Table of Contents
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Title of each class: |
Name of exchange on which registered: | |||
OTCBB SIX Swiss Exchange (SIX) | ||||
OTCBB |
| Large accelerated filer | ☐ | Accelerated filer | ☐ | ☒ | ||||||
| Emerging growth company | ||||||||||
† |
The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012. |
| U.S. GAAP ☐ | |
Other ☐ | ||||||
| by the International Accounting Standards Board | ☒ |
Table of Contents
TABLE OF CONTENTS
i
Table of Contents
ii
Table of Contents
INTRODUCTION
Unless otherwise indicated or the context otherwise requires, all references in this Annual Report on Form 20-F to the terms “Relief,” “Relief Therapeutics,” “the company,” “we,” “us” and “our” refer to RELIEF Therapeutics Holding SA together with its subsidiaries. Relief and its subsidiaries may also sometimes be referred to in this Form 20-F as the “Group.”
Our jurisdiction of incorporation is Switzerland, and our registered office is in Geneva, Canton of Geneva, Switzerland. A majority of our outstanding securities that are registered in our share register are owned by non-U.S. residents. Under the rules of the U.S. Securities and Exchange Commission, or the SEC, we are currently eligible for treatment as a foreign private issuer. As a foreign private issuer, we will not be required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. domestic issuers whose securities are registered under the Securities Exchange Act of 1934, as amended, referred to herein as the Exchange Act.
We own trademarks for Relief Therapeutics in Switzerland. All other trade names, trademarks and service marks of other companies appearing in this prospectus are the property of their respective holders. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend to use or display other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Our reporting currency is the Swiss franc. In this prospectus, unless otherwise specified, all monetary amounts are in Swiss francs, all references to “CHF” and “Swiss francs” mean Swiss francs and all references to “U.S. dollars,” “$,” “US$” and “USD” mean United States dollars. Throughout this prospectus, references to ADSs mean ADSs or ordinary shares represented by such ADSs, as the case may be.
We present our consolidated financial statements in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB. Readers of this prospectus should note that there may be certain differences between the presentation of our financial position, results of operations and cash flows under IFRS and U.S. generally accepted accounting principles.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report on Form 20-F contains forward-looking statements that involve substantial risks and uncertainties. All statements contained in this annual report, other than statements of historical fact, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, projects, plans and objections of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend”, “may,” “plan,” “predict,” “project,” “target,” “potential,” “would,” “could,” “should,” “continue,” and other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. The forward-looking statements in this annual report include, among other things, statements about:
| • | the success, cost and development of our clinical programs, including the progress of, and results from, our (and our partners’) clinical trial and preclinical programs; |
| • | the ability of our collaboration partners to obtain authorizations to market products that are the subject of the respective collaborations; |
| • | our ability or our collaboration partners’ abilities to obtain and maintain regulatory approval of our product candidates and any related restrictions, limitations or warnings on the label of any such product, if approved; |
| • | our plans to pursue research and development of product candidates we may obtain in the future; |
| • | our ability to compete with companies currently marketing or engaged in the development of treatments for indications that our product candidates are designed to target; |
| • | the potential advantages or disadvantages of our product candidates; |
| • | the rate and degree of market acceptance and clinical utility of our product candidates; |
| • | the success of our collaborations and partnerships with third parties; |
1
Table of Contents
| • | our estimates regarding the potential market opportunities for our product candidates; |
| • | our sales, marketing, and distribution capabilities and strategy; |
| • | our ability to establish and maintain arrangements for the manufacture of our product candidates; |
| • | our ability to protect and defend our intellectual property; |
| • | whether any of our product candidates (or our collaboration partners’ product candidates) will ever be approved for commercialization; |
| • | whether we will ever achieve cash flow positive operations or profitability; |
| • | whether we can obtain funding for our activities when required and on terms that are acceptable to us; |
| • | our estimates regarding expenses, future revenues, capital requirements and requirements for additional financing; |
| • | our expectations related to our use of our capital; |
| • | the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including, but not limited to our preclinical studies and clinical trials and our commercial activities; |
| • | the impact of government laws and regulations; and |
| • | our competitive position. |
You should read this annual report and the documents we have filed as exhibits to the annual report completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward- looking statements we make. You should refer to the sections of this annual report titled “Item 3. Key Information, D. Risk Factors,” “Item 4. Information on the Company.” and “Item 5. Operating and Financial Review and Prospects” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
This annual report on Form 20-F includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
2
Table of Contents
PART I
| ITEM 1 | IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
| A. | DIRECTORS AND SENIOR MANAGEMENT |
The following table sets forth the names, dates of birth and positions of our directors and senior management as of the date of this annual report:
Directors
| Name |
Date of Birth |
Position | ||
| Dr. Raghuram (Ram) Selvaraju | 11/25/1978 | Chairman | ||
| Dr. Tom Plitz | 08/09/1968 | Vice-Chairman | ||
| Dr. Patrice P. Jean | 04/06/1971 | Director | ||
| Paolo Galfetti | 05/03/1965 | Director | ||
| Michelle Lock | 11/21/1968 | Director | ||
| Senior Management
Name |
Date of Birth |
Position | ||
| Paolo Galfetti | 05/03/1965 | Chief Operating Officer | ||
| Jack Weinstein | 01/19/1956 | Chief Executive Officer | ||
| Jeremy Meinen | 02/21/1989 | Chief Financial Officer and Treasurer | ||
| Marco Marotta | 06/20/1985 | Chief Business Officer | ||
Directors
Raghuram (Ram) Selvaraju, Ph.D., MBA, serves as Chairman of our Board of Directors. Dr. Selvaraju Managing Director of Equity Research at H.C. Wainwright & Co., LLC whose research focuses on the healthcare sector. Dr. Selvaraju has over 16 years of experience on Wall Street and previously was a pharmaceutical researcher at Serono in Switzerland. In addition, Dr. Selvaraju has appeared numerous times on Bloomberg, CNBC, Business News Network and BTV where he discussed drug development trends, healthcare reform policy, and pharma and biotech M&A. Prior to joining Wainwright, Dr. Selvaraju held Senior Research positions at MLV & Co., Aegis Capital Corp. – Head of Healthcare Equity Research and Director of Equity Research, Hapoalim Securities U.S.A. and Rodman & Renshaw LLC. Dr. Selvaraju became the youngest-ever recipient of the Serono Pharmaceutical Research Institute’s Inventorship Award for exceptional innovation and creativity in 2003. Dr. Selvaraju earned his Ph.D. in cellular immunology and molecular neuroscience and an M.S. in molecular biology from the University of Geneva in Switzerland on the basis of his drug development research. He also holds an M.B.A. from the Cornell University accelerated one-year program for scientists and engineers and a B.S. in biological sciences and technical writing from Carnegie Mellon University.
Tom Plitz serves as Vice Chairman of our Board of Directors and is chairperson of the Nomination and Compensation Committee of the Board. He most recently served as Chief Executive Officer of Chord Therapeutics SA, a privately held biopharmaceutical firm based in Geneva, Switzerland, which was acquired by Merck KGaA in January 2022, for an undisclosed amount. Prior to Chord, Dr. Plitz worked as Chief Scientific Officer of the rare disease company, Wilson Therapeutics, which was acquired for USD 855 million by Alexion Pharmaceuticals in April 2018. Dr. Plitz’s previous assignments include senior roles at Serono, Merck, and Shire Pharmaceuticals, where he worked across multiple therapeutic areas including neuroinflammatory, metabolic, and rare diseases, completing more than two decades of experience in pharmaceutical R&D. He holds a Ph.D. from Technical University of Munich, Germany
Patrice Jean is a member of our Board of Directors and is chairperson of the Audit and Finance Committee. She is the Chair of the Life Sciences Practice at Hughes Hubbard & Reed, an international law firm based in New York City. She has over a decade of experience counselling and leading startup pharmaceutical, chemical and biotechnology companies in all areas of intellectual property law including asserting and defending patent rights underlying core technologies and innovations. Dr. Jean serves as Vice-President of the New York Intellectual Property Law Education Foundation and is a board member of the New York Intellectual Property Law Association.. She holds a Ph.D. in molecular biology from Princeton University, a J.D. from Columbia University School of Law, and a B.A. in biochemistry from Xavier University.
3
Table of Contents
Paolo Galfetti is a member of our Board of Directors, the chairperson of the Corporate Governance Committee, and the Chief Operating Officer of Relief. He has more than thirty years of management experience in the pharmaceutical sector including in the areas of business development and licensing, operational strategic management, clinical research and pharmaceutical discovery and development. Mr. Galfetti joined APR in 1995 as head of licensing and business development and was appointed Chief Executive Officer in 2002. Prior to joining APR, he was a founding partner, Chief Executive Officer and board member of the Institute for Pharmacokinetic and Analytical Studies AG (IPAS), a Swiss contract research organization focusing on Phase I and II clinical trials, as well as Chief Executive Officer and board member of Farma Resa s.r.l., an Italian contract research organization dedicated to Phase III and IV clinical trial on a contract basis. Mr. Galfetti is a Chartered Financial Analyst (CFA) and has a bachelor’s degree in economics from the Commercial University Bocconi, Milan, Italy.
Michelle Lock is a member of our Board of Directors. She is the Chief Operating Officer and Chief Commercial Officer of Covis Pharma Group, a Switzerland-based global specialty pharmaceutical company that markets therapeutic solutions for patients with life-threatening conditions and chronic illnesses. Ms. Lock’s broad biopharmaceutical industry experience spans nearly 30 years and includes leadership roles in commercialization across various therapeutic areas including oncology, hematology, cardiovascular and metabolic disease, liver disease, immunology, virology and neuroscience. Previously, Ms. Lock served as the Senior Vice President and Head of International organization at Acceleron Pharma Inc, a biopharmaceutical company dedicated to the discovery, development, and commercialization of therapeutics to treat serious and rare diseases. Before that, she was a consultant to biotechnology companies, providing leadership, guidance, and strategic support to managements seeking to establish or improve their international businesses based in Switzerland. Earlier, Ms. Lock was Senior Vice President & Head of International at Sage Therapeutics, a clinical-stage biopharmaceutical company committed to discovering, developing, and commercializing novel medicines to transform the lives of patients with life-altering central nervous system (CNS) disorders. During her career, Ms. Lock also spent 24 years with Bristol-Myers Squibb (BMS) in positions of increasing responsibility in sales, commercial, general management, regional leadership and business strategy. In her most recent role at BMS, she served as Vice President and General Manager for EU Country Clusters & Global Capabilities Hub leadership, Switzerland, driving the company’s leadership efforts in immuno-oncology. She has served as Honorary Ambassador between Switzerland and the U.S. since 2018, as well is a past member of the board of directors of the Swiss American Chamber of Commerce and the Interpharma Switzerland Pharmaceutical Industry. She earned a degree in Science/Nursing at Royal Melbourne University, Australia and studied General Management and Internal General Management at CEDEP, France.
Executive Officers
Paolo Galfetti. See biographical information above.
Jack Weinstein joined us in October 2020 and since December 2022 has served as our Chief Executive Officer. From October 2020 to December 2022, Mr. Weinstein served as our U.S. based Chief Financial Officer and Treasurer, and as the President of Relief U.S. Mr. Weinstein has nearly 40 years of wide-ranging executive management expertise, including as a CFO, investment banker and consultant in the biopharmaceutical and life sciences industries. Prior to joining Relief, Mr. Weinstein served as Managing Director and Head of Healthcare Investment Banking at Avalon NetWorth, an independent New York-based boutique investment bank. Prior to joining Avalon, Mr. Weinstein was CFO, Treasurer and Vice President of Business Development at Catalyst Pharmaceuticals, Inc.(Nasdaq: CPRX), a biopharmaceutical company developing therapies to treat rare diseases, where he led the Company through its Initial Public Offering. Prior to joining Catalyst, Mr. Weinstein was the President and founder of The Sterlington Group, Inc., a consulting firm providing strategic, business development, regulatory and “CFO” consulting services. Mr. Weinstein received his MBA from the Harvard Business School.
Jeremy Meinen became our Chief Financial Officer and Treasurer in December 2022. Previously Mr. Meinen had served as our Vice President Finance and Administration since October 2020 and our Chief Accounting Officer since December 2021. He joined Relief as ad-interim Chief Financial Officer in April 2020. Prior to joining Relief, Jeremy provided financial consulting, controlling and auditing services to companies in various industries. He began his career in an international audit firm, where he held positions of increasing responsibility and scope over more than six years. Mr. Meinen holds a Master of Science in finance from Bocconi University and a Bachelor of Arts degree in Business Administration from the University of Geneva. He is a Swiss certified public accountant and former licensed audit expert.
4
Table of Contents
Marco Marotta became our Chief Business Officer in December 2021. Mr. Marotta joined us as part of our acquisition of APR, where he served as Corporate Director, Business Development and Licensing. Mr. Marotta joined APR in January 2015, where he was initially in charge to reshape and optimize APR’s end-to-end supply chain process, and afterwards, he joined the licensing and business development department, establishing and consolidating APR’s presence in emerging markets like the Asia-Pacific and Latin American regions. From 2019, Mr. Marotta led APR Business Development as a director, with responsibility of out-licensing proprietary products worldwide, divesting non-strategic assets and maximizing monetization as well as merging APR’s business with Relief. Mr. Marotta received a Master of Science in Engineering from the University Federico II in Napoli and an Executive MBA from Commercial University Bocconi in Milan.
| B. | ADVISERS |
Our principal Swiss legal advisor is VISCHER AG, located at Schuetzengasse 1, PO Box, CH-8021 Zurich, Switzerland and our principal United States (U.S.) legal adviser is Akerman LLP, located at 201 East Las Olas Boulevard, Suite 1800, Fort Lauderdale, Florida 33301
| C. | AUDITORS |
MAZARS SA has been our auditor since 2017. The address for MAZARS SA is Chemin de Blandonnet 2, 1214 Vernier-Geneva, Switzerland.
| ITEM 2 | OFFER STATISTICS AND EXPECTED TIMELINE |
Not applicable.
| ITEM 3 | KEY INFORMATION |
| A. | SELECTED FINANCIAL DATA |
Summary Financial Information of Relief
We derived the selected consolidated financial data as of and for the years ended December 31, 2022 and 2021 from our audited consolidated financial statements included elsewhere in this annual report. We present our consolidated financial statements in accordance with International Financial Reporting Standards (IFRS), as promulgated by the International Accounting Standards Board (IASB). All amounts below are in Swiss Francs (CHF) and are in thousands.
The summary consolidated financial data below should be read together with those consolidated financial statements as well as the Item 5. “Operating and Financial Review and Prospects.” Our historical results for any prior period are not necessarily indicative of results to be expected in any future period, and our interim period results are not necessarily indicative of results to be expected for a full year or any other interim period.
| December 31, | ||||||||
| Statement of Operations Data |
2022 | 2021 | ||||||
| Revenue |
6,081 | 3,321 | ||||||
| Other gains |
9,921 | 1,171 | ||||||
| Total Income |
16,002 | 4,492 | ||||||
| Raw materials and consumables expenses |
(1,250 | ) | (750 | ) | ||||
| External selling and distribution expenses |
(3,307 | ) | (365 | ) | ||||
| External research and development expenses |
(12,393 | ) | (19,024 | ) | ||||
| Personnel expenses |
(12,998 | ) | (9,121 | ) | ||||
| Other administrative expense |
(7,747 | ) | (6,750 | ) | ||||
| Other losses |
(63 | ) | (752 | ) | ||||
| EBITDA |
(21,756 | ) | (32,270 | ) | ||||
| Impairment losses on intangible assets |
(26,424 | ) | — | |||||
| Amortization and depreciation expense |
(3,860 | ) | (2,036 | ) | ||||
| Operating Loss |
(52,040 | ) | (34,306 | ) | ||||
| Financial income |
18 | 97 | ||||||
| Financial expense |
(2,294 | ) | (1,316 | ) | ||||
| Result before income taxes |
(54,316 | ) | (35,525 | ) | ||||
| Income taxes |
3,526 | 820 | ||||||
| Results for the period |
(50,790 | ) | (34,705 | ) | ||||
5
Table of Contents
| Balance Sheet Data |
December 31, 2022 | |||
| Current Assets |
22,583 | |||
| Total Assets |
188,798 | |||
| Equity |
145,417 | |||
| Non-Current Liabilities |
32,665 | |||
| Current Liabilities |
10,716 | |||
| B. | CAPITALIZATION AND INDEBTEDNESS |
The table below sets forth our cash and cash equivalents and shows our capitalization as of December 31, 2022. You should read this table in conjunction with our unaudited condensed consolidated financial statements included in this annual report, together with the accompanying notes and the other information appearing under the heading “Item 5. Operating and Financial Review and Prospects.” All amounts below are in Swiss Francs (CHF) and are in thousands. Liabilities set forth below are unsecured and non-guaranteed.
| December 31, 2022 |
||||
| Cash |
||||
| Cash and Cash Equivalents |
19,237 | |||
|
|
|
|||
| Total cash |
19,237 | |||
| Debt |
||||
| Non-current liabilities |
32,665 | |||
| Current liabilities |
10,716 | |||
| Total debt |
43,381 | |||
|
|
|
|||
| Shareholder’s equity: |
||||
| Share capital |
56,163 | |||
| Treasury shares |
(12,108 | ) | ||
| Reserves |
220,961 | |||
| Accumulated loss |
(119,599 | ) | ||
| Total equity |
145,417 | |||
| Total liabilities and shareholder’s equity |
188,798 | |||
|
|
|
|||
As of April 30, 2023, we had cash and cash equivalents of approximately CHF 11.1 million. We will need to obtain additional funding to continue our operations beyond the third quarter of 2023.
On April 28, 2023, our stockholders approved a consolidation (or reverse split) of our ordinary shares at a 400 to 1 ratio. The consolidation occurred on May 5, 2023. Assuming we meet all listing requirements, we intend to seek a listing of our ordinary shares on the NASDAQ stock market. There can be no assurance that we will be successful in such listing.
| C. | REASONS FOR THE OFFER AND USE OF PROCEEDS |
Not applicable
6
Table of Contents
| D. | RISK FACTORS |
Risk Factors Summary
We are providing the following summary of the risk factors contained in our Form 20-F to enhance the readability and accessibility of our risk factor disclosures. We encourage our stockholders to carefully review the full risk factors contained in this Form 20-F in their entirety for additional information regarding the risks and uncertainties that could cause our actual results to vary materially from our recent results or from our anticipated future results.
Risks Related to Our Business
| • | We depend heavily on the success of our product candidates. If our clinical studies are unsuccessful, if we or our collaboration partners do not obtain regulatory approval or if we or our collaboration partners are unable to commercialize our product candidates, or if we experience significant delays in doing so, our business, financial condition and results of operations will be materially adversely affected. |
| • | Our business is subject to significant regulation from governments and regulatory bodies, including marketing approval requirements, which could lengthen the development time, increase the cost of developing our drug product candidates or delay, prevent or limit the commercialization of our product candidates. |
| • | We have a limited history of commercializing pharmaceutical products, which may make it difficult to evaluate our future viability. |
| • | Results of early clinical studies may not be predictive of future study results. |
| • | The successful commercialization of our product candidates will depend on the extent to which governmental authorities and health insurers establish adequate coverage and reimbursement levels and pricing policies. |
| • | Our products may not gain market acceptance, in which case we or our collaboration partners may not be able to generate product revenues, which would materially adversely affect our business, financial condition and results of operations. |
| • | We depend on enrollment of patients in our clinical studies for our product candidates. If we are unable to enroll patients in our clinical studies, our research and development efforts could be materially adversely affected. |
| • | If serious adverse, undesirable or unacceptable side effects are identified during the development of our product candidates or following approval, if any, we may need to abandon our development of such product candidates, the commercial profile of any approved label may be limited, or we may be subject to other significant negative consequences following marketing approval, if any. |
| • | We operate in highly competitive and rapidly changing industries, which may result in others discovering, developing or commercializing competing products before or more successfully than we do. |
| • | Our business is subject to additional risks associated with international operations. |
| • | The COVID-19 pandemic may impact our business. |
| • | Our future growth and ability to compete depends on retaining our key personnel and recruiting additional qualified personnel. |
| • | We may become exposed to costly and damaging liability claims, either when testing our product candidates or at the commercial stage or as a result of claims against our directors and officers, and our liability insurance may not cover all damages from such claims. |
| • | Business disruptions could seriously harm our future revenue and financial condition and increase our costs. |
7
Table of Contents
| • | Pressure on drug product third-party payor coverage, reimbursement and pricing may impair our ability to be reimbursed at prices or on terms sufficient to provide a viable financial outcome. |
| • | We cannot give any assurance that any of our product candidates in development will receive regulatory approval, which is necessary before they can be commercialized. |
| • | Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes. If clinical studies of our product candidates are prolonged or delayed, we may be unable to obtain required regulatory approvals, and therefore be unable to commercialize our product candidates on a timely basis or at all. |
| • | Even if we obtain and maintain approval for certain of our drug candidates from one jurisdiction, we may never obtain approval for our drug candidates in other jurisdictions, which would limit our market opportunities and adversely affect our business. |
| • | We have conducted and may in the future conduct clinical studies for our drug candidates in the U.S., Europe and Switzerland, and the FDA, EMA and Swissmedic and applicable foreign regulatory authorities may not accept data from such studies. |
| • | Even if certain of our product candidates obtain regulatory approval, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expenses. Additionally, our additional product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products. |
| • | Our business is subject to complex and evolving U.S. and international laws and regulations regarding clinical trials reimbursement and privacy and data protection. Many of these laws and regulations are subject to change and uncertain interpretation and could result in claims, changes to our business practices, penalties, increased cost of operations, or declines in user growth or engagement, or otherwise harm our business. |
| • | We could be subject to liabilities under environmental, health and safety laws or regulations, or fines, penalties or other sanctions, if we fail to comply with such laws or regulations or otherwise incur costs that could have a material adverse effect on the success of our business. |
Risks Related to Our Relationships with Third Parties
| • | If we fail to maintain our strategic relationships with any of our current or future collaboration or strategic partners, our business, commercialization prospects and financial condition may be materially adversely affected. |
| • | We may seek to form additional strategic alliances in the future with respect to our product candidates, and if we do not realize the benefits of such alliances, our business, financial condition, commercialization prospects and results of operations may be materially adversely affected. |
| • | We rely on third parties to conduct our nonclinical and clinical studies and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates, and our business could be substantially harmed. |
| • | We currently rely on third-party suppliers and other third parties for production of our product candidates and our dependence on these third parties may impair the advancement of our research and development programs and the development of our product candidates. |
Risks Related to Intellectual Property
| • | We may not have sufficient patent terms to protect our products and business effectively. |
8
Table of Contents
| • | We or our licensing or collaboration partners may become subject to intellectual property-related litigation or other proceedings to protect or enforce our patents or the patents of our licensors or licensees and collaborators, any of which could be expensive, time-consuming, and unsuccessful, and may ultimately result in our loss of ownership of intellectual property. |
| • | If we or our licensing or collaboration partners are unable to obtain and maintain effective patent rights for our technologies, product candidates or any future product candidates, or if the scope of the patent rights obtained is not sufficiently broad, our competitors could develop and commercialize products and technology similar or identical to ours, and our, or our collaboration partners’ ability to successfully commercialize our products and technology may be adversely affected. |
| • | We may be subject to claims challenging the inventorship of our patents and other intellectual property. |
| • | Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, thereby impairing our ability to protect our technologies and products. |
| • | If we are unable to maintain effective proprietary rights for our technologies, product candidates or any future product candidates, we may not be able to compete effectively in our markets. |
| • | Obtaining and maintaining our patent protection depends on compliance with various procedural, document-submission, fee-payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements. |
| • | The patent protection and patent prosecution for some of our product candidates could be dependent on third parties. |
| • | Third-party claims of intellectual property infringement may expose us to substantial liability or may prevent or delay our or our collaboration partners’ development and commercialization efforts. |
| • | We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers. |
| • | We may not be able to protect our intellectual property rights throughout the world. |
| • | We may be unable to protect our trade secrets, know-how and technologies. |
Risks Related to Our Financial Condition and Results of Operations
| • | We are a commercial-stage biopharmaceutical company with a history of operating losses. Our recurring losses, negative cash flows and significant accumulated deficit have raised substantial doubt regarding our ability to continue as a going concern. |
| • | If we fail to obtain additional funding, we may delay, reduce or eliminate our product development programs or commercialization efforts. |
| • | Raising additional capital will likely cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our intellectual property or future revenue streams. |
| • | Exchange rate fluctuations may materially affect our results of operations and financial condition. |
| • | The SIX Exchange Regulation AG has launched an investigation into Relief, the results of which are uncertain. |
9
Table of Contents
Risk Factors
You should carefully consider the risks and uncertainties described below and the other information in this Annual report before making an investment. Our business, financial condition or results of operations could be materially and adversely affected if any of these risks occur, and as a result, the market price of our shares could decline. This Annual report also contains forward-looking statements that involve risks and uncertainties. See “Forward-Looking Statements.” Our actual results could differ materially and adversely from those anticipated in these forward- looking statements as a result of certain factors.
Risks Related to Our Business
We depend heavily on the success of our product candidates. If our clinical studies are unsuccessful, if we or our collaboration partners do not obtain regulatory approval or if we or our collaboration partners are unable to commercialize our product candidates, or if we experience significant delays in doing so, our business, financial condition and results of operations will be materially adversely affected.
We currently have a small number of products approved for sale, all of which were acquired in the business combination with APR, generating a limited volume of sales. We have licensed OLPRUVA™ (ACER-001), and have added additional products to our portfolio through our acquisitions of AdVita and APR. Our ability to generate significantly higher product revenues will depend heavily on successful clinical development, obtaining regulatory approval and eventual commercialization of these product candidates. The success of our current and future product candidates will depend on several factors, including the following:
| • | completing preclinical studies and clinical studies that demonstrate the efficacy, safety and clinical utility of our product candidates; |
| • | receiving marketing approvals from applicable regulatory authorities; |
| • | developing product formulations with sufficiently long-term stability and chemistry, manufacturing and controls that meet governmental regulatory standards; |
| • | establishing commercial manufacturing capabilities; |
| • | launching commercial sales, marketing and distribution operations; |
| • | acceptance of our product candidates by patients, the medical community and third-party payors; |
| • | a continued acceptable safety profile following approval; |
| • | competing effectively with other therapies; and |
| • | obtaining, maintaining, enforcing and defending our intellectual property rights and claims and not infringing on third parties’ intellectual property rights. |
If we or our collaborators do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our current or future product candidates, which would materially adversely affect our business, financial conditions and results of operations.
Our business is subject to significant regulation from governments and regulatory bodies, including marketing approval requirements, which could lengthen the development time, increase the cost of developing our drug product candidates or delay, prevent or limit the commercialization of our product candidates.
When a medicinal product candidate receives regulatory approval, the approval can nonetheless be subject to limitations, e.g., with regard to the indications for which it may be marketed. The approval may also be given subject to conditions, such as additional proof of the medicinal product’s effectiveness and safety. Even after approval is granted, manufacturing, safety, efficacy, recordkeeping, labeling, marketing, sales and distribution of its product candidates are regulated by government agencies in countries where we intend to market our products. All these activities are subject to recurring scrutiny and regular inspections by the relevant agencies. As a consequence, if previously unknown problems are discovered in connection with an approved product, its manufacturer or the manufacturing facilities, this can result in restrictions on the product, the manufacturer or the manufacturing facilities, up to the requirement to withdraw the product from the market. In any event, changes in existing regulations or adoption of new regulations could prevent the Company and/or its commercialization partners from obtaining or maintaining, or affect the timing of, future regulatory approvals.
10
Table of Contents
These and other factors, alone or together, may have a material adverse effect on the Company’s business, financial condition, results of operations and growth prospects as well as the market price of our securities.
We have a limited history of commercializing pharmaceutical products, which may make it difficult to evaluate our future viability.
Our operations to date have mostly been limited to financing and staffing our company, developing our technology, and developing our product candidates. Until our acquisition of APR, we had not generated any revenue from product sales. While we began marketing commercial products with our acquisition of APR, our history of operating in the commercial market is short. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a longer history of successfully developing and commercializing pharmaceutical products. Factors that may affect our ability to commercialize our product candidates on our own include recruiting and retaining adequate numbers of effective sales and marketing personnel, obtaining access to or persuading adequate numbers of physicians to prescribe our drug candidates, and other unforeseen costs associated with creating an independent sales and marketing organization. Developing a sales and marketing organization requires significant investment, is time consuming and could delay the launch of our product candidates. Consequently, we may not be able to build an effective sales and marketing organization. Additionally, successful commercialization also requires an enhanced regulatory organization, which we currently do not have. If we are unable to build our own distribution and marketing capabilities, are unable to find suitable partners for the commercialization of our product candidates or do not successfully obtain the necessary regulatory capabilities, we may not generate sufficient revenues from them and as a result may not be able to reach or sustain profitability.
Results of early clinical studies may not be predictive of future study results.
Positive or timely results from preclinical or early-stage clinical studies do not ensure positive or timely results in late-stage clinical studies or product approval by the FDA, the EMA, or comparable foreign regulatory authorities. Products that show positive preclinical or early clinical results may not show sufficient safety or efficacy in later-stage clinical studies and therefore may fail to obtain regulatory approvals. In addition, preclinical and clinical data are often susceptible to varying interpretations and analyses. Many companies that believed their product candidates performed satisfactorily in preclinical and clinical studies have nonetheless failed to obtain marketing approval for the product candidates. The FDA, the EMA and comparable foreign regulatory authorities have substantial discretion in the approval process and in determining when or whether regulatory approval will be obtained for any of our product candidates. Even if we believe that the data collected from clinical studies of our product candidates are promising, such data may not be sufficient to support approval by the FDA, the EMA or any other regulatory authority.
In some instances, there can be significant variability in safety and/or efficacy results between different studies of the same product candidate due to numerous factors, including changes in study procedures set forth in protocols, differences in the size and type of the patient populations, adherence to the dosing regimen and other study protocols, and the rate of dropout among clinical study participants. In the case of our later-stage clinical product candidates, results may differ in general on the basis of the larger number of clinical study sites and the additional countries and languages involved in these clinical studies.
Clinical studies may include subject-reported outcomes, some of which may be captured with electronic diaries. We have no assurance and cannot rely on past experience that the high frequency of questioning is not influencing the measured outcome. In addition, low compliance with daily reporting requirements may impact the studies’ validity or statistical power. We cannot assure that any Phase 1, phase 2, phase 3 or other clinical studies that either we or our collaboration partners may conduct will demonstrate consistent or adequate efficacy and safety to obtain regulatory approval to market our product candidates.
If we or our collaboration partners are required to conduct additional clinical studies or other testing of any of our current or future product candidates that we or our collaboration partners develop, beyond the studies and testing that we or our collaboration partners contemplate, if we or our collaboration partners are unable to successfully complete clinical studies of our product candidates or other testing, if the results of these studies or tests are unfavorable or are only modestly favorable, or if there are safety concerns associated with our current or future product candidates, we may:
11
Table of Contents
| • | be delayed in obtaining marketing approval for our product candidates; |
| • | not obtain marketing approval; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings; |
| • | be subject to conditional approval or otherwise to additional post-marketing studies or other requirements; or |
| • | remove the product from market after obtaining marketing approval. |
Our product development costs will also increase if we experience delays in testing or receiving marketing approvals and we may be required to obtain additional funds to complete clinical studies. We cannot assure that our clinical studies will begin as planned or be completed on schedule, if at all, or that we will not need to amend our studies after they have begun. Significant clinical study delays could also shorten any periods during which we or our collaboration partners may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which may harm our business and results of operations. In addition, some of the factors that cause, or lead to, clinical study delays may ultimately lead to the denial of regulatory approval of our product candidates.
The successful commercialization of our product candidates will depend on the extent to which governmental authorities and health insurers establish adequate coverage and reimbursement levels and pricing policies.
The successful commercialization of our product candidates will depend, in part, on the extent to which coverage and reimbursement for our products will be available from government and health administration authorities, private health insurers and other third-party payors. To manage healthcare costs, many governments and third-party payors increasingly scrutinize the pricing of new technologies and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage. In light of such challenges to prices and the requirement for increasing levels of evidence of the benefits and clinical outcomes of new technologies, we cannot be sure that coverage will be available for any of our current or future product candidates that we or our collaboration partners will commercialize or, if available, that the reimbursement rates will be adequate in each respective region. If we are unable to obtain adequate levels of coverage and reimbursement for our product candidates, their marketability will be negatively and materially impacted.
Third-party payors may deny coverage and reimbursement status altogether for a given drug product or may cover the product but also establish prices at levels that are too low to enable us to realize an appropriate return on our investment in product development. Because the rules and regulations regarding coverage and reimbursement change frequently, in some cases at short notice, even when there is favorable coverage and reimbursement, future changes may occur that adversely impact the favorable status. Further, the net reimbursement for drug products may be subject to additional reductions in the future depending on policy changes enacted by the national regulatory bodies.
The unavailability or inadequacy and variability of third-party coverage and reimbursement could have a material adverse effect on the market acceptance of our product candidates and the future revenues we may expect to receive from those products. In addition, we are unable to predict what additional legislation or regulation relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future, or what effect such legislation or regulation would have on our business.
Our products may not gain market acceptance, in which case we or our collaboration partners may not be able to generate product revenues, which would materially adversely affect our business, financial condition and results of operations.
Even if the FDA, the EMA or any other regulatory authority approves the marketing of any product candidates that we develop, physicians, healthcare providers, patients or the medical community may not accept or use them. Efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may not be successful. If any of our current or future product candidates does not achieve an adequate level of acceptance, we or our collaboration partners may not generate significant product or royalty revenues or any profits from operations. The degree of market acceptance of our product candidates that are approved for commercial sale will depend on a variety of factors, including:
| • | how clinicians and potential patients perceive our novel products; |
12
Table of Contents
| • | the timing of market introduction; |
| • | the number and clinical profile of competing products; |
| • | our ability to provide acceptable evidence of safety and efficacy; |
| • | the prevalence and severity of any side effects; |
| • | relative convenience and ease of administration; |
| • | cost-effectiveness; |
| • | patient diagnostics and screening infrastructure in each market; |
| • | marketing and distribution support; |
| • | availability of coverage, reimbursement and adequate payment from third party payors, both public and private; and |
| • | other potential advantages over alternative treatment methods. |
If our product candidates fail to gain market acceptance, this will have a material adverse impact on our ability to generate revenues to provide a satisfactory, or any, return on our investments. Even if some products achieve market acceptance, the market may prove to not be large enough to allow us to generate significant revenues.
In addition, the potential market opportunity of our product candidates is difficult to estimate precisely. Our estimates of the potential market opportunity are predicated on several key assumptions such as industry knowledge and publications, third-party research reports and other surveys. These assumptions involve the exercise of significant judgment on the part of our management and are inherently uncertain, and the reasonableness of these assumptions could not have been assessed by an independent source in every detail. If any of the assumptions proves to be inaccurate, then the actual market for our product candidates could be smaller than our estimates of the potential market opportunity. If the actual market for our product candidates is smaller than we expect, or if any approved products fail to achieve an adequate level of acceptance by physicians, healthcare payors and patients, our product or royalty revenue may be limited, and it may be more difficult for us to achieve or maintain profitability.
We depend on enrollment of patients in our clinical studies for our product candidates. If we are unable to enroll patients in our clinical studies, our research and development efforts could be materially adversely affected.
If our product candidates are associated with serious adverse, undesirable or unacceptable side effects, we may need to abandon their development or limit development to certain uses or subpopulations in which such side effects are less prevalent, less severe or more acceptable from a risk–benefit perspective. Many compounds that initially showed promise in preclinical or early-stage testing were later found to cause side effects that restricted their use and prevented further development of the compound for larger indications.
Generally, the specific target population of patients and therapeutic time windows may make it difficult for us to enroll enough patients to complete clinical studies for our products in a timely and cost-effective manner. Delays in the completion of any clinical study of our product candidates will increase our costs, slow down our product candidate development and approval process, and delay or potentially jeopardize our or our collaboration partners’ ability to commence product sales and generate revenue. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical studies may also ultimately lead to the denial of regulatory approval of our product candidates.
If serious adverse, undesirable or unacceptable side effects are identified during the development of our product candidates or following approval, if any, we may need to abandon our development of such product candidates, the commercial profile of any approved label may be limited, or we may be subject to other significant negative consequences following marketing approval, if any.
If our product candidates are associated with serious adverse, undesirable or unacceptable side effects, we may need to abandon their development or limit development to certain uses or subpopulations in which such side effects are less prevalent, less severe or more acceptable from a risk–benefit perspective. Many compounds that initially showed promise in preclinical or early-stage testing were later found to cause side effects that restricted their use and prevented further development of the compound for larger indications.
13
Table of Contents
Occurrence of serious side effects could impede clinical study enrollment and receipt of marketing approval from the U.S. FDA, the EMA and comparable other national regulatory authorities. Adverse events (AEs) and/or serious adverse events (SAEs) could also adversely affect physician or patient acceptance of our product candidates.
Additionally, if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including the following:
| • | regulatory authorities may withdraw approvals of such product and require us or our collaboration partners to take any approved products off the market; |
| • | regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication or field alerts to physicians and pharmacies; |
| • | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; |
| • | we may be required to change the way the product is administered, to conduct additional studies or to change the labeling of the product; |
| • | we or our collaboration partners may be subject to limitations in how we promote the product; |
| • | sales of the product may decrease significantly; |
| • | we could be sued and held liable for harm caused to patients; and |
| • | our reputation and physician or patient acceptance of our products may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
We operate in highly competitive and rapidly changing industries, which may result in others discovering, developing or commercializing competing products before or more successfully than we do.
The biopharmaceutical and pharmaceutical industries are highly competitive and subject to significant and rapid technological change. Our success is highly dependent on our ability to discover, develop and obtain marketing approval for new and innovative products on a cost-effective basis and to market them successfully. In doing so, we face and will continue to face intense competition from a variety of businesses, including large, fully integrated pharmaceutical companies, specialty pharmaceutical companies and biopharmaceutical companies, academic institutions, government agencies and other private and public research institutions in Europe, the U.S. and other jurisdictions. Many of our potential competitors, alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments, and the commercialization of those treatments. Mergers and acquisitions in the pharmaceutical and biopharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors.
The highly competitive nature of and rapid technological changes in the pharmaceutical and biopharmaceutical industries could render our product candidates or our technology obsolete or noncompetitive. The commercial opportunity for our products could be reduced or eliminated if our competitors:
| • | develop and commercialize products that are safer, more effective, less expensive, or more convenient or easier to administer; |
| • | obtain quicker FDA or other regulatory approval for their products; |
| • | establish superior intellectual property and proprietary positions; |
14
Table of Contents
| • | have access to more manufacturing capacity; |
| • | implement more effective approaches to sales, marketing and distribution; or |
| • | form more advantageous strategic alliances. |
Should any of these occur, our business, financial condition and results of operations could be materially adversely affected.
Our business is subject to additional risks associated with international operations.
Our business is subject to risks associated with conducting business internationally. Accordingly, our future results could be harmed by a variety of factors, including:
| • | potentially reduced protection for intellectual property rights; |
| • | changes in a specific country’s or region’s political or economic environment; |
| • | negative consequences from changes in tax laws; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | difficulties associated with staffing and managing international operations, including differing labor relations; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
The COVID-19 pandemic may impact our business.
In December 2019, a novel strain of coronavirus, COVID-19, surfaced in Wuhan, Hubei Province, China. By March 2020, COVID-19 had spread to other countries, including Switzerland and the U.S., and was declared a pandemic by the World Health Organization on March 11, 2020. Since the beginning of the pandemic, governments, public institutions, and other organizations in countries and localities where COVID-19 cases have been identified have taken certain preventative or protective measures to combat the transmission of the virus, including implementation of travel restrictions or bans, closures of non-essential businesses, limitations of public gatherings, other social distancing and shelter-in-place measures, and delays or cancellations of elective surgeries. The COVID-19 pandemic continues to pose the risk that the Company, our employees, contractors, suppliers, and other partners may be prevented from conducting business activities for an indefinite period of time due to shutdowns that may be requested or mandated by state and federal governmental authorities.
As the COVID-19 pandemic continues, we may experience disruptions that could materially impact our business and planned clinical trials, including:
| • | delays or difficulties in conducting preclinical and clinical trials; |
| • | interruption in global manufacturing and shipping that may affect the manufacturing and/or transport of clinical trial materials and other materials, including testing equipment; and |
| • | changes in local regulations as a response to COVID-19 that may require us to change the way we perform our trials. |
15
Table of Contents
Our future growth and ability to compete depends on retaining our key personnel and recruiting additional qualified personnel.
Our success depends upon the continued contributions of our key management, scientific and technical personnel, many of whom have substantial experience with or been instrumental for us and our projects. The loss of our key managers and senior scientists could delay our research and development activities. Laws and regulations on executive compensation, including legislation in Switzerland, may restrict our ability to attract, motivate and retain the required level of qualified personnel. In Switzerland, legislation affecting public companies has been passed that, among other things, imposes an annual binding shareholder “say on pay” vote with respect to the compensation of the executive management, including executive officers and the members of the board of directors. In addition, the competition for qualified personnel in the pharmaceutical and biopharmaceutical field is intense, and our future success depends upon our ability to attract, retain and motivate highly skilled scientific, technical and managerial employees. We face competition for personnel from other companies, universities, public and private research institutions and other organizations. If our recruitment and retention efforts are unsuccessful in the future, it may be difficult for us to implement our business strategy, which could have a material adverse effect on our business.
We may become exposed to costly and damaging liability claims, either when testing our product candidates or at the commercial stage or as a result of claims against our directors and officers, and our liability insurance may not cover all damages from such claims.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical or biopharmaceutical products. Currently we have no products that have been approved for commercial sale; however, our current and future use of product candidates in clinical studies, and the sale of any approved products in the future, may expose us to liability claims. These claims might be made by patients that use the product, by healthcare providers, or by pharmaceutical or biopharmaceutical companies or others selling such products. Any claims against us, regardless of their merit, could be difficult and costly to defend and could materially adversely affect the market for our product candidates or any prospects for commercialization of our product candidates.
Although the clinical trial process is designed to identify and assess potential side effects, it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. If any of our product candidates were to cause adverse side effects during clinical studies or after approval of the product candidate, we may be exposed to substantial liabilities. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our product candidates.
We continuously seek to maintain appropriate and cost-effective liability insurance coverage in connection with our products and for purposes of indemnifying our directors and officers for claims against them. It is, however, possible that our liabilities could exceed our insurance coverage. For example, we intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for any of our product candidates. However, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy any liability that may arise. If a successful liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business operations could be impaired.
Should any of the events described above occur, this could have a material adverse effect on our business, financial condition and results of operations.
16
Table of Contents
Business disruptions could seriously harm our future revenue and financial condition and increase our costs.
Our operations and those of our third-party collaborators and other contractors and consultants, could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics, and other natural or man-made disasters or business interruptions, for which we are partly uninsured. In addition, we rely on our third-party research institution collaborators for conducting research and development of our product candidates, and they may be affected by government shutdowns or withdrawn funding. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce and process our product candidates. Our ability to obtain clinical supplies of our product candidates could be disrupted if the operations of these suppliers are affected by a man-made or natural disaster or by other business interruption.
Our operations are conducted internationally with employees, consultants and strategic vendors located in the U.S. and in Europe, including Switzerland, where the Company is headquartered. Damage or extended periods of interruption to our corporate, development or research facilities due to fire, natural disaster, power loss, communications failure, unauthorized entry or other events could cause us to cease or delay development of some or all of our product candidates. Although we maintain property damage and business interruption insurance coverage on these facilities, our insurance might not cover all losses under such circumstances and our business may be seriously harmed by such delays and interruption.
Pressure on drug product third-party payor coverage, reimbursement and pricing may impair our ability to be reimbursed at prices or on terms sufficient to provide a viable financial outcome.
The commercial success of our pharmaceutical products will depend substantially on the extent to which the cost of those products will be paid or reimbursed by government health administration authorities (such as Medicare and Medicaid in the U.S.), private health coverage providers and other third party payors. If reimbursement is not available, or is available only to limited levels, we may be unable to continue to successfully commercialize our products. Even if coverage is provided, the approved reimbursement amount may not be high enough to establish and maintain pricing sufficient to realize a meaningful return on our investment.
The healthcare industry is acutely focused on cost containment, both in the U.S. and elsewhere. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability to sell our products profitably. These payors may not view our products as cost-effective, and coverage and reimbursement may not be available to our customers, or may not be sufficient to allow our products, if any, to be marketed on a competitive basis. Cost-control initiatives could cause us to decrease the price we might establish for products, which could result in lower than anticipated product revenues. If the prices for our products decrease or if governmental and other third-party payors do not provide adequate coverage or reimbursement, our prospects for revenue and profitability will suffer.
We cannot give any assurance that any of our product candidates in development will receive regulatory approval, which is necessary before they can be commercialized.
We cannot be certain that any of our product candidates in development will be successful in clinical studies or receive regulatory approval. Applications for our product candidates could fail to receive regulatory approval for many reasons, including but not limited to the following:
| • | the FDA, EMA, Swissmedic or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical studies; |
| • | the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval; |
| • | the FDA, EMA, Swissmedic or comparable foreign regulatory authorities may disagree with our interpretation of data from nonclinical or clinical studies; |
| • | the data collected from clinical studies of our product candidates may not be sufficient to support the submission of a new drug application (NDA) or other submission or to obtain regulatory approval in the U.S., Switzerland or elsewhere; |
17
Table of Contents
| • | we may be unable to demonstrate to the FDA, EMA, Swissmedic or comparable foreign regulatory authorities that a product candidate’s benefit-risk ratio for its proposed indication is acceptable; |
| • | the FDA, EMA, Swissmedic or other regulatory authorities may fail to approve the manufacturing processes, test procedures and specifications, or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| • | the approval policies or regulations of the FDA, EMA, Swissmedic or comparable foreign regulatory authorities may change significantly in a manner rendering our clinical data insufficient for approval. |
We generally plan to seek regulatory approval to commercialize our product candidates in the U.S., the EU, Switzerland and in additional foreign countries where we have commercial and typically IP rights. To obtain regulatory approval in other countries, we must comply with numerous and varying regulatory requirements of such other countries regarding safety, efficacy, chemistry, manufacturing and controls, clinical studies, commercial sales, pricing, marketing and distribution of our product candidates. Even if we are successful in obtaining approval in one jurisdiction, we cannot ensure that we will obtain approval in any other jurisdictions. Failure to obtain marketing authorization for our product candidates will result in our being unable to market and sell such products, which would materially adversely affect our business, financial condition and results of operations. If we fail to obtain approval in any jurisdiction, the geographic market for our product candidates could be limited.
Similarly, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes. If clinical studies of our product candidates are prolonged or delayed, we may be unable to obtain required regulatory approvals, and therefore be unable to commercialize our product candidates on a timely basis or at all.
In order to commercialize any of our product candidates, we or our partners must obtain the necessary regulatory approvals to market and sell such product. To obtain that approval, we must demonstrate through extensive preclinical and clinical studies that our products are safe and effective in humans. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical study process. The results of preclinical and early clinical studies of our product candidates may not be predictive of the results of later-stage clinical studies. For example, the positive results generated to date in clinical studies for our product candidates do not ensure that later clinical studies will demonstrate similar results. Product candidates in later stages of clinical studies may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical studies. A number of companies in the pharmaceutical or biopharmaceutical industry, including us, have suffered significant setbacks in advanced clinical studies due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies. Our future clinical study results may not be successful.
Clinical studies must be conducted in accordance with the legal requirements, regulations or guidelines of the FDA, EMA, Swissmedic and comparable regulatory authorities, and are subject to oversight by these governmental agencies and institutional review boards (IRBs) at the medical institutions where the clinical studies are conducted. In addition, clinical studies must be conducted with supplies of our product candidates produced under cGMP and other requirements. We depend on medical institutions and CROs to conduct our clinical studies in compliance with cGCP standards. To the extent the CROs fail to enroll participants for our clinical studies, fail to conduct the study to cGCP standards or are delayed for a significant time in the execution of studies, including achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business.
The completion of clinical studies for our clinical product candidates may be delayed, suspended or terminated as a result of many factors, including but not limited to:
| • | the delay or refusal of regulators or IRBs to authorize us to commence or amend a clinical study at a prospective study site or changes in regulatory requirements, policies and guidelines; |
| • | delays or failure to reach agreement on acceptable terms with prospective CROs and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and study sites; |
| • | delays in patient enrollment and variability in the number and types of patients available for clinical studies; |
18
Table of Contents
| • | the inability to enroll a sufficient number of patients in studies to ensure adequate statistical power to detect statistically significant treatment effects; |
| • | negative or inconclusive results, which may require us to conduct additional preclinical or clinical studies or to abandon projects that we expected to be promising; |
| • | safety or tolerability concerns, which could cause us to suspend or terminate a study if we find that the participants are being exposed to unacceptable health risks; |
| • | regulators or IRBs requiring that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or safety concerns, among others; |
| • | lower than anticipated retention rates of patients and volunteers in clinical studies; |
| • | our CROs or clinical study sites failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, deviating from the protocol or dropping out of a study; |
| • | delays relating to adding new clinical study sites; |
| • | difficulty in maintaining contact with patients after treatment, resulting in incomplete data; |
| • | delays in establishing the appropriate dosage levels; |
| • | the quality or stability of the product candidate falling below acceptable standards; |
| • | the inability to produce or obtain sufficient quantities of the product candidate to complete clinical studies; and |
| • | exceeding budgeted costs due to difficulty in accurately predicting costs associated with clinical studies. |
Any delays in completing our clinical studies will increase our costs, slow our product candidate development and approval process, and jeopardize our ability to commence product sales and generate sales revenues. Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical studies may also ultimately lead to the denial of regulatory approval of certain of our product candidates.
Even if we obtain and maintain approval for certain of our drug candidates from one jurisdiction, we may never obtain approval for our drug candidates in other jurisdictions, which would limit our market opportunities and adversely affect our business.
If marketing authorization is obtained for certain of our product candidates, the products will remain subject to continual regulatory review and therefore authorization could be subsequently withdrawn or restricted. Any regulatory approvals that we receive for certain of our product candidates may also be subject to limitations on the approved indicated uses for which the products may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical studies, and surveillance to monitor the safety and efficacy of the product candidate. In addition, if the FDA or a comparable regulatory authority approves any of our product candidates in development, we will be subject to ongoing regulatory obligations and oversight by regulatory authorities, including with respect to the manufacturing processes, labeling, packing, distribution, adverse event reporting, storage, advertising and marketing restrictions, and record-keeping and, potentially, other post-marketing obligations, all of which may result in significant expense and limit our or our collaboration partners’ ability to commercialize such products. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMP and cGCP requirements for any clinical studies that we conduct post-approval. Later discovery of previously unknown problems with a product, including AEs of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, warning letters or holds on clinical studies; |
19
Table of Contents
| • | refusal by the FDA to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| • | regulatory constraints in promotion and distribution of drug products in various markets; |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
If any of these events occurs, our ability to sell such product may be impaired, and we may incur substantial additional expense to comply with regulatory requirements, which could materially adversely affect our business, financial condition and results of operations. Regulatory policies may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
We have conducted and may in the future conduct clinical studies for our drug candidates outside the U.S., Europe and Switzerland, and the FDA, EMA and Swissmedic and applicable foreign regulatory authorities may not accept data from such studies.
We, or our collaboration partners, have conducted and may in the future choose to conduct one or more of our clinical studies outside the U.S., Europe and Switzerland. The acceptance of study data from clinical studies conducted outside the U.S., Europe and Switzerland or another jurisdiction by the FDA, EMA and Swissmedic or applicable foreign regulatory authority may be subject to certain conditions. In cases where data from foreign clinical studies are intended to serve as the basis for marketing approval, for instance in the U.S., the FDA will not approve the application on the basis of foreign data alone unless the following are true: the data are applicable to the U.S. population and U.S. medical practice; the studies were performed by clinical investigators of recognized competence; and the data are considered valid without the need for an on-site inspection by the FDA or, if the FDA considers such an inspection to be necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means. Additionally, the FDA’s clinical study requirements, including sufficient size of patient populations and statistical powering, must be met. Many foreign regulatory bodies have similar requirements. In addition, such foreign studies would be subject to the applicable local laws of the foreign jurisdictions in which the studies are conducted. There can be no assurance that the FDA, EMA, Swissmedic or any applicable foreign regulatory authority will accept data from studies conducted outside of the U.S. or the applicable jurisdiction. If the FDA, EMA, Swissmedic or any applicable foreign regulatory authority does not accept such data, it would result in the need for additional studies, which would be costly and time-consuming and delay aspects of our business plan, and which may result in our drugs or drug candidates not receiving approval or clearance for commercialization in the applicable jurisdiction.
