UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number:
(Exact name of Registrant as specified in its charter)
Not Applicable
(Translation of Registrant’s name into English)
Kingdom of
(Jurisdiction of incorporation or organization)
(Address of principal executive offices)
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading Symbol |
| Name of each exchange on which registered |
Ordinary Share, no nominal value per share | The | |||
| The |
* |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None.
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None.
The number of outstanding shares of each of the issuer’s classes of capital or common stock as of December 31, 2023 was:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ☐ Yes ☒
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ | |
Non accelerated filer ☐ | Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ |
| as issued by the International Accounting Standards Board ☒ |
| Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. ☐ Yes ☐ No
TABLE OF CONTENTS
|
| Page | ||
3 | ||||
3 | ||||
3 | ||||
36 | ||||
54 | ||||
54 | ||||
76 | ||||
86 | ||||
88 | ||||
88 | ||||
89 | ||||
99 | ||||
101 | ||||
103 | ||||
Material Modifications to the Rights of Security Holders and Use of Proceeds | 103 | |||
103 | ||||
104 | ||||
104 | ||||
104 | ||||
105 | ||||
Purchases of Equity Securities by the Issuer and Affiliated Purchasers | 105 | |||
105 | ||||
105 | ||||
106 | ||||
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | 106 | |||
106 | ||||
107 | ||||
109 | ||||
109 | ||||
109 |
INTRODUCTION
Except as otherwise required by the context, references to (i) “Materialise,” “Company,” “we,” “us” and “our” are to Materialise NV and its subsidiaries, (ii) “ACTech” are to ACTech Holding GmbH and its subsidiaries, which we acquired in 2017, (iii) “Engimplan” are to Engimplan Engenharia De Implante Indústria E Comércio Ltda., in which we acquired a controlling interest in 2019 and in which we acquired the remaining interest in 2020, making us Engimplan’s sole shareholder (through our Brazilian subsidiary), (iv) “Materialise Motion” are to Materialise Motion NV, a joint venture we established in 2014 under the name “RSPrint Powered by Materialise” NV and in which we acquired the remaining interest in 2020, together with substantially all of the assets of RSScan International NV, or RS Scan, making us Materialise Motion’s sole shareholder, (v) “Link3D” are to Link3D Inc., which we acquired an option to buy in 2021, which we exercised in 2022, and which we subsequently merged into our U.S. subsidiary, Materialise USA, LLC, and (vi) “Identify3D” are to Identify3D, Inc., which we acquired in 2022 and subsequently merged into Materialise USA, LLC.
Our trademark portfolio contained 185 registered trademarks and 3 pending trademark application as of December 31, 2023. All other trademarks or trade names referred to in this annual report are the property of their respective owners. Solely for convenience, the trademarks and trade names in this annual report are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
All references in this annual report to “U.S. dollars” or “$” are to the legal currency of the United States and all references to “€” or “euro” are to the currency introduced at the start of the third stage of the European economic and monetary union pursuant to the treaty establishing the European Community, as amended.
SPECIAL NOTE REGARDING FORWARD-LOOKING INFORMATION
This annual report includes certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, concerning our business, operations and financial performance and condition as well as our plans, objectives and expectations for our business operations and financial performance and condition. Any statements that are not of historical facts may be deemed to be forward-looking statements. You can identify these forward-looking statements by words such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “aims,” or other similar expressions that convey uncertainty of future events or outcomes. Forward-looking statements appear in a number of places throughout this annual report and include statements regarding our intentions, beliefs, assumptions, projections, outlook, analyses or current expectations concerning, among other things, our intellectual property position, research and development projects, acquisitions, results of operations, cash needs, spending of the remaining net proceeds from our initial public offering, capital expenditures, financial condition, liquidity, prospects, growth and strategies, regulatory approvals and clearances, the markets and industry in which we operate and the trends and competition that may affect the markets, industry or us. In particular, under “Item 5. Operating and Financial Review and Prospects—D. Trend Information” of this annual report and in the notes to our audited consolidated financial statements, we discuss, based on our current assessment of the ongoing armed conflict in Ukraine, and other geopolitical tensions how our business, results of operations, and financial condition could be impacted during the year 2024 and beyond.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics and industry change, and depend on economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this annual report, we caution you that forward-looking statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. All of our forward-looking statements are subject to risks and uncertainties that may cause our actual results to differ materially from our expectations.
Actual results could differ materially from our forward-looking statements due to a number of factors, including, without limitation, risks related to:
| ● | the global political, economic, and macroeconomic climate, whether within our industry in general, or among specific types of customers or within particular geographies, including but not limited to, the impacts related to labor shortages, supply chain disruptions, actual or perceived instability in the global banking system, the results of local and national elections, a potential recession, inflation, and rising interest rates; |
| ● | our ability to enhance and adapt our software, products and services to meet changing technology and customer needs; |
1
| ● | fluctuations in our revenue and results of operations; |
| ● | impacts on our business, financial conditions and results of operations from the armed conflicts in Ukraine, Israel and the Middle East; |
| ● | impacts on our business, financial conditions and results of operations from increased geopolitical tensions, including the ongoing tensions between the United States and China; |
| ● | our ability to operate in a highly competitive and rapidly changing industry; |
| ● | our ability to adequately increase demand for our products and services; |
| ● | our collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships with third parties; |
| ● | our ability to integrate acquired businesses or technologies effectively; |
| ● | our dependence upon sales to certain industries; |
| ● | our relationships with suppliers; |
| ● | our ability to attract and retain employees and contractors; |
| ● | any disruptions to our service center operations, including by accidents, warfare, natural disasters or otherwise; |
| ● | our ability to raise additional capital on attractive terms, or at all, if needed to meet our growth strategy; |
| ● | our ability to adequately protect our intellectual property and proprietary technology; |
| ● | our international operations; |
| ● | our ability to comply with applicable governmental laws and regulations to which our products, services and operations are subject; and |
| ● | other risk factors as set forth under “Item 3. Key Information – D. Risk Factors.” |
Any forward-looking statements that we make in this annual report speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this annual report or to reflect the occurrence of unanticipated events. Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless expressed as such, and should only be viewed as historical data. You should, however, review the factors and risks we describe in the reports we will file from time to time with the U.S. Securities and Exchange Commission, or the SEC, after the date of this annual report. See “Item 10. Additional Information – H. Documents on Display.”
You should also read carefully the factors described in “Item 3. Key Information – D. Risk Factors” and elsewhere in this annual report to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this annual report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all.
2
PART I
ITEM 1.IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. | OFFER STATISTICS AND EXPECTED TIMETABLE |
Not applicable.
ITEM 3.KEY INFORMATION
| A. | [Reserved] |
| B. | Capitalization and Indebtedness |
Not applicable.
| C. | Reasons for the Offer and Use of Proceeds |
Not applicable.
| D. | Risk Factors |
Summary of Risk Factors
Risks Relating to Our Business
| ● | We may not be able to maintain or increase the market share or reputation of our software and other products and services that they need to remain or become a market standard. |
| ● | We may not be successful in continuing to enhance and adapt our software, products and services in line with developments in market technologies and demands. |
| ● | The research and development programs that we are currently engaged in, or that we may establish in the future, may not be successful and our significant investments in these programs may be lost. |
| ● | Existing and increased competition may reduce our revenue and profits. |
| ● | We rely on collaborations with users of our additive manufacturing and related solutions to be present in certain large-scale markets and, indirectly, to expand into potentially high-growth specialty markets. Our inability to continue to develop or maintain these relationships in the future could harm our ability to remain competitive in existing markets and expand into other markets. |
| ● | Our revenue and results of operations may fluctuate. |
| ● | Inflation has had and may continue to have an adverse effect on our results. |
| ● | Demand for additive manufacturing generally and our additive manufacturing software solutions, products and services in particular may not increase adequately, or at all. |
| ● | We are dependent upon sales to certain industries. |
| ● | If our relationships with suppliers, including with limited source suppliers of consumables, were to terminate or our manufacturing arrangements were to be disrupted, our business could be adversely affected. |
3
| ● | The dominant software subscription model in the industrial sector is changing, and we may not be successful in developing and deploying a cloud-based platform to offer our software. |
| ● | We may not be able to successfully adapt our software offering to the changing needs of the additive manufacturing market. |
| ● | We depend on the knowledge and skills of key personnel throughout our entire organization, and if we are unable to retain and motivate them or recruit additional qualified personnel, our operations could suffer. |
| ● | We may need to raise additional capital from time to time in order to meet our growth strategy and may be unable to do so on attractive terms, or at all. |
| ● | As a result of the armed conflict in Ukraine, our supporting operations in Kyiv are expected to continue to be subject to continuous reorganization, uncertainty and instability. |
| ● | Our international operations pose currency risks, which may adversely affect our results of operations and net income. |
| ● | Our international operations subject us to various risks, and our failure to manage these risks could adversely affect our results of operations. |
| ● | We may engage in acquisitions or investments that could disrupt our business, cause dilution to our shareholders and harm our financial condition and results of operations. |
| ● | We may enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships with third parties that may not result in the development of commercially viable products or the generation of significant future revenue. |
| ● | Failure to comply with applicable anti-corruption and trade sanctions legislation could result in fines, criminal penalties and an adverse effect on our business. |
| ● | Errors or defects in our software or other products could cause us to incur additional costs, lose revenue and business opportunities, damage our reputation and expose us to potential liability. |
| ● | We rely on our information technology systems to manage numerous aspects of our business and customer and supplier relationships, and a disruption of these systems could adversely affect our results of operations. |
| ● | A breach of security in our products or computer systems may compromise the integrity of our products, harm our reputation, create additional liability and adversely impact our financial results. |
| ● | If our service center operations are disrupted, sales of our 3D printing services, including the medical devices that we print, may be affected, which could have an adverse effect on our results of operations. |
| ● | Our failure to adequately address current and emerging sustainability risks, including environmental, social and governance (ESG) matters, could have a material adverse effect on our business, financial condition and results of operations. |
Risks Related to Our Materialise Medical Segment and Regulatory Environment
| ● | Our medical business, financial condition, results of operations and cash flows could be significantly and negatively affected by substantial government regulations. |
| ● | Our Materialise Medical segment’s 3D printing operations are required to operate within a quality management system that is compliant with the regulations of various jurisdictions, including the requirements of ISO 13485, and the U.S. Quality System Regulation, which is costly and could subject us to enforcement action. |
4
Risks Related to Our Intellectual Property
| ● | If we are unable to obtain patent protection for our products or otherwise protect our intellectual property rights, our business could suffer. |
Risks Related to the American Depositary Shares (ADSs)
| ● | We do not expect to be a passive foreign investment company for U.S. federal income tax purposes; however, there is a risk that we may be classified as a passive foreign investment company, which could result in materially adverse U.S. federal income tax consequences to U.S. investors. |
Risks Relating to Our Business
We may not be able to maintain or increase the market share or reputation of our software and other products and services that they need to remain or become a market standard.
The additive manufacturing, or 3D printing, industry is rapidly growing on a global scale and is subject to constant innovation and technological change. A variety of technologies compete against one another in our market, which is driven, in part, by technological advances and end-user requirements and preferences, as well as by the emergence of new standards and practices. As the additive manufacturing market evolves, the industry standards that are adopted and adhered to are a function of the inherent qualities of the technology as well as the willingness of members of the industry to adopt them. To remain competitive, we depend in large part on our ability to increase and maintain market share and influence in the industry in order to be recognized as a market standard. Nonetheless, in the future, our influence in setting standards for the additive manufacturing industry may be limited and the standards adopted by the market may not be compatible with our present or future products and services.
We may not be successful in continuing to enhance and adapt our software, products and services in line with developments in market technologies and demands.
Our present or future software, products and services could be rendered obsolete or uneconomical by technological advances by one or more of our present or future competitors, by other technologies or by new customer needs. Our ability to remain competitive will depend, in large part, on our ability to enhance and adapt our current software, product and services to developments in technologies and to new and changing customer needs (including the manufacturing of end use parts and the offering of cloud-based software solutions). We believe that to remain competitive we must continuously enhance and expand the functionality and features of our products, services and technologies. However, there can be no assurance that we will be able to:
| ● | maintain and enhance the market share of our current products, services and technologies; |
| ● | enhance our existing products, services and technologies; |
| ● | develop new products, services and technologies that address the increasingly sophisticated and varied needs of prospective end-users (including in the emerging market of using additive manufacturing for end use parts instead of prototypes and the trend of offering more cloud-enabled software solutions); |
| ● | respond to technological advances and emerging industry standards and practices on a cost-effective and timely basis; |
| ● | adequately protect our intellectual property as we develop new products, services and technologies and anticipate intellectual property claims from third parties. |
5
The research and development programs that we are currently engaged in, or that we may establish in the future, may not be successful and our significant investments in these programs may be lost.
To remain competitive, we invest, and intend to continue to invest, significant amounts in various research and development programs. There can be no assurances, however, that these research and development programs will improve our existing additive manufacturing software solutions, products and services or create new software, products or services. Even if some of these programs are successful, it is possible that the new software, products or services developed from such programs will not be commercially viable, that new 3D printing technologies that we, or others, develop will eventually supplant our current 3D printing technologies, that changes in the manufacturing or use of 3D printers will adversely affect the need or demand for our software, products or services or that our competitors will create or successfully market 3D printing technologies that will replace our solutions, products and services in the market. As a result, any of our software solutions, products or services may be rendered obsolete or uneconomical and our significant investments in all or some of our research and development programs may be lost.
Existing and increased competition may reduce our revenue and profits.
The market segments in which we operate, Materialise Software, Materialise Medical and Materialise Manufacturing, are characterized by vigorous competition, by the entry of competitors with innovative technologies, by consolidation of companies with complementary products, services and technologies, and by entry of large corporations in any one or more of our market segments.
In particular, the barriers to enter the software, medical and industrial markets with 3D printing solutions are decreasing rapidly.
In the Materialise Software segment, the availability of computing devices with continually expanding performance at progressively lower prices contributes to the ease of market entry. Additionally, there are certain open-source software applications that are being offered free of charge or for a nominal fee that can place additional competitive pressure on us. 3D printer manufacturers, which closely work with their customers, may also successfully bundle their own software solutions with their equipment, which may make our independent software solutions obsolete. In addition, companies that have greater financial, technical, sales and marketing and other resources, including market leaders with significant in-house capacities in software development, or existing computer-aided design, or CAD, or computer-aided manufacturing, or CAM, or manufacturing execution system, or MES, software providers, are entering the additive manufacturing market and may very rapidly gain a significant share of the markets that we target (including through the acquisition of startup and scale-up companies that are active in the development and sale of additive manufacturing software tools).
In the Materialise Medical segment, medical device companies are investing in 3D printing solutions that may compete with our software solutions, products and services. Companies that initially rely on us to enter the additive manufacturing market for medical applications may, as they gain experience and as 3D printing technology gains strategic importance, decide to develop their own in-house solutions and enter the market themselves with their own software, products or services, thus becoming competitors and denying us continued access to their distribution channels. In addition, startup and scale-up companies, as well as companies that have greater financial, technical, sales and marketing and other resources, are entering the additive manufacturing market and may very rapidly gain a significant share of the markets that we target.
In the Materialise Manufacturing segment, as additive manufacturing gains importance as a strategic technology, our customers are likely to bring 3D manufacturing in-house and reduce or even discontinue using our 3D printing services. In addition, competitors with more efficient or profitable business models, superior techniques or more advanced technologies may take market share away from us. Also, in certain specific markets that our Materialise Manufacturing segment targets, including, among others, the shoe wear, eyewear and fixtures markets, established players may develop their own competitive solutions or may engage in collaborations with competitors of ours, preventing us from gaining a viable position in these markets.
Because of these and other factors, competitive conditions in the industry are likely to intensify in the future. Increased competition could result in price reductions, reduced revenue and operating margins and loss of market share, any of which would likely harm our results of operations.
6
We rely on collaborations with users of our additive manufacturing and related solutions to be present in certain large-scale markets and, indirectly, to expand into potentially high-growth specialty markets. Our inability to continue to develop or maintain these relationships in the future could harm our ability to remain competitive in existing markets and expand into other markets.
Our strategy includes entering into collaborations with our customers in certain large-scale markets and leveraging these collaborations to enter into other underserved specialty markets. In the medical market, we have entered into collaborations with DePuy Synthes Companies of Johnson & Johnson, or DePuy Synthes, and Zimmer Biomet Holdings, Inc., or Zimmer Biomet, as well as with Encore Medical, L.P. (d/b/a Enovis), or Enovis, Limacorporate Spa, or Lima, Mathys AG, or Mathys (which is now part of the same group as Enovis), Smith & Nephew Inc., or Smith & Nephew, Corin Ltd, or Corin, Medtronic Inc., or Medtronic, and Abbott Laboratories Inc., or Abbott. Increased adoption of our software, products and services, especially in potentially high-growth specialty markets, will depend in part on our current and future collaborators’ willingness to continue to adopt our additive manufacturing and other solutions in their markets and on our ability to continue to collaborate with these and other players. Certain of our customers that have initially relied on our 3D printing software and services have announced their intention to bring their 3D printing operations in-house and enter the market themselves, and other customers may also do so in the future as they gain experience and as 3D printing technology gains strategic importance, thus denying us continued access to their distribution channels. In addition, a change of control of any of our collaboration partners may negatively impact our relationship. If we are not able to maintain our existing collaborations and develop new collaborative relationships, our foothold in larger markets and expansion into potentially high-growth specialty markets could be harmed significantly.
Our revenue and results of operations may fluctuate.
Our revenue and results of operations may fluctuate from quarter-to-quarter and year-to-year and are likely to continue to vary due to a number of factors, many of which are not within our control. You should not rely on our past results as an indication of our future performance.
Fluctuations in our results of operations and financial condition may occur due to a number of factors, including, but not limited to, those listed below and those identified throughout this annual report:
| ● | our ability to continue, renew or replace relationships with key customers; |
| ● | the degree of market acceptance of our software and our products; |
| ● | the mix of software, products and services that we sell during any period, as well as the mix of the various markets in which we make sales during said periods; |
| ● | a decline in new or renewed licenses or maintenance contracts for our software, including from customers refusing to transition from perpetual to annual licensing models for our software or disruptions related to our deployment of cloud-based software solutions; |
| ● | delays in the introduction of new features; |
| ● | the entry of new competitors into our market; |
| ● | the development and degree of market acceptance of new competitive systems or processes by others; |
| ● | changes in our pricing policies or those of our competitors, including our responses to price competition; |
| ● | changes in the amount we spend in our marketing and other efforts; |
| ● | delays between our expenditures to develop, acquire or license new technologies and processes, and the generation of sales related thereto; |
| ● | the amounts we spend on, and the success rate of, our research and development activities; |
7
| ● | changes in the regulatory environment, including changes in regulatory laws and regulations, and the interpretation thereof, applicable to our software programs, products or services; |
| ● | delays in obtaining regulatory approval for our products, services or software programs; |
| ● | interruptions to or other problems with our website and interactive user interface, information technology systems, manufacturing processes or other operations; |
| ● | general macroeconomic and industry conditions that affect end-user demand and end-user levels of product design and manufacturing, including the adverse effects of global macroeconomic uncertainties including those related to the armed conflicts in Ukraine, Israel and the Middle East and the ongoing geopolitical tensions between the United States and China; and |
| ● | changes in accounting rules and tax laws. |
Inflation has had and may continue to have an adverse effect on our results.
Inflationary pressures negatively impacted our operating margins and net income in fiscal 2022 and 2023, including increasing the costs of labor, energy, materials, and freight. We implemented price increases on many of our products and services in 2022 and 2023. In an effort to mitigate the effects of higher costs related to inflation. However, not all cost increases could be entirely offset, in part due to the delayed effect of price increases in multi-year agreements to which we are a party, where price increases can only be implemented at the renewal date. In addition, in Belgium, the salaries of our employees are indexed to inflation increases by law and, as a result, in can be difficult to keep our sales prices aligned with increases in our labor costs. If these inflationary pressures continue, our revenue, gross and operating margins and net income may be impacted in fiscal 2024 as well, which would harm our results of operations.
Demand for additive manufacturing generally and our additive manufacturing software solutions, products and services in particular may not increase adequately, or at all.
The industrial and medical industries are generally dominated by conventional production methods with limited use of additive manufacturing technology in certain specific instances. If additive manufacturing technology for the production of end use parts does not gain more mainstream market acceptance, the pace by which additive manufacturing technology gains market acceptance does not accelerate or if the marketplace adopts additive manufacturing based on a technology other than the technologies that we currently use or serve (including in the medical, eyewear, footwear and fixtures markets that we target), we may not be able to meet our growth objectives or increase or sustain the level of sales of our additive manufacturing software solutions, products and services, and our results of operations would be adversely affected as a result.
We are dependent upon sales to certain industries.
Our revenue from products is currently relatively concentrated in the industrial and medical industries, and particularly in the automotive/aerospace and orthopedic/cranio-maxillofacial segments within such industries, respectively, and we expect additional growth to come from certain other specific markets, such as the eyewear and footwear markets. To the extent any of these industries experience, or continue to experience, a downturn, our results of operations may be adversely affected. Additionally, if any of these industries or their respective suppliers or other providers of manufacturing services develop new technologies or alternatives to manufacture the products that are currently manufactured using our 3D printing software, products and services, it may adversely affect our results of operations.
If our relationships with suppliers, including with limited source suppliers of consumables, were to terminate or our manufacturing arrangements were to be disrupted, our business could be adversely affected.
We purchase consumables and other components that are used in our production from third party suppliers. We currently use only a limited number of suppliers for several of the raw materials that we use for our printing activities. Our reliance on a limited number of vendors involves a number of risks, including:
| ● | potential shortages of some key consumables or other components; |
8
| ● | printed material performance or quality shortfalls, if traceable to particular consumables or other components, since the supplier of the faulty consumable or component cannot readily be replaced; |
| ● | discontinuation of a consumable or other component on which we rely; |
| ● | potential insolvency of these vendors; and |
| ● | reduced control over delivery schedules, manufacturing capabilities, quality and costs. |
If certain suppliers were to decide to discontinue production, or the supply to us, of a consumable or other component that we use, the unanticipated change in the availability of supplies, or unanticipated supply limitations, could cause delays in, or loss of, sales, increased production or related costs and, consequently, reduced margins, and damage to our reputation. In addition, because we use a limited number of suppliers, and there is an increasing trend of consolidation among our existing suppliers, the increase in the prices charged by our suppliers may have an adverse effect on our results of operations, as we may be unable to find a supplier who can supply us at a lower price. As a result, the loss of a limited source supplier could adversely affect our relationships with our customers and our results of operations and financial condition.
The dominant software subscription model in the industrial sector is changing, and we may not be successful in developing and deploying a cloud-based platform to offer our software.
We offer most of our current software products through on-premises licensing (either on a perpetual or annual basis). We believe the industrial software market is evolving to Software as a Service, or SaaS, and other cloud-based models of software deployment where software providers typically license their applications to customers for use as a service on demand through web browser technologies. While we are deploying an increasing number of cloud-enabled platform components, through our CO-AM and Mimics Flow platforms to offer our software products either by means of a SaaS or a cloud-based subscription model, there is no guarantee that we will be able to complete this integration successfully or in a timely manner or that our platform will be adopted by customers over other platforms.
A SaaS or cloud-based software offering may differ significantly from the perpetual and annual licensing models that we have offered until recently. An increase in the prevalence of SaaS and cloud-based delivery models offered by us or our competitors could unfavorably impact the pricing of our on-premises software offerings and have a dampening impact on overall demand for our on-premises software product offerings, which could reduce our revenues and profitability. In addition, to the extent that demand for our SaaS or cloud-based offerings increases in the future, we may experience volatility in our reported revenues and operating results due to the differences in timing of revenue recognition between our perpetual and annual software licenses and our SaaS and cloud-based offering arrangements.
Furthermore, the SaaS and cloud-based software products we offer reside upon and are hosted by third party providers. A security breach, whether of our products, of our customers’ network security and systems or of third party hosting services, could disrupt access to our customers’ stored information and could lead to the loss of, damage to or public disclosure of our customers’ stored information.
We may not be able to successfully adapt our software offering to the changing needs of the additive manufacturing market.
While the current proto-typing market that we serve with our software solutions (in particular the Magics 3D Print Suite) is not expected to disappear, the main growth in additive manufacturing is expected to come from the use of 3D printing for the production of end use parts. While we are investing significantly in the expansion of our current software product portfolio to also serve the needs of this new and growing market (in particular, with the development of our CO-AM platform), there can be no certainty that our new software offering will adequately serve the needs of this new market, will be operational in time to address these market needs, will be well received by the market or will effectively compete with other players in this market.
9
We depend on the knowledge and skills of key personnel throughout our entire organization, and if we are unable to retain and motivate them or recruit additional qualified personnel, our operations could suffer.
Our success depends upon the continued service and performance of key personnel at all levels within our organization, including machine operators, engineers, designers, software developers, salespeople, product managers and senior management, and our ability to identify, hire, develop, motivate and retain qualified personnel in the future. Competition for key employees in our industry is intense and we cannot guarantee that we will be able to retain our personnel or attract new, qualified personnel. We may need to invest significant amounts of cash and equity to attract and retain new employees and we may not realize returns on these investments. The loss of the services of key personnel could prevent or delay the implementation and completion of our strategic objectives, could divert management’s attention to seeking certain qualified replacements or could adversely affect our ability to manage our company effectively. Each member of our personnel may resign at any time. Only some of the members of our personnel are subject to non-competition agreements, which may also be difficult to enforce. Accordingly, the adverse effect resulting from the loss of certain member of our key personnel could be compounded by our inability to prevent them from competing with us. We do not carry key-man insurance on any member of our senior management team or other key personnel. If we lose the ability to hire and retain key executives and employees with a diversity and high level of skills in appropriate domains (such as research and development and sales), it could have a material adverse impact on our business activities and results of operations.
In addition, the success of our acquisitions may depend in part on our ability to retain senior management and other key personnel of the acquired company following the acquisition and to continue to attract such persons to our company. For example, the companies we acquire may depend on small teams of founders and senior managers with extensive market knowledge and relationships or that exercise substantial influence over the acquired business. As result, the loss of such persons could adversely affect us.
We may need to raise additional capital from time to time in order to meet our growth strategy and may be unable to do so on attractive terms, or at all.
We intend to continue to make investments to support the growth of our business and may require additional funds to respond to business challenges, including the need to implement our growth strategy, increase market share in our current markets or expand into other markets, or broaden our technology, intellectual property or service capabilities. Accordingly, we may require additional investments of capital from time to time, and our existing sources of cash and any funds generated from operations may not provide us with sufficient capital. For various reasons, including the current macroeconomic environment or any noncompliance with existing or future lending arrangements, additional financing, may not be available when needed, or may not be available on terms favorable to us. If we fail to obtain adequate capital on a timely basis or if capital cannot be obtained on terms satisfactory to us, we may not be able to achieve our planned rate of growth, which will adversely affect our results of operations.
Our international operations subject us to various risks, and our failure to manage these risks could adversely affect our results of operations.
We face significant operational risks as a result of doing business internationally, including, among others:
| ● | fluctuations in foreign currency exchange rates; |
| ● | potentially longer sales and payment cycles; |
| ● | potentially greater difficulties in collecting accounts receivable; |
| ● | potentially adverse tax consequences, including liabilities imposed from inconsistent enforcement; |
| ● | challenges in providing solutions across a significant distance, in different languages and among different cultures; |
| ● | the impact of global public health crises, pandemics and epidemics; |
| ● | transportation delays; |
| ● | becoming subject to the different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations; |
10
| ● | reduced protection of, or significant difficulties in enforcing, intellectual property rights in certain countries; |
| ● | difficulties in staffing and managing foreign operations, particularly in new geographic locations; |
| ● | restrictions imposed by local labor practices and laws on our business and operations, including unilateral cancellation or modification of contracts; |
| ● | expropriation or nationalization of property; |
| ● | rapid changes in global government, economic and political policies and conditions, political or civil unrest or instability, terrorism or pandemics, epidemics and other similar outbreaks or events, such as the armed conflicts in Ukraine, Israel and the Middle East and the ongoing geopolitical tensions between the United States and China; |
| ● | operating in countries with a higher incidence of corruption and fraudulent business practices; |
| ● | seasonal reductions in business activity in certain parts of the world, particularly during the summer months in Europe; |
| ● | costs and difficulties of customizing products for foreign countries; and |
| ● | tariffs, export controls, trade barriers and other regulatory or contractual limitations on our ability to sell or develop our products in certain foreign markets. |
As a result of the armed conflict in Ukraine, our supporting operations in Kyiv are expected to continue to be subject to continuous reorganization, uncertainty and instability.
We have an office in Kyiv, Ukraine where more than 400 of our collaborators are mainly engaged in engineering, software development and IT support, as well as other staff functions. The invasion of Ukraine by the Russian Federation on February 24, 2022, has impacted our operations in Kyiv significantly.
Although our operations in Kyiv nearly ceased in the first quarter of 2022, we have since been able to gradually reorganize the internal services provided from that region through a combination of measures, including Ukrainian collaborators who have fled to other regions in their country now working from home, support provided by existing (and often enlarged) Materialise teams in other regions, the relocation of a number of Ukrainian collaborators outside of Ukraine, and, circumstances permitting, services provided from our Kyiv office, which we have re-opened and accommodated to try to cope with the challenges resulting from the continuous military strikes on key infrastructure in the country.
While our people in Ukraine have shown, and continue to show, incredible resilience and professionalism, the situation in Ukraine remains unstable and uncertain and is expected to continue to have an impact on our operations, both financially and operationally. We expect that, as long as the armed conflict continues (and possibly for a period thereafter), this impact will continue and may even worsen, depending on the developments both geo-politically and in Ukraine. The ongoing additional mobilization for the Ukrainian army may also impact our operations. Although we are presently determined to continue to flexibly support our operations in Kyiv and at present do not see any reason to revise that strategy, we constantly monitor and evaluate the situation. Any change in strategy may have an additional negative impact on our results of operations and financial condition.
We are unable to predict how the armed conflict in Ukraine will evolve and what the further political and economic repercussions will be. As a result, we are unable to assess with certainty its future impact on our business and operations, results of operations, financial condition, cash flows and liquidity. In particular, although we have included under “Item 5. Operating and Financial Review and Prospects—D. Trend Information” of this annual report a discussion, based on our current assessment of the armed conflict in Ukraine, of how our business, results of operations, and financial condition could be impacted during fiscal 2024, this discussion should be considered as uncertain. While we expect to suffer adverse effects, the severity is currently impossible to assess.
11
Our international operations pose currency risks, which may adversely affect our results of operations and net income.
Our results of operations may be affected by volatility in currency exchange rates and our ability to effectively manage our currency transaction risks. In general, we conduct our business, earn revenue and incur costs in the local currency of the countries in which we operate. During the year ended December 31, 2023, 66% of our revenue was generated, and approximately 77% of our total costs were incurred in euros. As we continue to expand internationally, our exposure to currency risks may increase. Historically, although we seek to monitor the ratio of revenues to expenses in certain foreign currencies, we have not managed all our foreign currency exposure in a manner that would eliminate the effects of changes in foreign exchange rates. Changes in exchange rates between the foreign currencies in which we do business and the euro will affect our revenue, cost of sales, and operating margins, and could result in exchange losses in any given reporting period.
Changes in tax laws, treaties or regulations could adversely affect our financial results.
Our future effective tax rates could be adversely affected by changes in tax laws, treaties and regulations, both internationally and domestically, including possible changes to the innovation income deduction regime in Belgium or the way it proportionately impacts our effective tax rate. An increase of our future effective tax rates could have a material adverse effect on our business, financial position, results of operations and cash flows.
We may engage in acquisitions or investments that could disrupt our business, cause dilution to our shareholders and harm our financial condition and results of operations.
In the past, we have acquired or invested in companies that we believe have products, services, competencies or capabilities that are a strategic or commercial fit with any of our businesses or that otherwise offer opportunities for us, and we intend to continue evaluating opportunities to do so.
In connection with acquisitions or investments, we may:
| ● | issue American Depositary Shares, or ADSs, or other forms of equity that would dilute our existing shareholders’ percentage of ownership; |
| ● | incur debt and assume liabilities; and/or |
| ● | incur amortization expenses related to intangible assets or incur large and immediate write-offs. |
If we complete an acquisition or investment, we cannot assure that it will ultimately strengthen our competitive position or that it will be viewed positively by customers, suppliers, employees, financial markets or investors. Furthermore, future acquisitions or investments could pose numerous additional risks to our operations, including:
| ● | problems integrating the purchased business, products, services or technologies; |
| ● | challenges in achieving strategic objectives, cost savings and other anticipated benefits; |
| ● | increases to our expenses; |
| ● | the potential write down of assets or goodwill acquired in the context of an acquisition or investment; |
| ● | due diligence investigations failing to discover undisclosed liabilities or risks affecting the acquired businesses; |
| ● | the assumption of significant liabilities that exceed the limitations of any applicable indemnification provisions or the financial resources of any indemnifying party; |
| ● | inability to maintain relationships with key customers, vendors and other business partners of our current or acquired businesses; |
| ● | diversion of management’s attention from their day-to-day responsibilities; |
12
| ● | difficulty in maintaining controls, procedures and policies during the transition and integration; |
| ● | entrance into marketplaces where we have no or limited prior experience and where competitors have stronger marketplace positions; |
| ● | potential loss of key employees, particularly those of the acquired entity; and |
| ● | historical financial information may no longer be representative or indicative of our results as a combined company. |
Alternatively, while certain acquisitions or investments may be of strategic importance for the execution of our business plan, we may not ultimately be able to complete such acquisitions or investments on favorable terms, or at all, which may in turn materially affect our ability to grow or even cause us to lose market share, and could have a material adverse effect on our business, financial condition and results of operations.
We may enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships with third parties that may not result in the development of commercially viable products or the generation of significant future revenue.
In the ordinary course of our business, we enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships to develop proposed products or services and to pursue new markets. Proposing, negotiating and implementing collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships may be a lengthy and complex process. Other companies, including those with substantially greater financial, marketing, sales, technology or other business resources, may compete with us for these opportunities or arrangements. We may not succeed in maintaining, renewing or extending existing collaborations or in identifying, securing, or completing any such new transactions or arrangements in a timely manner, on a cost-effective basis, on acceptable terms or at all. We may also not realize the anticipated benefits of any such transaction or arrangement. In particular, these collaborations may not result in the development of products or services that achieve commercial success or result in significant revenue and could be terminated prior to developing any products or services.
Additionally, we may not be in a position to exercise sole decision-making authority regarding the transaction or arrangement, which could create the potential risk of creating impasses on decisions, and our collaboration partners may have economic or business interests or goals that are, or that may become, inconsistent with our economic or business interests or goals. It is possible that conflicts may arise with our current or future collaboration partners, such as conflicts concerning the achievement of performance milestones, or the interpretation of terms under any agreement, such as those related to financial obligations, the ownership or license rights or control of intellectual property developed before or during the collaboration or indemnification. If any conflicts arise with our current or future collaboration partners, they may act in their self-interest, which may be adverse to our best interest, and they may breach their obligations to us. In addition, we have limited control over the amount and timing of resources that our current collaboration partners or any future collaboration partners devote to our collaboration partners’ or our future products or services. Disputes with our collaboration partners may result in litigation or arbitration that would increase our expenses and divert the attention of our management. Further, these transactions and arrangements are contractual in nature and may be terminated or dissolved under the terms of the applicable agreements and, in such event, we may not continue to have rights to the products or access to the markets relating to such transaction or arrangement or may need to purchase such rights at a premium.
Failure to comply with applicable anti-corruption and trade sanctions legislation could result in fines, criminal penalties and an adverse effect on our business.
We operate in a number of countries throughout the world and we are committed to doing business in accordance with applicable anti-corruption laws. We are subject, however, to the risk that our officers, directors, employees, agents and collaboration partners may take action determined to be in violation of such anti-corruption laws, as well as trade sanctions administered by the Office of Foreign Assets Control and the U.S. Department of Commerce. Any such violation could result in substantial fines, sanctions, civil and/or criminal penalties or curtailment of operations in certain jurisdictions and might adversely affect our results of operations. In addition, actual or alleged violations could damage our reputation and ability to do business.
13
Errors or defects in our software or other products could cause us to incur additional costs, lose revenue and business opportunities, damage our reputation and expose us to potential liability.
Sophisticated software and complex 3D printed products may contain errors, defects or other performance problems at any point in the life of the product. If errors or defects are discovered in our current or future software or other products, we may not be able to correct them in a timely manner, or provide an adequate response to our customers. We may therefore need to expend significant financial, technical and management resources, or divert some of our development resources, in order to resolve or work around those defects. We may also experience an increase in our service and warranty costs. Particularly in the medical sector, errors or defects in our software or products could lead to claims by patients against us and our customers and expose us to lawsuits that may damage our and our customers’ reputations. Claims may be made by individuals or by classes of users. Our product liability and related insurance policies may not apply or sufficiently cover any product liability lawsuit that arises from defective software or products. Customers such as our collaboration partners may also seek indemnification for third party claims allegedly arising from breaches of warranties under our collaboration agreements.
Errors, defects or other performance problems in our software or other products may also result in the loss of, or delay in, the market acceptance of our software, our products and related 3D printing or engineering services or postponement of customer deployment. Such difficulties could also cause us to lose customers and, particularly in the case of our largest customers, the potentially substantial associated revenue which would have been generated by our sales to companies participating in our customer’s supply chain. Technical problems, or the loss of a customer with a particularly important global reputation, could also damage our own business reputation and cause us to lose new business opportunities.
We rely on our information technology systems to manage numerous aspects of our business and customer and supplier relationships, and a disruption of these systems could adversely affect our results of operations.
We rely on our information technology systems and databases to manage numerous aspects of our business and to provide analytical information to management. Our information technology systems allow us to, among other things, optimize our software development and research and development efforts, organize our in-house 3D printing services logistics, efficiently purchase products from our suppliers, provide other procurement and logistic services, ship and invoice products to our customers on a timely basis, maintain cost-effective operations and generally provide service to our customers. Our information technology systems are an essential component of our business and growth strategies, and a disruption to or perceived failure in our information technology systems could significantly limit our ability to manage and operate our business efficiently. Although we take steps to secure our information technology systems, including our computer systems, intranet and internet sites, email and other telecommunications and data networks, the security measures we have implemented may not be effective and our systems may be vulnerable to, among other things, damage and interruption from power loss, including as a result of natural disasters, computer system and network failures, loss of telecommunication services, operator negligence, loss of data, security breaches, computer viruses and other disruptive events. Any such disruption could adversely affect our reputation, brand and financial condition.
In addition, during the next few years, we expect to gradually replace a number of our information technology systems with new, cloud-based systems. This transformation is intended to further increase our security and data integrity. Disruptions during the configuration, implementation or operation of, or during the migration to, these new systems may have an impact on our operations and could adversely affect us.
14
A breach of security in our products or computer systems may compromise the integrity of our products, harm our reputation, create additional liability and adversely impact our financial results.
We make significant efforts to maintain the security and integrity of our product source code and computer systems. The risk of a security breach or disruption, particularly through cyber-attack or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. These threats include identity theft, unauthorized access, DNS attacks, wireless network attacks, viruses and worms, advanced persistent threat, application centric attacks, peer-to-peer attacks, phishing, backdoor trojans and distributed denial of service attacks. Any of the foregoing could attack our products and computer systems. Despite significant efforts to create and continuously reinforce the security barriers to such programs, it is virtually impossible for us to entirely eliminate this risk. Like all software products and computer systems, our software products and computer systems are vulnerable to such cyber-attacks, and our computer systems have been subject to certain cyber security incidents in the past. The impact of cyber-attacks could disrupt the proper functioning of our software products and computer systems, cause errors in the output of our or our customers’ work, allow unauthorized access to sensitive, proprietary or confidential information of our company, our customers or the patients that we and our customers serve through our medical solutions. Moreover, as we continue to invest in new lines of products and services we are exposed to increased security risks and the potential for unauthorized access to, or improper use of, the information of our product and service users. If any of the foregoing occur, our reputation may suffer, customers may stop buying our products or services, we could face lawsuits and potential liability, and our results of operations could be adversely affected.
As noted above, any security compromise that causes an apparent privacy violation could also result in legal claims or proceedings; liability under various laws and regulations that regulate the privacy, security, or breach of personal information; and related regulatory penalties. See “—We face potential liability related to the privacy and security of personal information we collect.” below for more information. Moreover, the landscape of laws, regulations, and industry standards related to patient health and other private information, data privacy and cybersecurity is evolving globally. We may be subject to increased compliance burdens by regulators and our customers and the patients that we and our customers serve, as well as additional costs to oversee and monitor security risks. Many jurisdictions have enacted laws mandating companies to inform individuals, shareholders, regulatory authorities, and others of security breaches. For example, the SEC recently adopted cybersecurity risk management and disclosure rules, which require the disclosure of information pertaining to cybersecurity incidents and cybersecurity risk management, strategy, and governance. In addition, certain of our customer agreements may require us to promptly report security breaches involving their data on our systems or those of subcontractors processing such data on our behalf. This mandatory disclosure can be costly, harm our reputation, erode customer trust, and require significant resources to mitigate issues stemming from actual or perceived security breaches.
We rely on third-party technology, platform, carriers, server and hardware providers and as well as local servers, and a failure of service by these providers or by our local servers could adversely affect our business and reputation.
We use third party cloud providers to host a major part of our servers as well as to host our SaaS and cloud-based software applications. If these providers are unable to handle current or higher volumes of use, experience any interruption in operations or cease operations for any reason or if we are unable to agree on satisfactory terms for a continued hosting relationship, we would be forced to enter into a relationship with other service providers or assume these hosting responsibilities ourselves. Moreover, breaches of our customers’ data caused by errors, omissions or hostile acts of third parties within the third party hosted environment are beyond our control, yet we would remain responsible for such data security incidents from a regulatory standpoint, in some instances. We may also be limited in our remedies against our third party hosting providers in the event of a failure of service. A failure or limitation of service or available capacity by our third party hosting providers could adversely affect our business and reputation.
In addition to using third party cloud providers, we have also established local servers and infrastructure in multiple offices, including in Leuven. A failure of these local servers could adversely affect our business and reputation.
15
We develop and offer online software services through our SaaS and cloud-based software applications where we manage data we receive from our customers, and a cybersecurity breach of these online services could harm our customers and our reputation, expose us to liability, and adversely impact our business, financial condition and results of operations.
We are in an ongoing transition from distributing desktop software applications to developing and distributing online software services through our SaaS and cloud-based software applications. This transition comes with a shift in cybersecurity responsibilities from the customer to us, since we manage data we receive from our customers and may be responsible to our customers for breaches of their data. This shift in responsibilities requires us to implement appropriate internal changes and to invest in additional cybersecurity capabilities (including training, tooling, and processes). However, cybersecurity incidents and malicious internet-based activity continue to increase generally, and providers of cloud-based services have frequently been targeted by such attacks. We may be unable to anticipate or prevent techniques used to obtain unauthorized access or to sabotage systems because they change frequently and often are not detected until after an incident has occurred. If sensitive customer information is lost, improperly disclosed or threatened to be disclosed, our reputation could be harmed, we could incur significant costs associated with remediation and the implementation of additional security measures, we may incur significant liability and financial loss, and we may be subject to regulatory scrutiny, investigations, proceedings, and penalties. In addition, certain of our customers are large and highly regulated, and if any of them were to conclude that our systems and procedures are insufficiently rigorous, they could terminate their relationships with us, and our financial condition, results of operations and business could be adversely affected.
In addition, the SaaS and cloud-based software applications business is a highly dynamic market with rapidly evolving regulatory requirements, and we need to continually improve our cybersecurity controls to ensure continued compliance. We are investing in information security and privacy certifications to meet these evolving requirements. However, given the rapidly evolving nature of the regulatory landscape (e.g., the Cybersecurity Maturity Model Certification program of the U.S. Department of Defense, the EU-wide NIS2 directive, the upcoming EU-wide Cyber Resilience Act), we may be unable to ensure timely compliance with these requirements, which may adversely impact our business, financial condition and results of operations.
We may not be successful in our artificial intelligence and machine learnng initiatives, which could adversely affect our business, reputation or financial results.
We have recently begun incorporating generative artificial intelligence (or AI) and machine learning (or ML) into our programs and platforms, particularly in the Materialise Medical segment. As with many innovations, AI and ML present risks, challenges and unintended consequences that could impact our successful ability to incorporate the use of AI and ML in our business. For example, our algorithms may be flawed and not achieve sufficient levels of accuracy or contain biased information. In addition, our competitors or other third parties may incorporate AI and ML solutions into their platforms more successfully than us, and their AI and ML solutions may achieve higher market acceptance than ours, which may result in us failing to recoup our investments in developing ML and AI-powered offerings. We have made and expect to continue to make significant investments in our AI and ML technology. Our ability to employ AI and ML, or any ability of our competitors to do so more successfully, may negatively impact our business, impair our ability to compete effectively, result in reputational harm and have an adverse impact on our operating results.
Moreover, our use of AI and ML may give rise to litigation risk, including potential intellectual property or privacy liability. Because AI is an emerging technology, there is not a mature body of case law construing the appropriateness of certain of its uses of data – whether through the employment of large language models or other models leveraging data found on the internet – and the evolution of this law may limit our ability to exploit artificial intelligence tools, or expose us to litigation. Further, AI and ML presents emerging ethical issues and if our use of AI and ML algorithms draws controversy due to their perceived or actual impact on society, we may experience brand or reputational harm, competitive harm or legal liability.
In addition, given the complex nature of AI and ML technology, we face an evolving regulatory landscape and significant competition from other companies, some of which have longer operating histories and significantly greater financial, technical, marketing, distribution, professional services, or other resources than us. For example, the European Union’s Artifical Intelligence Act (or the AI Act) – the world’s first comprehensive AI law – is anticipated to enter into force in the spring of 2024 and, with some exceptions, become effective 24 months thereafter. This legislation imposes significant obligations on providers and deployers of high risk AI systems, and encourages providers and deployers of AI systems to account for E.U. ethical principles in their development and use of these systems. If we develop or use AI or ML systems that are governed by the AI Act, it may necessitate ensuring higher standards of data quality, transparency, and human oversight, as well as adhering to specific and potentially burdensome and costly ethical, accountability, and administrative requirements. Any of the foregoing could adversely affect our business, reputation, or financial results.
16
If businesses do not continue to adopt our platform for any of the reasons discussed above or for other reasons not contemplated, our sales would not grow as quickly as anticipated, or at all, and our business, operating results, and financial condition would be adversely affected.
Workplace accidents or environmental damage could result in substantial remedial obligations and damage to our reputation.
Accidents or other incidents that occur at our service centers and other facilities or involve our personnel or operations could result in claims for damages against us. In addition, in the event we are found to be financially responsible, as a result of environmental or other laws or by court order, for environmental damages alleged to have been caused by us or occurring on our premises, we could be required to pay substantial monetary damages or undertake expensive remedial obligations. The amount of any costs, including fines or damages payments that we might incur under such circumstances could substantially exceed any insurance we have to cover such losses. Any of these events, alone or in combination, could have a material adverse effect on our business, financial condition and results of operations and could adversely affect our reputation.
Our operations are subject to environmental laws and other government regulations that could result in liabilities in the future.
We are subject to local environmental laws and regulations governing our operations, including, but not limited to, emissions into the air and water and the use, handling, disposal and remediation of hazardous substances. A certain risk of environmental liability is inherent in our production activities. Under certain environmental laws, we could be held solely or jointly and severally responsible, regardless of fault, for the remediation of any hazardous substance contamination at our service centers and other facilities and the respective consequences arising out of human exposure to such substances or other environmental damage. We may not have been and may not be at all times in complete compliance with environmental laws, regulations and permits, and the nature of our operations exposes us to the risk of liabilities or claims with respect to environmental and worker health and safety matters. If we violate or fail to comply with environmental laws, regulations and permits, we could be subject to penalties, fines, restrictions on operations or other sanctions, and our operations could be interrupted. The cost of complying with current and future environmental, health and safety laws applicable to our operations, or the liabilities arising from past releases of, or exposure to, hazardous substances, may result in future expenditures. Any of these developments, alone or in combination, could have a material adverse effect on our business, financial condition and results of operations.
If our service center operations are disrupted, sales of our 3D printing services, including the medical devices that we print, may be affected, which could have an adverse effect on our results of operations.
We have seven 3D printing service centers in Europe, the United States, Brazil and Japan, including our principal 3D printing service center located in Leuven, Belgium. If the operations of these facilities are materially disrupted, whether by fires or other industrial accidents, extreme weather, natural disasters, labor stoppages, acts of terror, or otherwise, we would be unable to fulfill customer orders for the period of the disruption, we would not be able to recognize revenue on orders, we could suffer damage to our reputation, and we might need to modify our standard sales terms to secure the commitment of new customers during the period of the disruption and perhaps longer. In addition, extreme weather and other natural disasters may become more intense or more frequent as a result of climate change. Depending on the cause of the disruption, we could incur significant costs to remedy the disruption and resume providing 3D printing services. Such a disruption could have an adverse effect on our results of operations.
We could experience unforeseen difficulties in building and operating key portions of our 3D printing infrastructure.
We have designed and built our own 3D printing operations, some of the 3D printer platforms in use and other key portions of our technical infrastructure through which we serve our products and services, and we plan to continue to expand the size of our infrastructure through expanding our 3D printing facilities. The infrastructure expansion we may undertake may be complex, and unanticipated delays in the completion of these projects or availability of components may lead to increased project costs, operational inefficiencies, or interruptions in the delivery or degradation of the quality of our products. In addition, there may be issues related to this infrastructure that are not identified during the design and implementation phases, which may only become evident after we have started to fully utilize the underlying equipment, that could further degrade the user experience or increase our costs.
17
We may not have adequate insurance for potential liabilities, including liabilities arising from litigation.
In the ordinary course of business, we have been, and in the future may be, subject to various product and non-product related claims, lawsuits and administrative proceedings seeking damages or other remedies arising out of our commercial operations, including litigation related to defects in our software or other products. We maintain insurance to cover our potential exposure for a number of claims and losses. However, our insurance coverage is subject to various exclusions, self-retentions and deductibles, may be inadequate or unavailable to protect us fully, and may be cancelled or otherwise terminated by the insurer. Furthermore, we face the following additional risks related to our insurance coverage:
| ● | we may not be able to continue to obtain insurance coverage on commercially reasonable terms, or at all, including with respect to our activities in the medical industry; |
| ● | we may be faced with types of liabilities that are not covered under our insurance policies, such as environmental contamination, terrorist attacks or alleged infringements of third parties’ intellectual property rights, and that exceed any amounts that we may have reserved for such liabilities; |
| ● | the amount of any liabilities that we may face may exceed our policy limits; and |
| ● | we may incur losses resulting from the interruption of our business that may not be fully covered under our insurance policies. |
Even a partially uninsured claim of significant size, if successful or if settled for a substantial amount of money, could have a material adverse effect on our business, financial condition, results of operations and liquidity. However, even if we successfully defend ourselves against any such claim, we could be forced to spend a substantial amount of money in litigation expenses, our management could be required to spend valuable time defending these claims and our reputation could suffer, any of which could adversely affect our results of operations.
Current and future global macroeconomic uncertainties and political conditions may adversely affect our results of operations.
Our results of operations could be substantially affected not only by global economic conditions, but also by local operating, economic, public health or environmental conditions, which can vary substantially across regions. Unfavorable conditions can depress sales in a given market and may result in actions that adversely affect our margins, constrain our operating flexibility or result in charges that are unusual or non-recurring.
Certain macroeconomic events could have a wide-ranging and prolonged impact on the general business environment, which could also adversely affect us. Current macroeconomic events that could impact us include, but are not limited to the following:
| ● | geopolitical instability resulting from, among other factors, the armed conflicts in Ukraine Israel and the Middle East and the ongoing geopolitical tensions between the United States and China; |
| ● | the risk of potential recessions, continued rising interest rates, inflation and labor shortages in Europe and the United States; |
| ● | actual or perceived instability in the global banking system; |
| ● | disruptions caused by global health crises, pandemics and epidemics and related responses thereto in certain economies and markets; and |
| ● | in general, the economic and political challenges faced by, among others, China, certain Eurozone countries and the United States. |
We cannot predict the likely duration and severity of these economic and political developments, which could affect us in numerous ways, many of which we cannot predict. For example, the existence of inflation in certain economies has resulted in, and may continue to result in, rising interest rates and capital costs, supply shortages, increased costs of labor, components, manufacturing and freight costs, as well as weakening exchange rates and other similar effects. As a result of inflation, we have experienced and may continue to experience cost increases. Although we take measures to mitigate the effects of inflation, if these measures are not effective, our business, financial condition, results of operations and liquidity could be materially adversely affected. Even if such measures are effective, there could be a difference between the timing of when those beneficial actions impact our results or operations and when the cost of inflation is incurred.
18
In addition, political and economic developments, including as a result of political elections, could also result in changes to legislation or reformation of government policies, rules and regulations that adversely impact our business, such as changes in policies, rules and regulations related to taxation or trade. Such changes could have a significant impact on our business by increasing the cost of doing business, affecting our ability to sell our software, products and services and negatively impacting our profitability
We face potential liability related to the privacy and security of personal information we collect.
In particular, but not exclusively, in connection with our Materialise Medical segment and the personalized wearables business we are pursuing within our Materialise Manufacturing segment, we may have access to personal information that is subject to a number of U.S. federal and state, E.U. and other applicable foreign laws protecting the confidentiality of certain patient health or other private information, including patient records, and restricting the use and disclosure of that protected information. In addition, in our Materialise Software segment, we collect, transmit, process and store large amounts of proprietary or other sensitive data from our customers through our SaaS and cloud-based software applications, some of which are highly regulated.
In the United States, we are subject to the Health Insurance Portability and Accountability Act, or HIPAA, the Health Information Technology for Economic and Clinical Health Act of 2009, regulations issued pursuant to these statutes, state privacy and security laws and regulations. These statutes, regulations and contractual obligations impose numerous requirements regarding the use and disclosure of personal health information with which we must comply. In addition, we are subject to data privacy and cybersecurity laws such as the California Consumer Privacy Act, or CCPA, as amended and expanded by the California Privacy Rights Act, or CPRA. The CCPA, as amended by the CPRA, requires, among other things, covered companies, including us, to provide new disclosures to California consumers and afford such consumers the ability to opt out of certain sales of personal information. We are undertaking appropriate steps to modify our data processing practices and policies to comply with data privacy and cybersecurity laws and expect to incur substantial costs and expenses in an effort to comply with such laws, including in connection with our development and deployment of SaaS and cloud-based software solutions.
In the European Union, the General Data Protection Regulation, or the GDPR, was passed on May 24, 2016, and replaced the E.U. Data Protection Directive when it came into force on May 25, 2018. GDPR introduced new data protection requirements in the European Union, unprecedented regulatory risk for non-compliant data processors and controllers and sizeable penalties for serious breaches—up to €20 million or 4% of global turnover, whichever is higher. The GDPR also significantly expands the territorial reach of existing E.U. data protection and privacy rules. Our business processes have been and continue to be modified in order to incorporate the requirements of the GDPR. In addition, in connection with its withdrawal from the European Union, the United Kingdom implemented the GDPR as of January 1, 2021 (as it existed on December 31, 2020 but subject to certain U.K.-specific amendments), or U.K. GDPR.
In ensuring continued compliance with the E.U. regime, our transfer of any personal data from the European Union to the United States must be done in a manner which satisfies E.U. cross-border data transfer requirements. The E.U.-U.S. Privacy Shield, which had been adopted by the United States and the European Union as a framework for protecting the fundamental rights of anyone in the European Union whose personal data is transferred to the United States for commercial purposes, was subsequently invalidated by the European Court of Justice on July 16, 2020 for not meeting E.U. regulatory requirements. On July 10, 2023, the European Commission adopted its adequacy decision for the E.U.-U.S. Data Privacy Framework. The decision concludes that the United States ensures an adequate level of protection – comparable to that of the European Union – for personal data transferred from the European Union to U.S. companies under the new framework. On the basis of the new adequacy decision, personal data can flow safely from the European Union to U.S. companies participating in the E.U.-U.S. Data Privacy Framework, without having to put in place additional data protection safeguards. The adequacy decision followed the adoption of Executive Order on “Enhancing Safeguards for United States Signals Intelligence Activities” by U.S. President Biden on October 7, 2022, and a regulation issued by the U.S. Attorney General. These measures introduced new binding safeguards to address the points raised by Court of Justice of the European Union in its Schrems II decision of July 2020, ensuring that data can be accessed by U.S. intelligence agencies only to the extent necessary and proportionate and establishing an independent and impartial redress mechanism to handle and resolve complaints from Europeans concerning the collection of their data for national security purposes.
The safeguards that have been put in place by the U.S. government in the area of national security (including the redress mechanism) apply to all data transfers under the GDPR to companies in the United States, regardless of the transfer mechanisms used. These safeguards therefore also facilitate the use of other tools, such as standard contractual clauses.
We are investigating and are undertaking appropriate steps to mitigate the risks associated with these evolving data privacy laws and data transfer requirements.
19
In addition, the use and disclosure of personal health and other private information is subject to regulation in other jurisdictions in which we do business or expect to do business in the future. Those jurisdictions may attempt to apply such laws extraterritorially or through treaties or other arrangements with European governmental entities. We might unintentionally violate such laws, such laws may be modified and new laws may be enacted in the future which may increase the chance that we violate them. For example, each of the GDPR and the U.K. GDPR contains rules relating to the collection and processing of personal information, which are not identical to the current rules under national privacy laws and which contain more strict provisions. Any such developments, or developments stemming from enactment or modification of other laws, or the failure by us to comply with their requirements or to accurately anticipate the application or interpretation of these laws could create material liability to us, result in adverse publicity and negatively affect our medical business.
