UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
INFORMATION FOR SHAREHOLDERS (Mark One)
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
or
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
or
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
or
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number
(Exact name of Registrant as specified in its charter)
(Jurisdiction of incorporation or organization) |
(Address of principal executive offices)
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading Symbol |
| Name on each exchange on which registered |
| New York Stock Exchange* |
* Not for , but only in connection with the first registration of American Depositary Shares, pursuant to the requirements of the Securities and Exchange Commission.
Securities registered or to be registered pursuant to Section 12(g) of the Act: None.
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None.
Indicate the number of outstanding shares of each of the issuer’s class of capital or common stock as of the close of the period covered by the annual report:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Yes ◻
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days:
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer, “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Accelerated Filer ◻ | Non-accelerated filer ◻ | |
Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ◻
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
◻ U.S. GAAP |
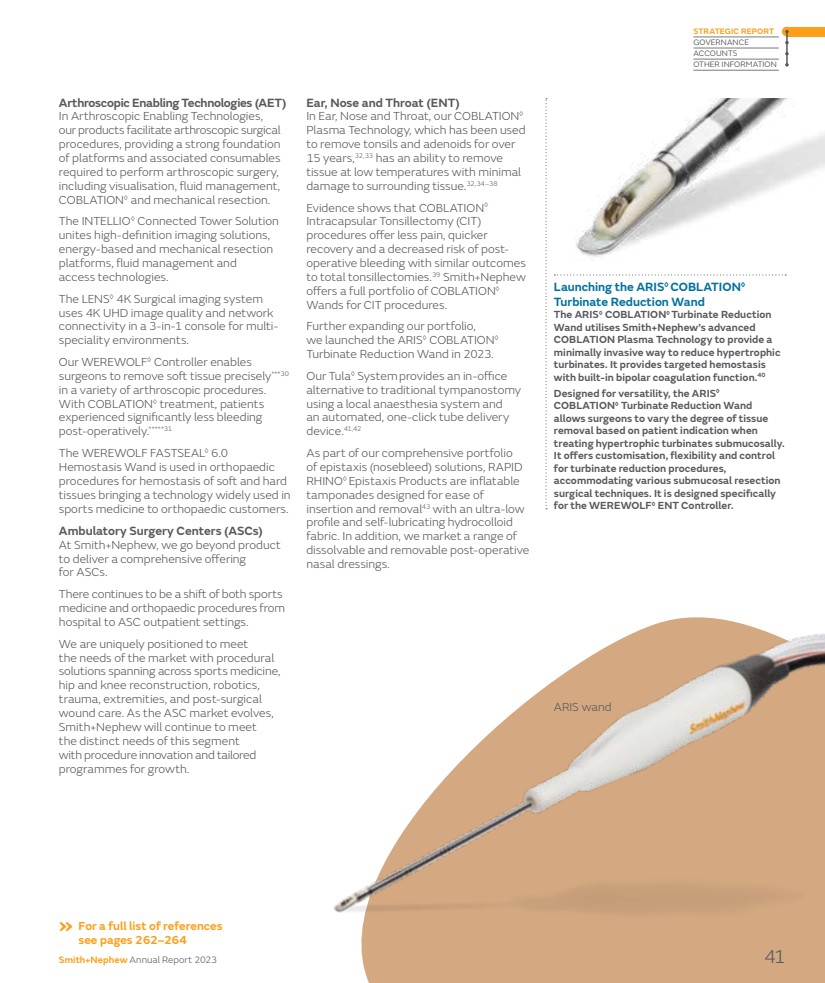
| ⌧ |
| ◻ Other |
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow: Item 17 ◻ Item 18 ◻
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
| Life Unlimited Annual Report 2023 |
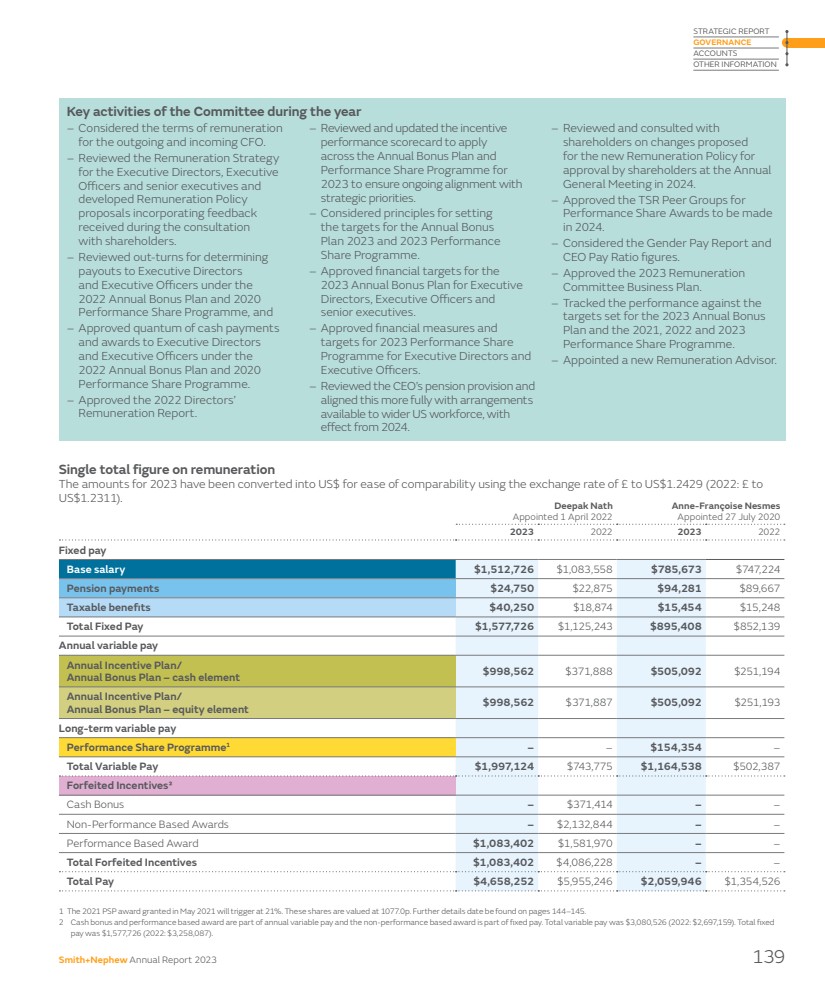
| Contents Our performance Strategic Report Our performance IFC Who we are 2 Chair’s statement 4 Chief Executive Officer’s review 8 Our marketplace 14 Our business model 16 Key Performance Indicators 18 Financial review 20 Creating value through innovation 26 Taking our innovation to market 34 Building a culture of belonging 46 Shaping a healthy and sustainable future 52 Risk report 67 Our stakeholders 82 Engaging with stakeholders 84 Governance Governance at a glance 88 Board leadership and Company purpose 90 Nomination & Governance Committee Report 102 Compliance & Culture Committee Report 111 Audit Committee Report 114 Directors’ Remuneration Report 121 Accounts Statement of Directors’ responsibilities 156 Report of Independent Registered Public Accounting Firm 157 Group income statement 172 Group statement of comprehensive income 172 Group balance sheet 173 Group cash flow statement 174 Group statement of changes in equity 175 Notes to the Group accounts 176 Company financial statements 227 Notes to the Company accounts 229 Other information Group information 235 Other information 236 Shareholder information 248 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. The images used throughout the report represent the ways that Smith+Nephew is taking the limits off living and helping patients live Life Unlimited. Images used are not photographs of our patients unless expressly indicated. $5,549m Group revenue 37.5¢ Unchanged +6.4% Dividend per share Reported +7.2% Underlying1 $425m -5.4% Operating profit 7.7% -90bps Operating profit margin $970m +7.6% Trading profit1 17.5% +20bps Trading profit margin1 30.2¢ +1.3% Earnings per share (EPS) 82.8¢ +18.2% Adjusted earnings per share1 (EPSA) $829m +42.7% Cash generated from operations $635m +43.0% Trading cash flow1 $339m -1.8% R&D investment 5.9% -70bps Return on invested capital1 (ROIC) |
| Physical health is never just about our body. It’s our mind, feelings and ambitions. When something holds us back, it’s our whole life on hold. We’re here to change that, to use technology to take the limits off living, and help other medical professionals do the same. So that patients can stare down fear, see that anything is possible, then go on stronger. Inspired by a simple promise. Two words that bring together all we do… Life Unlimited To learn more about our purpose visit www.smith-nephew.com Smith+Nephew Annual Report 2023 1 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Who we are Creating value through innovation Research & Development Developing new technology through our Research & Development (R&D) programme, and acquiring exciting technologies where we can add value. Medical education The Smith+Nephew Academy network supports the safe and effective use of our products and provides opportunities to learn innovative clinical techniques. Manufacturing Building resilient manufacturing and supply chains to ensure quality and competitiveness and support new product development. Shaping a healthy and sustainable future Our ESG strategy supports our Strategy for Growth and strengthens the foundation to help us serve customers over the long term. Our ESG strategy focuses on three areas: People, Planet and Products. » People: Creating a lasting positive impact on our communities. » Planet: Aiming to reduce our impact on the environment. » Products: Innovating sustainably. Building a culture of belonging We strive to create a culture of belonging where employees can bring their full selves and best ideas, which fosters innovation, delivers business success and strengthens engagement and personal fulfilment. Our culture is based on our values of Care, Courage and Collaboration. » Care: A culture of empathy and understanding for each other, our customers and their patients. » Courage: A culture of continuous learning, innovation and accountability. » Collaboration: A culture of teamwork based on mutual trust and respect. » See pages 26–29 » See pages 30–31 » See pages 32–33 » See pages 52–66 for information on our sustainability targets and progress » See pages 46–49 for more on how we are building our culture 168 year history 14+ million patients treated with our products 100+ countries served $339 million R&D investment 18,452 employees 20 new product launches We are a leading Key facts 2023 portfolio medical technology company. We exist to restore people’s bodies and their self-belief. 2 Smith+Nephew Annual Report 2023 |
| Taking our innovation to market We take our innovation to market through three global business units of Orthopaedics, Sports Medicine & ENT, and Advanced Wound Management. These business units are responsible for strategy and global marketing, and contain specialist sales and support teams dedicated to serving the specific requirements of our healthcare professional customers. Serving our customers through our sales force We pride ourselves on giving customers a high standard of service through our specialist sales and clinical support teams. Representatives in our surgical businesses have a detailed knowledge of the products and instruments that they sell and the surgical techniques they may be used for, and provide technical and logistical support to surgeons and hospitals. In Advanced Wound Management, sales representatives develop their knowledge of how clinicians seek to prevent and treat wounds, as well as support customers through their understanding of the economic benefits of using our products within treatment protocols. Sports Medicine & ENT Our Sports Medicine & Ear, Nose and Throat (ENT) businesses offer advanced products and instruments used to repair or remove soft tissue. They operate in growing markets where unmet clinical needs provide opportunities for procedural and technological innovation. Advanced Wound Management Our Advanced Wound Management portfolio provides a comprehensive set of products and services to meet broad and complex clinical needs across hard to heal wounds, delivering on our mission to shape what is possible in wound care. Orthopaedics Orthopaedics includes an innovative range of hip and knee implants used to replace diseased, damaged or worn joints, robotics-assisted and digital enabling technologies and services that empower surgeons, and Trauma & Extremities products used to stabilise severe fractures and correct hard tissue deformities, as well as a shoulder replacement system. 40% of Group revenue 31% of Group revenue 29% of Group revenue » See pages 34–37 » See pages 38–41 » See page 42–45 Smith+Nephew Annual Report 2023 3 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Chair’s statement Encouraged by the progress made, excited by our prospects for the future “Deepak has set out a confident outlook as he leads the business in the Strategy for Growth and the second year of delivery of the 12-Point Plan.” Rupert Soames Chair Dear Fellow Shareholder, It is a great honour to write to you for the first time as Chair of Smith+Nephew, and to share my reflections on the year just gone and the journey ahead. But before I do, I want to pay tribute to my predecessor Roberto Quarta who chaired the Company with great care and diligence for nine, sometimes difficult, years. Most recently, he as Chair and Marc Owen as Senior Independent Director, have supported my induction and transition to Chair with sensitivity and skill, for which I am grateful. Since joining the Board on 26 April 2023, I have been learning about the business: its products and services; its people, customers and competitors; its strengths and weaknesses, as well as the opportunities and threats it faces. In all these things I have been supported by Deepak Nath, our Chief Executive Officer, who has deep knowledge of, and experience in, the MedTech sector. Many of our larger investors have also been generous with their time, candid in their analysis, and speak from many years’ experience of both the sector and Smith+Nephew. In addition, I had the opportunity to meet some of our smaller investors at the Annual General Meeting in April, which was a pleasure to attend and a reminder that ultimately, in all we do, there are savers and pensioners who rely on us to grow the value of their investments. Setting clear priorities In a world in which stakeholders have different, and sometimes conflicting, views on how, and to what end, companies should be run, Boards have to be resolute in discharging their responsibilities in the best interests of the Company as a whole. This means they have to have priorities, and to be clear on what their job is. 4 Smith+Nephew Annual Report 2023 |
| The first priority of the Board is to hire and retain management who can lead Smith+Nephew to be the best business it can be; and then, watching closely, encourage, support, guide and challenge them in their work. As a Board, we are very well aware that Smith+Nephew has not performed to its full potential in recent years. The reasons for this, and more importantly, what management are doing about it, are set out in the Strategy and Operating Reviews, and I am pleased to say that in 2023 there were encouraging signs of progress. In Deepak Nath, we have an exceptionally talented CEO, and the Board is following closely the implementation of the 12-Point Plan he and his executive team developed to enable Smith+Nephew to create sustainable long-term value. Deepak has a rare combination of strategic vision and grasp of detail, and under his leadership the business has begun to gather forward momentum, including accelerating revenue growth. Joining Deepak from December 2023 is John Rogers, who will succeed Anne-Françoise Nesmes as Chief Financial Officer in the first quarter of 2024. John brings long experience as a former CFO of two FTSE 100 companies, and has also managed impressive transformations of companies’ operations. I would like to thank Anne-Françoise for her dedication and support to the business over the last three years, during which she has had to support a change of CEO and the significant impact of Covid on the business. The other priority of the Board is to serve our shareholders and wider stakeholders by governing the business effectively and in accordance with regulation and good practice, but with an emphasis on substance over form, simplicity over complexity, and transparency over opaqueness. Governance at Smith+Nephew embraces many different areas. In terms of risk management, Smith+Nephew shares a similar palette of risks to other manufacturers, but the application of medical devices, products and services in the treatment of people, the global scale of our operations, and the highly regulated environment in which we operate make monitoring of operating risk a key part of the Board’s responsibilities. In matters of corporate regulation and corporate governance, being dual-listed brings a degree of complexity. Smith+Nephew hews to the rules and regulations of both the London Stock Exchange, which is our primary listing, and the New York Stock Exchange. Our NYSE listing as a foreign private issuer brings us under the ambit of the Securities and Exchange Commission, and means that we have made the significant investment required to comply with US regulations including the applicable requirements of the Sarbanes-Oxley Act. Like many listed companies, Smith+Nephew works hard to adapt to a changing landscape of regulation and reporting requirements, all of which seem to have one result in common: significantly more lengthy Annual Reports. But whilst companies can adapt to evolving and greater reporting requirements, be it on audit or environmental or social issues, what they cannot manage is operating within a framework which does not allow them to recruit and retain the management they need to grow. Visiting our Advanced Wound Management R&D and manufacturing facility in Hull, UK 37.5¢ Dividend unchanged Smith+Nephew Annual Report 2023 5 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
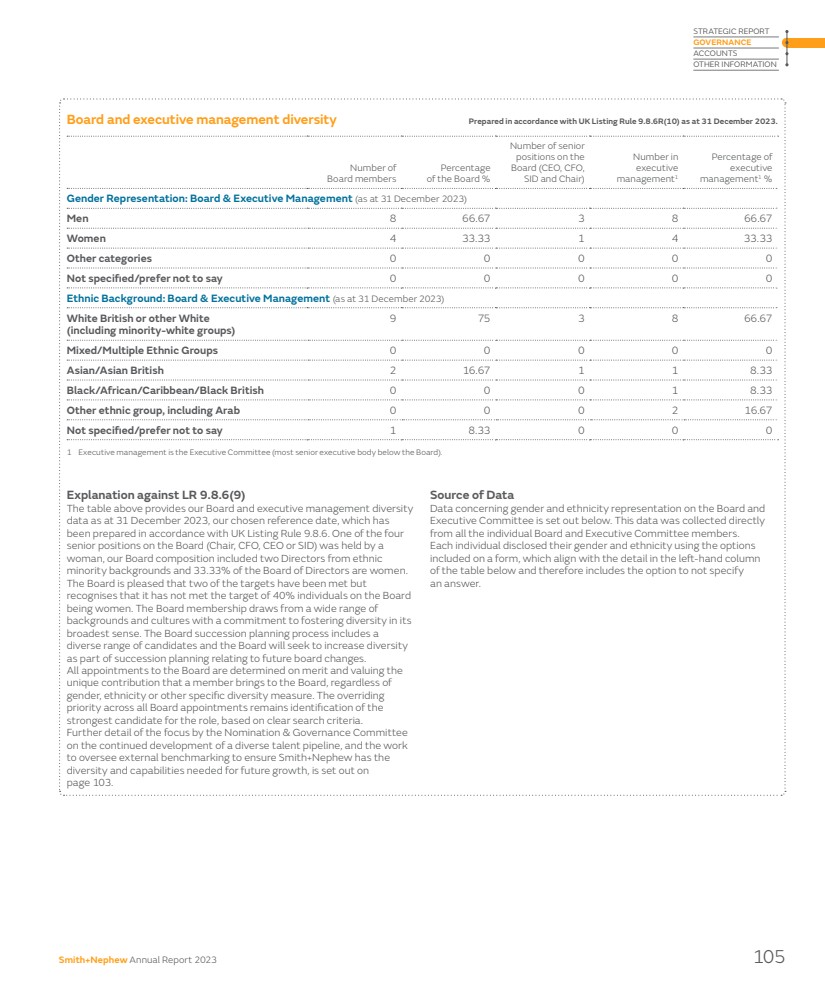
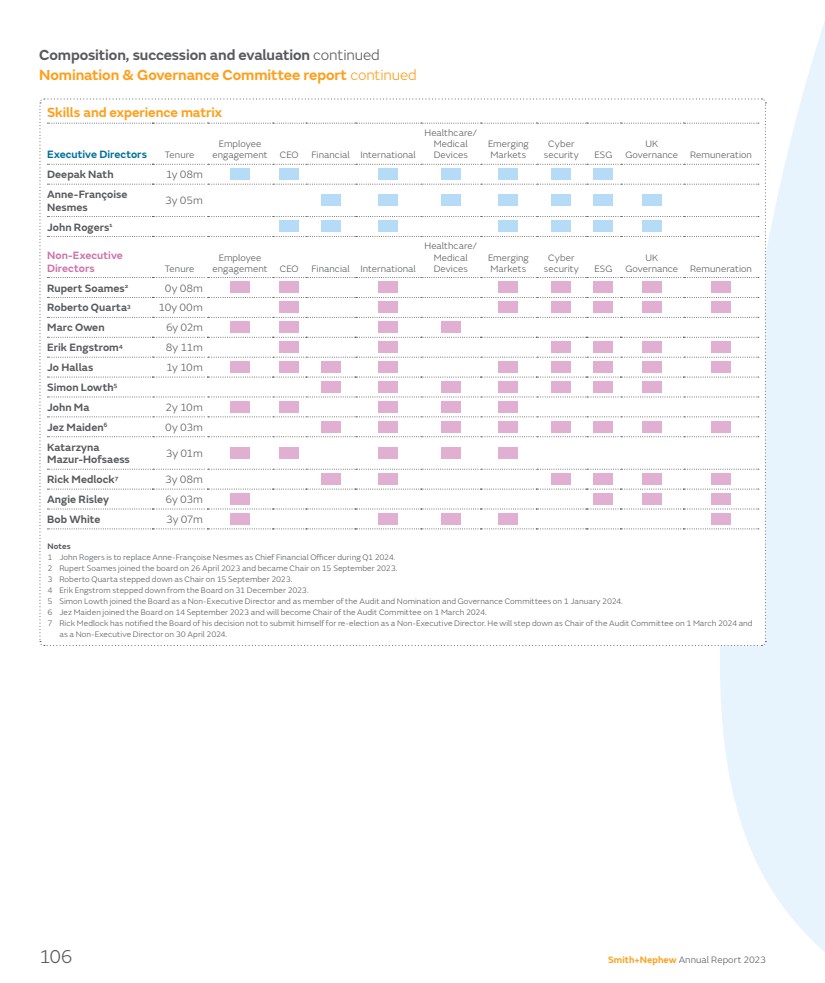
| Smith+Nephew has a proud British heritage – our Company was founded in Hull in 1856 and we have grown over the last 168 years to become a truly global organisation with over 18,000 employees operating in around 100 countries. As a result of that success and global growth, the UK accounts for around 3% of our revenues and 7% of our employees, whilst over 50% of our revenues arise in the US, and nearly all of our senior operational managers, including our CEO, are US citizens, and based in the US. Pay practices differ widely around the world, and it is axiomatic and uncontroversial that companies pay their management teams in line with the norms of the country where they live and work. This approach is accepted without qualm by stakeholders for the millions of people which businesses such as Smith+Nephew employ around the world, with one exception: Executive Directors who are expected to be paid by reference to the norms of the country in which their employer has its primary listing, irrespective of where they actually live, work, and pay tax. This is unique to a listed company environment, and of course does not apply to privately-owned businesses. Currently our remuneration policies for Executive Directors are aligned to the norms of people living and working in the UK; given the small proportion of our revenues that arise in the UK, and the fact that the centre of gravity of the MedTech industry is in the US, this is not sustainable if we are to attract and retain people who live and work in the US. It is for this reason that our Chair of Remuneration, Angie Risley, and I have had extensive consultations with our largest investors in recent months and they have confirmed their broad support for our proposals to give Smith+Nephew the ability to attract and retain senior executives in the United States, if we need to do so. Our 2024 Remuneration Policy proposes a package of long-term incentive plan adjustments for US-based executives to be more closely aligned with norms in the US in terms of structure and quantum, and a comprehensive discussion of our proposals is set out on pages 121–135 of our Remuneration Report. The Board strongly believes that these proposals are in the best interests of the Company and that they will help the Board to execute on its priority to ensure the Company is led by a first-class management team. In other issues pertaining to governance and people, we are committed to fostering diversity in its broadest sense and we continue to ensure that our Board membership draws from a wide range of backgrounds and cultures. Our Board is truly multi-cultural and includes members who are from, live, or work in the US, UK, China, India, Germany and Poland. We continue to review the composition of the Board on an ongoing basis; we actively review diversity in addition to skillsets and capabilities as part of our Board succession planning process and ensure that our candidate selection process for new Board members comprises a balanced slate of candidates for consideration. We consider diversity of candidates on every appointment and selection is based on ensuring we have the best person for the role. When Anne-Françoise steps down from the Board in 2024 our Board will continue to have three experienced female Directors (Angie Risley, Katarzyna Mazur-Hofsaess and Jo Hallas), acknowledging that our percentage of female Board members will, in the short term, reduce from 33.3% to 27.3%. Our Board succession plan will seek to address this as other NEDs step down from the Board. We have announced a number of other changes to our Board this year. I would like to thank Rick Medlock and Erik Engstrom for their highly-effective service. Erik stepped down after nine years on 31 December 2023 and Rick has confirmed to the Board that he will not submit himself for re-election at our AGM in May 2024. In their place, Jez Maiden and Simon Lowth, both of whom have extensive executive and non-executive experience within large and complex global companies, have joined the Board. Until recently, Jez was CFO of Croda International and has held a number of non-executive roles including as Senior Independent Director at Travis Perkins plc. As announced, Jez will assume the role of Chair of our Audit Committee with effect from 1 March 2024. Simon is CFO of BT Group and has previously served as a non-executive director of Standard Chartered. I am delighted that our Board has been able to attract such strong candidates to continue to encourage diversity of perspective and experience on its Board. Taken in the round, I believe that your Board has the skills, diversity, strength and experience to operate effectively in the interests of all stakeholders. You can find more information on our Board and Committees and their work in our Governance Report starting on page 88 of this Annual Report. The Board also places strong emphasis on being a good corporate citizen, supporting our communities and reducing our impact on the planet and its resources. During the year we reviewed progress across our ESG strategy, and welcomed the establishment of a new governance structure and strengthened leadership in this area. More information on our progress against our sustainability targets, including our roadmap to net zero, can be found on pages 52–66. Chair’s statement continued 6 Smith+Nephew Annual Report 2023 |
| 2023 performance 2023 saw Smith+Nephew make progress both in terms of operational performance and financial results. Revenue grew at 6.4% on a reported basis which equates to 7.2% on an underlying basis.1 Trading profit margin1 was slightly ahead of the prior year, but the strong top-line growth meant that trading profit1 grew 7.6% on a reported basis. Operating profit was $425 million, with an operating profit margin of 7.7%. Cash generation from operations improved over the prior year, but was below where it should be going forward. Having considered the performance in the round, and the ongoing investments, the Board is recommending a final dividend of 23.1¢ per share. Together with the interim dividend of 14.4¢ per share, this will give a total distribution of 37.5¢ per share, unchanged from 2022. Our colleagues Before looking ahead in the Outlook, I want to pay tribute on behalf of the Board to our Smith+Nephew colleagues. Having worked in several large and global businesses during some 40 years of executive life, I think I know what good looks like when it comes to corporate culture, and I have been deeply impressed by the resilience, commitment and skill of my colleagues. They have had many dragons to wrestle with in recent years, not the least of which has been a number of leadership changes with their attendant uncertainties and distractions. Throughout they have remained focused on their purpose of helping people to take the limits off living and restore and promote health and wellbeing. I know the Board respects and is deeply grateful for their hard work, and is proud to be part of the same team. Outlook: building momentum Deepak has set out a confident outlook as he leads the business in the Strategy for Growth and the second year of delivery of the 12-Point Plan, and the Board is encouraged by the accountability shown and the progress the business has made in 2023, and excited by the prospects for the future. We look forward to welcoming shareholders to our Annual General Meeting in person in May and to updating you further on the transformation underway at Smith+Nephew. Yours sincerely, Rupert Soames, OBE Chair »See page 88 for our Governance Report »See page 121 for our Remuneration Policy 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. Smith+Nephew Annual Report 2023 7 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Chief Executive Officer’s review Strong revenue growth and improved trading profit margin “The progress we are making is fundamental as we need to have a strong platform from which we will be able to build further shareholder value.” Deepak Nath, PhD Chief Executive Officer Dear Fellow Shareholder, In 2023 we delivered strong revenue growth and an improved trading profit margin1 as the results of our actions to transform Smith+Nephew started to come through. Our 12-Point Plan is on track, with progress beginning to translate into financial outcomes, and our innovation strategy is delivering a strong pipeline of new products that we expect to drive performance in the next few years and beyond. 2023 performance Group revenue in 2023 was $5,549 million, an increase of 7.2% on an underlying basis1 (6.4% reported). This growth was ahead of our full-year guidance published in February 2023 for underlying1 revenue growth between 5.0% and 6.0%, and reflects the strength of the portfolio, with all three business units delivering underlying1 growth above 5% for the full year. Operating profit was $425 million, with an operating profit margin of 7.7%. Trading profit1 for 2023 was up 7.6% on a reported basis to $970 million. The trading profit margin1 was 17.5%, a 20bps improvement on the prior year and in line with our full-year guidance. 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. Transforming Smith+Nephew In July 2022, we announced our 12-Point Plan to fundamentally change the way Smith+Nephew operates, accelerating delivery of our Strategy for Growth and transforming to a consistently higher-growth company. The 12-Point Plan is focused on: 1. Fixing Orthopaedics, to regain momentum across hip and knee implants, robotics and trauma, and win share with our differentiated technology; 2. Improving productivity, to support trading profit margin expansion; and 3. Further accelerating growth in our already well-performing Advanced Wound Management and Sports Medicine & ENT business units. Since inception we have measured our progress across the 12-Point Plan through a set of internal KPIs to drive accountability. The 12-Point Plan is on track and starting to deliver financial outcomes. Work will continue in 2024, with further financial progress expected to follow across the year and in 2025. 8 Smith+Nephew Annual Report 2023 |
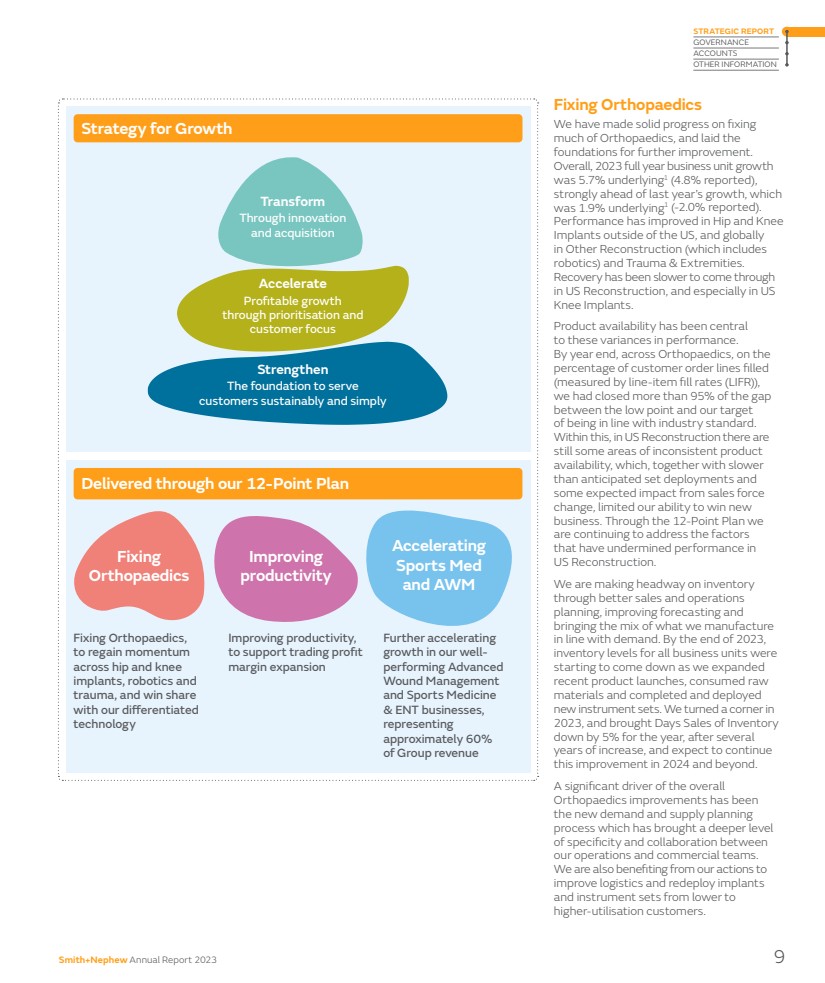
| Fixing Orthopaedics We have made solid progress on fixing much of Orthopaedics, and laid the foundations for further improvement. Overall, 2023 full year business unit growth was 5.7% underlying1 (4.8% reported), strongly ahead of last year’s growth, which was 1.9% underlying1 (-2.0% reported). Performance has improved in Hip and Knee Implants outside of the US, and globally in Other Reconstruction (which includes robotics) and Trauma & Extremities. Recovery has been slower to come through in US Reconstruction, and especially in US Knee Implants. Product availability has been central to these variances in performance. By year end, across Orthopaedics, on the percentage of customer order lines filled (measured by line-item fill rates (LIFR)), we had closed more than 95% of the gap between the low point and our target of being in line with industry standard. Within this, in US Reconstruction there are still some areas of inconsistent product availability, which, together with slower than anticipated set deployments and some expected impact from sales force change, limited our ability to win new business. Through the 12-Point Plan we are continuing to address the factors that have undermined performance in US Reconstruction. We are making headway on inventory through better sales and operations planning, improving forecasting and bringing the mix of what we manufacture in line with demand. By the end of 2023, inventory levels for all business units were starting to come down as we expanded recent product launches, consumed raw materials and completed and deployed new instrument sets. We turned a corner in 2023, and brought Days Sales of Inventory down by 5% for the year, after several years of increase, and expect to continue this improvement in 2024 and beyond. A significant driver of the overall Orthopaedics improvements has been the new demand and supply planning process which has brought a deeper level of specificity and collaboration between our operations and commercial teams. We are also benefiting from our actions to improve logistics and redeploy implants and instrument sets from lower to higher-utilisation customers. Transform Through innovation and acquisition Accelerate Profitable growth through prioritisation and customer focus Strengthen The foundation to serve customers sustainably and simply Strategy for Growth Fixing Orthopaedics, to regain momentum across hip and knee implants, robotics and trauma, and win share with our differentiated technology Improving productivity, to support trading profit margin expansion Further accelerating growth in our well-performing Advanced Wound Management and Sports Medicine & ENT businesses, representing approximately 60% of Group revenue Delivered through our 12-Point Plan Fixing Orthopaedics Improving productivity Accelerating Sports Med and AWM Smith+Nephew Annual Report 2023 9 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Chief Executive Officer’s review continued We have invested in improving our commercial execution. In 2023 we repositioned our offering and undertook deeper sales training for the Orthopaedics team, and enhanced our incentive plans to better align reward with performance, sales mix, robotic placement and implant pull-through. These steps are expected to help us address the performance in Hip and Knee Implants in the US, which remains a priority. At the same time, they will also ensure we sustain the progress we have delivered elsewhere. In Trauma & Extremities, where we have successfully addressed availability of product and instrument sets for our EVOS◊ Plating system, we are focused on maintaining the improved growth dynamic delivered in the second half of 2023. Improving productivity We have made good progress on our actions to improve productivity, contributing around 160bps to our 2023 trading profit margin1. Actions have included updating and standardising pricing strategies across our portfolio and reducing days sales outstanding. We are also making procurement savings to help mitigate cost inflation and drive productivity. During 2023, we deployed an enhanced supplier selection process to identify and award business to suppliers that better align to the global business unit strategies and long-term performance metrics, and better aligned global category strategies to unlock the Smith+Nephew buying power and leverage, helping to drive volume to the most preferred suppliers and reduce cost. In line with our plan, work on manufacturing optimisation is at an earlier stage, with the benefits from network simplification and cost and asset efficiencies expected to support our mid-term margin improvement targets. The underlying work is progressing, with KPIs tracking accordingly. For instance, conversion cost, which is total direct and indirect cost to convert raw materials into finished goods as a percentage of sales, started to come down in the second half. Better aligned supply and demand process has enabled us to critically assess our manufacturing capacity. From a network perspective we are reducing excess capacity, having exited one small site in France and announced the closures of two more in China and Germany. Over the last two years we have also reduced hiring and our reliance on contingent workers. Accelerating AWM and Sports Medicine The important third pillar of the 12-Point Plan is focused on building on our consistent above-market performance from our Advanced Wound Management and Sports Medicine & ENT business units. Progress is also coming through across this workstream. Our negative pressure wound therapy business is benefiting from focused additional resource behind our sales force, delivering strong growth in 2023 across both our traditional RENASYS◊ Negative Pressure Wound Therapy System and our single-use PICO◊ Negative Pressure Wound Therapy System. We are pleased with our progress across Ambulatory Surgical Centers (ASCs), as we more than tripled the pace of cross-business unit deals between our Orthopaedics and Sports Medicine businesses in 2023. Under the 12-Point Plan we have developed a coordinated approach across these business units overseen by a dedicated strategic sales team. We are building on the strong position established by our Sports Medicine business, which is already the preferred choice for a large proportion of the ASC market, and successfully introducing our Orthopaedics portfolio. Creating value through innovation Innovation through our R&D programme is central to our higher-growth ambitions. In 2023, approaching half of our full year underlying revenue growth came from products launched in the last five years. Encouragingly, some of our key growth platforms like our robotics-enabled CORI◊ Surgical System, our EVOS◊ trauma plating platform and our REGENETEN◊ Bioinductive Implant for biological healing are not only contributing to growth today, but also have multi-year runways still ahead of them as we expand applications and launch in new markets. In 2023 we delivered a good cadence of new product launches, completing 20 with development finished on a further two ahead of launch in 2024. These included expanding CORI◊ , adding functionality and AI powered planning tools. We introduced our AETOS◊ Shoulder System, an important part of our growth plans for Trauma & Extremities which will enable Smith+Nephew to compete effectively in the $1.7 billion shoulder market, which, at around 9% CAGR, is one of the fastest growing segments in Orthopaedics. In Advanced Wound Management, we are at the early stages of rolling out the new RENASYS◊ EDGE NPWT System. RENASYS◊ EDGE brings an important new option to customers looking for enhanced intuitiveness, simplicity and durability, especially important for home-care settings. We also continued to invest behind our Sports Medicine portfolio, for instance launching REGENETEN◊ in China, India and Japan. Acquisition of CartiHeal In recent years we have successfully augmented our R&D programmes with acquisitions of exciting technologies. During the year we announced another such acquisition, CartiHeal, the developer of the CARTIHEAL◊ AGILI-C◊ Cartilage Repair Implant, a novel sports medicine technology for cartilage regeneration in the knee. CARTIHEAL◊ AGILI-C◊ is an off-the-shelf one-step treatment for osteochondral (bone and cartilage) lesions with a broader indication than existing treatments. It is indicated to treat a wide patient population, including those with lesions in knees with mild to moderate osteoarthritis, a previously unaddressed condition. Our expertise in regenerative therapy and leadership in knee repair gives me great confidence that this will be a significant value creator for Smith+Nephew over the mid-term. You can read more about our R&D programme and CARTIHEAL◊ AGILI-C◊ on pages 26–29. 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. 10 Smith+Nephew Annual Report 2023 |
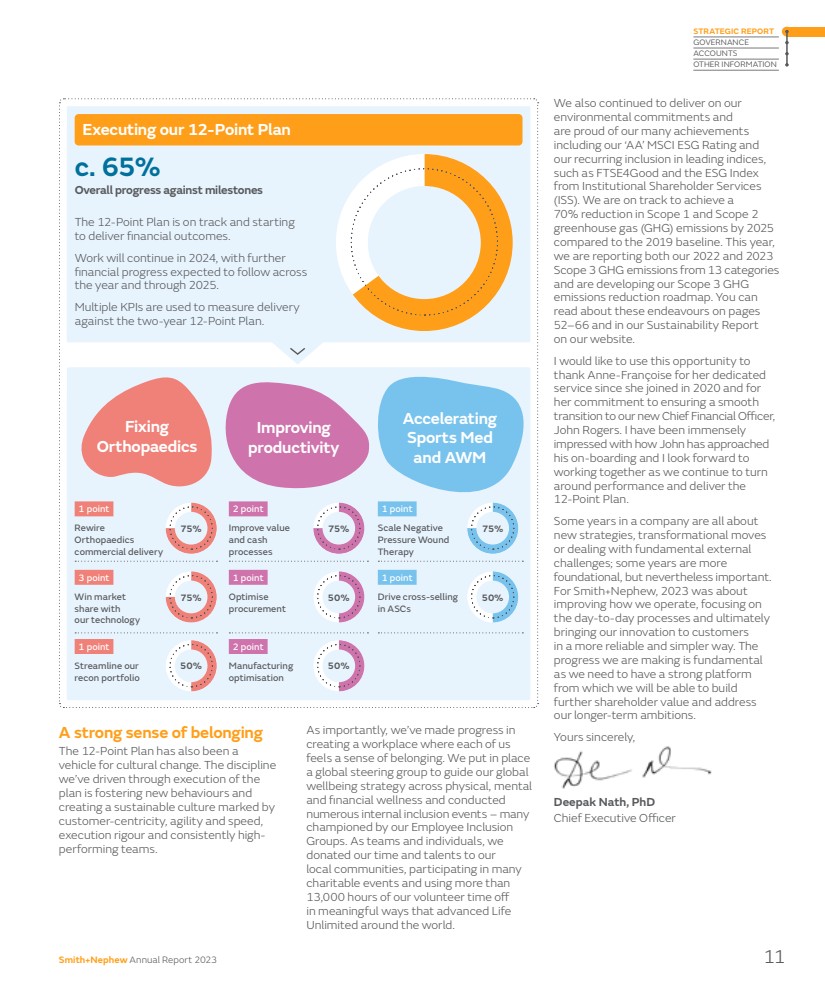
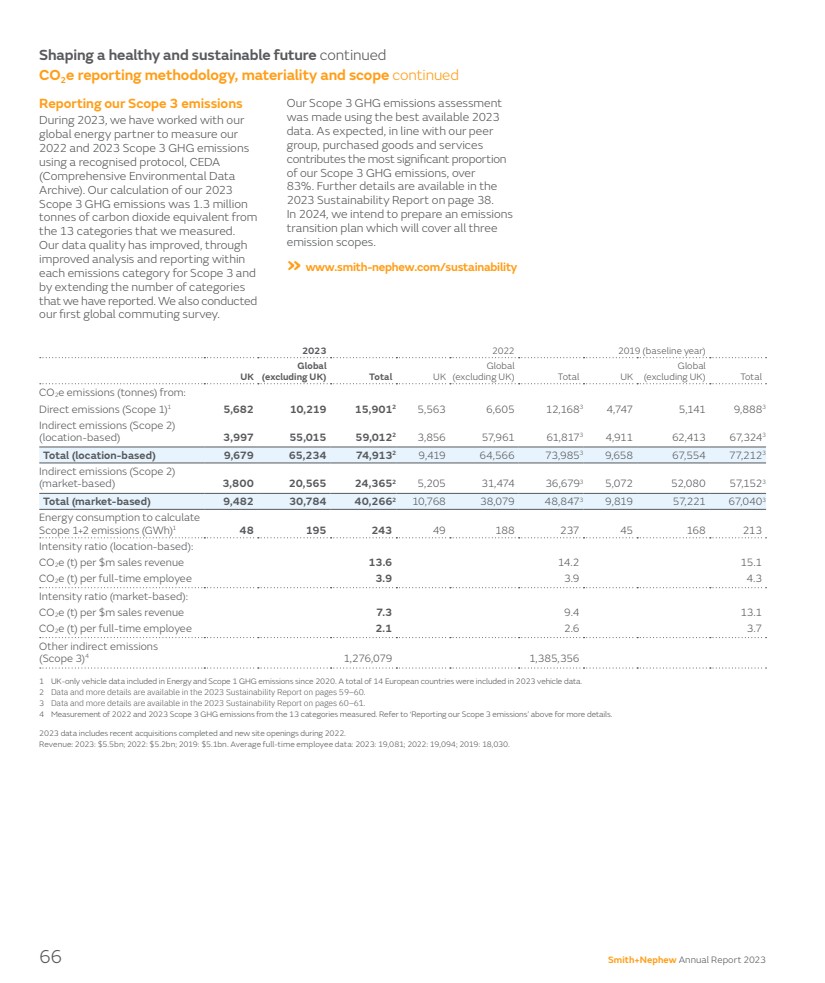
| We also continued to deliver on our environmental commitments and are proud of our many achievements including our ‘AA’ MSCI ESG Rating and our recurring inclusion in leading indices, such as FTSE4Good and the ESG Index from Institutional Shareholder Services (ISS). We are on track to achieve a 70% reduction in Scope 1 and Scope 2 greenhouse gas (GHG) emissions by 2025 compared to the 2019 baseline. This year, we are reporting both our 2022 and 2023 Scope 3 GHG emissions from 13 categories and are developing our Scope 3 GHG emissions reduction roadmap. You can read about these endeavours on pages 52–66 and in our Sustainability Report on our website. I would like to use this opportunity to thank Anne-Françoise for her dedicated service since she joined in 2020 and for her commitment to ensuring a smooth transition to our new Chief Financial Officer, John Rogers. I have been immensely impressed with how John has approached his on-boarding and I look forward to working together as we continue to turn around performance and deliver the 12-Point Plan. Some years in a company are all about new strategies, transformational moves or dealing with fundamental external challenges; some years are more foundational, but nevertheless important. For Smith+Nephew, 2023 was about improving how we operate, focusing on the day-to-day processes and ultimately bringing our innovation to customers in a more reliable and simpler way. The progress we are making is fundamental as we need to have a strong platform from which we will be able to build further shareholder value and address our longer-term ambitions. Yours sincerely, Deepak Nath, PhD Chief Executive Officer A strong sense of belonging The 12-Point Plan has also been a vehicle for cultural change. The discipline we’ve driven through execution of the plan is fostering new behaviours and creating a sustainable culture marked by customer-centricity, agility and speed, execution rigour and consistently high-performing teams. As importantly, we’ve made progress in creating a workplace where each of us feels a sense of belonging. We put in place a global steering group to guide our global wellbeing strategy across physical, mental and financial wellness and conducted numerous internal inclusion events – many championed by our Employee Inclusion Groups. As teams and individuals, we donated our time and talents to our local communities, participating in many charitable events and using more than 13,000 hours of our volunteer time off in meaningful ways that advanced Life Unlimited around the world. Improve value and cash processes Scale Negative Pressure Wound Therapy Optimise procurement Drive cross-selling in ASCs Manufacturing optimisation Executing our 12-Point Plan The 12-Point Plan is on track and starting to deliver financial outcomes. Work will continue in 2024, with further financial progress expected to follow across the year and through 2025. Multiple KPIs are used to measure delivery against the two-year 12-Point Plan. c. 65% Overall progress against milestones 75% 75% 75% 50% 50% 50% Rewire Orthopaedics commercial delivery 1 point 3 point Win market share with our technology Streamline our recon portfolio 2 point 1 point 1 point 2 point 1 point 1 point Fixing Orthopaedics Improving productivity Accelerating Sports Med and AWM 75% 50% Smith+Nephew Annual Report 2023 11 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| 12 Smith+Nephew Annual Report 2023 |
| Letting brothers enjoy their vacation together Life Unlimited 13 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 |
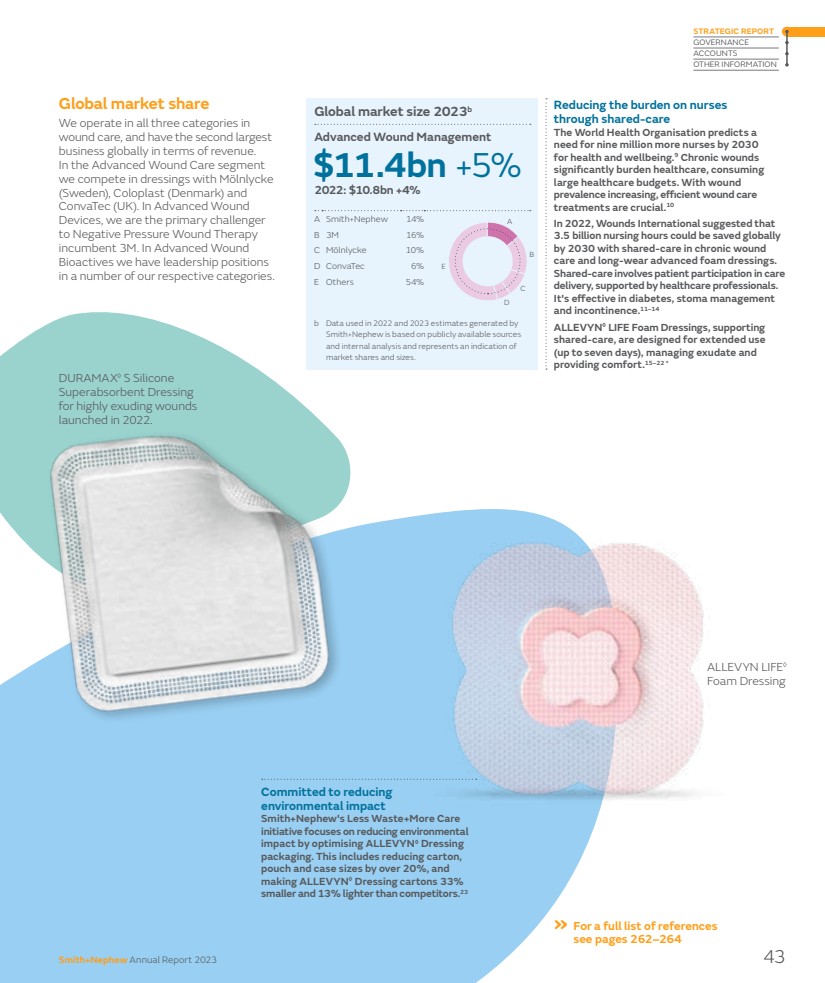
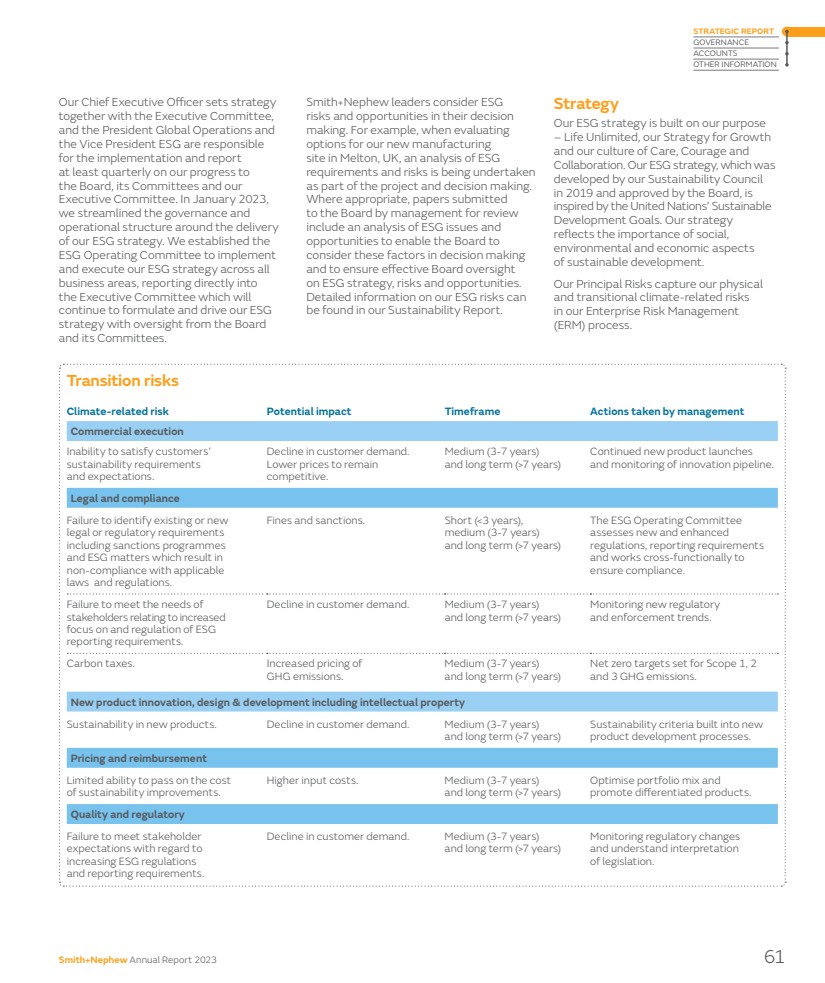
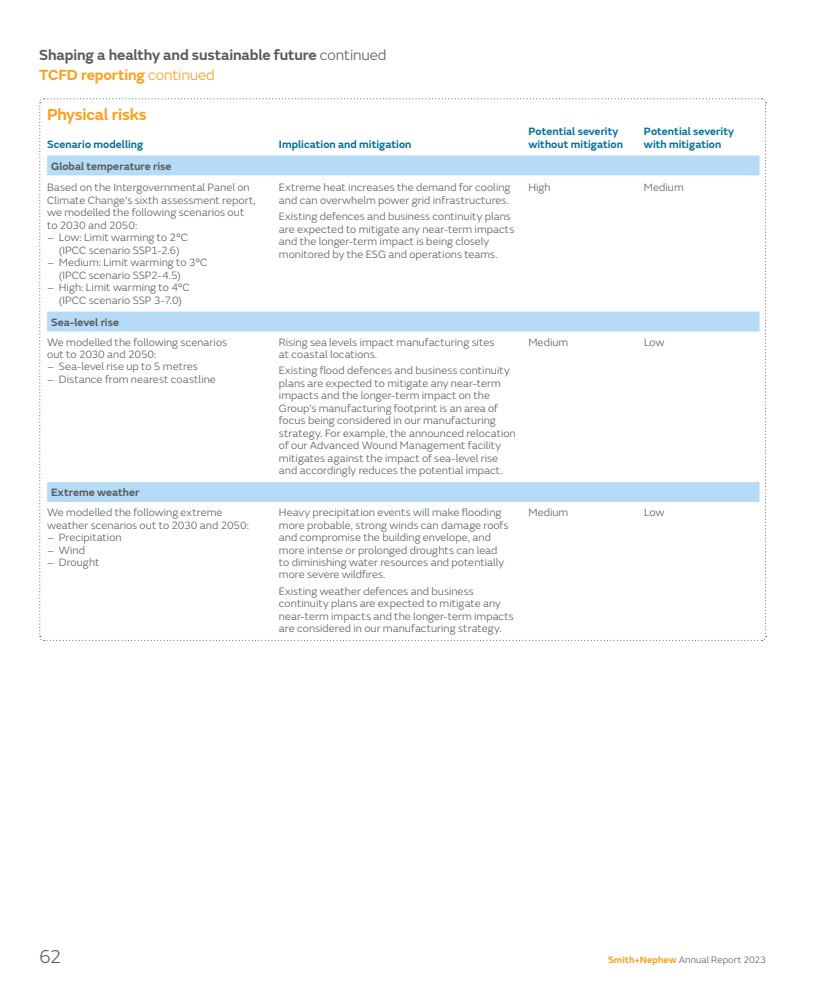
| Our marketplace Leading positions in attractive markets Smith+Nephew competes in global markets worth around $45 billion per annum. Long-term growth drivers Influencing the development of innovative treatments and the evolution of healthcare delivery. The medical technology industry is underpinned by compelling long-term growth drivers that make it an attractive market. Demographic trends, such as an ageing population and greater levels of physical activity later in life, continue to fuel demand for healthcare services. As the global population grows older, there is a natural increase in the prevalence of chronic diseases and age-related conditions, necessitating ongoing medical care. Other lifestyle-related health conditions, such as increasing prevalence of diabetes and obesity, create further demand. Advancements in medical technology are catalysts for long-term growth in healthcare. Breakthroughs in fields like artificial intelligence and biotechnology are leading to more effective and personalised healthcare solutions. This innovation enhances patient outcomes and creates new business opportunities supporting further growth. Emerging markets Increasing healthcare demand creates opportunities and challenges for healthcare providers. In emerging markets, the long-term growth drivers have been compounded by economic development including the emergence of an increasingly prosperous middle class driving demand for better healthcare services and products. As living standards improve, people seek access to higher-quality healthcare, including advanced medical treatments and medical devices. Additionally, emerging markets may have less mature healthcare infrastructure with a pressing need for investment in healthcare technology, which benefits companies offering innovative medical solutions. Emerging markets can be more receptive to novel healthcare solutions which fosters an environment where innovative and cost-effective approaches can gain rapid acceptance. Decentralised care Promoting accessible care outside traditional hospital settings. While the medical technology market has matured in recent years, changing customer and market dynamics have created new high-growth opportunities. In many countries care is becoming more decentralised, with more procedures moving to outpatient settings such as Ambulatory Surgery Centers (ASCs) in the US. This has long been a feature of the sports medicine market, but a growing percentage of orthopaedic joint replacement cases are now completed in such settings, bringing cost and time savings for healthcare providers. The trend towards outpatient care was accelerated by Covid as providers sought to keep patients out of hospitals and also tackle procedure backlogs. POLAR3◊ Total Hip Solution 14 Smith+Nephew Annual Report 2023 |
| Seasonality Seasonality necessitates agile strategies to navigate fluctuations in demand throughout the year. There tends to be a higher volume of orthopaedic and sports medicine procedures during the winter months in our markets, when accidents and sports-related injuries are more frequent. Elective procedures tend to slow down in the summer months due to holidays. Advanced Wound Management is less impacted by seasonality due to the nature of procedures and products. At Smith+Nephew, the majority of our business is in the northern hemisphere, including approximately 50% in the US and 20% in Europe. In the US, out-of-pocket costs for health insurance plans are tied to medical expenses in a calendar year. As a result, households that have reached their annual deductible amount and/or annual out-of-pocket cap before the year’s end will find it to be cost-effective to schedule necessary procedures later in that year rather than delaying into the next year. Cost of healthcare A pressing concern globally, necessitating comprehensive strategies for sustainable healthcare delivery. Governments are focused on reducing the cost of healthcare and are sensitive to price. Medical technology companies respond through new innovation and also provide evidence supporting both the clinical and economic benefits of products. Globally, countries are focused on increasing domestic production across critical sectors, including advanced technologies and life sciences. These actions include localisation policies and export restrictions that disrupt global supply chains. Simultaneously, many countries in key emerging markets are targeting measures to lower healthcare costs and broaden accessibility, implementing price-control policies with respect to government procurement of healthcare products. In China, we saw this reflected in the introduction of volume-based procurement in some of our segments. » See pages 67–79 for more details on risks in the Risk report High regulation Stringent regulations in the medical devices industry play a crucial role in ensuring product safety, efficacy, and quality. The medical device sector is one of the world’s most heavily regulated industries providing a high cost of entry for market participants. National regulatory authorities govern the design, development, approval, manufacture, labelling, marketing and sale of healthcare products. They also review data supporting the products to ensure they are safe and perform as intended. The majority of countries require products to be authorised or registered prior to entering the market, and such authorisation or registration needs to be subsequently maintained. Regulations and industry codes govern the way the industry interacts with healthcare professionals and government officials globally, including the AdvaMed Code of Ethics and the MedTech Europe Code of Ethical Business Practice. Companies establish global compliance programmes to help employees and third-party partners comply with laws, regulations and industry codes, and often have their own codes of conduct to guide behaviour. » See page 49 for more information on our approach to compliance Smith+Nephew Annual Report 2023 15 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
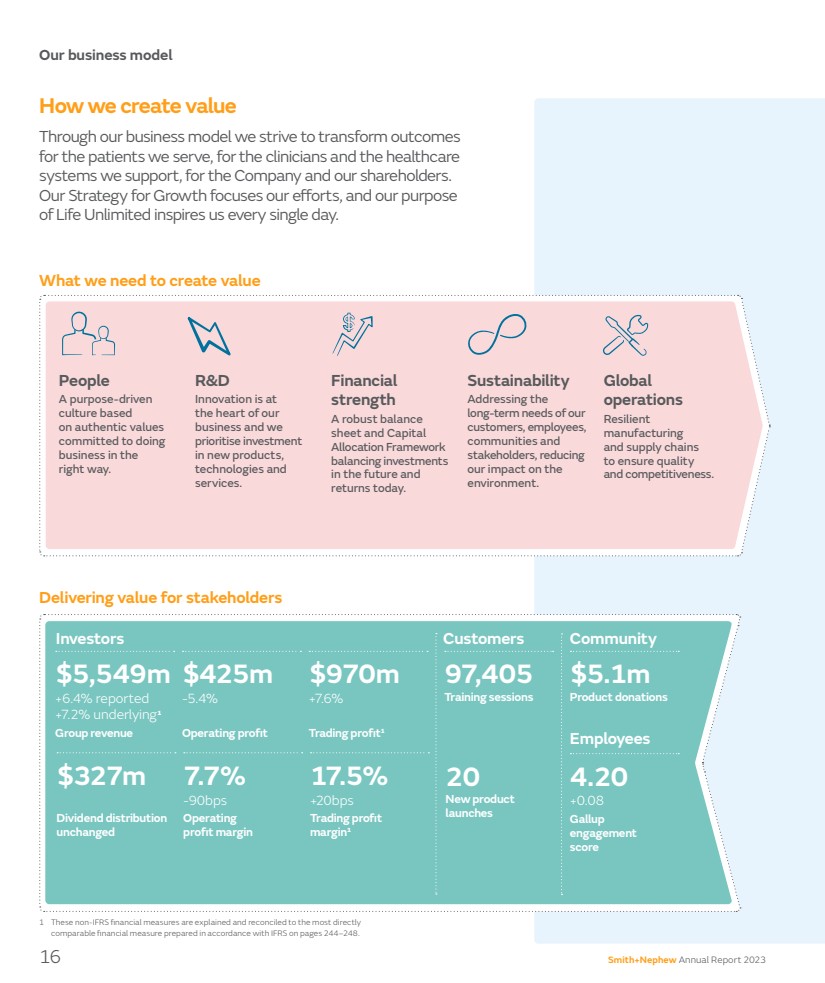
| Our business model How we create value Through our business model we strive to transform outcomes for the patients we serve, for the clinicians and the healthcare systems we support, for the Company and our shareholders. Our Strategy for Growth focuses our efforts, and our purpose of Life Unlimited inspires us every single day. 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. People A purpose-driven culture based on authentic values committed to doing business in the right way. R&D Innovation is at the heart of our business and we prioritise investment in new products, technologies and services. Financial strength A robust balance sheet and Capital Allocation Framework balancing investments in the future and returns today. Sustainability Addressing the long-term needs of our customers, employees, communities and stakeholders, reducing our impact on the environment. Global operations Resilient manufacturing and supply chains to ensure quality and competitiveness. What we need to create value Delivering value for stakeholders 17.5% +20bps Trading profit margin¹ 7.7% -90bps Operating profit margin $327m Dividend distribution unchanged $970m +7.6% Trading profit¹ $425m -5.4% Operating profit $5,549m +6.4% reported +7.2% underlying¹ Group revenue Investors Community $5.1m Product donations 4.20 +0.08 Gallup engagement score 97,405 Training sessions Employees Customers 20 New product launches 16 Smith+Nephew Annual Report 2023 |
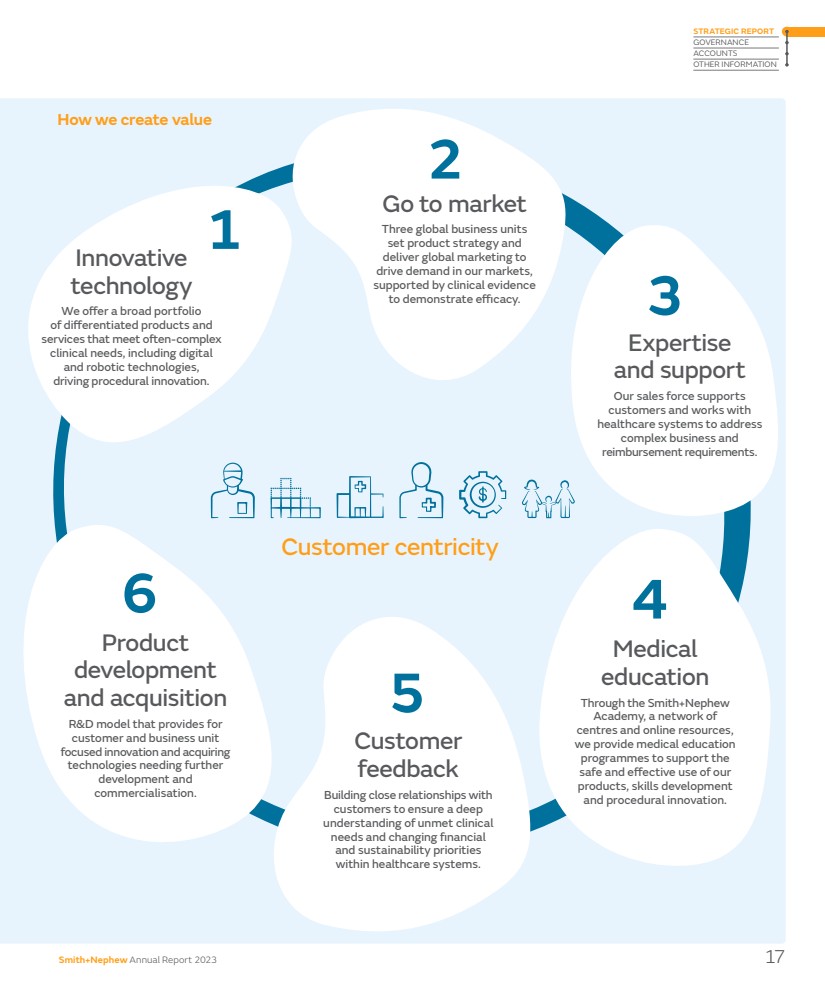
| How we create value Innovative technology We offer a broad portfolio of differentiated products and services that meet often-complex clinical needs, including digital and robotic technologies, driving procedural innovation. Product development and acquisition R&D model that provides for customer and business unit focused innovation and acquiring technologies needing further development and commercialisation. Expertise and support Our sales force supports customers and works with healthcare systems to address complex business and reimbursement requirements. Medical education Through the Smith+Nephew Academy, a network of centres and online resources, we provide medical education programmes to support the safe and effective use of our products, skills development and procedural innovation. Go to market Three global business units set product strategy and deliver global marketing to drive demand in our markets, supported by clinical evidence to demonstrate efficacy. Customer feedback Building close relationships with customers to ensure a deep understanding of unmet clinical needs and changing financial and sustainability priorities within healthcare systems. 6 2 5 3 4 1 Customer centricity Smith+Nephew Annual Report 2023 17 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
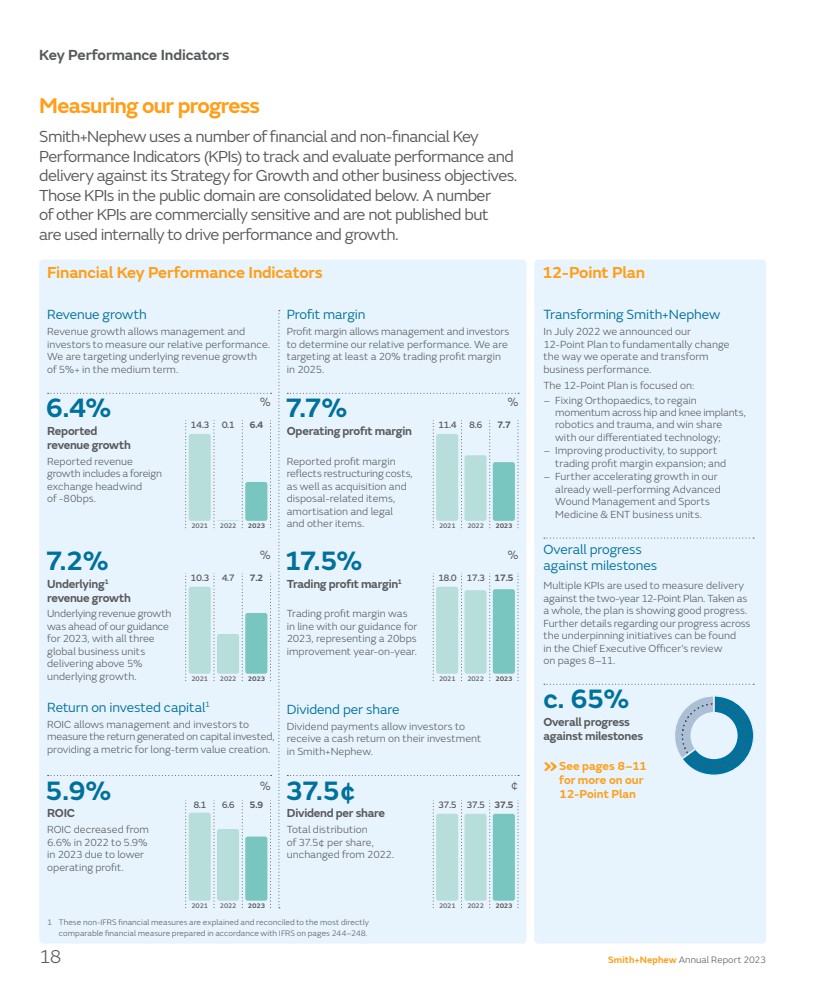
| 5.9% ROIC Key Performance Indicators Measuring our progress Smith+Nephew uses a number of financial and non-financial Key Performance Indicators (KPIs) to track and evaluate performance and delivery against its Strategy for Growth and other business objectives. Those KPIs in the public domain are consolidated below. A number of other KPIs are commercially sensitive and are not published but are used internally to drive performance and growth. Revenue growth Reported revenue growth includes a foreign exchange headwind of -80bps. Revenue growth allows management and investors to measure our relative performance. We are targeting underlying revenue growth of 5%+ in the medium term. Underlying revenue growth was ahead of our guidance for 2023, with all three global business units delivering above 5% underlying growth. Profit margin Reported profit margin reflects restructuring costs, as well as acquisition and disposal-related items, amortisation and legal and other items. Profit margin allows management and investors to determine our relative performance. We are targeting at least a 20% trading profit margin in 2025. Trading profit margin was in line with our guidance for 2023, representing a 20bps improvement year-on-year. Financial Key Performance Indicators Return on invested capital1 ROIC decreased from 6.6% in 2022 to 5.9% in 2023 due to lower operating profit. ROIC allows management and investors to measure the return generated on capital invested, providing a metric for long-term value creation. Dividend per share Total distribution of 37.5¢ per share, unchanged from 2022. Dividend payments allow investors to receive a cash return on their investment in Smith+Nephew. 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. 6.4% Reported revenue growth 7.7% Operating profit margin 7.2% Underlying1 revenue growth 17.5% Trading profit margin1 37.5¢ Dividend per share % % ¢ % % % 12-Point Plan Transforming Smith+Nephew In July 2022 we announced our 12-Point Plan to fundamentally change the way we operate and transform business performance. The 12-Point Plan is focused on: – Fixing Orthopaedics, to regain momentum across hip and knee implants, robotics and trauma, and win share with our differentiated technology; – Improving productivity, to support trading profit margin expansion; and – Further accelerating growth in our already well-performing Advanced Wound Management and Sports Medicine & ENT business units. »See pages 8–11 for more on our 12-Point Plan 2021 10.3 2023 7.2 2022 4.7 2021 18.0 2023 17.5 2022 17.3 2021 8.1 2023 5.9 2022 6.6 2021 14.3 2023 6.4 2022 0.1 2021 11.4 2023 7.7 2022 8.6 2021 37.5 2023 37.5 2022 37.5 c. 65% Overall progress against milestones Overall progress against milestones Multiple KPIs are used to measure delivery against the two-year 12-Point Plan. Taken as a whole, the plan is showing good progress. Further details regarding our progress across the underpinning initiatives can be found in the Chief Executive Officer’s review on pages 8–11. 18 Smith+Nephew Annual Report 2023 |
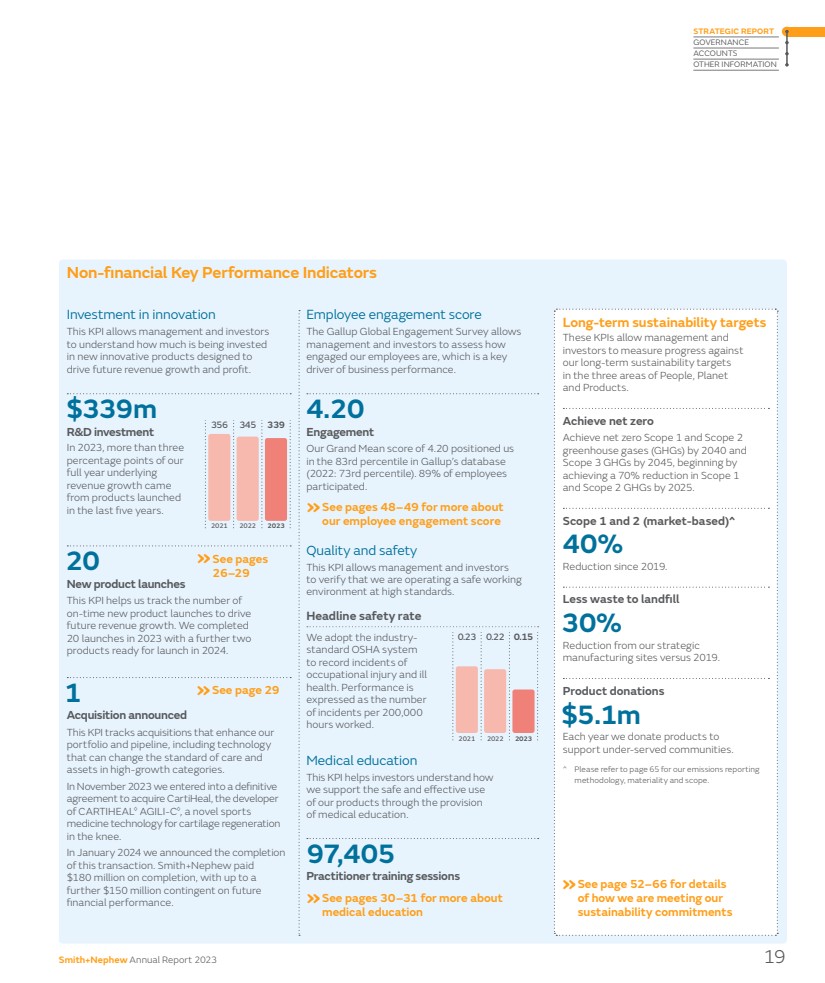
| Non-financial Key Performance Indicators Long-term sustainability targets These KPIs allow management and investors to measure progress against our long-term sustainability targets in the three areas of People, Planet and Products. Achieve net zero Achieve net zero Scope 1 and Scope 2 greenhouse gases (GHGs) by 2040 and Scope 3 GHGs by 2045, beginning by achieving a 70% reduction in Scope 1 and Scope 2 GHGs by 2025. Scope 1 and 2 (market-based) 40% Reduction since 2019. Less waste to landfill 30% Reduction from our strategic manufacturing sites versus 2019. Product donations $5.1m Each year we donate products to support under-served communities. Please refer to page 65 for our emissions reporting methodology, materiality and scope. Employee engagement score The Gallup Global Engagement Survey allows management and investors to assess how engaged our employees are, which is a key driver of business performance. 4.20 Engagement Our Grand Mean score of 4.20 positioned us in the 83rd percentile in Gallup’s database (2022: 73rd percentile). 89% of employees participated. We adopt the industry-standard OSHA system to record incidents of occupational injury and ill health. Performance is expressed as the number of incidents per 200,000 hours worked. This KPI helps investors understand how we support the safe and effective use of our products through the provision of medical education. Quality and safety This KPI allows management and investors to verify that we are operating a safe working environment at high standards. Headline safety rate Medical education 20 New product launches This KPI helps us track the number of on-time new product launches to drive future revenue growth. We completed 20 launches in 2023 with a further two products ready for launch in 2024. Investment in innovation In 2023, more than three percentage points of our full year underlying revenue growth came from products launched in the last five years. This KPI allows management and investors to understand how much is being invested in new innovative products designed to drive future revenue growth and profit. »See pages 26–29 »See pages 30–31 for more about medical education »See page 52–66 for details of how we are meeting our sustainability commitments »See pages 48–49 for more about our employee engagement score $339m R&D investment 1 Acquisition announced This KPI tracks acquisitions that enhance our portfolio and pipeline, including technology that can change the standard of care and assets in high-growth categories. In November 2023 we entered into a definitive agreement to acquire CartiHeal, the developer of CARTIHEAL◊ AGILI-C◊ , a novel sports medicine technology for cartilage regeneration in the knee. In January 2024 we announced the completion of this transaction. Smith+Nephew paid $180 million on completion, with up to a further $150 million contingent on future financial performance. 97,405 Practitioner training sessions »See page 29 2021 0.23 2023 0.15 2022 0.22 2021 356 2023 339 2022 345 Smith+Nephew Annual Report 2023 19 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
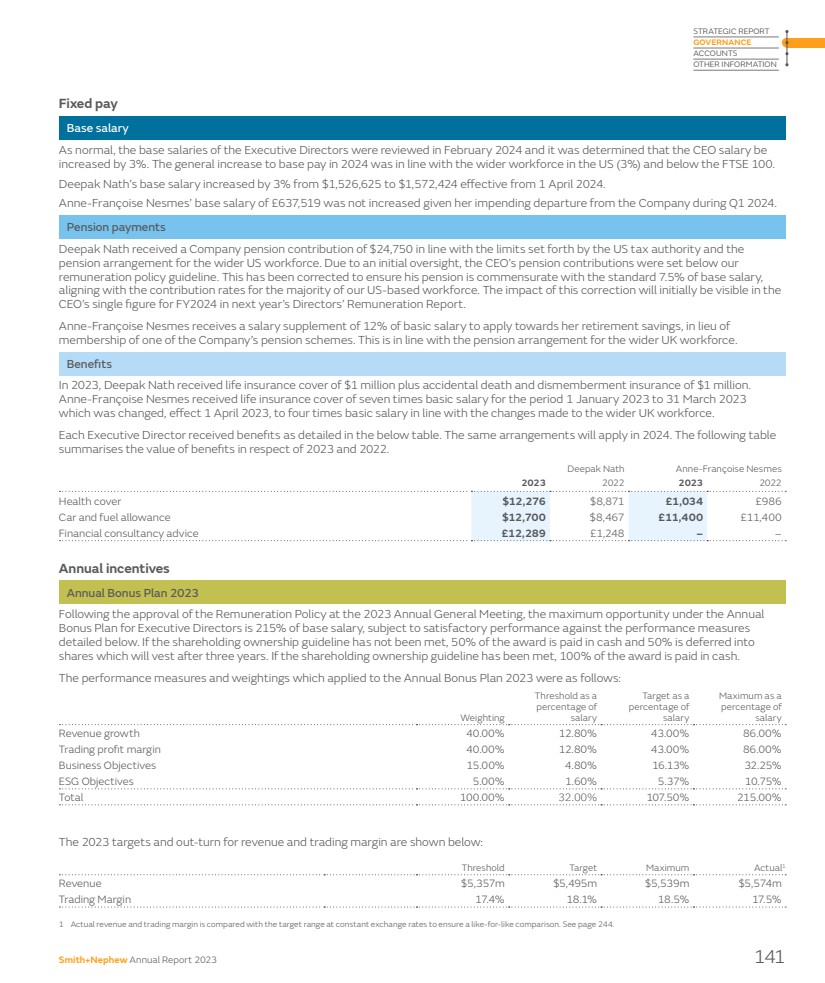
| Financial review 2023 performance Group revenue in 2023 was $5,549 million, an increase of 6.4% on a reported basis and 7.2% on an underlying basis1 excluding a 80bps headwind from foreign exchange, slightly above the revenue guidance range of 5.0% to 6.0% for 2023 we announced previously. The operating profit was $425 million (2022: $450 million) with an operating profit margin of 7.7% (2022: 8.6%) after acquisition and disposal related items, restructuring and rationalisation costs, amortisation and impairment of acquisition intangibles and legal and other items. Trading profit1 for 2023 was $970 million (2022: $901 million) with a trading profit margin1 of 17.5% (2022: 17.3%) reflecting improvement in revenue and productivity savings across the Group. The reported profit before tax was $290 million (2022: $235 million) after adjusting for an impairment related to Engage Surgical. We acquired this business in 2022 for a maximum consideration of $135 million payable in cash. The provisional fair value consideration was $131 million and included $32 million of contingent consideration. During 2023, management evaluated the commercial viability of Engage products and concluded that they should be discontinued. A total of $109 million of Engage’s assets and liabilities were written off as a result of this action. Efficiency and 12-Point Plan progress We have made significant progress in our 12-Point Plan to fundamentally change the way we operate and transform business performance, especially our activities to fix Orthopaedics and improve productivity. This was reflected in our improved financial performance for 2023. In 2023, restructuring costs totalled $220 million, including costs related to the efficiency and productivity under the 12-Point Plan. Overall, incremental benefits of around $68 million was recognised during the year. Strengthening our foundations Dear Fellow Shareholder, The 12-Point Plan was announced in July 2022 to improve execution and drive our Strategy for Growth. The plan focuses on fixing Orthopaedics, improving productivity and accelerating growth in Advanced Wound Management and Sports Medicine through 12 initiatives, which have underpinned our improved performance in 2023. “The 12-Point Plan is beginning to translate into improved financial outcomes in 2023.” Anne-Françoise Nesmes Chief Financial Officer 20 Smith+Nephew Annual Report 2023 |
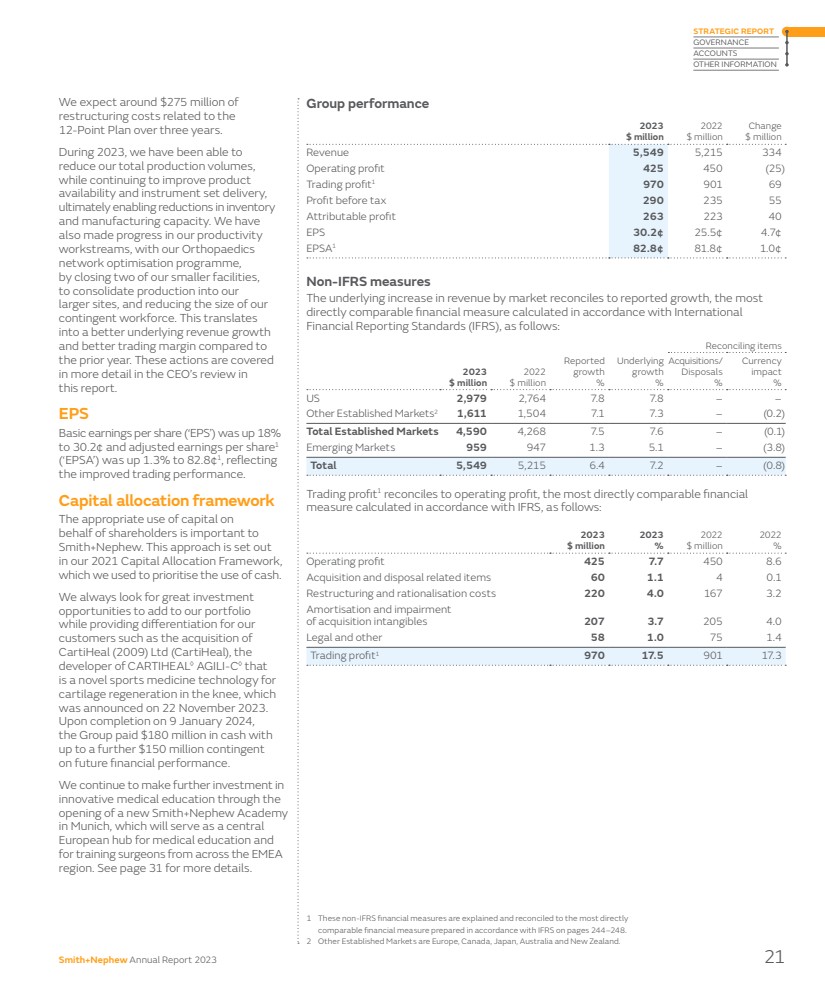
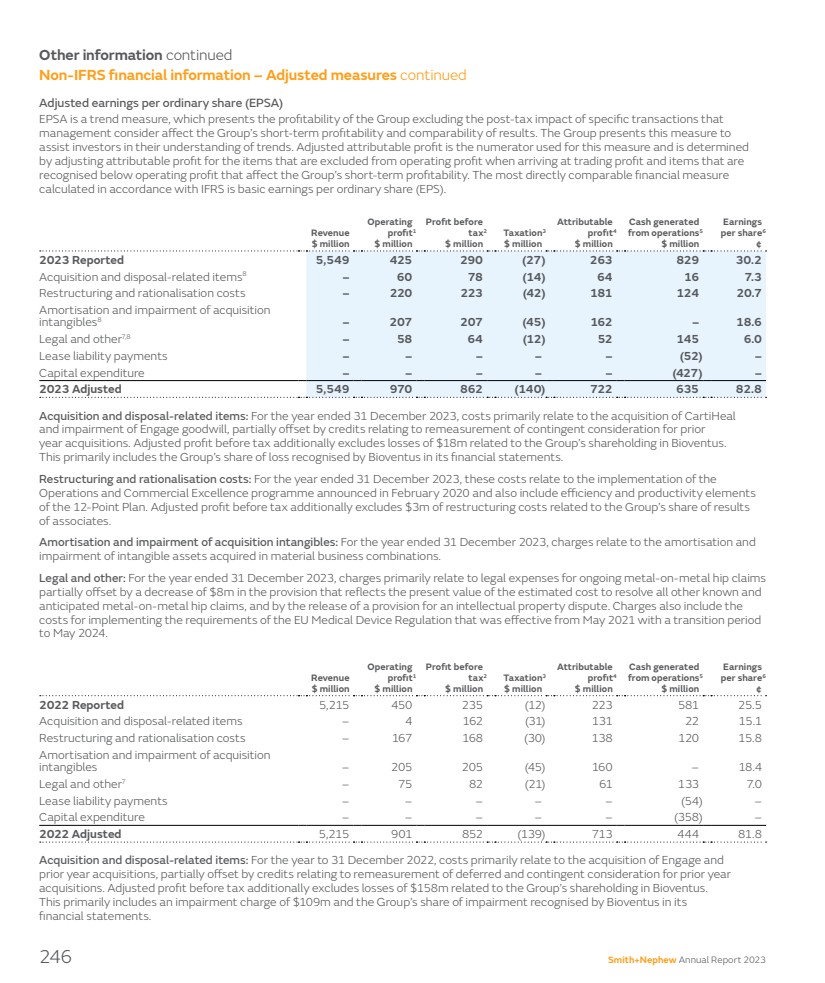
| Group performance 2023 $ million 2022 $ million Change $ million Revenue 5,549 5,215 334 Operating profit 425 450 (25) Trading profit1 970 901 69 Profit before tax 290 235 55 Attributable profit 263 223 40 EPS 30.2¢ 25.5¢ 4.7¢ EPSA1 82.8¢ 81.8¢ 1.0¢ Non-IFRS measures The underlying increase in revenue by market reconciles to reported growth, the most directly comparable financial measure calculated in accordance with International Financial Reporting Standards (IFRS), as follows: 2023 $ million 2022 $ million Reported growth % Underlying growth % Reconciling items Acquisitions/ Disposals % Currency impact % US 2,979 2,764 7.8 7.8 – – Other Established Markets2 1,611 1,504 7.1 7.3 – (0.2) Total Established Markets 4,590 4,268 7.5 7.6 – (0.1) Emerging Markets 959 947 1.3 5.1 – (3.8) Total 5,549 5,215 6.4 7.2 – (0.8) Trading profit1 reconciles to operating profit, the most directly comparable financial measure calculated in accordance with IFRS, as follows: 2023 $ million 2023 % 2022 $ million 2022 % Operating profit 425 7.7 450 8.6 Acquisition and disposal related items 60 1.1 4 0.1 Restructuring and rationalisation costs 220 4.0 167 3.2 Amortisation and impairment of acquisition intangibles 207 3.7 205 4.0 Legal and other 58 1.0 75 1.4 Trading profit1 970 17.5 901 17.3 We expect around $275 million of restructuring costs related to the 12-Point Plan over three years. During 2023, we have been able to reduce our total production volumes, while continuing to improve product availability and instrument set delivery, ultimately enabling reductions in inventory and manufacturing capacity. We have also made progress in our productivity workstreams, with our Orthopaedics network optimisation programme, by closing two of our smaller facilities, to consolidate production into our larger sites, and reducing the size of our contingent workforce. This translates into a better underlying revenue growth and better trading margin compared to the prior year. These actions are covered in more detail in the CEO’s review in this report. EPS Basic earnings per share (‘EPS’) was up 18% to 30.2¢ and adjusted earnings per share1 (‘EPSA’) was up 1.3% to 82.8¢1, reflecting the improved trading performance. Capital allocation framework The appropriate use of capital on behalf of shareholders is important to Smith+Nephew. This approach is set out in our 2021 Capital Allocation Framework, which we used to prioritise the use of cash. We always look for great investment opportunities to add to our portfolio while providing differentiation for our customers such as the acquisition of CartiHeal (2009) Ltd (CartiHeal), the developer of CARTIHEAL◊ AGILI-C◊ that is a novel sports medicine technology for cartilage regeneration in the knee, which was announced on 22 November 2023. Upon completion on 9 January 2024, the Group paid $180 million in cash with up to a further $150 million contingent on future financial performance. We continue to make further investment in innovative medical education through the opening of a new Smith+Nephew Academy in Munich, which will serve as a central European hub for medical education and for training surgeons from across the EMEA region. See page 31 for more details. 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. 2 Other Established Markets are Europe, Canada, Japan, Australia and New Zealand. Smith+Nephew Annual Report 2023 21 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
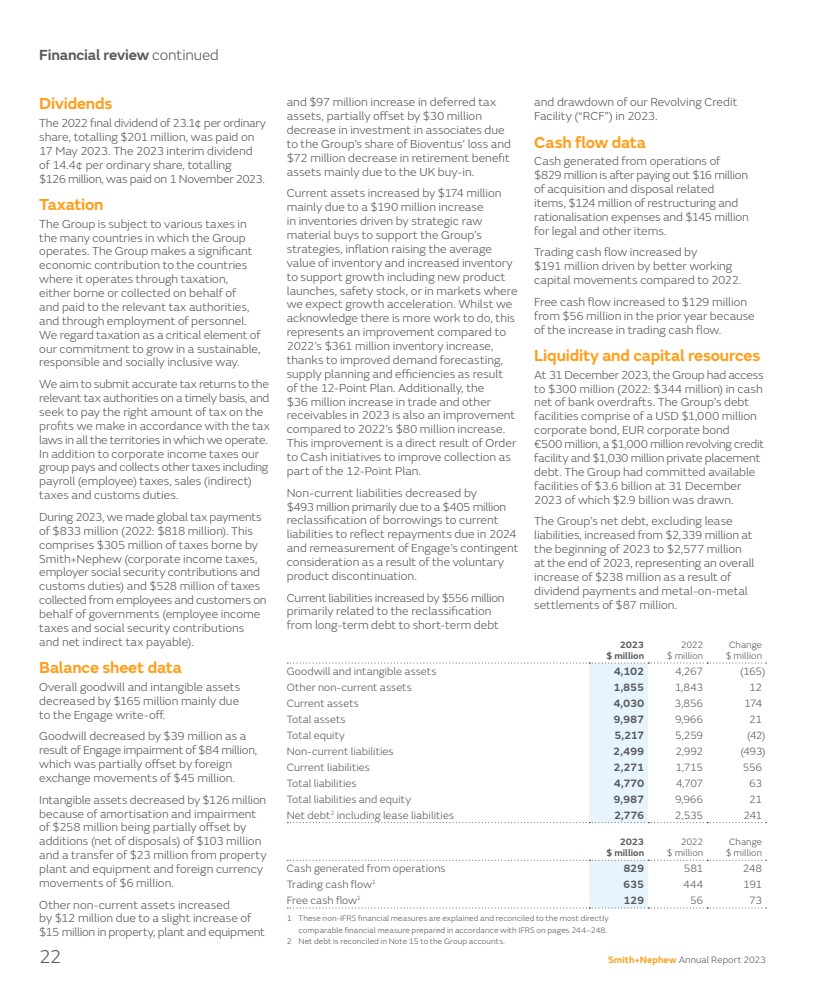
| Financial review continued 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. 2 Net debt is reconciled in Note 15 to the Group accounts. and $97 million increase in deferred tax assets, partially offset by $30 million decrease in investment in associates due to the Group’s share of Bioventus’ loss and $72 million decrease in retirement benefit assets mainly due to the UK buy-in. Current assets increased by $174 million mainly due to a $190 million increase in inventories driven by strategic raw material buys to support the Group’s strategies, inflation raising the average value of inventory and increased inventory to support growth including new product launches, safety stock, or in markets where we expect growth acceleration. Whilst we acknowledge there is more work to do, this represents an improvement compared to 2022’s $361 million inventory increase, thanks to improved demand forecasting, supply planning and efficiencies as result of the 12-Point Plan. Additionally, the $36 million increase in trade and other receivables in 2023 is also an improvement compared to 2022’s $80 million increase. This improvement is a direct result of Order to Cash initiatives to improve collection as part of the 12-Point Plan. Non-current liabilities decreased by $493 million primarily due to a $405 million reclassification of borrowings to current liabilities to reflect repayments due in 2024 and remeasurement of Engage’s contingent consideration as a result of the voluntary product discontinuation. Current liabilities increased by $556 million primarily related to the reclassification from long-term debt to short-term debt and drawdown of our Revolving Credit Facility (“RCF”) in 2023. Cash flow data Cash generated from operations of $829 million is after paying out $16 million of acquisition and disposal related items, $124 million of restructuring and rationalisation expenses and $145 million for legal and other items. Trading cash flow increased by $191 million driven by better working capital movements compared to 2022. Free cash flow increased to $129 million from $56 million in the prior year because of the increase in trading cash flow. Liquidity and capital resources At 31 December 2023, the Group had access to $300 million (2022: $344 million) in cash net of bank overdrafts. The Group’s debt facilities comprise of a USD $1,000 million corporate bond, EUR corporate bond €500 million, a $1,000 million revolving credit facility and $1,030 million private placement debt. The Group had committed available facilities of $3.6 billion at 31 December 2023 of which $2.9 billion was drawn. The Group’s net debt, excluding lease liabilities, increased from $2,339 million at the beginning of 2023 to $2,577 million at the end of 2023, representing an overall increase of $238 million as a result of dividend payments and metal-on-metal settlements of $87 million. Dividends The 2022 final dividend of 23.1¢ per ordinary share, totalling $201 million, was paid on 17 May 2023. The 2023 interim dividend of 14.4¢ per ordinary share, totalling $126 million, was paid on 1 November 2023. Taxation The Group is subject to various taxes in the many countries in which the Group operates. The Group makes a significant economic contribution to the countries where it operates through taxation, either borne or collected on behalf of and paid to the relevant tax authorities, and through employment of personnel. We regard taxation as a critical element of our commitment to grow in a sustainable, responsible and socially inclusive way. We aim to submit accurate tax returns to the relevant tax authorities on a timely basis, and seek to pay the right amount of tax on the profits we make in accordance with the tax laws in all the territories in which we operate. In addition to corporate income taxes our group pays and collects other taxes including payroll (employee) taxes, sales (indirect) taxes and customs duties. During 2023, we made global tax payments of $833 million (2022: $818 million). This comprises $305 million of taxes borne by Smith+Nephew (corporate income taxes, employer social security contributions and customs duties) and $528 million of taxes collected from employees and customers on behalf of governments (employee income taxes and social security contributions and net indirect tax payable). Balance sheet data Overall goodwill and intangible assets decreased by $165 million mainly due to the Engage write-off. Goodwill decreased by $39 million as a result of Engage impairment of $84 million, which was partially offset by foreign exchange movements of $45 million. Intangible assets decreased by $126 million because of amortisation and impairment of $258 million being partially offset by additions (net of disposals) of $103 million and a transfer of $23 million from property plant and equipment and foreign currency movements of $6 million. Other non-current assets increased by $12 million due to a slight increase of $15 million in property, plant and equipment 2023 $ million 2022 $ million Change $ million Cash generated from operations 829 581 248 Trading cash flow1 635 444 191 Free cash flow1 129 56 73 2023 $ million 2022 $ million Change $ million Goodwill and intangible assets 4,102 4,267 (165) Other non-current assets 1,855 1,843 12 Current assets 4,030 3,856 174 Total assets 9,987 9,966 21 Total equity 5,217 5,259 (42) Non-current liabilities 2,499 2,992 (493) Current liabilities 2,271 1,715 556 Total liabilities 4,770 4,707 63 Total liabilities and equity 9,987 9,966 21 Net debt2 including lease liabilities 2,776 2,535 241 22 Smith+Nephew Annual Report 2023 |
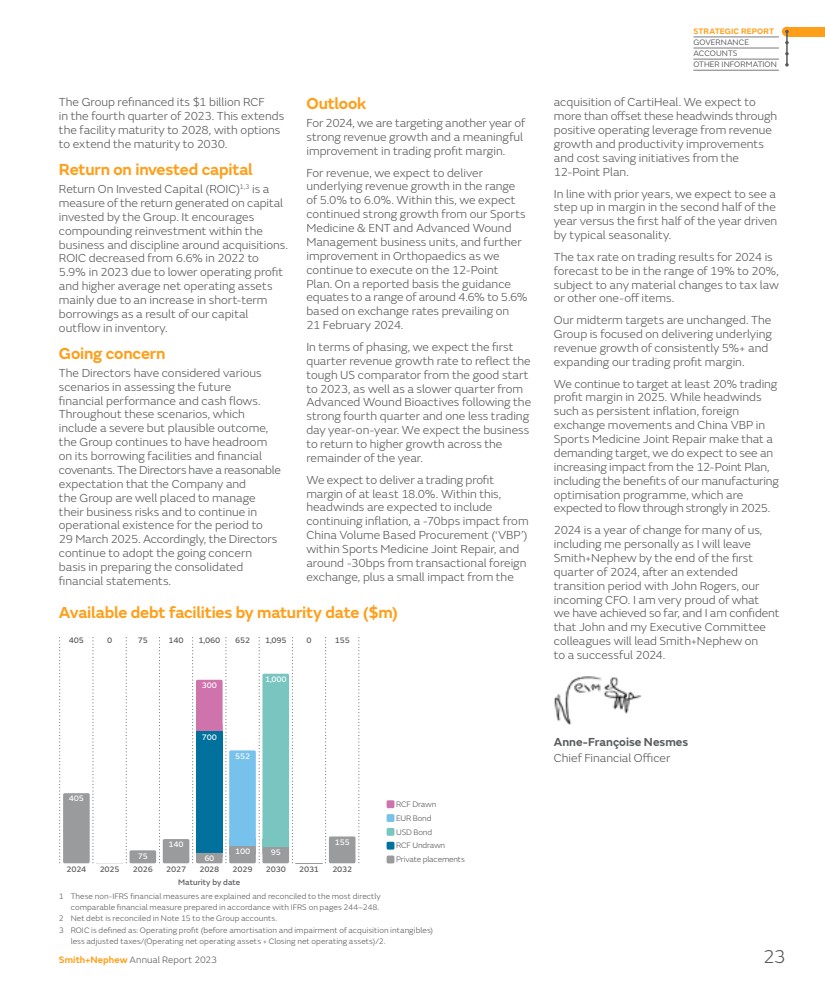
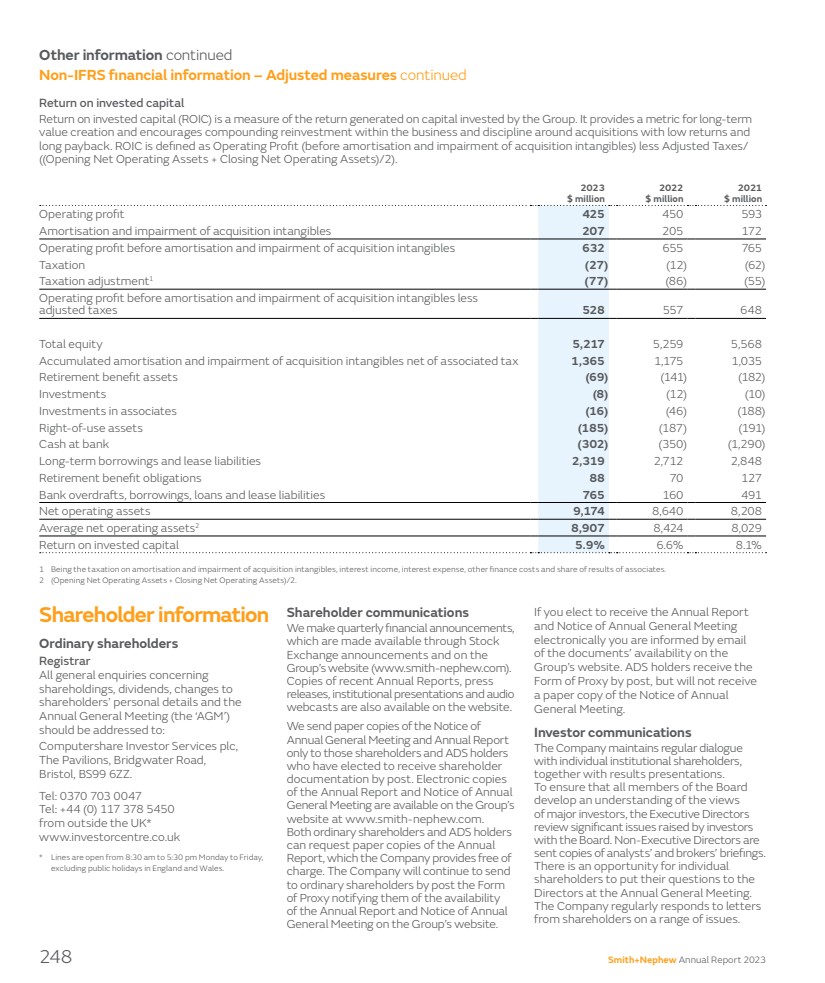
| 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. 2 Net debt is reconciled in Note 15 to the Group accounts. 3 ROIC is defined as: Operating profit (before amortisation and impairment of acquisition intangibles) less adjusted taxes/(Operating net operating assets + Closing net operating assets)/2. The Group refinanced its $1 billion RCF in the fourth quarter of 2023. This extends the facility maturity to 2028, with options to extend the maturity to 2030. Return on invested capital Return On Invested Capital (ROIC)1,3 is a measure of the return generated on capital invested by the Group. It encourages compounding reinvestment within the business and discipline around acquisitions. ROIC decreased from 6.6% in 2022 to 5.9% in 2023 due to lower operating profit and higher average net operating assets mainly due to an increase in short-term borrowings as a result of our capital outflow in inventory. Going concern The Directors have considered various scenarios in assessing the future financial performance and cash flows. Throughout these scenarios, which include a severe but plausible outcome, the Group continues to have headroom on its borrowing facilities and financial covenants. The Directors have a reasonable expectation that the Company and the Group are well placed to manage their business risks and to continue in operational existence for the period to 29 March 2025. Accordingly, the Directors continue to adopt the going concern basis in preparing the consolidated financial statements. Outlook For 2024, we are targeting another year of strong revenue growth and a meaningful improvement in trading profit margin. For revenue, we expect to deliver underlying revenue growth in the range of 5.0% to 6.0%. Within this, we expect continued strong growth from our Sports Medicine & ENT and Advanced Wound Management business units, and further improvement in Orthopaedics as we continue to execute on the 12-Point Plan. On a reported basis the guidance equates to a range of around 4.6% to 5.6% based on exchange rates prevailing on 21 February 2024. In terms of phasing, we expect the first quarter revenue growth rate to reflect the tough US comparator from the good start to 2023, as well as a slower quarter from Advanced Wound Bioactives following the strong fourth quarter and one less trading day year-on-year. We expect the business to return to higher growth across the remainder of the year. We expect to deliver a trading profit margin of at least 18.0%. Within this, headwinds are expected to include continuing inflation, a -70bps impact from China Volume Based Procurement (‘VBP’) within Sports Medicine Joint Repair, and around -30bps from transactional foreign exchange, plus a small impact from the acquisition of CartiHeal. We expect to more than offset these headwinds through positive operating leverage from revenue growth and productivity improvements and cost saving initiatives from the 12-Point Plan. In line with prior years, we expect to see a step up in margin in the second half of the year versus the first half of the year driven by typical seasonality. The tax rate on trading results for 2024 is forecast to be in the range of 19% to 20%, subject to any material changes to tax law or other one-off items. Our midterm targets are unchanged. The Group is focused on delivering underlying revenue growth of consistently 5%+ and expanding our trading profit margin. We continue to target at least 20% trading profit margin in 2025. While headwinds such as persistent inflation, foreign exchange movements and China VBP in Sports Medicine Joint Repair make that a demanding target, we do expect to see an increasing impact from the 12-Point Plan, including the benefits of our manufacturing optimisation programme, which are expected to flow through strongly in 2025. 2024 is a year of change for many of us, including me personally as I will leave Smith+Nephew by the end of the first quarter of 2024, after an extended transition period with John Rogers, our incoming CFO. I am very proud of what we have achieved so far, and I am confident that John and my Executive Committee colleagues will lead Smith+Nephew on to a successful 2024. Anne-Françoise Nesmes Chief Financial Officer Available debt facilities by maturity date ($m) 2025 0 0 405 2024 405 300 2026 75 75 2027 140 140 2028 1,060 700 300 60 2029 652 100 552 2030 1,095 95 1,000 2031 0 155 2032 155 EUR Bond RCF Drawn USD Bond RCF Undrawn Private placements Maturity by date Smith+Nephew Annual Report 2023 23 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Getting grandparents back to playing with their grandchildren Life Unlimited 24 Smith+Nephew Annual Report 2023 |
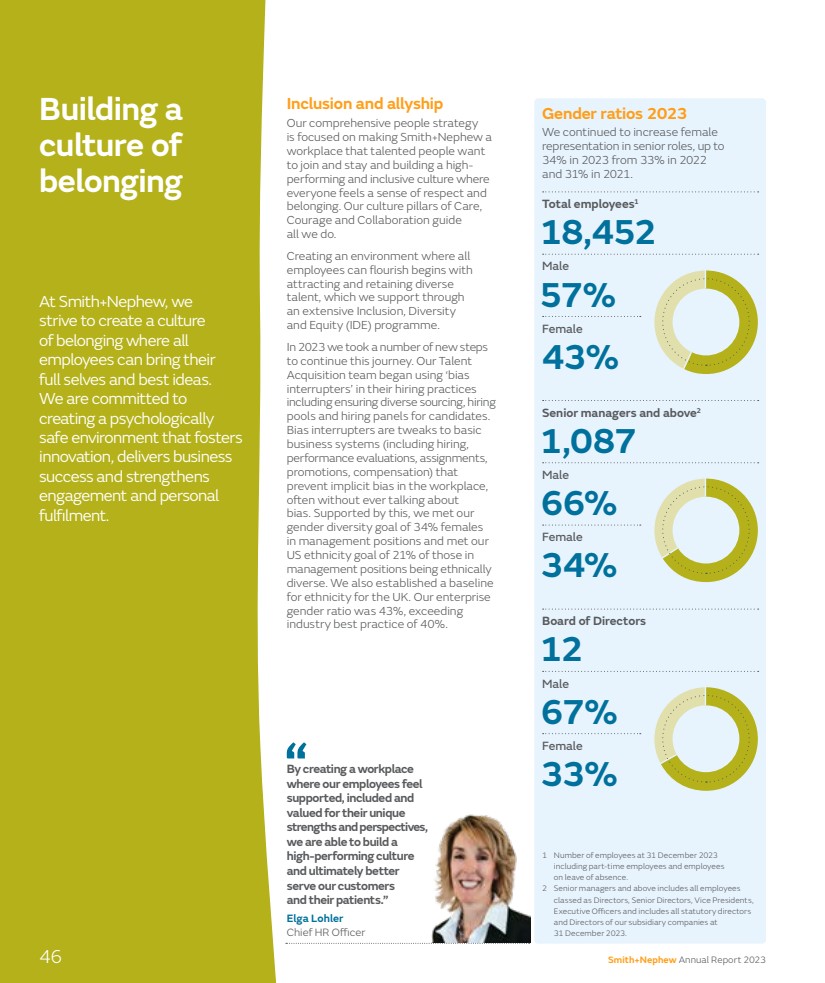
| Smith+Nephew Annual Report 2023 25 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Research & Development A long history of transformative innovation Creating value through innovation JOURNEY◊ II: JOURNEY II TKA has been demonstrated to restore anatomical shape, position and motion.*1,2 This anatomical restoration can provide superior clinical outcomes and higher patient satisfaction.** 3–7 Smith+Nephew has a long and proud history of transformative innovation, dating back to our founding in 1856. In the recent past we shaped clinical practice and helped to deliver our purpose of Life Unlimited to millions of patients. In Orthopaedics, products such as our kinematic knee, JOURNEY◊ II, have brought more natural motion to joint replacement. In Sports Medicine our products have been instrumental in enabling arthroscopic repair where previously open surgery was the standard of care. And in wound care, Smith+Nephew’s PICO◊ single-use Negative Pressure Wound Management System has revolutionised the availability of this important treatment option. Over decades, we have repeatedly brought technologies to market that have disrupted established approaches and changed the standard of care.” Vasant Padmanabhan President of Research & Development and ENT $339m Invested in R&D in 2023 20 New products launched in 2023 Smith+Nephew’s innovation pipeline is a material contributor to our revenue growth, with approaching 50% of our 2023 underlying revenue growth coming from recent product launches. We expect this trend to continue as we drive innovation across our business. » For a full list of references see pages 262–264 26 Smith+Nephew Annual Report 2023 |
| CORI◊ Digital Tensioner The CORI◊ Digital Tensioner was designed to address different demographics, making this product ergonomically suitable for female and male surgeons. This approach to innovation is helping remove barriers and limitations traditionally associated with orthopaedic surgical equipment. Addressing unmet clinical needs Today, there are still significant unmet clinical needs. These needs can be for the patient, in terms of satisfaction, clinical outcomes and reduction in complications, or for the healthcare system with costs of existing treatments or of unaddressed problems. For instance, in knee replacement, 80% of recipients state that their new knee feels ‘artificial’,8 while in Sports Medicine, the re-tear rates associated with repair of large full thickness rotator cuff tears exceeds 50%.9 In ENT, almost one in 16 children undergoing total tonsillectomies have post-procedure haemorrhages,10 and in wound care the treatment for surgical site infections costs the US healthcare system more than $3 billion per year.11 These challenges, and many others like them, inspire us to invest in developing the next generation of products and services that will continue to advance clinical practice and improve outcomes for patients and payers. We are helping to shape an innovation environment that is driven by four key trends. – Robotics and digital systems enable a degree of accuracy and personalisation of procedures that has not been possible in the past. – Biologics technology is developing rapidly and enables different types of treatments – including fully restoring tissue and function. – Procedural innovation is focused on less invasive and tissue sparing methods that can improve recovery times. – Healthcare costs require greater focus on delivering compelling value and health economic benefits. Smith+Nephew’s R&D team is focused on growth segments where we can deploy our expertise in such fast developing areas of innovation, and deliver novel solutions that address unmet clinical needs. Inspiration for new products comes from observing our customers, working with healthcare professionals on design and development, acquiring technologies needing further development and commercialisation, and our co-development partners. New products are developed using a rigorous phase-gate process starting with business case review and ending with launch readiness. We also strive to embed sustainability principles into our design and packaging. Designing a world-class user experience Users perform better when they believe they are working with best-in-class equipment. In the context of product design, devices are typically considered best-in-class when they are recognised as being developed by an industry-leading brand and exhibit a compelling and purposeful user experience. Within R&D our Human Factors team strives to bring a unique user experience to product development, encompassing a common high-quality look, feel and sound across the entire portfolio of instruments and digital systems. Their philosophy is that each product should be considered a Smith+Nephew brand ambassador, expressing excellence and encouraging ease-of-use and familiarity. Smith+Nephew Annual Report 2023 27 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Creating value through innovation continued Research & Development continued New products in 2023 Innovation is central to our higher-growth ambitions In 2023 we launched 20 new products, with development complete on a further two ahead of their launch in 2024. Investing in robotics and AI Our CORI◊ Surgical System is the only robotics-assisted system indicated for partial, total and revision knees. During 2023 we continued to add features and functionality. The CORI◊ Digital Tensioner is a proprietary device for soft tissue balancing in knee replacement, and the only tensioner for robotics-assisted surgery. This helps make planning more objective and eliminates inconsistencies in surgery from current manual or mechanical tools. Personalized Planning powered by AI and the RI.INSIGHTS◊ Data Visualization Platform on CORI◊ transform data into contextual intelligence by enabling surgeons to better understand how pre-operative surgical plans and intra-operative decision making link to post-operative outcomes. A new saw solution added versatility, appealing to a broader range of surgeons. CORI◊ is the only solution to offer robotics-assisted burring and saw bone-cutting options. This development was accelerated as part of our 12-Point Plan. New shoulder system In 2023 we launched our AETOS◊ Shoulder System. We acquired this technology in early 2021 and it is an important part of our growth plans for Trauma & Extremities. AETOS◊ is designed with both patient and surgeon benefits in mind. For example, the MetaStem aligns with the market trend towards minimally invasive short stem devices. Short stems are easier to implant, have improved bone preservation, and are a better fit to anatomy. AETOS◊ will enable Smith+Nephew to compete effectively in the $1.7 billion12 shoulder repair market, which, at around 9% compound annual growth rate, is one of the fastest growing segments in Orthopaedics. AETOS◊ Shoulder System CORI Surgical System 28 Smith+Nephew Annual Report 2023 |
| Accessing external innovation through M&A Smith+Nephew has a strong track record of using bolt-on acquisitions to enhance our portfolio and R&D pipeline. This includes technology that can change the standard of care and assets in higher-growth categories. We look to acquire assets where we can use our commercial expertise and channels to drive growth, and also use our R&D expertise to develop new iterations or indications to expand the addressable market. One example is the acquisition of Rotation Medical in 2017, which included REGENETEN◊ , a novel tissue regeneration technology for rotator cuff repair. To support this acquisition we built a specialist Sports Medicine sales force which has delivered strong growth in the US and Europe, and we have started to roll out into new markets such as Japan, India and China in 2023. The acquisition of BlueBelt Technologies brought a first-generation robotics system and considerable R&D expertise which we have leveraged to create a second-generation system CORI◊ which we continued to expand with new indications and enhancements. Through the acquisition of an Extremity Orthopaedics business we added a next generation shoulder replacement platform AETOS◊ to our pipeline, completing the development ahead of its launch in 2023. In November 2023 we announced a definitive agreement to acquire CartiHeal, the developer of the CARTIHEAL◊ AGILI-C◊ Cartilage Repair Implant, a novel sports medicine technology for cartilage regeneration in the knee. CARTIHEAL◊ AGILI-C◊ is an off-the-shelf one-step treatment for osteochondral (bone and cartilage) lesions with a broader indication than existing treatments. It is indicated to treat a wide patient population, including those with lesions in knees with mild to moderate osteoarthritis, a previously unaddressed condition, as well as the approximately 700,000 patients1 that receive cartilage repair annually in the US. The acquisition was completed in January 2024. Novel cartilage regeneration – challenging standard of care The CARTIHEAL◊ AGILI-C◊ Cartilage Repair Implant is a porous, biocompatible and resorbable scaffold which promotes natural regeneration of the articular cartilage and restoration of its underlying subchondral bone. The US Food and Drug Administration (FDA) granted CARTIHEAL◊ AGILI-C◊ Breakthrough Device designation status in 2020 and Premarket Approval (PMA) in March 2022. PMA approval was granted based on the results of a two-year randomised controlled trial (N=251) that confirmed the superiority of CARTIHEAL◊ AGILI-C◊ over the current standard of care – microfracture and debridement for the treatment of knee joint surface lesions, chondral and osteochondral defects. Study inclusion criteria included patients with mild and moderate osteoarthritis. At four-year follow-up the trial continues to show significant improvement of patient reported outcome scores, low surgical reintervention, and that the difference in improvement using CARTIHEAL◊ AGILI-C◊ compared to the standard of care is statistically significant – offering potential for a new standard of care in cartilage repair. Creating a winning edge The new RENASYS◊ EDGE Negative Pressure Wound Therapy System is designed to reduce inefficiency and complexity and features an improved user interface for enhanced intuitiveness and simplicity and a durable pump built to offer virtually maintenance-free use. RENASYS◊ EDGE We have shown with REGENETEN◊ that we have the market development and commercialisation expertise to take novel technologies and successfully establish a new standard of care. AGILI-C◊ is the perfect addition to our portfolio and we look forward to leveraging our expertise to transform cartilage repair outcomes for patients.” Scott Schaffner President Sports Medicine » For a full list of references see pages 262–264 Smith+Nephew Annual Report 2023 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION 29 |
| Creating value through innovation continued Medical education Providing opportunities to learn innovative clinical and surgical techniques Smith+Nephew is committed to educating and training healthcare professionals on the safe and effective use of our products. Every year we provide tens of thousands of surgeons and nurses with opportunities to evaluate the latest clinical evidence and learn innovative surgical techniques and the effective use of our products through our medical education programmes. Central to Smith+Nephew’s commitment to being a global leader in medical education and improving patient outcomes is providing a comprehensive accessible learning environment tailored to the needs of the healthcare professional. Through the Smith+Nephew Academy we are actively transforming the way we educate our customers around the world by surrounding them with cutting-edge technology, clinical content and scientific data. The multiple elements of the Smith+Nephew Academy offer a blended learning environment inclusive of state-of-the-art digital interactive learning, symposia, procedure-based education through hands-on experiences inclusive of Virtual Reality (VR) simulations, customised curriculum and programming specifically designed to meet the needs of the accomplished physician, resident, fellow and allied health professionals. Smith+Nephew Academy augments in-person training opportunities with a comprehensive online presence through Smith+Nephew Academy Online. We have three in-person Academies in the US in Memphis (Tennessee), Andover (Massachusetts) and Pittsburgh (Pennsylvania), as well as Academy London, Academy Singapore and, new in 2023, Academy Munich. In addition, we have smaller training facilities in the US in Phoenix (Arizona) and Austin (Texas). Our investment in S+N Academy Munich is part of a global commitment to drive innovation and learning in medical technology, creating an environment where the best healthcare providers can learn, collaborate and innovate in order to meet the needs of their patients.” Cynthia Walker Senior Vice President Medical Education 4,241 Education courses run by Smith+Nephew in 2023 97,405 Healthcare professional training sessions in 2023 30 Smith+Nephew Annual Report 2023 |
| Academy Munich A new centre for surgical innovation and training In October 2023, we opened S+N Academy Munich, a central European hub for surgeons from across Europe, the Middle East and Africa. Surgeons and other healthcare specialists will learn the latest surgical techniques using the most advanced technology available, and practise surgical techniques using both hands-on and fully immersive digital interactive experiences. S+N Academy Munich is expected to train more than 5,000 global healthcare providers each year. Additionally, S+N Academy Munich will also serve as a hub to connect healthcare professionals with our global marketing and Research & Development teams to test and validate new technologies. RCSEng accreditation Royal College of Surgeons of England (RCSEng) Centre Accreditation has been awarded to Smith+Nephew and is the highest level of accreditation. It is seen as a kite-mark of excellence and demonstrates external validation of the Medical Education training that we provide. S+N Academy Online S+N Academy Online S+N Academy Online is the global medical education platform used by healthcare professionals and caregivers to access the latest peer-to-peer scientific, education-based best practice; intended to deliver thought leadership and content across orthopaedic reconstruction, sports medicine, ENT, trauma and extremities, and wound management. Our S+N Academy Online platform supports personalised learning journeys and educational pathways, with evolving libraries, educational resources and on-demand educational activities (such as webinars, products and recorded courses) as well as e-learning modules (including faculty-led techniques, surgical videos, expert lectures, panel discussions, clinical data, evidence literature and course information) and access to online training such as live webinars and virtual classrooms. S+N Academy Online resources are available to all registered healthcare professionals. 670 Modules available on S+N Academy Online 10,504 Healthcare professionals used S+N Academy Online in 2023 Smith+Nephew Annual Report 2023 31 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Creating value through innovation continued Manufacturing Smith+Nephew takes great pride in our manufacturing expertise and commitment to distributing innovative, quality products globally. Our Global Operations team supports the delivery of the Group’s strategy by ensuring that we respond efficiently to demand, new product development and changing regulatory requirements. Supporting the 12-Point Plan Global Operations is integral to the delivery of our 12-Point Plan, specifically our activities to fix Orthopaedics and improve productivity. These activities require close collaboration with our commercial teams, and have been supported by a refreshed leadership team across Orthopaedics and Global Operations with area-specific experience and track record. During 2023 we have been able to reduce our total production, while continuing to improve product availability. This in turn will ultimately enable reductions in inventory and manufacturing capacity. A significant challenge has been a misalignment between Commercial and Operations. Our previous Sales, Inventory and Operations Planning, or SIOP process, was leading to over-ordering by our commercial organisations and the creation of excess capacity. We rolled out an improved and redesigned SIOP process in 2023. This has led to improved service, both on new sets and on replenishment. We have been working to optimise our manufacturing network for a number of years. Recent landmarks in this journey have included opening a new high-technology Orthopaedics manufacturing facility in Malaysia in 2022, and we are currently building a new Advanced Wound Management facility in the UK. We are also reviewing lean methodologies across our operations to simplify processes, drive greater standardisation, and reduce scrap. With renewed focus under the 12-Point Plan we have identified further opportunities in our network for simplification to bring cost and asset efficiencies. Important steps in 2023 included announcing the closure of two smaller facilities in China and Germany to consolidate production into our larger sites. We also reduced the size of our contingent workforce. 13 Global manufacturing sites Manufacturing and distributing innovative, quality products globally By delivering on the manufacturing and procurement initiatives within the 12-Point Plan we expect to support commercial growth and drive better efficiency on both fixed and variable costs.” Paul Connolly President Global Operations 32 Smith+Nephew Annual Report 2023 |
| The 12-Point Plan includes focus on improving productivity to support trading profit margin expansion. Areas of opportunity include driving lean methodologies across our manufacturing operations, further network optimisation and direct and indirect procurement savings. Improving procurement We are also targeting procurement savings to help mitigate cost inflation and drive productivity. We see opportunities where spend is fragmented between large numbers of suppliers, or where providers in high-cost countries are disproportionately used. During 2023 we deployed an enhanced supplier selection process to identify and award business to suppliers that better align to the global business unit strategies and long-term performance metrics, and better aligned global category strategies to unlock the Smith+Nephew buying power and leverage, helping to drive volume to the most preferred suppliers and reduce cost. We procure raw materials, components, finished products and packaging materials from suppliers globally. These include metal forgings and castings, optical and electronic sub-components, active ingredients and semi-finished goods, as well as packaging materials. During 2023 we improved supplier resilience, reducing back orders due to raw materials or components shortages to the lowest levels in more than two years. All our suppliers are subject to our Third-Party Guide to Working with Smith+Nephew, meaning they agree to conduct business on our behalf in an ethical manner that is compliant with all applicable laws, regulations and industry codes of conduct, and to manage their suppliers in accordance with the same standards. We outsource certain parts of our manufacturing processes where necessary to obtain specialised expertise or to lower cost without undue risk to our intellectual property or quality. We monitor suppliers through on-site assessments and performance audits to ensure the required levels of quality, service and delivery as well as compliance with our Third-Party Guide to Working with Smith+Nephew. Our manufacturing network We operate manufacturing facilities in countries across the globe, and have central distribution facilities in the US, Europe and Asia. Products for our Orthopaedics business unit are primarily manufactured at facilities in Memphis (US), Penang (Malaysia), Aarau (Switzerland) and Warwick (UK), as well as Tuttlingen (Germany) and Beijing (China), two facilities we are closing as described above. Sports Medicine products are primarily manufactured in the Alajuela (Costa Rica), Mansfield (US) and Oklahoma City (US) facilities. Our major manufacturing sites for Advanced Wound Management products are Hull (UK), Fort Worth (US), Columbia, Maryland (US) and Suzhou (China). Quality & Regulatory Affairs Our Quality & Regulatory Affairs function supports full product life cycle management of Smith+Nephew’s global product portfolio from design and development through manufacturing and post-market surveillance. These teams establish appropriate processes and procedures to facilitate compliance with complex global regulations and laws that govern the design, development, approval, manufacture, labelling, marketing and sale of healthcare products. The Quality & Regulatory Affairs teams directly support expansion of our global portfolio through the registration of new products and existing products in new markets, as well as ensuring compliance with regulatory reporting standards. The European Union Medical Device Regulation (EU MDR) is a significant regulatory change whereby medical devices carrying a CE mark, confirming conformity with relevant requirements, now face greater scrutiny than ever before to ensure they are effective and safe. We have made good progress with our respective submissions, with all files submitted to the Notified Bodies and 90% percent of respective product lines have received MDR certification. The Regulation allows devices certified under previous legislation (Medical Device Directive or MDD) to continue to be placed on the market in Europe until 31 December 2027 or 31 December 2028, dependent on risk classification. We closely monitor other Regulatory landscape changes. This includes changes in UK Medical Device Legislation and UKCA marking. These changes allow CE marked devices to be placed on the market in Great Britain until June 2030. Additionally, we are closely monitoring international regulatory trends that include an increased focus on cybersecurity in medical technology. 33 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Taking our innovation to market Orthopaedics Smith+Nephew’s Orthopaedics vision is to improve mobility and outcomes, with unique and differentiated technologies that allow patients to live a Life Unlimited. Our innovative implants seek to mimic natural movement, are manufactured using materials with a track record of longevity and performance, and are accompanied by our enabling robotic technologies. We are well positioned as the supplier of choice for surgeons across the globe. Smith+Nephew’s Orthopaedics business unit includes an innovative range of hip and knee implants used to replace diseased, damaged or worn joints, robotics-assisted enabling technologies that improve accuracy and facilitate precision during the surgical procedure, and trauma products used to stabilise fractures and correct bone deformities. In Orthopaedic Joint Reconstruction, we have a broad, clinically proven and differentiated portfolio that allows us to compete effectively across a market worth around $15.9 billion annually. This portfolio includes our proprietary OXINIUM◊ material which offers a clear advantage over competitors. In addition, our CORI◊ Surgical System is strongly positioned to take advantage of the trends towards robotic-assisted surgery and outpatient joint replacement seen across the segment. The Trauma & Extremities market is worth over $13.6 billion annually, and we are well positioned to compete effectively in this segment. The simplicity and efficiency of our complete EVOS◊ Plating System gives us an advantage in the largest segment in Trauma, and our TRIGEN◊ INTERTAN◊ Intertrochanteric Nail is backed by the clinical and economic data to position it as the standard of care for hip fracture,1,2 the second-largest segment. In Extremities, we launched our next generation shoulder implant, the AETOS◊ Shoulder System. 2023 performance Orthopaedics revenue increased 4.8% on a reported basis in 2023, including a 90bps headwind from foreign exchange. Underlying revenue growtha was 5.7%. Within this, all segments positively contributed to growth. In Knee Implants and Hip Implants our performance outside the US benefited from improved product supply and execution. Further work is required to address these challenges A leading portfolio of hip and knee implants, robotics and digital enabling technologies driving procedural innovation with Precision in Motion, and a strengthened Trauma & Extremities portfolio. Highlights Orthopaedics revenue $2,214m 2022: $2,113m Reported 4.8% Underlyinga 5.7% Orthopaedics trading profit $398m 2022: $383m 2023 Revenue 2023 Reported growth 2023 Underlying growtha Knee Implants $940m 4.7% 5.5% Hip Implants $599m 2.5% 3.8% Other Reconstruction $111m 27.8% 28.0% Trauma & Extremities $564m 3.7% 4.4% a These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. We strengthened performance across most segments in 2023, and we are clear on where we still need to improve with the necessary actions underway.” Brad Cannon President Orthopaedics & Americas We serve our markets through three global business units of Orthopaedics, Sports Medicine & ENT, and Advanced Wound Management. These business units are responsible for strategy and global marketing, and contain specialist sales and support teams dedicated to serving the specific requirements of healthcare systems. 34 Smith+Nephew Annual Report 2023 |
| in the US. Other Reconstruction grew strongly as we expanded our CORI◊ Surgical System, and Trauma & Extremities performed well in the US where we focused on improving availability of our EVOS◊ Plating System. Trading profita grew 3.9%, although the trading profit margina of 18.0% remains below that of our other business units. Fixing Orthopaedics A major area of focus for our 12-Point Plan is to fix Orthopaedics, to regain momentum across hip and knee implants, robotics and trauma, and win share with our differentiated technology. In 2023 we made good progress improving product availability, logistics and utilisation of implants and instrument sets. We also improved commercial execution, repositioning our offering, streamlining the organisation, simplifying our commercial process and investing in deeper sales training. We also enhanced our incentive plan to better reward performance, sales mix, robotic placement and implant pull-through. Our actions and progress under the 12-Point Plan are discussed further on pages 8–11. Strategy Our Orthopaedics business unit has an innovative portfolio that allows us to compete in joint reconstruction, robotics-enabled procedures, and Trauma & Extremities markets. We are building on our strong foundation in order to sustain profitable growth. Our areas of focus include advancing innovative surgical solutions and optimising the use of working capital. Our initiatives are designed to drive growth across the Orthopaedic business unit. In joint reconstruction and robotics, we aim to accelerate growth by focusing on robotically enabled knee procedures and navigated hip arthroplasty with the CORI◊ Surgical System. Additionally, we will continue to leverage the unique material properties in OXINIUM◊ across the knee and hip platform. For Trauma & Extremities, Smith+Nephew expects to globally scale the EVOS◊ Plating System portfolio to compete more broadly in trauma centres. In addition, the 2023 launch of our AETOS◊ Total Shoulder System is expanding our footprint in the shoulder replacement market. Global market share In our Orthopaedics business unit we are one of four leading players, competing against US-based companies Stryker, Zimmer Biomet and DePuy Synthes. A Smith+Nephew 10% B Zimmer Biomet 31% C Stryker 24% D DePuy Synthesc 19% E Others 16% A Smith+Nephew 4% B DePuy Synthesc 25% C Stryker 23% D Zimmer Biomet 11% E Others 37% Trauma & Extremities $13.6bn +7% 2022: $12.7bn +3% b Data used in 2022 and 2023 estimates generated by Smith+Nephew is based on publicly available sources and internal analysis and represents an indication of market shares and sizes. c A division of Johnson & Johnson. Global market size 2023b Hip and Knee Implants $15.9bn +8% 2022: $14.8bn +4.5% Reconstruction & Robotics Knee Implants In Knee Implants, Smith+Nephew’s specialised systems include leading products for total primary replacement and revision, as well as partial and patellofemoral joint resurfacing procedures, offering surgeons and patients the benefits of many proprietary technologies. These include a unique kinematic knee, the JOURNEY◊ II Total Knee Arthroplasty system, which features OXINIUM◊ Technology and has been shown to replicate normal knee shape, position and motion.*3,4 Our LEGION◊ CONCELOC◊ Cementless Total Knee System (TKS) uses innovative 3D printed cementless technology to achieve biological fixation, bringing efficiency and versatility to the OR.5 The JOURNEY II ROX◊ Total Knee Solution, is a reverse-hybrid procedural solution which aims to provide surgeons with the normal kinematics*3,4,7–9 of JOURNEY II TKA, the cementless technology of CONCELOC◊ Advanced Porous Titanium and the wear resistance10,11 of OXINIUM◊ Technology. Hip Implants The Hip Implants portfolio is headlined by the POLAR3◊ Total Hip Solution which has among the lowest revision rates in total hip arthroplasty.*12–16 Our OR3O◊ Dual Mobility System is the first system to use the latest OXINIUM◊ DH advanced bearing technology. Dual mobility hip implants are used in primary as well as revision procedures. In addition, we offer a full breadth of stems to address surgical needs, including the ANTHOLOGY◊ Hip System. For revisions, the REDAPT◊ Revision Hip System features CONCELOC◊ Technology. Bringing innovation to India In 2023 we launched our OR3O Dual Mobility System in India for use in primary and revision hip arthroplasty. » For a full list of references see pages 262–264 A B C D E A B C D E Smith+Nephew Annual Report 2023 35 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Taking our innovation to market continued Orthopaedics continued Our award-winning, advanced implant material for hip and knee arthroplasty OXINIUM◊ Technology is a strong, resilient and advanced implant material that is only found in Smith+Nephew’s portfolio of joint replacement systems. OXINIUM◊ Technology has established itself as the best performing bearing with the lowest risk of revision in total hip arthroplasty (THA) at 9–18 years,13–16,33 alongside strong clinical performance in knees.33 It has been used clinically for over 20 years as part of over two million procedures. Other Reconstruction Our Other Reconstruction business includes the CORI◊ Surgical System, one of the most advanced and efficient***17 solutions. CORI◊ is a smaller,****18 portable solution capable of performing robotic-assisted knee and computer-guided hip surgery on a single platform. In robotic-assisted knee procedures, CORI◊ utilises handheld precision milling which allows surgeons to execute TKA and UKA procedures with reproducible accuracy.*****19–23 Unlike other systems, the proprietary smart mapping feature creates a 3-D image of the patient’s anatomy in surgery, eliminating time, costs, and radiation exposure23 associated with preoperative CT scans. In 2024, we will introduce the CORIOGRAPH◊ pre-op planning and modelling service that delivers a unique surgical planning solution desired by surgeons. The addition of pre-op planning will make the CORI◊ System the most versatile and flexible robotic-assisted system on the market. The proprietary software allows the CORI ◊ System to utilise our proven image-free surface mapping and image-based planning solutions for the right indications. RI.HIP◊ NAVIGATION further expands indications on the CORI◊ System, bringing a computer-guided total hip application to a platform previously dedicated to robotic-assisted knee procedures. When combined with Smith+Nephew hip implants, like the POLAR3◊ Total Hip Solution and OR3O◊ Dual Mobility System, and complementary tools to assess spinopelvic mobility (RI.HIP MODELER). RI.HIP◊ on CORI◊ delivers a comprehensive solution for navigated total hip arthroplasty. RI.HIP◊ NAVIGATION and RI.HIP◊ MODELER are designed to help maximise accuracy and reproducibility by delivering patient-specific component alignment. With the addition of a first-in-market indication in the US for robotic-assisted revision knee using the LEGION◊ Revision Knee System, the CORI◊ System is currently the only solution indicated for robotic-enabled knee procedures across the full continuum of care – partial, total, and revision knee arthroplasty. Furthermore, indications for LEGION◊ CONCELOC◊ Cementless Total Knee System and RI.HIP◊ NAVIGATION are part of CORI◊ .. Further strengthening our portfolio, we introduced the first of its kind handheld digital tensioning device for robotically-enabled total knee arthroplasty in 2023. The CORI◊ Digital Tensioner is a purpose-built device that lets surgeons measure the ligament tension in a knee prior to cutting bone.24,25 By enabling a surgeon to quantify joint laxity in the native knee and achieve an optimal ligament tensioning force, the CORI◊ Digital Tensioner helps to reduce variability when balancing the knee in surgery.24–27 Helping personalise robotics-enabled surgery with AI In 2023 we introduced two key products that close the feedback loop for our robotics and digital surgery portfolio – Personalized Planning powered by AI and RI.INSIGHTS◊ Data Visualization Platform. These solutions transform data into contextual intelligence by enabling surgeons to see how pre-operative surgical plans and intra-operative decision-making link to post-operative outcomes. Personalized Planning powered by AI, guided by RI.INSIGHTS◊ data enables the surgeon to set the initial implant placement within the total knee arthroplasty procedure based on AI-guided reference values and the surgeon’s planning preferences for specific implants and patient-specific deformities. Through the RI.INSIGHTS◊ Data Visualization Platform, surgeons can reference individual case performance and benchmark that data against an anonymised global database. The platform was designed to give surgeons a simple and effective way to link patient reported outcome measures (PROMs) to pre-operative planning and intra-operative decisions in robotically enabled knee replacements. Surgeon-specific dashboards provide the ability to analyse procedure data, such as case times, resections and alignment, and ligament tensioning data from the CORI◊ Digital Tensioner. RI.INSIGHTS◊ delivers an elegant solution to visualise data, connect PROMs, address known challenges with information access and utilisation, and transform surgical insights into actionable information. GENESIS◊ II Knee RI.INSIGHTS◊ Data Visualization Platform In 2023, we expanded the CORI◊ Surgical System’s capability in knee replacement with Personalized Planning powered by AI, guided by RI.INSIGHTS◊ data. This new addition enables surgeons to set the initial implant placement within the total knee arthroplasty procedure based on AI-guided reference values and the surgeon’s planning preferences for specific implants and patient-specific deformities. 36 Smith+Nephew Annual Report 2023 |
| Driving procedural innovation with Precision in Motion Joint arthroplasty is dynamic, and future progress will be defined by the constant evolution of technology and its ability to accommodate unique patient circumstances, all while refining accuracy and reproducibility. Covering a broad range of indications and approaches – from primary and revision implant solutions to cutting-edge digital surgery and advanced bearing science – Precision in Motion embodies the ability of technology to: – Personalise surgery: Leveraging handheld robotic assistance, computer guided surgery and digital tensioning to help position implants based on individual patient anatomy, CORI Digital Tensioner is the first digital tensioning device with a robotic system that quantifies joint laxity prior to any bone resection and reduces variability of tensioning by 64%.24–27 – Advance efficiency: Aiming to make the complex as simple as possible by pioneering solutions to help restore joint anatomy and improve knee balance, especially in challenging cases like revision knee arthroplasty. The CORI System is the first robotic-assisted technology indicated for revision knee procedures. It has demonstrated operating room efficiency, such as a mean 56% reduction in trays28† thereby reducing sterilisation and OR time cost with an estimated savings of $1,500/ case in a single centre.29†† – Optimise performance: Combining innovations to facilitate surgeon preferences and help solve the challenges they face; such as CoCr-free modular dual mobility hip and truly unique bearing material science. Trauma & Extremities Smith+Nephew’s portfolio includes differentiated technology across the major categories of Plates and Screws, Intramedullary Nails, Hip Fracture, Limb Restoration, Extremities, and Shoulder Replacement. Leading products include the EVOS◊ Plating System which includes a wide range of clinical indications from mini and small to large fragment and periprosthetic. Designed to offer surgeons an all-inclusive, expansive plating portfolio, EVOS◊ provides the simplicity of logically organised instrumentation with advanced implant solutions that meets the demands and expectations of trauma surgeons. The portfolio also includes the TRIGEN◊ INTERTAN◊ Hip Fracture System, which is backed by many years of strong clinical evidence.2 For Extremities, SMART TSF◊ expands the capabilities of the TAYLOR SPATIAL FRAME◊ External Fixator. In 2023, we launched the AETOS◊ Shoulder System, indicated for both anatomic and reverse total shoulder arthroplasty. It is designed to restore patients’ range of motion34–37 and help minimise arthritic shoulder pain. The AETOS◊ Shoulder System is the latest solution in Smith+Nephew’s expanding Upper Extremity portfolio and complements our market-leading Sports Medicine shoulder repair and biologics solutions. The portfolio also includes the TRIGEN◊ INTERTAN◊ Hip Fracture System, which is backed by many years of strong clinical evidence.40,41 For Extremities, SMART TSF◊ expands the capabilities of the TAYLOR SPATIAL FRAME◊ External Fixator. This is our commitment. This is Precision in Motion » For a full list of references see pages 262–264 EVOS◊ Plating System Integrated solutions for fracture fixation The EVOS◊ Plating System, an evolutionary approach to simplify and unify into one plating system, offers surgeons the simplicity of one, comprehensive plating system that addresses all of their small fragment surgical needs. Smith+Nephew Annual Report 2023 37 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Taking our innovation to market continued Sports Medicine & ENT Smith+Nephew’s Sports Medicine & ENT business unit leads with innovative procedural solutions to elevate the standard of care in Sports Medicine & ENT. With a comprehensive offering and differentiated technologies backed by clinical evidence, we help healthcare professionals get their patients back to a Life Unlimited. Sports Medicine & ENT operates in growing markets where unmet clinical needs provide opportunities for procedural and technological innovation. Smith+Nephew holds a leadership position as a global player in the $5.8 billion annual Sports Medicine Market. Sports Medicine spans a broad patient population, including athletes. People of all ages are more active than ever before, and whenever they seek treatment for an injury or a degenerative condition, they expect a fast recovery and rapid return to activity. The surgeons who serve these patients want to treat them as efficiently and as minimally invasively as possible while ensuring the best possible outcomes. We have a rich history of product development, and our technologies, instruments and implants enable surgeons to perform minimally invasive surgery, treating soft tissue injuries and degenerative conditions of the shoulder, knee, hip and small joints. ENT is also an attractive, growing market segment offering the opportunity to address unmet needs with differentiated procedural solutions. The positive momentum is driven by emerging therapies, changes in the point of care, mainly to the office setting, and increasing global access for ENT procedures. We offer a portfolio of technologies focused on the unmet needs of some of the most common procedures general and paediatric ENT surgeons perform today. These include tonsillectomies, epistaxis (severe nose bleeds) and tympanostomies (insertion of ear tubes). Elevating the Standard of Care Highlights Sports Medicine & ENT revenue $1,729m 2022: $1,590m Reported 8.8% Underlyinga 10.0% Sports Medicine & ENT trading profit $503m 2022: $472m 2023 Revenue 2023 Reported growth 2023 Underlying growtha Sports Medicine Joint Repair $945m 8.7% 9.9% Arthroscopic Enabling Technologies $588m 3.7% 4.7% ENT $196m 28.1% 29.8% a These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. We delivered strong growth in 2023 as we built upon our leading portfolios in Joint Repair and Arthroscopic Enabling Technologies and expanded our exciting biological healing business.” Scott Schaffner President Sports Medicine Arthroscopy solutions for the OR We are driven to design products that enable better outcomes and improved quality of care. We work with customers to ensure their arthroscopy suite is complete, robust and ready to perform – providing and supporting comprehensive technologies for visualisation, fluid management, tissue resection (COBLATION◊) and patient positioning. Our INTELLIO◊ Connected Tower Solution provides sports medicine surgeons with a complete suite of enabling technologies in the operating room (OR). It uses a centralised app to wirelessly connect and control the major components of an arthroscopy surgical tower from outside the sterile field, helping to streamline procedure support. 38 Smith+Nephew Annual Report 2023 |