Even if certain of our product candidates obtain regulatory approval, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expenses. Additionally, our additional product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products.
If marketing authorization is obtained for certain of our product candidates, the products will remain subject to continual regulatory review and therefore authorization could be subsequently withdrawn or restricted. Any regulatory approvals that we receive for certain of our product candidates may also be subject to limitations on the approved indicated uses for which the products may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including phase 4 clinical studies, and surveillance to monitor the safety and efficacy of the product candidate. In addition, if the FDA or a comparable regulatory authority approves any of our product candidates in development, we will be subject to ongoing regulatory obligations and oversight by regulatory authorities, including with respect to the manufacturing processes, labeling, packing, distribution, adverse event reporting, storage, advertising and marketing restrictions, and record-keeping and, potentially, other post-marketing obligations, all of which may result in significant expense and limit our or our collaboration partners’ ability to commercialize such products. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMP and cGCP requirements for any clinical studies that we conduct post-approval. Later discovery of previously unknown problems with a product, including AEs of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
20
Table of Contents
| • | fines, warning letters or holds on clinical studies; |
| • | refusal by the FDA to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| • | regulatory constraints in promotion and distribution of drug products in various markets; |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
If any of these events occurs, our ability to sell such product may be impaired, and we may incur substantial additional expense to comply with regulatory requirements, which could materially adversely affect our business, financial condition and results of operations. Regulatory policies may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
Our business is subject to complex and evolving U.S. and international laws and regulations regarding clinical trials reimbursement and privacy and data protection. Many of these laws and regulations are subject to change and uncertain interpretation and could result in claims, changes to our business practices, penalties, increased cost of operations, or declines in user growth or engagement, or otherwise harm our business.
Regulatory authorities around the world have adopted laws and regulations and are continuing to consider a number of legislative and regulatory proposals, concerning privacy and data protection, including measures to ensure that encryption of users’ data does not hinder access of law enforcement agencies to that data. In addition, the interpretation and application of consumer and data protection laws in the U.S., Europe, Switzerland and elsewhere are often uncertain and in flux. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our data practices. These laws and regulations, and legislative and regulatory proposals, if adopted, and such interpretations could, in addition to the possibility of fines, result in an order requiring that we change our data practices, which could have an adverse effect on our business and results of operations. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices in a manner adverse to our business.
In the EU, new clinical trial regulations came into force on January 31, 2022. This new legislation enforces the centralization of clinical trial applications and approvals, New clinical trial sponsors must begin using the new system by January 31, 2023, and any previously approved trial sponsors must comply from January 31, 2025, but in some cases, this may extend timelines for clinical study approvals, due to potentially longer wait times enabling sponsors to apply for trial authorization in up to 30 European countries with a single online application. The General Data Protection Regulation (GDPR), which became effective in May 2018 in all EU member states, created a range of new compliance obligations for companies that process the personal data of EU residents. Although it is expected that the GDPR will provide consistency across the territory of the EU, it imposes more onerous requirements concerning consent and the obligations of sponsors of clinical trials (acting as data controllers), among other measures, which may increase the costs and extend the timelines of our product development efforts. Austerity measures in certain European nations may also affect the prices we are able to seek if our products are approved, as discussed below. Furthermore, the Brexit vote and the impact of the withdrawal of the UK may adversely affect business activity, political stability and economic conditions in the UK, the Eurozone, the EU and elsewhere. Specifically, Brexit and ongoing developments in the UK have created uncertainty with regard to data protection regulation in the UK. We may be required to comply with both the GDPR and the UK GDPR, exposing us to two parallel regimes with potentially divergent interpretations and enforcement actions for certain violations. The relationship between the UK and the EU in relation to certain aspects of data protection law remains unclear, for example, how data transfers between EU member states and the UK will be treated and the role of the UK’s Information Commissioner’s Office with respect to the EU following the end of the transitional period. Although we do not have material operations in the UK, we cannot rule out potential disruptions in relation to the clinical regulatory framework applicable to our clinical studies in the UK, and to data privacy and security rules with respect to personal data sharing with vendors and clinical investigators in the UK, and we cannot predict future implications.
Both in the U.S. and in the EU, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical and biopharmaceutical products. We do not know whether additional legislative changes will be enacted, whether the regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be.
21
Table of Contents
We could be subject to liabilities under environmental, health and safety laws or regulations, or fines, penalties or other sanctions, if we fail to comply with such laws or regulations or otherwise incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws, regulations, and permitting requirements, including those governing laboratory procedures, decontamination activities, and the handling, transportation, use, remediation, storage, treatment and disposal of hazardous materials, human substances and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials that produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials or wastes either at our sites or at third-party disposal sites. In the event of such contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties. Although we maintain workers’ compensation insurance to cover us for costs and expenses, we may incur due to injuries to our employees resulting from the use of hazardous materials, human substances or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws, regulations or permitting requirements. Such laws, regulations and requirements are becoming increasingly more stringent and may impair our research, development or production efforts. Failure to comply with these laws, regulations and permitting requirements also may result in substantial fines, penalties or other sanctions.
Our relationships with clinical centers, customers and payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which, if violated, could expose us to criminal sanctions, civil penalties, exclusion from government healthcare programs, contractual damages, reputational harm and diminished profits and future earnings.
| • | for instance, the U.S. healthcare Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under U.S. government healthcare programs such as Medicare and Medicaid; |
| • | for instance, the U.S. False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the U.S. government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | for instance, the Health Insurance Portability and Accountability Act (HIPAA), imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | for instance, the transparency requirements under the Health Care Reform Law require manufacturers of drugs, devices, biologics and medical supplies to report to the U.S. Department of Health and Human Services information related to payments and other transfers of value made by such manufacturers to physicians and teaching hospitals, and ownership and investment interests held by physicians or their immediate family members; and |
| • | in various other jurisdictions, analogous laws and regulations, such as state anti-kickback and false claims laws, will apply to sales or marketing arrangements, consultancy and service agreements, and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers, and some state laws require pharmaceutical and biopharmaceutical companies to comply with the pharmaceutical and biopharmaceutical industries’ voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, in addition to requiring manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under the U.S. federal Anti-Kickback Statute, it is possible that some of our future business activities could be subject to challenge under one or more of such laws. In addition, recent healthcare-reform legislation has strengthened these laws. For example, the Health Care Reform Law, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the Health Care Reform Law provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
22
Table of Contents
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from U.S. government-funded healthcare programs, such as Medicare and Medicaid, other foreign healthcare reimbursement and procurement programs, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business with is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
Risks Related to Our Relationships with Third Parties
If we fail to maintain our strategic relationships with any of our current or future strategic partners, our business, commercialization prospects and financial condition may be materially adversely affected.
We rely, in part, on our strategic partners. Good relationships with our strategic partners are important for our business prospects. If our relationships with our current or future strategic partners were to challenge our use of their intellectual property or our calculations of the payments we are owed under our agreements, our business, financial condition, commercialization prospects and results of operations could be materially adversely affected.
We may seek to form additional strategic alliances in the future with respect to our product candidates, and if we do not realize the benefits of such alliances, our business, financial condition, commercialization prospects and results of operations may be materially adversely affected.
We face significant competition in seeking appropriate collaborators. Collaborations are complex and time-consuming to negotiate, document and manage. Any delays in entering into new strategic partnership agreements related to our product candidates could delay the development and commercialization of our product candidates and reduce their competitiveness even if they reach the market. We may also be restricted under existing and future collaboration agreements from entering into strategic partnerships or collaboration agreements on certain terms with other potential collaborators. We may not be able to negotiate collaborations on acceptable terms, or at all, for any of our existing or future product candidates and programs because the potential partner may consider that our research and development pipeline is insufficiently developed to justify a collaborative effort, or that our product candidates and programs do not have the requisite potential to demonstrate safety and efficacy in the target population. If we are unsuccessful in establishing and maintaining a collaboration with respect to a particular product candidate, we may have to curtail the development of that product candidate, reduce the scope of or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of our sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense, for which we have not budgeted. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we will not be able to bring our product candidates to market and generate product revenue. Even if we are successful in establishing a new strategic partnership or entering into a collaboration agreement, we cannot be certain that, following such a strategic transaction or license, we will be able to progress the development and commercialization of the applicable product candidates as envisaged, or that we will achieve the revenues that would justify such transaction, and we could be subject to the following risks, each of which may materially harm our business, commercialization prospects and financial condition:
| • | we may not be able to control the amount and timing of resources that the collaboration partner devotes to the product development program; |
| • | the collaboration partner may experience financial difficulties; |
23
Table of Contents
| • | we may be required to grant or otherwise relinquish important rights such as marketing, distribution and intellectual property rights; or |
| • | business combinations or significant changes in a collaboration partner’s business strategy may adversely affect our willingness to continue any arrangement. |
We rely on third parties to conduct our nonclinical and clinical studies and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates, and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third-party clinical research organizations or contract research organizations (CROs), to monitor and manage data for our ongoing nonclinical and clinical programs, including the clinical studies of our product candidates. We rely on these parties for execution of our nonclinical and clinical studies and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards and our reliance on the clinical CROs does not relieve us of our regulatory responsibilities. We and our clinical CROs and other vendors are required to comply with current Good Manufacturing Practice (cGMP), current Good Clinical Practice (cGCP), and current Good Laboratory Practice (cGLP), which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the EU and comparable foreign regulatory authorities for our product candidates in nonclinical and clinical development (where applicable). Regulatory authorities enforce these regulations through periodic inspections of study sponsors, principal investigators, study sites and other contractors. If we or any of our clinical CROs or vendors fail to comply with applicable regulations, the data generated in our nonclinical and clinical studies may be deemed unreliable and the EMA, FDA, other regulatory authorities may require us to perform additional nonclinical and clinical studies before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that all of our clinical studies comply with cGCP regulations. In addition, our clinical studies must be conducted with products produced under cGMP regulations. Our failure to comply with these regulations may require us to repeat clinical studies, which would delay the regulatory approval process.
If any of our relationships with these third-party clinical CROs terminates, we may not be able to enter into arrangements with alternative clinical CROs or do so on commercially reasonable terms. In addition, our clinical CROs are not our employees, and except for remedies available to us under our agreements with such clinical CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing nonclinical and clinical programs. If clinical CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to our protocols, regulatory requirements, or for other reasons, our clinical studies may be extended, delayed, or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. Clinical CROs may also generate higher costs than anticipated. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
Switching or adding additional clinical CROs involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new clinical CROs commences work. As a result, delays occur, which could materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our clinical CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
We currently rely on third-party suppliers and other third parties for production of our product candidates and our dependence on these third parties may impair the advancement of our research and development programs and the development of our product candidates.
We currently rely on and expect to continue to rely on third parties for the manufacturing and supply of chemical and biological compounds and formulations for the clinical studies of our current and future product candidates. For the foreseeable future, we expect to continue to rely on such third parties for the manufacture of any of our product candidates on a clinical or commercial scale, if any of our product candidates receives regulatory approval. Reliance on third-party providers may expose us to different risks than if we were to manufacture product candidates ourselves. The facilities used by our contract manufacturers to manufacture our product candidates must be approved by the FDA or other regulatory authorities, pursuant to inspections that will be conducted after we submit our NDA or comparable marketing application to the FDA or other regulatory authority. We do not have control over a supplier’s or manufacturer’s compliance with these laws, regulations and applicable cGMP standards and other laws and regulations, such as those related to environmental health and safety matters. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control (QC), quality assurance (QA) and qualified personnel. If we are compelled or we wish to find alternative manufacturing facilities, this could significantly impact our ability to develop, obtain regulatory approval for or market our product candidates. Any failure to achieve and maintain compliance with these laws, regulations and standards could subject us to the risk that we may have to suspend the manufacturing of our product candidates or that obtained approvals could be revoked, which would adversely affect our business and reputation.
24
Table of Contents
Third-party providers may breach agreements they have with us because of factors beyond our control. Contract manufacturers often encounter difficulties involving production yields, QC and QA, as well as shortages of qualified personnel. They may also terminate or refuse to renew their agreements because of their own financial difficulties or business priorities, potentially at a time that is costly or otherwise inconvenient for us. If we are unable to find adequate replacement or another acceptable solution in time, our clinical studies could be delayed, or our commercial activities could be harmed.
In addition, the fact that we are dependent on our suppliers and other third parties for the manufacture, storage and distribution of our product candidates means that we are subject to the risk that our product candidates and, if approved, commercial products may have manufacturing defects that we have limited ability to prevent or control. The sale of products containing such defects could result in recalls or regulatory enforcement action that could adversely affect our business, financial condition and results of operations.
Growth in the costs and expenses of components or raw materials may also adversely influence our business, financial condition and results of operations. Supply sources could be interrupted from time to time and, if interrupted, we cannot be certain that supplies could be resumed (whether in part or in whole) within a reasonable timeframe and at an acceptable cost or at all. Our current and anticipated future dependence upon others for the manufacturing of our current and future product candidates may adversely affect our future profit margins and our, or our collaboration partners’, ability to commercialize any products that receive marketing approval on a timely and competitive basis.
Risks Related to Intellectual Property
We may not have sufficient patent terms to protect our products and business effectively.
Patents have a limited lifespan. In the U.S. and Europe, the natural expiration of a patent is generally 20 years after it is filed. Although various extensions or adjustments may be available, such as adjustments based on certain delays caused by the U.S. Patent and Trademark Office (the USPTO) or the European Patent Office (EPO) to the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned, co-owned and licensed patent portfolios may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours or otherwise provide us with a competitive advantage. Even if patents covering our product candidates are obtained and unchallenged, once the patent life has expired for a product, we may be open to competition from generic medications.
Although patent term extensions under the Hatch-Waxman Act in the U.S. and under supplementary protection certificates (SPCs) in Europe may be available to extend the patent exclusivity term for our products, we cannot provide any assurances that any such patent term extension will be obtained and, if so, for how long. The Hatch-Waxman Act permits a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended, and only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended. However, we may not be granted any extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. It is not possible to base an SPC in Europe on a patent in a European Member State if that patent expires before the market approval of the clinical product, protected by the patent, is obtained. As the “product” (active ingredient(s)) must be “protected by a basic patent in force”, only a granted patent that is in force, and remains in force until it reaches the end of its full term, can serve as a “basic patent” upon which an SPC can be based. Therefore, expired patents and pending patent applications cannot serve as the basis for an SPC. Given the relatively long clinical development timelines of biologicals and new chemical entities for therapeutic purpose, we may not be granted any patent extensions as we might fail to apply for the extensions prior to expiration of relevant patents. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or if the term of any such extension is less than we request, such result could have a material adverse effect on our business.
25
Table of Contents
We or our licensing or collaboration partners may become subject to intellectual property-related litigation or other proceedings to protect or enforce our patents or the patents of our licensors or licensees and collaborators, any of which could be expensive, time-consuming, and unsuccessful, and may ultimately result in our loss of ownership of intellectual property.
Competitors may infringe our patents or the patents of our licensors or collaborators. To counter such infringement, we may be required to file infringement claims against those competitors, which can be expensive and time-consuming. If we or one of our licensing or collaboration partners were to initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid or unenforceable or that the defendant’s products do not infringe our or our licensing collaborators’ patents or that we or our licensing collaborators infringe the defendant’s patents. In patent litigation in the U.S., defendant counterclaims alleging invalidity, unenforceability and non-infringement are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, obviousness-type double patenting, lack of written description, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or the EPO, or made a misleading statement, during prosecution. In addition, third parties may raise similar claims before administrative bodies in the U.S., in Europe or elsewhere, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, inter partes review, interference and derivation proceedings as well as equivalent proceedings in foreign jurisdictions, such as opposition proceedings in Europe. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Such proceedings or patent litigations could result in the revocation or cancellation of or amendment to our patents in such a way that they no longer cover our product candidates or otherwise provide any competitive advantage. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which the patent examiner and we or our licensing or collaboration partners were unaware during prosecution. A court may also refuse to stop a third party from using the technology in question on the grounds that our patents do not cover that technology. An adverse result in any proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly, which could have a material adverse effect on our business and financial condition.
Interference proceedings provoked by third parties or brought by us or declared by the USPTO or the EPO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors, licensees or collaborators. An unfavorable outcome could require us or our licensing or collaboration partners to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be materially harmed if the prevailing party does not offer us or our licensing or collaboration partners a license on commercially reasonable terms or at all. If we or our licensing or collaboration partners are unsuccessful in any interference proceedings, we may lose our ownership of intellectual property, or our patents may be narrowed or invalidated. There can be no assurance as to the outcome of the interference and opposition proceedings, and any of the foregoing could result in a material adverse effect on our business, financial condition, results of operations or prospects.
Our defense of litigation, interference proceedings or other intellectual property-related proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees from their normal responsibilities. Such litigation or proceedings could substantially increase our operating losses and could substantially reduce the funds necessary to continue our clinical studies and research programs or force us to license necessary technology from third parties or enter into development partnerships that would help us bring our product candidates to market. We may not be able to prevent, alone or with our licensing or collaboration partners, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the U.S.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, decisions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our Shares.
26
Table of Contents
If we or our licensing or collaboration partners are unable to obtain and maintain effective patent rights for our technologies, product candidates or any future product candidates, or if the scope of the patent rights obtained is not sufficiently broad, our competitors could develop and commercialize products and technology similar or identical to ours, and our, or our collaboration partners’ ability to successfully commercialize our products and technology may be adversely affected.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our technologies and product candidates. Our success depends in large part on our and our licensing or collaboration partners’ ability to obtain and maintain patent and other intellectual property protection in the U.S., the EU and other countries with respect to our proprietary technologies and product candidates. If such license is not granted or terminated, our licensing or collaboration partners may be required to cease development and commercialization of our product candidates, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
We have sought to protect our proprietary position by filing patent applications in the U.S. and other countries related to any of our novel technologies and products that are important to our business. This process is expensive, time-consuming, and complex, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost, in a timely manner or in all jurisdictions. It is also possible that we will fail to identify patentable aspects of our or our licensing or collaboration partners’ research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license to or from third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of pharmaceutical and biopharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unsolved. As a result, the inventorship, issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. The pending or future patent applications that we own, co-own or in-license may fail to issue, fail to result in issued patents with claims that cover our product candidates in the U.S. or in other countries, or fail to effectively prevent others from commercializing competitive technologies and product candidates. Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of our patents or narrow the scope of our patent protection.
We may not be aware of all third-party intellectual property rights potentially relating to our technologies or product candidates. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions remain confidential for a period of time after filing, and some remain so until issued. Therefore, we cannot be certain that we were the first to file any patent application related to our product candidates or technologies, or whether we were the first to make the inventions claimed in our owned or co-owned patents or pending patent applications, nor can we know whether those from whom we license patents were the first to make the inventions claimed or were the first to file.
There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our product candidates, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated, which could allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our or our collaboration partners’ inability to manufacture or commercialize products without infringing third-party patent rights. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our product candidates, prevent others from designing around our claims or provide us with a competitive advantage. Any of these outcomes could impair our ability to prevent competition from third parties, which may have a material adverse effect on our business.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest or title in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants, CROs, contract manufacturing organizations (CMOs), academic institutions or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or our ownership of our patents or other intellectual property. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or the right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
27
Table of Contents
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, thereby impairing our ability to protect our technologies and products.
Changes in either the patent laws or interpretation of the patent laws in the U.S., EU or elsewhere could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Assuming the other requirements for patentability are met, in the U.S. prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, whereas outside the U.S., the first to file a patent application was entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act (the Leahy-Smith Act), which was enacted on September 16, 2011, the U.S. moved to a first-to-file system. Under a first-to-file system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether a third party was the first to invent the invention. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications are prosecuted and may also affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by the USPTO administered during post grant proceedings, including re-examination proceedings, inter partes review, post-grant review and derivation proceedings. Therefore, the Leahy-Smith Act and its implementation increases the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, future actions by the U.S. Congress, the federal courts and the USPTO could cause the laws and regulations governing patents to change in unpredictable ways. Any of the foregoing could harm our business, financial condition and results of operations.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future in the U.S.
If we are unable to maintain effective proprietary rights for our technologies, product candidates or any future product candidates, we may not be able to compete effectively in our markets.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce, and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect and some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. For instance, the EU has introduced a new Directive on trade secrets increasing the standards for protection. Because we rely on our advisors, employees and third-party contractors and consultants to research and develop and to manufacture our product candidates, we must, at times, share our intellectual property with them. We seek to protect our intellectual property and other proprietary technology in part by entering into confidentiality agreements and master service agreements, if applicable, material transfer agreements, consulting agreements or other similar agreements with our advisors, employees, contractors, consultants, licensing and collaboration partners, and other third parties with confidentiality provisions. These agreements typically limit the rights of these third parties to use or disclose our confidential information, including our intellectual property and trade secrets. These agreements also typically restrict the ability of third parties to publish data potentially relating to our intellectual property, although our agreements may contain certain limited publication rights. For example, any academic institution that we may collaborate with in the future may expect to be granted rights to publish data arising out of such collaboration, provided that we may have the right to be notified in advance and given the opportunity to delay publication for a limited time period in order for us to secure patent protection of intellectual property rights arising from the collaboration, in addition to the opportunity to remove confidential or trade secret information from any such publication. We also conduct joint research and development programs that may require us to share intellectual property under the terms of our research and development or similar agreements. However, we cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or other confidential information or proprietary technology and processes, or that such agreements will not be breached or that our trade secrets or other confidential information will not otherwise be disclosed. Despite the contractual provisions employed when working with these advisors, employees and third-party contractors and consultants, the need to share intellectual property and other confidential information increases the risk that such confidential information becomes known by our competitors, is inadvertently incorporated into the product development of others or is disclosed or used in violation of these agreements. Additionally, our grant agreements typically provide for dissemination of results to academic institutions and to the general public. As a result, our information may be disseminated with the loss of protection status.
28
Table of Contents
We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining the physical security of our premises and the physical and electronic security of our information technology systems. Despite our efforts to protect our intellectual property, our competitors may discover our trade secrets through breach of our agreements by third parties, for which we may not have adequate remedies for any breach, or publication of information by any of our CROs, academic partners, funding organizations or our licensing or collaboration partners. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate by law, we may have insufficient recourse against third parties for misappropriating such trade secrets. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business. Moreover, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent such competitor or other third party from using that technology or information to compete with us. A competitor’s or other third party’s discovery of our intellectual property would impair our competitive position and have a material adverse effect on our business.
Further, the laws of different countries protect proprietary rights to a different extent or in a different manner. As a result, we may encounter significant problems in protecting and defending our intellectual property in different countries both in the U.S. and abroad. If we are unable to prevent material disclosure of the intellectual property related to our technologies to third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, financial condition and results of operations.
Despite confidentiality clauses within our employment agreements, we cannot ensure that departing employees will not breach any post-termination commitments in such agreements by allowing others to access our trade secrets.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document-submission, fee-payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on a patent and patent application are due to be paid to the USPTO and other national patent agencies in several stages over the lifetime of the patent and patent application. The USPTO, the EPO and various other governmental patent agencies require compliance with a number of procedural, documentary, fee-payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply with these requirements and we are also dependent on our licensors or collaboration partners to take the necessary action to comply with these requirements with respect to certain of our intellectual property. Although an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, nonpayment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
The patent protection and patent prosecution for some of our product candidates could be dependent on third parties.
Although we normally seek to obtain the right to control prosecution, maintenance and enforcement of the patents relating to our product candidates, there may be times when the filing and prosecution activities for patents relating to our product candidates are controlled by our licensors or collaboration partners. If any of our current or future licensing or collaboration partners fail to prosecute, maintain and enforce such patents and patent applications in a manner consistent with the best interests of our business, including by payment of all applicable fees for patents covering our product candidates, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, our or our collaboration partners’ ability to develop and commercialize those product candidates may be adversely affected and we may not be able to prevent competitors from making, using, and selling competing products. In addition, even where we have the right to control patent prosecution of patents and patent applications we have licensed to and from third parties, we may still be adversely affected or prejudiced by actions or inactions of our licensees, our licensors and their counsel that took place prior to the date upon which we assumed control over patent prosecution.
29
Table of Contents
Additionally, we may be adversely affected or prejudiced by actions or inactions of our external and internal patent counsels working solely on our projects or our joint patent counsels representing us and our collaboration partners.
Third-party claims of intellectual property infringement may expose us to substantial liability or may prevent or delay our or our collaboration partners’ development and commercialization efforts.
Numerous EU- and foreign-issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing product candidates. For example, we are aware of third-party patents or patent applications that may be construed to cover one or more of our product candidates. If these patents are asserted against us or our licensing or collaboration partners and either we or our licensing or collaboration partners are found to infringe any of these patents, and are unsuccessful in demonstrating that such patents are invalid or unenforceable, then we and our licensing or collaboration partners could be required to pay substantial monetary damages or cease further development or commercialization of one or more of our product candidates or be compelled to enter into onerous licenses with such third parties. There may also be other third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods of treatment related to the use or manufacture of our product candidates and technology. Although we generally conduct a freedom-to-operate search and review with respect to our product candidates, we cannot guarantee that our search and review is complete and thorough, nor can we be sure that we have identified each and every patent and pending application in the U.S. and abroad that is relevant or necessary to the manufacturing or commercialization of our product candidates or use of our technology. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may infringe. In addition, third parties may file and obtain additional patents in the future and claim that use of our technologies infringes upon these patents.
Third parties may assert infringement claims against us based on existing patents or on patents that may be granted in the future, regardless of merit. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, which could materially and adversely affect our or our collaboration partners’ ability to commercialize our product candidates or technologies covered by the asserted third-party patents.
Parties making claims against us may also obtain injunctive or other equitable relief, which could effectively block our or our collaboration partners’ ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. Any of the foregoing could have a material and adverse effect on our business, financial conditions, results of operations and prospects.
In addition, claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business, financial condition, results of operations and prospects.
There could also be public announcements of the results of hearings, motions, decisions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our Shares.
Some of our competitors may have substantially greater resources and more mature and developed intellectual property portfolios than we do and may be able to sustain the costs of complex intellectual property litigation to a greater degree and for longer periods of time than we could. In addition, patent-holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us. As the pharmaceutical and biopharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties. The uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
30
Table of Contents
We employ and utilize the services of individuals who were previously employed or provided services to universities or other pharmaceutical or biopharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employees’, consultants’ or independent contractors’ former employers or of other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
In addition, although it is our policy to require our employees, consultants and independent contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries may be less extensive than those in the U.S. or Europe. In addition, the laws of different countries do not protect intellectual property rights to the same extent as the laws in the U.S. or Europe. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S. or Europe, or from selling or importing products made using our inventions in and into the U.S., Europe or other jurisdictions. In the ordinary course of prosecution and maintenance activities, we determine whether to seek patent protection outside the U.S. and Europe and in which countries. This also applies to patents we have acquired or in-licensed from third parties. In some cases, we, or our predecessors in interest or licensors of patents within our portfolio, have sought patent protection in a limited number of countries for patents covering our product candidates. Competitors may use our technologies and products in jurisdictions where we have not obtained or are unable to adequately enforce patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection but enforcement is not as strong as that in the U.S. or Europe. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing, which would have a material adverse effect on our business and financial positions.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement, misappropriation or other violations of our intellectual property and proprietary rights. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We may be unable to protect our trade secrets, know-how and technologies.
We also rely on trade secrets and non-patentable know-how and technologies it seeks to protect, in part, by confidentiality agreements with our employees, consultants, suppliers, licensees and other contractual parties. Trade secrets and non-patentable know-how and technologies are difficult to protect. There can be no assurance that these agreements represent effective protection or that they will not be breached, that we would have adequate remedies for any breach, or that our trade secrets or non-patentable know-how and technologies will not otherwise become known or be independently developed by competitors and other third parties.
31
Table of Contents
These and other factors, alone or together, may have a material adverse effect on our business, financial condition, results of operations and growth prospects as well as the price of our shares.
Risks Related to Our Financial Condition and Results of Operations
We are a commercial-stage biopharmaceutical company with a history of operating losses. Our recurring losses, negative cash flows and significant accumulated deficit raise substantial doubt regarding our ability to continue as a going concern.
We incurred a net loss (defined as net loss attributable to owners of the Company) of approximately CHF 50.8 million for the year ended December 31, 2022 and had accumulated losses at consolidation level of approximately CHF 119.6 million as of December 31, 2022. We may continue to incur losses in the foreseeable future as development expenses and other operating expenses may exceed future revenue.
Our losses have resulted principally from research and development expenses and from general business and administrative expenses. We expect to continue to incur significant operating losses in the future as we continue our research and development efforts for our current and future product candidates and seek to obtain regulatory approval and commercialization of such product candidates. The amount of future losses is uncertain and our ability to achieve profitability, if ever, will depend on, among other things, us or partners successfully developing drug candidates, obtaining regulatory approval to market and commercialize drug candidates, manufacturing any approved products on commercially reasonable terms, growing our sales and marketing organization for any additional approved product and raising sufficient funds to finance our activities. We cannot guarantee that we will have sufficient funds available in the future to develop and commercialize our current or future drug candidates.
To date, we have financed our liquidity requirements primarily from equity financings and loans from our largest shareholder. Biopharmaceutical and pharmaceutical product development are highly speculative undertakings and involve a substantial degree of risk.
As of April 30, 2023, we have cash and cash equivalents of approximately CHF 11.1 million. We believe that our current cash and cash equivalents are sufficient to fund our operating expenses into the third quarter of 2023 and this raises substantial doubt about our ability to continue as a going concern. These factors individually and collectively indicate that a material uncertainty exists that may cast significant doubt about our ability to continue as a going concern. Our future viability is dependent on our ability to raise additional capital to finance our future operations. We may seek additional funding through public or private financings, debt financing or collaboration agreements. The inability to obtain funding, as and when needed, would have a negative impact on our financial condition and ability to pursue our business strategies. If we are unable to obtain the required funding to run our operations and to develop and commercialize our candidates, we could be forced to delay, reduce or eliminate some or all of our research and development programs, drug portfolio expansion or commercialization efforts, which could adversely affect our business prospects, or we may be unable to continue operations. Management continues to explore options to obtain additional funding. However, there is no assurance that we will be successful in raising funds, closing a collaboration agreement, obtaining sufficient funding on terms acceptable to us, or if at all, which could have a material adverse effect on our business, results of operations and financial conditions.
If we fail to obtain additional funding, we may delay, reduce or eliminate our product development programs or commercialization efforts.
We are currently advancing our product candidates through clinical development, either together with a collaboration partner or independently. We expect our research and development expenses to continue to increase in connection with our ongoing activities, particularly as we and/or our collaboration partners continue our ongoing studies and initiate new studies and initiate preclinical and clinical development of our product candidates.
We will require additional capital to develop and commercialize certain of our product candidates. If we receive regulatory approval for our current and future product candidates, and if we have not already licensed such product candidate to a collaboration partner and choose to commercialize such product candidate independently, we expect to incur significant commercialization expenses related to product manufacturing, sales, marketing, distribution and establishing a regulatory structure, depending on where we choose to commercialize. Additional funds may not be available on a timely basis, on favorable terms, or at all, and such funds, if raised, may not be sufficient to enable us to continue to implement our long-term business strategy. Additionally, we may be dependent on the status of the capital markets at the time such capital is sought. If we are not able to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
32
Table of Contents
Raising additional capital may cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our intellectual property or future revenue streams.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our liquidity needs through a combination of equity offerings, debt financings, grants, and license and development agreements in connection with collaborations. We do not have any material committed external source of funds. In the event we need to seek additional funds, we may raise additional capital through the sale of equity, convertible debt or other securities, and through drawdowns from our Share Subscription Facility in place with GEM Global Yield LLC SCS (GEM). In such an event, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of our Shares. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or proposing dividends to our shareholders.
If we raise additional funds through collaborations, strategic alliances, or marketing, distribution or licensing arrangements with third parties, we may have to grant or otherwise relinquish valuable rights to our intellectual property or future revenue streams.
Exchange rate fluctuations may materially affect our results of operations and financial condition.
As our reporting currency is the Swiss franc, transactions and balance sheet items denominated in foreign currencies are converted into Swiss francs at the applicable exchange rates. Our current expenses are denominated in Swiss francs, U.S. Dollars and Euros. In the future, we expect that the majority of our revenue and expenses will be in U.S. Dollars and Euros. Therefore, unfavorable developments in the value of the Swiss franc as compared to the U.S. Dollar and Euro could have a material adverse effect on our business, financial condition and results of operations.
The SIX Exchange Regulation AG has launched an investigation into Relief, the results of which are uncertain.
The SIX Exchange Regulation AG – the self-regulatory supervisory body for issuers listed on the SIX Swiss Exchange – has launched a formal investigation into Relief due to potential violations of the rules on ad-hoc publicity. While we do not believe that this investigation will have a material adverse effect on our business, there can be no assurance of that conclusion.
| ITEM 4 | INFORMATION ON THE COMPANY |
| A. | HISTORY AND DEVELOPMENT OF THE COMPANY |
Relief was formed in 2013 and became public in 2016 following a reverse merger with THERAMETRICS holding AG. The latter was formed in 2007 under the company name i-Mondo AG, which later in 2007 was changed to mondoRPHAN AG, in 2008 to mondoBIOTECH holding AG, in 2013 to THERAMETRICS holding AG, and to RELIEF THERAPEUTICS Holding SA in 2016. RELIEF THERAPEUTICS Holding SA and its predecessors have been listed on the SIX Swiss Exchange since 2009.
Our legal seat is located in Geneva, Switzerland. Our registered office is located at Avenue de Sécheron 15, 1202 Genève, Switzerland, and our telephone number is +41 22 545 11 16. Our website address is http://www.relieftherapeutics.com. The reference to our website is for textual reference only and information contained in, or that can be accessed through, our website or any other website cited in this annual report is not a part thereof.
| B. | BUSINESS OVERVIEW |
We are a Swiss, commercial-stage biopharmaceutical company committed to delivering innovative treatment options with the potential for transformative outcomes to benefit those suffering from rare debilitating conditions that have no or limited treatment options. Our cost-effective, capital-efficient approach to drug development and commercialization is focused on rare metabolic disorders, rare skin diseases, rare respiratory diseases and rare monogenetic diseases.
33
Table of Contents
Our portfolio offers a balanced mix of marketed, revenue-generating products, our proprietary, globally patented drug delivery platform technologies that have utility for development in other specialty or rare disease therapeutic areas and a highly targeted clinical development pipeline consisting of risk-mitigated assets that have been engineered for improvements in efficacy, safety or convenience to benefit the lives of patients. In addition, the Company is commercializing several legacy products via licensing and distribution partners.
We are actively pursuing a strategy to diversify our portfolio through the ongoing evaluation of potential in-licensing opportunities. To bring treatments to patients as quickly as possible, we are seeking partnerships with, or acquisitions of, companies that have late-stage clinical molecules with a strong human safety profile, allowing for relatively short, capital-efficient clinical trials with clear endpoints. We are also evaluating prospective opportunities that fit within our genetic medicine initiative for devastating, as-yet-unaddressed, rare monogenetic diseases.
We are led by a proven and seasoned management team of business leaders with significant experience in discovering, developing and commercializing important new medicines, delivering them to market and maximizing shareholder value. Collectively, the members of our management team have overseen research and development of products supporting regulatory approvals as well as commercial launches of marketed products.
In March 2021, we signed a Collaboration and License Agreement with Acer Therapeutics, Inc. (Acer) for the worldwide development and commercialization of ACER-001 (now OLPRUVA™) for the treatment of urea cycle disorders (UCDs) and maple syrup urine disease (MSUD). OLPRUVA™ is a proprietary powder formulation of sodium phenylbutyrate (NaPB) designed to be both taste-masked and immediate release. In August 2021, Acer submitted an NDA for ACER-001 to the FDA for use as a treatment of UCD, which submission was accepted for filing in November 2021 with a PDUFA decision date of June 5, 2022. However, on or about the PDUFA decision date, Acer received a complete response letter stating that a satisfactory inspection of Acer’s third-party contract manufacturer would be required before its NDA for ACER-001 could be approved. On December 27, 2022, Relief and Acer announced that, after resubmission, the FDA had approved ACER-001, under the trade name OLPRUVA™, for the treatment of UCDs. In addition, Acer has filed an IND to investigate OLPRUVA™ for the treatment of MSUD.
In June 2021, we signed and closed a definitive agreement to acquire all outstanding shares of APR Applied Pharma Research SA (APR), a privately held Swiss pharmaceutical company with over 25 years’ experience in identifying, developing and globally commercializing known molecules engineered with drug delivery systems in niche and rare diseases.
In July 2021, Relief acquired AdVita Lifescience GmbH (AdVita), a Germany-based privately held pharmaceutical company developing products for the treatment and diagnosis of rare lung diseases. AdVita’s capabilities helped the Company to further progress the development of RLF-100® for a range of lung diseases.
In July 2022, we executed a definitive agreement with Meta Healthcare Ltd. (Meta), acquiring the worldwide rights, except for the UK, for a novel dosage form of a prescription drug already approved by the FDA and intended for the treatment of patients with PKU. This improved product is expected to increase patient acceptance and compliance as well as enable easier, self or caregiver administered metered dosing and dispensing. According to the terms of the agreement, Meta shall transfer to Relief all data, know-how, as well as any intellectual property as developed or generated so far by Meta. Relief shall only be responsible for funding the remaining development work as well as for filing and prosecuting a NDA in all countries worldwide except for the UK where Relief Therapeutics shall grant a license back to Meta, enabling Meta to directly promote and commercialize the product in such country. Other than the initial acquisition payment and low double-digit royalty payments on net profit of the product in the various countries, Relief shall be under no obligation to fund or pay any other amount to Meta.
Previously, Relief partnered with NeuroRx, Inc. (NeuroRx) to seek to develop RLF-100® as a treatment of COVID-19. In September 2020, Relief entered into a binding collaboration agreement with NeuroRx (the Collaboration Agreement). The Collaboration Agreement established the terms under which we and NeuroRx collaborate and assist each other to maximize the revenues in our respective territories from the sale of aviptadil for intravenous and inhale use primarily for the treatment of COVID-19 related conditions.
Based on numerous breaches of the Collaboration Agreement by NeuroRx, in October 2021, we filed a lawsuit against NeuroRx and its then CEO, Dr. Jonathan Javitt, for multiple breaches of the Collaboration Agreement. The complaint was filed in the Supreme Court of the State of New York in Manhattan. On January 10, 2022, NeuroRx filed a complaint against Relief alleging that we were in breach of the Collaboration Agreement and have thus repudiated and cancelled the Collaboration Agreement. Additionally, NeuroRx’s claims included a count for defamation.
34
Table of Contents
On November 14, 2022, Relief and NRx issued a press release announcing that the parties had entered into a definitive settlement agreement to resolve all matters relating to the pending litigation. At the closing of this settlement, held on December 19, 2022, (i) NRx transferred to Relief all of the assets that it previously used in its aviptadil development program, including its regulatory filings, patent applications, clinical data, and the formulation of the aviptadil product it was previously developing, (ii) Relief has the exclusive right and control going forward to develop and commercialize an aviptadil product, (iii) Relief has agreed to use commercially reasonable efforts to continue the existing Right to Try Program for aviptadil in the U.S. for at least two years, (iv) Relief will pay NRx milestone payments if it can successfully obtain commercial approval of an aviptadil product (whether for COVID-19 or any other indication), (v) Relief will pay NRx royalties based on a percentage of future sales of an aviptadil product (whether for COVID-19 or any other indication), up to a maximum of $30 million in the aggregate, (vi) NRx Pharmaceuticals has agreed not to compete in the development of an aviptadil product in the future, and (vii) at the closing, Relief and NRx Pharmaceuticals have dismissed their pending litigation. Further, the parties, as part of the settlement, the parties cancelled the collaboration agreement and exchanged mutual releases with respect to matters that were the subject of the litigation.
On November 24, 2021, we announced that we had entered into a collaboration agreement with InveniAI LLC (InveniAI), a U.S. based company that has pioneered the application of artificial intelligence and machine learning across biopharma and other industries, in order to identify promising drug candidates to treat rare and specialty diseases (the InveniAI Collaboration Agreement). Under the terms of the InveniAI Collaboration Agreement, InveniAI will use its proprietary platform for the identification of potential pharmaceutical product opportunities using its Pharma Big Innovation Data Lab, consisting of (i) its proprietary AlphaMeld platform, a cloud-based artificial intelligence platform that uses its proprietary machine learning and deep learning based neural networks to identify product opportunities in therapeutic areas, (ii) its cross-functional teams at its Integrated Center of Excellence, and (iii) domain expertise, to generate novel pharmaceutical opportunities and the related development pathway for the development of such concepts.
Under the terms of the InveniAI Collaboration Agreement, we paid InveniAI an initial up-front fee of $500,000. We will be required to pay success milestones for any products brought to us in connection with the InveniAI Collaboration Agreement ranging from $200,000 per product candidate for which we exercise our option to acquire IP rights to $50 million for any required product reaching $1 billion per year in net sales. We will also be required to pay royalties on any such commercialized product in certain countries a royalty of approximately three percent. We are not currently developing any product brought to us by InveniAI, and there can be no assurance that our collaboration with InveniAI will result in the development of new product candidates or product concepts.
Capital Resources
As of December 31, 2022, we had cash and cash equivalents of approximately CHF 19.2 million. As of April 30, 2023, we had cash and cash equivalents of approximately CHF 11.1 million. Based on current financial current projections, we expect that we have sufficient resources to fund operations into the third quarter of 2023.
Our Strategy
Our mission is to provide therapeutic relief to those suffering from debilitating rare diseases that have limited or no treatment options to help them live their best possible lives and achieve their full potential. We target established products with a proven history of safety and efficacy and either initial human therapeutic activity, proof-of-concept or a strong scientific rationale, allowing for relatively short, capital-efficient clinical trials with clear endpoints. Our global skills and internal research and development (R&D) resources are directed toward optimizing the therapeutic potential of these assets to deliver improvements in efficacy, safety and convenience through the application of our proprietary platform technologies, drug delivery systems or novel dosage forms.
Our risk-mitigated, cost-effective approach to drug development and commercialization is being advanced by an international team of well-established biopharma industry leaders with extensive research, development and rare disease expertise. Focusing on rare diseases with significant unmet medical need allows us to maintain a lean and capital efficient organization where all key strategic functions are internalized, combined with an optimized network of outsourced service providers for various development activities. We have a direct commercial footprint in the U.S. and in Europe, coupled with a strong network of commercial partners in the other major territories.
35
Table of Contents
Our strategic plans are specifically centered around the therapeutic areas we know best, including:
| • | Rare Metabolic Disorders: |
| • | PKU GOLIKE® in phenylketonuria (PKU). We intend to maximize the commercial potential of the PKU GOLIKE® family of products in all major territories either directly or through distribution partners in order to create a steady and growing positive cash flow; PKU GOLIKE® is an approved, and fully reimbursed line of patented and differentiated medical food products for the dietary management of PKU. |
| • | OLPRUVA™ in urea cycle disorders (UCDs). Pursuant to the FDA approval granted in December 2022, we are expecting OLPRUVA™ to become commercially available in the U.S. from mid-June 2023 as a new prescription option for the treatment of UCDs; in parallel, together with our collaboration partner Acer Therapeutics Inc. (Acer), we are currently pursuing approval for use of OLPRUVA™ in Europe. |
| • | OLPRUVA™ in maple syrup urine disease (MSUD). Together with our collaboration partner Acer, we intend to pursue the development of OLPRUVA™ for the treatment of MSUD in the U.S. and Europe, especially considering this disease currently has no approved treatments available. |
| • | RLF-OD032 in PKU. We intend to complete the development of this prescription drug candidate intended as a new, patented and highly differentiated treatment option for PKU; we anticipate the completion of the development and the filing of a 505(b)(2) NDA before the FDA in 2024 and anticipate an expected approval sometime in Q3/2025. |
| • | Rare Skin Diseases: |
| • | RLF-TD011 in epidermolysis bullosa (EB). We intend to advance the development of this drug candidate and to complete the ongoing proof of concept, investigator initiated clinical trial sometime before the end of 2023 subject to patient enrolment pace. |
| • | RLF-TD011 in cutaneous T-cell lymphoma (CTCL). Subject to additional financing, we intend to conduct a proof of concept, investigator initiated clinical trial on CTCL patients at Northwestern University in the U.S. where clinical protocol has been already approved. |
| • | Rare Respiratory Diseases: |
In 2023, we completed the development of a shelf stable formulation of RLF-100® (aviptadil acetate) for both injectable intravenous (IV) and inhaled use.
The IV RLF-100® formulation is specifically designed and indicated for all COVID or non-COVID related acute respiratory distress syndromes (ARDSs) because (i) it allows a prompt and efficient delivery of the drug in patients with severe conditions (e.g., patients in ICUs) while (ii) keeping under control the possible side effects because administered in a hospital setting.
The Inhaled RLF-100® formulation is specifically designed and indicated for all the rare chronic lung diseases (CLDs) we are targeting because (i) it can maximize the clinical efficacy directly in the target organs (the lungs), (ii) while reducing the side effects of the drug (vasodilator and hypotension) because not systemically absorbed.
| • | RLF-100® in ARDSs. Subject to additional financing, we intend to advance the development of this drug candidate in ARDSs by entering into Phase 2 development. |
| • | RLF® in CLDs. Subject to additional financing, we intend to advance the development of this drug candidate in the targeted CLDs, namely sarcoidosis, berylliosis and checkpoint inhibitor-induced pneumonitis (CIP) by entering into Phase 2 development. |
36
Table of Contents
| • | Expansion of our pipeline: |
We intend to leverage our strength and experience to identify, acquire and bring to market drug candidates for therapeutic areas that align with our areas of focus.
Product Portfolio and Development Timeline
Relief Therapeutics’ portfolio offers a balanced mix of marketed, revenue-generating products, our proprietary, globally patented Physiomimic™ and TEHCLO™ drug delivery platform technologies and a highly targeted clinical development pipeline consisting of risk-mitigated assets focused in three core therapeutic areas: rare metabolic disorders, rare skin diseases and rare respiratory diseases where the company can best leverage its internal research and development (R&D) capabilities and track record.
In addition, Relief Therapeutics is commercializing several legacy products via licensing and distribution partners.
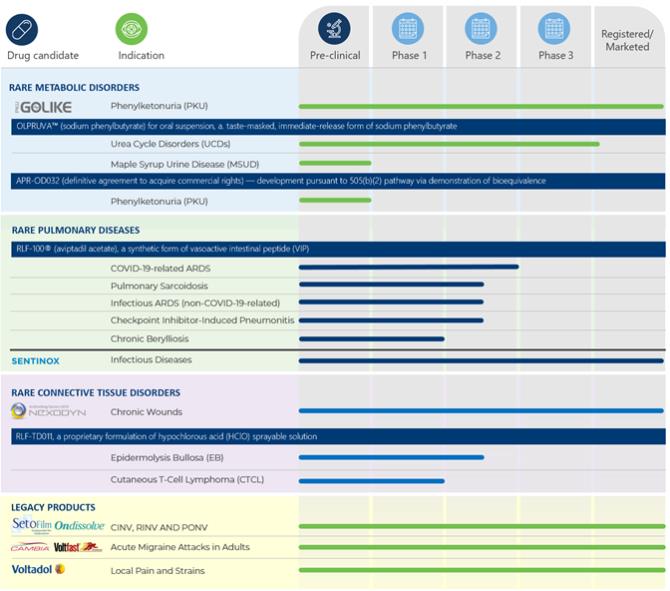
37
Table of Contents
DRUG DELIVERY PLATFORM TECHNOLOGIES
Our drug delivery platform technologies enable us to optimize the therapeutic potential of established products with proven efficacy, known safety profiles or where proof-of-concept exists. These platforms have utility for development in other specialty or rare disease therapeutic areas, partnerships and out-licensing.
TEHCLO NANOTECHNOLOGY™
Our TEHCLO Nanotechnology™ platform (TEHCLO™) consists of our proprietary, globally patent-protected electrode with nanocoating, the method for preparing and making highly stable aqueous solutions and our device for the electrolytic treatment of a fluid. TEHCLO™ was used to develop RLF-TD011, Nexodyn and some of our legacy products.
Our TEHCLO™ intellectual property portfolio consists of four patent families. The first three families include 107 granted patents world-wide directed to systems and methods for generating APR’s hypochlorous acid solution, compositions comprising APR’s hypochlorous acid solution and methods for treating ocular disorders. These patents expire between October 2026 and June 2030, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity. If granted, additional patents would expire no earlier than July 2040.
PHYSIOMIMIC TECHNOLOGY™
Our Physiomimic Technology™, used in the PKU GOLIKE® product line, is our globally patented, proprietary method to engineer amino acids to modify their release and absorption to mimic the physiological absorption of natural dietary proteins. This technology provides extended-release, taste and odor masking and increased absorption.
Our PKU GOLIKE® intellectual property portfolio consists of two patent families including 34 pending applications and 50 granted patents world-wide. Patents resulting from these families, if granted, will expire no earlier than 2036 and 2038, respectively, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity.
RARE METABOLIC DISORDERS
Phenylketonuria (PKU)
Phenylketonuria (PKU) is a rare metabolic disorder that hinders the body’s ability to break down the amino acid phenylalanine (Phe), resulting in a dangerous build-up of Phe when patients eat foods containing protein or aspartame. High levels of the Phe can lead to severe symptoms, including:
| • | permanent cognitive disorders and intellectual disability; |
| • | delays in development; |
| • | behavioral, emotional and social problems, and psychiatric disorders; |
| • | a musty odor in the breath, skin or urine, caused by too much Phe in the body; |
| • | neurological problems, which may include seizures; |
| • | skin rashes (eczema); |
| • | fair skin and blue eyes, because phenylalanine can’t transform into melanin, the pigment responsible for hair and skin tone; |
| • | abnormally small head (microcephaly); and |
| • | hyperactivity, |
In PKU patients, the enzyme needed to convert Phe is missing or severely reduced, which can result in high build-up of Phe and severe systemic damage, mainly in the brain. Treatment guidelines for these patients require a life-long strict and very limited, low protein diet combined with a Phe-free mix of amino acid (AA) supplementation (which can represent up to 75 percent of the total daily protein intake).
A further reduction of Phe levels is important before conception: pregnant women with PKU, including those with less severe forms of the disease, may place their unborn children at risk by not following the PKU diet. Children of woman with untreated PKU may have an unusually small head (microcephaly), congenital heart disease, developmental abnormalities or facial abnormalities. There is a strong relationship between the severity of these symptoms and high Phe levels in the mother.
38
Table of Contents
Diagnosis and Incidence
Diagnosis of PKU in Europe and the U.S. is conducted on each and every newborn through the new-born screening (NBS) programs which are mandatory in those countries. When diagnosed, newborns are to specialized referral centers/hospitals trained to deal with such rare metabolic disorders. According to a study published in August 2020 in the American Journal of Human Genetics, approximately 450,000 people suffer from PKU worldwide.
Current Treatment Options for PKU
While PKU is not curable, if diagnosed early enough, an affected newborn can grow up with normal brain development by managing and controlling Phe levels through a strict diet combined with AA supplementation or with available medications. Diet is composed by few amounts of natural food (based on severity of the disease) supplemented with AA mix with absence or low Phe content plus low protein foods. Diet is recommended for the entire life since it has been demonstrated that high Phe levels has an impact not only during growing but also in adulthood. In 2018, the FDA approved an enzyme substitute called pegvaliase, sold by BioMarin Pharmaceuticals under the brand name PALYNZIQ® (pegvaliase-pqpz). PALYNZIQ® is a derivative of the enzyme Phe ammonia-lyase that metabolizes Phe to reduce its blood levels (but it is not able to produce tyrosine as the natural enzyme, which still needs to be supplemented). Tetrahydrobiopterin (BH4), a cofactor for the oxidation of Phe, when taken by mouth, is also thought to reduce blood levels of Phe in some people. Along with PALYNZIQ®, BioMarin Pharmaceuticals also markets KUVAN® (sapropterin dihydrochloride) for the treatment of PKU. There are also other amino acid products, sold both by prescription and over the counter, that are marketed toward PKU patients.