Our failure to accurately anticipate the application or interpretation of these statutes, regulations and contractual obligations as we develop our medical and other products and services, a failure by us to comply with their requirements (e.g., evolving encryption and security requirements) or an allegation that defects in our medical or other products have resulted in noncompliance by our customers could create material civil and/or criminal liability for us, resulting in adverse publicity and negatively affecting our medical business. Any legislation or regulation in the area of privacy and security of personal information could affect the way we operate and could harm our business. The costs of compliance with, and the other burdens imposed by, these and other laws or regulatory actions may prevent us from selling our solutions or increase the costs associated with selling our products and services, and may affect our ability to invest in or jointly develop our products and services in the United States, the European Union and in foreign jurisdictions. Further, we cannot assure you that our privacy and security policies and practices will be sufficient to protect us from liability or adverse publicity relating to the privacy and security of personal information.
Our failure to adequately address current and emerging sustainability risks, including environmental, social and governance (ESG) matters, could have a material adverse effect on our business, financial condition and results of operations.
Our ability to ensure a resilient business that delivers long-term sustainable growth, is reliant on our ability to identify current and emerging sustainability risks and legislative requirements that could adversely impact our business and ensure appropriate strategies are in place to manage such risks and requirements. Some of the key risks and requirements include:
| ● | Growing expectations of how businesses respond to and address sustainability issues from customers, non-governmental organizations, ESG-focused investors and other stakeholders. The failure to meet these expectations can have adverse consequences, such as: active product delisting, negative non-governmental organization campaigns, loss of market share, omission from sustainability indices and adverse public perception or publicity; |
| ● | Increased mandatory sustainability due-diligence and non-financial reporting and disclosure obligations, requiring businesses to take appropriate action or face regulatory penalties. This includes the SEC’s recently adopted climate disclosure rules, as well as laws and regulations in the countries where we operate, such as the E.U. Corporate Sustainability Due Diligence Directive, the E.U. Corporate Sustainability Reporting Directive, the German Supply Chain Due Diligence Act, California’s Voluntary Carbon Market Disclosures Act, the Task Force on Climate Related Financial Disclosure (TCFD) and the proposed Task Force on Nature Related Financial Disclosures (TNFD). |
| ● | Physical risks of climate change, such as increased frequency of extreme weather and natural disasters, causing damage to physical assets within our operations and our supply chain. |
Any of the above risks, together with any others which relate to our inability to address increased and emerging sustainability risks, could have a material adverse effect on our business, financial condition and results of operations. Further, our efforts to address current and emerging sustainability requirements could result in increased costs and divert management’s attention and resources from our business.
20
Risks Related to Our Materialise Medical Segment and Regulatory Environment
Our medical business, financial condition, results of operations and cash flows could be significantly and negatively affected by substantial government regulations.
Our medical products are subject to rigorous regulation by the European Commission, the U.S. Food and Drug Administration, or the FDA, and numerous other applicable governmental authorities. In general, the development, testing, manufacturing and marketing of our medical products are subject to extensive regulation and review by numerous governmental authorities in the European Union, the United States, the United Kingdom, Canada, Brazil, Japan and Australia, and in other markets where we are currently active or may become active in the future. The regulatory process requires the expenditure of significant time, effort and expense to bring new medical products to market, and we cannot be certain that we will receive regulatory approvals, certifications or registrations in any country in which we plan to market our medical products.
The laws and regulations, including the requirements for approvals, certifications or registrations and the time required for regulatory review, vary from country to country. For example, to market our medical products within the member states of the European Union, we are required to comply with the European Medical Device Directive. Under the European Medical Device Directive, all medical devices except custom-made and investigational devices must bear the CE mark. To obtain authorization to affix the CE mark to our medical products, a recognized European notified body must assess our quality systems and the product’s conformity to the requirements of the European Medical Device Directive. This process has been impacted by the general lack of capacity of notified bodies properly designated under the E.U. Medical Device Regulation, which became effective on May 26, 2021. These issues may delay the (re)certification and commercialization of our new or updated medical products in the European Economic Area, or EEA. Similarly, in the United States, we are required to obtain clearance or approval from the FDA prior to marketing our medical products.
The regulatory approval process outside the European Union and the United States may include all of the risks associated with obtaining CE or FDA clearance or approval in addition to other risks. Clearance or approval by the FDA in the United States, or conformity assessment and affixing a CE mark in the EEA does not ensure approval or certification by regulatory authorities in other countries, and approval or certification by one foreign regulatory authority does not ensure approval by regulatory authorities in other countries. We may be required to perform additional pre-clinical or clinical studies even if FDA clearance or approval, or the right to bear the CE label, has been obtained. We may not obtain regulatory approvals or certifications outside the European Union and the United States on a timely basis, if at all. If we fail to receive necessary approvals to commercialize our medical products in jurisdictions outside the European Union and the United States on a timely basis, or at all, our medical business, financial condition and results of operations could be adversely affected.
As a manufacturer of medical devices, we participate in the Medical Device Single Audit Program, or MDSAP, which is a prerequisite for market entry in Canada, and which makes results from external audits by an accredited auditing organization available to the regulatory authorities of the United States, Canada, Brazil, Japan and Australia. A single audit is used in lieu of multiple separate audits or inspections by participating regulatory authorities or their representatives, reducing the overall number of audits or inspections. However, the auditing organization must inform regulatory authorities directly when certain non-conformity thresholds are reached, enabling participating regulatory authorities to immediately undertake actions appropriate for their jurisdictions.
In addition, we are required to implement and maintain stringent reporting, labelling and record keeping procedures and make our facilities and operations subject to periodic inspections, both scheduled and unannounced, by the regulatory authorities. The medical device industry is also subject to a myriad of complex laws and regulations governing reimbursement, which varies from jurisdiction to jurisdiction in the European Union and which includes Medicare and Medicaid reimbursement in the United States as well as healthcare fraud and abuse laws, with these laws and regulations being subject to interpretation. In many instances, the industry does not have the benefit of significant regulatory or judicial interpretation of these laws and regulations. In certain public statements, governmental authorities have taken positions on issues for which little official interpretation was previously available. Some of these positions appear to be inconsistent with common practices within the industry but that have not previously been challenged.
Various governmental agencies have become increasingly vigilant in recent years in their investigation of various business practices. Governmental and regulatory actions against us can result in various actions that could adversely impact our medical operations, including:
| ● | the recall or seizure of products; |
| ● | the suspension or revocation of the authority necessary for the production or sale of a product; |
21
| ● | the delay of our ability to introduce new products into the market; |
| ● | the suspension of shipments from particular manufacturing facilities; |
| ● | the issuance of warning letters or untitled letters; |
| ● | the imposition of operating restrictions; |
| ● | the imposition of injunctions, fines and penalties; |
| ● | the exclusion of our products from being reimbursed by healthcare programs in the European Union or U.S. federal and state healthcare programs (such as Medicare, Medicaid, Veterans Administration health programs and Civilian Health and Medical Program of the Uniformed Services); |
| ● | the delay or denial of customs clearance of our products for import in certain jurisdictions; and |
| ● | other civil or criminal sanctions against us. |
Failure to comply with applicable regulatory requirements could also result in civil actions against us and other unanticipated expenditures. Any of these actions, in combination or alone, or even a public announcement that we are under investigation for possible violations of these laws, could have a material adverse effect on our medical business, financial condition, results of operations and cash flows. If investigated, we cannot assure that the costs of defending or resolving those investigations or proceedings would not have a material adverse effect on our financial condition, results of operations and cash flows.
In many of the countries in which we market our medical products, we are subject to regulations affecting, among other things, clinical efficacy, product standards, packaging requirements, labelling requirements, import/ export restrictions, tariff regulations, duties and tax requirements. Many of the regulations applicable to our medical surgical guides, models, implants and software products in these countries are similar to those of the European Commission and the FDA. In addition, in many countries the national health or social security organizations require our medical products to be qualified before they can be marketed with the benefit of reimbursement eligibility. Failure to receive or delays in the receipt of relevant foreign qualifications also could have a material adverse effect on our medical business, financial condition, results of operations and cash flows.
As the government regulators in the European Union, United States and elsewhere have become increasingly stringent, we may be subject to more rigorous regulation by governmental authorities in the future.
Modifications to our medical products marketed in the United States may require new 510(k) clearances or premarket approvals, or may require us to cease marketing or recall the modified products until clearances are obtained.
Any modification to a 510(k)-cleared device that could significantly affect its safety or efficacy, or that would constitute a major change in its intended use, technology, materials, packaging and certain manufacturing processes, may require a new 510(k) clearance or, possibly, a premarket approval, or PMA. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) clearance or PMA in the first instance, but the FDA may (and often does) review the manufacturer’s decision. The FDA may not agree with a manufacturer’s decision regarding whether a new clearance or approval is necessary for a modification, and may retroactively require the manufacturer to submit a premarket notification requesting 510(k) clearance or an application for PMA. We have made modifications to our medical products in the past and may make additional modifications in the future that we believe did not or will not require additional clearances or approvals. No assurance can be given that the FDA will agree with any of our decisions not to seek 510(k) clearance or PMA. If the FDA requires us to cease marketing and recall the modified device until we obtain a new 510(k) clearance or PMA, our medical business, financial condition, results of operations and future growth prospects could be materially adversely affected. Further, our medical products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective. Any recall or FDA requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
22
Healthcare policy changes, including legislation to reform the U.S. healthcare system, could adversely affect us.
From time to time, legislation is drafted and introduced that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of a medical device. In addition, regulations and guidance are often revised or reinterpreted in ways that may significantly affect our medical business and our medical products. It is impossible to predict whether legislative changes will be enacted or regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
For instance, in 2010, the U.S. Patient Protection and Affordable Care Act, as amended by the U.S. Health Care and Education Reconciliation Act of 2010, or collectively, the PPACA, was enacted, which included, among other things, the following measures: a Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research; reporting and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to prescribers and other healthcare providers, effective March 30, 2013 (referred to as the Physician Sunshine Payment Act); payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models, beginning on or before January 1, 2013; and an independent payment advisory board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. Some of the provisions of the PPACA have yet to be fully implemented, while certain provisions have been subject to U.S. judicial and Congressional challenges. Efforts to repeal and replace the PPACA have been ongoing since the 2016 election, but it is unclear if these efforts will be successful. Since January 2017, former President Trump signed Executive Orders and other directives designed to delay, circumvent or loosen the implementation of certain provisions requirements mandated by the PPACA or otherwise circumvent some of the requirements for health insurance mandated by the PPACA. In addition, as part of the December 2017 Tax Cuts and Jobs Act, the “individual mandate,” which required individuals to purchase insurance, was repealed. Furthermore, in December 2018, a U.S. District Court Judge in the Northern District of Texas ruled that the PPACA is unconstitutional in its entirety because such individual mandate was repealed, although the U.S. District Court Judge and former President Trump, among others, had acknowledged the ruling would have no immediate effect pending appeal. Thus, the full impact of the PPACA, any law repealing or replacing elements of it, and the political uncertainty surrounding any repeal or replacement legislation on our business remains unclear.
We cannot predict what healthcare programs and regulations will be ultimately implemented at the U.S. federal or state level, or at the E.U. level or within the implementing legislation of the individual E.U. Member States, or the effect of any future legislation or regulation. However, these provisions as adopted could meaningfully change the way healthcare is delivered and financed, and may materially impact numerous aspects of our medical business. In particular, any changes that lower reimbursements or reduce medical procedure volumes could adversely affect our medical business and results of operations.
In addition, in the future there may continue to be additional proposals relating to the reform of the healthcare systems of the United States, the European Union, any individual Member State of the European Union or any other jurisdiction where we may operate. For example, the new E.U. Medical Device Regulation became effective on May 26, 2021. The Medical Device Regulation, among other things:
| ● | strengthens the rules on placing devices on the market and reinforce surveillance once they are available; |
| ● | establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| ● | improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; and |
| ● | strengthens rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
Transition from the regulation of our products under the current E.U. regulatory framework to regulation under the Medical Device Regulation may require a substantial transition effort by us. While we have taken the first steps to comply with the Medical Device Regulation’s requirements and obtained CE Certificates of Conformity, any future failure by us to keep our quality system and regulatory documentation in accordance with the Medical Device Regulation’s requirements could delay our further transition to compliance and delay or prevent us from obtaining new CE Certificates of Conformity. As a result, transition from compliance with the current E.U. regulatory framework to the Medical Device Regulation could result in disruption to our business in the European Economic Area, which could adversely affect our business, results of operation and financial condition.
23
Furthermore, initiatives sponsored by government agencies, legislative bodies and the private sector to limit the growth of healthcare costs, including price regulation and competitive pricing, are ongoing in markets where we do business. We could experience a negative impact on our results of operations due to increased pricing pressure in certain or all of the markets in which we operate. Governments, hospitals and other third party payors could reduce the amount of approved reimbursements for our products. Reductions in reimbursement levels or coverage or other cost-containment measures could unfavorably affect our future results of operations.
The use, including the misuse or off-label use, of our medical services and products may be deemed unauthorized use or improper promotion, which could harm our image in the marketplace or result in injuries that lead to product liability suits and could be costly to our business or result in regulatory sanctions.
Medical decisions may only be made and operations may only be executed by trained professionals who are authorized to do so in the jurisdictions in which they operate.
Our medical services and products are generally designed to support surgeons in the planning and performance of their operations. In our medical software products set up, training and engineering support, we make it very clear that responsibility for medical decisions rests exclusively with the responsible surgeon, who is responsible for carefully reviewing and explicitly approving the surgical plan and/or the design of the medical device that is proposed by our software and engineers. Nonetheless, we cannot assure that patients, hospitals, surgeons or other parties will not try to hold us responsible for all or a part of the medical decisions underlying the operations that we support, exposing us to potential litigation or civil and criminal liability for unauthorized medical decision-making. Such actions or liability could lead governmental agencies to conclude that our products or services are used improperly, all of which could significantly damage our reputation and could materially impair the continued adoption of our medical services and product offering in the market.
In the markets in which we operate, our medical promotional materials and training methods must comply with numerous applicable laws and regulations, including the prohibition on the promotion of a medical device for a use that has not been cleared or approved by the relevant regulator or supervisory body. Use of a device outside of its cleared or approved indication is known as “off-label” use. If a relevant governmental authority determines that our medical promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. In that event, our reputation could be damaged and adoption of our medical products would be impaired. Although we train our sales force not to promote our medical products for off-label uses, and our instructions for use in all markets specify that our products are not intended for use outside of those indications cleared for use, competent regulatory agency could conclude that we have engaged in off-label promotion. In addition, there may be increased risk of injury if surgeons attempt to use our medical products off-label.
Surgeons also may misuse our medical products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us. Any of these events could adversely affect our medical business, results of operations and reputation and our ability to attract and retain customers for our products and services.
If our marketed medical devices are defective or otherwise pose safety risks, the relevant governmental authorities could require their recall, or we may initiate a recall of our products voluntarily.
The relevant governmental authorities may require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. Manufacturers, on their own initiative, may recall a product if any material deficiency in a device is found. A government mandated or voluntary recall could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labelling defects or other deficiencies and issues. Recalls of any of our medical products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. Any recall could impair our ability to produce our medical products in a cost-effective and timely manner in order to meet our customers’ demands. We also may be required to bear other costs or take other actions that may have a negative impact on our future revenue and our ability to generate profits. We may initiate voluntary recalls involving our medical products in the future that we determine do not require notification of the relevant regulatory body. If a governmental agency disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our revenue. In addition, the relevant authority could take enforcement action for failing to report the recalls when they were conducted.
24
Alternative medical solutions could outperform the solutions we offer, rendering our solutions obsolete.
Our Materialise Medical segment products and services compete with other innovative technologies that offer similar medical solutions. In addition, many of our competitors are continuing to innovate in the subsegments of the market that we seek to address. For example, our 3D printed surgical guides compete with robotics and navigational solutions, which offer alternative methods to guide a surgeon during an intervention. These current and future alternative technological solutions could outperform the solutions we offer and render our solutions, obsolete.
If our Materialise Medical segment products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, or MDR, we are required to report to the FDA any incident in which our medical product has malfunctioned and would be likely to cause or contribute to a death or serious injury if the malfunction happened again. If we fail to report these events to the FDA within the required timeframes, or at all, the FDA could take enforcement action against us. Any adverse event involving our medical products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
In the EEA, we must comply with the E.U. Medical Device Vigilance System, the purpose of which is to improve the protection of health and safety of patients, users and others by reducing the likelihood of reoccurrence of incidents related to the use of a medical device. Under this system, incidents must be reported to the competent authorities of the Member States of the EEA. An incident is defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labelling or the instructions for use which, directly or indirectly, might lead to or might have led to the death of a patient or user or of other persons or to a serious deterioration in their state of health. Incidents are evaluated by the EEA competent authorities to whom they have been reported, and where appropriate, information is disseminated between them in the form of National Competent Authority Reports. The E.U. Medical Device Vigilance System is further intended to facilitate a direct, early and harmonized implementation of Field Safety Corrective Actions, or FSCAs, across the Member States of the EEA where the device is in use. A FSCA is an action taken by a manufacturer to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. A FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its legal representative to its customers and/or to the end users of the device through Field Safety Notices.
Our Materialise Medical segment’s 3D printing operations are required to operate within a quality management system that is compliant with the regulations of various jurisdictions, including the requirements of ISO 13485, and the U.S. Quality System Regulation, which is costly and could subject us to enforcement action.
We are subject to the regulations of various jurisdictions regarding the manufacturing process for our medical products, including the requirements of ISO 13485. Within the United States, we are required to comply with the Quality System Regulation, which covers, among other things, the methods of documentation of the design, testing, production, control, quality assurance, labelling, packaging, sterilization, storage and shipping of our medical products. Compliance with these regulations is costly and time-consuming. In addition, the FDA enforces the Quality System Regulation through periodic announced and unannounced inspections of manufacturing facilities. The failure by a manufacturer to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, any of the following enforcement actions:
| ● | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| ● | customer notifications or repair, replacement, refunds, recall, detention or seizure of our medical products; |
| ● | operating restrictions or partial suspension or total shutdown of production; |
| ● | refusing or delaying requests for 510(k) clearance or PMA of new products or modified products; |
| ● | withdrawing 510(k) clearances or PMAs that have already been granted; |
25
| ● | refusal to grant export approval for our medical products; or |
| ● | criminal prosecution. |
Any regulatory enforcement actions could impair our ability to produce our medical products in a cost-effective and timely manner in order to meet our customers’ demands. We also may be required to bear other costs or take other actions that may have a negative impact on our future revenue and our ability to generate profits. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our medical products on a timely basis and in the required quantities, if at all.
We may be subject to or otherwise affected by U.S. federal and state, European or other healthcare laws, including fraud and abuse and health information privacy and security laws, and could face substantial penalties if we are unable to fully comply with such laws.
Healthcare regulation by U.S. federal and state, European or other governments could significantly impact our medical business. Healthcare fraud and abuse and health information privacy and security laws potentially applicable to our medical operations include:
| ● | the U.S. federal Anti-Kickback Law, which constrains our marketing practices and those of our independent sales agencies, educational programs, pricing, bundling and rebate policies, grants for physician-initiated trials and continuing medical education, and other remunerative relationships with healthcare providers, by prohibiting, among other things, soliciting, receiving, offering or providing remuneration, intended to induce the purchase or recommendation of an item or service reimbursable under a U.S. federal healthcare program, such as the Medicare or Medicaid programs; |
| ● | U.S. federal false claims laws which prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third party payors that are false or fraudulent; |
| ● | HIPAA, and its implementing regulations, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of individually identifiable health information; |
| ● | U.S. state laws analogous to each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third party payor, including commercial insurers, and state laws governing the privacy and security of certain health information, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts; and |
| ● | similar foreign laws and regulations governing healthcare fraud and abuse, patient data privacy, interactions with healthcare professionals and related laws and regulations that apply to us in the countries in which we operate. |
If our past or present operations are found to be in violation of any of such laws or any other governmental regulations that may apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from U.S. federal healthcare programs and the curtailment or restructuring of our operations. Similarly, if the healthcare providers or entities with whom we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on us. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our medical business and our financial results. The risk of our company being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Further, the PPACA, among other things, amends the intent requirement of the U.S. federal anti-kickback and criminal health care fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the U.S. federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
26
Risks Related to Our Intellectual Property
If we are unable to obtain patent protection for our products or otherwise protect our intellectual property rights, our business could suffer.
We rely on a combination of patents, copyrights, trademarks, trade secrets, confidentiality and other contractual arrangements with our employees, end users and others to maintain our competitive position. Our success depends, in part, on our ability to obtain patent protection for or maintain as trade secrets our proprietary products, technologies and inventions and to maintain the confidentiality of our trade secrets and know-how, operate without infringing upon the proprietary rights of others and prevent others from infringing upon our business proprietary rights.
Despite our efforts to protect our proprietary rights, it is possible that competitors or other unauthorized third parties may obtain, copy, use or disclose or otherwise circumvent our technologies, software, inventions, processes or improvements. We cannot assure investors that any of our existing or future patents or other intellectual-property rights will be enforceable, will not be challenged, invalidated or circumvented, or will otherwise provide us with meaningful protection or any competitive advantage. In addition, our pending patent applications may not be granted, and we may not be able to obtain foreign patents or elect to file applications corresponding to our U.S., European or other patents. We intend to expand our business to certain countries that may not provide the same level of patent or other intellectual-property protection as the United States and the European Union. Even if we assert our patents or obtain additional patent or similar protection in such countries, effective enforcement of such patents or other rights may not be available. If our patents do not adequately protect our technology, our competitors may be able to offer products or services similar to ours or potential customers may gain illegal access to our proprietary technology. Our competitors may also be able to develop similar technology independently or design around our patents, and we may not be able to detect the unauthorized use of our proprietary technology or take appropriate steps to prevent such use. Any of the foregoing events would lead to increased competition and lower revenue or gross margins, which could adversely affect our results of operations.
Moreover, ongoing changes to the U.S. patent laws may impact our ability to obtain and enforce our intellectual-property rights. In recent years, the courts have interpreted U.S. patent laws and regulations differently, and in particular the U.S. Supreme Court has decided a number of patent cases and continues to actively review more patent cases than it has in the past. Some of these changes or potential changes may not be advantageous for us, and may make it more difficult to obtain adequate patent protection or to enforce our patents against parties using them without a license or payment of royalties. These changes could increase the costs and uncertainties surrounding the prosecution of our patent applications and the enforcement or defense of our patent rights, all of which could have a material adverse effect on our business and financial condition.
We may not be able to protect our trade secrets and intellectual property.
While some of our technology is licensed under patents belonging to others or is covered by process patents which are owned or applied for by us, much of our technology is not protected by patents. Furthermore, patents are jurisdictional in nature and therefore only protect us in certain markets, rather than globally. We have devoted substantial resources to the development of our technology, trade secrets, know-how and other unregistered proprietary rights. While we enter into confidentiality and invention assignment agreements intended to protect such rights, such agreements can be difficult and costly to enforce or may not provide adequate remedies if violated. Such agreements may be breached and confidential information may be willfully or unintentionally used or disclosed in violation of the agreements, or our competitors or other parties may learn of the information in some other way. We cannot legally prevent one or more other companies from developing similar or identical technology to our unpatented technology and accordingly, it is likely that, over time, one or more other companies may be able to replicate our technology, thereby reducing our technological advantages. If we do not protect our technology or are unable to develop new technology that can be protected by patents or as trade secrets, we may face increased competition from other companies, which may adversely affect our results of operations.
We may incur substantial costs enforcing or acquiring intellectual property rights and defending against third party claims as a result of litigation or other proceedings.
We have been and may in the future be subject or party, directly or indirectly, to claims, negotiations or complex, protracted litigation, arbitration or post-grant review proceedings in connection with the enforcement of our intellectual property and patent rights.
27
While we strive to avoid infringing the intellectual-property rights of third parties, we cannot provide any assurances that we will be able to avoid any claims, directed against us directly or against our collaboration partners or our other customers, that our products and technology, including the technology that we license from others, infringe the intellectual-property rights of third parties. Patent applications in the United States and most other countries are confidential for a period of time until they are published, and the publication of discoveries in scientific or patent literature typically lags behind the actual discoveries by several months or more. As a result, the nature of claims contained in unpublished patent filings around the world is unknown to us, and we cannot be certain that we were the first to conceive inventions covered by our patents or patent applications or that we were the first to file patent applications covering such inventions. Furthermore, it is not possible to know in which countries patent applicants may choose to extend their filings under the Patent Cooperation Treaty or other mechanisms, such as the European Patent Convention, or to predict the final scope of protection that may result from pending patent applications. Moreover, the patent landscape in the different fields in which we operate is heavily occupied and freedom to operate examinations are costly and time-consuming. We have not obtained extensive freedom to operate reports in the past for each and all of our products and services, nor do we intend to install on a general basis freedom to operate examinations for our future products and services. In addition, we may be subject to intellectual property infringement claims from non-practicing entities, individuals, vendors and other companies, including those that are in the business of asserting patents, but are not commercializing products or services in the different fields in which we operate, or our collaboration partners or our other customers may seek to invoke indemnification obligations to involve us in such intellectual-property infringement claims. Furthermore, although we maintain certain procedures to help to ensure that the items we 3D print on behalf of customers do not infringe upon the intellectual-property rights of others, we cannot be certain that our procedures will be effective in preventing any such infringement.
Intellectual-property disputes, litigation and arbitration, regardless of the merit or resolution, could cause us to incur significant costs in enforcing, or responding to, defending and resolving such claims. In addition, such claims can be costly and disruptive to our business operations by diverting attention and energies of management and key technical personnel, by prohibiting or otherwise impairing our ability to commercialize new or existing products or services and by increasing our costs of doing business. We may not prevail in any such dispute or litigation, and an adverse decision in any legal action involving intellectual-property rights, including any such action commenced by us, could limit the scope of our intellectual property rights and the value of the related technology. Third party claims of intellectual-property infringement successfully asserted against us may require us to redesign infringing technology or enter into costly settlement or license agreements on terms that are unfavorable to us, prevent us from manufacturing or licensing certain of our products, subject us to injunctions restricting our sale of products and use of infringing technology, cause severe disruptions to our operations or the markets in which we compete, impose costly damage awards or require indemnification of our sales agents and end-users. In addition, as a consequence of such claims, we may incur significant costs in acquiring the necessary third party intellectual-property rights for use in our products and services or developing non-infringing substitute technology. Any of the foregoing developments may have a material adverse effect on our business, financial condition and results of operations.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to governmental patent agencies, including the U.S. Patent and Trademark Office, or USPTO, in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late payment fee or by other means of redress in accordance with the applicable rules, there are situations in which noncompliance can result in definitive lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or our licensors fail to maintain the patents and patent applications covering our products and processes, our competitive position could be adversely affected.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets.
Certain of our past and present employees were previously employed at other companies, including our competitors or potential competitors. Some of these employees executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer.
28
We are not aware of any threatened or pending claims related to these matters, but in the future, litigation may be necessary to defend against such claims. If we fail to defend against any such claims, in addition to paying monetary damages, we may lose valuable personnel or intellectual property rights. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
If disputes arise, we could lose rights that are important to our business or be subject to restrictions on the conduct of our business.
We have license agreements with respect to certain intellectual property that is important to our business and that may include exclusivity and non-competition undertakings. Disputes may arise between the counterparties to these agreements and us that could result in termination of these agreements. If we fail to comply with our obligations under our intellectual property-related agreements, or misconstrue the scope of the rights granted to us or restrictions imposed on us under these agreements, the counterparties may have the right to terminate these agreements or sue us for damages or equitable remedies, including injunctive relief. Termination of these agreements, the reduction or elimination of our rights under these agreements, or the imposition of restrictions under these agreements that we have not anticipated may result in our having to negotiate new or reinstated licenses with less favorable terms, or to cease commercialization of licensed technology and products. This could materially adversely affect our business.
Certain technologies and patents have been developed with collaboration partners and we may face restrictions on this jointly developed intellectual property.
We have entered into collaborations with a number of industrial and medical device companies and academic institutions, including Zimmer Biomet, Enovis, DePuy Synthes, Lima, Mathys, Siemens, BASF 3D Printing Solutions GmbH and HOYA. We have, in some cases individually and in other cases along with our collaboration partners, filed for patent protection for a number of technologies developed under these agreements and may in the future file for further intellectual property protection and/or seek to commercialize such technologies. Under some of these agreements, certain intellectual-property developed jointly by us and the relevant partner may be subject to joint ownership by us and the partner and our commercial use of such intellectual-property may be restricted, or may require written consent from, or a separate agreement with, the partner. In other cases, we may not have any rights to use intellectual property solely developed and owned by the partner. If we cannot obtain commercial use rights for such jointly-owned intellectual property or partner-owned intellectual property, our future product development and commercialization plans may be adversely affected. For additional information, see “Item 4. Information on the Company—B. Business Overview—Intellectual Property.”
Our use of open source software may expose us to additional risks and harm our intellectual property.
Some of our proprietary software, including some of our 3D printing software, may use or incorporate open source software. Some open source software licenses require users who distribute open source software as part of their own software product to publicly disclose all or part of the source code to such software product or make available any derivative works of the open source code on unfavorable terms or at no cost. We monitor, on an ongoing basis, whether our proprietary software, including that in our 3D printing software, would make use of any open source software that could require us to disclose our proprietary source code, which could adversely affect our business.
Risks Related to the ADSs
The ADSs may experience price and volume fluctuations.
The stock market generally has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of listed companies. Broad market and industry factors may negatively affect the market price of the ADSs, regardless of our actual operating performance. The market price and liquidity of the market for the ADSs may be higher or lower than the price you paid and may be significantly affected by numerous factors, some of which are beyond our control. These factors include:
| ● | changes in macroeconomic or market conditions or trends in our industry or markets, such as inflation, recessions, the continued rise in interest rates, ongoing supply chain shortages, actual or perceived instability in the global banking system, the results of local and national elections, international currency fluctuations, epidemics and pandemics, corruption, political instability and acts of war, such as the armed conflicts in Ukraine, Israel and the Middle East, or terrorism; |
| ● | significant volatility in the market price and trading volume of securities of companies in our sector, which is not necessarily related to the operating performance of these companies; |
29
| ● | the mix of products that we sell, and related services that we provide, during any period; |
| ● | delays between our expenditures to develop and market new products and the generation of sales from those products; |
| ● | changes in the amount that we spend to develop, acquire or license new products, technologies or businesses; |
| ● | changes in our expenditures to promote our products and services; |
| ● | success or failure of research and development projects of us or our competitors; |
| ● | announcements of acquisitions by us or one of our competitors; |
| ● | the general tendency towards volatility in the market prices of shares of companies that rely on technology and innovation; |
| ● | changes in regulatory policies or tax guidelines; |
| ● | changes or perceived changes in earnings or variations in operating results; and |
| ● | any shortfall in revenue or net income from levels expected by investors or securities analysts. |
Any of these could result in a material decline in the price of the ADSs.
Members of our board of directors and senior management own a significant percentage of our ordinary shares and are able to exert significant influence over matters subject to shareholder approval.
Members of our board of directors and senior management beneficially owned approximately 57.66% of our outstanding ordinary shares (including ordinary shares represented by ADSs), as of March 26, 2024. These shareholders have significant influence over the election of members of our board of directors and the outcome of corporate actions requiring shareholder approval, including dividend policy, mergers, share capital increases, amendments of our restated articles of association and other extraordinary transactions. For example, these shareholders may be able to influence the outcome of elections of members of our board of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transactions. In addition, our restated articles of association provide that, as long as Wilfried Vancraen, our founder and a member of our board of directors, and Hilde Ingelaere, a member of our board of directors, who is also Mr. Vancraen’s spouse, and their three children, Linde, Sander (who is also a member of our board of directors) and Jeroen Vancraen, or collectively the Family Shareholders, control, directly or indirectly, in the aggregate at least 20% of the voting rights attached to our ordinary shares, a majority of our directors must be appointed by our shareholders from a list of candidates proposed by the Family Shareholders. This concentration of ownership within this group of shareholders and the rights of the Family Shareholders prevent or discourage unsolicited acquisition proposals or offers for our ordinary shares or ADSs that you may feel are in your best interest as one of our shareholders. The interests of these existing shareholders or the Family Shareholders may not always coincide with your interests or the interests of other shareholders, and they may act in a manner that advances their best interests and not necessarily those of other shareholders, including seeking a premium value for their ordinary shares, which might affect the prevailing market price for the ADSs.
The dilutive effect of our warrants could have an adverse effect on the future market price of the ADSs or otherwise adversely affect the interests of our shareholders.
Based on outstanding granted warrants, as of December 31, 2023, there were outstanding granted warrants to subscribe for an aggregate of 423,452 ordinary shares at a weighted average exercise price of €5.39 per share. The warrants likely will be exercised if the market price of the ADSs equals or exceeds the applicable exercise price. To the extent such securities are exercised, additional ordinary shares will be issued, which would dilute the ownership of existing shareholders.
30
You may not have the same voting rights as the holders of our ordinary shares and may not receive voting materials in time to be able to exercise your right to vote.
Except as described in the deposit agreement related to the ADSs, holders of ADSs are not able to exercise voting rights attaching to the ordinary shares evidenced by the ADSs on an individual basis. Under the terms of the deposit agreement, holders of ADSs may instruct the depositary to vote the ordinary shares underlying their ADSs, but only if we ask the depositary to ask for their instructions. Otherwise, holders of ADSs are not able to exercise their right to vote, unless they withdraw our ordinary shares underlying the ADSs they hold to vote them in person or by proxy. However, holders of ADSs may not know about the meeting far enough in advance to withdraw those ordinary shares. If we ask for the instructions of holders of ADSs, the depositary, upon timely notice from us, will notify holders of ADSs of the upcoming vote and arrange to deliver our voting materials to them. Upon our request, the depositary will mail to holders of ADSs a shareholder meeting notice which contains, among other things, a statement as to the manner in which voting instructions may be given, including an express indication that such instructions may be given or deemed given to the depositary to give a discretionary proxy to a person designated by us if no instructions are received by the depositary from holders of ADSs on or before the response date established by the depositary. However, no voting instruction shall be deemed given and no such discretionary proxy shall be given with respect to any matter as to which we inform the depositary that (i) substantial opposition exists, or (ii) such matter materially and adversely affects the rights of shareholders. We cannot guarantee that holders of ADSs will receive the voting materials in time to ensure that they can instruct the depositary to vote their shares. In addition, the depositary’s liability to holders of ADSs for failing to execute voting instructions or for the manner of executing voting instructions is limited by the deposit agreement. As a result, holders of ADSs may not be able to exercise their right to give voting instructions or to vote in person or by proxy and they may not have any recourse against the depositary or our company if their shares are not voted as they have requested or if their shares cannot be voted.
You may not receive distributions on our ordinary shares represented by the ADSs or any value for them if it is illegal or impractical to make them available to holders of ADSs.
Under the terms of the deposit agreement, the depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of our ordinary shares your ADSs represent. However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to take any other action to permit the distribution of the ADSs, ordinary shares, rights or anything else to holders of ADSs. This means that you may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical to make them available to you. These restrictions may have a material adverse effect on the value of your ADSs.
We have no present intention to pay cash dividends on our ordinary shares in the foreseeable future and, consequently, your only opportunity to achieve a return on your investment during that time is if the price of the ADSs appreciates.
We have no present intention to pay cash dividends on our ordinary shares in the foreseeable future. Any recommendation by our board of directors to pay cash dividends will depend on many factors, including our financial condition, results of operations, legal requirements and other factors. Furthermore, pursuant to Belgian law, the calculation of amounts available for distribution to shareholders, as dividends or otherwise, must be determined on the basis of our non-consolidated statutory financial statements prepared under generally accepted accounting principles in Belgium, or Belgian GAAP. In addition, in accordance with Belgian law and our restated articles of association, we must allocate each year an amount of at least 5% of our annual net profit under our statutory non-consolidated accounts (prepared in accordance with Belgian GAAP) to a legal reserve until the reserve equals 10% of our share capital. Our legal reserve currently does not meet this requirement. As a consequence of these facts, there can be no assurance as to whether dividends or other distributions will be paid out in the future or, if they are paid, their amount.
31
As a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and are permitted to file less information with the SEC than U.S. domestic issuers. This may limit the information available to holders of ADSs.
We are a “foreign private issuer,” as defined in the rules and regulations of the SEC and, consequently, we are not subject to all of the disclosure requirements applicable to U.S. domestic issuers. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, we are not required to file periodic reports and consolidated financial statements with the SEC as frequently or as promptly as U.S. domestic issuers. Accordingly, there may be less publicly available information concerning our company than there is for U.S. public companies. As a foreign private issuer, we file an annual report on Form 20-F within four months of the close of each year ended December 31 and furnish reports on Form 6-K relating to certain material events promptly after we publicly announce these events. However, although we intend to continue to issue quarterly financial information, because of the above exemptions for foreign private issuers, we are not required to do so, and, therefore, our shareholders will not be afforded the same protections or information generally available to investors holding shares in public companies organized in the United States.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As a foreign private issuer, we are not required to comply with all the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. Accordingly, we will next make a determination with respect to our foreign private issuer status on June 30, 2024. There is a risk that we will lose our foreign private issuer status in the future.
We would lose our foreign private issuer status if, for example, more than 50% of our assets are located in the United States and more than 50% of our outstanding ordinary shares are held of record by U.S. residents. As of December 31, 2023, 3% of our assets were located in the United States. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly greater than the costs we incur as a foreign private issuer. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive in certain respects than the forms available to a foreign private issuer. We would be required under current SEC rules to prepare our consolidated financial statements in accordance with U.S. GAAP and modify certain of our policies to comply with corporate governance practices associated with U.S. domestic issuers. Such conversion and modifications would involve significant additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers such as the ones described above and exemptions from procedural requirements related to the solicitation of proxies.
If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, we are required, under Section 404 of the Sarbanes-Oxley Act, to perform system and process evaluations and testing of our internal controls over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting. This assessment must include disclosure of any material weaknesses in our internal control over financial reporting identified by our management or our independent registered public accounting firm. A material weakness is a control deficiency, or combination of control deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim consolidated financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. Our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. Our remediation efforts may not enable us to avoid a material weakness in the future.
32
We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of the ADSs could decline, and we could be subject to sanctions or investigations by the Nasdaq Stock Market, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
We have incurred and will incur significant increased costs as a result of operating as a company whose ADSs are publicly traded in the United States, and our management is required to devote substantial time to new compliance initiatives.
As a company whose ADSs are publicly traded in the United States, we have incurred and will incur significant legal, accounting, insurance and other expenses that we did not incur prior to our initial public offering. In addition, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act and related rules implemented by the SEC and the Nasdaq Stock Market have imposed various requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls. These costs have increased now that we are no longer an emerging growth company eligible to rely on exemptions under the JOBS Act from certain disclosure and governance requirements. Our management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly. These laws and regulations could also make it more difficult and expensive for us to attract and retain qualified persons to serve on our board of directors or its committees. Furthermore, if we are unable to satisfy our obligations as a public company, we could be subject to delisting of the ADSs, fines, sanctions and other regulatory action and potentially civil litigation.
In order to satisfy our obligations as a U.S. public company, we may need to hire or engage additional qualified accounting and financial personnel and consultants with appropriate experience.
As a U.S. public company, we are required to establish and maintain effective internal controls over financial reporting and disclosure controls and procedures. In order to establish and maintain this control environment, we have hired accounting and financial personnel and engaged consultants with experience and technical accounting knowledge, but we may need to hire or engage additional personnel and consultants to further our efforts. It is difficult to recruit and retain qualified personnel and consultants, and our operating expenses and operations have been and may continue to be impacted by the costs of their employment or engagement. Further, these efforts may divert management’s attention from their day-to-day responsibilities.
You may be subject to limitations on the transfer of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems doing so expedient in connection with the performance of its duties. The depositary may close its books from time to time for a number of reasons, including in connection with corporate events such as a rights offering, during which time the depositary needs to maintain an exact number of ADS holders on its books for a specified period. The depositary may also close its books in emergencies, and on weekends and public holidays. The depositary may refuse to deliver, transfer or register transfers of the ADSs generally when our share register or the books of the depositary are closed, or at any time if we or the depositary thinks that it is advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason in accordance with the terms of the deposit agreement. As a result, you may be unable to transfer your ADSs when you wish to.
If securities or industry analysts do not publish research or reports about our business, or if they adversely change their recommendations regarding the ADSs, the market price for the ADSs and trading volume could decline.
The trading market for the ADSs is influenced by research or reports that industry or securities analysts publish about our business. If one or more analysts who cover us downgrade the ADSs, the market price for the ADSs would likely decline. If one or more of these analysts cease to cover us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for the ADSs to decline.
33
It may be difficult for investors outside Belgium to serve process on or enforce foreign judgments against us or our directors and senior management.
We are a Belgian limited liability company. None of the members of our board of directors and senior management is a resident of the United States. All or a substantial portion of the assets of such non-resident persons and most of our assets are located outside the United States. As a result, it may not be possible for investors to effect service of process upon such persons or on us or to enforce against them or us a judgment obtained in U.S. courts. Original actions or actions for the enforcement of judgments of U.S. courts relating to the civil liability provisions of the federal or state securities laws of the United States are not directly enforceable in Belgium. The United States and Belgium do not currently have a multilateral or bilateral treaty providing for reciprocal recognition and enforcement of judgments, other than arbitral awards, in civil and commercial matters. In order for a final judgment for the payment of money rendered by U.S. courts based on civil liability to produce any effect on Belgian soil, it is accordingly required that this judgment be recognized or be declared enforceable by a Belgian court in accordance with Articles 22 to 25 of the 2004 Belgian Code of Private International Law. Recognition or enforcement does not imply a review of the merits of the case and is irrespective of any reciprocity requirement. A U.S. judgment will, however, not be recognized or declared enforceable in Belgium if it infringes upon one or more of the grounds for refusal which are exhaustively listed in Article 25 of the Belgian Code of Private International Law. These grounds mainly require that the recognition or enforcement of the foreign judgment should not be a manifest violation of public policy, that the foreign courts must have respected the rights of the defense, that the foreign judgment should be final, and that the assumption of jurisdiction by the foreign court may not have breached certain principles of Belgian law. In addition to recognition or enforcement, a judgment by a federal or state court in the United States against us may also serve as evidence in a similar action in a Belgian court if it meets the conditions required for the authenticity of judgments according to the law of the state where it was rendered. The findings of a federal or state court in the United States will not, however, be taken into account to the extent they appear incompatible with Belgian public policy.
Holders of ADSs are not treated as shareholders of our company.
Holders of ADSs with underlying shares in a Belgian limited liability company are not treated as shareholders of our company, unless they withdraw our ordinary shares underlying the ADSs that they hold. The depository is the holder of the ordinary shares underlying the ADSs. Holders of ADSs therefore do not have any rights as shareholders of our company, other than the rights that they have pursuant to the deposit agreement.
We are a Belgian limited liability company but are not a listed company in Belgium, and shareholders of our company may have different and, in some cases, more limited shareholder rights than shareholders of a listed company in Belgium or of a U.S. listed corporation.
We are organized as a limited liability company (naamloze vennootschap / société anonyme) under the laws of Belgium. Our corporate affairs are governed by Belgian corporate law. From a Belgian corporate law point of view, we do not qualify as a listed company (genoteerde vennootschap / société cotée) because none of our securities are listed on any regulated market in the EEA. The Belgian corporate law provisions that are applicable to Belgian listed companies do therefore not apply to us. Furthermore, we are not subject to most of the disclosure obligations applicable to Belgian listed companies. As a result, shareholders of our company may not enjoy certain of the rights and protection generally afforded to shareholders of a Belgian listed company. You should also be aware that the rights provided to our shareholders under Belgian corporate law and our restated articles of association differ in certain respects from the rights that you would typically enjoy as a shareholder of a U.S. corporation under applicable U.S. federal and state laws.
Under Belgian corporate law, except in certain limited circumstances, our shareholders may not ask for an inspection of our corporate records, while under Delaware corporate law any shareholder, irrespective of the size of his or her shareholdings, may do so. Shareholders of a Belgian corporation are also unable to initiate a derivative action, a remedy typically available to shareholders of U.S. companies, in order to enforce a right of our company, in case we fail to enforce such right ourselves, other than in certain cases of director liability under limited circumstances. In addition, a majority of our shareholders may release a director from any claim of liability we may have, including if he or she has acted in bad faith or has breached his or her duty of loyalty, provided, in some cases, that the relevant acts were specifically mentioned in the convening notice to the shareholders’ meeting deliberating on the discharge. In contrast, most U.S. federal and state laws prohibit a company or its shareholders from releasing a director from liability altogether if he or she has acted in bad faith or has breached his or her duty of loyalty to the company. Finally, Belgian corporate law does not provide any form of appraisal rights in the case of a business combination. For additional information on these and other aspects of Belgian corporate law and our restated articles of association, see “Item 10. Additional Information—B. Memorandum and Articles of Association.” As a result of these differences between Belgian corporate law and our restated articles of association, on the one hand, and U.S. federal and state laws, on the other hand, in certain instances, you could receive less protection as a shareholder of our company than you would as a shareholder of a U.S. corporation.
34
As a foreign private issuer, we are not subject to certain Nasdaq Stock Market corporate governance rules applicable to U.S. listed companies.
We rely on provisions in the Listing Rules of the Nasdaq Stock Market that permit us to follow our home country corporate governance practices with regard to certain aspects of corporate governance. This allows us to follow Belgian corporate law and the Belgian Code of Companies and Associations, which differ in significant respects from the corporate governance requirements applicable to U.S. companies listed on the Nasdaq Global Select Market. See “Item 16G. Corporate Governance.”
Holders of ADSs or ordinary shares have limited rights to call shareholders’ meetings or to submit shareholder proposals, which could adversely affect their ability to participate in the governance of our company.
Except under limited circumstances, only the board of directors may call a shareholders’ meeting. Shareholders who collectively own at least 10% of the ordinary shares of our company may require the board of directors or the statutory auditor to convene a special or an extraordinary general meeting of shareholders. As a result, the ability of individual holders of the ADSs or ordinary shares to influence the governance of our company is limited.
Holders of the ADSs have limited recourse if we or the depositary fail to meet our respective obligations under the deposit agreement or if they wish to involve us or the depositary in a legal proceeding.
The deposit agreement expressly limits the obligations and liability of us and the depositary. Neither we nor the depositary will be liable to the extent that liability results from the fact that we:
| ● | are prevented or hindered in performing any obligation by circumstances beyond their control; |
| ● | exercise or fail to exercise discretion under the deposit agreement; |
| ● | perform our obligations without negligence or bad faith; |
| ● | take any action based upon advice of or information from legal counsel, accountants, any person presenting shares for deposit, any holder of the ADSs or any other qualified person; or |
| ● | rely on any documents we believe in good faith to be genuine and properly executed. |
In addition, neither we nor the depositary has any obligation to participate in any action, suit or other proceeding in respect of the ADSs which may involve it in expense or liability unless it is indemnified to its satisfaction. These provisions of the deposit agreement will limit the ability of holders of the ADSs to obtain recourse if we or the depositary fails to meet our respective obligations under the deposit agreement or if they wish to involve us or the depositary in a legal proceeding.
Investors may not be able to participate in equity offerings, and ADS holders may not receive any value for rights that we may grant.
In accordance with Belgian corporate law, our restated articles of association provide for preferential subscription rights to be granted to our existing shareholders to subscribe on a pro rata basis for any issue for cash of new shares, convertible bonds or warrants that are exercisable for cash, unless such rights are cancelled or limited by resolution of our shareholders’ meeting or the board of directors. Our shareholders’ meeting or board of directors may cancel or restrict such rights in future equity offerings. In addition, certain shareholders (including those in the United States, Australia, Canada or Japan) may not be entitled to exercise such rights even if they are not cancelled unless the rights and related shares are registered or qualified for sale under the relevant legislation or regulatory framework. As a result, there is the risk that investors may suffer dilution of their shareholding should they not be permitted to participate in preference right equity or other offerings that we may conduct in the future. We may also limit the exercise of rights by shareholders in certain jurisdictions if we distribute rights in connection with other changes to our capital structure, like a distribution of rights to tender our shares to us for redemption in connection with an issuer tender offer, resulting in such shareholders being unable to participate in such transactions.
If rights are granted to our shareholders, as the case may be, but if by the terms of such rights offering or other transaction, or for any other reason, the depositary may not either make such rights available to any ADS holders or dispose of such rights and make the net proceeds available to such ADS holders, then the depositary may allow the rights to lapse, in which case ADS holders will receive no value for such rights.
35
Shareholders in jurisdictions with currencies other than the euro face additional investment risk from currency exchange rate fluctuations in connection with their holding of our shares.
Any future payments of cash dividends on shares will be denominated in euro. The U.S. dollar—or other currency—equivalent of any dividends paid on our shares or received in connection with any sale of our shares could be adversely affected by the depreciation of the euro against these other currencies.
We do not expect to be a passive foreign investment company for U.S. federal income tax purposes; however, there is a risk that we may be classified as a passive foreign investment company, which could result in materially adverse U.S. federal income tax consequences to U.S. investors.
We do not expect to be a passive foreign investment company, or a PFIC. However, the application of complex U.S. federal income tax rules concerning the classification of our assets and income, and the application of these rules is uncertain in some respects. Additionally, certain aspects of the tests will be outside our control; therefore, no assurance can be given that we will not be classified as a PFIC for any taxable year. If you are a U.S. taxpayer and we are determined to be a PFIC at any time during your holding period, you may be subject to materially adverse consequences, including additional tax liability and tax filing obligations. See “Item 10. Additional Information—E. Taxation—U.S. Taxation—Passive Foreign Investment Company.”
Changes in our United States federal income tax classification, or that of our subsidiaries, could result in adverse tax consequences to our 10% or greater U.S. shareholders.
We do not believe that we, or any of our non-U.S. subsidiaries, are controlled foreign corporations, or CFCs, based upon the ADSs or shares owned directly by U.S. shareholders. However, we or certain of our non-U.S. subsidiaries may be classified as CFCs depending on the U.S. holdings of certain of our non-U.S. shareholders. This classification could cause significant and adverse U.S. tax consequences for our U.S. shareholders that own, or are considered to own, as a result of the attribution rules, 10% or more of the voting power or value of the stock of us or our non-U.S. subsidiaries, or a 10% U.S. shareholder, or any person who becomes a 10% U.S. shareholder under the U.S. Federal income tax law applicable to owners of CFCs. Therefore, we would advise our 10% U.S. shareholders (if any) and persons considering becoming 10% U.S. shareholders to consult their tax advisors regarding the U.S. Federal income tax law applicable to owners of CFCs.
ITEM 4.INFORMATION ON THE COMPANY
| A. | History and Development of the Company |
Materialise NV was incorporated in Belgium on June 28, 1990 as a limited liability company under Belgian company law.
Our principal executive and registered offices are located at Technologielaan 15, 3001 Leuven, Belgium. Our telephone number is +32 (16) 39 66 11. We are registered with the Register of Legal Entities of Leuven under the number 0441.131.254. Our agent for service of process in the United States is Materialise USA, LLC, located at 44650 Helm Ct., Plymouth, Michigan 48170, telephone number (734) 259-6445. Our internet website is www.materialise.com. The information contained on, or accessible through, our website is not incorporated by reference into this annual report and should not be considered a part of this annual report.
The SEC maintains an internet website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at www.sec.gov.
Capital Expenditures (Property Plant and Equipment and Intangible Assets)
Our capital expenditures amounted to € 11.8 million, € 24.8 million, and € 11.7 million for the years ended December 31, 2023, 2022, and 2021, respectively. In 2023, our main capital expenditures were€ 2.0 million for our new metal production facility in the United States, € 3.6 million for the expansion of our production capacity in Germany and € 1.6 million for our internal digital transformation program. In 2022, our main capital expenditures were € 7.3 million for our new metal production facility in the United States, € 7.9 million for the expansion of our production capacity in Germany and € 2.4 million for our internal digital transformation program. In 2021, our main capital expenditures were € 1.7 million for our internal digital transformation program, € 1.6 million for a new building in Germany and € 1.0 million for the transformation of our platform architecture which was partially impaired in 2022.
36
| B. | Business Overview |
Our Mission
Our mission is to innovate product development that results in a better and healthier world, through our software and hardware infrastructure, and an in-depth knowledge of additive manufacturing.
Our Company
We are a leading provider of additive manufacturing and medical software tools and of sophisticated 3D printing services. With our knowledge, products and services, we empower our customers’ use of additive manufacturing technology, in general, and we enable certain specific and significant applications of additive manufacturing, in particular. In both instances, we seek to empower the choice for sustainability through the use of additive manufacturing.
The customers of our general software tools and 3D printing services are active in a wide variety of industries, including healthcare, automotive, aerospace, art and design and consumer products. The significant additive manufacturing applications that we are more deeply and more directly involved in currently include applications for orthopedic, cranio maxillo facial, eyewear, footwear and measurement fixtures.
As of December 31, 2023, our team consisted of 2,437 full-time equivalent employees, or FTEs, and fully dedicated consultants. Our portfolio of intellectual property featured 476 patents and 101 pending patent applications as of December 31, 2023. For the year ended December 31, 2023, we generated € 256.1 million of revenue, representing a 10% increase over the prior year, a net profit of € 6.7 million and an Adjusted EBITDA of € 31.4 million. For a description of Adjusted EBITDA and a reconciliation of our net profit to our Adjusted EBITDA, see “Item 5. Operating and Financial Review and Prospects—A. Operating Results—Other Financial Information.”
Our Core Competencies
Our established and proven business model integrates our three research-based core competencies: (i) software development, (ii) 3D printing, and (iii) engineering for 3D printing, which act as complementary incubators for our new products and function as integrated support centers for our existing products. The interaction and synergies among our software development, 3D printing and engineering teams position us well to continuously develop and support innovative applications of 3D printing that often integrate all three core competencies.
Software Development (Software). Our expertise in developing 3D printing software originated from our efforts to enable 3D printing applications and to continually improve processes within our own additive manufacturing operations. As a result of our continued deployment over the course of 30 years of human, intellectual and economic capital to software development, a number of our products, including Magics and Mimics, have evolved into industry-leading flagship products. We have an established quality management system for the development of our software products that is ISO 9001:2015 certified. We are also ISO 13485:2016 certified for our medical applications and our medical applications comply with the regulatory requirements of several jurisdictions, including Europe and the United States. Additionally, we are ISO27001 certified for the secure operational management of the production environment of our cloud-based software for medical case management and medical image processing.
3D Printing (Hardware). As a pioneer in the additive manufacturing industry, we have an extensive history of 3D printing millions of parts utilizing a broad array of technologies, often in highly regulated environments, for thousands of commercial, industrial and medical customers. We operate some of the most sophisticated printing machines currently available on the market, as well as our own proprietary stereolithography-based technology, Mammoth, to provide a very broad range of technologies, sizes, materials and finishing degrees and to address the needs of customers across a large number of potential markets. Production is organized in multiple production lines that are dedicated to the Medical and the Industrial Production segments that we serve. Our 3D printing group operates in an ISO 13485:2016-certified system for the production of medical devices, in an EN9100:2018 as well as EASA Part 21G POA certified system for the production of plastic aerospace parts, and in an ISO 9001:2015-certified quality management system for all other markets. Further, our 3D printing group has its own maintenance and research team that utilizes an in-house laboratory facility where products can be tested. The wide variety of products that are processed by our multiple production lines are logistically streamlined through our proprietary database systems that manage the entire process from order intake to 3D printing to final shipment.
37
Engineering (Mindware). Our engineering expertise is integral to our entire business, as it enhances our software development and 3D printing expertise. Our engineers work in teams that support customers in different market segments. These teams work directly with our customers to identify new, and customize and refine existing, 3D printing applications and to increase productivity, efficiency and ease of use across all aspects of the solutions we provide. Our engineering teams have particular expertise in industrial and medical applications, including patient-specific surgical guides, models and implants with the applicable market clearances. Our teams are highly specialized, especially in the medical field, and include quality controllers, development researchers for new hardware concepts and trainers who bring new engineers to the required level of expertise. Our engineers operate within the framework of the aforementioned ISO 9001:2015 certified quality management system. Our engineering teams make extensive use of our proprietary software tools and have direct access to our 3D printing center where developments can be tested in an actual production environment.
Our Market Segments
We offer our products and services through a market oriented organization that is active across three principal market segments: (i) Materialise Software, (ii) Materialise Medical, and (iii) Materialise Manufacturing. We believe that our customers benefit significantly from the synergistic interplay between our core competencies and the three market segments on which we focus and which provide regular end-user feedback to the product development and support teams within our core competencies.
Our Materialise Software Segment
In our Materialise Software segment, we offer proprietary software worldwide through programs and platforms that enable companies to set up efficient, reliable and sustainable 3D printing production. Our software supports 3D printing service bureaus both large and small that are producing a variety of parts for their customers and addresses the needs of large corporations producing at volume, either through significant serial manufacturing or mass customization. In all of these environments, we believe our software enables both operational excellence and flexibility. We work directly with many 3D printing machine manufacturers to enable and enhance the functionality of 3D printers and of 3D printing operations. We have developed software that interfaces between almost all types of industrial 3D printers, and various software applications and capturing technologies, including CAD/CAM packages and 3D scanners, by enabling data preparation and process planning and execution. Our programs interface with machines manufactured by leading original equipment manufacturers, or OEMs, such EOS GmbH, HP Inc., DesktopMetal, Inc., Renishaw PLC, SLM Solutions Group AG, Stratasys Ltd., Trumpf GmbH & Co. KG, Uniontech Corporation, GE Additive and Voxeljet AG. In addition, we have entered into partnership agreements with leading CAD, CAM and product lifecycle management, or PLM, companies such as Siemens, HCL Technologies Ltd., and PTC, for the integration of our additive manufacturing technology into Siemens’ NX software, HCL’s CAMworks, and PTC’s Creo software. This enables the streamlining of the design to manufacturing process for products being produced by additive manufacturing. We have also established connectivity between our software and the software of other providers in the broader 3D printing ecosystem like AM Flow, PostProcess, Castor, AMT, Dyemansion, Additive Marking, Twikit and Trinkle. We offer software that enables our customers to more efficiently organize the entire workflow of a 3D printing operation with multiple 3D printing machines, many operators and complex data flow and logistical requirements. We believe that the capabilities of our software products and their unique compatibility with many 3D printing systems continue to set standards in the professional 3D printing software market. Customers operating machines from multiple OEMs and customers running large 3D printing operations are among those who can benefit the most from our software packages and we believe that in many cases those customers demand compatibility with our software from the systems of OEMs.
