)This grant was approved by the Board on April 15, 2021 and granted on November 29, 2021 after shareholder approval for the grant was received at the AGM.
(7)This grant was approved by the Board on May 23, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
Terms and conditions of share-based payment arrangements
The terms and conditions of each grant of options affecting remuneration in the current or a future reporting period are as follows:
121
Table 12 – Terms and conditions of share-based payment arrangements
| Grant date | Recipients of Grants | Vesting date | Expiry date | Exercise price A$ | Value per option at grant date A$ | |||||||||||||||||||||||||||
23-Nov-22(1) | Philip Krause | one third - 23-May-2023 one third - 23-May-2024 one third - 23-May-2025 | 22-May-29 | 1.01 | 0.60 | |||||||||||||||||||||||||||
23-Nov-22(2) | Jane Bell | one third - 24-Aug-2023 one third - 24-Aug-2024 one third - 24-Aug-2025 | 23-Aug-29 | 0.85 | 0.64 | |||||||||||||||||||||||||||
23-Nov-22(3) | Eric Rose | one third - 17-Oct-2023 one third - 17-Oct-2024 one third - 17-Oct-2025 | 16-Oct-29 | 1.03 | 0.61 | |||||||||||||||||||||||||||
23-Nov-22(3) | Silviu Itescu Eric Rose | Vesting in accordance with the following schedule, but only after achievement of performance milestones: one third - 17-Oct-2023 one third - 17-Oct-2024 one third - 17-Oct-2025 | 16-Oct-29 | 1.03 | 0.61 | |||||||||||||||||||||||||||
29-Nov-21(4) | Silviu Itescu | Vesting in accordance with the following schedule, but only after achievement of performance milestones: one third - 8-Sep-2022 one third - 8-Sep-2023 one third - 8-Sep-2024 | 7-Sep-28 | 1.77 | 0.42 | |||||||||||||||||||||||||||
29-Nov-21(5) | Philip Facchina | one third - 15 Apr 2022 one third - 15 Apr 2023 one third - 15 Apr 2024 | 14-Apr-28 | 2.28 | 1.11 | |||||||||||||||||||||||||||
24-Nov-20(6) | Silviu Itescu | Vesting in accordance with the following schedule, but only after achievement of performance milestones: one third - 16-Jul-2021 one third - 16-Jul-2022 one third - 16-Jul-2023 | 15-Jul-27 | 3.41 | 0.92 | |||||||||||||||||||||||||||
27-Nov-19(7) | Silviu Itescu | Vesting in accordance with the following schedule, but only after achievement of performance milestones: one third - 19-Jul-2020 one third - 19-Jul-2021 one third - 19-Jul-2022 | 19-Jul-26 | 1.47 | 1.03 | |||||||||||||||||||||||||||
27-Nov-19(7) | Silviu Itescu | one third - 19-Jul-2020 one third - 19-Jul-2021 one third - 19-Jul-2022 | 19-Jul-26 | 1.47 | 1.03 | |||||||||||||||||||||||||||
| 27-Nov-19 | William Burns Eric Rose | one third - 17 Nov 2020 one third - 17 Nov 2021 one third - 17 Nov 2022 | 17-Nov-26 | 1.83 | 0.94 | |||||||||||||||||||||||||||
| 27-Nov-19 | Joseph Swedish | one third - 4 Apr 2020 one third - 4 Apr 2021 one third - 4 Apr 2022 | 3-Apr-26 | 1.48 | 0.78 | |||||||||||||||||||||||||||
| 30-Nov-18 | William Burns Eric Rose Michael Spooner | one third - 30 Nov 2019 one third - 30 Nov 2020 one third - 30 Nov 2021 | 29-Nov-25 | 1.33 | 0.54 | |||||||||||||||||||||||||||
| 30-Nov-18 | Joseph Swedish | one third - 18 Jun 2019 one third - 18 Jun 2020 one third - 18 Jun 2021 | 17-Jun-25 | 1.52 | 0.85 | |||||||||||||||||||||||||||
| 30-Nov-18 | Shawn Tomasello | one third - 11 Jul 2019 one third - 11 Jul 2020 one third - 11 Jul 2021 | 10-Jul-25 | 1.56 | 0.78 | |||||||||||||||||||||||||||
(1)This grant was approved by the Board on May 23, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(2)This grant was approved by the Board on August 24, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
122
(3)This grant was approved by the Board on October 17, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
(4)This grant was approved by the Board on September 8, 2021 and granted on November 29, 2021 after shareholder approval for the grant was received at the AGM.
(5)This grant was approved by the Board on April 15, 2021 and granted on November 29, 2021 after shareholder approval for the grant was received at the AGM.
(6)This grant was approved by the Board on July 16, 2020 and granted on November 24, 2020 after shareholder approval for the grant was received at the AGM.
(7)This grant was approved by the Board on July 20, 2019 and granted on November 27, 2019 after shareholder approval for the grant was received at the AGM.
Table 13 - Shares provided to KMPs on the exercise of remuneration options
| No. of options exercised during the period | No. of ordinary shares in Mesoblast Limited issued | Exercise Date | Value per share at exercise date A$ | Exercise price per option A$ | |||||||||||||||||||||||||
| For the year ended June 30, 2023 | |||||||||||||||||||||||||||||
| Nil | — | — | — | — | — | ||||||||||||||||||||||||
| For the year ended June 30, 2022 | |||||||||||||||||||||||||||||
| Nil | — | — | — | — | — | ||||||||||||||||||||||||
KMP Shareholdings
The table below shows a reconciliation of ordinary shares held by each KMP from the beginning to the end of the 2023 financial year.
Table 14 – KMP Shareholdings
| Name | Balance at the start of the year | Received during the year upon exercise of options | Acquisitions/(Disposals) during the year | Balance at the end of the year | ||||||||||||||||||||||
| Silviu Itescu | 68,958,928 | — | — | 68,958,928 | ||||||||||||||||||||||
| Eric Rose | — | — | — | — | ||||||||||||||||||||||
| Jane Bell | — | — | 247,618 | 247,618 | ||||||||||||||||||||||
| William Burns | 63,000 | — | 22,000 | 85,000 | ||||||||||||||||||||||
| Philip Facchina | 273,224 | — | 1 | 273,225 | ||||||||||||||||||||||
| Philip Krause | — | — | 100,000 | 100,000 | ||||||||||||||||||||||
Michael Spooner(1) | 1,091,335 | — | — | 1,091,335 | ||||||||||||||||||||||
| Joseph Swedish | — | — | — | — | ||||||||||||||||||||||
(1)This total includes shareholdings of related parties, of this balance, Mr. Spooner has a relevant interest, as defined under the Corporations Act, of 1,069,000 ordinary shares.
123
Employment Agreements
The employment of our CEO and CMO are formalized in employment agreements, the key terms of which are as follows:
Table 15 – KMP Employment Agreements
| Name | Term | Notice period | Termination benefit | |||||||||||||||||
| Silviu Itescu (CEO) | Initial term of 3 years commencing April 1, 2014, and continuing subject to a 12 months’ notice period. | 12 months | 12 months base salary | |||||||||||||||||
| Eric Rose (CMO) | An ongoing employment agreement until notice is given by either party. | 3 months | 3 months base salary | |||||||||||||||||
On termination of employment our CEO, who is based in Australia, is entitled to receive his statutory entitlements of accrued annual and long service leave, together with any superannuation benefits.
On termination of employment our CMO, who is based in the United States, is entitled to participate in the Company’s healthcare plan during the severance period.
There is no entitlement to a termination payment in the event of resignation (except, in the case of the CMO, if the Company has materially reduced his role or benefits or materially moved office location) or removal for misconduct.
KMP Loans or other related transactions
Philip Krause was appointed to a formal strategic advisory role on June 4, 2023. The consulting agreement is in addition to Philip Krause's existing role as non executive director, the terms of which remain unchanged. He will provide specialist regulatory advisory services and will be remunerated at an hourly rate. The agreement is ongoing with either party able to terminate on 15 written days notice. The total aggregate fees paid to Philip Krause as of June 30, 2023 was US$110,383.
There were no loans or other related transactions with KMP during the financial year.
(End of Remuneration Report)
124
Employee Profile
As of June 30, 2023, we had 83 (2022:77) employees globally:

59% of our employees and a majority of our executives are based in the United States where Mesoblast operational activities are concentrated.
Australia is the corporate headquarters where 29% of the employees work. This includes the CEO and a portion of the executive team. The remaining 11% of employees are located in Singapore and 1% in Switzerland where research and development activities are primarily conducted.
125
Australian Disclosure Requirements
Options Granted as Remuneration
The following table presents options that have been granted over unissued shares during or since the end of the year ended June 30, 2023, to our Directors and our next 5 most highly remunerated officers.
Table 16 – Options Granted as Remuneration
| Name | Issue Date | Exercise Price A$ | Number of shares, under option | |||||||||||||||||
| Directors | ||||||||||||||||||||
| Silviu Itescu | 23-Nov-22(1) | 1.03 | 2,325,000 | |||||||||||||||||
| Eric Rose | 23-Nov-22(1) | 1.03 | 2,150,000 | |||||||||||||||||
| Non-Directors | ||||||||||||||||||||
| Dagmar Rose-Bjorkeson | 17-Oct-22 | 1.03 | 825,000 | |||||||||||||||||
| Kenneth Borow | 17-Oct-22 | 1.03 | 525,000 | |||||||||||||||||
| Michael Schuster | 17-Oct-22 | 1.03 | 600,000 | |||||||||||||||||
| Peter Howard | 17-Oct-22 | 1.03 | 675,000 | |||||||||||||||||
| Justin Horst | 17-Oct-22 | 1.03 | 450,000 | |||||||||||||||||
(1)This grant was approved by the Board on October 17, 2022 and granted on November 23, 2022 after shareholder approval for the grant was received at the AGM.
KMP Interests
The relevant interest of each KMP, as defined by section 608 of the Corporations Act, in the share capital of Mesoblast, as notified by the directors to the ASX in accordance with section 205G(1) of the Corporations Act, at the date of this report is as follows:
Table 17 – KMP Interests
| Director | Mesoblast Limited ordinary shares | Options over Mesoblast Limited ordinary shares | ||||||||||||
| Silviu Itescu | 68,958,928 | 6,960,334 | ||||||||||||
| Eric Rose | — | 2,370,000 | ||||||||||||
| Jane Bell | 247,618 | 200,000 | ||||||||||||
| William Burns | 85,000 | 220,000 | ||||||||||||
Philip Facchina(1) | 273,225 | 200,000 | ||||||||||||
| Philip Krause | 100,000 | 200,000 | ||||||||||||
| Michael Spooner | 1,069,000 | 100,000 | ||||||||||||
| Joseph Swedish | — | 500,000 | ||||||||||||
| Shawn Tomasello | — | 200,000 | ||||||||||||
(1)Mr Facchina also has a relevant interest in 68,306 warrants over ordinary shares.
126
Meeting of Directors
The number of meetings our board of directors (including committee meetings of directors) held during the year ended June 30, 2023 and the number of meetings attended by each director were:
Table 18 – Meeting of Directors
| Board of Directors | Audit and Risk Committee | Nomination and Remuneration Committee | ||||||||||||||||||||||||||||||||||||
| Director | A* | B* | A | B | A | B | ||||||||||||||||||||||||||||||||
| Silviu Itescu | 9 | 9 | — | — | 4 | 4 | ||||||||||||||||||||||||||||||||
| Eric Rose | 9 | 9 | — | — | 3 | 3 | ||||||||||||||||||||||||||||||||
| Jane Bell | 7 | 7 | 3 | 2 | 3 | 3 | ||||||||||||||||||||||||||||||||
| William Burns | 9 | 7 | — | — | 4 | 3 | ||||||||||||||||||||||||||||||||
| Philip Facchina | 9 | 9 | 4 | 4 | 4 | 4 | ||||||||||||||||||||||||||||||||
| Philip Krause | 9 | 7 | — | — | 4 | 4 | ||||||||||||||||||||||||||||||||
| Michael Spooner | 9 | 9 | 4 | 4 | 4 | 4 | ||||||||||||||||||||||||||||||||
| Joseph Swedish | 9 | 8 | 4 | 3 | 4 | 3 | ||||||||||||||||||||||||||||||||
| Shawn Tomasello | 2 | — | — | — | 1 | 1 | ||||||||||||||||||||||||||||||||
A = Number of meetings held during the time the director held office or was a member of the committee.
B = Number of meetings attended by board/committee members
* = This includes both meetings scheduled in the board calendar as well as teleconference meetings organized on an ad-hoc basis. For the most part, each director attended every scheduled meeting in the board calendar.
Shares under option
Unissued ordinary shares of Mesoblast Limited under option at the date of this Directors’ report are as follows:
| Grant date | Exercise price of options A$ | Expiry date of options | Number of shares under option | |||||||||||||||||
| 8/7/2020 | 2.86 | 8/7/2023 | 1,500,000 | |||||||||||||||||
| 6/12/2016 | 1.31 | 5/12/2023 | 533,000 | |||||||||||||||||
| 6/12/2016 | 1.19 | 5/12/2023 | 1,950,730 | |||||||||||||||||
| 16/9/2017 | 1.54 | 15/9/2024 | 50,000 | |||||||||||||||||
| 13/10/2017 | 1.94 | 12/10/2024 | 975,000 | |||||||||||||||||
| 13/10/2017 | 1.76 | 12/10/2024 | 902,425 | |||||||||||||||||
| 24/11/2017 | 1.41 | 23/11/2024 | 750,000 | |||||||||||||||||
| 24/11/2017 | 1.28 | 23/11/2024 | 750,000 | |||||||||||||||||
| 18/6/2018 | 1.52 | 17/6/2025 | 200,000 | |||||||||||||||||
| 11/7/2018 | 1.56 | 10/7/2025 | 200,000 | |||||||||||||||||
| 18/7/2018 | 1.87 | 17/7/2025 | 3,133,332 | |||||||||||||||||
| 18/7/2018 | 1.87 | 17/7/2025 | 350,000 | |||||||||||||||||
| 30/11/2018 | 1.33 | 29/11/2025 | 590,000 | |||||||||||||||||
| 19/1/2019 | 1.45 | 18/1/2026 | 3,333 | |||||||||||||||||
| 19/1/2019 | 1.45 | 18/1/2026 | 150,000 | |||||||||||||||||
| 4/4/2019 | 1.48 | 3/4/2026 | 300,000 | |||||||||||||||||
| 20/7/2019 | 1.62 | 19/7/2026 | 3,018,669 | |||||||||||||||||
| 20/7/2019 | 1.47 | 19/7/2026 | 2,833,332 | |||||||||||||||||
| 20/7/2019 | 1.47 | 19/7/2026 | 1,346,667 | |||||||||||||||||
| 20/7/2019 | 1.47 | 19/7/2026 | 538,667 | |||||||||||||||||
| 20/7/2019 | 1.47 | 19/7/2026 | 700,000 | |||||||||||||||||
127
| 25/11/2019 | 1.98 | 24/11/2026 | 20,000 | |||||||||||||||||
| 29/5/2019 | 1.48 | 28/5/2026 | 350,000 | |||||||||||||||||
| 17/11/2019 | 1.83 | 17/11/2026 | 200,000 | |||||||||||||||||
| 25/11/2019 | 1.80 | 24/11/2026 | 100,000 | |||||||||||||||||
| 25/11/2019 | 1.98 | 24/11/2026 | 150,000 | |||||||||||||||||
| 24/1/2020 | 3.38 | 23/1/2027 | 10,000 | |||||||||||||||||
| 18/5/2020 | 4.02 | 17/5/2027 | 1,200,000 | |||||||||||||||||
| 18/5/2020 | 3.65 | 17/5/2027 | 1,200,000 | |||||||||||||||||
| 16/7/2020 | 3.75 | 15/7/2027 | 3,253,333 | |||||||||||||||||
| 16/7/2020 | 3.41 | 15/7/2027 | 685,000 | |||||||||||||||||
| 16/7/2020 | 3.41 | 15/7/2027 | 1,050,000 | |||||||||||||||||
| 16/7/2020 | 3.41 | 15/7/2027 | 350,000 | |||||||||||||||||
| 16/7/2020 | 3.41 | 15/7/2027 | 300,000 | |||||||||||||||||
| 16/7/2020 | 3.41 | 15/7/2027 | 1,200,000 | |||||||||||||||||
| 11/9/2020 | 4.78 | 10/9/2027 | 200,000 | |||||||||||||||||
| 20/11/2020 | 3.60 | 19/11/2027 | 200,000 | |||||||||||||||||
| 20/11/2020 | 3.60 | 19/11/2027 | 100,000 | |||||||||||||||||
| 17/2/2021 | 2.67 | 16/2/2028 | 250,000 | |||||||||||||||||
| 15/4/2021 | 2.28 | 14/4/2028 | 200,000 | |||||||||||||||||
| 8/9/2021 | 1.95 | 7/9/2028 | 3,119,667 | |||||||||||||||||
| 8/9/2021 | 1.77 | 7/9/2028 | 2,000,000 | |||||||||||||||||
| 8/9/2021 | 1.77 | 7/9/2028 | 1,850,000 | |||||||||||||||||
| 8/9/2021 | 1.77 | 7/9/2028 | 1,550,000 | |||||||||||||||||
| 23/12/2021 | 1.42 | 22/12/2028 | 200,000 | |||||||||||||||||
| 17/10/2022 | 1.03 | 16/10/2029 | 1,250,000 | |||||||||||||||||
| 23/5/2022 | 1.01 | 22/5/2029 | 200,000 | |||||||||||||||||
| 24/8/2022 | 0.85 | 23/8/2029 | 200,000 | |||||||||||||||||
| 17/10/2022 | 1.13 | 16/10/2029 | 5,604,500 | |||||||||||||||||
| 17/10/2022 | 1.03 | 16/10/2029 | 4,350,000 | |||||||||||||||||
| 17/10/2022 | 1.13 | 16/10/2029 | 225,000 | |||||||||||||||||
| 17/10/2022 | 1.03 | 16/10/2029 | 3,225,000 | |||||||||||||||||
| 17/10/2022 | 1.03 | 16/10/2029 | 1,200,000 | |||||||||||||||||
| 8/8/2022 | 0.93 | 7/8/2029 | 100,000 | |||||||||||||||||
| 11/12/2020 | 4.60 | 10/12/2027 | 100,000 | |||||||||||||||||
| 21/11/2022 | 1.12 | 20/11/2029 | 100,000 | |||||||||||||||||
| 30/3/2023 | 1.03 | 29/3/2030 | 150,000 | |||||||||||||||||
| Grand Total | 57,217,655 | |||||||||||||||||||
No option holder has any right under the options plan to participate in any other of our share issues.
Shares issued on exercise of options during the year
There were no exercise of options during or since the end of the financial year.
Indemnification of Officers
During the financial year, we paid premiums in respect of a contract insuring our directors and company secretaries, and all of our executive officers. The liabilities insured are to the extent permitted by the Corporations Act 2001. Further disclosure required under section 300(9) of the Corporations Act 2001 is prohibited under the terms of the insurance contract.
128
Proceedings on Our Behalf
The Corporations Act 2001 allows specified persons to bring, or intervene in, proceedings on our behalf. No proceedings have been brought or intervened in on our behalf with leave of the Court under section 237 of the Corporations Act 2001.
Non-Audit Services
We may decide to employ the auditor on assignments additional to their statutory audit duties where the auditor’s expertise and experience are relevant and considered to be important.
The board of directors considers the position and in accordance with advice received from the audit committee, only permits the provision of the non-audit services compatible with the general standard of independence for auditors imposed by the Corporations Act 2001.
During both the current and prior financial years, no fees were paid or payable for non-audit services provided by the auditor of the parent entity, its related practices and non-related audit firms.
Auditor’s Independence Declaration
A copy of the auditor’s independence declaration under Section 307C of the Corporations Act in relation to the audit for the year ended June 30, 2023 is included in Exhibit 99.2 of this annual report on Form 20-F.
Rounding of Amounts
Our company is of a kind referred to in ASIC Corporations (Rounding in Financial/Directors’ Reports) Instrument 2016/191, issued by the Australian Securities and Investments Commission, relating to the ‘rounding off’ of amounts in the directors’ report. Unless mentioned otherwise, amounts within this report have been rounded off in accordance with that Legislative Instrument to the nearest thousand dollars, or in certain cases, to the nearest dollar.
The components of our directors’ report are incorporated in various places within this annual report on the Form 20-F. A table charting these components is included within ‘Exhibit 99.1 Appendix 4E’.
Directors’ Resolution
This report is made in accordance with a resolution of the directors.
| /s/ Joseph R Swedish | /s/ Silviu Itescu | |||||||
| Joseph R Swedish | Silviu Itescu | |||||||
| Chairman | Chief Executive Officer | |||||||
Dated: August 31, 2023
6.C Board Practices
Our board of directors currently consists of eight members: six non-executive directors and two executive directors, being our Chief Executive Officer and Dr. Rose, our Chief Medical Officer.
Our directors are generally elected to serve three-year terms in a manner similar to a “staggered” board of directors under Delaware law. No director, except the Managing Director (currently designated as our Chief Executive Officer, Silviu Itescu), may hold office for a period in excess of three years, or beyond the third annual general meeting
129
following the director’s last election, whichever is the longer, without submitting himself or herself for re-election. As a result of the staggered terms, not all of our directors will be elected in any given year.
| Name | First election at AGM | Last election at AGM | End of current term | |||||||||||||||||
| William Burns | 2014 | 2022 | 2025 | |||||||||||||||||
| Eric Rose | 2013 | 2022 | 2025 | |||||||||||||||||
| Michael Spooner | 2004 | 2021 | 2024 | |||||||||||||||||
| Joseph Swedish | 2018 | 2021 | 2024 | |||||||||||||||||
Shawn Cline Tomasello(1) | 2018 | 2021 | N/A | |||||||||||||||||
| Philip Facchina | 2021 | 2021 | 2024 | |||||||||||||||||
| Philip Krause | 2022 | 2022 | 2025 | |||||||||||||||||
Jane Bell(2) | 2022 | 2022 | 2025 | |||||||||||||||||
(1)Ms. Tomasello resigned from the board on August 18, 2022.
(2)Ms. Bell joined the board on August 18, 2022.
We believe that each of our directors has relevant industry experience. The membership of our board of directors is directed by the following requirements:
•our Constitution specifies that there must be a minimum of 3 directors and a maximum of 10, and our board of directors may determine the number of directors within those limits;
•we may appoint or remove any director by resolution passed in the general meeting of shareholders;
•our directors may appoint any person to be a director, and that person only holds office until the next general meeting at which time the director may stand for election by shareholders at that meeting;
•it is the intention of our board of directors that its membership consists of a majority of independent directors who satisfy the criteria for independence recommended by the ASX’s Corporate Governance Principles and Recommendations;
•the chairperson of our board of directors should be an independent director who satisfies the criteria for independence recommended by the ASX’s Corporate Governance Principles and Recommendations;
•Australia's Corporations Act requires that at least two of our directors must be resident Australians; and
•our board of directors should, collectively, have the appropriate level of personal qualities, skills, experience, and time commitment to properly fulfill its responsibilities or have ready access to such skills where they are not available.
Our board of directors is responsible for, and has the authority to determine, all matters relating to our corporate governance, including the policies, practices, management and operation. The principal roles and responsibilities of our board of directors are to:
•facilitate board of directors and management accountability to our company and its shareholders;
•ensure timely reporting to shareholders;
•provide strategic guidance to us, including contributing to the development of, and approving, the corporate strategy;
•oversee management and ensure there are effective management processes in place;
•monitor:
◦organizational performance and the achievement of our strategic goals and objectives;
◦financial performance including approval of the annual and half-year financial reports and liaison with our auditors;
◦progress of major capital expenditures and other significant corporate projects including any acquisitions or divestments;
130
◦compliance with our code of conduct;
◦progress in relation to our diversity objectives and compliance with its diversity policy;
•review and approve business plans, the annual budget and financial plans including available resources and major capital expenditure initiatives;
•approve major corporate initiatives;
•enhance and protect the reputation of the organization;
•oversee the operation of our system for compliance and risk management reporting to shareholders; and
•ensure appropriate resources are available to senior management.
Our non-executive directors do not have any service contracts with Mesoblast that provide for benefits upon termination of those services.
Committees
To assist our board of directors with the effective discharge of its duties, it has established a Nomination and Remuneration Committee and an Audit and Risk Management Committee. Each committee operates under a specific charter approved by our board of directors.
Nomination and Remuneration Committee. The members of our Nomination and Remuneration Committee for the full year ended June 30, 2023 to the date of this report unless otherwise noted are Messrs. Burns (Chairman), Swedish, Spooner, Facchina, Krause, Ms. Bell (from August 24, 2022) and Ms. Tomasello (resignation effective August 18, 2022). The remuneration committee is a committee of our board of directors, and is primarily responsible for making recommendations to our board of directors on:
•board appointments;
•non-executive director fees;
•the executive remuneration framework;
•remuneration of executive directors, including the CEO and other key executives;
•short-term and long-term incentive awards; and
•share ownership plans.
The committee’s objective is to ensure remuneration policies are fair and competitive and in line with similar industry benchmarks while aligned with our objectives. The remuneration committee seeks independent advice from remuneration consultants as and when it deems necessary. See “Management—Remuneration.”
Audit and Risk Management Committee. The members of our Audit and Risk Management Committee for the full year ended June 30, 2023 to the date of this report unless otherwise noted are Messrs. Spooner (Chairman), Facchina, Swedish and Ms. Bell (from August 24, 2022), all of whom are independent, non-executive directors. This committee oversees, reviews, acts on and reports on various auditing and accounting matters to our board of directors, including the selection of our independent accountants, the scope of our annual audits, fees to be paid to the independent accountants, the performance of our independent accountants and our accounting practices. In addition, the committee oversees, reviews, acts on and reports on various risk management matters to our board of directors.
The effective management of risk is central to our ongoing success. We have adopted a risk management policy to ensure that:
•appropriate systems are in place to identify, to the extent that is reasonably practical, all material risks that we face in conducting our business;
•the financial impact of those risks is understood and appropriate controls are in place to limit exposures to them;
•appropriate responsibilities are delegated to control the risks; and
131
•any material changes to our risk profile are disclosed in accordance with our continuous disclosure reporting requirements in Australia.
It is our objective to appropriately balance, protect and enhance the interests of all of our shareholders. Proper behavior by our directors, officers, employees and those organizations that we contract to carry out work is essential in achieving this objective.
We have established a code of conduct, which sets out the standards of behavior that apply to every aspect of our dealings and relationships, both within and outside Mesoblast. The following standards of behavior apply:
•patient well-being;
•comply with all laws that govern us and our operations;
•act honestly and with integrity and fairness in all dealings with others and each other;
•avoid or manage conflicts of interest;
•use our assets properly and efficiently for the benefit of all of our shareholders; and
•seek to be an exemplary corporate citizen.
Board Diversity
The Nasdaq’s rules require foreign private issuers, such as Mesoblast, to have, or explain why it does not have, at least two diverse directors, including one who self-identifies as female, and one who self-identifies as either female, LGBTQ+ or an underrepresented individual. The Company believes that it is presently in compliance with the diversity requirements pursuant to Nasdaq’s rules. The following matrix sets forth Board diversity information required by the Nasdaq’s rules.
| Board Diversity Matrix as at December 23, 2022 and June 30, 2023 | ||||||||||||||
| Country of Principal Executive Offices | Australia | |||||||||||||
| Foreign Private Issuer | Yes | |||||||||||||
| Disclosure Prohibited under Home Country Law | No | |||||||||||||
| Total Number of Directors | 8 | |||||||||||||
| Female | Male | Non-Binary | Did Not Disclose Gender | |||||||||||
| Part I: Gender Identity | ||||||||||||||
| Directors | 1 | 3 | — | 4 | ||||||||||
| Part II: Demographic Background | ||||||||||||||
| Underrepresented Individual in Home Country Jurisdiction | 1 | |||||||||||||
| LGBTQ+ | — | |||||||||||||
| Did not Disclose Demographic Background | 7 | |||||||||||||
6.D Employees
As of June 30, 2023, we had 83 employees, 49 of whom are based in the United States, 24 of whom are based in Australia, including our CEO and certain executive team members, 9 of whom are based in Singapore, and 1 of whom is based in Switzerland. We had 77 and 83 employees as of June 30, 2022 and 2021, respectively.
132
The table below sets forth the breakdown of the total year-end number of our employees by main category of activity and geographic area for the past three years:
| As of June 30, 2023 | Research & Development | Commercial | Manufacturing | Corporate | Total | |||||||||||||||||||||||||||
| USA | 35 | — | 5 | 9 | 49 | |||||||||||||||||||||||||||
| Australia | 8 | — | 1 | 15 | 24 | |||||||||||||||||||||||||||
| Singapore | 4 | — | 4 | 1 | 9 | |||||||||||||||||||||||||||
| Switzerland | 1 | — | — | — | 1 | |||||||||||||||||||||||||||
| Total | 48 | — | 10 | 25 | 83 | |||||||||||||||||||||||||||
| As of June 30, 2022 | Research & Development | Commercial | Manufacturing | Corporate | Total | |||||||||||||||||||||||||||
| USA | 32 | — | 4 | 8 | 44 | |||||||||||||||||||||||||||
| Australia | 8 | — | 1 | 15 | 24 | |||||||||||||||||||||||||||
| Singapore | 1 | — | 6 | 1 | 8 | |||||||||||||||||||||||||||
| Switzerland | 1 | — | — | — | 1 | |||||||||||||||||||||||||||
| Total | 42 | — | 11 | 24 | 77 | |||||||||||||||||||||||||||
| As of June 30, 2021 | Research & Development | Commercial | Manufacturing | Corporate | Total | |||||||||||||||||||||||||||
| USA | 35 | 1 | 1 | 11 | 48 | |||||||||||||||||||||||||||
| Australia | 9 | — | — | 16 | 25 | |||||||||||||||||||||||||||
| Singapore | 6 | — | 2 | 1 | 9 | |||||||||||||||||||||||||||
| Switzerland | 1 | — | — | — | 1 | |||||||||||||||||||||||||||
| Total | 51 | 1 | 3 | 28 | 83 | |||||||||||||||||||||||||||
We have no collective bargaining agreement with our employees. We have not experienced any work stoppages to date and consider our relations with our employees to be good.
See “Item 6.A Directors and Senior Management – Employee Profile”.
6.E Share Ownership
The table below sets forth information regarding the beneficial ownership of our ordinary shares based on 814,204,825 ordinary shares outstanding at August 31, 2023 by each of our directors and key management personnel.
We have determined beneficial ownership in accordance with the rules of the SEC. A person has a beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options that are exercisable within 60 days of August 31, 2023. Ordinary shares subject to options currently exercisable or exercisable within 60 days of August 31, 2023 are deemed to be outstanding for computing the percentage ownership of the person holding these options and the percentage ownership of any group of which the holder is a member, however are not deemed outstanding for computing the percentage of any other person.
Unless otherwise indicated, to our knowledge each shareholder possesses sole voting and investment power over the ordinary shares listed. None of our shareholders has different voting rights from other shareholders. Unless otherwise
133
indicated, the principal address of each of the shareholders below is c/o Mesoblast Limited, Level 38, 55 Collins Street, Melbourne 3000, Australia.
| Ordinary Shares beneficially owned | ||||||||||||||
| Name | Number | % | ||||||||||||
| Directors and key management personnel: | ||||||||||||||
Silviu Itescu(1) | 71,400,929 | 8.7 | % | |||||||||||
Eric Rose(2) | 936,667 | * | ||||||||||||
Jane Bell(3) | 314,285 | * | ||||||||||||
William Burns(4) | 305,000 | * | ||||||||||||
Philip Facchina(5) | 474,865 | * | ||||||||||||
Philip Krause(6) | 166,667 | * | ||||||||||||
Michael Spooner(7) | 1,169,000 | * | ||||||||||||
Joseph Swedish(8) | 500,000 | * | ||||||||||||
Shawn Tomasello(9) | 200,000 | * | ||||||||||||
| All directors and key management personnel as a group (9 persons) | 75,467,413 | 9.2 | % | |||||||||||
_______________
*Less than 1% of the outstanding ordinary shares.
(1)Includes (a) 67,756,838 ordinary shares owned by Dr. Itescu, (b) 487,804 ordinary shares owned by Josaka Investments Pty Ltd, the trustee of Dr. Itescu’s self-managed superannuation fund, (c) 714,286 ordinary shares owned by Tamit Nominees Pty Ltd, an Australian corporation owned by Dr. Itescu and (d) 2,442,001 ordinary shares subject to options of which; 1,212,001 exercisable at a price of A$1.47 per share until July 19, 2026, 720,000 exercisable at a price of A$3.41 per share until July 15, 2027 and 510,000 exercisable at a price of A$1.77 per share until September 7, 2028.
(2)Includes 936,667 ordinary shares subject to options of which; 120,000 are exercisable at a price of A$1.33 per share until November 29, 2025, 100,000 are exercisable at a price of A$1.83 per share until November 17, 2026 and 716,667 are exercisable at a price of A$1.03 per share until October 16, 2029.
(3)Includes (a) 247,618 ordinary shares owned by Ms. Bell and Mr. Geoffrey Arthur Bell as trustees for Ms. Bell's family trust, (b) 66,667 ordinary shares subject to options exercisable at a price of A$0.85 per share until August 23, 2029.
(4)Includes (a) 85,000 ordinary shares owned by Mr. Burns and (b) 220,000 ordinary shares subject to options of which; 120,000 exercisable at a price of A$1.33 per share until November 29, 2025 and 100,000 exercisable at a price of A$1.83 per share until November 17, 2026.
(5)Includes (a) 273,225 ordinary shares owned by HNP, LLC (held as American Depository Shares), (b) 68,306 warrants over ordinary shares owned by HNP, LLC and (c) 133,334 ordinary shares subject to options exercisable at a price of A$2.28 per share until April 14, 2028.
(6)Includes (a) 100,000 ordinary shares owned by Mr Krause (held as American Depository Shares) and (b) 66,667 ordinary shares subject to options exercisable at a price of A$1.01 per share until May 22, 2029.
(7)Includes (a) 1,060,000 ordinary shares owned by Mr. Spooner, (b) 9,000 ordinary shares owned by Mr. Spooner's family trust and (c) 100,000 ordinary shares subject to options exercisable at a price of A$1.33 per share until November 29, 2025.
(8)Includes 500,000 ordinary shares subject to options of which; 200,000 are exercisable at a price of A$1.52 per share until June 17, 2025 and 300,000 are exercisable at a price of A$1.48 per share until April 3, 2026.
(9)Includes 200,000 ordinary shares subject to options exercisable at a price of A$1.56 per share until July 10, 2025. On August 18, 2022, Ms. Tomasello resigned as director of the Company.
134
6.F Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
Not applicable.
Item 7. Major Shareholders and Related Party Transactions
7.A Major Shareholders
The following table and accompanying footnotes present certain information regarding the beneficial ownership of our ordinary shares based on 814,204,825 ordinary shares outstanding at August 31, 2023 by each person known by us to be the beneficial owner of more than 5% of our ordinary shares. Based upon information known to us, as of August 31, 2023 we had 38 shareholders (ordinary shares) in the United States. These shareholders held an aggregate of 227,102,101 of our ordinary shares, or approximately 28% of our outstanding ordinary shares. None of our shareholders has different voting rights from other shareholders.
| Ordinary Shares beneficially owned | ||||||||||||||
| Name | Number | % | ||||||||||||
| 5% or Greater Shareholders: | ||||||||||||||
Silviu Itescu(1) | 71,400,929 | 8.8 | % | |||||||||||
G to the Fourth Investments, LLC(2) | 73,197,402 | 9.0 | % | |||||||||||
(1)Includes (a) 67,756,838 ordinary shares owned by Dr. Itescu, (b) 487,804 ordinary shares owned by Josaka Investments Pty Ltd, the trustee of Dr. Itescu’s self-managed superannuation fund and (c) 714,286 ordinary shares owned by Tamit Nominees Pty Ltd, an Australian corporation owned by Dr. Itescu, (d) 1,212,001 ordinary shares subject to options exercisable at a price of A$1.47 per share until July 19, 2026, (e) 720,000 ordinary shares subject to options exercisable at a price of A$3.41 per share until July 15, 2027, and (f) 510,000 ordinary shares subject to options exercisable at a price of A$1.77 per share until September 7, 2028.