PKU GOLIKE® for the Dietary Management of PKU
Patients with PKU require supplementation of amino acid (AA)-based foods for special medical purposes (FSMPs or medical formula) to prevent protein deficiency and optimize metabolic control. Many of these FSMPs can result in poor dietary compliance due to their taste and odor. Further, the unpleasant odor and aftertaste of current AA supplements can become a barrier to social interaction for PKU patients.
In addition, proteins needed for normal growth and coming from natural food sources are broken down during the digestion process and are gradually absorbed, keeping blood AA levels sufficient stable over time. However, free-AA mix administered to PKU patients in the form of FSMPs are not comparable to natural proteins because they do not need to be broken down before they are absorbed, resulting in a rapid peak of absorption and rapid decrease of their concentration into the bloodstream.
This rapid peak in blood amino acids following ingestion of FSMPs impairs the ability of the body to process them properly and incorporate them into the body’s own tissue, through a process known as anabolism, resulting in a portion of unprocessed amino acids which are then oxidated and eliminated. This rapid elimination of unprocessed AAs that is associated with traditional FSMPs represents a fundamental unmet need for PKU patients especially during any prolonged fasting period.
In order to compensate for the low levels of AAs during fasting periods, the body is forced to initiate a process called catabolism, where the body will break down lean muscle mass to obtain the AAs or energy it requires.
PKU GOLIKE® products are Phe-free FSMPs for both children and adults. Developed with the Relief Therapeutics proprietary, patent-protected Physiomimic Technology™ drug delivery platform, PKU GOLIKE® products are the first prolonged-release AA FSMPs, characterized by a special coating that ensures physiological absorption of the AAs mirroring that of natural proteins. The special coating also masks the unpleasant taste, odor and aftertaste of the AAs. PKU GOLIKE® granules are flavorless and can be mixed with many foods. PKU GOLIKE® products contain all 19 amino acids that people with PKU need to maintain neurological and muscular health and is fortified with 27 essential vitamins and minerals, including ones normally found in protein-rich foods like iron, calcium and vitamin B12.
The PKU GOLIKE® line of products are available in convenient packets (PKU GOLIKE Plus® for ages 3-16 and ages 16+), medical food bars (PKU GOLIKE BAR®) and tablets to be chewed (PKU GOLIKE KRUNCH®). Relief Therapeutics plans to expand the PKU GOLIKE® commercial infrastructure beyond the current countries and aims to strengthen the commercial activities to increase and accelerate future growth. PKU GOLIKE® is currently promoted and marketed by a direct sales and marketing infrastructure in U.S., Germany, Italy, Switzerland and Austria; in addition, the product is marketed in the UK, Spain, Israel and Ecuador by local distributors under contract with APR.
39
Table of Contents
PKU GOLIKE® products have been commercially available in Europe since 2018. Relief Therapeutics launched the PKU GOLIKE® family of products in the U.S. in late October 2022, with its recently assembled commercial infrastructure and team. In early 2023, the company announced the U.S. and EU availability of the new red fruit and tropical fruit flavored PKU GOLIKE BAR®, which contain real fruit flavors, from natural ingredients. More flavors of the bars and other forms of PKU GOLIKE® are currently in development.
On August 23, 2022, APR was issued U.S. patent number 11,419,837, which covers certain formulations of PKU GOLIKE® and supplements the PKU GOLIKE® intellectual property portfolio, which includes U.S. patent number 10,500,180, which was issued on December 10, 2019. The patents will expire no earlier than September 27, 2036, with the payment of all prescribed maintenance fees.
In March 2023, Relief Therapeutics presented the findings from pre-clinical research evaluating the metabolic impact of PKU GOLIKE® on nitrogen balance, muscle strength and glucose in a poster session at the Society for Inherited Metabolic Disorders (SIMD) 44th Annual Meeting. The poster summarized the acute and long-term metabolic effects of PKU GOLIKE® supplementation on the utilization of AAs and glucose metabolism in a pre-clinical rat model using biomarkers for muscle metabolism, functional muscle performance and a glucose tolerance test. Due to the prolonged release of the AAs, beneficial effects were observed on AA oxidation, muscle metabolism, grip strength and glucose tolerance in healthy rats. BUN (blood urine nitrogen test) was significantly lower in the acute treatment with PKU GOLIKE® indicating the potential to improve AA utilization in PKU patients resulting in a reduction of catabolic episodes. The results from this pre-clinical research demonstrate the important body composition benefits of the physiological absorption of our prolonged-release AA supplement PKU GOLIKE®. Detailed results from this study are available on the Relief Therapeutics website.
Relief Therapeutics plans to expand the PKU GOLIKE® commercial infrastructure beyond the current countries to increase and accelerate future growth. This will be supported by newer formulations of PKU GOLIKE®.
In the U.S., PKU GOLIKE® was granted ODD by the FDA for its development as a prescription drug. We have decided not to pursue this program and will continue to market our PKU GOLIKE® line of products solely as FSMPs.
RLF-OD032 (formerly known as APR-OD32) for the Treatment of PKU
Through a definitive agreement with Meta Healthcare Ltd. (Meta), Relief Therapeutics has acquired the worldwide rights, title and interest, except in the UK, for a novel dosage form of a prescription drug already approved by the FDA and intended for the treatment of patients with PKU. This improved product is expected to increase patient acceptance and compliance as well as provide easy metered dosing and dispensing. Meta will provide the technology transfer package, while Relief Therapeutics will conduct clinical studies, manufacturing, regulatory submission and commercialization in the U.S. and EU.
According to the terms of the purchase agreement, Meta will transfer to Relief Therapeutics all data, know-how, as well as any intellectual property as developed or generated so far by Meta. Relief Therapeutics will only be responsible for funding the remaining development work as well as for filing and prosecuting NDA (or equivalent thereof) in all countries worldwide except for the UK, where we will grant a license back to Meta, enabling Meta to market the product in that country. Other than the initial acquisition payment and low double-digit royalty payments on net profits of the product in the various countries, we will be under no obligation to fund or pay any other amount to Meta.
Relief Therapeutics anticipates filing for registration approval in the U.S. and Europe through a 505(b)(2) NDA sometime in the second half of 2024.
ACER-001 / OLPRUVA™ (SODIUM PHENYLBUTYRATE) FOR ORAL SUSPENSION
In March 2021, Relief Therapeutics signed a collaboration and license agreement with Acer Therapeutics Inc. (Acer) for the worldwide development and commercialization of ACER-001 (sodium phenylbutyrate) for the treatment of various inborn errors of metabolism, urea cycle disorders (UCDs) and maple syrup urine disease (MSUD).
40
Table of Contents
ACER-001 is a proprietary, coated powder formulation of sodium phenylbutyrate (NaPB) designed to be both taste-masked and immediate release. ACER-001 was developed using a multiple coating process, and the microparticles consist of an inert core center, a coated layer of active drug and a final taste-masking coating that quickly dissolves in the stomach to avoid a bitter taste while still allowing for rapid systemic absorption. ACER-001’s taste-masked formulation is designed to improve the palatability of NaPB and could make it a compelling alternative to existing NaPB-based treatments, as the unpleasant taste associated with NaPB is cited as a major impediment to patient compliance with those treatments. Additionally, bioequivalence trials have shown ACER-001 to have similar relative bioavailability to BUPHENYL® under both fasted and fed conditions, along with significantly lower projected pricing compared to RAVICTI®.
On December 22, 2022, the FDA approved ACER-001 under the brand name OLPRUVA™ (sodium phenylbutyrate) for oral suspension as a prescription medicine as an adjunctive treatment for use in certain patients with UCDs.
Urea Cycle Disorders (UCDs)
The urea cycle is a series of biochemical reactions that occur primarily in the liver, which converts toxic ammonia produced by the breakdown of protein and other nitrogen-containing molecules in the human body into urea for excretion. Primary hyperammonemia is a term to describe an elevation of ammonia in blood or plasma due to a defect within the urea cycle, which is the pathway responsible for ammonia detoxification and arginine biosynthesis. Urea cycle disorders (UCDs) are rare diseases caused by genetic defects affecting any of the six enzymes or two transporters that are directly involved in the urea cycle function. The clinical situation is variable and largely depends on the time of onset. Newborns who are often affected by hyper-ammonaemic encephalopathy carry a potential risk of severe brain damage, which may lead to death. Outside the neonatal period, symptoms are very unspecific but most often neurological (with wide variability), psychiatric and/or gastrointestinal. Early identification of patients is extremely important to start effective treatment modalities immediately. The acute management includes detoxification of ammonia, which often requires extracorporeal means such as hemodialysis, and the use of intravenous drugs that work as nitrogen scavengers. Long-term management of patients with UCDs consists of a low-protein diet, which needs to be balanced and supplemented to avoid deficiencies of essential amino acids, trace elements or vitamins and the use of nitrogen scavengers. In cases where dietary management or medication is not effective, patients with UCD may require a liver transplant.
Diagnosis and Incidence
The diagnosis of UCDs is based on clinical observations, confirmed by biochemical and molecular genetic testing. A plasma ammonia concentration of 150 µmol/L or higher associated with a normal anion gap and a normal plasma glucose concentration is an indication for the presence of UCDs. Plasma quantitative amino acid analysis and measurement of urinary orotic acid can distinguish between the various types of UCDs. A definitive diagnosis of UCDs depends on either molecular genetic testing or measurement of enzyme activity. Molecular genetic testing is possible for all urea cycle defects. Studies suggest the incidence of UCDs in the U.S. and Europe is 1 in 35,000 live births. Approximately 2,100 patients suffer from UCDs in the U.S.
Current Treatment Options for UCDs
The current treatment of UCDs consists of dietary management to limit ammonia production in conjunction with medications that provide alternative pathways for the removal of ammonia from the bloodstream. Dietary protein must be carefully monitored, and some restriction is necessary; too much dietary protein causes excessive ammonia production. However, if protein intake is too restrictive or insufficient calories are consumed, the body will break down lean muscle mass to obtain the amino acids or energy it requires, which can also lead to excessive ammonia in the bloodstream. Dietary management may also include supplementation with special AA formulas developed specifically for UCDs, which can be prescribed to provide approximately 50 percent of the daily dietary protein allowance. Some patients may also require individual branched-chain amino acid supplementation.
Medications for UCDs primarily comprise nitrogen scavenger drugs, which are substances that provide alternative metabolic excretion pathways for nitrogen, thereby bypassing the urea cycle. The use of these alternative pathways for nitrogen removal is important for the management of acute episodes of hyperammonemia and are also included as part of a long-term treatment regime for UCD patients. Current nitrogen scavenger treatments for UCDs are based on sodium benzoate or phenylbutyrate, which conjugate with glycine and glutamine, respectively, allowing for urinary excretion of nitrogen as hippurate and phenylacetylglutamine, respectively.
41
Table of Contents
According to a 2016 study by Shchelochkov et al., published in Molecular Genetics and Metabolism Reports, while nitrogen scavenging medications are effective in helping to manage UCD, non-compliance with treatment is common. Reasons given for non-compliance include the unpleasant taste associated with available medications, the frequency with which medication must be taken and the high cost of the medication.
Sodium phenylbutyrate (NaPB) is currently approved in the U.S. and the EU to treat patients with UCDs, which is marketed as BUPHENYL® (sodium phenylbutyrate) Tablets, BUPHENYL® (sodium phenylbutyrate) Powder and RAVICTI® (glycerol phenylbutyrate) Oral Liquid. While a study provided by Horizon Therapeutics, Inc. in the RAVICTI® package insert involving 46 adults with UCD demonstrated that BUPHENYL® and RAVICTI® were similarly effective in controlling the blood level of ammonia over a 24-hour period, many patients who take their medicine orally prefer RAVICTI®, as it is significantly more palatable than BUPHENYL®. However, the very high annual treatment cost of RAVICTI®, based on patient weight, is often prohibitive. RAVICTI® and BUPHENYL® are registered trademarks owned by or licensed to Horizon Therapeutics plc. Phenylburate is also marketed in the U.S., Europe, Australia and New Zealand under the trade name PHEBURANE® (sodium phenylbutyrate) Oral Pellets. AMMONAPS (sodium phenylbutyrate) Tablets, another formulation of NaPB that claims to be tasteless and odor free is approved and marketed in Europe.
Rationale for OLPRUVA™ (formerly ACER-001) Treatment in UCDs
Two Phase 1 bridging studies that evaluated the bioavailability and bioequivalence of OLPRUVA™ (sodium phenylbutyrate) compared to sodium phenylbutyrate (BUPHENYL®) powder showed the bioequivalence of OLPRUVA™ administered as a suspension with sodium phenylbutyrate (BUPHENYL®) powder administered as a solution in healthy adult volunteers after a single dose under fasting and fed conditions (Steiner 2022).
Additionally, two Phase 1, open-label, repeated measures, taste assessment studies of polymer-coated sodium phenylbutyrate (OLPRUVA™) and sodium phenylbutyrate (BUPHENYL®) powder, conducted on healthy panelists who were required to complete a training program for a minimum of six months that educated panelists on the identification, description and quantification of sensory attributes of products, concluded that OLPRUVA™ had overall lower flavor intensity scores than sodium phenylbutyrate (BUPHENYL®) powder when administered within five minutes of preparation (Cedarbaum 2022).
Currently approved therapies for UCDs, including BUPHENYL® and RAVICTI®, are required to be administered with food. BUPHENYL is required to be administered in a fed state due to its aversive odor and taste, with side effects including nausea, vomiting and headaches, which can lead to discontinuation of treatment. Additionally, prescribing information states that the BUPHENYL food effect is unknown. RAVICTI PK and pharmacodynamic (PD) properties were determined to be indistinguishable in fed or fasted states. OLPRUVA™ is uniquely formulated with its multi-particulate, taste-masked coating to allow for administration in a fasted state, while still allowing for rapid systemic release.
Based on the results from the food effect study within the first OLPRUVA™ BE trial, Acer commissioned Rosa & Co. LLC to create a PhysioPD PK model to evaluate the potential food effect on exposure, tolerability and efficacy of OLPRUVA™ in UCDs patients. Results from this in silico model suggested that administration of OLPRUVA™ in a fasted state required approximately 30 percent less PBA to achieve comparable therapeutic benefit to that in a fed state. In addition, the model predicted that administration of OLPRUVA ™ in a fasted state compared to administration of BUPHENYL® or RAVICTI® (same amounts of PBA) in their required fed states would be expected to result in higher peak blood PBA, PAA and PAGN concentrations, which should achieve a 43 percent increase in urinary PAGN levels (a negative correlation between blood ammonia area under the curve and 24-hour urinary PAGN amount has been demonstrated).
42
Table of Contents

In February 2021, Acer announced topline results from its bioequivalence trial in which OLPRUVA™ (formerly ACER-001) showed similar relative bioavailability to BUPHENYL® (sodium phenylbutyrate) under fed conditions. The single-center, single-blind, randomized, single-dose crossover trial evaluated BE of OLPRUVA™ compared to BUPHENYL® when administered under fed conditions in 36 healthy adults. The topline data from this trial showed OLPRUVA™ to have similar PK profiles for both PBA and PAA compared to BUPHENYL® under fed conditions.
Registration Plan and FDA Approval of OLPRUVA™ (Sodium Phenylbutyrate) for Oral Suspension for UCDs
On December 22, 2022, the FDA approved ACER-001 under the brand name OLPRUVA™ (sodium phenylbutyrate) for oral suspension as a prescription medicine for use with certain therapy, including changes in diet, for the long-term management of adults and children weighing 44 pounds (20 kg) or greater and with a body surface area (BSA) of 1.2 m2 or greater, with UCDs, involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinic acid synthetase (AS). OLPRUVA™ is not used to treat rapid increase of ammonia in the blood (acute hyperammonemia), which can be life-threatening and requires emergency medical treatment. Please see Important Safety Information and full Prescribing Information, including Patient Information.
On March 15, 2023, Acer and Relief Therapeutics announced that, in support of a planned launch for commercial OLPRUVA™ in the third quarter of 2023, that Acer is actively adding resources to establish its commercial and medical affairs presence in the U.S. Acer has recently introduced its patient support service, OLPRUVA™ Navigator by Acer Therapeutics, designed to support UCD patients with support, access, education and patient adherence to treatment. Representatives are expected to begin accepting prescriptions late in the second quarter of 2023. Acer also reported that it is actively engaged in negotiations regarding access for OLPRUVA™ with the major commercial payers and state Medicaid organizations.
According to Acer, it has established a pricing strategy reflecting its commitment to deliver innovative treatments that are responsibly priced and accessible to those in need. Acer intends to price OLPRUVA™ competitively, at a significant discount to the currently available commercial product RAVICTI®, while implementing predictable pricing that will not increase beyond the rate of inflation. Acer indicated that it also plans to invest a portion of OLPRUVA™ revenue back into additional solutions aimed at improving outcomes for UCD patients.
43
Table of Contents
We intend to seek approval from the European Medicines Agency (EMA) in the EU and potentially other territories outside the U.S. Because the FDA has approved an NDA for BUPHENYL®, which is referred to as the reference listed drug (RLD), we intend to rely on the RLD’s preclinical and clinical safety and efficacy data, while supplementing the data with a bridging study that shows similar relative bioavailability of OLPRUVA™ to BUPHENYL®. In parallel or after initial potential FDA approval for administration under fed conditions, and subject to additional capital, we also plan to evaluate potential development of OLPRUVA™ for administration under fasted (pre-meal) conditions, which will likely require additional nonclinical and clinical studies to provide the necessary evidence of safety and efficacy of OLPRUVA™ to be considered for FDA approval for administration under fasted (pre-meal) conditions.
Maple Syrup Urine Disease (MSUD)
Maple syrup urine disease (MSUD) is a rare inherited disorder caused by defects in the mitochondrial branched-chain ketoacid dehydrogenase complex, which results in elevated blood levels of the branched-chain amino acids (BCAA), leucine, valine and isoleucine, as well as the associated branched-chain ketoacids (BCKA) in a patient’s blood. Left untreated, this can result in neurological damage, mental disability, coma, or death. The most severe presentation of MSUD, known as “classic” MSUD, accounts for 80 percent of cases and can result in neonatal onset with encephalopathy and coma. Although metabolic management of the disease is possible via a highly restrictive diet, the outcome is unpredictable, and a significant portion of affected individuals are mentally impaired or experience neurological complications.
Diagnosis and Incidence of MSUD
MSUD is typically diagnosed at birth via newborn screening. Studies indicate that MSUD affects an estimated 1 in 185,000 infants worldwide. The disorder occurs more frequently in the Old Order Mennonite population, with an estimated incidence of about 1 in 380 newborns, and the Ashkenazi Jewish population, with an estimated incidence of 1 in 26,000. Approximately 3,000 patients suffer from MSUD worldwide, of whom approximately 1,000 are located in the U.S.
Current Treatment Options in MSUD
There are currently no approved pharmacologic therapies in the U.S. or the EU for MSUD. Treatment of MSUD consists primarily of a severely restricted diet to limit the intake of BCAA, with aggressive medical interventions when blood-levels of BCAA or BCKA become elevated.
Rationale for OLPRUVA™ Treatment in MSUD
Therapy with NaPB in UCD patients has been associated with a selective reduction in BCAA despite adequate dietary protein intake.
Based on this clinical observation, investigators at Baylor College of Medicine (BCM) explored the potential of NaPB treatment to lower BCAA and their corresponding BCKA in patients with MSUD. The investigators found that BCAA and BCKA were both significantly reduced following NaPB therapy in control subjects and in patients with MSUD, although there was no simple correlation between the patients’ levels of residual enzymatic activity with the response of plasma BCAA and their BCKA to NaPB. NaPB showed a statistically significant reduction of BCAA leucine, in all three healthy subjects and in three out of the five MSUD patients who participated in the trial. The reduction in leucine, the most toxic of the BCAAs, in the three responsive MSUD patients ranged between 28-34 percent, which is considered by clinicians to be a clinically meaningful response.
Investigators at BCM further explored the mechanistic rationale for NaPB lowering BCAA/BCKA levels. NaPB was found to be an allosteric inhibitor of the branched-chain keto acid dehydrogenase complex kinase (BCKD-kinase), and enzyme that regulates the activity of the branched-chain keto acid dehydrogenase complex (BCKDC) enzyme that is responsible for the normal metabolism of BCKAs. By inhibiting the BCKD-kinase, the BCKDC is constitutively activated, thus the increased activity results in a reduction in the plasma levels of BCAA and BCKA in all people, including those with MSUD, suggesting that NaPB may be an effective treatment for people with MSUD, who experience elevated BCAA levels.
In November 2020, study results evaluating the effect of NaPB in the management of acute MSUD attacks in pediatric patients (n=10) were published in the Journal of Pediatric Endocrinology and Metabolism showing a significant reduction in leucine levels in MSUD patients experiencing an acute attack. However, verifying this outcome would require additional validation in a controlled trial. If OLPRUVA™ is approved for the treatment of chronic MSUD, we believe patients will not be required to interrupt their therapy in the event of an acute crisis.
44
Table of Contents
Registration Plan for MSUD
Acer has submitted an investigational new drug (IND) application to the FDA to evaluate the safety and efficacy of OLPRUVA™ for the treatment of MSUD, and although there can be no assurance, we expect that Acer will begin such study during the first quarter of 2023. Given its regulatory status with regard to UCDs, there is no requirement for Phase 1 studies in healthy volunteers. The timing of a Phase 2 clinical trial, if such a trial occurs, would be subject to completion of a commercial assessment of the opportunity, including, but not limited to, a possible pre-IND meeting with the FDA and/or the EMA, with an objective of validation and agreement on the primary and secondary clinical trial end-points, which remain important given that there are presently no approved treatment options for this disease nor are there guidelines to assess efficacy and safety of an investigational drug in this disease. If a successful clinical trial for OLPRUVA™ in MSUD is completed, along with our partner Acer, we plan to seek FDA approval to market OLPRUVA™ for the treatment of MSUD as an added indication in the U.S. by submitting a supplemental NDA (sNDA) incorporating the efficacy and safety data from the MSUD population, assuming OLPRUVA™ is approved for the treatment of UCDs prior to sNDA submission. We also intend to seek approval in the EU and other territories outside the U.S. after the sNDA for treatment of MSUD is filed, or simultaneously with the U.S. filing.
Royalty Sharing for OLPRUVA™
The companies will split net profits from OLPRUVA™ from Acer’s territories (consisting of the U.S., Canada, Turkey, Japan and Brazil) 60 percent to 40 percent in favor of Relief Therapeutics. In addition, Relief Therapeutics has licensed the rights for the rest of the world, where Acer will receive from Relief Therapeutics a 15 percent royalty on all net revenues received in the Relief Therapeutics territories.
RARE PULMONARY DISEASES
RLF-100® (AVIPTADIL ACETATE)
Aviptadil acetate is a synthetic form of vasoactive intestinal peptide (VIP) consisting of 28 amino acids which was first discovered in 1970. That same year, Nature published a short report entitled “Potent peripheral and splanchnic vasodilator peptide from normal gut,” authored by two young scientists working at the Karolinska Institute (Said, Mutt, 1970). Although named (or mis-named) for the intestinal tissue in which it was first isolated, human VIP is now known to be produced by neuroendocrine cells throughout the body by T-lymphocytes, B-lymphocytes and macrophages, primarily concentrated in the lungs. VIP is highly localized in the lung (Leys 1986, Virgolini 1995) but is a widely distributed that showed, in various models, effects in hemodynamics and coronary circulation (Feliciano 1998, Frase 1987, Henning 2001), kidney (Dimaline 1983, Calam 1983 and 1988), immune system (Gonzales-Rey 2007, Ganea 2015, Li 2013), intestinal tract (Iwasaki 2019) and reproduction (Fredericks 1983, Fraccaroli 2012).
VIP has a multimodal mechanism of action: decrease of inflammatory cytokines release leading to prevention of cytokine storm syndrome and viral replication, immunomodulating effect, vasodilating and bronchodilating effects and prevention of surfactant depletion. Seventy percent of VIP in the body is bound to a less common type of cell in the lung, the alveolar epithelial type II (AT2) cell, which is critical to the absorption of oxygen into the body. Five decades of subsequent research documented VIP’s role as a potent natural anti-cytokine that has unique capability to block pathways of cell death in ATII cells – the cell targeted by the SARS-CoV-2 virus.
Since RLF-100’s mechanism of action is not restricted to the protection of AT2 cells, we believe that its beneficial effects could extend to other types of acute lung injury (ALI) as supported by pre-clinical and preliminary clinical data in sepsis-induced ALI. It is our objective to establish our proprietary and patent-protected formulation of aviptadil, RLF-100® as the standard of care for the prevention and treatment of respiratory failure and its complications in both the acute intensive care and chronic ambulatory settings.
In April 2023, Relief Therapeutics announced positive 12-month stability data for the liquid and lyophilized preparations of RLF-100®, intended for intravenous (IV) and inhaled administration. RLF-100® is the company’s proprietary, patent protected investigational formulation of aviptadil acetate. The data from the stability study showed that both inhaled and IV RLF-100® demonstrated high purity levels at 12 months at all temperatures tested, including refrigerated and room temperature environments. The results are consistent with prior data observed at three- and six-month intervals. The stability testing study will continue to determine the maximum shelf life of RLF-100®.
45
Table of Contents
Based on the latest results, Relief Therapeutics intends to amend its previously filed provisional patent application for RLF-100® with the new findings. If granted, this patent could provide exclusivity for RLF-100® at least until 2042, without considering Hatch-Waxman extensions or other patent term adjustments. The Hatch-Waxman Act permits a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process.
Relief Therapeutics intends to develop the intravenous (IV) formulation of RLF-100® for targeted COVID or non-COVID acute respiratory distress syndromes (ARDSs) because (i) it allows a prompt and efficient delivery of the drug in severe conditions (e.g., patients in intensive care units (ICUs)) while (ii) keeping under control the possible side effects because administered in a hospital setting and (iii) it remains a dosage form easy to handle by healthcare professionals (HPCs).
At the same time, Relief Therapeutics intends to develop the inhaled formulation of RLF-100® for targeted chronic lung diseases (CLDs) such as sarcoidosis, berylliosis and checkpoint inhibitor-induced pneumonitis (CIP) because (i) it can maximize the clinical efficacy directly in the target organs (the lungs), (ii) while reducing the side effects of the drug (vasodilator and hypotension) because it is not systemically absorbed and (iii) it is a patient friendly dosage form as those CLDs are primarily treated at home and drug administered directly by the patients.
COVID-19 ARDS
Acute respiratory failure is the primary cause of death in COVID-19. In some cases, the injury is attributed to cytokine storm – i.e., a massive release of inflammatory cytokines that then cause destruction of pulmonary epithelium cells. However, the cytokine storm is only produced after the SARS-CoV2 virus enters the ATII cell through binding of its spike protein to angiotensin converting enzyme 2 (ACE2) surface receptors (Mason 2020). ACE2 is not present on type I alveolar cells, which comprise 95 percent of the pulmonary epithelium hence those cells are not infected by the coronavirus. Similarly, only the ATII cell expresses the VPAC1 receptor to which VIP binds. VIP is shown to prevent their apoptosis in models of lung injury (Ao 2011, Pakbaz 1993). Hence, VIP represents a highly specific approach to rescuing the lung from the overwhelming failure of oxygenation seen in COVID-19.
Pulmonary drugs are difficult to develop, given regulatory requirements for long-term toxicology studies in multiple species, including primates (Tepper 2016). The FDA has asserted that these preclinical toxicology requirements must be observed in the case of candidate drugs to treat COVID-19. VIP, on the other hand, have successfully completed four species toxicology and safety pharmacology studies in both intravenous (IV) and inhaled dosages. Phase 2 trials in sarcoidosis (Prasse 2010), pulmonary hypertension (Petkov 2003, Leuchte 2008), pulmonary fibrosis (unpublished data) and asthma (Bundgaard 1983, Morice 1983 and 1986, Altiere 1984, Barnes 1984, Crimi 1988, Morice 1986) document that VIP has no major toxicities when inhaled at doses of 300µg/day or infused at dose of 6 pmol/kg/min. VIP was first proposed as a modulator of lung inflammation by Said (Said 1988, 1991). It has demonstrated positive effects in clinical trials of sepsis-related acute respiratory distress syndrome (ARDS) (Youssef 2020 preprint) and sarcoidosis (Prasse 2010).
In March 2020, at the beginning of the first wave of the pandemic in the U.S., our former collaboration partner, NeuroRx Inc. (NeuroRx), submitted an IND application to the FDA for a Phase 2b/3 trial of intravenous (IV)aviptadil for the treatment of patients with critical COVID-19 respiratory failure. Within 24 hours, the FDA issued a “Study May Proceed” letter and the first patients were treated in April 2020 at Thomas Jefferson University Hospital in Philadelphia.
In late 2020, a Phase 2b/3 clinical study with aviptadil acetate IV in patients with COVID-19-induced acute respiratory distress syndrome (ARDS) was completed in the U.S. by NeuroRx. In its press release reporting those results, NeuroRx announced that across all patients and sites, the aviptadil acetate IV treated cohort met the primary endpoint for successful recovery from respiratory failure at days 28 (p=0.14) and 60 (p=0.13) and had a meaningful survival benefit after controlling for ventilation status and clinical site. However, they also reported that the trial did not demonstrate a statistically significant difference on the study’s primary endpoint without statistical adjustment for these pre- specified covariates. Based on these findings, NeuroRx announced on June 1, 2021, the company applied to the FDA for emergency use authorization (EUA) for aviptadil acetate IV for the treatment of acute respiratory failure due to critical COVID-19 and that it planned to submit an NDA with the FDA. On November 5, 2021, NeuroRx announced the FDA declined its application for EUA of aviptadil IV for the treatment of acute respiratory failure due to critical COVID-19. Subsequent applications filed by NeuroRx with the FDA seeking EUA for more limited use of the product for the treatment of COVID-19 and for breakthrough therapy designation for the product were also denied in the first half of 2022.
46
Table of Contents
In March 2021, NeuroRx announced that aviptadil acetate IV was included in a National Institutes of Health (NIH)-sponsored Phase 3 ACTIV-3b/TESICO clinical trial in severely ill patients with COVID-19. In May 2022, Relief Therapeutics learned that the ACTIV-3b/TESICO trial was discontinued by its Data Safety Monitoring Board (DSMB) based on futility.
Relief Therapeutics intends to obtain and review all available clinical data, including data from the NIH-sponsored trial to better understand the results observed, up to and including the point at which the study was discontinued.
While regulatory approval for aviptadil acetate IV to treat COVID-19-induced ARDS has not been granted in the U.S., an unrelated pharmaceutical company received approval for this indication in India in early 2022 for their formulation of aviptadil, thereby substantiating Relief Therapeutics’ original hypothesis.
Inhaled RLF-100® is being evaluated in an investigator-initiated trial at a site in Switzerland for the treatment of ARDS associated with COVID-19 (Leuppi/NCT04536350). While the study is in an advanced stage of recruitment, changing disease patterns have hindered the completion of patient recruitment. The lead investigator has reported that top-line data is now expected in the fourth quarter of 2023.
Non-COVID-19 ARDS
Infectious acute respiratory distress syndrome (ARDS) is a potentially life-threatening condition in which the lungs become severely inflamed, leading to buildup of fluid in the lungs, preventing oxygen from getting to the bloodstream and the rest of the body. Infectious ARDS results from an injury or an infection (such as pneumonia, severe flu, sepsis, etc.) of the air sacs in the lung. Plans for clinical trials of RLF-100 for the treatment of infectious ARDS are in development.
RLF-100® (AVIPTADIL ACETATE) IN CHRONIC LUNG DISEASES (CLDs)
Inhaled RLF-100® is under development for targeted CLDs, including pulmonary sarcoidosis, checkpoint inhibitor-induced pneumonitis (CIP) and chronic berylliosis.
Pulmonary sarcoidosis, chronic berylliosis and CIP are generally classified as granulomatous chronic lung diseases because they all have similar pathogenesis which lead to the formation of lung granulomas. It is a process driven by an exaggerated immune response where activation of CD4+ Th1 and Th17 cells causes the pro-inflammatory cytokine storms and lung granuloma formation.
47
Table of Contents
Inhaled RLF-100®, which is the synthetic form of VIP, is supposed to bind its receptor VPAC1 on CD4+ Th1 and Th17 immune cells, thus inhibiting NfKB. Thanks to this specific mechanism of action, RLF-100® is able to reduce the pro-inflammatory cytokines and increase anti-inflammatory cytokines, preventing granuloma formation and allowing disease resolution as detailed in the illustration below.

48
Table of Contents
Pulmonary Sarcoidosis
Sarcoidosis is an inflammatory disease characterized by the formation of granulomas—tiny clumps of inflammatory cells that can develop in any part of the body. When the disease occurs in the lungs, it is called pulmonary sarcoidosis and is a form of interstitial lung disease (ILD) which are a group of immune-mediated disorders that cause progressive fibrosis of the lung interstitium (the extravascular and extracellular space between cells in tissue).
The granulomas disrupt the intake of oxygen and can cause scarring on the lungs, preventing the lungs stretching fully, and therefore limiting their capacity. The prognosis for patients with pulmonary sarcoidosis ranges from benign and self-limiting to chronic, debilitating disease and death. Despite increasing advances in research, pulmonary sarcoidosis remains difficult to diagnose with limited treatment options to manage symptoms and no known cure. According to the Foundation for Sarcoidosis Research, approximately 200,000 Americans live with pulmonary sarcoidosis. Relief Therapeutics was granted ODD by the FDA for inhaled RLF-100® for the treatment of pulmonary sarcoidosis in August 2021.
Checkpoint Inhibitor-Induced Pneumonitis (CIP)
Checkpoint inhibitor-induced pneumonitis (CIP) is a rare, potentially fatal form of lung inflammation following treatment with immune checkpoint inhibitors (ICIs). ICIs are a type of immune therapy used to treat cancer. CIP can result in cough, dyspnea, fever, chest pain, and in severe cases, lack of oxygen in the lungs (hypoxia) and respiratory distress. The use of inhaled RLF-100® for this indication will be further evaluated to explore whether such use could enhance compliance with chemotherapy and improve outcomes for cancer patients. Relief Therapeutics received a Swiss method-of-use patent protection related to the inhaled formulation of RLF-100® for the potential treatment of CIP extending into at least 2039.
Berylliosis / Chronic Beryllium Disease (CBD)
Chronic beryllium disease (CBD) is an orphan lung disease caused by the inhalation of beryllium particles, dust or fumes in the workplace, resulting in severe inflammation of the lungs, coughing and increasing breathlessness (dyspnea). CBD is a clinical phenocopy of sarcoidosis. Currently there are no treatments approved for berylliosis. The ex-vivo effect of RLF-100® on mononuclear cells in the setting of CBD is currently being evaluated. Together with the results from the Phase 2b sarcoidosis trial, these results would justify the therapeutic use of inhaled RLF-100® in CBD, providing a rationale for the clinical trial design in this indication.
SENTINOX
Sentinox, is a novel, acid-oxidizing solution containing hypochlorous acid in a nasal spray formulation that was developed by APR. Sentinox was certified in Europe on February 16, 2021, as a Class III medical device (certificate number EPT 0477.MDD21/4200.1). Sentinox is intended for irrigation, cleansing and moistening of the nasal cavities and is indicated to reduce the risk of infections caused by bacteria and viruses, including SARS-CoV-2, by lowering the nasal microbial load; symptomatic nasal care; and nasal care in cases of minor lesions/alterations of the nasal mucosa.
On October 27, 2021, we reported positive interim results from our randomized, controlled clinical trial designed to evaluate the safety and efficacy of Sentinox in reducing viral load in the upper respiratory airways in recently SARS-CoV-2 infected patients. We also reported that data from the study suggest Sentinox could potentially be effective in reducing the SARS-CoV-2 viral load at the level of the nasal mucosa.
On March 17, 2022, Relief Therapeutics and APR reported the final data from APR’s clinical trial of nasal spray, Sentinox, in SARS-CoV-2 infected patients. The post-market, interventional, randomized, controlled clinical study (NCT04909996, clinicaltrials.gov) enrolled 57 patients who were randomized to receive Sentinox treatment 0.5 ml into each nostril, performed three times/day or five times/day for five days as add-on to the standard therapy, vs. no Sentinox treatment group. The study was designed to assess the efficacy and safety of Sentinox spray in terms of viral load reduction, negativization and infectivity in recently infected SARS-CoV-2 individuals. It was conducted by the Hygiene Unit of IRCCS Policlinico San Martino Hospital in Genoa, Italy, and coordinated by Professor Giancarlo Icardi.
Considering the small sample size and the high variability in the baseline viral load observed within study groups, the primary endpoint was not reached; however, the results of the study suggest the potential efficacy of Sentinox in the reduction of the nasal viral load, negativization and infectivity and confirmed its safety and tolerability.
49
Table of Contents
Additional analyses have been conducted in patients stratified according to baseline value of RT-PCR cycles: in the subgroup with medium (Ct 20-30) viral load, the use of Sentinox significantly reduced the viral load of 1.9761 Log10 (p=0.0178) at day five compared to the control group, suggesting a positive trend in the treatment effect. Further efficacy analyses on the ITT population showed that negativization in the Sentinox three times/day group started at day four; at day six patients with negative swab were almost two-fold compared to the control group (47 percent in Sentinox group versus 22 percent in no treatment group) (p=0.0005). Similar results were obtained in the analysis conducted in the 20-30 RT PCR cycles subpopulation.
Analysis on infectivity data was also conducted in the ITT population: patients were considered “not infectious” (patient likely not be able to spread virus to others) when the cycle threshold value of >35 cycles was achieved (Carrouel et al. 2021; Jang et al. 2021; Iwanami et al. 2021; Choudhuri et al. 2020). In the three times/day Sentinox group, 71 percent of patients were non-infectious versus 44 percent in the control group at day six (p<0.0001). Overall safety data monitored through clinical examination showed a good safety profile for Sentinox. This has been confirmed also by VAS and LIKERT scale results.
Relief Therapeutics initiated a confirmatory, controlled clinical trial in the prevention of viral and bacterial airborne infections in the fourth quarter of 2022. Completion of the clinical trial is subject to availability of capital.
RARE CONNECTIVE TISSUE DISORDERS
NEXODYN® Acid-Oxidizing Solution (AOS)
Nexodyn® Acid-Oxidizing Solution (AOS) was developed using APR’s proprietary, patent protected TEHCLO Nanotechnology® and is a solution of highly pure and stabilized hypochlorous acid (HClO >95% of free chlorine species), acidic pH (2.5 – 3.0) with high reduction-oxidation potential (ORP 1.000 – 1.200 mV). The product is a self-administered sprayable solution with ancillary antimicrobial properties intended for use in the debridement, irrigation, cleansing and moistening of acute and chronic wounds (e.g., diabetic foot ulcers, pressure ulcers and vascular ulcers), post-surgical wounds, burns and other lesions. The product is certified in the EU as a Class III medical device and is certified as a 510(k) medical device in the U.S.
Nexodyn® AOS is proven to restart healing in chronic wounds by creating an ideal microenvironment to sustain the physiological healing process. A wealth of evidence and real-world experience has consistently shown accelerated wound closure with reduced infection rates and less wound-associated pain.
The anti-microbial and anti-inflammatory properties of Nexodyn® AOS, along with its tolerability, could make this an attractive treatment candidate for the management of wounds in epidermolysis bullosa (EB), with the potential to be the only product approved for the control of wound infection in this disease, thereby reducing long term antibiotic use, while assisting wound healing and decreasing wound related pain, all of which would significantly benefit quality of life in patients with this genetic disorder.
RLF-TD011 (formerly referred to as APR-TD011 or APR-TM011)
RLF-TD011 is a differentiated acid oxidizing solution of hypochlorous acid (HCIO) that combines strong antimicrobial action with anti-inflammatory properties, thereby allowing for infection control, reduction of wound colonization, alleviation of pain and itching and improved wound healing.
Developed with APR’s proprietary, patent-protected TEHCLO Nanotechnology®, RLF-TD011 employs an exclusive combination of three physio-chemical properties—high-purity HCIO, hypotonic low pH and high oxidation-reduction potential (ORP), which is believed to support a faster physiological healing of wounds by creating a favorable wound microenvironment. HCIO is well known as a broad-spectrum, fast acting antimicrobial agent, which reinforced by low pH and high ORP contributes to the prevention and treatment of skin infections.
RLF-TD011 for the Potential Treatment of Epidermolysis Bullosa
RLF-TD011 is an investigational drug candidate with the potential to treat wounds in epidermolysis bullosa (EB) as it is a self-administered, sprayable solution enabling targeted application while avoiding skin contact and cross-contamination. EB, also known as “Butterfly Skin,” is a group of rare, genetic, life-threatening connective tissue disorders characterized by skin fragility and blistering, which may appear in response to minor injury, even from heat, rubbing or scratching. These widely distributed, painful, chronic wounds can easily become infected, resulting in an elevated risk of sepsis and death. A crucial element of patient management involves rigorous and timely wound care.
50
Table of Contents
There are four main types of EB, which are classified based on the depth, or level, of blister formation: EB simplex (EBS), junctional EB (JEB), dystrophic EB (DEB) and Kindler syndrome. In severe cases, the blisters may develop into chronic wounds or occur inside the body, such as the lining of the mouth or stomach. Patients with JEB and DEB are at increased risk for serious complications, including aggressive squamous cell carcinoma. The National Epidermolysis Bullosa Registry (NEBR) reports, based on 16 years of data, that the incidence of EB in the U.S. is 19.57 per 1 million live births and the prevalence is 11.07 per 1 million population. Worldwide, EB impacts 500,000 lives.
Currently there is no cure or approved treatments for EB in the U.S. RLF-TD011 could represent the first product specifically indicated for EB patients that provides a comprehensive solution to prevent or reduce wound colonization and infection. This, along with its anti-inflammatory action, could provide symptom relief and wound healing. The Company estimates the global market opportunity for EB to exceed USD 1.0 billion.
Subject to clinical demonstration of efficacy and safety in clinical trials, RLF-TD011 could play an important role in the reduction of inflammation by inhibiting the NF-kB pro-inflammatory pathway and, at the same time, may offer a faster wound healing in EB patients and by reducing the itching and pain linked to infections and inflammation. In a preliminary clinical trial, EB patients who administered RLF-TD011 demonstrated improvement in skin blistering and tissue repair within just two weeks of treatment, and the product candidate was shown to be well tolerated with a favorable safety profile.
In late 2019, RLF-TD011 was granted ODD by the FDA for the treatment of EB, which qualifies the sponsor of the treatment for certain development incentives, including seven-year marketing exclusivity after FDA marketing approval is received. Relief Therapeutics intends to seek qualified infectious disease product (QIDP) designation status for RLF-TD011, which may confer up to an additional five years of market exclusivity regardless of patent protection status. Good Manufacturing Practice (GMP) grade product is being prepared for clinical development under an FDA-authorized IND.
In February 2023, Relief Therapeutics announced the first three patients were enrolled in a proof-of-concept, investigator-initiated study to evaluate RLF-TD011 as a treatment for EB (NCT05533866). The primary aim of this study will be to assess changes in the skin microbiome before, during and after treatment with RLF-TD011. Patients with dystrophic or junctional EB whose wounds are colonized by staphylococcus aureus, pseudomonas aeruginosa or commensal organisms will be treated with RLF-TD011 for eight weeks followed by discontinuation of treatment for four weeks with assessment of their wound microbiome at each stage. All study participants will have the option to continue treatment in a six-month open label study extension. Results of this study are expected sometime between the fourth quarter of 2023 and the first quarter of 2024 depending on the enrollment and treatment pace.
RLF-TD011 for Potential Treatment in Oncology Supportive Care
RLF-TD011 is currently approved in Europe as a Class III medical device for the treatment of skin lesions and toxicities induced by cancer treatments, including anti-epidermal growth factor receptors (anti-EGFR) monoclonal antibodies, such as Cetuximab. The use of anti-EGFR inhibitors causes papulopustular manifestations due to their interference of epidermal growth factor receptor (EGFR) signaling in the skin with a high risk of secondary infections.
Following commercial assessment, the company is planning to conduct a follow-on clinical study to renew product approval in Europe as a Class III medical device beyond 2024, when the new EU device regulations will apply. This clinical study will be a multi-center, post-market, double-blinded, placebo-controlled trial to evaluate the efficacy, safety and tolerability of RLF-TD011 in the management of skin lesions and reactions resulting from anti-EGFR monoclonal antibodies and/or radiotherapy treatments in oncology patients. This study would follow a preliminary, proof of concept study completed by the company on 15 patients with head and neck cancer treated for 8-12 weeks with Cetuximab and showing a mean reduction of 94 percent of the lesion area compared to the standard of care
In January 2023, Relief Therapeutics announced that an independent institutional review board (IRB) approved the protocol of an investigator-initiated trial to evaluate RLF-TD011 as an adjunctive treatment for patients diagnosed with cutaneous t-cell lymphoma (CTCL) (NCT05728879). The study will evaluate the effect of RLF-TD011, on the microbiome of CTCL skin lesions and determine tolerability, symptom improvement, and potential for reducing lesion size and skin disease activity.
51
Table of Contents
Cutaneous t-cell lymphoma (CTCL) is a rare, heterogeneous group of non-Hodgkin’s lymphomas characterized by abnormal accumulation of malignant t-cells in the skin that can result in the development of rashes, plaques and tumors. Because CTCL is rare and often looks like eczema or another common skin disease, it can be difficult to diagnose. Advanced CTCL lesions harbor staphylococcus aureus, which release toxins that stimulate malignant cells and drive disease progression. This often leads to recurrent skin infections with a high risk for sepsis and death. Treatment of advanced CTCL remains a challenge, with five-year disease-specific survival rates ranging from 70 percent for early stage to 24 percent for advanced disease, with the greatest mortality stemming from bacterial infections.
While there are many types of CTCLs, the most common diagnoses are mycosis fungoides, primary CTCL and primary cutaneous anaplastic large cell lymphoma. The overall incidence rate of CTCL was 8.55 per 1 million with MF being the subtype with the highest incidence, at 5.42 per 1 million. The overall incidence of CTCL in the U.S. and Europe has increased, a reflection of better diagnostic tools and increased awareness among physicians and patients, which has led to improved disease detection.
According to Fortune Business Insights, the North American CTCL therapeutics market size is projected to reach an annual valuation of USD 587.4 million by 2028, registering a 13.6 percent compound annual growth rate (CAGR) in the 2021-2028 period. The market value was estimated to be worth USD 225.9 million in 2020 and reached USD 240.9 million in 2021. The increasing burden of CTCL in the region is slated to increase the demand for novel CTCL therapeutics solutions. Cleveland Clinic reports that more than 3,000 new CTCL patients are diagnosed in the U.S. each year and about 16,000-20,000 individuals suffer from mycosis fungoides, the most common form of CTCL that is linked to skin-localized immune cell stimulation.
LEGACY PRODUCTS
Our legacy products are revenue-generating, approved products marketed in various countries and regions of the world including the U.S. and Europe, originally developed and patented by APR and subsequently licensed to third parties for commercialization in the different territories. The rights on the legacy products were acquired by Relief Therapeutics as part of the 2021 acquisition of APR.
SETOFILM / ONDISSOLVE
SETOFILM is the first prescription-only medicine approved in Europe and Canada, developed as an orodispersible film (ODF) formulation. The product is available in 4 mg and 8 mg doses. Once placed on the tongue, it dissolves in a few seconds and is swallowed with saliva without the need for water. The innovative ODF form may reduce the patient pill burden and enable patients to take their medication virtually anywhere.
The product is indicated for radiotherapy induced nausea and vomiting (RINV), chemotherapy induced nausea and vomiting (CINV) as well as postoperative induced nausea and vomiting (PONV) in both adults and children 6 months of age or older. The product has been formulated and developed using the RapidFilm drug delivery technology and is the form of a soluble film to be placed on the tongue where it dissolves in few seconds thus greatly improving patient compliance and avoiding possible risks of suffocation in kids.
The product is approved in Europe and Canada as prescription drug and it is marketed by Norgine B.V. and Takeda Pharmaceuticals respectively under license from APR.
CAMBIA™
Diclofenac potassium is an off-patent, potent non-steroidal anti-inflammatory drug (NSAID) widely used for treating inflammatory conditions and pain management. By applying its patented Dynamic Buffering Technology (DBT), APR developed the first and only NSAID approved by the FDA for the treatment of acute migraine attacks with or without aura in adults. The product is currently marketed as CAMBIATM by Assertio Therapeutics Inc. (Nasdaq: ASRT) in the U.S. and Miravo Healthcare (formerly Nuvo Pharmaceuticals Inc.) in Canada, under an exclusive, royalty-bearing license agreement with APR.
In January 2022, APR received a notice of allowance from the U.S. Patent and Trademark Office (USPTO) for patent application number 16/713,052 entitled, “Ready to Use Diclofenac Packs” with an expiration date in 2039.
52
Table of Contents
On February 28, 2022, Unimedica Laboratories Pvt. Ltd., India, sent APR a Notice of Certification under the Federal Food, Drug, and Cosmetic Act (FFDCA) related to the filing of an abbreviated new drug application (ANDA) for CAMBIA. While there can be no assurance, it is unlikely that Unimedica will get accelerated approval, and we reserve the right to seek to enforce our patents.
DBT and CAMBIA are currently protected by a family of four patents listed in the FDA Orange Book, all expiring in 2026. In 2023, based on litigation settlements between Assertio and specific generic filers, generic versions at Cambia may become available. CAMBIA is currently available in the form of a dry powder packed into a single dose envelope to be poured and dissolved in water before administration.
VOLTADOL
Developed by APR using a patented matrix patch technology, Voltadol is a topical, locally applied and locally acting patch delivering diclofenac sodium, an off-patent, potent non-steroidal anti-inflammatory drug (NSAID) for the local treatment of painful, acute conditions such as muscle and joint strains. Unlike heat plaster, the patch contains an anti-inflammatory. It penetrates deep to the source of pain to provide powerful pain relief. The medicated patch provides up to two times more powerful deep pain relief, compared to a non-medicated, non-heated placebo patch. The patch also provides 12 hours continuous release of the active ingredient (diclofenac) to the site of pain. This means the patch only needs to be applied once in the morning and once in the evening to provide effective pain relief. The product is marketed in various countries as an over-the-counter medicine by GlaxoSmithKline (GSK) which recently spun-off the rights to Haleon.
NeuroRx Collaboration Agreement
On September 18, 2020, we entered into a binding collaboration agreement (the Collaboration Agreement) with NeuroRx. The Collaboration Agreement established the terms under which we will collaborate and assist with NeuroRx in order to maximize revenues in our respective territories from the sale of RLF-100® for intravenous and inhaled use primarily in the treatment of COVID-19 related conditions. The NeuroRx territory included the U.S., Canada, and Israel. The Relief territory comprised the rest of the world and includes the EU, Switzerland, Iceland, Norway, the UK, the Channel Islands, Liechtenstein, Monaco, Andorra, San Marino and Vatican City.
The Collaboration Agreement provided that we would fund the costs associated with the clinical trials and development of RLF-100® (aviptadil acetate) in the U.S., which development would be conducted and managed by NeuroRx. NeuroRx was responsible for ensuring that the costs of the clinical trials and development activities for RLF-100 IV did not exceed the budget contemplated by the parties by more than 30 percent. The Collaboration Agreement also provided options for the parties to treat health conditions outside COVID-19 and for the commercialization of RLF-100® outside of the above-described territories.
Dispute and Litigation with NeuroRx
On October 7, 2021, because of breaches of the Collaboration Agreement by NeuroRx, among others, we filed a lawsuit against NeuroRx and its Chief Executive Officer, Dr. Jonathan Javitt, for multiple breaches of the Collaboration Agreement (Complaint). The Complaint was filed in the Supreme Court in the State of New York in Manhattan. The suit alleged, among other matters, breaches of the covenant of good faith and fair dealing and tortious interference with prospective economic advantage. The Complaint, among other remedies, sought damages, an order compelling NeuroRx to comply with multiple provisions of the Collaboration Agreement, and a declaration directing NeuroRx to deliver the entire data set from the Phase 2b/3 clinical trial of intravenously administering aviptadil to Relief.
On January 10, 2022, NeuroRx, through its parent, NRx, filed a complaint against Relief. In the complaint, NeuroRx claimed damages in excess of $185 million and sought a ruling that the Collaboration Agreement is void. It also included a count for defamation.