As of December 31, 2023, our Materialise Software segment had a team of approximately 293 FTEs and fully dedicated consultants, with approximately 31% based at our headquarters in Belgium and the remaining employees distributed throughout our local field offices in China, Colombia, Germany, Japan, Malaysia, Ukraine, the United Kingdom and the United States.
Business Model. We generate revenue in our Materialise Software segment from our software licenses, maintenance contracts, hardware controller sales for our Materialise Controllers and custom software development services. Additionally, we offer consultancy and training services. We license our software products to our customers on either a time-based or perpetual basis, in which case we offer annual maintenance contracts that provide for software updates and support. In addition, we also provide a number of cloud-based solutions. Making use of, among others, our CO-AM platform, we are significantly accelerating the migration of our software solutions to the cloud, which we intend to offer along with our license-based solutions. We charge our custom software development services either on a time and material or on a fixed-cost basis. For the years ended December 31, 2023, 2022 and 2021, our Materialise Software segment generated revenue of € 44.4 million, €43.7 million and €42.9 million, respectively, representing 17.4% 18.8% and 20.9%, respectively, of our total revenue.
38
Software. We have a diversified portfolio comprised of software applications addressing different 3D printing market opportunities. Our decades of experience in the additive manufacturing industry are reflected in the sophisticated 3D printing software and business management tools we provide for our customers. We believe that each of our software applications is, or has the potential of becoming, one of the leading technologies in its domain. We believe that our neutral platform approach positions our software to drive greater innovation and choice across the 3D printer software ecosystem, and provides 3D printer users with more powerful and flexible printing capabilities.
In particular, we offer the following software applications:
| ● | Magics. Magics enables customers to import a wide variety of CAD formats and to the industry standard file formats 3MF and STL, as well as to the enriched BREP and MeshREP data format proprietary to us, ready for additive manufacturing. Magics’ applications include repairing and optimizing 3D models; analyzing parts; making process-related design changes on customers’ input files; designing support structures; documenting customer projects; nesting multiple parts in a single print run; and process planning. |
Our Magics product suite is enhanced with modules that further expand functionality and utility for our customers. For instance, the Magics Import Module plays an important role in efficiently moving CAD designs through to manufactured products by importing nearly all standard CAD formats into Magics. The Magics Structures Module was designed to help customers to reduce weight and material usage in their designs. We also have developed logistical modules such as the Magics SG Module, which offers tools for support structure design during the 3D printing process, and the Magics Sintermodule, which offers solutions for automated part nesting, protecting small and fragile parts and locating them after building. The Magics Simulation Module enables our users to simulate the build process virtually and optimizes the build preparation based on the results of such simulation, thus reducing build failures and improving the results.
In addition to offering state-of-the-art data preparation functionality to our users, our Magics product suite also focuses on automation and other productivity improvements and brings interconnectivity to machines and enterprise software platforms.
Specific versions of the Magics application were also brought to the market by us, including Magics Essentials (an entry-level package offering premium data preparation functionality), Magics Print (combining the most important build preparation tools and straightforward build file generation technology) and MiniMagics/MiniMagicsPro (providing viewing, communication and quoting solutions for our customers working in data preparation, or in quoting and quality control teams). Users of Magics Essentials and Magics Print can upgrade to our expert Materialise Magics product suite if they want the full data and build preparation functionality at their disposal in one package.
| ● | CO-AM. CO-AM is an additive workflow and digital manufacturing software platform that supports customers in major manufacturing industries and large AM service bureaus to scale and integrate their additive manufacturing operations across complex supply chains and IT environments. At the core of the CO-AM platform is the customers’ project data. The CO-AM platform provides a series of applications that are instrumental to organizations scaling their additive manufacturing capability. These solutions enable organizations to plan, manage, and optimize their operations. The platform includes centralized order management, quoting and costing, production planning, production scheduling, postproduction management, machine connectivity, quality management and manufacturing analytics. |
| ● | Streamics. Streamics is our legacy 3D Print planning system that we consider as the predecessor of the CO-AM platform. We are gradually migrating Streamics functionality to our CO-AM platform. Once the Streamics functionality is fully integrated in CO-AM, a transition plan will be set up to migrate existing Streamics customers to the Link3D platform over the coming years. In the meantime, we will continue to maintain and support Streamics and its customers. |
| ● | 3-matic. 3-matic is a versatile application that permits, among other things, design modification, design simplification, 3D texturing, re-meshing and forward engineering directly to standard additive manufacturing mesh files. Using Materialise consultancy services, targeted design automation solutions can be created for specific workflows. |
39
| ● | Build Processors. We work in close collaboration with a wide variety of 3D printer OEMs to develop customized and integrated solutions for their additive manufacturing machines. Our build processors automatically translate the 3D model data into layer data to provide sliced geometry and can link the latter with the appropriate build parameters to feed the machine control software. Another key benefit of our build processors is that they allow for a two-way communication between Magics and 3D printers. We also develop the metal build processors in Materialise Bremen and as a consequence we are able to cover a wide range of metal 3D printers. Furthermore, licensing and integrating our build processor framework, companies such as Siemens and PTC can also leverage the extensive ecosystem of build processors we have developed together with OEMs. Over the past years, we have transformed the architecture of our build processor to a cloud-native solution. Next to the standard build flows, the architecture and the availability of a BP-SDK (Software Development Kit) also allows for custom fit-for-purpose build pipelines to be scripted, enabling companies and 3D printer machine vendors alike to adapt and optimize the behavior and output of the build processor. This BP-SDK is available for customers to build their own build pipelines whilst having the possibility to integrate their proprietary IP in these pipelines. We believe this is very valuable in the context of volume production. |
| ● | e-Stage. e-Stage is a software solution that increases additive manufacturing productivity by automating support generation, optimizing the build process, and reducing the time our customers spend on finishing work such as build support removal and sanding. e-Stage is designed to allow our customers to use less material, to be able to 3D nest and to minimize failed builds. e-Stage for plastic has been commercially available since September 2007, and in the fall of 2017, we released e-Stage for metal. |
| ● | Materialise Controller. Materialise Controller controls and steers additive manufacturing machines using embedded Materialise software, and is fully integrated into the Materialise 3D printing software platform. It is engineered towards research and development applications, machine manufacturers and those who want to control or adapt the production process to their specific needs. |
| ● | Materialise Process Tuner. An intuitive online platform that helps manufacturing companies, service bureaus and machine builders speed up the process tuning that is required for mass-manufacturing 3D printed parts. |
| ● | Materialise Workflow Automation. This solution enables the user to leverage the full power of the Materialise Software technology in creating specific end-to-end workflows, which can be executed automatically and autonomously, or can be called from other software solutions like Magics through the Workflow Automation plugin function. The workflows can be executed in the cloud, on premise or on the user’s workstation. |
| ● | Identify3D. Identify3D is a suite of products that plugs into CO-AM and that allows customers to secure datasets throughout the full end-to-end process of 3D printing. Securing the data means adding a digital rights management tool on top of the part data, which protects the geometrical information of the data, but can be extended as well with process information (e.g., the number of times a file can be printed or the exact specifications how the file must be printed). Data security is gaining importance both because an increasing number of components are serially produced through additive manufacturing as well as with the growing importance of decentralized additive manufacturing production. |
| ● | Layer Analysis. Layer Analysis is a Machine Learning (ML) based tool that interprets images taken during the print of parts and looks for anomalies during the printing process. The tool combines the ML identified anomaly volumes with the to-be 3D files, allowing users to detect immediately after finishing a print if certain printed parts may show defects. In this way, unnecessary and expensive post-processing and (non-destructive) quality control can be avoided while it helps customers as well in defining allowable defects that do not affect the eventual part quality. |
Sales and Marketing. We market and distribute our software directly through our sales force as well as through our own website and third party distributors. Our Belgian team oversees our global marketing strategy and sales processes. Our local field office employees manage sales for particular markets and provide pre- and post-sales technical support to our customers. We also utilize a growing network of distributors and resellers to bring our solutions to specific regions or market segments. In addition, machine manufacturers and their local dealers often distribute our software products together with their 3D printers, with our software enhancing the printers’ value proposition and broadening the suite of applications available to the machines.
Customers. The customers for our Materialise Software segment include 3D printing machine manufacturers as well as production companies and contract manufacturers in a variety of industries, such as the automotive, aerospace, consumer goods and hearing aid industries, and external 3D printing service bureaus. Our Materialise Software segment customer base is spread across Asia, Europe and the Americas.
40
Competition. In our Materialise Software segment, we face indirect competition from the software developed by 3D printing OEMs, which are often more “closed ecosystem”-oriented (i.e., only focused on their own machines), and from companies that offer software that addresses one or more specific functional areas covered by our software solutions, such as providers of traditional CAD solutions. We compete directly with other providers of additive manufacturing management and machine control software, including open source software providers.
Growth Opportunities. We believe that 3D printing will be increasingly used for the manufacturing of complex or customized end use parts, and expect that the number of 3D printer manufacturers will increase accordingly, with certain new players initially focusing more on the hardware than on the software component of their 3D printers. Hence, we anticipate that the demand for highly performing industrial 3D printing software platforms will grow accordingly. The new products that we have developed and are developing, including the CO-AM platform, Process Tuner, Workflow Automation and fit-for-purpose build processors specifically address what we believe will be the needs of this growing end use part manufacturing market.
We believe that we can continue to expand our market penetration through expanding relationships with customers and OEMs, and through the continued innovation of our software products to adapt to and meet market demands. In order to be able to do so, we intend to bring our teams closer to our customer base worldwide, which will require continued investments in the expansion of our marketing and sales presence. In order to be able to meet the demands of new entrants on the market and to better address the needs of the end use parts market, we also intend to continue to invest significantly in the development of our software tools and solutions, including furthering their compatibility with as many 3D printers on the market as possible.
Our Materialise Medical Segment
In our Materialise Medical segment, our product and services offering addresses what we believe to be long-term trends in the medical industry towards personalized, functional and evidence-based medicine.
As of December 31, 2023, our Materialise Medical segment consisted of approximately 928 FTEs and fully dedicated consultants, with approximately 24.0% based at our headquarters in Belgium and the remaining employees distributed throughout our local offices in Australia, Brazil, China, Colombia, France, Germany, Japan, Malaysia, Ukraine, the United Kingdom and the United States.
Business Model. We generate revenue in our Materialise Medical segment through the sale of medical software and personalized medical devices. We sell licenses of our medical software packages and software maintenance contracts and sell medical devices that we customize and print for our customers. We also provide custom software development and engineering services, for which we charge either on a time and material or fixed-cost basis. The majority of the medical devices that we printed in 2023 were surgical guides (and related bone models) that were distributed to surgeons through our collaboration partners such as DePuy Synthes, Smith & Nephew, Stryker and Zimmer Biomet. We also print patient-specific implants that we sell directly to hospitals or distribute through partners such as DePuy Synthes. The customer base for our medical software products includes academic institutions, medical device companies and hospitals.
For the years ended December 31, 2023, 2022, and 2021, our Materialise Medical segment generated revenue of € 101.4 million, € 84.8 million and € 73.4 million, respectively, representing 39.6%, 36.6% and 35.7%, respectively, of our total revenue.
Medical Software. Our software allows medical-image based analysis, planning and engineering as well as patient-specific design and printing of surgical devices and implants. Our customers include leading research institutes, renowned hospitals and major medical device companies. Our medical software packages often serve as an introduction to our capabilities and in certain cases lead to custom software developments and clinical services opportunities. Our medical software packages are:
| ● | Materialise Mimics Innovation Suite. The Materialise Mimics Innovation Suite is a complete set of tools developed for biomedical professionals that allows them to perform a multitude of engineering operations based on medical imaging data. The suite consists of several complementary products and services, including Materialise Mimics, Materialise 3-matic, engineering services and medical models, as well as consultancy and custom software development. |
| ● | Materialise Mimics. Materialise Mimics is software addressing medical professionals specifically developed for medical image processing that can be used to segment accurate 3D models from medical imaging data (for example, from CT or MRI) to measure accurately in 2D and 3D and to export 3D models for additive manufacturing or to Materialise 3-matic. |
41
| ● | Materialise 3-matic. Materialise 3-matic focuses on anatomical design and is able to combine CAD tools with pre-processing capabilities directly on the anatomical data coming from Materialise Mimics. It enables our customers to conduct thorough 3D measurements and analysis, design a patient-specific implant, a surgical guide, or a benchtop model, and to prepare the anatomical data and/or resulting implants for simulation. |
| ● | Materialise OrthoView. Materialise OrthoView is a 2D digital pre-operative planning and templating solution for orthopedic surgeons. The software imports a digital X-ray image from a Picture Archiving and Communication System, or PACS, and positions the templates of suitable prostheses on the X-ray image at the correct scale. Materialise OrthoView currently serves more than 15,000 orthopedic surgeons in 60 countries globally, focusing primarily on joint replacements. We acquired OrthoView Holdings Limited in October 2014, and have included the OrthoView solution in our portfolio of pre-operative planning solutions. |
| ● | Materialise Mimics inPrint. With Materialise Mimics inPrint, clinicians can easily create files for 3D printing and use anatomically accurate models to help simulate or evaluate options for patient-specific surgical treatment. |
| ● | Materialise ProPlan CMF. Materialise ProPlan CMF is a software package developed for oral, maxillofacial, nose, throat and plastic surgeons. The software allows surgeons to pre-operatively plan their surgeries in 3D based on (CB)CT or MRI images using a set of tools to analyze, measure and reconstruct the patient’s anatomy. With the software the surgeon can also plan the movements (translations and rotations) of the mandible or maxilla and preplan the reconstruction of defects. |
| ● | Materialise Mimics Enlight. Materialise Mimics Enlight is a workflow-based planning software that enables companies, clinicians and hospitals to scale 3D planning for procedures. Mimics Enlight is based on the strengths of Materialise’s Mimics Innovation Suite and can be applied in various clinical fields such as structural heart or lung surgery. |
| ● | Materialise Surgicase. Materialise Surgicase is an online case management platform that enables medical device companies and hospitals to manage ordering and processing of personalized services and devices. |
Clinical Services and Personalized Medical Devices. Using our FDA-cleared and CE compliant medical software, we analyze 3D medical images of patients and provide doctors with virtual surgical planning services for their review and approval. In most cases, we also design and 3D print surgical guides that uniquely fit a specific patient and allow the surgeon to conduct the operation in accordance with the approved surgical plan. In certain circumstances, we deliver 3D printed customized patient-specific medical implants.
In our 3D printing centers in Belgium, Japan, Brazil, and the United States, we have separate production lines for our Materialise Medical segment.
We believe that our medical image-based simulation and planning software and 3D printing technology can assist hospitals and clinicians in providing personalized care to patients which can contribute to increased quality of life.
In many cases, surgeons using our clinical services work together with our clinical engineers to turn their patients’ medical image data into virtual surgical plans, and patient-specific 3D printed precise surgical and customized anatomical models to optimize intervention planning. For indications such as shoulder surgery, we have optimized and automated our 3D planning capabilities to provide surgical plans within a short timeframe and at a high quality that does not require an anatomical model to be provided. Utilizing our SurgiCase tool, surgeons upload CT or MRI medical image data and submit their cases to us, track their cases and review them as interactive virtual 3D models. In the framework of our collaborations with certain leading medical device companies, our SurgiCase tool is rebranded and adapted to the specific product offering and needs of our collaboration partners.
In many cases surgeons use personalized surgical guides or implants to translate the surgical plan into the operating room. Our 3D printed surgical guides include joint replacement guides for knee, shoulder and hip replacement surgeries, osteotomy guides and CMF guides, and our 3D printed implants include hip-revision implants, shoulder and CMF implants. The surgical guides and implants we print for U.S. based patients are FDA-cleared, and to the extent required by law, our medical devices for EEA-based patients bear the appropriate CE labels.
We address large surgical markets in orthopedics and CMF through collaboration agreements with leading medical device companies, including DePuy Synthes, Zimmer Biomet, Enovis, and Smith & Nephew. Pursuant to these agreements, we print joint replacement and/or CMF guides that our collaboration partners distribute under their own brands, together with their own implants, in the United States, Canada, South Africa, Latin America, Europe, China, Japan and Australia. We leverage our collaboration partners’ distribution capabilities to extend our reach into these large markets, and our collaboration partners utilize our 3D printing-related expertise to provide surgical planning and customized devices to surgeons.
42
We also address certain high value-added, specialty applications by providing the full solution ourselves, including the delivery of implants and guides directly to the hospital or surgeon. Such applications include customized CMF implants and guides, hip revision and shoulder implants in a patented porous matrix configuration and osteotomy guides. Through Engimplan, we distribute implants and instruments in Brazil, offering both traditional and 3D printed CMF products as well as a broader portfolio that includes product lines for trauma and sport medicine.
We also work with customers to print anatomical models that may be used for a wide range of applications such as sizing of medical devices, clinical trials, training, patient communications and marketing.
Sales and Marketing. We distribute our medical software through our direct sales force, our website and PACS partners (some of which partners also include our OrthoView solutions in their product offering to hospitals) and sell our medical devices through our agreements with collaboration partners such as Zimmer Biomet and Depuy Synthes. In specialty markets, we market and distribute our 3D printed medical devices and other clinical services through our experienced engineers who develop a close collaboration with key opinion leaders in each of these market segments.
All our activities in our Materialise Medical segment are coordinated and supervised from our headquarters in Belgium, which supervises product management and sales of our medical devices and software products.
Customers. The customers for our Materialise Medical segment mainly include medical device companies, hospitals, universities, research institutes and industrial companies. We have one individual customer that represents sales larger than 10% of our total revenue in 2023 (2022: 1; 2021: 1) from the Materialise Medical segment.
Collaboration Partners. We collaborate with leading medical device companies and academic institutions for the development and distribution of our surgical planning software, services, and products, such as Zimmer Biomet and DePuy Synthes, as well as Enovis, Integra, Lima, Mathys, Medtronic, Abbott and Corin. Pursuant to these arrangements, we develop and license software and sell surgical planning, guides and implants, including for use in the fields of knee and shoulder replacement, CMF and thoracic procedures that our collaboration partners may then distribute under their own brands, together with their own implants, mainly in the United States, Europe, Japan and Australia. In addition, we grant licenses to collaboration partners to use, market and distribute such software or surgical guides and implants. Some of the licenses we have granted to our products and software provide for exclusive rights, including with respect to a particular field of medicine or to the software or product developed during the collaboration, and certain collaboration partners may have rights of first refusal with respect to related products or collaborations. The compensation structures under these arrangements vary and may include an upfront fee, royalties, milestone payments linked to certain targets, and fees for the service, maintenance and training we provide in connection with our software and products.
Competition. In our Materialise Medical segment, we compete with a number of companies that provide image based software, 3D printed surgical models or medical devices, such as 3DSystems, Stratasys, Simpleware and Pie Medical as well as with medical device companies that develop and commercialize 3D printed medical devices and related software services.
Growth Opportunities. The Materialise Medical segment is the market where we believe we can most directly realize our mission statement and contribute to a healthier world. We believe that personalized surgical approaches, because they offer the potential of higher predictability and accuracy, lead to improved patient outcomes, fewer complications and increased long-term survival rates. Personalization also drives operational efficiencies by replacing a broad range of instrumentation with tailored versions. This makes surgery more efficient, but also lowers the cost of operational steps like sterilization. Personalized surgical approaches have benefits not only in complex interventions and we believe that personalized solutions will therefore see an increased adoption in the future.
As a result, we are currently investing significantly in the development of new product offerings and the optimization of existing offerings in terms of cost and lead times, as well as in the expansion of our distribution channel in the various sub-segments of our Materialise Medical segment and in new territories.
As a result of the trend that we see in the medical community towards more patient-specific devices and treatments, as well as towards more advanced planning, a growing number of academic, clinical and commercial researchers are focusing on personalized medical treatments. Because these new products and treatments can only be brought to the market in compliance with very strict regulatory requirements, we believe there is an opportunity for safe and stable medical software tools, such as our Mimics Innovation Suite, that can pass significant regulatory scrutiny. We also believe that increasing regulatory requirements provide opportunities for our clinical services as we can leverage our significant medical sector experience and strong quality management systems.
43
A growing number of hospitals have adopted personalized solutions and built 3D printing facilities on site for point-of-care printing of these personalized solutions. We believe that there is a growing opportunity to provide our clinical services as well as our software solutions and experience in establishing operations to design personalized solutions in compliance with regulatory requirements.
We are investing significantly in the development of new solutions of sub-markets other than orthopedics and CMF, including planning tools for the cardiovascular markets in the shorter term and the respiratory markets in the longer term.
Our Materialise Manufacturing Segment
In our Materialise Manufacturing segment, we primarily offer 3D printing services to industrial and commercial customers, the majority of which are located in Europe. In addition, we have identified, and provide 3D printing services to certain specialty growth markets in both the industrial and consumer marketplaces.
Many of the parts we print require functionality that cannot be delivered using other production processes. We believe that our industrial customers value the high quality, accuracy, complexity, durability, functionality and diversity in terms of size, scale and materials of the 3D printing services that we can offer. We deliver products to highly regulated industries, such as the aerospace, medtech, machine manufacturing, quality control equipment and consumer goods industries, where our applications, technology and hardware capabilities enable us to adhere to high quality standards in a certified production environment.
As of December 31, 2023, our Materialise Manufacturing segment consisted of 784 FTEs and fully dedicated consultants, with 31% based at our headquarters in Belgium and in Materialise Motion and RapidFit+. The remaining employees distributed throughout our local field offices in Austria, the Czech Republic, France, Germany, India, Italy, Poland, Spain, Ukraine, the United States and the United Kingdom.
Business Model. We generate a majority of our revenue in our Materialise Manufacturing segment through the sale of parts that we print for our customers. We generate a smaller portion of our revenue by the sale of scanners (e.g., foot scan plates for Materialise Motion) and software solutions in our eyewear and footwear business and consulting services that mainly help our customers to find applications for 3D printing.
For the years ended December 31, 2023, 2022, and 2021, our Materialise Manufacturing segment generated revenue of € 110.3 million, € 103.5 million, and € 89.2 million, respectively, representing 43.1%, 44.6%, and 43.4% respectively, of our total revenue.
Business-to-Business Services. We offer the following services in our Materialise Manufacturing segment:
Additive Manufacturing Solutions. We provide design and engineering services, rapid prototyping and additive manufacturing of production parts to customers serving the automotive, consumer goods industrial goods, semiconductor, art and architecture and aerospace markets. Our service centers offer a variety of 3D printing technologies including stereolithography, laser sintering, Filament Fusion, or FDM, PolyJet, Multi Jet Fusion, selective laser melting, or SLM, and vacuum casting. We have a dedicated production line for making aerospace-certified components using a number of technologies and materials. Along with this, we offer consulting services, which we bring to the market as Mindware, which helps customers to adopt 3D printing in their business before they can even start printing.
Specialty Industrial Solutions. We have developed additive manufacturing solutions that serve certain specialty industrial applications.
Our RapidFit+ business utilizes additive manufacturing to provide customers active in the automotive market with customized, highly precise and, in certain cases, patent protected measurement and fixturing tools. Using additive manufacturing technology, we believe that RapidFit+ fixtures provide more functionality and flexibility than the traditional fixtures that are currently widely used in the automotive industry. We also offer production tooling that we believe has substantially better ergonomics and improved functionality compared to traditional fixtures.
ACTech provides specialized solutions mainly for the automotive industry. In particular ACTech supplies prototyping of highly complex metal components through casting techniques that result in products that have a production grade performance. The casting is done using state-of-the-art 3D printed sand molds, while the final functionality of the components is achieved by a fully integrated post processing of the components in our CNC workshop.
44
Wearables initiatives in consumer industry. We have developed two wearables verticals for the consumer market. We believe 3D printing and design automation has great potential to help both consumers and healthcare professionals improve comfort, health and performance through personalized eyewear or footwear.
In our eyewear vertical, we offer a complete end-to-end solution for 3D-printed, often custom, eyewear frames. Based on a scan, patented technology identifies the critical parameters to automatically design eyewear that is customized to a person’s face. The resulting file can be printed in our eyewear production line, and we provide the necessary finishing, assembly steps and packaging.
Through Materialise Motion, we offer a full suite of solutions for footcare professionals. We offer digital measurement tools and personalized solutions to footcare professionals treating foot or gait problems. By means of our foot scan plates, we can capture a dynamic scan of a person’s foot sole and combined with our software tools, we create custom insoles based on this scan. The insoles are 3D printed, finished and assembled in a dedicated production line. Our research and product development teams aim to build a growing suite of solutions for patients with different types of motion problems.
Sales and Marketing. We market our services to our additive manufacturing solutions business customers using our sales force and through our website. Our more complex product offerings are addressed directly by our specialized sales teams who are located throughout Europe near our larger accounts and who align our customers’ needs with the wide range of 3D printing technologies or market-specific solutions that we offer. More straightforward products can be ordered directly by our customers through our “Materialise OnSite” or i.materialise web portals, a proprietary automated system that provides quotations, takes orders, and manages the printing process from start to finish, and allows customers to track the manufacturing and shipment process of their product online. Within our larger sales teams, specialized sales managers focus either on rapid prototyping, which is our traditional and well-established market, or the additive manufacturing of end-use production parts, which is the market where we see opportunities for significant growth. Our marketing team in Belgium oversees our global marketing strategy. In addition, employees at our Belgian headquarters and in our local field offices manage sales for particular markets and accounts and provide back office and production management support to our customers.
For our specialty markets and wearables initiatives we have separate sales teams that offer our customers the necessary expertise in their domain. Our sales teams have a direct approach to the market but in some cases we also work with partners or distributors locally to address specific market segments, such as the large segments of eyewear opticians or footcare professionals.
Customers. The customers for our Materialise Manufacturing segment are from a wide variety of industries, including the automotive, aerospace, medtech, semiconductor, industrial goods, art and design and consumer products industries. For these customers, we offer a complete set of services ranging from consultancy and co-creation, to design and engineering, rapid prototyping, and certified manufacturing of end-use parts.
Through our consultancy offering, which we brand as Materialise Mindware, we work together with customers to solve complex design challenges and to discuss how the introduction of 3D printing can affect product development, manufacturing workflow, business models and customer experiences. For example, a co-creation with HOYA, in collaboration with Hoet Design Studio, saw the launch of the world’s first vision-centric, 3D-tailored eyewear solution, Yuniku. Yuniku enables individualized lens and frame design through a sophisticated end-to-end digital supply chain, which includes a custom 3D scanner and software platform, co-created by us and HOYA, directly linked to our eyewear manufacturing factory.
Through our design and engineering service, we also service those customers looking for support in their initial concept design or with maximizing a design for 3D printing. Our design and engineering team, which is comprised of highly specialized designers and CAD engineers, offers dedicated design and software support for additive manufacturing, including remodeling and file preparation, as well as 3D scanning and measuring. Our team also offers training to engineering professionals active in various markets to accelerate the adoption of design for additive manufacturing.
The customers of both our Materialise OnSite and i.materialise platforms order through our website. Materialise OnSite customers tend to be industrial customers looking to rapid prototype parts quickly and reliably, often taking advantage of fast-lane machines to ensure short lead times for time-critical projects. For i.materialise, while there is a potential to address the wide consumer market with this platform, we prefer to describe our current customers as “home professionals.” Our i.materialise client base includes independent designers and CAD hobbyists that often sell their creations or their services to others. Through i.materialise’s APIs, companies can also partner with i.materialise to give their own customers a cloud-based, 3D-printing solution on their website, streamlining the ordering, manufacturing and shipping processes through a direct link to our factory for 3D printing. Since 2016, Microsoft has been using the i.materialise API to offer a cloud-based 3D print solution for Windows 10 users, and PTC did the same for Creo 4.0 software users.
45
Most of our straightforward additive manufacturing and rapid prototyping solutions are executed on the basis of single transaction contracts or purchase orders with the customer. These contracts and purchase orders lay out the pricing, delivery and other terms of the order. For our Additive Manufacturing service of end-use parts, an entirely new approach to ensure parts are made according to agreed standards is required, for which we have set processes to onboard new customers. An example of this is our dedicated aerospace manufacturing line, backed by certifications EN9100 and EASA Part 21G, through which we are currently manufacturing plastic parts for, among others, Airbus’s A350 XWB. We expect that as demand for our Certified Additive Manufacturing service grows, we may enter into more long-term agreements with customers.
For the automotive manufacturers and their suppliers that use our RapidFit+ service, the fixtures are custom engineered by dedicated teams. Our RapidFit+ customers, which include their quality departments, expect that fixtures meet high accuracy standards. Several automotive OEMs in Europe are currently considering our innovative solution as a potential new standard, while a solid base of automotive Tier 1 suppliers in Europe has embraced RapidFit+ as one of their fixture solutions.
Competition. In our additive manufacturing solutions business, we compete with a number of companies that provide industrial 3D printing services, including Sculpteo, Prototal, Protolabs and Quickparts. In addition, larger accounts tend to move their 3D printing production in-house once their orders have reached certain volumes, which not only creates opportunities for our Materialise Software segment but also for our Materialise Manufacturing segment in terms of capacity balancing services.
In the measurement and quality control fixture market addressed by RapidFit+, we are not aware of any direct competition coming from 3D printing companies. We do have competition, however, from a large group of smaller companies that are active in the more traditional tooling manufacturing.
Growth Opportunities. We believe that there is particular potential to grow our presence in the markets for additive manufacturing of complex and/or unique end products, including in particular certain parts for the aviation industry, medtech and eyewear and footwear products. In recent years, more companies have been using additive manufacturing for production across a broad range of industrial sectors, including aerospace, orthopedic implants, surgical guides, dental copings and hearing devices. For industrial end-use parts, we intend to continue to selectively invest in the expansion and creation of certified 3D manufacturing environments that meet the high standards of the specialized segments of the industrial production market that we focus on. In addition, we believe that our local sales teams, which are near our customers, as well as our engineering teams, which can bring in additional expertise where required, are important and rather unique assets in this market that are worthwhile to continue to invest in.
46
Manufacture and Supply. We produce our 3D printed products at our service centers in Belgium, Brazil, the Czech Republic, Germany, Poland, Japan and the United States. We print substantially all of products in-house using a variety of technologies, including stereolithography, laser sintering, FDM, PolyJet, powder binding, Multi Jet Fusion, Powder Bed Fusion and vacuum casting, and only subcontract the manufacture of products if certain other technologies (such as CNC machined components) are required or for capacity balancing purposes. As of December 31, 2023, we operated a total of 207 3D printers, five vacuum casting machines and 28 CNC machines at these service centers, which include distinct areas dedicated to the machinery, quality control, cleaning and labelling of our products. The table below provides selected information about our 3D printers and vacuum casting machines:
Technology |
| Size |
| Manufacturer |
| Number |
Stereolithography | Small/Medium Size | 3D Systems Corporation / Other | 42 | |||
Large Size | Stratasys | 2 | ||||
Mammoth | Materialise(1) | 15 | ||||
DLP | Small Size | Asiga/ Stratasys | 10 | |||
PolyJet | Connex | Stratasys Ltd. | 4 | |||
FDM | Small Size(2) | Stratasys Ltd. | 2 | |||
Medium Size(3) | Stratasys Ltd. | 16 | ||||
Large Size(4) | Stratasys Ltd. | 16 | ||||
Laser Sintering | Small Size | EOS GmbH | 14 | |||
Medium Size | 3D Systems Corporation/ EOS GmbH / Other | 25 | ||||
Large Size | EOS GmbH / Ricoh / Sindoh | 25 | ||||
Multi Jet Fusion | Medium Size | HP | 12 | |||
Sand Binding | Large Size | ExOne | 4 | |||
Vacuum Casting | MCP HEK GmbH | |||||
Medium Size | SCHUHL | 2 | ||||
Medium Size | MCP HEK GmbH | 1 | ||||
Large Size | 2 | |||||
Direct Metal Laser Sintering | Medium Size | EoS GmbH / GE Additive / SLM Solutions | 17 | |||
Large Size | SLM Solutions | 3 |
(1)We have proprietary stereolithography machines based on our patented curtain coat technologies. The original curtain coat machines had a medium sized build volume. These medium sized machines have subsequently been adapted to become the extra-large sized Mammoth machines.
(2)Small size machines are machines with a build volume of less than 250×250×250 mm.
(3)Medium size machines have a build volume of less than 500×500×500 mm.
(4)Large size machines have a build volume of more than 500×500×500 mm.
As of December 31, 2023, 50 printers produced parts exclusively for our Materialise Medical segment, while the other 157 printers and five vacuum casting machines produced parts for our Materialise Manufacturing segment.
As of December 31, 2023, all of our 3D printers and vacuum casting machines were either owned or held under a lease contract. At the end of the lease agreements (which are typically for a period of five years), we have an option to purchase the machines for a value of approximately 1.0% of their original value. We are responsible for the maintenance of such leased equipment.
47
We devote significant time and attention to the quality control of our products during the printing process by maintaining a comprehensive quality control program, which, among other things, includes the control and documentation of all material specifications, operating procedures, equipment maintenance and quality control methods. In addition, we inspect all of our raw materials to be used in our products throughout the printing process. We control our production orders through the use of labels or visual references on our internal database, bar-codes, controlled prints and routers, which enables us to trace our products during the printing process. Upon completion of the production process, we package and label our products.
The raw materials used in the printing of our products are mainly aluminum, titanium alloy and stainless steel powders, epoxy based photocurable resins, PA12 and thermoplastic polyurethane, or TPU, based powders and a suite of thermoplastic filaments like ABS, PC and Ultem and quartz sand and furanic resin binder.
With the exception of FDM, Stereolithography and PolyJet-materials, we believe that none of our other raw material requirements is limited to any significant extent by critical supply or price volatility. We continuously look for second sourcing of our raw materials in order not to be dependent on a single supplier in case a supply issue was to occur. We monitor the costs of our raw materials in order to optimize the cost/performance whilst not jeopardizing the expectations of our customers and the safe use of the materials in critical applications. With our strategic partnership with BASF 3D Printing Solutions GmbH, we are working towards offering to the market open solutions in terms of materials and software through which the user of additive manufacturing equipment can choose functionalities that best suit the user.
Our 3D printing operations for our patient-specific surgical guides, models and implants are subject to extensive regulation. We operate a certified quality management system in line with the U.S. Quality System Regulation, good manufacturing practice regulations and ISO 13485. We are registered with regulatory authorities in the United States, Europe, Canada, Australia and other jurisdictions. We CE mark our products where required. Our service centers are subject to periodic and sometimes unannounced inspections by regulatory authorities, including inspections by the FDA.
Research and Development
We have an ongoing research and development program to improve and expand the capabilities of our existing technology portfolio, which reflects our continued investments in a range of disciplines, including software development, industrial, and mechanical and biomedical engineering.
We have a long history of research and development through collaborations, which augment our internal development efforts. As of December 31, 2023, we were active in over 20 government funded research projects and we also employed multiple researchers with a publicly funded scholarship. With our platform technologies and strong track record in successful commercialization of scientific innovations, we receive many requests for participation in new development projects. While we strongly protect our intellectual property in our core competencies, many of our products require collaborations in order to create healthy ecosystems for their successful implementation.
As of December 31, 2023, we had more than 50 active research and development projects in various stages of completion and approximately 540 FTEs and fully dedicated consultants working on research and development in our facilities in Belgium, France, Germany, Spain, the United Kingdom, the United States, Colombia, Ukraine and Malaysia.
Our research and development projects include (but are not limited to) the following:
| 1. | various software development projects including projects related to engineering and design for 3D printing, and improving existing technological challenges (for example, the handling of large amounts of data and advanced image segmentation), which are expected to benefit both our Materialise Software and Materialise Medical segments; |
| 2. | research projects to understand and develop cutting-edge software tools for industrially relevant additive manufacturing technologies (powder bed fusion for plastics (laser sintering) and metal (laser melting and electron beam), stereolithography, FDM (also known as Filament Fusion), binder jetting power bed fusion, DLP-based printing and inkjet based technologies); |
| 3. | research projects in our Materialise Medical segment to develop patient specific surgical planning tools or surgical guides or implants for orthopedic, CMF and cardiovascular surgeries; |
48
| 4. | research projects on the use of virtual and augmented reality by our Materialise Medical segment; |
| 5. | research and development projects on smart digital technologies for the large-scale personalization of wearables; |
| 6. | various research projects on the use of artificial intelligence and (deep) machine learning in the fields of image processing and additive manufacturing; and |
| 7. | several research projects related to improving the maturity, reliability and quality of the additive manufacturing process, which are expected to benefit each of our three segments. |
We also regularly apply for research and development grants and subsidies under, among other, European, Belgian, British, French and German, grant rules. The majority of these grants and subsidies are non-refundable. We have received grants and subsidies from different authorities, including the Flemish government (VLAIO, or Vlaams Agentschap Innoveren en Ondernemen), the European Union (FP7 and H2020 framework programs) and BMBF, the German Federal Ministry of Education and Research.
We expect to continue to invest significantly in research and development in the future.
Intellectual Property
We regard our intellectual property rights as valuable to our business and protect our technology portfolio through a combination of patent, copyright, trademark, trade-secret and other intellectual property laws, confidentiality and other contractual provisions and other measures. The nature and extent of legal protection associated with each such intellectual property right depends on, among other things, the type of intellectual property right and the given jurisdiction in which such right arises.
As of December 31, 2023, our portfolio of intellectual property featured 476 issued patents and an additional 101 pending patent applications primarily in the United States, the European Union and Japan. Of these, our issued patents expire between approximately 2023 and 2040, while our currently pending patent applications will generally remain in effect for 20 years from the date of the initial applications. We believe that, while our patents provide us with a competitive advantage, our success depends primarily on our business development, applications know-how and ongoing research and development efforts. Accordingly, we believe that the expiration of any single patent, or the failure of any single patent application to result in an issued patent, would not be material to our business or financial position.
As is the case in the 3D printing industry generally, the development of our products, processes and materials has required considerable experience, manufacturing and processing know-how and research and development activities. We protect our proprietary products, processes and materials as trade secrets through nondisclosure and confidentiality agreements with our employees, consultants and customers.
In addition, we own the trademark registrations for “Materialise” and “ACTech” and trademark registrations and pending applications for many of our services and software solutions in those territories where we have substantial sales, including “CO-AM,” “Mimics,” “3-matic,” “Inspector,” “Magics,” “RapidFit+,” “Heartprint,” “ADaM,” “Surgicase,” “Enlight,” “Mindware,” “Streamics,” and “Phits,” among others.
We are party to various licenses and other arrangements that allow us to practice and improve our technology under a broad range of patents, patent applications and other intellectual property, including agreements with our collaboration partners, Zimmer Biomet, Enovis, DePuy Synthes, Lima, Mathys, Stryker, Corin, Siemens, Fluidda, HOYA and PTC.
There can be no assurance that the steps we take to protect our proprietary rights will be adequate or that third parties will not infringe or misappropriate such rights. We have been subject to claims and expect to be subject to legal proceedings and claims from time to time in the ordinary course of our business. In particular, we may face claims from third parties that we have infringed their patents, trademarks or other intellectual property rights. Such claims, even if not meritorious, could result in the expenditure of significant financial and managerial resources. Any unauthorized disclosure or use of our intellectual property could make it more expensive to do business and harm our operating results.
49
Seasonality
End markets such as healthcare, automotive, aerospace and consumer products may experience some seasonality. Historically, the revenue of our Materialise Software segment has been greater in the fourth quarter, as compared to the revenue of each of the other quarters. A number of our customers make their initial software purchase in the fourth quarter prior to the end of their annual budget cycle and tend to renew, extend or broaden the scope of their licenses on the anniversary date of their first purchase. In addition, we have in the past often brought new releases on the market in the third quarter of the calendar year, which may also have an impact on sales in the subsequent quarter.
Regulatory / Environmental Matters
Environmental Matters
Our facilities and operations are subject to extensive U.S. federal, state and local, European and other applicable foreign environmental and occupational health and safety laws and regulations. These laws and regulations govern, among other things, air emissions; wastewater discharges; the generation, storage, handling, use and transportation of hazardous materials; the handling and disposal of hazardous wastes; the clean-up of contamination; and the health and safety of our employees. Under such laws and regulations, we are required to obtain permits from governmental authorities for some of our operations. If we violate or fail to comply with these laws, regulations or permits, we could be fined or otherwise sanctioned by regulators. We could also be held responsible for costs and damages arising from any contamination at our past or present facilities or at third party waste disposal sites.
Our headquarters in Belgium, our manufacturing site in Poland, and ACTech’s headquarters in Germany, follow the ISO 14001:2015 criteria for an effective environmental management system. These sites are ISO 14001:2015 certified.
Compliance with laws and regulations relating to the discharge of materials into the environment or otherwise relating to the protection of the environment has not had a material impact on capital expenditures, earnings or the competitive position of our subsidiaries and us. We are not the subject of any legal or administrative proceedings relating to the environmental laws of Belgium or any country in which we have facilities. We have not received any notices of any violations of any such environmental laws.
Healthcare Regulatory Matters
In our Materialise Medical segment, we are subject to extensive and complex U.S. federal, state and local, European and other applicable foreign healthcare and medical devices laws and regulations.
Both before and after approval or clearance our medical products and product candidates are subject to extensive regulation. In the United States, the FDA under the Federal Food, Drug and Cosmetic Act primarily regulates us. In Europe and in other foreign jurisdictions in which we sell our medical products, many of the regulations applicable to our medical devices and products in these countries are similar to those of the FDA. Together, these regulations govern, among other things and where applicable, the following activities in which we are involved:
| ● | product development; |
| ● | product testing; |
| ● | product clinical trial compliance; |
| ● | product manufacturing; |
| ● | product labelling and instructions for use; |
| ● | product safety, product safety reporting, recalls and field corrective actions; |
| ● | product packaging and storage; |
| ● | product registration, market clearance or approval; |
50
| ● | product modifications; |
| ● | product marketing, advertising and promotion; |
| ● | product import and export, restrictions, tariff regulations, duties and tax requirements; |
| ● | product sales and distribution; |
| ● | post-market surveillance, including reporting of deaths or serious deterioration in the state of health and malfunctions that, if they were to recur, could lead to death or serious deterioration in the state of health; |
| ● | record keeping procedures; |
| ● | registration for reimbursement; and |
| ● | necessity of testing performed in country by distributors for licenses. |
Failure to comply with the Federal Food, Drug and Cosmetic Act could result in, among other things, warning letters, civil penalties, delays in approving or refusal to approve a medical device candidate, product recall, product seizure, interruption of production, operating restrictions, suspension or withdrawal of product approval, injunctions or criminal prosecution. Outside the United States, failure to comply with applicable laws and regulations could result in similar actions, and in the suspension or withdrawal of Quality Management System certification which may be a prerequisite to market medical devices.
The time required to obtain clearance required by foreign countries may be longer or shorter than that required for FDA clearance, and requirements for licensing a product in a foreign country may differ significantly from FDA requirements.
Moreover, these laws and regulations are subject to change. For example, on May 26, 2021, the Medical Devices Regulation became applicable in the European Union and replaced the Medical Device Directive. This required us to adopt a series of measures and we will continue to update our systems and product registrations during the provided transition period to comply with this new Regulation. For more information, see “Item 3. Key Information—D. Risk Factors—Risks Related to Our Materialise Medical Segment and Regulatory Environment—Healthcare policy changes, including legislation to reform the U.S. healthcare system, could adversely affect us.”
We have obtained MDSAP certification. This program allows an MDSAP-recognized auditing organization to conduct a single regulatory audit of a medical device manufacturer that satisfies the relevant requirements of the regulatory authorities participating in the program. To the extent that we do business in the participating jurisdictions, certain major non-conformities identified under this program may be escalated to the regulatory authorities of the United States, Canada, Japan, Australia and Brazil. The Canadian regulatory authority, Health Canada, has made participation in MDSAP a mandatory requirement for medical device manufacturers importing products to Canada. Failure to maintain certification under MDSAP may impact our capability to do business in Canada. In addition, failure to address escalated issues reported to the participating authorities may impact our capability to do business in the respective jurisdictions.
51
| C. | Organizational Structure |
The following illustrates our corporate structure as of the date of this annual report:
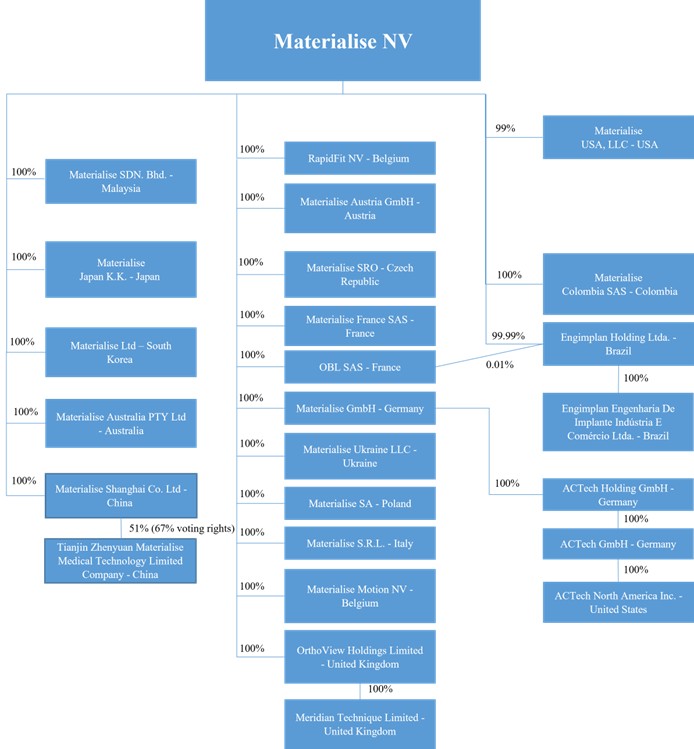
52
| D. | Property, Plants and Equipment |
Our corporate headquarters and our largest 3D printing service center are located in Leuven, Belgium. We currently own office and service spaces in Belgium as well as in the Czech Republic, France, Germany, Poland and the United States. We also lease other service centers and sales offices, which are located in Austria, Australia, Belgium, Brazil, China, Colombia, France, Germany, Italy, India, Japan, Malaysia, Spain, Ukraine, the United Kingdom, the United States, Poland, and South Korea. The aggregate annual lease payments for our facilities in 2023, 2022 and 2021 were € 2.2 million, € 2.0 million and € 2.1 million, respectively. The table below provides selected information regarding our facilities as of December 31, 2023.
Location |
| Ownership |
| Use |
| Approximate Area |
| Lease Expiration |
Leuven, Belgium |
| Owned |
| Corporate headquarters; production |
| 50,614.35 sq. m. |
| N/A |
Leuven, Belgium | Leased | Warehouse | 165 sq. m. | March 31, 2024 | ||||
Beringen, Belgium | Leased | Office; production | 2,848.25 sq. m. | October 31, 2030 | ||||
Plymouth, Michigan, United States |
| Owned |
| Office; production; parking |
| 3.89 acres |
| N/A |
Ann Arbor, Michigan, United States | Leased | Office; production | 2,771 sq. ft. | April 30, 2024 | ||||
Lexington, KY, United States | Leased | Office | 1,872 sq. ft. | August 31, 2027 | ||||
Princeton, NJ, United States | Leased | Office | 2,866 sq. ft. | March 31, 2025 | ||||
Lafayette, CO, United States | Leased | Office | 2,218 sq. ft. | February 28, 2025 | ||||
Saint Marcel les Valence, France |
| Owned |
| Office |
| 1,100 sq. m. |
| N/A |
Yokohama, Japan |
| Leased |
| Office |
| 515.58 sq. m. |
| March 31, 2024 |
Kawasaki, Japan |
| Leased |
| Production |
| 205 sq. m. |
| May 19, 2024 |
Ústí nad Labem, Czech Republic |
| Owned |
| Office; production |
| 16,013 sq. m. |
| N/A |
Vienna, Austria |
| Leased |
| Office |
| 44 sq. m. |
| December 31, 2025 |
Gilching, Germany |
| Leased |
| Office |
| 399 sq. m. |
| December 31, 2024 |
Bremen, Germany | Owned | Office | 6,724 sq. m. | N/A | ||||
Petaling Jaya, Malaysia |
| Leased |
| Office |
| 13,935 sq. ft. |
| May 31, 2029 |
Paris, France |
| Leased |
| Office |
| 564.40 sq. m. |
| May 31, 2028 |
Kyiv, Ukraine |
| Leased |
| Office |
| 2,532.6 sq. m. |
| February 29, 2024 under negotiation to extend every 6 months (due to war conditions) |
Rozdil, Ukraine | Leased | Office | 570.4 sq. m. | February 28, 2024- lease cancelled | ||||
Sheffield, United Kingdom |
| Leased |
| Office |
| 1,575 sq. ft. |
| No fixed end date |
Southampton, United Kingdom | Leased | Office | 2,046 sq. ft. | May 31, 2028 | ||||
Shanghai, China |
| Leased |
| Office |
| 1,200 sq. m. |
| June 8, 2024 |
Medellin, Colombia |
| Leased |
| Office |
| 248 sq. m. |
| May 31, 2024 |
Leased | Office | 64 sq. m. | January 31, 2025 | |||||
Leased | Office | 190 sq. m. | November 30, 2024 | |||||
Leased | Office | 59.79 sq. m. | March 15, 2024 | |||||
Leased | Office | 60.31 sq. m. | March 31, 2024 | |||||
Wroclaw, Poland |
| Owned |
| Office; production |
| 2.3975 hectare |
| N/A |
Gold Coast, Australia |
| Leased |
| Office |
| N/A |
| January 22, 2024 |
Milan, Italy |
| Leased |
| Office |
| 55 sq. m. |
| December 31, 2023 |
Milan, Italy |
| Leased |
| office |
| 131 sq. m. |
| March 31, 2029 |
Freiberg, Germany | Owned | Office, Production, Parking (Land) | 34,273 sq. m. | N/A | ||||
Freiberg, Germany | Owned | Office, warehouse, production, parking (Land) | 24,243 sq. m. | N/A | ||||
Bangalore, India | Leased | Office | 2,000 sq. ft. | December 31, 2024 | ||||
Rio Claro, Brazil | Leased | Corporate Offices, R&D Laboratory, Production | 4,092.27 sq. m. | August 5, 2029 | ||||
Seoul, South Korea | Leased | Shared workspace | N/A | January 31, 2025 | ||||
Tianjin, China | Leased | Office | 129 sq. m. | March 19, 2025 |
See also “—B. Business Overview—Manufacture and Supply” for information about the printers we operate, “—Regulatory / Environmental Matters—Environmental Matters” for information about environmental matters and “Item 5. Operating and Financial Review and Prospects —B. Liquidity and Capital Resources—Indebtedness” for information about indebtedness secured by mortgages.
53
ITEM 4A.UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 5.OPERATING AND FINANCIAL REVIEW AND PROSPECTS
This section contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those contained in forward-looking statements. Factors that could cause or contribute to such differences include, without limitation, those discussed in the sections entitled “Item 3. Key Information—D. Risk Factors,” “Special Note Regarding Forward-Looking Information” and “Item 4. Information on the Company—B. Business Overview” and elsewhere in this annual report.
A. | Operating Results |
Overview
Company Overview
We are a leading provider of additive manufacturing and medical software tools and of sophisticated 3D printing services. With our knowledge, products and services, we empower our customers to use additive manufacturing technology more effectively, in general, and we enable certain specific and significant applications of additive manufacturing, in particular. In both instances, we seek to empower the choice for sustainability through the use of additive manufacturing.
The customers of our general software tools and 3D printing services are active in a wide variety of industries, including healthcare, automotive, aerospace, art and design and consumer products. The significant additive manufacturing applications that we are more deeply and more directly involved in currently include applications for orthopedic, cranio maxillo facial, eyewear, footwear and measurement fixtures.
As of December 31, 2023, our team consisted of 2,437 FTEs and fully dedicated consultants. Our portfolio of intellectual property featured 476 issued patents and 101 pending patent applications as of December 31, 2023. For the year ended December 31, 2023, we generated € 256.1 million of revenue, representing 10% increase over the prior year, a net profit of € 6.7 million and an Adjusted EBITDA of € 31.4 million. For a description of Adjusted EBITDA and a reconciliation of our net profit to our Adjusted EBITDA, see “—Other Financial Information” below.
Public Offering
On July 6, 2021, we closed a follow-on public offering of a total of 4,600,000 ADSs at a public offering price of $24.00 per ADS for gross proceeds of $ 110.4 million.
We raised approximately $ 105.0 million (or € 92.7 million, based on the exchange rate as of December 31, 2021) in aggregate net proceeds from such follow-on offering.
Link3D Acquisition
On April 9, 2021, we acquired an option to buy Link3D Inc., which we exercised on November 15, 2021. We closed the acquisition on January 4, 2022. This acquisition was effected by our U.S. subsidiary, Materialise USA, LLC, by exercising the call option. As a result of this transaction, Materialise USA became the sole shareholder of Link3D, and subsequently Link3D was merged into Materialise USA. Link3D was an additive workflow and digital manufacturing software company. The acquisition of Link3D is intended to strengthen and accelerate the creation of the Materialise software platform.
Identify3D Acquisition
On September 1, 2022, we acquired Identify3D, a company that develops software to encrypt, distribute and trace the flow of digital parts across complex supply chains. This acquisition was effected by our U.S. subsidiary, Materialise USA, LLC, and subsequently Identify3D was merged into Materialise USA. The acquisition of Identify3D is intended to strengthen the security features of our CO-AM platform.
54
Growth Strategy
In general, our strategy is built on the development and sale of two different sets of product portfolios: our horizontal and our vertical solutions.
| ● | Each of our segments has what we call a horizontal product offering that addresses a broad set of needs of customers that make use of additive manufacturing: our market leading Magics Software platform and the CO-AM platform that we launched in 2022 in our Materialise Software segment, the Mimics Innovation Suite in our Materialise Medical segment and the additive manufacturing services that we offer through our Materialise Manufacturing segment. We believe that each of these horizontal platforms has the potential of continuing to grow as the adoption of additive manufacturing by our customers in each of our segments grows. |
| ● | Second, leveraging on the technological and market knowledge that we gain as we bring our horizontal offerings to the market, we have built a select number of what we call vertical applications of 3D printing. These vertical applications, which address the specific needs of a particular subset of customers in a much more specific manner, include our surgical knee guides and personalized CMF guides and implants in our Materialise Medical segment and our measurement fixtures and personalized foot and eyewear products in our Materialise Manufacturing segment. We believe that this more focused presence in a few applications of 3D printing has the potential to further boost our growth. |
Within the horizontal and vertical frameworks, each of our segments develops its own shorter term strategy.
In our Materialise Software segment, we intend to strengthen the market penetration of our software platform by (i) continuing to gradually grow the strong position of our Magics 3D Print Suite in the market for print preparation software tools, including by offering its functionality through the cloud, and (ii) bringing our CO-AM platform to the market, offering to our customers both proprietary and third party functionalities that focus on volume production, including manufacturing execution systems, or MES, automated workflows for additive manufacturing and solutions such as quality analysis tools and data security. The transition to a cloud-based software platform and associated subscription models will affect our revenue levels in the near term, but we believe it may ensure the continued strength of our business model going forward. Further, in order to be able to meet the demands that are associated with volume production, including mass customization, and to accelerate the development and roll out of our cloud-based software platform, where software as a service, big data technologies, and machine learning will be key drivers, we intend to continue to invest significantly in both research and development.
In our Materialise Medical segment, we believe that the growing trend of personalized patient care will boost the demand for digital planning tools as well as for personalized medical devices. In response to that trend, we intend to continue to increase the penetration of our existing software products in the hospital market and to expand our portfolio of planning tools into new areas such as cardiovascular and pulmonology. We also intend to continue to develop and grow the sales of our personalized medical device portfolio, both directly and indirectly and in existing as well as in new markets, including in particular in the CMF market.
In our Materialise Manufacturing segment, we believe that there is significant growth potential in the markets for additive manufacturing of end-use products. We therefore intend to continue to invest in the expansion and creation of certified 3D manufacturing environments that meet the high standards of the specialized segments of the industrial market, including the aerospace market. More particularly, we believe that our growth initiatives in the wearables market that have been incubated within Materialise Manufacturing may drive growth in the coming years. In the eyewear market, we are investing in back-end production facilities for the production of 3D printed eyewear, including customized frames and also invest in the introduction of advanced front-end digital technologies, such as virtual try-on and fitting solutions, In the footwear market, we will continue to invest in the development and commercial roll out of the pressure plate technology and related applications that we acquired from RS Scan and in the worldwide commercialization of our Phits customized 3D printed insoles.
Importantly, our applications and solutions focus on empowering our customers’ and partners’ choice for sustainability. In the applications that we support, additive manufacturing has the potential to not only replace traditional manufacturing technologies, but also to enable the digitization of customer journeys and supply chains, to localize manufacturing, to reduce inventories and the use of raw materials and to make end customers’ solutions more durable through personalization. We believe that this focus on the choice for sustainability will position us for long term sustainable growth, even if this may imply that we forego short term growth opportunities that do not fit this vision.
55
Recent Developments
See Note 27 to our audited consolidated financial statements for disclosure of significant transactions and significant changes in our financial condition or results of operations that occurred subsequent to December 31, 2023. In addition, see “—Trend Information” below.
