UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| For the fiscal year ended |
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF SECURITIES EXCHANGE ACT OF 1934 | |
| For the transition period from to |
Commission file number

(Exact name of Registrant as specified in its charter)
| ||
(State or other jurisdiction of incorporation or organization) |
| (IRS Employer Identification No.) |
| ||
(Address of principal executive offices) |
| (Zip Code) |
(
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
| Trading Symbol(s) |
| Name of Exchange on Which Registered |
| The |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer☐ | Accelerated filer☐ |
Smaller reporting company | |
Emerging growth company |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12(b)-2 of the Act. Yes
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $
As of March 25, 2024, the registrant had
NEUROBO PHARMACEUTICALS, INC.
Table of Contents
2
Unless the context requires otherwise, references in this Annual Report on Form 10-K for the fiscal year ended December 31, 2023 (this “Annual Report” or “Report”) to “we,” “us,” “the Company,” “NeuroBo” and “our” refer to NeuroBo Pharmaceuticals, Inc. (the “Company”) and its subsidiaries.
Special Note Regarding Forward-Looking Statements
This Annual Report contains “forward-looking statements” within the meaning of the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements that address future operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation, our expectations regarding our ability to execute on our commercial strategy, the timeline for regulatory submissions, regulatory steps and potential regulatory approval of our current and future product candidates, the ability to realize the benefits of the license agreement with Dong-A ST Co. Ltd., a related party, (“Dong-A”), including the impact on future financial and operating results of NeuroBo; the ability to integrate the product candidates into our business in a timely and cost-efficient manner; the cooperation of our contract manufacturers, clinical study partners and others involved in the development of our current and future product candidates; our ability to initiate clinical trials on a timely basis; our ability to recruit subjects for our clinical trials; costs related to the license agreement, known and unknown, including costs of any litigation or regulatory actions relating to the license agreement; changes in applicable laws or regulations; effects of changes to our stock price on the terms of the license agreement and any future fundraising and other risks and uncertainties described in our filings with the SEC. Forward-looking statements are based on management’s current expectations and assumptions about future events, which are inherently subject to uncertainties, risks and changes in circumstances that are difficult to predict. These statements may be identified by words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. In addition, statements that “we believe,” “we expect,” “we anticipate” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report and management believes that these forward-looking statements are reasonable as and when made. However, you should not place undue reliance on forward-looking statements because they speak only as of the date when made.
Forward-looking statements are based on management’s current expectations and assumptions about future events, which are inherently subject to uncertainties, risks and changes in circumstances that are difficult to predict. In some instances, you can identify forward-looking statements by words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. In addition, statements that “we believe,” “we expect,” “we anticipate” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report and management believes that these forward-looking statements are reasonable as and when made. However, you should not place undue reliance on forward-looking statements because they speak only as of the date when made. We undertake no obligation to update or revise forward-looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes to future operating results or expectations, except as required by law.
We operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for us to predict all risk factors and uncertainties. We may not actually achieve the plans, projections or expectations disclosed in forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including without limitation, the possibility that regulatory authorities do not accept our application or approve the marketing of our products, the possibility we may be unable to raise the funds necessary for the development and commercialization of our products, and those described in our filings with the SEC.
3
Summary Risk Factors
Our business is subject to a number of risks, as fully described in Part I, Item 1A. Risk Factors in this Annual Report. The principal factors and uncertainties include, among others:
| ● | NeuroBo expects to incur losses for the foreseeable future and may never achieve or maintain profitability; |
| ● | NeuroBo will need additional financings to fund operations and such additional financings may cause dilution to existing stockholders, restrict NeuroBo’s operations or require NeuroBo to relinquish its technologies; |
| ● | The timing and costs related to the clinical development of NeuroBo’s products are difficult to predict, and any delays in NeuroBo’s clinical trials may lead to a delay in commercialization; |
| ● | NeuroBo may be required to make significant payments under the Dong-A License Agreement and other existing license agreements; |
| ● | The regulatory review and approval processes of the United States Food and Drug Administration (“FDA”) and comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable; |
| ● | Undesirable side effects in current or future product candidates could delay or prevent their commercialization, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, and the development of such product candidates exposes NeuroBo to additional risks; |
| ● | NeuroBo may engage in future acquisitions, mergers, in-licenses of technology, strategic alliances or additional licensing arrangements that could disrupt its business, cause dilution to the organization’s stockholders, harm its financial condition and operating results or result in no benefits being realized from such engagement; |
| ● | Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside of NeuroBo’s control; |
| ● | NeuroBo faces substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than it does; |
| ● | NeuroBo’s commercial success depends upon attaining significant market acceptance of its product candidates, if approved, among hospitals, physicians, patients and healthcare payors; |
| ● | Product liability lawsuits against NeuroBo could cause it to incur substantial liabilities and could limit commercialization of any product candidate that it may develop; |
| ● | NeuroBo relies on third parties to develop NeuroBo’s preclinical studies, clinical trials, research programs and product candidates and to manufacture its product candidates and preclinical and clinical drug supplies. If these third parties do not successfully carry out their contractual duties or meet expected deadlines or if they engage in misconduct or other improper activities or if NeuroBo is unable to engage with these third parties, it could have a material adverse effect on NeuroBo’s business and NeuroBo’s obtaining regulatory approval and commercialization of its product candidates; |
| ● | Any product candidate for which NeuroBo obtains marketing approval could be subject to marketing restrictions or withdrawal from the market, and NeuroBo may be subject to penalties if it fails to comply with regulatory requirements or if it experiences unanticipated problems with these product candidates; |
| ● | NeuroBo or any of its potential collaborators may never receive regulatory approval to market NeuroBo’s product candidates within or outside of the United States (“U.S.”); |
| ● | Mechanisms that NeuroBo may utilize to expedite and/or reduce the cost for development or approval of its product candidates may not lead to faster or less expensive development, regulatory review or approval process; |
| ● | Legislation may increase the difficulty and cost to obtain marketing approval of and commercialize its product candidates, and governments outside the U.S. tend to impose strict price controls, which also may adversely affect NeuroBo’s revenues; |
| ● | NeuroBo’s compliance with legal standards related to foreign trade could impair its ability to compete in domestic and international markets, and NeuroBo could face criminal liability and other serious consequences for violations; |
4
| ● | Certain tax matters, including NeuroBo’s ability to use its NOLs to offset future taxable income may be subject to certain limitations, could impact its results of operations and financial conditions; |
| ● | Inadequate funding for the FDA and other government agencies could prevent those agencies from performing normal business functions on which the operation of NeuroBo’s business may rely, which could negatively impact NeuroBo’s business; |
| ● | If NeuroBo is unable to obtain, maintain and protect sufficient intellectual property rights, its competitive position could be harmed; |
| ● | NeuroBo may become involved in lawsuits to protect or enforce its intellectual property, which could be expensive, time consuming, unsuccessful and could distract NeuroBo’s personnel from their normal responsibilities; |
| ● | NeuroBo has identified material weaknesses in its internal control over financial reporting that could, if not remediated, result in material misstatements in its financial statements or impair its ability to produce accurate and timely consolidated financial statements; |
| ● | NeuroBo’s obtaining and maintaining patent protection could be reduced or eliminated for non-compliance with certain requirements imposed by governmental patent agencies; |
| ● | NeuroBo’s business and operations could suffer in the event of system failures or unplanned events; |
| ● | Any failure, inadequacy, interruption or security lapse of NeuroBo’s information technology could prevent NeuroBo from accessing critical information or expose NeuroBo to liability; |
| ● | If securities analysts do not publish research or reports about NeuroBo’s business or if they publish negative evaluations, the price of NeuroBo’s stock could decline; |
| ● | NeuroBo does not anticipate declaring or paying, in the foreseeable future, any cash dividends on its capital stock and, consequently, the ability of its stockholders to achieve a return on their investment will depend on appreciation in the price of NeuroBo’s common stock; |
| ● | NeuroBo’s Bylaws designate the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by NeuroBo’s stockholders, which could limit the ability of NeuroBo’s stockholders to obtain a favorable judicial forum for disputes with NeuroBo or its directors, officers or employees; |
| ● | Unstable market and economic conditions may have serious adverse consequences on NeuroBo’s business, financial condition and stock price; |
| ● | The liquidity and trading volume of NeuroBo’s common stock could be low due to concentration of ownership and the market price of its common stock may therefore be highly volatile; and |
| ● | NeuroBo’s common stock may be delisted from Nasdaq Capital Market LLC (“Nasdaq”) if it fails to comply with the continued listing requirements. |
5
Part I
Item 1.Business
Overview
We are a clinical-stage biotechnology company focused primarily on developing and commercializing novel pharmaceuticals to treat cardiometabolic diseases. NeuroBo has two programs focused primarily on treatment of metabolic dysfunction-associated steatohepatitis (“MASH”) and obesity. MASH was formerly known as non-alcoholic steatohepatitis (“NASH”). The American Association for the Study of Liver Diseases (“AASLD”) and its European and Latin American counterparts changed the name to metabolic dysfunction-associated steatohepatitis to reflect the complexity of the disease.
| ● | DA-1241 is a novel G-Protein-Coupled Receptor 119 (“GPR119”) agonist with development optionality as a standalone and/or combination therapy for both MASH and type 2 diabetes mellitus (“T2DM”). Agonism of GPR119 in the gut promotes the release of key gut peptides, glucagon-like peptide-1 (“GLP-1”), glucose-dependent insulinotropic polypeptide (“GIP”), and peptide YY (“PYY”). These peptides play a further role in glucose metabolism, lipid metabolism and weight loss. DA-1241 has beneficial effects on glucose, lipid profile and liver inflammation, supported by potential efficacy demonstrated during in vivo preclinical studies. The therapeutic potential of DA-1241 has been demonstrated in multiple preclinical animal models of MASH and T2DM where DA-1241 reduced hepatic steatosis, inflammation, fibrosis, and improved glucose control. |
| o | In Phase 1a and 1b human trials, DA-1241 was well tolerated in both healthy volunteers and those with T2DM. |
| o | We initiated a Phase 2a trial in 2023 with the goal of establishing the mechanism of action and efficacy of DA-1241 in the treatment of MASH and to evaluate trends for T2DM. This is the first-in-human MASH trial for DA-1241 and we are expecting top line results by the end of 2024. |
| ● | DA-1726 is a novel oxyntomodulin (“OXM”) analogue functioning as a GLP-1 receptor (“GLP1R”) and glucagon receptor (“GCGR”) dual agonist for the treatment of obesity that is to be administered once weekly subcutaneously. DA-1726 acts as a dual agonist of GLP1R and GCGR. Activating GLP1R may lead to weight loss through reduced appetite while activating GCGR may increase energy expenditure. DA-1726 has a well understood mechanism and, in preclinical mice models, resulted in improved weight loss compared to semaglutide and tirzepatide. |
| o | We received an Investigational New Drug (“IND”) approval from the U.S. Food and Drug Administration (“FDA”) for DA-1726 and we intend to initiate a Phase 1 clinical trial during the first half of 2024. |
While we focus our financial resources and management’s attention on the development of DA-1241 and DA-1726, we also have four legacy therapeutic programs designed to impact a range of indications in viral, neurodegenerative and cardiometabolic diseases which we continue to consider for out-licensing and divestiture opportunities:
| ● | ANA001, a proprietary oral niclosamide formulation for the treatment of patients with moderate COVID-19 |
| ● | NB-01 for the treatment for painful diabetic neuropathy (“PDN”) |
| ● | NB-02 for the treatment of cognitive impairment |
| ● | Gemcabene for the treatment of dyslipidemia |
Our operations have consisted principally of performing research and development (“R&D”) activities, preclinical developments, clinical trials, and raising capital. Our activities are subject to significant risks and uncertainties, including failing to secure additional funding before sustainable revenues and profit from operations are achieved and other risks listed in “Risks Related to our Operations and to Development, Marketing, Commercialization and Regulation of Our Product Candidates” in Item 1A. Risk Factor.
6
Our Strategy
Our goal is to discover, develop and commercialize novel therapeutics designed to impact a range of indications primarily in cardiometabolic diseases. The key elements of our business strategy to achieve this goal include:
| ● | Advance DA-1241 through the FDA regulatory process to obtain approval for the treatment of MASH. Successful completion of the Phase 2a trial will establish the mechanism of action and an early signal of efficacy in MASH and T2DM, which will allow us to seek initiation of Phase 2b trial as monotherapy or in combination with dipeptidyl peptidase-4 (“DPP4”) inhibitor, GLP1R or other therapeutic candidates. |
| ● | Pursuit for DA-1241 combination therapy. The Phase 2a Part 2 trial is designed to establish combination therapy of DA-1241 and Sitagliptin, a DPP4 inhibitor. With successful proof of concept in the Phase 2a trial, we will be exploring other combination therapies that can benefit from the mechanism of action of DA-1241 and expand the target efficacy of DA-1241 for the treatment of MASH. |
| ● | Advance DA-1726 through the FDA regulatory process to obtain approval for the treatment of obesity. Explore various avenues to advance DA-1726 to FDA approval, including seeking ways to expedite the clinical trials and conducting non-clinical studies. |
| ● | Pursue additional pipelines and/or other technologies. With both DA-1241 and DA-1726 in clinical trials, we will explore adding (i) clinical stage product candidates to diversify and enrich our pipeline and/or (ii) other technologies. |
Our Pipeline
Our focus is on two cardiometabolic assets. Our lead asset DA-1241, is a GPR119 agonist, in Phase 2a trial for treatment of MASH. Our second asset is DA-1726, a GLP-1 receptor and glucagon receptor dual agonist, for treatment of obesity. The Phase 1 trial for DA-1726 is expected to be initiated within the first half of 2024.
The following illustrates the current status of our assets as of filing date of this Annual Report.
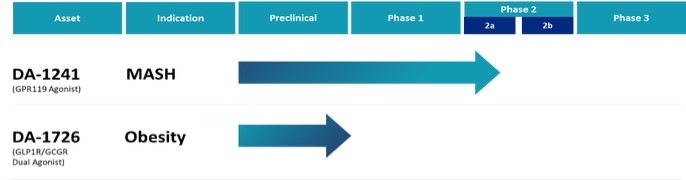
DA-1241 Treatment of MASH
DA-1241 is a potential first-in-class G protein-coupled receptor 119 (“GPR119”) candidate with therapeutic potential for MASH and T2DM that can be orally administered once a day. Two Phase 1 clinical trials for the treatment of T2DM have been completed and a Phase 2a trial for the treatment of MASH is ongoing in the U.S. with top line results expected by the end of 2024.
DA-1241 is a novel chemical drug candidate selectively activating GPR119 which has shown consistent target-related mechanisms and glucose-lowering effects from nonclinical studies in Phase 1b exploratory clinical trials in patients with T2DM in the U.S. GPR119 is known to be a regulator of both blood glucose and lipid levels. Non-clinical studies suggest DA-1241 selectively activates GPR119, stimulates the secretion of insulin and incretin hormones such as GLP-1, and thereby reduces plasma glucose levels without hypoglycemia risk and lowers plasma lipids levels of both triglycerides and cholesterol. Moreover, impaired insulin action and lipid metabolism which are frequently observed in T2DM patients are highly associated with the pathogenesis of steatosis and inflammation in MASH. Extensive non-clinical studies have shown DA-1241 has therapeutic potential for the reduction in hepatic steatosis, inflammation, fibrosis, and improved glucose control regardless of body weight reduction.
MASH Overview
MASH is a severe form of metabolic dysfunction-associated steatotic liver disease (“MASLD”) characterized by inflammation and fibrosis in the liver that can progress to cirrhosis, liver failure, hepatocellular carcinoma (“HCC”) and death. MASLD was formerly known as nonalcoholic fatty liver disease (“NAFLD”) and was changed by the AASLD and
7
its European and Latin American counterparts. Patients with MASH are at increased risk of liver damage and other complications. Fibrosis is generally reversible in its early-to-mid stages. However, late-stage fibrosis can be irreversible in the absence of therapy and prevents the liver from performing its natural functions.
The prevalence of MASLD, which affects approximately 25% of the global population, and MASH, which develops in approximately 12% to 14% of MASLD patients, is growing and is driven primarily by the worldwide obesity epidemic. The critical pathophysiologic mechanisms underlying the development and progression of MASH include reduced ability to handle lipids, increased insulin resistance, injury to hepatocytes and liver fibrosis in response to hepatocyte injury. Patients with MASH frequently have other significant metabolic co-morbidities such as obesity, hyperglycemia, dyslipidemia and systemic hypertension (a constellation of which is commonly referred to as metabolic syndrome) and these further contribute to the risk of cardiovascular disease. The number of MASH cases in the U.S. is projected to expand from 16.5 million in 2015 to 27 million in 2030, with similar prevalence growth expected in Europe. Diet and exercise are currently the standard of care for MASLD and MASH, but adherence to this treatment regimen is poor and there remains a high unmet need in the treatment of MASH.
DA-1241 Preclinical Development
Extensive preclinical pharmacology, Absorption, Distribution, Metabolism and Excretion (“ADME”), safety and toxicology studies have been completed for DA-1241. The pharmacokinetic characteristics of DA-1241 were identified through the full set of preclinical ADME studies. The safety and toxicology studies completed were: (i) central nervous system (“CNS”), cardiovascular (“CV”), and respiratory safety in rats and dogs; (ii) a single-dose, 4-week, 13-week and 26-week oral toxicity studies in rats; (iii) 4-week, 13-week and 39-week oral toxicity studies in dogs; (iv) pre-natal development studies in rats and rabbits; and (v) genotoxicity tests of in vitro bacterial reverse mutation, chromosome aberration, and in vivo micronucleus.
Comprehensive non-clinical studies demonstrated DA-1241 distinctively activates GPR119 across species, stimulates the secretion of insulin and GLP-1, a gut peptide hormone with various metabolic benefits, from the pancreas and intestine, respectively, and thereby reduces postprandial glucose and lipid levels after single administration to mice. The postprandial hypoglycemic response by DA-1241 observed in wild type mice disappeared in GPR119-deficient mice, demonstrating target engagement. Notably, DA-1241 treatment did not cause hypoglycemia < 50 mg/dl in overnight fasted mice.
In diabetic mice with hypertriglyceridemia, chronic treatment with DA-1241 lowered fasting and non-fasting blood glucose levels, in which DA-1241 prevented pancreatic beta cell loss and preserved pancreatic function. Moreover, DA-1241 treatment decreased hepatic lipid accumulation in addition to plasma triglycerides levels at the same dose levels. When a DPP4 inhibitor was cotreated with DA-1241 to prolong the biological half-life of plasma GLP-1, plasma concentrations of active GLP-1 increased more than those due to degradation blockade with DPP4 inhibitors, and thereby potentiation of GLP-1 action further improved glucose and lipid metabolism compared to each treatment alone.
In a non-diabetic mouse model with pre-established dyslipidemia, DA-1241 completely reduced plasma and hepatic triglycerides to normal control levels and also decreased plasma LDL-cholesterol, independent of glycemic control. Comprehensive mechanism studies have shown that the lipid-lowering effects of DA-1241 are due in part to inhibiting lipid synthesis in the liver and interfering with dietary lipid transport in the intestine.
With regard to the MASH indication, DA-1241 has been shown to improve fatty liver in various types of mouse models with metabolic diseases. Thereafter, therapeutic potential for treating MASH has been evaluated in several MASH mice models with different pathophysiology. Among them, the STAM-MASH mouse model exhibits mild fatty liver and moderate liver inflammation/fibrosis and is rapidly chemically induced. DA-1241 improved hepatic inflammation and fibrosis, showing a decrease in MASLD activity score (“NAS”) and relative fibrotic area of the liver compared to the vehicle-treated control. Diet-induced obesity (“DIO”)-MASH mice are chronically induced through a Western diet and are characterized by marked fatty liver and mild to moderate hepatic inflammation/fibrosis. In DIO-MASH mice, DA-1241 improved hepatic steatosis, inflammation, and fibrosis assessed by histological and biochemical methods regardless of body weight reduction. Of note, DA-1241 improved systemic inflammatory status with reduced plasma inflammatory cytokines (TNFα, IL6) and chemokines (CCL2, CXCL1, CXCL2, CXCL10) contributing to tissue damage. Therefore, DA-1241 treatment reduced the levels of plasma liver enzymes (ALT, AST), which were increased due to liver tissue damage in DIO-MASH mice. In mice with metabolic diseases, the effects of DA-1241 on the MASH phenotypes (steatosis, inflammation, and fibrosis in the liver) are enhanced by the co-treatment with a DPP4 inhibitor compared to each treatment alone due to potentiated GLP-1 actions.
8
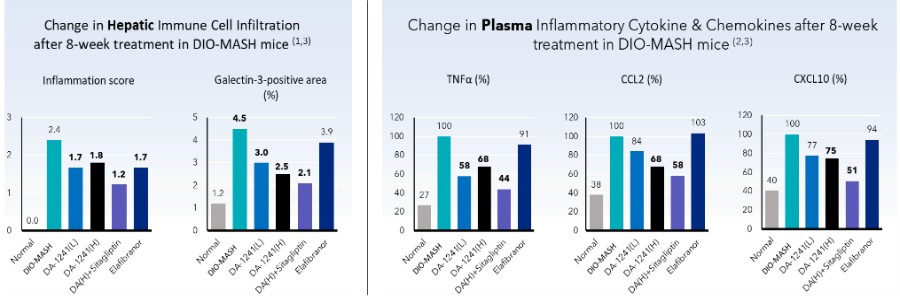
Differentiated Anti-Inflammatory Effect in MASH Mice
Result of Phase 1 U.S. Clinical Trial for DA-1241
Completed Phase 1a and 1b trials in the US healthy subjects. The first-in-humans Phase 1a study was a double-blind, placebo controlled, single ascending dose (“SAD”), single-center study in 60 healthy male volunteers to evaluate the safety, tolerability, pharmacokinetics (“PK”), pharmacodynamics (“PD”), and interaction effect with metformin. Each cohort was given a single oral dose of 12.5, 25, 50, 100, 200, and 400 mg DA-1241 or placebo tablets. The dose level of DA-1241 for the interaction effect (“IE”) assessment of metformin on the PK of DA-1241 was 100 mg. Therefore, the IE cohort had 2 separate treatment periods. Subjects in the IE cohort received DA-1241 100 mg or placebo alone in Treatment Period 1, and DA-1241 100 mg or placebo with 500 mg metformin (IR formulation) in Treatment Period 2. DA-1241 was well tolerated over a dose range of 12.5 mg to 400 mg. There was no effect of concomitant administration of metformin on DA-1241 PK parameters.
Phase 1b, Part 1 was a double-blind placebo-controlled, multiple-ascending dose (“MAD”), single-center study of DA-1241 in healthy subjects. Overall, 24 male subjects were blinded and randomized to receive DA-1241: 50, 100 or 200 mg or placebo, as single daily oral doses for 28 days. Safety data reviews and dose escalation decisions between cohorts took place after all subjects of an ongoing cohort had completed procedures through day 14. All doses tested were well tolerated. There were no Serious Adverse Events (“SAEs”) and no discontinuations due to Adverse Events (“AEs”).
Completed Phase 1b trial in the US T2DM patients. The Phase 1b study was designed as a placebo and active comparator (sitagliptin 100 mg)-controlled, double-blind, randomized, multi-center study with an objective of evaluating whether DA-1241 delivers improved glucose-lowering efficacy in 83 diabetic patients. Patients were treated with placebo, sitagliptin 100 mg or DA-1241 25 mg, 50 mg and 100 mg once daily for 8 weeks, in combination with stable doses of metformin (13~19 patients/group). In the mixed meal tolerance test to evaluate the ability to reduce postprandial glucose through GPR119 activation, the incremental AUE0-4h of plasma glucose (“iAUE”) upon nutrient ingestion was measured and compared. Eight-week treatment of DA-1241 25 mg, 50 mg and 100 mg showed the changes of +6.3%, -2.0% and -13.8% in iAUE levels from the baseline and DA-1241 100 mg showed similar blood glucose improvement with that of sitagliptin 100 mg (-9.0%), and it outperformed placebo (+10.5%).
Exploratory P1b Study in the U.S.: Glucose-Lowering Effects
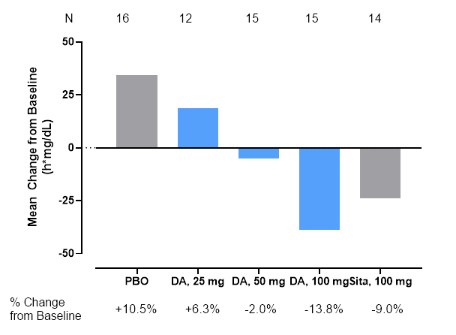
Mean Change in Postprandial Glucose Excursion at Week 8
9
In the parameters of glycemic variability measured with a Continuous Glucose Monitoring (“CGM”) system and fasting plasma glucose, the glucose-lowering efficacy by DA-1241 was similar to that of sitagliptin. Moreover, the time-in-range, the percentage of how long blood glucose value is within 70~180mg/dL, was increased by mitigating the hypoglycemia risk and duration of hyperglycemia whereas such time-in-range was reduced in the placebo group.
Single administration or 8-week repeated administration of DA-1241 increased secretion of gut peptide hormones such as GLP-1, GIP and PYY in gastrointestinal tracts after taking meals. The amount of secretion of such hormones increased in proportion to the extent of exposure to DA-1241.
Exploratory P1b Study in the U.S.: Target-related Biomarker Change
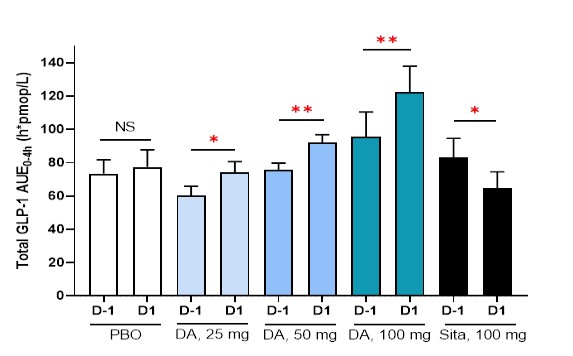
* & ** P<0.05 & P<0.01 versus corresponding baseline values; DA, DA-1241; Sita, Sitagliptin
Total GLP-1 Secretion during Mixed Meal Tolerance Test
In terms of safety, no clinically significant adverse events were observed following the 8-week treatment, confirming the tolerability of DA-1241, and the bodyweight showed a tendency to decrease.
DA-1241 Phase 2a Trial
We are currently conducting a Phase 2a trial in the U.S. MASH Phase 2a is a 16-week, multicenter, randomized, double-blind, placebo-controlled, parallel arm clinical trial to establish safety and an early signal of efficacy in MASH as a next-generation competitive oral agent while we follow the trend for T2DM. The trial opened enrollment in August 2023 and is expected to enroll a total of 87 subjects, with a planned maximum of 98 subjects to account for early discontinuations, who will be randomized into 4 treatment groups and will be dosed with: DA-1241 50 mg, DA-1241 100 mg, DA-1241 100 mg/Sitagliptin 100 mg, or Placebo in a 1:2:2:2 ratio. The primary efficacy endpoint for the study is the change from baseline in the alanine transaminase (“ALT”) levels at week 16. The secondary efficacy endpoints evaluate changes in the following at week 16 including: proportion of subjects with normalization of ALT level of < 30 IU/L; relative percent change liver fat fraction from baseline; absolute change in liver fat from baseline; proportion of subjects with a 30% or more reduction in liver fat from baseline; change in aspartate transaminase (“AST”), gamma glutamyl transpeptidase, and alkaline phosphatase from baseline; change in hemoglobin A1c (“HbA1c”) (%); change in NAFLD Fibrosis Score from baseline; liver stiffness measurement assessed by FibroScan® from baseline; and change in FAST (FibroScan - AST) from baseline. Safety will be evaluated by monitoring AEs including determination of SAEs and AEs leading to discontinuation and laboratory abnormalities as characterized by type, frequency, timing, severity (mild, moderate, severe), seriousness and relationship to DA-1241, vital signs measurements, clinical laboratory tests and electrocardiogram (“ECG”) assessments.
DA-1726 Treatment of Obesity
DA-1726 is a novel OXM analogue functioning as a GLP1R and GCGR dual agonist. It is a long-acting, novel peptide drug candidate, with a Phase 1 IND approved by the FDA with therapeutic potential for obesity. Activation of GLP1R contributes to central anorexic effect (appetite suppression) and activation of GCGR peripherally enhances basal metabolic rate. Accordingly, non-clinical studies have shown that DA-1726 not only reduces food intake but also increases energy expenditure even at the basal resting state, leading to persistent weight loss in diet-induced obese mice and rats. DA-1726 directly lowers blood glucose and lipid levels in addition to the accompanying metabolic improvement
10
by weight loss. Weight reduction is closely related to the alleviation of fatty liver. Having stabilized the fragile peptide through several unique modifications, DA-1726 is predicted to be available as a once-weekly regimen to humans.
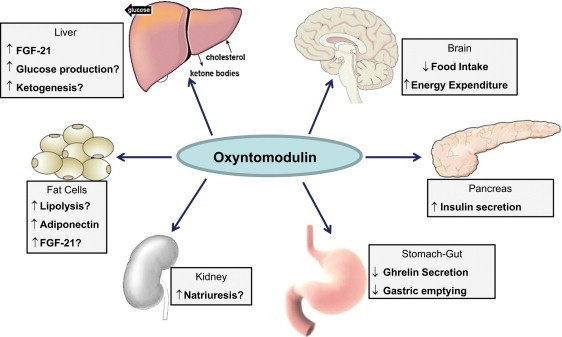
Physiological effects of oxyntomodulin
Background
Obesity is a disease caused by abnormal or excessive fat accumulation due to an imbalance in energy intake and consumption over a long period of time. According to the World Health Organization (“WHO”), more than 1.9 billion people worldwide are overweight with 650 million considered to be obese. The comorbidities of obesity include T2DM, cardiovascular disease, hypertension and MASH, and the risk of these diseases is higher in obese people than in non-obese people.
The treatment of obesity can be divided into three mechanisms: (i) appetite control, (ii) absorption inhibition, and (iii) increase of energy expenditure. Currently, there are a total of eight approved anti-obesity medications on the market, of which the most notable are Novo Nordisk semaglutide (WEGOVY®) and Eli Lilly tirzepatid (Zepbound®). However, there is still an unmet need in the market as there are no agents with a mechanism to reduce body weight by increasing energy expenditure in peripheral tissue.
Oxyntomodulin is a gut hormone released from intestinal L-cells after meal ingestion and represents dual agonism of the GLP-1 receptor and glucagon receptor. It increases energy expenditure through glucagon receptors and increases appetite suppression and insulin secretion through GLP-1 receptor activation, ultimately inducing weight loss and glycemic control. The furthest stage of development of any oxyntomodulin analogue are survudutide and mazdutide in Phase 3 trials for the treatment of obesity or MASH.
DA-1726 Preclinical Development
Animal toxicity studies of DA-1726 for the Phase 1 clinical trial have been completed. The toxicity studies included safety pharmacology studies and general toxicity studies.
The mode of action and pharmacological effects of DA-1726 were evaluated in various disease models. In high-fat diet-induced obese (“HF-DIO”) mice, DA-1726 showed more body weight loss and increasing energy expenditure than a pair-fed group.
11
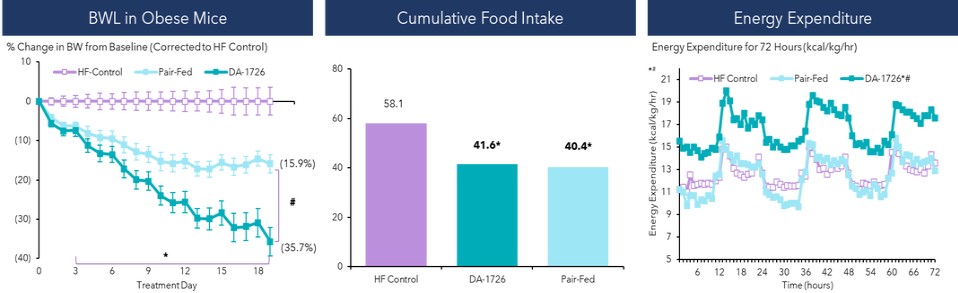
Mean energy expenditure:
DA-1726*# 16.6 kcal/kg/hr
Pair-Fed 12.4 kcal/kg/hr
HF Control 12.6 kcal/kg/hr
Mechanism of action
In comparison with GLP-1 analogue, DA-1726 represented superior body weight loss compared to semaglutide in HF-DIO obese mice. At the end of the study, DA-1726 significantly increased the expression of thermogenic genes (Ucp-1 and Ppargc1a) in epididymal fat and increased white adipose tissue browning was histologically confirmed. In addition, DA-1726 inhibited adipocyte differentiation in vitro. Taken together, it suggests the GCGR action of DA-1726 contributes to reduced adiposity by enhancing fat burning and inhibiting adipogenesis. DA-1726 effectively reduced postprandial glucose excursion in acute oral glucose tolerance test in normal mice. Notably, DA-1726 showed similar glycemic control and excellent weight loss to semaglutide in obese mice with hyperglycemia. Simultaneously, DA-1726 enhanced insulin sensitivity by significantly reducing fasting plasma insulin and glucose levels. Meanwhile, DA-1726 showed no hypoglycemia risk in overnight fasted normal mice, unlike semaglutide.
In comparison with GLP-1 receptor and glucagon-dependent insulinotropic polypeptide receptor (“GIP”) dual agonist tirzepatide in HF-DIO obese MASH mice, DA-1726 showed similar body weight while consuming significantly more food. In addition, DA-1726 reduced plasma clinical chemistry parameters (ALT, AST, ALP, T-BIL, glucose, and cholesterol) and hepatic fat accumulation.
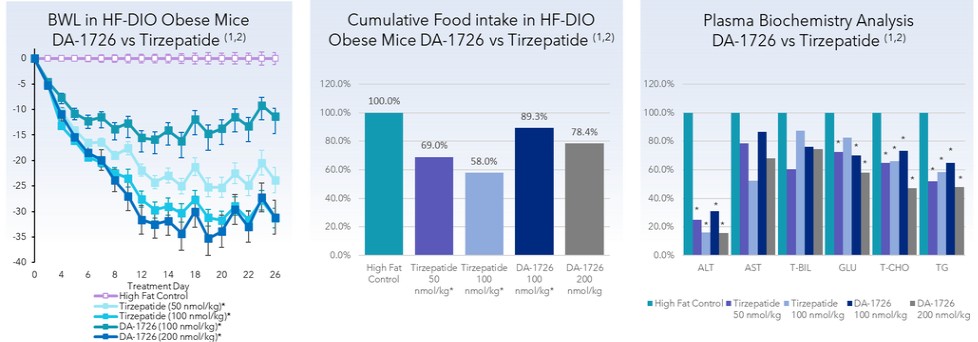
Weight loss and plasma biochemistry analysis
DA-1726 Phase 1 Trial
We have received Phase 1 trial IND approval from the FDA and are planning to initiate the Phase 1 trial within the first half of 2024. The Phase 1 trial, a first-in-human trial, is a randomized, placebo-controlled, double-blind, two-part study to investigate the safety, tolerability, PK, and PD of single and multiple ascending doses of DA-1726 in obese, otherwise healthy subjects. Part 1 is a SAD study, expected to enroll approximately 45 participants, randomized into one of five planned cohorts. Each cohort will be randomized in a 6:3 ratio of DA-1726 or placebo. Part 2 will be a MAD study, expected to enroll
12
approximately 36 participants, who will be randomized into four planned cohorts, each to receive 4 weekly administrations of DA-1726 or placebo.
The primary endpoint will assess the safety and tolerability of DA-1726 by monitoring AEs, SAEs, treatment emergent adverse events (“TEAEs”) and AEs leading to treatment discontinuation. Secondary endpoints include the PK of DA-1726, assessed via serum concentrations over time and metabolite profiling at the highest doses of DA-1726. Exploratory endpoints will include the effect of DA-1726 on metabolic parameters, cardiac parameters, fasting lipid levels, body weight, waist circumference and body mass index (“BMI”), among others.
Other Product Candidates
We are focusing our financial resources and management’s attention on the development of DA-1241 for MASH and DA-1726 for obesity. We also have four legacy therapeutic programs, ANA001, NB-01, NB-02 and Gemcabene, that we will not advance to any future clinical trials. We will continue to consider out-licensing and divestiture opportunities with respect to the following legacy programs.
ANA001 Treatment of COVID-19 Symptoms
ANA001 is a proprietary oral niclosamide formulation that was developed as a treatment for patients with moderate COVID-19 (patients not requiring ventilators). Niclosamide is a potential oral antiviral and anti-inflammatory agent with a long history of use and documented safety in humans. Niclosamide has demonstrated both antiviral and immunomodulatory activity with possible downstream effects on coagulation abnormalities observed in COVID-19. In preclinical research by an independent academic group published in Antimicrobial Agents and Chemotherapy, niclosamide inhibited viral replication in vitro and was more potent than remdesivir and chloroquine in the same assay.
We believe ANA001 has the potential to reduce the viral load and inflammation associated with cytokine dysregulation, acute respiratory distress syndrome (“ARDS”), and coagulation abnormalities and thus improve time to clinical improvement as defined as hospital discharge recorded using the WHO Ordinal Scale for Clinical Improvement.