On November 14, 2022, Relief and NRx issued a press release announcing that the parties had entered into a definitive settlement agreement to resolve all matters relating to the pending litigation. At the closing of this settlement, which was held on December 19, 2022, (i) NRx transferred to Relief all of the assets that it previously used in its aviptadil development program, including its regulatory filings, patent applications, clinical data, and the formulation of the aviptadil product it was previously developing, (ii) Relief has the exclusive right and control going forward to develop and commercialize an aviptadil product, (iii) Relief has agreed to use commercially reasonable efforts to continue the existing Right to Try Program for aviptadil in the U.S. for at least two years, (iv) Relief will pay NRx milestone payments if it can successfully obtain commercial approval of an aviptadil product (whether for COVID-19 or any other indication), (v) Relief will pay NRx royalties based on a percentage of future sales of an aviptadil product (whether for COVID-19 or any other indication), up to a maximum of $30 million in the aggregate, (vi) NRx Pharmaceuticals has agreed not to compete in the development of an aviptadil product in the future, and (vii) at the closing, Relief and NRx Pharmaceuticals dismissed their pending litigation. Further, the Collaboration Agreement was cancelled. Finally, as part of the settlement, the parties exchanged releases of all claims that could have been brought in the lawsuits between the parties.
53
Table of Contents
Patents and Licenses
Our success depends significantly on our ability to develop, obtain and maintain intellectual property rights for our product candidates, technology and know-how, to operate without infringing intellectual property rights of others and to prevent others from infringing our intellectual property rights. We seek to protect our proprietary position by, among other methods, filing patent applications in Europe, the U.S. and other relevant jurisdictions related to our proprietary technology, inventions and improvements that are vital to the development of our business, where patent protection is available. We also rely on trade secrets, know-how and in licensing opportunities to develop and maintain our proprietary position.
OLPRUVA™ License
We in-licensed from Acer the rights to commercialize OLPRUVA™ for the treatment of UCD and MSUD. Under the terms of our collaboration agreement, Acer received approximately $10 million cash payment (originally $14 million, offset by repayment of the $4 million outstanding balance of the prior loan, plus interest, from Relief to Acer). Relief will also pay Acer up to $20 million ($15 million which has been paid to-date) in U.S. development and commercial launch costs for the UCDs and MSUD indications. Acer will retain development and commercialization rights in the U.S., Canada, Brazil, Turkey, and Japan. The companies will split net profits from Acer’s territories 60 percent to 40 percent favor of Relief. In addition, Relief has licensed the rights for the rest of the world, where Acer will receive from Relief a 15 percent royalty on all revenues received in Relief’s territories. Acer may also receive a total of $6 million in development milestone payments following the first EU marketing approvals for UCDs and MSUD. Acer intends to submit the patent for listing by the FDA in the Approved Drug Products with Therapeutic Equivalence Evaluations, or Orange Book.
In parallel with Acer’s actions, Relief and Acer are pursuing similar claims in the European Patent Office to cover OLPRUVA™ as Relief continues to execute on its plan to submit a Marketing Authorization Application (MAA) for OLPRUVA™ for the treatment of patients with UCDs in Europe. There can be no assurance that Relief and Acer will be successful in those endeavors.
Acer maintains its own intellectual property portfolio. In August 2014, Acer was granted ODD by the FDA to sodium phenylbutyrate (OLPRUVA™) for the treatment of Maple Syrup Urine Disease.
As of the date of this annual report, Acer’s patent portfolio for ACER-001(OLPRUVA™) consists of three patent families. The first family includes 41 granted patents world-wide directed towards novel sodium phenylbutyrate particle formulations and methods of use. These patents have an expiration date of October 2036, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity. If granted, additional patents, would expire no earlier than October 2036. Acer’s patent portfolio further includes PCT/US2021/040760 and PCT/US2022/040082. Patents granting from applications claiming priority to PCT/US2021/040760 will expire in July 2041, excluding any patent term adjustments or extensions, or any form of potential exclusivity. Patents granted from applications claiming priority to PCT/US2022/040082 will expire in April 2042, excluding any patent term adjustments or extensions, or any form of potential exclusivity.
54
Table of Contents
OLPRUVA™’s patent applications worldwide are as follows:
| Summary Description of Patent or Patent Application |
United States or Foreign Jurisdiction |
Expiration Date | ||
| Palatable Compositions Including Sodium Phenylbutyrate and Uses Thereof | Granted: United States (Patent Nos. 11,154,521, 11,202,767, and 11,433,041), Albania, Austria, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Germany, Denmark, Estonia, European Patent Convention, Spain, Finland, France, United Kingdom, Greece, Croatia, Hungary, Ireland, Iceland, Italy, Lithuania, Luxembourg, Latvia, Monaco, North Macedonia, Malta, Mexico, Netherlands, Norway, Poland, Portugal, Romania, Serbia, Sweden, Slovenia, Slovakia, San Marino
Pending: Bahrain, Brazil, Canada, European Patent Convention, Israel*, Japan, Republic of Korea, Kuwait, Mexico, New Zealand*, Oman, Qatar, Saudi Arabia, United Arab Emirates |
October 17, 2036. Expiration of pending patents to be determined upon grant. | ||
| Administration of Sodium Phenylbutyrate in a Fasted State to Treat Urea Cycle Disorders | Pending: Patent Cooperation Treaty | Applications claiming priority to this PCT application, if granted, will expire no earlier than July 7, 2041. | ||
| Dosage Form for Improving Palatability of Drug Substance | Granted: China
Pending: China, Patent Cooperation Treaty |
August 24, 2031 (China). Applications claiming priority to the PCT application, if granted, will expire no earlier than April 12, 2042. | ||
| * | Two Patent Applications |
Aviptadil Acetate/RLF-100® Patents
As of the date of this annual report, Relief has two patent families covering formulations of aviptadil acetate. The first family includes a patent in the U.S. valid until at least July 2029, with extension opportunities up to five years, as well as patents in several countries in Europe and the rest of the world valid until at least 2026, excluding extension opportunities comparable to the U.S. The first family applications were filed in 2006 and granted between 2011 and 2012. The second family includes an unpublished provisional application. Relief’s aviptadil patents and patent applications worldwide are as follows:
| Summary Description of Patent or Patent Application |
United States or Foreign Jurisdiction |
Expiration Date | ||
| Formulation for Aviptadil | United States (No. 8,178,489), China, European Patent Convention, Mexico, India, Austria, Denmark, Switzerland/Lichtenstein, Germany, Spain, United Kingdom, Ireland, Netherlands, Turkey | July 3, 2029 (United States), March 7, 2026 (all other jurisdictions) | ||
| **Unpublished** | United States (provisional) | Expiration of any potential applications claiming priority to this provisional application to be determined upon grant. | ||
AdVita
As of the date of this annual report, AdVita has three patent families in various stages of prosecution. The first family includes 1 granted patent and 15 applications world-wide claiming priority to PCT/EP2020/062420. Patents granting from applications claiming priority to PCT/EP2020/062420 will expire in May 2040, excluding any patent term adjustments or extensions, or any form of potential exclusivity. The second family includes 15 applications world-wide claiming priority to PCT/EP2021/052151. Patents granted from applications claiming priority to PCT/EP2021/052151 will expire in January 2041, excluding any patent term adjustments or extensions, or any form of potential exclusivity. The last family includes an a PCT application , PCT/IB2022/053709. Each family of applications is directed to novel uses and/or formulations of Aviptadil for treating various conditions such as drug-induced pneumonitis. AdVita’s patents and patent applications worldwide are as follows:
55
Table of Contents
| Summary Description of Patent Application |
United States or Foreign Jurisdiction |
Expiration Date | ||
| Patent Family 1 | ||||
| Vasoactive Intestinal Peptide (VIP) for Use in the Treatment of Drug-Induced Pneumonitis | United States (Application No. 17/595,025), Australia, Brazil, Canada, Switzerland, China, European Patent Convention, Hong Kong, Israel, Japan, Republic of Korea, Mexico, New Zealand, Russian Federation, Singapore, South Africa | May 5, 2040 (Switzerland) Applications, if granted, will expire no earlier than May 5, 2040. | ||
| Patent Family 2 | ||||
| Human Anti-Inflammatory Peptides for the Inhalatory Treatment of Inflammatory Pulmonary Diseases | United States (Application No. 17/759,559), Australia, Brazil, Canada, China, European Patent Convention, Hong Kong, Israel, India, Japan, Republic of Korea, Mexico, New Zealand, Singapore, South Africa | Applications claiming priority to this PCT application, if granted, will expire no earlier than Jan 29, 2041. | ||
| Patent Family 3 | ||||
| Use of Aviptadil Alone or in Combination with Alpha Lipoic Acid as a Therapeutic Medicament for Post-Viral Infection Syndrome | Patent Cooperation Treaty | Expiration of any potential applications claiming priority to this PCT application to be determined upon grant. | ||
Partner Patents and Licenses
TECHLO Technology™
As of the date of this annual report, our TEHCLOTM portfolio consists of four patent families. The first three families include 107 granted patents world-wide directed to systems and methods for generating hypochlorous acid solution, compositions comprising Relief’s hypochlorous acid solution, and methods for treating ocular disorders. These patents expire between October 2026 and June 2030, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity. If granted, additional patents would expire no earlier than July 2040.
56
Table of Contents
| Summary Description of Patent or Patent Application |
United States or Foreign Jurisdiction |
Expiration Date | ||
| Patent Family 1 | ||||
| Electrolytic Water Treatment Device Having Sintered Nanoparticle Coated Electrode and Method for Making Acid or Basic Water Therewith | United States (Patent No. 8,277,634) | August 23, 2029 | ||
| Device Comprising an Electrode with Nanocoating for Preparing a Highly Stable Aqueous Solution and Method for Making this Aqueous Solution | Austria, Belgium, Bulgaria, Switzerland, Switzerland, Cyprus, Czechia, Germany, Denmark, Estonia, European Patent Convention, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Lithuania, Luxembourg, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, Turkey | October 24, 2026 (Luxembourg), October 23, 2026 (all other jurisdictions) | ||
| New Highly Stable Aqueous Solution, Electrode with Nanocoating for Preparing the Solution and Method for Making this Electrode | Australia, Canada, China, Israel, Mexico, New Zealand, Republic of Korea, Russian Federation, Singapore, South Africa | October 22, 2026 (China), October 23, 2026 (all other jurisdictions) | ||
| A Device for the Electrolytic Treatment of a Fluid | India | October 23, 2026 | ||
| Patent Family 2 | ||||
| Highly Stable Electrolytic Water with Reduced NMR Half Line Width | United States (Patent Nos. 8,709,495, 9,402,192, and 9,889,153), Austria, Australia, Belgium, Bulgaria, Brazil, Canada, Switzerland, Cyprus, Czechia, Germany*, Denmark, Estonia, European Patent Convention*, Spain*, Finland, France*, United Kingdom*, Greece, Croatia, Hungary, Ireland, Iceland, Italy*, Japan, Republic of Korea, Lithuania, Luxembourg, Latvia, Monaco, Malta, Mexico, Netherlands, Norway, New Zealand, Poland*, Portugal, Romania, Russian Federation, Sweden, Singapore, Slovenia, Slovakia, Turkey*, South Africa
* Two patents |
February 7, 2030 (United States Patent No. 8,709,495), April 24, 2028 (one United Kingdom patent), April 26, 2028 (Greece), April 25, 2028 (all other patents and jurisdictions) | ||
| Electrolytic Acid Water | India | April 25, 2028 | ||
| Patent Family 3 | ||||
| Methods of Treating Outer Eye Disorders Using High ORP Acid Water and Compositions Thereof | United States (Patent No. 8,691,289), Germany, European Patent Convention, Spain, France, United Kingdom, Italy, South Africa | June 15, 2030 (United Kingdom), March 13, 2032 (United States), June 16, 2030 (all other jurisdictions) | ||
| Patent Family 4 | ||||
| Therapeutic Uses of Oxidising Hypotonic Acid Solutions | United States (Application No. 17/597,220), United Arab Emirates, Australia, Brazil, Canada, China, Colombia, Egypt, European Patent Convention, Israel, Japan, Korea, Kuwait, Qatar, Russian Federation | Applications, if granted, will expire no earlier than July 2040. | ||
We were granted worldwide licenses for TECHLOTM to numerous regional and national pharmaceutical firms. None of the licenses, either individually or as a whole, currently represent a material amount of the revenues of the consolidated company.
57
Table of Contents
Physiomimic Technology™ - PKU GOLIKE®
As of the date of this annual report, the PKU GOLIKE® portfolio consists of two patent families including 34 pending applications and 50 granted patents world-wide. Patents resulting from these families, if granted, will expire no earlier than 2036 and 2038, respectively, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity.
| Summary Description of Patent or Patent Application |
United States or Foreign Jurisdiction |
Expiration Date | ||
| Patent Family 1 | ||||
| Modified Release Orally Administered Amino Acid Formulations | Granted: United States (Nos. 10,500,180 and 11,419,837), Armenia, Austria, Australia, Azerbaijan, Belgium, Bulgaria, Belarus, Switzerland, China, Colombia, Czechia, Germany, Denmark, Eurasian Patent Convention, European Patent Convention, Spain, Finland, France, United Kingdom, Greece, Croatia, Hungary, Indonesia, Ireland, Israel, Italy, Jordan, Kyrgyzstan, Kazakhstan, Lebanon, Lithuania, Macao, Malta, Mexico, Malaysia, Norway, Netherlands, Poland, Portugal, Romania, Russian Federation, Sweden, Slovenia, Slovakia, Tajikistan, Turkmenistan, Turkey, Taiwan, South Africa
Pending: United States (Application No. 17/660,999), Argentina, Australia, Brazil, Canada, Chile, China, Egypt, European Patent Convention, Gulf Cooperation Council, Hong Kong*, Israel, Iraq, Malaysia, Philippines, Pakistan, Saudi Arabia, Uruguay, Venezuela, Vietnam
* Two Applications |
September 25, 2036 (Jordan), September 28, 2036 (Taiwan), September 27, 2036 (all other jurisdictions).
Applications, if granted, will expire no earlier than September 27, 2036. | ||
| Patent Family 2 | ||||
| Methods of Normalizing Markers of Amino Acid Metabolism | Granted: Iraq | August 29, 2039 (Iraq) | ||
| Pending: United States (Application No. 16/543,437), Australia, Brazil, Canada, Chile, China, Colombia, European Patent Convention, Hong Kong, Israel, Pakistan, Saudi Arabia, Taiwan | Expiration of pending patents to be determined upon grant. | |||
We were granted licenses for PKU GOLIKE® in Spain, Portugal, the UK, Ireland, Brazil, Israel, Gaza territories and West Bank, Ecuador, Greece and Cyprus.. None of the licenses, either individually or as a whole, currently represent a material amount of the revenues of the consolidated company.
Dynamic Buffer Technology - Diclofenac
As of the date of this annual report, our diclofenac patent portfolio consists of multiple patent families comprising 49 granted patents world- wide, with expiration dates in either between 2026 and 2040, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity. The portfolio further includes 14 pending applications directed to new diclofenac formulations and methods of use. If granted, patents resulting from these pending applications will expire between 2026 and 2041, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity.
58
Table of Contents
| Summary Description of Patent or Patent Application |
United States or Foreign Jurisdiction |
Expiration Date | ||
| Patent Family 1 | ||||
| Diclofenac Formulations and Methods of Use | Granted: United States (Nos. 7,759,394, 8,097,651, 8,927,604 and 9,827,197), Australia, Canada*, Switzerland*, Germany**, European Patent Convention***, Spain*, France*, United Kingdom*, Greece*, Indonesia, Italy**, Jordan, Republic of Korea, Lebanon, Malta, Mexico, Norway, New Zealand, Pakistan, Poland, Portugal, Russian Federation, Turkey, South Africa
Pending: United States (Application No. 16/716,511), China, Egypt, Gulf Cooperation Council*, Hong Kong, Thailand
* Two Patents ** Three Patents *** Four Patents |
June 16, 2026 (all United States Patents), June 8, 2026 (Lebanon), June 14, 2026 (Malta), June 15, 2026 (United Kingdom), June 16, 2026 (all other jurisdictions). Expiration of pending applications to be determined upon grant. | ||
| Diclofenac Formulations | Granted: Germany, Spain, France, United Kingdom, Italy, | June 15, 2026 (United Kingdom), June 16, 2026 (All other jurisdictions) | ||
| Patent Family 2 | ||||
| Moisture Resistant Container Systems for Rapidly Bioavailable Dosage Forms | Granted: United States (Nos. 7,700,125 and 8,097,267) | February 7, 2026 (No. 8,097,267), October 11, 2026 (No. 7,700,125) | ||
| Patent Family 3 | ||||
| Substantially Sodium Free Diclofenac Potassium Oral Solutions | Granted: United States (No. 11,127,318)
Pending: United States (Application No. 17/463,154), European Patent Convention |
January 27, 2038 (United States Patent No. 11,127,318). Expiration of pending patents to be determined upon grant. | ||
| Patent Family 4 | ||||
| Ready to Use Diclofenac Stick Packs | Granted: United States (No. 11,260,026)
Pending: United States (Application No. 17/584,212), European Patent Convention, Hong Kong |
February 22, 2040 (United States Patent No. 11,260,026). Expiration of pending applications to be determined upon grant. | ||
| Patent Family 5 | ||||
| Bioavailable Sugar-Based Diclofenac Formulations | Patent Cooperation Treaty | Expiration of pending application to be determined upon grant. | ||
59
Table of Contents
We licensed Diclofenac to Assertio Therapeutics for its Cambia® product and to Novartis for its Voltaren®/Voltfast® product. We entered into a partnership agreement with Fidia Farmaceutici S.p.A. for diclofenac patches. We also entered into license and supply agreements with Actavis Group PTC ehf (MerckleGmbH) and Zentiva k.s..
We sold the IT Patent for Diclofenac to the Neilos s.r.l. (an affiliate of Shedir Pharma Group S.p.A) but retained a non-exclusive and perpetual license right on such patent for the production in the country of Italy of drops solution for oral administration containing Diclofenac Potassium as sole active ingredient in a concentration of 5 percent. The sale of this patent does not have an effect on the license and supply agreements described in this section.
We received a sublicense right in the territory of U.S. and China from Fidia Farmaceutici in relation to the following patents owned by IBSA Farmaceutici on Diclofenac transdermal patch:
| • | Chinese Patent No. CN101001616B; |
| • | U.S. Patent No. 10,328,034. |
We do not believe that this license agreement our material to its business.
Oral Disposable Film—Ondansetron
As of the date of this annual report, our Ondansetron patent portfolio consists of two patent families comprising two pending applications and six granted patents with expiration dates ranging from 2027 to 2031, exclusive of any patent term adjustments or extensions, or any form of potential exclusivity.
| Summary Description of Patent or Patent Application |
United States or Foreign Jurisdiction |
Expiration Date | ||
| Patent Family 1 | ||||
| Non-Mucoadhesive Film Dosage Forms | United States (Patent Nos. 8,580,830 and 9,682,037), Canada, Republic of Korea | November 22, 2029 (United States Patent No. 8,580,830), October 2, 2027 (All other patents) | ||
| Patent Family 2 | ||||
| Fast Dissolving Drug Delivery Systems | Granted: Russian Federation, South Africa
Pending: Brazil, Egypt |
March 23, 2031. Expiration of pending patents to be determined upon grant. | ||
APR has granted a license right on the abovementioned patents and patent applications to Takeda in Canada. This license does not represent a material amount of our revenues.
Other APR IP
In addition to the patents and applications described above, APR has several other pending applications and granted patents:
| • | U.S. Patent No. 8,039,024, entitled “Device and composition for the delivery of a preservative-free balsamic cream” and patents in Canada, Russia and Ukraine entitled “Adhesive Label with Bittering Agent and Fluidifying Agents for Natural Airway Secretions” claim and cover a preservative-free, OTC decongestant stick pack which is no longer marketed. |
| • | I.T. patent 102020000021223 and pending unpublished applications in the U.S. and Canada related to dermal compositions, entitled “Dermal Compositions Replicating the Vernix Caseosa”, cover and claim OTC formulations targeting atopic dermatitis as well as other moderate skin disorders. |
60
Table of Contents
Manufacturing and Supply
We do not own or operate facilities for the manufacture, packaging, labeling, storage or distribution of preclinical or clinical supplies of any of our drug candidates. We instead contract with and rely on third-party CMOs to manufacture, package, label, store test and distribute all preclinical development and clinical supplies of our drug candidates, and we plan to continue to do so for the foreseeable future. APR maintains laboratories for the testing of its products. Such laboratories are also used to develop new formulations.
Compliance with governing rules and quality requirements
The facilities used by our collaboration partners and CMOs to manufacture our product candidates are systematically audited by local authorities and occasionally inspected by competent authorities where the clinical studies are ongoing. The facilities where the commercial productions are performed must be approved by the FDA or other relevant regulatory authorities, pursuant to inspections that are conducted after we submit our NDA or comparable marketing applications. We perform periodic quality audits of the manufacturing facilities and CMOs to monitor their compliance with the regional laws, regulations and applicable cGMP standards and other laws and regulations, such as those related to environmental health and safety matters. The scope of our audits also involves monitoring the ability of our providers to maintain adequate QCs and QA systems including personnel qualification.
After manufacturing, our products are submitted to extensive characterization and QC testing plans performed by using properly developed analytical methods that are qualified or validated; this ensures the accuracy of the results generated and provides evidence of the quality of our products. In addition, our products are submitted to detailed and standardized stability programs aimed at demonstrating product stability during the storage period; this, in addition to guaranteeing the safety of the products, supports the definition of a suitable supply chain that may encompass the distribution of the products in different continents.
Contractual framework
We have established, with CMOs supplying drug substances or drug products under cGMP, quality agreements and master service agreements. Quality agreements define the quality standards required to develop, produce and supply the product, and also define the responsibilities related to the collaboration with regards to the quality related aspects. Manufacturing service agreements define the commercial and financial framework under which product manufacturing under cGMP is performed. Any failure to achieve and maintain compliance with the laws, regulations and standards, suspension of the manufacturing of our product candidates or revoke of cGMP permissions, which would adversely affect our business and reputation, are defined in the master service agreements and quality agreements. The risk that any third-party providers may breach the agreements they have with us because of factors beyond our control and the possibility that they may also terminate or refuse to renew their agreements because of their own financial difficulties or business priorities, potentially at a time that is costly or otherwise inconvenient for us, is managed by us with constant investments toward maintaining reserve stocks and in-depth process know-how.
Interaction with collaboration partners and CMOs
Finally, our partnership with CMOs is managed through an efficient project management platform in which teams are formed with the representatives of each key function from both parties. Meetings occur either through telephone conferences aimed at updating short-term actions or face-to-face conferences when mid- to long-term development plans are discussed.
Acquisition of APR Applied Pharma Research SA
On April 30, 2021, we entered into a binding term sheet with the then current shareholders of APR Applied Pharma Research SA, a privately held Swiss company with over 25 years of experience in identifying developing and commercializing known molecules engineered with drug delivery systems in niche and rare diseases, to acquire all of the outstanding shares of APR. On June 28, 2021, the former shareholders of APR and Relief signed and closed a definitive agreement for Relief to acquire all outstanding shares of APR. Under the terms of the agreement APR’s shareholders have received from Relief CHF 21.5 million in cash and 206,786,784 Consideration Shares at a value of CHF 45 million when the Consideration Shares were issued and listed. The APR shareholders were also eligible to receive possible future contingent milestone payments in the aggregate maximum amount of up to CHF 35 million, upon achievement of pre-agreed objectives involving (i) the execution of a definitive agreement for the commercialization of Sentinox (as such product is defined below), (ii) the launch of Sentinox in the first of France, Germany, Spain, Italy, and the UK, (iii) the launch of PKU GOLIKE® in the U.S., and (iv) the launch of RLF-TD011 (as such product is described below) in the first of France, Germany, Spain, Italy and the UK. The launch of PKU GOLIKE® in the U.S. on October 10, 2022 marked the completion of the third milestone, for which Relief issued a cash payment of CHF 2.8 million as well as 150,200,120 shares.
61
Table of Contents
Acquisition of AdVita Lifescience GmbH
On July 28, 2021, we announced the closing of a definitive agreement to acquire all of the outstanding shares of AdVita Lifescience GmbH. Under the agreement, the stockholders of AdVita received 135,741,063 of our ordinary shares, representing EUR 25 million (approximately CHF 27.4 million) in value based on a 60-day volume weighted average price (VWAP) of our ordinary shares and were also eligible to receive additional contingent payments in cash. In April 2022, we made an initial milestone payment of EUR 5 million (approximately CHF 5 million) upon completion of the first milestone.
As of the date of this annual report, the former shareholders of AdVita may receive up to EUR 10 million (approximately CHF 10 million) upon achievement of pre-agreed milestones involving (i) the approval in the U.S. or Europe of the inhaled form of aviptadil for the treatment of sarcoidosis or berylliosis, and (ii) the conduct of a phase II clinical study for the inhaled form of aviptadil in the treatment of checkpoint inhibitor-induced pneumonitis.
AdVita was founded in 2019 for the purpose of developing products and strategies to improve the therapy and diagnosis of rare lung diseases. Among AdVita’s assets are intellection property rights that may cover RLF-100 inhaled formulation specifications and the potential application of inhaled Aviptadil in the treatment of Acute Respiratory Distress Syndrome, Checkpoint Inhibitor-induced Pneumonitis and Sarcoidosis.
Collaboration Agreement with InveniAI LLC
On November 24, 2021, we announced that we had entered into a collaboration agreement with InveniAI LLC (InveniAI), a U.S. based company that has pioneered the application of artificial intelligence and machine learning across biopharma and other industries, in order to identify promising drug candidates to treat rare and specialty diseases (the InveniAI Collaboration Agreement).
Under the terms of the InveniAI Collaboration Agreement, InveniAI will use its proprietary platform for the identification of potential pharmaceutical product opportunities using its Pharma Big Innovation Data Lab, consisting of (i) its proprietary AlphaMeld platform, a cloud-based artificial intelligence platform that uses its proprietary machine learning and deep learning based neural networks to identify product opportunities in therapeutic areas, (ii) its cross-functional teams at its Integrated Center of Excellence, and (iii) domain expertise, to generate novel pharmaceutical opportunities and the related development pathway for the development of such concepts.
In the collaboration it is expected that InveniAI will use its platform to navigate the volume of data for all regulatory agency approved drugs and their associated active ingredients to identify potential rate and specialty disease indications for development and commercialization by us (product concepts). InveniAI will seek to prioritize top product concepts, associated diseases, scientific packages and evidence to support the potential drug development opportunities by us. We anticipate that InveniAI’s platform will complement APR’s existing capabilities in research and development and in drug reformulation. Based on product leads developed by InveniAI, we hope to develop proprietary versions of existing drugs, and to protect those drugs with long-lived intellectual property and defensible product claims.
Under the terms of the InveniAI Collaboration Agreement, we paid InveniAI an initial up-front fee of $500,000. We will be required to pay success milestones for any products brought to us in connection with the InveniAI Collaboration Agreement ranging from $200,000 per product candidate for which we exercise our option to acquire IP rights to $50 million for any required product reaching $1 billion per year in net sales. We will also be required to pay royalties on any such commercialized product in certain countries a royalty of approximately three percent.
We are not currently developing any product brought to us by InveniAI, and there can be no assurance that our collaboration with InveniAI will result in the development of new product candidates or product concepts.
62
Table of Contents
Regulation in the United States
The Company assumes that some of its product candidates will be submitted under an NDA and that approval of not only the products but also their manufacture is required before starting to market them. According to the definition of the U.S. Code of Federal Regulations, a drug product is approved only after demonstrating that it meets standards that assure the product’s safety, purity, effectiveness and potency.
The design, pre-clinical and clinical study, manufacture, labeling, packaging, storage, holding, sale, distribution, marketing, and promotion of pharmaceutical products – including biologic products – are subject to extensive and rigorous government regulation. The Federal Food, Drug, and Cosmetic Act (FFDCA) and other federal and state statutes and regulations govern or influence these activities. Non-compliance with applicable requirements can result in fines, recall or seizure of products, total or partial suspension of production and/or distribution, refusal of the government to enter into supply contracts or to approve NDAs, civil penalties and criminal prosecution.
Product Approval Process
Pharmaceutical products are subject to extensive regulation by the FDA. The FFDCA, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the U.S. typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
The FDA’s Center for Drug Evaluation and Research fosters early communications between sponsors and new drug review divisions to provide guidance on the data necessary to warrant IND submission, and a 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the IND to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice, or GCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators, and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
63
Table of Contents
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, after the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance, and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases the FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in instances where the study is a large multicenter trial demonstrating internal consistency and a statistically persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
In addition, the manufacturer of an investigational drug in a Phase 2 or Phase 3 clinical trial for a serious or life-threatening disease is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for expanded access.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S.. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls. The cost of preparing and submitting an NDA exceeds USD 3,000,000.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be filed based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. If the NDA submission is filed, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for standard review drug products are reviewed within ten to twelve months; most applications for priority review drugs are reviewed in six to eight months. Priority review can be applied to drugs that the FDA determines offer major advances in treatment or provide a treatment where no adequate therapy exists. The review process for both standard and priority review may be extended by the FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission.
The FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy, or REMS, to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
64
Table of Contents
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Quality Assurance
The FDA regulates the facilities, processes and procedures used to manufacture and market pharmaceutical products in the U.S. Manufacturing facilities, including those located outside the U.S., must be registered with the FDA and all products made in such facilities must be manufactured in accordance with cGMP regulations enforced by the FDA. Compliance with cGMP regulations requires the dedication of substantial resources and requires significant expenditures. These cGMP standards are particularly stringent for biologic products. The FDA periodically inspects manufacturing facilities and procedures to assure compliance. The FDA may cause a suspension or withdrawal of product approvals if regulatory standards are not maintained. In the event an approved manufacturing facility is required by the FDA to curtail or cease operations, or otherwise becomes inoperable, or a third party contract manufacturing facility faces manufacturing problems, obtaining the required FDA authorization to manufacture at the same or a different manufacturing site could result in production delays, which could adversely affect the Company’s business, results of operations, financial condition and cash flow.
The FDA conducts pre-approval inspections of facilities engaged in the development, manufacture, processing, packing, testing and holding of the products subject to INDs. If the FDA concludes that the facilities to be used do not or did not meet cGMP, GLP or GCP requirements, it will not approve an IND application. Corrective actions to remedy the deficiencies must be performed and are usually verified in a sub-sequent inspection. In addition, manufacturers of both pharmaceutical products and active pharmaceutical ingredients (APIs) used to formulate the product also ordinarily undergo a pre-approval inspection, although the inspection can be waived when the manufacturer has had a passing cGMP inspection in the immediate past. Failure of any facility to pass a pre-approval inspection will result in delayed approval and would have a material adverse effect on the Company’s business, results of operations, financial condition and cash flows.
The FDA also conducts periodic inspections of facilities to assess their cGMP status. If the FDA were to find serious cGMP non-compliance during such an inspection, it could take regulatory actions that could adversely affect the Company’s business, results of operations, financial condition and cash flows. Imported API and other components needed to manufacture products could be rejected by U.S. Customs, usually after conferring with the FDA. In respect to domestic establishments, the FDA could initiate product seizures or request product recalls and seek to enjoin a product’s manufacture and distribution. In certain circumstances, violations could support civil penalties and criminal prosecutions. In addition, if the FDA concludes that a company is not in compliance with cGMP requirements, sanctions may be imposed that include classifying that company as an “unacceptable supplier”, thereby disqualifying that company from selling products to federal agencies.
Marketing
Companies that market pharmaceutical products in the U.S. are subject to various federal and state laws pertaining to healthcare fraud and abuse, including prohibitions on the offer of payment or acceptance of kickbacks or other remuneration for the purchase of products, such as inducements to potential patients to request the company’s products. Specifically, the federal Anti-Kickback Statute prohibits persons or entities from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid pro-grams. Due to legislative changes, violations of the Anti-Kickback Statute also carry potential federal False Claims Act liability. Because of the sweeping language of the federal Anti-Kickback Statute, many potentially beneficial business arrangements would be prohibited if the statute were strictly applied. To avoid this outcome, the U.S. Department of Health and Human Services’ Office of Inspector General has published regulations—known as “safe harbors”—that identify exceptions or exemptions to the statute’s prohibitions. Arrangements that do not fit within the safe harbors are not automatically deemed to be illegal but must be evaluated on a case-by-case basis for compliance with the statute. Additionally, many states have adopted laws similar to the federal Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any third party payer, not only the Medicare and Medicaid programs, and do not contain identical safe harbors.
65
Table of Contents
The Company is unaware of any violations of these laws. However, due to the breadth of the statutory provisions and the absence of uniform guidance in the form of regulations or court decisions, there can be no assurance that its practices will not be challenged under anti-kickback or similar laws. Violations of such restrictions may be punishable by civil and/or criminal sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from participation in U.S. federal and state healthcare programs (including Medicaid and Medicare). Any liability from such a violation could have a material adverse effect on our business, financial condition, results of operations and cash flows.
In addition, the FDA has the authority to regulate the claims made by a manufacturer in marketing its products to ensure that such claims are true, not misleading, supported by scientific evidence and consistent with the products approved or cleared labeling. Failure to comply with FDA requirements in this regard could result in, among other things, suspensions or withdrawal of approvals, product seizures, injunctions against the manufacture, holding, distribution, marketing and sale of a product, civil and criminal sanctions.
Also, the federal False Claims Act prohibits persons from knowingly filing, or causing to be filed, a false claim to, knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay or transmit money or property to, or the knowing use of false statements to obtain payment from, the government. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. Various states have also enacted laws modeled after the federal False Claims Act. Federal and state authorities and private whistleblower plaintiffs have brought actions against pharmaceutical product manufacturers alleging that the manufacturers’ activities constituted causing healthcare providers to submit false claims, alleging that the manufacturers themselves made false or misleading statements to the federal government, or alleging that the manufacturers improperly promoted their products for “off-label” uses not approved by the FDA, or offered inducements to referral sources that are prohibited by the federal Anti-Kickback Statute. To the extent the Company becomes the subject of any such investigations or litigation, it could be time-consuming and costly to the Company and could have a material adverse effect on its business. In addition, if its activities are found to violate federal or state False Claims Act statutes, it could have a material adverse effect on its business, financial conditions, results of operations and cash flows.
Product Liability
There are potential liability risks that arise from the testing, manufacturing, marketing and sale of pharmaceutical products. In addition to direct expenditures for damages, settlement and defense costs, there is a possibility of adverse publicity as a result of product liability claims. Some plaintiffs have received substantial damage awards in some jurisdictions against pharmaceutical companies based upon claims for injuries allegedly caused by the use of their products. In addition, it may be necessary for the Company to voluntarily or mandatorily recall or withdraw products that do not meet approved specifications, or which subsequent data demonstrate may be unsafe or ineffective, which would also result in adverse publicity as well as in costs connected to the recall and loss of revenue.
Health Information Privacy and Security
There are potential liability risks that arise from the testing, manufacturing, marketing and sale of pharmaceutical products. In addition to direct expenditures for damages, settlement and defense costs, there is a possibility of adverse publicity as a result of product liability claims. Some plaintiffs have received substantial damage awards in some jurisdictions against pharmaceutical companies based upon claims for injuries allegedly caused by the use of their products. In addition, it may be necessary for the Company to voluntarily or mandatorily recall or withdraw products that do not meet approved specifications or which subsequent data demonstrate may be unsafe or ineffective, which would also result in adverse publicity as well as in costs connected to the recall and loss of revenue.
Legislative and regulatory initiatives at the state and federal levels address concerns about the privacy and security of health information. HITECH expands the health information privacy and security protections under HIPAA and imposes new obligations to notify individuals and the U.S. Department of Health and Human Services Office for Civil Rights (OCR), of breaches of certain unsecured health information. Compliance with these laws and regulations may require the Company to spend substantial sums, including, but not limited to, purchasing new information technology, which could negatively impact financial results. Additionally, if the Company fails to comply with the HIPAA privacy, security and breach notification standards, it could suffer civil penalties of up to USD 1,500,000 per calendar year for violations of an identical standard and criminal penalties of up to USD 250,000 and 10 years in prison for offenses committed with the intent to sell, transfer, or use individually identifiable health information for commercial advantage, personal gain or malicious harm. In addition, healthcare providers will continue to remain subject to any state laws that are more restrictive than the federal privacy regulations. These privacy laws vary by state and could impose additional penalties.
66
Table of Contents
The provisions of HIPAA criminalize situations that previously were handled exclusively civilly through repayments of overpayments, offsets and fines by creating new federal healthcare fraud crimes. Further, as with the federal laws, general state criminal laws may be used to prosecute healthcare fraud and abuse. A violation could subject the Company to penalties, fines and/or possible exclusion from Medicare or Medicaid. Such sanctions could significantly reduce its financial results. Future healthcare legislation and regulation or other changes in the administration of or interpretation of existing legislation or regulations regarding governmental healthcare pro-grams could have an adverse effect on the Company’s business the results of its operations.
Regulation in the European Union
Product development, the regulatory approval process, and safety monitoring of medicinal products and their manufacturers in the EU proceed in much the same manner as they do in the U.S.. Therefore, many of the issues discussed above apply similarly in the context of the EU. In addition, drugs are subject to the extensive price and reimbursement regulations of the various EU Member States.
In the EEA, which is comprised of the 27 Member States of the EU plus Norway, Iceland and Liechtenstein, medicinal products can only be commercialized after obtaining a marketing authorization. There are two types of marketing authorization: the Community Marketing Authorization, which is issued by the EC through the Centralized Procedure based on the opinion of the Committee for Medicinal Products for Human Use (CHMP), a body of the EMA, and which is valid throughout the entire territory of the EEA; and the National Marketing Authorization, which is issued by the competent authorities of the Member States of the EEA and authorizes marketing only in that Member State’s national territory and not the EEA as a whole.
The Centralized Procedure is compulsory for human medicines for the treatment of human immunodeficiency virus or acquired immune deficiency syndrome (AIDS), cancer, diabetes, neurodegenerative diseases, autoimmune and other immune dysfunctions, and viral diseases; for veterinary medicines for use as growth or yield enhancers; for medicines derived from biotechnology processes, such as genetic engineering; for advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines; and for officially designated ‘orphan medicines’ (medicines used for rare human diseases). The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation, or for products that are in the interest of public health in the EU. The National Marketing Authorization is for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National Marketing Authorization can be recognized in another Member State through the Mutual Recognition Procedure. If the product has not received a National Marketing Authorization in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure, an identical dossier is submitted to the competent authorities of each of the Member States in which the marketing authorization is sought, one of which is selected by the applicant as the Reference Member State (RMS). If the RMS proposes to authorize the product, and the other Member States do not raise objections, the product is granted a National Marketing Authorization in all the Member States in which the authorization was sought. Before granting the marketing authorization, the EMA or the competent authorities of the Member States of the EEA assesses the risk–benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
Clinical Studies
As is the case in the U.S., the various phases of preclinical and clinical research in the EU are subject to significant regulatory controls. The EU Clinical Trial Regulation (EU) No. 536/2014 (Clinical Trials Relation) on clinical trials and medicinal products for human use, repealed Directive 2001/20/EC. The Clinical Trials Regulation entered into application on January 31, 2022 and is intended to simplify the current rules for clinical trial authorization and standards of performance. For instance, there will be a streamlined application procedure via a single entry point, a EU portal and database. The new clinical trial portal and database will be maintained by the EMA in collaboration with the European Commission and the EU Member States. The objectives of the Clinical Trials Regulation include consistent rules for conducting trials throughout the EU, consistent data standards and adverse events listing, and consistent information on authorization status. Additionally, information on the conduct and results of each clinical trial carried out in the EU will be made publicly available.
67
Table of Contents
Marketing Approval
Marketing approvals under the EU regulatory system may be obtained through a centralized or decentralized procedure. The centralized procedure results in the grant of a single marketing authorization, which is valid for all (currently 27) EU Member States and the three European Free Trade Association (EFTA) members (Norway, Iceland and Liechtenstein).
Pursuant to Regulation (EC) No. 726/2004, as amended, the centralized procedure is mandatory for drugs developed by means of specified biotechnological processes, advanced-therapy medicinal products, drugs for human use containing a new active substance for which the therapeutic indication is the treatment of specified diseases, including but not limited to AIDS, neurodegenerative disorders, auto-immune diseases and other immune dysfunctions, as well as drugs designated as orphan drugs. The CHMP also has the discretion to permit other products to use the centralized procedure if it considers them sufficiently innovative or they contain a new active substance.
In the marketing authorization application, the applicant has to properly and sufficiently demonstrate the quality, safety and efficacy of the drug. Under the centralized approval procedure, the CHMP, possibly in conjunction with other committees, is responsible for drawing up the opinion of the EMA on any matter concerning the admissibility of the files submitted in accordance with the centralized procedure, such as an opinion on the granting, variation, suspension or revocation of a marketing authorization, and pharmacovigilance.
The CHMP and other committees are also responsible for providing guidelines and have published numerous guidelines that may apply to our product candidates. These guidelines provide additional guidance on the factors that the EMA will consider in relation to the development and evaluation of drug products and may include, among other things, the preclinical studies required in specific cases, the manufacturing and control information that should be submitted in a marketing authorization application, and the post-approval measures required to monitor patients and evaluate the long-term efficacy and potential adverse reactions. Although these guidelines are not legally binding, we believe that our compliance with them is likely to be necessary to gain approval for any of our product candidates.
The maximum timeframe for the evaluation of a marketing authorization application by the CHMP under the centralized procedure is 210 days after receipt of a valid application. This period will be suspended until such time as the supplementary information requested by the CHMP has been provided by the applicant. Likewise, this time limit will be suspended for the time allowed for the applicant to prepare oral or written explanations. When an application is submitted for a marketing authorization in respect of a drug that is of major interest from the viewpoint of public health and in particular therapeutic innovation, the applicant may request an accelerated assessment procedure. If the CHMP accepts such a request, the time limit of 210 days will be reduced to 150 days, but it is possible that the CHMP can revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment.
If the CHMP concludes that the quality, safety and efficacy of the product are sufficiently proven, it adopts a positive opinion. This is sent to the EC, which drafts a decision within approximately 67 days following the CHMP opinion. After consulting with the Member States, the EC adopts a decision and grants a marketing authorization, which is valid for the whole of the EEA. The marketing authorization may be subject to certain conditions, which may include, without limitation, the performance of post-authorization safety and/or efficacy studies.
The EMA has various programs, including accelerated assessment, conditional approval and Priority Medicines (PRIME), which are intended to increase agency interactions, expedite or facilitate the process for reviewing drug candidates, and/or provide for initial approval on the basis of surrogate endpoints. One or more of our product candidates may qualify for some of these expedited development and review programs. However, even if a drug candidate qualifies for one or more of these programs, the EMA may later decide that the drug candidate no longer meets the conditions for qualification. Eligibility to the PRIME scheme is limited to products considered to offer a major therapeutic advantage in populations with high unmet need. PRIME is a voluntary scheme aimed at enhancing interaction and early dialogue with developers of promising medicines through achieving the early appointment of the Rapporteur for the product, optimizing development plans and speeding up evaluation so these medicines can reach patients earlier. Products benefiting from PRIME can expect to be eligible for accelerated assessment at the time of application for a marketing authorization application.
68
Table of Contents
EU legislation also provides for a system of regulatory data and market exclusivity. According to Article 14(11) of Regulation (EC) No. 726/2004, as amended, and Article 10(1) of Directive 2001/83/EC, as amended, upon receiving marketing authorization, new chemical entities approved on the basis of a complete independent data package benefit from 8 years of data exclusivity and an additional 2 years of market exclusivity. Data exclusivity prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic (abbreviated) application. During the additional 2-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic medicinal product can be marketed until the expiration of the market exclusivity. The overall 10-year period will be extended to a maximum of 11 years if, during the first 8 years of those 10 years, the marketing authorization holder (MAH) obtains an authorization for one or more new therapeutic indications that, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the innovator can gain the period of data exclusivity, another company nevertheless could also market another version of the drug if such company obtained marketing authorization based on a marketing authorization application with a completely independent data package of pharmaceutical test, preclinical tests and clinical studies. However, products designated as orphan medicinal products enjoy, upon receiving marketing authorization, a period of 10 years of orphan market exclusivity. See also “Orphan drug regulation” below. Depending upon the timing and duration of the EU marketing authorization process, products may be eligible for an SPC of up to five years, pursuant to Regulation (EC) No. 469/2009. Such SPCs extend the rights under the basic patent for the drug.
In the EU, the pediatric regulation (Regulation (EC) No 1901/2006, as amended) requires sponsors to submit a pediatric investigation plan at the end of Phase 1. This plan will provide the details of the quality, non-clinical and clinical studies required to support the authorization of a pediatric indication. Additional rules apply to medicinal products for pediatric use under Regulation (EC) No. 1901/2006. Potential incentives include a six-month extension of any supplementary protection certificate granted pursuant to Regulation (EC) No. 469/2009, but not in cases in which the relevant product is designated as an orphan medicinal product pursuant to Regulation (EC) No. 141/2000, as amended. Instead, a medicinal product designated as an orphan medicinal product may enjoy an extension of the 10-year market exclusivity period granted under Regulation (EC) No. 141/2000 to 12 years subject to the conditions applicable to orphan drugs.
Orphan Drug Regulation
In the EU, Regulation (EC) No. 141/2000, as amended, states that a drug will be designated as an orphan drug if its sponsor can establish:
| • | that it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than 5 in 10,000 persons in the EU when the application is made, or that it is intended for the diagnosis, prevention or treatment of a life- threatening, seriously debilitating or serious and chronic condition in the EU and that without incentives it is unlikely that the marketing of the drug in the EU would generate sufficient return to justify the necessary investment; and |
| • | that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the EU or, if such method exists, that the drug will be of significant benefit to those affected by that condition. |
Regulation (EC) No. 847/2000 sets out further provisions for implementation of the criteria for designation of a drug as an orphan drug. An application for the designation of a drug as an orphan drug must be submitted at any stage of development of the drug before filing of a marketing authorization application.
If a EU-wide community marketing authorization in respect of an orphan drug is granted or if all the EU Member States have granted marketing authorizations in accordance with the procedures for mutual recognition, the EU and the Member States will not, for a period of 10 years, accept another application for a marketing authorization, or grant a marketing authorization or accept an application to extend an existing marketing authorization, for the same therapeutic indication, in respect of a similar drug. This period may, however, be reduced to 6 years if, at the end of the fifth year, it is established, with respect to the drug concerned, that the criteria for orphan-drug designation are no longer met; in other words, when it is shown on the basis of available evidence that the product is sufficiently profitable not to justify maintenance of market exclusivity. Notwithstanding the foregoing, a marketing authorization may be granted, for the same therapeutic indication, to a similar drug if:
| • | the holder of the marketing authorization for the original orphan drug has given its consent to the second applicant; |
| • | the holder of the marketing authorization for the original orphan drug is unable to supply sufficient quantities of the drug; or |
69
Table of Contents
| • | the second applicant can establish in the application that the second drug, although similar to the orphan drug already authorized, is safer, more effective or otherwise clinically superior. |
Other incentives available to orphan drugs in the EU include financial incentives such as a reduction of fees or fee waivers and protocol assistance. ODD does not shorten the duration of the regulatory review and approval process.
Manufacturing and Manufacturers’ License
Pursuant to Directive 2003/94/EC, as transposed into the national laws of the Member States, the manufacturing of investigational medicinal products and approved drugs is subject to a separate manufacturer’s license and must be conducted in strict compliance with cGMP requirements, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. Manufacturers must have at least one qualified person permanently and continuously at their disposal. The qualified person is ultimately responsible for certifying that each batch of finished product released onto the market has been manufactured in accordance with cGMP and the specifications set out in the marketing authorization or investigational medicinal product dossier. cGMP requirements are enforced through mandatory registration of facilities and inspections of those facilities. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenues, and subject the applicant to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action, or possible civil and criminal penalties.
Wholesale Distribution and License
Pursuant to Directive 2001/83/EC, the wholesale distribution of medicinal products is subject to the possession of an authorization to engage in activity as a wholesaler in medicinal products. Possession of a manufacturing authorization includes authorization to distribute by wholesale the medicinal products covered by that authorization. The distribution of medicinal products must comply with the principles and guidelines of cGDP.
Advertising
In the EU, the promotion of prescription medicines is subject to intense regulation and control, including EU and national legislation as well as self- regulatory codes (industry codes). Advertising legislation inter alia includes a prohibition on direct-to-consumer advertising. All advertising of prescription medicines must be consistent with the product’s approved Summary of Product Characteristics, and must be factual, accurate, balanced and not misleading. Advertising of prescription medicines pre-approval or off-label is not allowed. Some jurisdictions require that all promotional materials for prescription medicines be subjected to prior review and approval, either internal or regulatory.
Other Regulatory Requirements
A Marketing Authorization Holder (MAH) for a medicinal product is legally obliged to fulfill a number of obligations by virtue of its status as an MAH. The MAH can delegate the performance of related tasks to third parties, such as distributors or marketing partners, provided that this delegation is appropriately documented and the MAH maintains legal responsibility and liability.
An MAH for a medicinal product is legally obliged to fulfill a number of obligations by virtue of its status as an MAH. The MAH can delegate the performance of related tasks to third parties, such as distributors or marketing partners, provided that this delegation is appropriately documented and the MAH maintains legal responsibility and liability.
The obligations of an MAH include the following:
| • | Manufacturing and batch release. MAHs should guarantee that all manufacturing operations comply with relevant laws and regulations, applicable GMPs, and the product specifications and manufacturing conditions set out in the marketing authorization, and that each batch of product is subject to appropriate release formalities. |
| • | Availability and continuous supply. Pursuant to Directive 2001/83/EC, as transposed into the national laws of the Member States, the MAH for a medicinal product and the distributors of the said medicinal product actually placed on the market in a Member State shall, within the limits of their responsibilities, ensure appropriate and continued supplies of that medical product to pharmacies and persons authorized to supply medicinal products so that the needs of patients in the Member State in question are covered. |
70
Table of Contents
| • | Advertising and promotion. MAHs remain responsible for all advertising and promotion of their products, including promotional activities by other companies or individuals on their behalf, and in some cases must conduct internal or regulatory pre-approval of promotional materials. Regulation in this area also covers interactions with healthcare practitioners and/or patient groups, and in some jurisdictions legal or self-regulatory obligations to disclose such interactions exist. |
| • | Medical affairs/scientific service. MAHs are required to disseminate scientific and medical information on their medicinal products to healthcare professionals, regulators and patients. |
| • | Legal representation and distributor issues. MAHs are responsible for regulatory actions or inactions of their distributors and agents. |
| • | Preparation, filing and maintenance of the application and subsequent marketing authorization. MAHs must maintain appropriate records, comply with the marketing authorization’s terms and conditions, fulfill reporting obligations to regulators, submit renewal applications and pay all appropriate fees to the authorities. We may hold any future marketing authorizations granted for our product candidates in our own name or appoint an affiliate or a collaboration partner to hold marketing authorizations on our behalf. Any failure by an MAH to comply with these obligations may result in regulatory action against an MAH and ultimately threaten our ability to commercialize our products. |
International Regulation
In addition to regulations in the U.S. and Europe, a variety of foreign regulations govern clinical trials, commercial sales and distribution of product candidates. The approval process varies from country to country and the time to approval may be longer or shorter than that required for FDA or EMA approval.
Pharmaceutical Coverage, Pricing and Reimbursement
In both domestic and foreign markets, our or our collaboration partners’ sales of any approved products will depend in part on the availability of coverage and adequate reimbursement from third-party payors. Third-party payors include government authorities, managed care providers, private health insurers and other organizations. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products, if approved, unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Sales of our products will therefore depend substantially, both domestically and abroad, on the extent to which the costs of our products will be paid by third-party payors. These third-party payors are increasingly focused on containing healthcare costs by challenging the price and examining the cost-effectiveness of medical products and services.
