As filed with the Securities and Exchange Commission on March 20, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _______________________ to ______________________________
OR
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number
(Exact name of Registrant as specified in its charter)
(Jurisdiction of incorporation or organization)
Telephone: +32 10 22 23 55
(Address of principal executive offices)
Nyxoah SA
Telephone: +
Email:
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report: Ordinary shares, no nominal value per share:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ | Non-accelerated filer ☐ | |||
Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act.
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ |
| Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
Auditor Firm Id: 0 | Auditor Name: Bedrijfsrevisoren SRL/BV | Auditor Location: |
TABLE OF CONTENTS
| Page | |||
1 | ||||
1 | ||||
2 | ||||
3 | ||||
3 | ||||
| 3 | |||
3 | ||||
3 | ||||
3 | ||||
3 | ||||
3 | ||||
4 | ||||
58 | ||||
58 | ||||
59 | ||||
99 | ||||
100 | ||||
100 | ||||
100 | ||||
101 | ||||
109 | ||||
112 | ||||
112 | ||||
112 | ||||
113 | ||||
113 | ||||
115 | ||||
122 | ||||
127 | ||||
128 | ||||
128 | ||||
128 | ||||
130 | ||||
131 | ||||
131 | ||||
131 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
132 | ||||
133 | ||||
133 | ||||
133 | ||||
148 | ||||
i
148 | ||||
148 | ||||
149 | ||||
149 | ||||
150 | ||||
150 | ||||
150 | ||||
150 | ||||
150 | ||||
151 | ||||
151 | ||||
Material Modifications to the Rights of Security Holders and Use of Proceeds | 151 | |||
151 | ||||
151 | ||||
B. Management’s Annual Report on Internal Control over Financial Reporting | 151 | |||
C. Attestation Report of the Registered Public Accounting Firm | 152 | |||
153 | ||||
153 | ||||
153 | ||||
154 | ||||
155 | ||||
Purchases of Equity Securities by the Issuer and Affiliated Purchasers | 155 | |||
155 | ||||
155 | ||||
156 | ||||
Disclosure Regarding Foreign Jurisdictions That Prevent Inspections | 156 | |||
158 | ||||
158 | ||||
158 | ||||
158 |
ii
GENERAL INFORMATION
In this annual report on Form 20-F, or Annual Report, “Nyxoah,” “Nyxoah,” the “Company,” “we,” “us” and “our” refer to Nyxoah SA and its consolidated subsidiaries, except where the context otherwise requires.
“Nyxoah,” the Nyxoah logo, Genio and other trademarks or service marks of Nyxoah appearing in this Annual Report are the property of Nyxoah or its subsidiaries. Solely for convenience, the trademarks, service marks and trade names referred to in this Annual Report are listed without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their right thereto. All other trademarks, trade names and service marks appearing in this Annual Report are the property of their respective owners. We do not intend to use or display other companies’ trademarks and trade names to imply any relationship with, or endorsement or sponsorship of us by, any other companies.
PRESENTATION OF FINANCIAL AND OTHER DATA
The consolidated financial statement data as at December 31, 2023 and 2022 and for the years ended December 31, 2023, 2022 and 2021 have been derived from our consolidated financial statements, which have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB.
Our financial statements included in this Annual Report are presented in Euros and, unless otherwise specified, all monetary amounts are in Euros. All references in this Annual Report to “$”, “U.S. dollars,” and “dollars” are to U.S. dollars and all references to “€” and “Euro” are to Euros, unless otherwise noted.
1
INFORMATION REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains estimates and forward-looking statements, principally in the sections titled “Risk Factors,” “Operating and Financial Review and Prospects” and “Business.” Some of the matters discussed concerning our operations and financial performance include forward-looking statements and estimates within the meaning of the Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended. The words “anticipate,” “believe,” “can,” “could,” “estimate,” “expect,” “intend,” “is designed to,” “may,” “might,” “plan,” “potential,” “predict,” “objective,” “should,” or the negative of these and similar words are intended to identify forward-looking statements and estimates. Forward-looking statements include, but are not limited to, statements about:
| ● | timing, progress, completion and results of clinical trials and our research and development programs; |
| ● | the timing or likelihood of regulatory filings and approvals; |
| ● | our reliance on the success of our Genio system; |
| ● | our ability to achieve and maintain adequate levels of coverage or reimbursement for procedures performed with our products and any future products we may seek to commercialize; |
| ● | the commercialization of our products; |
| ● | estimates of our expenses, future revenues, capital requirements and our needs for additional financing; |
| ● | the scope of protection we are able to establish and maintain for intellectual property rights covering our products and technology; |
| ● | our ability to operate our business without infringing the intellectual property rights and proprietary technology of third parties; |
| ● | cost associated with defending intellectual property infringement, product liability and other claims; |
| ● | regulatory development in the U.S., Europe and other jurisdictions; |
| ● | the rate and degree of market acceptance of our products; |
| ● | our expectations about market trends; |
| ● | developments relating to our competitors and our industry, including competing products; |
| ● | our ability to accurately forecast customer demand and manage our inventory; |
| ● | our ability to effectively manage our anticipated growth; |
| ● | our ability to attract and retain qualified employees and key personnel; |
| ● | statements regarding future revenue, hiring plans, expenses, capital expenditures, capital requirements and share performance; |
| ● | our expected use of proceeds from the initial public offering on The Nasdaq Global Market; |
| ● | the future trading price of the ordinary shares and impact of securities analysts’ reports on these prices; |
| ● | the impact on our business, financial condition and results of operations from regional conflicts, geopolitical events and any pandemic, epidemic or outbreak of an infectious disease in the U.S. or worldwide; |
| ● | our plans to remediate our material weakness; and |
| ● | other risks and uncertainties, including those listed under the caption “Risk Factors.” |
These forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors that could cause our actual results of operations, financial condition, liquidity, performance, prospects, opportunities, achievements or industry results, as well as those of the markets we serve or intend to serve, to differ materially from those expressed in, or suggested by, these forward-looking statements. Factors that could cause actual results, financial condition, liquidity, performance, prospects, opportunities, achievements or industry results to differ materially include, but are not limited to, those discussed under “Risk Factors” in this Annual Report. Additional risks that we may currently deem immaterial or that are not presently known to us could also cause the forward-looking events discussed in this Annual Report not to occur. These forward-looking statements are based on assumptions regarding our present and future business strategies and the environment in which we expect to operate in the future.
Forward-looking statements and estimates speak only at the date they were made, and we undertake no obligation to update or to review any forward-looking statement or estimate because of new information, future events or other factors. Forward-looking statements and estimates involve risks and uncertainties and are not guarantees of future performance. Our future results may differ materially from those expressed in these forward-looking statements and estimates.
2
Additional factors that could cause actual results, financial condition, liquidity, performance, prospects, opportunities, achievements or industry results to differ materially include, but are not limited to, those discussed under “Risk Factors” in this Annual Report. Additional risks that we may currently deem immaterial or that are not presently known to us could also cause the forward-looking events discussed in this Annual Report not to occur. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect” and similar words are intended to identify estimates and forward-looking statements. Estimates and forward-looking statements speak only at the date they were made, and we undertake no obligation to update or to review any estimate and/or forward-looking statement because of new information, future events or other factors. Estimates and forward-looking statements involve risks and uncertainties and are not guarantees of future performance. Our future results may differ materially from those expressed in these estimates and forward-looking statements. In light of the risks and uncertainties described above, the estimates and forward-looking statements discussed in this Annual Report might not occur, and our future results and our performance may differ materially from those expressed in these forward-looking statements due to, inclusive of, but not limited to, the factors mentioned above. Because of these uncertainties, you should not make any investment decision based on these estimates and forward-looking statements.
WEBSITE DISCLOSURE
We maintain a public website at https://www.nyxoah.com and use our website as a routine channel of distribution of company information, including press releases, analyst presentations, and supplemental financial information, as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Our website includes an Investors section through which we make available, free of charge, our Annual Reports on Form 20-F, Reports on Form 6-K, as well as any amendments to those reports filed or furnished pursuant to the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Accordingly, investors should monitor our website in addition to following press releases, filings with the SEC, and public conference calls and webcasts.
None of the information provided on our website, in our press releases or public conference calls and webcasts or through social media is incorporated into, or deemed to be a part of, this Annual Report or in any other report or document we file with the SEC, and any references to such website is intended to be inactive textual references only.
PART I
Item 1.Identity of Directors, Senior Management and Advisers
Not Applicable.
Item 2.Offer Statistics and Expected Timetable
Not Applicable.
Item 3.Key Information
A.[Reserved]
B.Capitalization and Indebtedness
Not Applicable.
C.Reasons for the Offer and Use of Proceeds
Not Applicable.
3
D.Risk Factors
Our business has significant risks. You should carefully consider the risks and uncertainties described below, together with all of the other information in this Annual Report, including the matters addressed in the section of the Annual Report entitled “Information Regarding Forward-Looking Statements” and in our consolidated financial statements and related notes, before deciding whether to purchase our ordinary shares. If any of the following risks are realized, our business, financial condition, operating results and prospects could be materially and adversely affected. In that event, the market price of our ordinary shares could decline, and you could lose part or all of your investment. Additional risks and uncertainties not currently known to us or that we now deem immaterial may also harm us and adversely affect our business, results of operations and financial condition.
Summary of Risk Factors
An investment in our ordinary shares is subject to a number of risks, including risks related to our business and industry, risks related to development of our product candidates, and risks related to our ordinary shares. The following summarizes some, but not all, of these risks. Please carefully consider all of the information discussed in “Item 3. Key Information—D. Risk Factors” in this Annual Report for a more thorough description of these and other risks.
Risks Associated With Our Business
| ● | We have a limited operating history, have incurred losses in each period since our inception and may not be able to achieve or maintain profitability in the future. |
| ● | Our future financial performance depends on the commercial acceptance of the Genio system in target markets. |
| ● | We will require additional capital in the future, which may not be available to us on commercially favorable terms, or at all. More specifically, there is substantial doubt about our ability to continue as a going concern for a period of at least twelve months from the date of this Annual Report and our ability to continue as a going concern will depend on our ability to obtain additional funding, as to which no assurances can be given as of the date of this Annual Report. We refer to the disclosure in this respect in note 5.1 to our consolidated financial statements and the emphasis of matter paragraph in respect of going concern in the report of the Independent Registered Public Accounting Firm found elsewhere in this Annual Report. |
| ● | Even though we have obtained certification, a CE-Mark, in Europe for the Genio system based on first positive clinical trial results, there is no guarantee that we will be able to maintain our current certification or to obtain additional certification or marketing authorizations in other jurisdictions, including the United States, or that the results from our ongoing and planned clinical trials will be sufficient for us to obtain or maintain such certifications or authorizations. |
| ● | We may not receive, or may be delayed in receiving, the necessary marketing authorizations or certifications for our Genio system or any future product candidates, and failure to timely obtain necessary marketing authorizations or certifications for our product candidates would have a material adverse effect on our business. |
| ● | Even if we receive marketing authorizations, clearances or certifications in our target markets to commercialize the Genio system or any product candidate that we develop, the product may become subject to unfavorable pricing regulations, third-party payor reimbursement practices or healthcare reform initiatives that could harm our business. |
| ● | A pandemic, epidemic, or outbreak of an infectious disease, such as the COVID-19 pandemic, could materially and adversely affect our business and our financial results and cause a disruption to our research, development and commercialization efforts. |
| ● | A loss or degradation in performance of the suppliers on which we depend for services and components used in the production and assembly of the Genio system could have a material effect on our business, financial condition and results of operations. |
| ● | We may not be able to manufacture or outsource manufacturing of the Genio system in sufficient quantities, in a timely manner or at a cost that is economically attractive. |
| ● | Our products and operations are subject to extensive government regulation and oversight both in the United States. and abroad, and our failure to comply with applicable requirements could harm our business. |
| ● | The Genio system is still unapproved in certain significant markets, such as the United States market, and seeking and obtaining regulatory authorization or certification for active implantable medical devices can be a long, expensive and uncertain process. |
| ● | Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer. |
| ● | We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology or loss of data, including any cyber security incidents, could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability which could harm our ability to operate our business effectively and adversely affect our business and reputation. |
4
| ● | Our inability to fully protect and exploit our intellectual property and trade secrets may adversely affect our financial performance and prospects. |
| ● | The dual listing of our ordinary shares may adversely affect the liquidity and value of the ordinary shares. |
| ● | We or the third parties upon which we depend may be adversely affected by general political, unstable market and economic conditions and other events beyond our control and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster. |
| ● | Climate change or legal, regulatory or market measures to address climate change may negatively affect our business, results of operations, cash flows and prospects. |
| ● | In connection with our preparation and the audit of our consolidated financial statements as of and for the year ended December 31, 2023, we and our independent registered public accounting firm identified material weaknesses in our internal control over financial reporting. Additionally, we may identify additional material weaknesses in the future that may cause us to fail to meet our reporting obligations or result in material misstatements in our financial statements. If we fail to remediate our material weaknesses, we may not be able to report our financial results accurately or prevent fraud. |
Risks Related to Our Financial Position
We have a limited operating history, have incurred losses in each period since our inception and may not be able to achieve or maintain profitability in the future.
We were incorporated in 2009, obtained certification (CE-Mark) for our Genio system in March 2019, and had our first commercial sales in Germany in July 2020. In 2023 we generated €4.3 million of sales from the Genio system compared to €3.1 million in 2022. We have incurred operating losses and negative operating cash flows in each period since we were incorporated in 2009, including operating losses of €45.1 million and €32.5 million and negative operating cash flows of €44.8 million and €28.8 million for each of the years ended December 31, 2023 and December 31, 2022, respectively. As of December 31, 2023, we had an accumulated deficit of €160.8 million. These losses have resulted primarily from costs incurred in the development of our Genio system, as well as from general and administrative costs associated with our operations and manufacturing.
We expect that our operating expenses will continue to increase as we fund the continued development of our technology and the Genio product line, seek to expand manufacturing and sales and marketing capabilities, seek further regulatory clearances, certifications, approvals and marketing authorizations, particularly in the United States, for the Genio system, and as we incur the additional costs associated with being a public company in the United States. In June 2020, we obtained approval from the FDA under an investigational device exemption, or IDE, to begin our pivotal trial, the dual-sided hypoglossal nerve stimulation for the treatment of obstructive sleep apnea, or DREAM, trial. The aim of the DREAM trial, if the data are positive, is to support market authorization of the Genio system in the United States, as well as to support obtaining coverage and reimbursement more generally. We also plan to conduct additional clinical trials, and as a result, we expect clinical expenses will increase significantly over the next several years.
As a result, we expect to continue to incur operating losses for the foreseeable future, and we may never achieve profitability, which could impair our ability to sustain operations or obtain any required additional funding. Furthermore, even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis. If we do not achieve or sustain profitability in the future, we may suffer net losses or negative operating cash flows in subsequent periods.
Our future financial performance depends on the commercial acceptance of the Genio system in target markets.
The Genio system is currently our only commercial product, which we market in certain European countries, and our success depends entirely upon its market acceptance and adoption by physicians, payors and patients. The Genio system may not gain commercial acceptance in target markets. If we fail to gain and maintain commercial market acceptance of the Genio system in our target markets, for instance, because of insufficient price and reimbursement levels from government and third-party payors, competition, or the inability to demonstrate the benefits and cost-effectiveness of the Genio system compared to other products available on the market, the amount of revenue generated from sales of the Genio system in the future could continue to be limited, and could even decrease over time. In addition, the Genio system has not received marketing authorization in the United States, and our future financial performance will depend on the successful completion of our DREAM pivotal trial, which is intended to support an application for market authorization to commercialize the Genio system in the United States.
5
These and other factors present obstacles to commercial acceptance of the Genio system in target markets and could lead to our failure, or a substantial delay, in gaining significant market acceptance of the Genio system in target markets, which could affect our ability to generate revenue. Any failure of the Genio system to achieve meaningful market acceptance will harm our business and future prospects.
We will require additional capital in the future, which may not be available to us on commercially favorable terms, or at all. More specifically, there is substantial doubt about our ability to continue as a going concern for a period of at least twelve months from the date of this Annual Report and our ability to continue as a going concern will depend on our ability to obtain additional funding, as to which no assurances can be given as of the date of this Annual Report.
We expect to incur significant expenses and operating losses over the next few years, and we may need to raise additional capital in the future. We have so far been financed primarily by funds invested by our shareholders, including in connection with our initial public offering on Euronext Brussels in September 2020 and the listing of our ordinary shares on the Nasdaq Global Market in July 2021. Based on our current operating plan and our existing cash and cash equivalents of €21.6 million and financial assets of €36.1 million as of December 31,2023, we expect to be able to fund our operations until the beginning of the fourth quarter of 2024. However, we have based these estimates on assumptions that may prove to be incorrect, and we could spend our financial resources much faster than currently expected. Pursuant to the requirements of IAS 1.25-26, Presentation of Financial Statements - Going Concern, and as a result of our financial condition and other factors described herein, there is substantial doubt about our ability to continue as a going concern for a period of at least twelve months from the date of this Annual Report. See Note 5.1 to our consolidated financial statements found elsewhere in this Annual Report. Our ability to continue as a going concern will depend on our ability to obtain additional funding, as to which no assurances can be given. Our future success depends on our ability to raise capital and/or execute our current operating plan. Any future funding requirements will depend on many factors, including without limitation:
| ● | acceptance of our Genio system by patients, physicians, government payors, private payors, and the market generally in our target markets; |
| ● | the scope, rate of progress and cost of current or future clinical trials; |
| ● | the cost and timing of obtaining additional regulatory clearances, approvals, classifications, certifications or other marketing authorizations for the Genio system; |
| ● | the cost and timing of establishing additional sales and marketing capabilities; |
| ● | the cost of research and development activities; |
| ● | the cost of filing and prosecuting patent applications and other intellectual property rights and defending and enforcing our patents or other intellectual property rights in various jurisdictions; |
| ● | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; |
| ● | the cost associated with any complications or side effects related to the use of the Genio system; |
| ● | costs associated with any product recall that may occur; |
| ● | the effect of competing technological and market developments; |
| ● | the extent to which we acquire or invest in products, technologies and businesses, although we currently have no commitments or agreements relating to any of these types of transactions; and |
| ● | the costs of operating as a public company in Belgium and the United States. |
Any additional equity or debt financing that we raise may contain terms that are not favorable to us or our shareholders. If we raise additional funds by selling additional ordinary shares or other securities convertible into or exercisable or exchangeable for ordinary shares, the issuance of such securities will result in dilution to our shareholders.
6
In addition, any future debt financing into which we enter may impose upon us covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our ordinary shares, make certain investments and engage in certain merger, consolidation or asset sale transactions. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or products, or grant licenses on terms that are not favorable to us.
Furthermore, we cannot be certain that additional funding will be available on acceptable terms, if at all. We have no committed source of additional capital other than our at-the-market facility. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third-parties the rights to commercialize products or technologies that we would otherwise seek to commercialize ourselves. We also may have to reduce marketing, customer support or other resources devoted to our products or cease operations, or even terminate our operations, which may involve seeking bankruptcy protection.
Any loss or decrease of subsidies, reimbursable cash advances and tax reductions may affect our financial resources.
Since September 2011, we have received financial support from the Walloon Region in the form of recoverable cash advances and subsidies. In March 2018, in accordance with Section 27A of the Australian Industry Research and Development Act 1986, the Australian Government gave notice to Nyxoah Pty Ltd, our Australian subsidiary, of registration for the research and development, or R&D, tax incentive from the 2017/2018 income year. This incentive represents 43.5% of the yearly eligible R&D expenditure. In October 2023, we received confirmation from the Walloon Region that we can apply tax credits in Belgium on eligible R&D investments.
All these subsidies and reimbursable cash advances increased our financial resources to support R&D and clinical development projects. However, we cannot predict whether we or our subsidiaries will continue to benefit from such incentives and/or advantages and/or to what extent. The repayment obligations with respect to the financial support from the Walloon Region will also have the effect of reducing our profitability until fully repaid.
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults or nonperformance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and its financial condition and results of operations.
Actual events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, on March 10, 2023, Silicon Valley Bank, or SVB, was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation, or the FDIC, as receiver. Similarly, on March 12, 2023, Signature Bank and Silvergate Capital Corp. were each swept into receivership. If any of our counterparties to any credit agreements, letters of credit or certain other financial instruments that we may enter into in the future were to be placed into receivership, we may be unable to access such funds. In addition, if any parties with whom we conduct business are unable to access funds pursuant to such instruments or lending arrangements with such a financial institution, such parties’ ability to pay their obligations to us or to enter into new commercial arrangements requiring additional payments to us could be adversely affected. Similar impacts have occurred in the past, such as during the 2008-2010 financial crisis.
7
Inflation and rapid increases in interest rates have led to a decline in the trading value of previously issued government securities with interest rates below current market interest rates. There is no guarantee that the U.S. Department of Treasury, FDIC and Federal Reserve Board will provide access to uninsured funds in the future in the event of the closure of other banks or financial institutions, or that they would do so in a timely fashion.
Although we assess our banking relationships as we believe necessary or appropriate, our access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations could be significantly impaired by factors that affect us, the financial institutions with which we have arrangements directly, or the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit or liquidity agreements or arrangements, disruptions or instability in the financial services industry or financial markets, or concerns or negative expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions or financial services industry companies with which we have financial or business relationships, but could also include factors involving financial markets or the financial services industry generally.
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses, financial obligations or fulfill our other obligations, result in breaches of our financial and/or contractual obligations or result in violations of federal or state wage and hour laws. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
In addition, any further deterioration in the macroeconomic economy or financial services industry could lead to losses or defaults by parties with whom we conduct business, which in turn, could have a material adverse effect on our current and/or projected business operations and results of operations and financial condition. For example, a party with whom we conduct business may fail to make payments when due, default under their agreements with us, become insolvent or declare bankruptcy. Any bankruptcy or insolvency, or the failure to make payments when due, of any counterparty of ours, or the loss of any significant relationships, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
Risks Related to Development of Our Products and Product Candidates
Even though we have obtained certification, a CE-Mark, in Europe for the Genio system based on first positive clinical trial results, there is no guarantee that we will be able to maintain our current certification or to obtain additional certification or marketing authorizations in other jurisdictions, including the United States, or that the results from our ongoing and planned clinical trials will be sufficient for us to obtain or maintain such certifications or authorizations.
Even though we have obtained certification (CE-Mark) in Europe for the Genio system based on positive results from our BiLAteral hypoglossal nerve stimulation for treatment of Obstructive Sleep Apnea, or BLAST, clinical trial, there is no assurance that ongoing and future clinical trials we may conduct to support further marketing authorizations, certifications or clearances (or to maintain existing ones) will be successful and that the Genio system will perform as intended. We may be required to develop more clinical evidence than we currently anticipate before we are able to demonstrate to the satisfaction of the FDA or other regulatory authorities that the Genio system is safe and effective for its intended use, if ever. To obtain a certificate of conformity, manufacturers need to comply with the essential requirements of the EU Medical Devices Directive (Council Directive 93/42/EEC), the Active Implantable Medical Devices Directive (Council Directive 90/385/EEC) or Medical Device Regulation (EU) 2017/745 of the European Parliament, and in particular to demonstrate that devices are designed and manufactured in such a way that they will not compromise the clinical condition or safety of patients, or the safety and health of users and others (that the potential benefits outweigh potential risks). In addition, medical devices must achieve the performance intended by the manufacturer and be designed, manufactured and packaged in a suitable manner. However, if the Genio system causes or contributes to consumer injuries or other harm or other serious issues arise as to the device’s performance, it may be necessary to conduct further clinical trials to confirm the device can perform safely and effectively.
8
In particular, even if certification has been obtained in Europe, there is no guarantee for success in the United States of a pivotal trial to support a premarket submission to the FDA or for future U.S. marketing authorization. The FDA’s standard of review differs from that required to obtain a CE-Mark in Europe, which only indicates that the device in question is in full compliance with European legislation. Medical devices certified for marketing in the European Union need notably to demonstrate that they are designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users and others. On the other hand, before FDA approval of a medical device in the United States, a device must not only be shown to be safe, but also effective its intended use, or in the case of a 510(k) clearance, substantially equivalent to a predicate device.
We may not receive, or may be delayed in receiving, the necessary marketing authorizations or certifications for our Genio system or any future product candidates, and failure to timely obtain necessary marketing authorizations or certifications for our product candidates would have a material adverse effect on our business.
In the United States, before we can market a new medical device, or a new use of, or other significant modification to an existing, marketed medical device, we must first receive either clearance under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA, approval of a premarket approval, or PMA, application or grant of a De Novo classification request from the FDA, unless an exemption applies. In the 510(k) clearance process, before a device may be marketed, the FDA must determine that a proposed device is “substantially equivalent” to a legally marketed “predicate” device, which includes a device that has been previously cleared through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (pre amendments device), a device that was originally on the U.S. market pursuant to an approved PMA and later down-classified, or a 510(k) exempt device. To be “substantially equivalent,” the proposed device must have the same or similar intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness from the predicate device. Clinical data are sometimes required to support substantial equivalence. In the process of obtaining PMA approval, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life sustaining, life supporting or implantable devices. In the De Novo classification process, a manufacturer whose novel device under the FDA would otherwise be automatically classified as Class III and require the submission and approval of a PMA prior to marketing is able to request initial classification of the device as Class I or Class II based on evidence that the device in fact presents a low or moderate risk. If the FDA grants the De Novo classification request, the applicant receives authorization to market the device. If the De Novo process results in the classification of a device as Class II, the authorized device may be used subsequently as a predicate device for future 510(k) submissions.
The PMA approval, 510(k) clearance and De Novo classification processes can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process can take anywhere from three to 12 months or longer to complete. The process of obtaining a PMA or De Novo classification is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is submitted to the FDA. In addition, PMAs and De Novo classification requests generally require the applicant to have conducted one or more clinical trials. Despite the time, effort and cost expended in seeking a marketing authorization, there is no assurance that the FDA will grant it. Any delay or failure to obtain necessary regulatory marketing authorizations could harm our business. Furthermore, even if we are granted such marketing authorizations, they may include significant limitations on the indicated uses for the device, which may limit the potential commercial market for the device.
To date, we have not obtained authorization from the FDA to market any product candidate in the United States. However, we initiated a modular PMA application process for our Genio system and submitted the first module to the FDA in March 2023, the second module to the FDA in June 2023 and the third module to the FDA in November 2023. We anticipate submitting the fourth module to the FDA in the second quarter of 2024. In a modular PMA, the complete contents of a PMA are compiled as sections or “modules”, such as preclinical, clinical, and manufacturing, that together become a complete application. This method is used for products that are in early stages of clinical study. The FDA reviews each module separately as it is received to provide feedback during the review process. If the FDA requires us to go through a lengthier, more rigorous examination for our product than we currently expect, our product introduction could be delayed or prevented, which would have a material adverse impact on our business and prospects. Following completion of our DREAM pivotal trial, we expect to engage further with the FDA during the clinical module to discuss the clinical trial results in order to obtain marketing authorization in the United States. The Genio system cannot be legally marketed until the FDA reviews and approves the device based on all required modules of the PMA application. We may not be able to meet the requirements to obtain PMA approval, and even if we do obtain marketing authorization, the FDA may place significant limitations on any such marketing authorization depending on the available safety and effectiveness data for the Genio system for its intended uses.
9
In order to sell our products in member countries of the European Union, or the EU, our products must comply with the essential requirements of the EU Medical Devices Directive (Council Directive 93/42/EEC), the Active Implantable Medical Devices Directive (Council Directive 90/385/EEC) or Medical Device Regulation (EU) 2017/745 of the European Parliament. Compliance with these requirements is a prerequisite to be able to affix the European Conformity, or CE, mark to our products, without which they cannot be sold or marketed in the EU. To demonstrate compliance with the essential requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its (risks) classification. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the EU Medical Devices Directive (for devices entering the EU market prior to May 26, 2021) or the Medical Device Regulation (for devices entering the EU market on or after May 26, 2021), a conformity assessment procedure requires the intervention of an organization accredited or designated by a member state of the EU to conduct conformity assessments to the applicable legal requirements, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a certificate of conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This certificate entitles the manufacturer to affix the CE-Mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. If we fail to remain in compliance with applicable European laws and directives and corresponding EU member state laws, we would be unable to continue to affix the CE-Mark to our products, which would prevent us from selling them within the EU.
The aforementioned EU rules are generally applicable in the European Economic Area, or EEA, which consists of the 27 EU member states plus Norway, Liechtenstein and Iceland. Noncompliance with the above requirements would also prevent us from selling our products in these three countries.
Following the end of the “Brexit” transition period, from January 1, 2021 onwards, the UK Medicines and Healthcare products Regulatory Agency, or MHRA is responsible for the UK medical device market. The new regulations require medical devices to be registered with the MHRA, (but manufacturers are given a grace period of four to 12 months to comply with the new registration process). Manufacturers based outside the UK must appoint a UK Responsible Person to register devices with the MHRA in line with the grace periods. The UK will continue to recognize CE-Markings, as well as certificates issued by EU recognized Notified Bodies, until the earlier of June 30, 2028 or the expiration of the certificate for devices compliant with the Medical Devices Directive or the Active Implantable Medical Devices Directive, or until June 30, 2030 for devices compliant with the MDR. Devices marketed in the UK (England, Scotland, Northern Ireland and Wales) after such respective dates will require a UK Conformity Assessed (UKCA) mark. However, UKCA marking alone will not be recognized in the EU. The rules for placing medical devices on the Northern Ireland market will differ from those in the UK. Compliance with this legislation is a prerequisite to be able to affix the UKCA mark to our products, without which they cannot be sold or marketed in the UK.
In order to sell products in Switzerland, manufacturers based in the EU must appoint a Swiss Authorized Representative (CH-Rep).
The FDA or foreign regulatory authorities or Notified Bodies can delay, limit or deny marketing authorization or certification of a device for many reasons, including:
| ● | our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory entity or Notified Bodies that our products are safe and effective for their intended uses; |
| ● | the disagreement of the FDA, foreign regulatory authorities or other foreign (regulatory) body with the design or implementation of our clinical trials or the interpretation of data from non- clinical studies or clinical trials; |
| ● | serious and unexpected adverse device effects experienced by participants in our clinical trials; |
| ● | the data from our non-clinical studies and clinical trials may be insufficient to support clearance, certification, De Novo classification or approval, where required; |
| ● | our inability to demonstrate that the clinical and other benefits of the device outweigh the risks; |
10
| ● | the manufacturing process or facilities we use may not meet applicable requirements; and |
| ● | the potential for approval policies or regulations of the FDA or foreign regulatory authorities to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval. |
Our growth will depend, in part, on our ability to expand the indications for the Genio system, as well as to continue to development enhancements to the system and also develop and commercialize additional products.
Expanding indications for our Genio system and developing new products is expensive and time-consuming and could divert management’s attention away from our core business. We plan to continue to invest in pursuing additional indications for our Genio system and in improving the Genio system to develop next generation versions designed to improve patient comfort, efficacy and convenience. For example, in July 2022, we received FDA approval for an IDE to enable us to initiate a clinical trial, called ACCCESS, to evaluate the use of the Genio system for the treatment of adult patients with moderate-to-severe OSA with complete concentric collapse (CCC).
The success of any such product development efforts will depend on several factors, including our ability to do the following:
| ● | properly identify and anticipate physician and patient needs; |
| ● | develop and introduce new products and product enhancements in a timely manner; |
| ● | avoid infringing upon the intellectual property rights of third parties; |
| ● | obtain necessary licenses from or reach commercial agreements with third parties owning proprietary technologies or solutions; |
| ● | demonstrate, if required, the safety and efficacy of new products with data from preclinical studies and clinical trials; |
| ● | obtain the necessary regulatory authorizations and/or certifications for expanded indications, new products or product modifications; |
| ● | be fully compliant with requirements related to marketing of new devices or modified products; |
| ● | provide adequate training to potential users of our products; |
| ● | receive adequate coverage and reimbursement for procedures performed with our products; and |
| ● | develop an effective and dedicated sales and marketing team. |
If we are not successful in expanding indications and developing and commercializing new products and product enhancements, our ability to increase our revenue in the future may be impaired.
Clinical trials involve a lengthy and expensive process with an uncertain outcome. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
We obtained CE-Mark certification in Europe for the Genio system in March 2019, commenced sales of the Genio system in Germany in July 2020, and are pursuing marketing activities in advance of commencing selling efforts in several other European countries. In the United States, we received FDA approval of an IDE to commence our DREAM trial, which if successfully completed, we anticipate relying upon to support our application for marketing authorization of the Genio system in the U.S. market.
Before obtaining marketing clearance, approval or certification from regulatory authorities or Notified Bodies respectively for the sale of our Genio system, or any additional products we may develop, we expect to conduct clinical trials to demonstrate the safety and efficacy of the device in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing.
It is possible that even if the Genio system has a beneficial effect, that effect may not be detected during clinical evaluation, or may not be statistically significant, as a result of one or more of a variety of factors, including the size, duration, design, measurements, conduct or analysis of our clinical trials. Conversely, as a result of the same factors, our clinical trials may indicate an apparent positive effect that is greater than the actual positive effect, if any. Similarly, in our clinical trials we may fail to detect adverse effects caused by our Genio system, or mistakenly believe that our system caused certain adverse effects when that is not in fact the case. Also, the inclusion and exclusion criteria we define may not sufficiently capture a trial subject population that would be most appropriate for treatment with our Genio system.
11
The outcome of prior clinical trials may not be predictive of the success of later clinical trials. For example, the positive outcome of our BLAST clinical trial, based on which we obtained certification for the Genio system in the EU, does not ensure that our DREAM or our ACCCESS trials will be successful. Furthermore, interim results of a clinical trial do not necessarily predict final results. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy despite having progressed through non-clinical studies and earlier clinical trials. Many companies in the medical device industry have suffered significant setbacks in late-stage clinical trials after achieving positive results in earlier development, and we cannot be certain that we will not face such setbacks.
The design of a clinical trial can determine whether its results will support marketing authorization or certification of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced or completed. We have limited experience in designing clinical trials, and there is no certainty that the design of our ongoing clinical trials will ultimately support marketing authorization or certification. Even if we believe that the results of clinical trials for our product candidates warrant marketing authorization or certification, the FDA or comparable non-U.S. regulatory authorities and Notified Bodies may disagree and may not grant marketing authorization or certification of our product candidates.
In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the clinical trial protocols and the rate of dropout among clinical trial participants. Any pivotal or other clinical trials that we may conduct may not demonstrate the efficacy and safety to the degree necessary, if at all, to obtain regulatory approval to market our product candidates.
The initiation and completion of clinical trials may be prevented, delayed, or halted for numerous reasons. We may experience delays in our clinical trials for a number of reasons, which could adversely affect the costs, timing or successful completion of our clinical trials, including related to the following:
| ● | we may be required to submit additional IDEs to the FDA, which must become effective prior to commencing certain human clinical trials of medical devices, and the FDA may reject our IDE application and notify us that we may not begin clinical trials, or place restrictions on the conduct of such trials; |
| ● | regulators and other comparable foreign regulatory authorities may disagree as to the design or implementation of our clinical trials; |
| ● | regulators and/or institutional review boards, or IRBs, or other bodies may not authorize us or our investigators to commence a clinical trial, or to conduct or continue a clinical trial at a prospective or specific trial site; |
| ● | we may not reach agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
| ● | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| ● | we might have to suspend or terminate clinical trials for various reasons, including occurrence of adverse events or other findings that the subjects in our clinical trials are being exposed to unacceptable health risks; |
| ● | we may have to amend clinical trial protocols or conduct additional studies to reflect changes in regulatory requirements or guidance, which we may be required to submit to an IRB or other bodies and/or regulatory authorities for re-examination; |
| ● | regulators, IRBs, or other parties or bodies may require or recommend that we or our investigators suspend or terminate clinical research for various reasons, including safety signals or noncompliance with regulatory requirements; |
| ● | the cost of clinical trials may be greater than we anticipate; |
| ● | clinical sites may not adhere to the clinical protocol or may drop out of a clinical trial; |
| ● | we may be unable to recruit a sufficient number of clinical trial sites; |
| ● | regulators or other bodies may fail to approve or subsequently find fault with our manufacturing processes or facilities of third-party manufacturers with which we enter into agreement for clinical and commercial supplies, the supply of devices or other materials necessary to conduct clinical trials may be insufficient, inadequate or not available at an acceptable cost, or we may experience interruptions in supply; |
| ● | approval policies or regulations of FDA or applicable foreign regulatory agencies may change in a manner rendering our clinical data insufficient for approval; and |
12
| ● | our current or future products may have undesirable side effects or other unexpected characteristics. |
Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of any product candidate.
The U.S. Congress also recently amended the FDCA to require sponsors of a pivotal study of a new device to support marketing authorization, to design and submit a diversity action plan for such clinical trial. The action plan must describe appropriate diversity goals for enrollment, as well as a rationale for the goals and a description of how the sponsor will meet them. Our DREAM and ACCCESS trials are considered pivotal trials but were initiated before the diversity action plan requirement became effective. For any future pivotal trials we plan to conduct for the Genio system or any other product candidate, we must submit a diversity action plan to the FDA by the time we submit a pivotal study protocol to the agency for review, unless we are able to obtain a waiver for some or all of the requirements for a diversity action plan. It is unknown at this time how the diversity action plan may affect the planning and timing of any future pivotal trial for our product candidates or what specific information FDA will expect in such plans. However, initiation of such trials may be delayed if the FDA objects to our proposed diversity action plans for any future pivotal trial for our product candidates, and we may experience difficulties recruiting an adequately diverse population of patients in attempting to fulfill the requirements of any approved diversity action plan.
In addition, clinical trials must be conducted in accordance with the laws and regulations of the FDA and other applicable regulatory authorities’ legal requirements, regulations or guidelines, and are subject to oversight by these governmental agencies and IRBs or other bodies at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with supplies of our devices produced under current good manufacturing practice, or cGMP, requirements and other regulations. Furthermore, we rely on clinical trial sites, and we may in the future rely on CROs to ensure the proper and timely conduct of our clinical trials and while we have agreements governing their committed activities, we have limited influence over their actual performance. We depend on our collaborators and on medical institutions and we may in the future depend on CROs to conduct our clinical trials in compliance with good clinical practice, or GCP, requirements. To the extent our collaborators or the CROs fail to enroll participants for our clinical trials, fail to conduct the trial to GCP standards or are delayed for a significant time in the execution of trials, including achieving full enrollment, we may be affected by increased costs, program delays, regulatory enforcement actions or all three. In addition, conducting clinical trials in various countries may subject us to further delays and expenses as a result of increased shipment costs, additional regulatory requirements and the engagement of non-U.S. service providers, as well as expose us to risks associated with clinical investigators who are unknown to the FDA, and different standards of diagnosis, screening and medical care.
Interim, “top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more trial subject data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose interim, top-line or preliminary data from our clinical trials, which are based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular registry, trial or trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. Importantly, interim data from clinical trials are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. As a result, the interim, top-line or preliminary results that we report may differ from future results of the same trial, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Interim, top-line or preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the interim, top-line or preliminary data we previously published.
13
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general. In addition, the information we choose to publicly disclose regarding a particular non-clinical trial or clinical trial is based on what is typically extensive information, and others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure. If the interim top-line or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our products and product candidates may be harmed, which could harm our business, operating results, prospects or financial condition. Further, disclosure of interim data by us or by our competitors could result in volatility in the price of our common stock. For all of the foregoing reasons, interim, top-line and preliminary data should be viewed with caution until the final data are available.
Attracting patients to perform clinical trials and meeting clinical trial objectives can be more costly and time-consuming than expected and could be adversely affected by another health crisis.
In order conduct our clinical trials, we must recruit, screen and enroll eligible patients. Patients may be identified from the investigator’s own clinical practice or hospital or may be referred by another physician. Potential clinical trial participants must provide informed consent before undergoing certain clinical tests that are used to determine patient eligibility based on inclusion/exclusion criteria. As a result, at the time of informed consent, we do not know if a patient will be eligible to participate in the trial. For example, patients with CCC are excluded from our DREAM trial, and we cannot determine eligibility until after the patient has consented and undergone a drug-induced sleep endoscopy. To that end, we will need to screen many more patients than we intend to enroll in order to meet our enrollment criteria. After a patient is determined to be eligible and is enrolled in the clinical trial, they must comply with the trial requirements and undergo periodic time-consuming tests, including a sleep test in a sleep lab. Not all patients who undergo screening will ultimately be eligible for enrollment in our clinical trials. Moreover, some of the enrolled participants may not comply with the requirements of the trial, thereby leading to poor or unusable data, or some may withdraw from the trial, which may compromise the results of the clinical trial.
We may not be able to initiate, continue and/or complete in a timely manner clinical trials if we are unable to locate and enroll a sufficient number of eligible patients within the planned recruitment period to participate in these trials as required by the applicable regulatory authorities in the United States, Europe and any other applicable jurisdictions.
Delays in subject enrollment or failure of trial subjects to continue to participate in a clinical trial may delay commencement or completion of the clinical trial, cause an increase in the costs of the clinical trial and delays, or result in the failure of the clinical trial. Patient enrollment in our clinical trials may be affected by many factors including:
| ● | the fact that the Genio system is an implantable device requiring clinical trial subjects to undergo surgery; |
| ● | the existence of a competing device with FDA marketing authorization and long-term data supporting its safety and efficacy; |
| ● | clinicians’ and patients’ perceptions as to the potential advantages and risks of the Genio system in relation to other available therapies, including any new product candidates that may be approved for the indications we are investigating; |
| ● | the size and nature of the patient population; |
| ● | the severity of the disease under investigation; |
| ● | the eligibility criteria for the trial in question; |
| ● | subject compliance with the trial protocol; |
| ● | the design of the clinical trial; |
| ● | the referral practices of physicians; |
| ● | limitations placed on enrollment by regulatory authorities or other bodies; |
| ● | the ability to monitor trial subjects adequately during and after treatment; |
| ● | the proximity and availability of clinical trial sites for prospective subjects; |
| ● | the approval of other devices or therapeutics for the target indications; |
| ● | efforts to facilitate timely enrollment; |
| ● | other clinical trials competing for the same target patients as those of our clinical trials; and |
| ● | the necessity for the trial subjects to dedicate their time to multiple visits to the clinic and/or sleep lab for tests, including a sleep test in a lab, forming part of the clinical trial. |
14
Any difficulties in enrolling a sufficient number of subjects for any of our clinical trials, or any subjects withdrawing from the clinical trials or not complying with the trial protocols, could result in significant delays and could require us to abandon one or more clinical trials altogether. If our trial sites are restricted in performing elective surgeries or following up with their trial subjects, this may lead to missing information and may potentially impact clinical trial data quality and integrity. Enrollment delays and other issues with our clinical trials may result in increased research and development costs that may exceed the resources available to us and in delays to commercially launch the Genio system in target markets, if authorized for sale in such markets.
Serious adverse events, or SAEs, or undesirable side effects or other unexpected properties of our product candidates may be identified during development that could delay or prevent the product candidate’s marketing authorization or certification.
As is the case with implantable medical devices generally, it is likely that there may be side effects and adverse events associated with the use of our Genio system or any future product candidate. Results of our clinical trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics. SAEs or undesirable side effects caused by, or other unexpected properties of, our product candidates could cause us, an IRB or regulatory authorities or other bodies to interrupt, delay or halt clinical trials of one or more of our product candidates and could result in a more restrictive label or the delay or denial of marketing approval or certification by the FDA, Notified Bodies or comparable non-U.S. regulatory authorities. If any of our product candidates is associated with SAEs or undesirable side effects or has properties that are unexpected, we may need to abandon development or limit development of that product candidate to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many medical devices that initially showed promise in clinical or earlier stage testing have later been found to cause undesirable or unexpected side effects that prevented further development of the device. Additionally, if any of our product candidates, including the Genio system, receives marketing authorization from the FDA, the side effects observed in clinical trials could result in a more restrictive label than we anticipate.
Risks Related to Commercialization and Reimbursement
Even if we receive marketing authorizations, clearances or certifications in our target markets to commercialize the Genio system or any product candidate that we develop, the product may become subject to unfavorable pricing regulations, third-party payor reimbursement practices or healthcare reform initiatives that could harm our business.
The commercial success of the Genio system and any other product candidates we develop will depend substantially, both in the United States and abroad, on the extent to which coverage and reimbursement for our products and related procedures will be available from government health administration authorities, private health insurers and other third-party payors such as managed care and similar healthcare management organizations. Thus, our ability to commercialize the Genio system and any product candidates we develop will depend to a significant degree on which government authorities and third-party payors decide to cover our products and at what reimbursement levels. If reimbursement is not available, or is available only to a limited extent, we may not be able to successfully commercialize our products. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish and maintain pricing sufficient to realize a meaningful return on our investment.
There is significant uncertainty related to government and other third-party payor coverage and reimbursement of newly approved medical devices. Regulatory approvals and pricing and reimbursement for new device products vary widely from country to country. Some countries require approval of the sale price of a device before it can be marketed. In many countries, the pricing review period begins after marketing authorization or certification is granted. In some non-U.S. markets, pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing authorization or certification for a product in a particular country but then be subject to price regulations that delay commercial launch of the product, possibly for lengthy time periods, which may negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing authorization or certification.
15
The healthcare industry is acutely focused on cost containment, both in the United States and elsewhere. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medical products, which could affect our ability to sell our product candidates profitably. These payors may not view the Genio system or any other product candidates, if authorized for marketing, as cost-effective, and coverage and reimbursement may not be available to our customers, or may not be sufficient to allow our product candidates, if authorized for marketing, to be sold on a competitive basis. Cost-control initiatives could cause us to decrease the price we might establish for products, which could result in lower than anticipated product revenues. Further, if the prices for our product candidates, if authorized for marketing, decrease or if governmental and other third-party payors do not provide adequate coverage or reimbursement, our prospects for revenue and profitability will suffer. Marketing authorization or certification of a product does not guarantee sufficient reimbursement to achieve commercial success.
There may also be delays in obtaining coverage and reimbursement for newly approved products, and coverage may be more limited than the indications for which the product is authorized by the FDA or comparable non-U.S. regulatory authorities. Moreover, eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Reimbursement rates may vary, by way of example, according to the use of the product and the clinical setting in which it is used. Reimbursement rates may also be based on reimbursement levels already set for lower cost products or may be incorporated into existing payments for other services.
Obtaining and maintaining coverage and reimbursement can be a time-consuming process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of our products. Increasingly, third-party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging the prices charged. We may not be able to provide data sufficient to satisfy governmental and third-party payors that procedures using our products should be covered and reimbursed. We cannot be sure that coverage will be available for any product candidate that we commercialize and, if available, that the reimbursement rates will be adequate.
Outside the United States, reimbursement levels vary significantly by country and by region, particularly based on whether the country or region at issue maintains a single-payor system. Annual healthcare budgets generally determine the number of therapeutic devices like the Genio system that will be paid for by the payor in these single-payor system countries and regions. Some countries or regions may require us to gather additional clinical data before granting coverage and reimbursement for our products. We are currently working with payors in the EU to obtain coverage and reimbursement approval in countries and regions where it makes economic sense to do so; however, we may not obtain such coverage, which could have a material adverse effect on our business, financial condition and results of operations and impair our ability to grow our business.
We have limited experience marketing and selling our Genio system, and if we are unable to expand, manage and maintain our direct sales and marketing organization, we may not be able to generate revenue growth.
We have only limited experience in marketing and selling our Genio system. To achieve commercial success, we will need to keep expanding our internal sales and marketing organization to commercialize the Genio system in markets that we will target directly, such as Germany, Austria and Switzerland. Expanding our sales and marketing team further will entail recruiting additional managerial, operational, financial and other employees, which is expensive and time-consuming and could delay product launches.
For example, if we obtain regulatory authorization to market the Genio system in the United States, we intend to build a direct sales force. We have no experience marketing and selling the Genio system in the United States. To commence commercial launch will require us to hire, develop, grow and retain a U.S. marketing and sales organization. To do so will require significant investment in recruiting and training as we ramp up to a U.S. commercial launch. There is significant competition for marketing and sales personnel experienced in medical device sales. Once we hired such personnel, we expect to provide them with in-depth training, which can be lengthy, because it will require significant education for new marketing and sales representatives to achieve the level of clinical competency with the Genio system that physicians expect. Upon completion of training, our sales representatives will require lead time in the field to grow their network of accounts and achieve productivity levels we expect them to reach in any individual territory. If we are unable to attract, motivate, develop and retain a sufficient number of qualified sales personnel, and if our sales representatives do not achieve the productivity levels we expect them to reach, our revenue will not grow at the rate we expect and our financial performance will suffer.
16
If the commercial launch of the Genio system in the United States or another jurisdiction for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. In addition, our sales efforts may be hindered in target markets if we fail to develop complementary products.
We may also decide to target certain markets indirectly via distributors or other arrangements. If we are unable to find suitable distribution partners, lose these distribution partners or if our distribution partners fail to sell our products in sufficient quantities, on commercially viable terms or in a timely manner, the commercialization of the Genio system could be materially harmed, which could prevent us from achieving or maintaining profitability.
Hesitation to change or to undertake special training and economic, social, psychological and other concerns among physicians may limit general acceptance and adoption of the Genio system.
Even if the Genio system receives marketing authorization or certification from the appropriate regulatory authorities or Notified Bodies, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. Our efforts to educate the medical community and third-party payors regarding the benefits of the Genio system are expected to require significant resources and may not be successful.
Acceptance of the Genio system will depend on physicians being convinced of the distinctive characteristics, clinical performance, benefits, safety and cost-effectiveness of the device and being prepared to undertake special training in certain cases. Furthermore, physicians will likely only adopt the Genio system if they determine, based on experience, clinical data, and published peer-reviewed journal articles that the Genio system is an attractive treatment solution, and that third-party payors, such as government programs and private health insurance plans, will provide coverage and adequate reimbursement for its use. Regarding the Genio system, only two articles related to the BLAST OSA trial have been published in the European Respiratory Journal and Laryngoscope Investigative Otolaryngology.
The degree of market acceptance of the Genio system and any other product candidates we develop will depend on a number of social, psychological, economic and other factors and concerns, including
| ● | general conservatism about the adoption of new treatment practices and reluctance to switch their patients from existing therapies; |
| ● | personal history of adverse events and severe/serious adverse events; |
| ● | lack or perceived lack of long-term evidence supporting additional patient benefits; |
| ● | perceived liability risks associated with the use of new products and procedures; |
| ● | limited or lack of reimbursement and coverage within healthcare payment systems; |
| ● | costs associated with the purchase of new products and equipment; |
| ● | other procedures competing for physician time and attention; |
| ● | the fact that the Genio system contains an implantable device requiring surgery for implantation; |
| ● | the time commitment that may be required for special training; |
| ● | insufficient level of commercial attractiveness to physicians; |
| ● | the extent of ongoing support required by the clinician; and |
| ● | the extent of ongoing involvement of the patient in therapy. |
17
We may focus our financial and managerial resources on a particular market resulting in a failure to capitalize on markets that may be more profitable or for which there is a greater likelihood of success.
Taking into account our current financial and managerial resources, we will have to carefully prioritize the order in which we address of our target European markets for commercialization of the Genio system, based on parameters such as market size, market readiness, and competition, and then allocate our financial and managerial resources accordingly. In order to identify our primary target markets, we make projections on the number of people by target market. These projections are derived from a variety of sources, including, but not limited to, scientific literature, governmental statistics and market research, and are highly contingent on a number of variables that are difficult to predict and may prove to be too high. If as a result of these or other factors the market for the Genio system does not develop as currently anticipated, our ability to generate revenue could be materially adversely affected. Further, if we use our financial and managerial resources to promote a particular indication expansion that is not ultimately sufficiently commercially successful, this could result in a smaller population of patients who could benefit from the Genio system than we anticipate which would result in lower potential revenue.
Competition from medical device companies and medical device subsidiaries of large healthcare and pharmaceutical companies is intense and expected to increase.
The medical technology industry is highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. Our competitors have historically dedicated and will continue to dedicate significant resources to promoting their products or developing new products or methods to treat moderate to severe OSA. We compete as a second line therapy in the OSA treatment market for patients with moderate to severe OSA.
We consider other companies that have designed hypoglossal nerve stimulation technologies to treat OSA as direct competitors. We are aware of only one currently marketed nerve stimulation device for the treatment of OSA, the Inspire Medical system marketed by Inspire Medical Systems, Inc., and one other nerve stimulation system for the treatment of OSA currently not actively commercialized in Europe from ImThera/ LivaNova PLC. The Inspire Medical system is currently the only neuro stimulation system approved to treat moderate to severe OSA in the United States. Additionally, we also consider, as indirect competition, invasive surgical treatment options such as uvulopalatopharyngoplasty and maxillomandibular advancement surgery and, to a lesser extent, mandibular advancement devices, which are primarily used in the treatment of mild to moderate OSA.
In Europe, the Genio system is CE-Mark certified for use as a second-line therapy in the treatment of moderate to severe OSA in patients who do not tolerate, refused or failed positive airway pressure, or PAP, therapy. If one or more PAP device manufacturers successfully develop a PAP device that is better tolerated and demonstrates significantly higher compliance rates, or if improvements in other second-line therapies make them more effective, cost effective, easier to use or otherwise more attractive than the Genio system, these therapies could have a material adverse effect on our sales, financial condition and results of operations.
Companies against which we compete, directly or indirectly, may have competitive advantages with respect to primary competitive factors in the OSA treatment market, including:
| ● | greater company, product and brand recognition; |
| ● | a more extensive body of clinical data demonstrating product reliability and durability; |
| ● | more effective marketing to and education of patients, physicians and sleep centers; |
| ● | greater product ease of use and patient comfort; |
| ● | more sales force experience and greater market access; |
| ● | better product support and service; |
| ● | more advanced technological innovation, product enhancements and speed of innovation; |
| ● | more effective pricing and revenue strategies; |
| ● | lower procedure costs to patients; |
| ● | more effective reimbursement teams and strategies; |
| ● | dedicated practice development; and |
| ● | more effective clinical training teams. |
18
The commercial availability of any approved competing product could potentially inhibit recruitment and enrollment in our clinical trials. We may successfully conclude our clinical trials and obtain final regulatory authorization or certification, and nevertheless may fail to compete against competitors or alternative treatments that may be available or developed for the relevant indication. Alternative treatments include devices and surgery, as well as potential pharmacological treatments, among others. New treatment options may emerge yielding clinical results better than or equal to those achieved with the Genio system, possibly at a lower cost. Emergence of such new therapies may inhibit our ability to develop and grow the market for the Genio system. Furthermore, new entrants into the markets in which we operate could also decide to more aggressively compete on price, requiring us to reduce prices to maintain market share.
A pandemic, epidemic, or outbreak of an infectious disease, such as the COVID-19 pandemic, could materially and adversely affect our business and our financial results and cause a disruption to our research, development and commercialization efforts.
Public health crises such as pandemics or similar outbreaks could adversely impact our business. Notably, the COVID-19 pandemic continues to evolve. The extent to which COVID-19 impacts our operations or those of our collaborators, vendors and other material business relations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information that will emerge concerning the severity of the virus and the actions to contain it or treat its impact, among others.
While we previously implemented work-from-home policies to support the community efforts to reduce the transmission of COVID-19 and protect employees, these work-from-home policies continue in effect to the degree that we believe to be appropriate, tailored to the role of each team member, and continue to evolve as the specific conditions associated with COVID-19 evolve. We also implemented a number of measures to ensure employee safety and business continuity. While many restrictions in locations in which we have employees or independent contractors have been lifted or continue to be relaxed and phased re-openings were implemented, these restrictions may be re-implemented, or new restrictions imposed if rates or incidence of infection increase.
The spread of COVID-19 could also have adverse economic impacts to us. While the potential economic impact brought by, and the duration of, the COVID-19 pandemic, have been, and continue to be, difficult to assess or predict, the spread of COVID-19 has caused a broad impact globally. The ongoing COVID-19 pandemic continues to evolve. The extent to which the COVID-19 pandemic may impact our business continues to be highly uncertain and cannot be predicted with confidence.
Risks Related to Our Dependence on Third Parties and on Key Personnel
A loss or degradation in performance of the suppliers on which we depend for services and components used in the production and assembly of the Genio system could have a material effect on our business, financial condition and results of operations.
The Genio system requires customized components and services that are currently available from a limited number of sources. If these suppliers decide not to supply, are unable to supply, or if they provide us with components or services of insufficient quality, this could harm our reputation and business by affecting, for example, product availability and performance. Our suppliers might not be able or willing to continue to provide us with the components or services we need, at suitable prices or in sufficient quantity or quality. If any of our existing suppliers is unable or unwilling to meet our demand for components or services, or if the services or components that they supply do not meet quality and other specifications, clinical trials or sales of the Genio system could be delayed or halted, which could prevent us from achieving or maintaining profitability. For instance, we currently rely on a single source supplier for a number of critical components to the Genio system. We are seeking to qualify additional suppliers for certain of our components. The addition of a new supplier to the production process generally requires extensive evaluations, testing and regulatory approval, making it difficult and costly for us to diversify our exposure to single source suppliers. In addition, if we have to switch to a replacement supplier for any of our product components or for certain services required for the production and assembly of the Genio system such as, for example, the sterilization and coating of the product components, or if we have to commence our own manufacturing to satisfy market demand, we may face delays, and the manufacturing and delivery of the Genio system could be interrupted for an extended period of time, which could delay completion of our clinical trials or commercialization and prevent us from achieving or maintaining profitability. Alternative suppliers may be unavailable, may be unwilling to supply, may not have the necessary regulatory approvals or certifications, or may not have in place an adequate quality management system. Furthermore, modifications to a service or component made by a third-party supplier could require new approvals or certifications from the relevant regulatory authorities before the modified service or component may be used.
19
If we are required to change the manufacturer of a critical component of our implant systems, we will be required to verify that the new manufacturer maintains facilities, procedures and operations that comply with our quality specifications and applicable regulatory requirements, which could further impede our ability to manufacture our implant systems in a timely manner. If we encounter demand for our system in excess of our inventory and we need to contract with these additional suppliers, we will face challenges in meeting that demand. Transitioning to a new supplier could be time-consuming and expensive, may result in interruptions in our operations and product delivery, could affect the performance specifications of our implant systems or could require that we modify the design of those systems. If the change in manufacturer results in a significant change to any product, new marketing authorizations or certification from the FDA or similar regulatory authority may be necessary before we implement the change, which could cause substantial delays. The occurrence of any of these events could harm our ability to meet the demand for our products in a timely or cost-effective manner.
In addition, our suppliers may discontinue their supply of components or services upon which we rely before the end of the product life of the Genio system. The timing of a discontinuation may not allow us sufficient time to develop and obtain any regulatory authorizations or certifications as required for replacement components or service before we exhaust our inventory. If suppliers discontinue their supply of components or services, we may have to pay premium prices to our suppliers to keep their production or service lines open or to obtain alternative suppliers, buy substantial inventory to last until the scheduled end of life of the Genio system or through such time as we have an alternative component developed and authorized by the regulatory authorities, or temporarily cease supplying the Genio system once our inventory of the affected component is exhausted.
Any of these interruptions to the supply of services or components could result in a substantial reduction in our available inventory and an increase in our production costs.
We may be unable to attract and retain management and other personnel we need to succeed.
Given our current state of the development, reliance on the expertise and experience of our board of directors, management and other key employees, as well as contractors, in management, engineering, manufacturing, clinical and regulatory matters, sales and marketing, and other functions is crucial. The departure of any of these individuals without timely and adequate replacement or the loss of any of our senior management or other key employees would make it difficult for us to achieve our objectives in a timely manner, or at all. We might not be able to find and attract other individuals with similar levels of expertise and experience or similar relationships with commercial partners and other market participants. In addition, our competitive position could be compromised if a member of senior management transferred to a competitor.
We expect to expand our operations and grow our clinical development, manufacturing, administrative and commercial operations. This will require hiring a number of qualified clinical, scientific, commercial and additional administrative, sales and marketing personnel. Competition for skilled personnel is intense and may limit our ability to hire and retain highly qualified personnel on acceptable terms or at all. Competitors may have greater financial and other resources, different risk profiles and a longer history than we do. If we are unable to identify, attract, retain and motivate these highly skilled personnel, we may be unable to continue our development, commercialization or growth. Failure to retain or attract key personnel could have a material adverse effect on our business, results of operations, cash flows, financial condition and/or prospects.
We rely, or may rely in the future, on third parties to provide critical advice and conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of clinical trials. Third-party performance failure may increase our developments costs, delay granting of regulatory authorizations or certifications or delay or prevent commercialization.
We rely, and may rely in the future, on third parties to conduct certain clinical trials, perform data collection and analysis and provide marketing, manufacturing, regulatory advice and other services that are crucial to our business. In particular, our technology and product development activities or clinical trials conducted in reliance on third parties may be delayed, suspended, or terminated if the third parties do not devote a sufficient amount of time or effort to our activities or otherwise fail to successfully carry out their contractual duties or to meet regulatory obligations or expected deadlines; if we replace a third party; if the quality or accuracy of the data obtained by third parties is compromised due to their failure to adhere to clinical protocols, regulatory requirements, or for other reasons including the loss of data; or if the third party becomes bankrupt or enters into liquidation.
20
We may not always have the ability to control the performance of third parties in their conduct of their activities. Our agreements with these third parties generally allow the third party to terminate the agreement at any time, subject to standard notice terms. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, or agreements with such third parties are terminated for any reason, we would be required to find a replacement third party to conduct the required activities. We may be unable to enter into a new agreement with another third party on commercially acceptable terms, if at all. Furthermore, if the quality or accuracy of the data obtained by the third party is compromised, or if data are otherwise lost, we would be required to repeat the affected trial. Third-party performance failures may therefore increase our development costs, delay our ability to obtain regulatory approval, and delay or prevent the commercialization of the Genio system in target markets. In addition, our third-party agreements usually contain a clause limiting such third party’s liability, such that we may not be able to obtain full compensation for any losses that we may incur in connection with the third party’s performance failures.
Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we design our clinical trials and will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA and other regulatory authorities require us to comply with GCP regulations and international standards relating to the conduct, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties, over which we have limited control, to manage those operations does not relieve us of these responsibilities and requirements. Our failure or any failure by these third parties to comply with these regulations or to recruit a sufficient number of patients may require us to repeat clinical trials, which would delay the marketing authorization or certification process. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws. We also are required to register ongoing clinical trials and post the results of certain completed clinical trials on certain government-sponsored databases, such as ClinicalTrials.gov in the United States, within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties for any reason, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
Performance issues, service interruptions or price increases by our shipping carriers could adversely affect our business and harm our reputation and ability to supply our products on a timely basis.
Expedited, reliable shipping is essential to our operations since the components of the Genio system are manufactured to our specifications by third-party suppliers in various jurisdictions. While the initial assembly of the different electronic components is done by different external suppliers, the final assembly is performed in our facilities in Israel and Belgium. As a result, we rely heavily on providers of transport services for reliable and secure point-to-point transport of the key components of the Genio system to our facility and for tracking of these shipments. Should a carrier encounter delivery performance issues such as loss, damage or destruction of any components, it would be costly to replace such components in a timely manner and such occurrences, if they resulted in delays to the assembly and shipment of the completed Genio system to customers, may damage our reputation and lead to decreased demand for the Genio system and increased cost and expense to our business. In addition, any significant increase in shipping rates could adversely affect our operating margins and results of operations. Similarly, strikes, severe weather, natural disasters or other service interruptions affecting delivery services we use would adversely affect our ability to process orders for the Genio system on a timely basis.
21
Our employees, independent contractors, principal investigators, contract research organizations, consultants or vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, principal investigators, contract research organizations, consultants or vendors may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: FDA regulations, including those laws requiring the reporting of true, complete and accurate information to the FDA; manufacturing standards; federal and state healthcare fraud and abuse laws and regulations; or laws that require the true, complete and accurate reporting of financial information or data. In addition, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws also involve the improper use or misrepresentation of information obtained in the course of clinical trials or creating fraudulent data in our nonclinical studies or clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to the risk that a person could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished potential profits and future earnings, and curtailment of our operations, any of which could adversely affect our business, financial condition, results of operations or prospects.
Risks Related to the Countries in which We Operate
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, the UK Bribery Act 2010, and other anti- corruption laws, as well as export control laws, import and customs laws, trade and economic sanctions laws and other laws governing our business and operations.
Our operations are subject to anti-corruption laws, including the U.S. Foreign Corrupt Practices Act of 1977, or FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. §201, the U.S. Travel Act; the UK Bribery Act 2010, or the Bribery Act; and other anti-corruption laws that apply in countries where we do business. The FCPA, the Bribery Act, and these other laws generally prohibit us and our employees and intermediaries from authorizing, promising, offering, or providing, directly or indirectly, a financial or other advantage to government officials or other persons to induce them to improperly perform a relevant function or activity (or reward them for such behavior). U.S. authorities that enforce the FCPA, including the Department of Justice, deem most healthcare professionals and other employees of foreign hospitals, clinics, research facilities and medical schools in countries with public healthcare or public education systems to be “foreign officials” under the FCPA. When we interact with foreign healthcare professionals and researchers in testing and marketing our products abroad, we must have policies and procedures in place sufficient to prevent us and agents acting on our behalf from providing any bribe, gift or gratuity, including excessive or lavish meals, travel or entertainment in connection with marketing our products and services or securing required permits and approvals such as those needed to initiate clinical trials in foreign jurisdictions. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the maintenance of books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and the development and maintenance of an adequate system of internal accounting controls for international operations. The SEC is involved with the books and records provisions of the FCPA.
We are also subject to other laws and regulations governing our international operations, including regulations administered by the governments of the United Kingdom and the United States, and authorities in the EU, including applicable export control regulations, economic sanctions and embargoes on certain countries and persons, anti-money laundering laws, import and customs requirements and currency exchange regulations.
If we fail to comply with these laws and regulations, we could be subject to governmental investigations, prosecutions and penalties, including substantial fines and potential imprisonment of the individuals involved. Any such circumstances would carry the risk of substantial damage to our reputation for corporate integrity and would likely have a material adverse effect on our business and future prospects.
22
Risks Related to Manufacturing
We may not be able to manufacture or outsource manufacturing of the Genio system in sufficient quantities, in a timely manner or at a cost that is economically attractive.
Our revenue and other operating results will depend, in large part, on our ability to manufacture and sell the Genio system in sufficient quantities and quality, in a timely manner, and at a cost that is economically attractive.
We expect to be required to significantly increase manufacturing volumes as clinical trials on the Genio system are expanded and the Genio system is commercialized. The capacity of our manufacturing facilities in Tel Aviv, Israel, and Milmort, Belgium, along with our contract manufacturer in the United States, is expected to cover the Genio Implantable Stimulator and Genio External Stimulator demand for 2024. Manufacturing of the Genio Activation Chip and the Genio Charging Unit is mostly outsourced to a third party contract manufacturing organization. In order to support future demand for the Genio system, we may need to expand our manufacturing capacity, which could require opening a new facility or additional outsourcing to a third-party contract manufacturing organization. For example, if we obtain regulatory authorization to market the Genio system in the United States we would likely have to significantly increase our manufacturing capabilities in order to satisfy anticipated demand. We expect that this could include opening a manufacturing facility in the United States. Opening a new manufacturing facility could involve significant additional expenses, including for the construction of a new facility, the movement and installation of key manufacturing equipment, the modification of manufacturing processes and for the recruitment and training of new team members. In addition, we must also notify, and in most cases obtain approval from, regulatory authorities regarding any changes or modifications to our manufacturing facilities and processes, and the regulatory authorities might not authorize us to proceed or might delay the process significantly.
In addition, our current business expectation is that the cost of goods sold will decline over time as (i) internal efficiencies increase and (ii) the cumulative volume of Genio systems manufactured grows. However, we or our suppliers might not be able to increase yields and/or decrease manufacturing costs with time, and in fact costs may increase, which could prevent us from achieving or maintaining profitability.
Our results of operations could be materially harmed if we are unable to accurately forecast customer demand for our Genio system and manage our inventory.
To ensure adequate inventory supply of the Genio system in general and its components, we must forecast inventory needs and place orders with our suppliers based on our estimates of future demand for the Genio system and its components. To date, we have only commercialized the Genio system in limited quantities, mostly in Germany, and our ability to accurately forecast demand for our Genio system could be negatively affected by many factors, including failure to accurately manage our expansion strategy, product introductions by competitors, an increase or decrease in customer demand for the Genio system or for products of our competitors, failure to accurately predict customer acceptance of new products, unanticipated changes in general market conditions or regulatory matters, and weakening of economic conditions or consumer confidence in future economic conditions. Inventory levels in excess of customer demand may result in inventory write-downs or write-offs, which would cause our gross margin to be adversely affected and could impair the strength of the Genio brand. Conversely, if we underestimate customer demand for the Genio system, our third-party contract manufacturers may not be able to deliver products to meet our requirements, and this could result in damage to our reputation and customer relationships. In addition, if we experience a significant increase in demand, additional supplies of raw materials or additional manufacturing capacity may not be available when required on terms that are acceptable to us, or at all, or suppliers or third-party manufacturers might not be able to allocate sufficient capacity in order to meet our increased requirements, which could have an adverse effect on our ability to meet customer demand for the Genio system.
We intend to maintain sufficient levels of inventory in order to protect ourselves from supply interruptions. As a result, we will be subject to the risk that a portion of our inventory will become obsolete or expire, which could affect our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory.
23
Risks Related to Legal and Regulatory Compliance Matters
Our products and operations are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business.
Our Genio system is regulated as a medical device in the United States and other jurisdictions. We and our products are subject to extensive regulation in the United States and elsewhere, including by the FDA and its foreign counterparts. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices: design, development and manufacturing; testing, labeling, content and language of instructions for use and storage; clinical trials; product safety; establishment registration and device listing; marketing, sales and distribution; pre-market clearance, classification and approval; recordkeeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; post-market approval trials; and product import and export.
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. The FDA enforces its regulatory requirements through, among other means, periodic announced or unannounced inspections. We do not know whether we or our contract manufacturers will be found substantially compliant with applicable regulations in connection with any future FDA inspections. Failure to comply with applicable regulations could jeopardize our ability to sell the Genio system and any other product candidates, if they obtain marketing authorization, and result in enforcement actions such as: warning letters; fines; injunctions; civil penalties; termination of distribution; recalls or seizures of products; delays in the introduction of products into the market; total or partial suspension of production; refusal to grant future clearances or approvals; withdrawals or suspensions of clearances or approvals, resulting in prohibitions on sales of our products; and in the most serious cases, criminal penalties.
The Genio system is still unapproved in certain significant markets, such as the United States market, and seeking and obtaining regulatory authorization or certification for active implantable medical devices can be a long, expensive and uncertain process.
Applications for prior regulatory authorization in the countries where we intend to sell or market the Genio system and any other products we develop may require extensive non-clinical, clinical and performance testing, all of which must be undertaken in accordance with the requirements of regulations established by the relevant regulatory agencies, which are complex and have become more stringent over time. We may be adversely affected by potential changes in government policy or legislation applicable to implantable medical devices. At the date of this Annual Report, we have received certification to market the Genio system and the Genio 2.1 system in the EU member states through CE-Marking and Israeli Medical Devices and Accessories, or AMAR. CE-Marking is also valid in the European Economic Area, or EEA (which consists of the 27 EU member states plus Norway, Liechtenstein and Iceland).
In the United States, we are in the early stages of seeking FDA marketing authorization. We have received IDE approval from the FDA, which allows us to proceed with our DREAM and ACCCESS clinical trials of the Genio system in the United States, and we are in the process of determining the appropriate regulatory pathway to pursue for seeking marketing authorization for the device from the FDA. Even though we have received approval IDEs, the Genio system may not successfully obtain marketing authorization. In addition, there may be substantial and unexpected delays in the process, for example in the initiation and completion of clinical trial testing and evaluation.