(2)Includes (a) 39,242,597 ordinary shares owned by G to the Fourth Investments, LLC and Gregory George, (b) 2,500,000 ordinary shares owned by James George and Gregory George, (c) 2,405,000 ordinary shares owned by Grant George and Gregory George, (d) 22,219,203 ordinary shares owned by Gregory George, and (e) 6,830,602 ordinary shares subject to warrants.
To our knowledge, there have not been any significant changes in the ownership of our ordinary shares by major shareholders over the past three years, except as follows (which is based on substantial shareholder notices filed with the ASX and SEC).
•M&G Investment Group reported that as of September 3, 2020 in total it held 51,752,865 ordinary shares (including 908,090 ADSs, each representing 5 ordinary shares), or 8.84% of the total voting power as of that date. It reported that as of on August 12, 2022 in total it held 93,150,226 ordinary shares (including 1,320,000 ADSs, each representing 5 ordinary shares), or 12.64% of the total voting power as of that date. It reported that as of May 1, 2023 it held 86,251,092 ordinary shares (including 532,981 ADSs, each representing 5 ordinary shares), or 10.60% of the total voting power as of that date. It reported that as of July 27, 2023 in total it held 76,996,783 ordinary shares, or 9.46% of the total voting power as of that date. It reported that as of August 4, 2023 in total it held 58,312,858 ordinary shares, or 7.16% of the total voting power as of that date. It reported that as of August 8, 2023 in total it held 48,079,421 ordinary shares, or 5.91% of the total voting power as of that date. It reported that as of August 9, 2023 that it had ceased to be a substantial shareholder.
•G to the Fourth Investments, LLC reported that as of May 1, 2023 it held 53,920,195 ordinary shares, or 6.62% of the total voting power as of that date. It reported that as of August 24, 2023 in total it held 66,366,800 ordinary shares, or 8.15% of the total voting power as of that date.
7.B Related Party Transactions
The Company has not entered into any related party transactions during the year ended June 30, 2023 other than compensation and other services provided by Directors and other members of key management personnel, see “Item 6.B Compensation”.
135
7.C Interests of Experts and Counsel
Not applicable.
Item 8. Financial Information
8.A Consolidated Statements and Other Financial Information
See “Item 18. Financial Statements.”
Legal Proceedings
A class action proceeding in the Federal Court of Australia was served on the Company in May 2022 by the law firm William Roberts Lawyers on behalf of persons who, between February 22, 2018, and December 17, 2020, acquired an interest in Mesoblast shares, American Depository Receipts, and/or related equity swap arrangements. In June 2022, the firm Phi Finney McDonald commenced a second shareholder class action against the Company in the Federal Court of Australia asserting similar claims arising during the same period. Like the class action lawsuit from October 2020 filed in the U.S. Federal District Court for the Southern District of New York (which had court approval for settlement in August 2022), the Australian class actions relate to the Complete Response Letter released by the FDA in September 2020 in relation to the Company's GvHD product candidate; they also relate to certain representations made by the Company in relation to our COVID-19 product candidate and the decline in the market price of the Company's ordinary shares in December 2020. The Australian class actions have been consolidated into one lawsuit. The Company will continue to vigorously defend against the proceedings. The Company cannot provide any assurance as to the possible outcome or cost to us from the lawsuit, particularly as it is at an early stage, nor how long it may take to resolve such lawsuit. Thus, the Company has not accrued any amounts in connection with such legal proceedings.
Dividend policy
Since our inception, we have not declared or paid any dividends on our shares. We intend to retain any earnings for use in our business and do not currently intend to pay cash dividends on our ordinary shares. Dividends, if any, on our outstanding ordinary shares will be declared by and subject to the discretion of our board of directors, and subject to Australian law.
Any dividend we declare will be paid to the holders of ADSs, subject to the terms of the deposit agreement, to the same extent as holders of our ordinary shares, to the extent permitted by applicable law and regulations, less the fees and expenses payable under the deposit agreement. Any dividend we declare will be distributed by the depositary bank to the holders of our ADSs, subject to the terms of the deposit agreement. See “Item 12.D. Description of American Depositary Shares.”
8.B Significant Changes
In August 2023, the FDA provided a complete response to our BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data. In line with our overall commercial strategy to progress to adult populations, we intend to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality.
There were no other events that have arisen subsequent to June 30, 2023 and prior to the signing of this report that would likely have a material impact on the financial results presented.
Item 9. The Offer and Listing
9.A Offer and Listing Details
Our ordinary shares have been listed in Australia on the Australian Securities Exchange (ASX) since December 2004. Our ordinary shares have been trading under the symbol “MSB”.
136
American Depositary Shares (“ADSs”), each representing five ordinary shares, are available in the US through an American Depositary Receipts (“ADR”) program. This program was established under the deposit agreement which we entered into with JP Morgan Chase Bank N.A. as depositary and our ADR holders. Our ADRs have been listed on the Nasdaq Global Select Market since August 2015 and are traded under the symbol “MESO”.
9.B Plan of Distribution
Not applicable.
9.C Markets
See “Item 9.A Offer and Listing Details.”
9.D Selling Shareholders
Not applicable.
9.E Dilution
Not applicable.
9.F Expenses of the Issue
Not applicable.
Item 10. Additional Information
10.A Share Capital
Not applicable.
10.B Memorandum and Articles of Association
Our Constitution is similar in nature to the bylaws of a U.S. corporation. It does not provide for or prescribe any specific objectives or purposes of Mesoblast. Our Constitution is subject to the terms of the ASX Listing Rules and the Australian Corporations Act. It may be modified or repealed and replaced by special resolution passed at a meeting of shareholders, which a resolution is passed by at least 75% of the votes cast by shareholders (including proxies and representatives of shareholders) entitled to vote on the resolution.
Under Australian law, a company has the legal capacity and powers of an individual both within and outside Australia. The material provisions of our Constitution are summarized below. This summary is not intended to be complete nor to constitute a definitive statement of the rights and liabilities of our shareholders, and is qualified in its entirety by reference to the complete text of our Constitution, a copy of which is on file with the SEC.
Directors
Interested Directors
Except as permitted by the Corporations Act and the ASX Listing Rules, a director must not vote in respect of a matter that is being considered at a directors' meeting in which the director has a material personal interest according to our Constitution. Such director must not be counted in a quorum, must not vote on the matter and must not be present at the meeting while the matter is being considered, unless the non-interested directors resolve otherwise.
Pursuant to our Constitution, the fact that a director holds office as a director, and has fiduciary obligations arising out of that office will not require the director to account to us for any profit realized by or under any contract or arrangement entered into by or on behalf of Mesoblast and in which the director may have an interest.
Unless a relevant exception applies, the Corporations Act requires our directors to provide disclosure of certain interests and prohibits directors of companies listed on the ASX from voting on matters in which they have a material
137
personal interest and from being present at the meeting while the matter is being considered. In addition, unless a relevant exception applies, the Corporations Act and the ASX Listing Rules require shareholder approval of any provision of financial benefits (including the issue by us of ordinary shares and other securities) to our directors, including entities controlled by them and certain members of their families.
Borrowing Powers Exercisable by Directors
Pursuant to our Constitution, our business is managed by our board of directors. Our board of directors has the power to raise or borrow money, and charge any of our property or business or all or any of our uncalled capital, and may issue debentures or give any other security for any of our debts, liabilities or obligations or of any other person, and may guarantee or become liable for the payment of money or the performance of any obligation by or of any other person.
Election, Removal and Retirement of Directors
We may appoint or remove any director by resolution passed in a general meeting of shareholders. Additionally, our directors are elected to serve maximum three-year terms in a manner similar to a “staggered” board of directors under Delaware law. No director except the Managing Director (currently designated as our chief executive officer, Silviu Itescu) may hold office for a period in excess of three years, or beyond the third annual general meeting following the director’s last election, whichever is the longer, without submitting himself or herself for re-election.
A director who is appointed during the year by the other directors only holds office until the next general meeting at which time the director may stand for election by shareholders at that meeting.
In addition, provisions of the Corporations Act apply where at least 25% of the votes cast on a resolution to adopt our remuneration report (which resolution must be proposed each year at our annual general meeting) are against the adoption of the report at two successive annual general meetings. Where these provisions apply, a resolution must be put to a vote at the second annual general meeting to the effect that a further meeting, or a spill meeting, take place within 90 days. At the spill meeting, the directors in office when the remuneration report was considered at the second annual general meeting (other than the Managing Director) cease to hold office and resolutions to appoint directors (which may involve re-appointing the former directors) are put to a vote.
Voting restrictions apply in relation to the resolutions to adopt our remuneration report and to propose a spill meeting. These restrictions apply to our key management personnel and their closely related parties. See “Rights and Restrictions on Classes of Shares—Voting Rights” below.
Pursuant to our Constitution, a person is eligible to be elected as a director at a general meeting only if:
•the person is in office as a director immediately before the meeting, in respect of an election of directors at a general meeting that is a spill meeting as defined in section 250V(1) of the Corporations Act;
•the person has been nominated by the directors before the meeting;
•where the person is a shareholder, the person has, at least 35 business days but no more than 90 business days before the meeting, given to us a notice signed by the person stating the person's desire to be a candidate for election at the meeting; or
•where the person is not a shareholder, a shareholder intending to nominate the person for election at that meeting has, at least 35 business days but no more than 90 business days before the meeting, given to us a notice signed by the shareholder stating the shareholder's intention to nominate the person for election, and a notice signed by the person stating the person's consent to the nomination.
Share Qualifications
There are currently no requirements for directors to own our ordinary shares in order to qualify as directors.
Rights and Restrictions on Classes of Shares
Subject to the Corporations Act and the ASX Listing Rules, the rights attaching to our ordinary shares are detailed in our Constitution. Our Constitution provides that any of our ordinary shares may be issued with preferential, deferred or special rights, privileges or conditions, with any restrictions in regard to dividends, voting, return of share capital or
138
otherwise as our board of directors may determine from time to time. Subject to the Corporations Act, the ASX Listing Rules and any rights and restrictions attached to a class of shares, we may issue further ordinary shares on such terms and conditions as our board of directors resolve. Currently, our outstanding ordinary share capital consists of only one class of ordinary shares.
Dividend Rights
Our board of directors may from time to time determine to pay dividends to shareholders; however, no dividend is payable except in accordance with the thresholds set out in the Corporations Act.
Voting Rights
Under our Constitution, the general conduct and procedures of each general meeting of shareholders will be determined by the chairperson, including any procedures for casting or recording votes at the meeting whether on a show of hands or on a poll. A poll may be demanded by the chairman of the meeting; by at least five shareholders present and having the right to vote on at the meeting; or any shareholder or shareholders representing at least 5% of the votes that may be cast on the resolution on a poll. On a show of hands, each shareholder entitled to vote at the meeting has one vote regardless of the number of ordinary shares held by such shareholder. If voting takes place on a poll, rather than a show of hands, each shareholder entitled to vote has one vote for each ordinary share held and a fractional vote for each ordinary share that is not fully paid, such fraction being equivalent to the proportion of the amount that has been paid (not credited) of the total amounts paid and payable, whether or not called (excluding amounts credited), to such date on that ordinary share.
Under Australian law, an ordinary resolution is passed on a show of hands if it is approved by a simple majority (more than 50%) of the votes cast by shareholders present (in person or by proxy) and entitled to vote. If a poll is demanded or required, an ordinary resolution is passed if it is approved by holders representing a simple majority of the total voting rights of shareholders present (in person or by proxy) who (being entitled to vote) vote on the resolution. Special resolutions require the affirmative vote of not less than 75% of the votes cast by shareholders present (in person or by proxy) and entitled to vote at the meeting. Votes on resolutions set out in a notice of meeting must be voted on by poll.
Pursuant to our Constitution, each shareholder entitled to attend and vote at a meeting may attend and vote:
•in person physically or by electronic means;
•by proxy, attorney or by representative; or
•other than in relation to any clause which specifies a quorum, a member who has duly lodged a valid vote delivered to us by post, fax or other electronic means approved by the directors in accordance with the Constitution.
Under Australian law, shareholders of a public listed company are generally not permitted to approve corporate matters by written consent. Our Constitution does not specifically provide for cumulative voting.
Note that ADS holders may not directly vote at a meeting of the shareholders but may instruct the depositary to vote the number of deposited ordinary shares their ADSs represent. Under voting by a show of hands, multiple “yes” votes by ADS holders will only count as one “yes” vote and will be negated by a single “no” vote, unless a poll is demanded.
There are a number of circumstances where the Corporations Act or the ASX Listing Rules prohibit or restrict certain shareholders or certain classes of shareholders from voting. For example, key management personnel whose remuneration details are included elsewhere in this prospectus are prohibited from voting on the resolution that must be proposed at each annual general meeting to adopt our remuneration report, as well as any resolution to propose a spill meeting. An exception applies to exercising a directed proxy which indicates how the proxy is to vote on the proposed resolution on behalf of someone other than the key management personnel or their closely related parties; or that person is chair of the meeting and votes an undirected proxy where the shareholder expressly authorizes the chair to exercise that power. Key management personnel and their closely related parties are also prohibited from voting undirected proxies on remuneration related resolutions. A similar exception to that described above applies if the proxy is the chair of the meeting.
139
Right to Share in Our Profits
Subject to the Corporations Act and pursuant to our Constitution, our shareholders are entitled to participate in our profits by payment of dividends. The directors may by resolution declare a dividend or determine a dividend is payable, and may fix the amount, the time for and method of payment.
Rights to Share in the Surplus in the Event of Winding Up
Our Constitution provides for the right of shareholders to participate in a surplus in the event of our winding up.
Redemption Provisions
Under our Constitution and subject to the Corporations Act, the directors have power to issue and allot shares with any preferential, deferred or special rights, privileges or conditions; with any restrictions in regard to the dividend, voting, return of capital or otherwise; and preference shares which are liable to be redeemed or converted.
Sinking Fund Provisions
Our Constitution allows our directors to set aside any amount available for distribution as a dividend such amounts by way of reserves as they think appropriate before declaring or determining to pay a dividend, and may apply the reserves for any purpose for which an amount available for distribution as a dividend may be properly applied. Pending application or appropriation of the reserves, the directors may invest or use the reserves in our business or in other investments as they think fit.
Liability for Further Capital Calls
According to our Constitution, our board of directors may make any calls from time to time upon shareholders in respect of all monies unpaid on partly paid shares respectively held by them, subject to the terms upon which any of the partly paid shares have been issued. Each shareholder is liable to pay the amount of each call in the manner, at the time and at the place specified by our board of directors. Calls may be made payable by instalment.
Provisions Discriminating Against Holders of a Substantial Number of Shares
There are no provisions under our Constitution discriminating against any existing or prospective holders of a substantial number of our ordinary shares.
Variation or Cancellation of Share Rights
The rights attached to shares in a class of shares may only be varied or cancelled by a special resolution of shareholders, together with either:
•a special resolution passed at a separate meeting of members holding shares in the class; or
•the written consent of members with at least 75% of the votes in the class.
General Meetings of Shareholders
General meetings of shareholders may be called by our board of directors or, under the Corporations Act, by a single director. Except as permitted under the Corporations Act, shareholders may not convene a meeting. Under the Corporations Act, shareholders with at least 5% of the votes that may be cast at a general meeting may call and arrange to hold a general meeting. The Corporations Act requires the directors to call and arrange to hold a general meeting on the request of shareholders with at least 5% of the votes that may be cast at a general meeting. Notice of the proposed meeting of our shareholders is required at least 28 days prior to such meeting under the Corporations Act.
No business shall be transacted at any general meeting unless a quorum is present at the time when the meeting proceeds to business. Under our Constitution, the presence, in person or by proxy, attorney or representative, of two shareholders constitutes a quorum, or if we have less than two shareholders, then those shareholders constitute a quorum. If a quorum is not present within 30 minutes after the time appointed for the meeting, the meeting must be either dissolved if it was requested or called by shareholders or adjourned in any other case. A meeting adjourned for lack of a quorum is
140
adjourned to the same day in the following week at the same time and place, unless otherwise decided by our directors. The reconvened meeting is dissolved if a quorum is not present within 30 minutes after the time appointed for the meeting.
Change of Control
Takeovers of listed Australian public companies, such as Mesoblast, are regulated by the Corporations Act, which prohibits the acquisition of a “relevant interest” in issued voting shares in a listed company if the acquisition will lead to that person’s or someone else’s voting power in Mesoblast increasing from 20% or below to more than 20% or increasing from a starting point that is above 20% and below 90% (“Takeovers Prohibition”), subject to a range of exceptions.
Generally, a person will have a relevant interest in securities if the person:
•is the holder of the securities or the holder of an ADS over the shares;
•has power to exercise, or control the exercise of, a right to vote attached to the securities; or
•has the power to dispose of, or control the exercise of a power to dispose of, the securities (including any indirect or direct power or control)
If, at a particular time:-
•a person has a relevant interest in issued securities; and
•the person has:
◦entered or enters into an agreement with another person with respect to the securities;
◦given or gives another person an enforceable right, or has been or is given an enforceable right by another person, in relation to the securities; or
◦granted or grants an option to, or has been or is granted an option by, another person with respect to the securities; and
•the other person would have a relevant interest in the securities if the agreement were performed, the right enforced or the option exercised,
then, the other person is taken to already have a relevant interest in the securities.
There are a number of exceptions to the above Takeovers Prohibition on acquiring a relevant interest in issued voting shares above 20%. In general terms, some of the more significant exceptions include:
•when the acquisition results from the acceptance of an offer under a formal takeover bid;
•when the acquisition is conducted on market by or on behalf of the bidder during the bid period for a full takeover bid that is unconditional or only conditional on certain 'prescribed' matters set out in the Corporations Act;
•when the acquisition has been previously approved by resolution passed at general meeting by shareholders of Mesoblast;
•an acquisition by a person if, throughout the six months before the acquisition, that person or any other person has had voting power in Mesoblast of at least 19% and, as a result of the acquisition, none of the relevant persons would have voting power in Mesoblast more than three percentage points higher than they had six months before the acquisition;
•when the acquisition results from the issue of securities under a pro rata rights issue;
•when the acquisition results from the issue of securities under a dividend reinvestment plan or bonus share plan;
•when the acquisition results from the issue of securities under certain underwriting arrangements;
•when the acquisition results from the issue of securities through a will or through operation of law;
•an acquisition that arises through the acquisition of a relevant interest in another company listed on the ASX or other Australian financial market or a foreign stock exchange approved in writing by ASIC;
•an acquisition arising from an auction of forfeited shares; or
141
•an acquisition arising through a compromise, arrangement, liquidation or buy-back.
A formal takeover bid may either be a bid for all securities in the bid class or a fixed proportion of such securities, with each holder of bid class securities receiving a bid for that proportion of their holding. Under our Constitution, a proportionate takeover bid must first be approved by resolution of our shareholders in a general meeting before it may proceed.
Breaches of the takeovers provisions of the Corporations Act are criminal offenses. In addition, ASIC and, on application by ASIC or an interested party, such as a shareholder, the Australian Takeovers Panel have a wide range of powers relating to breaches of takeover provisions, including the ability to make orders cancelling contracts, freezing transfers of, and rights (including voting rights) attached to, securities, and forcing a party to dispose of securities including by vesting the securities in ASIC for sale. There are certain defenses to breaches of the takeover provisions provided in the Corporations Act.
Ownership Threshold
There are no provisions in our Constitution that require a shareholder to disclose ownership above a certain threshold. The Corporations Act, however, requires a substantial shareholder to notify us and the ASX once a 5% interest in our ordinary shares is obtained. Further, once a shareholder has (alone or together with associates) a 5% or greater interest in us, such shareholder must notify us and the ASX of any increase or decrease of 1% or more in its interest in our ordinary shares. In addition, the Constitution requires a shareholder to provide information to the Company in relation to its entry into any arrangement restricting the transfer or other disposal of shares, which are of the nature of arrangements that Mesoblast is required to disclose under the ASX Listing Rules. Following our initial public offering in the United States, our shareholders are also subject to disclosure requirements under U.S. securities laws.
Issues of Shares and Change in Capital
Subject to our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, we may at any time grant options over unissued shares and issue shares on any terms, with any preferential, deferred or special rights, privileges or conditions; with any restrictions in regard to dividend, voting, return of capital or otherwise, and for the consideration and other terms that the directors determine. Our power to issue shares includes the power to issue bonus shares (for which no consideration is payable to Mesoblast), preference shares and partly paid shares.
Subject to the requirements of our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, including relevant shareholder approvals, we may reduce our share capital (provided that the reduction is fair and reasonable to our shareholders as a whole, does not materially prejudice our ability to pay creditors and obtains the necessary shareholder approval) or buy back our ordinary shares including under an equal access buy-back or on a selective basis. Under the Constitution, the directors may do anything required to give effect to any resolution altering or approving the reduction of our share capital.
Access to and Inspection of Documents
Inspection of our records is governed by the Corporations Act. Any member of the public has the right to inspect or obtain copies of our share registers on the payment of a prescribed fee. Shareholders are not required to pay a fee for inspection of our share registers or minute books of the meetings of shareholders. Other corporate records, including minutes of directors’ meetings, financial records and other documents, are not open for inspection by shareholders. Where a shareholder is acting in good faith and an inspection is deemed to be made for a proper purpose, a shareholder may apply to the court to make an order for inspection of our books.
10.C Material Contracts
Manufacturing Service Agreements with Lonza Bioscience Singapore Pte. Ltd.
In September 2011, we entered into a manufacturing services agreement, or MSA, with Lonza Walkersville, Inc. and Lonza Bioscience Singapore Pte. Ltd., collectively referred to as Lonza, a global leader in biopharmaceutical manufacturing. Under the MSA, we pay Lonza on a fee for service basis to provide us with manufacturing process development capabilities for our product candidates, including formulation development, establishment and maintenance of master cell banks, records preparation, process validation, manufacturing and other services.
142
We have agreed to order a certain percentage of our clinical requirements and commercial requirements for MPC products from Lonza. Lonza has agreed not to manufacture or supply commercially biosimilar versions of any of our product candidates to any third party, during the term of the MSA, subject to our meeting certain thresholds for sales of our products.
We can trigger a process requiring Lonza to construct a purpose-built manufacturing facility exclusively for our product candidates. In return if we exercise this option, we will purchase agreed quantities of our product candidates from this facility. We also have a right to buy out this manufacturing facility at a pre-agreed price two years after the facility receives regulatory approval.
The MSA will expire on the three-year anniversary of the date of the first commercial sale of product supplied under the MSA, unless it is sooner terminated. We have the option of extending the MSA for an additional 10 years, followed by the option to extend for successive three-year periods subject to Lonza’s reasonable consent. We may terminate the MSA with two years prior written notice, and Lonza may terminate with five years prior written notice. The MSA may also terminate for other reasons, including if the manufacture or development of a product is suspended or abandoned due to the results of clinical trials or guidance from a regulatory authority. In the event we request that Lonza construct the manufacturing facility described above, neither we nor Lonza may terminate before the third anniversary of the date the facility receives regulatory approval to manufacture our product candidates, except in certain limited circumstances. Upon expiration or termination of the MSA, we have the right to require Lonza to transfer certain technologies and lease the Singapore facility or the portion of such facility where our product candidates are manufactured, subject to good faith negotiations.
We currently rely, and expect to continue to rely, on Lonza for the manufacture of our MPC product candidates for preclinical and clinical testing, as well as for commercial manufacture of our MPC product candidates if marketing approval is obtained.
In October 2019, we entered into an agreement with Lonza for commercial manufacture of remestemcel-L for pediatric SR-aGVHD. This agreement will facilitate inventory build ahead of the planned US market launch of remestemcel-L and commercial supply to meet Mesoblast’s long-term market projections. The agreement provides for Lonza to expand its Singapore cGMP facilities if required to meet long-term growth and capacity needs for the product. Additionally, it anticipates introduction of new technologies and process improvements which are expected to result in significant increases in yields and efficiencies.
Under the agreement, we agree to order a certain percentage of our commercial requirements for remestemcel-L from Lonza. The agreement is subject to standard provisions for termination and its effects, including termination by either party for uncured, material breach of the other, by us in the event of FDA related rejection or delay of approval of remestemcel-L and after a specified minimum period following the initiation date by either party, on advance notice to the other, which in the case Lonza is the terminating party is intended to provide us sufficient time to transfer the manufacture of the product to an alternative manufacturer.
License Agreement with Grünenthal GmbH
In September 2019, we entered into a strategic partnership with Grünenthal GmbH (Grünenthal) to develop and commercialize MPC-06-ID, the Company’s Phase 3 allogeneic cell therapy candidate for the treatment of chronic low back pain due to degenerative disc disease in patients who have exhausted conservative treatment options. The agreement was amended by the parties in June 2021. Under the partnership, Grünenthal will have exclusive commercialization rights to MPC-06-ID for Europe and Latin America. We may receive up to $112.5 million in upfront and milestone payments prior to product launch, inclusive of $17.5 million already received, if certain clinical and regulatory milestones are satisfied and reimbursement targets are achieved. Cumulative milestone payments could exceed $1.0 billion depending on the final outcome of Phase 3 studies and patient adoption. We will also receive tiered double-digit royalties on product sales. There cannot be any assurance as to the total amount of future milestone and royalty payments that Mesoblast will receive nor when they will be received.
Grünenthal is able to terminate the agreement with a specified period of notice without cause, or on shorter notice in the case of certain clinical, regulatory and commercial events. We have termination rights with respect to certain patent challenges by Grünenthal. Either party may terminate the agreement on material breach of the agreement if such breach is not cured within the specified cure period or if certain events related to bankruptcy of the other party occurs. For more information, see “Item 18. Financial Statements - Note 3 – Revenue recognition.”
143
Agreements with JCR Pharmaceuticals Co., Ltd.
In October 2013, we acquired all of Osiris Therapeutics, Inc.’s business and assets related to culture expanded MSCs. These assets included assumption of a collaboration agreement with JCR (“JCR Agreement”), which will continue in existence until the later of 15 years from the first commercial sale of any product covered by the agreement and expiration of the last Osiris patent covering any such product. JCR is a research and development oriented pharmaceutical company in Japan. Under the JCR Agreement we assumed from Osiris, JCR has the right to develop our MSCs in two fields for the Japanese market: exclusive in conjunction with the treatment of hematological malignancies by the use of HSCs derived from peripheral blood, cord blood or bone marrow, or the First JCR Field; and non-exclusive for developing assays that use liver cells for non-clinical drug screening and evaluation, or the Second JCR Field. Under the JCR Agreement, JCR obtained rights in Japan to our MSCs, for the treatment of aGVHD. JCR also has a right of first negotiation to obtain rights to commercialize MSC-based products for additional orphan designations in Japan. We retain all rights to those products outside of Japan.
JCR received full approval in September 2015 for its MSC-based product for the treatment of children and adults with aGVHD, TEMCELL. TEMCELL is the first culture-expanded allogeneic cell therapy product to be approved in Japan. It was launched in Japan in February 2016.
Under the JCR Agreement, JCR is responsible for all development and manufacturing costs including sales and marketing expenses. With respect to the First JCR Field, we have received all sales milestone payments, a total of $3.0 million. Ongoing we are entitled to escalating double-digit royalties in the twenties. These royalties are subject to possible renegotiation downward in the event of competition from non-infringing products in Japan. With respect to the Second JCR Field, we are entitled to a double digit profit share in the fifties.
Intellectual property is licensed both ways under the JCR Agreement, with JCR receiving exclusive and non-exclusive rights as described above from us and granting us non-exclusive, royalty-free rights (excluding in the First JCR Field and Second JCR Field in Japan) under the intellectual property arising out of JCR’s development or commercialization of MSC-based products licensed in Japan.
JCR has the right to terminate the JCR Agreement for any reason, and we have a limited right to terminate the JCR Agreement, including a right to terminate in the event of an uncured material breach by JCR. In the event of a termination of the JCR Agreement other than for our breach, JCR must provide us with its owned product registrations and technical data related to MSC-based products licensed in Japan and all licenses of our intellectual property rights will revert to us.
We have expanded our partnership with JCR in Japan for two new indications: for wound healing in patients with EB in October 2018, and for neonatal HIE, a condition suffered by newborns who lack sufficient blood supply and oxygen to the brain, in June 2019. We will receive royalties on TEMCELL product sales for EB and HIE, if and when such indications receive marketing approval in Japan.
We have the right to use all safety and efficacy data generated by JCR in Japan to support our development and commercialization plans for our MSC product candidate remestemcel-L in the United States and other major healthcare markets, including for GVHD, EB and HIE.
Loan Agreement with Oaktree
In November 2021, we entered into a $90.0 million five-year senior debt facility provided by funds associated with Oaktree. We drew the first tranche of $60.0 million on closing. We will seek to extend our option to draw the additional $30.0 million tranche beyond September 30, 2023, subject to us achieving certain milestones. The facility has a three-year interest only period, at a fixed rate of 9.75% per annum, after which time 40% of the principal amortizes over two years and a final payment is due no later than November 2026. The facility also allows us to make quarterly payments of interest at a rate of 8.0% per annum for the first two years, and the unpaid interest portion (1.75% per annum) will be added to the outstanding loan balance and shall accrue further interest at a fixed rate of 9.75% per annum. The loan agreement contains certain covenants, see “Item 5.B Liquidity and Capital Resource – Borrowings.”
In November 2021, Oaktree was granted warrants to purchase 1,769,669 American Depositary Shares (“ADSs”) at $7.26 per ADS, a 15% premium to the 30-day VWAP. The warrants were legally issued in January 2022 and may be exercised within 7 years of issuance.
144
In December 2022, we amended the terms of the loan agreement with Oaktree and in connection with the loan amendment, Oaktree was granted warrants to purchase 455,000 ADSs at $3.70 per ADS, a 15% premium to the 30-day VWAP. The warrants were legally issued in March 2023 and may be exercised within 7 years of issuance.
Loan Agreement with NovaQuest
In June 2018, we entered into an eight-year non-dilutive secured loan with NovaQuest for $40.0 million. We drew the first tranche of $30.0 million on closing. The loan term includes an interest only period of approximately four years through until July 8, 2022, then a four-year amortization period through until maturity on July 8, 2026.
All interest and principal payments will be deferred until after the first commercial sale of our allogeneic product candidate remestemcel-L for the treatment in pediatric patients with SR-aGVHD, in the United States and other geographies excluding Asia (“pediatric aGVHD”). Principal is repayable in equal quarterly instalments over the amortization period of the loan and is subject to the payment cap described below. Interest on the loan will accrue at a fixed rate of 15% per annum. If there are no net sales of remestemcel-L for pediatric SR-aGVHD, the loan is only repayable at maturity. We can elect to prepay all outstanding amounts owing at any time prior to maturity, subject to a prepayment charge, and may decide to do so if net sales of pediatric aGVHD are significantly higher than current forecasts.
Following approval and first commercial sales, repayments commence based on a percentage of net sales and are limited by a payment cap which is equal to the principal due for the next 12 months, plus accumulated unpaid principal and accrued unpaid interest. During the four-year period commencing July 8, 2022, principal amortizes in equal quarterly instalments payable only after approval and first commercial sales. If in any quarterly period, 25% of net sales of pediatric SR-aGVHD exceed the annual payment cap, we will pay the payment cap and an additional portion of excess sales which will be used towards the prepayment amount in the event there is an early prepayment of the loan. If in any quarterly period 25% of net sales of pediatric SR-aGVHD is less than the annual payment cap, then the payment is limited to 25% of net sales of pediatric SR-aGVHD. Any unpaid interest will be added to the principal amounts owing and will accrue further interest. At maturity date, any unpaid loan balances are repaid. The loan agreement contains certain covenants, see “Item 5.B Liquidity and Capital Resource – Borrowings.”
Osiris Acquisition—Continuing Obligations
In October 2013, we and Osiris entered into a purchase agreement, as amended, or the Osiris Purchase Agreement, under which we acquired all of Osiris’ business and assets related to culture expanded MSCs. Pursuant to the Osiris Purchase Agreement, we also agreed to make certain milestone and royalty payments to Osiris pertaining to remestemcel-L for the treatment of aGVHD and Crohn’s disease. Each milestone payment is for a fixed dollar amount and may be paid in cash or our ordinary shares or ADSs, at our option. The maximum amount of future milestone payments we may be required to make to Osiris is $40.0 million. Any ordinary shares or ADSs we issue as consideration for a milestone payment will be subject to a contractual one year holding period, which may be waived in our discretion. In the event that the price of our ordinary shares or ADSs decreases between the issue date and the expiration of any applicable holding period, we will be required to make an additional payment to Osiris equal to the reduction in the share price multiplied by the amount of issued shares under that milestone payment. This additional payment can be made either wholly in cash or 50% in cash and 50% in our ordinary shares, in our discretion. We have also agreed to pay varying earnout amounts as a percentage of annual net sales of acquired products, ranging from low single-digit to 10% of annual sales in excess of $750.0 million. These royalty payments will cease after the earlier of a ten year commercial sales period and the first sale of a relevant competing product. The first royalty payments were made in 2016.
Agreements with Tasly Pharmaceutical Group
In July 2018, we entered into a Development and Commercialization Agreement with Tasly.
The Development and Commercialization Agreement provides Tasly with exclusive rights to develop, manufacture and commercialize in China MPC-150-IM for the treatment or prevention of chronic heart failure and MPC-25-IC for the treatment or prevention of acute myocardial infarction. Tasly will fund all development, manufacturing and commercialization activities in China for MPC-150-IM and MPC-25-IC. On closing, we received a $20.0 million upfront technology access fee. Further, we will receive $25.0 million on product regulatory approvals in China. Mesoblast will receive double-digit escalating royalties on net product sales. Mesoblast is eligible to receive six escalating milestone payments upon the product candidates reaching certain sales thresholds in China.
145
The Development and Commercialization Agreement provides that Tasly can terminate this agreement with a specified amount of notice, on the later of (a) third anniversary of the agreement coming into effect and (b) receipt of marketing approval in China for each of MPC-150-IM or MPC-25-IC. Mesoblast has termination rights with respect to certain patent challenges by Tasly and if certain competing activities are undertaken by Tasly. Either party may terminate the agreement on material breach of the agreement if such breach is not cured within the specified cure period or if certain events related to bankruptcy of the other party occurs.
TiGenix NV – patent license for treatment of fistulae
In December 2017, we entered into a Patent License Agreement with TiGenix NV, now a wholly owned subsidiary of Takeda, which granted Takeda exclusive access to certain of our patents to support global commercialization of the adipose-derived mesenchymal stromal cell product Alofisel®, previously known as Cx601, a product candidate of Takeda, for the local treatment of fistulae. The agreement includes the right for Takeda to grant sub-licenses to affiliates and third parties.
As part of the agreement, we received $5.9 million (€5.0 million) before withholding tax as a non-refundable upfront payment and a further payment of $5.9 million (€5.0 million) before withholding tax 12 months after the patent license agreement date. We are entitled to further payments up to €10.0 million when Takeda reaches certain product regulatory milestones. Additionally, we receive single digit royalties on net sales of Alofisel®.
The agreement will continue in full force in each country (other than the United States) until the date upon which the last issued claim of any licensed patent covering Alofisel® expires in such country (currently expected to be 2029) or, with respect to the United States, until the later of (i) the date upon which the last issued claim of any licensed patent covering Alofisel® in the United States expires (currently expected to be around 2031) or (ii) the expiration of the regulatory exclusivity period in the United States with an agreed maximum term.
Either we or Takeda may terminate the agreement for any material breach that is not cured within 90 days after notice. We also have the right to terminate the agreement with a written notice in the event that Takeda file a petition in bankruptcy or insolvency or Takeda makes an assignment of substantially all of its assets for the benefit of its creditors.