In addition, significant uncertainty exists as to the coverage and reimbursement status of newly approved healthcare product candidates. The market for our product candidates for which we may receive regulatory approval will depend significantly on access to third-party payors’ drug formularies or lists of medications for which third-party payors provide coverage and reimbursement. The industry competition to be included in such formularies often lead to downward pricing pressures on pharmaceutical or biopharmaceutical companies. Additionally, third-party payors may refuse to include a particular branded drug in their formularies or otherwise restrict patient access to a branded drug when a less costly generic equivalent or another alternative is available. Because each third-party payor individually approves coverage and reimbursement levels, obtaining coverage and adequate reimbursement is a time-consuming, costly and sometimes unpredictable process. We may be required to provide scientific and clinical support for the use of any product to each third-party payor separately with no assurance that approval would be obtained, and we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. This process could delay the market acceptance of any product and could have a negative effect on our future revenues and operating results. We cannot be certain that our product candidates will be considered cost-effective. Because coverage and reimbursement determinations are made on a payor-by-payor basis, obtaining acceptable coverage and reimbursement from one payor does not guarantee we will obtain similar acceptable coverage or reimbursement from another payor. If we are unable to obtain coverage of, and adequate reimbursement and payment levels for, our product candidates from third-party payors, physicians may limit how much or under what circumstances they will prescribe or administer them and patients may decline to purchase them. This in turn could affect our ability to successfully commercialize our products and impact our profitability, results of operations, financial condition and future success.
71
Table of Contents
In the EU, the pricing and reimbursement mechanisms by private and public health insurers vary largely by country and even within countries. The public systems reimbursement for standard drugs is determined by guidelines established by the legislator or responsible national authority. The approach taken varies by Member State. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other Member States allow companies to fix their own prices for medicines but monitor and control company profits and may limit or restrict reimbursement. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers to the entry of new products are being erected and some EU countries require the completion of studies that compare the cost-effectiveness of a particular product candidate with that of currently available therapies in order to obtain reimbursement or pricing approval. Special pricing and reimbursement rules may apply to orphan drugs. Inclusion of orphan drugs in reimbursement systems tend to focus on the medical usefulness, need, quality and economic benefits to patients and the healthcare system as for any drug. Acceptance of any medicinal product for reimbursement may come with cost, use and often volume restrictions, which again can vary by country. In addition, results based rules of reimbursement may apply.
Environmental, Health, and Safety Laws and Regulations
We are subject to numerous environmental, health and safety laws and regulations and permitting requirements, including those governing laboratory procedures, decontamination activities, and the handling, transportation, use, remediation, storage, treatment, and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, and the risk of injury, contamination or noncompliance with environmental, health and safety requirements cannot be eliminated. Although compliance with such laws and regulations and permitting requirements has not had a material effect on our capital expenditures, earnings or competitive position, environmental, health and safety laws, and regulations and permitting requirements have tended to become increasingly stringent and, to the extent that legal or regulatory changes may occur in the future, they could result in, among other things, increased costs to us or the impairment of our research, development or production efforts.
| C. | ORGANIZATIONAL STRUCTURE |
We are a Swiss stock corporation (société anonyme). We were originally formed in 2013, with our registered office and domicile in Geneva, Switzerland. Our Swiss enterprise identification number is CHE-113.516.874. We are located in the Canton of Geneva, City of Geneva, at Avenue de Sécheron 15, 1202 Genève, Switzerland.
As of the date of this annual report we have the following direct subsidiaries:
| Name |
Domicile |
Percent Owned | ||
| Relief Therapeutics International SA |
Switzerland | 100 | ||
| Relief Therapeutics US, Inc. |
Connecticut (U.S.) | 100 | ||
| Relief Therapeutics, Inc. |
Delaware (U.S.) | 100 | ||
| APR Applied Pharma Research SA |
Switzerland | 100 | ||
| APR Applied Pharma Research Holding SA |
Switzerland | 100 | ||
| APR Applied Pharma Research – Italy s.r.l. |
Italy | 100 | ||
| APR Applied Pharma Research Deutschland GmbH |
Germany | 100 | ||
| AdVita Lifescience GmbH |
Germany | 100 | ||
| AdVita Lifescience AG |
Switzerland | 100 | ||
| AdVita Lifescience, Inc. |
New York (U.S.) | 100 |
| D. | PROPERTY, PLANT AND EQUIPMENT |
The Group leases approximately 1,800 square feet of office, lab space and representative offices located in Geneva and Balerna (Switzerland), Freiburg im Breisgau and Offenbach am Main (Germany), and Rome (Italy). Our headquarters are at Avenue de Sécheron 15, Geneva, Switzerland. We are not aware of any environmental issues or other constraints that would materially impact the intended use of our facilities. While we may require additional space and facilities our business expands, we believe that our current facilities are suitable and adequate to meet our current needs.
72
Table of Contents
| ITEM 4A | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 5 | OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
We are a company developing drugs via participation in active entities that have obtained intellectual properties through their own research activities, or via in-licensing, or via internal research and development activities. Historically, our development has focused primarily on clinical-stage projects based on molecules of natural origin (peptides and proteins) with a history of clinical testing and use in human patients or a strong scientific rationale.
The following discussion contains references to the financial statements of Relief Therapeutics Holding SA and its consolidated subsidiaries (also referred to as the Company). These financial statements consolidate the Company’s subsidiaries and include the Company’s interest in investments held at fair value. Subsidiaries are those entities over which the Company retains control. Where we have neither control nor significant influence for financial accounting purposes, we recognize our holding in such entity at fair value. For additional information regarding the accounting treatment of these entities, see Note 1 of our consolidated financial statements included in this annual report. For additional information regarding our operating structure, see “Basis of Presentation and Consolidation” below.
| A. | OPERATING RESULTS |
Overview
We are a Swiss, commercial-stage biopharmaceutical company committed to delivering innovative treatment options with the potential for transformative outcomes to benefit those suffering from rare debilitating conditions that have no or limited treatment options. Our cost-effective, capital-efficient approach to drug development and commercialization is focused on rare metabolic disorders, rare skin diseases, rare respiratory diseases and rare monogenetic diseases.
Our portfolio offers a balanced mix of marketed, revenue-generating products, our proprietary, globally patented drug delivery platform technologies that have utility for development in other specialty or rare disease therapeutic areas and a highly targeted clinical development pipeline consisting of risk-mitigated assets that have been engineered for improvements in efficacy, safety or convenience to benefit the lives of patients. In addition, the Company is commercializing several legacy products via licensing and distribution partners.
We are actively pursuing a strategy to diversify our portfolio through the ongoing evaluation of potential in-licensing opportunities. To bring treatments to patients as quickly as possible, we are seeking partnerships with, or acquisitions of, companies that have late-stage clinical molecules with a strong human safety profile, allowing for relatively short, capital-efficient clinical trials with clear endpoints. We are also evaluating prospective opportunities that fit within our genetic medicine initiative for devastating, as-yet-unaddressed, rare monogenetic diseases.
We are led by a proven and seasoned management team of business leaders with significant experience in discovering, developing and commercializing important new medicines, delivering them to market and maximizing shareholder value. Collectively, the members of our management team have overseen research and development of products supporting regulatory approvals as well as commercial launches of marketed products.
In March 2021, we signed a Collaboration and License Agreement with Acer Therapeutics, Inc. (Acer) for the worldwide development and commercialization of ACER-001 (now OLPRUVA™) for the treatment of urea cycle disorders (UCDs) and maple syrup urine disease (MSUD). OLPRUVA™ is a proprietary powder formulation of sodium phenylbutyrate (NaPB) designed to be both taste-masked and immediate release. In August 2021, Acer submitted an NDA for ACER-001 to the FDA for use as a treatment of UCD, which submission was accepted for filing in November 2021 with a PDUFA decision date of June 5, 2022. However, on or about the PDUFA decision date, Acer received a complete response letter stating that a satisfactory inspection of Acer’s third-party contract manufacturer would be required before its NDA for ACER-001 could be approved. On December 27, 2022, Relief and Acer announced that, after resubmission, the FDA had approved ACER-001, under the trade name OLPRUVA™, for the treatment of UCDs. In addition, Acer has filed an IND to investigate OLPRUVA™ for the treatment of MSUD.
In June 2021, we signed and closed a definitive agreement to acquire all outstanding shares of APR Applied Pharma Research SA (APR), a privately held Swiss pharmaceutical company with over 25 years’ experience in identifying, developing and globally commercializing known molecules engineered with drug delivery systems in niche and rare diseases.
73
Table of Contents
In July 2021, Relief acquired AdVita Lifescience GmbH (AdVita), a Germany-based privately held pharmaceutical company developing products for the treatment and diagnosis of rare lung diseases. AdVita’s capabilities helped the Company to further progress the development of RLF-100® for a range of lung diseases.
In July 2022, we executed a definitive agreement with Meta Healthcare Ltd. (Meta), acquiring the worldwide rights, except for the UK, for a novel dosage form of a prescription drug already approved by the FDA and intended for the treatment of patients with PKU. This improved product is expected to increase patient acceptance and compliance as well as enable easier, self or caregiver administered metered dosing and dispensing. According to the terms of the agreement, Meta shall transfer to Relief all data, know-how, as well as any intellectual property as developed or generated so far by Meta. Relief shall only be responsible for funding the remaining development work as well as for filing and prosecuting an NDA in all countries worldwide except for the UK where Relief Therapeutics shall grant a license back to Meta, enabling Meta to directly promote and commercialize the product in such country. Other than the initial acquisition payment and low double-digit royalty payments on net profit of the product in the various countries, Relief shall be under no obligation to fund or pay any other amount to Meta.
Previously, Relief partnered with NeuroRx, Inc. (NeuroRx) to seek to develop RLF-100® as a treatment of COVID-19. In September 2020, Relief entered into a binding collaboration agreement with NeuroRx (the Collaboration Agreement). The Collaboration Agreement established the terms under which we and NeuroRx collaborate and assist each other to maximize the revenues in our respective territories from the sale of aviptadil for intravenous and inhale use primarily for the treatment of COVID-19 related conditions.
Based on numerous breaches of the Collaboration Agreement by NeuroRx, in October 2021, we filed a lawsuit against NeuroRx and its then CEO, Dr. Jonathan Javitt, for multiple breaches of the Collaboration Agreement. The complaint was filed in the Supreme Court of the State of New York in Manhattan. On January 10, 2022, NeuroRx filed a complaint against Relief alleging that we were in breach of the Collaboration Agreement and have thus repudiated and cancelled the Collaboration Agreement. Additionally, NeuroRx’s claims included a count for defamation.
On November 14, 2022, Relief and NRx issued a press release announcing that the parties had entered into a definitive settlement agreement to resolve all matters relating to the pending litigation. At the closing of this settlement, held on December 19, 2022, (i) NRx transferred to Relief all of the assets that it previously used in its aviptadil development program, including its regulatory filings, patent applications, clinical data, and the formulation of the aviptadil product it was previously developing, (ii) Relief has the exclusive right and control going forward to develop and commercialize an aviptadil product, (iii) Relief has agreed to use commercially reasonable efforts to continue the existing Right to Try Program for aviptadil in the U.S. for at least two years, (iv) Relief will pay NRx milestone payments if it can successfully obtain commercial approval of an aviptadil product (whether for COVID-19 or any other indication), (v) Relief will pay NRx royalties based on a percentage of future sales of an aviptadil product (whether for COVID-19 or any other indication), up to a maximum of $30 million in the aggregate, (vi) NRx Pharmaceuticals has agreed not to compete in the development of an aviptadil product in the future, and (vii) at the closing, Relief and NRx Pharmaceuticals have dismissed their pending litigation. Further, as part of the settlement, the parties cancelled the Collaboration Agreement and exchanged mutual releases with respect to matters that were the subject of the litigation.
Material Agreements
We are party to certain agreements with third parties relating to licensing, collaboration, or other matters that are material to our business and performance.
NeuroRx
In September 2020, we entered into a collaboration agreement with NeuroRx for the global commercialization of RLF-100® (aviptadil acetate) and the selection of commercial partners. This partnership was designed to rapidly advance RLF-100® through clinical development so that it reaches COVID-19 patients worldwide as soon as possible. Under the agreement, NeuroRx was to lead commercialization in the U.S., Canada, and Israel, while we were to lead commercialization in Europe and the rest of the world.
On December 19, 2022, we finalized a settlement with NeuroRx by which the collaboration agreement was cancelled. For additional information, “Item 4. Information on the Company – B. Business Overview.”
74
Table of Contents
Acer Therapeutics
On January 25, 2021, we entered into an option agreement with Acer Therapeutics Inc providing exclusivity for the right to negotiate a potential collaboration and license agreement (CLA) for worldwide development and commercialization for ACER-001 (now OLPRUVA™).
Under the terms of the option agreement, we paid Acer a $1 million USD non-refundable payment in return for exclusivity until June 30, 2021 to negotiate and enter into a definitive collaboration and license agreement for the development of ACER-001. Further, in connection with entering into the option agreement, we made a $4 million USD secured loan to Acer.
On March 22, 2021, both companies announced the execution of the CLA. Acer since received a $10 million USD cash payment (originally $14 million USD, offset by repayment of the $4 million USD outstanding balance of the prior loan, plus interest, to Acer). We also committed to pay Acer up to $20 million USD ($15 million which has been paid to-date) in U.S. development and commercial launch costs of the molecule for the treatment of the UCD and MSUD indications. Acer will retain development and commercialization rights in the U.S., Canada, Brazil, Turkey and Japan. The companies will split net profits from Acer’s territories 60 percent to 40 percent in our favor. In addition, we have licensed the rights for the rest of the world, where Acer will receive from us a 15 percent royalty on all revenues received in our territories. Acer may also receive a total of $6 million USD in development milestone payments following the first EU marketing approvals for UCDs and MSUD.
GEM Global Yield LLC SCS
On January 20, 2021, we signed a binding agreement with our largest shareholder, GEM Global Yield LLC SCS (GEM) for the implementation of a new share subscription facility (SSF) in the amount of up to CHF 50 million.
Under the terms of the SSF, we have the right to periodically, during a timeframe of up to three years, issue and sell shares to GEM. Under the facility, GEM undertakes to subscribe to or acquire our ordinary registered shares upon our exercise of a drawdown notice. In accordance with the customary terms of the SSF agreement, we control the timing and maximum amount of any drawdown and retains the right, not the obligation, to draw down on the full commitment amount. Future subscription prices under the SSF will correspond to 90 percent of the average of the closing bid prices on the SIX Swiss Exchange during the reference period, which corresponds to fifteen trading days following Relief’s drawdown notice.
Under the terms of the SSF, we are required to pay GEM a commitment fee of CHF 1.25 million, which is currently outstanding as an interest bearing loan pursuant to the SSF. As of the date of this annual report, no amounts have been drawn on this facility.
APR Applied Pharma Research SA
On April 30, 2021, we entered into a binding term sheet with the then current shareholders of APR Applied Pharma Research SA, a privately held Swiss company with over 25 years of experience in identifying developing and commercializing known molecules engineered with drug delivery systems in niche and rare diseases, to acquire all of the outstanding shares of APR. On June 28, 2021, the former shareholders of APR and Relief signed and closed a definitive agreement for Relief to acquire all outstanding shares of APR. Under the terms of the agreement APR’s shareholders have received from Relief CHF 21.5 million in cash and 206,786,784 Consideration Shares at a value of CHF 45 million when the Consideration Shares were issued and listed. The APR shareholders were also eligible to receive possible future contingent milestone payments in the aggregate maximum amount of up to CHF 35 million, upon achievement of pre-agreed objectives involving (i) the execution of a definitive agreement for the commercialization of Sentinox (as such product is defined below), (ii) the launch of Sentinox in the first of France, Germany, Spain, Italy, and the UK, (iii) the launch of GOLIKE in the U.S., and (iv) the launch of RLF-TD011 (as such product is described below) in the first of France, Germany, Spain, Italy and the UK. The launch of PKU GOLIKE® in the U.S. on October 10, 2022 marked the completion of the third milestone, for which Relief issued a cash payment of CHF 2.8 million as well as 150,200,120 shares.
AdVita Lifescience GmbH
On July 28, 2021, we announced the closing of a definitive agreement to acquire all of the outstanding shares of AdVita Lifescience GmbH. Under the agreement, the stockholders of AdVita received 135,741,063 of our ordinary shares, representing EUR 25 million (approximately CHF 27.4 million) in value based on a 60-day Volume Weighted Average Price of our ordinary shares and were also eligible to receive additional contingent payments in cash. In April 2022, we made an initial milestone payment of EUR 5 million (approximately CHF 5 million) upon completion of the first milestone.
75
Table of Contents
As of the date of this annual report, the former shareholders of AdVita may receive up to EUR 10 million (approximately CHF 10 million) upon achievement of pre-agreed milestones involving (i) the approval in the U.S. or Europe of the inhaled form of aviptadil for the treatment of sarcoidosis or berylliosis, and (ii) the conduct of a phase II clinical study for the inhaled form of aviptadil in the treatment of checkpoint inhibitor-induced pneumonitis.
AdVita was founded in 2019 for the purpose of developing products and strategies to improve the therapy and diagnosis of rare lung diseases. Among AdVita’s assets are intellection property rights that may cover RLF-100® inhaled formulation specifications and the potential application of inhaled aviptadil in the treatment of acute respiratory distress syndrome, checkpoint inhibitor-induced pneumonitis and sarcoidosis.
InveniAI LLC Collaboration Agreement
On November 24, 2021, we announced that we had entered into a collaboration agreement with InveniAI LLC (InveniAI), a U.S. based company that has pioneered the application of artificial intelligence and machine learning across biopharma and other industries, in order to identify promising drug candidates to treat rare and specialty diseases (the InveniAI Collaboration Agreement).
Under the terms of the InveniAI Collaboration Agreement, InveniAI will use its proprietary platform for the identification of potential pharmaceutical product opportunities using its Pharma Big Innovation Data Lab, consisting of (i) its proprietary AlphaMeld platform, a cloud-based artificial intelligence platform that uses its proprietary machine learning and deep learning based neural networks to identify product opportunities in therapeutic areas, (ii) its cross-functional teams at its Integrated Center of Excellence, and (iii) domain expertise, to generate novel pharmaceutical opportunities and the related development pathway for the development of such concepts.
In the collaboration it is expected that InveniAI will use its platform to navigate the volume of data for all regulatory agency approved drugs and their associated active ingredients to identify potential rate and specialty disease indications for development and commercialization by us (“product concepts”) . InveniAI will seek to prioritize top product concepts, associated diseases, scientific packages and evidence to support the potential drug development opportunities by us. We anticipate that InveniAI’s platform will complement APR’s existing capabilities in research and development and in drug reformulation. Based on product leads developed by InveniAI, we hope to develop proprietary versions of existing drugs, and to protect those drugs with long-lived intellectual property and defensible product claims.
Under the terms of the InveniAI Collaboration Agreement, we paid InveniAI an initial up-front fee of $500,000. We will be required to pay success milestones for any products brought to us in connection with the InveniAI Collaboration Agreement ranging from $200,000 per product candidate for which we exercise our option to acquire IP rights to $50 million for any required product reaching $1 billion per year in net sales. We will also be required to pay royalties on any such commercialized product in certain countries a royalty of approximately three percent.
We are not currently developing any product brought to us by InveniAI, and there can be no assurance that our collaboration with InveniAI will result in the development of new product candidates or product concepts.
Basis of Preparation and Consolidation
Our consolidated financial statements have been prepared in accordance with IFRS, as issued by the IASB, and comply with Swiss law. They have been prepared under the historical cost convention, as modified by the revaluation of financial instruments at fair value, are presented in Swiss Francs (CHF), and all values are rounded to the nearest thousand (TCHF), except when otherwise indicated.
The consolidated financial statements comprise the financial statements of us and our subsidiaries as of December 31, 2022. Control is achieved when we are exposed, or have rights, to variable returns from our involvement with the investee and have the ability to affect those returns through our power over the investee.
Specifically, we control an investee if and only if we have:
| • | power over the investee (i.e., existing rights that give us the current ability to direct the relevant activities of the investee); |
| • | exposure, or rights, to variable returns from our involvement with the investee; and |
76
Table of Contents
| • | the ability to use our power over the investee to affect our returns. |
When we have less than a majority of the voting or similar rights over an investee, we consider all relevant facts and circumstances in assessing whether we have power over an investee, including:
| • | any contractual arrangement over the other vote holders of the investee; |
| • | rights arising from other contractual arrangements; and |
| • | our voting rights and potential voting rights. |
We reassess whether or not we control an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control. Consolidation of a subsidiary begins when we obtain control over the subsidiary and ceases when we lose control of the subsidiary. Assets, liabilities, income and expenses of a subsidiary acquired or disposed of during the year are included in the statement of comprehensive income from the date we gain control until the date we cease to control the subsidiary.
Profit or loss and each component of other comprehensive income are attributed to the equity holders of RELIEF THERAPEUTICS Holding SA and to the non-controlling interests, even if this results in the non-controlling interests having a deficit balance. When necessary, adjustments are made to the financial statements of subsidiaries to bring their accounting policies into line with our accounting policies. Inter-company transactions, balances and unrealized gains/losses on transactions between us and our subsidiaries are eliminated. The accounting policies of subsidiaries are consistent with the policies adopted by RELIEF THERAPEUTICS Holding SA.
Components of Our Results of Operations
Revenue and Other Gains
Revenue is primarily derived from our portfolio of marketed products and the provision of R&D services to third parties. We generate revenue from product sales, licensing fees, and royalties since the date of acquisition of APR in June 2021. Prior to the acquisition, Relief did not generate any revenue from commercial activities.
To date, our revenue has been substantially less than our operating expenses and does not significantly contribute to our cash needs. Accordingly, we rely on external funding to continue operations and fund our clinical and commercial development plan. We expect the expansion of our PKU GOLIKE® franchise as a medical food in the U.S. and other territories, as well as the commercialization of OLPRUVA™ by Acer (for which we may receive royalty repayments in the amount of 60 percent of net profits) will contribute to increases in future revenues. We do not expect to generate revenue from product candidates unless and until we complete their development and obtain regulatory approvals.
Other gains generally consist of gains on disposal of intangible assets, write-offs of liabilities and adjustments in fair value of certain assets and liabilities.
Raw Materials and Consumables Expenses
Raw materials and consumables expenses are comprised of expenditures incurred with third parties in relation to the purchase and manufacturing of drug products for sale, as well as laboratory supplies in connection with R&D services provided to customers.
External Selling and Distribution Expenses
External selling and distribution expenses are comprised of expenditures incurred with third parties in relation to advertising, marketing, sales promotion, shipping, distribution, and commission on sales, for the sale of products and R&D services.
External Research and Development Expenses
External research and development expenses include costs associated with outsourced clinical research organization activities, sponsored research studies, clinical trial costs, process development, drug candidate manufacturing expenses, license fees, and investigator-sponsored trials, including licensing fees and milestone payments charged by licensors or collaboration partners, as well as expenses related to laboratory supplies and materials.
77
Table of Contents
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using information from the clinical sites and our vendors. Costs associated with the development activity under collaboration agreements are recognized based on actual expenses reported by our collaboration partners.
Personnel Expenses
Personnel expenses consist of employee-related expenses, including salaries, benefits, share-based compensation, and other related costs.
Our benefits under our defined benefit (pension) plan are determined using the projected unit credit method, pursuant to which costs are recognized immediately in the statement of financial position with a corresponding debit or credit to retained earnings through other comprehensive income in the period in which they occur.
Past service costs are recognized in profit or loss on the earlier of: the date of the plan amendment or curtailment, or the date that the restructuring- related costs are recognized.
The following changes in the net defined benefit obligation are recognized under ‘personnel expense’ in the consolidated statement of comprehensive income:
| • | service costs comprising of current service costs, past-service costs, gains and losses on curtailments and non-routine settlements; and |
| • | net interest expense or income. |
The cost of share-based compensation is recognized over the period in which the performance and/or service conditions are fulfilled. The cumulative expense recognized for such transactions at each reporting date reflects the extent to which the performance and/or service conditions underlying the share award are earned. The financial statements in any period represent the movement in cumulative expense recognized at the beginning and the end of such period. No expense is recognized for awards that do not ultimately vest, except in certain cases.
Other Administrative Expenses
Other administrative expenses consist primarily of corporate facility costs, fees for legal and audit services, and consulting fees not otherwise included in research and development expenses.
Financial Income
Financial income consists mainly of foreign exchange net result, when positive. Foreign exchange net result is allocated to financial expense when negative.
Financial Expense
Financial expense consists mainly of interest expense associated with the discounting over time of provisions for contingent payments measured at fair value. The commitment fee that became due upon execution of our current share subscription facility agreement with GEM in January 2021 is expensed over the period of effectiveness of the instrument. In addition, we incurred negative interest charge on our Swiss franc and Euro cash deposits until year-end 2022.
Income Taxes
We are subject to corporate income taxation in Switzerland, the U.S., Italy, and Germany. We are also subject to corporate capital tax for our parent company and subsidiaries located in Switzerland. Unless and until the Group becomes profitable in certain tax jurisdictions, we expect income tax losses and gains will primarily arise from variations of deferred tax assets and liabilities.
78
Table of Contents
Relief Results of Operations
The following table, which has been derived from our audited financial statements for the years ended December 31, 2022 and 2021, together with changes from year to year.
| December 31, | Change | |||||||||||
| Statement of Operations Data |
2022 | 2021 | (2022 to 2021) | |||||||||
| Revenue |
6,081 | 3,321 | 2,760 | |||||||||
| Other gains |
9,921 | 1,171 | 8,750 | |||||||||
| Total Income |
16,002 | 4,492 | 11,510 | |||||||||
|
|
|
|
|
|
|
|||||||
| Raw materials and consumables expenses |
(1,250 | ) | (750 | ) | (500 | ) | ||||||
| External selling and distribution expenses |
(3,307 | ) | (365 | ) | (2,942 | ) | ||||||
| External research and development expenses |
(12,393 | ) | (19,024 | ) | 6,631 | |||||||
| Personnel expenses |
(12,998 | ) | (9,121 | ) | (3,877 | ) | ||||||
| Other administrative expenses |
(7,747 | ) | (6,750 | ) | (997 | ) | ||||||
| Other losses |
(63 | ) | (752 | ) | 689 | |||||||
|
|
|
|
|
|
|
|||||||
| EBITDA |
(21,756 | ) | (32,270 | ) | 10,514 | |||||||
| Reversal of impairment losses on intangible assets |
(26,424 | ) | — | (26,424 | ) | |||||||
| Amortization and depreciation expense |
(3,860 | ) | (2,036 | ) | (1,824 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(52,040 | ) | (34,306 | ) | (17,734 | ) | ||||||
| Financial income |
18 | 97 | (79 | ) | ||||||||
| Financial expenses |
(2,294 | ) | (1,316 | ) | (978 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Result before income taxes |
(54,316 | ) | (35,525 | ) | (18,791 | ) | ||||||
| Income taxes |
3,526 | 820 | 2,706 | |||||||||
|
|
|
|
|
|
|
|||||||
| Results for the period |
(50,790 | ) | (34,705 | ) | (16,085 | ) | ||||||
|
|
|
|
|
|
|
|||||||
Comparison of the Years Ended December 31, 2022 and 2021
Revenue and other gains
In 2022, we generated CHF 6.1 million in revenue from product sales, licensing fees, royalties and contract services, compared to CHF 3.3 million in 2021. Relief began generating revenue following the acquisition of APR at the end of June 2021, which generated net sales of CHF 3.2 million in the six-month period from July 1, 2021 to December 31, 2021. On an annualized basis, revenue decreased by 8.4 percent mainly due to a decrease in non-recurring license fees and in contract services revenue.
Other gains were CHF 9.9 million in 2022, compared to CHF 1.2 million in 2021. In the current period, other gains consisted mainly of a change in the fair value of provisions for contingent liabilities (CHF 8.9 million) and an impairment reversal (CHF 0.5 million) following the repayment of a loan issued to NeuroRx in 2020 and for which we had recorded a complete impairment allowance. In the comparative period, other gains were mainly related to write-offs of liabilities.
The 2022 one-off gain of CHF 8.9 million resulted from unfavorable changes in the estimated market potential and development programs for certain clinical stage assets acquired in the APR and AdVita business combinations. Under the acquisition agreements, Relief agreed to pay additional consideration upon completion of specific milestones. The fair value of the contingent consideration is recorded as a liability on our balance sheet and adjusted at the end of each reporting period based on the estimated probability of occurrence and the time factor. Any changes in fair value of the contingent liability due to assumption adjustments are recorded as ‘Other gains’ or ‘Other losses’. Refer to notes 7 and 18 of our consolidated financial statements for further information.
79
Table of Contents
Raw materials and consumables expenses, and external selling and distribution expenses
Raw materials and consumables expenses, and external selling and distribution expenses, were, respectively, CHF 1.3 million and CHF 3.3 million in 2022. We did not incur any such expenses prior to the acquisition of APR and its marketing activities. In addition, premarketing and marketing activities in relation to the launch of PKU GOLIKE® in the U.S. in October 2022 accounted for CHF 2.5 million in 2022.
External Research and Development Expenses
External research and development expenses were primarily driven by development activities for RLF-100® and expenses incurred by Acer for the development and intended commercialization of ACER-001. The decrease of CHF 6.6 million, to CHF 12.4 million in 2022 from CHF 19.0 million in 2021, was primarily due to a reduction of CHF 6.7 million in development expenses associated with RLF-100®. Expenditures associated with other in-process programs did not vary materially.
Contingent upon availability of funds, we plan to further increase our research and development expenses for the foreseeable future as we commence additional clinical trials and pursue discovery and development of new product candidates.
Personnel Expenses
Personnel expenses increased to CHF 13.0 million in 2022, compared to CHF 9.1 million in 2021, an increase of CHF 3.9 million mainly due to an increase in employee headcount resulting from the acquisitions of APR and AdVita and the establishment of our U.S. sales force. Non-cash remuneration in the form of stock options amounted to CHF 2.2 million and CHF 1.1 million in 2022 and 2021, respectively. As of December 31, 2022, Relief had 69 full-time equivalents on its payroll.
Other Administrative Expenses
Other administrative expenses increased to CHF 7.7 million in 2022, compared to CHF 6.7 million in 2021, an increase of CHF 1.0 million. The main drivers of this increase were higher expenses related to the maintenance and prosecution of intellectual property, travel of Company’s staff, and IT infrastructure and software primarily due to the addition of APR and AdVita. Legal fees remain flat as non-recurring costs incurred as part of Relief’s effort to list its securities on Nasdaq were offset by a reduction in costs incurred for other legal and regulatory matters.
Other Losses
Other losses were not material in 2022, compared to CHF 0.8 million in 2021. Other losses for the comparative period were mainly constituted by an impairment loss of CHF 0.4 million on the loan issued to NeuroRx in 2020.
Impairment Expense
We conducted impairment tests of intangible assets as of June 30 and December 31, 2022, and concluded that the carrying amount of certain assets, mainly intangible assets associated with PKU GOLIKE® and Sentinox™, exceeded their recoverable amount. As a result, we recognized a non-cash impairment charge on intangible assets of CHF 26.4 million in the current period. The impairment charge reflects a reduction of estimated future net cash flows from PKU GOLIKE® following changes in market assumptions, and, for Sentinox™, a one-year delay in the estimated launch date.
Amortization and Depreciation Expense
Amortization and depreciation expenses were CHF 3.9 million in 2022, compared to CHF 2.0 million in 2021. These expenses are mainly related to the amortization of our intangible assets. Prior to the acquisition of APR in June 2021, we did not have amortizable intangible assets nor material property, plant, and equipment assets on our balance sheet.
Financial Income
Financial income was not material in 2022, compared to CHF 0.1 million in 2021. Financial income for the comparative period was mainly constituted by a net foreign exchange gain on monetary assets and liabilities and transactions denominated in U.S. dollars and Euros. In 2022, the net foreign exchange result was a loss recorded in financial expense.
80
Table of Contents
Financial Expenses
Financial expenses increased to CHF 2.3 million in 2022, compared to CHF 1.3 million in 2021, an increase of CHF 1.0 million. The cost of unwinding of the time discount of provisions for contingent consideration for the acquisition of APR and AdVita increased to CHF 1.3 million in 2022 from CHF 0.7 million in 2021 as the period that elapsed was 12 months in 2022, compared to 6 months in 2021. Financial expenses also increased as the Company incurred a net foreign exchange loss of CHF 0.4 million in 2022, compared to a net foreign exchange gain in 2021.
Income Taxes
Income taxes were a gain of CHF 3.5 million in 2022, compared to a gain of CHF 0.8 million in 2021. The income tax gain resulted mainly from the amortization and impairment of intangible assets and a corresponding reduction in the temporary difference between the carrying amount of these assets and their tax base. In 2022, the Company also wrote off deferred tax assets for a total amount of CHF 1.5 million as the criteria for the recognition of these assets were not met as of December 31, 2022. The write-off charge was offset by a reduction in deferred tax liabilities of CHF 5.0 million.
Summary of critical accounting judgements and key sources of estimation uncertainty
Our operating and financial review and prospects were derived from our consolidated financial statements in conformity with IFRS as issued by the IASB. The preparation of the consolidated financial statements in conformity with IFRS requires management to make estimates and assumptions that affect the application of policies and reported amounts of assets, liabilities, income, expenses and related disclosures. The estimates and underlying assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making the judgments about carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. The estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are described below.
Critical accounting judgements and key sources of estimation uncertainty are described in note 4 of our 2022 consolidated financial statements included in this annual report.
| B. | LIQUIDITY AND CAPITAL RESOURCES |
Sources of Liquidity
To date, we have funded our operations primarily through private placements, at-the-market sales of treasury shares, and equity offerings and loans from our largest shareholder, GEM. We have never been profitable and have incurred operating losses in each year since inception. We have an accumulated deficit of CHF 119.6 million as of December 31, 2022 and expect to incur further losses over the foreseeable future as we develop our business. We have spent, and expect to continue to spend, a substantial amount of funds in connection with implementing our business strategy, including our planned product development and commercialization efforts.
As Relief continues to incur significant operating losses, our ability to pursue and finance our operations and our intended development plans depends on our ability to continue to raise additional financing. Our primary uses of capital are R&D expenses, personnel compensation expenses, and administrative expenses. We expect to continue to incur substantial expenses in connection with our product candidates at various stages of development and for working capital requirements. We expect to continue to raise financing through the sale of equity and debt financing. We intend to use future expected proceeds, together with cash on hand, to finance our development and commercial activities and the diversification of our pipeline, as well as to fund our outstanding liabilities and other commitments. We expect our expenses to increase in connection with our ongoing activities, particularly as we continue to advance our portfolio of product candidates, initiate further clinical trials, and seek marketing approval for our product candidates. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur additional commercialization expenses related to program sales, marketing, manufacturing, and distribution to the extent that such sales, marketing and distribution are not the responsibility of potential partners. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations.
81
Table of Contents
Going Concern
As of December 31, 2022, we had cash and cash equivalents of CHF 19.2 million. Based on current operating plans, we expect that we have sufficient resources to fund operations into the third quarter of 2023. This raises substantial doubt about our ability to continue as a going concern. Please refer to note 4.1 ‘going concern’ of our consolidated financial statements included in this annual report. Our consolidated financial statements do not include any adjustments or classifications that may result from our possible inability to continue as a going concern.
Our future capital requirements will depend on many factors, including:
| • | the scope, progress, results and costs of our ongoing and planned preclinical studies and clinical trials; |
| • | the number and development requirements of other product candidates that we may pursue; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the timing amount of milestone payments we may have to pay in relation to the acquisitions of APR and AdVita; |
| • | the extent to which we in-license or acquire other product candidates and technologies; |
| • | the costs and timing of future commercialization activities, including drug manufacturing, marketing, sales and distribution, for any of our product candidates for which we receive or have received marketing approval; |
| • | the timing of repayment of the Relief’s borrowings; and |
| • | the funding necessary to sustain our commercial operations until we attain the breakeven point. |
We will need to raise additional capital to fund continued operations beyond the third quarter of 2023. We may not be successful in our efforts to raise additional funds or achieve profitable operations. We continue to explore potential opportunities and alternatives to obtain the additional resources that will be necessary to support our ongoing operations beyond the third quarter of 2023, including raising additional capital through either private or public equity or debt financing, or additional program collaborations or non-dilutive funding, as well as using our treasury share sales program or our shares subscription facility with GEM.
If we are unable to obtain additional funding to support our current or proposed activities and operations, we may not be able to continue our operations as proposed, which may require us to suspend or terminate any ongoing development activities, modify our business plan, curtail various aspects of our operations, cease operations, or seek relief under applicable bankruptcy laws. In such event, our stockholders may lose a substantial portion or even all of their investment.
82
Table of Contents
Cash Flows
The following table summarizes our cash flows for each of the periods presented.
| December 31, | ||||||||
| In thousands |
2022 | 2021 | ||||||
| Cash flow from operating activities |
(24,126 | ) | (35,718 | ) | ||||
| Cash flow from investing activities |
(7,999 | ) | (30,262 | ) | ||||
| Cash flow from financing activities |
6,417 | 67,689 | ||||||
| Net (decrease) increase in cash and cash equivalents |
(25,708 | ) | 1,709 | |||||
| Cash and cash equivalents at end of period |
19,237 | 44,761 | ||||||
Operating Activities
Net cash used in operating activities consist of the net operating loss adjusted for changes in net working capital and for non-cash items such as impairment and depreciation, fair value adjustments, the value of share-based services and changes in post-employment benefits.
Net cash used in operating activities was CHF 24.1 million in 2022, compared to CHF 35.7 million in 2021. The decrease in cash used in operating activities of CHF 11.6 million was due to an increase in net loss of CHF 16.1 million, before adjustments for non-operating transactions, offset by an increase in non-cash items of CHF 19.0 million and an increase in change in net working capital of CHF 10.8 million.
Investing Activities
Net cash used in investing activities was CHF 8.0 million in 2022, compared to CHF 30.3 million in 2021.
In 2022, net cash used in investing activities consisted primarily of payments to the former shareholders of APR and AdVita in relation to the completion of contractual milestones.
In 2021, net cash used in investing activities consisted primarily in payments for the acquisition of APR and ACER 001 license.
Financing Activities
Net cash from financing activities was CHF 6.4 million in 2022, compared to CHF 67.7 million in 2021.
In 2022, net cash from financing activities consisted primarily of CHF 7.1 million gross proceeds from the offer of treasury shares into the trading market, partially offset by issuance costs of CHF 0.2 million and by debt repayments of CHF 0.5 million.
In 2021, net cash from financing activities consisted primarily of CHF 50.9 million gross proceeds from the offer of treasury shares into the trading market and private placements of CHF 25 million, partially offset by issuance costs of CHF 2.8 million and by debt repayments of CHF 5.6 million.
Quantitative and Qualitative Disclosure about Financial Risks
We are exposed to various financial risks such as credit risk, liquidity risk and market risk (including interest rate and currency risk). The following is an overview of the extent of such risks and our processes employed to handle those risks.
Credit Risk
Credit risk refers to the risk that a party will default on its contractual obligations to us, resulting in financial losses. We do not currently have any revenue and as a result we do not have credit risk with relation to our customers. Our financial assets consist of mainly cash, for which the risk is minimal as such deposits are at well-known banks in Switzerland with an “A” rating as per Standard & Poors.
83
Table of Contents
Liquidity Risk
Liquidity risk implicates our ability to maintain sufficient cash and cash equivalents to meet our financial obligations. Our management monitors our net liquidity through rolling forecasts of projected cash flows.
Interest Rate Risk
We are exposed to interest risk in respect of the Company’s cash deposits, bank loans and other interest-bearing liabilities. We deem the interest rate risk as low on its performance and its equity.
Currency Risk
We operate internationally and are exposed to currency risk arising from various exposures, primarily with respect to the Swiss francs, Euros and U.S. dollars. Currency risk arises from future transactions, recognized assets and liabilities and net investments in foreign operations. To manage such risk, we monitor our exposure by periodically assessing future spending needs in foreign currencies and maintain foreign currency cash balances to cover anticipated future requirements. We do not enter into any forward currency transactions.
While we consider our current exposure to foreign currency risk to be low, adverse changes in the value of the Swiss franc could still have a significant negative impact on our financial condition, results of operations, and future prospects.
JOBS Act Exemptions and Foreign Private Issuer Status
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (JOBS Act) in the U.S. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. This includes an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002. We may take advantage of this exemption for up to five years or such earlier time that we are no longer an emerging growth company. We will cease to be an emerging growth company if we have more than $1.07 billion in total annual gross revenue, have more than $700 million in market value of our ordinary shares held by non-affiliates or issue more than $1 billion of nonconvertible debt over a three- year period. We may choose to take advantage of some but not all of these provisions that allow for reduced reporting and other requirements.
Upon completion of the U.S. listing to which this annual report relates, we will report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| • | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| • | sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; |
| • | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events; and |
| • | Regulation FD, which regulates selective disclosures of material information by issuers. |
| C. | RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES, ETC. |
See “Item 4. Information on the Company – B. Business Overview” and “Item 5. Operating and Financial Review and Prospects – A. Operating Results”.
| D. | TREND INFORMATION |
See “Item 5. Operating and Financial Review and Prospects – A. Operating Results and B. Liquidity and Capital Resources”.
84
Table of Contents
| E. | OFF-BALANCE SHEET ARRANGEMENTS |
As of the date of this annual report, the Company had the contingent liabilities listed in note 36 of the 2022 consolidated financial statements included in this annual report. There were no other material off-balance sheet arrangements.
| F. | TABULAR DISCLOSURE OF CONTRACTUAL ARRANGEMENTS |
The following table summarizes our contractual commitments as of December 31, 2022:
| (TCHF) | Less than 1 year |
1 to 3 years |
4 to 5 years |
More than 5 years |
Total | |||||||||||||||
| Lease liabilities (1) |
340 | 309 | 140 | — | 789 | |||||||||||||||
| Financial borrowings (2) |
1’652 | 12 | 3 | — | 1’667 | |||||||||||||||
| Total |
||||||||||||||||||||
| (1) | Represents the minimum lease payments due under our operating leases for offices, laboratory space, company cars, and office and labs material. |
| (2) | Bank loans are classified as short term due to the absence of contractual repayment date that renders their repayment date uncertain and dependent on the creditor’s demand. |
Further, as described elsewhere in this document, we have committed to pay royalties on any revenue derived from the sale of aviptadil and OLPRUVA™, to NeuroRx and Acer Therapeutics, respectively, as well as royalties on net commercialization profit of a low double-digit percentage to Meta if we commercialize or license RLF-OD32. We currently do not generate any revenue from these three compounds and therefore have no current payment obligations.
We enter into contracts in the normal course of business with CROs, contract manufacturing organizations and other third parties for clinical trials, preclinical studies, manufacturing and other services and products for operating purposes. These contracts generally provide for termination upon notice, and therefore we believe that our non-cancelable obligations under these agreements are not material.
| G. | SAFE HARBOR |
This annual report contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act, and as defined in the Private Securities Litigation Reform Act of 1995. See “Special Note with Respect to Forward Looking Statements” included elsewhere in this annual report.
| ITEM 6 | DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
| A. | DIRECTORS AND SENIOR MANAGEMENT |
For information about our directors and senior management, see “Item 1. Identity of Directors, Senior Management and Advisers – A. Directors and Senior Management”.
| B. | COMPENSATION |
Compensation in General
We are subject to the Directive on Information Relating to Corporate Governance of the SIX Swiss Exchange (Corporate Governance Directive) and the Swiss Ordinance Against Excessive Compensation in Public Companies of November 20, 2013, as amended from time to time (Compensation Ordinance), and the Swiss Code of Obligations.
In line with the requirements of the Compensation Ordinance, the Company’s Articles of Association and the Organizational Regulation include provisions on the following governance and compensation-related matters:
| • | number of permissible mandates in the supreme governing bodies of other legal entities; |
| • | maximum terms of employment contracts and maximum notice period for members of the Executive Committee; |
85
Table of Contents
| • | principles of compensation applicable to the Board of Directors and Executive Committee; |
| • | shareholders’ binding vote on compensation of the Board of Directors and Executive Committee; |
| • | additional amount for members of the Executive Committee hired after the vote on compensation by the Annual General Meeting; and |
| • | loans, credit facilities and post-employment benefits for members of the Board of Directors and of the Executive Committee. |
Role of the Compensation Committee
The Nomination and Compensation Committee (NCC) assists the Board of Directors in all nomination and compensation matters. As detailed in the Organizational Rules of the Company, the NCC is responsible for ensuring the best possible leadership and management talent for the company and an appropriate compensation policy. In particular, the NCC is responsible for the following activities:
| • | identification of suitable candidates for positions on the Board of Directors and on the Executive Committee; |
| • | recommendation and proposal of compensation principles and programs, including share-based compensation plans; |
| • | recommendation and proposal of the compensation for the members of the Board of Directors and Executive Committee; and |
| • | recommendation and proposal of specific compensation packages for other members of management. |
The decision-making authorities in compensation matters are summarized in the table below:
| Level of Authority CEO* |
CEO |
Nomination |
Board of |
Annual | ||||
| Compensation policy including share-based plans | proposes | approves | ||||||
| Aggregate compensation of the Board of Directors | proposes | reviews | approves | |||||
| Individual remuneration of the Board of Directors members | proposes | approves | ||||||
| Aggregate compensation of the Executive Committee | proposes | reviews | approves | |||||
| Individual compensation of the CEO | proposes | approves | ||||||
| Individual compensation of Executive Committee members | proposes | reviews | approves | |||||
| Compensation report | proposes | approves |
The NCC consists of a minimum of one member of the Board. The members of the NCC are elected individually and annually by the Annual General Meeting (AGM) for the period until the following AGM. At the AGM 2022, Thomas Plitz (NCC Chairman) and Raghuram Selvaraju were elected members of the NCC.
The NCC meets as often as the business requires, but at least once a year. The NCC Chairman may invite the Chairman of the Board, the CEO or other members of the Executive Committee to join the meeting in an advisory capacity. However, the executives do not take part in the meeting, or parts of meeting, during which their own compensation is discussed. The NCC Chairman reports to the Board on the activities of the committee after each meeting. The NCC may retain external advisors to obtain support in fulfilling its duties.
Role of Shareholders and Say-On-Pay Vote
At the AGM to be held by the end of June 2023, a binding vote on the compensation amount of the Board and Executive Committee will be conducted. The AGM will vote on the maximum compensation amount of the Board for the period of office until the following AGM and on the maximum compensation amount of the Executive Committee for the next financial year. The prospective voting structure provides the Company and its management with the necessary level of planning certainty to operate efficiently.
86
Table of Contents
The AGM held on May 31, 2022, approved a maximum compensation amount of CHF 2’500’000 for the Board for the period from the AGM 2022 to the AGM 2023 and a maximum compensation amount of CHF 5’000’000 for the Executive Committee for the financial year 2023.
Method of Determination of Compensation
Based on the recommendation of the NCC, the Board of Directors decides upon the compensation of the Board of Directors and Executive Committee at its own discretion, which is ultimately approved by the AGM. When preparing the compensation proposals, the NCC takes the following factors into consideration:
| • | affordability and overall situation of the Company; |
| • | business financial results and individual performance; and |
| • | level of compensation paid by other companies that are deemed to be comparable in terms of industry (where they compete for talent) and complexity (defined by their size and geographic scope). |
The compensation of the Board of Directors and Executive Committee is reviewed annually on the basis of those factors; however, the review does not necessarily lead to any adjustment.
Based on the recommendation of the NCC, the Board decides on the compensation of the Board and Executive Committee at its own discretion, which is prospectively approved by the AGM. When preparing the compensation proposals, the NCC takes the following factors into consideration:
| • | Affordability and overall situation of the Company; |
| • | Achievement of corporate goals and individual objectives; and |
| • | Level of compensation paid by comparable companies in the biotech and pharmaceutical industry (where they compete for talent) and complexity (defined by their size and geographic scope). |
The compensation of the Board and Executive Committee is reviewed annually on the basis of those factors. However, the review does not necessarily lead to adjustments.
Executive Compensation
In accordance with the laws of Switzerland, we do not report executive compensation on an individualized basis. The disclosure below includes all forms of compensation given by us in exchange for services rendered in 2022 and 2021 by members of the Executive Committee.
In 2022 and 2021, members of the Executive Committee received total remuneration as follows:
Compensation of the Executive Committee for the 2022 Calendar Year, in CHF
| Fixed Compensation |
Cash Bonus |
Pension Benefits |
Other Benefits |
Options | Total (3) | |||||||||||||||||||
| Total Executive Committee (1) |
1,635,477 | 160,130 | 89,087 | 35,068 | — | 1,919,762 | ||||||||||||||||||
| (1) | The highest paid member of the Executive Committee in 2022 was our former Chief Financial Officer and later Chief Executive Officer, Jack Weinstein, who received CHF 481,521 of fixed compensation, CHF 50,130 of variable cash compensation, and CHF 10,929 in other benefits. |
| (2) | Does not include the Company’s mandatory contribution to social security (AHV) of CHF 128,696. |
87
Table of Contents
Compensation of the Executive Committee for the 2021 Calendar Year, in CHF
| Fixed Compensation |
Cash Bonus |
Pension Benefits |
Other Benefits |
Options (2) | Total (3) | |||||||||||||||||||
| Total Executive Committee (1) |
1,763,451 | 231,340 | 30,151 | — | 1,947,634 | 3,972,476 | ||||||||||||||||||
| (1) | The highest paid member of the Executive Committee in 2021 was our then Chief Financial Officer, Jack Weinstein, who received CHF 460,614 of fixed compensation, CHF 175,000 of variable cash compensation and CHF 1,096,799 of options. |
| (2) | Reflects the value of share-based payments in accordance with IFRS 2 at grant date independently of the vesting schedule. Such stock option values are theoretical values at grant date and do not reflect taxable income nor realized income. |
| (3) | Does not include the Company’s mandatory contribution to social security (AHV) of CHF 82,953 |
During the 2022 fiscal year, remuneration to the executive committee amounted to CHF 1,919,762. This was within the limit of CHF 5,000,000 approved by the AGM 2022.
Employment Arrangements
We have entered into employment letters with each of our executive officers. These agreements provide for a base salary and annual incentive cash and option bonus opportunities as well as payments upon termination. Agreements with our executive officers may be terminated upon two to twelve months’ advance notice.
Principles and Compensation Architecture
Our compensation principles are aligned with the Company’s strategy of becoming profitable by growing its business and increasing revenue. The compensation principles are as follows:
| • | Balance between competitiveness and affordability: within the Company’s financial ability, compensation levels are competitive and aligned with market practice for similar functions in comparable companies in the biotech and pharmaceutical industry; |
| • | Pay for performance: part of compensation is directly linked to the performance of the business and to the achievement of individual objectives; and |
| • | Alignment with shareholders’ interests: part of compensation is delivered in the form of stock options and thus is directly tied to the Company’s long-term share performance. |
The compensation of the members of the Executive Committee consists of fixed and variable remunerations. Fixed remuneration comprises the base salary and other benefits. Variable remuneration may comprise a performance-based cash bonus and equity grants.
Compensation Model for Executive Officers
| Vehicle |
Purpose |
Drivers |
Performance | |||||
| Fixed Base Salary | Monthly Cash | Attract & Retain | Market practice | — | ||||
| Performance bonus | Cash bonus | Pay for performance | Business and individual performance | Company’s profitability, individual performance | ||||
| Employee Participation Program | Share options | Align to shareholders’ interests | Level of the role | Share price | ||||
| Benefits | Pension/insurance plans | Protect against risk | Market practice | — |
Fixed Base Salary. The fixed base salary pays for the function and depends on our financial ability, the market value of the function and the profile of the individual in terms of qualifications and skill set.
Performance Bonus. The performance bonus rewards the effective and successful conduct of the business and the achievement of individual objectives. The target performance bonus is generally expressed as a percentage of the fixed base salary. At the discretion of the Board and the NCC, decision to grant a bonus may be taken. The bonus amount effectively paid out is then determined by the Board, based upon the proposal of the NCC. The performance bonus is usually paid in cash or options, usually at the end of the financial year.
88
Table of Contents
Employee Participation Program. The Employee Participation Program provides an incentive for management to make significant contributions towards our long-term success and aligns their interests to those of our shareholders. The Board of Directors determines the individual allocation of stock options at its own discretion, taking into account the level of the role and economic considerations. The value of the options for financial reporting purpose is calculated according to the Black-Scholes valuation model.
Benefits. Executive Officers participate in the regular pension and retirement plans applicable to all employees in their country of employment. The provisions of those pension and retirement plans are in line with local regulations and prevailing market practice. Further, executive officers may be entitled to benefits in kind, in line with local market practice, such as a company car or other benefits.
Contractual Provisions. The employment contracts of our executive officers are concluded for an indefinite period and without a notice period. They do not contain any agreement on severance provisions.