Key Income Statement Items
Revenue
Revenue is generated primarily by the sale of our software and 3D printed and complex manufactured products and services.
In our Materialise Software segment, we generate revenues from software licenses, maintenance contracts and custom software development services and sales of Materialise Controller.
In our Materialise Medical segment, we generate revenue through the sale of medical devices that we print or manufacture for our customers and from the sale of licenses on our medical software packages, software maintenance contracts and custom software development and engineering services.
In our Materialise Manufacturing segment, we generate most of our revenue through the sale of parts that we print or produce for our customers.
Software. Software revenue is comprised of perpetual and time-based licenses, maintenance revenue and software development service fees. Our software products are mainly licensed pursuant to one of two payment structures: (i) perpetual licenses, for which the customer pays an initial fee for a perpetual license and subsequently pays fees for maintenance under separate maintenance contracts, generally on an annual basis, or (ii) time-based licenses (generally annual licenses), for which the customer pays equal periodic fees to keep the license active. Perpetual licenses require the payment of fees for maintenance, technical support and product updates. Time-based licenses entitle the customer to corrective maintenance and product updates without additional charge. We generally recognize revenue from our time-based licenses and our maintenance revenue on a straight-line basis over the term of the applicable license or maintenance contracts. Our software revenue depends upon both incremental sales of software licenses to both new and existing customers and renewals of existing time-based licenses and maintenance contracts. Sales and renewals are also driven by our customers’ usage and budget cycle. Software development services are typically charged either on a time and materials basis or on a fixed fee basis.
3D printed products and services. 3D printed products revenue is derived from our network of 3D printing service centers. Our service centers not only utilize our 3D printing technology to print products but are also full-service operations that provide support and services such as pre-production collaboration prior to printing the product. Revenue from 3D printed products depends upon the volume of products that we print for our customers. Sales of these products are linked to the number of our 3D printing machines that are installed and active worldwide. We have dedicated teams and production lines for industrial applications and medical applications. All medical products require a highly regulated production environment. Whereas both segments use the same 3D printing technologies, the complex combination of our engineering and software solutions in connection with medical applications results in higher margins for our medical applications.
Production of limited runs of highly complex casted metal parts. Casted products revenue is derived from ACTech’s network, with its production unit in Freiberg, Germany. ACTech is utilizing casting technology, including 3D printing technology for mold making, and offers full-service project operations, including project and pre-production collaboration, and high-end complex finishing services.
Cost of Sales
Our cost of sales includes raw materials, external subcontracting services, labor costs, manufacturing overhead expenses, amortization and depreciation and reserves for inventory obsolescence. Our manufacturing overhead expenses include quality assurance, manufacturing engineering, material procurement, inventory control, facilities, equipment and information technology and operations supervision and management.
56
Research and Development Expenses
Our research and development activities primarily consist of engineering and research programs associated with our products under development as well as research and development activities associated with our core technologies and processes. Research and development expenses are primarily related to employee compensation, including salary, fringe benefits, share-based compensation and temporary employee expenses. We also incur expenses for software and materials, supplies, costs for facilities and equipment, depreciation, and outside design and outside research support.
Development expenditures on an individual project are recognized as an intangible asset when we can demonstrate:
| ● | the technical feasibility of completing the intangible asset so that the asset will be available for use or sale; |
| ● | the intention to complete and the ability to use or sell the asset; |
| ● | how the asset will generate future economic benefits; |
| ● | the availability of resources to complete the asset; and |
| ● | the ability to measure reliably the expenditure during development. |
We have determined that the conditions for recognizing internally generated intangible assets from proprietary software, guides and other product development activities are not met until shortly before the products are available for sale, unless either (i) we have strong evidence that the above criteria are met and a detailed business plan is available showing the asset will on a reasonable basis generate future economic benefits or (ii) the development is done based upon specific request of the customer, we have the intention to market the product to parties other than the customer, the development is subject to an agreement and the substance of the agreement is that the customer reimburses us for a significant portion of the development expenses incurred. As such, development expenditures not satisfying the above criteria and expenditures on the research phase of internal projects are recognized in the consolidated income statement as incurred.
Sales and Marketing Expenses
Our sales and marketing expenses primarily consist of employee compensation, including salary, fringe benefits and share-based compensation for our marketing, sales and business development functions. Other significant expenses include travel, depreciation, product demonstration samples, brochures, websites and trade show expenses.
General and Administrative Expenses
Our general and administrative expenses primarily consist of employee compensation, including salary, fringe benefits and share-based compensation for our executive, financial, human resources, information technology support and regulatory affairs and administrative functions. Other significant expenses include outside legal counsel, independent auditors and other outside consultants, insurance, facilities, depreciation and information technologies expenses.
Net Other Operating Income
Net other operating income consists primarily of withholding tax exemptions for qualifying researchers, development and government grants, partial funding of research and development projects, currency exchange results on purchase and sales transactions the amortization of intangible assets from business combinations, write-off of trade receivables, impairment of goodwill and intangible assets, and revaluation income or costs from participations.
Government grants are recognized when there is reasonable assurance that the grant will be received and all attached conditions will be complied with. When the grant relates to development costs or another expense, it is recognized as income over the grant period necessary to match the income on a systematic basis to the costs that it is intended to compensate. When the grant relates to the construction of buildings, it is recognized as income over the depreciation period of the related building.
57
Such grants have been received from the federal and regional governments and from the European Union in the forms of grants linked to certain of its research and development programs, reduced payroll taxes and the financing of the construction of an office building in Leuven, Belgium and in Freiberg, Germany.
Where retention of a government grant is related to assets or to income and dependent on the Company satisfying certain criteria, it is initially recognized as deferred income. When the criteria for retention have been satisfied, the deferred income balance is released to other operating income in the consolidated income statement on a systematic basis over the periods in which the entity recognizes as expenses the related costs for which the grants are intended to compensate.
Government grants are recognized when there is reasonable assurance that the grant will be received and all attached conditions will be complied with.
Financial Expenses
Our financial expenses primarily include costs associated with currency exchange differences and with interest payments on our debt.
Critical Accounting Policies and Accounting Estimates
The preparation of our consolidated financial statements requires management to make judgments, estimates and assumptions that affect the reported amounts of revenue, expenses, assets and liabilities, and the accompanying disclosures. Uncertainty about these assumptions and estimates could result in outcomes that require a material adjustment to the carrying amount of assets or liabilities for future periods.
On an ongoing basis, we evaluate our estimates, assumptions and judgments, including those related to revenue recognition, development expenses, share-based payment transactions, income taxes, impairment of goodwill, intangible assets and property, plant & equipment and business combinations.
We based our assumptions and estimates on parameters available when the consolidated financial statements were prepared. Existing circumstances and assumptions about future developments, however, may change due to market changes or circumstances arising beyond our control. Such changes are reflected in the assumptions when they occur.
Revenue Recognition
Our revenue recognition policies require management to make significant estimates. Management analyzes various factors, including a review of specific transactions, historical experience, creditworthiness of customers and current market and economic conditions. Changes in judgments based upon these factors could impact the timing and amount of revenue and cost recognized and thus affects our results of operations and financial condition. The significant estimates and judgments relate to:
| ● | assessing whether a performance obligation is distinct in a bundled sale(s) transaction; |
| ● | estimation of the variable considerations and the assessment of the revenue constraint limitation; |
| ● | estimation of the stand-alone selling prices for each distinct performance obligation; and |
| ● | the stage of completion of our custom development of software components for customers when revenues are satisfied over time. |
58
We make significant judgments when performing the assessment of whether a performance obligation is distinct from the other performance obligations in a contract, i.e., whether the good or service has a benefit to the customer on its own or together with readily available resources and/or whether the good or service is highly interrelated or constitutes a significant input with another good or service provided, or whether it significantly modifies or tailors another good or service. Relevant judgments include the following:
| ● | Whether the software license is distinct from the 3D printed guides – in most cases with contracts with collaborative partners in the Materialise Medical segment, the software license is combined with the manufacturing of the 3D printed guides as the software license has no benefit to the customer without the manufacturing services. We have also implemented a new “Plan Only” feature where the collaboration partners can benefit from a virtual plan produced with the software license without the manufacturing of any physical product. Such Plan Only features are recognized in revenue as a separate performance obligation based on the usage by the collaboration partner. |
| ● | Whether the development services are distinct from other performance obligations – in most cases, these performance obligations are distinct however for one contract with a collaboration partner in the Materialise Medical segment, the software license is combined with the license and the 3D printed guides as one “distinct” performance obligation. |
For the stand-alone selling prices, we use prices from price list or historical prices for similar transactions. However, in certain cases, such information may not be readily available, and in those cases, we estimate the stand-alone selling price based on a cost-plus mark-up or other estimate. If the stand-alone selling price of one or more goods or services in arrangements with multiple performance obligations is highly variable or uncertain, we estimate the stand-alone selling price with reference to the total transaction price less the sum of the observable stand-alone selling prices of other goods or services promised in the contract. In addition, for certain performance obligations such as development services, the stand-alone selling prices also require an estimate of the time required to complete the development.
Certain contracts include estimates of variable considerations within the transaction price and assessing the revenue constraint, such as:
| ● | quantities/volume sold at fixed prices related to, but not limited to, the manufacturing of 3D printed products, software licenses sold and maintenance renewals; |
| ● | contractual prices may vary based on volume purchased during a given period; |
| ● | FTE expenses for development or other services billed on a time & material basis; and |
| ● | volume rebates. |
The method used to estimate the variable consideration depends on the number of possible scenarios and the probability of each scenario. If there are many possible scenarios with a high probability probabilities (each less than 50)%, we will use the expected value method, while the most likely method is used when there is a scenario with a higher probability (more than 50)%.
Variable consideration is not a constraint when, based on historical experience, a high reliable business forecast and/or the timeframe of the estimates, we determine that there is a high probability that it will not result in a future reversal of revenue.
We determine the stage of completion for development contracts satisfied over time by comparing the labor hours incurred to date with the estimated total labor hours required to complete the project. We consider labor hours to be the most reliable, available measure of progress on these projects. Adjustments to estimates to complete are made in the periods when facts that give rise to a change become known. When the estimate indicates that a loss will be incurred, the loss is recorded in the relevant period. Significant judgments and estimates are involved in determining the percentage completion for each contract. Different assumptions can produce materially different results.
59
Development Expenses
Under International Accounting Standards 38, or IAS 38, internally generated intangible assets from the development phase are recognized if certain conditions are met. These conditions include the technical feasibility, the intention to complete, the ability to use or sell the asset under development, the availability of technical, financial and other resources to complete the asset, and the demonstration of how the asset will generate probable future economic benefits. The cost of a recognized internally generated intangible asset comprises all directly attributable cost necessary to make the asset capable of being used as intended by management. In contrast, all expenditures arising from the research phase are expensed as incurred.
Determining whether internally generated intangible assets from development are to be recognized as intangible assets requires significant judgment, particularly in determining whether the activities are considered research activities or development activities, whether the product enhancement is substantial, whether the completion of the asset is technically feasible considering a company-specific approach and the likelihood of future economic benefits from the sale or use.
We have determined that the conditions for recognizing internally generated intangible assets from proprietary software, guide and other product development activities are not met until shortly before the products are available for sale, unless either (i) we have strong evidence that the above criteria are met and a detailed business plan is available showing that the asset will generate future economic benefits on a reasonable basis or (ii) the development is done based upon specific request of the customer, we have the intention to market the product also to other parties than the customer, the development is subject to an agreement and the substance of the agreement is that the customer reimburses us for a significant portion of the development expenses incurred. As such, development expenditures not satisfying the above criteria and expenditures on the research phase of internal projects are recognized in the consolidated income statement as incurred. This assessment is monitored on a regular basis.
We have determined that the criteria for internally generated intangible assets were met for two projects in 2018: (1) the software development of a new planner for hospitals within the cardiovascular field and (2) the process to obtain FDA and E.U. approval for a 3D printed tracheal splint within the Materialise Medical segment. The first project was successfully completed in 2019. In 2021, we recognized an impairment of € 0.2 million as the business case no longer showed a positive result over the next 5 years. The main reason was a delay in revenue due to the ongoing COVID-19 pandemic. The second project obtained the Investigational Device Exemption, or IDE, approval from the FDA in the first quarter of 2024. Because the present value of the expected cash flows until 2030 remains negative we continue to report the R&D expenses related to this program in our income statement.
In the year ended December 31, 2020 we determined that the criteria for internally generated intangible assets were met for certain subprojects related to our internal digital transformation program. With this program, we are investing in our IT landscape and upgrading and/or standardizing part of our digital core business. We have further invested in 2023, and will continue to invest in 2024 in state-of-the-art technology that is available on the market to upgrade our CRM, ERP and license management software. Together with this implementation, we will also upgrade and further develop those internal software programs that are closely related to 3D printing and the specific needs that arise from 3D printing. The integration of both standard and internal systems in the digital chain of Materialise is crucial and requires deep analysis, development and technical validation. It is expected that it will not only streamline our processes internally and help us reduce costs in maintenance in the short term, but it also will allow us to learn from this and commercialize this knowledge by making our software even easier to integrate with standard systems. This competitive advantage should become important in the coming years where 3D printing will be more and more integrated on the traditional manufacturing floor. As of December 31, 2023, we capitalized €7.5 million as software and carried €0.4 million as assets under construction in respect of those projects.
60
In the year ended December 31, 2021 we determined that the criteria for internally generated intangible assets were met for our project on the transformation of the platform architecture to enable our software products to make the transition from desktop to (hybrid) cloud. As of December 31, 2021 we had capitalized K€975 in respect of this project. During 2022 we continued to invest in this project and added K€984 to the asset under construction. As of December 31, 2022, we recognized an impairment of K€672 in respect of this asset under construction, due to an overlap with the recently acquired Link3D technology and the fact that this Link3D technology was already in a more advanced stage. The remaining K€1,287 was transferred from assets under construction to software, and amortization on this asset started in 2022.
Share-Based Payment Transactions
We measure the cost of equity-settled transactions with employees based on the fair value of the equity instruments at the date at which they are granted and measures the cost of cash-settled transactions based on the fair value of the equity instrument at the date of reporting. We have applied the Black-Scholes valuation model to estimate fair value. The use of this model requires management to make assumptions regarding the volatility and expected life of the equity instruments. The assumptions used to determine fair value for share-based payment transactions are disclosed in Note 14 to our consolidated financial statements and are estimated as follows:
| ● | the dividend return is estimated by reference to the historical dividend payment. Currently, this is estimated to be zero as no dividends have been paid since inception; |
| ● | expected volatility is determined based on the average annualized volatility of our shares (until September 2016: of a number of quoted peers in the 3D printing industry and the volatility of our shares); |
| ● | estimated life of the warrant is determined to be until the first exercise period which is typically the month after vesting; and |
| ● | fair value of the shares is determined based on the share price of our ADSs on Nasdaq at the date of valuation. For the grants prior to the initial public offering, the fair value of the shares was estimated based on a discounted cash flow model with three-year cash flow projections and a multiple of EBITDA determined based on a number of quoted peers in the 3D printing industry. |
Income Taxes
Deferred tax assets are recognized only to the extent that it is probable that taxable profit will be available against which the deductible temporary difference can be utilized. Significant management judgment is required to determine the amount of deferred tax assets that may be recognized, based on the probable timing and level of future taxable profits, together with future tax planning strategies. As of December 31, 2023, we had current and non-current receivables related to tax credits for an amount of € 5 million (2022: € 5 million; 2021: € 5 million).
As of December 31, 2023, we had € 92 million (2022: € 88 million; 2021: € 58 million) of tax losses carried forward and Innovation Income Deductions, of which € 47 million related to Materialise NV (2022: € 45 million; 2021: € 36 million). These losses related to Materialise NV and subsidiaries that have a history of losses, in countries where these losses do not expire and may not be used to offset taxable income elsewhere in our consolidated group.
Under the Belgian Innovation Income Deduction system, companies can deduct up to 85% of their net innovation income from the taxable basis.
With respect to the tax losses carried forward and Innovation Income Deductions carried forward we recognized at December 31, 2023 a deferred tax asset of € 0.1 million for Materialise NV (2022: € 0.2 million, 2021: € 0.0 million) and € 1.0 million for Materialise USA (2022: € 1.6 million, 2021: € 0.0 million).
We have not recognized deferred tax assets on unused tax losses and Innovation Income Deduction totaling € 22 million as at December 31, 2023 (2022: € 19 million; 2021: € 12 million) given that it is not probable that sufficient positive taxable base will be available in the foreseeable future against which these tax losses and Innovation Income Deduction can be utilized. If all deferred tax assets related to unused tax losses carried forward and Innovation Income Deduction would meet the criteria for recognition, net result for the year would have improved by € 22 million in 2023 through a deferred tax benefit. This would represent the planned recovery of € 88 million of unused tax losses carried forward and Innovation Income Deduction in future periods. Further details on taxes are disclosed in Note 22.10 to our consolidated financial statements.
61
Impairment of Goodwill, Intangible Assets and Property, Plant and Equipment
We have goodwill for a total amount of € 43.2 million as of December 31, 2023 (2022: € 44.2 million; 2021: € 18.7 million) which has been subject to an impairment test. Goodwill is tested for impairment based on a discounted cash flow model with cash flows for the next five years derived from the budget, and a residual value considering a perpetual growth rate. The value in use is sensitive to the discount rate used for the discounted cash flow model as well as the expected future cash-inflows and the growth rate used for extrapolation purposes. The key assumptions used to determine the value in use for the different cash generating units, or CGUs, are disclosed and further explained in Note 5 to our consolidated financial statements.
When events or changes in circumstances indicate that the carrying amount of the intangible assets and property, plant and equipment may not be recoverable, we estimate the value in use for the individual assets, or when not possible, at the level of CGUs to which the individual assets belong. Total impairment charges recorded during 2023 were € 4.2 million (2022: € 0.7 million; 2021: € 0.2 million).
In 2021, we recorded a goodwill impairment of € 0.2 million on the CGU Metal in Belgium, formerly the company Aldema BV. We acquired all shares and voting rights of Aldema BV in 2015 for € 0.1 million. With the creation of the Materialise Metal Competence Center in Bremen, Germany, the recoverable amount of this asset decreased to zero.
In 2022, we recorded impairment charges of € 0.7 million related to the transformation of the platform architecture to enable our software products to make the transition from desktop to cloud-based.
In 2023, we recorded a goodwill impairment of € 4.2 million related to Materialise Motion and Engimplan. On the CGU Materialise Motion in Belgium, it was concluded that the value in use was lower than the carrying value, which resulted in a full impairment of the goodwill for an amount of € 1.2 million as well as a partial impairment on the intangible assets related to the partnership agreement, customer list, and developed technology for respectively € 0.9 million, € 0.1 million, and € 1.4 million. The key elements that led to the impairment loss for the Motion CGU was the delay in business growth versus what was initially foreseen. On the CGU Engimplan in Brazil, it was concluded that the value in use was lower than its carrying value, which resulted in a full impairment of the intangible assets related to customer list and trademarks for respectively € 0.4 million and € 0.1 million as well as a tangible asset 3D printer for € 0.1 million. The key elements that led to the impairment loss for the Engimplan CGU were related to a delay in business growth and to less benefits of synergies than initially anticipated.
Business Combinations
We determine and allocate the purchase price of an acquired business to the assets acquired and liabilities assumed as of the business combination date. The purchase price allocation process requires us to use significant estimates and assumptions, including:
| ● | estimated fair value of the acquired intangible assets; |
| ● | estimated fair value of property, plant and equipment; and |
| ● | estimated fair value of the contingent consideration. |
The contingent consideration as included in the financial statements is recorded at fair value at the date of acquisition and is reviewed on a regular basis, at least annually. The fair value of the contingent consideration is based on risk-adjusted future cash flows of different scenarios discounted using appropriate interest rates. The structure of the possible scenarios and the probability assigned to each one of them is reassessed by management at every reporting period and requires judgement from management about the outcome and probability of the different scenarios as well as the evolution of the variables.
While we are using our best estimates and assumptions as part of the purchase price allocation process to accurately value assets acquired and liabilities assumed at the date of acquisition, our estimates and assumptions are inherently uncertain and subject to refinement. Examples of critical estimates in valuing certain of the intangible assets we have acquired or may acquire in the future, include but are not limited to:
| ● | future expected cash flows from customer contracts and relationships, software license sales and maintenance agreements; |
| ● | the fair value of the plant and equipment; |
62
| ● | the fair value of the deferred revenue; |
| ● | discount rates; and |
| ● | the technology royalty rate. |
Provision for Expected Credit Losses, or ECLs, of Trade Receivables and Contract Assets
We use a provision matrix to calculate ECLs for trade receivables and contract assets. The provision rates are based on days past due for groupings of various customer segments that have similar loss patterns (i.e., by legal entity).
The provision matrix is initially based on our historical observed default rates. The matrix is calibrated to adjust the historical credit loss experience with forward-looking information. For example, if economic conditions (i.e., gross domestic product) are expected to deteriorate over the next year which can lead to an increased number of defaults, the historical default rates are adjusted. At each reporting date, the historical observed default rates are updated and changes in the forward-looking estimates are analyzed.
The assessment of the correlation between historical observed default rates, expectations of economic conditions and ECLs is a significant estimate. The amount of ECLs is sensitive to changes in circumstances and of forecast economic conditions. Our historical credit loss experience and expectations of economic conditions may also not be representative of a customer’s actual default in the future. Information about the ECLs on our trade receivables and contract assets is disclosed in Note 25 to our consolidated financial statements.
Convertible Loan Granted to Fluidda
We account for the convertible loan granted to Fluidda in January 2019, with a notional amount of € 2.5 million, at fair value. The carrying value of the convertible loan amounts to € 3.7 million at December 31, 2023. Fluidda is a private start-up company which offers turnkey contract research services for drug development and medical device development. Fluidda is currently loss-making. In determining the fair value of the convertible loan, we consider different contractual parameters such as the repayment and conversion scenarios and dates. In addition, we must make significant estimates such as (i) the discount rate, (ii) the probability and timing of each repayment and conversion scenario, and (iii) the amount of a qualified capital increase that will determine the conversion factor. The convertible loan has a duration of seven years with a 10% annual interest rate which is capitalized. We have applied a discount factor of 13.32% that is based on the estimated weighted average cost of capital of Fluidda, reflecting the uncertainty in relation to Fluidda’s ability to be successful and the applied estimates by our consolidated group.
Leases – Estimating the Discount Rate and Probability of Exercising Extension Options/Termination Options and Purchase Options
As we cannot always determine the interest rate implicit in lease contracts, we must estimate the incremental borrowing rate to measure certain lease liabilities such as buildings. For buildings, we use the property yield as a reference to determine the incremental borrowing rate. For other assets, we generally use the interest rate implicit in the lease contract or apply the incremental borrowing rate for a portfolio of similar assets. The incremental borrowing rate reflects what we “would have to pay”, which requires estimation when no observable rates are available or when they need to be adjusted to reflect the terms and conditions of the lease.
In addition, certain lease contracts may have extension options, termination options (in the case of property leases) and/or purchase options (in the case of leases). We estimate whether it is reasonably certain that such options will be exercised, which impacts the lease term in the case of extension options and termination options and the period over which the lease assets are depreciated in the case of purchase options.
Recent Accounting Pronouncements
The standards and interpretations that are issued, but not yet effective, up to the date of issuance of our financial statements are disclosed in our financial statements included elsewhere in this annual report. Of those standards that are not yet effective, none are expected to have a material impact on our financial statements in the period of initial application.
63
Results of Operations
Comparison of Years Ended December 31, 2023 and 2022
For the year ended December 31, |
| ||||||
in 000€, except percentages |
| 2023 |
| 2022 |
| % Change |
|
Revenue |
| 256,127 |
| 232,023 |
| 10.4 | % |
Cost of sales |
| (110,996) |
| (103,255) |
| 7.5 | % |
Gross profit |
| 145,131 |
| 128,768 |
| 12.7 | % |
Research and development expenses |
| (38,098) |
| (37,568) |
| 1.4 | % |
Sales and marketing expenses |
| (57,822) |
| (62,125) |
| (6.9) | % |
General and administrative expenses |
| (37,068) |
| (35,143) |
| 5.5 | % |
Net other operating income |
| (6,524) |
| 3,196 |
| ||
Operating profit |
| 5,619 |
| (2,872) |
| ||
Financial expenses |
| (3,865) |
| (4,420) |
| ||
Financial income |
| 5,019 |
| 6,114 |
| ||
Profit before taxes |
| 6,772 |
| (1,178) |
| ||
Income taxes |
| (78) |
| (975) |
| ||
Net profit |
| 6,695 |
| (2,153) |
| ||
Revenue. Revenue increased by € 24.1 million, or 10.4%, to € 256.1 million in the year ended December 31, 2023, from € 232.0 million in the year ended December 31, 2022.
Revenue by geographical area is presented as follows:
For the year ended December 31, | ||||
in 000€ |
| 2023 |
| 2022 |
Americas |
| 97,399 |
| 86,924 |
Europe & Africa |
| 138,741 |
| 125,138 |
Asia-Pacific |
| 19,988 |
| 19,960 |
Total |
| 256,127 |
| 232,023 |
Revenue generated in Europe increased by € 13.6 million, or 10.9%, in the year ended December 31, 2023, compared to the year ended December 31, 2022, due to higher revenue from our Materialise Medical, Materialise Manufacturing and Materialise Software segments. Revenue generated throughout the Americas increased by € 10.5 million, or 12.1%, in the year ended December 31, 2023, compared to the year ended December 31, 2022, due to higher revenue from our Materialise Medical, Materialise Manufacturing and Materialise Software segments. Revenue generated in Asia-Pacific remained consistent in the year ended December 31, 2023, compared to the year ended December 31, 2022, as revenue increased within our Materialise Manufacturing segment with offsetting decreases within our Materialise Medical and Materialise Software segments in this region.
During 2023, we saw an increased revenue in all three of our segments compared to 2022.
Revenue from our Materialise Software segment increased € 0.7 million, or 1.7%, from € 43.7 million in the year ended December 31, 2022, to € 44.4 million in the year ended December 31, 2023. Recurrent revenue, consisting of limited license fees and maintenance fees, increased by € 2.0 million, or 7.2%, in the year ended December 31, 2023. Non-recurrent revenue, mainly consisting of perpetual fees and services, decreased by € 1.2 million, or 7.6%, in the year ended December 31, 2023. Deferred revenue from license and maintenance fees amounted to € 0.8 million in the year ended December 31, 2023, compared to € 2.7 million in the year ended December 31, 2022.
64
Revenue from our Materialise Medical segment increased € 16.5 million, or 19.5%, from € 84.8 million in the year ended December 31, 2022, to € 101.4 million in the year ended December 31, 2023. Within our medical software department recurrent revenue from annual and renewed licenses and maintenance fees increased by € 4.7 million, or 20.3%, reflecting the implementation of our continued strategy focused on products with defined contractual periods. These recurrent revenues represented 87.2% of all medical software revenues in the year ended December 31, 2023, compared to 84.8% in the year ended December 31, 2022. Our non-recurrent revenue from perpetual licenses and services remained consistent in the year ended December 31, 2023, compared to the year ended December 31, 2022. Deferred revenue from license and maintenance fees amounted to € 0.7 million in the year ended December 31, 2023, compared to € 5.1 million in the year ended December 31, 2022. Revenues from medical devices and services grew by € 11.9 million, or 20.6%, in the year ended December 31, 2023, driven by growth in all of our sales channels across our different core markets. As of December 31, 2023, our Materialise Medical segment operated 50 3D printers, as compared to 49 as of December 31, 2022.
Revenue from our Materialise Manufacturing segment increased € 6.8 million, or 6.6%, from € 103.5 million in the year ended December 31, 2022, to € 110.3 million in the year ended December 31, 2023. Materialise Manufacturing operated 157 3D printers, 28 CNC machines and 5 vacuum casting machines as of December 31, 2023, compared to 156 3D printers, 22 CNC machines and 6 vacuum casting machines as of December 31, 2022, respectively.
Cost of sales. Cost of sales was € 111.0 million in the year ended December 31, 2023, compared to € 103.3 million in the year ended December 31, 2022, representing an increase of € 7.7 million, or 7.5%. This increase in cost of sales was related to increased sales volumes, increased subcontracting volumes and prices, and the impact of inflation related to energy, materials and compensation expenses.
Gross profit. Gross profit increased € 16.4 million from € 128.8 million in the year ended December 31, 2022, to € 145.1 million in the year ended December 31, 2023, mainly driven by increased revenue in all three Materialise segments, partially offset by higher production costs. The overall gross profit margin (gross profit divided by our revenue) amounted to 56.7% in the year ended December 31, 2023, compared to 55.5% in the year ended December 31, 2022.
Research and development, or R&D, sales and marketing, or S&M, and general and administrative, or G&A, expenses. R&D, S&M and G&A expenses decreased, in the aggregate, to € 133.0 million in the year ended December 31, 2023, compared to € 134.8 million in the year ended December 31, 2022. R&D expenses increased from € 37.6 million to € 38.1 million, or 1.4%.S&M expenses decreased from € 62.1 million to € 57.8 million, or 6.9%, driven by our Materialise Software segment sales reorganization. G&A expenses increased from € 35.1 million to € 37.1 million, or 5.5%. The G&A expenses included the roll-out of our ongoing internal digital transformation project.
Net other operating income. Net other operating income decreased to a negative € 6.5 million, in the year ended December 31, 2023, compared to a positive € 3.2 million net other operating income in the year ended December 31, 2022. The main drivers of this decrease were an arbitration settlement of € 5.2 million, an impairment loss related to intangible assets of € 3.0 million, an impairment loss related to goodwill of € 1.2 million, and an amortization expenses of acquired intangible assets, which represented an expense of € 4.0 million for the year ended December 31, 2023, compared to € 5.1 million for the year ended December 31, 2022. These expenses were partially offset by witholding tax exemptions (€ 3.0 million), grants received (€ 1.8 million), and R&D tax credits (€ 1.4 million).
Net financial income (financial income and financial expense). Net financial income was € 1.2 million in the year ended December 31, 2023, compared to a net income of € 1.7 million in the year ended December 31, 2022. In 2023, the net positive result was mainly due to increased interest income on short-term deposits from higher prevailing interest rates, partially offset by increased interest expense on our loans and borrowings.
Income taxes. Income taxes in the year ended December 31, 2023 resulted in an expense of € 0.1 million, which was a combination of deferred tax benefits amounting to € 2.3 million and current income tax expenses of € 2.4 million.
Net profit/loss. As a result of the factors described above, net profit amounted to € 6.7 million in the year ended December 31, 2023 compared to a net loss of € 2.2 million in the year ended December 31, 2022.
65
Other Financial Information
We believe EBITDA and Adjusted EBITDA are meaningful measures to our investors to enhance their understanding of our financial performance. Although EBITDA and Adjusted EBITDA are not necessarily a measure of our ability to fund our cash needs, we understand that it is frequently used by securities analysts, investors and other interested parties as a measure of financial performance and to compare our performance with the performance of other companies that report EBITDA or Adjusted EBITDA. Management believes these non-IFRS measures to be important measures as they exclude the effects of items which primarily reflect the impact of long-term investment and financing decisions, rather than the performance of our day-to-day operations. As compared to net profit, these measures are limited in that they do not reflect the periodic costs of certain capitalized tangible and intangible assets used in generating revenues in our business, or the charges associated with impairments. Management evaluates such items through other financial measures such as capital expenditures and cash flow provided by operating activities. We believe that these measurements are useful to measure a company’s ability to grow or as a valuation measurement. Our calculation of EBITDA and Adjusted EBITDA may not be comparable to similarly titled measures reported by other companies.
We calculate EBITDA as net profit (loss) plus income tax expense / (benefit), financial expenses (less financial income), depreciation and amortization, and share in loss of joint venture. We calculate Adjusted EBITDA by adding share-based compensation expenses, acquisition-related expenses of business combinations, impairments and re-valuation of fair value due to business combinations to EBITDA.
Disclosure in this annual report of EBITDA and Adjusted EBITDA, which are non-IFRS financial measures, is intended as a supplemental measure of our performance that is not required by, or presented in accordance with, IFRS. EBITDA and Adjusted EBITDA should not be considered as alternatives to net profit or any other performance measure derived in accordance with IFRS. Our presentation of EBITDA and Adjusted EBITDA should not be construed to imply that our future results will be unaffected by unusual or non-recurring items.
Reconciliation of Net Profit to Adjusted EBITDA (unaudited) on a Consolidated Basis
For the year ended December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Net profit (loss) |
| 6,695 |
| (2,153) |
| 13,145 |
Income tax expense / (benefit) |
| 78 |
| 975 |
| 591 |
Financial expenses |
| 3,865 |
| 4,420 |
| 4,101 |
Financial income |
| (5,019) |
| (6,114) |
| (5,620) |
Depreciation and amortization |
| 21,511 |
| 22,026 |
| 20,516 |
Share in loss of joint venture |
| — |
| — |
| — |
EBITDA (unaudited) |
| 27,130 |
| 19,154 |
| 32,733 |
Share-based compensation expenses(1) |
| 39 |
| (140) |
| (833) |
Acquisition-related expenses of business combinations(2) |
| — |
| — |
| 413 |
Impairments(3) |
| 4,228 |
| — |
| 177 |
Adjusted EBITDA (unaudited) |
| 31,397 |
| 19,014 |
| 32,490 |
| (1) | Share-based compensation expenses represent the cost of equity-settled and cash-settled share-based payments to employees. |
| (2) | Acquisition-related expenses of business combinations represent fees and costs in connection with the acquisition of Link3D on January 4, 2022. |
| (3) | Impairments represents the impairment of goodwill of Aldema BV (€ 0.2 million) in 2021, the impairment of goodwill (€ 1.2 million) and partial impairment of the intangible assets (€ 2.4 million) of Materialise Motion NV, and the impairment of intangible and tangible assets (€ 0.7 million) of Engimplan in 2023. |
EBITDA. As a result of the factors described above, our consolidated EBITDA was € 27.1 million in the year ended December 31, 2023, compared to € 19.2 million in the year ended December 31, 2022, an increase of € 8.0 million. During 2023, we continued to strategically invest in our growth businesses and progressed on our transition to cloud-based annual license revenue in our Materialise Software segment. In 2023, revenue increased by 10.4%. Our 2023 EBITDA included expenses of € 4.2 million from the impairment of goodwill (€ 1.2 million) and partial impairment of the intangible assets (€ 2.4 million) of Materialise Motion NV and the impairment of intangible and tangible assets (€ 0.7 million) of Engimplan. These expenses were, among others, not reflected in our Adjusted EBITDA. Our consolidated Adjusted EBITDA was € 31.4 million in the year ended December 31, 2023, compared to € 19.0 million in the year ended December 31, 2022, an increase of € 12.4 million.
66
Comparison of Years Ended December 31, 2023 and 2022 by Segment
| Materialise |
| Materialise |
| Materialise |
| Total |
|
|
| |||
in 000€ | Software | Medical | Manufacturing | segments | Unallocated (1) | Consolidated |
| ||||||
For the year ended December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
| |
Revenues |
| 44,442 |
| 101,376 |
| 110,310 |
| 256,127 |
| — |
| 256,127 | |
Segment Adjusted EBITDA |
| 7,450 |
| 26,544 |
| 7,537 |
| 41,530 |
| (10,133) |
| 31,397 | |
Segment Adjusted EBITDA % | 16.8 | % | 26.2 | % | 6.8 | % | 16.2 | % |
| 12.3 | % | ||
For the year ended December 31, 2022 |
|
|
|
|
|
|
|
| |||||
Revenues | 43,688 |
| 84,846 |
| 103,489 |
| 232,023 |
| — |
| 232,023 | ||
Segment Adjusted EBITDA | 1,514 |
| 18,822 |
| 8,229 |
| 28,565 |
| (9,551) |
| 19,014 | ||
Segment Adjusted EBITDA % | 3.5 | % | 22.2 | % | 8.0 | % | 12.3 | % |
| 8.2 | % |
| (1) | Unallocated Segment Adjusted EBITDA consists of corporate research and development, corporate headquarter costs and other operating income (expense) and the added share-based compensation expenses, acquisition related expenses of business combinations, impairment and fair value of business combinations that are included in Adjusted EBITDA when not attributable to a segment. |
Our Materialise Software segment’s Adjusted EBITDA was € 7.5 million in the year ended December 31, 2023, compared to € 1.5 million in the year ended December 31, 2022. This segment’s Adjusted EBITDA margin (the segment’s Adjusted EBITDA divided by the segment’s revenue) increased to 16.8% in the year ended December 31, 2023, from 3.5% for the year ended December 31, 2022. The increase in the Adjusted EBITDA margin was the result of cost containment efforts while further investing in R&D expenses.
Our Materialise Medical segment’s Adjusted EBITDA amounted to € 26.5 million in the year ended December 31, 2023, compared to € 18.8 million in the year ended December 31, 2022. The segment’s Adjusted EBITDA margin increased to 26.2% in the year ended December 31, 2022 from 22.2% in the year ended December 31, 2022. The increase in the segment’s Adjusted EBITDA margin was as a result of increased revenues while keeping costs under control.
Our Materialise Manufacturing segment’s Adjusted EBITDA amounts to € 7.5 million in the year ended December 31, 2023, from € 8.2 million in the year ended December 31, 2022. The Adjusted EBITDA margin of this segment decreased to 6.8% in the year ended December 31, 2023, from 8.0% in the year ended December 31, 2022, as a result of less favorable market conditions and continued investments in our growth business lines.
Reconciliation of Net Profit to Segment Adjusted EBITDA
For the year ended December 31, | ||||
in 000€ |
| 2023 |
| 2022 |
Net profit |
| 6,695 |
| (2,153) |
Income tax expense / (benefit) |
| 78 |
| 975 |
Financial expenses |
| 3,865 |
| 4,420 |
Financial income |
| (5,019) |
| (6,114) |
Operating profit / (loss) |
| 5,619 |
| (2,872) |
Depreciation and amortization |
| 21,511 |
| 22,026 |
Corporate research and development |
| 2,785 |
| 2,600 |
Corporate headquarters costs |
| 10,464 |
| 9,504 |
Net other operating (income) expense |
| (3,077) |
| (2,693) |
Impairments(1) |
| 4,228 |
| — |
Segment Adjusted EBITDA (unaudited) |
| 41,530 |
| 28,565 |
(1) | Impairments represent the impairment of goodwill and intangible assets of Materialise Motion (€ 3.6 million) and the impairment of tangible and intangible assets of Engimplan (€ 0.7 million). |
67
Comparison of Years Ended December 31, 2022 and 2021
For the year ended December 31, |
| ||||||
in 000€, except percentages |
| 2022 |
| 2021 |
| % Change |
|
Revenue |
| 232,023 |
| 205,450 |
| 12.9 | % |
Cost of sales |
| (103,255) |
| (87,278) |
| 18.3 | % |
Gross profit |
| 128,768 |
| 118,172 |
| 9.0 | % |
Research and development expenses |
| (37,568) |
| (26,891) |
| 39.7 | % |
Sales and marketing expenses |
| (62,125) |
| (49,151) |
| 26.4 | % |
General and administrative expenses |
| (35,143) |
| (33,315) |
| 5.5 | % |
Net other operating income (expenses) |
| 3,196 |
| 3,402 |
| (6.1) | % |
Operating profit |
| (2,872) |
| 12,217 |
| ||
Financial expenses |
| (4,420) |
| (4,101) |
| ||
Financial income |
| 6,114 |
| 5,620 |
| ||
Profit (loss) before taxes |
| (1,178) |
| 13,736 |
| ||
Income tax expense / (benefit) |
| (975) |
| (591) |
| ||
Net profit (loss) |
| (2,153) |
| 13,145 |
| ||
Revenue. Revenue was € 232.0 million in the year ended December 31, 2022 compared to € 205.5 million in the year ended December 31, 2021, an increase of € 26.6 million, or 13%.
Revenue by geographical area is presented as follows:
For the year ended December 31 | ||||
in 000€ |
| 2022 |
| 2021 |
Americas | 86,924 |
| 75,437 | |
Europe & Africa | 125,138 |
| 110,477 | |
Asia-Pacific | 19,960 |
| 19,536 | |
Total | 232,023 |
| 205,450 | |
Revenue generated in Europe increased by € 14.7 million, or 13.3 %, in the year ended December 31, 2022, compared to the year ended December 31, 2021, due to higher revenue from our Materialise Medical and Materialise Manufacturing segments. Revenue generated throughout the Americas increased by € 11.5 million, or 15.2%, in the year ended December 31, 2022, compared to the year ended December 31, 2021. In the Americas, revenue for the Materialise Medical segment and for the Materialise Software segment increased and revenue for the Materialise Manufacturing segment remained consistent. Revenue generated in Asia-Pacific increased by € 0.4 million, or 2.2%, in the year ended December 31, 2022, compared to the year ended December 31, 2021, as revenue increased within our Materialise Medical and Materialise Manufacturing segment and decreased within our Materialise Software segment in this region.
During 2022, we had increased revenue in all three of our segments compared to 2021.
Revenue from our Materialise Software segment increased € 0.8 million, or 1.8%, from € 42.9 million in the year ended December 31, 2021, to € 43.7 million in the year ended December 31, 2022. Recurrent revenue, consisting of limited license fees and maintenance fees, increased by € 4.6 million, or 19.7%, in the year ended December 31, 2022. Non-recurrent revenue, mainly consisting of perpetual fees and services, decreased by € 3.8 million, or 19.0%, in the year ended December 31, 2022. Deferred revenue from license and maintenance fees amounted to € 2.7 million in the year ended December 31, 2022, compared to € 1.9 million in the year ended December 31, 2021.
Revenue from our Materialise Medical segment increased € 11.5 million, or 15.6%, from € 73.4 million in the year ended December 31, 2021, to € 84.8 million in the year ended December 31, 2022. Within our medical software department recurrent revenue from annual and renewed licenses and maintenance fees increased by € 3.9 million, or 20.8%, reflecting the implementation of our continued strategy focused on products with defined contractual periods. These recurrent revenues represented 84.8% of all medical software revenues in the year ended December 31, 2022, compared to 83.0% in the year ended December 31, 2021. Our non-recurrent revenue from perpetual licenses and services increased by € 0.3 million, or 6.7%. Deferred revenue from license and maintenance fees amounted to € 5.1 million in the year ended December 31, 2022, compared to € 2.0 million in the year ended December 31, 2021. Revenues from medical devices and services grew by € 7.3 million, or 14.4%, in the year ended December 31, 2022, driven by growth in our CMF business line. As of December 31, 2022, our Materialise Medical segment operated 49 3D printers, as compared to 48 as of December 31, 2021.
68
Revenue from our Materialise Manufacturing segment increased € 14.3 million, or 16.0%, from € 89.2 million in the year ended December 31, 2021, to € 103.5 million in the year ended December 31, 2022. Materialise Manufacturing operated 156 3D printers, 22 CNC machines and 6 vacuum casting machines as of December 31, 2022, compared to 153 3D printers, 23 CNC machines and 6 vacuum casting machines as of December 31, 2021, respectively.
Cost of sales. Cost of sales was € 103.3 million in the year ended December 31, 2022, compared to € 87.3 million in the year ended December 31, 2021, representing an increase of € 16.0 million, or 18.3%. This increase in cost of sales was related to increased sales volumes, increased subcontracting volumes and prices, and the impact of inflation related to energy, materials and compensation expenses.
Gross profit. Gross profit increased € 10.6 million from € 118.2 million in the year ended December 31, 2021, to € 128.8 million in the year ended December 31, 2022, mainly driven by increased revenue in all three Materialise segments, while facing higher production costs. The overall gross profit margin (gross profit divided by our revenue) amounted to 55.5% in the year ended December 31, 2022, compared to 57.5% in the year ended December 31, 2021.
Research and development, or R&D, sales and marketing, or S&M, and general and administrative, or G&A, expenses. R&D, S&M and G&A expenses increased, in the aggregate, to € 134.8 million in the year ended December 31, 2022, compared to € 109.4 million in the year ended December 31, 2021. R&D expenses increased from € 26.9 million to € 37.6 million, or 39.7%, and included the accelerated investments in our Materialise Software CO-AM business which contained the expenditures of Link3D and Identify3D since their acquisition. S&M expenses increased from € 49.2 million to € 62.1 million, or 26.4%, driven by our Materialise Software segment, including severance pay. G&A expenses increased from € 33.3 million to € 35.1 million, or 5.5%. The G&A expenses included the roll-out of our ongoing internal digital transformation project.
Net other operating income. Net other operating income decreased to € 3.2 million, or 6.6%, in the year ended December 31, 2022, compared to € 3.4 million in the year ended December 31, 2021. The main driver for this decrease were the amortization expenses of the acquired intangible assets, which represented an expense of € 5.1 million for the year ended December 31, 2022, compared to € 2.5 million for the year ended December 31, 2021. This result for the year ended December 31, 2022 also contained an impairment loss related to capitalized development expenditure in the Materialise Software segment. These expenses were partly offset by a COVID-19 grant received by our German subsidiaries (€ 0.7 million) and a commercial indemnity fee (€ 0.5 million). Our net other operating income for 2021 included a € 0.2 million impairment loss on the goodwill from the acquisition of Aldema (Metal Belgium) in 2015.
Net financial income (financial income and financial expense). Net financial income was € 1.7 million in the year ended December 31, 2022, compared to a net income of € 1.5 million in the year ended December 31, 2021. In both years the net positive result was mainly due to positive foreign exchange differences, partially offset by interest expenses on our loans and borrowings.
Income taxes. Income taxes in the year ended December 31, 2022 resulted in an expense of € 1.0 million, which was a combination of deferred tax income amounting to € 1.0 million and current income tax expenses of € 2.0 million. This increase in current income tax expense compared to the prior year was mainly due to the fact that mark-ups and margins applied under our consolidated group’s transfer pricing arrangements were still temporarily waived during the first half year of 2021.
Net profit/loss. As a result of the factors described above, net loss amounted to € 2.2 million in the year ended December 31, 2022 compared to a net profit of € 13.1 million in the year ended December 31, 2021.
Other Financial Information
EBITDA. As a result of the factors described above, our consolidated EBITDA was € 19.2 million in the year ended December 31, 2022, compared to € 32.7 million in the year ended December 31, 2021, a decrease of € 13.6 million. During 2022, we prioritized the sustainability of our revenue growth over the maximization of short term EBITDA. In 2022, revenue increased by 13% and deferred revenues grew by 22%. We continued to strategically invest in our growth businesses despite significant inflationary pressures on labor, energy and materials costs and accelerated the consolidation of both Link3D and Identify3D, as a basis for our future cloud-based annual license revenue in our Materialise Software segment. These added expenses in addition to certain one-time items weighed on the overall profitability of our Adjusted EBITDA for the year, with Adjusted EBITDA decreasing to € 19.0 million.
69
Comparison of the Years Ended December 31, 2022 and 2021 by Segment
| Materialise |
| Materialise |
| Materialise |
| Total |
|
|
| |||
in 000€ | Software | Medical | Manufacturing | segments | Unallocated (1) | Consolidated |
| ||||||
For the year ended December 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
| |
Revenues |
| 43,688 |
| 84,846 |
| 103,489 |
| 232,023 |
| — |
| 232,023 | |
Segment Adjusted EBITDA |
| 1,514 |
| 18,822 |
| 8,229 |
| 28,565 |
| (9,551) |
| 19,014 | |
Segment Adjusted EBITDA % | 3.5 | % | 22.2 | % | 8.0 | % | 12.3 | % |
| 8.2 | % | ||
For the year ended December 31, 2021 |
|
|
|
|
|
|
| ||||||
Revenues | 42,902 |
| 73,368 |
| 89,180 |
| 205,450 |
| — |
| 205,450 | ||
Segment Adjusted EBITDA | 15,705 |
| 20,669 |
| 6,275 |
| 42,649 |
| (10,159) |
| 32,490 | ||
Segment Adjusted EBITDA % | 36.6 | % | 28.2 | % | 7.0 | % | 20.8 | % |
| 15.8 | % |
| (1) | Unallocated Segment Adjusted EBITDA consists of corporate research and development, corporate headquarter costs and other operating income (expense) and the added share-based compensation expenses, acquisition related expenses of business combinations, impairment and fair value of business combinations that are included in Adjusted EBITDA when not attributable to a segment. |
Our Materialise Software segment’s Adjusted EBITDA was € 15.7 million in the year ended December 31, 2021 compared to € 1.5 million in the year ended December 31, 2022, a decrease of € 14.2 million. This segment’s Adjusted EBITDA margin (the segment’s Adjusted EBITDA divided by the segment’s revenue) decreased to 3.5% in the year ended December 31, 2022, from 36.6% for the year ended December 31, 2021. The decrease in the Adjusted EBITDA margin was a result of our investments to strengthen and accelerate the creation of the Materialise cloud based software platform.
Our Materialise Medical segment’s Adjusted EBITDA amounted to € 18.8 million in the year ended December 31, 2022, compared to € 20.7 million in the year ended December 31, 2021. The segment’s Adjusted EBITDA margin decreased to 22.2% in the year ended December 31, 2022 from 28.2% in the year ended December 31, 2021. The decrease in the segment’s Adjusted EBITDA margin was due to our increased investment in research and development to position ourselves for further growth.
Our Materialise Manufacturing segment’s Adjusted EBITDA increased to € 8.2 million in the year ended December 31, 2022, from € 6.3 million in the year ended December 31, 2021. The Adjusted EBITDA margin of this segment increased to 8.0% in the year ended December 31, 2022, from 7.0% in the year ended December 31, 2021, as a result of the 16% revenue growth and improved production capacity levels, partly offset by the effects of inflation on compensation, energy and materials expenses.
Reconciliation of Net Profit to Segment Adjusted EBITDA
For the year ended December 31, | ||||
in 000€ |
| 2022 |
| 2021 |
Net profit (loss) |
| (2,153) |
| 13,145 |
Income tax expense / (benefit) |
| 975 |
| 591 |
Financial expenses |
| 4,420 |
| 4,101 |
Financial income |
| (6,114) |
| (5,620) |
Share in loss of joint venture |
| — |
| — |
Operating profit |
| (2,872) |
| 12,217 |
Depreciation and amortization |
| 22,026 |
| 20,516 |
Corporate research and development |
| 2,600 |
| 2,948 |
Corporate headquarters costs |
| 9,504 |
| 10,317 |
Net other operating income (expense) |
| (2,693) |
| (3,527) |
Impairments |
| — |
| 177 |
Segment Adjusted EBITDA (unaudited) |
| 28,565 |
| 42,648 |
70
B. | Liquidity and Capital Resources |
Prior to our initial public offering, we historically funded our operations principally from cash generated from operations and borrowings. From our initial public offering on June 30, 2014 through December 31, 2022, we have raised approximately $258.5 million in aggregate net proceeds from public offerings of our ADSs and a private placement of our ordinary shares. On July 6, 2021, we sold 4,600,000 ADSs in a follow-on public offering at a public offering price of $24.00 per ADS, and received net proceeds of approximately $105 million. As we continue to grow our business, we envision funding our operations through multiple sources, including the remaining proceeds from our equity offerings, and future earnings and cash flow from operations and borrowings. We may also seek to raise additional capital from offerings of our equity or debt securities on an opportunistic basis when we believe there are suitable opportunities for doing so.
We expect our main uses of cash in the future will be funding our business operations, capital expenditures, loan reimbursements, acquisitions and partnerships. Depending on market conditions, our liquidity requirements, contractual restrictions and other factors, we may also repurchase some of our outstanding ordinary shares and ADSs. We believe that we will have sufficient liquidity to satisfy the operating requirements of our business through the next 12 months.
In 2022, we entered into a credit facility agreement with KBC, which allows for a € 50 million delayed draw, that will allow funding of potential additional acquisitions, partnerships, and capital expenditures. The credit facility provides for a first draw between October 2022 and April 2025, repayable in full in April 2030. A second draw may be made between October 2022 and June 2025, repayable in full in June 2031. A third and final draw may be made between October 2022 and June 2026, repayable in full in June 2032.
Our liquidity plans are subject to a number of risks and uncertainties, including those described in the section of this annual report titled “Item 3. Key Information—D. Risk Factors,” some of which are outside of our control. Macro-economic conditions could hinder our business plans, which could, in turn, adversely affect our financing strategy.
Cash Flows
The table below summarizes our cash flows from operating activities, investing activities and financing activities for the years ended December 31, 2023, 2022 and 2021.
For the year ended December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Net cash flow from operating activities |
| 20,157 |
| 22,288 |
| 25,845 |
Net cash flow from/(used in) investing activities |
| (11,037) |
| (53,861) |
| (13,134) |
Net cash flow from/(used in) financing activities |
| (22,368) |
| (22,510) |
| 71,156 |
Net increase / (decrease) of cash and cash equivalents |
| (13,248) |
| (54,082) |
| 83,867 |
Comparison of Years Ended December 31, 2023 and 2022
Net cash flow from operating activities amounted to € 20.2 million in the year ended December 31, 2023 compared to € 22.3 million in the year ended December 31, 2022, a decrease of € 2.1 million, or 9.6%. In the year ended December 31, 2023, the net cash flow from operating activities was the result of the income statement cash result of € 32.8 million, decreased by working capital requirements of € 13.1 million, offset by increased deferred revenue of € 0.5 million.
Net cash flow used in investing activities was € 11.0 million in the year ended December 31, 2023 compared to € 53.9 million in the year ended December 31, 2022, a decrease of € 42.8 million, or 79.5%. The decrease was mainly due to the acquisition of Link3D and Identify3D (€ 29.3 million) in 2022 and no comparable acquisition activity occurring in 2023.
Net cash flow used in financing activities was € 22.4 million in the year ended December 31, 2023, compared to € 22.5 million in net cash flow from financing activities in the year ended December 31, 2022. In 2022, we entered into a new credit facility with KBC, which provides for a € 50 million delayed draw. No drawdowns were made under this new facility in 2023, while our repayment of borrowings and leases amounted to € 20.3 million.
71
Comparison of Years Ended December 31, 2022 and 2021
Net cash flow from operating activities amounted to € 22.3 million in the year ended December 31, 2022 compared to € 25.8 million in the year ended December 31, 2021, a decrease of € 3.6 million, or 14%. In the year ended December 31, 2022, the net cash flow from operating activities was the result of the income statement cash result of € 21.3 million, decreased by working capital requirements of € 9.2 million, offset by increased deferred revenue of € 10.3 million.
Net cash flow used in investing activities was € 53.9 million in the year ended December 31, 2022 compared to € 13.1 million in the year ended December 31, 2021, an increase of € 40.8 million, or 310%, of which € 29.3 million related to the acquisition of Link3D and Identify3D, and € 24.8 million related to capital expenditures.
Net cash flow used in financing activities was € 22.5 million in the year ended December 31, 2022, compared to € 71.2 million in net cash flow from financing activities in the year ended December 31, 2021. The positive cash flow in 2021 was driven by the net capital increase € 88.1 million. In 2022, we entered into a new credit facility with KBC, which provides for a € 50 million delayed draw. No drawdowns were made under this new facility in 2022, while our repayment of borrowings and leases amounted to € 21.1 million.
Investments in Property, Plant & Equipment and Intangible Assets
The table below describes cash paid for investments in property, plant & equipment and intangible assets for the years ended December 31, 2023, 2022 and 2021:
For the year ended December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Purchase of property, plant and equipment |
| 9,235 |
| 21,608 |
| 7,934 |
Purchase of intangible assets |
| 2,525 |
| 3,165 |
| 3,788 |
Total |
| 11,760 |
| 24,773 |
| 11,722 |
Indebtedness
As of December 31, 2023, we had loans and borrowings in the total amount of € 64.4 million, with mainly fixed interest rates. These loans include secured bank loans used to finance the acquisition of ACTech, the construction of office and production facilities in Belgium and Poland, the acquisition of production equipment and installations, and research and development projects.
The following table sets forth our principal indebtedness:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
K€50,000 KBC credit facility | — | — | — | |||
K€35,000 EIB bank loan |
| 21,667 |
| 27,500 |
| 33,333 |
K€28,000 acquisition bank loan |
| 10,000 |
| 12,559 |
| 15,604 |
K€17,700 secured bank loans |
| 14,904 |
| 16,165 |
| 16,592 |
K€12,300 bank loans ACTech |
| 3,546 |
| 5,860 |
| 8,160 |
K€5,000 other facility loan |
| 1,496 |
| 1,881 |
| 2,248 |
Bank investment loans - top 20 outstanding |
| 4,778 |
| 8,828 |
| 12,852 |
Bank investment loans - other |
| — |
| 606 |
| 1,569 |
Lease liabilities |
| 7,943 |
| 7,485 |
| 8,621 |
Related party loan |
| 64 |
| 96 |
| 128 |
Total loans and borrowings |
| 64,398 |
| 80,980 |
| 99,107 |
Current |
| 25,483 |
| 19,960 |
| 21,202 |
Non-Current |
| 38,915 |
| 61,020 |
| 77,905 |
K€50,000 KBC credit facility
In October 2022, we entered into a credit facility agreement with KBC, which allows for a € 50 million delayed draw. The credit facility provides for a first draw between October 2022 and April 2025, repayable in full in April 2030, with an interest rate of 3.56%. A second draw may be made between October 2022 and June 2025, repayable in full in June 2031, with an interest rate of 3.81%. A third and final draw may be made between October 2022 and June 2026, repayable in full in June 2032, with an interest rate of 3.87%.
72
All loan drawings were contracted at a fixed interest rate, and a reservation cost for the 3 tranches amounts is applicable at 0.15% per year. As of December 31, 2023, no amounts had been drawn under this facility.
K€35,000 EIB bank loan
On December 20, 2017, we entered into a finance contract with the European Investment Bank, or EIB, to finance future research and development programs. The contract provides a credit of up to € 35.0 million drawable in two tranches. As part of the first tranche, an amount of €10.0 million was drawn in July of 2018. The duration of the loan will be between six to eight years, and includes a two-year loan repayment grace period.