NB-01
NB-01 addresses a range of mechanisms that contribute to neuropathic pain and nerve degeneration in diabetic and other peripheral neuropathies. These include a decrease in key inflammatory markers, restoration of nerve growth factor (“NGF”) to normal levels, and reduction of advanced glycation end products (“AGEs”). Inflammation is a central factor in pain generation and other peripheral neurodegenerative diseases. NB-01 reduces levels of TNF-a and IL-6, both of which are markers of inflammation. NB-01 also reduces AGEs, which are implicated in diabetes-related complications. AGE inhibitors have been clinically tested as potential treatments for these complications. NB-01 also restores the neurotrophin NGF, which is involved in nerve growth, maintenance and repair. NB-01 has been shown in animal models to alleviate symptoms of PDN.
NB-02
NB-02 was being developed for the symptomatic and disease modifying treatment of neurodegenerative diseases, including Alzheimer's disease and tauopathies. In preclinical studies, we have observed the mechanisms of action of NB-02 to include inhibition of tau phosphorylation, acetylcholinesterase (“AChE”) inhibition, inhibition of Ab toxicity and amyloid plaque formation, and anti-inflammatory effects. Specifically, in both in vitro and in vivo models, NB-02 has demonstrated inhibition of AChE, as is the case with three of the current products on the market to treat the symptoms of Alzheimer's disease. It has also demonstrated inhibition of tau phosphorylation and of amyloid plaque formation, both mechanisms believed to contribute to the progression of neurodegenerative diseases.
Gemcabene
Gemcabene is a novel, once-daily, oral therapy designed to target known lipid metabolic pathways to lower levels of LDL-C, hsCRP and triglycerides. Gemcabene shares many of the attributes of statin therapy, including broad therapeutic applications, convenient route of administration and cost-effective manufacturing process, but does not appear to increase the reporting of myalgia when added to statin therapy. Gemcabene has also shown additive LDL-C lowering in combination with stable low, moderate or high-intensity statin therapy. As described below, we licensed global rights to Gemcabene from Pfizer in April 2011. Under the terms of the amended and restated license agreement with Pfizer, Pfizer may terminate the license if we have not made a commercial sale by April 2024.
13
License Agreements
License Agreement with Dong-A for DA-1241 and DA-1726
In September 2022, we entered into an exclusive license agreement (the “2022 License Agreement”) and a Securities Purchase Agreement with Dong-A (the “Securities Purchase Agreement”). Pursuant to the 2022 License Agreement and subject to the conditions set forth therein, we received an exclusive global license (excluding the Republic of Korea) to two proprietary compounds for specified indications. The 2022 License Agreement covers the rights to a compound referred to as DA-1241 for treatment of MASH and T2DM and a compound referred to as DA-1726 for treatment of obesity and MASH. The 2022 License Agreement became effective in November 2022.
Under the terms of the 2022 License Agreement, Dong-A (i) received an upfront payment which was settled in 2,200 shares of preferred stock of NeuroBo designated as “Series A Convertible Preferred Stock”, par value $0.001 per share (the “Series A Preferred Stock”), under the terms of the Securities Purchase Agreement (the “Upfront License Payment”); (ii) is eligible to receive single digit royalties on net sales received by us from the commercial sale of products covering DA-1241 or DA-1726; (iii) is eligible to receive commercial-based milestone payments, dependent upon the achievement of specific commercial developments; and (iv) is eligible to receive regulatory milestone payments of up to $178.0 million for DA-1726 and $138.0 million for DA-1241, dependent upon the achievement of specific regulatory developments. See “Liquidity and capital resources” in Part I, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations for discussion on the Securities Purchase Agreement.
Our obligation to pay royalties to Dong-A under the 2022 License Agreement continues on a product-by-product and country-by-country basis until the later of (i) the fifth anniversary of the first commercial sale of such product in such country, (ii) the expiration or termination of the last valid patent claim that covers a product in such country and (iii) the loss of regulatory exclusivity for such product in such jurisdiction. Either we or Dong-A may terminate the 2022 License Agreement (i) if the other party is in material breach of the agreement and has not cured or started to cure the breach within 60 days of notice of such breach; provided that if the breach cannot be cured within the 60-day period and the breaching party started to remedy the breach, if such breach is not cured within 90 days of receipt of written notice or (ii) if the other party is subject to a bankruptcy or insolvency event (subject to a 30-day cure period in the case of a petition for bankruptcy).
License Agreement with Dong-A for NB-01
In January 2018, we entered into an exclusive license agreement with Dong-A (the “2018 License Agreement”), which agreement was amended in April 2018 and July 2019. Under the terms of the 2018 License Agreement, we obtained an exclusive, royalty-bearing, worldwide (except for the Republic of Korea) license to make, use, offer to sell, sell and import products covered by certain Dong-A intellectual property rights in its proprietary compound designated as DA-9801 (NB-01). Our license rights cover any and all applications and markets for the therapeutic, health, nutrition or well-being of humans. We may grant sublicenses to any affiliate or third party. We are responsible for all future patent prosecution costs.
We are obligated to use commercially reasonable efforts to develop products for use in each of the U.S., the European Union, Japan and the People's Republic of China. If we terminate, discontinue or suspend, for longer than 12 months, the development of any product listed as a product under development in any development plan provided to Dong-A (other than for reasons of force majeure or requirements of applicable law), then we are deemed in breach of this development obligation, and Dong-A may terminate the 2018 License Agreement for cause after a 60-day cure period.
The term of the 2018 License Agreement continues on a country-by country and product-by-product basis until the later of the 12th anniversary of the first commercial sale of such product in such country or expiration or termination of the last valid claim within the patent rights covering the product. Either Dong-A or we may terminate the 2018 License Agreement if the other party is in material breach of the 2018 License Agreement and has not cured or started to cure the breach within 60 days of notice of such breach, or is subject to a bankruptcy or insolvency event. We may terminate the 2018 License Agreement at any time upon 90 days’ written notice.
Pfizer License Agreement
In August 2018, an Amended and Restated License Agreement with Pfizer (the “Pfizer Agreement”) for the research, development, manufacture and commercialization of Gemcabene went into effect. The Pfizer Agreement amended and restated the prior license agreement with Pfizer dated April 16, 2011. The Pfizer Agreement includes milestone payments to Pfizer and tiered royalties on a country-by-country basis based upon the annual amount of net sales as specified in the Pfizer Agreement.
The Pfizer Agreement will expire upon expiration of the last royalty term. Either party may terminate the Pfizer Agreement for the other party's material breach following a cure period or immediately upon certain insolvency events relating to the other
14
party. Pfizer may immediately terminate the Pfizer Agreement in the event that (i) we or any of our affiliates or sublicenses contests or challenges, or supports or assists any third party to contest or challenge, Pfizer's ownership of or rights in, or the validity, enforceability or scope of any of the patents licensed under the Pfizer Agreement or (ii) we or any of our affiliates or sublicensees fails to achieve the first commercial sale in at least one country by April 16, 2024.
License Agreement with Beijing SL
Pursuant to the terms and conditions of a License and Collaboration Agreement dated July 23, 2019 (the “Beijing SL License Agreement”), Beijing SL has an exclusive royalty-bearing license to research, develop, manufacture and commercialize pharmaceutical products comprising, as an active ingredient, Gemcabene in the territory comprised of mainland China, Hong Kong, Macau and Taiwan. We retain all rights to Gemcabene outside of the territory. The parties have agreed to collaborate with respect to development and commercialization activities under the Beijing SL License Agreement through a joint steering committee composed of an equal number of representatives of Beijing SL and us.
Pursuant to the Beijing SL License Agreement, Beijing SL made an upfront gross payment of $2.5 million. Additionally, with respect to each licensed product, Beijing SL will make payments for specified developmental and regulatory milestones and payments for specified global net sales milestones. Beijing SL will also be obligated to pay tiered royalties ranging from the mid-teens to twenty percent on the net sales of all licensed products in the territory until the latest of (a) the date on which any applicable regulatory exclusivity with respect to such Licensed Product expires in such region, (b) the expiration or abandonment of the last valid patent claim or joint patent claim covering such Licensed Product in each region and (c) the fifth anniversary of the first commercial sale of such Licensed Product in such region.
Either party may terminate the Beijing SL License Agreement (x) with written notice for the other party's material breach following a cure period or (y) if the other party becomes subject to certain insolvency proceedings. In addition, we may terminate the Beijing SL License Agreement in its entirety if Beijing SL or its affiliates or sublicensees commence a proceeding challenging the validity, enforceability or scope of any of our patents.
Manufacturing
Dong-A manufactures clinical quantities of DA-1241 and DA-1726 in accordance with the 2022 License Agreement and the Shared Services Agreement. As NeuroBo advances the product candidates through clinical development our current plans are to continue to use third parties including Dong-A to manufacture drug products for our trials. See “Shared Services Agreement” in Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence for additional information.
Among the conditions for FDA approval of a pharmaceutical product is the requirement that the manufacturer’s quality control and manufacturing procedures conform to cGMP, which must be followed at all times. The FDA typically inspects manufacturing facilities every two years. In complying with cGMP regulations, pharmaceutical manufacturers must expend resources and time to ensure compliance with product specifications as well as production, record keeping, quality control, reporting and other requirements.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions, governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
Some of our competitors may have significantly greater financial resources and expertise in R&D, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Other firms may also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient enrollment for clinical trials, as well as in acquiring technologies complementary to, or necessary for our programs. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors with us, particularly through collaborative arrangements with large and established companies.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize therapeutics that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain marketing approvals for their products more rapidly than we may obtain approval for our products, which could result in our competitors establishing a strong market position before we are able to
15
enter the market. In addition, our ability to compete may be affected because in some cases insurers or other third-party payors, including government programs, seek to encourage the use of generic products. This may have the effect of making branded products less attractive from a cost perspective to buyers.
DA-1241 MASH
There is only one approved treatment of MASH, Madrigal Pharmaceuticals’ thyroid hormone receptor beta agonist. However, various therapeutics are used off-label for the treatment of MASH, including vitamin E (an antioxidant), insulin sensitizers (e.g., metformin, pioglitazone), antihyperlipidemic agents (e.g., gemfibrozil), pentoxifylline and ursodeoxycholic acid (UDCA). There are several product candidates in Phase 3 or earlier clinical or preclinical development for the treatment of MASH, including Novo Nordisk’s GLP1 agonist semaglutide, Eli Lilly’s GLP1R and GIP dual agonist tirzepatide, Akero Therapeutics’s FGF21 analog efruxifermin, 89 Bio’s FGF21 analog pegaozafermin, Inventiva’s pan-peroxisome proliferator-activated receptor agonist, Boston Pharmaceuticals and Roche’s fibroblast growth factor 21 analogs, and farnesoid X receptor agonists from Intercept Pharmaceuticals Inc., among others. Additional pharmaceutical and biotechnology companies with product candidates in development for the treatment of MASH include AstraZeneca plc, Altimmune Inc., Boehringer Ingelheim GmbH, Bristol-Myers Squibb Company, Durect Corporation, Galectin Therapeutics Inc., Galmed Pharmaceuticals Ltd., Immuron Ltd., Ionis Pharmaceuticals, Inc., Islet Sciences, Inc., MediciNova, Inc., NGM Biopharmaceuticals, Inc., NuSirt Sciences Inc., Pfizer Inc., Viking Therapeutics, Inc. and Zydus Pharmaceuticals (USA) Inc. MASH is a complex disease and we believe that it is unlikely that any one therapeutic option will be optimal for every MASH patient.
DA-1726-Obesity
Due to the growing overweight and obesity epidemic and consumer demand, there are many competitors in the field of obesity treatment. Obesity treatments range from behavioral modification to drugs, medical devices and surgery, generally as a last resort. If DA-1726 were approved for obesity, our primary competition in the obesity treatment market would currently be from approved and marketed products, including semaglutide (WEGOVY®) and tirzepatide (Zepbound®). Further competition could arise from products currently in development, including among others, with GLP1R/GCGR dual agonists, Boehringer Ingelheim, Merck/Hanmi Pharmaceutical, AstraZeneca, Altimmune, Innovent Biologics/Eli Lilly, Carmot and D&D Pharma; with GLP1R/GCGR/GIP triple agonists, Hanmi Pharmaceutical and Eli Lilly; Amgen with its GLP-1 agonist/GIP antagonist antibody; and Novo Nordisk with Amylin and Amylin-GLP-1 combination. To the extent our product candidate is approved for obesity, the commercial success of our product will also depend on our ability to demonstrate benefits over the then-prevailing standard of care. Finally, morbidly obese patients sometimes undergo a gastric bypass procedure, with salutary effects on the many co-morbid conditions of obesity.
DA-1241 T2DM
There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for T2DM. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Intellectual Property
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries, including the U.S., the patent term is 20 years from the earliest filing date of a non-provisional patent application or a Patent Cooperation Treaty (“PCT”) application to which a U.S. application claims priority. In the U.S., a patent's term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office (“USPTO”) in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier filed patent. The term of a U.S. patent that covers a drug or biological product may also be eligible for patent term extension when approval from the FDA is granted, provided statutory and regulatory requirements are met. In the future, if our product candidates receive approval from the FDA or foreign regulatory authorities, we expect to apply for patent term extensions on issued patents covering those products, depending upon the length of the clinical trials for each drug and/or other factors. There can be no assurance that any of our pending patent applications will be issued or that we will benefit from any patent term extension or other favorable adjustment to the term of any patents.
16
As with other biotechnology and pharmaceutical companies, our ability to maintain and solidify our proprietary and intellectual property position for our product candidates, preclinical compounds, and core technologies will depend on our success in obtaining effective patent claims and enforcing those claims if granted. However, patent applications that we may file or license from third parties may not result in the issuance of patents. We also cannot predict the breadth of claims that may be allowed or enforced in our patents. Any issued patents that we may receive in the future may be challenged, invalidated or circumvented. For example, prior to March 16, 2013, in the U.S., patent applications were subject to a “first to invent” rule of law. Applications effectively filed on or after March 16, 2013, are subject to a “first to file” rule of law.
Discoveries reported in the scientific literature often lag the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We cannot be certain that any existing application will be subject to the “first to file” or “first to invent” rule of law, that we or our licensor were the first to make the inventions claimed in our existing patent portfolio subject to the prior laws, or that we or our licensor were the first to file for patent protection of such inventions subject to the new laws. If third parties prepare and file patent applications in the U.S. that also claim technology we have claimed in our patents or patent applications, we may have to participate in interference or derivation proceedings and/or invalidation proceedings in the USPTO, which could result in substantial costs to us, even if the eventual outcome is favorable. In addition, because of the extensive time required for clinical development and regulatory review of a product candidate we may develop, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of any such patent.
In addition to patents, we rely upon unpatented trade secrets, know-how, and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, by using confidentiality agreements with our collaborators, scientific advisors, employees and consultants, and invention assignment agreements with our employees. We also have agreements requiring assignment of inventions with selected consultants, scientific advisors and collaborators. Confidentiality agreements are designed to protect our proprietary information and, in the case of agreements or clauses requiring invention assignment, to grant us ownership of technologies that are developed under those agreements.
Our ability to commercialize product candidates depends in large part on our ability to obtain and maintain intellectual property protection for our product candidates. Our policy is to seek to protect our intellectual property position by, among other methods, filing U.S. and foreign patent applications related to the technology, inventions and improvements that are important to the development and implementation of our business strategy. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary position.
DA-1241
As of December 31, 2023, our exclusively licensed intellectual property portfolio for DA-1241 includes one U.S. patent directed to both composition of matter and a process of making the composition and three U.S. non-provisional patent applications directed to both composition of matter and use of the composition. The two issued U.S. patents are expected to expire in July 2035, excluding any additional term for patent term adjustments or patent term extensions. NeuroBo’s intellectual property portfolio for DA-1241 also includes 22 non-U.S. patents and 39 non-U.S. patent applications directed to composition of matter and/or use of the composition. The issued non-U.S. patents are expected to expire between 2035 and 2041, excluding any additional term for patent term adjustments or patent term extensions. The jurisdictions for the non-U.S. patents and applications include: Australia, Brazil, Canada, China, the European Patent Convention, Hong Kong, India, Israel, Japan, Mexico, New Zealand, Philippines, Republic of Korea, Russia, Saudi Arabia, and Singapore.
DA-1726
As of December 31, 2023, our exclusively licensed intellectual property portfolio for DA-1726 includes two U.S. patents directed to both composition of matter and use of the composition and one U.S. non-provisional patent application directed to both composition of matter and use of the composition. The issued U.S. patent is expected to expire in 2038, excluding any additional term for patent term adjustments or patent term extensions. Our intellectual property portfolio for DA-1726 also includes 13 non-US patents and 22 non-US patent applications directed to composition of matter and/or use of the composition. The issued non-U.S. patents is expected to expire between 2038 and 2041, excluding any additional term for patent term adjustments or patent term extensions. The jurisdictions for the non-U.S. patents and applications include: Australia, Brazil, Canada, China, the European Patent Convention, Japan, Philippines, Republic of Korea, Russia, and Singapore.
17
ANA001
As of December 31, 2023, our intellectual property portfolio for ANA001 included one US non-provisional application and two non-US applications (Argentina and Europe) directed to niclosamide formulation and one PCT application directed to combined use of niclosamide and gemcabene. Patent applications may be issued in the U.S. and any countries in which NeuroBo files national phase applications of the PCT application. The patents issued from the national phase applications are estimated to expire between 2041 and 2042.
NB-01 and NB-02
As of December 31, 2023, our intellectual property portfolio for NB-01 included four issued U.S. patents, comprised of one patent directed to composition of matter and three patents directed to use, and two pending U.S. non-provisional patent applications, comprised of one directed to composition of matter and another directed to use, and 62 granted foreign patents, all related to our NB-01 programs in peripheral neuropathy and neurological conditions. The issued patents have expiration dates ranging between October 2026 and June 2033. The patent issuing from the application, if any, is expected to expire December 2031. The jurisdictions for the foreign patents and application include: Brazil, Canada, China, the European Patent Convention (including Austria, Belgium, Finland, France, Germany, Greece, Hungary, Italy, Netherlands, Poland, Portugal, Romania, Spain, Switzerland, Turkey, and the United Kingdom), India, Japan, Mexico, the Republic of Korea, and Russia. One patent family including some of the above patents for NB-01 is assigned to University-Industry Cooperation Group of Kyung Hee University, and is exclusively licensed from Kyung Hee University to Dong-A and then from Dong-A to us pursuant to the terms of the corresponding agreements. The other two patent families including the other above patents and patent applications for NB-01 are assigned to Dong-A and exclusively licensed to us.
As of December 31, 2023, our intellectual property portfolio for NB-02 included three issued U.S. patents, one pending U.S. non-provisional patent application, 74 foreign granted patents, and 1 foreign patent application. Patents issuing from these applications, if any, are expected to expire around 2035. The issued patents have an expiration date in December 2035. The jurisdictions for the foreign patents and applications include: Brazil, Canada, China, the European Patent Convention (including Austria, Belgium, Finland, France, Germany, Greece, Hungary, Italy, Netherlands, Poland, Portugal, Romania, Spain, Switzerland, Turkey, and the United Kingdom), India, Japan, Mexico, the Republic of Korea, and Russia. All of the above patents and patent applications for NB-02 were assigned to us.
Gemcabene
As of December 31, 2023, our intellectual property portfolio relating to Gemcabene included six issued U.S. patents, three pending U.S. patent applications, 43 foreign-granted patents and 21 foreign patent applications directed to formulations, compositions, methods of use and methods of manufacturing. The Gemcabene intellectual property includes both owned and Pfizer-licensed issued and pending patents in the U.S. and foreign jurisdictions. The issued patents in the U.S. and foreign countries have expiration dates between December 2031 and November 2036. The patents in the U.S. and foreign countries that may be issued from pending applications, if any, are expected to expire between December 2031 and October 2039. The jurisdictions for the non-U.S. patents include Argentina, Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Japan, Mexico, New Zealand, Philippines, Korea, Russia, Singapore, South Africa, Taiwan and Thailand.
Government Regulation
Government authorities at the federal, state and local level in the U.S. and in other countries extensively regulate, among other things, the research, development, testing, manufacture (including any manufacturing changes), packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, import and export of pharmaceutical products, such as those we are developing.
U.S. FDA Regulation
In the U.S., pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act (“FDCA”) and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as imposition of clinical holds, refusal by the FDA to approve pending new drug applications (“NDAs”), warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement, civil penalties and criminal prosecution.
Pharmaceutical product development in the U.S. typically involves preclinical or other nonclinical laboratory and animal tests and the submission to the FDA of an IND application, which must become effective before clinical testing may commence.
18
For commercial approval, the sponsor must submit adequate tests by all methods reasonably applicable to show that the drug is safe for use under the conditions prescribed, recommended or suggested in the proposed labeling. The sponsor must also submit substantial evidence, generally consisting of adequate, well-controlled clinical trials to establish that the drug will have the effect it purports or is represented to have under the conditions of use prescribed, recommended or suggested in the proposed labeling. Satisfaction of the FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Nonclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal studies to assess the characteristics and potential safety and efficacy of the product. The conduct of the nonclinical tests must comply with federal requirements, including the FDA's good laboratory practice regulations and the regulations of the U.S. Department of Agriculture (“USDA”) implementing the Animal Welfare Act. The results of nonclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long-term nonclinical tests, such as animal studies of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not imposed a clinical hold on the IND or otherwise commented or questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations, (ii) in compliance with good clinical practice (“GCP”), an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors (some of which have been codified into U.S. federal regulations), and (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial either is not being conducted in accordance with the FDA requirements or presents an unacceptable risk to the clinical trial patients. The trial protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board (“IRB”) at each site where a trial will be conducted for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB's requirements or may impose other conditions. Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In general, in Phase 1, the initial introduction of the drug into healthy human volunteers or, in some cases, patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases, the FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. The FDA may, however, determine that a drug is effective based on one clinical trial plus confirmatory evidence. Only a small percentage of investigational drugs complete all three phases and obtain marketing approval. In some cases, the FDA may require post-market studies, known as Phase 4 studies, to be conducted as a condition of approval to gather additional information on the drug's effect in various populations and any side effects associated with long-term use. Depending on the risks posed by the drugs, other post-market requirements may be imposed.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical, and other testing and a compilation of data relating to the product's pharmacology, chemistry, manufacture, and controls. The cost of preparing and submitting an NDA is substantial. Under federal law, the submission of most NDAs is additionally subject to a substantial application user fee.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency's threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. Under the statute and implementing regulations, the FDA has 180 days (the initial review cycle) from the date of filing to issue either an approval letter or a complete response letter, unless the review period is adjusted by mutual agreement between the FDA and the applicant or as a result of the applicant submitting a major amendment. In practice, the performance goals established pursuant to the Prescription Drug User Fee Act have effectively
19
extended the initial review cycle beyond 180 days. The FDA's current performance goals call for the FDA to complete review of 90% of standard (non-priority) NDAs within 10 months of receipt and within six months for priority NDAs, but two additional months are added to standard and priority NDAs for a new molecular entity (“NME”) such that the 10-month and 6-month action goals for NME applications begin to run from the 60-day filing date rather than from receipt of the original NDA submission.
The FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an advisory committee, which is typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current good manufacturing practice (GMP) regulations is satisfactory, and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter (CRL) generally outlines the deficiencies in the submission and may require substantial additional testing or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA's satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing 90% of NDA resubmissions within two to six months depending on the type of information included in response to the deficiencies identified in the CRL.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy (“REMS”) to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for health care professionals, and/or elements to assure safe use (“ETASU”). ETASU can include, but is not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug's safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained, or problems are identified following initial marketing.
Fast Track Designation and Accelerated Approval
The FDA is authorized to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment, and which demonstrate the potential to address unmet medical needs for the condition. These programs include fast track designation, breakthrough therapy designation, priority review designation and other accelerated approvals.
Under the Fast Track Program, the sponsor of a new drug candidate that is intended to treat a serious condition may request that the FDA designate the drug candidate for a specific indication as a Fast Track drug concurrent with, or after, the filing of the IND for the drug candidate. The FDA must determine if the drug candidate qualifies for Fast Track designation within 60 days of receipt of the sponsor's request. In addition to other benefits such as the ability to engage in more frequent interactions with the FDA, the FDA may initiate review of sections of a Fast Track drug's NDA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA's time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, the Fast Track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
In 2012, Congress enacted the Food and Drug Administration Safety and Innovation Act (“FDASIA”). This law established a new regulatory program for products designated as “breakthrough therapies.” A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to designated breakthrough therapies, including: holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
The FDA may also designate a product for priority review if it is a drug that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines at the time that the marketing application is
20
submitted, on a case- by-case basis, whether the proposed drug represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting drug reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, or evidence of safety and effectiveness in a new subpopulation. A priority review designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA's goal for taking action on a marketing application from ten months to six months.
Under the FDA’s accelerated approval regulations, the FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. The accelerated approval regulations are codified within Title 21 of the Code of Federal Regulations, as Subpart H under Part 314, the part of the FDA regulations covering applications for FDA approval to market a new drug, and as such the accelerated approval pathway is sometimes referred to as approval under “Subpart H.”
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A drug candidate approved under Subpart H is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. Unless otherwise informed by the FDA, for an accelerated approval product an applicant must submit to the FDA for consideration during the preapproval review period copies of all promotional materials, including promotional labeling as well as advertisements, intended for dissemination or publication within 120 days following marketing approval. After 120 days following marketing approval, unless otherwise informed by the FDA, the applicant must submit promotional materials at least 30 days prior to the intended time of initial dissemination of the labeling or initial publication of the advertisement. The accelerated approval pathway is most often used in settings in which the course of a disease is long, and an extended period of time is required to measure the intended clinical benefit of a drug, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. For example, accelerated approval has been used extensively in the development and approval of drugs for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large clinical trials to demonstrate a clinical or survival benefit.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition—generally a disease or condition that affects fewer than 200,000 individuals. The U.S. Orphan Drug Designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity and trade name, if any, of the drug and its designated use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
Pediatric Information
Under the Pediatric Research Equity Act (“PREA”), NDAs or supplements to NDAs must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant full or partial waivers for submission of data, as well as deferrals for several reasons, including a finding that the drug is ready for approval for use in adults before pediatric studies are complete or that additional safety or effectiveness data needs to be collected before the pediatric studies begin. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which orphan designation has been granted.
The Best Pharmaceuticals for Children Act (“BPCA”) provides NDA holders a six-month extension of any exclusivity-patent or non-patent-for a drug if certain conditions are met. Conditions for exclusivity include the FDA's determination that
21
information relating to the use of a new drug in the pediatric population may produce health benefits in that population, the FDA making a written request for pediatric studies, and the applicant agreeing to perform, and reporting on, the requested studies within the statutory timeframe. Applications under the BPCA are treated as priority applications, with all of the benefits that designation confers.
Special Protocol Assessment
A company may reach an agreement with the FDA under the Special Protocol Assessment (“SPA”) process as to the required design and size of clinical trials intended to form the primary basis of an efficacy claim for a new drug product. According to its performance goals, the FDA seeks to evaluate the protocol within 45 days of the request to assess whether the proposed trial is adequate, and that evaluation may result in discussions and a request for additional information. An SPA request must be made before the proposed trial begins, and all open issues must be resolved before the trial begins. If a written agreement is reached, it will be documented and made part of the administrative record. Under the FDCA and FDA guidance implementing the statutory requirement, an SPA is generally binding on the FDA except in limited circumstances, such as if the FDA identifies a substantial scientific issue essential to determining safety or efficacy after the study begins, public health concerns emerge that were unrecognized at the time of the protocol assessment, the sponsor and the FDA agree to the change in writing, or if the study sponsor fails to follow the protocol that was agreed upon with the FDA.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of certain FDA-regulated products, including prescription drugs, are required to register and disclose certain clinical trial information on a public website maintained by the U.S. National Institutes of Health (NIH). Information related to the product, patient population, phase of investigation, study sites and investigator, and other aspects of the clinical trial is made public as part of the registration. Sponsors are also obligated to disclose the results of these trials after completion. Disclosure of the results of these trials can be delayed for up to two years if the sponsor certifies that it is seeking approval of an unapproved product or that it will file an application for approval of a new indication for an approved product within one year. Competitors may use this publicly available information to gain knowledge regarding the design and progress of the development programs. Failure to timely register a covered clinical study or to submit study results as provided for in the law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal government. Since the NIH's Final Rule on ClinicalTrials.gov registration and reporting requirements became effective in 2017, both NIH and FDA have signaled the government's willingness to begin enforcing those requirements against clinical trial sponsors who fail to meet those legal obligations, with FDA releasing a guidance document in August 2020 for certain procedural steps it intends to take when determining whether and how to assess civil monetary penalties against a non-compliant party.
Post-Approval Requirements
Drugs manufactured, marketed or distributed pursuant to FDA approval decisions are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion, and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to FDA review and approval before they can be implemented. There also are continuing, annual user fee requirements for any marketed products and related manufacturing facilities, as well as new application fees for supplemental applications.
In addition, drug manufacturers and other entities involved in the manufacture of approved drugs are required to register their facilities with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA for compliance with GMP requirements. Prescription drug distribution facilities are also subject to state licensure, including inspections, by the relevant local regulatory authority. Changes to the manufacturing process, specifications or container closure system for an approved drug are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from GMP and impose reporting and documentation requirements upon the sponsor and others involved in the drug manufacturing process. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain GMP compliance and ensure ongoing compliance with other statutory requirements the FDCA, such as the requirements for making manufacturing changes to an approved NDA.
Thus, even after new drug approval is granted, Regulatory authorities may withdraw that approval or request product recalls if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks;
22
or imposition of distribution or other restrictions under a REMS program. Other potential consequences of regulatory non-compliance include, among other things:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| ● | fines, warning letters or holds on post-approval clinical trials; |
| ● | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
| ● | product seizure or detention, or refusal to permit the import or export of products; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
As described further below, the FDA strictly regulates marketing, labeling, advertising and promotion of prescription drug products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant penalties.
The Hatch-Waxman Amendments
Orange Book Listing
In 1984, with passage of the Hatch-Waxman Amendments to the FDCA, Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. As part of the marketing application process when seeking approval for a new drug through an NDA, applicants are required to list with the FDA every patent of which claims cover the applicant's product or an approved method of using the product. Upon approval of a drug, approval information about the drug along with each of the applicant's listed patents is then published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the "Orange Book." Pursuant to the Hatch-Waxman Amendments, drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application (“ANDA”). An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the reference license drug (“RLD”) and has been shown through bioequivalence testing to be bioequivalent to the RLD. The FDA is responsible for determining that the generic drug is "bioequivalent" to the innovator drug, although under the statute, a generic drug is bioequivalent to a RLD if "the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug."
Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, preclinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are most often considered to be therapeutically equivalent to the RLD, are commonly referred to as "generic equivalents" to the RLD, and can often be substituted by pharmacists under prescriptions written for the original RLD in accordance with state law. Specifically, upon approval of an ANDA, the FDA indicates whether the generic product is "therapeutically equivalent" to the RLD in the Orange Book. By operation of certain state laws and numerous health insurance programs, the FDA's designation of therapeutic equivalence in the Orange Book often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or the patient.
The Hatch-Waxman Amendments also amended the FDCA to enact Section 505(b)(2) of the FDCA, which permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. A Section 505(b)(2) applicant may eliminate the need to conduct certain preclinical or clinical studies, if it can establish that reliance on studies conducted for a previously-approved product is scientifically appropriate. The FDA may also require companies to perform additional trials or measurements to support the change from the approved product. The FDA may then approve the new product for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant. With respect to listed patents, patent certification requirements, and the blocking of follow-on marketing applications for the drug product previously approved under an NDA and listed in the Orange Book-known as the reference listed drug (“RLD”)-505(b)(2) NDA applications and ANDAs are required under the statute and FDA's implementing regulations to follow similar procedures and are subject to similar conditions. However, only in some cases is a 505(b)(2) NDA-approved drug product determined by FDA to be therapeutically equivalent to the original innovator RLD.
As part of our own marketing application process, the ANDA/505(b)(2) applicant is required to certify to the FDA concerning any patents listed for the relevant RLD in the FDA's Orange Book. Specifically, the applicant must certify either that: (i) the
23
required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the generic product. The ANDA applicant may also elect to submit a Section VIII statement, certifying that our proposed ANDA or 505(b)(2) labeling does not contain (or carves out) any language regarding the patented method-of-use, rather than certify to a listed method-of-use patent.
If the ANDA/505(b)(2) applicant does not challenge the innovator's listed patents, or indicates that it is not seeking approval of a patented method of use, the ANDA/505(b)(2) application will not be approved by the FDA until all the listed patents claiming the referenced product have expired.
A certification that the new product will not infringe the already approved product's listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA/505(b)(2) applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of that Paragraph IV certification to the NDA sponsor and patent holders once FDA accepts the ANDA/505(b)(2) application for filing. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification, as provided for in the statute. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA/505(b)(2) NDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA/505(b)(2) applicant.
Non-Patent Exclusivity
Under the Hatch-Waxman Amendments, the FDA also may not approve an ANDA or 505(b)(2) NDA until any applicable period of non-patent exclusivity for the RLD has expired. The FDCA provides a period of five years of non-patent exclusivity for a new drug containing a new chemical entity (“NCE”) which is a drug that contains no active moiety that has been approved by the FDA in any other NDA. During these five years of marketing exclusivity, the FDA cannot receive any ANDA or 505(b)(2) application seeking approval of a drug that references a version of the NCE drug.
The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or the addition of a new indication. During this three-year period of exclusivity, the FDA cannot approve an ANDA or 505(b)(2) application that includes the change.
An ANDA or 505(b)(2) application may be submitted one year before NCE exclusivity expires if a Paragraph IV certification is filed. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification requirement, and in such situations, no ANDA or 505(b)(2) application may be filed before the expiration of the exclusivity period.
Patent Term Extension
After NDA approval, owners of relevant drug patents may apply for up to a five-year patent extension. The allowable patent term extension is calculated as half of the drug's testing phase—the time between IND submission and NDA submission—and all of the review phase—the time between NDA submission and approval up to a maximum of five years. The time can be shortened if the FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years from market approval.
For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the U.S. Patent and Trademark Office must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA has not been submitted.
Prescription Drug Marketing Act
As part of the sales and marketing process, pharmaceutical companies frequently provide samples of approved drugs to physicians. The Prescription Drug Marketing Act (“PDMA”) imposes requirements and limitations upon the provision of drug samples to physicians, as well as prohibits states from licensing distributors of prescription drugs unless the state licensing program meets certain federal guidelines that include minimum standards for storage, handling and record keeping. In addition, the PDMA sets forth civil and criminal penalties for violations.
24
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA and relevant regulatory authorities outside the U.S. In addition to new legislation, regulations and policies are often revised or interpreted by regulatory authorities in ways that may significantly affect our business and our product candidates. It is impossible to predict whether further legislative changes will be enacted or whether regulations, guidance, policies or interpretations will be changed or what the effect of such changes, if any, may be.
Other U.S. Healthcare Laws and Compliance Requirements
If we obtain regulatory approval of our product candidates and launch them commercially in the U.S., we may be subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. Some of the laws that may affect our future ability to operate include:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| ● | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| ● | HIPAA, as amended by the Health Information Technology and Clinical Health Act (“HITECH”) and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
| ● | the federal transparency requirements under the Physician Payments Sunshine Act require manufacturers of FDA-approved drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to the Department of Health and Human Services information related to payments and other transfers of value to physicians, teaching hospitals, and certain advanced non-physician health care practitioners and physician ownership and investment interests; and |
| ● | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Moreover, some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines, or the relevant compliance guidance promulgated by the federal government, in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures to the extent that those laws impose requirements that are more stringent than the Physician Payments Sunshine Act. |
Europe/Rest of World Government Regulation
In addition to regulations in the U.S., we are and will be subject, either directly or through our potential partners, to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products, if approved.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in non-U.S. countries prior to the commencement of clinical trials or marketing of the product in those countries.
The approval process varies from country to country and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
25
In the European Union, medicinal products are subject to extensive pre- and post-marketing regulation by regulatory authorities at both the European Union and national levels. Additional rules also apply at the national level to the manufacture, import, export, storage, distribution and sale of controlled substances. In many European Union. member states the regulatory authority responsible for medicinal products is also responsible for controlled substances. Responsibility is, however, split in some member states. Generally, any company manufacturing or distributing a medicinal product containing a controlled substance in the European Union will need to hold a controlled substances license from the competent national authority and will be subject to specific record-keeping and security obligations. Separate import or export certificates are required for each shipment into or out of the member state.
Clinical Trials and Marketing Approval
Certain countries outside of the U.S. have a process that requires the submission of a clinical trial application much like an IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application (“CTA”) must be submitted to the competent national health authority and to independent ethics committees in each country in which a company intends to conduct clinical trials. Once the CTA is approved in accordance with a country's requirements and a company has received favorable ethics committee approval, clinical trial development may proceed in that country.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country, even though there is already some degree of legal harmonization in the European Union member states resulting from the national implementation of underlying European Union. legislation. In all cases, the clinical trials must be conducted in accordance with the International Conference on Harmonization (“ICH”) guidelines on GCP and other applicable regulatory requirements.
To obtain regulatory approval to place a drug on the market in the European Union, we must submit a marketing authorization application. This application is similar to the NDA in the U.S., with the exception of, among other things, country-specific document requirements. All application procedures require an application in the common technical document (“CTD”) format, which includes the submission of detailed information about the manufacturing and quality of the product, and non-clinical and clinical trial information. Drugs can be authorized in the European Union by using (i) the centralized authorization procedure, (ii) the mutual recognition procedure, (iii) the decentralized procedure or (iv) national authorization procedures.