89
Table of Contents
NON-EMPLOYEE DIRECTOR COMPENSATION
The disclosure of compensation below includes all forms of compensation given by us in exchange for services rendered by members of our Board of Directors in 2022 and 2021, in CHF.
| Board of Directors |
Cash Fee 2022 |
Cash Fee 2021 |
Options 2022 (1) |
Options 2021 (1) |
Total 2022 (2) |
Total 2021 (2) | ||||||||||||||||||
| Raghuram Selvaraju Chairman since May 25, 2016 |
500,000 | 475,000 | — | 248,470 | 500,000 | 723,470 | ||||||||||||||||||
| Thomas Plitz Member since December 17, 2020 |
150,000 | 125,000 | — | 266,103 | 150,000 | 391,103 | ||||||||||||||||||
| Patrice Jean Member since June 18, 2021 |
150,000 | 76,998 | — | 16,330 | 150,000 | 93,329 | ||||||||||||||||||
| Paolo Galfetti (3) Member since June 18, 2021 |
150,000 | 79,722 | — | — | 150,000 | 79,722 | ||||||||||||||||||
| Michelle Lock Member since January 28, 2022 |
92,473 | — | — | — | 92,473 | — | ||||||||||||||||||
| Thomaz Burckhardt Member until 8 February 2021 |
— | 7,500 | — | — | — | 7,500 | ||||||||||||||||||
| Total |
1,042,473 | 764,220 | — | 530,903 | 1,042,473 | 1,295,123 | ||||||||||||||||||
| (1) | Reflects value of share-based payments in accordance with IFRS 2 at grant date independently of the vesting schedule. Such stock option values are theoretical values at grant date and do not reflect taxable income nor realized income. |
| (2) | Does not include the Company’s mandatory contribution to social security of CHF 35,515 (2021: 18,628). |
| (3) | For his executive role, Mr. Galfetti received an additional remuneration of CHF 442’990 in cash and CHF 64’636 in the form of pension contribution for the 2022 calendar year. His executive compensation is reported as part of the compensation of the Executive Committee. |
The figures in the table above cover the 2022 calendar year, as required by Swiss law. They differ from the period authorized by the AGM, which runs from AGM to AGM (the Authorization Period). Differences between calendar years and Authorization periods are shown in the tables below.
90
Table of Contents
During the current Authorization Period, members of the Board of Directors are expected to earn a total compensation of CHF 1,141,667. This is within the limit of CHF 2,500,000 approved at the EGM 2022.
| Calendar Year 2022 | Authorization period 2023/2022 | |||||||||||||||||||
| Compensation (CHF) |
Period | Amount | Period | Amount | (1) | Approved | ||||||||||||||
| Cash Fee |
|
January 2022- December 2022 |
1,042,473 | |
July 2022-June 2023 |
|
1,141,667 | |||||||||||||
| Options |
|
January 2022- December 2022 |
— | |
July 2022-June 2023 |
|
— | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total |
1,042,473 | 1,141,667 | 2,500,000 | |||||||||||||||||
|
(1) As this period is not yet ended as of the date of this report, the amount includes actual to date and an estimate of the compensation to be earned over the remaining period to the AGM 2023. |
| |||||||||||||||||||
| Calendar Year 2021 | Authorization period 2022/2021 | |||||||||||||||||||
| Compensation (CHF) |
Period | Amount | Period | Amount | (1) | Approved | ||||||||||||||
| Cash Fee |
|
January 2021- December 2021 |
764,220 | |
July 2021- June 2022 |
922,110 | ||||||||||||||
| Options |
|
January 2021- December 2021 |
530,903 | |
July 2021- June 2022 |
264,800 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Total |
1,295,123 | 1,186,911 | 2,500,000 | |||||||||||||||||
In 2022 and 2021, no compensation was granted to former members of the Board of Directors or related parties.
Option Grants to Members of the Board of Directors and Executive Committee
During 2022 and 2021, the following options to purchase shares of the Company’s ordinary stock as listed on the SIX Swiss Exchange were granted to members of the Board of Directors and Executive Committee:
| Date of Grant |
Options Granted | Strike Price (in CHF) |
Expiration Date | |||||||
| January 1, 2021 |
100,000 | 0.2690 | December 31, 2025 | |||||||
| May 30, 2021 |
8,500,000 | 0.0100 | 1/2 on each of May 29, 2027 and 2028 | |||||||
| September 12, 2021 |
|
1,900,000 3,200,000 |
|
|
0.0100 0.1540 |
|
1/3 on each of September 12, 2027, 2028 and 2029 | |||
| December 20, 2021 |
3,000,000 | 0.0100 | December 27, 2027 | |||||||
| December 30, 2021 |
|
15,500,000 11,000,000 |
|
|
0.0610 0.0100 |
|
1/3 on each of December 27, 2027, 2028 and 2029 | |||
| C. | BOARD PRACTICES |
Our Board of Directors currently consists of five members. As a foreign private issuer, we are not required to have independent directors on our Board of Directors, except that our audit committee is required to consist fully of independent directors, subject to certain phase-in schedules.
91
Table of Contents
Overview
The Board of Directors is self-constituting and determines our internal organization. The Chairman convenes the Board as often as our affairs require and presides (or in his absence, another Board member specifically designated by the majority of the other members present at the meeting) over the Board meetings. Each Board member is entitled to request to the Chairman, in writing, a meeting of the Board by indicating the grounds for such a request. The Chairman decides on the agenda items and motions. Every Director is entitled to request to the Chairman, in writing, the inclusion of a specific agenda item by indicating the grounds for such a request.
To pass a valid resolution, the majority of the Board members has to attend the meeting. Meetings may also be held by telephone or video conference, to which all the Board members are invited. No quorum is required for confirmatory resolutions and adaptations of the Articles in connection with capital increases. The Board of Directors passes its resolutions by way of simple majority. The members of the Board may only vote in person, not in proxy. In the event of a tie vote, the Chairman has the deciding vote. Minutes of deliberations and resolutions are kept and signed by the Chairman and the designated Secretary.
The Board has established the following permanent committees to further strengthen our corporate governance structure.
Audit and Finance Committee
The Audit and Finance Committee (AFC) advises the Board of Directors in the performance of its supervisory duties. In particular, the AFC reviews the financial reporting to shareholders and the general public as well as the relationship with the external auditors; satisfies itself that our financial risk management and our internal controls are of an appropriate standard; ensures that its activities are consistent and compliant with the Organizational Regulations; assesses adherence to the relevant ‘best practice’ corporate governance provisions, to the extent such practice has effect on the activities and the functions of the AFC; satisfies itself that our overall fraud prevention procedures are of an appropriate standard and ensures that appropriate procedures to enable employees to confidentially and anonymously submit their concerns regarding accounting, internal controls or auditing matters are in place.
As of the date of this annual report, the AFC is composed of Patrice Jean (Chair), Michelle Lock and Thomas Plitz.
Nomination and Compensation Committee
The Nominating and Compensation Committee (NCC) advises the Board of Directors in the performance of its supervisory duties related to nomination and compensation matters. It is responsible for ensuring the best possible leadership and management of the Company and for determining compensation policies, including share-based incentive programs, for the Company’s top management and Board of Directors.
As of the date of this annual report, the NCC is composed of Thomas Plitz (Chair) and Raghuram Selvaraju.
Corporate Governance Committee
The Corporate Governance Committee (CGC) advises the Board on all matters of corporate governance. It is responsible for carrying out in-depth analysis of specific corporate governance-related matters and monitors compliance with corporate governance principles and policies.
As of the date of this annual report, the CGC is composed of Paolo Galfetti (Chair) and Patrice Jean.
Other Corporate Governance Matters
The Sarbanes-Oxley Act of 2002, as well as related rules subsequently implemented by the SEC, requires foreign private issuers, including our company, to comply with various corporate governance practices. In addition, Nasdaq rules provide that foreign private issuers may follow home country practice in lieu of the Nasdaq corporate governance standards, subject to certain exceptions and except to the extent that such exemptions would be contrary to U.S. federal securities laws.
92
Table of Contents
We intend to take all actions necessary for us to maintain compliance as a foreign private issuer under the applicable corporate governance requirements of the Sarbanes-Oxley Act of 2002, the rules adopted by the SEC and Nasdaq’s listing standards.
Because we are a foreign private issuer, our directors and senior management are not subject to short-swing profit and insider trading reporting obligations under Section 16 of the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act. They will, however, be subject to the obligations to report changes in share ownership under Section 13 of the Exchange Act and related SEC rules.
Indemnification of Officers and Directors
Under Swiss law, a corporation may indemnify its officers and directors against losses and expenses, except for such losses and expenses arising from willful misconduct or negligence (although some legal scholars advocate that at least gross negligence be required), including attorney’s fees, judgments, fines, and settlement amounts actually and reasonably incurred in a civil or criminal action, suit or proceeding by reason of having been the representative of, or serving at the request of, the corporation.
Subject to Swiss law, our articles of association provide for indemnification of the exiting and former members of our board of directors, executive management, and the heirs, executors and administrators, against liabilities arising in connection with the performance of their duties in such capacity, and permits us to advance the expenses of defending any act, suit or proceeding to members of our board of directors and executive management. In addition, under general principles of Swiss employment law, an employer may be required to indemnify an employee against losses and expenses incurred by such employee in the proper execution of their duties under their employment agreement with the company.
We have entered into indemnification agreements with each of the members of our board of directors and with our executive officers.
| D. | EMPLOYEES |
At December 31, 2022, we had a total of 69 employees. Our relationship with our employees is good and no employees are covered by a labor union. We did not employ any temporary employees during 2022.
| E. | SHARE OWNERSHIP |
For information about share ownership by our directors and senior management, see “Item 7. Major Shareholders and Related Party Transactions – A. Major Shareholders”.
| ITEM 7 | MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
| A. | MAJOR SHAREHOLDERS |
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of April 30, 2023 by:
| • | each of our executive officers; |
| • | each of our directors; and |
| • | each person, or group of affiliated persons, who is known by us to beneficially own more than 3 percent of our outstanding ordinary shares. |
These share holding numbers have not been adjusted for the 400:1 reverse split which occurred on May 5, 2023.
93
Table of Contents
Beneficial ownership is determined in accordance with the rules and regulations of the SEC and includes voting or investment power with respect to our ordinary shares. Ordinary shares subject to options that are currently exercisable or exercisable within 60 days after calculation date are considered outstanding and beneficially owned by the person holding the options for the purpose of calculating the percentage ownership of that person but not for the purpose of calculating the percentage ownership of any other person. Except as otherwise noted, the persons and entities in this table have sole voting and investment power with respect to all of the ordinary shares beneficially owned by them, subject to community property laws, where applicable. Except as otherwise set forth below, the address of the beneficial owner is c/o the Company. The information in the table below is based on information known to us or ascertained by us from public filings made by the shareholders. We have also set forth below information known to us regarding any significant change in the percentage ownership of our ordinary shares by any major shareholders during the past three years. The major shareholders listed below do not have voting rights with respect to their ordinary shares that are different from the voting rights of other holders of our ordinary shares.
| Name | Shares | Option | Percentage | |||||||||
| 5 Percent Stockholders |
||||||||||||
| GEM Global Yield LLC SCS† |
1,155,899,126 | 0 | 20.58 | %* | ||||||||
| Executive Officers and Directors |
||||||||||||
| Jack Weinstein |
185,000 | 18,600,000 | * | * | ||||||||
| Jeremy Meinen |
140,655 | 1,100,000 | * | * | ||||||||
| Marco Marotta |
0 | 1,500,000 | * | * | ||||||||
| Raghuram Selvaraju |
0 | 8,963,197 | * | * | ||||||||
| Thomas Plitz |
500,000 | 1,500,000 | * | * | ||||||||
| Patrice Jean |
140,000 | 200,000 | * | * | ||||||||
| Paolo Galfetti |
31,237,437 | 12,500,000 | * | * | ||||||||
| Michelle Lock |
0 | 0 | * | * | ||||||||
| † | As set forth in a filing with the SIX Swiss Exchange on April 7, 2023. Pursuant to such filing, Christopher Brown, a resident of the State of New York, is the person who can exercise the voting rights at his own discretion. |
| * | Calculated at the date of notification based on the number of outstanding shares registered at the Commercial Register of Geneva. |
| ** | Less than one percent. |
We are not aware of any arrangement, the operation of which may result in a change of control of the Company.
| B. | RELATED PARTY TRANSACTIONS |
The following is a description of related party transactions we have entered into with the beneficial owners of 10 percent or more of our ordinary shares, which are our only voting securities, senior management and members of our Board of Directors, from January 1, 2021, to December 31, 2022.
GEM Global Yield LLC SCS
On January 20, 2021, we signed a binding agreement with our largest shareholder, GEM Global Yield LLC SCS (GEM) for the implementation of a new share subscription facility (SSF) in the amount of up to CHF 50 million.
Under the terms of the SSF, we have the right to periodically, during a timeframe of up to three years, issue and sell shares to GEM. Under the facility, GEM undertakes to subscribe to or acquire ordinary registered shares of the Company upon the Company’s exercise of a drawdown notice. In accordance with the customary terms of the SSF agreement, the Company controls the timing and maximum amount of any drawdown and retains the right, not the obligation, to draw down on the full commitment amount. Future subscription prices under the SSF will correspond to 90 percent of the average of the closing bid prices on the SIX Swiss Exchange during the reference period, which corresponds to fifteen trading days following Relief’s drawdown notice.
Under the terms of the SSF, we are required to pay GEM a commitment fee of CHF 1.25 million, which is currently outstanding as an interest bearing loan pursuant to the SSF. As of the date of this annual report, no amounts have been drawn on this facility.
94
Table of Contents
Management and Board of Directors
The following additional transactions were carried out with our executive officers and members of our board of directors
| Key Management Compensation, in TCHF | 2022 | 2021 | ||||||
| Fees, salaries and other short-term employee benefits |
2,873 | 2,759 | ||||||
| Post-employment benefits |
89 | 30 | ||||||
| Share-based compensation |
— | 814 | ||||||
| Total compensation for key management |
2,962 | 3,603 | ||||||
| C. | INTERESTS OF EXPERTS AND COUNSEL |
None.
| ITEM 8 | FINANCIAL INFORMATION |
| A. | CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION |
Consolidated Financial Statements
Our audited financial statements for the fiscal years ended December 31, 2022 and 2021 are included in Item 18 of this annual report.
Legal Proceedings
From time to time, we may become involved in legal, governmental or arbitration proceedings or be subject to claims arising in the ordinary course of our business. Except as set forth therein or elsewhere in this Annual Report, we are not presently a party to any legal, governmental or arbitration proceeding. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Dividend Distribution Policy
We have not paid any dividends since our incorporation. Even if future operations lead to significant levels of distributable profits, we currently intend that any earnings will be reinvested in our business and that dividends will not be paid until we have an established revenue stream to support continuing dividends. Under our articles of association, the declaration of dividends requires a resolution passed by a simple majority of the votes cast at a shareholders’ meeting regardless of abstentions and empty or invalid votes. The proposal to pay future dividends to shareholders will in addition effectively be at the discretion of our board of directors after considering various factors including our business prospects, liquidity requirements, financial performance and new product development. In addition, payment of future dividends is subject to certain limitation pursuant to Swiss law or by our articles of association. Accordingly, investors cannot rely on dividend income from our ordinary shares and any returns on an investment in our ordinary shares will likely depend entirely upon any future appreciation in the price of our ordinary shares.
| B. | SIGNIFICANT CHANGES |
Except as otherwise disclosed above or elsewhere in this annual report, there has been no significant change since the date of the most recent financial statements included in this annual report.
| ITEM 9 | THE OFFER AND LISTING |
| A. | OFFER AND LISTING DETAILS |
Not applicable.
95
Table of Contents
| B. | PLAN OF DISTRIBUTION |
Not applicable.
| C. | MARKETS |
Our ordinary shares trade on the SIX Swiss Exchange (SIX) under the symbol “RLF” and over the counter in the U.S. under the symbol “RLFTF”. Our ADRs trade in the over-the-counter market under the symbol “RLFTY.”
| D. | SELLING SHAREHOLDERS |
Not applicable.
| E. | DILUTION |
Not applicable.
| F. | EXPENSES OF THE ISSUE |
Not applicable.
| ITEM 10 | ADDITIONAL INFORMATION |
| A. | SHARE CAPITAL |
Issued Share Capital
Our issued share capital as of December 31, 2022 was 5,616,334,617 shares with a par value of CHF 0.01 per share, including 1,210,809,431 shares held in treasury.
On April 28, 2023, the extraordinary general meeting of shareholders approved the consolidation (reverse split) of the Company’s ordinary shares at a 400 to 1 ratio. On May 5, 2023, the reverse split was implemented in accordance with the resolution of the extraordinary general meeting. Following the consolidation, our issued share capital was 14,040,837 shares with a par value of CHF 4.00 per share, including 3,022,336 shares held in treasury.
Unless stated otherwise, all numbers of shares throughout this annual report are pre-reverse split and shall be divided by 400 to obtain the equivalent in post-reverse split shares. The same applies to disclosures related to stock options and derivative instruments.
Each issued ordinary share is fully paid. We currently have no deferred shares in our issued share capital.
Ordinary Shares
To the extent dividends are paid, holders of ordinary shares are entitled to receive dividends in proportion to the number of ordinary shares held by them and according to the amount paid up on such ordinary shares during any portion or portions of the period in respect of which the dividend is paid. Holders of ordinary shares are entitled, in proportion to the number of ordinary shares held by them and to the amounts paid up thereon, to share in any surplus in the event of our winding up. The holders of ordinary shares are entitled to receive notice of, attend either in person or by proxy or, being a corporation, by a duly authorized representative, and vote at general meetings of shareholders.
| B. | MEMORANDUM AND ARTICLES OF ASSOCIATION |
Please read this section in conjunction with our Articles of Association, which are attached to this annual report as Exhibit 3.1.
Increase of Share Capital
Under Swiss law, we may increase our share capital (capital-actions) with a resolution of the general meeting of shareholders (ordinary capital increase) that must be carried out by the board of directors within three months of the respective general meeting in order to become effective. Under our articles of association and Swiss law, in the case of subscription and increase against payment of contributions in cash, a resolution passed by an absolute majority of the shares represented at the general meeting of shareholders is required. In the case of subscription and increase against contributions in kind or to fund acquisitions in kind, when shareholders’ statutory pre-emptive subscription rights or advance subscription rights are limited or withdrawn or where transformation of freely disposable equity into share capital is involved, a resolution passed by two-thirds of the shares represented at a general meeting of shareholders and the absolute majority of the par value of the shares represented is required.
96
Table of Contents
Furthermore, under the Swiss Code of Obligations (CO), our shareholders, by a resolution passed by two-thirds of the shares represented at a general meeting of shareholders and the absolute majority of the par value of the shares represented, may empower our board of directors to issue shares of a specific aggregate par value up to a maximum of 50 percent of the share capital in the form of:
| • | conditional share capital (capital-actions conditional) for the purpose of issuing shares in connection with, among other things, (i) option and conversion rights granted in connection with bonds and similar debt instruments of the Company or one of our subsidiaries or (ii) grants of rights to employees, members of our board of directors or consultants or to our subsidiaries or other persons providing services to the Company or a subsidiary to subscribe for new shares (conversion or option rights); or |
| • | authorized share capital (capital-actions autorisé) to be utilized by the board of directors within a period determined by the shareholders but not exceeding two years from the date of the shareholder approval. |
Pre-Emptive and Advance Subscription Rights and Pre-Emptive Rights
Pursuant to the CO (but subject to the articles of association), shareholders have subscription rights (droits de souscription) to subscribe for new issuances of shares in connection with ordinary and authorized capital increases. With respect to conditional capital in connection with the issuance of bonds or similar debt instruments, shareholders have pre-emptive rights (droit de souscrire préalablement) for the subscription of such bonds or similar debt instruments.
A resolution passed at a general meeting of shareholders by two-thirds of the shares represented and the absolute majority of the par value of the shares represented may authorize our board of directors to withdraw or limit subscription rights or pre-emptive rights in certain circumstances.
If preemptive rights are granted, but not exercised, the board of directors may allocate the unexercised preemptive rights at its discretion.
Our Authorized Share Capital
As of December 31, 2022, our board of directors was authorized under our articles of association to increase at any time our ordinary share capital, until May 30, 2024, by a maximum amount of CHF 10,000,000.00 by issuing up to 1,000,000,000 registered shares to be fully paid up with a par value of CHF 0.01 each.
Increases in partial amounts are permitted. The board of directors has the power to determine the type of contributions, the issue price and the date on which the dividend entitlement starts.
With respect to our authorized share capital, the board of directors is authorized by our articles of association to withdraw restrict or to exclude limit the pre-emptive subscription rights of shareholders, and to allocate them to third parties or to us, in the event that the newly issued shares are issued under the following circumstances:
| • | for the acquisition of companies, parts of companies or participations, for the acquisition of products, intellectual property or licenses, or for investment projects or for the financing or refinancing of such transactions through a placement of shares; |
| • | for the purpose of broadening the shareholder constituency or in connection with a listing of shares on domestic or foreign stock exchanges; |
| • | for the participation of employees, members of the Board of Directors and consultants of the Company or its subsidiaries in accordance with one or more regulations adopted by the Board of Directors; |
| • | for investment projects and/or financial instruments which are used in national or international capital markets, or for raising capital in a fast and flexible manner, which would hardly be achieved without the exclusion of the statutory subscription rights of the existing shareholders; or |
| • | for other valid grounds pursuant to Article 652b, paragraph 2 of the Swiss Code of Obligations. |
97
Table of Contents
This authorization is exclusively linked to the particular available authorized share capital set out in the respective article. If the period to increase our share capital out of authorized share capital lapses without having been used by the board of directors, the authorization to withdraw or to limit the pre-emptive subscription rights lapses simultaneously with such capital.
Our Conditional Share Capital
As of December 31, 2022, we had available conditional capital of 1,668,769,814 shares with a nominal value of CHF 0.01 each in the aggregate nominal value of CHF 16,687,698.14, as follows:
| • | 1,563,000,000 registered shares to be fully paid up, each with a nominal value of CHF 0.01 to the nominal value of CHF 15,630,000.00 through the exercise of conversion or option rights granted to entitled parties in connection with bonds and similar financial instruments or loans of the Company or its subsidiaries that allow for conversion into shares of the Company, or option rights granted to existing and/or new shareholders in connection with capital increases. |
| • | 105,769,814 registered shares to be fully paid up, each with a nominal value of CHF 0.01 to a nominal value of CHF 1,057,689.14 through the exercise of options granted to employees, members of the Board of Directors and consultants of the Company or its subsidiaries. |
As to the conditional share capital described in the first bullet point above, the subscription rights of shareholders are excluded. The Board of Directors determines the conversion and option terms, the issue price and the date of dividend entitlement. The Board of Directors is authorized to limit or exclude the preemptive rights of existing shareholders in the event: (1) of the financing or refinancing of the acquisition of enterprises, parts of enterprises, participations or investments, (2) of the financing or refinancing of the Company or its subsidiaries, (3) of the issuance of convertibles and/or option bonds for the purpose of placement on national or international capital markets (including private placements), (4) for purposes of the underwriting of such bonds and other financial instruments by one or more banks with subsequent public offer; or if the issuance occurs in national or international capital markets, or (5) through a private placement.
As to the conditional share capital described in the second bullet point above, the rights of pre-emption and subscription rights of shareholders are excluded in connection with the issuance of any shares, options or subscription rights thereof.
To the extent that the advance subscription rights are withdrawn or limited, (i) the convertible bonds, warrants or similar instruments are to be issued at market conditions; (ii) the term to exercise the convertible bonds, warrants or similar instruments may not exceed ten years from the date of issue of the respective instrument and (iii) the conversion, exchange or exercise price of the convertible bonds, warrants or similar instruments has to be set with reference to or be subject to change based upon the valuation of the Company’s equity or market conditions.
The extraordinary general meeting of shareholders held on April 28, 2023 approved a revision of the terms of the conditional and authorized capitals under the articles of association for consistency with the consolidation of our ordinary share capital at a ratio of 400 to 1.
Uncertificated Securities
Our shares are in the form of uncertificated securities (droits-valeurs, within the meaning of Article 973c of the CO) and are established as book-entry securities within the meaning of the Swiss Federal Act on Securities held with an Intermediary of 2008, as amended. In accordance with Article 973c of the CO, we will maintain a non-public register of uncertificated securities (registre des droits-valeurs). We may at any time convert uncertificated securities into share certificates (including global certificates), one kind of certificate into another, or share certificates (including global certificates) into uncertificated securities. Following entry in the share register, a shareholder may at any time request from us a written confirmation in respect of his or her shares. Shareholders are not entitled, however, to request the conversion and/or printing and delivery of share certificates. We may print and deliver certificates for shares at any time.
98
Table of Contents
General Meeting of Shareholders
Ordinary/Extraordinary Meetings, Powers
The general meeting of shareholders is our supreme corporate body. Under Swiss law, an annual general meeting of shareholders must be held annually within six months after the end of a corporation’s financial year. In our case, this generally means on or before June 30. In addition, extraordinary general meetings of shareholders may be held.
The following powers are vested exclusively in the general meeting of shareholders:
| • | adopting and amending the articles of association, including the change of a company’s purpose or domicile; |
| • | electing the members of the board of directors, the chairman of the board of directors, the members of the compensation committee, the auditors and the independent proxy; |
| • | approving the business report, the annual statutory and consolidated financial statements, and deciding on the allocation of profits as shown on the balance sheet, in particular with regard to dividends; |
| • | approving the aggregate amount of compensation of members of the board of directors and the executive committee; |
| • | discharging the members of the board of directors and the executive committee from liability with respect to their conduct of business; |
| • | dissolving a company with or without liquidation; and |
| • | deciding matters reserved to the general meeting of shareholders by law or the articles of association or submitted to it by the board of directors. |
An extraordinary general meeting of shareholders may be called by a resolution of the board of directors or the general meeting of shareholders or, under certain circumstances, by a company’s auditor, liquidator or the representatives of bondholders, if any. In addition, the board of directors is required to convene an extraordinary general meeting of shareholders if shareholders representing at least 10 percent of our share capital request such general meeting of shareholders in writing. Such request must set forth the items to be discussed and the proposals to be acted upon. The board of directors must convene an extraordinary general meeting of shareholders and propose financial restructuring measures if, based on our stand-alone annual statutory balance sheet, half of our share capital and statutory reserves are not covered by our assets.
Voting and Quorum Requirements
Shareholder resolutions and elections (including elections of members of the board of directors) require the affirmative vote of the absolute majority of shares represented at the general meeting of shareholders, unless otherwise stipulated by law or our articles of association.
Under Swiss law and our articles of association, a resolution of the general meeting of the shareholders passed by two-thirds of the shares represented at the meeting, and the absolute majority of the par value of the shares represented is required for:
| • | a change in our corporate purpose; |
| • | the introduction and abolition of voting shares; |
| • | restrictions on the transferability of registered shares; |
| • | authorized or conditional capital increase; |
| • | capital increase from equity for investment in kind or for the purpose of acquisition of assets and the granting of special privileges; |
| • | the restriction or abolition of subscription rights; |
| • | the transfer of our headquarters; |
99
Table of Contents
| • | facilitating or waiving restrictions on transferability of registered shares; or |
| • | the dissolution of the company. |
The same voting requirements apply to resolutions regarding transactions among corporations based on Switzerland’s Federal Act on Mergers, Demergers, Transformations and the Transfer of Assets of 2003, as amended (Swiss Merger Act).
In accordance with Swiss law and generally accepted business practices, our articles of Nasdaq do not provide quorum requirements generally applicable to general meetings of shareholders. To this extent, our practice varies from NASDAQ listing standards, which require an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting shares.
Notice
General meetings of shareholders must be convened by the board of directors at least 20 days before the date of the meeting. The general meeting of shareholders is convened by way of a notice appearing in our official publication medium, currently the Swiss Official Gazette of Commerce. Registered shareholders may also be informed by ordinary mail or e-mail. The notice of a general meeting of shareholders must state the items on the agenda, the motions to the shareholders and, in case of elections, the names of the nominated candidates. A resolution on a matter which is not on the agenda may not be passed at a general meeting of shareholders, except for motions to convene an extraordinary general meeting of shareholders or to initiate a special investigation, on which the general meeting of shareholders may vote at any time. No previous notification is required for motions concerning items included in the agenda or for debates that do not result in a vote.
All of the owners or representatives of our shares may, if no objection is raised, hold a general meeting of shareholders without complying with the formal requirements for convening general meetings of shareholders (a universal meeting). This universal meeting of shareholders may discuss and pass binding resolutions on all matters within the purview of the general meeting of shareholders, provided that the owners or representatives of all the shares are present at the meeting.
Agenda Requests
Pursuant to Swiss law and our articles of association, one or more shareholders, whose combined shareholdings represent the lower of (i) one tenth of our share capital and (ii) an aggregate par value of at least CHF 1,000,000 may request that an item be included in the agenda for a general meeting of shareholders. To be timely, the shareholder’s request must be received by us generally at least 45 calendar days in advance of the meeting. The request must be made in writing and shall specify the agenda item and the shareholders’ proposals.
In addition, if the shareholder intends to solicit proxies from the shareholders of a company, such shareholder shall notify the company of this intent in accordance with SEC Rule 14a-4 and/or Rule 14a-8.
Our business report, the compensation report and the auditor’s report must be made available for inspection by the shareholders at our registered office no later than 20 days prior to the general meeting of shareholders. Shareholders of record must be notified of this in writing.
Voting Rights
Each of our ordinary shares entitles a holder to one vote. The ordinary shares are not divisible. The right to vote and the other rights of share ownership may only be exercised by shareholders (including any nominees) who are entered in the share register at a cut-off date determined by the board of directors. Those entitled to vote in the general meeting of shareholders may be represented by the independent proxy holder (annually elected by the general meeting of shareholders), by its legal representative, who does not have to be a shareholder, or by another shareholder with voting rights. The chairman has the power to decide whether to recognize a power of attorney.
Dividends and Other Distributions
Our board of directors may propose to shareholders that a dividend or other distribution be paid but cannot itself authorize the distribution. Dividend payments require a resolution passed by an absolute majority of the shares represented at a general meeting of shareholders. In addition, our auditors must confirm that the dividend proposal of our board of directors conforms to Swiss statutory law and our articles of association.
100
Table of Contents
Under Swiss law, we may pay dividends only if we have sufficient distributable profits from the previous business year (bénéfice de l’exercice) or brought forward from the previous business years (report des bénéfices), or if we have distributable reserves (réserves à libre disposition), each as evidenced by the Company’s audited stand-alone statutory balance sheet prepared pursuant to Swiss law, and after allocations to reserves required by Swiss law and by the articles of association have been deducted. We are not permitted to pay interim dividends out of profit of the current business year.
Distributable reserves are generally booked either as “free reserves” (réserves libres) or as “reserve from capital contributions” (apports de capital). Under the CO, if our general reserves (réserve générale) amount to less than 20 percent of our share capital recorded in the Commercial Register (i.e., 20 percent of the aggregate par value of our issued capital), then at least five percent of our annual profit must be retained as general reserves. In addition, if our general reserves amount to less than 50 percent of our share capital recorded in the Commercial Register, 10 percent of the amounts distributed beyond payment of a dividend of five percent must be retained as general reserves. The CO permits us to accrue additional general reserves. Further, a purchase of our own shares (whether by us or a subsidiary) reduces the distributable reserves in an amount corresponding to the purchase price of such own shares. Finally, the CO under certain circumstances requires the creation of revaluation reserves which are not distributable.
Distributions out of issued share capital (i.e., the aggregate par value of our issued shares) are not allowed and may be made only by way of a share capital reduction. Such a capital reduction requires a resolution passed by an absolute majority of the shares represented at a general meeting of shareholders. The resolution of the shareholders must be recorded in a public deed and a special audit report must confirm that claims of our creditors remain fully covered despite the reduction in our share capital recorded in the Commercial Register. Our share capital may be reduced below CHF 100,000 only if and to the extent that at the same time the statutory minimum share capital of CHF 100,000 is reestablished by sufficient new fully paid-up capital. Upon approval by the general meeting of shareholders of the capital reduction, the board of directors must give public notice of the capital reduction resolution in the Swiss Official Gazette of Commerce three times and notify creditors that they may request, within two months of the third publication, satisfaction of or security for their claims. The reduction of our share capital may be implemented only after expiration of this time limit.
Our board of directors determines the date on which the dividend entitlement starts. Dividends are usually due and payable shortly after the shareholders have passed the resolution approving the payment, but shareholders may also resolve at the annual general meeting of shareholders to pay dividends in quarterly or other installments.
Transfer of Shares
For as long as the shares are in uncertificated form (droits-valeurs) and registered as book-entry securities, any transfer of the shares has to be made in accordance with the FISA. The transfer of book-entry securities by way of assignment is excluded. The company maintains, through Computershare, a share register, in which the full name, address and nationality (in the case of legal entities, the company name and registered office) of the shareholders and usufructuaries are recorded.
Voting rights may be exercised only after a shareholder has been entered in the share register (registre des actions) with his or her name and address (in the case of legal entities, the registered office) as a shareholder with voting rights.
Inspection of Books and Records
Under the CO, a shareholder has a right to inspect the share register with respect to his or her own shares and otherwise to the extent necessary to exercise his or her shareholder rights. No other person has a right to inspect the share register. Our books and correspondence may be inspected with the express authorization of the general meeting of shareholders or by resolution of the board of directors and subject to the safeguarding of our business secrets and other legitimate interests.
Special Investigation
If the shareholders’ inspection rights as outlined above prove to be insufficient in the judgment of the shareholder, any shareholder may propose to the general meeting of shareholders that specific facts be examined by a special examiner in a special investigation. If the general meeting of shareholders approves the proposal, we or any shareholder may, within 30 calendar days after the general meeting of shareholders, request a court at our registered office in Geneva, Switzerland to appoint a special examiner. If the general meeting of shareholders rejects the request, one or more shareholders representing at least 10 percent of our share capital or holders of shares in an aggregate par value of at least CHF 2,000,000 may request that the court appoint a special examiner. The court will issue such an order if the petitioners can demonstrate that the board of directors, any member of the board of directors or our executive committee infringed the law or our articles of association and thereby caused damages to the Company or the shareholders. The costs of the investigation would generally be allocated to us and only in exceptional cases to the petitioners.
101
Table of Contents
Compulsory Acquisitions; Appraisal Rights
Business combinations and other transactions that are governed by the Swiss Merger Act (i.e., mergers, demergers, transformations and certain asset transfers) are binding on all shareholders. A statutory merger or demerger requires approval of two-thirds of the shares represented at a general meeting of shareholders and the absolute majority of the par value of the shares represented.
If a transaction under the Swiss Merger Act receives all of the necessary consents, all shareholders are compelled to participate in such transaction.
Swiss corporations may be acquired by an acquirer through the direct acquisition of the shares of the Swiss corporation. The Swiss Merger Act provides for the possibility of a so-called “cash-out” or “squeeze-out” merger with the approval of holders of 90 percent of the issued shares. In these limited circumstances, minority shareholders of the corporation being acquired may be compensated in a form other than through shares of the acquiring corporation (for instance, through cash or securities of a parent corporation of the acquiring corporation or of another corporation). For business combinations effected in the form of a statutory merger or demerger and subject to Swiss law, the Swiss Merger Act provides that if equity rights have not been adequately preserved or compensation payments in the transaction are unreasonable, a shareholder may request the competent court to determine a reasonable amount of compensation.
In addition, under Swiss law, the sale of “all or substantially all of our assets” by us may require the approval of two-thirds of the number of shares represented at a general meeting of shareholders and the absolute majority of the par value of the shares represented. Whether a shareholder resolution is required depends on the particular transaction, including whether the following test is satisfied:
| • | a core part of our business is sold without which it is economically impracticable or unreasonable to continue to operate the remaining business; |
| • | our assets, after the divestment, are not invested in accordance with our corporate purpose as set forth in the articles of association; and |
| • | the proceeds of the divestment are not earmarked for reinvestment in accordance with our corporate purpose but, instead, are intended for distribution to our shareholders or for financial investments unrelated to our corporate purpose. |
A shareholder of a Swiss corporation participating in certain major corporate transactions may, under certain circumstances, be entitled to appraisal rights. As a result, such shareholder may, in addition to the consideration (be it in shares or in cash) receive an additional amount to ensure that the shareholder receives the fair value of the shares held by the shareholder. Following a statutory merger or demerger, pursuant to the Swiss Merger Act, shareholders can file an appraisal action against the surviving company. If the consideration is deemed inadequate, the court will determine an adequate compensation payment.
Board of Directors
Pursuant to Swiss law and according to our articles of association, the board of directors shall consist of at least one member. Swiss law requires that any listed company exceeding two of the three thresholds specified in art. 727 para.1 no. 2 of the CO in two successive financial years shall have each gender represented by at least 30 percent on the board of directors and 20 percent on the executive management team. If a company fails to comply, it must be disclosed in the remuneration report, including an explanation and a designation of measures to be taken to reconcile the failed compliance. For our board of directors, this rule will apply, subject to meeting the thresholds required under the CO, from the business year 2026, whereas for the executive management from the business year 2031. The triggering thresholds are (i) a balance sheet total of 20 million CHF, (ii) sales revenue of 40 million CHF and (iii) an average of 250 full-time employees per year.
The members of our board of directors are elected by the general meeting of shareholders for a term of one year. A year within the meaning of this provision is the period between two ordinary general meetings of shareholders. If a member of the board of directors retires or is replaced, his successor shall continue in office until the end of his predecessor’s term. Each member of our board of directors must be elected individually.
102
Table of Contents
Powers
The board of directors has the following non-delegable and inalienable powers and duties:
| • | the ultimate direction of the business of the Company and issuing of the relevant directives; |
| • | laying down the organization of the Company; |
| • | formulating accounting procedures, financial controls and financial planning; |
| • | nominating and removing persons entrusted with the management and representation of the Company and regulating the power to sign for the Company; |
| • | the ultimate supervision of those persons entrusted with management of the Company, with particular regard to adherence to law, our articles of association, and regulations and directives of the Company; |
| • | issuing the business report and the compensation report, and preparing for the general meeting of shareholders and carrying out its resolutions; and |
| • | informing the court in case of over-indebtedness. |
The board of directors may, while retaining such non-delegable and inalienable powers and duties, delegate some of its powers, in particular direct management, to a single or to several of its members, committees or to third parties (such as executive officers) who need be neither members of the board of directors nor shareholders. Pursuant to Swiss law and our articles of association, details of the delegation and other procedural rules such as quorum requirements have been set in the organizational rules established by the board of directors.
Indemnification of Executive Officers and Directors
We continuously seek to maintain appropriate and cost-effective liability insurance coverage in connection with our products and for purposes of indemnifying our directors and officers for claims against them. In addition, under general principles of Swiss employment law, an employer may be required to indemnify an employee against losses and expenses incurred by such employee in the proper execution of his or her duties under the employment agreement with the employer. See “Differences in Corporate Law—Indemnification of Directors and Executive Management and Limitation of Liability.”
Conflict of Interest
Swiss law does not have a general provision regarding conflicts of interest. However, the CO contains a provision that requires our directors and executive officers to safeguard the Company’s interests and imposes a duty of loyalty and duty of care on our directors and executive officers. This rule is generally understood to disqualify directors and executive officers from participation in decisions that directly affect them. Our directors and executive officers are personally liable to us for breaches of these obligations. In addition, Swiss law contains provisions under which directors and all persons engaged in the Company’s management are liable to the Company, each shareholder and the Company’s creditors for damages caused by an intentional or negligent violation of their duties. Furthermore, Swiss law contains a provision under which payments made to any of the Company’s shareholders or directors or any person related to any such shareholder or director, other than payments made at arm’s length, must be repaid to the Company if such shareholder or director acted in bad faith.
Our board of directors has adopted a Code of Business Conduct and Ethics and will adopt, upon the closing of this offering, other policies that will cover a broad range of matters, including the handling of conflicts of interest.
Principles of the Compensation of the Board of Directors
Pursuant to Swiss law, our shareholders must annually, upon becoming a public company whose shares are listed for trade by the public, approve the compensation of the board of directors and the persons whom the board of directors has, fully or partially, entrusted with the management of the Company. The board of directors must issue, on an annual basis, a written compensation report that must be reviewed together with a report on our business by our auditor. The compensation report must disclose all compensation, loans and other forms of indebtedness granted by the Company, directly or indirectly, to current or former members of the board of directors and executive management to the extent related to their former role within the Company or not on customary market terms.
103
Table of Contents
The disclosure concerning compensation, loans and other forms of indebtedness must include the aggregate amount for the board of directors and the executive management as well as the particular amount for each member of the board of directors and executive officer, specifying the name and function of each respective person.
Certain forms of compensation are prohibited for members of our board of directors and executive management, such as:
| • | severance payments provided for either contractually or in the articles of association (compensation due until the termination of a contractual relationship does not qualify as severance payment); |
| • | advance compensation; |
| • | incentive fees for the acquisition or transfer of corporations or parts thereof by the Company or by companies being, directly or indirectly, controlled by us; |
| • | loans, other forms of indebtedness, pension benefits not based on occupational pension schemes and performance-based compensation not provided for in the articles of association; and |
| • | equity securities and conversion and option rights awards not provided for in the articles of association. |
Compensation to members of the board of directors and executive management for activities in entities that are, directly or indirectly, controlled by the Company is prohibited if the compensation (i) would have been prohibited if it was paid directly by the Company, (ii) is not provided for in the articles of association or (iii) has not been approved by the general meeting of shareholders.
The general meeting of shareholders votes on the compensation received directly or indirectly by the board of directors, the executive management and the advisory board. The general meeting of shareholders must vote annually on the compensation of its board of directors, executive management and the advisory board, and accordingly, at such a meeting, the vote of the general meeting of shareholders shall have a binding effect.
In the event that the general meeting of shareholders votes prospectively on the compensation of the executive management, the articles of association may provide for an additional amount for the compensation of the members of the executive management appointed after the vote.
The additional amount may only be used if the total amount of the compensation of the executive management decided by the general meeting of shareholders is not sufficient for the compensation of the new members until the next vote of the general meeting of the shareholders.
The general meeting of shareholders shall not vote on the additional amount of compensation.
Borrowing Powers
Neither Swiss law nor our articles of association restrict in any way our power to borrow and raise funds. The decision to borrow funds is made by or under the direction of our board of directors, and no approval by the shareholders is required in relation to any such borrowing.
Repurchases of Shares and Purchases of Own Shares and Other Limitations on the Right to Own Shares
The CO limits our right to purchase and hold our own shares. We and our subsidiaries may purchase shares only if and to the extent that (i) freely disposable equity capital is available in the required amount; and (ii) the combined nominal value of all such shares does not exceed 10 percent of the share capital. Pursuant to Swiss law, where shares are acquired in connection with a transfer restriction set out in the articles of association, the foregoing upper limit is 20 percent. We currently do not have any transfer restriction in our articles of association. If we own shares that exceed the threshold of 10 percent of our share capital, the excess must be sold or cancelled by means of a capital reduction within a reasonable time.
Shares held by us or our subsidiaries are not entitled to vote at the general meeting of shareholders but are entitled to the economic benefits applicable to the shares generally, including dividends and pre-emptive rights in the case of share capital increases.
Swiss law and/or our articles of association do not impose any restrictions on the exercise of voting or any other shareholder rights by shareholders residing outside of Switzerland.
104
Table of Contents
Notification and Disclosure of Substantial Share Interests, Opting Out of Mandatory Tender Offer
The Swiss Federal Financial Market Infrastructure Act, as amended from time to time, (FMIA) requires persons who directly, indirectly or in concert with other parties acquire or dispose of listed shares of a Swiss company or purchase or sale rights or obligations relating to the listed shares, and, thereby, directly, indirectly or in concert with other parties reach, exceed or fall below a threshold of 3, 5, 10, 15, 20, 25, 331/3, 50 or 662/3 percent of the company’s voting rights (whether exercisable or not) to notify the company and SIX Exchange Regulation AG of such acquisition or disposal in writing within four trading days. Within two trading days after the receipt of such notification, the company must publish such information through SIX Exchange Regulation AG’s electronic reporting and publishing platform.
As of the date of this Prospectus, our articles of association contain an opting-out provision. Hence, a purchaser of shares in the Company is not obliged to make a public tender offer in accordance with the provisions of the FMIA.
Pursuant to Article 663c of the CO, Swiss corporations whose shares are listed on a stock exchange must specify the significant shareholders and their shareholdings in the notes to the balance sheet, where these are known or ought to be known. Significant shareholders are defined as shareholders and groups of shareholders linked through voting rights who own more than five percent of all voting rights.
Changes to Swiss Corporate Law
On January 1, 2023, new amendments to Swiss corporate law came into force. These changes were geared towards greater flexibility for share capital and equity distributions, enhancement of shareholders’ rights in terms of better corporate governance, and modernization of shareholders’ meetings. Companies have a two-year transition period in order to adapt these regulations.
Share Capital and Nominal Value
Under the new amendment, existing companies may state their share capital to a currency other than Swiss Francs at the end of a business year, provided that (i) the chosen currency is the most relevant currency to the company’s activities, (ii) the share capital in the foreign currency corresponds to an equivalent value of at least CHF 100,000, (iii) the accounting and financial reporting are done in the same currency, and (iv) the foreign currency is one in which the Swiss Federal Council has declared as suitable for the purpose (namely the Swiss Franc, U.S. Dollar, Euro, British Pound and Japanese Yen). Further, the nominal value of shares may be set below the current minimum of CHF 0.01 to any amount higher than zero.
Intended Acquisition of Assets and Repayment Obligations
Currently, in the case of an anticipated acquisition of assets (beabsichtigte Sachübernahme), where a company intends or undertakes to acquire certain assets soon after incorporation or a capital increase, current Swiss law imposes a qualified majority for the shareholders’ meeting and strict transparency rules. This has led to questions regarding when an acquisition meets the thresholds of a relevant acquisition of assets. The amendments have removed these rules; however, transparency for acquisition of assets remain in place, and the new law provides for a repayment obligation in case of an unfair advantage or disadvantage.
Where an acquisition of assets is made in connection with a contribution in kind or another manner in which a shareholder contributes in kind where the value of the contributed asset exceeds the shareholder’s capital contribution obligation and, in consideration, the company grants another consideration in return to the shareholder on top of issuing shares. In such cases, the respective consideration must be disclosed in the articles of association and in the commercial register.
Participation Capital
The amendments will allow listed companies limited by shares to provide for participation capital (non-voting stock) up to ten times the amount of she share capital. Furthermore, the amendment distinguishes between shareholders and holders of participation capital when it comes to calculating the thresholds necessary to reach a quorum for certain decisions. By way of example, under the new amendments, shareholders of listed companies can initiate a special investigation if they hold at least five percent of the share capital or voting rights, even if the share capital does not exceed the participation capital.
105
Table of Contents
Arbitration
A company’s articles of association may contain an arbitration clause regarding corporate disputes, which is binding on all parties, including the company and its shareholders. The seat of the arbitration tribunal must be in Switzerland.
Virtual Shareholders Meetings
Swiss companies may now hold virtual shareholders meetings, provided that the possibility of virtual shareholders meetings is spelled out in the company’s articles of association.
Stock Exchange Listing
Our ordinary shares are listed on the SIX Swiss Exchange under the symbol “RLF” and over the counter in the U.S. under the symbol “RLFTF.” Our ADSs trade in the U.S. over-the-counter market under the symbol “RLFTY.”
106
Table of Contents
COMPARISON OF SHAREHOLDER RIGHTS
The Swiss laws applicable to Swiss corporations and their shareholders differ from laws applicable to U.S. corporations and their shareholders. The following table summarizes significant differences in shareholder rights between the applicable provisions of the CO and the Compensation Ordinance (as a company incorporated in Switzerland and listed on a stock exchange) and the Delaware General Corporation Law, applicable to companies incorporated in Delaware and their shareholders. Please note that this is only a general summary of certain provisions applicable to companies in Delaware. Certain Delaware companies may be permitted to exclude certain of the provisions summarized below in their charter documents.
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Mergers and similar arrangements | ||
| Under the Delaware General Corporation Law, with certain exceptions, a merger, consolidation, sale, lease or transfer of all or substantially all of the assets of a corporation must be approved by the board of directors and a majority of the outstanding shares entitled to vote thereon. A shareholder of a Delaware corporation participating in certain major corporate transactions may, under certain circumstances, be entitled to appraisal rights pursuant to which such shareholder may receive cash in the amount of the fair value of the shares held by such shareholder (as determined by a court) in lieu of the consideration such shareholder would otherwise receive in the transaction. The Delaware General Corporation Law also provides that a parent corporation, by resolution of its board of directors, may merge with any subsidiary of which it owns at least 90.0% of each class of capital stock without a vote by the shareholders of such subsidiary. Upon any such merger, dissenting shareholders of the subsidiary would have appraisal rights. | Under Swiss law, with certain exceptions, a merger or a division of the corporation or a sale of all or substantially all of the assets of a corporation must be approved by two-thirds of the shares represented at the respective general meeting of shareholders as well as the absolute majority of the share capital represented at such shareholders’ meeting. The articles of association may increase the voting threshold. A shareholder of a Swiss corporation participating in a statutory merger or demerger pursuant to the Swiss Merger Act can file an appraisal right lawsuit against the surviving company. As a result, if the consideration is deemed “inadequate,” such shareholder may, in addition to the consideration (be it in shares or in cash) receive an additional amount to ensure that such shareholder receives the fair value of the shares held by such shareholder. Swiss law also provides that a parent corporation, by resolution of its board of directors, may merge with any subsidiary of which it owns at least 90.0% of the shares without a vote by shareholders of such subsidiary, if the shareholders of the subsidiary are offered the payment of the fair value in cash as an alternative to shares. | |
| Shareholder Lawsuits | ||
| Class actions and derivative actions generally are available to shareholders of a Delaware corporation for, among other things, breach of fiduciary duty, corporate waste and actions not taken in accordance with applicable law. In such actions, the court has discretion to permit the winning party to recover attorneys’ fees incurred in connection with such action. | Class actions and derivative actions as such are not available under Swiss law. Nevertheless, certain actions may have a similar effect. A shareholder is entitled to bring suit against directors for breach of, among other things, their fiduciary duties and claim the payment of the company’s damages to the corporation. Likewise, an appraisal lawsuit won by a shareholder will indirectly compensate all shareholders. Under Swiss law, the winning party is generally entitled to recover attorneys’ fees incurred in connection with such action, provided, however, that the court has discretion to permit the shareholder whose claim has been dismissed to recover attorneys’ fees incurred to the extent he acted in good faith. | |
107
Table of Contents
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Shareholder Vote on Board and Management Compensation | ||
| Under the Delaware General Corporation Law, the board of directors has the authority to fix the compensation of directors, unless otherwise restricted by the certificate of incorporation or bylaws. | Pursuant to the Compensation Ordinance, applicable to Swiss companies traded on exchanges, such as Nasdaq, the general meeting of shareholders has the non-transferable right, amongst others, to vote separately (in a binding vote) on the aggregate compensation due to the board of directors, executive management and advisory boards. | |
| Annual vote on Board Renewal | ||
| Unless otherwise specified in the certificate of incorporation or bylaws, the annual meeting of shareholders elects annually (i.e., until the next annual meeting), by a plurality of votes, the shareholders on a date and at a time designated by or in the manner provided members of the board of directors (including the chairman) and the in the bylaws. Re-election is possible. | The general meeting of shareholders elects annually (i.e., until the following general meeting of shareholders), by a plurality of votes, the members of the board of directors (including the chairman) and the members of the compensation committee individually for a term of office of one year. Re-election is possible. | |
| Indemnification of Directors and Executive Management and Limitation of Liability | ||
| The Delaware General Corporation Law provides that a certificate of incorporation may contain a provision eliminating or limiting the personal liability of directors, officers, employees or agents of the corporation for monetary damages for breach of a fiduciary duty as a director, except no provision in the certificate of incorporation may eliminate or limit the liability of a director for:
• any breach of a director’s duty of loyalty to the corporation or its shareholders;
• acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
• statutory liability for unlawful payment of dividends or unlawful share purchase or redemption; or
• any transaction from which the director derived an improper personal benefit. |
Under Swiss corporate law, an indemnification of a director or member of the executive management in relation to potential personal liability is not effective to the extent the director or member of the executive management intentionally or negligently violated his or her corporate duties towards the corporation (certain views advocate that at least a grossly negligent violation is required to exclude the indemnification). Most violations of corporate law are regarded as violations of duties towards the corporation rather than towards the shareholders. In addition, indemnification of other controlling persons is not permitted under Swiss corporate law, including shareholders of the corporation.