Since the Genio system is a wireless medical device, additional complications may arise with respect to obtaining marketing authorization in the United States. For example, the Federal Communications Commission must also determine that wireless medical devices, such as the Genio system, are compatible with other uses of the spectrum on which the device operates, and that power levels and the frequency spectrum of the wireless energy transfer comply with applicable regulations.
24
Even if we obtain marketing authorization or certification for our product candidates, the terms of such authorizations or certifications and ongoing regulation of our products may limit how we manufacture and market our products. Compliance with such requirements may involve substantial resources, which could materially impair our ability to generate revenue.
Even if marketing authorization, certification or approval of a product candidate is received, commercial products and their manufacturers are subject to ongoing review and extensive regulation, including with respect to the manufacture, medical device reporting, import, export, registration, listing of devices and post-market surveillance of the product. For example, medical device manufacturers must submit periodic reports to the FDA after obtaining marketing authorization. These reports include information about failures and certain adverse events associated with the device after its marketing authorization.
Failure to submit such reports, or failure to submit the reports in a timely manner, could result in enforcement action by the FDA. Following its review of the periodic reports, the FDA might ask for additional information or initiate further investigation. Accordingly, assuming we receive marketing authorization or certification for one or more of our product candidates, we and our contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance, and quality control. If we are not able to comply with post-market regulatory requirements, we could have any marketing authorizations or certifications we have obtained for our products withdrawn by regulatory authorities and our ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Thus, the cost of compliance with post-market regulations may have a negative effect on our operating results and financial condition.
Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA and other regulatory authorities, which may include, among other things: untitled letters or warning letters; fines, injunctions, consent decrees and civil penalties; recalls, termination of distribution, administrative detention, or seizure of our products; customer notifications or repair, replacement or refunds; operating restrictions or partial suspension or total shutdown of production; delays in or refusal to grant our requests for future clearances, De Novo classifications, approvals, certifications or other marketing authorizations of new products, new intended uses, or modifications to existing products; withdrawals or suspensions of any granted marketing authorizations or certifications, resulting in prohibitions on sales of our products; or criminal prosecution. Any of these sanctions could have a material adverse effect on our reputation, business, financial condition and results of operations.
In addition, the FDA may change its marketing authorization policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay marketing authorization of any product candidate under development or impact our ability to modify any products authorized for market on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain marketing authorizations, increase the costs of compliance or restrict our ability to maintain any marketing authorizations we have obtained.
Failure to comply with the significant regulations and approvals to which our manufacturing facilities and those of our third-party suppliers are subject to may affect our business.
We currently manufacture the Genio system and have entered into relationships with third-party suppliers to manufacture and supply certain components of the Genio system. Our manufacturing practices and the manufacturing practices of our third-party suppliers are subject to ongoing regulation and periodic inspection. In the United States, the methods used in, and the facilities used for, the manufacture of medical devices must comply with the FDA’s Quality System Regulation, or QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, and servicing of medical devices. Furthermore, we will be required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality standards and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors. The Genio system is also subject to similar state regulations and various laws and regulations of other countries governing manufacturing.
Any failure to follow and document the adherence to regulatory requirements (including having in place an adequate quality management system in line with the most up-to-date standards and regulations) by us or our third-party suppliers may lead to significant delays in the availability of the Genio system for commercial sale or clinical trials, may result in the termination of or a hold on a clinical trial, or may delay or prevent filing or approval or maintenance of marketing applications for the Genio system.
25
In the United States, the FDA and other federal and state agencies, including the U.S. Department of Justice, closely regulate compliance with all requirements governing medical device products, including requirements pertaining to marketing and promotion of devices in accordance with the provisions of the approved labeling and manufacturing of products in accordance with cGMP requirements. Violations of such requirements may lead to investigations alleging violations of the FDCA and other statutes, including the False Claims Act and other federal and state healthcare fraud and abuse laws as well as state consumer protection laws. Our failure to comply with all regulatory requirements, and later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, may yield various results, including:
| ● | litigation involving patients using our products; |
| ● | restrictions on our products, manufacturers or manufacturing processes; |
| ● | restrictions on the labeling or marketing of a product; |
| ● | restrictions on product distribution or use; |
| ● | requirements to conduct post-marketing studies or clinical trials; |
| ● | untitled or warning letters; |
| ● | fines, restitution or disgorgement of profits or revenues; |
| ● | consent decrees; |
| ● | total or partial suspension or clinical hold of one or more of our clinical trials; |
| ● | total or partial suspension or withdrawal regulatory approvals; |
| ● | total or partial suspension of production or distribution; |
| ● | delay of or refusal to approve pending applications or supplements to approved applications or to provide future market authorizations, certifications or approvals; |
| ● | mandatory communications with physicians and other customers about concerns related to actual or potential safety, efficacy, and other issues involving us; |
| ● | withdrawal of the products from the market; |
| ● | mandatory product recalls or seizure of products; |
| ● | damage to relationships with any potential collaborators; |
| ● | unfavorable press coverage and damage to our reputation; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
Any of the foregoing actions could be detrimental to our reputation or result in significant costs or loss of revenues. Any of these actions could significantly and negatively affect supply of the Genio system, if authorized for sale by the FDA. If any of these events occurs, we could be exposed to product liability claims and we could lose customers and experience reduced sales and increased costs.
Our ability to continue sales of our product in the EU may be materially impaired if we do not take necessary steps to comply with the certification requirements of the new EU Medical Devices Regulation.
On May 25, 2017, the EU Medical Devices Regulation 2017/745, or the MDR, entered into force, repealing and replacing Council Directive 93/42/EEC, or the Medical Devices Directive, and Council Directive 90/385/EEC, or the AIMD Directive. Unlike directives, which must be implemented into the national laws of the EU member states, regulations are directly applicable (i.e., without the need for adoption of EU member state laws implementing them) in all EU member states from their effective applicability date and are intended to eliminate differences in the regulation of medical devices among EU member states. The MDR, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EU for medical devices and ensure a high level of safety and health while supporting innovation.
26
The MDR became effective on May 26, 2021. Devices lawfully placed on the market pursuant to the EU Medical Devices Directive, or MDD, and the AIMD Directive, or AIMDD, prior to May 26, 2021 could initially continue to be made available on the market or put into service until May 26, 2025. Nevertheless, the European Parliament very recently adopted legislation to extend this transitional period to give manufacturers more time to switch from the previously applicable provisions to the new certification requirements for medical devices as laid down by the MDR. For high risk, class III and class IIb implantable devices the transitional period is extended until December 31, 2027. For medium and low risk, class IIb devices and class IIa, Im, Is and Ir devices the transition period is extended until December 31, 2028. The MDR among other things:
| ● | Strengthens the rules on placing devices on the market and reinforces surveillance once they are available; |
| ● | Establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| ● | Improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| ● | Sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and |
| ● | Strengthens the rules for the assessment of certain high-risk devices, which may have to undergo an additional check by experts before they are placed on the market. |
These modifications may have an effect on the way we design and manufacture product and products candidates and conduct our business in the EU and EEA. For example, as a result of the transition towards the new regime, Notified Body review times have lengthened, and product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business. While our Genio system has been certified under the AIMDD and can therefore remain on the EU market until the extended deadline of December 31, 2027, we are evaluating the implementation of the new requirements of the MDR. We cannot exclude unexpected regulatory hurdles and possible delays while transitioning towards the new regime.
The EU-UK Trade and Cooperation Agreement, or TCA, came into effect on January 1, 2021. The TCA does not specifically refer to medical devices. However, as a result of Brexit, the MDR will not be implemented in the UK, and previous legislation that mirrored the MDR in the UK law has been revoked. The regulatory regime for medical devices in the UK will continue to be based on the requirements derived from current EU legislation, and the UK may choose to retain regulatory flexibility or align with the MDR going forward. CE-markings will continue to be recognized in the UK, and certificates issued by EU-recognized Notified Bodies will be valid in the UK, until the earlier of June 30, 2028 or the expiration of the certificate for devices compliant with the MDD or AIMDD or until June 30, 2030 for devices compliant with the MDR. For medical devices placed on the UK market after this period, the UK Conformity Assessed, or UKCA, marking will be mandatory. In contrast, UKCA marking and certificates issued by UK Notified Bodies will not be recognized on the EU market. The TCA does provide for cooperation and exchange of information in the area of product safety and compliance, including market surveillance, enforcement activities and measures, standardization related activities, exchanges of officials, and coordinated product recalls (or other similar actions). For medical devices that are locally manufactured but use components from other countries, the “rules of origin” criteria will need to be reviewed. Depending on which countries products will be ultimately sold in, manufacturers may start seeking alternative sources for components if this would allow them to benefit from no tariffs. The rules for placing medical devices on the Northern Ireland market will differ from those in the UK. These modifications may have an effect on the way we design and manufacture products and we conduct our business in these countries.
Seeking, obtaining and maintaining certification in the EU under the MDR, with the CE-Mark to be re-certified before December 31, 2027, can be an uncertain process and Notified Bodies have limited resources and may experience backlogs.
Devices such as our Genio system currently on the market in the EU that have been granted a CE-Mark under the AIMD Directive, will need to be re-evaluated and re- certified in accordance with the MDR. Any modification to an existing CE-Marked medical device will also require review and certification under the MDR. Under normal circumstances, medical device manufacturers must undergo on-site audits by Notified Bodies in order to maintain their CE-Mark certifications per the requirements of the EU Medical Devices Directive. As many CE-Mark certifications will become void as part of the transition to the MDR, Notified Bodies also have to start certifying medical devices in accordance with the MDR.
27
The MDR also requires a re-designation of the Notified Bodies, the organizations designated by the EU member state in which they are based that are responsible for assessing whether medical devices and manufacturers of medical devices meet the applicable regulatory requirements in the EU. To be re-designated, Notified Bodies must demonstrate increased technical expertise in their scope of designation, as well as improved quality management systems. This re-designation process has caused backlogs in the assessment of medical devices and medical device manufacturers during the transition period leading up to May 26, 2021, the effective date of the MDR. In the European Union, currently 42 Notified Bodies have been re-designated, including one for Belgium.
To be able to continue to place our Genio device on the EU market, if we decide to do so, the CE-Mark obtained in 2019 for our Genio system will have to be re-certified under the MDR before the extended deadline of December 31, 2027. To benefit from the extended transitional period, the manufacturer or its authorized representative need to have submitted an application for MDR certification by May 26, 2024 and needs to have signed a written proposal/agreement with the Notified Body by September 26, 2024. The re-certification requires us to present documentation and other evidence demonstrating that the performance and the safety of the system has been maintained and that the system continues to meet existing regulations and standards. Otherwise, the marketing and sale of the Genio system in EU member states may be temporarily or permanently prohibited. Significant modifications to the Genio system, if any, will require certification under the MDR and cannot be implemented during the transition period from AIMDD to MDR.
The overall backlogs experienced by the Notified Bodies having already been re-designated (including the Dutch company DEKRA Certification B.V., which issued the CE-Mark and an ISO 13485:2016 certificate to us under the AIMD Directive) might have a negative impact on the re-certification of the Genio system. We believe, however, that we are on track to meet the new requirements by the deadlines set forth in the MDR.
Any third-party entities that we rely upon for distribution of our products in the EU, such as our local distributor in Spain, also need to be compliant with the MDR. If a distributor in the EU fails to meet the MDR requirements, on a timely basis or at all, the marketing and sale of our Genio products by such distributor may be temporarily or permanently prohibited.
Any delay or failure to comply with the MDR could result in the sale of our Genio products being temporarily or permanently prohibited in EU member states and affect our reputation, business, financial condition, results of operations and prospects.
Compliance with regulations for quality systems for medical device companies is difficult, time consuming and costly.
We have developed and maintains a quality management system for medical devices intended to ensure quality of our products and activities. The system is designed to be in compliance with regulations in many different jurisdictions, including the QSR mandated by the FDA in the United States and the requirements of the AIMD Directive in the European Union, including the international standard ISO13485 required by the member states in Europe that recognize the CE-Mark, as well as Israel, New Zealand and Australia. The FDA issued a Notice of Proposed Rulemaking in February 2022 describing revisions to the QSR to harmonize it with ISO13485. However, it is not clear when the FDA plans to issue a Final Rule to implement the harmonized regulations.
Compliance with regulations for quality management systems for medical device companies is time consuming and costly, and there are changes in such regulations from time to time. For example, the latest version of ISO13485, ISO13485:2016, aims to harmonize the requirements of ISO13485 with the requirements of the AIMD Directive. While management believes that we are compliant with existing quality management system regulations for medical device companies as of the date of this Annual Report, it is possible that we may be found to be noncompliant with new or existing regulations in the future. In addition, we may be found to be noncompliant as a result of future changes in, or interpretation of, the regulations for quality systems. If we do not achieve compliance or subsequently become noncompliant, the regulatory authorities may require that we take appropriate action to address non- conformance issues identified in a regulatory audit, and may, if we do not take such corrective actions in a timely manner, withdraw marketing clearance, or require product recall or take other enforcement action.
Our external vendors must, in general, also comply with the quality systems regulations and ISO13485. Any of our external vendors may become noncompliant with quality systems regulations or ISO13485, which could result in enforcement action by regulatory authorities, including, for example a warning letter from the FDA or a requirement to withdraw from the market or suspend distribution, or export or use of products manufactured by one or more of our vendors.
28
Any change or modification to a device (including changes to the manufacturing process) may require supplemental filings to regulatory authorities or new submissions for marketing authorization or certification (depending on the jurisdiction) and must be made in compliance with appropriate quality system regulations (such as the QSR for the United States and the AIMD Directive and the MDR for Europe), which may cause interruption to or delays in the marketing and sale of our products. Regulations and laws regarding the manufacture and sale of AIMDs are subject to future changes, as are administrative interpretation and policies of regulatory agencies. If we fail to comply with such laws and regulations where we would intend to market the Genio system, we could be subject to enforcement action including recall of our device, withdrawal of approval, authorization, certification or clearance and civil and criminal penalties. If any of these events occur, it may materially and adversely affect our business, financial condition, results of operations and prospects.
Active implantable medical devices such as the Genio system carry risks associated with the surgical procedure for implant or removal of the device, use of the device, or the therapy delivered by the device.
The Genio system is a medical device with complex electronic circuits and software and includes a component that is implanted in the patient through a surgical procedure. It is not possible to design and build electronic implantable medical devices that are 100% reliable, since all electronic devices carry a risk of failure. Furthermore, all surgical procedures carry risks, and the effectiveness of any medical therapy varies between patients. The consequences of failure of the Genio system include complications arising from product use and associated surgical procedures and could range from minor to life-threatening effects and even death.
All medical devices have associated risks. Regulatory authorities regard active implantable medical devices, or AIMDs, as the highest risk category of medical devices and, accordingly, AIMDs are subject to a high level of scrutiny when seeking regulatory approval or other marketing authorization. The Genio system was reviewed, classified and the certificate of conformity as an AIMD was issued by our European Notified Body allowing us to affix the CE-Mark. A CE-Mark in Europe indicates that the device in question is in full compliance with European legislation. Medical devices authorized for marketing in the European Union need to comply with the essential requirements laid down in the AIMD Directive and in particular to demonstrate that they are designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users and others (that the potential benefits outweigh potential risks). In addition, medical devices must achieve the performance intended by the manufacturer and be designed, manufactured, and packaged in a suitable manner. Devices authorized first in the European Union may be associated with an increased risk of post-marketing safety alerts and recalls. On the other hand, before FDA premarket approval of a medical device in the United States, a device must be shown to be safe and effective per its intended use. The risks associated with medical devices and the therapy delivered by them, include, among others, risks associated with any surgical procedure, such as infection, allergic reaction, and consequences of anesthesia and risks associated with any implantable medical device such as device movement, electromagnetic interference, device failure, tissue damage including nerve damage, pain and psychological side effects associated with the therapy or the surgical procedure.
Adverse events associated with these risks may lead some patients to blame us, the physician or other parties for such occurrences. This may result in product liability lawsuits, medical malpractice lawsuits, investigations by regulatory authorities, adverse publicity, criminal charges or other harmful circumstances for us. Any of those circumstances may have a material adverse effect on our ability to conduct our business, to continue selling the Genio system, to achieve revenue objectives, or to develop future products.
If our products are defective, or otherwise pose safety risks, the relevant governmental authorities could require their recall, or we may need to initiate a recall of our products voluntarily.
AIMDs are characterized by a complex manufacturing process, requiring adherence to demanding product specifications. The Genio system uses many disciplines including electrical, mechanical, software, biomaterials, and other types of engineering. Device failures discovered during the clinical trial phase may lead to suspension or termination of the trial. In addition, device failures and malfunctions may result in a recall of the product, which may relate to a specific manufacturing lot or may affect all products in the field. Recalls may occur at any time during the life cycle of a device after regulatory authorization has been obtained for the commercial distribution of the device. For example, engineers employed by us undertaking development or manufacturing activities may make an incorrect decision or make a decision during the engineering phase without the benefit of long-term experience, and the impact of such wrong decisions may not be felt until well into a product’s life cycle.
29
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. Product defects or other errors may occur in the future.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new marketing authorizations for the device before we may market or distribute the corrected device. Seeking such authorizations may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties or civil or criminal fines.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could also harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
Recalls of the Genio system would divert managerial and financial resources and could result in damaged relationships with regulatory authorities and lead to loss of market share to competitors. In addition, any product recall may result in irreparable harm to our reputation. Any product recall could impair our ability to produce products in a cost-effective and timely manner in order to meet customer demand. We may also be required to bear other costs or take other actions that may have a negative impact on future revenue and could prevent us from achieving or maintaining profitability.
The misuse or off-label use of our product candidates may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
Any marketing authorization or certification we may receive for our Genio system or other product candidates will be limited to specified indications for use, and we must also comply with requirements concerning advertising and promotion of the system. Promotional communications with respect to medical devices are subject to a variety of legal and regulatory restrictions and must be consistent with the device’s intended use, data from any clinical trials, and established specifications. Thus, we will not be able to promote the Genio system for indications or uses for which they are not authorized. We plan to train our marketing personnel and direct sales force not to promote the Genio system for uses outside of the authorized indications for use, known as “off-label uses.” We cannot, however, prevent a physician from using our devices off-label, when in the physician’s independent professional medical judgment he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use our devices off-label, which could harm our reputation in the marketplace among physicians and patients.
If the FDA or any other regulatory authority determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance or imposition of a warning letter or an untitled letter (which is used to notify regulated entities of violations that do not necessitate a warning letter) injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or other enforcement authorities might take action under other regulatory authority, such as false claims laws, if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment of our operations.
30
In addition, physicians may misuse our products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If our devices are misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizeable damage awards against us that may not be covered by insurance.
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing and marketing of medical devices. The Genio system is designed to be implanted in the body and to affect important bodily functions and processes. As with any other complex medical device, there exists the reasonable certainty that, over time, one or more components of some Genio systems will malfunction. As a medical device manufacturer, we are exposed to the product liability claims arising from the Genio system failures and malfunctioning, product use and associated surgical procedures. This risk exists even if the Genio system is certified or authorized for commercial sale by regulatory authorities or Notified Bodies and manufactured in facilities licensed and regulated by the applicable regulatory authority or Notified Body. The medical device industry has historically been subject to extensive litigation over product liability claims, and we may face product liability suits if the Genio system causes, or merely appears to have caused, patient injury or death. In addition, an injury that is caused by the activities of our suppliers, such as those who provide us with components and raw materials, may be the basis for a claim against us. Product liability claims may be brought against us by patients, healthcare providers or others selling or otherwise being exposed to the Genio system, among others. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in one or more of the following:
| ● | costs of litigation; |
| ● | distraction of management’s attention from our primary business; |
| ● | the inability to commercialize the Genio system or new products; |
| ● | decreased demand for the Genio system; |
| ● | damage to our reputation; |
| ● | product recalls or withdrawals from the market; |
| ● | withdrawal of clinical trial participants; |
| ● | substantial monetary awards to patients or other claimants; or |
| ● | loss of sales. |
While we may attempt to manage our product liability exposure by proactively recalling or withdrawing from the market any defective products, any recall or market withdrawal of our products may delay the supply to our customers and may impact our reputation. We may not be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future and these efforts may not have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Such recalls and withdrawals may also be used by our competitors to harm our reputation for safety or be perceived by patients as a safety risk when considering the use of our products, either of which could have a material adverse effect on our business, financial condition and results of operations.
Although we maintain product liability and clinical trial liability insurance at levels we believe are appropriate, this insurance is subject to deductibles and coverage limitations. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, coverage may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at an acceptable cost or on acceptable terms or otherwise protect against potential product liability claims, we could be exposed to significant liabilities, including claims for amounts in excess of insured liabilities. As of the date of the Annual Report, there are no product liability claims against us.
31
We bear the risk of warranty claims on the Genio system.
We bear the risk of warranty claims on the Genio system. We may not be successful in claiming recovery under any warranty or indemnity provided to us by our suppliers or vendors in the event of a successful warranty claim against us by a customer, and any such recovery from a vendor or supplier may be inadequate to fully compensate us. In addition, warranty claims brought by our customers related to third-party components may arise after our ability to bring corresponding warranty claims against such suppliers expires, which could result in costs to us. As of the date of the Annual Report, there are no warranty claims against us.
We are and will be subject to healthcare fraud and abuse laws and other laws applicable to our business activities and if we are unable to comply with such laws, we could face substantial penalties.
We are subject to various federal, state and local laws pertaining to healthcare fraud and abuse laws, including anti-kickback, false claims and transparency laws. Many EU member states have adopted specific anti-gift statutes that further limit commercial practices for medical devices, in particular vis-à-vis healthcare professionals and organizations. Additionally, there has been a recent trend of increased regulation of payments and transfers of value provided to healthcare professionals or entities. In addition, many EU member states have adopted national “Sunshine Acts” which impose reporting and transparency requirements (often on an annual basis) on medical device manufacturers, similar to the requirements in the United States. For instance, pursuant to the Belgian Act of December 18, 2016 and its implementing Royal Decree of June 14, 2017, which entered into force on June 23, 2017, manufacturers of medical devices are required to document and disclose all direct or indirect premiums and benefits granted to healthcare professionals, healthcare organizations and patient organizations with a practice or a registered office in Belgium. Also, under Article 10 of the Belgian Act of March 25, 1964, it is prohibited (subject to limited exceptions) in the context of the supply of medical devices to offer or grant any advantage or benefit in kind to amongst others healthcare professionals and healthcare organizations. In addition, certain countries also mandate implementation of commercial compliance programs.
Healthcare laws and regulations in the United States may constrain the business or financial arrangements and relationships through which we research, market, sell and distribute any products for which we obtain marketing approval. The healthcare laws and regulations that may affect our ability to operate include, but are not limited to:
| ● | the U.S. federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
| ● | the U.S. federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment by a federal government program, or making a false statement or record material to payment of a false claim or avoiding, decreasing or concealing an obligation to pay money to the federal government. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti- Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. Private individuals can bring False Claims Act “qui tam” actions, on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in amounts paid by the entity to the government in fines or settlement; |
| ● | the U.S. federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including in some circumstances mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| ● | the U.S. federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
32
| ● | the U.S. federal transparency requirements under the Physician Payments Sunshine Act require manufacturers of FDA-authorized drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to the Department of Health and Human Services information related to payments and other transfers of value to physicians, certain advanced non-physician healthcare practitioners, and teaching hospitals as well as ownership and investment interests held by physicians and their immediate family members; and |
| ● | analogous foreign and state laws and regulations such as state anti-kickback and false claims laws and analogous non-U.S. fraud and abuse laws and regulations, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require medical device companies to comply with the device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring device manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. State and non-U.S. laws, including the EU General Data Protection Regulation, or GDPR, also govern the privacy and security of health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices, including our financial arrangements with physicians, some of whom receive compensation in the form of stock options, which could be viewed as influencing the purchase of or use of our products in procedures they perform and may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations.
Any action brought against us for violations of these laws or regulations, even if successfully defended, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. We may be subject to private qui tam actions brought by individual whistleblowers on behalf of the federal or state governments, with potential liability under the federal False Claims Act including mandatory treble damages and significant per-claim penalties. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Any of the foregoing consequences will negatively affect our business, financial condition and results of operations.
Healthcare policy changes, including legislation or regulations aiming to reform the U.S. healthcare system, could harm our business, financial condition and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. Current and future legislative proposals to further reform healthcare or reduce healthcare costs may limit coverage of or lower reimbursement for the procedures associated with the use of our product candidates, if authorized for marketing. The cost containment measures that payors and providers are instituting and the effect of any healthcare reform initiative implemented in the future could impact our revenue from the sale of our products.
In December 2022, the U.S. Congress enacted the Consolidated Appropriations Act for 2023, an omnibus appropriations bill, which included amendments to the FDCA under the Food and Drug Omnibus Reform Act of 2022, or FDORA. In addition to the requirement that sponsors of pivotal trials submit diversity action plans for pivotal trials (see “Government Regulation—Regulatory Landscape in the United States—Device Clinical Studies”), FDORA included new requirements for cyber devices, defined as any medical device that is or includes software that is validated, installed, or authorized by the manufacturer; can connect to the internet; and may be vulnerable to cybersecurity threats. Under the FDORA amendments to the FDCA, any application for marketing authorization of the cyber device must include a software bill of materials and a cybersecurity plan describing the methods by which the manufacturer will monitor, identify and address cybersecurity vulnerabilities. Any failure by a cyber device manufacturer to comply with applicable cybersecurity requirements is considered a violation of the FDCA and will subject the manufacturer to enforcement actions and possibly legal sanctions.
33
We expect additional state and federal healthcare policies and reform measures to be adopted in the future, any of which could limit reimbursement for healthcare products and services or otherwise result in reduced demand for our product candidates, if approved, or additional pricing pressure and have a material adverse effect on our industry generally and on our customers. We cannot predict what other healthcare programs and regulations will ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the United States may negatively affect our business, financial condition and results of operations. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare may adversely affect:
| ● | our ability to set a price that we believe is fair for the Genio system; |
| ● | our ability to generate revenue and achieve or maintain profitability; and |
| ● | the availability of capital. |
Any changes of, or uncertainty with respect to, future coverage or reimbursement rates could affect demand for our product candidates, if approved, which in turn could impact our ability to successfully commercialize our device and could have a material adverse effect on our business, financial condition and results of operations.
We are subject to, or may in the future become subject to, federal, state, and foreign laws and regulations imposing obligations on how we collect, store, use and process information collected from or about patients or their procedures using our products. Our actual or perceived failure to comply with such obligations could harm our business. Ensuring compliance with such legal requirements could also impair our efforts to maintain and expand our customer base, and thereby decrease our revenue.
The collection and use of personal health data in the European Union and European Economic Area, or EEA, is governed by the GDPR. Since we are located in the European Union, we are subject to the GDPR when we process personal data from anywhere in the world for purposes of our business in the EEA. The territorial reach of the GDPR also includes the activities of businesses located outside of the EEA that relate to the businesses’ provision of goods or services to residents in the EEA, or monitoring the behavior of people in the EEA. We are therefore also subject to the GDPR even where our data processing activities take place outside of the European Union and relate only to our business outside of the European Union to the extent that such activities involve the personal data of individuals located in the European Union and relate to our offer of goods or services to them, or to our monitoring of their behavior. The GDPR imposes strict requirements on controllers and processors of personal data, including special protections for “sensitive personal data” which includes health and genetic information of data subjects and we may be required to put in place additional mechanisms to ensure compliance with the new data protection rules This may be onerous and may interrupt or delay our development activities, and adversely affect our business, financial condition, results of operations and prospects.
The GDPR also regulates the transfer of personal data subject to the GDPR to so-called third countries that have not been found by the European Commission to provide an adequate level of data protection, including the United States. As from 2020, legal developments in Europe have created complexity and uncertainty regarding such transfers.
34
For instance, on July 16, 2020, the Court of Justice of the European Union, or CJEU, invalidated, by means of the so-called Schrems II-judgment, the EU-U.S. Privacy Shield Framework, or the Privacy Shield, under which personal data could be transferred from the EEA to U.S. entities who had self-certified under the Privacy Shield scheme. While the CJEU upheld the adequacy of the standard contractual clauses (a standard form of contract approved by the European Commission as an adequate third country personal data transfer mechanism and potential alternative to the Privacy Shield), it made clear that reliance on such clauses alone may not necessarily be sufficient in all circumstances. The impact of the Schrems II-judgment is thus not confined to US personal data transfers, but to any transfer of personal data to third countries that are not deemed by the European Commission to provide for an adequate level of data protection. Both the EU controller (we) and the third country recipient need to verify whether the destination country’s laws will allow compliance with the GDPR, the transfer tool itself and also the EU Charter on Fundamental Rights (essentially equivalent level of protection to that guaranteed within the EU by the GDPR). If this is not the case, notably if third country mass surveillance legislation leads in practice (case by case analysis taking into account all relevant aspects to evaluate whether the mass surveillance legislation will apply and the impact thereof) to noncompliance, then we need to assess whether this can be remedied by supplementary measures (organizational, technical (encryption, pseudonymization) and contractual). The European Data Protection Board, or EDPB, regrouping all data protection authorities in the EU, has issued guidance in this respect; see EDPB Opinion 01/2020). If not, such transfer should be suspended or ended. If we are however intending to keep transferring personal data despite this conclusion, then we must notify the competent data protection authority. Nevertheless, there have been recent developments towards more certainty in respect of EU-US personal data transfers. On July 10, 2023, the European Commission adopted an adequacy decision for a new mechanism for transferring data from the EU to the United States – the EU-US Data Privacy Framework, which provides EU individuals with several new rights, including the right to obtain access to their data, or obtain correction or deletion of incorrect or unlawfully handled data, and allows US companies to self-certify to the U.S. Department of Commerce their compliance with a set of agreed privacy principles in order to freely receive EU personal data. The adequacy decision followed the signing of an executive order introducing new binding safeguards to address the points raised in the Schrems II-judgment. Notably, the new obligations were geared to ensure that data can be accessed by U.S. intelligence agencies only to the extent necessary and proportionate and to establish an independent and impartial redress mechanism to handle complaints from Europeans concerning the collection of their data for national security purposes. The European Commission will continually review developments in the United States along with its adequacy decision. Adequacy decisions can be adapted or even withdrawn in the event of developments affecting the level of protection in the applicable jurisdiction. On a general note, failure to comply with the GDPR could result in penalties for noncompliance (including possible fines of up to the greater of €20 million and 4% of our global annual turnover for the preceding financial year for the most serious violations, as well as the right to compensation for financial or non-financial damages claimed by individuals under Article 82 of the GDPR). If any of these events were to occur, our business and financial results could be significantly disrupted and adversely affected
In addition, the GDPR provides that European Union member states may further “implement” the GDPR in certain areas; in respect of the processing of genetic, biometric or health data, the GDPR for instance allows for member states to maintain or introduce further conditions, including limitations, which leads to additional uncertainties. By means of example, the Belgian legislator has made use of this option and makes the processing of such types of personal data subject to additional requirements (Art. 9 of the GDPR Act of 30 July 2018).
In addition to the GDPR, the European Commission has another draft regulation in the approval process that focuses on a person’s right to conduct a private life. The proposed legislation, known as the Regulation on Privacy and Electronic Communications, or ePrivacy Regulation, would replace the current ePrivacy Directive. While the text of the ePrivacy Regulation is still under development, the 2019 Planet49 - judgment of the Court of Justice of the European Union and regulators’ recent guidance and increased enforcement activity are driving increased attention to cookies and other tracking technologies. Some regulators have started to enforce the strict approach in recent guidance; the Belgian Data Protection Authority for instance has been proactively looking for cookie infringements on (press) websites and placed those websites under scrutiny in recent case law. Moreover, the EDPB has published the Cookie Banner Taskforce Report, which identifies common minimum thresholds for the data protection authorities in relation to cookies. Compliance with existing and future rules concerning cookies and other tracking technologies could lead to substantial costs, require significant systems changes, limit the effectiveness of our marketing activities, divert the attention of our technology personnel, adversely affect our margins, increase costs and subject us to additional liabilities. Regulation of cookies and similar technologies may lead to broader restrictions on our marketing and personalization activities and may negatively impact our efforts to understand users.
35
Further, in March 2017, the United Kingdom formally notified the European Council of its intention to leave the European Union pursuant to Article 50 of the Treaty on European Union. The United Kingdom ceased to be a European Union Member State on January 31, 2020, but enacted a Data Protection Act substantially implementing the GDPR, effective in May 2018, which was further amended to align more substantially with the GDPR following Brexit. Currently, the data protection laws of the UK and the EU remain closely aligned, which means that the UK also requires additional analysis of local laws and additional measures for transfers of personal data out of the UK to countries (including the U.S.) that have not been deemed by the UK to have adequate data protection laws. In relation to UK-EEA personal data transfers, these can freely continue provided that on the one hand the UK has deemed that the EEA has adequate data protection laws (i.e. we can freely transfer personal data from the U.K. to our business in Belgium or elsewhere in the EEA) and on the other hand the European Commission adopted on 28 June 2021 an adequacy decision for the UK. The adequacy decision does however have a limited duration of four years, so that we need to re-evaluate our transfers from the EEA to the UK in 2025 (or earlier if the European Commission intervenes following a lowering of the level of data protection in the UK. For the period between 31 January 2020 (date the UK left the EU) until 28 June 2021, transfers of personal data from the EEA to the UK could continue unrestricted by virtue of the temporary Trade and Cooperation Agreement. We are required to comply with both the GDPR and the UK GDPR, with each regime having the ability to fine up to the greater of €20 million (in the case of the GDPR) or £17,5 million (in the case of the UK GDPR) and 4% of total annual revenue. We may need to appoint a local representative in the UK, and incur other additional costs and risks as a result of the UK and the EU having separate data protection regimes.
In addition, in the conduct of our business, we may at times process personal data, including health-related personal data. When conducting clinical trials, we face risks associated with collecting trial participants’ data, especially health data, in a manner consistent with applicable laws and regulations. In the EU and the UK, certain guidance issued by the organization representing the national data protection supervisory authorities may conflict with the requirements or guidelines of the entities that oversee clinical trials, creating uncertainty, increased compliance costs and potential delays in the process of gaining approval to conduct our clinical trials.
We also face risks inherent in handling and in protecting the security of personal data, including health- related data. In addition to specific healthcare laws and regulations, the U.S. federal government and various states have adopted or proposed laws, regulations, guidelines, and rules with respect to the collection, distribution, use, and storage of personal information of patients. For example, HIPAA imposes requirements on certain healthcare providers, health plans and healthcare clearinghouses, or Covered Entities, as well as their business associates that perform services for them that involve the use or disclosure of individually identifiable health information, called Protected Health Information, or PHI, under HIPAA, relating to the privacy and security of PHI, including the use of mandatory contractual terms, or Business Association Agreements, in some circumstances, as well as privacy and security standards and breach notification requirements. Failure to comply with the HIPAA privacy and security standards can result in significant civil monetary penalties and, in certain circumstances, criminal penalties. HIPAA also imposes penalties on third parties that wrongfully obtain PHI. State attorneys general can also bring a civil action to enjoin a HIPAA violation or to obtain statutory damages on behalf of residents of his or her state.
In addition, state privacy and security laws and regulations vary from state to state, constantly evolve, and remain subject to significant change. In some cases, such laws and regulations can impose more restrictive requirements than HIPAA and other U.S. federal laws, thus complicating compliance efforts. By way of example, California and Virginia have enacted significant privacy laws that give residents of those states expanded rights to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. New privacy laws will also become effective in Florida, Montana and Texas in 2024, in Tennessee and Iowa in 2025, and in Indiana in 2026 and numerous other states are considering new privacy laws. Furthermore, other U.S. states, such as New York, Massachusetts, and Utah have enacted stringent data security laws and numerous other states have proposed similar privacy laws. Failure to comply with these state privacy laws could result in penalties and present unresolved compliance issues. In addition, the enactment of a U.S. federal privacy law is possible. The changing number of U.S. state or federal privacy laws may increase our compliance costs and potential liability. New privacy and data security laws have been proposed in more than half of the states in the U.S. and in the U.S. Congress, reflecting a trend toward more stringent privacy legislation in the U.S., which trend may accelerate with increasing concerns about individual privacy. The existence of comprehensive privacy laws in different states in the country will make our compliance obligations more complex and costly and may require us to modify our data processing practices and policies and to incur substantial costs and potential liability in an effort to comply with such legislation. In addition to fines and penalties that may be imposed for failure to comply with state law, some states also provide for private rights of action to patients for misuse of or unauthorized access to personal information.
36
We are not subject to HIPAA, but our customers, research collaborators and others in the United States with whom we do business are. Accordingly, we must ensure that any business arrangements that we have with Covered Entities are structured to comply with HIPAA and ensure that we have the authority to obtain any PHI that may be disclosed to us. Some countries also are considering or have enacted legislation requiring local storage and processing of data that could increase the cost and complexity of delivering our services. Any actual or perceived failure by us or the third parties with whom we work to comply with privacy or security laws, policies, legal obligations, or industry standards, or any security incident that results in the unauthorized release or transfer of PHI, may result in governmental enforcement actions and investigations by U.S. federal and state regulatory authorities, fines and penalties, claims, litigation, and/or adverse publicity, including by consumer advocacy groups and other private parties, and could cause our customers, their patients and other healthcare professionals to lose trust in us, which could harm our reputation and have a material adverse effect on our business, financial condition, and results of operations.
In addition, many jurisdictions outside of Europe are also considering and/or enacting comprehensive data protection legislation. For example, as of August 2020, the Brazilian General Data Protection Law imposes stringent requirements similar to GDPR with respect to personal information collected from individuals in Brazil.
In China, there have also been recent significant developments concerning privacy and data security. The Data Security Law of the People’s Republic of China (“Data Security Law”), which took effect on September 1, 2021, requires data processing (which includes the collection, storage, use, processing, transmission, provision and publication of data), to be conducted in a legitimate and proper manner. The Data Security Law imposes data security and privacy obligations on entities and individuals carrying out data processing activities and also introduces a data classification and hierarchical protection system based on the importance of data in economic and social development and the degree of harm it may cause to national security, public interests, or legitimate rights and interests of individuals or organizations if such data are tampered with, destroyed, leaked, illegally acquired or illegally used. The appropriate level of protection measures is required to be taken for each respective category of data.
Also in China, the Personal Information Protection Law, which took effect on November 1, 2021, introduced stringent protection requirements for processing personal information, which are in many ways akin to the requirements of the GDPR. We may be required to make further significant adjustments to our business practices to comply with the personal information protection laws and regulations in China including the Personal Information Protection Law.
We also continue to see jurisdictions imposing data localization laws. These regulations may interfere with our intended business activities, inhibit our ability to expand into those markets or prohibit us from continuing to offer services in those markets without significant additional costs.
Any failure, or perceived failure, by us to comply with privacy and data protection laws, rules and regulations could result in proceedings or actions against us by governmental entities or others. These proceedings or actions may subject us to significant penalties and negative publicity, require us to change our business practices, increase our costs and severely disrupt our business.
37
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
We and certain third parties that we rely on for our operations collect and store confidential and sensitive information, and our and their operations are highly dependent on information technology systems, including internet-based systems, which may be vulnerable to damage or interruption from earthquakes and hurricanes, fires, floods and other natural disasters, and attacks by computer viruses, unauthorized access, terrorism, and war, as well as telecommunication and electrical failures. Damage or extended periods of interruption to our corporate, development or research facilities due to fire, natural disaster, power loss, communications failure, unauthorized entry or other events could also cause us to cease or delay our manufacturing of the Genio systems. If such an event were to occur and cause interruptions in our operations, it could have a material adverse effect on our business. For example, the loss of clinical trial data from completed, ongoing or planned trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Since the Genio system is a wireless medical device, additional complications may arise with respect to the wireless, RF, technology used for the communication between the system parts. While we have reviewed and determined the integrity of the Genio system and the communication protocol, use of wireless technology imposes a risk that third parties might attempt to access our system. An additional risk is related to interruption or distortion of communication by other devices that might be used in the vicinity of the system, especially when in use by the user, which might have an effect on the effectiveness of the therapy delivered by the system. Any disruption or security breach or other security incident that resulted in a loss of or damage to our data or applications, or the inappropriate access to or disclosure of personal, confidential, or proprietary information could delay our product development, clinical trials, or commercialization efforts, result in increased overhead costs and damage our reputation, all of which could negatively affect our business, financial condition and operating results.
The secure processing, maintenance and transmission of our confidential business information and other information maintained or processed in our business, including sensitive or confidential patient or employee data, is critical to our operations. Such information includes, among other things, intellectual property and proprietary information, the confidential information of any of our future collaborators and licensees, the personal data of our employees, and personal data from patients using the Genio system, which falls into the specially protected category of health data, for which additional safeguards are required under applicable laws. Unauthorized access to or disclosure of any sensitive or confidential patient, trial participant, or employee data, including whether through breach of computer systems, systems failure, employee negligence, fraud or misappropriation, or otherwise, or unauthorized access to or through our information systems and networks, whether by our employees or third parties, or the perception that this has occurred, could result in negative publicity, legal liability and damage to our reputation and could also expose us to sanctions for violations of laws and regulations relating to privacy and data security. Although we have general liability and cybersecurity insurance coverage, our insurance may not cover all claims, continue to be available to us on reasonable terms or be sufficient in amount to cover one or more large claims; additionally, the insurer may disclaim coverage as to any claim. The successful assertion of one or more large claims against us that exceed or are not covered by our insurance coverage or changes in our insurance policies, including premium increases or the imposition of large deductible or co- insurance requirements, could have a material adverse effect on our business, prospects, operating results and financial condition.
Despite our security measures, our information technology systems and infrastructure may be vulnerable to attacks by hackers or internal bad actors, or breached due to employee error, a technical vulnerability, malfeasance or other disruptions. Phishing attempts, social engineering, and other attacks upon our information technology systems are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. In addition to unauthorized access to or acquisition of personal information, confidential information, or other sensitive information, such attacks could include the deployment of harmful malware and ransomware, and may use a variety of methods, including denial-of-service attacks, social engineering and other means, to attain such unauthorized access or acquisition or otherwise affect service reliability and threaten the confidentiality, integrity and availability of information. Because the techniques used to obtain unauthorized access, disable or degrade service, or sabotage systems change frequently and often are not foreseeable or recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures. Any such access, disclosure, or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, significant regulatory penalties, and such an event could disrupt our operations, damage our reputation, and cause a loss of confidence in us and our ability to commercialize our products and conduct clinical trials, which could adversely affect our reputation and delay our commercialization strategy for our Genio system and clinical development of our current and future products.
38
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology or loss of data, including any cyber security incidents, could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability which could harm our ability to operate our business effectively and adversely affect our business and reputation.
Our ability to execute our business plan and maintain operations depends on the continued and uninterrupted performance of our information technology, or IT, systems, some of which are in our control and some of which are in the control of third parties. In the ordinary course of our business, we collect and store sensitive data, including personally identifiable information about our employees, intellectual property, and proprietary business information (confidential information). We manage and maintain our applications and data utilizing on-site systems and we also have outsourced elements of our operations to third parties, and as a result we manage a number of third-party vendors who may or could have access to our confidential information. These applications and data encompass a wide variety of business-critical information including research and development information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy. Despite the implementation of security measures, our IT systems are vulnerable to risks and damages from a variety of sources, including telecommunications or network failures, cyber-attacks, computer viruses, ransomware attacks, phishing schemes, breaches, unauthorized access, interruptions due to employee error or malfeasance or other disruptions, damage from natural disasters, terrorism, war and telecommunication and electrical failures, or other attempts to harm or access our systems. Moreover, despite network security and back-up measures, some of our servers and those of our business partners are potentially vulnerable to physical or electronic break-ins, including cyber-attacks, computer viruses and similar disruptive problems. These events could lead to the unauthorized access, disclosure and use of confidential information. Breaches resulting in the compromise, disruption, degradation, manipulation, loss, theft, destruction, or unauthorized disclosure or use of confidential information, or the unauthorized access to, disruption of, or interference with our products and services, can occur in a variety of ways, including but not limited to, negligent or wrongful conduct by employees or others with permitted access to our IT systems and information, or wrongful conduct by hackers, competitors, or certain governments. Our third party vendors and business partners face similar risks.
Cyber-attacks come in many forms, including the deployment of harmful malware or ransomware, exploitation of vulnerabilities, phishing and other use of social engineering, and other means to compromise the confidentiality, integrity, and availability of our IT systems and confidential information. The techniques used by criminal elements to attack computer systems are sophisticated, change frequently and may originate from less regulated or remote areas of the world. As a result, we may not be able to address these techniques proactively or implement adequate preventative measures. There can be no assurance that we will promptly detect or intercept any such disruption or security breach, if at all. If our computer systems are compromised, we could be subject to fines, damages, reputational harm, litigation and enforcement actions, and we could lose trade secrets, the occurrence of which could harm our business, in addition to possibly requiring substantial expenditures of resources to remedy. For example, any such event that leads to unauthorized access, use or disclosure of personal information, including personal information regarding our patients or employees, could harm our reputation, require us to comply with breach notification laws under GDPR and other legal equivalents, and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information. In addition, the loss of data from clinical trials of the Genio system could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce data, and a cybersecurity breach could adversely affect our reputation and could result in other negative consequences, including disruption of our internal operations, increased cyber security protection costs, lost revenues or litigation. Despite precautionary measures to prevent unanticipated problems that could affect our IT systems, sustained or repeated system failures that interrupt our ability to generate and maintain data could adversely affect our ability to operate our business.
The costs related to significant security breaches or disruptions could be material and could exceed the limits of the cybersecurity insurance we maintain, if any, against such risks. If the information technology systems of our third-party vendors and other contractors and consultants become subject to disruptions or security breaches, we may have insufficient recourse against such third parties and may have to expend significant resources to mitigate the impact of such an event, and to develop and implement protections to prevent future events of this nature from occurring.
39
We cannot assure you that our data protection efforts and our investment in IT will prevent significant breakdowns, data leakages, breaches in our systems, or those of our third-party vendors and other contractors and consultants, or other cyber incidents that could have a material adverse effect upon our reputation, business, operations, or financial condition. For example, if such an event were to occur and cause interruptions in our operations, or those of our third-party vendors and other contractors and consultants, it could result in a material disruption of our programs and the development of our services and technologies could be delayed. Furthermore, significant disruptions of our internal information technology systems or those of our third-party vendors and other contractors and consultants, or security breaches could result in the loss, misappropriation, and/or unauthorized access, use, or disclosure of, or the prevention of access to, confidential information (including trade secrets or other intellectual property, proprietary business information, and personal information), which could result in financial, legal, business, and reputational harm to us. For instance, any such event that leads to unauthorized access, use, or disclosure of personal information, including personal information regarding our customers or employees, could harm our reputation directly, compel us to comply with federal and/or state breach notification laws and foreign law equivalents, subject us to mandatory corrective action, and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information, which could result in significant legal and financial exposure and reputational damages that could potentially have an adverse effect on our business.
Although we take measures to protect sensitive data from unauthorized access, use or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to personnel error, malfeasance, or other malicious or inadvertent disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, manipulated, publicly disclosed, lost, or stolen.
Any such access, breach, or other loss of information could result in legal claims or proceedings, liability under domestic or foreign privacy, data protection and data security laws such as HIPAA and the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and penalties. Notice of certain security breaches must be made to affected individuals, the Secretary of Department of Health and Human Services (“HHS”), and for extensive breaches, notice may need to be made to the media or state attorneys general. Such notice could harm our reputation and our ability to compete. Although we have implemented security measures, such data is currently accessible through multiple channels, and there is no guarantee we can protect our data from breach. Unauthorized access, loss or dissemination could also damage our reputation or disrupt our operations, including our ability to conduct our analyses, conduct research and development activities, collect, process and prepare company financial information, and manage the administrative aspects of our business.
Penalties for violations of these laws vary. For instance, penalties for failure to comply with a requirement of HIPAA and HITECH vary significantly, and include significant civil monetary penalties and, in certain circumstances, criminal penalties with fines up to $250,000 per violation and/or imprisonment. A person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty of up to $50,000 and up to one-year imprisonment. The criminal penalties increase if the wrongful conduct involves false pretenses or the intent to sell, transfer or use identifiable health information for commercial advantage, personal gain or malicious harm.
Further, various states, such as California and Massachusetts, have implemented similar privacy laws and regulations, such as the California Confidentiality of Medical Information Act, that impose restrictive requirements regulating the use and disclosure of health information and other personally identifiable information. These laws and regulations are not necessarily preempted by HIPAA, particularly if a state afford greater protection to individuals than HIPAA. Where state laws are more protective, we have to comply with the stricter provisions. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. California’s patient privacy laws, for example, provide for penalties of up to $250,000 and permit injured parties to sue for damages. Similarly, the California Consumer Privacy Act (“CCPA”) allows consumers a private right of action when certain personal information is subject to unauthorized access and exfiltration, theft or disclosure due to a business’ failure to implement and maintain reasonable security procedures. The interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and data we receive, use and share, potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify. Changes in laws or regulations associated with the enhanced protection of certain types of sensitive data, for the treatment of genetic data, along with increased customer demands for enhanced data security infrastructure, could greatly increase our cost of providing our products, decrease demand for our products, reduce our revenues and/or subject us to additional liabilities.
40
Artificial intelligence presents risks and challenges that can impact our business including by posing security risks to our confidential information, proprietary information, and personal data.
Issues in the development and use of artificial intelligence, combined with an uncertain regulatory environment, may result in reputational harm, liability, or other adverse consequences to our business operations. As with many technological innovations, artificial intelligence presents risks and challenges that could impact our business. We may adopt and integrate certain general artificial intelligence tools for specific use cases reviewed by legal and information security Our vendors may incorporate generative artificial intelligence tools into their offerings without disclosing this use to us, and the providers of these generative artificial intelligence tools may not meet existing or rapidly evolving regulatory or industry standards with respect to privacy and data protection and may inhibit our or our vendors’ ability to maintain an adequate level of service and experience. If we, our vendors, or our third-party partners experience an actual or perceived breach or privacy or security incident because of the use of generative artificial intelligence, we may lose valuable intellectual property and confidential information and our reputation and the public perception of the effectiveness of our security measures could be harmed. Further, bad actors around the world use increasingly sophisticated methods, including the use of artificial intelligence, to engage in illegal activities involving the theft and misuse of personal information, confidential information, and intellectual property. Any of these outcomes could damage our reputation, result in the loss of valuable property and information, and adversely impact our business.
Changes in or inadequate funding for, or disruptions caused by global health concerns impacting, the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the FDA have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new medical devices to be reviewed and/or authorized by necessary government agencies, which would adversely affect our business. For example, in recent years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities.
If a prolonged government shutdown or slowdown occurs or if global health concerns prevent the FDA or other regulatory authorities or bodies from conducting business as usual or conducting inspections or other regulatory activities, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Risks Related to Intellectual Property
The inability to fully protect and exploit our intellectual property and trade secrets may adversely affect our financial performance and prospects.
Our success will depend significantly on our ability to protect our proprietary and licensed in rights, including in particular the intellectual property and trade secrets related to the Genio system. We rely on a combination of patent(s) (applications), trademarks, designs and trade secrets, and use non-disclosure, confidentiality and other contractual agreements to protect our technology. If we are unable to obtain and maintain sufficient intellectual property protection for the Genio system or other product candidates that we may identify, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors and other third parties could develop and commercialize product candidates similar or identical to ours, and our ability to successfully commercialize the Genio system and other product candidates that we may pursue may be impaired.
41
We generally seek patent protection where possible for those aspects of our technology and products that we believe provide significant competitive advantages. However, obtaining, maintaining, defending and enforcing pharmaceutical patents is costly, time consuming and complex, and we may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce and license any patents that may issue from such patent applications, at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Under certain of our license or collaboration agreements, we may not have the right to control the preparation, filing, prosecution and maintenance of patent applications, or to maintain the rights to patents licensed to or from third parties. Further, we cannot be certain that patents will be issued with respect to our pending or future patent applications. In addition, we do not know whether any issued patents will be upheld as valid or proven enforceable against alleged infringers or whether they will prevent the development of competitive patents or provide meaningful protection against competitors or against competitive technologies.
The patent position of medical device companies generally is uncertain, involves complex legal, technological and factual questions. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States, or vice versa. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain. The subject matter claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Therefore, our pending and future patent applications may not result in patents being issued in relevant jurisdictions that protect the Genio system or our product candidates, in whole or in part, or which effectively prevent others from commercializing competitive product candidates, and even if our patent applications issue as patents in relevant jurisdictions, they may not issue in a form that will provide us with any meaningful protection for our product candidates or technology, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Additionally, our competitors may be able to circumvent our patents by developing similar or alternative product candidates or technologies in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. We may be subject to a third-party preissuance submission of prior art to the United States Patent and Trademark Office, or the USPTO, or become involved in opposition, derivation, revocation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others, or other proceedings in the USPTO or applicable foreign offices that challenge priority of invention or other features of patentability. An adverse determination in any such submission, proceeding or litigation could result in loss of exclusivity or freedom to operate, patent claims being narrowed, invalidated or held unenforceable, in whole or in part, limit the scope or duration of the patent protection of the Genio system or our product candidates, all of which could limit our ability to stop others from using or commercializing similar or identical product candidates or technology to compete directly with us, without payment to us, or result in our inability to manufacture or commercialize product candidates or approved products (if any) without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates, or could have a material adverse effect on our ability to raise funds necessary to continue our research programs or clinical trials. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
In addition, our intellectual property rights might be challenged, invalidated, circumvented or rendered unenforceable. Our competitors or other third parties may successfully challenge and invalidate or render unenforceable our issued patents, including any patents that may be issued in the future. This could prevent or limit our ability to stop competitors from marketing products that are identical or substantially equivalent to the Genio system. In addition, despite the broad definition of our concepts and inventions in our portfolio, as is common in technological progress, competitors may be able to design around our patents or develop products that provide outcomes that are comparable to the Genio system but that are not covered by our patents. Much of our value is in our intellectual property, and any challenge to our intellectual property portfolio (whether successful or not) may affect our value.
We could become subject to intellectual property litigation.
The medical device industry is characterized by rapidly changing products and technologies and there is intense competition to establish intellectual property and proprietary rights covering the use of these new products and the related technologies. This vigorous pursuit of intellectual property and proprietary rights has resulted and will continue to result in extensive litigation and administrative proceedings over patent and other intellectual property rights. Whether a product and/or a process infringes a patent involves complex legal and factual issues, and the outcome of such disputes is often uncertain.
42
There may be existing patents of which we are unaware that are inadvertently infringed by the Genio system. We cannot guarantee that any of our patent searches or analyses, including the identification of relevant patents, the scope of patent claims or the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending patent application in the United States and abroad that is relevant to or necessary for the commercialization of our product candidates in any jurisdiction. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our product candidates could have been filed by third parties without our knowledge. Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our product candidates or the use of our product candidates. The scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect, which may negatively impact our ability to market our product candidates.
We may incorrectly determine that our product candidates are not covered by a third-party patent or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the United States or abroad that we consider relevant may be incorrect, which may negatively impact our ability to develop and market our product candidates. Our failure to identify and correctly interpret relevant patents may negatively impact our ability to develop and market the Genio system and our product candidates.
Any infringement claim against us, even if without merit, may cause us to incur substantial costs, and could place a significant strain on our financial resources and/or divert the time and efforts of management from the conduct of our business. In addition, any intellectual property litigation could force us to do one or more of the following: (i) stop selling the Genio system or using technology that contains the allegedly infringing intellectual property; (ii) forfeit the opportunity to license our patented technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property rights against others; (iii) pay substantial damages to the party whose intellectual property rights we may be found to be infringing; or (iv) redesign those products that contain or utilize the allegedly infringing intellectual property. As of the date of this Annual Report, there is no intellectual property litigation pending against us.
Additionally, competitors and other third parties may infringe or otherwise violate our issued patents or other intellectual property or the patents or other intellectual property of our licensors. In addition, our patents or the patents of our licensors may become involved in inventorship or priority disputes. Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. To counter infringement or other unauthorized use, we may be required to file infringement claims, which can be expensive and time- consuming. Our ability to enforce patent rights also depends on our ability to detect infringement. It may be difficult to detect infringers who do not advertise the components or methods that are used in connection with their products and services. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product or service. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents or that our patents are invalid or unenforceable. In a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology. An adverse result in any litigation proceeding could put one or more of our owned or licensed patents at risk of being invalidated, held unenforceable or interpreted narrowly. We may find it impractical or undesirable to enforce our intellectual property against some third parties.
Patent terms may be inadequate to protect our competitive position with respect to the Genio system and our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product candidate, we may be open to competition from competitive devices. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing product candidates similar or identical to ours for a meaningful amount of time, or at all.
43
Depending upon the timing, duration and conditions of any FDA marketing approval of our product candidates, one or more of our owned or licensed U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Act, and similar legislation in the European Union and certain other countries. The Hatch-Waxman Act permits a patent term extension of up to five years for a patent covering an approved product as compensation for effective patent term lost during product development and the FDA regulatory review process. However, we may not receive an extension if we fail to exercise due diligence during the testing phase or regulatory review process, fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the extension could be less than we request. Only one patent per approved product can be extended, the extension cannot extend the total patent term beyond 14 years from approval and only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for the applicable product candidate will be shortened and our competitors may obtain approval to market competing products sooner. As a result, our revenue from applicable products could be reduced. Further, if this occurs, our competitors may take advantage of our investment in development and trials by referencing our clinical and nonclinical data and launch their product earlier than might otherwise be the case, and our competitive position, business, financial condition, results of operations and prospects could be materially harmed.
If we are unable to protect the confidentiality of our proprietary information, our business and competitive position would be harmed.
We rely upon unpatented confidential and proprietary information, including technical information, know- how, and other trade secrets to develop and maintain our competitive position with respect to the Genio system. While we generally enter into non-disclosure or confidentiality agreements with our employees and other third parties to protect our intellectual property and trade secrets, we cannot guarantee that we have entered into such agreements with each party that may have or has had access to our proprietary information. Further, despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, and we may not be able to obtain adequate remedies for such breaches. Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our product candidates that we consider proprietary. Monitoring unauthorized uses and disclosures is difficult, and we do not know whether the steps we have taken to protect our proprietary information will be effective. If any of our proprietary information is disclosed to or independently developed by a competitor or other third party, our competitive position would be materially and adversely harmed.
We depend on exclusive licenses and agreements with third parties, which might not provide adequate protection for our technology.
We rely on licensing agreements providing us exclusivity in the field of our practice. While we have ensured through multiple robust agreements acquisition of exclusive licenses and freedom to operate for our technology, as with any agreement, under unexpected or unpredictable circumstances, these could be under a risk of being terminated despite companies’ efforts and diligence in ensuring integrity of the agreement. Should the agreements be found invalid or licenses revoked and the licensor decide to sue us for infringement of its patents rights, this could expose us to risks of litigation. In addition, any intellectual property litigation could force us to do one or more of the following: (i) stop selling the Genio system or using technology that contains the allegedly infringing intellectual property; (ii) forfeit the opportunity to license our patented technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property rights against others; (iii) pay substantial damages to the party whose intellectual property rights we may be found to be infringing; or (iv) redesign those products that contain or utilize the allegedly infringing intellectual property. The requirement to obtain licenses to third party intellectual property could also arise in the future. If we need to license in any third-party intellectual property, we could be required to pay lump sums or royalties on our products. In addition, if we are required to obtain licenses to third party intellectual property, we might not be able to obtain such licenses on commercially reasonable terms or at all.
We may be subject to claims by third parties asserting that we or our employees have infringed upon, misappropriated or otherwise violated their intellectual property rights, or claiming ownership of what we regard as our own intellectual property.
Although we try to ensure that our employees, consultants and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these individuals have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s former employer. We may also be subject to claims that patents and applications we have filed to protect inventions of our employees, consultants and advisors, even those related to one or more of our product candidates, are rightfully owned by their former or concurrent employer. Litigation may be necessary to defend against these claims.
44
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs, delay development of our product candidates and be a distraction to management. Any of the foregoing events would harm our business, financial condition, results of operations and prospects.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. We may license our trademarks and trade names to third parties, such as distributors. Though these license agreements may provide guidelines for how our trademarks and trade names may be used, a breach of these agreements or misuse of our trademarks and tradenames by our licensees may jeopardize our rights in or diminish the goodwill associated with our trademarks and trade names. Our efforts to enforce or protect our proprietary rights related to trademarks, trade names, and domain names, or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
| ● | others may be able to make products that are similar to any product candidates we may develop or utilize similar technology but that are not covered by the claims of the patents that we license or may own in the future; |
| ● | we, or our current or future licensors might not have been the first to make the inventions covered by the issued patent or pending patent application that we license or may own in the future; |
| ● | we, or our current or future licensors might not have been the first to file patent applications covering certain of our or their inventions; |
| ● | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights; |
| ● | it is possible that our pending owned or licensed patent applications or those that we may own or license in the future will not lead to issued patents; |
| ● | issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors; |
| ● | our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| ● | we may not develop additional proprietary technologies that are patentable; |
| ● | the patents of others may harm our business; and |
| ● | we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property. |
Should any of these events occur, they could harm our business, financial condition, results of operations and prospects.
45
Risks Related to the Ordinary Shares
The dual listing of our ordinary shares may adversely affect the liquidity and value of the ordinary shares.
Our ordinary shares trade on both Euronext Brussels and the Nasdaq Global Market. Trading of the ordinary shares in these markets will take place in different currencies (U.S. dollars on the Nasdaq Global Market and € on Euronext Brussels), and at different times (resulting from different time zones, different trading days and different public holidays in the United States and Belgium). The trading prices of our ordinary shares on these two markets may differ due to these and other factors. Any decrease in the price of our ordinary shares on Euronext Brussels could cause a decrease in the trading price of the ordinary shares on the Nasdaq Global Market. Investors could seek to sell or buy our ordinary shares to take advantage of any price differences between the markets through a practice referred to as arbitrage. Any arbitrage activity could create unexpected volatility in both the trading prices on one exchange and the ordinary shares available for trading on the other exchange. However, the dual listing of the ordinary shares may reduce the liquidity of these securities in one or both markets and may adversely affect the development of an active trading market for the ordinary shares in the United States.
The trading price of our equity securities may be volatile due to factors beyond our control, and holders of our ordinary shares could incur substantial losses.
The market prices of the ordinary shares may be volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their ordinary shares or shares at or above the price originally paid for the security. The market price for the ordinary shares may be influenced by many factors, including:
| ● | actual or anticipated fluctuations in our financial condition and operating results; |
| ● | the release of new data from our DREAM and other clinical trials; |
| ● | actual or anticipated changes in our growth rate relative to our competitors; |
| ● | competition from existing products or new products that may emerge; |
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
| ● | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| ● | issuance of new or updated research or reports by securities analysts; |
| ● | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| ● | currency fluctuations; |
| ● | additions or departures of key management or scientific personnel; |
| ● | disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| ● | changes to coverage policies or reimbursement levels by commercial third-party payors and government payors and any announcements relating to coverage policies or reimbursement levels; |
| ● | announcement or expectation of additional debt or equity financing efforts; |
| ● | uncertainty caused by the ongoing COVID-19 pandemic; |
| ● | issuances or sales of the ordinary shares by us, our insiders or our other shareholders; and |
| ● | general economic and market conditions, including inflation, higher interest rates and potential recession. |
These and other market and industry factors may cause the market price and demand for the ordinary shares to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their shares or ordinary shares and may otherwise negatively affect the liquidity of the trading market for ordinary shares.
46
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the price of the ordinary shares and their trading volume could decline.
The trading market for the ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. If no or only limited securities or industry analysts cover our company, the trading price for the ordinary shares could be negatively impacted. If one or more of the analysts who covers us downgrades our equity securities or publishes inaccurate or unfavorable research about our business, the price of ordinary shares would likely decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, or downgrades our securities, demand for ordinary shares could decrease, which could cause the price of the ordinary shares or their trading volume to decline.
We intend to retain all available funds and any future earnings and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of the ordinary shares.
We have never declared or paid any cash dividends on our shares, and we intend to retain all available funds and any future earnings to fund the development and expansion of our business. Therefore, you are not likely to receive any dividends on your ordinary shares for the foreseeable future and the success of an investment in ordinary shares will depend upon any future appreciation in their value. Consequently, investors may need to sell all or part of their holdings of ordinary shares after price appreciation, which may never occur, as the only way to realize any future gains on their investment. There is no guarantee that the ordinary shares will appreciate in value or even maintain the price at which our investors have purchased them. Investors seeking cash dividends should not purchase the ordinary shares.
In addition, if we choose to pay dividends in the future, exchange rate fluctuations may affect the amount of Euros that we are able to distribute, and the amount in U.S. dollars that our shareholders receive upon the payment of cash dividends or other distributions we declare and pay in euros, if any. Any dividends will generally be subject to Belgian withholding tax. See the section of this Annual Report titled “Material Belgian Income Tax Consequences” for a more detailed description of Belgian taxes on dividends. These factors could harm the value of the ordinary shares.
Investors should be aware that the rights provided to our shareholders under Belgian corporate law and our articles of association differ in certain respects from the rights that you would typically enjoy as a shareholder of a U.S. company under applicable U.S. federal and state laws.
We are a Belgian company with limited liability. Our corporate affairs are governed by our articles of association and by the laws governing companies incorporated in Belgium. The rights of shareholders and the responsibilities of members of our board of directors may be different from the rights and obligations of shareholders and boards of directors in companies governed by the laws of U.S. jurisdictions. In the performance of its duties, our board is required by Belgian law to consider the interests of our company, its shareholders, its employees and other stakeholders. It is possible that some of these parties will have interests that are different from, or in addition to, the interests of our shareholders.
If we issue ordinary shares in future financings, shareholders may experience dilution and, as a result, our ordinary share price may decline.
We may from time to time issue additional ordinary shares at a discount from the trading price of our ordinary shares. As a result, our shareholders would experience immediate dilution upon the issuance of any of our ordinary shares at such discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preference shares or shares. If we issue ordinary shares or securities convertible into ordinary shares of our share capital, our shareholders would experience additional dilution and, as a result, our ordinary share price may decline.
47
It may be difficult for investors outside Belgium to serve process on, or enforce foreign judgments against, us or our directors and senior management.
We are a Belgian public limited liability company. Less than a majority of the members of our board of directors and members of our executive management team are residents of the United States. All or a substantial portion of the assets of such non-resident persons and most of our assets are located outside the United States. As a result, it may not be possible for investors to effect service of process upon such persons or on us or to enforce against them or us a judgment obtained in U.S. courts. Original actions or actions for the enforcement of judgments of U.S. courts relating to the civil liability provisions of the federal or state securities laws of the United States are not directly enforceable in Belgium.
The United States and Belgium do not currently have a multilateral or bilateral treaty providing for reciprocal recognition and enforcement of judgments, other than arbitral awards, in civil and commercial matters. In order for a final judgment for the payment of money rendered by U.S. courts based on civil liability to produce any effect on Belgian soil, it is accordingly required that this judgment be recognized or be declared enforceable by a Belgian court in accordance with Articles 22 to 25 of the 2004 Belgian Code of Private International Law. Recognition or enforcement does not imply a review of the merits of the case and is irrespective of any reciprocity requirement. A U.S. judgment will, however, not be recognized or declared enforceable in Belgium, unless (in addition to compliance with certain technical provisions) the Belgian courts are satisfied of the following:
| ● | the effect of the enforcement judgment is not manifestly incompatible with Belgian public policy; |
| ● | the judgment did not violate the rights of the defendant’ |
| ● | the judgment was not rendered in a matter where the parties transferred rights subject to transfer restrictions with the sole purpose of avoiding the application of the law applicable according to Belgian international private law; |
| ● | the judgment is not subject to further recourse under U.S. law; |
| ● | the judgment is not incompatible with a judgment rendered in Belgium or with a subsequent judgment rendered abroad that might be recognized in Belgium; |
| ● | the claim was not filed outside Belgium after the same claim was filed in Belgium, while the claim filed in Belgium is still pending; |
| ● | the Belgian courts did not have exclusive jurisdiction to rule on the matter; |
| ● | the U.S. court did not accept its jurisdiction solely on the basis of the presence of the plaintiff or the location of goods not direct linked to the dispute in the United States; |
| ● | the judgment did not concern the deposit or validity of intellectual property rights when the deposit or registration of those intellectual property rights was requested, done or should have been done in Belgium pursuant to international treaties; |
| ● | the judgment did not relate to the validity, operation, dissolution, or liquidation of a legal entity that has its main seat in Belgium at the time of the petition of the U.S. court; |
| ● | if the judgment relates to the opening, progress or closure of insolvency proceedings, it is rendered on the basis of the European Insolvency Regulation (EC Regulation No. 1346/2000 of May 29, 2000) or, if not, that (a) a decision in the principal proceedings is taken by a judge in the state where the most important establishment of the debtor was located or (b) a decision in territorial proceedings was taken by a judge in the state where the debtor had another establishment than its most important establishment; |
| ● | the judgment submitted to the Belgian court is authentic under the laws of the state where the judgment was issued; in case of a default judgment, it can be shown that under locally applicable laws the invitation to appear in court was properly served on the defendant; a document can be produced showing that the judgment is, under the rules of the state where it was issued, enforceable and was properly served on the defendant. |
In addition to recognition or enforcement, a judgment by a federal or state court in the United States against us may also serve as evidence in a similar action in a Belgian court if it meets the conditions required for the authenticity of judgments according to the law of the state where it was rendered. The findings of a federal or state court in the United States will not, however, be taken into account to the extent they appear incompatible with Belgian public policy.
Based on the lack of a treaty as described above, U.S. investors may not be able to enforce against us or members of our board of directors or our executive management any judgments obtained in U.S. courts in civil and commercial matters, including judgments under the U.S. federal securities laws.
48
We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, the ordinary shares may be less attractive to investors.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. As an emerging growth company, we are required to report only two years of financial results and selected financial data compared to three and five years, respectively, for comparable data reported by other public companies. We may take advantage of these exemptions until we are no longer an emerging growth company. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the aggregate market value of our ordinary shares held by non-affiliates exceeds $700 million as of the end of our second fiscal quarter before that time, in which case we would no longer be an emerging growth company as of the following December 31st (the last day of our fiscal year). We cannot predict if investors will find the ordinary shares less attractive because we may rely on these exemptions. If some investors find the ordinary shares less attractive as a result, there may be a less active trading market for the ordinary shares and the price of the ordinary shares may be more volatile.
As a foreign private issuer and as permitted by the listing requirements of Nasdaq, we rely on certain home country corporate governance practices rather than the corporate governance requirements of Nasdaq.
We qualify as a foreign private issuer and our ordinary shares have been approved for listing on Nasdaq. As a result, in accordance with the listing requirements of Nasdaq, we rely on home country governance requirements and certain exemptions thereunder rather than relying on the corporate governance requirements of Nasdaq. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act, including the U.S. proxy rules under Section 14 of the Exchange Act. In addition, our officers and directors are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, while we currently publish annual and semi-annual reports on our website pursuant to the rules of Euronext Brussels and expect to file such financial reports with the SEC, we will not be required to file periodic reports with the SEC as frequently or as promptly as U.S. public companies. Specifically, we are not required to file quarterly reports on Form 10-Q or current reports on Form 8-K that a domestic company would be required to file under the Exchange Act. Accordingly, there may be less publicly available information concerning our company than there would be if we were not a foreign private issuer.
In addition, the Listing Rules of the Nasdaq Stock Market require a majority of the directors of a listed U.S. company to be independent, whereas in Belgium, only three directors need to be independent. The Listing Rules of the Nasdaq Stock Market further require that each of the nominating, compensation and audit committees of a listed U.S. company be comprised entirely of independent directors. However, the Belgian Corporate Governance Code recommends only that a majority of the directors on the nomination committee meet the technical requirements for independence under Belgian corporate law. At present, our audit committee is composed of three independent directors out of three members, whereas our nomination and remuneration committees are composed of two independent directors out of three members. Our board of directors has no plan to change the composition of our audit committee and nomination and remuneration committee, and we intend to follow home country practice to the maximum extent possible. Therefore, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers.