The European Commission created the centralized procedure for the approval of human drugs to facilitate marketing authorizations that are valid throughout the European Union and, by extension (after national implementing decisions) in Iceland, Liechtenstein and Norway, which, together with European Union member states, comprise the European Economic Area (“EEA”). Applicants file marketing authorization applications with the EMA, where a relevant scientific committee reviews them, in most cases the Committee for Medicinal Products for Human Use (“CHMP”). The EMA forwards CHMP opinions to the European Commission, which uses them as the basis for deciding whether to grant a marketing authorization. This procedure results in a single marketing authorization granted by the European Commission that is valid across the European Union, as well as in Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for human drugs that are: (i) derived from biotechnology processes, such as genetic engineering, (ii) contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative diseases, autoimmune and other immune dysfunctions and viral diseases, (iii) officially designated "orphan drugs" (drugs used for rare human diseases) and (iv) advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines. The centralized procedure may, at the voluntary request of the applicant, also be used for human drugs which do not fall within the above-mentioned categories if the CHMP agrees that (a) the human drug contains a new active substance not yet approved on November 20, 2005; (b) it constitutes a significant therapeutic, scientific or technical innovation or (c) authorization under the centralized procedure is in the interests of patients at the European Union level.
Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of a marketing authorization application by the EMA is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP), with adoption of the actual marketing authorization by the European Commission thereafter. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest from the point of view of therapeutic innovation, defined by three cumulative criteria: the seriousness of the disease to be treated, the absence of an appropriate alternative therapeutic approach, and anticipation of exceptional high therapeutic benefit. In this circumstance, the EMA ensures that the evaluation for the opinion of the CHMP is completed within 150 days and the opinion issued thereafter.
For those medicinal products for which the centralized procedure is not available, the applicant must submit marketing authorization applications to the national medicines regulators through one of three procedures: (i) the mutual recognition procedure (which must be used if the product has already been authorized in at least one other European Union member state, and in which the European Union member states are required to grant an authorization recognizing the existing authorization
26
in the other European Union member state, unless they identify a serious risk to public health), (ii) the decentralized procedure (in which applications are submitted simultaneously in two or more European Union member states) or (iii) national authorization procedures (which results in a marketing authorization in a single European Union member state).
Mutual Recognition Procedure
The mutual recognition procedure (“MRP”) for the approval of human drugs is an alternative approach to facilitate individual national marketing authorizations within the European Union. Basically, the MRP may be applied for all human drugs for which the centralized procedure is not obligatory. The MRP is applicable to the majority of conventional medicinal products and must be used if the product has already been authorized in one or more member states.
The characteristic of the MRP is that the procedure builds on an already—existing marketing authorization in a member state of the European Union that is used as a reference in order to obtain marketing authorizations in other European Union member states. In the MRP, a marketing authorization for a drug already exists in one or more member states of the European Union and subsequently marketing authorization applications are made in other European Union member states by referring to the initial marketing authorization. The member state in which the marketing authorization was first granted will then act as the reference member state. The member states where the marketing authorization is subsequently applied for act as concerned member states. The concerned member states are required to grant an authorization recognizing the existing authorization in the reference member state unless they identify a serious risk to public health.
The MRP is based on the principle of mutual recognition by the European Union member states of their respective national marketing authorizations. Based on a marketing authorization in the reference member state, the applicant may apply for marketing authorizations in other member states. In such case, the reference member state shall update its existing assessment report about the drug in 90 days. After the assessment is completed, copies of the report are sent to all member states, together with the approved summary of product characteristics, labeling and package leaflet. The concerned member states then have 90 days to recognize the decision of the reference member state and the summary of product characteristics, labeling and package leaflet. National marketing authorizations shall be granted within 30 days of acknowledgement of the agreement.
If any European Union member state refuses to recognize the marketing authorization by the reference member state, on the grounds of potential serious risk to public health, the issue will be referred to a coordination group. Within a timeframe of 60 days, member states shall, within the coordination group, make all efforts to reach a consensus. If this fails, the procedure is submitted to an EMA scientific committee for arbitration. The opinion of this EMA Committee is then forwarded to the European Commission for the start of the decision-making process. As in the centralized procedure, this process entails consulting various European Commission Directorates General and the Standing Committee on Human Medicinal Products.
Data and Market Exclusivity in the European Union
In the European Union, NCEs qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator's data to assess a generic (abbreviated) application for eight years, after which generic marketing authorization can be submitted, and the innovator's data may be referenced, but not approved for two years. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization (“MA”) holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a NCE and the sponsor is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the drug if such company can complete a full marketing authorization application (“MAA”) with a complete database of pharmaceutical test, preclinical studies and clinical trials and obtain marketing approval of its product.
Pharmaceutical Coverage, Pricing and Reimbursement
Sales of pharmaceutical products approved for marketing in the U.S. by the FDA will depend, in part, on the extent to which the costs of the products will be covered by third-party payers, such as government health programs, and commercial insurance and managed health care organizations. These third-party payers are increasingly challenging the prices charged for medical products and services. Additionally, the containment of health care costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, utilization management and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our operating results. If these third-party payers do not consider our products to be cost-effective compared to other available therapies, they may not cover
27
our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (“MMA”) imposed requirements for the distribution and pricing of prescription drugs for Medicare beneficiaries and included a major expansion of the prescription drug benefit under Medicare Part D. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities that provide coverage of outpatient prescription drugs. Part D is available through both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Parts A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee.
Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval in the U.S. However, any negotiated prices for our products covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payers often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payers.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the “ACA”), was enacted with the goal of expanding coverage for the uninsured while at the same time containing overall health care costs. With regard to pharmaceutical products, among other things, the ACA expanded and increased industry rebates for drugs covered under Medicaid programs and made changes to the coverage requirements under the Medicare Part D program.
Individual states in the U.S. have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs and suppliers will be included in their healthcare programs Furthermore, there has been increased interest by third party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
In the U.S., Medicare covers certain drug purchases by the elderly and eligible disabled people and introduced a reimbursement methodology based on average sales prices for physician-administered drugs. In addition, Medicare may limit the number of drugs that will be covered in any therapeutic class. Ongoing cost reduction initiatives and future laws could decrease the coverage and price that we will receive for any approved products. While Medicare beneficiaries are limited to most elderly and certain disabled individuals, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates.
Among the provisions of the ACA of importance or potential importance to our product candidates are the following:
| ● | an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic products; |
| ● | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| ● | expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; |
| ● | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices; |
| ● | extension of manufacturers' Medicaid rebate liability; |
| ● | expansion of eligibility criteria for Medicaid programs; |
| ● | expansion of the entities eligible for discounts under the Public Health Service Act's pharmaceutical pricing program; |
28
| ● | new requirements to report financial arrangements with physicians and teaching hospitals (i.e., the Federal Physician Payment Sunshine Act, which has since been expanded to cover additional specified healthcare providers); |
| ● | a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and |
| ● | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we will receive for any approved product. Any reduction in payments from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products.
There remain judicial and political challenges to certain aspects of the ACA. In June 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special enrollment period from February 2021 to August 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to health care, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments to the statute, including the Bipartisan Budget Act of 2018, will remain in effect through 2030, with the exception of a temporary suspension from May 2020 through March 2022, unless additional Congressional action is taken. In addition, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. The likelihood of success of these and other measures is uncertain.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, some European Union jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines but monitor and control company profits. Such differences in national pricing regimes may create price differentials between European Union member states. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the U.S. In the European Union, the downward pressure on healthcare costs in general, particularly prescription medicines, has become intense. As a result, barriers to entry of new products are becoming increasingly high and patients are unlikely to use a drug product that is not reimbursed by their government.
Human Capital
As of March 25, 2024, we had eight full-time employees, of which four were engaged in R&D functions, three were engaged in general and administrative functions, and one was engaged in both R&D and general and administrative functions. We have no collective bargaining agreements with our employees and have not experienced any work stoppages. We consider our relationships with our employees to be good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new full-time employees. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder
29
value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Corporate Information
NeuroBo was incorporated under the laws of the State of Delaware in October 2014. Our principal executive offices are located at 545 Concord Avenue, Suite 210, Cambridge, Massachusetts, 02138. Our website address is www.neurobopharma.com. The information contained on or that can be accessed through our website is not a part of this report.
Item 1A.Risk Factors
Our business, prospects, financial condition or results of operations could be materially adversely affected by any of the risks and uncertainties set forth below, as well as in any amendments or updates reflected in subsequent filings with the Securities and Exchange Commission (the “SEC”). In assessing these risks, you should also refer to other information contained in this report, including our financial statements and related notes.
Risks Related to our Operations and to Development, Marketing, Commercialization and Regulation of Our Product Candidates
We have incurred losses since inception, we anticipate that we will incur continued losses for the foreseeable future. We require additional financing to accomplish our long-term business plan and failure to obtain necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our operations.
We have experienced net losses and negative cash flows from operating activities since our inception and have an accumulated deficit of $108.3 million as of December 31, 2023. It is possible we will never generate revenue or profit.
Although we are exploring financing opportunities and carefully monitoring the capital markets, we do not yet have any commitments for additional financing and may not be successful in our efforts to raise additional funds. There can be no assurances that additional financing will be available to us on satisfactory terms, or at all. If we are unable to raise sufficient additional capital (which is not assured at this time, particularly as a result of recent depressed capital market conditions), our long-term business plan may not be accomplished, and we may be forced to cease, reduce, or delay operations. For more information about our liquidity and capital resources, see “Liquidity and Capital Resources” in Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Raising additional capital may cause dilution to existing stockholders, restrict our operations or require us to relinquish rights to our technologies.
Existing stockholders could suffer dilution or be negatively affected by fixed payment obligations we may incur if we raise additional funds through the issuance of additional equity securities or debt. Furthermore, these securities may have rights senior to those of our common stock and could contain covenants or protective rights that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we need to secure additional financing, such additional fundraising efforts may divert our management and research efforts from our day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates.
Adverse global economic conditions could have a negative effect on our business, results of operations and financial condition and liquidity.
A general slowdown in the global economy, including a recession, or in a particular region or industry, an increase in trade tensions with U.S. trading partners, inflation or a tightening of the credit markets could negatively impact our business, financial condition and liquidity. Adverse global economic conditions have from time to time caused or exacerbated significant slowdowns in the industries and markets in which we operate, which have adversely affected our business and results of operations. Macroeconomic weakness and uncertainty also make it more difficult for us to accurately forecast revenue, gross margin and expenses, and may make it more difficult to raise or refinance debt.
Worldwide economic and social instability could adversely affect our revenue, financial condition, or results of operations.
The health of the global economy, and the credit markets and the financial services industry in particular, as well as the stability of the social fabric of our society, affects our business and operating results. The general economic and capital market conditions, both in the U.S. and worldwide, have been volatile in the past and at times have adversely affected our access to capital and increased the cost of capital. The capital and credit markets may not be available to support future capital raising
30
activity on favorable terms. If economic conditions decline, our future cost of equity or debt capital and access to the capital markets could be adversely affected. Our vendors and development partners may experience financial difficulties or be unable to borrow money to fund their operations, which may adversely impact their ability to purchase our products or to pay for our products on a timely basis, if at all. These economic conditions make it more difficult for us to accurately forecast and plan our future business activities.
Conditions in the banking system and financial markets, including the failure of banks and financial institutions, could have an adverse effect on our operations and financial results.
Actual events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, on March 10, 2023 and March 12, 2023, the Federal Deposit Insurance Corporation took control and was appointed receiver of Silicon Valley Bank, Signature Bank and Silvergate Capital Corp, respectively, after each bank was unable to continue their operations. Since then, additional financial institutions have experienced similar failures and have been placed into receivership. It is possible that other banks will face similar difficulty in the future.
Although we do not maintain any deposit accounts, credit agreements or letters of credit with any financial institution currently in receivership, we are unable to predict the extent or nature of the impacts of these evolving circumstances at this time. If, for example, other banks and financial institutions enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our existing cash, cash equivalents and investments may be threatened. While it is not possible at this time to predict the extent of the impact that the failure of these financial institutions or the high market volatility and instability of the banking sector could have on economic activity and our business in particular, the failure of other banks and financial institutions and the measures taken by governments, businesses and other organizations in response to these events could adversely impact our business, financial condition and results of operations.
We are developing DA-1241 for the treatment of MASH, an indication for which there is only one approved product. This makes it difficult to predict the timing and costs of the clinical development of DA-1241.
Our R&D efforts are focused in part on developing DA-1241 for the treatment of MASH, an indication for which there is only one approved product. The regulatory approval process for novel product candidates, such as DA-1241 for MASH, can be more expensive and take longer than for other, better known or extensively studied product candidates. In addition to Madrigal Pharmaceuticals’ approved product, other companies are in later stages of clinical trials for their potential MASH therapies, and we expect that the path for regulatory approval for MASH therapies may continue to evolve in the near term as these other companies refine their regulatory approval strategies and interact with regulatory authorities. Such evolution may impact our future clinical trial designs, including trial size and endpoints, in ways that we cannot predict today. Our anticipated development costs would likely increase if development of DA -1241 or any future product candidate is delayed because the FDA requires us to perform studies or trials in addition to, or different from, those that we currently anticipate. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to predict the timing or amount of any increase in our anticipated development costs.
We may be required to make significant payments under the 2022 License Agreement.
We have exclusive rights (other than in the Republic of Korea) to DA-1241 and DA-1726 for the specific indications provided in the 2022 License Agreement. Under the 2022 License Agreement, in consideration for the license, we made an upfront payment of 2,200 shares of our Series A Convertible Preferred Stock. As additional consideration for the license, we are required to pay Dong-A milestone payments upon the achievement of specified regulatory milestones and milestone payments upon the achievement of specified commercial milestones. Commencing on the first commercial sale of licensed products, we are obligated to pay royalties of single-digit percentages on annual net sales of the products covered by the license. If milestone or other non-royalty obligations become due, we may not have sufficient funds available to meet our obligations, which may materially adversely affect our business operations and financial condition.
Even if we obtain favorable clinical results, we may not be able to obtain regulatory approval for, or successfully commercialize DA-1241 and DA-1726
We are not permitted to market DA-1241 or DA-1726 in the U.S. until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. As a condition to submitting an NDA to the FDA for DA-1241 or DA-1726, we must successfully complete several clinical trials demonstrating efficacy and safety. DA-1241 and DA-1726 may not be successful in clinical trials or receive regulatory approval. Further, DA-1241 and DA-1726 may
31
not receive regulatory approval even if they are successful in clinical trials. Obtaining approval of an NDA is a complex, lengthy, expensive and uncertain process that typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, the policies or regulations, or the type and amount of clinical data necessary to gain approval, may change during a product candidate’s clinical development and may vary among jurisdictions. Our development activities could be harmed or delayed by a partial shutdown of the U.S. government, including the FDA. We have not obtained regulatory approval for any product candidate, and it is possible that DA-1241 and DA-1726 will never obtain regulatory approval. The FDA may delay, limit or deny approval of DA-1241 or DA-1726 for many reasons, including, among others:
| ● | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA for marketing approval; |
| ● | the FDA may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| ● | the FDA may not approve the formulation, labeling or specifications of DA-1241 or DA-1726; |
| ● | the FDA may require that we conduct additional clinical trials; |
| ● | the contract research organizations (“CROs”) or the clinical investigators that we retain to conduct our clinical trials may take actions outside of our control that materially adversely impact our clinical trials; |
| ● | we, our CROs or clinical investigators may fail to perform in accordance with the FDA’s good clinical practice (“GCP”) requirements; |
| ● | the FDA may disagree with our interpretation of data from our preclinical studies and clinical trials; |
| ● | the FDA may find deficiencies with the manufacturing processes or facilities of third-party manufacturers with which we contract; or |
| ● | the policies or regulations of the FDA may significantly change in a manner that renders our clinical data insufficient for approval or may require that we amend or submit new clinical protocols. |
In addition, similar reasons may cause the EMA or other regulatory authorities to delay, limit or deny approval of DA-1241 or DA-1726 outside the U.S. Any of these factors, many of which are beyond our control, could jeopardize our ability to obtain regulatory approval for and successfully market DA-1241 and DA-1726.
Alternatively, even if we obtain regulatory approval, that approval may be for indications or patient populations that are not as broad as we intend or desire or may require labeling that includes significant use or distribution restrictions or safety warnings. We may also be required to perform additional, unanticipated clinical trials to obtain approval or be subject to additional post marketing testing requirements to maintain regulatory approval. In addition, regulatory authorities may withdraw their approval of a product, or the FDA may require a risk evaluation and mitigation strategy (“REMS”) for a product, which could impose restrictions on our distribution. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
We may not be able to successfully obtain regulatory or marketing approval for, or successfully commercialize, any of our product candidates.
Although we currently have no drug product for sale and may never be able to develop marketable drug products, our business depends heavily on the successful clinical development, regulatory approval and commercialization of our product candidates.
The clinical trials of our product candidates are, and the manufacturing and marketing of our product candidates will be, subject to extensive and rigorous review and regulation by government authorities in the U.S. and in other countries where we intend to test and, if approved, market any product candidate. Before obtaining regulatory approvals for the commercial sale of any product candidate as a pharmaceutical product, we must successfully meet a number of critical developmental milestones, including:
| ● | developing dosages that will be well-tolerated, safe and effective; |
| ● | completing the development and scale-up to permit manufacture of our product candidates in commercial quantities and at acceptable costs; |
| ● | demonstrating through pivotal clinical trials that the product candidate is safe and effective in patients for the intended indication; |
32
| ● | establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers; and |
| ● | obtaining and maintaining exclusive rights, including patent and trade secret protection and non-patent exclusivity for our product candidates. |
The time necessary to achieve these developmental milestones for any individual product candidate is long and uncertain, and we may not successfully complete these milestones for any product candidates that we may develop.
We are continuing to test and develop our product candidates and may explore possible design or formulation changes to address safety, efficacy, manufacturing efficiency and performance issues to the extent any arise. The design of a clinical trial may be able to determine whether its results will support approval of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced or completed. There is no assurance that we will be able to design and complete a clinical trial to support marketing approval. Moreover, nonclinical and clinical data are often susceptible to multiple interpretations and analyses. A number of companies in the pharmaceutical and biotechnology industries have experienced significant setbacks in advanced clinical trials, even after promising results in earlier trials.
We may not be able to complete development of any product candidates that demonstrate safety and efficacy and that will have a commercially reasonable treatment and storage period. If we are unable to complete development of DA-1241, DA-1726 or any other product candidates that we may develop, we will not be able to commercialize and earn revenue from them.
The regulatory review and approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable, our business will be substantially harmed.
Of the large number of drugs in development in the U.S., only a small percentage receive FDA regulatory approval and are commercialized in the U.S. We are not permitted to market DA-1241, DA-1726 or any other product candidate as a pharmaceutical drug in the U.S. until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries or jurisdictions, such as the MAA in the European Union from the European Medicines Agency (“EMA”).
Successfully completing clinical trials and obtaining approval of an NDA is a complex, lengthy, expensive and uncertain process, and the FDA, or a comparable foreign regulatory authority, may delay, limit or deny approval of an NDA for many reasons, including, among others:
| ● | disagreement with the design or implementation of our clinical trials; |
| ● | disagreement with the sufficiency of our clinical trials; |
| ● | failure to demonstrate the safety and efficacy of the product candidate for the proposed indications; |
| ● | failure to demonstrate that any clinical and other benefits of the product candidate outweigh their safety risks; |
| ● | a negative interpretation of the data from our nonclinical studies or clinical trials; |
| ● | insufficient data collected from clinical trials or changes in the approval requirements that render our nonclinical and clinical data insufficient to support the filing of an NDA or to obtain regulatory approval; or |
| ● | changes in clinical practice in our approved products available for the treatment of the target patient population that could have an impact on the indications that we are pursuing for our product candidates. |
The FDA or a comparable foreign regulatory authority may also require more information, including additional nonclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or cause us to abandon the development program. Even if we obtain regulatory approval, our product candidates may be approved for fewer or more limited indications than we request, such approval may be contingent on the performance of costly post-marketing clinical trials, or we may not be allowed to include the labeling claims necessary or desirable for the successful commercialization of such product candidate.
Product candidates may cause undesirable side effects that could delay or prevent their marketing approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any, including marketing withdrawal.
Undesirable side effects caused by any of our product candidates that we may develop or acquire could cause us or the FDA or other regulatory authorities to interrupt, delay or halt our clinical trials and could result in more restrictive labels or the delay or denial of marketing approval by the FDA or other regulatory authorities of such product candidates. Results of our clinical
33
trials could reveal a high and unacceptable severity and prevalence of these or other side effects. In such an event, our trials could be suspended or terminated, and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. In addition, any drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly.
Further, clinical trials by their nature utilize a sample of the potential patient population. With a limited number of patients, rare and severe side effects of our product candidates may only be uncovered with a significantly larger number of patients exposed to the product candidate. If our product candidates receive marketing approval and we or others identify undesirable side effects caused by such product candidates (or any other similar drugs) after such approval, a number of potentially significant negative consequences could result, including:
| ● | regulatory authorities may withdraw or limit their approval of such product candidates; |
| ● | regulatory authorities may require the addition of labeling statements, such as a "boxed" warning or a contraindication; |
| ● | we may be required to recall the product, change the way such product candidates are distributed or administered, conduct additional clinical trials or change the labeling of the product candidates; |
| ● | regulatory authorities may require a Risk Evaluation and Mitigation Strategy (REMS) plan to mitigate risks, which could include medication guides to be distributed to patients, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools; |
| ● | we may be subject to regulatory investigations and government enforcement actions; |
| ● | we may be subject to fines, injunctions or the imposition of civil or criminal penalties; |
| ● | we may decide to remove such product candidates from the marketplace after they are approved; |
| ● | the product may be rendered less competitive, and sales may decrease; |
| ● | we could be sued and held liable for injury caused to individuals exposed to or taking our product candidates; and |
| ● | our reputation may suffer. |
We believe that any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidates and could substantially increase the costs of commercializing our product candidates, if approved, and significantly impact our ability to successfully commercialize our product candidates and generate revenues.
Delays in our clinical trials may lead to a delay in the submission of marketing approval applications and jeopardize our ability to potentially receive approvals and generate revenues from the sale of our products.
We may experience delays in ongoing and planned clinical trials. We do not know whether planned clinical trials will begin or enroll subjects on time, need to be redesigned or be completed on schedule, if at all. Clinical trials may be delayed, suspended or terminated for a variety of reasons, such as:
| ● | delay or failure in reaching agreement with the FDA or a comparable foreign regulatory authority on a trial design that we are able to execute; |
| ● | delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial; |
| ● | inability, delay or failure in identifying and maintaining a sufficient number of trial sites, many of which may already be engaged in competing clinical trial programs; |
| ● | issues with the manufacture of drug substance for use in clinical trials; |
| ● | delay or failure in recruiting and enrolling suitable subjects to participate in a trial; |
| ● | delay or failure in having subjects complete a trial or return for post-treatment follow-up; |
| ● | clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial; |
34
| ● | delay or failure in reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | delay or failure in obtaining IRB approval to conduct a clinical trial at each site; |
| ● | delays resulting from negative or equivocal findings of the Data Safety Monitoring Board (“DSMB”), if any; |
| ● | ambiguous or negative results; |
| ● | decision by the FDA, a comparable foreign regulatory authority, or recommendation by a DSMB to suspend or terminate clinical trials at any time for safety issues or for any other reason; |
| ● | conflicts affecting clinical trial sites and regions where clinical trials are being completed; |
| ● | lack of adequate funding to continue the product development program; or |
| ● | changes in governmental regulations or requirements. |
Any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
We may develop DA-1241 and DA-1726, and potentially future product candidates, in combination with other therapies, which exposes us to additional risks.
We may develop DA-1241 and DA-1726 and future product candidates in combination with one or more currently approved therapies. Even if any product candidate we develop were to receive marketing approval or be commercialized for use in combination with other existing therapies, we would continue to be subject to the risks that the FDA or similar regulatory authorities outside of the U.S. could revoke approval of the therapy used in combination with our product candidate or that safety, efficacy, manufacturing or supply issues could arise with these existing therapies. This could result in our own products being removed from the market or being less successful commercially.
We may also evaluate DA-1241 and DA-1726 or any other future product candidates in combination with one or more other therapies that have not yet been approved for marketing by the FDA or similar regulatory authorities outside of the U.S. We will not be able to market and sell DA-1241 and DA-1726 or any product candidate we develop in combination with any such unapproved therapies that do not ultimately obtain marketing approval. If the FDA or similar regulatory authorities outside of the U.S. do not approve these other drugs or revoke their approval of, or if safety, efficacy, manufacturing, or supply issues arise with, the drugs we choose to evaluate in combination with DA-1241 and DA-1726 or any other product candidate we develop, we may be unable to obtain approval of or market DA-1241 and DA-1726 or any other product candidate we develop.
Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside our control, including difficulties in identifying patients with MASH and significant competition for recruiting such patients in clinical trials.
Identifying and qualifying patients to participate in our clinical trials is critical to our success. We may encounter delays in enrolling, or be unable to enroll, a sufficient number of patients to complete any of our clinical trials, and even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials. In particular, as a result of the inherent difficulties in diagnosing MASH and the significant competition for recruiting patients with MASH in clinical trials, there may be delays in enrolling the patients we need to complete clinical trials on a timely basis, or at all. This risk may be more significant for us than other companies conducting clinical trials for the treatment of patients with MASH because we are enrolling only patients with a biopsy-confirmed diagnosis of MASH in our clinical trials.
Factors that may generally affect patient enrollment include:
| ● | the size and nature of the patient population; |
| ● | the number and location of clinical sites we enroll; |
| ● | competition with other companies for clinical sites or patients; |
| ● | the eligibility and exclusion criteria for the trial; |
35
| ● | the design of the clinical trial; |
| ● | inability to obtain and maintain patient consents; |
| ● | risk that enrolled participants will drop out before completion; and |
| ● | competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. |
In addition, if any significant adverse events or other side effects are observed in any of our future clinical trials, it may make it more difficult for us to recruit patients to our clinical trials and patients may drop out of our trials, or we may be required to abandon the trials or our development efforts of one or more product candidates altogether. Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays, which would increase our costs and have an adverse effect on our company.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new products is highly competitive. Our future success depends on our ability to demonstrate and maintain a competitive advantage with respect to the development and commercialization of our product candidates. Our objective is to develop and commercialize new products with superior efficacy, convenience, tolerability and safety. In many cases, the products that we commercialize will compete with existing, market-leading products.
Many of our potential competitors have significantly greater financial, manufacturing, marketing, drug development, technical and human resources than we do. Large pharmaceutical companies, in particular, have extensive experience in clinical testing, obtaining regulatory approvals, recruiting patients and in manufacturing pharmaceutical products. In particular, these companies have greater experience and expertise in securing government contracts and grants to support their R&D efforts, conducting testing and clinical trials, obtaining regulatory approvals to market products, manufacturing such products on a broad scale and marketing approved products. These companies also have significantly greater research and marketing capabilities than we do and may also have products that have been approved or are in late stages of development, and have collaborative arrangements in our target markets with leading companies and research institutions. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel compounds or to in-license novel compounds that could make the product that we develop obsolete. As a result of all of these factors, our competitors may succeed in obtaining patent protection and/or FDA approval or discovering, developing and commercializing products before, or more effectively than, we do. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. If we are not able to compete effectively against potential competitors, our business will not grow, and our financial condition and operations will suffer.
MASH
There is only one approved treatment of MASH, Madrigal Pharmaceuticals’ thyroid hormone receptor beta agonist. However, various therapeutics are used off-label for the treatment of MASH, including vitamin E (an antioxidant), insulin sensitizers (e.g., metformin, pioglitazone), antihyperlipidemic agents (e.g., gemfibrozil), pentoxifylline and ursodeoxycholic acid (UDCA). There are several product candidates in Phase 3 or earlier clinical or preclinical development for the treatment of MASH, including Novo Nordisk’s GLP1 agonist semaglutide, Eli Lilly’s GLP1R and GIP dual agonist tirzepatide, Akero Therapeutics’s FGF21 analog efruxifermin, 89 Bio’s FGF21 analog pegaozafermin, Inventiva’s pan-peroxisome proliferator-activated receptor agonist, Boston Pharmaceuticals and Roche’s fibroblast growth factor 21 analogs, and farnesoid X receptor agonists from Intercept Pharmaceuticals Inc., among others. Additional pharmaceutical and biotechnology companies with product candidates in development for the treatment of MASH include AstraZeneca plc, Altimmune Inc., Boehringer Ingelheim GmbH, Bristol-Myers Squibb Company, Durect Corporation, Galectin Therapeutics Inc., Galmed Pharmaceuticals Ltd., Immuron Ltd., Ionis Pharmaceuticals, Inc., Islet Sciences, Inc., MediciNova, Inc., NGM Biopharmaceuticals, Inc., NuSirt Sciences Inc., Pfizer Inc., Viking Therapeutics, Inc. and Zydus Pharmaceuticals (USA) Inc. MASH is a complex disease and we believe that it is unlikely that any one therapeutic option will be optimal for every MASH patient.
Obesity
Due to the growing overweight and obesity epidemic and consumer demand, there are many competitors in the field of obesity treatment. Obesity treatments range from behavioral modification to drugs and medical devices, and surgery,
36
generally as a last resort. If DA-1726 were approved for obesity, our primary competition in the obesity treatment market would currently be from approved and marketed products, including semaglutide (WEGOVY®) and tirzepatide (Zepbound®). Further competition could arise from products currently in development, including among others, with GLP1R/GCGR dual agonists, Boehringer Ingelheim, Merck/Hanmi Pharmaceutical, AstraZeneca, Altimmune, Innovent Biologics/Eli Lilly, Carmot and D&D Pharma; with GLP1R/GCGR/GIP triple agonists, Hanmi Pharmaceutical and Eli Lilly; Amgen with its GLP-1 agonist/GIP antagonist antibody; and Novo Nordisk with Amylin and Amylin-GLP-1 combination. To the extent any of our product candidates are approved for obesity, the commercial success of our product will also depend on our ability to demonstrate benefits over the then-prevailing standard of care. Finally, morbidly obese patients sometimes undergo a gastric bypass procedure, with salutary effects on the many co-morbid conditions of obesity.
T2DM
There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for T2DM. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among hospitals, physicians, patients and healthcare payors.
Even if we obtain regulatory approval for any of our product candidates that we may develop or acquire in the future, the product may not gain market acceptance among hospitals, physicians, health care payors, patients and the medical community. Market acceptance of any of our product candidates for which we receive regulatory approval depends on a number of factors, including:
| ● | the clinical indications for which the product candidate is approved; |
| ● | acceptance by major operators of hospitals, physicians and patients of the product candidate as a safe and effective treatment, particularly the ability of our product candidates to establish themselves as a new standard of care in the treatment paradigm for the indications that we are pursuing; |
| ● | the potential and perceived advantages of our product candidates over alternative treatments as compared to the relative costs of the product candidates and alternative treatments; |
| ● | the willingness of physicians to prescribe, and patients to take, a product candidate that is based on a botanical source; |
| ● | the prevalence and severity of any side effects with respect to our product candidates, and any elements that may be imposed by the FDA under a REMS program that could discourage market uptake of the products; |
| ● | the availability of adequate reimbursement and pricing for any approved products by third party payors and government authorities; |
| ● | inability of certain types of patients to take our product; |
| ● | demonstrated ability to treat patients and, if required by any applicable regulatory authority in connection with the approval for target indications, to provide patients with incremental cardiovascular disease benefits, as compared with other available therapies; |
| ● | the relative convenience and ease of administration of our product candidates, including as compared with other treatments available for approved indications; |
| ● | limitations or warnings contained in the labeling approved by the FDA; |
| ● | availability of alternative treatments already approved or expected to be commercially launched in the near future; |
| ● | the effectiveness of our sales and marketing strategies; |
| ● | guidelines and recommendations of organizations involved in research, treatment and prevention of various diseases that may advocate for alternative therapies; |
37
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage; |
| ● | physicians or patients may be reluctant to switch from existing therapies even if potentially more effective, safe or convenient; |
| ● | efficacy, safety, and potential advantages compared to alternative treatments; |
| ● | the ability to offer our product for sale at competitive prices; |
| ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| ● | any restrictions on the use of our product together with other medications; |
| ● | interactions of our product with other medicines patients are taking; and |
| ● | the timing of market introduction of our products as well as competitive products. |
There may be delays in getting our product candidates, if approved, on hospital or insurance formularies or limitations on coverages that may be available in the early stages of commercialization for newly approved drugs. If any of our product candidates are approved but fail to achieve market acceptance among hospitals, physicians, patients or health care payors, we will not be able to generate significant revenues, which would have a material adverse effect on our business, prospects, financial condition and results of operations.
Even if we are able to commercialize a future pharmaceutical drug candidate, the profitability of such product candidate will likely depend in significant part on third-party reimbursement practices, which, if unfavorable, would harm our business.
Our ability to commercialize a drug successfully will depend in part on the extent to which coverage and adequate reimbursement will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that coverage will be available for any product candidate that we commercialize and, if coverage is available, whether the level of reimbursement will be adequate. Assuming we obtain coverage for our product candidates, if approved, by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Patients are unlikely to use a product candidate, if approved, unless coverage is provided, and reimbursement is adequate to cover all or a significant portion of the cost of our products. Therefore, coverage and adequate reimbursement is critical to new product acceptance. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which a product candidate is approved by the FDA or similar regulatory authorities outside the U.S. Moreover, eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for a new product, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the product and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost medicines and may be incorporated into existing payments for other services. Net prices for products may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of medicines from countries where they may be sold at lower prices than in the U.S. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. However, no uniform policy requirement for coverage and reimbursement for drug products exists among third-party payors in the U.S. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
38
Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products that we develop could have an adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any product candidate that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any product that we may develop. Product liability claims might be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with any of our products or future product candidate during product testing, manufacturing, marketing or sale. For example, we may be sued on allegations that a product candidate caused injury or that the product is otherwise unsuitable. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, including as a result of interactions with alcohol or other drugs, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts.
If we cannot successfully defend against claims that our product caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| ● | decreased demand for any product candidate that we are developing; |
| ● | injury to our reputation and significant negative media attention; |
| ● | withdrawal of clinical trial participants; |
| ● | increased FDA warnings on product labels; |
| ● | significant costs to defend the related litigation; |
| ● | substantial monetary awards to trial participants or patients; |
| ● | distraction of management's attention from our primary business; |
| ● | loss of revenue; |
| ● | the inability to commercialize any product candidate that we may develop; |
| ● | the removal of a product from the market; and |
| ● | increased insurance costs. |
If we or our third-party manufacturers fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have an adverse effect on the success of our business.
Our R&D activities involve the controlled use of potentially hazardous substances, including chemical and biological materials, by us and our third-party manufacturers. Our manufacturers are subject to federal, state and local laws and regulations in the U.S. and abroad governing laboratory procedures and the use, manufacture, storage, handling and disposal of medical and hazardous materials. Although we believe that our manufacturers' procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. As a result of any such contamination or injury, we may incur liability or local, city, state or federal authorities may curtail the use of these materials and interrupt our business operations. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources. We do not have any insurance for liabilities arising from medical or hazardous materials. Although we maintain workers' compensation insurance to cover us for costs and expenses, we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. Compliance with applicable environmental, health and safety laws and regulations are expensive, and current or future environmental regulations may impair our research, development and production efforts, which could harm our business, prospects, financial condition or results of operations
39
We rely and will continue to rely on collaborative partners regarding the development of our research programs and product candidates.
We are and expect to continue to be dependent on collaborations with partners relating to the development and commercialization of our existing and future research programs and product candidates. In particular, we rely on Dong-A to provide services with respect to our development of DA-1241 and DA-1726. In addition, we have had, have and will continue to have discussions on potential partnering opportunities with various pharmaceutical companies. If we fail to enter into or maintain collaborative agreements on reasonable terms or at all, our ability to develop our existing or future research programs and product candidates could be delayed, the commercial potential of our products could change, and our costs of development and commercialization could increase.
Our dependence on collaborative partners subjects it to a number of risks, including, but not limited to, the following:
| ● | We may not be able to control the amount or timing of resources that collaborative partners devote to our research programs and product candidates; |
| ● | We may be required to relinquish significant rights, including intellectual property, marketing and distribution rights; |
| ● | We rely on the information and data received from third parties regarding our research programs and product candidates and will not have control of the process conducted by the third party in gathering and composing such data and information. We may not have formal or appropriate guarantees from our contract parties with respect to the quality and the completeness of such data; |
| ● | A collaborative partner may develop a competing product either by itself or in collaboration with others, including one or more of our competitors; |
| ● | Our collaborative partners’ willingness or ability to complete their obligations under our collaboration arrangements may be adversely affected by business combinations or significant changes in a collaborative partner’s business strategy; and/or |
| ● | We may experience delays in, or increases in the costs of, the development of our research programs and product candidates due to the termination or expiration of collaborative R&D arrangements. |
If, in the future, we are unable to establish sales and marketing capabilities or to selectively enter into agreements with third parties to sell and market our product candidates, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product for which we retain sales and marketing responsibilities, we must either develop a sales and marketing organization or outsource these functions to other third parties. In the future, we may choose to build a focused sales and marketing infrastructure to sell some of our product candidates if and when they are approved.
There are risks involved both with establishing our own sales and marketing capabilities and with entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
| ● | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| ● | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future pharmaceutical products; and |
| ● | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenue or the profitability of these product revenue may be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product
40
candidates or may be unable to do so on terms that are favorable to us. We may have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
Any product candidate for which we obtain marketing approval could be subject to marketing restrictions or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products.
Any pharmaceutical product candidate for which we obtain marketing approval will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements, quality assurance and corresponding maintenance of records and documents and requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the medicine. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure that they are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing and/or promotion.