| 2023 performance Sports Medicine & ENT delivered revenue growth on a reported basis of 8.8% including a 120bps headwind from foreign exchange. Underlying growtha was 10.0%. Performance was impacted as distributors reduced inventory in anticipation of volume based procurement in China. Sports Medicine Joint Repair delivered a strong performance, in line with previous years, led by the REGENETEN◊ Bioinductive Implant. Arthroscopic Enabling Technologies improved year-on-year as we benefited from improved supply. ENT grew strongly led by our tonsil and adenoid business. Business unit trading profita was up 6.6% with a trading profit margina of 29.1%. Strategy We have a strong Sports Medicine & ENT business and are well positioned for long-term leadership and delivering our vision of advancing standards of care. Our business unit is driven by the three strategic priorities – innovation, market development and commercial execution. Smith+Nephew’s Sports Medicine & ENT business is founded on procedural innovation, with differentiated technologies that shape clinical outcomes across the globe. Our portfolio continues to demonstrate strong growth across key segments, and we have an innovative pipeline in development. In line with our vision, our emphasis on market development will help shift standards of care to technologies and procedures that deliver on the promise of Life Unlimited. We are committed to investments in key areas such as clinical evidence, medical education and surgeon training for continued market development around key procedures. Our commercial initiatives reflect balanced selling across segments and regions, aligned priorities and a customer-centric, winning mentality. Global market share In Sports Medicine, Smith+Nephew holds a leading position behind Arthrex (US), and also competes against Stryker and DePuy Mitek. A Smith+Nephew 28% B Arthrex 33% C Stryker 12% D DePuy Mitekd 10% E Others 17% Global market size 2023b Sports Medicinec $5.8bn +7% 2022: $5.5bn +4% b Data used in 2022 and 2023 estimates generated by Smith+Nephew is based on publicly available sources and internal analysis and represents an indication of market shares and sizes. c Representing repair products and arthroscopic enabling technologies, and excluding ENT. d A division of Johnson & Johnson. UltraTRAC Sports Medicine advanced procedural innovation in 2023 by launching the QUADTRAC◊ Quadriceps Tendon Harvest Guide System and expanded family of ULTRABUTTON◊ Adjustable Fixation Devices for anterior cruciate ligament (ACL) reconstruction. ULTRABUTTON◊ TIB Adjustable fixation device QUADTRAC◊ Quadricepts tendon harvest guide system X-WING Graft preparation system◊ ULTRABUTTON◊ QUAD Adjustable fixation device A B C D E » For a full list of references see pages 262–264 Smith+Nephew Annual Report 2023 39 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Taking our innovation to market continued Sports Medicine & ENT continued Key products by segment Sports Medicine Joint Repair Our Sports Medicine Joint Repair business offers innovative procedural solutions for repairing soft tissue injuries including systems of specialised implants and instruments to facilitate arthroscopic procedures across Sports Medicine for knees, shoulders, hips and small joints. For shoulder repair, we develop products for Rotator Cuff Repair (RCR) and instability repair to help address pain and restore function. Advanced Healing Solutions for RCR include the innovative REGENETEN◊ Implant. With at least 12 published clinical studies including more than 700 patients,1,2,13–22 the REGENETEN◊ Implant has been shown to change the course of tear progression in early studies,1,3,4,6,23,24 aid return to normal activity13 and reduce re-tears versus conventional surgery.7,16,17,25–28 The HEALICOIL◊ Platform of Shoulder Anchors features an open architecture design to facilitate healing8 and is available in our REGENESORB◊ material which is designed to be absorbed and replaced by bone within 24 months.****10–12 In knee repair, arthroscopic repair techniques have become more prevalent and widely recognised for the treatment of meniscal tears in recent years.29 Our All Tears, All Repairs Meniscal Repair Portfolio provides surgeons with unsurpassed options and possibilities for meniscal repair. In November 2023 we announced a definitive agreement to acquire CartiHeal, developer of the CARTIHEAL◊ AGILI-C◊ Cartilage Repair Implant, a novel sports medicine technology for cartilage regeneration in the knee. CARTIHEAL◊ AGILI-C◊ is a porous, biocompatible and resorbable scaffold which promotes natural regeneration of the articular cartilage and restoration of its underlying subchondral bone. See page 29 for further information. We also offer a comprehensive ligament portfolio of high-quality products and thoughtful techniques to address the full spectrum of ligament pathologies and concomitant injuries. Building upon our trusted legacy of data-driven solutions, we continue to innovate in this space. In 2023 we introduced the UltraTRAC◊ QUAD ACL Reconstruction Technique which consists of the new QUADTRAC◊ Quadriceps Tendon Harvest Guide System, X-WING◊ Graft Preparation System and a family of ULTRABUTTON◊ Adjustable Fixation Devices. These technologies work together to provide an innovative procedural solution, expanding Smith+Nephew’s ability to address surgeon graft preference. Our hip preservation portfolio contains a comprehensive offering of technologies and techniques, establishing Smith+Nephew as a leader and innovator in the hip repair segment. The CAP-FIX◊ Capsular Management Family addresses all capsular management needs, from open to close. We are committed to Redefining Healing Potential in gluteus medius repairs, with the use of the REGENETEN◊ Implant.** In 2023 we launched new procedure solutions in the foot and ankle soft tissue repair segment, entering the market with focused techniques and procedural kits for ankle instability and Achilles reconstruction. Our core platform technology is designed specifically for the foot and ankle surgeon and provides a significant opportunity for growth. In addition, with the REGENETEN◊ Implant, we offer an innovative biologic solution that can be used to augment insertional or midsubstance Achilles repair.** Advanced Healing Solutions At Smith+Nephew, we are redefining healing potential with our portfolio of innovative products and materials. The REGENETEN◊ Implant supports the body’s natural healing response to promote the growth of tendon-like tissue and change the course of tear progression.**1–6 Derived from highly purified bovine Achilles tendon, it creates an environment that is conducive to healing.1,3 When used in Rotator Cuff Repair, the results of a new randomised controlled trial showed that the addition of our REGENETEN◊ Implant delivered a significant reduction in rotator cuff re-tear rates at 12 months.7 In addition, the unique open-architecture design of HEALICOIL◊ anchors reduces the amount of implanted material in the shoulder from that of solid-core anchors and may provide a biologic healing advantage.8,9 Our REGENESORB◊ material is designed to provide a jump start in bone healing and formation by full absorption and bone replacement in 24 months.****10–12 40 Smith+Nephew Annual Report 2023 |
| Arthroscopic Enabling Technologies (AET) In Arthroscopic Enabling Technologies, our products facilitate arthroscopic surgical procedures, providing a strong foundation of platforms and associated consumables required to perform arthroscopic surgery, including visualisation, fluid management, COBLATION◊ and mechanical resection. The INTELLIO◊ Connected Tower Solution unites high-definition imaging solutions, energy-based and mechanical resection platforms, fluid management and access technologies. The LENS◊ 4K Surgical imaging system uses 4K UHD image quality and network connectivity in a 3-in-1 console for multi-speciality environments. Our WEREWOLF◊ Controller enables surgeons to remove soft tissue precisely***30 in a variety of arthroscopic procedures. With COBLATION◊ treatment, patients experienced significantly less bleeding post-operatively.*****31 The WEREWOLF FASTSEAL◊ 6.0 Hemostasis Wand is used in orthopaedic procedures for hemostasis of soft and hard tissues bringing a technology widely used in sports medicine to orthopaedic customers. Ambulatory Surgery Centers (ASCs) At Smith+Nephew, we go beyond product to deliver a comprehensive offering for ASCs. There continues to be a shift of both sports medicine and orthopaedic procedures from hospital to ASC outpatient settings. We are uniquely positioned to meet the needs of the market with procedural solutions spanning across sports medicine, hip and knee reconstruction, robotics, trauma, extremities, and post-surgical wound care. As the ASC market evolves, Smith+Nephew will continue to meet the distinct needs of this segment with procedure innovation and tailored programmes for growth. Launching the ARIS◊ COBLATION◊ Turbinate Reduction Wand The ARIS◊ COBLATION◊ Turbinate Reduction Wand utilises Smith+Nephew’s advanced COBLATION Plasma Technology to provide a minimally invasive way to reduce hypertrophic turbinates. It provides targeted hemostasis with built-in bipolar coagulation function.40 Designed for versatility, the ARIS◊ COBLATION◊ Turbinate Reduction Wand allows surgeons to vary the degree of tissue removal based on patient indication when treating hypertrophic turbinates submucosally. It offers customisation, flexibility and control for turbinate reduction procedures, accommodating various submucosal resection surgical techniques. It is designed specifically for the WEREWOLF◊ ENT Controller. Ear, Nose and Throat (ENT) In Ear, Nose and Throat, our COBLATION◊ Plasma Technology, which has been used to remove tonsils and adenoids for over 15 years,32,33 has an ability to remove tissue at low temperatures with minimal damage to surrounding tissue.32,34–38 Evidence shows that COBLATION◊ Intracapsular Tonsillectomy (CIT) procedures offer less pain, quicker recovery and a decreased risk of post-operative bleeding with similar outcomes to total tonsillectomies.39 Smith+Nephew offers a full portfolio of COBLATION◊ Wands for CIT procedures. Further expanding our portfolio, we launched the ARIS◊ COBLATION◊ Turbinate Reduction Wand in 2023. Our Tula◊ Systemprovides an in-office alternative to traditional tympanostomy using a local anaesthesia system and an automated, one-click tube delivery device.41,42 As part of our comprehensive portfolio of epistaxis (nosebleed) solutions, RAPID RHINO◊ Epistaxis Products are inflatable tamponades designed for ease of insertion and removal43 with an ultra-low profile and self-lubricating hydrocolloid fabric. In addition, we market a range of dissolvable and removable post-operative nasal dressings. ARIS wand » For a full list of references see pages 262–264 Smith+Nephew Annual Report 2023 41 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Taking our innovation to market continued Advanced Wound Management Smith+Nephew’s Advanced Wound Management vision is to Shape What’s Possible in Wound Care. Through our extensive portfolio, designed to meet broad and complex clinical needs, we help healthcare professionals solve the challenges of preventing and healing wounds. The global wound care market is worth around $11.4 billion globally per annum. Long-term growth has been driven by the needs of an ageing population in many markets and as we experience lifestyle-related health conditions, such as increasing prevalence of obesity, diabetes and vascular disease. These conditions are key drivers of wound prevalence which contribute to the pressure on healthcare spending. In Advanced Wound Management, we seek to help healthcare systems through innovation in products and services, to deliver accelerated healing or preventing wounds, and to do more with less, such as enabling patients to be treated faster requiring fewer resources, or moved from acute to homecare settings. We do this across our three segments of Advanced Wound Care (AWC), Advanced Wound Bioactives (AWB) and Advanced Wound Devices (AWD). 2023 performance Advanced Wound Management delivered revenue growth on a reported basis of 6.2% including a 20bps headwind from foreign exchange. Underlying growtha was 6.4%. Within this, Advanced Wound Care’s performance included growth from our major categories of foams, films and infection management. Advanced Wound Bioactives' performance was driven by strong growth from SANTYL◊ .. Advanced Wound Devices was driven by both our traditional RENASYS◊ Negative Pressure Wound Therapy System and our single-use PICO◊ Negative Pressure Wound Therapy System. Business unit trading profita was up 8.3% with a trading profit margina of 29.4%. Strategy Our vision of shaping what's possible in wound care is delivered through innovation in product with strong clinical evidence and digital tools that enable protocol compliance to ensure optimal patient outcomes. Innovation includes new product development, line extensions and acquisitions as well as digital services for both clinicians and patients. To drive ever-improving commercial execution we seek to inspire, engage and align on our global strategy across all regions and functions as efficiently as possible. Through these strategic priorities we are driving performance and supporting delivery of Smith+Nephew’s global Strategy for Growth to Strengthen, Accelerate and Transform through the 12-Point Plan. Shaping What’s Possible in Wound Care Highlights Advanced Wound Management revenue $1,606m 2022: $1,512m Reported 6.2% Underlyinga 6.4% Advanced Wound Management trading profit $472m 2022: $436m 2023 Revenue 2023 Reported growth 2023 Underlying growtha Advanced Wound Care $725m 1.8% 2.1% Advanced Wound Bioactives $553m 6.3% 6.2% Advanced Wound Devices $328m 17.0% 17.6% We are pleased with our 2023 performance, led by our Negative Pressure Wound Therapy portfolio where we focused on accelerating growth, delivering on the 12-Point Plan.” Rohit Kashyap President Advanced Wound Management & Global Commercial Operations GRAFIX◊ Placental Membranes from our skin substitute product range. a These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. 42 Smith+Nephew Annual Report 2023 |
| A Smith+Nephew 14% B 3M 16% C Mölnlycke 10% D ConvaTec 6% E Others 54% Global market size 2023b Advanced Wound Management $11.4bn +5% 2022: $10.8bn +4% b Data used in 2022 and 2023 estimates generated by Smith+Nephew is based on publicly available sources and internal analysis and represents an indication of market shares and sizes. Global market share We operate in all three categories in wound care, and have the second largest business globally in terms of revenue. In the Advanced Wound Care segment we compete in dressings with Mölnlycke (Sweden), Coloplast (Denmark) and ConvaTec (UK). In Advanced Wound Devices, we are the primary challenger to Negative Pressure Wound Therapy incumbent 3M. In Advanced Wound Bioactives we have leadership positions in a number of our respective categories. Reducing the burden on nurses through shared-care The World Health Organisation predicts a need for nine million more nurses by 2030 for health and wellbeing.9 Chronic wounds significantly burden healthcare, consuming large healthcare budgets. With wound prevalence increasing, efficient wound care treatments are crucial.10 In 2022, Wounds International suggested that 3.5 billion nursing hours could be saved globally by 2030 with shared-care in chronic wound care and long-wear advanced foam dressings. Shared-care involves patient participation in care delivery, supported by healthcare professionals. It's effective in diabetes, stoma management and incontinence.11–14 ALLEVYN◊ LIFE Foam Dressings, supporting shared-care, are designed for extended use (up to seven days), managing exudate and providing comfort.15–22 * ALLEVYN LIFE◊ Foam Dressing DURAMAX◊ S Silicone Superabsorbent Dressing for highly exuding wounds launched in 2022. Committed to reducing environmental impact Smith+Nephew's Less Waste+More Care initiative focuses on reducing environmental impact by optimising ALLEVYN◊ Dressing packaging. This includes reducing carton, pouch and case sizes by over 20%, and making ALLEVYN◊ Dressing cartons 33% smaller and 13% lighter than competitors.23 A B C D E » For a full list of references see pages 262–264 Smith+Nephew Annual Report 2023 43 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Taking our innovation to market continued Advanced Wound Management continued Key products by segment Advanced Wound Care Smith+Nephew started as a wound care company and through our Advanced Wound Care business we have grown to be a leader in the segment. Today our portfolio includes products that are designed to manage exudate and infection, protect the skin and help prevent pressure injuries. In exudate management, our products provide appropriate wound fluid handling and absorption to help promote an optimal wound healing environment.24–26 Our ALLEVYN◊ LIFE Foam Dressing is uniquely differentiated, with its EXUMASK◊ change indicator and hyper-absorbent lock-away layer, with EXULOCK◊ technology for odour control and fluid lock-in.26–28 The effectiveness of the ALLEVYN Dressing range has been demonstrated across 138 publications in 19 countries on over 12,000 patients and volunteers.29 In 2023, sales of the DURAMAX◊ S Silicone Superabsorbent Dressing, launched last year, continued to grow. Our key silver-based ACTICOAT◊ Antimicrobial Barrier Dressings, DURAFIBER◊ Ag Absorbent Gelling Silver Fibrous Dressing, ALLEVYN◊ Ag Antimicrobial Foam Dressing, as well as our range of IODOSORB◊ Cadexomer Iodine products provide clinicians with a range of solutions to help patients with complex wounds, managing exudate as well as providing a barrier to bacterial penetration.30–40 We were successful in receiving US 510(k) clearance for our improved range of ALLEVYN◊ Ag Antimicrobial Foam Dressings in 2023, giving access to expanding market segments in the future. Advanced Wound Bioactives Our Advanced Wound Bioactives portfolio provides a unique approach to debridement, dermal repair and tissue substitutes with considerable evidence supporting their clinical application. Collagenase SANTYL◊ Ointment (250 units/ gram) is the only FDA-approved enzymatic debridement agent indicated for debriding both chronic dermal ulcers and severely burned areas available in the US market, with a unique mechanism of action that removes necrotic collagen and contributes to the formation of healthy collagen in chronic wounds and severely burned areas. REGRANEX◊ (becaplermin) Gel 0.01% is the only FDA-approved Platelet-Derived Growth Factor for the treatment of diabetic neuropathic ulcers, formulated to act as a first-line treatment following effective ulcer care. In our skin substitute product range, GRAFIX◊ Placental Membranes and STRAVIX◊ Umbilical Tissues retain the extracellular matrix, growth factors and native placental components to support wound closure.41–42 They are intended for application directly to acute and chronic wounds and as a surgical cover or barrier. In addition, we offer OASIS®** Matrix and OASIS MICRO products, which are naturally derived scaffolds of extracellular matrix (ECM), composed of porcine small intestinal submucosa (SIS) and indicated for the management of a wide range of acute and chronic wounds, burns and surgical interventions.43 Advanced Wound Devices In Advanced Wound Devices, our portfolio helps improve healing outcomes in chronic wounds, reduces surgical site complications and facilitates preventative care for pressure injuries. Within the negative pressure wound therapy (NPWT) category, we offer single-use and traditional (cannister-based) solutions offering customers a one-stop shop with great flexibility. The future of pressure injury prevention Hospital-acquired pressure injuries (HAPIs) are on the rise. Despite a decrease in other hospital-acquired conditions, HAPIs are up 6%.1†† Each year, complications from pressure injuries result in an estimated 60,000 deaths in the US. The average incremental cost of treating a pressure injury is $21,767. Smith+Nephew’s LEAF◊ Patient Monitoring System promotes adherence to patient turning procedures.2,3 Visual alerts in the patient room and at the nurses’ station make it easy for the whole team to see who needs to be turned and when.4 Plus, the LEAF◊ System’s Integrated Positioning Technology is the first tool that measures the quality and effectiveness of patient turning, including patient turn frequency and turn angle. Smith+Nephew's ALLEVYN◊ LIFE Dressings and SECURA◊ skincare products are designed to help prevent pressure injuries, aiding in evidence-based protocol adherence for HAPI prevention.5–8 These products showcase the Company's commitment to improved healthcare practices. 44 Smith+Nephew Annual Report 2023 |
| Our technology takes the limits off living Smith+Nephew’s Advanced Wound Management Business Unit is also focused on utilising digitally enabled technologies and pioneering data services to provide new forms of value to our customers. We aim to help optimise clinical practice, prevent unnecessary wounds and complications, support patient care and self-management where appropriate and drive the transition to value-based business models. Building from the launch of the award-winning WOUND COMPASS◊ Clinical Support App in 2022, we are investing in a digital health portfolio that leverages the latest advancements in connectivity, artificial intelligence and data-driven health services. These technologies aim to support our customers in delivering more accessible, efficient and effective wound care for patients. PICO PICO◊ Single Use Negative Pressure Wound Therapy System (sNPWT) is cost effective and improved outcomes compared with standard care to help prevent surgical site complications in patients with surgically closed incisions. A systematic literature review and meta-analysis of 19 studies involving 4,530 patients showed a 63% reduction in the odds of developing surgical site infections with the prophylactic use of PICO◊ sNPWT compared with standard care.45 Hospital-acquired pressure injuries (HAPIs) are on the rise1 Despite a decrease in other hospital-acquired conditions, HAPIs are +6%1†† Each year, complications from pressure injuries result in an estimated 60,000 deaths in the US54 The average incremental cost of treating a pressure injury is $21,76755 Our PICO◊ range of single-use NPWT systems, with their proprietary AIRLOCK◊ Technology layer, has demonstrated significant healing outcomes for chronic wounds44*** and in the reduction of surgical site complications in closed incisions,45† in a highly portable form that allows patients to return to their daily lives.46,47 Our traditional RENASYS◊ NPWT Systems are easy to use platforms with a range of accessories to treat a wide variety of wounds and patients – across all care settings.48,49 The RENASYS◊ portfolio has been enhanced with the recent addition of EDGE, our latest innovation in NPWT designed to be clinically easy to use,50 alleviating the daily patient burden of living with a wound,51 whilst delivering higher efficiency and utility for healthcare systems.50,52,53 AWD also includes the LEAF◊ Patient Monitoring System that supports a hospital’s pressure injury prevention strategy. In 2023, we gained 510(k) clearance and introduced into the US the VERSAJET◊ III Hydrosurgery System, a surgical debridement device. » For a full list of references see pages 262–264 Smith+Nephew Annual Report 2023 45 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Building a culture of belonging At Smith+Nephew, we strive to create a culture of belonging where all employees can bring their full selves and best ideas. We are committed to creating a psychologically safe environment that fosters innovation, delivers business success and strengthens engagement and personal fulfilment. Inclusion and allyship Our comprehensive people strategy is focused on making Smith+Nephew a workplace that talented people want to join and stay and building a high-performing and inclusive culture where everyone feels a sense of respect and belonging. Our culture pillars of Care, Courage and Collaboration guide all we do. Creating an environment where all employees can flourish begins with attracting and retaining diverse talent, which we support through an extensive Inclusion, Diversity and Equity (IDE) programme. In 2023 we took a number of new steps to continue this journey. Our Talent Acquisition team began using ‘bias interrupters’ in their hiring practices including ensuring diverse sourcing, hiring pools and hiring panels for candidates. Bias interrupters are tweaks to basic business systems (including hiring, performance evaluations, assignments, promotions, compensation) that prevent implicit bias in the workplace, often without ever talking about bias. Supported by this, we met our gender diversity goal of 34% females in management positions and met our US ethnicity goal of 21% of those in management positions being ethnically diverse. We also established a baseline for ethnicity for the UK. Our enterprise gender ratio was 43%, exceeding industry best practice of 40%. Gender ratios 2023 We continued to increase female representation in senior roles, up to 34% in 2023 from 33% in 2022 and 31% in 2021. Total employees1 18,452 Male 57% Female 43% Senior managers and above2 1,087 Male 66% Female 34% Board of Directors 12 Male 67% Female 33% 1 Number of employees at 31 December 2023 including part-time employees and employees on leave of absence. 2 Senior managers and above includes all employees classed as Directors, Senior Directors, Vice Presidents, Executive Officers and includes all statutory directors and Directors of our subsidiary companies at 31 December 2023. By creating a workplace where our employees feel supported, included and valued for their unique strengths and perspectives, we are able to build a high-performing culture and ultimately better serve our customers and their patients.” Elga Lohler Chief HR Officer 46 Smith+Nephew Annual Report 2023 |
| In 2023 we introduced a new workspace on our learning platform to educate our employees on allyship in the workplace. Allyship refers to actions, behaviours and practices that support, amplify and advocate with others, especially with individuals who do not belong to the same social identity group. With this, we are teaching our employees how to advocate for and elevate the experience of all their colleagues, particularly those who may be under-represented. Allyship is a strong theme within our Employee Inclusion Groups (EIGs) and will be the guiding theme for our IDE programme in 2024. Smith+Nephew’s EIGs are voluntary, employee-led groups that foster an inclusive, diverse workplace. They are aligned with our purpose, culture pillars, and business objectives and empower our employees to share their experiences, find advocacy, support and strength. To amplify their impact, in 2023 we have brought our woman-focused EIGs including the Society for Women Engineers and Women’s Inspired Network, under a combined group: The Women’s Network. Sponsored by our Group General Counsel & Company Secretary, Helen Barraclough, this group includes more than 800 members globally. Additionally, our EMPOWER EIG, which is focused on employees affected by or living with a visible or invisible disability, chronic health condition and/ or mental health difficulty, launched the Neurodiversity Network. Created and managed by our passionate neurodiverse members, the Neurodiversity Network has a wealth of tools and resources aimed at supporting neurodivergent individuals in the workplace to recognise their strengths and celebrate their unique skills and perspectives. The Neurodiversity Network also helps educate colleagues on the different types of neurodiversity, promoting the value of our neurodiverse colleagues, and raising awareness of their unique challenges. Smith+Nephew is committed to amplifying the inclusion, influence and achievements of women employees by fostering professional development, advocacy and networking. Our Women’s Network is at the centre of our efforts.” Helen Barraclough Group General Counsel & Company Secretary, Executive Sponsor Women’s Network Ethnic diversity In 2023 we met our US ethnicity goal of 21% of those in management positions being ethnically diverse. We also established a baseline for ethnicity across our UK-based management. US management1 UK management1 1 Data correct as 31 December 2023. White 76.2% Ethnically Diverse 21.4% Unknown 2.4% White 88.8% Ethnically Diverse 10.9% Unknown 0.3% +800 Members globally who are part of our EIG Women’s Network Smith+Nephew Annual Report 2023 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION 47 |
| Building a culture of belonging continued Promoting wellbeing Wellness – physical, mental and financial – plays a critical part in enabling employees to engage and focus on delivering their objectives. In 2023 we expanded and improved our global wellness programme. Our wellbeing strategy has five components: – Embed wellness into our culture. – Raise awareness and usage of global wellness resources. – Strengthen employee engagement. – Increase employee wellness and health. – Increase employee productivity through increased engagement and overall health. Promoting wellness of body and mind Individuals whose physical and mental health needs are cared for are three times more likely to be engaged at work, according to Gallup. At Smith+Nephew, our wellness offerings care for the whole person, not just the employee. This year we’ve focused our global wellness steering group to guide our global wellbeing strategy and established a global champion network to help better understand what employees want and what is already available. During 2023, we expanded our mental health first aiders network. We now have 150 trained first aiders across 11 countries. These colleagues are trained to help identify when help is needed, the level of support required, and signpost people towards doctors, helplines or organisations that may offer counselling, professional support and treatments. Privacy is always respected, and conversations are never shared with direct managers. Our mental health champions are often just ‘someone to talk to’. Underscoring the importance of wellness, we replaced our previous Employee Assistance Plan with a new provider, Spring Health, available to all employees and their household members. The new service includes therapy sessions, coaching, a wide range of diverse providers, as well as legal assistance, financial services and referrals for child and elder care. In the US, for the first time, Smith+Nephew was one of only 50 employers recognised with a Best Employer Award for creating a healthy work culture through a well-established, progressive and measurable employee wellbeing and engagement programme. The Business Group on Health is made up of large employers interested in ensuring their wellness and benefit programmes are benchmarked and appropriate for their employees. The group supports collaborating and sharing information on vendors and best practices in wellness and benefits, including diversity, inclusion and health equity. In the US, we also won the Cigna Healthy Workforce Designation: Gold Level award for our focus on the vitality and wellbeing of our workforce and for helping employees to be healthier, more productive and engaged. At Smith+Nephew we promote flexibility in where, how and when we work. This means looking at the spaces in which we work, the ways we work and our work patterns. We believe our approach is an important differentiator, and helps our employees balance work and home life. Our Global Flexibility Principles serve as the guiding philosophy for identifying flexible work solutions that foster productivity and wellbeing while supporting our culture. While the principles are consistent globally, specific flexibility options will vary depending upon the individual, role and site/ country/region. Continuous improvement Creating a culture of belonging, where our employees are highly engaged, is a continuous journey. We measure our progress using the Gallup Q12 as the tool for our annual employee engagement survey. The Q12 survey tool focuses strongly on the role of the people leader in engaging their team. People leaders are provided with their individual survey scores and conduct team sessions where the results are discussed and actions agreed – both to improve on opportunity areas and to maintain strengths. These action plans continued throughout the year and are assessed at our annual Gallup Accountability Check-in Survey to determine whether employees are seeing improvements. In our fifth year using the survey, we again saw an improvement in our ‘grand mean’, putting Smith+Nephew in the top half of participating companies and well above the Gallup average. Since the first administration of the survey, our grand mean has increased every year and we are trending on the improvement trajectory of Gallup’s top quartile clients. Our participation rate in this year’s survey was 89%. Our most meaningful improvements were in employees feeling supported in their progress at work and being recognised for their contributions. Once again, our strongest area was connection to our purpose of Life Unlimited. We also use Gallup to measure our progress in fostering Equity and Inclusion. We made solid progress across all eight categories in 2023, and overall. In Equity our greatest improvement came in employee recognition that we encourage their progress and development. In Inclusion we improved most significantly in the category recognising that Smith+Nephew is committed to building the strengths of each employee. Smith+Nephew supports a culture of wellbeing that provides benefits, resources and programmes for employees across the globe to provide an opportunity to improve their overall wellbeing so that they can bring their best self to work each day. Physical Financial Social Emotional 48 Smith+Nephew Annual Report 2023 |
| Achieving results with responsibility Our global compliance programme helps our business to comply with applicable laws, regulations and industry code requirements in the markets in which we operate. Our comprehensive programme includes policies, guidance, role-based training, monitoring and validation processes supported by data analytics and reporting channels. Our compliance teams work closely with business partners to ensure that our programme evolves in parallel with business changes and emerging risks in the sector. Data privacy is an integral part of our programme and regulation in this area continues to increase. We are committed to helping our employees and third-party partners to do business in the right way through simplification of compliance programme requirements and by embedding key compliance controls into business processes. We regularly review our global policies and use an interactive tool and other resources to guide employees to make decisions that comply both with the law and our Code of Conduct. Our business models require that we work closely with third-party partners, and in many countries these partners sell products on our behalf. We have a well-established risk-based third party compliance programme which includes ongoing due diligence, training and oversight of these partners. An ethical employer Creating an environment where employees feel safe and that fosters innovation means building trust by operating ethically and compliantly. We have multiple levels of ethics and compliance oversight, including a Board Compliance & Culture Committee, to ensure managers, employees and business partners act with integrity. Data privacy has now been fully integrated into the compliance governance framework. We ensure appropriate oversight of significant interactions with healthcare professionals or government officials, and we comply with all national and state transparency reporting laws which require reporting of physician compensation. All employees have a responsibility to report violations of our Code. This may be done via their manager, directly to Compliance, HR or Legal functions, or through an externally managed reporting channel where anonymous reports may be made. At Smith+Nephew, we recruit, employ and promote employees on the sole basis of the qualifications and abilities needed for the work to be performed. We do not tolerate discrimination on any grounds and provide equal opportunity based on merit. Smith+Nephew gives individuals with disabilities fair consideration for all vacancies against the requirements of the role. Where possible, for any employee who has a disability or who becomes disabled while working for us, we make reasonable adjustments and provide appropriate training to ensure that they are supported in their career. We are committed to providing equal opportunities in recruitment, promotion and career development for all employees, including those with disabilities. We do not use any form of forced, compulsory or child labour. Smith+Nephew supports the Universal Declaration of Human Rights of the United Nations, respecting the human rights, dignity and privacy of individuals and their right to freedom of association, freedom of expression and the right to be heard. As a global medical technology business, we recognise our responsibility to take a robust approach to preventing slavery and human trafficking. Smith+Nephew is committed to preventing such activities in all of its corporate operations and in its supply chains. We comply with applicable laws and regulations globally in terms of our interactions with labour unions. +0.19 +0.10 +0.08 +0.07 +0.08 +0.04 -0.04 +0.32 +0.12 +0.07 +0.05 +0.04 2019 2020 2021 2022 2023 GrandMean Change (Average from Baseline) Smith+Nephew Gallup clients average Top 25% Gallup clients Smith+Nephew’s culture trajectory is well above average Source: Gallup In 2023, Smith+Nephew conducted its fifth administration of our Global Employee Survey using the Gallup Q12 engagement tool. We saw a significant uptick in engagement from year one (2019) to year two (2020) following the launch of our purpose, culture pillars and brand refresh. Smith+Nephew’s engagement trajectory (in orange) has remained well above the Gallup average (grey) and is tracking towards the top quartile of the Gallup database (blue). » Our Code of Conduct and Business Principles and Modern Slavery Statements are available at www.smith-nephew.com Smith+Nephew Annual Report 2023 49 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Giving friends back their freedom to enjoy the slopes Life Unlimited 50 Smith+Nephew Annual Report 2023 |
| Smith+Nephew Annual Report 2023 51 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Protecting the future Through our Strategy for Growth we are working to strengthen the foundation of our business to serve customers sustainably and simply, to accelerate profitable growth through prioritisation and customer focus, and to transform our business through innovation. Our Strategy for Growth is underpinned by our Capital Allocation Framework, which has as one of its priorities investing in innovation and our ESG agenda. You can read more about our Strategy for Growth on pages 8–11, and our Capital Allocation Framework on page 21. We strive to deliver our ESG strategy in the communities where we live and work through the application of our values: – We demonstrate Care by respecting our global resources and striving to protect the safety and wellbeing of our employees. – We demonstrate Courage by setting ambitious goals to increase our volunteerism, reducing waste and greenhouse gas emissions, and by operating responsibly and sustainably. – We demonstrate Collaboration by working together with our partners who share our commitment and contributing to our communities through individual and team volunteerism. Our ESG strategy supports these value drivers by helping us to address the requirements of our stakeholders, creating a lasting positive difference to our communities, and protecting our environment. Our ESG strategy, inspired by the United Nations’ Sustainable Development Goals (SDGs), takes into account the social, environmental and economic aspects of our business and reflects the fact that sustainability and financial performance are closely linked. As a profit-seeking business, we aim to meet our economic objectives whilst at the same time managing the social and environmental impacts of our business activities. Our ESG strategy focuses on three areas: People, Planet and Products. Our objectives and progress against these are summarised on pages 54–59. Shaping a healthy and sustainable future Our ESG strategy is built on our purpose – Life Unlimited, our Strategy for Growth and our culture of Care, Courage and Collaboration. Our ESG and sustainability programme is intended to drive business value for our stakeholders, while inspiring our employees and empowering our teams to make a positive impact on society and the planet.” Katya Hantel Vice President ESG People Creating a lasting positive impact on our communities Planet Aiming to reduce our impact on the environment Products Innovating sustainably 52 Smith+Nephew Annual Report 2023 |
| Our stakeholders’ priorities Through our ESG strategy we are addressing the needs and expectations of our stakeholders. Customers and suppliers Building ESG principles into the delivery of healthcare is of growing importance to our customers. Increasingly, customers require us to provide details of our ESG strategy and objectives. Customers place increasing importance on these responses when making contract decisions. Our Third Party Guide to Working with Smith+Nephew requires our suppliers to conduct business in a way which fits with the values and ethics of Smith+Nephew and provides our customers with further insight into how we work with suppliers to drive our ESG strategy. Employees Employees are looking for companies with strong values and cultures, that operate with integrity, transparency and accountability, and offer satisfying career opportunities for all. Living our values and being a force for positive change is part of our ESG strategy. Investors Investors are prioritising investments based on corporate ESG programmes and outputs. Our ESG programme provides evidence of our progress in these areas. Governments and regulators ESG regulation is increasing at pace globally. Our ESG strategy and governance focuses on ensuring compliance with existing and emerging regulation on sustainability matters. Our Compliance & Culture Committee reviews, tracks and monitors our compliance and progress towards our ESG objectives aligned with applicable regulations and our Strategy for Growth. Our senior management engage with industry bodies and interest groups such as AdvaMed, MedTech Europe and similar organisations on ESG matters which have the potential to impact our organisation. Environment and communities The communities where we are located want to see support for local education, health and volunteering programmes from businesses which operate there. Our ESG strategy prioritises giving back to local communities, for example through employee volunteering programmes. More information on our ESG activities can be found in our 2023 Sustainability Report, available on our website. www.smith-nephew.com Stakeholders want to understand the impact of our ESG strategy on People, Planet and Products to understand how we are driving and implementing strategy to reduce our impact on the planet and its resources and enabling us to innovate sustainably. ESG governance In January 2023, we streamlined the governance and operational structure around the delivery of our ESG strategy. We established the ESG Operating Committee to implement and execute our ESG strategy across all business areas, reporting directly to the Executive Committee. The Executive Committee will continue to formulate and drive our ESG strategy with oversight from the Board and its Committees. The Board reviews the ESG strategy, key risks and opportunities and progress on a regular basis and three Board Committees review its implementation: Compliance & Culture Committee, Audit Committee and Remuneration Committee. For further information on our governance see the Governance Report from page 88 and our Task Force on Climate-related Financial Disclosures (TCFD) reporting on pages 60–64. Our ESG strategy focuses on three areas: People, Planet and Products. Within these three areas, we have developed comprehensive objectives to help us deliver on our sustainability ambitions. Each year we measure and report progress against these objectives. During 2023, we adjusted several of our objectives to better reflect the challenges we face and to ensure they remain meaningful. We are proud of our many achievements over the years, including our ‘AA’ MSCI ESG Rating and our recurring inclusion in leading indices, such as FTSE4Good, the ESG Index from Institutional Shareholder Services (ISS) and the Dow Jones Sustainability Index. This year, we are reporting both our 2022 and 2023 Scope 3 greenhouse gas (GHG) emissions from 13 categories and are developing our Scope 3 GHG emissions reduction roadmap and strategy for the short to medium term. Climate change During 2023, we have continued to consider the potential impact of climate change on our business operations. Our physical assets and supply chains are vulnerable to weather and climate change, for example through sea-level rise, more frequent extreme weather events and more severe extreme weather events. Patients are vulnerable to a potential rise in infectious disease propagation. Governments and corporations alike are under increasing pressure to mitigate the expected effects of climate change, potentially resulting in infrastructure projects which would require large capital outlays and further increase pressure on healthcare payments. In 2021, we made a commitment to achieve net zero Scope 1 and Scope 2 GHG emissions by 2040 and net zero Scope 3 GHG emissions by 2045, beginning by achieving a 70% reduction in Scope 1 and Scope 2 GHG emissions by 2025. We are on track to achieve a 70% reduction in Scope 1 and Scope 2 GHG emissions by 2025 compared to the 2019 baseline. We aim to minimise the disruption to our manufacturing and distribution network. We understand how important it is to balance environmental initiatives with business activities, and strive to reduce emissions through new technology development, renewable energy use and other measures. Smith+Nephew Annual Report 2023 53 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Shaping a healthy and sustainable future continued People Creating a lasting positive impact on our communities Our objectives Our progress in 2023 Volunteering We are committed to living our culture in our communities by providing 8 hours of paid volunteer time to all employees and enabling at least 50 community/charity events across our sites each year from 2023 to 2030. 95 Events in first year Giving Between 2020 and 2030, donate $125 million in products to underserved communities. $5.1m ($16.2m since 2020) Inclusion Empower and promote the inclusion of all. 4,200+ Supporters across our seven Global Employee Inclusion Groups and sub-groups Our facilities in Memphis (US) and Malaysia sourced renewable electricity for the 2023 calendar year. In the final three months of 2023, we started procuring green energy at all our UK sites. This is set to continue through 2024. Our reporting against the TCFD framework and the Sustainability Accounting Standards Board (SASB) framework for our sector of Medical Equipment and Supplies can be found on pages 60–64 and 258–259 respectively. The Compliance & Culture Committee and the Audit Committee received updates on TCFD and SASB reporting during 2023. As part of our Enterprise Risk Management process, we have a sustainability risk register and a business resilience process review built into our review of our Principal Risks (see pages 69–77). Our Principal Risks capture our physical and transitional climate-related risks in our Enterprise Risk Management process. Climate change is an element of our Global Supply Chain Principal Risk, as increasingly frequent climate events increase the likelihood and impact of disruptions to our supply chain. Delivering on our sustainability ambitions In 2023, we continued to focus on our three priority areas: People, Planet and Products. Within these areas we have refined our objectives so that we can measure and report clearly on our progress. With an emphasis on increasing participation in EIGs and Life Councils, eight hours of paid volunteer time continues to be available to and promoted for uptake by all employees. In addition, in 2023, we focused on site-wide and community engagement activities, enabling us to combine individual efforts and maximise our impact through organised events. We reviewed and refined our packaging objective in light of industry and customer engagement, expanding its scope to include broader themes. In 2024 and beyond, we will continue to review and adapt our strategy and objectives to ensure they are current and that we make meaningful progress. » Read our TCFD reporting on pages 60–64 54 Smith+Nephew Annual Report 2023 |
| People are at the heart of our purpose – Life Unlimited Putting people first will help us to achieve our vision of a world where healthcare professionals are able to help restore health to patients, wherever they are. We prioritise people in three ways: First, we help improve patients’ wellbeing and empower the healthcare professionals who treat them. Second, we engage with the communities where we operate, encouraging our people to volunteer in local communities, offering paid volunteer time and matching employee charitable donations. And third, we support our own employees’ wellbeing by ensuring their work environment is healthy and safe. We also continue to build employee wellness programmes that enable healthy life choices. Our giving activities during the year totalled donations of $5.2 million. These consisted of $5.1 million in product donations and $88,000 from matching employee gifts to qualified charities. Since 2020, our product donation strategies have been held back by the impacts of Covid; however, we are seeing the return of medical missions and continue to support, as needed. Celebrating our partnership with IHP – helping thousands in need For over 20 years Smith+Nephew has partnered with International Health Partners (IHP) to donate products to help treat people in need in over 30 countries worldwide. Most recently, we donated more than 40,000 products from our Advanced Wound Management portfolio to Ukraine, where medical supplies are desperately needed. To celebrate their impact across the world, in 2023 IHP held an event in London for all their valued partners and supporters, which Smith+Nephew attended. The infographic shows some of the places that our support has helped through our partnership with IHP. 95 volunteer events in 2023. $5.1m of product donations in 2023. 2013 Democratic Republic of the Congo, Sierra Leone, Zimbabwe 2014 Gambia, Gaza, Honduras, Philippines, Sri Lanka. Turkey, West Bank 2015 Haiti, Iraq 2016 Afghanistan, Gambia, Haiti, Honduras, Iraq, Jamaica, Nicaragua, Ukraine 2017 Iraq, Nicaragua 2018 El Salvador, Iraq, Sierra Leone 2019 Benin, Myanmar, Nicaragua 2020 Lebanon 2021 Jamaica 2022 Ukraine 2023 Ukraine Employee engagement is important to us and is measured by the Gallup Global Engagement Survey (see pages 48–49). In 2023, we combined the four Employee Inclusion Groups focused on women into one group. » See pages 48–49 Smith+Nephew Annual Report 2023 55 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Planet Aiming to reduce our impact on the environment We recognise the need to protect our planet and help mitigate against the impacts of climate change. In response, we manage resources efficiently, reduce our emissions where possible and are mindful of the impact our decisions have on the environment. In 2023, as the impacts of the global pandemic started to recede, many colleagues chose to adopt remote or hybrid working. Accordingly, some offices continued to see lower occupancy levels and we are adapting to those situations. Our ESG strategy extends upstream to our suppliers and downstream to our customers. This means that we want to work with partners who are making efforts to reduce their own environmental impacts. We are also working to deliver products and services that have less impact on the environment and are taking steps to better understand the extended footprints of our top-selling products. This helps us focus our resources where they will produce the most positive impact. To help achieve improvements in this area, we are collaborating with our key suppliers where there are more opportunities. We are mindful of the importance of biodiversity, particularly in some of the countries in which we operate including Costa Rica and Malaysia. The impact on local biodiversity is one of our considerations when we approve capital expenditure within our Global Operations business. Reducing our GHG emissions Our approach to cutting emissions is three-fold: tackling energy efficiency, generating our own renewable energy on-site and sourcing lower-carbon energy through green tariffs and procuring renewable energy certificates. To achieve this, we are evaluating new ideas and investing in technological solutions at many of our sites. Our aim is to achieve net zero status in line with our objectives. During 2023, we calculated both our 2022 and 2023 Scope 3 GHG emissions for 13 categories, and are beginning to develop a roadmap for reduction. See page 66 for more details. Shaping a healthy and sustainable future continued Our objectives Our progress in 2023 Progress since 2019 baseline Net zero Achieve net zero Scope 1 and Scope 2 GHG emissions by 2040 and Scope 3 GHG emissions by 2045, beginning by achieving a 70% reduction in Scope 1 and Scope 2 GHG emissions by 2025. A carbon reduction roadmap for Scopes 1 and 2 through 2025 has been developed and a roadmap for Scope 3 is being developed. We have calculated our Scope 3 GHG emissions data for 2022 and 2023. Scopes 1 and 2 (total) 40,266 tonnes CO2e emitted (market-based)1 Our manufacturing sites in Malaysia and Suzhou are generating on-site renewable energy. Scopes 1 and 2 (total) 40% reduction CO2e emitted (market-based)1 Emissions have been reduced by undertaking energy efficiency projects, on-site renewables and procurement of renewable energy and purchase of RECs. Scope 3 1.3 million tonnes CO2e emitted in 2023 Scope 3 Now reporting 13 categories, up from 8 in 2021. Waste Achieve zero waste to landfill2 at our manufacturing facilities in Memphis and Malaysia by 2025 and at all our strategic manufacturing facilities by 2030. Our Malaysia facility has achieved zero waste to landfill. 849 tonnes sent to landfill from Memphis manufacturing facilities, representing 38% of total waste from those facilities. 1,411 tonnes sent to landfill from the Group.1 32% reduction Less waste was sent to landfill from Memphis manufacturing facilities during 2023 compared to 2019. 30% reduction Less waste was sent to landfill from all our strategic manufacturing facilities during 2023 compared to 2019. 1 Data and more details are available in the 2023 Sustainability Report on pages 59–60. 2 We define zero waste to landfill as a landfill diversion rate of 90% or greater. 56 Smith+Nephew Annual Report 2023 |
| 2025 Begin by achieving a 70% reduction in Scope 1 and Scope 2 GHG emissions by 2025. 2040 Achieve net zero Scope 1 and Scope 2 GHG emissions by 2040. 2045 Achieve net zero Scope 3 GHG emissions by 2045. Our net zero targets What we have completed in 2023 – Conducted a detailed analysis of our energy usage data. – Actioned our carbon reduction roadmap. – Measured and reported our 2022 and 2023 Scope 3 GHG emissions from 13 categories, an increase from 8 categories reported for 2021. – Sourced renewable electricity for our manufacturing facility in Memphis (US) and started to generate renewable electricity from solar photovoltaic panels in Malaysia and Suzhou (China). What we are currently doing – Preparing a carbon reduction roadmap to reduce Scope 3 GHG emissions. – Sourcing renewable energy opportunities at all our strategic manufacturing sites. – Converting our European and UK leased car fleet to electric vehicles (EVs). – Expanding our supplier engagement through CDP. – Promoting a salary sacrifice scheme in the UK to enable employees to drive EVs. Roadmap to net zero What we expect to do next – Implement renewable electricity at all our strategic manufacturing sites by 2025. – Convert our remaining global leased car fleet to electric vehicles. – Actively engage with our suppliers and encourage them to set their own net zero targets. We encourage all our employees and supply chain partners to take responsibility for minimising their energy use. We make efforts to motivate staff to actively care about the environment, providing them with guidance and access to information to enable them to make a real difference. To identify and reduce our Scope 3 GHG emissions, we are working with our suppliers to identify opportunities and then build our roadmap for emissions reduction. We continued to source renewable wind energy for all our locations in Memphis (US). We also sourced hydroelectric energy for our manufacturing location in Malaysia. These were both achieved through the purchase of renewable energy certificates (RECs). From October 2023, all our UK sites began sourcing renewable energy through the UK Green Tariff. We have installed solar photovoltaic panels at our Suzhou and Penang sites. Both systems began operating in early 2023 and combined the two solar-powered systems reduced our Scope 2 GHG emissions by over 2,000 tonnes of CO2e in 2023. Sourcing renewable energy reduces our market-based GHG emissions, i.e. the emissions from the electricity we purchase. Our roadmap to net zero is outlined below. These are our current actions, which will be updated in the coming years as our plans develop. In accordance with the California Voluntary Carbon Market Disclosures Act (AB1305), detailed information is available on pages 36–39 of the 2023 Sustainability Report. Net zero In line with the Paris Agreement, which aims to hold the increase in the global average temperature to well below 2°C above pre-industrial levels, and pursue efforts to limit the temperature increase to 1.5°C above pre-industrial levels, we are committed to net zero. ‘Net zero’ means that the activities within a company’s value chain result in no net impact on the climate from GHGs. It is in our roadmap to achieve net zero Scope 1 and Scope 2 GHG emissions by 2040 and Scope 3 GHG emissions by 2045, for more details see the table below and page 36 of the 2023 Sustainability Report. We are on track to achieve a 70% reduction in Scope 1 and Scope 2 GHG emissions by 2025 compared to a 2019 baseline. Highlights 3.5 GWh We are now generating renewable energy in China and Malaysia in 2023. 2000 tCO2e GHG emissions prevented from going into the atmosphere by these projects. Smith+Nephew Annual Report 2023 57 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Shaping a healthy and sustainable future continued Products Innovating sustainably We aim to develop products with sustainable attributes, increase access to care, improve our environmental impact and reduce costs. Along with our customers and other stakeholders, we are focused on the environmental footprint of our products and services. Manufacturing and supplying safe and effective products is at the heart of our business. Our people, processes and technology are structured to support progress towards the objective of innovating sustainably. We are applying sustainability attributes to both our new products and their packaging to support delivery of our ESG objectives and those of our customers. We have integrated sustainability as a specific topic in our New Product Development phase review process to drive consideration of sustainability and efficiency in our product design, specifically: 1) material and energy usage during production; 2) reduced product footprint for shipping/ transportation; and 3) recyclability of waste products (e.g. packaging). Our customers are increasingly requesting information on the chemical components and recyclability of our products and packaging. Our focus on products will assist our customers in reaching their sustainability goals. Packaging sustainability to minimise environmental impact, both for new products and our existing portfolio, continues to be a key area of opportunity, as does moving to digital Instructions For Use (IFU). Our 2022 initiative to reduce packaging dimensions for our foam dressings has continued in 2023 and is resulting in reduced volumes of packaging materials being used and lower GHG emissions. By 2025, we aim to have completed a focused risk-based due diligence of our Tier 1 suppliers, including a risk-based analysis of sub-tier suppliers. Supplier risk criteria include country, commodity and spend, and we have updated our global process for managing Corporate Social Responsibility (CSR) supplier risk. In 2023, we completed internal screening due diligence with 100% of our Tier 1 suppliers with additional due diligence with identified potential high-risk Tier 1 suppliers. Our objectives Our progress in 2023 New products Include sustainability review in New Product Development (NPD) for all new products and product acquisitions. Sustainability is now embedded into our NPD phase review process, ensuring that we discuss, consider and implement sustainability when we design new products. Packaging We are committed to reducing our packaging and designing with reusable, recyclable and/or renewable resources which are sustainably sourced. We have refined and updated our packaging objective. We have continued to improve sustainable sourcing, including our ‘regionalisation strategy’ to purchase more packaging materials from local suppliers. We continue to use our electronic Instructions For Use platform, minimising paper instructions issued where possible. Supply chain By 2025, complete a focused risk-based due diligence of our Tier 1 suppliers, including risk-based analysis of sub-tier suppliers, to assure compliance with our sustainability requirements. We have completed due diligence and assessments of all Tier 1 suppliers according to our risk-based procedure. We have continued our supplier on-site audit programme for suppliers identified through risk-based analysis. On-site audits include worker interviews and practical assessment of the implementation of supplier policies and procedures to assure compliance with modern slavery, human trafficking, HSE and sustainability requirements. 58 Smith+Nephew Annual Report 2023 |
| RENASYS◊ EDGE The future starts now The RENASYS◊ EDGE Negative Pressure Wound Therapy System has been designed with healthcare professionals and patients in mind. RENASYS◊ EDGE features years of innovation built into a format that is more easily portable and has a smaller footprint compared to RENASYS◊ TOUCH and RENASYS◊ GO products.1 The size and weight of the device is designed to allow patients to continue with their daily lives and supports patient privacy.2 RENASYS◊ EDGE incorporates an intuitive, user-friendly interface for easy to learn operation and troubleshooting.3 Our step-by-step user interface guidance is aimed at supporting clinicians’ training and increasing their confidence with therapy application.3 RENASYS◊ EDGE offers a short, user-friendly and time-efficient on-board guide for cleaning in between patient use.3,4 The modularised design enables easy and low-cost repair.4 RENASYS◊ EDGE is sturdy, reliable and durable, with the aim of minimising the need for device returns.5 34 kWh energy saved compared to RENASYS◊ TOUCH over its lifetime, enough to charge a mobile phone over 2,800 times.7 3× RENASYS◊ EDGE more energy efficient than RENASYS◊ TOUCH.6 13 kg C02 emissions saved compared to RENASYS◊ TOUCH over its lifetime.8 RENASYS◊ TOUCH See page 264 for references. Smith+Nephew Annual Report 2023 59 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| TCFD reporting Pages 60–64 set out Smith+Nephew’s disclosures which are consistent with the recommendations of the Task Force on Climate-related Financial Disclosures (TCFD) framework. By this we mean the four TCFD recommendations and the 11 recommended disclosures set out in Figure 6 of Section B of the report entitled ‘Implementing the Recommendations of the Task Force on Climate-related Financial Disclosures’ published in October 2021 by the TCFD. Governance The way in which we evaluate, manage and embed sustainability within our business and culture is directly linked to our Strategy for Growth through a focus on People, Planet and Products. Oversight of our ESG strategy is one of the Matters Reserved to the Board. The Board reviews the ESG strategy, key risks and opportunities, and progress on a regular basis and approves the Sustainability Report annually, and reviews and approves the ESG, TCFD and SASB reporting in the Annual Report. Three Board Committees are also closely involved in reviewing the elements of sustainability that impact the key areas of our business. All Committees receive regular updates on ESG strategy, implementation, objectives and targets, and climate-related financial risks and opportunities. The Committee Chairs report to the Board at each Board meeting: – The Compliance & Culture Committee, chaired by Marc Owen, assesses how we implement our ESG strategy in the core areas of People, Planet and Products, encompassing the Group’s impact on employees, the environment, the local communities in which it operates, customers, suppliers and other key stakeholders. The Compliance & Culture Committee also tracks progress of the delivery on ESG objectives and metrics, including a regular review of our net zero emissions progress at each Committee meeting. – The Audit Committee, chaired by Rick Medlock, is responsible for ensuring oversight of the process by which risks relating to the Group and its operations are managed and reported. The Audit Committee assesses the extent to which climate change and other ESG risks are likely to have a material impact upon our financial statements by reviewing the possible impact of different scenarios related to climate change. The Audit Committee also has oversight of the TCFD reporting in the Annual Report. – The Remuneration Committee, chaired by Angie Risley, is responsible for ensuring that the Remuneration Policy and related incentive schemes incorporate ESG targets and metrics where appropriate to do so. Executive Committee: – Driven by the Chief Executive Officer, determination and management of ESG strategy, with the President Global Operations and Vice President ESG accountable for leading on implementation. – Ensures that ESG risks and opportunities are included in decision making as part of each project, initiative and the 12-Point Plan. ESG Operating Committee: – Established in January 2023. – Supports the Executive Committee in the execution and delivery of the ESG strategy. – Membership includes Global Operations, ESG, Global Manufacturing, Research & Development, Global Procurement, Public Policy & Government Affairs, Finance and Human Resources. Board: – Oversight of ESG strategy and risk management programme. Remuneration Committee: – Oversight and review of ESG metrics within Remuneration Policy, and compensation and incentive plans generally. – Approval of ESG percentage and measures within short-term and long-term incentive plans. In 2023, the Committee approved 5% of the Annual Bonus Plan for Executive Directors would be dependent on the achievement of ESG objectives and in 2024, 5% of the Annual Bonus Plan and 10% of the Performance Share Plan for Executive Directors and Executive Officers are dependent on the achievement of ESG objectives. Audit Committee: – Oversight of the risk management process and reviewing its operating effectiveness. – Receives regular updates on ESG and climate-related financial risks and opportunities. – Assesses whether climate change has a material impact on our financial statements. – Ensures the Company reports in line with the recommendations of the TCFD framework. Compliance & Culture Committee: – Oversight of ESG policy and performance versus targets, with reviews undertaken at each committee meeting. – Receives regular updates on ESG and climate-related risks and opportunities, people and culture objectives including IDE and ethics, compliance, quality and regulatory matters. Shaping a healthy and sustainable future continued 60 Smith+Nephew Annual Report 2023 |
| Our Chief Executive Officer sets strategy together with the Executive Committee, and the President Global Operations and the Vice President ESG are responsible for the implementation and report at least quarterly on our progress to the Board, its Committees and our Executive Committee. In January 2023, we streamlined the governance and operational structure around the delivery of our ESG strategy. We established the ESG Operating Committee to implement and execute our ESG strategy across all business areas, reporting directly into the Executive Committee which will continue to formulate and drive our ESG strategy with oversight from the Board and its Committees. Smith+Nephew leaders consider ESG risks and opportunities in their decision making. For example, when evaluating options for our new manufacturing site in Melton, UK, an analysis of ESG requirements and risks is being undertaken as part of the project and decision making. Where appropriate, papers submitted to the Board by management for review include an analysis of ESG issues and opportunities to enable the Board to consider these factors in decision making and to ensure effective Board oversight on ESG strategy, risks and opportunities. Detailed information on our ESG risks can be found in our Sustainability Report. Strategy Our ESG strategy is built on our purpose – Life Unlimited, our Strategy for Growth and our culture of Care, Courage and Collaboration. Our ESG strategy, which was developed by our Sustainability Council in 2019 and approved by the Board, is inspired by the United Nations’ Sustainable Development Goals. Our strategy reflects the importance of social, environmental and economic aspects of sustainable development. Our Principal Risks capture our physical and transitional climate-related risks in our Enterprise Risk Management (ERM) process. Climate-related risk Potential impact Timeframe Actions taken by management Commercial execution Inability to satisfy customers’ sustainability requirements and expectations. Decline in customer demand. Lower prices to remain competitive. Medium (3-7 years) and long term (>7 years) Continued new product launches and monitoring of innovation pipeline. Legal and compliance Failure to identify existing or new legal or regulatory requirements including sanctions programmes and ESG matters which result in non-compliance with applicable laws and regulations. Fines and sanctions. Short (<3 years), medium (3-7 years) and long term (>7 years) The ESG Operating Committee assesses new and enhanced regulations, reporting requirements and works cross-functionally to ensure compliance. Failure to meet the needs of stakeholders relating to increased focus on and regulation of ESG reporting requirements. Decline in customer demand. Medium (3-7 years) and long term (>7 years) Monitoring new regulatory and enforcement trends. Carbon taxes. Increased pricing of GHG emissions. Medium (3-7 years) and long term (>7 years) Net zero targets set for Scope 1, 2 and 3 GHG emissions. New product innovation, design & development including intellectual property Sustainability in new products. Decline in customer demand. Medium (3-7 years) and long term (>7 years) Sustainability criteria built into new product development processes. Pricing and reimbursement Limited ability to pass on the cost of sustainability improvements. Higher input costs. Medium (3-7 years) and long term (>7 years) Optimise portfolio mix and promote differentiated products. Quality and regulatory Failure to meet stakeholder expectations with regard to increasing ESG regulations and reporting requirements. Decline in customer demand. Medium (3-7 years) and long term (>7 years) Monitoring regulatory changes and understand interpretation of legislation. Transition risks Smith+Nephew Annual Report 2023 61 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Scenario modelling Implication and mitigation Potential severity without mitigation Potential severity with mitigation Global temperature rise Based on the Intergovernmental Panel on Climate Change's sixth assessment report, we modelled the following scenarios out to 2030 and 2050: – Low: Limit warming to 2°C (IPCC scenario SSP1-2.6) – Medium: Limit warming to 3°C (IPCC scenario SSP2-4.5) – High: Limit warming to 4°C (IPCC scenario SSP 3-7.0) Extreme heat increases the demand for cooling and can overwhelm power grid infrastructures. Existing defences and business continuity plans are expected to mitigate any near-term impacts and the longer-term impact is being closely monitored by the ESG and operations teams. High Medium Sea-level rise We modelled the following scenarios out to 2030 and 2050: – Sea-level rise up to 5 metres – Distance from nearest coastline Rising sea levels impact manufacturing sites at coastal locations. Existing flood defences and business continuity plans are expected to mitigate any near-term impacts and the longer-term impact on the Group’s manufacturing footprint is an area of focus being considered in our manufacturing strategy. For example, the announced relocation of our Advanced Wound Management facility mitigates against the impact of sea-level rise and accordingly reduces the potential impact. Medium Low Extreme weather We modelled the following extreme weather scenarios out to 2030 and 2050: – Precipitation – Wind – Drought Heavy precipitation events will make flooding more probable, strong winds can damage roofs and compromise the building envelope, and more intense or prolonged droughts can lead to diminishing water resources and potentially more severe wildfires. Existing weather defences and business continuity plans are expected to mitigate any near-term impacts and the longer-term impacts are considered in our manufacturing strategy. Medium Low Physical risks Shaping a healthy and sustainable future continued TCFD reporting continued 62 Smith+Nephew Annual Report 2023 |
| We address climate-related risk primarily through business strategies in our global operations functions including facilities, health and safety, business continuity and global supply chain management. Severe weather patterns as a result of climate change may cause damage to manufacturing or distribution facilities, potentially impacting our ability to meet customer demand over the long term. Refer to the risk management section on page 64 and the Risk report on page 67 for more details on our risk management process. Climate-related opportunities Climate-related opportunities are identified and addressed through our ESG strategy and programmes. Through this process we have identified a number of climate-related opportunities relating to energy sourcing, energy efficiency, on-site renewable energy generation, engagement through the CDP Supply Chain programme and packaging reduction initiatives. In 2020, all our locations in Memphis (US) began sourcing electricity from renewable wind energy via the procurement of renewable energy certificates (RECs) and this has continued through 2023. We completed construction of our Malaysia facility in 2021 and photovoltaic panels started generating renewable energy on-site at the beginning of 2023. Similarly, at our facility in Suzhou (China), solar photovoltaic panels commenced generating on-site renewable energy in early 2023. All UK sites have sourced a green tariff for the supply of electricity from renewable sources from October 2023. In 2021, we aligned with the recommendations of the Intergovernmental Panel on Climate Change and published our commitment to achieve net zero Scope 1 and Scope 2 GHG emissions by 2040 and Scope 3 GHG emissions by 2045, beginning by achieving a 70% reduction in Scope 1 and Scope 2 GHG emissions by 2025. We understand how important it is to balance environmental initiatives with business activities and strive to reduce emissions through new technology. We have conducted a review of our current state and captured related business risks in our risk register. Energy efficiency audits have been carried out at sites in the UK and Germany in 2023 with the recommendations added to improvement action plans. The new UK site at Melton, on the outskirts of Hull, will be designed to high ESG standards with a focus on energy and resource efficiency. The site aims to generate on-site renewable energy. Scenario analysis The scenario analysis undertaken in 2023 was supported by a third party and included more than 30 locations. The modelling focused on the material impacts on our business and was based on our current business activities and assumed no mitigation. As outlined on pages 61–62, our physical and transition risks are captured in our ERM process. Refer to our Risk report on page 67 for further details. Based on the modelling undertaken, the highest potential impact (without mitigation) is in relation to global temperature rise. The potential impact of sea-level rise has decreased from the prior year modelling with the announced plans to build a new Advanced Wound Management facility at Melton, on the outskirts of Hull, which sits at a higher elevation and is further inland than the current facility. The Group closely monitors climate-related physical risks and is taking mitigating measures such that the net impact to the business with these measures in place is not expected to be material. Smith+Nephew Annual Report 2023 63 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Risk management Climate-related risks are managed through our comprehensive risk governance framework. At the top of our structure, the Board sets our risk appetite and monitors the application of our risk framework, including strategy, execution and outputs of risk reviews by the business and the Group Risk team. The Board cascades our risk appetite throughout our organisation through the Executive Committee, the risk owner community and our management group. A formal ‘bottom-up’ exercise ensures that risks are escalated back through the process to our Board and are reflected in our Principal Risks as appropriate. Refer to pages 67–77 for more detail. Climate-related risks We identify climate-related risks based on short-, medium- and long-term horizons. We consider short term to be within one to three years (in line with our annual budget and three-year plan cycles), medium term to be within three to seven years (in line with scenario modelling to 2030 and typical product life cycles) and long term to be greater than seven years. Short-term risks are captured in our financial planning process; medium- and long-term risks are captured within our global footprint planning process. Our annual and three-year financial planning, and our capital expenditure planning processes require climate-related risk information and specific ESG considerations. We maintain a separate sustainability risk register where risk owners consider how ESG and climate risks affect our Principal Risks. These are managed through our ERM process. Detailed information on our ERM process can be found on pages 67–68 of the Annual Report and in our Sustainability Report. Metrics and targets We have published an annual Sustainability Report since 2001 detailing progress against our global objectives. We have objectives in each of our priority areas: People, Planet and Products. Our key climate-related metrics are greenhouse gas emissions and waste to landfill. Our key objectives in relation to these metrics are net zero greenhouse gas emissions by 2045 and zero waste to landfill at our strategic manufacturing facilities by 2030. Detailed information about our objectives and progress made against those objectives can be found on pages 54–59 of the Annual Report and in our Sustainability Report. In 2023, the Remuneration Committee approved 5% of the Annual Bonus Plan for Executive Directors would be dependent on the achievement of ESG objectives linked to our ESG strategy and in 2024, 5% of the Annual Bonus Plan and 10% of the Performance Share Plan for Executive Directors and Executive Officers are dependent on the achievement of ESG objectives. We have mapped our Scope 1 and Scope 2 GHG emissions, and during 2022 we also began to map our Scope 3 GHG emissions in order to meet our objective of reducing total life cycle GHG emissions to net zero by 2045. In 2021, we also established interim carbon reduction objectives for 2025. See our 2023 Sustainability Report for details on our Scope 1 and Scope 2 net zero roadmap. In 2022, we published our 2021 baseline Scope 3 GHG emissions, including data from eight of the 15 categories. In 2023, we reported both our 2022 and 2023 Scope 3 GHG emissions from 13 of the 15 categories. We have carbon reduction roadmaps for our Scope 1 and Scope 2 GHG emissions to show our pathway to meet our objectives. Our Scope 1, 2 and 3 GHG emissions data are provided on page 66 of the Annual Report with more detailed information also available in our Sustainability Report. In 2023, our combined Scope 1 and market-based Scope 2 GHG emissions reduced by 40% compared to our 2019 baseline year. We sent 30% less waste to landfill from our strategic manufacturing facilities compared to 2019. In January 2023, we launched a salary sacrifice scheme to make electric vehicles available to all employees in the UK. With electric vehicle chargers in place at the majority of our UK offices and manufacturing facilities, all employees are being encouraged to commute with more consideration for the environment. This initiative will help to lower our GHG emissions arising from employee commuting. Shaping a healthy and sustainable future continued TCFD reporting continued 64 Smith+Nephew Annual Report 2023 |
| Our focus is on the areas of largest environmental impact, including manufacturing sites, warehouses, R&D sites and offices. Smaller locations representing less than 2% of our overall emissions are not included. Acquisitions completed before 2023 are included in the data, with more recent ones excluded. This is in line with our established policy for the integration of acquired assets. Our GHG emissions reporting represents our core business operations and facilities that fall within the scope of our consolidated financial statements. Primary data from energy suppliers has been used wherever possible. We report our emissions in three scopes: – Scope 1: Direct sources of emissions which mainly comprise the fuels we use on-site, such as gas and heating oil, and fugitive emissions arising mainly from the losses of refrigerant gases. We have included UK vehicle emissions from leased cars since 2020. In 2023, we widened this to include 14 European countries in our leased vehicle reporting. – Scope 2: Indirect sources of emissions such as purchased electricity and steam we use at our sites. – Scope 3: Indirect value chain emissions that arise as a result of activities from assets or processes not owned or controlled by Smith+Nephew; these can be further divided into upstream and downstream emissions and fall into 15 defined categories. During 2023, we have data available for 13 categories. We have also targeted the use of online ‘real-time’ data to monitor energy usage to make savings. We have a programme to replace older inefficient equipment with highly efficient equipment, such as compressors, chillers, pumps, fans and motors. This year we also continued to convert our company car fleet in Europe to electric vehicles where appropriate. In Memphis during 2023, we continued to purchase RECs through Green Flex, a voluntary renewable energy programme. Certified by Green-e Energy, North America’s leading certification programme for renewable energy, Green Flex RECs are based on wind power generated in the Midwest US. Purchasing RECs gives buyers the right to renewable energy and also makes it possible to track ownership of it. Our participation in this scheme underscores our commitment to supporting renewable energy and helps to reduce our market-based carbon emissions footprint. We also sourced hydroelectric energy for our manufacturing location in Malaysia through the purchase of RECs. From October 2023, all our UK sites began sourcing renewable power. Our sites in Suzhou and Penang are now generating electricity on-site using solar PVs. CO2e reporting methodology, materiality and scope We report the carbon footprint of our Scope 1, Scope 2 and Scope 3 GHG emissions in tonnes of CO2 equivalent from our business operations for the year ended 31 December 2023. We are including UK-specific energy and emissions data to satisfy the Streamlined Energy and Carbon Reporting (SECR) requirements. Location-based emissions are calculated in compliance with the WRI/WBCSD GHG Protocol Corporate Accounting and Reporting Standard and have been calculated using carbon conversion factors published by the UK Government Department for Energy Security & Net Zero and the Department for Environment, Food & Rural Affairs (Defra) for 2023. We have applied the emission factors most relevant to the source data, including Defra 2023 (for UK locations), IEA 2021 (for overseas locations) and for the US we have used the most recently available US EPA ‘Emissions & Generation Resource Integrated Database’ (eGRID) for the regions in which we operate. All other emission factors for gas, oil, steam and fugitive emissions are taken from Defra 2023. In line with dual-reporting we also report market-based emissions. These are contractual or supplier-specific emission factors that can be applied when procuring low-carbon energy or siting facilities in areas with lower emissions but also recognising that this might be higher than the grid average in some cases. Where market-based factors were not available, we have used ‘Residual Mix’ data for the EU locations and IEA data for all other countries, except for the remaining US locations where the eGRID factors were applied. Smith+Nephew Annual Report 2023 65 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Shaping a healthy and sustainable future continued CO2e reporting methodology, materiality and scope continued Reporting our Scope 3 emissions During 2023, we have worked with our global energy partner to measure our 2022 and 2023 Scope 3 GHG emissions using a recognised protocol, CEDA (Comprehensive Environmental Data Archive). Our calculation of our 2023 Scope 3 GHG emissions was 1.3 million tonnes of carbon dioxide equivalent from the 13 categories that we measured. Our data quality has improved, through improved analysis and reporting within each emissions category for Scope 3 and by extending the number of categories that we have reported. We also conducted our first global commuting survey. Our Scope 3 GHG emissions assessment was made using the best available 2023 data. As expected, in line with our peer group, purchased goods and services contributes the most significant proportion of our Scope 3 GHG emissions, over 83%. Further details are available in the 2023 Sustainability Report on page 38. In 2024, we intend to prepare an emissions transition plan which will cover all three emission scopes. 2023 2022 2019 (baseline year) UK Global (excluding UK) Total UK Global (excluding UK) Total UK Global (excluding UK) Total CO2e emissions (tonnes) from: Direct emissions (Scope 1)1 5,682 10,219 15,9012 5,563 6,605 12,1683 4,747 5,141 9,8883 Indirect emissions (Scope 2) (location-based) 3,997 55,015 59,0122 3,856 57,961 61,8173 4,911 62,413 67,3243 Total (location-based) 9,679 65,234 74,9132 9,419 64,566 73,9853 9,658 67,554 77,2123 Indirect emissions (Scope 2) (market-based) 3,800 20,565 24,3652 5,205 31,474 36,6793 5,072 52,080 57,1523 Total (market-based) 9,482 30,784 40,2662 10,768 38,079 48,8473 9,819 57,221 67,0403 Energy consumption to calculate Scope 1+2 emissions (GWh)1 48 195 243 49 188 237 45 168 213 Intensity ratio (location-based): CO2e (t) per $m sales revenue 13.6 14.2 15.1 CO2e (t) per full-time employee 3.9 3.9 4.3 Intensity ratio (market-based): CO2e (t) per $m sales revenue 7.3 9.4 13.1 CO2e (t) per full-time employee 2.1 2.6 3.7 Other indirect emissions (Scope 3)4 1,276,079 1,385,356 1 UK-only vehicle data included in Energy and Scope 1 GHG emissions since 2020. A total of 14 European countries were included in 2023 vehicle data. 2 Data and more details are available in the 2023 Sustainability Report on pages 59–60. 3 Data and more details are available in the 2023 Sustainability Report on pages 60–61. 4 Measurement of 2022 and 2023 Scope 3 GHG emissions from the 13 categories measured. Refer to ‘Reporting our Scope 3 emissions’ above for more details. 2023 data includes recent acquisitions completed and new site openings during 2022. Revenue: 2023: $5.5bn; 2022: $5.2bn; 2019: $5.1bn. Average full-time employee data: 2023: 19,081; 2022: 19,094; 2019: 18,030. » www.smith-nephew.com/sustainability 66 Smith+Nephew Annual Report 2023 |
| Risk report Like all businesses, we face risks and uncertainties. Smith+Nephew has developed an enterprise risk management framework, together with supporting policies and procedures, to support risk management and value creation. Our risk management process Successful identification and management of existing and emerging risks is critical to the achievement of strategic objectives and to the long-term success of any business. Risk management is therefore an integral component of our corporate governance. As in previous years, our Enterprise Risk Management (ERM) process is based on a holistic approach to risk management. Our belief is that the strategic and operational benefits of proactively managing risk are achieved when ERM is aligned with the strategic and operational goals of the organisation. Our process and governance structure achieve this. 2023 has seen a further maturing of risk management. Our quarterly Risk Champion workshops focused on topics such as Business Continuity and Business Change, Pricing and Reimbursement, Cybersecurity, and external risk trends and developments. This increased awareness of external and internal risks and management actions across the Group. We enhanced our reporting dashboards to share regular ERM insights with Risk Champions and our executive management. Executive Committee risk owners report and discuss Principal Risk trends from an operational perspective in monthly Executive Committee meetings. We further enhanced the annual top-down Executive Committee risk assessment process to ensure it considers a wide range of external and internal themes, trends and benchmarking information. The Group Risk Team also enhanced their quality checks to improve alignment between top-down and bottom-up processes. Emerging risks Executive Committee risk owners continue to scan the horizon for new and emerging risks and these are discussed and considered in the top-down risk discussion. Emerging risks that were identified this year include: – Our customers, investors and other internal and external stakeholders are increasingly focused on our approach to Environmental, Social and Governance (ESG) matters and how we embed ESG considerations into all areas of our business. In 2023, we have responded to this increased interest by enhancing our ESG governance structure, ensuring that ESG considerations are taken into account in decision-making processes and are reflected within each of our Principal Risks as appropriate. – Advances in Artificial Intelligence (AI), machine learning, robotics, and other technologies create opportunities for the Group when used within a clear governance framework. These technologies can help us to innovate to meet unmet patient needs and earn and retain market share through improved productivity and customer service. The use of AI technology should be implemented with clear guidance on usage and risk management in order to mitigate the risk of employees or third parties inadvertently disclosing proprietary information or confidential or sensitive data. As many AI tools are limited by the information within the data sets that they are trained on, human oversight is required in order to manage risk and avoid outputs that are inherently biased or untrue. The Group has an internal policy that defines the governance and controls required to ensure the use of AI is appropriate, transparent, and properly implemented and monitored. 1. Risk identification 2. Gross (inherent) risk assessment 3. Current control identification 4. Net (residual) risk assessment 5. Risk response planning 6. Risk reporting 7. Monitoring and review Our risk management process Smith+Nephew Annual Report 2023 67 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Risk report continued 2024 Risk Management Plan Our work will continue to evolve in 2024 with a particular focus on strengthening cross-functional risk management in alignment with the 12-Point Plan. This will include deep-dive sessions into specific risks with cross-functional teams. We will work with the new head of sustainability to further develop the risk register in this area. The Group Risk team will also continue to influence decision making through effective challenge to risk owners and Risk Champions in the quarterly review process. Our risk governance framework At the very top of our structure is our Board with responsibility for oversight of risk management, setting our risk appetite and monitoring the application of our risk framework including strategy, execution, and outputs of risk reviews by the business and Group Risk team. The Board cascades our risk appetite throughout our organisation through the Executive Committee, risk owner community and our management Group. A formal ‘bottom-up’ risk management exercise ensures that risks are escalated back through the process to our Board and are reflected in our Principal Risks as appropriate. Providing guidance and rigour across this process is our Executive Committee and the Group Risk team. At the third line of defence is our Internal Audit function, providing an annual opinion on the effectiveness of our Risk Management process to the Executive Committee, chaired by the Chief Executive Officer, and then to the Board and its Committees. Business Area Risk Champions – Carry out day-to-day risk management activities. – Identify and assess risk. – Implement strategy and mitigating actions to treat risk within Business Area. – Lead regular risk register updates. Executive Committee – Identifies and ensures the management of risks that would prevent the Company from achieving our strategic objectives. – Appoints Business Area Risk Champions who are accountable for applying the Enterprise Risk Management Policy and Framework to produce the risk deliverables. – Reviews external/ internal environment for emerging risks. – Reviews risk register updates from Business Area Risk Champions. – Identifies significant risks and assesses effectiveness of mitigating actions. Board of Directors and Board Committees – The Board is responsible for oversight of risk management, for our annual strategic risk review and for determining the risk appetite the organisation is willing to take in achieving its strategic objectives. – The Board monitors risks through Board processes (Strategy Review, Disclosures, M&A, Investments, Disposals) and Committees (Audit and Compliance & Culture). – The Audit Committee is responsible for ensuring oversight of the process by which risks relating to the Company and its operations are managed and for reviewing the operating effectiveness of the Group’s Risk Management process. Group Risk Team – Manages all aspects of the Group’s approach to Enterprise Risk Management including design and implementation of processes, tools, and systems to identify, assess, measure, manage, monitor, and report risks. – Facilitates implementation and co-ordination through Business Area Risk Champions. – Provides resources and training to support process. – Reports regularly on risk to the Executive Committee. – Prepares Board and Group Risk Committee reports. Internal Audit – Provides independent assurance to the Board and Audit Committee on the effectiveness of the Group’s Risk Management process. – Provides annual assessment of effectiveness of Enterprise Risk Management. Group Risk Team Internal Audit Board of Directors and Board Committees Executive Committee Business Area Risk management life cycle Annual improvement and refinement of our risk management process ensures that it remains aligned with strategy and operations. Our Risk Management Policy, sponsored by our Chief Executive Officer, is driven by an Enterprise Risk Management Manual and the Group Risk team providing training to Business Area Risk Champions. As in prior years, risks continue to be managed through a ‘top-down’ and ‘bottom-up’ process, with regular oversight from the Executive Committee and quarterly reports to the Board Committees. An overview of our risk management life cycle is illustrated below. 68 Smith+Nephew Annual Report 2023 |
| Increased risk Reduced risk No change Our Strategy for Growth 1Strengthen the foundation to serve customers sustainably and simply 2 Accelerate profitable growth through prioritisation and customer focus 3 Transform our business through innovation and acquisition » See pages 8–11 for further information on our Strategy for Growth A Audit Committee N Nomination & Governance Committee R Remuneration Committee C Compliance & Culture Committee B Board Compliance and Reputation – Legal and Compliance. – Quality and Regulatory. External – Political and Economic. Financial – Foreign Exchange. – Pricing and Reimbursement. Operational – Cybersecurity. – Global Supply Chain. – Mergers and Acquisitions. – New Product Innovation, Design & Development including Intellectual Property. – Strategy and Commercial Execution. People – Talent Management. 2023 Principal Risks We assess our Principal Risks in terms of their potential impact on our ability to deliver our business strategy. We have grouped our Principal Risks into five categories: Compliance and Reputation, External, Financial, Operational and People. The Principal Risks are presented in alphabetical order according to their grouping below. Risk grouping Risk change from 2022 Risk oversight Risk key 1 2 3 Smith+Nephew Annual Report 2023 69 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Quality and regulatory Examples of risks – Transition to EU MDR impacts ability to meet customer demand. – Time required by Notified Bodies to review product submissions and site quality systems’ certification time for new products impacts ability to meet customer demand. – Defects in design or manufacturing of products supplied to, and sold by, the Group could lead to product recalls or product removal or result in loss of life or major injury. – Significant non-compliance with policy, regulations or standards governing products and operations regarding registration, design, manufacturing, distribution, sales or marketing. – Failure to obtain proper approvals for products or processes. – Stringent local requirements for clinical data across various markets globally. – Failure to meet stakeholder expectations with regard to increasing sustainability regulations and reporting requirements. Actions taken by management – The Quality departments within each Business Unit regularly monitor activities to comply with new requirements, including EU MDR. – Regular engagement with Notified Bodies, MHRA and regulatory representatives to monitor regulatory changes and understand interpretation of legislation. – Comprehensive and documented product quality processes and controls from design to customer distribution in place, with the addition of cybersecurity to new product development projects for relevant products. – Standardised monitoring and compliance with quality management practices through our Global Quality and Regulatory Affairs organisation. – Incident management teams in place to provide a timely response in the event of an incident relating to patient safety. – Governance framework in place for reporting, investigating and responding to instances of product safety and complaints. – Local clinical evidence requirements are included in global new product development projects. Global regulatory bodies continue to increase their expectations of manufacturers and distributors of medical devices not only in respect of quality and regulation of products but also in respect of sustainability requirements. Our products are used in the human body and therefore patient safety is of paramount importance. The European Medical Device Regulation (EU MDR), and multiple other global regulations and changes in standards have increased the focus on clinical and technical evidence, supplier controls and product performance transparency. Our customers and other stakeholders also require us to explain our approach to and demonstrate compliance with increasing sustainability regulations and reporting requirements. Legal and compliance Examples of risks – Failure to act in an ethical manner consistent with our Code of Conduct and Business Principles. – Violation of anti-corruption or healthcare laws, breach by employee or third-party representative. – Misuse or loss of personal information of patients, employees, research subjects, consumers or customers results in violations of data privacy laws, including General Data Protection Regulations. – The development, manufacture and sale of medical devices entail risk of product liability claims or recalls. – Failure to identify changes in or new legal or regulatory requirements including sanctions programmes and ESG matters which result in non-compliance with applicable laws and regulations. – Failure to meet needs of stakeholders relating to increased focus on and regulation of ESG reporting requirements. Actions taken by management – Board Compliance & Culture Committee oversees our ethical and compliance practices. – Global compliance programme, policies and procedures in place and regularly updated. – Annually all employees required to undertake training and certify compliance with our Code of Conduct and Business Principles. – Group monitoring and auditing programmes in place. – Launched enhanced confidential independent reporting channels for employees and third parties to report concerns. – Trade compliance programme, policies and procedures. – Appointed a new head of ESG – The ESG Operating Committee assesses new and enhanced regulations and reporting requirements and works cross-functionally to ensure compliance. – Monitoring new regulatory and enforcement trends. We are committed to doing business with integrity and believe that ‘doing the right thing’ is part of our mandate to operate. We operate in multiple countries and regulatory authorities in each jurisdiction enforce an increasingly complex pattern of laws and regulations that govern the design, development, approval, manufacture, labelling, marketing, sale and operation of both traditional and digital healthcare products and services. Operating across this complex and dynamic legal and compliance environment, which includes regulations on bribery, corruption, privacy, sustainability and trade compliance, increases the risk of fines, penalties, and reputational damage. We mitigate this through policies, procedures, training and practices designed to prevent and detect violations of law, regulations and industry codes. We conduct risk-based oversight to monitor compliance with our Code of Conduct and associated policies. Oversight Link to Strategy 1. Strengthen 2. Accelerate Change from 2022 3. Transform Oversight Link to Strategy 1. Strengthen 2. Accelerate Change from 2022 Risk report continued 2023 Principal Risks continued Compliance and reputation risks C C 1 2 3 1 2 3 70 Smith+Nephew Annual Report 2023 |
| Political and economic Examples of risks – Global or regional recession and increasing macroeconomic controls impact on customer financial strength. – Global political and economic uncertainty and conflict, including in Ukraine and the Middle East. – Failure to meet the sustainability targets and public policy changes. – Failure to pivot on business strategy in light of increased sanction programmes globally. – Market access rights. – Increases in import and labour costs. – Increases in tariffs and restrictions on global trade. – Inflationary pressures impacting raw materials, freight, salaries and wages. – Potential for significant tax rate changes and/or base broadening measures in key jurisdictions where we operate including OECD proposals and US tax reform. – Failure to comply with current tax laws. – Transfer pricing policy not correctly implemented or monitored. – Changing legislation in the US and other key markets may require changes to our operating model. Actions taken by management – Built sustainability strategy on our purpose, business strategy, and culture pillars, and tracked and benchmarked targets within the industry. – Our ESG Operating Committee implements and operationalises ESG strategy and provides data and metrics to monitor implementation. – Continued engagement with governments, administrations, and regulatory bodies to enhance education and advocacy efforts with policymakers. – Global trade compliance programme, policies and procedures. – Business continuity plans developed with alternative source options identified for critical suppliers and increased safety inventory levels for critical products affected by the conflict in Ukraine and disruptions of travel through the Red Sea and Suez Canal. – Actively participate in trade associations to enhance education and advocacy efforts with policymakers. – Ongoing engagement and monitoring/ lobbying on localisation initiatives. – The Group Tax team continually monitors developments in tax legislation and obtain external advice where relevant. – The Group Tax team, supported by external advisers, works closely with the business to implement agreed processes and procedures. – Seeking appropriate independent third-party advice when required. We operate a global business and are exposed to the effects of political and economic risks, changes in the regulatory and competitive landscape, trade policies and trade compliance requirements, war, political upheaval, changes in government policy regarding healthcare priorities and sustainability expectations, increasing inflationary pressure and tax rates, preference for local suppliers, import quotas, economic sanctions and terrorist activities. Oversight Link to Strategy 1. Strengthen 2. Accelerate Change from 2022 External risks B 1 2 3 Smith+Nephew Annual Report 2023 71 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Pricing and reimbursement Examples of risks – Reduced reimbursement levels and increasing pricing pressures. – Systemic challenge on number of elective procedures. – Lack of compelling health economics data to support reimbursement requests. – Unilateral price controls/reductions imposed on medical devices. – Price-driven tendering/ procurement processes. – Volume-based procurement in China and other markets. – Limited access to non-clinical decision makers. – Limited ability to pass on increased costs such as raw materials, freight, sustainability improvements and the cost of compliance with regulations to our customers. Actions taken by management – Our 12-Point Plan includes an initiative which focuses on pricing strategy and execution in order to mitigate some of the impact of inflation. – Developed innovative economic product and service solutions for both Established and Emerging Markets. – Incorporated health economic components into the design and development of new products. – Sales training to improve capability to communicate the clinical and economic value proposition to non-clinical decision makers. – Implementing innovative contracting models designed to lessen the risk of adoption and coverage for healthcare providers and payers. – Increased engagement with payer bodies to influence reimbursement mechanisms to reward innovation. – Optimise portfolio mix and promote differentiated products. – Consideration of price increases. Our success depends on our ability to sell our products profitably, despite increasing inflation and costs associated with improving the sustainability of our products, pricing pressures from customers and the availability of and access to adequate government funding and reimbursement to meet increasing demands for our products arising from patient demographic trends. The prices we charge are therefore impacted by budgetary constraints and our ability to persuade customers and governments of the economic value of our products, based on clinical data, cost, patient outcomes and comparative effectiveness. Market developments such as China volume-based procurement, consolidation of customers into buying groups, inflation, increasing professionalisation of procurement departments and the commoditisation of entire product groups, continue to challenge prices. We mitigate this through price increases to counteract the impact of inflation where possible, portfolio mix and promotion of differentiated products, including a compelling clinical and economic value proposition. Foreign exchange Examples of risks – Risk of adverse trading margins due to fluctuating foreign currency exchange rates between our main manufacturing operations (the US, UK, Costa Rica, Malaysia and China) and where our products are sold. Actions taken by management – A foreign exchange hedging programme is operated and is overseen centrally by the Group Treasury team. – The Finance and Banking Committee monitors ongoing treasury matters including foreign exchange exposure. We operate a global business and are therefore exposed to exchange rate volatility. Volatility in foreign currency exchange rates can impact our results and it may not be possible to fully mitigate against them. Oversight Link to Strategy 1. Strengthen Change from 2022 Oversight Link to Strategy 1. Strengthen 2. Accelerate Change from 2022 Risk report continued 2023 Principal Risks continued Financial risks A B 1 2 3 1 2 3 72 Smith+Nephew Annual Report 2023 |