49
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As a foreign private issuer, we are not required to comply with all the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. The determination of foreign private issuer status will be made annually on the last business day of our most recently completed second fiscal quarter. Accordingly, we will next make a determination with respect to our foreign private issuer status on June 30, 2024. There is a risk that we will lose our foreign private issuer status in the future.
We would lose our foreign private issuer status if, for instance more than 50% of our ordinary shares are owned by U.S. residents or persons and more than 50% of our assets are located in the United States and we continue to fail to meet additional requirements necessary to maintain our foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly greater than the costs we incur as a foreign private issuer. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive in certain respects than the forms available to a foreign private issuer. We would be required under current SEC rules to prepare our financial statements in accordance with U.S. GAAP and modify certain of our policies to comply with corporate governance practices associated with U.S. domestic issuers. Such conversion and modifications would involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers, which could also increase our costs.
U.S. Holders may suffer adverse tax consequences if we are characterized as a passive foreign investment company, or PFIC.
In general, a non-U.S. corporation is a PFIC for U.S. federal income tax purposes for any taxable year in which (i) 50% or more of the average value of its assets (generally determined on a quarterly basis) consists of assets that produce, or are held for the production of, passive income, or (ii) 75% or more of its gross income consists of passive income. For purposes of the above calculations, a non-U.S. corporation that owns, directly or indirectly, at least 25% by value of the shares of another corporation is treated as if it held its proportionate share of the assets of the other corporation and received directly its proportionate share of the income of the other corporation. Passive income generally includes dividends, interest, investment gains and certain rents and royalties. Cash is generally a passive asset for these purposes. The value goodwill is generally treated as an active asset if it is associated with business activities that produce active income.
If we are a PFIC for any taxable year during which a U.S. holder (as defined below under “Certain Material U.S. Federal Income Tax Considerations to U.S. holders”) holds ordinary shares, we will generally continue to be treated as a PFIC with respect to such U.S. holder in all succeeding years during which the U.S. holder owns the ordinary shares regardless of whether we continue to meet the PFIC test described above, unless the U.S. holder makes a specified election once we cease to be a PFIC. If we are classified as a PFIC for any taxable year during which a U.S. holder holds ordinary shares, the U.S. holder may be subject to adverse tax consequences regardless of whether we continue to qualify as a PFIC, including ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements.
Based on the current estimates, and expected future composition, of our income and the value of our assets, including goodwill, we do not expect to be a PFIC for our current taxable year. However, our PFIC status for any taxable year is an annual determination that can be made only after the end of that year and will depend on the composition of our income and assets and the value of our assets from time to time. The determination of whether we are a PFIC is fact-intensive and the applicable law is subject to varying interpretation. There can be no assurance that the United States Internal Revenue Service, or IRS, will agree with our conclusion or that the IRS will not successfully challenge our position including our classification of certain income and assets as non-passive or our valuation of our tangible and intangible assets.
A U.S. holder may in certain circumstances mitigate the adverse tax consequences of the PFIC rules by filing an election to treat the PFIC as a QEF, or, if shares of the PFIC are “marketable stock” for purposes of the PFIC rules, by making a mark-to-market election with respect to the shares of the PFIC. However, we do not currently intend to provide the information necessary for U.S. holders to make a QEF election if we were treated as a PFIC for any taxable year and prospective investors should assume that a QEF election will not be available. Furthermore, if a U.S. holder were to make a mark-to-market election with respect to its ordinary shares, the U.S. holder would be required to include annually in its U.S. federal taxable income (taxable at ordinary income rates) an amount reflecting any year end increase in the value of its ordinary shares. For further discussion of the PFIC rules and the adverse U.S. federal income tax consequences in the event we are classified as a PFIC, see the section titled “Certain Material U.S. Federal Income Tax Considerations to U.S. holders.”
50
The U.S. federal income tax rules relating to PFICs are very complex. Prospective U.S. holders are strongly urged to consult their own tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of ordinary shares, the consequences to them of an investment in a PFIC, any elections available with respect to the ordinary shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of ordinary shares of a PFIC.
If a U.S. Holder is treated as owning at least 10% of our ordinary shares, such holder may be subject to adverse U.S. federal income tax consequences.
If a U.S. holder (as defined below under “Certain Material U.S. Federal Income Tax Considerations to U.S. Holders”) is treated as owning, directly, indirectly or constructively, at least 10% of the value or voting power of our ordinary shares, such U.S. holder may be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group, if any. Because our group currently includes at least one U.S. subsidiary, under current law, any of our current non-U.S. subsidiaries and any future newly formed or acquired non-U.S. subsidiaries will be treated as controlled foreign corporations, regardless of whether we are treated as a controlled foreign corporation. A United States shareholder of a controlled foreign corporation may be required to annually report and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income” and investments in U.S. property by controlled foreign corporations, regardless of whether we make any distributions. An individual that is a United States shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a United States shareholder that is a U.S. corporation. Failure to comply with controlled foreign corporation reporting obligations may subject a United States shareholder to significant monetary penalties. We cannot provide any assurances that we will furnish to any United States shareholder information that may be necessary to comply with the reporting and tax paying obligations applicable under the controlled foreign corporation rules of the Code. U.S. holders should consult their tax advisors regarding the potential application of these rules to their investment in ordinary shares. See section titled “Certain Material U.S. Federal Income Tax Considerations to U.S. holders” for a more detailed discussion.
Our business may become subject to economic, political, regulatory and other risks associated with international operations.
As a company based in Belgium, our business is subject to risks associated with conducting business internationally. Many of our suppliers and collaborative and clinical trial relationships are located outside the United States. Accordingly, our future results could be harmed by a variety of factors, including:
| ● | economic weakness, including inflation, or political instability in particular non-U.S. economies and markets; |
| ● | differing and changing regulatory requirements for drug approvals in non-U.S. countries; |
| ● | differing jurisdictions could present different issues for securing, maintaining or obtaining freedom to operate in such jurisdictions; |
| ● | potentially reduced protection for intellectual property rights; |
| ● | difficulties in compliance with non-U.S. laws and regulations; |
| ● | changes in non-U.S. regulations and customs, tariffs and trade barriers; |
| ● | changes in non-U.S. currency exchange rates of the pound sterling, the euro and currency controls; |
| ● | changes in a specific country’s or region’s political or economic environment, including the implications of the United Kingdom’s withdrawal from the European Union; |
| ● | trade protection measures, import or export licensing requirements or other restrictive actions by U.S. or non-U.S. governments; |
| ● | differing reimbursement regimes and price controls in certain non-U.S. markets; |
| ● | negative consequences from changes in tax laws; |
| ● | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| ● | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| ● | difficulties associated with staffing and managing international operations, including differing labor relations; |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
| ● | the potential for economic or political instability resulting from the ongoing conflicts between Russian and Ukraine and Israel and Hamas, including any impacts to energy prices or the supply chain; and |
| ● | business interruptions resulting from geo-political actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
51
We or the third parties upon which we depend may be adversely affected by general political, unstable market and economic conditions and other events beyond our control and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
We have become increasingly subject to the risks arising from adverse changes in market and economic and political conditions, both domestically and globally, including trends toward protectionism and nationalism, other unfavorable changes in economic conditions as well as disruptions in global credit and financial markets, such as inflation, failures and instability in U.S. and international banking systems, downgrades of the U.S. credit rating, rising interest rates, slower economic growth or a recession, and other events beyond our control, such as natural disasters, pandemics such as the COVID-19 (coronavirus), epidemics, political instability, and armed conflicts and wars, including the ongoing conflict between Russia and Ukraine, the war between Israel and Hamas.
Increases in inflation could raise our costs for commodities, labor, materials and services and other costs required to grow and operate our business, and failure to secure these on reasonable terms may adversely impact our financial condition. Additionally, increases in inflation, along with the uncertainties surrounding geopolitical developments and global supply chain disruptions, have caused, and may in the future cause, global economic uncertainty and uncertainty about the interest rate environment. A failure to adequately respond to these risks could have a material adverse impact on our financial condition, results of operations or cash flows. In response to high levels of inflation and recession fears, the U.S. Federal Reserve, the European Central Bank, and the Bank of England have raised, and may continue to raise, interest rates and implement fiscal policy interventions. Even if these interventions lower inflation, they may also reduce economic growth rates, create a recession, and have other similar effects.
The U.S. debt ceiling and budget deficit concerns have increased the possibility of credit-rating downgrades and economic slowdowns, or a recession in the United States. Although U.S. lawmakers have previously passed legislation to raise the federal debt ceiling on multiple occasions, there is a history of ratings agencies lowering or threatening to lower the long-term sovereign credit rating on the United States given such uncertainty. On August 1, 2023, Fitch Ratings downgraded the United States’ long-term foreign currency issuer default rating to AA+ from AAA as a result of these repeated debt ceiling and budget deficit concerns. The impact of this or any further downgrades to the U.S. government’s sovereign credit rating or its perceived creditworthiness could adversely affect the U.S. and global financial markets and economic conditions.
If the equity and credit markets deteriorate, it may make any necessary equity or debt financing more difficult to secure, more costly or more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could harm our growth strategy, financial performance and stock price and could require us to delay or abandon plans with respect to our business, including clinical development plans. Further, recent developments in the banking industry could adversely affect our business. If the financial institutions with which we do business enter receivership or become insolvent in the future, there is no guarantee that the Department of the Treasury, the Federal Reserve and the Federal Deposit Insurance Corporation (“FDIC”) will intercede to provide us and other depositors with access to balances in excess of the $250,000 FDIC insurance limit, that we would be able to access our existing cash, cash equivalents and investments, that we would be able to maintain any required letters of credit or other credit support arrangements, or that we would be able to adequately fund our business for a prolonged period of time or at all, any of which could have a material adverse effect on our business, financial condition and results of operations. We cannot predict the impact that the high market volatility and instability of the banking sector more broadly could have on economic activity and our business in particular. In addition, there is a risk that one or more of our current service providers, manufacturers or other third parties with which we conduct business may not survive difficult economic times, including the current global situation resulting from the COVID-19 pandemic, the ongoing conflict between Russia and Ukraine, the war between Israel and Hamas, the instability of the banking sector, and the uncertainty associated with current worldwide economic conditions, which could directly affect our ability to attain our operating goals on schedule and on budget.
52
The short and long-term implications of Russia’s invasion of Ukraine are difficult to predict at this time. We continue to monitor any adverse impact that the outbreak of war in Ukraine and the subsequent institution of sanctions against Russia by the United States and several European and Asian countries may have on the global economy in general, on our business and operations and on the businesses and operations of our suppliers and customers. For example, a prolonged conflict may result in challenges associated with timely receipt of customer payments and banking transactions, supply-chain issues, increased inflation, escalating energy prices and constrained availability, and thus increasing costs, of raw materials. We will continue to monitor this fluid situation and develop contingency plans as necessary to address any disruptions to our business operations as they develop. To the extent the war in Ukraine may adversely affect our business as discussed herein, it may also have the effect of heightening many of the other risks described herein. Such risks include, but are not limited to, adverse effects on macroeconomic conditions, including inflation; disruptions to our global technology infrastructure, including through cyber-attack, ransom attack, or cyber-intrusion; adverse changes in international trade policies and relations; our ability to maintain or increase our product prices; disruptions in global supply chains; our exposure to foreign currency fluctuations; and constraints, volatility, or disruption in the capital markets, any of which could negatively affect our business and financial condition.
Our research and development facility and all manufacturing facilities are located in Tel Aviv, Israel. In addition, the majority of our employees and some officers are residents of Israel. Accordingly, political, economic and military conditions in Israel, including the ongoing conflict between Israel and Hamas, may directly adversely affect our business. Any armed conflicts, terrorist activities, political instability in the region or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our business conditions in general and harm our results of operations. Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Although Israeli legislation requires the Israeli government to cover the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure that this government coverage will be maintained, or if maintained, will be sufficient to fully compensate us if any damages are incurred. Any losses or damages incurred by us could have a material adverse effect on our business.
The effects of current and future economic and political conditions and other events beyond our control on us, patients, our third party vendors, including clinical trial sites, and our partners could severely disrupt our operations and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as the manufacturing facilities of our third-party contract manufacturers, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place may prove inadequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business.
Climate change or legal, regulatory or market measures to address climate change may negatively affect our business, results of operations, cash flows and prospects.
We believe that climate change has the potential to negatively affect our business and results of operations, cash flows and prospects. We are exposed to physical risks (such as extreme weather conditions or rising sea levels), risks in transitioning to a low-carbon economy (such as additional legal or regulatory requirements, changes in technology, market risk and reputational risk) and social and human effects (such as population dislocations and harm to health and well-being) associated with climate change. These risks can be either acute (short-term) or chronic (long-term).
The adverse impacts of climate change include increased frequency and severity of natural disasters and extreme weather events such as hurricanes, tornados, wildfires (exacerbated by drought), flooding, and extreme heat. Extreme weather and sea-level rise pose physical risks to our facilities as well as those of our suppliers. Such risks include losses incurred as a result of physical damage to facilities, loss or spoilage of inventory, and business interruption caused by such natural disasters and extreme weather events. Other potential physical impacts due to climate change include reduced access to high-quality water in certain regions and the loss of biodiversity, which could impact future product development. These risks could disrupt our operations and supply chains, which may result in increased costs.
53
New legal or regulatory requirements may be enacted to prevent, mitigate, or adapt to the implications of a changing climate and its effects on the environment. These regulations, which may differ across jurisdictions, could result in us being subject to new or expanded carbon pricing or taxes, increased compliance costs, restrictions on greenhouse gas emissions, investment in new technologies, increased carbon disclosure and transparency, upgrade of facilities to meet new building codes, and the redesign of utility systems, which could increase our operating costs, including the cost of electricity and energy used by us. Our supply chain would likely be subject to these same transitional risks and would likely pass along any increased costs to us.
We are exposed to changes in foreign currency exchange rates.
We incur some of our expenses, and derive certain of our revenues, in currencies other than the Euro. In particular, as we expand our operations and conduct additional clinical trials in the United States, we will incur additional expenses in U.S. dollars. As a result, we are exposed to foreign currency exchange risk as our results of operations and cash flows are subject to fluctuations in foreign currency exchange rates.
We currently do not engage in hedging transactions to protect against uncertainty in future exchange rates between particular foreign currencies and the Euro. Therefore, an unfavorable change in the value of the Euro against the U.S. dollar could have a negative impact on our revenue and earnings growth. We cannot predict the impact of foreign currency fluctuations, and foreign currency fluctuations in the future may adversely affect our financial condition, results of operations and cash flows. Our ordinary shares in the U.S. trade in U.S. dollars on Nasdaq, while our ordinary shares trade in Euro on Euronext Brussels. Our financial statements are prepared in euro. Therefore, fluctuations in the exchange rate between the Euro and the U.S. dollar will also affect, among other matters, the value of our ordinary shares.
We could also sign contracts denominated in currencies other than the euro, which would increase our exposure to currency risk. In accordance with our business decisions, our exposure to this type of risk could change depending on:
| ● | the currencies in which we receive our revenues; |
| ● | the currencies chosen when agreements are signed, such as licensing agreements, or co-marketing or co-development agreements; |
| ● | the location of clinical trials; and |
| ● | our policy for insurance cover. |
At present, we have not put any specific hedging arrangements in place to address these risks. Should any of these risks materialize, this could have a material adverse effect on our business, prospects, financial condition and results of operations.
Shareholders outside Belgium may be subject to exchange rate risk.
Our ordinary shares are denominated in euros. Accordingly, an investment in the ordinary shares by an investor whose principal currency is not the Euro may expose such investor to foreign currency exchange rate risk. Any depreciation of the Euro against such foreign currency would reduce the value of the investment in the ordinary shares in terms of such foreign currency.
We incur significant increased costs as a result of operating as a company that is publicly listed on both Nasdaq in the United States and Euronext Brussels in Belgium, and our management is required to devote substantial time to new compliance initiatives.
As a U.S. public company listed on Nasdaq, we incur legal, accounting, and other expenses that we would not incur if we were only listed on Euronext Brussels. We are subject to the reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the Nasdaq listing requirements and other applicable securities rules and regulations. Continued compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company” and/or a foreign private issuer. The Exchange Act requires that, as a public company, we file annual, semi-annual and current reports with respect to our business, financial condition and result of operations. However, as a foreign private issuer, we are not required to file quarterly and current reports with respect to our business and results. In 2023, we made annual, semiannual and quarterly reporting with respect to our listing on Euronext Brussels.
54
Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management personnel or members for our board of directors.
However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Further, being a U.S. listed company and a Belgian public company with ordinary shares admitted to trading on Euronext Brussels impacts the disclosure of information and requires compliance with two sets of applicable rules. From time to time, this may result in uncertainty regarding compliance matters and result in higher costs necessitated by legal analysis of dual legal regimes, ongoing revisions to disclosure and adherence to heightened governance practices. As a result of the enhanced disclosure requirements of the U.S. securities laws, business and financial information that we report is broadly disseminated and highly visible to investors, which we believe may increase the likelihood of threatened or actual litigation, including by competitors and other third parties, which could, even if unsuccessful, divert financial resources and the attention of our management from our operations.
As a result of becoming a U.S. public company, we are subject to additional regulatory compliance requirements, including Section 404, and if we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud.
Pursuant to Section 404, our management is required to assess and attest to the effectiveness of our internal control over financial reporting in connection with issuing our consolidated financial statements as of and for the year ending December 31, 2023. See “Item 15. Controls and Procedures.” Section 404 also requires an attestation report on the effectiveness of internal control over financial reporting be provided by our independent registered public accounting firm beginning with our annual report following the date on which we are no longer an “emerging growth company”, which may be up to five fiscal years from the date of our listing of ordinary shares on Nasdaq.
Complying with Section 404 is costly and challenging, and management’s attention may be diverted from other business concerns, which could adversely affect our results. We have hired employees and engaged outside consultants, and may need to continue to hire employees and engage outside consultants, to comply with these requirements, which may further increase expenses. If we fail to comply with the requirements of Section 404, we may be subject to sanctions or investigations by regulatory authorities, including the SEC and Nasdaq. Furthermore, our inability to attest to the effectiveness of our internal control over financial reporting may result in the loss of investor confidence in the accuracy and completeness of our financial reports, and the market price of our ordinary shares may decline. Our failure to implement or maintain effective internal control over financial reporting could also restrict our future access to the capital markets and subject each of us, our directors and our officers to both significant monetary and criminal liability.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expense and a diversion of management’s time and attention from revenue generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business, financial position, results and prospects may be adversely affected.
55
If we fail to implement and maintain effective internal controls over financial reporting, our ability to produce accurate financial statements on a timely basis could be impaired.
We are subject to reporting obligations under U.S. securities laws and the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). Section 404 of the Sarbanes-Oxley Act requires that we include a report from management on the effectiveness of our internal control over financial reporting. As a result of our failure to remediate the material weaknesses identified herein, our management has concluded that our internal control over financial reporting was not effective as of December 31, 2023. This conclusion could adversely impact the market price of our ordinary shares due to a loss of investor confidence in the reliability of our reporting processes and the accuracy or completeness of our reported financial information.
We are required to perform system and process evaluations and testing of our internal controls over financial reporting to allow our management to report on the effectiveness of our internal control over financial reporting. In addition, our compliance with Section 404 of the Sarbanes-Oxley Act will require that we incur substantial accounting expense, spend significant management effort, continue to hire additional accounting and financial staff with the appropriate experience and technical accounting knowledge, and compile the system and process documentation necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or any subsequent testing by our independent registered public accounting firm, may reveal additional deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. We cannot assure you that there will not be additional material weaknesses or significant deficiencies in our internal control over financial reporting in the future.
For as long as we are an “emerging growth company” under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We could be an “emerging growth company” for up to five years. At the time when we are no longer an emerging growth company, we may report that there continue to exist material weaknesses and our independent registered public accounting firm may issue a report that is adverse in the event if the level at which our controls are documented, designed or operating is not satisfactory. Our remediation efforts may not enable us to avoid a material weakness in the future. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur remediation costs. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
In connection with our preparation and the audit of our consolidated financial statements as of and for the year ended December 31, 2023, we identified material weaknesses in our internal control over financial reporting. Additionally, we may identify additional material weaknesses in the future that may cause us to fail to meet our reporting obligations or result in material misstatements in our financial statements. If we fail to remediate our material weaknesses, we may not be able to report our financial results accurately or prevent fraud.
As a public company, we are operating in an increasingly demanding regulatory environment that requires us to comply with, among other things, the Sarbanes-Oxley Act and related rules and regulations of the SEC’s substantial disclosure requirements, accelerated reporting requirements and complex accounting rules. Company responsibilities required by the Sarbanes-Oxley Act include establishing corporate oversight and adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud.
In connection with our preparation and the audit of our consolidated financial statements as of and for the year ended December 31, 2023, we identified material weaknesses in our internal control over financial reporting. As defined in the standards established by the PCAOB, a “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
56
The material weaknesses identified during the audit of our consolidated financial statements as of and for the year ended December 31, 2023 relate to
| ● | Insufficient accounting and supervisory personnel who have the appropriate level of technical accounting experience and training, as well as a lack of sufficient oversight of external advisors; |
| ● | Lack of a comprehensive risk based control assessment to establish an effective structure of internal controls, which leads to insufficient procedures and controls, including IT General Controls, to ensure that accurate financial statements can be prepared and reviewed on a timely basis for annual reporting purposes. |
To address the material weaknesses identified, we have taken, and continue to take, several remedial actions, including engaging an external professional advisor that is evaluating and validating the design effectiveness of our internal control framework and based on the outcomes of such evaluation, we are continuing to adapt our internal controls. Our remediation plan is underway, however, it had not sufficiently advanced by December 31, 2023 to remediate these material weaknesses.
If we are unable to successfully remediate our identified ongoing material weaknesses, or if we discover additional material weaknesses, we would be required to continue disclosing such material weaknesses in future filings with the SEC, which could adversely impact investor confidence in our company and the market price of our ordinary shares, and could subject us to litigation or regulatory enforcement actions.
We may be subject to securities litigation, which is expensive and could divert management’s attention.
The market price of the ordinary shares may be volatile and, in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
We are a Belgian public limited liability company, and shareholders of our company may have different and in some cases more limited shareholder rights than shareholders of a U.S. listed corporation.
We are a Belgian company with limited liability. Our corporate affairs are governed by our articles of association and by the laws governing companies incorporated in Belgium. The rights of shareholders and the responsibilities of members of our board of directors may be different from the rights and obligations of shareholders and boards of directors in companies governed by the laws of U.S. jurisdictions. In the performance of its duties, our board is required by Belgian law to consider the interests of our company, its shareholders, its employees and other stakeholders, in all cases with due observation of the principles of reasonableness and fairness. It is possible that some of these parties will have interests that are different from, or in addition to, the interests of our shareholders.
Investors resident in countries other than Belgium may suffer dilution if they are unable to participate in future preferential subscription rights offerings.
Under Belgian law and our constitutional documents, shareholders have a waivable and cancellable preferential subscription right to subscribe pro rata to their existing shareholdings to the issuance, against a contribution in cash, of new ordinary shares or other securities entitling the holder thereof to new ordinary shares, unless such rights are limited or cancelled by resolution of our general shareholders’ meeting or, if so authorized by a resolution of such meeting, our board of directors. The exercise of preferential subscription rights by certain shareholders not residing in Belgium (including those in the United States, Australia, Israel, Canada or Japan and taking into account the current shareholding and international network of our current board of directors) may be restricted by applicable law, practice or other considerations, and such shareholders may not be entitled to exercise such rights, unless the rights and ordinary shares are registered or qualified for sale under the relevant legislation or regulatory framework. In particular, we may not be able to establish an exemption from registration under the U.S. Securities Act, and we are under no obligation to file a registration statement with respect to any such preferential subscription rights or underlying securities or to endeavor to have a registration statement declared effective under the U.S. Securities Act. Shareholders in jurisdictions outside Belgium who are not able or not permitted to exercise their preferential subscription rights in the event of a future preferential subscription rights, equity or other offering may suffer dilution of their shareholdings.
57
Takeover provisions in the national law of Belgium may make a takeover difficult.
Public takeover bids on our shares and other voting securities, such as warrants or convertible bonds, if any, are subject to the Belgian Act of April 1, 2007 on public takeover bids, as amended and implemented by the Belgian Royal Decree of April 27, 2007, or Royal Decree, and to the supervision by the Belgian Financial Services and Markets Authority, or FSMA. Public takeover bids must be made for all of our voting securities, as well as for all other securities that entitle the holders thereof to the subscription to, the acquisition of or the conversion into voting securities. Prior to making a bid, a bidder must issue and disseminate a prospectus, which must be approved by the FSMA. The bidder must also obtain approval of the relevant competition authorities, where such approval is legally required for the acquisition of our company. The Belgian Act of April 1, 2007 provides that a mandatory bid will be required to be launched for all of our outstanding shares and securities giving access to ordinary shares if a person, as a result of its own acquisition or the acquisition by persons acting in concert with it or by persons acting on their account, directly or indirectly holds more than 30% of the voting securities in a company that has its registered office in Belgium and of which at least part of the voting securities are traded on a regulated market or on a multilateral trading facility designated by the Royal Decree. The mere fact of exceeding the relevant threshold through the acquisition of one or more shares will give rise to a mandatory bid, irrespective of whether or not the price paid in the relevant transaction exceeds the current market price.
There are several provisions of Belgian company law and certain other provisions of Belgian law, such as the obligation to disclose important shareholdings and merger control, that may apply to us and which may make an unfriendly tender offer, merger, change in management or other change in control, more difficult. These provisions could discourage potential takeover attempts that third parties may consider and thus deprive the shareholders of the opportunity to sell their shares at a premium (which is typically offered in the framework of a takeover bid).
Item 4.Information on the Company
A.History and Development of the Company
We were incorporated on July 15, 2009 as a company with limited liability (naamloze vennootschap/ société anonyme) incorporated and operating under the laws of Belgium. We are registered with the legal entities register (Brabant Wallon) under enterprise number 0817.149.675. We were publicly listed on Euronext Brussels in September 2020 and we were publicly listed on The Nasdaq Global Market in July 2021.
We have four wholly owned subsidiaries: Nyxoah Ltd, an Israeli limited company incorporated in January 2008 under the name M.L.G. Madaf G. Ltd and our subsidiary since October 2009, Nyxoah Pty Ltd, an Australian limited company incorporated in 2017, Nyxoah, Inc., a Delaware corporation incorporated in May 2020, and Nyxoah GmbH, a German limited liability company incorporated in May 2023 under the name Blitz F23-668 GmbH and our subsidiary since July 2023. Our headquarters and principal executive offices are located at Rue Edouard Belin 12, 1435 Mont-Saint-Guibert, Belgium, and our telephone number is +32 10 22 23 55. Our website address is www.nyxoah.com. Our website and the information contained on or accessible through our website are not part of this Annual Report. Our authorized representative in the United States is Nyxoah, Inc. Our agent for service of process in the United States is Corporation Service Company, 1090 Vermont Avenue N.W., Washington D.C. 20005.
We file reports and other information with the SEC. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC (http://www.sec.gov).
Capital Expenditures
Our capital expenditures amounted to €10.5 million, €16.3 million and €11.8 million for the years ended December 31, 2023, 2022 and 2021, respectively.
For the year ended December 31, 2023, our principal capital expenditure mainly related to capitalized development expenses, establishment of U.S. production line and purchases of laboratory equipment and investments in the construction of new clean rooms.
For the year ended December 31, 2022, our principal capital expenditures mainly related to capitalized development expenses and to laboratory equipment and furniture and office equipment.
58
For the year ended December 31, 2021, our principal capital expenditures mainly related to purchases of laboratory equipment and investments in the construction of new clean rooms.
B.Business
Overview
We are a medical technology company focused on the development and commercialization of innovative solutions to treat Obstructive Sleep Apnea, or OSA. Our lead solution is the Genio system, a CE-Marked, patient-centric, minimally invasive, next generation hypoglossal neurostimulation, or HGNS, therapy for the treatment of moderate to severe OSA. OSA is the world’s most common sleep disordered breathing condition and is associated with increased mortality risk and comorbidities including cardiovascular diseases, depression and stroke. Our innovative technology platform is a first-of-its-kind HGNS device designed to treat OSA through bilateral stimulation, by maintaining an open airway for a restful night’s sleep. We started generating revenue from the sale of the Genio system in Europe in July 2020, and we are currently conducting our DREAM pivotal trial designed to support marketing authorization in the United States. We are developing a significant body of clinical evidence to further support the strong value proposition of the Genio system and its ability to improve the health and quality of life of OSA patients.
OSA occurs due to the relaxation of the soft tissue, throat and tongue muscles in a patient’s airway, which causes an obstruction that temporarily prevents breathing during sleep. In patients with OSA, the airway repeatedly becomes partially or completely blocked, thereby limiting the airflow reaching the lungs from sufficiently oxygenating the blood. Approximately 425 million people between the ages of 30 and 69 globally suffer from moderate to severe OSA. This chronic disease negatively affects a patient’s health and quality of life.
Published scientific literature estimates that there are currently approximately 23.8 million individuals with moderate to severe OSA in our initial target markets in Europe. Based on published scientific literature, we estimate that approximately 2.6 million patients are diagnosed annually in those countries and that approximately 80% of diagnosed patients are prescribed a continuous positive airway pressure, or CPAP, device. Published scientific literature reports non-compliance rates to CPAP between 29% and 83%. Based on these data, and for purposes of calculating the total addressable market in Europe for the Genio system, we estimate that approximately 35% of patients that are prescribed CPAP in those countries are not compliant with the therapy. Additionally, certain patients possess anatomical characteristics, including higher body-mass-index or increased tongue fat deposition that make them ineligible for HGNS. Taking that into account, we estimate that approximately 70% of those non-compliant patients are eligible for HGNS based on their anatomical characteristics. As a result, we believe the total addressable market in Europe for the Genio system is at least 515,000 patients which represents an estimated annual market opportunity of approximately $10 billion based on our current pricing for the Genio system. We also plan to enter the United States market, assuming we obtain marketing authorization in the United States, where published scientific literature estimates that there are approximately 23.7 million individuals with moderate to severe OSA. Based on the same assumptions set out above, we estimate a target market of approximately 510,000 patients in the United States, which represents an estimated annual total addressable market of approximately $10 billion based on our current pricing for the Genio system.
The standard of care first-line therapy for patients with moderate to severe OSA is CPAP. CPAP is a treatment whereby air, at a constant or automated pressure, is pushed into the upper airway via a facial or nasal mask that the patient must wear during sleep. Despite its proven efficacy, CPAP has been associated with many limitations, making compliance a serious challenge. Second-line treatments, such as mandibular oral devices, are more suitable to treat mild-to-moderate OSA, and other therapies, such as anatomical surgical procedures, are highly invasive. In recent years, neurostimulation technology has emerged as a viable second-line therapy to treat patients suffering from moderate to severe OSA. This technology is centered on stimulating the hypoglossal nerve, which activates the genioglossus muscle resulting in a forward protrusion of the tongue. HGNS therapies have proven to be a safe and effective treatment for those suffering from moderate to severe OSA. Systems competing with our Genio system consist of multiple incisions and implantable components, including an implantable pulse generator with a battery and one or more leads. In addition, competing systems exclude a substantial subset of the OSA patient population. OSA patients diagnosed with complete concentric collapse at the level of the soft palate, or CCC, are currently contraindicated for other HGNS OSA therapies. Unlike other HGNS technologies indicated for treating OSA that provide unilateral stimulation of the hypoglossal nerve, our Genio system provides bilateral stimulation that we believe results in a stronger muscle contraction, a more symmetric tongue movement and a wider opening of the airway, which we believe has the potential to provide better clinical outcomes. Further, we believe that bilateral stimulation enables the Genio system to potentially address moderate to severe OSA patients with CCC, who are currently contraindicated for, or unable to be treated with, existing HGNS OSA therapies.
59
In order to diagnose CCC, a drug induced sleep endoscopy, or DISE, procedure is required. During this procedure, the patient receives propofol and/or midazolam to artificially induce sleep, and the pharyngeal collapse patterns are visualized using a flexible fiber optic nasopharyngoscope, a soft and flexible endoscope which is inserted in the patient’s nose to visualize the pharyngeal area and assess the level, direction and degree of the collapsed area. Currently, the only HGNS therapy approved in the United States requires all patients seeking HGNS OSA therapy to undergo a DISE procedure. It is estimated that approximately 35% of moderate to severe OSA patients are affected by CCC and are therefore unable to receive currently available neurostimulation treatment in the United States.
Our Genio system includes the first battery-free, leadless and minimally invasive neurostimulator, capable of delivering bilateral HGNS for moderate to severe OSA patients who did not tolerate, have failed or refused conventional positive airway pressure, or PAP, therapy. We developed the Genio system with a patient-centric approach, designed for comfort and safety, to increase compliance and improve quality of life. The Genio system includes a single implanted device that can be placed through a minimally invasive, single-incision surgery under the chin. The power source for the stimulator is external. Unlike competing HGNS therapies, the lack of an implantable battery or additional leads limits the need for complex tunneling and only requires a single incision for implantation. This minimally invasive procedure is typically completed in approximately one hour and allows patients to recover quickly and resume normal activities typically within a week. Patients return to the physician approximately six weeks later for device titration, which typically involves an in-lab sleep trial to analyze breathing frequency. Further, the external activation chip eliminates the need for additional surgical procedures to replace depleted batteries and enables software, firmware or external hardware updates and upgrades to be implemented without the need for surgical intervention thereby limiting potential infection risk due to an additional procedure.
We continue to develop a substantial body of clinical evidence on the Genio system. In 2019, we completed our BiLAteral hypoglossal nerve STimulation for treatment of Obstructive Sleep Apnea, or BLAST OSA, trial, a prospective, open label, non-randomized, single arm treatment trial involving 27 implanted participants. Twenty-two patients completed the protocol, and the trial met all primary, secondary and exploratory endpoints. In the six-month data, the mean individual reduction in the Apnea-Hypopnea Index, or AHI, events per hour was 47.3%. Participants’ AHI decreased from 23.7±12.2 to 12.9±10.1, representing a mean change of 10.8 events per hour. The results of the trial were published in the European Respiratory Journal in October 2019 and were the basis for receiving CE-Mark on the Genio system.
We are seeking to expand indications of the Genio system by obtaining clinical evidence through our ongoing multicenter, prospective, open-label BilatEral Hypoglossal Nerve StimulaTion for TreatmEnt of ObstRuctive SLEEP Apnoea With and Without Complete Concentric Collapse clinical trial in Australia and New Zealand, or the BETTER SLEEP trial, to evaluate the effectiveness of the Genio system for patients suffering from CCC. We believe that positive results from this trial may eliminate the need for Genio system patients to be selected based on a DISE procedure prior to implantation of the Genio system, thereby leading to a potential indication expansion in Europe. In June 2021, we announced initial top-line results from the six-month data for the BETTER SLEEP trial. Based on this data, in October 2021, the EU Notified Body granted CE-Marked indication to include OSA patients with CCC for the Genio system in Europe, which should eliminate the need for a DISE procedure. Additionally, in September 2021, we received breakthrough device designation in the United States for the Genio system from the Food and Drug Administration, or FDA, for the treatment of OSA with CCC, based on the initial clinical evidence from the BETTER SLEEP trial. We plan to continue to obtain authorization in additional target markets and are currently conducting our Dual-sided Hypoglossal neRvE stimulAtion for the treatMent of Obstructive Sleep Apnea clinical trial, or DREAM trial, a multicenter, prospective, open-label, pivotal Investigational Device Exemption, or IDE, trial designed to support marketing authorization in the United States. Additionally, we presented 12-month data on the first 34 DREAM patients reaching 12-month follow-up as a late-breaking abstract at SLEEP 2023, a joint meeting of the American Academy of Sleep Medicine and the Sleep Research Society, demonstrating a 65% AHI responder rate, a 76% ODI responder rate and safety in line with expectations. These data are preliminary and not conclusive of final success of the DREAM trial. On March 19, 2024, we issued a press release announcing that the DREAM U.S. pivotal trial met its primary endpoints. For more information see “—Clinical Results and Studies—Pivotal DREAM Trial” below. We expect to apply for marketing authorization in the United States with the aim of being commercially available in the United States in late 2024.
In July 2022, we announced that the FDA approved an IDE to enable us to initiate a clinical trial, called ACCCESS, to evaluate the use of the Genio system for the treatment of adult patients with moderate-to-severe OSA with CCC that have failed, did not tolerate, or refused PAP. In the ACCCESS trial, we plan to implant up to 106 subjects with co-primary efficacy endpoints of AHI responder rate, per the Sher criteria, and ODI responder rate, both assessed at twelve months post-implant. The first enrolled subjects have been implanted, and we anticipate completing implantation in late 2024.
60
We are initially targeting markets in Europe where we have identified a country- specific reimbursement pathway or execution strategy. We began our commercial launch in Germany in July 2020. After obtaining reimbursement approval in Germany through the existing HGNS special innovation funding program, or NUB, we generated our first revenue in the second half of 2020. In 2021, we successfully obtained reimbursement in Germany under a dedicated DRG code for HGNS and obtained reimbursement under an OSA-specific DRG code in Switzerland from the Federal Statistic Office, or BFS. The reimbursement coverage in both Germany and Switzerland includes the cost of the Genio system, implant procedure, hospital stay and follow-up care. In 2021, we began marketing products in Switzerland and also secured first revenue in Spain and we began commercialization in Finland in 2022. We generated our first revenue in Austria in 2023. Based on market access activities conducted by us over the past several years, we have developed tailored reimbursement strategies using assessments of the local requirements of target countries. In countries where there is existing reimbursement coverage in place, we plan to piggyback on existing coding and reimbursement, acting as a fast follower. In countries where there is no existing reimbursement coverage, we will seek to be the first in that market to obtain reimbursement coverage. In countries without existing reimbursement coverage, the strategy could include (i) making the Genio system commercially available for patients through country specific innovation funding pathways for procedures and products that would not yet be covered by an existing code, (ii) supporting case-by-case funding submission in focus hospitals that can use their budget to fund the therapy, (iii) entering into specific commercial deals with privately funded hospital groups, or (iv) out-of-pocket payment.
We have established a systematic approach to commercializing the Genio system in our target markets, focusing on active engagement, education and market development across patients, physicians and hospitals. We currently market our therapy to physicians and hospitals where ear, nose, and throat doctors, or ENTs, sleep doctors and general practitioners see, diagnose and treat patients with OSA. We are actively expanding our current European sales and marketing organization with country-specific sales teams established in connection with obtaining reimbursement. Our sales teams are focused on prioritizing high volume ENT centers and sleep centers, and on building long-standing relationships with key physicians such as sleep doctors, ENTs and general practitioners who have strong connections to the OSA patient population that may be eligible for our therapy. We also seek to establish long-term partnerships with key opinion leaders, or KOLs, and patient associations that are oriented towards the needs of our patients. Our sales and marketing organization is focused on building physician awareness through referral network development, education, targeted KOL development and training, and direct-to-consumer marketing.
In addition to our ongoing clinical studies, we are also committed to continuing our research and development efforts related to the Genio system, with an emphasis on improving clinical outcomes, optimizing patient adoption and comfort, increasing access for a greater number of patients, and allowing more physicians to perform the implantation procedure. The primary focus of our research and development efforts in the near-term will be the continued technological advancement of the Genio system. Some of these improvements include features aimed at enhancing a physician’s ability to monitor patient compliance and therapeutic efficacy. The Genio 2.1 system further reflects such improvements and is designed to improve patient comfort and compliance with a new smartphone application and an upgraded external activation chip. The Genio 2.1 system offers patients daily feedback on therapy usage and the autonomy to adjust stimulation amplitude within pre-defined boundaries. Physicians can also fine-tune stimulation amplitude to determine the optimal level of comfort for patients without compromising therapy efficacy. In the long term, including through our partnership with Vanderbilt University, we intend to provide new neurostimulation technologies for OSA patients. We continue to enhance our scalable technology platform to allow for quick and streamlined release of new features and functionalities through software, firmware and hardware updates and upgrades and therapy enhancement, and anticipate making regulatory submissions relating to our Genio 3.1 system in late 2024.
61
Our Competitive Strengths
We are focused on transforming the lives of patients who suffer from moderate to severe OSA by continuing to develop, clinically validate, manufacture and commercialize our innovative Genio system. We believe the Genio system offers a compelling solution for a large and significantly underpenetrated global patient population and that our focus and experience in treating patients with OSA, combined with the following strengths, will allow us to build our business and potentially expand our market opportunity:
| ● | Disruptive, patient-centric neurostimulation solution to treat moderate to severe OSA. We specifically designed the Genio system with the goal of advancing a therapy to treat moderate to severe OSA and providing a safe and effective patient-centric solution offering significant benefits to address the unmet needs of patients. The Genio system includes the first battery-free, leadless, neurostimulator designed to be implanted in a minimally invasive procedure using a single incision. The Genio system delivers bilateral HGNS for patients who suffer from moderate to severe OSA and did not tolerate, failed or refused standard first-line therapy, including CPAP. We believe that bilateral stimulation could lead to better therapeutic performance and address more therapeutic indications compared to other HGNS-based technologies. While other commercially available neurostimulation platforms require implantation of leads and a pulse generator containing a battery, our Genio system only requires implantation of a battery-free neurostimulator. Due to its unique design, the Genio system’s implantable stimulator is the only neurostimulation-based OSA therapy that has received CE-Mark conditional labeling for 1.5T and 3T full-body MRI scans. CE-Mark conditional labeling for MRI scans have become more and more important for physicians and patients due to the growing need and incidence of MRI scans. Implantable medical devices that have not been tested and approved with MR conditional labeling are considered as MR unsafe, and MR scans are contra-indicated for these patients. We believe our Genio system technology has the potential to become the leading neurostimulation solution for many of the estimated 425 million diagnosed and undiagnosed OSA patients worldwide suffering from moderate to severe OSA. |
| ● | Growing body of clinical data and long-term clinical strategy. The Genio system is predicated on a well-established mechanism of action of electrically stimulating the hypoglossal nerve. Our BLAST OSA trial provided positive data for the Genio system, demonstrating that treatment with the Genio system resulted in statistically significant improvements in sleep apnea symptoms and quality of life measures. These data results were also associated with high therapy compliance. The trial’s results supported receipt of the CE-Mark in 2019 and have been published in peer-reviewed journals, including the European Respiratory Journal. We are continuing our clinical research to evaluate the efficacy of the Genio system on a longer-term basis through our post- market clinical trial for the treatment of OSA in adults, or the EliSA trial. In December 2020, we implanted the first patient in the DREAM trial, which is designed to support marketing authorization in the United States. In addition, in June 2021, we announced initial top-line results from the six-month data for the BETTER SLEEP trial. Based on this data, in October 2021, we expanded the CE-Marked indication to include OSA patients with CCC, which should eliminate the need for a DISE procedure. In September 2021, we received breakthrough device designation in the United States for the Genio system from the FDA for the treatment of OSA with CCC, based on the initial clinical evidence from the BETTER SLEEP trial. Further, in June 2022, we announced that the FDA approved the use of our next generation Genio 2.1 system for use in the DREAM trial. In June 2023, we presented 12-month data on the first 34 DREAM patients reaching 12-month follow-up as a late-breaking abstract at SLEEP 2023, a joint meeting of the American Academy of Sleep Medicine and the Sleep Research Society, demonstrating a 65% AHI responder rate, a 76% ODI responder rate and safety in line with expectations. On March 19, 2024, we issued a press release announcing that the DREAM trial met its primary endpoints. For more information see “—Clinical Results and Studies—Pivotal DREAM Trial”below. These data are preliminary and not conclusive of final success of the DREAM trial. Additionally, in July 2022, we announced that the FDA approved an IDE to enable us to initiate a clinical trial, called ACCCESS, to evaluate the use of the Genio system for the treatment of adult patients with moderate-to-severe OSA with CCC that have failed, did not tolerate, or refused PAP. |
62
| ● | Significant product development and new indication pipeline. The Genio system is a scalable-technology platform that allows for future external hardware, software and firmware updates to enhance therapeutic capabilities without requiring additional surgical procedures. We continue to invest in improving the Genio system to develop next generation products with features designed to improve patient comfort and compliance, efficacy and patient and market acceptance. Some of these improvements include features aimed at enhancing the physician’s ability to monitor patient compliance and therapeutic efficacy, including sensor technology to monitor a patient’s sleep position. We are also committed to expanding current treatment options for moderate to severe OSA patients by developing next generation neurostimulation-based technologies. We previously entered into a licensing agreement with Vanderbilt University pursuant to which we are exploring additional neurostimulation technologies. Under the agreement, we have an exclusive, worldwide license to make, use, sell or distribute products for treating sleep disordered breathing covered by certain patent rights owned, or that may be owned, by Vanderbilt. We will also work together with Vanderbilt University to continue prosecution of patent applications made by Vanderbilt. |
| ● | Platform technology protected by comprehensive and broad intellectual property. Our platform technology is supported by a strong and growing portfolio of intellectual property rights, which includes utility and design patents, know-how and trade secrets, including therapy protocols, electrodes and methods. As of December 31, 2023, we had 199 granted or pending patent applications (with 54 issued or allowed U.S. patents), and 40 pending patent applications, ten of which are U.S. pending patent applications and hold six trademark registrations (with three U.S. trademark registrations). Additionally, we operate a manufacturing facility responsible for silicone overmolding and select assembly of external components, which provides us with enhanced proprietary know-how and control of the supply chain to meet future demand. |
| ● | Strong and experienced team. Our senior management team has many years of experience in the healthcare and medical device industry. Specifically, our team has extensive operating experience in product development, clinical, regulatory approval and commercialization activities as well as established relationships with industry leaders in the academic, clinical and commercial neuromodulation industries. Members of our management team have served in leadership positions with well-regarded medical technology companies such as St. Jude Medical Inc., Medtronic Inc., Stryker Corp and Nevro Corp. Since our founding, we have been supported by a seasoned Board of Directors with extensive industry and public company experience and a Scientific Advisory Committee that consists of industry-relevant KOLs. |
Our Strategy
Our mission is to become a global leader in providing innovative, clinically proven solutions to treat patients suffering from OSA. The key elements of our strategy to achieve this goal and promote future growth include:
| ● | Obtaining marketing authorization in the United States. We are conducting clinical trials to further evaluate the efficacy and safety of the Genio system for treating patients with moderate to severe OSA. We are currently conducting the DREAM trial, a pivotal trial designed to support marketing authorization for the Genio system in the United States via a premarket approval, or PMA, application. The DREAM trial is a multicenter, prospective, open-label trial designed to enroll 115 patients in approximately 20 centers in the United States and internationally. The trial aims to evaluate the safety and effectiveness of the Genio system to treat patients with moderate to severe OSA who either did not tolerate, failed or refused first-line PAP therapy. In June 2022, we announced that the FDA approved the use of our next generation Genio 2.1 system for use in the DREAM trial. On March 19, 2024, we issued a press release announcing that the DREAM U.S. pivotal trial met its primary endpoints. For more information see “—Clinical Results and Studies—Pivotal DREAM Trial” below. We expect to apply for marketing authorization in the United States with the aim of being commercially available in the United States in late 2024. |
63
| ● | Promoting awareness of the Genio system among physicians, patients and payors to accelerate market adoption. We believe that the Genio system has the potential to become the leading neurostimulation solution for moderate to severe OSA patients. To accomplish this, we intend to raise market awareness and educate physicians, payors and patients on the negative impact of OSA and position the Genio system as a safe and effective treatment for moderate to severe OSA patients. We currently offer education and training programs to sleep centers and surgeons, which we believe provide a better understanding of the Genio system’s benefits and increase surgeons’ confidence implanting our technology. In addition, we provide programs targeted towards patients who use the Genio system to promote and increase their engagement, long-term observance, quality of life and well-being. We intend to establish long-term partnerships with KOLs, ENTs and sleep scientific societies and patient associations that are built on mutual trust and oriented towards the needs of OSA patients and their families. Finally, we intend to establish relationships with government and commercial payors to help reduce barriers to treating OSA by highlighting our clinical data, costs affiliated with untreated OSA patients and the clinical benefit of the Genio system. We plan to build upon this multi-pronged approach with direct-to-consumer marketing initiatives that help to educate patients and can frequently result in patient leads. |
| ● | Continuing to enhance the Genio system and expand its indications. We continue to invest in our solutions and services to further improve the implantation procedure and enhance the patient experience and product features. Potential feature improvements could include design alterations, information driven integrated capabilities, diagnostics or monitoring, sleep apnea testing or various other technological advancements. We believe that bilateral stimulation could lead to better therapeutic performance and address more therapeutic indications compared to other hypoglossal nerve stimulation-based technologies. In June 2021, we announced initial top-line results from the six-month data for the BETTER SLEEP clinical trial. Based on this data, in October 2021, the EU Notified Body granted CE-Marked indication to include OSA patients with CCC for the Genio system in Europe. Currently, CCC patients are contraindicated for other HGNS OSA therapies. Further, in June 2022, we announced that the FDA approved the use of our next generation Genio 2.1 system for use in the DREAM trial. In July 2022, we obtained the CE-Mark for the Genio 2.1 system. In addition, we may look for strategic opportunities, including partnerships or collaborations, to broaden our capabilities and expertise in line with our patient-centric vision. |
| ● | Pursuing and establishing favorable reimbursement coverage of the Genio system. While there is general consensus among physicians and payors of the medical necessity to treat OSA and increase the number of HGNS therapy coverage decisions, we continue to develop further clinical evidence intended to demonstrate a long-term meaningful improvement in health outcomes for patients meeting the specified criteria. We are initially targeting markets in Europe where we have identified a clear reimbursement pathway or execution strategy. In Germany, we have successfully obtained reimbursement under a dedicated DRG code for HGNS. In Switzerland, we obtained reimbursement under an OSA-specific DRG code by the Federal Statistic Office, or BFS. Each of these reimbursement coverages includes the cost of the Genio system, implant procedure, hospital stay and follow-up care. We expect that the outcomes of the ongoing pivotal DREAM trial, if positive, will support marketing authorization and reimbursement in the United States. We believe that establishing and maintaining reimbursement will be important in achieving broad acceptance of our system by healthcare providers in these markets. |
64
| ● | Continuing to build a commercial infrastructure in selected geographies. We have grown our commercial team to include a sales and marketing organization of over a dozen representatives with substantial medical device sales, education and clinical experience to support commercialization of the Genio system. Our initial strategy is to employ a targeted approach to increase therapy penetration within specific physician practice groups instead of a broad outreach strategy to physicians in general. Our sales and marketing organization is focused on prioritizing high volume centers that are strategically located and building long-standing relationships with key physicians with strong connections to the population of OSA patients indicated for the Genio system. We are focusing our efforts on developing Centers of Excellence in each of our commercial markets, where we plan to invest in developing the Genio system as the preferred treatment option for indicated moderate to severe OSA patients. Using a direct commercialization model in most of our target countries, we plan to utilize account managers to support these Centers of Excellence to strengthen the referral physician network, guiding new patients to these Centers of Excellence. We expect to gradually scale up our commercial organization in line with market entry and access in the various countries that we are targeting. Based on our experience gained from the commercial roll-out in Europe, but also taking into account particular dynamics of the local markets, we will determine and prepare what we believe to be the optimal sales and marketing structure for commercial launch in the United States if we obtain marketing authorization. |
Market Overview
Overview of Obstructive Sleep Apnea
OSA is the most prevalent sleep disordered breathing condition. It is estimated that OSA currently affects approximately 936 million people globally between the ages of 30 and 69, of which approximately 425 million people suffer from moderate to severe OSA and require treatment. Every year, there are over 5.3 million new patients diagnosed with moderate to severe OSA, representing approximately 2.6 million in the United States and 2.6 million in our initial target markets in Europe.
OSA occurs due to the relaxation of the soft tissue, throat and tongue muscles in a patient’s airway causing an obstruction that temporarily prevents breathing during sleep. In patients with OSA, the airway repeatedly becomes partially or completely blocked thereby limiting the airflow reaching the lungs to sufficiently oxygenate the blood. During an obstruction, the patient’s oxygen level in the blood, or SpO2, drops, causing an increase of their Oxygen Desaturation Index, or ODI, leading to significant and repeated sleep interruptions. The lack of airflow can last anywhere from ten seconds to more than a minute and, in severe cases, may occur 30 or more times during an hour of sleep. When the airway becomes blocked, the brain detects a stress signal from various biological sources including the chest muscles, lungs and, at times, a drop in blood oxygen content that causes the individual to awaken unconsciously, just enough to tighten the airway muscles and allow normal breathing to resume. A hypopnea is a partially blocked airway; apnea is a fully blocked airway. While regular breathing is restored temporarily, the obstruction typically occurs again, which restarts the apnea cycle. This cycle of obstructions and waking can repeat dozens of times per hour throughout the night, disrupting the rapid eye movement and deep, restorative sleep that are critical to maintaining good health. The overall quality of a patient’s sleep, health and quality of life are diminished.
The total number of apneas and hypopneas per hour of sleep is referred to as the Apnea-Hypopnea Index, or AHI. The severity of OSA is based on the following four AHI categories and corresponding events per hour:
Categories |
| AHI Range |
|
Normal Range | <5 Events Per Hour | ||
Mild OSA | 5-14 Events Per Hour | ||
Moderate OSA | 15-30 Events Per Hour | ||
Severe OSA | >30 Events Per Hour |
65
The figure below illustrates the physiologic blockage experienced by patients with OSA.
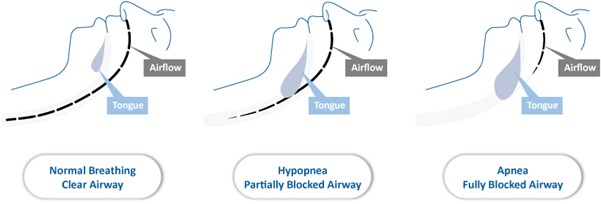
Moderate to severe OSA patients require a dedicated therapy according to published guidelines by sleep doctors’ scientific societies such as the American Academy of Sleep Medicine. If left untreated, OSA is associated with increased mortality risk and significant comorbidities, including cardiovascular diseases, depression and stroke.
Symptoms and Diagnosis of OSA
OSA is a serious and chronic sleep breathing disorder that negatively impacts a patient’s sleep, health and quality of life. Due to the poor quality and lack of sleep, OSA patients often feel tired and fatigued during the day. They may find it difficult to concentrate and experience emotional stress, including depression. Patients struggling with OSA are typically unaware of their condition as OSA remains significantly underdiagnosed. While sleep apnea has traditionally been perceived as a lifestyle disease, with snoring and tiredness as the main implications, it is now known to be a major underlying risk factor and disease progression accelerator for most cardiovascular diseases and many cognitive and neurodegenerative diseases. Despite the increased availability of diagnostic technology, approximately 80% of people in the United States suffering from sleep apnea are undiagnosed. In recent years, increased awareness of the importance of sleep and the devastating potential consequences of sleep apnea have been on the rise in medical communities, and among patients and patient association groups.
Common first indicators of OSA are a patient’s heavy snoring, excessive daytime sleepiness, headaches, depression, memory or concentration problems, nighttime gasping and dry mouth or sore throat. The impact of heavy snoring creates unrest for both the patient and the patient’s bed partner and often drives the patient to obtain medical advice and potential diagnosis. Once a physician makes a preliminary diagnosis, the patient may undergo an in-lab sleep trial, or a home sleep apnea test, or HSAT, to obtain a clinically validated diagnosis of OSA. An in-lab sleep trial requires the patient to stay overnight at a sleep center, where nasal air tubes and sensors, electrodes and wires are attached to various parts of the body, including the head, chest and abdomen. The system of monitors and sensors measure the patient’s airflow, sleep quality, blood oxygen levels and breathing patterns. More recently, sleep doctors and cardiologists have begun prescribing HSAT in lieu of an in-lab sleep trial to help diagnose OSA. HSATs are low-cost, self- administered portable devices that allow patients to be tested in the comfort of their own homes while offering greater ease of use. Data is collected, downloaded and interpreted by a board-certified sleep physician. While an in-lab sleep trial is currently considered the standard of care for OSA diagnosis, we expect HSAT acceptance and utilization to continue to increase thereby reducing the percentage of undiagnosed OSA patients.
66
Comorbidities Associated with OSA
OSA may also be associated with severe medical comorbidities, including coronary artery disease, cardiac arrhythmias such as atrial fibrillation, heart failure, hypertension, obesity, stroke and Type 2 diabetes.
The following graphic summarizes the high prevalence of OSA in key chronic diseases.
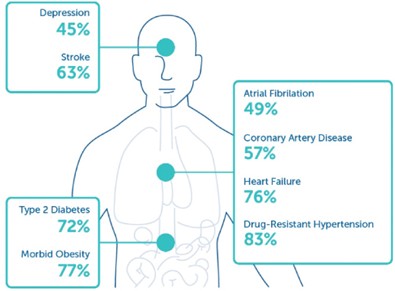
Several peer-reviewed, published studies have shown that sleep apnea is a direct contributing factor to the incidence of various forms of cardiovascular disease. Cardiovascular disease is highly prevalent and, often, a severe and potentially fatal medical condition. There is increasing awareness among cardiologists and the general population of the importance of sleep apnea in the causation or promotion of hypertension, coronary artery disease, heart failure, atrial arrhythmias and strokes, and, consequently, a predictor of premature cardiovascular disease.
Published clinical literature, including an 18-year mortality follow-up trial at the University of Wisconsin, has demonstrated strong correlation between OSA and the risk of mortality. Based on the 1,522-person Wisconsin Sleep Cohort sample, participants with untreated moderate and severe OSA experienced a significant impact on mortality with survival rates of approximately 85% and 60%, respectively. Untreated OSA can be deadly, as untreated OSA patients have two times more risk of suffering stroke, two and a half times more risk of heart failure and five times more risk of cardiovascular mortality. Numerous studies have demonstrated the correlation between efficient OSA therapy and the reduction of mortality and comorbidities.
67
The chart below illustrates the significant impact on mortality and survival rates over time based on severity of OSA.
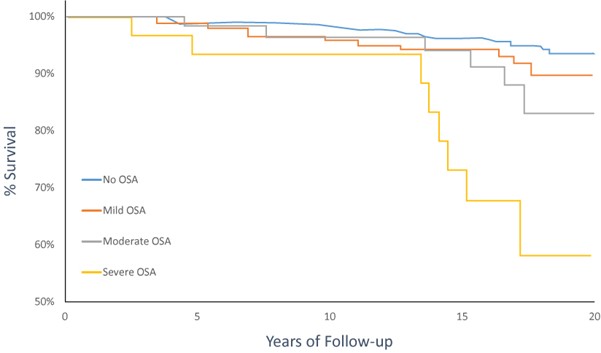
Economic Costs of Untreated OSA
The socio-economic burden of OSA stems from both direct and indirect health costs. According to a Harvard Medical School trial published in December 2010, the annual economic cost of unmanaged OSA was estimated to be $67 billion to $165 billion based on total direct healthcare costs, incremental healthcare costs from comorbidities, non-medical traffic accident-related costs due to increased fatigue, non-medical workplace accident-related costs, OSA-driven job absenteeism costs and other family-related and other societal costs. This amount is greater than the estimated annual economic cost in the United States of asthma, heart failure, stroke and hypertensive disease, each estimated at $20 to $80 billion annually.
The direct costs associated with OSA include the costs for diagnosis and treatment and associated medical conditions, several of which also result in impaired work productivity and road traffic accidents that give rise to indirect health costs. People with unmanaged OSA are about two to three times more likely to have a traffic accident. OSA is associated with an increase in the rate and severity of motor vehicle accidents, increased healthcare utilization, reduction of work performance and occupational injuries.
Existing Treatments for OSA
There are several treatment options available to OSA patients, including medical management, involving lifestyle changes such as weight loss, CPAP therapy, mandibular advancement devices, or MADs, surgical interventions, and advanced neuromodulation devices.
CPAP
CPAP is a treatment whereby air, at a constant or automated pressure, is pushed into the upper airway via a facial or nasal mask that the patient must wear all night. CPAP has demonstrated efficacy in reducing AHI, as well as improving patient sleep quality and daytime sleepiness. Since its introduction in the 1980’s, CPAP therapy has been the first-line therapy for OSA patients of all severities. However, the efficacy of CPAP therapy is directly correlated to the number of hours of use per night and its long-term compliance.
68
Poor patient compliance and discomfort have been major factors in the efficacy of CPAP treatment. Patients often struggle with sustained regular use of the CPAP device due to mask discomfort, mask leakage, pressure intolerance, skin irritation, nasal congestion, nasal drying, nosebleeds, claustrophobia and lack of intimacy. In addition, the airway pressure can cause severe dryness in the nose and mouth, resulting in the sense of suffocation and nasal congestion. Medicare defines compliance as using a CPAP device at least four hours a night for 70% of nights during any consecutive 30-day period within the first three months of initial usage. CPAP non-compliance is estimated to be between 29% and 83%, and we estimate the CPAP non-compliance rate to be approximately 35%.
Oral Appliances — Mandibular Advancement Devices
MADs are similar to orthodontic retainers and are intended to diminish restrictions that occur in the back of the throat by moving the jaw and tongue forward to increase the size of the upper airway and reduce the air resistance that leads to snoring. These devices are utilized nightly by patients. Due to their form factor, MADs have multiple limitations, including tooth and jaw pain, potential tooth displacement and recurrent dental follow-ups. According to published literature, MADs generally provide unpredictable therapy efficacy.
Surgery to Remove or Reposition Patient Tissue or Bone
For patients who have difficulties utilizing or complying with CPAP and MADs, invasive surgical procedures for the nose, throat or mandible, such as uvulopalatopharyngoplasty, or UPPP, and maxillomandibular advancement, or MMA, can be beneficial alternatives. Surgery is suggested to patients with specific anatomical conditions, but this is a highly invasive procedure that irreversibly alters the patient’s anatomy, requires extended and painful recovery periods, and has only moderate efficacy. For example, MMA involves enlarging the airway by surgically moving the upper jaw (maxilla) and lower jaw (mandible) forward. The surgical procedure can last up to four or five hours and the patient can only return to work after four to five weeks. Therefore, surgical procedures are often considered as last resort options, due to their invasiveness, cost, the high incidence of side effects and varying responder rates, which are estimated to be between 30% and 60%.
Hypoglossal Nerve Stimulation, a Proven Strategy to Treat OSA
Over the last decade, technologies focused on the stimulation of the hypoglossal nerve have emerged as an alternative treatment option for moderate to severe OSA patients who refused, do not tolerate or are not compliant with conventional CPAP therapy. The hypoglossal nerve controls the tongue and airway muscles. By stimulating the hypoglossal nerve, these therapies trigger the contraction of the tongue muscles and thereby help maintain an open airway during sleep.
Inspire Medical Systems, Inc. is a publicly-traded medical technology company offering FDA-approved unilateral neurostimulation technology. Inspire Medical’s STAR trial demonstrated an approximately 70% reduction in AHI from a baseline of 29.3 events per hour to 9.0 events per hour at 12 months following initial treatment and a 66% responder rate, defined as the rate of patients that achieved a decrease in AHI of at least 50% and a residual AHI of less than 20 events per hour. Inspire Medical published its pivotal five-year STAR trial in 2018. At five years, the STAR trial reported a 75% responder rate, defined as the rate of patients that achieved a decrease in AHI of at least 50% and a residual AHI of less than 20 events per hour. At five years, median AHI in patients with moderate to severe OSA remained low at 6.2 events per hour.
Many countries, including the Netherlands, Germany, Switzerland and the United States, already recognize the benefits of HGNS as a therapy for moderate to severe OSA and have provided requisite reimbursement for the therapy.
Limitations of Competing Hypoglossal Nerve Stimulation Devices
We are aware of two competing HGNS devices for use in treating patients with OSA. The most widely-used HGNS device is the Inspire system. The Inspire system consists of a remote control and three implantable components: a pressure sensing lead, which detects when the patient is attempting to breathe; a neurostimulator, which houses the electronics and battery power for the device; and a stimulation lead, which delivers electrical stimulation to one branch of the hypoglossal nerve. The other device is similar to the Inspire system, but its implantable pulse generator uses only one lead and contains a rechargeable battery.
69
While the benefits of HGNS have been well-recognized, we believe competing HGNS solutions suffer from several limitations, including:
| ● | Neurostimulator with Internal Battery |
| o | Competing neurostimulation systems for the treatment of OSA rely on an implanted neurostimulator that includes an internal battery. |
| o | In most cases, the internal battery cannot be recharged and, once depleted, the neurostimulator must be replaced in a further surgical procedure. In some cases, the battery can be recharged by the patient, but will eventually become depleted and require surgery to be replaced. Additional procedures may result in an increased risk of infection at the incision site. |
| o | The neurostimulator has been designed to be large enough to accommodate the additional space necessary for the battery. As a result, the neurostimulator is positioned in a subcutaneous pocket, and the device may be palpable or visible in the chest area. |
| o | Given the design of the implanted neurostimulator used by competing systems, those systems have either received 1.5T MRI clearance only or don’t have MRI clearance. |
| ● | Multiple Implantable Components Requiring Multiple Surgical Incisions |
| o | Competing systems require multiple parts to be implanted including leads and a cuff electrode; |
| o | Competing systems require multiple surgical incisions and subcutaneous lead tunneling including bringing a lead from the pulse generator to the neck and bringing a lead from the pulse generator to the respiratory system, which monitors breathing. These multiple steps during implantation lead to an average implantation procedure of approximately 2 hours, which, combined with additional incisions, can result in an increased risk of surgical infection. |
| ● | Unilateral Stimulation |
| o | Unilateral stimulation delivers stimulation to only one branch of the hypoglossal nerve, which limits options for nonresponding or contraindicated patients, including patients with CCC. |
The Genio System Market Opportunity
Despite the availability of diagnostics and device-based and surgical treatments, the market for OSA therapy remains highly underpenetrated. OSA is the world’s most common sleep disordered breathing condition, affecting approximately 936 million people between 30 and 69 years of age globally, of which an estimated 425 million suffer from moderate to severe OSA and require treatment. According to the 2019 Lancet Respiratory Medicine Journal, the estimated prevalence of moderate to severe OSA patients between 30 and 69 years of age in our targeted commercial markets is approximately 63 million people.
The figure below summarizes the prevalence of moderate to severe OSA patients among our targeted initial commercial markets.
ESTIMATED MODERATE TO SEVERE OSA PREVALENCE BY COUNTRY
Prevalence of | Percentage of |
| |||||
moderate-to- | moderate-to- |
| |||||
Population | severe OSA in | severe OSA in |
| ||||
aged | 30 – 69Y old | 30 – 69Y old |
| ||||
30 – 69 years | population | population |
| ||||
United States |
|
|
|
|
|
| |
United States |
| 163,246,772 |
| 23,678,109 |
| 14.50 | % |
INITIAL TARGET MARKETS |
|
|
|
|
|
| |
European Countries |
|
|
|
|
|
| |
Germany |
| 43,751,645 |
| 14,393,964 |
| 32.90 | % |
Spain |
| 26,158,266 |
| 4,233,728 |
| 16.20 | % |
Netherlands |
| 9,050,266 |
| 2,582,583 |
| 28.50 | % |
Belgium |
| 5,917,763 |
| 931,859 |
| 15.7 | % |
Switzerland |
| 4,518,615 |
| 1,654,232 |
| 36.60 | % |
Total Initial Target Markets |
|
|
|
|
|
| |
Total |
| 89,396,555 |
| 23,796,366 |
| 26.62 | % |
70
OSA therapy is a large and growing market. We believe there is a significant population in the United States with moderate to severe OSA who are unable to use or achieve the intended clinical benefit from CPAP and who would be eligible for the Genio system upon approval. Published scientific literature estimates that there are currently approximately 23.8 million individuals with moderate to severe OSA in our initial target markets in Europe. Based on published scientific literature, we estimate that approximately 2.6 million patients are diagnosed annually in those countries and that approximately 80% of diagnosed patients are prescribed a CPAP device. Published scientific literature reports non-compliance rates to CPAP between 29% and 83%. Based on these data, and for purposes of calculating the total addressable market in Europe for the Genio system, we estimate that approximately 35% of patients that are prescribed CPAP in those countries are not compliant with the therapy. Additionally, certain patients possess anatomical characteristics, including higher body-mass index or increased tongue fat deposition that make them ineligible for HGNS. Taking that into account, we estimate that approximately 70% of those non-compliant patients are eligible for HGNS based on their anatomical characteristics. As a result, we believe the total addressable market in Europe for the Genio system is at least 515,000 patients annually, which represents an estimated annual market opportunity of approximately $10 billion based on our current pricing for the Genio system. We also plan to enter the United States market, assuming we obtain marketing authorization in the United States, where published scientific literature estimates there are approximately 23.7 million individuals with moderate to severe OSA. Based on the same assumptions set out above, we estimate a target market of approximately 510,000 patients in the United States, which represents an estimated annual total addressable market of approximately $10 billion based on our current pricing for the Genio system.
Our Solution
We developed the Genio system to provide patients suffering from moderate to severe OSA with an alternative HGNS system that addresses their unmet needs. We believe our minimally invasive and clinically proven solution has the potential to become the leading neurostimulation solution for many patients suffering from moderate to severe OSA, including patients with CCC. The Genio system has obtained CE-Mark and we are currently pursuing FDA marketing authorization.
Overview of the Genio system
The Genio system is the first neurostimulation system for the treatment of OSA to include a battery-free and leadless neurostimulator capable of delivering bilateral HGNS. The system includes an implanted component that can be implanted in a minimally invasive procedure requiring only a single incision. We developed the system using a patient-centric approach to offer patients a convenient alternative design to overcome the limitations of competing neurostimulation devices.