In July 2019, the second tranche of € 25.0 million was drawn. Similar to the first tranche, the duration of the loan will be between six to eight years, and includes a two-year loan repayment grace period.
Loans under the contract are made at a fixed rate, based on the Euribor rate at the time of the borrowing, plus a variable margin. The applied rate for the first tranche is initially equal to 2.4%. The applied rate for the second tranche is initially equal to 2.72% and varies in function of certain EBITDA levels and debt ratios. The contract contains customary security, covenants and undertakings.
K€28,000 Acquisition loan
This bank loan was concluded in October 2017 to finance the acquisition of ACTech. The loan includes a portion of € 18.0 million, repayable monthly over seven years, and a bullet portion of € 10.0 million, payable at once in October 2024. The interest rate is fixed for the duration of the loan, and amounts to 1% on average for both portions. The bank loans are secured with a business pledge mandate, a share pledge on Materialise Germany GMBH, and debt covenants.
K€17,700 secured bank loans
The € 17.7 million loan has been concluded in 2016 in two agreements to finance the construction of new facilities in Leuven (Belgium) and in Poland, both maturing in 2032. The agreement for the Belgian facility financing amounts to € 11.7 million, and repayments started in June 2023. The agreement for the Polish facility financing amounts to € 6.0 million (fully drawn per end of 2020), and repayments started in June 2019. The average interest rate of both agreements amounts to 1%. The bank loan is secured with a mortgage mandate on the Belgian facility buildings.
K€12,300 bank loans
In March 2018, three bank loans originating from the acquired ACTech business were refinanced in their entirety for an aggregate amount of € 9.3 million, with the maturity adjusted to May 2025 and the first repayments beginning in August 2020. The interest rate was fixed at approximately 1.6%, and pledges including a € 4.7 million mortgage on ACTech’s facilities and guaranteed by Materialise NV. In addition, a new investment credit of € 3.0 million was obtained from Commerzbank in June 2018, repayable as from January 2019 and with a fixed interest rate of 1.5%.
K€5,000 - Other facility loan
A facility loan was contracted in 2012 for the construction of Leuven office and production facilities. The balance of this loan amounted to € 1.5 million as of December 31, 2023. This loan has a repayment schedule of 15 years and interest rate is fixed at 4.61%.
Bank investment loans
The 20 largest of these investment loans outstanding as of December 31, 2023 amount to a balance of € 4.8 million. They were agreed in 2018, 2017 and prior years to finance various investments in machinery, printers, equipment, and software tools. The vast majority of the loans have a repayment period over seven years, and are at fixed interest rates with weighted average below 1%.
K€7,943 Lease liabilities
We have had several lease obligations, mainly with financial institutions and related to the financing of buildings and various other items of plant and equipment such as 3D printers. As of December 31, 2023 the balance of these lease obligations amounts to € 7.9 million, and are mostly at fixed interest rates with weighted average below 1%.
73
Related party loan
Ailanthus NV previously granted us a loan at a fixed interest rate of 4.23% that matures in 2025. Prior to the merger between Ailanthus NV and Materialise NV on December 31, 2020, Ailanthus NV was demerged into Lunebeke NV, a newly incorporated company. As a result of this demerger, the loan was transferred from Ailanthus NV to Lunebeke NV. For more information on the merger and related demerger, see “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions.” The purpose of the loan was to finance the purchase of a building in France. The amounts outstanding as of December 31, 2023 were K€64 (2022: K€96; 2021: K€128). The interest expense for the year ended December 31, 2023 was K€3 (2022: K€5; 2021: K€5).
Material Unused Sources of Liquidity
Our cash and cash equivalents as of December 31, 2023, 2022 and 2021 were € 127.6 million, € 140.9 million and € 196.0 million, respectively. We have one undrawn line of credit at December 31, 2023, a credit facility agreement with KBC, which provides for a € 50 million delayed draw. For more information, see “—K€50,000 KBC credit facility” above.
Reservation cost for all 3 tranches amounts to 0.15% per year.
Transfers from Subsidiaries
The amount of dividends payable by our subsidiaries to us is subject to general limitations imposed by the corporate laws and certain restrictions in the jurisdictions that we operate in. For example, China has very specific approval regulations for all capital transfers to or from the country, certain capital transfers to and from Ukraine are subject to obtaining a specific permit and current legislation in Brazil permits the Brazilian government to impose temporary restrictions on remittances of foreign capital abroad in the event of a serious imbalance or an anticipated serious imbalance in Brazil’s balance of payments. Dividends paid to us by certain of our subsidiaries may also be subject to withholding taxes in certain jurisdictions. Of our cash and cash equivalents held outside of Belgium as of December 31, 2023, 2022 and 2021, the amount of cash that would have been subject to withholding taxes if transferred to us by way of dividends and the amount of cash that could not have been transferred by law, or the transfer of which would have been subject to prior approval that was beyond our control, was in each case immaterial.
Contractual Obligations
Our contractual commitments will have an impact on our future liquidity. The table below sets forth our contractual obligations as of December 31, 2023, which represents contractually committed future obligations:
|
| Less than 1 |
|
|
| More than 5 | ||||
in 000€ | Total | year | 2-3 years | 4-5 years | years | |||||
Loans and borrowings |
| 56,455 |
| 22,873 | 18,585 | 7,989 | 7,008 | |||
Lease Liabilities |
| 7,943 |
| 2,610 | 2,757 | 1,778 | 798 | |||
Scheduled interest payments(1) |
| 3,200 |
| 1,270 | 1,335 | 441 | 154 | |||
Purchase obligations |
| 31,597 |
| 22,798 | 8,617 | 182 | 0 | |||
Total |
| 99,195 |
| 49,551 | 31,294 | 10,390 | 7,960 |
| (1) | Scheduled interest payments comprise the interest payable on loans and borrowings and lease commitments. No interest is payable on the other contractual obligations in the above table. |
As of December 31, 2023 we had purchase commitments of K€9,330 related to property, plant and equipment. We did not have any significant purchase commitments related to property, plant and equipment as of December 31, 2022 and 2021.
| C. | Research and Development, Patents and Licenses |
For information regarding our research and development program, see “Item 4. Information on the Company—B. Business Overview—Research and Development.”
74
| D. | Trend Information |
Impact of the armed conflict in Ukraine
As discussed in more detail in “Item 3. Key Information—D. Risk Factors” of this annual report, we have an office in Kyiv, employing over 400 collaborators who are mainly engaged in engineering, software development and supporting IT and staff functions. As a result of the armed conflict in Ukraine, our operations from our Kyiv office operate in very difficult, uncertain and unstable circumstances.
To-date, most of our personnel from the office in Kyiv have continued to work for us throughout the armed conflict, either remotely from Ukraine or other neighboring countries, from our Wroclaw office or, circumstances permitting, from our office in Kyiv, while others remain unable to perform their work. As of the date of this annual report, we have been able to continue servicing our customers without significant disruption or delay, as personnel with similar skills and competencies located elsewhere in the world have increased their roles and responsibilities to assist displaced personnel.
As the armed conflict with Russia continues, it is impossible to predict how much of our Ukrainian workforce will be able or willing to continue working for us. As we are unable to predict how the armed conflict in Ukraine will evolve, we cannot exclude that delays or disruption in certain of our services may occur or that a more radical, temporary shift of certain operations to other jurisdictions may become necessary, which could impact our business and operations, results of operations, financial condition, cash flows and liquidity.
We have incurred, and will continue to incur, expenses related to hiring additional and more expensive resources outside Ukraine.
It is uncertain to what extent some of the development projects of our Materialise Software and Materialise Medical segments, and to a lesser extent our Materialise Manufacturing segment, will be impacted by the ongoing armed conflict in Ukraine. As a result of such impact, some of our anticipated product releases may be delayed, which may adversely affect our revenue.
As of the date of this annual report, we are unable to predict how the armed conflict in Ukraine will evolve or what the impact of any political and direct and indirect economic repercussions will be on the global economy and our business. Indirect economic repercussions could, for example, come from continued or further increased inflation, or currencies instability. As a result, we are unable to assess with certainty its impact on our business and operations, results of operations, financial condition, cash flows and liquidity.
| E. | Critical Accounting Estimates |
For information regarding our critical accounting estimates, see “—Operating Results—Critical Accounting Policies and Accounting Estimates” above.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
75
ITEM 6.DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
The following tables set forth certain information with respect to the current members of our board of directors and senior management:
Directors:
Name | Age | Time served as director | Position | |||
Wilfried Vancraen | 62 | Since 1990 (34 years) | Founder & Director | |||
Peter Leys | 59 | Since 2013 (11 years) | Director | |||
A Tre C CVOA, represented by Johan De Lille | 61 | Since 2006 (18 years) | Director | |||
Hilde Ingelaere | 62 | Since 1997 (27 years) | Director | |||
Sander Vancraen | 33 | Since 2020 (4 years) | Director | |||
Jürgen Ingels | 53 | Since 2013 (11 years) | Director | |||
Jos Vander Sloten | 61 | Since 2007 (17 years) | Director | |||
Lieve Verplancke | 64 | Since 2015 (9 years) | Director | |||
Bart Luyten | 47 | Since 2017 (7 years) | Director | |||
Volker Hammes | 60 | Since 2018 (6 years) | Director |
Senior Management and Executive Committee Members:
Name |
| Age |
| Position |
Seaquence BV, represented by Johan Pauwels | 56 | Executive Vice President, Chief Operating Officer (COO) | ||
BEspired BV, represented by Bart Van der Schueren | 57 | Chief Strategy and Technology Officer | ||
Finstraco B.V. represented by Koen Berges | 48 | Chief Financial Officer | ||
De Vet Management BV, represented by Brigitte de Vet-Veithen | 53 | Chief Executive Officer (CEO) | ||
Level 5 BV, represented by Jurgen Laudus | 45 | Vice President, Materialise Manufacturing segment | ||
SoHo services, represented by Conny Hooghe | 58 | Vice President, Human Resources | ||
Super Mare & Park BV, represented by Carla Van Steenbergen | 48 | Executive Vice President, Director Corporate Affairs | ||
Udo Eberlein | 56 | Vice President, Software Segment |
The term of the directorship of each member of our board of directors will expire at the 2024 annual general meeting of shareholders. The business address of the members of our board of directors is the same as our business address: Technologielaan 15, 3001 Leuven, Belgium.
Our board of directors has determined that Jürgen Ingels, Bart Luyten, Volker Hammes and Lieve Verplancke are independent under both Belgian law and the Nasdaq Stock Market Listing Rules. The Belgian law definition of independence differs from the definition of independence under Nasdaq Stock Market Listing Rules. In particular, under Belgian law, A Tre C CVOA (represented by Johan De Lille) and Jos Vander Sloten are no longer deemed independent by virtue of its term of office exceeding 12 years. However, the Nasdaq Stock Market Listing Rules do not have a similar requirement, and our board of directors has determined that A Tre C CVOA (represented by Johan De Lille) and Jos Vander Sloten continue to be independent under the Nasdaq Stock Market Listing Rules.
76
The following is a brief summary of the business experience of the current members of our board of directors:
Wilfried Vancraen. Wilfried Vancraen has served as one of our directors since founding our company in July 1990. Mr. Vancraen previously served as our Chief Executive Officer from July 1990 until December 31, 2023. Mr. Vancraen previously worked as a research engineer and consultant at the Research Institute of the Belgian Metalworking Industry, where he was introduced to 3D printing. Passionate about this new technology and firm in his belief that it could help create a better and healthier world, he founded Materialise in July 1990. Mr. Vancraen holds several patents related to the technical and medical applications of 3D printing and remains committed to using the technology to make positive changes in people’s lives. In recent years, Mr. Vancraen has been awarded the RTAM/SME Industry Achievement Award, the highest honor in the 3D printing industry, has been selected as the most influential person in additive manufacturing by industry professionals and TCT Magazine, and has been listed one of the five leading players in his sector by the Financial Times. He is also the recipient of a 2013 Visionaries! Award from the Museum of Art and Design in New York. Mr. Vancraen holds a Master of Science in Electro-Mechanical Engineering and a Master in Business Administration from KU Leuven. Wilfried Vancraen was chosen in the TCT Hall of Fame in 2017 for his contributions to the 3D printing industry. In 2018, he was chosen by the Additive Manufacturing Users Group (AMUG) as the Innovators Showcase and received the Industry Dino Award. In 2019, Mr. Vancraen was appointed as a faculty honorary professor at the Faculty of Engineering, KU Leuven on the recommendation of the Department of Mechanical Engineering because of his role as founder and CEO of our company.
Peter Leys. Peter Leys has served as one of our directors since 2013. Mr. Leys previously served as our Executive Chairman from 2013 until December 31, 2023. Previously, from 1990 to 2013, Mr. Leys was at the Brussels office of Baker & McKenzie CVBA, where he focused on mergers and acquisitions, and capital markets. Mr. Leys lectures a contract negotiation course at the KU Leuven. Mr. Leys holds a Candidacy Degree in Philosophy from KU Leuven and Master of Law degrees from KU Leuven and the University of Georgia.
Johan De Lille. Johan De Lille has represented A Tre C CVOA as one of our directors since July 2006. Mr. De Lille started his professional career as an auditor at Arthur Andersen LLP in 1988. In 1994, he became Vice President & Group Controller of Ackermans & van Haaren NV, a Belgian public holding company. In 1999, he became Chief Financial Officer of Easdaq/Nasdaq Europe and took on the role of Chief Financial Officer of Option NV, a Belgian public technology company, in 2001. Mr. De Lille joined Delhaize Group, a Belgian public company, as Vice President & Controller in September 2002, and later became Chief Internal Auditor of the Delhaize Group in August 2006, and Chief Financial Officer of Delhaize Belgium in January 2009. Since 2013, Mr. De Lille has acted as Chief Financial & Information Officer of BMT Group, an industrial family owned holding company active in high-precision machining. Mr. De Lille serves as an independent director on the board of directors of Boma NV, a Belgian private company specializing in cleaning products. In 1988, Mr. De Lille was the award winner for the best final paper of the Department of Economics from KU Leuven. In 2010, he received the CFO Magazine Award for the Best Finance Team of the year for Working Capital in Belgium. Mr. De Lille holds a Master’s degree in Economics, with a major in Econometrics and Mathematical Economics, from KU Leuven.
Hilde Ingelaere. Hilde Ingelaere co-founded Materialise in 1990, together with Wilfried Vancraen, and has served as one of our directors since 1997. In her early years at Materialise, Ms. Ingelaere managed several staff departments including human resources, finance and legal, and she served as Executive Vice President of Materialise until December 31, 2023. Mrs. Ingelaere continues to play an important role in supporting our South American operations and in strategic negotiations with a focus on partnerships. Prior to joining Materialise, Ms. Ingelaere conducted cardiovascular clinical research at Bristol-Myers Squibb from 1986 to 1989. She then worked as a business analyst with Plant Genetic Systems from 1989 to 1992. Ms. Ingelaere holds a Master’s degree in Bioengineering from KU Leuven, where she focused on Biotechnology, and a Master’s degree in Business Administration from KU Leuven.
Sander Vancraen. Sander Vancraen has served as one of our directors since 2020. Mr. Vancraen holds a Bachelor’s degree in Aerospace Engineering from Delft University of Technology, with a thesis on a GES (Gravity Explorer Satellite), providing data on temporal changes in Earth’s gravity field for scientific use at low cost. He also holds a Master’s degree in Aerospace Engineering, track Space Exploration, from Delft University of Technology, with a thesis on aCOTS GNSS Receiver, testing of an onboard receiver for the Indian Space Research Organization. In 2013, he did a three month internship at Materialise USA in Plymouth, MI, supporting the clinical engineering team. From 2013 to 2018, he managed a guesthouse, Intermezzo. Since October 2018, he has been a design engineer for the EASA DOA of TUI fly, a charter airline.
77
Jürgen Ingels. Jürgen Ingels has served as one of our independent directors since November 2013. Mr. Ingels is Founder and Managing Partner of Smartfin, a growth stage private equity fund that was set up in December 2014. In October 2014, Mr. Ingels sold Clear2Pay NV/S.A., a global innovative payments software technology company he founded in 2000, to FIS Global. The clients of Clear2Pay include global and major regional financial institutions such as ING Group, Banco Santander, S.A., Crédit Agricole S.A., BNP Paribas, The U.S. Federal Reserve, Royal Bank of Scotland, The People’s Bank of China (PBOC). Mr. Ingels started his career in private equity in 1997 at Dexia NV/S.A., where his role was focused on investing in technology companies. Mr. Ingels currently serves as a director on the board of directors for Guardsquare NV, Projective Group NV, Itineris NV, Willemen Groep, Ghelamco NV and WDP (Euronext). In 2015, Mr. Ingels co-founded The Glue, a provider of infrastructure solutions for financial institutions. In 2018, Mr. Ingels founded Scale-Ups.eu and organized Supernova, a four-day technology event in Antwerp with over 30.000 visitors. Mr. Ingels holds a Master’s degree in Business Administration and a Master’s degree in Political and Social Sciences from the University of Antwerp.
Jos Vander Sloten. Jos Vander Sloten has served as one of our directors since January 2007. Mr. Vander Sloten is a full professor at the Faculty of Engineering Science, KU Leuven and chaired the Division of Biomechanics for two terms from 2006 to 2014. He chaired the Leuven Medical Technology Centre (L-MTC), which he founded in 2008 until the end of his two terms in 2016. Mr. Vander Sloten teaches engineering mechanics, problem solving and engineering design, computer integrated surgery systems, and medical device design including regulatory affairs. From 2006 to 2012, he served as program director of the Master in Biomedical Engineering at KU Leuven. His research interests are computer applications in musculoskeletal biomechanics and computer integrated surgery, on which he authored more than 160 journal papers. Mr. Vander Sloten is a Founding Fellow of the European Alliance for Medical and Biological Engineering and Science, where he previously served as president in 2006, president-elect in 2005 and secretary-general from 2003 to 2004. In 2015, he was elected as a member of the International Academy for Medical and Biological Engineering. Mr. Vander Sloten holds a Master’s degree in Mechanical Engineering and a PhD in Mechanical Engineering – Biomedical Engineering from KU Leuven. Since 2016, he is Vice-Dean for International Affairs at the Faculty of Engineering Science, KU Leuven.
Lieve Verplancke. Lieve Verplancke has served as one of our independent directors since June 2015. Ms. Verplancke began her career in 1984 with The Beecham Group (now part of GlaxoSmithKline), and has since held key management positions with Merck & Co., as well as Bristol-Myers Squibb, where she served as Managing Director, leading their Belgian/GDL subsidiary until 2012. Ms. Verplancke has also served as a board member for Brussels-based Europe Hospitals, the Imelda Hospital in Bonheiden, the Euronext fund, Quest for Growth, MDxHealth and the Stichting tegen Kanker. She is also the founder and managing director of Qaly@Beersel, an elderly care center in Belgium. In addition to being a medical doctor (MD – KU Leuven), Ms. Verplancke holds a postgraduate degree in Economics and a Master in Business Administration from the University of Antwerp. She has also completed courses at INSEAD, CEDEP, Columbia University and the Vlerick Business School, and is a certified Executive Coach (PCC).
Bart Luyten. Bart Luyten has served as one of our independent directors since June 2017 and also previously served as representative of one of our directors from 2012 to 2015. Mr. Luyten is Founder and Managing Partner of SmartFin, a private equity fund platform investing in early- and growth stage technology companies through four investment entities under the SmartFin brand. Previously, Mr. Luyten was the Founder and Managing Director of Sniper Investments NV, a B2B technologies fund that was set up in 2010. Mr. Luyten has experience as Investment Director of Partners At Venture, Managing Partner of Privast Capital Partners and General Partner of Nausicaa Ventures, all Belgian-based private equity and venture capital funds with a focus on B2B technology investments. Mr. Luyten currently holds positions on the boards of directors of a number of European B2B technology companies such as Betty Blocks, Recharge and Eyesee. Mr. Luyten holds a Master of Science degree in Applied Economics from the University of Antwerp and a postgraduate Master degree in SME management from VIZO Brussels.
Volker Hammes. Volker Hammes, has served as one of our directors since November 2018. Mr. Hammes has served as a Managing Director of BASF New Business GmbH, a subsidiary of BASF SE, the German chemical conglomerate (FWB: BAS), since January 2016 as well as first as Managing Director and then as Chairman of BASF 3D Printing Solutions GmbH, another subsidiary of BASF, since August 2017 and June 2019 respectively. Between 2012 and 2016, Mr. Hammes also served as director or officer of various BASF affiliates, including as Chief Executive Officer and Managing Director, Head of Business Center Turkey, Middle East and North Africa of BASF Turk Kimya San. Ltd. Sti. In addition, Mr. Hammes has served as a director on the board of directors of Essentium Inc. and Evolve Additive Solutions, both providers of industrial 3D printing solutions, since December 2017 and January 2021 respectively. Mr. Hammes holds a Master of Science degree in Mechanical Engineering, Polymer Technology from RWTH Aachen.
78
Our board of directors has established an Executive Committee. The following is a brief summary of the professional experience of the members of our Executive Committee, which was established effective as of January 1, 2017:
Johan Pauwels. Johan Pauwels, as permanent representative of Seaquence BV, has served as an Executive Vice President and Chief Operating Officer of our company since January 2011 and has been with our company since our founding. In 1990, Mr. Pauwels completed his Master’s thesis on stereolithography on the very first 3D printing machine at Materialise. After graduating in 1991, Mr. Pauwels stayed on with our company, focusing on software development to support our 3D printing services. Throughout his career with our company, Mr. Pauwels has held several positions, including Software Sales Manager and Director of Sales, and is currently an Executive Vice President responsible for global sales organization and our sales offices around the world. As of 2021, Mr. Pauwels is also the Chief Operating Officer of our company. Mr. Pauwels holds a Master’s degree in Electro-Mechanical Engineering from KU Leuven.
Bart Van der Schueren. Bart Van der Schueren has served as an Executive Vice President of our company since January 2011 and as our Chief Strategy and Technology Officer since 2016. In February 2022 he also assumed the position of Vice President of the Materialise Software segment. As permanent representative of BEspired BV, Mr. Van der Schueren serves as Chief Strategy and Technology Office since January 2024. Prior to joining Materialise, Mr. Van der Schueren was at KU Leuven as a liaison engineer for the newly founded Materialise and established the basic research activities for the company while also founding the research activities in 3D printing at the KU Leuven. Mr. Van der Schueren then went on to obtain a PhD in selective laser metal sintering. In 1995, Mr. Van der Schueren officially joined Materialise and ran the service bureau. Over the years, his dedication and expertise has grown the service bureau from a regional player to one of the most prominent additive manufacturing facilities in Europe. In 2011, Mr. Van der Schueren became an Executive Vice President of our company, responsible for the Materialise Manufacturing segment and focusing on production and engineering services. Since 2018, Mr. Van der Schueren is globally responsible for the research activities of Materialise, and between 2022 until the end of 2023 he was also responsible for the activities of the Materialise Software segment. Mr. Van der Schueren holds a PhD in Selective Laser Metal Sintering and a Master’s degree in Mechanical Engineering from KU Leuven.
Koen Berges. Koen Berges, as permanent representative of Finstraco BV, has served as our Chief Financial Officer since May 2023. Mr. Berges brings more than 20 years of experience in financial leadership positions in various business environments ranging from large multinational corporations to leading family holdings and to fast-growing private equity-backed services companies. Mr. Berges joined Materialise from Cheops Technology NV, a managed service provider in secure IT infrastructures and cloud computing, where he served as Chief Financial Officer and where he was also a member of the Executive Committee from May 2019 until April 2023. Mr. Berges started his professional career at PwC Consulting and subsequently also held various international finance leadership roles at ExxonMobil and investment group Alcopa. Mr. Berges holds a Master of Science in Business Engineering, International Management from the University of Antwerp.
Brigitte de Vet-Veithen. Brigitte de Vet-Veithen has represented De Vet Management BV and has served as our Chief Executive Officer since January 2024. Prior to that Ms. De Vet-Veithen served as Vice President of the Materialise Medical segment since June 2016. Mrs. de Vet-Veithen has more than 20 years of experience in the Healthcare and Life Sciences Sector. She has worked in various management roles for Johnson & Johnson, ultimately serving as Vice President for the EMEA region of Cordis Neurovascular and General Manager of Cordis in Germany. Before joining Materialise she has held various leadership roles as representative of De Vet Management BV including the role of Chief Executive Officer of Acertys group, a provider of medical devices, software, services and supplies to hospitals and medical professionals. Mrs. de Vet-Veithen holds a Master of Business Administration with a Major in Engineering from HEC Liege and an MBA from INSEAD.
Jurgen Laudus. Jurgen Laudus, as permanent representative of Level 5 B.V., serves as Vice President of our Materialise Manufacturing segment. Mr. Laudus joined us in August 2001 as project manager and continued to our U.K. office to become Rapid Tooling manager in 2003. For two years, Mr. Laudus was responsible for both our Rapid Tooling sales support and production management. In 2005, Mr. Laudus returned to Belgium to become international production manager for our additive manufacturing services and later on sales manager, playing an active role in the growth of the additive manufacturing production activities of Materialise. Mr. Laudus holds a Master of Science degree in Engineering from the KU Leuven.
Conny Hooghe. Conny Hooghe represented SoHo Services as our Vice President of Human Resources since September 2017. She holds a Master of Industrial Psychology from the University of Ghent. Previously she has held several human resources management positions within technological oriented or IT companies like Wolters Kluwer, Fujitsu Services and Atos Origin.
79
Carla Van Steenbergen. Carla Van Steenbergen, as permanent representative of Super Mare & Park BV, has served as our Executive Vice President and Director, Corporate Affairs, supporting the company’s legal and procurement department and M&A and partnership transactions since January 2024. Prior to that, Ms. Van Steenbergen served as our in-house counsel since 2003, and her role has gradually evolved into our Chief Legal Officer. Ms. Van Steenbergen has served as our Compliance Officer since June 2014, and is a member of our Executive Committee in addition to being secretary to the board of directors. Ms. Van Steenbergen graduated from the law faculty of KU Leuven in 1999. After having worked for three years at Brussels’ based law firm Marx Van Ranst Vermeersch & Partners, she temporarily moved to London to earn a LLM degree at King’s College London. Upon her return to Belgium, she started working as in-house legal counsel for our company, a position which she holds to this day.
Udo Eberlein. Udo Eberlein, has served as our Vice President of Software, since November 2023. Prior to that, in February 2021 Mr. Eberlein co-founded Goldn, an online working space for cosmetic creators and suppliers and he also works in Chemovator supporting startups in their business journey. Mr Eberlein is a seasoned software technology executive with successfully building and leading large and mid-scale technology organizations in complex global markets. Throughout his career, he has acquired a diverse range of skills and accomplishments spanning various fields, such as internet services, digital transformation, digital media software, IoT, SaaS, marketplaces, corporate development, strategic advisory, and venture capital, among others. He holds a degree in Logistics and Business Administration from Stuttgart University.
Family Relationships
Wilfried Vancraen and Hilde Ingelaere are spouses. Sander Vancraen is the son of Wilfried Vancraen and Hilde Ingelaere. No other family relationship exists between any members of our board of directors or senior management.
Board Diversity Disclosures
In accordance with Nasdaq Listing Rule 5606, each company must disclose annually information on each director’s voluntary self-identified characteristics. The table below includes information on the diversity of our board of directors based upon information voluntarily provided by each director for each of the years ended December 31, 2022 and 2023.
Board Diversity Matrix | ||||
Country of Principal Executive Offices: | Belgium | |||
Foreign Private Issuer | Yes | |||
Disclosure Prohibited under Home Country Law | No | |||
Total Number of Directors | 10 | |||
Female | Male | Non-Binary | Did Not | |
Part I: Gender Identity | ||||
Directors | 2 | 8 | 0 | 0 |
Part II: Demographic Background | ||||
Underrepresented Individual in Home Country Jurisdiction | 0 | |||
LGBTQ+ | 0 | |||
Did Not Disclose Demographic Background | 0 | |||
80
| B. | Compensation |
Compensation of Directors
Our Remuneration and Nomination Committee recommends the level of remuneration for directors. These recommendations are subject to approval by our board of directors and, subsequently, by our shareholders at the annual general meeting. During the year ended December 31, 2023, only the directorships of Mr. De Lille, Mr. Vander Sloten, Mr. Ingels, Mr. Luyten, Ms. Verplancke, Mr. Sander Vancraen and Mr. Hammes were remunerated. The directorships of Mr. Wilfried Vancraen, Mr. Leys and Ms. Ingelaere were not remunerated because these individuals were remunerated in their capacity as senior management. During the year ended December 31, 2023, Mr. De Lille, Mr. Vander Sloten, Mr. Ingels, Mr. Luyten, Ms. Verplancke, Mr. Sander Vancraen and Mr. Hammes each received annual remuneration equal to € 11,000. In addition, Mr. De Lille, Mr. Vander Sloten, Mr. Ingels, Mr. Luyten, Ms. Verplancke, Mr. Sander Vancraen and Mr. Hammes each received a remuneration of € 1,375 per physical board meeting that he or she attended and € 687.5 for each board meeting held via conference call (lasting more than one hour) that he or she attended.
In addition, the Chairman of the Audit Committee received an annual remuneration of € 8,250. Each independent member (including the Chairman) of the Audit Committee or the Remuneration and Nomination Committee received a remuneration of € 1,375 for each physical committee meeting that he or she attended, and € 687.5 for each committee meeting held via conference call (lasting more than one hour) and that he or she attended. The Remuneration and Nomination Committee benchmarks directors’ compensation against peer companies to ensure that it is competitive. In addition, our board of directors sets and revises, from time to time, the rules and level of compensation for directors carrying out a special mandate or sitting on one or more of the board of directors committees and the rules for reimbursement of directors’ business-related out-of-pocket expenses.
Compensation of Senior Management and Executive Committee
In 2023, the aggregate total gross compensation of our senior management amounted to € 2.6 million, which included base salary, bonus payments, company car allowance and other benefits. This amount also includes the compensation for the members of the Executive Committee. During 2023, the directorships of Mr. Wilfried Vancraen, Mr. Leys and Ms. Ingelaere were not remunerated.
We have entered into services agreements (Contracts for Paid Office as a member of the Executive Committee) with each member of our Executive Committee. The terms of these agreements are substantially similar. These agreements generally provide for an annual base salary. In addition to the fixed remuneration components, under the terms of these agreements, members of our Executive Committee are entitled to certain additional benefits (including mobile phone and director and officer liability insurance) and reimbursement of necessary and reasonable expenses. These services agreements with members of our Executive Committee provide for payments and benefits (including upon termination of employment) that we believe are in line with customary market practice for similar companies who are operating in our industry.
Service Contracts
Except as described above under “—B. Compensation—Compensation of Senior Management and Executive Committee,” we do not have service contracts with any member of our board of directors or Executive Committee.
Board of Directors Practices
Decisions are generally made by our board of directors as a whole. However, decisions on certain matters may be delegated to committees of our board of directors or to the Executive Committee to the extent permitted by law and our restated articles of association. The chairperson, or if he or she is prevented from doing so, the vice chairperson, chairs the meetings of our board of directors.
Our board of directors transferred management powers to the Executive Committee, except for the general policy of the company and other powers which are reserved by Belgian company law to the board of directors. The Executive Committee is supervised by our board of directors. The following actions are comprised under general policy of our company and are thus excluded from the powers of the Executive Committee:
| ● | mergers and acquisitions; |
81
| ● | transfer and waive of intellectual property rights to third parties; |
| ● | granting of exclusivity rights to third parties with an important impact on the freedom of a particular business segment; |
| ● | nomination and removal of members of the Executive Committee; |
| ● | opening of offices abroad and nomination and removal of managers thereof; |
| ● | conclusion of financial loans; |
| ● | sale and purchase of real estate; and |
| ● | cancellation of a particular product line. |
As from January 1, 2024, our board of directors entrusted the daily management of the company to De Vet Management BV, represented by Brigitte de Vet-Veithen, our Chief Executive Officer, in conformity with article 7:121of the Belgian Companies and Associations Code. Until December 31, 2023, this position was held by Wilfried Vancraen.
Pursuant to our restated articles of association, our board of directors may form committees from among its members and charge them with the performance of specific tasks. The committees’ tasks, authorizations and processes are determined by our board of directors. Where permissible by law and our restated articles of association, important powers of our board of directors may also be transferred to committees.
Audit Committee
The Audit Committee consists of three members: Johan De Lille (Chairman), Bart Luyten and Jürgen Ingels. Our board of directors has determined that Messrs. De Lille, Luyten and Ingels are independent under Rule 10A-3 of the Exchange Act and the applicable rules of the Nasdaq Stock Market and that each of Messrs. De Lille, Luyten and Ingels qualifies as an “audit committee financial expert” as defined under the Exchange Act.
Our Audit Committee assists our board of directors in overseeing the accuracy and integrity of our accounting and financial reporting processes and audits of our consolidated financial statements, the implementation and effectiveness of an internal control system and our compliance with legal and regulatory requirements, the independent auditors’ qualifications and independence and the performance of the independent auditors.
The Audit Committee’s duties and responsibilities to carry out its purposes include, among others:
| ● | the review of our accounting processes; |
| ● | the review of the effectiveness of our internal systems of control, risk management and compliance; |
| ● | the consideration and recommendation of the nomination, compensation, retention and termination of the Company’s statutory auditor for Belgian company law purposes and the Company’s independent auditor for SEC purposes, the commissioning of the auditors to conduct audits, agreeing on additional services to be provided by the auditors under their respective engagements, the establishment of the scope and the main review points of the audit and oversight of the auditors’ work (including resolution of disagreements with the auditors); |
| ● | the preparation of our board of directors’ resolution on our consolidated financial statements; |
| ● | reviewing our interim consolidated financial statements that are made public or otherwise filed with any securities regulatory authority; |
| ● | discussing any flaws relating to our internal control systems, as reported by our board of directors to the audit committee; |
| ● | monitoring our bookkeeping and records; and |
82
| ● | the establishment of procedures for (i) the receipt, retention and treatment of complaints we receive regarding accounting, internal accounting controls or auditing matters and (ii) the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters. |
Our Audit Committee is entitled to review information on any point it wishes to verify, and is authorized to acquire such information from any of our employees. It is also authorized to obtain independent advice, including legal advice, if this is necessary for an inquiry into any matter under its responsibility. It is entitled to call on the resources that would be needed for this task. It is entitled to receive reports directly from the auditors, including reports with recommendations on how to improve our control processes.
Remuneration and Nomination Committee
Our Remuneration and Nomination Committee consists of three members: Wilfried Vancraen, Jozef Vander Sloten and Lieve Verplancke. As from January 1, 2024, Hilde Ingelaere has replaced Wilfried Vancraen as a member of our Remuneration and Nomination Committee. Our board of directors has determined that Ms. Verplancke is independent under the applicable rules of the Nasdaq Stock Market.
Our Remuneration and Nomination Committee assists our board of directors in its decisions relating to the remuneration policy and individual remuneration packages for our board of directors, the appointment of directors, the Chief Executive Officer and the other members of senior management.
The Remuneration and Nomination Committee’s duties and responsibilities to carry out its purposes include, among others:
| ● | identifying individuals qualified to become members of our board of directors, consistent with criteria approved by our board of directors; |
| ● | recommending to our board of directors the director nominees for each annual general meeting, taking into account any nomination rights that certain shareholders may have under our restated articles of association; |
| ● | recommending to our board of directors director nominees to fill vacancies; |
| ● | recommending to our board of directors qualified and experienced directors for service on the committees of the board of directors; |
| ● | recommending to our board of directors the compensation of the members of senior management; |
| ● | recommending to our board of directors any incentive compensation plans and equity-based plans, and awards thereunder, and profit-sharing plans for our employees; |
| ● | evaluating the performance of our Chief Executive Officer; and |
| ● | advising our board of directors on other compensation issues. |
83
The table below sets out information about the number of FTEs and fully dedicated consultants, which consultants included individual professionals who are registered as private entrepreneurs in Ukraine. Due to the war in Ukraine, some private entrepreneurs have been relocated to Poland, though they continue to work exclusively with our company. FTEs who are a part of one or more of our three core competencies are allocated to one of our segments and therefore included in our segment reporting.
At December 31, | ||||||
| 2023 |
| 2022 |
| 2021 | |
Total |
| 2,437 |
| 2,439 |
| 2,332 |
Segments: |
|
|
|
|
|
|
Materialise Software |
| 293 |
| 339 |
| 281 |
Materialise Medical |
| 928 |
| 888 |
| 861 |
Materialise Manufacturing |
| 784 |
| 760 |
| 752 |
Additional staff |
| 432 |
| 452 |
| 438 |
We currently do not have a workers’ council or trade union delegation. We have a health and safety committee entitled to certain information and consultation rights under Belgian law, at our Belgian headquarters. We consider our employee relations to be good and have never experienced a work stoppage.
The following table sets forth information relating to beneficial ownership of our ordinary shares, for each member of our board of directors and senior management as of March 26th, 2024:
Ordinary Shares Beneficially | ||||
Owned as of 26 March 2024 | ||||
Name of beneficial owner(1) |
| Number(2) |
| Percent(2) |
Wilfried Vancraen (3) |
| 33,325,821 | 56.42 | |
Peter Leys (4) |
| 320,459 | * | |
A Tre C CVOA, represented by Johan De Lille (5) |
| — | — | |
Sander Vancraen |
| — | — | |
Jürgen Ingels |
| — | — | |
Jos Vander Sloten (6) |
| 12,000 | * | |
Lieve Verplancke |
| — | — | |
Hilde Ingelaere (3) |
| 33,325,821 | 56.42 | |
Bart Luyten |
| — | — | |
Volker Hammes (7) |
| 2,500 | — | |
Johan Pauwels (8) |
| 151,545 | * | |
Bart Van der Schueren (9) |
| 143,346 | * | |
Conny Hooghe | — | — | ||
Jurgen Laudus (10) |
| 45,145 | * | |
Carla Van Steenbergen (11) |
| 28,635 | * | |
Brigitte de Vet - Veithen (12) |
| 27,793 | * | |
Koen Berges (13) | 2,780 | * | ||
Udo Eberlein | — | — | ||
* | Less than 1% |
| (1) | Except as otherwise indicated, the address for each of the persons named above is Technologielaan 15, 3001 Leuven, Belgium. |
84
| (2) | Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days of March 26, 2024, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person. Except as otherwise indicated, we believe the persons named in this table have sole voting and investment power with respect to all ordinary shares shown as beneficially owned by them, subject to community property laws where applicable and to the information contained in the footnotes to this table. |
| (3) | Consists of (i) 110,545 ordinary shares and 27,135 ADSs held by Mr. Vancraen, (ii) 110,545 ordinary shares and 27,135 ADSs held by Ms. Ingelaere. (iii) 30,858,964 ordinary shares and 2,171,497 ADSs jointly held by Mr. Vancraen and Ms. Ingelaere through Idem, a partnership (maatschap) that is controlled and managed by Mr. Vancraen and Ms. Ingelaere and (iv) 20,000 ADSs jointly held (directly) by Mr. Vancraen and Ms. Ingelaere. |
| (4) | Consists of (i) 320,459 ADSs and ordinary shares held by Peter Leys. 307,419 of these ADS and ordinary shares are subject to shared voting and investment power and are owned by: Mountain View (maatschap) as 75,000 ADS and 101,781 ordinary shares. (ii) Riverside (maatschap) holds 22,862 ADSs and (iii) Els Kindt, the spouse of Peter Leys, holds 4,215 ADS and 103,561 ordinary shares. Both Mountain View and Riverside are jointly controlled by Peter Leys and Els Kindt. |
| (5) | The address for A Tre C CVOA is Timmermansstraat 32, 8340 Damme, Belgium. |
| (6) | Consists of 12,000 shares held by Mr. Vander Sloten. |
| (7) | Consists of 2,500 ADSs held by Mr. Hammes. |
| (8) | Consists of (i) 40.000 ordinary shares held by Mr. Pauwels (ii) 100,000 ordinary shares held by Sorelle, a civil partnership that is controlled and managed by Mr. Pauwels and Ms Van Muylder (iii) 1,000 ADS in an investment account in the name of Sorelle and (iv) 10,545 ADS held by Mr. Pauwels. |
| (9) | Consists of 143,346 ordinary shares held by Mr. Van der Schueren. |
| (10) | Consists of 45,145 ADSs held by Mr. Laudus. |
| (11) | Consists of 28,635 ordinary shares held by Ms. Van Steenbergen. |
| (12) | Consists of 27,793 ADSs held by Ms. de Vet-Veithen. |
| (13) | Consist of 2,780 ADS held by Mr. Berges. |
F. | Disclosure of a registrant’s action to recover erroneously awarded compensation |
Not applicable.
85
ITEM 7.MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
| A. | Major Shareholders |
The following table sets forth information relating to beneficial ownership of our ordinary shares, as of March 26, 2024, for each person who is known by us to own beneficially 5% or more of our outstanding ordinary shares:
Ordinary Shares Beneficially | ||||
Owned as of March 26, 2024 | ||||
Name of Beneficial Owner(1) |
| Number(2) |
| Percent(2) |
Wilfried Vancraen(3) |
| 33,325,821 |
| 56.42 |
Hilde Ingelaere(3) |
| 33,325,821 |
| 56.42 |
ARK Investment Management LLC(4) |
| 3,592,979 |
| 6.08 |
(1)Except as otherwise indicated, the address for each of the persons named above is Technologielaan 15, 3001 Leuven, Belgium.
(2) | Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days of March 26, 2024, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person. Except as otherwise indicated, we believe the persons named in this table have sole voting and investment power with respect to all ordinary shares shown as beneficially owned by them, subject to community property laws where applicable and to the information contained in the footnotes to this table. |
(3) | Consists of (i) 110,545 ordinary shares and 27,135 ADSs held by Mr. Vancraen, (ii) 110,545 ordinary shares and 27,135 ADSs held by Ms. Ingelaere and (iii) 30,858,964 ordinary shares and 2,171,497 ADSs jointly held by Mr. Vancraen and Ms. Ingelaere through Idem, a partnership (maatschap) that is controlled and managed by Mr. Vancraen and Ms. Ingelaere. |
(4)Based on a Schedule 13G/A filed with the SEC on February 10, 2023 by ARK Investment Management LLC or ARK. ARK is an investment advisor and in the Schedule 13G/A filed by ARK it is reported that ARK has (a) sole voting power with respect to 3,592,979 ADSs; (b) shared voting with respect to 71,296 ADSs; and (c) sole dispositive power with respect to 3,415,360 ADSs.
None of our shareholders have different voting rights from other shareholders, except that as long as the Family Shareholders control, directly or indirectly, in the aggregate at least 20% of the voting rights attached to our ordinary shares, a majority of our directors must be appointed by our shareholders from a list of candidates proposed by the Family Shareholders. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
As of March 26, 2024, there were 30 individual holders of record entered in our share register. The number of individual holders of record is based exclusively upon our share register and does not address whether a share or shares may be held by the holder of record on behalf of more than one person or institution who may be deemed to be the beneficial owner of a share or shares in our company. As of March 26, 2024, 53.60% of our outstanding ordinary shares were held directly by 30 holders of record, and we believe that at least 23 of such shareholders (representing 53.60% of our outstanding ordinary shares), are residents of Belgium. As of March 26, 2024, assuming that all of our ordinary shares represented by ADSs are held by residents of the United States, approximately 46.39% of our outstanding ordinary shares were held in the United States by one holder of record, the Bank of New York Mellon, depositary of the ADSs. At such date, there were outstanding 27,399,403 ADSs, each representing one of our ordinary shares, and in the aggregate representing approximately 46.39% of our outstanding ordinary shares. The actual number of holders is greater than these numbers of record holders, and includes beneficial owners whose ADSs are held in street name by brokers and other nominees. This number of holders of record also does not include holder whose shares may be held in trust by other entities.
| B. | Related Party Transactions |
Since January 1, 2023, there has not been, nor is there currently proposed, any material transaction or series of similar material transactions to which we were or are a party in which any of the members of our board of directors or senior management, holders of more than 10% of any class of our voting securities, or any member of the immediate family of any of the foregoing persons, had or will have a direct or indirect material interest, other than the compensation and shareholding arrangements we describe in “Item 6. Directors, Senior Management and Employees” and “—A. Major Shareholders,” and the transactions we describe below.
86
Lunebeke NV
In the past, Ailanthus NV, which was a shareholder of our company up until it was merged into our company (which we refer to as the “Merger”) and which was owned and controlled by Mr. Vancraen and Ms. Ingelaere, had provided several loans and financial leases to us for the purchase of machinery and a portion of our office and production buildings.
Ailanthus NV had granted us a loan at a fixed interest rate of 4.23% that matures in 2025. The purpose of the loan was to finance the purchase of a building in France. Prior to the Merger, Ailanthus NV was demerged into Lunebeke NV, a newly incorporated company. All of Ailanthus NV’s assets and liabilities were transferred to Lunebeke NV, with the exception of (i) the ordinary shares of our company held by Ailanthus NV and (ii) the corresponding accounting equity components. As such, the loan granted by Ailanthus NV was also transferred from Ailanthus NV to Lunebeke NV. For additional information about the loan, see Note 15 to our audited consolidated financial statements.
We used to rent apartments on a regular basis from Ailanthus NV in order to host our employees from foreign subsidiaries who were visiting our headquarters in Leuven. This activity was also transferred from Ailanthus NV to Lunebeke NV as a result of Ailanthus’s demerger. In 2023, we incurred K€97 of rent expense to Lunebeke NV.
Indemnification Agreement
In connection with and prior to the Merger, we entered into an indemnification agreement with Ailanthus NV and with Wilfried Vancraen, Hilde Ingelaere and Lunebeke NV (which we refer to collectively as the “indemnifying parties”). Pursuant to the indemnification agreement, among other things, the indemnifying parties agreed to reimburse us for: (i) costs incurred by us in connection with the Merger, (ii) possible liabilities of our company as a result of the Merger, and (iii) possible negative tax consequences, if any, for certain of our shareholders. The obligation to reimburse our shareholders applies to shareholders who were shareholders prior to April 30, 2021 (which we refer to as “qualifying shareholders”).
The term of the indemnification agreement expires on December 31, 2030. However, we and any qualifying shareholders have the right to make claims against the indemnifying parties for a period of 10 years following the occurrence giving rise to the claim.
Registration Rights Agreement
On September 15, 2016, we entered into a registration rights agreement with certain holders of our ordinary shares, warrants and convertible bonds, including certain of our directors, senior management and consultants, which we refer to as the Registration Rights Agreement. In accordance with the terms of the Registration Rights Agreement, we filed a shelf registration statement on Form F-3 registering certain ordinary shares represented by ADSs to be sold by the selling shareholders from time to time. These ordinary shares consisted of ordinary shares previously issued to and ordinary shares issuable upon exercise of warrants or conversion of convertible bonds held by the selling shareholders, as well as ordinary shares underlying ADSs that were acquired by the selling shareholders on the Nasdaq Global Select Market.
Letter Agreement Regarding Shares Issuance and Registration Rights
In connection with the Merger, we entered into a letter agreement, dated December 31, 2020, with Wilfried Vancraen and Hilde Ingelaere pursuant to which, among other things, we granted certain demand and “piggyback” registration rights to Wilfried Vancraen and Hilde Ingelaere in respect of the new ordinary shares that were issued to them in connection with the Merger.
| C. | Interests of Experts and Counsel |
Not applicable.
87
ITEM 8.FINANCIAL INFORMATION
See “Item 18. Financial Statements.”
Legal or Arbitration Proceedings
From time to time, we may be subject to various claims or legal or arbitration proceedings that arise in the ordinary course of our business.
In May 2023, the Belgian Center for Arbitration and Mediation issued a decision in the arbitration proceedings filed by Zimmer Biomet against Materialise, pursuant to which we were ordered to pay an amount of € 5,0000,000 plus interest to Zimmer Biomet. No amounts had been accrued for this loss contingency.
We are currently not a party to any other legal or arbitration proceedings, which, in the opinion of our management, is likely to have or could reasonably possibly have a material adverse effect on our business, financial condition or results of operations.
Policy on Dividend Distribution
We have never declared or paid any cash dividends on our shares, and we have no present intention of declaring or paying any cash dividends in the foreseeable future. Any recommendation by our board of directors to pay cash dividends, subject to compliance with applicable law and any contractual provisions that restrict or limit our ability to pay dividends, including under agreements for indebtedness that we may incur, will depend on many factors, including our financial condition, results of operations, legal requirements, capital requirements, business prospects and other factors that our board of directors deems relevant.
All of the shares represented by the ADSs have the same dividend rights as all of our other outstanding shares. In general, distributions of dividends proposed by our board of directors require the approval of our shareholders at a shareholders’ meeting, although our board of directors may declare interim dividends without shareholder approval.
Furthermore, pursuant to Belgian law, the calculation of amounts available for distribution to shareholders, as dividends or otherwise, must be determined on the basis of our non-consolidated statutory Belgian GAAP financial statements. In addition, in accordance with Belgian law and our restated articles of association, we must allocate each year an amount of at least 5% of our annual net profit under our statutory non-consolidated accounts (prepared in accordance with Belgian GAAP) to a legal reserve until the reserve equals 10% of our share capital. As a consequence of these facts there can be no assurance as to whether dividends or other distributions will be paid out in the future or, if they are paid, their amount.
For information regarding the Belgian withholding tax applicable to dividends and related U.S. reimbursement procedures, see “Item 10. Additional Information—E. Taxation—Belgian Taxation.”
B.Significant Changes
None.
ITEM 9.THE OFFER AND LISTING
A.Offer and Listing Details
Price History
The ADSs, each representing one ordinary share, have been listed on the Nasdaq Global Select Market under the symbol “MTLS” since June 25, 2014. Prior to that date, there was no public trading market for ADSs or our ordinary shares.
B.Plan of Distribution
Not applicable.
88
C.Markets
The ADSs have been listed on the Nasdaq Global Select Market under the symbol “MTLS” since June 25, 2014.
D.Selling Shareholders
Not applicable.
E.Dilution
Not applicable.
F.Expenses of the Issue
Not applicable.
ITEM 10.ADDITIONAL INFORMATION
A.Share Capital
Not applicable.
B.Memorandum and Articles of Association
The information called for by this item was previously reported in Exhibit 2.3 (Description of Securities) to our Annual Report on Form 20-F for the year ended December 31, 2020, which exhibit is incorporated herein by reference, and is supplemented by the following additional information related to changes in our share capital. The share capital of Materialise NV was increased following the exercise of warrants previously issued under our 2007 Warrant Plan on November 27, 2014, with € 4,336.77 (excluding an issuance premium of € 69,359.23) against the issuance of 75,200 new ordinary shares.
On March 5, 2015, the board of directors increased the share capital of Materialise NV by €4,626.50 (excluding an issuance premium of € 574,290.50) against the issuance of 80,182 new ordinary shares.
The share capital of Materialise NV was increased following the exercise of warrants previously issued under our 2007 Warrant Plan on November 20, 2015, with € 5,647.15 (excluding an issuance premium of € 90,392.85) against the issuance of 98,000 new ordinary shares. The 2007 Warrant Plan 2007 is now terminated. There are no outstanding warrants issued under this plan.
On December 18, 2015, the board of directors adopted a new Warrant Plan, our 2015 Warrant Plan, and issued 1,400,000 warrants, which warrants are exercisable for 1,400,000 new ordinary shares. As of December 31, 2020, 352,000 of the warrants were granted.
On March 30, 2018, the board of directors increased the share capital of Materialise NV by € 5,931.68 (excluding an issuance premium of € 201,331.37) against the issuance of 102,856 new ordinary shares.
On July 19, 2018, the board of directors increased the share capital of Materialise NV by € 112,636.20 (excluding an issuance premium of € 21,418,670.32) against the issuance of 1,953,125 new ordinary shares.
On July 18, 2018, the board of directors decided to increase the share capital of Materialise NV, which capital increase was confirmed on July 26 and July 27, 2018, by € 173,009.19 (excluding an issuance premium of € 33,188,838.54) and € 25,951.38 (excluding an issuance premium of € 4,967,220.35), respectively, against the issuance of 3,000,000 and 450,000 new ordinary shares, respectively.
On December 28, 2018, the board of directors increased the share capital of Materialise NV following the exercise of warrants previously issued under the 2013 Warrant Plan and the 2014 Warrant Plan by € 1,102.07 (excluding an issuance premium of € 39,676.43) and € 2,321.96 (excluding share premium of € 352,210.06), respectively, against the issuance of 19,100 and 40,242 new ordinary shares, respectively.
89
On November 29, 2019, the board of directors increased the share capital of Materialise NV following the exercise of warrants previously issued under the 2013 Warrant Plan and the 2014 Warrant Plan by € 10,274.68 (excluding an issuance premium of € 345,325.58) and € 5,973.90 (excluding an issuance premium of € 906,636.38), respectively, against the issuance of 178,164 and 103,588 new ordinary shares, respectively.
On April 16, 2020, the board of directors increased the share capital of Materialise NV following the exercise of warrants previously issued under the 2015 Warrant Plan by € 1,254.32 (excluding an issuance premium of € 139,033.18) against the issuance of 21,750 new ordinary shares.
On October 9, 2020, the board of directors increased the share capital of Materialise NV following the conversion of the convertible bonds held by Peter Leys and his spouse by € 1,000,000 against the issuance of 508,904 new ordinary shares.
On November 13, 2020, the board of directors increased the share capital of Materialise NV following the exercise of warrants previously issued under the 2013 Warrant Plan, the 2014 Warrant Plan and the 2015 Warrant Plan by € 2,180.98 (excluding an issuance premium of 231,347.86), € 15,212.54 (excluding an issuance premium of € 1,757,042.30) and € 11,324.48 (excluding an issuance premium of € 954,563.02) against the issuance of 115,176, 201,164 and 149,750 new ordinary shares, respectively.
On December 31, 2020, in the context of the merger between Materialise NV and Ailanthus NV, the extraordinary general meeting of shareholders decided to increase the share capital of Materialise NV and in the same notarial deed of the same date, decided to decrease the share capital of Materialise NV by the same amount. As a result, the share capital of Materialise NV did not change as a result of the aforementioned merger.
On May 5, 2021, the board of directors increased the share capital of Materialise NV following the exercise of warrants previously issued under the 2015 Warrant Plan by € 102,09 (excluding an issuance premium of € 8.605,41) against the issuance of 1.350 new ordinary shares.
On June 9, 2021, the board of directors decided to increase the share capital of Materialise NV, which capital increase was confirmed on June 14, 2021 and July 6, 2021, by € 320.000,00 (excluding an issuance premium of € 78.484.793,95) and € 48.000,00 (excluding an issuance premium of € 11.772.719,09), respectively, against the issuance of 4,000,000 and 600,000 new ordinary shares, respectively.
On November 23, 2021, the board of directors increased the share capital of Materialise NV following the exercise of warrants previously issued under the 2014 Warrant Plan and the 2015 Warrant Plan by € 13.655,81 (excluding an issuance premium of 1.570.065,03) and € 8.595,46 (excluding an issuance premium of € 721.222,04) against the issuance of 179.764 and 113.150 new ordinary shares, respectively.
On December 28, 2022, the board of directors increased the share capital of Materialise NV following the exercise of warrants previously issued under the 2014 Warrant Plan and the 2015 Warrant Plan by € 65,71 (excluding an issuance premium of 7,554.94) and € 212.70 (excluding an issuance premium of € 17.847,30) against the issuance of 865 and 2,800 new ordinary shares, respectively.
On October 25, 2023, the board of directors adopted a new Warrant Plan, our 2023 Warrant Plan, and issued 500,000 warrants, which warrants are exercisable for 500,000 new ordinary shares. As of December 31, 2023, 350,000 warrants were granted.
C.Material Contracts
We have not entered into any material contracts in the prior two years other than in the ordinary course of business and other than those described elsewhere in this annual report, including under “—B. Memorandum and Articles of Association,” “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions.”
D.Exchange Controls
There are no Belgian exchange control regulations that impose limitations on our ability to make, or the amount of, cash payments to residents of the United States. See “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Transfers from Subsidiaries” for a discussion of various restrictions applicable to transfers of funds by our subsidiaries.
90
E.Taxation
Belgian Taxation
The following paragraphs are a summary of material Belgian tax consequences of the ownership of ADSs by an investor. The summary is based on laws, treaties and regulatory interpretations in effect in Belgium on the date of this document, all of which are subject to change, including changes that could have retroactive effect.
The summary only discusses Belgian tax aspects which are relevant to U.S. holders of ADSs (“Holders”). This summary does not address Belgian tax aspects which are relevant to persons who are residents in Belgium or engaged in a trade or business in Belgium through a permanent establishment or a fixed base in Belgium. This summary does not purport to be a description of all of the tax consequences of the ownership of ADSs, and does not take into account the specific circumstances of any particular investor, some of which may be subject to special rules, or the tax laws of any country other than Belgium. This summary does not describe the tax treatment of investors that are subject to special rules, such as banks, insurance companies, collective investment undertakings, dealers in securities or currencies, persons that hold, or will hold, ADSs in a position in a straddle, share-repurchase transaction, conversion transactions, synthetic security or other integrated financial transactions. Investors should consult their own advisers regarding the tax consequences of an investment in ADSs in the light of their particular circumstances, including the effect of any state, local or other national laws, double tax treaties and regulatory interpretation thereof.
In addition to the assumptions mentioned above, it is also assumed in this discussion that for purposes of the Belgian tax legislation, the owners of ADSs will be treated as the owners of the ordinary shares represented by such ADSs. However, the assumption has not been confirmed by or verified with the Belgian Tax Administration.
For the purposes of this summary, ADSs or ordinary shares means ordinary shares represented by ADSs. Both terms are used interchangeably.