Takeda has the right to terminate its obligation to pay royalties for net sales in a specific country if it is of the opinion that there is no issued claim of any licensed patent covering Alofisel® in such country, subject to referral of the matter to the joint oversight/cooperation committee established under the agreement if we disagree.
10.D Exchange Controls
The Australian dollar is freely convertible into U.S. dollars. In addition, there are currently no specific rules or limitations regarding the export from Australia of profits, dividends, capital or similar funds belonging to foreign investors, except that certain payments to non-residents must be reported to the Australian Transaction Reports and Analysis Centre (“AUSTRAC”), which monitors such transaction, and amounts on account of potential Australian tax liabilities may be required to be withheld unless a relevant taxation treaty can be shown to apply.
Regulation of acquisition by foreign entities
Under Australian law, in certain circumstances foreign persons are prohibited from acquiring more than a limited percentage of the shares in an Australian company without approval from the Australian Treasurer. These limitations are set forth in the Australian Foreign Acquisitions and Takeovers Act 1975. These limitations are in addition to the more general overarching Takeovers Prohibition of an acquisition of more than a 20% interest in a public company (in the absence of an applicable exception) under the takeovers provisions of Australia’s Corporations Act by any person whether foreign or otherwise.
Under the Foreign Acquisitions and Takeovers Act, as currently in effect, any foreign person, together with associates, or parties acting in concert, is prohibited from acquiring 20% or more of the shares in any company having consolidated total assets of or that is valued at A$281.0 million or more (or A$1,216.0 million or more in case of U.S. investors or investors from certain other countries). No asset threshold applies in the case of foreign government investors. Different rules apply to sensitive industries (such as media, telecommunications, and encryption and security technologies),
146
companies owning land or that are agribusinesses. “Associates” is a broadly defined term under the Foreign Acquisitions and Takeovers Act and includes in relation to any person:
•any relative of the person;
•any person with whom the person is acting or proposes to act in concert;
•any person with whom the person carries on a business in partnership;
•any entity of which the person is a ‘senior officer’ (such as a director or executive);
•if the person is an entity, any holding entity or any senior officer of the entity;
•any entity whose senior officers are accustomed or obliged to act in accordance with the directions, instructions or wishes of the person or if the person is an entity, its senior officers or vice versa;
•any corporation in which the person holds a ‘substantial interest’ (i.e., 20%) or any person holding a substantial interest in the person if a corporation;
•a trustee of a trust in which the person holds a substantial interest or if the person is the trustee of a trust, a person who holds a substantial interest in the trust;
•if the person is a foreign government, a separate government entity or a foreign government investor in relation to a foreign country, any other person that is a foreign government, a separate government entity or foreign government investor, in relation to that country.
The Australian Treasurer also has power in certain circumstances to make an order specifying that two or more persons are associates.
In addition, a foreign person may not acquire shares in a company having consolidated total assets of or that is valued at A$281 million or more (or A$1,216 million or more in case of U.S. investors or investors from certain other countries) if, as a result of that acquisition, the total holdings of all foreign persons and their associates will exceed 40% in aggregate without the approval of the Australian Treasurer. If the necessary approvals are not obtained, the Treasurer may make an order requiring the acquirer to dispose of the shares it has acquired within a specified period of time. The same rule applies if the total holdings of all foreign persons and their associates already exceeds 40% and a foreign person (or its associate) acquires any further shares, including in the course of trading in the secondary market of the ADSs. Different rules apply to government investors, and acquisitions of interests in sensitive business acquisitions, agribusiness and land owning entities.
Each foreign person seeking to acquire holdings in excess of the above caps (including their associates, as the case may be) would need to complete an application form setting out the proposal and relevant particulars of the acquisition/shareholding and pay the relevant application fees. The Australian Treasurer then has 30 days to consider the application and make a decision. However, the Australian Treasurer may extend the period by up to a further 90 days by publishing an interim order. The Australian Foreign Investment Review Board, an Australian advisory board to the Australian Treasurer has provided a guideline titled Australia’s Foreign Investment Policy which provides an outline of the policy. As for the risk associated with seeking approval, the policy provides, among other things, that the Treasurer will reject an application if it is contrary to the national interest.
If the level of foreign ownership in Mesoblast exceeds 40% at any time, we would be considered a foreign person under the Foreign Acquisitions and Takeovers Act. In such event, we would be required to obtain the approval of the Australian Treasurer for our company, together with our associates, to acquire (i) more than 20% of an Australian company or business having total assets of, or that is valued at, A$281 million or more; or (ii) any direct or indirect ownership in Australian land; or (iii) any ‘direct interest’ in any agribusiness.
The percentage of foreign ownership in our company may also be included in determining the foreign ownership of any Australian company or business in which we may choose to invest. Since we have no current plans for any such acquisition and do not own any property, any such approvals required to be obtained by us as a foreign person under the Takeovers Act will not affect our current or future ownership or lease of property in Australia.
Our Constitution does not contain any additional limitations on the right to hold or vote our securities by reason of being a non-resident.
147
Australian law requires the transfer of shares in our company to be made in writing or electronically through the Clearing House Electronic Sub-register System.
10.E Taxation
The following summary of the material Australian and U.S. federal income tax consequences of an investment in our ADSs or ordinary shares is based upon laws and relevant interpretations thereof in effect as of the date of this Form 20-F, all of which are subject to change, possibly with retroactive effect. This summary does not deal with all possible tax consequences relating to an investment in our ADSs or ordinary shares, such as the tax consequences under U.S. state, local and other tax laws other than Australian and U.S. federal income tax laws.
Certain Material U.S. Federal Income Tax Considerations to U.S. Holders
The following summary describes certain material U.S. federal income tax consequences to U.S. holders (as defined below) of the ownership and disposition of our ordinary shares and ADSs as of the date hereof. Except where noted, this summary deals only with our ordinary shares or ADSs acquired and held as capital assets within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”). This section does not discuss the tax consequences to any particular holder, nor any tax considerations that may apply to holders subject to special tax rules, such as:
•banks, insurance companies, regulated investment companies and real estate investment trusts;
•financial institutions;
•individual retirement and other tax-deferred accounts;
•certain former U.S. citizens or long-term residents;
•brokers or dealers in securities or currencies;
•traders that elect to use a mark-to-market method of accounting;
•partnerships and other entities treated as partnership or pass through entities for U.S. federal income tax purposes, and partners or investors in such entities;
•tax-exempt organizations (organizations that would be exempt from tax under U.S. law, including public charities and private foundations);
•persons that may have been subject to the alternative minimum tax;
•persons that hold or dispose of ordinary shares or ADSs as a position in a straddle or as part of a hedging, constructive sale, conversion or other integrated transaction;
•persons that have a functional currency other than the U.S. dollar;
•persons that own (directly, indirectly or constructively) 10% or more of the vote or value of our equity;
•persons subject to special tax accounting rules as a result of any item of gross income with respect to ordinary shares or ADSs being taken into account in an applicable financial statement;
•persons who acquire ordinary shares or ADSs pursuant to the exercise of any employee share option or otherwise as compensation; or
•persons that are not U.S. holders (as defined below).
In this section, a “U.S. holder” means a beneficial owner of ordinary shares or ADSs, other than a partnership or other entity treated as a partnership for U.S. federal income tax purposes, that is, for U.S. federal income tax purposes:
•an individual who is a citizen or resident of the United States (for U.S. federal income tax purposes);
•a corporation (or other entity classified for purposes of and pursuant to U.S. federal income tax laws as a corporation) created or organized in or under the laws of the United States or any state thereof or the District of Columbia;
•an estate the income of which is includable in gross income for U.S. federal income tax purposes regardless of its source; or
148
•a trust (i) the administration of which is subject to the primary supervision of a court in the United States and for which one or more U.S. persons have the authority to control all substantial decisions or (ii) that has an election in effect under applicable U.S. income tax regulations to be treated as a U.S. person.
The discussion below is based upon the provisions of the Code, and the U.S. Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be replaced, revoked or modified, possibly with retroactive effect, so as to result in U.S. federal income tax consequences different from those discussed below. In addition, this summary is based, in part, upon the terms of the deposit agreement and assumes that the deposit agreement, and all other related agreements, will be performed in accordance with their terms.
If a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes acquires, owns or disposes of ordinary shares or ADSs, the U.S. federal income tax treatment of a partner generally will depend on the status of the partner and the activities of the partnership. Partners of partnerships that acquire, own or dispose of ordinary shares or ADSs should consult their tax advisors.
You are urged to consult your own tax advisor with respect to the U.S. federal, as well as state, local and non-U.S., tax consequences to you of acquiring, owning and disposing of ordinary shares or ADSs in light of your particular circumstances, including the possible effects of changes in U.S. federal income and other tax laws and the effects of any tax treaties.
ADSs
Assuming the deposit agreement and all other related agreements will be performed in accordance with their terms, a U.S. holder of ADSs will be treated as the beneficial owner for U.S. federal income tax purposes of the underlying shares represented by the ADSs. The U.S. Treasury has expressed concerns that parties to whom American depositary shares are released before shares are delivered to the depositary, or intermediaries in the chain of ownership between holders of American depositary shares and the issuer of the security underlying the American depositary shares, may be taking actions that are inconsistent with claiming foreign tax credits by holders of American depositary shares. These actions would also be inconsistent with claiming the reduced rate of tax, described below, applicable to dividends received by certain non-corporate holders. Accordingly, the creditability of any foreign taxes and the availability of the reduced tax rate for dividends received by certain non-corporate U.S. holders, each described below, could be affected by actions taken by such parties or intermediaries.
Distributions
Subject to the passive foreign investment company, or PFIC, rules discussed below, U.S. holders generally will include as gross dividend income the U.S. dollar value of the gross amount of any distributions of cash or property, other than certain pro rata distributions of ordinary shares, with respect to ordinary shares or ADSs to the extent the distributions are made from our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. Subject to the PFIC rules, a U.S. holder may be permitted to credit the taxes withheld, subject to a limitation, or deduct the taxes withheld. A U.S. holder will include the dividend income on the day actually or constructively received: (i) by the holder, in the case of ordinary shares, or (ii) by the depositary, in the case of ADSs. To the extent, if any, that the amount of any distribution by us exceeds our current and accumulated earnings and profits, as so determined, the excess with respect to any share (ordinary share or ADS) will be treated first as a tax-free return of the U.S. holder’s tax basis in such ordinary share or ADS and thereafter as capital gain on such share. Notwithstanding the foregoing, we do not intend to determine our earnings and profits on the basis of U.S. federal income tax principles. Consequently, any distributions generally will be reported as dividend income for U.S. information reporting purposes. See “—Backup Withholding Tax and Information Reporting Requirements” below. Dividends paid by us will not be eligible for the dividends-received deduction generally allowed to U.S. corporate shareholders.
The U.S. dollar amount of dividends received by an individual, trust or estate with respect to the ordinary shares or ADSs will be subject to taxation at preferential rates if the dividends are “qualified dividends.” Dividends paid on ordinary shares or ADSs will be treated as qualified dividends if (i)(a) we are eligible for the benefits of a comprehensive income tax treaty with the United States that the Secretary of the Treasury of the United States determines is satisfactory for this purpose and includes an exchange of information program or (b) the dividends are with respect to ordinary shares (or ADSs in respect of such shares) which are readily tradable on a U.S. securities market; (ii) certain holding period requirements are met; and (iii) we are not classified as a PFIC for the taxable year in which the dividend is paid or for the preceding taxable year. The Agreement between the Government of the United States of America and the Government of
149
Australia for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, or the Treaty, has been approved for the purposes of the qualified dividend rules, and we expect to qualify for benefits under the Treaty. In addition, our ADSs are listed on the Nasdaq Global Select Market, and as such U.S. Treasury Department guidance indicates that our ADSs will be readily tradable on an established U.S. securities market. Thus, we believe that as long as we are not a PFIC, dividends we pay generally should be eligible for the preferential tax rates on qualified dividends. However, the determination of whether a dividend qualifies for the preferential tax rates must be made at the time the dividend is paid. U.S. holders should consult their own tax advisors regarding the availability of the preferential tax rates on dividends.
Includible distributions paid in Australian dollars, including any Australian withholding taxes, will be included in the gross income of a U.S. holder in a U.S. dollar amount calculated by reference to the spot exchange rate in effect on the date of actual or constructive receipt, regardless of whether the Australian dollars are converted into U.S. dollars at that time. If Australian dollars are converted into U.S. dollars on the date of actual or constructive receipt, the tax basis of the U.S. holder in those Australian dollars will be equal to their U.S. dollar value on that date and, as a result, a U.S. holder generally should not be required to recognize any foreign currency exchange gain or loss. If Australian dollars so received are not converted into U.S. dollars on the date of receipt, the U.S. holder will have a basis in the Australian dollars equal to their U.S. dollar value on the date of receipt. Any foreign currency exchange gain or loss on a subsequent conversion or other disposition of the Australian dollars generally will be treated as ordinary income or loss to such U.S. holder and generally will be income or loss from sources within the United States for foreign tax credit limitation purposes.
Dividends received by a U.S. holder with respect to ordinary shares (or ADSs in respect of such shares) will be treated as foreign source income, which may be relevant in calculating the holder’s foreign tax credit limitation. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to ADSs or ordinary shares will generally constitute “passive category income” but could, in the case of certain U.S. holders, constitute “general category income.”
Subject to certain complex limitations, including the PFIC rules discussed below, a U.S. holder generally will be entitled, at such holder’s option, to claim either a credit against such holder’s U.S. federal income tax liability or a deduction in computing such holder’s U.S. federal taxable income in respect of any Australian taxes withheld. If a U.S. holder elects to claim a deduction, rather than a foreign tax credit, for Australian taxes withheld for a particular taxable year, the election will apply to all foreign taxes paid or accrued by or on behalf of the U.S. holder in the particular taxable year.
The availability of the foreign tax credit and the application of the limitations on its availability are fact specific and are subject to complex rules. You are urged to consult your own tax advisor as to the consequences of Australian withholding taxes and the availability of a foreign tax credit or deduction. See “—Australian Tax Considerations Australian—Income Tax—Taxation of Dividends” below.
Sale, Exchange or Other Disposition of Ordinary Shares or ADSs
Subject to the PFIC rules discussed below, a U.S. holder generally will, for U.S. federal income tax purposes, recognize capital gain or loss, if any, on a sale, exchange or other disposition of ordinary shares or ADSs equal to the difference between the amount realized on the disposition and the U.S. holder’s tax basis (in U.S. dollars) in the ordinary shares or ADSs. This recognized gain or loss will generally be long-term capital gain or loss if the U.S. holder has held the ordinary shares or ADSs for more than one year. Generally, for U.S. holders who are individuals (as well as certain trusts and estates), long-term capital gains are subject to U.S. federal income tax at preferential rates. For foreign tax credit limitation purposes, gain or loss recognized upon a disposition generally will be treated as from sources within the United States. The deductibility of capital losses is subject to limitations for U.S. federal income tax purposes.
You should consult your own tax advisor regarding the tax consequences if a foreign tax is imposed on a disposition of ADSs or ordinary shares, including availability of a foreign tax credit or deduction in respect of any Australian tax imposed on a sale or other disposition of ordinary shares or ADSs. See “—Australian Tax Considerations—Australian Income Tax—Tax on Sales or Other Dispositions of Shares—Capital Gains Tax.”
Passive Foreign Investment Company
As a non-U.S. corporation, we will be a PFIC for any taxable year if either: (i) 75% or more of our gross income for the taxable year is passive income (such as certain dividends, interest, rents or royalties and certain gains from the sale
150
of shares and securities or commodities transactions, including amounts derived by reason of the temporary investment of funds raised in offerings of our ordinary shares or ADSs); or (ii) the average quarterly value of our gross assets during the taxable year that produce passive income or are held for the production of passive income is at least 50% of the value of our total assets. For purposes of the PFIC asset test, passive assets generally include any cash, cash equivalents and cash invested in short-term, interest bearing debt instruments or bank deposits that are readily convertible into cash. If we own at least 25% (by value) of the stock of another corporation, we will be treated, for purposes of the PFIC income and asset tests, as owning our proportionate share of the other corporation’s assets and receiving our proportionate share of the other corporation’s income.
We do not believe that we were a PFIC for the taxable year ending June 30, 2023. However, if there is a change in the type or composition of our gross income, or our actual business results do not match our projections, it is possible that we may become a PFIC in future taxable years. Investors should be aware that our gross income for purposes of the PFIC income test depends on the receipt of Australian research and development tax incentive credits and other revenue, and there can be no assurances that such tax incentive credit programs will not be revoked or modified, that we will continue to conduct our operations in the manner necessary to be eligible for such incentives or that we will receive other gross income that is not considered passive for purposes of the PFIC income test. The value of our assets for purposes of the PFIC asset test will generally be determined by reference to our market capitalization, which may fluctuate. The composition of our income and assets will also be affected by how, and how quickly, we spend the cash raised in offerings of our ordinary shares or ADSs. Under circumstances where our gross income from activities that produce passive income significantly increases relative to our gross income from activities that produce non-passive income or where we decide not to deploy significant amounts of cash for active purposes, our risk of becoming classified as a PFIC may substantially increase. Since a separate factual determination as to whether we are or have become a PFIC must be made each year (after the close of such year), we cannot assure you that we will not be or become a PFIC in the current year or any future taxable year. There can be no assurance that we will not be a PFIC for any taxable year, as PFIC status is determined each year and depends on the composition of our income and assets and the value of our assets in such year. If we are a PFIC for any taxable year, upon request, we intend to provide U.S. holders with the information necessary to make and maintain a “Qualified Electing Fund” election, as described below.
Default PFIC Rules
If we are a PFIC for any taxable year during which you own our ordinary shares or ADSs, unless you make the mark-to-market election or the Qualified Electing Fund election described below, you will generally be (and remain) subject to additional taxes and interest charges, regardless of whether we remain a PFIC in any subsequent taxable year, (i) on certain “excess distributions” we may make; and (ii) on any gain realized on the disposition or deemed disposition of your ordinary shares or ADSs. Distributions in respect of your ordinary shares (or ADSs in respect of such shares) during the taxable year will generally constitute “excess” distributions if, in the aggregate, they exceed 125% of the average amount of distributions in respect of your ordinary shares (or ADSs) over the three preceding taxable years or, if shorter, the portion of your holding period before such taxable year.
To compute the tax on “excess” distributions or any gain: (i) the “excess” distribution or the gain will be allocated ratably to each day in your holding period for the ADSs or the ordinary shares; (ii) the amount allocated to the current taxable year and any taxable year before we became a PFIC will be taxed as ordinary income in the current year; (iii) the amount allocated to other taxable years will be taxable at the highest applicable marginal rate in effect for that year; and (iv) an interest charge at the rate for underpayment of taxes will be imposed with respect to any portion of the “excess” distribution or gain described under (iii) above that is allocated to such other taxable years. In addition, if we are a PFIC or, with respect to a particular U.S. holder, we are treated as a PFIC for the taxable year in which the distribution was paid or the prior taxable year, no distribution that you receive from us will qualify for taxation at the preferential rate for non-corporate holders discussed in “—Distributions” above. You should consult with your own tax advisor regarding the application of the default PFIC rules based on your particular circumstances.
If we are a PFIC for any taxable year during which a U.S. holder holds our ADSs or ordinary shares and any of our non-U.S. subsidiaries is also a PFIC (i.e., a lower-tier PFIC), such U.S. holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be subject to the rules described above on certain distributions by the lower-tier PFIC and our disposition of shares of the lower-tier PFIC, even though such U.S. holder may not receive the proceeds of those distributions or dispositions. You should consult with your own tax advisor regarding the application to you of the PFIC rules to any of our subsidiaries if we are a PFIC.
151
Mark-to-Market Election
If we are a PFIC for any taxable year during which you own our ADSs or ordinary shares, you will be able to avoid the rules applicable to “excess” distributions or gains described above if the ordinary shares or ADSs are “marketable” and you make a timely “mark-to-market” election with respect to your ordinary shares or ADSs. The ordinary shares or ADSs will be “marketable” stock as long as they remain regularly traded on a national securities exchange, such as the Nasdaq Global Select Market, or a foreign securities exchange regulated by a governmental authority of the country in which the market is located and which meets certain requirements, including that the rules of the exchange effectively promote active trading of listed stocks. If such stock is traded on such a qualified exchange or other market, such stock generally will be “regularly traded” for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter, but no assurances can be given in this regard. Our ordinary shares are traded on the ASX, which may qualify as an eligible foreign securities exchange for this purpose.
If you are eligible to make a “mark-to-market” election with respect to our ordinary shares or ADSs and you make this election in a timely fashion, you will generally recognize as ordinary income or ordinary loss the difference between the fair market value of your ordinary shares or ADSs on the last day of any taxable year and your adjusted tax basis in the ordinary shares or ADSs. Any ordinary income resulting from this election will generally be taxed at ordinary income rates. Any ordinary losses will be deductible only to the extent of the net amount of previously included income as a result of the mark-to-market election, if any. Your adjusted tax basis in the ordinary shares or ADSs will be adjusted to reflect any such income or loss. Any gain recognized on the sale or other disposition of your ordinary shares or ADSs in a year when we are a PFIC will be treated as ordinary income, and any loss will be treated as an ordinary loss (but only to the extent of the net amount previously included as ordinary income as a result of the mark-to-market election).
Because a mark-to-market election cannot be made for any lower-tier PFICs that we may own, a U.S. holder may continue to be subject to the PFIC rules with respect to such holder’s indirect interest in any investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes, including shares in any of our subsidiaries that are treated as PFICs.
You should consult with your own tax advisor regarding the applicability and potential advantages and disadvantages to you of making a “mark-to-market” election with respect to your ordinary shares or ADSs if we are or become a PFIC, including the tax issues raised by lower-tier PFICs that we may own and the procedures for making such an election.
QEF Election
Alternative rules to those set forth under “Default PFIC Rules” above apply if an election is made to treat us as a “Qualified Electing Fund,” or QEF, under Section 1295 of the Code. A QEF election is available only if a U.S. holder receives an annual information statement from us setting forth such holder’s pro rata share of our ordinary earnings and net capital gains, as calculated for U.S. federal income tax purposes.
Upon request from a U.S. holder, we will endeavor to provide to the U.S. holder within 90 days after the request an annual information statement, in order to enable the U.S. holder to make and maintain a QEF election for us or for any of our subsidiaries that is or becomes a PFIC. However, there is no assurance that we will have timely knowledge of our or our subsidiaries’ status as a PFIC in the future or of the required information to be provided. You should consult your own tax advisor regarding the availability and tax consequences of a QEF election with respect to the ordinary shares or ADSs or with respect to any lower-tier PFIC that we may own under your particular circumstances.
Reporting
If we are a PFIC for any taxable year during which you own our ordinary shares or ADSs, as a U.S. holder, you will generally be required to file IRS Form 8621 on an annual basis and other reporting requirements may apply. The PFIC rules are complex and you should consult with your own tax advisor regarding whether we or any of our subsidiaries are a PFIC, the tax consequences of any elections that may be available to you, and how the PFIC rules may affect the U.S. federal income tax consequences of the receipt, ownership, and disposition of our ordinary shares or ADSs.
Tax on Net Investment Income
Certain non-corporate U.S. holders will be subject to a 3.8% tax on the lesser of (i) the U.S. holder’s “net investment income” for the relevant taxable year; and (ii) the excess of the U.S. holder’s modified adjusted gross income
152
for the taxable year over a certain threshold. A U.S. holder’s net investment income will generally include dividends received on the ordinary shares or ADSs and net gains from the disposition of ordinary shares or ADSs, unless such dividend income or net gains are derived in the ordinary course of the conduct of a trade or business (other than a trade or business that consists of certain passive or trading activities). A U.S. holder that is an individual, estate or trust should consult the holder’s tax advisor regarding the applicability of the tax on net investment income to the holder’s dividend income and gains in respect of the holder’s investment in the ordinary shares or ADSs.
Backup Withholding Tax and Information Reporting Requirements
U.S. backup withholding tax and information reporting requirements generally apply to payments to non-corporate holders of ordinary shares or ADSs. Information reporting will apply to payments of dividends on, and to proceeds from the disposition of, ordinary shares or ADSs by a paying agent within the United States to a U.S. holder, other than U.S. holders that are exempt from information reporting and properly certify their exemption. A paying agent within the United States will be required to withhold at the applicable statutory rate, currently 24%, in respect of any payments of dividends on, and the proceeds from the disposition of, ordinary shares or ADSs within the United States to a U.S. holder (other than U.S. holders that are exempt from backup withholding and properly certify their exemption) if the holder fails to furnish its correct taxpayer identification number or otherwise fails to comply with applicable backup withholding requirements. U.S. holders who are required to establish their exempt status generally must provide a properly completed IRS Form W-9.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. holder’s U.S. federal income tax liability. A U.S. holder generally may obtain a refund of any amounts withheld under the backup withholding rules in excess of such holder’s U.S. federal income tax liability by filing the appropriate claim for refund with the IRS in a timely manner and furnishing any required information.
Certain U.S. holders may be required to report (on IRS Form 8938) information with respect to such holder’s interest in “specified foreign financial assets” (as defined in Section 6038D of the Code), including stock of a non-U.S. corporation that is not held in an account maintained by a U.S. “financial institution”. Persons who are required to report specified foreign financial assets and fail to do so may be subject to substantial penalties. U.S. holders are urged to consult their own tax advisors regarding foreign financial asset reporting obligations and their possible application to the holding of ordinary shares or ADSs.
The discussion above is a general summary only. It is not intended to constitute a complete analysis of all tax considerations applicable to an investment in our ADSs or ordinary shares. You should consult with your own tax advisor concerning the tax consequences to you of an investment in our ADSs or ordinary shares in light of your particular circumstances.
Australian Tax Considerations
In this section, we discuss the material Australian income tax, stamp duty and goods and services (“GST”) tax considerations related to the acquisition, ownership and disposal by the absolute beneficial owners of the ordinary shares or ADSs. It is based upon existing Australian tax law as of the date of this annual report, which is subject to change, possibly retrospectively. This discussion does not address all aspects of Australian tax law which may be important to particular investors in light of their individual investment circumstances, such as shares held by investors subject to special tax rules (for example, financial institutions, insurance companies, tax exempt organizations or employee share scheme participants). In addition, this summary does not discuss any non-Australian tax considerations. Prospective investors are urged to consult their tax advisors regarding the Australian and foreign income and other tax considerations of the acquisition, ownership and disposition of the shares. This summary is based upon the premise that the holder is not an Australian tax resident and is not carrying on business in Australia through a permanent establishment (referred to as a “Foreign Shareholder” in this summary).
Australian Income Tax
Nature of ADSs for Australian Taxation Purposes
Ordinary shares represented by ADSs held by a U.S. holder will be treated for Australian income tax purposes as held under a “bare trust” for such holder. Consequently, the underlying ordinary shares will be regarded as owned by the ADS holder for Australian income tax (including capital gains tax (“CGT”)) purposes. Dividends paid on the underlying
153
ordinary shares will also be treated as dividends paid to the ADS holder, as the person beneficially entitled to those dividends.
Taxation of Dividends
Australia operates a dividend imputation system under which dividends may be declared to be “franked” to the extent of tax paid on company profits. Fully franked dividends paid to Foreign Shareholders are not subject to dividend withholding tax. Dividends paid to Foreign Shareholders are generally subject to dividend withholding tax, to the extent that the dividends are not foreign (i.e. non-Australian) sourced and declared to be “conduit foreign income” (“CFI”), and are unfranked. Dividend withholding tax will be imposed at 30%, unless a Foreign Shareholder is a resident of a country with which Australia has a double taxation agreement ("DTA") and qualifies for the benefits of the DTA. Under the provisions of the current DTA between Australia and the United States (“Australia-U.S. DTA”), the rate of tax Australian tax to be withheld on unfranked dividends paid by Mesoblast Limited (the “Company”) (which are not declared to CFI) to which a resident of the United States is beneficially entitled, is generally limited to 15% if the U.S. resident holds less than 10% of the voting power in the Company.
If a Foreign Shareholder that is a company and is a resident of the United States holds 10% or more of the voting power in the Company and is beneficially entitled to dividends from the Company, the rate of Australian dividend withholding tax is limited to 5%. In limited circumstances, the rate of withholding can be reduced to zero.
Tax on Sales or Other Dispositions of Shares – CGT
A Foreign Shareholder will not be subject to Australian CGT on any gain made on the sale or other disposal of ordinary shares in the Company, unless broadly it, together with associates, holds 10% or more of the issued capital in the Company, at the time of disposal or for 12 months of the last 2 years prior to disposal.
A Foreign Shareholder who, together with associates, owns a 10% or more interest would be subject to Australian CGT on the sale of that interest if more than 50% of the Company’s assets (held directly or indirectly and determined by reference to market value), consists of interests in Australian real property, which includes land and leases of land, as well as mining, quarrying or prospecting rights (this is referred to as “taxable Australian property” (“TAP”)). Relief from Australian CGT is unlikely to be provided by the Australian-U.S. DTA. Australian CGT applies to net capital gains of Foreign Shareholders at the Australian tax rates for non-Australian residents, which start at a marginal rate of 32.5% for individuals. Net capital gains are calculated after reduction for capital losses (including carry forward net capital losses provided that the relevant loss utilization tests have been satisfied), noting that capital losses may only be offset against capital gains.
The 50% CGT discount is not available to non-Australian residents on gains that accrued after May 8, 2012. Companies, whether Australian resident or not, are not entitled to the CGT discount.
Broadly, where there is a disposal of TAP, the purchaser will be required to withhold and remit to the Australian Taxation Office (“ATO”) 12.50% of the proceeds from the sale. A transaction is excluded from the withholding requirements in certain circumstances, including where the value of the TAP is less than A$750,000, the transaction is an on-market transaction conducted on an approved stock exchange, a securities lending arrangement, or the transaction is conducted using a broker operated crossing system. There is also an exception to the requirement to withhold where the Commissioner issues a clearance certificate which broadly certifies that the vendor is not a foreign person. The Foreign Shareholder may be entitled to receive a tax credit for the tax withheld by the purchaser which they may claim in their Australian income tax return.
Tax on Sales or Other Dispositions of Shares – Shareholders Holding Shares on Revenue Account
Some Foreign Shareholders may hold ordinary shares on “revenue” account rather than on capital account – for example, share traders. These shareholders may have the gains made on the sale or other disposal of the ordinary shares included in their assessable income under the ordinary income or trading stock provisions of the income tax law, if the gains are sourced in Australia.
Foreign Shareholders assessable under these ordinary income provisions in respect of gains made on ordinary shares held on revenue account would be assessed for such gains at the Australian tax rates for non-Australian residents,
154
which start at a marginal rate of 32.5% for individuals. Relief from Australian income tax may be available to such Foreign Shareholders under the Australia-U.S. DTA.
The comments above in “Tax on Sales or Other Dispositions of Shares—Capital Gains Tax” regarding a purchaser being required to withhold 12.5% tax on the acquisition of TAP equally applies where the disposal of the Australian real property asset by a foreign resident is likely to generate gains on revenue account, rather than a capital gain.
Australian Death Duty
Australia does not have estate or death duties. As a general rule, no CGT liability is realized upon the inheritance of a deceased person’s ordinary shares. The disposal of inherited ordinary shares by beneficiaries may, however, give rise to a CGT liability if the gain falls within the scope of Australia’s jurisdiction to tax (as discussed above).
Stamp Duty
Generally, no Australian stamp duty is payable by Australian residents or non-Australian residents on the issue, agreement to transfer, transfer, surrender of, or other dealing in, the ADSs or the ordinary shares in the Company, provided that at the time of such dealing, all of the ADSs and ordinary shares in the Company are quoted on Nasdaq and ASX and the dealing does not result in a person or entity acquiring or commencing to hold or being beneficially entitled to (together with associates and having regard to any associated transactions) 90% or more of the total issued shares in the Company.
GST
The supply of ADSs and/or ordinary shares in the Company will not be subject to Australian GST.
10.F Dividends and Paying Agents
Not applicable.
10.G Statement by Experts
Not applicable.
10.H Documents on Display
Any statement in this Form 20-F about any of our contracts or other documents is not necessarily complete. If the contract or document is filed as an exhibit to the Form 20-F the contract or document is deemed to modify the description contained in this Form 20-F. You must review the exhibits themselves for a complete description of the contract or document.
In addition, the SEC maintains a website at http://www.sec.gov that contains reports and other information regarding issuers that file electronically with the SEC. These SEC filings are also available to the public from commercial document retrieval services.
We are required to file or furnish reports and other information with the SEC under the Securities Exchange Act of 1934 and regulations under that act. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the form and content of proxy statements and our officers, directors and principal shareholders are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the Exchange Act.
10.I Subsidiary Information
For information about our subsidiaries, see “Item 18. Financial Statements – Note 12.”
10.J Annual Report to Security Holders
Not applicable.
155
Item 11. Quantitative and Qualitative Disclosures about Market Risk
For information about our exposure to market risk and how we manage this risk, see “Item 18. Financial Statements – Note 10.”
Item 12. Description of Securities Other than Equity Securities
12.A Debt Securities
Not applicable.
12.B Warrants and Rights
Not applicable.
12.C Other Securities
Not applicable.
12.D American Depositary Shares
Fees Payable by ADR Holders
Holders of our ADRs may have to pay our ADS depositary, JPMorgan Chase Bank N.A. (JPMorgan), fees or charges up to the amounts described in the following table:
| Persons depositing or withdrawing ordinary shares or ADS holders must pay: | Description of service | |||||||
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | •Issuance of ADSs, including issuances pursuant to a deposits of shares, share or rights distributions, stock dividend, stock split, merger or any other transactions affecting the issuance of ADSs •Cancellation of ADSs for the purpose of withdrawal of deposited securities | |||||||
| $0.05 (or less) per ADS | •Cash distribution to ADS holders | |||||||
| $1.50 per ADR | •Transfers of ADRs | |||||||
| $0.05 (or less) per ADS per calendar year | •Administrative services performed by the depositary | |||||||
Fees Payable by the Depositary to the Issuer
From time to time, the depositary may make payments to us to reimburse and/or share revenue from the fees collected from ADS holders, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS program. In performing its duties under the deposit agreement, the depositary may use brokers, dealers or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
156
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Not applicable.
Item 15. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our interim Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of June 30, 2023. “Disclosure controls and procedures,” as defined in Rules 13a-15(e) under the Exchange Act, are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and (ii) accumulated and communicated to the company’s management, including its principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Based on the evaluation of our disclosure controls and procedures, our Chief Executive Officer and interim Chief Financial Officer concluded that our disclosure controls and procedures were effective as of June 30, 2023.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act. Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of June 30, 2023 based on the criteria set forth in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the assessment, our management has concluded that its internal control over financial reporting was effective as of June 30, 2023. Our auditor, PricewaterhouseCoopers, an independent registered public accounting firm, have provided an attestation report on our internal control over financial reporting, which is included herein, see “Item 18. Financial Statements.”
Changes in Internal Control over Financial Reporting
There were no changes to our internal control over financial reporting that occurred during the period covered by this Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Internal Control
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Item 16. [Reserved]
Item 16A. Audit Committee Financial Expert
The Board of Directors of Mesoblast Ltd has determined that Michael Spooner possesses specific accounting and financial management expertise and is an Audit Committee Financial Expert as defined by the SEC. The Board of Directors has also determined that Joseph Swedish, Philip Facchina and Jane Bell, members of the Audit and Risk Management Committee, have sufficient experience and ability in finance and compliance matters to enable them to
157
adequately discharge their responsibilities. All members of the Audit and Risk Management Committee are “independent” according to the listing standards of the Nasdaq Global Select Market.
Item 16B. Code of Ethics
Our Code of Conduct covers conflicts of interest, confidentiality, fair dealing, protection of assets, compliance with laws and regulations, whistle blowing, security trading and commitments to stakeholders. In summary, the code requires that at all times all Company personnel act with the utmost integrity, objectivity and in compliance with the letter and the spirit of the law and Company policies. This document is accessible on our internet website at: http://www.mesoblast.com/company/corporate-governance/code-of-conduct-and-values.