Components of the Genio system
| ● | Implantable Stimulator. The implantable stimulator consists of a saddle-like antenna with two legs, each containing two metal pads, called paddle electrodes. The paddle electrodes are placed in contact with both branches of the hypoglossal nerve and deliver bilateral stimulation to the hypoglossal nerve. Pulses from the stimulator trigger a slight forward movement of the posterior portion of the tongue in order to maintain an open airway throughout the night. The implantable stimulator is FDA and CE labeled as MR conditional for 1.5T and 3T full body MRI scans. |
| ● | Activation chip. The activation chip is a detachable, external power source for the implantable stimulator and is composed of a chipset, which provides the patient’s personalized therapy program, and a rechargeable battery. The chipset is programmable, which allows us to make future updates and upgrades, or to provide additional services to the Genio system without having to replace the implantable stimulator during an additional surgery. We advise that patients charge the activation chip with the charging unit after use. |
| ● | Disposable patch. The disposable patch is a single-use, medical grade adhesive patch, which also contains a transmitting coil. The patch is placed on the skin under the chin each time before the patient goes to sleep. The patient attaches the activation chip to the disposable patch, which then activates the implantable stimulator. After use, the patient detaches the activation chip from the chin, places it in the charging unit, and disposes of the patch. |
| ● | Charging unit. The charging unit and its power adapter are used to charge the activation chip’s battery. A fully depleted activation chip can be charged on the charging unit within 3 hours. |
71
| ● | External stimulator. In addition to the patient-use components described above, the system includes an external stimulator which is a disposable single-use device that is used during the implantation procedure by the surgeon to test activation and function of the implantable stimulator. |
Benefits of the Genio System
We designed the Genio system to advance patient care and provide a convenient treatment option to the large and underpenetrated patient population suffering from OSA. We believe the following factors offer meaningful benefits for patients, physicians and payors that have the potential to drive broad adoption of our system:
| ● | Patient-centric therapeutic option. The results of our BLAST OSA trial demonstrated safety and effectiveness of the Genio system for patients suffering from moderate to severe OSA, and the data were sufficient to obtain a CE-Mark from the European Notified Body. These results showed significant benefits in the following patient-centered outcomes: |
| ● | Attractive safety profile. The results from the BLAST OSA trial demonstrated that the Genio system was well tolerated with no device-related serious adverse events, or SAEs, reported during the first 6-months of the trial. |
| ● | Compelling clinical data. Clinical data suggest that the Genio system is a clinically effective therapy for patients eligible for HGNS treatment. The BLAST OSA trial found a 47.3% reduction in mean individual AHI (p-value<0.0001) and a decrease in mean individual ODI of 43.3% (p-value<0.0001) at six months following implantation, compared to their baseline measurements, for patients using the Genio system. In statistics, a p-value is a number calculated from a statistical test. It provides the probability that a null hypothesis (e.g., there is no treatment effect) is true for the particular set of observations being tested. The smaller the p-value (typically p-value < 0.05), the stronger the evidence that the null hypothesis should be rejected in favor of an alternative hypothesis (e.g., there is a treatment effect greater than a given threshold). A p-value less than 0.05 is said to be statistically significant. It indicates strong evidence against the null hypothesis, as there is less than a 5% probability that the null hypothesis is correct. |
| ● | Convenient therapy leading to strong compliance. Our device is designed to be convenient for patients to use, once implanted and optimized, requiring no additional programming or therapy titration. The BLAST OSA data reported that 91% of patients used the system more than five nights per week over a period of six months following implantation. |
| ● | Improved quality of life. Results from the BLAST OSA trial demonstrated that patients’ quality of life significantly improved as assessed using the FOSQ-10 questionnaire, with an increase in mean score by 1.9 units (p-value=0.0157) and a decrease on the Epsworth Sleepiness Scale, or ESS, score, by a mean of 3.3 units (p-value=0.0113). Additionally, the number of sleep partners who reported that their partner did not snore, or snored only softly, increased from 4.2% at baseline to 65.0%. |
| ● | Bilateral hypoglossal nerve stimulation. The Genio system was designed to provide bilateral stimulation of the hypoglossal nerve. We believe bilateral stimulation results in a stronger muscle contraction, a more symmetric tongue movement and a wider opening of the airway, which we believe has the potential to provide better clinical outcomes. We also believe that the bilateral stimulation of the Genio system has the potential to treat moderate to severe OSA in patients with CCC. These patients are currently contraindicated for other HGNS systems. |
| ● | Minimally invasive implant procedure and design. The Genio system only has one implantable, low-profile component, which is leadless and battery-free, and only requires a single incision for implantation. The surgical implantation occurs during an outpatient procedure that lasts approximately one hour. Importantly, our system relies on our proprietary duty cycle stimulation algorithm to control the frequency and strength of the neurostimulation. As a result, our system does not require the implantation of a sensing lead to monitor breathing. We believe that the minimally invasive procedure enables patients to recover quickly and resume normal activities within a week. We also believe that our single-incision implantation process will facilitate adoption by a growing number of physicians and surgeons. |
72
| ● | External activation chip and battery. The Genio system’s power source is located in the external activation chip, requiring no battery to be implanted in the patient. Similarly, the external activation chip also includes the software for each user’s personalized therapy and can be updated or upgraded without the need for an additional surgical intervention. By eliminating the need for additional surgeries to replace a depleted battery and by enabling updates without additional surgeries, we believe the Genio system may offer a potential reduction in systematic healthcare costs. |
Treating patients with the Genio system
Patient selection
Under CE-Mark approval, the Genio system is indicated for adult patients suffering from moderate to severe OSA with an AHI equal to or greater than 15, but less than 65 events/hour. The Genio system is intended as a second-line therapy for patients who do not tolerate, or who fail or refuse CPAP therapy.
A variety of considerations are required to assess if a patient is eligible for the Genio system. Patients may only have a body mass index, or BMI, of up to 35kg/m². Additionally, patients cannot have any medical illness or condition that contraindicates a surgical procedure under general anesthesia or that would prevent the implantation. Current contraindications for the device include: major craniofacial abnormalities that narrow the airway or the implantation site or that would impair the functioning of the hypoglossal nerve stimulator and congenital malformations of the larynx, tongue and throat.
Once a patient is diagnosed with moderate to severe OSA and either fails, does not tolerate or refuses CPAP treatment, they become eligible for HGNS.
Implantation
A surgeon implants the implantable stimulator of the Genio system during a minimally invasive procedure that requires only one incision and typically lasts approximately one hour in an out-patient setting under general anesthesia. During implantation, the surgeon makes a small curvilinear incision approximately six centimeters in length under the chin to expose the genioglossus muscle and the left and right hypoglossal nerve branches through dissection of multiple muscle layers. The Genio system’s specifically designed and unique paddle electrodes allow the surgeon to position the implant stimulator over both genioglossus muscles facing both medial left and right branches of the hypoglossal nerve to allow bilateral stimulation. During surgery, the surgeon applies the disposable, single use external stimulator to test activation and function of the implantable stimulator. Once function is verified, the surgeon sutures the implantable stimulator to the muscle to secure fixation. After fixing the stimulator, the physician closes the incision. Patients are typically discharged the same day. While patients may experience mild discomfort or swelling at the incision site, often associated with minimally invasive procedures, this can be managed with over-the-counter pain medications. Patients can return home after completion of the procedure and generally recover within a few days and are able to resume normal activities within a week.
Therapy activation and optimization
Within approximately six weeks following implantation, the patient returns to the physician for a follow-up visit where the physician activates the Genio system. The physician also provides appropriate patient training on how to use the different components of the device and to activate the therapy. Once activated, the patient can start using the Genio system during sleep.
73
The exact level of stimulation varies between patients based on the response of their hypoglossal nerve to the Genio system. Once activated, the patient enters the first phase of the therapy process, during which the device operates using low stimulation parameters that allow the patient to acclimate to the sensation and tongue movement of stimulation. Once the patient is acclimated to therapy, the second phase of therapy begins. This phase is designed to identify the patient’s individual and specific therapeutic levels and patterns of stimulation during wakeful titration and studies performed in a sleep lab. The goal of the wakeful titration is to identify the optimal tongue contraction characteristics including direction and intensity using nasal endoscopy. Therapy titration is typically completed in one or two visits. The Genio system delivers stimulation at a programmed rate determined by the physician based on the patient’s breathing frequency. To determine the appropriate rate, the patient’s breathing frequency is initially analyzed during an in-lab sleep trial, and the stimulation pattern is adjusted using our proprietary duty cycle algorithm, which provides timely, alternative cycles of stimulation with patient-specific targeted therapy. Once the physician determines the desired titration and stimulation pattern, the physician programs the Genio activation chip to deliver patient-specific therapy based on those levels and patterns. At the optimal titration setting, the physician aims to keep the upper airway open during sleep resulting in blood oxygen saturation, and sleep continuity without waking the patient.
The figure below illustrates the algorithmic, alternating stimulation cycle that is designed to maximize the Genio system’s efficacy.
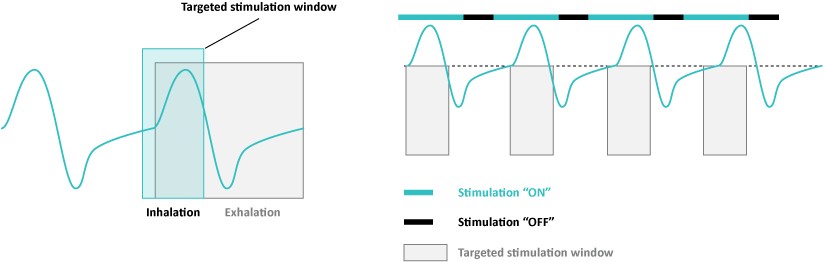
Daily home stimulation and use
Once the Genio system is activated and optimized, the patient uses the system at home while asleep to alleviate the symptoms of their moderate to severe sleep apnea. We recommend that the patient visit their physician once a year for a routine follow up where therapy efficacy can be evaluated and adjustments made as needed.
Clinical Results and Studies
We continue to invest in developing a substantial body of clinical evidence to support the safety and efficacy of the Genio system. Our clinical strategy consists of obtaining authorization in our target markets, demonstrating long-term clinical data for the Genio system and expanding authorized indications to reach a broader patient population, including patients with CCC. We have completed one clinical trial and are conducting three clinical trials globally with the goal of generating compelling and reproducible results with the s
74
BLAST OSA Trial
Overview
The BLAST OSA trial was a prospective, open-label, non-randomized, multicenter, single-arm trial initiated in April 2017 with enrollment completed in February 2018. The objective of this trial was to evaluate and assess the safety, performance and efficacy of the Genio system in adult patients with moderate to severe OSA. The trial measured safety and efficacy endpoints at six months following five months of treatment. The primary safety endpoint was the incidence of device-related SAEs recorded during the trial over a period of six months post implantation. The primary efficacy endpoint was the mean change in the AHI score from baseline to six months post implantation measured by the number of apneas and hypopneas events per hour during an overnight sleep trial. The secondary performance endpoint was the change in the ODI score from baseline to six months post implantation. ODI score was measured by the number of desaturation episodes per hour during an overnight sleep trial. A desaturation period occurs when the patient stops breathing resulting in a decrease in blood oxygen.
Performance measures included changes in the sleep-related quality of life, evaluated by the level of daytime sleepiness using the Epworth Sleepiness Scale, or ESS, and the Functional Outcomes of Sleep Questionnaire, or FOSQ-10, as well as supplementary objective measures evaluated in an in-lab sleep trial, such as therapy response rate. The ESS measures the propensity for daytime sleepiness and the FOSQ-10 questionnaire measures sleep-related quality of life. Therapy response was defined based on the Sher success criteria as a reduction in AHI from baseline to six months of 50% or more, a remaining AHI score at six months of less than 20. The study also evaluated the change in the percentage of time spent at an oxygen desaturation state below 90% (SaO2<90%). Response rate was a percentage of patients passing the Sher success criteria at six months. Sleep partner-reported snoring and nightly usage of the system were also evaluated.
In 2019, the BLAST OSA trial protocol was amended to include a long-term safety follow-up phase. All participants who received the Genio system were eligible to enroll in the long-term follow-up phase of the trial. While the long-term follow-up phase was not initiated, subjects were nevertheless followed up for an additional 36 months before the study was closed out.
BLAST OSA Results
The BLAST OSA results were published in the European Respiratory Journal in October 2019. Screening exclusion criteria included in-lab sleep study test results, AHI that was above 60 or below 20 based on the 2014 American Academy of Sleep Medicine recommended scoring guidelines, or a patient having a non- supine AHI less than 10. Another 18% of patients were excluded from the trial due to CCC. A total of 27 participants underwent the implantation procedure of the Genio system. Of these participants, 63% (17/27) were men with a mean age of 55.9 ± 12.0 years and a mean body mass index of 27.4 ± 3.0 kg/m2. Twenty-two patients completed the protocol, and the trial met all primary, secondary and exploratory endpoints. In the six-month data, the mean individual reduction in AHI events per hour decreased 47.3%. Participants’ AHI decreased from 23.7 ± 12.2 to 12.9 ± 10.1, representing a mean change of 10.8 events/ hour (p-value<0.0001). In statistics, a p-value is a number calculated from a statistical test. It provides the probability that a null hypothesis (e.g., there is no treatment effect) is true for the particular set of observations being tested. The smaller the p-value (typically < 0.05), the stronger the evidence that the null hypothesis should be rejected in favor of an alternative hypothesis (e.g., there is a treatment effect greater than a given threshold). A p-value less than 0.05 is said to be statistically significant. It indicates strong evidence against the null hypothesis, as there is less than a 5% probability that the null hypothesis is correct.
Safety Results
Four SAEs related to the surgical procedure (but not device-related) were reported in three of the 27 patients implanted during the six-month post-implantation period. These included two participants at the same hospital who developed local infections at the surgical site that resulted in removal of the implanted device. The fourth SAE was impaired swallowing, which led to one day prolongation of implantation-related hospitalization. Two patients were kept in the hospital for overnight observation. All SAEs were successfully resolved. The most frequent procedure-related adverse events, or AEs, that occurred in implanted patients were impairment or painful swallowing (30% of participants), dysarthria, or speech- slurring, (26% of participants), hematoma (19% of participants) and swelling or bruising around the incision site (19% of participants).
75
No device-related SAEs occurred during the six-month post-implantation period. The majority of device- related AEs were reported as mild and resolved within days. The most frequent device-related AE was a temporary and mild local skin irritation due to use of the disposable patch (30% of participants). This AE was generally resolved with the application of skin lotion to the irritated skin, and there was no discontinuation of therapy within implanted devices. Additional device related AEs that occurred in 11% of the patients included tongue abrasion, tongue fasciculation, discomfort due to electrical stimulation and abnormal scarring. The adverse reaction to stimulation discomfort was typically resolved by reprogramming the stimulation parameters.
Trial Performance Results
Six months post-implantation, the mean individual reduction in AHI events per hour decreased 47.3%. Participants’ mean AHI decreased from 23.7±12.2 to 12.9±10.1, representing a mean change of 10.8 events/ hour (p-value<0.0001).
AHI at Screening and 6-month for Patients that Reached the 6-month Visit
A reduction in the ODI score was demonstrated between baseline and six-month post-implantation, dropping from a mean of 19.1±11.2 to 9.8±6.9, representing a mean change of 9.3 events/hour (p-value<0.001).
Both the propensity for daytime sleepiness, as measured by the Epworth Sleepiness Scale, and sleep-related quality of life, as assessed using FOSQ-10, significantly improved. The ESS decreased from 11.0±5.3 to 8.0±5.4, representing a mean change of 3.3 units (95% CI 0.8-5.7, p-value=0.0113), whereas the FOSQ-10 score increased from 15.3±3.3 to 17.2±3.0, representing a mean change of 1.9 units (95% CI 0.4-3.4, p-value=0.0157). The FOSQ-10 objective is to demonstrate a change in sleep-related quality of life at the 6-month visit compared to baseline. A FOSQ-10 score greater than 17 is considered clinically significant. A score below 8 for the Epworth Sleepiness Scale is considered clinically significant. Finally, the arousal index (measures shift from deep sleep to light sleep) significantly decreased from 28.7±11.5 to 16.0±8.0 (p- value<0.0001), representing a mean change of 12.7 events per hour.
76
The following chart sets forth the various outcome measures for the intent to treat patient population:
Baseline | 6-months | Mean | ||||||
Outcome | (n=22) | (n=22) | Difference (95% CI) | P-value | ||||
AHI, events/hour |
| 23.7 ± (12.2) |
| 12.9 ± (10.1) |
| 10.8 ± (14.6 to 7.0) |
| <0.0001 |
ODI, events/hour |
| 19.1 ± (11.2) |
| 9.8 ± (6.9) |
| 9.3 ± (13.1 to 5.5) |
| <0.0001 |
FOSQ-10 |
| 15.3 ± (3.3) |
| 17.2 ± (3.0) |
| 1.9 ± (0.4 to 3.4) |
| 0.0157 |
ESS |
| 11.0 ± (5.3)* |
| 8.0 ± (5.4) |
| 3.0 ± (5.7 to 0.8) |
| 0.0113 |
SaO2<90%, % time |
| 5.0 ± (6.0) |
| 2.1 ± (3.0) |
| 2.9 ± (4.6 to 1.3) |
| 0.0015 |
Arousal Index, events per hour |
| 28.7 ± (11.5) |
| 16.0 ± (8.0) |
| 12.7 ± (16.6 to 8.9) |
| <0.0001 |
Sleep efficiency (%) |
| 84.0 ± (10.8) |
| 87.3 ± (8.9) |
| 3.2 ± (0-01 to 6.4) |
| 0.0494 |
Responder rate (Sher Criteria) at 6-month |
| 11 patients out of 22 (50)% |
| NA | ||||
Legend
Data are mean (Standard Deviation) unless otherwise specified. Arousal Index is the number of arousals and awakenings registered during the sleep trial. SaO2 < 90% is the proportion of the night spent at an oxygen saturation below 90%. Sleep efficiency is the ratio of total time spent asleep in a night compared to the total amount of time spent in bed. ESS is the Epworth Sleepiness Scale. FOSQ10 is the 10 — item Functional Outcomes of Sleep Questionnaire. * means n=21.
Other Metrics and Outcomes
The reported snoring intensity was reduced, with 65.0% of patients’ sleep partners reporting no snoring or soft snoring at the six-month post-implantation visit compared to only 4.2% at baseline. Additionally, 91% of patients reported using the Genio system more than five days a week, of whom 77% reported a nightly use of more than five hours per night.
The BLAST OSA trial demonstrated that the Genio system’s therapy was well-tolerated, met its performance endpoints, and was associated with high compliance. The trial showed significant reduction of OSA severity and improvement of sleepiness and quality of life, while being well-tolerated.
BETTER SLEEP Trial
We are currently conducting the BETTER SLEEP trial, a multicenter, prospective, open-label, two-group clinical trial, designed to assess the long-term safety and performance of the Genio system for the treatment of adult OSA patients with and without CCC over a period of 36 months post- implantation. The BETTER SLEEP trial includes a subgroup of CCC patients, which is a patient population that is contraindicated for unilateral HGNS.
Patients with moderate to severe AHI scores (15 ≤ AHI < 65) and aged between 21 and 75 years were eligible for enrollment if they failed, refused or did not tolerate PAP treatment. Patients with a body mass index above 32 kg/m² were excluded. The trial has been authorized by the Australian and New Zealand regulatory authorities and is being conducted in eight local medical centers.
In the BETTER SLEEP trial, 42 patients were implanted with the Genio system, 18 of which have CCC (or 42.9% of the total implanted population) and 24 who were classified as non-CCC. Three patients in each arm did not complete their six-month polysomnography, and as a result, the analysis was calculated based on 36 patients (15 CCC, 21 non-CCC). Of these 36 patients, there were 23 responders (64%), including nine of the 15 CCC patients (60%) and 14 of the 21 non-CCC patients (67%), at six months.
The primary safety endpoint included the incidence of device-related serious adverse events (SAEs) from consent to 6 months post-implant.
Primary and exploratory efficacy endpoints were defined as a mean reduction in AHI (4% oxygen desaturation AHI4) at six months post-implant for the entire cohort and for the CCC subgroup, respectively. Scoring followed the American Academy of Sleep Medicine 2014 acceptable guidelines. Secondary efficacy endpoints included the oxygen desaturation index scored at 4% desaturation (ODI4). Statistical significance was assessed at p<0.05 using paired t-tests.
77
The overall reduction was statistically significant with an 11-point reduction (p<0.001), with statistically significant reductions of 10 points (p=0.001) in the CCC cohort and 11 points (p<0.001) in the non-CCC cohort. In addition, mean AHI4 reduction exceeded 70% among responders in both CCC and non-CCC cohorts. These results are subject to final review and validation.
With respect to the primary safety endpoint, no device-related SAEs up to six months post-implant were reported by the site investigators. The clinical events committee (CEC) identified two device-related SAEs (device migration, infection). Final review and adjudication of SAEs and AEs have not yet been completed by an independent CEC and as a result the characterization of SAEs or AEs could be subject to change.
We expect to announce additional data with respect to the trial as further analyses are conducted and we seek to publish the full data set from the trial in a peer-reviewed publication. There will be no additional enrollment in the BETTER SLEEP trial. However, we will continue to monitor patients in the evaluable patient population and plan to continue evaluating over the course of three years following implantation.
In October 2021, Nyxoah received CE-mark indication approval to treat OSA patients with CCC, based on clinical evidence from the BETTER SLEEP trial.
Additionally, in September 2021, we received breakthrough device designation in the United States for the Genio system from the FDA for the treatment of OSA with CCC, based on the initial clinical evidence from the BETTER SLEEP trial.
EliSA Trial
After having obtained certification in Europe for the Genio system in March 2019, we initiated the EliSA post-marketing trial in Europe for the treatment of OSA in adult patients with moderate to severe OSA. The primary objective of this trial is to evaluate the long-term safety and clinical efficacy of the Genio system in adult patients suffering from moderate to severe OSA. The trial is expected to follow patients over a five-year period. EliSA is a multicenter prospective single-arm post market clinical follow-up trial and is expected to enroll at least 110 patients across approximately 25 investigational centers in Europe.
Pivotal DREAM Trial
In June 2020, the FDA approved our IDE application, allowing us to commence our pivotal DREAM trial of the Genio system. In June 2022, we announced that the FDA approved the use of the Genio 2.1 system in our DREAM trial. Our DREAM trial is a multicenter, prospective, open-label trial in which each participant who undergoes implantation of the Genio system will be followed for five years post-implantation to assess the safety and efficacy of the system in patients with moderate to severe OSA. We initiated the DREAM trial as an IDE pivotal trial to support an application seeking FDA marketing authorization and ultimately, reimbursement in the United States for bilateral HGNS for the treatment of moderate to severe OSA. The trial enrolled 115 patients who have all been implanted as of the date of this Annual Report, with 12-month effectiveness and safety primary endpoints. We have identified 20 centers for the trial, including 15 in the United States. Fifteen of them were active and enrolling patients as of December 2023.
The primary safety endpoint is incidence of device-related SAEs at 12 - months post implantation. One of the co-primary effectiveness endpoints is the percentage of responders with at least a 50% reduction in AHI with hypopneas associated with a 4% oxyhemoglobin desaturation and a remaining AHI with hypopneas associated with a 4% oxyhemoglobin desaturation less than 20, together with a 25% reduction of ODI between baseline and 12month visits. Patients with moderate to severe OSA (AHI score between 15 and 65) and aged between 22 and 75 years are eligible for enrollment if they failed, did not tolerate or refused PAP treatment. Patients with a body mass index above 32 kg/m², a CCC observed during a drug induced sleep endoscopy and combined central and mixed AHI above 25% at baseline polysomnography are to be excluded. - We presented 12-month data on the first 34 DREAM patients reaching 12-month follow-up as a late-breaking abstract at SLEEP 2023, a joint meeting of the American Academy of Sleep Medicine and the Sleep Research Society, demonstrating a 65% AHI responder rate, a 76% ODI responder rate and safety in line with expectations. These data are preliminary and not conclusive of final success of the DREAM trial.
78
On March 19, 2024, we announced that our DREAM trial achieved a statistically significant reduction in the co-primary endpoints of 12-month AHI responder rate, per the Sher criteria, and ODI responder rate, both on an ITT basis. Study participants entered the DREAM trial with a mean AHI of 28.0, mean ODI of 27.0 and mean body mass index of 28.5. At 12 months, 73 subjects were determined to be AHI responders, per the Sher criteria, resulting in an ITT AHI responder rate of 63.5% (p=0.002), and 82 subjects were determined to be ODI responders, resulting in an ODI responder rate of 71.3% (p<0.001). Subjects demonstrated a median 12-month AHI reduction of 70.8%, with similar AHI improvements in supine and non-supine sleeping positions. The safety results for the investigational treatment were favorable, with 11 serious SAEs in ten subjects resulting in an SAE rate of 8.7%. Out of the 11 SAEs, three were device related and there were three explants.
ACCCESS Trial
In July 2022, we announced that the FDA approved an IDE to enable us to initiate a clinical trial, called ACCCESS, to evaluate the use of the Genio system for the treatment of adult patients with moderate-to-severe OSA with CCC that have failed, did not tolerate, or refused PAP. In the ACCCESS trial, we plan to implant up to 106 subjects with co-primary efficacy endpoints of AHI responder rate, per the Sher criteria, and ODI responder rate, both assessed at twelve months post-implant. The first enrolled subjects have been implanted, and we anticipate completing implantation in late 2024.
Sales and Marketing
We have grown our commercial team to more than 15 individuals, including sales representatives, field engineers and marketing professionals, who collectively bring substantial medical device sales, education and clinical experience to support commercialization of the Genio system. We are initially targeting markets in Europe where we have identified a clear reimbursement pathway or execution strategy. In Germany, we have successfully obtained reimbursement under a dedicated DRG code for HGNS, and, in Switzerland, we recently obtained reimbursement under an OSA-specific DRG code by the BFS. Each of these reimbursement coverages includes the cost of the Genio system, implant procedure, hospital stay and follow-up care. We began our commercial launch of the Genio system in July 2020. Our sales team in Germany consists of one country director and several representatives and field engineers, with support provided by our corporate team. We began marketing products in Switzerland and also secured first revenue in Spain in 2021 and we began commercialization in Finland in 2022 and in Austria in 2023.
We have established a systematic approach to commercializing the Genio system in select European countries which centers on active engagement and market development across patients, physicians and hospitals. Our Genio System has CE-Mark for OSA in patients with moderate to severe OSA in Europe. We market our Genio System to physicians and hospitals where ENTs, sleep doctors and general practitioners who see, diagnose and treat patients with OSA. We have developed a methodical marketing strategy to educate and develop the market and a commercial strategy tailored to suit local market needs in order to maximize therapy penetration and patient base expansion.
Our initial strategy is to employ a targeted approach to increase therapy penetration within specific physician practice groups instead of a broad outreach strategy to physicians. Our sales and marketing organization is focused on prioritizing high volume centers that are strategically located and building long-standing relationships with key physicians with strong connectivity to the population of OSA patients indicated for the Genio system. We are focusing our efforts on developing “Centers of Excellence”, where we plan to invest in developing the Genio system as the preferred treatment option for appropriate moderate to severe OSA patients in need of an alternative to conventional first-line therapies. Using a direct commercialization model in most of our target countries, we plan to utilize account managers to support the Centers of Excellence to strengthen the referral physician network, guiding new patients to these Centers of Excellence. We expect to gradually scale up in line with market entry and access in the various countries that we are targeting. Based on our experience we will have gained from our initial commercial roll-out in Europe, but also taking into account particular aspects of local markets, we will determine and prepare what we believe to be the optimal sales and marketing structure for commercial launch in the United States if we obtain U.S. marketing authorization.
79
Our direct sales representatives and field engineers, which we refer to as our market development team, generally have substantial experience, specifically with patients, physicians and payors in the ENT or neurostimulation space. Our market development team is focused on prioritizing high volume ENT centers, sleep centers, and building long-standing relationships with key physicians such as sleep doctors, ENT and general practitioners who have strong connectivity to the OSA patient population that may be eligible for the Genio system. Additionally, we target cardiac electrophysiologists, cardiologists, cardiovascular surgeons and dentists, which are a second OSA patient referral base for ENT physicians. We support our physicians through all aspects of the patient journey, starting from initial diagnosis through surgical support and post implantation patient follow-up.
We seek to establish long-term partnerships with key opinion leaders and patient associations that are built on mutual trust and oriented towards the needs of our patients and customers. Our marketing organization is focused on building physician awareness through referral network development, education, and targeted KOL development and training. Additionally, we have established and implemented a dedicated direct-to-patient marketing strategy aligned with local regulations in selected countries. Through targeted digital and offline media campaigns, we are raising awareness, engaging and driving patients eligible to the Genio system to our active centers of excellence. We have developed dedicated education and training programs leading to a certification delivered by an approved proctor. These education and training programs offer sleep centers and implanting surgeons excellent training pertaining to the Genio system technology, the latest and most up-to-date insights on the implantation procedure and on therapy optimization as well as on the subject of HGNS science. Additionally, these education and training programs promote a better understanding of OSA, which we believe will result in maximizing outcomes for Genio users, a better understanding of the technology’s benefits and risks and increasing confidence in the safety of the technology.
Additionally, we build awareness of the Genio system through digital social networks. The objective of this outreach is to target these patients and make them aware of our education webinars and website, where they can find a wealth of information on OSA and the purpose and benefits of the Genio system, based on our approved labeling. In addition to driving broad awareness and increasing physician and patient education, our marketing team has developed the in-house resources necessary to assist patients and physicians in the process of obtaining reimbursement approval for their procedures.
Research and Development
In addition to our ongoing clinical studies, we are also committed to continuing our research and development efforts related to the Genio system, with an emphasis on improving clinical outcomes, optimizing patient adoption and comfort, increasing access for a greater number of patients and allowing more physicians to perform the procedure. The primary focus of our research and development efforts in the near-term will be the continued technological advancement of the Genio system. Some of these improvements include features aimed at enhancing a physician’s ability to monitor patient compliance and therapy efficacy. We continue to enhance our scalable technology platform to potentially enable quick and streamlined release of new features and functionalities through software, firmware, hardware updates and upgrades and therapy enhancement. In January 2021, we entered into an exclusive license agreement with Vanderbilt University in order to further develop new neurostimulation technologies for the treatment of sleep disordered breathing conditions. We expect that these potential new treatments will focus on stimulating the ansa cervicalis, the efferent fiber of the glossopharyngeal nerve or nerves that innervate the palatoglossus and/or the palatopharyngeus muscle. Additionally, in June 2022, we announced that the FDA approved the use of our next generation Genio 2.1 system, which is designed to improve patient comfort and compliance with a new smartphone application and an upgraded external activation chip, for use in the DREAM trial. In July 2022, we obtained the CE-Mark for the Genio 2.1 system.
Further improvements or a next generation product may also bring additional features or services to the Genio system, potentially opening opportunities to generate revenue from data collected. For example, we expect the future generation of our products to focus on the capability to assess variables related to the patient’s sleep quality including monitoring patient respiratory flow, snoring, movement and sleep position as well as the ability for the Genio system to be connected to the cloud. We believe this information may enable us to monitor and better understand the patient’s quality of sleep and respiratory status, which we could consider sharing with key stakeholders. For example, we are considering developing solutions designed to enhance patient compliance by letting patients follow up regularly regarding the quality of the treatment received with healthcare connectivity tools. We are also exploring future tools that would provide sleep specialists with access to detailed patient therapy status via a digital care management platform, enabling them, on a remote and potentially reimbursable basis, to assess patient status and adjust Genio system treatment parameters. We believe the Genio system’s location close to the airway is optimal for detection and analysis of sleep and respiratory variables.
80
We intend to build a scalable technology platform allowing quick and streamlined release of new features and functionalities through software, firmware, hardware updates and upgrades and therapy enhancement. We believe that the external Genio system Activation Chip could allow for external enhancements to the Genio system without the need for additional surgical intervention.
Competition
The industry in which we operate is subject to rapid change and is highly sensitive to the introduction of new products and technologies of current or new industry participants. Our primary focus of OSA treatment is as a second line therapy for patients with moderate to severe OSA. If we are not successful in convincing others of the merits of our products or educating them on the use of our products, they may not use our products or use them effectively and we may be unable to increase our sales.
We consider our primary competition to be other device-based neurostimulation therapies designed to treat patients with moderate to severe OSA. Outside the United States, in addition to Inspire, we also are aware of the LivaNova’s ImThera device which received CE-Mark approval to market an open-loop neurostimulation device in Europe. In the United States, the Inspire system is the only FDA-approved closed- loop neurostimulation device for moderate to severe OSA. In the United States, LivaNova announced in 2021 the initiation of an IDE study to supporting FDA approval and commercialization of the ImThera device. We believe other emerging businesses are in the early stages of developing neurostimulation devices.
We also compete with invasive surgical treatment options such as UPPP, MMA and, to a lesser extent, MADs, which are primarily used in the treatment of mild to moderate OSA. We do not believe we directly compete with CPAP or other types of positive airway pressure devices because CPAP is typically a first line therapy for patients with OSA.
We believe that the primary competitive factors in the OSA treatment market are:
| ● | product safety, reliability and durability; |
| ● | company and brand recognition; |
| ● | ease of implantation and procedure time; |
| ● | quality of clinical data; |
| ● | adoption by patients, physicians and sleep centers; |
| ● | adequate reimbursement for our device; |
| ● | procedure costs to patients; |
| ● | product ease of use and patient comfort; |
| ● | sales force expansion, experience and access; |
| ● | product availability, support and service; |
| ● | manufacturing and supply chain; |
| ● | technological innovation and product enhancements; and |
| ● | intellectual property portfolio. |
In addition, our competitors may have greater financial resources or more established distribution networks than we do or may be acquired by enterprises that have more established distribution networks than we do. Our competitors may also develop and patent processes or products earlier than we can or obtain domestic or international regulatory clearances or approvals for competing products more rapidly than we can, which could impair our ability to develop and commercialize similar products. We also compete with our competitors in acquiring technologies and technology licenses complementary to our products or advantageous to our business. We also compete with other medical technology companies to recruit and retain qualified sales, training and other personnel.
Manufacturing and Supply
We rely on third-parties to manufacture and supply all the components of the Genio system to our specifications. Most components are supplied by single-source suppliers. Our principal suppliers of components are Meko, Medistri SA, Resonetics, VSI Parylene, Reinhardt Microtech GmbH (Cicor), Abatec (previously Lust Hybrid), Specialty Coating Systems (SCS), VSI Parylene, Resonetics, Medistri SAMeko, and S&D Tech SRL. The raw materials used by our suppliers are purchased in the open market. We continue to look for additional or replacement suppliers for the currently single-source components and we plan to maintain a sufficient level of inventory of such components to enable continued production for a limited period, such as during a supplier transition phase.
81
We work with third parties to manufacture and supply the components of the implantable stimulator and external stimulator. The initial assembly of the different electronics components is done by different external suppliers. The final assembly of the external stimulator and the final manufacturing step of the implantable stimulator, the silicone molding, are done internally by our manufacturing teams in the clean rooms at our facilities in Tel Aviv, Israel, and Milmort, Belgium. The capacity of our facilities in Tel Aviv and Milmort is expected to cover our expected clinical and European commercial product demand for 2024. We are working with a U.S. third party manufacturer to cover our expected future U.S. commercial product demand.
We work with third parties to manufacture and supply the electronic and plastic components of the activation chip and charging unit. In Tel Aviv, the final assembly of these parts is done by our manufacturing team in our facility. In Belgium, we have outsourced the assembly of the activation chip and charging unit to an external supplier. The manufacturing of the disposable patch is fully outsourced to the third party-supplier based in Israel.
Collaboration and License Agreements
Cochlear Collaboration Agreement
We and Cochlear Limited, or Cochlear, have entered into a collaboration agreement, dated November 2018, under which we and Cochlear agree to collaborate to further develop and progress commercialization of implantable treatments for sleep disordered breathing conditions. Cochlear has significant expertise in the development of implantable devices. The specific contributions and services to be used, applied and provided by both parties are further detailed in a document called “Statement of Work” that may be agreed upon by the parties from time to time. The initial Statement of Work was agreed upon by us and Cochlear on November 7, 2018 and is now complete. According to this Statement of Work, Cochlear evaluated various packaging technologies support us in the assessment of encapsulation technologies for the implantable stimulator. Additional Statements of Work were entered into on June 8, 2020 and January 30, 2023 and were both completed in 2023. Under the June 8, 2020 Statement of Work, Cochlear worked with us in developing and enhancing the next generation implantable stimulator, and we spent approximately €5.3 million on these efforts, of which €1.3 million was paid in June 2020, €1.4 million in September 2021, €1.2 million in February 2022 and €1.4 million in May 2023. Under the January 30, 2023 Statement of Work, Cochlear provided support in a transfer project to a U.S. third party manufacturer, for which we spent approximately €584,000. As no new Statement of Work was entered into and parties do not currently have the intention to enter into additional Statements of Work, the collaboration agreement can be considered as ended.
There are currently no IP licenses granted by Cochlear or by us to the other party.
Man & Science Agreement
We, Man & Science SA, Cephalix SA, Glucobel SA and Surgical Electronics SA, among others, have entered into a multiparty agreement regarding their respective ownership and licensing rights in relation to multiple inventions, including but not limited to inventions generally related to implantable, flexible neurostimulators and inventions for specific medical indications including sleep disordered breathing, head pain, glucose monitoring, hypertension and other indications. This agreement provides that (i) we fully own all rights in relation to the inventions specifically related to the sleep disordered breathing field, which we believe includes sleep disordered breathing conditions such as sleep apnea and snoring, and comorbidities of these conditions and (ii) Man & Science SA is the owner of the generic inventions and granted a fully paid-up, exclusive and worldwide, license with respect to these inventions to several parties, including us in the field of sleep disordered breathing. Pursuant to the terms of the agreement, no party may terminate the licenses.
In June 2016, we, Cephalix SA, Surgical Electronics SA, and Man & Science SA entered into a confirmatory addendum, aiming to confirm that (i) we fully own all rights in relation to the inventions specifically related to the sleep disordered breathing field as further detailed in the agreement, (ii) Man & Science SA granted an exclusive, worldwide, fully paid-up, royalty free and transferable license to us covering certain patents in the sleep disordered breathing field, and (iii) we granted an exclusive, fully paid-up, royalty free, transferable license to use certain of those patents outside the sleep disordered breathing field, namely to Cephalix SA in the head pain field, Surgical Electronics SA in the hypertension field and Man & Science SA outside the head pain field and the hypertension field.
82
In February 2020, we entered into a clarification of the Confirmatory Addendum, or Clarification, with Man & Science SA. The Clarification confirms that the license granted to us by Man & Science SA under the agreement and the Addendum are irrevocable, transferable, fully paid up, royalty-free and include the right to grant sublicenses in the sleep disordered breathing field, which are retroactive as from the filing date of the oldest of the patents and patent applications and will continue in effect until the last to expire patent, which is expected to occur in 2032 (excluding any potential patent term extension). We have no current or future financial obligation to Man & Science SA pursuant to the agreement.
Intellectual Property
Our intellectual property and the rights underlying the same are valuable and important in the medical device and health tech industry in which we operate. Our success depends, in part, on our ability to obtain and maintain intellectual property protection for our product candidates, to defend and enforce our intellectual property rights, to preserve the confidentiality of our know-how and proprietary information, and to operate without infringing upon the proprietary rights of others. We seek to protect our products and product candidates by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We rely heavily on our patent and design portfolio to maintain competitive technological advantage, as well as on our trademarks that support our brand identity.
We have implemented an intellectual property protection policy with the objective of obtaining protection for key aspects of the technology embodied in the Genio system and certain methods of use.
We may, from time to time, file patent applications for inventions that may be of importance to our future business. We may license or acquire rights to patents, patent applications, or other intellectual property owned by third parties, academic partners or commercial companies which are of interest to us. Further, we may decide, from time to time, to license our intellectual property to other parties, for example, in exchange for cash, marketing collaboration, or other valuable consideration to us.
We continuously review our development activities to assess the novelty and patentability of new intellectual property being developed. In addition to patents, we also rely on a combination of trade secrets, design rights, copyright laws, non-disclosure agreements and other contractual provisions and technical measures that help us maintain and develop our competitive position with respect to intellectual property. Despite our efforts to protect our intellectual property rights, third parties might invalidate, engineer around these or challenge our rights in court or patent offices.
Our policy is that our employees and contractors execute a propriety information and inventions assignment agreement, which protects proprietary information, and which assigns to us all inventions created by an employee during the term of employment. Where possible and appropriate, agreements with third parties (e.g. consultants and vendors) contain language designed to protect our intellectual property and confidential information, and to assign to us new inventions related to our business.
As of December 31, 2023, we have 199 granted or pending patent applications (both utility and design) comprised of 54 issued or allowed U.S. patents, 12 pending U.S. non-provisional applications, 1 pending international patent applications filed under the Patent Cooperation Treaty, or PCT, and 40 pending patent applications and 92 granted patents in jurisdictions outside the United States, including Australia, Canada, China, Europe, Hong Kong, Israel and Japan. The exclusivity terms of our patents depend upon the laws of the countries in which they are obtained. In the countries in which we currently file, the patent term is 20 years from the earliest date of filing of a non-provisional patent application. Current issued patents and patent applications covering our Genio system will expire on dates ranging from 2032 to 2034, if the applications are issued.
83
In addition to the patent portfolio owned by us, we hold exclusive licenses granting us a fully paid-up, transferrable and sub-licensable, worldwide, irrevocable license and royalty free in the field of sleep disordered breathing in relation to multiple inventions, including but not limited to inventions generally related to implantable flexible neuro-stimulators. Such licenses were granted to us by Man & Science SA (a company held and governed by Robert Taub, TOGETHER Partnership, Jürgen Hambrecht and Noshaq SA). We also hold an exclusive worldwide license from Vanderbilt University, to develop, use, grant sublicense and commercialize products, with a different mechanism of action than the Genio system, in the field of sleep disordered breathing conditions and comorbidities of such conditions. We will also work together with Vanderbilt University to continue prosecution of patent applications made by Vanderbilt. Under the agreement, we paid to Vanderbilt an upfront license issue fee of approximately $650,000. We may be required to pay earned royalties in the mid-single digits on net sales of licensed products that are covered by the patent rights owned by Vanderbilt. After the second anniversary of the agreement, we may terminate the obligation to pay further earned royalties to Vanderbilt on net sales of licensed products in exchange for a one-time royalty buyout payment. We may be required to make minimum annual royalty payments to Vanderbilt of up to $250,000 in 2024 and 2025, up to $500,000 in 2026 and 2027, and up to $1,000,000 in 2028 and each year thereafter, which are creditable against the earned royalties owed to Vanderbilt for the same calendar year. Additionally, Vanderbilt may be entitled to milestone payments of up to an aggregate of $13,750,000 in connection with patent issuance, clinical studies, regulatory approvals and net sales milestones. We may also be required to pay Vanderbilt a low to mid double-digit percentage, not to exceed 40% of any non-royalty sublicensing revenue we receive. The Vanderbilt Agreement, including the royalty obligations thereunder, will continue on a licensed product-by- licensed product and country-by-country basis until the expiration date of the last-to expire licensed patent in each country. Either we or Vanderbilt may terminate the Vanderbilt Agreement in connection with the other party’s insolvency. Vanderbilt may also terminate the Vanderbilt Agreement in the event we fail to make a payment to Vanderbilt, breach or default our diligence obligations or breach or default on any other material term, and if we fail to make such payment or cure such breach or default within 60 days of written notice from Vanderbilt. We may terminate the agreement by providing 120 days’ advance notice to Vanderbilt.
With respect to trademarks, we use our corporate name, Nyxoah, and associated logo as well as the tagline, in creating awareness of our expertise and in marketing our Genio system technology. We use the trademark Genio to identify our Genio system. We have obtained registration for the Nyxoah name and the Genio trademark in seven jurisdictions around the globe.
Government Regulation
Governmental authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions, including the European Union, extensively regulate, among other things, the research, development, testing, manufacture, quality control, certification, authorization, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of medical device products. As a medical device manufacturer, our operations are subject to such laws and regulations in the jurisdictions in which we or our research and development partners or affiliates do business.
The processes for obtaining marketing approvals in the United States and in other countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations, as well as policies and rules established by regulatory authorities, require the expenditure of substantial time and financial resources. In addition, the laws and regulations governing our business and interpretations of those laws and regulations and are subject to frequent change, and we must, therefore, devote significant resources to monitoring developments in legislation, enforcement, and regulation in such areas. Our ability to operate profitably will depend in part upon our ability, and that of our research and development partners and affiliates, to operate in compliance with applicable laws and regulations. As the applicable laws and regulations change, we are likely to make conforming modifications in our business processes from time to time. We cannot provide assurance that a review of our business by courts or regulatory authorities will not result in determinations that could adversely affect our operations or that the regulatory environment will not change in a way that restricts our operations.
Regulatory Landscape in the European Union
The EU has adopted specific directives and subsequently regulations regulating the design, manufacture, clinical investigations, labeling, conformity assessment, post-market surveillance and vigilance reporting for medical devices. EU directives, which had to be implemented into the national laws of the EU member states, led to certain variations between national laws of the different member states. Regulations are directly applicable in all EU member states from their effective applicability date and are intended to eliminate differences in the regulation of medical device requirements among EU member states. The EU rules listed below are generally applicable in the EEA. Other countries, such as Switzerland, have entered into Mutual Recognition Agreements and allow the marketing of medical devices that meet EU requirements.
84
Prior to May 26, 2021, all medical devices placed on the EU market had to meet the relevant essential requirements laid down in Council Directive 93/42/EEC, or the Medical Devices Directive (MDD) and the Council Directive 90/385/EEC, or the Active Implantable Medical Devices Directive (AIMDD). Active Implantable Medical Devices, or AIMDs, are defined as medical devices that rely on a source of electrical energy or any source of power other than that generated by the body, which are totally or partially introduced, either surgically or medically, into the human body and intended to remain after the procedure.
On May 26, 2021, the MDR became effective, repealing and replacing the MDD and the AIMDD. The MDR is directly applicable in all EU member states. The MDR changed several aspects of the regulatory framework for medical device marketing in Europe in order to increase regulatory oversight of all medical devices marketed in the EU (which, in turn, increased the costs, time and requirements to place innovative or high-risk medical devices on the European market). The MDR among other things:
| ● | strengthens the rules on placing devices on the market and reinforces surveillance once they are available; |
| ● | establishes explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| ● | improves the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| ● | sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the European Union, or EU; and |
| ● | strengthens the rules for the assessment of certain high-risk devices, which may have to undergo an additional check by experts before they are placed on the market. |
An overarching requirement under the MDR is that any device must be designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users and others. In addition, the device must meet the performance specifications intended by the manufacturer and be designed, manufactured and packaged in a suitable manner. To that effect, the European Commission has adopted various standards applicable to medical devices. These include standards governing common requirements, such as sterilization and safety of medical electrical equipment and product standards for certain types of medical devices. There are also harmonized standards relating to design and manufacture. A harmonized standard is a European standard developed by a recognized European Standards Organization. While not mandatory, compliance with harmonized standards is a way for manufacturers to demonstrate that products comply with relevant EU legislation.
To demonstrate compliance with the General Safety and Performance Requirements, or GSPRs, laid down in the MDR, medical device manufacturers must undergo a conformity assessment procedure, which varies according to the type of medical device and its (risk) classification. Conformity assessment procedures require an assessment of the technical documentation, including the device description, the design stages, the manufacturing process, available clinical evidence, literature data for the product, and post-market experience in respect of similar products already marketed. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can self-declare the conformity of its products with the GSPRs (except for any parts which relate to sterility or metrology), a conformity assessment procedure requires the intervention of a Notified Body. To that effect, manufacturers of medical devices must make an application to a Notified Body. Notified Bodies are independent organizations designated by EU member states to assess the conformity of devices before being placed on the market. A Notified Body would typically audit and examine a product’s technical dossiers and the manufacturers’ quality management system (which must, in particular, comply with ISO 13485 related to Medical Devices Quality Management Systems). If satisfied that the AIMD or other medical device conforms to the relevant GSPRs, the Notified Body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity. The manufacturer may then apply the CE-Mark to the device, allowing the device to be legally marketed throughout the EU.
Notified Body certificates of conformity are valid for a fixed duration (which shall not exceed five years).
Throughout the term of the certificate, the manufacturer will be subject to periodic surveillance audits to verify continued compliance with the applicable requirements. In particular, there will be a new audit by the Notified Body before it renews the relevant certificate(s).
85
As a general rule, demonstration of conformity of medical devices with the GSPRs must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. In order to demonstrate safety and effectiveness for their AIMDs and other medical devices, manufacturers must, save limited exceptions, conduct clinical investigations in accordance with the requirements of Annex VII and Annex XV to the MDR. Clinical investigations for medical devices usually require the approval of an ethics committees and approval by the national regulatory authorities. Both regulators and ethics committees also require the submission of periodic safety reports during a study and may request a copy of the final study report.
Once the product has been placed on the market in the EU, the manufacturer must comply with the vigilance reporting requirements under the MDR. In accordance with these vigilance reporting requirements, serious incidents must be reported to the relevant authorities of the EU member states, and manufacturers are required to take Field Safety Corrective Actions, or FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. A serious incident is defined as any malfunction or deterioration in the characteristics or performance of a device made available on the market, including use-error due to ergonomic features, as well as any inadequacy in the information supplied by the manufacturer and any undesirable side-effect that directly or indirectly led, might have led or might lead to death, temporary or permanent serious deterioration of health state, or a serious public health threat. An FSCA can include the withdrawal of the device from the market, or a recall thereof. FSCAs must be communicated by the manufacturer or its legal representative to the users of the device through Field Safety Notices. Furthermore, the manufacturer must report any statistically significant increase in the frequency or severity of incidents that are not serious incidents or that are expected undesirable side-effects that could have a significant impact on the benefit-risk analysis and which have led or may lead to risks to the health or safety of patients, users or other persons that are unacceptable when weighed against the intended benefits. The significant increase shall be established in comparison to the foreseeable frequency or severity of such incidents in respect of the device, or category or group of devices, in question during a specific period as specified in the technical documentation and product information.
The advertising and promotion of medical devices is subject to some general principles set forth by EU directives. Directive 2006/114/EC concerning misleading and comparative advertising and Directive 2005/29/EC on unfair commercial practices. While the aforementioned directives are not specific to the advertising of medical devices, the provisions of national law transposing them must also be complied with and contain general rules, for example requiring that advertisements are evidenced, balanced and not misleading. Specific requirements are defined at national level. EU member states laws related to the advertising and promotion of medical devices, which vary between jurisdictions, may limit or restrict the advertising and promotion of products to the general public and may impose limitations on promotional activities with healthcare professionals.
Many EU member states have adopted specific anti-gift statutes that further limit commercial practices for medical devices, in particular vis-à-vis healthcare professionals and organizations. Additionally, there has been a recent trend of increased regulation of payments and transfers of value provided to healthcare professionals or entities. In addition, many EU member states have adopted national “Sunshine Acts” which impose reporting and transparency requirements (often on an annual basis), similar to the requirements in the United States, on medical device manufacturers. Certain countries also mandate implementation of commercial compliance programs.
Devices lawfully placed on the market pursuant to the MDD and the AIMDD prior to May 26, 2021 could initially continue to be made available on the market or put into service until May 26, 2025. Nevertheless, the European Parliament very recently adopted legislation to extend this transitional period to give manufacturers more time to switch from the previously applicable provisions to the new certification requirements for medical devices as laid down by the MDR. For high risk, class III and class IIb implantable devices the transitional period is extended until December 31, 2027. For medium and low risk, class IIb devices and class IIa, Im, Is and Ir devices the transition period is extended until December 31, 2028.
We obtained the CE-Mark for the Genio system under the AIMDD in March 2019. Additionally, we obtained the CE-Mark for the Genio 2.1 system under the MDR in July 2022. DEKRA Certification B.V., a Notified Body designated for regulatory review of medical devices and their manufacturers under applicable EU regulations, conducted the assessment of the manufacturers’ quality system and the technical dossiers for the Genio system and Genio 2.1 system and issued the certificate of conformity for the device.
86
Based on an agreement signed between the Company and DEKRA Certification B.V. in January 2024, the validity of the certificates issued by DEKRA Certification B.V. in accordance with the AIMDD in March 2019 in relation to the Genio system (granting CE-Mark for the Genio system), is extended until December 31, 2027.
In addition, our manufacturing facility is certified as compliant with ISO 13584:2016 and we are registered as a legal manufacturer in Belgium under the MDR. We also invest significant efforts to maintain compliance with the most updated and harmonized standards, as well as with local and international regulatory requirements.
United Kingdom Regulatory Framework and Operational Impacts Post-Brexit
The United Kingdom left the European Union on January 31, 2020 (commonly referred to as “Brexit”), with a transitional period that expired on December 31, 2020. The United Kingdom and the European Union entered into a trade agreement known as the Trade and Cooperation Agreement, or TCA, which came into effect on January 1, 2021. The TCA does not specifically refer to medical devices. However, as a result of Brexit, the MDR will not be implemented in the UK, and previous legislation that mirrored the MDR in the UK law has been revoked. The regulatory regime for medical devices in the UK will continue to be based on the requirements derived from previous EU legislation, and the UK may choose to retain regulatory flexibility or align with the MDR going forward. CE-Markings will continue to be recognized in the UK, and certificates issued by EU recognized Notified Bodies will be valid in the UK, until the earlier of June 30, 2028 or the expiration of the certificate for devices compliant with the MDD or AIMDD or until June 30, 2030 for devices compliant with the MDR. For medical devices placed on the UK market after this period, the UK Conformity Assessed, or UKCA, marking will be mandatory. In contrast, UKCA marking and certificates issued by UK Notified Bodies will not be recognized on the EU market. The TCA does provide for cooperation and exchange of information in the area of product safety and compliance, including market surveillance, enforcement activities and measures, standardization related activities, exchanges of officials, and coordinated product recalls (or other similar actions). For medical devices that are locally manufactured but use components from other countries, the “rules of origin” criteria will need to be reviewed. Depending on which countries products will ultimately be sold in, manufacturers may start seeking alternative sources for components if this would allow them to benefit from no tariffs. The rules for placing medical devices on the Northern Ireland market will differ from those in Great Britain. It remains to be seen how the UK rules will impact regulatory requirements for our product candidates and our product in the United Kingdom. We continue to evaluate the potential impacts on our business of the new Trade and Cooperation Agreement.
Such outcomes could make it more difficult and expensive for us to do business in Europe, complicate our clinical, manufacturing and regulatory strategies and impair our ability to obtain and maintain regulatory approval for, and, if approved, commercialize, our products and product candidates in Europe.
Regulatory Landscape in the United States
Medical devices are strictly regulated by the FDA in the United States. Under the Federal Food, Drug, and Cosmetic Act, or FDCA, a medical device is defined as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component, part or accessory which is, among other things: intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals; or intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.” This definition provides a clear distinction between a medical device and other FDA regulated products such as drugs. If the primary intended use of a medical product is achieved through chemical action or by being metabolized by the body, the product is usually a drug or biologic. If not, it is generally a medical device.
The Genio system is regulated by the FDA as a medical device under the FDCA, as implemented and enforced by the FDA. The FDA regulates the development, testing, manufacturing, labeling, packaging, storage, installation, servicing, advertising, promotion, marketing, distribution, import, export, and market surveillance of our medical devices. The Genio system is not yet approved or cleared for marketing in the United States.
87
Device Premarket Regulatory Requirements
Before being introduced into the U.S. market, each medical device must obtain marketing authorization from the FDA through the premarket notification, or 510(k), process, the De Novo classification process or the premarket approval, or PMA, process, unless they are determined to be Class I devices or to otherwise qualify for an exemption from one of these available forms of premarket review and authorization by the FDA. Under the FDCA, medical devices are classified into one of three classes — Class I, Class II or Class III — depending on the degree of risk associated with each medical device and the extent of controls needed to provide reasonable assurance of safety and effectiveness. Classification of a device is important because the class to which a device is assigned determines, among other things, the necessity and type of FDA review required prior to marketing the device. Class I devices are those for which reasonable assurance of safety and effectiveness can be maintained through adherence to general controls, which include compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, as well as regulations requiring facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. The Class I designation also applies to devices for which there is insufficient information to determine that general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device or to establish special controls to provide such assurance, but that are not life-supporting or life-sustaining or for a use which is of substantial importance in preventing impairment of human health, and that do not present a potential, unreasonable risk of illness or injury.
Class II devices are those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish “special controls.” These special controls can include performance standards, post-market surveillance requirements, patient registries and FDA guidance documents describing device-specific special controls. While most Class I devices are exempt from the premarket notification requirement, most Class II devices require a premarket notification prior to commercialization in the United States; however, the FDA has the authority to exempt Class II devices from the premarket notification requirement under certain circumstances. As a result, manufacturers of most Class II devices must submit premarket notifications to the FDA under Section 510(k) of the FDCA (21 U.S.C. § 360(k)) in order to obtain the necessary authorization to market or commercially distribute such devices. To obtain 510(k) clearance, manufacturers must submit to the FDA adequate information demonstrating that the proposed device is “substantially equivalent” to a “predicate device” that is already on the market. A predicate device is a legally marketed device that is not subject to PMA, meaning, (i) a device that was legally marketed prior to May 28, 1976, or preamendments device, and for which a PMA is not required, (ii) a device that has been reclassified from Class III to Class II or I, or (iii) a device that was found substantially equivalent through the 510(k) process.
Following receipt of a premarket notification for a device, the FDA determines whether the submission is sufficiently complete to permit a substantive review. According to the most recent medical device user fee goals, the FDA will attempt to issue a decision within 90 days of receipt on 95% of all 510(k) submissions accepted for review. However, the FDA may stop the review clock for up to 180 days to request that the applicant respond to the agency’s requests for additional information about the proposed device. If the FDA agrees that the device is substantially equivalent to the predicate device identified by the applicant in a premarket notification submission, the agency will grant 510(k) clearance for the new device, permitting the applicant to commercialize the device. Premarket notifications are subject to user fees, unless a specific exemption applies.
After a medical device receives 510(k) clearance, any modification that could significantly affect the device’s safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) submission or could require a PMA. The FDA requires each manufacturer to make the determination of whether a device modification requires a new 510(k) or PMA in the first instance, but the FDA may review any such decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance or PMA for a particular change, the FDA may retroactively require the manufacturer to submit a 510(k) or PMA application. The FDA may also require the manufacturer to cease its marketing activities for the modified device in the United States and/or recall the device until the appropriate marketing authorization for the modification is obtained.
If there is no adequate predicate to which a manufacturer can compare its proposed device, the proposed device is automatically classified as a Class III device. In such cases, a device manufacturer must then fulfill the more rigorous PMA requirements or can request a risk-based classification determination for its device in accordance with the De Novo classification process.
88
Devices that are intended to be life sustaining or life supporting, devices that are implantable, devices that present a potential unreasonable risk of harm or are of substantial importance in preventing impairment of health, and devices that are not substantially equivalent to a predicate device and for which safety and effectiveness cannot be assured solely by the general controls and special controls are placed in Class III. Such devices generally require FDA approval through the PMA process, unless the device is a preamendments device not yet subject to a regulation requiring premarket approval. The PMA process is more demanding than the 510(k) process. For a PMA, the manufacturer must demonstrate through extensive data, including data from preclinical studies and one or more clinical studies, that the device is safe and effective for its proposed indication. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA submission, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review and determine whether the proposed device can be approved for commercialization, although in practice, PMA reviews often take significantly longer, and it can take up to several years for the FDA to issue a final decision. Before approving a PMA, the FDA generally also performs an on-site inspection of manufacturing facilities for the product to ensure compliance with the QSR.
The FDA may refer any PMA, including applications for novel device candidates or device candidates that present difficult questions of safety or efficacy, to an advisory committee for review. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations when making final decisions on approval.
If the FDA’s evaluation of the PMA application and inspection of the manufacturing facility is favorable, the FDA may issue an approval order authorizing commercial marketing of the device, or an “approvable letter,” which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been met to the satisfaction of the FDA, the agency will issue a PMA approval order, subject to the conditions of approval and the limitations established in the approval order. If the FDA’s evaluation of a PMA application or manufacturing facility is not favorable, the FDA will deny approval of the PMA or issue a “not approvable letter.” The FDA may also determine that additional studies are necessary, in which case the PMA approval may be delayed for several months or years while such additional studies are conducted and data is submitted in an amendment to the PMA. The PMA process can be expensive, uncertain and lengthy, and each PMA submission is subject to a substantial user fee unless a specific exemption applies. PMA approval may also be granted with post-approval requirements such as the need for additional patient follow-up or requirements to conduct additional clinical trials.
New PMA applications or PMA supplements may be required for any modifications to the manufacturing process, labeling, device specifications, materials or design of a device that is approved through the PMA process. PMA supplements often require submission of the same type of information as an initial PMA application, except that the supplements are limited to information needed to support any changes from the device covered by the approved PMA application and may or may not require as extensive clinical data or the convening of an advisory committee.
The De Novo classification process allows a manufacturer whose novel device is automatically classified into Class III to request that the FDA classify such device as Class I or Class II based on evidence that the device in fact presents low or moderate risk, instead of following the typical Class III device pathway requiring the submission and approval of a PMA application. Under the Food and Drug Administration Safety and Innovation Act of 2012, or FDASIA, the FDA is required to classify a device within 120 days following receipt of the De Novo classification request from an applicant; however, the process may take significantly longer. For example, the most recent FDA user fee goals state that in fiscal year 2023, the FDA will attempt to issue a decision within 150 days of receipt on 70% of all De Novo classification requests received during the year. If the manufacturer seeks reclassification into Class II, the classification request must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. If FDA grants the De Novo request, the device may be legally marketed in the United States. However, the FDA may reject the classification request if it identifies a suitable legally marketed predicate device that provides a reasonable basis for review of substantial equivalence or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and adequate special controls cannot be developed. De Novo classification requests are subject to user fees, unless a specific exemption applies.
89
Device Clinical Studies
Clinical studies are almost always required to support PMA applications and are sometimes required to support 510(k) and De Novo classification submissions. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s good clinical practice, or GCP, regulations, including the investigational device exemption, or IDE, regulations that govern investigational device labeling, prohibit promotion of investigational devices, and specify recordkeeping, reporting and monitoring responsibilities of trial sponsors and investigators. If the device presents a “significant risk,” as defined by the FDA, the agency requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical studies. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a patient. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that the device has a safety profile appropriate for human testing and that the trial protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA expressly approves or denies the application in writing or notifies the sponsor that the investigation is on hold and may not begin until the sponsor provides supplemental information about the investigation that satisfies the agency’s concerns. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the trial, the FDA may permit a clinical trial to proceed under a conditional approval or the sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. In addition, the trial must be approved by, and conducted under the oversight of, an institutional review board, or IRB, for each clinical site. If the device presents a non-significant risk to the patient according to criteria established by FDA as part of the IDE regulations, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate authorization from the FDA, but must still comply with abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
As part of its clinical trial oversight responsibilities, an IRB must review and approve, among other things, the trial protocol and informed consent information to be provided to clinical trial subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical studies, including details of the protocol and eventually trial results, also must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on the ClinicalTrials.gov data registry. Information related to the product, patient population, phase of investigation, trial sites and investigators and other aspects of the clinical trial is made public as part of the registration of the clinical trial. Sponsors are also obligated to disclose the results of their clinical studies after completion. Disclosure of the results of these studies can be delayed in some cases for up to two years after the date of completion of the trial. Failure to timely register a covered clinical study or to submit study results as provided for in the law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal government. The NIH Final Rule on ClinicalTrials.gov registration and reporting requirements became effective in 2017, and the government has brought enforcement actions against non-compliant clinical trial sponsors.
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and more frequently if unanticipated serious adverse events, or SAEs, occur. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the clinical protocol, GCP, or other IRB requirements or if the investigational product has been associated with unexpected serious harm to patients.
In the Consolidated Appropriations Act for 2023, Congress amended the FDCA to require the sponsor of any pivotal clinical trial that will be used to demonstrate the safety and effectiveness of a medical device marketing authorization submission to develop a diversity action plan for such trial, and if submission of an IDE application is required, to submit such diversity action plan to the FDA. The action plan must include the sponsor’s diversity goals for enrollment, as well as a rationale for the goals and a description of how the sponsor will meet them. The FDA may grant a waiver for some or all of the requirements for a diversity action plan. It is unknown at this time how the diversity action plan may affect device pivotal clinical trial planning and timing or what specific information FDA will expect in such plans, but if FDA objects to a sponsor’s diversity action plan and requires the sponsor to amend the plan or take other actions, it may delay trial initiation.
90
Post-Marketing Restrictions and Enforcement
After a device is cleared or approved for marketing by the FDA, numerous medical device regulatory requirements continue to apply, such as:
| ● | establishment registration and device listing with the FDA; |
| ● | QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; |
| ● | labeling and marketing regulations, which require that advertising and promotional materials about the cleared or approved device are truthful, not misleading, fairly balanced and provide adequate directions for use and that all claims are substantiated, and also prohibit the promotion of devices for uncleared or unapproved or “off -label” uses and impose other restrictions on labeling; |
| ● | medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device marketed by the manufacturer would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur; |
| ● | correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; |
| ● | the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
| ● | post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device; and |
| ● | periodic scheduled or unscheduled inspections by the FDA to assess compliance with applicable regulations, which could result in the shutdown of, or restrictions on, our manufacturing operations and the recall or seizure of our products. |
The medical device reporting requirements also extend to healthcare facilities that use medical devices in providing care to patients, or “device user facilities,” which include hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, or outpatient treatment facilities, but not physician offices. A device user facility must report any device-related death to both the FDA and the device manufacturer, or any device-related serious injury to the manufacturer (or, if the manufacturer is unknown, to the FDA) within 10 days of the event. Device user facilities are not required to report device malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur but may voluntarily report such malfunctions through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program.
The FDA also has the authority to require the recall of commercialized medical device products in the event of material deficiencies or defects in design or manufacture. The authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious adverse health consequences or death. A manufacturer may, under its own initiative, recall one or more of its products if any distributed devices fail to meet established specifications, are otherwise misbranded or adulterated under the FDCA, or if any other material deficiency is found. A device manufacturer must report to the FDA any correction, removal or recall of its devices, if such action is taken to reduce a risk to health posed by such devices or to remedy a violation of the FDCA caused by such devices that may present a risk to health, within 10 working days after the recall is initiated.
The failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| ● | warning letters, fines, injunctions or civil penalties; |
| ● | recalls, detentions or seizures of products; |
| ● | operating restrictions; |
| ● | delays in the introduction of products into the market; |
| ● | total or partial suspension of production; |
| ● | delay or refusal of the FDA or other regulators to grant 510(k) clearance, PMA approvals, or other marketing authorization to new products; |
| ● | withdrawals of marketing authorizations; or |
| ● | in the most serious cases, criminal prosecution. |
91
To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled or unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of contract manufacturers. Manufacturing processes for medical devices are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. Failure to maintain compliance with the QSR requirements could result in the shutdown of, or restrictions on, manufacturing operations and the recall or seizure of marketed products.
Breakthrough Device Designation
The 21st Century Cures Act, which was signed into law on December 13, 2016, established and directed FDA to implement the Breakthrough Devices Program. Under the program, device manufacturers may voluntarily request breakthrough designation for devices that may provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions over currently available technology and that meet at least one of the following criteria:
| ● | the device represents breakthrough technology; |
| ● | there are no approved or cleared alternatives for the device; |
| ● | the device offers significant advantages over existing approved or cleared alternatives; or |
| ● | availability of the device is in the best interest of patients. |
The goal of the Breakthrough Devices Program is to expedite the development and prioritize the review of certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. A breakthrough device designation offers multiple benefits to the device manufacturer, including prioritized review of the pre-market submission for the device, opportunities to interact directly with FDA’s experts throughout the process, and engagement of FDA senior management.
Federal Trade Commission Regulatory Oversight
Our advertising for our products is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission, or FTC, as well as comparable state consumer protection laws. Under the Federal Trade Commission Act, or FTC Act, the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
Federal Communications Commission Regulation
The Genio system includes a wireless radio frequency transmitter and receiver and, therefore, is subject to equipment authorization requirements in the United States. The Federal Communications Commission, or FCC, requires advance clearance of all radio frequency devices before they can be imported into, sold or marketed in the United States. These clearances ensure that the proposed products comply with FCC radio frequency emission and power level standards and will not cause interference.
92
Healthcare Law and Regulation
If the Genio system is approved in the United States, we will have to comply with various U.S. federal and state laws, rules and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws, false claims laws and price transparency reporting laws, rules and regulations. Violations of the fraud and abuse laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid. These laws include the following:
| ● | the federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the Anti-Kickback Statute or specific intent in order to violate it; |
| ● | the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act; |
| ● | HIPAA imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| ● | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| ● | the federal transparency requirements under the Physician Payments Sunshine Act require manufacturers of FDA-approved drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to the Centers for Medicare and Medicaid Services, information related to payments and other transfers of value to physicians, teaching hospitals, and certain advanced non-physician health care practitioners, as well as ownership and investment interests held by physicians and their immediate family members; and |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers. |
The majority of states also have statutes or regulations similar to the aforementioned federal laws, some of which are broader in scope and apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Some state laws require medical device companies to comply with the relevant industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring device manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures.
93
State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. We also may be subject to, or may in the future become subject to, U.S. federal and state, and foreign laws and regulations imposing obligations on how we collect, use, disclose, store and process personal information. Nyxoah S.A. is not a covered entity or a business associate under HIPAA; however, it is indirectly affected by HIPAA because the protected health information held by investigators conducting Nyxoah clinical trials is subject to HIPAA and can only be used for Nyxoah research consistent with HIPAA requirements imposed on those investigators. In addition, state laws, such as the California Consumer Privacy Act (CCPA), govern the privacy and security of the personal information of individuals residing in such states, and may in certain circumstances, apply to health information. In addition to California, other states have implemented laws protecting identifiable health and personal information, and many of these laws differ from each other in significant ways and may not be preempted by HIPAA, thus complicating compliance efforts.
Violation of any of the federal and state healthcare laws may result in penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government programs, such as Medicare and Medicaid, injunctions, private “qui tam” actions brought by individual whistleblowers in the name of the government, or refusal to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of operations. Our actual or perceived failure to comply with healthcare and data privacy laws could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base and thereby decrease our future revenues.
Coverage and Reimbursement
Sales of the Genio system and any product candidates, if approved, will depend, in part, on the extent to which the procedures using the Genio system and any product candidates are covered by third-party payors, such as government healthcare programs, commercial insurance and managed healthcare organizations. Third-party payors are increasingly limiting coverage and reducing reimbursements for medical products and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, and restrictions on coverage and reimbursement. Third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical devices and medical services, in addition to questioning their safety and efficacy. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net sales and results.
Moreover, the process for determining whether a third-party payor will provide coverage for a product or procedure may be separate from the process for establishing the reimbursement rate that such a payor will pay for the product or procedure. A payor’s decision to provide coverage for a product or procedure does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product or procedure does not assure that other payors will also provide coverage. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to ensure profitability.
Healthcare Reform
The FDA’s and other regulatory authorities’ policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory authorization of our product candidates. For example, Congress must reauthorize the FDA’s user fee programs every five years and often makes changes to those programs in addition to policy or procedural changes that may be negotiated between the FDA and industry stakeholders as part of this periodic reauthorization process. Congress most recently reauthorized the user fee programs in September 2022 but without any substantive policy changes. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing authorization that we otherwise may have obtained, and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations.
94
In December 2022, the U.S. Congress enacted the Consolidated Appropriations Act for 2023, an omnibus appropriations bill, which included amendments to the FDCA under the Food and Drug Omnibus Reform Act of 2022, or FDORA. In addition to the requirement that sponsors of pivotal trials submit diversity action plans for pivotal trials (see “Government Regulation—Regulatory Landscape in the United States—Device Clinical Studies”), FDORA included new requirements for cyber devices, defined as any medical device that is or includes software that is validated, installed, or authorized by the manufacturer; can connect to the internet; and may be vulnerable to cybersecurity threats. Under the FDORA amendments to the FDCA, any application for marketing authorization of the cyber device must include a software bill of materials and a cybersecurity plan describing the methods by which the manufacturer will monitor, identify and address cybersecurity vulnerabilities. Any failure by a cyber device manufacturer to comply with applicable cybersecurity requirements is considered a violation of the FDCA and will subject the manufacturer to enforcement actions and possibly legal sanctions.
In the United States, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, (collectively, the “ACA”) was signed into law and substantially changed the way healthcare is financed by both governmental and private insurers in the United States. The ACA contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement adjustments and fraud and abuse changes. Additionally, the ACA provided incentives to programs that increase the federal government’s comparative effectiveness research, and implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models.
Legislative and regulatory changes under the ACA remain possible, although it is unknown what form any such changes or any law would take, and how or whether it may affect the medical device industry as a whole or our business in the future. We expect that changes or additions to the ACA, the Medicare and Medicaid programs and changes stemming from other healthcare reform measures, especially with regard to healthcare access, financing or other legislation in individual states, could have a material adverse effect on the healthcare industry in the United States.
Moreover, there has recently been heightened governmental scrutiny, including increasing legislative and enforcement interest, over the manner in which manufacturers set prices for their marketed healthcare products, which has resulted in several Congressional inquiries and proposed and enacted legislation designed, among other things, to bring more transparency to healthcare product pricing, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for healthcare products. Individual states in the United States have also become increasingly active in implementing regulations designed to control healthcare product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, mechanisms to encourage importation of healthcare products from other countries. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
Data Privacy and Security
Data privacy and security is governed by both European and national legislation.
At the European Union level, data protection is regulated by Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016 (General Data Protection Regulation, “GDPR”) and—specifically with respect to electronic communication—by the Directive 2002/58/EC of the European Parliament and of the Council of July 12, 2002 (Directive on Privacy and Electronic Communications, “e-privacy Directive”)).