In addition, later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| ● | restrictions on such products, manufacturers or manufacturing processes; |
| ● | restrictions on the labeling, marketing, distribution or use of a product; |
| ● | requirements to conduct post-approval clinical trials; |
| ● | warning or untitled letters; |
| ● | withdrawal of the products from the market; |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; |
| ● | recall of products; |
| ● | fines, restitution or disgorgement of profits or revenue; |
| ● | suspension or withdrawal of marketing approvals for the drug products; |
| ● | refusal to permit the import or export of our products; |
| ● | product seizure; and |
| ● | injunctions or the imposition of civil or criminal penalties. |
We or any potential collaborator may never receive regulatory approval to market our product candidates outside of the U.S.
The activities associated with the development and commercialization of pharmaceutical drugs are subject to comprehensive regulation by the FDA, other regulatory agencies in the U.S. and by comparable authorities in other countries. Failure to obtain regulatory approval for our product candidates will prevent us or any potential collaborator from commercializing our product candidates as pharmaceutical drugs. We have not received regulatory approval to market any of our product candidates in any jurisdiction, and we do not expect to obtain FDA or any other regulatory approvals to market any of our product candidates for the foreseeable future, if at all. The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon the type, complexity and novelty of the product candidates involved.
41
We may seek to avail ourselves of mechanisms to expedite and/or reduce the cost for development or approval of any of our product candidates or product candidates we may pursue in the future, such as fast track designation or orphan drug designation, but such mechanisms may not actually lead to a faster or less expensive development or regulatory review or approval process.
We may seek fast track designation, priority review, orphan drug designation, or accelerated approval for any product candidate we may pursue in the future. For example, if a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply for FDA fast track designation. However, the FDA has broad discretion with regard to these mechanisms, and even if we believe a particular product candidate is eligible for any such mechanism, we cannot assure you that the FDA would decide to grant it. Even if we obtain fast track or priority review designation or pursue an accelerated approval pathway, we may not experience a faster and/or less costly development process, review or approval compared to conventional FDA procedures. The FDA may withdraw a particular designation if it believes that the designation is no longer supported by data from our clinical development program.
Current and future legislation may increase the difficulty and cost of obtaining marketing approval of and commercialization of our product candidates and affect the prices we may obtain.
In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval. See the section titled “Government Regulation” in above Item 1. Business.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals, if any, of our product candidates may be. In addition, increased scrutiny by the U.S. Congress of the FDA's approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing conditions and other requirements.
Governments outside the U.S. tend to impose strict price controls, which may adversely affect our revenues, if any.
In some countries, particularly the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
Our relationships with healthcare providers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings, among other penalties and consequences.
Healthcare providers and third-party payors will play a primary role in the recommendation and prescription of any product candidate for which we obtain marketing approval. Our arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any product candidate for which we obtain marketing approval. Restrictions and obligations under applicable federal and state healthcare laws and regulations are noted in “Government Regulation” in above Item 1. Business.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to it, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
42
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department's Office of Foreign Assets Controls, the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other partners from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties for clinical trials outside of the U.S. to sell our products abroad and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other partners, even if it does not explicitly authorize or have actual knowledge of such activities. Our violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
Our ability to use our NOLs to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of Internal Revenue Code of 1986, as amended (the “Code”), a corporation that undergoes an "ownership change" is subject to limitations on our ability to utilize our carryforwards to offset future taxable income. Our existing net operating loss (“NOL”) carryforwards were subject to limitation arising from an ownership change related to the Dong-A Financing and the underwritten public offering we closed on in November 2022 (the “2022 Public Offering”). Future changes in our stock ownership, some of which are outside of our control, could result in further ownership changes under Section 382 of the Code. There is also a risk that due to regulatory changes, such as suspensions on the use of NOL carryforwards, or other unforeseen reasons, our existing and any future NOL carryforwards could expire or otherwise be unavailable to offset future income tax liabilities.
Tax matters, including the changes in corporate tax rates, disagreements with taxing authorities and imposition of new taxes could impact the results of our operations and financial condition.
We are subject to income and other taxes in the U.S. and our operations, plans and results are affected by tax and other initiatives. On December 22, 2017, comprehensive changes to the Code were signed into law, informally titled the Tax Cuts and Jobs Act (the “Tax Act”). The Tax Act included significant changes that could materially impact the taxation of corporations, like us, including among other things, changes to the corporate income tax rate, limitation of the tax deduction for interest expense to business interest income plus 30% of adjusted taxable income (except for certain small businesses), immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifying or repealing many business deductions and credits (including changes to the orphan drug tax credit and changes to the deductibility of research and experimental expenditures that will be effective in the future). The Tax Act also included a limitation of the deduction for net operating losses (“NOLs”) generated in tax years beginning after December 31, 2017 to 80% of current year taxable income and the general elimination of carrybacks of NOLs generated in taxable years ending after December 31, 2017. However, the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) signed into law in March 2020, provided that NOLs generated in a taxable year beginning in 2018, 2019 or 2020, may now be carried back five years. In addition, the 80% taxable income limitation is temporarily removed, allowing NOLs to fully offset net taxable income. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the Tax Act and any future tax reform is uncertain and our business and financial condition could be adversely affected. The impact of the Tax Act and any future tax reform on holders of our common stock is likewise uncertain and could be adverse.
We are also subject to regular reviews, examinations, and audits by the IRS and other taxing authorities with respect to our taxes. Although we believe our tax estimates are reasonable, if a taxing authority disagrees with the positions we have taken, we could face additional tax liability, including interest and penalties. There can be no assurance that payment of such additional amounts upon final adjudication of any disputes will not have a material impact on our results of operations and financial position.
We also need to comply with new, evolving or revised tax laws and regulations. The enactment of or increases in tariffs, or other changes in the application or interpretation of the Tax Act, or on specific products that we may ultimately sell or with which our products compete, may have an adverse effect on our business or on our results of operations.
43
Inadequate funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, the ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies on which the combined organization's operations may rely, including those that fund R&D activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Federal legislation and actions by state and local governments may permit reimportation of drugs from foreign countries into the U.S., including foreign countries where the drugs are sold at lower prices than in the U.S., which could adversely affect our operating results.
We may face competition for our product candidates, if approved, from cheaper alternatives sourced from foreign countries that have placed price controls on pharmaceutical products. The Medicare Modernization Act contains provisions that may change U.S. importation laws and expand pharmacists' and wholesalers' ability to import cheaper versions of an approved drug and competing products from Canada, where there are government price controls. These changes to U.S. importation laws will not take effect unless and until the Secretary of Health and Human Services (“HHS”) certifies that the changes will pose no additional risk to the public's health and safety and will result in a significant reduction in the cost of products to consumers. On August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022 (“IRA”), which, among other provisions, included several measures intended to lower the cost of prescription drugs and related healthcare reforms. Further, the Biden administration released an additional executive order on October 14, 2022, directing HHS to submit a report on how the Center for Medicare and Medicaid Innovation can be further leveraged to test new models for lowering drug costs for Medicare and Medicaid beneficiaries.
It is unclear whether these executive orders or similar policy initiatives will be implemented in the future. Individual states in the U.S. have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. It remains to be seen how these actions will affect NeuroBo and the pharmaceutical industry as a whole.
Risks Related to Dependence on Third Parties
We have relied and will rely on third-party clinical research organizations (CROs) to conduct our preclinical studies and clinical trials. If these CROs do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon CROs and clinical data management organizations to monitor and manage data for our ongoing preclinical and clinical programs. Although we control only certain aspects of their activities, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We also rely on third parties to conduct our preclinical studies in accordance with Good Laboratory Practice (“GLP”) requirements and the Laboratory Animal Welfare Act of 1966 requirements. We, our CROs and our clinical trial sites are required to comply with regulations and current Good Clinical Practices (“GCP”), and comparable foreign requirements to ensure that the health, safety and rights of patients are protected in clinical trials, and that data integrity is assured. Regulatory authorities ensure compliance with GCP requirements through periodic inspections of trial sponsors and trial sites. If we, any of our CROs or our clinical trial sites fail to comply with applicable GCP requirements, the clinical data generated in our clinical trials, or a specific site may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications.
44
Our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical programs. If CROs do not successfully carry out their contractual obligations or meet expected timelines or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
We rely on third parties to manufacture our product candidates and preclinical and clinical drug supplies.
We have no experience manufacturing our product candidates on a large clinical or commercial scale and have no manufacturing facility. We currently have no plans to build our own clinical or commercial scale manufacturing capabilities. We currently work exclusively with Dong-A as the sole manufacturer for the production of DA-1241 and DA-1726. To meet our projected needs for clinical supplies to support our activities for DA-1241 and DA-1726 through regulatory approval and commercial manufacturing, Dong-A will need to provide sufficient scale of production for these projected needs. If any issues arise in the manufacturing and we are unable to arrange for alternative third-party manufacturing sources, we are unable to find an alternative third party capable of reproducing the existing manufacturing method or we are unable to do so on commercially reasonable terms or in a timely manner, we may not be able to complete development of our product candidates, or market or distribute them.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured our product candidates and preclinical and clinical drug supplies, including:
| ● | reliance on the third party for regulatory compliance and quality assurance; |
| ● | the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control (including a failure to synthesize and manufacture our product candidates or any products that we may eventually commercialize in accordance with our specifications); |
| ● | the possibility of termination or nonrenewal of the agreement by the third party, based on our own business priorities, at a time that is costly or damaging to us; |
| ● | delay in, or failure to obtain, regulatory approval of any of our product candidates because of the failure by our third-party manufacturer to comply with cGMP or failure to scale up manufacturing processes; and |
| ● | current manufacturer and any future manufacturers may not be able to manufacture our product candidates at a cost or in quantities or in a timely manner necessary to make commercially successful products. |
If third-party manufacturers do not successfully carry out their contractual obligations or meet expected timelines or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates.
We may engage in future acquisitions, mergers or in-licenses of technology that could disrupt our business, cause dilution to the organization's stockholders and harm our financial condition and operating results.
We may, in the future, make acquisitions or licenses of, or investments in, companies, products or technologies that we believe are a strategic or commercial fit with our current product candidates and business or otherwise offer opportunities for us. In connection with these acquisitions, mergers or investments, the organization may:
| ● | issue stock that would dilute our stockholders' percentage of ownership; |
| ● | expend cash; |
| ● | incur debt and assume liabilities; and |
| ● | incur amortization expenses related to intangible assets or incur large and immediate write-offs. |
We also may be unable to find suitable acquisition, merger or license candidates and we may not be able to complete acquisitions, mergers or licenses on favorable terms, if at all. If we do complete an acquisition, merger or license, we cannot assure you that it will ultimately strengthen our competitive position or that it will not be viewed negatively by customers,
45
financial markets or investors. Further, future acquisitions, mergers or licenses could also pose numerous additional risks to our operations, including:
| ● | problems integrating the purchased or licensed business, products or technologies; |
| ● | increases to our expenses; |
| ● | the failure to have discovered undisclosed liabilities of the acquired or licensed asset or company; |
| ● | diversion of management's attention from their day-to-day responsibilities; |
| ● | harm to our operating results or financial condition; |
| ● | entrance into markets in which we have limited or no prior experience; and |
| ● | potential loss of key employees, particularly those of the acquired entity. |
We may not be able to complete one or more acquisitions or mergers or effectively integrate the operations, products or personnel gained through any such acquisition without a material adverse effect on our business, financial condition and results of operations.
We may form or seek strategic alliances or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
We may form or seek strategic alliances, create joint ventures or collaborations or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our products and any future product candidates that we may develop. Any strategic alliance or collaboration may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. Our likely collaborators include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. If we enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our products or any future product candidate. Our ability to generate revenues from these arrangements will depend on our collaborators' abilities to successfully perform the functions assigned to them in these arrangements. We cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction.
Collaborations involving our product candidates, or any future product candidate pose the following risks to us:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| ● | collaborators may not perform their obligations as expected; |
| ● | collaborators may not pursue development and commercialization or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator's strategic focus or available funding or external factors such as an acquisition that diverts resources or creates competing priorities; |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive; |
| ● | a collaborator with marketing and distribution rights to one or more product candidates may not commit sufficient resources to the marketing and distribution of any such product candidate; |
| ● | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
46
| ● | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| ● | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidate or that result in costly litigation or arbitration that diverts management’s attention and resources; |
| ● | we may lose certain valuable rights under circumstances identified in our collaborations, including if we undergo a change of control; |
| ● | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates; |
| ● | collaborators may learn about our discoveries and use this knowledge to compete with us in the future; |
| ● | the results of collaborators' preclinical or clinical studies could harm or impair other development programs; |
| ● | there may be conflicts between different collaborators that could negatively affect those collaborations and potentially others; |
| ● | the number and type of our collaborations could adversely affect our attractiveness to future collaborators or acquirers; |
| ● | collaboration agreements may not lead to development or commercialization of our product candidate in the most efficient manner or at all. If our present or future collaborator were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program under such collaboration could be delayed, diminished or terminated; and |
| ● | collaborators may be unable to obtain the necessary marketing approvals. |
If future collaboration partners fail to develop or effectively commercialize our product candidates or any future product candidate for any of these reasons, such product candidate may not be approved for sale and our sales of such product candidate, if approved, may be limited, which would have an adverse effect on our operating results and financial condition.
Our employees, principal investigators, CROs and consultants may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk that our employees, principal investigators, CROs and consultants may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, to provide accurate information to the FDA or comparable foreign regulatory authorities, to comply with manufacturing standards we have established, to comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee or third-party misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity, such as employee training, may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending such action or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Risks Related to Intellectual Property
If we are unable to obtain and maintain sufficient intellectual property rights, our competitive position could be harmed.
We depend on our ability to protect our proprietary technology. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing and other agreements with employees and third parties, all of which offer only limited protection. Our success depends in large part on our ability to obtain and maintain patent protection in the U.S. and other countries with
47
respect to our proprietary technology and products. Where we have the right to do so under our license agreements, we seek to protect our proprietary position by filing patent applications in the U.S. and abroad related to our novel technologies and products that are important to our business.
The patent positions of pharmaceutical and biotechnology companies generally are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patents, including those patent rights licensed to us by third parties, are highly uncertain.
The steps we have taken to police and protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the U.S. The rights already granted under any of our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages that we are seeking. If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
With respect to patent rights, we do not know whether any of our pending patent applications for any of our product candidates will result in the issuance of patents that protect our technology or products, or which will effectively prevent others from commercializing competitive technologies and products. Our pending applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent is issued from such applications. Further, the examination process may require us or our licensors to narrow the claims, which may limit the scope of patent protection that may be obtained. Although our license agreement with Dong-A includes a number of issued patents that are exclusively licensed to us, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, issued patents that we own or have licensed from third parties may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents, or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products. Protecting against the unauthorized use of our patented technology, trademarks and other intellectual property rights is expensive, difficult and may, in some cases, not be possible. In some cases, it may be difficult or impossible to detect third party infringement or misappropriation of our intellectual property rights, even in relation to issued patent claims, and proving any such infringement may be even more difficult.
Laws and rulings by U.S. courts make it difficult to predict how patents will be issued or enforced in the biotechnology industry.
Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. There have been numerous changes to the patent laws and to the rules of the USPTO which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For example, the Leahy-Smith America Invents Act, which was signed into law in 2011, includes a transition from a "first-to-invent" system to a "first- to-file" system, and changes the way issued patents are challenged. Certain changes, such as the institution of inter partes review proceedings, came into effect in September 2012. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and, if obtained, to enforce or defend them in litigation or post-grant proceedings, all of which could harm our business.
Furthermore, the patent positions of companies engaged in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Two cases involving diagnostic method claims and "gene patents" have been decided by the Supreme Court. In March 2012, the Supreme Court issued a decision in Mayo Collaborative Services v. Prometheus Laboratories, Inc. (“Prometheus”), a case involving patent claims directed to measuring a metabolic product in a patient to optimize a drug dosage amount for the patient. According to the Supreme Court, the addition of well-understood, routine or conventional activity such as "administering" or "determining" steps was not enough to transform an otherwise patent ineligible natural phenomenon into patent eligible subject matter. In July 2012, the USPTO issued guidance indicating that process claims directed to a law of nature, a natural phenomenon or an abstract idea that do not include additional elements or steps that integrate the natural principle into the claimed invention such that the natural principle is practically applied and the claim amounts to significantly more than the natural principle itself should be rejected as directed to non-statutory subject matter. In June 2013, the Supreme Court issued its decision in Association for Molecular Pathology v. Myriad Genetics, Inc., (“Myriad”), a case involving patent claims held by Myriad Genetics, Inc. relating to the breast cancer susceptibility genes BRCA1 and BRCA2. Myriad held that isolated segments of naturally occurring DNA, such as the DNA constituting the BRCA1 and BRCA2 genes, is not patent eligible subject matter, but that complementary DNA, which is an artificial construct that may be created from RNA transcripts of genes, may be patent eligible.
48
We cannot assure you that our current patent protection and our efforts to seek patent protection for our technology and products will not be negatively impacted by the decisions described above, rulings in other cases or changes in guidance or procedures issued by the USPTO.
Moreover, although the Supreme Court has held in Myriad that isolated segments of naturally occurring DNA are not patent-eligible subject matter, certain third parties could allege that activities that we may undertake infringe other gene-related patent claims, and we may deem it necessary to defend against these claims by asserting non-infringement and/or invalidity positions, or pay to obtain a license to these claims. In any of the foregoing or in other situations involving third-party intellectual property rights, if we are unsuccessful in defending against claims of patent infringement, we could be forced to pay damages or be subjected to an injunction that would prevent us from utilizing the patented subject matter. Such outcomes could harm our business.
We may not be able to protect or practice our intellectual property rights throughout the world.
In jurisdictions where we have not obtained patent protection, competitors may use our intellectual property to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where it is more difficult to enforce a patent as compared to the U.S. competitor products may compete with our product candidates, if approved, or any future product candidate in jurisdictions where we do not have issued or granted patents or where we issued or granted patent claims or other intellectual property rights are not sufficient to prevent competitor activities in these jurisdictions. The legal systems of certain countries, particularly certain developing countries, make it difficult to enforce patents and such countries may not recognize other types of intellectual property protection, particularly that relating to pharmaceuticals. This could make it difficult for us to prevent the infringement of our patents or marketing of competing products in violation of our proprietary rights generally in certain jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the U.S., and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we, or our licensors, encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished, and we may face additional competition from others in those jurisdictions. Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we, or any of our licensors, are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position in the relevant jurisdiction may be impaired and our business and results of operations may be adversely affected.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
In addition to the possibility of litigation relating to infringement claims asserted against us, we may become a party to other patent litigation and other proceedings, including inter partes review proceedings, post-grant review proceedings, derivation proceedings declared by the USPTO and similar proceedings in foreign countries, regarding intellectual property rights with respect to our current or future technologies or product candidates or products. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace.
Competitors may infringe or otherwise violate our intellectual property, including patents that may be issued to or be licensed by us. As a result, we may be required to file claims in an effort to stop third-party infringement or unauthorized use. Any such claims could provoke these parties to assert counterclaims against us, including claims alleging that we infringe their patents or other intellectual property rights. This can be prohibitively expensive, particularly for a company of our size, and time-consuming, and even if we are successful, any award of monetary damages or other remedy we may receive may not be commercially valuable. In addition, in an infringement proceeding, a court may decide that our asserted intellectual property is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our intellectual property does not cover our technology. An adverse determination in any litigation or defense proceedings could put our intellectual property at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not being issued.
49
If the breadth or strength of our patent or other intellectual property rights is compromised or threatened, it could allow third parties to commercialize our technology or products or result in our inability to commercialize our technology and products without infringing third-party intellectual property rights. Further, third parties may be dissuaded from collaborating with us.
Interference or derivation proceedings brought by the USPTO or its foreign counterparts may be necessary to determine the priority of inventions with respect to our patent applications, and we may also become involved in other proceedings, such as re-examination proceedings, before the USPTO or its foreign counterparts. Due to the substantial competition in the pharmaceutical space, the number of such proceedings may increase. This could delay the prosecution of our pending patent applications or impact the validity and enforceability of any future patents that we may obtain. In addition, any such litigation, submission or proceeding may be resolved adversely to us and, even if successful, may result in substantial costs and distraction to our management.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. Moreover, intellectual property law relating to the fields in which we operate is still evolving and, consequently, patent and other intellectual property positions in our industry are subject to change and are often uncertain. We may not prevail in any of these suits or other efforts to protect our technology, and the damages or other remedies awarded, if any, may not be commercially valuable. During the course of this type of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, the market price for the combined organization's common stock could be significantly harmed.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates, and to use our proprietary technologies without infringing the proprietary rights of third parties. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference and various post grant proceedings before the USPTO or non-U.S. opposition proceedings. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future.
As a result of any such infringement claims, or to avoid potential claims, we may choose or be compelled to seek intellectual property licenses from third parties. These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us likely would be nonexclusive, which would mean that our competitors also could obtain licenses to the same intellectual property. Ultimately, we could be prevented from commercializing a product candidate or technology or be forced to cease some aspect of our business operations if, as a result of actual or threatened infringement claims, we are unable to enter into licenses of the relevant intellectual property on acceptable terms. Further, if we attempt to modify a product candidate or technology or to develop alternative methods or products in response to infringement claims or to avoid potential claims, we could incur substantial costs, encounter delays in product introductions or interruptions in sales. Ultimately, such efforts could be unsuccessful.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Litigation or other legal proceedings relating to intellectual property claims, with or without merit, is unpredictable and generally expensive and time consuming and is likely to divert significant resources from our core business, including distracting our technical and management personnel from their normal responsibilities. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock and negatively impact our ability to raise additional funds. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities.
We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating or from successfully challenging our intellectual property rights. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
50
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Our employees and consultants have been previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we are not aware of any claims currently pending against us, we may be subject to claims that these employees, or we have, inadvertently or otherwise used or disclosed trade secrets or other proprietary information or intellectual property of the former employers of our employees. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying money claims, we may lose valuable intellectual property rights or personnel. A loss of key personnel or their work product could hamper or prevent our ability to commercialize product candidates, which would adversely affect our commercial development efforts.
Our trade secrets are difficult to protect and if we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technologies and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality, non-competition, non-solicitation, and invention assignment agreements with our employees and consultants that obligate them to assign to us any inventions developed in the course of their work for us. However, we cannot guarantee that we have executed these agreements with each party that may have or have had access to our trade secrets or that the agreements we have executed will provide adequate protection. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to seek patent protection on technology relating to our product candidates or obtain adequate remedies for such breaches. As a result, we may be forced to bring claims against third parties, or defend claims that they bring against us, to determine ownership of what we regard as our intellectual property. Monitoring unauthorized disclosure is difficult and we do not know whether the procedures that we have followed to prevent such disclosure are or will be adequate. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the U.S. may be less willing or unwilling to protect trade secrets.
Furthermore, if any of the technology or information that we protect as trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to, or independently developed by, a competitor, our competitive position would be harmed.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO, and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedurals, documentary, fee payment and other requirements during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or our licensors fail to maintain the patents and patent applications covering our product candidates, our competitors might be able to enter the market, which would have a material adverse effect on our business.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
| ● | others may be able to make product candidates that are similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed; |
51
| ● | we or our future licensors or collaborators might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed; |
| ● | we or our future licensors or collaborators might not have been the first to file patent applications covering certain of our inventions; |
| ● | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| ● | it is possible that our pending patent applications will not lead to issued patents; |
| ● | issued patents that we own or have exclusively licensed may be held invalid or unenforceable, as a result of legal challenges by our competitors; |
| ● | our competitors might conduct R&D activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| ● | we may not develop additional proprietary technologies that are patentable; and |
| ● | the patents of others may have an adverse effect on our business. |
Should any of these events occur, they could significantly harm our business, results of operations and prospects.
Risks Related to Operations, Employee Matters and Managing Growth
We currently have a small number of employees and consultants, and our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel.
Because of the specialized scientific nature of our business, we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. We are highly dependent upon current members of our management and scientific team. We intend to increase our technical and management staff as needs arise and supporting resources become available, but the loss of one or more of our senior executive officers could be detrimental to us if we cannot recruit suitable replacements in a timely manner. The competition for qualified personnel in the pharmaceutical field is intense and as a result, we may be unable to continue to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
We may need to increase the size of our organization, and we may experience difficulties in managing this growth.
As of March 25, 2024, we had eight full-time employees. As our development and commercialization plans and strategies develop, or as a result of any future acquisitions, we may need additional managerial, operational, development, sales, marketing, financial and other resources. Our management, personnel and systems currently in place may not be adequate to support our future growth. Future growth would impose significant added responsibilities on our employees, including:
| ● | managing our clinical trials effectively; |
| ● | identifying, recruiting, maintaining, motivating and integrating additional employees; |
| ● | managing our internal development efforts effectively while complying with our contractual obligations to licensors, contractors and other third parties; and |
| ● | improving our managerial, development, operational and finance systems |
As our operations expand, we will need to manage additional relationships with various CROs, strategic partners, and other third parties. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, administrative, R&D, and sales and marketing personnel. We may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing NeuroBo.
52
We intend to market our product candidates outside of the U.S., and if we do, we will be subject to the risks of doing business outside of the U.S.
Because we intend to market our product candidates, if approved, outside of the U.S., our business is subject to risks associated with doing business outside of the U.S. Accordingly, our business and financial results in the future could be adversely affected due to a variety of factors, including:
| ● | failure to develop an international sales, marketing and distribution system for our products; |
| ● | changes in a specific country's or region's political and cultural climate or economic condition; |
| ● | unexpected changes in foreign laws and regulatory requirements; |
| ● | difficulty of effective enforcement of contractual provisions in local jurisdictions; |
| ● | inadequate intellectual property protection in foreign countries; |
| ● | inadequate data protection against unfair commercial use; |
| ● | trade-protection measures, import or export licensing requirements such as Export Administration Regulations promulgated by the U.S. Department of Commerce and fines, penalties or suspension or revocation of export privileges; |
| ● | the effects of applicable foreign tax structures and potentially adverse tax consequences; and |
| ● | significant adverse changes in foreign currency exchange rates. |
The pharmaceutical industry is highly competitive and is subject to rapid and significant technological change, which could render our technologies and products obsolete or uncompetitive.
The pharmaceutical industry is highly competitive and is subject to rapid and significant technological change, which could render certain of our products obsolete or uncompetitive. This is particularly true in the development of therapeutics for indications where new products and combinations of products are rapidly being developed that change the treatment paradigm for patients. There is no assurance that our product candidates will be the most effective, have the best safety profile, be the first to market, or be the most economical to make or use. The introduction of competitive therapies as alternatives to our product candidates could dramatically reduce the value of those development projects or chances of successfully commercializing those product candidates, which could have a material adverse effect on our long-term financial success.
We will compete with companies in the U.S. and internationally, including major pharmaceutical and chemical companies, specialized CROs, R&D firms, universities and other research institutions. Many of our competitors have greater financial resources and selling and marketing capabilities, greater experience in clinical testing and human clinical trials of pharmaceutical products and greater experience in obtaining FDA and other regulatory approvals than we do. In addition, some of our competitors may have lower development and manufacturing costs.
Risks Related to our Common Stock
The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for holders of our common stock.
The trading price of our common stock has been and is likely to continue to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume. In addition to the factors discussed in this "Risk Factors" section, these factors include:
| ● | adverse results or delays in preclinical studies, clinical trials, regulatory decisions or the development status of our product candidates or any product candidates we may pursue in the future; |
| ● | our ability to raise sufficient additional funds necessary for the continued development of our product candidates whether through potential collaborative, partnering or other strategic arrangements or otherwise; |
| ● | the terms and timing of any future collaborative, licensing or other strategic arrangements that we may establish; |
| ● | our inability to comply with the minimum listing requirements of Nasdaq; |
53
| ● | the timing of achievement of, or failure to achieve, our, or any potential collaborator’s clinical, regulatory and other milestones, such as the commencement of clinical development, the completion of a clinical trial or the receipt of regulatory approval; |
| ● | decisions to initiate a clinical trial, not initiate a clinical trial, or terminate an existing clinical trial; |
| ● | adverse regulatory decisions, including failure to receive regulatory approval for our product candidates or regulatory actions requiring or leading to a delay or stoppage of any clinical trials; |
| ● | the commercial success of any product approved by the FDA or its foreign counterparts; |
| ● | changes in applicable laws, rules or regulations; |
| ● | adverse developments concerning our manufacturers, suppliers, collaborators and other third parties; |
| ● | occurrence of health epidemics or contagious diseases, and potential effects on our business, clinical trial sites, supply chain and manufacturing facilities; |
| ● | our failure to commercialize our product candidates; |
| ● | the success of competitive drugs; |
| ● | if our patents covering our product candidates expire or are invalidated or are found to be unenforceable, or if some or all of our patent applications do not result in issued patents or result in patents with narrow, overbroad, or unenforceable claims; |
| ● | additions or departures of key scientific or management personnel; |
| ● | unanticipated safety concerns related to the use of any product candidates; |
| ● | our announcements or our competitor's announcements regarding new products, enhancements, significant contracts, acquisitions or strategic partnerships and investments; |
| ● | the size and growth of our target markets; |
| ● | our, or companies perceived to be similar to us, failure to meet external expectations or management guidance; |
| ● | fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar to us; |
| ● | publication of research reports about us or our industry, recommendations, earning results or estimates or withdrawal of research coverage by securities analysts; |
| ● | changes in the market valuations of similar companies; |
| ● | changes in general economic, political and market conditions in any of the regions in which we conduct our business; |
| ● | changes in our capital structure or dividend policy, future issuances of securities, sales of common stock by officers, directors and significant stockholders or our incurrence of debt; |
| ● | trading volume of our common stock; |
| ● | changes in accounting practices and ineffectiveness of our internal controls; |
| ● | disputes, litigation or developments relating to proprietary rights; |
| ● | timing of milestones and royalty payments; and |
| ● | other events or factors, many of which are beyond our control. |
In addition, the stock market in general, Nasdaq, and the stock of biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against companies following
54
periods of volatility in the market price of a company's securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management's attention and resources, which would harm our business, operating results or financial condition.
We may enter into financing transactions that are dilutive to our stockholders, impose material restrictions on our business and/or require us to relinquish valuable rights.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances, and marketing, distribution or licensing arrangements with third parties. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of current stockholders may be materially diluted, and the terms of such securities could include liquidation or other preferences that adversely affect the rights of our current stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specified actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, scale back or discontinue the development and commercialization of one or more of our product candidates or delay our pursuit of potential in-licenses or acquisitions.
Our largest shareholder may use its significant interest to take actions not supported by our other shareholders.
As of March 25, 2024, our largest shareholder, Dong-A beneficially owned 57.2% of our voting rights. As a result, Dong-A is able to exert a significant influence on the outcome of corporate actions requiring shareholder approval, including mergers, share capital increases and other extraordinary items.
In addition, pursuant to the Investor Rights Agreement between us and Dong-A, Dong-A has the right to appoint a number of our directors commensurate with its percentage holding of our common stock, which may result in Dong-A controlling both the determinations of the Board and the vote of all matters submitted to a vote of our shareholders, which enables them to control all corporate decisions. This concentration of ownership may delay, deter or prevent acts that would be favored by our other shareholders. The interests of Dong-A may not always coincide with our interests or the interests of our other shareholders. For as long as Dong-A owns shares of our common stock and the Investor Rights Agreement is effective, Dong-A will have significant influence on our management, business plans and policies, including the appointment and removal of members of our board of directors (“Board”), decisions on whether to raise future capital and amending our certificate of incorporation and bylaws, which govern the rights attached to our common stock. In particular, with a significant ownership percentage of our stock, Dong-A will be able to cause or prevent a change of control of us or a change in the composition of our Board and could preclude any unsolicited acquisition of us. The concentration of ownership could deprive you of an opportunity to receive a premium for your shares of common stock as part of a sale of NeuroBo and ultimately might affect the market price of our common stock. In addition, this concentration of ownership may adversely affect the trading price of our common stock because investors may perceive disadvantages in owning shares in a company with significant stockholders.
Dong-A and its affiliates engage in a broad spectrum of activities, including investments in the healthcare industry generally. In the ordinary course of its business activities, Dong-A and its affiliates may engage in activities where their interests conflict with our interests or those of our other shareholders, such as investing in or advising businesses that directly or indirectly compete with certain portions of our business or are suppliers or customers of ours. Nothing in our certificate of incorporation provides that neither Dong-A or any of their affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both her or his director and officer capacities) or its affiliates have any duty to refrain from engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. Dong-A also may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. In addition, Dong-A may have an interest in pursuing acquisitions, divestitures and other transactions that, in their judgment, could enhance its investment, even though such transactions might involve risks to our stockholders.
We are a controlled company within the meaning of the Nasdaq listing requirements and as a result, may rely on exemptions from certain corporate governance requirements. To the extent we rely on such exemptions, you will not have the same protections afforded to stockholders of companies that are subject to such corporate governance requirements.
Because of the voting power over our Company held by Dong-A and the Investor Rights Agreement between such parties, we are considered a controlled company for the purposes of the Nasdaq listing requirements. As such, we are exempt from the corporate governance requirements that our Board, compensation committee, and nominating and corporate governance committee meet the standard of independence established by those corporate governance requirements. The independence
55
standards are intended to ensure that directors who meet the independence standards are free of any conflicting interest that could influence their actions as directors.
We do not currently utilize the exemptions afforded to a controlled company, though we are entitled to do so. To the extent we utilize these exemptions, you will not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
Provisions in our corporate charter documents and under Delaware law may make an acquisition of NeuroBo, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and the bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which our stockholders might otherwise receive a premium for their shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by stockholders to replace or remove their current management by making it more difficult for stockholders to replace members of our board. Among other things, these provisions:
| ● | establish a classified Board such that not all members of the board are elected at one time; |
| ● | allow the authorized number of our directors to be changed only by resolution of our Board; |
| ● | limit the manner in which our stockholders can remove directors from the Board; |
| ● | establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our Board; |
| ● | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
| ● | prohibit our stockholders from calling special meetings; |
| ● | authorize our board to issue preferred stock without stockholder approval, which preferred stock may include rights superior to the rights of the holders of common stock, and which could be used to institute a shareholder rights plan, or so-called "poison pill," that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board; and |
| ● | require the approval of the holders of at least two-thirds of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with it for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
We may fail to comply with the continued listing requirements of the Nasdaq, such that our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
Our common stock is listed for trading on Nasdaq. We must satisfy Nasdaq’s continued listing requirements, including, among other things, a minimum closing bid price requirement of $1.00 per share for thirty consecutive business days (the “Minimum Bid Price Requirement”). We have in the past been notified by Nasdaq that we were not in compliance with the Minimum Bid Price Requirement, and while we have regained compliance, there can be no assurance that we will remain compliant with the Minimum Bid Price Requirement, or any other Nasdaq continued listing requirements.
A delisting of our common stock from Nasdaq could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, employees and fewer business development opportunities.
56
We are a "smaller reporting company" and we cannot be certain if the reduced reporting requirements applicable to such companies could make our common stock less attractive to investors.
We are a "smaller reporting company", as defined in the Exchange Act. For as long as we continue to be an smaller reporting company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not "emerging growth companies", including exemption from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley Act), only being required to provide two years of audited financial statements in our annual reports and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We have identified material weaknesses in our internal control over financial reporting that could, if not remediated, result in material misstatements in our financial statements or impair our ability to produce accurate and timely consolidated financial statements.
We concluded that there were material weaknesses relating to our internal control over financial reporting relating to a lack of segregation of duties over certain financial processes, management review over financial reporting and logical access to financial reporting systems. For more information about these material weaknesses, see Part II, Item 9A. Controls and Procedures of this report. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
Although we have begun to take measures to remediate these material weaknesses, the measures we have taken, and expect to take, to improve our internal controls may not be sufficient to address the issues identified, to ensure that our internal controls are effective or to ensure that the identified material weaknesses will not result in a material misstatement of our annual or interim consolidated financial statements. If we are unable to correct material weaknesses or deficiencies in internal controls in a timely manner, our ability to record, process, summarize and report financial information accurately and within the time periods specified in the rules and forms of the SEC will be adversely affected. This failure could negatively affect the market price and trading liquidity of our common stock, cause investors to lose confidence in our reported financial information, subject us to civil and criminal investigations and penalties, and materially and adversely impact our business and financial condition.
General Risk Factors
Our business and operations may suffer in the event of system failures or unplanned events.
Despite the implementation of security measures, our internal computer systems and those of our current and future contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we are not aware of any such material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed.
Furthermore, any unplanned event, such as flood, fire, explosion, tornadoes, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunication failure or other natural or manmade accidents or incidents that result in us being unable to fully utilize the facilities, may have an adverse effect on our ability to operate the business, particularly on a daily basis, and have significant negative consequences on our financial and operating conditions. Loss of access to these facilities may result in increased costs, delays in the development of our product candidates or interruption of our business operations.
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology or loss of data, including any cyber security incidents, may compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability which could harm our ability to operate our business effectively and adversely affect our business and reputation.
In the ordinary course of our business, our contract research organizations and other third parties on which we rely collect and store sensitive data, including legally protected patient health information, personally identifiable information about our
57
employees, intellectual property, and proprietary business information. We manage and maintain our applications and data utilizing on-site systems. These applications and data encompass a wide variety of business-critical information, including R&D information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy. Despite the implementation of security measures, our internal computer systems and those of third parties with which we contract are vulnerable to damage from cyber-attacks, computer viruses, breaches, unauthorized access, interruptions due to employee error or malfeasance or other disruptions, or damage from natural disasters, terrorism, war and telecommunication and electrical failures. Any such event could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. We have measures in place that are designed to detect and respond to such security incidents and breaches of privacy and security mandates. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, government enforcement actions and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to conduct research, development and commercialization activities, process and prepare Company financial information, manage various general and administrative aspects of our business and damage our reputation, in addition to possibly requiring substantial expenditures of resources to remedy, any of which could adversely affect our business. The loss of clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. In addition, there can be no assurance that we will promptly detect any such disruption or security breach, if at all. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our research, development and commercialization efforts could be delayed.