Item 16C. Principal Accountant Fees and Services
Pre-Approval of Audit and Non-Audit Services
The Audit and Risk Management Committee’s pre-approval is required for all services provided by PwC. These services may include audit services, audit-related services, tax services and permissible non-audit services, and are subject to a specific budget. The Audit and Risk Management Committee uses a combination of two approaches – general pre-approval and specific pre-approval – in considering whether particular services or categories of services are consistent with the SEC’s rules on auditor independence. Under general pre-approval proposed services may be pre-approved without consideration of specific case-by-case services.
Audit and Non-Audit Services Fees
See “Item 18. Financial Statements – Note 18”. For the purpose of SEC classification, there were no audit-related, tax or other fees that were paid or payable to PwC that were not pre-approved by the Audit and Risk Management Committee during the years ended June 30, 2023 and 2022.
Item 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
Item 16F. Change in Registrant’s Certifying Accountant
Not applicable.
Item 16G. Corporate Governance
Under Nasdaq Stock Market Rule 5615(a)(3), foreign private issuers, such as our company, are permitted to follow certain home country corporate governance practices instead of certain provisions of the Nasdaq Stock Market Rules. For example, we may follow home country practice with regard to certain corporate governance requirements, such as the composition of the board of directors and quorum requirements applicable to shareholders’ meetings. In addition, we may follow home country practice instead of the Nasdaq Stock Market Rules requirement to hold executive sessions and to obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions or private placements of securities. Further, we may follow home country practice instead of the Nasdaq Stock Market Rules requirement to obtain shareholder approval prior to the establishment or amendment of certain share option, purchase or other compensation plans. A foreign private issuer that elects to follow a home country practice instead of any Nasdaq rule must submit to Nasdaq, in advance, a written statement from an independent counsel in such issuer’s home country certifying that the issuer’s practices are not prohibited by the home country’s laws. We submitted such a written statement to Nasdaq.
Other than as set forth below, we currently intend to comply with the corporate governance listing standards in the Nasdaq Stock Market Rules to the extent possible under Australian law. However, we may choose to change such practices to follow home country practice in the future.
158
The Nasdaq Stock Market Rules require that a listed company specify that the quorum for any meeting of the holders of share capital be at least 33 1/3% of the outstanding shares of the company’s common voting stock. We follow our home country practice, rather than complying with this rule. Consistent with Australian law, our bylaws do not require a quorum of at least 33 1/3% of the issued voting shares of Mesoblast for any general meeting of its shareholders. Our constitution provides that a quorum for a general meeting of our shareholders constitutes two shareholders present in person, by proxy, by attorney, or, where the shareholders is a body corporate, by representative. This provision and our practice of holding meetings with this quorum are not prohibited by the ASX Listing Rules or any other Australian law.
Item 16H. Mine Safety Disclosure
Not applicable.
Item 16I. Disclosure Regarding Foreign Jurisdiction that Prevent Inspections
Not applicable.
Item 16J. Insider Trading Policies
We have a Share Trading Policy (“Policy”) which sets out the policy and procedures governing the purchase, sale and other dispositions of the Company’s securities and applies to directors, officers, employees, contractors and consultants of the Company and its subsidiaries ("Mesoblast Personnel").
The Policy aims to (i) restrict Mesoblast Personnel in possession of "inside information" from trading in our securities and (ii) ensure compliance with all applicable securities laws, rules and regulations, and listing standards.
We have filed the Policy as an exhibit to this Annual Report on Form 20-F.
PART III
Item 17. Financial Statements
See “Item 18. Financial Statements”.
Item 18. Financial Statements
The following financial statements are filed as part of this Annual Report on Form 20-F.
Australian Disclosure Requirements
All press releases, financial reports and other information are available on our website: www.mesoblast.com.
159
Index to Financial Statements
Report Of Independent Registered Public Accounting Firm ( | |||||
160
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Mesoblast Limited
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheet of Mesoblast Limited and its subsidiaries (the “Company”) as of June 30, 2023 and 2022, and the related consolidated income statement and statements of comprehensive income, changes in equity and cash flows for each of the three years in the period ended June 30, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of June 30, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2023 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board and Australian equivalent International Financial Reporting Standards as issued by the Australian Accounting Standards Board. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1(i) to the consolidated financial statements, the Company has net cash outflows from operating activities and is dependent upon implementing cost containment and deferment strategies and obtaining additional funding from one or more sources to meet the Company’s projected expenditure consistent with its business strategy, and has stated that these events or conditions result in material uncertainty that may cast significant doubt (or raise substantial doubt as contemplated by PCAOB standards) on the Company’s ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1(i). The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under Item 15. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial
161
statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Impairment assessment of in-process research and development intangible assets and goodwill
As described in Note 6(c) to the consolidated financial statements, the Company’s consolidated in-process research and development (“IPRD”) intangible assets balance and consolidated goodwill balance were $427.8 million and $134.5 million as of June 30, 2023, respectively. Management tests the IPRD intangible
162
assets and goodwill balances for impairment on an annual basis or more frequently if events or changes in circumstances indicate that they might be impaired. The receipt of the complete response from the United States Food and Drug Administration (“FDA”) in August 2023 to the Company’s Biologics License Application (“BLA”) for remestemcel-L was considered to be an impairment indicator, and after completing an updated impairment assessment, management concluded that there was no impairment. The recoverability of the carrying values of IPRD intangible assets and goodwill are estimated by management using future cash flow projections and assumptions related to the outcome of research and development activities. These significant judgments and assumptions made by management are specific to the nature of the Company’s activities including estimates of market populations, product pricings, launch timings, probabilities of success and discount rates.
The principal considerations for our determination that performing procedures relating to the impairment assessment of IPRD intangible assets and goodwill is a critical audit matter are there were significant judgments made by management in estimating the recoverable amount of the Company’s IPRD intangible assets and goodwill. This in turn led to a high degree of auditor judgment, subjectivity and effort in performing procedures to evaluate management’s cash flow projections and significant assumptions, including estimates of market populations, product pricings, launch timings, probabilities of success and discount rates. In addition, the audit effort involved the use of professionals with specialized skill and knowledge to assist in performing these procedures and evaluating the audit evidence obtained.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s impairment assessments, including controls over significant assumptions. These procedures also included, among others, testing the Company’s process used to develop the fair value estimate, which included (i) evaluating the appropriateness of the valuation methodology and discounted cash flow models used to estimate the recoverable amount of the Company’s IPRD intangible assets and goodwill; (ii) testing the completeness, accuracy and relevance of the underlying data used in the models; and (iii) evaluating the reasonableness of significant assumptions used by management including estimates of market populations, product pricings, launch timings, probabilities of success and discount rates. Evaluating the significant assumptions relating to the estimates of the recoverable amount of IPRD intangible assets and goodwill involved evaluating whether the significant assumptions used by management were reasonable considering consistency with (i) external market and industry data; (ii) the outcome of clinical trials; (iii) formal communications from regulatory authorities; (iv) announcements made by the Company; and (v) other comparable estimates of the Company’s valuation released by securities analysts. Professionals with specialized skill and knowledge were used to assist in the evaluation of the reasonableness of the Company’s discount rate assumptions.
Fair value measurement of the provision for contingent consideration
As described in Note 5(g) to the consolidated financial statements, the Company had a balance of $17.2 million as of June 30, 2023 for the provision for contingent consideration, which management determined using an internal valuation with a discounted cash flow model requiring the use of inputs classified as level 3 in the fair value hierarchy. Significant assumptions used by management to value the provision for contingent consideration included probabilities of success and probability of payment, including the impact from the complete response from the FDA on the Company’s BLA for remestemcel-L.
The principal considerations for our determination that performing procedures relating to the fair value measurement of contingent consideration is a critical audit matter are there were significant judgments made by management in estimating the fair value of the provision for contingent consideration. This in turn
led to high degree of auditor judgment, subjectivity and effort in performing procedures to evaluate management’s cash flow projections and significant assumptions, including probabilities of success and probability of payment.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s process for valuing the provision for contingent consideration, including controls over significant assumptions. These procedures also included, among others, testing the Company’s process used to develop the fair value estimate, which included (i) evaluating the appropriateness of the valuation methodology and discounted cash flow model used to estimate the value of the provision; (ii) testing the completeness, accuracy and relevance of the underlying data used in the model; and (iii) evaluating the reasonableness of significant assumptions used by management, including probabilities of success and probability of payment. Evaluating the significant assumptions relating to the estimate of the fair value measurement of the provision for contingent consideration involved evaluating whether the assumptions used by management were reasonable considering consistency with (i) external market and industry data; (ii) the outcome of
163
clinical trials; (iii) formal communications from regulatory authorities; (iv) announcements made by the Company; and (v) evidence obtained from our procedures over the impairment assessment of IPRD intangible assets and goodwill.
/s/ PricewaterhouseCoopers
Melbourne, Australia
August 31, 2023
We have served as the Company’s auditor since 2008.
164
Mesoblast Limited
Consolidated Income Statement
| Year Ended June 30, | |||||||||||||||||||||||
| (in U.S. dollars, in thousands, except per share amount) | Note | 2023 | 2022 | 2021 | |||||||||||||||||||
| Revenue | 3 | ||||||||||||||||||||||
| Research & development | ( | ( | ( | ||||||||||||||||||||
| Manufacturing commercialization | ( | ( | ( | ||||||||||||||||||||
| Management and administration | ( | ( | ( | ||||||||||||||||||||
| Fair value remeasurement of contingent consideration | 3 | ||||||||||||||||||||||
| Fair value remeasurement of warrant liability | 3 | ( | |||||||||||||||||||||
| Other operating income and expenses | 3 | ( | |||||||||||||||||||||
| Finance costs | 3 | ( | ( | ( | |||||||||||||||||||
| Loss before income tax | 3 | ( | ( | ( | |||||||||||||||||||
| Income tax benefit/(expense) | 4 | ||||||||||||||||||||||
| Loss attributable to the owners of Mesoblast Limited | ( | ( | ( | ||||||||||||||||||||
| Losses per share from continuing operations attributable to the ordinary equity holders of the Group: | Cents | Cents | Cents | ||||||||||||||||||||
| Basic - losses per share | 19 | ( | ( | ( | |||||||||||||||||||
| Diluted - losses per share | 19 | ( | ( | ( | |||||||||||||||||||
The above consolidated income statement should be read in conjunction with the accompanying Notes.
165
Mesoblast Limited
Consolidated Statement of Comprehensive Income
| Year Ended June 30, | |||||||||||||||||||||||
| (in U.S. dollars, in thousands) | Note | 2023 | 2022 | 2021 | |||||||||||||||||||
| Loss for the period | ( | ( | ( | ||||||||||||||||||||
| Other comprehensive (loss)/income | |||||||||||||||||||||||
| Items that may be reclassified to profit and loss | |||||||||||||||||||||||
| Exchange differences on translation of foreign operations | 7(b) | ( | ( | ||||||||||||||||||||
| Items that will not be reclassified to profit and loss | |||||||||||||||||||||||
| Financial assets at fair value through other comprehensive income | 7(b) | ( | ( | ||||||||||||||||||||
| Other comprehensive (loss)/income for the period, net of tax | ( | ( | ( | ||||||||||||||||||||
| Total comprehensive losses attributable to the owners of Mesoblast Limited | ( | ( | ( | ||||||||||||||||||||
The above consolidated statement of comprehensive income should be read in conjunction with the accompanying Notes.
166
Mesoblast Limited
Consolidated Statement of Changes in Equity
| (in U.S. dollars, in thousands) | Note | Issued Capital | Share Option Reserve | Investment Revaluation Reserve | Foreign Currency Translation Reserve | Warrant Reserve | Retained Earnings/ (accumulated losses) | Total | |||||||||||||||||||||||||||||||||||||||
| Balance as of July 1, 2020 | ( | ( | — | ( | |||||||||||||||||||||||||||||||||||||||||||
| Loss for the period | — | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||
| Other comprehensive income/(loss) | — | — | ( | — | — | ( | |||||||||||||||||||||||||||||||||||||||||
| Total comprehensive income/(loss) for the period | — | — | ( | — | ( | ( | |||||||||||||||||||||||||||||||||||||||||
| Transactions with owners in their capacity as owners: | |||||||||||||||||||||||||||||||||||||||||||||||
| Contributions of equity net of transaction costs | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| Tax credited / (debited) to equity | — | ( | — | — | — | — | ( | ||||||||||||||||||||||||||||||||||||||||
| Transfer of exercised options | ( | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| Fair value of share-based payments | 17 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| Issuance of warrants | 7(b) | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Balance as of June 30, 2021 | 7(a) | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||||
| Balance as of July 1, 2021 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||||||||
| Loss for the period | — | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||
| Other comprehensive (loss)/income | — | — | ( | — | — | ( | |||||||||||||||||||||||||||||||||||||||||
| Total comprehensive (loss)/income for the period | — | — | ( | — | ( | ( | |||||||||||||||||||||||||||||||||||||||||
| Transactions with owners in their capacity as owners: | |||||||||||||||||||||||||||||||||||||||||||||||
| Contributions of equity net of transaction costs | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| Tax credited / (debited) to equity | — | ( | — | — | — | — | ( | ||||||||||||||||||||||||||||||||||||||||
| Transfer of exercised options | ( | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| Fair value of share-based payments | 17 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Balance as of June 30, 2022 | 7(a) | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||||
| Balance as of July 1, 2022 | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||||||||
| Loss for the period | — | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||
| Other comprehensive (loss)/income | — | — | ( | ( | — | — | ( | ||||||||||||||||||||||||||||||||||||||||
| Total comprehensive (loss)/income for the period | — | — | ( | ( | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||
| Transactions with owners in their capacity as owners: | |||||||||||||||||||||||||||||||||||||||||||||||
| Contributions of equity net of transaction costs | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| Tax credited / (debited) to equity | — | ( | — | — | — | — | ( | ||||||||||||||||||||||||||||||||||||||||
| Fair value of share-based payments | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||
| Balance as of June 30, 2023 | 7(a) | ( | ( | ( | |||||||||||||||||||||||||||||||||||||||||||
The above consolidated statement of changes in equity should be read in conjunction with the accompanying Notes.
167
Mesoblast Limited
Consolidated Balance Sheet
| As of June 30, | |||||||||||||||||
| (in U.S. dollars, in thousands) | Note | 2023 | 2022 | ||||||||||||||
| Assets | |||||||||||||||||
| Current Assets | |||||||||||||||||
| Cash & cash equivalents | 5(a) | ||||||||||||||||
| Trade & other receivables | 5(b) | ||||||||||||||||
| Prepayments | 5(b) | ||||||||||||||||
| Total Current Assets | |||||||||||||||||
| Non-Current Assets | |||||||||||||||||
| Property, plant and equipment | 6(a) | ||||||||||||||||
| Right-of-use assets | 6(b) | ||||||||||||||||
| Financial assets at fair value through other comprehensive income | 5(c) | ||||||||||||||||
| Other non-current assets | 5(d) | ||||||||||||||||
| Intangible assets | 6(c) | ||||||||||||||||
| Total Non-Current Assets | |||||||||||||||||
| Total Assets | |||||||||||||||||
| Liabilities | |||||||||||||||||
| Current Liabilities | |||||||||||||||||
| Trade and other payables | 5(e) | ||||||||||||||||
| Provisions | 6(d) | ||||||||||||||||
| Borrowings | 5(f) | ||||||||||||||||
| Lease liabilities | 6(b) | ||||||||||||||||
| Warrant liability | 5(f) | ||||||||||||||||
| Total Current Liabilities | |||||||||||||||||
| Non-Current Liabilities | |||||||||||||||||
| Provisions | 6(d) | ||||||||||||||||
| Borrowings | 5(f) | ||||||||||||||||
| Lease liabilities | 6(b) | ||||||||||||||||
| Deferred consideration | 6(f) | ||||||||||||||||
| Total Non-Current Liabilities | |||||||||||||||||
| Total Liabilities | |||||||||||||||||
| Net Assets | |||||||||||||||||
| Equity | |||||||||||||||||
| Issued Capital | 7(a) | ||||||||||||||||
| Reserves | 7(b) | ||||||||||||||||
| Accumulated losses | ( | ( | |||||||||||||||
| Total Equity | |||||||||||||||||
The above consolidated balance sheet should be read in conjunction with the accompanying Notes.
168
Mesoblast Limited
Consolidated Statement of Cash Flows
| Year Ended June 30, | |||||||||||||||||||||||
| (in U.S. dollars, in thousands) | Note | 2023 | 2022 | 2021 | |||||||||||||||||||
| Cash flows from operating activities | |||||||||||||||||||||||
| Commercialization revenue received | |||||||||||||||||||||||
| Government grants and tax incentives received | |||||||||||||||||||||||
| Payments to suppliers and employees (inclusive of goods and services tax) | ( | ( | ( | ||||||||||||||||||||
| Interest received | |||||||||||||||||||||||
| Income taxes received /(paid) | ( | ( | |||||||||||||||||||||
| Net cash (outflows) in operating activities | 8(b) | ( | ( | ( | |||||||||||||||||||
| Cash flows from investing activities | |||||||||||||||||||||||
| Investment in fixed assets | ( | ( | ( | ||||||||||||||||||||
| Receipts from investment in sublease | |||||||||||||||||||||||
| Payments for licenses | ( | ( | |||||||||||||||||||||
| Net cash (outflows) in investing activities | ( | ( | ( | ||||||||||||||||||||
| Cash flows from financing activities | |||||||||||||||||||||||
| Proceeds from borrowings | |||||||||||||||||||||||
| Repayment of borrowings | ( | ||||||||||||||||||||||
| Payment of transaction costs from borrowings | ( | ( | ( | ||||||||||||||||||||
| Interest and other costs of finance paid | ( | ( | ( | ||||||||||||||||||||
| Proceeds from issue of shares | |||||||||||||||||||||||
| Proceeds from issue of warrants | |||||||||||||||||||||||
| Payments for share issue costs | ( | ( | ( | ||||||||||||||||||||
| Payments for lease liabilities | ( | ( | ( | ||||||||||||||||||||
| Net cash inflows/(outflows) by financing activities | ( | ||||||||||||||||||||||
| Net increase/(decrease) in cash and cash equivalents | ( | ||||||||||||||||||||||
| Cash and cash equivalents at beginning of period | |||||||||||||||||||||||
| FX (loss)/gain on the translation of foreign bank accounts | ( | ( | |||||||||||||||||||||
| Cash and cash equivalents at end of period | 8(a) | ||||||||||||||||||||||
The above consolidated statement of cash flows should be read in conjunction with the accompanying Notes.
169
Mesoblast Limited
Notes to Consolidated Financial Statements
Mesoblast Limited (“the Company”) and its subsidiaries (“the Group”) are primarily engaged in the development of regenerative medicine products. The Group’s primary proprietary regenerative medicine technology platform is based on specialized cells known as mesenchymal lineage cells. The Company was formed in 2004 as an Australian company and has been listed on the Australian Securities Exchange (the “ASX”) since 2004. In November 2015, the Company listed in the United States of America (“U.S.”) on the Nasdaq Global Select Market (“Nasdaq”) and from this date has been dual-listed in Australia and the U.S.
These financial statements and notes are presented in U.S. dollars (“$” or “USD” or “US$”), unless otherwise noted, including certain amounts that are presented in Australian dollars (“AUD” or “A$”) and Singapore dollars (“SGD” or “S$”).
1. Basis of preparation
The general purpose financial statements of Mesoblast Limited and its subsidiaries have been prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board and Australian equivalent International Financial Reporting Standards, as issued by the Australian Accounting Standards Board. Mesoblast Limited is a for-profit entity for the purpose of preparing the financial statements. Certain comparative amounts have been reclassified to facilitate comparison to the current period. There was no material impact to the financial statements.
The financial statements cover Mesoblast Limited and its subsidiaries. The financial statements were authorized for issue by the board of directors on August 31, 2023. The directors have the power to amend and reissue the financial statements.
(i) Going concern
The Group has continued its focus on maintaining tight control of net cash usage for operating activities, which was $63.3 million for the year ended June 30, 2023. As of June 30, 2023, the Group held total cash reserves of $71.3 million. The Group is implementing various cost containment and deferment strategies, including the reprioritization of projects and operational streamlining to manage net operating cash usage.
In August 2023, the FDA provided a complete response to the Group's BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data. In line with the Group's overall commercial strategy to progress to adult populations, the Group intends to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. In conjunction with implementing cost containment and deferment strategies, additional inflows from royalty monetization, capital markets, strategic partnerships or product specific financing will be required to meet the Group's projected expenditure consistent with the Group's business strategy over at least the next 12 months. As a result of these matters, there is material uncertainty related to events or conditions that may cast significant doubt (or raise substantial doubt as contemplated by Public Company Accounting Oversight Board (“PCAOB”) standards) on the Group’s ability to continue as a going concern and, therefore, that the Group may be unable to realize its assets and discharge its liabilities in the normal course of business. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
(ii) Historical cost convention
These financial statements have been prepared under the historical cost convention, as modified by the revaluation of financial assets at fair value through other comprehensive income and financial assets and liabilities (including derivative instruments) at fair value through profit or loss, certain classes of property, plant and equipment and investment property.
(iii) New and amended standards adopted by the Group
There were no new or amended standards adopted by the Group in the year ended June 30, 2023. These financial statements follow the same accounting policies as compared to the June 30, 2022 consolidated financial statements and related notes as filed with the Australian Securities Exchange and the Securities and Exchange Commission.
170
(iv) New accounting standards and interpretations not yet adopted by the Group
There were no new accounting standards and interpretations not yet adopted by the Group for the June 30, 2023 reporting period that are expected to materially impact the Group.
(v) Use of estimates
The preparation of these consolidated financial statements requires the Group to make estimates and judgments that affect the reported amounts of assets, liabilities, income and expenses and related disclosures. On an ongoing basis, the Group evaluates its significant accounting policies and estimates. Estimates are based on historical experience and on various market-specific and other relevant assumptions that the Group believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities.
(vi) Impact of after effects of COVID-19 and geopolitical instability
Estimates are assessed each period and updated to reflect current information, such as the economic considerations related to the after effects of the COVID-19 pandemic could have on the Group’s significant accounting estimates.
The Group is having to account for the after effects of the COVID-19 pandemic on healthcare network, which have been and may continue to be impacted by the pandemic with respect to patient care, operations/staffing, financials, and health and safety protocols. These impacts change the way that Mesoblast will have to engage with the relevant collaborators.
Due to the effects of the COVID-19 pandemic, and recent geopolitical instability, countries in which the Group has operations have experienced some challenges in the ability of the Group’s suppliers and contractors to source, supply or acquire raw materials or components needed for its manufacturing process and supply chain. As a result, the manufacturing and commercialization of remestemcel-L and other product candidates could be adversely affected.
2. Significant changes in the current reporting period
(i) Significant events
The financial position and performance of the Group was affected by the following events during the year ended June 30, 2023:
•On April 25, 2023, the Group completed a global private placement primarily to existing major shareholders raising approximately $40.0 million, net of transaction costs.
•In January 2023, the Group resubmitted to the FDA the Biologics License Application ("BLA") for approval of remestemcel-L in the treatment of children with SR-aGVHD and in March 2023, the FDA accepted the Company's BLA resubmission and set a Prescription Drug User Fee Act ("PDUFA") goal date of August 2, 2023. In August 2023, the FDA provided a complete response to the Group's BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data. In line with the Group's overall commercial strategy to progress to adult populations, the Group intends to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. Assumptions associated with SR-aGVHD are included within the impairment assessment of Osiris MSC products within in-process research and development, contingent consideration, pre-launch inventory and the NovaQuest borrowings on the consolidated balance sheet and forecast net operating cash usage. The Group has assessed and included the impact of the FDA's complete response to the Group's BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD in these areas. Refer to Note 15 for more information.
•In December 2022, the Group amended the terms of the loan agreement with Oaktree and in connection with the loan amendment, Oaktree was granted warrants to purchase 455,000 American Depositary Shares (ADSs) at $3.70 per ADS, a 15 % premium to the 30-day VWAP. The Group determined that an obligation to issue the warrants had arisen from the time the debt facility was signed; consequently, a liability for the warrants was recognized in December 2022. The warrants were legally issued on March 8, 2023 and may be exercised within 7 years of issuance. Refer to Note 5(g)(vi) for more details on warrants issued.
171
3. Loss before income tax
| Year Ended June 30, | |||||||||||||||||||||||
| (in U.S. dollars, in thousands) | Note | 2023 | 2022 | 2021 | |||||||||||||||||||
| Revenue | |||||||||||||||||||||||
| Commercialization revenue | |||||||||||||||||||||||
| Milestone revenue | |||||||||||||||||||||||
| Total Revenue | |||||||||||||||||||||||
| Clinical trial and research & development | ( | ( | ( | ||||||||||||||||||||
| Manufacturing production & development | ( | ( | ( | ||||||||||||||||||||
| Employee benefits | |||||||||||||||||||||||
| Salaries and employee benefits | ( | ( | ( | ||||||||||||||||||||
| Defined contribution superannuation expenses | ( | ( | ( | ||||||||||||||||||||
Equity settled share-based payment transactions(1) | ( | ( | ( | ||||||||||||||||||||
| Total Employee benefits | ( | ( | ( | ||||||||||||||||||||
| Depreciation and amortization of non-current assets | |||||||||||||||||||||||
| Plant and equipment depreciation | ( | ( | ( | ||||||||||||||||||||
| Right of use asset depreciation | ( | ( | ( | ||||||||||||||||||||
| Intellectual property amortization | ( | ( | ( | ||||||||||||||||||||
| Total Depreciation and amortization of non-current assets | ( | ( | ( | ||||||||||||||||||||
| Other Management & administration expenses | |||||||||||||||||||||||
| Overheads & administration | ( | ( | ( | ||||||||||||||||||||
| Consultancy | ( | ( | ( | ||||||||||||||||||||
| Legal, patent and other professional fees | ( | ( | ( | ||||||||||||||||||||
| Intellectual property expenses (excluding the amount amortized above) | ( | ( | ( | ||||||||||||||||||||
| Total Other Management & administration expenses | ( | ( | ( | ||||||||||||||||||||
| Fair value remeasurement of contingent consideration | |||||||||||||||||||||||
| Remeasurement of contingent consideration | 5(g)(iii) | ||||||||||||||||||||||
| Total Fair value remeasurement of contingent consideration | |||||||||||||||||||||||
| Fair value remeasurement of warrant liability | |||||||||||||||||||||||
| Remeasurement of warrant liability | 5(g)(vi) | ( | |||||||||||||||||||||
| Total Fair value remeasurement of warrant liability | ( | ||||||||||||||||||||||
| Other operating income and expenses | |||||||||||||||||||||||
Research and development tax incentive income(2) | |||||||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Foreign exchange (losses)/gains | ( | ( | |||||||||||||||||||||
| Derecognition of right-of-use asset | |||||||||||||||||||||||
172
| Foreign withholding tax paid | ( | ||||||||||||||||||||||
| Government grant revenue | |||||||||||||||||||||||
| Total Other operating income and expenses | ( | ||||||||||||||||||||||
| Finance (costs)/gains | |||||||||||||||||||||||
| Remeasurement of borrowing arrangements | ( | ( | |||||||||||||||||||||
| Interest expense | ( | ( | ( | ||||||||||||||||||||
| Total Finance costs | ( | ( | ( | ||||||||||||||||||||
| Total loss before income tax | ( | ( | ( | ||||||||||||||||||||
(1)Share-based payment transactions
For the years ended June 30, 2023, 2022 and 2021, share-based payment transactions have been reflected in the Consolidated Statement of Comprehensive Income functional expense categories as follows:
| Year Ended June 30, | |||||||||||||||||
| (in U.S. dollars) | 2023 | 2022 | 2021 | ||||||||||||||
| Research and development | |||||||||||||||||
| Manufacturing and commercialization | ( | ||||||||||||||||
| Management and administration | |||||||||||||||||
| Equity settled share-based payment transactions | |||||||||||||||||
(2) Research and development tax incentive
The Group's research and development activities are eligible under the Australian government's Innovation Australia Research and Development Tax Incentive program for research and development activities conducted in relation to qualifying research that meets the regulatory criteria. Management has assessed these activities and expenditures to determine which costs are likely to be eligible under the incentive scheme. The Group assesses, on an annual basis, the quantum of previous research and development tax claims and on-going eligibility to claim this tax incentive in Australia.
173
4. Income tax benefit/(expense)
| Year Ended June 30, | ||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | 2021 | |||||||||||||||||
| (a) | Reconciliation of income tax to prima facie tax payable | |||||||||||||||||||
| Loss from continuing operations before income tax | ( | ( | ( | |||||||||||||||||
Tax benefit at the Australian tax rate of | ( | ( | ( | |||||||||||||||||
| Tax effect of amounts which are not deductible/(exempt) in calculating taxable income: | ||||||||||||||||||||
| Share-based payments expense | ||||||||||||||||||||
| Research and development tax concessions | ( | ( | ( | |||||||||||||||||
| Foreign exchange translation gains/(losses) | ||||||||||||||||||||
| Contingent consideration | ( | ( | ( | |||||||||||||||||
| Other sundry items | ( | |||||||||||||||||||
| Subtotal | ( | ( | ( | |||||||||||||||||
| Adjustments for current tax of prior periods | ( | ( | ||||||||||||||||||
| Differences in overseas tax rates | ||||||||||||||||||||
| Tax benefit not recognized | ||||||||||||||||||||
Change in tax rate on Deferred tax assets(1) | ( | ( | ||||||||||||||||||
Change in tax rate on Deferred tax liability(1) | ||||||||||||||||||||
| Income tax benefit attributable to loss before income tax | ( | ( | ( | |||||||||||||||||
| (1) | On June 30, 2022, there was a change in the expected tax rate applicable on future taxable profits in Singapore. The Group was expecting to benefit from concessionary tax rates (tax holiday) in Singapore under the tax incentives granted to the Group by the Singapore Economic Development Board, however at June 30, 2022 the Group had not met the conditions under the agreement to access the concessionary tax rates and therefore have recognized a change in the expected tax rate in Singapore to reflect the statutory tax rate of | ||||
| Year Ended June 30, | ||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | 2021 | |||||||||||||||||
| (b) | Income tax (benefit)/expense | |||||||||||||||||||
| Current tax | ||||||||||||||||||||
| Current tax | ||||||||||||||||||||
| Total current tax (benefit)/expense | ||||||||||||||||||||
| Deferred tax | ||||||||||||||||||||
| (Increase)/decrease in deferred tax assets | ( | ( | ||||||||||||||||||
| (Decrease)/increase in deferred tax liabilities | ( | |||||||||||||||||||
| Total deferred tax (benefit)/expense | ( | ( | ( | |||||||||||||||||
| Income tax (benefit)/expense | ( | ( | ( | |||||||||||||||||
Deferred tax assets have been brought to account only to the extent that it is foreseeable that they are recoverable against future tax liabilities.
Deferred tax assets are recognized for unused tax losses to the extent that it is probable that future taxable profit will be available against which the unused tax losses can be utilized. Deferred tax assets are offset against taxable temporary differences (deferred tax liabilities) when the deferred tax balances relate to the same tax jurisdiction in accordance with our accounting policy.
174
Deferred taxes are measured at the rate in which they are expected to settle within the respective jurisdictions, which can change based on factors such as new legislation or timing of utilization and reversal of associated assets and liabilities.
| Year Ended June 30, | ||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | 2021 | |||||||||||||||||
| (c) | Amounts that would be recognized directly in equity if brought to account | |||||||||||||||||||
| Aggregate current and deferred tax arising in the reporting period and not recognized in net loss or other comprehensive income but which would have been directly applied to equity had it been brought to account: | ||||||||||||||||||||
| Current tax recorded in equity (if brought to account) | ( | ( | ( | |||||||||||||||||
| Deferred tax recorded in equity (if brought to account) | ||||||||||||||||||||
| ( | ||||||||||||||||||||
| Year Ended June 30, | ||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | 2021 | |||||||||||||||||
| (d) | Amounts recognized directly in equity | |||||||||||||||||||
| Aggregate current and deferred tax arising in the reporting period and not recognized in net loss or other comprehensive income but debited/credited to equity | ||||||||||||||||||||
| Current tax recorded in equity | ||||||||||||||||||||
| Deferred tax recorded in equity | ||||||||||||||||||||
| Year Ended June 30, | ||||||||||||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | 2021 | |||||||||||||||||
| (e) | Deferred tax assets not brought to account | |||||||||||||||||||
| Unused tax losses | ||||||||||||||||||||
| Potential tax benefit at local tax rates | ||||||||||||||||||||
| Other temporary differences | ||||||||||||||||||||
| Potential tax benefit at local tax rates | ||||||||||||||||||||
| Other tax credits | ||||||||||||||||||||
| Potential tax benefit at local tax rates | ||||||||||||||||||||
The Group has not brought to account $553.0 million (2022: $477.8 million, 2021: $424.9 million) of gross tax losses, which includes the benefit arising from tax losses in overseas countries. As of June 30, 2023 $553.0 million of tax losses not brought to account have an indefinite life. Gross tax losses of $44.5 million recognized as deferred tax asset expire within a range of 9 to 15 years. The benefits of unused tax losses will only be brought to account when it is probable that they will be realized.
This benefit of tax losses will only be obtained if:
•the Group derives future assessable income of a nature and an amount sufficient to enable the benefit from the deductions for the losses to be realized;
•the Group continues to comply with the conditions for deductibility imposed by tax legislation; and
•no changes in tax legislation adversely affect the Group in realizing the benefit from the deductions for the losses.
175
5. Financial assets and liabilities
This note provides information about the Group's financial instruments, including:
•an overview of all financial instruments held by the Group;
•specific information about each type of financial instrument;
•accounting policies; and
•information used to determine the fair value of the instruments, including judgments and estimation uncertainty involved.
The Group holds the following financial instruments:
| Financial assets (in U.S. dollars, in thousands) | Notes | Assets at FVOCI(1) | Assets at FVTPL(2) | Assets at amortized cost | Total | ||||||||||||||||||||||||
| As of June 30, 2023 | |||||||||||||||||||||||||||||
| Cash & cash equivalents | 5(a) | — | — | ||||||||||||||||||||||||||
| Trade & other receivables | 5(b) | — | — | ||||||||||||||||||||||||||
| Financial assets at fair value through other comprehensive income | 5(c) | — | — | ||||||||||||||||||||||||||
| Other non-current assets | 5(d) | — | — | ||||||||||||||||||||||||||
| — | |||||||||||||||||||||||||||||
| As of June 30, 2022 | |||||||||||||||||||||||||||||
| Cash & cash equivalents | 5(a) | — | — | ||||||||||||||||||||||||||
| Trade & other receivables | 5(b) | — | — | ||||||||||||||||||||||||||
| Financial assets at fair value through other comprehensive income | 5(c) | — | — | ||||||||||||||||||||||||||
| Other non-current assets | 5(d) | — | — | ||||||||||||||||||||||||||
| — | |||||||||||||||||||||||||||||
(1)Fair value through other comprehensive income
(2)Fair value through profit or loss
| Financial liabilities (in U.S. dollars, in thousands) | Notes | Liabilities at FVOCI(1) | Liabilities at FVTPL(2) | Liabilities at amortized cost | Total | ||||||||||||||||||||||||
| As of June 30, 2023 | |||||||||||||||||||||||||||||
| Trade and other payables | 5(e) | — | — | ||||||||||||||||||||||||||
| Borrowings | 5(f) | — | — | ||||||||||||||||||||||||||
| Contingent consideration | 5(g)(iii) | — | — | ||||||||||||||||||||||||||
| Warrant liability | 5(g)(vi) | — | — | ||||||||||||||||||||||||||
| — | |||||||||||||||||||||||||||||
| As of June 30, 2022 | |||||||||||||||||||||||||||||
| Trade and other payables | 5(e) | — | — | ||||||||||||||||||||||||||
| Borrowings | 5(f) | — | — | ||||||||||||||||||||||||||
| Contingent consideration | 5(g)(iii) | — | — | ||||||||||||||||||||||||||
| Warrant liability | 5(g)(vi) | — | — | ||||||||||||||||||||||||||
| — | |||||||||||||||||||||||||||||
(1)Fair value through other comprehensive income
(2)Fair value through profit or loss
176
The Group’s exposure to various risks associated with the financial instruments is discussed in Note 10. The maximum exposure to credit risk at the end of the reporting period is the carrying amount of each class of financial assets mentioned above.
a. Cash and cash equivalents
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Cash at bank | |||||||||||
Deposits at call(1) | |||||||||||
| (1) | As of June 30, 2023 and June 30, 2022, interest-bearing deposits at call include amounts of $ | ||||
(i) Classification as cash equivalents
Term deposits are presented as cash equivalents if they have a maturity of three months or less from the date of acquisition.
b. Trade and other receivables and prepayments
(i) Trade and other receivables
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Trade debtors | |||||||||||
| Tax incentives recoverable | |||||||||||
| Foreign withholding tax recoverable | |||||||||||
| U.S. Tax credits | |||||||||||
| Net investment in sublease | |||||||||||
| Interest receivables | |||||||||||
| Other recoverable taxes (Goods and services tax and value-added tax) | |||||||||||
| Trade and other receivables | |||||||||||
(ii) Prepayments
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Clinical trial research and development expenditure | |||||||||||
| Prepaid insurance and subscriptions | |||||||||||
| Other | |||||||||||
| Prepayments | |||||||||||
(iii) Classification as trade and other receivables
Trade receivables and other receivables represent the principal amounts due at balance date less, where applicable, any provision for expected credit losses. The Group uses the simplified approach to measuring expected credit losses, which uses a lifetime expected credit loss allowance. Debts which are known to be uncollectible are written off in the consolidated income statement. All trade receivables and other receivables, with the exception of the net investment in sublease, are recognized at the value of the amounts receivable, as they are due for settlement within 60 days and therefore
177
do not require remeasurement. The net investment in sublease is recognized at the present value of minimum lease payments receivable over the remaining life of the lease.