Nevertheless, a corporation may obtain and pay for directors’ and officers’ liability insurance, which typically covers negligent acts as well. | |
108
Table of Contents
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Indemnification of Directors and Executive Management and Limitation | ||
| A Delaware corporation may indemnify any person who was or is a party or is threatened to be made a party to any proceeding, other than an action by or on behalf of the corporation, because the person is or was a director or officer, against liability incurred in connection with the proceeding if the director or officer acted in good faith and in a manner reasonably believed to be in, or not opposed to, the best interests of the corporation; and the director or officer, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Unless ordered by a court, any foregoing indemnification is subject to a determination that the director or officer has met the applicable standard of conduct:
• by a majority vote of the directors who are not parties to the proceeding, even though less than a quorum;
• by a committee of directors designated by a majority vote of the eligible directors, even though less than a quorum;
• by independent legal counsel in a written opinion if there are no eligible directors, or if the eligible directors so direct; or by the shareholders. |
||
| Moreover, a Delaware corporation may not indemnify a director or officer in connection with any proceeding in which the director or officer has been adjudged to be liable to the corporation unless and only to the extent that the court determines that, despite the adjudication of liability but in view of all the circumstances of the case, the director or officer is fairly and reasonably entitled to indemnity for those expenses which the court deems proper. | ||
109
Table of Contents
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Directors’ Fiduciary Duties | ||
| A director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty.
The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties.
Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the transaction, and that the transaction was of fair value to the corporation. |
A director of a Swiss corporation has a fiduciary duty to the corporation only. This duty has two components: the duty of care and the duty of loyalty.
The duty of care requires that a director act in good faith, with the care that an ordinarily prudent director would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose, all material information reasonably available regarding a significant transaction.
The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interest of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits in principle self-dealing by a director and mandates that the best interest of the corporation take precedence over any interest possessed by a director or officer.
The burden of proof for a violation of these duties is with the corporation or with the shareholder bringing a suit against the director.
Directors also have an obligation to treat shareholders equally proportionate to their share ownership. | |
110
Table of Contents
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Shareholder Action by Written Consent | ||
| A shareholder of a Delaware corporation has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings. | At any general meeting of shareholders any shareholder may put proposals to the meeting if the proposal is part of an agenda item. Unless the articles of association provide for a lower threshold or for additional shareholders’ rights:
• one or several shareholders representing 10.0% of the share capital may ask that a general meeting of shareholders be called for specific agenda items and specific proposals; and
• one or several shareholders representing 10.0% of the share capital or CHF 1.0 million of nominal share capital may ask that an agenda item including a specific proposal be put on the agenda for a regularly scheduled general meeting of shareholders, provided such request is made with appropriate notice.
Any shareholder can propose candidates for election as directors without prior written notice. In addition, any shareholder is entitled, at a general meeting of shareholders and without advance notice, to (i) request information from the Board on the affairs of the company (note, however, that the right to obtain such information is limited), (ii) request information from the auditors on the methods and results of their audit and (iii) request, under certain circumstances and subject to certain conditions, a special audit. | |
| Cumulative Voting | ||
| Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation provides for it. | A Swiss corporation may remove, with or without cause, any director at any time with a resolution passed by an absolute majority of the shares represented at a general meeting of shareholders. The articles of association may provide for a qualified majority for the removal of a director. | |
111
Table of Contents
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Removal of Directors | ||
| Unless there is cumulative voting or there is a classified board, generally a director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors | A Swiss corporation may remove, with or without cause, any director at any time with a resolution passed by an absolute majority of the shares represented at a general meeting of shareholders. The articles of association may provide for a qualified majority for the removal of a director. | |
| Transactions with Interested Shareholders | ||
| The Delaware General Corporation Law generally prohibits a Delaware corporation from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or group who or which owns or owned 15.0% or more of the corporation’s outstanding voting shares within the past three years. | No such rule applies to a Swiss corporation. | |
| Variation of Rights of Shares | ||
| A Delaware corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. | The general meeting of shareholders of a Swiss corporation may resolve that preference shares be issued or that existing shares be converted into preference shares with a resolution passed by a majority of the shares represented at the general meeting of shareholders. Where a company has issued preference shares, further preference shares conferring preferential rights over the existing preference shares may be issued only with the consent of both a special meeting of the adversely affected holders of the existing preference shares and of a general meeting of all shareholders, unless otherwise provided in the articles of association.
Shares that are granted more voting power are not regarded as a special class for these purposes. | |
112
Table of Contents
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Amendment of Governing Documents | ||
| A Delaware corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. | By way of a public deed, the articles of association of a Swiss corporation may be amended with a resolution passed by an absolute majority of the shares represented at such meeting, unless otherwise provided in the articles of association. There are a number of resolutions, such as an amendment of the stated purpose of the corporation and the introduction of authorized and conditional capital, that require the approval by two-thirds of the votes and an absolute majority of the nominal value of the shares represented at a shareholders’ meeting. The articles of association may increase the voting thresholds. | |
| Inspection of Books and Records | ||
| Shareholders of a Delaware corporation, upon written demand under oath stating the purpose thereof, have the right during the usual hours for business to inspect for any proper purpose, and to obtain copies of list(s) of shareholders and other books and records of the corporation and its subsidiaries, if any, to the extent the books and records of such subsidiaries are available to the corporation. | Shareholders of a Swiss corporation may only inspect books and records if the general meeting of shareholders or the board of directors approved such inspection. The inspection right is limited in scope and only extends to information required for the exercise of shareholder rights and does not extend to confidential information. The right to inspect the share register is limited to the right to inspect that shareholder’s own entry in the share register. | |
| Payment of Dividends | ||
| The board of directors may approve a dividend without shareholder approval. Subject to any restrictions contained in its certificate of incorporation, the board may declare and pay dividends upon the shares of its capital stock either:
• out of its surplus, or
• in case there is no such surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year. |
Dividend payments are subject to the approval of the general meeting of shareholders. The board of directors may propose to shareholders that a dividend shall be paid but cannot itself authorize the distribution.
Payments out of the Company’s share capital (in other words, the aggregate nominal value of the Company’s registered share capital) in the form of dividends are not allowed and may be made by way of a capital reduction only. Dividends may be paid only from the profits brought forward from the previous business years or if the Company has distributable reserves, each as will be presented on the Company’s audited annual stand-alone balance sheet. The dividend may be determined only after the allocations to reserves required by the law and the articles of association have been deducted. | |
113
Table of Contents
| DELAWARE CORPORATE LAW |
SWISS CORPORATE LAW | |
| Creation and Issuance of New Shares | ||
| Shareholder approval is required to authorize capital stock in excess of that provided in the charter. The corporation must file a certificate of amendment to its certificate of incorporation before the creation of additional authorized shares may become effective.
The board of directors may, without shareholder consent, authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation, or any combination thereof. |
All creation of shares requires a shareholders’ resolution documented by way of a public deed. The creation of authorized or conditional share capital requires at least two-thirds of the voting rights represented at the general meeting of shareholders and an absolute majority of the nominal value of shares represented at such meeting. The board of directors may issue shares out of the authorized share capital during a period of up to two years. Shares are created and issued out of conditional share capital through the exercise of options or of conversion rights that the board of directors may grant in relation to, e.g., debt instruments or to employees. | |
114
Table of Contents
| C. | MATERIAL CONTRACTS |
Except as otherwise disclosed in this annual report on Form 20-F (including the exhibits hereto), we are not currently, and have not been in the past two years, party to any material contract, other than contracts entered into in the ordinary course of business.
| D. | EXCHANGE CONTROLS |
There are no Swiss governmental laws, decrees or regulations that restrict, in a manner material to us, the export or import of capital, including any foreign exchange controls, or that generally affect the remittance of dividends or other payments to non-residents or non-citizens of Switzerland who hold our ordinary shares.
| E. | TAXATION |
The following summary contains a description of the material Swiss and U.S. federal income tax consequences of the acquisition, ownership and disposition of ordinary shares, but it does not purport to be a comprehensive description of all the tax considerations that may be relevant to a decision to purchase ordinary shares. The summary is based upon the tax laws of Switzerland and regulations thereunder and on the tax laws of the U.S. and regulations thereunder as of the date hereof, which are subject to change.
Swiss tax considerations
This summary of material Swiss tax consequences is based on Swiss law and regulations and the practice of the Swiss tax administration as in effect on the date hereof, all of which are subject to change (or subject to changes in interpretation), possibly with retroactive effect. The summary does not purport to consider the specific circumstances of any particular shareholder or potential investor and does not relate to persons in the business of buying and selling ordinary shares or other securities. The summary is not intended to be, and should not be interpreted as, legal or tax advice to any particular potential shareholder, and no representation with respect to the tax consequences to any particular shareholder is made.
Current and prospective shareholders are advised to consult their own tax advisors in light of their particular circumstances as to the Swiss tax laws, regulations and regulatory practices that could be relevant to them in connection with the acquiring, owning and selling or otherwise disposing of ordinary shares and receiving dividends and similar cash or in-kind distributions on ordinary shares (including dividends on liquidation proceeds and stock dividends) or distributions on ordinary shares based upon a capital reduction (remboursements de la valeur nominale) or reserves paid out of capital contributions (réserves issues d’apports en capital) and the consequences thereof under the tax laws, regulations and regulatory practices of Switzerland.
Taxation
We are, through Relief, subject to corporate Swiss federal, cantonal and communal taxation in Switzerland, Canton of Geneva, City of Geneva, respectively, and through APR, we are subject to corporate Swiss federal, cantonal and communal taxation in Switzerland, Canton of Ticino, City of Balerna.
We are entitled under Swiss laws to carry forward any losses incurred for a period of seven years and can offset our losses carried forward against future taxable profit. As of December 31, 2022, we had consolidated tax loss carry-forwards totaling CHF 145 million. There is no certainty that we will make sufficient profits to be able to utilize these tax loss carry-forwards in full.
The effective corporate income tax rate (federal, cantonal and communal) where we are domiciled in the Canton of Geneva is currently 13.99 percent and is 18.47 percent in the Canton of Ticino.
Federal, cantonal and communal individual income tax and corporate income tax
Non-resident shareholders
Shareholders who are not resident in Switzerland for tax purposes, and who, during the relevant taxation year, have not engaged in a trade or business carried on through a permanent establishment or fixed place of business situated in Switzerland for tax purposes (all such shareholders for purposes of this section termed, “Non-resident shareholders”), will not be subject to any Swiss federal, cantonal and communal income tax on dividends and similar cash or in-kind distributions on Shares (including liquidation proceeds and stock dividends) (for the purposes of this section, “dividends”), distributions based upon a capital reduction capped to the nominal value (e.g. réduction de la valeur nominale des actions), and distributions paid out of reserves from capital contributions (réserves issues d’apports en capital) on shares, or capital gains realized on the sale or other disposition of shares (see, however, “Swiss federal withholding tax” below for a summary of Swiss federal withholding tax on dividends.)
115
Table of Contents
Resident private shareholders
Swiss-resident individuals who hold their shares as private assets are required to include dividends, but not distributions based upon a capital reduction capped to the nominal value (e.g. réduction de la valeur nominale des actions), and distributions paid out of reserves from capital contributions (réserves issues d’apports en capital), in their personal income tax return and are subject to Swiss federal, cantonal and communal income tax on any net taxable income for the relevant taxation period, including the dividends, but not the distributions based upon a capital reduction capped to the nominal value (e.g. réduction de la valeur nominale des actions), and distributions paid out of reserves from capital contributions (réserves issues d’apports en capital). Shareholders holding at least 10 percent of the share capital of the Company may be able to deduct their taxable dividends at 30 percent at the federal level and up to 50 percent at the cantonal level, depending on their respective cantonal rates, as partial relief from economic double taxation. Capital gains resulting from the sale or other disposition of shares are, subject to a few exceptions, not subject to Swiss federal, cantonal and communal income tax, and conversely, capital losses are not tax-deductible for resident private shareholders (the shareholders referred to in this paragraph for the purposes of this section, “Resident private shareholders”). See “Domestic commercial shareholders” below for a summary of the taxation treatment applicable to Swiss-resident individuals, who, for income tax purposes, are classified as “professional securities dealers” or are otherwise deemed to hold Company shares in their commercial wealth.
Domestic commercial shareholders
Corporate and individual shareholders who are resident in Switzerland for tax purposes, and corporate and individual shareholders who are not resident in Switzerland, and who, in each case, hold their shares as part of a trade or business carried on in Switzerland, in the case of corporate and individual shareholders not resident in Switzerland, through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, are required to recognize dividends, distributions based upon a capital reduction capped to the nominal value (e.g. réduction de la valeur nominale des actions) and distributions paid out of reserves from capital contributions (réserves issues d’apports en capital) received on shares and capital gains or losses realized on the sale or other disposition of shares in their income statement for the relevant taxation period and are subject to Swiss federal, cantonal and communal individual or corporate income tax, as the case may be, on any net taxable earnings for such taxation period. The same taxation treatment also applies to Swiss-resident private individuals who, for income tax purposes, are classified as “professional securities dealers” for reasons of, inter alia, frequent dealing, or leveraged investments, in shares and other securities (the shareholders referred to in this paragraph for purposes of this section, “Domestic commercial shareholders”). Domestic commercial shareholders who are corporate taxpayers may be eligible for tax relief (réduction pour participations) in respect of dividends and distributions based upon a capital reduction capped to the nominal value (e.g. réduction de la valeur nominale des actions), and distributions paid out of reserves from capital contributions (réserves issues d’apports en capital), as well as capital gains on sales of shares, if the shares held by them as part of a Swiss business have an aggregate market value of at least CHF 1 million or represent 10 percent or more of the outstanding share capital of the Company (in the case of capital gains, if the shares have been held for at least one year).
Swiss cantonal and communal private wealth tax and capital tax
Non-resident shareholders
Non-resident shareholders are not subject to Swiss cantonal and communal private wealth tax or capital tax.
Resident private shareholders and domestic commercial shareholders
Resident private shareholders and domestic commercial shareholders who are individuals are required to report their shares as part of their private wealth or their Swiss business assets, as the case may be, and will be subject to Swiss cantonal and communal private wealth tax on any net taxable wealth (including shares), in the case of domestic commercial shareholders to the extent the aggregate taxable wealth is allocable to Switzerland. Domestic commercial shareholders who are corporate taxpayers are subject to Swiss cantonal and communal capital tax on taxable capital to the extent the aggregate taxable capital is allocable to Switzerland.
Swiss federal withholding tax
Dividends that the Company pays on the shares are subject to Swiss Federal withholding tax (impôt anticipé) at a rate of 35 percent on the gross amount of the dividend. The Company is required to withhold the Swiss federal withholding tax from the dividend and remit it to the Swiss Federal Tax Administration. Distributions based upon a capital reduction capped to the nominal value (e.g. réduction de la valeur nominale des actions) and distributions paid out of reserves from contributions (réserves issues d’apports en capital) are not subject to Swiss federal withholding tax.
116
Table of Contents
The Swiss federal withholding tax on a dividend will be refundable in full to a resident private shareholder and to a domestic commercial shareholder, who, in each case, inter alia, as a condition to a refund, duly reports the dividend in his individual income tax return as income or recognizes the dividend in his income statement as earnings, as applicable.
A Non-resident shareholder may be entitled to a partial or full refund, as the case may be, of the Swiss federal withholding tax on a dividend if the country of his or her residence for tax purposes has entered into an international treaty for the avoidance of double taxation with Switzerland and the conditions of such treaty are met. Such shareholders should be aware that the procedures for claiming treaty benefits (and the time required for obtaining a refund) might differ from country to country. For example, a shareholder who is a resident of the U.S. for the purposes of the bilateral tax treaty between the U.S. and Switzerland is eligible for a partial refund of the amount of the withholding tax in excess of the 15 percent treaty rate, provided such shareholder: (i) qualifies for benefits under this treaty and qualifies as beneficial owner of the dividends; (ii) holds, directly or indirectly, less than 10 percent of the voting stock of the Company; (iii) does not qualify as a pension scheme or retirement arrangement for the purpose of the bilateral treaty; and (iv) does not conduct business through a permanent establishment or fixed base in Switzerland to which the shares are attributable. Such an eligible U.S. shareholder may apply for a refund of the amount of the withholding tax in excess of the 15 percent treaty rate. The applicable refund request form may be filed with the Swiss Federal Tax Administration following receipt of the dividend and the relevant deduction certificate, however no later than 31 December of the third year following the calendar year in which the dividend was payable.
Swiss federal stamp taxes
The Company will be subject to and pay to the Swiss Federal Tax Administration a one percent Swiss federal issuance stamp duty (droit de timbre d’émissions) on the consideration received for the issuance of the shares less certain costs incurred in connection with the issuance, where the share capital increase exceeds the nominal value of the shares. The issuance and delivery of the shares to the initial shareholders at the offering price is not subject to Swiss federal securities transfer stamp duty (droit de timbre de négociation).
Any subsequent dealings in the shares, for which a bank or another securities dealer in Switzerland, as defined in the Swiss Federal Stamp Tax Act, acts as an intermediary, or is a party, to the transaction, are, subject to certain exemptions provided for in the Swiss Federal Stamp Tax Act, subject to Swiss securities transfer stamp duty tax at an aggregate tax rate of up to 0.15 percent of the consideration paid for such shares.
Material U.S. Federal Income Tax Considerations for U.S. Holders
The following summary of the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our ordinary shares or ADRs is based upon current law and does not purport to be a comprehensive discussion of all the tax considerations that may be relevant to a decision to purchase our ordinary shares or ADRs. This summary is based on current provisions of the Internal Revenue Code of 1986, as amended, or the Code, existing, final, temporary and proposed U.S. Treasury Regulations, administrative rulings and judicial decisions, in each case as available on the date of this annual report. All of the foregoing are subject to change, which change could apply retroactively and could affect the tax consequences described below.
This section summarizes the material U.S. federal income tax consequences to U.S. holders and certain non-U.S. holders, each as defined below, of our ordinary shares or ADRs. This summary addresses only the U.S. federal income tax considerations for holders that acquire our ordinary shares or ADRs at their original issuance and hold our ordinary shares or ADRs as capital assets. This summary does not address all U.S. federal income tax matters that may be relevant to a particular holder. Each prospective investor should consult a professional tax advisor with respect to the tax consequences of the acquisition, ownership or disposition of our ordinary shares or ADRs. This summary does not address tax considerations applicable to a holder of our ordinary shares or ADRs that may be subject to special tax rules including, without limitation, the following:
| • | banks or other financial institutions; |
| • | insurance companies; |
| • | dealers or traders in securities, currencies, or notional principal contracts; |
| • | tax-exempt entities, including an “individual retirement account” or “Roth IRA” retirement plan; |
117
Table of Contents
| • | regulated investment companies or real estate investment trusts; |
| • | “qualified foreign pension funds,” or entities wholly owned by a “qualified foreign pension fund”; |
| • | persons who have elected to mark securities to market; |
| • | persons that hold the ordinary shares as part of a hedge, straddle, conversion, constructive sale or similar transaction involving more than one position; |
| • | holders (whether individuals, corporations or partnerships) that are treated as expatriates for some or all U.S. federal income tax purposes; |
| • | persons who acquired the ADRs as compensation for the performance of services; |
| • | persons holding the ADRs in connection with a trade or business conducted outside of the U.S.; |
| • | holders that own (or are deemed to own) 10 percent or more of our ordinary shares or ADRs, by vote or value; and |
| • | holders that have a “functional currency” other than the U.S. dollar. |
Further, this summary does not address any aspects of any U.S. state, local or non-U.S. tax law, alternative minimum tax, gift or estate consequences, the rules regarding qualified small business stock within the meaning of Section 1202 of the Code, any election to apply Section 1400Z-2 of the Code to gains recognized with respect to our ordinary shares, any other U.S. federal tax other than the income tax or the indirect effects on the holders of equity interests in entities that own our ordinary shares or ADRs.
For the purposes of this summary, a “U.S. holder” is a beneficial owner of ordinary shares or ADRs that is (or is treated as), for U.S. federal income tax purposes:
| • | an individual who is either a citizen or resident of the U.S.; |
| • | a corporation, or other entity that is treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the U.S. or any state thereof or of the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust, if a court in the U.S. is able to exercise primary supervision over its administration and one or more U.S. persons has the authority to control all of the substantial decisions of such trust or has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person. |
If a partnership holds ordinary shares or ADRs, the tax treatment of a partner will generally depend upon the status of the partner and upon the activities of the partnership. This discussion does not address the tax treatment of partnerships or other entities that are pass-through entities for U.S. federal income tax purposes or persons that hold their ordinary shares or ADRs through partnerships or other pass-through entities. A partner in a partnership or other pass-through entity that will hold our ordinary shares or ADRs should consult his, her or its tax advisor regarding the tax consequences of acquiring, holding and disposing of our ordinary shares or ADRs through a partnership or other pass-through entity, as applicable.
We will not seek a ruling from the U.S. Internal Revenue Service, or IRS, with regard to the U.S. federal income tax treatment of an investment in our ordinary shares or ADRs, and we cannot assure you that the IRS will agree with the conclusions set forth below.
PERSONS CONSIDERING AN INVESTMENT IN OUR ORDINARY SHARES OR ADSs SHOULD CONSULT THEIR TAX ADVISORS AS TO THE PARTICULAR TAX CONSEQUENCES APPLICABLE TO THE ACQUISITION, OWNERSHIP AND DISPOSITION OF THE ORDINARY SHARES OR ADRs, INCLUDING THE APPLICABILITY OF U.S. FEDERAL, STATE AND LOCAL TAXES.
118
Table of Contents
Ownership of ADRs
For U.S. federal income tax purposes, a holder of ADRs will generally be treated as the owner of the ordinary shares represented by such ADRs. Gain or loss will generally not be recognized on account of exchanges of ordinary shares for ADRs, or of ADRs for ordinary shares. References to ordinary shares in this discussion are deemed to include ADRs, unless context otherwise required.
Taxation of distributions
As discussed above, we do not currently expect to make distributions on our ordinary shares. In the event that we do make distributions of cash or other property, subject to the PFIC rules described below, distributions paid on ordinary shares, other than certain pro rata distributions of ordinary shares, will generally be treated as dividends to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Because we do not maintain calculations of our earnings and profits under U.S. federal income tax principles, we expect that distributions generally will be reported to U.S. holders as dividends. For so long as our ordinary shares are listed on Nasdaq or we are eligible for benefits under the Treaty, dividends paid to certain non-corporate U.S. holders will be eligible for taxation as “qualified dividend income” and therefore, subject to applicable limitations, will be taxable at rates not in excess of the long-term capital gain rate applicable to such U.S. holder.
U.S. holders should consult their tax advisors regarding the availability of the reduced tax rate on dividends in their particular circumstances. The amount of a dividend will include any amounts withheld by us in respect of Swiss income taxes. The amount of the dividend will be treated as foreign-source dividend income to U.S. holders and will not be eligible for the dividends-received deduction generally available to U.S. corporations under the Code. Dividends will be included in a U.S. holder’s income on the date of the U.S. holder’s actual or constructive receipt of the dividend. The amount of any dividend income paid in Swiss Francs will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of actual or constructive receipt, regardless of whether the payment is in fact converted into U.S. dollars at that time. If the dividend is converted into U.S. dollars on the date of actual or constructive receipt, a U.S. holder should not be required to recognize foreign currency gain or loss in respect of the dividend income. A U.S. holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of actual or constructive receipt. Such gain or loss would generally be treated as U.S.-source ordinary income or loss.
Subject to certain significant conditions, some of which vary depending upon the U.S. holder’s particular circumstances, Swiss income taxes withheld from dividends on ordinary shares at a rate not exceeding the rate provided by the U.S.–Swiss Treaty and not refundable to a U.S. holder may be creditable against the U.S. holder’s U.S. federal income tax liability. Swiss taxes withheld in excess of the rate applicable under the U.S.-Swiss Treaty will not be eligible for credit against a U.S. holder’s federal income tax liability. The rules governing foreign tax credits are complex and U.S. holders should consult their tax advisors regarding the creditability of foreign taxes in their particular circumstances. In lieu of claiming a foreign tax credit, U.S. holders may, at their election, deduct foreign taxes, including any Swiss income tax, in computing their taxable income, subject to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year.
Sale or other disposition of ordinary shares
Subject to the PFIC rules described below, gain or loss realized on the sale or other disposition of ordinary shares will be capital gain or loss and will be long-term capital gain or loss if the U.S. holder held the ordinary shares for more than 1 year. The amount of the gain or loss will equal the difference between the U.S. holder’s tax basis in the ordinary shares disposed of and the amount realized on the disposition, in each case as determined in U.S. dollars. This gain or loss will generally be U.S.-source gain or loss for foreign tax credit purposes. The deductibility of capital losses is subject to various limitations.
Passive foreign investment company (PFIC) rules
Under the Code, we will be a PFIC for any taxable year in which, after the application of certain “look-through” rules with respect to subsidiaries, either (i) 75 percent or more of our gross income consists of “passive income,” or (ii) 50 percent or more of the average quarterly value of our assets consists of assets that produce, or are held for the production of, “passive income.” For purposes of the above calculations, we will be treated as if we hold our proportionate share of the assets of, and directly receive our proportionate share of the income of, any other corporation in which we directly or indirectly own at least 25 percent, by value, of the shares of such corporation. Passive income for this purpose generally includes, among other things, interest, dividends, rents, certain non-active royalties and capital gains.
119
Table of Contents
If we are classified as a PFIC, a U.S. holder will generally be treated as owning stock owned by us in any direct or indirect subsidiaries that are also PFICs and will be subject to similar adverse rules with respect to distributions to us by, and dispositions by us of, the stock of such subsidiaries. A mark-to market election is not permitted for shares of any subsidiary of ours that is also classified as a PFIC.
The determination of whether we are, or will be, a PFIC for a taxable year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to various interpretations. The tests for determining PFIC status are subject to a number of uncertainties. These tests are applied annually, and it is difficult to accurately predict future income, assets and activities relevant to this determination. In addition, because the market price for the ordinary shares is likely to fluctuate, the market price may affect the determination of whether we will be considered a PFIC. There can be no assurance that we will not be considered a PFIC for any taxable year. Prospective investors should consult their tax advisors regarding our PFIC status.
If we were deemed to be a PFIC in any year during which a U.S. investor held or holds ordinary shares (assuming such U.S. holder has not made a timely mark-to-market election, as further described below), any gain recognized by a U.S. holder on a sale or other disposition (including certain pledges) of the ordinary shares would be allocated ratably over the U.S. holder’s holding period for the ordinary shares. The amounts allocated to the taxable year of the sale or other disposition and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to any other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge would be imposed on the amount allocated to that taxable year. Further, to the extent that any distribution received by a U.S. holder on its ordinary shares exceeds 125 percent of the average of the annual distributions on the ordinary shares received during the preceding three years or the U.S. holder’s holding period, whichever is shorter, that distribution would be subject to taxation in the same manner as gain, described immediately above.
If we are a PFIC for any year during which a U.S. holder holds common shares, we generally will continue to be treated as a PFIC with respect to the U.S. holder for all succeeding years during which the U.S. holder holds common shares, even if we cease to meet the threshold requirement for PFIC status.
A U.S. holder can avoid certain of the adverse rules described above by making a mark-to-market election with respect to its ordinary shares, provided that the ordinary shares are “marketable.” Ordinary shares will be marketable if they are “regularly traded” on a “qualified exchange” or other market within the meaning of applicable Treasury regulations. If a U.S. holder makes the mark-to-market election, it generally will recognize as ordinary income any excess of the fair market value of the ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. holder makes this election, the holder’s tax basis in the ordinary shares will be adjusted to reflect the income or loss amounts recognized. Any gain recognized on the sale or other disposition of ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). Once made, the election cannot be revoked without the consent of the IRS unless the ordinary shares cease to be marketable.
In addition, in order to avoid the application of the foregoing rules, a U.S. person who owns stock in a PFIC for U.S. federal income tax purposes may make a “qualified electing fund” (QEF) election with respect to such PFIC if the PFIC provides the information necessary for such election to be made. If a U.S. person makes a QEF election with respect to a PFIC, the U.S. person will be currently taxable on their pro rata share of the PFIC’s ordinary earnings and net capital gain (at ordinary income and capital gain rates, respectively) for each taxable year that the entity is classified as a PFIC and will not be required to include such amounts in income when actually distributed by the PFIC. We do not intend to provide the information necessary for U.S. holders to make QEF elections.
Under proposed U.S. Treasury Regulations, if a U.S. holder has an option, warrant or other right to acquire stock of a PFIC (such as the Warrants), such option, warrant or right is considered to be PFIC stock also subject to the rules discussed above. However, a U.S. holder generally may not make a QEF election or mark-to-market election with respect to warrants. In addition, under proposed U.S. Treasury Regulations, if a U.S. holder holds an option, warrant or other right to the acquired stock of a PFIC, the holding period with respect to shares of stock of the PFIC acquired upon exercise of such option, warrant or other right will include the period that the option, warrant or other right was held. Thus, this will impact the availability of a timely QEF election and mark-to-market election with respect to such shares as discussed below. Because of the complexity and uncertainty of the treatment of the Warrants under the PFIC rules, each U.S. holder should consult his own tax advisor regarding the application of the PFIC rules to the common shares acquired upon an exercise of common warrants and the availability of, and procedure for making, a qualifying election with respect to common warrants.
If we were a PFIC or, with respect to particular U.S. holder, were treated as a PFIC for the taxable year in which we paid a dividend or for the prior taxable year, the preferential dividend rates discussed above with respect to dividends paid to certain non-corporate U.S. holders would not apply.
120
Table of Contents
If a U.S. holder owns ordinary shares or Warrants during any year in which we are a PFIC, the holder generally must file annual reports containing such information as the U.S. Treasury may require on IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) (or any successor form) with respect to us, generally with the holder’s federal income tax return for that year.
U.S. holders should consult their tax advisors concerning our potential PFIC status and the potential application of the PFIC rules.
Net investment income tax
Certain U.S. holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8 percent tax on all or a portion of their net investment income, which may include their gross dividend income and net gains from the disposition of the ordinary shares. If you are a U.S. holder that is an individual, estate or trust, you should consult your tax advisors regarding the applicability of this net investment income tax to your income and gains in respect of your investment in the common shares..
Information reporting and backup withholding
Payments of dividends and sales proceeds that are made within the U.S. or through certain U.S.-related financial intermediaries generally are subject to information reporting, and may be subject to backup withholding, unless (i) the U.S. holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. holder provides a correct taxpayer identification number and certifies that they are not subject to backup withholding.
The amount of any backup withholding from a payment to a U.S. holder will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle the U.S. holder to a refund, provided that the required information is furnished in a timely manner to the IRS.
Information with respect to foreign financial assets
Certain U.S. holders who are individuals (and, under proposed regulations, certain entities) may be required to report information relating to an interest in our ordinary shares, subject to certain exceptions (including an exception for ordinary shares held in accounts maintained by certain U.S. financial institutions). U.S. holders should consult their tax advisors regarding the effect, if any, of this legislation on their ownership and disposition of the ordinary shares.
| F. | DIVIDENDS AND PAYING AGENTS |
Not applicable.
| G. | STATEMENT BY EXPERTS |
The consolidated financial statements of RELIEF THERAPEUTICS Holding SA contained herein have been included in reliance on the report of MAZARS SA, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
| H. | DOCUMENTS ON DISPLAY |
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers, and under those requirements file reports with the SEC. The SEC maintains a website at http://www.sec.gov from which filings may be accessed.
| I. | SUBSIDIARY INFORMATION |
For information about our subsidiaries, see “Item 4. Information on the Company – C. Organizational Structure.”
| ITEM 11 | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
For information about our quantitative and qualitative disclosures about market risk, see “Item 5. Operating and Financial Review and Prospects – B. Liquidity and Capital Resources.”
121
Table of Contents
| ITEM 12 | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
| A. | DEBT SECURITIES |
Not Applicable.
| B. | WARRANTS AND RIGHTS |
Not Applicable.
| C. | OTHER SECURITIES |
Not applicable.
| D. | AMERICAN DEPOSITARY RECEIPTS |
J.P. Morgan Chase Bank, N.A. (J.P. Morgan) is acting as the depositary bank for our American Depositary Receipts. J.P. Morgan’s depositary offices are located at 383 Madison Avenue, Floor 11, New York, New York 10179. American Depositary Shares are sometimes referred to as ADSs and represent ownership interests in securities that are on deposit with the depositary bank. ADSs may be represented by certificates that are commonly known as American Depositary Receipts or ADRs. The depositary bank typically appoints a custodian to safekeep the securities on deposit. In this case, the custodian is The Corporation Trust Company, located at 1209 Orange Street, Wilmington, Delaware 19801.
A copy of the deposit agreement, as amended, is on file with the SEC under cover of a Registration Statement on Form F-6. You may obtain a copy of the deposit receipt from the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549 and from the SEC’s website at www.sec.gov. Please refer to Relief Therapeutics Holding SA and Registration Number 333-260712 when retrieving such copy.
We are providing you with a summary description of the material terms of the ADSs and of your material rights of a holder of ADSs. Please remember that summaries by their nature lack the precision of the information. summarized and that the rights and obligations of an owner of ADSs will be determined by reference to the terms of the deposit agreement and not by this summary. We urge you to review the deposit agreement in its entirety.
Each ADS represents the right to receive, and to exercise the beneficial ownership interests in, ordinary shares that are on deposit with the depositary bank and/or custodian. As of the date of this prospectus, each ADS represents the ownership of one of our ordinary shares. An ADS also represents the right to receive, and to exercise the beneficial interests in, any other property received by the depositary bank or the custodian on behalf of the owner of the ADS but that has not been distributed to the owners of ADSs because of legal restrictions or practical considerations. We and the depositary bank may agree to change the ADS-to-Share ratio by amending the deposit agreement. This amendment may give rise to, or change, the depositary fees payable by ADS owners. The custodian, the depositary bank and their respective nominees will hold all deposited property for the benefit of the holders and beneficial owners of ADSs. The deposited property does not constitute the proprietary assets of the depositary bank, the custodian or their nominees. Beneficial ownership in the deposited property will under the terms of the deposit agreement be vested in the beneficial owners of the ADSs. The depositary bank, the custodian and their respective nominees will be the record holders of the deposited property represented by the ADSs for the benefit of the holders and beneficial owners of the corresponding ADSs. A beneficial owner of ADSs may or may not be the holder of ADSs. Beneficial owners of ADSs will be able to receive, and to exercise beneficial ownership interests in, the deposited property only through the registered holders of the ADSs, the registered holders of the ADSs (on behalf of the applicable ADS owners) only through the depositary bank, and the depositary bank (on behalf of the owners of the corresponding ADSs) directly, or indirectly, through the custodian or their respective nominees, in each case upon the terms of the deposit agreement.
If you become an owner of ADSs, you will become a party to the deposit agreement and therefore will be bound to its terms and to the terms of any ADS that represents your ADSs. The deposit agreement and the ADS specify our rights and obligations as well as your rights and obligations as owner of ADSs and those of the depositary bank. As an ADS holder you appoint the depositary bank to act on your behalf in certain circumstances. The deposit agreement and the ADSs are governed by New York law. However, our obligations to the holders of ordinary shares will continue to be governed by Swiss law which may be different from the laws of the U.S..
In addition, applicable laws and regulations may require you to satisfy reporting requirements and obtain regulatory approvals in certain circumstances. You are solely responsible for complying with such reporting requirements and obtaining such approvals. Neither the depositary bank, the custodian, us or any of their or our respective agents or affiliates shall be required to take any actions whatsoever on your behalf to satisfy such reporting requirements or obtain such regulatory approvals under applicable laws and regulations.
122
Table of Contents
As an owner of ADSs, we will not treat you as one of our shareholders and you will not have direct shareholder rights. The depositary bank will hold on your behalf the shareholder rights attached to the ordinary shares underlying your ADSs. As an owner of ADSs you will be able to exercise the shareholders rights for the ordinary shares represented by your ADSs through the depositary bank only to the extent contemplated in the deposit agreement. To exercise any shareholder rights not contemplated in the deposit agreement you will, as an ADS owner, need to arrange for the cancellation of your ADSs and become a direct shareholder.
The manner in which you own the ADSs (e.g., in a brokerage account vs. as registered holder, or as holder of certificated vs. uncertificated ADSs) may affect your rights and obligations, and the manner in which, and extent to which, the depositary bank’s services are made available to you. As an owner of ADSs, you may hold your ADSs either by means of an ADS registered in your name, through a brokerage or safekeeping account, or through an account established by the depositary bank in your name reflecting the registration of uncertificated ADSs directly on the books of the depositary bank (commonly referred to as the direct registration system or DRS). The direct registration system reflects the uncertificated (book-entry) registration of ownership of ADSs by the depositary bank. Under the direct registration system, ownership of ADSs is evidenced by periodic statements issued by the depositary bank to the holders of the ADSs. The direct registration system includes automated transfers between the depositary bank and The Depository Trust Company DTC, the central book-entry clearing and settlement system for equity securities in the U.S.. If you decide to hold your ADSs through your brokerage or safekeeping account, you must rely on the procedures of your broker or bank to assert your rights as ADS owner. Banks and brokers typically hold securities such as the ADSs through clearing and settlement systems such as DTC. The procedures of such clearing and settlement systems may limit your ability to exercise your rights as an owner of ADSs. Please consult with your broker or bank if you have any questions concerning these limitations and procedures. All ADSs held through DTC will be registered in the name of a nominee of DTC. This summary description assumes you have opted to own the ADSs directly by means of an ADS registered in your name and, as such, we will refer to you as the “holder.” When we refer to “you,” we assume the reader owns ADSs and will own ADSs at the relevant time.
The registration of the ordinary shares in the name of the depositary bank or the custodian shall, to the maximum extent permitted by applicable law, vest in the depositary bank or the custodian the record ownership in the applicable ordinary shares with the beneficial ownership rights and interests in such ordinary shares being at all times vested with the beneficial owners of the ADSs representing the ordinary shares. The depositary bank or the custodian shall at all times be entitled to exercise the beneficial ownership rights in all deposited property, in each case only on behalf of the holders and beneficial owners of the ADSs representing the deposited property.
Intended termination of our ADR program
Subject to the listing of our ordinary shares on the Nasdaq Stock Market or another regulated U.S. exchange, we intend to terminate the ADR program on or around the date of such listing.
Dividends and Distributions
As a holder of ADSs, you generally have the right to receive the distributions we make on the securities deposited with the custodian. Your receipt of these distributions may be limited, however, by practical considerations and legal limitations. Holders of ADSs will receive such distributions under the terms of the deposit agreement in proportion to the number of ADSs held as of the specified record date, after deduction of the applicable fees, taxes and expenses.
Distributions of Cash
Whenever we make a cash distribution for the securities on deposit with the custodian, we will deposit the funds with the custodian. Upon receipt of confirmation of the deposit of the requisite funds, the depositary bank will arrange for the funds received in a currency other than U.S. dollars to be converted into U.S. dollars and for the distribution of the U.S. dollars to the holders, subject to the laws and regulations of Switzerland.
The conversion into U.S. dollars will take place only if practicable and if the U.S. dollars are transferable to the U.S. The depositary bank will apply the same method for distributing the proceeds of the sale of any property (such as undistributed rights) held by the custodian in respect of securities on deposit.
The distribution of cash will be made net of the fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. The depositary bank will hold any cash amounts it is unable to distribute in a non-interest-bearing account for the benefit of the applicable holders and beneficial owners of ADSs until the distribution can be affected or the funds that the depositary bank holds must be escheated as unclaimed property in accordance with the laws of the relevant states of the U.S.
123
Table of Contents
Distributions of Shares
Whenever we make a free distribution of ordinary shares for the securities on deposit with the custodian, we will deposit the applicable number of ordinary shares with the custodian. Upon receipt of confirmation of such deposit, the depositary bank will either distribute to holders new ADSs representing the ordinary shares deposited or modify the ADS-to-ordinary shares ratio, in which case each ADS you hold will represent rights and interests in the additional ordinary shares so deposited. Only whole new ADSs will be distributed. Fractional entitlements will be sold and the proceeds of such sale will be distributed as in the case of a cash distribution.
The distribution of new ADSs or the modification of the ADS-to-ordinary shares ratio upon a distribution of ordinary shares will be made net of the fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. In order to pay such taxes or governmental charges, the depositary bank may sell all or a portion of the new ordinary shares so distributed.
No such distribution of new ADSs will be made if it would violate a law (e.g., the U.S. securities laws) or if it is not operationally practicable. If the depositary bank does not distribute new ADSs as described above, it may sell the ordinary shares received upon the terms described in the deposit agreement and will distribute the proceeds of the sale as in the case of a distribution of cash.
Distributions of Rights
Whenever we intend to distribute rights to subscribe for additional ordinary shares, we will give prior notice to the depositary bank and we will assist the depositary bank in determining whether it is lawful and reasonably practicable to distribute rights to subscribe for additional ADSs to holders.
The depositary bank will establish procedures to distribute rights to subscribe for additional ADSs to holders and to enable such holders to exercise such rights if it is lawful and reasonably practicable to make the rights available to holders of ADSs, and if we provide all of the documentation contemplated in the deposit agreement (such as opinions to address the lawfulness of the transaction). You may have to pay fees, expenses, taxes and other governmental charges to subscribe for the new ADSs upon the exercise of your rights. The depositary bank is not obligated to establish procedures to facilitate the distribution and exercise by holders of rights to subscribe for new ordinary shares other than in the form of ADSs.
The depositary bank will not distribute the rights to you if:
| • | We do not timely request that the rights be distributed to you or we request that the rights not be distributed to you; |
| • | We fail to deliver satisfactory documents to the depositary bank; or |
| • | It is not reasonably practicable to distribute the rights. |
The depositary bank will sell the rights that are not exercised or not distributed if such sale is lawful and reasonably practicable. The proceeds of such sale will be distributed to holders as in the case of a cash distribution. If the depositary bank is unable to sell the rights, it will allow the rights to lapse.
Elective Distributions
Whenever we intend to distribute a dividend payable at the election of shareholders either in cash or in additional shares, we will give prior notice thereof to the depositary bank and will indicate whether we wish the elective distribution to be made available to you. In such case, we will assist the depositary bank in determining whether such distribution is lawful and reasonably practicable.
The depositary bank will make the election available to you only if it is reasonably practicable and if we have provided all of the documentation contemplated in the deposit agreement. In such case, the depositary bank will establish procedures to enable you to elect to receive either cash or additional ADSs, in each case as described in the deposit agreement.
If the election is not made available to you, you will receive either cash or additional ADSs, depending on what a shareholder in Switzerland would receive upon failing to make an election, as more fully described in the deposit agreement.
Other Distributions
Whenever we intend to distribute property other than cash, ordinary shares or rights to subscribe for additional ordinary shares, we will notify the depositary bank in advance and will indicate whether we wish such distribution to be made to you. If so, we will assist the depositary bank in determining whether such distribution to holders is lawful and reasonably practicable.
124
Table of Contents
If it is reasonably practicable to distribute such property to you and if we provide to the depositary bank all of the documentation contemplated in the deposit agreement, the depositary bank will distribute the property to the holders in a manner it deems practicable.
The distribution will be made net of fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. In order to pay such taxes and governmental charges, the depositary bank may sell all or a portion of the property received.
The depositary bank will not distribute the property to you and will sell the property if:
| • | We do not request that the property be distributed to you or if we request that the property not be distributed to you; |
| • | We do not deliver satisfactory documents to the depositary bank; or |
| • | The depositary bank determines that all or a portion of the distribution to you is not reasonably practicable. The proceeds of such a sale will be distributed to holders as in the case of a cash distribution. |
Redemption
Whenever we decide to redeem any of the securities on deposit with the custodian, we will notify the depositary bank in advance. If it is practicable and if we provide all of the documentation contemplated in the deposit agreement, the depositary bank will provide notice of the redemption to the holders.
The custodian will be instructed to surrender the shares being redeemed against payment of the applicable redemption price. The depositary bank will convert into U.S. dollars, upon the terms of the deposit agreement, the redemption funds received in a currency other than U.S. dollars and will establish procedures to enable holders to receive the net proceeds from the redemption upon surrender of their ADSs to the depositary bank. You may have to pay fees, expenses, taxes and other governmental charges upon the redemption of your ADSs. If less than all ADSs are being redeemed, the ADSs to be retired will be selected by lot or on a pro rata basis, as the depositary bank may determine.
Changes Affecting Ordinary Shares
The ordinary shares held on deposit for your ADSs may change from time to time. For example, there may be a change in nominal or par value, split-up, cancellation, consolidation or any other reclassification of such ordinary shares or a recapitalization, reorganization, merger, consolidation or sale of assets of the company.
If any such change were to occur, your ADSs would, to the extent permitted by law and the deposit agreement, represent the right to receive the property received or exchanged in respect of the ordinary shares held on deposit.
The depositary bank may in such circumstances deliver new ADSs to you, amend the deposit agreement, the ADSs and the applicable Registration Statement(s) on Form F-6, call for the exchange of your existing ADSs for new ADSs and take any other actions that are appropriate to reflect as to the ADSs the change affecting the ordinary shares. If the depositary bank may not lawfully distribute such property to you, the depositary bank may sell such property and distribute the net proceeds to you as in the case of a cash distribution.
Issuance of ADSs upon Deposit of Ordinary Shares
The depositary bank may create ADSs on your behalf if you or your broker deposit ordinary shares with the custodian. The depositary bank will deliver these ADSs to the person you indicate only after you pay any applicable issuance fees and any charges and taxes payable for the transfer of the ordinary shares to the custodian. Your ability to deposit ordinary shares and receive ADSs may be limited by U.S. and English legal considerations applicable at the time of deposit.
The issuance of ADSs may be delayed until the depositary bank or the custodian receives confirmation that all required approvals have been given and that the ordinary shares have been duly transferred to the custodian. The depositary bank will only issue ADSs in whole numbers.
When you make a deposit of ordinary shares, you will be responsible for transferring good and valid title to the depositary bank. As such, you will be deemed to represent and warrant that:
| • | The ordinary shares are duly authorized, validly issued, fully paid, non-assessable and legally obtained. |
| • | All preemptive (and similar) rights, if any, with respect to such ordinary shares have been validly waived or exercised. |
| • | You are duly authorized to deposit the ordinary shares. |
125
Table of Contents
| • | The ordinary shares presented for deposit are free and clear of any lien, encumbrance, security interest, charge, mortgage or adverse claim, and are not, and the ADSs issuable upon such deposit will not be, “restricted securities” (as defined in the deposit agreement). |
| • | The deposit of ordinary shares does not violate any provisions of our articles of association or any of the applicable laws and regulations of Switzerland. |
If any of the representations or warranties are incorrect in any way, we and the depositary bank may, at your cost and expense, take any and all actions necessary to correct the consequences of the misrepresentations.
Transfer, Combination and Split Up of ADSs
As an ADS holder, you will be entitled to transfer, combine or split up your ADSs and the ADSs evidenced thereby. For transfers of ADSs, you will have to surrender the ADSs to be transferred to the depositary bank and also must:
| • | ensure that the surrendered ADS is properly endorsed or otherwise in proper form for transfer; |
| • | provide such proof of identity and genuineness of signatures as the depositary bank deems appropriate; |
| • | provide any transfer stamps required by state or federal law; or |
| • | pay all applicable fees, charges, expenses, taxes and other governmental charges payable by ADS holders pursuant to the terms of the deposit agreement, upon a combination or split up of ADSs. |
To have your ADSs either combined or split up, you must surrender the ADSs in question to the depositary bank with your request to have them combined or split up, and you must pay all applicable fees, charges and expenses payable by ADS holders, pursuant to the terms of the deposit agreement, upon a combination or split up of ADSs.
Withdrawal of Ordinary Shares Upon Cancellation of ADSs
As a holder, you will be entitled to present your ADSs to the depositary bank for cancellation and then receive the corresponding number of underlying ordinary shares at the custodian’s offices. Your ability to withdraw the ordinary shares held in respect of the ADSs may be limited by U.S. and Swiss law considerations applicable at the time of withdrawal. In order to withdraw the ordinary shares represented by your ADSs, you will be required to pay to the depositary bank the fees for cancellation of ADSs and any charges and taxes payable upon the transfer of the ordinary shares. You assume the risk for delivery of all funds and securities upon withdrawal. Once canceled, the ADSs will not have any rights under the deposit agreement.
If you hold ADSs registered in your name, the depositary bank may ask you to provide proof of identity and genuineness of any signature and such other documents as the depositary bank may deem appropriate before it will cancel your ADSs. The withdrawal of the ordinary shares represented by your ADSs may be delayed until the depositary bank receives satisfactory evidence of compliance with all applicable laws and regulations. Please keep in mind that the depositary bank will only accept ADSs for cancellation that represent a whole number of securities on deposit.
You will have the right to withdraw the securities represented by your ADSs at any time except for:
| • | Temporary delays that may arise because (i) the transfer books for the ordinary shares or ADSs are closed, or (ii) ordinary shares are immobilized on account of a shareholders’ meeting or a payment of dividends. |
| • | Obligations to pay fees, taxes and similar charges. |
| • | Restrictions imposed because of laws or regulations applicable to ADSs or the withdrawal of securities on deposit. |
The deposit agreement may not be modified to impair your right to withdraw the securities represented by your ADSs except to comply with mandatory provisions of law.
Each holder and beneficial owner of ADSs agrees to provide such information as the company may request in a disclosure notice given pursuant to the Swiss Code of Obligations (CO), or the Articles of Association. Each holder and beneficial owner of ADSs acknowledges that it understands that failure to comply with such request may result in the imposition of sanctions against the holder of the ordinary shares in respect of which the non-complying person is or was, or appears to be or has been, interested as provided in the CO and the Articles of Association which currently include, the withdrawal of the voting rights of such Shares and the imposition of restrictions on the rights to receive dividends on and to transfer such Shares.
126
Table of Contents
Voting Rights
As a holder, you generally have the right under the deposit agreement to instruct the depositary bank to exercise the voting rights for the ordinary shares represented by your ADSs. The voting rights of holders of ordinary shares are described in “Description of Share Capital and Articles of Association— Articles of Association.”
At our request, the depositary bank will distribute to you any notice of shareholders’ meeting received from us together with information explaining how to instruct the depositary bank to exercise the voting rights of the securities represented by ADSs. In lieu of distributing such materials, the depositary bank may distribute to holders of ADSs instructions on how to retrieve such materials upon request.
If the depositary bank timely receives voting instructions from a holder of ADSs, it will endeavor to vote the securities (in person or by proxy) represented by the holder’s ADSs as follows:
| • | In the event of voting by show of hands, the depositary bank will vote (or cause the custodian to vote) all ordinary shares held on deposit at that time in accordance with the voting instructions received from a majority of holders of ADSs who provide timely voting instructions. |
| • | In the event of voting by poll, the depositary bank will vote (or cause the Custodian to vote) the ordinary shares held on deposit in accordance with the voting instructions received from the holders of ADSs. |
Securities for which no voting instructions have been received will not be voted (except as otherwise contemplated in the deposit agreement). Please note that the ability of the depositary bank to carry out voting instructions may be limited by practical and legal limitations and the terms of the securities on deposit. We cannot assure you that you will receive voting materials in time to enable you to return voting instructions to the depositary bank in a timely manner.