An active trading market for our common stock may not be maintained.
Our common stock is currently traded on Nasdaq, but we can provide no assurance that we will be able to maintain an active trading market for our shares on Nasdaq or any other exchange in the future. If there is no active market for our common stock, it may be difficult for our stockholders to sell shares without depressing the market price for the shares or at all.
If securities analysts do not publish research or reports about our business or if they publish negative evaluations of our business, the price of our stock could decline.
If one or more analysts cover our business and downgrade their evaluations of our stock or publish inaccurate or unfavorable research about our business, the price of our stock could decline. If one or more of these analysts cease to cover our stock, we could lose visibility in the market for our stock, which in turn could cause our stock price and trading volume to decline.
We incur increased costs as a result of operating as a public company and our management is required to devote substantial time to compliance initiatives.
The Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the stock exchange upon which our common stock is listed, and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly. However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
We are a "smaller reporting company", as defined in the Exchange Act. For as long as we continue to be an smaller reporting company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not "emerging growth companies", including exemption from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley Act), only being required to provide two years of audited financial statements in our annual reports and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. If (i) the market value of our voting and non-voting ordinary shares held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) (a) our annual revenue is less than $100.0 million during the most recently completed fiscal year and (b) the market value of our voting and non-voting ordinary shares held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter,
58
if before such date, we opt to no longer take advantage of the applicable exemption, we will be required to include an opinion from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting.
To achieve compliance with Section 404, we are required to engage in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we must dedicate internal resources, hire additional finance and accounting personnel, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting.
During the course of our review and testing, we may identify deficiencies and be unable to remediate them before we must provide the required reports. We or our independent registered public accounting firm may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting, which could harm our operating results, cause investors to lose confidence in our reported financial information and cause the trading price of our stock to fall.
In addition, as a public company we are required to timely file accurate quarterly and annual reports with the SEC under the Exchange Act. In order to report the results of our operations and financial position on an accurate and timely basis, we will depend on CROs to provide timely and accurate notice of their costs to us. Any failure to report our financial results on an accurate and timely basis could result in sanctions, lawsuits, delisting of our shares from Nasdaq or other adverse consequences that would materially harm our business.
We do not anticipate declaring or paying, in the foreseeable future, any cash dividends on our capital stock and, consequently, the ability of our stockholders to achieve a return on their investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividend on our capital stock and do not currently intend to do so in the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which you purchased them.
Our bylaws designate the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders' ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will generally be the sole and exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, as amended, the certificate of incorporation or the bylaws or any other action asserting a claim governed by the internal affairs doctrine. This provision does not apply to claims arising under the Securities Act and the Exchange Act or any claim for which the federal courts have exclusive jurisdiction. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and to have consented to the provisions of the bylaws described above. This choice of forum provision may limit a stockholder's ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and employees. Alternatively, if a court were to find this provision inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
The global credit and financial markets have experienced extreme volatility and disruptions in the past several years, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. We cannot assure you that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, or do not improve, it may make any necessary debt or equity financing more difficult, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require it to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service
59
providers, manufacturers and other partners may not survive these difficult economic times, which could directly affect our ability to attain our operating goals on schedule and on budget.
Item 1B.Unresolved Staff Comments
None.
Item 1C.Cybersecurity
Risk Management
We recognize the importance of assessing, identifying, and managing risks associated with cybersecurity threats. These risks include, among other things, operational risks; intellectual property theft; fraud; extortion; harm to employees, violation of privacy or security laws and other litigation and legal risk; and reputational risks. We are committed to maintaining robust governance and oversight of these risks and to implementing mechanisms, controls, technologies, and processes designed to help us assess, identify, and manage these risks. While we have not, as of the date of this Form 10-K, experienced a cybersecurity threat or incident that resulted in a material adverse impact to our business or operations, there can be no guarantee that we will not experience such an incident in the future.
We aim to incorporate industry best practices throughout our cybersecurity program. Our cybersecurity strategy focuses on implementing effective and efficient controls, technologies, and other processes to assess, identify, and manage material cybersecurity risks. Our cybersecurity program is designed to be aligned with applicable industry standards. We work with a third-party provider to monitor for threats and potential cybersecurity breaches.
We have processes in place to assess, identify, manage, and address material cybersecurity threats and incidents. These include, among other things: ongoing security awareness training for employees; mechanisms to detect and monitor unusual network activity; and containment and incident response tools. We monitor issues that are internally discovered or reported by our third-party monitoring service that may affect our information services, and have processes to assess those issues for potential cybersecurity impact or risk. We impose security requirements upon our suppliers and CROs, including maintaining an effective security management program; abiding by information handling and asset management requirements; and notifying us in the event of any known or suspected cyber incident.
Governance
Our Board has ultimate oversight of cybersecurity risk, which it manages as part of our enterprise risk management program. That program is utilized in making decisions with respect to company priorities, resource allocations, and oversight structures. The Board is assisted by the audit committee, which reviews our cybersecurity program with management and reports to the Board.
The audit committee is central to the Board’ oversight of cybersecurity risks and bears the primary responsibility for this domain. The audit committee is composed of board members with diverse expertise including risk management, technology, and finance, equipping them to oversee cybersecurity risks effectively.
Our chief executive officer, chief financial officer and corporate controller have operational experience in assessing and managing cybersecurity risk. Our chief executive officer plays a pivotal role in informing the audit committee on cybersecurity risks. They provide comprehensive briefings to the audit committee on a regular basis, with a minimum frequency of once per year. These briefings encompass a broad range of topics, including:
| ● | Current cybersecurity landscape and emerging threats; |
| ● | Status of ongoing cybersecurity initiatives and strategies; |
| ● | Incident reports and learnings from any cybersecurity events; and |
| ● | Compliance with regulatory requirements and industry standards. |
In addition to our scheduled meetings, the audit committee and chief executive officer maintain an ongoing dialogue regarding emerging or potential cybersecurity risks. Together, they receive updates on any significant developments in the cybersecurity domain, ensuring the board’s oversight is proactive and responsive. The audit committee actively participates in strategic decisions related to cybersecurity, offering guidance and approval for major initiatives. This involvement ensures that cybersecurity considerations are integrated into the broader strategic objectives of NeuroBo.
60
Our chief financial officer is informed by our third-party monitoring service of any cybersecurity incidents, who will then escalate the incident to our chief executive officer, if necessary. Furthermore, significant cybersecurity matters and strategic risk management decisions are escalated to the Board, ensuring that they have comprehensive oversight and can provide guidance on critical cybersecurity issues.
Item 2. | Properties |
We currently lease 2,441 square feet of office space in Cambridge, Massachusetts as our new corporate headquarters. The initial lease term is for three years with an option to renew for an additional two-year term. The lease commenced in September 2023 and expires in August 2026.
We believe that our leased properties are adequate for our purposes and to pursue our strategy.
Item 3. | Legal Proceedings |
From time to time, we may be involved in various claims and legal proceedings arising out of our ordinary course of business. We are not currently a party to any claims or legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business and consolidated financial statements. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 4. | Mine Safety Disclosures |
Not applicable.
Part II
Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Common stock
Our common stock is listed on Nasdaq under the symbol “NRBO.”
In December 2023, we completed a one-for-eight reverse stock split of our common stock (the “2023 Reverse Stock Split”). As a result, every eight shares of our issued and outstanding common stock were combined, converted and changed into one share of our common stock. Any fraction of a share of our common stock that was created as a result of the 2023 reverse stock split was rounded down to the next whole share and the stockholder received cash equal to the market value of the fractional share, determined by multiplying such fraction by the closing sales price of our common stock as reported on Nasdaq on the last trading day before the reverse stock split. The 2023 Reverse Stock Split was initially approved by our stockholders at the annual meeting of stockholders in June 2023. At the annual meeting, the stockholders approved a proposal to amend our certificate of incorporation to affect a reverse split of our outstanding common stock at a ratio in the range of one-for-five to one-for-eight to be determined at the discretion of our Board. Following the annual meeting, our Board approved a one-for-eight reverse stock split of our issued and outstanding shares of common stock.
In September 2022, we completed a 1-for-30 reverse stock split of our common stock (the “2022 Reverse Stock Split”). As a result, every thirty shares of our issued and outstanding common stock were combined, converted and changed into one share of our common stock. Any fraction of a share of our common stock that was created as a result of the reverse stock split was rounded down to the next whole share and the stockholder received cash equal to the market value of the fractional share, determined by multiplying such fraction by the closing sales price of our common stock as reported on Nasdaq on the last trading day before the reverse stock split. The 2022 Reverse Stock Split was initially approved by our stockholders at the annual meeting of stockholders in June 2022. At the annual meeting, the stockholders approved a proposal to amend our certificate of incorporation to affect a reverse split of our outstanding common stock at a ratio in the range of one-for-five to one-for-thirty-five to be determined at the discretion of our Board. Following the annual meeting, our Board approved a one-for-thirty reverse stock split of our issued and outstanding shares of common stock.
Neither the 2023 Reverse Stock Split nor the 2022 Reverse Stock Split impacted the number of authorized shares of common stock of 100,000,000 shares. For each of the reverse stock splits, a proportionate adjustment was made to the per share exercise price and the number of shares issuable upon the exercise of all outstanding stock options, and warrants to purchase shares of our common stock, the number of shares issuable upon vesting of restricted stock units (“RSUs”) and the number of shares reserved for issuance pursuant to our equity incentive compensation plans. Specifically, for the Series A and B warrants issued
61
in November 2022 that were outstanding on December 20, 2023, the number of outstanding warrants did not change; instead, the warrants have an exchange ratio of eight warrants for one share of our common stock.
In this Annual Report, all historical numbers of shares of common stock and per share data have been adjusted to give effect to the 2023 Reverse Stock Split and the 2022 Reverse Stock Split. Additionally, since the common stock par value was unchanged, historical amounts for common stock and additional paid-in capital have been adjusted to give effect to the 2023 Reverse Stock Split and the 2022 Reverse Stock Split.
Stockholders
On March 25, 2024, we had 4,906,032 shares of common stock outstanding and 52 holders of record of our common stock. The transfer agent and registrar for our common stock is Equiniti Trust Company, LLC.
Dividend policy
We have never declared or paid any dividends on our common stock, and we do not currently intend to pay any dividends on our common stock for the foreseeable future. Any future determination to pay dividends on our common stock will be, subject to applicable law, at the discretion of our Board and will depend upon, among other factors, our results of operations, financial condition, capital requirements, and contractual restrictions in loan or other agreements.
Item 6. | [Reserved] |
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the information set forth under our consolidated financial statements and the notes to those financial statements, included elsewhere in this Annual Report. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements. Our actual results may differ materially from those contained in or implied by any forward-looking statements as a result of various factors, including, but not limited to, the risks and uncertainties described under “Risk Factors” elsewhere in this Annual Report.
Certain amounts in the following discussion and analysis may not add due to rounding, and all percentages have been calculated using unrounded amounts.
Overview
We are a clinical-stage biotechnology company focused primarily on developing and commercializing novel pharmaceuticals to treat cardiometabolic diseases. NeuroBo has two programs focused primarily on treatment of metabolic dysfunction-associated steatohepatitis (“MASH”) and obesity. MASH was formerly known as non-alcoholic steatohepatitis (“NASH”). The American Association for the Study of Liver Diseases (“AASLD”) and its European and Latin American counterparts changed the name to metabolic dysfunction-associated steatohepatitis to reflect the complexity of the disease.
| ● | DA-1241 is a novel G-Protein-Coupled Receptor 119 (“GPR119”) agonist with development optionality as a standalone and/or combination therapy for both MASH and type 2 diabetes. Agonism of GPR119 in the gut promotes the release of key gut peptides GLP-1, GIP, and PYY. These peptides play a further role in glucose metabolism, lipid metabolism and weight loss. DA-1241 has beneficial effects on glucose, lipid profile and liver inflammation, supported by potential efficacy demonstrated during in vivo preclinical studies. |
| ● | DA-1726 is a novel oxyntomodulin analogue functioning as a GLP-1 receptor (“GLP1R”) and glucagon receptor (“GCGR”) dual agonist for the treatment of obesity that is to be administered once weekly subcutaneously. DA-1726 acts as a dual agonist of GLP1R and GCGR. |
Our operations have consisted principally of performing research and development (“R&D”) activities, clinical development and raising capital. Our activities are subject to significant risks and uncertainties, such as failing to secure additional funding before sustainable revenues and profit from operations are achieved. For more information on our business and product candidates, see Part I, Item 1. Business of this Annual Report.
62
Key operating information
Research and development expenses
Our R&D expenses were $9.2 million and $2.8 million for 2023 and 2022, respectively. R&D expenses consist primarily of costs incurred in connection with the development of our product candidates. These expenses include:
| ● | employee-related expenses, including salaries, related benefits and stock-based compensation, for employees engaged in research and development functions; |
| ● | expenses incurred in connection with the clinical development of our product candidates, including under agreements with third parties, such as consultants and CROs; |
| ● | the cost of manufacturing and storing drug products for use in our preclinical studies and clinical trials, including under agreements with third parties, such as consultants and Clinical Manufacturing Organizations (“CMOs”); |
| ● | facilities, depreciation and other expenses, which include direct or allocated expenses for rent and maintenance of facilities and insurance; |
| ● | costs related to compliance with regulatory requirements; and |
| ● | payments made under third-party licensing agreements. |
We recognize external development costs based on an evaluation of the progress toward completion of specific tasks using information provided to us by our service providers. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf, and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual costs. Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. Such amounts are recognized as an expense when the goods have been delivered or the services have been performed, or when it is no longer expected that the goods will be delivered, or the services rendered.
Our direct research and development expenses consist primarily of external costs, such as fees paid to outside consultants, CROs, CMOs and research laboratories in connection with our clinical development, quality assurance and quality control processes, manufacturing, and clinical development activities. Our direct research and development expenses also include fees incurred under third-party license agreements. We use our employee and infrastructure resources across multiple research and development projects. We do not allocate employee costs and costs associated with our facilities, including depreciation or other indirect costs, to specific product candidates because these costs are deployed across multiple programs and, as such, are not separately classified. We use internal resources to manage CMO and CRO activities. These employees work across multiple programs. We do not track our costs by product candidate.
Clinical development activities are central to our business model. We do not believe that our historical costs are indicative of the future costs associated with these programs, nor do they represent the costs of other future programs we may initiate. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We have some control over the timing of these expenses, but costs may be difficult to control once clinical trials have commenced.
The successful development and commercialization of our product candidates are highly uncertain. At this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the preclinical and clinical development of any of our product candidates. Additionally, because of the risks inherent in novel treatment discovery and development, we cannot reasonably estimate or know:
| ● | the timing and progress of preclinical and clinical development activities; |
| ● | the number and scope of clinical programs that we decide to pursue; |
| ● | our ability to maintain our current development programs and to establish new ones; |
| ● | establishing an appropriate safety profile with IND-enabling studies; |
| ● | successful patient enrollment in, and the initiation and completion of, clinical trials; |
63
| ● | the successful completion of clinical trials with safety, tolerability and efficacy profiles that are satisfactory to the FDA or any comparable foreign regulatory authority; |
| ● | the receipt of regulatory approvals from applicable regulatory authorities; |
| ● | the timing, receipt and terms of any marketing approvals from applicable regulatory authorities; |
| ● | our ability to establish new licensing or collaboration arrangements; |
| ● | establishing agreements with third-party manufacturers for clinical supply for our clinical trials and commercial manufacturing, if any of our product candidates is approved; |
| ● | development and timely delivery of clinical-grade and commercial-grade drug formulations that can be used in our clinical trials and for commercial launch; |
| ● | obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights; |
| ● | launching commercial sales of our product candidates, if approved, whether alone or in collaboration with others; |
| ● | maintaining a continued acceptable safety profile of the product candidates following commercialization; or |
| ● | the effect of competing technological and market developments. |
A change in the outcome of any of these variables with respect to the development of our product candidates could significantly change the costs and timing associated with the development of that product candidate.
Acquired in-process research and development expenses
We had no acquired in-process research and development (“IPR&D”) expenses for 2023, compared to $8.2 million of acquired IPR&D expenses for 2022. We include costs to acquire or in-license product candidates in acquired IPR&D. When we acquire the right to develop and commercialize a new product candidate, any up-front payments, or any future milestone payments that relate to the acquisition or licensing of such a right are immediately expensed as acquired in-process research and development in the period in which they are incurred. These costs are immediately expensed provided that the payments do not also represent processes or activities that would constitute a “business,” or provided that the product candidate has not achieved regulatory approval for marketing and, absent obtaining such approval, has no alternative future use. Royalties owed on future sales of any licensed product will be expensed in the period the related revenues are recognized.
General and administrative expenses
Our general and administrative expenses were $6.7 million and $8.6 million for 2023 and 2022, respectively. General and administrative expenses consist primarily of salaries and related costs, including stock-based compensation, for personnel in executive, finance and administrative functions. General and administrative expenses also include direct and allocated facility-related costs as well as professional fees for legal, patent, consulting, investor and public relations, accounting, and audit services.
We anticipate that our general and administrative expenses will increase in the future as a result of accounting, audit, legal, regulatory, compliance, and director and officer insurance costs as we pursue the development of our product pipeline, as well as investor and public relations expenses associated with being a public company.
Income taxes
Our provision for income taxes was zero for 2023 and 2022. We have had significant pre-tax losses since our inception, and we have not yet generated revenues and face significant challenges to becoming profitable. Accordingly, we recorded a valuation allowance on the deferred tax assets attributable to the NOL we have incurred in each year or for our earned R&D credits. We will continue to monitor all positive and negative evidence until we believe it is more likely than not that the valuation allowance is no longer necessary, resulting in an income tax benefit in the period such determination is made.
As of December 31, 2023 and 2022, our U.S. federal NOL carryforwards were $8.8 million and $1.5 million, respectively. We had U.S. federal R&D credit carryforwards of $24 thousand as of December 31, 2023 and 2022. Since our U.S. federal net operating losses were incurred after December 31, 2017, U.S NOL and R&D credit carryforwards will not expire. As of December 31, 2023 and 2022, we had state NOL carryforwards of $4.4 million and $0.9 million, respectively. We had state R&D credit carryforwards of $2 thousand as of December 31, 2023 and 2022, respectively. Our state NOL and R&D credit carryforwards will begin to expire in 2042, if not utilized. Lastly, since the foreign subsidiary that generated the foreign losses
64
was dissolved and liquidated in June 2023, the recorded value of foreign NOL and related deferred tax asset have been reduced to zero. Accordingly, we have no had foreign NOL carryforwards as of December 31, 2023 and $0.7 million of foreign NOL carryforwards as of December 31, 2022.
Net loss
We have incurred significant operating losses since our inception. Our ability to generate product revenue sufficient to achieve profitability will depend on the successful development and eventual commercialization of one or more of our current or future product candidates. Our net loss was $12.5 million and $14.0 million for 2023 and 2022, respectively. To date, we have not generated any revenue from product sales, collaborations with other companies, government grants or any other source, and do not expect to generate any revenue in the foreseeable future.
Accrual for R&D costs related to clinical trial activities
As part of the process of preparing our consolidated financial statements, we are required to record an accrual for R&D costs related to clinical trial activities. We recorded an accrual for external R&D costs of $3.8 million and $0.1 million as of December 31, 2023 and 2022, respectively. This process involves reviewing open contracts and purchase orders, communicating with applicable personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual costs. Certain of our service providers invoice us in arrears for services performed, on a pre-determined schedule or when contractual milestones are met; however, some service providers require advance payments. We make estimates of our accrued and prepaid expenses as of each balance sheet date in the consolidated financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of these estimates with the service providers and make adjustments, if necessary. Examples of estimated accrued R&D expenses include fees paid to:
| ● | vendors in connection with preclinical development activities; |
| ● | CROs and investigative sites in connection with preclinical studies and clinical trials; and |
| ● | CMOs in connection with the production of preclinical and clinical trial materials. |
We base the expense recorded related to external R&D on our estimates of the services received and efforts expended pursuant to quotes and contracts with multiple CMOs and CROs that supply, conduct and manage preclinical studies and clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the expense. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we adjust the accrual or the amount of prepaid expenses accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in reporting amounts that are too high or too low in any particular period.
Accumulated deficit
As of December 31, 2023 and 2022, we had an accumulated deficit of $108.3 million and $95.8 million, respectively. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We expect that our expenses and capital requirements will increase substantially in connection with our ongoing activities, particularly if and as we:
| ● | pursue clinical development for our current product candidates; |
| ● | initiate preclinical studies and clinical trials with respect to our current product candidates and indications and any future product candidates or indications that we may pursue; |
| ● | acquire or in-license other product candidates and/or technologies; |
| ● | develop, maintain, expand and protect our intellectual property portfolio; |
| ● | hire additional clinical, scientific and commercial personnel; |
65
| ● | establish a commercial manufacturing source and secure supply chain capacity sufficient to provide commercial quantities of any product candidates for which we may obtain regulatory approval; |
| ● | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| ● | establish a sales, marketing and distribution infrastructure and/or enter into partnership arrangements to commercialize any products for which we may obtain regulatory approval; or |
| ● | add administrative, operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts, and to support our being a public reporting company. |
Results of Operations
2023 compared to 2022
The following table summarizes our results of operations for 2023 and 2022 (in thousands, other than share and per share amounts):
Year Ended December 31, | ||||||
| 2023 | 2022 | ||||
Operating expenses: | ||||||
Research and development | $ | 9,158 | $ | 2,778 | ||
Acquired in-process research and development | — | 8,210 | ||||
General and administrative | 6,728 | 8,640 | ||||
Total operating expenses |
| 15,886 |
| 19,628 | ||
Loss from operations |
| (15,886) |
| (19,628) | ||
Other income (expense): | ||||||
Change in fair value of warrant liabilities | 2,955 | 7,935 | ||||
Interest income | 461 | — | ||||
Financing expense | — | (2,191) | ||||
Other expense | — | (83) | ||||
Total other income | 3,416 | 5,661 | ||||
Loss before income taxes | (12,470) | (13,967) | ||||
Provision for income taxes | — | — | ||||
Net loss | $ | (12,470) | $ | (13,967) | ||
Loss per share of common stock, basic and diluted | $ | (2.46) | $ | (43.42) | ||
Weighted average shares of common stock, basic and diluted |
| 5,071,101 |
| 321,703 | ||
Operating expenses and loss from operations
Our total operating expenses and loss from operations for 2023 were $15.9 million, a decrease of $3.7 million, or 19.1%, compared to 2022. This decrease was attributable to (i) $8.2 million in lower acquired IPR&D expenses and (ii) $1.9 million in lower general and administrative expenses, partially offset by $6.4 million in higher R&D expenses.
Our R&D expenses were $9.2 million for 2023, an increase of $6.4 million, or 229.7%, compared to 2022. This increase was primarily related to increased development activities for DA-1241 and DA-1726. Specifically, the $6.4 million increase in R&D expenses was primarily attributable to (i) $6.3 million in higher expenditures for investigational drug manufacturing costs, non-clinical and preclinical services, clinical trials and consulting, (ii) $0.1 million in higher stock-based compensation, and (iii) $0.1 million in higher employee compensation and benefits. Included in R&D expenses for 2023 was $2.4 million of investigational drug manufacturing costs, non-clinical and preclinical expenses incurred under the Shared Services Agreement with Dong-A as compared to none in 2022.
We had no acquired IPR&D expenses for 2023, compared to $8.2 million for 2022. The 2022 acquired IPR&D expenses were attributable to the acquisition of intellectual property rights under the 2022 License Agreement. Given that no processes or activities constituting a “business” were acquired and since none of the rights underlying the 2022 License Agreement had alternative future uses or had reached a stage of technological feasibility, the acquisition was recorded as acquired IPR&D expense and was based on the fair value of the 2,200 shares of Series A Preferred Stock issued to Dong-A pursuant to the terms and conditions of the 2022 License Agreement.
66
Our general and administrative expenses were $6.7 million for 2023, a decrease of $1.9 million, or 22.1%, compared to 2022. This decrease in general and administrative expenses was primarily attributable to (i) $0.9 million in lower insurance cost, (ii) $0.7 million in lower stock-based compensation, (iii) $0.3 million in lower legal and professional fees, and (iv) $0.3 million in lower employee compensation and benefits. The decreases were partially offset by an increase of $0.2 million in state non-income taxes and fees as well as public company costs.
Other income
Our other income for 2023 was $3.4 million, a decrease of $2.2 million, or 39.7%, compared to 2022. This decrease was attributable to a decrease of $5.0 million in gain related to the change in fair value of warrant liabilities as compared to 2022. This decrease was partially offset by $0.5 million of interest income recorded in 2023, of which there was none in 2022, and $2.3 million of financing and other expenses incurred in 2022.
The change in fair value of warrant liabilities resulted in a gain of $3.0 million for 2023, compared to a gain of $7.9 million for 2022. This change was primarily a result of (i) a decrease in the number of outstanding warrants as of December 31, 2023 due to various cashless conversion of warrants to common stock during 2023 and (ii) the impact of our common stock’s lower underlying stock price since December 31, 2022.
Interest income of $0.5 million for 2023 was primarily related to interest earned on our cash balance. We did not have any interest income for 2022.
We did not incur any financing expenses for 2023, compared to $2.2 million of financing expenses for 2022, which represents the portion of the transaction costs allocated to the issuance of the Series A Warrants and Series B Warrants, described further below.
We did not incur any other non-operating expenses for 2023, compared to $0.1 million of non-operating other expenses for 2022, which was primarily related to the loss on sale of fixed assets and losses on translations of foreign currency.
Provision for income taxes
Our effective tax rate for 2023 and 2022 was zero percent as we have recorded a full valuation allowance for the income tax benefits attributable to our pre-tax losses.
Net loss
For 2023, we had a net loss of $12.5 million, or $2.46 per share of basic and diluted common stock, compared to a net loss of $14.0 million, or $43.42 per share of basic and diluted common stock for 2022.
Liquidity and capital resources
Our primary use of cash is to fund our R&D activities and clinical development activities. We have funded our operations primarily through public offerings of our common stock and private placements of equity. As of December 31, 2023, we had cash totaling $22.4 million. We maintain cash at financial institutions that at times may exceed the Federal Deposit Insurance Corporation (“FDIC”) insured limits of $250 thousand per bank. Our cash balance includes liquid insured deposits, which are obligations of the program banks in which the deposits are held and qualify for FDIC insurance protection per depositor in each recognized legal category of account ownership in accordance with the rules of the FDIC. To date, we have not experienced any losses related to these funds.
We believe that our existing cash will be sufficient to fund our operations into the fourth quarter of 2024. We plan to continue to fund our operations through a combination of equity offerings, debt financings, or other sources, potentially including collaborations, out-licensing and other similar arrangements. There can be no assurance that we will be able to obtain any sources of financing on acceptable terms, or at all. To the extent that we can raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact our ability to conduct our business. If we are unable to raise additional capital, we may slow down or stop our ongoing and planned clinical trials until such time as additional capital is raised and this may have a material adverse effect on us.
2022 Transactions
Transactions with Dong-A
In September 2022, in connection with the 2022 License Agreement with Dong-A, we entered into the Securities Purchase Agreement with Dong-A. Pursuant to the Securities Purchase Agreement, upon the consummation of the 2022 License Agreement and a Qualified Financing (as defined in the Securities Purchase Agreement), which occurred in November 2022
67
as a result of the 2022 Public Offering, (i) Dong-A received the Upfront License Payment and (ii) Dong-A purchased 1,500 shares of Series A Preferred Stock and warrants to purchase 1,250,000 shares of our common stock substantially equivalent to those issued to investors in respect of the Qualified Financing (the “Dong-A Warrants”) for a purchase price of $15.0 million (the “Dong-A Financing”).
In December 2022, our stockholders approved the conversion of the Series A Preferred Stock and the exercise of the Dong-A warrants (the “Stockholder Approval”) and all of the Series A Preferred Stock converted into 1,541,667 shares of our common stock.
Public Offering
In November 2022, we closed on the 2022 Public Offering and received gross proceeds of $17.3 million. The 2022 Public Offering was comprised of (1) 3,147,003 Class A Units, priced at a public offering price of $3.00 per Class A Unit, with each Class A Unit consisting of (a) one-eighth (1/8) share of common stock (as adjusted for the 2023 Reverse Stock Split), which equates to 393,375 shares of common stock, (b) one Series A Warrant (the “Series A Warrants”) to purchase one-eighth (1/8) share of common stock for a purchase price of $3.00 per warrant that expires on the one year anniversary following the initial exercise date and (c) one Series B Warrant (the “Series B Warrants”) to purchase one-eighth (1/8) share of common stock, for a purchase price of $3.00 per warrant, that expires on the five year anniversary following the initial exercise date, and (2) 2,602,997 Class B Units, priced at a public offering price of $3.00 per Class B Unit, with each Class B Unit consisting of (a) one share of Series B convertible preferred stock (the “Series B Preferred Stock”), convertible into one-eighth (1/8) share of common stock (as adjusted for the 2023 Reverse Stock Split), (b) one Series A Warrant and (c) one Series B Warrant. The Series A Warrants and the Series B Warrants, (collectively, the “Public Warrants”) were to only be exercisable upon stockholder approval, and each Warrant was to be exchangeable for one share of common stock for no additional consideration. Following the closing of the 2022 Public Offering, all of the shares of Series B Preferred Stock were converted into shares of our common stock.
The 2022 Public Offering met the definition of a Qualified Financing, as defined by the Securities Purchase Agreement; therefore, also in November 2022, the license under the 2022 License Agreement became effective, and we issued 2,200 shares of Series A Preferred Stock to Dong-A. In addition, we closed on the Dong-A Financing, and issued an additional (i) 1,500 shares of Series A Preferred Stock, (ii) 5,000,000 warrants substantially similar to the Series A Warrants and (iii) 5,000,000 warrants substantially similar to the Series B Warrants (the “Dong-A Warrants”). We received gross proceeds in the amount of $15.0 million in connection with the Dong-A Financing. In 2023, all of the Dong-A Warrants were converted into shares of our common stock.
Net proceeds of the 2022 Public Offering and Dong-A Financing after deducting underwriter’s fees and related offering expenses were $28.6 million.
Cash Flows
The principal use of cash in operating activities is to fund our current expenditures in support of our R&D activities and clinical development activities. Financing activities currently represent the principal source of our cash flow.
The following table reflects the major categories of cash flows for each of the periods presented (in thousands).
Year Ended December 31, | ||||||
| 2023 | 2022 | ||||
Net cash used in operating activities | $ | (10,799) | $ | (11,712) | ||
Net cash (used in) provided by investing activities |
| (50) | 8 | |||
Net cash (used in) provided by financing activities |
| (80) | 28,681 | |||
Net (decrease) increase in cash | $ | (10,929) | $ | 16,977 | ||
Operating Activities
Net cash used in operating activities was $10.8 million for 2023, a decrease of $0.9 million, or 7.8%, compared to $11.7 million for 2022. Net cash used in operating activities of $10.8 million for 2023 consisted of (i) net loss of $12.5 million and (ii) net non-cash income of $2.7 million, partially offset by net cash provided operating assets and liabilities of $4.4 million. Net cash used in operating activities of $11.7 million for 2022 consisted of (i) net loss of $14.0 million and (ii) net cash used by operating assets and liabilities of $1.2 million, partially offset by net non-cash expenses of $3.4 million.
68
Investing Activities
Net cash used in investing activities for 2023 was $50 thousand, compared to net cash provided by investing activities of $8 thousand for 2022. Net cash used in investing activities for 2023 was related to purchases of property and equipment. Net cash provided by investing activities for 2022 was related to the sale of property and equipment.
Financing Activities
Net cash used in financing activities was $80 thousand, compared to net cash provided by financing activities of $28.7 million for 2022. Net cash used in financing activities for 2023 was related to payment of certain issuance costs related to the 2022 Public Offering. Net cash provided by financing activities for 2022 consisted of proceeds of $32.3 million from the issuance of common stock, preferred stock and warrants in connection with the 2022 Public Offering, partially offset by the payment of certain issuance costs of $3.6 million related to the 2022 Public Offering.
For additional details, see the consolidated statements of cash flows in the consolidated financial statements included elsewhere in this Annual Report.
Going concern
The determination as to whether we can continue as a going concern contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Our consolidated financial statements, included elsewhere in this Annual Report, have been prepared assuming that we will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty. This basis of accounting contemplates the recovery of our assets and the satisfaction of our liabilities in the normal course of business.
As reflected in the consolidated financial statements, we had $22.4 million in cash as of December 31, 2023. We have experienced net losses and negative cash flows from operating activities since our inception and had an accumulated deficit of $108.3 million as of December 31, 2023. We have incurred a net loss of $12.5 million and used cash of $10.8 million for operating activities for the year ended December 31, 2023. Due in large part to the ongoing Phase 2a clinical trial for DA-1241 and Phase 1 clinical trial for DA-1726, we expect to continue to incur net losses and negative cash flows from operating activities for the foreseeable future. These conditions raise substantial doubt about our ability to continue as a going concern.
We believe that our existing cash will be sufficient to fund our operations into the fourth quarter of 2024. We plan to continue to fund our operations through a combination of equity offerings, debt financings, or other sources, potentially including collaborations, licenses and other similar arrangements. There can be no assurance that we will be able to obtain any sources of financing on acceptable terms, or at all. To the extent that we can raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact our ability to conduct our business. If we are unable to raise additional capital, we may slow down or stop our ongoing and planned clinical trials until such time as additional capital is raised and this may have a material adverse effect on us.
Contractual obligations, purchase commitments and employment agreements
Contractual obligations
In August 2023, we entered into a non-cancelable operating lease for our new corporate headquarters in Cambridge, Massachusetts (the “Cambridge Headquarters Lease”). The initial lease term is for three years with an option to renew for an additional two-year term. The lease commenced in September 2023 and expires in August 2026. The following table sets forth our contractual obligations under our operating lease as of December 31, 2023:
Operating | |||
Lease | |||
2024 | $ | 86 | |
2025 | 89 | ||
2026 | 60 | ||
Total lease payments | $ | 235 | |
In the ordinary course of business, we enter into agreements with third parties that include indemnification provisions, which, in our judgment, are normal and customary for companies in our industry sector. These agreements are typically with business partners, clinical sites, and suppliers. Pursuant to these agreements, we generally agree to indemnify, hold harmless, and reimburse indemnified parties for losses suffered or incurred by the indemnified parties with respect to our products or product candidates, use of such products or product candidates, or other actions taken or omitted by us. The maximum potential amount of future payments we could be required to make under these indemnification provisions is sometimes unlimited. We have not
69
incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. As a result, the estimated fair value of liabilities relating to these provisions is minimal. Accordingly, we have no liabilities recorded for these provisions as of December 31, 2023 and 2022.
In the normal course of business, we may be confronted with issues or events that may result in contingent liability. These generally relate to lawsuits, claims, environmental actions, or the actions of various regulatory agencies. We consult with counsel and other appropriate experts to assess the claim. If, in our opinion, we have incurred a probable loss, an estimate is made of the loss and the appropriate accounting entries are reflected in our financial statements.
We are party to license agreements with respect to certain of our product candidates that would obligate us to pay royalties with respect to revenue from such product candidates and milestone payments upon achievement of certain development milestones. As of the date hereof, we do not expect to achieve such milestones in the near term, but we would have to obtain additional capital to pay such milestone payments.
Additional information regarding contingent payments and license agreements is in “Note 5. Related party” and "Note 6. Commitments and contingencies" to the consolidated financial statements included in this Annual Report.
Purchase commitments
Information regarding purchase commitments is in "Note 6. Commitments and contingencies" to the consolidated financial statements included in this Annual Report.
Employment agreements
Information regarding employment agreements is in "Note 6. Commitments and contingencies" to the consolidated financial statements included in this Annual Report.
Critical Accounting Estimates
Our consolidated financial statements included in this Annual Report have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses, and related disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of expenses during the reporting period. The most significant estimates in our consolidated financial statements relate to accrued expenses and the fair value of stock-based compensation and warrants. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results could differ from those estimates. Changes in estimates are reflected in reported results in the period in which they become known.
Our critical accounting estimates and judgements relate to the following items: (i) clinical trial costs and accruals, (ii) fair value of stock-based compensation, (iii) fair value of warrants, and (iv) cash forecast for conclusion about NeuroBo’s ability to continue as a going concern. For information on our estimates and judgements used in clinical trial costs and accruals, fair value of stock-based compensation and fair value of warrants, see “J. Research and development costs” in “Note 1. Business, basis of presentation, new accounting standards and summary of significant accounting policies,” “Note 8. Stock-based compensation,” and “11. Fair value measurements,” respectively, to the consolidated financial statements included elsewhere in this Annual Report. Our cash forecast for the 12-month period from the filing date of this Annual Report utilize current cash balance less estimated payments for future clinical trials and G&A costs plus forecasted cash inflows
Recent accounting pronouncements
Information regarding (i) adoption of new accounting standards during 2023 and (ii) accounting standards issued but not yet adopted is included in "Note 1. Business, basis of presentation, new accounting standards and summary of significant accounting policies " to the consolidated financial statements included in this Annual Report.