(iv) Fair values of trade and other receivables
Due to the short-term nature of the current receivables, their carrying amount is assumed to be the same as their fair value.
(v) Impairment and risk exposure
Information about the impairment of trade and other receivables, their credit quality and the Group’s exposure to credit risk, foreign currency risk and interest rate risk can be found in Note 10(a) and (b).
c. Financial assets at fair value through other comprehensive income
Financial assets at fair value through other comprehensive income include the following classes of financial assets:
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Unlisted securities: | |||||||||||
| Equity securities | |||||||||||
(i) Classification of financial assets at fair value through other comprehensive income
Financial assets at fair value through other comprehensive income comprises equity securities which are not held for trading, and which the Group has irrevocably elected at initial recognition to recognize in this category. These are strategic investments and the Group considers this classification to be more relevant.
The financial assets are presented as non-current assets unless they mature, or management intends to dispose of them within 12 months of the end of the reporting period.
(ii) Impairment indicators for financial assets at fair value through other comprehensive income
Impairment losses (and reversal of impairment losses) on equity investments measured at FVOCI are not reported separately from other changes in fair value. See Note 23(m)(iv) for further details about the Group’s impairment policies for financial assets.
(iii) Amounts recognized in other comprehensive income
For the years ended June 30, 2023, 2022 and 2021, the Group recognized in statement of comprehensive income a nil gain/loss, a loss of $0.3 million and a gain of $0.2 million respectively, for change in fair value of the financial assets through other comprehensive income.
(iv) Fair value, impairment and risk exposure
Information about the methods and assumptions used in determining fair value is provided in Note 5(g). None of the financial assets through other comprehensive income are either past due or impaired.
All financial assets at fair value through other comprehensive income are denominated in US$.
178
d. Other non-current assets
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Bank guarantee | |||||||||||
| Net investment in sublease | |||||||||||
| Letter of credit | |||||||||||
| Security deposit | |||||||||||
(i) Classification of financial assets as other non-current assets
Bank guarantee
These funds are held in an account named Mesoblast Limited at National Australia Bank according to the terms of a Bank Guarantee which is security for the sublease agreement for our occupancy of Level 38, 55 Collins Street, Melbourne, Victoria, Australia. The Bank Guarantee is security for the full and faithful performance and observance by the subtenant of the terms, covenants and conditions of the sublease. The Bank Guarantee continues in force until it is released by the lessor.
Letter of credit
These funds held in an account named Mesoblast, Inc. at the Bank of America according to the terms of an irrevocable standby letter of credit which is security for the sublease agreement for our occupancy of 505 Fifth Avenue, New York, New York, United States of America. The letter of credit is security for the full and faithful performance and observance by the subtenant of the terms, covenants and conditions of the sublease. The letter of credit is deemed to automatically extend without amendment for a period of one year at each anniversary.
(ii) Impairment and risk exposure
Information about the impairment of other non-current assets and their credit quality and the Group’s exposure to credit risk can be found in Note 10(b).
e. Trade and other payables
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Trade payables and other payables | |||||||||||
| Trade and other payables | |||||||||||
The carrying amounts of trade and other payables are assumed to be the same as their fair values, due to their short-term nature.
179
f. Borrowings
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Borrowings | |||||||||||
| Secured liabilities: | |||||||||||
| Borrowing arrangements | |||||||||||
| Less: transaction costs | ( | ( | |||||||||
| Amortization of carrying amount, net of payments made | |||||||||||
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Borrowings | |||||||||||
| Current | |||||||||||
| Borrowings - NovaQuest | |||||||||||
| Borrowings - Oaktree | |||||||||||
| Non-current | |||||||||||
| Borrowings - NovaQuest | |||||||||||
| Borrowings - Oaktree | |||||||||||
(i) Borrowing arrangements
Funds associated with Oaktree Capital Management, L.P. (“Oaktree”)
In November 2021, the Group entered into a $90.0 million five-year senior debt facility provided by funds associated with Oaktree. The Group drew the first tranche of $60.0 million on closing. The conditions required to draw down the additional $30.0 million tranche have not been met. The facility has a three-year interest only period, at a fixed rate of 9.75 % per annum, after which time 40 % of the principal amortizes over two years and a final payment is due no later than November 2026. The facility also allows the Group to make quarterly payments of interest at a rate of 8.0 % per annum for the first two years , and the unpaid interest portion (1.75 % per annum) will be added to the outstanding loan balance and currently accrues further interest at a fixed rate of 9.75 % per annum.
On November 19, 2021, Oaktree were granted warrants to purchase 1,769,669 American Depositary Shares (“ADSs”) at US$7.26 per ADS, a 15 % premium to the 30-day VWAP. The Group determined that an obligation to issue the warrants arose from the time the debt facility was signed; consequently, a liability for the warrants was recognized in November 2021. The warrants were legally issued on January 11, 2022 and may be exercised within 7 years of issuance. On the issuance date of the Oaktree facility and the warrants, the warrants were initially measured at fair value and the Oaktree borrowing liability measured as the difference between the $60.0 million received from the Oaktree facility and the fair value of the warrants. In December 2022, the Group amended the terms of the loan agreement with Oaktree and in connection with the loan amendment, Oaktree was granted warrants to purchase 455,000 ADSs at $3.70 per ADS, a 15 % premium to the 30-day VWAP. The Group determined that an obligation to issue the warrants arose from the time the first amendment to the loan agreement was signed; consequently, a liability for the warrants was recognized in December 2022. The warrants were legally issued on March 8, 2023 and may be exercised within 7 years of issuance. Refer to Note 5(g)(vi) for more details on warrants issued.
In the year ended June 30, 2023, the Group recognized a loss of $1.6 1.6 1.0 million relates to the remeasurement due to additional warrants being issued to Oaktree as a result of the first amendment to the loan agreement and $0.6 million relates to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facility. In the year ended June 30, 2022, the Group recognized a minimal gain in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in
180
relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facility. No remeasurement of borrowing arrangements was recognized in the year ended June 30, 2021.
The Group has pledged substantially all of its assets as collateral under the loan facility with Oaktree.
NovaQuest Capital Management, L.L.C.
On June 29, 2018, the Group entered into an eight-year , $40.0 million loan and security agreement with NovaQuest before drawing the first tranche of $30.0 million of the principal in July 2018. The loan term includes an interest only period of approximately four years through until July 8, 2022, then a four-year amortization period through until maturity on July 8, 2026. All interest and principal payments will be deferred until after the first commercial sale of remestemcel-L for the treatment in pediatric patients with SR-aGVHD, in the United States and other geographies excluding Asia (“pediatric SR-aGVHD”). Principal is repayable in equal quarterly instalments over the amortization period of the loan and is subject to the payment cap described below. The loan has a fixed interest rate of 15 % per annum. If there are no net sales of remestemcel-L for pediatric SR-aGVHD, the loan is only repayable at maturity. The Group can elect to prepay all outstanding amounts owing at any time prior to maturity, subject to a prepayment charge, and may decide to do so if net sales of remestemcel-L for pediatric SR-aGVHD are significantly higher than current forecasts.
Following approval and first commercial sales, repayments commence based on a percentage of net sales and are limited by a payment cap which is equal to the principal due for the next 12 months, plus accumulated unpaid principal and accrued unpaid interest. During the four-year period commencing July 8, 2022, principal amortizes in equal quarterly instalments payable only after approval and first commercial sales. If in any quarterly period, 25 % of net sales of remestemcel-L for pediatric SR-aGVHD exceed the annual payment cap, the Group will pay the payment cap and an additional portion of excess sales which will be used towards the prepayment amount in the event there is an early prepayment of the loan. If in any quarterly period 25 % of net sales of remestemcel-L for pediatric SR-aGVHD is less than the annual payment cap, then the payment is limited to 25 % of net sales of remestemcel-L for pediatric SR-aGVHD. Any unpaid interest will be added to the principal amounts owing and shall accrue further interest. At maturity date, any unpaid loan balances are repaid.
Because of this relationship of net sales and repayments, changes in our estimated net sales may trigger an adjustment of the carrying amount of the financial liability to reflect the revised estimated cash flows. The carrying amount adjustment is recalculated by computing the present value of the revised estimated future cash flows at the financial instrument’s original effective interest rate. The adjustment is recognized in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in the period the revision is made.
In the year ended June 30, 2023, the Group recognized a gain of $0.9 million in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows as a net result of changes to the key assumption in development timelines. In the years ended June 30, 2022 and 2021, respectively, the Group recognized gains of $0.5 million and $4.8 million in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in relation to the adjustment of the carrying amount of the Group's financial liability to reflect the revised estimated future cash flows from our credit facility.
The Group recognizes a liability as current based on repayments linked to estimates of sales of remestemcel-L. However, if sales of remestemcel-L are higher than estimated, actual repayments will exceed this amount, subject to the annual payment cap described above.
The carrying amount of the loan and security agreement with NovaQuest is subordinated to the Group’s fixed rate loan with the senior creditor, Oaktree. The Group have pledged a portion of our assets relating to the SR-aGVHD product candidate as collateral under the loan facility with NovaQuest.
(ii) Compliance with loan covenants
Our loan facilities with Oaktree and NovaQuest contain a number of covenants that impose operating restrictions on us, which may restrict our ability to respond to changes in our business or take specified actions. The Group has an operating objective to at all times maintain unrestricted cash reserves in excess of six months liquidity. The objective aligns with our loan and security agreement with Oaktree where the Group is currently obliged to maintain a minimum unrestricted cash balance in the United States of $35.0 million.
181
The Group has complied with the financial and other restrictive covenants of its borrowing facilities during the year ended June 30, 2023 and during the year ended June 30, 2022.
(iii) Net debt reconciliation
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Cash and cash equivalents | |||||||||||
| Borrowings | ( | ( | |||||||||
| Lease liabilities | ( | ( | |||||||||
| Warrant liability | ( | ( | |||||||||
Net Debt(1) | ( | ( | |||||||||
| Cash and cash equivalents | |||||||||||
| Gross debt - fixed interest rates | ( | ( | |||||||||
| Gross debt - variable interest rates | |||||||||||
| Warrant liability | ( | ( | |||||||||
Net Debt(1) | ( | ( | |||||||||
(1)Net debt amount includes leases and borrowing arrangements
| Liabilities from financing activities | Other assets | ||||||||||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | Borrowings | Leases | Warrant liability | Sub-total | Cash and cash equivalents | Total | |||||||||||||||||||||||||||||
| Net Debt as at June 30, 2022 | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
Cash Flows(1) | |||||||||||||||||||||||||||||||||||
| Remeasurement adjustments | — | ( | ( | — | ( | ||||||||||||||||||||||||||||||
Other Changes(2) | ( | ( | — | ( | — | ( | |||||||||||||||||||||||||||||
| Issuance of warrants | — | — | ( | ( | — | ( | |||||||||||||||||||||||||||||
| Acquisition – leases | — | — | — | ||||||||||||||||||||||||||||||||
| Foreign exchange adjustments | — | ( | ( | ||||||||||||||||||||||||||||||||
| Net Debt as at June 30, 2023 | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
(1)Cash flows include the payments of borrowings, lease liabilities, interest and debt transaction costs which are presented as financing cash flows in the statement of cash flows.
(2)Other changes include modification of leases and accrued interest expenses for borrowings and leases.
(i)Fair values of borrowing arrangements
The carrying amount of the borrowings at amortized cost in accordance with our accounting policy is a reasonable approximation of fair value.
182
g. Recognized fair value measurements
(i) Fair value hierarchy
The following table presents the Group's financial assets and financial liabilities measured and recognized at fair value as of June 30, 2023 and June 30, 2022 on a recurring basis, categorized by level according to the significance of the inputs used in making the measurements:
| As of June 30, 2023 | |||||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | Notes | Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||||||
| Financial Assets | |||||||||||||||||||||||||||||
Financial assets at fair value through other comprehensive income: | |||||||||||||||||||||||||||||
| Equity securities - biotech sector | 5(c) | ||||||||||||||||||||||||||||
| Total Financial Assets | |||||||||||||||||||||||||||||
| Financial Liabilities | |||||||||||||||||||||||||||||
| Financial liabilities at fair value through profit or loss: | |||||||||||||||||||||||||||||
| Contingent consideration | 5(g)(iii) | ||||||||||||||||||||||||||||
| Warrant liabilities | 5(g)(vi) | ||||||||||||||||||||||||||||
| Total Financial Liabilities | |||||||||||||||||||||||||||||
There were no transfers between any of the levels for recurring fair value measurements during the period.
| As of June 30, 2022 | |||||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | Notes | Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||||||
| Financial Assets | |||||||||||||||||||||||||||||
Financial assets at fair value through other comprehensive income: | |||||||||||||||||||||||||||||
| Equity securities - biotech sector | 5(c) | ||||||||||||||||||||||||||||
| Total Financial Assets | |||||||||||||||||||||||||||||
| Financial Liabilities | |||||||||||||||||||||||||||||
| Financial liabilities at fair value through profit or loss: | |||||||||||||||||||||||||||||
| Contingent consideration | 5(g)(iii) | ||||||||||||||||||||||||||||
| Warrant liabilities | 5(g)(vi) | ||||||||||||||||||||||||||||
| Total Financial Liabilities | |||||||||||||||||||||||||||||
The Group’s policy is to recognize transfers into and transfers out of fair value hierarchy levels as at the end of the reporting period.
Level 1: The fair value of financial instruments traded in active markets (such as publicly traded derivatives, and trading and financial assets at fair value through other comprehensive income securities) is based on quoted market prices at the end of the reporting period. The quoted market price used for financial assets held by the Group is the current bid price. These instruments are included in level 1.
Level 2: The fair value of financial instruments that are not traded in an active market (for example, foreign exchange contracts) is determined using valuation techniques which maximize the use of observable market data and rely as little as possible on entity-specific estimates. If all significant inputs required to fair value an instrument are observable, the instrument is included in level 2.
Level 3: If one or more of the significant inputs is not based on observable market data, the instrument is included in level 3. This is the case for provisions (contingent consideration), equity securities (unlisted) and warrant liabilities.
183
(ii) Valuation techniques used.
The Group did not
The Group’s level 3 assets consists of an investment in unlisted equity securities in the biotechnology sector. Level 3 assets were 100 100
(iii) Fair value measurements using significant unobservable inputs (level 3)
The following table presents the changes in the contingent consideration balances within the level 3 instruments for the years ended June 30, 2023 and June 30, 2022:
| (in U.S. dollars, in thousands) | Contingent consideration provision | ||||
| Opening balance - July 1, 2021 | |||||
| Amount used during the period | ( | ||||
| Charged/(credited) to consolidated income statement: | |||||
Remeasurement(1) | ( | ||||
| Closing balance - June 30, 2022 | |||||
| Opening balance - July 1, 2022 | |||||
| Reclassification during the period | |||||
| Charged/(credited) to consolidated income statement: | |||||
Remeasurement(2) | ( | ||||
| Closing balance - June 30, 2023 | |||||
| (1) | In the year ended June 30, 2022, a gain of $ | ||||
| (2) | In the year ended June 30, 2023, a gain of $ | ||||
184
(iv) Valuation inputs and relationship to fair value
The following table summarizes the quantitative information about the significant unobservable inputs used in level 3 fair value measurements:
Range of inputs (weighted average) | ||||||||||||||||||||||||||||||||||||||||||||
(in U.S. dollars, in thousands, except percent data) Description | Fair value as of June 30, 2023) | Fair value as of June 30, 2022) | Valuation technique | Unobservable inputs(1) | Year Ended June 30, 2023 | Year Ended June 30, 2022 | Relationship of unobservable inputs to fair value | |||||||||||||||||||||||||||||||||||||
| Contingent consideration provision | Discounted cash flows | Risk adjusted discount rate | ( | ( | Year ended June 30, 2023: A change in the discount rate by Year ended June 30, 2022: A change in the discount rate by | |||||||||||||||||||||||||||||||||||||||
| Expected unit sales price | Various | Various | Year ended June 30, 2023: A change in the price assumptions by Year ended June 30, 2022: A change in the price assumptions by | |||||||||||||||||||||||||||||||||||||||||
| Expected sales volumes | Various | Various | Year ended June 30, 2023: A change in the volume assumptions by Year ended June 30, 2022: A change in the volume assumptions by | |||||||||||||||||||||||||||||||||||||||||
| Probability of success and payment | Various | Various | Year ended June 30, 2023: A change in the probability of success and payment assumptions by Year ended June 30, 2022: A change in the probability of success and payment assumptions by | |||||||||||||||||||||||||||||||||||||||||
(1)There were no significant inter-relationships between unobservable inputs that materially affect fair values.
(v) Valuation processes
In connection with the Osiris acquisition, on October 11, 2013 (the “acquisition date”), an independent valuation of the contingent consideration was carried out by an independent valuer.
For the years ended June 30, 2023 and June 30, 2022, the Group has adopted a process to value contingent consideration internally. This valuation has been completed by the Group’s internal valuation team and reviewed by the interim Chief Financial Officer (the "CFO"). The valuation team is responsible for the valuation model. The valuation team also manages a process to continually refine the key assumptions within the model. This is done with input from the relevant business units. The key assumptions in the model have been clearly defined and the responsibility for refining those assumptions has been assigned to the most relevant business units. For each indication we determine the probability
185
of success based on the current development status within each jurisdiction and payment provisions within the agreement. Cash flows relevant to each jurisdiction are discounted appropriately based on the discount rate assumed. The remeasurement charged to the consolidated income statement in the year ended June 30, 2023 was a net result of changing the key assumptions of the contingent consideration valuation such as probability of payment, development timelines and the increase in valuation as the time period shortens between the valuation date and the potential settlement dates of contingent consideration, including the impact from the complete response from the FDA on the BLA for remestemcel-L for the treatment of pediatric SR-aGVHD in August 2023. The assumptions relating to development timelines have been updated to reflect current expectations as a result of the complete response, as discussed in Note 15. Future discussions with the FDA could lead to a change in the assumptions associated with SR-aGVHD and a further remeasurement of contingent consideration, up or down, could occur.
| As of June 30, | |||||||||||
The fair value of contingent consideration (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Fair value of cash or stock payable, dependent on achievement of future late-stage clinical or regulatory targets | |||||||||||
| Fair value of royalty payments from commercialization of the intellectual property acquired | |||||||||||
The main level 3 inputs used by the Group are evaluated as follows:
| Risk adjusted discount rate: | The discount rate used in the valuation has been determined based on required rates of returns of listed companies in the biotechnology industry (having regards to their stage of development, their size and number of projects) and the indicative rates of return required by suppliers of venture capital for investments with similar technical and commercial risks. This assumption is reviewed as part of the valuation process outlined above. | ||||
| Expected unit sales prices: | Expected market sale price giving consideration to comparable products available in the market place and a value based pricing assessment. This assumption is reviewed as part of the valuation process outlined above. | ||||
| Expected sales volumes: | Expected sales volumes of the most comparable products currently available in the market place. This assumption is reviewed as part of the valuation process outlined above. | ||||
| Probability of success and payment: | Expected cash flows used to measure contingent consideration are risk adjusted for the probability of successful development of products. This assumption is reviewed as part of the valuation process outlined above. | ||||
(vi) Warrant liability
| (in U.S. dollars, in thousands) | As of June 30, | ||||||||||
| Warrant liability | 2023 | 2022 | |||||||||
| Opening balance | |||||||||||
| Warrants fair value at grant date - November 19, 2021 | |||||||||||
| Warrants fair value at grant date - December 22, 2022 | |||||||||||
| Remeasurement of warrant liability | ( | ||||||||||
| Closing Balance | |||||||||||
On November 19, 2021, in connection with the $60.0 million drawdown of the Oaktree debt, Oaktree was granted the right to warrants to purchase 1,769,669 ADSs at US$7.26 per ADS, a 15 % premium to the 30-day VWAP. Given that Oaktree received an unconditional right to the warrants on November 19, 2021, this date has been determined as the measurement date. The warrant instruments were issued on January 11, 2022, following the required administrative process, and these warrants may be exercised within 7 years of issuance of the warrant instruments. The warrants do not confer any rights to dividends or a right to participate in a new issue without exercising the warrant.
186
On December 21, 2022, the Group amended the terms of the loan agreement with Oaktree and in connection with the loan amendment, Oaktree was granted warrants to purchase 455,000 ADSs at $3.70 per ADS, a 15 % premium to the 30-day VWAP. The Group determined that an obligation to issue the warrants arose from the time the first amendment to the loan agreement was signed; consequently, a liability for the warrants was recognized in December 2022. The warrants were legally issued on March 8, 2023 and may be exercised within 7 years of issuance. The warrants do not confer any rights to dividends or a right to participate in a new issue without exercising the warrant.
The exercise price of the warrants will be received in US$, which is different to Mesoblast Limited’s functional currency of A$ which gives rise to variability in the cash flow. As a result, the warrants are classified as a financial liability in accordance with IAS32 Financial Instruments: Presentation. The financial liability is recorded in warrant liability at fair value at grant date and subsequently remeasured at each reporting period with changes being recorded in the Consolidated Income Statement as remeasurement of warrant liability. The warrant liabilities are considered level 3 liabilities as the determination of fair value includes various assumptions about the share prices and historical volatility as inputs.
As of June 30, 2023 and 2022, the fair value of warrant liability was $5.4 million and $2.2 million, respectively. During the year ended June 30, 2023, a remeasurement loss of $2.2 million was recognized on the remeasurement of warrant liability. During the year ended June 30, 2022, a remeasurement gain of $5.9 million was recognized on the remeasurement of warrant liability.
(vii) Fair value of warrants
The warrants granted are not traded in an active market and therefore the fair value has been estimated by using the Black-Scholes valuation method based on the following assumptions. Key terms of the warrants are included below. The following assumptions were based on observable market conditions that existed as of June 30, 2023 and 2022.
| (in U.S. dollars, except percent data and as otherwise noted) Assumption | As of June 30, 2023 | As of June 30, 2022 | Rationale | |||||||||||||||||
| Share Price | $ | $ | Closing share price on valuation date from external market source | |||||||||||||||||
| Exercise Price | $ | $ | As per subscription agreement | |||||||||||||||||
| Expected Term | As per subscription agreement | |||||||||||||||||||
| Dividend Yield | Based on Company’s nil dividend history | |||||||||||||||||||
| Expected Volatility | Based on historical volatility data for the Company | |||||||||||||||||||
| Risk Free Interest Rate | Based on the closing U.S. treasury issued 7 year bonds on valuation date | |||||||||||||||||||
| Fair value per warrant | $ | $ | Determined using Black-Scholes valuation model with the inputs above | |||||||||||||||||
| Fair value | $ | $ | Fair value of | |||||||||||||||||
187
6. Non-financial assets and liabilities
a. Property, plant and equipment
| (in U.S. dollars, in thousands) | Plant and Equipment | Office Furniture and Equipment | Computer Hardware and Software | Total | |||||||||||||||||||
| Year Ended June 30, 2022 | |||||||||||||||||||||||
| Opening net book amount | |||||||||||||||||||||||
| Additions | |||||||||||||||||||||||
| Exchange differences | ( | ( | ( | ||||||||||||||||||||
| Depreciation charge | ( | ( | ( | ( | |||||||||||||||||||
| Closing net book value | |||||||||||||||||||||||
| As of June 30, 2022 | |||||||||||||||||||||||
| Cost | |||||||||||||||||||||||
| Accumulated depreciation | ( | ( | ( | ( | |||||||||||||||||||
| Net book value | |||||||||||||||||||||||
| Year Ended June 30, 2023 | |||||||||||||||||||||||
| Opening net book amount | |||||||||||||||||||||||
| Additions | |||||||||||||||||||||||
| Exchange differences | ( | ( | ( | ||||||||||||||||||||
| Depreciation charge | ( | ( | ( | ( | |||||||||||||||||||
| Closing net book value | |||||||||||||||||||||||
| As of June 30, 2023 | |||||||||||||||||||||||
| Cost | |||||||||||||||||||||||
| Accumulated depreciation | ( | ( | ( | ( | |||||||||||||||||||
| Net book value | |||||||||||||||||||||||
(i) Depreciation methods and useful lives
Depreciation is calculated using the straight-line method to allocate their cost or revalued amounts, net of their residual values, over the estimated useful lives. The estimated useful lives are:
•Plant and equipment 3 – 15 years
•Office furniture and equipment 5 – 10 years
•Computer hardware and software 3 – 5 years
See Note 23(o) for other accounting policies relevant to property, plant and equipment.
188
b. Leases
(i) Amounts recognized on the consolidated balance sheet
Right-of-use assets
| (in U.S. dollars, in thousands) | Buildings | Manufacturing | Total | ||||||||||||||
| Year Ended June 30, 2022 | |||||||||||||||||
| Opening net book amount | |||||||||||||||||
| Additions | — | ||||||||||||||||
| Reassessment | |||||||||||||||||
| Exchange differences | ( | — | ( | ||||||||||||||
| Depreciation charge | ( | ( | ( | ||||||||||||||
| Closing net book value | |||||||||||||||||
| As of June 30, 2022 | |||||||||||||||||
| Cost | |||||||||||||||||
| Accumulated depreciation | ( | ( | ( | ||||||||||||||
| Net book value | |||||||||||||||||
| Year Ended June 30, 2023 | |||||||||||||||||
| Opening net book amount | |||||||||||||||||
| Additions/(derecognition) | ( | — | ( | ||||||||||||||
| Reassessment | ( | ||||||||||||||||
| Exchange differences | ( | — | ( | ||||||||||||||
| Depreciation charge | ( | ( | ( | ||||||||||||||
| Closing net book value | |||||||||||||||||
| As of June 30, 2023 | |||||||||||||||||
| Cost | |||||||||||||||||
| Accumulated depreciation | ( | ( | ( | ||||||||||||||
| Net book value | |||||||||||||||||
Lease liabilities
| As of June 30, | |||||||||||
| 2023 | 2022 | ||||||||||
| Current | |||||||||||
| Non-current | |||||||||||
| Lease liabilities included in the balance sheet | |||||||||||
The lease liability is measured at the present value of the fixed and variable lease payments net of cash lease incentives that are not paid at the balance date. Lease payments are apportioned between the finance charges and reduction of the lease liability using the incremental borrowing rate to achieve a constant rate of interest on the remaining balance of the liability. Lease payments for buildings exclude service fees for cleaning and other costs. The interest expense (included in finance costs) for leases was $0.5 million for the year ended June 30, 2023, and $0.6 3.2 million and $3.4 million, respectively.
Payments associated with short-term leases with a lease term of 12 months or less, contracts that contain lease and non-lease components that are cancellable within 12 months and leases of low-value assets are recognized on a straight-line
189
basis as an expense in profit or loss. The expense relating to short term leases was $1.1 million for the year ended June 30, 2023 and $3.2 million for the year ended June 30, 2022.
(ii) Depreciation methods and useful lives of right-of use assets
Depreciation is calculated using the straight-line method to allocate their cost or revalued amounts, net of their residual values, over the estimated useful lives. Depreciation for leases for the years ended June 30, 2023, 2022 and 2021 was $1.7 million, $1.7 million and $1.7 million, respectively.
(iii) Extension and termination options
Extension options and termination options may be included in the right-of-use asset leases across the Group. These are used to maximize operational flexibility in terms of managing the assets used in the Group’s operations.
In determining the lease term, management considers all facts and circumstances that create an economic incentive to exercise an extension option, or not exercise a termination option. Extension options and periods after termination options are only included in the lease term if the lease is reasonably certain to be extended or not terminated.
A right-of-use asset and lease liability has been recognized in relation to the manufacturing service agreement entered into with Lonza in October 2019 for the supply of commercial product for the potential approval and launch of remestemcel-L for the treatment of SR-aGVHD in the US market. Management has determined that this agreement has a non-cancellable lease term expiring within 2 years from June 30, 2023, which is cancellable in limited circumstances.
As of June 30, 2023, the anticipated future contractual cash flows relating to the lease component of the Lonza agreement are $3.0 million on an undiscounted basis, as included within lease liabilities in Note 10(c). The anticipated future contractual cash flows exclude cashflows beyond the non-cancellable lease term as it is not reasonably certain the Group will extend the agreement. At the Group's discretion, the minimum financial commitment relating to the lease component under this manufacturing services agreement can be reduced by $1.1 million under certain conditions.
See Note 23(v) for other accounting policies relevant to lease accounting.
190
c. Intangible assets
| (in U.S. dollars, in thousands) | Goodwill | Acquired licenses to patents | In-process research and development acquired | Current marketed products | Total | ||||||||||||||||||||||||
| Year Ended June 30, 2022 | |||||||||||||||||||||||||||||
| Opening net book amount | |||||||||||||||||||||||||||||
| Additions/reversals | — | ( | — | — | ( | ||||||||||||||||||||||||
| Exchange differences | — | — | |||||||||||||||||||||||||||
| Amortization charge | — | ( | — | ( | ( | ||||||||||||||||||||||||
| Closing net book amount | |||||||||||||||||||||||||||||
| As of June 30, 2022 | |||||||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||
| Accumulated amortization | — | ( | — | ( | ( | ||||||||||||||||||||||||
| Accumulated impairment | — | — | ( | — | ( | ||||||||||||||||||||||||
| Net book amount | |||||||||||||||||||||||||||||
| Year Ended June 30, 2023 | |||||||||||||||||||||||||||||
| Opening net book amount | |||||||||||||||||||||||||||||
| Additions | — | — | — | ||||||||||||||||||||||||||
| Exchange differences | — | — | |||||||||||||||||||||||||||
| Amortization charge | — | ( | — | ( | ( | ||||||||||||||||||||||||
| Closing net book amount | |||||||||||||||||||||||||||||
| As of June 30, 2023 | |||||||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||
| Accumulated amortization | — | ( | — | ( | ( | ||||||||||||||||||||||||
| Accumulated impairment | — | — | ( | — | ( | ||||||||||||||||||||||||
| Net book amount | |||||||||||||||||||||||||||||
(i) Carrying value of in-process research and development acquired by product
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
Cardiovascular products(1) | |||||||||||
Intravenous products for metabolic diseases and inflammatory/immunologic conditions(2) | |||||||||||
MSC products(3) | |||||||||||
(1)Includes MPC-150-IM for the treatment or prevention of chronic heart failure and MPC-25-IC for the treatment or prevention of acute myocardial infarction
(2)Includes MPC-300-IV for the treatment of biologic-refractory rheumatoid arthritis and diabetic nephropathy
For all products included within the above balances, the underlying currency of each item recorded is US$.
191
(ii) Amortization methods and useful lives
The Group amortizes intangible assets with a finite useful life using the straight-line method over the following periods:
•Acquired licenses to patents 7 – 16 years
•Current marketed products 15 – 20 years
See Note 23(p) for the other accounting policies relevant to intangible assets and Note 23(j) for the Group’s policy regarding impairments.
(iii) Significant estimate: Impairment of goodwill and assets with an indefinite useful life
The Group tests annually, or more frequently if events or changes in circumstances indicate that they might be impaired, whether goodwill and its assets with indefinite useful lives have suffered any impairment in accordance with its accounting policy stated in Note 23(j). The recoverable amounts of these assets and cash-generating units have been determined based on fair value less costs to dispose calculations, which require the use of market-participant assumptions that are based on development strategies using external data sources as well as past experience. The full annual impairment assessment was performed at March 31, 2023 and no
In August 2023, as disclosed in Note 15, the FDA issued a complete response to the Group's BLA for remestemcel-L for the treatment of pediatric SR-aGVHD and the Group has considered this to be an impairment indicator that could cause the carrying amount of its intangible assets to exceed its recoverable amounts. As a result, the Group completed an impairment assessment on its MSC products intangible asset and goodwill. The assumptions used in the impairment assessment were updated from the March 31, 2023 impairment assessment and included changes to the market population and penetration, product pricing and launch timings. There were no other significant changes in key assumptions, including the probability of success. No impairment to the in-process research and development and goodwill was identified.
(iv) Impairment tests for goodwill and intangible assets with an indefinite useful life
The Group has recognized goodwill as a result of two separate acquisitions. Goodwill of $118.4 million was recognized on acquisition of Angioblast Systems Inc. in 2010, $13.9 million was recognized on the acquisition of the MSC assets from Osiris (“MSC business combination”) in 2013 and $2.1 million was recognized on finalization of the MSC business combination of Osiris in 2015. In all cases the goodwill recognized represented excess in the purchase price over the net identifiable assets and in-process research and development acquired in the transaction.
On acquisition, goodwill was not able to be allocated to the cash generating unit (“CGU”) level or to a group of CGU given the synergies of the underlying research and development. For the purpose of impairment testing, goodwill is monitored by management at the operating segment level. The Group is managed as one operating segment, being the development of cell technology platform for commercialization.
IFRS requires that acquired in-process research and development be measured at fair value upon acquisition and carried as an indefinite life intangible asset subject to annual impairment reviews. The Group have recognized in-process research and development as a result of two separate acquisitions. In-process research and development of $387.0 million was recognized on the acquisition of Angioblast Systems Inc. in 2010 and $126.7 million was recognized on the acquisition of assets from Osiris in 2013 and $24.0 million was reclassified to current marketed products upon the TEMCELL asset becoming available for use in Japan. In 2016, the Group fully impaired $61.9 million of in-process research and development relating to our product candidates, MPC-MICRO-IO for the treatment of age-related macular degeneration and MPC-CBE for the expansion of hematopoietic stem cells within cord blood, as the Group suspended further patient enrollment of the Phase IIa MPC-MICRO-IO clinical trial and the Phase III MPC-CBE clinical trial as the Group prioritized the funding of its lead product candidates.
The Group still believe these product candidates remain viable upon further funding, or partnership, and accordingly these products should not be regarded as abandoned, where typically, abandoned programs would be closed down and the related research and development efforts are considered impaired and the asset is fully expensed. The remaining carrying amount of in-process research and development as at June 30, 2023 and June 30, 2022 was $427.8
192
In-process research and development acquired is considered to be an indefinite life intangible asset on the basis that it is incomplete and cannot be used in its current form (see Note 23(p)(iii)). The intangible asset’s life will remain indefinite until such time it is completed and commercialized or impaired. The carrying value of in-process research and development is a separate asset which has been subject to impairment testing at the cash generating unit level, which has been determined to be at the product level.