| Cybersecurity Examples of risks – Loss of confidential or sensitive information, intellectual property and/or data privacy breach. – Inadequate consideration of cybersecurity in the design of new products, systems and/or processes. – Disruption to business operations due to a significant cybersecurity incident. – Increased government focus on cybersecurity and changes in regulatory environment. – Increasing demand for cybersecurity expertise could impact our ability to attract and retain cybersecurity talent. – Disruption to the business due to critical system infrastructure and applications being unavailable. Actions taken by management – Ensured every user has access to and is using a secure Virtual Private Network (VPN) when connecting to Smith+Nephew networks to safeguard remote working. – Continued security awareness activities including email communications, intranet posts, visuals, videos and more email phishing training activities. – Multi-factor authentication tools reduce the likelihood of remote attacks. – Security information and event management (SIEM) in place to provide real-time analysis of security alerts generated by applications and network hardware. – Regular penetration testing and frequent vulnerability scanning undertaken. – Endpoint protection and intrusion detection/prevention implemented. – Security governance structure in place including a Security & Privacy Steering Committee. – Monitor developments from governments and raise changes and developments with Global IT Security. – Cybersecurity Maturity Programme monitored by the Audit Committee. – IT disaster recovery policy in place. We depend on a wide variety of information systems, programmes and technology to run our business effectively. We also develop and sell certain digitally enabled products that connect to proprietary and third-party networks and/or the internet. Our systems and the systems of the entities we acquire may be vulnerable to a cyber-attack, theft of intellectual property, malicious intrusion, data privacy breaches or other significant disruption. We have a layered security approach in place to prevent, detect and respond, to minimise the risk and disruption of any intrusions and to monitor our systems on an ongoing basis for current or potential threats. Oversight Link to Strategy 1. Strengthen 3. Transform Change from 2022 Operational risks A 1 2 3 Smith+Nephew Annual Report 2023 73 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Risk report continued 2023 Principal Risks continued Global supply chain Examples of risks – Disruption to manufacturing at a single source facility (lack of manufacturing redundancy), including from natural disaster. – Manufacturing and supply chain capacity not adequate to support growth. – Constrained supplier sterilisation capacity due to increased regulation and enforcement. – Risks associated with the transition of warehouse and distribution activities to external supplier impacting inbound and outbound logistics. – Supplier failure impacts ability to meet customer demand (single source supplier). – Inadequate sales and operational planning impacts ability to meet customer demand for product. – Excess inventory due to incorrect demand forecasts, inaccurate demand signals and unexpected changes in demand. – Failure of suppliers and distribution partners to achieve and maintain regulatory compliance. – Increasing costs of raw materials and freight. – Increasing salary and wage costs for manufacturing and distribution employees and contractors. – Severe weather patterns, global temperature rise and sea-level rise caused by climate change or natural disaster causes damage to manufacturing or distribution facilities, impacting ability to meet customer demand. – Disruption to the business due to critical system infrastructure and applications being unavailable. – Critical material shortages leading to supply challenges. – Increased freight cycle times due to conflict in the Middle East, resulting in disruptions of travel through the Red Sea and Suez Canal. – Labour attrition and delays in backfilling. – Failure to transform to achieve our sustainability targets. Actions taken by management – Our 12-Point Plan includes initiatives to improve product availability and inventory, enhance procurement and management of transportation costs, focus on lean manufacturing and quality and optimise our manufacturing network. – Delivering Global Operations transformation programme to optimise manufacturing and distribution centres and reduce single source limitations. – Global Operations project management governance and toolkits to support successful execution of transformation programmes. – Risk-based review programmes undertaken for critical suppliers. – Business continuity plans developed with alternative source options identified for critical suppliers and increased safety inventory levels for critical products. – Implemented an enhanced Sales Inventory and Operations (SI&OP) process to improve demand and supply planning. – Executive oversight of sales and operational planning. – Increased co-ordination between commercial, supply chain and logistics to improve forecast accuracy. – Comprehensive product quality processes in place from design to customer supply. – Supplier contract agreements achieve and manage regulatory compliance. – Initiatives to improve manufacturing efficiency and reduce overhead costs. – IT disaster recovery policy in place. – Leadership taskforce established to resolve cumulative impact of global supply chain events. – Global, regional, and local crisis management governance in place. – Emergency and incident management and business recovery plans in place at major facilities and for key products and key suppliers. – Appointed a new head of ESG. – An ESG Operating Committee implements and operationalises ESG strategy and provides data and metrics to monitor implementation. – Investment in flood defences at our operations in Hull and building of a new R&D and manufacturing facility for Advanced Wound Management in Melton West. Our ability to make, distribute and sell medical products to customers in over 100 countries involves complex manufacturing and supply chain processes. Increased outsourcing, sophisticated materials, and the speed of technological change in an already complex manufacturing process leads to greater potential for disruption in our supply chain. Lack of availability of raw materials and components compound supply and business disruption. Capacity constraints and the regulatory environment, including the increased focus on global regulation of sustainability, increase our exposure to supply chain disturbance. Increasingly frequent climate events increase the likelihood and impact of disruptions to our supply chain. Increased inflationary pressure on production, freight and warehousing and distribution costs increases our risk of failing to achieve accelerated profitable growth. Our business depends on our ability to plan for and be resilient in the face of events that threaten one or more of our key locations. Damage caused by environmental and climate change factors, including natural disasters and severe weather, can and do threaten our critical sites. Oversight Link to Strategy 1. Strengthen 2. Accelerate Change from 2022 Operational risks continued B 1 2 3 74 Smith+Nephew Annual Report 2023 |
| New product innovation, design & development including intellectual property Examples of risks – Failure to develop, partner or acquire a competitively differentiated innovation. – Insufficient long-term planning to respond to competitor and disruptive entries into the marketplace. – Inadequate innovation due to low Research & Development (R&D) investment, R&D skills gap or ineffective product development execution. – Loss of market share due to critical gaps in product portfolio not filled. – Loss of proprietary data due to natural disasters or failure of Product Lifecycle Management (PLM) systems. – Competitors may assert patents or other intellectual property rights against the Group or fail to respect the Group’s intellectual property rights. – Failure to ensure sustainability in new products. Actions taken by management – Our 12-Point Plan includes an initiative to reposition our knee and hip portfolio. – Continued product and technology acquisitions and product launches and effective implementation of new product launches. – Global R&D organisation and governance framework providing strategic direction for allocation of R&D investment across all businesses. Clear stage-gate process to continually evaluate R&D investment decisions and development of new products. – Cross-functional New Product Design and R&D processes focused on identifying new products and potentially disruptive technologies and solutions. – Replacing global Product Lifecycle Management systems. – Monitored external market trends and collated customer insights to develop product strategies. – Ongoing monitoring of competitor patent portfolios post product launch. – Ongoing intellectual property training for business counterparts. – Sustainability criteria built into new product development processes. Our product innovation pipeline is becoming broader in scope and increasingly complex, as we focus our efforts on procedure innovation using digital technologies such as connectivity, machine learning, and artificial intelligence. Our focus on high growth and profitable markets requires us to better understand unmet customer needs, drivers of surgical efficiency and patient outcomes, and new country/ regional regulations including requirements related to cybersecurity and sustainability. Our innovation pipeline needs to be sufficiently differentiated from our competition in order for us to deliver our commercial ambition. If Smith+Nephew fails to protect and enforce its intellectual property rights successfully, its competitive position could suffer, which could impact profitable, sustainable growth. Mergers and acquisitions Examples of risks – Failure to identify appropriate acquisitions. – Failure to conduct effective acquisition due diligence. – Failure to integrate newly acquired businesses effectively, including integration with Group standards, policies and financial controls. – Failure to deliver on plans to achieve the acquisition business case. Actions taken by management – Acquisition activity aligned with corporate strategy and prioritised towards products, business units and markets identified to have the greatest long-term potential. – Clearly defined investment appraisal process based on range of valuation metrics including return on invested capital, in accordance with Capital Allocation Framework and comprehensive post-acquisition review programme. – Detailed and comprehensive cross-functional due diligence undertaken prior to acquisitions by experienced internal and external experts (including the integration management office). – Compliance and other risks included as part of due diligence reviews, integration plans and reporting for acquisitions. – Integration committee review, approval of integration plans and monitoring of ongoing process. – Board has annual post-deal review session. As the Group grows to meet the needs of our customers and patients, we recognise that we are not able to develop all the products and services required using internal resources and therefore need to undertake mergers and acquisitions in order to expand our offering and to complement our existing business. In other areas, we may divest businesses or products which are no longer core to our activities. It is crucial for our long-term success that we make the right choices around acquisitions and divestments. Failure to identify appropriate acquisition targets, to conduct adequate due diligence or to integrate them successfully or to deliver on the acquisition business case would have an adverse impact on our competitive position and profitability. Oversight Link to Strategy 3. Transform Change from 2022 Oversight Link to Strategy 3. Transform Change from 2022 B B 1 2 3 1 2 3 Smith+Nephew Annual Report 2023 75 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Risk report continued 2023 Principal Risks continued Strategy and commercial execution Examples of risks – Failure to execute our strategy adequately from high-level ambition to specific actions to make the ambition a reality. – Multiple change initiatives, including those within our 12-Point Plan could distract management from delivering business-as-usual objectives. – Inability to keep pace with significant product innovation and technical advances to develop commercially viable products. – Failure to engage effectively with our key stakeholders to meet their evolving needs leading to loss of customers. – Failure to manage distributors effectively leading to stocking and compliance issues. – Inability to satisfy customers’ sustainability requirements and expectations. – Limited healthcare professional access to medical education. – Failure to achieve potential from acquisitions due to integration challenges. – Failure to effectively implement core elements of business change prevents our projects and programmes achieving the intended benefits and disrupts existing business activities. Actions taken by management – Dedicated Acceleration Office and Executive Steering Committee led by our CEO to monitor the successful delivery of the 12-Point Plan. – Changed our commercial operating model from a franchise and regions model to business unit model. – Executive oversight of changes to our commercial operating model. – Strategic planning process clearly linked to business and Group risk. – Continued new product launches and monitoring of innovation pipeline. – Implemented an enhanced Sales Inventory and Operations (SI&OP) process to improve demand and supply planning. – Enhanced accessible digital sales information and training modules for sales staff. – Enhanced Virtual Medical Education platforms and opening of the Smith+Nephew Academies in Singapore and Munich. – Integration committee to review/ approve integration plans and monitor ongoing processes. – Our 12-Point Plan includes an initiative to reposition our knee and hip portfolio. – Continued product and technology acquisitions and product launches and effective implementation of new product launches. – Project management governance, toolkits and project steering committee oversight to support successful execution of programme and projects. The long-term success of our business depends on setting the right strategic priorities such as the 12-Point Plan and our three-year strategic plan and executing on our plans to deliver priority initiatives in highly competitive markets. This requires effective communication and engagement both internally on a cross-functional basis (for example, in order to drive procedure-based selling models) and with our customers, suppliers and other stakeholders. We must also successfully embed the right governance structures, accountability and capabilities across the Group and ensure we adjust and refine strategic priorities and business models when necessary. The pace and scope of our business change initiatives may increase execution risk for the change programmes as well as for our business-as-usual activities. Failure to set and execute on priorities and drive cross-functional accountability within our business will impact our ability to continue to grow our business profitably and sustainably and to serve our customers. Oversight Link to Strategy 1. Strengthen 2. Accelerate Change from 2022 3. Transform Operational risks continued B 1 2 3 76 Smith+Nephew Annual Report 2023 |
| Talent management Examples of risks – Loss of key talent, high attrition and lack of appropriate succession planning in context of required skillsets for future business needs. – In the event that the Company’s remuneration strategies, quantum and structure particularly in terms of long term incentives for US executives are not adequately addressed to better align to local market norms, the Company may not be able to effectively compete for, attract and retain talent, which may impact management stability, internal talent pipeline development and the ability for management to drive value creation. – Loss of competitive advantage due to an inability to attract and retain top talent. – Loss of intellectual capital due to poor retention of talent. – Failure to attract talented and capable candidates. – Increased talent movement globally due to shifting personal work-life balance priorities. – Increased salaries globally. Actions taken by management – Our 2024 Remuneration Policy proposes a package of long-term incentive plan adjustments for US executives to be more closely aligned with norms in the US in terms of structure and quantum. Our draft Remuneration Policy and a comprehensive discussion of our proposals is set out on pages 126–135 of our Remuneration Report. – Talent planning and people development processes well established across the Group. – Talent and succession planning discussed annually by the Board and regularly by the Executive Committee and Nomination & Governance Committee. – Identification of high-value roles and ensuring that these roles are filled with our high-performance individuals with strong succession plans in place. – Developed strategic skills resourcing plan by functional areas. – Provided employees with access to tools and resources to manage their emotional, physical, and mental wellness. – Enhanced Inclusion, Diversity and Equity (IDE) policy, including establishment of Employee Inclusion Groups (EIGs) and IDE Inclusion Council in order to foster culture of belonging within the organisation and promote engagement, attraction and retention of top talent. – Ongoing segmentation of specific job roles and applying focused rewards to ensure we are competitive and attractive to candidates. In the current market, recruitment and retention of top talent and minimising attrition is a critical risk which requires a strong engagement process. We recognise that people leadership, effective succession planning and the ability to engage, retain and attract talent is a key lever of success for our business. Failure to do so places our ability to execute the Group strategy and to be effective in the chosen market/discipline at risk. People Oversight B Link to Strategy 1. Strengthen 2. Accelerate Change from 2022 1 2 3 Smith+Nephew Annual Report 2023 77 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Risk report continued Our Viability Statement How we assess our prospects During the year, the Board has carried out a robust assessment of the Principal Risks affecting the Company, particularly those which could threaten the business model. These risks, and the actions being taken to manage or mitigate them, are explained in detail on pages 67–77 of this Annual Report. In reaching our Viability Statement conclusion, we have undertaken the following process: – The Audit Committee reviewed the Risk Management process at their meetings in February, April, July and December, receiving presentations from the Group Risk team, explaining the processes followed by management in identifying and managing risk throughout the business. – In July and September 2023, the Executive Committee met to review the 2023 Principal Risks (the top-down risk review process). The Executive Committee was asked to consider the significant risks which they believed could seriously impact the profitability and prospects of the Group and the Principal Risks that would threaten its business model, future performance, solvency or liquidity. – All Executive Committee members nominated the Risk Champions and have worked with them to prepare risk registers. The Risk Champions nominated by the Executive Committee are senior employees and in risk management. – Using the outputs from the Business Area ‘bottom-up’ risk identification completed in September 2023 and following ‘top-down’ discussions with the Executive Committee, the most significant risks affecting our organisation were presented to the Executive Committee for approval in November as the draft 2023 Principal Risks facing the Company and again in January 2024 as final disclosures. – The Executive Committee decided to streamline the 12 Principal Risks from 2022 into 11 Principal Risks with amendments to the descriptions within each Principal Risk to reflect the macro and internal factors to be taken into account in 2023. – In assessing our TCFD risks we concluded that climate-related risks are not significant in our viability horizon of three years. Nonetheless, the impact of extreme weather events have been considered in our operational risk scenarios. – All relevant executives have attested alignment to the Group’s Enterprise Risk Management Process as part of the annual certification on governance, risk, and compliance. – The Board debated and agreed the risk appetite for each of the Principal Risks in February 2023. – Final Principal Risks were presented to the Audit Committee and the Board in February 2023 for their consideration and approval. – Throughout the year, a number of reviews into different risks were conducted by the Board, the Audit Committee and the Compliance & Culture Committee looking into the nature of the risks and how they were mitigated. Assessment period The Board have determined that the three-year period to December 2026 is an appropriate period over which to provide its Viability Statement. This period is aligned to the Group’s Strategic Planning process and reflects the Board’s best estimate of the future viability of the business. Scenario testing To test the viability of the Company, we have undertaken a robust scenario assessment of the Principal Risks, which could threaten the viability or existence of the Group. These have been modelled as follows: – In carrying out scenario modelling of the Principal Risks on the following page we have also evaluated the impact of a severe but plausible combination of these risks occurring over the three-year period. We have considered and discussed a report setting out the terms of our current financing arrangements and potential capacity for additional financing should this be required in the event of one of the scenarios modelled occurring. – We are satisfied that we have robust mitigating actions in place as detailed on pages 67–77 of this Annual Report. We recognise, however, that the long-term viability of the Group could also be impacted by other, as yet unforeseen, risks or that the mitigating actions we have put in place could turn out to be less effective than intended. Viability Statement Having assessed the Principal Risks, the Board has determined that we have a reasonable expectation that the Group will be able to continue in operation and meet its liabilities as they fall due over a period of three years from 1 January 2024. In our long-term planning we consider horizons of between five and 10 years. However, as most of our efforts are focused on the coming three years, we have chosen this period when considering our viability. Our conclusion is based on the Strategic Plan reviewed and approved by the Board in December 2023. We will continue to evaluate any additional risks which might impact the business model. By order of the Board, on 26 February 2024. Helen Barraclough Company Secretary 78 Smith+Nephew Annual Report 2023 |
| 2023 Scenarios modelled Scenario 1: Global economic downturn Significant global economic recession, leading to sustained lower healthcare spending across both public and private systems. Action taken: We have modelled 10% lower revenue throughout 2024 and 5% lower revenue throughout 2025. Reduced reimbursement levels and increasing pricing pressures. Action taken: We have modelled annual price erosion of 1% impacting all product lines, along with a full drop through impact on profit in each of the periods 2024–2026. Link to strategy – Accelerate profitable growth through prioritisation and customer focus. Link to Principal Risks – Global supply chain. – Strategy and commercial execution. – Political and economic. – Pricing and reimbursement. Scenario 2: Operational risk Inability to keep pace with significant product, innovation, and technical advances to develop commercially viable products, losing significant market share to the competition. Action taken: We have modelled 1% lower growth than planned for a key product range in the US, along with a full drop through impact on profit in each of the periods 2024–2026. Disruption to a Global Distribution Centre (GDC) preventing our ability to supply our customers with all products from the applicable GDC for one quarter. Action taken: We have modelled an inability to supply products from one of our GDCs for one quarter of 2025. Key supplier disruption – resulting in our inability to manufacture and supply a few key products for a full year. Action taken: We have modelled an interruption to receiving goods from a key supplier for a period of one year in 2025. Increases in raw materials, freight and labour costs. Action taken: We have modelled an increase in our input costs by an additional 5% in each of the periods 2024–2026, due to continued inflationary pressures. Product liability claim. Action taken: We have modelled a group of product liability claims resulting in a settlement agreement requiring cash payment in each of the periods 2024–2026, without any insurance coverage. Link to strategy – Strengthen the foundation to serve customers sustainably and simply. – Transform our business through innovation and acquisition. Link to Principal Risks – Strategy and commercial execution. – New product innovation, design & development including intellectual property. – Global supply chain. – Legal and compliance. – Political and economic. – Talent management. Scenario 3: Foreign exchange, legal, regulatory and compliance risks Data privacy failure – giving rise to a significant fine or loss. Action taken: We have modelled a one-off significant fine from regulator of 2% of revenue or loss resulting from a data privacy issue in 2025. Failure to obtain proper regulatory approvals for products or processes impacting our ability to sell products. Action taken: We have modelled the complete loss of revenue from a key product effective in mid-2024 for two years, and returning to lower volumes in mid-2026. Risk of adverse trading margins due to fluctuating foreign currency exchange rates across our markets. Action taken: We have modelled a reduction in profitability in 2025 and 2026 due to a weakening in other currencies relative to the US Dollar by 5%. Link to strategy – Strengthen the foundation to serve customers sustainably and simply. Link to Principal Risks – Legal and compliance. – Quality and regulatory. – Foreign exchange. Scenario 4: Cybersecurity Disruption to business operations due to a significant cybersecurity incident. Action taken: We have modelled one of our key regions being unable to invoice also affecting shipping and tracking of deliveries for one month due to a disruption to our IT infrastructure in 2024. Link to strategy – Strengthen the foundation to serve customers sustainably and simply. Link to Principal Risks – Cybersecurity. Scenario 5: Mergers and acquisitions Failure to integrate newly acquired business effectively to achieve expected growth. Action taken: We have modelled a scenario of zero growth in a recently acquired business in 2024. Link to strategy – Transform our business through innovation and acquisition. Link to Principal Risks – Mergers and acquisitions. Smith+Nephew Annual Report 2023 79 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| 80 Smith+Nephew Annual Report 2023 |
| Enabling those moments that bring balance together Life Unlimited Smith+Nephew Annual Report 2023 81 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Our stakeholders Section 172 statement We are a leading portfolio medical technology company and Our Purpose is Life Unlimited – we exist to restore people’s bodies and their self-belief. » See pages 13–17 for more on Life Unlimited We live our Purpose through our culture pillars of Care, Courage and Collaboration to use technology to take the limits off living, and help other medical professionals do the same. » See pages 46–49 for more on our culture Our ambition is to transform into a structurally higher growth company through our Strategy for Growth: Strengthen the foundations in commercial and manufacturing to enable us to serve customers sustainably and simply, and deliver the best from our core portfolio. Accelerate our growth profitably through robust prioritisation of resources and investment, and with continuing customer focus. Transform ourselves for higher long-term growth, through investment in innovation and acquisitions. » Read more in the Chief Executive Officer’s Review pages 8–11, and the Governance report on the Board activities on pages 100–101. In accordance with section 172 of the Companies Act 2006 and the UK Corporate Governance Code 2018, the Board considers the potential impact on the Company’s key stakeholders and takes their views and interests into account when making decisions. The pages referenced in each of the sections below form part of this statement and provide examples of our approach to stakeholder engagement and how the Board considers their views and the impact of decisions on key stakeholder groups. The Board is committed to taking a long-term view in order to deliver sustainable value creation for shareholders and other stakeholders. The Board understands the importance of ensuring that the views and interests of all stakeholders are considered in the delivery and oversight of the Company’s strategy and culture. Although members of the Board engage directly with stakeholders as part of site visits or employee engagement meetings, engagement with stakeholders mostly takes place at an operational level and the Board forms its views through reports and information presented to it by management. Management are asked to outline and present the potential impacts on stakeholders to the Board where appropriate during the review, discussion and decision making process. Employees Our Employees are crucial to the success of our business. Creating a culture of belonging and an environment that fosters innovation, delivers business success and strengthens engagement and development is core to everything we do. » See page 84 82 Smith+Nephew Annual Report 2023 |
| Investors Our Investors are the owners of our business. The Board seeks to engage and understand their perspectives on performance, value, risk and governance. Our Investor presentations are available to download on our website: www.smith-nephew.com. » See page 85 Customers and suppliers Healthcare Professionals and patients are at the centre of everything we do. Working in partnership with our suppliers ensures we have the right resources to support our growth and that those who partner with us are committed to doing business in a way which is consistent with our values. » See page 87 Governments and regulators We focus on product safety, compliance and doing business the right way in order to achieve the full potential of our portfolio. We engage through industry bodies and similar organisations with focus on key issues impacting our organisation and the MedTech industry more broadly. » See page 86 Environment and communities People, Planet and Products are at the heart of our ESG strategy aiming to create a positive impact on our communities, reducing the impact on our environment and enabling us to innovate sustainably. » See page 86 Our Stakeholders Smith+Nephew Annual Report 2023 83 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Focus for 2024 – Management have taken actions to continue to focus on communicating on innovation, linking strategy and commitments, talent development and ESG/IDE strategic initiatives in global webcasts, leadership team meetings and through internal communications and the Company intranet S+N Life. – Board members will follow up on the outcomes and impact of the actions taken to address the 2023 employee feedback from Board listening sessions. – The Chair and Non-Executive Directors will be provided with additional opportunities to visit certain key Company sites as part of their induction. – The 2024 Board Listening Sessions have been amplified to include additional Non-Executive Directors to enable the Board to hear from a broader group of employees. The 2024 sessions will focus on several key topics including the new Global Business Unit model, new leaders, remuneration, 12-Point Plan initiatives within Operations and the ways in which corporate functions enable success. – Induction and refresher sessions will continue to be developed and delivered programmatically to Non-Executive Directors depending on their areas of interest. – Continue to report on and review workforce equity initiatives and programmes to support the wider workforce. – Two full board sessions planned for 2024 focusing on talent, retention and succession planning for senior leaders. » See pages 46–49 for People » See pages 111–113 for Compliance & Culture Committee Engaging with our stakeholders The Board has oversight of the initiatives being undertaken to strengthen, accelerate and transform the Company in line with our Strategy for Growth, driving the creation of value whilst ensuring we are doing business in the right way. Engagement with our stakeholders, including employees, investors, customers and suppliers, governments and regulators and our local communities provides valuable feedback and insight for the Board into what matters to stakeholders most, and helps to foster greater understanding of the impact of decisions on each of our key stakeholder groups. In matters brought to the Board for discussion and approval, the Board considers the likely consequences and impact on stakeholders in the longer term, and carefully considers their interests as part of the decision-making process in the interests of the Company as a whole. Employees 2023 Outcome/impact – The Board received valuable feedback from the 2023 listening sessions with employees wanting to hear more from management on innovation, the link from our strategy to our Commitments and culture, talent development and ESG and IDE initiatives. – The site visit to Costa Rica provided an opportunity for direct engagement with EIGs and leadership teams in Operations and GBS functions which enable the Board to measure and monitor the Culture of the organisation. – Feedback from the Chair and new Non-Executive Directors has been positive on the breadth and depth of the induction programme. Please see page 107 for our Q&A with Jez Maiden. – The review programmes relating to compensation adjustment for markets materially impacted by high inflation have continued and have been reported to the Remuneration Committee and CCC. – Board listening sessions, site visits and succession planning reviews have provided confidence for the Board that the Company is focusing its efforts on developing a strong internal talent pipeline and visibility on succession planning for high value roles within the Company. Areas of interest – Purpose, Strategy and Culture. – Leadership and succession planning. – Talent, retention and development. – Employee wellbeing and cost of living. – Inclusion, Diversity and Equity (IDE). How we engage – Direct engagement through Board/employee listening sessions. A number of Non-Executive Directors lead these sessions in order to understand more about the Company culture, employee engagement and IDE. – Meeting with employees informally during visits to our sites and during our Board meetings. – Board Inductions enable the Board to hear directly from employees on Purpose, Strategy and Culture. – The Remuneration Committee and the Compliance & Culture Committee (CCC) receive updates at each meeting on the activities of our Employee Inclusion Groups (EIGs), and initiatives relating to IDE, wellbeing and community together with the initiatives relating to ongoing review of programmes to support the wider workforce. – The Board and its Committees are provided with updates on leadership and talent development and succession planning for senior executives. Engaging with our stakeholders 84 Smith+Nephew Annual Report 2023 |
| Focus for 2024 – Continued focus and support from the Board on strategy, performance and operational excellence to support delivery of value creation for investors. – Continued engagement by the Chair and Board on remuneration matters in order to ensure that the Company remains competitive in key markets to attract and retain talent. – Continue to seek opportunities for direct Board engagement with Investors on key topics of investor focus. – Continued engagement by the Chair and Board with Investors on key matters throughout the year which feed through to the Board agenda. » See pages 248–256 for Shareholder information Investors Areas of interest – Strategy and Performance. – Capital Allocation and Dividend. – Leadership and Succession planning. – Remuneration. – Sustainability and ESG. How we engage – Chair engagement with investors, groups and teams covering strategy and operational excellence, remuneration, succession planning and ESG matters. – Senior Independent Director engages with investors and governance teams on topics of investor interest including Board composition, diversity and ESG matters. – Our Chief Executive Officer and our Chief Financial Officer engage regularly with investors as part of an ongoing dialogue throughout the year. – US Executive Director Remuneration Consultation. – Meet the Management Day enabled direct engagement with investors for members of the Board. – The Board receives analyst reports, reviews the share register and receives reports on investor meetings and investor perceptions of the Company through external advisor sessions. 2023 Outcome/impact – The Chair and management received consistent feedback from investors to reiterate investor perspectives and expectations on performance improvement, value creation and cost management. The Board and senior management have addressed the feedback provided to the extent possible through investor communications and other engagement. – The Meet the Management event was attended by Non-Executive Directors either virtually or in person, which provided further context and insight into investor areas of focus such as operational excellence, cost management, improvement in inventory and cash and talent retention, which in turn shape Board agendas and discussions. – Engagement on US Executive Director Remuneration proposals shaped the proposed 2024 Remuneration Policy (see pages 126–135). I have had the opportunity to meet some of our larger investors who have been generous with their time and speak from many years’ experience of both the sector and Smith + Nephew. I also had the opportunity to meet some of our smaller investors at the AGM in April 2023 which was a pleasure to attend and a reminder that ultimately, in all we do, there are savers and pensioners who rely on us to grow the value of their investments.” Rupert Soames Chair Smith+Nephew Annual Report 2023 85 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Focus for 2024 – The Board and the CCC will continue to receive reports on product safety, compliance with applicable laws and regulations and will receive updates on Company interactions with governments and regulators as appropriate. – The Chief Executive Officer and senior leadership will continue to engage and participate in discussions with industry bodies in order to advocate for and amplify issues which are of importance to the organisation and the MedTech industry more broadly. » See page 33 for Quality & Regulatory Governments and regulators Areas of interest – Product safety. – Compliance with applicable legal and regulatory requirements. – Promotion of fair competition. – Social and economic concerns. How we engage – Updates are provided on our Global Compliance programme with applicable metrics and monitoring at each CCC meeting. – The Board and CCC receive updates on product quality and regulatory matters and compliance with applicable laws and regulations. – The Chief Executive Officer and other senior leaders engage through industry bodies such as AdvaMed, Medtech Europe and similar organisations in order to advocate for and provide perspectives on core issues which are of critical importance to the MedTech industry. – The Chief Executive Officer and senior management meet with governments and regulators, as applicable. 2023 Outcome/impact – The Chief Executive Officer and other senior leaders participated in a number of industry meetings and interest groups in order to drive issues of critical importance to both the organisation and the MedTech industry. – The CCC received reports on product and regulatory audits which provide comfort and confidence that product safety is being managed and maintained effectively. – The CCC and Board also receive updates from the Group General Counsel under legal privilege relating to any material legal matters of which the Board should be aware. Focus for 2024 – Board site visits and induction programmes will continue to include further information and on key areas of focus within ESG for the Company. – The Board will continue to engage with EIGs as appropriate on site visits and report on Board listening sessions. – The CCC will continue to track progress against key objectives and metrics in terms of transition plan and other objectives. – The Audit Committee will continue to review key disclosures and reporting obligations around sustainability risks. – The Remuneration Committee will continue to review key elements of ESG metrics for remuneration purposes. – Board members and the Company Secretary will continue to attend industry discussions on ESG matters of importance to the Company. » See pages 111–113 for Compliance & Culture Committee Environment and communities Areas of interest – Investment and innovation in local communities. – Understanding how the Company’s business impacts local communities and global business. How we engage – The Board approves the ESG Strategy annually and receives updates on core ESG initiatives at each meeting as appropriate. – Updates on performance and progress on key environment and social metrics are provided at each CCC meeting. – Updates on reporting and disclosures are included at each Audit Committee meeting. – Remuneration Committee determines ESG metrics for remuneration purposes liaising closely with the CCC to ensure that metrics are quantifiable and measurable. – The Chair, Chief Executive Officer and Company Secretary attend industry roundtable and panel discussions on ESG matters which impact the Company. 2023 Outcome/impact – At the Costa Rica site visit, the Board received detailed presentations on the environmental and community initiatives being undertaken by our employees locally and had direct engagement with members of our EIGs. – Proposed Remuneration Policy for 2024 includes ESG metrics for both short-term and long-term incentive plans. Engaging with our stakeholders continued 86 Smith+Nephew Annual Report 2023 |
| Focus for 2024 – Continued focus at Board level on innovation pipeline and product portfolio to meet customer requirements. – Continued focus from the Board and its Committees on risk management and ensuring product quality, compliance with applicable laws and regulations and doing business the right way. – The Board will monitor ongoing network transformation initiatives and supply chain focus on excellence and productivity. – The Board and CCC will continue to focus on customer requirements in the area of People, Planet and Products. – See also Sustainability Report for further details on how we plan to continue to consider areas of importance to our customers. » See pages 111–113 for Compliance & Culture Committee Report Customers and suppliers 2023 Outcome/impact – Please see pages 26–29 on innovation highlighting initiatives designed to support unmet customer needs. – The Board and CCC received regular reports on quality audits as part of ongoing monitoring. – The CCC continues to monitor company response to new regulations impacting our products, quality and regulatory matters and FDA and other regulatory engagement and reports to the Board at each Board meeting. – Monitoring of supply chain and procurement matters is reviewed regularly by the Board with a focus on outcomes of the 12-Point Plan initiatives and metrics. – Please also see pages 9 and 45–47 of our Sustainability Report which highlight our customer and supplier focus. – Board review of our Sustainability Strategy ensures a clear link to stakeholders and issues of importance to customers. – The Board and CCC receive reports at each meeting on sustainability matters which take into account the views and requirements of our customers and in turn how we engage with our suppliers to reflect customer approach. Areas of interest – Innovation and improved outcomes. – Ensuring product quality, compliant with regulations and doing business the right way. – Partnering with suppliers to ensure business is done the right way. – ESG. How we engage – The Board reviews the portfolio strategy throughout the year, together with acquisition pipeline for key assets to accelerate innovation and respond to customer and patient unmet needs. – The Board and CCC are provided with updates on product quality, regulatory matters and complaints and legal, compliance and ethical matters. – We continue to work with our suppliers and explain our expectations in our Third-Party Guide to our Code of Conduct. – Our customers continue to focus on ensuring that ESG and sustainability are taken into account in our decision making aligned with their own policies and procedures. Further information about our relationship with other stakeholders, including the local communities in which we operate and the impact of climate change on our business, can be found in the Sustainability Report on pages 9 and 33. The Strategic Report comprising pages IFC–81 was approved by the Board on 26 February 2024. Deepak Nath, PhD Chief Executive Officer Smith+Nephew Annual Report 2023 87 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION |
| Governance at a glance 1. Board leadership and Company purpose The Board’s primary focus is the long-term success of the Company. It ensures the right resources and culture are in place to deliver on its objectives, and is responsible for effective engagement with stakeholders. 2. Division of responsibilities The Board ensures a diverse balance of Executive and Non-Executive Directors with clear definition of their respective roles and responsibilities. Statement of Compliance The Board is committed to the highest standards of corporate governance. We comply with the provisions and principles of the UK Corporate Governance Code 2018 (2018 Code). The Company’s American Depositary Shares and bonds are listed on the New York Stock Exchange (NYSE) and we are therefore subject to the rules of the NYSE as well as to US securities laws and the rules of the Securities and Exchange Commission (SEC) applicable to foreign private issuers. We comply with the requirements of the NYSE and SEC and have no significant differences to report between the US and UK corporate governance standards. We explain in this ‘Governance’ section how we comply with and have applied the 2018 Code during the year. The 2018 Code can be found at www.frc.org.uk/getattachment/88bd8c45-50ea-4841-95b0-d2f4f48069a2/2018-UK-Corporate-Governance-Code-FINAL.pdf. We also explain how we have complied with the Financial Conduct Authority’s (FCA) Listing Rules and Disclosure & Transparency Rules (DTRs) throughout the year. In a world in which stakeholders have different, and sometimes conflicting, views on how, and to what end, companies should be run, Boards have to be resolute in discharging their responsibilities in the best interests of the Company as a whole.” Rupert Soames, OBE Chair »See pages 96–99 Board meeting attendance »See pages 84–87 and 90–95 Key Activities – Purpose and culture – Strategy and innovation – Operations and commercial excellence – Stakeholders – Risk and internal controls Board Audit Nomination & Governance Compliance & Culture Remuneration 94% 100% 88% 95% 96% This section provides an overview of our corporate governance structure, our policies and practices, as well as the key activities undertaken by the Board and its Committees to ensure effective leadership and the implementation of strong corporate governance at Smith+Nephew. 88 Smith+Nephew Annual Report 2023 |
| 3. Composition, succession and evaluation The Board maintains an appropriate balance of skills, experience and knowledge to ensure that it can discharge its responsibilities. The evaluation of Board performance and succession plans are crucial to ensure that the Board operates effectively. 5. Remuneration The Remuneration Committee designs policies and practices to support strategy and stability thereby promoting the long-term sustainable success of the Company. In doing so, it ensures these align with the remuneration and related policies across the Group’s workforce. 4. Audit, risk and internal control and Compliance and Culture The Audit Committee’s assurance that the financial reporting is fair, balanced and understandable is important for stakeholders to determine the Company’s performance. The Compliance & Culture Committee assists the Board in monitoring ethics and compliance, quality and regulatory, culture and sustainability matters across the Group. Board ethnicity Board tenure »See pages 121–154 »See pages 111–120 »See pages 102–110 Deepak Nath $4,658,252 Anne-Françoise Nesmes $2,059,946 Total remuneration 33.33% female 66.67% male Board gender diversity White British or other White (including minority-white groups) 9 Asian/Asian British 2 Not specified/prefer not to say 1 0-2 yrs 5 3-5 yrs 4 6+ yrs 3 Deepak Nath Anne-Françoise Nesmes Single figure remuneration Deepak Nath Anne-Françoise Nesmes Salary $1,512,726 $785,673 Pension & Benefits $65,000 $109,735 Bonus $1,997,124 $1,010,184 LTI Nil $154,354 Forfeited Incentives $1,083,402 Nil Employee engagement CEO Financial International Healthcare/Medical Devices Emerging Markets Cybersecurity ESG UK Governance Remuneration 67% 58% 42% 92% 58% 67% 58% 67% 58% 58% Board experience STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 89 |
| Rupert Soames Chair Appointed as an Independent Non-Executive Director in April 2023 and as Chair in September 2023 Board leadership and Company purpose Board of Directors Key skills and competencies: Rupert has extensive global leadership experience, a proven track record of delivering shareholder value and a deep understanding of UK corporate governance. Current external appointments: – Chair of the Confederation of British Industry. Previous experience: Rupert stepped down in December 2022 after nine years as Group Chief Executive from Serco Group plc, the specialist services business in Health, Defence, Transport and Immigration. Previously, he was Chief Executive Officer of Aggreko plc for 11 years and prior to that Chief Executive of Misys plc’s Banking and Securities Division. Rupert was Senior Independent Director and a member of the Audit, Remuneration and Nomination Committees for both DS Smith and Electrocomponents plc (now RS Group). Nationality: British Anne-Françoise Nesmes Chief Financial Officer Appointed Chief Financial Officer in July 2020 and stepping down from the Board in Q1 2024 Key skills and competencies: Anne-Françoise has worked as a senior finance executive in global FTSE listed companies for many years, which alongside a strong business acumen and deep sector knowledge provides her with the experience required to be part of the Smith+Nephew leadership team. She demonstrates a high competency for delivering operational excellence across different geographic markets and leading large teams who are responsible for significant budgets. She has an impressive and diverse background and her ability to translate financial insights into results helps guide Smith+Nephew. Current external appointments: – Senior Independent Director and Chair of the Audit Committee at Compass Group plc. Previous experience: Anne-Françoise joined GlaxoSmithKline plc in 1997 where she worked for 16 years, holding multiple senior finance roles including Senior Vice President, Global Vaccines. Anne-Françoise served as Chief Financial Officer for Dechra Pharmaceuticals plc in 2013 where she successfully implemented financial strategies to support the growth of the business. She was Chief Financial Officer of Merlin Entertainments Limited (formerly Merlin Entertainments plc) from 2016 to 2020, leaving after successfully completing the transaction to take the company private. Nationality: British/French Key skills and competencies: Marc is a proven leader with an astute strategic vision, capable of building significant international healthcare businesses. He has strong commercial healthcare expertise. Marc is responsible for ESG through his role as Chair of the CCC. Current external appointments: None. Previous experience: Marc commenced his healthcare and technology career at McKinsey & Company where he progressed to senior partner and eventually a founding partner of McKinsey’s Business Technology Office. In 2001, Marc joined McKesson Corporation and served as Executive Vice President and member of their Executive Committee. He delivered strategic objectives and led over 40 acquisitions and divestments over a 10-year period. In late 2011, he headed McKesson Speciality Health, which operates over 130 cancer centres across the US and provides market intelligence, supply chain services, patient access to therapy, provider and patient engagement and clinical trial support. In 2014, he was appointed Chair of the European Management Board at Celesio AG. He retired in March 2017 once he had improved operations, set the strategy and recruited his successor. Nationality: British/American Marc Owen Senior Independent Director Appointed Independent Non-Executive Director in October 2017 and Senior Independent Director in September 2022 N R Committee key Committee Chair Member of the Audit Committee Member of the Remuneration Committee Member of the Nomination & Governance Committee Member of the Compliance & Culture Committee C A R N C Deepak Nath Chief Executive Officer Appointed Chief Executive Officer in April 2022 Key skills and competencies: Deepak brings global leadership and risk-management expertise and has a track record of driving growth at major healthcare companies through delivering a significant improvement in execution and building a strong results-focused culture. Current external appointments: None. Previous experience: He began his career as a scientist in computational physics at Lawrence Livermore National Laboratory and holds a BSc and MSc in Mechanical Engineering and a PhD in Theoretical Mechanics from the University of California, Berkeley. Prior to joining Siemens Healthineers, he held roles at both Amgen and McKinsey and spent 10 years at Abbott Laboratories, Inc. culminating in his appointment as President of Abbott Vascular. At Siemens Healthineers (2018–2022) he was President of the Diagnostics business responsible for $6 billion of revenue and 15,000 employees. Nationality: American A C N 90 Smith+Nephew Annual Report 2023 |
| Erik Engstrom Independent Non-Executive Director Appointed Independent Non-Executive Director in January 2015. Stepped down from the Board on 31 December 2023 Key skills and competencies: Erik has successfully reshaped RELX Group’s business in terms of portfolio and geographies. He brings a deep understanding of how technology can be used to transform a business and insight into the development of new commercial models that deliver attractive economics. His experience as a Chief Executive Officer of a global company gives him valuable insights as a member of our Audit and Nomination & Governance Committees. Current external appointments: – Chief Executive Officer of RELX Group. Previous experience: Erik commenced his career at McKinsey & Company and then worked in publishing, latterly as President and Chief Operating Officer of Random House Inc. and as President and Chief Executive Officer of Bantam Doubleday Dell, North America. In 2001, he moved on to be a partner at General Atlantic Partners, a private equity investment firm. Between 2004 and 2009, he was Chief Executive Officer of Elsevier, the division specialising in scientific and medical information and then from 2009 Chief Executive Officer of RELX Group, the division specialising in scientific and medical information and then from 2009 Chief Executive Officer of RELX Group. Nationality: Swedish Jo Hallas Independent Non-Executive Director Appointed Independent Non-Executive Director in February 2022 Key skills and competencies: Jo has extensive international experience focused on business transformation through both organic and acquisitive growth in global industrial and consumer sectors. She brings valuable expertise which will help Smith+Nephew build upon and achieve our strategic ambitions. Current external appointments: None. Previous experience: Jo commenced her career at Procter & Gamble based in Germany, the US, Thailand and the Netherlands. She then joined Bosch where she held a business unit leadership role in their Power Tools division followed by Invensys in 2009 where she ran their global heating controls business unit including launching its first smart home offer. She then moved to Spectris plc, where she had responsibility for a portfolio of global industrial technology businesses, as well as for the Group’s digital strategy. From April 2019 to April 2023, Jo served as Chief Executive Officer for Tyman plc where she made sustainability a core foundation of the group’s strategy. Jo was also previously Chair of the Remuneration Committee for Norcros plc. Nationality: British John Ma Independent Non-Executive Director Appointed Independent Non-Executive Director in February 2021 Key skills and competencies: John has an impressive track record in medical device businesses and his contribution provides value as Smith+Nephew continues to develop innovative ways to grow and serve our markets with a focus towards Asia Pacific regions. He is an established healthcare leader and has strong experience of driving market entry and growth within emerging markets. Current external appointments: – Founder, Chair and Chief Executive of Ronovo Surgical. Previous experience: In 2000, John joined GE Healthcare and became Vice President and General Manager of their Global Product Company in China. John has also held a number of senior positions as President of Asia Pacific regions at Pentair Inc., Vice President of Express Scripts Inc., and Global Partner of Fosun Group. He initially joined Fosun Pharma to lead their medical device business and in 2014 became President of Fosun Healthcare Holdings. He served as a key member of their healthcare investment committee which went on to establish a global presence across the US, Europe, Israel and China. In 2017, John joined Intuitive Surgical as their Senior Vice President of Strategic Growth Initiatives. He has previously served as a NED for both Haier Electronics Group and Clinical Innovations LLC. Nationality: American Katarzyna Mazur-Hofsaess Independent Non-Executive Director Appointed Independent Non-Executive Director in November 2020 Key skills and competencies: Katarzyna demonstrates a true passion for customer focus and maintains an impressive track record in senior leadership within the MedTech industry. She is a qualified medical doctor (PhD) and has a wealth of experience in medical devices and orthopaedic sectors. Her Chief Executive Officer experience of a global company and valuable industry knowledge will help drive innovation and ensure the continued development of Smith+Nephew. Current external appointments: – Chief Executive Officer, Care Enablement (MedTech segment), at Fresenius Medical Care AG and a member of the Management Board. Previous experience: Katarzyna commenced her corporate career at Roche in Poland, was later recruited by Abbott Laboratories to manage their diabetes care division in Poland and became Country General Manager. Her career progressed to General Manager of Molecular Diagnostics Division for EMEA and eventually to Divisional Vice President Abbott Diagnostics for Europe. In 2010, she became President EMEA region at Zimmer, following the Biomet acquisition and led the integration in the region and served as President EMEA for Zimmer Biomet, leading orthopaedic company. In 2018, she joined Fresenius Medical Care, the renal company, as CEO EMEA and Member of the Management Board. Effective January 2022, Katarzyna took over responsibility for the globally operating Care Enablement segment in which Fresenius Medical Care AG has consolidated its €5.5 billion healthcare products business into one MedTech organization. Her responsibility includes research and development, quality and regulatory, manufacturing, supply chain and commercial operations. Nationality: German/Polish A N A C C STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 91 |
| A R Board leadership and Company purpose continued Board of Directors continued Angie Risley Independent Non-Executive Director Appointed Independent Non-Executive Director in September 2017 Bob White Independent Non-Executive Director Appointed Independent Non-Executive Director in May 2020 Key skills and competencies: Angie has gained experience in a wide range of sectors, including a regulated environment. This diversity of experience is welcomed by the Board and the Remuneration Committee. Angie is also an additional resource and sounding board for Smith+Nephew’s own internal Human Resources function. Current external appointments: – Non-Executive Director and Chair of the Remuneration Committee at InterContinental Hotels Group plc. Previous experience: From 2007 to 2013 Angie was the Group HR Director for Lloyds Banking Group and was Group HR Director of Sainsbury plc and a member of their Operating Board from January 2013 to May 2023. Over the years, Angie has been a member of the Low Pay Commission and has held a number of Non-Executive Directorships with Biffa plc, Arriva and Serco Group plc. At Serco Group plc she was the Chair of the Remuneration Committee. Previously she has attended Remuneration Committees of Whitbread plc and Lloyds Bank. Nationality: British Key skills and competencies: Bob is an experienced leader with more than 25 years’ worth of industry relevant experience. He is an influential and well-known figure in the medical technology sector and has an impressive track record in delivering growth and fostering innovation. He brings valuable global medical technology insight to the Board, which will prove fundamental in helping to shape and develop the future strategic direction of Smith+Nephew healthcare expertise. Current external appointments: None Previous experience: Bob has held a number of senior Vice President positions throughout his career, most recently as Executive Vice President and President at Medtronic plc. He was also senior Vice President at Chemdex Corporation, Accelrys Inc., SourceOne Healthcare Technologies, Inc., GE Healthcare and Covidien as President for Emerging Markets and President for Respiratory and Monitoring Solutions. He then became Senior Vice President and President of Medtronic Asia Pacific, having led the integration of Covidien Asia Pacific when it was acquired by Medtronic plc in 2015. Nationality: American C R Rick Medlock Independent Non-Executive Director Appointed Independent Non-Executive Director in April 2020. Stepping down as Chair of the Audit Committee on 1 March 2024 and will not submit for re-election at the AGM Jez Maiden Independent Non-Executive Director Appointed Independent Non-Executive Director in September 2023 and as a member of the Audit and Remuneration Committee. Will become Chair of the Audit Committee from 1 March 2024 Key skills and competencies: Rick has extensive experience and a deep understanding of technology focused R&D businesses. He has driven value and transformation throughout his executive career which will further reinforce the ability of Smith+Nephew to grow and develop into new and existing markets. Rick brings significant financial and risk management expertise as a well-regarded former FTSE 100 Chief Financial Officer, NED and Audit Committee Chair. Current external appointments: – NED and member of the Audit, Risk and Compliance Committee at Datatec Ltd. – NED and Chair of the Audit Committee at Deliveroo. – NED and Chair of the Board at British Engineering Services Limited. – NED and Chair of the Board at Alaska TopCo Limited, the parent company of Nomentia Oy (the software cash and treasury solutions provider). Previous experience: Rick has had a highly successful career as a strong commercial Chief Financial Officer in the technology industry, working for a range of international FTSE 100 and NASDAQ listed businesses during periods of high growth. He has held a number of Chief Financial Officer positions throughout his career, including at NDS Group plc, Inmarsat plc and Worldpay Group plc. Rick brings a wealth of experience as a former NED and Audit Committee Chair of several technology driven businesses, such as Sophos Group plc, Edwards Vacuum, and Thus plc. Rick was also previously Chair of BluJay Solutions Ltd, Chair of Momondo Group and Chair of the Audit Committee for LoveFilm UK Limited. Nationality: British Key skills and competencies: Jez has extensive financial experience across a diverse range of industries and sectors. Jez brings more than 15 years of global experience both as a FTSE Chief Financial Officer and as a Non-Executive Director on boards of companies addressing strategic and operational challenges across a number of different industries, including life-sciences and healthcare. He has had oversight of large operations in the US, Europe and Asia in highly regulated industries. Current external appointments: – Senior Independent Director, Travis Perkins plc. – Non-Executive Director and member of the Audit Committee at Intertek Group plc. Previous experience: Jez retired in 2023 as Group Finance Director at Croda International plc, the FTSE 100 global speciality chemicals company, and previously held similar roles at National Express Group plc and Northern Foods Limited. He has served as the Senior Independent Director at Synthomer PLC and at both PZ Cussons plc and Synthomer PLC he chaired the Audit Committee and served on the Remuneration Committee. He is a fellow of the Chartered Institute of Management Accountants. Nationality: British A C R N 92 Smith+Nephew Annual Report 2023 |
| Key skills and competencies: Helen is a qualified Solicitor admitted in England & Wales and a Chartered Governance Professional. She also serves as the Chief Risk Officer for Smith+Nephew. Previous experience: Helen started her career with Allen & Overy LLP and prior to joining Smith+Nephew held senior legal roles at WPP plc and Nomura International plc. Nationality: British John Rogers Chief Financial Officer Designate Joining the Board as Chief Financial Officer in Q1 2024 Key skills and competencies: John has extensive financial and commercial leadership experience across a range of sectors and on a global basis, as well as a track record of delivering complex international transformation programmes. Current external appointments: – Non-Executive Director of Grab Holdings Limited. Previous experience: He has served as the Chief Financial Officer at WPP plc, where he successfully led the implementation of their global transformation programme, and as Chief Financial Officer at J Sainsbury plc where he also served as Chief Executive Officer of Argos, Habitat and Sainsbury’s clothing and general merchandise businesses. Nationality: British Directors who have joined the Board since 31 December 2023 Simon Lowth Independent Non-Executive Director Appointed as Independent Non-Executive Director on 1 January 2024 Key skills and competencies: Simon has extensive experience in finance, accounting, risk, corporate strategy as well as mergers and acquisitions and brings a wealth of expertise across a wide range of sectors, including within regulated industries. Having served as the CFO in four FTSE 100 companies, he has deep experience of capital markets, implementing strategic change, cost transformation and performance improvement programmes as well as understanding how technology can be used to transform a business. Current external appointments: – Group Chief Financial Officer of BT Group. Previous experience: Simon was previously Group Chief Financial Officer at AstraZeneca and Scottish Power. Before joining Scottish Power, he led the Industrial Practice of McKinsey in the UK. He previously served as a Non-Executive Director on the Board of Standard Chartered. Nationality: British A N Helen Barraclough Group General Counsel and Company Secretary Appointed Company Secretary in April 2022 Board member whose tenure ceased during the year Roberto Quarta, Chair, stepped down from the Board on 15 September 2023. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 93 |
| » See page 90 for CEO and CFO biographies Mizanu Kebede Chief Quality & Regulatory Affairs Officer Nationality: American Location: Georgia, US Mizanu brings more than 20 years of leadership experience in Quality and Regulatory Affairs. Prior to Smith+Nephew, Mizanu held senior roles at Avanos Medical, Life Technologies Corporation, Johnson & Johnson and STERIS Corporation. Phil Cowdy Chief Corporate Development & Corporate Affairs Officer Nationality: British Location: Watford, UK Prior to joining Smith+Nephew, Phil served as a senior Director at Deutsche Bank AG for 13 years specialising in corporate finance and equity capital markets. Phil serves as the representative of Smith+Nephew on the Board of Bioventus Inc. Rohit Kashyap President Advanced Wound Management and Global Commercial Operations Nationality: American Location: Fort Worth, US Rohit brings more than 20 years’ experience across wound care, surgical management, business development and global commercial leadership. Prior to joining Smith+Nephew, Rohit worked at Acelity, a global advanced wound care company, most recently as President, Global Commercial and at MIMEDX as President of the Wound and Surgical business and as Chief Commercial Officer. Executive Committee The CEO, with support from the CFO, leads the Executive Committee of Smith+Nephew which is responsible for the day-to-day operational management of the Group and executing its strategy. Brad Cannon President Orthopaedics & Americas Nationality: American Location: Andover, US Brad brings more than 25 years of experience across medical devices and medtech. Prior to Smith+Nephew, Brad worked in Medtronic plc’s Spine and Biologics division and previously served as Chief Marketing Officer and President of Europe and Canada at Smith+Nephew. Paul Connolly President Global Operations Nationality: American/Irish Location: Andover, US Paul brings more than 30 years of global manufacturing and supply chain experience at multinational companies with a strong track record in delivering operational excellence and transformation programmes. Prior to joining Smith+Nephew, Paul held senior roles at Goodyear, DePuy, Inc., and other Johnson & Johnson family companies. Board leadership and Company purpose continued 94 Smith+Nephew Annual Report 2023 |
| Vasant Padmanabhan President Research & Development and ENT Nationality: American Location: Andover, US Vasant has over 25 years of global med-tech leadership experience. Prior to Smith+Nephew, Vasant held senior roles at Thoratec Corporation and Medtronic plc as Vice President of Connected Care R&D and Operations and Vice President of Product Development for the Implantable Defibrillator Business. Helen Barraclough Group General Counsel and Company Secretary Nationality: British Location: Watford, UK Prior to joining Smith+Nephew, Helen started her career at Allen & Overy LLP and held senior roles at WPP plc and Nomura International plc. She is a qualified Solicitor admitted in England & Wales and a Chartered Governance Professional. She also serves as the Chief Risk Officer for Smith+Nephew. Alison Parkes Chief Compliance Officer Nationality: British Location: Hull, UK Prior to moving into her current role, Alison served as the Compliance Officer for the Global Advanced Wound Management business, APAC and Emerging Markets and established and led the Global Compliance Programme Effectiveness & Improvement team. Scott Schaffner President Sports Medicine Nationality: American Location: Austin, US Scott has more than 30 years experience across the medical device industry, including cardiac rhythm management, neuromodulation, spine, and sports medicine. Prior to moving into his current role, Scott served as Executive Vice President, Global Marketing and US Commercial, Sports Medicine, Senior Vice President, Global Marketing, Sports Medicine and Vice President, Sports Medicine. Elga Lohler Chief HR Officer Nationality: American/South African Location: Fort Worth, US Prior to joining Smith+Nephew, Elga held Human Resources roles at Transnet SOC Ltd, Sensormatic (now Tyco International plc) and Advanced Tissue Sciences, Inc. (acquired by Smith+Nephew in 2002). Executive Officers whose tenures ceased and recent appointments Simon Fraser, President Advanced Wound Management and Global Commercial Operations, served until 2 June 2023. Myra Eskes, President APAC Region, served until 1 November 2023. Brad Cannon, President Orthopaedics & Americas, served until 4 March 2024. Craig Gaffin was appointed President Orthopaedics effective as of 4 March 2024. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 95 |
| Company Secretary Helen Barraclough – Supports the Chair and ensures Board members have access to the information required to perform their duties. – Advises the Board on legal and corporate governance matters and supports the Board in applying the 2018 Code and complying with UK listing obligations, and other statutory and regulatory requirements. – Provides a channel for Board and Committee communications and a link between the Board and management. Chief Financial Officer Anne-Françoise Nesmes – Supports the Chief Executive Officer in developing and implementing Group strategy. – Responsible for ensuring effective financial reporting, investor relations, tax, treasury and financial controls are in place within the Group. – Provides information and participates in Board discussions regarding financial matters. – Leads global finance function, developing key finance talent and succession planning. Chief Executive Officer Deepak Nath – Responsible for delivering and implementing Group strategy and management of the organisation as a whole. Provides information and participates in Board discussions regarding Group management and operational matters. – Leads the Executive Committee and ensures its effectiveness in managing the overall operations and resources of the Group. – Sets tone at the top with regard to culture, compliance and sustainability matters. – Ensures the Chair and Board are updated regularly regarding key matters and maintains relationships with shareholders, advising the Board accordingly. Senior Independent Director Marc Owen – Acts as a sounding board for the Chair and as an intermediary for other Directors and stakeholders as necessary. – As a member of the Nomination & Governance Committee, leads the Board evaluation process and searches for Chair and Independent Non-Executive Directors to ensure effective succession. – Acts as an alternative contact for stakeholders to raise concerns (in addition to Chair and senior management). Chair Rupert Soames – Responsible for the effective leadership and operation of the Board and for facilitating the review of its composition, effectiveness and development. – Promotes effective board relationships, encouraging constructive challenge and facilitating effective communication between Board members and supporting a culture of openness, challenge and debate. – Ensures that the Board understands the views and needs of the Company’s stakeholders and facilitates effective communication and dialogue, whilst maintaining an appropriate balance between stakeholders. – Leads relations with shareholders in order to understand their views on governance and performance against strategy. – Responsible for promoting high standards of governance by the Board and its Committees. The Chair achieves this through effective chairing of Board meetings; setting a board agenda which focuses on strategy, performance, value creation, risk management, culture, stakeholders and accountability; enabling an annual review of Board effectiveness; holding discussions with Board members both inside and outside the boardroom and ensuring appropriate Board induction and development programmes are in place. Division of responsibilities Roles and composition of the Board Independent Non-Executive Directors Jo Hallas, John Ma, Katarzyna Mazur-Hofsaess, Erik Engstrom, Jez Maiden, Rick Medlock, Angie Risley and Bob White – Comprise more than half of Board membership in order to meet the independence criteria set out in the 2018 Code. – Ensure that no individual/ small group can dominate the Board’s decision making. – Provide constructive challenge, give strategic guidance, offer specialist advice and hold executive management to account. 96 Smith+Nephew Annual Report 2023 |
| At the opening and close of each Board meeting, the Full Board meets for a short closed session discussion. At the end of each Board meeting, the Chair meets with Non-Executive Directors in the absence of the Executive Directors. The Chair also holds one to one discussions with each Board member throughout the year. Independence of Directors We require our Non-Executive Directors to remain independent from management so that they are able to exercise independent oversight and effectively challenge management. We therefore continually assess the independence of each of our Non-Executive Directors. The Executive Directors have determined that all our Non-Executive Directors are independent in accordance with both UK and US requirements. None of our Non-Executive Directors or their immediate families has ever had a material relationship with the Group. None of them receive additional remuneration apart from Directors’ fees, nor do they participate in the Group’s share plans or pension schemes. None of them serve as directors of any companies or affiliates in which any other Director is a director. The Board considers all external directorships prior to appointment, reviewing any potential conflict of interests and time commitment for both Executive Directors and Non-Executive Directors. Management of conflicts of interest None of our Directors or their connected persons, has any family relationship with any other Director or Officer, nor has a material interest in any contract to which the Company or any of its subsidiaries are, or were, a party during the year or up to 16 February 2024. Each Director has a duty under the Companies Act 2006 to avoid a situation in which they have or may have a direct or indirect interest that conflicts or might conflict with the interests of the Company. This duty is in addition to the existing duty owed to the Company to disclose to the Board any interest in a transaction or arrangement under consideration by the Company. If any Director becomes aware of any situation which might give rise to a conflict of interest, they must, and do, inform the rest of the Board immediately and the Board is then permitted under the Company’s Articles of Association to authorise such conflict. This information is then recorded in the Company’s Register of Conflicts, together with the date on which authorisation was given. In addition, each Director certifies on an annual basis that the information contained in the Register of Conflicts is correct. When the Board decides whether or not to authorise a conflict, only the Directors who have no interest in the matter are permitted to participate in the discussion and a conflict is only authorised if the Board believes that it would not have an impact on the Board’s ability to promote the success of the Company in the long term. Additionally, the Board may determine that certain limits or conditions must be imposed when giving authorisation. No actual conflicts have been identified, which have required approval by the Board. However, the situations that could potentially give rise to a conflict of interest have been identified and duly authorised by the Board and are reviewed at least on an annual basis. Outside directorships We encourage our Executive Directors to serve as Non-Executive Directors of external companies. We believe that the work they do as Non-Executive Directors of other companies has benefits for their executive roles with the Company, giving them a fresh insight into the role of a Non-Executive Director. Anne-Françoise Nesmes is the Senior Independent Director and Chair of the Audit Committee at Compass Group plc which is listed on the London Stock Exchange. Re-appointment of Directors In accordance with the 2018 Code, all Directors offer themselves to shareholders for re-election annually, except those who are retiring immediately after the Annual General Meeting. Each Director may be removed at any time by the Board or the shareholders. Board support Together with the Chief Executive Officer and the Group General Counsel and Company Secretary, the Chair ensures that the Board is kept properly informed. Each Director has access to the Group General Counsel and Company Secretary, who helps to ensure that Board procedures and good corporate governance practices are followed. Directors are permitted to take independent professional advice at the Company’s expense if required in order to enable them to fulfil their duties. Each Director is covered by appropriate directors’ and officers’ liability insurance and there are also Deeds of Indemnity in place between the Company and each Director. These Deeds of Indemnity mean that the Company indemnifies Directors in respect of any proceedings brought by third parties against them personally in their capacity as Directors of the Company. The Company would also fund ongoing costs in defending a legal action as they are incurred rather than after judgment has been given. In the event of an unsuccessful defence in an action against them, individual Directors would be liable to repay the Company for any damages and to repay defence costs to the extent funded by the Company. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 97 |
| Division of responsibilities continued Corporate governance framework Our Board The Board is accountable to shareholders for the performance and long-term sustainable success of the Company. It approves the strategy of the Group, evaluates and monitors the management of risk, and oversees the implementation of the strategy in order to achieve sustainable growth. The Board delegates certain matters to the Audit, Remuneration, Nomination & Governance and Compliance & Culture committees which support the Board in carrying out its responsibilities. Full details of the Matters Reserved to the Board can be found on the Company’s website. www.smith-nephew.com Finance & Banking Committee A Committee comprising senior executives which approves banking and treasury matters, guarantees and Group structure changes relating to mergers, acquisitions and disposals. Disclosures Committee A Committee comprising senior executives which oversees and approves public announcements and communications to investors and Stock Exchanges. Reviews communications and reporting requirements in respect of market sensitive information. Compliance & Culture Committee »pages 111–113 Reviews and monitors and has oversight of ethics and compliance, quality and regulatory, culture, sustainability matters and metrics, stakeholder relationships and related legal matters across the Group. Audit Committee »pages 114–120 Ensures the integrity of the Company’s financial reporting, systems and controls. Oversight of risk management process. Reviews and monitors climate change disclosures and related ESG financial reporting obligations. Monitors the Group’s cyber resilience. Ensures effectiveness of internal and external audit functions. Nomination & Governance Committee »pages 102–110 Reviews size, skills, experience, knowledge and composition of the Board, succession planning, diversity and governance matters. Remuneration Committee »pages 121–154 Determines Remuneration Policy and packages for Executive Directors and senior management, having regard to pay across our workforce. Ensures reward strategy aligns with our purpose, values and long-term strategy. Executive Committee »pages 94–95 The Board delegates the day-to-day operational management and implementation of Group strategy to the Chief Executive Officer and Executive Committee). The Executive Committee recommends, and following Board approval, implements strategy, budget and three-year strategic plan within the Group. It ensures cross-functional alignment in order to deliver on strategy and reviews major investments, divestments and capital expenditure proposals. The Executive Committee also focuses on people and organisational culture, reviewing recruitment, attrition and development initiatives within the Company and developing and monitoring succession planning and talent pipeline below Board level. The Executive Committee meets at least 10 times per year to review commercial and operating results against budget, key initiatives, KPIs and performance metrics aligned to deliver Group strategy. The Executive Committee forms sub-committees including those listed below: Group Ethics & Compliance Committee ESG Operating Committee Mergers & Acquisitions Investment Committee Global Benefits Committee 12-Point Plan Steering Committee Global Crisis Management Team New Product Development Review Committee Inclusion, Diversity and Equity Council Security and Privacy Steering Committee 98 Smith+Nephew Annual Report 2023 |
| Board and Committee attendance Total meetings Board WIP Audit Remuneration Nomination & Governance Compliance & Culture Attendees Appointed 8 7 9 6 4 Roberto Quarta1 December 2013 4/5 – 4/5 3/4 – Rupert Soames2 April 2023 6/6 – 6/6 4/5 – Deepak Nath April 2022 8/8 – – – – Erik Engstrom3 January 2015 8/8 7/7 – 5/6 – Jez Maiden4 September 2023 3/3 3/3 4/4 – – John Ma5 February 2021 7/8 – – – 4/4 Katarzyna Mazur-Hofsaess6 November 2020 7/8 – – – 3/4 Rick Medlock7 April 2020 7/8 7/7 – – – Anne-Françoise Nesmes July 2020 8/8 – – – – Marc Owen October 2017 8/8 7/7 – 6/6 4/4 Angie Risley8 September 2017 7/8 – 9/9 6/6 4/4 Bob White May 2020 8/8 – 9/9 – 4/4 Jo Hallas February 2022 8/8 7/7 – – – 1 Roberto Quarta stepped down from the Board on 15 September 2023 and of the five meetings held during his tenure in 2023, he was unable to attend the Board meeting in April as he was recuperating from a surgical procedure. Roberto was unable to attend the Nomination & Governance Committee meeting in August and the Remuneration Committee Meeting in February due to travel disruption. 2 Rupert Soames was appointed as an Independent Non-Executive Director and Chair Designate on 26 April 2023. Rupert was unable to join the Nomination & Governance Committee in July due to a pre-existing commitment prior to appointment as Chair Designate. 3 Erik Engstrom retired from the Board on 31 December 2023. Erik was unable to join the Nomination & Governance Committee in October due to executive management commitments. 4 John Ma was unable to attend the July Board meeting due to travel disruption. 5 Jez Maiden was appointed to the Board as an Independent Non-Executive Director and a member of the Audit and Remuneration Committees on 14 September 2023. 6 Katarzyna Mazur-Hofsaess was not in attendance at the December Board meetings due to a prior statutory commitment. She provided comments to the Chair in advance of the meeting. 7 Rick Medlock was not in attendance at the January Board Meeting due to a prior professional commitment. He provided his comments to the Chair in advance of the meeting. 8 Angie Risley was not in attendance at the January Board Meeting due to a prior professional commitment. She provided her comments to the Chair in advance of the meeting. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 99 |
| Strategy and Innovation – Reviewing and monitoring progress against the 12-Point Plan and related metrics, global business unit reorganisation and infrastructure and network optimisation projects in support of the Group strategy. – Reviewing and approving three-year strategic plan, with a focus on innovation, transformation and portfolio. – Setting priorities for capital investment across the Group with a view to ensuring systemic transformation. – Determining the dividend policy and dividend recommendations. – Reviewing the implementation of cost management programmes and monitoring outcomes against objectives. – Approving annual budget and financial plan and half-year, full-year and trading updates. – Approving major borrowings and finance and banking arrangements, such as the refinancing of the Group’s $1bn USD revolving credit facility in October 2023. – Approving changes to the composition of the Board, its Committees and the Executive Committee. » Chair’s Statement (pages 4–7) » Financial Review (pages 20–23) Group Purpose and Culture – Reviewing and monitoring Group strategy in alignment to the Purpose of Life Unlimited and culture pillars of Care, Collaboration and Courage. – Monitoring and ensuring the scope and focus of strategic projects and initiatives support the Group’s purpose and culture pillars. – Review of initiatives on people, leadership and development of internal talent pipeline. – Review of Sustainability strategy, climate-related disclosures and key performance metrics with input from Audit, Remuneration and Compliance & Culture Committee Chairs. – Review of initiatives to strengthen and embed Inclusion, Diversity and Equity throughout the Group, including receiving reports on employee engagement, employee interest groups and Board listening sessions. – Approving Group policies relating to Code of Conduct and Business Principles, Code of Share Dealing and other reserved matters. » Employees (pages 46–49) » Compliance & Culture Committee report (pages 111–113) Division of responsibilities continued Board activities 1 2 3 4 5 1 2 3 4 5 Link to our strategic priorities Link to stakeholder groups Employees Investors Customers/Suppliers Governments/Regulators Environment/Communities 1 2 3 5 4 The following pages provide an overview of the key topics reviewed, monitored, considered and debated by the Board in the year to 31 December 2023. – Agendas for each Board meeting focus on matters within the core areas of strategy and risk, innovation and portfolio, capital allocation and operational excellence and are agreed in advance by the Chair, CEO and CFO with the support of the Company Secretary. – The Board receives the 12-Point Plan updates on performance against key metrics, operating and financial reports from the CEO and CFO on strategic and business developments, as well as financial performance and forecasts at each meeting. – Presentations led by Exco members and their direct reports and senior leaders are also included on key topics of interest to the Board, such as Sustainability, Risk Management, Cybersecurity and AI. – The Chairs of each Committees update the Board on the proceedings of those meetings, including key topics and areas of concern. – At the end of each meeting the Chair holds a closed session with Board members providing further opportunity for the Non-Executive Directors to assess the performance of management in an atmosphere conducive to transparent and collaborative debate. 3 2 1 100 Smith+Nephew Annual Report 2023 |
| Operations and Commercial Excellence – Strategic deep dives on global business unit plans in Orthopaedics, Sports Medicine & ENT and Advanced Wound Management aligned to 12-Point Plan initiatives and broader long-term strategic initiatives. – Monitoring Group operations updates on inventory management, asset utilisation, network optimisation, and response to external and internal challenges in line with 12-Point Plan key metrics and deliverables. – Review of global innovation pipeline and product portfolio with a focus on differentiation and delivery for our customers, patients and stakeholders. – Overseeing succession planning at Board and senior management level to ensure development of internal talent pipeline and stability for the Group. – Review of performance and return on investment of acquisitions and integration planning. – Continuing review and monitoring of impact of external factors such as inflation, supply constraints, cybersecurity and business continuity on ability to deliver on strategic objectives. » Compliance & Culture Committee report (pages 111–113) » Audit Committee report (pages 114–120) Stakeholders – Overseeing and maintaining relationships with stakeholders including employees, customers, suppliers, investors, regulators, governments and local communities. Further details of Board interactions with stakeholders can be found on pages 84–87. – Review of external and investor perceptions of the Company including feedback on investor engagement and Meet the Management sessions. – Reviewing Executive Director and Executive Officer talent pipeline and succession planning. – Reviewing employee engagement scores and performance/trends within the Company. – Review of diversity metrics and gender pay gap data and reporting. – Engaging with shareholders on strategy, operational performance, governance, remuneration, succession planning, ESG and Governance matters. » Our stakeholders (pages 82–83) » Engaging with Stakeholders (pages 84– 87) Risk Oversight, Management and Controls – Evaluation of risks and opportunities with regard to strategic initiatives such as the 12-Point Plan, global business unit reorganisation, operations and network optimisation, innovation and portfolio opportunities, AI strategic initiatives and Sustainability. – Oversight of the Group’s risk management strategy, programme and related processes. See pages 67–77 for further details. – Review and approval of the Principal Risks of the Group and Board appetite for risk. – Board consideration of key risks and opportunities to manage risk and create value in including cybersecurity and business continuity, succession and talent pipeline, IT investment and infrastructure and ESG considerations. – Discussion at Board and Committee meetings on key macro topics including impact of inflation, regulatory changes and portfolio competitiveness, geopolitical outlook and global conflicts, operational challenges and global talent outlook. – Approving the appointment and removal of the External Auditor on the recommendation of the Audit Committee. – Approving significant changes to accounting policies or practices. – Approving the use of the Company’s shares for the Company’s Share Plans. » Chair’s Statement (pages 4–7) » Financial Review (pages 20–23) Our Investor presentations are available to download on our website www.smith-nephew.com 1 2 3 4 5 1 2 3 4 5 1 2 3 4 5 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 101 |
| Composition, succession and evaluation Nomination & Governance Committee Report Dear Fellow Shareholder, In my first Annual Report as your Chair of the Nomination & Governance Committee, I am pleased to present this report within the governance section of our Annual Report. The Committee has been busy over the past 12 months managing the appointment of four new Directors to the Board: Chair of the Board, two Non-Executive Directors, and a Chief Financial Officer. This will result in a Board which, over the last 18 months, is substantially refreshed, and I would like to recognise in particular the contribution of our Senior Independent Director, Marc Owen, who has diligently and effectively led the Chair and NED searches. In addition, the Committee carried out its other responsibilities supporting Board induction, development programmes and strong corporate governance. Board and Executive appointments in 2023 Jez Maiden was appointed as a Non-Executive Director to the Board on 14 September 2023 and as a member of the Audit Committee and Remuneration Committee. Jez brings more than 15 years of experience both as a FTSE Chief Financial Officer and as a Non-Executive Director in businesses involved in manufacturing, science and technology and has already demonstrated that he is a valuable addition to the Board and its Committees. We welcomed Simon Lowth on 1 January 2024 as a Non-Executive Director and member of the Audit Committee and Nomination & Governance Committee following Erik Engstrom’s completion of his 9 year tenure. Simon brings a wealth of expertise across a wide range of sectors, including within regulated industries. His experience of capital markets, implementing strategic change, cost transformation and performance improvement programmes as well as understanding how technology can be used to transform a business, will be very helpful to the Board. Following Anne-Françoise notifying the Board in August 2023 of her intention to step down from her role as Chief Financial Officer, John Rogers joined the Company as Chief Financial Officer designate on 1 December 2023 and will join the Board during the first quarter of 2024. John is a highly regarded Chief Financial Officer with a proven track record operating around the world and across a number of industry sectors. His extensive experience in transformation and capital markets is especially important given Smith+Nephew’s focus on driving greater shareholder value and we look forward to welcoming him to the Board. New Director appointments and process For our new Board appointments in 2023, the Committee followed the process outlined in the table on page 103 and considered the shortlist of candidates for each position taking into account: (i) the purpose, values and culture of the business and the Company’s strategic priorities; (ii) the key skills and experience which may be required on the Board and its Committees; and (iii) the importance of diversity including gender, personal strengths, and social and ethnic backgrounds. With all of our new appointments, we had a diverse slate of candidates taking into account diversity in its broadest sense. In our appointments, we will always ensure we select the most qualified candidate for the role in the best interests of the organisation as a whole. 1 Rupert Soames joined the Committee with effect from 26 April 2023 and was appointed as Chair on 15 September 2023. 2 Erik Engstrom was unable to join the Nomination & Governance Committee in October due to executive management commitments. 3 Roberto Quarta stepped down from the Board on 15 September 2023. Membership Member from Meetings attended Rupert Soames (Chair)1 April 2023 4/5 Erik Engstrom2 April 2023 5/6 Roberto Quarta3 April 2014 3/4 Marc Owen March 2020 6/6 Angie Risley Sept 2022 6/6 www.smith-nephew.com/ investor-centre/about-us/ governance/corporate-documents-and-policies/ terms-of-reference/ The Terms of Reference for the Nomination & Governance Committee describe the role and responsibilities of the Nomination & Governance Committee more fully and can be found on our website. Rupert Soames Chair of the Nomination & Governance Committee 102 Smith+Nephew Annual Report 2023 |
| Board and Executive Succession Planning Succession planning is a key focus for the Board from both a leadership and governance perspective. The Committee engaged in a review throughout the year of Board and Committee composition and skillsets to ensure alignment with the Company’s strategic objectives and culture pillars to enable effective succession planning for Non-Executive and Executive Directors. The full Board also reviewed the Board Skills Composition Matrix (please see table on page 106) which sets out the tenure, skills, competencies and diversity of the Board. The Board composition and skills matrix feeds into a formal rolling succession plan for Directors. The Committee starts board recruitment well ahead of retirements, understanding the competitiveness of the market. Priorities for recruiting and succession planning include the ability to respond to evolving strategic imperatives for the Company, adding and enhancing Board skills including in the areas of healthcare sector perspectives, finance, operational and digital/cyber experience and ESG and enhancing diversity in the boardroom. The Board discusses succession plans with management for senior executives and this will receive enhanced focus in 2024, with a biannual Board review of talent pipeline and development programmes (see page 110 Board Effectiveness) in addition to the focus already provided by the Compliance & Culture Committee on employee engagement and the Remuneration Committee on executive compensation. These plans include consideration and monitoring of diversity in the executive pipeline. Pages 94–95 give details of the members of the Executive Committee, 33.3% of whom are female, one of whom is of African heritage and one of Asian ethnicity. Following the leadership changes at executive level this year, the Committee is aware that management are focused on ensuring that there are development plans in progress to enable a broader range of candidates to be considered within the internal succession pipeline for senior management roles. Diversity The Committee believes that a Board and management team which has a range of diverse skills, background and experience is best equipped to take the decisions which will deliver sustainable value to shareholders and other stakeholders. We are therefore committed to fostering diversity in its broadest sense and we continue to ensure that our Board membership draws from a wide range of backgrounds and cultures. When Anne-Françoise steps down from the Board in 2024, our Board will continue to have three experienced female Directors and our succession planning process will continue to ensure that we have a diverse slate of candidates and will seek to increase diversity within the Board as and when the opportunity arises. We will also have a diverse range of ethnicities, experience and backgrounds on the Board. The Committee will continue to appoint Board members on merit, valuing the unique contribution that they will bring to the Board, regardless of gender, ethnicity or with other specific diversity measure. Our diversity statement is located on our website: www.smith-nephew.com/en/ about-us/corporate-governance/diversity-statements. During 2023, the Board has benefited from the diversity of experience, background and global and regional expertise of its members. The Committee believes the Board’s composition gives us the necessary balance of diversity, skills, experience, independence and knowledge to ensure continued effectiveness in running the business and delivery of sustainable growth. Yours sincerely, Rupert Soames, OBE Nomination & Governance Committee Chair * Russell Reynolds was appointed as the search firm in respect of the appointments of Rupert Soames, Jez Maiden and Simon Lowth. Spencer Stuart was engaged for the Chief Financial Officer appointment. 1 Before any appointment is made, the Committee evaluates the balance of skills, knowledge, experience, independence and diversity on the Board. In light of this evaluation, the Committee prepares a description of the role and capabilities required for a particular appointment and works with external advisors, as appropriate, to compile a shortlist of candidates based on the role description. The Committee (together with external advisors*) then compiles a shortlist including a broad slate of candidates from a wide range of backgrounds to ensure diversity. The Committee evaluates the shortlist of candidates on merit and against objective criteria, taking care to ensure that appointees have sufficient time to devote to the position in light of their other commitments. The Committee also assesses any actual or potential conflicts of interest as part of the process. Members of the Committee interview key candidates from the shortlist. Other Board members are also involved in the interview process as appropriate. For example, where a candidate is required to have a requisite level of financial expertise, the Audit Committee Chair and Chief Financial Officer would be involved in the interview process. The Committee reviews and considers the feedback provided based on the interview process, reference checks and due diligence in arriving at a decision on a candidate to recommend to the Board. 3 4 5 6 2 Board appointment process STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 103 |
| Composition, succession and evaluation continued Nomination & Governance Committee report continued Focus and Actions for 2024 – Continued oversight of succession planning at and below Board level, with biannual discussion at Board level as well as at Compliance & Culture and Remuneration Committees on senior management talent pipeline planning, attraction, retention and development. – Ongoing review of Board structure, size and composition with a view to ensuring that the Board continues to demonstrate the right balance of skills, knowledge and diversity in its broadest sense and to evaluate potential opportunities to increase diversity within the Board and the timeline for doing so. – Implementing comprehensive induction programmes for our new Board members to enable them to gain strong insight into our business and high levels of engagement with our Purpose, Culture Pillars and strategic objectives over the short, medium and longer term. Responsibilities of the Nomination & Governance Committee Board composition – Reviewing the structure, size and composition of the Board. – Overseeing Board succession plans including engaging external search consultancies and making recommendations on appointments to the Board. – Recommending the appointment of Directors and Company Secretary. – Monitoring the range of skills, knowledge, experience, independence and diversity of the Board. – Monitoring Board diversity in its broadest sense. Corporate governance – Overseeing governance aspects of the Board and its Committees. – Overseeing the review into the effectiveness of the Board. – Considering and updating the Schedule of Matters Reserved to the Board and the Terms of Reference of the Board Committees. – Overseeing the Induction process for new Directors and the Board Development Programme to support the ongoing development of all Board members. – Considering the continued independence of the Non-Executive Directors and any conflict of interest. – Overseeing the annual Board Evaluation process led either externally or internally by the Senior Independent Director. – Approving external directorships to be held by the Board. Highlights in 2023: – Appointment of Rupert Soames as a Non-Executive Director and Chair designate on 26 April 2023. Rupert became Chair of both the Board and the Committee effective 15 September 2023 following a transition from Roberto Quarta. – Appointment of Jez Maiden on 14 September 2023 as Independent Non-Executive Director and a member of the Audit and Remuneration Committees. – Appointment of John Rogers as Chief Financial Officer designate announced on 2 November 2023. John joined the Company on 1 December 2023 and will be appointed as an Executive Director during the first quarter of 2024. – Appointment of Simon Lowth effective 1 January 2024 as Independent Non-Executive Director and a member of the Audit and Nomination & Governance Committees following the departure of Erik Engstrom. – Devising and implementing comprehensive induction and development programmes for our new Board members. – Annual review of conflict process and Directors’ external appointments. – Approval of re-appointment of Directors and assessment of continued independence of Non-Executive Directors. Board tenure* Board ethnicity* White British or other White (including minority-white groups) 9 Asian/Asian British 2 Not specified/ prefer not to say 1 British 5 American 3 British/American 1 Swedish 1 British/French 1 Polish/German 1 0–2 yrs 5 3–5 yrs 4 6+ yrs 3 Board nationality* 66.67% male 33.33% female FTSE 350 companies to have at least one woman in the Chair or Senior Independent Director role on the Board, and/or one woman in the Chief Executive or Finance Director role in the company by the end of 2025. Year achieved 2020 Anne-Françoise Nesmes was appointed Chief Financial Officer in 2020. Board gender diversity * This information is at 31 December 2023. 104 Smith+Nephew Annual Report 2023 |
| Number of Board members Percentage of the Board % Number of senior positions on the Board (CEO, CFO, SID and Chair) Number in executive management1 Percentage of executive management1 % Gender Representation: Board & Executive Management (as at 31 December 2023) Men 8 66.67 3 8 66.67 Women 4 33.33 1 4 33.33 Other categories 0 0 0 0 0 Not specified/prefer not to say 0 0 0 0 0 Ethnic Background: Board & Executive Management (as at 31 December 2023) White British or other White (including minority-white groups) 9 75 3 8 66.67 Mixed/Multiple Ethnic Groups 0 0 0 0 0 Asian/Asian British 2 16.67 1 1 8.33 Black/African/Caribbean/Black British 0 0 0 1 8.33 Other ethnic group, including Arab 0 0 0 2 16.67 Not specified/prefer not to say 1 8.33 0 0 0 1 Executive management is the Executive Committee (most senior executive body below the Board). Board and executive management diversity Prepared in accordance with UK Listing Rule 9.8.6R(10) as at 31 December 2023. Explanation against LR 9.8.6(9) The table above provides our Board and executive management diversity data as at 31 December 2023, our chosen reference date, which has been prepared in accordance with UK Listing Rule 9.8.6. One of the four senior positions on the Board (Chair, CFO, CEO or SID) was held by a woman, our Board composition included two Directors from ethnic minority backgrounds and 33.33% of the Board of Directors are women. The Board is pleased that two of the targets have been met but recognises that it has not met the target of 40% individuals on the Board being women. The Board membership draws from a wide range of backgrounds and cultures with a commitment to fostering diversity in its broadest sense. The Board succession planning process includes a diverse range of candidates and the Board will seek to increase diversity as part of succession planning relating to future board changes. All appointments to the Board are determined on merit and valuing the unique contribution that a member brings to the Board, regardless of gender, ethnicity or other specific diversity measure. The overriding priority across all Board appointments remains identification of the strongest candidate for the role, based on clear search criteria. Further detail of the focus by the Nomination & Governance Committee on the continued development of a diverse talent pipeline, and the work to oversee external benchmarking to ensure Smith+Nephew has the diversity and capabilities needed for future growth, is set out on page 103. Source of Data Data concerning gender and ethnicity representation on the Board and Executive Committee is set out below. This data was collected directly from all the individual Board and Executive Committee members. Each individual disclosed their gender and ethnicity using the options included on a form, which align with the detail in the left-hand column of the table below and therefore includes the option to not specify an answer. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 105 |
| Skills and experience matrix Executive Directors Tenure Employee engagement CEO Financial International Healthcare/ Medical Devices Emerging Markets Cyber security ESG UK Governance Remuneration Deepak Nath 1y 08m Anne-Françoise Nesmes 3y 05m John Rogers1 Non-Executive Directors Tenure Employee engagement CEO Financial International Healthcare/ Medical Devices Emerging Markets Cyber security ESG UK Governance Remuneration Rupert Soames2 0y 08m Roberto Quarta3 10y 00m Marc Owen 6y 02m Erik Engstrom4 8y 11m Jo Hallas 1y 10m Simon Lowth5 John Ma 2y 10m Jez Maiden6 0y 03m Katarzyna Mazur-Hofsaess 3y 01m Rick Medlock7 3y 08m Angie Risley 6y 03m Bob White 3y 07m Notes 1 John Rogers is to replace Anne-Françoise Nesmes as Chief Financial Officer during Q1 2024. 2 Rupert Soames joined the board on 26 April 2023 and became Chair on 15 September 2023. 3 Roberto Quarta stepped down as Chair on 15 September 2023. 4 Erik Engstrom stepped down from the Board on 31 December 2023. 5 Simon Lowth joined the Board as a Non-Executive Director and as member of the Audit and Nomination and Governance Committees on 1 January 2024. 6 Jez Maiden joined the Board on 14 September 2023 and will become Chair of the Audit Committee on 1 March 2024. 7 Rick Medlock has notified the Board of his decision not to submit himself for re-election as a Non-Executive Director. He will step down as Chair of the Audit Committee on 1 March 2024 and as a Non-Executive Director on 30 April 2024. Composition, succession and evaluation continued Nomination & Governance Committee report continued 106 Smith+Nephew Annual Report 2023 |