Since European Union regulation supersedes congruent national data privacy laws, the GDPR is binding in its entirety and directly applicable in each member state. It was primarily intended to harmonize data protection law in the European Union, to improve data protection enforcement and to strengthen the internal market. Nevertheless, the GDPR contains a number of opening clauses that allow EU member states to create specific national laws relating to individual data processing activities or requirements, such as the protection of employee data. Accordingly, EU member states have enacted national implementation acts which accompany the GDPR.
95
Under the GDPR, the regulatory requirements include that personal data may only be collected for specified, explicit and legitimate purposes based on a lawful basis. Personal data may only be collected and processed in a manner consistent with those purposes. Personal data must also be adequate, relevant and limited to what is necessary in relation to the purposes for which it is processed. It must be processed in a manner that ensures transparency to the data subject (i.e., an identified or identifiable natural person to whom the personal data relates). The GDPR stipulates strict requirements regarding the processing of special categories of personal data (such as data concerning health, genetic and biometric information), on the duties to prepare documentation and to furnish proof of compliance with the requirements of the GDPR. The rights of data subjects have been strengthened and include, among others, a right to require information about their data being processed, the right to “data portability” as well as the right to restrict certain processing of their data as well as a “right to be forgotten” pursuant to which data subjects may require that their data is to be deleted when there is a problem with the underlying legality of the processing or where they withdraw their consent. The GDPR also provides restrictive requirements as regards automated decision making and profiling activities, which could impact marketing activities based on such processing of data.
Among other requirements, the GDPR regulates the transfer of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States and the efficacy and longevity of current transfer mechanisms between the EU and the United States remains uncertain. Recent legal developments in Europe have created complexity and uncertainty regarding such transfers. For instance, on July 16, 2020, (so-called Schrems II decision, C - 311/18) the Court of Justice of the European Union, or the CJEU, invalidated the EU-U.S. Privacy Shield Framework, or the Privacy Shield, under which personal data could be transferred from the European Union to U.S. entities who had self-certified under the Privacy Shield scheme. While the CJEU upheld the adequacy of the standard contractual clauses (a standard form of contract approved by the European Commission as an adequate personal data transfer mechanism and alternative to the Privacy Shield), it made clear that reliance on such clauses for personal data transfers from the European Union to so-called third countries (i.e., countries outside the EEA), such as the United States or Russia alone may not necessarily be sufficient in all circumstances. Use of the standard contractual clauses must now be assessed on a case-by-case basis taking into account the legal regime applicable in the destination country, including, in particular, applicable surveillance laws and rights of individuals, and additional measures and/or contractual provisions may need to be put in place; however, the nature of these additional measures is currently uncertain. The CJEU went on to state that if a competent supervisory authority believes that the standard contractual clauses cannot be complied with in the destination country and that the required level of protection cannot be secured by other means, such supervisory authority is under an obligation to suspend or prohibit that transfer. Nevertheless, there have been recent developments towards more certainty in respect of EU-US personal data transfers. On July 10, 2023, the European Commission adopted an adequacy decision for a new mechanism for transferring data from the EU to the United States – the EU-US Data Privacy Framework, which provides EU individuals with several new rights, including the right to obtain access to their data, or obtain correction or deletion of incorrect or unlawfully handled data, and allows US companies to self-certify to the U.S. Department of Commerce their compliance with a set of agreed privacy principles in order to freely receive EU personal data. The adequacy decision followed the signing of an executive order introducing new binding safeguards to address the points raised in the Schrems II-judgment. Notably, the new obligations were geared to ensure that data can be accessed by U.S. intelligence agencies only to the extent necessary and proportionate and to establish an independent and impartial redress mechanism to handle complaints from Europeans concerning the collection of their data for national security purposes. The European Commission will continually review developments in the United States along with its adequacy decision. Adequacy decisions can be adapted or even withdrawn in the event of developments affecting the level of protection in the applicable jurisdiction.
In addition, the GDPR requires us to implement appropriate technical and organizational measures to ensure a level of security appropriate to the organization’s processing requirements and risk. Accordingly, certain cyber security requirements must be fulfilled to ensure that data is processed and stored safely. Organizations must notify the relevant supervisory authority about data breaches within 72 hours and in some instances, provide notification to data subjects. The GDPR provides for substantial fines of up to 4% of the total worldwide group turnover of the preceding fiscal year or up to €20 million (whichever is higher), considerable civil claims for material and immaterial damages (e.g., for infringements of privacy rights) and a general burden of proof for companies. Individual EU member state implementation laws such as the BDSG also provide criminal sanctions for specific violations.
96
Privacy regulations, like the GDPR, concerning the use of web analysis are particularly relevant to our online platform. Web analysis technologies (e.g., processing of cookies or tracking records such as through Google Analytics) process personal data in order to enable the operator of a website to personalize its offers and marketing to better match the client’s interests. Most web analysis tools anonymize or pseudonymize collected data, but the use of such tools is nonetheless regulated by data privacy laws. For example, the use of cookies is regulated by the Directive 2002/58/EC on Privacy and Electronic Communications, or the ePrivacy Directive, that provides for an opt-in regime pursuant to which the use of technically non-necessary cookies and comparable tracking technologies requires an informed consent of the end-user of a device.
However, the ePrivacy Directive needs to be adapted to align with the GDPR. On February 10, 2021 the EU member states agreed on a negotiating mandate regarding the draft proposal for a Regulation on Privacy and Electronic Communications, or the ePrivacy Regulation, which will replace the ePrivacy Directive. The EU Council recently agreed on a draft for the ePrivacy Regulation and, as soon as enacted, the ePrivacy Regulation could lead to stricter requirements and could further impact our online platform. Furthermore, based on its data strategy, the European Union plans to comprehensively revise the legal framework for the handling of data, for example through the recently adopted Digital Markets Act and the Digital Services Act.
Data Privacy and Security Laws in Belgium
In Belgium, the legislator adopted secondary legislation following the GDPR. Notably, the Act of July 30, 2018 on the Protection of Natural Persons with regard to the Processing of Personal Data, or the Data Protection Act, which addresses various national substantive aspects of the GDPR and introduces several specifications and derogations. The Data Protection Act stipulates 13 years old as the age from which children may provide consent for the use of an information service. This is lower than the age of 16 set by the GDPR. Furthermore, the Data Protection Act imposes additional security measures in relation to sensitive data. An entity processing genetic data, biometric data, data concerning health or data related to criminal convictions and offences must maintain a list of the categories of persons who have access to that data, together with a description of their function related to processing such data. When requested, the list must be disclosed to the competent supervisory authority.
The ePrivacy Directive regulates, among other things, the processing of traffic and location data, unsolicited commercial communications and online targeting of consumers by storing information on the equipment of end-users (e.g., cookies). These requirements have been implemented in Belgium in the Act of June 13, 2005 on Electronic Communications, or the Electronic Communications Act. As regards cookies, Article 129 of the Electronic Communications Act follows the wording of the ePrivacy Directive closely. As a result, Article 129 of the Electronic Communication Act requires prior informed consent and does not allow for the user’s consent to be expressed by usage of the appropriate settings of a browser or other application. Furthermore, consumer data may be stored and processed only as long as this is necessary for the provision of services to that consumer.
With regards to cookies, the Belgian Data Protection Authority also indicated that they will treat this as a top priority. The Belgian Data Protection Authority has been proactively looking for cookie infringements on (press) websites and placed those websites under scrutiny in recent case-law. Moreover, the EDPB has published the Cookie Banner Taskforce Report, which identifies common minimum thresholds for the data protection authorities in relation to cookies.
Data Privacy and Security Laws in the United States
Medical device companies may be subject to U.S. federal and state health information privacy, security and data breach notification laws, which may govern the collection, use, disclosure and protection of health- related and other personal information.
HIPAA imposes privacy, security and breach reporting obligations with respect to individually identifiable health information upon “covered entities” (health plans, healthcare clearinghouses and certain health care providers), and their respective business associates, individuals or entities that create, received, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HIPAA mandates the reporting of certain breaches of health information to the U.S. Department of Health and Human Services, or HHS, affected individuals and if the breach is large enough, the media. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, or PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance.
97
Even when HIPAA does not apply, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Personally identifiable health information is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule.
Various states, such as California and Massachusetts, have implemented similar privacy laws and regulations, such as the California Confidentiality of Medical Information Act, that impose restrictive requirements, in some cases more stringent than HIPAA, regulating the use and disclosure of health information and other personally identifiable information. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. Furthermore, California enacted the California Consumer Privacy Act, or CCPA, which took effect on January 1, 2020, became enforceable by the California Attorney General on July 1, 2020, and has been dubbed the first “GDPR-like” law in the United States. The CCPA gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing and receive detailed information about how their personal information is used by requiring covered companies to provide new disclosures to California consumers (as that term is broadly defined). The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. In addition, the California Privacy Rights Act, or CPRA, recently passed in California. The CPRA will impose additional data protection obligations on companies doing business in California, including additional consumer rights processes, limitations on data uses, new audit requirements for high-risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions became effective on January 1, 2023, and additional compliance investment and potential business process changes may be required. Although the CCPA currently exempts certain health-related information, including clinical trial data, the CCPA and the CPRA may increase our compliance costs and potential liability. Similar laws have been adopted in other states (for example, Nevada, Virginia, Connecticut, Utah, Delaware, Florida, Indiana, Iowa, Montana, Oregon, Tennessee, Texas, and Colorado) or proposed in other states and at the federal level, and if passed, such laws may have potentially conflicting requirements that would make compliance challenging.
The interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and our affiliates and partners and potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify.
The legislative and regulatory landscape for privacy and data security continues to evolve, and there has been an increasing focus on privacy and data security issues which may affect our business. Failure to comply with current and future laws and regulations could result in government enforcement actions (including the imposition of significant penalties), criminal and civil liability for us and our officers and directors, private litigation and/or adverse publicity that negatively affects our business.
FCPA and Other Anti-Bribery and Anti-Corruption Laws.
Our operations are subject to anti-corruption laws, including FCPA; the Bribery Act; and other anti-corruption laws that apply in countries where we do business. The FCPA, the Bribery Act, and these other laws generally prohibit Nyxoah and our employees and intermediaries from authorizing, promising, offering, or providing, directly or indirectly, a financial or other advantage to government officials or other persons to induce them to improperly perform a relevant function or activity (or reward them for such behavior).
In general, the FCPA prohibits offering to pay, paying, promising to pay, or authorizing the payment of money or anything of value to a foreign official in order to influence any act or decision of the foreign official in his or her official capacity or to secure any other improper advantage in order to obtain or retain business for or with, or in order to direct business to, any person. The prohibitions apply not only to payments made to “any foreign official,” but also those made to “any foreign political party or official thereof,” to “any candidate for foreign political office” or to any person, while knowing that all or a portion of the payment will be offered, given, or promised to anyone in any of the foregoing categories. “Foreign officials” under the FCPA include officers or employees of a department, agency, or instrumentality of a foreign government. The term “instrumentality” is broad and can include state- owned or state-controlled entities.
98
Importantly, United States authorities that enforce the FCPA, including the Department of Justice, deem most healthcare professionals and other employees of foreign hospitals, clinics, research facilities and medical schools in countries with public healthcare or public education systems to be “foreign officials” under the FCPA. When we interact with foreign healthcare professionals and researchers in testing and marketing our products abroad, we must have policies and procedures in place sufficient to prevent us and agents acting on our behalf from providing any bribe, gift or gratuity, including excessive or lavish meals, travel or entertainment in connection with marketing our products and services or securing required permits and approvals such as those needed to initiate clinical studies in foreign jurisdictions.
The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the maintenance of books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and the development and maintenance of an adequate system of internal accounting controls for international operations. The SEC is involved with the books and records provisions of the FCPA.
We are also subject to other laws and regulations governing our international operations, including regulations administered by the governments of the United Kingdom and the United States, and authorities in the EU, including applicable export control regulations, economic sanctions and embargoes on certain countries and persons, anti-money laundering laws, import and customs requirements and currency exchange regulations.
Employees and Human Capital Resources
As of December 31, 2023, we employed 146.8 full-time equivalents (including employees and consultants), of which 61.4 were based in Europe (Belgium and Germany), 46.4 were based in Israel, 4 were based in Australia and 35 were based in the United States. None of our employees are represented by labor unions or covered by company specific bargaining agreements.
We believe that one of our key strengths is our employee base, which has extensive know-how across research, manufacturing, quality-control, engineering software programming and marketing and sales. We also believe that developing a diverse, equitable and inclusive culture is critical to continuing to attract and retain the top talent necessary for our long-term success and strategy. We value diversity at all levels and continue to focus on extending our diversity and inclusion initiatives across our entire workforce, including the expansion of individuals with diverse backgrounds in leadership.
Our principles of accountability, honesty, integrity and customer-focused, serve as our cultural pillars. We focus our efforts on creating a collaborative environment where our colleagues feel respected and valued. We provide our employees with competitive compensation, opportunities for equity ownership and a robust employment package, including health care, disability and long-term planning insurance, retirement planning and paid time off. In addition, we regularly interact with our employees to gauge employee satisfaction and identify areas of focus.
Legal Proceedings
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our business, results of operations, financial condition or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
C.Organizational Structure
The following is a list of our significant subsidiaries:
| Country of |
|
| Percent | ||
Name of undertaking |
| registration |
| Activity |
| holding |
Nyxoah Ltd | Israel | Medical Technology Research and Development | 100 | |||
Nyxoah Pty Ltd |
| Australia |
| Medical Technology Research and Development |
| 100 |
Nyxoah, Inc. | United States | Medical Technology Research and Development; pre-commercialization activities | 100 | |||
Nyxoah GmbH | Germany | Commercialization of medical devices | 100 |
99
D.Property, Plant and Equipment
Type |
| Location |
| Size (square meters) |
| Expiry |
Executive office |
| Mont-Saint-Guibert, Belgium |
| 570 | June 30, 2029 | |
Offices |
| Milmort, Belgium |
| 120 | December 31, 2026 | |
Manufacturing |
| Milmort, Belgium |
| 140 | N.A. (indefinite duration) | |
Executive office/Storage |
| Tel Aviv, Israel |
| 1,356 | December 31, 2027 |
All of our property is leased. We believe that our office facilities are sufficient to meet our current needs.
Facilities
We operate out of a leased site in Mont-Saint-Guibert, Belgium, which consists of 570 square meters (approximately 6,135 square feet) of office space and is our corporate headquarters and home to our commercial, therapy development and marketing, and clinical activities. The lease for the site in Mont-Saint-Guibert, Belgium expires on June 30, 2029.
We operate out of a leased site in Milmort, Belgium, which consists of 140 square meters (approximately 1,500 square feet) of manufacturing space (cleanroom) and 90 square meters (approximately 970 square feet) of office space and is home to our manufacturing activities. The lease for the office space in Milmort, Belgium expires on December 31, 2026. We can terminate the contract at any time with a notice period of six months. The lease for the manufacturing space in Milmort, Belgium is for an indefinite duration. We can terminate it as from January 1, 2026 with a notice period of eighteen months.
Nyxoah Ltd operates out of a leased site in Tel Aviv, Israel, which consists of 1,306 square meters (approximately 11,830 square feet) of office space and 50 square meters (approximately 540 square feet) of additional storage space and is home to our research and development and manufacturing activities. The lease for the site in Tel Aviv, Israel expires on December 31, 2025 and may be renewed for two additional years to bring the lease to December 31, 2027. We expect the lease extension pursuant to the renewal to occur, and the landlord may only reject the exercise of the lease extension by us if the landlord plans to pull down the building to construct a new one. The landlord must provide us notice of the decision not to extend the lease 120 days prior to the end of the lease period or prior to the planned demolition, whichever is earlier.
Item 4A.Unresolved Staff Comments
None.
Item 5. | Operating and Financial Review and Prospects |
You should read the following discussion and analysis of financial condition and operating results together with the information in “Selected Consolidated Financial Data” and our consolidated financial statements and the related notes to those statements included elsewhere in this Annual Report. We present our consolidated financial statements in EUR and in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB, which may differ in material respects from generally accepted accounting principles in other jurisdictions, including generally accepted accounting principles in the United States, or U.S. GAAP.
The statements in this discussion regarding industry outlook, our expectations regarding our future performance, liquidity and capital resources and other non-historical statements are forward-looking statements. These forward-looking statements are subject to numerous risks and uncertainties, including the risks and uncertainties described in the section titled “Risk Factors.” Our actual results may differ materially from those contained in or implied by any forward-looking statements.
100
A.Operating Results
Overview
We are a medical technology company focused on the development and commercialization of innovative solutions to treat OSA. Our lead solution is the Genio system, a CE-Marked, patient-centric, minimally invasive, next generation hypoglossal neurostimulation therapy for the treatment of moderate to severe OSA. OSA is the world’s most common sleep disordered breathing condition and is associated with increased mortality risk and comorbidities including cardiovascular diseases, depression and stroke. Our innovative technology platform is a first-of-its-kind hypoglossal nerve stimulation device designed to treat OSA through bilateral stimulation, by maintaining an open airway for a restful night’s sleep. We started generating revenue from the sale of the Genio system in Europe in July 2020, and during first quarter of 2023 enrollment in the Dream trial designed to support marketing authorization in the United States was completed. we are currently conducting our DREAM pivotal trial. We are developing a significant body of clinical evidence to further support the strong value proposition of the Genio system and its ability to improve the health and quality of life of OSA patients.
To date, our primary sources of capital have been private placements and public offerings of our common stock, debt financing agreements, and revenue from the sale of our products. Since inception, we have raised equity financing of €200.0 million. As of December 31, 2023, we had cash and cash equivalents of €21.6 million and financial assets of € 36.1 million, long-term debt of € 11.5 million and an accumulated deficit of €160.8 million. We project that our existing cash and cash equivalents and marketable securities should be sufficient to fund operations until the beginning of the fourth quarter of 2024. To meet our future working capital needs, management is actively exploring different financing avenues, including the public or private issuance of equity and debt financing. Additional funds are pivotal for diverse activities, in particular to launch the Genio system in the U.S. and the ongoing progression of research and development projects. This raises, however, a substantial doubt in respect of going concern as the current funds are not sufficient to cover a period of 12 months following the date of the Annual Report. We have devoted substantially all our resources to research and development activities related to our Genio system, including clinical and regulatory initiatives to obtain marketing approvals and have more recently begun to build our European commercialization infrastructure. During the year ended December 31, 2023, we generated revenue of €4.3 million and our net loss was €43.2 million. We expect that our, research and development and selling, general and administrative and other expenses will continue to increase as we expand our marketing efforts to increase sales of the Genio system, conduct clinical trials, including our pivotal DREAM trial and ACCCESS trial, seek for additional regulatory approvals and clearances and continue to invest in research and development to create product enhancements and enhance our product offering.
Key Factors and Trends
Obtaining regulatory approval in additional significant markets
We must successfully obtain timely approval or clearances and introduce new markets that gain acceptance with physicians. We are currently approved to market in Europe. And for our sales to grow, we will also need to receive FDA marketing authorization for the Genio system. We finalized the enrollment of the DREAM trial, which is our pivotal trial that we intend to rely on to receive marketing authorization in the United States. On March 19, 2024, we issued a press release announcing that the DREAM U.S. pivotal trial met its primary endpoints. For more information see “Item 4—Business—Clinical Results and Studies—Pivotal DREAM Trial” above. We expect to apply to the FDA for marketing authorization for the Genio system in the United States with the aim of being commercially available in the United States in late 2024. Our ability to expand the list of countries in which we are able to market and sell our system will significantly impact our revenue growth and the costs we incur in anticipation of such growth. Seeking for and obtaining regulatory approval for the Genio system in any of these countries is a long, expensive and uncertain process that can be impacted by numerous risks which are outside our control.
101
Growing and supporting our commercial organization.
We are committed to making additional investments in, and will continue to invest in recruiting, training and retaining experience and specialized sales teams. As of December 31, 2023, our European training, commercial and marketing team consists of eight professionals, who have substantial medical device sales and marketing, training and education as well as clinical experience and are operating from our headquarter in Belgium. Since the Genio system began being reimbursed under a dedicated DRG code in Germany, we have additionally invested in building up a dedicated direct sales and marketing organization of ten individuals, led by a country director in Germany. In order to grow our business with existing and new accounts, we will need to continue making significant investments in educating physicians, hospitals and patients in the advantages of the Genio system for the treatment of moderate to severe OSA.
Continuing to invest in developing clinical support.
Publication of clinical results by us can have a significant influence on whether the Genio system is used by physicians. We intend to continue investing in clinical studies on the Genio system. We are initially targeting markets in Europe where we have identified a country-specific reimbursement pathway or execution strategy. We obtained reimbursement coverage and began marketing in Germany in 2020 and Switzerland in 2021.We generated our first revenue in Spain in 2021 through local funding and we began commercialization in Finland in 2022. We generated our first revenue in Austria in 2023.
Increasing physician adoption and acceptance of the Genio System
The growth of our business depends on our ability to gain broader acceptance of the Genio system by continuing to make physicians aware of the benefits of the Genio system in order to generate increased demand and frequency of use and, thus, increase sales to our customers. Our ability to grow our business will also depend on our ability to expand our customer base in existing and new target markets. To date, the Genio system is our only product on the market. The Genio system has not yet received marketing approval in the United States, however. Accordingly, our future financial performance will depend on the successful completion of our planned pivotal trial in the United States.
Securing additional coverage and reimbursement by third-party payors
The level of reimbursement from third-party payors for procedures performed using the Genio system could have a substantial impact on the prices we are able to charge for the Genio system and how widely it is accepted. In many countries, payment for the Genio system will be dependent on obtaining a reimbursement code or codes for the procedure and the Genio system. Obtaining a reimbursement code can be a lengthy process that varies from country to country. While there is general consensus among physicians and payors of the medical necessity to treat OSA and increase the number of hypoglossal nerve stimulation therapy coverage decisions, we continue to develop further clinical evidence demonstrating a long-term meaningful improvement in net health outcomes for patients meeting the specified criteria. We believe that establishing and maintaining reimbursement will be important in achieving broad acceptance of our system by healthcare providers in these markets. For our sales to grow, we will also need to receive FDA marketing authorization for the Genio system. We expect that the outcomes of the ongoing pivotal DREAM trial, if favorable, will support marketing approval and reimbursement in the United States.
Continuing to invest in innovation and growth
We continue to invest in, and innovate with respect to, our existing Genio system to further improve future generations, as well as clinical outcomes, enhance the patient and physician experience and broaden the patient population that can be treated. We are also investing in building our pipeline of new products through our partnership with Vanderbilt University to expand the current neurostimulation options to treat moderate to severe OSA. While developing new products and technologies can be time consuming and costly, we believe that a pipeline of new technologies and next generation products is important for supporting increased adoption of our products. In the short term, we expect these activities to increase our net losses, but in the longer term, we anticipate they will positively impact our business and results of operations.
102
Due to these and other factors and trends, we expect to experience meaningful variability in our financial performance for the foreseeable future, including, but not limited to: costs, benefits and timing of new product introductions; the availability and cost of components and raw materials; and fluctuations in foreign currency exchange rates. Additionally, we experience quarters in which operating expenses, in particular research and development expenses, fluctuate depending on the stage and timing of product development.
While these factors may present significant opportunities for us, they also pose significant risks and challenges that we must address. See the section titled “Risk Factors” for more information.
Need for additional capital
We expect to incur significant expenses and operating losses over the next few years, and we may need to raise additional capital in the future. We have so far been financed primarily by funds invested by our shareholders, including in connection with our initial public offering on Euronext Brussels in September 2020 and the listing of our ordinary shares on the Nasdaq Global Market in July 2021. Based on our current operating plan and our existing cash and cash equivalents of €21.6 million and financial assets of €36.1 million as of December 31,2023, we expect to be able to fund our operations until the beginning of the fourth quarter of 2024. However, we have based these estimates on assumptions that may prove to be incorrect, and we could spend our financial resources much faster than currently expected. Pursuant to the requirements of IAS 1.25-26, Presentation of Financial Statements - Going Concern, and as a result of our financial condition and other factors described herein, there is substantial doubt about our ability to continue as a going concern for a period of at least twelve months from the date of this Annual Report. See the report of our independent registered public accounting firm and Note 5.1 to our consolidated financial statements found elsewhere in this Annual Report. Our ability to continue as a going concern will depend on our ability to obtain additional funding, as to which no assurances can be given. See “Risk Factors—We will require additional capital in the future, which may not be available to us on commercially favorable terms, or at all. More specifically, there is substantial doubt about our ability to continue as a going concern for a period of at least twelve months from the date of this Annual Report and our ability to continue as a going concern will depend on our ability to obtain additional funding, as to which no assurances can be given as of the date of this Annual Report.”
Financial Operations Overview
Revenue
We currently derive all of our revenue from the sale of our proprietary Genio system, which we have started commercializing in Europe and, more specifically, in Germany. We sell the Genio system to both hospitals and distributors. Revenue is recognized at a point in time upon satisfaction of the performance obligation, being the moment control over the Genio® system is transferred to the customer, which is in general at delivery at customer site or a predefined location in the country of the customer. For certain customers, control may be transferred upon shipment to the customer in case the incoterms are Ex-Works. The revenue from the Genio® system consists of a kit of products delivered at the same point in time, and as such revenue does not need to be allocated over the different products. The revenue is then recognized at an amount that reflects the consideration to which the Company expects to be entitled in exchange of the Genio® system. In determining the transaction price for the sale of the Genio® system, the Company considers the effects of variable consideration.
Cost of Goods Sold
Cost of goods sold consists primarily of third-party manufacturing costs that we incur to obtain the components necessary to manufacture our Genio system. Direct costs from our third-party manufacturers includes costs for raw materials plus the mark-up for the assembly of the components. Cost of goods sold also includes allocated overhead for indirect labor, depreciation and information technology, certain direct costs such as those incurred for shipping our products, and personnel costs, including salary and share-based compensation.
Gross Profit and Gross Margin
We calculate gross profit as revenue less cost of goods sold, and gross margin as gross profit divided by revenue. Our gross margin has and will continue to be affected by a variety of factors, primarily average selling prices, production and ordering volumes, third-party manufacturing costs and cost-reduction strategies. We expect our gross profit to increase in the foreseeable future as our revenue grows. Our gross margin may increase over the long-term to the extent our production volume increases as our fixed manufacturing costs would be spread over a larger number of units.
103
Operating Expenses
Research and Development Expenses (in aggregate)
Research and development expenses (in aggregate) consist primarily of employee compensation, consulting and contractor’s fees, quality assurance and regulatory expenses, manufacturing and outsourced development expenses, clinical expenses, legal fees related patents and other related expenses, to support our products and the next generation of the Genio system. We expect to continue to invest in research and development expenses as we develop the next generation of the Genio system, investing in building a new product pipeline, continue to enroll additional patients in EliSA, and ACCCESS studies.
Selling, General and Administrative (in aggregate)
Selling, general and administrative expenses (in aggregate) include the following components: general and administrative expenses, therapy development expenses and other operating income/expenses.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation for personnel, including salaries, bonuses, benefits, and stock-based compensation, spending related to direct sale force, market access and reimbursement activities to support the commercialization of Genio system in Europe, consulting fees and spending related to finance, information technology, legal and human resource functions, as well as professional services fees (including legal, audit and tax fees), insurance costs, general corporate expenses and allocated facilities-related expenses to support the scale up of the Company. We expect that our general and administrative expenses will increase as commercial activities will grow over time and scale up of the Company in legal, finance, tax and IT matters.
Other Operating Income/Expenses
Other operating income/expenses consist of the impact of the initial measurement and re-measurement of financial debt and the Australian R&D incentive subsidies receive by our subsidiary in Australia and as from 2023 the tax incentive in Belgium as well.
Results of Operations
Comparison of Year Ended December 31, 2022, and 2023
The following table summarizes our results of operations for the periods presented below:
For the year ended | Year-Over-Year |
| |||||||||
December 31 | Change |
| |||||||||
(In Thousands) | 2023 | 2022 | Euro Change | % Change |
| ||||||
Revenue |
| € | 4,348 |
| 3,084 |
| 1,264 |
| 41 | % | |
Cost of goods sold |
| (1,656) |
| (1,150) |
| (506) |
| 44 | % | ||
Gross Profit | € | 2,692 | € | 1,934 |
| 758 |
| 39 | % | ||
Research and development expenses |
| (26,651) |
| (15,861) |
| (10,790) |
| 68 | % | ||
General and administrative expenses |
| (21,687) |
| (18,855) |
| (2,832) |
| 15 | % | ||
Other operating income/(expenses) |
| 544 |
| 283 |
| 261 |
| 92 | % | ||
Operating loss for the period |
| (45,102) |
| (32,499) |
| (12,603) |
| 39 | % | ||
Financial income |
| 4,174 |
| 6,763 |
| (2,589) |
| (38) | % | ||
Financial expense |
| (3,729) |
| (4,320) |
| 591 |
| (14) | % | ||
Loss for the period before taxes |
| (44,657) |
| (30,056) |
| (14,601) |
| 49 | % | ||
Taxes |
| 1,445 |
| (1,169) |
| 2,614 |
| (224) | % | ||
Loss for the period |
| (43,212) |
| (31,225) |
| (11,987) |
| 38 | % | ||
104
Revenue
Revenue was €4.3 million for the year ended December 31, 2023, compared to €3.1 million for the year ended December 31, 2022. The increase in revenue was attributable to our growing commercialization of the Genio system, including in additional markets, such as Austria.
Cost of Goods Sold
Cost of goods sold was €1.7 million for the year ended December 31, 2023, compared to €1.2 million for the year ended December 31, 2022. The increase in cost of goods sold was attributable to our growing commercialization of the Genio system, including in additional markets, such Austria.
Research and Development Expenses (in aggregate)
Research and development expenses (in aggregate) increased by € 10.8 million, or 68.0%, from €15.9 million in 2022 to €26.7 million in 2023 due to the factors discussed below with respect to each component of research and development expenses (in aggregate).
Staff costs Expenses. Staff cost expenses increased by € 2.7 million, or 24%, from €11.1 million in 2022 to € 13.8 million in 2023 mainly due to an increase in staff to support our clinical, R&D and manufacturing activities.
Consulting and contractors’ fees Expenses. Consulting and contractors’ fees expenses increased by € 0.2 million, or 8%, from €2.6 million in 2022 to € 2.8 million in 2023 due to an increase in consulting and contractors’ fees to support our clinical, R&D and manufacturing activities.
Clinical Expenses. Clinical expenses decreased by €3.7 million, or 57%, from €8.6 million in 2022, to € 4.9 million in 2023. The decrease in clinical expense was mainly due to a decrease in clinical study activities due to finalizing enrollment of Dream Study.
Manufacturing and outsourced development Expenses. Manufacturing and outsourced development expenses increased by € 1.5 million, or 30%, from €5.0 million in 2022 to € 6.5 million in 2023 due to an increase in manufacturing and outsourced development expenses to support our clinical, R&D and manufacturing activities.
IP costs Expenses. Legal fee expenses increased by € 0.5 million, or 125%, from €0.4 million for the year ended December 31, 2022, to € 0.9 million for the year ended December 31, 2023. The increase is mainly due to the payment for in-licensing agreement with Vanderbilt University during 2023.
IT Expenses. IT expenses increased by € 1.8 million, or 100%, from €0.0 million for the year ended December 31, 2022, to € 1.8 million for the year ended December 31, 2023. The increase is mainly due to the start of a new ERP implementation.
Selling, General and Administrative Expenses (in aggregate)
Selling, general and administrative expenses (in aggregate) increased by € 2.8 million, or 15 %, from €18.9 million in 2022 to €21.7 million in 2023 due to the factors discussed below with respect to each component of selling, general and administrative expenses (in aggregate).
Staff costs Expenses. Staff costs expenses increased by €0.9 million, or 12 %, from €7.8 million in 2022 to €8.7 million in 2023 mainly due an increase in staff costs to support commercialization of Genio system in Europe and scale up of the Company in legal, finance, tax and IT matters.
Consulting and contractors’ fees Expenses. Consulting and contractors’ fees expenses increased by € 2.3 million, or 50%, from €4.5 million in 2022 to € 6.8 million in 2023 mainly due mainly due an increase in consulting and contractors’ fees costs to support commercialization of Genio system in Europe and scale up of the Company in legal, finance, tax and IT matters.
105
Legal fee Expenses. Legal fee expenses decreased by € 0.3 million, or 25%, from €1.0 million for the year ended December 31, 2022, to € 0.8 million for the year ended December 31, 2023. This decrease is mainly due to transaction costs for an amount of €494,000 related to the shelf registration and “at-the-market” offering (the “ATM”) in 2022.
Insurance fees Expenses. Insurance fees expenses decreased by € 0.5 million, or 35%, from €1.5 million for the year ended December 31, 2022, to € 1.0 million for the year ended December 31, 2023. The insurances fees expenses decreased mainly due to renegotiation of terms.
IT Expenses. IT expenses increased by € 0.7 million, or 130%, from €0.5 million for the year ended December 31, 2022, to € 1.2 million for the year ended December 31, 2023. The increase is mainly due to the start of a new ERP implementation.
Operating Loss
The increase of operating loss from €32.5 million in 2022 to € 45.1 million in 2023, or a change of € 12.6 million, was mainly due to increases of activities in all departments. We are currently conducting three clinical trials to continue gathering clinical data and obtain regulatory approvals. During first quarter of 2023 enrollment in the Dream trial designed to support marketing authorization in the United States was completed. We continue to enroll additional patients in EliSA, and ACCCESS studies. In line with its strategy, we continue to invest in research and development to improve and develop the next generation of the Genio system and prepare for scaling-up of production capacities.
Comparison of Year Ended December 31, 2021, and 2022
The following table summarizes our results of operations for the periods presented below:
For the year ended | Year-Over-Year |
| ||||||||||
December 31 | Change |
| ||||||||||
(In Thousands) | 2022 | 2021 | Euro Change | % Change |
| |||||||
Revenue |
| € | 3,084 |
| € | 852 |
| 2,232 |
| 262 | % | |
Cost of goods sold |
| (1,150) |
| (303) |
| (847) |
| 280 | % | |||
Gross Profit | € | 1,934 | € | 549 |
| 1,385 |
| 252 | % | |||
Research and development expenses |
| (15,861) |
| (12,344) |
| (3,517) |
| 28 | % | |||
General and administrative expenses |
| (18,855) |
| (14,712) |
| (4,143) |
| 28 | % | |||
Other operating income/(expenses) |
| 283 |
| 265 |
| 18 |
| 7 | % | |||
Operating loss for the period |
| (32,499) |
| (26,242) |
| (6,257) |
| 24 | % | |||
Financial income |
| 6,763 |
| 3,675 |
| 3,088 |
| 84 | % | |||
Financial expense |
| (4,320) |
| (2,072) |
| (2,248) |
| 108 | % | |||
Loss for the period before taxes |
| (30,056) |
| (24,639) |
| (5,417) |
| 22 | % | |||
Taxes |
| (1,169) |
| (2,980) |
| 1,811 |
| 61 | % | |||
Loss for the period |
| (31,225) |
| (27,619) |
| (3,606) |
| 13 | % | |||
Revenue
Revenue was €3.1 million for the year ended December 31, 2022, compared to €0.9 million for the year ended December 31, 2021. The increase in revenue was attributable to our growing commercialization of the Genio system, including in additional markets, such as Spain, Switzerland, Belgium, and Finland.
Cost of Goods Sold
Cost of goods sold was €1.2 million for the year ended December 31, 2022, compared to €303,000 for the year ended December 31, 2021. The increase in cost of goods sold was attributable to our growing commercialization of the Genio system, including in additional markets, such as Spain, Switzerland, Belgium, and Finland.
106
Research and Development Expenses (in aggregate)
Research and development expenses (in aggregate) increased by € 3.6 million, or 28.5%, from €12.3 million in 2021 to €15.9 million in 2022 due to the factors discussed below with respect to each component of research and development expenses (in aggregate).
Staff costs Expenses. Staff cost expenses increased by € 3.1 million, or 39%, from €8.0 million in 2021 to € 11.1 million in 2022 mainly due to an increase in staff to support our clinical, R&D and manufacturing activities.
Consulting and contractors’ fees Expenses. Consulting and contractors’ fees expenses increased by € 0.6 million, or 30%, from €2.0 million in 2021 to € 2.6 million in 2022 due to an increase in consulting and contractors’ fees to support our clinical, R&D and manufacturing activities.
Clinical Expenses. Clinical expenses increased by €4.6 million, or 115%, from €4.0 million in 2021, to € 8.6 million in 2022. The increase in clinical expense was mainly due to an increase in study activities of the on-going DREAM IDE in the United States, completion of the BETTER SLEEP trial implantations, continuous recruitment for the EliSA trial and launching the ACCCESS U.S. pivotal trial.
Other Expenses. Other expenses increased by €0.6 million, or 60%, from €1.0 million in 2021, to € 1.6 million in 2022. The increase in other expense was mainly due to an increase in supporting activities related to the scale up of the Company.
Legal fee Expenses. Legal fee expenses decreased by € 0.7 million, or 64%, from €1.1 million for the year ended December 31, 2021, to € 0.4 million for the year ended December 31, 2022. The decrease is mainly due to the payment for in-licensing agreement with Vanderbilt University during 2021.
Selling, General and Administrative Expenses (in aggregate)
Selling, general and administrative expenses (in aggregate) increased by € 4.1 million, or 28.2 %, from €14.7 million in 2021 to €18.9 million in 2022 due to the factors discussed below with respect to each component of selling, general and administrative expenses (in aggregate).
Staff costs Expenses. Staff costs expenses increased by €4.1 million, or 111 %, from €3.7 million in 2021 to €7.8 million in 2022 mainly due an increase in staff costs to support commercialization of Genio system in Europe and scale up of the Company in legal, finance, tax and IT matters.
Consulting and contractors’ fees Expenses. Consulting and contractors’ fees expenses decreased by € 2.0 million, or 31.8%, from €6.6 million in 2021 to € 4.5 million in 2022 due to variable compensations for an amount of €1.9 million for the year ended December 31, 2021 related to a cash-settled share based payment transaction.
Legal fee Expenses. Legal fee expenses increased by € 0.6 million, or 150%, from €0.4 million for the year ended December 31, 2021, to € 1.0 million for the year ended December 31, 2022. This increase is mainly due to transaction costs for an amount of €494,000 related to the shelf registration and “at-the-market” offering (the “ATM”).
Travel Expenses. Travel expenses increased by € 0.8 million, or 267%, from €0.3 million for the year ended December 31, 2021, to € 1.1 million for the year ended December 31, 2022. The travel expenses increased mainly due to the ongoing commercialization in Europe and the scale up of the Company.
Insurance fees Expenses. Insurance fees expenses increased by € 0.6 million, or 67%, from €0.9 million for the year ended December 31, 2021, to € 1.5 million for the year ended December 31, 2022. The insurances fees expenses increased mainly due to full year for Directors & Officers insurance following initial public offering in the United States in July 2021.
107
Other Operating Income / (Expenses). The other operating income contains the R&D Incentive (Australia) that relates to an incentive to be received on development expenses incurred by the subsidiary in Australia. The R&D incentive for the year ended December 31, 2022 includes a correction for 2021. For the year ended December 31, 2022, €123,000 has been deducted from the expenses capitalized and for the year ended December 31, 2021, €0.6 million has been deducted from the expenses capitalized in relation to this R&D Incentive.
Operating Loss
The increase of operating loss from €26.2 million in 2021 to € 32.5 million in 2022, or a change of € 6.3 million, was mainly due to increases of activities in all departments. We are currently conducting three clinical trials to continue gathering clinical data and obtain regulatory approvals. In July 2022, we obtained IDE approval to start the ACCCESS trial in the United States. In line with its strategy, we continue to invest in research and development to improve and develop the next generation of the Genio system and prepare for scaling-up of production capacities.
Recently Issued Accounting Pronouncements
We applied for the first-time certain interpretations and amendments, which are effective for annual periods beginning on or before January 1, 2023. The new standards and amendments that apply for the first time in 2023 are not expected to have a material impact on our financial position, results of operations or cash flows and are described in Note 2.2 to our consolidated financial statements found elsewhere in this Annual Report.
Emerging Growth Company and Foreign Private Issuer Status
Emerging Growth Company Status
As a company with an annual revenue under $1.07 billion, we qualify as an “emerging growth company” as defined in the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies.
These provisions include:
| ● | the ability to present only two years of audited financial statements in addition to any required interim financial statements and correspondingly reduced disclosure in management’s discussion and analysis of financial condition and results of operations in this Annual Report; |
| ● | exemption from the auditor attestation requirement of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, in the assessment of our internal controls over financial reporting; and |
| ● | to the extent that we no longer qualify as a foreign private issuer, (i) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (ii) exemptions from the requirements of holding a non-binding advisory vote on executive compensation, including golden parachute compensation. |
We may take advantage of these exemptions for up to five years or until such earlier time that we are no longer an emerging growth company. We will cease to be an emerging growth company upon the earliest to occur of (i) the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; (ii) the date we qualify as a “large accelerated filer” with at least $700 million of equity securities held by non-affiliates; (iii) the issuance, in any three-year period by our company of more than $1.0 billion in non-convertible debt securities held by non-affiliates; and (iv) the last day of the fiscal year ending after the fifth anniversary of this public offering of our ordinary shares.
We may choose to take advantage of some but not all of these reduced burdens. For example, we intend to take advantage of the exemption from the auditor attestation on the effectiveness of our internal control over financial reporting. Accordingly, the information that we provide shareholders may be different than you might obtain from other public companies.
108
In addition, Section 107 of the JOBS Act provides that an emerging growth company can use the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Given that we currently report and expect to continue to report under IFRS as issued by the IASB, we have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the IASB. Since IFRS makes no distinction between public and private companies for purposes of compliance with new or revised accounting standards, the requirements for our compliance as a private company and as a public company are the same.
Foreign Private Issuer Status
We currently report under the Exchange Act as a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| ● | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| ● | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; |
| ● | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10 - Q containing unaudited financial and other specified information, or current reports on Form 8 - K, upon the occurrence of specified significant events; and |
| ● | Regulation FD, which regulates selective disclosures of material information by issuers. |
B.Liquidity and Capital Resources
Overview
To date, our primary sources of capital have been private placements and public offerings of our common stock and debt financing agreements. Since inception, we have raised equity financing of €200.0 million. In September 2020, we raised €103.6 million as a result of the initial public offering of new shares on the Euronext. All of our shares were admitted to trading on the regulated market of Euronext Brussels under the symbol “NYXH”. In July 2021, we raised €75.0 million net of transaction costs as a result of the initial public offering of new shares on The Nasdaq Global Market. In December 2022, we entered into an “at-the-market” (“ATM”) sales agreement with Cantor Fitzgerald & Co. (“Cantor”) pursuant to which we may sell from time to time ordinary shares having an aggregate offering price of up to $50.0 million through Cantor, acting as our sales agent. Sales of our ordinary shares pursuant to this ATM program are subject to certain conditions specified in the sales agreement. Sales under the ATM program are registered on a shelf registration statement on From F-3 that we filed with the SEC in December 2022, and which permits the offering, issuance and sale by us of up to a maximum aggregate offering price of $200.0 million of our securities, inclusive of our ordinary shares sold under the ATM program. During 2023 we carried out several capital raises for a total amount of €18.9 million. All of our shares were admitted to trading on The Nasdaq Global Market under the symbol “NYXH”. As of December 31, 2023, we had cash and cash equivalents of €21.6 million and financial assets of €36.1 million and an accumulated deficit of €160.8 million. We project that our existing cash and cash equivalents and marketable securities should be sufficient to fund operations until the beginning of the fourth quarter of 2024. To meet our future working capital needs, management is actively exploring different financing avenues, including the public or private issuance of equity and debt financing. Additional funds are pivotal for diverse activities, in particular to launch the Genio product in the U.S. and the ongoing progression of research and development projects. This raises, however, a substantial doubt in respect of going concern as the current funds are not sufficient to cover a period of 12 months following the date of the Annual Report. See the report of our independent registered public accounting firm and Note 5.1 to our consolidated financial statements found elsewhere in this Annual Report.
109
Cash Flows
The following table summarizes the results of our cash flows for the years ended December 31, 2022 and 2023.
Year ended December 31, | ||||||
2023 | 2022 | |||||
(In thousands) | ||||||
Net cash used in operating activities |
| € | (44,778) |
| € | (28,756) |
Net cash from (used in) investing activities |
| 32,011 |
| (89,946) | ||
Net cash from (used in) financing activities |
| 16,858 |
| (983) | ||
Effects of exchange rate changes |
| (369) |
| 2,064 | ||
Change in cash and cash equivalents | € | 3,722 | € | (117,621) | ||
Operating activities. Net cash used in operations was €44.8 million in 2023 compared to €28.8 million in 2022. The increase of cash used in operations of €16.0 million was primarily due to higher losses of €14.6 million that were mainly attributable to increased research and development expenses and selling, general and administrative general expenses, as described in more detail above. This increase was offset by a negative variation in the working capital and other non-cash adjustments.
Investing activities. Net cash from investing activities was €32.0 million in 2023 compared to €90.0 million in 2022. The change of €122.0 million compared to 2022 is mainly due to the fact that last year purchases of term accounts amounted to € 102.6 million offset by € 28.9 million term accounts reaching their maturity (after which the term deposit is held as cash), whilst in current year purchases of term accounts amounted to € 80 million offset by € 120.7 million term accounts reaching their maturity.
Financing activities. Net cash from financing activities in 2023, was €16.9 million compared to €(1.0) million in 2022. The increase was primarily derived from several capital increases during 2023. See Note 15 to our consolidated financial statements found elsewhere in this Annual Report.
The following table summarizes the results of our cash flows for the years ended December 31, 2021 and 2022.
Year ended December 31, | ||||||
2022 | 2021 | |||||
(In thousands) | ||||||
Net cash used in operating activities |
| € | (28,756) |
| € | (25,336) |
Net cash used in investing activities |
| (89,946) |
| (11,817) | ||
Net cash from (used in) financing activities |
| (983) |
| 76,472 | ||
Effects of exchange rate changes |
| 2,064 |
| 3,890 | ||
Change in cash and cash equivalents | € | (117,621) | € | 43,209 | ||
Operating activities. Net cash used in operations was €28.8 million in 2022 compared to €25.3 million in 2021. The increase of cash used in operations of €3.4 million was primarily due to higher losses of €5.4 million that were mainly attributable to increased research and development expenses and selling, general and administrative general expenses, as described in more detail above. This increase was offset by a negative variation in the working capital and other non-cash adjustments.
Investing activities. Net cash from investing activities in 2022, was € 89.9million compared to €11.8 million in 2021. The increase in net cash from investing activities related mainly due to term accounts of €77 million recorded as financial assets
Financing activities. Net cash from financing activities in 2022, was €(0.1) million compared to €76.5 million in 2021. The decrease was primarily derived from capital raise from the Nasdaq IPO in 2021.
110
Operating and Capital Expenditure Requirements
We use our cash to fund our operations, which primarily include the cost of manufacturing our Genio system, as well operating expenses and related personnel costs. We expect research and development expenses to increase for the foreseeable future as we continue to hire personnel and invest in next-generation innovations of the Genio system and related products. In addition, we expect our general and administrative expenses to increase for the foreseeable future as we hire personnel and expand our infrastructure to both drive and support the anticipated growth in our organization. We will also incur additional expenses as a result of operating as a dual listed public company and also expect to increase the size of our administrative function to support the growth of our business. The timing and amount of our operating expenditures will depend on many factors, including:
| ● | acceptance of our therapy by patients, physicians, government payers, private payers, and the market generally; |
| ● | the scope, rate of progress and cost of current or future clinical studies; |
| ● | the cost of research and development activities; |
| ● | the cost associated with any complications or side effects related to the use of the Genio system; |
| ● | the cost of filing and prosecuting patent applications and other intellectual property rights and defending and enforcing our patents or other intellectual property rights in various jurisdictions; |
| ● | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; |
| ● | the cost and timing of additional regulatory clearances or approvals; |
| ● | the cost and timing of establishing additional sales and marketing capabilities; |
| ● | costs associated with any product recall that may occur; |
| ● | the effect of competing technological and market developments; |
| ● | the extent to which we acquires or invests in products, technologies and businesses; and |
| ● | the costs of operating as a dual listed public company. |
We have consistently operated with deficits and sustained negative cash flows since our inception considering the significant research and development expenses incurred for the development and regulatory approval of the Genio device. As of December 31, 2023, our statement of financial position includes an accumulated loss of € 160.8 million and total assets of € 124.2 million. Current assets as of December 31, 2023 total €68.4 million, comprising €21.6 million in available cash and cash equivalents, and €36.1 million in marketable securities, primarily derived from previous public offerings.
Our current operating plan indicates that it will continue to incur losses from operations and generate negative cash flows from operating activities given ongoing expenditures related to the completion of its clinical trials only partially offset by our revenue generating activities outside the U.S. (which were which were €4.3 million in 2023 in the EU). Substantial revenue generation is expected to start following the launch of the Genio product in the U.S., which is dependent on obtaining marketing authorization in the United States for the Genio product from the FDA.
111
We project that our existing cash and cash equivalents and marketable securities should be sufficient to fund operations until the beginning of the fourth quarter of 2024. To meet our future working capital needs, management is actively exploring different financing avenues, including the public or private issuance of equity and debt financing. Additional funds are pivotal for diverse activities, in particular to launch the Genio product in the U.S. and the ongoing progression of research and development projects. This raises, however, a substantial doubt in respect of going concern as the current funds are not sufficient to cover a period of 12 months following the date of the Annual Report.
Although the additional funds have not been raised yet, given the positive outcome from the DREAM trial, we are confident that raising sufficient funding to continue our operations for at least 12 months following the date of the Annual Report should not pose significant challenges.
The accompanying consolidated financial statements have therefore been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
Contractual Obligations
The following table sets out our contractual obligations and commitments due by period as of December 31, 2023:
2023 | |||||||
(in EUR 000) | Lease Liability | Financial Debt | Trade & Other Payable | ||||
Less than 1 year |
| 990 |
| 378 |
| 11,240 | |
1 - 5 years |
| 2,729 |
| 8,488 |
| — | |
5+ years |
| 748 |
| 4,608 |
| — | |
TOTAL | € | 4,467 |
| 13,474 |
| 11,240 | |
C.Research and Development, Patents and Licenses, etc.
Full details of our research and development activities and expenditures are given in “Item 4. Information on the Company–B. Business” and “Item 5A. Operating Results” within this Annual Report.
D.Trend Information
See “Item 5A. Operating Results” within this Annual Report.
E.Critical Accounting Estimates
See Note 5 to our consolidated financial statements found elsewhere in this Annual Report for a discussion on the critical accounting policies, estimates, assumptions, and judgments that we believe to have the most significant impact on our consolidated financial statements.
112
Item 6.Directors, Senior Management and Employees
A.Directors and Senior Management
The following table sets forth the names, ages and positions of our executive officers and directors as of the date of this Annual Report:
Name |
| Age |
| Position |
| Term (1) |
|
Executive Officers | |||||||
Olivier Taelman | 52 | Chief Executive Officer and Director | — | ||||
Loïc Moreau | 44 | Chief Financial Officer | — | ||||
Non-Executive Directors | |||||||
Robert Taub | 75 | Non-Executive Director and Chairman | 2024 | ||||
Kevin Rakin | 63 | Non-Executive Director | 2024 | ||||
Pierre Gianello, M.D. | 67 | Non-Executive Director | 2024 | ||||
Jürgen Hambrecht, Ph.D. | 77 | Non-Executive Director | 2024 | ||||
Rita Johnson-Mills | 64 | Non-Executive Director | 2024 | ||||
Virginia Kirby | 68 | Non-Executive Director | 2024 | ||||
Wildman Ventures LLC | 67 | Non-Executive Director | 2024 |
| (1) | The term of the mandates of the non-executive directors will expire immediately after the annual shareholders’ meeting held in the year set forth next to the director’s name. |
Executive Officers
Olivier Taelman has served as an executive director since September 2020 and our Chief Executive Officer since November 2019. Mr. Taelman joined our company in July 2019 as Chief Operating and Commercial Officer. Prior to joining our Company, Mr. Taelman was Vice President Europe at Autonomic Technologies, Inc., a U.S. medical device company, where he focused on clinical, market access and commercialization of SPG Neuromodulation to treat patients with severe headache and developed strong relationships with global key opinion leaders and managed investor relations. Prior to that, Mr. Taelman was Business Director, Neuromodulation at Nevro, Corp. (NYSE: NVRO) a neuromodulation company, where he led the development of the company’s European commercial structure. Prior to Nevro, Mr. Taelman served for 10 years in various roles at Medtronic plc (NYSE: MDT), leading the neuromodulation department in Western European countries. Mr. Taelman holds an executive MBA from the Wharton University and a bachelor’s degree in Biology and Physics from Hasselt University.
Loïc Moreau has served as our Chief Financial Officer since January 2022. From 2009 through 2021, he held various senior roles at GlaxoSmithKline plc. (GSK), including roles in Mergers and Acquisitions, Corporate Development and Country- Chief Financial Officer across different geographies. Prior to GSK, Mr. Moreau built his career at Ernst & Young Global Limited (External Audit) and PricewaterhouseCoopers (Corporate Finance). Mr. Moreau holds an Executive Master from the École Supérieure des Sciences Commerciales d’Angers School of Management, France, and a Master of Finance from Solvay University, Belgium.
113
Non-Executive Directors
Robert Taub is the founder of our company and has served as Chairman of our Board of Directors since our inception in July 2009. He also served as our Chief Executive Officer from July 2009 to September 2016. Mr. Taub is an entrepreneur, investing in the pharmaceutical and medical fields. Prior to founding our Company, he co-founded and co-managed Octapharma AG, a human plasma protein company, from 1983 to 1995. He also founded and managed Omrix Biopharmaceuticals, Inc. through its initial public offering and listing on Nasdaq and its acquisition by Johnson & Johnson in 2008. Prior to that, Mr. Taub held various general management and sales and marketing positions with The Monsanto Company, Baxter Travenol Laboratories and the Revlon Health Care Group. Mr. Taub holds an MBA at INSEAD. Currently, Robert is the Chairman of Aya Gold and Silver (TSX: AYA.TO).
Kevin Rakin has served as a non-executive director since June 2016. Since October 2013, Mr. Rakin has been a co-founder and partner of HighCape Capital and he brings more than 30 years of experience as an executive and investor in the life sciences industry. He served as the President of Shire Regenerative Medicine, Inc. from June 2011 to November 2012. Mr. Rakin was the chairman and chief executive officer of Advanced BioHealing from 2007 until its acquisition by Shire in 2011. Before that, he served as an Executive-in-Residence at Canaan Partners, a venture capital firm. Until its merger with Clinical Data in 2005, Mr. Rakin was the co-founder, President and Chief Executive Officer of Genaissance Pharmaceuticals, Inc., a pharmacogenomics company. He is currently on the boards of a number of private companies as well as Aziyo Biologics, Inc. (NASDAQ: AZYO), where he serves as the chairman of the board, Oramed Pharmaceuticals, Inc. (NASDAQ: ORMP) and Quantum-SI (NASDAQ: QSI). Mr. Rakin received an MBA from Columbia University and a B.Com. (Hons) from the University of Cape Town, South Africa.
Pierre Gianello, M.D. has served as a non-executive director since 2018, and as a medical advisor to the Company since 2010. Dr. Gianello is the general coordinator of Research of the Health Sciences Sector at the Université Catholique de Louvain, Brussels, or UCL, and councilor of the vice-rector in research and international relationships between UCL and others international universities for student exchange at the UCL. In 1997, Dr. Gianello became head of the Laboratory of Experimental Surgery and Transplantation at Université Catholique de Louvain and in 2005, he obtained the title of full Professor. From 2006 to 2009, he served as Dean of Research and from 2009 to 2011 as Vice-Rector. Professor Gianello has received ten scientific awards, including the Horlait-Dapsens Foundation (1986), Association “Professor Jean Morelle” Award (1989), “Claude Simon” Award (1989), Euroliver Foundation Prize (2001), Saint-Luc “Foundation” (2012). He is the author of more than 200 published manuscripts in peer reviewed scientific journals. Dr. Gianello was awarded a Doctor in Medicine, Surgery and Obstetrics at the Université Catholique de Louvain (Belgium) and completed his post-doc training at the Massachusetts General Hospital, Harvard Medical School in the Transplant Biology Research Centre managed by Prof. David Sachs.
Dr. Jürgen Hambrecht, Ph.D. served as a non-executive director from 2016 to 2017, and re-joined our Board of Directors in 2020. Dr. Hambrecht served BASF SE, a German company, in various responsibilities around the world for almost 45 years, lastly as CEO then Chairman of Supervisory Board until 2020. He has been member of the Supervisory Boards of Daimler AG, Daimler Truck AG, Fuchs Petrolub SE, Trumpf SE, Bilfinger SE and Lufthansa AG a.o. Dr. Hambrecht is member of the Board of AYA Gold&Silver (TSX: AYA.TO). He earned his doctorate in Chemistry from the University of Tuebingen, Germany.
Rita Johnson-Mills has served as a non-executive director since August 2021. Since January 2018, Ms. Johnson-Mills has been a founder and Chief Executive Officer of consulting firm RJM Enterprises and she brings a combined 30 years of direct health care experience from the federal, state and private industry, 15 years of which she was directly responsible for profitability and growth of healthcare organizations. She served as President and Chief Executive Officer of UnitedHealthcare Community Plan of Tennessee from August 2014 to December 2017, after having previously served as Senior Vice President, Performance Excellence and Accountability for UnitedHealthcare Community & State since 2006. Before that, she served as the Director of Medicaid Managed Care for the Centers for Medicare and Medicaid Services and as Chief Executive Officer of Managed Health Services Indiana and Buckeye Health Plan, wholly owned subsidiaries of Centene Corporation. She currently serves on the Board of Directors of Quest Analytics, LLC, Ellipsis Health Inc., and Ownes & Minor, Inc. and previously served on the Board of Directors of Brookdale Senior Living Inc. Ms. Johnson-Mills received dual Master’s degrees from Ohio State University, Master of Public Policy and Master of Labor/Human Resources. She is also a Hogan certified executive coach and a National Association of Corporate Directors Governance Fellow.
114
Virginia Kirby has served as a non-executive director since June 8, 2022. Ms. Kirby is currently a consultant with Virginia M. Kirby Consulting, a strategic consulting company that provides advisory services in regulatory strategy and operations, and has served in such role since April 2013. Additionally, Ms. Kirby is an Executive-in-Residence for the Officer of Technology Commercialization, Discovery Launch Pad at the University of Minnesota, and has served in such role since March 2020. Prior to serving in such roles, she served as the Senior Vice President of Clinical and Regulatory Affairs for Huinno, Inc. from March 2016 to October 2017, the Vice President of Clinical and Regulatory Affairs at Apnex Medical, Inc. from 2007 to 2013, and the Vice President of Clinical Affairs and Reimbursement at both EnteroMedics, Inc. from 2005 to 2006, and at ev3, Inc. from 2003 to 2005. She also held various roles of increasing seniority at Medtronic, Inc. (NYSE: MDT) from 1997 to 2003, and at 3M Company (NYSE: MMM) from 1983 to 1996. Ms. Kirby currently serves as a member of the Board of Directors of the Minneapolis Heart Institute Foundation, a non-profit cardiovascular research and education foundation, and has served in such role since April 2021. Ms. Kirby received a Bachelor of Science degree in Speech and Hearing Science from the University of Minnesota, a Master of Science degree in Psychoacoustics/Audiology from Purdue University and a Master of Science degree in Management of Technology from the University of Minnesota, Carlson School of Management/Institute of Technology.
Wildman Ventures LLC, as represented by Daniel Wildman, has served as a non-executive director since January 8, 2023. Mr. Wildman is currently the President and Chief Executive Officer of Wildman Ventures, LLC, a strategic consulting company that provides advisory services to several medical device and pharmaceutical companies, and has served in such role since January 2019. Additionally, Mr. Wildman is the Chairman of the Board of Progenerative Medical, Inc., where he has served in such role since March 2022, and also currently serves as a Strategic Advisor for PanTher Therapeutics, Inc., where he has served in such role since February 2022. Prior to serving in such roles, Mr. Wildman served in various roles at Johnson & Johnson (NYSE: JNJ), or J&J, from 2000 to January 2019, where he most recently led the Digital Surgery Strategy Initiative that developed an integrated strategy for robotic surgery. From 1990 to 2000, Mr. Wildman served in a variety of sales, marketing, operations and strategic planning roles at Boston Scientific Corporation (NYSE: BSX). Mr. Wildman has served as a member of the Board of Directors of Urogen Pharma, Ltd. (NASDAQ: URGN) since November 2022 and previously served as an Independent Director of Precision Healing, Inc. from June 2020 to April 2022. Mr. Wildman received a Bachelor of Arts degree in Economics from St. Lawrence University.
Family Relationships
There are no family relationships among any of our executive officers or directors.
B.Compensation
The following discussion provides the amount of compensation paid, and benefits in kind granted, by us and our subsidiaries to our directors and members of management for services in all capacities to us and our subsidiaries for the year ended December 31, 2023, as well as the amount contributed by us or our subsidiaries into money purchase plans for the year ended December 31, 2023 to provide pension, retirement or similar benefits to, our directors and members of the executive management board.
Directors’ and Executive Officers’ Compensation
Directors’ Compensation
Upon recommendation and proposal of the remuneration committee, our board of directors determines the remuneration of the directors to be proposed to the general shareholders’ meeting.
Pursuant to Belgian law, the general shareholders’ meeting approves the remuneration of the directors, including inter alia, each time as relevant:
(i) | in relation to the remuneration of executive and non-executive directors, the exemption from the rule that share based awards can only vest after a period of at least three years as of the grant of the awards (Article 7:91, first subsection of the Belgian CCA); |
115
(ii) | in relation to the remuneration of executive directors, the exemption from the rule that (unless the variable remuneration is less than a quarter of the annual remuneration) at least one quarter of the variable remuneration must be based on performance criteria that have been determined in advance and that can be measured objectively over a period of at least two years and that at least another quarter of the variable remuneration must be based on performance criteria that have been determined in advance and that can be measured objectively over a period of at least three years (Article 7:91, second to fourth subsection of the Belgian CCA); |
(iii) | in relation to the remuneration of non-executive directors, any variable part of the remuneration (independent directors can never receive a variable remuneration) (Article 7:92, fourth and fifth subsection of the Belgian CCA); and |
(iv) | any provisions of service agreements to be entered into with executive directors providing for severance payments exceeding twelve months’ remuneration and if the severance payments exceed eighteen months’ remuneration, only with the prior recommendation of the remuneration committee (Article 7:92, first subsection of the Belgian CCA). |
Notwithstanding points (i) and (ii) above, pursuant to our articles of association, our board of directors is explicitly authorized to deviate from the provisions of article 7:91 of the Belgian CCA.
The following annual remuneration and compensation of the directors applied for the year ended December 31, 2023:
| Annual | |
Fixed Fee | ||
(€) | ||
Chairman – Non-Executive Director |
| 82,000 |
Independent Director |
| 45,000 |
Non-Executive Director |
| 45,000 |
Additional fee for Audit Committee Member |
| 9,000 |
Additional fee for Remuneration Committee Member |
| 4,500 |
Additional fee for Science & Technology Committee Member |
| 4,500 |
Additional fee for Nominating and Corporate Governance Committee Member |
| 4,500 |
Additionally, for the year ended December 31, 2023, the chairman of the Audit Committee received runemeration and compensation at a rate equal to an annual fixed fee of €18,000, and the chairpersons of the Remuneration Committee, the Nomination and Corporate Governance Committee and the Science and Technology Committee received runemeration and compensation at a rate equal to an annual fixed fee of €9,000.
We also reimburse reasonable out-of-pocket expenses of directors (including travel and hotel expenses) incurred in performing the mandate of director.
Mr. Taelman, our chief executive officer and a member of our board of directors, does not receive any compensation for his service as a director. Additionally, there are no benefits upon the resignation of a director.
116
For the year ended December 31, 2023, the following remuneration or compensation (including reimbursement for out-of-pocket expenses) was due to the directors (excluding Olivier Taelman):
| Fees Earned | |
(€) | ||
Robert Taub |
| 127,633 |
Kevin Rakin |
| 68,593 |
Pierre Gianello, M.D. |
| 175,634 |
Jürgen Hambrecht, Ph.D. |
| 58,500 |
Rita Johnson-Mills |
| 63,621 |
Virginia Kirby |
| 59,218 |
Wildman Ventures LLC (represented by Daniel Wildman) (1) |
| 71,278 |
(1)Member of board of directors as of January 8, 2023.
In addition, in the year ended December 31, 2023, all non-executive directors were granted 25,000 warrants under our 2021 Warrants Plan (except that Wildman Ventures LLC received 11,389 warrants under our 2021 Warrants Plan and 13,602 warrants under our 2022 Warrants Plan).
The table below provides an overview as of December 31, 2023, of the warrants held by our non-executive directors.
Warrant Awards | ||||||
Number of | ||||||
Ordinary | ||||||
Shares | Warrant | |||||
Underlying | Exercise Price | Warrant | ||||
Warrants | (€) | Expiration Date | ||||
Robert Taub |
| 25,000 |
| 12.95 |
| June 8, 2027 |
Robert Taub | 25,000 | 7.19 | June 14, 2028 | |||
Kevin Rakin | 25,000 | 12.95 | June 8, 2027 | |||
Kevin Rakin |
| 25,000 |
| 7.19 | June 14, 2028 | |
Pierre Gianello, M.D. | 25,000 | 12.95 | June 8, 2027 | |||
Pierre Gianello, M.D. |
| 25,000 |
| 7.19 | June 14, 2028 | |
Jürgen Hambrecht, Ph.D. | 25,000 | 12.95 | June 8, 2027 | |||
Jürgen Hambrecht, Ph.D. |
| 25,000 |
| 7.19 | June 14, 2028 | |
Rita Johnson-Mills |
| 25,000 |
| 12.95 | June 8, 2027 | |
Rita Johnson-Mills | 25,000 | 7.19 | June 14, 2028 | |||
Virginia Kirby | 25,000 | 12.95 | June 8, 2027 | |||
Virginia Kirby | 25,000 | 7.19 | June 14, 2028 | |||
Wildman Ventures, LLC |
| 25,000 |
| 7.19 | June 14, 2028 | |
Executive Officers’ Compensation
The remuneration of the chief executive officer and the other members of our executive management is based on recommendations made by our remuneration committee. The chief executive officer participates in the meetings of the remuneration committee in an advisory capacity each time the remuneration of another member of the executive management is being discussed.
The remuneration is determined by our board of directors, in accordance with our remuneration policy.
As an exception to the foregoing rule, Belgian law provides that the general shareholders’ meeting must approve, as relevant:
(i) | in relation to the remuneration of members of the executive management and other executives, an exemption from the rule that share-based awards can only vest after a period of at least three years as of the grant of the awards (Article 7:121, last subsection jo. Article 7:91, first subsection of the Belgian CCA); |
117
(ii) | in relation to the remuneration of members of the executive management and other executives, an exemption from the rule that (unless the variable remuneration is less than a quarter of the annual remuneration) at least one quarter of the variable remuneration must be based on performance criteria that have been determined in advance and that can be measured objectively over a period of at least two years and that at least another quarter of the variable remuneration must be based on performance criteria that have been determined in advance and that can be measured objectively over a period of at least three years (Article 7:121, last subsection jo. Article 7:91, second to fourth subsection of the Belgian CCA); and |
(iii) | any service agreements to be entered into with members of the executive management and other executives (as the case may be) providing for severance payments exceeding twelve months’ remuneration (or, subject to an opinion by the remuneration committee, eighteen months’ remuneration) (Article 7:121, last subsection jo. Article 7:92, first subsection of the Belgian CCA). |
Notwithstanding points (i) and (ii) above, our board of directors has been explicitly authorized in the Articles of Association to deviate from the provisions of Article 7:91 CCA.
An appropriate proportion of the remuneration package should be structured so as to link rewards to corporate and individual performance, thereby aligning the interest of the executive management with the interests of our shareholders. Our board of directors will determine whether the targets for the variable remuneration of the members of the executive management, as set by our board of directors, are met.
The remuneration of the executive management currently consists of the following main remuneration components:
| ● | Base remuneration: annual base salary/fee (fixed); |
| ● | Fringe benefits: includes a company car, laptop, phone and representation allowance; |
| ● | Age and risk provisions: includes a pension plan with a fixed contribution and health insurance; |
| ● | Short-term incentives: includes a yearly performance bonus. If a target is reached, the member receives the full bonus, but if the target is not reached, they receive less or no payout; and |
| ● | Long-term incentives: includes participation in warrant incentive plans. |
The target proportion of fixed base salary, short-term and long-term incentives is: one third fixed base remuneration, one third short-term incentives (if all targets are reached) and one third long-term incentives. The short-term and long-term incentives are detailed in the table below:
Short-term incentive plan: yearly performance bonus
| |||
Main provisions | Short description | ||
Performance cycle | One calendar year | ||
Target bonus | NA | ||
Performance criteria and corresponding payout levels | One or more performance criteria (objectives) are determined. For each performance criterion, a target and corresponding payout level are determined: · If objective is 100% achieved: full payout of targeted payout level · If objective is achieved <75%: in principle no payout (but Board can decides otherwise) · If objective is achieved >75% and <125%: payout between 75% and 125%, based on linear calculation · If objective is achieved >125%: board can decide payout >125 | % | |
Calculation of bonus | The total bonus is composed of the sum of the payout levels related to the various performance criteria (if more than one) | ||
Payment modalities | Payment in cash or equivalent 100% of the bonus is paid at once |
118
Long-term incentive plan: share option plans
Main provisions |
| Short description | |
Frequency of offer | No pre-set frequency | ||
Performance cycle | NA | ||
Target number of offered share options | NA | ||
Exercise price | Value of underlying shares at date of offer of share options | ||
Exercise period | Five years from date of offer of share options | ||
Performance criteria and corresponding offering levels | NA | ||
Calculation of number of offered share options | NA | ||
Vesting | Options issued prior to 2021: vesting in three tranches: -1/3 of offered share options vests upon offer -1/3 of offered share options vests on first anniversary of offer -1/3 of offered share options vests on second anniversary of offer Options issued pursuant to 2021 Warrants Plan: vesting in four tranches: -1/4 of offered share options vests upon offer -1/4 of offered share options vests on first anniversary of offer -1/4 of offered share options vests on second anniversary of offer -1/4 of offered share options vests on third anniversary of offer | ||
Retention | NA |
The following table sets forth information regarding compensation paid by us to Olivier Taelman, our chief executive officer, for the year ended December 31, 2023:
Compensation | ||
| (€) | |
Base salary | 436,351 | |
Performance bonus |
| 301,500 |
Pension contributions |
| 33,188 |
Fringe benefits(1) |
| 36,057 |
(1) | Fringe benefits consist of company car (€4,599), laptop and mobile phone (€156), representation allowance (€4,200), health insurance (€5,824), life insurance (€19,860) and meal vouchers (€1,418). |
In addition, in 2023, Mr. Taelman was granted 25,000 warrants under our 2021 Warrants Plan. There are no benefits upon termination of employment.
The following table sets forth information regarding compensation paid by us to Loïc Moreau, our chief financial officer, for the year ended December 31, 2023:
Compensation | ||
| (€) | |
Base salary |
| 258,877 |
Performance bonus |
| 183,807 |
Pension contributions |
| 15,750 |
Fringe benefits(1) |
| 10,253 |
(1) | Fringe benefits consist of company car (€2,939), laptop and mobile phone (€156), representation allowance (€3,000), health insurance (€2,490), sectoral premium and eco-vouchers (€250) and meal vouchers (€1,418). |
In addition, in 2023, Mr. Moreau was granted 15,284 warrants under our 2021 Warrants Plan. There are no benefits upon termination of employment.