Belgian Dividend Withholding Tax
As a general rule, a Belgian dividend withholding tax of 30% is levied on the gross amount of dividends paid on or attributed to the ordinary shares represented by the ADSs, subject to such relief as may be available under applicable domestic or double tax treaty provisions. Dividends subject to the dividend withholding tax include all benefits attributed to the ordinary shares represented by the ADSs, irrespective of their form. A reimbursement of paid-up capital made in accordance with the Belgian Code of Companies and Associations is in principle partly considered to be a dividend distribution from a Belgian tax perspective stemming from the existing taxed reserves (irrespective whether incorporated into the capital or not) and/or the tax-free reserves incorporated into the capital. The proportion of the deemed dividend distribution for tax purposes is determined on the basis of the ratio between (A) the sum of (i) certain taxed reserves and (ii) tax-free reserves incorporated into the capital on the one hand and (B) the aggregate of such reserves and the fiscal paid-up capital on the other hand. In principle, fiscal paid-up capital includes paid-up statutory share capital, and subject to certain conditions, the paid-up issue premiums and the cash amounts subscribed to at the time of the issue of profit sharing certificates.
In case of a redemption by us of own shares represented by ADSs, the redemption distribution (after deduction of the portion of fiscal paid-up capital represented by the redeemed shares) will be treated as a dividend which in certain circumstances may be subject to a Belgian dividend withholding tax of 30%, subject to such relief as may be available under applicable domestic or double tax treaty provisions. In case of a liquidation of our Company, any amounts distributed in excess of the fiscal paid-up capital will be subject to a 30% dividend withholding tax, subject to such relief as may be available under applicable domestic or double tax treaty provisions.
For non-residents, the Belgian dividend withholding tax will be the only tax on dividends in Belgium, unless the non-resident holds ADSs in connection with a business conducted in Belgium, through a fixed base in Belgium or a Belgian permanent establishment.
91
Relief of Belgian Dividend Withholding Tax
Under the Belgium-United States Double Tax Treaty (the “Treaty”), there is a reduced Belgian dividend withholding tax rate of 15% on dividends paid by us to a U.S. resident which beneficially owns the dividends and is entitled to claim the benefits of the Treaty under the limitation of benefits article included in the Treaty, (a “Qualifying Holder”). If such Qualifying Holder is a company that owns directly at least 10% of our voting stock, the Belgian dividend withholding tax rate is further reduced to 5%. No Belgian dividend withholding tax is however applicable if the Qualifying Holder, is: (i) a company that is a resident of the United States that has owned directly ADSs representing at least 10% of our capital for a 12-month period ending on the date the dividend is declared, or (ii) a pension fund that is a resident of the United States, provided that such dividends are not derived from the carrying on of a business by the pension fund or through an associated enterprise.
Under the normal procedure, we or our paying agent must withhold the full Belgian withholding tax, i.e. 30% (without taking into account the Treaty rate). Qualifying Holders may make a claim for reimbursement for amounts withheld in excess of the rate defined by the Double Tax Treaty. The reimbursement form (Form 276 Div-Aut.) may be obtained from the Centre Etrangers, Team 6, Kruidtuinlaan 50, PO 3429, 1000 Brussels, Belgium or online on the website of the Belgian tax authorities. Qualifying Holders may also, subject to certain conditions, obtain the reduced Treaty rate at source. Qualifying Holders should deliver a duly completed Form 276 Div-Aut. no later than ten days after the date on which the dividend is attributed. U.S. holders should consult their own tax advisors in Belgium as to whether they qualify for reduction in Belgian withholding tax upon payment or attribution of dividends, and as to the procedural requirements for obtaining a reduced Belgian withholding tax upon the payment of dividends or for making claims for reimbursement.
Withholding tax is also not applicable, pursuant to Belgian tax law, on dividends paid to certain U.S. pension funds provided that the U.S. pension fund (i) qualifies as a non-resident saver for Belgian withholding tax purposes (i.e., it has a separate legal personality and fiscal residence outside of Belgium and without a permanent establishment or fixed base in Belgium), (ii) has a corporate purpose that consists solely in managing and investing funds collected in order to pay legal or complementary pensions, (iii) has activity that is limited to the investment of funds collected in the exercise of its statutory purpose, without any profit making activity and (iv) is exempt from income taxes in the United States. Furthermore, such pension fund may not contractually be obligated to redistribute the dividends to any beneficial owner of such dividends for whom it would manage the ADSs nor obligated to pay a manufactured dividend with respect to the ADSs under a securities borrowing transaction (save in certain particular cases as described in Belgian law) and subject to certain procedural formalities.
Under Belgian domestic tax law, a dividend withholding tax exemption is available to dividends paid to a non-resident corporate shareholder (located in a Member State of the European Union or in a country with which Belgium has entered in a double tax treaty including sufficient information exchange provisions) provided that (i) at the date of payment or attribution of the dividend it holds a participation in our company representing at least 10% of our share capital, (ii) this holding is held or will be held for an uninterrupted period of at least one year, (iii) this non-resident corporate shareholder is tax resident of the country where it is established according to the tax laws of and the bilateral tax treaties established by such country, (iv) this non-resident corporate shareholder is subject to a corporate income tax regime similar to Belgian corporate income tax regime without benefitting from a tax regime that derogates from the ordinary tax regime and (v) its legal form is (similar to one of the legal forms) listed in the annex of the E.U. directive dated 23 July 1990 (90/435/EC) as amended by the directive of 22 December 2003 (2003/123/EC). This reduced withholding tax will apply provided that certain procedural formalities are complied with.
Finally, a dividend withholding tax exemption is available, pursuant to Belgian tax law, to dividends paid to a non-resident corporate shareholder (located in the European Economic Area or in a country with which Belgium has entered in a double tax treaty including sufficient information exchange provisions) to the extent that at the date of payment or attribution of the dividend it holds a participation in our company representing less than 10% of our share capital but the acquisition value of which is at least €2.5 million and provided that certain other conditions are met, i.e., that (i) this holding is held or will be held in full ownership for an uninterrupted period of at least one year (ii) this non-resident corporate shareholder is subject to a corporate income tax regime similar to Belgian corporate income tax regime without benefitting from a tax regime that derogates from the ordinary tax regime, and (iii) its legal form is (similar to one of the legal forms) listed in the annex I, part A, of the E.U. directive dated 30 November 2011 (2011/96/EU) as amended by the directive of 8 July 2014 (2014/86/EU). This reduced withholding tax will apply only if and to the extent that the ordinary Belgian withholding tax cannot be credited or reimbursed to the non-resident corporate shareholder referred to above and subject to certain procedural formalities.
92
Capital Gains and Losses
Pursuant to the Belgium-US Double Tax Treaty, capital gains and/or losses realized by a Qualifying Holder from the sale, exchange or other disposition of ADSs do not fall within the scope of application of Belgian tax law.
Capital gains realized on ADSs by a corporate Holder which is not entitled to claim the benefits of the Treaty under the limitation of benefits article included in the Treaty are generally not subject to taxation in Belgium unless the corporate Holder is acting through a Belgian permanent establishment or a fixed place in Belgium to which the ADSs are effectively connected. Capital losses are not deductible.
Private individual Holders who are not entitled to claim the benefits of the Treaty under the limitation of benefits article included in the Treaty and which are holding ADSs as a private investment will, as a rule, not be subject to tax on any capital gains arising out of a disposal of ADSs. Losses will, as a rule, not be deductible in Belgium.
However, if the gain realized by such individual Holders on ADSs is deemed to be realized outside the scope of the normal management of such individual’s private wealth and the capital gain is obtained or received in Belgium, the gain will in principle be taxable at 33% in Belgium if and to the extent that such private individual is actually subject to Belgian non-resident personal tax based on Belgian domestic tax law. The Official Commentary to the Belgian Income Tax Code 1992 stipulates that occasional transactions on a stock exchange regarding ADSs should not be considered as transactions realized outside the scope of normal management of one’s own private wealth.
Capital gains realized by such individual Holders on the disposal of ADSs for consideration, outside the exercise of a professional activity, to a non-resident company (or a body constituted in a similar legal form), to a foreign state (or one of its political subdivisions or local authorities) or to a non-resident legal entity who is established outside the European Economic Area, are in principle taxable at a rate of 16.5% in Belgium if, at any time during the five years preceding the sale, such individual Holders has owned directly or indirectly, alone or with his/her spouse or with certain relatives, a substantial shareholding in us (that is, a shareholding of more than 25% of our shares).
Capital gains realized by a Holder upon the redemption of ADSs or upon our liquidation will generally be taxable as a dividend. See section “Belgian Dividend Withholding Tax.”
Belgian Estate and Gift Tax
There is no Belgian estate tax on the transfer of ADSs upon the death of a Belgian non-resident.
Donations of ADSs made in Belgium may or may not be subject to gift tax in Belgium depending on the modalities under which the donation is carried out.
Belgian Tax on Stock Exchange Transactions
A tax on stock exchange transactions (“taxe sur les opérations de bourse” in French / “taks op de beursverrichtingen” in Dutch) is generally levied on the purchase and the sale and on any other acquisition and transfer for consideration of existing ADSs on the secondary market carried out by a Belgian resident investor through a professional intermediary if (i) executed in Belgium through a professional intermediary, or (ii) deemed to be executed in Belgium, which is the case if the order is directly or indirectly made to a professional intermediary established outside of Belgium, either by private individuals having their usual residence in Belgium, or legal entities for the account of their seat or establishment in Belgium.
The applicable rate for ordinary shares in principle amounts to 0.35% of the consideration paid but with a cap of € 1,600 per transaction and per party. The tax is due separately from each party to any such transaction, i.e., the seller (transferor) and the purchaser (transferee), both collected by the professional intermediary.
However, if the intermediary is established outside of Belgium, the tax will in principle be due by the ordering private individual or legal entity, unless that individual or entity can demonstrate that the tax has already been paid. Professional intermediaries established outside of Belgium can, subject to certain conditions and formalities, appoint a Belgian representative for tax purposes, which will be liable for the tax on stock exchange transactions in respect of the transactions executed through the professional intermediary.
93
Belgian non-residents who purchase or otherwise acquire or transfer, for consideration, ADSs in Belgium for their own account through a professional intermediary may be exempt from the tax on stock exchange transactions if they deliver a sworn affidavit to the intermediary in Belgium confirming their non-resident status.
No stock exchange tax, nor tax on repurchase transactions is payable by: (i) professional intermediaries described in Article 2, 9° and 10° of the Law of August 2, 2002 acting for their own account, (ii) insurance companies described in Article 2, §1 of the Law of 9 July 1975 acting for their own account, (iii) professional retirement institutions referred to in Article 2, 1° of the Law of October 27, 2006 relating to the control of professional retirement institutions acting for their own account, (iv) collective investment institutions acting for their own account, or (v) regulated real estate companies (for the stock exchange tax only).
No stock exchange tax, nor tax on repurchase transactions will thus be due by Holders on the subscription, purchase or sale of ADSs, if the Holders are acting for their own account. In order to benefit from this exemption, the Holders must file with the professional intermediary in Belgium a sworn affidavit evidencing that they are non-residents for Belgian tax purposes.
Belgian Annual Tax on Securities Accounts
Pursuant to the Law of February 17, 2021 introducing a new annual tax on securities accounts due on securities accounts held through an intermediary if the average value of the taxable financial instruments held on this securities account exceeds €1 million during a reference period of 12 consecutive months. A new annual tax on securities accounts has been introduced because the previous tax on securities accounts was annulled by the Belgian Constitutional Court.
The annual tax on securities accounts is due irrespective of whether the holder of a securities account is a physical person or a legal entity. If the holder of a securities account is a Belgian resident, the annual tax on securities accounts will be applicable both to securities accounts held in Belgium as well as securities accounts held abroad. For non-residents, only securities accounts held in Belgium fall in scope of the annual tax on securities accounts. A double tax treaty could prevent Belgium to levy the annual tax on securities accounts.
Certain exemptions exist to mitigate the impact of the annual tax on securities accounts on the financial sector. As such, securities accounts held by certain financial undertakings are exempt.
All securities held on a securities account are targeted, such as shares, bonds, participations in investment funds and investment companies, but also derived products, such as index trackers, turbo’s, real estate certificates and cash. The rate of the annual tax on securities accounts amounts to 0.15% on securities accounts of which the average value exceeds €1 million during a reference period of 12 consecutive months. In order to avoid that the payment of the tax would result in a decrease of the average value below the €1 million threshold, the rate is limited to 10% of the difference between the taxable base and €1 million in those cases. The reference period is a subsequent period of 12 months starting on October 1 and ending September 30 of the subsequent year or (i) any earlier date when the account is closed; (ii) the moment when the account holder becomes a resident of a state with which Belgium has concluded a tax treaty and the tax treaty allocates the taxing rights to the other state, etc. The average value is calculated by taking the average of the securities accounts values on December 31, March 31, June 30 and September 30.
The tax must be declared and paid by the Belgian resident intermediary with whom the securities account is held. If a securities account is held with a non-resident intermediary, the holder of the securities account itself is responsible for the declaration and the payment of the annual tax on securities accounts. Alternatively, the foreign intermediary could also voluntarily appoint a recognized responsible representative in Belgium to declare and pay the tax.
In case of non-declaration, late, inaccurate or incomplete declaration, as well as non-payment or late payment, a penalty varying from 10% to 200% of the tax due can be imposed. Every holder of the securities account is jointly and severally liable to pay these penalties. The Law furthermore includes a general anti-abuse provision pursuant to which a rebuttable presumption of tax abuse applies in the following situations (non-exhaustive list): (i) distributing taxable financial instruments over different securities accounts to avoid the threshold of €1 million for an individual account, (ii) converting taxable financial instruments into nominative securities (the latter are out of scope of the tax); and (iii) transferring a securities account to a foreign legal entity which then transfers the securities to a foreign securities account, etc.
Prospective Holders should consult their own tax advisors as to whether they are subject to the new annual tax on securities accounts.
94
Proposed EU Financial Transactions Tax
On February 14, 2013, the European Commission published a proposal for a Directive for a common financial transactions tax (“FTT”) in Belgium, Germany, Greece, Spain, France, Italy, Austria, Portugal, Slovenia, Estonia and Slovakia (collectively, the “Participating Member States”).
The proposed FTT has a very broad scope and could, if introduced in its current form, apply to certain dealings in ADSs in certain circumstances. The FTT could apply in certain circumstances to persons both within and outside of the Participating Member States. Generally, it would apply to certain dealings in ADSs where at least one party is a financial institution, and at least one party is established in a Participating Member State.
A financial institution may be, or be deemed to be, “established” in a Participating Member State in a broad range of circumstances, including by transacting with a person established in a Participating Member State.
Currently, the proposed FTT remains subject to further negotiations between the Participating Member States. It may therefore be adjusted prior to any implementation, of which the timing and fate remains unclear. Moreover, additional E.U. Member States could decide to participate or drop out of the negotiations. Prospective Holders of ADSs are advised to seek their own professional advice in relation to the FTT. In June 2023, the European Commission stated that “the prospects of reaching an agreement on the FTT in the future are limited given that the last substantial discussions took place under the Portuguese Council Presidency in 2021” adding there was “little expectation that any proposal would be agreed in the short term.”
U.S. Taxation
The following is a discussion of the material U.S. federal income tax considerations to U.S. holders (as defined below) of acquiring, holding and disposing of the ADSs. The following discussion applies only to U.S. holders that purchase ADSs, will hold ADSs as capital assets for U.S. federal income tax purposes (generally, assets held for investment) and that are not residents of, or ordinarily resident in, Belgium for tax purposes nor hold their ADSs as part of a permanent establishment in Belgium. The discussion also does not address any aspect of U.S. federal taxation other than U.S. federal income taxation. In particular, this summary does not address all tax considerations applicable to investors that own (directly or by attribution) 10% or more of our stock by vote or value, nor does this summary discuss all of the tax considerations that may be relevant to certain types of investors subject to special treatment under the U.S. federal income tax laws (such as financial institutions, insurance companies, real estate investment trusts, regulated investment companies, investors liable for the alternative minimum tax, certain U.S. expatriates, individual retirement accounts and other tax-deferred accounts, partnerships or other pass-through entities for U.S. federal income tax purposes, tax-exempt organizations, dealers in securities or currencies, securities traders that elect mark-to-market tax accounting, investors that will hold the ADSs as part of constructive sales, straddles, hedging, integrated or conversion transactions for U.S. federal income tax purposes or investors whose “functional currency” is not the U.S. dollar). Further, this discussion is limited to U.S. holders that hold our ADSs or ordinary shares as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment) at all relevant times and does not address all U.S. federal income tax consequences relevant to a U.S. holder’s particular circumstances, including the impact of the Medicare tax on net investment income.
The following summary is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), U.S. Treasury Regulations thereunder, published rulings of the U.S. Internal Revenue Service (the “IRS”), the Treaty, and judicial and administrative interpretations thereof, in each case as available on the date of this prospectus supplement. Changes to any of the foregoing, or changes in how any of these authorities are interpreted, may affect the tax consequences set out below, possibly retroactively. No ruling will be sought from the IRS with respect to any statement or conclusion in this discussion, and there can be no assurance that the IRS will not challenge such statement or conclusion in the following discussion or, if challenged, a court will uphold such statement or conclusion.
For purposes of the following summary, a “U.S. holder” is a beneficial owner of ADSs that is for U.S. federal income tax purposes: (i) a citizen or individual resident of the United States, (ii) a corporation or other entity treated as a corporation for U.S. federal income tax purposes created or organized in or under the laws of the United States or any state thereof (including the District of Columbia), (iii) an estate, the income of which is subject to U.S. federal income taxation regardless of its source or (iv) a trust if (x) a court within the United States is able to exercise primary supervision over its administration and (y) one or more United States persons (as defined in the Code) have the authority to control all of the substantial decisions of such trust.
95
If a partnership (including any entity treated as a partnership for U.S. federal income tax purposes) holds ADSs, the U.S. federal income tax consequences to the partners of such partnership will depend on the activities of the partnership and the status of the partners. A partnership considering an investment in ADSs, and partners in such partnership, should consult their own tax advisers about the consequences of the investment.
We do not expect to be a PFIC, and the discussion under “—Distributions by Us” and “—Proceeds from the Sale, Exchange or Retirement of the ADSs” below assumes we will not be a PFIC. See “—Passive Foreign Investment Company” discussion below.
Prospective purchasers of ADSs should consult their own tax advisers with respect to the U.S. federal, state, local and non-U.S. tax consequences to them in their particular circumstances of acquiring, holding, and disposing of, ADSs.
Ownership of ADSs in General
The discussion below is based, in part, on representations by the Depositary and assumes that each obligation under the deposit agreement and any related agreement will be performed in accordance with its terms.
For U.S. federal income tax purposes, an owner of ADSs generally will be treated as the owner of the ordinary shares represented by such ADSs. However, the U.S. Treasury has expressed concerns that parties to whom interests such as the ADSs are delivered in transactions similar to pre-release transactions may be taking actions that are inconsistent with the claiming of foreign tax credits for U.S. holders of ADSs. Accordingly, the analysis of the creditability of Belgian taxes could be affected by actions taken by parties to whom the ADSs are pre-released. No gain or loss will be recognized if you exchange ADSs for the ordinary shares represented by those ADSs. Your tax basis in such ordinary shares will be the same as your tax basis in such ADSs, and the holding period in such ordinary shares will include the holding period in such ADSs.
Distributions by Us
Subject to the application of the PFIC rules discussed below, the U.S. dollar value of distributions paid by us (including the amount of any taxes withheld) out of its earnings and profits, as determined under U.S. federal income tax principles, will be subject to tax as foreign source ordinary dividend income and will be includible in your gross income upon receipt by the Depositary. However, we do not maintain calculations of its earnings and profits in accordance with U.S. federal income tax accounting principles. U.S. holders should therefore assume that any distribution by us with respect to ordinary shares or ADSs will constitute ordinary dividend income. Subject to applicable limitations, so long as the ADSs are regularly traded on the Nasdaq Global Select Market, we expect that dividends paid by us will be classified as “qualified dividend income” generally subject to tax at lower rates than other items of ordinary income when received by individuals and other non-corporate U.S. holders. Any dividends we pay with respect to the ADSs or ordinary shares will constitute foreign source income for foreign tax credit purposes.
The U.S. dollar value of distributions paid by us will be calculated by reference to the exchange rate in effect on the date the dividend distribution is received by the Depositary, regardless of when the Depositary converts the payments into U.S. dollars. If the foreign currency is converted by the Depositary on a later date, a U.S. holder will be required to recognize foreign currency gain or loss in respect of the foreign currency based on the difference between the rate at which it is converted and the rate on the date the dividend was received by the Depositary.
Subject to certain limitations, Belgian withholding tax, if any, paid in connection with any distribution with respect to ordinary shares or ADSs may be claimed as a credit against your U.S. federal income tax liability if you elect not to take a deduction for any non-U.S. income taxes for that taxable year otherwise, such Belgian withholding tax may be taken as a deduction. If you are eligible for benefits under the Treaty or are otherwise entitled to a refund for the taxes withheld, you will not be entitled to a foreign tax credit or deduction for the amount of any Belgian taxes withheld in excess of the maximum rate under the Treaty or for the taxes with respect to which you can obtain a refund from the Belgian taxing authorities. As the relevant rules are very complex, you should consult your own tax advisor concerning the availability and utilization of the foreign tax credit or deductions for non-U.S. taxes in your particular circumstances.
96
Proceeds from the Sale, Exchange or Retirement of the ADSs
Upon the sale, exchange or retirement of ADSs, a U.S. holder will generally recognize U.S. source capital gain or loss equal to the difference, if any, between the U.S. dollar amount realized on the sale, exchange or retirement and the U.S. holder’s tax basis in the ADSs (generally their cost in U.S. dollars). Any gain or loss generally will be long-term capital gain or loss if the ADSs have been held for more than a year. If you are a non-corporate U.S. holder, including an individual U.S. holder, you may be eligible for reduced U.S. federal income tax rates for long-term capital gains. The deductibility of capital losses is subject to limitations.
Gain or loss you recognize on the sale, exchange or retirement of ADSs will generally be treated as U.S. source income or loss for foreign tax credit purposes.
Passive Foreign Investment Company
We believe that we were not a PFIC for the tax year ended December 31, 2023, and we do not expect to be classified as a PFIC for U.S. federal income tax purposes for the current tax year ending December 31, 2024, or for the foreseeable future. However, PFIC status is a factual determination for each taxable year that cannot be made until after the close of each such year and will depend to a large degree on the market price of our ADSs, which could fluctuate significantly. Therefore, we cannot assure you that we will not be considered a PFIC for the taxable year ended December 31, 2023 or in any subsequent taxable year. If we are a PFIC at any time during the holding period of a U.S. holder, the U.S. holder would be subject to potentially materially greater amounts of tax and subject to additional U.S. tax form filing requirements. In addition, a non-corporate U.S. holder will not be eligible for qualified dividend income treatment on dividends received from us if we are treated as a PFIC for the taxable year in which the dividends are received or for the preceding taxable year.
A non-U.S. corporation is a PFIC in any taxable year in which, after taking into account certain look-through rules, either (i) at least 75% of its gross income is passive income or (ii) at least 50% of the average value (determined on a quarterly basis) of its assets is attributable to assets that produce or are held to produce passive income. Passive income generally includes dividends, interest, rents, royalties, gross income from certain commodities transactions, and capital gains. If a non-U.S. corporation owns at least 25% by value of the stock of another corporation, the foreign corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation, and as receiving directly its proportionate share of the other corporation’s income. The same general look-through rule applies when a foreign corporation owns at least 25% by value of the partnership (a look-through partnership) - the foreign corporation is treated as owning its share of the partnership’s assets and deriving its share of the partnership’s income, characterized as passive or active at the partnership level. In the case the foreign corporation satisfies an “active partner” test, the foreign corporation may treat less-than-25% owned partnerships as look-through partnerships, unless the foreign corporation elects otherwise. Although the determination of whether a non-U.S. corporation is a PFIC for a given taxable year is based on its income and assets for that taxable year, as determined under the PFIC rules, once a non-U.S. corporation is a PFIC for any taxable year, it generally remains a PFIC for any investors that owned interests in all or a portion of such taxable year even if it would not otherwise qualify as a PFIC in later taxable years. We do not undertake to monitor our PFIC status on an ongoing basis.
The Code imposes additional taxes on gains from the sale or other disposition of, and “excess distributions” with respect to, shares of a PFIC owned directly (or deemed to be owned directly or indirectly under certain attribution rules) by a U.S. holder. In general, an excess distribution is any distribution to the U.S. holder that is greater than 125% of the average annual distributions received by the U.S. holder (including return of capital distributions) during the three preceding taxable years or, if shorter, the U.S. holder’s holding period for the ADSs. If we were a PFIC in any year in which a U.S. holder held the ADSs (i) the gain or excess distribution would be allocated ratably over the U.S. holder’s holding period for the ADSs, (ii) the amount allocated to the taxable year in which the gain or excess distribution was realized and to any year before we became a PFIC would be taxable as ordinary income, (iii) the amount allocated to each other prior year would be subject to tax at the highest rate in effect for that year and (iv) the interest charge generally applicable to underpayments of tax would be imposed in respect of the tax allocated to each such year. For these purposes, a U.S. holder who uses the ADSs as collateral for a loan would be treated as having disposed of such ADSs.
97
The PFIC rules provide for certain elections that can, in certain circumstances, alter the tax consequences of PFIC status as generally described above, thereby mitigating the adverse tax consequences that generally apply under the PFIC rules as described above. One such election, the “qualified electing fund” or “QEF” election, allows a U.S. holder to include in income its share of the corporation’s income on a current basis and it requires (among other things) that the U.S. holder include with its U.S. federal income tax return a “PFIC Annual Information Statement” provided by the foreign corporation and disclosing to the U.S. Holder its pro rata share of the corporation’s “ordinary earnings” and “net capital gain” as determined under U.S. federal income tax principles. A QEF election also can, in certain circumstances, cause the “excess distribution” regime described above not to apply, generally resulting in more favorable tax consequences upon receipt of PFIC excess distributions or the recognition of gain on sale of PFIC shares (or ADSs). However, we do not intend to calculate our “ordinary earnings” or “net capital gain,” nor do we intend to supply U.S. holders with the required “PFIC Annual Information Statement.” Therefore, it generally will not be possible for you to make a QEF election if we are, or if we become, a PFIC.
A different election, the “mark-to-market” election could be available if our ADSs or ordinary shares, as applicable, are considered “marketable stock” as defined under applicable U.S. Treasury Regulations. This election can be made if the ADSs are considered to be “marketable securities” for purposes of the PFIC rules. The ADSs should be marketable securities for these purposes to the extent they are “regularly traded” on the Nasdaq Global Select Market. Generally, shares are treated as “regularly traded” in any calendar year in which more than a de minimis quantity of the shares are traded on a qualified exchange on at least 15 days during each calendar quarter. Subject to certain limitations, a U.S. holder that makes a valid mark-to-market election with respect to the ADSs would be required to take into account the difference, if any, between the fair market value at the end of each taxable year and the fair market value at the end of the preceding taxable year (or the acquisition price in the first year the election is in effect) of those ADSs, as ordinary income or ordinary loss (but only to the extent of the net amount previously included as income by the U.S. holder as a result of the mark-to-market election). A U.S. holder’s basis in the ADSs will be increased by the amount of any ordinary income inclusion and decreased by the amount of any ordinary loss taken into account under the mark-to-market rules. Gains from an actual sale or other disposition of the ADSs for which this election has been properly made would be treated as ordinary income, any losses incurred on a sale or other disposition of the ADSs would be treated as an ordinary loss to the extent of any net mark-to-market gains for prior years and any additional loss would be capital loss.
Even if a valid mark-to-market election is made with respect to the ADSs, there is a significant risk that indirect interests in any of our subsidiaries that are PFICs will not be covered by this election but will be subject to the excess distribution rules described above. Under these rules, distribution from, and dispositions of interests in, these subsidiaries, as well as certain other transactions, generally will be treated as a distribution or disposition subject to the discussion above regarding excess distributions.
Prospective U.S. holders are urged to consult their own tax advisers about the consequences of holding the ADSs if we are considered a PFIC in any taxable year, including the availability of the mark-to-market election, and whether making the election would be advisable in their particular circumstances. In particular, U.S. holders should consider carefully the impact of a mark-to-market election with respect to their ADSs given that there is a significant risk that we will have subsidiaries that are classified as PFICs.
Medicare Tax
Certain U.S. holders who are individuals, estates and trusts will be required to pay an additional 3.8% tax on some or all their “net investment income,” which generally includes its dividend income and net gains from the disposition of the ADSs. U.S. holders should consult their own tax advisors regarding the applicability of this additional tax on their particular situation.
Information Reporting and Backup Withholding
Information returns may be filed with the IRS in connection with distributions on the ADSs and the proceeds from the sale or other disposition of the ADSs unless a U.S. holder establishes that it is exempt from the information reporting rules. A U.S. holder may be subject to backup withholding on these payments if it fails to provide its tax identification number to the paying agent and comply with certain certification procedures. The amount of any backup withholding from a payment to a U.S. holder will be allowed as a credit against its U.S. federal income tax liability and may entitle the U.S. holder to a refund, provided that the required information is timely furnished to the IRS.
98
Tax Return Disclosure Requirement
U.S. federal income tax law requires certain U.S. investors to disclose information relating to investments in securities of a non-U.S. issuer. Failure to comply with applicable disclosure requirements could result in the imposition of substantial penalties. U.S. holders should consult their own tax advisors regarding any disclosure obligations.
F.Dividends and Paying Agents
Not applicable.
G.Statement by Experts
Not applicable.
H.Documents on Display
We previously filed with the SEC our registration statement on Form F-1 (Registration No. 333-194982), as amended, and our registration statement on Form F-3 (Registration No. 333-258949), including the prospectuses contained therein, to register our ordinary shares. We have also filed with the SEC a related registration statement on F-6 (Registration No. 333-196734) to register the ADSs.
We are subject to the periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Our annual reports on Form 20-F are due within four months after each fiscal year end. We are not required to disclose certain other information that is required from U.S. domestic issuers. Also, as a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing of proxy statements to shareholders and our directors, senior management and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
Our SEC filings, including the registration statement, are available to you on the SEC’s website at http://www.sec.gov.
We have filed our restated articles of association and all other deeds that are to be published in the annexes to the Belgian State Gazette with the clerk’s office of the Commercial Court of Leuven (Belgium), where they are available to the public. A copy of our restated articles of association is also publicly available as an exhibit to this annual report, as well as on the website of the Royal Federation of Belgian Notaries (only in Dutch, French or German, https://statuten.notaris.be/costa_v1/enterprises/search). This website address is included in this annual report as an inactive textual reference only, and the information and other content appearing on this website are not incorporated by reference into this annual report. In accordance with Belgian law, we must prepare audited annual statutory and consolidated financial statements. The audited annual statutory and consolidated financial statements and the reports of our board and statutory auditor relating thereto are filed with the Belgian National Bank, where they are available to the public.
I.Subsidiary Information
Not applicable.
ITEM 11.QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risk from fluctuations in interest rates and foreign currency exchange rates which may adversely affect our results of operations and financial condition. We seek to minimize these risks through regular operating and financing activities.
Interest Rate Risk
Although we mainly have loans outstanding with a fixed interest rate, some of the loans have been contracted with variable interest rates. The most significant loans with variable interest rates have been secured by means of a variable to fixed interest rate swap. We therefore believe that we are not materially affected by changes in interest rates. For information with respect to the interest rate swaps, see Note 20 to our audited consolidated financial statements.
99
Foreign Exchange Rate Risk
We transact business globally and are subject to risks associated with fluctuating foreign exchange rates. The geographic areas outside of the Eurozone to which we sell our products and services are generally not considered to be subject to a substantially higher inflation than in the Eurozone. In the years ended December 31, 2023, 2022 and 2021, 34%, 39%, and 35% of our revenue, respectively, were derived from sales in a currency different from the euro. Receivables denominated in a foreign currency are initially recorded at the exchange rate at the transaction date and subsequently re-measured in euro based on period-end exchange rates. Transaction gains and losses that arise from exchange rate fluctuations are charged to income. We primarily have exposure to the U.S. dollar, British pound, Japanese yen and Brazilian real as foreign currency.
If the U.S. dollar (rate for €1) would have appreciated by 10%, the operating result would have been € 0.9 million higher, excluding the effect of the cash and term accounts held in U.S. dollars. If the U.S. dollar (rate for €1) would have depreciated by 10%, the net result would have been € 0.8 million lower, excluding the effect of the cash and term accounts held in U.S. dollars.
To limit the exposure to foreign currency rate fluctuations on the U.S. dollar, we have entered into currency rate swaps. As of December 31, 2023, we had hedge agreements in place for $ 11.2 million, all maturing before year-end 2024. Refer to note 20 to our consolidated financial statements for the related fair value of these derivatives.
Additionally, we are exposed to credit risk, liquidity risk and challenges related to capital management.
Inflation Risk
We transact business globally and are subject to risks associated with fluctuating inflation. The risk exists that, if inflation increases our costs of remuneration, materials, services, energy, and capital expenditures, we may not be able to offset such costs fully by increasing our selling prices. As such, in a high inflationary environment, our results of operations and financial condition may be adversely affected.
Credit Risk
Credit risk is the risk that third parties may not meet their contractual obligations resulting in a loss for us. We are exposed to credit risk from our operating activities and from our financing activities, which are mainly deposits with financial institutions. We limit this exposure by contracting with credit-worthy business partners or with financial institutions which meet high credit rating requirements. In addition, the portfolio of receivables is monitored on a continuous basis.
Customer credit risk is managed by each business unit subject to our established policy, procedures and controls relating to customer credit risk management. An impairment analysis is performed at each reporting date using a provision matrix to measure ECLs. The provision rates are based on days past due for groupings of various customer segments with similar loss patterns (i.e., by legal entity). The calculation reflects the probability-weighted outcome, the time value of money and reasonable and supportable information that is available at the reporting date about past events, current conditions and forecasts of future economic conditions. Generally, trade receivables are written-off if past due for more than one year and are not subject to enforcement activity. The maximum exposure to credit risk at the reporting date is the carrying value of each class of financial assets at amortized cost or fair value, as disclosed in Note 20 to our consolidated financial statements. We do not hold collateral as security.
We evaluate the concentration of risk with respect to trade receivables as low, as our customers are located in several jurisdictions and industries and operate in largely independent markets.
Liquidity Risk
The liquidity risk is that we may not have sufficient cash to meet our payment obligations. This risk is countered by day-by-day liquidity management at corporate level. We have historically entered into financing and lease agreements with financial institutions to finance significant projects and certain working capital requirements. At December 31, 2023, we had cash and cash equivalents of € 127.6 million, while € 25.5 million of our € 64.4 million gross debt was short term. At December 31, 2023, we had an undrawn line of credit of € 50 million, as more fully described in Note 15 to our consolidated financial statements.
100
Capital Management
The primary objective of our capital management strategy is to ensure we maintain healthy capital ratios to support our business and maximize shareholder value. Capital is defined as our shareholders’ equity.
We consistently review our capital structure and make adjustments in light of changing economic conditions. We made no changes to our capital management objectives, policies or processes during the years ended December 31, 2023, 2022 and 2021.
ITEM 12.DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A.Debt Securities
Not applicable.
B. | Warrants and Rights |
Not applicable.
C.Other Securities
Not applicable.
D.American Depositary Shares
Bank of New York Mellon serves as the depositary for the ADSs. Each ADS represents one ordinary share (or a right to receive one ordinary share) deposited with the principal Amsterdam office of ING Securities Services, Inc., as custodian for the depositary. Each ADS also represents any other securities, cash or other property which may be held by the depositary. The depositary’s corporate trust office at which the ADSs are administered is located at 240 Greenwich Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at 240 Greenwich Street, New York, New York 10286.
A deposit agreement among us, the depositary and the ADS holders sets out the ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. A copy of the deposit agreement is incorporated by reference as an exhibit to this annual report.
101
Pursuant to the terms of the deposit agreement, you, as an ADS holder, will be required to pay the following fees to the depositary:
Persons depositing or withdrawing ordinary shares or ADS holders must pay to the depositary: |
| For: |
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
| Issuance of ADSs, including issuances resulting from a distribution of ordinary shares or rights or other property |
|
| |
|
| Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
|
| |
$0.05 (or less) per ADS |
| Any cash distribution to you |
|
| |
A fee equivalent to the fee that would be payable if securities distributed to you had been ordinary shares and the shares had been deposited for issuance of ADSs |
| Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to you |
|
| |
$0.05 (or less) per ADS per calendar year |
| Depositary services |
|
| |
Registration or transfer fees |
| Transfer and registration of ordinary shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
|
| |
Expenses of the depositary |
| Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) converting foreign currency to U.S. dollars |
|
| |
Taxes and other governmental charges the depositary or the custodian has to pay on any ADS or ordinary shares underlying an ADS, such as share transfer taxes, stamp duty or withholding taxes |
| As necessary |
|
| |
Any charges incurred by the depositary or its agents for servicing the deposited securities |
| As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing ordinary shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-based services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse or share revenue from the fees collected from ADS holders, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS program. In performing its duties under the deposit agreement, the depositary may use brokers, dealers or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
102
PART II
ITEM 13.DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14.MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Material Modifications to the Rights of Security Holders
None.
Use of Proceeds
Not applicable.
ITEM 15.CONTROLS AND PROCEDURES
a) | Disclosure Controls and Procedures |
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost benefit relationship of possible controls and procedures. Based on this evaluation, management concluded as of December 31, 2023, that our disclosure controls and procedures were effective.
b) | Management’s Annual Report on Internal Control over Financial Reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer and effected by our management and other personnel to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external reporting purposes in accordance with IFRS. Internal control over financial reporting includes policies and procedures that pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; provide reasonable assurance that transactions are recorded as necessary to permit preparation of our financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with the authorization of our board of directors and management; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with our policies and procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023 using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in the Internal Control-Integrated Framework, 2013 (the “COSO 2013 Framework”).
Based on its assessment, our management, including our Chief Executive Officer and our Chief Financial Officer, has concluded that our internal control over financial reporting was effective as of December 31, 2023.
103
c) | Attestation Report of the Registered Public Accounting Firm |
The effectiveness of internal control over financial reporting as of December 31, 2023 has been audited by KPMG Bedrijfsrevisoren BV / KPMG Réviseurs d’Entreprises SRL, our independent registered public accounting firm. Their audit report, including their opinion on management’s assessment of internal control over financial reporting, is included with our consolidated financial statements in this annual report.
d) | Changes in Internal Control over Financial Reporting |
There were no changes in our internal control over financial reporting that occurred during the period covered by this report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16A.AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors has determined that each of the members of our audit committee, Johan De Lille, Jürgen Ingels and Bart Luyten, is an “audit committee financial expert” as defined in Item 16A of Form 20-F under the Exchange Act and is independent under Rule 10A-3 under the Exchange Act.
ITEM 16B.CODE OF ETHICS
We have adopted a written code of conduct and ethics that outlines the principles of legal and ethical business conduct under which we do business. The code of conduct and ethics applies to all of our directors, senior management, consultants and other employees, including our Chief Executive Officer and Chief Financial Officer. We have posted this code of conduct and ethics on our website at www.materialise.com under the “Investors” section, “Governance – Documents”. This website address is included in this annual report as an inactive textual reference only, and the information and other content appearing on our website are not incorporated by reference into this annual report. We have not granted any waivers from any provision of our code of conduct and ethics since its adoption.
ITEM 16C.PRINCIPAL ACCOUNTANT FEES AND SERVICES
For the year ended December 31 | ||||
in 000€ |
| 2023 |
| 2022 |
Audit Fees | 1,162 | 1,284 | ||
Audit-Related Fees |
| 15 |
| 12 |
All Other Fees |
| — |
| — |
Total |
| 1,177 |
| 1,296 |
Audit Fees
Audit fees consist of the aggregate fees billed in connection with the audit of our annual consolidated and statutory financial statements and internal controls.
Audit-Related Fees
Audit-related fees are fees for services that are traditionally performed by the independent accountants and in the table above primarily related to the quarterly attestation reports for EIB.
All Other Fees
No non-audit related fees were paid to KPMG Bedrijfsrevisoren BV / KPMG Réviseurs d’Entreprises SRL or its affiliates for the fiscal years ended December 31, 2023 and 2022.
104
Audit Committee Pre-Approval Policies and Procedures
The pre-approval of the Audit Committee or member thereof, to whom pre-approval authority has been delegated, is required for the engagement of our independent auditors to render audit or non-audit services. Audit Committee pre-approval of audit and non-audit services will not be required if the engagement for the services is entered into pursuant to pre-approval policies and procedures established by our audit committee regarding our engagement of the independent auditors, provided the policies and procedures are detailed as to the particular service, our audit committee is informed of each service provided and such policies and procedures do not include delegation of the Audit Committee’s responsibilities under the Exchange Act to our management. Audit Committee pre-approval of non-audit services (other than review and attest services) also will not be required if such services fall within available exceptions established by the SEC.
All audit fees, audit related fees and tax fees for the fiscal years ended December 31, 2023 and 2022 were pre-approved under the pre-approval policies of the Audit Committee.
ITEM 16D.EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
None.
ITEM 16E.PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
None.
ITEM 16F.CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G.CORPORATE GOVERNANCE
The Listing Rules of the Nasdaq Stock Market include certain accommodations in the corporate governance requirements that allow foreign private issuers, such as us, to follow “home country” corporate governance practices in lieu of the otherwise applicable corporate governance standards of the Nasdaq Stock Market. The application of such exceptions requires that we disclose each noncompliance with the Nasdaq Stock Market Listing Rules and describe the Belgian corporate governance practices we do follow in lieu of the relevant Nasdaq Stock Market corporate governance standard. We follow Belgian corporate governance practices in lieu of the corporate governance requirements of the Nasdaq Stock Market in respect of the following:
| ● | Quorum at Shareholder Meetings. Nasdaq Stock Market Listing Rule 5620(c) requires that for any meeting of shareholders, the quorum must be no less than 33% or 1/3 of the outstanding ordinary shares. There is no quorum requirement under Belgian law for our shareholders’ meetings, except as provided for by law in relation to decisions regarding certain matters. |
| ● | Independent Director Majority on Board/Meetings. Nasdaq Stock Market Listing Rules 5605(b)(1) and (2) require that a majority of the board of directors must be comprised of independent directors and that independent directors must have regularly scheduled meetings at which only independent directors are present. We are not required under Belgian law to have any independent directors on our board of directors. However, our restated articles of association provide that our board of directors must be comprised of at least seven and no more than 11 directors, of which at least three directors must be independent directors under Belgian law. The Belgian law definition of independence differs from the definition of independence under the Nasdaq Stock Market Listing Rules.We do not intend to require our independent directors to meet separately from the full board of directors on a regular basis or at all although the board of directors is supportive of its independent members voluntarily arranging to meet separately from the other members of our board of directors. |
105
| ● | Director Nominations/Remuneration and Nomination Committee Composition. Nasdaq Stock Market Listing Rule 5605(d)(2) requires that compensation of officers must be determined by, or recommended to, the board of directors for determination, either by a majority of the independent directors, or a compensation committee comprised solely of independent directors. Nasdaq Stock Market Listing Rule 5605(e) requires that director nominees be selected, or recommended for selection, either by a majority of the independent directors or a nominations committee comprised solely of independent directors. Under Belgian law, we are not subject to any such requirements. In particular, we are not required by Belgian law to set up any compensation or nominations committees within our board of directors, and are therefore not subject to any Belgian legal requirements as to the composition of such committees either. However, our restated articles of association provide that our board of directors may form committees from among its members. See “Item 6. Directors, Senior Management and Employees—C. Board Practices —Board of Directors Practices.” Our board of directors has set up and appointed a Remuneration and Nomination Committee. Our Remuneration and Nomination Committee is currently comprised of three directors, one of whom is independent. In addition, as long as the Family Shareholders control, directly or indirectly, in the aggregate at least 20% of the voting rights attached to our ordinary shares, a majority of our directors must be appointed by our shareholders from a list of candidates proposed by the Family Shareholders. |
| ● | Shareholder Approval of Equity Compensation Plans. Nasdaq Stock Market Listing Rule 5635(c) requires shareholder approval prior to the issuance of securities in connection with equity-based compensation of officers, directors, employees or consultants. In lieu of the Nasdaq Stock Market Listing Rule 5635(c), we have historically followed Belgian law regarding the issuance of shares or securities in connection with the remuneration of the directors and/or the employees of a Belgian company. |
Under Belgian company law, a Belgian company may issue shares or grant rights to acquire shares pursuant to a resolution of the general meeting of shareholders or, within certain limits, pursuant to a resolution of the board of directors if so authorized by the shareholders’ meeting (the so-called authorized capital). By resolution of our extraordinary shareholders’ meeting of November 5, 2020, which entered into force on November 9, 2020, our shareholders authorized our board of directors, for a period of five years from November 9, 2020, to increase our share capital, in one or more transactions (including through the issuance of warrants), up to a maximum amount of € 4,067,700.72.
The board of directors is authorized to limit or cancel the preferential subscription right of current shareholders (for example, when it decides to issue warrants), if this is in the interest of our company. The board of directors can do this for the benefit of one or more specific persons, even if these persons are not personnel of our company or our subsidiaries.
Pursuant to this authorization, our board of directors may determine to adopt other equity-based compensation plans for our officers, directors, employees or consultants.
ITEM 16H.MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I.DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable
ITEM 16J.INSIDER TRADING POLICIES
Not applicable.
106
ITEM 16K.CYBERSECURITY
Our board of directors recognizes the critical importance of maintaining the trust and confidence of our customers, clients, shareholders, business partners, and employees. While everyone at Materialise plays a part in managing our cybersecurity risk management strategy, primary cybersecurity oversight responsibility is shared by our board of directors, and members of our senior management as part of our Executive Committee, as supported by our Governance Bureau. The Executive Committee is actively involved in oversight of the Materialise risk management program and supports our board of directors in the development and continual improvement of our information security management system. Our cybersecurity policies, standards, processes, and practices are fully integrated into our operational processes and are based on recognized frameworks established by the International Organization for Standardization (including ISO 27001 and 27701) and other applicable industry standards (including TISAX). We address cybersecurity risks through a comprehensive, cross-functional approach that is focused on safeguarding the confidentiality, integrity, and availability of our products and services, our customers’ and our own data, and our supporting IT infrastructure. We apply the principle of “defense in depth” and focus both on preventing the occurrence of cybersecurity incidents, and providing an effective response to cybersecurity incidents when they do occur.
Risk Management and Strategy
As one of the critical elements of our overall corporate risk management approach, the information security program is focused on the following key areas:
| ● | Governance: As discussed in more detail under the heading “Governance,” our board of directors’ oversight of cybersecurity risk management is supported by our Executive Committee which consists of eight members.The Executive Committee is also supported by our Governance Bureau, which consists of our Chief Executive Officer, Chief Operating Officer, and the Director of Internal Audit, and regularly invites the Chief Information Security Officer and other members executive management to closely follow up on open cybersecurity risks. |
| ● | Defense in depth: We have implemented a comprehensive, cross-functional approach to identifying, preventing, and mitigating cybersecurity threats and incidents, while also implementing controls and procedures that provide for the prompt escalation of certain cybersecurity incidents so that decisions regarding the public disclosure and reporting of such incidents can be made by management in a timely manner. |
| ● | Technical Safeguards: We deploy technical safeguards that are designed to protect our information systems and online environments from cybersecurity threats, including firewalls, intrusion prevention and detection systems, anti-malware functionality and access controls, which are evaluated and improved through vulnerability assessments and cybersecurity threat intelligence. |
| ● | Incident Response and Recovery Planning: We have established and we maintain incident response procedures and recovery plans to ensure our ability to timely respond to and recover from a cybersecurity incident. These procedures and plans are tested and evaluated on a regular basis. Cybersecurity incidents are assessed and handled according to risk-based priority levels. |
| ● | Third Party Risk Management: We maintain a comprehensive risk-based approach to identifying and overseeing cybersecurity risks presented by third parties, including vendors, service providers, and third-party systems that could adversely impact our business in the event of a cybersecurity incident affecting those third-party systems. |
| ● | Education and Awareness: Materialise provides continual training and awareness for personnel regarding cybersecurity threats to equip personnel with effective tools to address those threats, and to communicate evolving information security policies, standards, processes, and practices. |
107
We engage in the periodic assessment and testing of policies, implemented standards, processes, and practices that are designed to address cybersecurity threats and incidents. These efforts include a wide range of activities, including internal audits, assessments, vulnerability scanning, security testing and disaster recovery exercises focused on evaluating the effectiveness of our cybersecurity measures and planning. We regularly engage third parties to perform assessments on our cybersecurity measures, including phishing simulations, security penetration testing, and external compliance audits. The results of such assessments, audits, and reviews are handled according to our internal nonconformity and risk management processes and reported to the Executive Committee or Governance Bureau. We continually improve our cybersecurity policies, standards, processes, and practices as necessary based on the information provided by these assessments, audits, and reviews.
Governance
The Executive Committee, in coordination with the Governance Bureau, oversees the corporate management system, which includes information security management. Twice a year, the Executive Committee receives an update from the relevant members of senior management as part of the corporate management review, including recent developments of relevant cybersecurity risks, evolving standards, the threat environment, technological trends and information security considerations arising with respect to Materialise’s customers and peers. The Governance Bureau also receives prompt and timely information regarding any rapidly evolving cybersecurity risks or incidents that meet established reporting thresholds, as well as ongoing updates regarding any such topics until they have been addressed. The Executive Committee, provides updates to the board of directors with respect to cybersecurity risks, which address a wide range of topics, including recent developments, evolving standards, vulnerability assessments, third-party and independent reviews, the threat environment, technological trends and information security considerations arising with respect to the Company’s peers and third parties. Further, on an annual basis, the corporate information security roadmap is updated to account for the evolving threat landscape and strategic cybersecurity priorities for Materialise and its customers and presented to the Governance Bureau for approval.
Moreover, the CISO, in coordination with the Governance Bureau and Executive Committee, works collaboratively across the company to implement a program designed to protect Materialise’s information systems from cybersecurity threats and to promptly respond to any cybersecurity incidents in accordance with established incident response and recovery plans. Through ongoing communications with these teams, the CISO monitors the prevention, detection, mitigation, and remediation of cybersecurity threats and incidents, and reports such threats and incidents to the Governance Bureau when appropriate.
The CISO has served in various roles in product security and information security management for over 15 years. The CISO holds undergraduate and graduate degrees in computer science and has a Ph.D. in secure software engineering.
Although we are subject to ongoing and evolving cybersecurity threats, we are not aware of any material risks from cybersecurity threats in 2023, including as a result of any previous cybersecurity incidents, that have materially affected or are reasonably likely to affect us, including our business strategy, results of operations or financial condition. If we were to experience a material cybersecurity incident in the future, such incident may have a material effect, including on our business strategy, operating results, or financial condition. For more information regarding cybersecurity risks that we face and potential impacts on our business related thereto, see the risk factors disclosures in Item 3 of this Annual Report on Form 20-F titled “We rely on our information technology systems to manage numerous aspects of our business and customer and supplier relationships, and a disruption of these systems could adversely affect our results of operations,” “A breach of security in our products or computer systems may compromise the integrity of our products, harm our reputation, create additional liability and adversely impact our financial results”, “We rely on third-party technology, platform, carriers, server and hardware providers and as well as local servers, and a failure of service by these providers or by our local servers could adversely affect our business and reputation” and “We develop and offer online software services through our SaaS and cloud-based software applications where we manage data we receive from our customers, and a cybersecurity breach of these online services could harm our customers and our reputation, expose us to liability, and adversely impact our business, financial condition and results of operations.”
108
PART III
ITEM 17.FINANCIAL STATEMENTS
Not applicable.
ITEM 18.FINANCIAL STATEMENTS
See our consolidated financial statements beginning on page F-1 of this annual report.
ITEM 19.EXHIBITS
1.1 |
| |
2.1 | ||
2.2 | Form of American Depositary Receipt (included in Exhibit 2.1). | |
2.3 | ||
4.1 | ||
4.2 | ||
4.3 | ||
4.4 | ||
4.5* | ||
4.6 | ||
4.7 | ||
4.8 | ||
4.9 | ||
4.10 | ||
8.1* | ||
12.1* | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
12.2* | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
13.1** | ||
13.2** |
109
23.1* | ||
97.1* |
101.INS |
| Inline XBRL Instance Document |
101.SCH | Inline XBRL Taxonomy Extension Schema | |
101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase | |
101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase | |
101.LAB | Inline XBRL Taxonomy Extension Label Linkbase | |
101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase | |
104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). | |
* | Filed herewith. | |
** | Furnished herewith. |
110
Signatures
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
MATERIALISE NV | |||
By: | /s/ Brigitte de Vet-Veithen | ||
Name: | Brigitte de Vet-Veithen De Vet Management BV | ||
Title: | Chief Executive Officer | ||
Date: April 12, 2024 | |||
111
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Consolidated Financial Statements for the Years Ended December 31, 2023, 2022 and 2021
F-2 | |
F-4 | |
F-5 | |
F-6 | |
F-8 | |
F-9 | |
F-11 |
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Materialise NV:
Opinions on the Consolidated Financial Statements and Internal Control Over Financial Reporting
We have audited the accompanying consolidated statements of financial position of Materialise NV and subsidiaries (the Company) as of December 31, 2023, 2022 and 2021, the related consolidated income statements, consolidated statements of comprehensive income, consolidated statements of changes in equity, and consolidated cash flow statements for each of the years in the three-year period ended December 31, 2023 and the related notes (collectively, the consolidated financial statements). We also have audited the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023, 2022 and 2021 and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2023, in conformity with IFRS Accounting Standards as issued by the International Accounting Standards Board. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023 based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting in Item 15 (b). Our responsibility is to express an opinion on the Company’s consolidated financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
F-2
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Impairment analysis for the Engimplan and the Materialise Motion cash generating units
As discussed in Note 3 to the consolidated financial statements, the Company performs impairment testing on an annual basis and whenever events or changes in circumstances indicate that the carrying amount of a cash generating unit (CGU) may not be recoverable. The Company determined the recoverable amounts of the Engimplan and the Materialise Motion CGUs based on the value in use using a discounted cash flow model. As discussed in Note 5 to the consolidated financial statements, the Company recorded an impairment of K€ 657 related to the Engimplan CGU and K€ 3,572 related to the Materialise Motion CGU at December 31, 2023.
We identified the evaluation of the impairment analysis for the Engimplan and the Materialise Motion CGUs as a critical audit matter. A high degree of subjective auditor judgment and specialized skills and knowledge was required to evaluate the Engimplan and Materialise Motion CGUs’ respective forecasted year-on-year growth rate of revenue and gross margin, the perpetual growth rate and the discount rate, as changes in these assumptions could cause significant changes in the value in use of the Engimplan and Materialise Motion CGUs.
The following are the primary procedures we performed to address this critical audit matter:
— | We evaluated the design and tested the operating effectiveness of an internal control related to the Company’s impairment process including the evaluation of the assumptions for the forecasted year-on-year growth rate of revenue and gross margin, the perpetual growth rate and the discount rate used to determine the value in use of the Engimplan and Materialise Motion CGUs. |
— | We evaluated the forecasted year-on-year growth rates of revenue and gross margins by comparing them to the CGUs’ historical performances. |
— | We assessed management’s ability to accurately forecast by comparing forecasts made by management in the prior year to the actual performances of the Engimplan and Materialise Motion CGUs. |
— | We also involved valuation professionals with specialized skills and knowledge, who assisted in: |
| ● | evaluating the Company’s discount rates, by comparing them against discount rate ranges that were independently developed using publicly available market data for comparable entities; and |
| ● | evaluating the Company’s perpetual growth rates, by comparing them against the CGUs’ historical performances and to external market and industry data. |
KPMG Bedrijfsrevisoren BV / KPMG Réviseurs d’Entreprises SRL
/s/ Gotwin Victor Jaak Jackers
We have served as the Company’s auditor since 2020.
Zaventem, Belgium
April 12, 2024
F-3
Consolidated income statements
|
| For the year ended December 31, | ||||||
in 000€, except per share data | Notes |
| 2023 |
| 2022 |
| 2021 | |
Revenue |
| 22.1 |
| |
| |
| |
Cost of sales |
| 22.2 |
| ( |
| ( |
| ( |
Gross profit |
| |
| |
| | ||
Research and development expenses |
| 22.3 |
| ( |
| ( |
| ( |
Sales and marketing expenses |
| 22.4 |
| ( |
| ( |
| ( |
General and administrative expenses |
| 22.5 |
| ( |
| ( |
| ( |
Net other operating income |
| 22.6 |
| ( |
| |
| |
Operating profit (loss) |
| |
| ( |
| | ||
Financial expenses |
| 22.8 |
| ( |
| ( |
| ( |
Financial income |
| 22.9 |
| |
| |
| |
Profit (loss) before taxes |
| |
| ( |
| | ||
Income tax benefit/(expense) |
| 22.10 |
| ( |
| ( |
| ( |
Net profit (loss) for the year |
| |
| ( |
| | ||
Net profit (loss) attributable to: |
|
|
|
|
|
|
| |
The owners of the parent |
| |
| ( |
| | ||
Non-controlling interest |
| ( |
| ( |
| ( | ||
Earnings per share attributable to the owners of the parent |
|
|
|
|
|
|
| |
Basic |
| 23 |
| |
| ( |
| |
Diluted |
| 23 |
| |
| ( |
| |
The accompanying notes from page F-11 to page F-62 form an integral part of these consolidated financial statements.