| Q&A with Jez Maiden, Independent Non-Executive Director There is an openness to discuss challenging and difficult questions, living up to our culture pillars of Care, Courage and Collaboration. Most of all, the people I have met are committed to Smith+Nephew and to doing the right thing, with quality, innovation and trust.” Jez Maiden Independent Non-Executive Director How effective has your induction been? Induction is an ongoing process – as Directors, we are always learning about the business through our interactions and visits. For my first five months with Smith+Nephew, I started with an immersion in the MedTech market, gaining external perspectives on competitors and our strengths and weaknesses. This was followed by a two-day deep dive into our strategy and one-to-one meetings with all of the Executives, benefiting from their knowledge and expertise in the MedTech sector. Reflecting my forthcoming move to chair the Audit Committee, I spent a day at Croxley with the Finance Team, which will be really useful in helping the Committee execute its responsibilities to ensure accountability and effectiveness in reporting and control. I have also spent time with our external advisors – corporate brokers, remuneration advisor and auditors, particularly valuable as Deloitte will become our new external auditor in 2024 as I take over the Audit Committee Chair. Building on my previous IT experience, I have also had valuable sessions with Smith+Nephew’s application development and information security management teams, a key area of both systems opportunity and cyber risk. As a manufacturing guy, I was excited by my visit in February to the Advanced Wound Management production site in Hull, UK. As a resident of Yorkshire for over 30 years, I was already familiar with the heritage and reputation attached to Smith+Nephew’s foundation in the region, and the chance to see the site, and learn more on the development of the new state-of-the-art facility, was immensely rewarding. I am looking forward to meeting the Ortho and Sports Medicine teams as part of the forthcoming Board visit to the US in 2024. Crucial to Board members is the chance to hear about life at the ‘coal face’. I am looking forward to meeting more Smith+Nephew employees as part of our Employee Listening sessions in 2024. What most interested you in joining S+N? Most of my executive career has been spent in manufacturing businesses and I now help connect government innovation funding to growing process manufacturing businesses. I believe that innovative manufacturing must and will continue to play a key role in driving the global economy. Smith+Nephew is creating value through innovation, combining successful R&D with high-quality, efficient manufacturing. I am excited by the opportunity to support the team from a lifetime of manufacturing experience, hopefully leveraging insights in lean manufacture, inventory management and improving productivity. With many years experience helping companies develop in Life Sciences and Health Care, I am also excited by the opportunity to help drive success in a new but adjacent space, the MedTech market. Smith+Nephew has a broad, clinically proven and differentiated portfolio of MedTech products and services. Its Purpose is clear, Life Unlimited. I hope to bring my experience in R&D-driven, highly regulated, attractive growth markets to good use in helping Smith+Nephew deliver its strategy and 12-Point Plan. But perhaps the most exciting element for me in joining Smith+Nephew is the opportunity to help grow our global leading businesses, in Sports Medicine & ENT and Advanced Wound Management, combined with the significant benefits of fixing Orthopaedics, to create value for all stakeholders. I am convinced that companies such as Smith+Nephew with a clear purpose, embedded values and a focused ESG strategy will ultimately be the winners. What have your first impressions of S+N been? My first few months have been really fulfilling. Joining as Rupert took over as Chair and benefiting from Deepak’s deep knowledge of the MedTech sector, it is clear that we have a great opportunity to take the business forward in delivering its strategy and 12-Point Plan. I have been particularly struck by the Board’s balance, blending a strong combination of MedTech and general industry expertise. There is an openness to discuss challenging and difficult questions, living up to our culture pillars of Care, Courage and Collaboration. Most of all, the people I have met are committed to Smith+Nephew and to doing the right thing with quality, innovation and trust. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 107 |
| Board development Board Induction and Development Programme Our Board induction and development programmes are customised to address the specific needs and interests of each of our Directors. We focus the induction and development sessions on facilitating a greater awareness and understanding of our business, our stakeholders and the regulatory frameworks within which we operate. During 2023, we implemented induction programmes for our new Chair Rupert Soames as well as for Jez Maiden, Simon Lowth and John Rogers whose appointments to the Board were announced during the year. Induction programmes are tailored to each Board member’s individual skills and experiences and their roles on the Board and its Committees and include: – One-to-one meetings with senior executives to understand the organisation, the roles and responsibilities of our senior employees and specifically how we do things at Smith+Nephew; – Meetings with our external advisers including brokers, external counsel, remuneration consultants and auditors, to explain the legal and regulatory background to their role on our Board and how these matters are approached at Smith+Nephew; – Strategic presentations and site visits tailored to Executive and Non-Executive needs respectively in order to provide a strong foundation to learn about the organisation, its history, current and future opportunities and challenges and to give Board members an opportunity to ask questions and interact with our wider workforce. On an ongoing basis, we provide our Directors with both virtual and in-person opportunities to understand more about our business and the healthcare industry and support engagement with our teams and internal/external resources as appropriate, for example: – A number of Board Members have enjoyed holding employee listening sessions throughout the year both physically and virtually, where they have talked with employees and heard their views on what it means to work for Smith+Nephew. These sessions are discussed in more detail on page 113. – In November 2023, Board Members were invited to our Meet the Management session in London and were able to attend sessions virtually and in person which provided further insight into global product innovation strategy across each of our business units and our differentiated product pipeline, together with the opportunity to meet our investors. – All Board members have access to a library of Board induction and development internal materials within our Board resource portal as well as external thought leadership articles, materials, webinars and other resources. – We have arranged sessions on external perspectives on the Healthcare industry and macro trends/insights on topics of interest/relevance to the Board. The Chair regularly reviews the development needs of individual Directors and the Board as a whole. Chair Induction 2023 – Our 2023 Chair Induction programme was tailored with a strategic overview and introduction to Medtech and Medical Devices coupled with an immersive introduction to our Purpose, Culture Pillars and People through various meetings, visits and presentations from our Executive Committee and its direct reports. – External session on Global Healthcare Context and Trends. – External session on Medical Devices. – One-to-one sessions with each member of the Executive Committee, Investor Relations and Finance Global Leadership Team. – Visits to Hull, Croxley, Poland, Memphis, Pittsburgh and Andover sites in addition to the full Board Costa Rica site visit. – Informal office touchpoints with employees at the UK Group Head Office – Introduction at Global Senior Leadership Virtual Meeting. – Subject matter expert sessions on Medical Device Regulation, Healthcare Compliance, Enterprise Risk Management and Inventory/ Asset Utilisation. – Introductory sessions with external advisors, auditors, brokers and consultants. – Additional internal and external sessions upon request based on interest. Rupert Soames Chair of the Nomination & Governance Committee Composition, succession and evaluation continued Nomination & Governance Committee report continued 108 Smith+Nephew Annual Report 2023 |
| Costa Rica – Site Visit The Board also heard from teams on Sustainability and innovation at the site, highlighting success in sustainability and recycling programmes, quality frameworks, network footprint performance, engineering and quality innovation and business continuity and resilience in our Sports Medicine business and supply chain. The business focus of the afternoon was on accelerating growth in our Sports Medicine business. The sessions were framed to provide the Board with an overview of the impact of these projects on key stakeholders including employees, suppliers, customers, regulators, government, investors, local communities and the environment. The Board visited the Smith+Nephew Service Center Site on the second day of the site visit which is home to the GBS Costa Rica teams. The morning highlighted the S+N Service Center transformation journey, reviewing the GBS business model and transformation. Over lunch, Board members hosted two of our EIG groups (SWE and Pride) with rich discussions on remote working challenges, achieving gender balance and equality and diversity more broadly. Aligned with the ESG strategy, the Board heard more about the volunteering and other efforts that are supporting embedding culture and engagement priorities within the Costa Rica sites. In addition to the Chair Induction programme, the June 2023 Board site visit to Costa Rica focused on strategic and operational initiatives aligned with key priorities for the Board, including core business strategy, value creation opportunities, culture and workforce, operational transformation and ESG and stakeholder considerations. The visit began at our Coyol Facility with a tour of the Coyol Free Zone Business Park to orient Board members within the Medtech hub. The Board were provided with an overview of Costa Rica, its political, economic and social history and the business and Medtech context together with a history of the site. The Operations Site Tour and Product Demonstrations allowed Board members to see our clean rooms, microbiology lab and quality and operations facilities. The Board also enjoyed Product demonstrations for PICO◊ 14, WEREWOLF◊ and a number of other core product lines manufactured or assembled on site. Following the tour, Board members engaged in listening sessions with our teams with presentations on Operational Excellence in Costa Rica focusing on the One Smith+Nephew approach to site governance, culture and behaviours aligned with our purpose of Life Unlimited and culture pillars of Care, Courage and Collaboration. It was great to see the passion, authenticity, commitment and professionalism of the local teams in Costa Rica living our values. The organisation is working hard to capture and cascade best practice evidenced in this facility to other sites/teams.” Rupert Soames Chair The Costa Rica site is a world class facility and I was impressed by the pride, accountability and energy of the teams to drive continuous improvement.” John Ma Independent Non-Executive Director The site visit agenda was thoughtful and represented a good mix of listening sessions, presentations, product experience and customer voice. It was an insightful and uplifting experience that provided Board members with a great opportunity to understand more about the culture of the organisation and stakeholder perspectives.” Jo Hallas Independent Non-Executive Director STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 109 |
| Board effectiveness Evaluation Process The 2023 Review was conducted internally by the Senior Independent Director Marc Owen supported by the Company Secretary and sought to review all aspects of Board effectiveness. Questionnaire responses were provided by Board members (including both scores and narrative responses) in advance of one-to-one discussions between our Senior Independent Director and: (i) each Board Member; and (ii) the Company Secretary. Findings were summarised and presented to the Board for discussion in December 2023 with progress benchmarked against reviews from 2021 and 2022, noting the significant changes to the Board and management teams during the last 24 months. 2023 Progress and Conclusions Overall, the Smith+Nephew Board believes it is operating effectively as assessed both holistically and against the areas of focus for 2022: – During the year, the Board has spent more time on strategic discussions around the shape of portfolio, capital allocation and opportunities to drive longer-term strategic value creation. – The Board has regularly evaluated risks, opportunities and progress against key metrics within the 12-Point Plan, the Company’s restructuring programmes and systems and network optimisation, noting that considerable progress has been made over the last 18 months supported by stronger insights provided by management on the extent of the challenges and an ambitious plan on how to address them. – Board and Committees have discussed management succession planning, including monitoring of employee engagement scores and internal talent pipeline and development framework in particular for high-value roles within the Company. – Members of Exco and their direct reports have spent time over the year with Board members during inductions, site visits and strategic presentations fostering constructive discussion and continuing to build trust and credibility to strengthen governance. – Composition of the Board has been reviewed by the full Board and Nomination & Governance Committee and is considered appropriate with good progress made during the year on recruitment of two NEDs and CFO designate. Board members feel that views are properly heard and discussions allow individual members to have an impact. – The Board has had further discussions on the macro challenges, regulations and trends globally within healthcare and the Board and its Committees have been provided with additional sessions/materials from external experts to enhance understanding of the industry and the frameworks within which the Company operates. Areas of strength and focus for 2024 Several areas of strength around the operation of the Board and each Committee, induction process for new Board members, ESG strategy and risk management were noted. The areas of focus for 2024 are set out below: – Longer-term strategic drivers to deliver value creation: Continued focus on core areas of innovation, operational excellence/cost productivity, capital deployment and returns on capital and portfolio strategy. Formal opening session and informal closing session with Executive Directors at each Board meeting to provide more focused discussion and detail. – Succession Planning: Enhanced focus on management succession planning to attract, retain and develop senior leaders. Actions will include a biannual review of talent pipeline and gap analysis at Board level in addition to reports to Compliance & Culture and Remuneration Committees to review long-term people strategy with an emphasis on developing strong pipelines of senior leaders. Board listening sessions will also include talent attraction, retention and development topics as appropriate. – Commercial and operational transformation: Progress already made should continue, monitored by the Board through sessions with Exco and management. – Reporting: Further refinement of Board papers to focus on insights and Q&A and financial reporting to provide further analysis and insight on performance, risks and opportunities. – The 2024 review will be facilitated externally, with reviews in 2025 and 2026 to be facilitated internally and led by the Senior Independent Director, supported by the Company Secretary. Composition, succession and evaluation continued Nomination & Governance Committee report continued 110 Smith+Nephew Annual Report 2023 |
| Compliance & Culture Committee Report Our focus for 2024 will include: – Continued evaluation of ethics and compliance, regulatory, quality and cultural activities and trends and impact on the Strategy for Growth and 12-Point Plan. – Continued monitoring of the Company’s progress against our ESG strategy and plan, measuring actions against objectives and metrics and evaluating implementation. – Continued focus on stakeholder impact on Committee and Board decision making. – Monitoring the progress of the Company’s commitment to its net zero roadmap by 2045. – Monitoring the actions taken by management following the Board/employee listening sessions in 2023. – Amplification of the Board/employee listening sessions to include additional Non-Executive Board members to enable the full Board to hear from employees across the organisation and to monitor the corporate culture globally. – Evaluation of employee feedback gathered through the annual survey and other mechanisms to ensure the Board is aware of employee views and any resulting actions required by management. Recent survey results are discussed on pages 48–49. – Deeper understanding and focus on stakeholder needs and requests relating to ESG matters which are of interest to specific stakeholder groups. Responsibilities of the Compliance & Culture Committee Ethics and Compliance – Overseeing ethics and compliance programmes, strategies and plans. – Monitoring ethics and compliance process improvements and enhancements. – Assessing compliance performance based on monitoring, auditing and internal and external investigations data. – Discussion of allegations of significant potential compliance issues. – Receiving reports from the Chief Compliance Officer on ethics and compliance matters. – Reviewing implementation of the global data privacy compliance framework and other regulatory developments which impact our business. Sustainability – Overseeing the implementation of our ESG strategy and reviewing performance against targets and metrics, including the Scope 3 roadmap and ESG dashboard and metrics. – Receiving and discussing reports from the ESG Operating Committee focused on alignment of our ESG strategy with stakeholder requirements and our Strategy for Growth. Culture – Oversight of our relationship with stakeholders, including the employee voice and sustainability. – Receiving and assessing performance against Purpose and Culture, Talent, Engagement and Inclusion, Diversity and Equity (IDE). Quality and Regulatory Affairs (QRA) – Monitoring trends, activities and plans relating to regulatory and quality risks and events within the organisation aligned to our Strategy for Growth. – Receiving and assessing regular functional reports and presentations from the Chief Quality & Regulatory Affairs Officer on QRA strategy and operations. www.smith-nephew.com/ investor-centre/about-us/ governance corporate-documents-and-policies/ terms-of-reference/ The Terms of Reference for the Compliance & Culture Committee describe the role and responsibilities of this Committee more fully and can be found on our website. Membership Member from Meetings attended Marc Owen (Chair) March 2018 4/4 John Ma December 2021 4/4 Katarzyna Mazur-Hofsaess1 April 2021 3/4 Angie Risley April 2020 4/4 Bob White July 2020 4/4 In 2023, the Committee held four meetings. The Chief Executive Officer, Chief Financial Officer, Group General Counsel and Company Secretary, the Chief Compliance Officer, the Chief Quality & Regulatory Affairs Officer, Chief HR Officer, President of Global Operations and VP ESG also attended all or part of the meetings by invitation. 1 Katarzyna Mazur-Hofsaess was not present at the meeting held on 4 December 2023 due to a prior statutory commitment. The Board is committed to a strong focus on ethics and compliance, regulatory, quality and culture to support our Strategy for Growth and 12-Point Plan.” Marc Owen Chair of the Compliance & Culture Committee Compliance & Culture STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 111 |
| Ethics and Compliance As stated in the Code of Conduct, the sustainability of our business depends on doing business the right way and ensuring that we work with third parties who adhere to business principles consistent with our own. The Chief Compliance Officer provided regular reports to enable the Committee to evaluate the effectiveness of the Global Compliance programme and understand the audit, monitoring and continuous improvement activities undertaken to ensure our ethics and compliance programme continues to evolve aligned to our Strategy for Growth and the 12-Point Plan. The Committee is provided with updates on allegations of potentially significant issues which are raised through the Company’s hotline or to our Compliance team and the Company’s response to such matters. It also receives an annual review of investigations, actions taken to address substantiated matters and developing trends. The Committee received updates on potentially significant findings from compliance audits and oversight actions, including detail of mitigating actions to address findings. On an annual basis the Committee receives a trend analysis of audit findings and root cause analysis with details of any program changes required to address evolving trends. The Committee continues to receive a report on the annual self-assessment of the Compliance programme against the US Department of Justice Evaluation of Corporate Compliance programs guidance. The reports to the Committee demonstrate that the organisation has established mature processes and controls over compliance and ethics reporting and investigations. During 2023, the Committee also received updates with a regional focus on our Compliance programmes in China and the US which demonstrate how the global programme is adapted to mitigate market specific risks. Sustainability/ESG In 2023, sustainability and ESG matters more broadly received a refined focus and scrutiny from the Committee. The Committee reviewed the Company’s ESG strategy early in the year to ensure alignment with our Strategy for Growth, the 12-Point Plan and key stakeholder expectations. The Committee received updates throughout the year from the President Global Operations and newly appointed Vice President ESG on our performance against People, Planet and Product initiatives. Utilising enhanced dashboards and reporting following the establishment of the ESG Operating Committee, the Committee monitors management actions taken and tracks progress against the organisation’s ESG objectives through KPIs, metrics and leading indicators. Driven by increasing requirements by our Vice President ESG and the ESG Operating Committee for the organisation to align with and demonstrate shared sustainability objectives with a number of our stakeholders, the Committee reviewed our sustainability objectives holistically to align with strategy and approved updates to volunteering and sustainable packaging. During the site visit to Costa Rica in June 2023, the Committee was able to learn more about our sustainability initiatives in both our manufacturing operations and corporate facilities. In August 2023, the Committee discussed annual performance metrics as a member of the Dow Jones Sustainability Index to understand how the Company benchmarks against others in the industry. In December 2023, the Committee analysed and discussed the proposed remuneration ESG metrics for our Performance Share Programme and engaged in further discussions on stakeholder priorities to inform the organisation’s global ESG strategy in 2024. The Committee Chair continues to engage with investors, governance teams and other stakeholders on sustainability topics. Since the year end, the Committee has also approved the 2023 Sustainability Report. Quality and Regulatory Affairs Product safety and effectiveness is at the foundation of our business. Regulatory authorities across the world implement and enforce a complex series of laws and regulations that govern the design, development, approval, manufacture, labelling, marketing and sale of healthcare products. The Committee received and reviewed summary reports at each meeting of the Company’s performance against internal and external KPIs and metrics in order to ensure oversight of the quality and regulatory activities of our business aligned to our Strategy for Growth and the 12-Point Plan. At each meeting, the Committee received a briefing on key quality and regulatory matters from the Chief Quality & Regulatory Affairs Officer. The Committee reviewed results of external regulatory inspections and audits conducted by the FDA and other regulatory agencies including the progress being made on continuous improvement programs and activities. The Committee also discussed results of internal quality audits and key performance metrics associated with critical quality and regulatory compliance processes. The Committee received reports regarding preparation for emerging regulations applicable to our business and also received an update on the closure of our EU Medical Device Regulation program and implementation of the compliance framework. During the year, the Committee reviewed progress in areas of focus including quality assurance program improvements at key manufacturing sites across the business and our continued efforts on Quality System simplification and optimisation leading to continued efficiency across our network. Compliance & Culture continued Compliance & Culture Committee report continued 112 Smith+Nephew Annual Report 2023 |
| Culture The Company’s core purpose of Life Unlimited and the supporting culture pillars of Care, Courage and Collaboration continue to drive performance and accountability throughout the organisation globally. Our strategic objectives and culture pillars provide alignment across our business and stronger understanding by employees of their role in supporting our collective success. At each meeting, the Committee received briefings and updates on culture from the Chief HR Officer demonstrating progress in key areas. The specific focus for 2023 centred around the Employee Experience, People Leader Capabilities and Organisational Effectiveness and Embedding Change. The Committee was pleased to receive updates on the Employee Experience focused on workplace flexibility initiatives, wellbeing plans, IDE strategy and implementation and tracking of internal and external KPIs and metrics and employee engagement through the annual survey. Discussions at the Committee on People Leader Capabilities focused on refreshed leadership programmes aligned to our Commitments and the People Leader hub was launched to provide enhanced resources for manager development and self-led learning. The Committee also evaluated the impact of culture on 12-Point Plan engagement and change management and the way in which our Commitments were embedded to focus on driving strategy and performance. The Committee was made aware of the positive impact of and events held by our Employee Inclusion Groups (EIGs) including the consolidation of women employee EIGs into the S+N Women’s Network, the expansion of the ethnicity EIG Unite to include a Latin Heritage EIG, as well as continued progress for the EIGs focused on generations, veterans, mental health and physical wellbeing, the differently abled and LGBTQ+. The 2023 Gallup global employee survey results were shared with the Committee. These results, which allow Smith+Nephew to benchmark against similar companies in our industry, showed a strong employee response rate of 88%. The Committee was pleased to see that the survey highlighted overall strengths in employee connection to the purpose of Life Unlimited and an overall upward trend of our results compared with last year. For specific issues where employees may not feel comfortable articulating their views, we have a whistle-blowing policy and confidential line, as outlined in the Ethics and Compliance section of this report. Employees The Board proactively supports and further reinforces the Purpose of Life Unlimited and culture pillars of Care, Courage and Collaboration through informal board listening sessions. These sessions give the Board the opportunity to hear directly from employees and understand thoughts and perspectives on a number of topics in connection with our purpose and culture. Five Board listening sessions were held in 2023 with key enterprise-wide themes being raised and discussed such as portfolio strategy, innovation and simplification; embedding our leadership commitments and accountability; simplification, agility and speed of decision-making; celebrating purpose, talent, culture and organisational achievements; talent development and ESG engagement across the enterprise. One of the sessions was hosted by John Ma with the Greater China team in order to hear their views on strategy, operations, culture and people. The Committee will continue to track and monitor the implementation of actions arising from these listening sessions as part of its responsibilities in 2024. The Board listening session programme has been amplified for 2024 to include additional Non-Executive Directors in addition to Committee members and will focus on several key topics including the new Global Business Unit model, new leaders, remuneration, 12-Point Plan initiatives within operations and the ways in which corporate functions enable success. How we monitor culture 2023 Interactions and Engagement Looking ahead to 2024 The Employee Experience The Committee received updates on workplace flexibility, wellbeing plans and initiatives, IDE Strategy and IDE Council and annual engagement survey results and plans. Listening sessions aligned to hear employee voices in key areas of focus. The Committee will continue to engage and focus on understanding Smith+Nephew’s well-being plan and initiatives and Allyship as part of the IDE strategy and plan. People Leader Capabilities The Committee received updates on leadership and management programmes which were refreshed to align to our Commitments and the launch and implementation of the People Leader Hub provided further resources for manager self learning. Listening sessions for business unit and regional leadership teams facilitated discussions on leadership and our commitments. The Committee to receive updates on the programmes Smith+Nephew will deliver to improve leadership capabilities and how Smith+Nephew will continue to reinforce our Leadership Commitments and behaviours. Organisational Effectiveness and Embedding Change Updates provided to the Committee on culture and change management relating to 12-Point Plan engagement and delivery embedding our Commitments; focus on talent development pipelines. Listening sessions on simplification and speed of decision making supported discussions on organisational effectiveness and pace of change. Discussions with the Committee to include details on operationalising the discipline of the 12-Point Plan, embedding the new organisational structure, tracking key internal and external metrics for ESG and review of performance enablement strategy. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 113 |
| Audit, Risk and Internal Control Audit Committee Report 1 Designated financial expert under the SEC Regulations or recent and relevant financial experience under the UK Corporate Governance Code. 2 Erik Engstrom stepped down from the Board and as a member of the Committee on 31 December 2023. 3 Jez Maiden was appointed to the Board and as a member of the Committee on 14 September 2023 and will become Chair of the Committee on 1 March 2024. 4 Simon Lowth became a member of the Committee on 1 January 2024. * All members of the Committee are deemed to be independent Directors. www.smith-nephew.com The Terms of Reference of the Audit Committee describe the role and responsibilities more fully and can be found on our website. Dear Fellow Shareholder, During 2023, outside of the routine matters undertaken by the Committee (as set out in its Terms of Reference), the Committee has focused on the following: – Monitored progress on and enhancement of our ESG reporting plan including TCFD. – Continued oversight of the governance and maturity plan for our IT framework and controls. – Reviewed the Group’s cyber resilience. – Monitored the transition of the Group’s External Auditors. – Received progress reports on the change in the finance operating model. – Assessed the proposals within the Non-Financial Reporting Review, the changes to the UK Corporate Governance Code and the minimum standards for Audit Committees. Membership* Member from Meetings attended Rick Medlock (Chair)1 April 2020 7/7 Erik Engstrom2 Jan 2015 7/7 Jez Maiden3 Sept 2023 3/3 Marc Owen Oct 2017 7/7 Jo Hallas Sept 2022 7/7 The Committee met seven times during the year, with meetings timed to coincide with the financial and reporting cycles of the Company. In addition, the Committee met with both the Company’s external auditor and Group Head of Internal Audit without management present. Focus for 2024: – Continued oversight of the governance and maturity of our IT framework and controls, and of the planned upgrading of Enterprise Resource Planning (ERP) systems within the Group – Monitoring of the continuing investment in cyber resilience – Continued focus on the Group’s Enterprise Risk Management framework and the evolution of the principal and emerging risks we face – Supporting and monitoring the transition of the external audit to the Deloitte team – Developing the existing framework of controls across the Group in order to meet new requirements under the UK Corporate Governance Code, particularly the monitoring and review of the effectiveness of material controls from 2026 – Ensuring that the Committee is compliant with the UK FRC’s Minimum Standard: Audit Committees and the External Audit – Oversee adoption of a new internal reporting structure and financial operating model within the Group, ensuring that reporting and controls remain effective – Supporting new people in key roles within the Finance function, including a new CFO and new Head of Group Internal Audit. Responsibilities of the Audit Committee The Committee’s key roles are to: – Ensure the integrity of the Company’s financial reporting to shareholders and any announcements relating to the Group’s financial performance. – Ensure financial statements comply with UK and US statutory requirements. – Review the content of the Annual Report and advise the Board on whether, taken as a whole, it is fair, balanced and understandable and provides the information necessary for shareholders to assess the Company’s performance, business model and strategy. – Monitor the effectiveness of internal controls and compliance with the 2018 UK Corporate Governance Code and the SOX Act. – Ensure the effectiveness of the internal audit function, agree audit plans and consider outcomes of internal audits. – Review the operation of the Group’s risk management framework. – On behalf of the Board, carry out a robust assessment of the principal and emerging risks facing the Group. – Ensure the effectiveness of the external audit function, agree the scope of the audits (including materiality thresholds and areas of risk for focus) and the auditor’s fees and terms of engagement. – Consider any reported frauds and any concerns raised by the Company’s whistle-blowing process. – Oversee other matters, including cybersecurity, IT governance, ESG reporting, tax and treasury. As announced, I stepped down as Chair of this Committee on 1 March. I therefore take this opportunity to thank all of the Committee members for their support and focus during my tenure and to Erik Engstrom for his 9-year service to the Committee. We welcomed Simon Lowth to the Committee in January and I wish Jez Maiden every success in his new role as Chair of the Committee.” Rick Medlock Audit Committee Chair 114 Smith+Nephew Annual Report 2023 |
| Significant matters related to the financial statements We considered the following key areas of judgement in relation to the 2023 financial statements and at each half year and quarterly trading report, which we discussed in all cases with management and the External Auditor: Valuation of inventories A feature of the Orthopaedics business unit (which accounts for approximately 66% of the Group’s total inventory and approximately 82% of the total provision for excess and obsolete inventory) is the high level of product inventory required, some of which is located at customer premises and is available for customers’ immediate use. Complete sets of products, including large and small sizes, have to be made available in this way. These sizes are used less frequently than standard sizes and towards the end of the product life cycle are inevitably in excess of requirements. Adjustments to carrying value are therefore required to be made to orthopaedic inventory to anticipate this situation. These adjustments are calculated in accordance with a formula based on levels of inventory compared with historical usage. This formula is applied on an individual product line basis and typically is first applied when a product group has been on the market for two years. This method of calculation is considered appropriate based on experience, but it does involve management estimation of customer demand, effectiveness of inventory deployment, length of product lives and phase-out of old products. Our action At each quarter end, we received reports from, and discussed with, management the level of provisioning and material areas at risk. The provisioning level was 21% at 31 December 2023 (2022: 21%). We challenged the basis of the provisions and concluded that the proposed levels were appropriate and have been consistently estimated. Liability provisioning The recognition of provisions for legal disputes is subject to a significant degree of estimation. Provision is made for loss contingencies when it is considered probable that an adverse outcome will occur and the amount of the loss can be reasonably estimated. In making its estimates, management takes into account the advice of internal and external legal counsel and uses third-party actuarial modelling where appropriate. Provisions are reviewed regularly and amounts updated where necessary to reflect developments in the disputes. The ultimate liability may differ from the amount provided depending on the outcome of court proceedings and settlement negotiations or if investigations bring to light new facts. Our action As members of the Board, we receive regular updates from the Group General Counsel & Company Secretary. These updates form the basis for the level of provisioning. The Group carries a provision relating to potential liabilities arising on its portfolio of metal-on-metal hip products of $149 million as of 31 December 2023. We received detailed reports from management on this position, including the actuarial model used to estimate the provision, and challenged the key assumptions including the number of claimants and projected value of each claim. The provisions for legal matters have decreased by $105 million during the year, primarily due to utilisation of the metal-on-metal provision. We have determined that the proposed levels of provisioning at year end of $159 million included within ‘provisions’ in Note 17.1 in 2023 (2022: $264 million) were appropriate in the circumstances. Impairment In carrying out impairment reviews of goodwill and acquisition intangible assets, a number of significant assumptions have to be made when preparing cash flow projections. These include the future rate of market growth, discount rates, the market demand for the products acquired, the future profitability of acquired businesses or products, levels of reimbursement and success in obtaining regulatory approvals. If actual results should differ or changes in expectations arise, impairment charges may be required, which would adversely impact operating results. Our action We reviewed management’s reports on the key assumptions with respect to goodwill and acquisition intangible assets – particularly the forecast future cash flows and discount rates used to make these calculations. We reviewed in detail management’s conclusion that the goodwill and acquisition intangible assets related to Engage Surgical and agreed that they should be fully impaired. We had a particular focus on goodwill impairment testing for the Orthopaedics CGU. Although the level of headroom has increased, it is sensitive to a reasonably possible change in assumptions. We challenged the downside sensitivity analyses undertaken and concluded that the carrying value of these assets, excluded for Engage, is reasonable and appropriately supported by the cash flow projections. We have also considered the disclosure surrounding these reviews, and concluded that the review and disclosure were appropriate. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 115 |
| Audit, Risk and Internal Control continued Audit Committee report continued Other matters related to the financial statements As well as the identified significant matters, other matters that the Audit Committee considered during 2023 were: Going concern The impact of a global economic recession has been considered as part of the adoption of the going concern basis in these financial statements. We reviewed three-year projections as part of the Group’s Strategic Plan, and also more detailed cash flow scenarios to 29 March 2025 for going concern purposes and concurred with management that the continued adoption of the going concern basis is appropriate. Taxation The Group operates in numerous tax jurisdictions around the world and is subject to factors that may affect future tax charges. We annually review policies and approve the principles for management of tax risks. We review quarterly reports from management evaluating the existing tax profile, tax risks and tax provisions. Based on a thorough report from management of tax liabilities and our challenge of the basis of any tax provisions recorded, we concluded that the levels of provisions and disclosures were appropriate. Post-retirement benefits The Group has post-retirement defined benefit pension schemes, which require estimation in setting the assumptions. We received a report from management setting out their proposed assumptions for the UK and US schemes and concurred with management that these assumptions were appropriate. We also reviewed the assumptions, accounting and disclosures for the UK scheme buy-in and US scheme buy-out and deemed them appropriate. Climate change The impact of climate change has been considered as part of our review of the impairment testing of goodwill and acquired intangible assets, and the going concern assessment. We have also considered the disclosures on climate change and considered them appropriate. Since the year end We have reviewed the results for the full year 2023 and the Annual Report 2023, and have concluded that they are fair, balanced and understandable. In coming to this conclusion, we have considered the description of the Group’s strategy and key risks, the key elements of the business model, which is set out on pages 16–17, risks and the key performance indicators and their link to the strategy. External auditor Independence of external auditor Following a competitive tender in 2014, KPMG was appointed external auditor of the Company in 2015. We are satisfied that KPMG is fully independent from the Company’s management and free from conflicts of interest. Our Auditor Independence Policy, which ensures that this independence is maintained, is available on the Company’s website. We believe that the implementation of this policy helps ensure that auditor objectivity and independence is safeguarded. The policy also governs our approach when we require our external auditor to carry out non-audit services, and all such services are strictly governed by this policy. The Auditor Independence Policy also governs the policy regarding audit partner rotation with the expectation that the audit partner will rotate at least every five years. Paul Nichols was appointed as our senior lead audit partner on 1 January 2022. The Audit Committee confirms it has complied with the provision of the Competition and Markets Authority (CMA) Order 2014. Effectiveness of external auditor We conducted a review into the effectiveness of the external audit as part of the 2023 year-end process, in line with previous years. We sought the views of the Committee and key members of the finance management team, considered the feedback from this process and shared it with management. During the year, we also considered the inspection reports from the Audit Oversight Board in the UK and determined that we were satisfied with the audit quality provided by KPMG. The Audit Committee receives feedback from KPMG at each meeting where management present their summary of critical accounting estimates as at each quarter end and during the Committee’s private sessions with the auditors which are held throughout the year. Overall, therefore, we concluded that KPMG had carried out their audit for 2023 effectively. Change in Auditor Deloitte LLP has made good progress on the transition process further to the accelerated audit tender process reported last year. They are to be appointed auditors of the Company from 1 January 2024, subject to shareholders’ approval at the Annual General Meeting in May 2024. KPMG has been our auditor since 2015 and 2023 is their last year as our auditor. We would like to thank them for their service during their time as our auditor. Appointment of external auditor at Annual General Meeting Resolutions will be put to the Annual General Meeting to be held on 1 May 2024 for the appointment of Deloitte LLP as the Company’s auditor and authorising the Board to determine its remuneration, on the recommendation of the Audit Committee in accordance with the CMA Order 2014. 116 Smith+Nephew Annual Report 2023 |
| Disclosure of information to the auditor In accordance with Section 418 of the Companies Act 2006, the Directors serving at the time of approving the Directors’ Report confirm that, to the best of their knowledge and belief, there is no relevant audit information of which the auditor, KPMG, is unaware and the Directors also confirm that they have taken reasonable steps to be aware of any relevant audit information and, accordingly, to establish that the auditor is aware of such information. Non-audit fees paid to the auditor Non-audit fees are subject to approval in line with the Auditor Independence Policy which is reviewed annually and forms part of the Terms of Reference of the Audit Committee. The Audit Committee recognises the importance of the independence of the external auditor and ensures that the auditor’s independence should not be breached. The Audit Committee ensures that the auditor does not receive a fee from the Company or its subsidiaries that would be deemed large enough to impact its independence or be deemed a contingent fee. The total fees for permitted non-audit services shall be no more than 70% of the average of the fees paid in the last three consecutive financial years for the statutory audits of the Company and its subsidiaries. Any pre-approved aggregate or individual amounts up to $25,000 may be authorised by the Group Treasurer and SVP Group Finance respectively and amounts up to $50,000 by the Chief Financial Officer. Any individual amount over $50,000 must be pre-approved by the Chair of the Audit Committee. If unforeseen additional permitted services are required, or any which exceed the amounts approved, again pre-approval by the Chair of the Audit Committee is required. The following reflects the non-audit fees incurred with KPMG in 2023, which were approved by the Chair of the Audit Committee. 2023 $ million 2022 $ million Audit-related services 0.3 0.4 Audit-related fees in 2023 primarily consisted of routine services and were deemed by the Committee not to infringe auditor objectivity or independence. The ratio of non-audit fees to audit fees for the year ended 31 December 2023 is 0.03. The ratio of non-audit fees to audit fees for the year ended 31 December 2022 was 0.04. Full details are shown in Note 3.2 to the Notes to the Group accounts. Audit fees paid to the auditor Fees for professional services provided by KPMG, the Group’s independent auditor in each of the last two fiscal years, in each of the following categories were: 2023 $ million 2022 $ million Audit fees 10.0 9.4 Audit-related fees 0.3 0.4 Total 10.3 9.8 Internal audit The internal audit team, which reports functionally to the Audit Committee, carries out risk-based reviews across the Group. These reviews examine the management of risks and controls over financial, operational, commercial, IT and transformation programme activities. The audit team, led by the Group Head of Internal Audit, consists of appropriately qualified and experienced employees. Third parties may be engaged to support audit work as appropriate. The Group Head of Internal Audit has direct access to, and has regular meetings with, the Audit Committee Chair and prepares formal reports for Audit Committee meetings on the activities and key findings of the function, together with the status of management’s implementation of recommendations. The Audit Committee has unrestricted access to all internal audit reports, should it wish to review them. During the year, the team completed 35 audits and reviews across the Group. These covered significant aspects of all 11 Principal Risks and included: financial controls effectiveness reviews across the EMEA, APAC, US and LATAM regions; IT and various programme assurance reviews ranging from IT disaster recovery planning and cyber maturity; and an ERP pre-implementation review in Japan. Group-level reviews included enterprise risk management effectiveness, business continuity management arrangements, ESG governance, field inventory controls, trade compliance activities, 12-Point Plan governance and fraud risk management effectiveness. Management have taken swift action to implement Internal Audit’s recommendations. The team was able to travel to a number of locations, following the relaxing of Covid-related restrictions and there was continued use of data extraction and analysis techniques during all work. The function carries out its work in accordance with the standards and guidelines of the Institute of Internal Auditors. Its performance is annually assessed using a structured questionnaire, allowing non-executive, executive and senior management, plus the external auditor, to comment on key aspects of the function’s performance. In addition, in early 2022, Grant Thornton carried out an evaluation of the function and concluded that it was operating effectively. The Audit Committee, which re-approved the function’s charter in December 2023, has satisfied itself that adequate, objective internal audit standards and procedures exist within the Group and that the Internal Audit function is effective. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 117 |
| Audit, Risk and Internal Control continued Audit Committee report continued Risk management programme Whilst the Board is responsible for ensuring oversight of strategic risks relating to the Company, determining an appropriate level of risk appetite, and monitoring risks through a range of Board and Board Committee processes, the Audit Committee is responsible for ensuring oversight of the processes by which operational risks, relating to the Company and its operations are managed and for reviewing financial risks and the operating effectiveness of the Group’s Risk Management process. During the year, we reviewed our Risk Management processes and progress was discussed at our meetings in February, April, July, and December. We approved the Risk Management programme for 2023 and monitored performance against that programme, specifically reviewing the work undertaken by the risk champions across the Group, identifying the risks which could impact their areas of our business. The Risk Management programme followed the risk management policy and manual communicated company-wide in 2023. This programme combines a ‘bottom-up’ approach (whereby risks are identified within business areas by local risk champions working with their leadership teams), with a ‘top-down’ approach (when the Executive Committee meets as the Risk Committee to consider the risks facing the Group at an enterprise level). Throughout the year, the Audit Committee maintained oversight of this programme. We reviewed the Principal Risks identified and the heat maps prepared by management showing how these risks were being managed. We considered where the risk profile was changing. Since the year end, we have reviewed a report from the Group Head of Internal Audit into the effectiveness of the Risk Management programme throughout the year. We considered the Principal Risks, the actions taken by management to review those risks and the Board risk appetite in respect of each risk. We concluded that the Risk Management process during 2023 and up to the date of approval of this Annual Report was effective. Work will continue in 2024 and beyond to continue to enhance the process. » Risk Report (pages 67–77) Viability Statement We also reviewed management’s work in conducting a robust assessment of those risks which would threaten our business model and the future performance or liquidity of the Company, including its resilience to the threats of viability posed by those risks in severe but plausible scenarios. Management have considered various scenarios in assessing the impact of a global economic recession, with the key judgement applied being the speed and sustainability of the return to a normal volume of elective procedures in key markets. This assessment included stress and sensitivity analyses of these risks to enable us to evaluate the impact of a severe but plausible combination of risks. We then considered whether additional financing would be required in such eventualities. Based on this analysis, we recommended to the Board that it could approve and make the Viability Statement on page 78. Going concern The Group’s business activities, together with the factors likely to affect its future development, performance and position are set out in the financial review on pages 20–23 and the Principal Risks on pages 69–77. The financial position of the Group, its cash flows, liquidity position and borrowing facilities are described on pages 20–23. In addition, the Notes to the Group accounts include: the Group’s objectives, policies and processes for managing its capital; its financial risk management objectives; details of its financial instruments and hedging activities; and its exposure to credit risk and liquidity risk. The Group has considerable financial resources and its customers and suppliers are diversified across different geographic areas. As a consequence, the Directors believe that the Group is well placed to manage its business risk successfully despite the ongoing uncertain economic outlook. The Group has considered several scenarios (refer to Viability Statement on page 78 and 79) including the continued uncertainty as to the future impact on the financial performance and cash flows of the Group as a result of a global economic recession as part of the adoption of the going concern basis in these financial statements. The Directors have a reasonable expectation that the Group has adequate resources to continue in operational existence for the foreseeable future. Thus they continue to adopt the going concern basis for accounting in preparing the annual financial statements. Management also believes that the Group has sufficient working capital for its present requirements. Evaluation of internal controls Management are responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a–15(f) and 15d–15(f) under the US Securities Exchange Act of 1934. There is an established system of internal control throughout the Group and our country business units. The main elements of the internal control framework include: – The management of each country and Group function is responsible for the establishment, maintenance and review of effective financial controls within their business unit or function. – The Group’s IT organisation is responsible for the establishment of effective IT controls within the core financial systems and underlying IT infrastructure. – The Financial Controls & Compliance Group has responsibility for the review of the effectiveness of controls operating in the countries, functions and IT organisation, by either: performing testing directly, reviewing testing performed in-country, or utilising a qualified third party to perform this management testing on its behalf. – The Group Finance Manual sets out financial and accounting policies, and is updated regularly. The Group’s Minimum Acceptable Practices (MAPs) internal control framework is updated annually to adjust to changing business processes or to leverage leading practices. The business is required to self-assess their level of compliance with the MAPs on a monthly basis and remediate any gaps. 118 Smith+Nephew Annual Report 2023 |
| – MAPs compliance is validated through spot-checks conducted by the Financial Controls & Compliance Group and Internal Audit, as well as during wider Internal Audit reviews performed throughout the year. We continue to leverage a technology solution to facilitate the real time monitoring of the operation and testing of controls and have established KPIs for control performance. – There are clearly defined lines of accountability and delegations of authority. – The Internal Audit function executes a risk-based annual work plan, as approved by the Audit Committee. The Audit Committee reviews reports from Internal Audit on their findings on internal financial controls, including compliance with MAPs and from the SVP Group Finance and the heads of the Financial Controls & Compliance, Taxation and Treasury functions. – The Audit Committee reviews regular reports from the Financial Controls & Compliance Group with regard to compliance with the SOX (Sarbanes Oxley) Act. Additional complementary elements of our control environment include the following: – Business continuity planning, including preventative and contingency measures, back-up capabilities and the purchase of insurance. – Risk management policies and procedures including segregation of duties, transaction authorisation, monitoring, financial and managerial review and comprehensive reporting and analysis against approved standards and budgets. – A treasury operating framework and Group treasury team, accountable for treasury activities, which establishes policies and manages liquidity and financial risks, including foreign exchange, interest rate and counterparty exposures. Treasury policies, risk limits and monitoring procedures are reviewed regularly by the Audit Committee or the Finance & Banking Committee, on behalf of the Board. – Our published Group tax strategy which details our approach to tax risk management and governance, tax compliance, tax planning, the level of tax risk we are prepared to accept and how we deal with tax authorities, which is reviewed by the Audit Committee on behalf of the Board. – The Audit Committee reviews the Group whistle-blower procedures to ensure they are effective. This system of internal control has been designed to manage rather than eliminate material risks to the achievement of our strategic and business objectives and can provide only reasonable, and not absolute, assurance against material misstatement or loss. Because of inherent limitation, our internal controls over financial reporting may not prevent or detect all misstatements. In addition, our projections of any evaluation of effectiveness in future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Entities where the Company does not hold a controlling interest have their own processes of internal controls. We have reviewed the effectiveness of the Company’s internal controls over financial reporting. The Company’s assessment included documenting, evaluating and testing the design and operating effectiveness of its internal controls over financial reporting. Based on this evaluation, we have satisfied ourselves that we are meeting the required standards and that our internal control over financial reporting is effective both for the year ended 31 December 2023 and up to the date of approval of this Annual Report. No concerns were raised with us in 2023 regarding possible improprieties in matters of financial reporting. This process complies with the FRC’s ‘Guidance on Risk Management, Internal Control and Related Financial and Business Reporting’ under the UK Corporate Governance Code and additionally contributes to our compliance with the obligations under the SOX Act and other internal assurance activities. There has been no change during the period covered by this Annual Report that has materially affected, or is reasonably likely to materially affect, the Group’s internal control over financial reporting. The Board is responsible overall for reviewing and approving the adequacy and effectiveness of the risk management framework and the system of internal controls over financial, operational (including quality management and ethical compliance) processes operated by the Group. The Board has delegated responsibility for this review to the Audit Committee. The Audit Committee, through the Internal Audit function, reviews the adequacy and effectiveness of internal control procedures and identifies any significant weaknesses and ensures these are remediated within agreed timelines. The latest review covered the financial year to 31 December 2023 and included the period up to the approval of this Annual Report. The main elements of this review are as follows: – The Chief Executive Officer and the Chief Financial Officer evaluated the effectiveness of the design and operation of the Group’s disclosure controls and procedures as at 31 December 2023. Based upon the evaluation, the Chief Executive Officer and Chief Financial Officer concluded on 26 February 2024 that the disclosure controls and procedures were effective as at 31 December 2023. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 119 |
| Audit, Risk and Internal Control continued Audit Committee report continued – Management are responsible for establishing and maintaining adequate internal control over financial reporting. Management assessed the effectiveness of the Group’s internal control over financial reporting as at 31 December 2023 in accordance with the requirements in the US under section 404 of the SOX Act. In making that assessment, they used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013). Based on their assessment, management concluded and reported that, as at 31 December 2023, the Group’s internal control over financial reporting was effective based on those criteria. Having received the report from management, the Audit Committee reports to the Board on the effectiveness of controls. KPMG, an independent registered public accounting firm, audited the financial statements included in the 2023 Annual Report, containing the disclosure required by this item, issued an attestation report on the Group’s internal control over financial reporting as at 31 December 2023. Code of Ethics for Senior Financial Officers We have adopted a Code of Ethics for Senior Financial Officers, which applies to the Chief Executive Officer, the Chief Financial Officer, the SVP Group Finance and the Group’s senior financial officers. There have been no waivers to any of the Code’s provisions nor have there been any substantive amendments to the Code during 2023 or up until 26 February 2024. A copy of the Code of Ethics for Senior Financial Officers can be found on our website. In addition, every individual in the finance function certifies to the Chief Financial Officer that they have complied with the Finance Code of Conduct. Rick Medlock Chair of the Audit Committee The Committee has satisfied itself that the Smith & Nephew plc 2023 Annual Report is fair, balanced and understandable. The Committee therefore supports the Board in making its formal statement on page 156. 120 Smith+Nephew Annual Report 2023 |
| Remuneration 1 Roberto Quarta stepped down from the Board and as a member of the Committee on 15 September 2023. 2 Rupert Soames joined the Board and the Committee on 26 April 2023. 3 Jez Maiden joined the Board and as a member of the Committee on 14 September 2023. Directors’ Remuneration Report Angie Risley Chair of the Remuneration Committee Focus for 2024 – Propose to shareholders a package of changes to our Remuneration Policy in respect of US Executive Directors’ long-term incentive plans, with the objective of enabling the Company to effectively compete for, attract and retain the best people. – Continue to monitor the remuneration and initiatives to support the wider workforce, making interventions where required. – Focus on development of internal talent pipeline to ensure longer-term succession, promote stability and drive value creation. – Approving the ESG metrics for the 2024 Performance Share Programme. – Monitor the performance versus targets for the short and long-term awards under the Performance Share Programme and Annual Bonus Plan, rewarding pay for performance. The Committee’s role The Committee’s role is to ensure that our Remuneration Policy and practices are aligned to the business strategy and promote long-term sustainable success. We make sure that the Remuneration of our Executive Directors and leadership team is aligned to the Company’s purpose and values and is clearly linked to the successful delivery of business performance and drives value creation. We engage with shareholders as appropriate to ensure that the Committee hears and understands their views which in turn assists the Committee to shape its proposals. Membership Member from Meetings attended Angie Risley (Chair) Sept 2017 9/9 Roberto Quarta1 April 2014 4/5 Rupert Soames2 April 2023 6/6 Jez Maiden3 Sept 2023 4/4 Bob White July 2020 9/9 Dear Fellow Shareholder, The core focus of the Remuneration Committee this year has been on attraction, retention and development of talent across the organisation and understanding the needs of the wider workforce more broadly, particularly in the context of the cost of living globally. With our focus on ensuring longer-term stability to drive value creation for the organisation, the Committee has identified a pressing need to take proactive steps to ensure the organisation is able to compete for, attract and retain key talent in priority markets, most notably the US, in support of the delivery of our strategy and the 12-Point Plan. The Board is cognisant of the fact that stability and continuity of our people is critical to deliver on our commitments. This will enable senior management to successfully develop the internal talent pipeline and succession plans in the longer-term. The Company has seen several changes at Executive Director level during my six years as Chair of the Committee; Deepak Nath is the Company’s fourth CEO during a five-year period and with every CEO change there has been an increase in downstream senior leadership attrition across the business. These CEO and senior management changes have led to loss of talent in our internal pipeline which has had a longer-term negative impact on the Company. The Board believes that the Company needs to be able to offer employees who are normally resident in the US, including Executive Directors, remuneration packages which can compete with the Company’s US Medtech peers of a similar size and provides them with an opportunity to be compensated in accordance with US market norms. The global MedTech market is very heavily weighted to the US. Although our primary listing is in London, less than 4% of our revenues arise in the UK, and over 50% arise in the US. Currently, our CEO and key senior operational leaders, are STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 121 |
| Remuneration continued Directors’ Remuneration Report continued based in the US. In terms of our global employees, 8% are based in the UK and around 40% in the US. Such a balance is common in the MedTech world but makes the Company an outlier within the FTSE and is the basis on which we believe the Company must be able to differentiate itself in terms of the remuneration packages it needs to offer. As a result of detailed work undertaken by the Remuneration Committee during 2023 with support from our remuneration advisers and the Board, it became apparent that both in terms of quantum and structure, whilst our policies are appropriate in most countries in which the Company operates, they were not aligned with long-term incentive plans for MedTech executives normally resident in the US. Under the current Remuneration Policy, the target value of Smith+Nephew’s total direct compensation package for the role of CEO falls materially below the lower quartile of Smith+Nephew’s Global MedTech peer group, which is based on comparably sized competitors in the industry with most based in the US. Further, the competitiveness gap with Global MedTech peers is not solely a matter of quantum. These peers predominantly apply a portfolio approach to LTI (long-term incentives) design, operating a combination of performance shares, restricted shares, and share options (the majority of which do not have performance conditions or an underpin). Based on our review, 18 of 21 Global MedTech peers use restricted shares, or options, or both, and these plans usually vest on a phased annual basis over three years, rather than vesting on a cliff edge basis at the end of three years, followed by a 2-year post-vesting holding period, as with Smith+Nephew’s Performance Share Programme (PSP). Annual Bonus deferral of any kind is also very rare among Global MedTech peers, and they do not typically operate post-vesting holding periods on LTI. In combination with the phased vesting schedule for LTI, senior executives at Global MedTech peers can typically expect to receive vested equity and cash far earlier than is the case at FTSE-listed companies like Smith+Nephew, all of which raises the perceived value of the package independently of quantum. The changes proposed in our Policy on LTIs for US Executive Directors are intended to move some way towards addressing these gaps in competitiveness with Global MedTech peers, both in terms of quantum and design. We would emphasise that the proposals do not seek to match the prevalent Global MedTech practice and in terms of quantum would only raise the target value of CEO total direct compensation to a level around the lower quartile of this market. Shareholder consultation on the 2024 Remuneration Policy Upon becoming Chair, Rupert met with a significant number of our larger investors and highlighted his concerns on the ability of the Company to compete for talent in the US. The great majority of those investors supported engagement in a formal consultation. With full support from the Board and Committee members on our approach, Rupert and I engaged or corresponded with 52 of our largest shareholders, comprising over 67% of the share capital of the Company, and key proxy advisors. We heard their views and comments on the proposed package of measures which helped us to shape the proposals that we are putting to the shareholder vote. During the consultation we were pleased to receive support and positive feedback from the majority of those we engaged with directly. Investors were aligned with the Board desiring to achieve greater stability within senior management. Whilst there was an acknowledgment that our proposals do not fit squarely within the four corners of the current UK Corporate Governance framework, investor governance teams acknowledged the compelling rationale for the Company to differentiate itself from other companies within the FTSE given the size and scale of its business and operational leadership in the US, its prior history of management attrition due to reported issues on pay and the driving need to be able to compete for, attract and retain talent in the US to ensure longer-term stability for the organisation. At the 2024 Annual General Meeting, we are therefore seeking shareholder approval for a new Remuneration Policy which reflects investor feedback. We understand that some investors would ideally wish to see financial performance meeting investor expectations in advance of increasing LTI, but in the Board’s view the changes must be implemented in the short-term to incentivise long-term stability. The new package of proposed measures for US Executive Directors will move our practices and structures nearer to, but not equivalent to, US market norms. The management team also has the ambition to cascade a similar market-competitive model to other relevant organisational levels below the Executive Director level. Overview of proposed changes for US Executive Directors We have determined that there is a clear business need to make changes to our remuneration policy in respect of US Executive Directors for the reasons set out above. The proposed changes are set out below: LTIP quantum and structure: In addition to a proposed increase to the maximum opportunity under the performance share programme (PSP), we plan to introduce a new restricted stock programme (RSP) for US Executive Directors to ensure our LTI structure more closely mirrors LTIP design found in the US market and MedTech peers. The proposed quantum and structure of the two plans are as follows: – Performance Share Programme (PSP): We are proposing a change to the quantum of the PSP for US Executive Directors to align with continued Board and investor focus on driving performance globally across the organisation. Performance will continue to be assessed over a three-year period with an additional post-vesting two year holding period. Maximum opportunity will increase from 275% to 300%. – Restricted Share Programme (RSP): We propose the introduction of a new RSP of 125% of base salary for US Executive Directors. Awards will vest in three equal tranches over a three-year period, contingent on a reasonable judgement underpin being met as determined by the Committee. Please see page 130 for further details on the reasonable judgement underpin. 122 Smith+Nephew Annual Report 2023 |
| Executive Directors who are not normally resident in the US will not participate in the RSP and will only participate in the PSP; they will not receive any additional quantum under the LTIP than that already offered under the current Policy in accordance with market norms in the countries where they live and work. It should be noted that the Company has, in the past, used RSPs for executives and senior leaders on a case-by-case basis, to support retention. We are proposing to add a reasonable judgement underpin which is not normal practice in the US, to allay concerns on fully guaranteed additional pay. The introduction of restricted stock is critical to enable effective competition with MedTech peers, as RSPs are a standard component in US leadership remuneration packages. In order to achieve a balance of measures which drive longer-term stability and value creation and support a longer-term holding period, we propose to increase the share ownership guidelines for US Executive Directors from 300% to 500% and retain bonus deferral at 30% after share ownership guidelines are met. The Board considered the following alternatives in arriving at the proposal to introduce the RSP: – Increase in base salary for US Executive Directors: Unlikely to be acceptable to investors and wider stakeholders. Based on the analysis completed, base compensation is not the primary issue for the Company; longer-term incentives are out of line with US packages. – Reduce PSP on introduction of RSP: The Board feels strongly that there should be continued focus on driving performance and reduction of the PSP is not ideologically aligned with this goal. The PSP is global for all senior executives and therefore a reduction would not support organisational performance objectives. In addition, the reduction of PSP would not achieve the objective of increasing the overall quantum in order to become more competitive with US norms. – Increase in PSP only: This would not align to US norms and packages offered by our major competitors. We are proposing to add a reasonable judgement underpin to the RSP to allay concerns on full guaranteed additional pay. – Introduce an RSP at a lower quantum of 50% of salary: The Board seeks to ensure that our US talent are remunerated in line with the structures used in the local market. An RSP of 125% still only takes the CEO into the lower quartile when benchmarked against US peers. We understand that under UK market norms, this would not fall within commonly accepted practice, but failure to make these increases will not achieve the dual objective of becoming more competitive in the US market or attracting and retaining US talent to ensure longer term stability. – Introduce the 2024 Policy changes for all Executive Directors: The strategic rationale for the 2024 Policy changes for US Executive Directors is to ensure that the Board has a compensation framework to remunerate US Executive Directors in the jurisdiction in which they live and work. New Policy in the context of the wider workforce Although this report deals primarily with the remuneration of our Executive Directors, as outlined in my introduction, the focus of the Committee has and will continue to be on reviewing compensation and initiatives across the wider workforce. ShareSave Plans are operated in the UK and 31 other countries internationally. As Company financial performance improves, we anticipate seeing an increase in participation in our ShareSave Plans. In January, I chaired a Board listening session with some of our employees from our UK teams to explain our Executive Director’s Remuneration Policy and how Element Current Policy for US Executive Directors Change/No Change to Policy for US Executive Directors Updated Remuneration Proposal January 2024 for US Executive Directors Base salary CEO: $1,526,625 No change CEO: $1,572,424 Annual Bonus Maximum: 215% of base salary Target: 50% of maximum Threshold: 15% of maximum No change Maximum: 215% of base salary Target: 50% of maximum Threshold: 15% of maximum Long-term incentives Maximum PSP: 275% of base salary 3 years performance period + 2 year holding Increase maximum opportunity under PSP to 300% of base salary. Introduce an opportunity under a new RSP of 125% of base salary with a 3-year phased vesting with Committee underpin based on reasonable judgement. Holding period doesn’t apply to RSP. Maximum PSP: 300% of base salary 3 years performance period + 2 year holding Maximum RSP: 125% of base salary. Annual vest in three equal tranches over a 3-year period, contingent on Committee reasonable judgement underpin. Share Ownership Guidelines (SOG) 300% of base salary Increase to 500% 500% of base salary Post-cessation SOG 100% of SOG or actual holding (if lower) for 2 years No change 100% of SOG or actual holding (if lower) for 2 years Bonus deferral 50% paid in cash, 50% deferred for 3 years Reduction for all Executive Directors, of bonus deferral from 50% to 30% when SOG is met. 50% paid in cash, 50% deferred for 3 years 70% paid in cash, 30% deferred for 3 years when SOG is met. Malus and clawback Malus and clawback provisions apply No change Malus and clawback provisions apply Pension Capped at 7.5% of salary No change Capped at 7.5% of salary STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 123 |
| Remuneration continued Directors’ Remuneration Report continued it aligns to the Company’s purpose, values and delivery of the Company’s long-term strategy. We also discussed the impact of the cost of living crisis and fall in disposable incomes. In response to the continued challenges relating to the cost of living in a number of markets globally, the Company continued to monitor the situation and again, as in 2022, undertook an off-cycle salary adjustment for employees in markets where inflation had a material impact. This was determined by thorough review of external inflation rates across markets and was applied to employees below senior management level where the gap between their 2022 annual pay increase and the rate of inflation was above a certain level. We also offered our employees in Costa Rica an opportunity to convert their salaries back into Costa Rican Colóns from US Dollars to minimise the impact of devaluing currency. Pay equity is key in our commitment to our people. We have a globally consistent approach to managing pay equity, with clear accountability and regular reporting to ensure we are delivering on our commitment. We review annually the gender pay ratio in the UK and we continue to make positive progress to address the adjusted pay gap and drive pay equity on our global agenda. The Committee monitors the pay philosophy for the wider workforce throughout the year to ensure our people are paid fairly and equitably for the work they do. Smith+Nephew is committed to developing our talent globally and offers a variety of multi-level programmes and network events in support of continued growth and retention. Some of the key initiatives offered in 2023 include: – A broad range of self-paced learning options for our employees globally via our online learning library, the Accelerated Leadership Collection. Following completion of a particular learning module, employees present thoughts and insights on the module to their teams in order to share best practice and learning; – Continuing to deliver and expand our Diverse Talent Sponsorship Program. Previous cohorts provided positive feedback and we have noted a positive impact on career progression and talent retention for participants in the program. Following the first S+N sponsorship initiative in 2020/21, we have seen 71% of the 24 participant cohort achieve an internal promotion and there has been an 88% retention rate within the cohort. The second cohort in 2022/23 has seen 64% of the 11 participant cohort achieve lateral moves or promotions in line with their talent development plans and there has been a 92% retention rate within the second cohort to date; – Coaching for wellbeing, mental health, leadership and professional development through our Healthcare provider across key jurisdictions; – Facilitation of opportunities for employees and leaders to learn from each other via structured reflection sessions built into most of our programs; – Launching the leader transition toolkit to accelerate the impact of successors moving into broader roles aligned to their development plans; and – Career Conversation guides and micro-learnings modules are available to all employees and managers globally to build self-awareness and capability in engaging in career development conversations. » See more on pages 46–49 Introducing ESG measures in PSP alongside ABP As reported in 2022, the Committee recognises that ESG performance forms an important part of Smith+Nephew’s short-term and long-term strategic priorities. The current approach for our Annual Bonus Plan is to allocate 5% of the total opportunity under the plan to ESG performance. For 2024, we propose to allocate 5% out of the 15% weighting on the business objectives element to ESG, focusing on environmental measures aligned with carbon reduction targets and diversity measures within our People. For awards granted under the Performance Share Programme in 2024 we are introducing a 10% ESG allocation with primary focus on carbon reductions and diversity measures. Balancing performance scorecards to support our business strategy Together with developing the policy proposals above, and introducing ESG measures in PSP, the Committee has reviewed the mix of performance measures within the scorecards for the ABP and the PSP to ensure they continue to drive the implementation of business strategy. To maintain the balance in each incentive programme in the context of the adjustments to ESG performance measurement: – Cash flow performance will be removed from the PSP scorecard and introduced in the ABP scorecard in the form of a trading cash flow conversion metric to ensure stronger focus. – In both plans, the mix of measures will be slightly reweighted to accommodate these changes and the inclusion of ESG. Review of 2023 performance In 2023 the Group delivered strong revenue growth in line with its guidance issued in February 2023 and an improved trading profit margin⁴. Revenue was $5,549 million, up 6.4% on a reported basis and 7.2% on an underlying basis.⁴ Operating profit was $425 million, and the trading profit⁴ was $970 million with a trading profit margin⁴ of 17.5%. The strong revenue growth and improved trading profit margin in 2023 were built upon the early benefits from our actions to transform Smith+Nephew. The 12-Point Plan is on track, with progress beginning to translate into financial outcomes, and our innovation strategy is delivering a strong pipeline of new products that we expect will drive future performance. Remuneration outcomes for 2023 Annual Bonus Plan Performance against the financial targets under the Annual Bonus Plan was above maximum for revenue and between threshold and target for trading margin, resulting in an aggregated payout of 124.51% of target in respect of the financial objectives. The Remuneration Committee reviewed the performance of the Executive Directors against their individual business objectives and determined the rating as follows: Deepak achieved his objectives in terms of what he delivered and exceeded in how he performed and therefore received an above target payout in relation to this element of his bonus. 124 Smith+Nephew Annual Report 2023 |
| Compliance statement We have prepared this Directors’ Remuneration report (the Report) in accordance with The Enterprise and Regulatory Reform Act 2012–2013 (clauses 81–84), sections 420 to 422 of the Companies Act 2006 and The Large and Medium-Sized Companies and Groups (Accounts and Reports) (Amendment) Regulations 2013 (the Regulations), The Companies (Directors’ Remuneration Policy and Directors’ Remuneration Report) Regulations 2019 and The Companies (Miscellaneous Reporting) Regulations 2018. The Report also meets the relevant requirements of the Financial Conduct Authority (FCA) Listing Rules. Pages 138–154 is the annual report on remuneration (the Implementation Report). The Implementation Report will be put to shareholders for approval as an advisory vote at the Annual General Meeting on 1 May 2024. The Implementation Report explains how the Remuneration Policy was implemented during 2023. The Policy Report describes our Remuneration Policy as it relates to the Directors of the Company. All payments we make in relation to Directors of the Company will be in accordance with this Remuneration Policy. The Policy will be put to shareholders’ vote at the Annual General Meeting on 1 May 2024. Anne-Françoise achieved her objectives both in terms of what she delivered and how she performed and therefore received an on-target payout in relation to this element of her bonus. These ratings combined with performance against the financial objectives resulted in an overall bonus amounting to 130.8% of base salary for Deepak and 127.5% of base salary for Anne-Françoise. We appreciate that during 2023, the share price decreased by 4%, but having considered the progress against the 12-Point Plan, and that there have been no material risk or reputational events, we determined that the bonus outcomes are a fair representation of the performance of the Company and the Executive Directors in 2023. There is no need to apply any discretion to these formulaic outcomes. Performance Share Programme Similarly, the Remuneration Committee reviewed performance over the past three years against the targets determined in 2021 for the Performance Share Programme and determined that these awards should vest at 21% (see pages 144–145 for further details). This reflects performance against the targets over the three-year performance period since 1 January 2021. Note: As Deepak joined in 2022 he did not receive a PSP 2021 award. I would like to thank our shareholders, employees and other stakeholders for their engagement and support over the past year. Our shareholders have helped us to shape the proposed Remuneration Policy for 2024 through their engagement and constructive feedback and we are very grateful for their continued support and investment in the Company. Angie Risley Chair of the Remuneration Committee 4 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measures prepared in accordance with IFRS on pages 244–248. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 125 |
| Directors’ Remuneration Policy Compliance with the UK Corporate Governance Code The new Remuneration Policy has been developed taking into account the following principles set out in Provision 40 of the Code: – Simple and clear: Our remuneration structure is straightforward and transparent with Executive Directors’ variable pay consisting of an annual bonus and a single long-term incentive plan. – Aligned to culture, purpose and strategy: The remuneration structure has been designed to support our culture and business purpose with particular attention being paid to remuneration throughout the organisation to ensure that arrangements are appropriate in the context of our approach to reward for the wider workforce. Performance measures used in the incentive plans are aligned with key strategic objectives and the principle of long-term sustainable value creation. – Predictability: Incentive awards are capped so that the maximum potential award under each plan is transparent. The charts on page 132 provide an illustration of the potential total reward opportunity for the Executive Directors. – Proportionality and mitigating risk: Our variable remuneration arrangements are designed to provide a fair and proportionate link between Group performance and reward whilst mitigating risk where appropriate. The Committee has overriding discretion that allows it to adjust formulaic annual bonus or PSP outcomes so as to prevent disproportionate results and Policy provisions allow for the application of malus and/or clawback in specific circumstances. Additionally, there is a clear link between executive remuneration and the longer-term performance of the Group through a combination of bonus deferral into shares, five-year release periods for PSP awards and stretching shareholding requirements that apply during and post employment. Changes to policy The new Policy contains no changes to the 2023 Remuneration Policy for Executive Directors who reside outside the US. The changes proposed in the new Policy are summarised below. In designing the Directors’ remuneration policy set out on pages 126–135, the Committee followed a robust process which included detailed Committee discussions on approach and content of the Policy, engagement by the Committee Chair and Chair of the Board with 52 shareholders comprising over 67% of the share capital of the Company and proxy advisors, and further discussions following shareholder and proxy feedback culminating in the Proposal being put to the shareholder vote. In order to avoid any conflicts of interest, the Committee is composed entirely of independent Non-Executive Directors. The Committee considered input from management, while ensuring that conflicts of interest were suitably mitigated, and our independent advisors, and sought the views of Smith & Nephew plc (the Company) major shareholders and other stakeholders, including employees. If approved by shareholders, the Policy will take effect from the date of that approval. Proposed implementation of new Policy in 2024 Base salary – 2023 salaries: CEO $1,526,625; CFO £637,519 – 2024 salaries: CEO $1,572,424; Incoming CFO: £725,000. For context, the average 2023 increase for the US workforce was 3% and 3.65% for the UK workforce. Pension – CEO: 7.5% of base salary. The contribution is capped in accordance with plan rules and regulations (aligned with the US-based workforce), see page 141. – CFO: 12% of salary (aligned with UK employees). Annual Bonus – 2024 opportunity for Executive Directors: 215% of salary (unchanged from 2023). – Executive Directors receive 50% paid in cash, 50% deferred in shares for three years. 70% paid in cash, 30% deferred in shares once the shareholding guidelines have been met. – Performance measures:* 35% revenue growth, 35% trading profit margin, 15% trading cash flow conversion, 15% business objectives (including 5% on ESG metrics). Performance Share Programme – 2024 award for US Executive Directors increased to 300%. UK Executive Directors remains at 275% of salary. – Three-year performance period plus two-year holding period. – Performance measures: 30% relative TSR, 30% ROIC, 30% revenue growth and 10% ESG metrics. Restricted Share Plan – 2024 award for US Executive Directors at 125% of salary. – Awards will vest in 3 equal tranches over a 3-year period contingent on reasonable judgement underpins being met. Shareholding guideline – Whilst in employment, build up and maintain shareholding worth at least 500%/200% of salary for US Executive Director/Non-US Executive Director. – After ceasing employment, remain compliant with their ‘in employment’ guideline for two years after stepping down as Director. Remuneration continued * These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measures prepared in accordance with IFRS on pages 244–248. 126 Smith+Nephew Annual Report 2023 |
| Future policy table – Executive Directors Base salary and benefits Base salary Core element of remuneration, paid for doing the expected day-to-day job to recruit and retain Executive Directors of the calibre required to deliver the Company’s strategy. How the component operates Maximum levels of payment Framework in which performance is assessed Salaries are normally reviewed annually with any increase usually applying from 1 April. Salary levels and increases take into account: – scope and responsibility of position; – skill/experience and performance of the individual Executive Director; – general economic conditions in the relevant geographical market; – average increases awarded across the Company, with particular regard to increases in the market in which the Executive Director is based; and – market movements seen among relevant peer companies. While there is no maximum salary level, any increases will normally not exceed the typical increase for the wider employee population within the relevant geographic area. Higher increases may be made under certain circumstances at the Committee’s discretion. For example, this may include: – increase in the scope and/or responsibility of the individual’s role; and – development of the individual within the role. A full explanation will be provided in the Implementation Report should higher increases be approved in exceptional cases. In addition, where an Executive Director has been appointed to the Board at a lower than typical salary, larger increases may be awarded to move them closer to market practice as their experience develops. Performance in the prior year is one of the factors taken into account and poor performance is likely to lead to a zero salary increase. Pension and payment in lieu of pension Provide Executive Directors with an allowance for retirement planning to recruit and retain Executive Directors of the calibre required to deliver the Company’s strategy. How the component operates Maximum levels of payment Framework in which performance is assessed Executive Directors receive a cash allowance in lieu of membership of a Company-run pension scheme. In jurisdictions where the local law requires employees to participate in a Company-run pension scheme, Executive Directors participate in the local pension scheme. Base salary is the only component of remuneration which is pensionable. The maximum pension allowance for an Executive Director will be no more than the percentage of salary contribution paid in respect of the majority of our UK workforce (currently 12% of salary) unless the percentage of salary contribution paid in respect of the majority of the workforce in the Executive Director’s home country or the country in which the Executive Director is based is lower, in which case that lower percentage of salary contribution would usually be offered. None. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 127 |
| Remuneration continued Directors’ Remuneration Policy continued Benefits Provide Executive Directors with a market competitive benefits package to recruit and retain Executive Directors of the calibre required to deliver the Company’s strategy. How the component operates Maximum levels of payment Framework in which performance is assessed A wide range of benefits may be provided depending on the benefits provided for comparable roles in the location in which the Executive Director is based. These benefits will include, as a minimum: healthcare cover, life assurance, long-term disability, annual medical examinations, company car or car allowance. The Committee retains the discretion to provide additional benefits, where necessary or relevant in the context of the Executive Director’s location, or, in connection with an Executive Director’s recruitment, the country from which the Executive Director is recruited. Where applicable, relocation costs may be provided in-line with the Company’s relocation policy for senior executives, which may include, amongst other items: removal costs, assistance with accommodation, living expenses for self and family and financial, tax and/or legal consultancy advice. In some cases, such payments may be grossed up. While no maximum level of benefits is prescribed, they are set at an appropriate market competitive level, taking into account a number of factors, which may include: – the jurisdiction in which the individual is based. – the level of benefits provided for other employees within the Company. – market practice for comparable roles within appropriate pay comparators. The actual amount payable will depend on the cost of providing such benefits to an employee in the location at which the Executive Director is based. The Committee regularly reviews the benefit policy and benefit levels. None. All-employee arrangements All-employee share plans To enable Executive Directors to participate in all-employee share plans on a similar basis as other employees. How the component operates Maximum levels of payment Framework in which performance is assessed ShareSave Plans are operated in the UK and 31 other countries internationally. In the US, an Employee Stock Purchase Plan is operated. These plans enable employees to save on a regular basis and then buy shares in the Company. Executive Directors are able to participate in such plans on a similar basis to other employees, depending on where they are based. Executive Directors may currently invest up to £500 per month in the UK ShareSave Plan, in-line with UK participants. The Committee may exercise its discretion to increase this amount up to the maximum permitted by HM Revenue & Customs. Similar limits will apply in different locations. None. 128 Smith+Nephew Annual Report 2023 |
| Annual incentives Annual Bonus Plan Incentivises delivery of the business plan on an annual basis. Rewards performance against key performance indicators which are critical to the delivery of our business strategy. How the component operates Maximum levels of payment Framework in which performance is assessed The Annual Bonus Plan is designed to reward performance over the year against financial and business objectives. The Committee determines pay out levels based on the extent to which performance against these objectives has been achieved. The Committee retains discretion, in exceptional circumstances, to pay bonuses in respect of the half year and/or full year. The Committee has full discretion to adjust outcomes under the Annual Bonus Plan where: (i) the occurrence of certain events would unfairly advantage or disadvantage participants, in the reasonable opinion of the Committee and/or (ii) the amount that a participant would/could receive under an award would result in the participant receiving an amount which the Committee considers cannot be justified or which the Committee considers to unfairly disadvantage or advantage a participant. In exercising this discretion, the Committee may consider all circumstances, including (but not limited to): the financial performance of the Company; any changes in the Company’s share price; and the performance, conduct and contribution of the participant. Malus and clawback provisions apply, as detailed in the notes to this table. Normally, where the in-employment shareholding guideline of an Executive Director has not been met, half of the award is paid in cash after the end of the performance year and half is deferred into an award of shares under the Deferred Share Bonus Plan (DBP), which normally vests after three years. The bonus deferral reduces from 50% to 30% of base salary once shareholding guidelines have been met. The Committee has full discretion to authorise the payment of dividend equivalent payments on DBP awards to the extent they vest. The maximum opportunity is 215% of base salary. 50% of maximum is payable for on-target performance. Up to 15% of maximum is payable for threshold performance. The Committee will determine the appropriate performance measures for each financial year, in order to ensure that the Annual Bonus Plan focuses on key business priorities for the Company. Typically, at least 80% of the annual bonus will be based on financial performance measures. The remainder will usually be based on business objectives linked to key areas of strategic focus. The Committee retains the discretion to adjust the relative weightings of the financial and strategic components and to adopt any performance measure that is relevant to the Company. Under whatever measures are chosen, the Committee will set appropriately challenging maximum performance targets and additionally, where appropriate, targets for threshold and/or on-target performance. In doing so, they will take into account a number of internal and external reference points, including the Company’s key strategic objectives. The Committee may amend the performance conditions applicable to an award in accordance with the terms of the performance conditions or if events happen which cause the Committee to consider that it fails to fulfil its original purpose and would result in participants being unfairly advantaged or disadvantaged. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 129 |
| Remuneration continued Directors’ Remuneration Policy continued Long-term incentives Performance Share Programme (PSP) and Restricted Share Programme (RSP) To motivate and reward performance linked to the long-term strategy and share price of the Company. The performance measures which determine the level of vesting of the PSP awards are linked to our corporate strategy. How the component operates Maximum levels of payment Framework in which performance is assessed Awards are granted pursuant to the terms of the PSP and RSP. Awards are normally made in the form of conditional share awards, but may be awarded in other forms if appropriate, including nil cost options or a combination of awards. PSP awards usually vest after three years, subject to the achievement of stretching performance targets linked to the Company’s strategy. The performance period for the PSP is usually three years. RSP awards usually vest in equal annual tranches over the three-year vesting period. The Committee has full discretion to adjust outcomes under the PSP and RSP where: (i) the occurrence of certain events would unfairly advantage or disadvantage participants in the reasonable opinion of the Committee; and/ or (ii) the amount that a participant would/ could receive under an Award would result in the participant receiving an amount which the Committee considers cannot be justified or which the Committee considers to unfairly disadvantage or advantage a participant. In exercising this discretion, the Committee may consider all circumstances, including (but not limited to): the financial performance of the Company; any changes in the Company’s share price; and the performance, conduct and contribution of the participant. Participants may receive an additional number of shares (or, exceptionally, cash) equivalent to the amount of dividends payable on ordinary shares subject to the award that vest during the period up to vesting. On vesting, a number of shares are sold to cover the tax liability. The remaining shares are usually required to be held by the Executive Director for a further two year holding period. Malus and clawback provisions apply as detailed in the notes to this table. PSP Awards for Executive Directors not resident in the US will consist of performance shares only with a maximum annual opportunity of 275% of base salary. US Executive Directors will receive a mix of performance shares and restricted shares with a maximum performance shares annual opportunity of 300% of base salary. For on-target levels of performance, 50% of the award vests. For threshold levels of performance, 25% of the award vests. RSP US Executive Directors awards will consist of a mix of performance shares and restricted shares, with the annual grant of Restricted Shares comprising no more than 125% of salary. PSP The Committee aims to align the PSP performance measures with the Company’s key long-term strategic objectives. In this manner, strong performance against the measures should lead to long-term sustainable value creation for our shareholders. Measures used will typically include: – Financial measures – to reflect the financial performance of our business and a direct and focused measure of Company success. – Shareholder return measures – a measure of the ultimate delivery of shareholder returns, providing direct alignment. – Strategic measures – aligned with the Company’s long-term strategy The make-up and weighting of each measure will be determined by the Committee each year to reflect the particular strategic objectives over the relevant performance period. Maximum pay-outs will only be made for significant outperformance. Under whatever performance measures are chosen, the Committee will set appropriately challenging maximum performance targets and additionally, where appropriate, targets for threshold and/or on-target performance. In doing so, they will take into account a number of internal and external reference points, including the Company’s key strategic objectives. The Committee may amend the performance conditions applicable to an award in accordance with the terms of the performance conditions or if events happen which cause the Committee to consider it appropriate to do so provided that this would not result in, in the Committee’s reasonable opinion, an unfair benefit to the Executive Director. RSP Awards under the RSP are not subject to financial performance conditions and will vest to the extent the Committee determines in its discretion that the reasonable judgement underpin has been met. In determining the extent to which an award will vest, the Committee’s will consider multiple factors relating to the vesting period including market movements, shareholder experience, the impact of the regulatory environment and reputational factors. The Committee retains full discretion following the grant of an award to make adjustments to the vesting outcome if full vesting is not considered to be appropriate. Any awards granted to Executive Directors must be in line with the Directors’ Remuneration Policy. 130 Smith+Nephew Annual Report 2023 |
| Notes to future policy table – Executive Directors Share awards The Committee may, in the event of any variation of the Company’s share capital, demerger, delisting, or other event which may affect the value of awards, adjust or amend the terms of DBP, PSP, or RSP awards in accordance with the plan rules. Malus and clawback At any time prior to the vesting of a PSP, RSP, or DBP award or payment of a cash bonus, the Committee may determine that an unvested award or part of an award may not vest, including to zero on the occurrence of a Trigger Event (as defined below), regardless of whether or not the performance conditions have been met). At any time up to three years after the vesting of a PSP, RSP, or DBP award or payment of a cash bonus, the Committee may determine that any cash bonus, vested shares, or their equivalent value in cash be returned to the Company on the occurrence of a Trigger Event. A Trigger Event will occur if any of the following matters is discovered where: – There has been a misstatement of the Company’s financial results which has resulted in a material overpayment to participants, which is in the form of awards under the applicable programme or otherwise, irrespective of whether the relevant participants are at fault; – There has been an error in determining the size of the award or to the extent to which the performance conditions have been satisfied, or erroneous or misleading data, which has resulted in the vesting of an award which would not otherwise have vested or which would otherwise have vested to a materially lesser extent; – There has been a significant adverse change in the financial performance or reputation of the Company, including corporate failure and/or any significant loss at a general level or in respect of a global business unit or function in which a participant worked; and/or – The Committee determines that the conduct, capability or performance of a participant or any team, business area or profit centre warrants a review. These provisions will apply under the Global Share Plan 2020, the Annual Bonus Plan and the Deferred Bonus Shares Plan 2020. On 25 September 2023, the Board adopted the Financial Statement Compensation Recoupment Policy (the “Clawback Policy”) providing for the recovery of certain incentive-based compensation from current and former executive officers of the Company in the event the Company is required to restate its financial statements filed with the SEC in order to correct an error that is material to its financial statements. The Clawback Policy is in addition to the rights granted to the SEC under applicable legislation and the malus and clawback provisions set forth in the Global Share Plan 2020 which permit the Remuneration Committee to reduce or clawback awards in specific circumstances. Legacy matters The Committee can make remuneration payments and payments for loss of office outside of the Policy set out above where the terms of the payment were agreed (i) before the Policy came into effect, provided the terms of the payment were consistent with any applicable policy in force at the time they were agreed or the terms were agreed before the date on which the Company first obtained shareholder approval for a Directors’ remuneration policy; or (ii) at a time when the relevant individual was not an Executive Director of the Company (or other person to whom the Policy set out above applies) and that, in the opinion of the Committee, the payment was not in consideration for the individual becoming an Executive Director of the Company (or such other person). This includes the exercise of any discretion available to the Committee in connection with such payments. For these purposes, payments include the Committee satisfying awards of variable remuneration and, in relation to an award over shares, the terms of the payment are agreed at the time the award is granted. The Policy set out above applies equally to any individual who would be required to be treated as an Executive Director under the applicable regulations. The Committee can make remuneration payments and payments for loss of office outside of the Policy set out above if such payments are required by law in a relevant country. Consideration of employment conditions elsewhere in the Group and differences between arrangements for Executive Directors and workforce as a whole When setting the Policy for Director’s Remuneration, the Committee discusses, and takes into account of pay arrangements and employment conditions of employees across the Group when determining the pay of Executive Directors in the following ways: Base salary Increases to Executive Director base salaries will generally not exceed base salary budgets in the geography in which the Executive Director is based, although the Committee will also have oversight of base salary budgets across the Company more generally when making the decision. Pension contributions and payments in lieu of a pension A range of different pension arrangements operate across the Group depending on location and/or length of service. Executive Directors either participate in pension arrangements relevant to wider workforce in their local market or receive a cash allowance payable in lieu of a pension at a percentage of base salary in line with the wider workforce in the geography in which they are based. Benefits Benefit packages vary across the world depending on local market practice. Executive Directors receive a range of benefits in line with the standard executive benefits package available to the wider executive workforce in the geography in which they are based. Annual Bonus Plan Nearly all employees are eligible to receive performance-based pay, primarily in form of the Annual Bonus. Employees at different levels throughout the Group participate in Annual Bonus Plans with different payment outcomes. The annual performance objectives are cascaded down to all employees from the objectives set at the beginning of the year for the Executive Directors and Executive Officers, to ensure that the performance of all employees is linked to the Company’s strategy and the objectives of the Executive Directors and senior management as applicable. In 2023, Executive Officers and senior executives STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 131 |