119
The table below provides an overview as of December 31, 2023 of the warrants held by the members of executive management.
Warrant Awards | ||||||
Number of | ||||||
Ordinary | ||||||
Shares | Warrant | |||||
Underlying | Exercise Price | Warrant | ||||
Warrants | (€) | Expiration Date | ||||
Olivier Taelman |
| 25,000 (2021 plan) |
| 5.42 |
| March 24, 2028 |
| 24,930 (2021 plan) |
| 5.42 | March 24, 2028 | ||
| 8,310 (2021 plan) |
| 25.31 | September 17, 2026 | ||
320,000 (2020 plan) | 11.94 | April 7, 2025 | ||||
16,500 (2018 plan) | 11.93 | April 7, 2025 | ||||
| 33,500 (2018 plan) |
| 6.52 | July 29, 2024 | ||
Loïc Moreau |
| 15,284 (2021 plan) |
| 5.42 | March 24, 2023 | |
45,000 (2021 plan) | 5.42 | March 24, 2028 | ||||
| 15,000 (2021 plan) |
| 17.76 | February 21, 2027 | ||
Insurance and Indemnification
Under Belgian law, the directors of a company may be liable for damages to our company in case of improper performance of their duties. Our directors may be liable to our company and to third parties for infringement of our articles of association or Belgian company law. Under certain circumstances, directors may be criminally liable. We maintain liability insurance for the benefit of our directors and members of our executive management team.
We maintain liability insurance for our directors and officers, including insurance against liability under the Securities Act of 1933, as amended, and we intend to enter into agreements with our directors and executive officers to provide contractual indemnification. With certain exceptions and subject to limitations on indemnification under Belgian law, these agreements will provide for indemnification for damages and expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding arising out of his or her actions in that capacity.
These agreements may discourage shareholders from bringing a lawsuit against our directors and executive officers for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and executive officers, even though such an action, if successful, might otherwise benefit us and our shareholders. Furthermore, a shareholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these insurance agreements.
Certain of our non-employee directors may, through their relationships with their employers or partnerships, be insured and/or indemnified against certain liabilities in their capacity as members of our board of directors.
Warrant Plans
We have established a number of warrant plans, under which we have granted warrants to our employees, officers, directors, consultants and advisors.
The warrants issued on December 12, 2018 gives the holder thereof right to acquire 500 ordinary shares, which accounts for the 500:1 share split which occurred on February 21, 2020. As of December 31, 2023, there were still 100 of such warrants outstanding which entitle the holders thereof to an aggregate of 50,000 of our ordinary shares. Each of the warrants issued on February 21, 2020, September 8, 2021 and December 28, 2022 gives the holder thereof to subscribe to one of our ordinary shares. As of December 31, 2023, there were still 2,229,750 of such warrants outstanding which entitle the holders thereof to an aggregate of 2,229,750 of our ordinary shares.
120
The duration of all our outstanding warrants is the shorter of ten years from the date of issuance or five years from the date of grant. With respect to warrants issued on December 12, 2018 and February 21, 2020, one-third of such warrants granted to and accepted by a beneficiary vested upon the date of the grant, after which one third of the warrants granted to and accepted by a beneficiary vested on each of the first and second anniversary of the grant date. With respect to warrants issued on December 12, 2018 and February 21, 2020, such warrants vested and became exercisable ten business days prior to the closing of our initial public offering on Euronext Brussels in September 2020. With respect to warrants issued on September 8, 2021 and December 28, 2022, in principle one-fourth of such warrants granted to and accepted by a beneficiary vest upon the date of the grant, after which one fourth of the warrants granted to and accepted by a beneficiary vest on each of the first, second and third anniversary of the grant date. With respect to the warrants granted to directors on June 8, 2022 and on June 14, 2023, such warrants vest in full and become exercisable on the first anniversary of the grant date.
The table below sets forth the details of all warrants granted under the warrant plans in force as of December 31, 2023, including the plan under which the warrants were granted, the offer date, exercise price, expiry date, number of warrants exercised, number of warrants voided and number of warrants outstanding. Aside from the warrants set forth in the below table, there are currently no other stock options, options to purchase securities, or other rights to subscribe for or purchase outstanding securities.
Aggregate | ||||||||||||||||
Number of | number | |||||||||||||||
Warrants | and type of | |||||||||||||||
lapsed, | Number | Shares | ||||||||||||||
exercised | and type of | issuable | ||||||||||||||
or no | Exercise | Shares | upon | |||||||||||||
Number of | longer | Number of | Price | issuable | exercise of | |||||||||||
Warrants | available | Warrants | Issue | Expiration | Warrant | per ESOP | outstanding | |||||||||
Name of Warrants Plan |
| Issued |
| for grant |
| outstanding |
| date |
| date |
| (€) |
| Warrant |
| Warrants |
2018 Warrants Plan |
| 525 |
| 425 |
| 100 |
| 12/12/2018 |
| 12/12/2028 |
| 3,259.91 (1) |
| 500 Ordinary Shares |
| 50,000 Ordinary Shares |
| 5,966.59 (2) | |||||||||||||||
2020 Warrants Plan |
| 550,000 |
| 139,500 |
| 410,500 |
| 02/21/2020 |
| 02/21/2030 |
| 11.94 |
| 1 Ordinary Share |
| 410,500 Ordinary Shares |
2021 Warrants Plan |
| 1,400,000 |
| 280,750 |
| 1,119,250 |
| 09/08/2021 |
| 09/08/2031 |
| 25.31 (3) |
| 1 Ordinary Share |
| 1,119,250 Ordinary Shares |
| 17.76 (4) | |||||||||||||||
| 13.82 (5) | |||||||||||||||
| 12.95 (6) | |||||||||||||||
9.66(7) | ||||||||||||||||
5.42(8) | ||||||||||||||||
6.36(9) | ||||||||||||||||
| 7.19(10) | |||||||||||||||
2022 Warrants Plan |
| 700,000 |
| — |
| 700,000 |
| 12/28/2022 |
| 12/28/2032 |
| 7.19(11) |
| 1 Ordinary Share |
| 700,000 Ordinary Shares |
5.92(12) | ||||||||||||||||
| Total |
| 2,279,250 Ordinary Shares |
(1) | For 67 2018 Warrants granted in July 2019. This results in a subscription price of €6.52 (rounded) per new share. |
(2) | For 33 2018 Warrants granted in April 2020. This results in a subscription price of €11.93 (rounded) per new share. |
(3) | For 25,185 2021 Warrants granted and accepted in 2021 and 2022. |
(4) | For 97,375 2021 Warrants granted and accepted in 2022. |
(5) | For 58,875 2021 Warrants granted and accepted in 2022. |
(6) | For 150,000 2021 Warrants granted and accepted in 2022. |
(7) | For 6,250 2021 Warrants granted and accepted in 2022. |
(8) | For 595,167 2021 Warrants granted and accepted in 2021, 2022 and 2023. |
(9) | For 25,000 2021 Warrants granted and accepted in 2023. |
(10) | For 161,398 2021 Warrants granted and accepted in 2023. |
(11) | For 13,602 2022 Warrants granted and accepted in 2023. |
(12) | For 42, 254 2022 Warrants granted and accepted in 2023. |
121
C.Board Practices
Board Composition and Director Independence
As a foreign private issuer, under the listing requirements and rules of Nasdaq, we are not required to have a board of directors comprised of a majority of independent directors, except that our audit committee is required to consist fully of independent directors, subject to certain phase-in schedules. However, our board of directors has determined that, under current listing requirements and rules of Nasdaq and taking into account any applicable committee independence standards, Jürgen Hambrecht, Kevin Rakin, Rita Johnson-Mills, Virginia Kirby and Wildman Ventures LLC, as represented by Daniel Wildman, are “independent directors.” In making such determination, our board of directors considered the relationships that each non-executive director has with us and all other facts and circumstances our board of directors deemed relevant in determining each director’s independence, including the number of ordinary shares beneficially owned by the director and his or her affiliated entities (if any).
Under Belgian law, a director will only qualify as an independent director if he or she meets at least the criteria set out in provision 3.5 of the Belgian Code on Corporate Governance, which can be summarized as follows:
| ● | Not be an executive, or exercising a function as a person entrusted with the daily management of the company or a related company or person, and not have been in such a position for the previous three years before their appointment. Alternatively, no longer enjoying stock options of the company related to this position. |
| ● | Not have served for a total term of more than twelve years as a non-executive board member. |
| ● | Not be an employee of the senior management (as defined in article 19,2° of the law of 20 September 1948 regarding the organization of the business industry) of the company or a related company or person, and not have been in such a position for the previous three years before their appointment. Alternatively, no longer enjoying stock options of the company related to this position. |
| ● | Not be receiving, or having received during their mandate or for a period of three years prior to their appointment, any significant remuneration or any other significant advantage of a patrimonial nature from the company or a related company or person, apart from any fee they receive or have received as a non-executive board member. |
| ● | Not hold shares, either directly or indirectly, either alone or in concert, representing globally one tenth or more of the company’s capital or one tenth or more of the voting rights in the company at the moment of appointment. |
| ● | Not having been nominated, in any circumstances, by a shareholder fulfilling the conditions covered under (e). |
| ● | Not maintain, nor have maintained in the past year before their appointment, a significant business relationship with the company or a related company or person, either directly or as partner, shareholder, board member, member of the senior management (as defined in article 19, 2° of the law of 20 September 1948 regarding the organization of the business industry) of a company or person who maintains such a relationship. |
| ● | Not be or have been within the last three years before their appointment, a partner or member of the audit team of the company or person who is, or has been within the last three years before their appointment, the external auditor of the company or a related company or person. |
| ● | Not be an executive of another company in which an executive of the company is a non- executive board member, and not have other significant links with executive board members of the company through involvement in other companies or bodies. |
| ● | Not have, in the company or a related company or person, a spouse, legal partner or close family member to the second degree, exercising a function as board member or executive or person entrusted with the daily management or employee of the senior management (as defined in article 19, 2° of the law of 20 September 1948 regarding the organization of the business industry), or falling in one of the other cases referred to in a) to i) above, and as far as point b) is concerned, up to three years after the date on which the relevant relative has terminated their last term. |
Role of the Board in Risk Oversight
Our board of directors is responsible for the oversight of our risk management activities and has delegated to the audit committee the responsibility to assist our board in this task. While our board of directors oversees our risk management, our management is responsible for day-to-day risk management processes. Our board of directors expects our management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the board of directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face.
122
Powers, Responsibilities and Functioning of the Board of Directors
We have a “one tier” governance structure whereby our board of directors is the ultimate decision making body, with the overall responsibility for the management and control of our company, and is authorized to carry out all actions that are considered necessary or useful to achieve our company’s purpose. Our board of directors has all powers except for those reserved to the general shareholders’ meeting by law or our articles of association. Our board of directors acts as a collegiate body.
Our board of directors has the power to appoint and remove the chief executive officer. The role of the chief executive officer is to implement the mission, strategy and targets set by the board of directors and to assume responsibility our the day-to-day management. The chief executive officer reports directly to the board of directors.
Pursuant to the Belgian CCA, and our articles of association, the board of directors must consist of at least three directors. Our corporate governance charter provides that the composition of the board of directors should ensure that decisions are made in the corporate interest. It should be determined on the basis of diversity, as well as complementary skills, experience and knowledge. Pursuant to the Belgian Code on Corporate Governance, a majority of the directors must be non-executive and at least three directors must be independent in accordance with the criteria set out in the Belgian Code on Corporate Governance. By January 1, 2026, at least one third of the members of the board of directors must be of the opposite gender.
Our directors are elected by our general shareholders’ meeting. The term of the directors’ mandates cannot exceed four years. Resigning directors can be re-elected for a new term. Proposals by the board of directors for the appointment or re-election of any director must be based on a recommendation by the nomination committee. In the event the office of a director becomes vacant, the remaining directors can appoint a successor temporarily filling the vacancy until the next general shareholders’ meeting.
The general shareholders’ meeting can dismiss the directors at any time.
The board of directors elects a chairperson from among its members on the basis of his or her knowledge, skills, experience and mediation strength. The chairperson is responsible for the leadership and the proper and efficient functioning of the board of directors. As of the date of this Annual Report, Mr. Taub is chairperson of the board of directors and Mr. Taelman is the chief executive officer. If the board of directors envisages appointing a former chief executive officer as chairperson, it will carefully consider the positive and negative aspects of such a decision and disclose why such appointment is in our best interest.
The board of directors meets as frequently as our interests require, or at the request of one or more directors. In principle, the board of directors will meet sufficiently regularly. The decisions of the board of directors are made by a simple majority of the votes cast. In case votes are tied, the chairperson of the board of directors will have a casting vote.
There are no arrangements or understanding between us and any of the members of our board of directors providing for benefits upon termination of their service.
Committees of our Board of Directors
Our board of directors is assisted by a number of committees in relation to specific matters. The committees advise the board of directors on these matters, but the decision making remains with the board of directors as a whole.
Our board of directors has established four board committees, which are responsible for assisting the board of directors and making recommendations in specific fields: (a) the audit committee (in accordance with article 7:99 of the Belgian CCA and provisions 4.10 and following of the Belgian Code on Corporate Governance), (b) the remuneration committee (in accordance with article 7:100 of the Belgian CCA and provisions 4.17 and following of the Belgian Code on Corporate Governance), (c) the nomination committee (in accordance with provisions 4.19 and following of the Belgian Code on Corporate Governance) and (d) the science & technology committee. The terms of reference of these board committees are primarily set out in the Corporate Governance Charter.
Audit Committee
As of the date of this Annual Report, our audit committee consists of three directors: Kevin Rakin (Chairman), Jürgen Hambrecht and Wildman Ventures, LLC, as represented by Daniel Wildman.
123
According to the Belgian CCA, all members of the audit committee must be non-executive directors, and at least one member must be independent within the meaning of provision 3.5 of the Belgian Code on Corporate Governance. Our board of directors has determined that all three members of our audit committee are independent under Rule 10A-3 of the Exchange Act and the applicable listing standards of Nasdaq and all three members of our audit committee are independent under the applicable rules of the Belgian Code on Corporate Governance.
The members of the audit committee must have a collective competence in our business activities, as well as in accounting, auditing and finance, and at least one member of the audit committee must have the necessary competence in accounting and auditing, including qualifying as an “audit committee financial expert” as defined under the Exchange Act. Our board of directors has determined that (i) Kevin Rakin, Jürgen Hambrecht and Wildman Ventures, LLC, as represented by Daniel Wildman are independent under Rule 10A-3 of the Exchange Act and the applicable rules of NASDAQ, (ii) the members of the audit committee satisfy the competency requirement, and (iii) Kevin Rakin qualifies as an “audit committee financial expert” as defined under the Exchange Act.
The audit committee will be governed by a charter that complies with Nasdaq listing rules and the Belgian Code on Corporate Governance. The role of the audit committee is to:
| ● | inform our board of directors of the result of the audit of the financial statements and the manner in which the audit has contributed to the integrity of the financial reporting and the role that the audit committee has played in that process; |
| ● | monitor the financial reporting process, and to make recommendations or proposals to ensure the integrity of the process, |
| ● | monitor the effectiveness of our internal control and risk management systems, and our internal audit process and its effectiveness; |
| ● | monitor the audit of the financial statements, including the follow-up questions and recommendations by the statutory auditor; |
| ● | assess and monitor the independence of the statutory auditor, in particular with respect to the appropriateness of the provision of additional services. More specifically, the audit committee analyses, together with the statutory auditor, the threats for the statutory auditor’s independence and the security measures taken to limit these threats, when the total amount of fees exceeds the criteria specified in article 4 §3 of Regulation (EU) No 537/2014; and |
| ● | make recommendations to our board of directors on the selection, appointment and remuneration of our statutory auditor in accordance with article 16 §2 of Regulation (EU) No 537/2014. |
The audit committee has at least four regularly scheduled meetings each year. The audit committee regularly reports to our board of directors on the exercise of its missions, and at least when the board of directors approves the financial statements and the condensed or short form financial information that will be published. The members of the audit committee have full access to the executive management and to any other employee to whom they may require access in order to carry out their responsibilities.
Without prejudice to the statutory provisions which determine that the statutory auditor must address reports or warnings to our corporate bodies, the statutory auditor must discuss, at the request of the statutory auditor, or at the request of the audit committee or of our board of directors, with the audit committee or with the board of directors, essential issues which are brought to light in the exercise of the statutory audit of the financial statements, which are included in the additional statement to the audit committee, as well as any meaningful shortcomings discovered in our internal financial control system.
Remuneration Committee
As of the date of this Annual Report, our remuneration committee consists of three directors: Robert Taub (Chairman), Rita Johnson-Mills and Wildman Ventures, LLC, as represented by Daniel Wildman.
In line with the Belgian CCA and the Belgian Code on Corporate Governance (i) all members of the remuneration committee are non-executive directors, (ii) the remuneration committee consists of a majority of independent directors and (iii) the remuneration committee is chaired by the chairperson of our board of directors or another non-executive director appointed by the committee. Our board of directors has determined that two members of our remuneration committee are independent under the applicable listing standards of Nasdaq and two members of our remuneration committee are independent under the applicable rules of the Belgian Code on Corporate Governance.
Pursuant to the Belgian CCA, the remuneration committee must have the necessary expertise in terms of remuneration policy. Our board of directors has determined that the members of the remuneration committee satisfy this requirement.
124
The role of the remuneration committee is to make recommendations to the board of directors with regard to the remuneration of directors and members of the executive management and, in particular, to:
| ● | make proposals to the board of directors on the remuneration policy of directors, the persons in charge of the management, and the persons in charge of the daily management, as well as, where applicable, the resulting proposals that the board of directors must submit to the general shareholders’ meeting; |
| ● | make proposals to the board of directors on the individual remuneration of the directors, the other persons in charge of the management, and the persons in charge of day-to-day management, including variable remuneration and long-term performance premiums, whether or not tied to shares, in the form of stock options or other financial instruments, and of severance payments, and where applicable, the resulting proposals that the board of directors must submit to the general shareholders’ meeting; |
| ● | prepare the remuneration report; and |
| ● | explain the remuneration report at the annual general shareholders’ meeting. |
Pursuant to the Belgian CCA, the chief executive officer participates in the meetings of the remuneration committee in an advisory capacity each time the remuneration of another member of the executive management is being discussed.
Nomination Committee
As of the date of this Annual Report, our nomination committee consists of three directors: Rita Johnson-Mills (Chairwoman), Robert Taub and Jürgen Hambrecht.
In line with the Belgian Code on Corporate Governance (i) the nomination committee consists of a majority of independent directors and (iii) the nomination committee is chaired by the chairperson of our board of directors or another non-executive director appointed by the committee. Our board of directors has determined that two members of our nomination committee are independent under the applicable standards of Nasdaq and two members of our nomination committee are independent under the applicable rules of the Belgian Code on Corporate Governance.
The role of the nomination committee is to:
| ● | make recommendations to our board of directors with regard to the appointment of directors and members of the executive management; |
| ● | make recommendations to our board of directors in relation to the assignment of responsibilities to the executives; |
| ● | prepare plans for the orderly succession of board members; |
| ● | lead the reappointment process of board members; |
| ● | ensure that sufficient and regular attention is paid to the succession of executives; and |
| ● | ensure that appropriate talent development programs and programs to promote diversity in leadership are in place. |
Science & Technology Committee
As of the date of this Annual Report, our science & technology committee consists of four directors: Pierre Gianello (Chairman), Robert Taub and Virginia Kirby.
The role of science & technology committee is to assist our board of directors in all matters relating to:
| ● | strategic direction of our technology, research and product development programs; |
| ● | monitoring and evaluating existing and future trends in technology that may affect the our strategic plans, including monitoring of overall industry trends; |
| ● | the innovation and technology acquisition process to assure ongoing business growth; |
| ● | IT risk management and cyber security strategy; and |
| ● | measurement and tracking systems in place to monitor the performance of our technology in support of overall business strategy and to achieve successful innovation. |
125
Board Diversity
The table below provides certain information regarding the diversity of our board of directors as of the date of this Annual Report.
Board Diversity Matrix (as of March 20, 2024) | ||||
Country of Principal Executive Offices: | Belgium | |||
Foreign Private Issuer | Yes | |||
Disclosure Prohibited under Home Country Law | No | |||
Total Number of Directors | ||||
Female | Male | Non- Binary | Did Not | |
Part I: Gender Identity | ||||
Directors | 2 | 6 | ||
Part II: Demographic Background | ||||
Underrepresented Individual in Home Country Jurisdiction | 1 | |||
LGBTQ+ | 0 | |||
Did Not Disclose Demographic Background | 1 | |||
Corporate Governance Code
We adopted a corporate governance charter that is in line with the Belgian Code on Corporate Governance. The corporate governance charter describes the main aspects of the corporate governance of our company, including our governance structure, the terms of reference of our board of directors and its committees and other important topics. The corporate governance charter must be read together with our articles of association.
The Belgian Code on Corporate Governance is based on a “comply or explain” system: Belgian listed companies are expected to follow the Belgian Code on Corporate Governance, but can deviate from specific provisions and guidelines (though not the principles) provided they disclose the justification for such deviations. We apply the ten corporate governance principles contained in the Belgian Code on Corporate Governance and comply with the corporate governance provisions set forth in the Belgian Code on Corporate Governance, except in relation to the following:
| ● | In deviation of provision 4.14 of the Belgian Code on Corporate Governance, no independent internal audit function has been established. This deviation is explained by our size. Our audit committee will regularly assess the need for the creation of an independent internal audit function and, where appropriate, will call upon external persons to conduct specific internal audit assignments and will inform the board of directors of their outcome. |
| ● | We do not exclude awarding share-based incentives to the non-executive directors. This is contrary to provision 7.6 of the Belgian Code on Corporate Governance that provides that no stock options should be granted to non-executive board members. We believe that this provision of the Belgian Code on Corporate Governance is not appropriate and adapted to take into account the realities of companies in the biotech and life sciences industry that are still in a development phase. Notably, the ability to remunerate non-executive directors with share options allows us to limit the portion of remuneration in cash that we would otherwise need to pay to attract or retain renowned experts with the most relevant skills, knowledge and expertise. We are of the opinion that granting non-executive directors the opportunity to be remunerated in part in share-based incentives rather than all in cash strengthens the alignment of their interests with the interests of our shareholders. This is in our interest and the interest of our stakeholders. Furthermore, this is customary for directors active in companies in the life sciences industry. |
| ● | In deviation of provision 7.6 of the Belgian Code on Corporate Governance, the non-executive members of our board of directors do not systematically receive part of their remuneration in the form of shares. This deviation is explained by the fact that the interests of the non-executive members of our board of directors are considered to be sufficiently oriented to the creation of long-term value for our company, taking into account that some of the non-executive members of our board of directors will from time to time hold shares or warrants under our outstanding stock-based incentive plans, the value of which is based on the value of the shares. Therefore, a regular payment in shares is not deemed necessary. |
126
| ● | Pursuant to article 7:91 of the Belgian CCA and provisions 7.6 and 7.11 of the Belgian Code on Corporate Governance, shares should not vest and share options should not be exercisable within three years as of their granting. Our board of directors has been explicitly authorized in our articles of association to deviate from this rule in connection with stock-based incentive plans, compensations, awards and issuances to our employees, directors and service providers and/or our subsidiaries (from time to time). We are of the opinion that this allows for more flexibility when structuring share-based awards. |
| ● | In deviation of provision 7.9 of the Belgian Code on Corporate Governance, no minimum threshold of shares to be held by members of our executive management team is set. This deviation is explained by the fact that the interests of the members of the executive management team are considered to be sufficiently oriented to the creation of long-term value for our company, taking into account that some of them will from time to time hold shares or warrants under our outstanding stock-based incentive plans, the value of which is based on the value of the shares. Therefore, setting a minimum threshold of shares to be held by them is not deemed necessary. |
| ● | In deviation of provision 7.12 of the 2020 Code, the board of directors does not include, in the contracts with the CEO and other members of executive management, provisions that would enable the Company to recover variable remuneration paid, or withhold the payment of variable remuneration, and specify the circumstances in which it would be appropriate to do so, insofar as enforceable by law. The Company believes that this provision of the 2020 Code is not appropriate and adapted to take into account the realities of companies in the life sciences industry that are still in a development phase nor considers that it is necessary to apply claw-back provisions as (i) the pay-out of the short-term variable remuneration, based on the achievement of one or more individual objectives and one or more Company objectives as set by the board of directors, is paid only upon achievement of those objectives, and (ii) the Company does not apply any other performance-based remuneration or variable compensation. Furthermore, the ESOP warrant plans set up by the Company contain bad leaver provisions that can result in the unexercised share options, whether vested or not, automatically and immediately becoming null and void if the agreement or other relationship between the holder and the (relevant subsidiary of the) Company is terminated for “cause”. Notwithstanding the Company’s position that warrants are not to be qualified as variable remuneration (when not depending on performance criteria), the board of directors is of the opinion that such bad leaver provisions sufficiently protect the Company’s interests and that it is therefore currently not necessary to provide for additional contractual provisions that give the Company a contractual right to reclaim any (variable) remuneration from the members of the executive management. For those reasons, there are no contractual provisions in place between the Company and the members of the executive management that give the Company a contractual right to reclaim from said executives any variable remuneration that would be awarded. |
What constitutes good corporate governance will evolve with the changing circumstances of a company and with the standards of corporate governance globally, and must be tailored to meet those changing circumstances. Our board of directors intends to update the corporate governance charter as often as required to reflect changes to our corporate governance.
Our articles of association and the corporate governance charter are available on our website (www.nyxoah.com) and can be obtained free of charge at our registered office. Information contained on our website does not constitute part of this Annual Report.
D.Employees
The number of employees by function and geographic location as of the end of the period for our fiscal years ended December 31, 2023, 2022 and 2021 was as follows:
As at December 31, | ||||||
2023 | 2022 | 2021 | ||||
By Function: |
|
|
|
|
|
|
Sales, General & Administration |
| 40.4 |
| 34.9 |
| 27.6 |
Research & Development |
| 106.4 |
| 102.6 |
| 78.2 |
Total |
| 146.8 |
| 137.5 |
| 105.8 |
By Geography: |
|
|
|
|
| |
Europe (Belgium & Germany) |
| 61.4 |
| 55.9 |
| 37.8 |
Israel |
| 46.4 |
| 44.6 |
| 46 |
Australia |
| 4 |
| 6 |
| 7 |
United States |
| 35 |
| 31 |
| 15 |
Total |
| 146.8 |
| 137.5 |
| 105.8 |
127
None of our employees are represented by labor unions or covered by company specific bargaining agreements.
We believe that one of our key strengths is our employee base, which has extensive know-how across research, manufacturing, quality-control, engineering software programming and marketing and sales. We also believe that developing a diverse, equitable and inclusive culture is critical to continuing to attract and retain the top talent necessary for our long-term success and strategy. We value diversity at all levels and continue to focus on extending our diversity and inclusion initiatives across our entire workforce, including the expansion of individuals with diverse backgrounds in leadership.
Our principles of accountability, honesty, integrity and customer-focused, serve as our cultural pillars. We focus our efforts on creating a collaborative environment where our colleagues feel respected and valued. We provide our employees with competitive compensation, opportunities for equity ownership and a robust employment package, including health care, disability and long-term planning insurance, retirement planning and paid time off. In addition, we regularly interact with our employees to gauge employee satisfaction and identify areas of focus.
E.Share Ownership
For information regarding the share ownership of our directors and executive officers, see “Item 6.B—Compensation” and “Item 7.A—Major Shareholders.”
F.Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
Not applicable.
Item 7.Major Shareholders and Related Party Transactions
A.Major Shareholders
The following table and related footnotes set forth information with respect to the beneficial ownership of our ordinary shares as of March 18, 2024 by:
· | each of our directors and executive officers; and |
· | each person beneficially owning more than 3% of our share capital; and |
· | all of our directors and executive officers as a group. |
To our knowledge and assuming that all of our ordinary shares listed on the Nasdaq Global Market are held by residents of the United States, as of March 18, 2024, we estimate that approximately 19.86% of our outstanding ordinary shares are held of record by seven residents of the United States. The actual number of holders is greater than these numbers of record holders and includes beneficial owners whose ordinary shares are held in street name by brokers and other nominees. This number of holders of record also does not include holders whose shares may be held in trust by other entities.
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including ordinary shares that can be acquired within 60 days of March 18, 2024. Ordinary shares subject to derivative securities currently exercisable or exercisable within 60 days of March 18, 2024 are deemed to be outstanding for computing the percentage ownership of the person holding these securities and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person.
The percentage ownership information shown in the table is based on 28,682,635 ordinary shares outstanding as of March 18, 2024.
Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all ordinary shares shown that they beneficially own, subject to community property laws where applicable. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Sections 13(d) and 13(g) of the Securities Act.
128
Except as otherwise indicated in the table below, addresses of the directors, members of the executive management team and named beneficial owners are in care of Nyxoah SA, Rue Edouard Belin 12, 1435 Mont-Saint-Guibert, Belgium.
Ordinary Shares |
| ||||
Beneficially |
| ||||
Owned |
| ||||
Name of beneficial owner | Number | Percent |
| ||
3% or Greater Shareholders: |
|
|
|
| |
Cochlear Investments Pty Ltd(1) |
| 5,090,779 |
| 17.75 | % |
Robert Taub(2) |
| 3,415,514 |
| 11.90 | % |
Entities affiliated with Gilde Healthcare(3) |
| 3,153,822 |
| 11.00 | % |
TOGETHER Partnership(4) |
| 2,948,285 |
| 10.28 | % |
Resmed Inc.(5) |
| 1,619,756 |
| 5.65 | % |
Jürgen Hambrecht(6) |
| 1,369,000 |
| 4.77 | % |
Executive Officers and Directors: |
|
|
|
| |
Robert Taub(2) |
| 3,415,514 |
| 11.90 | % |
Kevin Rakin(7) |
| 148,500 |
| * | |
Virginia Kirby(8) |
| 30,560 |
| * | |
Pierre Gianello(9) |
| 36,560 |
| * | |
Wildman Ventures LLC (as represented by Daniel Wildman) |
| — |
| * | |
Jürgen Hambrecht(6) |
| 1,369,000 |
| 4.77 | % |
Rita Johnson-Mills(10) |
| 30,560 |
| — | |
Olivier Taelman(11) |
| 419,930 |
| 1.44 | % |
Loïc Moreau (12) |
| 63,369 |
| * | |
All current directors and executive management as a group (9 persons)(13) |
| 5,513,993 |
| 19.19 | % |
* Represents beneficial ownership of less than one percent.
(1) | Consists of 5,090,779 ordinary shares held by Cochlear Investments Pty Ltd. The principal business address of Cochlear Investments Pty Ltd. is 1 University Avenue, Macquarie University, NSW 2109 (Australia). 100% of the share capital of Cochlear Investments Pty Ltd is owned by Cochlear Limited, a company which is listed on the Australian Securities Exchange and is not a controlled company. |
(2) | Consists of (i) 2,127,030 ordinary shares held by Robert Taub, a member of our board of directors, (ii) 696,000 ordinary shares held by Robelga SRL, a company controlled by Mr. Taub, (iii) 567,484 ordinary shares held by BMI Estate, a company controlled by Mr. Taub, and (iv) 25,000 ordinary shares issuable upon the exercise of warrants held by Mr. Taub that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(3) | Consists of (i) 1,576,911 ordinary shares held by Coöperatieve Gilde Healthcare III Sub-Holding U.A., or Gilde Sub-Holding, and (ii) 1,576,911 ordinary shares held by Coöperatieve Gilde Healthcare III Sub-Holding 2 U.A., or Gilde Sub-Holding 2. The principal business address of each of Gilde Sub-Holding and Gilde Sub-Holding 2 is Newtonlaan 91, 3584 BP Utrecht, The Netherlands. Gilde Healthcare III Management BV is the management company of Gilde Sub-Holding and Gilde Sub-Holding 2. Gilde Healthcare III Management BV exercises the voting rights attached to our ordinary shares at its discretion. Gilde Healthcare III Management BV is controlled by Gilde Healthcare Holding BV. Gilde Healthcare Holding BV is not a controlled entity. |
(4) | Consists of 2,948,285 ordinary shares held by TOGETHER Partnership. The principal business address of TOGETHER Partnership is Van Putlei 31, 2018 Antwerp, Belgium. TOGETHER Partnership is not a controlled entity. |
(5) | Consists of 1,619,756 ordinary shares held by Resmed Inc. The principal business address of Resmed Inc. is 9001 Spectrum Center Boulevard., San Diego, CA 92123. Resmed Inc. is a public company that is listed on the New York Stock Exchange and is not a controlled company. |
129
(6) | Consists of (i) 1,052,589 ordinary shares held by Dr. Hambrecht, a member of our board of directors, (ii) 291,411 ordinary shares held by JH Capital GmbH, a company controlled by Dr. Hambrecht, and (iii) 25,000 ordinary shares issuable upon the exercise of warrants held by Dr. Hambrecht that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(7) | Consists of (i) 78,030 ordinary shares held by Mr. Rakin, (ii) 45,470 ordinary shares held by Kevin L. Rakin Irrevocable Trust, and (iii) 25,000 ordinary shares issuable upon the exercise of warrants held by Mr. Rakin that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(8) | Consists of (i) 5,560 ordinary shares held by Ms. Kirby, and (ii) 25,000 ordinary shares issuable upon the exercise of warrants held by Ms. Kirby that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(9) | Consists of (i) 11,560 ordinary shares held by Mr. Gianello, and (ii) 25,000 ordinary shares issuable upon the exercise of warrants held by Mr. Gianello that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(10) | Consists of (i) 5,560 ordinary shares held by Ms. Johnson-Mills, and (ii) 25,000 ordinary shares issuable upon the exercise of warrants held by Mr. Johnson-Mills that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(11) | Consists of 419,930 ordinary shares issuable upon the exercise of warrants held by Mr. Taelman that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(12) | Consists of 50,142 ordinary shares issuable upon the exercise of warrants held by Mr. Moreau that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
(13) | Consists of (i) 4,893,921 ordinary shares and (ii) 620,072 ordinary shares issuable upon the exercise of warrants that are immediately exercisable or exercisable within 60 days of March 18, 2024. |
To our knowledge, other than as provided in the table above, our other filings with the SEC and this Annual Report, there has been no significant change in the percentage ownership held by any major shareholder since January 1, 2019.
The major shareholders listed above do not have voting rights with respect to their ordinary shares that are different from the voting rights of other holders of our ordinary shares.
B.Related Party Transactions
The following is a description of related party transactions we have entered into since January 1, 2022 with any members of our board of directors or executive officers or the holders of more than 3% of our share capital.
Consulting Agreement with Olivier Taelman
Effective September 1, 2021, the Company and Olivier Taelman decided by mutual agreement to terminate the employment contract of Olivier Taelman with the Company and to enter into an agreement, pursuant to which Mr. Taelman will perform his functions as CEO of the Company on a self-employed basis going forward. Pursuant to the terms of this agreement, as amended effective as of January 1, 2022, Mr. Taelman will be entitled to receive an annual fee equal to the euro equivalent of $450,000, as well as a short term incentive and a long term incentive (in the form of the grant of warrants) in accordance with the Company’s remuneration policy as approved from time to time by the shareholders’ meeting of the Company. Mr. Taelman will continue to benefit from a company car, a laptop, a mobile phone, an occupational pension scheme and a hospitalization insurance. The consulting agreement has an indefinite term and can be terminated by either us or Mr. Taelman at any time subject to a notice period of three months, supplemented with one month per completed year of services under the Agreement, with a maximum total notice period of nine months. We can immediately terminate the consulting agreement in case of serious cause.
130
Employment Agreement with Loïc Moreau
We are party to an employment agreement, dated October 8, 2021, with Loïc Moreau, our chief financial officer since January 1, 2022. Pursuant to the terms of his employment agreement, Mr. Moreau receives a base salary of €259,000, which amount is subject to increase, as well as a short term incentive and a long term incentive (in the form of the grant of warrants) in accordance with the Company’s remuneration policy as approved from time to time by the shareholders’ meeting of the Company. The employment agreement has an indefinite term and can be terminated by either us or Mr. Moreau at any time subject to prior notice in accordance with Belgian law. We can immediately terminate the employment agreement in case of serious cause.
Warrants to Our Board Directors and Executive Management
We have granted warrants to certain members of our board of directors and executive management. For more information regarding the warrants granted to our board of directors and executive Management, see “—Directors’ and Executive Officers’ Compensation.”
Policies and Procedures for Related Person Transactions
We have adopted a related person transaction policy requiring that all related person transactions required to be disclosed by a foreign private issuer pursuant to the Exchange Act be approved by the audit committee or another independent body of our board of directors.
C.Interests of Experts and Counsel
Not Applicable.
Item 8.Financial Information
A.Consolidated Statements and Other Financial Information
See “Item 18. Financial Statements.”
Legal Proceedings
For more information see “Information on the Group—B. Business Overview—Legal Proceedings”.
Dividend Policy
We have never declared or paid any cash dividends on our ordinary shares. We do not anticipate paying cash dividends on our equity securities in the foreseeable future and intend to retain all available funds and any future earnings for use in the operation and expansion of our business. All of the ordinary shares offered by this prospectus will have the same dividend rights as all of our other outstanding ordinary shares. In general, distributions of dividends proposed by our board of directors require the approval of our shareholders at a meeting of shareholders with a simple majority vote, although our board of directors may declare interim dividends without shareholder approval, subject to the terms and conditions of the Belgian Code of Companies and Associations, or CCA.
Our ability to distribute dividends is subject to availability of sufficient distributable profits as defined under Belgian law on the basis of our stand-alone statutory accounts prepared in accordance with Belgian GAAP. In particular, dividends can only be distributed if following the declaration and issuance of the dividends the amount of our net assets on the date of the closing of the last financial year as follows from the statutory non-consolidated financial statements (i.e., summarized, the amount of the assets as shown in the balance sheet, decreased with provisions and liabilities, all in accordance with Belgian accounting rules), and, save in exceptional cases, to be mentioned and justified in the notes to the annual accounts, decreased with the non-amortized costs of incorporation and extension and the non-amortized costs for research and development, does not fall below the amount of the paid-up capital (or, if higher, the issued capital), increased with the amount of non-distributable reserves (which include, as the case may be, the unamortized part of any revaluation surpluses).
131
In addition, pursuant to Belgian law and our Articles of Association, we must allocate an amount of 5% of our Belgian GAAP annual net profit to a legal reserve in its stand-alone statutory accounts, until the legal reserve amounts to 10% of our share capital. Our legal reserve currently does not meet this requirement nor will it meet the requirement at the time of the closing. Accordingly, 5% of our Belgian GAAP annual net profit during future years will need to be allocated to the legal reserve, further limiting our ability to pay out dividends to its shareholders.
For information regarding the Belgian withholding tax applicable to dividends and related U.S. reimbursement procedures, see “Material United States Federal Income and Belgian Tax Considerations — Material Belgian Tax Consequences.”
B.Significant Changes
Other than the information set forth herein, there have been no significant changes since December 31, 2023.
Item 9.The Offer and Listing
A.Offer and Listing Details
Our ordinary shares are listed on The Nasdaq Global Market under the symbol “NYXH” and the on Euronext Brussels under the symbol “NYXH.”
B.Plan of Distribution
Not Applicable.
C.Markets
Our ordinary shares are listed on The Nasdaq Global Market under the symbol “NYXH” and the on Euronext Brussels under the symbol “NYXH.”
D.Selling Shareholders
Not Applicable.
E.Dilution
Not Applicable.
F.Expenses of the Issue
Not Applicable.
Item 10.Additional Information
A.Share Capital
Not Applicable.
B.Articles of Association
The information set forth in our Registration Statement on Form F-1/A (File No. 333-257000), filed with the SEC on June 28, 2021 and declared effective by the SEC on June 30, 2021, under the heading “Description of Share Capital and Articles of Association” is incorporated herein by reference.
132
C.Material Contracts
Except as otherwise disclosed in this Annual Report (including the exhibits thereto), we are not currently, and have not been in the last two years, party to any material contract, other than contracts entered into in the ordinary course of our business.
D.Exchange Controls
There are no Belgian exchange control regulations that impose limitations on our ability to make, or the amount of, cash payments to residents of the United States.
We are in principle under an obligation to report to the National Bank of Belgium certain cross-border payments, transfers of funds, investments and other transactions in accordance with applicable balance-of- payments statistical reporting obligations. Where a cross-border transaction is carried out by a Belgian credit institution on our behalf, the credit institution will in certain circumstances be responsible for the reporting obligations.
E.Taxation
Material U.S. Federal Income Tax Considerations
Certain Material U.S. Federal Income Tax Considerations to U.S. Holders
The following is a summary of certain material U.S. federal income tax considerations relating to ownership and disposition of ordinary shares by a U.S. holder (as defined below) that is based on the U.S. Internal Revenue Code of 1986, as amended, or the Code; existing, proposed and temporary U.S. Treasury Regulations promulgated thereunder, administrative and judicial interpretations thereof; and the U.S.-Belgium Tax Treaty (as defined below) in each case as of and available on the date hereof. All the foregoing is subject to change, which change could apply retroactively, and to differing interpretations, all of which could affect the tax considerations described below. There can be no assurances that the U.S. Internal Revenue Service, or the IRS, will not take a contrary or different position concerning the tax consequences of ownership and disposition of the ordinary shares or that such a position would not be sustained. Holders should consult their own tax advisers concerning the U.S. federal, state, local and non-U.S. tax consequences of owning, and disposing of the ordinary shares in their particular circumstances.
This summary addresses only the U.S. federal income tax considerations for U.S. holders of our ordinary shares and that will hold such ordinary shares as capital assets for U.S. federal income tax purposes. This summary does not address all U.S. federal income tax matters that may be relevant to a particular U.S. holder. This summary does not address all tax considerations that may be applicable to a holder of ordinary shares that may be subject to special tax rules including, without limitation, the following:
| ● | banks, financial institutions or insurance companies; |
| ● | brokers, dealers or traders in securities, currencies, commodities, or notional principal contracts; |
| ● | tax-exempt entities or organizations, including an “individual retirement account” or “Roth IRA” as defined in Section 408 or 408A of the Code (as defined below), respectively; |
| ● | real estate investment trusts, regulated investment companies or grantor trusts; |
| ● | persons that hold the ordinary shares as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle” for U.S. federal income tax purposes; |
| ● | partnerships (including entities classified as partnerships for U.S. federal income tax purposes) or other pass-through entities (including S Corporations), or persons that will hold the ordinary shares through such an entity; |
| ● | persons that received our ordinary shares as compensation for the performance of services; |
| ● | certain former citizens or long-term residents of the United States; |
| ● | persons that hold our ordinary shares through a permanent establishment or fixed base outside the United States; |
| ● | holders that own directly, indirectly, or through attribution 10% or more of the voting power or value of our ordinary shares; and |
| ● | holders that have a “functional currency” for U.S. federal income tax purposes other than the |
| ● | U.S. dollar. |
133
Further, this summary does not address the U.S. federal estate, gift, or alternative minimum tax considerations, or any U.S. state, local, or non-U.S. tax considerations of the ownership and disposition of the ordinary shares.
If a partnership (or any other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds ordinary shares, the U.S. federal income tax consequences relating to an investment in our ordinary shares will depend in part upon the status of the partner and the activities of the partnership. Such a partner or partnership should consult its tax advisor regarding the U.S. federal income tax considerations of owning and disposing of our ordinary shares in its particular circumstances.
| ● | For the purposes of this summary, a “U.S. holder” is a beneficial owner of ordinary shares that is (or is treated as), for U.S. federal income tax purposes: |
| ● | an individual who is a citizen or resident of the United States; |
| ● | a corporation, or other entity that is treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District |
| ● | of Columbia; |
| ● | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| ● | a trust, if a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) have the authority to control all of the substantial decisions of such trust or has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a United States person. |
As indicated below, this discussion is subject to U.S. federal income tax rules applicable to a “passive foreign investment company,” or a PFIC.
This discussion is for informational purposes only and is not tax advice. Persons considering an investment in our ordinary shares should consult their own tax advisors as to the particular tax consequences applicable to them relating to ownership and disposition of our ordinary shares, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
134
Distributions. Although we do not currently plan to pay dividends, and subject to the discussion under “— Passive Foreign Investment Company Considerations” below, the gross amount of any distribution (before reduction for any amounts withheld in respect of Belgian withholding tax) actually or constructively received by a U.S. holder with respect to ordinary shares will be taxable to the U.S. holder as a dividend to the extent of the U.S. holder’s pro rata share of our current and accumulated earnings and profits as determined under U.S. federal income tax principles. Distributions in excess of earnings and profits will be non-taxable to the U.S. holder to the extent of, and will be applied against and reduce, the U.S. holder’s adjusted tax basis in the ordinary shares. Distributions in excess of earnings and profits and such adjusted tax basis will generally be taxable to the U.S. holder as either long-term or short-term capital gain depending upon whether the U.S. holder has held the ordinary shares for more than one year as of the time such distribution is received. However, since we do not calculate our earnings and profits under U.S. federal income tax principles, it is expected that any distribution will be reported as a dividend, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. Non-corporate U.S. holders may qualify for the preferential rates of taxation with respect to dividends on ordinary shares applicable to long-term capital gains (i.e., gains from the sale of capital assets held for more than one year) and applicable to qualified dividend income (as discussed below) if we are a “qualified foreign corporation” and certain other requirements (discussed below) are satisfied. A non-U.S. corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information provision, or (b) with respect to any dividend it pays on ordinary shares which are readily tradable on an established securities market in the United States. The ordinary shares are listed on the Nasdaq Global Market, or Nasdaq, which is an established securities market in the United States, and we expect the ordinary shares to be readily tradable on Nasdaq. However, there can be no assurance that the ordinary shares will be considered readily tradable on an established securities market in the United States in later years. We are incorporated under the laws of Belgium, and we believe that we qualify as a resident of Belgium for purposes of, and are eligible for the benefits of, The Convention between the Government of the United States of America and the Government of the Kingdom of Belgium for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, signed on November 27, 2006, or the U.S.-Belgium Tax Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-Belgium Tax Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange-of- information program. Therefore, subject to the discussion under “— Passive Foreign Investment Company Considerations” below, such dividends will generally be “qualified dividend income” in the hands of individual U.S. holders, provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the 121-day period beginning 60 days before the ex-dividend date) and certain other requirements are satisfied. The dividends will not be eligible for the dividends- received deduction generally allowed to corporate U.S. holders.
A U.S. holder generally may claim the amount of any Belgian withholding tax as either a deduction from gross income or a credit against U.S. federal income tax liability. However, the foreign tax credit is subject to numerous complex limitations that must be determined and applied on an individual basis. Generally, the credit cannot exceed the same proportion of a U.S. holder’s U.S. federal income tax liability which such U.S. holder’s “foreign source” taxable income bears to such U.S. holder’s worldwide taxable income. In applying this limitation, a U.S. holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source.” In addition, this limitation is calculated separately with respect to specific categories of income. The amount of a distribution with respect to the ordinary shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Belgian income tax purposes, potentially resulting in a reduced foreign tax credit for the U.S. holder. Furthermore, Belgian income taxes that are withheld in excess of the rate applicable under the U.S.-Belgium Tax Treaty or that are refundable under Belgian law will not be eligible for credit against a U.S. holder’s federal income tax liability. Recently issued U.S. Treasury regulations may restrict the availability of foreign tax credits based on the nature of the tax imposed by foreign taxes without regard to such restrictions for taxable years ending prior to the year further guidance is issued. Each U.S. holder should consult its own tax advisors regarding the foreign tax credit rules.
In general, the amount of a distribution paid to a U.S. holder in a foreign currency will be the dollar value of the foreign currency calculated by reference to the spot exchange rate on the day the U.S. holder receives the distribution, regardless of whether the foreign currency is converted into U.S. dollars at that time. Any foreign currency gain or loss a U.S. holder realizes on a subsequent conversion of foreign currency into U.S. dollars will be U.S. source ordinary income or loss. If dividends received in a foreign currency are converted into U.S. dollars on the day they are received, a U.S. holder should not be required to recognize foreign currency gain or loss in respect of the dividend.
135
Sale, Exchange or Other Taxable Disposition of the Ordinary Shares. A U.S. holder will generally recognize gain or loss for U.S. federal income tax purposes upon the sale, exchange or other taxable disposition of ordinary shares in an amount equal to the difference between the U.S. dollar value of the amount realized from such sale or exchange and the U.S. holder’s tax basis for those ordinary shares. Subject to the discussion under “— Passive Foreign Investment Company Considerations” below, this gain or loss will generally be a capital gain or loss. The adjusted tax basis in the ordinary shares generally will be equal to the cost of such ordinary shares. Capital gain from the sale, exchange or other taxable disposition of ordinary shares of a non-corporate U.S. holder is generally eligible for a preferential rate of taxation applicable to capital gains, if the non-corporate U.S. holder’s holding period determined at the time of such sale, exchange or other taxable disposition for such ordinary shares exceeds one year (i.e., such gain is a long-term capital gain). The deductibility of capital losses for U.S. federal income tax purposes is subject to limitations. Any such gain or loss that a U.S. holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes.
For a cash basis taxpayer, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the settlement date of the purchase or sale. In that case, no foreign currency exchange gain or loss will result from currency fluctuations between the trade date and the settlement date of such a purchase or sale. An accrual basis taxpayer, however, may elect the same treatment required of cash basis taxpayers with respect to purchases and sales of the ordinary shares that are traded on an established securities market, provided the election is applied consistently from year to year. Such election may not be changed without the consent of the IRS. For an accrual basis taxpayer that does not make such an election, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the trade date of the purchase or sale. Such an accrual basis taxpayer may recognize exchange gain or loss based on currency fluctuations between the trade date and the settlement date. Any foreign currency gain or loss a U.S. holder realizes will be U.S. source ordinary income or loss.
Net Investment Income Tax. Certain U.S. holders that are individuals, estates or trusts are subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend income and net gains from the disposition of ordinary shares. Each U.S. holder that is an individual, estate or trust is urged to consult its tax advisors regarding the applicability of the Net Investment Income tax to its income and gains in respect of its investment in the ordinary shares.
Passive Foreign Investment Company Considerations. If we are a PFIC for any taxable year, a U.S. holder would be subject to special rules generally intended to reduce or eliminate any benefits from the deferral of U.S. federal income tax that a U.S. holder could derive from investing in a non-U.S. company that does not distribute all of its earnings on a current basis.
A corporation organized outside the United States generally will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which, after applying certain look-through rules with respect to the income and assets of its subsidiaries, either: (i) at least 75% of its gross income is “passive income” or (ii) at least 50% of the average quarterly value of its total gross assets, for which purpose, assuming we are treated as a publicly traded company pursuant to Section 1297(e)(3) of the Code, the total value of our assets may be determined in part by reference to the market value of its ordinary shares, which is subject to change) is attributable to assets that produce “passive income” or are held for the production of “passive income.”
Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income, and includes amounts derived by reason of the temporary investment of cash, including the funds raised in offerings of the ordinary shares. If a non-U.S. corporation owns directly or indirectly at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation’s income for purposes of the PFIC tests. If we are classified as a PFIC for any year with respect to which a U.S. holder owns ordinary shares, we will generally continue to be treated as a PFIC with respect to such U.S. holder in all succeeding years during which the U.S. holder owns ordinary shares, regardless of whether we continue to meet the tests described above.
136
Whether we are a PFIC for any taxable year will depend on the composition of our income and the projected composition and fair market values of our assets in each year, and because this is a factual determination made annually after the end of each taxable year, there can be no assurance that we will not be considered a PFIC for any taxable year. The market value of our assets is generally determined in large part by reference to the market price of the ordinary shares, which is likely to fluctuate. Based on the foregoing, with respect to our 2024 taxable year, we do not anticipate that we will be a PFIC based upon the expected value of our assets, including any goodwill, and the expected composition of our income and assets, however, as previously mentioned, we cannot provide any assurances regarding our PFIC status for the current or future taxable years. Accordingly, our U.S. counsel expresses no opinion with respect to our PFIC status for the current or any future taxable year.
If we are a PFIC for any taxable year, then unless you make one of the elections described below, a special tax regime will apply to both (a) any “excess distribution” by us to you (generally, your ratable portion of distributions in any year which are greater than 125% of the average annual distribution received by you in the shorter of the three preceding years or your holding period for the ordinary shares) and (b) any gain realized on the sale or other disposition of the ordinary shares. Under this regime, any excess distribution and realized gain will be treated as ordinary income and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over your holding period, (b) the amount deemed realized in each year had been subject to tax in each year of that holding period at the highest marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax at the U.S. holder’s regular ordinary income rate for the current year and would not be subject to the interest charge discussed below), and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable in those years. In addition, dividend distributions made to you will not qualify for the lower rates of taxation applicable to long-term capital gains discussed above under “— Distributions.”
If we are a PFIC for any year during which a U.S. holder holds our ordinary shares, we must generally continue to be treated as a PFIC by that U.S. holder for all succeeding years during which the U.S. holder holds our ordinary shares, unless we cease to meet the requirements for PFIC status and the U.S. holder makes a “deemed sale” election with respect to our ordinary shares. If such election is made, the U.S. holder will be deemed to have sold our ordinary shares it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain from such deemed sale would be subject to the consequences applicable to sales of PFIC shares described above. After the deemed sale election, the U.S. holder’s ordinary shares with respect to which the deemed sale election was made will not be treated as shares in a PFIC unless we subsequently become a PFIC.
Certain elections exist that would result in an alternative treatment (such as mark-to-market treatment) of the ordinary shares. If a U.S. holder makes the mark-to-market election, the U.S. holder generally will recognize as ordinary income any excess of the fair market value of the ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. holder makes the election, the U.S. holder’s tax basis in the ordinary shares will be adjusted to reflect these income or loss amounts. Any gain recognized on the sale or other disposition of ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). The mark-to-market election is available only if we are a PFIC and our ordinary shares are “regularly traded” on a “qualified exchange.” Our ordinary shares will be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of our ordinary shares are traded on a qualified exchange on at least 15 days during each calendar quarter (subject to the rule that trades that have as one of their principal purposes the meeting of the trading requirement as disregarded). Nasdaq is a qualified exchange for this purpose and, consequently, if our ordinary shares are regularly traded, the mark-to-market election will be available to a U.S. holder. However, even if a U.S. holder validly makes a mark-to-market election with respect to our ordinary shares, the U.S. holder may continue to be subject to PFIC rules (described above) with respect to its indirect interest in any of our investments that are lower-tier PFICs (as defined below). In addition, it is possible that a mark-to-market election in our ordinary shares may result in a U.S. holder being taxed on the earnings and profits of a lower-tier PFIC that will result in a double counting of the same income.
The tax consequences that would apply if we were a PFIC would also be different from those described above if a U.S. holder were able to make a valid “qualified electing fund,” or QEF, election. However, we do not currently intend to provide the information necessary for U.S. holders to make a QEF election if we were treated as a PFIC for any taxable year and prospective investors should assume that a QEF election will not be available. U.S. holders should consult their tax advisors to determine whether any of these above elections would be available and if so, what the consequences of the alternative treatments would be in their particular circumstances.
137
If we are determined to be a PFIC, the general tax treatment for U.S. holders described in this section would apply to indirect distributions and gains deemed to be realized by U.S. holders in respect of any of our subsidiaries that also may be determined to be PFICs, or lower-tier PFICs.
If a U.S. holder owns ordinary shares during any taxable year in which we are a PFIC, the U.S. holder generally will be required to file an IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to the company and any lower-tier PFICs, generally with the U.S. holder’s federal income tax return for that year. If our company were a PFIC for a given taxable year, then you should consult your tax advisor concerning your annual filing requirements.
The U.S. federal income tax rules relating to PFICs are complex. Prospective U.S. investors are urged to consult their own tax advisers with respect to ownership and disposition of our ordinary shares, the consequences to them of an investment in a PFIC, any elections available with respect to our ordinary shares and the IRS information reporting obligations with respect to ownership and disposition of the ordinary shares.
Backup Withholding and Information Reporting. U.S. holders generally will be subject to information reporting requirements with respect to dividends on ordinary shares and on the proceeds from the sale, exchange or disposition of ordinary shares that are paid within the United States or through U.S.-related financial intermediaries, unless the U.S. holder is an “exempt recipient.” In addition, U.S. holders may be subject to backup withholding on such payments, unless the U.S. holder provides a correct taxpayer identification number and a duly executed IRS Form W-9 or otherwise establishes an exemption. Backup withholding is not an additional tax, and the amount of any backup withholding will be allowed as a credit against a U.S. holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Asset Reporting. Certain U.S. holders who are individuals and certain entities controlled by individuals may be required to report information relating to an interest in our ordinary shares, subject to certain exceptions (including an exception for shares held in accounts maintained by U.S. financial institutions) by filing IRS Form 8938 (Statement of Specified Foreign Financial Assets) with their federal income tax return. U.S. holders are urged to consult their tax advisors regarding their information reporting obligations, if any, with respect to their ownership and disposition of our ordinary shares.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN ORDINARY SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
Material Belgian Tax Consequences
The paragraphs below present a summary of certain material Belgian federal income tax consequences of the acquisition, ownership and disposal of ordinary shares by an investor, but does not address all tax consequences of the ownership and disposal of ordinary shares, and does not take into account the specific circumstances of particular investors, some of which may be subject to special rules, or the tax laws of any country other than Belgium. The following does not describe the tax treatment of investors that are subject to special rules, such as banks, insurance companies, collective investment undertakings, dealers in securities or currencies, persons that hold, or will hold, ordinary shares as a position in a straddle, share-repurchase transaction, conversion transactions, synthetic security or other integrated financial transactions. The summary is based on laws, treaties and regulatory interpretations in effect in Belgium on the date of this Annual Report, all of which are subject to change, including changes that could have retroactive effect. Investors should appreciate that, as a result of evolutions in law or practice, the eventual tax consequences may be different from what is stated below.
A Belgian resident is (i) an individual subject to Belgian personal income tax (i.e. an individual who has his domicile in Belgium or has the seat of his wealth in Belgium, or a person assimilated to a Belgian resident), (ii) a company subject to Belgian corporate income tax, i.e. a company that has its principal establishment, administrative seat or effective place of management in Belgium (and that is not excluded from the scope of the Belgian corporate income tax) (A company having its registered seat in Belgium shall be presumed, unless the contrary is proved, to have its principal establishment, administrative seat or effective place of management in Belgium), (iii) an Organization for Financing Pensions, or an OFP, subject to Belgian corporate income tax (i.e., a Belgian pension fund incorporated under the form of an OFP), or (iv) a legal entity subject to the Belgian tax on legal entities (i.e. a legal entity other than a company subject to the corporate income tax that has its principal establishment, administrative seat or effective place of management in Belgium). A Belgian non-resident is a person that is not a Belgian resident.
138
Investors are encouraged to consult their own advisers as to the tax consequences of the acquisition, ownership and disposal of the shares in light of their particular circumstances, including the effect of any state, local or other national laws.
Belgian taxation of dividends on ordinary shares
For Belgian income tax purposes, the gross amount of all distributions made by the company to its shareholders is generally taxed as a dividend distribution, except for the repayment of capital carried out in accordance with the Belgian Code on Companies and Associations to the extent that such repayment is imputed to the “fiscal” capital. The fiscal capital includes, in principle, the actual paid-up statutory capital and, subject to certain conditions, the paid issue premiums and the amounts subscribed to at the time of the issue of profit sharing certificates. Note that Article 18 of the Belgian Income Tax Code 1992, or ITC, provides that for any decision of capital reduction taken in accordance with the Belgian Code on Companies and Associations, the amount of the capital reduction will be deemed to be derived proportionally (a) from our fiscal capital, on the one hand and (b) on the other hand, from the total of (i) certain taxed reserves incorporated in our capital, (ii) certain taxed reserves not incorporated into our capital and (iii) certain untaxed reserves incorporated into our capital (it being understood that the imputation of the capital reduction on these different categories of reserves will be made in that order of priority). The part of the capital reduction that is deemed to be derived from the abovementioned taxed and untaxed reserves will be treated as a dividend distribution from a tax perspective and be subject to Belgian withholding tax, if applicable. The part of the capital reduction that is deemed to derive from the abovementioned untaxed reserves may additionally give rise to a corporate income tax charge at the level of the company.
In general, a Belgian withholding tax of (currently) 30% is levied on dividends.
In the case of a redemption of ordinary shares, the redemption price (after deduction of the part of the paid-up fiscal capital represented by the ordinary shares redeemed) will be treated as dividend that is subject to a Belgian withholding tax of 30%, subject to such relief as may be available under applicable domestic or tax treaty provisions. No withholding tax will be triggered if such redemption is carried out on a stock exchange and meets certain conditions. In the event of a liquidation, a withholding tax of 30% will be levied on any distributed amount exceeding the paid-up fiscal capital, subject to such relief as may be available under applicable domestic or tax treaty provisions.
Belgian tax law provides for certain exemptions from Belgian withholding tax on Belgian source dividends. If there is no exemption applicable under Belgian domestic tax law, the Belgian withholding tax can potentially be reduced or exempted for investors who are non-residents pursuant to the treaties regarding the avoidance of double taxation concluded between the Kingdom of Belgium and the state of residence of the non-resident shareholder (see below).
Belgian income tax
Belgian resident individuals
Belgian resident individuals who acquire and hold our ordinary shares as a private investment do not have to declare the dividend income in their personal income tax return since the 30% Belgian withholding tax fully discharges their personal income tax liability. If (and only if) the dividend income would be declared in the personal income tax return, it will be taxed at 30% or, if lower, at the progressive personal income tax rates applicable to the taxpayer’s overall declared income. The first €833 (amount applicable for income year 2024 per year and per taxpayer) of reported ordinary dividend income will be exempt from tax, subject to certain conditions. For the avoidance of doubt, all reported dividends (hence, not only dividends distributed on the shares) are taken into account to assess whether said maximum amount is reached.
If the dividends are declared in the personal income tax return, the Belgian withholding tax paid can be credited against the final personal income tax liability of the investor and may also be reimbursed to the extent that it exceeds the final personal income tax liability, provided that the dividend distribution does not result in a reduction in value of, or capital loss on, the shares. The latter condition is not applicable if the Belgian individual can demonstrate that he has had full ownership of the ordinary shares during an uninterrupted period of 12 months prior to the attribution of the dividends.
139
Belgian resident individuals who acquire and hold the shares for professional purposes must always declare the dividend income in their personal income tax return and will be taxable at the individual’s personal income tax rate increased with local surcharges. Withholding tax withheld at source may be credited against the personal income tax due and is reimbursable to the extent that it exceeds the personal income tax due, subject to two conditions: (i) the taxpayer must own the shares in full legal ownership on the day the beneficiary of the dividend is identified, and (ii) the dividend distribution may not result in a reduction in value of or a capital loss on the shares. The latter condition is not applicable if the individual can demonstrate that he has held the full legal ownership of the ordinary shares for an uninterrupted period of 12 months prior to the payment or attribution of the dividends.
Belgian resident companies
For Belgian resident companies, the dividend withholding tax does not fully discharge the corporate income tax liability. For such companies, the gross dividend income (including the Belgian withholding tax and excluding the foreign withholding tax, if any) must be declared in the corporate income tax return and will be added to their taxable income, which is, in principle, taxed at the ordinary corporate income tax rate of 25%. In certain circumstances and subject to certain conditions, a reduced corporate income tax rate of 20% applies to small companies and Medium Sized Enterprises (as defined by Article 1:24, §1 to §6 of the Belgian Code on Companies and Associations) on the first €100,000 of taxable profits.
Belgian resident companies can generally (although subject to certain conditions) deduct up to 100% of the gross dividend received from their taxable income, i.e. the dividend received deduction, provided that at the time of a dividend payment or attribution: (1) the Belgian resident company holds shares representing at least 10% of the share capital of the company or a participation in the company with an acquisition value of at least €2,500,000; (2) the shares have been held or will be held in full legal ownership for an uninterrupted period of at least one year; and (3) the conditions relating to the taxation of the underlying distributed income, as described in article 203 of the ITC are met (together the “Conditions for the application of the dividend received deduction regime”).
For qualifying investment companies (within the meaning of art. 2, §1, 5°, f) ITC), certain of the aforementioned conditions with respect to the dividend received deduction do not apply. The Conditions for the application of the dividend received deduction regime depend on a factual analysis and for this reason the availability of this regime should be verified upon each dividend distribution.
The Belgian withholding tax may, in principle, be credited against the Belgian corporate income tax due and is reimbursable to the extent that it exceeds such corporate income tax due, subject to the two following conditions: (i) the taxpayer must own the shares in full legal ownership on the day the beneficiary of the dividend is identified and (ii) the dividend distribution may not give rise to a reduction in the value of, or a capital loss on, the ordinary shares. The latter condition is not applicable if the company proves that it held the shares in full legal ownership during an uninterrupted period of 12 months prior to the attribution of the dividends or if, during that period, the shares never belonged to a taxpayer other than a resident company or a non-resident company which has, in an uninterrupted manner, invested the ordinary shares in a Belgian establishment.
No Belgian withholding tax will be due on dividends paid by us to a Belgian resident company provided that the resident company owns, at the time of the distribution of the dividend, at least 10% of our share capital for an uninterrupted period of at least one year and, provided further, that the resident company provides us or our paying agent with a certificate as to its status as a resident company and as to the fact that it has owned a 10% shareholding for an uninterrupted period of one year. For those companies owning a share participation of at least 10% in our share capital for less than one year, we will levy the withholding tax but will not transfer it to the Belgian Treasury provided that the Belgian resident company certifies its qualifying status, the date from which it has held such minimum participation, and its commitment to hold the minimum participation for an uninterrupted period of at least one year. The Belgian resident company must also inform us or our paying agent if the one-year period has expired or if its shareholding will drop below 10% of our share capital before the end of the one-year holding period. As soon as the investor owns the share participation of at least 10% in our capital for one year, it will receive the amount of this temporarily levied withholding tax.
140
Please note that the above described dividend received deduction and withholding tax exemption will not be applicable to dividends which are connected to an arrangement or a series of arrangements (“rechtshandeling of geheel van rechtshandelingen”/“acte juridique ou un ensemble d’actes juridiques”) for which the Belgian tax administration, taking into account all relevant facts and circumstances, has proven, unless evidence to the contrary, that this arrangement or this series of arrangements is not genuine (“kunstmatig”/“non authentique”) and has been put in place for the main purpose or one of the main purposes of obtaining the dividend received deduction, the above dividend withholding tax exemption or one of the advantages of the EU Parent-Subsidiary Directive of 30 November 2011 (2011/96/EU), or Parent-Subsidiary Directive, in another EU Member State. An arrangement or a series of arrangements is regarded as not genuine to the extent that they are not put into place for valid commercial reasons which reflect economic reality.
Belgian resident organizations for financing pensions
For Belgian pension funds incorporated under the form of an Organization for Financing Pensions (“organismen voor de financiering van pensioenen”/“organismes de financement de pensions”) within the meaning of article 8 of the Belgian Act of 27 October 2006, the dividend income from the ordinary shares is generally tax exempt.
Subject to certain limitations, any Belgian dividend withholding tax levied at source may be credited against the corporate income tax due and is reimbursable to the extent that it exceeds the corporate income tax due.
Belgian (or foreign) OFPs not holding the ordinary shares — which give rise to dividends — for an uninterrupted period of 60 days in full ownership amounts to a rebuttable presumption that the arrangement or series of arrangements (“rechtshandeling of geheel van rechtshandelingen”/“acte juridique ou un ensemble d’actes juridiques”) which are connected to the dividend distributions, are not genuine (“kunstmatig”/“non authentique”). The withholding tax exemption will in such case not apply and/or any Belgian dividend withholding tax levied at source on the dividends will in such case not be credited against the corporate income tax, unless counterproof is provided by the OFP that the arrangement or series of arrangements are genuine.
Other Belgian resident legal entities subject to Belgian legal entities tax
Belgian resident legal entities will be subject to the Belgian withholding tax on the dividends distributed by us. Under the current Belgian tax rules, Belgian withholding tax will represent the final tax liability and the dividends should, therefore, not be included in the tax returns of the legal entities.
Non-resident individuals and companies
For non-resident individuals and companies, the dividend withholding tax will be the only tax on dividends in Belgium, unless the non-resident holds the shares in connection with a business conducted in Belgium through a fixed base in Belgium or a Belgian permanent establishment.
If the ordinary shares are acquired by a non-resident in connection with a business in Belgium, the investor must report any dividends received, which will be taxable at the applicable non-resident individual or corporate income tax rate, as appropriate. Belgian withholding tax levied at source may be credited against non- resident individual or corporate income tax and is reimbursable to the extent that it exceeds the income tax due, subject to two conditions: (1) the taxpayer must own the shares in full legal ownership on the day the beneficiary of the dividend is identified and (2) the dividend distribution may not result in a reduction in value of or a capital loss on the shares. The latter condition is not applicable if (a) the non-resident individual or the non-resident company can demonstrate that the shares were held in full legal ownership for an uninterrupted period of 12 months prior to the payment or attribution of the dividends or (b) with regard to non-resident companies only, if, during the relevant period, the ordinary shares have not belonged to a taxpayer other than a resident company or a non-resident company which has, in an uninterrupted manner, invested the ordinary shares in a Belgian establishment.
Non-resident companies whose ordinary shares are invested in a Belgian permanent establishment may deduct 100% of the gross dividends received from their taxable income if, at the date the dividends are paid or attributed, the Conditions for the application of the dividend received deduction regime are met (see supra). Application of the dividend received deduction regime depends, however, on a factual analysis to be made upon each distribution and its availability should be verified upon each distribution.
141
Belgian dividend withholding tax relief for non-residents
Dividends paid or attributed to non-resident individuals who do not use the ordinary shares in the exercise of a professional activity, may, subject to certain conditions and formalities, be eligible for the tax exemption with respect to ordinary dividends in an amount of up to €833 (amount applicable for income year 2024) per year and per taxpayer. For the avoidance of doubt, all dividends paid or attributed to such non-resident individual (and hence not only dividends paid or attributed on the ordinary shares) are taken into account to assess whether said maximum amount is reached. Consequently, if Belgian withholding tax has been levied on dividends paid or attributed to the ordinary shares, such non-resident individual may request in its Belgian non-resident income tax return that any Belgian withholding tax levied on up to such an amount be credited and, as the case may be, reimbursed. However, if no Belgian non- resident income tax return has to be filed by the non-resident individual, any Belgian withholding tax levied on up to such an amount could in principle be reclaimed by filing a request thereto addressed to the tax official (“Adviseur-generaal Centrum Buitenland”/“Conseiller-général du Centre Étranger”) appointed by article 206/1 of the Royal Decree implementing the ITC. Such a request has to be made at the latest on December 31 of the calendar year following the calendar year in which the relevant dividend(s) have been received, together with an affidavit confirming the non-resident individual status and certain other formalities determined in the Royal Decree.
Belgian tax law provides for certain exemptions from withholding tax on Belgian source dividends distributed to non-resident investors. Under Belgian tax law, withholding tax is not due on dividends paid to a foreign pension fund which satisfies the following conditions: (i) it is a non-resident saver within the meaning of Article 227, 3° ITC which implies that it has separate legal personality and has its tax residence outside of Belgium; (ii) whose corporate purpose consists solely in managing and investing funds collected in order to pay legal or complementary pensions; (iii) whose activity is limited to the investment of funds collected in the exercise of its corporate purpose, without any profit making aim; (iv) which is exempt from income tax in its country of residence; and (v) provided that it is not contractually obliged to redistribute the dividends to any ultimate beneficiary of such dividends for whom it would manage the ordinary shares, nor obliged to pay a manufactured dividend with respect to the ordinary shares under a securities borrowing transaction. The exemption will only apply if the foreign pension fund provides a certificate confirming that it is the full legal owner or usufruct holder of the Shares and that the above conditions are satisfied. The pension fund must then forward that certificate to us or our paying agent. As indicated above, Belgian (or foreign) OFPs not holding the ordinary shares — which give rise to dividends — for an uninterrupted period of 60 days in full ownership amounts to a rebuttable presumption that the arrangement or series of arrangements (“rechtshandeling of geheel van rechtshandelingen”/“acte juridique ou un ensembled’actes juridiques”) which are connected to the dividend distributions, are not genuine (“kunstmatig”/“non authentique”). In such case the withholding tax exemption will not apply.