F-4
Consolidated statements of comprehensive income
|
| For the year ended December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 | ||
Net profit (loss) for the year |
| |
| ( |
| | ||
Other comprehensive income/(loss) |
|
|
|
|
|
|
|
|
Items that are or may be reclassified subsequently to profit or loss |
|
|
|
|
|
|
|
|
Exchange differences on translation of foreign operations |
| |
| ( |
| | ||
Items that will not be reclassified to profit or loss |
|
|
|
|
|
|
|
|
Fair value adjustment through OCI - Equity instruments |
| 10 |
| ( |
| ( |
| ( |
Other comprehensive loss, net of taxes |
| |
| ( |
| ( | ||
Total comprehensive income/(loss), net of taxes |
| |
| ( |
| | ||
Total comprehensive (loss)/ income attributable to: |
|
|
|
|
|
|
|
|
The owners of the parent |
| |
| ( |
| | ||
Non-controlling interest |
| ( |
| ( |
| ( | ||
F-5
Consolidated statements of financial position
|
| As of December 31, | ||||||
in 000€ | Notes |
| 2023 |
| 2022 |
| 2021 | |
Assets |
|
|
|
|
|
|
|
|
Non-current assets |
|
|
|
|
|
|
|
|
Goodwill |
| 5 |
| |
| |
| |
Intangible assets |
| 6 |
| |
| |
| |
Property, plant & equipment |
| 7 |
| |
| |
| |
Right-of-use assets |
| 7 |
| |
| |
| |
Deferred tax assets |
| 22.10 |
| |
| |
| |
Investments in convertible loans |
| 10 |
| |
| |
| |
Investments in non-listed equity instruments |
| 10 |
| |
| |
| |
Other non-current assets |
| 10 |
| |
| |
| |
Total non-current assets |
| |
| |
| | ||
Current assets |
|
|
|
|
|
|
| |
Inventories and contracts in progress |
| 9 |
| |
| |
| |
Trade receivables |
| 11 |
| |
| |
| |
Other current assets |
| 10 |
| |
| |
| |
Cash and cash equivalents |
| 12 |
| |
| |
| |
Total current assets |
| |
| |
| | ||
Total assets |
| |
| |
| | ||
F-6
Consolidated statements of financial position
|
| As of December 31, | ||||||
in 000€ | Notes |
| 2023 |
| 2022 |
| 2021 | |
Equity and liabilities |
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
Share capital |
| 13 |
| |
| |
| |
Share premium |
| 13 |
| |
| |
| |
Retained earnings |
| 13 |
| |
| ( |
| |
Other reserves |
| 13 |
| ( |
| ( |
| ( |
Equity attributable to the owners of the parent |
| |
| |
| | ||
Non-controlling interest |
| 13 |
| ( |
| ( |
| |
Total equity |
| |
| |
| | ||
Non-current liabilities |
|
|
|
|
|
|
| |
Loans & borrowings |
| 15 |
| |
| |
| |
Lease liabilities |
| 15 |
| |
| |
| |
Deferred tax liabilities |
| 22.10 |
| |
| |
| |
Deferred income |
| 18 |
| |
| |
| |
Other non-current liabilities |
| 16 |
| |
| |
| |
Total non-current liabilities |
| |
| |
| | ||
Current liabilities |
|
|
|
|
|
|
| |
Loans & borrowings |
| 15 |
| |
| |
| |
Lease liabilities |
| 15 |
| |
| |
| |
Trade payables |
| |
| |
| | ||
Tax payables |
| 17 |
| |
| |
| |
Deferred income |
| 18 |
| |
| |
| |
Other current liabilities |
| 19 |
| |
| |
| |
Total current liabilities |
| |
| |
| | ||
Total equity and liabilities |
| |
| |
| | ||
F-7
Consolidated statements of changes in equity
|
| Attributable to the owners of the parent | ||||||||||||||
Non- | ||||||||||||||||
Share | Share | Retained | Other | controlling | Total | |||||||||||
in 000€ | Notes |
| capital |
| premium |
| earnings |
| reserves |
| Total |
| interest |
| equity | |
At January 1, 2023 |
|
| |
| |
| ( |
| ( |
| |
| ( |
| | |
Net profit (loss) for the year |
| — |
| — |
| |
| — |
| |
| ( |
| | ||
Other comprehensive income (loss) |
| — |
| — |
| — |
| |
| |
| |
| | ||
Total comprehensive income (loss) |
| — |
| — |
| |
| |
| |
| ( |
| | ||
Capital increase through exercise of warrants |
| 13 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
Equity-settled share-based payment expense |
| 14 |
| — |
| |
| — |
| — |
| |
| — |
| |
At December 31, 2023 |
| |
| |
| |
| ( |
| |
| ( |
| | ||
|
| Attributable to the owners of the parent |
|
| ||||||||||||
Non- | ||||||||||||||||
Share | Share | Retained | Other | controlling | Total | |||||||||||
in 000€ | Notes |
| capital |
| premium |
| earnings |
| reserves |
| Total |
| interest |
| equity | |
At January 1, 2022 |
|
| |
| |
| |
| ( |
| |
| |
| | |
Net profit (loss) for the year |
| — |
| — |
| ( |
| — |
| ( |
| ( |
| ( | ||
Other comprehensive income (loss) |
| — |
| — |
| — |
| ( |
| ( |
| — |
| ( | ||
Total comprehensive income (loss) |
| — |
| — |
| ( |
| ( |
| ( |
| ( |
| ( | ||
Capital increase through exercise of warrants |
| 13 |
| ( |
| |
| — |
| — |
| |
| — |
| |
Equity-settled share-based payment expense |
| 14 |
| — |
| — |
| — |
| — |
| — |
| — |
| — |
At December 31, 2022 |
| |
| |
| ( |
| ( |
| |
| ( |
| | ||
|
| Attributable to the owners of the parent | ||||||||||||||
Non- | ||||||||||||||||
Share | Share | Retained | Other | controlling | Total | |||||||||||
in 000€ | Notes |
| capital |
| premium |
| earnings |
| reserves |
| Total |
| interest |
| equity | |
At January 1, 2021 | | ( | ( | | — | | ||||||||||
Net profit (loss) for the year |
| — |
| — |
| |
| — |
| |
| ( |
| | ||
Other comprehensive income (loss) |
| — |
| — |
| — |
| ( |
| ( |
| — |
| ( | ||
Total comprehensive income (loss) |
| — |
| — |
| |
| ( |
| |
| ( |
| | ||
Capital increase initial public offering | 13 | | | ( | — | | — | | ||||||||
Capital increase through exercise of warrants |
| 13 |
| |
| |
| — |
| — |
| |
| — |
| |
Incorporation NCI Tianjin Zhenyuan Materialise Medical Technology Ltd | 13 | — | — | — | — | — | | | ||||||||
Equity-settled share-based payment expense |
| 14 |
| — |
| |
| — |
| — |
| |
| — |
| |
At December 31, 2021 |
| |
| |
| |
| ( |
| |
| |
| | ||
F-8
Consolidated cash flow statements
|
| For the year ended December 31, | ||||||
in 000€ | Notes |
| 2023 |
| 2022 |
| 2021 | |
Operating activities |
|
|
|
|
|
|
|
|
Net profit (loss) for the year |
| |
| ( |
| | ||
Non-cash and operational adjustments |
|
|
|
|
|
|
|
|
Depreciation of property, plant & equipment |
| 7 |
| |
| |
| |
Amortization and impairment of intangible assets |
| 6 |
| |
| |
| |
Impairment of goodwill and intangible assets from business combinations |
| 5; 6 |
| |
| — |
| |
Share-based payment expense |
| 14 |
| |
| ( |
| ( |
Loss (gain) on disposal of property, plant & equipment |
| 7 |
| ( |
| |
| |
Movement in provisions |
|
| ( |
| |
| | |
Movement in reserve for bad debt and slow moving inventory |
|
| |
| ( |
| | |
Financial income |
| 22.9 |
| ( |
| ( |
| ( |
Financial expense |
| 22.8 |
| |
| |
| |
Impact of foreign currencies |
|
| ( |
| ( |
| | |
Share in loss of joint venture (equity method) |
| 8 |
| — |
| — |
| — |
Income taxes and deferred taxes |
| 22.10 |
| |
| |
| |
Working capital adjustment and income tax paid |
|
|
|
|
|
|
|
|
Decrease (increase) in trade receivables and other current assets |
| ( |
| ( |
| ( | ||
Decrease (increase) in inventories and contracts in progress |
| ( |
| ( |
| ( | ||
Increase in trade payables and other payables |
| ( |
| |
| | ||
Income tax paid |
| ( |
| ( |
| ( | ||
Interest received |
| |
| |
| | ||
Net cash flow from operating activities |
| |
| |
| | ||
F-9
Consolidated cash flow statements
|
| For the year ended December 31, | ||||||
in 000€ | Notes |
| 2023 |
| 2022 |
| 2021 | |
Investing activities |
|
|
|
|
|
|
|
|
Purchase of property, plant & equipment |
| 7 |
| ( |
| ( |
| ( |
Purchase of intangible assets |
| 6 |
| ( |
| ( |
| ( |
Proceeds from the sale of property, plant, equipment and intangibles (net) |
| |
| |
| | ||
Acquisition of subsidiary (net of cash) |
| 4 |
| — |
| ( |
| ( |
Convertible loan granted |
| 10 |
| — |
| — |
| ( |
Net cash flow used in investing activities |
| ( |
| ( |
| ( | ||
Financing activities |
|
|
|
|
|
|
|
|
Repayment of loans & borrowings |
| 15 |
| ( |
| ( |
| ( |
Repayment of leases |
| 15 |
| ( |
| ( |
| ( |
Capital increase in parent company |
| 13 |
| — |
| |
| |
Interest paid |
| ( |
| ( |
| ( | ||
Other financial income (expense), net |
| ( |
| |
| | ||
Net cash flow from financing activities |
| ( |
| ( |
| | ||
Net increase/(decrease) of cash and cash equivalents |
| ( |
| ( |
| | ||
Cash and cash equivalents at beginning of the year |
| 12 |
| |
| |
| |
Exchange rate differences on cash and cash equivalents |
| ( |
| ( |
| | ||
Cash and cash equivalents at end of the year |
| 12 |
| |
| |
| |
F-10
Notes to the consolidated financial statements
1Corporate information
Materialise NV is a limited liability company with its office at Technologielaan 15, 3001 Leuven, Belgium. The consolidated financial statements comprise Materialise NV (the “Company” or “Parent”) and its subsidiaries (collectively, the “Group” or “we,” “us” and “our”). See Note 28 for a list of subsidiaries of the Company.
We are a leading provider of additive manufacturing and medical software and of sophisticated 3D printing services. Our products and services are offered through a market oriented organization that is active across three principal market segments: (i) Materialise Software, (ii) Materialise Medical, and (iii) Materialise Manufacturing. We sell our products and services in Europe, the Americas, Africa and Asia-Pacific.
The consolidated financial statements of the Group for the year ended December 31, 2023 were approved and authorized for issue on April 12, 2024, in accordance with a resolution of the Company’s board of directors.
2Basis of preparation
The consolidated financial statements of the Group for the three years ended December 31, 2023, 2022 and 2021 are prepared in accordance with the International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB) (collectively “IFRS”).
These consolidated financial statements have been prepared on a historical cost basis, except for the assets and liabilities that have been acquired as part of a business combination, which have been initially recognized at fair value, and certain financial assets such as the non-listed equity instruments and the convertible loan receivable which are both included in the other non-current assets and the share appreciation rights which are measured at fair value.
The financial statements are prepared on a going concern basis. The consolidated financial statements are presented in thousands of euros (K€ or thousands of €) and all “currency” values are rounded to the nearest thousand (€000), except when otherwise indicated.
The preparation of financial statements in compliance with IFRS requires the use of certain critical accounting estimates. It also requires Group management to exercise judgment in applying the Group’s accounting policies. The areas where significant judgment and estimates have been made in preparing the financial statements and their effect are disclosed in Note 3.
New standards, interpretations and amendments adopted by the Group
The following amendments and interpretations issued by the IASB and IFRIC apply for the first time in 2023, but do not have a significant impact on the consolidated financial statements of the Group.
| ● | IFRS 17 Insurance Contracts (issued on 18 May 2017); including Amendments to IFRS 17 (issued on 25 June 2020) |
| ● | Amendments to IAS 8 Accounting policies, Changes in Accounting Estimates and Errors: Definition of Accounting Estimates (issued on 12 February 2021) |
| ● | Amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2: Disclosure of Accounting policies (issued on 12 February 2021) |
| ● | Amendments to IAS 12 Income Taxes: Deferred Tax related to Assets and Liabilities arising from a Single Transaction (issued on 7 May 2021) |
| ● | Amendments to IFRS 17 Insurance contracts: initial application of IFRS 17 and IFRS 9 – Comparative information (issued on 9 December 2021) |
| ● | Amendments to IAS 12 Income taxes: International Tax Reform – Pillar Two Model Rules (issued on 23 May 2023) |
F-11
Standards and Interpretations issued but not yet effective in the current period
No amendments to standards that are issued but not yet effective are considered to materially affect the Company’s accounting policies or any of the disclosures when applied for the first time. The Company has not early adopted any of the below.
Amendments to IAS 1 ‘Presentation of Financial Statements: Classification of Liabilities as current or non-current’ (effective January 1, 2024), affect only the presentation of liabilities in the statement of financial position — not the amount or timing of recognition of any asset, liability income or expenses, or the information that entities disclose about those items.
The amendments:
| ● | clarify that the classification of liabilities as current or non-current should be based on rights that are in existence at the end of the reporting period and align the wording in all affected paragraphs to refer to the “right” to defer settlement by at least twelve months and make explicit that only rights in place “at the end of the reporting period” should affect the classification of a liability; |
| ● | clarify that classification is unaffected by expectations about whether an entity will exercise its right to defer settlement of a liability; and make clear that settlement refers to the transfer to the counterparty of cash, equity instruments, other assets or services; and |
| ● | clarify how conditions with which an entity must comply within 12 months after the reporting period, such as covenants, affect the corresponding liability’s classification. |
Amendments to IFRS 16 Leases: Lease Liability in a Sale and Leaseback (effective January 1, 2024). The amendments explain how an entity accounts for a sale and leaseback after the date of the transaction, specifically where some or all the lease payments are variable lease payments that do not depend on an index or rate. They state that, in subsequently measuring the lease liability, the seller-lessee determines ‘lease payments’ and ‘revised lease payments’ in a way that does not result in the seller-lessee recognizing any amount of the gain or loss that relates to the right of use it retains. Any gains and losses relating to the full or partial termination of a lease continue to be recognized when they occur as these relate to the right of use terminated and not the right of use retained.
Amendments to IAS 7 ‘Statement of Cash Flows’ and IFRS 7 ‘Financial Instruments: Disclosures’1: Supplier Finance Arrangements (effective 1 January 2024). The amendments add disclosure requirements, and ‘signposts’ within existing disclosure requirements, that ask entities to provide qualitative and quantitative information about supplier finance arrangements.
The amendments:
| ● | Do not define supplier finance arrangements. |
| ● | Add two disclosure objectives. Entities will have to disclose in the notes information that enables users of financial statements to assess how supplier finance arrangements affect an entity’s liabilities and cash flows and to understand the effect of supplier finance arrangements on an entity’s exposure to liquidity risk and how the entity might be affected if the arrangements were no longer available to it. |
| ● | Complement current requirements in IFRSs by adding to IAS 7 additional disclosure requirements about the type and effect of non-cash changes in the carrying amounts of the financial liabilities that are part of the arrangement and the terms and conditions of the supplier finance arrangements; for the arrangements, as at the beginning and end of the reporting period: |
| o | the carrying amounts of financial liabilities that are part of the arrangement and the associated line item presented; |
| o | the carrying amount of financial liabilities disclosed under a) for which suppliers have already received payment from the finance providers; |
| o | the range of payment due dates (for example, 30 to 40 days after the invoice date) of financial liabilities disclosed under a) and comparable trade payables that are not part of a supplier finance arrangement. |
F-12
Amendments to IAS 21 ‘The Effects of Changes in Foreign Exchange Rates: Lack of Exchangeability’1, (effective 1 January 2025). The amendments to IAS 21 The Effects of Changes in Foreign Exchange Rates require disclosure of information that enables users of financial statements to understand the impact of a currency not being exchangeable.
The amendments:
| ● | Specify when a currency is exchangeable into another currency and when it is not. |
| ● | Specify how an entity determines the exchange rate to apply when a currency is not exchangeable. |
| ● | Require the disclosure of additional information when a currency is not exchangeable. |
3Material accounting policies
Basis for consolidation
The consolidated financial statements comprise the financial statements of the Group and its subsidiaries.
Entities are fully consolidated from the date of acquisition, which is the date when the Group obtains control, and continue to be consolidated until the date when such control ceases. The financial statements of the entities are prepared for the same reporting period as the parent company, using consistent accounting policies.
Foreign currency translation
The Group’s consolidated financial statements are presented in euros, which is also the parent company’s functional currency. For each entity, the Group determines the functional currency, and items included in the financial statements of each entity are measured using the functional currency.
Financial statements of foreign subsidiaries
Foreign subsidiaries use the local currencies of the country where they operate. The statement of financial position is translated into euro at the closing rate on the reporting date and their income statement is translated at the average exchange rate at each month-end. Differences resulting from the translation of the financial statements of said subsidiaries are recognized in other comprehensive income as “exchange differences on translation of foreign operations”.
Business combinations and goodwill
Business combinations are accounted for using the acquisition method at the acquisition date, which is the date at which the Group obtains control over the entity. The cost of an acquisition is measured as the amount of the consideration transferred to the seller, measured at the acquisition date fair value, and the amount of any non-controlling interest in the acquiree.
Acquisition costs incurred are expensed and included in general and administrative expenses.
F-13
Property, plant & equipment
Property, plant and equipment is stated at cost, net of accumulated depreciation and/or accumulated impairment losses, if any. Repair and maintenance costs are recognized in the income statement as incurred.
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets as follows:
● Buildings: |
|
| |
● Machinery: | |||
● IT assets: | |||
● Fixtures & Furniture: | |||
● Vehicles: | |||
● Leasehold Building Improvements: |
Land is not depreciated.
An item of property, plant and equipment and any significant part initially recognized is derecognized upon disposal or when no future economic benefits are expected from its use or disposal. Any gain or loss arising on derecognition of the asset (calculated as the difference between the net disposal proceeds and the carrying amount of the asset) is included in the income statement when the asset is derecognized.
The assets’ residual values, useful lives and methods of depreciation are reviewed at each financial year-end and adjusted prospectively, if appropriate.
Right-of-use assets and related liabilities
Right-of-use assets:
The Group recognizes right-of-use assets at the commencement date of the lease (i.e., the date the underlying asset is available for use). Unless the Group is reasonably certain to obtain ownership of the leased asset at the end of the lease term, the recognized right-of-use assets are depreciated on a straight-line basis over the shorter of its estimated useful life and the lease term:
● Property leased Assets: | Lease terms up to | ||
● Leased machines: | Lease terms up to | ||
● Leased vehicles: | Lease terms up to | ||
Right-of-use assets are subject to impairment review whenever there is an indication that the right-of-use asset may be impaired.
Lease liabilities:
In calculating the present value of lease payments, the Group uses the incremental borrowing rate at the lease commencement date. After the commencement date, the amount of lease liabilities is measured at amortized cost using the effective interest rate method.
In addition, the carrying amount of lease liabilities is remeasured when there is a change in future lease payments arising from a change in an index or a rate, if there is a change in the Group’s estimate of the amount expected to be payable under a residual value guarantee, if the Group changes its assessment of whether it will exercise a purchase, extension or termination option or if there is a revised in-substance fixed lease payment. When the lease liability is remeasured in this way, a corresponding adjustment is made to the carrying amount of the right-of-use asset, or is recorded in profit or loss if the carrying amount of the right-of-use asset has been reduced to zero.
F-14
Short-term leases and leases of low-value assets:
The Group applies the short-term lease recognition exemption to its short-term leases of machinery and equipment (i.e., those leases that have a lease term of 12 months or less from the commencement date and do not contain a purchase option) however this exemption is not applied for property leases. It also applies the lease of low-value assets recognition exemption to leases of office equipment that are considered of low value (i.e., below € 5k). Lease payments on short-term leases and low-value assets are recognized in the income statement when incurred.
Research and development
Research and development includes the costs incurred by activities related to the development of software solutions (new products, updates and enhancements), guides and other products.
Development activities involve the application of research findings or other knowledge to a plan or a design of new or substantially improved (software) products before the start of the commercial use.
Development expenditures on an individual project are recognized as an intangible asset when the Group can demonstrate:
| ● | the technical feasibility of completing the intangible asset so that the asset will be available for use or sale; |
| ● | its intention to complete and its ability to use or sell the asset; |
| ● | how the asset will generate future economic benefits; |
| ● | the availability of resources to complete the asset; and |
| ● | the ability to measure reliably the expenditure during development. |
The Group has determined that the conditions for recognizing internally generated intangible assets from proprietary software, guide and other product development activities are not met until shortly before the products are available for sale, unless either (i) the Group has strong evidence that the above criteria are met and a detailed business plan is available showing the asset will on a reasonable basis generate future economic benefits or (ii) the development is done based upon specific request of the customer, it is highly likely that the Group will be able to market the product also to other parties than the customer, the development is subject to an agreement and the substance of the agreement is that the customer reimburses the Group for a significant portion, but not all, of the development expenses incurred. As such, development expenditures not satisfying the above criteria and expenditures on the research phase of internal projects are recognized in the consolidated income statement as incurred. Internally generated intangible assets from proprietary software are amortized over their useful lives, starting from the moment they are ready for use/available for sale.
Following initial recognition of the development expenditure as an asset, the asset is carried at cost less any accumulated amortization and accumulated impairment losses. Amortization of the asset begins when development is complete and the asset is available for use. It is amortized over the period of expected future benefit, which is determined on a project-by-project basis. Amortization is recorded in research and development expenditure. During the period of development, the asset is tested for impairment at least annually or whenever there is an indication of impairment.
Intangible assets other than goodwill and capitalized development expenditures
Intangible assets comprise acquired technology and customer portfolio, patents and licenses and technology and customers acquired in connection with business combinations. Those intangible assets are measured on initial recognition at cost, except for the acquired technology and customers arising from business combinations, which are measured initially at fair value. Following initial recognition, intangible assets other than goodwill are carried at cost less any accumulated amortization and accumulated impairment losses, if any.
F-15
The useful life of the intangible assets is as follows:
● Software: |
|
| |
● Perpetual licences for ERP & front end software: | |||
● Software with subscription license: | subscription term | ||
● Patents and licenses: | |||
● Acquired customers and technology: |
The intangible assets with finite lives are amortized over their useful economic life and assessed for impairment whenever there is an indication that the intangible asset may be impaired. The amortization period and the amortization method for an intangible asset with a finite useful life are reviewed at least at the end of each reporting period. The amortization expense on intangible assets with finite lives acquired through business combination is recognized in the consolidated income statement in the line “net other operating income”.
Impairment of goodwill and other non-financial assets (excluding inventories and deferred tax assets)
Impairment tests on goodwill, assets under construction or capitalized development expenses which are not amortized yet, are undertaken annually at the financial year end. Other non-financial assets and goodwill are subject to impairment tests whenever events or changes in circumstances indicate that their carrying amount may not be recoverable. Where the carrying value of an asset exceeds its recoverable amount (i.e. the higher of value in use and fair value less costs to sell), the asset is written down accordingly.
Where it is not possible to estimate the recoverable amount of an individual asset, the impairment test is carried out on the smallest Group of assets to which it belongs for which there are separately identifiable cash flows: its cash generating units (CGUs). Goodwill is allocated on initial recognition to each of the Group’s CGUs that are expected to benefit from the synergies of the combination giving rise to the goodwill.
The Group bases its impairment calculation on detailed budgets and forecast calculations, which are prepared separately for each of the Group’s CGUs to which the individual assets are allocated. These budgets and forecast calculations cover a period of five years. For longer periods, a long-term growth rate is calculated and applied to future cash flows projected after the fifth year.
Impairment charges are included in profit or loss. An impairment loss recognized for goodwill is not reversed.
Inventories and Contracts in progress
Inventories are valued at the lower of cost and net realizable value. Costs incurred in bringing each product to its present location and condition are accounted for as follows:
| ● | raw materials: purchase cost on a first in, first out basis; and |
| ● | finished goods and work in progress: cost of direct materials and labor and a proportion of manufacturing overheads based on the normal operating capacity, but excluding borrowing costs. |
Net realizable value is the estimated selling price in the ordinary course of business, less estimated costs of completion and the estimated costs necessary to make the sale.
A write-off of inventories is estimated based on an ageing or rotation analysis.
Work in progress relates to production of inventory for which a customer has not yet been secured, while contracts in progress are contract assets that relate to production for specific customers in performance of a signed contract. We refer also to the accounting policy on revenue recognition.
Financial assets
Trade receivables and debt instruments issued are initially recognized when they are originated. All other financial assets are initially recognized when the Group become a party to the contractual provisions of the instrument.
F-16
Financial assets are classified at initial recognition, and subsequently measured either at amortized cost, either fair value through other comprehensive income (OCI), and fair value through profit or loss. The classification of financial assets at initial recognition depends on the financial asset’s contractual cash flow characteristics and the Group’s business model for managing them.
Except for trade receivables that do not contain a significant financing component or for which the Group has applied the practical expedient, the Group initially measures a financial asset at its fair value plus transaction costs, in the case of a financial asset not at fair value through profit or loss. Trade receivables that do not contain a significant financing component or for which the Group has applied the practical expedient are measured at the transaction price.
For purposes of subsequent measurement, financial assets are classified in four categories:
| ● | Financial assets at amortized cost; |
| ● | Financial assets at fair value through OCI with recycling of cumulative gains and losses (debt instruments); |
| ● | Financial assets designated at fair value through OCI with no recycling of cumulative gains and losses upon derecognition (equity instruments); and |
| ● | Financial assets at fair value through profit or loss. |
Financial assets measured at amortized cost
This category is the most relevant to the Group. The Group measures financial assets at amortized cost if both of the following conditions are met:
| ● | the financial asset is held within a business model with the objective to hold financial assets in order to collect contractual cash flows; and |
| ● | the contractual terms of the financial asset give rise on specified dates to cash flows that are solely payments of principal and interest on the principal amount outstanding. |
Financial assets, trade and other receivables, cash and cash equivalents at amortized cost are subsequently measured using the effective interest rate (EIR) method and are subject to impairment. Gains and losses are recognized in profit or loss when the asset is derecognized, modified or impaired.
Financial assets at fair value through OCI with recycling of cumulative gains and losses (debt instruments)
The Group currently does not have financial assets at fair value through OCI with recycling of cumulative gains and losses.
Financial assets designated at fair value through OCI with no recycling of cumulative gains and losses upon derecognition (equity instruments)
The Group has irrevocably elected at initial recognition to classify the minority equity investment in the non-listed company AM-Flow BV and Essentium, as disclosed in Note 10 and Note 20, as financial asset designated at fair value through OCI as this measurement is most representative of the business model for these assets. Gain and losses on these financial assets are never recycled to profit and loss.
Financial assets measured at fair value through profit or loss
The Group has the following financial assets classified as financial assets at fair value through profit or loss:
| ● | derivatives as disclosed in Note 10; |
| ● | a convertible loan granted to the company Fluidda as disclosed in Note 10. |
F-17
Those financial assets are carried in the statement of financial position at fair value with changes recognized in the income statement in the lines financial income/expense.
Impairment of financial assets
Further disclosures relating to impairment of financial assets are also provided in Note 3 Significant accounting judgments, estimates and assumptions.
The Group recognizes an allowance for expected credit losses (ECLs) for all debt instruments not held at fair value through profit or loss.
For trade receivables and contract assets, the Group applies a simplified approach in calculating ECLs. A loss allowance is recognized at each reporting date based on lifetime ECLs. The Group established a provision matrix that is based on its historical loss experience, adjusted for forward-looking factors specific to the debtors and the economic environment.
Financial liabilities
All financial liabilities are recognized initially at fair value and, in the case of loans and borrowings and payables, net of directly attributable transaction costs.
The Group’s financial liabilities include trade and other payables, loans and borrowings including bank overdrafts and derivative financial instruments.
Financial liabilities at amortized cost
The trade and other payables, and loans and borrowings are classified as financial liabilities at amortized cost.
Those financial liabilities are measured at amortized cost using the effective interest rate method. Gains and losses are recognized in the income statement when the liabilities are derecognized as well as through the effective interest rate method amortization process.
Financial liabilities at fair value through profit and loss
The derivative financial instruments are classified as financial liabilities at fair value through profit and loss.
Share capital
Financial instruments issued by the Group are classified as equity only to the extent that they do not meet the definition of a financial liability or financial asset. The Group’s ordinary shares are classified as equity instruments.
Pension benefits
The Group has a defined contribution obligation where the Group pays contributions based on salaries to an insurance company, in accordance with the laws and agreements in each country.
The Belgian defined contribution pension plans are by law with variable minimum returns based on the Belgian government bonds, with a minimum of
These plans qualify as defined benefit plans. Contributions are recognized as expenses for the period in which employees perform the corresponding services. Outstanding payments at the end of the period are shown as other current liabilities.
Share based payments
Directors and employees (including senior executives) of the Group receive remuneration in the form of share-based payments, whereby employees render services as consideration for equity instruments (equity-settled transactions). The Group currently has only warrants and share-appreciation rights as share-based payments.
F-18
Equity-settled transactions
Equity-settled share-based payments to employees and others providing similar services are measured, indirectly, at the fair value of the equity instruments granted. The cost of equity-settled transactions is recognized, together with a corresponding increase in other capital reserves in equity, over the period in which the performance and/or service conditions are fulfilled. The cumulative expense recognized for equity-settled transactions at each reporting date until the vesting date reflects the extent to which the vesting period has expired and the Group’s best estimate of the number of equity instruments that will ultimately vest. The income statement expense or credit for a period represents the movement in cumulative expense recognized at the beginning and end of that period and is recognized as employee benefits expense.
The Group does currently only have equity-settled share-based payments that have service-based vesting conditions and no instruments with market vesting conditions.
No expense is recognized for awards that do not ultimately vest.
Other long-term employee benefits
The Group’s net obligation for long-term employee benefits is equal to the value of future benefits acquired by personnel in exchange for services rendered in the current and prior periods.
Revenue from contracts with customers
The Group’s revenue, which is presented net of sales taxes, is primarily generated by the sale of our software and 3D printed products and services. Software revenue is comprised of perpetual and periodic licenses, maintenance revenue and software development service fees. Perpetual license holders may opt to take an annual maintenance contract, which leads to annual fees. Periodic licenses entitle the customer to maintenance, support and product updates without additional charge. Revenue from prototypes and end products involving 3D printing technology is derived from our network of production centers and may include support and services such as pre-production collaboration prior to the actual production.
The Group sells its products and software through its direct sales force and through authorized distributors.
Software license revenue, maintenance and/or software development service fees may be bundled in one arrangement or may be sold separately.
The Group recognizes revenue for goods including software based on the five-step model per the requirements of IFRS 15.
Revenue from contracts with customers is recognized when control of the goods or services is transferred to the customer at an amount that reflects the consideration to which the Group is expected to be entitled in exchange from those goods and services.
If the consideration in a contract includes a variable amount, the Group estimates the amount of consideration to which it will be entitled in exchange for transferring the goods to the customer. The variable consideration is estimated at contract inception and constrained until it is highly probable that a significant revenue reversal in the amount of cumulative revenue recognized will not occur when the associated uncertainty with the variable consideration is subsequently resolved. Variable consideration is mainly related to quantities sold, volume (step-based) rebates and development time spent.
Prototypes and end products involving 3D printing technology
The Group recognizes revenue on the sale of goods to the customer or distributor at a point in time when control of the asset is transferred, generally upon shipment or delivery considering the shipment terms (usually Ex-works or FOB Time of Shipment Incoterms (International Commercial Terms)).
Perpetual licensed software
The sale and/or license of software products is deemed to have occurred at a point in time, i.e. when a customer either has taken possession of or has the ability to take immediate possession of the software and the software key.
F-19
Most of the perpetual software licenses include one year maintenance and support services as a separate performance obligation. The Group sells these maintenance services also on a stand-alone basis and is therefore capable of determining their stand-alone selling price. On this basis, the amount of the embedded maintenance is separated from the fee for the perpetual license and is recognized ratably over the period to which they relate.
Time-based licensed software
The time-based license agreements include the use of a software license for a fixed term and maintenance and support services during the same period. The Group does not sell time-based licenses without maintenance and support services and therefore revenues are satisfied over time for the entire arrangements and are recognized ratably over the term.
Maintenance and support services
Maintenance and support services are satisfied over time and as such, the Group recognizes this revenue ratably on a straight-line basis over the term that the maintenance service is provided. In general, maintenance services are not automatically renewed.
A maintenance and support contract may include a reinstatement for previous years when the customer did not have a maintenance and support contract previously. Revenue from reinstatements is recognized immediately when the maintenance and support services commence.
Software development services (SDS)
SDS include customized development of software components for customers. Revenue from SDS agreements when distinct from other performance obligations is satisfied over time or at a point in time, depending whether one of the IFRS 15.35 criteria for performance obligations to be satisfied over time is met or not. In case of recognition over time, revenue is recognized either on time and material basis or on the stage of completion of each service when the percentage of completion can be measured reliably.
The Group determines the percentage-of-completion by comparing labor hours incurred to-date to the estimated total labor hours required to complete the project. The Group considers labor hours to be the most reliable available measure of progress on these projects. Adjustments to the Group’s estimates of the time to completion are made when facts resulting in a change become known. When the estimate indicates that a loss will be incurred, such loss is recognized immediately.
In case of recognition at a point in time revenue is recognized when control over the product is transferred to the customer.
Contracts with multiple performance obligations
The Group has entered into a number of contracts with multiple performance obligations, such as when selling perpetual licenses that may include maintenance and support (included in the price of perpetual licenses) and time-based licenses (that include embedded maintenance and support, both of which may be sold with software development services, training, and other product sales). In some cases, the Group delivers software development services bundled with the sale of the software.
The Group evaluates whether each performance obligation is distinct from each other, i.e. the customer can benefit from the good or service on its own, or with readily available resources. Certain development services significantly modify and/or enhance the software license and as such are not considered distinct and combined with the software license.
In those contracts, whether sold to end-customers or to collaboration partners, the Group uses either price list, historical pricing information or management’s best estimate of selling prices (e.g. also using a cost-plus method) to determine the stand-alone selling price for each distinct performance obligation, including software and software-related services such as maintenance and support. In general, elements in such arrangements are also sold on a stand-alone basis and stand-alone selling prices are readily available. If the stand-alone selling price of one or more goods or services in such arrangements is highly variable or uncertain, the Group estimates the stand-alone selling price with reference to the total transaction price less the sum of the observable stand-alone selling prices of other goods or services promised in the contract.
Revenue is allocated to each distinct performance obligation (“PO”) based on the relative percentage of the stand-alone selling price for each PO compared to the total of stand-alone selling prices for all PO over the total transaction price and is recognized when the revenue recognition criteria described above are met.
F-20
Contracts with collaboration partners in the medical segment also include multiple elements such as software, maintenance and support services, training, software development services, 3D printed products and royalties. Revenue from those contracts is determined and recognized consistent with other multiple element arrangements.
For certain contracts with collaboration partners, the Group receives up-front fees, paid by customers for certain exclusivity rights, which may be bundled with transfer of title, rights and ownership of certain software products and maintenance and support services. In case the up-front fees do not relate to already delivered good or services, the Group includes the up-front fees in the total transaction price which is then allocated to all the distinct performance obligations. Other contracts with collaboration partners include prepaid fees to purchase a maximum number of “Plan Only” cases or case ‘bundles’ during a 12-month period. In this case, the prepaid fees are recognized over the period of 12 months based on the expected number of cases that will be purchased.
Contract assets
A contract asset is the right to consideration in exchange for goods or services transferred to the customer. If the Group performs by transferring goods or services to a customer before the customer pays consideration or before payment is due, a contract asset is recognized for the earned consideration that is conditional. Contract assets are only contracts in progress that are disclosed with the line inventory and contracts in progress in the statement of financial position. We refer to our accounting policies regarding Inventories and Contracts in Progress.
Contract liabilities
A contract liability is the obligation to transfer goods or services to a customer for which the Group has received consideration (or an amount of consideration is due) from the customer. If a customer pays consideration before the Group transfers goods or services to the customer, a contract liability is recognized when the payment is made or the payment is due (whichever is earlier). Contract liabilities are recognized as revenue when the Group performs under the contract. Contract liabilities are presented as deferred income in the statement of financial position.
Government grants
Government grants are recognized when there is reasonable assurance that the grant will be received and all attached conditions will be complied with. When the grant relates to development costs or another expense, it is recognized as income over the grant period necessary to match the income on a systematic basis to the costs that it is intended to compensate. When the grant relates to the construction of buildings, it is recognized as income over the depreciation period of the related building.
Such grants have been received from the federal and regional governments and from the European Union in the forms of grants linked to certain of its research and development programs, reduced payroll taxes and the financing of the construction of an office building in Leuven (Belgium) and in Freiberg (Germany).
Where retention of a government grant related to assets or to income, is dependent on the Group satisfying certain criteria, it is initially recognized as deferred income. When the criteria for retention have been satisfied, the deferred income balance is released to other operating income in the consolidated income statement on a systematic basis over the periods in which the entity recognizes as expenses the related costs for which the grants are intended to compensate.
Other financial income and expenses
Other financial income and expenses include mainly foreign currency gains or losses on financial transactions and bank related expenses.
Taxes
Current income tax
Income tax assets and liabilities for the current period are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted, at the reporting date.
F-21
Current income tax relating to items that are recognized directly in equity is recognized in equity and not in the income statement. Management periodically evaluates positions taken in the tax returns with respect to situations in which applicable tax regulations are subject to interpretation and establishes provisions where appropriate.
Deferred tax
Deferred tax is calculated using the liability method on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes.
Deferred tax liabilities are recognized for all taxable temporary differences. Deferred tax assets are recognized for all deductible temporary differences, carry forward of unused tax credits and unused tax losses, to the extent that it is probable that taxable profit will be available against which the deductible temporary differences or the carry forward of unused tax credits and unused tax losses can be utilized. In order for any deferred tax assets to be recognized, and at a minimum, the respective Materialise entity should have recorded a taxable profit in the current year and it should be probable that a taxable profit will be achieved in the subsequent year.
The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized. Unrecognized deferred tax assets are reassessed at each reporting date and are recognized to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been (substantively) enacted at the reporting date.
Deferred tax assets and deferred tax liabilities are offset, if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred taxes relate to the same taxable entity and the same taxation authority.
Significant accounting judgments, estimates and assumptions
The preparation of the Group’s consolidated financial statements requires management to make judgments, estimates and assumptions that affect the reported amounts of revenue, expenses, assets and liabilities, and related disclosures. Uncertainty about these assumptions and estimates could lead to outcomes that require a material adjustment to the carrying amount of assets or liabilities for future periods.
The Group reviews its estimates, assumptions and judgments on an ongoing basis, including those related to revenue recognition, development expenses, share-based payment transactions, income taxes, impairment of goodwill, intangible assets and property, plant & equipment and business combinations, provisions for expected credit losses, convertible loans, equity instruments, useful lives of certain assets and leases.
The Group has based its assumptions and estimates on the parameters that were available when the consolidated financial statements were prepared. However, existing conditions and assumptions about future developments may change due to market changes or circumstances beyond the Group’s control. Such changes are incorporated into the assumptions as they occur.
Revenue recognition
Our revenue recognition policy requires management to make significant estimates. Management analyzes various factors, including an evaluation of specific transactions, historical experience, creditworthiness of customers and current market and economic conditions. Changes in judgments based upon these factors may affect the timing and amount of revenues and expenses recognized and, consequently, the results of operations and financial condition. Significant estimates and judgments relate to:
| ● | assessing whether a performance obligation is distinct in a bundled sales transactions; |
| ● | estimation of the variable considerations and the revenue constraint; |
| ● | estimation of stand-alone selling prices for each distinct performance obligation; and |
| ● | the stage of completion of our custom development of software components for customers when revenues are satisfied over time. |
F-22
The Group makes significant judgments when performing the assessment of whether a performance obligation is distinct from the other performance obligations in a contract, i.e. whether the good or service has a benefit to the customer in its own or together with readily available resources and/or whether the good or service is highly interrelated or constitutes a significant input with another good or service provided, or whether it significantly modifies or tailors another good or service. The relevant assessments include but are not limited to the following:
| ● | Whether the software license is distinct from the 3D printed guides - in most cases with contracts with collaborative partners in the Materialise Medical segment, the software licenses are combined with the manufacturing of the 3D printed guides, as the software license has no benefit to the customer without the manufacturing services. |
| ● | Whether the development services are distinct from other performance obligations - in most cases these performance obligations are distinct but for certain contracts, the software license may be combined with the license and the 3D printed guides as one distinct performance obligation. |
For stand-alone selling prices, the Group uses prices from price lists or historical prices for similar transactions. However, in certain cases such information is not readily available and in those cases the Group estimates the stand-alone selling price based on a cost plus mark-up or other estimate. In addition, for certain performance obligations such as development services, the stand-alone selling prices also require an estimate of the time required to complete the development. If the Group determines that the stand-alone selling price of one or more goods or services in a multiple element arrangement is highly variable or uncertain, the Group estimates the stand-alone selling price with reference to the total transaction price less the sum of the observable stand-alone selling prices of other goods or services promised in the contract.
Certain contracts include estimates of variable considerations within the transaction price and assessing the revenue constraint, such as:
| ● | quantities/volume sold at fixed prices related to, but not limited to, the manufacturing of 3D printed products, software licenses sold, maintenance renewals; |
| ● | contractual prices may vary based on volume purchased during a given period; |
| ● | FTE expenses for development or other services billed on a time and material basis; and |
| ● | volume rebates. |
The method used to estimate the variable consideration depends on the number of possible scenarios and the probability of each scenario. If there are many possible scenarios with a high probability (each less than 50%), the Group will use the expected value method, while the most likely method is used when there is a scenario with a higher probability (more than 50%).
Variable consideration is not constrained when the Group determines, based on historical experience, a high reliable business forecast and/or the time frame of the estimates, that there is a high probability that it will not result in a future reversal of revenue.
We determine the stage of completion for development contracts satisfied over time by comparing the labor hours incurred to date with the estimated total labor hours required to complete the project. We consider labor hours to be the most reliable, available measure of progress on these projects. Adjustments to estimates are made in the period when facts that give rise to a change become known. When the estimate indicates that a loss will be incurred, the loss is recorded in the relevant period. Significant judgments and estimates are involved in determining the percentage of completion for each contract. Different assumptions can produce materially different results.
Development expenses
Determining whether internally generated intangible assets from development should be recognized as intangible assets requires significant judgment, particularly in determining whether the activities are considered research activities or development activities, whether the product enhancement is substantial, whether completion of the asset is technically feasible considering a company-specific approach, the likelihood of future economic benefits from sale or use, including an assessment of whether FDA approval will be obtained.
F-23
The Group has determined that the conditions for recognizing internally generated intangible assets from its own software, guides and other product development activities are not met until shortly before the products are available for sale, unless either (i) the Group has strong evidence that the above criteria are met and a detailed business plan is available showing that the asset will generate future economic benefits on a reasonable basis or (ii) the development is done at the specific request of the customer, the Group intends to market the product to other parties than the customer, the development is subject to an agreement and the substance of the agreement is that the customer will reimburse the Group for a significant portion of the development costs incurred. As such, development expenditures that do not meet the above criteria and expenditures for the research phase of internal projects are recognized in the consolidated income statement as incurred. This assessment is monitored by the Group on a regular basis.
The Group capitalized a total of K€
Income taxes
Deferred tax assets are recognized only to the extent that it is probable that taxable profit will be available against which the deductible temporary difference can be utilized. Significant management judgment is required to determine the amount of deferred tax assets that may be recognized, based on the probable timing and level of future taxable profits, together with future tax planning strategies. As of December 31, 2023, the Group had current and non-current receivables related to tax credits for an amount of K€
For any deferred tax assets to be recognized, and at a minimum, the respective Materialise entity should have recorded a taxable profit in the current year and it should be probable that a taxable profit will be achieved in the subsequent year.
Impairment of goodwill, intangible assets and property, plant & equipment and determination of the cash-generating-unit.
The Group has goodwill for a total amount of K€
Also, as part of the impairment analysis, the Group needs to determine the different CGUs at the lowest non-aggregated level which requires the Group to make judgments about application of the criteria to determine the CGUs based on the facts and circumstances how the entities and business units within the CGU and within the Group operate and are monitored. The level of CGU may also have an impact on certain assumptions to make with regard to transfer pricing.
The key assumptions used to determine the value in use for the different CGUs are disclosed and further explained in Note 5.
During 2023 impairment charges have been recorded for K€
Business combinations
We determine and allocate the purchase price of an acquired business to the assets acquired and liabilities assumed as of the business combination date. Business combinations are discussed further in Note 4. The purchase price allocation process requires us to make significant estimates and assumptions, including:
F-24
While we are using our best estimates and assumptions as part of the purchase price allocation process to accurately value assets acquired and liabilities assumed at the date of acquisition, our estimates and assumptions are inherently uncertain and subject to refinement. Examples of critical estimates in valuing certain of the intangible assets we have acquired or may acquire in the future include but are not limited to:
| ● | future expected cash flows from customer contracts and relationships, software license sales and maintenance agreements; |
| ● | the fair value of the plant and equipment |
| ● | the fair value of the deferred revenue; and |
| ● | discount rates. |
Convertible debt instruments
At December 31, 2023 the Group holds a convertible debt instrument issued by Fluidda which is measured at fair value through profit & loss. In determining the fair value of those convertible debt instruments, the Group considers different contractual parameters such as the repayment and conversion scenarios and dates. In addition, the Group needs to make significant estimates such as (i) the discount rate, (ii) the probabilities for each repayment and conversion scenario, (iii) the amount of a qualified capital increase that will determine the conversion factor and (iv) the timing for each repayment and conversion scenario.
The convertible loan granted to Fluidda in January 2019 has a notional amount of K€
The Group previously granted a convertible loan to AM Flow in January 2020 with a notional amount of K€
Equity investment held in Essentium
The Group acquired an equity investment of K$
Leases – estimating the discount rate and probability of exercising extension options/termination options and purchase options
The Group cannot always determine the interest rate implicit in the lease contract and therefore, the Group has to estimate the incremental borrowing rate to measure certain lease liabilities such as buildings. The Group uses for buildings the property yield as reference to determine the incremental borrowing rate. For other assets, the Group generally uses the interest rate implicit in the lease contract or applies the incremental borrowing rate for a portfolio of similar assets. The incremental borrowing rate reflects what the Group “would have to pay”, which requires estimation when no observable rates are available or when they need to be adjusted to reflect the terms and conditions of the lease.
F-25
In addition, certain lease contracts may have extension options, termination options in case of property leases and/or purchase options in case of leases. The Group estimates whether it is reasonably certain or not, whether those options will be exercised or not, which impact the lease term in case of extension options and termination options and the period over which the lease assets are depreciated in case of purchase options.
4Business Combinations
Acquisitions in 2023
The Group did not effect any business combinations in the course of 2023.
Acquisitions in 2022
Materialise Link3D, Inc.
On April 9, 2021, the Group acquired an option to buy Link3D, Inc. (“Link3D”) On November 15, 2021, Materialise provided notice to Link3D of its intention to exercise the option. The acquisition was completed on January 4, 2022. This acquisition was realized by the Group’s U.S. subsidiary, Materialise USA, LLC by exercising our call option. As a result of this transaction, Materialise USA became the sole shareholder of Link3D. On January 4, 2022, the Group completed the acquisition and obtained control of Link3D. Link3D is an additive workflow and digital manufacturing software company. The Group acquired
The acquisition of Link3D is expected to strengthen and accelerate the creation of the Materialise software platform, particularly for companies that are scaling up their additive manufacturing operations to volume production. By integrating Link3D’s additive MES (Manufacturing Execution System) solution with the Materialise Magics software suite into a unified, cloud-based software platform, manufacturers will be able to run and continuously improve the most efficient, repeatable, automated and controlled processes to mass-produce identical or customized products. This process extends beyond the actual 3D printing operations and creates a closer alignment between 3D printing and conventional manufacturing, signaling the removal of the wall between both production environments.
On October 1, 2022, Link3D was merged into parent entity Materialise USA.
F-26
The fair value of the identifiable assets and liabilities at the date of acquisition was assessed at:
| Carrying |
|
| |||
value at | Fair value | |||||
acquisition | Fair value | at acquisition | ||||
in 000€ | date | adjustments | date | |||
Assets | ||||||
Brands and trademarks |
| — |
| |
| |
Software |
| — |
| |
| |
IT, Furniture & Vehicles |
| |
| — |
| |
Right-of-use assets |
| |
| — |
| |
Deferred tax assets |
| |
| |
| |
Trade receivables |
| |
| — |
| |
Other current assets |
| |
| — |
| |
Cash & cash equivalents |
| |
| — |
| |
Total Assets |
| |
| |
| |
Liabilities |
|
|
|
|
|
|
Long-term borrowings & Leases |
| ( |
| — |
| ( |
Other non-current liabilities |
| — |
| — |
| — |
Short-term borrowings & Leases |
| ( |
| — |
| ( |
Deferred tax liability |
| — |
| ( |
| ( |
Trade payables |
| ( |
| — |
| ( |
Payroll-related payables |
| ( |
| — |
| ( |
Deferred revenue |
| ( |
| |
| ( |
Other current liabilities |
| ( |
| — |
| ( |
Total Liabilities |
| ( |
| ( |
| ( |
Total identified assets and liabilities |
| ( |
| |
| |
Goodwill |
| — |
| |
| |
Acquisition price |
| — |
| — |
| |
The fair value of the identified assets and liabilities included in our consolidated financial statements at the acquisition date was K€
The goodwill recognized is primarily attributable to the trained and knowledgeable workforce and to the expected synergies that will be realized at the level of development, manufacturing and the existing customer base. The goodwill is not deductible for income tax purposes.
The accounting for the business combination resulted in fair values at date of acquisition of K€
The Link3D revenue included in the consolidated financial statement between acquisition date of January 4, 2022 and merger date October 1, 2022 amounted to K€
There are
F-27
Materialise Identify3D, Inc.
On September 1, 2022, the Group executed a share purchase agreement and acquired
With the acquisition of Identify3D the Group wants to address growing data security and integrity requirements and market interest, and to make CO-AM the most secure software platform for distributed manufacturing. This acquisition will allow manufacturers to secure the flow of digital parts and maintain a competitive advantage.
On December 31, 2022, Identify3D was merged into parent entity Materialise USA.
The fair value of the identifiable assets and liabilities at the date of acquisition was assessed at:
| Carrying |
|
| |||
value at | Fair value | |||||
acquisition | Fair value | at acquisition | ||||
in 000€ | date | adjustments | date | |||
Assets | ||||||
Brands and trademarks |
| — |
| |
| |
Software |
| — |
| |
| |
Deferred tax assets |
| |
| — |
| |
Cash & cash equivalents |
| |
| — |
| |
Total Assets |
| |
| |
| |
Liabilities |
|
|
|
|
|
|
Long-term borrowings |
| ( |
| — |
| ( |
Deferred tax liability |
| — |
| ( |
| ( |
Trade payables |
| ( |
| — |
| ( |
Payroll-related payables |
| ( |
| — |
| ( |
Total Liabilities |
| ( |
| ( |
| ( |
Total identified assets and liabilities |
| ( |
| |
| |
Goodwill |
| — |
| |
| |
Acquisition price |
| — |
| — |
| |
The fair value of the identified assets and liabilities included in our consolidated financial statements at the acquisition date was K€
The goodwill recognized is primarily attributable to the trained and knowledgeable workforce and to the expected synergies that will be realized at the level of development, manufacturing and the existing customer base. The goodwill is not deductible for income tax purposes.
The accounting for the business combination resulted in fair values at date of acquisition of K€
The amount of revenue included in the consolidated financial statement between acquisition date of September 1, 2022 and the merger date of December 31, 2022 was K€
There are
F-28
Acquisitions in 2021
The Group did not effect any business combinations in the course of 2021.
5Goodwill
The goodwill has been allocated to the cash generating units (“CGU”) as follows:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
CGU: MAT Software | | | ||||
CGU: e-Prototypy | | | ||||
CGU: ACTech | | | ||||
CGU: OrthoView | | | ||||
CGU: Engimplan | — | — | — | |||
CGU: Materialise Motion | — | | | |||
Total | | | ||||
The changes in the carrying value of the goodwill can be presented as follows for the years 2023, 2022 and 2021:
in 000€ |
| Gross |
| Impairment |
| Total |
At January 1, 2021 |
| |
| ( |
| |
Additions |
| — |
| — |
| — |
Impairment | — | ( | ( | |||
Currency translation |
| |
| — |
| |
At December 31, 2021 |
| |
| ( |
| |
Additions |
| |
| — |
| |
Impairment |
| — |
| — |
| — |
Currency translation |
| ( |
| — |
| ( |
At December 31, 2022 |
| |
| ( |
| |
Additions |
| — |
| — |
| — |
Impairment |
| — |
| ( |
| ( |
Currency translation |
| |
| — |
| |
At December 31, 2023 |
| |
| ( |
| |
The goodwill of MAT Software, Orthoview and e-Prototypy include respectively K€
The Group has performed an impairment test for all CGUs, estimating the Value-in-Use based on a discounted cash flow model with cash flows for the next
CGU: MAT Software
The goodwill allocated to the CGU MAT software relates to the goodwill from the acquisition of Cenat in 2015, the goodwill related to the acquisition of Marcam in 2011 (DE-3D Printing Software), the goodwill from the acquisition of Link3D in 2022 and the goodwill from the acquisition of Identify3D in 2022.
F-29
The impairment test is based on the discounted cash flows resulting from the CGU MAT Software, considering a period of
CGU e-Prototypy
The goodwill relates to the acquisition of the Polish entity e-Prototypy. The impairment test on the CGU e-Prototypy is based on the discounted cash flows considering a period of
CGU ACTECH
The impairment test on the CGU ACTech is based on the discounted cash flows, considering a period of
CGU Orthoview
The goodwill relates to the acquisition of Orthoview. The impairment test on the CGU Orthoview is based on the discounted cash flows considering a period of
The Orthoview business is integrated in the existing software business within our Materialise Medical segment. Synergies that are expected from joined product lines are not taken into account in the current impairment review as management believes that Orthoview can be considered a separate cash generating unit.
F-30
CGU Engimplan
The impairment test on the CGU Engimplan is based on the discounted cash flows, considering a period of
The key events that led to the impairment loss for the CGU Engimplan were related to a delay of business growth and to less advantage of synergies than initially foreseen.
A sensitivity analysis was performed to assess the impact of changes in the key assumptions used on the current estimated value-in-use and can be summarized as follows:
Sensitivity analysis Engimplan impairment |
| As of December 31,2023 | ||
Evolution of the | ||||
Change | value-in-use | |||
Relevant assumption | applied | (in 000€) | ||
WACC |
| + | % | ( |
WACC |
| - | % | |
Perpetual Growth |
| - | % | ( |
CGU Materialise Motion
The impairment test on the CGU Materialise Motion is based on the discounted cash flows, considering a period of
The key event that led to the impairment loss for the CGU Materialise Motion was a delay in business growth versus what was initially foreseen.
A sensitivity analysis was performed to assess the impact of changes in the key assumptions used on the current estimated value-in-use and can be summarized as follows:
Sensitivity analysis Materialise Motion impairment |
| As of December 31,2023 | ||
Evolution of the | ||||
Change | value-in-use | |||
Relevant assumption | applied | (in 000€) | ||
WACC |
| + | % | ( |
WACC |
| - | % | |
Perpetual Growth |
| - | % | ( |
F-31
6Intangible assets
The changes in the carrying value of the intangible assets can be presented as follows for the years 2023, 2022 and 2021:
Acquired | Developed | |||||||||
customers, | technology and | |||||||||
Patents and | technology and | software under | ||||||||
in 000€ |
| licenses |
| Software |
| order backlog |
| construction |
| Total |
Acquisition value |
|
|
|
|
| |||||
At January 1, 2021 |
| |
| |
| |
| |
| |
Additions |
| |
| |
| — |
| |
| |
Acquisition of a subsidiary |
| — |
| — |
| — |
| — |
| — |
Disposals |
| ( |
| ( |
| — |
| ( |
| ( |
Transfer between accounts |
| |
| |
| |
| ( |
| ( |
Currency translation |
| |
| |
| |
| — |
| |
Other |
| — |
| — |
| — |
| — |
| — |
At December 31, 2021 |
| |
| |
| |
| |
| |
Additions |
| |
| |
| — |
| |
| |
Acquisition of a subsidiary |
| |
| — |
| |
| — |
| |
Disposals |
| ( |
| ( |
| — |
| — |
| ( |
Transfer between accounts |
| |
| |
| — |
| ( |
| |
Currency translation |
| ( |
| |
| ( |
| |
| ( |
Other |
| — |
| — |
| — |
| — |
| — |
At December 31, 2022 |
| |
| |
| |
| |
| |
Additions |
| | | — | | | ||||
Acquisition of a subsidiary |
| — | — | — | — | — | ||||
Disposals |
| ( | ( | — | ( | ( | ||||
Transfer between accounts |
| | | — | ( | ( | ||||
Currency translation |
| | | | | | ||||
Other |
| — | — | — | — | — | ||||
At December 31, 2023 |
| | | | | |
F-32
Acquired | Developed | |||||||||
customers, | technology and | |||||||||
Patents and | technology and | software under | ||||||||
in 000€ |
| licenses |
| Software |
| order backlog |
| construction |
| Total |
Amortization & Impairments |
|
|
|
|
| |||||
At January 1, 2021 |
| ( |
| ( |
| ( |
| ( |
| ( |
Amortization charge for the year |
| ( |
| ( |
| ( |
| — |
| ( |
Impairments | — | ( | — | — | ( | |||||
Disposals |
| |
| |
| — |
| — |
| |
Transfer between accounts |
| ( |
| ( |
| ( |
| |
| ( |
Currency translation |
| ( |
| ( |
| ( |
| — |
| ( |
Other |
| — |
| ( |
| — |
| — |
| ( |
At December 31, 2021 |
| ( |
| ( |
| ( |
| ( |
| ( |
Amortization charge for the year |
| ( |
| ( |
| ( |
| — |
| ( |
Impairments |
| ( |
| ( |
| — |
| — |
| ( |
Disposals |
| |
| |
| — |
| — |
| |
Transfer between accounts |
| — |
| — |
| — |
| — |
| — |
Currency translation |
| |
| ( |
| |
| — |
| |
Other |
| — |
| — |
| — |
| — |
| — |
At December 31, 2022 |
| ( |
| ( |
| ( |
| ( |
| ( |
Amortization charge for the year |
| ( | ( | ( | — | ( | ||||
Impairments |
| — | — | ( | — | ( | ||||
Disposals |
| |
| |
| — |
| — |
| |
Transfer between accounts |
| — |
| — |
| — |
| — |
| — |
Currency translation |
| ( |
| ( |
| ( |
| — |
| ( |
Other |
| — |
| — |
| — |
| — |
| — |
At December 31, 2023 |
| ( | ( | ( | ( | ( | ||||
Net carrying value |
|
|
|
|
| |||||
At December 31, 2023 |
| | | | | | ||||
At December 31, 2022 |
| |
| |
| |
| |
| |
At December 31, 2021 |
| |
| |
| |
| |
| |
At January 1, 2021 |
| |
| |
| |
| |
| |
Patent and licenses include only the directly attributable external costs incurred in registering the patent and obtaining the license. Software relates to purchased software for internal use and in-house developed technology. The remaining amortization period is
The ‘Acquired customers, technology and other intangibles’ have been recognized as part of the acquisition of Materialise Motion, Engimplan, ACTech, E-Prototypy, OrthoView, Cenat, Link3D and Identify3D (see Note 4). At December 31, 2023, the remaining amortization period for the acquired customers is
The net book value of developed technology and software under construction at December 31, 2023 relates primarily to the internal digitalization program.