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We are exposed to market risks in the ordinary course of business. Some potential market risks are discussed below:
70
Market risk
Strategic and operational risks arise if we fail to carry out business operations and/or raise sufficient equity and/or debt financing. These strategic opportunities or threats arise from a range of factors that might include changing economic and political circumstances and regulatory approvals and competitor actions. The risk is mitigated by consideration of other potential development opportunities and challenges which management may undertake.
Currency risk
Our operating results and financial position are reported in U.S. dollars. Some of our financial transactions are denominated in currencies other than the U.S. dollar. Accordingly, our results of operations are subject to currency transaction risks.
We have no hedging agreements in place with respect to foreign exchange rates. We have not entered into any agreements or purchased any instruments to hedge possible currency risks at this time.
Interest rate risk
Interest rate risk is the risk that the fair value or the future cash flows of a financial instrument will fluctuate as a result of changes in market interest rates. Cash bears interest at market rates.
Inflation risk
If our costs become subject to significant inflationary pressures, it could harm our business, financial condition, and operating results.
Item 8.Financial Statements and Supplementary Data
Reference is made to the financial statements, the notes thereto, and the report thereon, commencing on page F-1 of this Annual Report, which financial statements, notes and report are incorporated herein by reference.
Item 9.Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A.Controls and Procedures
Evaluation of disclosure controls and procedures
As required by Rules 13a-15(b) and 15d-15(b) under the Exchange Act, our management, with the participation of our principal executive officer (“PEO”) and principal financial officer (“PFO”), evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report. Based upon that evaluation, our PEO and PFO concluded that our disclosure controls and procedures were not effective as of the end of the period covered by this Annual Report, as a result of material weaknesses in our internal control over financial reporting, which are discussed further below.
Management’s annual report on internal control over financial reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and Board; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2023. The scope of management’s assessment regarding the Company’s internal control over financial reporting includes the criteria set forth by the Internal Control Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. As a result of material weaknesses, management has concluded that our internal control over financial reporting was not effective as of December 31, 2023.
71
In connection with the preparation of the audited financial statements included elsewhere in this Annual Report, management has identified the following material weaknesses: (i) lack of segregation of duties over cash disbursements and financial reporting, (ii) logical access over computer applications, and (iii) lack of supervision and review over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Specifically, there was a lack of segregation of duties involved in the execution of wire transfers, preparing journal entries, and review over clinical trial accruals, and certain individuals in the accounting department have administrative access to the financial reporting systems. See “Remediation efforts to address the material weaknesses” below for steps we are taking to correct these material weaknesses.
Remediation efforts to address the material weaknesses
We are in the process of remediating, but have not yet remediated, the material weaknesses, as described above, related to lack of segregation of duties over cash disbursements and financial reporting, logical access over computer applications, and lack of supervision and review over financial reporting. Under the oversight of the audit committee, management has developed a detailed plan and timetable for the implementation of appropriate remedial measures to address the material weaknesses. As of the date of this report, we have taken the following actions:
| ● | we have added additional personnel to the accounting department to allow for increased segregation of duties; |
| ● | we have implemented a change management review process for access to systems used for financial reporting systems |
| ● | we have enhanced the controls over disbursements, separating the functions of initiating and approving to two separate individuals; and |
| ● | we have implemented enhanced controls relating to the review and oversight of financial reporting, including the preparation of journal entries, and clinical trial accruals. |
Management is working to fully remediate these material weaknesses during 2024.
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. The management’s report was not subject to attestation by our registered public accounting firm pursuant to the rules of the SEC that permit us to provide only the management’s report in this Annual Report.
Inherent limitations of disclosure controls and procedures and internal control over financial reporting
Our management, including our PEO and PFO, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Changes in internal control over financial reporting
Other than the remediation activities listed above, there have been no changes in our internal control over financial reporting during the quarter ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B.Other Information
Bylaws Amendment
On March 27, 2024, our Board approved and adopted amended and restated bylaws of the Corporation (as so amended and restated, the “Third Amended and Restated Bylaws”), effective as of such date.
72
The Third Amended and Restarted Bylaws were adopted to:
| ● | Expressly provide for stockholder meetings by remote communications; |
| ● | Address the universal proxy rules adopted by the SEC by clarifying that no person may solicit proxies in support of a director nominee other than the board’s nominees unless such person has complied with Rule 14a-19 under the Exchange Act, including the applicable notice and solicitation requirements; |
| ● | Enhance certain procedural mechanics and disclosure requirements in connection with stockholder submissions of proposals regarding other business at annual meetings of stockholders, including requiring additional background information and disclosures related to proposing stockholders, proposed nominees and business proposals, and other persons related to a stockholder’s solicitation of proxies; |
| ● | Provide that if a stockholder does not comply with Rule 14a-19, we will disregard proxies and votes for such stockholders’ nominees; and |
| ● | Require a stockholder, or group of stockholders, calling a special meeting in order to nominate a person to the board to hold 10% of the votes at the meeting, to be a stockholder at the time of the notice, at the record date of the meeting and to comply with the relevant provisions of the Third Amended and Restated Bylaws with regards to stockholder disclosure about the nominee and the proposing stockholders. |
The foregoing summary of the amendments effected by the Third Amended and Restated Bylaws does not purport to be complete and is qualified in its entirety by reference to the complete text of the Amended and Restated Bylaws, which are filed as Exhibit 3.5 hereto and are incorporated herein by reference.
Trading Plans
During the three months ended December 31, 2023, none of our directors or Section 16 officers
Item 9C.Disclosure Regarding Foreign Jurisdictions that Prevents Inspections
None.
Part III
Item 10.Directors, Executive Officers and Corporate Governance
Directors and Executive Officers
The Board is divided into three classes. Members of each class serve staggered three-year terms. The terms of office of directors in Class I, Class II and Class III expire at the annual meetings of stockholders to be held in 2026, 2024 and 2025, respectively. The following table provides information as to each person who is, as of the filing hereof, a director and/or executive officer of NeuroBo.
Name | Position(s) | Age |
Mark A. Glickman | Class III Director | 58 |
Jason L. Groves | Class II Director | 53 |
Hyung Heon Kim | Chief Executive Officer, President and Class II Director | 48 |
Andrew Koven | Class II Director and Chair of the Board | 66 |
Michael Salsbury | Class III Director | 74 |
D. Gordon Strickland | Class I Director | 77 |
James P. Tursi | Class I Director | 59 |
Marshall H. Woodworth | Chief Financial Officer | 66 |
73
Business Experience and Background of Directors and Executive Officers
Mr. Mark A. Glickman has served as a member of the Board since May 2023. Since December 2023, Mr. Glickman has served as President and Chief Executive Officer of BioFlorida Inc., an association representing life sciences and research organizations based in Florida. Previously, Mr. Glickman served as the Co-Chief Executive officer for TherapeuticsMD, Inc. (Nasdaq: TXMD), a women’s healthcare product company, from September 2022 through the sale of the assets of TXMD to Mayne Therapeutics in January 2023. Mr. Glickman also served as Chief Business Officer, Commercial of TherapeuticsMD, Inc. since June 2021 through the sale of assets of TXMD. Previously, Mr. Glickman served as the Chief Commercial Officer for Esperion Therapeutics, Inc. (Nasdaq: ESPR) from 2018 until December 2020, where he developed and led the commercial division in the launch of the company’s first cardiovascular prescription therapy. From June 2015 to March 2018, Mr. Glickman served as the Chief Commercial Officer for Aralez Pharmaceuticals, where he built out and led the first commercial effort for a previously clinical organization. Prior to June 2015, Mr. Glickman was Executive Vice President of Sales and Marketing for Auxilium (Endo), where he led all commercial efforts for a portfolio of thirteen pharmaceutical products. Mr. Glickman's previous positions include Senior Vice President of Sales and Marketing and Vice President of Medical Devices for Otsuka America Pharmaceuticals Inc. and Marketing Head, Regional Sales Director and Vice President of Sales and Operations at Kos Pharmaceuticals (Abbott Labs), where he expanded his skills in the commercial products area. Mr. Glickman received a Bachelor of Arts degree in Political Science from S.U.N.Y Oswego, and a Master of Business Administration in Finance and International Management from the N.Y.U. Stern School of Business. The Board believes that Mr. Glickman’s 30 years of experience in the pharmaceutical and medical device industry qualifies him to serve as a director.
Mr. Jason L. Groves, Esq. has served as a member of the Board since December 2019. Since July 2022, Mr. Groves has served as the Chief Legal Officer and Corporate Secretary of Medifast, Inc. (NYSE: MED), a publicly-held leading manufacturer and distributor of clinically-proven, healthy-living products and programs. After joining Medifast in 2009, Mr. Groves has held several executive management positions, most recently serving as Executive Vice President and General Counsel of Medifast, Inc. from 2011 to July 2022. Mr. Groves was a Medifast, Inc. director from 2009 to 2015, serving on the Audit Committee from 2009 to 2011. Prior to joining Medifast, Mr. Groves was Assistant Vice President of Government Affairs for Verizon Maryland, a telecommunications company, where he was responsible for the company’s legislative policy and government affairs. A U.S. Army veteran, Mr. Groves was a direct-commissioned Judge Advocate in the U.S. Army Judge Advocate General’s (JAG) Corps. As a JAG officer, he practiced law and had the distinction of prosecuting criminal cases in the District Court of Maryland as a Special Assistant U.S. Attorney. Over the course of three years, he received two Army Achievement Medals and one Army Commendation Medal. Mr. Groves completed nine years with the Anne Arundel Medical Center Board of Trustees, chairing their international captive insurance company board for eight years. Mr. Groves received his Bachelor of Science degree, cum laude, in Hospitality Management from Bethune-Cookman University, and obtained his Juris Doctor from North Carolina Central University School of Law. The Board believes that Mr. Grove’s experience serving as an independent director, audit committee member, and chief legal officer of a large public corporation while assisting with the initial international introduction of such corporation’s products qualify him to serve as a director.
Mr. Hyung Heon Kim has served as a member of the Board since July 2021 and was appointed as our President and Chief Executive Officer in August 2023. Previously, Mr. Kim was the General Counsel and a Vice President of Dong-A ST and Dong-A Socio Group, a Korean-based group of companies mainly engaged in the research, development, production and sale of pharmaceuticals, medical devices and APis. Mr. Kim served as General Counsel of Dong-A ST from January 2018 until August 2023 and as a Vice President of Dong-A ST from December 2020 until August 2023. Mr. Kim previously served as Executive Director of Dong-A ST from January 2018 through December 2020. Prior to his roles with Dong-A ST, Mr. Kim was Head of International Legal Affairs for Dong-A Socio Holdings Co., Ltd., a Korean-based holdings company for the Dong-A Socio group of companies from 2012 to 2018. Since April 2021, Mr. Kim has served as a director of AnaPath Services GmbH, a private Swiss-based provider of scientific research and development services, and STP America Research Corp, a private New Jersey-based research and development company. Prior to joining Dong-A Socio Group, Mr. Kim served as legal counsel to SK Energy Co., Ltd. and SK Innovation Co., Ltd. from 2008 to 2011. Mr. Kim received his Bachelor of Law degree from Soongshil University in Korea, and obtained his Juris Doctor from Washington University School of Law. The Board believes that Mr. Kim’s experiences gained as General Counsel and Head of International Legal Affairs to an established pharmaceutical group of companies qualify him to serve as a director. In addition, his day-to-day leadership of NeuroBo gives him critical insights into our operations, strategy and competition, and he facilitates the Board’s ability to perform its oversight function.
74
Mr. Andrew I. Koven has served as a member of the Board since July 2021, and Chair of the Board since January 2022. Mr. Koven is the Lead Independent Director of Kala Pharmaceuticals, Inc. (Nasdaq: KALA), a public biopharmaceutical company focused on the discovery, development and commercialization of innovative therapies for diseases of the eye. He has served as the Lead Independent Director of Kala Pharmaceuticals, Inc. since December 2018 and as a member of the Kala board of directors since September 2017. Mr. Koven serves as Chair of Kala’s nominating and corporate governance committee and compensation committee. Mr. Koven was, until his retirement in January 2019, the President and Chief Business Officer of Aralez Pharmaceuticals Inc., or Aralez, a public specialty pharmaceutical company, and served in that role with the company’s predecessor, Pozen Inc., commencing in June 2015. Prior to joining Pozen, Mr. Koven served as Executive Vice President, Chief Administrative Officer and General Counsel of Auxilium Pharmaceuticals Inc., a public specialty biopharmaceutical company, from February 2012 until January 2015, when it was acquired by Endo International plc. Mr. Koven served as President and Chief Administrative Officer and a member of the board of directors of Neurologix, Inc., a company focused on the development of multiple innovative gene therapy development programs, from September 2011 to November 2011. Before Neurologix, Mr. Koven served as Executive Vice President and Chief Administrative and Legal Officer of Inspire Pharmaceuticals, Inc., a public specialty pharmaceutical company, from July 2010 until May 2011 when it was acquired by Merck & Co., Inc. Previously, Mr. Koven served as Executive Vice President, General Counsel and Corporate Secretary of Sepracor Inc. (now Sunovion), a public specialty pharmaceutical company, from March 2007 until February 2010 when it was acquired by Dainippon Sumitomo Pharma Co., Ltd. Prior to joining Sepracor, Mr. Koven served as Executive Vice President, General Counsel and Corporate Secretary of Kos Pharmaceuticals, Inc., a public specialty pharmaceutical company, from August 2003 until its acquisition by Abbott Laboratories (now AbbVie) in December 2006. Mr. Koven began his career in the pharmaceutical industry first as an Assistant General Counsel and then as Associate General Counsel at Warner-Lambert Company from 1993 to 2000, followed by his role as Senior Vice President and General Counsel at Lavipharm Corporation from 2000 to 2003. From 1986 to 1992, he was a corporate associate at Cahill, Gordon & Reindel in New York. From 1992 to 1993, he served as Counsel, Corporate and Investment Division, at The Equitable Life Assurance Society of the U.S. Mr. Koven holds a Master of Laws (LL.M.) Degree from Columbia University School of Law and a Bachelor of Laws (LL.B.) Degree and B.A. Degree in Political Science from Dalhousie University. The Board believes that Mr. Koven’s extensive experience in the pharmaceutical industry qualifies him to serve as a director.
Mr. Michael Salsbury has served as a member of the Board since December 2019. He has served as Counsel to Current Health Inc., a provider of remote care management products and services since May 2021. Current Health was acquired by Best Buy Co., Inc. (BBY) in November 2021. From September 2017 to May 2022, Mr. Salsbury has served as Counsel to Verisma Systems, Inc., a provider of cloud-based automated disclosure management systems; and from February 2013 to July 2017, he served as Secretary and General Counsel to Best Doctors, Inc., a provider of expert medical opinions. Best Doctors was acquired by Teladoc Health, Inc. (TDOC) in July 2017. Mr. Salsbury has more than 25 years’ experience as a senior executive with public and private companies and private law practice. Mr. Salsbury received a J.D. and M.B.A. from the University of Virginia and a B.A. from Dartmouth College. The Board believes that Mr. Salsbury’s legal expertise and his experience serving as general counsel and secretary of a Fortune 100 corporation qualifies him to serve as a director.
Mr. D. Gordon Strickland has served as a member of the Board since January 2022. He served as Chair of Ampex Corporation, a publicly-traded technology company, from March 2012 until June 2019. He also served as Ampex’s Chief Executive Officer from February 2007 to March 2012. Prior to Ampex, he served as President and Chief Executive Officer of Cardiff Holdings, a privately held producer of credit, debit, loyalty and other cards by Brookside Equity Partners from March 2012 to August 2013. Prior to Cardiff Holdings, Mr. Strickland was the Chair of Medical Resources, a public operator of diagnostic imaging centers. Mr. Strickland was also president and CEO of MCSi, Inc, a technical integrator of audio-visual products, from March 2003 until March 2004. Prior to MCSi, Mr. Strickland was the president and CEO of Capitol Wire, Inc, an internet-based news and information service provider from September 1999 until August 2002 and had leadership roles with Kerr Group, a manufacturer of glass containers and plastic packaging, from June 1986 until August 1997, including serving as the president and CEO, and as Senior Vice President, Finance and Chief Financial Officer. Mr. Strickland has over 35 years of experience as a senior executive and board member with public and private companies. Mr. Strickland received an M.B.A. from the Wharton School of the University of Pennsylvania and a B.A from Yale University. The Board believes that Mr. Strickland’s experience serving as Chair and Chief Executive Officer of a publicly-traded company, Ampex, qualifies him to serve as a director.
75
Dr. James P. Tursi was appointed to the Board in November 2023. Dr. Tursi has served as Executive Vice President – Global R&D for Endo International plc (Nasdaq: ENDP) since January 2022. From April 2020 until January 2022, Dr. Tursi served as Chief Scientific Officer US for Ferring Pharmaceuticals. From August 2018 until April 2020, Dr. Tursi served as Executive Vice President, R&D for Antares Pharma Inc. Prior to August 2018, Dr. Tursi served as Chief Medical Officer at Aralez Pharmaceuticals, Chief Medical Officer and Vice President of Clinical R&D for Auxilium Pharmaceuticals, and held positions of increasing responsibility at GlaxoSmithKline and Procter & Gamble Pharmaceuticals. Dr. Tursi practiced medicine and surgery for over 10 years and created a medical education company, I Will Pass®, which assisted physicians in the process of board certification. He holds a Bachelor of Science degree in Chemistry and Biology from Ursinus College; a Doctor of Medicine from Medical College of Pennsylvania and performed his residency in Gynecology and Obstetrics at the Johns Hopkins Hospital. The Board believes Dr. Tursi’s pharmaceutical industry and senior leadership experience qualifies him to serve as a director.
Mr. Marshall Woodworth served as our Acting Chief Financial Officer from October 25, 2023 until his appointment as our Chief Financial Officer on March 1, 2024. Previously, Mr. Woodworth served as the Chief Financial Officer of Nevakar Inc. and its respective subsidiaries (Nevakar Injectables Inc. and Vyluma Inc.) from May 2017 through May 2023, where Mr. Woodworth was responsible for the accounting, financing, legal and human resources functions. From October 2015 through October 2016, Mr. Woodworth served as the Chief Financial Officer of Braeburn Pharmaceuticals Inc., where Mr. Woodworth led and coordinated the accounting, finance and treasury functions. From May 2014 to July 2015, Mr. Woodworth served as the Chief Financial Officer of Aerocrine AB, where Mr. Woodworth had responsibility for directing and coordinating the accounting and finance, FRS (Swedish SEC) reporting, investor relations, human resources and legal aspects of the company. From January 2010 through February 2014, Mr. Woodworth served as Chief Financial Officer of Furiex Pharmaceuticals, Inc. (Nasdaq: FURX), where Mr. Woodworth led a multi-disciplinary team and managed accounting, finance, SEC reporting, financial planning, analysis and reporting, and treasury functions. Mr. Woodworth received a Bachelor of Science degree from the University of Maryland and a Master of Business Administration degree in Finance from Indiana University.
Family Relationships
None of our directors or executive officers has a family relationship as defined in Item 401 of Regulation S-K.
Involvement in Certain Previous Legal Proceedings
Mr. Glickman served as Chief Commercial Officer at Aralez Pharmaceuticals Inc. (“Aralez”) from June 2016 to March 2018, Mr. Koven served as President and Chief Business Officer of Aralez’s predecessor, Pozen, Inc. (“Pozen”) and then at Aralez from June 2015 to January 2019, and Dr. Tursi served as Chief Medical Officer of Pozen and then Aralez from 2015 until August 2018, and has served as Executive Vice President – Global R&D for Endo International plc (Nasdaq: ENDP) since January 2022. Each of Aralez and Endo International plc and certain of their respective affiliates filed a voluntary petition for relief under Chapter 11 of the U.S. Bankruptcy Code on August 10, 2018 and August 16, 2022, respectively.
Code of Business Conduct and Ethics
Our Board has adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including our Chief Executive Officer, Chief Financial Officer and other executive officers, as applicable. We intend to disclose future amendments to certain provisions of our code of business conduct and ethics, or waivers of these provisions, on our website. The full text of our code of conduct is posted on the investor relations section of our website at http://neurobopharma.com under “Investors & News-Corporate Governance-Highlights”.
Audit Committee
Our Board has established an audit committee, which is comprised of Mr. Strickland, Mr. Koven and Mr. Glickman, with Mr. Strickland serving as chair of the committee. Each member of our audit committee meets the requirements for independence under the current Nasdaq and SEC rules and regulations and is financially literate. In addition, our Board has determined that Messrs. Glickman and Strickland each qualifies as an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K promulgated under the Securities Act based on his serving as chief executive officer of multiple companies as described above. This designation does not impose on either of them any duties, obligations or liabilities that are greater than are generally imposed on members of our audit committee and our Board.
76
Item 11.Executive Compensation
Executive Officer Compensation
Summary Compensation Table for 2023 and 2022
The following table presents summary information regarding the total compensation for services rendered in all capacities that was earned by our named executive officers for 2023 and 2022.
Salary | Bonus | Stock Awards (1) | Option Awards (1) | All Other Compensation | Total | |||
Name and Principal Position | Year | ($) | ($) | ($) | ($) | ($) | ($) | |
Hyung Heon Kim, President and Chief Executive Officer (2) | 2023 | 174,009 | 55,000 | 386,915 | — | 48,131 | (3) | 664,055 |
2022 | — | — | — | 6,663 | 54,217 | (3) | 60,880 | |
Marshall Woodworth, Chief Financial Officer (4) | 2023 | — | — | — | — | 154,500 | (5) | 154,500 |
2022 | — | — | — | — | — | — | ||
Gil Price, Former President and Chief Executive Officer (6) | 2023 | 16,667 | — | — | — | 100,140 | (7) | 116,807 |
2022 | 400,000 | 100,000 | — | — | 1,579 | (7) | 501,579 | |
Joseph Hooker, Former Interim President and Chief Executive Officer (8) | 2023 | — | — | — | — | 432,000 | (9) | 432,000 |
2022 | — | — | — | — | — | — |
(1) | Amounts for 2023 reported reflect the aggregate grant date fair value of RSUs, whose grant date fair value was determined based on the closing sales price of our common stock as reported on Nasdaq on the date of grant. The amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. Amounts for 2022 reported reflect the aggregate grant date fair value of options, whose grant date fair value was estimated using the Black Scholes valuation model and the assumptions used for valuation can be found in Note 8. Stock-based compensation of the Notes to Consolidated Financial Statements, included elsewhere in this Annual Report. The amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. | |||
(2) | Mr. Kim was appointed as our President and Chief Executive Officer in August 2023. | |||
(3) | Other compensation paid to Mr. Kim was related to (i) $33,000 fees earned in 2023 as a director prior to Mr. Kim’s appointment as an executive officer in August 2023, (ii) $15,131 for health and welfare benefits paid by NeuroBo in 2023, and (iii) $54,217 fees earned in 2022 as a director. | |||
(4) | Mr. Woodworth was appointed as our Acting Chief Financial Officer in October 2023 and was appointed as Chief Financial Officer in March 2024. | |||
(5) | While serving as our Acting Chief Financial Officer, Mr. Woodworth was employed by WhiteCap Search Holdings, LLC (“WhiteCap”) and was contracted to us from October 2023 until March 2024. We paid $154,500 in consulting fees to WhiteCap for Mr. Woodworth’s services for 2023. | |||
(6) | Dr. Price was appointed as our President and Chief Executive Officer in November 2021. Dr. Price resigned as our President and Chief Executive Officer in January 2023. | |||
(7) | Other compensation paid to Dr. Price was related to (i) a severance payment of $100,000 in 2023 and (ii) health and welfare benefits paid by NeuroBo in 2023 and 2022. | |||
(8) | Mr. Hooker was appointed as our interim President and Chief Executive Officer in January 2023. Mr. Hooker stepped down as the Interim President and Chief Executive Officer in August 2023. | |||
(9) | While serving as our Interim President and Chief Executive Officer, Mr. Hooker was employed by Korn Ferry US (“Korn Ferry”) and was contracted to us from January 2023 until August 2023. We paid $432,000 in consulting fees to Korn Ferry for Mr. Hooker’s services for 2023. | |||
77
Narrative Disclosure to Summary Compensation Table
Agreements with Our Named Executive Officers
We had entered into a written agreement with Dr. Price who served as our President and Chief Executive Officer through January 2023. We entered into an engagement letter with Korn Ferry (the “KF Agreement”) pursuant to which Mr. Joseph Hooker was appointed in January 2023 to serve as our Interim President and Chief Executive Officer until August 2023. We entered into an employment agreement with Mr. Kim in connection with his appointment as our President and Chief Executive Officer in August 2023. In October 2023, Mr. Woodworth was appointed as the Acting Chief Financial Officer of NeuroBo, pursuant to an engagement agreement with WhiteCap Search Holdings, LLC (“WhiteCap”).
Hyung Heon Kim
We entered into an employment agreement with Mr. Kim in connection with his appointment as our Chief Executive Officer and President in August 2023 (the “Kim Employment Agreement”). Under the terms of Kim Employment Agreement, we agreed to provide Mr. Kim: (i) an annual base salary of $450,000, reviewed annually; (ii) an annual discretionary bonus targeted at 50% of his base salary, as determined in the sole discretion of the Board or committee thereof; (iii) the right to participate in the benefit programs and arrangements that we make available to our employees, including paid vacation and sick leave, contributory and non-contributory welfare and benefit plans, disability plans, and medical, death benefit and life insurance plans for which Mr. Kim is eligible under the terms of those plans; and (iv) a RSU award for 625,064 shares of our common stock pursuant to the terms of a RSU grant notice and form award agreement (the “Kim RSU Award”) under our 2022 Equity Incentive Plan. The Kim RSU Award vests as to 50% of the shares underlying the Kim RSU Award on the first anniversary of Mr. Kim’s employment with NeuroBo and, the remaining shares subject to the Kim RSU Award, shall vest and become exercisable in equal monthly installments on the last day of each full month over the twelve (12) months following the first anniversary of Mr. Kim’s employment with us.
In the event of Mr. Kim’s death during the employment period or a termination due to disability, Mr. Kim or his beneficiaries or legal representatives shall be entitled to receive (i) any annual base salary earned, but unpaid, for services rendered to NeuroBo on or prior to the date on which the employment period ends, (ii) unreimbursed expenses and (iii) certain other benefits provided for in the employment agreement (the “Kim Unconditional Entitlements”). In the event of termination for cause by NeuroBo or the termination of employment as a result of resignation without good reason, Mr. Kim shall be provided the Kim Unconditional Entitlements.
In the event of a resignation by Mr. Kim for good reason or the exercise by NeuroBo of its right to terminate Mr. Kim other than for cause, death or disability, Mr. Kim will receive the Kim Unconditional Entitlements and, subject to Mr. Kim signing and delivering to us and not revoking a general release of claims in favor of NeuroBo and certain related parties, we shall pay a severance amount to Mr. Kim equal to fifty percent (50%) of Mr. Kim’s then-current base salary (the “Severance Amount”) and pay for Mr. Kim’s continued health insurance coverage under the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (known as COBRA) for a period of six (6) months (the “Kim Conditional Benefits”).
In the event of a resignation by Mr. Kim for good reason, the exercise by NeuroBo of its right to terminate Mr. Kim other than for cause, death or disability, in each case, within twelve (12) months following or three (3) months prior to the effective date of a change in control, Mr. Kim will receive the following: (i) the Kim Unconditional Entitlements and the Kim Conditional Benefits less the Severance Amount; (ii) an amount equal to the product of 1.0 times the sum of Mr. Kim’s annual base salary and target annual cash bonus, less the Non-Compete Amount as defined in the Kim Employment Agreement (if applicable); and (iii) accelerated vesting of all equity awards that were assumed, continued or substituted by the surviving or acquiring corporation in the change in control and remain subject to time-based vesting conditions, if any.
In addition, Mr. Kim entered into an Employee Proprietary Information and Invention Assignment Agreement that applies during the term of Mr. Kim’s employment and thereafter.
Marshall Woodworth
In October 2023, Mr. Woodworth was appointed as the Acting Chief Financial Officer of NeuroBo, pursuant to an engagement agreement with WhiteCap, dated February 3, 2023. Mr. Woodworth received his compensation and benefits from WhiteCap Search Holdings, LLC. In connection with the appointment of Mr. Woodworth as our Acting Chief Financial Officer, we paid WhiteCap approximately $375.00 per hour under the engagement agreement for services rendered to NeuroBo by Mr. Woodworth.
In March 2024, we entered into an employment agreement with Mr. Woodworth in connection with his appointment as Chief Financial Officer of NeuroBo (the “Woodworth Employment Agreement”). The Woodworth Employment Agreement has an initial term of two (2) years beginning on March 1, 2024 and automatically renews for an additional one-year period at the end
78
of the Initial Term and each anniversary thereafter provided that at least 60 days prior to the expiration of the Initial Term or any Renewal Term the Board does not notify Mr. Woodworth of its intention not to renew.
Under the terms of Woodworth Employment Agreement, we agreed to provide Mr. Woodworth: (i) an annual base salary of $380,000, reviewed annually; (ii) an annual discretionary bonus targeted at 40% of his base salary, as determined in the sole discretion of the Board or committee thereof; (iii) the right to participate in the benefit programs and arrangements that we make available to our employees, including paid vacation and sick leave, contributory and non-contributory welfare and benefit plans, disability plans, and medical, death benefit and life insurance plans for which Mr. Woodworth is eligible under the terms of those plans; and (iv) a RSU award for 33,496 shares of our common stock pursuant to the terms of a RSU grant notice and form award agreement (the “Woodworth RSU Award”) under our 2022 Equity Incentive Plan. The Woodworth RSU Award vests as follows: (i) 30% of the shares underlying the Woodworth RSU Award on the first anniversary of the grant; (ii) 30% of the shares underlying the Woodworth RSU Award on the second anniversary of the grant date; and (iii) the remaining shares subject to the Woodworth RSU Award, shall vest and become exercisable in equal monthly installments on the last day of each full month over the twelve (12) months following the first anniversary of grant date.
If during the period Mr. Woodworth is employed by NeuroBo, we consummate a change in control (as defined in the Woodworth Employment Agreement) and the Woodworth RSU Award is not assumed, continued or substituted by the surviving corporation or acquiring corporation (or the surviving or acquiring corporation’s parent company) in such change in control in the manner contemplated by the 2022 Plan, then 100% of the unvested portion of the Woodworth RSU Award shall fully vest immediately prior to the effectiveness of such change in control.
In the event of Mr. Woodworth’s death during the employment period or a termination due to disability, Mr. Woodworth or his beneficiaries or legal representatives shall be entitled to receive any annual base salary earned, but unpaid, for services rendered to NeuroBo on or prior to the date on which the employment period ends, unreimbursed expenses and certain other benefits provided for in the Woodworth Employment Agreement (the “Unconditional Entitlements”). In the event of termination for cause by NeuroBo or the termination of employment as a result of resignation without good reason, Mr. Woodworth shall be provided the Unconditional Entitlements.
In the event of a resignation by Mr. Woodworth for good reason or the exercise by NeuroBo of its right to terminate Mr. Woodworth other than for cause, death or disability, Mr. Woodworth will receive the Unconditional Entitlements and, subject to Mr. Woodworth signing and delivering to NeuroBo and not revoking a general release of claims in favor of the NeuroBo and certain related parties, we shall pay a severance amount to Mr. Woodworth equal to twenty-five percent (25%) of Mr. Woodworth’s then-current annual base salary and pay for Mr. Woodworth’s continued health insurance coverage under the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (known as COBRA) for a period of three (3) months (the “Conditional Benefits”).
In the event of a resignation by Mr. Woodworth for good reason or the exercise by NeuroBo of its right to terminate Mr. Woodworth other than for cause, death or disability, in each case, within twelve (12) months following or three (3) months prior to the effective date of a change in Control, Mr. Woodworth will receive the following: (i) the Unconditional Entitlements and the Conditional Benefits less the Severance Amount; (ii) an amount equal to the product of 0.50 times the sum of Mr. Woodworth’s annual base salary and target annual cash bonus, less the Non-Compete Amount as defined in the Woodworth Employment Agreement (if applicable); and (iii) accelerated vesting of all equity awards that were assumed, continued or substituted by the surviving or acquiring corporation in the Change in Control and remain subject to time-based vesting conditions, if any.
Gil Price
In November 2021, we entered into an employment agreement with Dr. Price (the “Price Employment Agreement”). The Price Employment Agreement had an initial term of one year beginning on November 3, 2021 and automatically renewed for an additional one-year period at the end of the initial term. Dr. Price resigned in January 2023. In connection with Dr. Price’s departure, we entered into a Separation and Release Agreement with Dr. Price, pursuant to which Dr. Price received severance pay of $100,000 and $100,000 as Dr. Price’s annual bonus for 2022.
Joseph Hooker
We entered into the KF Agreement pursuant to which Mr. Joseph Hooker was appointed in January 2023 to serve as our Interim President and Chief Executive Officer. In connection with the appointment of Mr. Kim as our Chief Executive Officer and President as described above, in August 2023, we terminated the KF Agreement and Mr. Hooker ceased to serve as our Interim Chief Executive Officer, President and Chief Financial Officer.
79
Outstanding Equity Awards at Fiscal Year-End 2023
The following table sets forth information regarding outstanding stock option and RSU awards held by our named executive officers as of December 31, 2023:
Name | Grant Date | Number Of Securities Underlying Unexercised Options Exercisable (#) | Option Exercise Price ($) | Option Expiration Date | Number Of Shares Of Stock That Have Not Vested (#) | Market Value Of Shares Of Stock That Have Not Vested ($) | ||||||
Hyung Heon Kim | June 9, 2022 | 83 | 14.18 | June 9, 2032 | — | — | ||||||
Hyung Heon Kim | August 10, 2023 | — | — | — | 78,133 (1) | 288,858 | ||||||
Non-Employee Director Compensation
Our non-employee directors receive a mix of cash and share-based compensation intended to encourage non-employee directors to continue to serve on the Board, further align the interests of the directors and stockholders, and attract new non-employee directors with outstanding qualifications. Directors who are employees or officers of NeuroBo do not receive any additional compensation for Board service.
The following table provides compensation information for 2023 for each non-employee member of the Board.
Fees Earned | |||
or Paid | Stock | ||
in Cash | Awards (1) | Total | |
Name | ($) | ($) | ($) |
Mark A. Glickman (2) | 31,481 | 18,988 | 50,469 |
Jason Groves (3) | 49,988 | 50,363 | 100,351 |
Richard Kang (4) | 10,000 | — | 10,000 |
Hyung Heon Kim (5) | 33,000 | — | 33,000 |
Na Yeon (Irene) Kim (6) | 34,757 | 50,363 | 85,120 |
Andrew Koven (7) | 90,473 | 50,363 | 140,836 |
Michael Salsbury (8) | 52,000 | 50,363 | 102,363 |
D. Gordon Strickland (9) | 60,351 | 50,363 | 110,714 |
James P. Tursi (10) | 7,459 | 14,467 | 21,926 |
(1) | Amounts reported reflect the aggregate grant date fair value of RSUs, whose grant date fair value was determined based on the closing sales price of our common stock as reported on Nasdaq on the date of grant. The amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. |
(2) | Mr. Glickman was appointed to the Board in May 2023. As of December 31, 2023, Mr. Glickman had 3,126 outstanding unvested RSUs. |
(3) | As of December 31, 2023, Mr. Groves had 333 outstanding exercisable options and 7,032 outstanding unvested RSUs. |
(4) | Dr. Kang resigned from the Board in March 2023. |
(5) | Other compensation paid to Mr. Kim was related to (i) $33,000 fees earned as a director prior to Mr. Kim’s appointment as an executive officer in 2023 and 2022 and (ii) $15,131 for health and welfare benefits paid by NeuroBo in 2023. |
(6) | Ms. Kim resigned from the Board in September 2023. |
80
(7) | As of December 31, 2023, Mr. Koven had 333 outstanding options, of which 271 options were exercisable, and 12,501 outstanding RSUs, of which 5,469 RSUs were vested with common stock issuance deferred under the terms of the RSU award, and the remaining 7,032 RSUs were unvested. |
(8) | As of December 31, 2023, Mr. Salsbury had 333 outstanding exercisable options and 7,032 outstanding unvested RSUs. |
(9) | As of December 31, 2023, Mr. Strickland had 250 outstanding options, of which 189 options were exercisable, and 7,032 outstanding unvested RSUs. |
(11) | Dr. Tursi was appointed to the Board in November 2023. As of December 31, 2023, Dr. Tursi had 2,458 outstanding unvested RSUs. |
Non-Employee Director Compensation Policy
In June 2023, the compensation committee recommended, and the Board approved our Amended and Restated Non-Employee Director Compensation Policy (the “Amended Non-Employee Director Compensation Policy”). Under the Amended Non-Employee Director Compensation Policy, all of our non-employee directors receive an annual cash retainer of $40,000 for Board service except for the Chair of the Board who receives an annual cash retainer of $75,000. Additionally, directors receive an additional cash retainer for serving as a committee chair or member as follows:
| | | Audit Committee | | | Compensation Committee | | | Nominating and Corporate Governance Committee |
Committee Chair | | | $18,000 | | | $12,000 | | | $10,000 |
Committee Member (other than the Chair) | | | 9,000 | | | 6,000 | | | 5,000 |
Legacy grant: On the thirtieth (30th) day following the effective date of the Amended Non-Employee Director Compensation Policy, each person who was then serving as a non-employee member of the Board who had continuously served as non-employee member of the Board during 2022 was automatically granted a RSU award for 10,937 (the “Legacy Director RSU”) shares of the Company’s common stock, of which 50% of each Legacy Director RSU vested as of the date of grant and the remainder will vest in two equal installments on each subsequent anniversary of the date of grant, subject to such non-employee director’s continuous service with the Company on each vesting date.