Dividends distributed to non-resident qualifying parent companies established in a Member State of the EU or in a country with which Belgium has concluded a double tax treaty that includes a qualifying exchange of information clause, will, under certain conditions, be exempt from Belgian withholding tax provided that the ordinary shares held by the non-resident company, upon payment or attribution of the dividends, amount to at least 10% of our share capital and such minimum participation is held or will be held during an uninterrupted period of at least one year. A non-resident company qualifies as a parent company provided that (i) for companies established in a Member State of the EU, it has a legal form as listed in the annex to the EU Parent-Subsidiary Directive, as amended from time to time, or, for companies established in a country with which Belgium has concluded a qualifying double tax treaty, it has a legal form similar to the ones listed in such annex; (ii) it is considered to be a tax resident according to the tax laws of the country where it is established and the double tax treaties concluded between such country and third countries; and (iii) it is subject to corporate income tax or a similar tax without benefiting from a tax regime that derogates from the ordinary tax regime. In order to benefit from this exemption, the non- resident company must provide us or our paying agent with a certificate confirming its qualifying status and the fact that it meets the required conditions.
If the non-resident company holds such a minimum participation for less than one year at the time the dividends are attributed to the ordinary shares, we must levy the withholding tax but do not need to transfer it to the Belgian Treasury provided that the non-resident company provides us or our paying agent with a certificate confirming, in addition to its qualifying status, the date as of which it has held the minimum participation, and its commitment to hold the minimum participation for an uninterrupted period of at least one year. The non-resident company must also inform us or our paying agent when the one-year period has expired or if its shareholding drops below 10% of our share capital before the end of the one year holding period. Upon satisfying the one-year holding requirement, the dividend withholding tax which was temporarily withheld, will be refunded to the non-resident company.
142
Please note that the above withholding tax exemption will not be applicable to dividends which are connected to an arrangement or a series of arrangements (“rechtshandeling of geheel van rechtshandelingen”/“acte juridique ou un ensemble d’actes juridiques”) for which the tax Belgian tax administration, taking into account all relevant facts and circumstances, has proven, unless evidence to the contrary, that this arrangement or this series of arrangements is not genuine (“kunstmatig”/“non authentique”) and has been put in place for the main purpose or one of the main purposes of obtaining the dividend received deduction, the above dividend withholding tax exemption or one of the advantages of the Parent-Subsidiary Directive in another EU Member State. An arrangement or a series of arrangements is regarded as not genuine to the extent that they are not put into place for valid commercial reasons which reflect economic reality.
Dividends distributed by a Belgian company to non-resident companies on a share participation of less than 10% will under certain conditions be subject to an exemption from withholding tax, provided that the non- resident companies (i) are either established in another Member State of the EEA or in a country with which Belgium has concluded a double tax treaty, where that treaty, or any other treaty concluded between Belgium and that jurisdiction, includes a qualifying exchange of information clause; (ii) have a legal form as listed in Annex I, Part A to the Parent-Subsidiary Directive as amended from time to time, or a legal form similar to the legal forms listed in the aforementioned annex and which is governed by the laws of another Member State of the EEA or a similar legal form in a country with which Belgium has concluded a double tax treaty; (iii) hold a share participation in the Belgian dividend distributing company, upon payment or attribution of the dividends, of less than 10% of our share capital but with an acquisition value of at least €2.5 million; (iv) hold or will hold the ordinary shares which give rise to the dividends in full legal ownership during an uninterrupted period of at least one year; and (v) are subject to the corporate income tax or a tax regime similar to the corporate income tax without benefiting from a tax regime which deviates from the ordinary regime. The exemption from withholding tax is only applied to the extent that the Belgian withholding tax, which would be applicable absent the exemption, could not be credited nor reimbursed at the level of the qualifying, dividend receiving, company. The non-resident company must provide us or our paying agent with a certificate confirming, in addition to its full name, legal form, address and fiscal identification number (if applicable), its qualifying status and the fact that it meets the required conditions mentioned under (i) to (v) above, and indicating to which extent the withholding tax, which would be applicable absent the exemption, is in principle creditable or reimbursable on the basis of the law as applicable on December 31 of the year preceding the year during which the dividend is paid or attributed.
If there is no exemption applicable under Belgian domestic tax law, the Belgian dividend withholding tax can potentially be reduced or exempted for investors who are non-residents pursuant to the treaties regarding the avoidance of double taxation concluded between the Kingdom of Belgium and the state of residence of the non-resident shareholder. Belgium has concluded tax treaties with more than 95 countries, reducing the dividend withholding tax rate to 15%, 10%, 5% or 0% for residents of those countries, depending on conditions, among others, related to the size of the shareholding and certain identification formalities.
Belgium and the United States have concluded a double tax treaty concerning the avoidance of double taxation (the “U.S. — Belgium Treaty”). The U.S. — Belgium Treaty reduces the applicability of Belgian withholding tax to 15%, 5% or 0% for U.S. taxpayers, provided that the U.S. taxpayer meets the limitation of benefits conditions imposed by the U.S. — Belgium Treaty. The Belgian withholding tax is generally reduced to 15% under the U.S. — Belgium Treaty. The 5% withholding tax applies in case where the U.S. shareholder (beneficial owner) is a company which owns directly at least 10% of our ordinary shares. A 0% Belgian withholding tax applies when the shareholder is a company (beneficial owner) which has owned directly at least 10% of our ordinary shares during at least 12 months, or is, subject to certain conditions, a U.S. pension fund. The U.S. shareholders are encouraged to consult their own tax advisers to determine whether they can invoke the benefits and meet the limitation of benefits conditions as imposed by the U.S. — Belgium Treaty.
Prospective holders are encouraged to consult their own tax advisers to determine whether they qualify for an exemption or a reduction of the withholding tax rate upon payment of dividends and, if so, the procedural requirements for obtaining such an exemption or a reduction upon the payment of dividends or making claims for reimbursement.
Capital gains and losses on ordinary shares
Belgian resident individuals
Belgian resident individuals acquiring the ordinary shares as a private investment should not be subject to Belgian capital gains tax on the disposal of the ordinary shares and capital losses are not tax deductible. However, capital gains realized by a private individual are taxable at 33% (plus local surcharges) if the capital gain is deemed to be realized outside the scope of the normal management of the individual’s private estate. Capital losses incurred in such transactions are generally not tax deductible.
143
Capital gains realized by Belgian resident individuals on the disposal of the ordinary shares for consideration, outside the exercise of a professional activity, to a non-resident company (or a body constituted in a similar legal form), to a foreign state (or one of its political subdivisions or local authorities) or to a non-resident legal entity, each time established outside the EEA, are in principle taxable at a rate of 16.5% (plus local surcharges) if, at any time during the five years preceding the sale, the Belgian resident individual has owned directly or indirectly, alone or with his/her spouse or with certain relatives, a substantial shareholding in us (i.e., a shareholding of more than 25% in us). Capital losses are, however, not tax deductible in such event.
Capital gains realized by Belgian resident individuals upon redemption of the ordinary shares or upon our liquidation will generally be taxable as a dividend. See “— Belgian taxation of dividends on ordinary shares — Belgian income tax — Belgian resident individuals.”
Belgian resident individuals who hold ordinary shares for professional purposes are taxed at the ordinary progressive income tax rates increased by the applicable local surcharges on any capital gains realized upon the disposal of the ordinary shares, except for the shares held for more than five years, which are taxable at a separate rate of 10% (capital gains realized in the framework of the cessation of activities under certain circumstances) or 16.5% (other), plus local surcharges. Capital losses on the shares incurred by Belgian resident individuals who hold the ordinary shares for professional purposes are in principle tax deductible.
Belgian resident companies
Belgian resident companies are normally not subject to Belgian capital gains taxation on gains realized upon the disposal of the ordinary shares provided that the Conditions for the application of the dividend received deduction regime are met. If one or more of the Conditions for the application of the dividend received deduction regime are not met, any capital gain realized would be taxable at the standard corporate income tax rate of 25%, unless the reduced corporate income tax rate of 20% for small companies and Medium Sized Enterprises applies (see supra).
Capital losses on the ordinary shares incurred by Belgian resident companies are as a general rule not tax deductible.
However, ordinary shares held in the trading portfolios of Belgian qualifying credit institutions, investment enterprises and management companies of collective investment undertakings are subject to a different regime. In general, the capital gains on such ordinary shares are taxable at the corporate income tax rate of 25% and capital losses on such ordinary shares are tax deductible. Internal transfers to and from the trading portfolio are assimilated to a realization.
Capital gains realized by Belgian resident companies upon redemption of the ordinary shares or upon our liquidation will, in principle, be subject to the same taxation regime as dividends.
Belgian resident organizations for financing pensions
Belgian pension funds incorporated under the form of an OFP are, in principle, not subject to Belgian capital gains taxation on the disposal of the ordinary shares, and capital losses are not tax deductible.
Capital gains realized by Belgian OFPs upon the redemption of ordinary shares or upon our liquidation will in principle be taxed as dividends.
Other Belgian resident legal entities subject to Belgian legal entities tax
Capital gains realized upon disposal of the ordinary shares by Belgian resident legal entities are in principle not subject to Belgian income tax and capital losses are not tax deductible.
Capital gains realized upon disposal of (part of) a substantial participation in a Belgian company (i.e. a participation representing more than 25% of our share capital at any time during the last five years prior to the disposal) may, however, under certain circumstances be subject to income tax in Belgium at a rate of 16.5%.
Capital gains realized by Belgian resident legal entities upon redemption of the ordinary shares or upon our liquidation will, in principle, be subject to the same taxation regime as dividends (see above).
144
Non-resident individuals, non-resident companies or non-resident entities
Non-resident individuals, companies or entities are, in principle, not subject to Belgian income tax on capital gains realized upon disposal of the ordinary shares, unless the ordinary shares are held as part of a business conducted in Belgium through a fixed base in Belgium or a Belgian permanent establishment. In such a case, the same principles apply as described with regard to Belgian individuals (holding the ordinary shares for professional purposes), Belgian companies, Belgian resident organizations for financing pensions or other Belgian resident legal entities subject to Belgian legal entities tax.
Non-resident individuals who do not use the ordinary shares for professional purposes and who have their fiscal residence in a country with which Belgium has not concluded a tax treaty or with which Belgium has concluded a tax treaty that confers the authority to tax capital gains on the shares to Belgium, might be subject to tax in Belgium if the capital gains are obtained or received in Belgium and arise from transactions which are to be considered speculative or beyond the normal management of one’s private estate or in case of disposal of a substantial participation in a Belgian company as mentioned in the tax treatment of the disposal of the ordinary shares by Belgian individuals (see supra). Such non-resident individuals might therefore be obliged to file a tax return and should consult their own tax adviser.
Capital gains realized by non-resident individuals or non-resident companies upon redemption of the ordinary shares or upon our liquidation will, in principle, be subject to the same taxation regime as dividends (see above).
Belgian Tax on Stock Exchange Transactions
A tax on stock exchange transactions (“Taxe sur les opérations de bourse”/“Taks op de beursverrichtingen”) at the rate of 0.35% (subject to a maximum amount of EUR 1,600 per party and per transaction) will in principle be levied upon the sale and purchase and any other acquisition or transfer for consideration of the ordinary shares on the secondary market if (i) it is entered into or carried out in Belgium through a professional intermediary, or (ii) deemed to be entered into or carried out in Belgium, which is the case if the order is directly or indirectly made to a professional intermediary established outside of Belgium, either by private individuals with habitual residence (“gewone verblijfplaats”/“residence habituelle”) in Belgium, or legal entities for the account of their seat or establishment in Belgium (both, a “Belgian Investor”). A separate tax is due from each of the seller and the purchaser, both collected by the professional intermediary. No tax on stock exchange transactions will be due on the issuance of the ordinary shares (primary market transaction).
However, if the order is directly or indirectly made to a professional intermediary established outside of Belgium by a Belgian Investor, the tax on stock exchange transactions will in principle be due by this Belgian Investor (who will be responsible for the filing of a stock exchange tax return and for the timely payment of the amount of stock exchange tax due), unless that Belgian Investor can demonstrate that the tax on stock exchange transactions due has already been paid by the professional intermediary established outside of Belgium. In such a case, the foreign professional intermediary also has to provide each client (which gives such intermediary an order) with a qualifying order statement (“bordereau”/“borderel”) at the latest on the business day after the day the transaction concerned was realized. The qualifying order statements must be numbered in series and a duplicate must be retained by the professional intermediary. The duplicate can be replaced by a qualifying day-to-day listing, numbered in series. Alternatively, professional intermediaries established outside of Belgium could appoint a stock exchange tax representative in Belgium, subject to certain conditions and formalities, or Stock Exchange Tax Representative. Such Stock Exchange Tax Representative will then be liable towards the Belgian Treasury for the tax on stock exchange transactions due on behalf of clients that fall within one of the aforementioned categories (provided that these clients do not qualify as exempt persons for stock exchange tax purposes — see below) and for complying with the reporting obligations and the obligations relating to the order statement (“bordereau”/“borderel”) in that respect. If such a Stock Exchange Tax Representative would have paid the tax on stock exchange transactions due, the Belgian Investor will, as per the above, no longer be the debtor of the tax on stock exchange transactions.
No tax on stock exchange transactions is due on transactions entered into by the following parties, provided they are acting for their own account: (i) professional intermediaries described in article 2, 9° and 10° of the Belgian Law of 2 August 2002 on the supervision of the financial sector and financial services; (ii) insurance companies described in article 2, §1 of the Belgian Law of 9 July 1975 on the supervision of insurance companies; (iii) pension institutions referred to in article 2,1° of the Belgian Law of 27 October 2006 concerning the supervision of pension institutions; (iv) undertakings for collective investment; (v) regulated real estate companies; and (vi) Belgian non-residents provided they deliver a certificate to their financial intermediary in Belgium confirming their non-resident status.
145
As stated below, the tax on stock exchange transactions should be abolished once the FTT enters into force. The proposal is still subject to negotiation between the participating Member States and therefore may be changed at any time.
Other Income Tax Considerations
In addition to the income tax consequences discussed above, we may be subject to tax in one or more other jurisdictions where we conduct activities. The amount of any such tax imposed upon our operations may be material.
Annual tax on securities accounts
The tax on securities accounts is an annual tax of levied at a rate of 0.15% that is levied on securities accounts of which the average value of taxable financial instruments exceeds EUR 1 million during a reference period of twelve consecutive months (in principle) starting on October 1 and ending on September 30 of the subsequent year. The taxable base is determined based on four reference dates: December 31, March 31, June 30 and September 30. If applicable, the amount of tax due is limited to 10% of the difference between the said average value of the taxable financial instruments and the threshold of €1 million.
The tax targets securities accounts held by resident individuals subject to Belgian personal income tax, resident companies subject to Belgian corporate income tax, companies and resident legal entities subject to Belgian legal entities tax, irrespective as to whether these accounts are held with a financial intermediary which is established or located in Belgium or abroad. The tax also applies to securities accounts held by non-resident individuals, companies and legal entities with a financial intermediary established or located in Belgium (individuals, companies and legal entities subject to Belgian non-resident tax). Securities accounts that form part of the business property of a Belgian establishment of a non-resident as referred to in Article 229 ITC, wherever the intermediary is incorporated or established (in Belgium or abroad), are also subject to the annual tax. The financial instruments envisaged include not only shares, bonds and notes, but also derivatives. Each securities account would be assessed separately.
There are various exemptions, such as securities accounts held by specific types of regulated entities for their own account.
A financial intermediary is defined as (i) the National Bank of Belgium, the European Central Bank and foreign central banks performing similar functions, (ii) a central securities depository included in article 198/1, §6, 12°ITC, (iii) a credit institution or a stockbroking firm as defined by Article 1, §3 of the Law of 25 April 2014 on the status and supervision of credit institutions and investment companies and (iv) the investment companies as defined by Article 3, §1 of the Law of 25 October 2016 on access to the activity of investment services and on the legal status and supervision of portfolio management and investment advice companies, which are, pursuant to national law, admitted to hold financial instruments for the account of customers.
A Belgian intermediary is an intermediary incorporated under Belgian law as well as an intermediary established in Belgium.
The Belgian intermediary in principle withholds, declares and pays the tax. In all other cases, the holder will declare and pay the tax himself, unless he can prove that the tax has already been declared and paid by an intermediary, irrespective as to whether the intermediary is incorporated or established in Belgium or abroad. When multiple holders hold a securities account, each holder may fulfil the declaration requirements for all holders and each holder shall be jointly and severally liable for the payment of the tax. An intermediary not incorporated or established in Belgium, when managing a securities account subject to the tax, may have a representative established in Belgium recognized by or on behalf of the Minister of Finance. The representative shall be jointly and severally liable towards to Belgian State to declare and pay the tax, as well as to perform all obligations to which an intermediary is bound.
A general anti-abuse provision is also included to counter certain actions to avoid the application of the tax.
Investors are advised to consult their tax advisors about the consequences of the tax on securities accounts on their own tax situation.
146
Common Reporting Standard
Following recent international developments, the exchange of information is governed by the Common Reporting Standard, or CRS. On March 7, 2024, 122 jurisdictions have signed the multilateral competent authority agreement, or MCAA. The MCAA is a multilateral framework agreement to automatically exchange financial and personal information, with the subsequent bilateral exchanges coming into effect between those signatories that file the subsequent notifications.
49 jurisdictions, including Belgium, have committed to a specific and ambitious timetable leading to the first automatic information exchanges in 2017, relating to income year 2016, or early adopters. More than 50 jurisdictions have committed to exchange information as from 2018, one jurisdiction as from 2019, six jurisdictions as from 2020, two jurisdictions as from 2021 and three jurisdictions as from 2022.
Under CRS, financial institutions resident in a CRS country are required to report, according to a due diligence standard, financial information with respect to reportable accounts, which includes interest, dividends, account balance or value, income from certain insurance products, sales proceeds from financial assets and other income generated with respect to assets held in the account or payments made with respect to the account. Reportable accounts include accounts held by individuals and entities (which includes trusts and foundations) with fiscal residence in another CRS country. The standard includes a requirement to look through passive entities to report on the relevant controlling persons.
On December 9, 2014, EU Member States adopted Directive 2014/107/EU on administrative cooperation in direct taxation, or DAC2, which provides for mandatory automatic exchange of financial information as foreseen in CRS. DAC2 amends the previous Directive on administrative cooperation in direct taxation, Directive 2011/16/EU.
The mandatory automatic exchange of financial information by EU Member States as foreseen in DAC2 started as of September 30, 2017 (as of September 30, 2018 for Austria).
The Belgian government has implemented said Directive 2014/107/EU, respectively the Common Reporting Standard, per the Law of December 16, 2015 regarding the exchange of information on financial accounts by Belgian financial institutions and by the Belgian tax administration, in the context of an automatic exchange of information on an international level and for tax purposes.
As a result of the Law of December 16, 2015, the mandatory automatic exchange of information applies in Belgium (i) as of income year 2016 (first information exchange in 2017) towards the EU Member States, (ii) as of income year 2014 (first information exchange in 2016) towards the US and (iii), with respect to any other non-EU States that have signed the MCAA, as of the respective date as determined by the Royal Decree of June 14, 2017. The Royal Decree provides that (i) for a first list of 18 countries, the mandatory exchange of information applies as of income year 2016 (first information exchange in 2017) and for a second list of 44 countries, the mandatory automatic exchange of information applies as of income year 2017 (first information exchange in 2018), (iii) as from 2019 (for the 2018 financial year) for another single jurisdiction and (iv) as from 2020 (for the 2019 financial year) for a third list of 6 jurisdictions.
Investors who are in any doubt as to their position should consult their professional advisers
The proposed financial transactions tax
On February 14, 2013, the European Commission published a proposal, or the “Commission’s Proposal, for a Directive for a common financial transaction tax, or FTT, to be levied on transactions in financial instruments by financial institutions if at least one of the parties to the transaction is located in the ‘FTT- zone’ as defined in the Commission’s Proposal. It was approved by the European Parliament in July 2013. Originally, the adopted Commission’s Proposal foresaw the financial transaction tax for 11 “Participating Member States” (Belgium, Germany, Estonia, Greece, Spain, France, Italy, Austria, Portugal, Slovenia and Slovakia). However, on March 16, 2016 Estonia formally withdrew from the group of states willing to introduce the FTT. The actual implementation date of the FTT would depend on the future approval of the European Council and consultation of other EU institutions, and the subsequent transposition into local law.
147
If the financial transaction tax is introduced, under current published proposals financial institutions and certain other parties would be required to pay tax on transactions in financial instruments with parties (including, with respect to the EU-wide proposal, its affiliates) located in the FTT-zone. The proposed FTT has very broad scope and could, if introduced in its current form, apply to certain dealings in the ordinary shares in certain circumstances. It is a tax on derivatives transactions (such as hedging activities) as well as on securities transactions, i.e. it applies to trading in instruments such as shares and bonds. The initial issue of instruments such as shares and bonds is exempt from financial transaction tax in the current Commission’s Proposal. This means that the issuance and subscription of the ordinary shares should not become subject to financial transaction tax. Under current proposals the FTT could apply in certain circumstances to persons both within and outside of the participating Member States. Generally, it would apply to certain dealings in the ordinary shares where at least one party is a financial institution, and at least one party is established in a participating Member State. A financial institution may be, or be deemed to be, “established” in a participating Member State in a broad range of circumstances, including (a) by transacting with a person established in a participating Member State or (b) where the financial instrument which is subject to the dealings is issued in a participating Member State.
In 2019, Finance Ministers of the Member States participating in the enhanced cooperation indicated that they were discussing a new FTT proposal based on the French model of the tax and the possible mutualisation of the tax as a contribution to the EU budget.
According to the latest draft of this new FTT proposal (submitted by the German government), the FTT would be levied at a rate of at least 0.2 per cent. of the consideration for the acquisition of ownership of shares (including ordinary and any preference shares) admitted to trading on a trading venue or a similar third country venue, or of other securities equivalent to such shares, or Financial Instruments, or similar transactions (e.g. an acquisition of Financial Instruments by means of an exchange of Financial Instruments or by means of a physical settlement of a derivative). The FTT would be payable to the Participating Member State in whose territory the issuer of a Financial Instrument has established its registered office. According to the latest draft of the new FTT proposal, the FTT would not apply to straight notes. Like the Commission’s Proposal, the latest draft of the new FTT proposal also stipulates that once the FTT enters into force, the Participating Member States shall not maintain or introduce taxes on financial transactions other than the FTT (or VAT as provided in the Council Directive 2006/112/EC of November 28, 2006 on the common system of value added tax).
As a consequence, Belgium should abolish the tax on stock exchange transactions once the FTT enters into force. However, the FTT Commission’s Proposal remains subject to negotiation between the participating Member States. Further, its legality is at present uncertain. It may therefore be altered prior to any implementation, the timing of which remains unclear. Additional EU Member States may decide to participate. Prospective Noteholders are advised to seek their own professional advice in relation to the FTT.
F.Dividends and Paying Agents
Not Applicable.
G.Statement by Experts
Not Applicable.
H.Documents on Display
We are subject to the informational requirements of the Exchange Act. Accordingly, we are required to file reports and other information with the SEC, including annual reports on Form 20-F and reports on Form 6-K. You may inspect and copy reports and other information filed with the SEC at the Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-732-0330. In addition, the SEC maintains an Internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
We also make available on our website, free of charge, our Annual Report and the text of our reports on Form 6-K, including any amendments to these reports, as well as certain other SEC filings, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. Our website address is www.nyxoah.com. The information contained on our website is not incorporated by reference in this Annual Report.
148
I.Subsidiary Information
Not Applicable.
Item 11.Quantitative and Qualitative Disclosures About Market Risk
Market risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate because of changes in market prices. Our activities may expose us to changes in foreign currency exchange rates and interest rates. We are not exposed to any equity price risk or commodity price risk as we do not invest in these classes of investments.
Credit Risk
The credit risk arises mainly from trade receivables, cash and cash equivalents and deposits with banks and financial institutions. We only work with international reputable commercial banks and financial institutions.
Furthermore, we are not exposed to any material credit risk as other receivables are mainly due by the governments in Australia and the Walloon Region and there is no risk associated to this receivable.
Foreign Exchange Risk
We are minimally exposed to currency risk on a limited number of expenses that are denominated in currencies other than the functional currency of our subsidiaries, primarily the U.S. dollar, or USD, Israeli new shekel, or NIS, or Australian dollars, or AUD.
Additionally, earnings variability arises from the translation of monetary assets and liabilities denominated in currencies other than the functional currency of subsidiaries at the rate of exchange at each closing date, the impact of which is reported as a foreign exchange gain or loss in the consolidated statements of comprehensive income. Most foreign exchange transactions were denominated in USD, NIS, or AUD for the subsidiaries that have functional currency in Euro. For the years ended December 31, 2023 and 2022, if the USD strengthened by 5% against the Euro with all other variables held constant, net loss for the year then ended would have been with zero impact and €54,000 lower, respectively. For the years ended December 31, 2023 and 2022, if the USD weakened by 5% against the Euro with all other variables held constant, net loss for the year then ended would have been with zero impact and €60,000 higher, respectively. For the years ended December 31, 2023 and 2022, if the NIS strengthened by 5% against the Euro with all other variables held constant, net loss for the years then ended would have been €29,000 lower and €122,000 lower, respectively. For the years ended December 31, 2023 and 2022, if the NIS weakened by 5% against the Euro with all other variables held constant, net loss for the years then ended would have been €29,000 higher and €79,000 higher, respectively. For the years ended December 31, 2023 and 2022, if the AUD strengthened by 5% against the Euro with all other variables held constant, net loss for the years then ended would have been €33,000 lower and €73,000 lower, respectively. For the years ended December 31, 2023 and 2022, if the AUD weakened by 5% against the Euro with all other variables held constant, net loss for the years then ended would have been €36,000 higher and €80,000 higher, respectively.
We do not generally enter into arrangements to hedge our currency risk exposure.
149
Item 12.Description of Securities Other than Equity Securities
A.Debt Securities
Not Applicable.
B.Warrants and Rights
Not Applicable.
C.Other Securities
Not Applicable.
D.American Depositary Shares
Not Applicable.
150
PART II
Item 13.Defaults, Dividend Arrearages and Delinquencies
None.
Item 14.Material Modifications to the Rights of Security Holders and Use of Proceeds
Initial Public Offering
In July 2021, we raised gross proceeds of $97.8 million in our initial public offering of 3,260,250 ordinary shares on The Nasdaq Global Market, which includes 425,250 ordinary shares issued in connection with the exercise of the underwriters’ option to purchase additional shares, at a price of $30.00 per ordinary share.
The net proceeds to us, after deducting underwriting discounts and commissions and offering expenses, were approximately $91.9 million. The offering closed on July 7, 2021, and the closing of the exercise of the underwriters’ option to purchase additional shares occurred on July 9, 2021. Piper Sandler, Stifel and Cantor acted as joint book-running managers for the offering. Degroof Petercam acted as a co-manager.
The net proceeds from our initial public offering on The Nasdaq Global Market have been used, and are expected to continue to be used, as described in the final prospectus for the global offering filed with the U.S. Securities and Exchange Commission on July 6, 2021. None of the net proceeds of our global offering were paid directly or indirectly to any director, officer, general partner of ours or to their associates, persons owning 10% or more of any class of our equity securities, or to any of our affiliates.
Item 15.Controls and Procedures
A.Disclosure Controls and Procedures
We maintain disclosure controls and procedures as such term is defined in Rules 13(a)-15(e) and 15(d)-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. Disclosure controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives.
Our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer) have carried out an evaluation of the effectiveness of our disclosure controls and procedures as of December 31, 2023. Based on that evaluation, our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer) concluded that our disclosure controls and procedures were not effective as of December 31, 2023 due to the fact that the material weaknesses described below under “Management’s Annual Report on Internal Control over Financial Reporting” continued to exist at December 31, 2023, as discussed below.
B.Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Exchange Act. Our internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS as issued by the IASB. We have a program for the review of our internal control over financial reporting to ensure compliance with the requirements of the Exchange Act and Section 404 of the Sarbanes-Oxley Act. Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
151
As defined in the standards established by the PCAOB, a “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
Our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of the internal control over financial reporting as of December 31, 2023 using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control – Integrated Framework (2013). Based on the evaluation performed, management concluded that the material weaknesses reported in previous years still exists as of December 31, 2023 and are related to
| ● | Insufficient accounting and supervisory personnel who have the appropriate level of technical accounting experience and training, as well as a lack of sufficient oversight of external advisors; |
| ● | Lack of a comprehensive risk based control assessment to establish an effective structure of internal controls, which leads to insufficient procedures and controls, including IT General Controls, to ensure that accurate financial statements can be prepared and reviewed on a timely basis for annual reporting purposes. |
To address the material weaknesses identified, our management has taken, and continues to take, several remedial actions. In 2023 we implemented a new ERP system that went live on January 1, 2024. We have designed and validated a set of basic ITGC controls in 2023 and will implement the full ITGC scope in the new ERP system in 20024. We continue to engage an external professional advisor that is evaluating and validating the design effectiveness of our internal control framework and, based on the outcomes of such evaluation, we are continuing to adapt our internal controls.
While our management has taken, and continues to take, steps to remediate the material weaknesses, the material weaknesses have not yet been resolved as of December 31, 2023, and remediation is ongoing as described above.
The process of designing and implementing an effective financial reporting system is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a financial reporting system that is adequate to satisfy our reporting obligations. Our failure to correct these material weaknesses or our failure to discover and address any other control deficiencies could result in inaccuracies in our financial statements and could also impair our ability to comply with applicable financial reporting requirements and make related regulatory filings on a timely basis. As a result, our business, financial condition, results of operation and prospects, as well as the trading price of our ordinary shares, may be materially and adversely affected. See “Risk Factors— In connection with our preparation and the audit of our consolidated financial statements as of and for the year ended December 31, 2023, we identified material weaknesses in our internal control over financial reporting. Additionally, we may identify additional material weaknesses in the future that may cause us to fail to meet our reporting obligations or result in material misstatements in our financial statements. If we fail to remediate our material weaknesses, we may not be able to report our financial results accurately or to prevent fraud.”
As a result of the material weaknesses described above that have not yet been resolved as of December 31, 2023, we have concluded that our internal control over financial reporting was not effective as of December 31, 2023.
Notwithstanding these material weaknesses and management’s assessment that internal control over financial reporting was ineffective as of December 31, 2023, our management, including our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer), believes that the consolidated financial statements contained in this Annual Report present fairly, in all material respects, our financial position, results of operations and cash flows for the periods presented in conformity with IFRS.
C.Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include a report from our Registered Public Accounting Firm regarding internal control over financial reporting of due to our Emerging Growth Company status and due to the transition period established by rules of the SEC for newly public companies.
152
D.Changes in Internal Control Over Financial Reporting
The following changes in our internal control over financial reporting occurred during the period covered by this Annual Report that have materially affected our internal control over financial reporting:
| ● | We hired additional personnel with sufficient experience with U.S. listed companies and knowledge of the Sarbanes-Oxley Act, IFRS, SEC and PCAOB requirements to meet our needs and further bolstered existing staffs’ technical knowledge and expertise; and |
| ● | We performed a gap analysis and updated the design of our internal control framework and continue to have the control design effectiveness evaluated and validated by a third party. |
These actions were taken based on the following material weaknesses previously identified by Management as of December 31, 2022: (i) insufficient procedures and controls, including IT General Controls, to ensure that accurate financial statements can be prepared and reviewed on a timely basis for annual reporting purposes and; (ii) insufficient accounting and supervisory personnel with the appropriate level of technical accounting experience and training.
As of December 31, 2023, Management concluded that the material weaknesses reported in previous years still exists as of December 31, 2023 and are related to
| ● | Insufficient accounting and supervisory personnel who have the appropriate level of technical accounting experience and training, as well as a lack of sufficient oversight of external advisors; |
| ● | Lack of a comprehensive risk based control assessment to establish an effective structure of internal controls, which leads to insufficient procedures and controls, including IT General Controls, to ensure that accurate financial statements can be prepared and reviewed on a timely basis for annual reporting purposes. |
Item 16A.Audit Committee Financial Expert
Our Audit Committee is comprised of three of our non-executive directors, Kevin Rakin, Jürgen Hambrecht and Wildman Ventures LLC, as represented by Daniel Wildman, and each of these members is an “independent director” as such term is defined in Rule 10A-3 under the Exchange Act and under the listing standards of Nasdaq. Mr. Rakin serves as chair of this committee. Our Board has determined that Mr. Rakin is an “audit committee financial expert” as defined in Item 16A of Form 20-F.
Item 16B.Code of Ethics
Our Corporate Code of Conduct and Ethics and Whistleblower Policy is applicable to all of our employees, officers and directors and is available on our website at http://www.nyxoah.com. We expect that any amendment to this code, or any waivers of its requirements, will be disclosed on our website. Information contained on, or that can be accessed through, our website is not incorporated by reference into this Annual Report, and you should not consider information on our website to be part of this Annual Report.
153
Item 16C.Principal Accountant Fees and Services
Our financial statements have been prepared in accordance with IFRS and are audited by EY Reviseurs d’Entreprises / EY Bedrijfsrevisoren SRL/BV,
EY Reviseurs d’Entreprises / EY Bedrijfsrevisoren SRL/BV has served as our independent registered public accountant for each of the years ended December 31, 2021, December 31, 2022 and December 31, 2023 for which audited statements appear in this Annual Report.
The following table shows the aggregate fees billed to us, including some of our subsidiaries, for services rendered by EY Reviseurs d’Entreprises / EY Bedrijfsrevisoren SRL/BV.
Year ended December 31, | ||||||
2023 | 2022 | |||||
(in thousands) | ||||||
Audit Fees |
| € | 433 |
| € | 567.0 |
Audit-Related Fees (1) |
| 80 |
| 45.0 | ||
Tax Fees(2) |
| 20 |
| 20.6 | ||
All Other Fees (3) |
| 13 |
| 18.0 | ||
Total | € | 546 | € | 650.6 | ||
(1) | Audit-Related Fees are primarily for services related to SEC filings, including comfort letters, consents and comment letters. |
(2) | Tax Fees are the aggregate fees billed for professional services rendered by the principal accountant for tax compliance, tax advice and tax planning related services. |
(3) | All Other Fees please describe the nature of the products and/or services provided by the principal accountant, other than the serves reported in the footnotes above. |
Our Audit Committee reviews and pre-approves the scope and the cost of audit services related to us and permissible non-audit services performed by the independent auditors, other than those for de minimis services which are approved by the Audit Committee prior to the completion of the audit. All of the services (100%) related to our company provided by EY Reviseurs d’Entreprises / EY Bedrijfsrevisoren SRL/BV during the last two fiscal years have been approved by the Audit Committee in accordance with Regulation S-X, Rule 2-01, paragraph (c)(7)(i).
154
Item 16D.Exemptions From the Listing Standards For Audit Committees
Not Applicable.
Item 16E.Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not Applicable.
Item 16F.Change in the Registrant’s Certifying Accountant
Not Applicable.
Item 16G.Corporate Governance
The listing rules of the Nasdaq Stock Market include certain accommodations in the corporate governance requirements that allow foreign private issuers, to follow “home country” corporate governance practices in lieu of the otherwise applicable corporate governance standards of the Nasdaq Stock Market. The application of such exceptions requires that we disclose each noncompliance with the Nasdaq Stock Market listing rules that we do not follow and describe the Belgian corporate governance practices we do follow in lieu of the relevant Nasdaq Stock Market corporate governance standard.
We intend to continue to follow Belgian corporate governance practices in lieu of the corporate governance requirements of the Nasdaq Stock Market in respect of the following:
| ● | Quorum at Shareholder Meetings. Nasdaq Stock Market Listing Rule 5620(c) requires that for any meeting of shareholders, the quorum must be no less than 33.33% of the outstanding ordinary shares. There is no general quorum requirement under Belgian law for ordinary meetings of shareholders, except in relation to decisions regarding certain matters. See “Description of Share Capital and Articles of Association — Description of the Rights and Benefits Attached to Our Shares — Right to Attend and Vote at Our Meetings of Shareholders — Quorum and Majority Requirements”. |
| ● | Compensation Committee and Nomination Committee. Nasdaq Stock Market Listing Rule 5605(d)(2) requires that compensation of officers must be determined by, or recommended to, the board of directors for determination, either by a majority of the independent directors, or a compensation committee comprised solely of independent directors. Nasdaq Stock Market Listing Rule 5605(e) requires that director nominees be selected, or recommended for selection, either by a majority of the independent directors or a nominations committee comprised solely of independent directors. Under Belgian law, we are not subject to any such requirements. In particular, we are not required by Belgian law to set up any compensation or nominations committees within our board of directors, and are therefore not subject to any Belgian legal requirements as to the composition of such committees either. However, our articles of association provide that our board of directors may form committees from among its members. Our board of directors has set up and appointed a nomination committee and a remuneration committee. Pursuant to article 7:100 of the Belgian CCA, only a majority of the members of the remuneration committee should in principle meet the independence criteria referred to in article 7:87 of the Belgian CCA and set out in provision 3.5 of the Belgian Code on Corporate Governance. Pursuant to provision 4.19 of the Belgian Code on Corporate Governance, only a majority of the members of the remuneration committee must qualify as independent. |
| ● | Independent Director Majority. Nasdaq Stock Market Listing Rules 5605(b)(1) and (2) require that a majority of the board of directors must be comprised of independent directors and that independent directors must have regularly scheduled meetings at which only independent directors are present. We are not required under Belgian law to have a majority of independent directors on our board of directors. However, our articles of association provide that our board of directors must be comprised of at least three directors, of which, pursuant to our corporate governance charter and provision 3.4 of the Belgian Code on Corporate Governance, at least three directors must be independent directors under Belgian law. We do not intend to require our independent directors to meet separately from the full board of directors on a regular basis or at all. |
| ● | Executive Session. NASDAQ Stock Market Listing Rule 5605(b)(2) requires that independent directors must have regularly scheduled meetings at which only independent directors are present. We do not intend to require our independent directors to meet separately from the full board of directors on a regular basis or at all, although the board of directors is supportive of its independent members voluntarily arranging to meet separately from the other members of our board of directors when and if they wish to do so. |
155
| ● | Charters. NASDAQ Stock Market Listing Rules 5605(c)(1), (d)(1) and (e)(2) require that each committee of the board of directors must have a formal written charter. Pursuant to the Belgian Corporate Governance Code, our board of directors has drawn up a corporate governance charter including, amongst others, the internal rules of our committees. |
Item 16H.Mine Safety Disclosure
Not Applicable.
Item 16I.Disclosure Regarding Foreign Jurisdictions That Prevent Inspections
Not Applicable.
Item 16J.Insider Trading Policy
Not applicable.
Item 16K.Cybersecurity
We recognize the critical importance of maintaining the trust and confidence of customers, patients, business partners and employees toward our business and are committed to protecting the confidentiality, integrity and availability of our business operations and systems. Our board of directors is involved in oversight of our risk management activities, and cybersecurity represents an important element of our overall approach to risk management. Our cybersecurity policies, standards, processes and practices are based on recognized frameworks established by the National Institute of Standards and Technology, or NIST, and other applicable industry standards. In general, we seek to address cybersecurity risks through a comprehensive, cross-functional approach that is focused on preserving the confidentiality, security and availability of the information that we collect and store by identifying, preventing and mitigating cybersecurity threats and effectively responding to cybersecurity incidents when they occur.
Cybersecurity Risk Management and Strategy; Effect of Risk
We face risks related to cybersecurity such as unauthorized access, cybersecurity attacks and other security incidents, including as perpetrated by hackers and unintentional damage or disruption to hardware and software systems, loss of data, and misappropriation of confidential information. To identify and assess material risks from cybersecurity threats, we maintain a comprehensive cybersecurity program to ensure our systems are effective and prepared for information security risks, including regular oversight of our programs for security monitoring for internal and external threats to ensure the confidentiality and integrity of our information assets. We consider risks from cybersecurity threats alongside other company risks as part of our overall risk assessment process. We employ a range of tools and services, including regular network and endpoint monitoring, audits, vulnerability assessments and threat modeling to inform our risk identification and assessment. As discussed in more detail under “Cybersecurity Governance” below, our board of directors provides oversight of our cybersecurity risk management and strategy processes, which are led by our Chief Financial Officer.
We also identify our cybersecurity threat risks by comparing our processes to standards set by the National Institute of Standards and Technology, or NIST. To provide for the availability of critical data and systems, maintain regulatory compliance, manage our material risks from cybersecurity threats, and protect against and respond to cybersecurity incidents, we undertake the following activities:
| ● | monitor emerging data protection laws and implement changes to our processes that are designed to comply with such laws; |
| ● | through our policies, practices and contracts (as applicable), require employees, as well as third parties that provide services on our behalf, to treat confidential information and data with care; |
| ● | employ technical safeguards that are designed to protect our information systems from cybersecurity threats, including firewalls, intrusion prevention and detection systems, anti-malware functionality and access controls, which are evaluated and improved through vulnerability assessments and cybersecurity threat intelligence; |
156
| ● | provide regular, mandatory training for our employees and contractors regarding cybersecurity threats as a means to equip them with effective tools to address cybersecurity threats, and to communicate our evolving information security policies, standards, processes and practices; |
| ● | conduct cybersecurity management and incident training for employees involved in our systems and processes that handle sensitive data; |
| ● | leverage the NIST incident handling framework to help us identify, protect, detect, respond and recover when there is an actual or potential cybersecurity incident; and |
| ● | carry information security risk insurance that provides protection against the potential losses arising from a cybersecurity incident; and |
Our incident response plan coordinates the activities we take to prepare for, detect, respond to and recover from cybersecurity incidents, which include processes to triage, assess severity for, escalate, contain, investigate and remediate the incident, as well as to comply with potentially applicable legal obligations and mitigate damage to our business and reputation.
Our processes also address cybersecurity threat risks associated with our use of third-party service providers, including our suppliers and manufacturers or who have access to patient and employee data or our systems. In addition, cybersecurity considerations affect the selection and oversight of our third-party service providers. We perform diligence on third parties that have access to our systems, data or facilities that house such systems or data, and continually monitor cybersecurity threat risks identified through such diligence. Additionally, we generally require those third parties that could introduce significant cybersecurity risk to us to agree by contract to manage their cybersecurity risks in specified ways, and to agree to be subject to cybersecurity audits, which we conduct as appropriate.
We describe whether and how risks from identified cybersecurity threats, including as a result of any previous cybersecurity incidents, have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition, under the heading “We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology or loss of data, including any cyber security incidents, could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability which could harm our ability to operate our business effectively and adversely affect our business and reputation,” which disclosures are incorporated by reference herein.
We have not experienced any material cybersecurity incidents and the expenses we have incurred from cybersecurity incidents were immaterial.
Cybersecurity Governance; Management
Cybersecurity is an important part of our risk management processes and an area of focus for our board of directors and management. Our board of directors, as assisted by our science and technology committee, is responsible for the oversight of risks from cybersecurity threats.
Our board of directors and science and technology committee receive updates from management of our cybersecurity threat risk management and strategy processes covering topics such as data security posture, progress towards pre-determined risk-mitigation-related goals, and material cybersecurity threat risks or incidents and developments, as well as the steps management has taken to respond to such risks. Our board of directors and science and technology committee also receive prompt and timely information regarding any material cybersecurity incident, as well as ongoing updates regarding any such incident until it has been addressed.
Our cybersecurity risk management and strategy processes, which are discussed in greater detail above, are led by our Chief Financial Officer. Our Chief Financial Officer is informed about and monitors the prevention, mitigation, detection, and remediation of cybersecurity incidents through his management of, and participation in, the cybersecurity risk management and strategy processes described above. As discussed above, our Chief Financial Officer reports to our board of directors and science and technology committee about cybersecurity threat risks, among other cybersecurity related matters.
157
PART III
Item 17. | Financial Statements |
We have elected to provide financial statements pursuant to Item 18.
Item 18.Financial Statements
The financial statements are filed as part of this Annual Report beginning on page F-1.
Item 19.Exhibits
Exhibit |
| Description |
| Schedule/ Form |
| File Number |
| Exhibit |
| File Date |
|
|
|
|
|
|
|
|
|
|
|
1.1* |
|
| ||||||||
|
|
| ||||||||
2.1* |
| Articles of Association of Nyxoah SA (English Translation) (included in Exhibit 1.1) |
| |||||||
|
|
| ||||||||
2.2* | ||||||||||
4.1+ |
| Form F-1 | 333-257000 | 10.1 |
| 06/10/2021 | ||||
|
|
| ||||||||
4.2+ |
| Form F-1 | 333-257000 | 10.2 |
| 06/10/2021 | ||||
|
|
| ||||||||
4.3+ |
| Form F-1 | 333-257000 | 10.3 |
| 06/10/2021 | ||||
|
|
| ||||||||
4.4# |
| Form F-1 | 333-257000 | 10.4 |
| 06/10/2021 | ||||
|
|
| ||||||||
4.5# |
| Form F-1 | 333-257000 | 10.5 |
| 06/10/2021 | ||||
|
|
| ||||||||
4.6# |
| Form F-1 | 333-257000 | 10.6 |
| 06/10/2021 | ||||
|
|
| ||||||||
4.7# |
| Form F-1 | 333-257000 | 10.7 |
| 06/10/2021 | ||||
4.8# | Form S-8 | 333-261233 | 99.5 | 11/19/2021 | ||||||
4.9# | Form S-8 | 333-269410 | 99.1 | 01/25/2023 | ||||||
|
|
|
158
4.10# | Sales Agreement, dated as of December 22, 2022, by and between Nyxoah SA and Cantor Fitzgerald & Co. | Form S-3 | 333-268955 | 1.2 | 12/22/2022 | |||||
4.11* | ||||||||||
4.12*+ | ||||||||||
8.1* |
|
| ||||||||
|
|
| ||||||||
12.1* |
|
| ||||||||
|
|
| ||||||||
12.2* |
|
| ||||||||
|
|
| ||||||||
13.1** |
|
| ||||||||
|
|
| ||||||||
13.2** |
|
| ||||||||
|
|
| ||||||||
15.1* |
|
| ||||||||
97.1* | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
101.INS XBRL Instance Document |
|
|
|
|
|
|
|
| ||
101.SCH XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
| ||
101.CAL XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
|
| ||
101.DEF XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
| ||
101.LAB XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
|
|
| ||
101.PRE XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
|
|
| ||
104 Cover Page Interactive Data File (embedded within the Inline XBRL document) | ||||||||||
* | Filed herewith. |
** | Furnished herewith. |
# | Indicates management contract or compensatory plan. |
+ | Confidential treatment previously requested and granted as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission. |
159
Signature
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
NYXOAH SA | ||
By: | /s/ Olivier Taelman | |
Name: Olivier Taelman | ||
Title: Chief Executive Officer | ||
Date: March 20, 2024
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Nyxoah S.A.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Nyxoah SA (the Company) as of December 31, 2023 and 2022, the related consolidated statements of loss and other comprehensive loss, changes in equity, and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 5.1 to the financial statements, the Company has suffered recurring losses from operations, sustained negative cash flows since its inception and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 5.1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ EY Réviseurs d’Entreprises / EY Bedrijfsrevisoren SRL/BV
We have served as the Company’s auditors since 2016
Diegem, Belgium
March 20, 2024
F-2
NYXOAH SA
CONSOLIDATED BALANCE SHEETS
(in thousands)
As at December 31 | ||||||||
| Notes |
| 2023 |
| 2022 | |||
ASSETS | ||||||||
Non-current assets |
|
|
|
|
|
| ||
Property, plant and equipment |
| 7 |
| € | |
| € | |
Intangible assets |
| 8 |
| |
| | ||
Right of use assets |
| 9 |
| |
| | ||
Deferred tax asset |
| 29 |
| |
| | ||
Other long-term receivables |
| 10 |
| |
| | ||
|
|
| € | |
| € | | |
Current assets |
|
|
|
|
|
| ||
Inventory |
| 11 |
| |
| | ||
Trade receivables |
| 12 |
| |
| | ||
Other receivables |
| 12 |
| |
| | ||
Other current assets |
|
| |
| | |||
Financial assets |
| 14 |
| |
| | ||
Cash and cash equivalents |
| 13 |
| |
| | ||
|
|
| € | |
| € | | |
Total assets |
|
|
| € | |
| € | |
|
|
|
|
|
| |||
EQUITY AND LIABILITIES |
|
|
|
|
|
| ||
Capital and reserves |
|
|
|
|
|
| ||
Capital |
| 15 |
| |
| | ||
Share premium |
| 15 |
| |
| | ||
Share based payment reserve |
| 16 |
| |
| | ||
Other comprehensive income |
| 15 |
| |
| | ||
Retained loss |
|
|
| ( |
| ( | ||
Total equity attributable to shareholders |
|
|
| € | |
| € | |
|
|
|
|
| ||||
LIABILITIES |
|
|
|
|
| |||
Non-current liabilities |
|
|
|
|
| |||
Financial debt |
| 17 |
| |
| | ||
Lease liability |
| 9 |
| |
| | ||
Pension liability |
| 26 |
| |
| | ||
Provisions |
|
|
| |
| | ||
Deferred tax liability |
| 29 |
| |
| | ||
|
|
| € | |
| € | | |
Current liabilities |
|
|
|
|
| |||
Financial debt |
| 17 |
| |
| | ||
Lease liability |
| 9 |
| |
| | ||
Trade payables |
| 18 |
| |
| | ||
Current tax liability |
| 29 |
| |
| | ||
Other payables |
| 19 |
| |
| | ||
|
|
| € | |
| € | | |
Total liabilities |
|
|
| € | |
| € | |
Total equity and liabilities |
|
|
| € | |
| € | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
NYXOAH SA
CONSOLIDATED STATEMENTS OF LOSS AND OTHER COMPREHENSIVE LOSS
(in thousands)
For the year ended December 31 | |||||||||||
| Notes |
| 2023 |
| 2022 |
| 2021 | ||||
Revenue | 20 | € | | € | | € | | ||||
Cost of goods sold |
| 20 | ( |
| ( |
| ( | ||||
Gross profit |
| € | | € | | € | | ||||
Research and Development Expense |
| 22 |
| ( |
| ( |
| ( | |||
Selling, General and Administrative Expense |
| 23 |
| ( |
| ( |
| ( | |||
Other income/(expense) |
| 24 |
| |
| |
| | |||
Operating loss for the period |
| ( | ( | ( | |||||||
Financial income |
| 27 |
| |
| |
| | |||
Financial expense |
| 28 |
| ( |
| ( |
| ( | |||
Loss for the period before taxes |
| ( | ( | ( | |||||||
Income taxes |
| 29 |
| |
| ( |
| ( | |||
Loss for the period |
| ( | ( | ( | |||||||
|
|
|
| ||||||||
Loss attributable to equity holders |
| ( | ( | ( | |||||||
|
|
|
| ||||||||
Other comprehensive income/(loss) |
|
|
|
| |||||||
Items that may not be subsequently reclassified to profit or loss (net of tax) |
|
|
|
| |||||||
Remeasurements of post-employment benefit obligations, net of tax |
| 26 | | | ( | ||||||
Items that may be subsequently reclassified to profit or loss (net of tax) |
| ||||||||||
Currency translation differences |
|
| ( |
| ( |
| | ||||
Total other comprehensive income/(loss) |
| € | ( | € | ( | € | | ||||
Total comprehensive loss for the year, net of tax |
| € | ( | € | ( | € | ( | ||||
Loss attributable to equity holders | € | ( | € | ( | € | ( | |||||
Basic loss per share (in EUR) | 30 | € | ( | € | ( | € | ( | ||||
Diluted loss per share (in EUR) | 30 | € | ( | € | ( | € | ( | ||||
The accompanying notes are an integral part of these consolidated financial statements
F-4
NYXOAH SA
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(in thousands)
Attributable to owners of the parent | ||||||||||||||||||||
Share | ||||||||||||||||||||
based | Other | |||||||||||||||||||
Common | Share | payment | comprehensive | Retained | ||||||||||||||||
| Notes |
| shares |
| premium |
| reserve |
| income |
| loss |
| Total | |||||||
Balance at January 1, 2021 |
| € | | € | | € | | € | | € | ( | € | | |||||||
Loss for the period |
|
| — |
| — |
| — |
| — |
| ( |
| ( | |||||||
Other comprehensive income for the period |
|
| — |
| — |
| — |
| |
| — |
| | |||||||
Total comprehensive income/(loss) for the period |
|
| — |
| — |
| — | € | | € | ( | € | ( | |||||||
Equity-settled share-based payments |
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
Granted during the period |
|
|
| — |
| — |
| |
| — |
| — |
| | ||||||
Exercised during the period |
|
|
| |
| |
| ( |
| — |
| |
| | ||||||
Issuance of shares for cash |
|
|
| |
| |
| — |
| — |
| — |
| | ||||||
Transaction cost | — | ( | — | — | — | ( | ||||||||||||||
Total transactions with owners of the company recognized directly in equity |
|
| |
| |
| |
| — |
| |
| | |||||||
Balance at December 31, 2021 |
| € | | € | | € | | € | | € | ( | € | | |||||||
Attributable to owners of the parent | ||||||||||||||||||||
Share | ||||||||||||||||||||
based | Other | |||||||||||||||||||
Common | Share | payment | comprehensive | Retained | ||||||||||||||||
| Notes |
| shares |
| premium |
| reserve |
| income |
| loss |
| Total | |||||||
Balance at January 1, 2022 | € | | € | | € | | € | | € | ( | € | | ||||||||
Loss for the period |
| — |
| — |
| — |
| — |
| ( |
| ( | ||||||||
Other comprehensive loss for the period |
| — |
| — |
| — |
| ( |
| — |
| ( | ||||||||
Total comprehensive loss for the period |
| — |
| — |
| — | € | ( | € | ( | € | ( | ||||||||
Equity-settled share-based payments |
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Granted during the period | 16 |
| — |
| — |
| |
| — |
| — |
| | |||||||
Exercised during the period | 15 |
| |
| |
| ( |
| — |
| |
| | |||||||
Issuance of shares for cash | 15 |
| |
| — |
| — |
| — |
| — |
| | |||||||
Total transactions with owners of the company recognized directly in equity |
| |
| |
| |
| — |
| |
| | ||||||||
Balance at December 31, 2022 | € | | € | | € | | € | | € | ( | € | | ||||||||
F-5
Attributable to owners of the parent | ||||||||||||||||||||
Share | ||||||||||||||||||||
based | Other | |||||||||||||||||||
Common | Share | payment | comprehensive | Retained | ||||||||||||||||
| Notes |
| shares |
| premium |
| reserve |
| income |
| loss |
| Total | |||||||
Balance at January 1, 2023 | € | | € | | € | | € | | € | ( | € | | ||||||||
Loss for the period |
| — |
| — |
| — |
| — |
| ( |
| ( | ||||||||
Other comprehensive income for the period |
| — |
| — |
| — |
| ( |
| — |
| ( | ||||||||
Total comprehensive loss for the period |
| — |
| — |
| — | € | ( | € | ( | € | ( | ||||||||
Equity-settled share-based payments |
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Granted during the period | 16 | — | — | | — | — | | |||||||||||||
Exercised during the period | 16 |
| |
| |
| ( |
| — |
| |
| | |||||||
Expired during the period | 16 | — | — | ( | — | | — | |||||||||||||
Transaction cost | 15 | — | ( | — | — | — | ( | |||||||||||||
Issuance of shares for cash | 15 | | | — | — | — | | |||||||||||||
Total transactions with owners of the company recognized directly in equity |
| |
| |
| |
| — |
| |
| | ||||||||
Balance at December 31, 2023 | € | | € | | € | | € | | € | ( | € | | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
NYXOAH SA
CONSOLIDATED STATEMENTS OF CASH FLOW
(in thousands)
For the year ended December 31 | |||||||||||
| Notes |
| 2023 |
| 2022 |
| 2021 | ||||
CASH FLOWS FROM OPERATING ACTIVITIES | |||||||||||
Loss before tax for the year |
|
| € | ( | € | ( | € | ( | |||
Adjustments for |
|
|
|
| |||||||
Finance income |
| 27 |
| ( |
| ( | ( | ||||
Finance expenses |
| 28 |
| |
| | | ||||
Depreciation and impairment of property, plant and equipment and right-of-use assets |
| 7,9 |
| |
| | | ||||
Amortization of intangible assets |
| 8 |
| |
| | | ||||
Share-based payment transaction expense |
| 16 |
| |
| | | ||||
Remeasurement of recoverable cash advances | 17 | ( | ( | ( | |||||||
Increase/(decrease) in provisions |
|
|
| |
| | ( | ||||
Other non-cash items |
|
|
| ( |
| ( | ( | ||||
Cash generated before changes in working capital |
|
| € | ( | € | ( | € | ( | |||
(Increase)/decrease in inventory |
|
| ( |
| ( | ( | |||||
(Increase)/decrease in trade and other receivables |
|
| ( |
| | ( | |||||
Increase/(decrease) in trade and other payables |
|
| |
| | | |||||
Cash generated from changes in operations |
| ( | ( | ( | |||||||
Interests received |
|
|
| |
| | | ||||
Income tax paid |
|
| ( | ( | ( | ||||||
Net cash generated from / (used in) operating activities |
|
| ( | ( | ( | ||||||
CASH FLOWS FROM INVESTING ACTIVITIES |
|
|
| ||||||||
Purchases of property, plant and equipment |
| 7 |
| ( |
| ( | ( | ||||
Capitalization of intangible assets | 8 | ( | ( | ( | |||||||
Purchase of financial assets - current |
|
| ( |
| ( | | |||||
Proceeds from sale of financial assets - current |
|
| | | | ||||||
Interest income on financial assets |
|
|
| |
| | | ||||
Net cash generated from / (used in) investing activities |
| € | | € | ( | € | ( | ||||
CASH FLOWS FROM FINANCING ACTIVITIES |
|
|
|
| |||||||
Payment of principal portion of lease liabilities |
| 9 |
| ( |
| ( | ( | ||||
Repayment of other loan |
| 17 |
| ( |
| ( | ( | ||||
Interests paid |
|
| ( |
| ( | ( | |||||
Repayment of recoverable cash advance |
| 17 |
| ( |
| ( | ( | ||||
Proceeds from issuance of shares, net of transaction costs |
| 15 | | | | ||||||
Other financial costs |
|
| ( |
| ( | ( | |||||
Net cash generated from / (used in) financing activities |
| | ( | | |||||||
Movement in cash and cash equivalents | | ( | | ||||||||
Effect of exchange rates on cash and cash equivalents | ( | | | ||||||||
Cash and cash equivalents at January 1 | 13 | | | | |||||||
Cash and cash equivalents at December 31 | 13 | € | | € | | € | | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
NYXOAH SA
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1.General information
Nyxoah SA (the “Company”) is a public listed company with limited liability (naamloze vennootschap/société anonyme) incorporated and operating under the laws of Belgium and is domiciled in Belgium. Nyxoah SA is registered with the legal entities register (Brabant Walloon) under enterprise number 0817.149.675. The Company’s registered office is in Rue Edouard Belin 12, 1435 Mont-Saint-Guibert, Belgium.
The Company is a medical technology company focused on the development and commercialization of innovative solutions to treat Obstructive Sleep Apnea, or OSA. Our lead solution is the Genio® system, a CE-Marked, patient-centric, minimally invasive, next generation hypoglossal neurostimulations therapy for OSA. OSA is the world’s most common sleep disordered breathing condition and is associated with increased mortality risk and comorbidities including cardiovascular diseases, depression and stroke.
The Genio® system is the first neurostimulation system for the treatment of OSA to include a battery-free and leadless neurostimulator capable of delivering bilateral hypoglossal nerve stimulation to keep the upper airway open. The product is intended to be used as a second-line therapy to treat moderate to severe OSA patients who have either not tolerated, failed or refused conventional therapy, including Continuous Positive Airway Pressure, or CPAP, which, despite its proven efficacy, is associated with many limitations, meaning compliance is a serious challenge. In addition, other second-line treatments are more suitable to treat mild to moderate OSA (such as oral devices) or highly invasive. Compared to other hypoglossal nerve stimulation technologies for the treatment of OSA, the Genio® system is a disruptive, differentiating technology that targets a clear unmet medical need thanks to its minimally invasive and quick implantation technique, its external battery and its ability to stimulate the two branches of the hypoglossal nerve.
Obstructive sleep apnea is the world’s most common sleep disordered breathing condition. OSA occurs when the throat and tongue muscles and soft tissues relax and collapse. It makes a person stop breathing during sleep, while the airway repeatedly becomes partially (hypopnea) or completely (apnea) blocked, limiting the amount of air that reaches the lungs. During an episode of apnea or hypopnea, the patient’s oxygen level drops, which leads to sleep interruptions.
Nyxoah SA has four wholly owned subsidiaries: Nyxoah Ltd, a subsidiary of the Company since October 21, 2009 (located in Israel and incorporated on January 10, 2008 under the name M.L.G. Madaf G. Ltd), Nyxoah Pty Ltd since February 1, 2017 (located in Australia), Nyxoah Inc. since May 14, 2020 (located in the USA) and Nyxoah GmbH since July 26, 2023 (located in Germany).
These consolidated financial statements have been authorized for issue on March 20, 2024 by the Board of Directors of the Company.
2.Material accounting policies
2.1.Basis of Preparation and Going Concern
Basis of Preparation
The Company’s consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (IASB) and as endorsed by the European Union.
The consolidated financial statements are presented in thousands of Euros (€) and all values are rounded to the nearest thousand, except when otherwise indicated (e.g. € million).
The preparation of the consolidated financial statements requires the use of certain critical accounting estimates. It also requires management to exercise its judgment in the process of applying the Company’s accounting policies. The areas involving a higher degree of judgement or complexity, are areas where assumptions and estimates are significant to the consolidated financial statements.
F-8
Going concern principle
The consolidated financial statements have been prepared on a going concern basis. Please refer to note 5.1 for the detailed explanation of the going concern.
The Company confirms that despite the conflict between Israel and Hamas, operations are continuing notably regarding R&D and production with no major impact and the assets are currently safeguarded. The Company is not suffering impact of this conflict.
2.2.New and amended standards and interpretations applicable
Effective for the annual periods beginning on January 1, 2023
The Company has not early adopted any standard, interpretation or amendment that has been issued but is not yet effective. Several amendments and interpretations apply for the first time in 2023, but do not have an impact on the consolidated financial statements of the Company:
| ₋ | IFRS 17 Insurance Contracts (applicable for annual periods beginning on or after January 1, 2023) |
| ₋ | Amendments to IFRS 17 Insurance contracts: Initial Application of IFRS 17 and IFRS 9 – Comparative Information (applicable for annual periods beginning on or after January 1, 2023) |
| ₋ | Amendments to IAS 8 Accounting policies, Changes in Accounting Estimates and Errors: Definition of Accounting Estimates (applicable for annual periods beginning on or after January 1, 2023) |
| ₋ | Amendments to IAS 12 Income Taxes: Deferred Tax related to Assets and Liabilities arising from a Single Transaction (applicable for annual periods beginning on or after January 1, 2023) |
| ₋ | Amendments to IAS 12 Income taxes: International Tax Reform – Pillar Two Model Rules (effective immediately – disclosures are required for annual periods beginning on or after 1 January 2023). The Company has adopted these amendments, however they are not yet applicable for the current reporting year as the Company’s consolidated revenue is currently below the threshold of € |
The following amendments have had an impact on the Company’s disclosures of accounting policies, but not on the measurement, recognition or presentation of any items in the Company’s financial statements.
| ₋ | Amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2: Disclosure of Accounting Policies (applicable for annual periods beginning on or after January 1, 2023) |
New standards not yet effective
The standards and interpretations that are issued, but not yet effective, up to the date of issuance of the Company’s financial statements are disclosed below. The Company intends to adopt these standards and interpretations, if applicable, when they become effective.
- | Amendments to IAS 1 Presentation of Financial Statements: Classification of Liabilities as Current or Non-current and Non-current Liabilities with Covenants (applicable for annual periods beginning on or after 1 January 2024). |
- | Amendments to IFRS 16 Leases: Lease Liability in a Sale and Leaseback (applicable for annual periods beginning on or after January 1, 2024). |
- | Amendments to IAS 7 Statement of Cash Flows and IFRS 7 Financial Instruments: Disclosures: Supplier Finance Arrangements (applicable for annual periods beginning on or after 1 January 2024, but not yet endorsed in the EU). |
- | Amendments to IAS 21 The Effects of Changes in Foreign Exchange Rates: Lack of Exchangeability (applicable for annual periods beginning on or after 1 January 2025, but not yet endorsed in the EU). |
F-9
None of the IFRS standards issued, but not yet effective are expected to have a material impact on the Company’s financials.
2.3.Basis of Consolidation
The consolidated financial statements comprise the financial statements of the Company and its subsidiaries as at December 31, 2023, 2022 and 2021.
Subsidiaries are all entities (including structured entities) over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. They are deconsolidated from the date control ceases.
Inter-company transactions, balances and unrealized gains on transactions between group companies are eliminated.
2.4.Foreign Currency Translations
The consolidated financial statements are presented in Euro, which is the Company’s functional and presentation currency. For each subsidiary, the Company determines the functional currency. Items included in the financial statements of each subsidiary are measured using that functional currency.
Transactions in foreign currencies are recorded at their respective foreign exchange rate prevailing at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are translated at the foreign exchange rates prevailing at the closing date. Exchange differences arising on the settlement of monetary items or on reporting monetary items at rates different from those at which they were initially recorded during the period or in previous periods, are recognized in the consolidated income statement. Non-monetary items that are measured in terms of historical cost in a foreign currency are translated using the exchange rates as at the date of the initial transactions.
On consolidation, the assets and liabilities of foreign operations are translated into euros at the rate of exchange prevailing at the reporting date and the income statement is translated at the average rate of the year. The exchange differences arising on the translation are recognized in other comprehensive income. On disposal of a foreign operation, the component of other comprehensive income relating to that particular foreign operation is recognized in the income statement.
2.5.Intangible Assets
Patents
Patents relate to direct attributable expenditure incurred for obtaining patent rights related to the Genio® system and are carried at costs less accumulated amortization and accumulated impairment losses. Patents costs are amortized as from January 2021 together with the related Genio® system capitalized development costs.
Research and Development Costs
Research costs are expensed as incurred. Development expenditures on an individual project are recognized as an intangible asset when the Company can demonstrate:
| ● | the technical feasibility of completing the intangible asset so that it will be available for use or sale; |
| ● | the intention to complete the intangible asset and use or sell it; |
| ● | the ability to use or sell the intangible asset; |
| ● | how the intangible asset will generate probable future economic benefits; |
F-10
| ● | the availability of adequate technical, financial and other resources to complete the development and to use or sell the intangible asset; and |
| ● | the ability to measure reliably the expenditure attributable to the intangible asset during its development. |
The Company started recognizing the development expenditure as an asset since March 2019 triggered by obtaining CE mark for the first generation of the Genio® system. As from July 2020, the Company started recognizing the development expenditure as an asset for the improved second generation of the Genio® system. The asset is carried at cost less any accumulated amortization and accumulated impairment losses. Development costs include employee compensation and outsourced development expenses. Amortization of the asset begins when development is complete and the asset is available for use. The asset is depreciated on a straight-line basis over the estimated useful life of
2.6.Property, Plant and Equipment
Property, plant and equipment are initially recorded in the statement of financial position at their acquisition cost, which includes the costs directly attributable to the acquisition and installation of the asset.
Property, plant and equipment are subsequently measured at their historical cost less accumulated depreciation and impairment, if any.
Property, plant and equipment are depreciated on a straight-line basis over their estimated useful life. The estimated useful life of each category of property, plant and equipment is as follows:
● IT equipment |
| |
● Furniture and office equipment | ||
● Laboratory equipment | ||
● Leasehold improvements | The shorter of lease term and |
Assets under construction are not depreciated until the date that the asset is available for use.
Property, plant and equipment are derecognized upon disposal or when no future economic benefits are expected from its use or disposal. Any gain or loss arising on derecognition of the asset, which is the difference between the net disposal proceeds and the carrying amount of the asset, is included in the income statement when the asset is derecognized.
The residual values, useful lives and methods of depreciation of property, plant and equipment are reviewed at each financial year end and adjusted prospectively, if appropriate.
2.7.Impairment of Intangible Assets and Property, Plant and Equipment
At each reporting date, the Company assesses whether there is an indication that property, plant and equipment and intangible assets with a definite useful life may be impaired. If an indication of impairment exists, or at least annually when impairment test is required in case of intangible assets with an indefinite useful life or intangible assets not yet for use, the Company estimates the asset’s recoverable amount. The recoverable amount of an asset is the higher of the assets or cash-generating units (CGU) fair value less costs to sell and its value in use.
The recoverable amount is determined based on the value in use of the individual asset or the CGU. In assessing value in use, the estimated future pre-tax cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
A previously recognized impairment loss is reversed only if there has been a change in the assumptions used to determine the asset’s recoverable amount since the last impairment loss was recognized. The reversal is limited so that the carrying amount of the asset does not exceed its recoverable amount, nor exceeds the carrying amount that would have been determined, net of depreciation, had no impairment loss has been recognized for the asset in prior years. Such reversal is recognized in the consolidated income statement.
F-11
2.8.Financial Assets
Financial assets include mainly other long-term receivables, trade receivables, other receivables, term accounts with an initial maturity longer than 3 months but less than 12 months and cash and cash equivalents, and are measured at amortized cost using the effective interest method, less impairment allowance. Interest income is recognized by applying the effective interest rate, except for short-term receivables when the effect of discounting is immaterial.
Derecognition
A financial asset is derecognized when the contractual rights to receive cash flows from the asset have expired or when the Company transferred its rights to receive cash flows and substantially all risks and rewards of ownership of the financial asset to another party.
Impairment of Financial Assets
For trade receivables and other receivables, the Company applies a simplified approach in calculating Expected Credit Losses (“ECL”). Therefore, the Company does not track changes in credit risk, but instead recognizes a loss allowance based on lifetime ECLs at each reporting date. The Company has established a provision matrix that is based on its historical credit loss experience, adjusted for forward-looking factors specific to the debtors and the economic environment.
The carrying amount of the asset is reduced through the use of an allowance account and the loss is recognized in the income statement.
2.9.Financial Liabilities
The financial liabilities include financial debt, derivative liabilities, trade payables and other payables.
Liabilities at amortized cost
Those financial liabilities, except for the derivative liabilities, are measured at amortized cost using the effective interest rate method. Amortized cost is calculated by taking into account any discount or premium on acquisition and fees or costs that are an integral part of the effective interest rate. The effective interest rate amortization is included as financial cost in the consolidated income statement. When the estimated contractual cash flows are modified, the entity recalculates the gross carrying amount of the financial liability as the present value of the modified cash flows discounted at the original effective interest rate. The difference between the recalculated carrying amount and the initial carrying amount is included in other operating income & expense in the consolidated income statement.
Liabilities at fair value with changes in fair value through profit and loss
The Company has derivative liabilities consisting of foreign currency options to hedge its contingency risk exposure to certain foreign currencies. Those derivative financial instruments are initially recorded at fair value and derivative financial instruments are subsequently remeasured at their fair value with changes in fair value recorded in the income statement under “Financial income/financial expenses”. Any transactions costs incurred are immediately recognized in the consolidated income statement.
The Company does not apply hedge accounting to those derivative financial liabilities.
The fair value of a hedging derivative financial instrument is classified as a non-current liability when the remaining maturity of the hedged item is more than
Derecognition
The Company derecognizes financial liabilities when, and only when, the Company’s obligations are discharged, cancelled or they expire. The difference between the carrying amount of the financial liability derecognized and the consideration paid and payable is recognized in income statement.