The total amortization charge for 2023 is K€
F-33
7Property, plant & equipment
The changes in the carrying value of the property, plant & equipment can be presented as follows for the year 2023, 2022 and 2021:
| Land and |
| Plant and |
| Right-of-use |
| Construction |
| ||
in 000€ | buildings | equipment | assets | in progress | Total | |||||
Acquisition value | ||||||||||
At January 1, 2021 |
| |
| |
| |
| |
| |
Additions |
| |
| |
| |
| |
| |
Disposals |
| — |
| ( |
| ( |
| ( |
| ( |
Transfers |
| |
| |
| ( |
| ( |
| |
Currency Translation |
| |
| |
| |
| |
| |
At December 31, 2021 |
| |
| |
| |
| |
| |
Additions |
| |
| |
| |
| |
| |
Acquired from business combinations |
| — |
| |
| |
| — |
| |
Disposals |
| ( |
| ( |
| ( |
| ( |
| ( |
Transfers |
| |
| |
| ( |
| ( |
| ( |
Currency Translation |
| |
| |
| |
| ( |
| |
At December 31, 2022 |
| | | | | | ||||
Additions |
| | | | | | ||||
Disposals |
| — | ( | ( | — | ( | ||||
Transfers |
| | | ( | ( | ( | ||||
Currency Translation |
| | | ( | ( | | ||||
At December 31, 2023 |
| | | | | | ||||
Depreciation |
| — |
| — |
| — |
| — |
| — |
At January 1, 2021 |
| ( |
| ( |
| ( |
| — |
| ( |
Depreciation charge for the year |
| ( |
| ( |
| ( |
| — |
| ( |
Disposals |
| — |
| |
| |
| — |
| |
Transfers | ( | ( | | — | ( | |||||
Currency Translation |
| ( |
| ( |
| ( |
| — |
| ( |
At December 31, 2021 |
| ( |
| ( |
| ( |
| — |
| ( |
Depreciation charge for the year |
| ( |
| ( |
| ( |
| — |
| ( |
Disposals |
| — |
| |
| |
| — |
| |
Transfers |
| — |
| — |
| — |
| — |
| — |
Currency Translation |
| ( |
| |
| |
| — |
| |
At December 31, 2022 |
| ( | ( | ( | — | ( | ||||
Depreciation charge for the year |
| ( | ( | ( | — | ( | ||||
Impairment | — | ( | — | — | ( | |||||
Disposals | — | | | — | | |||||
Transfers |
| — | ( | | — | | ||||
Currency Translation |
| ( | ( | | — | ( | ||||
At December 31, 2023 |
| ( | ( | ( | — | ( | ||||
Net book value |
|
|
|
|
|
|
|
|
|
|
At December 31, 2023 | | | | | | |||||
At December 31, 2022 | | | | | | |||||
At December 31, 2021 | | | | | | |||||
At January 1, 2021 | | | | | |
F-34
The investments in property, plant & equipment and right-of-use assets in 2023 amounted to K€
The Group realized a net gain on disposal of property, plant and equipment of K in 2023 (2022: a net loss of K€
Impairments of property, plant and equipment amounted to K€ (
Assets under construction
Per December 31, 2023 the main assets under construction were related to the extension and expansion of capacity in Germany for K€
F-35
The right of use assets can be presented as follows:
The carrying value of Right-of-Use assets at December 31, 2023 was K€
in 000€ |
| Buildings |
| Vehicles |
| Equipment |
| Total |
Acquisition value | ||||||||
At January 1, 2021 | | | | | ||||
Additions | | | | | ||||
Disposals | ( | ( | ( | ( | ||||
Currency Translation | | | | | ||||
Transfers | ( | ( | ( | ( | ||||
At December 31, 2021 |
| | | | | |||
Additions |
| | | | | |||
Acquired from business combinations |
| | — | — | | |||
Disposals |
| ( | ( | ( | ( | |||
Currency Translation |
| | | ( | | |||
Transfers |
| ( | ( | ( | ( | |||
At December 31, 2022 |
| | | | | |||
Additions |
| | | | | |||
Disposals |
| ( | ( | ( | ( | |||
Currency Translation |
| ( | | | ( | |||
Transfers |
| ( | ( | ( | ( | |||
At December 31, 2023 |
| | | | | |||
Depreciation |
| |||||||
At January 1, 2021 | ( | ( | ( | ( | ||||
Depreciation charge for the year | ( | ( | ( | ( | ||||
Disposals | | | | | ||||
Currency Translation | ( | ( | | ( | ||||
Transfers | | | | | ||||
At December 31, 2021 |
| ( | ( | ( | ( | |||
Depreciation charge for the year |
| ( | ( | ( | ( | |||
Disposals |
| | | | | |||
Currency Translation |
| | ( | | | |||
Transfers |
| | | | | |||
At December 31, 2022 |
| ( | ( | ( | ( | |||
Depreciation charge for the year |
| ( | ( | ( | ( | |||
Disposals |
| | | | | |||
Currency Translation |
| | ( | ( | | |||
Transfers |
| | | | | |||
At December 31, 2023 |
| ( | ( | ( | ( | |||
Net book value |
| |||||||
At December 31, 2023 | | | | | ||||
At January 1, 2023 | | | | |
The following amounts related to leases are recognized in profit & loss
As of December 31, | ||||||
(in 000€) |
| 2023 |
| 2022 |
| 2021 |
Depreciation expense |
| ( | ( | ( | ||
Interest expense on lease liabilities |
| ( | | ( | ||
Expenses related to short-term leases/ low-value assets/ variable lease payments |
| ( | | ( | ||
F-36
The Group has negotiated several contracts with extension and termination options because of common practice in the country or for the asset. Management has exercised significant judgments in determining whether these extension and termination options are reasonably certain to be exercised. The potential future cash flows beyond the period following the exercise of the extension and termination option that are not included in the lease term are presented in the following table:
As of December 31, | ||||||
(in 000€) |
| 2023 |
| 2022 |
| 2021 |
Potential (non-discounted) cash flows for terminations options that are not reasonably certain to be exercised: |
| | | | ||
Potential (non-discounted) cash flows for extensions options that are reasonably certain to be exercised |
| | | | ||
Pledges
Land and buildings (including buildings under construction) with a carrying amount of K€
8Investments in joint ventures
Materialise had
9Inventories and contracts in progress
Inventories and contracts in progress include the following:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Raw materials |
| |
| |
| |
Work in progress |
| |
| |
| |
Finished goods |
| |
| |
| |
Contracts in progress |
| |
| |
| |
Total inventories and contracts in progress |
| |
| |
| |
Inventory written-off on the balance sheet amounted to K€
The Group has contracts in progress and advances from customers. The total costs incurred is K€
10Other assets
Other non-current assets
Other non-current assets include the following:
Investments in convertible loans | As of December 31, | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Convertible loan |
| |
| |
| |
Total |
| |
| |
| |
F-37
The Group granted a convertible loan to Fluidda in January 2019, with a notional amount of K€
Investments in non-listed equity instruments | As of December 31, | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Non-listed equity investments |
| — |
| |
| |
Total |
| — |
| |
| |
At December 31, 2023, the Group remeasured the fair value of its investment in AM Danube BV (holding company for AM Flow Holding BV) to zero, recognizing a K€
At December 31, 2022, the Group remeasured the fair value of its investment in African Drive NV to zero, recognizing a K€
At December 31, 2021, the Group remeasured the fair value of its equity investment in Essentium, Inc. to zero, recognizing a K€
Other non-current assets | As of December 31, | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Tax credits |
| |
| |
| |
Guarantees and deposits |
| |
| |
| |
Loan to Link3D incl capitalized interest |
| — |
| — |
| |
LT deferred charges |
| — |
| — |
| |
Other |
| |
| |
| |
Total |
| |
| |
| |
The non-current tax credits mainly relate to Belgian R&D tax credits, recoverable between 2025 and 2029.
Other current assets
Other current assets include the following:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Deferred charges |
| |
| |
| |
Tax credits |
| |
| |
| |
Accrued income |
| |
| |
| |
Other tax receivables |
| |
| |
| |
Grants |
| |
| |
| |
Other non-trade receivables |
| |
| |
| |
Derivatives | | | | |||
Total other current assets |
| |
| |
| |
The other tax receivables included Value Added Tax (VAT) receivables and corporate tax receivables.
11Trade receivables
The trade receivables include the following:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Trade receivables | | | | |||
Allowance for doubtful accounts |
| ( |
| ( |
| ( |
Total |
| |
| |
| |
F-38
Trade receivables are non-interest bearing and are generally on payment terms of to
As of December 31, 2023, trade receivables of an initial value of K€
in 000€ |
| |
At January 1, 2021 |
| ( |
Addition |
| ( |
Usage |
| |
Reversal |
| |
At December 31, 2021 |
| ( |
Addition |
| ( |
Usage |
| |
Reversal |
| |
At December 31, 2022 |
| ( |
Addition |
| ( |
Usage |
| |
Reversal |
| |
At December 31, 2023 |
| ( |
12Cash and cash equivalents
Cash and cash equivalents include the following:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Cash at bank | | | | |||
Cash equivalents |
| |
| |
| |
Total |
| |
| |
| |
For the year ended December 31, 2023, cash at banks earned a net interest income of €
There were
13Equity
Share capital
The share capital of the parent company Materialise NV consists of
| Total |
| Total |
| ||
number of | shareholders’ | Total | ||||
in 000€, except share data | ordinary shares | capital | share premium | |||
Outstanding at January 1, 2021 |
| |
| |
| |
Capital increase through exercise of warrants |
| |
| |
| |
Capital increase through exercise of convertible bonds | | | | |||
Equity settled share-based payments expense | — | — | | |||
Outstanding on December 31, 2021 |
| |
| |
| |
Capital increase through exercise of warrants |
| |
| ( |
| |
Outstanding on December 31, 2022 |
| |
| |
| |
Equity settled share-based payments expense |
| |
| |
| |
Outstanding on December 31, 2023 |
| |
| |
| |
F-39
Share premium
In Belgium, the portion of the capital increase in excess of par value is typically allocated to share premium.
The carrying value of the share premium is K€
The change in 2022 is the result of the capital increase via exercise of warrants of K€
The change in 2021 is the result of the share-based payments expense of K€
Other reserves
The nature and purpose of the other reserves is as follows:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Legal reserve |
| |
| |
| |
Other reserves |
| |
| |
| |
Equity-settled share-based payment expense |
| |
| |
| |
Other Comprehensive Income (loss) |
| ( |
| ( |
| ( |
Other reserves |
| ( |
| ( |
| ( |
Based on the statutory result and after final result allocation approved by the annual shareholders meeting the legal reserve is increased by reserving
The Group did not pay any dividend during 2023, 2022 and 2021.
Other comprehensive loss
Other comprehensive loss consists of the following:
| Currency |
| Fair value |
| Total OCI | |
Translation | adjustment | attributable to | ||||
Differences | equity | the | ||||
in ’000€ | & Other | investments | shareholder | |||
At January 1, 2021 |
| ( |
| |
| ( |
Currency translation impact | | — | | |||
Fair value adjustment | — | ( | ( | |||
At December 31, 2021 |
| ( |
| ( |
| ( |
Currency translation impact |
| ( |
| — |
| ( |
Fair value adjustment |
| — |
| ( |
| ( |
At December 31, 2022 |
| ( |
| ( |
| ( |
Currency translation impact |
| |
| — |
| |
Fair value adjustment |
| — |
| ( |
| ( |
At December 31, 2023 |
| ( |
| ( |
| ( |
F-40
Non-controlling interest
As of June 22, 2021, the Group, together with Zhenyuan (Tianjin) Medical Appliances Technology Co., Ltd., incorporated a new subsidiary with the name Tianjin Zhenyuan Materialise Medical Technology Limited Company. This entity will be responsible for all regulatory requirements regarding the Materialise Mimics Enlight Lung Software on the Chinese market. Both Materialise and Zhenyuan will work on development and distribution, in a collaborating manner. Materialise holds
14Share-based payment plans
Share-based payment plans of the parent
The changes of the year for the warrant plans are as follows:
| 2023 |
| 2022 |
| 2021 | |
Outstanding at January 1 |
| |
| |
| |
Granted |
| |
| — |
| — |
Forfeited / Cancelled |
| ( |
| ( |
| ( |
Exercised |
| — |
| ( |
| ( |
Outstanding at December 31 |
| |
| |
| |
Exercisable at December 31 |
| |
| |
| |
The Group’s share-based payment plans are all equity-settled except for the IPO warrants that have been granted to certain employees in certain countries due to legal requirements which are cash-settled. The outstanding amount includes stock appreciation rights (“SARs”) issued under cash-settled share-based payment plans.
In all outstanding warrant plans
Equity-settled share-based payment plans
The Group has several plans in place which each have slightly different characteristics as described below.
IPO warrant plan
Each warrant gives the right to the holder to
The Group granted
The status of the IPO warrant plan at December 31 is as follows:
| 2023 |
| 2022 |
| 2021 | |
Outstanding at January 1 |
| |
| |
| |
Granted |
| — |
| — |
| — |
Forfeited / Cancelled |
| ( |
| ( |
| ( |
Exercised |
| — |
| ( |
| ( |
Outstanding at December 31 |
| |
| |
| |
Exercisable at December 31 |
| |
| |
| |
F-41
Warrant plan 2015
The board of directors decided on December 18, 2015 on a new plan (“2015 warrant plan”) by which it can grant up to
The Group granted
The status of the 2015 warrant plan at December 31 is as follows:
| 2023 |
| 2022 |
| 2021 | |
Outstanding at January 1 |
| | | | ||
Granted |
| — | — | — | ||
Forfeited / Cancelled |
| — | — | ( | ||
Exercised |
| — | ( | ( | ||
Outstanding at December 31 |
| | | | ||
Exercisable at December 31 |
| | | |
Warrant plan 2023
The board of directors decided on September 25, 2023 on a new plan (“2023 warrant plan”) by which it can grant up to
The Group granted
The status of the 2023 warrant plan at December 31 is as follows:
| 2023 |
| 2022 |
| 2021 | |
Outstanding at January 1 |
| — |
| — |
| — |
Granted |
| |
| — |
| — |
Forfeited / Cancelled |
| — |
| — |
| — |
Exercised |
| — |
| — |
| — |
Outstanding at December 31 |
| |
| — |
| — |
Exercisable at December 31 |
| — |
| — |
| — |
Fair value
The fair value of the warrants is estimated at the grant date using the Black-Scholes option pricing model, taking into account the terms and conditions upon which the warrants were granted.
F-42
The following table provides the input to the Black-Scholes model for the IPO warrant plan, 2015 warrant plan and the 2023 warrant plan:
| 2023 |
| 2023 |
| 2015 |
| 2015 |
| IPO 2014 |
| IPO 2014 |
| |
(Nov) | (Oct) | (Sept 16) | (Nov) | (Nov) | (June) | ||||||||
Return dividend |
| | % | | % | | % | | % | | % | | % |
Expected volatility |
| | % | | % | | % | | % | | % | | % |
Risk-free interest rate |
| | % | | % | | % | | % | | % | | % |
Expected life |
| |
| |
| |
| |
| |
| | |
Exercise price (in €) |
| |
| |
| |
| |
| |
| | |
Stock price (in €) |
| |
| |
| |
| |
| |
| | |
Fair value warrant (in €) |
| |
| |
| |
| |
| |
| |
The above input for the Black-Scholes model have been determined based on the following:
| ● | the dividend return is estimated by reference to the historical dividend payments of the Group. Currently, this is estimated to be |
| ● | expected volatility is estimated based on the average annualized volatility of the Group’s stock (until September 2016: of a number of quoted peers in the 3D printing industry and the volatility of the Group’s stock); |
| ● | risk-free interest rate is based on the interest rate applicable for the 10Y Belgian government bond at the grant date; |
| ● | estimated life of the warrant is determined to be until the first exercise period which is typically the month after vesting; and |
| ● | fair value of the shares is determined based on the share price of the Group on Nasdaq at the date of valuation. For the grants prior to the initial public offering, the fair value of the shares was estimated based on a discounted cash flow model with 3-year cash flow projections and a multiple of EBITDA determined based on a number of quoted peers in the 3D printing industry. |
The expense arising from share-based payment transactions for the warrant plans mentioned above was K€
The weighted average fair value for the warrants outstanding at the end of 2023 was €
Cash-settled share-based payment plans
The Group has issued
The status of this plan is as follows:
| 2023 |
| 2022 |
| 2021 | |
Outstanding at January 1 |
| |
| |
| |
Granted |
| — |
| — |
| — |
Forfeited / Cancelled |
| — |
| ( |
| ( |
Exercised |
| — |
| — |
| ( |
Outstanding at December 31 |
| |
| |
| |
Exercisable at December 31 |
| |
| |
| |
The SAR plan grants the bearer the right to a cash payment equal to the difference between the exercise price and the stock price at the exercise date. This plan is considered a cash settled share based payment and is as such recorded as a liability (see Note 16).
F-43
The SARs have a contractual term of
The fair value of the SAR is estimated at each reporting date using the Black-Scholes option pricing model, taking into account the terms and conditions upon which the warrants were granted.
The following table lists the input used for the Black-Scholes model:
| 2023 |
| 2022 |
| 2021 |
| |
Return dividend |
| | % | | % | | % |
Expected volatility |
| | % | | % | | % |
Risk-free interest rate |
| | % | | % | | % |
Expected life |
| |
| |
| | |
Exercise price (in €) |
| |
| |
| | |
Stock price (in €) |
| |
| |
| | |
Fair value SAR (in €) |
| |
| |
| |
The expense arising from share-based payment transactions for the SARs plan was K€
Share-based payment plans of RapidFit+
The subsidiary RapidFit+ has issued a warrant plan on August 23, 2013 where a maximum of
The changes for the year for the RapidFit+ warrant plan are as follows:
| 2023 |
| 2022 |
| 2021 | |
Outstanding at January 1 |
| |
| |
| |
Granted |
| — |
| — |
| — |
Forfeited / Cancelled |
| ( |
| ( |
| — |
Exercised |
| — |
| — |
| — |
Outstanding at December 31 |
| — |
| |
| |
Exercisable at December 31 |
| — |
| |
| |
The following table lists the input to the Black-Scholes model for the RapidFit+ warrant plan:
| 2014 |
| |
Return dividend |
| | % |
Expected volatility |
| | % |
Risk-free interest rate |
| | % |
Expected life |
| | |
Exercise price |
| | |
Fair value warrant |
| |
The expense arising from share-based payment transactions for RapidFit+ warrant plan was K€
F-44
15Loans and borrowings
The loans and borrowings include the following:
As of December 31 | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
K€50,000 KBC credit facility | — | — | — | |||
K€35,000 EIB bank loan |
| |
| |
| |
K€28,000 acquisition bank loan |
| |
| |
| |
K€17,700 secured bank loans |
| |
| |
| |
K€12,300 bank loans ACTech |
| |
| |
| |
K€5,000 other facility loan |
| |
| |
| |
Bank investment loans - top 20 outstanding |
| |
| |
| |
Bank investment loans - other |
| — |
| |
| |
Lease liabilities |
| |
| |
| |
Related party loan |
| |
| |
| |
Total loans and borrowings |
| |
| |
| |
Current |
| |
| |
| |
Non-Current |
| |
| |
| |
K€
In October 2022 the Group entered into a credit facility agreement with KBC which allows for a €
Reservation cost for all
K€
On December 20, 2017 the Group entered into a finance contract with the European Investment Bank, or EIB, to finance future research and development programs. As part of a first tranche, an amount of K€
On June 29, 2020, the European Investment Bank temporarily waived the compliance obligation of the covenants “Total gross Debt to Adjusted EBITDA” (until December 31, 2022), and “Adjusted EBITDA to Net financial charges” (until 31 December 2020) under the condition that the covenant “Total net debt to Adjusted EBITDA” will be met for the period. In addition, the European Investment Bank agreed not to recalculate the interest rate until January 3, 2022 for the first tranche and until January 17, 2022 for the second tranche. Finally, the European Investment Bank waived “the subsidiary financial indebtedness” covenant for the calculation period ending on June 30, 2020. For the periods thereafter this covenant has been eased. These covenants were waived in order to allow the Group to continue investing in its growth programs, even under stressed COVID-19 scenarios. At December 31, 2023, The Group was in compliance with all debt covenants.
K€
This bank loan has been concluded in October 2017 to finance the acquisition of ACTech. The loan includes a portion of K€
F-45
K€
The K€
K€
In March 2018,
K€
This facility loan was contracted in 2012 for the construction of Leuven office and production facilities. The balance of this loan amounts to K€
Miscellaneous investment loans
The 20 largest of these loans outstanding as of December 31, 2023 amount to a balance of K€
K€
The Group has several lease obligations mainly with financial institutions and related to the financing of buildings and various other items of plant and equipment such as 3D printers. As of December 31, 2023 the balance of these lease agreements amounts to K€
The total cash outflow from the lease liabilities amounts to K€
Related party loan
Lunebeke NV, a related party of the Group as discussed in Note 26, has granted the Group a loan of K€
Changes of liabilities for financing activities:
The following table presents the changes of the liabilities for financing activities:
For the year ended December 31 | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
At January 1, | | | | |||
Repayment of loans & borrowings |
| ( |
| ( |
| ( |
New leases |
| |
| |
| |
Repayment of leases |
| ( |
| ( |
| ( |
Loans acquired from business combination |
| — |
| |
| — |
Net foreign exchange movements |
| ( |
| ( |
| ( |
At December 31, |
| |
| |
| |
F-46
16Other non-current liabilities
The other non-current liabilities consist of the following:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Advances received on contracts |
| |
| |
| |
Provisions |
| |
| |
| |
Other |
| |
| |
| |
Total |
| |
| |
| |
Provisions mainly relate to retention bonuses for our employees.
The advances received on contracts as of December 31, 2021 related to advances received from a customer in the context of a long term contract for medical devices.
The other amount relates to a commitment for a multi-year license contract.
In Belgium, the Group contributes to a Sector Plan for eligible employees and to a “Branch 21” pension plan for a limited group of management staff. Under both plans, the Group pays contributions expressed as a percentage of a reference salary. These plans are administered by third party insurance companies and are not material to the consolidated financial statements.
17Tax payables
The tax payables amount to K€
18Deferred income
Deferred income consists of the following:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Deferred maintenance and license revenue |
| |
| |
| |
Deferred (project) fees |
| |
| |
| |
Deferred government grants |
| |
| |
| |
Total |
| |
| |
| |
current |
| |
| |
| |
non-current |
| |
| |
| |
The deferred maintenance and license revenue consists of maintenance and license fees paid up-front which are deferred and recognized in earnings over the maintenance period or the duration of the license, respectively. Deferred maintenance and license revenue grew to K€
We refer to Note 22.1.2 for more detail on the contract liabilities.
F-47
19Other current liabilities
Other current liabilities include the following:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Payroll-related liabilities | | | | |||
Non-income tax payables |
| |
| |
| |
Accrued charges |
| |
| |
| |
Advances received |
| |
| |
| |
Derivatives |
| |
| |
| |
Cash settled share-based payment plan |
| |
| |
| |
Other current liabilities |
| |
| |
| |
Total |
| |
| |
| |
The non-income tax payables mainly relate to VAT payables and payroll taxes.
20Fair value
Financial assets
The carrying value and fair value of the financial assets as of December 31, 2023, 2022 and 2021 are as follows:
Carrying value | Fair value | |||||||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
| 2023 |
| 2022 |
| 2021 |
Financial assets | ||||||||||||
Financial assets measured at amortized cost | ||||||||||||
Trade receivables (current) |
| |
| |
| |
| |
| |
| |
Other financial assets (non-current) |
| |
| |
| |
| |
| |
| |
Other current non-trade receivables |
| |
| |
| |
| |
| |
| |
Cash & cash equivalents |
| |
| |
| |
| |
| |
| |
Total financial assets measured at amortized cost |
| |
| |
| |
| |
| |
| |
Financial assets at fair value through profit or loss | ||||||||||||
Derivatives |
| |
| |
| | ||||||
Convertible loan |
| |
| |
| | ||||||
Total financial assets measured at fair value through profit and loss |
| |
| |
| | ||||||
Financial assets at fair value through OCI | ||||||||||||
Non-listed equity investments |
| — |
| |
| | ||||||
Total financial assets at fair value through OCI |
| — |
| |
| | ||||||
The fair value of the financial assets has been determined on the basis of the following methods and assumptions:
| ● | the carrying value of the cash and cash equivalents and the current receivables approximate their fair value due to their short term character; |
| ● | the fair value of the derivatives has been determined based on a mark-to-market analysis prepared by the bank based on observable market inputs (level 2 inputs); |
| ● | other current non-trade receivables are being evaluated on the basis of their credit risk and interest rate. Their fair value is not different from their carrying value on December 31, 2023, 2022 and 2021 |
| ● | other non-current financial assets are being evaluated on the basis of their credit risk and interest rate which are considered as level 2 inputs. Their fair value is not considered different from their carrying value given the related interest rate is revised on a regular basis. |
F-48
| ● | for the non-listed equity investment in AM Flow, as of December 31, 2023, Materialise recorded a remeasurement of fair value to |
| ● | for the non-listed equity investment in Essentium, as of December 31, 2021, Materialise recorded a remeasurement of fair value to |
| ● | the convertible loan granted to Fluidda is measured at fair value. As of December 31, 2023, management determined the fair value based upon level 3 inputs as follows: |
| o | The Group determined that the fair value of the convertible loan as of December 31, 2023 amounted to K€ |
In assessing the fair value, the Group has made significant estimates with regard to the discount rate, the probability of each repayment and conversion scenario and related timing, the amount of the qualified capital increase. Changes in the assumptions may lead to a significant increase/decrease in the fair value of the convertible loan. A increase/decrease in the applied discount rate for Fluidda by
Financial liabilities:
The carrying value and fair value of the financial liabilities as of December 31, 2023, 2022 and 2021 can be presented as follows:
Carrying value | Fair value | |||||||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
| 2023 |
| 2022 |
| 2021 |
Financial liabilities measured at amortized cost | ||||||||||||
Loans & Borrowings including lease liabilities | | |||||||||||
Trade payables | | |||||||||||
Other liabilities excl. written put option on NCI | | | ||||||||||
Total financial liabilities measured at amortized cost | | | ||||||||||
Financial liabilities measured at fair value | ||||||||||||
Cash settled share based payments |
| |
| |
| |
| |||||
Derivatives |
| |
| |
| |
| |||||
Total financial liabilities measured at fair value | | | | |||||||||
Total non-current |
| |
| |
| |
| |||||
Total current |
| |
| |
| |
| |||||
The fair value of the financial liabilities has been determined on the basis of the following methods and assumptions:
| ● | The carrying value of current liabilities approximates their fair value due to the short term character of these instruments; |
| ● | Loans and borrowings are evaluated based on their interest rates and maturity date. Most interest bearing debts have fixed interest rates and their fair value is subject to changes in interest rates and individual creditworthiness; |
| ● | The fair value of the derivatives has been determined based on a mark-to-market analysis prepared by the bank based on observable market inputs (level 2 inputs); |
| ● | The fair value of the written put option on non-controlling interest has been determined based on the present value of the redemption amount (level 3 inputs); |
| ● | The fair value of the cash-settled share based payments has been determined based on a Black-Scholes model using inputs that are level 1 (stock-price and risk-free interest rate) as well as level 2 (e.g. volatility). We refer to Note 14. |
F-49
Fair value hierarchy
The Group uses the following hierarchy for determining and disclosing the fair value of financial instruments by valuation technique:
| ● | Level 1: quoted (unadjusted) prices in active markets for identical assets and liabilities; |
| ● | Level 2: other techniques for which all inputs which have a significant effect on the recorded fair value are observable, either directly or indirectly; and |
| ● | Level 3: techniques which use inputs that have a significant effect on the recorded fair value that are not based on observable market data. |
Fair value hierarchy 3 evolution
Convertible Loans Ditto & Fluidda | Fair Value Evolution | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
As of 1 January, |
| |
| |
| |
Addition |
| |
| |
| |
Remeasurement |
| |
| ( |
| |
Capitalized interest |
| |
| |
| |
Reimbursement Ditto convertible loan |
| |
| |
| ( |
As of 31 December, |
| |
| |
| |
Written Put Option on NCI RapidFit+ | Fair Value Evolution | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
As of 1 January, |
| |
| |
| |
Remeasurement |
| |
| |
| |
Payout put-option PMV |
| |
| |
| ( |
As of 31 December, |
| |
| |
| |
21Segment information
For management purposes, the Group is organized into segments based on their products, services and industry and has the following three reportable segments:
| ● | The Materialise Medical segment, which develops and delivers medical software solutions, medical devices and other related products and services; |
| ● | The Materialise Manufacturing segment, which delivers 3D printed products and related services; and |
| ● | The Materialise Software segment, which develops and delivers additive manufacturing software solutions and related services. |
The measurement principles used by the Group in preparing this segment reporting are also the basis for segment performance assessment and are in conformity with IFRS. The Chief Executive Officer of the Group acts as the chief operating decision maker. As a performance indicator, the chief operating decision maker controls the performance by the Group’s revenue and Adjusted EBITDA.
F-50
The following table summarizes the segment reporting for each of the reportable periods ending December 31. Corporate research and development, headquarters’ function, financing and income taxes are managed on a Group basis and are not allocated to operating segments. As management’s controlling instrument is mainly revenue-based, the reporting information does not include assets and liabilities by segment and is as such not available per segment.
| Materialise |
| Materialise |
| Materialise |
| Total |
|
|
| |||
in 000€ | Software | Medical | Manufacturing | segments | Unallocated | Consolidated |
| ||||||
For the year ended December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
| ||
Revenues | |
| |
| |
| | | | ||||
Segment Adjusted EBITDA | |
| |
| |
| | ( | | ||||
Segment Adjusted EBITDA % | | % | | % | | % | | % | | % | |||
For the year ended December 31, 2022 |
|
|
| ||||||||||
Revenues | |
| |
| |
| | | | ||||
Segment Adjusted EBITDA | |
| |
| |
| | ( | | ||||
Segment Adjusted EBITDA % | | % | | % | | % | | % | | % | |||
For the year ended December 31, 2021 |
|
|
| ||||||||||
Revenues | |
| |
| |
| | — | | ||||
Segment Adjusted EBITDA | |
| |
| |
| | ( | | ||||
Segment Adjusted EBITDA % | | % | | % | | % | | % | | % |
The segment Adjusted EBITDA is reconciled with the consolidated net profit (loss) for the year as follows:
For the year ended December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Net profit (loss) for the year |
| |
| ( |
| |
Share in loss of joint venture |
| |
| |
| |
Income taxes |
| |
| |
| |
Financial income |
| ( |
| ( |
| ( |
Financial expenses |
| |
| |
| |
Operating (loss)/ profit | |
| ( |
| | |
Impairments |
| |
| |
| |
Other operating income (expense) |
| ( |
| ( |
| ( |
Corporate headquarter costs |
| |
| |
| |
Corporate research and development |
| |
| |
| |
Depreciation, amortization and impairment |
| |
| |
| |
Segment Adjusted EBITDA |
| |
| |
| |
The Group has 1 individual customer that represents sales larger than
Entity-wide disclosures.
The revenue by geographical area is as follows:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
United States of America |
| |
| |
| |
Americas other than USA |
| |
| |
| |
Belgium |
| |
| |
| |
Germany |
| |
| |
| |
France |
| |
| |
| |
Switzerland |
| |
| |
| |
United Kingdom |
| |
| |
| |
Italy |
| |
| |
| |
Netherlands |
| |
| |
| |
Other Europe |
| |
| |
| |
Asia Pacific |
| |
| |
| |
Total |
| |
| |
| |
F-51
The total revenue realized in the country of domicile (Belgium) in 2023 amounts to K€
The total non-current assets, other than financial instruments and deferred tax assets, by geographical area are as follows:
As of December 31, | ||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
United States of America (USA) |
| |
| |
| |
Americas other than USA |
| |
| |
| |
Belgium |
| |
| |
| |
Germany |
| |
| |
| |
Poland |
| |
| |
| |
Rest of Europe |
| |
| |
| |
Asia-Pacific |
| |
| |
| |
Total |
| |
| |
| |
The totals of the above table include goodwill, intangible assets, property, plant & equipment and Right-of-Use Assets as disclosed in the consolidated statements of financial position
22Income and expenses
22.1 Revenue
22.1.1 Disaggregated revenue information
For the year ended December 31, 2023 | ||||||||||||
| Materialise |
| Materialise |
| Materialise |
| Total |
|
| |||
in 000€ | Software | Medical | Manufacturing | segments | Unallocated | Consolidated | ||||||
Geographical markets |
|
|
|
|
|
|
|
|
|
|
|
|
United States of America (USA) |
| | | | | — | | |||||
Americas other than USA |
| | | | | — | | |||||
Europe (without Belgium) & Africa |
| | | | | — | | |||||
Belgium |
| | | | | — | | |||||
Asia Pacific |
| | | | | — | | |||||
Total revenue from contracts with customers |
| | | | | — | | |||||
Type of goods or service |
| |||||||||||
Software revenue (non-medical) |
| | — | — | | — | | |||||
Software revenue (medical) |
| — | | — | | — | | |||||
Medical devices and services |
| — | | — | | — | | |||||
Manufacturing |
| — | — | | | — | | |||||
Other |
| — | — | — | — | — | — | |||||
Total revenue from contracts with customers |
| | | | | — | | |||||
Timing of revenue recognition |
| |||||||||||
Goods/Services transferred at a point in time |
| | | | | — | | |||||
Goods/Services transferred over time |
| | | | | — | | |||||
Total revenue from contracts with customers |
| | | | | — | | |||||
F-52
For the year ended December 31, 2022 | ||||||||||||
| Materialise |
| Materialise |
| Materialise |
| Total |
|
| |||
in 000€ | Software | Medical | Manufacturing | segments | Unallocated | Consolidated | ||||||
Geographical markets |
|
|
|
|
|
|
|
|
|
|
|
|
United States of America (USA) |
| | | | | — | | |||||
Americas other than USA |
| | | | | — | | |||||
Europe (without Belgium) & Africa |
| | | | | — | | |||||
Belgium |
| | | | | — | | |||||
Asia Pacific |
| | | | | — | | |||||
Total revenue from contracts with customers |
| | | | | — | | |||||
Type of goods or service |
| |||||||||||
Software revenue (non-medical) |
| | — | — | | — | | |||||
Software revenue (medical) |
| — | | — | | — | | |||||
Medical devices and services |
| — | | — | | — | | |||||
Manufacturing |
| — | — | | | — | | |||||
Other |
| — | — | — | — | — | — | |||||
Total revenue from contracts with customers |
| | | | | — | | |||||
Timing of revenue recognition |
| |||||||||||
Goods/Services transferred at a point in time |
| | | | | — | | |||||
Goods/Services transferred over time |
| | | | | — | | |||||
Total revenue from contracts with customers |
| | | | | — | | |||||
The revenue per type of good or service including the previous years is as follows:
| For the year ended December 31 | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Software revenue (non-medical) |
| |
| |
| |
Software revenue (medical) |
| |
| |
| |
Medical devices and services |
| |
| |
| |
Manufacturing |
| |
| |
| |
Total |
| |
| |
| |
22.1.2 Contract balances
The following table provides information about receivables, contracts in progress (contract assets) and deferred income (contract liabilities) from contracts with customers.
| As of December 31, | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Trade receivables, included in ‘trade and other receivables’ |
| |
| | | |
Contract assets / contracts in progress |
| |
| | | |
Contract liabilities / deferred income / advances received on contracts |
| |
| | | |
We refer to Note 18 for a detail of the deferred income. Note 18 includes a split of the deferred income in current and non-current. Non-current deferred income, representing mainly maintenance contracts with terms more than
The relation between the timing of satisfaction of the performance obligations and the timing of billing resulting in contract assets and liabilities is as follows:
| ● | Maintenance services: maintenance services are typically billed at the beginning of the maintenance period resulting in deferred income that is recognized on a straightline basis over the maintenance period. |
| ● | Software licenses: certain software licenses may have been billed prior to the delivery of the software key or time-based software licenses may have been billed up-front resulting in a deferred income balance. |
F-53
| ● | Certain agreements in the medical segment include up-front fees such as step-in fees or milestone payments which are billed at inception of the contract but which are allocated to performance obligations which are satisfied at a later time in the contract term or which have not been recognized considering the revenue constraint (i.e. may have to be credited when customer achieves certain volume targets). In addition, certain contracts include prepaid fees for volume “Plan Only” purchases for which the purchased services are only delivered during a one year period. Those fees result in deferred income which are recognized as revenue when services/products are delivered and revenue is not constrainted. |
| ● | Certain development services are satisfied while the services can only billed at certain pre-defined points in time or when the services are fully satisfied resulting in contracts in progress / contract assets. |
22.2 Cost of sales
Cost of sales includes the following selected information:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Purchase of goods and services |
| ( |
| ( |
| ( |
Amortization and depreciation |
| ( |
| ( |
| ( |
Payroll expenses |
| ( |
| ( |
| ( |
Work in Progress |
| |
| |
| |
Total |
| ( |
| ( |
| ( |
22.3 Research and development expenses
Research and development expenses include the following selected information:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Purchase of goods and services |
| ( |
| ( |
| ( |
Amortization and depreciation |
| ( |
| ( |
| ( |
Payroll expenses |
| ( |
| ( |
| ( |
Other |
| |
| — |
| — |
Total |
| ( |
| ( |
| ( |
22.4 Sales and marketing expenses
Sales and marketing expenses include the following selected information:
| For the year ended December 31 | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Purchase of goods and services |
| ( |
| ( |
| ( |
Amortization and depreciation |
| ( |
| ( |
| ( |
Payroll expenses |
| ( |
| ( |
| ( |
Total |
| ( |
| ( |
| ( |
22.5 General and administrative expenses
General and administrative expenses include the following selected information:
| For the year ended December 31 | |||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
Purchase of goods and services |
| ( |
| ( |
| ( |
Amortization and depreciation |
| ( |
| ( |
| ( |
Payroll expenses |
| ( |
| ( |
| ( |
Total |
| ( |
| ( |
| ( |
F-54
22.6 Net other operating income
The net other operating income can be detailed as follows:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Government grants |
| |
| |
| |
Amortization intangibles purchase price allocation |
| ( |
| ( |
| ( |
Allowance for doubtful debtors |
| ( |
| |
| ( |
Capitalized expenses (asset construction) |
| — |
| — |
| |
Tax credits |
| |
| |
| |
Arbitration settlement | ( | — | — | |||
Impairment of intangible assets (Note 6) and PP&E (Note 7) | ( | — | ( | |||
Impairment of goodwill (Note 5) | ( | — | — | |||
Indemnity fee from commercial agreement | — | | — | |||
COVID support Germany | — | | — | |||
Other |
| |
| |
| |
Total |
| ( |
| |
| |
The Company has received government grants from the Belgian federal and regional governments and from the European Community in the forms of grants linked to certain of its research and development programs and reduced payroll taxes.
In May 2023, the Belgian Center for Arbitration and Mediation issued a decision in the arbitration proceedings filed by ZimmerBiomet against Materialise, pursuant to which we were ordered to pay an amount of €
22.7 Payroll expenses
The following table shows the breakdown of payroll expenses for 2023, 2022 and 2021:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Short-term employee benefits |
| ( |
| ( |
| ( |
Social security expenses |
| ( |
| ( |
| ( |
Expenses defined contribution plans |
| ( |
| ( |
| ( |
Other employee expenses |
| ( |
| ( |
| ( |
Total |
| ( |
| ( |
| ( |
Total registered employees at the end of the period |
| |
| |
| |
22.8 Financial expenses
Financial expenses includes the following selected information:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Interest expense |
| ( |
| ( |
| ( |
Foreign exchange losses |
| ( |
| ( |
| ( |
Other financial expenses |
| ( |
| ( |
| ( |
Total |
| ( |
| ( |
| ( |
F-55
22.9 Financial income
Financial income includes the following selected information:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Interest income |
| |
| |
| |
Foreign exchange gains |
| |
| |
| |
Other finance income |
| |
| |
| |
Total |
| |
| |
| |
22.10 Income taxes and deferred taxes
Current income tax
The following table shows the breakdown of the tax expense for 2023, 2022 and 2021:
| As of December 31, | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Current income tax |
| ( |
| ( |
| ( |
Deferred income taxes |
| |
| |
| |
Total income taxes for the period |
| ( |
| ( |
| ( |
The current tax expense is equal to the amount of income tax owed to the tax authorities for the year, under the applicable tax laws and rates in effect in the various countries.
Deferred tax
Deferred tax is presented in the statement of financial position under non-current assets and non-current liabilities, as applicable. The following table shows the breakdown of the deferred tax assets, deferred tax liabilities and the deferred tax expense for 2023, 2022 and 2021:
| Asset/(liability) |
| Income/(expense) | |||||||||
in 000€ |
| 2023 |
| 2022 |
| 2021 |
| 2023 |
| 2022 |
| 2021 |
Tax losses, patent and innovation income deduction, and other tax credits |
| |
| |
| |
| — |
| — |
| — |
Amortization development assets and other intangible assets |
| |
| |
| |
| — |
| — |
| — |
Depreciation property, plant & equipment |
| |
| |
| |
| — |
| — |
| — |
Leases |
| |
| |
| |
| — |
| — |
| — |
Other items |
| |
| — |
| |
| — |
| — |
| — |
Total deferred tax assets |
| |
| |
| |
| |
| |
| |
Property, plant & equipment |
| ( |
| ( |
| ( |
| — |
| — |
| — |
Intangible assets |
| ( |
| ( |
| ( |
| — |
| — |
| — |
Deferred income | ( | ( | — | — | — | — | ||||||
Investment grants |
| ( |
| ( |
| ( |
| — |
| — |
| — |
Inventory valuation |
| — |
| — |
| — |
| — |
| — |
| — |
Total deferred tax liabilities |
| ( |
| ( |
| ( |
| ( |
| ( |
| ( |
Netting |
| |
| |
| |
| — |
| — |
| — |
Total deferred tax assets, net |
| |
| |
| |
| — |
| — |
| — |
Total deferred tax liabilities, net |
| ( |
| ( |
| ( |
| — |
| — |
| — |
Total deferred tax income (expense) |
| — |
| — |
| — |
| |
| |
| |
The Group has unused tax losses carried forward and Innovation Income Deduction of K€
Under the Belgian Innovation Income Deduction system, companies can deduct up to
F-56
With respect to the tax losses carried forward and Innovation Income Deductions carried forward we recognized at December 31, 2023 a deferred tax asset of €
The deferred tax liability of K€
Relationship between Tax Expense and Accounting Profit
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Profit (loss) before taxes |
| |
| ( |
| |
Income tax at statutory rate of 25% |
| ( |
| |
| ( |
Effect of different local tax rate |
| ( |
| |
| |
Tax adjustments to the previous period |
| ( |
| |
| |
Non-deductible expenses |
| ( |
| ( |
| ( |
Research and development tax credits |
| |
| |
| |
Innovation income deduction |
| |
| — |
| |
Non recognition of deferred tax asset |
| ( |
| ( |
| ( |
Recognition of previously unrecognized tax losses |
| |
| |
| — |
Non-taxable income |
| |
| |
| |
Use of previous years’ tax losses and tax credits for which no deferred tax assets were recognized |
| — |
| |
| |
Taxes on other basis |
| ( |
| ( |
| ( |
Other |
| |
| ( |
| ( |
Income tax benefit (expense) as reported in the consolidated income statement |
| ( |
| ( |
| ( |
23Earnings per share
Basic earnings per share amounts are calculated by dividing the net profit (loss) for the year attributable to ordinary equity holders of the parent company by the weighted average number of ordinary shares outstanding during the year.
Diluted earnings per share amounts are calculated by dividing the net profit (loss) attributable to ordinary equity holder of the parent company by the weighted average number of ordinary shares outstanding during the year plus the weighted average number of ordinary shares that would be issued on conversion of all warrants and the weighted average number of ordinary shares that would be issued on conversion of the convertible debt. If there is a net loss after taxes, the number of diluted shares is equal to the basic shares.
The net profit (loss) for the year used for the basic and diluted earnings per share are reconciled as follows:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Net profit (loss) attributable to ordinary equity holders of the parent for basic earnings |
| |
| ( |
| |
Net profit (loss) attributable to ordinary equity holders of the parent adjusted for the effect of dilution |
| |
| ( |
| |
The warrants are dilutive at December 31, 2023. The warrants were antidilutive as per December 31, 2022 and were dilutive as per December 31, 2021.
F-57
The following reflects the share data used in the basic and diluted earnings per share computations:
| For the year ended December 31 | |||||
in 000 |
| 2023 |
| 2022 |
| 2021 |
Weighted average number of ordinary shares for basic earnings per share |
| |
| |
| |
Effect of dilution: |
|
|
| |||
Warrants |
| |
| — |
| |
Weighted average number of ordinary shares adjusted for effect of dilution |
| |
| |
| |
The earnings per share are as follows:
| For the year ended December 31 | |||||
2023 |
| 2022 |
| 2021 | ||
Earnings per share attributable to the owners of the parent |
|
|
|
|
|
|
Basic |
| |
| ( |
| |
Diluted |
| |
| ( |
| |
24Commitments and contingent liabilities
Mortgages and pledges
The Group has several loans secured by a mortgage on the building. The carrying value of related property, plant & equipment (including buildings under construction) is K€
Included in the above, the Group also has pledges on the business goodwill (“fonds de commerce”) of the Company for a total amount of K€
Other commitments
At December 31, 2023, the Group has outstanding non-cancellable contracts with a future commitment of K€
Legal Proceedings
The Group is currently not a party to any legal or arbitration proceedings, which, in the opinion of the management, is likely to have or could reasonably possibly have a material adverse effect on the business, financial position or results of operations.
25Risks
Foreign exchange risk
The Group transacts business globally and is subject to risks associated with fluctuating foreign exchange rates. The geographic areas outside of the Eurozone to which it sells its products and services are generally not considered to be highly inflationary. In the years ended December 31, 2023, 2022 and 2021,
The Group has primarily exposure to the USD, GBP, BRL, PLN and JPY as foreign currency. The exposure on MYR and CZK is limited. There is only a limited portion of turnover in local currency.
F-58
If the U.S. dollar (rate for €1) would have appreciated by
To limit the exposure to foreign currency rate fluctuations on the U.S. dollar, the Group has entered into currency rate swaps. As of December 31, 2023 the Group had hedge agreements in place for $
Inflation risk
We transact business globally and are subject to risks associated with fluctuating inflation. The risk exists that, if inflation increases our costs of remuneration, materials, services, energy, and capital expenditures, we may not be able to offset such costs fully by increasing our selling prices. As such, in a high inflationary environment, our results of operations and financial condition may be adversely affected.
Liquidity risk
The liquidity risk is that the Group may not have sufficient cash to meet its payment obligations. This risk is countered by day-by-day liquidity management at the corporate level. The Group has historically entered into financing and lease agreements with financial institutions to finance significant projects and certain working capital requirements. At December 31, 2023, we held cash and cash equivalents of €
The range of contracted obligations are as follows (incl. interest):
| Less than 1 |
|
|
| More than 5 |
| ||||
in 000€ | year | 2 to 3 years | 4-5 years | years | Total | |||||
At December 31, 2023 | ||||||||||
Loans & borrowings |
| | | | | | ||||
Lease liabilities |
| | | | | | ||||
Trade payables |
| | | | | | ||||
Other liabilities |
| | | | | | ||||
Total |
| | | | | |
| Less than 1 |
|
| More than 5 |
| |||||
year | 2 to 3 years |
| 4-5 years | years | Total | |||||
At December 31, 2022 | ||||||||||
Loans & borrowings |
| |
| |
| |
| |
| |
Lease liabilities |
| |
| |
| |
| |
| |
Trade payables |
| |
| |
| |
| |
| |
Other current liabilities |
| |
| |
| |
| |
| |
Total |
| |
| |
| |
| |
| |
| Less than 1 |
|
| More than 5 |
| |||||
year | 2 to 3 years |
| 4-5 years | years | Total | |||||
At December 31, 2021 | ||||||||||
Loans & borrowings |
| |
| |
| |
| |
| |
Lease liabilities |
| |
| |
| |
| |
| |
Trade payables |
| |
| |
| |
| |
| |
Other current liabilities |
| |
| |
| |
| |
| |
Total |
| |
| |
| |
| |
| |
Interest rate risk
Although the Group mainly has loans outstanding with a fixed interest rate, some of the loans have been contracted with variable interest rates. The most significant loans with variable interest rates have been secured by means of a variable to fixed interest rate swap. We therefore believe that the Group is not subject to immediate changes in interest rates.
F-59
Credit risk
Credit risk is the risk that third parties may not meet their contractual obligations resulting in a loss for the Group. The Group is exposed to credit risk from its operating activities (primarily trade receivables) and from its financing activities, which are mainly deposits with financial institutions. The Group limits this exposure by contracting with credit-worthy business partners or with financial institutions which meet high credit rating requirements. In addition, the portfolio of receivables is monitored on a continuous basis.
Trade receivables and contracts in progress
Customer credit risk is managed by each business unit subject to the Group’s established policy, procedures and controls relating to customer credit risk management.
An impairment analysis is performed at each reporting date per company and using a provision matrix per company to measure expected credit losses. The provision rates are based on days past due for groupings of various customer segments with similar loss patterns (i.e., by legal entity).
The calculation reflects the probability-weighted outcome, the time value of money and reasonable and supportable information that is available at the reporting date about past events, current conditions and forecasts of future economic conditions. Generally, trade receivables are written-off if past due for more than one year and are not subject to enforcement activity. The maximum exposure to credit risk at the reporting date is the carrying value of each class of financial assets at amortized cost or fair value through OCI as disclosed in Note 20. The Group does not hold collateral as security.
The Group evaluates the concentration of risk with respect to trade receivables as low, as its customers are located in several jurisdictions and industries and operate in largely independent markets.
Set out below is the information about the credit risk exposure on the Group’s trade receivables using a provision matrix:
|
|
| Less than 30 |
|
| More than | ||||||||
in 000€ | Total | Non-due | days | 31-60 days |
| 61-90 days |
| 91-180 days | 181 days | |||||
December 31, 2023 |
| |
| |
| |
| | | | | |||
December 31, 2022 |
| |
| |
| |
| | | | | |||
December 31, 2021 |
| |
| |
| |
| | | | |
Capital management
The primary objective of the Group’s shareholders’ capital management strategy is to ensure it maintains healthy capital ratios to support its business and maximize shareholder value. Capital is defined as the Group shareholder’s equity.
The Group consistently reviews its capital structure and makes adjustments in light of changing economic conditions. The Group made no changes to its capital management objectives, policies or processes during the years ended December 31, 2023, 2022 and 2021.
26Related party transactions
The compensation of key management personnel of the Group is as follows:
| For the year ended December 31 | |||||
in 000€ | 2023 |
| 2022 |
| 2021 | |
Short-term employee benefits |
| | | | ||
Post-employment benefits |
| | | | ||
Total |
| | | | ||
Warrants granted |
| | | | ||
Warrants outstanding |
| | | | ||
The amounts disclosed in the table are the amounts recognized as an expense during the reporting period related to key management personnel (senior management and executive committee members). In the year ending December 31, 2023, a total of
F-60
The following table provides the total amount of transactions that have been entered into with related parties for the relevant financial year:
| Sale of |
| Purchases |
|
| Interest |
| Right-of- |
|
| Lease |
| Other | |||
in 000€ | goods to | from | Depreciation | expense | Use Assets | Receivables | liabilities | liabilities | ||||||||
Non-executive directors of the Group |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
| |
| |
| |
| |
| |
| |
| |
| |
2022 |
| |
| |
| |
| |
| |
| |
| |
| |
2021 |
| |
| |
| |
| |
| |
| |
| |
| |
Shareholders of the Group |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
| |
| |
| |
| |
| |
| |
| |
| |
2022 |
| |
| |
| |
| |
| |
| |
| |
| |
2021 |
| |
| |
| |
| |
| |
| |
| |
| |
Joint ventures |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
| |
| |
| |
| |
| |
| |
| |
| |
2022 |
| |
| |
| |
| |
| |
| |
| |
| |
2021 |
| |
| |
| |
| |
| |
| |
| |
| |
Non-controlling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
| |
| |
| |
| |
| |
| |
| |
| |
2022 |
| |
| |
| |
| |
| |
| |
| |
| |
2021 |
| |
| |
| |
| |
| |
| |
| |
| |
Related party – Lunebeke NV / Ailanthus NV
Lunebeke NV is owned by a shareholder and director of the Group and was established on December 29, 2020 following a partial demerger of Ailanthus NV (a former related party of the Group that merged with Materialise NV subsequent to a partial demerger). The activities taken over by Lunebeke NV through the partial demerger of Ailanthus NV were taken over from Ailanthus NV with retro-active effect as of October 1st, 2021. The Group rents apartments on a regular basis from Lunebeke NV in order to host our employees from foreign subsidiaries who are visiting our headquarters in Leuven. The total amount paid to Lunebeke NV for rent in 2023 was K€
27Events subsequent to the statement of financial position date
No events subsequent to the date of the statement of financial position have occurred that would require adjustment to, or disclosure in, the consolidated financial statements.
F-61
28Overview of consolidated entities
| Country of |
|
|
|
|
|
| ||
Name | incorporation | % equity interest* |
| ||||||
| 2023 |
| 2022 |
| 2021 | ||||
Materialise NV |
|
| | % | | % | | % | |
Materialise SAS |
|
| | % | | % | | % | |
Materialise GmbH |
|
| | % | | % | | % | |
Materialise Japan K.K. |
|
| | % | | % | | % | |
Materialise s.r.o. |
|
| | % | | % | | % | |
Materialise USA, LLC |
|
| | % | | % | | % | |
OBL SAS |
|
| | % | | % | | % | |
Materialise Austria GmbH |
|
| | % | | % | | % | |
MATERIALISE SDN. BHD |
|
| | % | | % | | % | |
Materialise Ukraine LLC |
|
| | % | | % | | % | |
RapidFit NV |
|
| | % | | % | | % | |
Meridian Technique Limited |
|
| | % | | % | | % | |
OrthoView Holdings Limited |
|
| | % | | % | | % | |
Materialise SA |
|
| | % | | % | | % | |
Materialise Colombia SAS |
|
| | % | | % | | % | |
Materialise Motion NV |
|
| | % | | % | | % | |
Materialise Shanghai Co.Ltd |
|
| | % | | % | | % | |
Engimplan Engenharia de Implante Industria E Comércio Ltda |
|
| | % | | % | | % | |
Engimplan Holding Ltda |
|
| | % | | % | | % | |
Materialise Limited |
|
| | % | | % | | % | |
Materialise Australia PTY Ltd |
|
| | % | | % | | % | |
Materialise S.R.L. |
|
| | % | | % | | % | |
ACTech GmbH |
|
| | % | | % | | % | |
ACTech Holding GmbH |
|
| | % | | % | | % | |
ACTech North America, Inc. |
|
| | % | | % | | % | |
Tianjin Zhenyuan Materialise Medical Technology Ltd |
|
| | % | | % | | % | |
*The overview provides the equity interest held as of 31 December of each respective year.
The entities Materialise GmbH, Gilching, Germany, ACTech Holding GmbH, Freiberg / Saxony, Germany and ACTech GmbH, Freiberg / Saxony, Germany, have taken advantage of the exemption regulations of § 264 (3) HGB (German Commercial Code) for the financial year ending December 31, 2021, 2022 and 2023.
29 | Non-IFRS Measures |
EBITDA and Adjusted EBITDA is used in the Note 21 Segments as one of the basis of the Segments performance measurement. We calculate EBITDA as net profit plus income taxes, financial expenses (less financial income), depreciation and amortization, and share in loss of joint venture. Adjusted EBITDA is determined by adding back share-based compensation expenses, acquisition-related expenses of business combinations, impairments and fair value remeasurements due to business combinations to EBITDA.
F-62