Initial grant: For each non-employee director who was first elected or appointed to the Board in 2023 prior to the effective date of the Amended Non-Employee Director Compensation Policy or on or following the effective date of the Amended Non-Employee Director Compensation Policy, at the close of business on the thirtieth day following the date of such non-employee director’s initial election or appointment to the Board or on the thirtieth day following the effective date of the Amended Non-Employee Director Compensation Policy with respect to any non-employee director that was first elected or appointed to the Board in 2023 prior to the effective date of the Amended Non-Employee Director Compensation Policy, each such non-employee director was granted an RSU award for 3,125 shares of the common stock (each, an “Initial Grant”). 50% of each Initial Grant vested as of the date of grant and the remainder will vest in two equal installments on each subsequent anniversary of the date of grant, subject to such non-employee director’s continuous service with the Company on each vesting date.
Annual grant and prorated annual grant: On the thirtieth day following the first annual meeting of the Company’s stockholders following the effective date of the Amended Non-Employee Director Compensation Policy and on the date of each subsequent annual meeting of the Company’s stockholders (each, an “Annual Meeting”), each person who is then a non-employee director will be automatically be granted an RSU award for 1,562 shares of the common stock (each, an “Annual Grant”).
In addition, for each non-employee director who is first elected or appointed to the Board after the first annual meeting of the Company’s stockholders following the effective date of the Amended Non-Employee Director Compensation Policy on a date other than the date of an annual meeting of the Company’s stockholders, at the close of business on the thirtieth (30th) day following such non-employee director’s initial election or appointment to the Board, such non-employee director will be automatically granted an RSU award for 1,562 shares of the common stock, multiplied by a fraction, the numerator of which
81
equals 365 minus the total number of days, as of the grant date of such RSU award, that have occurred since the last Annual Meeting and the denominator of which equals 365, rounded down to the nearest whole unit (each, a “Prorated Annual Grant”).
Each Annual Grant and Prorated Annual Grant will vest in full on the earlier of (i) the one-year anniversary of the grant date of the Annual Grant or Prorated Annual Grant, as applicable, and (ii) the date immediately prior to the date of the Annual Meeting next following the grant date of such Annual Grant or Prorated Annual Grant, as applicable, subject to such non-employee director’s continuous service with the Company on each vesting date.
Retainer grant: Each non-employee director may elect to forego receiving payment of all (but not less than all) of the annual cash retainers described above that he or she is otherwise eligible to receive for the period during the Company’s fiscal year that the election applies commencing on the first day of such fiscal year (or if the non-employee director makes the election in the Company’s fiscal year that the election applies, on the first day of the Company’s fiscal quarter next following the Company’s fiscal quarter in which the election is made) and ending on the last day of such fiscal year and instead receive an RSU award (the “Retainer Grant”), provided such election is timely made and complies with certain other requirements specified in the Amended Non-Employee Director Compensation Policy. If a non-employee director timely makes the election described above in accordance with the Amended Non-Employee Director Compensation Policy, on the first day of the Company’s fiscal year that the election applies (or if the non-employee director makes the election in the Company’s fiscal year that the election applies, on the first day of the Company’s fiscal quarter following the Company’s fiscal quarter in which the election is made), the non-employee director will be automatically granted a Retainer Grant covering a number of RSUs equal to the (i) aggregate amount of the annual cash retainers that the non-employee director is eligible to receive under the Amended Non-Employee Director Compensation Policy for the applicable period to which the election applies divided by (ii) the average fair market value of a share of the Company’s common stock for the 30 consecutive market trading days ending on and including the last market trading day prior to the grant date of such Retainer Grant, rounded down to the nearest whole unit. Each Retainer Grant will vest in equal quarterly installments over the period commencing on the grant date of the Retainer Grant and ending on the last day of the fiscal year in which the Retainer Grant is granted, subject to the non-employee director’s continued service on each vesting date.
Deferral of settlement of RSU awards: Each non-employee director may elect to defer the delivery of shares in settlement of any RSU award granted under the Amended Non-Employee Director Compensation Policy that would otherwise be delivered to such non-employee director on or following the date such award vests pursuant to the terms of a deferral election such non-employee director makes in accordance with the Amended Non-Employee Director Compensation Policy.
Change of Control; Death; Disability: Each RSU award held by a non-employee director that is granted under the Amended Non-Employee Director Compensation Policy, including the awards described above, will fully vest upon such non-employee director’s death or disability (as defined in the Company’s 2022 Equity Incentive Plan), or immediately prior to the consummation of a change in control (as defined in the Company’s 2022 Equity Incentive Plan), in each case to extent such award is outstanding immediately prior to the occurrence of such event.
Non-employee director compensation limit: The aggregate value of all compensation granted or paid, to any non-employee director with respect to any fiscal year of the Company, including awards granted and cash fees paid by the Company to such non-employee director, will not exceed the limits set forth in the Company’s 2022 Equity Incentive Plan, currently, (1) $750,000 in total value or (2) if such non-employee director first joins the Board during such fiscal year, $1,500,000 in total value.
All RSU awards shall be issued pursuant to the terms of the Company’s 2022 Equity Incentive Plan.
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The following table sets forth information regarding beneficial ownership of our common stock, as of March 25, 2024 by:
| ● | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; |
| ● | each of our named executive officers; |
| ● | each of our directors; and |
| ● | all of our current executive officers and directors as a group. |
The table lists applicable percentage ownership based on 4,906,032 shares of common stock outstanding as of March 25, 2024. In addition, the rules include shares of our common stock issuable pursuant to the exercise of stock options and warrants that are either immediately exercisable or exercisable within 60 days of March 25, 2024. These shares are deemed to be outstanding
82
and beneficially owned by the person holding those options for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Except as otherwise noted below, the address for each person or entity listed in the table is c/o NeuroBo Pharmaceuticals, Inc., 545 Concord Avenue, Suite 210, Cambridge, Massachusetts, 02138.
| Shares |
|
|
| |||||||
Name Of Beneficial Owner | Number (1) | Percent (2) | |||||||||
Greater than 5% stockholders | |||||||||||
Dong-A (3) | 2,803,699 | 57.2 | % | ||||||||
Directors and Named Executive Officers | |||||||||||
Mark A. Glickman, Director | 1,562 | * | |||||||||
Jason Groves, Director (4) | 5,801 | * | |||||||||
Andrew Koven, Chair of the Board of Directors (5) | 5,766 | * | |||||||||
Hyung Heon Kim, Chief Executive Officer, President, and Director (6) | 83 | * | |||||||||
Michael Salsbury, Director (4) | 5,801 | * | |||||||||
D. Gordon Strickland, Director (7) | 5,680 | * | |||||||||
James P. Tursi, Director | 1,563 | * | |||||||||
Marshall H. Woodworth, Chief Financial Officer | — | * | |||||||||
Joseph Hooker, Former Interim Chief Executive Officer and President (8) | — | * | |||||||||
Gil Price, Former President and Chief Executive Officer (9) | — | * | |||||||||
All current executive officers and directors as a group (8 persons) | 26,256 | * | |||||||||
* | Represents beneficial ownership of less than one percent. |
(1) | Includes shares underlying (i) options that are exercisable and (ii) RSUs that are vested or will become vested within 60 days of March 25, 2024. |
(2) | Applicable percentage of ownership is based on 4,906,032 shares of common stock outstanding as of March 25, 2024, as adjusted for each stockholder. |
(3) | Represents shares of common stock owned by Dong-A, a South Korean corporation, with an address of Dong-A ST Co., Ltd. is 64, Cheonho-daero, Dongdaemun-gu, Seoul, Republic of Korea. |
(4) | Includes 333 shares of common stock issuable upon exercise of outstanding options within 60 days of March 25, 2024. |
(5) | Includes 297 shares of common stock issuable upon exercise of outstanding options within 60 days of March 25, 2024 and 5,469 of vested RSUs whose common stock issuance was deferred under the terms of the RSU award. |
(6) | Includes 83 shares of common stock issuable upon exercise of outstanding options within 60 days of March 25, 2024. |
(7) | Includes 212 shares of common stock issuable upon exercise of outstanding options within 60 days of March 25, 2024. |
(8) | Mr. Hooker served as our Interim President and Chief Executive Officer from January 2023 to August 2023. |
(9) | Dr. Price resigned as our President and Chief Executive Officer in January 2023. |
83
Securities Authorized for Issuance Under Equity Compensation Plans
The following table presents information as of December 31, 2023 with respect to compensation plans under which shares of our common stock may be issued.
Plan Category |
| Number of securities to be issued upon exercise of outstanding options, warrants and rights (#) (a) |
| Weighted average exercise price of outstanding options, warrants and rights |
| Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (#)(c) |
| |||
Equity compensation plans approved by security holders |
|
| 171,063 | 398.80 | 469,657 | (1)(2) | ||||
Equity compensation plans not approved by security holders |
|
| — | — | 4,166 | (3) | ||||
| | | | |||||||
Total |
|
| 171,063 | 398.80 | 469,823 |
| ||||
| (1) | The number of shares of common stock remaining available for future issuance represents shares available for issuance under the 2022 Plan. |
| (2) | The 2022 Plan provides that the number of shares that may be issued under the 2022 Plan shall be increased on the first day of each fiscal year by an amount equal to the lesser of (i) 5% of the number of outstanding shares of common stock on such date and (ii) an amount determined by the plan administrator. |
| (3) | Our only equity compensation plan not approved by our security holders is our 2021 Inducement Plan. A total of 4,166 shares of our common stock have been reserved for issuance under the Inducement Plan, subject to adjustment for stock dividends, stock splits, or other changes in our common stock or capital structure. The Inducement Plan was approved by the Compensation Committee without stockholder approval pursuant to Nasdaq Stock Market Listing Rule 5635(c)(4), and is to be utilized exclusively for the grant of stock awards to individuals who were not previously an employee or non-employee director of NeuroBo (or following a bona fide period of non-employment with NeuroBo) as an inducement material to such individual’s entry into employment with NeuroBo, within the meaning of Nasdaq Listing Rule 5635(c)(4). The 2021 Inducement Plan is administered by the Board. Stock awards under the Inducement Plan may only be granted by: (i) the Compensation Committee or (ii) another committee of the Board composed solely of at least two members of the Board who meet the requirements for independence under the Nasdaq Stock Market Listing Rules (the foregoing subsections (i) and (ii) are collectively referred to as the “Committee”). Under the 2021 Inducement Plan, the Committee may choose to grant (i) non-statutory stock options, (ii) stock appreciation rights, (iii) restricted stock awards, (iv) restricted stock unit awards, (v) performance stock awards, (vi) performance cash awards, and (vii) other stock awards to eligible recipients, with each grant to be evidenced by an award agreement setting forth the terms and conditions of the grant as determined by the Committee in accordance with the terms of the Inducement Plan. |
Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The following includes a summary of transactions since January 1, 2022 to which we have been a party, in which the amount involved in the transaction exceeded the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years, and in which any of our directors, nominees for director, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
2022 License Agreement
In September 2022, we entered into the 2022 License Agreement with Dong-A. See “License Agreements” in Part I, Item 1. Business for additional information.
84
Securities Purchase Agreement
In September 2022, in connection with the 2022 License Agreement, we entered into a Securities Purchase Agreement with Dong-A. See “Liquidity and capital resources” in Part I, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations for additional information.
Shared Services Agreement
In September 2022, in conjunction with the 2022 License Agreement, we entered into a shared services agreement with Dong-A (the “Shared Services Agreement”), relating to DA-1241 and DA-1726. The Shared Services Agreement provides that Dong-A may provide technical support, preclinical development, and clinical trial support services on terms and conditions acceptable to both parties. In addition, the Shared Services Agreement provides that Dong-A will manufacture all of our clinical requirements of DA-1241 and DA-1726 under the terms provided in the Shared Services Agreement.
Either party may terminate the Shared Services Agreement for the other party’s material breach that is not cured within 30 days of notice. Dong-A may also terminate the Shared Services Agreement in part on a service-by-service or product-by-product basis upon a breach by us which is not cured within 30 days.
Registration Rights Agreement
In connection with the Securities Purchase Agreement, in September 2022, we entered into a registration rights agreement with Dong-A and the other selling stockholders party thereto (the “Registration Rights Agreement”). The Registration Rights Agreement provides Dong-A with demand and piggyback registration rights, including the right to two long-form registration statements. In addition, we agreed to file, within 30 days following the stockholder approval of the conversion of the Series A Preferred Stock (“Stockholder Approval”), which occurred on December 22, 2022, a registration statement to: (i) register the shares of common stock issuable upon the conversion of the Series A Preferred Stock; (ii) register the shares of common stock issuable upon the exercise of the warrants; and (iii) register any other common stock held by the parties to the Registration Rights Agreement (the “Registrable Securities”); and to use commercially reasonable efforts to cause each registration statement to be declared effective under the Securities Act, as promptly as possible after the filing thereof, but in any event no later than the 60th day after Stockholder Approval (or in case the SEC reviews the registration statement, the 90th date after Stockholder Approval); provided that if we are notified that the registration statement is not being reviewed or is no longer subject to comment, we are required to make the registration statement effective by the fourth trading day after such date. We agreed to use our commercially reasonable efforts to keep such registration statement continuously effective under the Securities Act until the date that all Registrable Securities covered by such registration statement have been sold or are otherwise able to be sold pursuant to Rule 144.
Investor Rights Agreement
In September 2022, we entered into an investor rights agreement with Dong-A (the “Investor Rights Agreement”) pursuant to which, following the conversion of the Series A Preferred Stock into common stock, Dong-A will have the right, subject to the terms thereof, to designate for appointment to the Board that number of directors commensurate with Dong-A’s and its affiliates’ beneficial ownership of the common stock, with the number of directors that Dong-A is entitled to designate rounded up to the nearest whole number (the “DA Designees”). To the extent necessary to permit the designation of the DA Designees, the size of the Board shall be increased to that number of directors that would permit Dong-A to designate a number of directors to fill the vacancies created thereby that is commensurate with Dong-A’s and its affiliates’ collective beneficial ownership of the common stock outstanding at such time (taking into account any DA Designees already serving on the Board at such time). The compensation (including equity-based compensation) and rights to indemnity of, and reimbursement of expenses incurred by, the DA Designees that are members of the Board will be the same as those provided to other non-employee directors generally. When evaluating a prospective DA Designee for membership on the Board, the Board and the nominating and corporate governance committee shall apply the same review processes and standards as each of them, respectively, applies to other prospective non-employee directors generally.
Furthermore, for so long as Dong-A has the right to designate any DA Designee to the Board, Dong-A will vote their shares of the common stock in favor of any Company Director (as defined in the Investor Rights Agreement) or any nominee designated by the nominating and corporate governance committee of the Board and against the removal of any Director, in each case, at any meeting of the stockholders.
Director Independence
Our common stock is listed on the Nasdaq. Because Dong-A ST Co., Ltd. holds a majority of the voting power of our outstanding common stock, we are a “controlled company” under the listing rules of Nasdaq. As a controlled company, we are exempt from certain Nasdaq governance requirements that would otherwise apply to the composition and function of our Board.
85
For example, we are not required to comply with certain rules that would otherwise require, among other things, (i) our Board to have a majority of independent directors, (ii) the compensation of our executive officers to be determined by a majority of the independent directors or a committee of independent directors, and (iii) director nominees to be selected or recommended either by a majority of the independent directors or a committee of independent directors. Notwithstanding our status as a controlled company, we remain subject to the requirements that our independent directors hold regular executive sessions and that our Audit Committee consist entirely of independent directors. Under the rules of Nasdaq, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors or any other board of directors committee: (i) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries.
Notwithstanding our status as a controlled company, the Board has undertaken a review of the independence of each director and considered whether each director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. As a result of this review, the Board affirmatively determined that Mark A. Glickman, Jason Groves, Andrew Koven, Michael Salsbury, D. Gordon Strickland, James Tursi and Na Yeon (Irene) Kim, who served as a director until September 2023, are “independent directors” as defined under the applicable rules and regulations of the SEC and the listing requirements and rules of Nasdaq. The Board determined that Hyung Heon Kim, our Chief Executive Officer and President, who serves as a director, and Richard Kang, who served as our former Chief Executive Officer and as a director until March 2023, are not independent. In making this determination, the Board considered the current and prior relationships that each non-employee director has with us and all other facts and circumstances that the Board deemed relevant in determining each non-employee director’s independence, including the participation by our non-employee directors, or their affiliates, in certain financing transactions by us and the beneficial ownership of the common stock by each non-employee director. See “Certain Relationships and Related-Party Transactions” and “Security Ownership of Certain Beneficial Owners and Management.”
Additionally, all of our committees are comprised solely of independent directors under the current Nasdaq and SEC rules and regulations, with Messrs. Glickman, Koven and Strickland serving on our audit committee, Messrs. Glickman, Salsbury and Strickland serving on our compensation committee, and Messrs. Groves, Koven and Tursi serving on our nominating and corporate governance committee.
Item 14. | Principal Accountant Fees and Services |
Service Fees Paid to the Independent Registered Public Accounting Firms
The Audit Committee has considered the scope and fee arrangements for all services provided by BDO USA, P.C. (“BDO”), taking into account whether the provision of non-audit-related services is compatible with maintaining BDO independence. The following table presents fees for professional audit services rendered by BDO for the audit of the annual financial statements for 2023 and 2022.
Year Ended December 31, | ||||||
Fee Category |
| 2023 |
| 2022 | ||
Audit fees |
| $ | 436,704 |
| $ | 677,037 |
Audit-related fees |
| — |
| — | ||
Tax fees |
| 42,400 |
| — | ||
All other fees |
| — |
| — | ||
Total fees |
| $ | 479,104 |
| $ | 677,037 |
Audit fees consist of fees billed for services relating to the audit of our annual financial statement and review of our quarterly financial statements, services that are normally provided in connection with statutory and regulatory filings or engagements, comfort letters, reports on an issuer's internal controls, and review of documents to be filed with the SEC (e.g. periodic filings, registration statements, and company responses to SEC comment letters).
Tax fees relate to permissible services for technical tax advice related to federal and state income tax matters.
86
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firm
Our audit committee generally pre-approves all audit and permitted non-audit and tax services provided by the independent registered public accounting firm. Pre-approval is detailed as to the particular service or category of services and is generally subject to a specific budget. The independent registered public accounting firm and management are required to periodically report to the audit committee regarding the extent of services provided by the independent registered public accounting firm in accordance with this pre-approval, and the fees for the services performed to date. Our audit committee may also pre-approve particular services on a case-by-case basis. All of the services relating to the fees described in the table above were approved by our audit committee.
Part IV
Item 15. | Exhibits and Financial Statement Schedules |
(a)Financial statements and financial statements schedules
(1)Financial Statements are listed in the Index to Financial Statements on page F-1 of this Annual Report.
(2)No financial statement schedules are included because such schedules are not applicable, are not required, or because required information is included in the consolidated financial statements or notes thereto
(b)Exhibits
Exhibit | Description of Document | |
3.1 | ||
3.2 | ||
3.3 | ||
3.4 | ||
|
| |
3.5 | ||
3.6* | ||
3.7 | ||
3.8 | ||
4.1 | ||
|
| |
4.2 | ||
|
| |
87
4.3 | ||
4.4 | ||
4.5 | ||
4.6 | ||
4.7 | ||
4.8 | ||
4.9 | ||
10.1# | ||
10.2 | ||
10.3# | ||
10.4# | ||
10.5# | ||
10.6# | ||
10.7# | ||
10.8# | ||
10.9# | ||
10.10# | ||
10.11# | ||
10.12# | ||
88
10.13 | ||
10.14 | ||
10.15 | ||
10.16 | ||
10.17# | ||
10.18 | ||
10.19 | ||
10.20 | ||
10.21 | ||
10.22 | ||
10.23 | ||
10.24+ | ||
|
| |
10.25+++ | ||
10.26++ | ||
10.27 | ||
21.1* | ||
23.1* | Consent of Independent Registered Public Accounting Firm (BDO USA, P.C.). | |
89
31.1* | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) or 15d-14(a). |
31.2* | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) or 15d-14(a). |
32.1** | Certification of Chief Executive Officer under Section 906 of the Sarbanes-Oxley Act of 2002. |
32.2** | Certification of Chief Financial Officer under Section 906 of the Sarbanes-Oxley Act of 2002. |
97.1* | NeuroBo Pharmaceuticals, Inc. Policy for the Recovery of Erroneously Awarded Compensation. |
101.INS* | Inline XBRL Instance Document. |
101.SCH* | Inline XBRL Taxonomy Extension Schema Document. |
101.CAL* | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
101.DEF* | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase Document. |
101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
|
|
# | Indicates management contract or compensatory plan. |
* | Filed herewith. |
** | Furnished herewith. |
+ | Registrant has omitted and filed separately with the SEC portions of the exhibit pursuant to a confidential treatment request under Rule 406 promulgated under the Securities Act. |
++ | Certain schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request. |
+++ | Certain schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request. Certain portions of the exhibits that are not material and would be competitively harmful if publicly disclosed have been redacted pursuant to Item 601(b)(10)(iv) of Regulation S-K. Copies of the unredacted exhibits will be furnished to the SEC upon request. |
Item 16.Form 10-K Summary
None
90
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 28, 2024.
NEUROBO PHARMACEUTICALS, INC. | |||||||||
|
|
| |||||||
/s/ Hyung Heon Kim | |||||||||
Hyung Heon Kim |
| ||||||||
President and Chief Executive Officer |
| ||||||||
/s/ Marshall H. Woodworth | |||||||||
Marshall H. Woodworth | |||||||||
Chief Financial Officer | |||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report has been signed below by the following persons on behalf of the registrant and in the capacities indicated on March 28, 2024.
SIGNATURE |
| TITLE | ||
|
|
| ||
/s/ Hyung Heon Kim |
| President, Chief Executive Officer and Director (Principal Executive Officer) | ||
Hyung Heon Kim |
| |||
/s/ Marshall H. Woodworth | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | |||
Marshall H. Woodworth |
| |||
/s/ Mark A. Glickman |
| Director | ||
Mark A. Glickman |
|
| ||
|
| |||
/s/ Jason L. Groves |
| Director | ||
Jason L. Groves |
|
| ||
|
| |||
/s/ Andrew I. Koven |
| Chair of the Board | ||
Andrew I. Koven |
| |||
/s/ Michael Salsbury |
| Director | ||
Michael Salsbury |
| |||
/s/ D. Gordon Strickland |
| Director | ||
D. Gordon Strickland |
| |||
/s/ James P. Tursi | Director | |||
James P. Tursi | ||||
91
Index to Financial Statements
Report of Independent Registered Public Accounting Firm ( |
| F-2 |
F-4 | ||
Consolidated Statements of Operations and Comprehensive Loss | F-5 | |
Consolidated Statements of Mezzanine Equity and Stockholders' Equity | F-6 | |
F-7 | ||
F-8 |
F-1
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
NeuroBo Pharmaceuticals, Inc.
Boston, Massachusetts
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of NeuroBo Pharmaceuticals, Inc. (the “Company”) as of December 31, 2023 and 2022, the related consolidated statements of operations and comprehensive loss, mezzanine equity and stockholders’ equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered net losses and negative cash flows from operating activities that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Accrual for Research and Development Costs Related to Clinical Trial Activities
As described in Notes 1 and 4 to the consolidated financial statements, the Company’s accrued external research and development costs balance was approximately $3.8 million at December 31, 2023. This accrual includes liabilities for clinical trial and related clinical manufacturing expenses, contract services and other outside expenses. Expenses incurred for certain research and development activities are recognized based on an evaluation of the progress or completion of specific tasks using either time-based measures or data such as information provided to the Company by its vendors on actual activities completed or costs incurred.
F-2
We identified the determination of the accrual for research and development costs related to clinical trial activities as a critical audit matter. When estimating clinical trial accruals, the Company considered several factors including clinical trial budgets, contract amendments and the progress toward completion. Auditing these elements involved especially challenging auditor judgment due to the nature and extent of audit effort required to address this matter.
The primary procedures we performed to address the critical audit matter included:
| ● | For certain contract research organizations, inspecting, on a sample basis, invoices received from and payments made to such organizations in the development of the clinical trial accruals. |
| ● | For certain clinical trial studies, assessing the Company's estimates of the activities completed to date through (i) inspection of original contract terms, change orders and the expected timeline for the related study, (ii) discussion of the current status of the clinical trials with certain members of management and project teams (iii) confirmation of patient enrollment information and (iv) evaluation of the payments made and the invoices received after December 31, 2023 for proper application in the determination of the accruals. |
| ● | Testing the completeness of the Company’s clinical trial accruals by inspection of i) Company press releases and public databases that track clinical trials and ii) review of board of directors’ materials regarding the status of clinical trials. |
/s/ BDO USA, P.C.
We have served as the Company's auditor since 2019.
Boston, Massachusetts
March 28, 2024
F-3
NeuroBo Pharmaceuticals, Inc.
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
As of December 31, | ||||||
2023 | 2022 | |||||
Assets | ||||||
Current assets: | ||||||
Cash | $ | | $ | | ||
Prepaid and other current assets | | | ||||
Total current assets |
| |
| | ||
Property and equipment, net |
| |
| | ||
Right-of-use asset | | — | ||||
Other assets | | — | ||||
Total assets | $ | | $ | | ||
Liabilities and stockholders’ equity | ||||||
Current liabilities: | ||||||
Accounts payable | $ | | $ | | ||
Accrued liabilities (including related party payable of $ |
| |
| | ||
Warrant liabilities | | | ||||
Lease liability, short-term | | — | ||||
Total current liabilities |
| |
| | ||
Lease liability, long-term | | — | ||||
Total liabilities |
| |
| | ||
Commitments and contingencies (Note 6) | ||||||
Stockholders’ equity | ||||||
Preferred stock, $ | ||||||
Common stock, $ |
| |
| | ||
Additional paid–in capital |
| |
| | ||
Accumulated deficit |
| ( |
| ( | ||
Total stockholders’ equity |
| |
| | ||
Total liabilities and stockholders’ equity | $ | | $ | | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
NeuroBo Pharmaceuticals, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
Year Ended December 31, | ||||||
2023 | 2022 | |||||
Operating expenses: |
|
| ||||
Research and development | $ | | $ | | ||
Acquired in-process research and development | — | | ||||
General and administrative | | | ||||
Total operating expenses |
| |
| | ||
Loss from operations |
| ( |
| ( | ||
Other income (expense): | ||||||
Change in fair value of warrant liabilities | | | ||||
Interest income | | — | ||||
Financing expense | — | ( | ||||
Other expense | — | ( | ||||
Total other income | | | ||||
Loss before income taxes | ( | ( | ||||
Provision for income taxes | |
| | |||
Net loss | $ | ( | $ | ( | ||
Loss per share of common stock, basic and diluted | $ | ( | $ | ( | ||
Weighted average shares of common stock, basic and diluted |
| |
| | ||
Comprehensive loss: | ||||||
Net loss | $ | ( | $ | ( | ||
Other comprehensive loss, net of tax |
| — |
| ( | ||
Comprehensive loss | $ | ( | $ | ( | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
NeuroBo Pharmaceuticals, Inc.
Consolidated Statements of Mezzanine Equity and Stockholders’ Equity
(In thousands, except share amounts)
Mezzanine Equity | Stockholders’ Equity | ||||||||||||||||
Series A | Series B | Additional | Accumulated | ||||||||||||||
Preferred Stock | Preferred Stock | Common Stock | Paid–In | Comprehensive | Accumulated | Total | |||||||||||
Shares | Amount | Shares | Amount | Shares | Amount | Capital | Income | Deficit | Equity | ||||||||
As of January 1, 2022 | — | $ | — | — | $ | — | | $ | — | $ | | $ | | $ | ( | $ | |
Issuance of Series A preferred stock in private offering and license agreement | | | — | — | — | — | — | — | — | | |||||||
Issuance of common stock and Series B preferred stock in public offering | — | — | | | | — | | — | — | | |||||||
Transaction costs in connection with private offering and public offering | — | ( | — | — | — | — | ( | — | — | ( | |||||||
Conversion of Series A preferred stock to common stock | ( | ( | — | — | | | | — | — | — | |||||||
Conversion of Series B preferred stock to common stock | — | — | ( | ( | | — | | — | — | — | |||||||
Issuance of stock from exercise of warrants | — | — | — | — | | | | — | — | | |||||||
Stock–based compensation | — | — | — | — | — | — | | — | — | | |||||||
Foreign currency translation adjustment | — | — | — | — | — | — | — | ( | — | ( | |||||||
Net loss | — | — | — | — | — | — | — | — | ( | ( | |||||||
As of December 31, 2022 | — | — | — | — | | | | — | ( | | |||||||
Issuance of stock from exercise of warrants | — | — | — | — | | | | — | — | | |||||||
Issuance of stock for vested restricted stock units | — | — | — | — | | — | — | — | — | — | |||||||
Stock–based compensation | — | — | — | — | — | — | | — | — | | |||||||
Net loss | — | — | — | — | — | — | — | — | ( | ( | |||||||
As of December 31, 2023 | — | $ | — | — | $ | — | | $ | | $ | | $ | — | $ | ( | $ | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
NeuroBo Pharmaceuticals, Inc.
Consolidated Statements of Cash Flows
(In thousands)
Year Ended December 31, | ||||||
2023 | 2022 | |||||
Operating activities | ||||||
Net loss | $ | ( | $ | ( | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||
Stock-based compensation |
| |
| | ||
Non-cash lease expense | | | ||||
Depreciation |
| |
| | ||
Loss on sale of property and equipment | — | | ||||
Change in fair value of warrant liabilities | ( | ( | ||||
Acquired in-process research and development | — | | ||||
Transaction costs allocated to issuance of warrants | — | | ||||
Change in operating assets and liabilities: | ||||||
Prepaid expenses and other assets |
| |
| | ||
Accounts payable |
| |
| ( | ||
Accrued and other liabilities |
| |
| ( | ||
Net cash used in operating activities |
| ( |
| ( | ||
Investing activities | ||||||
Purchases of property and equipment |
| ( |
| — | ||
Sale of property and equipment | — | | ||||
Net cash (used in) provided by investing activities |
| ( |
| | ||
Financing activities | ||||||
Proceeds from issuance of common stock, preferred stock and warrants | — | | ||||
Payment of issuance costs | ( | ( | ||||
Net cash (used in) provided by financing activities |
| ( |
| | ||
Net (decrease) increase in cash |
| ( |
| | ||
Cash at beginning of period |
| |
| | ||
Cash at end of period | $ | | $ | | ||
Supplemental non-cash investing and financing transactions: | ||||||
Right-of-use assets obtained in exchange for new operating lease liabilities | $ | | $ | — | ||
Modification of right-of-use asset and associated liability | $ | — | $ | | ||
Unpaid deferred issuance costs | $ | — | $ | | ||
Reclassification of warrant liabilities upon exercise of warrants | $ | | $ | — | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
(In thousands, except share and per share amounts)
1. Business, basis of presentation, new accounting standards and summary of significant accounting policies
General
NeuroBo Pharmaceuticals, Inc. (the “Company), a Delaware corporation, and its subsidiaries are referred to collectively in these notes to the financial statements of the Company as “NeuroBo,” “we,” “our” and “us.” We are a clinical-stage biotechnology company focused primarily on developing and commercializing novel pharmaceuticals to treat cardiometabolic diseases. NeuroBo has two programs focused primarily on treatment of metabolic dysfunction-associated steatohepatitis (“MASH”) and obesity. MASH was formerly known as non-alcoholic steatohepatitis (“NASH”). The American Association for the Study of Liver Diseases (“AASLD”) and its European and Latin American counterparts changed the name to metabolic dysfunction-associated steatohepatitis to reflect the complexity of the disease.
| ● | DA-1241 is a novel G-Protein-Coupled Receptor 119 (“GPR119”) agonist with development optionality as a standalone and/or combination therapy for both MASH and type 2 diabetes. Agonism of GPR119 in the gut promotes the release of key gut peptides GLP-1, GIP, and PYY. These peptides play a further role in glucose metabolism, lipid metabolism and weight loss. DA-1241 has beneficial effects on glucose, lipid profile and liver inflammation, supported by potential efficacy demonstrated during in vivo preclinical studies. |
| ● | DA-1726 is a novel oxyntomodulin analogue functioning as a GLP-1 receptor (“GLP1R”) and glucagon receptor (“GCGR”) dual agonist for the treatment of obesity that is to be administered once weekly subcutaneously. DA-1726 acts as a dual agonist of GLP1R and GCGR. |
While we primarily focus our financial resources and management’s attention on the development of DA-1241 and DA-1726, we also have four legacy therapeutic programs designed to impact a range of indications in viral, neurodegenerative and cardiometabolic diseases which we continue to consider for out-licensing and divestiture opportunities.
Our operations have consisted principally of performing research and development (“R&D”) activities, preclinical developments, clinical trials, and raising capital. Our activities are subject to significant risks and uncertainties, including failing to secure additional funding before sustainable revenues and profit from operations are achieved.
Common stock reverse stock splits
In December 2023, we completed a reverse stock split of our common stock (the “2023 Reverse Stock Split”). As a result, every eight shares of our issued and outstanding common stock were combined, converted and changed into one share of our common stock. Any fraction of a share of our common stock that was created as a result of the reverse stock split was rounded down to the next whole share and the stockholder received cash equal to the market value of the fractional share, determined by multiplying such fraction by the closing sales price of our common stock as reported on Nasdaq Capital Market LLC (“Nasdaq”) on the last trading day before the reverse stock split. The 2023 Reverse Stock Split was initially approved by our stockholders at the annual meeting of stockholders in June 2023. At the annual meeting, the stockholders approved a proposal to amend our certificate of incorporation to affect a reverse split of our outstanding common stock at a ratio in the range of to to be determined at the discretion of our Board of Directors (“Board”). Following the annual meeting, our Board approved a one-for-eight reverse stock split of our issued and outstanding shares of common stock.
In September 2022, we completed a reverse stock split of our common stock (the “2022 Reverse Stock Split”). As a result, every thirty shares of our issued and outstanding common stock were combined, converted and changed into one share of our common stock. Any fraction of a share of our common stock that was created as a result of the reverse stock split was rounded down to the next whole share and the stockholder received cash equal to the market value of the fractional share, determined by multiplying such fraction by the closing sales price of our common stock as reported on Nasdaq on the last trading day before the reverse stock split. The 2022 Reverse Stock Split was initially approved by our stockholders at the annual meeting of stockholders in June 2022. At the annual meeting, the stockholders approved a proposal to amend our certificate of incorporation to affect a reverse split of our outstanding common stock at a ratio in the range of to to be determined at the discretion of our Board. Following the annual meeting, our Board approved a one-for-thirty reverse stock split of our issued and outstanding shares of common stock.
Neither the 2023 Reverse Stock Split nor the 2022 Reverse Stock Split impacted the number of authorized shares of common stock of
F-8
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
reserved for issuance pursuant to our equity incentive compensation plans. Specifically, for the Series A and B warrants issued in November 2022 that were outstanding on December 20, 2023, the number of outstanding warrants did not change; instead, the warrants have an exchange ratio of eight warrants for one share of our common stock.
In the accompanying consolidated financial statements and these notes to consolidated financial statements, all historical numbers of shares of common stock and per share data have been adjusted to give effect to the 2023 Reverse Stock Split and the 2022 Reverse Stock Split. Additionally, since the common stock par value was unchanged, historical amounts for common stock and additional paid-in capital have been adjusted to give effect to the 2023 Reverse Stock Split and the 2022 Reverse Stock Split.
Going concern
The determination as to whether we can continue as a going concern contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Our consolidated financial statements have been prepared assuming that we will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty. This basis of accounting contemplates the recovery of our assets and the satisfaction of our liabilities in the normal course of business.
As reflected in the consolidated financial statements, we had $
We believe that our existing cash will be sufficient to fund our operations into the fourth quarter of 2024. We plan to continue to fund our operations through a combination of equity offerings, debt financings, or other sources, potentially including collaborations, out-licensing and other similar arrangements. There can be no assurance that we will be able to obtain any sources of financing on acceptable terms, or at all. To the extent that we can raise additional funds by issuing equity securities, our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact our ability to conduct our business. If we are unable to raise additional capital, we may slow down or stop our ongoing and planned clinical trials until such time as additional capital is raised and this may have a material adverse effect on us.
A.Basis of presentation
Our consolidated financial statements include a South Korean subsidiary, NeuroBo Co., Ltd., a wholly owned subsidiary which was dissolved and liquidated in June 2023. The accompanying financial statements were prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Our fiscal year-end is as of and for the year ended December 31st for each year presented. All significant intercompany accounts and transactions have been eliminated in the preparation of the financial statements.
B.New accounting standards
Adoption of new accounting standards
New accounting standards or accounting standards updates were assessed and determined to be either not applicable or did not have a material impact on our consolidated financial statements or processes.
Accounting standards issued but not yet adopted
In October 2021, the Financial Accounting Standard Board (“FASB”) issued Accounting Standards Update (“ASU”) 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers. This ASU requires that an entity (acquirer) recognize, and measure contract assets and contract liabilities acquired in a business combination in accordance with Topic 606. At the acquisition date, an acquirer should account for the related revenue contracts in accordance with Topic 606 as if it had originated the contracts. To achieve this, an acquirer may assess how the acquiree applied Topic 606 to determine what to record for the acquired revenue contracts. Generally, this should result in an acquirer recognizing and measuring the acquired contract assets and contract liabilities consistent with how they were recognized and measured in the acquiree’s financial statements (if the acquiree prepared financial statements in accordance with generally accepted accounting principles). The amendments in this ASU are effective for annual and interim
F-9
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
periods beginning after December 15, 2023, although early adoption is permitted. We do not expect the adoption of this ASU will have a material impact on our consolidated financial statements.