| Remuneration continued Directors’ Remuneration Policy continued participated in the Annual Bonus Plan on the same basis as the Executive Directors, subject to lower limits. All Employee Share Plans We operate two all-employee share plan arrangements depending on the most appropriate arrangement for different geographies. In 2023, US employees participated in the Employee Stock Purchase Plan. In 2023, UK and international employees from 31 other countries, participated in the ShareSave Plan. Executive Directors, executive officers and senior executives participated in these plans aligned to the geography in which they are based. Long term incentives Executive Officers and senior executives participate in the PSP and RSP on the same basis as the Executive Directors subject to lower limits. Shareholding requirements Executive Officers and senior executives who participate in the Annual Bonus Plan, the PSP, and RSP are also required to build a significant shareholding in the Company. Corporate events If there is a takeover of the Company, awards under the PSP and DBP will normally vest early at the time of the transaction. DBP awards will normally vest in full. The extent to which awards under the PSP and RSP vest will be determined by the Committee, taking into account, where considered to be appropriate in all the circumstances, the actual or likely achievement of the relevant performance conditions and, unless the Committee determines otherwise, the awards will be time pro-rated by reference to the proportion of the relevant performance period that has elapsed. Any post-vesting holding requirements will normally cease to apply. In these circumstances, the Committee reserves the discretion to treat the payment of annual bonuses for the financial year in which the takeover takes place in such manner as it considers appropriate (subject to the limit set out in the Policy table above). If there is a demerger or other transaction that is likely to materially affect the Company’s share price, the Committee may allow awards to vest and bonus to be paid early on the same basis as set out above for a takeover. Illustrations of the application of the Remuneration Policy 2024 The following charts show the potential split between the different elements of the Executive Directors’ remuneration under four different performance scenarios: Chief Executive Officer 100 $1,731k 22 22 56 $7,745k 15 29 22 44 22 57 $11,794k 11 $15,136k Minimum % Target % Maximum % Maximum+ %* Current * + 50% Chief Financial Officer share price growth Fixed pay Annual bonus LTIP LTIP – share price appreciation 100 £824k 32 30 38 £2,600k 19 29 37 36 19 46 £4,377k 15 £5,374k Minimum % Target % Maximum % Maximum+ %* Current * + 50% share price growth Fixed pay Annual bonus LTIP LTIP – share price appreciation 100 £824k 32 30 38 £2,600k 19 29 37 36 19 46 £4,377k 15 £5,374k Minimum % Target % Maximum % Maximum+ %* Current * + 50% share price growth Fixed pay Annual bonus LTIP LTIP – share price appreciation Assumed performance Assumptions used for proposed Policy Fixed pay All performance scenarios – Consists of total fixed pay, including base salary and pension allowance (as at 1 April 2024) and benefits (as received during 2023). – Pro-rated for Deepak Nath. Variable pay Minimum Performance – No pay out under the Annual Bonus Plan. – No vesting under the PSP. Target Performance – 50% of maximum pay out under the Annual Bonus Plan (i.e. 107.5% of salary). – 50% vesting under the PSP (i.e. 137.5% of salary). Maximum Performance – 100% of the maximum pay out under the Annual Bonus Plan (i.e. 215% of salary). – 100% vesting under the PSP (i.e. 300% of salary for the CEO and 275% for the CFO). Maximum performance + 50% share price growth – As maximum performance but this column assumes that the face value of the PSP award increases by 50% as a result of share price growth. PSP awards have been shown at face value with no discount rate assumptions. The charts provide illustrative values of the remuneration package in 2023. Actual outcomes may differ from those shown. 132 Smith+Nephew Annual Report 2023 |
| Policy on recruitment arrangements Our policy on the recruitment of Executive Directors is to pay a fair remuneration package for the role being undertaken and the experience of the Executive Director appointed. In terms of base salary, we will seek to pay a salary comparable, in the opinion of the Committee, to that which would be paid for an equivalent position elsewhere. The Committee will determine a base salary in line with the Policy and having regard to the parameters set out in the Future Policy Table. Incoming Executive Directors will be entitled to pension (or cash payment in lieu of pension), benefits and incentive arrangements aligned with those set out in the Policy table above. On that basis, the aggregate annual opportunity under their incentive arrangements would not exceed 490% of base salary if based outside the US or 640% of base salary if based in the US. We recognise that in the event that we require a new Executive Director to relocate to take up a position with the Company, we may also pay relocation and related costs, in line with the relocation arrangements we operate across the Group. In addition, where a new Executive Director requires legal or other professional advice related to the appointment with the Company, we may agree to pay directly or reimburse the Executive Director for fees and expenses reasonably and properly incurred including the provision of advice to enable the Executive Director to understand the obligations, duties and legal and regulatory requirements of the new role. The Committee also has the discretion to determine whether a new Executive Director should be subject to a different set of criteria for annual and/or long-term incentive performance measures during the first 12 months following appointment. For external appointments, the Committee may award compensation for the forfeiture of remuneration awards or compensation arrangements from a previous employer. In doing so, the Committee would aim to structure the replacement awards in a like-for-like manner to the extent possible, taking into account relevant factors, including: – the form of the forfeited awards (e.g. cash or shares); – any performance conditions attached to them and the likelihood of these conditions being satisfied; and – the proportion of the vesting and/or performance period remaining. The Committee will have regard to the best interests of both Smith+Nephew and its shareholders and is conscious of the need to pay no more than is necessary, particularly when determining buy-out arrangements. In making buy-out awards to new appointments, the Committee may grant awards under the relevant provision in the Financial Conduct Authority Listing Rules, which allows for the granting of awards specifically to facilitate, in unusual circumstances, the recruitment of an Executive Director, without seeking prior shareholder approval. The overall approach outlined above would also apply to internal appointments, with the proviso that any commitments entered into before promotion which are inconsistent with the Policy will continue to be honoured. Service contracts We employ Executive Directors on rolling service contracts with notice periods of up to 12 months from the Company and up to 12 months from the Executive Director. On termination of the contract, we may require the Executive Director not to work their notice period and pay them (in phased instalments or as a lump sum) an amount equivalent to the base salary, contributions to a pension or equivalent savings plan (or payment in lieu thereof) and benefits they would have received if they had been required to work their notice period. The Executive Directors may become entitled to additional/alternative sums if termination occurs within 12 months of a change in control (as further described in the following section ‘Policy for payment for loss of office’). Directors’ service contracts are available for inspection at the Company’s registered office: Building 5, Croxley Park, Hatters Lane, Watford, Hertfordshire WD18 8YE, United Kingdom. Policy for payment for loss of office Our usual policy regarding termination payments to departing Executive Directors is to limit severance payments to pre-established contractual terms. Where necessary to comply with the mandatory laws of the jurisdiction in which the Executive Director is resident, the Committee may authorise remuneration payments or payments for loss of office in excess of the pre-established contractual terms. In the event that the employment and/or office of an Executive Director ends, any compensation payable will be determined in accordance with the terms of the service contract between the Company and the Executive Director, as well as the rules of any incentive plans and the Policy. In addition, the Committee will have the discretion to make payments in discharge of an existing legal obligation (or by way of damages for breach of such obligation) or by way of settlement of any claim arising in connection with the cessation of office or employment. Under normal circumstances (excluding termination for gross misconduct and certain other terminations for ‘cause’) all leavers are entitled to receive a termination payment (in phased instalments or as a lump sum) in lieu of notice equal to base salary, pension contributions (or payment in lieu of pension) and benefits. The leaver may also be paid a payment in lieu of accrued but untaken holiday leave. Payments may also include (but are not limited to) costs associated with relocation/repatriation, the costs of legal advice, financial (including tax) advice and outplacement services in connection with cessation of office or employment. In the event of termination in connection with a change in control of the Company, in circumstances where there is a diminution of status, a reduction in salary or benefits, a mandatory relocation or where termination results from the change in control, the payment in lieu of notice will be payable as a lump sum, the Committee will consider to what extent an annual bonus award should be made, and the leaver will receive reasonable outplacement costs. In the event that an Executive Director dies or ceases to be an employee because of ill-health, injury, disability, redundancy, retirement with the agreement with the Company, the sale of their employing company or business out of the Group, or for any other reason for which the Committee determines that good leaver treatment is appropriate: – They may be eligible to receive an annual bonus on a time pro-rated basis for the period of the year that they have worked. – The annual bonus will typically be subject to business and individual performance in the same manner as for the continuing Executive Directors, and paid at the usual time. The annual bonus may be paid in such proportion of cash and shares and subject to such deferral arrangements as the Committee may determine. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 133 |
| Remuneration continued Directors’ Remuneration Policy continued The Committee will have the discretion to take into account performance over the full financial year or up to the date of cessation of employment based on appropriate performance measures determined by the Committee in line with the Policy. – Outstanding PSP and RSP awards will typically, unless the Committee determines otherwise, be pro-rated for the proportion of the relevant performance period (in the case of the PSP) or the vesting period (in the case of the RSP) that has elapsed at the time Executive Director leaves, and be tested for performance at the end of the performance period (in the case of the PSP), unless the Committee determines to test performance otherwise. The two-year post-vesting holding period for the PSP will, unless the Committee determines otherwise, continue to be enforced. If an Executive Director dies, awards will normally vest early and only be time pro-rated if the Committee considers it appropriate. Any outstanding awards under the PSP and RSP will remain subject to the same terms and conditions (including, malus and clawback) as applied at time of grant. For participants who leave for any other reason, outstanding PSP or RSP awards will lapse in full. – If an Executive Director leaves for any reason other than dismissal or any other reason that the Committee determines, any outstanding DBP awards will remain subject to the same terms and conditions (including malus and clawback) as applied at time of grant and vest as if the Executive Director had not left. In the event of termination in connection with a change in control of the Company or, if an Executive Director dies, any outstanding DBP awards will vest. In any other circumstances any unvested DBP awards will lapse. One-off awards granted on appointment will normally lapse on leaving except in cases of death, retirement, redundancy or ill-health. The Committee has discretion to permit such awards to vest in other circumstances or to agree to make a cash payment in respect of such an award and will be subject to satisfactorily meeting applicable performance conditions. We will supply details via an announcement to the London Stock Exchange of a departing Executive Director’s termination arrangements as soon as is practicable. Policy on shareholding requirements The Committee believes that one of the best ways our Executive Directors’ interests can be aligned with that of shareholders is for them to hold a significant number of shares in the Company. If based outside the US, the Chief Executive Officer is expected to build a holding of Smith+Nephew shares worth three times base salary and the Chief Financial Officer is expected to build a holding of two times base salary. If based in the US, the Chief Executive Officer is expected to build a holding of Smith+Nephew shares worth five times base salary and the Chief Financial Officer is expected to build a holding of two times base salary. Executive Directors are required to retain at least 50% of the shares (after tax) vesting under Company incentive plans decreasing to 30% once share ownership guidelines are met, recognising that differing international tax regimes affect the pace at which Executive Directors may fulfil the shareholding requirement, unless the Committee determines otherwise. When calculating whether or not this requirement has been met, Ordinary Shares or ADRs held by the Executive Directors and their immediate family are included, as are unvested awards under the DBP (on a net-of-tax basis), but not PSP awards. Ordinarily we would expect Executive Directors to achieve their shareholding requirement within a period of five years from the date of appointment. Executive Directors are also usually required to hold any shares vesting under the PSP for a period of two years after vesting. The Executive Officers and senior executives who participate in the Annual Bonus Plan and PSP are also required to build a significant shareholding in the Company, extending the principle of alignment with our shareholders across the senior management team. Policy on post cessation shareholding Executive Directors are usually required to retain any shareholding up to the applicable shareholding requirement (or their actual holding on departure if lower) for a period of two years after cessation of employment. This post employment holding requirement does not apply to shares purchased by an Executive Director in the market which have not been awarded as part of remuneration. In order to reinforce this expectation, and to the extent that the shareholding requirement has not been reached, all relevant vested DBP, PSP, and RSP shares will be held in a vested share plan account, which will not usually be accessible until two years post cessation of employment. In addition, former Executive Directors will be required to seek permission to deal during this period. The Committee retains the discretion to adjust or waive all or part of the post-employment shareholding requirement in appropriate circumstances. In exercising this discretion, the Committee may consider circumstances including (but not limited to) the performance, conduct and contribution of the participant. Limited discretion to make minor amendments to Policy The Committee retains the discretion to make minor amendments to the Policy as may be required or reasonably necessary for administrative reasons or to the extent required or reasonably necessary to comply with applicable laws and regulations. Consultation with employees relating to Executive Director remuneration While the Committee does not directly consult with our employees as part of the process of determining executive pay, the Chair provided an overview of the compensation of Executive Officers at one of our Board Listening Sessions. No comments were raised by the employees attending that session. Statement of consideration of shareholder views Angie Risley, the Committee Chair, engaged with shareholders during development of the Policy. The feedback received was presented to and discussed by the Committee and informed the final shape of the proposed Policy which is being put to the 2024 Annual General Meeting. The Committee Chair and shareholders appreciated the engagement and the Committee took all comments received on board during its subsequent discussions and ensured further clarity was included in the narrative detailing the proposed changes to the new Policy (see page 126). 134 Smith+Nephew Annual Report 2023 |
| Notes to future policy table – Non-Executive Directors Additional duties undertaken by Non-Executive Directors In the event that the Chair or a Non-Executive Director is required to undertake significant executive duties in order to support the Executive Directors during a period of absence due to illness or a gap prior to the appointment of a permanent Executive Director, the Committee is authorised to determine an appropriate level of fees which will be payable. These fees will not exceed the amounts which would normally be paid to a permanent Executive Director undertaking such duties and will not include participation in short or long-term incentive arrangements or benefit plans. Additional benefits The Committee will have the discretion to approve such additional benefits for Non-Executive Directors as may be required or reasonably necessary in connection with the performance of their duties, including without limitation expenses and associated taxes. Policy on recruitment arrangements Any new Non-Executive Director will be paid in accordance with the current fee levels on appointment, in-line with the Policy set out above. With respect to the appointment of a new Chair, fee levels will take account of market rates, the individual’s profile and experience, the time required to undertake the role and general business conditions. In addition, the Committee retains the right to: (i) authorise the payment of relocation assistance or an accommodation allowance in the event of the appointment of a Chair not currently based in the UK; and (ii) authorise the payment of a contribution towards ongoing administrative support services as may be required or reasonably necessary to enable the Chair to fulfil the required duties and obligations of the role. Terms of appointment The Chair and Non-Executive Directors have letters of appointment which set out the terms under which they provide their services to the Company. These are available for inspection at the Company’s registered office: Building 5, Croxley Park, Hatters Lane, Watford, Hertfordshire WD18 8YE, United Kingdom. The appointment of the Non-Executive Directors is not subject to a notice period, nor is there any compensation payable on loss of office, for example, should they not be re-elected at an Annual General Meeting. The Committee has the discretion to waive all or a portion of the notice period of six months applicable for the Chair. The Chair and Non-Executive Directors are encouraged to acquire a shareholding in the Company equivalent in value to their basic fee within two years of their appointment to the Board. Future policy table – Chair and Non-Executive Directors The following table and accompanying notes explain the different elements of remuneration we pay to our Chair and Non-Executive Directors. No element of their remuneration is subject to performance. All payments made to the Chair are determined by the Committee, whilst payments made to the Non-Executive Directors are determined by those Directors who are not themselves Non-Executive Directors, currently the Chair, Chief Executive Officer and Chief Financial Officer. Annual fees Basic annual fee To attract and retain Directors by setting fees at rates comparable to what would be paid in an equivalent position elsewhere. A proportion of the fees is usually paid in shares in the third quarter of each year in order to further align Non-Executive Directors’ fees with the interests of shareholders. Where appropriate, the Chair or Non-Executive Director may be provided with an alternative option of receiving their fee wholly in cash in return for them entering into a commitment to separately purchase the required number of shares to comply with the above requirement. How the component operates Maximum levels of payment Fees will be reviewed on an annual basis. Any increase will usually be paid in shares until 25% of the total fees is paid in shares. Fees are set in-line with market practice for companies of a similar size and complexity. Annual fees are set and paid in UK Sterling or US Dollars depending on the location of the Non-Executive Director. If appropriate, fees may be set and paid in alternative currencies and exchange rate fluctuation will be taken into account when determining fees to be paid in alternative currencies. Whilst it is not usually expected to increase the fees paid to the Non-Executive Directors and the Chair by more than the increases paid to employees generally, in certain circumstances (including periodic and substantial increases in activity or time commitment), higher fees might become payable. The total maximum aggregate fees payable to the Non-Executive Directors will not exceed the limit set out in the Company’s Articles of Association. Additional Fees To compensate Non-Executive Directors for additional responsibilities such as Committee Chair or Senior Independent Director reflecting additional time involved in such roles. How the component operates Maximum levels of payment A fixed fee is paid, which is reviewed annually. The aggregate amount of fees payable to the Non-Executive Directors may not exceed the limit set out in the Company’s Articles of Association. Intercontinental travel To compensate Non-Executive Directors for the time spent travelling to attend meetings in another continent. How the component operates Maximum levels of payment A fixed fee is paid, which is reviewed annually. The aggregate amount of fees payable to the Non-Executive Directors may not exceed the limit set out in the Company’s Articles of Association. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 135 |
| Remuneration continued Remuneration at a glance Our at a glance summary sets out the total remuneration paid to our Executive Directors in 2023 We aim to align the total remuneration for our Executive Directors to our key performance measures through a combination of fixed pay, bonus and long-term incentives. Remuneration principles 2023 in numbers Performance Remuneration across the Group Chief Executive Officer remuneration Remuneration principles – supporting long-term success and sustainable value – We will materially differentiate reward according to performance. – Performance targets will be relevant, stretching and aligned to our business strategy. – Rewards will be compatible with the Group’s risk policies and systems, with malus and clawback applied to all forms of variable pay. – We will provide a balance between attracting, retaining and motivating talented people as well as supporting equal opportunity and diversity of talent. – Remuneration outcomes will be clear and explainable, avoiding paying more than the Committee considers necessary. Base salary Pension and benefits Annual bonus (AIP) »See more on page 141 »See more on page 141 Long-term incentive plan (PSP) »See more on pages 144–145 $425m Operating profit (2022: $450m) $970m 7.6% Trading profit1 (2022: $901m) -25.7% Relative TSR (2022: -33.7%) 30.2c EPS (2022: 25.5c) $1.7bn Total pay bill (2022:$1.6bn) 3.0% US Base Salary Increase (2022: 6.5%) $4.6m Single figure (2022: $5.9m) 21.0% 2021 PSP (2022: 2020 PSP 0%) 61.37% Annual bonus percentage of max (2022: 31.27%) 3.0% Base Salary Increase (2022: 3.5%) 1 These non-IFRS financial measures are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS on pages 244–248. »See more on page 141 136 Smith+Nephew Annual Report 2023 |
| Total Pay Deepak Nath Chief Executive Officer $4,658,252 Anne-Françoise Nesmes Chief Financial Officer $2,059,946 »See more on page 139 Annual Bonus Plan (ABP) Forfeited Incentives: Performance share award: Total bonus payout (% of maximum): Deepak Nath 61.37% Anne-Françoise Nesmes 59.80% Total vesting Deepak Nath 99.98% Long Term incentive Plan (2021 PSP) Total vesting (% of maximum): Anne-Françoise Nesmes 21% TSR performance Cumulative free cash flow Weighting (%) Vesting (%) 25% 0% Global revenue Pension and Benefits Deepak Nath received a Company pension contribution of $24,750 in line with the tax authority limits and wider US workforce arrangements. Anne-Françoise Nesmes receives a salary supplement of 12% of basic salary to apply towards her retirement savings, in lieu of membership of one of the Company’s pension schemes. This is in line with the pension arrangement for the wider UK workforce. Other benefits include life insurance, health cover, car and fuel allowance and financial consultancy advice. »See more on page 141 Return on invested capital (ROIC) 25% 0% 25% 21% 25% 0% Total 21% Single figure of remuneration Deepak Nath Anne-Françoise Nesmes Salary $1,512,726 $785,673 Pension & Benefits $65,000 $109,735 Bonus $1,997,124 $1,010,184 LTI Nil $154,354 Forfeited Incentives $1,083,402 Nil »See more on pages 141–144 »See more on page 140 »See more on pages 144–145 »See more on page 139 Deepak Nath $4,658,252 Anne-Françoise Nesmes $2,059,946 $0k $500k $1,000k $1,500k $2,000k $2,500k $3,000k $3,500k $4,000k $4,500k $5,000k Salary Pension & Benefits Bonus LTI Forfeited Incentives STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 137 |
| Remuneration Implementation Report Work of the Remuneration Committee in 2023 In 2023, we held nine meetings. The Chief Executive Officer and the Chief Human Resources Officer, key members of the HR and Finance functions, the Company Secretary and Deputy Company Secretary also attended all or part of some of the meetings, except when their own remuneration was being discussed. Attendance by the members of the Committee at each meeting is set out on page 121 of this Annual Report. We also met with the independent remuneration consultants, Deloitte LLP (Deloitte) and Willis Towers Watson plc (WTW), who both contributed as remuneration advisors to the Committee during the year. The work carried out by the Committee during the year is set out on pages 121–125. Since the year end, we have reviewed the financial results for 2023 against the targets under the short-term and long-term incentive arrangements jointly with the Audit Committee. We have also determined base salary increases for Executive Directors and Executive Officers with effect from April 2024 and have determined the payouts under the 2023 Annual Bonus Plan and the vesting under the Performance Share Programme 2021. Independent Remuneration Committee advisors During the year, the Committee received information and advice from Deloitte and WTW. Both are global firms and provide many services to the Company, including tax, data and consultancy services. WTW replaced Deloitte as advisors to the Remuneration Committee at the conclusion of the Annual General Meeting in April 2023 further to Deloitte being appointed external auditors of the Company. Remuneration continued Role of the Remuneration Committee Main Responsibilities – Determination of Remuneration Policy for the Chair, Executive Directors, Executive Officers and senior executives. – Approval of individual remuneration packages for Executive Directors and Executive Officers, at least annually, and any major changes to individual packages throughout the year. – Consideration of remuneration policies and practices across the Group in particular relating to CEO Pay Ratio and Gender Pay. – Approval of appropriate performance measures for short-term and long-term incentive plans for Executive Directors, Executive Officers and senior executives. – Determination of payouts under short-term and long-term incentive plans for Executive Directors, Executive Officers and senior executives. – Engage with major shareholders and ensure their views are sought and considered when determining the Remuneration Policy. During the year, WTW provided advice on market trends and remuneration in general, attended Committee meetings, assisted in the review of the Directors’ Remuneration Policy and Implementation report, undertook calculations relating to the TSR performance conditions and advised on annual bonus plan measures. The fees paid to WTW for advice to the Committee during 2023, charged on a time and expense basis, were £188,500 (US$234,287). WTW complies with the Code of Conduct in relation to Executive Remuneration Consulting in the UK and the Committee is satisfied that their advice is objective and independent. Matters of a routine nature considered by the Committee – Received updates on the external market context and data. – Noted grants of awards under the Company’s Share Plans. – Monitored dilution limits and the number of shares available for use in respect of discretionary and all-employee share plans. – Monitored adherence to shareholding guidelines for Executive Directors. Executive Officers and senior executives. – Received regulatory/best practice updates from WTW and other consulting groups. – Reviewed and approved the Committee’s Terms of Reference. The Remuneration Committee presents the Annual Report on Remuneration (the Implementation Report) which will be put to shareholders for an advisory vote at the Annual General Meeting to be held on 1 May 2024. The Terms of Reference of the Remuneration Committee describe our role and responsibilities more fully and can be found on our website: www.smith-nephew.com 138 Smith+Nephew Annual Report 2023 |
| Key activities of the Committee during the year – Considered the terms of remuneration for the outgoing and incoming CFO. – Reviewed the Remuneration Strategy for the Executive Directors, Executive Officers and senior executives and developed Remuneration Policy proposals incorporating feedback received during the consultation with shareholders. – Reviewed out-turns for determining payouts to Executive Directors and Executive Officers under the 2022 Annual Bonus Plan and 2020 Performance Share Programme, and – Approved quantum of cash payments and awards to Executive Directors and Executive Officers under the 2022 Annual Bonus Plan and 2020 Performance Share Programme. – Approved the 2022 Directors’ Remuneration Report. – Reviewed and updated the incentive performance scorecard to apply across the Annual Bonus Plan and Performance Share Programme for 2023 to ensure ongoing alignment with strategic priorities. – Considered principles for setting the targets for the Annual Bonus Plan 2023 and 2023 Performance Share Programme. – Approved financial targets for the 2023 Annual Bonus Plan for Executive Directors, Executive Officers and senior executives. – Approved financial measures and targets for 2023 Performance Share Programme for Executive Directors and Executive Officers. – Reviewed the CEO’s pension provision and aligned this more fully with arrangements available to wider US workforce, with effect from 2024. – Reviewed and consulted with shareholders on changes proposed for the new Remuneration Policy for approval by shareholders at the Annual General Meeting in 2024. – Approved the TSR Peer Groups for Performance Share Awards to be made in 2024. – Considered the Gender Pay Report and CEO Pay Ratio figures. – Approved the 2023 Remuneration Committee Business Plan. – Tracked the performance against the targets set for the 2023 Annual Bonus Plan and the 2021, 2022 and 2023 Performance Share Programme. – Appointed a new Remuneration Advisor. Single total figure on remuneration The amounts for 2023 have been converted into US$ for ease of comparability using the exchange rate of £ to US$1.2429 (2022: £ to US$1.2311). Deepak Nath Appointed 1 April 2022 Anne-Françoise Nesmes Appointed 27 July 2020 2023 2022 2023 2022 Fixed pay Base salary $1,512,726 $1,083,558 $785,673 $747,224 Pension payments $24,750 $22,875 $94,281 $89,667 Taxable benefits $40,250 $18,874 $15,454 $15,248 Total Fixed Pay $1,577,726 $1,125,243 $895,408 $852,139 Annual variable pay Annual Incentive Plan/ Annual Bonus Plan – cash element $998,562 $371,888 $505,092 $251,194 Annual Incentive Plan/ Annual Bonus Plan – equity element $998,562 $371,887 $505,092 $251,193 Long-term variable pay Performance Share Programme1 – – $154,354 – Total Variable Pay $1,997,124 $743,775 $1,164,538 $502,387 Forfeited Incentives² Cash Bonus – $371,414 – – Non-Performance Based Awards – $2,132,844 – – Performance Based Award $1,083,402 $1,581,970 – – Total Forfeited Incentives $1,083,402 $4,086,228 – – Total Pay $4,658,252 $5,955,246 $2,059,946 $1,354,526 1 The 2021 PSP award granted in May 2021 will trigger at 21%. These shares are valued at 1077.0p. Further details date be found on pages 144–145. 2 Cash bonus and performance based award are part of annual variable pay and the non-performance based award is part of fixed pay. Total variable pay was $3,080,526 (2022: $2,697,159). Total fixed pay was $1,577,726 (2022: $3,258,087). STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 139 |
| Remuneration continued Remuneration Implementation Report continued Base salary The actual salary receivable for the year. Pension payments The value of the salary supplement in lieu of pension or contribution to any pension scheme made by the Company. Taxable benefits The gross value of all taxable benefits (or benefits that would be taxable in the UK) received in the year. Annual Incentive Plan – cash element/Annual Bonus Plan The value of the cash incentive payable for performance in respect of the relevant financial year. Annual Incentive Plan – equity element/Annual Bonus Plan The value of the equity element awarded in respect of performance in the relevant financial year as described on pages 141–144 of this report. Performance Share Programme The value of shares vesting that were subject to performance over the three-year period ending on 31 December in the relevant financial year.(includes dividend shares accrued during the performance period). For awards vesting in early 2024 this is based on the closing mid-market share price on 29 December 2023 which was 1077.0p. Total The sum of the above elements. All data is presented in our reporting currency of US Dollars (USD). Amounts for Anne-Françoise Nesmes have been converted from Sterling (GBP) using 12 month average exchange rates. Given currency movements in 2023, this may give the impression of changes that are misleading. Data is presented in local currency in the subsequent sections in the interests of full transparency. Forfeited Incentives These relate to buy-out awards which vested during the year. These were granted to Deepak Nath in respect of outstanding incentives he forfeited on leaving his former company. Full details of the buy-out awards can be found on page 129 of the 2021 Annual Report and in the stock exchange announcement released to the market on 3 May 2022. During the year ended 31 December 2023, the following such awards vested: – Partial vesting of Restricted Stock Unit (“RSU”) award granted over a total of 12,061 shares: 3,015 shares vested on 8 November 2023. – Partial vesting of a further RSU award granted over a total of 8,716 shares: 4,358 shares vested on 8 November 2023 – Partial vesting of RSU award over a total of 14,364 shares: 4,788 shares vested on 13 November 2023. Note: The total value of the RSUs granted to Deepak Nath was previously disclosed in the 2022 Single Figure Table ($2,132,844). – Partial vesting of a Performance Share Award granted over a total of 84,868. Following confirmation of performance against the targets attached to the original award, 84,855 shares vested on 21 December 2023 with the remaining balance of 13 shares lapsing on the same date. The shares are valued at 1027.25p being the S+N mid-market share price as at the 2023 year-end date of his former company (30 September 2023). 140 Smith+Nephew Annual Report 2023 |
| Fixed pay Base salary As normal, the base salaries of the Executive Directors were reviewed in February 2024 and it was determined that the CEO salary be increased by 3%. The general increase to base pay in 2024 was in line with the wider workforce in the US (3%) and below the FTSE 100. Deepak Nath’s base salary increased by 3% from $1,526,625 to $1,572,424 effective from 1 April 2024. Anne-Françoise Nesmes’ base salary of £637,519 was not increased given her impending departure from the Company during Q1 2024. Pension payments Deepak Nath received a Company pension contribution of $24,750 in line with the limits set forth by the US tax authority and the pension arrangement for the wider US workforce. Due to an initial oversight, the CEO’s pension contributions were set below our remuneration policy guideline. This has been corrected to ensure his pension is commensurate with the standard 7.5% of base salary, aligning with the contribution rates for the majority of our US-based workforce. The impact of this correction will initially be visible in the CEO’s single figure for FY2024 in next year’s Directors’ Remuneration Report. Anne-Françoise Nesmes receives a salary supplement of 12% of basic salary to apply towards her retirement savings, in lieu of membership of one of the Company’s pension schemes. This is in line with the pension arrangement for the wider UK workforce. Benefits In 2023, Deepak Nath received life insurance cover of $1 million plus accidental death and dismemberment insurance of $1 million. Anne-Françoise Nesmes received life insurance cover of seven times basic salary for the period 1 January 2023 to 31 March 2023 which was changed, effect 1 April 2023, to four times basic salary in line with the changes made to the wider UK workforce. Each Executive Director received benefits as detailed in the below table. The same arrangements will apply in 2024. The following table summarises the value of benefits in respect of 2023 and 2022. Deepak Nath Anne-Françoise Nesmes 2023 2022 2023 2022 Health cover $12,276 $8,871 £1,034 £986 Car and fuel allowance $12,700 $8,467 £11,400 £11,400 Financial consultancy advice £12,289 £1,248 – – Annual incentives Annual Bonus Plan 2023 Following the approval of the Remuneration Policy at the 2023 Annual General Meeting, the maximum opportunity under the Annual Bonus Plan for Executive Directors is 215% of base salary, subject to satisfactory performance against the performance measures detailed below. If the shareholding ownership guideline has not been met, 50% of the award is paid in cash and 50% is deferred into shares which will vest after three years. If the shareholding ownership guideline has been met, 100% of the award is paid in cash. The performance measures and weightings which applied to the Annual Bonus Plan 2023 were as follows: Weighting Threshold as a percentage of salary Target as a percentage of salary Maximum as a percentage of salary Revenue growth 40.00% 12.80% 43.00% 86.00% Trading profit margin 40.00% 12.80% 43.00% 86.00% Business Objectives 15.00% 4.80% 16.13% 32.25% ESG Objectives 5.00% 1.60% 5.37% 10.75% Total 100.00% 32.00% 107.50% 215.00% The 2023 targets and out-turn for revenue and trading margin are shown below: Threshold Target Maximum Actual1 Revenue $5,357m $5,495m $5,539m $5,574m Trading Margin 17.4% 18.1% 18.5% 17.5% 1 Actual revenue and trading margin is compared with the target range at constant exchange rates to ensure a like-for-like comparison. See page 244. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 141 |
| Financial objectives The revenue target for 2023 is set at 5.7% by reference to our expectations for growth for the year. Threshold was set at 3.0 % growth over 2022 out-turn and maximum was set at 6.5% over 2022 out-turn. The trading margin target was set at 18.1% for the year. Threshold was set at 17.4% and maximum at 18.5% of trading profit margin, divided by threshold and maximum revenue respectively. Performance resulted in an overall payout of 133.8% of target against the financial objectives. Business and ESG objectives In determining performance against the business and ESG objectives, the Executive Directors have been assessed on the same basis as applies to all employees across the Group using a four-point rating scale reflecting both what has been achieved and how it has been achieved. At the beginning of the year, specific objectives were determined relating to achievement of the corporate strategy. For 2023, these objectives were Growth, People and Business processes as in 2022. Performance against these business objectives was considered alongside how the Executive Directors performed in respect of our culture pillars of Care, Collaboration and Courage. This includes consideration of performance against sustainability, compliance, quality and specific ESG metrics of building a more diverse and inclusive workforce as well as delivering planet and project plan objectives. Their overall performance has been assessed according to the extent to which the Executive Directors have met the expectations of the Board. 20% of the Annual Bonus Plan which is attributable to business and ESG objectives will be paid out as follows: Performance % of base salary Below expectations Nil Partially met expectations 6.4% In line with expectations (100% of target) 21.5% Above expectations 43% When setting objectives for the upcoming year, the Board looks not only at the expected financial performance for the year, but also at the actions it expects the Executive Directors to carry out in the year to build a solid foundation for financial performance over the longer term. In reviewing performance against these objectives at the end of the year, the Board is mindful that there is not always a necessary correlation between financial performance and the achievement of business and ESG objectives. The table below sets out how the Chair and the Board have assessed how Deepak Nath and Anne-Françoise Nesmes have performed against the objectives of Growth, People and Business Processes. Accordingly, the following amounts have been earned by Deepak Nath and Anne-Françoise for 2023 under the Annual Bonus Plan. Deepak Nath $1,997,124 Anne-Françoise Nesmes £812,763 As well as considering the monetary outcome of the formulaic calculation of these awards, the Committee considered that this performance fairly represented the overall financial performance during the year. Remuneration continued Remuneration Implementation Report continued 142 Smith+Nephew Annual Report 2023 |
| Annual incentives continued Annual Bonus Plan 2023 Deepak Nath Anne-Françoise Nesmes People and Process – Achieved above target to strengthen Executive Committee competency, effectiveness and accountability to drive consistent execution, successfully recruiting two high-quality external executives and promoting and expanding two internal executive roles as a result of an enhanced executive talent assessment, succession and development process. – Achieved above target to execute 12-Point Plan and cost-reduction programme, specifically fixing Orthopaedics, including supply and inventory, Improve Productivity and Accelerate Advanced Wound Management and Sports Medicine. Actively engaged shareholders with exceptional cadence of updates on the delivery of 12-Point Plan delivery and ability to meet guidance. – Achieved against target to increase employee engagement and embed Finance Competency Model through creation and execution of strategic communication programme, development of Finance career path and integration of Finance Competency Model in performance management process. This has resulted in an increase in engagement as measured by annual survey as well as increases in internal hires for high-value roles, retention and gender diversity in management roles. – Achieved against target to support the 12-Point Plan through delivery of Order-to-Cash and Pricing initiatives, tracking of plan delivery and value creation, alignment of enterprise IT strategy to the plan, and clear communication to progress to shareholders. – Progressed target to drive business accountability through better reporting and insights through franchise P&L balance sheets, earlier communication of budget targets for improved planning, and improvement of control environment with emphasis on cyber risks. ESG – Achieved against target to progress building a more inclusive and diverse workforce, exceeding targets for female leaders in people management and female to male leaders in people management. Increased ethnic minorities in people management in both the US and UK markets. – Exceeded against target to increase employee engagement as measured by our Gallup annual survey. – Achieved above target to deliver 2023 milestones to reduce scope 1 & 2 greenhouse gases by 70% by 2025, and reduction of waste to landfill including attainment of zero waste to landfill at newest manufacturing facility in Malaysia. Outlined clear scope 3 plan and milestones. – Achieved against target to ensure ongoing compliance with disclosure requirements and started to create a profile of the investments required to meet stakeholder commitments. Customer – Achieved against target to continue merger and acquisition activities that strengthen growth and complement core businesses through seamless integration of acquisition and delivery of integration milestones. – Achieved above target to build and strengthen S+N’s innovation pipeline, including attainment of target for successful delivery of launches and on-time delivery of NPD programme milestones. – Achieved against target to simplify the Finance Operating Model to deliver better customer support through completion of end-to-end restructuring of global business services and Group Finance Controller Teams, as well as alignment of wider Finance team to new vertical Business Unit commercial operating model. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 143 |
| Therefore the total amount earned by Executive Directors in 2023 under the Annual Bonus Plan 2023 is: Amount earned in respect of financial objectives Amount earned in respect of business objectives Total amount earned Total as percentage of target Total as percentage of salary Deepak Nath $1,620,822 $376,301 $1,997,124 122.73% 132.02% Anne-Françoise Nesmes £676,855 £135,908 £812,763 119.61% 128.58% The Board has reviewed the formulaic calculation of these figures. We acknowledged that during 2023, the share price decreased by 4%, that the Company had partially delivered against its 2023 financial targets and that there had been no material risk or reputational events. We therefore determined that these outcomes were a fair representation of performance and there was no need to apply discretion to these formulaic outcomes. 50% of the total amount earned will be paid in cash and the remaining 50% will be deferred into shares which will vest after three years. 2024 Annual Bonus The maximum opportunity under the Annual Bonus Plan for Executive Directors will be 215% of base salary, subject to satisfactory performance against the performance measures detailed below. 50% of the award will be paid in cash and 50% will be deferred into shares which will vest after three years in accordance with the share ownership guidelines. Following the Remuneration Committee’s review of the incentive scorecard during 2023, the performance measures and weightings which apply to the Annual Bonus Plan 2024 are as follows: Weighting Threshold as a percentage of salary Target as a percentage of salary Maximum as a percentage of salary Revenue 35% 11.287% 37.625% 75.250% Trading margin 35% 11.287% 37.625% 75.250% Business objectives (including ESG) 15% 4.837% 16.125% 32.250% Trading cash flow conversion 15% 4.837% 16.125% 32.250% Total 100% 32.248% 107.500% 215.000% For reasons of commercial sensitivity no 2024 ABP targets can be disclosed at this stage. They will be disclosed retrospectively in the 2024 Annual Report, when performance against those targets is determined. Long-term incentives Performance Share Programme (PSP) Scheme Interests Vesting during the Year: PSP 2021 Since the end of the year, the Committee has reviewed the vesting of the conditional award made to the CFO in 2021 under the Global Share Plan 2020. Vesting of the conditional award made in 2021 was subject to performance against four equally weighted performance measures – TSR, global revenue growth, cumulative free cash flow and return on invested capital – measured over a three-year period commencing 1 January 2021. TSR performance 25% of the award was based on the Company’s TSR performance relative to two equally weighted peer groups against which the Company’s TSR performance was measured as follows: – A sector-based peer group based on those companies classified as the S&P 1200 Global Healthcare subset comprising medical devices, equipment and supplies companies (official industry classifications of ‘Health Care Equipment and Supplies, Life Sciences Tools & Services and Health Care Technology’). The Company’s TSR was -25.7% against an index threshold TSR for the peer group of -16.3%. – FTSE 100 constituents excluding financial services and commodities companies. This is in response to shareholders who assess our performance not based on sector, but instead based on the index we operate in. The Company’s TSR was -25.7% against an index threshold TSR for the peer group of 15.4%. In aggregate, therefore, the Company’s TSR performance results in a final vesting outcome of 0% out of the 25% target. Remuneration continued Remuneration implementation Report continued 144 Smith+Nephew Annual Report 2023 |
| Long-term incentives continued Performance Share Programme continued Global revenue growth 25% of the award was based on global revenue growth. The threshold set in 2021 was $15,799 million with a target of $17,173 million and a maximum of $18,547m. Over the three-year period, the adjusted revenues in Global Revenue Growth were $16,732 million. These adjustments include translational foreign exchange and Board-approved M&A. This part of the award therefore vested at 21% out of the 25% target. Cumulative free cash flow performance 25% of the award was based on cumulative cash flow performance. The threshold set in 2021 was $1,370m with a target of $1,713 million and a maximum of $2,055 million. Over the three-year period, the adjusted cumulative free cash flow was $629 million which was below threshold. These adjustments include translational foreign exchange and Board-approved M&A and restructuring programmes. This part of the award therefore vested at 0% out of the 25% target. Return on invested capital (ROIC) 25% of the award was based on return on invested capital defined as follows: Operating profit1 less adjusted taxes2 (Opening net operating assets + closing net operating assets)3 ÷ 2 1 Operating Profit is as disclosed in the Group income statement in the Annual Report less amortisation of acquired intangible assets. 2 Adjusted taxes represents our taxation charge per the Group income statement adjusted for the impact of tax on items not included in Adjusted Operating Profit notably amortisation of acquired intangible assets, interest income and expense, other finance costs and share of results of associates. 3 Net Operating Assets comprises net assets from the Group balance sheet (Total assets less total liabilities) excluding the following items: accumulated amortisation of acquired intangible assets, investments, investments in associates, retirement benefit assets and liabilities, long-term borrowings, bank overdrafts, borrowings and loans, IFRS 16 lease liabilities and right-of-use assets, and cash at bank. The threshold set in 2021 was 9.8% with a target of 11.8% and a maximum at 13.8%. The adjusted ROIC measurement was 6.7%. These adjustments include Board-approved M&A. This part of the award therefore vested at 0% of the 25% target. In summary, therefore, the Performance Share Programme award made in 2021 vested at 21% of target as follows: Threshold Target Maximum Actual Percentage Vesting TSR Equal to Index – 8% Above Index Below Index 0% Global revenue growth $15,799m $17,173m $18,547m $16,732m 21% Cumulative free cash flow $1,370m $1,713m $2,055m $508m 0% Return on invested capital 9.8% 11.8% 13.8% 6.7% 0% As well as considering the monetary outcome of the formulaic calculation of these awards, the Committee considered whether discretion should be applied to override these formulaic outcomes and concluded that the monetary outcomes were aligned with the financial performance of the Company during the performance period and the intention of the Remuneration Policy. Scheme Interests Granted during the Year: PSP 2023 In accordance with the Remuneration Policy approved by shareholders at the 2023 Annual General Meeting, performance share awards were granted to the Executive Directors under the Global Share Plan 2020 to a maximum value of 275% of salary (137.5% for target performance) measured over the three financial years commencing 1 January 2023 against four equally weighted performance measures: Indexed TSR, Global Revenue Growth, ROIC and Cumulative Free Cash Flow. The performance conditions for these awards were determined in February 2023 and the awards were made in March 2023. The maximum payout under each element will only be for significant outperformance. On vesting, sufficient shares will be sold to cover taxation obligations and the Executive Directors will be required to hold the net shares for a further period of two years. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 145 |
| TSR performance 25% of the award is based on the Company’s TSR performance measured against two equally weighted peer groups as defined for the awards made in 2020. TSR performance is relative to the two separate indices as follows: Relative TSR Award vesting as % of salary at date of grant Sector Based Peer Group FTSE 100 Peer Group Below the index Nil Nil Equalling the index (Threshold vesting at 50% of target) 8.6% 8.6% 8% above the index (Maximum vesting at 200% of target) 34.4% 34.4% Awards vest on a straight-line basis between these points. The maximum has been set significantly above target reflecting the maximum opportunity for outperformance. Global revenue growth 25% of the award is based on global revenue growth against the following targets: Revenue growth over three-year period commencing 1 January 2023 Award vesting as % of salary Below Threshold Nil Threshold (–8% of target) 17.2% Target – set by reference to our expectations 34.4% Maximum or above (+8% of target) 68.8% It is not possible to disclose precise targets for sales growth as this will give commercially sensitive information to our competitors concerning our growth plans and is considered to be potentially price-sensitive information. This target however will be disclosed in the 2026 Annual Report, when the Committee will discuss performance against the target. The maximum has been set significantly above target reflecting the increased maximum opportunity for outperformance. Return on invested capital (ROIC) 25% of the award is based on ROIC, as defined for the awards made in 2020, with the following targets: Return on Invested Capital (three-year average) Award vesting as % of salary Below Threshold 8.5% Nil Threshold 8.5% 17.2% Target 9.5% (+1.0% of threshold) 34.4% Maximum or above 10.5% (+1.0% of target) 68.8% Awards vest on a straight-line basis between these points Cumulative free cash flow 25% of the award is based on cumulative cash flow performance defined for the awards made in 2020, with the following targets: Cumulative free cash flow Award vesting as % of salary Below $1,233m Nil $1,233 (–20% of target) 17.2% $1,541m 34.4% $1,695m (target) or more (+10% of target) 68.8% The maximum has been set significantly above target reflecting the maximum opportunity for out-performance. Awards vest on a straight-line basis between these points. Remuneration continued Remuneration Implementation Report continued 146 Smith+Nephew Annual Report 2023 |
| Scheme Interests To be Granted Post Year End: PSP 2024 The Remuneration Committee reviewed the incentive scorecard during 2023, taking into account its prior commitment to introduce ESG metrics into the Performance Share Programme (“PSP”) from 2024. In early 2024, the Committee considered the performance framework and determined the targets for the PSP awards due to be made in 2024. In line with the results of its review, it was agreed that performance would be measured under a slightly modified set of performance measures and weightings compared to those applied in 2023. The measures for 2024 are indexed TSR, Global Revenue Growth, ROIC, and ESG Objectives, as set out below. The Executive Directors will be granted an award under the PSP 2024 with a maximum opportunity for the CEO of 300% of base salary (subject to shareholder approval at the AGM) and a maximum opportunity for the CFO of 275% of base salary. The award for the CFO will be granted in March 2024 and the award for the CEO will be granted following shareholder approval in accordance with the rules of the Global Share Plan 2020. TSR performance 30% of the award will be based on the Company’s TSR performance. The Committee have made refinements to both peer groups in order to remove outliers. The targets remain the same as the awards made in 2023. Revenue growth 30% of the award will be based on global revenue growth. It is not possible to disclose precise targets for sales growth as this will give commercially sensitive information to our competitors concerning our growth plans and is considered to be potentially price-sensitive information. ROIC 30% of the award will be based on ROIC as defined for the awards made in 2023. Targets will be 8.5% at Threshold, 9.5% at Target and 10.5% at Maximum. ESG objectives 10% of the award will be based on objectives relating to strategic priorities in this area. Details of outstanding awards made under the Performance Share Programme Details of conditional awards over shares granted to Executive Directors subject to performance conditions are shown below. These awards were granted under the Global Share Plan 2020. The performance conditions and performance periods applying to these awards are detailed below: Date granted Outstanding number of ordinary shares under award at maximum Date of vesting Deepak Nath 9 March 2023 283,748 9 March 2026 Deepak Nath 20 May 2022 259,422 20 May 2025 Anne-Françoise Nesmes 9 March 2023 140,106 9 March 2026 Anne-Françoise Nesmes 20 May 2022 134,648 20 May 2025 Anne-Françoise Nesmes 21 May 2021 102,936 21 May 2024 1 The award granted on 21 May 2021 will vest at 21%. Summary of scheme interests awarded during the financial year Director Deepak Nath Anne-Françoise Nesmes Number of shares Face value Number of shares Face value Performance Share Programme award at maximum (see pages 145–146) 283,748 £3,430,513.32 140,106 £1,693,881.54 Deferred Share Bonus Plan award (2022 bonus) 26,014 £314,509.26 16,877 £204,042.93 Please see Policy Table contained within the Annual Report 2022 on pages 119–128 on our website at www.smith-nephew.com for details of how the above plans operate. The number of shares is calculated using the closing share price on the day before grant, which for the Performance Share Programme award granted on 9 March 2023 was 1,209p. The Deferred Share Bonus Plan award granted on 9 March 2023 is calculated using the closing share price on the day before grant being 1209.0p. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 147 |
| Restricted Share Programme 2024 In line with the new Remuneration Policy being presented for shareholder approval, the Remuneration Committee intends to make an award in 2024 to the CEO under a new long-term incentive plan for which only US-based Executive Directors are eligible. It is expected that the CEO will be granted an award under the RSP, to the value of 125% of his base salary, subject to shareholder approval in accordance with the rules of the Restricted Share Programme. The award will vest in equal tranches on the first, second and third anniversaries of the grant date. Single total figure on remuneration Chair and Non-Executive Directors Director Basic annual fee1 Committee Chair/ Senior Independent Director fee Intercontinental travel fee Total 2023 2022 2023 2022 2023 2022 2023 2022 Rupert Soames2 £138,340 – – – £3,500 – £141,840 – Roberto Quarta3 £335,085 £428,645 – – – £3,500 £335,085 £432,145 Jo Hallas7 £69,500 £64,250 – – £3,500 £3,500 £73,000 £67,750 Erik Engstrom £69,500 £69,500 – – – £69,500 £69,500 Robin Freestone⁴ – £53,750 – £15,000 – – – £68,750 Jez Maiden5 £20,583 – – – – – £20,583 – John Ma $129,780 $129,780 – – $42,000 $21,000 $171,780 $150,780 Katarzyna Mazer-Hofsaess8 £69,500 £69,500 – – £3,500 £3,500 £73,000 £73,000 Rick Medlock £69,500 £69,500 £20,000 £20,000 £3,500 £3,500 £93,000 £93,000 Marc Owen6 $129,780 $129,780 $35,000 $35,000 $42,000 $21,000 $206,780 $185,780 Angie Risley £69,500 £69,500 £20,000 £20,000 £3,500 £3,500 £93,000 £93,000 Bob White $129,780 $129,780 – – $35,000 $7,000 $164,780 $136,780 1 The basic annual fee includes shares purchased for the Non-Executive Directors and previous Chair (Roberto Quarta) in lieu of part of the annual fee, details of which can be found on the table below. Rupert Soames fee does not include a “share” element. See below disclosure “Chair and Non-Executive Director fees”. 2 Rupert Soames joined the Board on 26 April 2023, becoming the Chair on 15 September 2023. 3 Roberto Quarta stepped down from the Board on 15 September 2023. 4 Robin Freestone stepped down from the Board on 30 September 2022. 5 Jez Maiden joined the Company on 14 September 2023. 6 Marc Owen waives his right to receive $35,000 in relation to his role of Senior Independent Director (effective from 1 October 2022). 7 Jo Hallas joined the Board on 1 February 2022. 8 Katarzyna Mazur-Hofsaess joined the Board on 1 November 2020. Chair and Non-Executive Director fees In February 2024, the fees paid to the Chair and the Non-Executive directors were reviewed. It was determined that the Non-Executive Director annual fees and the fee for the Senior Independent Director and Committee Chair roles be increased with effect from 1 April 2024. There was no change in the fee for the Chair or to the intercontinental travel fees. Annual fee paid to the Chair¹ £450,000 Annual fee paid to Non-Executive Directors £72,250 of which £6,757 paid in shares or $135,000 of which $10,173 paid in shares Intercontinental travel fee (per meeting) £3,500 or $7,000 Fee for Senior Independent Director and Committee Chair £20,800 or $36,400 1 The Chair is required, each year, to purchase shares worth at least 25% of his post-tax annual fee. On 27 April 2023, he purchased 9,040 shares at a price of 12.94p per share. Payments made to former Directors Roland Diggelmann ceased to be Chief Executive Officer and a member of the Board on 31 March 2022. As detailed in the 2021 Remuneration Report, in accordance with his employment agreement and with the Remuneration Policy approved by shareholders on 9 April 2020, Roland Diggelmann continued to receive his base salary of CHF1,380,000, pension payments and benefits up to 28 February 2023. Accordingly, he received a total of CHF255,238 for the period 1 January 2023 to 28 February 2023. Roland Diggelmann holds an award over 42,113 shares under the Deferred Share Bonus Plan (“DBP”) which was granted on 9 March 2022. This represented 50% of his 2021 bonus which vests after three years in line with the Remuneration Policy. He received a further award over 6,678 shares under the DBP on 9 March 2023 to the value of 50% of his 2022 annual bonus. Roland also holds awards (in aggregate) over 191,048 shares at maximum under the Performance Share Programme, exclusive of dividend equivalents. These shares were pro-rated to his date of leaving and vest subject to achievement of the relevant performance conditions. His prorated 2021 PSP award will vest at 21% on 21 May 2024. Remuneration continued Remuneration Implementation Report continued 148 Smith+Nephew Annual Report 2023 |
| Service contracts Existing Executive Directors are employed on rolling service contracts with notice periods of up to 12 months from the Company and six months from the Executive Director. In line with our updated 2024 Remuneration Policy, future appointments to Executive Director positions will apply rolling service contracts with notice periods of up to 12 months from the Company and 12 months from the Executive Director. Further information can be found on page 126 of the Policy Report contained within the 2022 Annual Report. Directors’ interests in ordinary shares Beneficial interests of the Executive Directors in the ordinary shares of the Company are as follows: Deepak Nath Anne-Françoise Nesmes 1 January 2023 31 December 2023 16 February 20241 1 January 2023 31 December 2023 16 February 20241 Ordinary shares 97,784 159,850 160,667 – – – Share options – – – 1,621 1,621 1,621 Deferred Share Bonus Plan award (2022 bonus) – 26,014 26,014 24,169 41,046 41,046 Buy-out award agreement 205,208 108,179 108,179 – – – Performance Share Programme awards2 259,422 543,170 543,170 280,310 377,690 377,690 1 The latest practicable date for this Annual Report. 2 These share awards are subject to further performance conditions before they may vest. The performance conditions attached to the award granted to the CFO on 21 May 2021 will vest at 21% on 20 May 2024 (see pages 144–145 for further details). The beneficial interest of each Executive Director is less than 1% of the ordinary share capital of the Company. Beneficial interests of the Directors in the ordinary shares of the Company are as follows: Director 1 January 2023 (or date of appointment if later) 31 December 2023 (or date of retirement if earlier) 16 February 20241 Shareholding as % of annual salary/fee2,3,8 Rupert Soames5 – 9,040 9,040 71.19 Roberto Quarta6 73,300 78,813 78,813 262.58 Erik Engstrom 16,774 17,097 17,097 274.78 Jo Hallas 5,332 5,655 5,655 86.53 John Ma4 924 1,500 1,500 12.25 Jez Maiden7 – 1,000 1,000 54.27 Katarzyna Mazur-Hofsaess 880 1,368 1,368 20.93 Rick Medlock 3,564 3,917 3,917 47.05 Deepak Nath4 97,784 159,850 160,667 164.03 Anne-Françoise Nesmes – – – 38.92 Marc Owen4 16,478 16,858 16,858 114.38 Angie Risley 5,343 5,666 5,666 68.05 Bob White4 7,284 7,860 7,860 66.92 1 The latest practicable date for this Annual Report. 2 Calculated using the closing share price of 1,117.0p per ordinary share and $28.06 per ADS on 16 February 2024, and an exchange rate of £1:$1.2583. 3 Due to their length of service some Non-Executive Directors have not met their shareholding requirements, but this will continue to be monitored in accordance with the Remuneration Policy. 4 John Ma, Marc Owen and Bob White hold their shares in the form of ADRs. Deepak Nath also holds some of his shares in the form of ADRs. 5 Rupert Soames joined the Board on 26 April 2023 and assumed chairmanship on 15 September 2023. 6 Roberto Quarta stepped down from the Board as Chairman on 15 September 2023; his shareholding stated is therefore as at 15 September 2023. 7 Jez Maiden was appointed Non-Executive Director on 14 September 2023. 8 For the purposes of calculating an Executive Director’s performance against their shareholding requirement, ordinary shares or ADRs held by the individual and their immediate family are included as are unvested awards under the DBP (on a net of tax basis) but not awards subject to an ongoing performance condition. The percentages in this column are consistent with this methodology. The beneficial interest of each Non-Executive Director is less than 1% of the ordinary share capital of the Company. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 149 |
| Chief Executive Officer remuneration compared to employees generally The percentage change in the remuneration of the Chief Executive Officer between 2021, 2022 and 2023 compared to that of employees generally was as follows: % change 2022/2023 % change 2021/2022 % change 2020/2021 Salary/fees Taxable benefits Annual incentive Salary/fees Taxable benefits Annual incentive Salary/fees Taxable benefits Annual incentive Executive Directors CEO Deepak Nath1 39.61% 55.81% 168.5% 0.00% -55.54% 44.89% 0.00% 0.00% – CFO Anne-Françoise Nesmes 4.18% 3.60% 129.6% 4.62% 3.97% -29.50% 0.00% 0.00% – Chairman Rupert Soames2 100.00% – – – – – – – – Former Chairman Roberto Quarta3 -22.46% – – 0.82% – – 0.37% – – Non-Executive Directors Erik Engstrom4 0.00% – – 0.00% – – 0.00% – – Angie Risley 0.00% – – 3.91% – – 0.00% – – Marc Owen5 11.30% – – 8.15% – – 0.00% – – Rick Medlock 0.00% – – 3.91% – – 51.58% – – Bob White5 20.47% – – 5.39% – – 44.55% – – Katarzyna Mazur-Hofsaess 0.00% – – 5.04% – – 561.90% – – Jo Hallas 7.75% – – 272.81% – – – – – John Ma5 13.93% – – 32.88% – – – – – Jez Maiden5,6 0.00% – – – – – – – – Average of all employees 5.18% – – 5.95% – – 1.64% – – 1 Deepak Nath was appointed CEO on 1 April 2022. 2 Rupert Soames joined the Board as a Non-Executive Director and Chair Designate on 26 April 2023 and was appointed Chair of the Board on 15 September 2023. 3 Roberto Quarta stepped down as Chair of the Board on 15 September 2023. 4 Erik Engstrom stepped down from the Board as a Non-Executive Director on 31 December 2023. 5 The change in benefits is due to changes in travel spend during the year. 6 Jez Maiden joined the Board as a Non-Executive Director on 14 September 2023. The average cost of wages and salaries for employees generally increased by 7.54% in 2023 (see Note 3.1 to the Group accounts). Figures for annual cash bonuses are included in the numbers. When considering remuneration arrangements for our Executive Directors, the Committee takes into account pay across the Group in the following ways: – Salary levels and increases for all employees including Executive Directors take account of the scope and responsibility of position, the skills, experience and performance of the individual and general economic conditions within the relevant geographical market. When considering increases to Executive Director base salaries, the Committee considers the average pay increases in the market where the Executive Director is based. – All employees including the Executive Directors have performance objectives determined at the beginning of the year which cascade down from the Strategic Imperatives for the Group. – The level of variable pay determined for all employees, whether in the form of shares or cash is dependent on performance against these imperatives, both financially and personally. – Executive Directors participate in benefits plans and arrangements comparable to benefits paid to other senior executives in the relevant geography. Executive Directors participate in the same senior executive incentive plans (currently the Annual Bonus Plan and the Performance Share Programme) as other Executive Officers and senior executives. The level of award reflects the differing seniority of participants and the market where the Executive is located. Performance conditions for the Performance Share Programme are the same for Executive Directors and Executive Officers. Executives, however, have only three measures with no reference to ROIC. For the Annual Bonus Plan (ABP) Performance Measures apply to all Executives consistently, however, weighting between Financials and Non-Financials differs based on the position. Remuneration continued Remuneration Implementation Report continued 150 Smith+Nephew Annual Report 2023 |
| Chief Executive Officer pay ratio The regulations provide three options which may be used to calculate the pay for the employees at the 25th percentile, median and 75th percentile. We have used option A (as set out in the Companies (Miscellaneous Reporting) Regulations 2018), following guidance issued by some proxy advisers and institutional shareholders. The ratio has been calculated by comparing against the full-time equivalent pay of all UK employees within the Group including both our entities Smith & Nephew UK Limited and T.J. Smith and Nephew, Limited. Option A calculates pay for all employees on the same basis as the single figure for remuneration calculated for Executive Directors. The period for which the employee pay has been calculated under Option A is the calendar year 2023. Figures are calculated by reference to 31 December 2023 using actual pay data from 1 January 2023 to 31 December 2023. The single figure for remuneration for each employee includes earned salary, annual incentive, allowance, pension and benefits for 2023. Part-time employees have been excluded for the purpose of calculations. Comparisons have been made with employees at median (P50), lower (P25) and upper (P75) quartiles. We have used the actual salaries paid to our employees in the UK. The values were listed lowest to highest and three percentiles were identified. We are confident this methodology gives us the most reflective pay at the median. The Committee is satisfied that the individuals identified in the employee comparison group appropriately reflect the employee pay profile at those quartiles, and that the overall picture presented by the ratios is consistent with our pay, reward and progression policies for UK employees. The table below sets out the ratio at the median, lower and upper quartiles: Year P25 (lower quartile) P50 (median) P75 (upper quartile) 2019 116:1 81:1 51:1 2020 42:1 29:1 19:1 2021 71:1 49:1 32:1 2022 160:1 107.1 70:1 2023 102:1 72:1 46:1 In 2023, the ratio was impacted by the vesting of the performance award under the 2022 buy-out award agreement made to Deepak Nath. Excluding this one-off arrangement, the median ratio would have been 55:1. The table below provides the total pay figure used for each quartile employee, and the salary component within this. Component CEO P25 (lower quartile) P50 (median) P75 (upper quartile) Salary $1,512,726 $45,600 $51,244 $77,454 Total pay $4,658,252 $45,600 $64,627 $101,369 Relative importance of spend on pay When considering remuneration arrangements for our Executive Directors and employees as a whole, the Committee also takes into account the overall profitability of the Company and the amounts spent elsewhere, particularly in returning profits to shareholders in the form of dividends and share buy-backs. The following table sets out the total amounts spent in 2023 and 2022 on remuneration, the attributable profit for each year and the dividends declared and paid in each year. For the year to 31 December 2023 For the year to 31 December 2022 % change Attributable profit for the year $263m $223m 18% Dividends paid during the year $327m $327m 0% Share buy-back1 $0m $158m -100% Total Group spend on remuneration² $1,683m $1,565m 7.5% 1 Shares are bought in the market in respect of shares issued as part of the executive and employee share plans. In December 2021 we announced an updated capital allocation policy to prioritise the use of cash. The 2022 share buyback programme commenced on 22 February 2022 and $150 million was completed by 31 August 2022. As macroeconomic conditions continued to be uncertain, including higher cost inflation, the Board decided it was prudent to delay further buy-backs until conditions improved. We remain committed to returning surplus cash to shareholders over time. 2 See note 3.1 staff costs and employee numbers. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 151 |
| Total Shareholder Return A graph of the Company’s TSR performance compared to that of the FTSE 100 index, of which the Company, is a constituent, is shown below in accordance with Schedule 8 to the Regulations. Dec 2013 Dec 2014 Dec 2015 Dec 2016 Dec 2017 Dec 2018 Dec 2019 Dec 2020 Dec 2021 Dec 2022 Dec 2023 Source: S&P Capital IQ Smith+Nephew FTSE 100 Ten-year Total Shareholder Return (measured in UK Sterling, based on monthly spot values) 300 250 200 150 100 50 0 As we also compare the Company’s performance to a tailored sector peer group of medical devices companies (see page 144), when considering TSR performance in the context of the Global Share Plan 2010 and Global Share Plan 2020, we feel that the following graph showing the TSR performance of this peer group is also of interest. Source: S&P Capital IQ Medical Devices comparators that are still trading for awards made since 2012 Dec 2013 Dec 2014 Dec 2015 Dec 2016 Dec 2017 Dec 2018 Dec 2019 Dec 2020 Dec 2021 Dec 2022 Dec 2023 Smith+Nephew S&P Medical Devices Ten-year Total Shareholder Return (measured in US Dollars, based on monthly spot values) 700 600 500 400 300 200 100 0 Remuneration continued Remuneration Implementation Report continued 152 Smith+Nephew Annual Report 2023 |
| Table of historic data The following table details information about the pay of the Chief Executive Officer in the previous 10 years: Year Long-term incentive vesting rates against maximum opportunity Chief Executive Officer Single figure of total remuneration $ Annual Cash Incentive payout against maximum % Performance Share Programme shares % 2023 Deepak Nath $4,658,252 61 – 2022 Deepak Nath1 $5,955,246 32 – 2022 Roland Diggelmann $603,103 24 – 2021 Roland Diggelmann $3,102,426 41 – 2020 Roland Diggelmann $1,697,773 05 – 2019 Roland Diggelmann2 $265,814 – – 2019 Namal Nawana3 $4,489,374 716 – 2018 Namal Nawana $2,883,632 69 – 2018 Olivier Bohuon4 $2,383,582 63 46.5 2017 Olivier Bohuon $5,116,689 61 54 2016 Olivier Bohuon $3,332,850 30 8 2015 Olivier Bohuon $5,342,377 75 33.5 2014 Olivier Bohuon $6,785,121 43 57 1 Appointed Chief Executive Officer on 1 April 2022. 2 Appointed Chief Executive Officer on 1 November 2019 and stepped down on 31 March 2022. 3 Appointed Chief Executive Officer on 7 May 2018 and resigned on 31 October 2019. 4 Retired as Chief Executive Officer on 7 May 2018. 5 Due to the impact of Covid upon the Chief Executive Officer’s financial targets, a cash award of 0% was achieved. 6 Calculated as 106.7% for Namal Nawana (disclosed on page 108 of the Company’s Annual Report for the year ended 31 December 2019), divided by the maximum potential payout of 150%. Gender pay ratio In 2023, the Committee reviewed our UK gender pay ratio. It was noted that today our gender pay gap is greater than we would like it to be, but we are seeing improvements year-on-year. Both our mean pay gap and median pay gap have decreased from 16% in 2022 to 14% in 2023. We shall continue to review these figures. Shareholding requirements If based outside the US, the Chief Executive Officer is expected to build a holding of Smith+Nephew shares worth three times base salary and the Chief Financial Officer is expected to build a holding of two times base salary. If based in the US, the Chief Executive Officer is expected to build a holding of Smith+Nephew shares worth four times base salary and the Chief Financial Officer is expected to build a holding of three times base salary. Executive Directors have five years from their appointment within which to meet that holding requirement. Due to the tenure of the Executive Directors neither have met their shareholding requirements, but this will continue to be monitored in accordance with the Remuneration Policy. Post-cessation shareholding requirements In addition, Executive Directors are expected to hold vested shares for up to two years post-vesting of the Performance Share Programme and Deferred Share Bonus Plan. They are expected to hold up to their shareholding requirement only. These shares are held in the vested Share Plan Account provided by the Company’s share plan administrator. Statement of voting at Annual General Meeting At the Annual General Meeting held on 26 April 2023, votes cast by proxy and at the meeting and votes withheld in respect of the votes on the Directors’ Remuneration Report are noted below. In addition, votes cast by proxy and at the meeting and votes withheld in respect of the votes on the Directors’ Remuneration Policy, which was last approved by shareholders on 26 April 2023, are noted below: Resolution Votes for % for Votes against % against Total votes validly cast Votes withheld Approval of the Directors’ Remuneration Report (excluding policy) 641,046,658 94.20 39,445,391 5.80 680,492,049 435,096 Approval of the Directors’ Remuneration Policy at the 2023 Annual General Meeting 643,583,465 94.55 37,067,165 5.45 680,650,630 276,215 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 153 |
| Senior management remuneration The Group’s administrative, supervisory and management body (senior management) comprises, for US reporting purposes, Executive Directors, Non-Executive Directors and Executive Officers. Details of the current Executive Directors, Non-Executive Directors and Executive Officers are given on pages 90–95. Compensation paid to senior management in respect of 2021, 2022 and 2023 was as follows: 2023 2022 2021 Total compensation (excluding pension emoluments, but including cash payments under the performance-related incentive plans) $18,890,117 $17,211,000 $15,795,000 Total compensation for loss of office $1,659,101 – – Aggregate increase in accrued pension scheme benefits – – – Aggregate amounts provided for under supplementary pension schemes $1,332,505 $1,626,000 $1,454,000 As at 16 February 2024, senior management owned 619,051 shares and 11,912 ADSs, constituting less than 0.074% of the share capital of the Company. For this purpose, the Group is defined as the Executive Directors, members of the Executive Committee, including the Company Secretary and their Persons Closely Associated. Details of share awards granted during the year and held as at 16 February 2024 by members of senior management are as follows: Share awards granted during the year Total share awards held as at 16 February 2024 Equity Incentive Programme awards 0 28,648 Deferred Share Bonus Plan awards 113,017 159,442 Performance Share Programme awards at maximum 1,501,680 2,943,708 Performance Share Programme – Supplementary awards 0 0 Conditional Share Awards under the Global Share Plan 2020 0 77,399 Sign-on Awards under the Global Share Plan 2020 69,604 69,604 Buy-Out Award Agreement 0 108,179 Options under Employee ShareSave plans 0 3,756 The Smith+Nephew Employee Share Trust Note 19.2 of these accounts states the movement in Treasury Shares and the Trust during 2023. No more shares are held within the Trust than are required for the next twelve months’ of anticipated vestings. Any unvested shares held in the Trust are not voted upon at shareholder meetings. No more than 5% of the issued share capital at 31 December 2023 is held within the Trust. At 31 December 2023 shares were held in the Trust representing 0.28% of the issued share capital. Dilution headroom The Remuneration Committee ensures that at all times the number of new shares which may be issued under any share-based plans, including all-employee plans, does not exceed 10% of the Company’s issued share capital over any rolling 10-year period (of which up to 5% may be issued to satisfy awards under the Company’s discretionary plans). The Company monitors headroom closely when granting awards over shares taking into account the number of options or shares that might be expected to lapse or be forfeited before vesting or exercise. In the event that insufficient new shares are available, there are processes in place to purchase shares in the market to satisfy vesting awards and to net-settle option exercises. Over the previous 10 years (2014 to 2023), the number of new shares issued under our share plans has been as follows: All-employee share plans 6,461,742 (0.74% of issued share capital as at 16 February 2024) Discretionary share plans 9,340,452 (1.07% of issued share capital as at 16 February 2024) By order of the Board, on 26 February 2024 Angie Risley Chair of the Remuneration Committee Remuneration continued Remuneration Implementation Report continued 154 Smith+Nephew Annual Report 2023 |
| Accounts Statement of Directors’ responsibilities 156 Report of Independent Registered Public Accounting Firm 157 Group financial statements 172 Notes to the Group accounts 176 Company financial statements 227 Notes to the Company accounts 229 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 155 |
| The Directors are responsible for preparing the Annual Report and Form 20-F and the Group and Parent Company financial statements in accordance with applicable law and regulations. Company law requires the Directors to prepare Group and Parent Company financial statements for each financial year. Under that law they are required to prepare the Group financial statements in accordance with UK-adopted international accounting standards and applicable law and have elected to prepare the Parent Company financial statements in accordance with UK accounting standards and applicable law, including FRS 101 Reduced Disclosure Framework. In addition the Directors have also chosen to prepare the Group financial statements in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB). Under company law the Directors must not approve the financial statements unless they are satisfied that they give a true and fair view of the state of affairs of the Group and Parent Company and of their profit or loss for that period. In preparing each of the Group and Parent Company financial statements, the Directors are required to: – Select suitable accounting policies and then apply them consistently; – Make judgements and estimates that are reasonable, relevant, reliable and prudent; – For the Group financial statements, state whether they have been prepared in accordance with UK-adopted international accounting standards and IFRS Accounting Standards as issued by the IASB; – For the Parent Company financial statements, state whether applicable UK Accounting Standards have been followed, subject to any material departures disclosed and explained in the Parent Company financial statements; – Assess the Group and Parent Company’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern; and – Use the going concern basis of accounting unless they either intend to liquidate the Group or the Parent Company or to cease operations, or have no realistic alternative but to do so. In accordance with their obligations under the Companies Act 2006, the Directors are responsible for keeping adequate accounting records that are sufficient to show and explain the Parent Company’s transactions and disclose with reasonable accuracy at any time the financial position of the Parent Company and enable them to ensure that its financial statements comply with the Companies Act 2006. They are responsible for such internal control as they determine is necessary to enable the preparation of financial statements that are free from material misstatement, whether due to fraud or error, and have general responsibility for taking such steps as are reasonably open to them to safeguard the assets of the Group and to prevent and detect fraud and other irregularities. Under applicable law and regulations, the Directors are also responsible for preparing a Strategic Report, Directors’ Report, Directors’ Remuneration Report and Corporate Governance Statement that comply with that law and those regulations. The Directors are responsible for the maintenance and integrity of the corporate and financial information included on the Company’s website. Legislation in the UK governing the preparation and dissemination of financial statements may differ from legislation in other jurisdictions. In accordance with Disclosure Guidance and Transparency Rule (“DTR”) 4.1.16R, the financial statements will form part of the annual financial report prepared under DTR 4.1.17R and 4.1.18R. The auditor’s report on these financial statements provides no assurance over whether the annual financial report has been prepared in accordance with those requirements. Responsibility statement of the Directors in respect of the Annual Report We confirm that to the best of our knowledge: – The financial statements, prepared in accordance with the applicable set of accounting standards, give a true and fair view of the assets, liabilities, financial position and profit or loss of the Company and the undertakings included in the consolidation taken as a whole; and – The Strategic Report and Directors’ Report include a fair review of the development and performance of the business and the position of the issuer and the undertakings included in the consolidation taken as a whole, together with a description of the principal risks and uncertainties that they face. The Strategic Report, which has been prepared in accordance with the requirements of the Companies Act 2006, comprises pages IFC–87. The Directors’ Report, prepared in accordance with the requirements of the Companies Act 2006 and the UK Listing Authority’s Listing Rules, and Disclosure Guidance and Transparency Rules, comprising pages 7, 22–23, 34–45, 46–49, 52–66, 67–79, 82–87, 88, 98–99, 108, 111–113, 114–120, 205–211, 226, 231–234 and 248–256, was approved by the Board and signed on its behalf. We consider the Annual Report and financial statements, taken as a whole, are fair, balanced and understandable and provide the information necessary for shareholders to assess the Group’s position and performance, business model and strategy. By order of the Board, on 26 February 2024. Helen Barraclough Company Secretary Statement of Directors’ responsibilities in respect of the Annual Report and Financial Statements 156 Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Smith & Nephew plc:
Opinions on the Consolidated Financial Statements and Internal Control Over Financial Reporting
We have audited the accompanying Group balance sheets of Smith & Nephew plc and subsidiaries (the “Group”) as of 31 December 2023 and 2022, the related Group income statements, Group statements of comprehensive income, Group cash flow statements and Group statements of changes in equity for each of the years in the three-year period ended 31 December 2023, and the related notes (collectively, the “consolidated financial statements”). We also have audited the Group’s internal control over financial reporting as of 31 December 2023, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Group as of 31 December 2023 and 2022, and the results of its operations and its cash flows for each of the years in the three-year period ended 31 December 2023, in conformity with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board. Also in our opinion, the Group maintained, in all material respects, effective internal control over financial reporting as of 31 December 2023 based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Basis for Opinions
The Group’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Evaluation of Internal Controls. Our responsibility is to express an opinion on the Group’s consolidated financial statements and an opinion on the Group’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Group in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgements. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Smith+Nephew Annual Report 2023 | 157 |
Recoverability of Orthopaedics CGU goodwill |
As discussed in Notes 1.2 and 8 to the consolidated financial statements, the goodwill balance as of 31 December 2023 was $2,992 million, of which $915 million related to the Orthopaedics cash generating unit (CGU). The Group performs an impairment test for goodwill annually and additionally whenever an indicator of impairment is identified. The recoverable amounts are based on value-in-use which is calculated from pre-tax cash flow projections for three years using data from the Group’s budget and strategic planning process and extrapolated for a further two years. We identified the evaluation of the recoverability of Orthopaedics CGU goodwill as a critical audit matter. Significant auditor judgement was required to evaluate the key assumptions used in the Group’s impairment test, specifically the revenue growth rates and trading profit margins. Minor changes to the trading profit margins would have a significant effect on the Group’s assessment of the recoverable amount of the goodwill of Orthopaedics CGU. The following are the primary procedures we performed to address this critical audit matter. — We evaluated the design and tested the operating effectiveness of certain internal controls over the Group’s goodwill impairment process, including controls over the key assumptions listed above. — We assessed the revenue growth rates and trading profit margins assumptions by comparing them to external industry forecasts and analysts’ reports. — We evaluated the Group’s ability to forecast the cash flow projections by comparing historical actual results to the approved budgets in the previous years. — We performed a sensitivity analysis over the key assumptions listed above to assess the impact on the value in use. |
Provision for excess and obsolescence (E&O) for Orthopaedics inventory |
As discussed in Notes 1.2 and 12 to the consolidated financial statements, the Group’s total E&O provision is $544 million, approximately 82% of which is related to Orthopaedics. The Group has high levels of Orthopaedics inventory that is available for customers’ immediate use. Complete sets of products including large and small sizes of inventory (which are used less frequently) have to be available to customers at their premises. An assessment is made by the Group to identify excess or obsolete inventory. The key input into this provision is the estimate of the forecasted usage of inventory on hand which is based on assumptions of historical sales of inventory adjusted for other internal or external factors such as effectiveness of inventory deployment, length of product lives and planned phase out of product which may impact the demand for the product. We identified the evaluation of the provision for excess and obsolescence for Orthopaedics inventory as a critical audit matter. A high degree of auditor judgement was required in assessing management’s estimate of forecasted usage of inventory, including the assessment of adjustments made to historical sales data. The following are the primary procedures we performed to address this critical audit matter. — We evaluated the design and tested the operating effectiveness of the internal control over the Group’s process for assessing the E&O provision, specifically the Group’s control over the key assumptions used to determine forecasted usage of Orthopaedics inventory. — We assessed and challenged the key assumptions listed above through a combination of interviews of both finance and operations personnel and inspection of internal budgets, including a selection of product plans to assess the impact of plans for phasing out product lines on forecasted usage of Orthopaedics inventory. — We evaluated the Group’s ability to accurately estimate the E&O provision by comparing historically recorded provisions to actual inventory write-offs and historic estimates of forecasted usage to actual usage. — We assessed the sensitivity of the key assumptions listed above, incorporating the recent volatility in sales of inventory, to consider their impact on the Group’s determination of the provision recognised. |
Provision for metal-on-metal hip products |
As discussed in Note 17.1 to the consolidated financial statements, the Group holds a provision of $149 million relating to the present value at 31 December 2023 of the estimated costs to resolve all other known and anticipated metal-on-metal hip claims globally. The estimate for this provision requires the Group to use an actuarial model and make a number of key assumptions relating to the number of claimants and settlement outcomes. We identified the evaluation of the provision for metal-on-metal hip products for these potential liabilities as a critical audit matter because especially challenging auditor judgement and specialised skills and knowledge was required in assessing the key assumptions above. Minor changes to these assumptions would have a significant effect on the provision. |
158 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
The following are the primary procedures we performed to address this critical audit matter. — We evaluated the design and tested the operating effectiveness of certain internal controls over the Group’s legal provision process. This included controls related to the Group’s review, challenge and assessment of the metal–on-metal provision and related key assumptions listed above. — We obtained correspondence directly from the Group’s external counsel on the status of open metal-on-metal court proceedings and settlement negotiations. We compared the number of open metal-on-metal claims per the Group's records against this correspondence, and considered any relevant information provided in our evaluation of the related exposure. — We involved actuarial professionals with specialised skills and knowledge, who assisted in challenging the number of claimants and settlement outcomes used in statistical projections in determining the provision, as well as the range of reasonably possible outcomes determined by the Group, by reference to historical data including settlement amounts, number of new claimants and experience of other cases. In addition, the actuarial professionals assisted in evaluating the statistical model applied by the Group with actuarial professional standards and industry practice for similar product liability claims. — We evaluated the scope, competency, and objectivity of the Group’s experts involved in developing the actuarial model used in the determination of the provision by considering the work they were engaged to perform, their professional qualifications, and reporting lines. |
/s/
We have served as the Group’s auditor since 2015.
26 February 2024
PCAOB ID
Smith+Nephew Annual Report 2023 | 159 |
THIS PAGE LEFT INTENTIONALLY BLANK
160 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
THIS PAGE LEFT INTENTIONALLY BLANK
Smith+Nephew Annual Report 2023 | 161 |
THIS PAGE LEFT INTENTIONALLY BLANK
162 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
THIS PAGE LEFT INTENTIONALLY BLANK
Smith+Nephew Annual Report 2023 | 163 |
THIS PAGE LEFT INTENTIONALLY BLANK
164 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
THIS PAGE LEFT INTENTIONALLY BLANK
Smith+Nephew Annual Report 2023 | 165 |
THIS PAGE LEFT INTENTIONALLY BLANK
166 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
THIS PAGE LEFT INTENTIONALLY BLANK
Smith+Nephew Annual Report 2023 | 167 |
THIS PAGE LEFT INTENTIONALLY BLANK
168 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
THIS PAGE LEFT INTENTIONALLY BLANK
Smith+Nephew Annual Report 2023 | 169 |
THIS PAGE LEFT INTENTIONALLY BLANK
170 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
THIS PAGE LEFT INTENTIONALLY BLANK
Smith+Nephew Annual Report 2023 | 171 |
Group financial statements
Group income statement
|
| Year ended |
| Year ended |
| Year ended | ||
31 December | 31 December | 31 December | ||||||
2023 | 2022 | 2021 | ||||||
| Notes |
| $ million |
| $ million |
| $ million | |
Revenue | 2 | | | | ||||
Cost of goods sold |
| ( | ( | ( | ||||
Gross profit |
| | | | ||||
Selling, general and administrative expenses | 3 | ( | ( | ( | ||||
Research and development expenses | 3 | ( | ( | ( | ||||
Operating profit | 2 & 3 | | | | ||||
Interest income | 4 | | | | ||||
Interest expense | 4 | ( | ( | ( | ||||
Other finance costs | 4 | ( | ( | ( | ||||
Share of results of associates | 11 | ( | ( | | ||||
Gain on disposal of interest in associate | – | – | | |||||
Profit before taxation |
| | | | ||||
Taxation | 5 | ( | ( | ( | ||||
Attributable profit for the year1 |
| | | | ||||
Earnings per ordinary share1 | 6 |
|
|
| ||||
Basic |
| |||||||
Diluted |
|
Group statement of comprehensive income
|
| Year ended |
| Year ended |
| Year ended | ||
31 December | 31 December | 31 December | ||||||
2023 | 2022 | 2021 | ||||||
| Notes |
| $ million |
| $ million |
| $ million | |
Attributable profit for the year1 |
| | | | ||||
Other comprehensive income: |
|
|
|
| ||||
Items that will not be reclassified to income statement |
|
|
|
| ||||
Remeasurement of net retirement benefit obligations | 18 | ( | | | ||||
Taxation on other comprehensive income | 5 | | ( | ( | ||||
Total items that will not be reclassified to income statement |
| ( | | | ||||
Items that may be reclassified subsequently to income statement |
|
|
|
| ||||
Cash flow hedges – forward foreign exchange contracts |
|
|
|
| ||||
Gains arising in the year |
| | | | ||||
(Gains)/losses transferred to inventories for the year |
| ( | ( | | ||||
Exchange differences on translation of foreign operations |
| | ( | ( | ||||
Taxation on other comprehensive income | 5 | – | | ( | ||||
Total items that may be reclassified subsequently to income statement |
| | ( | ( | ||||
Other comprehensive (loss)/income for the year, net of taxation |
| ( | ( | | ||||
Total comprehensive income for the year1 |
| | | |
| 1 |
The Notes on pages 176–226 are an integral part of these accounts.
172 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Group balance sheet
At | At | |||||
31 December | 31 December | |||||
2023 | 2022 | |||||
| Notes |
| $ million |
| $ million | |
Assets |
|
|
| |||
Non-current assets |
|
|
| |||
Property, plant and equipment | 7 | | | |||
Goodwill | 8 | | | |||
Intangible assets | 9 | | | |||
Investments | 10 | | | |||
Investments in associates | 11 | | | |||
Other non-current assets | 13 | | | |||
Retirement benefit assets | 18 | | | |||
Deferred tax assets | 5 | | | |||
| | | ||||
Current assets |
|
|
| |||
Inventories | 12 | | | |||
Trade and other receivables | 13 | | | |||
Current tax receivable | | | ||||
Cash at bank | 15 | | | |||
| | | ||||
Total assets |
| | | |||
|
|
| ||||
Equity and liabilities |
|
|
| |||
Equity attributable to owners of the Company |
|
|
| |||
Share capital | 19 | | | |||
Share premium |
| | | |||
Capital redemption reserve |
| | | |||
Treasury shares | 19 | ( | ( | |||
Other reserves |
| ( | ( | |||
Retained earnings |
| | | |||
Total equity |
| | | |||
Non-current liabilities |
|
|
| |||
Long-term borrowings and lease liabilities | 15 | | | |||
Retirement benefit obligations | 18 | | | |||
Other payables | 14 | | | |||
Provisions | 17 | | | |||
Deferred tax liabilities | 5 | | | |||
| | | ||||
Current liabilities |
|
|
| |||
Bank overdrafts, borrowings, loans and lease liabilities | 15 | | | |||
Trade and other payables | 14 | | | |||
Provisions | 17 | | | |||
Current tax payable | | | ||||
| | | ||||
Total liabilities |
| | | |||
Total equity and liabilities |
| | |
The accounts were approved by the Board and authorised for issue on 26 February 2024 and are signed on its behalf by:
Rupert Soames, OBE | Deepak Nath, PhD | Anne-Françoise Nesmes |
Chair | Chief Executive Officer | Chief Financial Officer |
The Notes on pages 176–226 are an integral part of these accounts.
Group financial statements continued
Group cash flow statement
Year ended | Year ended | Year ended | ||||||
31 December | 31 December | 31 December | ||||||
2023 | 2022 | 2021 | ||||||
|
| Notes |
| $ million |
| $ million |
| $ million |
Cash flows from operating activities |
|
|
|
| ||||
Profit before taxation |
| | | | ||||
Net interest expense | 4 | | | | ||||
Depreciation, amortisation and impairment |
| | | | ||||
Loss on disposal of property, plant and equipment and software |
| | | | ||||
Share-based payments expense (equity-settled) | 22 | | | | ||||
Share of results of associates | 11 | | | ( | ||||
Gain on disposal of interest in associate | – | – | ( | |||||
Net movement in post-retirement benefit obligations |
| | | – | ||||
Increase in inventories |
| ( | ( | ( | ||||
Increase in trade and other receivables |
| ( | ( | ( | ||||
(Decrease)/increase in trade and other payables and provisions |
| ( | ( | | ||||
Cash generated from operations1 |
| | | | ||||
Interest received |
| | | | ||||
Interest paid |
| ( | ( | ( | ||||
Income taxes paid |
| ( | ( | ( | ||||
Net cash inflow from operating activities |
| | | | ||||
Cash flows from investing activities |
|
|
|
| ||||
Acquisitions, net of cash acquired | ( | ( | ( | |||||
Capital expenditure | ( | ( | ( | |||||
Purchase of investments | – | ( | ( | |||||
Distribution from associate | 11 | – | | | ||||
Net cash used in investing activities |
| ( | ( | ( | ||||
Cash flows from financing activities |
|
|
|
| ||||
Proceeds from issue of ordinary share capital | 20 | – | | | ||||
Purchase of own shares | 20 | – | ( | – | ||||
Payment of capital element of lease liabilities | 20 | ( | ( | ( | ||||
Proceeds from borrowings due within one year | 20 | | – | – | ||||
Settlement of borrowings due within one year | 20 | ( | ( | ( | ||||
Proceeds from borrowings due after one year | 20 | – | | – | ||||
Settlement of borrowings due after one year | 20 | – | ( | – | ||||
Proceeds from own shares | 20 | – | | | ||||
Settlement of currency swaps | 20 | | | ( | ||||
Equity dividends paid | 19 | ( | ( | ( | ||||
Net cash used in financing activities |
| ( | ( | ( | ||||
Net decrease in cash and cash equivalents |
| ( | ( | ( | ||||
Cash and cash equivalents at beginning of year | 20 | | | | ||||
Exchange adjustments | 20 | ( | ( | ( | ||||
Cash and cash equivalents at end of year2 |
| | | |
| 1 |
| 2 |
The Notes on pages 176–226 are an integral part of these accounts.
174 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Group statement of changes in equity
Capital | ||||||||||||||
Share | Share | redemption | Treasury | Other | Retained | Total | ||||||||
capital | premium | reserve | shares2 | reserves3 | earnings4 | equity | ||||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
At 31 December 2020 | | | | ( | ( | | | |||||||
Attributable profit for the year1 | – | – | – | – | – | | | |||||||
Other comprehensive income | – | – | – | – | ( | | | |||||||
Equity dividends declared and paid | – | – | – | – | – | ( | ( | |||||||
Share-based payments recognised | – | – | – | – | – | | | |||||||
Taxation on share-based payments | – | – | – | – | – | ( | ( | |||||||
Cost of shares transferred to beneficiaries | – | – | – | | – | ( | | |||||||
Issue of ordinary share capital5 | – | | – | – | – | – | | |||||||
At 31 December 2021 | | | | ( | ( | | | |||||||
Attributable profit for the year1 | – | – | – | – | – | | | |||||||
Other comprehensive income | – | – | – | – | ( | | ( | |||||||
Equity dividends declared and paid | – | – | – | – | – | ( | ( | |||||||
Share-based payments recognised | – | – | – | – | – | | | |||||||
Taxation on share-based payments | – | – | – | – | – | ( | ( | |||||||
Purchase of own shares | – | – | – | ( | – | – | ( | |||||||
Cost of shares transferred to beneficiaries | – | – | – | | – | ( | | |||||||
Cancellation of treasury shares | ( | – | | | – | ( | – | |||||||
Issue of ordinary share capital5 | – | | – | – | – | – | | |||||||
At 31 December 2022 | | | | ( | ( | | | |||||||
Attributable profit for the year1 | – | – | – | – | – | | | |||||||
Other comprehensive income | – | – | – | – | | ( | ( | |||||||
Equity dividends declared and paid | – | – | – | – | – | ( | ( | |||||||
Share-based payments recognised | – | – | – | – | – | | | |||||||
Cost of shares transferred to beneficiaries | – | – | – | | – | ( | – | |||||||
At 31 December 2023 | | | | ( | ( | | |
| 1 | Attributable to equity holders of the Company and wholly derived from continuing operations. |
| 2 |
| 3 |
| 4 |
| 5 |
The Notes on pages 176–226 are an integral part of these accounts.