The recoverable amount of both goodwill and in-process research and development was assessed as of March 31, 2023 based on the fair value less costs to dispose. Management assess for indicators of impairment as at June 30, 2023 including considering events up to the date of the approval of the financial statements. As a result of the receipt of the complete response from the FDA in August 2023, an impairment assessment was performed over the MSC products in-process research and development asset and goodwill. No
(v) Key assumptions used for fair value less costs to dispose calculations
In determining the fair value less costs to dispose the Group has given consideration to the following internal and external indicators:
•discounted expected future cash flows of programs valued by the Group’s internal valuation team and reviewed by the interim CFO. The valuation team also manages a process to continually refine the key assumptions within the model. This is done with input from the relevant business units. The key assumptions in the model have been clearly defined and the responsibility for refining those assumptions has been assigned to the most relevant business units. When determining key assumptions, the business units refer to both external sources and past experience as appropriate. The valuation is considered to be level 3 in the fair value hierarchy due to unobservable inputs used in the valuation;
•the scientific results and progress of the trials since acquisition;
•the market capitalization of the Group on the ASX (ASX:MSB); and
•the valuation of the Group’s assets from an independent valuation. An independent valuation was obtained for all assets at March 31, 2023 and for the MSC products for the impairment assessment performed as a result of the receipt of the complete response.
Costs of disposal were assumed to be immaterial.
Discounted cash-flows used a real post-tax discount rate range of 13.8 % to 15.5 %, and include estimated real cash inflows and outflows for each program through to expected patent expiry which ranges from 9 to 25 years.
In relation to cash outflows consideration has been given to cost of goods sold, selling costs and clinical trial schedules including estimates of numbers of patients and per patient costs. Associated expenses such as regulatory fees and patent maintenance have been included as well as any further preclinical development if applicable.
In relation to cash inflows consideration has been given to product pricing, market population and penetration, sales rebates and discounts, launch timings and probability of success in the relevant applicable markets.
There are no standard growth rates applied, other than our estimates of market penetration which increase initially, plateau and then decline.
The assessment of the recoverable amount of each product has been made in accordance with the discounted cash-flow assumptions outlined above. The assessments showed that the recoverable amount of each product exceeds the carrying amount and therefore there is no impairment.
The assessment of goodwill showed the recoverable amount of the Group's operating segment, including goodwill and remaining in-process research and development, exceeds carrying amounts, and therefore there is no impairment.
(vi) Impact of possible changes in key assumptions
The Group has considered and assessed reasonably possible changes in the key assumptions and has not identified any instances that could cause the carrying amount of our intangible assets to exceed its recoverable amount.
193
Whilst there is no impairment, the key sensitivities in the valuation are dependent on the continued successful development of our technology platforms. Future discussion with the FDA as discussed in Note 15, could change the assumptions used within the impairment assessments. If the Group is unable to successfully develop our technology platforms, an impairment of the carrying amount of our intangible assets may result.
d. Provisions
| As of June 30, 2023 | As of June 30, 2022 | ||||||||||||||||||||||||||||||||||
| (in U.S. dollars, in thousands) | Current | Non-current | Total | Current | Non-current | Total | |||||||||||||||||||||||||||||
| Contingent consideration | |||||||||||||||||||||||||||||||||||
| Employee benefits | |||||||||||||||||||||||||||||||||||
| Provision for license agreements | |||||||||||||||||||||||||||||||||||
(i) Information about individual provisions and significant estimates
Contingent consideration
The contingent consideration provision relates to the Group’s liability for certain milestones and royalty achievements pertaining to the acquired MSC assets from Osiris. Further disclosures can be found in Note 5(g)(iii).
Employee benefits
The provision for employee benefits relates to the Group’s liability for annual leave, short term incentives and long service leave.
Employee benefits include accrued annual leave. As of June 30, 2023 and 2022, the entire amount of the annual leave accrual was $1.1 million and $1.0 million respectively, and is presented as current, since the Group does not have an unconditional right to defer settlement for any of these obligations.
(ii) Movements
The contingent consideration provision relates to the Group’s liability for certain milestones and royalty achievements. Refer to Note 5(g)(iii) for movements in contingent consideration for the years ended June 30, 2023 and 2022.
e. Deferred tax balances
(i) Deferred tax balances
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Deferred tax assets | |||||||||||
| The balance comprises temporary differences attributable to: | |||||||||||
| Tax losses | |||||||||||
| Other temporary differences | |||||||||||
| Total deferred tax assets | |||||||||||
| Deferred tax liabilities | |||||||||||
| The balance comprises temporary differences attributable to: | |||||||||||
| Intangible assets | |||||||||||
| Total deferred tax liabilities | |||||||||||
| Net deferred tax liabilities | |||||||||||
194
(ii) Movements
| (in U.S. dollars, in thousands) | Tax losses(1) (DTA) | Other temporary differences(1) (DTA) | Intangible assets (DTL) | Total (DTL) | |||||||||||||||||||
| As of June 30, 2021 | ( | ( | |||||||||||||||||||||
| Charged/(credited) to: | |||||||||||||||||||||||
| - profit or loss | ( | ( | |||||||||||||||||||||
| - directly to equity | ( | — | |||||||||||||||||||||
| As of June 30, 2022 | ( | ( | |||||||||||||||||||||
| Charged/(credited) to: | |||||||||||||||||||||||
| - profit or loss | ( | ( | ( | ||||||||||||||||||||
| - directly to equity | — | ||||||||||||||||||||||
| As of June 30, 2023 | ( | ( | |||||||||||||||||||||
(1)Deferred tax assets are netted against deferred tax liabilities.
f. Deferred consideration
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
Opening balance(1) | |||||||||||
| Amount recognized as revenue during the period | |||||||||||
| Balance as of the end of the period | |||||||||||
| (1) | The $ | ||||
7. Equity
a. Contributed equity
(i) Share capital
| As of June 30, | |||||||||||||||||||||||||||||||||||
| 2023 | 2022 | 2021 | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||
| Shares No. | (U.S. dollars, in thousands) | ||||||||||||||||||||||||||||||||||
| Contributed equity | |||||||||||||||||||||||||||||||||||
| (i) Share capital | |||||||||||||||||||||||||||||||||||
| Ordinary shares | |||||||||||||||||||||||||||||||||||
| Less: Treasury Shares | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Total Contributed Equity | |||||||||||||||||||||||||||||||||||
195
(ii) Movements in ordinary share capital
| As of June 30, | As of June 30, | ||||||||||||||||||||||||||||||||||
| 2023 | 2022 | 2021 | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||
| Shares No. | (U.S. dollars, in thousands) | ||||||||||||||||||||||||||||||||||
| Opening balance | |||||||||||||||||||||||||||||||||||
| Issues of ordinary shares during the period | |||||||||||||||||||||||||||||||||||
Exercise of share options(1) | — | — | — | — | |||||||||||||||||||||||||||||||
Transfer to employee share trust(1) | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Share based compensation for services rendered | — | — | |||||||||||||||||||||||||||||||||
Placement of shares under a share placement agreement(2)(3) | — | — | |||||||||||||||||||||||||||||||||
| Transaction costs arising on share issue | — | — | — | ( | ( | ||||||||||||||||||||||||||||||
Total contributions of equity during the period | |||||||||||||||||||||||||||||||||||
| Share options reserve transferred to equity on exercise of options | — | — | — | — | |||||||||||||||||||||||||||||||
| Ending balance | |||||||||||||||||||||||||||||||||||
| (1) | Options are issued to employees, directors and consultants in accordance with the Mesoblast Employee Share Option Plan. Unpaid shares are issued to the share trust to enable future option exercises to be settled. On exercise of options, the proceeds of the exercise are recorded in ordinary share capital in Mesoblast Limited and the exercise is settled by transfer of the shares from the share trust to the employee. | ||||
| (2) | In March 2021, | ||||
| (3) | In August 2022, | ||||
196
(iii) Movements of shares in share trust
| As of June 30 | As of June 30 | ||||||||||||||||||||||||||||||||||
| 2023 | 2022 | 2021 | 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||
| Shares No. | (U.S. dollars, in thousands) | ||||||||||||||||||||||||||||||||||
Opening balance(1) | |||||||||||||||||||||||||||||||||||
| Movement of shares in share trust | |||||||||||||||||||||||||||||||||||
Transfer to employee share trust(2) | |||||||||||||||||||||||||||||||||||
Exercise of share options(2) | ( | ( | |||||||||||||||||||||||||||||||||
| Ending balance | |||||||||||||||||||||||||||||||||||
| (1) | In July 2020, the Group formed the Mesoblast Employee Share Trust, being a new trust formed to administer the Group’s employee share scheme. Prior to forming the new trust, the Group had been using the Mesoblast Limited Employee Share Trust for administering some aspects of the Group’s employee share scheme. In July 2020, | ||||
| (2) | Options are issued to employees, directors and consultants in accordance with the Mesoblast Employee Share Option Plan. Unpaid shares are issued to the share trust to enable future option exercises to be settled. On exercise of options, the proceeds of the exercise are recorded in ordinary share capital in Mesoblast Limited and the exercise is settled by transfer of the shares from the share trust to the employee. | ||||
(iv) Ordinary shares
Ordinary shares participate in dividends and the proceeds on winding up of the Group in equal proportion to the number of shares held. At shareholders meetings each ordinary share is entitled to one vote when a poll is called, otherwise each shareholder has one vote on a show of hands. Ordinary shares have no par value and the Company does not have a limited amount of authorized capital.
(v) Employee share options
Information relating to the Group’s employee share option plan, including details of shares issued under the scheme, is set out in Note 17.
b. Reserves
(i) Reserves
| As at June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Share-based payments reserve | |||||||||||
| Investment revaluation reserve | ( | ( | |||||||||
| Foreign currency translation reserve | ( | ( | |||||||||
| Warrants reserve | |||||||||||
197
(ii) Reconciliation of reserves
| (in U.S. dollars, in thousands) | As at June 30, | ||||||||||
| Share-based payments reserve | 2023 | 2022 | |||||||||
| Opening balance | |||||||||||
| Tax credited / (debited) to equity | ( | ( | |||||||||
| Transfer to ordinary shares on exercise of options | ( | ||||||||||
| Share-based payment expense for the year | |||||||||||
| Closing Balance | |||||||||||
| Investment revaluation reserve | |||||||||||
| Opening balance | ( | ( | |||||||||
| Changes in the fair value of financial assets through other comprehensive income | ( | ( | |||||||||
| Closing Balance | ( | ( | |||||||||
| Foreign currency translation reserve | |||||||||||
| Opening balance | ( | ( | |||||||||
Currency gain/(loss) on translation of foreign operations net assets | ( | ||||||||||
| Closing Balance | ( | ( | |||||||||
| Warrant reserve | |||||||||||
| Opening balance | |||||||||||
| Movements during the period | |||||||||||
| Closing Balance | |||||||||||
(iii) Nature and purpose of reserves
Share-based payment reserve
The share-based payments reserve is used to recognize:
•the fair value(1) of options issued but not exercised; and
•the fair value(1) of deferred shares granted but not yet vested.
(1)The fair value recognized is determined at the acceptance date, which is the date at which the entity and the employee agree to a share-based payment arrangement, being when the entity and the employee have a shared understanding of the terms and conditions of the arrangement or when they are approved by shareholders when this is required.
Foreign currency translation reserve
Exchange differences arising on translation of a foreign controlled entity are recognized in other comprehensive income and accumulated in a separate reserve within equity. The cumulative amount is reclassified to profit or loss when the net investment is disposed of.
Warrants reserve
In March 2021, the Group completed a A$138.0 million (US$110.0 million) private placement of 60,109,290 new fully-paid ordinary shares at a price of A$2.30 . As part of this placement, the Group also issued one warrant for every four ordinary shares issued in the placement, which resulted in a further 15,027,327 warrants issued. Each warrant has an exercise price of A$2.88 per share and a 7 year term. The Group has a right to compel exercise of the warrants at any time, subject to the price of the Group’s ordinary shares trading at least A$4.32 for 45 consecutive days on the ASX. The warrants do not confer any rights to dividends or a right to participate in a new issue without exercising the warrant.
198
The terms of the warrants include certain anti-dilution clauses, which adjust the exercise price or conversion ratio in the event of a rights issue or bonus issue. Management analyzed these clauses and determined the fixed-for-fixed requirement was still satisfied because the relative rights of shareholders and warrant holders were maintained. Therefore the warrants were classified as equity. The warrants were initially measured in equity at fair value, which was determined using a Monte Carlo simulation (refer to Note 7(b)(iv)), with the residual consideration being attributed to the ordinary shares issued in the same transaction. The warrants are not remeasured for subsequent changes in fair value.
(iv) Fair value of warrants
The warrants granted are not traded in an active market and therefore the fair value has been estimated by using the Monte Carlo pricing model based on the following assumptions. Key terms of the warrants are included above. The following assumptions were based on observable market conditions that existed at the issue date.
| (in U.S. dollars, except percent data and as otherwise noted) Assumption | At Issue date - March 18, 2021 | Rationale | ||||||||||||
| Share Price | A$ | Closing share price on valuation date from external market source | ||||||||||||
| Exercise Price | A$ | As per subscription agreement | ||||||||||||
| Expected Term | As per subscription agreement | |||||||||||||
| Dividend Yield | Based on Company’s nil dividend history | |||||||||||||
| Expected Volatility | Based on historical volatility data for the Company | |||||||||||||
| A$-US$ FX Spot Rate | Closing FX rate on valuation date from the Reserve Bank of Australia historical foreign exchange rate tables | |||||||||||||
| Risk Free Interest Rate | Based on the mid-point of the Australian Government issued | |||||||||||||
| Fair value per warrant | $ | Determined using Monte Carlo pricing models with the inputs above | ||||||||||||
| Fair value | $ | Fair value of | ||||||||||||
199
8. Cash flow information
| (in U.S. dollars, in thousands) | As of June 30, | ||||||||||||||||
| (a) Reconciliation of cash and cash equivalents | 2023 | 2022 | 2021 | ||||||||||||||
| Cash at bank | |||||||||||||||||
| Deposits at call | |||||||||||||||||
| (in U.S. dollars, in thousands) | As of June 30, | ||||||||||||||||
| (b) Reconciliation of net cash flows used in operations with loss after income tax | 2023 | 2022 | 2021 | ||||||||||||||
| Loss for the period | ( | ( | ( | ||||||||||||||
| Add/(deduct) net loss for non-cash items as follows: | |||||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Foreign exchange losses/(gains) | ( | ||||||||||||||||
| Finance costs | |||||||||||||||||
| Remeasurement of contingent consideration | ( | ( | ( | ||||||||||||||
| Remeasurement of warrant liabilities | ( | ||||||||||||||||
| Equity settled share-based payment | |||||||||||||||||
| Deferred tax benefit | ( | ( | ( | ||||||||||||||
| Gain on derecognition of right-of-use assets | ( | ||||||||||||||||
| Change in operating assets and liabilities: | |||||||||||||||||
| Decrease/(increase) in trade and other receivables | ( | ( | |||||||||||||||
| Decrease/(increase) in prepayments | ( | ||||||||||||||||
| Increase/(decrease) in trade creditors and accruals | ( | ( | |||||||||||||||
| Decrease/(increase) in tax incentive recoverable | ( | ||||||||||||||||
| Increase/(decrease) in provisions | ( | ( | ( | ||||||||||||||
| Net cash outflows used in operations | ( | ( | ( | ||||||||||||||
9. Significant estimates, judgments and errors
The preparation of financial statements requires the use of accounting estimates which, by definition, will seldom equal the actual results. Management also needs to exercise judgment in applying the Group’s accounting policies.
This note provides an overview of the areas that involved a higher degree of judgment or complexity, and of items which are more likely to be materially adjusted due to estimates and assumptions turning out to be wrong. Detailed information about each of these estimates and judgments is included in Notes 1 to 8 together with information about the basis of calculation for each affected line item in the financial statements. In addition, this note also explains where there have been actual adjustments this year as a result of an error and of changes to previous estimates.
Significant estimates and judgments
The areas involving significant estimates or judgments are:
•recognition of revenue (Note 3 and Note 23(e));
•fair value of contingent liabilities and contingent purchase consideration in a business combination (Note 5(g) and 13);
•recoverable amount of goodwill and other intangible assets including in-process research and development (Note 6(c));
•useful life of intangible assets (Note 6(c));
•recognition of deferred tax assets and deferred tax liabilities (Note 4);
•fair value of share-based payments (Note 17);
200
•remeasurement of borrowings due to change in estimated cash flows (Note 5(f));
•recognition of pre-launch inventory costs (Note 23(f)); and
•fair value of warrant liability (Note 5(g)).
10. Financial risk management
This note explains the Group’s exposure to financial risks and how these risks could affect the Group’s future financial performance. Current year profit and loss information has been included where relevant to add further context.
| Risk | Exposure arising from | Measurement | Management | |||||||||||||||||
| Market risk – currency risk | Future commercial transactions Recognized financial assets and liabilities not denominated in the functional currency of each entity within the Group | Cash flow forecasting Sensitivity analysis | The future cash flows of each currency are forecast and the quantum of cash reserves held for each currency are managed in line with future forecasted requirements. Cross currency swaps are undertaken as required. | |||||||||||||||||
| Market risk – interest rate risk | Term deposits at fixed rates | Sensitivity analysis | Vary length of term deposits, utilize interest bearing accounts and periodically review interest rates available to ensure we earn interest at market rates. | |||||||||||||||||
| Market risk – price risk | Long-term borrowings | Sensitivity analysis | Forecasts of net sales of the product underlying the NovaQuest borrowing arrangement are updated on a quarterly basis to evaluate the impact on the carrying amount of the financial liability. | |||||||||||||||||
| Credit risk | Cash and cash equivalents, trade and other receivables and other non-current assets | Aging analysis Credit ratings | Transact primarily with the best risk rated banks available in each region giving consideration to the products required, the quantum of cash reserves held and future forecasted requirements | |||||||||||||||||
| Liquidity risk | Cash and cash equivalents, borrowings, trade payables, lease liabilities and contingent consideration | Rolling cash flow forecasts | Future cash flows requirements are forecasted and capital raising strategies are planned to ensure sufficient cash balances are maintained to meet the Group’s future commitments. | |||||||||||||||||
201
a. Market risk
(i) Currency risk
The Group has foreign currency amounts owing relating to clinical, regulatory and overhead activities and foreign currency deposits held primarily in the Group’s Australian based entity, whose functional currency is the A$. The Group also has foreign currency amounts owing in the Group’s Swiss and Singapore based entities, whose functional currencies are the US$. The Group also has foreign currency amounts owing in various other non-US$ currencies in A$ and US$ functional currency entities in the Group relating to clinical, regulatory and overhead activities. These foreign currency balances give rise to a currency risk, which is the risk of the exchange rate moving, in either direction, and the impact it may have on the Group’s financial performance.
Currency risk is minimized by ensuring the proportion of cash reserves held in each currency matches the expected rate of spend of each currency.
As of June 30, 2023, the Group held 67 % of its cash in US$, and 33 % in A$. As of June 30, 2022 the Group held 97 % of its cash in US$, and 3 % in A$.
The balances held at the end of the year that give rise to currency risk exposure are presented in US$ in the following table, together with a sensitivity analysis which assesses the impact that a change of +/-20% in the exchange rate as of June 30, 2023 and June 30, 2022 would have had on the Group’s reported net profits/(losses) and/or equity balance. The bank balances held at the end of the year that are presented in the following table give rise to currency risk exposure as they are not in the functional currency of the entity in which it is held.
| +20% | -20% | ||||||||||||||||
| (in U.S. dollars, in thousands, unless otherwise noted) As of June 30, 2023 | Foreign currency balance held | Profit/(Loss) US$ | Profit/(Loss) US$ | ||||||||||||||
| Bank accounts – USD | US$ | $ | $ | ( | |||||||||||||
| Bank accounts – CHF | CHF | $ | $ | ( | |||||||||||||
| Bank accounts – SGD | S$ | $ | $ | ( | |||||||||||||
| Bank accounts – EUR | EUR | $ | $ | ( | |||||||||||||
| Trade and other receivables - USD | US$ | $ | $ | ( | |||||||||||||
| Trade and other receivables - SGD | S$ | $ | $ | ( | |||||||||||||
| Trade and other receivables - CHF | CHF | $ | $ | ( | |||||||||||||
| Trade and other receivables - EUR | EUR | $ | $ | ( | |||||||||||||
| Trade payables and accruals - USD | (US$ | $ | ( | $ | |||||||||||||
| Trade payables and accruals - AUD | (A$ | $ | ( | $ | |||||||||||||
| Trade payables and accruals - SGD | (S$ | $ | ( | $ | |||||||||||||
| Trade payables and accruals - GBP | (GBP | $ | ( | $ | |||||||||||||
| Trade payables and accruals - EUR | (EUR | $ | ( | $ | |||||||||||||
| Trade payables and accruals - CHF | (CHF | $ | ( | $ | |||||||||||||
| Provisions – USD | (US$ | $ | ( | $ | |||||||||||||
| $ | ( | $ | |||||||||||||||
202
| +20% | -20% | ||||||||||||||||
| (in U.S. dollars, in thousands, unless otherwise noted) As of June 30, 2022 | Foreign currency balance held | Profit/(Loss) US$ | Profit/(Loss) US$ | ||||||||||||||
| Bank accounts – USD | US$ | $ | $ | ( | |||||||||||||
| Bank accounts – CHF | CHF | $ | $ | ( | |||||||||||||
| Bank accounts – SGD | S$ | $ | $ | ( | |||||||||||||
| Bank accounts – EUR | EUR | $ | $ | ( | |||||||||||||
| Trade and other receivables - SGD | S$ | $ | $ | ( | |||||||||||||
| Trade and other receivables - CHF | CHF | $ | $ | ( | |||||||||||||
| Trade and other receivables - EUR | EUR | $ | $ | ( | |||||||||||||
| Trade payables and accruals - USD | (US$ | $ | ( | $ | |||||||||||||
| Trade payables and accruals - AUD | (A$ | $ | ( | $ | |||||||||||||
| Trade payables and accruals - SGD | (S$ | $ | ( | $ | |||||||||||||
| Trade payables and accruals - GBP | (GBP | $ | ( | $ | |||||||||||||
| Trade payables and accruals - EUR | (EUR | $ | ( | $ | |||||||||||||
| Trade payables and accruals - CHF | (CHF | $ | ( | $ | |||||||||||||
| Provisions – USD | (US$ | $ | ( | $ | |||||||||||||
| Provisions – SGD | (S$ | $ | ( | $ | |||||||||||||
| $ | ( | $ | |||||||||||||||
(1)We have corrected for immaterial errors in the year ended June 30, 2022 within the financial risk management disclosure above. We do not believe this correction is material to the consolidated financial statements in any period presented.
(ii) Cash flow and interest rate risk
The Group is exposed to interest rate movements which impacts interest income earned on its deposits and at call accounts. The interest rate risk is managed by spreading the maturity date of our deposits across various periods. The Group ensures that sufficient funds are available, in at call accounts, to meet the working capital requirements of the Group.
The deposits held which derive interest revenue are described in the table below, together with the maximum and minimum interest rates being earned as of June 30, 2023 and June 30, 2022. The effect on profit is shown if interest rates change by 10
| As of Jun 30, 2023 | As of June 30, 2022 | ||||||||||||||||||||||||||||||||||
| (in U.S. dollars, in thousands, except percent data) | Low | High | US$ | Low(1) | High(1) | US$ | |||||||||||||||||||||||||||||
| Funds invested – US$ | % | % | % | % | |||||||||||||||||||||||||||||||
Rate increase by | % | % | % | % | |||||||||||||||||||||||||||||||
Rate decrease by | % | % | ( | % | % | ( | |||||||||||||||||||||||||||||
| (in Australian dollars, in thousands, except percent data) | Low | High | A$ | Low | High | A$ | |||||||||||||||||||||||||||||
| Funds invested – A$ | % | % | % | % | |||||||||||||||||||||||||||||||
Rate increase by | % | % | % | % | |||||||||||||||||||||||||||||||
Rate decrease by | % | % | ( | % | % | ( | |||||||||||||||||||||||||||||
| (1) | The interest rate was | ||||
203
(iii) Price risk
Price risk is the risk that future cash flows derived from financial instruments will be altered as a result of a market price movement, which is defined as movements other than foreign currency rates and interest rates. The Group is exposed to price risk which arises from long-term borrowings under its facility with NovaQuest, where the timing and amounts of principal and interest payments is dependent on net sales of remestemcel-L for the treatment of SR-aGVHD in pediatric patients in the United States and other territories excluding Asia. As net sales of remestemcel-L for the treatment of SR-aGVHD in pediatric patients in these territories increase/decrease, the timing and amount of principal and interest payments relating to the financing arrangement will also fluctuate, resulting in an adjustment to the carrying amount of financial liability. The adjustment is recognized in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in the period the revision is made.
The exposure of the Group’s borrowing to price rate changes are as follows:
| As of Jun 30, 2023 | As of June 30, 2022 | ||||||||||||||||||||||
| (in U.S. dollars, in thousands, except percent data) | Total | % of total borrowings | Total | % of total borrowings | |||||||||||||||||||
| Financial liabilities | |||||||||||||||||||||||
| Current borrowings | |||||||||||||||||||||||
| Borrowings – NovaQuest | % | % | |||||||||||||||||||||
| Non-current borrowings | |||||||||||||||||||||||
| Borrowings – NovaQuest | % | % | |||||||||||||||||||||
| % | % | ||||||||||||||||||||||
As at June 30, 2023, all other factors held constant, a +/-20 % change in the forecast net sales of remestemcel-L for the treatment of SR-aGVHD in pediatric patients in the United States and other territories excluding Asia would have a minimal impact on non-current borrowings and profit.
The Group is also exposed to price risk on contingent consideration provision balances, as expected unit revenues are a significant unobservable input used in the level 3 fair value measurements. As at June 30, 2023, all other factors held constant, the increase/decrease in price assumptions adopted in the fair value measurements of the contingent consideration provision are discussed in Note 5(g)(iv).
The Group does not consider it has any exposure to price risk other than those already described above.
204
b. Credit risk
Credit risk is the risk that one party to a financial instrument will fail to discharge its obligation and cause financial loss to the other party. The maximum exposure to credit risk at the end of the reporting period is the carrying amount of each class of financial assets. The Group’s receivables are tabled below.
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Cash and cash equivalents | |||||||||||
| Deposits at call (Note 5(a)) - minimum A rated | |||||||||||
| Cash at bank (Note 5(a)) - minimum A rated | |||||||||||
| Trade and other receivables | |||||||||||
| Receivable from other parties (non-rated) | |||||||||||
| Receivable from the Australian Government (Income Tax) | |||||||||||
| Receivable from the United States Government (U.S. tax credits) | |||||||||||
| Receivable from the Australian Government (Foreign Withholding Tax) | |||||||||||
| Receivable from the Australian Government (Goods and Services Tax) | |||||||||||
| Receivable from the Singapore Government (Goods and Services Tax) | |||||||||||
| Receivable from the United States Government (Foreign Withholding Tax) | |||||||||||
| Receivable from minimum A rated bank deposits (interest) | |||||||||||
| Receivable from the Swiss Government (Value-Added Tax) | |||||||||||
| Receivable from the United States Government (Income Tax) | |||||||||||
| Other non-current assets | |||||||||||
| Minimum A rated bank deposits (held as security) | |||||||||||
c. Liquidity risk
Liquidity risk is the risk that the Group will not be able to pay its debts as and when they fall due. Liquidity risk has been assessed in Note 1(i).
All financial liabilities, excluding contingent consideration, borrowings and lease liabilities held by the Group as of June 30, 2023 and June 30, 2022 mature within 6
As of June 30, 2023, the maturity profile of the anticipated future contractual cash flows, on an undiscounted basis and removing probability adjustments as applicable for contingent consideration, and which, therefore differs from the carrying value, is as follows:
| (in U.S. dollars, in thousands) | Within 1 year | Between 1-2 years | Between 2-5 years | Over 5 years | Total contractual cash flows | Carrying amount | |||||||||||||||||||||||||||||
Borrowings(1)(2) | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
| Trade payables | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Lease liabilities | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
Contingent consideration(3) | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
| ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||
(1)Contractual cash flows include payments of principal, interest and other charges. Interest is calculated based on debt held at June 30, 2023 without taking into account drawdowns of further tranches.
205
11. Capital management
12. Interests in other entities
The Group’s subsidiaries as of June 30, 2023 and 2022 are set out below. Unless otherwise stated, they have share capital consisting solely of ordinary shares that are held directly by the Group, and the proportion of ownership interests held equals the voting rights held by the Group. The country of incorporation or registration is also their principal place of business, aside from BeiCell Ltd, which was incorporated on November 15, 2018 in the Cayman Islands however operates in Hong Kong.
| Country of incorporation | Class of shares | Equity holding | |||||||||||||||||||||
| As of June 30, | |||||||||||||||||||||||
| 2023 | 2022 | ||||||||||||||||||||||
| % | % | ||||||||||||||||||||||
| Mesoblast, Inc. | USA | Ordinary | |||||||||||||||||||||
| Mesoblast International Sàrl (includes Mesoblast International Sàrl Singapore Branch) | Switzerland | Ordinary | |||||||||||||||||||||
| Mesoblast Australia Pty Ltd | Australia | Ordinary | |||||||||||||||||||||
| Mesoblast UK Ltd | United Kingdom | Ordinary | |||||||||||||||||||||
| BeiCell Ltd | Cayman Islands | Ordinary | |||||||||||||||||||||
13. Contingent assets and liabilities
a. Contingent assets
The Group did not
b. Contingent liabilities
(i) Central Adelaide Local Health Network Incorporated (“CALHNI”) (formerly Medvet)
The Group acquired certain intellectual property relating to our MPCs, or Medvet IP, pursuant to an Intellectual Property Assignment Deed, or IP Deed, with Medvet Science Pty Ltd, or Medvet. Medvet’s rights under the IP Deed were transferred to Central Adelaide Local Health Network Incorporated, or CALHNI, in November 2011. In connection with its use of the Medvet IP, on completion of certain milestones the Group will be obligated to pay CALHNI, as successor in interest to Medvet, (i) certain aggregated milestone payments of up to $2.2 million and single-digit royalties on net sales of products covered by the Medvet IP, for cardiac muscle and blood vessel applications and bone and cartilage regeneration and repair applications, subject to minimum annual royalties beginning in the first year of commercial sale of those products and (ii) single-digit royalties on net sales of the specified products for applications outside the specified fields.
206
(ii) Other contingent liabilities
The Group has entered into a number of other agreements with other third parties pertaining to intellectual property. Contingent liabilities may arise in the future if certain events or developments occur in relation to these agreements. As of June 30, 2023, the Group has assessed these contingent liabilities to be remote and specific disclosure is not required.
14. Commitments
a. Capital commitments
The Group did not
b. Purchase commitments
In December 2019, the Group commenced production under its manufacturing service agreement with Lonza for the supply of commercial product for the potential approval and launch of remestemcel-L for the treatment of pediatric SR-aGVHD in the US market. This agreement contains lease and non-lease components. As of June 30, 2023, the agreement contains a minimum remaining financial commitment of the non-lease component of $16.8 million, payable until December 2024, which is cancellable in limited circumstances. The Group has accounted for the lease component within the agreement as a lease liability separately from the non-lease components. As of June 30, 2023, the lease component is $3.0 million on an undiscounted basis, as disclosed within the total contractual cash flows as lease liabilities in Note 10(c). At the Group's discretion, the minimum financial commitment under this manufacturing services agreement can be reduced by $12.2 million under certain conditions, with $1.1 million of this reduction relating to the lease component and $11.1 million relating to the non-lease component of the agreement.
The group have agreements with third parties related to contract manufacturing and other goods and services. As of June 30, 2023, the Group had $9.1 million of non-cancellable purchase commitments related to raw materials, manufacturing agreements and other goods and services. This amount represents our minimum contractual obligations, including termination fees. Certain agreements provide for termination rights subject to termination fees. Under such agreement, the Group are contractually obligated to make certain payments, mainly, to reimburse them for their unrecoverable outlays incurred prior to cancellation.
15. Events occurring after the reporting period
In August 2023, the FDA provided a complete response to the Group's BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD and requires more data to support marketing approval, including potency assay or clinical data. In line with the Group's overall commercial strategy to progress to adult populations, the Group intends to conduct a targeted, controlled study in the highest-risk adults with the greatest mortality. Assumptions associated with SR-aGVHD are included within the impairment assessment of Osiris MSC products within in-process research and development and goodwill, contingent consideration, pre-launch inventory and the NovaQuest borrowings on the consolidated balance sheet and forecast net operating cash usage. The Group has assessed and included the impact of the FDA's complete response to the Group's BLA resubmission for remestemcel-L for the treatment of pediatric SR-aGVHD in these areas. Future discussions with the FDA could lead to a change in the assumptions associated with SR-aGVHD within the impairment assessment of Osiris MSC products within in-process research and development and goodwill, contingent consideration, pre-launch inventory and the NovaQuest borrowings on the consolidated balance sheet and forecast net operating cash usage.
There were no other events that have occurred after June 30, 2023 and prior to the signing of this financial report that would likely have a material impact on the financial results presented.
16. Related party transactions
a. Parent entity
The parent entity within the Group is Mesoblast Limited.
207
b. Subsidiaries
Details of interests in subsidiaries are disclosed in Note 12 to the financial statements.
c. Key management personnel compensation
The aggregate compensation made to Directors and other members of key management personnel of the Group is set out below:
| Year Ended June 30, | |||||||||||
| (in U.S. dollars) | 2023 | 2022 | |||||||||
| Short-term employee benefits | |||||||||||
| Long-term employee benefits | |||||||||||
| Post-employment benefits | |||||||||||
| Share based payments | |||||||||||
The aggregate other service payments made to Directors and other members of key management personnel of the Group is set out below:
Philip Krause was appointed to a formal strategic advisory role on June 4, 2023. The consulting agreement is in addition to Philip Krause's existing role as non executive director, the terms of which remain unchanged. He will provide specialist regulatory advisory services and will be remunerated at an hourly rate. The agreement is ongoing with either party able to terminate on 15 written days notice. The total aggregate fees paid to Philip Krause as of June 30, 2023 was $110,383 .
There were no loans or other related transactions with KMP during the financial year.
d. Transactions with other related parties
Accounts receivable from revenues, accounts payable to expenses and loans from subsidiaries as at the end of the fiscal year have been eliminated on consolidation of the Group.
e. Terms and conditions
All other transactions were made on normal commercial terms and conditions and at market rates, except that there are no fixed terms for the repayment of loans between the parties.
Outstanding balances are unsecured and are repayable in cash.
17. Share-based payments
The Company has adopted an Employee Share Option Plan (“ESOP”) to foster an ownership culture within the Company and to motivate senior management and consultants to achieve performance targets. Selected directors, employees and consultants may be eligible to participate in ESOP at the absolute discretion of the board of directors, and in the case of directors, upon approval by shareholders.
Grant policy
In accordance with the Company’s policy, options are typically issued in three equal tranches. The length of time from grant date to expiry date is typically 7 years.
Options issued to employees generally vest based on performance or time conditions, or both. In the year ended June 30, 2023, senior executives were issued options that vest based on performance and time conditions. These options are required to satisfy certain pre-specified performance conditions and time-based vesting conditions prior to vesting. Time-based conditions restrict vesting to a maximum of one third at 12 months, two thirds at 24 months and full grant at 36 months, but only if the pre-specified performance conditions have been met. For time-based vesting options, the first
208
tranche typically vests 12 months after grant date, the second tranche 24 months after grant date, and the third tranche 36 months after grant date.