In June 2022, the FASB issued ASU 2022-03, Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions. This ASU clarifies that a contractual restriction on the sale of an equity security is not considered part of the unit of account of the equity security and, therefore, is not considered in measuring fair value. The amendments also clarify that an entity cannot, as a separate unit of account, recognize and measure a contractual sale restriction. The amendments in this ASU are effective for annual and interim periods beginning after December 15, 2023, although early adoption is permitted. We do not expect the adoption of this ASU will have a material impact on our consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. This ASU amends the guidance in Accounting Standards Codification (“ASC’) 740, Income Taxes, to improve the transparency of income tax disclosures by amending the required rate reconciliation disclosures as well as requiring disclosure of income taxes paid disaggregated by jurisdiction. As amended, the rate reconciliation disclosure will be required to be presented in both percentages and reporting currency amounts, with consistent categories and greater disaggregation of information. This ASU also includes amendments intended to improve the effectiveness of income tax disclosures and eliminate certain existing disclosure requirements related to uncertain tax positions and unrecognized deferred tax liabilities. The amendments are effective for fiscal years beginning after December 15, 2024 and should be applied prospectively. Early adoption is permitted. We are currently evaluating the amendments to identify potential impacts to our notes to the consolidated financial statements and processes.
Other recently issued accounting standards not yet adopted by us are not expected, upon adoption, to have a material impact on our consolidated financial statements.
C.Estimates and assumptions
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses, and related disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of expenses during the reporting period. The most significant estimates in our consolidated financial statements relate to accrued expenses and the fair value of stock-based compensation and warrants. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results could differ from those estimates. Changes in estimates are reflected in reported results in the period in which they become known.
D.Cash
We maintain cash at financial institutions that at times may exceed the Federal Deposit Insurance Corporation (“FDIC”) insured limits of $250 per bank. Our cash balance includes liquid insured deposits, which are obligations of the program banks in which the deposits are held and qualify for FDIC insurance protection per depositor in each recognized legal category of account ownership in accordance with the rules of the FDIC. To date, we have not experienced any losses related to these funds.
E.Property and equipment
Property and equipment are recorded at cost and reduced by accumulated depreciation. Depreciation expense is recognized over the estimated useful lives of the assets using the straight-line method. The estimated useful life for property and equipment ranges from to
F.Leases
We assess our contracts at inception to determine whether the contract contains a lease, including evaluation of whether the contract conveys the right to control an explicitly or implicitly identified asset for a period of time. We have recognized right-of-use assets and lease liabilities that represent the net present value of future operating lease payments utilizing a discount rate corresponding to our incremental borrowing rate and amortized over the remaining terms of the leases. For operating leases of
F-10
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
a short-term nature, i.e., those with a term of less than twelve months, we recognize lease payments as an expense on a straight-line basis over the remaining lease term.
G.Warrant liabilities
Warrants are accounted for as liabilities at their fair value if equity accounting treatment is precluded due to provisions existing within the warrant agreements. The change in fair value of the warrant liabilities is recognized as a fair value change in warrant liabilities in the consolidated statements of operations and comprehensive loss and as an operating item in the statement of cash flows. Additionally, issuance costs associated with warrants initially classified as liabilities are expensed as incurred and reflected as financing costs in the accompanying consolidated statements of operations and comprehensive loss.
H.Fair value of financial instruments
Our financial instruments principally include cash, prepaid expenses, right of use assets, accounts payable, accrued liabilities, lease liabilities and warrant liabilities. The carrying amounts of cash, prepaid expenses and other current assets, accounts payable, and accrued liabilities are reasonable estimates of their fair value because of the short maturity of these items.
I.Segment reporting
We manage and operate as one business, which is principally the business of development and commercialization of pharmaceutical products. Our business operations are managed by a single executive leadership team, which is led by our chief executive officer. We do not operate separate lines of business with respect to any of our pharmaceutical products being studied in a clinical environment, and we do not prepare discrete financial information with respect to our pharmaceutical products. Accordingly, we view our business as
J.Research and development costs
R&D expenditures for clinical development, including upfront licensing fees and milestone payments associated with products that have not yet been approved by the United States Food and Drug Administration, are charged to R&D expense as incurred. These expenses consist of costs incurred in performing development activities, including salaries and benefits, equity-based compensation expense, preclinical expenses, clinical trial and related clinical manufacturing expenses, contract services and other outside expenses.
Expenses incurred for certain R&D activities, including expenses associated with particular activities performed by contract research organizations, investigative sites in connection with clinical trials and contract manufacturing organizations, are recognized based on an evaluation of the progress or completion of specific tasks using either time-based measures or data such as information provided to us by our vendors on actual activities completed or costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of expense recognition. Expenses for R&D activities incurred that have yet to be invoiced by the vendors that perform the related activities are reflected in the consolidated financial statements as accrued R&D development expenses. Advance payments for goods or services to be received in the future for R&D activities are deferred and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed.
K.Acquired in-process research and development
We include costs to acquire or in-license product candidates in acquired in-process R&D (“IPR&D”). When we acquire the right to develop and commercialize a new product candidate, any up-front payments, or any future milestone payments that relate to the acquisition or licensing of such a right are immediately expensed as acquired IPR&D in the period in which they are incurred. These costs are immediately expensed provided that the payments do not also represent processes or activities that would constitute a “business” as defined under GAAP, or provided that the product candidate has not achieved regulatory approval for marketing and absent obtaining such approval, has no alternative future use. Royalties owed on future sales of any licensed product will be expensed in the period the related revenues are recognized.
L.General and administrative expenses
General and administrative expenses consist primarily of personnel-related costs, including salaries and stock-based compensation costs, for personnel in functions not directly associated with R&D activities. Other significant costs include legal fees related to intellectual property and corporate matters and professional fees for accounting and other services.
F-11
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
M.Patent costs
Costs related to filing and pursuing patent applications are expensed as incurred, as recoverability of such expenditures is uncertain. These costs are included in general and administrative expenses.
N.Stock-based compensation
Compensation costs related to equity instruments granted are recognized at the grant-date fair value, which is amortized as compensation expense on a straight-line basis over the service period (generally, the vesting period) for both graded and cliff vesting awards. We have elected to account for forfeitures as they occur.
O.Income taxes
Income taxes are accounted for under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating loss and income tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in income tax rates is recorded as a component of the income tax provision in the period that includes the enactment date.
Regular assessments are made on the likelihood that our deferred tax assets will be recovered from our future taxable income. Our evaluation is based on estimates, assumptions, and includes an analysis of available positive and negative evidence, giving weight based on the evidence’s relative objectivity. Sources of positive evidence include estimates of future taxable income, future reversal of existing taxable temporary differences, taxable income in carryback years, and available tax planning strategies. Sources of negative evidence include current and cumulative losses in recent years, losses expected in early future years, any history of operating losses or tax credit carryforwards expiring unused, and unsettled circumstances that, if unfavorably resolved, would adversely affect future profit levels.
The remaining carrying value of our deferred tax assets, after recording the valuation allowance on our deferred tax assets, is based on our present belief that it is more likely than not that we will be able to generate sufficient future taxable income to utilize such deferred tax assets. The amount of the remaining deferred tax assets considered recoverable could be adjusted if our estimates of future taxable income during the carryforward period change favorably or unfavorably. To the extent we believe that it is more likely than not that some or all the remaining deferred tax assets will not be realized, we must establish a valuation allowance against those deferred tax assets, resulting in additional income tax expense in the period such determination is made. To the extent a valuation allowance currently exists, we will continue to monitor all positive and negative evidence until we believe it is more likely than not that it is no longer necessary, resulting in an income tax benefit in the period such determination is made.
Our policy is to recognize both interest and penalties related to uncertain tax positions as part of the income tax provision. A significant judgment is required in evaluating our tax positions, and in determining our provisions for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We establish reserves when, despite our belief that the income tax return positions are fully supportable, certain positions are likely to be challenged and we may ultimately not prevail in defending those positions.
P.Foreign currency translation
Our foreign subsidiary, dissolved and liquidated in June 2023, used the South Korean Won (KRW) as its functional currency. We translated the assets and liabilities of our foreign operation into United States (“U.S.”) dollars based on the rates of exchange in effect as of the transaction date. The resulting adjustments from the translation process are included in accumulated other comprehensive (loss) income in the accompanying consolidated balance sheets.
Certain transactions are settled in foreign currency and are thus translated to U.S. dollars at the rate of exchange in effect at the end of each month. Gains and losses resulting from the translation are included in other income or expense in the accompanying consolidated statements of operations and comprehensive loss.
Q.Comprehensive loss
Comprehensive loss is comprised of net loss and other comprehensive income or loss. Comprehensive loss includes net loss as well as other changes in stockholders' equity that result from transactions and economic events other than those with stockholders. Comprehensive loss currently consists of net loss and changes in foreign currency translation adjustments.
F-12
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
R.Loss per share of common stock
Basic earnings or loss per share of common stock is computed by dividing net income or loss available to stockholders of common stock by the weighted average number of shares of common stock. Diluted earnings per share of common stock is computed by dividing net income or loss available to stockholders of common stock by the sum of the weighted average number of shares of common stock and the number of additional shares of common stock that would have been outstanding if our outstanding potentially dilutive securities had been issued. Potentially dilutive securities include convertible preferred stock, outstanding stock options, non-vested RSUs and outstanding warrants. The dilutive effect of potentially dilutive securities is reflected in diluted earnings per share of common stock by application of the treasury stock method, except if its impact is anti-dilutive. Under the treasury stock method, an increase in the fair market value of our common stock can result in a greater dilutive effect from potentially dilutive securities.
S.Concentrations
Credit Risk
Financial instruments that potentially subject us to concentration of credit risk consist of cash. Our cash is held by two financial institutions in the U.S. We believe that the two financial institutions are financially sound, and accordingly, minimal credit risk exists with respect to the two financial institutions. Subsequently, in January 2024, we eliminated one of the financial institutions holding our cash to one.
Supplier Risk
In 2022, we entered into an exclusive license agreement (the “2022 License Agreement”) with Dong-A ST Co., Ltd. (“Dong-A”), a related party, which requires Dong-A to be the sole manufacturer for the production of DA-1241 and DA-1726. If any issues arise in the manufacturing and we are unable to arrange for alternative third-party manufacturing sources, or unable to find an alternative third party capable of reproducing the existing manufacturing method or unable to do so on commercially reasonable terms or in a timely manner, we may not be able to complete development of DA-1241 or DA-1726.
T.Loss contingencies
In determining whether an accrual for a loss contingency is required, we first assess the likelihood of occurrence of the future event or events that will confirm the loss. When a loss is probable (the future event or events are likely to occur) and the amount of the loss can be reasonably estimated, the estimated loss is accrued. If the reasonable estimate of the loss is a range and an amount within the range appears to be a better estimate than any other amount within the range, that amount should be accrued. However, if no amount within the range is a better estimate, the minimum amount in the range should be accrued.
When a loss is reasonably possible (the chance of the future event or events occurring is more than remote but less than likely), no accrual is recognized.
2. Prepaid and other current assets
Prepaid and other current assets consist of the following:
As of December 31, | ||||||
2023 | 2022 | |||||
Clinical Costs | $ | | — | |||
Other |
| |
| | ||
Total | $ | | $ | | ||
3. Property and equipment
Property and equipment consist of the following:
As of December 31, | ||||||
2023 | 2022 | |||||
Office equipment | $ | | $ | | ||
Less accumulated depreciation |
| ( |
| ( | ||
Property and equipment, net | $ | | $ | | ||
F-13
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
We recorded depreciation expense of $
4. Accrued liabilities
Accrued liabilities consist of the following:
As of December 31, |
| ||||||
2023 | 2022 | ||||||
External R&D costs (including related party payable of $ | $ | | $ | | |||
Employee related costs |
| |
| | |||
Professional service fees | | | |||||
Other |
| |
| | |||
Total | $ | | $ | | |||
5.Related party
License agreement with Dong-A for DA-1241 and DA-1726
In September 2022, we entered into a license agreement with Dong-A pursuant to which NeuroBo received an exclusive global license (except for the territory of the Republic of Korea and certain other jurisdictions) to two proprietary compounds for specified indications (the “2022 License Agreement”) upon meeting certain financing milestones. The 2022 License Agreement covers the rights to DA-1241 for treatment of MASH and DA-1726 for treatment of obesity and MASH. The 2022 License Agreement also provides that we may develop DA-1241 for the treatment of T2DM.
Under the terms of the 2022 License Agreement, we agreed to pay Dong-A an upfront payment to be settled with
Also, Dong-A will be eligible to receive (i) regulatory milestone payments of up to $
The term of the 2022 License Agreement continues on a product-by-product and country-by country basis until the later of (i) the fifth anniversary of the first commercial sale of such product in such country, (ii) the expiration or termination of the last valid patent claim that covers a product in such country and (iii) the loss of regulatory exclusivity for such product in such jurisdiction. Either Dong-A or NeuroBo may terminate the 2022 License Agreement (i) if the other party is in material breach of the agreement and has not cured or started to cure the breach within
As of December 31, 2023, there were no potential milestones under the 2022 License Agreement that were yet considered probable; therefore,
As of December 31, 2023, Dong-A owns approximately
F-14
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Shared services agreement with Dong-A
In September 2022, in conjunction with the 2022 License Agreement, we entered into a shared services agreement with Dong-A (the “Shared Services Agreement”), relating to DA-1241 and DA-1726. The Shared Services Agreement provides that Dong-A may provide technical support, preclinical development, and clinical trial support services on terms and conditions acceptable to both parties. In addition, the Shared Services Agreement provides that Dong-A will manufacture all of our clinical requirements of DA-1241 and DA-1726 under the terms provided in the Shared Services Agreement.
Either party may terminate the Shared Services Agreement for the other party’s material breach that is not cured within
We incurred R&D expenses of $
License agreement with Dong-A for NB-01 (a legacy therapeutic program)
In January 2018, we entered into an exclusive license agreement with Dong-A, (the “2018 License Agreement”) which agreement was amended in April 2018 and July 2019. Under the terms of the 2018 License Agreement, we obtained an exclusive, royalty-bearing, worldwide (except for the Republic of Korea) license to make, use, offer to sell, sell and import products covered by certain Dong-A intellectual property rights in its proprietary compound designated as DA-9801 (NB-01). Our license rights cover any and all applications and markets for the therapeutic, health, nutrition or well-being of humans. We may grant sublicenses to any affiliate or third party. We are responsible for all future patent prosecution costs.
6. Commitments and contingencies
Operating lease
In August 2023, we entered into a non-cancelable operating lease for our new corporate headquarters in Cambridge, Massachusetts. The initial lease term is for
As of December 31, 2023, our lease liability, which represents the net present value of future lease payments, was calculated utilizing a discount rate of
The following table reconciles the undiscounted lease liabilities to the total lease liabilities recognized on the consolidated balance sheet as of December 31, 2023:
Operating | |||
Lease | |||
2024 | $ | | |
2025 | | ||
2026 | | ||
Total lease payments | | ||
Less effect of discounting | ( | ||
Total | | ||
Short-term portion | | ||
Long-term portion | $ | | |
Additionally, we had short-term operating leases for our former corporate headquarters located in Boston, Massachusetts and a former facility in Korea. The lease for our former corporate headquarters and former Korean facility terminated in January 2024 and April 2022, respectively. In the aggregate, we recorded rental expense of $
F-15
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Contingent payments and license agreements
We have certain contractual contingent payments under various merger or license agreements executed between 2019 and 2020 for our legacy therapeutic programs. Since we have discontinued the clinical development of our legacy therapeutic programs, we believe any contractual payments to be paid by us or to be received by us under these agreements are remote. Therefore, as of December 31, 2023,
Legal proceedings
From time to time, we may be involved in various claims and legal proceedings arising out of our ordinary course of business. We are not currently a party to any claims or legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business and consolidated financial statements. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Purchase commitments
We enter into contracts in the normal course of business with various third parties for clinical trials, preclinical research studies, and testing, manufacturing, and other services and products for operating purposes. These contracts provide for termination upon notice. Payments due upon cancellation generally consist only of payments for services provided or expenses incurred, including non-cancellable obligations of our service providers, up to the date of cancellation. There are no minimum purchase requirements under these contracts.
Off-balance sheet arrangements
As of December 31, 2023 and 2022 we had no off-balance sheet arrangements that have had or are reasonably likely to have current or future effects on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Employment agreements
We have entered into employment agreements with certain of our executives that provide for compensation and certain other benefits. Under certain circumstances, including a change in control, some of these agreements provide for severance or other payments, if those circumstances occur during the term of the employment agreement.
In January 2023, our former chief executive officer and president (“former executive”) resigned from NeuroBo, and we entered into a Separation and Release Agreement (the “Separation Agreement”) with the former executive. Pursuant to the terms and conditions of the Separation Agreement, the former executive received (i) severance pay of $
Employee benefit plan
We adopted a 401(k) defined contribution plan in November 2018, which became effective in January 2019, for all employees over age
7.Stockholders’ equity
Common stock
The voting, dividend, and liquidation rights of the holders of the common stock are subject to and qualified by the rights, powers, and preferences of the holders of the preferred stock when outstanding. The holders of the common stock are entitled to
F-16
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Preferred stock
The rights of the designated Series A Preferred Stock and designated Series B Preferred Stock (as defined further below), collectively, the “Preferred Stock,” while outstanding were as follows:
Dividends. Holders of the Preferred Stock were entitled to receive dividends on shares of the Preferred Stock equal (on an as-if-converted-to common-stock basis) to the amount paid on shares of the common stock.
Voting Rights. The Preferred Stock had no voting rights. However, as long as any shares of Preferred Stock were outstanding, we could not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Preferred Stock or amend its Certificate of Designation, (b) amend other charter documents in any manner that could have adversely affected any rights of the holders, (c) increase the number of authorized shares of Preferred Stock, or (d) enter into any agreement with respect to any of the foregoing. In addition, the Series A Preferred Stock could have voted on any matter that authorized, created and/or issued any funded indebtedness (other than indebtedness already incurred); sold or transferred, other than in the ordinary course of business, mortgaged, assigned, pledged, leased, granted a security interest in, or encumbered any of our assets.
Liquidation. The Preferred Stock while outstanding in the event of a liquidation, dissolution or winding-up of NeuroBo (collectively a “Liquidation”) had the following rights:
Series A Preferred Stock: Upon any Liquidation of NeuroBo, whether voluntary or involuntary, after the satisfaction in full of our debts and the payment of any liquidation preference owed to the holders of shares of our capital stock ranking senior to the Series A Preferred Stock upon liquidation, but before any distribution or payment out of our assets shall be made to the holders of any junior securities, including common stock, an amount in cash per share equal to the amount per share in cash payable to the holder if the shares of Series A Preferred Stock were converted immediately prior to the Liquidation into shares of common stock.
Series B Preferred Stock: Upon any Liquidation of NeuroBo, whether voluntary or involuntary, the holders would be entitled to receive out of our assets, whether capital or surplus, the same amount that a holder of common stock would receive if the Series B Preferred Stock were fully converted (disregarding for such purposes any conversion limitations hereunder) to common stock which amounts shall be paid pari passu with all holders of common stock.
Conversion.
Series A Preferred Stock:
| ● | Automatic Conversion. On the first Trading Day after we were to obtain the stockholder approval, all outstanding shares of the Series A Preferred Stock would, without any further action by holders and whether or not any certificates representing such shares are surrendered to us or the Transfer Agent, automatically be converted into such number of shares of common stock as determined by dividing the stated value of $ |
Special Cash Payout Provisions: Unless and until stockholder approval was obtained, the holder did not have the right to acquire shares of common stock issuable upon conversion of the Series A Preferred Stock, and we were not required to issue shares of common stock issuable upon conversion of the Series A Preferred Stock in excess of the Share Cap as defined (the "Conversion Restriction"). Notwithstanding the foregoing, if the Automatic Conversion had not occurred by the nine (9)-month anniversary of the original issuance date, the holder would have been entitled to submit a request to us for the conversion of all, but not less than all, of holder's shares of Series A Preferred Stock that were subject to the Conversion Restriction that would have exceeded the Share Cap; provided, that, in lieu of the Conversion Shares that would have otherwise been deliverable upon conversion but for the Conversion Restriction, we would have instead delivered to such holder for each share of common stock that would have been so otherwise delivered an amount of cash equal to the volume weighted average price (“VWAP”) per share of common stock on the trading day immediately preceding the date such request is made. If we failed to make any required cash payment by the required deadline on any share of Series A Preferred Stock, then the holder thereof would have been entitled to receive cumulative cash dividends on each such share at a rate
F-17
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
per annum of
In December 2022, all shares of the Series A Preferred Stock were converted into
Series B Preferred Stock
| ● | Conversions at Option of Holder. Each share of Series B Preferred Stock was convertible, at any time at the option of the holder thereof, into that number of shares of common stock (subject to certain limitations) determined by dividing the stated value of $ |
In December 2022, all shares of the Series B Preferred Stock were converted into
Fundamental Transaction. If, at any time while shares of the Preferred Stock were outstanding, NeuroBo, directly or indirectly, in one or more related transactions effected any merger or consolidation of NeuroBo, the holder of the Preferred Stock would have had the right to receive, for each conversion share that would have been issuable upon such conversion immediately prior to the occurrence of such Fundamental Transaction as defined, the number of shares of common stock of the successor or acquiring company or of NeuroBo, if it is the surviving company, and any additional consideration (the "Alternate Consideration'') receivable as a result of such Fundamental Transaction by a holder of the number of shares of the common stock for which shares of the Preferred Stock would have been convertible immediately prior to such Fundamental Transaction. If holders of the common stock were given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the holder of the Preferred Stock would have been given the same choice as to the Alternate Consideration it would have received upon any conversion of shares of the Preferred Stock following such Fundamental Transaction.
2022 private placement and public offering
In September 2022, we entered into a Securities Purchase Agreement with Dong-A (the “Securities Purchase Agreement”). Pursuant to the Securities Purchase Agreement, Dong-A agreed to purchase shares of our Series A Preferred Stock and warrants to purchase shares of our common stock, equivalent to those to be issued in the Qualified Financing (as hereafter defined), (collectively, the “Dong-A Financing”) concurrent with and contingent upon a Qualified Financing resulting in gross proceeds of at least $
In November 2022, we closed on an underwritten public offering (the “2022 Public Offering”) and received gross proceeds of $
The 2022 Public Offering met the definition of a Qualified Financing, as defined by the Securities Purchase Agreement; therefore, also in November 2022, the license under the Dong-A License Agreement became effective, and we issued
F-18
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
amount of $
The Series A Preferred Stock was initially classified outside of stockholders’ equity due to a contingent redemption feature if stockholder approval of the Series A Preferred Stock had not been secured within nine months from the date of issuance. Stockholder approval was secured in December 2022, at which time, all of the Series A Preferred Stock was automatically converted into shares of our common stock.
The Series B Convertible Preferred Stock was classified in stockholders’ equity upon issuance as there were no other provisions precluding equity treatment. In December 2022, all of the Series B Preferred Stock had been converted into shares of our common stock.
The public Warrants and the Dong-A Warrants (together, the “2022 Warrants”), upon their issuance were not exercisable unless and until stockholder approval was obtained as required under Nasdaq rules. We recorded these warrants as a liability at their fair value as certain provisions precluded equity accounting treatment for these instruments.
As the 2022 License Agreement and Dong-A Financing were both contingent and based on the terms of a Qualified Financing, we combined these two transactions along with the 2022 Public Offering (collectively, the “2022 Transaction”) when allocating gross consideration and issuance costs.
The table below lists the aggregate consideration received by us in the 2022 Transaction:
| Consideration |
| |||
Received | Classification | ||||
Dong-A License Agreement | $ | | Acquired IPR&D | ||
Dong-A Financing | | Cash | |||
2022 Public Offering | | Cash | |||
Total | $ | ||||
The consideration received was first allocated to the 2022 Warrants at their estimated fair value on the date of issuance. The remainder of the consideration from the 2022 Transaction was allocated to the Series A Preferred Stock and Series B Preferred Stock (collectively the “Preferred Stock”), and to the common stock based on their relative fair values on the date of issuance. Issuance costs in connection with 2022 Transaction in the amount of $
The fair value of the Series A Preferred Stock was probability weighted for stockholder approval to convert to common stock. In the approval scenario, the fair value was calculated using the underlying stock price multiplied by the number of shares of common stock to be issued on conversion as adjusted for a
The fair value of the Series B Preferred Stock was equal to the underlying common stock fair value as the shares were readily convertible at the time of issuance on a one-for-one basis.
The estimated fair value for the 2022 Warrants was equal to the trading market price of our common stock due to the cashless exercise provision of the 2022 Warrants, which rendered the warrant exercise price to zero, prior to taking into account the probability factor of stockholder approval factor at
F-19
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Warrants
The following warrants were outstanding as of December 31, 2023 and 2022:
Shares of Common | ||||||||||
Stock Issuable for | ||||||||||
Outstanding Warrants | ||||||||||
as of December 31, | Exercise | Expiration | ||||||||
Warrant Issuance | 2023 | 2022 | Price | Date | ||||||
July 2018 | (1) | | | $ | July 2028 | |||||
April 2020 | (1) | | | $ | April 2025 | |||||
January 2021 | (1) | | | $ | July 2026 | |||||
October 2021 | (1) | | | $ | April 2025 | |||||
November 2022 Series A | (2) | — | | $ | - | December 2023 | ||||
November 2022 Series B | (2) | | | $ | - | December 2027 | ||||
Total | | | ||||||||
| (1) | The number of outstanding warrants was adjusted for the impact of each of the common stock reverse stock splits of 2023 and 2022. Accordingly, the number of outstanding warrants is equal to the number of shares of common stock issuable for outstanding warrants. |
| (2) | The number of outstanding warrants was not impacted by the 2023 Reverse Stock Split. Accordingly, the number of outstanding warrants is equal to |
The outstanding warrants are all exercisable as of December 31, 2023. Additionally, the 2022 Warrants have a cashless exercise provision whereby
8.Stock-based compensation
Stock-based compensation expense was included in general and administrative as follows in the accompanying consolidated statements of operations and comprehensive loss:
Year Ended December 31, | ||||||
2023 | 2022 | |||||
General and administrative | $ | | $ | | ||
Research and development | | - | ||||
Total stock-based compensation | $ | | $ | | ||
Unrecognized stock-based compensation cost for stock options and RSUs granted under the 2019 Plan, the 2021 Inducement Plan and the 2022 Plan is $
Stock-based award plans
In December 2019, in connection with the 2019 Merger, we assumed a previously adopted stock option plan (the “2018 Plan”) and adopted the 2019 Equity Incentive Plan (the “2019 Plan”). Additionally, we adopted the 2021 Inducement Plan in November 2021 and the 2022 Equity Incentive Plan (the “2022 Plan”) in December 2022. The 2018 Plan, 2019 Plan, 2021 Inducement Plan and 2022 Plan provide for the grant of stock options, restricted stock, RSUs and other equity awards of our common stock to employees, officers, consultants, and directors. Stock options granted under any of these plans expire within a period of not more than
F-20
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Under the 2022 Plan, the shares reserved automatically increase on January 1st of each year, for a period of not more than
Stock options
The following table summarizes the status of our outstanding and exercisable options and related transactions for each for the following years:
Outstanding | Exercisable | |||||||||||||||||||
Shares of | Weighted | Shares of | Weighted | |||||||||||||||||
Common | Weighted | Average | Common | Weighted | Average | |||||||||||||||
Stock | Average | Remaining | Aggregate | Stock | Average | Remaining | Aggregate | |||||||||||||
Issuable | Exercise | Contractual | Intrinsic | Issuable | Exercise | Contractual | Intrinsic | |||||||||||||
for Options | Price | Term (years) | Value | for Options | Price | Term (years) | Value | |||||||||||||
As of January 1, 2022 | | $ | | $ | — | | $ | | $ | — | ||||||||||
Granted | | | — | — | ||||||||||||||||
Forfeited/Cancelled | ( | | — | — | ||||||||||||||||
As of December 31, 2022 | | | — | | | — | ||||||||||||||
Granted | | | — | — | ||||||||||||||||
Forfeited/Cancelled | ( | | — | — | ||||||||||||||||
As of December 31, 2023 | | $ | | $ | — | | $ | | $ | — | ||||||||||
The weighted average fair value per share of stock options granted for 2023 and 2022 was $
The assumptions used in the Black-Scholes option-pricing model are as follows:
Year Ended December 31, | |||||||
| 2023 | 2022 | |||||
Expected stock price volatility | % | % | |||||
Expected life of stock options (years) | |||||||
Expected dividend yield | — | % | — | % | |||
Risk free interest rate | % | % | |||||
F-21
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Restricted Stock Units
The following table summarizes the status of our RSUs and related transactions:
Outstanding | Vested | |||||||||||||||
Shares of | Average | Shares of | Average | |||||||||||||
Common Stock | Grant Date | Aggregate | Common Stock | Grant Date | Aggregate | |||||||||||
Issuable | Fair Value | Intrinsic | Issuable | Fair Value | Intrinsic | |||||||||||
for RSUs | Price | Value | for RSUs | Price | Value | |||||||||||
As of January 1, 2023 | — | $ | — | $ | — | — | $ | — | $ | — | ||||||
Granted | | | — | — | ||||||||||||
Vested and released | ( | | | — | — | |||||||||||
Vested and not released | — | — | | | ||||||||||||
Forfeited/Cancelled | ( | | — | — | ||||||||||||
As of December 31, 2023 | | $ | | $ | | | $ | | $ | | ||||||
We estimated the grant date fair value of restricted stock units granted to employees, consultants, and directors based on the closing sales price of our common stock as reported on Nasdaq on the date of grant.
9.Income taxes
Our loss before income taxes is as follows:
Year Ended December 31, | ||||||
2023 | 2022 | |||||
United States | $ | ( | $ | ( | ||
Foreign | ( | | ||||
Loss before income taxes | $ | ( | $ | ( | ||
The components of our income tax provision are as follows:
Year Ended December 31, | ||||||
2023 | 2022 | |||||
Income tax (benefit) provision: | ||||||
Current | ||||||
United States | $ | — | $ | — | ||
Foreign | — | — | ||||
Total current income tax provision | — | — | ||||
Deferred | ||||||
United States | ( | | ||||
Foreign | | | ||||
Total deferred income tax (benefit) provision | ( | | ||||
Change in valuation allowance - United States | | ( | ||||
Change in valuation allowance - Foreign | ( | ( | ||||
Income tax provision | $ | — | $ | — | ||
F-22
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Our effective tax rate for 2023 and 2022 was
Year Ended December 31, | ||||||
2023 | 2022 | |||||
Income tax (benefit) provision at federal statutory rate | | % | | % | ||
State income tax, net of federal benefit | | | ||||
Change in state tax rate | ( | — | ||||
Change in fair value of warrant liabilities | | | ||||
R&D credits | — | | ||||
Transaction costs | — | ( | ||||
Section 382 limitation adjustment attributes | — | ( | ||||
Other | ( | ( | ||||
Valuation allowance | ( | | ||||
Effective tax rate | — | % | — | % | ||
The significant components of our deferred tax assets and liabilities are as follows:
As of December 31, | ||||||
2023 | 2022 | |||||
Gross deferred income tax assets: | ||||||
Federal and state operating loss carryforwards | $ | | $ | | ||
Foreign operating loss carryforwards | — | | ||||
Acquired intangibles | | | ||||
Stock-based compensation | | | ||||
Lease liability | | - | ||||
Capitalized R&D expenses | | | ||||
Other | | | ||||
R&D credit carryforwards | | | ||||
Total gross deferred income tax assets | | | ||||
Valuation allowance - U.S. federal | ( | ( | ||||
Valuation allowance - foreign | — | ( | ||||
Gross deferred tax assets, net of valuation allowance | | — | ||||
Gross deferred tax liabilities: | ||||||
ROU asset | ( | — | ||||
Other | ( | — | ||||
Gross deferred income tax liabilities | ( | — | ||||
Deferred income tax assets, net | $ | — | $ | — | ||
The realization of our deferred income tax assets is primarily dependent upon future taxable income, if any, and such income is uncertain in both amount and timing. We have had significant pre-tax losses since our inception, and we have not yet generated revenues and face significant challenges to becoming profitable. Accordingly, we have recorded a valuation allowance of $
As of December 31, 2023 and 2022, our U.S. federal net operating loss (“NOL”) carryforwards were $
F-23
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Our ability to utilize our NOL and R&D credit carryforwards have been and may be substantially limited due to the ownership changes that have occurred or that could occur in the future, as provided by Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), and similar state provisions. These ownership changes may limit the amount of NOL and R&D credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an “ownership change,” as defined by Section 382 of the Code, results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50 percent of the outstanding stock of a company by certain stockholders or public groups. We completed a Section 382 study in 2022, and we determined that, as a result of the public stock offering and private placement offering during the fourth quarter of 2022, a Section 382 ownership change occurred with an annual limitation of $0. Because of the 2022 ownership change and corresponding limitation, $
We recognize interest and/or penalties related to uncertain tax positions in income tax expense. There were
Our U.S. corporate tax returns are subject to examination beginning with the 2019 tax year for U.S. federal and state jurisdictions, and beginning with the 2019 tax year for one foreign jurisdiction.
10.Loss per share of common stock
The following table sets forth the computation of basic and diluted loss per share of common stock for the periods presented
Year Ended December 31, | ||||||
2023 | 2022 | |||||
Numerator: | ||||||
Net loss | $ | ( | $ | ( | ||
Denominator: | ||||||
Weighted average shares of common stock, basic | ||||||
Effect of dilutive securities | — | — | ||||
Weighted average shares of common stock, diluted | ||||||
Loss per share of common stock, basic and diluted | $ | ( | $ | ( | ||
Our basic weighted average shares of common stock include the 2022 Warrants given that these instruments are exchangeable into common stock for which no additional consideration is required from the holder. Since we reported a net loss for 2023 and 2022, our potentially dilutive securities are deemed to be anti-dilutive, accordingly, there was no effect of dilutive securities. Therefore, our basic and diluted loss per share of common stock and our basic and diluted weighted average shares of common stock are the same for 2023 and 2022.
The following table sets forth the outstanding securities as of the periods presented which were not included in the calculation of diluted earnings per share of common stock during 2023 and 2022:
As of December 31, | ||||
| 2023 |
| 2022 | |
Stock options | | | ||
RSUs | | — | ||
Warrants (excluding 2022 Warrants) | | | ||
11.Fair value measurements
Fair value is a market-based measurement, not an entity specific measurement and is defined as “the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.” Fair value measurements are defined on a three-level hierarchy:
Level 1: | Unadjusted quoted prices for identical assets or liabilities in active markets; |
F-24
NeuroBo Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements - Continued
(In thousands, except share and per share amounts)
Level 2: | Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, whether directly or indirectly, for substantially the full term of the asset or liability; |
Level 3: Unobservable inputs that reflect our own assumptions about the assumptions market participants would use in pricing the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date.
The fair value of financial instruments measured on a recurring basis as of December 31, 2023 and 2022 are as follows:
As of December 31, 2023 | As of December 31, 2022 | |||||||||||||||||||||||
Description |
| Total |
| Level 1 |
| Level 2 |
| Level 3 |
| Total |
| Level 1 |
| Level 2 |
| Level 3 | ||||||||
Liabilities: | ||||||||||||||||||||||||
Warrant liabilities | $ | | $ | — | $ | | $ | — | $ | | $ | — | $ | — | $ | | ||||||||
Total liabilities at fair value | $ | | $ | — | $ | | $ | — | $ | | $ | — | $ | — | $ | | ||||||||
In 2023, we estimated the fair value of the 2022 Warrants using the trading market price of our common stock due to the cashless exercise provision of the 2022 Warrants, which rendered the warrant exercise price to zero. Accordingly, as of December 31, 2023, our warrant liabilities were considered to be Level 2 of the fair value hierarchy.
In 2022, we estimated the fair value of the 2022 Warrants using a Monte Carlo simulation. This valuation technique involves a significant amount of estimation and judgment. In general, the assumptions used in calculating the fair value of the common stock warrant liability represent management’s best estimate, but the estimate involves inherent uncertainties and the application of significant management judgment. Accordingly, as of December 31, 2022, our warrant liabilities were considered to be Level 3 of the fair value hierarchy. Input assumptions used in the Monte Carlo simulation were as follows:
As of | ||||
December 31, 2022 | ||||
Stock price | $ | |||
Exercise price (1) | $ | — | ||
Risk free interest rate | % | |||
Volatility | % | |||
Remaining term (years) | ||||
| (1) | Due to the cashless exercise provision of the 2022 Warrants rendering the exercise price effectively at zero, the calculated price per share of the 2022 Warrants was equal to that of a share of common stock. |
The following table provides a roll-forward of the warrant liabilities measured at fair value 2023 and 2022:
Year Ended December 31, | ||||||
2023 | 2022 | |||||
As of the beginning of period | $ | | $ | — | ||
Issuance of 2022 warrants | — | | ||||
( | ( | |||||
Reclassification of warrant liabilities upon exercise of warrants | ( | ( | ||||
As of the end of period | $ | | $ | | ||
12.Subsequent events
Management has evaluated subsequent events to determine if events or transactions occurring through the filing date of this Annual Report on Form 10-K require adjustment to or disclosure in the consolidated financial statements. There were no events that require adjustment to or disclosure in the consolidated financial statements.
F-25