Smith+Nephew Annual Report 2023 | 175 |
Group financial statements continued
Notes to the Group accounts
1 Basis of preparation
Smith & Nephew plc (the “Company”) is a public limited company incorporated in England and Wales. In these accounts, the ‘Group’ means the Company and all its subsidiaries. The principal activities of the Group are to develop, manufacture, market and sell medical devices and services.
The Group has prepared its accounts in accordance with UK-adopted International Accounting Standards. The Group has also prepared its accounts in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) effective as at 31 December 2023. IFRS as adopted in the UK differs in certain respects from IFRS Accounting Standards as issued by the IASB. However, the differences have no impact for the periods presented.
The preparation of accounts in conformity with IFRS requires management to use estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the accounts and the reported amounts of revenues and expenses during the year. The accounting policies requiring management to use significant estimates and assumptions are: valuation of inventories, liability provisions and impairment. These are discussed in Note 1.2 below. Although these estimates are based on management’s best knowledge of current events and actions, actual results ultimately may differ from those estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to estimates are recognised prospectively.
The uncertainties as to the future impact on the financial performance and cash flows of the Group as a result of the current challenging economic environment have been considered as part of the Group’s adoption of the going concern basis in these financial statements, in which context the Directors reviewed cash flow forecasts prepared for a period of at least 12 months from the date of approval of these financial statements. Having carefully reviewed those forecasts, the Directors concluded that it was appropriate to adopt the going concern basis of accounting in preparing these financial statements for the reasons set out below.
The Group had access to $
The Group has $
The Directors have considered various scenarios in assessing the impact of the economic environment on future financial performance and cash flows, with the key judgement applied being the speed and sustainability of the return to a normal volume of elective procedures in key markets, including the impact of a significant global economic recession, leading to lower healthcare spending across both public and private systems. Throughout these scenarios, which include a severe but plausible outcome, the Group continues to have headroom on its borrowing facilities and financial covenants.
The Directors have a reasonable expectation that the Company and the Group are well placed to manage their business risks, have sufficient funds to continue to meet their liabilities as they fall due and to continue in operational existence for a period of at least 12 months from the date of the approval of these financial statements. The financial statements have therefore been prepared on a going concern basis.
Accordingly, the Directors continue to adopt the going concern basis (in accordance with the guidance ‘Guidance on Risk Management, Internal Control and Related Financial and Business Reporting’ issued by the FRC) in preparing these financial statements.
New accounting standards effective 2023
A number of new amendments to standards are effective from 1 January 2023 but they do not have a material effect on the Group’s financial statements except for Deferred Tax related to Assets and Liabilities arising from a Single Transaction - Amendment to IAS 12, which the Group has adopted. The amendments narrow the scope of the initial recognition exemption to exclude transactions that give rise to equal and offsetting temporary differences such as leases.
The Group previously accounted for deferred tax on leases where the deferred tax asset or liability was recognised on a net basis. Following the amendments, the Group has recognised a separate deferred tax asset in relation to its lease liabilities and a deferred tax liability in relation to its right-of-use assets. However, there is no impact on the balance sheet because the balances qualify for offset under paragraph 74 of IAS 12. There was also no impact on the opening retained earnings as at 1 January 2023 as a result of the change. The policy for recognising and measuring income taxes is consistent with that applied in the comparative years except for the changes outlined above as a result of the Group’s adoption of the amendments to IAS 12. The change in accounting policy will also be reflected in the Group’s consolidated financial statements for the year ending 31 December 2023.
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Accounting standards issued but not yet effective
A number of new standards and amendments to standards are effective for annual periods beginning after 1 January 2024 and earlier application is permitted; however, the Group has not adopted them early in preparing these Financial Statements.
The Group is adopting the mandatory temporary exception from the recognition and disclosure of deferred taxes arising from the jurisdictional implementation of the Pillar Two model rules which will take effect for the Group from 1 January 2024.
1.1 Consolidation
The Group accounts include the accounts of Smith & Nephew plc and its subsidiaries for the periods during which they were members of the Group.
Subsidiaries are entities controlled by the Group. The Group controls an entity when it is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are consolidated in the Group accounts from the date that the Group obtains control and continue to be consolidated until the date that such control ceases. Intra-group balances and transactions, and any unrealised income and expenses arising from intra-group transactions, are eliminated on consolidation. All subsidiaries have year ends which are coterminous with the Group’s, with the exception of jurisdictions whereby a different year end is required by local legislation.
When the Group loses control over a subsidiary, it derecognises the assets and liabilities of the subsidiary and any related components of equity. Any resulting gain or loss is recognised in profit or loss. Any retained interest in the former subsidiary is measured at fair value.
1.2 Critical judgements and estimates
The Group prepares its consolidated financial statements in accordance with IFRS Accounting Standards as issued by the IASB and IFRS adopted in the UK, the application of which often requires judgements and estimates to be made by management when formulating the Group’s financial position and results. Under IFRS, the Directors are required to adopt those accounting policies most appropriate to the Group’s circumstances for the purpose of presenting fairly the Group’s financial position, financial performance and cash flows.
The Group’s accounting policies do not include any critical judgements. The Group’s accounting policies are set out in Notes 1–23 of the Notes to the Group accounts. Of those, the policies which require the most use of management’s estimation are outlined below. The critical estimates are consistent with 31 December 2022. Management have considered the impact of the uncertainties around the current challenging economic environment below.
Valuation of inventories
A feature of the Orthopaedics business unit (which accounts for approximately
Current economic environment impact assessment: In assessing the increase in provision for excess and obsolete inventory, management have considered the impact of higher input cost inflation on increased inventory levels. Management have not changed their accounting policy since 31 December 2022, nor is a change in the key assumptions underlying the methodology expected in the next 12 months. Primarily due to inventory growth, the provision has increased from $
Smith+Nephew Annual Report 2023 | 177 |
Group financial statements continued
Notes to the Group accounts continued
1 Basis of preparation continued
Liability provisioning
The recognition of provisions for legal disputes related to metal-on-metal cases is subject to a significant degree of estimation. Provision is made for loss contingencies when it is considered probable that an adverse outcome will occur and the amount of the loss can be reasonably estimated. In making its estimates, management takes into account the advice of internal and external legal counsel. Provisions are reviewed regularly and amounts updated where necessary to reflect developments in the disputes. The value of provisions may require future adjustment if experience such as number, nature or value of claims or settlements changes. Such a change may be material in 2024 or thereafter. The ultimate liability may differ from the amount provided depending on the outcome of court proceedings and settlement negotiations or if investigations bring to light new facts. See Note 17 for further details.
Current economic environment impact assessment: Management considered whether there had been any changes to the number and value of claims due to current challenging economic environment and to date have not identified any significant changes in trends. If the experience changes in the future, the value of provisions may require adjustment.
Impairment
In carrying out impairment reviews of intangible assets and goodwill, a number of significant assumptions have to be made when preparing cash flow projections. These include the future rate of market growth, discount rates, the market demand for the products acquired, the future profitability of acquired businesses or products, levels of reimbursement and success in obtaining regulatory approvals. If actual results should differ or changes in expectations arise, impairment charges may be required which would adversely impact operating results. There has been an increase in the level of headroom in relation to goodwill impairment testing for the Orthopaedics CGU which is still sensitive to a reasonably possible change in assumptions. In 2023, the Group impaired $
Current economic environment impact assessment: Management have assessed the non-current assets held by the Group at 31 December 2023 to identify any indicators of impairment as a result of current economic environment. Where an impairment indicator has arisen, impairment reviews have been undertaken by comparing the expected recoverable value of the asset to the carrying value of the asset. The recoverable amounts are based on cash flow projections using the Group’s base case scenario in its going concern models, which was reviewed and approved by the Board.
1.3 Climate change considerations
The impact of climate change has been considered as part of the assessment of estimates and judgements in preparing the Group accounts. The climate change scenario analyses undertaken this year in line with TCFD recommendations did not identify any material financial impact. The following considerations were made in respect of the financial statements:
| - | The impact of climate change on the going concern assessment and the viability of the Group over the next three years. |
| - | The impact of climate change on the cash flow forecasts used in the impairment assessments of non-current assets including goodwill. |
| - | The impact of climate change on the carrying value and useful economic lives of property, plant and equipment. |
1.4 Foreign currencies
Functional and presentation currency
The Group accounts are presented in US Dollars. The Company’s functional currency is US Dollars.
Foreign currency transactions
Transactions in foreign currencies are translated to the respective functional currencies of Group companies at exchange rates at the dates of the transactions. Monetary assets and liabilities denominated in foreign currencies are retranslated to the functional currency at the exchange rate as at the reporting date. Non-monetary items are not retranslated.
178 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Foreign operations
Balance sheet items of foreign operations, including goodwill and fair value adjustments arising on acquisition, are translated into US Dollars on consolidation at the exchange rates at the reporting date. Income statement items and the cash flows of foreign operations are translated at average rates as an approximation to actual transaction rates, with actual transaction rates used for large one-off transactions.
Foreign currency differences are recognised in ‘Other comprehensive income’ and accumulated in ‘Other reserves’ within equity. These include: exchange differences on the translation at closing rates of exchange of non-US Dollar opening net assets; the differences arising between the translation of profits into US Dollars at actual (or average, as an approximation) and closing exchange rates; to the extent that the hedging relationship is effective, the difference on translation of foreign currency borrowings or swaps that are used to finance or hedge the Group’s net investments in foreign operations; and the movement in the fair value of forward foreign exchange contracts used to hedge forecast foreign exchange cash flows.
The exchange rates used for the translation of currencies into US Dollars that have the most significant impact on the Group results were:
| 2023 |
| 2022 |
| 2021 | |
Average rates |
|
|
| |||
Sterling | | | | |||
Euro | | | | |||
Swiss Franc | | | | |||
Year end rates | ||||||
Sterling | | | | |||
Euro | | | | |||
Swiss Franc | | | |
2 Business segment information
The Group’s operating structure is organised around
Business unit presidents have responsibility for upstream marketing, driving product portfolio and technology acquisition decisions, full commercial responsibility and for the implementation of their business unit strategy globally.
The Executive Committee (‘ExCo’) comprises the Chief Financial Officer (‘CFO’), the business unit presidents and certain heads of function, and is chaired by the Chief Executive Officer (‘CEO’). ExCo is the body through which the CEO uses the authority delegated to him by the Board of Directors to manage the operations and performance of the Group. All significant operating decisions regarding the allocation and prioritisation of the Group’s resources and assessment of the Group’s performance are made by ExCo, and while the members have individual responsibility for the implementation of decisions within their respective areas, it is at the ExCo level that these decisions are made. Accordingly, ExCo is considered to be the Group’s chief operating decision maker as defined by IFRS 8 Operating Segments.
In making decisions about the prioritisation and allocation of the Group’s resources, ExCo reviews financial information for the
Smith+Nephew Annual Report 2023 | 179 |
Group financial statements continued
Notes to the Group accounts continued
2 Business segment information continued
2.1 Revenue by business segment and geography
Accounting policy
Revenue is recognised as the performance obligations to deliver products or services are satisfied and is recorded based on the amount of consideration expected to be received in exchange for satisfying the performance obligations. Revenue is recognised primarily when control is transferred to the customer, which is generally when the goods are shipped or delivered in accordance with the contract terms, with some transfer of services taking place over time. Substantially all performance obligations are fulfilled within one year. There is no significant revenue associated with the provision of services. Payment terms to our customers are based on commercially reasonable terms for the respective markets while also considering a customer’s credit rating. Appropriate provisions for returns, trade discounts and rebates are deducted from revenue. Rebates primarily comprise chargebacks and other discounts granted to certain customers. Chargebacks are discounts that occur when a third-party purchases product from a wholesaler at its agreed price plus a mark-up. The wholesaler in turn charges the Group for the difference between the price initially paid by the wholesaler and the agreed price. The provision for chargebacks is based on expected sell-through levels by the Group’s wholesalers to such customers, as well as estimated wholesaler inventory levels.
Orthopaedics and Sports Medicine & ENT (Ear, Nose & Throat)
Orthopaedics and Sports Medicine & ENT consists of the following businesses: Knee Implants, Hip Implants, Other Reconstruction, Trauma & Extremities, Sports Medicine Joint Repair, Arthroscopic Enabling Technologies and ENT. Sales of inventory located at customer premises and available for customers’ immediate use are recognised when notification is received that the product has been implanted or used. Substantially all other revenue is recognised when control is transferred to the customer, which is generally when the goods are shipped or delivered in accordance with the contract terms. Revenue is recognised for the amount of consideration expected to be received in exchange for transferring the products or services.
In general our business in Established Markets is direct to hospitals and ambulatory surgery centers whereas in the Emerging Markets we generally sell through distributors.
Advanced Wound Management
Advanced Wound Management consists of the following businesses: Advanced Wound Care, Advanced Wound Bioactives and Advanced Wound Devices. Substantially all revenue is recognised when control is transferred to the customer, which is generally when the goods are shipped or delivered in accordance with the contract terms. Revenue is recognised for the amount of consideration expected to be received in exchange for transferring the products or services. Appropriate provisions for returns, trade discounts and rebates are deducted from revenue, as explained above.
The majority of our Advanced Wound Management business, and in particular products used in community and homecare facilities, is through wholesalers and distributors. When control is transferred to a wholesaler or distributor, revenue is recognised accordingly. The proportion of sales direct to hospitals is higher in our Advanced Wound Devices business in Established Markets.
Segment revenue reconciles to statutory revenues from continuing operations as follows:
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Reportable segment revenue |
|
|
| |||
Orthopaedics | | | | |||
Sports Medicine & ENT | | | | |||
Advanced Wound Management | | | | |||
Revenue from external customers | | | |
180 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Disaggregation of revenue:
The following table shows the disaggregation of Group revenue by product by business unit:
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Revenue by product from continuing operations |
|
|
| |||
Knee Implants | | | | |||
Hip Implants | | | | |||
Other Reconstruction | | | | |||
Trauma & Extremities | | | | |||
Orthopaedics | | | | |||
Sports Medicine Joint Repair | | | | |||
Arthroscopic Enabling Technologies | | | | |||
ENT (Ear, Nose and Throat) | | | | |||
Sports Medicine & ENT | | | | |||
Advanced Wound Care | | | | |||
Advanced Wound Bioactives | | | | |||
Advanced Wound Devices | | | | |||
Advanced Wound Management | | | | |||
Consolidated revenue from continuing operations | | | |
The following table shows the disaggregation of Group revenue by geographic market and product category. The disaggregation of revenue into the two product categories below reflects that in general the products in the Advanced Wound Management business unit are sold to wholesalers and intermediaries, while products in the other business units are sold directly to hospitals, ambulatory surgery centers and distributors. The further disaggregation of revenue by Established Markets and Emerging Markets reflects that in general our products are sold through distributors and intermediaries in the Emerging Markets while in the Established Markets, with the exception of the Advanced Wound Care and Bioactives products, which are in general sold direct to hospitals and ambulatory surgery centers. The disaggregation by Established Markets and Emerging Markets also reflects their differing economic factors including volatility in growth and outlook.
2023 | 2022 | 2021 | ||||||||||||||||
Established | Emerging | Total | Established | Emerging | Total | Established | Emerging | Total | ||||||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
Orthopaedics, Sports Medicine & ENT | | | | | | | | | | |||||||||
Advanced Wound Management | | | | | | | | | | |||||||||
Total | | | | | | | | | |
| 1 | Established Markets comprises the US, Australia, Canada, Europe, Japan and New Zealand. |
Sales are attributed to the country of destination. US revenue for 2023 was $
Smith+Nephew Annual Report 2023 | 181 |
Group financial statements continued
Notes to the Group accounts continued
2 Business segment information continued
Contract assets and liabilities
The nature of our products and services do not generally give rise to contract assets as we do not typically incur costs to fulfil a contract before a product or service is provided to the customer. The Group generally satisfies performance obligations within one year from the contract inception date. There was no material revenue recognised in the current reporting period that related to carried-forward contract liabilities (deferred income) or performance obligations satisfied in the previous year. There is no material revenue that is likely to arise in future periods from unsatisfied performance obligations at the balance sheet date. Therefore, there are no associated significant accrued income and deferred income balances at 31 December 2023. As of 31 December 2023, contract assets principally comprise trade receivables and contract liabilities principally comprise rebates (as described in the accounting policy above). The accrual for rebates at 31 December 2023 was $
Major customers
2.2 Trading and operating profit by business segment
Trading profit is a trend measure which presents the profitability of the Group excluding the impact of specific transactions that management considers affect the Group’s short-term profitability and the comparability of results. The Group presents this measure to assist investors in their understanding of trends. The Group has identified the following items, where material, as those to be excluded from operating profit when arriving at trading profit: acquisition and disposal-related items; significant restructuring programmes; amortisation and impairment of acquisition intangibles; gains and losses arising from legal disputes; and other significant items. Further detail is provided in Notes 2.3, 2.4, 2.5 and 2.6.
Segment trading profit is reconciled to the statutory measure below:
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Segment profit | ||||||
Orthopaedics | | | | |||
Sports Medicine & ENT | | | | |||
Advanced Wound Management | | | | |||
Segment trading profit | | | | |||
Corporate costs | ( | ( | ( | |||
Group trading profit | | | | |||
Acquisition and disposal-related items1 | ( | ( | ( | |||
Restructuring and rationalisation expenses | ( | ( | ( | |||
Amortisation and impairment of acquisition intangibles1 | ( | ( | ( | |||
Legal and other1 | ( | ( | ( | |||
Group operating profit | | | |
| 1 | During 2023, management evaluated the commercial viability of Engage products and concluded that they should be discontinued. A total of $ |
2.3 Acquisition and disposal-related items
For the year ended 31 December 2023, costs primarily relate to the acquisition of CartiHeal and impairment of Engage goodwill, partially offset by credits relating to remeasurement of contingent consideration from prior year acquisitions.
For the year ended 31 December 2022, costs primarily relate to the acquisition of Engage and prior year acquisitions, partially offset by credits relating to remeasurement of deferred and contingent consideration for prior year acquisitions.
For the year ended 31 December 2021, costs primarily relate to the acquisition of Extremity Orthopaedics and prior year acquisitions, partially offset by credits relating to remeasurement of contingent consideration for prior year acquisitions.
2.4 Restructuring and rationalisation costs
For the year ended 31 December 2023 and 2022, these costs include efficiency and productivity elements of the 12-Point Plan.
For the years ended 31 December 2023, 2022 and 2021, these costs also relate to the Operations and Commercial Excellence programme.
For the years ended 31 December 2021, these costs also include the implementation of the Accelerating Performance and Execution (APEX) programme that was announced in February 2018.
182 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
2.5 Amortisation and impairment of acquisition intangibles
For the years ended 31 December 2023, 2022 and 2021, these costs relate to the amortisation and impairment of intangible assets acquired in material business combinations.
2.6 Legal and other
For the year ended 31 December 2023, charges primarily relate to legal expenses for ongoing metal-on-metal hip claims partially offset by an decrease of $
For the year ended 31 December 2022, charges primarily relate to legal expenses for ongoing metal-on-metal hip claims. These charges in the year to 31 December 2022 were partially offset by a credit of $
For the year ended 31 December 2021, charges primarily relate to legal expenses for ongoing metal-on-metal hip claims. These charges in the year to 31 December 2021 were partially offset by a credit of $
The years ended 31 December 2023, 2022 and 2021 also include costs for implementing the requirements of the EU Medical Device Regulation which came into effect in May 2021 with a transition period to May 2024.
2.7 Non-current assets by geography
The following table presents the non-current assets of the Group based on their location:
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
United Kingdom | | | | |||
United States of America | | | | |||
Other | | | | |||
Total non-current assets of the consolidated Group1 | | | |
1 | Non-current assets exclude retirement benefit assets and deferred tax assets. |
3 Operating profit
Accounting policy Research and development Research expenditure is expensed as incurred. Internal development expenditure is only capitalised if the recognition criteria in IAS 38 Intangible Assets have been satisfied. The Group considers that the regulatory, technical and market uncertainties inherent in the development of new products mean that in most cases development costs should not be capitalised as intangible assets until products receive approval from the appropriate regulatory body. Payments to third parties for research and development projects are accounted for based on the substance of the arrangement. If the arrangement represents outsourced research and development activities the payments are generally expensed except in limited circumstances where the respective development expenditure would be capitalised under the principles established in IAS 38. By contrast, the payments are capitalised if the arrangement represents consideration for the acquisition of intellectual property developed at the risk of the third party. Capitalised development expenditures are amortised on a straight-line basis over their useful economic lives from product launch. Advertising costs Advertising costs are expensed as incurred. |
Smith+Nephew Annual Report 2023 | 183 |
Group financial statements continued
Notes to the Group accounts continued
3 Operating profit continued
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Revenue | | | | |||
Cost of goods sold1 | ( | ( | ( | |||
Gross profit | | | | |||
Research and development expenses2 | ( | ( | ( | |||
Selling, general and administrative expenses: |
|
|
| |||
Marketing, selling and distribution expenses | ( | ( | ( | |||
Administrative expenses3,4,5,6 | ( | ( | ( | |||
( | ( | ( | ||||
Operating profit | | | |
| 1 | 2023 includes $ |
| 2 | 2023 includes $ |
| 3 | 2023 includes $ |
| 4 | 2023 includes $ |
| 5 | 2023 includes $ |
| 6 | 2023 includes $ |
Note that items detailed in 1, 2, 4, 5 and 6 are excluded from the calculation of trading profit, the segments’ profit measure.
Operating profit is stated after charging/(crediting) the following items:
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Other operating income | – | ( | ( | |||
Amortisation of intangible assets | | | | |||
Impairment of intangible assets1 | | | | |||
Impairment of Engage's goodwill | | – | – | |||
Impairment of property, plant and equipment | | | | |||
Fair value remeasurement of trade investments | | – | | |||
Depreciation of property, plant and equipment2 | | | | |||
Loss on disposal of property, plant and equipment and intangible assets | | | | |||
Advertising costs | | | |
| 1 | The 2023 impairment of intangible assets includes Engage’s intangible assets of $ |
| 2 | The 2023 depreciation charge includes $ |
In 2023, other operating income comprises insurance recoveries for ongoing metal-on-metal hip claims of $
184 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
3.1 Staff costs and employee numbers
Staff costs during the year amounted to:
2023 | 2022 | 2021 | ||||||
| Notes |
| $ million |
| $ million |
| $ million | |
Wages and salaries |
| | | | ||||
Social security costs |
| | | | ||||
Pension costs (including retirement healthcare) | 18 | | | | ||||
Share-based payments | 22 | | | | ||||
| | | |
During the year ended 31 December 2023, the average number of employees was
3.2 Audit Fees – information about the nature and cost of services provided by the auditor
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Audit services: |
|
|
| |||
Group accounts | | | | |||
Local statutory audit pursuant to legislation | | | | |||
Other services: | ||||||
Audit-related services | | | | |||
Total auditor’s remuneration | | | | |||
Arising: |
|
|
| |||
In the UK | | | | |||
Outside the UK | | | | |||
| | |
4 Interest and other finance costs
4.1 Interest income/(expense)
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Interest income | | | | |||
Interest expense: |
| |||||
Bank borrowings | ( | ( | ( | |||
Private placement notes | ( | ( | ( | |||
Lease liabilities | ( | ( | ( | |||
Corporate bond | ( | ( | ( | |||
Other1 | ( | ( | ( | |||
( | ( | ( | ||||
Net interest expense | ( | ( | ( |
| 1 | Other interest expenses included mainly swap interest expenses in 2023. |
4.2 Other finance costs
2023 | 2022 | 2021 | ||||||
| Notes |
| $ million |
| $ million |
| $ million | |
Retirement benefit net interest expense | 18 | ( | ( | ( | ||||
Unwinding of discount |
| ( | ( | ( | ||||
Other |
| – | | ( | ||||
Other finance costs |
| ( | ( | ( |
Smith+Nephew Annual Report 2023 | 185 |
Group financial statements continued
Notes to the Group accounts continued
5 Taxation
Accounting policy
The charge for current taxation is based on the results for the year as adjusted for items which are non-assessable or non-deductible. It is calculated using tax rates that have been enacted or substantively enacted as at the balance sheet date.
The Group operates in numerous tax jurisdictions around the world. At any given time, the Group typically is involved in tax audits and other disputes and will have other tax returns potentially subject to audit. Significant issues may take several years to resolve. In estimating the probability and amount of any tax charge, management takes into account the views of internal and external advisers and updates the amount of tax provision where considered appropriate. The ultimate tax liability may differ from the amount provided depending on factors including interpretations of tax law and settlement negotiations.
Deferred tax is recognised in respect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes.
Deferred tax is not recognised: for temporary differences related to investments in subsidiaries and associates where the Group is able to control the timing of the reversal of the temporary difference and it is probable that this will not reverse in the foreseeable future; on the initial recognition of non-deductible goodwill; and on the initial recognition of an asset or liability in a transaction that is not a business combination and that, at the time of the transaction, does not affect the accounting or taxable profit.
Deferred tax assets are recognised to the extent that it is probable that future taxable profits will be available against which they can be used. Deferred tax assets are reviewed at each reporting date taking into account the recoverability of the deferred tax assets, future profitability and any restrictions on use. The Group considers available evidence to assess future profitability over a reasonably foreseeable time period, depending on the circumstances and typically a minimum of five years. Any material unrecognised deferred tax assets are disclosed in Note 5.2.
Deferred tax is measured on an undiscounted basis, and at the tax rates that have been enacted or substantively enacted as at the balance sheet date that are expected to apply in the periods in which the asset or liability is settled. It is recognised in the income statement except when it relates to items credited or charged directly to other comprehensive income or equity, in which case the deferred tax is also recognised within other comprehensive income or equity respectively.
Deferred tax assets and liabilities are offset when they relate to income taxes levied by the same taxation authority, the Group intends to settle its current tax assets and liabilities on a net basis, offset is permissible according to the relevant jurisdiction’s tax laws and that authority permits the Group to make a single net payment.
In 2023, the Group has adopted IAS 12-Deferred Tax related to Assets and Liabilities arising from a Single Transaction amendments, which narrow the scope of the initial recognition exemption to exclude transactions that give rise to equal and offsetting temporary differences such as leases. The Group previously accounted for deferred tax on leases where the deferred tax asset or liability was recognised on a net basis. Following the amendments, the Group has recognised a separate deferred tax asset in relation to its lease liabilities and a deferred tax liability in relation to its right-of-use assets. However, there is no impact on the balance sheet because the balances qualify for offset under paragraph 74 of IAS 12. There was also no impact on the opening retained earnings as at 1 January 2023 as a result of the change.
5.1 Taxation charge attributable to the Group
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Current taxation: |
|
|
| |||
UK corporation tax at | | | | |||
Overseas tax | | | | |||
Current income tax charge | | | | |||
Adjustments in respect of prior periods | ( | ( | ( | |||
Total current taxation | | | | |||
Deferred taxation: |
|
|
| |||
Origination and reversal of temporary differences | ( | ( | ( | |||
Changes in tax rates | ( | ( | ( | |||
Adjustments to estimated amounts arising in prior periods | | ( | | |||
Total deferred taxation | ( | ( | ( | |||
Total taxation as per the income statement | | | | |||
Taxation in other comprehensive income | ( | | | |||
Taxation in equity | – | | | |||
Taxation charge attributable to the Group | | | |
186 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The 2023, 2022 and 2021 net prior period adjustments of $
The total taxation charge of $
Factors affecting future tax charges
The Group operates in numerous tax jurisdictions around the world and is subject to factors that may affect future tax charges including transfer pricing, tax rate changes, tax legislation changes, tax authority interpretation, expiry of statute of limitations, tax litigation, and resolution of tax audits and disputes.
At any given time, the Group has unagreed years outstanding in various countries and is involved in tax audits and disputes, some of which may take several years to resolve. Provisions are based on best estimates and management’s judgements concerning the likely ultimate outcome of any audit or dispute. Management considers the specific circumstances of each tax position and takes external advice, where appropriate, to assess the range of potential outcomes and estimate additional tax that may be due. Total tax liabilities include $
The Group believes that it has made adequate provision in respect of additional tax liabilities that may arise from unagreed years, tax audits and disputes, the majority of which relate to transfer pricing matters, as would be expected for a Group operating internationally. However, the actual liability for any particular issue may be higher or lower than the amount provided, resulting in a negative or positive effect on the tax charge in any given year. A reduction in the tax charge may also arise for other reasons such as an expiry of the relevant statute of limitations. Depending on the final outcome of tax audits which are currently in progress, statute of limitations expiry, and other factors, an impact on the tax charge could arise. Whilst such an impact can vary from year to year, these releases depend on factors which are uncertain, both as to outcome and timing. However, at the current time, we believe the possibility of a material impact on the tax charge for 2024 is unlikely.
Pillar Two
The OECD Pillar Two GloBE Rules (Pillar Two) introduce a global minimum corporation tax rate of 15% applicable to multinational enterprise groups with global revenue over €750m. All participating OECD members are required to incorporate these rules into national legislation. The Pillar Two rules will apply to the Group for its accounting period commencing 1 January 2024. On 23 May 2023, the International Accounting Standards Board (IASB) amended IAS 12 to introduce a mandatory temporary exception to the accounting for deferred taxes arising from the jurisdictional implementation of the Pillar Two model rules. On 19 July 2023 the UK Endorsement Board adopted the IASB amendments to IAS 12.
The Group has performed an assessment of its potential exposure to Pillar Two income taxes based on 2023 financial data and considers that the rules will result in an increase in the Group tax rate. The main jurisdictions which would give rise to Pillar Two income tax are Switzerland, Singapore and Costa Rica; all jurisdictions in which the Group has substantial operations. It is estimated that the Pillar Two income tax would increase the Group tax rate by around
The Group is adopting the mandatory temporary exception from the recognition and disclosure of deferred taxes arising from the jurisdictional implementation of the Pillar Two model rules.
The Group does not meet the threshold for application of the Pillar One transfer pricing rules.
Smith+Nephew Annual Report 2023 | 187 |
Group financial statements continued
Notes to the Group accounts continued
5 Taxation continued
The UK standard rate of corporation tax for 2023 is
The UK corporation tax rate increased to
2023 | 2022 | 2021 | ||||
| $ million |
| $ million |
| $ million | |
Profit before taxation | | | | |||
Expected taxation at UK statutory rate of | | | | |||
Differences in overseas taxation rates | ( | ( | ( | |||
Innovation reliefs | ( | ( | ( | |||
Tax losses and other deferred tax assets not recognised | – | – | | |||
Recognition of previously unrecognised tax losses | ( | ( | ( | |||
Expenses not deductible for tax purposes1 | | | | |||
Change in tax rates | ( | ( | ( | |||
Withholding tax on unremitted earnings | | | ( | |||
Adjustments in respect of prior years2 | ( | ( | ( | |||
Total taxation charge as per the income statement | | | |
| 1 | In 2023, this includes a $ |
| 2 | The adjustment in respect of prior years are explained on page 187. |
5.2 Deferred taxation
Movements in the main components of deferred tax assets and liabilities were as follows:
Inventory, | ||||||||||||
Accelerated | Retirement | Losses | provisions | |||||||||
tax | benefit | and other | and other | |||||||||
depreciation | Intangibles | obligations | tax attributes | differences | Total | |||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
At 31 December 2021 | ( | ( | ( | | | | ||||||
Exchange adjustment | – | | | – | ( | ( | ||||||
Movement in income statement – current year | ( | | | | | | ||||||
Movement in income statement – prior years | – | | | | | | ||||||
Movement in other comprehensive income | – | – | ( | – | | ( | ||||||
Movement in equity | – | – | – | – | ( | ( | ||||||
Changes in tax rate | ( | ( | – | – | | | ||||||
At 31 December 2022 | ( | ( | ( | | | | ||||||
Exchange adjustment | – | ( | ( | | | ( | ||||||
Movement in income statement – current year | ( | | – | | | | ||||||
Movement in income statement – prior years | – | | – | ( | ( | ( | ||||||
Movement in other comprehensive income | – | – | | – | – | | ||||||
Changes in tax rate | ( | – | – | | ( | | ||||||
At 31 December 2023 | ( | ( | ( | | | |
Represented by:
2023 | 2022 | |||
| $ million |
| $ million | |
Deferred tax assets | | | ||
Deferred tax liabilities | ( | ( | ||
Net position at 31 December | | |
188 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The deferred tax asset of $
The Group has gross unused trading and non-trading tax losses of $
A deferred tax asset of $
Management will reassess the recoverability of deferred tax assets at each balance sheet date by taking into account all relevant and available information. The Group assesses the likelihood of these being recovered within a reasonably foreseeable time frame, being typically a minimum of
6 Earnings per ordinary share
Accounting policy
Earnings per share
Basic earnings per share is calculated by dividing the profit attributable to equity holders by the weighted average number of ordinary shares in issue during the year, excluding shares held by the Company in the Employees’ Share Trust or as treasury shares.
Diluted earnings per share
Diluted earnings per share is calculated by adjusting the basic earnings per share for the effect of conversion to ordinary shares associated with dilutive potential ordinary shares, which comprise share options and awards granted to employees.
Adjusted earnings per share
Adjusted earnings per share (or adjusted basic earnings per share) is a trend measure which presents the long-term profitability of the Group excluding the impact of specific transactions that management considers affects the Group’s short-term profitability. The Group presents this measure to assist investors in their understanding of trends. Adjusted attributable profit is the numerator used for this measure. The Group has identified the following items as those to be excluded when arriving at adjusted attributable profit: acquisition and disposal-related items including amortisation and impairment of acquisition intangible assets; significant restructuring programmes; significant gains and losses arising from legal disputes and other significant items (including UK tax litigation) and taxation thereon. Adjusted diluted earnings per share is calculated by adjusting the adjusted basic earnings per share for the effect of conversion to ordinary shares associated with dilutive potential ordinary shares, which comprise share options and awards granted to employees.
Smith+Nephew Annual Report 2023 | 189 |
Group financial statements continued
Notes to the Group accounts continued
6 Earnings per ordinary share continued
The calculations of the basic, diluted and adjusted earnings per ordinary share are based on the following attributable profit and numbers of shares:
2023 | 2022 | 2021 | ||||||
| $ million |
| $ million |
| $ million | |||
Earnings | ||||||||
Attributable profit for the year | | | | |||||
Adjusted attributable profit (see below) | | | |
Attributable profit is reconciled to adjusted attributable profit as follows:
2023 | 2022 | 2021 | ||||||
| Notes |
| $ million |
| $ million |
| $ million | |
Attributable profit for the year | | | | |||||
Acquisition and disposal-related items1 | | | ( | |||||
Restructuring and rationalisation costs2 | 3 | | | | ||||
Amortisation and impairment of acquisition intangibles3 | 9 | | | | ||||
Legal and other4 | | | | |||||
Taxation on excluded items | 5 | ( | ( | ( | ||||
Adjusted attributable profit | | | |
1 | Acquisition and disposal-related items includes a $ |
2 | Restructuring and rationalisation costs include a $ |
3 | In 2023, amortisation and impairment of acquisition intangibles includes a $ |
4 | Legal and other in 2023 includes $ |
The numerators used for basic and diluted earnings per ordinary share are the same. The denominators used for all categories of earnings per ordinary share are as follows:
| 2023 |
| 2022 |
| 2021 | |||
Number of shares (millions) | ||||||||
Basic weighted number of shares | | | | |||||
Dilutive impact of share incentive schemes outstanding | | | | |||||
Diluted weighted average number of shares | | | | |||||
Earnings per ordinary share | ||||||||
Basic | ||||||||
Diluted | ||||||||
Adjusted: | ||||||||
Basic | ||||||||
Diluted |
190 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
7 Property, plant and equipment
Accounting policy
Property, plant and equipment
Owned assets
Items of property, plant and equipment are stated at cost less accumulated depreciation and any accumulated impairment losses.
Depreciation is calculated to write off the cost of items of property, plant and equipment less their estimated residual values using the straight‑line method over their estimated useful lives, and is ultimately recognised in profit or loss. Leased assets are depreciated over the shorter of the lease term and their useful lives unless it is reasonably certain that the Group will obtain ownership by the end of the lease term. Freehold land is not depreciated. The estimated useful lives of items of property, plant and equipment is
Assets in course of construction are not depreciated until they are available for use.
Depreciation methods, useful lives and residual values are reviewed at each reporting date and adjusted if appropriate.
Finance costs relating to the purchase or construction of property, plant and equipment and intangible assets that take longer than one year to complete are capitalised based on the Group weighted average borrowing costs. All other finance costs are expensed as incurred.
Leased assets
The assessment of whether a contract is or contains a lease takes place at the inception of the contract. The assessment involves whether the Group obtains substantially all the economic benefits from the use of that asset and whether the Group has the right to direct the use of the asset. The Group allocates the consideration in the contract to each lease and non-lease component. The non-lease component, where it is separately identifiable, is not included in the right-of-use asset.
The Group leases many assets including properties, motor vehicles and office equipment. The Group availed itself of the exemptions for short-term leases and leases of low-value items for leases other than those for properties and motor vehicles. The use of these exemptions does not have a material impact. The Group recognises a right-of-use asset and a lease liability at the commencement of the lease. The right-of-use asset is initially measured based on the present value of lease payments that are not paid at the commencement date plus initial direct costs less any incentives received. The lease payments are discounted using an incremental borrowing rate which is country-specific and reflective of the lease term. The right-of-use asset is depreciated over the shorter of the lease term or the useful life of the underlying asset.
Cash flows arising on lease interest payments are included in operating cash flows whereas cash flows arising on the capital repayments of the lease liability are included in financing cash flows.
Impairment of assets
The carrying values of property, plant and equipment are reviewed for impairment when events or changes in circumstances indicate the carrying value may be impaired. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of impairment loss. Where it is not possible to estimate the recoverable amount of an individual asset, the Group estimates the recoverable amount of the cash-generating unit to which it belongs.
An asset’s recoverable amount is the higher of an asset’s or cash-generating unit’s fair value less costs to sell and its value-in-use. In assessing value-in-use, its estimated future cash flow is discounted to its present value using a pre-tax discount rate that reflects the current market assessment of the time value of money and the risks specific to the asset.
Smith+Nephew Annual Report 2023 | 191 |
Group financial statements continued
Notes to the Group accounts continued
7 Property, plant and equipment continued
Plant and equipment | Assets in | |||||||||||
Land and | course of | |||||||||||
buildings | Instruments | Other | construction | Total | ||||||||
| Notes |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
Cost | ||||||||||||
At 1 January 2022 |
| | | | | | ||||||
Exchange adjustment |
| ( | ( | ( | ( | ( | ||||||
Acquisitions | 21 | – | | – | – | | ||||||
Additions |
| | | | | | ||||||
Disposals | ( | ( | ( | ( | ( | |||||||
Impairment | – | – | – | ( | ( | |||||||
Transfers |
| | | | ( | – | ||||||
At 31 December 2022 |
| | | | | | ||||||
Exchange adjustment |
| | | | | | ||||||
Additions |
| | | | | | ||||||
Disposals | ( | ( | ( | ( | ( | |||||||
Impairment | – | – | – | ( | ( | |||||||
Reclassification | | – | – | – | | |||||||
Transfers |
| | | | ( | ( | ||||||
At 31 December 2023 |
| | | | | | ||||||
Depreciation and impairment |
| |||||||||||
At 1 January 2022 |
| | | | – | | ||||||
Exchange adjustment |
| ( | ( | ( | – | ( | ||||||
Charge for the year |
| | | | – | | ||||||
Impairment |
| | | | – | | ||||||
Disposals |
| ( | ( | ( | – | ( | ||||||
Transfers |
| – | | ( | – | – | ||||||
At 31 December 2022 |
| | | | – | | ||||||
Exchange adjustment |
| | | | – | | ||||||
Charge for the year |
| | | | – | | ||||||
Impairment |
| | | | – | | ||||||
Disposals | ( | ( | ( | – | ( | |||||||
Reclassification | | – | – | – | | |||||||
Transfers |
| – | ( | | – | – | ||||||
At 31 December 2023 |
| | | | – | | ||||||
Net book amounts |
|
|
|
|
|
| ||||||
At 31 December 2023 | | | | | | |||||||
At 31 December 2022 |
| | | | | | ||||||
Land and buildings includes land with a cost of $
Information about the Group’s right-of-use assets is outlined below:
Land and | Plant and | |||
2023 |
| $ million |
| $ million |
Additions | | | ||
Depreciation charge in the year | | | ||
Net book value at 31 December | | |
192 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
8 Goodwill
Accounting policy
Goodwill is not amortised but is reviewed for impairment annually. Goodwill is allocated to the cash-generating unit (CGU) that is expected to benefit from the acquisition. The goodwill is tested annually for impairment by comparing the recoverable amount to the carrying value of the CGUs. The CGUs identified by management are at the aggregated product operating levels of Orthopaedics, Sports Medicine, ENT and Advanced Wound Management, in the way the core assets are used to generate cash flows.
If the recoverable amount of the CGU is less than its carrying amount then an impairment loss is determined to have occurred. Any impairment losses that arise are recognised immediately in the income statement and are allocated first to reduce the carrying amount of goodwill and then to the carrying amounts of the other assets of the CGU.
When an acquired business included within a CGU ceases to operate permanently, then the acquired business no longer forms part of the CGU and is therefore tested for impairment on a standalone basis. The portion of goodwill allocated to this acquired business is measured based on its relative value within the CGU, unless another method is considered more appropriate.
In carrying out impairment reviews of goodwill, a number of significant assumptions have to be made when preparing cash flow projections. These include the future rate of market growth, discount rates, the market demand for the products acquired, the future profitability of acquired businesses or products, levels of reimbursement and success in obtaining regulatory approvals. If actual results should differ, or changes in expectations arise, impairment charges may be required which would adversely impact operating results.
When the composition of CGUs changed, goodwill would be allocated using a relative value approach at the date of the reorganisation similar to that used when an operation within a CGU is disposed of or a method that could provide a better allocation of goodwill to the reorganised units.
2023 | 2022 | |||||
|
| Notes |
| $ million |
| $ million |
Cost and net book value |
|
| ||||
At 1 January | | | ||||
Exchange adjustment | | ( | ||||
Impairment | ( | – | ||||
Acquisitions | 21 | – | | |||
At 31 December | | |
Management has identified
In 2023, ENT was identified as a separate operating segment following change in the Group’s management structure. Therefore, ENT was identified as a new CGU for goodwill impairment reviews.
For the purpose of goodwill impairment testing, the Advanced Wound Care & Devices and Bioactives CGUs have been aggregated (Advanced Wound Management), as this is the level at which goodwill is monitored and level at which the economic benefits relating to the goodwill within these CGUs is realised.
During 2023, management evaluated the commercial viability of Engage products and concluded that they should be discontinued. The goodwill related to Engage of $
Goodwill is allocated to the Group’s CGUs as follows:
2023 | 2022 | |||
|
| $ million |
| $ million |
Orthopaedics | | | ||
Sports Medicine1 | | | ||
ENT1 | | – | ||
Advanced Wound Management | | | ||
| |
1 In 2022, Sports Medicine and ENT was combined CGU whereas ENT is identified as a separate CGU in 2023.
Impairment reviews were performed as of September 2023 and September 2022 by comparing the recoverable amount of each CGU with its carrying amount, including goodwill. These were reviewed during December, taking into account any significant events that occurred between September and December.
Smith+Nephew Annual Report 2023 | 193 |
Group financial statements continued
Notes to the Group accounts continued
8 Goodwill continued
The current challenging economic environment, including inflation, was considered in the goodwill impairment reviews. Additionally, severe downside sensitivity analyses have been undertaken on the base case scenario. Although the headroom for the Orthopaedics CGU has increased it is still considered sensitive to a reasonably possible change in assumptions,
For each CGU, the recoverable amounts are based on value-in-use which is calculated from pre-tax cash flow projections for
The discount rates used in the value-in-use calculations reflect management’s assessment of risks specific to the assets of each CGU. Our determination of the discount rates is based on Group’s weighted average cost of capital (WACC) which includes a risk-free rate, based on market participant’s cost of equity, an equity risk premium specifically adjusted to the medical technology industry and after-tax cost of debt and reflects the risks inherent in the cash flows adjusted for CGU specific risk.
8.1 Orthopaedics CGU
The cash flows used in the value-in-use calculation for the Orthopaedics CGU, which includes the Reconstruction and Trauma businesses, reflects management’s distinctive orthopaedic reconstruction strategy, which combines cutting-edge innovation, disruptive business models and a strong Emerging Markets platform to drive our performance.
The headroom for the Orthopaedics CGU has increased from $
8.2 Sports Medicine CGU
The cash flows used in the value-in-use calculation for the Sports Medicine CGU reflects growth rates and cash flows consistent with management’s strategy to maintain growth in Sports Medicine.
The weighted average growth rate used to extrapolate the cash flows beyond the
8.3 ENT CGU
The cash flow used in the value-in-use calculation for the ENT CGU reflects growth rates and cash flows consistent with management’s strategy.
The weighted average growth rate used to extrapolate the cash flows beyond the
8.4 Advanced Wound Management CGU
The aggregated Advanced Wound Management CGU comprises the Advanced Wound Care & Devices and Bioactives CGUs.
In performing the value-in-use calculation for this combined CGU, management considered the Group’s focus across the wound product, focusing on widening access to the customer, the higher added value sectors of healing chronic wounds and tissue repair using bioactives, and by continuing to improve efficiency.
The weighted average growth rate used to extrapolate the cash flows beyond the
194 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
8.5 Sensitivity to changes in assumptions used in value-in-use calculations
Management have performed a sensitivity analysis of the value-in-use calculations for the identified CGUs and there was no impact on the reported amounts of goodwill as a result of this review for the Sports Medicine, ENT and Advanced Wound Management CGUs. Management do not believe a reasonably possible change in assumptions used for the Orthopeadics CGU value-in-use, other than trading profit margin, could result in a material impairment. Management’s consideration of this sensitivity is set out below:
Trading profit margin – management has considered the impact of a decrease in the trading profit margin. This sensitivity analysis shows that for the recoverable amount of the Orthopaedics CGU to be less than its carrying value, the terminal period and year 5 trading profit margin would have to decrease by more than
9 Intangible assets
Accounting policy
Intangible assets
Intangible assets acquired separately from a business combination (including purchased patents, know-how, trademarks, licences and distribution rights) are initially measured at cost. The cost of intangible assets acquired in a material business combination (referred to as acquisition intangibles) is the fair value as at the date of acquisition. Following initial recognition, intangible assets are carried at cost less any accumulated amortisation and any accumulated impairment losses. All intangible assets are amortised on a straight-line basis over their estimated useful economic lives. The estimated useful economic life of software ranges between and . The estimated useful economic life of technology assets ranges between
Impairment of intangible assets
The carrying values of intangible assets are reviewed for impairment when events or changes in circumstances indicate the carrying value may be impaired. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of impairment loss. Where it is not possible to estimate the recoverable amount of an individual asset, the Group estimates the recoverable amount of the CGU to which it belongs. An asset’s recoverable amount is the higher of an asset’s or CGU’s fair value less costs to sell and its value-in-use. In assessing value-in-use, its estimated future cash flow is discounted to its present value using a pre-tax discount rate that reflects the current market assessments of the time value of money and the risks specific to the asset.
In carrying out impairment reviews of intangible assets, a number of significant assumptions have to be made when preparing cash flow projections. These include the future rate of market growth, discount rates, the market demand for the products acquired, the future profitability of acquired businesses or products, levels of reimbursement and success in obtaining regulatory approvals. If actual results should differ, or changes in expectations should arise, impairment charges may be required which would adversely impact operating results.
Smith+Nephew Annual Report 2023 | 195 |
Group financial statements continued
Notes to the Group accounts continued
9 Intangible assets continued
Customer and | Assets | |||||||||||||
Product- | distribution- | in course of | ||||||||||||
Technology | related | related | Software | construction | Total | |||||||||
Notes |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | ||
Cost | ||||||||||||||
At 1 January 2022 | | | | | | | ||||||||
Exchange adjustment | ( | ( | ( | ( | ( | ( | ||||||||
Acquisitions | 21 | – | | – | – | – | | |||||||
Additions | – | | | | | | ||||||||
Disposals | – | – | ( | ( | – | ( | ||||||||
Impairment | – | – | – | – | ( | ( | ||||||||
Transfers | – | – | – | | ( | – | ||||||||
At 31 December 2022 | | | | | | | ||||||||
Exchange adjustment | | | ( | | | | ||||||||
Additions | – | | | | | | ||||||||
Disposals | ( | ( | – | ( | – | ( | ||||||||
Transfers | ( | | – | | | | ||||||||
At 31 December 2023 | | | | | | | ||||||||
Amortisation and impairment |
| |||||||||||||
At 1 January 2022 | | | | | – | | ||||||||
Exchange adjustment | ( | ( | ( | ( | – | ( | ||||||||
Charge for the year | | | | | – | | ||||||||
Impairment | | | – | | – | | ||||||||
Disposals | – | – | – | ( | – | ( | ||||||||
At 31 December 2022 | | | | | – | | ||||||||
Exchange adjustment | | | – | | – | | ||||||||
Charge for the year | | | | | – | | ||||||||
Impairment | – | | – | – | – | | ||||||||
Disposals | – | ( | – | ( | – | ( | ||||||||
At 31 December 2023 | | | | | – | | ||||||||
Net book amounts |
| |||||||||||||
At 31 December 2023 | | | | | | | ||||||||
At 31 December 2022 | | | | | | |
Transfers into software and assets in course of construction includes $
Amortisation and impairment of acquisition intangibles is set out below:
2023 | 2022 | |||
|
| $ million |
| $ million |
Technology | | | ||
Product-related | | | ||
Customer and distribution-related | | | ||
Total | | |
In 2023, the Group impaired $
196 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Management have assessed the acquisition intangible assets held by the Group to identify any indicators of impairment as of September 2023. These were updated during December to take into account any significant events that occurred between September and December. Where an impairment indicator has arisen, impairment reviews have been undertaken by comparing the expected recoverable value of assets to the carrying value of assets.
The table below provides further detail on the largest intangible assets and their remaining amortisation period:
Remaining | ||||
Carrying value | amortisation | |||
$ million |
| period | ||
Intangibles acquired as part of the ArthroCare acquisition | | |||
Intangibles acquired as part of the Osiris acquisition | | |||
Intangibles acquired as part of the Healthpoint acquisition | |
10 Investments
Accounting policy
Investments, other than those related to associates, are initially recorded at fair value plus any directly attributable transaction costs on the trade date. The Group has investments in unquoted entities and an entity that holds mainly unquoted equity securities, which by their nature have no fixed maturity date or coupon rate. These investments are classed as fair value through profit or loss. The fair value of these investments is based on the underlying fair value of the equity securities: marketable securities are valued by reference to closing prices in the market; non-marketable securities are estimated considering factors including the purchase price; prices of recent significant private placements of securities of the same issuer; and estimates of liquidation value. Changes in fair value based on externally observable valuation events are recognised in profit or loss.
2023 | 2022 | |||||
|
| $ million |
| $ million | ||
At 1 January | | | ||||
Additions | – | | ||||
Fair value remeasurement | ( | – | ||||
At 31 December | | |
11 Investments in associates
Accounting policy
Investments in associates, being those entities over which the Group has a significant influence and which is neither a subsidiary nor a joint venture, are accounted for using the equity method, with the Group recording its share of the associates’ profit and loss and other comprehensive income. The Group’s share of associates’ profit or loss is included in one separate income statement line and is calculated after deduction of their respective taxes.
The carrying amounts of investments in associates are reviewed for impairment as at the balance sheet date. For the purposes of impairment testing, the recoverable amounts of these investments would be based on their observable market value. Any impairment loss is subsequently reversed only to the extent that the recoverable amounts of the investments increase.
At 31 December 2023, the Group holds
The loss after taxation recognised in the income statement relating to Bioventus was $
The Group did not identify any impairment indicator for Bioventus as part of 2023 impairment assessment. In 2022, Bioventus’ trading share price decreased significantly and the company disclosed a substantial doubt about their ability to continue as a going concern. Given these impairment indicators, management recorded an impairment loss of $
Smith+Nephew Annual Report 2023 | 197 |
Group financial statements continued
Notes to the Group accounts continued
11 Investments in associates continued
The amounts recognised in the balance sheet and income statement for associates are as follows:
2023 | 2022 | |||
|
| $ million |
| $ million |
Balance sheet | | | ||
Income statement loss | ( | ( | ||
Impairment of interest in associate | – | ( |
Summarised financial information for significant associates
Set out below is the summarised financial information for Bioventus, adjusted for differences with Group accounting policies. For the 2023 financial year, full-year information for Bioventus has not been released at the date of approval of these financial statements and is market sensitive given Bioventus is a publicly traded company. Accordingly, the summary financial information for 2023 is presented for a nine-month period, with adjustments made for any significant transactions or events which occur in the fourth quarter.
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
Summarised statement of comprehensive income |
|
| ||
Revenue | | | ||
Attributable loss for the year | ( | ( | ||
Group adjustments1 | | | ||
Total comprehensive loss | ( | ( | ||
Group share of loss for the year at | ( | ( |
2023 |
| 2022 | ||
|
| $ million |
| $ million |
Summarised balance sheet |
|
| ||
Non-current assets | | | ||
Current assets | | | ||
Non-current liabilities | ( | ( | ||
Current liabilities | ( | ( | ||
Net assets | | | ||
Net equity attributable to owners | | | ||
Group’s share of net assets at | | | ||
Group adjustments1,2 | ( | | ||
Impairment loss | – | ( | ||
Group’s carrying amount of investment at | | |
| 1 | Group adjustments include adjustments to align with Group policy. |
| 2 | In 2023, Group adjustments also include impairment loss of share in associates of $ |
The investment in Bioventus had a fair value less costs of disposal of $
During the year, the Group received a $
At 31 December 2023, the Group held equity investments in
198 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
12 Inventories
Accounting policy
Finished goods and work-in-progress are valued at factory cost, including appropriate overheads, on a first-in first-out basis. Raw materials and bought-in finished goods are valued at purchase price. All inventories are reduced to net realisable value where lower than cost. Inventory acquired as part of a business acquisition is valued at selling price less costs to sell and a profit allowance for selling efforts.
Orthopaedic instruments are generally not sold but provided to customers and distributors for use in surgery. They are recorded as inventory until they are deployed at which point they are transferred to plant and equipment and depreciated over their useful economic lives of between and .
A feature of the orthopaedic business is the high level of product inventory required, some of which is located at customer premises and is available for customers’ immediate use (referred to as consignment inventory). Complete sets of product, including large and small sizes, have to be made available in this way. These outer sizes are used less frequently than standard sizes and towards the end of the product life cycle are inevitably in excess of requirements. Adjustments to carrying value are therefore required to be made to orthopaedic inventory to anticipate this situation. These adjustments are calculated in accordance with a formula based on levels of inventory compared with historical or forecast usage. This formula is applied on an individual product line basis and is first applied when a product group has been on the market for . This method of calculation is considered appropriate based on experience but it involves management judgements on effectiveness of inventory deployment, length of product lives, phase-out of old products and efficiency of manufacturing planning systems.
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
Raw materials and consumables | | | ||
Work-in-progress | | | ||
Finished goods and goods for resale | | | ||
| |
Management have not changed their policy for calculating the provision since 31 December 2022, nor is a change in the key assumptions underlying the methodology expected in the next 12 months. As a result of increased inventory levels, the provision has increased from $
The cost of inventories recognised as an expense and included in cost of goods sold amounted to $
In 2023, management wrote off $
Notwithstanding inventory acquired within acquisitions, no inventory is carried at fair value less costs to sell in any year.
Smith+Nephew Annual Report 2023 | 199 |
Group financial statements continued
Notes to the Group accounts continued
13 Trade and other receivables
Accounting policy
Trade and other receivables are carried at amortised cost, less any allowances for uncollectable amounts. They are included in current assets, except for maturities greater than 12 months after the balance sheet date when they are classified as non-current assets.
The Group manages credit risk through credit limits which require authorisation commensurate with the size of the limit and which are regularly reviewed. Credit limit decisions are made based on available financial information and the business case. Significant receivables are regularly reviewed and monitored at Group level. The Group has no significant concentration of credit risk, with exposure spread over a large number of customers and geographies. Furthermore, the Group’s principal customers are backed by government and public or private medical insurance funding, which historically represent a lower risk of default. The maximum exposure to credit risk at the reporting date is the fair value of each class of receivable. The Group does not hold any collateral as security. Allowance losses are calculated by reviewing lifetime expected credit losses using historic and forward-looking data on credit risk. The Group performed the calculation of expected credit loss rates separately for customer groups which were segmented based on common risk characteristics such as credit risk grade and type of customer (such as government and non-government).
| 2023 | 2022 | ||
| $ million |
| $ million | |
Trade and other receivables due within one year | ||||
Trade receivables | | | ||
Less: loss allowance | ( | ( | ||
Trade receivables – net | | | ||
Derivatives – forward foreign exchange, currency swaps and interest rate contracts | | | ||
Other receivables | | | ||
Prepayments | | | ||
| | |||
Due after more than one year | ||||
Other non-current assets | | | ||
| |
Other non-current assets primarily relate to long-term prepayments and in 2023 interest rate contracts. Management considers that the carrying amount of trade and other receivables approximates the fair value. Allowance losses are calculated by reviewing lifetime expected credit losses using historic and forward-looking data on credit risk. The loss allowance relating to other receivables is de minimis.
The loss allowance expense for the year was $
The following table provides information about the ageing of and expected credit losses for trade receivables:
2023 Weighted | 2023 Loss | 2023 Gross | 2022 Gross | |||||
| % |
| $ million |
| $ million |
| $ million | |
Not past due | - | ( | | | ||||
Past due not more than 3 months | - | ( | | | ||||
Past due more than 3 months | - | ( | | | ||||
Past due more than 6 months | - | ( | | | ||||
( | | | ||||||
Loss allowance | ( | ( | ||||||
Trade receivables – net | | |
200 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The Group’s expected credit loss accounting policy includes guidance on how the expected credit loss percentages should be determined; it does not include present limits as the customer groups and risk profiles are not consistent across all of our markets. Each market determines their own percentages based on historic experience and future expectations, and in line with the general guidance in the Group’s policy.
Movements in the loss allowance were as follows:
| 2023 |
| 2022 | |
| $ million |
| $ million | |
At 1 January |
| |
| |
Exchange adjustment |
| |
| ( |
Net receivables provided during the year |
| |
| |
Utilisation of provision |
| ( |
| ( |
At 31 December |
| |
| |
Trade receivables include amounts denominated in the following major currencies:
| 2023 |
| 2022 | |
| $ million |
| $ million | |
US Dollar | |
| | |
Sterling | |
| | |
Euro | |
| | |
Other | |
| | |
Trade receivables – net | |
| |
14 Trade and other payables
| 2023 | 2022 | ||
|
| $ million |
| $ million |
Trade and other payables due within one year |
|
| ||
Trade and other payables | | | ||
Derivatives – forward foreign exchange, currency swaps and interest rate contracts | | | ||
Acquisition consideration | | | ||
| | |||
Other payables due after one year |
|
| ||
Acquisition consideration | | | ||
Derivatives – forward foreign exchange, currency swaps and interest rate contracts | – | | ||
Other payables | | | ||
| |
The acquisition consideration includes $
The acquisition consideration due after more than one year is expected to be payable as follows: $
Smith+Nephew Annual Report 2023 | 201 |
Group financial statements continued
Notes to the Group accounts continued
15 Cash and borrowings
15.1 Net debt
Net debt comprises borrowings and credit balances on currency swaps less cash at bank.
| 2023 | 2022 | ||
|
| $ million |
| $ million |
Bank overdrafts, borrowings and loans due within one year | | | ||
Corporate bond | | | ||
Private placement notes | | | ||
Borrowings | | | ||
Cash at bank | ( | ( | ||
Credit balance on derivatives – currency swaps | | – | ||
(Debit)/credit balance on derivatives – interest rate swaps | ( | | ||
Net debt | | | ||
Non-current lease liabilities | | | ||
Current lease liabilities | | | ||
Net debt including lease liabilities | | |
Borrowings are repayable as follows:
Within | Between | Between | Between | Between |
| |||||||||
one year or | one and | two and | three and | four and | After | |||||||||
on demand | two years | three years | four years | five years | five years | Total | ||||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
At 31 December 2023 |
|
|
|
|
|
|
| |||||||
Bank loans | | – | – | – | – | – | | |||||||
Bank overdrafts | | – | – | – | – | – | | |||||||
Corporate bond | – | – | – | – | – | | | |||||||
Private placement notes | | – | | | | | | |||||||
Lease liabilities1 | | | | | | | | |||||||
| | | | | | | ||||||||
At 31 December 2022 |
|
|
|
|
|
|
| |||||||
Bank overdrafts | | – | – | – | – | – | | |||||||
Corporate bond | – | – | – | – | – | | | |||||||
Private placement notes | | | – | | | | | |||||||
Lease liabilities1 | | | | | | | | |||||||
| | | | | | |
| 1 | The lease liabilities presented above of $ |
202 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
15.2 Liquidity risk exposures
The Board has established a set of policies to manage funding and currency risks. The Group only uses derivative financial instruments to manage the financial risks associated with underlying business activities and their financing. Liquidity risk is the risk that the Group is not able to settle or meet its obligations on time or at a reasonable price. The Group’s policy is to ensure that there is sufficient funding and facilities in place to meet foreseeable borrowing requirements. The Group manages and monitors liquidity risk through regular reporting of current cash and borrowing balances and periodic preparation and review of short-and medium-term cash forecasts, having regard to the maturities of investments and borrowing facilities. The Group has available committed facilities of $
The interest payable on borrowings under committed facilities is either at fixed or floating rates. Euro floating rates are typically based on EURIBOR and US Dollar rates are typically based on the Term Secured Overnight Financing Rate (Term SOFR). The Company is subject to financial covenants under its private placement agreements. The financial covenants are tested at the end of each half year for the 12 months ending on the last day of the testing period. As of 31 December 2023, the Company was in compliance with these covenants. The facilities are also subject to customary events of default, none of which are currently anticipated to occur.
The Group refinanced its $
The Group’s committed facilities at 31 December 2023 are:
Facility |
| Date due |
$ | January 2024 | |
$ | November 2024 | |
$ | January 2026 | |
$ | June 2027 | |
$ | June 2028 | |
$ | October 2028 | |
$ | June 2029 | |
€ | October 2029 | |
$ | June 2030 | |
$ | October 2030 | |
$ | June 2032 |
Smith+Nephew Annual Report 2023 | 203 |
Group financial statements continued
Notes to the Group accounts continued
15 Cash and borrowings continued
15.3 Year end financial liabilities by contractual maturity
The table below analyses the Group’s year end financial liabilities by contractual maturity date, including contractual interest payments and excluding the impact of netting arrangements:
| Within one | Between | Between |
| ||||||
year or on | one and | two and | After | |||||||
demand | two years | five years | five years | Total | ||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
At 31 December 2023 |
|
|
|
|
|
| ||||
Non-derivative financial liabilities: |
|
|
|
|
|
| ||||
Bank overdrafts and loans |
| | – | – | – | | ||||
Corporate bond |
| | | | | | ||||
Trade and other payables |
| | – | – | – | | ||||
Private placement notes |
| | | | | | ||||
Acquisition consideration |
| | | | | | ||||
Derivative financial instruments: |
| |||||||||
Currency swaps/forward foreign exchange contracts – outflow |
| | – | – | – | | ||||
Currency swaps/forward foreign exchange contracts – inflow |
| ( | – | – | – | ( | ||||
| | | | | | |||||
At 31 December 2022 |
|
|
|
|
|
| ||||
Non-derivative financial liabilities: |
|
|
|
|
|
| ||||
Bank overdrafts and loans |
| | – | – | – | | ||||
Corporate bond | | | | | | |||||
Trade and other payables |
| | – | – | – | | ||||
Private placement notes |
| | | | | | ||||
Acquisition consideration |
| | | | – | | ||||
Derivative financial instruments: |
| |||||||||
Currency swaps/forward foreign exchange contracts – outflow |
| | – | – | – | | ||||
Currency swaps/forward foreign exchange contracts – inflow |
| ( | – | – | – | ( | ||||
| | | | | |
The amounts in the tables above are undiscounted cash flows, which differ from the amounts included in the balance sheet where the underlying cash flows have been discounted.
15.4 Liquidity and capital resources
The Group’s policy is to ensure that it has sufficient funding and facilities to meet foreseeable borrowing requirements.
At 31 December 2023, the Group held $
The principal variations in the Group’s borrowing requirements result from the timing of dividend payments, acquisitions and disposals of businesses, timing of capital expenditure and working capital fluctuations. Smith+Nephew believes that its capital expenditure needs and its working capital funding for 2024, as well as its other known or expected commitments or liabilities, can be met from its existing resources and facilities. The Group’s net debt including leases increased from $
204 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
16 Financial instruments and risk management
Accounting policy
Derivative financial instruments
Derivative financial instruments are initially recognised at fair value on the date a derivative contract is entered into and are subsequently remeasured at their fair value at subsequent balance sheet dates. Changes in the fair value of derivative financial instruments that are designated and effective as cash flow hedges of forecast third-party transactions are recognised in other comprehensive income until the associated asset or liability is recognised. Amounts taken to other comprehensive income are transferred to the income statement in the period in which the hedged transaction affects profit and loss. Where the hedged item is the cost of a non-financial asset, the amounts taken to other comprehensive income are transferred to the initial carrying value of the asset.
On adoption of IFRS 9 on 1 January 2018, the Group elected to continue to apply the hedge accounting guidance in IAS 39 Financial Instruments: Recognition and Measurement. Changes in the fair values of hedging instruments that are designated and effective as net investment hedges are matched in other comprehensive income against changes in value of the related net assets. Interest rate derivatives transacted to fix interest rates on floating rate borrowings are accounted for as cash flow hedges and changes in the fair values resulting from changes in market interest rates are recognised in other comprehensive income. Amounts taken to other comprehensive income are transferred to the income statement when the hedged transaction affects profit and loss. Interest rate derivatives transacted to convert fixed rate borrowings into floating rate borrowings are accounted for as fair value hedges and changes in the fair values resulting from changes in market interest rates are recognised in the income statement. Any ineffectiveness on hedging instruments and changes in the fair value of derivative financial instruments that do not qualify for hedge accounting are recognised in the income statement within other finance costs as they arise.
Hedge accounting is discontinued when the hedging instrument expires or is sold, terminated or exercised, or no longer qualifies for hedge accounting. At that point in time, any cumulative gain or loss on the hedging instrument recognised in other comprehensive income is retained there until the forecast transaction occurs. If a hedged transaction is no longer expected to occur, the net cumulative gain or loss recognised in other comprehensive income is transferred to the income statement.
16.1 Foreign exchange risk management
The Group operates in many countries and as a consequence has transactional and translational foreign exchange exposure. It is the Group’s policy for operating units not to hold material unhedged monetary assets or liabilities other than in their functional currencies.
Foreign exchange variations affect trading results in two ways. Firstly, on translation of overseas sales and profits into US Dollars and secondly, transactional exposures arising where some, or all of the costs of sale are incurred in a different currency from the sale. The principal transactional exposures arise as the proportion of costs in US Dollars, Sterling and Swiss Francs exceed the proportion of sales in each of these currencies and correspondingly the proportion of sales in Euros exceeds the proportion of costs in Euros.
The impact of currency movements on the cost of purchases is partly mitigated by the use of forward foreign exchange contracts. The Group uses forward foreign exchange contracts, designated as cash flow hedges, to hedge forecast third-party trading cash flows up to
Smith+Nephew Annual Report 2023 | 205 |
Group financial statements continued
Notes to the Group accounts continued
16 Financial instruments and risk management continued
If the US Dollar were to weaken by
A
The Group’s policy is to hedge all actual foreign exchange exposures and the Group’s forward foreign exchange contracts are designated as cash flow hedges. The net impact of transaction-related foreign exchange on the income statement from a movement in exchange rates on the value of forward foreign exchange contracts is not significant. In addition, the movements in the fair value of other financial instruments used for hedging such as currency swaps for which hedge accounting is not applied offset movements in the values of assets and liabilities and are recognised through the income statement. Hedge ineffectiveness is caused by actual cash flows in foreign currencies varying from forecast cash flows.
16.2 Interest rate risk management
The Group is exposed to interest rate risk on cash, borrowings and certain currency and interest rate swaps which are at floating rates. When required the Group uses interest rate derivatives to meet its objective of protecting borrowing costs within parameters set by the Board. These interest rate derivatives are accounted for as cash flow hedges and, as such, changes in fair value resulting from changes in market interest rates are recognised in other comprehensive income and accumulated in the hedging reserve, with the fair value of the interest rate derivatives recorded in the balance sheet. Additionally, the Group uses interest rate swaps to reduce the overall level of fixed rate debt, within parameters set by the Board. When used in this way, interest rate derivatives are accounted for as fair value hedges. The fair value movement of the derivative is offset in the income statement against the fair value movement in the underlying fixed rate debt.
In 2022, the Group entered into a new €
Based on the Group’s gross borrowings and cash as at 31 December 2023, if interest rates were to increase by
16.3 Credit risk management
The Group limits exposure to credit risk on counterparties used for financial instruments through a system of internal credit limits. The financial exposure of a counterparty is determined as the total of cash and deposits, plus the risk on derivative instruments, assessed as the fair value of the instrument plus a risk element based on the nominal value and the historic volatility of the market value of the instrument. The Group does not anticipate non-performance of counterparties and believes it is not subject to material concentration of credit risk as the Group operates within a policy of counterparty limits designed to reduce exposure to any single counterparty.
The maximum credit risk exposure on derivatives at 31 December 2023 was $
206 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The amounts relating to items designated as hedging instruments were as follows:
Carrying | Carrying | Changes in | Hedge | Amounts reclassified | ||||||||||
Nominal | amount | amount | fair value | ineffectiveness | from hedging reserve | |||||||||
amount | assets | liabilities | in OCI | in profit or loss | to profit or loss | Line item in | ||||||||
| million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| profit or loss | |
At 31 December 2023 |
|
|
|
|
|
|
| |||||||
Foreign currency risk | ||||||||||||||
Forward exchange contracts1 | | | ( | ( | – | ( | Cash flow hedges | |||||||
Interest rate risk | ||||||||||||||
Interest rate swaps2 | ( | | – | – | – | – | Fair value hedge | |||||||
At 31 December 2022 |
| |||||||||||||
Foreign currency risk |
|
|
|
|
|
|
| |||||||
Forward exchange contracts1 | | | ( | ( | – | ( | Cash flow hedges | |||||||
Interest rate risk | ||||||||||||||
Interest rate swaps2 | ( | – | ( | – | – | – | Fair value hedge |
| 1 | Presented in Trade and other receivables and Trade and other payables on the Balance Sheet. The nominal amount is in $ million. |
| 2 | Presented in Non-current other receivables in 2023 and in Non-current other payables in 2022 on the Balance Sheet. The nominal amount is in € million. |
16.4 Net investment hedge
Part of the Group’s net investment in its Euro subsidiaries is hedged by €
To assess hedge effectiveness, the Group determines the economic relationship between the hedging instrument and the hedged item by comparing changes in the carrying amount of the debt that is attributable to a change in the spot rate with changes in the investment in the foreign operation due to movements in the spot rate (the offset method). The Group’s policy is to hedge the net investment only to the extent of the debt principal. Hedge ineffectiveness occurs if the value of the Euro-denominated Corporate Bond exceeds the value of the Euro subsidiaries.
16.5 Currency and interest rate profile of interest bearing liabilities and assets
Short-term receivables and payables are excluded from the following disclosures.
Currency and interest rate profile of interest bearing liabilities:
Fixed rate liabilities | ||||||||||||||||
|
|
|
|
|
|
|
| Weighted | ||||||||
average | ||||||||||||||||
Interest | Weighted | time | ||||||||||||||
Gross | Currency | rate | Total | Floating | Fixed rate | average | for which | |||||||||
borrowings | swaps | swaps | liabilities | rate liabilities | liabilities | interest rate | rate is fixed | |||||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| % |
| Years | |
At 31 December 2023 |
|
|
|
|
|
|
|
| ||||||||
US Dollar | ( | ( | – | ( | ( | ( | | |||||||||
Other | ( | ( | – | ( | ( | ( | ||||||||||
Total interest bearing liabilities | ( | ( | – | ( | ( | ( |
|
| ||||||||
At 31 December 2022 |
|
|
|
|
|
|
|
| ||||||||
US Dollar | ( | ( | – | ( | ( | ( | | |||||||||
Other | ( | ( | ( | ( | ( | ( | ||||||||||
Total interest bearing liabilities | ( | ( | ( | ( | ( | ( |
|
| ||||||||
In 2023, the Group also had liabilities due for deferred and contingent acquisition consideration (denominated in US Dollars, Swiss Francs and Euros) totalling $
Smith+Nephew Annual Report 2023 | 207 |
Group financial statements continued
Notes to the Group accounts continued
16 Financial instruments and risk management continued
Currency and interest rate profile of interest bearing assets:
| Cash | Currency | Interest rate |
|
| Floating | Fixed | |||||
at bank | swaps | swaps | Total assets | rate assets | rate assets | |||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
At 31 December 2023 | ||||||||||||
US Dollar | | | – | | | – | ||||||
Other | | | | | | – | ||||||
Total interest bearing assets | | | | | | – | ||||||
At 31 December 2022 | ||||||||||||
US Dollar | | | – | | | – | ||||||
Other | | | – | | | – | ||||||
Total interest bearing assets | | | – | | | – |
Floating rates on assets are typically based on the short-term deposit rates relevant to the currency concerned.
16.6 Fair value of financial assets and liabilities
Accounting policy
Measurement of fair values
A number of the Group’s accounting policies and disclosures require the measurement of fair values, for both financial assets and liabilities and non-financial assets acquired in a business combination (see Note 21).
When measuring the fair value of an asset or liability, the Group uses market observable data as far as possible. Fair values are categorised into different levels in the fair value hierarchy based on the inputs used in the valuation techniques as follows: Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities; Level 2: inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (ie as prices) or indirectly (ie derived from prices); and Level 3: inputs for the asset or liability that are not based on observable data (unobservable inputs).
The Group recognises transfers between the levels of the fair value hierarchy at the end of the reporting period during which the change has occurred.
There has been no change in the classification of financial assets and liabilities, the method and assumptions used in determining fair value and the categorisation of financial assets and liabilities within the fair value hierarchy from those disclosed in the Annual Report for the year ended 31 December 2022.
The Group enters into derivative financial instruments with financial institutions with investment grade credit ratings. The fair value of forward foreign exchange contracts is calculated by reference to quoted market forward exchange rates for contracts with similar maturity profiles. The fair value of currency swaps is determined by reference to quoted market spot rates. As a result, foreign forward exchange contracts and currency swaps are classified as Level 2 within the fair value hierarchy. The changes in counterparty credit risk had no material effect on the hedge effectiveness for derivatives designated in hedge relationships and other financial instruments recognised at fair value. The fair value of investments is based upon third-party pricing models for share issues. As a result, investments are considered Level 3 in the fair value hierarchy. There were
Long-term borrowings are measured in the balance sheet at amortised cost. The corporate bonds issued in October 2020 and October 2022 are publicly listed and a market price is available. The Group’s other long-term borrowings are not quoted publicly, their fair values are estimated by discounting future contractual cash flows to net present values at the current market interest rates available to the Group for similar financial instruments as at the year end. The fair value of the private placement notes is determined using a discounted cash flow model based on prevailing market rates.
208 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The following table shows the carrying amounts and fair values of financial assets and financial liabilities, including their levels in the fair value hierarchy. It does not include fair value information for financial assets and financial liabilities not measured at fair value.
Carrying | Fair value | |||||||||||||||||
Fair value – | Amortised | Fair value | Fair value | Other | Total | Level 2 | Level 3 | Total | ||||||||||
At 31 December 2023 |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
Financial assets measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Forward foreign exchange contracts | | – | – | – | – | | | – | | |||||||||
Investments | – | – | – | | – | | – | | | |||||||||
Contingent consideration receivable | – | – | – | | – | | – | | | |||||||||
Interest rate swaps | | – | – | – | – | | | – | | |||||||||
Currency swaps | – | – | | – | – | | | – | | |||||||||
| – | | | – | |
| ||||||||||||
Financial liabilities measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Acquisition consideration | – | – | – | ( | – | ( | – | ( | ( | |||||||||
Forward foreign exchange contracts | ( | – | – | – | – | ( | ( | – | ( | |||||||||
Currency swaps | – | – | ( | – | – | ( | ( | – | ( | |||||||||
( | – | ( | ( | – | ( |
|
|
| ||||||||||
Financial assets not measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Trade and other receivables | | – | – | – | – | |
|
|
| |||||||||
Cash at bank | – | | – | – | – | |
|
|
| |||||||||
| | – | – | – | |
|
|
| ||||||||||
Financial liabilities not measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Acquisition consideration | – | – | – | – | ( | ( |
|
|
| |||||||||
Bank overdrafts | – | – | – | – | ( | ( |
|
|
| |||||||||
Bank loans | – | – | – | – | ( | ( | ||||||||||||
Corporate bond not in a hedge relationship | – | – | – | – | ( | ( | ||||||||||||
Corporate bond in a hedge relationship | – | – | – | – | ( | ( |
|
|
| |||||||||
Private placement debt not in a hedge relationship | – | – | – | – | ( | ( | ||||||||||||
Trade and other payables | – | – | – | – | ( | ( |
|
|
| |||||||||
– | – | – | – | ( | ( |
|
|
|
At 31 December 2023, the book value and market value of the USD corporate bond were $
Smith+Nephew Annual Report 2023 | 209 |
Group financial statements continued
Notes to the Group accounts continued
16 Financial instruments and risk management continued
During the year ended 31 December 2023, acquisition consideration decreased by $
Carrying | Fair value | |||||||||||||||||
Fair value – | Amortised | Fair value | Fair value | Other | Total | Level 2 | Level 3 | Total | ||||||||||
At 31 December 2022 |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
Financial assets measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Forward foreign exchange contracts | | – | – | – | – | | | – | | |||||||||
Investments | – | – | – | | – | | – | | | |||||||||
Contingent consideration receivable | – | – | – | | – | | – | | | |||||||||
Currency swaps | – | – | | – | – | | | – | | |||||||||
| – | | | – | |
| ||||||||||||
Financial liabilities measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Acquisition consideration | – | – | – | ( | – | ( | – | ( | ( | |||||||||
Forward foreign exchange contracts | ( | – | – | – | – | ( | ( | – | ( | |||||||||
Interest rate swaps | ( | – | – | – | – | ( | ( | – | ( | |||||||||
Currency swaps | – | – | ( | – | – | ( | ( | – | ( | |||||||||
( | – | ( | ( | – | ( |
|
|
| ||||||||||
Financial assets not measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Trade and other receivables | | – | – | – | – | |
|
|
| |||||||||
Cash at bank | – | | – | – | – | |
|
|
| |||||||||
| | – | – | – | |
|
|
| ||||||||||
Financial liabilities not measured at fair value |
|
|
|
|
|
|
|
|
| |||||||||
Acquisition consideration | – | – | – | – | ( | ( |
|
|
| |||||||||
Bank overdrafts | – | – | – | – | ( | ( |
|
|
| |||||||||
Corporate bond not in a hedge relationship | – | – | – | – | ( | ( |
|
|
| |||||||||
Corporate bond in a hedge relationship | – | – | – | – | ( | ( |
|
|
| |||||||||
Private placement debt not in a hedge relationship | – | – | – | – | ( | ( | ||||||||||||
Trade and other payables | – | – | – | – | ( | ( |
|
|
| |||||||||
– | – | – | – | ( | ( |
|
|
|
The fair value of contingent acquisition consideration is estimated using a discounted cash flow model. The valuation model considers the present value of risk adjusted expected payments, discounted using a risk-free discount rate. The expected payment is determined by considering the possible scenarios, which relate to the achievement of established milestones and targets, the amount to be paid under each scenario and the probability of each scenario. As a result, contingent acquisition consideration is classified as Level 3 within the fair value hierarchy.
210 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The fair value of investments is based upon third-party pricing models for share issues. As a result, investments are considered Level 3 in the fair value hierarchy.
The movements in 2023 and 2022 for financial instruments measured using Level 3 valuation methods are presented below:
2023 | 2022 | |||
| $ million |
| $ million | |
Investments | ||||
At 1 January | | | ||
Additions | – | | ||
Fair value remeasurement | ( | – | ||
At 31 December | | | ||
Contingent consideration receivable | ||||
At 1 January | | | ||
Receipts | – | ( | ||
At 31 December | | | ||
Acquisition consideration liability | ||||
At 1 January | ( | ( | ||
Arising on acquisitions | – | ( | ||
Payments | | | ||
Remeasurements | | | ||
Discount unwind | – | ( | ||
At 31 December | ( | ( |
17 Provisions and contingencies
Accounting policy
In the normal course of business the Group is involved in various legal disputes. Provisions are made for loss contingencies when it is deemed probable that an adverse outcome will occur and the amount of the losses can be reasonably estimated. Where the Group is the plaintiff in pursuing claims against third parties, legal and associated expenses are charged to the income statement as incurred. The recognition of provisions for legal disputes is subject to a significant degree of estimation. In making its estimates, management takes into account the advice of internal and external legal counsel. Provisions are reviewed regularly and amounts updated where necessary to reflect developments in the disputes. The ultimate liability may differ from the amount provided depending on the outcome of court proceedings or settlement negotiations or as new facts emerge. Insurance recoveries are recognised when the inflow of benefits is virtually certain and are presented within other receivables.
A provision for onerous contracts is recognised when the expected benefits to be derived by the Group from a contract are lower than the unavoidable cost of meeting its obligations under the contract.
A provision for restructuring and rationalisation is recognised when the Group has approved a detailed and formal restructuring plan and the restructuring either has commenced or has been announced publicly. Future operating losses are not provided for.
Smith+Nephew Annual Report 2023 | 211 |
Group financial statements continued
Notes to the Group accounts continued
17 Provisions and contingencies continued
17.1 Provisions
| Restructuring |
|
|
| ||||||
and | ||||||||||
rationalisation | Legal and other | |||||||||
provisions | Metal-on-metal | provisions | Total | |||||||
|
| $ million |
| $ million |
| $ million |
| $ million | ||
At 1 January 2022 | | | | | ||||||
Charge to income statement | | | | | ||||||
Release to income statement | ( | – | ( | ( | ||||||
Unwinding of discount | – | | – | | ||||||
Utilised | ( | ( | ( | ( | ||||||
Exchange adjustment | ( | – | – | ( | ||||||
At 31 December 2022 | | | | | ||||||
Charge to income statement | | – | | | ||||||
Release to income statement | – | ( | ( | ( | ||||||
Unwinding of discount | – | | – | | ||||||
Utilised | ( | ( | ( | ( | ||||||
Exchange adjustment | | – | – | | ||||||
At 31 December 2023 | | | | | ||||||
Provisions – due within one year | | | | | ||||||
Provisions – due after one year | – | | | | ||||||
At 31 December 2023 | | | | | ||||||
Provisions – due within one year | | | | | ||||||
Provisions – due after one year | – | | | | ||||||
At 31 December 2022 | | | | |
The principal elements within restructuring and rationalisation provisions relate to the Operations and Commercial Excellence programme announced in February 2020 and the efficiency and productivity elements of the 12-Point Plan.
The Group has estimated a provision of $
The legal and other provisions mainly relate to various other product liability and intellectual property litigation matters. The Group carries considerable product liability insurance, and will continue to defend claims vigorously.
All provisions are expected to be substantially utilised within
212 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
17.2 Contingencies
The Company and its subsidiaries are party to various legal proceedings, some of which include claims for substantial damages. The outcome of these proceedings cannot readily be foreseen, but except as described herein management believes none of them is likely to result in a material adverse effect on the financial position of the Group. The Group provides for outcomes that are deemed to be probable and can be reliably estimated. There is no assurance that losses will not exceed provisions or will not have a significant impact on the Group’s results of operations in the period in which they are realised.
17.3 Legal proceedings
Product liability claims
The Group faces claims from time to time for alleged defects in its products and has on occasion recalled or withdrawn products from the market. Such claims are endemic to the medical device industry. The Group maintains product liability insurance subject to limits and deductibles that management believes are reasonable. All policies contain exclusions and limitations, however, and there can be no assurance that insurance will be available or adequate to cover all claims.
This includes matters raising concerns about possible adverse effects of hip implant products with metal-on-metal (MoM) bearing surfaces for which the Group has incurred and will continue to incur expenses to defend claims in this area.
As of December 2023, approximately
Through the end of 2023, entities of the Group have entered into several group, as well as individual, MoM related settlements without admitting liability. The Group requested indemnity from its product liability insurers for most of these MoM hip implant settlements and insurers have indemnified the Group to the limits of their respective applicable policies.
Litigation outcomes are difficult to predict and defence costs can be significant. The Group takes care to monitor the clinical evidence relating to its products, including its metal hip implant products, to help ensure that its product offerings are designed to serve patients’ interests.
Intellectual property disputes
The Group engages, as both plaintiff and defendant, in litigation with various competitors and others over claims of patent infringement and other intellectual property matters. These disputes are heard in courts in the US and other jurisdictions and also before agencies that examine patents. Outcomes are rarely certain and costs are often significant.
Arthrex asserted suture anchor patents against Smith+Nephew in 2014 and 2015 in the US District Court for the Eastern District of Texas. In February 2017, the parties reached a settlement resulting in the dismissal of all patent litigation. Smith+Nephew agreed to pay additional payments contingent on the outcome of patent validity proceedings pending at the US Patent & Trademark Office. In August 2019, the Court of Appeals for the Federal Circuit affirmed US Patent & Trademark Office ruling invalidating one of the asserted Arthrex patents. In October 2019, the Court of Appeals for the Federal Circuit vacated an earlier US Patent & Trademark Office ruling invalidating the other asserted Arthrex patent. The United States Supreme Court granted certiorari. The Supreme Court ruling allowed Arthrex to petition the Director of the US Patent & Trademark Office to review the decision invalidating the second asserted Arthrex patent. The US Patent & Trademark Office declined Arthrex’s rehearing request in October 2021. In May 2022, the Court of Appeals for the Federal Circuit affirmed the US Patent & Trademark Office’s invalidity ruling and its denial of Arthrex’s rehearing request. Arthrex petitioned the United States Supreme Court to review the US Patent & Trademark Office’s denial of Arthrex’s rehearing request. On 22 March 2023, the US Supreme Court denied Arthrex’s cert petition and on 7 June 2023, the US Patent & Trademark Office issued an IPR certificate officially cancelling all the claims of Arthrex’s patent.
17.4 Tax matters
At any given time the Group has unagreed years outstanding in various countries and is involved in tax audits and disputes, some of which may take several years to resolve. Provisions are based on best estimates and management’s judgements concerning the likely ultimate outcome of any audit or dispute. Management considers the specific circumstances of each tax position and takes external advice, where appropriate, to assess the range of potential outcomes and estimate additional tax that may be due. The Group believes that it has made adequate provision in respect of additional tax liabilities that may arise. See Note 5 for further details.
Smith+Nephew Annual Report 2023 | 213 |
Group financial statements continued
Notes to the Group accounts continued
18 Retirement benefit obligations
Accounting policy
The Group sponsors defined benefit plans in a number of countries. A defined benefit pension plan defines an amount of pension benefit that an employee will receive on retirement or a minimum guaranteed return on contributions, which is dependent on various factors such as age, years of service and final salary. The Group’s obligation is calculated separately for each plan by discounting the estimated future benefit that employees have earned in return for their service in the current and prior periods. The fair value of any plan assets is deducted to arrive at the net liability.
The calculation of the defined benefit obligation is performed annually by external actuaries using the projected unit credit method. Remeasurements arising from defined benefit plans comprise actuarial gains and losses and the return on the plan assets in excess of the discount rate net of the costs of managing the plan assets. The Group recognises these immediately in other comprehensive income (OCI) and all other expenses, such as service cost, net interest cost, administration costs and taxes, are recognised in the income statement.
A number of key assumptions are made when calculating the fair value of the Group’s defined benefit pension plans. These assumptions impact the balance sheet asset and liabilities, operating profit, finance income/costs and other comprehensive income. The most critical assumptions are the discount rate, the rate of inflation and mortality assumptions to be applied to future pension plan liabilities. The discount rate is based on the yield at the reporting date on bonds that have a credit rating of AA, denominated in the currency in which the benefits are expected to be paid and have a maturity profile approximately the same as the Group’s obligations. In determining these assumptions management takes into account the advice of professional external actuaries and benchmarks its assumptions against external data.
The Group determines the net interest expense/income on the net defined benefit liability/asset for the period by applying the discount rate used to measure the defined benefit obligation at the beginning of the annual period to the net defined benefit liability/asset.
The Group also operates a number of defined contribution plans. A defined contribution plan is a pension plan under which the Group and employees pay fixed contributions to a third-party financial provider. The Group has no further payment obligations once the contributions have been paid. Contributions are recognised as an employee benefit expense when they are due.
The Group’s retirement benefit assets/(obligations) comprise:
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
Funded plans: |
|
| ||
UK Plan | | | ||
US Plan | | | ||
Other plans | ( | ( | ||
| | |||
Unfunded plans: |
|
| ||
Other plans | ( | ( | ||
Retirement healthcare | ( | ( | ||
( | | |||
Amount recognised on the balance sheet – liability | ( | ( | ||
Amount recognised on the balance sheet – asset | | |
The Group sponsors defined benefit pension plans for its employees or former employees in
214 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The Group’s
The UK Plan operates under trust law and responsibility for its governance lies with a Board of Trustees. This Board is composed of representatives of the Group, plan participants and an independent trustee, who act on behalf of members in accordance with the terms of the Trust Deed and Rules and relevant legislation. The UK Plan’s assets are held by the trust. Annual increases on benefits in payment are dependent on inflation.
The 2018 and 2020 court cases in relation to Guaranteed Minimum Pensions do not impact the UK Plan as members were not contracted out of the State Earnings-Related Pension Scheme (SERPS) between 1990 and 1997.
In June 2023, the Trustee with the support of the Company concluded a full buy-in of the Main Fund with Rothesay Life. The total transaction value was £
When the full UK Fund buy-in was concluded in June 2023 no decision on a future buy-out had been reached by the Company. Whilst the contract between the Life Insurer (Rothesay) and the Trustee allows for a buy-out, a number of steps would need to be concluded before this could be achieved. Not least, the conclusion of the due diligence process with the Life Insurer which is expected to continue into the second half of 2024. Thereafter, the Trustee and the Company could not act unilaterally to move to a buy-out and the UK Fund governance structure lays out a number of steps the Company would be required to conclude for a buy-out decision. The transaction resulted in a $
The US Plan is governed by a US Pension Committee which comprises representatives of the Group. In the US, the Pension Protection Act (2006) established both a minimum required contribution and a maximum deductible contribution. Failure to contribute at least the minimum required amount will subject the Company to significant penalties, and contributions in excess of the maximum deductible have negative tax consequences. The minimum funding requirement is intended to fully fund the present value of accrued benefits over
In October 2022, US Pension Plan members were notified that Smith & Nephew Inc. (SNI) would begin the termination process for the US Plan. In December 2023, Fidelity & Guaranty Life was selected to take over responsibility for the remaining US Pension Plan obligation and administration upon termination. A premium amount of $
There is no legislative minimum funding requirement in the UK. The Trust Deed of the UK Plan and the Plan Document of the US Plan provide the Group with a right to a refund of surplus assets assuming the full settlement of plan liabilities in the event of a plan wind-up. Furthermore, in the ordinary course of business the UK Board of Trustees and US Pension Committee have no rights to unilaterally wind up, or otherwise augment the benefits due to members of the Plans. Based on these rights, any net surplus in the UK and US Plans is recognised in full.
Smith+Nephew Annual Report 2023 | 215 |
Group financial statements continued
Notes to the Group accounts continued
18 Retirement benefit obligations continued
18.2 Reconciliation of retirement benefit obligations and pension assets
The movement in the Group’s pension benefit obligation and pension assets is as follows:
2023 | 2022 | |||||||||||
| Obligation |
| Asset |
| Total |
| Obligation | Asset | Total | |||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
Amounts recognised on the balance sheet at beginning of the period |
| ( |
| |
| |
| ( |
| |
| |
Income statement expense: |
|
|
|
|
|
|
|
| ||||
Current service cost |
| ( | – | ( |
| ( | – |
| ( | |||
Settlements | | ( | ( | | ( | – | ||||||
Interest (expense)/income |
| ( | | |
| ( | |
| | |||
Administration costs and taxes |
| ( | – | ( |
| ( | – |
| ( | |||
Costs recognised in income statement |
| |
| ( |
| ( |
| ( |
| |
| ( |
Remeasurements: |
|
|
|
|
|
|
|
|
|
|
|
|
Actuarial (loss)/gain due to liability experience |
| ( | – | ( |
| ( | – |
| ( | |||
Actuarial gain due to financial assumptions change |
| ( | – | ( |
| | – |
| | |||
Actuarial gain due to demographic assumptions |
| | – | |
| | – |
| | |||
Return on plan assets (less)/greater than discount rate |
| – | ( | ( |
| – | ( |
| ( | |||
Remeasurements recognised in OCI |
| ( |
| ( |
| ( |
| |
| ( |
| |
Cash: |
|
|
|
|
|
|
|
|
|
|
|
|
Employer contributions |
| – | | |
| – | |
| | |||
Employee contributions |
| ( | | – |
| ( | |
| – | |||
Benefits paid directly by the Group |
| | – | |
| | – |
| | |||
Benefits paid, taxes and administration costs paid from scheme assets |
| | ( | ( |
| | ( |
| ( | |||
Net cash |
| |
| ( |
| |
| |
| ( |
| |
Exchange movements |
| ( | | |
| | ( |
| ( | |||
Amount recognised on the balance sheet |
| ( |
| |
| ( |
| ( |
| |
| |
Amount recognised on the balance sheet – liability |
| ( | | ( |
| ( | |
| ( | |||
Amount recognised on the balance sheet – asset |
| ( | | |
| ( | |
| |
Represented by:
2023 | 2022 | |||||||||||
| Obligation |
| Asset |
| Total |
| Obligation |
| Asset |
| Total | |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
UK Plan | ( | | | ( | | | ||||||
US Plan | ( | | | ( | | | ||||||
Other Plans | ( | | ( | ( | | ( | ||||||
Total | ( | | ( | ( | | |
The actuarial loss on obligation of $
All benefits are vested at the end of each reporting period. The weighted average duration of the defined benefit obligation at the end of the reporting period is
216 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
18.3 Plan assets
The market value of the US, UK and Other Plans assets are as follows:
| 2023 |
| 2022 |
| 2021 | |
| $ million |
| $ million |
| $ million | |
UK Plan: |
|
|
| |||
Assets with a quoted market price: |
|
|
| |||
Cash and cash equivalents | | | | |||
Equity securities | – | | | |||
Other bonds | – | | | |||
Short dated credit fund | – | | | |||
Liability driven investments | – | | | |||
Diversified growth funds | – | | | |||
| | | ||||
Other assets: |
|
|
| |||
Insurance contract | | | | |||
Market value of assets | | | | |||
US Plan: |
|
|
| |||
Assets with a quoted market price: |
|
| ||||
Cash and cash equivalents | | | | |||
Equity securities | – | – | | |||
Government bonds – fixed interest | – | | | |||
Corporate bonds | – | | | |||
Market value of assets | | | | |||
Other Plans: |
|
|
| |||
Assets with a quoted market price: |
|
| ||||
Cash and cash equivalents | | | | |||
Equity securities | | | | |||
Government bonds – fixed interest | | | | |||
Government bonds – index linked | – | – | | |||
Corporate and other bonds | | | | |||
Insurance contracts | | | | |||
Property | | | | |||
Other quoted securities | | | | |||
| | | ||||
Other assets: |
|
|
| |||
Insurance contracts | | | | |||
Market value of assets | | | | |||
Total market value of assets | | | |
No plans invest directly in property occupied by the Group or in financial securities issued by the Group.
Both the UK and US Plans hold predominantly matching assets. The UK Plan is comprised of annuity policies purchased by the Trustee. The US Plan in 2023 held predominantly matching assets. In 2024, following the scheme termination, the investment risks have been transferred to a US Life Insurer.
Smith+Nephew Annual Report 2023 | 217 |
Group financial statements continued
Notes to the Group accounts continued
18 Retirement benefit obligations continued
18.4 Expenses recognised in the income statement
The total expense relating to retirement benefits recognised for the year is $
The cost charged in respect of the Group’s defined contribution plans represents contributions payable to these plans by the Group at rates specified in the rules of the Plans. These were charged to operating profit in costs of goods sold, selling, general and administrative expenses, and research and development expenses. There were $
Defined benefit plan costs comprise service cost which is charged to operating profit in selling, general and administrative expenses and net interest cost and administration costs and taxes which are reported as other finance costs.
The defined benefit pension costs charged for the UK and Plans are $
18.5 Principal actuarial assumptions
The following are the principal financial actuarial assumptions used at the reporting date to determine the UK and US defined benefit obligations and expense.
| 2023 | 2022 | 2021 | |||
|
| % per annum |
| % per annum |
| % per annum |
UK Plan: |
|
|
| |||
Discount rate | | | | |||
Future salary increases | n/a | n/a | n/a | |||
Future pension increases | | | | |||
Inflation (RPI) | | | | |||
Inflation (CPI) | | | | |||
US Plan: | ||||||
Discount rate | | | | |||
Future salary increases | n/a | n/a | n/a | |||
Inflation | n/a | n/a | n/a |
Actuarial assumptions regarding future mortality are based on mortality tables. The UK uses the S3NA with projections in line with the CMI 2022 table, which places partial weight on post pandemic experience, and the US uses the PRI-2012 table with MP-2021 scale. The Directors will continue to monitor any potential future impact on the mortality assumptions used.
The current longevities underlying the values of the obligations in the defined benefit plans are as follows:
| 2023 | 2022 | 2021 | |||
|
| years |
| years |
| years |
Life expectancy at age 60 |
|
|
| |||
UK Plan: |
|
|
| |||
Males | ||||||
Females | ||||||
US Plan: | ||||||
Males | ||||||
Females | ||||||
Life expectancy at age 60 in 20 years’ time |
|
|
| |||
UK Plan: |
|
|
| |||
Males | ||||||
Females | ||||||
US Plan: | ||||||
Males | ||||||
Females |
218 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
18.6 Sensitivity analysis
The calculation of the defined benefit obligation is sensitive to the assumptions used. The following table summarises the increase/ decrease on the UK and US defined benefit obligation and pension costs as a result of reasonably possible changes in some of the assumptions while holding all other assumptions consistent. The sensitivity to the inflation assumption change includes corresponding changes to the future pension increase assumptions. The analysis does not take into account the full distribution of cash flows expected under the Plan.