F-12
2.10. Fair value measurement
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value measurement is based on the presumption that the transaction to sell the asset or transfer the liability takes place either in the principal market for the asset or liability or in the absence of a principal market, in the most advantageous market for the asset or liability. The principal or the most advantageous market must be accessible by the Company. The fair value of an asset or liability is measured using the assumptions that market participants would use when pricing the asset or liability, assuming that the market participants act in their economic best interest.
All assets and liabilities for which fair value is measured or disclosed in the consolidated financial statements are categorized within the fair value hierarchy, described as follows, based on the lowest level input that is significant to the fair value measurement as a whole:
Level 1 quoted (unadjusted) market prices in active markets for identical assets or liabilities;
Level 2 valuation techniques for which the lowest level input that is significant to the fair value measurement is directly or indirectly observable; and
Level 3 valuation techniques for which the lowest level input that is significant to the fair value measurement is unobservable.
2.11. Inventory
Inventories consist of raw materials, work-in-progress and finished goods of the Genio® System and related components. Inventories are valued at the lower of cost and net realizable value. Costs incurred in bringing each product to its present location and condition are accounted for as follows: cost of direct materials and labor and a proportion of manufacturing overheads based on the normal operating capacity, but excluding borrowing costs. The cost is assigned using the FIFO (“first-in-first-out”) method. Net realizable value is the estimated selling price in the ordinary course of business, less estimated costs of completion and the estimated costs necessary to make the sale.
2.12. Cash and Cash Equivalents
Cash and cash equivalents include cash in hand, deposits held at call with banks, other short-term deposits with a maturity of or less than
2.13. Income Taxes
Income taxes include current income tax and deferred income tax.
Current Income Tax
Current income tax assets and liabilities are measured at the amount expected to be recovered from or paid to the tax authorities. Tax rates and tax laws that are considered to determine the amount of tax assets or liabilities are those that are enacted or substantially enacted, at the reporting date.
The current income tax liability includes a liability for tax positions subject to uncertainty over income tax treatment when it is probable that an outflow of economic resources will occur. Measurement of the liability for tax positions subject to uncertainty over income tax treatment is based on either the most likely amount method or the expected value method based on the Company’s best estimate of the underlying risk.
Deferred Income Tax
Deferred tax is provided using the liability method on temporary differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes at reporting date. Deferred tax liabilities are recognized for all taxable temporary differences, except when the deferred tax liability arises from the initial recognition of an asset or liability in a transaction that at the time of the transaction affects neither the accounting profit nor taxable profit or loss.
F-13
Deferred tax assets are recognized for all deductible temporary differences, the carry forward of unused tax credits and any unused tax losses. Deferred tax assets are recognized to the extent that is probable that taxable profit will be available against which the deductible temporary differences, and the carry forward of unused tax credits and unused tax losses can be utilized, except when the deferred tax asset relating to the deductible temporary differences arises from the initial recognition of an asset or liability in a transaction that at the time of the transaction affects neither accounting profit nor taxable profit or loss.
The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized. Unrecognized deferred tax assets are re-assessed at each reporting date and are recognized to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered.
Deferred tax assets and tax liabilities are measured at the tax rates that are expected to apply in the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantially enacted at the reporting date.
Deferred tax assets and deferred tax liabilities are offset if a legally enforceable right exists to set off current tax assets against current tax liabilities and the deferred taxes relate to the same taxation authority.
2.14. Employee Benefits
Short-Term Employee Benefits
Short-term employee benefits include salaries and social security taxes, paid vacation and bonuses. They are recognized as expenses for the period in which employees perform the corresponding services. Outstanding payments at the end of the period are presented within current liabilities (other payables).
Post-Employment Benefits
Post-employment benefits include pensions and retirement benefits for employees, which are covered by contributions of the Company.
The Company has set up a pension plan for its employees which qualifies as Defined Benefit pension plan under IAS 19. In the view of the minimum legal returns guaranteed under such scheme, those plans qualify as Defined Benefits plans. Such pension scheme is treated in accordance with IAS 19 “Employee Benefits” as a defined benefit plan. For defined benefit plans, the amount recognized in the Statement of financial position as a net liability (asset) corresponds to the difference between the present value of future obligations and the fair value of the plan assets.
The present value of the obligation and the costs of services are determined by using the “projected unit credit method” and actuarial valuations are performed at the end of each reporting period. The actuarial calculation method implies the use of actuarial assumptions by the Company, involving the discount rate, evolution of wages, employee turnover and mortality tables. These actuarial assumptions correspond to the best estimations of the variables that will determine the final cost of post-employment benefits. The discount rate reflects the rate of return on high quality corporate bonds with a term equal to the estimated duration of the post-employment benefits obligations. The actuarial calculations of post-employment obligations are performed by independent actuaries.
Remeasurement, comprising actuarial gains and losses, the effect of the changes to the asset ceiling (if applicable) and the return on plan assets (excluding interest), is reflected immediately in the consolidated statement of financial position with a charge or credit recognized in other comprehensive income in the period in which they occur. Remeasurement recognized in other comprehensive income is reflected immediately in retained loss and will not be reclassified to profit or loss.
2.15. Share-Based Compensation
Equity-settled share-based compensation
The Company operates an equity-based compensation plan, whereby warrants are granted to directors, management and selected employees and non-employees. The warrants are accounted for as equity-settled share-based payment plans since the Company has no legal or constructive obligation to repurchase or settle the warrants in cash.
F-14
Each warrant gives the beneficiaries the right to subscribe to one or several common share of the Company. The warrants are granted for free and have an exercise price which is determined by the Board of Directors of the Company.
The fair value of the employee services received in exchange for the grant of stock options or warrants is determined at the grant date using a Black & Scholes valuation model.
The costs of equity-settled transactions are recognized in employee benefit expense. The total amount to be expensed over the vesting period, if any, with a corresponding increase in the « share-based payment reserve » within equity, is determined by reference to the fair value of the stock options or warrants granted, excluding the impact of any non-market vesting conditions. The cumulative expense recognized for equity-settled transactions at each reporting date until the vesting date reflects the extent to which the vesting period has expired and the entity’s best estimate of the number of equity instruments that will ultimately vest. At each closing date, the entity revises its estimates of the number of stock options that are expected to become exercisable. It recognizes the impact of the revision of original estimates, if any, in the income statement, and a corresponding adjustment to equity over the remaining vesting period.
The proceeds received net of any directly attributable transaction costs are credited to share capital when the stock options or the warrants are exercised. When warrants granted under a share-based compensation plan are exercised or when they are not exercised and have expired, the amount previously recognized under the share-based payment reserve is reclassified to the caption retained loss, within equity.
2.16. Provisions
A provision is set up by the Company if, at the reporting date, the Company has a present obligation, either legal or constructive, as a result of past events, when it is probable that an outflow of resources will be required to settle the obligation and when a reliable estimate of the amount can be made.
2.17. Leases
The Company assesses at contract inception whether a contract is, or contains, a lease. That is, if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration.
Right-of-use assets
The Company recognizes right-of-use assets at the commencement date of the lease (i.e., the date the underlying asset is available for use). Right-of-use assets are measured at cost, less any accumulated depreciation and impairment losses, and adjusted for any remeasurement of lease liabilities. The cost of right-of-use assets includes the amount of lease liabilities recognized, initial direct costs incurred, and lease payments made at or before the commencement date less any lease incentives received. Unless the Company is reasonably certain to obtain ownership of the leased asset at the end of the lease term, the recognized right-of-use assets are depreciated on a straight-line basis over the shorter of its estimated life and the lease term. Right-of-use assets are subject to impairment, but no impairment has been identified in fiscal year 2021, 2022 and 2023.
Lease liabilities
At the commencement date of the lease, the Company recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in-substance fixed payments) less any lease incentives receivable, variable lease payments that depend on an index or a rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option reasonably certain to be exercised by the Company and payments of penalties for terminating a lease, if the lease term reflects the Company exercising the option to terminate. The variable lease payments that do not depend on an index or a rate are recognized as expense in the period in which the event or condition that triggers the payment occurs.
F-15
In calculating the present value of lease payments, the Company uses the incremental borrowing rate at the lease commencement date if the interest rate implicit in the lease is not readily determinable. After the commencement date, the amount of lease liabilities is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of lease liabilities is remeasured if there is a modification, a change in the lease term, a change in the in-substance fixed lease payments or a change in the assessment to purchase the underlying asset.
Short-term leases and leases of low-value assets
The Company applies the short-term lease recognition exemption to its short-term leases of machinery, equipment and buildings (i.e., those leases that have a lease term of
2.18. Revenue
The Company continues the commercialization of its Genio® system in Europe, targeting customers primarily comprised of hospitals and distributors. Our internal sales force directly serves hospitals, while distributors are used in locations without a direct commercial presence. Revenue recognition aligns with the satisfaction of performance obligations under contractual agreements, with contracts having a single, short-term performance obligation.
Performance obligation is deemed fulfilled at a specific point in time when the customer gains control of the Genio® system, either upon product shipment or delivery, per the terms outlined in contractual agreements. The Genio® system, delivered as a kit of products simultaneously, is treated as a singular performance obligation.
Variable consideration including volume rebates
Revenue undergoes adjustments for variable consideration and other factors influencing the transaction price. Notably, some contracts may entail a volume discount, offering a free Genio® system for meeting or exceeding a specified purchase volume over a generally
The Company provides customers with a limited right of return for products in case of non-conformity or performance issues. Since product returns have been historically de-minimis, we haven’t factored in a revenue reduction related to variable considerations for returns.
Warranty obligations
The Company provides a three-year warranty on the Genio® system for general repairs of defects that existed at the time of sale. The assurance-type warranties are accounted for as warranty provisions which is currently not material.
2.19. Recoverable cash advances and other government grants
The Company received the support from a governmental agency, in this case the Walloon Region (“Region”), under the form of recoverable cash advances. Recoverable cash advances are aimed at supporting specific development programs. As part of this support, an agreement is concluded with the Region consisting in three distinct phases being a research phase, a decision phase and an exploitation phase. During the research phase, the Company receives funds from the Region based on eligible expenses incurred by the Company.
F-16
At the end of the research phase, there is a decision phase of six months, allowing the Company to decide whether or not it will use the results of the research phase.
| ● | If the Company decides not to use the results of the research phase, it has to notify the Region and transfer to the Region the rights associated with the research phase. Accordingly, the advances received are not to be reimbursed. |
| ● | If the Company decides to use the results of the research phase, it will enter into the exploitation phase. In such a situation, the advances received become refundable through a fixed repayment part ( |
| ● | Reimbursements (fixed and variable) to be made by the Company (interests included) may represent up to 2 times the amount of cash advance received, depending on the level and the timing of the sales. |
At inception, recoverable cash advances are recognized as financial liability at fair value when received. To determine the fair value of the cash advances received, the Company estimates future cash outflows considering (i) assumptions regarding the estimation of the timing and the probability of the future sales or (ii) the probability that the Company will notify the Walloon Region whether it will decide or not to use the results of the research phase and (iii) an appropriate discount rate.
At inception, if the fair value of the liability exceeds the amounts of the cash received, the difference is recognized in the income statement as operating expenses. If the amount of cash received would exceed the fair value of the liability, the difference would be considered as a government grant, being recognized in the income statement as operating income on a systematic basis in order to match the expenses incurred.
Subsequently, at each closing date, the financial liability is measured at amortized cost. When the estimated contractual cash flows are modified, the entity recalculates the gross carrying amount of the financial liability as the present value of the modified cash flows discounted at the original effective interest rate. The difference between the recalculated carrying amount and the initial carrying amount is included in the caption “other operating income/expenses” in the consolidated income statement and in the financial expenses for the impact of the discounting. When modifying the estimated contractual cash flows, the Company reviews if there are indicators, either positive or negative, influencing the estimation of the timing and level of the future sales of the products benefiting from the support of the Walloon Region.
When repayment of recoverable cash advances may be forgiven, the liability component of recoverable cash advances is treated as a government grant and taken to income only when there is reasonable assurance that the entity will meet the terms for forgiveness of the advance.
Government grants are recognized where there is reasonable assurance that the grant will be received and all attached conditions will be complied with. When the grant relates to an expense item, it is recognized as income on a systematic basis over the periods that the related costs, for which it is intended to compensate, are expensed. When the grant relates to an asset, it is deducted from the carrying amount of the asset and is reflected in the income statement as a reduction in the amortization expense of the asset concerned on a systematic basis over the life of the asset.
2.20. Segment Reporting
Based on the organizational structure, as well as the nature of financial information available and reviewed by the Company’s chief operating decision makers to assess performance and make decisions about resource allocations, the Company has concluded that its total operations represent
2.21. Significant events and transactions of the reporting period
On March 29, 2023, the Company issued
F-17
On March 30, 2023, the Company raised €
On April 17, 2023, the Company issued
On July 14, 2023, the Company issued
On August 29, 2023, the Company issued
Consequently, on December 31, 2023, the Company’s registered capital amounts to €
3.Capital Management
The Company’s objectives when managing capital are to maintain sufficient liquidity to meet its working capital requirements and fund capital investment in order to safeguard its ability to continue operating as a going concern. The capital structure of the Company consists of equity attributable to the shareholders, such as share capital, share premium, reserves and retained loss, and of borrowings. The capital of Nyxoah SA amounts to €
The Company monitors capital regularly to ensure that its ability to continue operating as a going concern (we refer to 5.1) and the legal capital requirements are met and may propose capital increases to the Shareholders’ Meeting to ensure the necessary capital remains intact.
4.Management of Financial Risks
The Company’s activities expose it to a variety of financial risks. The Company’s finance department identifies and evaluates the financial risks in co-operation with the operating units.
4.1.Market Risk
Market risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate because of changes in market prices. The Company’s activities may expose it to changes in foreign currency exchange rates and interest rates. The Company is not exposed to any equity price risk or commodity price risk as it does not invest in these classes of investments.
4.2.Credit risk
The credit risk arises mainly from trade receivables, cash and cash equivalents and deposits with banks and financial institutions. The Company only works with international reputable commercial banks and financial institutions.
Furthermore, the Company is not exposed to any material credit risk from trade receivables or other receivables. Other receivables are mainly due by the tax incentive in Australia and Belgium and there is limited risk associated to this receivable.
F-18
4.3.Foreign Exchange Risk
The Company is exposed to currency risk primarily due to the expected future USD, AUD and NIS expenses that will be incurred as part of the ongoing and planned marketing, clinical trials and other related expenses. A financial risk management policy has been approved to i) generate yields on liquidity and ii) reduce the exposure to currency fluctuations with a timeline up to
Additionally, earnings variability arises from the translation of monetary assets and liabilities denominated in currencies other than the functional currency of the Company’s subsidiaries at the rate of exchange at each closing date, the impact of which is reported as a foreign exchange gain or loss in the consolidated statements of comprehensive income.
2023 rates | 2022 rates | 2021 rates | ||||||||||
Currency |
| Closing |
| Average |
| Closing |
| Average |
| Closing |
| Average |
NIS |
| |
| |
| |
| |
| |
| |
AUD |
| |
| |
| |
| |
| |
| |
USD |
| |
| |
| |
| |
| |
| |
Based on the Company’s foreign currency exposures at the level of the consolidated income statement, varying the above foreign exchange rates to reflect positive and negative changes of
(in EUR 000) | Effect on loss (before tax) | Effect on pretax equity | ||||||||||||
Change in foreign exchange rate |
| NIS |
| USD |
| AUD |
| NIS |
| USD | AUD | |||
2023 |
| 5 | % | |
| — |
| |
| |
| |
| |
| (5) | % | ( |
| — |
| ( |
| ( |
| ( |
| ( | |
2022 |
| 5 | % | |
| |
| |
| |
| |
| |
| (5) | % | ( |
| ( |
| ( |
| ( |
| ( |
| ( | |
2021 |
| 5 | % | |
| |
| |
| |
| |
| |
| (5) | % | ( |
| ( |
| ( |
| ( |
| ( |
| ( | |
4.4.Interest rate risk
The Company has a significant amount of cash in EUR and USD for which the EUR cash position may be subject to negative interest rates above a certain level. The EUR cash balance at December 31, 2023 amounts to €
Without taking into account the impact of the FX vanilla options on the interest rate risk, an increase (decrease) in the interest rate by
4.5.Liquidity Risk
The Company’s main sources of cash inflows are obtained through capital increases, recoverable cash advances and grants. Cash is invested in low risk investments such as short-term bank deposits or savings accounts. The Company mainly makes use of liquid investment in current accounts (in Euro) or short-term deposit accounts.
The ability of the Company to maintain adequate cash reserves to support its activities in the medium term is highly dependent on the Company’s ability to raise additional funds. As a consequence, the Company is exposed to significant liquidity risk in the medium term.
Please refer to note 5.1 on going concern consideration.
F-19
Contractual undiscounted maturities of financial liabilities at December 31, are as follows:
As at December 31 | ||||||||||||
2023 | 2022 | |||||||||||
| Lease |
| Financial |
| Trade & |
| Lease |
| Financial |
| Trade & | |
(in EUR 000) | Liability | Debt | Other Payable | Liability | Debt | Other Payable | ||||||
Less than 1 year |
| |
| |
| |
| |
| |
| |
1 - 5 years |
| |
| |
| — |
| |
| |
| — |
5+ years |
| |
| |
| — |
| |
| |
| — |
Total |
| |
| |
| |
| |
| |
| |
4.6.Fair Value
The carrying amount of cash and cash equivalents, trade receivables, other receivables, financial assets and other current assets approximate their value due to their short-term character.
The carrying value of current liabilities approximates their fair value due to the short-term character of these instruments. The fair value of non-current liabilities (financial debt and other non-current liabilities), excluding the derivative financial liabilities, is evaluated based on their interest rates and maturity date. These instruments have fixed interest rates and their fair value measurements are subject to changes in interest rates. The fair value measurement is classified as level 3. Please refer to note 2.9 for information on the valuation of non-current liabilities.
The derivative financial liabilities and assets which consists of foreign currency swaps are measured at fair value through profit and loss. Fair value is determined by the financial institution and is based on foreign currency swap rates and the maturity of the instrument.
Carrying value | Fair value | |||||||
As at December 31 | As at December 31 | |||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2023 |
| 2022 |
Financial Assets |
|
|
|
|
|
|
|
|
Other long-term receivables (level 3) |
| |
| |
| |
| |
Trade and other receivables (level 3) |
| |
| |
| |
| |
Foreign currency swaps (level 2) |
| |
| |
| |
| |
Other current assets (level 3) |
| |
| |
| |
| |
Cash and cash equivalents (level 1) |
| |
| |
| |
| |
Financial Assets (level 1) |
| |
| |
| |
| |
Financial liabilities |
|
|
|
|
|
|
|
|
Financial debt (level 3) |
| |
| |
| |
| |
Foreign currency swaps (level 2) | | | | | ||||
Recoverable cash advances (level 3) |
| |
| |
| |
| |
Trade and other payables (level 1 and 3) |
| |
| |
| |
| |
5.Critical accounting estimates and assumptions
When preparing the consolidated financial statements, judgments, estimates and assumptions are made that affect the carrying amount of certain assets, liabilities and expenses. These include the going concern assessment, the share-based payment transactions, the accounting for research and development expenses, the recoverable cash advances and deferred taxes. These judgments, estimates and assumptions have been reviewed for each year and are reviewed on a regular basis, taking into consideration past experience and other factors deemed relevant under the then prevailing economic conditions. Changes in such conditions might accordingly result in different estimates in the Company’s future consolidated financial statements.
F-20
5.1.Critical Judgments
Going Concern
The Company has consistently operated with deficits and sustained negative cash flows since its inception considering the significant research and development expenses incurred for the development and regulatory approval of the Genio device. As of December 31, 2023, the Company’s statement of financial position includes an accumulated loss of €
The Company’s current operating plan indicates that it will continue to incur losses from operations and generate negative cash flows from operating activities given ongoing expenditures related to the completion of its clinical trials only partially offset by the Company’s revenue generating activities outside the U.S. (which were €
The Company projects that its existing cash and cash equivalents and marketable securities should be sufficient to fund operations until the beginning of the fourth quarter of 2024. To meet the Company’s future working capital needs, management is actively exploring different financing avenues, including the public or private issuance of equity and debt financing. Additional funds are pivotal for diverse activities, in particular to launch the Genio product in the U.S. and the ongoing progression of research and development projects. This raises, however, a substantial doubt in respect of going concern as the current funds are not sufficient to cover a period of 12 months following the date of the Annual Report.
Although the additional funds have not been raised yet, given the positive outcome from the DREAM trial, the Company is confident that raising sufficient funding to continue its operations for at least 12 months following the date of the Annual Report should not pose significant challenges.
The accompanying consolidated financial statements have therefore been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
Income tax
The tax laws applicable to the Company are complex and are subject to changes in tax landscapes, new laws, guidance, and rulings issued by the tax authorities. The Company may need to make a significant judgment whether certain tax positions taken in the tax filings are uncertain and whether it is probable that those tax positions may be challenged by the tax authorities in case of a tax audit. In making this judgment, the Company considers also third-party tax advice it has obtained.
When measuring the tax liability for uncertain tax positions, the Company need to assess the likelihood that the tax position will be challenged and determine the most likely amount (or expected value amount) that may have to be paid when the tax position is not accepted, considering any penalties and late interests payable.
5.2.Critical Accounting Estimates and Assumptions
Recoverable Cash Advances
The Company benefits from recoverable cash advances granted by the Walloon Region. These are in substance financial liabilities of the Company towards the Region. The determination of the amount of the financial liability is subject to a high degree of subjectivity and requires the Company to make estimates of the future sales it will derive in the future from the products that benefited from the support of the Region.
Based on these estimates, it may be concluded that the amount of the cash advance that the Company has received from the Region exceeds the amount of the financial liability estimated by the Company. In such a situation, the difference is considered as a government grant. Subsequent re-estimation of the timing of the cash outflows of the financial liability is accounted for in profit and loss.
F-21
Management estimates the fair value of the liability of the future payment to be made to the Walloon Region based on a forecasted volume of sales. The estimation of the fair value is dependent on the discount rate applied. The fixed part to be reimbursed has been discounted with a discount rate of
Development Expenses capitalized and related impairment testing
The Company capitalizes costs for product development projects. Initial capitalization of costs is based on management’s judgement that technological and economic feasibility is confirmed, usually when a product development project has reached a defined milestone according to an established project management model.
At December 31, 2019, for the first time the Company capitalized amount of development costs for the first generation of the Genio® System. This amount includes costs related to the development of the Genio® System which received CE Mark approval in March 2019 and related improvements. Therefore, the Company is of the opinion that, from March 2019, development expenditures do meet capitalization criteria. The Company uses an estimate for certain research and development expenses related to the Genio® System and related improvements to determine the amount to be capitalized or recorded as an expense. Accordingly, the costs incurred for the first generation of the Genio® System have been recognized as development assets for a total amount of €
The development expenses capitalized have to be tested annually for impairment during the development period, prior to the start of its amortization. The Company performs the impairment test on the smallest group of assets to which it belongs for which there are separately identifiable cash flows: its cash-generating units (“CGU’s”). Where the carrying value of an asset exceeds its recoverable amount (i.e. the higher of value in use and fair value less costs to sell), the asset is written down accordingly. The Company is a one product line company and the capitalized development expenses are only related to this product (Genio® System).The Company determined that it has two cash generating units, Genio® system launched in Europe and Genio® system launched in the United States, for which a value in use analysis has been performed.
When performing the impairment test, management needs to make significant judgments, estimates and assumptions. The Company bases its impairment calculation on detailed budgets and forecast calculations generally covering a period of three years (since the Company is in an early commercial stage). For longer periods, a growth rate is calculated and applied to future cash flows projected. See note 8.
Share-Based Payments
The Company has equity-settled share-based payment plans in place. Estimating fair value for share-based payment transactions requires determination of the most appropriate valuation model, which is dependent on the terms and conditions of the option plan. This estimate also requires determination of the most appropriate inputs to the valuation model including the expected life of the share option, volatility and dividend yield and making assumptions about them. The assumptions and models used for estimating the fair-value for share-based payment transactions are disclosed in note 16.
6.Subsidiaries
For all years ended as at December 31, 2023, 2022 and 2021 respectively, the Company owns
The Company also owns
The Company also owns
The Company also owns
F-22
7.Property, Plant and Equipment
| Furniture and |
|
|
|
| |||||
office | Leasehold | Laboratory | Assets under | |||||||
(in EUR 000) | equipment | improvements | equipment | construction | Total | |||||
Cost |
|
|
|
|
|
|
|
|
|
|
Opening Gross value January 1, 2022 |
| |
| |
| |
| |
| |
Additions | |
| |
| |
| |
| | |
Exchange differences |
| ( |
| ( |
| ( |
| — |
| ( |
Cost at December 31, 2022 |
| |
| |
| |
| |
| |
Additions |
| |
| |
| |
| |
| |
Transfers | — | | | ( | — | |||||
Other | — | — | ( | — | ( | |||||
Exchange differences |
| ( |
| ( |
| ( |
| — |
| ( |
Cost at December 31, 2023 |
| |
| |
| |
| |
| |
Depreciation |
|
|
|
|
|
|
|
|
| |
Opening accumulated depreciation January 1, 2022 |
| ( |
| ( |
| ( |
| — |
| ( |
Depreciation charge |
| ( |
| ( |
| ( |
| — |
| ( |
Exchange differences |
| |
| |
| |
| — |
| |
Depreciation at December 31, 2022 |
| ( |
| ( |
| ( |
| — |
| ( |
Depreciation charge |
| ( |
| ( |
| ( |
| — |
| ( |
Exchange differences |
| |
| |
| |
| — |
| |
Depreciation at December 31, 2023 |
| ( |
| ( |
| ( |
| — |
| ( |
Net book value at December 31, 2022 |
| |
| |
| |
| |
| |
Net book value at December 31, 2023 |
| |
| |
| |
| |
| |
In 2023, acquisitions were mainly related to the US production line under construction for an amount of €
There has been a transfer from asset under construction for an amount of €
The line Other relates to tax incentive in Belgium on the investments of 2022. We refer to note 10 for more details.
The depreciation charge amounts to €
F-23
8.Intangible assets
Development | Patents and | |||||
(in EUR 000) |
| cost |
| licenses |
| Total |
Cost |
|
|
|
|
|
|
Opening value at January 1, 2022 |
| |
| |
| |
Additions |
| |
| — |
| |
Cost at December 31, 2022 |
| |
| |
| |
Additions |
| |
| — |
| |
Other | ( | — | ( | |||
Cost at December 31, 2023 |
| |
| |
| |
Amortization |
|
|
| |||
Opening amortization at January 1, 2022 |
| ( |
| ( |
| ( |
Amortization |
| ( |
| ( |
| ( |
Amortization at December 31, 2022 |
| ( |
| ( |
| ( |
Amortization |
| ( |
| ( |
| ( |
Amortization at December 31, 2023 |
| ( |
| ( |
| ( |
Net book value at December 31, 2022 |
| |
| |
| |
Net book value at December 31, 2023 |
| |
| |
| |
There is only one development project: The Genio® system. The Company started amortizing the first-generation Genio® system in 2021. The amortization amounted to €
The Company continues to incur in 2023 development expenses with regard to the improved second-generation Genio® system and clinical trials to obtain additional regulatory approvals in certain countries or to be able to sell the Genio® System in certain countries. The total capitalized development expenses amounted to €
The line Other relates to tax incentive in Belgium. We refer to note 10 for more details.
In accordance with the accounting principle, the intangible assets are tested annually for impairment during the development period. The Genio® system is currently a unique product line developed by the Company and the Company determined that it has two cash generating units, Genio® system in Europe and Genio® system in the United States, for which a value in use analysis has been performed. The discount rates over the expected term that the assets will generate economic benefits are:
| Europe |
| US |
| |
Discount rate |
| | % | | % |
The discount rates have been determined by reference to the analyst reports covering the Company which are available.
Based on the current operating budget as approved by the Board of Directors, the Company’s management prepared cash flow forecasts, which covers a 3-year period and an appropriate extrapolation of cash flows beyond 2026. A sensitivity analysis has been performed concluding that a reasonable change in the WACC and/or forecasted growth rate would not lead to an impairment.
F-24
9.Right of use assets and lease liabilities
The Company has lease contracts for buildings and vehicles used in its operations. Leases of building have lease terms between and
The carrying amounts of right-of-use assets recognized and the movements during the period is as follows:
|
| Motor |
| |||
(in EUR 000) | Building | vehicles | Total | |||
Cost |
|
|
|
|
|
|
Opening value at January 1, 2022 |
| |
| |
| |
Additions |
| |
| |
| |
Disposal |
| — |
| ( |
| ( |
Exchange difference |
| ( |
| — |
| ( |
Cost at December 31, 2022 |
| |
| |
| |
Additions |
| — |
| |
| |
Disposal |
| — |
| ( |
| ( |
Lease modification | | | | |||
Exchange difference |
| ( |
| — |
| ( |
Cost at December 31, 2023 |
| |
| |
| |
Depreciation |
|
|
|
|
|
|
Opening accumulated depreciation at January 1, 2022 |
| ( |
| ( |
| ( |
Depreciation charge |
| ( |
| ( |
| ( |
Disposal |
| — |
| |
| |
Exchange difference |
| |
| — |
| |
Depreciation at December 31, 2022 |
| ( |
| ( |
| ( |
Depreciation charge |
| ( |
| ( |
| ( |
Disposal |
| — |
| |
| |
Exchange difference |
| |
| — |
| |
Depreciation at December 31, 2023 |
| ( |
| ( |
| ( |
Net book value at December 31, 2022 |
| |
| |
| |
Net book value at December 31, 2023 |
| |
| |
| |
In 2023, the Company did enter into new lease agreements for €
For the year ended December 31, 2023, the Company recognized
F-25
The maturity analysis of lease liabilities is disclosed in note 4.5.
(in EUR 000) |
| 2023 |
| 2022 |
Lease debt at January 1 |
| |
| |
New lease debts |
| |
| |
Rent expense paid |
| ( |
| ( |
Accretion of interest |
| |
| |
Lease modification |
| |
| — |
Exchange differences |
| ( |
| ( |
Lease debt at December 31 |
| |
| |
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Non-current lease liabilities |
| |
| |
Current lease liabilities |
| |
| |
Total |
| |
| |
10. Other long-term receivables
The increase in other long-term receivables is due to a tax incentive in Belgium for an amount of €
The incentive recorded as at December 31, 2023 relates to 2022 as well as 2023 investments both on tangible and intangible assets. The amount related to investments made in 2022 mainly related to intangible assets and is expected to be received in cash in 2027. The amount related to investments made in 2023 mainly related to intangible assets and is expected to be received in cash in 2028.
11.Inventory
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Raw materials | | | ||
Work in progress |
| |
| |
Finished goods |
| |
| |
Total Inventory |
| |
| |
The increase in inventory is due to increasing activities to prepare for the commercialization and further scale-up of the Company in 2024. For the year ended December 31, 2023 and 2022 the Company did not recognize any expenses for inventory write-offs since the inventory level as per year-end is expected to be sold in the foreseeable future.
12.Trade and Other receivables
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Trade receivables |
| |
| |
R&D incentive receivable (Australia) |
| |
| |
VAT receivable |
| |
| |
Current tax receivable |
| |
| |
Foreign currency swaps |
| |
| |
Other |
| |
| |
Total trade and other receivables |
| |
| |
F-26
The increase of €
The Company can include unbilled receivables in its accounts receivable balance. Generally, these receivables represent earned revenue from products delivered to customers, which will be billed in the next billing cycle. All amounts are considered collectible and billable. As at December 31, 2023 and December 31, 2022, there were
R&D incentive receivables relates to incentives received in Australia as support to the clinical trials and the development of the Genio® system.
The current tax receivable relates to excess payment of corporate income tax in Israel, US and Belgium. The increase can mainly be explained by an increase in Belgium by €
We refer to note 19.1 for more details on the foreign currency swaps.
13.Cash and cash equivalents
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Short term deposit |
| |
| |
Current accounts |
| |
| |
Total cash and cash equivalents |
| |
| |
Cash and cash equivalents increased to €
14.Financial assets
Current financial assets relate to term accounts with an initial maturity longer than 3 months but less than 12 months measured at amortized costs.
In 2023, the Company entered into USD term deposits and US Treasury bills for a total amount $US
The current financial assets consists of $US
15.Capital, Share Premium, Reserves
15.1. Capital and share premium
The number of shares and the par value in the paragraph below take into account resolutions adopted by the shareholders’ meeting of February 21, 2020. All existing preferred shares were converted into common shares, and then a share split of
As part of the IPO on September 21, 2020, the Company incurred direct-attributable transaction costs of €
F-27
As part of the IPO on July 7, 2021, the Company incurred direct-attributable transaction costs of €
As of December 31, 2022, the share capital of the Company amounts to €
As of December 31, 2023, the share capital of the Company amounts to €
Evolution of the share capital and share premium ended December 31, 2023 and 2022:
| Common |
| Total of |
| Par value |
| Share capital |
| Share premium | |
(Number of shares except otherwise stated) | shares | shares | (in EUR) | (in EUR 000) | (in EUR 000) | |||||
January 1, 2022 |
| |
| |
| |
| |
| |
February 10, 2022 - Exercise warrants |
| |
| |
| |
| |
| |
June 8, 2022 - Capital increase in cash |
| |
| |
| |
| |
| — |
September 30, 2022 - Exercise warrants |
| |
| |
| |
| |
| |
December 31, 2022 | | | | | | |||||
March 29, 2023 - Capital increase in cash |
| |
| |
| |
| |
| |
March 30, 2023 - Capital increase in cash |
| |
| |
| |
| |
| |
April 17, 2023 - Capital increase in cash | | | | | | |||||
July 14, 2023 - Exercise warrants |
| |
| |
| |
| — |
| |
August 29, 2023 - Exercise warrants |
| |
| |
| |
| |
| |
December 31, 2023 | | | | | |
On February 10, 2022, pursuant to the exercise of warrants, the Company issued
On June 8, 2022, the Company issued
On September 30, 2022, pursuant to the exercise of warrants, the Company issued
On March 29, 2023, the Company issued
On March 30, 2023, the Company raised €
On April 17, 2023, the Company issued
As part of above capital increases, the Company incurred direct-attributable transaction costs of €
On July 14, 2023, pursuant to the exercise of warrants, the Company issued
F-28
On August 29, 2023, pursuant to the exercise of warrants, the Company issued
15.2. Reserves
The reserves include the share-based payment reserve (see note 16), other comprehensive income and the retained loss. Retained loss is comprised of primarily of accumulated losses, other comprehensive income is comprised of currency translation reserves and remeasurements of post-employment benefit obligations.
The movement in other comprehensive income for the year ended December 31, 2023 and 2022 is detailed in the table below:
Post- | ||||||
Currency | employment | |||||
translation | benefit | |||||
(in EUR 000) |
| reserve |
| obligations |
| Total |
Opening value at January 1, 2022 |
| |
| ( |
| |
Currency translation differences |
| ( |
| — |
| ( |
Remeasurements of post-employment benefit obligations |
| — |
| |
| |
Total other comprehensive income at December 31, 2022 |
| |
| |
| |
Currency translation differences |
| ( |
| — |
| ( |
Remeasurements of post-employment benefit obligations |
| — |
| |
| |
Total other comprehensive income at December 31, 2023 |
| |
| |
| |
16.Share-Based compensation
As per December 31, 2023, the Company has
In accordance with the terms of the various plans, all warrants that had not yet vested before, vested on September 7, 2020, i.e. ten business days prior to the closing of the IPO on September 21, 2020.
The changes of the year for the equity-settled warrant plans are as follows:
Number of shares (after share split) warrants give right to across all plans |
| 2023 |
| 2022 |
Outstanding at January 1 |
| |
| |
Granted |
| |
| |
Forfeited |
| ( |
| ( |
Exercised |
| ( |
| ( |
Expired | ( | ( | ||
Outstanding at December 31 |
| |
| |
Exercisable at December 31 |
| |
| |
16.1. Description of the equity-settled share-based incentive plans
2016 Plan
On November 3, 2016, the shareholders’ meeting of the Company approved the issuance of
F-29
The total amount of warrant holders under the 2016 Plan cannot exceed
The status of the 2016 warrant plan at December 31 is as follows:
Number of shares (after share split) warrants give right to for Plan 2016 |
| 2023 |
| 2022 |
Outstanding at January 1 |
| |
| |
Granted |
| — |
| — |
Forfeited |
| — |
| — |
Exercised |
| ( |
| ( |
Expired | ( | — | ||
Outstanding at December 31 |
| — |
| |
Exercisable at December 31 |
| — |
| |
With respect to the warrants exercised in 2023, a total of
2018 Plan
On December 12, 2018, the shareholders’ meeting of the Company approved the issuance of
The total amount of warrant holders under the 2018 Plan cannot exceed
In April 2020,
F-30
The status of the 2018 warrant plan at December 31 is as follows:
Number of shares (after share split) warrants give right to for Plan 2018 |
| 2023 |
| 2022 |
Outstanding at January 1 |
| |
| |
Granted |
| — |
| — |
Forfeited |
| — |
| — |
Exercised |
| — |
| — |
Expired | — | — | ||
Outstanding at December 31 |
| |
| |
Exercisable at December 31 |
| |
| |
2020 Plan
On April 7, 2020, the shareholders’ meeting of the Company approved the issuance of
The total number of warrant holders under the 2020 Plan cannot exceed
The status of the 2020 warrant plan at December 31 is as follows:
Number of shares/warrants give right to for Plan 2020 |
| 2023 |
| 2022 |
Outstanding at January 1 |
| |
| |
Granted |
| — |
| — |
Forfeited |
| — |
| — |
Exercised |
| — |
| ( |
Expired | ( | ( | ||
Outstanding at December 31 |
| |
| |
Exercisable at December 31 |
| |
| |
A total of
2021 Plan
On September 8, 2021, the Board of Directors, within the framework of the authorized capital, issued
F-31
The total number of warrant holders under the 2021 Plan cannot exceed
On February 21, 2022
On March 24, 2023, the Company reduced the exercise price of
On March 24, 2023,
The status of the 2021 warrant plan at December 31 is as follows:
Number of shares/warrants give right to for Plan 2021 |
| 2023 |
| 2022 |
Outstanding at January 1 |
| |
| |
Granted |
| |
| |
Forfeited |
| ( |
| ( |
Exercised |
| ( |
| — |
Expired | ( | ( | ||
Outstanding at December 31 |
| |
| |
Exercisable at December 31 |
| |
| |
In 2023, a total of
2022 Plan
On December 28, 2022, the Board of Directors, within the framework of the authorized capital, issued
The total number of warrant holders under the 2022 Plan cannot exceed
F-32
On June 14, 2023 and October 20, 2023 respectively
The status of the 2022 warrant plan at December 31 is as follows:
Number of shares/warrants give right to for Plan 2022 |
| 2023 |
| 2022 |
Outstanding at January 1 |
| — |
| — |
Granted |
| |
| — |
Forfeited |
| — |
| — |
Exercised |
| — |
| — |
Expired |
| — |
| — |
Outstanding at December 31 |
| |
| — |
Exercisable at December 31 |
| |
| — |
16.2. Accounting for Equity-settled Share-Based Payment
The fair value of the plan is expensed over the vesting period. As a result of the exercise price reduction on March 24, 2023 of the warrants previously granted to warrant holders under the 2021 Warrants Plan, the Company determined the fair value of the options at the date of the modification (March 24, 2023). The incremental fair value of the re-priced warrants is recognised as an expense over the period from the modification date to the end of the vesting period. For the warrants already vested at the date of modification, the incremental fair value is fully recognised as an expense at date of modification.
The share-based compensation expense for all vested warrants recognized in the income statement was €
Total |
| 2023 |
| 2022 |
| 2021 |
Exercisable Warrants at December 31 |
| |
| |
| |
Shares representing the Exercisable Warrants at December 31 |
| |
| |
| |
Weighted average exercise price per share |
| |
| |
| |
Weighted average share price at the date of exercise |
| |
| |
| |
16.3. Fair value
The fair value of each option or subscription right is estimated on the date of grant using the Black & Scholes model based on the following:
| ● | The dividend return is estimated by reference to the historical dividend payment of the Group. Currently, this is estimated to be zero as no dividend have been paid since inception; |
| ● | Expected volatility is estimated based on a sample of similar companies based on the healthcare products sector of the Damodaran dataset; |
| ● | Risk-free interest rate is based on the yield of EUR bonds with an equivalent term to liquidation event; |
| ● | The expected life of the share options is based on current expectations and is not necessarily indicative of exercise patterns that may occur. |
Fair value of the shares is estimated based on the market approach using publicly traded companies and acquisitions of private held companies within the same industry as Nyxoah. (Prior to the initial public offering)
F-33
The following table provides the input to the Black-Scholes model for warrants granted in 2018, 2020, 2021, 2022 and 2023 related to the 2016 warrant plan, the 2018 warrant plan, the 2020 warrant plan, the 2021 warrant plan and the 2022 warrant plan. The table and notes uses as a basis, the number of shares the warrants give right to across all plans.
|
|
|
|
| Plan 2021 |
| |||||
Plan 2016 | Plan 2018 | Plan 2018 | Plan 2020 | (grant Sept 17 | |||||||
(grant 2018) | (grant 2018) | (grant 2020) | (grant 2020) | 2021) |
| ||||||
Return Dividend |
| | % | | % | | % | | % | | % |
Expected volatility |
| | % | | % | | % | | % | | % |
Risk-free interest rate |
| | % | ( | % | ( | % | ( | % | ( | % |
Expected life |
| |
| |
| |
| |
| | |
Exercise price |
| |
| |
| |
| |
| | |
Stock price |
| |
| |
| |
| |
| | |
Fair value |
| |
| |
| |
| |
| |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 | ||
(grant Oct 27 | (grant Feb 21 | (grant Feb 21 | (grant Feb 21 | (grant May 14 | |||||||
| 2021) |
| 2022) |
| 2022) |
| 2022) |
| 2022) | ||
Return Dividend |
| | % | | % | | % | | % | | % |
Expected volatility |
| | % | | % | | % | | % | | % |
Risk-free interest rate |
| ( | % | | % | | % | | % | | % |
Expected life |
| |
| |
| |
| |
| |
|
Exercise price |
| |
| |
| |
| |
| |
|
Stock price |
| |
| |
| |
| |
| |
|
Fair value |
| |
| |
| |
| |
| |
|
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| |
(grant June 8 | (grant Aug 8 | (grant Aug 8 | (grant March 24 | (grant April 12 |
| ||||||
2022) | 2022) | 2022) | 2023) | 2023) |
| ||||||
Return Dividend |
| | % | | % | | % | | % | | % |
Expected volatility |
| | % | | % | | % | | % | | % |
Risk-free interest rate |
| | % | | % | | % | | % | | % |
Expected life |
| |
| |
| |
| |
| | |
Exercise price |
| |
| |
| |
| |
| | |
Stock price |
| |
| |
| |
| |
| | |
Fair value |
| |
| |
| |
| |
| |
| Plan 2021 |
| Plan 2022 |
| Plan 2022 |
| |
(grant June 14 | (grant June 14 | (grant Oct 20 |
| ||||
2023) | 2023) | 2023) |
| ||||
Return Dividend |
| | % | | % | | % |
Expected volatility |
| | % | | % | | % |
Risk-free interest rate |
| | % | | % | | % |
Expected life |
| |
| |
| | |
Exercise price |
| |
| |
| | |
Stock price |
| |
| |
| | |
Fair value |
| |
| |
| |
F-34
As a result of the exercise price reduction on March 24, 2023 of the warrants previously granted to warrant holders under the 2021 Warrants Plan, the Company determined the fair value of the options at the date of the modification (March 24, 2023). The fair value of the modified warrants was determined using the same models and principles as described above, with the following model inputs:
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| |
(grant Sept 17 | (grant Oct 27 | (grant Feb 21 | (grant Feb 21 |
| |||||
2021) | 2021) | 2022) | 2022) |
| |||||
Return Dividend |
| | % | | % | | % | | % |
Expected volatility |
| | % | | % | | % | | % |
Risk-free interest rate |
| | % | | % | | % | | % |
Expected life |
| |
| |
| |
| | |
Exercise price |
| |
| |
| |
| | |
Stock price |
| |
| |
| |
| | |
Fair value |
| |
| |
| |
| | |
Incremental Fair value |
| |
| |
| |
| |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| Plan 2021 |
| |
(grant Feb 21 | (grant May 14 | (grant Aug 8 | (grant Aug 8 |
| |||||
2022) | 2022) | 2022) | 2022) |
| |||||
Return Dividend |
| | % | | % | | % | | % |
Expected volatility |
| | % | | % | | % | | % |
Risk-free interest rate |
| | % | | % | | % | | % |
Expected life |
| |
| |
| |
| | |
Exercise price |
| |
| |
| |
| | |
Stock price |
| |
| |
| |
| | |
Fair value |
| |
| |
| |
| | |
Incremental Fair value |
| |
| |
| |
| |
The weighted average fair value of warrants granted during the year was €
17.Financial Debt
Financial debt consists of recoverable cash advances, and other loans. The related amounts as at December 31, 2023 and 2022, can be summarized as follows:
| As at December 31 | |||
(in EUR 000) |
| 2023 |
| 2022 |
Recoverable cash advances - Non-current | | | ||
Recoverable cash advances - Current |
| |
| |
Total Recoverable cash advances |
| |
| |
Other loan - Non-current |
| — |
| |
Other loan - Current |
| |
| |
Total other loans |
| |
| |
Non-current |
| |
| |
Current |
| |
| |
Total Financial Debt |
| |
| |
F-35
17.1. Financial debt related to recoverable cash advances
Recoverable cash advances received
As at December 31, 2023, the details of recoverable cash advances received can be summarized as follows:
| Contractual |
| Advances |
| Fixed |
| Variable | |
(in EUR 000) | advances | received | reimbursements* | reimbursements* | ||||
Sleep apnea device (6472) |
| |
| |
| |
| |
First articles (6839) |
| |
| |
| |
| |
Clinical trial (6840) |
| |
| |
| |
| |
Activation chip improvements (7388) |
| |
| |
| |
| |
Total |
| |
| |
| |
| |
* Excluding interests
| ● | The Convention 6472 “Sleep apnea device” for a total amount of € |
| ● | The Convention 6839 “First Articles” for a total amount of € |
| ● | The Convention 6840 “Clinical Trial” for a total amount of € |
| ● | The Convention 7388 “Implant for Obstructive Sleep Apnea, “Activation Chip Improvements” for a total amount of € |
Evolution of the financial debt in the financial statements
The determination of the amount to be reimbursed to the Walloon Region under the signed agreements is subject to a degree of uncertainty as it depends on the amount of the future sales that the Company will generate or not in the future. To determine the fair value of those advances, management of the Company has considered the possible outcomes of the program currently benefiting from the support of the Walloon Region. Management has considered that the probability to have to reimburse the
The Management performed an initial recognition of the financial debt for the variable part using a discount rate of
F-36
The table below details the remaining undiscounted cash flows resulting from the reimbursement of the recoverable cash advances. The initial recognition of the liability reflects a reimbursement up to 2 times the amount of cash advance received.
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Recoverable cash advances received |
| |
| |
Amounts to be reimbursed |
| |
| |
Amounts reimbursed at year-end (interests included) |
| ( |
| ( |
Total Recoverable cash advances (undiscounted) |
| |
| |
Based on expected timing of sales and after discounting, the financial debt related to the recoverable cash advances is as follows:
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Contract 6472 |
| |
| |
Contract 6839 |
| |
| |
Contract 6840 |
| |
| |
Contract 7388 |
| |
| |
Total recoverable cash advances |
| |
| |
Non-current |
| |
| |
Current |
| |
| |
Total recoverable cash advances |
| |
| |
The amounts recorded under “Current” caption correspond to the sales-independent amounts (fixed repayment) and sales-dependent reimbursements (variable repayment) estimated to be repaid to the Walloon Region in the next 12-month period. The estimated sales-independent (fixed repayment) as well as sales-dependent reimbursements (variable repayment) beyond 12-months are recorded under “Non-current” liabilities. Changes in the recoverable cash advances can be summarized as follows:
(in EUR 000) |
| 2023 |
| 2022 |
As at January 1 |
| |
| |
Advances reimbursed (excluding interests) |
| ( |
| ( |
Interests paid | ( | ( | ||
Initial measurement and re-measurement |
| ( |
| ( |
Discounting impact |
| |
| |
As at December 31 |
| |
| |
The discounting impact is included and presented in the financial expenses and amounted to €
A sensitivity analysis of the carrying amount of recoverable cash advances has been done to assess the impact of a change in assumptions. The Company tested reasonable sensitivity to changes in revenue projections of +/-
Fair Value of Liabilities as of end of 2023 (in EUR 000) | Variation of revenue projections | |||||
Variation of discount rates * |
| (25)% |
| 0% |
| 25% |
(25)% | | | | |||
0% | |
| |
| | |
25% | |
| |
| | |
* A change of -
F-37
An increase of
An increase of
17.2. Other Loans
The Company has contracted a loan of €
18.Trade payables
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Payables |
| |
| |
Invoices to be received |
| |
| |
Total trade payables |
| |
| |
The increase in total trade payables of €
The Company normally settles its trade payables in 30 days.
19.Other payables
| As at December 31 | |||
(in EUR 000) |
| 2023 |
| 2022 |
Holiday pay accrual | | | ||
Salary |
| |
| |
Accrued expenses |
| |
| |
Foreign currency option - current |
| |
| |
Other |
| |
| |
Total other payables |
| |
| |
The decrease of €
19.1.Derivatives
The Company is exposed to currency risk primarily due to the expected future USD, AUD and NIS expenses that will be incurred as part of the ongoing and planned marketing, clinical trials and other related expenses. A financial risk management policy has been approved to i) generate yields on liquidity and ii) reduce the exposure to currency fluctuations with a timeline up to and by means of foreign currency swaps. There have not been any transfers of level 3 categories during the year.
F-38
The Company has also entered into several foreign currency swaps for which the notional amounts are detailed in the table below:
As at December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Foreign currency swaps EUR - NIS (in EUR) |
| | | |
Foreign currency swaps EUR - NIS (in NIS) |
| | | |
Foreign currency swaps NIS - EUR (in NIS) | | — | ||
Foreign currency swaps NIS - EUR (in EUR) | | — | ||
Foreign currency swaps EUR - USD (in EUR) |
| | | |
Foreign currency swaps EUR - USD (in USD) |
| | | |
The following table shows the carrying amount of derivative financial instruments measured at fair value in the statement of the financial position including their levels in the fair value hierarchy:
As at December 31, 2023 | ||||||||
(in EUR 000) |
| Level I |
| Level II |
| Level III |
| Total |
Financial assets |
|
|
|
|
|
|
|
|
Foreign currency swaps |
| — |
| |
| — |
| |
Financial liabilities |
|
|
|
|
|
|
| — |
Foreign currency swaps |
| — |
| |
| — |
| |
The fair value is determined by the financial institution and is based on foreign currency swaps rates and the maturity of the instrument. All foreign currency put and call options and foreign currency swaps are classified as current as their maturity date is within the next twelve months.
The change in the balance of the financial asset is detailed as follows:
(in EUR 000) |
| 2023 |
| 2022 |
Opening value at January 1 |
| |
| — |
Settled contracts |
| ( |
| — |
Fair value adjustments |
| |
| |
Closing value at December 31 |
| |
| |
The change in the balance of the financial liability is detailed as follows:
(in EUR 000) |
| 2023 |
| 2022 |
Opening value at January 1 |
| |
| |
Fair value adjustments |
| |
| |
Settled contracts |
| ( |
| — |
Exchange rate difference |
| — |
| |
Settlement foreign currency put and call contracts | — | ( | ||
Recognition premium income | — | ( | ||
Closing value at December 31 |
| |
| |
F-39
20.Revenue and cost of goods sold
For the year ended December 31, 2023, the Company generated revenue for the amount of €
The sales based on country of customer for the year ended December 31, 2023, 2022 and 2021:
| For the year ended December 31 | |||||
(in EUR 000) | 2023 |
| 2022 |
| 2021 | |
Sales Germany |
| |
| |
| |
Sales Finland |
| — |
| |
| — |
Sales Spain |
| |
| |
| |
Sales Switzerland |
| |
| |
| — |
Sales Austria |
| |
| — |
| — |
Sales Belgium |
| — |
| — |
| |
Total sales |
| |
| |
| |
For the year ended December 31, 2023, the Company has
Cost of goods sold for the year ended December 31, 2023, 2022 and 2021:
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Purchases of goods and services |
| | | | ||
Inventory movement |
| ( | ( | ( | ||
Total cost of goods sold |
| | | | ||
21.Operating expenses
The tables below detail the operating expenses for the year ended December 31, 2023, 2022 and 2021:
Operating | ||||||
expense for the | ||||||
(in EUR 000) |
| Total cost |
| Capitalized |
| year |
Research and development |
| |
| ( |
| |
Selling, general and administrative expenses |
| |
| — |
| |
Other income and expenses |
| ( |
| |
| ( |
For the year ended December 31, 2023 |
| |
| ( |
| |
|
|
| Operating | |||
expense for the | ||||||
(in EUR 000) | Total cost | Capitalized | year | |||
Research and development |
| |
| ( |
| |
Selling, general and administrative expenses |
| |
| — |
| |
Other income and expenses |
| ( |
| |
| ( |
For the year ended December 31, 2022 |
| |
| ( |
| |
F-40
|
|
| Operating | |||
expense for the | ||||||
(in EUR 000) | Total cost | Capitalized | year | |||
Research and development |
| |
| ( |
| |
Selling, general and administrative expenses |
| |
| — |
| |
Other income and expenses |
| ( |
| |
| ( |
For the year ended December 31, 2021 |
| |
| ( |
| |
22.Research and Development expenses
Research and development expenses consist primarily of product development, engineering to develop and support our products, testing, consulting services and other costs associated with the next generation of the Genio® system. These expenses primarily include employee compensation, consulting and contractor’s fees and outsourced development expenses.
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Staff costs |
| | | | ||
Consulting and contractors’ fees |
| | | | ||
Q&A regulatory |
| | | | ||
Depreciation and amortization expense |
| | | | ||
Travel |
| | | | ||
Manufacturing and outsourced development |
| | | | ||
Clinical studies |
| | | | ||
Other expenses |
| | | | ||
IP costs | | | | |||
IT | | — | — | |||
Capitalized costs |
| ( | ( | ( | ||
Total research and development expenses |
| | | | ||
Before capitalization of €
Before capitalization of €
F-41
23.Selling, General and Administrative expenses
Selling, general and administrative expenses consist primarily of payroll and personnel related costs, and spending related to finance, information technology and human resource functions. Other general and administrative expenses include travel expenses, professional services fees, audit fees, insurance costs and general corporate expenses, including facilities-related expenses.
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Staff costs |
| | | | ||
Consulting and contractors’ fees |
| | | | ||
Legal fees |
| | | | ||
Rent |
| | | | ||
Facilities | | | | |||
Depreciation and amortization expense |
| | | | ||
IT |
| | | | ||
Travel |
| | | | ||
Insurance fees |
| | | | ||
Recruitment | | | | |||
Other |
| | | | ||
Total selling, general and administrative expenses |
| | | | ||
Selling, General and Administrative expenses increased by €
Selling, General and Administrative expenses increased by €
24.Other operating income and expenses
The Company had other operating income of €
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Recoverable cash advances |
|
| ||||
Initial measurement and re-measurement |
| | | | ||
R&D incentives |
| | | | ||
Capitalization of R&D incentive |
| ( | ( | ( | ||
Other income/(expenses) |
| ( | | ( | ||
Total Other Operating Income |
| | | | ||
The other operating income contains the R&D Incentive in Australia and as from 2023 the tax incentive in Belgium as well. The incentives to be received relate to development expenses incurred by the subsidiary in Australia and Belgium. Refer to note 10 for more information on the tax incentive in Belgium. For the year ended December 31, 2023, €
F-42
25.Employee Benefits
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Salaries |
| | | | ||
Social charges |
| | | | ||
Fringe benefits |
| | | | ||
Defined contribution plan |
| | | | ||
Holiday pay |
| | | | ||
Share-based payment (see note 16) |
| | | | ||
Other |
| | | | ||
Total employee benefits |
| | | | ||
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Selling, general and administrative expenses |
| | | | ||
Research & Development expenses |
| | | | ||
Total employee benefits |
| | | | ||
As at December 31, 2023, the Company employed
As at December 31 | ||||||
(in FTE’s) |
| 2023 |
| 2022 |
| 2021 |
Selling, General & Administration | | | | |||
Research & Development |
| |
| |
| |
Total |
| |
| |
| |
As at December 31, 2023, the Company had
26. | Pension Schemes |
26.1. | Defined contribution plan |
The Company offers Defined Contribution Plan funded through group insurances to its employees of the Israel entity. The total expense recognized in the consolidated income statement for contributions under this plan amounts to €
26.2. | Defined benefit plan |
The Company offers a pension plan with a minimum return guaranteed by law to its employees of the Belgian entity. The contributions to this plan amount to minimum
As a consequence of the law of December 18, 2015, minimum returns guaranteed by the employers are as follows:
| ● | For the contributions paid as from January 1, 2016, a new variable return based on OLO rates comprised between |
| ● | For the contributions paid until end December 2015, the previously applicable legal returns of |
F-43
The insurance companies managing these plans for the Company also guarantee a minimum return on the reserves as well as on future contributions for some portions of the plan. They have evolved as follows:
The weighted average duration until the pension age for the Belgian plan is
A complete actuarial calculation has been performed for this plan by external actuaries based on the “Projected Unit Credit Method without future contribution” according to the IAS 19,115 as follows:
| ● | Projection of the minimum return guaranteed by the law till the retirement date and discounting of this amount with the discount rate used for the valuation (rate of high-quality corporate bonds); |
| ● | The discounted net obligation is the maximum between this discounted projection and the projection of the accrued reserves discounted at the discount rate used for the valuation (rate of high-quality corporate bonds). |
The net defined benefit obligation was established at €
(in EUR 000) |
| 2023 |
| 2022 |
Net defined benefit liability at January 1 |
| |
| |
Defined benefit cost included in profit or loss |
| |
| |
Total remeasurement included in OCI |
| ( |
| ( |
Employer contributions |
| ( |
| ( |
Net defined benefit liability at December 31 |
| |
| |
The gross defined benefit liability is as follows:
(in EUR 000) |
| 2023 |
| 2022 |
Gross defined benefit liability at January 1 |
| |
| |
Current service cost |
| |
| |
Interest cost |
| |
| |
Administrative expenses |
| ( |
| ( |
Taxes on contributions |
| ( |
| ( |
Insurance premiums for risk benefits |
| ( |
| ( |
Actuarial gain due to change in financial assumptions |
| |
| ( |
Actuarial loss due to change in experience assumptions |
| ( |
| |
Gross defined benefit liability at December 31 |
| |
| |
F-44
The fair value of the plan assets is as follows:
(in EUR 000) |
| 2023 |
| 2022 |
Fair value plan assets at January 1 |
| |
| |
Interest income |
| |
| |
Employer contributions |
| |
| |
Administrative expenses |
| ( |
| ( |
Taxes on contributions |
| ( |
| ( |
Insurance premiums for risk benefits |
| ( |
| ( |
Actuarial gain on fair value of the plan assets |
| ( |
| |
Fair value plan assets at December 31 |
| |
| |
The number of members and the average age of the members is as follows:
For the year ended December 31 | ||||
| 2023 |
| 2022 | |
Active members |
| |
| |
Average age |
| |
| |
All plan assets are invested in an insurance contract with guaranteed interest rate (branch 21 product). The defined benefit calculation has been performed based on the below assumptions:
For the year ended December 31 |
| ||||
| 2023 |
| 2022 |
| |
Discount rate |
| | % | | % |
Inflation rate |
| | % | | % |
Salary increase (in excess of inflation) |
| | % | | % |
Withdrawal rate based on age (minimum) |
| | % | | % |
Withdrawal rate based on age (maximum) |
| | % | | % |
The discount rate was derived from the EIOPA term structure on each valuation date, considering the weighted average duration of liabilities. The inflation rate is based on the long-term objective of the European Central Bank. Retirement age assumption is in line with current legal requirements. The withdrawal rate and the salary increase rate reflect the expectations of the company on a long-term basis.
A sensitivity with reasonable possible changes on the discount rate will impact the net defined benefit liability as follows (positive = increase net defined benefit liability / negative = decrease of net defined benefit liability):
For the year ended December 31 | ||||
| 2023 |
| 2022 | |
Increase of 0.25% in the discount rate |
| ( |
| — |
Decrease of 0.25% in the discount rate |
| |
| — |
The expected employer contributions for the year 2024 amount to €
The total expected benefit payments are:
As at | ||
December 31, | ||
(in EUR 000) |
| 2023 |
In the next 12 months |
| |
Between 2 and 5 years |
| |
Between 6 and 10 years |
| |
Expected total benefit payments |
| |
F-45
27.Financial income
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Interests |
| | | | ||
Exchange differences |
| | | | ||
Fair value adjustment | | | — | |||
Other |
| | | | ||
Total financial income |
| | | | ||
The financial income decreased by €
For the year ended December 31, 2023, the total interest income amounted to €
The fair value adjustment relates to the fair value adjustment on financial instruments. More information can be found in note 19.1.
For the year ended December 31, 2022 other financial income mainly to consists of premiums received on foreign currency options.
28.Financial Expense
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Fair value adjustment |
| | | — | ||
Recoverable cash advances, Accretion of interest |
| | | | ||
Interest and bank charges |
| | | | ||
Interest on lease liabilities |
| | | | ||
Exchange differences |
| | | | ||
Other |
| — | — | | ||
Total Financial expense |
| | | | ||
The financial expenses decreased by €
The discounting impact of the recoverable cash advances is further detailed in note 17.1 above.
F-46
29.Income taxes and deferred taxes
The major components of income tax expense for the years ended December 31, 2023, 2022 and 2021 are as follows:
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Current tax income/(expense) |
| |
| ( |
| ( |
Deferred tax income/(expense) |
| |
| |
| |
Total Income tax income/(expense) |
| |
| ( |
| ( |
As of January 1, 2022, new tax regulations are in place in the US in which R&D expenses could no longer be deducted when incurred but instead they should be capitalized only for tax purposes and amortized over a
The current tax income mainly relates to (i) reversal of tax liability in US for an amount of €
The current tax liability of €
The deferred tax relates to a subsidiary where some payroll accruals are temporary differences in the determination of the taxable income. These temporary differences generate deferred tax income/(expense) of €
The income tax expenses can be reconciled to the Company’s Belgian statutory income tax rate of
For the year ended December 31 |
| ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
|
Loss for the period before taxes |
| ( |
| ( |
| ( | |
Company statutory income tax rate |
| % | % | % | |||
Income tax at company statutory tax rate |
| |
| |
| | |
Foreign tax rate differential |
| |
| |
| | |
Unrecognized DTA on tax losses and temporary differences |
| ( |
| ( |
| ( | |
Non deductible expenses |
| ( |
| ( |
| ( | |
Share based payments |
| ( |
| ( |
| ( | |
Income not subject to tax |
| |
| |
| | |
Tax adjustments to the previous period |
| |
| |
| ( | |
Local income taxes |
| |
| |
| ( | |
Other |
| |
| ( |
| | |
Income tax at company effective tax rate |
| |
| ( |
| ( | |
Company effective income tax rate |
| | % | ( | % | ( | % |
The tax adjustments to the previous period relates to the reversal of the current tax liability in the US subsidiary due to the R&D tax credit study (see above for more information).
The local income taxes in the effective tax rate reconciliation mainly relates to the theoretical tax exposure on R&D costs in the Australian subsidiary.
F-47
The Belgian entity, the Australian entity and German entity have historical losses that can be carried forward to future taxable income. The Belgian entity has tax losses for €
Deferred tax assets and liabilities are detailed below by nature of temporary differences for the year ended December 31, 2023 and 2022:
As at December 31, 2023 | ||||||
(in EUR 000) |
| Assets |
| Liabilities |
| Net |
Intangible assets |
| |
| |
| |
Property, plant and equipment | | — | | |||
Right-of-use assets |
| |
| ( |
| ( |
Other current assets |
| |
| ( |
| ( |
Financial debt (Recoverable Cash Advances and derivatives) |
| |
| |
| |
Lease liabilities |
| |
| |
| |
Retirement benefit obligations | | — | | |||
Other current liabilities |
| |
| ( |
| |
Tax-losses carried forward |
| |
| |
| |
Total gross deferred tax assets/(liabilities) |
| |
| ( |
| |
Netting by tax entity |
| ( |
| |
| |
Unrecognized deferred tax assets |
| ( |
| |
| ( |
Total deferred tax assets/(liabilities) |
| |
| ( |
| |
As at December 31, 2022 | ||||||
(in EUR 000) |
| Assets |
| Liabilities |
| Net |
Intangible assets |
| |
| — |
| |
Property, plant and equipment | — | ( | ( | |||
Right-of-use assets |
| |
| ( |
| ( |
Other current assets | | — | | |||
Financial debt (Recoverable Cash Advances and derivatives) |
| |
| ( |
| |
Lease liabilities |
| |
| |
| |
Other current liabilities |
| |
| ( |
| ( |
Tax-losses carried forward |
| |
| |
| |
Total gross deferred tax assets/(liabilities) |
| |
| ( |
| |
Netting by tax entity |
| ( |
| |
| |
Unrecognized deferred tax assets |
| ( |
| |
| ( |
Total deferred tax assets/(liabilities) |
| |
| — |
| |
The Company accumulates tax losses that are carried forward indefinitely for offset against future taxable profits of the Company. As stated above, the entities accumulating tax losses are not expected to generate significant profits in the near future so no deferred tax assets on tax losses carried forward and temporary differences have been recognized at this stage. The recognized deferred tax assets and liabilities in the consolidated balance sheets of the Company are positions that arise from temporary differences between statutory and IFRS account balances in the subsidiary in Israel and US.
F-48
30.Loss Per Share (EPS)
The Basic Earnings Per Share and the Diluted Earnings Per Share are calculated by dividing earnings for the year by the weighted average number of shares outstanding during the year. As the Company is incurring net losses, outstanding warrants have no dilutive effect. As such, there is no difference between the Basic and Diluted EPS.
| 2023 |
| 2022 |
| 2021 | |
As at December 31, after conversion and share split |
|
| ||||
Outstanding common shares at period-end |
| | | | ||
Weighted average number of common shares outstanding |
| | | | ||
Potential number of shares resulting from the exercise of warrants |
| | | |
Basic and Diluted EPS for the periods ended December 31, 2023, 2022 and 2021 based on weighted average number of shares outstanding after conversion and share split are as follows:
For the period ended December 31 | ||||||
| 2023 |
| 2022 |
| 2021 | |
Loss of year attributable to common holders (in EUR) | ( | ( | ( | |||
Loss of year attributable to preferred holders (in EUR) | — | — | — | |||
Loss of year attributable to equity holders (in EUR) |
| ( | ( | ( | ||
Weighted average number of common shares outstanding (in units) |
| | | | ||
Basic earnings per share in EUR (EUR/unit) |
| ( | ( | ( | ||
Diluted earnings per share in EUR (EUR/unit) |
| ( | ( | ( | ||
31.Other commitments
31.1. Capital commitments
There are no commitments related to capital expenditures at the closing date.
31.2. Lease expenses
The lease expense recognized in the income statement related to low-value leases and short-term leases amounts to:
For the year ended December 31 | ||||||
(in EUR 000) |
| 2023 |
| 2022 |
| 2021 |
Expense |
| |
| |
| |
Total |
| |
| |
| |
31.3. Other commitments
The Company has granted in 2022 an amount of €
F-49
32.Related Party Transactions
Transactions between the Company and its subsidiaries have been eliminated in consolidation and are not disclosed in the notes. Related party transactions are disclosed below.
32.1. Remuneration of Key Management
The remuneration of the senior management consists of the remuneration of the CEO of the Company for the period ended December 31:
For the period ended | ||||
December 31 | ||||
(in EUR 000) |
| 2023 |
| 2022 |
Short-term remuneration & compensation |
| | | |
Post-employment benefits | | | ||
Share based payment |
| | | |
Total |
| | | |
32.2. Transactions with Non-Executive Directors and Shareholders:
For the period ended December 31, 2023 | For the period ended December 31, 2022 | |||||||||||
R&D | Consulting | Board | R&D | Consulting | Board | |||||||
(in EUR 000) |
| Collaboration |
| services |
| Remuneration |
| Collaboration |
| services |
| Remuneration |
Cochlear |
| |
| |
| |
| |
| |
| |
Robelga SRL (formerly MINV SA) |
| |
| |
| |
| |
| |
| |
Donald Deyo |
| |
| |
| |
| |
| |
| |
Robert Taub |
| |
| |
| |
| |
| |
| |
Kevin Rakin |
| |
| |
| |
| |
| |
| |
Pierre Gianello |
| |
| |
| |
| |
| |
| |
Jan Janssen |
| |
| |
| |
| |
| |
| |
Jurgen Hambrecht |
| |
| |
| |
| |
| |
| |
Rita Mills |
| |
| |
| |
| |
| |
| |
Giny Kirby | — | — | | — | — | | ||||||
Raymond Cohen | — | — | — | — | — | | ||||||
Wildman Venturees LLC | — | — | | — | — | — | ||||||
Total |
| |
| — |
| |
| |
| |
| |
Amounts outstanding at year-end |
| — |
| — |
| |
| |
| |
| |
The Company and Cochlear Limited, or Cochlear, have entered into a collaboration agreement, dated November 2018, under which they agreed to collaborate to further develop and progress commercialization of implantable treatments for sleep disordered breathing conditions. A new Statement of Work was entered into on June 8, 2020. Under this agreement, Cochlear is working with the Company in developing and enhancing the next generation implantable stimulator. This collaboration agreement lead to financial impact of €
On September 28, 2023, the Company announced a partnership with ResMed in Germany to increase OSA awareness and therapy penetration in the German market. The Company and ResMed Germany will establish a continuum of care that will educate and guide OSA patients in the German market from diagnosis through treatment. Together, the companies will work to accelerate patient identification and better support patient set-up on the appropriate therapy.
F-50
32.3. Transactions with related parties
The following is a description of related party transactions we have entered into with any members of our board of directors or executive officers or the holders of more than
Consulting Agreement with Olivier Taelman
Effective September 1, 2021, the Company and Olivier Taelman decided by mutual agreement to terminate the employment contract of Olivier Taelman with the Company and to enter into an agreement, pursuant to which Mr. Taelman will perform his functions as CEO of the Company on a self-employed basis going forward. Pursuant to the terms of this agreement, Mr. Taelman will be entitled to receive an annual fee equal to the euro equivalent of $
Employment Agreement with Loïc Moreau
We are party to an employment agreement, dated October 8, 2021, with Loïc Moreau, our chief financial officer since January 1, 2022. Pursuant to the terms of his employment agreement, Mr. Moreau receives a base salary of €
Consulting Arrangements
Robelga SRL (formerly MINV SA) Consulting Agreements
On June 9, 2021, we entered into a consulting agreement with MINV SA, pursuant to which MINV SA (i) assisted our executive management during investor meetings in connection with our initial public offering on Nasdaq and (ii) provided various consultancy services, including to support our executive management in business development activities. . On June 23, 2021, pursuant to a “merger by absorption” of MINV SA by Robelga SRL, all rights and obligations of MINV SA were transferred to Robelga SRL For the year ended December 31, 2023, we paid Robelga SRL a total fee of €
Warrants to Our Board Directors and Executive Management
We have granted warrants to certain members of our board of directors and executive management.
Policies and Procedures for Related Person Transactions
We have adopted a related person transaction policy requiring that all related person transactions required to be disclosed by a foreign private issuer pursuant to the Exchange Act be approved by the audit committee or another independent body of our board of directors.
33.Events after the Balance-Sheet Date
On March 6, 2024, the Company issued
F-51