The exercise price is determined by reference to the Company policy. Generally the exercise price is the higher of the volume weighted average share price of the five ASX trading days up to Board approval of the grant, and the last closing price of an ordinary share on the ASX at Board approval. In the case of options that have time-based vesting conditions only, the board of directors adds a 10 % premium to the market price. Options with performance based vesting conditions are issued with no premium. The board of directors’ policy is not to issue options at a discount to the market price.
The aggregate number of options which may be issued pursuant to the ESOP must not exceed 10,000,000 with respect to US incentive stock options, and with respect to Australian residents, the limit imposed under the Australian Securities and Investments Commission Class Order 14/1000.
a. Reconciliation of outstanding share based payments
| Series | Grant Date(1) | Expiry Date | Exercise Price | Opening Balance | Granted No. (during the year) | Exercised No. (during the year) | Lapsed/Forfeited* No. (during the year) | Closing Balance | Vested and exercisable No (end of year) | |||||||||||||||||||||||
| 34 | 27-Apr-16 | 06-Mar-23 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 34b | 31-Oct-16 | 06-Mar-23 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 35a | 08-Jul-20 | 08-Jul-23 | A$ | — | — | — | ||||||||||||||||||||||||||
| 36 | 06-Dec-16 | 05-Dec-23 | A$ | — | — | — | ||||||||||||||||||||||||||
| 36a | 06-Dec-16 | 05-Dec-23 | A$ | — | — | — | ||||||||||||||||||||||||||
| 38 | 16-Sep-17 | 15-Sep-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 38a | 16-Sep-17 | 15-Sep-24 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 39 | 13-Oct-17 | 12-Oct-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 39a | 13-Oct-17 | 12-Oct-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 40 | 24-Nov-17 | 23-Nov-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 40a | 24-Nov-17 | 23-Nov-24 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 41 | 18-Jun-18 | 17-Jun-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 42 | 11-Jul-18 | 10-Jul-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 43 | 18-Jul-18 | 17-Jul-25 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 43b | 18-Jul-18 | 17-Jul-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 45 | 30-Nov-18 | 29-Nov-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 46 | 19-Jan-19 | 18-Jan-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 47 | 19-Jan-19 | 18-Jan-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 48 | 04-Apr-19 | 03-Apr-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 49 | 20-Jul-19 | 19-Jul-26 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 49 | 20-Jul-19 | 19-Jul-26 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 49a | 20-Jul-19 | 19-Jul-26 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 49a | 20-Jul-19 | 19-Jul-26 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 49b | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 49c | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 50 | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 50a | 20-Jul-19 | 19-Jul-26 | A$ | — | — | ( | * | — | — | |||||||||||||||||||||||
| 52 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 53 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 54 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 55 | 29-May-19 | 28-May-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 56 | 18-Nov-19 | 17-Nov-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 57 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 58 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 58 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 59 | 24-Jan-20 | 23-Jan-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 63 | 18-May-20 | 17-May-27 | A$ | — | — | — | ||||||||||||||||||||||||||
209
| 63a | 18-May-20 | 17-May-27 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 63a | 18-May-20 | 17-May-27 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 64 | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 64 | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 64a | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 64c | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 64d | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 64e | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 65 | 26-Aug-20 | 25-Aug-27 | A$ | — | — | ( | — | |||||||||||||||||||||||||
| 65 | 26-Aug-20 | 25-Aug-27 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 66 | 11-Sep-20 | 10-Sep-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 68 | 20-Nov-20 | 19-Nov-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 69 | 20-Nov-20 | 19-Nov-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 71 | 17-Feb-21 | 16-Feb-28 | A$ | — | — | — | ||||||||||||||||||||||||||
| 72 | 15-Apr-21 | 14-Apr-28 | A$ | — | — | — | ||||||||||||||||||||||||||
| 74 | 08-Sep-21 | 07-Sep-28 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 74 | 08-Sep-21 | 07-Sep-28 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 74a | 08-Sep-21 | 07-Sep-28 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 74b | 08-Sep-21 | 07-Sep-28 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 74c | 08-Sep-21 | 07-Sep-28 | A$ | — | — | ( | * | — | — | |||||||||||||||||||||||
| 75 | 23-Dec-21 | 22-Dec-28 | A$ | — | — | — | ||||||||||||||||||||||||||
| 76 | 17-Oct-22 | 16-Oct-29 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 77 | 23-May-22 | 22-May-29 | A$ | — | — | — | ||||||||||||||||||||||||||
| 78 | 24-Aug-22 | 23-Aug-29 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 79 | 17-Oct-22 | 16-Oct-29 | A$ | — | — | ( | * | — | ||||||||||||||||||||||||
| 79a | 17-Oct-22 | 16-Oct-29 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 79b | 17-Oct-22 | 16-Oct-29 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 79c | 17-Oct-22 | 16-Oct-29 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 79d | 17-Oct-22 | 16-Oct-29 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 80 | 08-Aug-22 | 07-Aug-29 | A$ | — | — | — | ||||||||||||||||||||||||||
| 81 | 11-Dec-20 | 10-Dec-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 82 | 21-Nov-22 | 20-Nov-29 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 83 | 30-Mar-23 | 29-Mar-30 | A$ | — | — | ( | * | — | ||||||||||||||||||||||||
| June 30, 2023 | ( | |||||||||||||||||||||||||||||||
| Weighted average share purchase price | A$ | A$ | A$ | A$ | A$ | A$ | ||||||||||||||||||||||||||
(1)The dates presented in the grant date column represent the date on which board approval was obtained. For valuation dates per IFRS 2, refer to Note 17(c).
210
| Series | Grant Date(1) | Expiry Date | Exercise Price | Opening Balance | Granted No. (during the year) | Exercised No. (during the year) | Lapsed/Forfeited* No. (during the year) | Closing Balance | Vested and exercisable No (end of year) | |||||||||||||||||||||||
| 32 | 10-Jul-15 | 30-Jun-22 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 33 | 26-Aug-15 | 16-Aug-22 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 34 | 27-Apr-16 | 06-Mar-23 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 34b | 31-Oct-16 | 06-Mar-23 | A$ | — | — | — | ||||||||||||||||||||||||||
| 35a | 08-Jul-20 | 08-Jul-23 | A$ | — | — | — | ||||||||||||||||||||||||||
| 36 | 06-Dec-16 | 05-Dec-23 | A$ | — | ( | ( | ||||||||||||||||||||||||||
| 36a | 06-Dec-16 | 05-Dec-23 | A$ | — | — | — | ||||||||||||||||||||||||||
| 38 | 16-Sep-17 | 15-Sep-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 38a | 16-Sep-17 | 15-Sep-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 39 | 13-Oct-17 | 12-Oct-24 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 39a | 13-Oct-17 | 12-Oct-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 40 | 24-Nov-17 | 23-Nov-24 | A$ | — | — | — | ||||||||||||||||||||||||||
| 40a | 24-Nov-17 | 23-Nov-24 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 41 | 18-Jun-18 | 17-Jun-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 42 | 11-Jul-18 | 10-Jul-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 43 | 18-Jul-18 | 17-Jul-25 | A$ | — | ( | ( | ||||||||||||||||||||||||||
| 43b | 18-Jul-18 | 17-Jul-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 44 | 15-Jul-18 | 14-Jul-25 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 45 | 30-Nov-18 | 29-Nov-25 | A$ | — | — | — | ||||||||||||||||||||||||||
| 46 | 19-Jan-19 | 18-Jan-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 47 | 19-Jan-19 | 18-Jan-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 48 | 04-Apr-19 | 03-Apr-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 49 | 20-Jul-19 | 19-Jul-26 | A$ | — | ( | ( | ||||||||||||||||||||||||||
| 49 | 20-Jul-19 | 19-Jul-26 | A$ | — | ( | * | ||||||||||||||||||||||||||
| 49a | 20-Jul-19 | 19-Jul-26 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 49a | 20-Jul-19 | 19-Jul-26 | A$ | — | ( | * | ||||||||||||||||||||||||||
| 49b | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 49c | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 50 | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 50a | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 51 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | ( | * | — | — | |||||||||||||||||||||||
| 52 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 53 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 54 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 54 | 25-Nov-19 | 24-Nov-26 | A$ | — | ( | * | ||||||||||||||||||||||||||
| 55 | 29-May-19 | 28-May-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 56 | 18-Nov-19 | 17-Nov-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 57 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 58 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | — | ||||||||||||||||||||||||||
| 59 | 24-Jan-20 | 23-Jan-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 61 | 17-Apr-20 | 16-Apr-27 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 61 | 17-Apr-20 | 16-Apr-27 | A$ | — | ( | * | ||||||||||||||||||||||||||
| 63 | 18-May-20 | 17-May-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 63a | 18-May-20 | 17-May-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 64 | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | ||||||||||||||||||||||||||
| 64 | 16-Jul-20 | 15-Jul-27 | A$ | ( | * | |||||||||||||||||||||||||||
| 64a | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | * | |||||||||||||||||||||||||
| 64b | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | * | — | — | |||||||||||||||||||||||
| 64c | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 64d | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | — | |||||||||||||||||||||||||
211
| 64e | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 65 | 26-Aug-20 | 25-Aug-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 66 | 11-Sep-20 | 10-Sep-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 67 | 08-Oct-20 | 07-Oct-27 | A$ | — | — | ( | — | — | ||||||||||||||||||||||||
| 67 | 08-Oct-20 | 07-Oct-27 | A$ | — | ( | * | ||||||||||||||||||||||||||
| 68 | 20-Nov-20 | 19-Nov-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 69 | 20-Nov-20 | 19-Nov-27 | A$ | — | — | — | ||||||||||||||||||||||||||
| 71 | 17-Feb-21 | 16-Feb-28 | A$ | — | — | — | ||||||||||||||||||||||||||
| 72 | 15-Apr-21 | 14-Apr-28 | A$ | — | — | — | ||||||||||||||||||||||||||
| 73 | 30-Jun-21 | 30-Aug-21 | A$ | — | ( | — | — | — | ||||||||||||||||||||||||
| 74 | 08-Sep-21 | 07-Sep-28 | A$ | — | — | ( | * | — | ||||||||||||||||||||||||
| 74a | 08-Sep-21 | 07-Sep-28 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 74b | 08-Sep-21 | 07-Sep-28 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 74c | 08-Sep-21 | 07-Sep-28 | A$ | — | — | — | — | |||||||||||||||||||||||||
| 75 | 23-Dec-21 | 22-Dec-28 | A$ | — | — | — | — | |||||||||||||||||||||||||
| June 30, 2022 | ( | ( | ||||||||||||||||||||||||||||||
| Weighted average share purchase price | A$ | A$ | A$ | A$ | A$ | A$ | ||||||||||||||||||||||||||
(1)The dates presented in the grant date column represent the date on which board approval was obtained. For valuation dates per IFRS 2, refer to Note 17(c).
| Series | Grant Date(1) | Expiry Date | Exercise Price | Opening Balance | Granted No. (during the year) | Exercised No. (during the year) | Lapsed/Forfeited* No. (during the year) | Closing Balance | Vested and exercisable No (end of year) | ||||||||||||||||||||||||||
| 32 | 10-Jul-15 | 30-Jun-22 | US$ | — | ( | — | |||||||||||||||||||||||||||||
| 33 | 26-Aug-15 | 16-Aug-22 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 34 | 27-Apr-16 | 6-Mar-23 | A$ | — | ( | ( | |||||||||||||||||||||||||||||
| 34a | 27-Apr-16 | 17-Apr-23 | A$ | — | ( | ( | — | — | |||||||||||||||||||||||||||
| 34b | 31-Oct-16 | 6-Mar-23 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 35 | 30-Jun-16 | 30-Jun-22 | (2) | A$ | — | ( | — | — | — | ||||||||||||||||||||||||||
| 35a | 8-Jul-20 | 8-Jul-23 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 36 | 6-Dec-16 | 5-Dec-23 | A$ | — | ( | — | |||||||||||||||||||||||||||||
| 36a | 6-Dec-16 | 5-Dec-23 | A$ | — | ( | ( | * | ||||||||||||||||||||||||||||
| 36b | 13-Jan-17 | 12-Jan-24 | A$ | — | ( | — | — | — | |||||||||||||||||||||||||||
| 37 | 28-Jun-17 | 27-Jun-24 | A$ | — | ( | — | — | — | |||||||||||||||||||||||||||
| 38 | 16-Sep-17 | 15-Sep-24 | A$ | — | ( | — | |||||||||||||||||||||||||||||
| 38a | 16-Sep-17 | 15-Sep-24 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 39 | 13-Oct-17 | 12-Oct-24 | A$ | — | ( | — | |||||||||||||||||||||||||||||
| 39a | 13-Oct-17 | 12-Oct-24 | A$ | — | ( | — | |||||||||||||||||||||||||||||
| 40 | 24-Nov-17 | 23-Nov-24 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 40a | 24-Nov-17 | 23-Nov-24 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 41 | 18-Jun-18 | 17-Jun-25 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 42 | 11-Jul-18 | 10-Jul-25 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 43 | 18-Jul-18 | 17-Jul-25 | A$ | — | ( | ( | * | ||||||||||||||||||||||||||||
| 43b | 18-Jul-18 | 17-Jul-25 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 44 | 15-Jul-18 | 14-Jul-25 | A$ | — | ( | — | |||||||||||||||||||||||||||||
| 45 | 30-Nov-18 | 29-Nov-25 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 46 | 19-Jan-19 | 18-Jan-26 | A$ | — | ( | — | |||||||||||||||||||||||||||||
| 47 | 19-Jan-19 | 18-Jan-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 48 | 4-Apr-19 | 3-Apr-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 49 | 20-Jul-19 | 19-Jul-26 | A$ | — | ( | ( | |||||||||||||||||||||||||||||
| 49 | 20-Jul-19 | 19-Jul-26 | A$ | — | ( | * | |||||||||||||||||||||||||||||
| 49a | 20-Jul-19 | 19-Jul-26 | A$ | — | ( | ( | * | ||||||||||||||||||||||||||||
| 49b | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
212
| 49c | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 50 | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 50a | 20-Jul-19 | 19-Jul-26 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 51 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 52 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 53 | 29-Aug-19 | 28-Aug-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 54 | 25-Nov-19 | 24-Nov-26 | A$ | — | ( | ( | |||||||||||||||||||||||||||||
| 54 | 25-Nov-19 | 24-Nov-26 | A$ | — | ( | * | |||||||||||||||||||||||||||||
| 55 | 29-May-19 | 28-May-26 | A$ | — | ( | — | |||||||||||||||||||||||||||||
| 56 | 18-Nov-19 | 17-Nov-26 | A$ | — | — | ||||||||||||||||||||||||||||||
| 57 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 58 | 25-Nov-19 | 24-Nov-26 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 59 | 24-Jan-20 | 23-Jan-27 | A$ | — | — | ( | * | ||||||||||||||||||||||||||||
| 60 | 17-Apr-20 | 16-Apr-27 | A$ | — | — | ( | * | — | — | ||||||||||||||||||||||||||
| 61 | 17-Apr-20 | 16-Apr-27 | A$ | — | — | ( | * | ||||||||||||||||||||||||||||
| 63 | 18-May-20 | 17-May-27 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 63a | 18-May-20 | 17-May-27 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 64 | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | * | — | |||||||||||||||||||||||||||
| 64a | 16-Jul-20 | 15-Jul-27 | A$ | — | — | ( | * | — | |||||||||||||||||||||||||||
| 64b | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 64c | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 64 | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 64e | 16-Jul-20 | 15-Jul-27 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 65 | 26-Aug-20 | 25-Aug-27 | A$ | — | — | ( | * | — | |||||||||||||||||||||||||||
| 66 | 11-Sep-20 | 10-Sep-27 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 67 | 8-Oct-20 | 7-Oct-27 | A$ | — | — | ( | * | — | |||||||||||||||||||||||||||
| 68 | 20-Nov-20 | 19-Nov-27 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 69 | 20-Nov-20 | 19-Nov-27 | A$ | — | — | — | |||||||||||||||||||||||||||||
| 71 | 17-Feb-21 | 16-Feb-28 | A$ | — | — | — | — | ||||||||||||||||||||||||||||
| 73 | 30-Jun-21 | 30-Aug-21 | A$ | — | — | — | |||||||||||||||||||||||||||||
| June 30, 2021 | ( | ( | |||||||||||||||||||||||||||||||||
| Weighted average share purchase price | A$ | A$ | A$ | A$ | A$ | A$ | |||||||||||||||||||||||||||||
(1)The dates presented in the grant date column represent the date on which board approval was obtained. For valuation dates per IFRS 2, refer to Note 17(c).
The weighted average share price at the date of exercise of options exercised during the years ended June 30, 2023, 2022 and 2021 were Nil , A$1.82 and A$4.42 respectively. The weighted average remaining contractual life of share options outstanding as of June 30, 2023, 2022 and 2021 were 4.13 years, 4.16 years and 4.49 years, respectively.
b. Existing share-based payment arrangements
General terms and conditions attached to share based payments
Share options pursuant to the employee share option plan are generally granted in three equal tranches. The length of time from grant date to expiry date is typically seven years . Vesting occurs based on achievement of performance conditions and/or progressively over the life of the option with the first tranche vesting one year from grant date, the second tranche two years from grant date, and the third tranche three years from grant date. On cessation of employment the Company’s board of directors determines if a leaver is a bad leaver or not. If a participant is deemed a bad leaver, all rights, entitlements and interests in any unexercised options held by the participant will be forfeited and will lapse immediately. If a leaver is not a bad leaver they may retain vested options, however, they must be exercised within 60 days of cessation of employment (or within a longer period if so determined by the Company’s board of directors), after which time they will lapse. Unvested options will normally be forfeited and lapse.
213
This policy applies to all issues shown in the above table with the exception of the following:
| 35 | Incentive rights granted pursuant to the Equity Facility Agreement with Kentgrove Capital, dated June 30, 2016, had fully vested on the agreement date and will expire thirty six months after the date of the issue of the incentive right. The terms of this agreement were amended on July 30, 2019. Under the amended terms, these incentive rights will expire thirty six months after the effective date of July 1, 2019. | |||||||
| 35a | Additional incentive rights granted pursuant to the Amendment Deed of the Equity Facility Agreement with Kentgrove Capital, dated July 30, 2019, had fully vested on the agreement date and will expire thirty six months after the date of the issue of the incentive right. | |||||||
| 36a & 36b | Options were granted in | |||||||
| 49a, 49b, 50, 50a, 53, 64b, 64c, 64d, 64e, 71, 74a, 74b, 74c, 79c | Options were granted | |||||||
| 38a, 40a, 57 & 66 | Options were granted in | |||||||
| 39a | Options were granted in | |||||||
| 51 & 75 | Options were granted in | |||||||
| 55 | Options were granted in | |||||||
| 63a | Options were granted in | |||||||
| 64a | Options were granted in | |||||||
| 69, 73, 80 & 81 | Options were granted in one tranche and vested on the date on which board approval was obtained | |||||||
| 79a, 79d | Options were granted in two, three, four or five tranches and will vest on the date that the option holder has direct involvement (to the reasonable satisfaction of the Company's board of directors) in the Company achieving certain confidential commercial objectives. Time-based conditions restrict vesting to a maximum of one third at 12 months, two thirds at 24 months and full grant at 36 months, but only if the pre-specified performance conditions have been met. | |||||||
Modifications to share-based payment arrangements
There were no
214
c. Fair values of share based payments
The weighted average fair value of share options granted during the years ended June 30, 2023, 2022 and 2021 were A$0.66 , A$0.56 and A$1.42 , respectively.
The fair value of all shared-based payments made has been calculated using the Black-Scholes model. This model requires the following inputs:
Share price at acceptance date
The share price used in valuation is the share price at the date at which the entity and the employee agree to a share-based payment arrangement, being when the entity and the employee have a shared understanding of the terms and conditions of the arrangement or at shareholder approval date where this approval is required. This price is generally the volume weighted average share price for the five trading days leading up to the date.
Exercise price
The exercise price is a known value that is contained in the agreements.
Share price volatility
The model requires the Company’s share price volatility to be measured. In estimating the expected volatility of the underlying shares our objective is to approximate the expectations that would be reflected in a current market or negotiated exchange price for the option. Historical volatility data is considered in determining expected future volatility.
Life of the option
The life is generally the time period from grant date through to expiry. Certain assumptions have been made regarding “early exercise” i.e. options exercised ahead of the expiry date. These assumptions have been based on historical trends for option exercises within the Company and take into consideration exercise trends that are also evident as a result of local taxation laws.
Dividend yield
The Company has yet to pay a dividend so it has been assumed the dividend yield on the shares underlying the options will be 0 %.
Risk free interest rate
This has been sourced from the Reserve Bank of Australia historical interest rate tables for government bonds.
215
Model inputs
The model inputs for the valuations of options approved and granted during the year ended June 30, 2023 are as follows:
| Series | Valuation date(1) | Exercise price per share A$ | Share price at valuation date A$ | Expected share price volatility | Life(2) | Dividend yield | Risk-free interest rate | ||||||||||||||||
| 76 | 23-Nov-22 | ||||||||||||||||||||||
| 77 | 23-Nov-22 | ||||||||||||||||||||||
| 78 | 23-Nov-22 | ||||||||||||||||||||||
| 79 | 09-Dec-22 | ||||||||||||||||||||||
| 79a | 21-Jun-23 | ||||||||||||||||||||||
| 79b | 16-Mar-23 | ||||||||||||||||||||||
| 79c | 23-Nov-22 | ||||||||||||||||||||||
79d(3) | 30-Jun-23 | ||||||||||||||||||||||
| 80 | 18-Nov-22 | ||||||||||||||||||||||
| 81 | 18-Nov-22 | ||||||||||||||||||||||
| 82 | 30-Dec-22 | ||||||||||||||||||||||
| 83 | 06-Apr-23 | ||||||||||||||||||||||
(1)Valuation date is the date at which the entity and the employee agree to a share-based payment arrangement, being when the entity and the employee have a shared understanding of the terms and conditions of the arrangement.
(2)Expected life after factoring likely early exercise.
(3)Fair value estimated at June 30, 2023 as the valuation date under AASB2 has not been met as of June 30, 2023.
The closing share market price of an ordinary share of Mesoblast Limited on the ASX as of June 30, 2023 was A$1.14 .
The model inputs for the valuations of options approved and granted during the year ended June 30, 2022 are as follows:
| Series | Valuation date(1) | Exercise price per share A$ | Share price at valuation date A$ | Expected share price volatility | Life(2) | Dividend yield | Risk-free interest rate | ||||||||||||||||
| 72 | 05-May-21 | ||||||||||||||||||||||
| 74 | 10-Nov-21 | ||||||||||||||||||||||
| 74a | 07-Nov-22 | ||||||||||||||||||||||
| 74b | 07-Nov-22 | ||||||||||||||||||||||
| 74c | 15-Feb-22 | ||||||||||||||||||||||
| 75 | 17-Mar-22 | ||||||||||||||||||||||
(1)Valuation date is the date at which the entity and the employee agree to a share-based payment arrangement, being when the entity and the employee have a shared understanding of the terms and conditions of the arrangement.
(2)Expected life after factoring likely early exercise.
The closing share market price of an ordinary share of Mesoblast Limited on the ASX as of June 30, 2022 was A$0.61 .
216
The model inputs for the valuations of options approved and granted during the year ended June 30, 2021 are as follows:
| Series | Valuation date(1) | Exercise price per share A$ | Share price at valuation date A$ | Expected share price volatility | Life(2) | Dividend yield | Risk-free interest rate | ||||||||||||||||
| 61 | 28-Jul-20 | ||||||||||||||||||||||
| 63 | 08-Apr-21 | ||||||||||||||||||||||
| 63a | 05-Jul-21 | ||||||||||||||||||||||
| 64 | 28-Jul-20 | ||||||||||||||||||||||
| 64a | 05-Jul-21 | ||||||||||||||||||||||
| 64b | 30-Jun-21 | ||||||||||||||||||||||
| 64c | 25-Nov-20 | ||||||||||||||||||||||
| 64d | 30-Jun-22 | ||||||||||||||||||||||
| 64e | 05-Jul-21 | ||||||||||||||||||||||
| 65 | 25-Sep-20 | ||||||||||||||||||||||
| 66 | 16-Oct-20 | ||||||||||||||||||||||
| 67 | 10-Nov-20 | ||||||||||||||||||||||
| 68 | 24-Dec-20 | ||||||||||||||||||||||
| 69 | 24-Dec-20 | ||||||||||||||||||||||
| 71 | 29-Mar-21 | ||||||||||||||||||||||
| 73 | 30-Jun-21 | ||||||||||||||||||||||
(1)Valuation date is the date at which the entity and the employee agree to a share-based payment arrangement, being when the entity and the employee have a shared understanding of the terms and conditions of the arrangement.
(2)Expected life after factoring likely early exercise.
The closing share market price of an ordinary share of Mesoblast Limited on the ASX as of June 30, 2021 was A$1.98 .
18. Remuneration of auditors
During the year the following fees were paid or payable for services provided by the auditor of the parent entity, its related practices and non-related audit firms:
| Year Ended June 30, | |||||||||||||||||
| (in U.S. dollars) | 2023 | 2022 | 2021 | ||||||||||||||
| a. PricewaterhouseCoopers Australia | |||||||||||||||||
| Audit and other assurance services | |||||||||||||||||
| Audit and review of financial reports | |||||||||||||||||
Other audit services(1) | |||||||||||||||||
| Total remuneration of PricewaterhouseCoopers Australia | |||||||||||||||||
| b. Network firms of PricewaterhouseCoopers Australia | |||||||||||||||||
| Audit and other assurance services | |||||||||||||||||
| Audit and review of financial reports | |||||||||||||||||
Total remuneration of Network firms of PricewaterhouseCoopers Australia | |||||||||||||||||
Total auditors' remuneration(2) | |||||||||||||||||
217
(1)Other audit services relates to services performed in connection with the filing of registration statements on the Form S-8 and F-3.
(2)All services provided are considered audit fees for the purpose of SEC classification.
19. Losses per share
| Years Ended June 30, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| (Losses) per share | |||||||||||||||||
| (in cents) | |||||||||||||||||
| (a) Basic (losses) per share | |||||||||||||||||
From continuing operations attributable to the ordinary equity holders of the company | ( | ( | ( | ||||||||||||||
Total basic (losses) per share attributable to the ordinary equity holders of the company | ( | ( | ( | ||||||||||||||
| (b) Diluted (losses) per share | |||||||||||||||||
From continuing operations attributable to the ordinary equity holders of the company | ( | ( | ( | ||||||||||||||
Total basic (losses) per share attributable to the ordinary equity holders of the company | ( | ( | ( | ||||||||||||||
| (c) Reconciliation of (losses) used in calculating (losses) per share | |||||||||||||||||
| (in U.S. dollars, in thousands) | |||||||||||||||||
| Basic (losses) per share | |||||||||||||||||
(Losses) attributable to the ordinary equity holders of the company used in calculating basic (losses) per share: | |||||||||||||||||
| From continuing operations | ( | ( | ( | ||||||||||||||
| Diluted (losses) per share | |||||||||||||||||
(Losses) from continuing operations attributable to the ordinary equity holders of the company: | |||||||||||||||||
| Used in calculating basic (losses) per share | ( | ( | ( | ||||||||||||||
(Losses) attributable to the ordinary equity holders of the company used in calculating diluted losses per share | ( | ( | ( | ||||||||||||||
| 2023 Number | 2022 Number | 2021 Number | |||||||||||||||
Weighted average number of ordinary shares used as the denominator in calculating basic losses per share | |||||||||||||||||
Weighted average number of ordinary shares and potential ordinary shares used in calculating diluted losses per share | |||||||||||||||||
Options granted to employees and warrants (see Note 17) are considered to be potential ordinary shares. These securities have been excluded from the determination of basic losses per shares in the years ended June 30, 2023, 2022 and 2021. Shares that may be paid as contingent consideration have also been excluded from basic losses per share. They have also been excluded from the calculation of diluted losses per share because they are anti-dilutive for the years ended June 30, 2023, 2022 and 2021.
218
20. Parent entity financial information
a. Summary financial information
The parent entity financial information disclosure is an Australian Disclosure Requirement as required by Corporations Regulations 2001. The individual financial statements for the parent entity show the following aggregate amounts:
| As of June 30, | |||||||||||
| (in U.S. dollars, in thousands) | 2023 | 2022 | |||||||||
| Balance Sheet | |||||||||||
| Current Assets | |||||||||||
| Total Assets | |||||||||||
| Current Liabilities | |||||||||||
| Total Liabilities | |||||||||||
| Shareholders' Equity | |||||||||||
| Issued Capital | |||||||||||
| Reserves | |||||||||||
| Foreign Currency Translation Reserve | ( | ( | |||||||||
| Share Options Reserve | |||||||||||
| Warrant Reserve | |||||||||||
| (Accumulated losses)/retained earnings | ( | ( | |||||||||
| Loss for the period | ( | ( | |||||||||
| Total comprehensive loss for the period | ( | ( | |||||||||
b. Contingent liabilities of the parent entity
(i) Central Adelaide Local Health Network Incorporated (“CALHNI”) (formerly Medvet)
21. Segment information
219
22. Legal proceedings
23. Summary of significant accounting policies
This note provides the principal accounting policies adopted in the preparation of these consolidated financial statements as set out below. These policies have been consistently applied to all the years presented, unless otherwise stated. The financial statements are for the consolidated entity consisting of Mesoblast Limited and its subsidiaries.
b. Principles of consolidation
i. Subsidiaries
The consolidated financial statements incorporate the assets and liabilities of all subsidiaries of Mesoblast Limited (“Company” or “Parent Entity”) as of June 30, 2023 and the results of all subsidiaries for the year then ended. Mesoblast Limited and its subsidiaries together are referred to in this financial report as the Group or the consolidated entity.
Subsidiaries are all entities (including structured entities) over which the Group has control. The Group controls an entity when the Group is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity.
Subsidiaries are fully consolidated from the date on which control is transferred to the Group. They are deconsolidated from the date that control ceases.
The acquisition method of accounting is used to account for business combinations by the Group.
Intercompany transactions, balances and unrealized gains on transactions between Group companies are eliminated. Unrealized losses are also eliminated unless the transaction provides evidence of the impairment of the asset transferred. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Group.
ii. Employee share trust
The Group has formed a trust to administer the Group’s employee share scheme. This trust is consolidated, as the substance of the relationship is that the trust is controlled by the Group.
c. Segment reporting
The Group operates in one segment as set out in Note 21.
220
d. Foreign currency translation
(i) Functional and presentation currency
Items included in the financial statements of each of the Group’s entities are measured using the currency of the primary economic environment in which the entity operates (the “functional currency”). The functional currency of Mesoblast Limited is A$. The consolidated financial statements are presented in US$, which is the Group’s presentation currency.
(ii) Translations and balances
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the transaction at period end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in net loss, except when they are deferred in equity as qualifying cash flow hedges and qualifying net investment hedges or attributable to part of the net investment in a foreign operation.
Non-monetary items that are measured at fair value in a foreign currency are translated using the exchange rates at the date when the fair value was determined. Translation differences on assets and liabilities carried at fair value are reported as part of the fair value gain or loss. For example, translation differences on non-monetary assets and liabilities such as equities held at fair value through profit or loss are recognized in net loss as part of the fair value gain or loss and translation differences on non-monetary assets such as equities classified as financial assets at fair value are recognized in other comprehensive income.
(iii) Group companies
The results and financial position of all the Group entities (none of which has the currency of a hyperinflationary economy) that have a functional currency different from the presentation currency are translated into the presentation currency as follows:
•assets and liabilities for the consolidated balance sheets presented are translated at the closing rate at the date of that consolidated balance sheets;
•income and expenses for the statements of comprehensive income are translated at average exchange rates (unless this is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case income and expenses are translated at the dates of the transactions); and
•all resulting exchange differences are recognized in other comprehensive income.
(iv) Other
On consolidation, exchange differences arising from the translation of any net investment in foreign entities, and of borrowings and other financial instruments designated as hedges of such investments, are recognized in other comprehensive income. When a foreign operation is sold or any borrowings forming part of the net investment are repaid, the associated exchange differences are reclassified to net loss, as part of the gain or loss on sale.
Goodwill and fair value adjustments arising on the acquisition of a foreign entity are treated as assets and liabilities of the foreign entities and translated at the closing rate.
e. Revenue recognition
Revenue from contracts with customers is measured and recognized in accordance with the five step model prescribed by IFRS 15 Revenue from Contracts with Customers.
First, contracts with customers within the scope of IFRS 15 are identified. Distinct promises within the contract are identified as performance obligations. The transaction price of the contract is measured based on the amount of consideration the Group expect to be entitled from the customer in exchange for goods or services. Factors such as requirements around variable consideration, significant financing components, noncash consideration, or amounts payable to customers also determine the transaction price. The transaction is then allocated to separate performance obligations in the contract based on relative standalone selling prices. Revenue is recognized when, or as, performance obligations are satisfied, which is when control of the promised good or service is transferred to the customer.
221
Revenues from contracts with customers comprise commercialization and milestone revenue.
(i) Commercialization and milestone revenue
Commercialization and milestone revenue generally includes non-refundable upfront license and collaboration fees; milestone payments, the receipt of which is dependent upon the achievement of certain clinical, regulatory or commercial milestones; as well as royalties on product sales of licensed products, if and when such product sales occur; and revenue from the supply of products. Payment is generally due on standard terms of 30 to 60 days.
Amounts received prior to satisfying the revenue recognition criteria are recorded as deferred revenue or deferred consideration in our consolidated balance sheets, depending on the nature of arrangement. Amounts expected to be recognized as revenue within the 12 months following the consolidated balance sheet date are classified within current liabilities. Amounts not expected to be recognized as revenue within the 12 months following the consolidated balance sheet date are classified within non-current liabilities.
Milestone revenue
The Group applies the five-step method under the standard to measure and recognize milestone revenue.
The receipt of milestone payments is often contingent on meeting certain clinical, regulatory or commercial targets, and is therefore considered variable consideration. The Group estimate the transaction price of the contingent milestone using the most likely amount method. The Group include in the transaction price some or all of the amount of the contingent milestone only to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the contingent milestone is subsequently resolved. Milestone payments that are not within the control of the Company, such as regulatory approvals, are not considered highly probable of being achieved until those approvals are received. Any changes in the transaction price are allocated to all performance obligations in the contract unless the variable consideration relates only to one or more, but not all, of the performance obligations.
When consideration for milestones is a sale-based or usage-based royalty that arises from licenses of IP (such as cumulative net sales targets), revenue is recognized at the later of when (or as) the subsequent sale or usage occurs, or when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied).
Licenses of intellectual property
When licenses of IP are distinct from other goods or services promised in the contract, the Group recognize the transaction price allocated to the license as revenue upon transfer of control of the license to the customer. The Group evaluate all other promised goods or services in the license agreement to determine if they are distinct. If they are not distinct, they are combined with other promised goods or services to create a bundle of promised goods or services that is distinct.
The transaction price allocated to the license performance obligation is recognized based on the nature of the license arrangement. The transaction price is recognized over time if the nature of the license is a “right to access” license. This is when the Group undertake activities that significantly affect the IP to which the customer has rights, the rights granted by the license directly expose the customer to any positive or negative effects of our activities, and those activities do not result in the transfer of a good or service to the customer as those activities occur. When licenses do not meet the criteria to be a right to access license, the license is a “right to use” license, and the transaction price is recognized at the point in time when the customer obtains control over the license.
Sales-based or usage-based royalties
Licenses of IP can include royalties that are based on the customer’s usage of the IP or sale of products that contain the IP. The Group apply the specific exception to the general requirements of variable consideration and the constraint on variable consideration for sales-based or usage-based royalties promised in a license of IP. The exception requires such revenue to be recognized at the later of when (or as) the subsequent sale or usage occurs and the performance obligation to which some or all of the sales-based or usage-based royalty has been allocated has been satisfied (or partially satisfied).
222
Grünenthal arrangement
In September 2019, the Group entered into a strategic partnership with Grünenthal for the development and commercialization in Europe and Latin America of the Group’s allogeneic mesenchymal precursor cell (“MPC”) product, MPC-06-ID, receiving exclusive rights to the Phase 3 allogeneic product candidate for the treatment of low back pain due to degenerative disc disease.