Changes to the inflation assumption will not have any effect on the US Pension Plan as it was closed to future accrual in 2014 and it has no other inflation-linked assumptions.
Increase/(decrease) in pension obligation | Increase /(decrease) in pension cost | |||||||
$ million |
| +50bps/+1yr |
| -50bps/-1yr |
| +50bps/+1 yr |
| -50bps/-1yr |
UK Plan: |
| |||||||
Discount rate |
| ( | | – | – | |||
Inflation |
| | ( | – | – | |||
Mortality |
| | ( | – | – | |||
US Plan: |
| |||||||
Discount rate |
| ( | | – | – | |||
Mortality |
| | ( | – | – | |||
18.7 Risk
The pension plans expose the Group to the following risks:
Interest rate risk | Volatility in financial markets can change the calculations of the obligation significantly as the calculation of the obligation is linked to yields on AA rated corporate bonds. A decrease in the bond yield will increase the measure of plan liabilities, although this will be partially offset by increases in the value of matching plan assets such as bonds and insurance contracts. The UK buy-in in June 2023 removed all remaining material pension liability exposure from the balance sheet, hence, eliminating the interest rate risk for the UK Plan. Following the completion of the US buy-out on 4 January 2024, no further interest risk is linked to the valuation of liability for the US Plan as no liability will remain in the Plan. |
Inflation risk | The UK Plan is linked to inflation. A high rate of inflation will lead to a higher liability. This risk is managed by holding inflation-linked bonds and an inflation-linked insurance contract in respect of some of the obligation. In the UK, the liability matching portfolio held in conventional and index-linked gilts was transferred into liability driven investments in order to reduce inflation risk. The UK Plan is closed to future accrual which reduces the exposure to this risk. The US Plan is also closed to future accrual and has no other inflation-linkage thus eliminating the exposure to this risk. Following the full UK Pension buy-in in 2023, the residual inflation risks associated with the UK Plan have been transferred to the UK Plan’s Life Insurance Partners. |
Investment risk | If the return on plan assets is below the discount rate, all else being equal, there will be an increase in the Plan deficit. In the UK, following the full buy-in for the UK Plan, the investment risk has been transferred to the UK Plan’s Life Insurer Partners. The US Plan has a dynamic de-risking policy to shift plan assets from return-seeking (growth) assets to liability matching assets over time. The US Pension Plan has an established glide path that is designed to stabilise funding status by reducing the Plan’s exposure to return-seeking assets. Following the completion of the US buy-out on 4 January 2024, no further investment risk is linked to the valuation of liability for the US Plan as no liability will remain in the Plan. |
Longevity risk | The present value of the Plan’s defined benefit liability is calculated by reference to the best estimate of the mortality of the Plan participants both during and after their employment. An increase in the life expectancy of plan participants above that assumed will increase the benefit obligation. Following the full buy-in, the UK Plan has entered into insurance contract which covers all of the pensioners’ obligations. Following the completion of the US buy-out on 4 January 2024, there is no further longevity risk linked to the valuation of liability for the US Plan as no liability will remain in the Plan. |
Smith+Nephew Annual Report 2023 | 219 |
Group financial statements continued
Notes to the Group accounts continued
18 Retirement benefit obligations continued
18.8 Funding
A full valuation is performed by actuaries for the Trustees/Pension Committee of each plan to determine the level of funding required. Employer contribution rates, based on these full valuations, are agreed between the Trustees/Pension Committee of each plan and the Group. The assumptions used in the actuarial valuations used for funding purposes may differ from the accounting assumptions set out above.
UK Plan
The most recent full actuarial valuation of the UK Plan was undertaken as at 30 September 2020. Future accruals to the UK Plan ceased as at 31 December 2016. Contributions to the UK Plan in 2023 were $
Following the completion of the 30 September 2020 valuation, a dynamic contribution mechanism was agreed. Under that dynamic contribution mechanism, no further contributions were required in 2022 or 2023.
In 2023, the Trustees concluded a full buy-in of the UK Defined Benefit Fund. The transaction resulted in a $
US Plan
The most recent full actuarial valuation of the US Plan was undertaken as at 1 January 2022. Future accruals to the US Plan ceased as at 31 March 2014. Contributions to the US Plan were $
There are
A premium amount of $
19 Equity
Accounting policy
Incremental costs directly attributable to the issue of ordinary shares, net of any tax effects, are recognised as a deduction from equity.
When shares recognised as equity are repurchased, the amount of the consideration paid, which includes directly attributable costs, net of any tax effects, is recognised as a deduction from equity. Repurchased shares are classified as treasury shares and are presented in the treasury share reserve. When treasury shares are sold or reissued subsequently, the amount received is recognised as an increase in equity and the resulting surplus or deficit on the transaction is presented within share premium.
19.1 Share capital
Ordinary shares ( | Deferred shares (£ | Total | ||||||||
| Thousand |
| $ million |
| Thousand |
| $ million |
| $ million | |
Authorised |
|
|
|
|
| |||||
At 31 December 2021 | | | | – | | |||||
At 31 December 2022 | | | | – | | |||||
At 31 December 2023 | | | | – | | |||||
Allotted, issued and fully paid |
|
|
|
|
| |||||
At 1 January 2021 | | | | – | | |||||
Share options | | – | – | – | – | |||||
Shares cancelled | – | – | – | – | – | |||||
At 31 December 2021 | | | | – | | |||||
Share options | | – | – | – | – | |||||
Shares cancelled | ( | ( | – | – | ( | |||||
At 31 December 2022 | | | | – | | |||||
Share options | | – | – | – | – | |||||
At 31 December 2023 | | | | – | | |||||
220 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The deferred shares were issued in 2006 in order to comply with English Company law. They are not listed on any stock exchange and have extremely limited rights and effectively have no value. These rights are summarised as follows:
| - | The holder shall not be entitled to participate in the profits of the Company; |
| - | The holder shall not have any right to participate in any distribution of the Company’s assets on a winding-up or other distribution except that after the return of the nominal amount paid up on each share in the capital of the Company of any class other than the deferred shares and the distribution of a further $ |
| - | The holder shall not be entitled to receive notice, attend, speak or vote at any general meeting of the Company; and |
| - | The Company may create, allot and issue further shares or reduce or repay the whole or any part of its share capital or other capital reserves without obtaining the consent of the holders of the deferred shares. |
The Group’s objectives when managing capital are to ensure the Group has adequate funds to continue as a going concern and sufficient flexibility within the capital structure to fund the ongoing growth of the business and to take advantage of business development opportunities including acquisitions.
The Group determines the amount of capital taking into account changes in business risks and future cash requirements. The Group reviews its capital structure on an ongoing basis and uses share buy-backs, dividends and the issue of new shares to adjust the retained capital.
The Group considers the capital that it manages to be as follows:
| 2023 | 2022 | 2021 | |||
|
| $ million |
| $ million |
| $ million |
Share capital | | | | |||
Share premium | | | | |||
Capital redemption reserve | | | | |||
Treasury shares | ( | ( | ( | |||
Retained earnings and other reserves | | | | |||
| | |
19.2 Treasury shares
Treasury shares represent the holding of the Company’s own shares in respect of the Smith & Nephew Employees’ Share Trust and shares bought back as part of the share buy-back programme. In 2022, the Group purchased a total of
The Smith & Nephew 2004 Employees’ Share Trust (the Trust) was established to hold shares relating to the long-term incentive plans. The Trust is administered by an independent professional trust company resident in Jersey and is funded by a loan from the Company. The cost of the Trust is charged to the income statement as it accrues. A dividend waiver is in place in respect of those shares held under the long-term incentive plans. The Trust only accepts dividends in respect of nil-cost options and deferred bonus plan shares. The waiver represents less than
Smith+Nephew Annual Report 2023 | 221 |
Group financial statements continued
Notes to the Group accounts continued
19 Equity continued
The movements in Treasury shares and the Employees’ Share Trust are as follows:
|
| Employees’ | ||||
Treasury | Share Trust | Total | ||||
|
| $ million |
| $ million |
| $ million |
At 1 January 2022 | | | | |||
Shares purchased | | | | |||
Shares transferred from treasury | ( | | – | |||
Shares transferred to Group beneficiaries | ( | ( | ( | |||
Shares cancelled | ( | – | ( | |||
At 31 December 2022 | | | | |||
Shares transferred from treasury | ( | | – | |||
Shares transferred to Group beneficiaries | ( | ( | ( | |||
At 31 December 2023 | | | |
|
| Employees’ | ||||
Treasury | Share Trust | Total | ||||
Number | Number | Number | ||||
of shares | of shares | of shares | ||||
|
| million |
| million |
| million |
At 1 January 2022 | | | | |||
Shares purchased | | | | |||
Shares transferred from treasury | ( | | – | |||
Shares transferred to Group beneficiaries | ( | ( | ( | |||
Shares cancelled | ( | – | ( | |||
At 31 December 2022 | | | | |||
Shares transferred from treasury | ( | | – | |||
Shares transferred to Group beneficiaries | ( | ( | ( | |||
At 31 December 2023 | | | |
19.3 Dividends
| 2023 | 2022 | 2021 | |||
|
| $ million |
| $ million |
| $ million |
The following dividends were declared and paid in the year: |
|
|
| |||
Ordinary final of for 2022 (2021: , 2020: ) paid 17 May 2023 | | | | |||
Ordinary interim of for 2023 (2022: , 2021: ) paid 1 November 2023 | | | | |||
| | |
A final dividend for 2023 of
222 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
20 Cash flow statement
Accounting policy
In the Group cash flow statement, cash and cash equivalents includes cash at bank, other short-term liquid investments with original maturities of three months or less and bank overdrafts. In the Group balance sheet, bank overdrafts are shown within bank overdrafts, borrowings, loans and lease liabilities under current liabilities.
Analysis of net debt including lease liabilities
Borrowings | ||||||||||||||
Cash | Overdrafts | Due within | Due after | Net | Net | Total | ||||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
At 1 January 2021 |
| |
| ( |
| ( |
| ( |
| – |
| |
| ( |
Net cash flow/debt movement |
| ( | | ( | | | – |
| ( | |||||
Exchange adjustment |
| ( | ( | – | | ( | ( |
| | |||||
Corporate bond issuance expense | – | – | – | ( | – | – | ( | |||||||
IFRS 16 lease liabilities movement |
| – | – | | | – | – | | ||||||
At 31 December 2021 |
| |
| ( |
| ( |
| ( |
| – |
| – |
| ( |
Net cash flow/debt movement |
| ( | | | | ( | – |
| ( | |||||
Exchange adjustment | ( | ( | | | | ( | | |||||||
Corporate bond issuance expense | – | – | – | | – | – | | |||||||
IFRS 16 lease liabilities movement |
| – | – | | ( | – | – |
| | |||||
At 31 December 2022 |
| |
| ( |
| ( |
| ( |
| – |
| ( |
| ( |
Net cash flow/debt movement |
| ( | | ( | | ( | – |
| ( | |||||
Exchange adjustment |
| – | ( | – | ( | | |
| ( | |||||
Corporate bond issuance expense |
| – | – | – | | – | – | | ||||||
IFRS 16 lease liabilities movement | – | – | ( | | – | – | ( | |||||||
Net debt including lease |
| |
| ( |
| ( |
| ( |
| ( |
| |
| ( |
Reconciliation of net cash flow to movement in net debt including lease liabilities
| 2023 | 2022 | 2021 | |||
|
| $ million |
| $ million |
| $ million |
Net cash flow from cash net of overdrafts | ( | ( | ( | |||
Settlement of currency swaps | ( | ( | | |||
Net cash flow from borrowings | ( | | | |||
Change in net debt from net cash flow | ( | ( | ( | |||
IFRS 16 lease liabilities | ( | | | |||
Exchange adjustment | ( | | | |||
Corporate bond issuance expense | | | ( | |||
Change in net debt in the year | ( | ( | ( | |||
Opening net debt | ( | ( | ( | |||
Closing net debt | ( | ( | ( |
Cash and cash equivalents
For the purposes of the Group cash flow statement, cash and cash equivalents at 31 December 2023 comprise cash at bank net of bank overdrafts.
| 2023 | 2022 | 2021 | |||
|
| $ million |
| $ million |
| $ million |
Cash at bank | | | | |||
Bank overdrafts | ( | ( | ( | |||
Cash and cash equivalents | | | |
Smith+Nephew Annual Report 2023 | 223 |
Group financial statements continued
Notes to the Group accounts continued
20 Cash flow statement continued
The Group operates in over
Cash outflows/(inflows) arising from financing activities
Repayment | Borrowing | Proceeds from | Repayment | Cash outflow/ | Proceeds from own | |||||||||||||
| of bank |
| of bank |
| Corporate |
| of lease |
| (inflow) |
|
| Purchase of |
| shares/issue of |
| |||
loans1 | loans1 | Bond issue | liabilities | from other | Dividends | own shares | ordinary shares | Total | ||||||||||
2023 | $ million | $ million | $ million | $ million | $ million | $ million | $ million | $ million | $ million | |||||||||
Debt | | ( | – | | ( | – | – | – | ( | |||||||||
Equity |
| – | – | – | – | – | | – | – |
| | |||||||
Total |
| |
| ( |
| – |
| |
| ( |
| |
| – |
| – |
| |
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Debt | | – | ( | | ( | – | – | – | | |||||||||
Equity | – | – | – | – | – | | | ( |
| | ||||||||
Total | | – | ( | | ( | | | ( |
| | ||||||||
2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Debt | | – | – | | | – | – | – | | |||||||||
Equity |
| – | – | – | – | – | | – | ( | | ||||||||
Total |
| | – | – | | | | – | ( | |
1 | This includes drawdown and repayment of the syndicated revolving credit facility. |
21 Acquisitions
Accounting policy
The Group accounts for business combinations using the acquisition method when control is transferred to the Group. The consideration transferred in the acquisition is measured at fair value, as are the identifiable net assets acquired. Any goodwill that arises is tested annually for impairment. Any gain on a bargain purchase is recognised in profit or loss immediately. Transaction costs are expensed as incurred, except if related to the issue of debt or equity securities.
Any contingent consideration payable is measured at fair value at the acquisition date. If the contingent consideration is classified as equity, then it is not remeasured and settlement is accounted for within equity. Otherwise, subsequent changes in the fair value of the contingent consideration are recognised in profit or loss.
Year ended 31 December 2023
During 2023, management evaluated the commercial viability of Engage products and concluded that they should be discontinued (see Note 2.2 for further details).
Year ended 31 December 2022
On 18 January 2022, the Group completed the acquisition of
224 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
The fair value of assets acquired and liabilities assumed is set out below:
|
| Engage |
|
| $ million |
Intangible assets – Product-related | | |
Property, plant and equipment | | |
Inventory | | |
Trade and other payables | ( | |
Net assets | | |
Goodwill | | |
Consideration (net of $nil cash acquired) | |
The product-related intangible assets were valued using a relief-from-royalty methodology with the key inputs being revenue, profit and discount rate. The cash outflow from acquisitions of $
The carrying value of goodwill increased from $
For the year ended 31 December 2022, the contribution from Engage Surgical to revenue and to profit was immaterial. If the business combination had occurred at the beginning of the year the contribution to revenue and profit would not have been materially different.
Year ended 31 December 2021
On 4 January 2021, the Group completed the acquisition of the Extremity Orthopaedics business of Integra LifeSciences Holdings Corporation (‘Extremity Orthopaedics’). The acquisition significantly strengthens the Group’s extremities business by adding a combination of a focused sales channel, complementary shoulder replacement and upper and lower extremities portfolio, and a new product pipeline. The transaction comprised the acquisition of the entire issued share capital of
The goodwill represents the control premium, the acquired workforce and the synergies expected from integrating Extremity Orthopaedics into the Group’s existing business, and is expected to be partly deductible for tax purposes.
The fair value of assets acquired and liabilities assumed is set out below:
|
| Extremity |
$ million | ||
Intangible assets – Product-related | | |
Intangible assets – Customer-related | | |
Property, plant and equipment | | |
Inventory | | |
Other payables | ( | |
Net deferred tax asset | ( | |
Net assets | | |
Goodwill | | |
Consideration (net of $nil cash acquired) | |
The product-related intangible assets were valued using an excess earnings methodology with the key inputs being revenue, profit and discount rate. The cash outflow from acquisitions of $
The carrying value of goodwill increased from $
Smith+Nephew Annual Report 2023 | 225 |
Group financial statements continued
Notes to the Group accounts continued
22 Other notes to the accounts
22.1 Share-based payments
Accounting policy
The Group operates a number of equity-settled executive and employee share plans. For all grants of share options and awards, the fair value at the grant date is calculated using appropriate option pricing models. The grant date fair value is recognised over the vesting period as an expense, with a corresponding increase in retained earnings.
The Group operates the following equity-settled executive and employee share plans: Smith & Nephew Global Share Plan 2010, Smith & Nephew Global Share Plan 2020, Smith & Nephew Share Save Plan (2012) and Smith & Nephew International Share Save Plan (2012). At 31 December 2023,
At 31 December 2023, the maximum number of shares that could be awarded under the Group’s long-term incentive plans was
The expense charged to the income statement for share-based payments for the year is $
22.2 Related party transactions
Trading transactions
In the course of normal operations, the Group traded with its associates detailed in Note 11. The aggregated transactions, which have not been disclosed elsewhere in the financial statements, are $
Key management personnel
The remuneration of Executive Officers (including Non-Executive Directors) during the year is summarised below:
| 2023 |
| 2022 |
| 2021 | |
| $ million |
| $ million |
| $ million | |
Short-term employee benefits | | | | |||
Share-based payments expense | | | | |||
Pension and post-employment benefit entitlements | | | | |||
| | |
Retirement benefit schemes
Details of the Group’s retirement benefit schemes are set out in Note 18.
23 Post balance sheet events
On 9 January 2024, the Group completed the acquisition of
This acquisition will be treated as a business combination under IFRS 3-Business Combinations. The provisional value of acquired net tangible assets is not material and is not expected to have material fair value adjustments. The remaining consideration will be allocated between identifiable intangible assets (product-related) and goodwill, with the majority expected to be goodwill representing the control premium, the acquired workforce and the synergies expected from integrating CartiHeal into the Group’s existing business.
In October 2022, US Pension Plan members were notified that Smith & Nephew Inc. (SNI) would begin the termination process for the US Plan. In December 2023, Fidelity & Guaranty Life was selected to take over responsibility for the remaining US Pension Plan obligation and administration upon termination. A premium amount of $
226 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Company financial statements
Company balance sheet
|
| At 31 December |
| At 31 December | ||
2023 | 2022 | |||||
|
| Notes |
| $ million |
| $ million |
Non-current assets |
|
|
|
|
|
|
Investments |
| 2 |
| 7,092 |
| 7,092 |
Debtors | 3 | 7 | – | |||
Current assets |
|
|
|
| ||
Debtors |
| 3 |
| 3,317 |
| 2,991 |
Cash at bank |
| 5 |
| 82 |
| 190 |
|
|
| 3,399 |
| 3,181 | |
Creditors: amounts falling due within one year |
|
|
|
|
| |
Borrowings |
| 5 |
| (711) |
| (109) |
Other creditors |
| 4 |
| (1,210) |
| (947) |
|
|
| (1,921) |
| (1,056) | |
Net current assets |
|
|
| 1,478 |
| 2,125 |
Total assets less current liabilities |
|
|
| 8,577 |
| 9,217 |
Creditors: amounts falling due after one year |
|
|
|
|
|
|
Borrowings |
| 5 |
| (2,168) |
| (2,565) |
Other creditors |
| 4 |
| – |
| (13) |
|
|
| (2,168) |
| (2,578) | |
Total assets less total liabilities |
|
|
| 6,409 |
| 6,639 |
Equity shareholders’ funds |
|
|
|
|
|
|
Share capital |
|
|
| 175 |
| 175 |
Share premium |
|
|
| 615 |
| 615 |
Capital redemption reserve |
|
|
| 20 |
| 20 |
Capital reserve |
|
|
| 2,266 |
| 2,266 |
Treasury shares |
|
|
| (94) |
| (118) |
Exchange reserve |
|
|
| (52) |
| (52) |
Profit and loss account |
|
|
| 3,479 |
| 3,733 |
Shareholders’ funds |
|
|
| 6,409 |
| 6,639 |
The attributable profit for the year dealt with in the accounts of the Company is $58m (2022: $80m).
The accounts were approved by the Board and authorised for issue on 26 February 2024 and signed on its behalf by:
Rupert Soames, OBE | Deepak Nath, PhD | Anne-Françoise Nesmes |
Chair | Chief Executive Officer | Chief Financial Officer |
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
Smith+Nephew Annual Report 2023 | 227 |
Company financial statements continued
Company statement of changes in equity
Capital | Total | |||||||||||||||
Share | Share | redemption | Capital | Treasury | Exchange | Profit and | shareholders’ | |||||||||
capital | premium | reserve | reserve | shares | reserve | loss account | funds | |||||||||
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million |
| $ million | |
At 1 January 2022 | 177 |
| 614 |
| 18 |
| 2,266 |
| (120) |
| (52) |
| 4,095 |
| 6,998 | |
Attributable profit for the year |
| – | – | – | – | – | – | 80 |
| 80 | ||||||
Equity dividends paid in the year |
| – |
| – |
| – |
| – |
| – |
| – |
| (327) |
| (327) |
Share-based payments recognised1 |
| – |
| – |
| – |
| – |
| – |
| – |
| 40 |
| 40 |
Cost of shares transferred to beneficiaries |
| – |
| – |
| – |
| – |
| 31 |
| – |
| (26) |
| 5 |
New shares issued on exercise of share options |
| – |
| 1 |
| – |
| – |
| – |
| – |
| – |
| 1 |
Cancellation of treasury shares |
| (2) |
| – |
| 2 |
| – |
| 129 |
| – |
| (129) |
| – |
Treasury shares purchased |
| – |
| – |
| – |
| – |
| (158) |
| – |
| – |
| (158) |
At 31 December 2022 | 175 |
| 615 |
| 20 |
| 2,266 |
| (118) |
| (52) |
| 3,733 |
| 6,639 | |
Attributable profit for the year |
| – | – | – | – | – | – | 58 |
| 58 | ||||||
Equity dividends paid in the year |
| – |
| – |
| – |
| – |
| – |
| – |
| (327) |
| (327) |
Share-based payments recognised1 |
| – |
| – |
| – |
| – |
| – |
| – |
| 39 |
| 39 |
Cost of shares transferred to beneficiaries |
| – |
| – |
| – |
| – |
| 24 |
| – |
| (24) |
| – |
At 31 December 2023 |
| 175 |
| 615 |
| 20 |
| 2,266 |
| (94) |
| (52) |
| 3,479 |
| 6,409 |
| 1 | The Company operates a number of equity-settled executive and employee share plans. For all grants of share options and awards, the fair value as at the date of grant is calculated using an appropriate option pricing model and the corresponding expense is recognised over the vesting period. Subsidiary companies are recharged for the fair value of share options that relate to their employees. The disclosure relating to the Company is detailed in Note 22.1 of the Notes to the Group accounts. |
Further information on the share capital of the Company can be found in Note 19.1 of the Notes to the Group accounts.
The total distributable reserves of the Company are $3,333m (2022: $3,563m). In accordance with the exemption permitted by Section 408 of the Companies Act 2006, the Company has not presented its own profit and loss account.
Fees paid to KPMG LLP for audit and non-audit services to the Company itself are not disclosed in the individual accounts because Group financial statements are prepared which are required to disclose such fees on a consolidated basis. The fees for the consolidated Group are disclosed in Note 3.2 of the Notes to the Group accounts.
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
228 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Notes to the Company accounts
1 Basis of preparation
Smith & Nephew plc (the “Company”) is a public limited company incorporated in England and Wales.
The separate accounts of the Company are presented as required by the Companies Act 2006. These financial statements and accompanying notes have been prepared in accordance with the Financial Reporting Standard 101 Reduced Disclosure Framework (‘Reduced Disclosure Framework’) for all periods presented. The financial information for the Company has been prepared on the same basis as the consolidated financial statements, applying identical accounting policies as outlined throughout the Notes to the Group accounts. The Directors have determined that the preparation of the Company financial statements on a going concern basis is appropriate as the Company receives dividend cash receipts from its subsidiary undertakings which enable it to meet its liabilities as they fall due.
In applying these policies, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the accounts and the reported amounts of revenues and expenses during the reporting period. Although these estimates are based on management’s best knowledge of current events and actions, actual results ultimately may differ from those estimates.
In these financial statements, the Company has applied the exemptions available under FRS 101 in respect of the following disclosures:
| - | A cash flow statement and related notes; |
| - | Comparative period reconciliations for share capital and tangible fixed assets; |
| - | Disclosures in respect of transactions with wholly-owned subsidiaries; |
| - | Disclosures in respect of capital management; |
| - | The effects of new but not yet effective IFRSs; and |
| - | Disclosures in respect of the compensation of key management personnel. |
As the consolidated financial statements include the equivalent disclosures, the Company has also taken the exemptions under FRS 101 available in respect of the following disclosures:
| - | IFRS 2 Share Based Payments in respect of Group-settled share-based payments; and |
| - | Certain disclosures required by IFRS 13 Fair Value Measurement and the disclosures required by IFRS 7 Financial Instrument Disclosures. |
The Company proposes to continue to adopt the Reduced Disclosure Framework of FRS 101 in its next financial statements.
2 Investments
Accounting policy Investments in subsidiaries are stated at cost less provision for impairment. |
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
At 1 January and 31 December |
| 7,092 |
| 7,092 |
Investments represent holdings in subsidiary undertakings. In accordance with Section 409 of the Companies Act 2006, a listing of all entities invested in by the consolidated Group is provided in Note 8.
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
Smith+Nephew Annual Report 2023 | 229 |
Company financial statements continued
Notes to the Company accounts continued
3 Debtors
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
Amounts falling due within one year: |
|
|
|
|
Amounts owed by subsidiary undertakings |
| 3,284 |
| 2,896 |
Prepayments and accrued income |
| 6 |
| 6 |
Current asset derivatives – forward foreign exchange contracts |
| 25 |
| 46 |
Current asset derivatives – forward foreign exchange contracts – subsidiary undertakings |
| – |
| 42 |
Current asset derivatives – currency swaps |
| 2 |
| 1 |
3,317 | 2,991 | |||
Amounts falling due after one year | ||||
Non-current asset derivatives - interest rate swaps | 7 | – | ||
| 3,324 |
| 2,991 |
Allowance losses on amounts owed by subsidiary undertakings are calculated by reviewing 12-month expected credit losses using historic and forward-looking data on credit risk. The loss allowance expense for the year was de minimis (2022: de minimis).
4 Other creditors
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
Amounts falling due within one year: |
|
|
|
|
Amounts owed to subsidiary undertakings |
| 1,158 |
| 837 |
Other creditors |
| 24 |
| 21 |
Current liability derivatives – forward foreign exchange contracts |
| – |
| 42 |
Current liability derivatives – forward foreign exchange contracts – subsidiary undertakings |
| 25 |
| 46 |
Current liability derivatives – currency swaps |
| 3 |
| 1 |
| 1,210 |
| 947 | |
Amounts falling due after one year: | ||||
Non-current liability derivatives – interest rate swaps |
| – |
| 13 |
| – |
| 13 |
5 Cash and borrowings
Accounting policy Financial instruments Currency swaps are used to match foreign currency assets with foreign currency liabilities. They are initially recorded at fair value and then for reporting purposes remeasured to fair value at exchange rates and interest rates at subsequent balance sheet dates. |
Changes in the fair value of derivative financial instruments are recognised in the profit and loss account as they arise.
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
Bank loans, borrowing and overdrafts due within one year or on demand |
| 711 | 109 | |
Borrowings due after one year |
| 2,168 | 2,565 | |
Borrowings |
| 2,879 | 2,674 | |
Cash at bank |
| (82) | (190) | |
(Debit)/credit balance on derivatives – interest rate swaps |
| (7) | 13 | |
Net debt |
| 2,790 | 2,497 |
All currency swaps are stated at fair value. Gross US Dollar equivalents of $548m (2022: $369m) receivable and $549m (2022: $369m) payable have been netted. Currency swaps comprise foreign exchange swaps and were used in 2023 and 2022 to hedge intra-group loans.
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
230 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
6 Contingencies
| 2023 |
| 2022 | |
|
| $ million |
| $ million |
Guarantees in respect of subsidiary undertakings |
| – |
| – |
The Company gives guarantees to banks to support liabilities and cross guarantees to support overdrafts.
The Company operated defined benefit pension plans in 2004 but at the end of 2005 its pension plan obligations were transferred to Smith & Nephew UK Limited. The Company has provided guarantees to the trustees of the pension plans to support future amounts due from participating employers (see Note 18 of the Notes to the Group accounts).
7 Deferred taxation
The Company has gross unused capital losses of $79m (2022: $75m) available for offset against future chargeable gains. No deferred tax asset has been recognised on these unused losses as they are not expected to be realised in the foreseeable future.
8 Group companies
In accordance with Section 409 of the Companies Act 2006, a full list of subsidiaries, associates, joint arrangements, joint ventures and partnerships are listed below as at 31 December 2023, including their country of incorporation. All companies are 100% owned, unless otherwise indicated. The share capital disclosed comprises ordinary shares which are indirectly held by Smith & Nephew plc, unless otherwise stated.
Company name |
| Country of |
| Registered |
| Company name |
| Country of |
| Registered |
|---|---|---|---|---|---|---|---|---|---|---|
UK |
| Smith & Nephew UK Pension Fund Trustee Limited2 | England & Wales | Watford | ||||||
Additive Instruments Limited | England & Wales | Watford | ||||||||
Michelson Diagnostic Limited3 (6.4%) | England & Wales | Nottingham | Smith & Nephew USD Limited1 | England & Wales | Watford | |||||
Neotherix Limited3 (24.9%) | England & Wales | York | Smith & Nephew USD One Limited1 | England & Wales | Watford | |||||
Smith & Nephew (Overseas) Limited1,4 | England & Wales | Watford | T.J.Smith and Nephew, Limited | England & Wales | Hull | |||||
Smith & Nephew Beta Limited2 | England & Wales | Watford | The Albion Soap Company Limited2 | England & Wales | Watford | |||||
Smith & Nephew China Holdings | England & Wales | Watford | TP Limited1 | Scotland | Edinburgh | |||||
UK Limited1 | Rest of Europe | |||||||||
Smith & Nephew Employees Trustees Limited2 | England & Wales | Watford | Smith & Nephew GmbH | Austria | Vienna | |||||
Smith & Nephew ESN Limited2 | England & Wales | Watford | Smith & Nephew S.A.-N.V | Belgium | Zaventem | |||||
Smith & Nephew Extruded Films Limited2 | England & Wales | Hull | Smith & Nephew A/S | Denmark | Kobenhavn | |||||
Smith & Nephew Finance2 | England & Wales | Watford | Smith & Nephew Oy | Finland | Helsinki | |||||
Smith & Nephew Finance Oratec2 | England & Wales | Watford | Smith & Nephew France SAS1 | France | Neuilly-sur-Seine | |||||
Smith & Nephew Healthcare Limited2 | England & Wales | Hull | Smith & Nephew S.A.S. | France | Neuilly-sur- Seine | |||||
Smith & Nephew Investment Holdings Limited1 | England & Wales | Watford | Smith & Nephew Business Services GmbH & Co. KG1 | Germany | Hamburg | |||||
Smith & Nephew Lilia Limited2 | England & Wales | Watford | Smith & Nephew Business Services Verwaltungs GmbH | Germany | Hamburg | |||||
Smith & Nephew Medical Fabrics Limited2 | England & Wales | Watford | Smith & Nephew Deutschland (Holding) GmbH1 | Germany | Hamburg | |||||
Smith & Nephew Medical Limited | England & Wales | Hull | Smith & Nephew GmbH | Germany | Hamburg | |||||
Smith & Nephew Nominee Company Limited2 | England & Wales | Watford | Smith & Nephew Orthopaedics GmbH | Germany | Tuttlingen | |||||
Smith & Nephew Nominee Services Limited2 | England & Wales | Watford | Smith & Nephew Robotics GmbH | Germany | Munich | |||||
Smith & Nephew Orthopaedics Limited | England & Wales | Watford | Smith & Nephew (Ireland) Trading Limited | Ireland | Dublin | |||||
Smith & Nephew Pharmaceuticals Limited2 | England & Wales | Hull | Smith & Nephew S.r.l. | Italy | Milan | |||||
Smith & Nephew Raisegrade Limited1,2 | England & Wales | Watford | Smith & Nephew International S.A.1 | Luxembourg | Luxembourg | |||||
Smith & Nephew Rareletter Limited2 | England & Wales | Watford | Smith & Nephew (Europe) B.V.1 | Netherlands | Amsterdam, 2132NP | |||||
Smith & Nephew Trading Group Limited1 | England & Wales | Watford | Smith & Nephew B.V. | Netherlands | Amsterdam, 2132NP | |||||
Smith & Nephew UK Executive Pension Scheme Trustee Limited2 | England & Wales | Watford | Smith & Nephew Nederland CV | Netherlands | Amsterdam, 2132NP | |||||
Smith & Nephew UK Limited1,4 | England & Wales | Watford |
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
Smith+Nephew Annual Report 2023 | 231 |
Company financial statements continued
Notes to the Company accounts continued
8 Group companies continued
Company name |
| Country of |
| Registered |
| Company name |
| Country of |
| Registered |
Smith & Nephew Operations B.V. | Netherlands | Amsterdam, 2132NP | Africa, Asia, Australasia and Other Americas | |||||||
Serda B.V.3 (48.32%) | Netherlands | Amsterdam, 1105BP | Smith & Nephew Argentina S.R.L.2 | Argentina | Buenos Aires | |||||
Smith & Nephew AS | Norway | Oslo | Smith & Nephew Pty Limited | Australia | Macquarie Park | |||||
Smith & Nephew sp. z.o.o. | Poland | Warsaw | Smith & Nephew Surgical Holdings Pty Limited1,2 | Australia | Macquarie Park | |||||
Smith & Nephew Lda | Portugal | Forte da Casa | Smith & Nephew Surgical Pty Limited2 | Australia | Macquarie Park | |||||
S&N ORION PRIME, S.A. | Portugal | Coimbra | Smith & Nephew Comercio de Produtos Medicos LTDA | Brazil | São Paulo | |||||
DC LLC | Russian Federation | Puschino | Smith & Nephew Comercio de Produtos Medicos LTDA, Diadema Branch5 | Brazil | Diadema | |||||
Smith & Nephew LLC | Russian Federation | Moscow | Smith & Nephew Comercio de Produtos Medicos LTDA, Rio de Janeiro Branch5 | Brazil | Rio de Janeiro | |||||
Smith & Nephew S.A.U | Spain | Barcelona | Smith & Nephew Comercio de Produtos Medicos LTDA, São José dos Campos Branch5 | Brazil | São José | |||||
Smith & Nephew Aktiebolag | Sweden | Molndal | Smith & Nephew Inc.1 | Canada | Ontario | |||||
Lumina Adhesives AB3 (3.04%) | Sweden | Gothenburg | Smith & Nephew Finance Holdings Limited4 | Cayman Islands | George Town 1104 | |||||
Atracsys Sàrl | Switzerland | Puidoux | TEAMfund, LP3 (6.765%) | Cayman Islands | George Town 9008 | |||||
Plus Orthopedics Holding AG1 | Switzerland | Zug | Smith & Nephew Chile SpA2 | Chile | Chile | |||||
Smith & Nephew Manufacturing AG | Switzerland | Aarau | Plus Orthopedics (Beijing) Co. Limited2 | China | Shunyi District, Beijing | |||||
Smith & Nephew Orthopaedics AG1 | Switzerland | Zug | Smith & Nephew Medical (Shanghai) Limited | China | Shanghai Ao Na Rd | |||||
Smith & Nephew Schweiz AG | Switzerland | Zug | Smith & Nephew Medical (Shanghai) Limited Beijing Branch5 | China | Dong Cheng | |||||
Smith & Nephew AG | Switzerland | Zug | Smith & Nephew Medical (Shanghai) Limited Chengdu Branch5 | China | Wu Hou | |||||
Smith & Nephew Orthopaedics AG Aarau Branch5 | Switzerland | Aarau | Smith & Nephew Medical (Shanghai) Limited Guangzhou Branch5 | China | Yue Xiu | |||||
Smith & Nephew Medical (Shanghai) Limited Shanghai Branch5 | China | Jing’an | ||||||||
US | Smith & Nephew Medical (Shanghai) Limited Shanghai Second Branch5 | China | Shanghai Xin Jin Qiao Rd | |||||||
Arthrocare Corporation | United States | Wilmington | Smith & Nephew Medical (Suzhou) Limited | China | Suzhou City | |||||
Ascension Orthopedics, Inc. | United States | Wilmington Centreville | Smith & Nephew Orthopaedics (Beijing) Co., Ltd | China | Kechuang Dongliujie | |||||
Austin Miller Trauma LLC | United States | Wilmington | S&N Holdings SAS1 | Colombia | Bogota | |||||
Bioventus Inc.3,6 (27.96%) | United States | Wilmington | Smith & Nephew Colombia S.A.S | Colombia | Bogota | |||||
Orthopaedics Biosystem Ltd, Inc | United States | Phoenix | ArthroCare Costa Rica Srl | Costa Rica | Alajuela | |||||
Bioventus LLC3,7 (20.05%) | United States | Wilmington | Smith & Nephew Curaçao N.V.2 | Curaçao | Willemstad | |||||
Blue Belt Technologies, Inc. | United States | Philadelphia | Smith & Nephew Beijing Holdings Limited1 | Hong Kong | Hong Kong | |||||
Ceterix Orthopaedics, Inc. | United States | Wilmington | Smith & Nephew Limited | Hong Kong | Hong Kong | |||||
Engage Uni LLC | United States | Wilmington 19808 | Smith & Nephew Suzhou Holdings Limited1 | Hong Kong | Hong Kong | |||||
Integrated Shoulder Collaboration, Inc. | United States | Wilmington 19808 | Smith & Nephew GBS Private Limited | India | Pune | |||||
Leaf Healthcare Inc. | United States | Wilmington | Smith & Nephew Healthcare Private Limited | India | Mumbai | |||||
Miach Orthopaedics, Inc³ (8.76%) | United States | Dover GD | Smith & Nephew KK | Japan | Tokyo | |||||
Osiris Therapeutics, Inc. | United States | Columbia | Smith & Nephew Chusik Hoesia | Korea, Republic of | Seoul | |||||
Rotation Medical, Inc. | United States | Wilmington 19808 | ||||||||
Sinopsys Surgical, Inc.3 (1.44%) | United States | Wilmington | ||||||||
Smith & Nephew Consolidated, Inc.1 | United States | Wilmington | ||||||||
Smith & Nephew, Inc.1 | United States | Wilmington | ||||||||
IntraFuse LLC Investment3 (42.16%) | United States | Utah | ||||||||
Trice Medical Inc.3 (0.5%) | United States | Wilmington 19808 | ||||||||
Tusker Medical, Inc. | United States | Wilmington 19808 | ||||||||
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
232 | Smith+Nephew Annual Report 2023 |
STRATEGIC REPORT |
| |
GOVERNANCE | ||
ACCOUNTS | ||
OTHER INFORMATION |
Company name |
| Country of |
| Registered |
| Registered Office addresses | ||||
|---|---|---|---|---|---|---|---|---|---|---|
Neuilly-sur-Seine | 40-52, Boulevard du Parc, 92200 Neuilly-sur-Seine, France | |||||||||
Smith & Nephew Services Sdn. Bhd | Malaysia | Kuala Lumpur | Hamburg | Friesenweg 30, 22763, Hamburg, Germany | ||||||
Smith & Nephew Operations Sdn. Bhd | Malaysia | Kuala Lumpur | Munich | Rosenheimer Straße 116, Munich, 81669, Germany | ||||||
Smith & Nephew Services Sdn. Bhd | Malaysia | Kuala Lumpur | Tuttlingen | Alemannenstrasse 14, 78532, Tuttlingen, Germany | ||||||
Smith & Nephew S.A. de C.V. | Mexico | Mexico City | Dublin | 9 Clare Street, Dublin 2, D02 HH30, Ireland | ||||||
Smith & Nephew Limited1 | New Zealand | Auckland | Milan | Sesto San Giovanni (MI) Viale T. Edison 110 CAP 20099 Italy | ||||||
Smith & Nephew Superannuation Scheme Limited | New Zealand | Auckland | Luxembourg | 1A, rue Jean Piret, L-2350, Luxembourg, Luxembourg | ||||||
Smith & Nephew (Overseas) Limited Philippines Branch2,5 | Philippines | Manila | Amsterdam 2132NP | Bloemlaan 2, 2132NP, Hoofddorp, The Netherlands | ||||||
Smith & Nephew, Inc. | Puerto Rico | San Juan | Amsterdam 1105BP | Paasheuvelweg 25, 1105BP, Amsterdam, The Netherlands | ||||||
Smith & Nephew USD Limited Office for Technical & Scientific Services | Saudi Arabia | Riyadh | Oslo | Snaroyveien 36, FORNEBU, 1364, Norway | ||||||
Smith & Nephew Asia Pacific Pte. Limited1 | Singapore | Singapore | Warsaw | Ul Osmanska 12, 02-823, Warsaw, Poland | ||||||
Smith & Nephew Pte Limited | Singapore | Singapore | Forte da Casa | Rua do Parque Tejo, numbers 7, 7-A and 7-B 2625-437 Forte da Casa, Povoa de Santa Iria and Forte da Casa, Vila Franca de Xira, Portugal | ||||||
Smith & Nephew (Pty) Limited1 | South Africa | Westville | Coimbra | Rua Pedro Nunes, Instituto Pedro Nunes, Edificio IPN-D, 3030-199, Coimbra, Portugal | ||||||
Smith & Nephew Pharmaceuticals (Proprietary) Limited2 | South Africa | Westville | Moscow | 2nd Syromyatnichesky Lane, Moscow, 105120, Russian Federation | ||||||
Smith & Nephew (Overseas) Limited Taiwan Branch5 | Taiwan | Taipei | Puschino | 8/1 Stroiteley Street, 142290, City of Puschino, Moscow Region, Russian Federation | ||||||
Smith & Nephew Limited | Thailand | Huai Khwang District, Bangkok | Barcelona | Edificio Conata I, c/Fructuos Gelabert 2 y 4, San Joan Despi – 08970, Barcelona, Spain | ||||||
Smith ve Nephew Medikal Cihazlar Ticaret Limited Sirketi | Turkey | Istanbul | Molndal | Krokslatts fabriker 39 431 37 Molndal, Sverige, Sweden | ||||||
Smith & Nephew FZE | United Arab Emirates | Jebel Ali, | Gothenburg | Varbergsgatan 2A/412 65 Göteborg, Sweden | ||||||
Smith & Nephew FZE (DHCC Branch)5 | United Arab Emirates | HealthCare City, Dubai | Puidoux | Route du Verney 20, 1070, Puidoux, Switzerland | ||||||
The Representative Office Of Smith & Nephew Asia Pacific Pte. Limited | Vietnam | Ho Chi Minh City | Zug | Theilerstrasse 1A, 6300, Zug, Switzerland | ||||||
Aarau | Schachenallee 29, 5000, Aarau, Switzerland | |||||||||
1 Holding company. | ||||||||||
2 Dormant company. | ||||||||||
3 Not 100% owned by Smith & Nephew Group. | US | |||||||||
4 Directly owned by Smith & Nephew plc. | Wilmington | CT Corporation, 1209 Orange Street, Wilmington DE 19801, USA | ||||||||
5 Branch of a company in Smith & Nephew Group. | Wilmington Centreville | Corporation Services Company, Suite 400, 2711, Centreville Road, Wilmington DE, USA | ||||||||
6 Represents 27.96% voting rights and 7.91% economic interest. | ||||||||||
7 Represents 20.05% economic interest. | Philadelphia | CT Corporation 1515 Market Street, Philadelphia, PA 19102, USA | ||||||||
Wilmington 19808 | 251 Little Falls Drive, Wilmington DE 19808, USA | |||||||||
Dover GD | 160 Greentree Drive, Suite 101, Dover, DE, 19904, USA | |||||||||
Registered Office addresses | Pennsylvania | 63 Burke Road, Cranberry Township, Butler County PA 16066, USA | ||||||||
UK | Columbia | 7015 Albert Einstein Dr., Columbia, Howard County MD 21046 USA | ||||||||
Watford | Building 5, Croxley Park, Hatters Lane, Watford, Hertfordshire, WD18 8YE | Utah | P.O. Box 6008, North Logan, UT 84341, USA | |||||||
Nottingham | 80 Mount Street, Cumberland Court, Nottingham, NG1 6HH | Phoenix | CT Corporation System, 3800 North Central Avenue, Suite 460, Phoenix AZ 85012, United State | |||||||
York | 25, Carr Lane, York, YO26 5HT | Africa, Asia, Australasia and Other Americas | ||||||||
Hull | 101 Hessle Road, Hull, HU3 2BN | Buenos Aires | Maipu 1300, 13th Floor, Buenos Aires, Argentina | |||||||
Edinburgh | 4th Floor, 115 George Street, Edinburgh, EH2 4JN | Macquarie Park | Suite 1.01, Level 1, Building B, Pinnacle Office Park, 4 Drake Avenue, Macquarie Park, NSW 2113, Australia | |||||||
Rest of Europe | São Paulo | Av. das Nações Unidas, 14171- 23º andar – Torre C- Crystal, Vila Gertrudes, São Paulo, CEP 043794-000, Brazil | ||||||||
Vienna | Concorde Business Park, C3, 2320, Schwechat, Austria | |||||||||
Zaventem | Ikaroslaan 45, 1930 Zaventem, Belgium | |||||||||
Kobenhavn | Kay Fiskers Plads 9,1. 2300. Kobenhavn S, Denmark | |||||||||
Helsinki | Lentäjäntie 1, 01530 Vantaa, Finland | |||||||||
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
Smith+Nephew Annual Report 2023 | 233 |
Company financial statements continued
Notes to the Company accounts continued
8 Group companies continued
Registered Office addresses | Registered Office addresses | |||||
|---|---|---|---|---|---|---|
Diadema | Avenida Fagundes de Oliveira, 538, Piraporinha, Mbigucci Diadema Business Park, Module B21 and B22, City of Diadema São Paulo CEP 09950-300 Brazil | Alajuela | Building B32, 50 meters South of Revisión Téchnica Vehicular, Province de Alajuela, Canton Alajuela, Coyol Free Zone, District San José, Costa Rica | |||
Rio de Janeiro | Rua Francisco de Sousa e Melo, 1590, Galpao 3 Armazem 103 parte, Bairro Cordovil, Rio de Janeiro, CEP 21010-900, Brazil | Willemstad | Pietermaai 15, PO Box 4905, Curaçao | |||
São José | Rua Dionizio Chinelato Nr. 100 – Complemento Galpão 01 – Sala o1 CEP 12.238-578 Bairro – Eldorado, Municipio São José dos Campos SP | Hong Kong | Unit 813 – 816, 8/F, Delta House, 3 On Yiu Street, Shatin, New Territories, Hong Kong | |||
Ontario | 2280 Argentia Road, , Mississauga ON L5N 6H8, Canada | Pune | Podium Floor Tower 4, World Trade Center S No1 Kharadi, Pune, Maharashtra-MH, 411014, India | |||
Chile | Alonso de Cordova 5320 OF 1401 PS14, Las Condes, Rol 751-76, Santiago, Chile | Mumbai | 501-B – 509-B Dynasty Business Park, Andheri Kurla Road, Andheri East, Mumbai-59, Maharashtra, India | |||
Georgetown 1104 | c/o Maples Corporate Services Limited, P.O. Box 309, Ugland house, Grand Cayman, KY1-1104, Cayman Islands | Tokyo | Shiba Park blg A-3F , 2-4-1, Shiba -Koen , Minato -Ku, Tokyo, 105-0011, Japan | |||
Georgetown 9008 | Walkers Corporate Limited, Cayman Corporate Centre, 27 Hospital Road, George Town, Grand Cayman, KY1 -9008, Cayman Islands | Seoul | 13th Floor, ASEM Tower, Gangnam-gu 13th Floor, ASEM Tower, 159-1 Samsung-dong, Seoul, Korea | |||
Chao Yang District, Beijing | Room 17-021, Internal B17 floor, B3-24th floor, No 3 Xin Yuan South Rd, Chao Yang District, Beijing, China | Kuala Lumpur | Level 25, Menara Hong Leong, NO. 6 Jalan Damanlela Bukit Damansara Kuala Lumpur W.P. 50490 Kuala Lumpur, Malaysia | |||
Shunyi District, Beijing | 22 Linhe Avenue, Linhe Economic Development Zone, Shunyi District, Beijing, 101300, China | Mexico City | Av. Insurgentes Sur, numero 1602, Piso No.7, Oficina 702, Colonia Credito, Constructor, Delegacion Benito Juarez, C.P. 03940, Mexico | |||
Shanghai Ao Na Rd | Part B, 4th Floor, Tong Yong Building, No 188 Ao Na Rd, Shanghai Free Trade Test Zone, Shanghai, China | Auckland | 621 Rosebank Road, Avondale, Auckland, 1026, New Zealand | |||
Dong Cheng District | Unit B1, 2/F, Tower A, East Gate Plaza No.9, Dongshong Street Dong Cheng District, Beijing, China | Manila | 6/F Alfaro St, Salcedo Village, Makati City, Metro Manila, Philippines | |||
Wu Hou District | No 5. 15th Floor, Unit 1, Building, 1 Li Bao Building, No 62 North Ke Hua Rd, Wu Hou District, Chengdu, China | San Juan | Edificio Cesar Castillo, Calle Angel Buonomo #361, Hato Rey, 00917, Puerto Rico | |||
Yue Xiu District | Room 2503, No 33, 6th Jian She Rd, Yue Xiu District, Guang Zhou, China | Singapore | 29 Media Circle, #06-05, Alice@Mediapolis, Singapore, 138565, Singapore | |||
Jing'an District | Unit 09, Nominal Level 12 (Actual Level 11), Central Section of Bohua Square Office Tower, No. 669 Xinzha Road, Jing’an District, Shanghai, China | Westville | 30 The Boulevard, Westway Office Park, Westville, 3629, South Africa | |||
Shanghai Xin Jin Qiao Rd | Room 102, Floor 1, Building 3 (B1), No. 1599, Xin Jin Qiao Road China (Shanghai) Pilot Free Trade Zone, Shanghai, China | Taipei | 9F-2, No. 50, Sec. 1, Xinsheng South Road, Zhongzheng District Taipei City 10059, Taiwan | |||
Suzhou City | 12, Wuxiang Road, West Area of Comprehensive Bonded Zone, Suzhou Industrial Park, Suzhou City, SIP, Jiangsu Province, China | Huai Khwang District, Bangkok | 16th Floor Building A, 9th Tower Grand Rama 9, 33/4 Rama 9 Road, Huai Khwang District, Bangkok, 10310, Thailand | |||
Riyadh | Business Gate Exit 8 Airport Road, Riyadh, Saudi Arabia | Istanbul | Mahmutbey Mahallesi, 2538. Sokak, Kısık Plaza Apt. No:6/Z1, Istanbul, Bağcılar, Turkey | |||
Kechuang Dongliujie | No. 98 Kechuang Dongliujie, Beijing Economic and Technical Development Area, Beijing, China | Jebel Ali, Dubai | PO Box 16993 LB02016, Jebel Ali, Dubai, United Arab Emirates | |||
Bogota | Calle 100 No. 7 – 33 to 1 P3, Bogota D.C., Colombia | HealthCare City, Dubai | Floor 1, Building 52, Dubai Healthcare City, Dubai, United Arab Emirates | |||
Ho Chi Minh City | Room 02, 18th floor, TNR building, 180-192, Nguyen Cong Tru street, Nguyen Thai Binh Ward, District 1, Ho Chi Minh City, Vietnam | |||||
9 Subsidiary undertakings exempt from audit
The following UK subsidiaries will take advantage of the audit exemption set out within Section 479A of the Companies Act 2006 for the year ended 31 December 2023:
| – | Additive Instruments Limited (Registration number: 12323687) |
| – | Smith & Nephew China Holdings UK Limited (Registration number: 9152387) |
| – | Smith & Nephew Investment Holdings Limited (Registration number: 384546) |
| – | Smith & Nephew Trading Group Limited (Registration number: 681256) |
| – | Smith & Nephew USD One Limited (Registration number: 10428326) |
| – | TP Limited (Registration number: SC005366) |
Excluding Note 8 ‘Group Companies’, the Parent Company financial statements of Smith & Nephew plc on pages
229–234 do not form part of the Smith & Nephew plc Annual Report on Form 20-F as filed with the SEC.
234 | Smith+Nephew Annual Report 2023 |
| Properties The table below summarises the main properties which the Group uses and their approximate areas. Approximate area (square feet 000’s) Group head office and surgical training facility in Watford, UK 60 Manufacturing and office facilities in Memphis, Tennessee, US 923 Wound management manufacturing, research and office facility in Hull, UK 473 Surgical training and office facilities in Memphis, Tennessee, US 292 Manufacturing facility in Suzhou, China 288 Manufacturing facility in Penang, Malaysia 277 Manufacturing facility in Alajuela, Costa Rica 270 Manufacturing facility in Oklahoma City, Oklahoma, US 155 Manufacturing, Office facilities and laboratory space in Fort Worth, Texas, US 139 Research & development and office facility in Austin, Texas, US 125 Manufacturing facility in Aarau, Switzerland 116 Logistic facility in Lawrenceville, US 115 Office facilities in Andover, Massachusetts, US 112 Manufacturing facility in Beijing, China 109 Manufacturing facility in Mansfield, Massachusetts, US 98 Business services centre in Pune, India 74 Research & development facility in Pittsburgh, Pennsylvania, US 65 Manufacturing, Office facility in Tuttlingen, Germany 64 Manufacturing facility in Columbia, Maryland, US 61 The Group Global Operations strategy includes ongoing assessment of the optimal facility footprint. The Orthopaedics manufacturing facilities in Memphis are largely freehold, a portion of Tuttlingen and the Advanced Wound Management facilities in Hull are freehold while other principal locations are leasehold. The Group has freehold and leasehold interests in real estate in other countries throughout the world, but no other is individually significant to the Group. Where required, the appropriate governmental authorities have approved the facilities. Business overview and Group history Smith+Nephew’s operations have been organised into three global business units (previously franchises) (Orthopaedics, Sports Medicine & ENT and Advanced Wound Management) within the medical technology industry. The Group has a history dating back more than 160 years to the family enterprise of Thomas James Smith who opened a small pharmacy in Hull, UK, in 1856. Following his death in 1896, his nephew Horatio Nelson Smith took over the management of the business. By the late 1990s, Smith+Nephew had expanded into being a diverse healthcare company with operations across the globe, producing various medical devices, personal care products and traditional and advanced wound care treatments. In 1998, Smith+Nephew announced a major restructuring to focus management attention and investment on three global business units – Advanced Wound Management, Endoscopy and Orthopaedics – which offered high growth and margin opportunities. In 2011, the Endoscopy and Orthopaedics businesses were brought together to create an Advanced Surgical Devices division. In 2015, the Advanced Wound Management and Advanced Surgical Devices divisions were brought together to form a global business across nine product franchises. Smith+Nephew was incorporated and listed on the London Stock Exchange in 1937 and in 1999 the Group was also listed on the New York Stock Exchange. In 2001, Smith+Nephew became a constituent member of the FTSE 100 index in the UK. This means that Smith+Nephew is included in the top 100 companies traded on the London Stock Exchange measured in terms of market capitalisation. Today, Smith+Nephew is a public limited company incorporated and headquartered in the UK and carries out business around the world. Related party transactions Except for transactions with associates (see Note 22.2 of Notes to the Group accounts), no other related party had material transactions or loans with Smith+Nephew over the last three financial years. Group information Other information STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 235 |
| Other information continued Cybersecurity risk management and governance The Group has a dedicated information and cybersecurity function of 49 employees, led by an ISC2 Certified Information Systems Security Professional (CISSP) certified Chief Information Security Officer (CISO) with over 25 years of experience in this field. The CISO actively participates in Audit Committee and Executive Committee meetings. They are also responsible for offering updates and oversight on the information and cybersecurity strategy and reporting material cybersecurity risks and mitigation strategies to the Board and its subcommittees. Additionally, the CISO chairs a subcommittee comprised of business stakeholders, including, but not limited to legal, compliance, finance, internal audit, risk management and human resources. The committee has overall approval and sign-off of security and privacy policies, which allows for focused discussions and strategy alignment for both security and privacy. The committee provides necessary updates to the Board where required. The Group’s cybersecurity risk management processes, which include assessment, documentation and treatment, have been integrated into our overall enterprise risk management system. The cybersecurity function has well-defined processes for handling information security and cybersecurity incidents incorporating analysis and prioritisation mechanisms aligned with enterprise risk management. During the handling of an incident, the information security team will continuously monitor and assess the impact to the organisation. Thresholds have been set, which once triggered will bring information security, legal and compliance together as a subcommittee. The subcommittee will own the management assessment of materiality, invocation of crisis management, Board notification and the drafting of any regulatory notifications. In the event of a major cybersecurity incident, including those with a material impact on the Group, the CISO maintains the engagement with the executive and crisis management teams and the Board if required. The information and cybersecurity function conducts an annual mandatory information security awareness training programme for all users, covering topics such as physical security, email security, data privacy, ransomware guidance, phishing and general online safety. Regular security campaigns are also implemented to educate and raise awareness among users about emerging cyber threats. Our cybersecurity team adopts a hybrid model to ensure coverage and expertise across all areas; this includes utilising a small set of managed security service providers where required, including but not limited to security assessments, 24x7 monitoring and service enhancements. The Group uses a wide variety of information systems, programs, and technology to manage its business. The Group also develops and sells digitally enabled products some of which connect to networks and/or the internet. Recognising cybersecurity as a multifaceted discipline covering people, process, and technology, the Group emphasises a continuous improvement approach, measured via annual security assessments and audits using a dedicated 24x7 security platform scoring service and our own internal audit function. A layered security strategy is implemented to prevent, detect, and respond to threats in an effort to minimise the risk and disruption of intrusions. Robust governance practices are in place across the information security and cyber function, including an assessment of suppliers and vendors security and compliance posture prior to the onboarding and activation of any service. Active monitoring of third-party providers is implemented once live on a 24x7 basis, by utilising a dedicated service via a market-leading third party, reducing the risk of supply chain attacks. While the Group strives for effective governance and measures, there is no absolute assurance against future interruptions that could potentially disrupt business operations and materially adversely affect the organisation’s performance. Throughout the year 2023, there were no cybersecurity incidents identified which materially affected or are reasonably likely to materially affect the Group’s business strategy, results of operations or financial condition and no incidents have been reported to regulatory authorities during this period. However, despite our efforts, we cannot eliminate all risks from cybersecurity threats, or provide assurances that we have not experienced an undetected cybersecurity incident. For more information about these risks, please see page 240 ‘Risk Factors – Cybersecurity’ in this Annual Report on Form 20-F. 236 Smith+Nephew Annual Report 2023 |
| Risk factors There are known and unknown risks and uncertainties relating to Smith+Nephew’s business. The factors listed on pages 237–243 could cause the Group’s business, financial position and results of operations to differ materially and adversely from expected and historical levels. In addition, other factors not listed here that Smith+Nephew cannot presently identify or does not believe to be equally significant could also materially adversely affect Smith+Nephew’s business, financial position or results of operations. Global supply chain The Group’s manufacturing production is concentrated at several main facilities including Memphis, Mansfield, Columbia and Oklahoma City in the US, Hull in the UK, Aarau in Switzerland, Suzhou in China, Penang in Malaysia and Alajuela in Costa Rica. If major physical disruption or unavailability of critical system infrastructure and applications took place at any of these sites, it could adversely affect the results of operations. Disruptions to our supply chain as a result of geopolitical events such as the war in Ukraine and conflict in Gaza on the access to and cost of supply channels, freight, raw materials and components have had and may continue to have an adverse effect on the Group’s results of operations. Physical loss and consequential loss insurance carried to cover major physical disruption to these sites is subject to limits and deductibles, generally does not cover pandemic or war related disruptions, and may not be sufficient to cover catastrophic loss. Management, forecasting and production planning for inventory is complex and failures in operational execution could lead to excess inventory or individual product shortages. Further, as the Group continues to transform its supply chain network and operationalise its warehouse and distribution functions, there is a risk that, if the transition, transformation and ongoing operations do not go as planned, the supply of products may be disrupted and impact performance. The Group is reliant on certain key suppliers of raw materials, components, finished products and packaging materials or in some cases on a single supplier. Disruptions in the supply chain and operations of the Group’s suppliers, increased freight costs and cycle times due to disruptions to shipping routes (for example most recently through the Red Sea and Suez Canal) and increased sanctions, import and export controls flowing from geopolitical events such as the war in Ukraine and conflict in Gaza could result in a further increase in the Group’s costs of production and distribution. Suppliers must provide materials and perform services in compliance with legal and regulatory requirements and in accordance with the Group’s standard quality requirements. A supplier’s failure to comply with legal or regulatory requirements or otherwise meet expected quality standards could create reputational harm and liability for the Group and adversely affect Group sales. The Group may be forced to pay higher prices to obtain raw materials and/or to sterilise its products and may not be able to pass on those costs to its customers in the form of increased prices for its finished products. This risk is particularly relevant in the medical devices sector due to complex supply chains, increasing regulation and enforcement and the potential for healthcare budgets globally to be reduced. As certain raw materials may become unavailable and/or capacity for sterilisation services may become increasingly constrained beyond current capacity levels, in particular due to supply challenges and increased regulation and enforcement, there can be no assurance that the Group will be able to obtain suitable and cost-effective substitutes. Interruption of supply caused by these or other factors has had and may continue to have a negative impact on Smith+Nephew’s revenue and operating profit. The Group will, from time to time, including as part of the Operations and Commercial Excellence programme, outsource or insource the manufacture of components and finished products to or from third parties and will periodically relocate the manufacture of product and/or processes between existing and/or new facilities. Failure to effectively execute on these programmes may negatively impact the Group’s performance, revenue and profit margin. Natural disasters, weather and climate change-related events and unavailability of critical system infrastructure and applications can also lead to manufacturing and supply delays, product shortages, excess inventory, unanticipated costs, lost revenues and damage to reputation. In addition, the pace of development and expansion of environmental and sustainability regulations globally, coupled with more active enforcement of regulations, can impact the Group’s ability to manufacture, sterilise and supply product. In addition, the Group’s physical assets and supply chains are also vulnerable to weather and climate change (e.g. sea level rise, increased frequency and severity of extreme weather events, and stress on water resources). Where such events impact a manufacturing facility, the Group may be unable to manufacture products. In this case, if there are insufficient manufacturing alternatives for the relevant products, the Group may not be able to supply those products to its customers. The Group is exposed to increasing salary and wage costs for its employees and contractors due to global inflation and the cost of living crisis. This, combined with labour attrition and longer cycle times to backfill roles, may adversely impact the Group’s performance. Requirements of global regulatory agencies have become more stringent in recent years and the Group expects this to continue. The Group’s Quality and Regulatory Affairs team has implemented its programme to transition to the EU Medical Devices Regulation (MDR) regulatory regime, which includes requirements for the manufacture, supply and sale of all CE marked products sold in Europe (i.e. those products that conform with health, safety and environmental protection standards within the European Economic Area). MDR requires the re-registration of all medical devices, regardless of where they are manufactured. There continue to be significant capacity constraints in implementing MDR given the small number of notified bodies certified under MDR. This could continue to cause delays for medical device approvals for the industry more broadly and may result in delays for patients. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 237 |
| Other information continued Risk factors continued The European Commission has taken some important steps to aid implementation, including delaying the EU database (EUDAMED) and providing a longer implementation timeline for certain Class lR devices (ie. reusable surgical instruments). More recently the EU Commission has implemented transitional requirements to support products to continue to be made available. This supports both the Group as well as supporting capacity constraints within the Notified Bodies. The Group operates with a global remit and the speed of technological change in an already complex manufacturing process leads to greater potential for disruption. Additional risks to supply include inadequate sales and operational planning and inadequate supply chain or manufacturing capacity to support customer demand and growth. Widespread outbreaks of infectious diseases, and restrictions and lockdowns arising therefrom, could create uncertainty and challenges for the Group. These include, but are not limited to, declines in and cancellations of elective procedures at medical facilities, reduction in staffing and other support within institutions, disruptions at manufacturing facilities and disruptions in supply and other commercial activities due to travel restrictions and government restrictions on exports. Strategy and commercial execution Strong commercial execution requires effective cross-functional alignment, accountability, engagement and communication across the Group within embedded governance structures and frameworks. Effective engagement with customers, suppliers and other stakeholders is also a crucial factor to ensure strong commercial execution. Failure to effectively implement the Group’s programmes within appropriate governance frameworks or failure to understand or take into account customer, supplier and stakeholder needs and requirements could adversely affect the Group’s performance. Additional commercial execution risks include medical facilities stopping or severely restricting sales representative access due to increased post-pandemic pressure on these facilities and their staff. The Group’s business requires continuous improvement and depends on its ability to execute business change programmes such as the 12-Point Plan at pace, whilst continuing to operate business as usual. The pace and scope of the Group’s business change initiatives may increase execution risk for the change programmes as well as for the Group’s business-as-usual activities. The Group’s business depends on its ability to plan for and be resilient in the face of events that threaten one or more of its key locations. Highly competitive markets The Group competes across a diverse range of geographic and product markets. Each market in which the Group operates contains a broad range of competitors, including specialised and international corporations. Significant product innovations, technical advances or the intensification of price competition by competitors could adversely affect the Group’s operating results. Some competitors may have greater financial, marketing and other resources than Smith+Nephew. These competitors may be able to initiate technological advances in the field, deliver products on more attractive terms, more aggressively market their products or invest larger amounts of capital and research and development (R&D) into their businesses. Further consolidation of competitors could adversely affect the Group’s ability to compete with larger companies due to insufficient financial resources. If any of the Group’s businesses were to lose market share or achieve lower than expected revenue growth, there could be a disproportionate adverse impact on the Group’s share price and its strategic options. Competition exists among healthcare providers to gain patients on the basis of quality, service and price. There has been some consolidation in the Group’s customer base and this trend is expected to continue. Some customers have joined group purchasing organisations or introduced other cost containment measures that could lead to downward pressure on prices or limit the number of suppliers in certain business areas, which could adversely affect Smith+Nephew‘s results of operations and hinder its growth potential. Relationships with healthcare professionals The Group seeks to maintain effective and ethical working relationships with physicians and medical personnel who assist in the development of new products or improvements to its existing product range and in product training and medical education. If the Group is unable to maintain these relationships, this may affect its ability to innovate, meet patients’ needs and ensure its products are used safely and effectively. Customer and other stakeholder sustainability expectations The Group’s customers continue to develop more stringent sustainability requirements that they request or expect the Group to implement or adhere to in addition to the laws and regulations applicable to the Group. A failure to meet customers’ requirements or expectations may adversely impact the Group’s financial performance. Increased investment related to customer requests in this area may impact profit margin. Acquisitions Challenges in integration of new acquisitions may arise following completion of the deal, including external macro factors and geopolitical events. This may lead to the Group not achieving the planned synergies and results from the acquisition. Pricing and reimbursement Dependence on government and other funding In most global markets, expenditure on medical devices is ultimately controlled to a large extent by governments and healthcare systems. Funds may be made available or withdrawn from healthcare budgets depending on government policy. The Group is therefore dependent on future governments providing increased funds commensurate with the increased demand arising from demographic trends. Pricing of many of the Group’s products is governed in most markets by governmental reimbursement authorities. Increasing numbers of initiatives sponsored by government agencies, legislative bodies and the private sector to relieve the 238 Smith+Nephew Annual Report 2023 |
| pressure on healthcare budgets and limit the growth of healthcare costs, including price regulation on products or entire procedures, value and volume-based procurement initiatives, excise taxes and competitive pricing are being implemented at pace in markets where the Group has operations. The Group is exposed to government policies favouring locally sourced or manufactured products in many markets in which it operates, impacting its ability to compete effectively and gain share which can negatively impact Group revenues and profit margins. The Group is increasingly exposed to changes in reimbursement policy, tax policy and pricing, including as a result of financial pressure on governments and hospitals caused by recession and inflation in its markets, which may have an adverse impact on revenue and operating profit. Reimbursement codes are increasingly more widely interpreted to provide for remote delivery of healthcare services indicating a continued trend to shift site of care and manage related healthcare budgets away from traditional inpatient treatment. There may also be an increased risk of adverse changes to government funding policies arising from deterioration in macroeconomic conditions from time to time in the Group’s markets. The Group must adhere to the rules laid down by government agencies that fund or regulate healthcare, including extensive and complex rules in the US. Failure to do so could result in fines, litigation, reputational damage and/or loss of customers and future funding. Procurement and supply chain verification processes Global recessionary and inflationary pressures and the commoditisation of entire product groups have led to an increase globally in price-driven approaches to customer procurement processes and tenders, such as the value-based procurement programme in China and further consolidation of customer buying groups. Non-clinical staff are often key decision-makers in customers’ procurement processes, with access to these decision-makers being limited for some customers. These factors can adversely impact the pricing that the Group achieves for its products. Due to geopolitical conflicts and events and increased regulation relating to sustainability, supplier verification and trade compliance, procurement processes are now required to evaluate and demonstrate the provenance of raw materials, components and products at many levels in the medical product supply chain. Given the high level of complexity and multiple tiers within the industry supply chain, there is a risk that the Group is unable to verify the ultimate provenance of certain materials which may result in fines, penalties, seizure of goods, reputational harm and impact to performance of the Group. New product innovation, design and development, including intellectual property Development and introduction of new products The medical devices industry has a high level of innovation and new product introduction. In order to remain competitive, the Group must continue to develop innovative products that satisfy customer needs and preferences, meet unmet needs, and/or provide cost or other advantages. Developing new products is a costly, lengthy and uncertain process. The Group may fail to innovate due to insufficient R&D investment, an R&D skills gap or poor product development. A potential product may not be brought to market or not succeed in the market for any number of reasons, including failure to work optimally, failure to receive regulatory approval, failure to be cost-competitive, infringement of patents or other intellectual property rights and changes in consumer demand. The Group’s products and technologies are also subject to marketing challenge by competitors. Furthermore, new products that are developed and marketed by the Group’s competitors may affect price levels in the various markets in which the Group operates. If the Group’s new products do not remain competitive with those of competitors, the Group’s revenue could decline. The Group maintains reserves for excess and obsolete inventory resulting from the potential inability to sell its products at prices in excess of current carrying costs. Marketplace changes resulting from the introduction of new products or surgical procedures may cause some of the Group’s products to become obsolete. The Group makes estimates regarding the future recoverability of the costs of these products and records a provision for excess and obsolete inventories based on historical experience, expiration of sterilisation dates and expected future trends. If actual product life cycles, product demand or acceptance of new product introductions are less favourable than projected by management, additional inventory write-downs may be required. All new products that the Group develops need to be designed and manufactured in a sustainable manner. A failure in this aspect may impact the willingness of customers to purchase the new products and adversely impact the Group’s ability to continue selling the product. Where the Group has critical gaps in its product portfolio that are not filled by new products there is a risk that the Group will lose market share to competitors that can offer a more innovative or broader product portfolio. Proprietary rights and patents Due to the technological nature of medical devices and the Group’s emphasis on serving its customers with innovative products, the Group has been subject to patent infringement claims and is subject to the potential for additional claims. Claims asserted by third parties regarding infringement of their intellectual property rights, if successful, could require the Group to expend time and significant resources to engage in dispute resolution and, if unsuccessful, pay damages, develop non-infringing products or obtain licences to the products which are the subject of such litigation, affecting the Group’s growth and profitability. Smith+Nephew protects its intellectual property and opposes third-party patents and trademarks where it deems appropriate. If Smith+Nephew fails to protect and enforce its intellectual property rights effectively, its competitive position could suffer, which could negatively impact performance. In addition, intellectual property rights may not be protectable or enforceable to the same extent in all countries in which the Group operates. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 239 |
| Cybersecurity Reliance on information technology and cybersecurity The Group uses a wide variety of information systems, programmes and technology to manage its business. The Group also develops and sells certain products that are or will be digitally enabled including connection to networks and/or the internet. The Group’s systems and the systems of the entities it acquires are vulnerable to a cyber-attack, theft of intellectual property, malicious intrusion, loss of data privacy or other significant disruption. The Group’s systems have been and will continue to be the target of such threats, including as a result of remote working. There is increasing government focus on cybersecurity including changes in the regulatory environment. Cybersecurity is a multifaceted discipline covering people, process and technology. It is also an area where more can always be done; it is a continually evolving practice. There is no assurance that the Group’s ongoing commitment to prevent, detect and respond to cyber incidents and potential threats will prove effective. As a result, the Group could lose customers, have disputes with healthcare professionals, suffer regulatory sanctions or penalties, experience increases in operating expenses or an impairment in its ability to conduct its operations, patients or employees could be exposed to financial or medical identity theft or suffer a loss of product functionality, and the reputation and performance of the Group could be materially adversely affected. Although the Company maintains insurance coverage for various business continuity risks, all costs or losses incurred would not be fully insured. Legal and compliance risks including international regulation, product liability claims and loss of reputation Global regulation The Group operates globally and is subject to extensive complex legislation, regulation, and reporting requirements, including without limitation in respect of anti-bribery and corruption, data protection, trade compliance and corporate governance and sustainability in each country in which the Group operates. The Group’s global operations are governed by the UK Bribery Act and the US Foreign Corrupt Practices Act which prohibit the Group or its representatives from making or offering improper payments to government officials and other persons or accepting payments for the purpose of obtaining or maintaining business. The Group’s international operations which operate through distributors and agents increase our Group exposure to these risks. The Group undertakes investigations into allegations of possible violations of laws and regulations, supported by external counsel where appropriate. It is not possible to predict the nature, scope or outcome of investigations, including the extent to which, if at all, this could result in any liability or reputational harm to the Group. The Group is required to comply with the requirements of data privacy laws and regulations in the markets in which it operates which impose obligations regarding the handling of personal data. As privacy and data protection continue to be a focus for regulators and consumers, new and enhanced privacy and data protection laws and regulations and enforcement frameworks, continue to develop globally. Geopolitical events such as the war in Ukraine have led to an increase in sanctions and trade compliance programmes with which the Group is required to comply and which often require evaluation and implementation at pace. Increased stakeholder focus from customers, suppliers, investors, regulators and governments on environmental, social and governance matters and AI means that the Group is required to evaluate and ensure compliance with laws, regulations and reporting requirements in these areas. Ensuring compliance with all evolving laws, regulations, and reporting requirements on a global basis may require the Group to change or develop its current business models and practices and may increase its cost of doing business. Despite efforts to manage and mitigate legal and compliance risk across the organisation, there is a risk that the Group may be subject to fines and penalties, litigation and reputational harm in connection with its activities where breaches are found to have occurred. Failure to comply with the requirements of laws, regulations and reporting requirements could adversely affect the Group’s business, reputation, financial condition or results of operations. Operating in multiple jurisdictions also subjects the Group to local laws and regulations including without limitation relating to tax, pricing, reimbursement, regulatory requirements, product safety, and varying levels of protection of intellectual property. This exposes the Group to additional risks and potential costs. Product liability claims and loss of reputation The development, manufacture and sale of medical devices entails risk of product liability claims or recalls. Design and manufacturing defects with respect to products sold by the Group or by companies it has acquired could damage, or impair the repair of, body functions. The Group may become subject to liability, which could be substantial, because of actual or alleged defects in its products. In addition, product defects could lead to the need to recall from the market existing products, which may be costly and harmful to the Group’s reputation. There can be no assurance that customers, particularly in the US, the Group’s largest geographical market, will not bring product liability or related claims that would have a material adverse effect on the Group’s financial position or results of operations in the future, or that the Group will be able to resolve such claims within insurance limits. As at 31 December 2023, a provision of $149m is recognised relating to the present value of the estimated costs to resolve all unsettled known and unknown anticipated metal-on-metal hip implant claims globally. See Note 17 to the Group accounts for further details. Financial reporting, compliance and control The Group’s financial results depend on its ability to comply with financial reporting and disclosure requirements, comply with tax laws, appropriately manage treasury activities and avoid significant transactional errors and customer defaults Other information continued Risk factors continued 240 Smith+Nephew Annual Report 2023 |
| (the risk of which has been heightened post-pandemic). Failure to comply with the Group’s financial reporting requirements or relevant tax laws can lead to litigation and regulatory penalties and sanction and ultimately to potential material loss to the Group. Potential risks include failure to report accurate financial information in compliance with accounting standards and applicable legislation, failure to comply with current tax laws, failure to manage treasury risk effectively and failure to operate adequate financial controls over business operations. Political and economic World economic conditions Demand for the Group’s products is driven by demographic trends, including the ageing population and the incidence of osteoporosis and obesity. Supply of, use of and payment for the Group‘s products are also influenced by world economic conditions which could place increased pressure on demand and pricing, adversely impacting the Group’s ability to deliver revenue and margin growth. The conditions could favour larger, better capitalised groups, with higher market shares and margins. As a consequence, the Group’s prosperity is linked to general economic conditions and there is a risk of deterioration of the Group’s performance and finances during adverse macroeconomic conditions. The impact of geopolitical conditions such as the war in Ukraine and the conflict in Gaza on global economies and financial markets may trigger a recession or slowdown in various markets in which the Group operate which would significantly reduce customer capital spending and customer financial strength. Economic conditions worldwide continue to create several challenges for the Group, including the US Administration’s approach to trade policy, increased global sanctions and countersanctions in response to local or global conflicts, heightened inflation and pricing pressure (arising across the costs of raw materials, freight and employee salaries and wages), increasing tax rates, significant declines in capital equipment expenditures at hospitals and increased uncertainty over the collectability of government debt. These factors could have an increased impact on growth in the future. The Group is increasingly seeing sustainability targets and public policies being promulgated in the markets in which the Group operates as well as by its customers, suppliers and other stakeholders. A failure to meet these targets and policies could impact the Group’s sales and growth in those markets. Political uncertainties The Group operates on a worldwide basis and has distribution channels, agents and purchasing entities in over 100 countries. Political upheaval in some of those countries or in surrounding regions may impact the Group’s results of operations. Political changes in a country could prevent the Group from receiving remittances of profit from a member of the Group located in that country or from selling its products or investments in that country. Furthermore, changes in government policy regarding preference for local suppliers, import quotas, taxation or other matters could adversely affect the Group’s revenue and operating profit. War and conflict such as in Ukraine and Gaza, economic sanctions, terrorist activities or other conflict could also adversely impact the Group whether in terms of increased compliance resources and cost to serve, increased freight cycle times, market exit, disruption to operations and/or reputational damage. There remains a level of political and regulatory uncertainty in the UK following the exit from the European Union and the introduction of new legislation in the UK. Taxation The Group operates a global business and is therefore required to comply with tax legislation in multiple jurisdictions. There is the potential for an adverse impact on the Group’s financial performance due to significant tax rate changes, or broadening of the tax base, in key jurisdictions in which the Group operates. These include OECD Pillar Two (as outlined on page 187) and US tax reform proposals. These external factors may require the Group to adjust its operating model. Quality and regulatory Regulatory standards and compliance in the healthcare industry Business practices in the healthcare industry are subject to regulation and review by various government authorities. In general, the trend in many countries in which the Group does business is towards higher expectations and increased enforcement activity by governmental authorities. While the Group is committed to doing business with integrity and welcomes the trend to higher standards in the healthcare industry, the Group and other companies in the industry have been subject to investigations and other enforcement activity that have incurred and may continue to incur significant expense. Under certain circumstances, if the Group were found to have violated the law, its ability to sell its products to certain customers may be restricted. Regulatory approval The international medical device industry is highly regulated. Regulatory requirements are a major factor in determining whether substances and materials can be developed into marketable products and the amount of time and expense that should be allotted to such development. National regulatory authorities administer and enforce a complex series of laws and regulations that govern the design, development, approval, manufacture, labelling, marketing and sale of healthcare products. They also review data supporting the safety and efficacy of such products. Of particular importance is the requirement in many countries that products be authorised or registered prior to manufacture, marketing or sale and that such authorisation or registration be subsequently maintained. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 241 |
| The major regulatory agencies for Smith+Nephew’s products include the Food and Drug Administration (FDA) in the US, the Medicines and Healthcare products Regulatory Agency in the UK, the Ministry of Health, Labour and Welfare in Japan, the National Medical Products Administration in China and the Australian Therapeutic Goods Administration. At any time, the Group is awaiting a number of regulatory approvals which, if not received, could adversely affect results of operations. Following the entry into force in May 2017 of the MDR, the increase in the time required by Notified Bodies to review product submissions and site quality systems’ certification time has had and may continue to have an adverse impact on the Group’s ability to meet customer demand. The trend is towards more stringent regulation and higher standards of technical appraisal and there are increasingly stringent local requirements for clinical data across many of the markets globally in which the Group operates. Such controls have become increasingly demanding to comply with and management believes that this trend will continue. Privacy, environmental and sustainability laws and regulations have also been developed and implemented at pace globally and have become more stringent, supported by enhanced enforcement frameworks and resources. There is also an increase in regulation relating to labelling and reporting in the markets in which the Group operates which results in increased resourcing and cost to the Group. Regulatory requirements may also entail inspections for compliance with appropriate standards, including those relating to Quality Management Systems or Good Manufacturing Practices regulations. All manufacturing and other significant facilities within the Group are subject to regular internal and external audit for compliance with national medical device regulation and Group policies. Payment for medical devices may be governed by reimbursement tariff agencies in a number of countries. Reimbursement rates may be set in response to perceived economic value of the devices, based on clinical and other data relating to cost, patient outcomes and comparative effectiveness. They may also be affected by overall government budgetary considerations. The Group believes that its emphasis on innovative products and services should contribute to success in this environment. Failure to comply with these regulatory requirements could have a number of adverse consequences, including withdrawal of approval to sell a product in a country, temporary closure of a manufacturing facility, fines and potential damage to Company reputation. Mergers and acquisitions Failure to make successful acquisitions A key element of the Group’s strategy for continued growth is to make acquisitions or alliances to complement its existing business. Failure to identify appropriate acquisition targets or failure to conduct adequate due diligence or to integrate them successfully would have an adverse impact on the Group’s competitive position and profitability. This could result from the diversion of management resources from the acquisition or integration process, challenges of integrating organisations of different geographic, cultural and ethical backgrounds, as well as the prospect of taking on unexpected or unknown liabilities. In addition, the availability of global capital and increased interest rates may make financing less attainable or more expensive and could result in the Group failing in its strategic aim of growth by acquisition or alliance. Talent management The Group’s continued ability to deliver business objectives depends on its ability to hire, successfully engage and retain highly skilled talent with particular expertise and knowledge in each business unit and market in which it operates. This is critical, particularly in general management, new product development and in data analytics and insights. Since the Covid-19 pandemic, employee priorities have shifted in terms of work-life balance resulting in increased global movement of talent and higher requirement for flexibility from both our current talent and external candidates. Attracting and retaining talent efforts continue across all disciplines and geographies to ensure that we mitigate impacts on revenue and operating profits. Additionally, if the Group is unable to attract, develop and engage talent this could have an impact on effective succession planning, it may not be able to meet its strategic business objectives, and may lose competitive advantage and intellectual capital. Environment and sustainability Climate change and sustainability-related risks have the potential to impact the Group’s business model and performance. The impacts of climate change on the Group’s business may arise from new regulations and requirements to obtain certain sustainability standards, international sustainability accords and agreements, and changing business practices and trends to accommodate climate change risks. Further, the Group will be exposed to the physical impacts of climate change, which may impact the manufacture of its products and the supply chain to deliver them to its markets. The Group may need to adapt its business model and processes to accommodate the changes brought about by climate-related issues and increased focus and regulation of sustainability requirements by governments, regulators, customers, investors and other stakeholders. If the Group does not achieve the climate change and sustainability targets and objectives set by the Group, or set by the governments and regulators in the markets where it operates, or by its customers, there may be an impact on the Group’s performance and ability to grow. Foreign exchange The Group operates a global business and is therefore exposed to exchange rate volatility. There is the potential for an adverse impact on the Group’s financial performance due to currency fluctuations. Currency fluctuations Smith+Nephew’s results of operations are affected by transactional exchange rate movements in that they are subject to exposures arising from revenue in a currency different from the related costs and expenses. The Group‘s manufacturing cost base is situated principally in the US, the UK, China, Costa Rica, Malaysia and Switzerland, from which finished products Other information continued Risk factors continued 242 Smith+Nephew Annual Report 2023 |
| are exported to the Group’s selling operations worldwide. Thus, the Group is exposed to fluctuations in exchange rates between the US Dollar, Sterling and Swiss Franc and the currency of the Group’s selling operations, particularly the Euro, Chinese Yuan, Australian Dollar, Malaysian Ringgit and Japanese Yen. If the US Dollar, Sterling or Swiss Franc should strengthen against the Euro, Australian Dollar and the Japanese Yen, the Group’s trading margin could be adversely affected. The Group manages the impact of exchange rate movements on operating profit by a policy of transacting forward foreign currency contracts when firm commitments exist. In addition, the Group’s policy is for forecast transactions to be covered between 50% and 90% for up to one year. However, the Group is still exposed to medium to long-term adverse movements in the strength of currencies compared to the US Dollar. The Group uses the US Dollar as its reporting currency. The US Dollar is the functional currency of Smith & Nephew plc. The Group’s revenues, profits and earnings are also affected by exchange rate movements on the translation of results of operations in foreign subsidiaries for financial reporting purposes. See ‘Liquidity and capital resources’ on page 204. Artificial Intelligence Advances in Artificial Intelligence (AI), machine learning, robotics, and other technologies create opportunities for the Group when used within a clear governance framework. These technologies can help us to innovate to meet unmet patient needs and earn and retain market share through improved productivity and customer service. The use of AI technology should be implemented with clear guidance on usage and risk management in order to mitigate the risk of employees or third parties inadvertently disclosing proprietary information or confidential or sensitive data. As many AI tools are limited by the information within the data sets that they are trained on, human oversight is required in order to manage risk and avoid outputs that are inherently biased or untrue. Disruptor products Innovative products in the wider healthcare industry have the potential to disrupt the medical device industry, especially as the pharmaceutical sector looks to accelerate research and development through the use of AI. Investor perception of the impact of compounds, such as glucagon-like peptide-1 (GLP-1) receptor agonists, on the medical device industry could have a negative impact on the industry as a whole as well as a potential negative impact on the strategy and financial performance of the Group. Factors affecting results of operations Government economic, fiscal, monetary and political policies are all factors that materially affect the Group’s operation or investments of shareholders. Other factors include sales trends, currency fluctuations and innovation. Each of these factors is discussed further in the Taking our innovation to market section on pages 34–45, the Manufacturing section on pages 32–33, the Financial review on pages 20–23 and the Taxation information for shareholders on pages 251–253. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 243 |
| These financial statements include financial measures that are not prepared in accordance with International Financial Reporting Standards (IFRS). These measures, which include trading profit, trading profit margin, trading profit before tax, adjusted attributable profit, tax rate on trading results (trading tax expressed as a percentage of trading profit before tax), EPSA, ROIC, trading cash flow, free cash flow, trading profit to trading cash conversion ratio, leverage ratio and underlying revenue growth, exclude the effect of certain cash and non-cash items that Group management believe are not related to the underlying performance of the Group. These non-IFRS financial measures are also used by management to make operating decisions because they facilitate internal comparisons of performance to historical results. Non-IFRS financial measures are presented in these financial statements as the Group’s management believe that they provide investors with a means of evaluating performance of the business segments and the consolidated Group on a consistent basis, similar to the way in which the Group’s management evaluate performance, that is not otherwise apparent on an IFRS basis, given that certain non-recurring, infrequent, non-cash and other items that management does not otherwise believe are indicative of the underlying performance of the consolidated Group may not be excluded when preparing financial measures under IFRS. These non-IFRS measures should not be considered in isolation from, as substitutes for, or superior to financial measures prepared in accordance with IFRS. Payments of lease liabilities are included in trading cash flow. IFRS 16 right-of-use assets and IFRS 16 lease liabilities are included in net operating assets in arriving at ROIC. Underlying revenue growth ‘Underlying revenue growth’ is used to compare the revenue in a given year to the previous year on a like-for-like basis. This is achieved by adjusting for the impact of sales of products acquired in material business combinations or disposed of and for movements in exchange rates. Underlying revenue growth is considered by the Group to be an important measure of performance as it excludes those items considered to be outside the influence of local management. The Group’s management use this non-IFRS measure in their internal financial reporting, budgeting and planning to assess performance on both a business and a consolidated Group basis. Revenue growth at constant currency is important in measuring business performance compared to competitors and compared to the growth of the market itself. The Group considers that revenue from sales of products acquired in material business combinations results in a step-up in growth in revenue in the year of acquisition that cannot be wholly attributed to local management’s efforts with respect to the business in the year of acquisition. Depending on the timing of the acquisition, there will usually be a further step change in the following year. A measure of growth excluding the effects of business combinations also allows senior management to evaluate the performance and relative impact of growth from the existing business and growth from acquisitions. The process of making business acquisitions is directed, approved and funded from the Group corporate centre in line with strategic objectives. The material limitation of the underlying revenue growth measure is that it excludes certain factors, described above, which ultimately have a significant impact on total revenues. The Group compensates for this limitation by taking into account relative movements in exchange rates in its investment, strategic planning and resource allocation. In addition, as the evaluation and assessment of business acquisitions is not within the control of local management, performance of acquisitions is monitored centrally until the business is integrated. The Group’s management consider that the non-IFRS measure of underlying revenue growth and the IFRS measure of growth in revenue are complementary measures, neither of which management use exclusively. Underlying revenue growth reconciles to reported revenue growth, the most directly comparable financial measure calculated in accordance with IFRS, by making two adjustments, the ‘constant currency exchange effect’ and the ‘acquisitions and disposals effect’, described below. The ‘constant currency exchange effect’ is a measure of the increase/decrease in revenue resulting from currency movements on non-US Dollar sales and is measured as the difference between: 1) the increase/decrease in the current year revenue translated into US Dollars at the current year average exchange rate and the prior revenue translated at the prior year rate; and 2) the increase/ decrease being measured by translating current and prior year revenues into US Dollars using the prior year closing rate. The ‘acquisitions and disposals effect’ is the measure of the impact on revenue from newly acquired material business combinations and recent material business disposals. This is calculated by comparing the current year, constant currency actual revenue (which includes acquisitions and excludes disposals from the relevant date of completion) with prior year, constant currency actual revenue, adjusted to include the results of acquisitions and exclude disposals for the commensurate period in the prior year. These sales are separately tracked in the Group’s internal reporting systems and are readily identifiable. Non-IFRS financial information – Adjusted measures Other information continued 244 Smith+Nephew Annual Report 2023 |
| Reported revenue growth, the most directly comparable financial measure calculated in accordance with IFRS, reconciles to underlying revenue growth as follows: Reconciling items 2023 Reported growth Underlying growth Acquisitions/disposals Currency impact Consolidated revenue by business unit % % % % Knee Implants 4.7 5.5 – (0.8) Hip Implants 2.5 3.8 – (1.3) Other Reconstruction 27.8 28.0 – (0.2) Trauma & Extremities 3.7 4.4 – (0.7) Orthopaedics 4.8 5.7 – (0.9) Sports Medicine Joint Repair 8.7 9.9 – (1.2) Arthroscopic Enabling Technologies 3.7 4.7 – (1.0) ENT (Ear, Nose and Throat) 28.1 29.8 – (1.7) Sports Medicine & ENT 8.8 10.0 – (1.2) Advanced Wound Care 1.8 2.1 – (0.3) Advanced Wound Bioactives 6.3 6.2 – 0.1 Advanced Wound Devices 17.0 17.6 – (0.6) Advanced Wound Management 6.2 6.4 – (0.2) Total 6.4 7.2 – (0.8) Reconciling items 2022 Reported growth Underlying growth Acquisitions/disposals Currency impact Consolidated revenue by business unit % % % % Knee Implants 2.5 6.8 – (4.3) Hip Implants (4.4) (0.2) – (4.2) Other Reconstruction (5.6) (1.8) – (3.8) Trauma & Extremities (5.7) (2.6) – (3.1) Orthopaedics (2.0) 1.9 – (3.9) Sports Medicine Joint Repair 3.6 8.7 – (5.1) Arthroscopic Enabling Technologies (3.8) 0.9 – (4.7) ENT (Ear, Nose and Throat) 17.1 20.4 – (3.3) Sports Medicine & ENT 1.9 6.7 – (4.8) Advanced Wound Care (2.6) 5.2 – (7.8) Advanced Wound Bioactives 4.9 5.4 – (0.5) Advanced Wound Devices 4.3 11.6 – (7.3) Advanced Wound Management 1.1 6.4 – (5.3) Total 0.1 4.7 – (4.6) Trading profit, trading profit margin, trading cash flow and trading profit to trading cash conversion ratio Trading profit, trading profit margin (trading profit expressed as a percentage of revenue), trading cash flow and trading profit to trading cash conversion ratio (trading cash flow expressed as a percentage of trading profit) are trend measures, which present the profitability of the Group. The adjustments made exclude the impact of specific transactions that management consider affect the Group’s short-term profitability and cash flows, and the comparability of results. The Group has identified the following items, where material, as those to be excluded from operating profit and cash generated from operations, the most directly comparable IFRS measures, when arriving at trading profit and trading cash flow, respectively: acquisition and disposal related items arising in connection with business combinations, including amortisation of acquisition intangible assets, impairments and integration costs; restructuring events; and gains and losses resulting from legal disputes and uninsured losses. In addition to these items, gains and losses that materially impact the Group’s profitability or cash flows on a short-term or one-off basis are excluded from operating profit and cash generated from operations when arriving at trading profit and trading cash flow. The cash contributions to fund defined benefit pension schemes that are closed to future accrual are excluded from cash generated from operations when arriving at trading cash flow. Payment of lease liabilities is included within trading cash flow. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 245 |
| Adjusted earnings per ordinary share (EPSA) EPSA is a trend measure, which presents the profitability of the Group excluding the post-tax impact of specific transactions that management consider affect the Group’s short-term profitability and comparability of results. The Group presents this measure to assist investors in their understanding of trends. Adjusted attributable profit is the numerator used for this measure and is determined by adjusting attributable profit for the items that are excluded from operating profit when arriving at trading profit and items that are recognised below operating profit that affect the Group’s short-term profitability. The most directly comparable financial measure calculated in accordance with IFRS is basic earnings per ordinary share (EPS). Operating Profit before Attributable Cash generated Earnings Revenue profit1 tax2 Taxation3 profit4 from operations5 per share6 $ million $ million $ million $ million $ million $ million ¢ 2023 Reported 5,549 425 290 (27) 263 829 30.2 Acquisition and disposal-related items8 – 60 78 (14) 64 16 7.3 Restructuring and rationalisation costs – 220 223 (42) 181 124 20.7 Amortisation and impairment of acquisition intangibles8 – 207 207 (45) 162 – 18.6 Legal and other7,8 – 58 64 (12) 52 145 6.0 Lease liability payments – – – – – (52) – Capital expenditure – – – – – (427) – 2023 Adjusted 5,549 970 862 (140) 722 635 82.8 Acquisition and disposal-related items: For the year ended 31 December 2023, costs primarily relate to the acquisition of CartiHeal and impairment of Engage goodwill, partially offset by credits relating to remeasurement of contingent consideration for prior year acquisitions. Adjusted profit before tax additionally excludes losses of $18m related to the Group’s shareholding in Bioventus. This primarily includes the Group’s share of loss recognised by Bioventus in its financial statements. Restructuring and rationalisation costs: For the year ended 31 December 2023, these costs relate to the implementation of the Operations and Commercial Excellence programme announced in February 2020 and also include efficiency and productivity elements of the 12-Point Plan. Adjusted profit before tax additionally excludes $3m of restructuring costs related to the Group’s share of results of associates. Amortisation and impairment of acquisition intangibles: For the year ended 31 December 2023, charges relate to the amortisation and impairment of intangible assets acquired in material business combinations. Legal and other: For the year ended 31 December 2023, charges primarily relate to legal expenses for ongoing metal-on-metal hip claims partially offset by a decrease of $8m in the provision that reflects the present value of the estimated cost to resolve all other known and anticipated metal-on-metal hip claims, and by the release of a provision for an intellectual property dispute. Charges also include the costs for implementing the requirements of the EU Medical Device Regulation that was effective from May 2021 with a transition period to May 2024. Operating Profit before Attributable Cash generated Earnings Revenue profit1 tax2 Taxation3 profit4 from operations5 per share6 $ million $ million $ million $ million $ million $ million ¢ 2022 Reported 5,215 450 235 (12) 223 581 25.5 Acquisition and disposal-related items – 4 162 (31) 131 22 15.1 Restructuring and rationalisation costs – 167 168 (30) 138 120 15.8 Amortisation and impairment of acquisition intangibles – 205 205 (45) 160 – 18.4 Legal and other7 – 75 82 (21) 61 133 7.0 Lease liability payments – – – – – (54) – Capital expenditure – – – – – (358) – 2022 Adjusted 5,215 901 852 (139) 713 444 81.8 Acquisition and disposal-related items: For the year to 31 December 2022, costs primarily relate to the acquisition of Engage and prior year acquisitions, partially offset by credits relating to remeasurement of deferred and contingent consideration for prior year acquisitions. Adjusted profit before tax additionally excludes losses of $158m related to the Group’s shareholding in Bioventus. This primarily includes an impairment charge of $109m and the Group’s share of impairment recognised by Bioventus in its financial statements. Other information continued Non-IFRS financial information – Adjusted measures continued 246 Smith+Nephew Annual Report 2023 |