Fees and Charges
As an ADS holder, you will be required to pay the following fees under the terms of the deposit agreement:
| Service |
Fees | |
| Cash distributions made, or elective cash/stock options offered, pursuant to the Deposit Agreement | Up to $0.05 per ADS issued. | |
| Distribution or sale of securities under the Deposit Agreement | An amount equal to the fee for the execution and delivery of ADSs which would have been charged as a result of the deposit of such securities, but which securities or the net cash proceeds from the sale thereof are instead distributed by the depositary to the holders entitled thereto. | |
| Services performed by the Depositary in administering the ADSs | Up to $0.05 per ADS per calendar year | |
As an ADS holder you may also be responsible to pay certain charges such as:
| • | Reimbursement of fees, charges and expenses incurred by the Depositary and/or any of its agents in connection with the servicing of the shares or deposited securities, the delivery of deposited securities or otherwise in connection with the Depositary’s or the Custodian’s compliance with applicable law, rule or regulation; |
| • | Stock transfer or other taxes and other governmental charges; |
| • | SWIFT, cable, telex and facsimile transmission and delivery charges incurred at the request of persons depositing, or holders delivering shares, ADSs or deposited securities; and |
127
Table of Contents
| • | Transfer or registration fees for the registration or transfer of deposited securities on any applicable register in connection with the deposit or withdrawal of deposited securities. |
ADS fees and charges for (i) the issuance of ADSs, and (ii) the cancellation of ADSs are charged to the person for whom the ADSs are issued (in the case of ADS issuances) and to the person for whom ADSs are cancelled (in the case of ADS cancellations). In the case of ADSs issued by the depositary bank into DTC, the ADS issuance and cancellation fees and charges may be deducted from distributions made through DTC, and may be charged to the DTC participant(s) receiving the ADSs being issued or the DTC participant(s) holding the ADSs being cancelled, as the case may be, on behalf of the beneficial owner(s) and will be charged by the DTC participant(s) to the account of the applicable beneficial owner(s) in accordance with the procedures and practices of the DTC participants as in effect at the time. ADS fees and charges in respect of distributions and the ADS service fee are charged to the holders as of the applicable ADS record date. In the case of distributions of cash, the amount of the applicable ADS fees and charges is deducted from the funds being distributed. In the case of (i) distributions other than cash and (ii) the ADS service fee, holders as of the ADS record date will be invoiced for the amount of the ADS fees and charges and such ADS fees and charges may be deducted from distributions made to holders of ADSs. For ADSs held through DTC, the ADS fees and charges for distributions other than cash and the ADS service fee may be deducted from distributions made through DTC, and may be charged to the DTC participants in accordance with the procedures and practices prescribed by DTC and the DTC participants in turn charge the amount of such ADS fees and charges to the beneficial owners for whom they hold ADSs. In the case of (i) registration of ADS transfers, the ADS transfer fee will be payable by the ADS holder whose ADSs are being transferred or by the person to whom the ADSs are transferred, and (ii) conversion of ADSs of one series for ADSs of another series, the ADS conversion fee will be payable by the holder whose ADSs are converted or by the person to whom the converted ADSs are delivered.
In the event of refusal to pay the depositary bank fees, the depositary bank may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary bank fees from any distribution to be made to the ADS holder. Certain depositary fees and charges (such as the ADS services fee) may become payable shortly after the purchase of ADSs. Note that the fees and charges you may be required to pay may vary over time and may be changed by us and by the depositary bank. You will receive prior notice of such changes. The depositary bank may reimburse us for certain expenses incurred by us in respect of the ADS program, by making available a portion of the ADS fees charged in respect of the ADS program or otherwise, upon such terms and conditions as we and the depositary bank agree from time to time.
Amendments and Termination
We may agree with the depositary bank to modify the deposit agreement at any time without your consent. We undertake to give holders 30 days’ prior notice of any modifications that would materially prejudice any of their substantial rights under the deposit agreement. We will not consider to be materially prejudicial to your substantial rights any modifications or supplements that are reasonably necessary for the ADSs to be registered under the Securities Act or to be eligible for book-entry settlement, in each case without imposing or increasing the fees and charges you are required to pay. In addition, we may not be able to provide you with prior notice of any modifications or supplements that are required to accommodate compliance with applicable provisions of law.
You will be bound by the modifications to the deposit agreement if you continue to hold your ADSs after the modifications to the deposit agreement become effective. The deposit agreement cannot be amended to prevent you from withdrawing the ordinary shares represented by your ADSs (except as permitted by law).
We have the right to direct the depositary bank to terminate the deposit agreement. Similarly, the depositary bank may in certain circumstances on its own initiative terminate the deposit agreement. In either case, the depositary bank must give notice to the holders at least 30 days before termination. Until termination, your rights under the deposit agreement will be unaffected.
Termination
After termination, the depositary bank will continue to collect distributions received (but will not distribute any such property until you request the cancellation of your ADSs) and may sell the securities held on deposit. After the sale, the depositary bank will hold the proceeds from such sale and any other funds then held for the holders of ADSs in a non-interest bearing account. At that point, the depositary bank will have no further obligations to holders other than to account for the funds then held for the holders of ADSs still outstanding (after deduction of applicable fees, taxes and expenses).
In connection with any termination of the deposit agreement, the depositary bank may make available to owners of ADSs a means to withdraw the ordinary shares represented by ADSs and to direct the depositary of such ordinary shares into an unsponsored American depositary share program established by the depositary bank. The ability to receive unsponsored American depositary shares upon termination of the deposit agreement would be subject to satisfaction of certain U.S. regulatory requirements applicable to the creation of unsponsored American depositary shares and the payment of applicable depositary fees.
128
Table of Contents
Books of Depositary
The depositary bank will maintain ADS holder records at its depositary office. You may inspect such records at such office during regular business hours but solely for the purpose of communicating with other holders in the interest of business matters relating to the ADSs and the deposit agreement.
The depositary bank will maintain in New York facilities to record and process the issuance, cancellation, combination, split-up and transfer of ADSs. These facilities may be closed from time to time, to the extent not prohibited by law.
Limitations on Obligations and Liabilities
The deposit agreement limits our obligations and the depositary bank’s obligations to you. Please note the following:
| • | We and the depositary bank are obligated only to take the actions specifically stated in the deposit agreement without negligence or bad faith. |
| • | The depositary bank disclaims any liability for any failure to carry out voting instructions, for any manner in which a vote is cast or for the effect of any vote, provided it acts in good faith and in accordance with the terms of the deposit agreement. |
| • | The depositary bank disclaims any liability for any failure to determine the lawfulness or practicality of any action, for the content of any document forwarded to you on our behalf or for the accuracy of any translation of such a document, for the investment risks associated with investing in ordinary shares, for the validity or worth of the ordinary shares, for any tax consequences that result from the ownership of ADSs, for the credit-worthiness of any third party, for allowing any rights to lapse under the terms of the deposit agreement, for the timeliness of any of our notices or for our failure to give notice. |
| • | We and the depositary bank will not be obligated to perform any act that is inconsistent with the terms of the deposit agreement. |
| • | We and the depositary bank disclaim any liability if we or the depositary bank are prevented or forbidden from or subject to any civil or criminal penalty or restraint on account of, or delayed in, doing or performing any act or thing required by the terms of the deposit agreement, by reason of any provision, present or future of any law or regulation, or by reason of present or future provision of any provision of our Articles of Association, or any provision of or governing the securities on deposit, or by reason of any act of God or war or other circumstances beyond our control. |
| • | We and the depositary bank disclaim any liability by reason of any exercise of, or failure to exercise, any discretion provided for in the deposit agreement or in our Articles of Association or in any provisions of or governing the securities on deposit. |
| • | We and the depositary bank further disclaim any liability for any action or inaction in reliance on the advice or information received from legal counsel, accountants, any person presenting Shares for deposit, any holder of ADSs or authorized representatives thereof, or any other person believed by either of us in good faith to be competent to give such advice or information. |
| • | We and the depositary bank may rely without any liability upon any written notice, request or other document believed to be genuine and to have been signed or presented by the proper parties. |
| • | We and the depositary bank also disclaim liability for any consequential or punitive damages for any breach of the terms of the deposit agreement. |
| • | No disclaimer of any Securities Act liability is intended by any provision of the deposit agreement. |
| • | Nothing in the deposit agreement gives rise to a partnership or joint venture, or establishes a fiduciary relationship, among us, the depositary bank and you as ADS holder. |
| • | Nothing in the deposit agreement precludes the depositary bank (or its affiliates) from engaging in transactions in which parties adverse to us or the holders or beneficial owners of ADS have interests, and nothing in the deposit agreement obligates the depositary bank to disclose those transactions, or any information obtained in the course of those transactions, to us or to the holders or beneficial owners of ADS, or to account for any payment received as part of those transactions. |
129
Table of Contents
Taxes
You will be responsible for the taxes and other governmental charges payable on the ADSs and the securities represented by the ADSs. We, the depositary bank and the custodian may deduct from any distribution the taxes and governmental charges payable by holders and may sell any and all property on deposit to pay the taxes and governmental charges payable by holders. You will be liable for any deficiency if the sale proceeds do not cover the taxes that are due.
The depositary bank may refuse to issue ADSs, to deliver, transfer, split and combine ADSs or to release securities on deposit until all taxes and charges are paid by the applicable holder. The depositary bank and the custodian may take reasonable administrative actions to obtain tax refunds and reduced tax withholding for any distributions on your behalf. However, you may be required to provide to the depositary bank and to the custodian proof of taxpayer status and residence and such other information as the depositary bank and the custodian may require to fulfill legal obligations. You are required to indemnify us, the depositary bank and the custodian for any claims with respect to taxes based on any tax benefit obtained for you.
Foreign Currency Conversion
The depositary bank will arrange for the conversion of all foreign currency received into U.S. dollars if such conversion is practical, and it will distribute the U.S. dollars in accordance with the terms of the deposit agreement. You may have to pay fees and expenses incurred in converting foreign currency, such as fees and expenses incurred in complying with currency exchange controls and other governmental requirements.
If the conversion of foreign currency is not practical or lawful, or if any required approvals are denied or not obtainable at a reasonable cost or within a reasonable period, the depositary bank may take the following actions in its discretion:
| • | Convert the foreign currency to the extent practicable and lawful and distribute the U.S. dollars to the holders for whom the conversion and distribution is lawful and practicable. |
| • | Distribute the foreign currency to holders for whom the distribution is lawful and practical. |
| • | Hold the foreign currency (without liability for interest) for the applicable holders. |
Governing Law / Waiver of Jury Trial
The deposit agreement, the ADSs, and the ADSs will be interpreted in accordance with the laws of the State of New York. The rights of holders of ordinary shares (including ordinary shares represented by ADSs) are governed by the laws of Switzerland.
AS A PARTY TO THE DEPOSIT AGREEMENT, YOU IRREVOCABLY WAIVE, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, YOUR RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF, OR RELATING TO, THE DEPOSIT AGREEMENT OR THE ADSs, OR ANYTHING CONTAINED THEREIN AGAINST U.S. AND/OR THE DEPOSITARY.
The deposit agreement provides that, to the extent permitted by law, ADS holders waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our ordinary shares, the ADSs or the deposit agreement, including any claim under U.S. federal securities laws. If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable in the facts and circumstances of that case in accordance with applicable case law. However, you will not be deemed, by agreeing to the terms of the deposit agreement, to have waived our or the depositary’s compliance with U.S. federal securities laws and the rules and regulations promulgated thereunder.
PART II
| ITEM 13 | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
Not applicable.
| ITEM 14 | MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
Not applicable.
130
Table of Contents
| ITEM 15 | CONTROLS AND PROCEDURES |
Not applicable.
| ITEM 16 | RESERVED |
| ITEM 16A. | AUDIT COMMITTEE FINANCIAL EXPERT |
The Board of Directors has determined that Michelle Lock qualifies as an Audit Committee Financial Expert as defined in Regulation S-K under the Exchange Act. The listing rules of the Nasdaq Stock Market, where the Company intends to list its ordinary shares, require the audit committee to be comprised only of independent directors. All of the members of the Audit and Finance Committee, including Ms. Lock, are independent directors.
| ITEM 16B. | CODE OF ETHICS |
We have adopted a Code of Business Conduct and Ethics, which covers a broad range of matters including the handling of conflicts of interest, compliance issues and other corporate policies such as insider trading and equal opportunity and non-discrimination standards. Our Code of Business Conduct & Ethics applies to all of our directors, executive officers and employees. We have published our Code of Business Conduct and Ethics on our website, https://relieftherapeutics.com/investor-relations. The information contained on our website is not a part of this Annual Report.
| ITEM 16C. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Not applicable.
| ITEM 16D. | EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
Not applicable.
| ITEM 16E. | PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
In 2022, no purchases of our equity securities were made by or on behalf of the Company or any affiliated purchaser.
| ITEM 16F. | CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
None.
| ITEM 16G. | CORPORATE GOVERNANCE |
Summary of significant corporate governance differences from Nasdaq Listing Standards
Our corporate governance practices differ in certain respects from those that U.S. companies must adopt in order to maintain a Nasdaq listing. A brief, general summary of those differences is provided as follows.
Independent Directors
Swiss law does not require that a majority of our board of directors consist of independent directors. Our board of directors therefore may include fewer independent directors than would be required if we were subject to Nasdaq Listing Rule 5605(b)(1). In addition, we are not subject to Nasdaq Listing Rule 5605(b)(2), which requires that independent directors must regularly have scheduled meetings at which only independent directors are present.
Nomination and Compensation, Audit and Corporate Governance Committees
As Swiss law requires that we have a compensation committee (and we have in line with best practice installed a nomination and compensation committee as well as an audit committee and a corporate governance committee), we will follow home country requirements with respect to such committees. As a result, our practice will vary from the requirements of Nasdaq Listing Rule 5605(d), which sets forth certain requirements as to the responsibilities, composition and independence of the nomination and compensation, audit and corporate governance committees.
131
Table of Contents
Quorum Requirements
In accordance with Swiss law and generally accepted business practices, our articles of association do not provide quorum requirements generally applicable to general meetings of shareholders. Our practice thus varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock.
Solicitation of Proxies
Our articles of association provide for an independent proxy holder elected by our shareholders, who may represent our shareholders at a general meeting of shareholders, and we must provide shareholders with an agenda and other relevant documents for the general meeting of shareholders. However, Swiss law does not have a regulatory regime for the solicitation of proxies, and company solicitation of proxies is prohibited for public companies in Switzerland. Thus, our practice will vary from the requirement of Nasdaq Listing Rule 5620(b), which sets forth certain requirements regarding the solicitation of proxies.
| ITEM 16H. | MINE SAFETY DISCLOSURE |
Not applicable.
132
Table of Contents
PART III
| ITEM 17 | FINANCIAL STATEMENTS |
We have elected to furnish financial statements and related information in Item 18.
| ITEM 18 | FINANCIAL STATEMENTS |
See pages F-1 through F-41 of this Annual Report.
| ITEM 19 | EXHIBITS |
The Exhibits listed in the Exhibit Index at the end of this Annual Report are filed as Exhibits hereto.
133
Table of Contents
EXHIBIT INDEX
134
Table of Contents
| Exhibit |
Description of Exhibit |
Form | File Number | Date of Filing |
Exhibit |
Filed Herewith | ||||||
| 12.2 | Certification of Jeremy Meinen, Chief Financial Officer of the Company, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 13.1 | Certification of Jack Weinstein, Chief Financial officer of the Company, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 13.2 | Certification of Jeremy Meinen, Chief Financial Officer of the Company, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 101.INS | XBRL Instance Document | X | ||||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document | X | ||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | X | ||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | X | ||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document and included in Exhibit 101) | X | ||||||||||
| * | Contains information that has been excluded from this exhibit because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed. |
135
Table of Contents
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
Date: May 12, 2023
| RELIEF THERAPEUTICS HOLDING SA | ||
| By: | /s/ Jack Weinstein | |
| Jack Weinstein | ||
| Chief Executive Officer | ||
| By: | /s/ Jeremy Meinen | |
| Jeremy Meinen | ||
| Chief Financial Officer | ||
136
Table of Contents

RELIEF THERAPEUTICS Holding SA
Geneva
Independent Auditor’s Report
Consolidated Financial Statements
December 31, 2022
Table of Contents
F-2 |
||||
F-3 |
||||
F-4 |
||||
F-5 |
||||
F-6 |
||||
F-7 |
 |
Mazars SA Chemin de Blandonnet 2 CH-1214 Vernier-Geneva Tel: +41 22 708 10 80 www.mazars.ch |
Franck Paucod Licensed Audit Expert |
Yoann Bois Licensed Audit Expert |
|||
(Auditor in Charge) |
| in CHF thousands |
Notes |
December 31, 2022 |
December 31, 2021 |
|||||||
| ASSETS |
||||||||||
| Intangible assets |
8 | |||||||||
| Right-of-use |
9 | |||||||||
| Property and equipment |
||||||||||
| Other non-current assets |
||||||||||
| Deferred tax assets |
29 | |||||||||
| |
|
|
|
|||||||
| Non-current assets |
||||||||||
| Inventories |
10 | |||||||||
| Trade receivables |
11 | |||||||||
| Other current assets |
12 | |||||||||
| Cash and cash equivalents |
13 | |||||||||
| |
|
|
|
|||||||
| Current assets |
||||||||||
| |
|
|
|
|||||||
| Total assets |
||||||||||
| |
|
|
|
|||||||
| EQUITY AND LIABILITIES |
||||||||||
| Share capital |
14 | |||||||||
| Reserves |
15 | |||||||||
| Treasury shares |
( |
) | ( |
) | ||||||
| Accumulated losses |
( |
) | ( |
) | ||||||
| |
|
|
|
|||||||
| Equity |
||||||||||
| |
|
|
|
|||||||
| Non-current lease liabilities |
9 | |||||||||
| Non-current borrowings |
16 | |||||||||
| Defined benefit obligations |
17 | |||||||||
| Provisions |
18 | |||||||||
| Deferred tax liabilities |
29 | |||||||||
| |
|
|
|
|||||||
| Non-current liabilities |
||||||||||
| Current lease liabilities |
9 | |||||||||
| Current borrowings |
16 | |||||||||
| Trade payables |
||||||||||
| Financial liabilities due to related parties |
19 | |||||||||
| Provisions |
18 | |||||||||
| Other current payables and liabilities |
20 | |||||||||
| |
|
|
|
|||||||
| Current liabilities |
||||||||||
| |
|
|
|
|||||||
| Total equity and liabilities |
||||||||||
| |
|
|
|
|||||||
| in CHF thousands |
Notes |
2022 |
2021 |
|||||||
| Revenue |
6 | |||||||||
| Other gains |
21 | |||||||||
| |
|
|
|
|||||||
| Total income |
||||||||||
| Raw materials and consumables expenses |
22 | ( |
) | ( |
) | |||||
| External selling and distribution expenses |
22 | ( |
) | ( |
) | |||||
| External research and development expenses |
23 | ( |
) | ( |
) | |||||
| Personnel expenses |
24 | ( |
) | ( |
) | |||||
| Other administrative expenses |
25 | ( |
) | ( |
) | |||||
| Other losses |
26 | ( |
) | ( |
) | |||||
| |
|
|
|
|||||||
| EBITDA |
( |
) |
( |
) | ||||||
| Impairment losses on intangible assets |
8 | ( |
) | — | ||||||
| Amortization and depreciation expense |
27 | ( |
) | ( |
) | |||||
| |
|
|
|
|||||||
| Operating result |
( |
) |
( |
) | ||||||
| Financial income |
28 | |||||||||
| Financial expenses |
28 | ( |
) | ( |
) | |||||
| |
|
|
|
|||||||
| Net loss before taxes |
( |
) |
( |
) | ||||||
| Income taxes |
29 | |||||||||
| |
|
|
|
|||||||
| Net loss for the period |
( |
) |
( |
) | ||||||
| OTHER COMPREHENSIVE INCOME |
||||||||||
| Remeasurement of defined benefit obligation |
17 | |||||||||
| |
|
|
|
|||||||
| Items that will not be reclassified to profit or loss |
||||||||||
| Currency translation differences |
15.3 | |||||||||
| |
|
|
|
|||||||
| Items that may be reclassified to profit or loss |
||||||||||
| Other comprehensive income for the period, net of tax |
||||||||||
| |
|
|
|
|||||||
| Total comprehensive result for the period |
( |
) |
( |
) | ||||||
| |
|
|
|
|||||||
| EARNINGS PER SHARE |
||||||||||
| Basic and diluted loss per share (in CHF) |
31 | ( |
) | ( |
) | |||||
| |
|
|
|
|||||||
| in CHF thousands |
Notes |
2022 |
2021 |
|||||||
| Net loss for the period |
||||||||||
| Adjustments for: |
( |
) | ( |
) | ||||||
| Income tax (income)/expense |
29.1 | ( |
) | ( |
) | |||||
| Depreciation and amortization expense |
27 | |||||||||
| Impairment of intangible assets |
8 | — | ||||||||
| Impairment of receivables |
11 | |||||||||
| Reversal of impairment loss on receivables |
21 | ( |
) | — | ||||||
| Loss from disposal of other financial assets |
14 | — | ||||||||
| Gain from loan forgiveness |
21 | — | ( |
) | ||||||
| Gain from fair value adjustments to contingent payments |
18 | ( |
) | — | ||||||
| Finance expenses |
28 | |||||||||
| Finance income |
28 | ( |
) | ( |
) | |||||
| Interest expenses paid on borrowings and lease liabilities |
( |
) | ( |
) | ||||||
| Loss on disposal of property and equipment |
— | |||||||||
| Change in defined benefit obligations |
17 | ( |
) | |||||||
| Share-based payment expense |
30 | |||||||||
| Changes in working capital: |
||||||||||
| Decrease/(Increase) in inventories |
( |
) | ||||||||
| Decrease/(Increase) in trade receivables |
( |
) | ( |
) | ||||||
| Decrease/(Increase) in other assets |
( |
) | ||||||||
| (Decrease)/increase in trade payables |
( |
) | ( |
) | ||||||
| (Decrease)/increase in provisions |
( |
) | ||||||||
| (Decrease)/increase in other payables and liabilities |
( |
) | ( |
) | ||||||
| |
|
|
|
|||||||
| Cash flow used in operating activities |
( |
) |
( |
) | ||||||
| |
|
|
|
|||||||
| Payments for property, plant and equipment |
( |
) | — | |||||||
| Payments for intangible assets |
8 | ( |
) | ( |
) | |||||
| Payments for other financial assets |
( |
) | ( |
) | ||||||
| Proceeds from sale of right-of-use |
— | |||||||||
| Net cash outflow on acquisition of subsidiaries |
7 | — | ( |
) | ||||||
| Milestone payments related to acquisition of subsidiaries |
18 | ( |
) | — | ||||||
| Proceeds from other financial assets |
||||||||||
| Interest received |
||||||||||
| |
|
|
|
|||||||
| Cash flow used in investing activities |
( |
) |
( |
) | ||||||
| |
|
|
|
|||||||
| Proceeds from capital increase |
14 | |||||||||
| Equity transaction costs |
15 | ( |
) | ( |
) | |||||
| Repayment of lease liabilities |
( |
) | ( |
) | ||||||
| Repayment of borrowings |
( |
) | ( |
) | ||||||
| |
|
|
|
|||||||
| Cash flow from financing activities |
||||||||||
| |
|
|
|
|||||||
| Net increase in cash and cash equivalents |
( |
) |
||||||||
| Cash and cash equivalents at beginning of period |
||||||||||
| Exchange difference on cash and cash equivalents |
( |
) | ||||||||
| |
|
|
|
|||||||
| Cash and cash equivalents at end of period |
||||||||||
| |
|
|
|
|||||||
| in CHF thousands |
Notes |
Share capital |
Treasury shares |
Reserves |
Accumulated loss |
Total equity |
||||||||||||||||||
| Balance at January 1, 2021 |
— |
( |
) |
|||||||||||||||||||||
| Result for the period |
— | — | — | ( |
) | ( |
) | |||||||||||||||||
| Other comprehensive income for the period |
— | — | ||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total comprehensive result for the period |
— |
— |
( |
) |
( |
) | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Issuance of treasury shares |
14 | ( |
) | — | — | — | ||||||||||||||||||
| Direct Share Placement program |
14 | — | — | |||||||||||||||||||||
| Private placements |
14 | — | — | |||||||||||||||||||||
| Acquisition payments |
7 | — | — | |||||||||||||||||||||
| Exercise of options |
14 | — | — | |||||||||||||||||||||
| Share-based compensation cost |
30 | — | — | — | ||||||||||||||||||||
| Transaction cost in relation to capital increases |
15 | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2021 |
( |
) |
( |
) |
||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at January 1, 2022 |
( |
) |
( |
) |
||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Result for the period |
— | — | — | ( |
) | ( |
) | |||||||||||||||||
| Other comprehensive income for the period |
— | — | ||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total comprehensive result for the period |
— |
— |
( |
) |
( |
) | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Issuance of treasury shares |
14 | ( |
) | — | — | — | ||||||||||||||||||
| Direct Share Placement program |
14 | — | — | |||||||||||||||||||||
| Acquisition milestone share payments |
18 | — | — | |||||||||||||||||||||
| Exercise of options |
14 | — | — | |||||||||||||||||||||
| Share-based compensation cost |
30 | — | — | — | ||||||||||||||||||||
| Transaction cost in relation to capital increases |
15 | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2022 |
( |
) |
( |
) |
||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| • | Narrow-scope amendments to IFRS 3, IAS 16, IAS 8, IAS 37 and IFRS 16; and |
| • | Annual improvements of IFRS 1, IFRS 9 and IAS 41. |
| • | Amendments to IAS 1 ‘Presentation of financial statements’ on classification of liabilities; and |
| • | Narrow-scope amendments to IAS 8 and IAS 12. |
| • | power over the investee (i.e., existing rights that give it the current ability to direct the relevant activities of the investee); |
| • | exposure, or rights, to variable returns from its involvement with the investee; and |
| • | the ability to use its power over the investee to affect its returns. |
| • | any contractual arrangement with the other vote holders of the investee; |
| • | rights arising from other contractual arrangements; and |
| • | the Group’s voting rights and potential voting rights. |
| • | expected to be realized or intended to be sold or consumed in a normal operating cycle, which is twelve months; |
| • | held primarily for the purpose of trading; |
| • | expected to be realized within twelve months after the reporting period; or |
| • | cash or cash equivalents unless restricted from being exchanged or used to settle a liability for at least twelve months after the reporting period. |
| • | it is expected to be settled in a normal operating cycle, which is twelve months; |
| • | it is held primarily for the purpose of trading; |
| • | it is due to be settled within twelve months after the reporting period; or |
| • | there is no unconditional right to defer the settlement of the liability within twelve months after the reporting period. The Group classifies all other liabilities as non-current. |
| • | fixed lease payments (including in-substance fixed payments), less any lease incentives; |
| • | variable lease payments that depend on an index or rate, initially measured using the index or rate at the commencement date; |
| • | the amount expected to be payable by the lessee under residual value guarantees; |
| • | the exercise price of purchase options, if the lessee is reasonably certain to exercise the options; and |
| • | payments of penalties for terminating the lease if the lease term reflects the exercise of an option to terminate. |
| • | the contractual rights to the cash flows from the asset have expired; or |
| • | the Group has transferred its rights to receive cash flows from the asset or has assumed an obligation to pay the received cash flows in full without material delay to a third party under a pass-through arrangement; and either (a) the Group has transferred substantially all the risks and rewards of the asset, or (b) the Group has neither transferred nor retained substantially all the risks and rewards of the asset but has transferred control of the asset. |
| • | in the principal market for the asset or liability, or |
| • | in the absence of a principal market, in the most advantageous market for the asset or liability. |
| • | the date of the plan amendment or curtailment, or |
| • | the date that the Group recognizes restructuring-related costs. |
| • | service costs comprising current service costs, past-service costs, gains and losses on curtailments and non-routine settlements; and |
| • | net interest expense or income. |
Name |
Country |
Location |
Equity interest 2022 2021 |
|||||||||
Relief Therapeutics International SA |
% | % | ||||||||||
Relief Therapeutics US, Inc. |
% | % | ||||||||||
Relief Therapeutics, Inc. |
% | % | ||||||||||
APR Applied Pharma Research SA |
% | % | ||||||||||
APR Applied Pharma Research Holding SA |
% | % | ||||||||||
APR Applied Pharma Research - Italy s.r.l. |
% | % | ||||||||||
APR Applied Pharma Research Deutschland GmbH |
% | % | ||||||||||
AdVita Lifescience GmbH |
% | % | ||||||||||
AdVita Lifescience AG |
% | % | ||||||||||
AdVita Lifescience, Inc. |
% | % | ||||||||||
TCHF |
2022 |
2021* |
||||||
Revenue streams |
||||||||
Royalties |
||||||||
Product sales |
||||||||
License fees |
||||||||
Revenue from research & development services |
||||||||
Total revenue |
||||||||
Geographical area |
||||||||
Switzerland |
||||||||
Europe (excluding Switzerland) |
||||||||
North America |
||||||||
Rest of the world |
||||||||
Total revenue |
||||||||
Timing of revenue recognition |
||||||||
Point in time |
||||||||
Over time |
||||||||
Total revenue |
||||||||
| * | Revenue recognized since the acquisition of APR, i.e., from July 1, 2021, to December 31, 2021. The Group did |
TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
Switzerland |
||||||||
Rest of the world |
||||||||
Total non-current assets * |
||||||||
| * | Without financial assets and deferred tax assets. |
Consideration transferred |
||||
TCHF |
||||
Cash |
||||
Non-cash (Relief shares) |
||||
Contingent consideration |
||||
Total consideration transferred |
||||
TCHF |
||||
Non-current assets |
||||
Right-of-use |
||||
Property and equipment |
||||
Intangible assets |
||||
Deferred tax assets |
||||
Other non-current assets |
||||
Current assets |
||||
Inventories |
||||
Trade receivables |
||||
Other current assets and other receivables |
||||
Cash and cash equivalents |
||||
Non-current liabilities |
||||
Non-current lease liabilities |
( |
) | ||
Defined benefit obligation |
( |
) | ||
Deferred tax liabilities |
( |
) | ||
Current liabilities |
||||
Current lease liabilities |
( |
) | ||
Current borrowings |
( |
) | ||
Trade payables |
( |
) | ||
Other current liabilities |
( |
) | ||
Net assets acquired |
||||
TCHF |
||||
Consideration transferred |
||||
Fair value of identifiable net assets |
( |
) | ||
Goodwill |
||||
TCHF |
||||
Cash and cash equivalent balance acquired |
||||
Consideration paid in cash and cash equivalents |
( |
) | ||
Net cash outflow |
( |
) | ||
TCHF |
||||
Cash |
||||
Non-cash (Relief shares) |
||||
Contingent consideration |
||||
Total consideration transferred |
||||
TCHF |
||||
Non-current assets |
||||
Tangible assets |
||||
Right-of-use |
||||
Intangible assets |
||||
Current assets |
||||
Trade receivables |
||||
Inventory |
||||
Other current assets |
||||
Cash and cash equivalents |
||||
Non-current liabilities |
||||
Non-current lease liabilities |
( |
) | ||
Other non-current borrowings |
( |
) | ||
Deferred tax liabilities |
( |
) | ||
Current liabilities |
||||
Current lease liabilities |
( |
) | ||
Other current borrowings |
||||
Trade payables |
( |
) | ||
Provisions |
( |
) | ||
Other current liabilities |
( |
) | ||
Net assets acquired |
||||
TCHF |
||||
Cash and cash equivalent balance acquired |
||||
./. Loan due to Relief by the acquired subsidiary |
( |
) | ||
./. Consideration paid in cash and cash equivalents |
||||
Net cash outflow |
( |
) | ||
TCHF |
Technologies, patents and trademarks |
Licenses |
In-process research and development |
Goodwill |
Total |
|||||||||||||||
Historical cost |
||||||||||||||||||||
January 1, 2021 |
— |
— |
— |
|||||||||||||||||
Addition |
— | — | — | |||||||||||||||||
Business combination |
— | |||||||||||||||||||
December 31, 2021 |
||||||||||||||||||||
Addition |
— | — | ||||||||||||||||||
December 31, 2022 |
||||||||||||||||||||
Accumulated amortization and impairment |
||||||||||||||||||||
January 1, 2021 |
— |
— |
— |
— |
— |
|||||||||||||||
Amortization |
( |
) | — | — | — | ( |
) | |||||||||||||
December 31, 2021 |
( |
) |
— |
— |
— |
( |
) | |||||||||||||
Amortization |
( |
) | — | — | — | ( |
) | |||||||||||||
Impairment |
( |
) | — | ( |
) | ( |
) | ( |
) | |||||||||||
December 31, 2022 |
( |
) |
— |
( |
) |
( |
) |
( |
) | |||||||||||
Carrying amount per class |
||||||||||||||||||||
December 31, 2021 |
||||||||||||||||||||
December 31, 2022 |
||||||||||||||||||||
Carrying amount per asset |
||||||||||||||||||||
PKU Golike |
— | — | — | |||||||||||||||||
Diclofenac |
— | — | ||||||||||||||||||
ACER-001 |
— | — | ||||||||||||||||||
RLF-100 |
— | — | ||||||||||||||||||
RLF-TD011 |
— | — | ||||||||||||||||||
Sentinox |
— | — | — | |||||||||||||||||
RLF-OD32 |
— | — | — | |||||||||||||||||
Other |
— | — | — | — | — | |||||||||||||||
December 31, 2022 |
||||||||||||||||||||
PKU Golike |
— | — | ||||||||||||||||||
Diclofenac |
— | — | ||||||||||||||||||
ACER-001 |
— | — | ||||||||||||||||||
RLF-100 |
— | — | ||||||||||||||||||
RLF-TD011 |
— | — | ||||||||||||||||||
Sentinox |
— | — | ||||||||||||||||||
Other |
— | — | ||||||||||||||||||
December 31, 2021 |
||||||||||||||||||||
| • | PKU Golike ® , an amino acid mix product commercialized by Relief for the dietary management of phenylketonuria. |
| • | Diclofenac, a product line indicated for the treatment of inflammatory conditions and pain management. The active ingredient diclofenac is combined with Relief’s proprietary technologies in products with immediate release formulation, or in the form of a topical patch. These products are commercialized by third parties under different brand names, including Cambia ® , Voltfast® and Voltadol® . |
| • | RLF-100 ® , a medicinal product candidate under development in inhaled and intravenous formulations to prevent and resolve respiratory failure and its complications. It was initially acquired in 2016 in the business combination between Relief Therapeutics SA and THERAMetrics Holding AG. The Group gained additional expertise and intellectual property rights around the inhaled formulation of aviptadil with the acquisition of AdVita in 2021. Relief is developing RLF-100 for the treatment of acute respiratory distress syndrome (“ARDS”) associated with COVID-19, non-COVID-19-related |
| • | RLF-TD011, a phase 2 clinical-stage drug candidate for the management of wounds in patients with epidermolysis bullosa. Manufactured using the Group’s proprietary TEHCLO Nanotechnology™ , RLF-TD011 is a differentiated acid oxidizing solution of hypochlorous acid with an anti-microbial and anti-inflammatory activity with the potential to treat wound colonization, reduce local inflammation, alleviate symptoms and hasten wound healing in epidermolysis bullosa. |
| • | Sentinox ™ , a near-to-market |
| • | RLF-OD32, a novel dosage form of a prescription drug already approved by the U.S. Food and Drug Administration and intended for the treatment of patients with phenylketonuria. In July 2022, the Group executed a definitive agreement with Meta Healthcare Ltd., acquiring the worldwide rights, except for the United Kingdom, for the in-development product. The acquisition cost of TCHF |
| • | Cash flow projections were based on a financial forecast developed by management, which includes projections for net sales, cost of sales, and development costs. These projections are periodically reviewed and updated by management. |
| • | Revenue projections were based on a product-specific analysis that considered relevant market sizes, disease prevalence, incidence rates, expected market share, expected patent life, and the expected year of regulatory approval for unapproved product candidates based on the current stage of development and expected development plan. |
| • | Forecast periods were defined on a product basis and based on product life cycles. For on-market products, cash flows were projected for each CGU over a period of |
| • | Probabilities of success for in-process projects to reach final development and commercialization ranged from |
| • | Pre-tax discount rate was |
| • | The intangible assets associated with RLF-TD11 had an estimated recoverable amount that approximated the carrying amount of TCHF one-year delay in regulatory approval, or a reduction of |
| • | The intangible assets associated with RLF-100 had an estimated recoverable amount that exceeded by TCHF pre-tax discount rate by two-year market launch delay would result in an impairment. |
| • | The intangible assets associated with Diclofenac had an estimated recoverable amount that approximated the carrying amount of TCHF |
| • | The intangible assets associated with PKU Golike and Sentinox had estimated recoverable amounts that exactly matched their carrying amounts due to impairment at the end of the current reporting period. They are inherently sensitive to any changes in assumptions which would result in future impairments. |
| TCHF |
Building |
Equipment |
Total |
|||||||||
| Historical cost |
||||||||||||
| January 1, 2021 |
||||||||||||
| |
|
|
|
|
|
|||||||
| Business combination |
||||||||||||
| Disposal |
— | ( |
) | ( |
) | |||||||
| Foreign exchange difference |
( |
) | ( |
) | ( |
) | ||||||
| December 31, 2021 |
||||||||||||
| |
|
|
|
|
|
|||||||
| Addition |
— | |||||||||||
| Foreign exchange difference |
( |
) | ( |
) | ( |
) | ||||||
| December 31, 2022 |
||||||||||||
| |
|
|
|
|
|
|||||||
| Accumulated depreciation |
||||||||||||
| January 1, 2021 |
||||||||||||
| |
|
|
|
|
|
|||||||
| Depreciation |
( |
) | ( |
) | ( |
) | ||||||
| Foreign exchange difference |
— | |||||||||||
| December 31, 2021 |
( |
) |
( |
) |
( |
) | ||||||
| |
|
|
|
|
|
|||||||
| Depreciation |
( |
) | ( |
) | ( |
) | ||||||
| Foreign exchange difference |
— | |||||||||||
| December 31, 2022 |
( |
) |
( |
) |
( |
) | ||||||
| |
|
|
|
|
|
|||||||
| Carrying amount |
||||||||||||
| at December 31, 2021 |
||||||||||||
| at December 31, 2022 |
||||||||||||
| |
|
|
|
|
|
|||||||
| TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
| < 1 year |
||||||||
| 1-5 years |
||||||||
| > 5 years |
||||||||
| |
|
|
|
|||||
| Total |
||||||||
| |
|
|
|
|||||
| TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
| Lease expense for short-term and low value leases |
||||||||
| Depreciation expense on right-of-use |
||||||||
| Interest expense on lease liabilities (note 28) |
||||||||
| TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
| Raw material |
||||||||
| Finished goods |
||||||||
| |
|
|
|
|||||
| Gross inventories |
||||||||
| Valuation allowance |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Total |
||||||||
| |
|
|
|
|||||
| TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
| Current receivables |
||||||||
| Expected credit loss allowance |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Total |
||||||||
| |
|
|
|
|||||
| TCHF |
2022 |
2021 |
||||||
| Balance at beginning of year |
( |
) | ||||||
| Acquired through business combination |
— | ( |
) | |||||
| Impairment losses recognized |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Balance at end of year |
( |
) |
( |
) | ||||
| |
|
|
|
|||||
| TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
| Prepaid expenses |
||||||||
| Accrued revenue |
||||||||
| VAT receivable |
||||||||
| Deposits |
||||||||
| Indemnification asset (note 18) |
— | |||||||
| Other current receivables |
||||||||
| |
|
|
|
|||||
| Total |
||||||||
| |
|
|
|
|||||
Number of shares |
||||||||||||
Common shares |
Treasury shares |
Total |
||||||||||
Balance at January 1, 2021 |
— |
|||||||||||
Issuance of treasury shares |
( |
) | — | |||||||||
Direct Share Placement program |
— | |||||||||||
Private placements |
— | |||||||||||
Acquisition payments |
— | |||||||||||
Exercises of options |
— | |||||||||||
Balance at December 31, 2021 |
( |
) |
||||||||||
Balance at January 1, 2022 |
( |
) |
||||||||||
Issuance of treasury shares |
( |
) | — | |||||||||
Direct Share Placement program |
— | |||||||||||
Milestone payments |
— | |||||||||||
Exercises of options |
— | |||||||||||
Balance at December 31, 2022 |
( |
) |
||||||||||
| • | DSP program: sale of |
| • | Exercises of options: issuance upon exercise of |
| • | Private placement in March 2021: sale of |
| • | Private placement in July 2021: sale of |
| • | DSP program: sale of |
| • | Exercises of options: issuance upon exercise of |
TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
Share premium (note 15.1) |
||||||||
Share-based payment reserve (note 15.2) |
||||||||
Foreign currency translation reserve (note 15.3) |
||||||||
Total |
||||||||
TCHF |
2022 |
2021 |
||||||
Balance at beginning of year |
||||||||
Additional paid-in capital from capital increases |
||||||||
Transaction cost in relation to capital increases |
( |
) | ( |
) | ||||
Balance at end of year |
||||||||
TCHF |
2022 |
2021 |
||||||
Balance at beginning of year |
||||||||
Share-based payments (note 30) |
||||||||
Balance at end of year |
||||||||
| TCHF |
2022 |
2021 |
||||||
| Balance at beginning of year |
— | |||||||
| Exchange differences arising on translating foreign operations |
||||||||
| |
|
|
|
|||||
| Balance at end of year |
||||||||
| |
|
|
|
|||||
| TCHF |
December 31, 2022 |
December 31, 2021 |
||||||||||||||
Non-current |
Current |
Non-current |
Current |
|||||||||||||
| Bank loans |
||||||||||||||||
| Other financial liability |
— | — | — | |||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
| Present value of pension benefit obligation |
||||||||
| Fair value of pension plan assets |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Net pension defined benefit obligation |
||||||||
| Present value of other benefit obligations |
||||||||
| |
|
|
|
|||||
| Total defined benefit obligations |
||||||||
| |
|
|
|
|||||
| TCHF |
2022 |
2021 |
||||||
| Current service cost |
||||||||
| Net interest expense |
||||||||
| Administration cost excl. cost for managing plan assets |
||||||||
| |
|
|
|
|||||
| Expense recognized in profit or loss |
||||||||
| |
|
|
|
|||||
| TCHF |
2022 |
2021 |
||||||
| Remeasurement (gain)/loss on defined benefit obligation due to changes in financial assumptions |
( |
) | ( |
) | ||||
| due to changes in experience adjustments |
( |
) | ||||||
| Return on plan assets excl. interest income |
||||||||
| |
|
|
|
|||||
| (Income) recognized in other comprehensive income |
( |
) |
( |
) | ||||
| |
|
|
|
|||||
| TCHF |
2022 |
2021 |
||||||
| Opening defined benefit obligation |
||||||||
| Current service cost |
||||||||
| Past service cost |
( |
) | ||||||
| Interest expense on defined benefit obligation |
||||||||
| Contributions from plan participants |
||||||||
| Benefits (paid)/deposited |
( |
) | ||||||
| Remeasurement (gain)/loss due to changes in financial assumptions |
( |
) | ( |
) | ||||
| Remeasurement (gain)/loss due to changes in experience adjustments |
( |
) | ||||||
| Acquired through business combinations |
||||||||
| |
|
|
|
|||||
| Closing defined benefit obligation |
||||||||
| |
|
|
|
|||||
| TCHF |
2022 |
2021 |
||||||
| Opening fair value of plan assets |
||||||||
| Interest income on plan assets |
||||||||
| Return on plan assets excluding interest income |
( |
) | ( |
) | ||||
| Contributions from the employer |
||||||||
| Contributions from plan participants |
||||||||
| Benefits (paid)/deposited |
( |
) | ||||||
| Administration cost |
( |
) | ( |
) | ||||
| Acquisition through business combination |
||||||||
| |
|
|
|
|||||
| Closing fair value of plan assets |
||||||||
| |
|
|
|
|||||
| TCHF |
2022 |
2021 |
||||||
| Discount rates |
% | % | ||||||
| Expected rates of salary increase |
% | % | ||||||
| • | A |
| • | If the expected salary growth were to increase (decrease) by |
TCHF |
Contingent liabilities (i) |
Legal and regulatory (ii) |
Total |
|||||||||
Balance at January 1, 2022 |
||||||||||||
Reversal of provision |
— | ( |
) | ( |
) | |||||||
Payment upon reaching milestones |
( |
) | — | ( |
) | |||||||
Unwinding of discount on provisions |
— | |||||||||||
Variation due to assumption adjustment |
( |
) | ( |
) | ||||||||
Foreign exchange difference |
( |
) | — | ( |
) | |||||||
Balance at December 31, 2022 |
||||||||||||
thereof current |
||||||||||||
thereof non-current |
— | |||||||||||
TCHF |
December 31, 2022 |
December 31, 2021 |
||||||
Accrued expenses |
||||||||
Payroll tax and social security liabilities |
||||||||
Stamp duty and capital tax liabilities |
||||||||
Deferred revenue |
— | |||||||
Other current liabilities |
||||||||
Total |
||||||||
TCHF |
2022 |
2021 |
||||||
Gain from adjustment in fair value of contingent liabilities (note 18) |
||||||||
Gain from reversal of impairment on financial assets (i) |
||||||||
Reversal of impairment losses on receivables |
||||||||
Income from sublease agreements |
||||||||
Write-off of liabilities due to a former subsidiary |
||||||||
Write-off of old liabilities |
||||||||
Various other |
||||||||
Total other gains |
||||||||
| (i) | In 2020, the Group had provided a loan of TUSD RLF-100 as part of a collaboration agreement. The loan was repaid in April 2022 pursuant to its terms. The impairment allowance, which was recognized in prior periods, was reversed in 2022 resulting in a gain of TCHF |
TCHF |
2022 |
2021 |
||||||
Salaries and social security |
||||||||
Independent contractors fees |
||||||||
Share-based payment expense (note 30) |
||||||||
Service cost for other benefit obligations (note 17) |
( |
) | ||||||
Total personnel expenses |
||||||||
TCHF |
2022 |
2021 |
||||||
Professional services |
||||||||
Other administrative expenses |
||||||||
Total other administrative expenses |
||||||||
TCHF |
2022 |
2021 |
||||||
Losses on financial assets at fair value through profit or loss |
||||||||
Impairment losses on loans to third parties |
||||||||
Various other |
||||||||
Total other losses |
||||||||
TCHF |
2022 |
2021 |
||||||
Amortization of intangible assets (note 8) |
||||||||
Depreciation of rights-of-use |
||||||||
Depreciation of property and equipment |
||||||||
Total amortization and depreciation expense |
||||||||
TCHF |
2022 |
2021 |
||||||
Interest income |
||||||||
Foreign exchange gain, net |
||||||||
Total financial income |
||||||||
Unwinding of discount on provisions (note 18) |
( |
) | ( |
) | ||||
SSF commitment fee (note 19) |
( |
) | ( |
) | ||||
Negative interest on cash deposits |
( |
) | ( |
) | ||||
Interest expense related to leases |
( |
) | ( |
) | ||||
Other interest expenses |
( |
) | ( |
) | ||||
Bank charges |
( |
) | ( |
) | ||||
Foreign exchange loss, net |
( |
) | ||||||
Total financial expenses |
( |
) |
( |
) | ||||
TCHF |
2022 |
2021 |
||||||
Current tax |
||||||||
Current tax expense for the year |
||||||||
Adjustments in current tax of prior years |
||||||||
Deferred tax |
||||||||
Deferred tax (income)/expense recognized in the year |
( |
) | ( |
) | ||||
Write-down of deferred tax assets |
||||||||
( |
) |
( |
) | |||||
Net income tax gain |
( |
) |
( |
) | ||||
TCHF |
2022 |
2021 |
||||||
Loss before tax |
( |
) | ( |
) | ||||
Income tax expense calculated at |
( |
) | ( |
) | ||||
Unrecognized deferred tax assets during the year |
||||||||
Write-down of deferred tax assets |
||||||||
Effect of deferred tax balances due to difference in applicable tax rates |
( |
) | ( |
) | ||||
Effect of net (income)/expense that is not added/(deductible) |
( |
) | ( |
) | ||||
Income tax recognized in the current year |
( |
) |
( |
) | ||||
2022 TCHF |
Opening balance |
Recognized in OCI |
Recognized in profit or loss |
Closing balance |
||||||||||||
Tax losses |
( |
) | ||||||||||||||
Defined benefit obligation |
( |
) | ||||||||||||||
Intangible assets |
( |
) | ||||||||||||||
Leases |
( |
) | ||||||||||||||
Total deferred tax assets |
— | ( |
) |
|||||||||||||
Intangible assets |
— | ( |
) | |||||||||||||
Total deferred tax liabilities |
— |
( |
) |
|||||||||||||
2021 TCHF |
Opening balance |
Business combination |
Recognized in OCI |
Recognized in profit or loss |
Closing balance |
|||||||||||||||
Tax losses |
||||||||||||||||||||
Defined benefit obligation |
( |
) | ||||||||||||||||||
Intangible assets |
( |
) | ||||||||||||||||||
Financial instruments |
( |
) | ||||||||||||||||||
Leases |
||||||||||||||||||||
Total deferred tax assets |
( |
) |
||||||||||||||||||
Intangible assets |
— |
( |
) | |||||||||||||||||
Total deferred tax liabilities |
— |
( |
) |
|||||||||||||||||
TCHF |
2022 |
2021 |
||||||
Within one year |
||||||||
Later than one year and not later than five years |
||||||||
More than five years |
||||||||
Total tax losses carry forward |
||||||||
2022 |
2021 |
|||||||
At beginning of the year |
||||||||
Granted |
||||||||
Exercised 1 |
( |
) | ( |
) | ||||
Forfeited |
( |
) | ( |
) | ||||
At end of the year |
||||||||
1 |
In 2022, the weighted average exercise price was CHF |
Expiration year |
December 31, 2022 |
December 31, 2021 |
||||||
2022 |
||||||||
2023 |
||||||||
2024 |
||||||||
2025 |
||||||||
2026 |
||||||||
2027 |
||||||||
2028 |
||||||||
2029 |
||||||||
2030 |
||||||||
Weighted average remaining contractual life in months |
||||||||
2022 |
2021 |
|||||||
Loss attributable to shareholders (in TCHF) |
( |
) | ( |
) | ||||
Weighted average number of shares |
||||||||
Basic and diluted loss per share (in CHF) |
( |
) |
( |
) | ||||
December 31, 2022 TCHF |
Financial assets at amortised cost |
Financial liabilities at amortised cost |
Financial liabilities at FVTPL |
Total |
||||||||||||
Other non-current assets |
— | — | ||||||||||||||
Trade receivables |
— | — | ||||||||||||||
Other current assets and receivables |
— | — | ||||||||||||||
Cash and cash equivalents |
— | — | ||||||||||||||
Total financial assets |
— | — | ||||||||||||||
Non-current lease liabilities |
— | — | ||||||||||||||
Non-current borrowings |
— | — | ||||||||||||||
Current lease liabilities |
— | — | ||||||||||||||
Current borrowings |
— | — | ||||||||||||||
Provisions for milestone payments |
— | |||||||||||||||
Trade payables |
— | — | ||||||||||||||
Financial liabilities due to related parties |
— | — | ||||||||||||||
Other current payables and liabilities |
— | — | ||||||||||||||
Total financial liabilities |
— | |||||||||||||||
December 31, 2021 TCHF |
Financial assets at amortized cost |
Financial liabilities at amortized cost |
Financial liabilities at FVTPL |
Total |
||||||||||||
Other non-current assets |
— | — | ||||||||||||||
Trade receivables |
— | — | ||||||||||||||
Other current assets and receivables |
— | — | ||||||||||||||
Cash and cash equivalents |
— | — | ||||||||||||||
Total financial assets |
— |
— |
||||||||||||||
Non-current lease liabilities |
— | — | ||||||||||||||
Non-current borrowings |
— | — | ||||||||||||||
Current lease liabilities |
— | — | ||||||||||||||
Current borrowings |
— | — | ||||||||||||||
Provisions for milestone payments |
— | |||||||||||||||
Trade payables |
— | — | ||||||||||||||
Financial liabilities due to related parties |
— | — | ||||||||||||||
Other current payables and liabilities |
— | — | ||||||||||||||
Total financial liabilities |
— |
|||||||||||||||
Non cash-changes |
||||||||||||||||||||||||
2022 TCHF |
Opening balance |
Financing cash flows |
New leases |
Accrued interest |
Foreign exchange |
Closing balance |
||||||||||||||||||
Lease liabilities (note 9.2) |
( |
) | ( |
) | ||||||||||||||||||||
Borrowings (note 16) |
( |
) | ( |
) | ||||||||||||||||||||
Due to related parties (note 19) |
||||||||||||||||||||||||
Total |
( |
) |
( |
) |
||||||||||||||||||||
Non-cash changes |
||||||||||||||||||||||||||||
2021 TCHF |
Opening balance |
Financing cash flows |
Gain on settlement |
Business Combinat. |
Accrued interest |
Foreign exchange |
Closing balance |
|||||||||||||||||||||
Lease liabilities |
( |
) | ( |
) | ||||||||||||||||||||||||
Borrowings (note 16) |
( |
) | ( |
) | ||||||||||||||||||||||||
Due to third parties |
( |
) | ||||||||||||||||||||||||||
Due to related parties (note 19) |
||||||||||||||||||||||||||||
Total |
( |
) |
( |
) |
( |
) |
||||||||||||||||||||||
TCHF |
2022 |
2021 |
||||||
Short-term employee benefits |
||||||||
Post-employment benefits |
||||||||
Share-based compensation |
||||||||
Compensation to key management |
||||||||