The Group received a non-refundable upfront payment of $15.0 million in October 2019, on signing of the contract with Grünenthal. The Group received a milestone payment in December 2019 of $2.5 million in relation to meeting a milestone event as part of the strategic partnership with Grünenthal.
In June 2022, the Group announced its intention to leverage the results from a planned US trial to support potential product approvals in both the US and EU by including 20 % EU patients in order to provide regulatory harmonization, cost efficiencies and streamlined timelines, without initiating an EU trial. As a result, the strategic partnership with Grünenthal has been amended and milestone payments relating to R&D and CMC services and other development services which were linked to the Europe trial have been removed, instead the Group is eligible to receive payments up to US$112.5 million prior to product launch in the EU, inclusive of US$17.5 million already received, if certain clinical and regulatory milestones are satisfied and reimbursement targets are achieved. Cumulative milestone payments could reach US$1 billion depending on the final outcome of Phase 3 studies and patient adoption. The Group will also receive tiered double-digit royalties on product sales as per the original agreement.
The $2.5 million milestone payment received in December 2019 from Grünenthal was considered deferred consideration as of June 30, 2023. The performance obligation for the $2.5 million was previously satisfied under the original agreement, however under the amended agreement with Grünenthal it is subject to repayment to Grünenthal. Revenue will be recognized when the clinical trial has recruited the required amount of European patients, as the $2.5 million will no longer be subject to repayment to Grünenthal. There was no
Tasly arrangement
In July 2018, the Group entered into a strategic alliance with Tasly for the development, manufacture and commercialization in China of the Group’s allogeneic mesenchymal precursor cell MPC products, MPC-150-IM and MPC-25-IC. Tasly received all exclusive rights for MPC-150-IM and MPC-25-IC in China and Tasly will fund all development, manufacturing and commercialization activities in China.
The Group received a $20.0 million upfront technology access fee from Tasly upon closing of this strategic alliance in October 2018. The Group recognized $10.0 million from this $20.0 million upfront technology fee in milestone revenue at closing in October 2018 and the remaining $10.0 million was recognized in milestone revenue in February 2020. The Group is also entitled to receive $25.0 million on product regulatory approvals in China, double-digit escalating royalties on net product sales and up to six escalating milestone payments when the product candidates reach certain sales thresholds in China.
For the years ended June 30, 2023, 2022 and 2021, no
TiGenix arrangement
In December 2017, the Group entered into a patent license agreement with TiGenix, now a wholly owned subsidiary of Takeda, which granted Takeda exclusive access to certain of our patents to support global commercialization of the adipose-derived MSC product, Alofisel® a registered trademark of TiGenix, previously known as Cx601, for the local treatment of fistulae. The agreement includes the right for Takeda to grant sub-licenses to affiliates and third parties. The Group is entitled to further payments up to €10.0 million when Takeda reaches certain product regulatory milestones. Additionally, the Group will receive single digit royalties on net sales of Alofisel®.
In the years ended June 30, 2023, 2022 and 2021, the Group earned $0.4 million, $0.3 million and $0.2 million, respectively, of royalty income on sales of Alofisel® in Europe by our licensee Takeda.
223
was recognized with regards to the €1.0 million regulatory milestone payment receivable from Takeda given Takeda received approval to manufacture and market Alofisel® (darvadstrocel) in Japan for the treatment of complex perianal fistulas in patients with non-active or mildly active luminal Crohn’s Disease. No milestone revenue was recognized in the year ended June 30, 2021.
JCR arrangement
In October 2013, the Group acquired all of the culture-expanded, MSC-based assets from Osiris. These assets included assumption of a collaboration agreement with JCR, a research and development oriented pharmaceutical company in Japan. Revenue recognized under this agreement is limited to the amount of cash received or for which the Group is entitled, as JCR has the right to terminate the agreement at any time.
Under the JCR Agreement, JCR is responsible for all development and manufacturing costs including sales and marketing expenses. Under the JCR Agreement, JCR has the right to develop our MSCs in two fields for the Japanese market: exclusive in conjunction with the treatment of hematological malignancies by the use of hematopoietic stem cells derived from peripheral blood, cord blood or bone marrow, or the First JCR Field; and non-exclusive for developing assays that use liver cells for non-clinical drug screening and evaluation, or the Second JCR Field. With respect to the First JCR Field, the Group are entitled to payments when JCR reaches certain commercial milestones and to escalating double-digit royalties. These royalties are subject to possible renegotiation downward in the event of competition from non-infringing products in Japan. With respect to the Second JCR Field, the Group are entitled to a double-digit profit share. The Group expanded our partnership with JCR in Japan for two new indications: for wound healing in patients with Epidermolysis Bullosa (“EB”) in October 2018, and for hypoxic ischemic encephalopathy (“HIE”), a condition suffered by newborns who lack sufficient blood supply and oxygen to the brain, in June 2020. The Group will receive royalties on TEMCELL product sales for EB and HIE, if and when JCR begins selling TEMCELL for such indications in Japan. The Group applies the sales-based and usage-based royalty exception for licenses of intellectual property and therefore recognizes royalty revenue at the later of when the subsequent sale or usage occurs and the associated performance obligation has been satisfied.
In the years ended June 30, 2023, 2022 and 2021, the Group recognized $7.1 million, $8.7 million, and $7.2 million in commercialization revenue, respectively, relating to royalty income earned on sales of TEMCELL in Japan by our licensee JCR. These amounts were recorded in revenue as there are no further performance obligations required in regards to these items.
(ii) Interest income
Interest income is accrued on a time basis by reference to the principal outstanding and at the effective interest rate applicable, which is the rate that exactly discounts estimated future cash receipts through the expected life of the financial asset to that asset’s net carrying amount.
(iii) Research and development tax incentive
Tax incentives comprise payments from the Australian government’s Innovation Australia Research and Development Tax Incentive program for research and development activities conducted in relation to our qualifying research that meets the regulatory criteria. The research and development tax incentive credit is available for the Group's research and development activities in Australia. Eligible companies can receive a refundable tax offset for a percentage of their research and development spending.
The research and development tax incentive credit is available for the Group’s research and development activities in Australia as well as research and development activities outside of Australia to the extent such non-Australian based activities relate to intellectual property owned by our Australian resident entities do not exceed half the expenses for the relevant activities and are approved by the Australian government. Eligible companies can receive a refundable tax offset for a percentage of their research and development spending. In October 2020, the Australian Government introduced new legislation for the refundable tax offset applicable to eligible companies for income tax years commencing from July 1, 2021. Per the new legislation, the refundable tax offset for companies with an aggregated turnover of A$20.0 million or more is the Company’s corporate tax rate plus a rate between 8.5 % and 16.5 % depending on the proportion of research and development expenditures in relation to total expenditures. For companies with an aggregated turnover below A$20.0 million, the refundable research and development tax offset is 18.5 % above the Company's tax rate.
224
f. Inventories
Inventories are included in the financial statements at the lower of cost (including raw materials, direct labour, other direct costs and related production overheads) and net realizable value. Pre-launch inventory is held as an asset when there is a high probability of regulatory approval for the product in accordance with IAS 2 Inventories. Before that point, a provision is made against the carrying value to its recoverable amount in accordance with IAS 37 Provisions, Contingent Liabilities and Contingent Assets; the provision is then reversed at the point when a high probability of regulatory approval is determined.
The Group considers a number of factors in determining the probability of the product candidate realizing future economic benefit, including the product candidate’s current status in the regulatory approval process, results from the related pivotal clinical trial, results from meetings with relevant regulatory agencies prior to the filing of regulatory applications, the market need, historical experience, as well as potential impediments to the approval process such as product safety or efficacy, commercialization and market trends.
When a provision is made against the carrying value of pre-launch inventory the costs are recognized within Manufacturing Commercialization expenses. When the high probability threshold is met, the provision will be reversed through Manufacturing Commercialization expenses.
All inventory costs are currently fully provided for and are recognized within Manufacturing Commercialization expenses. Where it is determined that the pre-launch inventory will be used within a clinical trial, that amount is removed from the cost of pre-launch inventory. There is no impact on the consolidated income statement as the carrying value has been previously fully provided for.
As of June 30, 2023 and June 30, 2022, there was $22.4 million and $28.9 million of pre-launch inventory recognized on the consolidated balance sheet that was fully provided for, respectively. In the year ended June 30, 2023, the Group reclassified $10.0 million of costs within pre-launch inventory for use in clinical trials. The future commercial use of the remaining pre-launch inventory recognized on the consolidated balance sheet will be dependent on future discussions with the FDA and remains fully provided for.
g. Research and development undertaken internally
The Group currently does not have any capitalized development costs. Research expenditure is recognized as an expense as incurred. Costs incurred on development projects, which consist of preclinical and clinical trials, manufacturing development, and general research, are recognized as intangible assets when it is probable that the project will, after considering its commercial and technical feasibility, be completed and generate future economic benefits and its costs can be measured reliably.
The expenditure capitalized comprises all directly attributable costs, including costs of materials, services, direct labor and an appropriate proportion of overheads. Other development costs that do not meet these criteria are expensed as incurred. Development costs previously recognized as expenses, are not recognized as an asset in a subsequent period and will remain expensed. Capitalized development costs are recorded as intangible assets and amortized from the point at which the asset is ready for use on a straight-line basis over its useful life.
225
h. Income tax
The income tax expense or benefit for the period is the tax payable on the current period’s taxable income based on the applicable income tax rate for each jurisdiction adjusted by changes in deferred tax assets and liabilities attributable to temporary differences and to unused tax losses.
The current income tax charge is calculated on the basis of the tax laws enacted or substantively enacted at the end of the reporting period in the countries where the Group’s subsidiaries and associates operate and generate taxable income. Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is subject to interpretation. It establishes provisions where appropriate on the basis of amounts expected to be paid to the tax authorities.
Deferred income tax is provided in full, using the liability method, on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the consolidated financial statements. However, the deferred income tax is not accounted for if it arises from initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects neither accounting, nor taxable profit or loss. Deferred income tax is determined using tax rates (and laws) that have been enacted or substantially enacted by the end of the reporting period and are expected to apply when the related deferred income tax asset is realized or the deferred income tax liability is settled.
Deferred tax assets are recognized for deductible temporary differences and unused tax losses only if it is probable that future taxable amounts will be available to utilize those temporary differences and losses. Deferred tax assets are only recognized to the extent that there are sufficient deferred tax liabilities unwinding.
Deferred tax liabilities and assets are not recognized for temporary differences between the carrying amount and tax bases of investments in controlled entities where the parent entity is able to control the timing of the reversal of the temporary differences and it is probable that the differences will not reverse in the foreseeable future.
Deferred tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets and liabilities and when the deferred tax balances relate to the same taxation authority. Current tax assets and tax liabilities are offset where the entity has a legally enforceable right to offset and intends either to settle on a net basis, or to realize the asset and settle the liability simultaneously.
Current and deferred tax is recognized in net loss, except to the extent that it relates to items recognized in other comprehensive income or directly in equity. In this case, the tax is also recognized in other comprehensive income or directly in equity, respectively.
i. Business combinations
The acquisition method of accounting is used to account for all business combinations, regardless of whether equity instruments or other assets are acquired. The consideration transferred for the acquisition of a subsidiary comprises the fair values of the assets transferred, the liabilities incurred and the equity interests issued by the Group. The consideration transferred also includes the fair value of any asset or liability resulting from a contingent consideration arrangement and the fair value of any pre-existing equity interest in the subsidiary. Acquisition-related costs are expensed as incurred. Identifiable assets acquired and liabilities and contingent liabilities assumed in a business combination are, with limited exceptions, measured initially at their fair values at the acquisition date. On an acquisition-by-acquisition basis, the Group recognizes any noncontrolling interest in the acquiree either at fair value or at the non-controlling interest’s proportionate share of the acquiree’s net identifiable assets.
The excess of the consideration transferred and the amount of any non-controlling interest in the acquiree over the fair value of the net identifiable assets acquired is recorded as goodwill. If those amounts are less than the fair value of the net identifiable assets of the subsidiary acquired and the measurement of all amounts has been reviewed, the difference is recognized directly in net loss as a bargain purchase.
Where settlement of any part of cash consideration is deferred, the amounts payable in the future are discounted to their present value as at the date of exchange. The discount rate used is the entity’s incremental borrowing rate, being the rate at which a similar borrowing could be obtained from an independent financier under comparable terms and conditions.
226
j. Impairment of assets
Goodwill and intangible assets that have an indefinite useful life are not subject to amortization and are tested annually for impairment or more frequently if events or changes in circumstances indicate that they might be impaired. Other assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs to dispose and value in use. For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash inflows which are largely independent of the cash inflows from other assets or groups of assets (cash-generating units). Non-financial assets (other than goodwill) that have suffered impairment are reviewed for possible reversal of the impairment at the end of each reporting period.
Management maintains internal valuations of each asset annually (or more frequently should indicators of impairment be identified) and valuations from independent experts are requested periodically, within every three year period. The internal valuations are continually reviewed by management and consideration is given as to whether there are indicators of impairment which would warrant impairment testing. An external valuation of our assets was carried out by an independent expert as at March 31, 2023 with the recoverable amount of each asset exceeding its carrying amount.
k. Cash and cash equivalents
For the purpose of presentation in the statement of cash flows, cash and cash equivalents includes cash on hand, deposits held at call with financial institutions, other short-term and highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
l. Trade and other receivables
Trade receivables and other receivables represent the principal amounts due at balance date less, where applicable, any provision for expected credit losses. The Group uses the simplified approach to measuring expected credit losses, which uses a lifetime expected credit loss allowance. Debts which are known to be uncollectible are written off in the consolidated income statement. All trade receivables and other receivables are recognized at the value of the amounts receivable, as they are due for settlement within 60 days and therefore do not require remeasurement.
m. Investments and other financial assets
(i) Classification
The Group classifies its financial assets in the following measurement categories:
•those to be measured subsequently at fair value (either through OCI or through profit or loss); and
•those to be measured at amortized cost
The classification depends on the Group’s business model for managing the financial assets and the contractual terms of the cash flow. For assets measured at fair value, gains and losses will either be recorded in profit or loss or OCI. For investments in equity instruments that are not held for trading, this will depend on whether the group has made an
227
irrevocable election at the time of initial recognition to account for the equity investment at fair value through other comprehensive income (FVOCI). See Note 5 for details about each type of financial asset.
(ii) Recognition and derecognition
Regular way purchases and sales of financial assets are recognized on trade-date, the date on which the Group commits to purchase or sell the asset. Financial assets are derecognized when the rights to receive cash flows from the financial assets have expired or have been transferred and the Group has transferred substantially all the risks and rewards of ownership.
(iii) Measurement
At initial recognition, the Group measures a financial asset at its fair value plus, in the case of a financial asset not at fair value through profit or loss (FVPL), transaction costs that are directly attributable to the acquisition of the financial asset. Transaction costs of financial assets carried at FVPL are expensed in profit or loss. Financial assets with embedded derivatives are considered in their entirety when determining whether their cash flows are solely payment of principal and interest.
Details on how the fair value of financial instruments is determined are disclosed in Note 5(g).
Equity instruments
The group subsequently measures all equity investments at fair value. Where the Group has elected to present fair value gains and losses on equity investments in OCI, there is no subsequent reclassification of fair value gains and losses to profit or loss following the derecognition of the investment. Dividends from such investments continue to be recognized in profit or loss as other income when the group’s right to receive payments is established.
Changes in the fair value of financial assets at FVPL are recognized in other gains/(losses) in the statement of profit or loss as applicable. Impairment losses (and reversal of impairment losses) on equity investments measured at FVOCI are not reported separately from other changes in fair value.
(iv) Impairment
For trade receivables, the group applies the simplified approach permitted by IFRS 9, which requires expected lifetime losses to be recognized from initial recognition of the receivables, see Note 5(b) for further details.
n. Derivatives
Derivatives are initially recognized at fair value on the date a derivative contract is entered into and are subsequently remeasured to their fair value at the end of each reporting period. As at June 30, 2023 and 2022, the Group did not have any derivative instruments that qualified for hedge accounting.
Derivatives that do not qualify for hedge accounting
Certain derivative instruments do not qualify for hedge accounting. Changes in the fair value of any derivative instrument that does not qualify for hedge accounting are recognized immediately in profit or loss and are included in other income or other expenses.
o. Property, plant and equipment
Plant and equipment are stated at historical cost less accumulated depreciation and impairment. Cost includes expenditure that is directly attributable to the acquisition of the item.
Subsequent cost are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associates with the item will flow to the Group and the cost of the item can be measured reliably. All other repairs and maintenance are charged to profit and loss during the reporting period in which they are incurred.
228
Property, plant and equipment, other than freehold land, are depreciated over their estimated useful lives using the straight line method (see Note 6(a)).
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at the end of each reporting period.
An asset’s carrying amount is written down immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount.
Gains and losses on disposal of plant and equipment are taken into account in determining the profit for the year.
p. Intangible assets
(i) Goodwill
Goodwill is measured as described in Note 23(i). Goodwill on acquisition of subsidiaries is included in intangible assets (Note 6(c)). Goodwill is not amortized but it is tested for impairment annually or more frequently if events or changes in circumstances indicate that it might be impaired, and is carried at cost less accumulated impairment losses. Gains and losses on the disposal of an entity include the carrying amount of goodwill relating to the entity sold.
Goodwill is tested for impairment in accordance with IAS 36 Impairment of Assets which requires testing be performed at any time during an annual period, provided the test is performed at the same time every year. The Group tests for impairment annually in the third quarter of each year. Additionally, assets must be tested for impairment if there is an indication that an asset may be impaired. The recoverable amounts of our assets and cash-generating units have been determined based on fair value less costs to sell calculations, which require the use of certain assumptions.
Goodwill is allocated to cash generating units for the purpose of impairment testing. The allocation is made to those cash generating units or groups of cash generating units that are expected to benefit from the business combination in which the goodwill arose, identified according to operating segments (Note 21).
(ii) Acquired licenses to patents
Acquired licenses have a finite useful life and are carried at cost less accumulated amortization and impairment losses. Each asset is amortized through to the estimated patent expiry date which is reviewed and adjusted as patent extensions are granted.
Payments made to third parties to acquire licenses to patents, including initial upfront and subsequent milestone payments are capitalized. For subsequent payments under existing license agreements payments are capitalized if they meet the definition of an intangible asset. Management reviews the substance of the payment to determine its classification. Generally, payments made for a verifiable outcome, such as completion of a clinical trial, regulatory approvals and sales target milestones would be accumulated into the cost of the intangible.
The Group periodically evaluates whether current facts or circumstances indicate that the carrying value of its acquired intangibles may not be recoverable. If such circumstances are determined to exist, an estimate of the undiscounted future cash flow of these assets, or appropriate assets grouping is compared to the carrying value to determine whether an impairment exists. If the asset is determined to be impaired, the loss is measured based on the differences between the carrying value of the intangible asset and its fair value, which is determined based on the net present value of estimated future cash flows.
Royalty payments under license and sublicense agreements are expensed.
(iii) In-process research and development acquired
In-process research and development that has been acquired as part of a business acquisition is considered to be an indefinite life intangible asset on the basis that it is incomplete and cannot be used in its current form. Indefinite life intangible assets are not amortized but rather are tested for impairment annually in the third quarter of each year, or whenever events or circumstances present an indication of impairment.
229
In-process research and development will continue to be tested for impairment until the related research and development efforts are either completed or abandoned. Upon completion of the related research and development efforts, management determines the remaining useful life of the intangible assets and amortizes them accordingly. In order for management to determine the remaining useful life of the asset, management would consider the expected flow of future economic benefits to the entity with reference to the product life cycle, competitive landscape, obsolescence, market demand, any remaining patent useful life and various other relevant factors. At the time of completion, when the asset becomes available for use, all costs recognized in in-process research and development that related to the completed asset are transferred to the intangible asset category, current marketed products, at the asset’s historical cost.
In the case of abandonment, the related research and development efforts are considered impaired and the asset is fully expensed.
(iv) Current marketed products
Current marketed products contain products that are currently being marketed. The assets are recognized on our consolidated balance sheet as a result of business acquisitions or reclassifications from In-process research and development upon completion. Upon completion, when assets become available for use, assets are reclassified from in-process research and development to current marketed products at the historical value that they were recognized at within the in-process research and development category.
Upon reclassification to the current market products category management determines the remaining useful life of the intangible assets and amortizes them from the date they become available for use. In order for management to determine the remaining useful life of the asset, management would consider the expected flow of future economic benefits to the entity with reference to the product life cycle, competitive landscape, obsolescence, market demand, any remaining patent useful life and any other relevant factors.
q. Trade and other payables
Payables represent the principal amounts outstanding at balance date plus, where applicable, any accrued interest. Liabilities for payables and other amounts are carried at cost which approximates fair value of the consideration to be paid in the future for goods and services received, whether or not billed. The amounts are unsecured and are usually paid within 30 to 60 days of recognition.
r. Borrowings
Borrowings are initially recognized at fair value, net of transaction costs incurred. Borrowings are subsequently measured at amortized cost. Any difference between the proceeds (net of transaction costs) and the redemption amount is recognized in profit or loss over the period of the borrowings using the effective interest method.
Borrowings are removed from the consolidated balance sheet when the obligation specified in the contract is discharged, cancelled or expired. The difference between the carrying amount of a financial liability that has been extinguished or transferred to another party and the consideration paid, including any non-cash assets transferred of liabilities assumed, is recognized in profit or loss as other income or finance costs.
Borrowings are classified as current liabilities unless the Group has an unconditional right to defer settlement of the liability for at least 12 months after the reporting period
Funds associated with Oaktree Capital Management, L.P. (“Oaktree”)
In November 2021, the Group entered into a $90.0 million five-year senior debt facility provided by funds associated with Oaktree. The Group drew the first tranche of $60.0 million on closing. The conditions required to draw down the additional $30.0 million tranche have not been met. The facility has a three-year interest only period, at a fixed rate of 9.75 % per annum, after which time 40 % of the principal amortizes over two years and a final payment is due no later than November 2026. The facility also allows the Group to make quarterly payments of interest at a rate of 8.0 % per
230
annum for the first two years , and the unpaid interest portion (1.75 % per annum) will be added to the outstanding loan balance and shall accrue further interest at a fixed rate of 9.75 % per annum.
On November 19, 2021, Oaktree was granted warrants to purchase 1,769,669 American Depositary Shares (“ADSs”) at US$7.26 per ADS, a 15 % premium to the 30-day VWAP. The Group determined that an obligation to issue the warrants has arisen from the time the debt facility was signed; consequently, a liability for the warrants was recognized in November 2021. The warrants were legally issued on January 11, 2022 and may be exercised within 7 years of issuance. On the issuance date of the Oaktree facility and the warrants, the warrants were initially measured at fair value and the Oaktree borrowing liability measured as the difference between the $60.0 million received from the Oaktree facility and the fair value of the warrants. In December 2022, the Group amended the terms of the loan agreement with Oaktree and in connection with the loan amendment, Oaktree was granted warrants to purchase 455,000 ADSs at $3.70 per ADS, a 15 % premium to the 30-day VWAP. The Group determined that an obligation to issue the warrants arose from the time the first amendment to the loan agreement was signed; consequently, a liability for the warrants was recognized in December 2022. The warrants were legally issued on March 8, 2023 and may be exercised within 7 years of issuance. Refer to Note 5(g)(vi) for more details on warrants issued.
In the year ended June 30, 2023, the Group recognized a loss of $1.6 1.6 1.0 million relates to the remeasurement due to additional warrants being issued to Oaktree as a result of the first amendment to the loan agreement and $0.6 million relates to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facility. In the year ended June 30, 2022, the Group recognized a minimal gain in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows from our credit facility. No remeasurement of borrowing arrangements was recognized in the year ended June 30, 2021.
The Group has pledged substantially all of its assets as collateral under the loan facility with Oaktree.
NovaQuest
On June 29, 2018, the Group entered into an eight-year , $40.0 million loan and security agreement with NovaQuest before drawing the first tranche of $30.0 million of the principal in July 2018. The loan term includes an interest only period of approximately four years through until July 8, 2022, then a four-year amortization period through until maturity on July 8, 2026. All interest and principal payments will be deferred until after the first commercial sale of remestemcel-L for the treatment in pediatric patients with SR-aGVHD. Principal is repayable in equal quarterly instalments over the amortization period of the loan and is subject to the payment cap described below. The loan has a fixed interest rate of 15 % per annum. If there are no net sales of remestemcel-L for pediatric SR-aGVHD, the loan is only repayable at maturity. The Group can elect to prepay all outstanding amounts owing at any time prior to maturity, subject to a prepayment charge, and may decide to do so if net sales of remestemcel-L for pediatric SR-aGVHD are significantly higher than current forecasts.
Following approval and first commercial sales, repayments commence based on a percentage of net sales and are limited by a payment cap which is equal to the principal due for the next 12 months, plus accumulated unpaid principal and accrued unpaid interest. During the four-year period commencing July 8, 2022, principal amortizes in equal quarterly instalments payable only after approval and first commercial sales. If in any quarterly period, 25% of net sales of remestemcel-L for pediatric SR-aGVHD exceed the annual payment cap, the Group will pay the payment cap and an additional portion of excess sales which will be used towards the prepayment amount in the event there is an early prepayment of the loan. If in any quarterly period 25% of net sales of remestemcel-L for pediatric SR-aGVHD is less than the annual payment cap, then the payment is limited to 25% of net sales of remestemcel-L for pediatric SR-aGVHD. Any unpaid interest will be added to the principal amounts owing and shall accrue further interest. At maturity date, any unpaid loan balances are repaid.
Because of this relationship of net sales and repayments, changes in our estimated net sales may trigger an adjustment of the carrying amount of the financial liability to reflect the revised estimated cash flows. The carrying amount is recalculated by computing the present value of the revised estimated future cash flows at the financial instrument’s original effective interest rate. The adjustment is recognized in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in the period the revision is made.
231
In the years ended June 30, 2023 and 2022, the Group recognized a gain of $0.9 million and $0.5 million, respectively, in the Consolidated Income Statement as remeasurement of borrowing arrangements within finance costs in relation to the adjustment of the carrying amount of our financial liability to reflect the revised estimated future cash flows as a net result of changes to the key assumptions in development timelines.
The Group recognizes a liability as current based on repayments linked to estimates of sales of remestemcel-L. However, if sales of remestemcel-L are higher than estimated, actual repayments will exceed this amount, subject to the annual payment cap described above.
s. Provisions
Provisions are recognized when the Group has a present legal obligation as a result of a past event, it is probable that the Group will be required to settle the obligation, and a reliable estimate can be made of the amount of the obligation.
Provisions are measured at the present value of management’s best estimate of the expenditure required to settle the present obligation at the end of the reporting period. The discount rate used to determine the present value is a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the liability. The increase in the provision due to the passage of time is recognized as interest expense.
Provisions are recorded on acquisition of a subsidiary, to the extent they relate to a subsidiary’s contingent liabilities, if it relates to a past event, regardless of whether it is probable the amount will be paid.
t. Employee benefits
A liability is recognized for benefits accruing to employees in respect of wages and salaries, bonuses, annual leave and long service leave.
Liabilities recognized in respect of employee benefits which are expected to be settled within 12 months after the end of the period in which the employees render the related services are measured at their nominal values using the remuneration rates expected to apply at the time of settlement.
Liabilities recognized in respect of employee benefits which are not expected to be settled within 12 months after the end of the period in which the employees render the related services are measured as the present value of the estimated future cash outflows to be made by the Group in respect of services provided by employees up to reporting date.
The obligations are presented as current liabilities in the consolidated balance sheet if the entity does not have an unconditional right to defer settlement for at least twelve months after the reporting period, regardless of when the actual settlement is expected to occur.
Termination benefits are payable when employment is terminated by the Group before the normal retirement date, or when an employee accepts voluntary redundancy in exchange for these benefits. The Group recognizes termination benefits at the earlier of the following dates: when the Group can no longer withdraw the offer of those benefits and when the entity recognizes costs for a restructuring that is within the scope of IAS 37 and involves the payment of termination benefits.
u. Share-based payments
Share-based payments are provided to eligible employees, directors and consultants via the Employee Share Option Plan (“ESOP”) and the Australian Loan Funded Share Plan (“LFSP”). The terms and conditions of the LFSP are in substance the same as the employee share options and therefore they are accounted for on the same basis.
Equity-settled share-based payments with employees and others providing similar services are measured at the fair value of the equity instrument at acceptance date. Fair value is measured using the Black-Scholes model. The expected life used in the model has been adjusted, based on management’s best estimate, for the effects of non-transferability, exercise
232
restrictions, and behavioral considerations. It does not make any allowance for the impact of any service and non-market performance vesting conditions. Further details on how the fair value of equity-settled share-based transactions has been determined can be found in Note 17.
The fair value determined at the acceptance date of the equity-settled share-based payments is expensed on a straight-line basis over the vesting period, based on management’s estimate of shares that will eventually vest, with a corresponding increase in equity. At the end of each period, the entity revises its estimates of the number of shared-based payments that are expected to vest based on the non-market vesting conditions. It recognizes the impact of the revision to original estimates, if any, in profit or loss, with a corresponding adjustment to equity.
v. Leases
Leases are recognized as a right-of-use asset and a corresponding liability at the date at which the leased asset is available for use by the Group. Assets and liabilities arising from a lease are initially measured on a present value basis. Lease liabilities include the net present value of the following lease payments:
•fixed payments (including in-substance fixed payments), less any lease incentives receivable;
•variable lease payment that are based on an index or a rate;
•amounts expected to be payable by the lessee under residual value guarantees;
•the exercise price of a purchase option if the lessee is reasonably certain to exercise that option; and
•payments of penalties for terminating the lease, if the lease term reflects the lessee exercising that option.
Variable lease payments that are not based on an index or a rate are not included in the initial measurement of the lease liability and are expensed in the Consolidated Income Statement when incurred. There were no
For certain contracts that contain lease and non-lease components, the Group accounts for each lease component within the contract as a lease separately from non-lease components of the contract. The Group identifies a separate lease component if there is an explicit or implicit identified asset in the contract and if the Group controls use of the identified asset.
The lease payments are discounted using the interest rate implicit in the lease, if that rate can be determined, or the Group’s incremental borrowing rate.
Right-of-use assets are measured at cost comprising the following:
•the amount of the initial measurement of lease liability;
•any lease payments made at or before the commencement date, less any lease incentives received;
•any initial direct costs; and
•restoration costs.
Payments associated with short-term leases with a lease term of 12 months or less, contracts that contain lease and non-lease components that are cancellable within 12 months and leases of low-value assets are recognized on a straight-line basis as an expense in profit or loss. Low-value assets comprise IT-equipment and small items of office furniture.
233
x. Contributed equity
Ordinary shares are classified as equity.
Transaction costs arising on the issue of equity instruments are recognized separately in equity. Transaction costs are the costs that are incurred directly in connection with the issue of those equity instruments and which would not have been incurred had those instruments not been issued.
y. Loss per share
(i) Basic losses per share
Basic losses per share is calculated by dividing:
•the loss attributable to equity holders of the Group, excluding any costs of servicing equity other than ordinary shares;
•by the weighted average number of ordinary shares outstanding during the fiscal year, adjusted for bonus elements in ordinary shares issued during the year.
(ii) Diluted losses per share
Diluted losses per share adjusts the figures used in the determination of basic earnings per share to take into account
•the after income tax effect of interest and other financing costs associated with dilutive potential ordinary shares; and
•the weighted average number of shares assumed to have been issued for no consideration in relation to dilutive potential ordinary shares.
z. Goods and services tax (“GST”)
Revenues, expenses and assets are recognized net of the amount of GST except where the GST incurred on a purchase of goods and services is not recoverable from the taxation authority, in which case the GST is recognized as part of the cost of acquisition of the asset or as part of the expense.
Receivables and payables are stated with the amount of GST included. The net amount of GST recoverable from, or payable to, the taxation authority is included as part of receivables or payables in the Consolidated Balance Sheet.
Cash flows are included in the statement of cash flow on a gross basis. The GST component of cash flows arising from investing and financing activities, which is recoverable from, or payable to, the taxation authority, are classified as operating cash flows.
234
Australian Disclosure Requirements
Directors’ Declaration
In the directors’ opinion:
(a)the financial statements and Notes set out on pages 165 to 234 are in accordance with the Corporations Act 2001, including:
(i)Complying with Accounting Standards, the Corporations Regulations 2001 and other mandatory professional reporting requirements, and
(ii)Giving a true and fair view of the consolidated entity’s financial position as at June 30, 2023 and of its performance for the fiscal year ended on that date, and
(b)There are reasonable grounds to believe that the Group will be able to pay its debts as and when they become due and payable.
Note 1 ‘Basis of preparation’ confirms that the financial statements also comply with International Financial Reporting Standards as issued by the International Accounting Standards Board.
The directors have been given the declarations by the chief executive officer and chief financial officer required by section 295A of the Corporations Act 2001.
This declaration is made in accordance with a resolution of the directors.
| /s/ Joseph Swedish | /s/ Silviu Itescu | |||||||
| Joseph Swedish | Silviu Itescu | |||||||
| Chairman | Chief Executive Officer | |||||||
Melbourne, August 31, 2023
235
This page is intentionally left blank.
236
This page is intentionally left blank.
237
This page is intentionally left blank.
238
This page is intentionally left blank.
239
This page is intentionally left blank.
240
This page is intentionally left blank.
241
Item 19. Exhibits
| Item | ||||||||
| 1.1 | ||||||||
| 1.2 | ||||||||
| 2.1* | ||||||||
| 4.1 | ||||||||
| 4.2 | ||||||||
| 4.3† | ||||||||
| 4.4 | ||||||||
| 4.5 | ||||||||
| 4.6† | ||||||||
| 4.7† | ||||||||
| 4.8# | ||||||||
| 4.9#* | ||||||||
| 4.10 | ||||||||
| 4.11 | ||||||||
| 4.12† | ||||||||
| 4.13† | ||||||||
| 4.14† | ||||||||
| 4.15✓ | ||||||||
| 4.16✓ | ||||||||
| 4.17 | ||||||||
242
| 4.18✓ | ||||||||
| 4.19✓ | ||||||||
| 4.20✓ | ||||||||
| 4.21 | ||||||||
| 4.22✓ | Loan Agreement and Guaranty between Mesoblast Limited, Mesoblast UK Limited, Mesoblast, Inc., Mesoblast International Sàrl and Oaktree Fund Administration, LLC, dated November 19, 2021 (incorporated by reference to Exhibit 4.32 to the Company's Annual Report on From 20-F filed with the SEC on August 31, 2022). | |||||||
| 4.23* | ||||||||
| 4.24*✓ | ||||||||
| 4.25* | ||||||||
| 4.26*✓ | ||||||||
| 8.1* | ||||||||
| 12.1* | ||||||||
| 12.2* | ||||||||
| 13.1* | ||||||||
| 13.2* | ||||||||
| 15.1* | ||||||||
| 15.2* | ||||||||
| 99.1* | ||||||||
| 99.2* | ||||||||
| 101.INS | Inline XBRL Instance Document | |||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | |||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | |||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | |||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |||||||
| 104 | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) | |||||||
| # | Indicates management contract or compensatory plan. | |||||||
| * | Filed herewith. | |||||||
| † | Confidential treatment has been requested for portions of this exhibit. These portions have been omitted and have been filed separately with the Securities and Exchange Commission. | |||||||
| ✓ | Certain confidential portions of this exhibit were omitted by means of marking such portions with brackets (“[***]”) because the identified confidential portions are not material and are the type that the registrant treats as private or confidential. | |||||||
243
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Mesoblast Limited | ||||||||
| By: | /s/ Joseph R Swedish | |||||||
| Name: | Joseph R Swedish | |||||||
| Title: | Chairman | |||||||
| By: | /s/ Silviu Itescu | |||||||
| Name: | Silviu Itescu | |||||||
| Title: | Chief Executive Officer | |||||||
Dated: August 31, 2023
244