| Restructuring and rationalisation costs: For the year to 31 December 2022, these costs relate to the implementation of the Operations and Commercial Excellence programme announced in February 2020 and also include efficiency and productivity elements of the 12-Point Plan. Adjusted profit before tax additionally excludes $1m of restructuring costs related to the Group’s share of results of associates. Amortisation and impairment of acquisition intangibles: For the year to 31 December 2022, charges relate to the amortisation and impairment of intangible assets acquired in material business combinations. Legal and other: For the year ended 31 December 2022, charges primarily relate to legal expenses for ongoing metal-on-metal hip claims and an increase of $19m in the provision that reflects the present value of the estimated cost to resolve all other known and anticipated metal-on-metal hip claims. Charges also include the costs for implementing the requirements of the EU Medical Device Regulation that was effective from May 2021 with a transition period to May 2024. These charges in the year to 31 December 2022 were partially offset by a credit of $7m relating to insurance recoveries for ongoing metal-on-metal hip claims. 1 Represents a reconciliation of operating profit to trading profit. 2 Represents a reconciliation of reported profit before tax to trading profit before tax. 3 Represents a reconciliation of reported tax to trading tax. 4 Represents a reconciliation of reported attributable profit to adjusted attributable profit. 5 Represents a reconciliation of cash generated from operations to trading cash flow. 6 Represents a reconciliation of basic earnings per ordinary share to adjusted earnings per ordinary share (EPSA). 7 The ongoing funding of defined benefit pension schemes is not included in management’s definition of trading cash flow as there is no defined benefit service cost for these schemes. 8 During 2023, management evaluated the commercial viability of Engage products and concluded that they should be discontinued. A total of $109m of Engage’s assets and liabilities were written off as a result of this action, which includes goodwill of $84m (included in acquisition and disposal-related items), intangible assets of $37m (included in amortisation and impairment of acquisition intangibles), inventory of $21m (included in legal and other), partially offset by remeasurement of contingent consideration of $33m (included in acquisition and disposal-related items). Free cash flow Free cash flow is a measure of the cash generated for the Group to use after capital expenditure according to its Capital Allocation Framework, it is defined as the cash generated from operations less capital expenditure and cash flows from interest and income taxes. A reconciliation from cash generated from operations, the most comparable IFRS measure, to free cash flow is set out below: 2023 2022 2021 $ million $ million $ million Cash generated from operations1 829 581 1,048 Capital expenditure (427) (358) (408) Interest received 8 7 6 Interest paid (104) (73) (80) Payment of lease liabilities (52) (54) (59) Income taxes paid (125) (47) (97) Free cash flow 129 56 410 1 See Group cash flow statement on page 174. Leverage ratio The leverage ratio is net debt including lease liabilities to adjusted EBITDA. Net debt is reconciled in Note 15 to the Group accounts. Adjusted EBITDA is defined as trading profit before depreciation and impairment of property, plant and equipment and amortisation and impairment of other intangible assets, goodwill and trade investments. The calculation of the leverage ratio is set out below: 2023 2022 $ million $ million Net debt including lease liabilities 2,776 2,535 Trading profit 970 901 Depreciation of property, plant and equipment 306 319 Amortisation of other intangible assets, impairment of goodwill and trade investments 139 56 Impairment of property, plant and equipment 31 30 Impairment of other intangible assets – 7 Adjustment for items already excluded from trading profit (119) (31) Adjusted EBITDA 1,327 1,282 Leverage ratio (x) 2.1 2.0 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 247 |
| Return on invested capital Return on invested capital (ROIC) is a measure of the return generated on capital invested by the Group. It provides a metric for long-term value creation and encourages compounding reinvestment within the business and discipline around acquisitions with low returns and long payback. ROIC is defined as Operating Profit (before amortisation and impairment of acquisition intangibles) less Adjusted Taxes/ ((Opening Net Operating Assets + Closing Net Operating Assets)/2). 2023 2022 2021 $ million $ million $ million Operating profit 425 450 593 Amortisation and impairment of acquisition intangibles 207 205 172 Operating profit before amortisation and impairment of acquisition intangibles 632 655 765 Taxation (27) (12) (62) Taxation adjustment1 (77) (86) (55) Operating profit before amortisation and impairment of acquisition intangibles less adjusted taxes 528 557 648 Total equity 5,217 5,259 5,568 Accumulated amortisation and impairment of acquisition intangibles net of associated tax 1,365 1,175 1,035 Retirement benefit assets (69) (141) (182) Investments (8) (12) (10) Investments in associates (16) (46) (188) Right-of-use assets (185) (187) (191) Cash at bank (302) (350) (1,290) Long-term borrowings and lease liabilities 2,319 2,712 2,848 Retirement benefit obligations 88 70 127 Bank overdrafts, borrowings, loans and lease liabilities 765 160 491 Net operating assets 9,174 8,640 8,208 Average net operating assets2 8,907 8,424 8,029 Return on invested capital 5.9% 6.6% 8.1% 1 Being the taxation on amortisation and impairment of acquisition intangibles, interest income, interest expense, other finance costs and share of results of associates. 2 (Opening Net Operating Assets + Closing Net Operating Assets)/2. Shareholder information Ordinary shareholders Registrar All general enquiries concerning shareholdings, dividends, changes to shareholders’ personal details and the Annual General Meeting (the ‘AGM’) should be addressed to: Computershare Investor Services plc, The Pavilions, Bridgwater Road, Bristol, BS99 6ZZ. Tel: 0370 703 0047 Tel: +44 (0) 117 378 5450 from outside the UK* www.investorcentre.co.uk * Lines are open from 8:30 am to 5:30 pm Monday to Friday, excluding public holidays in England and Wales. Shareholder communications We make quarterly financial announcements, which are made available through Stock Exchange announcements and on the Group’s website (www.smith-nephew.com). Copies of recent Annual Reports, press releases, institutional presentations and audio webcasts are also available on the website. We send paper copies of the Notice of Annual General Meeting and Annual Report only to those shareholders and ADS holders who have elected to receive shareholder documentation by post. Electronic copies of the Annual Report and Notice of Annual General Meeting are available on the Group’s website at www.smith-nephew.com. Both ordinary shareholders and ADS holders can request paper copies of the Annual Report, which the Company provides free of charge. The Company will continue to send to ordinary shareholders by post the Form of Proxy notifying them of the availability of the Annual Report and Notice of Annual General Meeting on the Group’s website. If you elect to receive the Annual Report and Notice of Annual General Meeting electronically you are informed by email of the documents’ availability on the Group’s website. ADS holders receive the Form of Proxy by post, but will not receive a paper copy of the Notice of Annual General Meeting. Investor communications The Company maintains regular dialogue with individual institutional shareholders, together with results presentations. To ensure that all members of the Board develop an understanding of the views of major investors, the Executive Directors review significant issues raised by investors with the Board. Non-Executive Directors are sent copies of analysts’ and brokers’ briefings. There is an opportunity for individual shareholders to put their questions to the Directors at the Annual General Meeting. The Company regularly responds to letters from shareholders on a range of issues. Other information continued Non-IFRS financial information – Adjusted measures continued 248 Smith+Nephew Annual Report 2023 |
| UK capital gains tax For the purposes of UK capital gains tax, the price of the Company’s ordinary shares on 31 March 1982 was 35.04p. Smith & Nephew plc share price The Company’s ordinary shares are quoted on the London Stock Exchange under the symbol SN. The Company’s share price is available on the Group’s website (www.smith-nephew.com) and at www.londonstockexchange.com where the live financial data is updated with a 15-minute delay. American Depositary Shares (‘ADSs’) and American Depositary Receipts (‘ADRs’) In the US, the Company’s ordinary shares are traded in the form of ADSs, evidenced by ADRs, on the New York Stock Exchange under the symbol SNN. Each American Depositary Share represents two ordinary shares. J.P. Morgan Chase Bank N.A. is the authorised depositary bank for the Company’s ADR programme. ADS enquiries All enquiries regarding ADS holder accounts and payment of dividends should be addressed to: EQ Shareowner Services P.O. Box 64504 St Paul, MN 55164-0504 US toll free phone: +1-800-990-1135 Online: Visit www.shareowneronline.com and select ‘Contact Us’. Smith & Nephew plc ADS price The Company’s ADS price can be obtained from the official New York Stock Exchange website at www.nyse.com and the Group’s website (www.smith-nephew.com) where the live financial data is updated with a 15-minute delay, and is quoted daily in the Wall Street Journal. ADS payment information The Company hereby discloses ADS payment information for the year ended 31 December 2023 in accordance with the Securities and Exchange Commission rules 12.D.3 and 12.D.4 relating to Form 20-F filings by foreign private issuers. The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors, including payment of dividends by the Company by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deductions from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide fee-attracting services until its fee for those services is paid. During 2023, a fee of 1 US cent per ADS was collected by J.P. Morgan Chase Bank N.A. on the 2022 final dividend paid in May 2023 and a fee of 1 US cent per ADS was collected on the 2023 interim dividend paid in November. In the period 1 January 2023 to 16 February 2024, the total programme payments made by J.P. Morgan Chase Bank N.A. was $787,719.19. Dividend history Smith & Nephew plc has paid dividends on its ordinary shares in every year since 1937. Following the capital restructuring and dividend reduction in 2000, the Group adopted a policy of increasing its dividend cover (the ratio of EPSA, as set out in the ‘Selected financial data’, to ordinary dividends declared for the year). This was intended to increase the financing capability of the Group for acquisitions and other investments. From 2000 to 2004, the dividend increased in line with inflation and, in 2004, dividend cover stood at 4.1 times. Having achieved this level of dividend cover the Board changed its policy, from that of increasing dividends in line with inflation, to that of increasing dividends for 2005 and after by 10%. Following the redenomination of the Company’s share capital into US Dollars, the Board reaffirmed its policy of increasing the dividend by 10% a year in US Dollar terms. On 2 August 2012, the Board announced its intention to pursue a progressive dividend policy, with the aim of increasing the US Dollar value of ordinary dividends over time broadly based on the Group’s underlying growth in earnings, while taking into account capital requirements and cash flows. At the time of the full-year results, the Board reviews the appropriate level of total annual dividend each year. The Board intends that the interim dividend will be set by a formula and will be equivalent to 40% of the total dividend for the previous year. Dividends will continue to be declared in US Dollars with an equivalent amount in Sterling payable to those shareholders whose registered address is in the UK, or who have validly elected to receive Sterling dividends. An interim dividend in respect of each fiscal year is normally declared in July or August and paid in October or November. Persons depositing or withdrawing shares must pay For $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) $0.05 (or less) per ADS – Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property – Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates – Any cash distribution to ADS registered holders, including payment of dividend $0.05 (or less) per ADS per calendar year Registration or transfer fees – Depositary services – Transfer and registration of shares on our share register to or from the name of the depositary or its agent when shares are deposited or withdrawn Taxes and other governmental charges the depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes – As necessary Any charges incurred by the depositary or its agents for servicing the deposited securities – As necessary STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 249 |
| Shareholder information continued Dividends per share Years ended 31 December 2023 2022 2021 2020 2019 Pence per share: Interim 11.89 12.91 10.50 11.07 11.19 Final 18.361 19.07 18.40 16.62 18.66 Total 30.25 31.98 28.90 27.69 29.85 US cents per share: Interim 14.40 14.40 14.40 14.40 14.40 Final 23.10 23.10 23.10 23.10 23.10 Total 37.50 37.50 37.50 37.50 37.50 1 Translated at the Bank of England rate on 16 February 2024. A final dividend will be recommended by the Board of Directors and paid subject to approval by shareholders at the Company’s Annual General Meeting. Future dividends of Smith & Nephew plc will be dependent upon: future earnings; the future financial condition of the Group; the Board’s dividend policy; and the additional factors that might affect the business of the Group set out in ‘Special note regarding forward-looking statements’ and ‘Risk Factors’. Dividends per share The table below sets out the dividends per ordinary share in the last five years. Dividends below £1,000 per tax year are tax free for UK income tax purposes and dividends above £1,000 per tax year are subject to UK personal income tax at the rate of 8.75% for basic rate taxpayers, 33.75% for higher rate taxpayers and 39.35% for additional rate taxpayers. If you need to pay UK tax, how you pay depends upon the amount of dividend income you receive in a year. For the tax year 2024–2025 and subsequent tax years, the £1,000 dividend nil rate will be reduced to £500. If your dividend income is up to £10,000 you can request HMRC to change your tax code so that the tax will be taken from your wages or pension or you can complete a self-assessment tax return. If your dividend income is over £10,000 in the tax year, you will need to complete a self-assessment tax return. This will apply to both cash and dividend reinvestment plan (‘DRiP’) dividends, although dividends paid on shares held within pensions and ISAs will be unaffected, remaining tax free. Since the second interim dividend for 2005, all dividends have been declared in US cents per ordinary share. £50,000 of shares in Sterling in order to comply with English law. These were issued as deferred shares, which are not listed on any stock exchange. They have extremely limited rights and therefore effectively have no value. These shares are held by the Company Secretary, although the Board reserves the right to transfer them to a member of the Board should it so wish. Shareholdings As at 16 February 2024, to the knowledge of the Group, there were 11,560 registered holders of ordinary shares, of whom 90 had registered addresses in the US and held a total of 163,350 ordinary shares (0.018% of the total issued). Because certain ordinary shares are registered in the names of nominees, the number of shareholders with registered addresses in the US is not representative of the number of beneficial owners of ordinary shares resident in the US. As at 16 February 2024, 38,222,517 ADSs equivalent to 76,445,034 ordinary shares or approximately 8.7% of the total ordinary shares in issue, were outstanding and were held by 86 registered ADS holders. Major shareholders As far as is known to Smith+Nephew, the Group is not directly or indirectly owned or controlled by another corporation or by any Government and the Group has not entered into arrangements, the operation of which may at a subsequent date result in a change in control of the Group. As at 16 February 2024, the Company is not aware of any person who has a significant direct or indirect holding of securities in the Company, as defined in the Disclosure and Transparency Rules (DTRs) of the Financial Conduct Authority (FCA), other than as shown on page 251, and is not aware of any persons holding securities which may control the Company. There are no securities in issue which have special rights as to the control of the Company. The table on page 251 shows the last notification(s) received by the Company, in accordance with the FCA’s DTRs relating to notifiable interests in the voting rights in the Company’s issued share capital. Purchase of ordinary shares on behalf of the Company At the AGM, the Company will be seeking a renewal of its current permission from shareholders to purchase up to 10% of its own shares. The Company did not purchase In respect of the proposed final dividend for the year ended 31 December 2023 of 23.1 US cents per ordinary share, the record date will be 2 April 2024 and the payment date will be 22 May 2024. The Sterling equivalent per ordinary share will be set following the record date. Shareholders may elect to receive their dividend in either Sterling or US Dollars and the last day for election will be 30 April 2024. The ordinary shares will trade ex-dividend on both the London and New York Stock Exchanges from 28 March 2024. The proposed final dividend of 23.1 US cents per ordinary share, which together with the interim dividend of 14.4 US cents, makes a total for 2023 of 37.5 US cents. Share capital The principal trading market for the ordinary shares is the London Stock Exchange. The ordinary shares were listed on the New York Stock Exchange on 16 November 1999, trading in the form of ADSs evidenced by ADRs. Each ADS represents two ordinary shares from 14 October 2014, before which time one ADS represented five ordinary shares. The ADS facility is sponsored by J.P. Morgan Chase Bank N.A. acting as depositary. All the ordinary shares, including those held by Directors and Executive Officers, rank pari passu with each other. On 23 January 2006, the ordinary shares of 122/9p were redenominated as ordinary shares of US 20 cents (following approval by shareholders at the Extraordinary General Meeting in December 2005). The new US Dollar ordinary shares carry the same rights as the previous ordinary shares. The share price continues to be quoted in Sterling. In 2006, the Company issued 250 Smith+Nephew Annual Report 2023 |
| any shares during 2023 nor during the period to 16 February 2024. Exchange controls and other limitations affecting security holders There are no UK governmental laws, decrees or regulations that restrict the export or import of capital or that affect the payment of dividends, interest or other payments to non-resident holders of Smith & Nephew plc’s securities, except for certain restrictions imposed from time to time by His Majesty’s Treasury of the United Kingdom pursuant to legislation, such as the United Nations Act 1946 and the Emergency Laws Act 1964, against the Government or residents of certain countries. There are no limitations, either under the laws of the UK or under the Articles of Association of Smith & Nephew plc, restricting the right of non-UK residents to hold or to exercise voting rights in respect of ordinary shares, except that where any overseas shareholder has not provided to the Company a UK address for the service of notices, the Company is under no obligation to send any notice or other document to an overseas address. It is, however, the current practice of the Company to send every notice or other document to all shareholders regardless of the country recorded in the register of members, with the exception of details of the Company’s dividend reinvestment plan, which are not sent to shareholders with recorded addresses in the US and Canada. Taxation information for shareholders The comments below are of a general and summary nature and are based on the Group’s understanding of certain aspects of current UK and US federal income tax law and practice relevant to the ADSs and ordinary shares not in ADS form. The comments address the material US and UK tax consequences generally applicable to a person who is the beneficial owner of ADSs or ordinary shares and who, for US federal income tax purposes, is a citizen or resident of the US, a corporation (or other entity taxable as a corporation) created or organised in or under the laws of the US (or any State therein or the District of Columbia), or an estate or trust the income of which is included in gross income for US federal income tax purposes regardless of its source (each a US Holder). The comments set out below do not purport to address all tax consequences of the ownership of ADSs or ordinary shares that may be material to a particular holder and in particular do not deal with the position of US Holders who directly, indirectly or constructively own 10% or more of the Company’s issued ordinary shares. This discussion does not apply to (i) US Holders whose holding of ADSs or ordinary shares is effectively connected with or pertains to either a permanent establishment in the UK through which a US Holder carries on a business in the UK or a fixed base from which a US Holder performs independent personal services in the UK, or (ii) US Holders whose registered address is inside the UK. This discussion does not apply to certain US Holders subject to special rules, such as certain financial institutions, tax-exempt entities, insurance companies, broker-dealers and traders in securities that elect to use the mark-to-market method of tax accounting, partnerships or other entities treated as partnerships for US federal income tax purposes, US Holders holding ADSs or ordinary shares as part of a hedging, conversion or other integrated transaction or US Holders whose functional currency for US federal income tax purposes is other than the US Dollar. In addition, the comments below do not address the potential application of the provisions of the US Internal Revenue Code known as the Medicare contribution tax, any alternative minimum tax consequences, any US federal tax other than income tax or any US state, local or non-US (other than UK) taxes. The summary deals only with US Holders who hold ADSs or ordinary shares as capital assets for tax purposes. The summary is based on current UK and US law and practice which is subject to change, possibly with retroactive effect. US Holders are recommended to consult their tax advisers as to the particular tax consequences to them of the ownership of ADSs or ordinary shares. The Company believes, and this discussion assumes, that the Company was not a passive foreign investment company for its taxable year ended 31 December 2023. This discussion assumes that each obligation under the deposit agreement and any related agreement will be performed in accordance with its terms. For purposes of US federal income tax law, US Holders of ADSs will generally be treated as owners of the ordinary shares represented by the ADSs. Taxation of distributions in the UK and the US The UK does not currently impose a withholding tax on dividends paid by a UK corporation, such as the Company. For US federal income tax purposes, distributions paid by the Company will generally be foreign source dividends to the extent paid out of the Company’s current or accumulated earnings and profits as determined for US federal income tax purposes. Because the Company does not maintain calculations of its earnings and profits under US federal income tax principles, it is expected that distributions generally will be reported to US Holders as dividends. Such dividends will not be eligible for the dividends-received deduction generally allowed to corporate US Holders. Dividends paid to certain non-corporate US Holders of ordinary shares or ADSs may be subject to US federal income tax Major shareholders As at 31 December 2023 %* 2022 %* 2021 %* 16 February 2024 %* BlackRock, Inc. 5.2 5.2 5.2 5.2 As at 31 December 16 February 2024 ’000 2022 ’000 2021 ’000 2020 ’000 BlackRock, Inc. 46,427 46,427 46,427 46,427 * Percentage of ordinary shares in issue, excluding Treasury shares. Purchase of ordinary shares on behalf of the Company Total shares purchased 000’s Average price paid per share pence Approximate value of shares purchased $ million 2023 – – – STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 251 |
| Shareholder information continued of a permanent establishment of an enterprise situated in the UK. US information reporting and backup withholding Payments of dividends on, or proceeds from the sale of, ADSs or ordinary shares that are made within the US or through certain US-related financial intermediaries generally will be subject to US information reporting, and may be subject to backup withholding, unless a US Holder is an exempt recipient or, in the case of backup withholding, provides a correct US taxpayer identification number and certain other conditions are met. Any backup withholding deducted may be credited against the US Holder’s US federal income tax liability, and, where the backup withholding exceeds the actual liability, the US Holder may obtain a refund by timely filing the appropriate refund claim with the US Internal Revenue Service. US Holders who are individuals or certain specified entities may be required to report information relating to securities issued by a non-US person (or foreign accounts through which the securities are held), subject to certain exceptions (including an exception for securities held in accounts maintained by US financial institutions). US Holders should consult their tax advisers regarding their reporting obligations with respect to the ADSs or ordinary shares. UK stamp duty and stamp duty reserve tax UK stamp duty is charged on documents and in particular instruments for the transfer of registered ownership of ordinary shares. Transfers of ordinary shares in certificated form will generally be subject to UK stamp duty at the rate of ½% of the consideration given for the transfer with the duty rounded up to the nearest £5. UK stamp duty reserve tax (SDRT) arises when there is an agreement to transfer shares in UK companies ‘for consideration in money or money’s worth’, and so an agreement to transfer ordinary shares for money or other consideration may give rise to a charge to SDRT at the rate of ½% (rounded up to the nearest penny). The charge of SDRT will be cancelled, and any SDRT already paid will be refunded, if within six years of the agreement an instrument of transfer is produced to HM Revenue & Customs and the appropriate stamp duty paid. Transfers of ordinary shares into CREST (an electronic transfer system) are exempt from stamp duty so long as the transferee is a member of CREST who will hold the ordinary shares as a nominee for the transferor and the transfer is in a form that will ensure that the securities become held in uncertificated form within CREST. Paperless transfers of ordinary shares within CREST for consideration in money or money’s worth are liable to SDRT rather than stamp duty. SDRT on relevant transactions will be collected by CREST at ½%, and this will apply whether or not the transfer is effected in the UK and whether or not the parties to it are resident or situated in the UK. UK legislation provides for a charge to stamp duty or SDRT to be payable at the rate of 1.5% of the consideration (or, in some cases, the value of the shares concerned) where ordinary shares are issued or transferred to the depositary or to certain persons providing a clearance service (or their nominees or agents) for the conversion into ADRs and will generally be payable by the depositary or person providing clearance service. In accordance with the terms of the Deposit Agreement, any tax or duty payable by the depositary on deposits of ordinary shares will be charged by the depositary to the party to whom ADRs are delivered against such deposits. However, such transfers to the depository or to certain persons providing a clearance service (or their nominees or agents) will not attract stamp duty or SDRT where they satisfy the conditions of an exemption, including exemptions which can apply to certain capital raising or qualifying listing arrangements. The discussion above assumes that the Finance Bill currently proceeding through the UK Parliament (provision of which, broadly, provide for the repeal of certain 1.5% SDRT charges on the issue of securities by a UK company to depositary receipt issuers and clearance services) is enacted in substantively the same form as currently published and has retroactive effect from 1 January 2024. Until the Finance Bill receives Royal Ascent (which is likely to be later in 2024) relevant provisions affecting stamp duty and SDRT have been given provisional statutory effect, as if they were contained in an Act of Parliament, under ( in the case of SDRT) the Provisional Collection of Taxes at lower rates than those applicable to other types of ordinary income if certain conditions are met. Non-corporate US Holders should consult their own tax advisers to determine whether they are subject to any special rules that limit their ability to be taxed at these favourable rates. Taxation of capital gains US Holders, who are not resident for tax purposes in the UK, will not generally be liable for UK capital gains tax on any capital gain realised upon the sale or other disposition of ADSs or ordinary shares unless the ADSs or ordinary shares are held in connection with a trade carried on in the UK through a permanent establishment (or in the case of individuals, through a branch or agency). Furthermore, UK resident individuals who acquire ADSs or ordinary shares before becoming temporarily non-UK residents may remain subject to UK taxation of capital gains on gains realised while non-resident. For US federal income tax purposes, gains or losses realised upon a taxable sale or other disposition of ADSs or ordinary shares by US Holders generally will be US source capital gains or losses and will be long-term capital gains or losses if the ADSs or ordinary shares were held for more than one year. The amount of a US Holder’s gain or loss will be equal to the difference between the amount realised on the sale or other disposition and such holder’s tax basis in the ADSs, or ordinary shares, each determined in US Dollars. Inheritance and estate taxes HM Revenue & Customs imposes inheritance tax on capital transfers which occur on death and in the seven years preceding death. HM Revenue & Customs considers that the US/UK Double Taxation Convention on Estate and Gift Tax applies to inheritance tax. Consequently, a US citizen who is domiciled in the US and is not a UK national or domiciled in the UK will not be subject to UK inheritance tax in respect of ADSs and ordinary shares. A UK national who is domiciled in the US will be subject to UK inheritance tax but will be entitled to a credit for any US federal estate tax charged in respect of ADSs and ordinary shares in computing the liability to UK inheritance tax. Special rules apply where ADSs and ordinary shares are business property 252 Smith+Nephew Annual Report 2023 |
| Act 1968 and (in the case of stamp duty) the Finance Act 1973, through resolutions of the House of Commons passed on 27 November 2023. Specific professional advice should be sought before paying the 1.5% SDRT or stamp duty charge in any circumstances. No liability for stamp duty or SDRT will arise on any transfer of, or agreement to transfer, an ADS or beneficial ownership of an ADS, provided that the ADS and any instrument of transfer or written agreement to transfer remains at all times outside the UK, and provided further that any instrument of transfer or written agreement to transfer is not executed in the UK and the transfer does not relate to any matter or thing done or to be done in the UK (the location of the custodian as a holder of ordinary shares not being relevant in this context). In any other case, any transfer of, or agreement to transfer, an ADS or beneficial ownership of an ADS could, depending on all the circumstances of the transfer, give rise to a charge to stamp duty or SDRT. Any UK stamp duty or SDRT imposed upon transfers of ADSs or ordinary shares will not be treated as a creditable foreign tax for US federal income tax purposes. US Holders should consult their tax advisers regarding whether any such UK stamp duty or SDRT may be deductible or reduce the amount of gain (or increase the amount of loss) recognised upon a sale or other disposition of the ADSs or ordinary shares. Charitable and Political Donations The Group made no political donations during the year (2022: $nil). Details of charitable donations can be found on page 54. Suppliers’ Payment Policy Terms of payment are agreed with individual suppliers prior to supply. The Group aims to pay its creditors promptly, in accordance with terms agreed for payment. Further information can be obtained from the government payment practice reporting portal. Articles of Association The following summarises certain material rights of holders of the Company’s ordinary shares under the material provisions of the Company’s Articles of Association, being those which were adopted at the 2021 Annual General Meeting and English law. This summary is qualified in its entirety by reference to the Companies Act and the Company’s Articles of Association. In the following description, a ‘shareholder’ is the person registered in the Company’s register of members as the holder of an ordinary share. The Company is incorporated under the name Smith & Nephew plc and is registered in England and Wales with registered number 324357. The Company’s ordinary shares may be held in certificated or uncertificated form. No holder of the Company’s shares will be required to make additional contributions of capital in respect of the Company’s shares in the future. In accordance with English law, the Company’s ordinary shares rank equally. Directors Under the Company’s Articles of Association, a Director may not vote in respect of any contract, arrangement, transaction or proposal in which he or she, or any person connected with him or her, has any interest which is to his or her knowledge a material interest other than by virtue of his interests in securities of, or otherwise in or through, the Company. This is subject to certain exceptions relating to proposals (a) indemnifying him in respect of obligations incurred on behalf of the Company, (b) indemnifying a third party in respect of obligations of the Company for which the Director has assumed responsibility under an indemnity or guarantee, (c) relating to an offer of securities in which he will be interested as an underwriter, (d) concerning another body corporate in which the Director is beneficially interested in less than 1% of the issued shares of any class of shares of such a body corporate, (e) relating to an employee benefit in which the Director will share equally with other employees and (f) relating to any insurance that the Company is empowered to purchase for the benefit of Directors of the Company in respect of actions undertaken as Directors (and/or officers) of the Company. A Director shall not vote or be counted in any quorum present at a meeting in relation to a resolution on which he/she is not entitled to vote. The Board is empowered to exercise all the powers of the Company to borrow money, subject to the limitation that the aggregate amount of all monies borrowed after deducting cash and current asset investments by the Company and its subsidiaries shall not exceed the sum of $8,500,000,000. Any Director who has been appointed by the Board since the previous Annual General Meeting of shareholders, either to fill a casual vacancy or as an additional Director, holds office only until the conclusion of the next Annual General Meeting (notice of which was given after his or her appointment) and then shall be eligible for re-election by the shareholders. The Company’s Articles of Association provide that all Directors are subject to annual re-election in accordance with the UK Corporate Governance Code. If not re-appointed, a Director retiring at a meeting shall retain office until the meeting appoints someone in his place, or if it does not do so, until the conclusion of the meeting. The Directors are subject to removal with or without cause by the Board or the shareholders. Directors are not required to hold any shares of the Company by way of qualification. Under the Company’s Articles of Association and English law, a Director may be indemnified out of the assets of the Company against liabilities he or she may sustain or incur in the execution of his or her duties. Rights attaching to ordinary shares Under English law, dividends are payable on the Company’s ordinary shares only out of profits available for distribution, as determined in accordance with accounting principles generally accepted in the UK and by the Companies Act 2006. Holders of the Company’s ordinary shares are entitled to receive final dividends as may be declared by the Directors and approved by the shareholders in a general meeting, rateable according to the amounts paid up on such shares, provided that the dividend cannot exceed the amount recommended by the Directors. The Company’s Board of Directors may declare such interim dividends as appear to them to be justified by the Company’s financial position. If authorised by an ordinary resolution of the shareholders, the Board may also make a direct payment of a dividend in whole or in part by the distribution of STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 253 |
| specific assets (and in particular of paid-up shares or debentures of the Company). Any dividend unclaimed after 12 years from the date the dividend was declared, or became due for payment, will be forfeited and will revert to the Company. Provided that during this 12-year period, at least three dividends whether interim or final on or in respect of the share in question have become payable, and provided further the Company has taken steps which the Board considers reasonable during this 12-year period to trace the shareholder (including, if appropriate, engaging a professional tracing agent) and has sent notice of the Board’s intention to sell the shares, the Board can sell the shares and use such proceeds for any purpose that the Board thinks fit. There were no material modifications to the rights of shareholders under the Company’s Articles of Association during 2023. Voting rights of ordinary shares The Company’s Articles of Association provide that voting at any General Meeting of shareholders is by a show of hands unless a poll, which is a written vote, is duly demanded and held. On a show of hands, every shareholder who is present in person at a General Meeting has one vote regardless of the number of shares held. On a poll, every shareholder who is present in person or by proxy has one vote for each ordinary share held by that shareholder. A poll may be demanded by any of the following: – The Chair of the meeting; – At least five shareholders present or by proxy entitled to vote on the resolution; – Any shareholder or shareholders representing in the aggregate not less than one-tenth of the total voting rights of all shareholders entitled to vote on the resolution; or Any shareholder or shareholders holding shares conferring a right to vote on the resolution on which there have been paid-up sums in aggregate equal to not less than one-tenth of the total sum paid up on all the shares conferring that right. A Form of Proxy will be treated as giving the proxy the authority to demand a poll, or to join others in demanding one, as above. It is the Company’s usual practice to vote by poll at Annual General Meetings. The necessary quorum for a General Meeting is two shareholders present in person or by proxy carrying the right to vote upon the business to be transacted. Matters are transacted at General Meetings of the Company by the processing and passing of resolutions of which there are two kinds: ordinary and special resolutions: – Ordinary resolutions include resolutions for the re-election of Directors, the approval of financial statements, the declaration of dividends (other than interim dividends), the appointment and re-appointment of auditors or the grant of authority to allot shares. An ordinary resolution requires the affirmative vote of a majority of the votes of those persons voting at the meetings at which there is a quorum. – Special resolutions include resolutions amending the Company’s Articles of Association, dis-applying statutory pre-emption rights or changing the Company’s name; modifying the rights of any class of the Company’s shares at a meeting of the holders of such class or relating to certain matters concerning the Company’s winding-up. A special resolution requires the affirmative vote of not less than three-quarters of the votes of the persons voting at the meeting at which there is a quorum. Annual General Meetings must be convened upon advance written notice of 21 days. Other General Meetings must be convened upon advance written notice of at least 14 clear days. The days of delivery or receipt of notice are not included. The notice must specify the nature of the business to be transacted. Meetings are convened by the Board. Members with 5% of the ordinary share capital of the Company may requisition the Board to convene a meeting. Any two Members may call a General Meeting in order to appoint one or more additional Directors in the event that there are insufficient Directors to be able to call a General Meeting, or where they are unwilling to do so. Variation of rights If, at any time, the Company’s share capital is divided into different classes of shares, the rights attached to any class may be varied, subject to the provisions of the Companies Act, with the consent in writing of holders of three-quarters in nominal value of the issued shares of that class or upon the adoption of a special resolution passed at a separate meeting of the holders of the shares of that class. At every such separate meeting, all the provisions of the Articles of Association relating to proceedings at a General Meeting apply, except that the quorum is to be the number of persons (which must be two or more) who hold or represent by proxy not less than one-third in nominal value of the issued shares of the class and at any such meeting a poll may be demanded in writing by any person or their proxy who hold shares of that class. Where a person is present by proxy or proxies, he or she is treated as holding only the shares in respect of which the proxies are authorised to exercise voting rights. Rights in a winding-up Except as the Company’s shareholders have agreed or may otherwise agree, upon the Company’s winding-up, the balance of assets available for distribution: – After the payment of all creditors including certain preferential creditors, whether statutorily preferred creditors or normal creditors; – Subject to any special rights attaching to any other class of shares; and – Is to be distributed among the holders of ordinary shares according to the amounts paid-up on the shares held by them. This distribution is generally to be made in US Dollars. A liquidator may, however, upon the adoption of any extraordinary resolution of the shareholders and any other sanction required by law, divide among the shareholders the whole or any part of the Company’s assets in kind. Limitations on voting and shareholding There are no limitations imposed by English law or the Company’s Articles of Association on the right of non-residents or foreign persons to hold or vote the Company’s ordinary shares or ADSs, other than the limitations that would generally apply to all of the Company’s shareholders. Transfers of shares The Board may refuse to register the transfer of shares held in certificated form which: – Are not fully paid (provided that it shall not exercise this discretion in such a way as to prevent stock market dealings in the shares of that class from taking place on an open and proper basis); Shareholder information continued 254 Smith+Nephew Annual Report 2023 |
| – Are not duly stamped or duly certified or otherwise shown to the satisfaction of the Board to be exempt from stamp duty, lodged at the Transfer Office or at such other place as the Board may appoint and (save in the case of a transfer by a person to whom no certificate was issued in respect of the shares in question) accompanied by the certificate for the shares to which it relates, and such other evidence as the Board may reasonably require to show the right of the transferor to make the transfer and, if the instrument of transfer is executed by some other person on his or her behalf, the authority of that person so to do; – Are in respect of more than one class of shares; or – Are in favour of more than four transferees. Deferred shares Following the re-denomination of share capital on 23 January 2006, the ordinary shares’ nominal value became 20 US cents each. There were no changes to the rights or obligations of the ordinary shares. In order to comply with the Companies Act 2006, a new class of Sterling shares was created, deferred shares, of which 50,000 shares of £1 each were issued and allotted in 2006 as fully paid to the Chief Executive Officer. These shares were subsequently transferred and are now held by the Company Secretary, although the Board reserves the right to transfer them to a member of the Board should it so wish. These deferred shares have no voting or dividend rights and on winding-up are only entitled to repayment at nominal value only if all ordinary shareholders have received the nominal value of their shares plus an additional US$1,000 each. Amendments The Company does not have any special rules about amendments to its Articles of Association beyond those imposed by law. About Smith+Nephew The Smith+Nephew Group (the Group) is a portfolio medical technology business with leadership positions in Orthopaedics, Advanced Wound Management and Sports Medicine, and revenue of approximately $5.5bn in 2023. Smith & Nephew plc (the Company) is the Parent Company of the Group. It is an English public limited company with its shares listed on the premium list of the UK Listing Authority and traded on the London Stock Exchange. Shares are also traded on the New York Stock Exchange in the form of American Depositary Shares (ADSs). This is the Annual Report of Smith & Nephew plc for the year ended 31 December 2023. It comprises, in a single document, the Annual Report and Accounts of the Company in accordance with UK requirements and the Annual Report on Form 20-F in accordance with the regulations of the United States Securities and Exchange Commission (SEC). Smith+Nephew operates on a worldwide basis and has distribution channels in over 100 countries. The Group is engaged in a single business activity, being the development, manufacture and sale of medical technology products and services. In 2023, Smith+Nephew’s operations were organised into three global business units (Orthopaedics, Sports Medicine & ENT, and Advanced Wound Management) within the medical technology industry. Smith+Nephew’s corporate website, www.smith-nephew.com, gives additional information on the Group, including an electronic version of this Annual Report. Information made available on this website, or other websites mentioned in this Annual Report, are not and should not be regarded as being part of, or incorporated into, this Annual Report. The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise. For the convenience of the reader, a Glossary of terms used in this document is included on page 260. The product names referred to in this document are identified by use of capital letters and the ◊ symbol (on first occurrence on a particular page) and are trademarks owned by or licensed to members of the Group. Presentation The Group’s fiscal year end is 31 December. References to a particular year in this Annual Report are to the fiscal year, unless otherwise indicated. Except as the context otherwise requires, ‘ordinary share’ or ‘share’ refer to the ordinary shares of Smith & Nephew plc of 20 US cents each. The Group Accounts of Smith & Nephew plc in this Annual Report are presented in US Dollars. Solely for the convenience of the reader, certain parts of this Annual Report contain translations of amounts in US Dollars into Sterling at specified rates. These translations should not be construed as representations that the US Dollar amounts actually represent such Sterling amounts or could be converted into Sterling at the rate indicated. Unless stated otherwise, the translation of US Dollars and cents to Sterling and pence in this Annual Report has been made at the Bank of England exchange rate on the date indicated. On 16 February 2024, the latest practicable date for this Annual Report, the Bank of England rate was US$1.2584 per £1.00. The results of the Group, as reported in US Dollars, are affected by movements in exchange rates between US Dollars and other currencies. The Group applied the average exchange rates prevailing during the year to translate the results of companies with functional currency other than US Dollars. The currencies which most influenced these translations in the years covered by this report were Sterling, Swiss Franc and the Euro. The Accounts of the Group in this Annual Report are presented in millions (m) unless otherwise indicated. Change in auditor KPMG will conclude their engagement as our auditors with effect from 1 May 2024. The audit opinions provided by KPMG for the financial years ended 31 December 2022 and 2023 did not include an adverse opinion or disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles. Given that the Group was approaching the 10 year period when a competitive tender would be required, the Group chose to engage in a rigorous auditor selection process and invited other audit firms to submit detailed proposals for a new engagement. Following a thorough review of the proposals submitted by prospective audit firms, Deloitte LLP was selected as the new auditor of the Group with effect from 1 May 2024 and the appointment of Deloitte LLP as the Group’s auditors was recommended by the Audit Committee and approved by the Board. For the financial years 2022 and 2023 STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 255 |
| there were no material disagreements (as defined in Item 16F(a)(1)(iv) of Form 20-F) with KPMG on matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreement(s), if not resolved to the satisfaction of KPMG, would have caused KPMG to make reference to the subject matter of the disagreement(s) in connection with its report. During the financial years ended 31 December 2022 and 2023, there were no reportable events as defined under Item 16F(a)(1)(v). During the financial years ended 31 December 2022 and 2023, the Group did not consulted Deloitte LLP regarding: – The application of accounting principles to a specified transaction, either completed or proposed; – The type of audit opinion that might be rendered on the Group’s financial statements, and either a written report was provided to the registrant or oral advice was provided that Deloitte LLP concluded was an important factor considered by the registrant in reaching a decision as to the accounting, auditing or financial reporting issue; or – Any matter that was either the subject of a disagreement (as defined in Item 16F(a)(1)(iv) of Form 20-F) or a reportable event (as described in Item 16F(a)(1)(v) of Form 20-F) between Smith+Nephew and KPMG The Group has requested that KPMG LLP furnish it with a letter addressed to the SEC stating whether or not it agrees with the above statements. A copy of such letter is filed as an Exhibit to this Annual Report. Special note regarding forward-looking statements The Group’s reports filed with, or furnished to, the US Securities and Exchange Commission (SEC), including this document and written information released, or oral statements made, to the public in the future by or on behalf of the Group, contain ‘forward-looking statements’ within the meaning of the US Private Securities Litigation Reform Act of 1995, that may or may not prove accurate. For example, statements regarding expected revenue growth and trading profit margins discussed in the ‘Strategic Report’, market trends and our product pipeline are forward-looking statements. Phrases such as ‘aim’, ‘plan’, ‘intend’, ‘anticipate’, ‘well-placed’, ‘believe’, ‘estimate’, ‘expect’, ‘target’, ‘consider’ and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results, to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to factors such as the conflicts in Ukraine and the Middle East; economic and financial conditions in the markets we serve, especially those affecting healthcare providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal and financial compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; disruptions due to natural disasters, weather and climate change related events; changes in customer and other stakeholder sustainability expectations; changes in taxation regulations; effects of foreign exchange volatility; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature; relationships with healthcare professionals; reliance on information technology and cybersecurity. Specific risks faced by the Group are described under ‘Risk factors’ on pages 237–243 of this Annual Report. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew’s expectations. Product data Product data and product share estimates throughout this report are derived from a variety of sources including publicly available competitors’ information, internal management information and independent market research reports. Documents on display It is possible to read and copy documents referred to in this Annual Report at the Registered Office of the Company. Documents referred to in this Annual Report that have been filed with the Securities and Exchange Commission in the US may be read and copied at the SEC’s public reference room located at 450 Fifth Street, NW, Washington DC 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms and their copy charges. The SEC also maintains a website at www.sec.gov that contains reports and other information regarding registrants that file electronically with the SEC. This Annual Report on Form 20-F and some of the other information submitted by the Group to the SEC may be accessed through the SEC website. Corporate headquarters and registered office The corporate headquarters is in the UK and the registered office address is: Smith & Nephew plc, Building 5, Croxley Park, Hatters Lane, Watford, Hertfordshire, WD18 8YE, United Kingdom. Registered in England and Wales No. 324357. Tel. +44 (0)1923 477 100 www.smith-nephew.com Shareholder information continued 256 Smith+Nephew Annual Report 2023 |
| Cross-reference to Form 20-F Part I Page Item 1 Identity of Directors, Senior Management and Advisers n/a Item 2 Offer Statistics and Expected Timetable n/a Item 3 Key Information A – (Reserved) n/a B – Capitalisation and Indebtedness n/a C – Reason for the Offer and Use of Proceeds n/a D – Risk Factors 237–243 Item 4 Information on the Company A – History and Development of the Company 8–11, 14–23, 26–45, 58–59, 172–228, 224–225, 231–235, 246, 248–251, 255–256, 266, IBC B – Business Overview 2–79, 180–183, 235–243 C – Organisational Structure 197–198, 231–234 D – Property, Plants and Equipment 191–192, 235 Item 4A Unresolved Staff Comments None Item 5 Operating and Financial Review and Prospects A – Operating Results IFC, 16, 18–23, 237–243 B – Liquidity and Capital Resources 23, 202–204, 223–224 C – Research and Development, Patents and Licences, etc. 11, 16–17, 19, 75, 183, 239 D – Trend Information 7, 23, 26–45, 58–59, 237–243 E – Critical Accounting Estimates 159–171 Item 6 Directors, Senior Management and Employees A – Directors and Senior Management 88–97 B – Compensation 121–154, 214–220 C – Board Practices 88–93, 96–154 D – Employees 46–49, 185 E – Share Ownership 136–140, 144–145, 146–149, 153–154, 220–222, 226, 228 F – Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation n/a Item 7 Major Shareholders and Related Party Transactions A – Major Shareholders 250–251, 253–255 B – Related Party Transactions 226, 235 C – Interests of Experts and Counsel n/a Item 8 Financial information A – Consolidated Statements and Other Financial Information 155–231, 249–250 Legal Proceedings 211–213 Dividends 249–250 B – Significant Changes None Item 9 The Offer and Listing A – Offer and Listing Details 88, 248–250 This table provides a cross-reference from the information included in this Annual Report to the requirements of Form 20-F. Part I Page B – Plan of Distribution n/a C – Markets 5, 88, 248–250 D – Selling Shareholders n/a E – Dilution n/a F – Expenses of the Issue n/a Item 10 Additional Information A – Share Capital n/a B – Memorandum and Articles of Association 253–255 C – Material Contracts None D – Exchange Controls 251 E – Taxation 251–253 F – Dividends and Paying Agents n/a G – Statement by Experts n/a H – Documents on Display 256 I – Subsidiary Information 231–234 Item 11 Quantitative and Qualitative Disclosure about Market Risk 205–211 Item 12 Description of Securities other than Equity Securities A – Debt Securities n/a B – Warrants and Rights n/a C – Other Securities n/a D – American Depositary Shares 249–250 Part II Page Item 13 Defaults, Dividend Arrearages and Delinquencies None Item 14 Material Modifications to the Rights of Security Holders and Use of Proceeds None Item 15 Controls and Procedures 114, 116–120, 156–171 Item 16 (Reserved) n/a A – Audit Committee Financial Expert 92, 114 B – Code of Ethics 120 C – Principal Accountant Fees and Services 116–117, 185 D – Exemptions from the Listing Standards for Audit Committees n/a E – Purchases of Equity Securities by the Issuer and Affiliated Purchasers 226, 251 F – Change in Registrant’s Certifying Accountant 107, 116–117, 137, 255–256 G – Corporate Governance 88 H – Mine Safety Disclosure n/a I – Disclosure Regarding Foreign Jurisdictions that Prevent Inspections n/a J – Insider Trading Policies K – Cybersecurity 73, 236, 240 Part III Page Item 17 Financial Statements n/a Item 18 Financial Statements 172–228 Item 19 Exhibits STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 257 |
| Topic Metric 2023 Reporting Code Affordability and pricing Ratio of weighted average rate of net price increases (for all products) to the annual increase in the US Consumer Price Index. Not reported. HC-MS-240a.1 Description of how price information for each product is disclosed to customers or to their agents. Smith+Nephew uses several methods to disseminate price information to customers, including quotes, agreements, responses to requests for proposal, tender bid submissions, discount and rebate reporting and through large group purchasing organisation/ integrated delivery network customers to their members. HC-MS-240a.2 Product safety Number of recalls issued, total units recalled. In 2023, Smith+Nephew reported 14 recalls globally. A total of 136,848 units were impacted globally. All impacted products were either removed from the market or corrected per the applicable regulations and/or standards. HC-MS-250a.1 List of products listed in the FDA’s MedWatch Safety Alerts for Human Medical Products database. Smith+Nephew reports all data as required by the FDA. The MedWatch database is available at https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program HC-MS-250a.2 Number of fatalities related to products as reported in the FDA Manufacturer and User Facility Device Experience (MAUDE). Smith+Nephew reports all data as required by the FDA. The FDA MAUDE database is available at https://www.accessdata.fda. gov/scripts/cdrh/cfdocs/cfmaude/ search.cfm HC-MS-250a.3 Number of FDA enforcement actions taken in response to violations of current Good Manufacturing Practices (cGMP), by type. In 2023, Smith+Nephew received: – 1 Form 483 (1 observation in total). – 0 Warning letters. – 0 Seizures. – 7 Recalls (FDA reportable events). – 0 Consent decrees. HC-MS-250a.4 Ethical marketing Description of code of ethics governing promotion of off-label use of products. See the Product Promotion and Scientific Disclosures section of our Code of Conduct and Business Principles (http://www.smith-nephew.com/ compliance) and the Acting with Integrity section of our Sustainability Report for additional information. HC-MS-270a.2 SASB reporting 258 Smith+Nephew Annual Report 2023 |
| Topic Metric 2023 Reporting Code Product design and lifecycle management Discussion of process to assess and manage environmental and human health considerations associated with chemicals in products, and meet demand for sustainable products. Sustainability reviews are incorporated in New Product Development phase reviews for new products and acquisitions. Additionally, regulatory changes regarding chemicals in products are tracked and actioned, as appropriate. See our Sustainability Report for more information. HC-MS-410a.1 Total amount of products accepted for takeback and reused, recycled, or donated, broken down by: (1) devices and equipment and (2) supplies. Smith+Nephew operates takeback schemes where required by law. Smith+Nephew does not measure the amount of products reused or recycled for our business purposes. See the People section of our Sustainability Report for information on product donations. HC-MS-410a.2 Supply chain management Percentage of (1) entity’s facilities and (2) Tier 1 suppliers’ facilities participating in third-party audit programmes for manufacturing and product quality. All Smith+Nephew direct manufacturing locations participate in the Medical Device Single Audit Program (MDSAP). All Smith+Nephew direct and third-party manufacturing locations are certified to ISO13485. Additionally, all Tier 1 material suppliers are compliant with ISO13485. HC-MS-430a.1 Description of efforts to maintain traceability within the distribution chain. All Smith+Nephew products are labelled with either Unique Device Identifiers or HIBC barcodes to maintain traceability. HC-MS-430a.2 Description of the management of risks associated with the use of critical materials. Supply chain risks are captured within Smith+Nephew’s Enterprise Risk Management process and Global Supply Chain is identified as one of our Principal Risks. See our Risk Report on page 67 and our Conflict Minerals Disclosure Report on our website (www.smith-nephew.com) for additional information. HC-MS-430a.3 Business ethics Total amount of monetary losses as a result of legal proceedings associated with bribery or corruption. In 2023, Smith+Nephew did not have monetary losses due to legal proceedings associated with bribery or corruption. HC-MS-510a.1 Description of code of ethics governing interactions with healthcare professionals. See our website (www.smith-nephew.com) for our Code of Conduct and Business Principles, our Anti-Bribery Policy, our Annual Report, and also the Acting with Integrity section of our Sustainability Report for additional information. HC-MS-510a.2 Activity metric Number of units sold by product category. Not reported. HC-MS-000.A You can learn more about our sustainability targets and strategy in our 2023 Sustainability Report at www.smith-nephew.com/sustainability STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 259 |
| Term Meaning ADR In the US, the Company’s ordinary shares are traded in the form of American Depositary Shares evidenced by American Depositary Receipts (ADRs). ADS In the US, the Company’s ordinary shares are traded in the form of American Depositary Shares (ADSs). Arthroscopic Enabling Technologies (AET) A product group which includes a variety of technologies such as fluid management equipment for surgical access, high definition cameras, digital image capture, scopes, light sources and monitors to assist with visualisation inside the joints, radio frequency, electromechanical and mechanical tissue resection devices, and hand instruments for removing damaged tissue. Advanced Wound Bioactives (AWB) A product group which includes biologics and other bioactive technologies that provide unique approaches to debridement and dermal repair/regeneration, and regenerative medicine products including skin, bone graft and articular cartilage substitutes. Advanced Wound Care (AWC) A product group which includes products for the treatment and prevention of acute and chronic wounds, including leg, diabetic and pressure ulcers, burns and post-operative wounds. Advanced Wound Devices (AWD) A product group which includes traditional and single-use Negative Pressure Wound Therapy, a patient monitoring system for pressure injury prevention and patient mobility monitoring, and hydrosurgery systems. AGM Annual General Meeting of the Company. Arthroscopy Endoscopy of the joints is termed ‘arthroscopy’, with the principal applications including the knee and shoulder. ASC Ambulatory Surgery Center. Basis Point One hundredth of one percentage point. Chronic wounds Chronic wounds are those with long or unknown healing times including leg ulcers, pressure sores and diabetic foot ulcers. Company Smith & Nephew plc or, where appropriate, the Company’s Board of Directors, unless the context otherwise requires. Companies Act Companies Act 2006, as amended, of England and Wales. Emerging Markets Emerging Markets include Latin America, Asia (excluding Japan), Middle East, Africa and Russia. EPSA Adjusted earnings per ordinary share as defined on page 246. Endoscopy Through a small incision, surgeons are able to see inside the body using a monitor and identify and repair defects. ENT Ear, Nose and Throat. Established Markets Established Markets are United States of America, Europe, Australia, New Zealand, Canada and Japan. Euro or € References to the common currency used in the majority of the countries of the European Union. FDA US Food and Drug Administration. Financial statements Refers to the consolidated Group Accounts of Smith & Nephew plc. FTSE 100 Index of the largest 100 listed companies on the London Stock Exchange by market capitalisation. Group or Smith+Nephew Used for convenience to refer to the Company and its consolidated subsidiaries, unless the context otherwise requires. Health economics A branch of economics concerned with issues related to efficiency, effectiveness, value and behaviour in the production and consumption of health and healthcare. Hip Implants A product group which includes specialist products for reconstruction of the hip joint. IFC Inside Front Cover. IBC Inside Back Cover. Term Meaning IFRS International Financial Reporting Standards issued by the International Accounting Standards Board. Knee implants A product group which includes an innovative range of products for specialised knee replacement procedures. LSE London Stock Exchange. MDR Medical Device Regulation. MHRA The Medicines and Healthcare products Regulatory Agency in the UK. Negative Pressure Wound Therapy (NPNT) A technology used to treat chronic wounds such as diabetic ulcers, pressure sores and post-operative wounds through the application of sub-atmospheric pressure to an open wound. NHS The UK National Health Service. NYSE New York Stock Exchange. Orthopaedic products Orthopaedic reconstruction products include joint replacement systems for knees, hips and shoulders and support products such as computer-assisted surgery and minimally invasive surgery techniques. Orthopaedic trauma devices are used in the treatment of bone fractures including rods, pins, screws, plates and external frames. Other Reconstruction A product group which includes robotics-assisted surgery, bone cement and accessory products. OXINIUM OXINIUM material is an advanced load bearing technology. It is created through a proprietary manufacturing process that enables zirconium to absorb oxygen and transform to a ceramic on the surface, resulting in a material that incorporates the features of ceramic and metal. Management believes that OXINIUM material used in the production of components of knee and hip implants exhibits unique performance characteristics due to its hardness, low-friction and resistance to roughening and abrasion. Parent Company Smith & Nephew plc. Pound Sterling, Sterling, £, pence or p References to UK currency. 1p is equivalent to one hundredth of £1. SEC US Securities and Exchange Commission. Sports Medicine Joint Repair Sports Medicine Joint Repair includes instruments, technologies and implants necessary to perform minimally invasive surgery of joints. Trading results Trading profit, trading profit margin (trading profit expressed as a percentage of revenue), trading cash flow and trading profit to trading cash conversion ratio (trading cash flow expressed as a percentage of trading profit) are trend measures, which present the profitability of the Group. The adjustments made exclude the impact of specific transactions that management considers affect the Group’s short-term profitability and cash flows, and comparability of results. Refer to page 245 for further information. Trauma & Extremities A product group which includes internal and external devices used in the stabilisation of severe fractures and deformity correction procedures. UK United Kingdom of Great Britain and Northern Ireland. Underlying growth Growth after adjusting for the effects of currency translation and the inclusion of the comparative impact of acquisitions and exclusion of disposals. US United States of America. US Dollars, $, or cents or ¢ References to US currency. 1 cent is equivalent to one hundredth of US$1. Unless the context indicates otherwise, the following terms have the meanings shown below: Glossary 260 Smith+Nephew Annual Report 2023 |
| Accounting policies 176–226 Accounts presentation 255 Acquisitions 9, 10, 17–19, 20–23, 29, 42, 65, 69, 75, 82, 87, 98, 224–226, 246–248 Acquisition and disposal related items 18, 182, 190, 246–248 American Depositary Shares 249 Articles of Association 253–255 Audit fees 117, 185 Board 90–93 Business overview 2–3, 235 Business segment information 34–45, 179–183 Cash and borrowings 202–204 Chair’s statement 4–7 Chief Executive Officer’s review 8–11 Company balance sheet 227 Company notes to the accounts 229–234 Contingencies 211–213, 231 Critical judgements and estimates 177–178 Cross-reference to Form 20-F 257 Currency fluctuations 242–243 Currency translation 178–179 Deferred taxation 188–189 Directors’ Remuneration Report 121–154 Directors’ responsibility statement 156 Dividends 21, 222 Earnings per share 21, 172, 189–190 Employee share plans 226 Executive team 94–95 Factors affecting results of operations 243 Financial instruments 205–211 Financial review 20–23 Free cash flow 247 Glossary of terms 260 Goodwill 193–195 Group balance sheet 173 Group cash flow statement 174 Group companies 231–234 Group history 235 Group income statement 172 Group notes to the accounts 176–226 Group overview 2–3, 235 Group statement of changes in equity 175 Group statement of comprehensive income 172 Independent auditor’s report 157–171 Intangible assets 195–197 Intellectual property disputes 213 Interest and other finance costs 185 Inventories 199 Investments 197 Investment in associates 197–198 Key Performance Indicators 18–19 Legal and other 183, 247 Legal proceedings 212–213 Leverage ratio 247 Liquidity and capital resources 22, 203 Manufacturing and quality 32–33 Medical education 2, 7, 30–31 Net debt 202 New accounting standards 176 Operating profit 183–184 Other finance costs 185 Our approach to stakeholders 82, 82–87 Our global markets 35, 39, 43 Outlook and trend information 7, 23, 237–241 People/Employees 54 Post balance sheet events 226 Provisions 212–213 Property, plant and equipment 191–192 Regulation 15, 33, 53, 70 Related party transactions 226, 235 Research & development 75 Restructuring and rationalisation expenses 182, 246–247 Retirement benefit obligations 214–220 Return on invested capital (ROIC) 18, 248 Risk factors 237–243 Risk report 67–78 SASB reporting 258–259 Share-based payments 226 Share capital 221 Shareholder information 248–256 Staff costs and employee numbers 185 Stakeholder statement 82–87 Statement of compliance 88 Strategy for Growth 8–11 Sustainability 52–66 Taxation 186–189 Taxation information for shareholders 251–253 TCFD reporting 60–64 Total shareholder return 152 Trade and other payables 201 Trade and other receivables 200–201 Treasury shares 221–222 Index STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 261 |
| References from Innovation (pages 26–33) 1 Iriuchishima T, Ryu K. A Comparison of Rollback Ratio between Bicruciate Substituting Total Knee Arthroplasty and Oxford Unicompartmental Knee Arthroplasty. J Knee Surg. 2018;31(6):568–572. 2 Murakami K, Hamai S, Okazaki K, et al. Knee kinematics in bi-cruciate stabilized total knee arthroplasty during squatting and stair climbing activities. J Orthop. 2018;15(2):650–654. 3 Nodzo SR, Carroll KM, Mayman DJ. The Bicruciate Substituting Knee Design and Initial Experience. Techniques in Orthopaedics. 2018;33(1):37–41 4 Murakami K, Hamai S, Okazaki K, et al. In vivo kinematics of gait in posterior-stabilized and bicruciate-stabilized total knee arthroplasties using image-matching techniques. Int Orthop. 2018;42(11):2573–2581. 5 Mayman DJ, Patel AR, Carroll KM. Hospital related clinical and economic outcomes of a bicruciate knee system in total knee arthroplasty patients. Poster presented at: ISPOR Symposium; May 19–23, 2018; Baltimore, Maryland, USA. 6 Takubo A, Ryu K, Iriuchishima T, Tokuhashi Y. Comparison of muscle recovery following bicruciate substituting versus posterior stabilized total knee arthroplasty in an Asian population. J Knee Surg. 2017;30(7):725–729. 7 Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function? Clin Orthop Relat Res. 2005(431):157–165 8 Collins M, Lavigne M, Girard J, Vendittoli PA. Joint perception after hip or knee replacement surgery. Orthop Traumatol Surg Res.2012;98:275–280. 9 Rashid MS, Cooper C, Cook J, et al. Increasing age and tear size reduce rotator cuff repair healing rate at 1 year. Acta Orthop. 2017;88:606–611. 10 Data from a recent study, Mitchell RB et. al. Clinical practice guideline: Tonsillectomy in children (Update). Otolaryngology – Head and Neck Surgery. 2019;160(1 Suppl):S1–S42. 11 National Healthcare Safety Network report on surgical site infections, January 2023 12 SmartTrak Report, 2023 * Compared to non-JOURNEY™ II knees; Based on BCS evidence ** Based on BSC evidence References from Orthopaedics (pages 34–37) 1 Smith+Nephew. Evidence Outcomes Report EO.TRA. PCS001.v1. 2021. 2 Quartley M, Chloros G, Papakostidis K, Saunders C, Giannoudis PV. Stabilisation of AO OTA 31-A unstable proximal femoral fractures: Does the choice of intramedullary nail affect the incidence of post-operative complications? A systematic literature review and meta-analysis. Injury. 2022;53(3):827–840 3 Iriuchishima T, Ryu K. A Comparison of Rollback Ratio between Bicruciate Substituting Total Knee Arthroplasty and Oxford Unicompartmental Knee Arthroplasty. J Knee Surg. 2018;31(6):568–572. 4 Murakami K, Hamai S, Okazaki K, et al. Knee kinematics in bi-cruciate stabilized total knee arthroplasty during squatting and stair climbing activities. J Orthop. 2018;15(2):650–654. 5 Yayac M, Harrer S, Hozack WJ, Parvizi J, Courtney M. The use of cementless components does not significantly increase procedural costs in total knee arthroplasty. J Arthroplasty. 2020;35:407–712. 6 Nodzo SR, Carroll KM, Mayman DJ. The Bicruciate Substituting Knee Design and Initial Experience. Techniques in Orthopaedics. 2018;33(1):37–41 7 Grieco TF, Sharma A, Dessinger GM, Cates HE, Komistek RD. In Vivo Kinematic Comparison of a Bicruciate Stabilized Total Knee Arthroplasty and the Normal Knee Using Fluoroscopy. J Arthroplasty. 2018;33(2):565–571. 8 Murakami K, Hamai S, Okazaki K, et al. In vivo kinematics of gait in posterior-stabilized and bicruciate-stabilized total knee arthroplasties using image-matching techniques. Int Orthop. 2018;42(11):2573–2581. 9 Smith LA, Nachtrab J, LaCour M, et al. In Vivo Knee Kinematics: How Important Are the Roles of Femoral Geometry and the Cruciate Ligaments? J Arthroplasty. 2021;36:1445–1454. 10 Parikh A, Hill P, Pawar V, Sprague J. Long-term Simulator Wear Performance of an Advanced Bearing Technology for THA. Poster presented at: 2013 Annual Meeting of the Orthopaedic Research Society. Poster no. 1028 11 Papannagari R, Hines G, Sprague J, Morrison M. Long-term wear performance of an advanced bearing technology for TKA. Poster presented at: 2011 Annual Meeting of the Orthopaedic Research Society. Poster no. 1141. 12 National Joint Registry for England, Wales, Northern Ireland and the Isle of Man: 20th Annual Report. 2023. 13 Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2022 Annual Report. Adelaide: AOA, 2022. 14 Peters RM, Van Steenbergen LN, Stevens M, Rijk PC, Bulstra SK, Zijlstra WP. The effect of bearing type on the outcome of total hip arthroplasty. Acta Orthop. 2018:89;163–169. 15 Atrey A, Ancarani C, Fitch D, Bordini B. Impact of bearing couple on long-term component survivorship for primary cementless total hip replacement in a large arthroplasty registry. Poster presented at: Canadian Orthopedic Association; June 20–23, 2018; Victoria, British Columbia, Canada. 16 Davis ET, Pagkalos J, Kopjar B. Bearing surface and survival of cementless and hybrid total hip arthroplasty in the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man. JBJS OA. 2020;5:e0075. 17 Smith+Nephew 2020. NAVIO Technical Specification Comparison. March 2020. Internal Report ER0488 REVB. 18 Smith+Nephew 2020. Comparison of operating room footprint for robotic-assisted knee arthroplasty systems. Internal Report. EO.REC.PCS015.002.v1. 19 Gregori A, Picard F, Bellemans J, Smith JR, Simone A. Handheld Precision Sculpting Tool for Unicondylar Knee Arthroplasty. A Clinical Review. Poster presented at: 15th EFORT Congress; 4–6 June, 2014; London, UK. 20 Bollars P, Boeckxstaens A, Mievis J, Janssen D. The Learning Curve and Alignment Assessment of an Image-Free Handheld Robot in TKA: The First Patient Series in Europe. Poster presented at: 19th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery 2019; New York, USA. 21 Kopjar B, Schwarzkopf R, Chow J, et al. NAVIO Robotic Assisted Surgical System for Total Knee Arthroplasty Using JOURNEY II Guided-Motion Total Knee System. Poster presented at: ISTA 2–5 October, 2019; Toronto, Canada. 22 Geller JA, Rossington A, Mitra R, Jaramaz B, Khare R, Netravali NA. Rate of learning curve and alignment accuracy of an image-free handheld robot for total Knee Arthroplasty. European Knee Society Arthroplasty Conference;2019; Valencia, Spain. 23 Ponzio DY, Lonner JH. Preoperative Mapping in Unicompartmental Knee Arthroplasty Using Computed Tomography Scans Is Associated with Radiation Exposure and Carries High Cost. J Arthroplasty. 2015;30(6):964–967 24 Smith+Nephew 2022. Optimus TKA Tensioner Gap Assessment Verification Report. Internal Report. 10059269. 25 Smith+Nephew 2021. Tensioner Design Verification Test Report. Internal Report. TR100123 26 Smith+Nephew 2022. Tensioner KPC: Tensioner Calibration Check. Internal Report. TR100116, Rev.B 27 Smith+Nephew 2023. 37753 V2 CORI Digital Tensioner Evidence in focus White paper 0923. 28 Smith+Nephew 2023. Surgical tray and instrumentation data collection for conventional and robotic knee surgeries. Clinical Activity Report. 29 Seyler MT. Revision total knee arthroplasty with an imageless, 2nd generation robot system. Podium Presentation at: 2023 Members Meeting of The Knee Society; September 7–9, 2023; Monterey, California, US. 30 Mayman DJ, Patel AR, Carroll KM. Hospital related clinical and economic outcomes of a bicruciate knee system in total knee arthroplasty patients. Poster presented at: ISPOR Symposium; May 19–23, 2018; Baltimore, Maryland, USA. 31 Takubo A, Ryu K, Iriuchishima T, Tokuhashi Y. Comparison of muscle recovery following bicruciate substituting versus posterior stabilized total knee arthroplasty in an Asian population. J Knee Surg. 2017;30(7):725–729. 32 Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function? Clin Orthop Relat Res. 2005(431):157–165. 33 The Orthopaedic Data Evaluation Panel (ODEP). www.odep.org.uk. Accessed 1 December 2023. 34 Smith+Nephew 2023. AETOS Inlay Design Features. Internal Report. ER-04-0990-0017. 35 Arenas-Miquelez A, Murphy R, Rosa A, Caironi D, Zumstein M. Impact of humeral and glenoid component variations on range of motion in reverse geometry total shoulder arthroplasty. A standardised computer model study. (8214). Swiss Medical Weekly. 2020;150(SUPPL 244):2S. 36 Kalouche I, Sevivas N, Wahegaonker A, Sauzieres P, Katz D, Valenti P. Reverse shoulder arthroplasty: Does reduced medialisation improve radiological and clinical results? Acta Orthopaedica Belgica. 2009;75(2):158–166. 37 Lädermann A, Tay E, Collin P, et al. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res. 2019;8(8):378–386. 38 Harmer L, Throckmorton T, Sperling JW. Total shoulder arthroplasty: are the humeral components getting shorter? Curr Rev Musculoskelet Med. 2016;9(1):17–22. 39 SmartTrak Report, 2023. Accessed 1 December 2023. * Based on BSC evidence ** We thank the patients and staff of all the hospitals in England, Wales and Northern Ireland who have contributed data to the National Joint Registry. We are grateful to the Healthcare Quality Improvement Partnership (HQIP), the NJR Steering Committee and staff at the NJR Centre for facilitating this work. The views expressed represent those of the authors and do not necessarily reflect those of the National Joint Registry Steering Committee or the Health Quality Improvement Partnership (HQIP) who do not vouch for how the information is presented. *** Compared to NAVIO™ Handheld Robotics **** Compared to Mako and ROSA ***** With use of handpiece † All data from US-based CORI system surgeons (rTKa, n=8). †† Cost savings estimated based on single surgeon, single center experience and may not be representative. Savings based on surgical tray sterilisation cost reductions and decreased OR Time. Average sterilization cost of $58.18 per tray, with a reduction from 13 to 4 trays per case. OR time decreased by average 25 minutes/case, with OR time estimated to cost $40/minute. ††† Compared to conventional techniques. †††† Compared to the NAVIO◊ Surgical System and previous software versions. 29% faster resection demonstrated in total knee cadaver studies 2005 ASM International Engineering Materials Achievement Award References from business unit sections 262 Smith+Nephew Annual Report 2023 |
| 35 Roje Z, Racic G, Dogas Z, Pesutić Pisac V, Timms M. Postoperative morbidity and histopathologic characteristics of tonsillar tissue following coblation tonsillectomy in children: A prospective randomized single-blind study. Coll Antropol. 2009;33:293–298. 36 Smith+Nephew 2010. PROCISE LW & MLW, Thermal Measurement and Comparison to CO2 and KTP Laser Systems. Internal Report. P/N 86257 Rev. A. 37 Smith+Nephew 2010. PROcise XP Comparative Thermal Measurement Bench-Top Study. Internal Report. P/N 60736–01 Rev. A. 38 Magdy EA, Elwany S, El-Daly AS, Abdel-Hadi M, Morshedy MA. Coblation tonsillectomy: A prospective, double-blind, randomised, clinical and histopathological comparison with dissection-ligation, monopolar electrocautery and laser tonsillectomies. J Laryngol Otol. 2008;122:282–290. 39 Sedgwick MJ, Saunders C, Bateman N. Intracapsular Tonsillectomy Using Plasma Ablation Versus Total Tonsillectomy: A Systematic Literature Review and Meta-Analysis. OTO open. 2023;7(1):e22. 40 Smith+Nephew 2023.ARIS Targeted Hemostasis. Internal Memo. 10094398 Rev A. 41 Lustig LR, Ingram A, Vidrine M, et. al. In-Office Tympanostomy Tube Placement in Children Using Iontophoresis and Automated Tube Delivery. Laryngoscope. 2020;130:S1–S9, 2020. 42 IFU007011, available at www.tulatubes.com/IFU 43 Adam J. Singer, MD Michelle Blanda, MD, Kerry Cronin, MD, et al. Comparison of Nasal Tampons for the Treatment of Epistaxis in the Emergency Department: A Randomized Controlled Trial. The American College of Emergency Physicians. Volume 45, no. 2 : February 2005. doi:10.1016/j. annemergmed. 2004.10.002 * Compared to predicate device. ** The REGENETEN Implant is cleared for use on any tendon where there is not substantial loss of tendon tissue. REGENETEN Bone Anchors are only indicated for use in rotator cuff repair. Published clinical outcomes are for rotator cuff. The REGENETEN Implant is currently approved for use in treating Gluteus Medius and Achilles tears only in the US. *** As demonstrated ex vivo **** Demonstrated clinically and in vivo ***** As compared to mechanical debridement for knee chondroplasty; n=60; p<0.001 References from Advanced Wound Management (pages 42–45) 1 National Scorecard on Hospital-Acquired Conditions, Agency for Healthcare Research and Quality (AHRQ). January 2019 update. AHRQ National Scorecard on Hospital-Acquired Conditions Updated Baseline Rates and Preliminary Results 2014–2017. 2 Schutt SC, Tarver C, Pezzani M. Pilot study: Assessing the effect of continual position monitoring technology on compliance with patient turning protocols. Nurs Open. 2017;5(1):21–28. 3 Pickham D, Berte N, Pihulic M, Valdez A, Mayer B, Desai M. Effect of a wearable patient sensor on care delivery for preventing pressure injuries in acutely ill adults: A pragmatic randomized clinical trial (LS-HAPI study). Int J Nurs Stud. 2018;80:12–19. 4 Klaeb M, Krafft K, Walters B, Lowe J, Cooley A. The Influence of Wearable Technology on Nursing Attitudes and Adherence to Patient Turning and Repositioning. Poster presented at: Patient Handling and Mobility Annual Conference; March 5–March 7, 2019; Orlando, Florida, USA. 5 Forni C, D’alessandro F, Gallerani P, et al. Effectiveness of using a new polyurethane foam multi-layer dressing in the sacral area to prevent the onset of pressure ulcer in the elderly with hip fractures: A pragmatic randomised controlled trial. Int Wound J. 2018;15(3):1–8. 6 Smith+Nephew 2018.Pressure Redistribution Testing of ALLEVYN Life vs Mepilex Border and Optifoam Gentle SA. Internal Report. DS/18/351/R45. References from Sports Medicine & ENT (pages 38–41) 1 Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J 2016;6(1):16–25. 2 Arnoczky SP, Bishai SK, Schofield B, Sigman S, Bushnell BD, Hommen JP, Van Kampen C. Histologic Evaluation of Biopsy Specimens Obtained 3 Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg. 2018 27(2):242–251. 4 Bokor DJ, Sonnabend DH, Deady L, et al. Healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a highly porous collagen implant: a 5-year clinical and MRI follow-up. Muscles, Ligaments Tendons J. 2019;9(3):338–347. 5 Van Kampen C, Arnoczky S, Parks P, et al. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep. Muscles Ligaments Tendons J. 2013;3(3):229–235. 6 McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA, 3rd. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491–500. 7 Ruiz Iban MA et al. The effect on healing rate of the addition of a bioinductive implant to a rotator cuff repair. The results of a randomized controlled trial in 124 subjects. ISAKOS 2023. 8 Chahla J, Liu JN, Manderle B, et al. Bony ingrowth of coil-type open-architecture anchors compared with screw-type PEEK anchors for the medial row in rotator cuff repair: a randomized controlled trial. Arthroscopy. 2019 Dec 3. 2020;36(4):952–961. Epub ahead of print 9 Smith+Nephew 2021. Technical Report, HEALICOIL Implant Volume Comparison. Internal Report. 15010823 Rev A 10 Vonhoegen J, John D, Hägermann C. Osteoconductive resorption characteristics of a novel biocomposite suture anchor material in rotator cuff repair. Orthop Traumatol Surg Res. 2019;14(1):12. 11 Smith+Nephew 2010. Micro-CT and histological evaluation of specimens from resorbable screw study (RS-II/OM1-08) 24-month post-implantation. Internal Report WRP-TE045-700-08. 12 Smith+Nephew 2016. Healicoil Regenesorb Suture Anchor – a study to assess implant replacement by bone over a 2 year period. NCS248. 13 McIntyre LF, McMillan S, Trenhaile SW, Bishai SK, Bushnell BD. Full-Thickness Rotator Cuff Tears Can Be Safely Treated With Resorbable Bioinductive Bovine Collagen Implant: One-Year Results of a Prospective, Multicenter Registry. Arthrosc Sports Med Rehabil. 2021 Aug 20;3(5):e1473– e1479. 14 Bushnell BD, Connor P, Harris HW, Ho CP, Trenhaile SW, Abrams JS. Two-year outcomes with a bioinductive collagen implant used in augmentation of arthroscopic repair of full-thickness rotator cuff tears: Final results of a prospective multi-center study. J Shoulder Elbow Surg. 2022 Jul 1:S1058–2746. 15 Micheloni GM, Salmaso G, Zecchinato G, Giaretta S, Barison E, Momoli A. Bio-inductive implant for rotator cuff repair: our experience and technical notes. Acta Biomed. 2020 Dec 30;91(14–S). 16 Thon SG, O’Malley L 2nd, O’Brien MJ, Savoie FH 3rd. Evaluation of Healing Rates and Safety With a Bioinductive Collagen Patch for Large and Massive Rotator Cuff Tears: 2-Year Safety and Clinical Outcomes. Am J Sports Med. 2019 Jul;47(8):1901–1908. 17 Camacho-Chacon JA, Cuenca-Espierrez J, Roda-Rojo V, Martin-Martinez A, Calderon-Meza JM, Alvarez-Alegret R, Martin-Hernandez C. Bioinductive collagen implants facilitate tendon regeneration in rotator cuff tears. J Exp Orthop. 2022 Jun 8;9(1):53. 18 Bushnell BD, Bishai SK, Krupp RJ, McMillan S, Schofield BA, Trenhaile SW, McIntyre LF. Treatment of Partial-Thickness Rotator Cuff Tears With a Resorbable Bioinductive Bovine Collagen Implant: 1-Year Results From a Prospective Multicenter Registry. Orthop J Sports Med. 2021 Aug 13;9(8). 19 Dai A, Campbell A, Bloom D, Baron S, Begly J, Meislin R. Collagen-Based Bioinductive Implant for Treatment of Partial Thickness Rotator Cuff Tears. Bull Hosp Jt Dis (2013). 2020 Sep;78(3):195–201. 20 Schlegel TF, Abrams JS, Angelo RL, Getelman MH, Ho CP, Bushnell BD. Isolated bioinductive repair of partial-thickness rotator cuff tears using a resorbable bovine collagen implant: two-year radiologic and clinical outcomes from a prospective multicenter study. J Shoulder Elbow Surg. 2021 Aug;30(8):1938–1948. 21 Yeazell S, Lutz A, Bohon H, Shanley E, Thigpen CA, Kissenberth MJ, Pill SG. Increased stiffness and reoperation rate in partial rotator cuff repairs treated with a bovine patch: a propensity-matched trial. J Shoulder Elbow Surg. 2022 Jun;31(6S):S131–S135. 22 Bokor DJ, Sonnabend D, Deady L et al. Preliminary investigation of a biological augmentation of rotator cuff repairs using a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J. 2015;5(3):144–150. 23 McIntyre L, Bishai SK, Brown PB, Bushnell BD, Trenhaile SW. Patient-Reported Outcomes Following Use of a Bioabsorbable Collagen Implant to Treat Partial and Full-Thickness Rotator Cuff Tears. Arthroscopy. 2019 35(8):2262–2271. 24 Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J. 2016;6(1):16–25. 25 Bokor DJ, Sonnabend D, Deady L et al. Preliminary investigation of a biological augmentation of rotator cuff repairs using a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J. 2015;5(3):144–150. 26 Smith+Nephew 2019. An overview of the outcomes associated with the standard of care for the surgical treatment of rotator cuff tears. Internal Report EO/SPM/ REGENETEN/005/v1. 27 Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear Rates After Arthroscopic Single-Row, Double-Row, and Suture Bridge Rotator Cuff Repair at a Minimum of 1 Year of Imaging Follow-up: A Systematic Review. Arthroscopy. 2015;31(11):2274–2281. 28 Bushnell BD, Connor PM, Harris HW, Ho CP, Trenhaile SW, Abrams JS. Retear rates and clinical outcomes at 1 year after repair of full-thickness rotator cuff tears augmented with a bioinductive collagen implant: a prospective multicenter study. JSES Int. 2021;5(2):228–237 29 Konan S, Haddad F. Outcomes of Meniscal Preservation Using All-inside Meniscus Repair Devices. Clin Orthop Relat Res. 2010;468:1209–1213. 30 ArthroCare 2014.Comparative Performance of the FLOW 50 Wand and the Predicate Wands in Tissue Models. P/N 52918-01. 31 Spahn G, Kahl E, Muckley T, Hofmann GO, Klinger HM. Arthroscopic knee chondroplasty using a bipolar radiofrequency-based device compared to mechanical shaver: results of a prospective, randomized, controlled study. Knee Surg Sports Traumatol Arthrosc. 2008;16(6):565–573. 32 Smith+Nephew 2017. Coblation Dissection Versus Monopolar Dissection – A Systematic Review and Meta-analysis P/N 91999 Rev. A. 33 Temple RH, Timms MS. Paediatric coblation tonsillectomy. Int J Pediatr Otorhinolaryngol. 2001;61(3):195–198. 34 Smith+Nephew 2010. Temperature Study – PEAK PlasmaBlade® TnA and Covidien EDGE®. Internal Report. PN 86791 Rev. 1. STRATEGIC REPORT GOVERNANCE ACCOUNTS OTHER INFORMATION Smith+Nephew Annual Report 2023 263 |
| 7 Smith+Nephew 2019. Properties of ALLEVYN LIFE advanced wound care dressing that can contribute to the effective use as part of a Pressure Injury Prevention protocol. Internal Report. RD/19/177. 8 European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. Emily Haesler (Ed.) EPUAP/NPIAP/PPPIA: 2019. 9 State of the world’s nursing 2020: investing in education, jobs and leadership. Geneva: World Health Organization; 2020. 10 Moore, Z and Probst, S. Building the business case for shared wound care: A cost-benefit case for service providers. Wounds International 2023. 2023; 4(3) 10–15. 11 Moore Z et al. Wounds International. 2022;13(2):32–8. 12 USC University of Southern California. What Does Self-Care Mean for Individuals With Diabetes? Accessed May 19, 2022. https://nursing.usc.edu/blog/self-care-with-diabetes/ 13 di Gesaro A. Self-care and patient empowerment in stoma management. Gastrointestinal Nursing. 2012;10(2):19–23. 14 Spinks J. Self care in urinary incontinence. SelfCare. 2011;2(6):160–166. 15 Smith+Nephew 2016.Wound Model Testing of New ALLEVYN◊ Life Gen2 wcl Dressing using Horse Serum at a Flow Rate Modelling that of a Moderately Exuding Wound. DS/14/303/R. 16 Smith+Nephew 2016. Product Performance of Next Generation ALLEVYN Life Internal Report. (HVT080) GMCA-DOF/08. 17 Lisco C. Evaluation of a new silicone gel-adhesive hydrocellular foam dressing as part of a pressure ulcer prevention plan for ICU patients. In: WOCN; 2013. 18 Rossington A, Drysdale K, Winter R. Clinical performance and positive impact on patient wellbeing of ALLEVYN Life. Wounds UK. 2013;9(4):91–95. 19 Stephen-Haynes J, Bielby A, Searle R. The clinical performance of a silicone foam in an NHS community trust. Journal of Community Nursing. 2013;27(5):50–59. 20 Simon D, Bielby A. A structured collaborative approach to appraise the clinical performance of a new product. Wounds UK. 2014;10(3):80–87. 21 Smith+Nephew 2012. Simulated Wound Model Testing of ALLEVYN Life and Mepilex Border. Internal Report. DS/12/130/DOF. 22 Smith+Nephew. Subjective comparison of masking ability of the New ALLEVYN LIFE versus Current ALLEVYN LIFE by Healthcare Professionals. Internal Report. 2016; DS/16/061/R. 23 Smith+Nephew 2023. Calculation of global packaging comparison for ALLEVYN Dressings compared to leading competitors. Internal report CSD.AWM.23.010. 24 Smith+Nephew 2016.New ALLEVYN Life Gen2 wcl – Physical Testing. Internal Report. DS/15/025/R. 25 Smith+Nephew 2018. Use of Moisture Vapour Permeability* (MVP) and Moisture Vapour Transmission Rate** (MVTR) data to support product claims referring to moist wound healing. Internal Report. EO.AWM.PCSgen.001.v2. 26 Rossington A, Drysdale K, Winter R. Clinical performance and positive impact on patient wellbeing of ALLEVYN Life. Wounds UK. 2013;9(4):91–95. 27 Smith+Nephew 20 June 2016.A Randomised Cross-Over Clinical Evaluation to Compare Performance of ALLEVYN™ Life and Mepilex® Border Dressings on Patient Wellbeing-Related Endpoints. Internal Report. CE/047/ALF. 28 Smith+Nephew 14 June 2012. Odour reducing properties of ALLEVYN Life. Internal Report. DS/12/127/DOF. 29 Smith+Nephew 2021. Internal Report. EA/AWM/ ALLEVYN/001v4. 30 Smith+Nephew. Internal Report. 151008. 31 Nherera LM et al. Wound Repair Regen. 2017;25(4):707–721. 32 Smith+Nephew. Internal Report. RR-WMP07330-10-03. 33 Skog E et al. British Journal of Dermatology. 1983;109:77–83. 34 Fitzgerald DJ et al. Wound Repair Regen. 2016;25(1):13–24. 35 Roche ED, Woodmansey EJ, Yang Q, et al. Cadexomer iodine effectively reduces bacterial biofilm in porcine wounds ex vivo and in vivo. Int Wound J. 2019;16(3):674–83. 36 Smith+Nephew 2007. Antimicrobial Activity of Allevyn Ag Non-Adhesive Dressing against a Broad Spectrum of Microorganisms. Internal Report. DOF 0703006. 37 Smith+Nephew 2007. Antimicrobial activity of ALLEVYN Ag dressings against a broad spectrum of wound pathogens using a dynamic shake flask method. Internal Report. DOF 0707052. 38 Smith+Nephew 2008. A multi-centre in-market evaluation of ALLEVYN Ag dressings. Internal Report. SR/CIME/009. 39 Smith+Nephew 2018. PMCF Research for Allevyn Ag Adhesive. Internal Report. PMS-273-01. 40 Smith+Nephew 2007. Antimicrobial Activity of ALLEVYN Ag Adhesive Dressing Against a Broad Spectrum of Microorganisms. Internal Report. DOF 0703007. 41 Lavery et al. Int Wound J. 2014; 11(5): 554–560. 42 McGinness K, Kurtz Phelan DH. Wounds. 2018; 30(4): 90–95. 43 Nherera et al. Ostomy Wound Manage. 2017;63(12):38–47. 44 Dowsett C, Hampton K, Myers D, Styche T. Use of PICO to improve clinical and economic outcomes in hard-to-heal wounds. Wounds International. 2017;8(2):5258 45 Saunders C, Nherera LM, Horner A, Trueman P. Single-Use negative-pressure wound therapy versus conventional dressings for closed surgical incisions: systematic literature review and meta-analysis. BJS Open. 2021;0(0):1–8. 46 Gilchrist B, Robinson M, Jaimes H. Performance, safety, and efficacy of a single use negative pressure wound therapy system for surgically closed incision sites and skin grafts: A prospective multi-centre follow-up study. Paper presented at: SAWC; 2020; Virtual. 47 Hurd T, Trueman P, Rossington A. Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series. Ostomy Wound Manage. 2014;60(3):30–36. 48 Forlee M, van Zyl L, Louw V, Nel J, Fourie N, Hartley R. A randomised controlled trial to compare the clinical efficacy and acceptability of adjustable intermittent and continuous Negative Pressure Wound Therapy (NPWT) in a new portable NPWT system. Paper presented at: EWMA; 2018; Krakow, Poland. 49 Forlee M, Richardson J, Rossington A, Cockwill J, Smith J. An interim analysis of device functionality and usability of RENASYS TOUCH – a new portable Negative Pressure Wound Therapy (NPWT) system. Paper presented at: Wounds UK; 2016; Harrogate, UK. 50 Smith+Nephew 2022. RENASYS EDGE System Human Factors Summative Report Summary. Internal Report. CSD.AWM.22.071. 51 Smith+Nephew 2022. Summary of footprint, portability, wearability, weight and audible noise for the RENASYS EDGE system. Internal Report. CSD.AWM.22.067. 52 Smith+Nephew 2022. Summary of RENASYS EDGE pump mechanical and electronic reliability testing. Internal Report. CSD.AWM.22.069. 53 Smith+Nephew 2022. Summary of RENASYS EDGE pump cleaning, self-test and maintenance. Internal Report. CSD.AWM.22.068. 54 Agency for Healthcare Research and Quality website. Preventing pressure ulcers in hospitals: a toolkit for improving quality of care. Updated October 2014. Accessed February 2021. https://www.ahrq.gov/professionals/ systems/hospital/pressureulcertoolkit/putool1.html 55 Wassel C, Delhougne G, Gayle J et al. Risk of readmissions, mortality, and hospital-acquired conditions across hospital-acquired pressure injury (HAPI) stages in a US National Hospital discharge database. Int Wound J. 2020;1–11. * Up to 5 days for the sacral area. ** OASIS is manufactured by Cook Biotech, Inc. ***Compared to baseline trajectory, n=52 wounds; p<0.006. † Compared to care with standard dressings; p<0.00001; meta-analysis of 29 studies (odds ratio (OR): 0.37). †† Between 2014 and 2017 in the US. For detailed product information, including the indications for use, contraindications, effects, precautions and warnings, please consult the product’s Instructions for Use (IFU) prior to use. References from products (page 59) 1 Smith+Nephew 2022. Summary of footprint, portability, wearability, weight and audible noise for the RENASYS EDGE system. Internal Report. CSD.AWM.22.067 2 Smith+Nephew 2022. How the RENASYS EDGE Negative Pressure Wound Therapy System provides continuity of care to the patient Internal Report. EO.AWM. PCS270.003.V1. 3 Smith and Nephew 2022. RENASYS EDGE System Human Factors Summative Report Summary. Internal Report. CSD. AWM.22.071. 4 Smith+Nephew 2022. Summary of RENASYS EDGE pump cleaning, self-test and maintenance. Internal Report. CSD. AWM.22.068. 5 Smith+Nephew 2022. Summary of RENASYS EDGE pump mechanical and electronic reliability testing. Internal Report. CSD.AWM.22.069. 6 When EDGE device operates at a standard -125mmHg with a power consumption of 1.14W/hour vs when TOUCH operates at a standard -120mmHg with a power consumption of 3.75W/hour. 7 Amount of electricity saved by RENASYS EDGE instead of RENASYS TOUCH being equivalent to charging an iPhone 13 Pro over 2,866 times with a 12Wh battery e.g. iPhone13 Pro is 11.97Wh (https://en.wikipedia.org/ wiki/IPhone_13_Pro, accessed Nov 7th 2023) over a 5-year period assuming a RENASYS EDGE utilisation of 30%. 5 years is the device’s lifetime as per design input assuming a 30% utilisation rate equivalent to 13,149 run hours. 8 Over a 5-year period of use in the U.S. assuming a RENASYS EDGE utilisation of 30% and U.S. national Grid intensity of 0.857 lbCO2/kWh. (Source: https://www.epa.gov/system/ files/documents/2023-01/eGRID2021_summary_tables. pdf, accessed Nov 7th 2023). References from business unit sections continued 264 Smith+Nephew Annual Report 2023 |
| Financial calendar Annual General Meeting The Company’s Annual General Meeting (‘AGM’) will be held on Wednesday, 1 May 2024 at 12:00pm at Smith+Nephew Academy London, Building 5, Croxley Park, Hatters Lane, Watford, Hertfordshire, WD18 8YE. Please refer to the Notice of Meeting for detailed information on how to vote and submit your questions. The meeting will commence at 12:00pm with doors opening from 11.00am. Registered shareholders have been sent either a Notice of Annual General Meeting or notification of availability of the Notice of Annual General Meeting. This report was printed by Park Communications, a certified carbon neutral print company, on Magno Satin an FSC® certified paper. The FSC® label on this product ensures responsible use of the world’s forest resources. Park works to the EMAS standard and its Environmental Management System is certified to ISO 14001. This publication has been manufactured using 100% offshore wind electricity sourced from UK wind. 100% of the inks used are vegetable oil based, 95% of press chemicals are recycled for further use and, on average 99% of any waste associated with this production will be recycled and the remaining 1% used to generate energy. This is a climate neutral print product for which carbon emissions have been calculated and offset by supporting recognised carbon offset projects. Designed and Produced by Radley Yeldar. 2024 Annual General Meeting 1 May First quarter Trading Report 1 May Payment of 2023 final dividend 22 May Half-year results announced 1 August1 Third quarter Trading Report 31 October Payment of 2024 interim dividend October/November 2025 Full year results announced February¹ Annual Report available February/March Annual General Meeting April 1 Dividend declaration dates. CBP023542 |
| www.smith-nephew.com Smith & Nephew plc Building 5, Croxley Park, Hatters Lane, Watford, Hertfordshire, WD18 8YE, United Kingdom. Tel. +44 (0)1923 477 100 Registered in England and Wales No. 324357. enquiries@smith-nephew.com www.smith-nephew.com |
|
|
STRATEGIC REPORT |
|
|
GOVERNANCE |
|
|
ACCOUNTS |
|
|
OTHER INFORMATION |
EXHIBIT INDEX
|
Exhibit No. |
Description of Document |
Incorporated Herein by Reference To |
Filed
|
|
|
|
|
|
|
|
|
1 |
|
Form 20-F for the year ended December 31, 2021 filed on March 7, 2022 (File No.1-14978) |
|
|
|
|
|
|
|
|
|
2 |
|
Smith & Nephew plc is not party to any single instrument relating to long-term debt pursuant to which a total amount of securities exceeding 10% of Smith & Nephew plc’s total assets (on a consolidated basis) is authorized to be issued. Smith & Nephew plc hereby agrees to furnish to the SEC, upon its request, a copy of any instrument defining the rights of holders of its long-term debt or the rights of holders of the long-term debt of any of its subsidiaries for which consolidated or unconsolidated financial statements are required to be filed with the SEC |
|
|
|
|
|
|
|
|
|
2 |
(c) |
Exhibit 4.1 to the Form 6-K filed on October 14, 2020 (File No.1-14978) |
|
|
|
|
|
|
|
|
|
2 |
(d) |
Description of securities registered under section 12 of the exchange act |
|
X |
|
|
|
|
|
|
|
4 |
(a) (i) |
Form 20-F for the year ended December 31, 2014 filed on March 5, 2015 (File No.1-14978) |
|
|
|
|
|
|
|
|
|
|
(ii) |
X |
||
|
|
|
|
|
|
|
|
(iii) |
Form 20-F for the year ended December 31, 2019 filed on March 2, 2020 (File No.1-14978) |
|
|
|
|
||||
|
(iv) |
Form 20-F for the year ended December 31, 2019 filed on March 2, 2020 (File No.1-14978) |
|
||
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
Exhibit No. | Description of Document | Incorporated Herein by Reference To | Filed | |
4 | (c) (i) | Form 20-F for the year ended December 31, 2013 filed on March 6, 2014 (File No.1-14978) | ||
(ii) | Form 20-F for the year ended December 31, 2014 filed on March 5, 2015 (File No.1-14978) | |||
(iii) | Form 20-F for the year ended December 31, 2015 filed on March 4, 2016 (File No.1-14978) | |||
(iv) | Form 20-F for the year ended December 31, 2016 filed on March 6, 2017 (File No.1-14978) | |||
(v) | Form 20-F for the year ended December 31, 2012 filed on February 28, 2013 (File No.1-14978) | |||
(vi) | Form 20-F for the year ended December 31, 2012 filed on February 28, 2013 (File No.1-14978) | |||
(vii) | Letter of Appointment of Robin Freestone as Audit Committee Chairman | Form 20-F for the year ended December 31, 2016 filed on March 6, 2017 (File No.1-14978) | ||
(viii) | Form 20-F for the year ended December 31, 2016 filed on March 6, 2017 (File No.1-14978) | |||
(ix) | Form 20-F for the year ended December 31, 2017 filed on March 5, 2018 (File No.1-14978) | |||
(x) | Form 20-F for the year ended December 31, 2017 filed on March 5, 2018 (File No.1-14978) | |||
(xi) | Form 20-F for the year ended December 31, 2017 filed on March 5, 2018 (File No.1-14978) | |||
(xii) | Form 20-F for the year ended December 31, 2017 filed on March 5, 2018 (File No.1-14978) | |||
(xiii) | Form 20-F for the year ended December 31, 2018 filed on March 4, 2019 (File No.1-14978) | |||
(xiv) | Form 20-F for the year ended December 31, 2019 filed on March 2, 2020 (File No.1-14978) | |||
Exhibit No. | Description of Document | Incorporated Herein by Reference To | Filed | |
4 | (c)(xv) | Form 20-F for the year ended December 31, 2019 filed on March 2, 2020 (File No.1-14978) | ||
(xvi) | Form 20-F for the year ended December 31, 2019 filed on March 2, 2020 (File No.1-14978) | |||
(xvii) | Form 20-F for the year ended December 31, 2019 filed on March 2, 2020 (File No.1-14978) | |||
(xviii) | Form 20-F for the year ended December 31, 2019 filed on March 2, 2020 (File No.1-14978) | |||
(xix) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xx) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxi) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxii) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxiii) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxiv) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxv) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxvi) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxvii) | Form 20-F for the year ended December 31, 2020 filed on March 1, 2021 (File No.1-14978) | |||
(xxviii) | Form 20-F for the year ended December 31, 2021 filed on March 7, 2022 (File No.1-14978) | |||
STRATEGIC REPORT | |
GOVERNANCE | |
ACCOUNTS | |
OTHER INFORMATION |
Exhibit No. | Description of Document | Incorporated Herein by Reference To | Filed | |
(xxix) | Form 20-F for the year ended December 31, 2021 filed on March 7, 2022 (File No.1-14978) | |||
(xxx) | Form 20-F for the year ended December 31, 2021 filed on March 7, 2022 (File No.1-14978) | |||
4 | (c)(xxxi) | Form 20-F for the year ended December 31, 2021 filed on March 7, 2022 (File No.1-14978) | ||
(xxxii) | Form 20-F for the year ended December 31, 2022 filed on March 6, 2023 (File No.1-14978) | |||
(xxxiii) | Form 20-F for the year ended December 31, 2022 filed on March 6, 2023 (File No.1-14978) | |||
(xxxiv) | Form 20-F for the year ended December 31, 2022 filed on March 6, 2023 (File No.1-14978) | |||
(xxxv) | X | |||
(xxxvi) | X | |||
(xxxvii) | X | |||
8 | X | |||
12 | (a) | Certification of Deepak Nath filed pursuant to Exchange Act Rule 13a -14(a) | X | |
(b) | Certification of Anne-Francoise Nesmes filed pursuant to Exchange Act Rule 13a -14(a) | X | ||
13 | (a) | X | ||
15.1 | Consent of KPMG LLP, Independent Registered Public Accounting Firm | X | ||
15.2 | X | |||
97 | X | |||
Exhibit No. | Description of Document | Incorporated Herein by Reference To | Filed | |
101.INS | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | |||
101.SCH | XBRL Taxonomy Extension Schema Document | |||
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |||
101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |||
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |||
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |||
104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |||
SIGNATURE
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
Smith & Nephew plc | |
(Registrant) | |
By: | /s/ Helen Barraclough |
Helen Barraclough | |
Company Secretary |
Watford, England
March 11, 2024